Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/11/01. The contractual start date was in January 2014. The draft report began editorial review in October 2014 and was accepted for publication in April 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Pickett et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the underlying health problem
Chronic inflammatory skin diseases are commonly encountered conditions in dermatology. They include commonly reported conditions, such as eczema, psoriasis and acne, which are associated with skin inflammation. This inflammation can range in severity from mild to severe and, in some patients, can have associated health complications. The focus of this report is on chronic inflammatory skin diseases that have lasted for at least 12 weeks and may have caused significant tissue destruction and, potentially, impacted the individual significantly. Our initial scoping of this project identified a number of conditions that come under the term chronic inflammatory skin diseases; however, only the most commonly studied in educational intervention studies are discussed in the background of this report.
Classification of disease
Dermatologists in the UK use a disease classification based on aetiology and anatomical site which has been developed by the British Association of Dermatologists. 1 This is a very detailed and comprehensive system used to obtain information about skin diseases and, as such, has not been described here. Common types of chronic inflammatory skin conditions include eczema (a general term covering a range of conditions, which may or may not be atopic), psoriasis and acne vulgaris (commonly known as acne). There is also a wide range of other types of skin condition such as rosacea, lichen planus, hidradenitis suppurativa, cutaneous lupus erythematosus, lichen sclerosus and seborroeic dermatitis. The working definitions, causes and epidemiology for the three most common conditions are summarised below.
Eczema
Eczema (of which atopic dermatitis eczema is the most common type) is a common skin condition that presents as red, dry, itchy skin, often on the elbow, knee or face, but sometimes all over the body. 2,3 Often associated with atopy (a predisposition to developing hypersensitivity reactions), the predominant symptom is itching. 4 In some people, the skin can weep or blister and become thickened. In the chronic form of the condition there can also be altered skin pigmentation and exaggerated surface markings. 2 Eczema can start at any age, but is most common in children. It is considered to be caused by a combination of genetic and environmental factors. 2 Concurrent illness and psychological factors such as stress can also function as a trigger. 5 For the purpose of this report, we refer to the general term ‘eczema’ unless an individual study specifies that the condition is atopic dermatitis or atopic eczema.
Psoriasis
Psoriasis is a skin disease that is typically characterised by pink or red lesions which are covered with scales. 6 These lesions are well delineated and can vary in extent and shape, and the severity of psoriasis typically follows a relapsing and remitting course. 6,7 The most common form, plaque psoriasis, occurs in approximately 90% of people with the condition. Other types include guttate psoriasis and pustular forms. 7 The cause of psoriasis is thought to be a complex interplay between genetic and environmental factors, with the immune system having an important role in the disease process. 8
Acne vulgaris
Acne vulgaris (commonly known as acne) is a common inflammatory skin disease, which usually starts during puberty. It is characterised by a combination of comedones (blackheads and whiteheads), papules, pustules, nodules and scarring. 9 Genetic and hormonal causes are some of the key factors that trigger the condition. 10
Epidemiology
Scholfied and colleagues1 undertook a health-care needs assessment in 2009 and reported that 55% of the overall population in the UK have had some form of skin disease, and that in previous studies 23–33% of people have had a skin problem that could benefit from medical care (i.e. a moderate or severe condition). This section presents a brief overview of the incidence and prevalence across the different inflammatory skin conditions discussed above.
Eczema
A recent review noted that, although there are several studies considering the epidemiology of atopic eczema in children, a wide range of prevalence estimates are available given differences in study populations, the definitions used and the survey methods applied. 5 It is generally estimated that atopic eczema affects one in every five children in the UK at some stage3 and it is the most commonly diagnosed dermatological disorder in children and adolescents. 11 The prevalence of atopic eczema appears to be increasing, although the reasons for this are unclear. 2,5
A recent guideline5 reported an increasing trend in point prevalence of the skin disease in the 1990s and early 2000s. Numerous studies, mostly of low-level evidence, were reviewed. In this overview, we have focused on those from the UK where available. The point prevalence rates differed between the studies; in five UK-based studies, the guideline reports that this ranged from 5.9% in 3- to 11-year olds to 14.2% in 4-year olds. Trends in point prevalence for those who had ever had eczema increased. 5
One-year period prevalence was reported in two studies; this was reported as 11.5% in 3- to 11-year olds12 and 16.5% in 1- to 5-year olds. 13 In a birth cohort study that followed children until the age of 10 years, it was reported that the period prevalence of atopic eczema was 9.6% at 1 year of age, which increased up to 10.3% at 2 years of age; 11.9% at 4 years and 14.3% at 10 years. 14 In one UK study, period prevalence rates were also reported; these were highest, at 25.6%, for children aged 6–18 months, followed by those aged 18–23 months at 23.2%, those aged between 0–6 months at 21%, and those aged 30–42 months at 19.9%. 5,15
The prevalence of atopic eczema varies across the world. A recent systematic review of incidence and prevalence studies published between 1990 and 2010 included 69 studies. Evidence suggested that the prevalence is increasing in many regions of the world, including the UK. 16
In one UK study reviewed in the recent guideline, the incidence of atopic eczema in children aged up to 2.5 years born in 1991 and 1992 was found to be highest at 21% during the first 6 months of life, declining to 11.2% by the age of 6–18 months, and to 3.8% by the age of 30 months. 15 In another birth cohort study set up in 1982–4 to monitor the natural history of allergic diseases for 23 years, eczema usually remitted between 1 and 7 years of age; the prevalence of eczema was more likely to persist if a child was atopic, especially in girls. 4
Psoriasis
Psoriasis is estimated to affect around 1.3–2.2% of the population in the UK. It occurs equally in men and women, at any age, although it is uncommon in children, and can persist for up to 50 years. 6,7,17
Gelfand and colleagues18 estimated the overall prevalence of psoriasis in the UK from 1987 to 2002 to be 1.5%, with the prevalence increasing more rapidly in young female patients compared with their male counterparts. As the population ages, the prevalence is similar between sexes. Furthermore, the prevalence declines significantly in people aged 70 years and above, regardless of sex. In studies reviewed in a recent systematic review,17 similar trends were noted. Another study by Seminara and colleagues19 showed an overall prevalence of 1.9% based on the electronic records of The Health Improvement Network (THIN) database, which contained medical records of around 4.6% of the UK’s total population. The database presented prevalence data based on age groups and sex. Females had a slightly higher prevalence rate compared with males (1.9% vs. 1.8%). 19 Table 1 presents the UK-based prevalence rates reported by Gelfand and colleagues18 and Seminara and colleagues. 19
| GPRD by age group and sex (1987–2002) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence/10,000 | Age group (years) | ||||||||||
| 0–9 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | ≥ 90 | Total | |
| Male | 48.6 | 118.6 | 149.1 | 186.6 | 219.1 | 232.3 | 226.3 | 168.4 | 89.6 | 46.4 | 152.7 |
| Female | 61.8 | 154.8 | 152.6 | 169.8 | 187.9 | 213.7 | 225.7 | 156.6 | 87.9 | 47.6 | 151.4 |
| Total | 55.0 | 137.4 | 151.0 | 178.0 | 203.4 | 222.8 | 226.0 | 161.4 | 88.4 | 47.3 | 152.0 |
| THIN database by age19 | |||||||||||
| Prevalence (%) | Age group (years) | ||||||||||
| < 10 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | > 90 | ||
| 0.1 | 0.6 | 1.5 | 1.7 | 2.1 | 2.5 | 3.0 | 3.0 | 2.6 | 1.4 | ||
Recent estimates from a 2013 systematic review of global epidemiology of psoriasis17 found that the UK had a prevalence of between 1% and 3% (three studies) in adults. The review authors state that these rates were lower than estimates from other countries. 17 No UK-specific studies were identified in children, but the European prevalence rates were reported to be up to 0.71%. 17 A recent Norwegian population-based cohort study, following patients for 30 years, showed that the self-reported lifetime prevalence rose from 4.8% in 1979–80 to 11.4% in 2007–8. 20
No studies on the UK incidence of psoriasis specifically in children or adults were identified in the 2013 systematic review. 17 One UK study was identified that reported the combined incidence in all ages. The reported incidence rate was 140/100,000 person years. 21 A retrospective cohort study from the UK conducted in 2008 has since reported results that indicate an incidence of psoriasis in adults of 28/100,000 person years. 22
Acne
Epidemiological studies of acne show broad ranges of incidence and prevalence. 23 Acne affects up to 80% of people at some point in their lives, predominantly between the ages of 15 and 17 years. 24 Chronic acne can persist, however, into adulthood,24 and approximately 14% of people with acne are thought to consult their general practitioner (GP) (3.5 million visits annually). 9 A study suggests that this condition is prevalent in up to 50% of 14- to 16-year-olds in a community sample, and up to 30% of these teenagers had acne of sufficient severity to require medical treatment. 25 The prevalence of moderate-to-severe acne is likely to increase with age during puberty. 23 The incidence of the condition is similar in both men and women, with the numbers peaking in adults up to 25 years of age. 25
Other conditions
Other chronic inflammatory skin conditions include rosacea, lichen planus, hidradenitis suppurativa, cutaneous lupus erythematosus, lichen sclerosus and seborrhoeic dermatitis. Rosacea may affect up to 1 in 10 people; however, epidemiological data are scarce and controversial. The reported prevalence rates have ranged from 0.09% to 22% in the UK. 26 Lichen planus is reported to affect 1–2% of the population. Although the condition can occur at any age, it typically arises in females of middle age. A study reported that the condition primarily occurred in women aged over 45 years, with an annual incidence of 27/100,000 people in 2003. Hidradenitis suppurativa may have a prevalence of approximately 1% in the UK and is more prevalent in females than males. 27 Cutaneous lupus erythematosus is an uncommon autoimmune disorder, which has a variety of types. Those most commonly affected are women aged 20 to 50 years, although children, the elderly and males may also be affected. 28 Lichen sclerosus is a lymphocyte-mediated dermatosis that occurs in the genital skin and affects both sexes. The incidence is thought to be higher in females (peak ages of presentation are in prepubertal girls and postmenopausal women) than males. 29 Seborrhoeic dermatitis is a common form of dermatitis affecting areas rich in sebaceous glands such as the face, scalp and centre of the chest. 30 It is thought to affect between 3% and 5% of the global population and is more common in younger adults.
Impact of the diseases
People with inflammatory skin conditions can experience symptoms including itching (and sometimes pain), dry skin and changes in skin appearance, to varying degrees of severity and bodily involvement. 5,7,29 The symptoms can be distressing for patients and their carers31,32 and, in some cases, can lead to functional impairments, particularly when conditions affect the face, genitalia, hands and feet. 7 In adults, reduced levels of employment and income have been noted in psoriasis7 and more severe acne vulgaris. 9 Patients can feel stigmatised by their condition owing to visible skin symptoms and changes in appearance,31,33,34 which may contribute to distress35 and impact on their social interactions,33,34,36 normal activities (e.g. going to a public swimming pool or to the hairdressers) and relationships with people, including sexual relationships. 34 Sleep quality can also be affected owing to itching and scratching which can be particularly intense at night, and the sleep of carers can also be disrupted through dealing with symptoms at night. 31
Chronic inflammatory skin conditions are associated with high levels of psychological comorbidities, including depression and anxiety,31,35,37–39 and reduced health-related quality of life (HRQoL),9,33,39,40 which does not always correlate with disease severity. 41–43 Psychological difficulties and stress, along with symptoms (such as itching), undergoing treatment and concerns about appearance may negatively impact patients’ HRQoL. 33,39,40 Self-managing a long-term skin condition, with a relapsing and remitting course, is demanding for patients and their carers, and patients may feel that they lack control owing to the unpredictability of the disease on a weekly, or even daily, basis. 31 Poor psychological health can lead to a vicious cycle in patients where symptoms can be exacerbated by stress44 and reduced HRQoL may lead to less adherence to treatment regimens, reducing the effectiveness of treatment and resulting in greater use of health-care resources. 45
HRQoL is a commonly used outcome measure in health care to evaluate the impact of disease on patients’ lives. It is defined as a person’s subjective experience and perception of the impact that their health status has on their physical, psychological and social functioning. 46,47 HRQoL instruments measure various dimensions of these three domains, including physical symptoms, social activity, mental health, ability to carry out normal activities, life satisfaction and perceived health status,34,46,48 although the specific dimensions measured vary according to the instrument used. 48 HRQoL is a distinct concept from psychological distress (e.g. depression or anxiety), although, as described above, experiencing psychological distress is associated with poorer HRQoL in chronic inflammatory skin conditions.
Measurement of disease
A wide range of validated instruments are used to measure HRQoL and disease severity in patients with chronic inflammatory skin diseases. The Dermatology Life Quality Index (DLQI) is one of the most frequently used instruments in studies in dermatology. It has been used extensively in studies of over 40 different skin conditions, although the most common conditions it has been used for are psoriasis, atopic eczema and acne. 49 Other common measures include the Infants’ Dermatitis Quality of Life Index (IDQoL), which is aimed at children aged 0–4 years old,50 the Children’s Dermatology Life Quality Index (CDLQI), which is aimed at children aged 4–16 years, the Quality of life in Primary Caregivers of children with Atopic Dermatitis (QPCAD), and the World Health Organization Quality of Life-26 items (WHOQOL-26). These instruments cover a range of dimensions of HRQoL, including the severity of the condition, quality of sleep, coping, adherence with treatment, satisfaction and the impacts on family members, partners or carers. Some of the instruments that have been developed specifically for use in children (such as CDLQI and Quality of Life in Children Aged 14–16 Years) use proxy judgements made by someone else, such as a parent or carer.
Some of the common measures of disease severity include the Psoriasis Area Severity Index (PASI), the Self-Administered Psoriasis Area Severity Index (SAPASI), the Patient-Oriented Eczema Measure (POEM), the SCORing Atopic Dermatitis (SCORAD) and the Psoriasis Disability Index (PDI).
Appendix 1 gives an overview of some of these common instruments, including definitions of clinically meaningful changes where this is known (that is, the degree of change that is considered to be of benefit to the patient and their disease management, regardless of whether the change is statistically significant46).
Impact on the NHS
The need to improve patients’ HRQoL and for clinicians to take a holistic approach to managing patients’ skin conditions has been advocated in the research literature34 and clinical guidelines. 5,7 This might benefit both patients and the NHS through reduced use of health-care resources. It was estimated (using 2005/6 data) that 2.23% of the total NHS expenditure (£140M) was spent on diseases of the skin and subcutaneous diseases. 1 This included prescribing costs, outpatient and inpatient costs, but not primary care consultations, which could add another £395M. 1 In a national statistical report published by the Health and Social Care Information Centre51 on prescriptions dispensed in the community in England from 2003–13, the costs of emollient prescriptions in the year 2013 were estimated at £105,000, although these were prescribed for a range of conditions and not just for inflammatory skin diseases.
A UK-based study1 estimated the direct costs of skin diseases to the individual and to the NHS. It reported a year-on-year increase in over-the-counter (OTC) sales of the skin disease treatments in the UK from 2001 to 2007. The OTC sales for such conditions were £413.9M in 2007; this was 18% of the total OTC sales. Of the total prescribing budget, 2.85% (£237.7M) was for prescribing costs for skin disease in England in 2007. The study reported an estimated cost of about £395M per year, or 4.4% of the General Medical Services budget for GP consultations in England and Wales. In the year 2005/6, the overall direct costs (including medical care and products) of providing care for people with skin diseases was reported as about £1819M in England and Wales. It was observed that the direct cost of skin disease to the NHS was relatively low despite the conditions being very common. 1
Current service provision
A brief overview of the management of different chronic inflammatory skin diseases with relevance to the UK is discussed in the context of the relevant national guidelines as outlined below, including the role of education in the treatment of these conditions. Educational interventions are typically defined as providing patients with information about, and training in, skills for managing their condition. 52 In its simplest sense, education can be thought of as the provision of information that is intended to influence a specified outcome. In general, educational interventions involve encounters between teachers and learners for one or more of the following purposes: to raise awareness, to enhance or improve knowledge, or to change behaviour. 53
Relevant national guidelines
Of all the chronic inflammatory skin diseases prevalent in the UK, national guidelines have been published on only two of the conditions: atopic eczema5,54 and psoriasis. 7 In addition, National Institute for Health and Care Excellence (NICE) quality standards for psoriasis and atopic eczema are available and these provide quality statements describing best clinical practice, including pathways for assessment and treatment. The details of the guidelines are presented below.
Atopic eczema
Atopic eczema in children: management of atopic eczema in children from birth up to the age of 12 years. NICE Clinical guidelines, CG57. Issue date: December 2007. 5 In addition to covering the management of atopic eczema in children from birth up to 12 years, this guideline provides guidance on diagnosis and assessment, management, and providing information and education for children and their parents and carers.
Management of atopic eczema in primary care: a national clinical guideline. March 2011. Scottish Intercollegiate Guidelines Network (SIGN) guideline for atopic eczema. 54 Similarly to the NICE clinical guideline, the SIGN guideline also provides recommendations based on current evidence for the management of atopic eczema in children as well as adults in primary care in Scotland.
Psoriasis
The assessment and management of psoriasis. NICE Clinical guidelines, CG153. Issue date: October 2012. 7 This guideline provides evidence-based advice on the assessment and management of psoriasis in adults, young people and children.
Management of disease
A range of treatment options are available for inflammatory skin diseases and current recommendations for best practice are summarised here. Although these vary from condition to condition, they typically fall into topical treatments, which are applied directly to the skin; systemic pharmacological treatments (intravenous, subcutaneous or oral); bandaging techniques; and, for some conditions, phototherapy. 5,7 In addition, patients are encouraged to practise self-care (such as using emollients as indicated, avoiding known triggers) to minimise environmental triggers of their disease, to monitor their condition, maintain adherence to treatments, and to seek support groups. Education is an important way to help individuals manage the symptoms of chronic diseases55 and, in recent years, educational interventions for people with inflammatory skin disease have been seen as a useful adjunct to usual medical care with topical and pharmacological therapies.
Atopic eczema
The NICE clinical guideline on atopic eczema5 provides guidance on management of the condition during and between flares, in children from birth up to the age of 12 years. The guideline suggests that a holistic approach should be adopted by health-care professionals (HCPs) when assessing a child’s atopic eczema. Potential trigger factors, such as irritants (e.g. soaps and detergents), skin infections, contact allergens, food allergens and inhalant allergens should be sought by the HCPs while clinically assessing children with the condition. With respect to treatment of the condition, NICE recommend the use of a stepped approach whereby the treatment step was tailored to the severity of the atopic eczema. Emollients are recommended to form the basis of atopic eczema management.
The current NICE guideline5 states that parents and carers, along with the children with atopic eczema, should be offered information on how to recognise the symptoms and signs of bacterial infection and how to access appropriate treatment when a child’s atopic eczema becomes infected. Furthermore, children, along with their parents and/or carers, should be educated about the health condition and its treatment. In addition, HCPs should provide both verbal and written information, along with practical demonstrations on quantity and frequency of the treatment to use. NICE recommends that the information should be tailored to suit an individual child’s cultural practice relating to skin and the way they bathe. The NICE guideline acknowledges the impact that the disease can have on a patient’s HRQoL and makes a recommendation that the effect of the condition on HRQoL should be taken into account at consultations and treatment decisions. 5 Future research recommendations were advocated by NICE to assess the clinical effectiveness and cost-effectiveness of different models of educational programmes in the management of atopic eczema in children, as lack of education about therapy could lead to poor adherence, thereby leading to treatment failure.
In addition to the above guideline, NICE Quality Standards 4456 include a reference to the provision of education in Statement 2, that children with atopic eczema should be treated using a stepped-care plan on the basis of the recorded disease severity and that this care plan should be supported by education. The NICE pathways for atopic eczema state that quality of life (QoL) should be assessed by practitioners when making treatment decisions.
The SIGN guideline54 identifies a Cochrane review that examined the effect of parent educational interventions on severity of eczema in children. This review showed heterogeneity with respect to the format, content and settings of the interventions and, although two of the four included studies found the intervention to be effective in reducing clinical severity scores, no recommendations in the SIGN guideline were made owing to the lack of consistency seen in the trials included. 54
The SIGN guideline provides a checklist of information that patients and carers should have access to at the different stages of diagnosis and treatment. These largely focus on explanation of the condition, what treatment options there are, how to self-manage their conditions and look for changes in their condition. There is some limited reference to providing patients and carers with the contact details of organisations that may be able to provide advice and support. There is no specific advice regarding any educational interventions. 54
There are no published audits of how well the national guidelines are being adhered to in clinical practice with regard to education. The general impression of our Advisory Group was that this is variable.
From a patient and carer perspective, the need to establish which is the most effective route to manage eczema has been identified by the James Lind Alliance Eczema Priority Setting Partnership. 57 One of the top research priorities for HCPs identified by the group is to establish which of the following management approaches is the most effective: education programmes, GP care, nurse-led care, dermatologist-led care or multidisciplinary care.
Psoriasis
The NICE guideline on psoriasis7,58 provides recommendations on the management of all types of psoriasis across all age groups: children aged up to 12 years, young people and adults aged 18 years and above.
The NICE guideline7 recommends that for people with any type of psoriasis, disease severity should be assessed, along with the impact of disease on physical, psychological and social well-being, and whether they have psoriatic arthritis or presence of comorbidities. The guideline states reasons for referral to a dermatology specialist for a number of reasons, including uncertainty over the diagnosis, severe psoriasis, psoriasis that cannot be controlled with topical therapy or psoriasis that has a major impact on a person’s physical, psychological or social well-being.
It was recommended to discuss risk factors for cardiovascular comorbidities with people who have any type of psoriasis and with their families and/or carers.
With regard to treatment, practical support and advice about the use and application of topical treatments should be provided by trained and competent HCPs. For adults with trunk or limb psoriasis, NICE recommends the use of a potent corticosteroid applied once daily plus vitamin D or a vitamin D analogue applied once daily for up to 4 weeks as initial treatment. However, people with plaque or guttate-pattern psoriasis that cannot be treated with topical treatments alone should be offered narrowband ultraviolet B phototherapy two or three times a week depending on patient preference. Systemic non-biological therapy should be offered to people with any type of psoriasis if the disease cannot be controlled with topical therapies, if it has a significant impact on the patient in terms of physical, psychological or social well-being, or if it is associated with significant functional impairment. 7
In citing the principles of care for patients with the condition and their families or carers, the guideline recommends the provision of support and information to be provided to meet the requirements of each individual. Areas of focus for information should be an understanding of their diagnosis, the available treatments (including their safe and effective use), what the associated risk factors are, when and how to seek support, and information on strategies to deal with the impact on physical, psychological and social well-being. 7 The NICE guideline also recognises the potential impact on a patient’s HRQoL and notes that HRQoL should be taken into account during patient consultations and when making treatment decisions. The NICE pathways for psoriasis state that psychological well-being should be assessed by practitioners at diagnosis and when assessing response to treatments.
Similarly to the situation with eczema guidelines, there are no published audits of how well the NICE psoriasis guideline is adhered to in clinical practice with regard to education. The Advisory Group to this review believed that adherence is currently variable.
Overall, although these guidelines for eczema and psoriasis outline to some extent where patient education, information and advice sit in clinical practice, the guidance tends to focus on education relating to patients’ use of treatments, self-care and understanding of the disease. It is not currently clear where educational interventions that more directly address HRQoL could be placed in the clinical pathway, including which patient groups should be targeted for these kinds of interventions. The guidance also does not currently clearly state how any type of education is best provided in primary or secondary care, such as whether it should be provided in a structured, planned way or more informally by practitioners during routine medical consultations.
Description of the technology under assessment
As stated, educational interventions are typically defined as providing patients with information about and training in skills for managing their condition (see Current service provision). 52 People with chronic skin conditions and their carers have several educational needs. These include an understanding of the condition (typically chronic and relapsing, with no cure at present, but in general manageable), an opportunity to try treatments to find those that suit them best, reassurance that many treatments are generally safe and effective, guidance on how best to apply topical treatments, and motivation to continue treatment when the disease is in remission. 59 Educational interventions have traditionally been based on what health-care experts believed patients need to know about their conditions rather than patients’ expressed needs. 60,61 Recently, however, there has been a movement in medicine towards greater patient involvement in treatment and patient-centred care. 62 A more patient-empowering subset of educational interventions are self-management educational interventions, which focus on enabling patients to develop problem-solving skills and teach patients actions that they can take to resolve issues (including emotional and psychosocial issues) relating to their condition when they arise. 52 Self-management educational interventions will often involve the creation of patient action plans52 and represent a collaborative approach between HCPs and patients. 60 Patient activation is also an important part of promoting self-management in chronic diseases. 63 Patient activation is defined as the extent to which patients believe and have confidence that they can play an active part in managing their health and the extent to which they carry out and maintain activities which can positively impact their condition and health more broadly. Patient activation is associated with better QoL in patients in general64 and potentially could be improved through patient education.
Educational interventions may be delivered in a variety of ways, may include a number of inter-related elements that either singly or together may bring about change in an outcome and some tailoring to individual patient or carers’ needs, and therefore could be considered ‘complex interventions’. 65 Medical Research Council (MRC) guidance on the development and evaluation of complex interventions emphasises the importance of early groundwork in developing an intervention. 65 This should include specification of a clear theoretical basis for the intervention that outlines its rationale and how it might bring about change in the outcomes of interest, drawing on available evidence and theoretical models. This is because theory-based interventions tend to be more effective than those that are not theory-based. The MRC guidance additionally recommends carrying out research, such as qualitative work, with potential users, deliverers or creators of the intervention to supplement existing theory and evidence, if needed, to further inform intervention development. The guidance also recommends that systematic reviews of current evidence for the effectiveness of the intervention and pilot studies (e.g. to evaluate feasibility) are carried out.
As another consideration in intervention development and delivery, it is also recommended in the wider literature that educational interventions in dermatology should be sensitive to and take account of patients’ social and cultural backgrounds, including their education level, literacy and preferred language, and their favoured learning methods. 66,67 Some degree of tailoring of the intervention to individual needs might also be beneficial, as it has been found in health care generally that tailored interventions result in better outcomes than more generic ones. 68 Consideration might also be given to the characteristics of the intervention deliverer, because it has been suggested that interventions delivered by people who have similar characteristics to the intervention participants (such as similar social and ethnic backgrounds) might be more effective than those delivered by people who differ to the participants. 70 Ideally, evaluations of complex interventions should include process evaluations to gain insight into contextual factors during intervention delivery that may impact its effectiveness. 65
Research studies have investigated a wide variety of approaches for educating patients with chronic inflammatory skin diseases – mostly those with eczema and psoriasis, and, in some cases, their carers or families also. 2,36,55,70 The existing evidence for educational interventions in general shows variability in whether these interventions are effective in improving HRQoL, but some studies have shown positive effects. 55,60,71 A systematic review of educational interventions for children with eczema and their parents suggests that programmes for parents that are delivered by either a nurse or a multidisciplinary team may lead to improvements in infant and child HRQoL and disease severity. 71 However, there is currently little understanding overall about which elements of educational interventions make them effective in improving HRQoL and other outcomes. 60,61
Our project Advisory Group of patients, HCPs and researchers indicated that educational interventions are generally not widely used in UK clinical practice to supplement medical treatment for patients with chronic inflammatory skin conditions. The Group suggested that education in primary care is especially limited and will generally involve verbal instructions on medication use, provision of information leaflets and sign-posting to other information sources. The Group stated that educational interventions are more commonplace in secondary care and are often nurse-led and more likely to be planned and structured. These tend to involve information giving and advice, combined with paper- or web-based information. The Group stated that, overall, educational interventions are not sufficiently individualised and the creation of action plans is rare.
As part of educational interventions, patients with chronic inflammatory skin conditions are often provided with information about their condition and the use of treatments. However, it has been suggested that the impact of standard education in dermatology on HRQoL could be enhanced by the additional inclusion of elements that address issues related to HRQoL. This review focuses on these additional elements as per the commissioning brief. Our Advisory Group was aware of only two UK educational programmes that specifically aim to improve HRQoL. One is The Eczema Education Programme,72 which is a nurse-led programme delivered in the community and a specialised centre, which aims to improve parents’ and carers’ management of their child’s eczema and parents’ QoL. Elements covered in the programme include: understanding the disease, trigger factors and treatment; enhancing parental confidence in using treatments; action planning; and practical strategies for reducing itching and sleep problems. A before- and after- study evaluation of the programme73 found improvements in infant, child and parental HRQoL, indicating that this may be a potentially successful approach. The other programme is an online intervention delivered through the Supporting Parents and Carers of Children with Eczema (SPaCE) website for carers of children with eczema. 74
As with educational interventions in general, it is currently unclear which specific elements of these interventions may enhance HRQoL or what factors should be targeted to lead to improvements in HRQoL. Where complex educational interventions for chronic skin diseases involve multiple interacting components it may not be possible to identify which intervention components are responsible for observed effects on outcomes. 75,76 However, taxonomies of intervention techniques may be used, if appropriate, to map which intervention components may be related to improved outcomes. 69,77 In line with the MRC complex interventions guidance,65 authors of some studies evaluating educational interventions for childhood eczema aimed at improving HRQoL have provided information on the underlying theory of change. These studies have hypothesised that educational interventions may improve HRQoL through enhancing the ability to manage the disease,72,74,78 increasing self-efficacy72,78 (that is, patients’ or carers’ confidence in their ability to successfully manage the condition79), promoting positive outcome beliefs and behavioural capability78 and through the use of a number of other behaviour-change techniques. 74 As far as the authors of this review are aware, however, a clear theoretical basis for such interventions, including potential underlying mechanisms of change, has not been adequately outlined in the literature.
Based on opinion from our Advisory Group, addressing the following factors may differentiate educational interventions aimed at improving HRQoL from general educational interventions: unpredictability of the disease, interaction of multiple factors, impact of everyday life (e.g. family, tiredness and personal and work life) on the condition, mental well-being, negative thinking, coping skills (e.g. to cope with the disease or for managing psychological distress), problem-solving and action planning, and management of medication side effects. Additionally, the Group suggested that it may also be important to cover issues relating to parental guilt associated with passing on a genetic disease, and parents’ or carers’ empathy and appreciation of the impact of the disease. The Group also stated that educational interventions that improve condition management may also result in improvements in HRQoL and emphasised the potential importance of theory-based interventions in enhancing the effects of an intervention on this (e.g. through the incorporation of elements hypothesised to improve self-efficacy from social cognitive theory80). The literature shows that experiencing psychological difficulties, such as depression, is associated with poor HRQoL in chronic inflammatory skin diseases, among other factors,33,39,40 and patients experiencing a high psychosocial burden from their disease have been identified as a (psoriasis) subgroup requiring additional support. 81 Based on a model of delivery of psychosocial interventions for skin conditions generally,82 recommended nurse-delivered interventions for patients experiencing mild to moderate psychosocial distress include training in relaxation techniques, scratching habit reversal, problem solving and ways of camouflaging skin. It may be reasonable to assume that if these aspects are part of educational interventions, they may also help enhance HRQoL.
In light of limited existing evidence, there is uncertainty about best practice methods for educational interventions. Therefore, systematic reviews of clinical effectiveness as well as cost-effectiveness of educational interventions aimed at improving QoL in people with chronic inflammatory skin diseases are required.
Overall aims and objectives of assessment
The aim of this health technology assessment is to undertake systematic reviews of the clinical effectiveness and cost-effectiveness of educational interventions for people with chronic inflammatory skin diseases.
The main objectives are:
-
to conduct systematic reviews of the clinical effectiveness and cost-effectiveness of educational interventions for improving HRQoL in patients with chronic inflammatory skin diseases
-
if data permit, to adapt an existing economic model or construct a de novo model from the perspective of the UK NHS to estimate the cost-effectiveness of educational programmes for chronic inflammatory skin diseases
-
to identify deficiencies in current knowledge and to generate recommendations for future research.
Chapter 2 Methods
The a priori methods for systematically reviewing the evidence of clinical effectiveness and cost-effectiveness were described in a research protocol which was sent to our expert Advisory Group for comment. Although helpful comments were received relating to the general content of the research protocol, none of the comments identified specific problems with the methodology of the review. The methods outlined in the protocol are briefly summarised below.
During data extraction, we made a modification to our protocol to exclude post hoc those papers that otherwise met our inclusion criteria, but which did not report a study’s results in sufficient detail to be informative in the review.
Identification of studies
A comprehensive search strategy was developed, tested and refined by an experienced information specialist. Separate searches were conducted to identify studies of clinical effectiveness and cost-effectiveness. Sources of information and search terms are provided in Appendix 2. The most recent searches were undertaken in July 2014.
Literature was sourced from 12 electronic databases, the bibliographies of included articles and relevant systematic reviews, and our expert Advisory Group were contacted to identify any additional studies. All databases were searched from inception and limited to the English language. The following electronic databases were searched:
-
The Cochrane Library, including the Cochrane Database of Systematic Reviews (CDSR), the Cochrane Central Register of Controlled Trials, Centre for Reviews and Dissemination (University of York) Database of Abstracts of Reviews of Effectiveness (DARE), the NHS Economic Evaluation Database (NHS EED) and the Health Technology Assessment (HTA) database
-
MEDLINE (Ovid)
-
EMBASE (Ovid)
-
MEDLINE In-Process & Other Non-Indexed Citations (Ovid)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus with full text (EBSCOhost)
-
PsycINFO
-
Web of Science: Science Citation Index Expanded (SCIE) and Conference Proceedings Citation Index – Science (CPCI) (ISI Web of Knowledge)
-
Global Resource for Eczema Trials (GREAT) database (University of Nottingham).
A comprehensive database of relevant published and unpublished articles was constructed using Reference Manager (Thomson ReseachSoft, San Francisco, CA, USA) software. Research-in-progress databases were searched for any ongoing studies of relevance.
Searches for ongoing studies were undertaken in the following databases: UK Clinical Research Network (UKCRN), controlled-trials.com, clinicaltrials.gov, World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), Centre for Evidenced Based Dermatology (University of Nottingham), UK Clinical Trials Gateway (UKCTG).
Inclusion and exclusion criteria
Studies were eligible for inclusion in the systematic reviews if they met the criteria outline below.
Population
Adults, young people and children with a chronic inflammatory skin condition and/or their carers.
Intervention
Educational interventions that either specifically aimed to improve HRQoL or could improve HRQoL [e.g. by targeting patients’ ability to cope with the negative effects of chronic skin disease or by targeting compliance with therapy (with the aim of reducing the degree of skin affected)]. That is, to be included in the review, the educational interventions needed explicitly to state that the aim of the intervention was to improve HRQoL or, in the absence of this, the content of the intervention needed to focus on more than just information about the specific skin disease and its treatment, and needed to address patients’ ability to cope with the negative effects of the disease (e.g. by providing education on stress management, coping with itch or addressing the psychosocial effects of the disease, such as feelings of stigmatisation attributable to changes in skin appearance) or adherence to therapy.
Any type of educational technique was permitted provided that effects of education on outcomes could be isolated from effects of any non-educational intervention components that may also be present in the intervention. Therefore, any interventions that were purely psychological approaches (e.g. to manage distress) and did not seem to include an element of education were excluded.
Comparators
Any comparator was eligible. This could include treatment as usual, waiting-list controls, or other educational interventions.
Outcomes
-
HRQoL: only studies that measured HRQoL as an outcome, using a validated measure, were included.
The following outcomes, where reported in the included studies, were also included:
-
disease severity
-
disease control
-
scratching behaviour (where applicable)
-
health-care utilisation
-
depression
-
anxiety
-
patient or carer self-efficacy regarding disease management (self-efficacy is defined as a person’s level of confidence in their ability to perform particular behaviours to achieve desired outcomes,79 such as successful self-management of a condition)
-
process evaluations, including adherence to therapy, attitudes and knowledge.
Patient-reported outcome measures were included if assessed by validated tools. Reviewers considered that a measure was validated if the publication stated that it was or, where this information was not available, if a consultation of the wider literature determined that the measure fulfilled at least one validity criterion.
For the systematic review of cost-effectiveness, studies reporting measures of cost-effectiveness [e.g. cost per quality-adjusted life-year (QALY), cost per life-year saved] were eligible.
Study design
For each skin disease, relevant randomised controlled trials (RCTs) were sought. If no RCT evidence existed for a given disease, prospective trials with concurrent control group(s) were eligible.
The identified systematic reviews were used as sources of references only.
Studies were included in the systematic review of cost-effectiveness if they were full economic evaluations (cost-effectiveness, cost–utility or cost–benefit analyses) that reported both measures of costs and consequences or if they were cost–consequence or cost analyses.
Studies published as abstracts or conference presentations were only included if sufficient details were presented to allow an appraisal of the methodology and the assessment of results to be undertaken.
Study selection and data extraction strategy
Studies were selected for inclusion in the systematic review of clinical effectiveness through a two-stage process using the predefined and explicit criteria specified above. Titles and abstracts from the literature search results were independently screened by two reviewers to identify all citations that possibly met the inclusion criteria. Full papers of relevant studies were retrieved and assessed by one reviewer and checked by a second reviewer using a standardised eligibility form. As far as possible, full papers or abstracts describing the same study were linked together, with the article reporting key outcomes designated as the primary publication. Any disagreements between reviewers were resolved by consensus or if necessary by arbitration by a third reviewer.
Titles and abstracts identified by the search strategy for the systematic review of cost-effectiveness were assessed for potential eligibility by two reviewers using the predetermined inclusion criteria. Full papers were formally assessed for inclusion by one reviewer with respect to their potential relevance to the research question and this was checked by another reviewer. Any disagreements between reviewers were resolved by consensus or if necessary by discussion with a third reviewer.
Data were extracted by one reviewer using a standard data extraction form and checked by a second reviewer. At each stage, any disagreements between reviewers were resolved by consensus or, if necessary, by arbitration by a third reviewer.
In the systematic review of clinical effectiveness, for each study, results for outcomes measured at the immediate end of the intervention and the longest follow-up time point (where this differed) were extracted and presented. That is, results for any intermediary time points were not extracted. During data extraction, a modified version of Schulz and colleagues’69 intervention taxonomy was used to structure how and which information was extracted about the educational interventions in the included studies. The taxonomy characterises the different elements of interventions. Based on this, the following intervention components were extracted for each study: where it was delivered; whether it was a form of self-help; whether it was individual- or group-based; mode; materials used; provider; duration and intensity; scripting (use of a protocol guiding interaction between the interventionist and participants); sensitivity to participant characteristics; interventionist characteristics and training; content and topics (including educational strategies used); tailoring; and theoretical basis. Reviewers additionally extracted the following: intervention overview and aims; stated target group; ongoing support provided; and whether individuals’ preferred learning styles were taken into account.
Critical appraisal strategy
The methodological quality and the quality of reporting of the included clinical effectiveness studies were assessed using risk of bias criteria based on those recommended by Cochrane83 (see Appendix 3). Quality criteria were applied by one reviewer and checked by a second reviewer, with any differences in opinion resolved by consensus or by arbitration by a third reviewer.
Quality assessment for the systematic review of cost-effectiveness was based on a checklist for economic evaluation publications. 84
Method of data synthesis
Studies of clinical effectiveness and cost-effectiveness were synthesised through a narrative review with tabulation of results of included studies. In the systematic review of clinical effectiveness, studies were grouped according to the condition being considered and the general age group of the participants.
It was not considered appropriate to combine the studies in a meta-analysis owing to the heterogeneity between studies in patient characteristics (ages, conditions, duration of disease, severity of disease), the interventions and the comparators.
Advisory group
The Advisory Group informed the protocol and provided comments on the near complete draft report. In addition, members of the Advisory Group provided responses to specific questions around the current use of educational interventions for chronic inflammatory skin disease, which informed the background section of the report.
Chapter 3 Results of the systematic review of clinical effectiveness
Quantity and quality of research available
Figure 1 shows the flow of studies through the review. Eight studies74,76,85–90 met the inclusion criteria. Of these, seven were RCTs74,76,85–89 and one was a controlled clinical trial (CCT). 90 The CCT90 evaluated an educational intervention for psoriasis. In line with our review protocol, which pre-specified that CCTs would only be included if no RCT evidence existed for a particular condition, the CCT is not discussed further because RCT evidence was available for psoriasis.
FIGURE 1.
Flow chart for the identification of studies in the review of clinical effectiveness. CCT, controlled clinical trial.
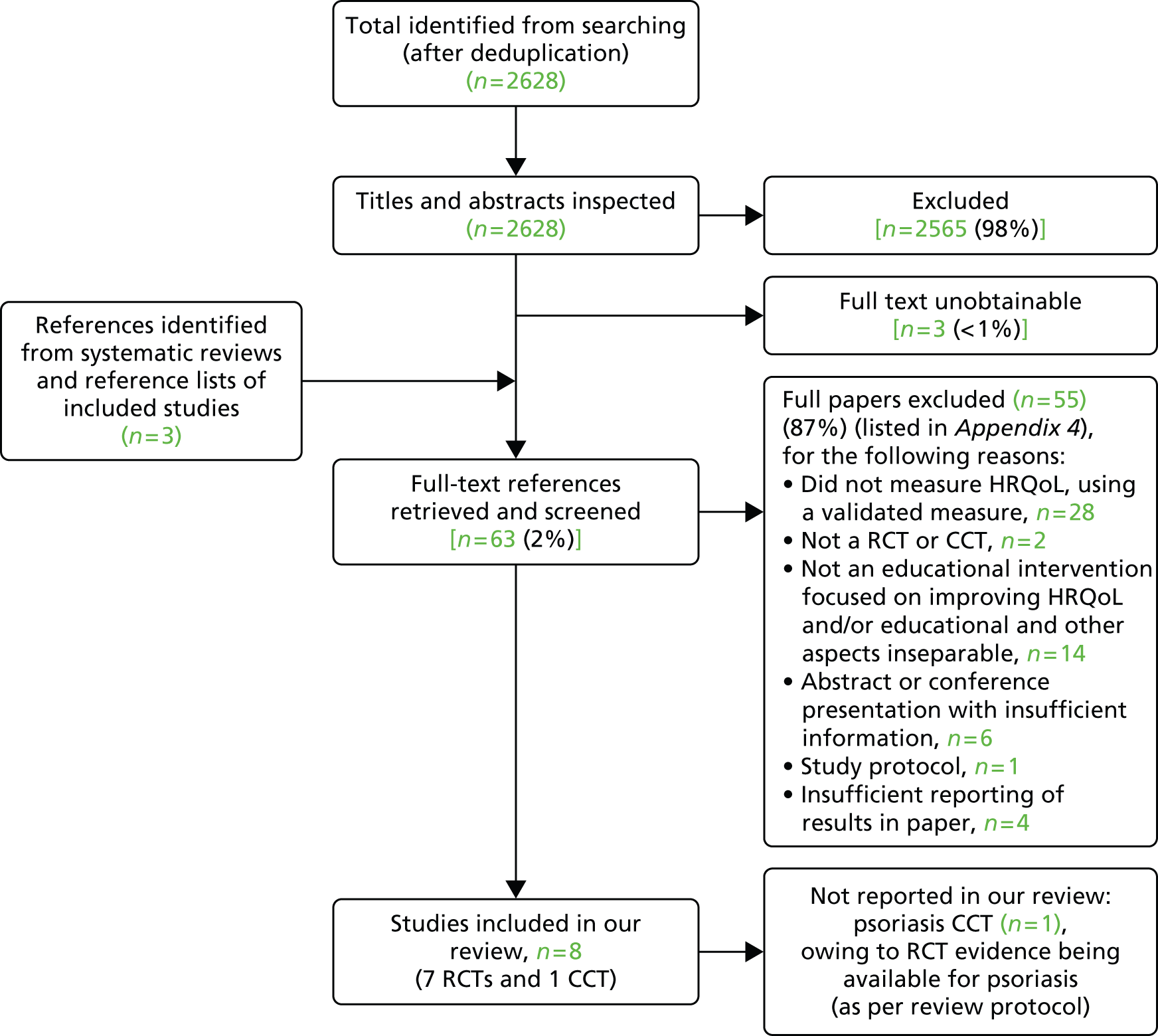
Searches identified 2628 records, of which 2565 were excluded at title and abstract screening. At the full-text screening stage of the review, 63 references were reviewed and 55 were excluded. The main reasons for exclusion were that studies did not measure HRQoL using a validated measure (n = 28) or that the intervention was not an educational intervention that aimed to, or could, improve HRQoL, and/or the effect of the educational component of the intervention on outcomes could not be isolated from the effect of other non-educational components of the intervention (n = 14) (see Appendix 5 for a full list of reasons for specific exclusions). Three studies, reported in four publications,78,91–93 were excluded for not reporting results in sufficient detail to be informative in the review. Of these, one reported in Staab and colleagues93 and Wenninger and colleagues78 was a RCT examining a theory-based programme called the ‘Berlin education programme’ for parents of children with atopic dermatitis, aimed at improving HRQoL and the disease course, which covered medical, nutritional and psychological issues. The authors reported limited numerical data in the results. For example, data were reported for only one of the five dimensions of a disease-specific HRQoL measure. The other two excluded studies were Jaspers and colleagues91 and Lora and colleagues,92 which were RCTs of a combined psychoeducational and dermatological treatment programme delivered by a multidisciplinary team for young adults with atopic dermatitis and a psychoeducation programme for patients with psoriasis, respectively. Jasper and colleagues91 provided numerical results for only one domain of the Short Form questionnaire-36 items (SF-36) (HRQoL measure) at 10 weeks post intervention and narratively reported changes for other domains at 10, 20 and 40 weeks post intervention as non-significant. Lora and colleagues92 presented no results for the HRQoL measure they used. Such exclusions as a result of poor quality reporting are important to note, because these studies otherwise met the review inclusion criteria and formed around one-third of the relevant evidence-base – this issue is considered further below (see Discussion).
Characteristics of the included trials
Table 2 provides an overview of the characteristics of the seven included RCTs. Detailed information about the educational intervention(s) evaluated in each RCT is provided in Table 3, with full details also provided in Appendix 6. The full data extraction forms are shown in Appendix 4. Two RCTs, by Balato and colleagues85 and Ersser and colleagues,86 assessed the effects of daily text message education over 12 weeks and a one-off nurse-delivered educational session for adults with psoriasis; one RCT by Matsuoka and colleagues88 focused on instructions in make-up use and skin care from a dermatologist to adult women with acne; and two RCTS, by Staab and colleagues87 and Santer and colleagues,74 focused on children (and adolescents in one trial87) with atopic dermatitis and their parents or carers, evaluating a 6-week educational programme delivered by a multidisciplinary team and an educational website (the ‘SPaCE website’), respectively. The educational interventions in the remaining two RCTs, by van Os-Medendorp and colleagues89 and Bostoen and colleagues,76 were delivered to people with a mixture of skin conditions. van Os-Medendorp and colleagues89 included people with chronic pruritic skin diseases (including eczema, atopic dermatitis, pruritus, prurigo, psoriasis and chronic urticaria) and evaluated a ‘Coping with itch’ programme, and Bostoen and colleagues76 included people with either psoriasis or atopic dermatitis in an evaluation of a 3-month programme delivered by a multidisciplinary team.
| Reference and design | Skin condition(s) | Population: patients (general age group), parents or carers | Intervention(s)/comparator(s) | Setting, country | Number of participants (randomised) | Length of follow-up |
|---|---|---|---|---|---|---|
| Balato et al., 201385 RCT (pilot study) |
Plaque psoriasis | Patients (adults) |
|
Home-based, Italy | 40 | 12 weeks (intervention end) |
| Ersser et al., 201186 Cluster RCT (pilot study) |
Psoriasis | Patients (adults) |
|
Primary care, UK | 64 | 6 weeksa |
| Bostoen et al., 201276 RCT |
Psoriasis or atopic dermatitis | Patients (adults) |
|
Setting not reported (reviewers inferred this as likely to be outpatients), Belgium | 50 | 9 months (from baseline) |
| Santer et al., 201474 RCT (pilot study) |
Eczema | Patients (children aged ≤ 5 years) and parents/carers |
|
Primary care, south-west England, UK | 149 | 3 months (from baseline) |
| Staab et al., 200687 RCT |
Atopic dermatitis | Patients (children and adolescents) and parents |
|
Setting not reported, Germany | 992 | 12 monthsa |
| Matsuoka et al., 200688 RCT |
Acne vulgaris | Patients (adults; women aged > 16 years) |
|
Outpatient clinic, Kagawa, Japan | 50 | 4 weeksa |
| van Os-Medendorp et al., 200789 RCT |
Chronic pruritic skin diseases | Patients (adults) |
|
Secondary care, The Netherlands | 120 | 9 months (from baseline) |
| Study | Balato et al., 201385 | Ersser et al., 201186 | Bostoen et al., 201276 | Santer et al., 201474 | Staab et al., 200687 (further information provided by Wenninger et al., 200078) | Matsuoka et al., 200688 | van Os-Medendorp et al., 200789 |
|---|---|---|---|---|---|---|---|
| Skin condition(s), population | Psoriasis, patients (adults) | Psoriasis, patients (adults) | Psoriasis or atopic dermatitis, patients (adults) | Eczema (children aged ≤ 5 years) and parents/carers | Atopic dermatitis, patients (children and adolescents) and parents | Acne, patients (adults; women aged > 16 years) | Chronic pruritic skin diseases, patients (adults) |
| Overview | Text message education | A theory-based self-management educational intervention | Educational programme for patients with psoriasis and atopic dermatitis | Two educational intervention groups:
|
Group-based educational programme, with different educational sessions for (a) parents of children aged 3 months to 7 years; (b) children aged 8–12 years and their parents; and (c) adolescents aged 13–18 years (parents optional for selected sessions) | Instructions on skin care and how to use make-up from a dermatologist | ‘Coping with itch’ programme – individual sessions with dermatology nurse, including educational and cognitive behavioural interventions |
| Intervention aim(s) | Not stated explicitly but implicit from the paper that aim was to use text messaging to improve treatment adherence and patient outcomes including HRQoL | To support self-management in psoriasis | Not explicitly stated. Study aimed to examine if the educational intervention ‘added value to medical therapy’ (p. 1025) and examine the effects on disease severity and QoL | Aimed to improve carers’ management of their child’s eczema by increasing regular use of emollients. Ultimate aim of intervention was to improve HRQoL through enhancing carers’ management of the condition | Not reported in primary publication, but the intervention for children aged 3 months to 7 years was based on one reported in Staab et al.,93 and Wenninger et al.,78 the aim of which was to improve parents’ ability to manage their child’s disease and thus improve disease course and families’ QoL | Not explicitly stated, but part of the aim of the study was to examine if instructions in make-up use from a dermatologist could affect female acne patients’ QoL | To reduce itch and to help patients cope with itch |
| Mode, including if individual- or group-based mode | Text messaging, individual-based | Face to face, written and audiovisual materials, individual telephone consultation. Group-based | Face-to-face workshop, group-based |
|
Face-to-face, group-based sessions | Face to face, with supporting videotape instructions and detailed leaflets/prescriptions. Not reported if individual- or group-based | Face to face, individual-based |
| Provider | Not reported | Nurse-led | Multidisciplinary team |
|
Multiprofessional team | Dermatologist | Dermatology nurse |
| Duration and intensity | One text message per day for a period of 12 weeks | Group session: one-off 2-hour session Telephone consultation: one-off 20-minute session |
3-month programme, with two, 2-hour sessions a week |
|
One 2-hour session a week, over 6 weeks | Not reported | Patients visited the itch clinic a mean of 2.9 times (median 3 times, range 1–6 times); duration of sessions not reported |
| Content and topics | Covered frequently asked questions about psoriasis drugs (e.g. administration and adverse effects) and general recommendations for taking care of overall health. Educational topics included daily care statements, healthy lifestyle statements, prompts about the use of treatments, and one statement about the psychosocial effects of psoriasis. Reminders reinforced many of the same principles | Practical element (no details provided), individual action planning, stress reduction (through provision of relaxation materials), feedback on action plans (through telephone consultation) | Information on specific skin disease and skin care sessions, healthy lifestyle and stress-reducing techniques, feedback sessions. Further details of the intervention provided in a separate publication94 |
|
Intervention for parents of 3-month- to 7-year-olds was based on the one reported in Staab et al.87 and Wenninger et al.78 Across the three groups, the educational sessions covered medical, nutritional and psychological issues. Participants were encouraged to share experiences and to put new skills into practice. A manual specified content | Patients received instructions on use of skin care and make-up products. Instructions included general skin care and how to use ‘point make-up’ (e.g. eyeliner and lipstick) | Education about: itch causes, consequences and treatment; patient advocacy groups; avoiding triggers; diet; interventions to relieve itching and scratching and their consequences. Cognitive behavioural therapy including diary-based awareness training, habit reversal to reduce scratching, and relaxation. Based on an initial itch medical history assessment taken by the nurse and structured according to an individual-based nursing care plan |
| Ongoing support | None reported | Nurse provided one 20-minute follow-up telephone consultation 1 month after the group session (based on outline script) | Not reported | Not reported | Not reported | Not reported | Individual counselling and ‘support’ (not defined) provided as required (no details given) |
Five of the included RCTs74,76,86,88,89 compared educational programmes with standard care for the skin condition. Balato and colleagues85 and Staab and colleagues87 compared the text message education and 3-month educational programme with a ‘control intervention’ and a ‘no education control’, respectively, but did not provide details about what, if any, treatment was given to the control conditions. In six RCTs74,76,85,86,88,89 the educational interventions were delivered as an adjunct to standard medical care, and it was unclear if it was an adjunct in the remaining trial evaluating the 6-week programme including children with atopic dermatitis and their carers. 82 Only one RCT74 compared two different approaches to the education delivery, in addition to comparing each educational approach with standard care. This trial compared education delivered through the SPaCE website only with education delivered through the same website, but with additional support through a one-off appointment with a HCP who promoted engagement with the website and supported participants in completing some of the modules.
Only two UK studies were identified. 74,86 One examined the effects of a one-off educational session for patients with psoriasis in primary care86 and the other was also set in primary care, but provided education mainly through the SPaCE website. 74 The other RCTs were conducted in a range of countries and settings, including secondary care in the Netherlands89 and Japan88 and home-based education in Italy. 85 In the remaining RCT,76 conducted in Belgium, the setting was unclear. The generalisability of these studies to the UK setting is, therefore, unclear.
No studies of any other chronic inflammatory skin diseases were identified in our searches.
All tables are ordered by condition and then by patient age group within condition.
The largest RCT was of 992 children and adolescents with atopic dermatitis and their parents. 87 One other RCT of mixed skin conditions had a sample size of 120 participants89 and another of children with eczema and their parents or carers had a sample size of 149. 74 Sample sizes in the four remaining trials,76,85,86,88 including all the RCTs of patients with psoriasis, ranged from 40 to 64 participants. One study measured outcomes at the end of the intervention only,85 with the other six74,76,86–89 employing various lengths of post-intervention follow-up, ranging from 4 weeks88 to 12 months. 87 Three studies76,87,89 had a follow-up of a reasonable duration to capture the clinical effects of the intervention (≥ 3 months post intervention; which the review Advisory Group suggested would be the minimum follow-up time necessary in studies to measure the effect and durability of patient benefits from an intervention).
Aims, content and structure of the educational interventions in the included trials
During data extraction, a modified version of a taxonomy of elements of interventions developed by Schulz and colleagues69 was used to structure how information about the interventions in each trial was recorded (see Methods for more information about this). Following this principle, an overview is provided here of the educational interventions in each trial, with summary details shown in Table 3. More detailed information about the interventions, covering all elements extracted, can be found in Appendix 6.
The aims, content and structure of the educational interventions were heterogeneous across the seven included RCTs. Only one of the included studies,74 of the SPaCE website intervention, explicitly reported that the aim of the intervention was to improve HRQoL (although another, by Staab and colleagues,87 was in part based on the ‘Berlin education programme’ reported in the excluded Staab and colleagues93 and Wenninger and colleagues78 publications, and these linked publications state that the aim of the intervention was to improve HRQoL; see Table 3 for more details). The other studies were included in the review because it was inferred from the content that the intervention could improve HRQoL (e.g. it included aspects that targeted compliance or patients’ ability to cope with the negative effects of their disease).
Three of the studies reported that the educational interventions were theory-based. The one-off educational session for patients with psoriasis in Ersser and colleagues86 and the 6-week programme for atopic dermatitis in Staab and colleagues87 were based on social cognitive theory. The SPaCE website intervention in the trial by Sanler and colleagues74 incorporated 20 of the 26 behaviour-change techniques listed in Abraham and Michie’s77 taxonomy of behaviour-change techniques. The design of the intervention in Ersser and colleagues86 was additionally informed by findings of previous qualitative research on the self-management needs of individuals with psoriasis. Similarly, the design and content of the SPaCE website in Santer and colleagues74 was based on qualitative interviews and input from a patient support group, as well as evidence-based patient information leaflets, ‘think-aloud’ interviews with users of a draft version of the website and feedback from other parents and HCPs.
The educational content and strategies used in the interventions varied across the studies. All the trials except one86 reported that participants were provided with information about the skin disease, its treatment, skin care, or the causes, consequences and treatment of itch. The interventions also commonly included education about stress-reducing or relaxation techniques (reported in five RCTs74,76,86,87,89) and living a healthy lifestyle and/or nutritional issues (reported in five RCTs74,76,85,87,89). Other psychosocial aspects included education on coping with the disease and managing itching, scratching and sleep,74,87,89 managing the psychosocial consequences of the disease,85 and provision of information about patient support groups. 89 The SPaCE website for carers of children aged 5 years or younger with eczema also contained information about how to involve their child in their treatment and guidance for carers on how to manage a consultation with their GP. 74 The intervention in the trial by Matsuoka and colleagues,88 in which patients with acne were provided with instructions on skin care and make-up use, could be considered to help patients manage appearance concerns and the feelings of stigma associated with their condition. As well as information provision, the reported education strategies used included encouraging participants to share experiences,87 discussion of difficulties in transferring newly learnt skills into their everyday lives,87 individual action planning or creation of (self-)management plans,74,86,87,89 feedback sessions,76 a 2-week challenge involving short message service (SMS) text alerts for setting goals, monitoring and rehearsing behaviours,74 and cognitive behavioural therapy, including habit reversal to reduce scratching. 89 Where reported, the duration and intensity of the educational interventions ranged from two 20-minute compulsory online modules (plus optional additional modules)74 to a 3-month programme consisting of two 3-hour sessions a week. 76
In all the RCTs except one,85 educational interventions were delivered at least in part through face-to-face sessions, and in three trials it was also delivered in groups. Group sizes across and within the studies ranged from 5 to 23 participants. 76,86,87 In the RCT by Santer and colleagues,74 the intervention was mainly delivered online (via the SPaCE website), but one group also received additional support from a HCP in a face-to-face appointment. Participants could also opt to take part in a 2-week challenge, which involved receiving behavioural prompts by text message. It is unclear if the instructions in skin care and using make-up were provided to patients individually or as part of groups in the RCT by Matsuoka and colleagues. 88 In one trial85 education was provided to individuals solely by daily text messages, over a period of 12 weeks. The educational interventions were delivered by a multidisciplinary team in two RCTs,76,87 a nurse in another two RCTs86,89 and a dermatologist in one RCT. 88 In the Santer and colleagues RCT,74 the SPaCE website, which had been developed by medical experts and informed by patient support groups, was the main delivery mechanism. The HCPs who provided support to one group varied across the general practices taking part and were the practice nurse in 11 practices, a health-care assistant in one practice and a GP in one practice. Only one of the HCPs was dermatology trained. In the remaining RCT, of text message education,88 it was unclear who provided the intervention. Three trials74,86,87 reported information about the interventionist characteristics, with all stating that the interventionists received training prior to delivering the programmes (although this was minimal in Santer and colleagues74 and consisted of 1 hour for the HCP to familiarise themselves with the SPaCE website). None of the RCTs provided information about whether the people delivering the educational interventions had similar characteristics to the patients taking part, such as similar social and ethnic backgrounds.
Ersser and colleagues,86 Bostoen and colleagues76 and Staab and colleagues87 reported that the interventionist was required to follow a protocol, syllabus or content manual, respectively, to standardise delivery. Delivery of the text message85 and website education74 was also standardised (but with some optional as well as compulsory modules on the website). In Ersser and colleagues,86 there was flexibility for the intervention to be tailored to participants’ needs. Another two RCTs74,89 also reported that, to some extent, the educational interventions were tailored to patients’ individual needs. The interventions in two trials85,87 were sensitive to participants’ characteristics to some extent, by providing parent and child education according to age group87 and ensuring that text messages were written in simple language. 85 None of the interventions took into account individuals’ preferred learning styles.
Outcomes assessed in the included trials
As per our inclusion criteria, all seven RCTs measured HRQoL using one or more validated measures. All seven trials also measured disease severity as an outcome. Other measured outcomes were: depression, stress, lifestyle (measured by a set of unvalidated questions in one trial76), health resource use, medication and ointment use, cost-effectiveness, itching behaviour, itch-related coping, skin-related psychosocial morbidity, general psychosocial morbidity, patient–physician relationship, attitudes and perceptions of ability to manage the condition, and treatment adherence. Only one trial88 measured adverse events. In line with our protocol, patient-reported outcome measures were extracted and included in the review only if assessed by validated tools. HRQoL was a primary outcome in four of the trials76,86,87,89 and a secondary outcome in one trial. 74 The remaining two trials85,88 did not specify if outcomes were primary or secondary. Three RCTs74,85,86 included process measures that could be regarded as ‘process evaluations’ (e.g. assessing patients’ perceptions of the usefulness of the intervention).
Four of the included trials76,85,86,88 measured HRQoL using the DLQI, which is a dermatology-specific, self-report measure. 95 van Os-Medendorp and colleagues89 measured HRQoL using the QoL subscale of the Adjustment to Chronic Skin Diseases Questionnaire (ACS), which measures skin-related psychosocial morbidity. Staab and colleagues87 measured parents’ HRQoL using the German questionnaire ‘Quality of life in parents of children with atopic dermatitis’. Santer and colleagues74 measured the impact of the website for carers on each family’s QoL using the Dermatitis Family Impact (DFI) questionnaire and also measured the impact on the carers’ children’s HRQoL using the IDQoL (for children aged ≤ 4 years) and the CDLQI (for children aged ≥ 5 years). Other HRQoL measures used in the RCTs were: Skindex-29,76 PDI,76 Quality of Life Index for Atopic Dermatitis (QoLIAD)76 and WHOQOL-26. 88 For more information about the most commonly used measures and how they are interpreted, please see Appendix 1.
Disease severity was assessed using a range of measures, including the PASI (used in all three trials that included patients with psoriasis76,85,86), the SCORAD (used in two studies including patients with atopic dermatitis76,87) and the POEM (used in a study of children with eczema and their carers74). Other measures used were the Plewig and Kligman’s grade measure of acne severity88 and a patient-reported measure of the frequency and intensity of itching. 89 For more information about the most commonly used measures and how they are interpreted, see Appendix 1.
Participants’ baseline characteristics
Table 4 shows the baseline characteristics of the participants randomised in each of the trials. Generally, across the trials, 50% or more of the participants in each study arm were women. The RCT by Matsuoka and colleagues,88 which evaluated skin care and make-up instructions for patients with acne, focused exclusively on women. Most carers of children randomised in the trial by Santer and colleagues74 were women. The overall mean age of participants in the studies of adult patients ranged from 3885 to 59 years,86 except in the trial by Matsuoka and colleagues88 where the mean age of the women was 24 years and 25 years in each arm. In the trial of children and adolescents with atopic dermatitis,87 the baseline mean ages were around 2 years in the 3 months to 7 years age group, 10 years in the 8–12 years age group and 15 years in the 13–18 years age group. In Santer and colleagues,74 the majority of carers were aged between 26 and 40 years and the ages of the children were between 0 to 5 years, as per the study inclusion criteria. None of the trials reported the participants’ ethnicity. Across the four trials reporting participants’ socioeconomic characteristics,74,76,85,89 there was a mixture of levels of education and employment status, see Table 4.
| Study, skin condition (population) | Sex, % female | Age (years), mean (SD) | Disease duration, mean (SD) years | HRQoL, mean score (SD) | Disease severity, mean score (SD) (unless stated) | Socioeconomic characteristics, % of participants |
|---|---|---|---|---|---|---|
| Balato et al., 2013,85 plaque psoriasis (adults) | I: 50; C: 40 | I: 38.4 (9.5); C: 39.3 (10.2) | I: 10.7 (5.3); C: 12.1 (5.8) | DLQI I: 7.9 (3.2); C: 7 (3) |
PASI I: 10.64 (4.2); C: 10.13 (4.7) SAPASI I: 11 (6.6); C: 10.90 (5.9) BSA I: 16 (7.5); C: 14.2 (8) PGA I: 2.6 (1.04); C: 2.3 (1.3) |
Education level and employment: Middle school I: 25; C: 30 High school I: 60; C: 50 Some college attendance I: 0; C: 5 College graduate I: 15; C: 15 Currently employed I: 80; C: 75 |
| Ersser et al., 2011,86 psoriasis (adults) | I: 71; C: 45 | I: 56.86 (12.67); C: 59.03 (13.53) | I: 22.68 (17.99); C: 24.17 (18.63) | DLQI I: 4.86 (5.14); C: 4.18 (3.19) |
PASI I: 2.34 (2.66); C: 3.22 (2.26) |
Not reported |
| Bostoen et al., 2012,76 psoriasis or atopic dermatitis (adults) | I: 52; C: 52 | I: 38.5 (12.3); C: 40.6 (12.2) | I: 18.9 (11.0); C: 20.1 (11.4) | DLQI I: 9.7 (6.0); C: 7.5 (5.0) Skindex-29 (total) I: 45.5 (16.1); C: 43.3 (17.7) QoLIAD (atopic dermatitis subgroup only) I: 9.1 (5.6); C: 9.6 (6.1) |
PASI I: 8.4 (CI 6.0 to 10.8); C: 7.1 (CI 4.8 to 9.4) SCORAD I: 38.9 (18.0); C: 38.8 (15.5) EASI I: 11.9 (10.9); C: 10.4 (8.1) |
Education level: Low I: 4; C: 4 Medium I: 22; C: 52 High I: 74; C: 44 |
| Santer et al., 201474 | Carers: Website: 96 Website + HCP: 98 Usual care: 98 |
Age of carer: the majority of participants (72–84%) across each arm were aged between 26 and 40 years Age of child: all between 0 and 5 years |
Not reported | DFI Website: 5.3 (5.3) Website + HCP: 6.4 (5.6) Usual care: 5.2 (5.9) |
POEM Website: 10.3 (7.0) Website + HCP: 9.4 (6.2) Usual care: 7.47 (6.2) |
Age carer left education: across study arms, between 12% and 16% left school between the ages of 15 and 16 years |
| Staab et al., 2006,87 atopic dermatitis (children, adolescents and parents) | 3 months to 7 years I: 48; C: 48 8–12 years I: 60; C: 52 13–18 years I: 59; C: 64 |
3 months to 7 years: I: 2.4 (1.8); C: 2.4 (1.9) 8–12 years: I: 9.5 (1.6); C: 9.5 (1.5) 13–18 years: I: 14.9 (1.7); C: 14.8 (1.7) |
Not reported | Reports baseline results for parents’ HRQoL across a number of HRQoL measure subscales; see the data extraction form in Appendix 4 for baseline values | SCORAD:a 3 months to 7 years I: 41.1 (16.6); C: 40.6 (15.2) 8–12 years I: 41.8 (16.6); C: 40.4 (15.1) 13–18 years I: 43.1 (14.7); C: 40.4 (13.9) |
Not reported |
| Matsuoka et al., 2006,88 acne vulgaris (adults) | I: 100; C: 100 | I: 24 (3); C: 25 (5) | I: 7 (4); C: 4 (4) | DLQI I: 8.24 (5.06); C: 6.24 (6.06) WHOQOL-26 (total mean score) I: 3.27 (0.54); C: 3.36 (0.44) |
Not reported | Not reported |
| van Os-Medendorp et al., 2007,89 chronic pruritic skin disease (adults) | I: 55; C: 72 | I: 57 (17.3); C: 55.7 (17.2) | I: 14.6 (14.4); C: 17.4 (18.3) | Impact on QoL (ACS subscale) I: 12.08 (5.00); C: 12.56 (4.93) |
Patient-reported frequency and intensity of itching and scratching, n (%): High frequency I: 18 (72); C: 21 (66) High intensity I: 20 (80); C: 25 (78) |
Education level: Low I: 46; C: 51 Medium I: 21; C: 31 High I: 32; C: 17 |
Five trials reported the length of time participants had had their skin condition. 76,85,86,88,89 In four trials, the disease duration ranged from a mean of around 11 to 24 years. 76,85,86,89 Patients with acne in the trial by Matsuoka and colleagues88 had been diagnosed with their condition for a slightly shorter duration (for a mean of 7 years and 4 years in each study arm). Only two studies reported participants’ comorbidities, and these included hypertension, dyslipidaemia, type 2 diabetes and unspecified ‘complications’. 85,88 Where measured with the same tool, baseline disease severity was similar across trials of the same condition. An exception was that participants in the trial by Ersser and colleagues86 had milder psoriasis than the participants in the two other psoriasis trials (this may be because the participants in Ersser and colleagues were recruited from primary care). 76,85
Four of the trials,76,85,86,88 including all three of patients with psoriasis,76,85,86 used the DLQI to measure HRQoL. On this measure, participants’ baseline HRQoL was comparable across three of the studies, with mean scores in each study arm ranging from 6.24 to 9.7. 76,85,88 According to the DLQI website,96 these scores indicate a moderate disease effect on patients’ HRQoL. In the remaining trial86 of patients with psoriasis, mean scores were 4.86 and 4.18 in the intervention and control groups, respectively, indicating a small effect on patients’ HRQoL. 96 Participants in this trial therefore had generally experienced less impact on their HRQoL than the participants in the other psoriasis trials. In addition to the DLQI, Matsuoka and colleagues88 used the WHOQOL-26 to measure HRQoL in patients with acne. The authors cite a normative mean score of 3.33 for healthy Japanese women aged 20–29 years, and state that the baseline scores of the participants were similar to this, suggesting that, on this measure, their HRQoL had not been extensively adversely affected by their condition. In the three trials using less common methods to assess HRQoL,74,87,89 the meaning of the baseline scores is unclear, because authors did not provide this information.
Baseline characteristics and baseline scores for outcome measures were generally similar between the intervention and comparator groups in all the trials. Exceptions were that in the RCT by Ersser and colleagues86 there were proportionally more women in the intervention than the control group and that in the Santer and colleagues trial74 POEM baseline scores were slightly higher in the website and website + HCP support groups than the usual care group. In the Bostoen and colleagues trial,76 rates of depression were higher in the intervention group than the control group.
Quality of reporting and methodology of the included trials
The quality of the reporting and methodology of the included trials was generally poor (Table 5), with only two studies86,88 judged not to be at high risk of bias on at least one domain, and all RCTs including at least one domain judged to be at unclear risk of bias, with few instances of low risk of bias. Five trials74,76,85–87 reported adequate random sequence generation, but only one of the seven included trials clearly reported how allocation was concealed and, therefore, the remaining six were judged to be at an unclear risk of bias. Most trials were judged to be at an unclear risk of performance and detection bias, because they did not report if participants, study personnel or outcome assessors were blinded to treatment allocation. Balato and colleagues85 and Staab and colleagues87 were judged to be at high risk of performance bias; in Balato and colleagues,85 although it was noted that physicians were blinded to group assignment until the end of the study, no details were provided about the blinding of participants, and in Staab and colleagues87 it was stated that participants and trainers were not blinded to treatment allocation. The trial by Staab and colleagues87 was also judged to be at high risk of detection bias (on self-reported measures) because participants were not blinded, and it was unclear if the investigators who rated eczema severity were blinded to treatment allocation. However, it may be difficult to blind participants to the fact they are taking part in an educational intervention rather than just receiving standard medical care, but this criterion is still appropriate for demonstrating potential risks of bias.
| Study, skin condition (population) | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other bias |
|---|---|---|---|---|---|---|---|
| Balato et al., 2013,85 plaque psoriasis (adults) | Low risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Low risk |
| Ersser et al., 2011,86 psoriasis (adults) | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Bostoen et al., 2012,76 psoriasis or atopic dermatitis (adults) | Low risk | Unclear risk | Unclear risk | Unclear risk | High risk | Unclear risk | Unclear risk |
| Santer et al., 2014,74 eczema (children and parents/carers) | Low risk | Low risk | Unclear risk | Unclear risk | Unclear risk | High risk | Unclear risk |
| Staab et al., 2006,87 atopic dermatitis (children, adolescents and parents) | Low risk | Unclear risk | High risk | High risk | High risk | Unclear risk | Low risk |
| Matsuoka et al., 2006,88 acne vulgaris (adults) | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk |
| van Os-Medendorp et al., 2007,89 chronic pruritic skin disease (adults) | Unclear risk | Unclear risk | Unclear risk | Unclear risk | High risk | Unclear risk | Low risk |
Incomplete outcome data were adequately addressed in three trials85,86,88 either because all participants completed the trial and were analysed according to their randomised groups,85,88 or because attrition was balanced across arms with similar reasons for drop-out and was therefore considered unlikely to bias the results. 86 One trial74 was rated as being at an unclear risk of bias on this criterion, because, although attrition rates were small and balanced across groups, clear reasons were not reported for the attrition, and, in addition, one participant was randomised and excluded from the analysis because technical difficulties resulted in baseline data not being available for this participant. The other three trials76,87,89 were considered to be at high risk of attrition bias, either because drop-outs were unbalanced between groups76,87 or because there was a high overall rate of attrition89 (with no exact reasons provided other than that participants did not return study measures at particular time points and that four participants in the control group received the intervention and therefore were excluded) and no intention-to-treat (ITT) analyses were used. In addition, one participant in the educational intervention group in Bostoen and colleagues76 was excluded from the analysis owing to experiencing extreme stress at work during the study.
Two trials were assessed as being at low risk of selective reporting, because the authors reported results for all the outcome measurements specified in the methods section of the paper. 85,86 Four were considered to be at an unclear risk of bias for various reasons, including narratively reporting results for many of the outcomes without providing supporting numerical data;76 converting a continuous measure to a dichotomous measure for a severity of itching and scratching outcome;89 reporting within-group p-values for two HRQoL outcomes, with between-group p-values reported for only one of the measures;88 and measuring outcomes at 6 and 12 months’ follow-up, but not reporting the 6-month outcomes. 87 The RCT by Santer and colleagues was considered to be at high risk of selective reporting bias, because results for the IDQoL and CDLQI measures of HRQoL were not reported. 74 Results were also not reported for a number of other measures [specifically, self-report measure of emollient use; Problematic Experiences of Therapy Scale (PETS); attitudes measure; and Patient Enablement Instrument].
Other potential sources of bias were identified in three trials where this was rated as ‘unclear’. In Bostoen and colleagues76 most results were presented as subgroup analyses for atopic dermatitis and psoriasis patients and it was unclear if these were adequately powered or pre-specified. In Ersser and colleagues86 a statistically significant higher proportion of participants in the intervention group compared with the control group were women. In Santer and colleagues,74 there were baseline imbalances in disease severity across groups, with both website groups having slightly more severe disease at baseline than the usual care group, and it was unclear if this had been adjusted for in the data analysis. In Santer and colleagues,74 statistical analyses were also reported inconsistently; specifically, mean change in the POEM score was presented for the combined website groups versus usual care, not individual website groups versus usual care (therefore this result was not data extracted or presented in this review).
Completeness of reporting of the interventions
During data extraction, a modified version of a taxonomy of elements of interventions69 was used to structure how information about the interventions in each trial was recorded. Table 6 shows the elements extracted and whether or not each trial reported details for each. The seven included trials generally provided adequately detailed descriptions of the educational interventions, particularly the content and topics, mode of delivery, providers, materials used and duration and intensity. Few trials explicitly reported the intervention aim, whether the intervention was theory-based, where it was delivered, whether elements were tailored to participants’ needs, the extent to which it was sensitive to participants’ characteristics, the interventionist characteristics and training, or whether ongoing support was provided after the intervention ended.
| Study, skin condition (population) | Intervention aim(s) | Where delivered | Self-help, individual- and/or group-based | Mode | Materials | Provider | Duration and intensity | Scriptinga | Sensitivity to participant characteristics | Interventionist characteristics and training | Content and topics | Tailoring | Ongoing support | Theory |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balato et al., 2013,85 plaque psoriasis (adults) | b | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Ersser et al., 2011,86 psoriasis (adults) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Bostoen et al., 2012,76 psoriasis or atopic dermatitis (adults) | b | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Santer et al., 2014,74 eczema (children and parents/carers) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Staab et al., 2006,87 atopic dermatitis (children, adolescents and parents) | b | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Matsuoka et al., 2006,88 acne vulgaris (adults) | b | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| van Os-Medendorp et al., 2007,89 chronic pruritic skin disease (adults) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Statistical issues
All the trials were superiority trials (where the aim is to demonstrate that one treatment is more effective than another). Only two trials76,87 reported power calculations. Despite this, in both it is unclear if the analyses that generated the results were adequately powered. In Bostoen and colleagues76 the results were mainly presented for subgroup analyses of patients with either psoriasis or atopic dermatitis and the power calculation was for the total sample size on an unspecified outcome [note that the subgroup results are presented separately below under each condition-specific section; results for two outcomes [stress and European Quality of Life-5 Dimensions (EQ-5D™) HRQoL measure] were reported for the total group, but only narratively and, therefore, they have not been included in this review]. In Staab and colleagues,87 the analyses of the results for the 3 months to 7 years age group appeared to be adequately powered (power calculation was based on the primary outcome, eczema severity), but it is unclear if the analyses of the results for the 8–12 years and 13–18 years age groups were adequately powered, because fewer participants per group were analysed than the 125 per group calculated as being needed. Three of the studies that did not report power calculations, including two psoriasis studies, were pilot studies. 74,85,86 Overall, with the exception of the analyses of the 3 months to 7 years age group in the Staab and colleagues RCT,87 it is unclear if any of the analyses presented in the trials and included in this review were adequately powered. It should also be noted that all the evidence available for psoriasis was based on either pilot studies85,86 or subgroup analyses. 76
Total attrition rates in five trials ranged from 0% to 26%,74,76,85–88 with four of these74,85,86,88 having no attrition or low attrition rates. The attrition rate in the remaining trial,89 in which the ‘Coping with itch’ programme was delivered to patients with chronic pruritic skin diseases during a median of three clinic visits, was particularly high at 58% overall, with 63% and 51% of patients in the education and control groups, respectively, not completing the trial. None of the trials reported use of ITT analyses, except Santer and colleagues,74 but reviewers note that the analyses in Santer and colleagues74 were not true ITT analyses, as not all randomised carers were included in the analyses. As stated above, the analyses in two trials85,88 can be regarded as ITT, as all patients were analysed according to their randomised groups.
Generally, the trials reported results as point estimates (means) for outcomes in the intervention and control groups, and provided measures of variability around these as either standard deviations (SDs) or 95% confidence intervals (CIs). In a number of instances, findings were reported only narratively, with no supporting numerical data provided (see the results sections for each skin condition in Assessment of effectiveness for specific details of where this occurred). Trials mostly reported the statistical significance of between-group differences, either narratively or by providing p-values. Only two trials86,87 reported CIs around between-group differences. Four of the trials provided some commentary in the publications on the definitions of clinically meaningful change in particular outcomes (specifically, the DLQI,76 PASI,86 SCORAD87 and POEM74), but only two directly applied these definitions to the interpretation of the results in the trial. Of these, one study,74 of the SPaCE website, reported the proportion of children with eczema who had experienced a clinically meaningful improvement in disease severity. The other,87 of a 6-week programme for atopic dermatitis, commented generally on whether the average improvement in disease severity among children and adolescents with atopic dermatitis seen in the study could be considered clinically significant.
Generalisability to UK clinical practice
The majority of the included trials are considered to be of limited generalisability to UK clinical practice, because most were small-scale studies conducted across a range of countries. The largest UK study was Santer and colleagues’74 evaluation of education delivered through the SPaCE website to 149 carers of children with eczema, most of whom were managed in primary care. Reviewers considered this trial to be of good generalisability to patients treated in primary care in the UK, because participants were recruited from 31 general practices and a range of socioeconomic areas. The other included UK study86 was a small pilot study in primary care, conducted in patients with psoriasis. In this study, participants’ baseline HRQoL scores indicated that their psoriasis had had, on average, a small effect on their HRQoL and the participants appeared to have, on average, milder disease at baseline compared with participants in the other psoriasis trials. Given this, it is also of some generalisability to UK clinical practice in primary care and to patients whose condition has had only a small impact on their HRQoL. The large-scale German trial of an educational intervention for children and adolescents with atopic dermatitis and their parents87 could be considered of some relevance to patients with moderate to severe atopic dermatitis seen in secondary care because it was conducted in seven centres with a range of different age groups and should thus capture a wider group of patients. However, the health-care system is likely to be different from the UK. In general, around 50% or more of the patients included in each of the arms of the studies were female, and this is reasonably representative of the chronic inflammatory skin condition populations in the UK. For example, psoriasis tends to affect equal proportions of males and females (see Epidemiology). 17 Acne also affects equal proportions of males and females,25 but the one included study of acne focused exclusively on women aged over 16 years and, therefore, its results may apply only to women in this age group and are unlikely to generalise to men (given that the intervention is, in part, focused on make-up use) or younger adolescents, who are commonly affected by this condition (see Epidemiology). Overall, therefore, the results presented below for educational interventions for children with eczema and their carers are the most generalisable to the UK.
Assessment of effectiveness
Trials of educational interventions for psoriasis
Three small trials, by Balato and colleagues,85 Bostoen and colleagues (a subgroup),76 and Ersser and colleagues,86 examined educational interventions that could improve HRQoL in patients with psoriasis, as an adjunct to standard medical care. Participants in the trial by Balato and colleagues85 were provided with educational text messages over 12 weeks. In the trial by Bostoen and colleagues,76 patients took part in an educational programme delivered in twice-weekly, 2-hour sessions over 3 months by a multidisciplinary team. In Ersser and colleagues,86 a shorter (a single, 2-hour session, supplemented by a 20-minute follow-up telephone call), theory-based, nurse-delivered self-management programme, targeted at individuals with mild to moderate plaque psoriasis, was delivered in primary care. The educational interventions in both Bostoen and colleagues76 and Ersser and colleagues,86 were compared with usual medical care alone. In the trial by Balato and colleagues,85 the text message education intervention was compared with an unspecified control intervention.
Balato and colleagues85 reported results for outcomes at 12 weeks (the end of the intervention), Bostoen and colleagues76 at 3 months (the end of the intervention), 6 months (although this was not data extracted and not reported here) and 9 months, and Ersser and colleagues86 at 6 weeks’ follow-up. At baseline, participants included in the trial by Ersser and colleagues86 had generally milder disease and had experienced less impact on their HRQoL than the participants in the other two trials. 76,85 (see Table 4). The results presented were from either pilot studies85,86 or subgroup analyses of patients with psoriasis who were part of a larger trial (see Statistical issues). 76
Health-related quality-of-life outcomes
All three trials used the DLQI to measure HRQoL. Bostoen and colleagues76 additionally used the PDI and Skindex-29. Skindex-29 results were reported only narratively, with no supporting data provided. Balato and colleagues,85 and Bostoen and colleagues76 found that patients who received the text message education and the 3-month educational intervention, respectively, had better DLQI scores at the end of the interventions than those who had received the control interventions (Table 7). However, this effect was not maintained at 9 months from baseline in Bostoen and colleagues. 76 In Bostoen and colleagues, the 3-month educational intervention group also had better PDI scores at the end of the intervention, and this was maintained at 9 months. 76 No statistically significant differences between groups were found on the Skindex-29. Ersser and colleagues86 found no statistically significant difference between the one-off education session and usual care alone groups in change in DLQI scores between baseline and 6 weeks’ follow-up.
| Study | Measure, time point | Score, mean (95% CI) unless otherwise stated | ||
|---|---|---|---|---|
| Intervention | Control | Difference between groups | ||
| Balato et al., 201385 | DLQI, mean (SD) | |||
| Baseline | 7.9 (3.2) (n = 20) | 7 (3) (n = 20) | ||
| 12 weeks (intervention end) | 4.2a (n = 20) | 5.8a (n = 20) | p-value < 0.05 | |
| Bostoen et al., 201276 | DLQI | (n unclear for all time points) | (n unclear for all time points) | |
| Baseline | 8.4 (5.6, 11.2)a | 6.6 (3.9, 9.3)a | ||
| 3 months (intervention end) | 4.4 (1.3, 7.4) | 6.4 (3.6, 9.2) | p-value = 0.019 | |
| 9 months | 4.0 (0.6, 7.4) | 5.8 (2.9, 8.8) | p-value = 1.00 | |
| PDI | ||||
| Baseline (SD) [95% CI] | 9.0 (6.8) [5.0 to 13.0]b | 7.6 (7.8) [3.8 to 11.5]b | ||
| 3 months (intervention end) | 4.3 (0.1, 8.4)b | 6.7 (2.9, 10.6)b | p-value = 0.015 | |
| 9 months | 4.9 (0.3, 9.5) | 7.4 (3.3, 11.6) | p-value = 0.021 | |
| Ersser et al., 201186 | DLQI, mean (SD) | |||
| Baseline | 4.86 (5.14) (n = 26) | 4.18 (3.19) (n = 33) | ||
| 6 weeks | 4.58 (5.05) (n = 26) | 3.70 (3.71) (n = 33) | ||
| Change from baseline | 0.28 (2.16)c (n = 26) | 0.48 (3.02)c (n = 33) | 95% CI: –1.20 to 1.61;d p-value = 0.772 | |
Disease severity outcomes
All three trials measured disease severity using the PASI. Balato and colleagues85 additionally used the SAPASI, body surface area (BSA) and physician’s global assessment (PGA). On all the disease severity measures employed in Balato and colleagues85 and Bostoen and colleagues,76 participants who received the text message education and 3-month educational intervention, respectively, showed statistically significant better scores (i.e. less severe disease) than the control groups at the end of the interventions (Table 8). However, Bostoen and colleagues76 found no statistically significant difference between groups on the PASI at 9 months after the start of the intervention. Ersser and colleagues86 found no statistically significant differences in change in disease severity between the one-off education session and usual care alone groups at the 6-week follow-up assessment.
| Study | Measure, time point | Score, mean (SD) (unless otherwise stated) | ||
|---|---|---|---|---|
| Intervention | Control | Difference between groups | ||
| Balato et al., 201385 | PASI | |||
| Baseline | 10.64 (4.2) (n = 20) | 10.13 (4.7) (n = 20) | ||
| 12 weeks (intervention end) | 5.8a (n = 20) | 6.8a (n = 20) | p-value < 0.05 | |
| SAPASI | ||||
| Baseline | 11 (6.6) (n = 20) | 10.90 (5.9) (n = 20) | ||
| 12 weeks (intervention end) | 5.9a (n = 20) | 8a (n = 20) | p-value < 0.05 | |
| BSA | ||||
| Baseline | 16 (7.5) (n = 20) | 14.2 (8) (n = 20) | ||
| 12 weeks (intervention end) | 5.8a (n = 20) | 8a (n = 20) | p-value < 0.05 | |
| PGA | ||||
| Baseline | 2.6 (1.04) (n = 20) | 2.3 (1.3) (n = 20) | ||
| 12 weeks (intervention end) | 0.7a (n = 20) | 1.5a (n = 20) | p-value < 0.05 | |
| Bostoen et al., 201276 | PASI, mean (95% CI) | (n unclear for all time points) | (n unclear for all time points) | |
| Baseline | 8.4 (6.0 to 10.8)b | 7.1 (4.8 to 9.4)b | ||
| 3 months (intervention end) | 6.8 (4.3 to 9.3)b | 8.1 (5.8 to 10.4)b | p-value = 0.036 | |
| 9 months | 7.0 (3.8 to 10.3) | 7.0 (3.8 to 10.3) | p-value = 0.116 | |
| Ersser et al., 201186 | PASI | |||
| Baseline | 2.34 (2.66) (n = 26) | 3.22 (2.26) (n = 33) | ||
| 6 weeks | 1.78 (1.62) (n = 26) | 2.82 (2.20) (n = 33) | Not reported | |
| Change from baseline | 0.56 (1.42)c (n = 26) | 0.40 (1.06)c (n = 33) | 95% CI: –0.81 to 0.49;d p-value = 0.619 | |
Other measured outcomes
The RCT by Bostoen and colleagues76 was the only psoriasis trial to measure other outcomes in addition to HRQoL and disease severity, namely, depression as measured by the Beck Depression Inventory (BDI) (Table 9) and medication use. The 3-month education programme group had a statistically significantly higher mean depression score at the end of the intervention (3 months) than the usual medical care group, but a statistically significant lower mean depression score at 9 months after the start of the intervention. The end of intervention result for this outcome might be explained by a baseline imbalance in mean depression scores, which was higher in the education than the control group. Regarding medication use, Bostoen and colleagues76 narratively reported that there were no differences between the groups at 3 and 9 months in the number of participants using topical, systemic or combined medication or the number not using any medication (for full results data, see the Bostoen and colleagues76 data extraction form in Appendix 4). Tests of statistical significance were not conducted for this outcome, because the data were not suitable for conducting tests of significance.
| Study | Measure, time point (n unclear for all time points) | Mean score (95% CI) | ||
|---|---|---|---|---|
| Intervention | Control | Difference between groups | ||
| Bostoen et al., 201276 | BDI (depression), mean (95% CI) | (n unclear for all time points) | (n unclear for all time points) | |
| Baseline | 12.3 (8.3 to 16.4) | 7.4 (3.5 to 11.3) | ||
| 3 months (intervention end) | 10.5 (6.1 to 14.9) | 6.3 (2.3 to 10.3) | p-value < 0.05 | |
| 9 months | 6.1 (1.7 to 10.5) | 7.3 (3.2 to 11.3) | p-value = 0.029 | |
Process evaluation findings
The trials by Balato and colleagues85 and Ersser and colleagues86 included measures that explored intervention processes. In the Balato and colleagues85 trial, participants were asked about how useful they had found the educational text messages. A total of 85% of the participants reported that they found the text messaging useful, with 75% stating that they would recommend it to a friend and 75% also stating that they would like to continue using it. Only 15%, however, agreed that they would be willing to pay for the service.
Ersser and colleagues86 obtained qualitative and quantitative data on participants’ perceptions of the usefulness of the education programme and recorded attendance. Qualitative data showed participants thought that the intervention was practical (not too time-consuming), convenient and that it provided follow-up care. Quantitative data were collected on participants’ perceptions of the usefulness of each intervention component, including the group learning session, digital versatile disc (DVD), workbook and telephone consultation. The majority of the participants (54–96%) rated each of these elements to be either moderately useful or very useful, with few (4–8%) rating them as not useful. A further 8% and 42% of the participants did not provide a response to the questions about the usefulness of the telephone consultation and DVD, respectively (there was a 100% response rate to the questions about the usefulness of the group learning session and workbook). There was good attendance at the group session, with 26 of the 28 randomised participants attending. Only 15 of the randomised participants, however, watched the DVD. Others reported that they had technical difficulties with it, did not like the DVD format and/or found that a workbook was more convenient to use periodically. One point of feedback from the research team also noted by Ersser and colleagues86 was that the smaller group sessions resulted in more interaction between participants than the larger sessions.
Trials of educational interventions for eczema/atopic dermatitis
Adults
One trial, by Bostoen and colleagues,76 examined a group-based educational intervention that could improve HRQoL in adults with atopic dermatitis. This trial also included patients with psoriasis and results were reported separately for the two patient subgroups (for psoriasis subgroup results, see Trials of educational interventions for psoriasis). Twenty-one patients were in the atopic dermatitis subgroup. The baseline severity of atopic dermatitis was considered ‘moderate’ by the authors. Patients took part in an educational programme, delivered as an adjunct to usual medical care, and delivered in twice-weekly, 2-hour sessions over 3 months by a multidisciplinary team. The educational intervention was compared with medical therapy alone. Outcomes were assessed at baseline, 3 months (i.e. the end of the intervention) and at 9 months (i.e. 6 months post intervention). Outcomes were mostly described narratively, with no CIs or p-values reported.
Health-related quality-of-life outcomes
Health-related quality of life was specified as a primary outcome. However, apart from QoLIAD scores at baseline, no quantitative HRQoL results were reported. The authors stated narratively that there were no significant differences between the educational intervention and control groups for the DLQI or QoLIAD at 3 and 9 months.
Disease severity outcomes
Bostoen and colleagues76 also reported baseline disease severity outcomes in adults with atopic dermatitis, but did not provide any quantitative data for follow-up assessments. The authors stated narratively that there were no significant differences between the educational intervention and control groups for the Eczema Area and Severity Index (EASI) or SCORAD measures of disease severity at 3 and 9 months.
Other measured outcomes
Bostoen and colleagues76 reported medication use and depression outcomes. The numbers of patients in the intervention and control groups who used each type of medication (topical, systemic, both, none or total medications) did not differ significantly between the groups (p-values were not reported) (Table 10). The authors narratively reported that there were no significant differences in depression between groups over time.
| Study | Measure, time pointa | Number of patients | ||
|---|---|---|---|---|
| Intervention (n = 10)b | Control (n = 11)b | Difference between groups | ||
| Bostoen et al., 201276 | Topical medications | |||
| Baseline | 8 | 9 | ||
| 3 months | 7 | 8 | Stated NS | |
| 9 months | 7 | 7 | Stated NS | |
| Systemic medications | ||||
| Baseline | 0 | 0 | ||
| 3 months | 0 | 0 | Stated NS | |
| 9 months | 0 | 0 | Stated NS | |
| Combination medications | ||||
| Baseline | 2 | 2 | ||
| 3 months | 1 | 2 | Stated NS | |
| 9 months | 1 | 1 | Stated NS | |
| No medications | ||||
| Baseline | 0 | 0 | ||
| 3 months | 1 | 0 | Stated NS | |
| 9 months | 0 | 0 | Stated NS | |
| Total medications | ||||
| Baseline | 10 | 11 | ||
| 3 months | 9 | 10 | Stated NS | |
| 9 months | 8 | 8 | Stated NS | |
Process evaluation findings
No evaluations of process were reported.
Children, adolescents and their parents
Two RCTs included children with eczema/atopic dermatitis and their parents or carers. 74,87 One study, by Staab and colleagues,87 investigated the effectiveness of a group-based educational intervention that could improve HRQoL in children or adolescents with atopic dermatitis and their carers, which was delivered by a multiprofessional team. The study included three subgroups with atopic dermatitis: children aged 3 months to 7 years; children aged 8–12 years; and adolescents aged 13–18 years. Patients’ carers participated in the interventions for the 3 months to 7 years and 8–12 years age groups, and could participate optionally in some sessions for the 13–18 years age group. The other study, by Santer and colleagues,74 was a pilot RCT that evaluated the SPaCE website, which provided education for carers of children with eczema aged 5 years or under recruited from primary care in the UK. There were three groups in this study: website only, website + HCP support and usual care alone. Both the website intervention groups also received usual care. Outcomes were measured at 12 months’ follow-up in Staab and colleagues87 and 3 months following baseline in Santer and colleagues. 74 Overall, the trial by Staab and colleagues87 was of a reasonable size, but it may not have been powered to detect intervention effects in the subgroups, especially the adolescent subgroup. Similarly, the Santer and colleagues74 trial was of a reasonable size, but it was unclear if this pilot RCT was adequately powered. The trial by Santer and colleagues was the only trial included in the review explicitly stating that the aim of the intervention was to improve QoL, although publications78,93 linked to Staab and colleagues87 provided information that the intervention for 3-month- to 7-year-olds was based on an intervention of which part of the aim was to improve the family’s HRQoL.
Health-related quality-of-life outcomes
Health-related quality of life was specified as a primary outcome in the study by Staab and colleagues87 and was assessed using the German questionnaire ‘Quality of Life in Parents of Children with Atopic Dermatitis’ (Table 11). Parental HRQoL results were reported for the two children subgroups (3 months to 7 years and 8–12 years of age) for five questionnaire domains (psychosomatic well-being, effects on social life, confidence in medical treatment, emotional coping, and acceptance of disease). In both age groups the scores for all five questionnaire domains increased in both the intervention and control groups, but the increase was consistently larger in the intervention group than in the control group (the authors did not provide any guidance on how to interpret this measure). Apart from the psychosomatic well-being and effects on social life domains in the 8–12 years subgroup, the differences between intervention and control groups were statistically significant (see Table 11). Overall, these findings suggest that the educational intervention contributed to improving HRQoL among the parents of children in both age subgroups. Santer and colleagues74 measured the impact of the online interventions for carers on the family’s QoL using the DFI questionnaire (Table 12). Across all three arms, DFI questionnaire scores were slightly lower at 3 months than at baseline (i.e. they had slightly improved), but the authors did not report if there were any statistically significant differences between groups in DFI questionnaire scores or changes in DFI questionnaire scores at 3 months. The authors suggest that, as scores were low at baseline (i.e. showing reasonably good QoL), the follow-up scores represent floor effects (i.e. there was no room for improvement). Santer and colleagues74 also measured the impact of the interventions on children’s HRQoL using the IDQoL and CDLQI measures, but do not report these results.
| Study: Staab et al., 200687 | Questionnaire item | Change in ‘Quality of Life in Parents of Children with Atopic Dermatitis’ score from baseline to 12 months (covariance analysis), mean (95% CI)a | ||
|---|---|---|---|---|
| Intervention (n = 274) | Control (n = 244) | Difference between groups | ||
| Age group: 3 months to 7 years | Psychosomatic well-being | 4.4 (3.6 to 5.2) | 3.1 (2.2 to 3.9) | 1.4 (0.2 to 2.5); p-value = 0.004 |
| Effects on social life | 1.8 (1.4 to 2.3) | 1.0 (0.6 to 1.5) | 0.8 (0.2 to 1.4); p-value < 0.0001 | |
| Confidence in medical treatment | 4.0 (3.5 to 4.5) | 1.9 (1.4 to 2.4) | 2.1 (1.4 to 2.8); p-value < 0.0001 | |
| Emotional coping | 3.1 (2.7 to 3.5) | 1.1 (0.7 to 1.6) | 1.9 (1.3 to 2.5); p-value < 0.0001 | |
| Acceptance of disease | 1.1 (0.8 to 1.3) | 0.5 (0.3 to 0.8) | 0.6 (0.2 to 0.9); p-value < 0.0001 | |
| Age group: 8–12 years | Questionnaire item | Intervention (n = 102) | Control (n = 83) | Difference between groups |
| Psychosomatic well-being | 3.2 (1.9 to 4.5) | 2.6 (1.4 to 3.8) | 0.6 (–1.2 to 2.4); p-value = 0.36 | |
| Effects on social life | 1.1 (0.4 to 1.8) | 0.9 (0.2 to 1.6) | 0.2 (–0.8 to 1.2); p-value = 0.94 | |
| Confidence in medical treatment | 3.1 (2.2 to 3.9) | 0.1 (–0.7 to 1.0) | 2.9 (1.7 to 4.1); p-value < 0.0001 | |
| Emotional coping | 2.7 (2.0 to 3.4) | 0.9 (0.2 to 1.6) | 1.8 (0.9 to 2.8); p-value = 0.002 | |
| Acceptance of disease | 0.8 (0.4 to 1.2) | 0.2 (–0.2 to 0.6) | 0.6 (0 to 1.2); p-value = 0.031 | |
| Study: Santer et al., 201474 | Mean (SD) | |||
|---|---|---|---|---|
| Measure, time point | Intervention 1: website only (n = 44) | Intervention 2, website + HCP support (n = 50) | Control: usual care alone (n = 50) | Difference between groups |
| DFI | ||||
| Baseline | 5.3 (5.3) | 6.4 (5.6) | 5.2 (5.9) | |
| 3 months | 4.0 (4.2) (n = 44) | 5.9 (5.3) (n = 50) | 4.4 (5.5) (n = 50) | Not reported |
Disease severity outcomes
Disease severity was assessed by Staab and colleagues87 for all three age groups (i.e. 3 months to 7 years, 8–12 years, and 13–18 years) using the objective and total SCORAD scores and the Skin Detective subjective score (Table 13). In each of these age groups, the scores on all three severity measures decreased from baseline to 12 months in both the educational intervention group and the control group. In all cases, the decreases were statistically significantly larger in the intervention group than in the control group. These findings suggest that the educational intervention consistently improved (i.e. reduced) disease severity irrespective of patients’ age. In Santer and colleagues,74 disease severity, as measured by the POEM score, improved in all three arms at 3 months in comparison to baseline, but the authors did not report if there were any statistically significant differences between arms (Table 14). The authors also reported the proportion of children from each trial group who showed a clinically significant change in disease severity at 3 months (defined as a change in the POEM score of ≤ 2). There were no statistically significant differences between groups in the proportion of children who showed a clinically significant improvement in their eczema.
| Study: Staab et al., 200687 | Change in disease severity score from baseline to 12 months (covariance analysis), mean (95% CI)a | |||
|---|---|---|---|---|
| Age group: 3 months to 7 years | Measure | Intervention (n = 274) | Control (n = 244) | Difference between groups |
| SCORAD: total severity score | –17.5 (–19.6 to –15.3) | –12.2 (–14.3 to –10.1) | –5.2 (–8.2 to –2.2), p-value = 0.0002 | |
| SCORAD: objective severity score | –13.0 (–14.8 to –11.2) | –8.7 (–10.5 to –7.0) | –4.2 (–6.8 to –1.7), p-value = 0.0009 | |
| ‘Skin Detective’ subjective severity score | –3.3 (–3.9 to –2.8) | –2.2 (–2.7 to –1.6) | –1.1 (–1.9 to –0.3), p-value < 0.001 | |
| Age group: 8–12 years | Measure | Intervention (n = 102) | Control (n = 83) | Difference between groups |
| SCORAD: total severity score | –16.0 (–20.0 to –12.0) | –7.8 (–11.4 to –4.3) | –8.2 (–13.6 to –2.8), p-value = 0.003 | |
| SCORAD: objective severity score | –12.3 (–15.6 to –8.9) | –5.6 (–8.7 to –2.5) | –6.7 (–11.2 to –2.1), p-value = 0.005 | |
| ‘Skin Detective’ subjective severity score | –3.7 (–4.6 to –2.7) | –1.6 (–2.5 to –0.7) | –2.1 (–3.4 to –0.8), p-value < 0.001 | |
| Age group: 13–18 years | Measure | Intervention (n = 70) | Control (n = 50) | Difference between groups |
| SCORAD: total severity score | –19.7 (–23.7 to –15.7) | –5.2 (–10.5 to 0.1) | –14.5 (–21.2 to –7.9), p-value < 0.0001 | |
| SCORAD: objective severity score | –15.0 (–18.4 to –11.6) | –5.1 (–9.5 to –0.6) | –9.9 (–15.5 to –4.3), p-value < 0.0001 | |
| ‘Skin Detective’ subjective severity score | –3.1 (–4.1 to –2.2) | –1.0 (–2.1 to 0.1) | –2.1 (–3.5 to –0.7), p-value < 0.0002 | |
| Study: Santer et al., 201474 | Mean (SD) (unless stated) | |||
|---|---|---|---|---|
| Measure, time point | Intervention 1: website only (n = 44) | Intervention 2, website + HCP support (n = 50) | Control: usual care alone (n = 49) | Difference between groups |
| POEM score | ||||
| Baseline | 10.3 (7.0) | 9.4 (6.2) | 7.47 (6.2) | |
| 3 months | 7.6 (6.1) | 8.7 (7.0) | 7.1 (6.6) | Not reported |
| Clinically significant change in POEM score between baseline and 3 months, n/N (%) | 23/42 (55) | 18/47 (38) | 16/49 (33) | p-value = 0.09 |
Other measured outcomes
Santer and colleagues74 measured other outcomes, including adherence to emollient use, adherence to the intervention, attitudes, and participants’ perceptions of their abilities to manage eczema, but did not report findings for these outcomes. Staab and colleagues87 assessed itching behaviour in two subgroups using the Juckreiz-Kognitions-Fragebogen Kinder (JUCKKI) questionnaire for 8- to 12-year-old children and the Juckreiz-Kognitions-Fragebogen Jugendliche (JUCKKU) questionnaire for adolescents aged 13–18 years. These measures yielded scores for itching catastrophisation (how the individual experiences the itching) and coping with itching (Table 15). In both age groups, the scores for itching catastrophisation decreased (i.e. improved) from baseline to 12 months, both in the educational intervention and control groups, with the decrease being statistically significantly larger in the intervention group than in the control group. In contrast, an improvement in coping with itching was only seen in the 8–12 years age group participants. Overall, these findings suggest that the educational intervention had an effect on reducing itch-related catastrophising, irrespective of the patient age group, but had less impact on coping with itch, which appears to have improved only in the 8- to 12-year-old group.
| Study: Staab et al., 200687 | Change in score from baseline to 12 months (covariance analysis), mean (95% CI)a | |||
|---|---|---|---|---|
| Age group: 8–12 years | Measure: JUCKKI | Intervention (n = 102) | Control (n = 83) | Difference between groups |
| Itching behaviour: catastrophisation | –7.0 (–8.9 to –5.1) | –1.8 (–3.5 to –0.2) | –5.2 (–7.7 to –2.7), p-value < 0.0001 | |
| Itching behaviour: coping | 1.0 (–0.3 to 2.3) | –0.4 (–1.6 to 0.8) | 1.5 (–0.3 to 3.2), p-value = 0.047 | |
| Age group: 13–18 years | Measure: JUCKKU | Intervention (n = 70) | Control (n = 50) | Difference between groups |
| Itching behaviour: catastrophisation | –6.8 (–8.6 to –5.0) | –2.0 (–3.9 to –0.2) | –4.7 (–7.3 to –2.2), p-value = 0.0002 | |
| Itching behaviour: coping | –0.2 (–1.9 to 1.5) | 0.4 (–1.2 to 2.1) | –0.6 (–3.0 to 1.7), p-value = 0.875 | |
Process evaluation findings
Only Santer and colleagues74 carried out what could be considered a process evaluation. Quantitative data were collected on website use and uptake of the HCP appointment. Qualitative data were obtained via interviews with and feedback from participants and the HCPs. As Table 16 shows, carers in the website + HCP support group spent more time using the website than carers in the website only group and proportionally more made three or more visits to the website. There did not appear to be any other differences between the two groups in their website use. (Note, however, that differences did not appear to have been statistically tested and the reviewers’ interpretation of these data differ to the study authors’,74 who stated that there were no differences between groups.) Overall, the findings show a reasonably high completion rate of the core modules, and some use of other aspects of the intervention, but that the median time carers spent using the website in the website only group was less than intended (median of 34 minutes; it was intended that participants would spend at least 40 minutes completing at least two compulsory modules). In the website + HCP support group, 23 of the 50 participants (46%) took up the offer of a HCP appointment. Reasons for non-uptake included the participant declining the appointment (n = 12), the participant not being contactable, so an appointment could not be arranged (n = 9), and non-attendance at the appointment (n = 6).
| Study: Santer et al., 201474 | n/N carers (%) (unless stated) | |
|---|---|---|
| Measure | Intervention 1: website only | Intervention 2: website plus HCP support |
| Time spent on website (minutes), median (IQR) | 34 (20–50) | 45 (26–70) |
| Completion of core modules | 38/44 (86) | 37/49 (76) |
| Three or more website visits | 16/44 (36) | 29/49 (59) |
| Watched ≥ 1 video | 16/44 (36) | 17/49 (35) |
| Took part in 2-week challenge text alerts | 18/44 (41) | 18/49 (37) |
Feedback from the carers about the website included that participants found it easy to use and useful. Only five of the 26 participants interviewed stated that they did not find it useful, and their reasons included that they had previously needed more support than now, and that their child’s eczema was mild. There was more variation in the perceived value of the HCP support from the carers who had received this. Of those who found the support useful, reasons reported included that it had helped carers engage more with the website, it had increased their confidence for consulting with HCPs in the future, it had been an opportunity to discuss other health problems, it had been an opportunity to obtain emollient samples (not part of the intervention) and they felt more comfortable consulting with an HCP than using the website. Reasons for not finding HCP support useful included carers feeling that they did not need help in looking after their child’s eczema and already feeling confident in their ability to source information from the internet. Feedback from the HCPs included that they felt pleased to play a part in helping carers to manage eczema, but they had initial reservations about not being knowledgeable enough about eczema, as most were not eczema specialists. There were some concerns that they had also received minimal training for the role. However, they reported that the consultations were useful, but perceived that some carers found it more useful than others and that some did not need HCP support.
Trials of educational interventions for acne
One small trial, by Matsuoka and colleagues,88 examined the effects of an educational intervention in which 25 women with acne received instructions from a dermatologist on skin care and make-up use, plus acne treatment with topical therapy and/or oral medications. The educational intervention group was compared with a control group in which 25 women received acne treatment with topical and/or oral medication with no specific skin care instructions from a dermatologist. Outcomes were measured at 4 weeks post intervention.
Health-related quality-of-life outcomes
Matsuoka and colleagues88 measured HRQoL using the DLQI and WHOQOL-26, and provided results for the total mean scores for both measures as well as scores for the various subscales of each. Table 17 presents results for the total mean scores, and the findings for the subscales are narratively summarised here (for the full subscale results, see the data extraction form in Appendix 4). Matsuoka and colleagues88 reported only the statistical significance of between-group differences for the DLQI and only one subscale of the WHOQOL-26 (the ‘overall quality of life (QoL)’ subscale). No statistically significant differences were found between the educational intervention and control groups’ DLQI total scores at 4 weeks’ follow-up. The only statistically significant difference between groups on the DLQI subscales was on the work/school aspect, with the intervention group showing significantly more disability than the control group at 4 weeks. However, as alpha levels were not adjusted for in multiple comparisons, this may be a chance finding. On the WHOQOL-26, at 4 weeks, the intervention group had a slightly lower total mean score than the control group (indicating poorer HRQoL). However, this was also the case at baseline and it was not reported if the difference at 4 weeks was statistically significant. Differences on the ‘overall QOL’ sub-scale of the WHOQOL-26 at 4 weeks were reported to be non-significant (p-value not provided).
| Study | Measure, time point | Mean (SD) score | ||
|---|---|---|---|---|
| Intervention (n = 25) | Control (n = 25) | Difference between groups | ||
| Matsuoka et al., 200688 | DLQI: total mean score | |||
| Baseline | 8.24 (5.06) | 6.24 (6.06) | ||
| 4 weeks | 3.88 (2.79) | 3.24 (4.36) | NS | |
| WHOQOL-26: total mean score | ||||
| Baseline | 3.27 (0.54) | 3.36 (0.44) | ||
| 4 weeks | 3.39 (0.45) | 3.44 (0.46) | Not reported | |
Disease severity outcomes
Disease severity in Matsuoka and colleagues88 was measured by Plewig and Kligman’s grade method, which assessed the degree of improvement in acne severity on the right and left side of the face. No statistically significant association was found between trial arm and changes in acne severity at 4 weeks (Table 18).
| Study | Measure, time point | N (%) | ||
|---|---|---|---|---|
| Intervention (n = 25) | Control (n = 25) | Difference in change categories between groups (chi-squared test) | ||
| Matsuoka et al., 200688 | Acne severity (Plewig and Kligman’s grade method): degree of improvement in acne severity on right side of face at 4 weeks | |||
| Markedly improved | 5 (20) | 8 (32) | ||
| Improved | 10 (40) | 8 (32) | ||
| Unchanged | 10 (40) | 9 (36) | ||
| Exacerbated | 0 (0) | 0 (0) | p-value = 0.62 | |
| Acne severity (Plewig and Kligman’s grade method): degree of improvement in acne severity on left side of face at 4 weeks | ||||
| Markedly improved | 9 (36) | 7 (28) | ||
| Improved | 8 (32) | 9 (36) | ||
| Unchanged | 8 (32) | 9 (36) | ||
| Exacerbated | 0 (0) | 0 (0) | p-value = 0.83 | |
Other measured outcomes
The only other outcome measured by Matsuoka and colleagues88 was adverse events. The authors narratively noted that no side effects from using cosmetics or conventional medicine were reported in either group.
Process evaluation findings
Matsuoka and colleagues88 did not include a process evaluation.
Trials of educational interventions for mixed chronic inflammatory skin conditions
van Os-Medendorp and colleagues89 examined the effects of an educational intervention (the ‘Coping with itch’ programme) for patients with chronic pruritic skin disease. The focus of the intervention was to reduce itch and help participants cope with itch. The educational intervention group was compared with a control group who had normal care, which consisted of outpatient consultations with a dermatologist and therapeutic interventions such as emollients and topical steroids.
Health-related quality-of-life outcomes
van Os-Medendorp and colleagues89 measured HRQoL through the ‘Impact on QoL’ subscale of the ACS. Outcomes were reported at 3 and 9 months (the latter time point corresponding with the programme duration). For the purpose of the present review, the 9-month outcomes are reported. Table 19 presents the results for the QoL subscale, where lower scores reflect lower skin-related psychosocial morbidity. The trial authors stated that no statistically significant differences were found between the educational intervention and control groups on this measure (neither p-values nor CIs were not reported). No power analysis was provided in the publication and there were high rates of attrition, which may have some relevance to the reported outcome as there were small numbers analysed. Other subscales of the ACS were reported in the trial; these are discussed separately below as they are not HRQoL subscales.
| Study | Measure, time point | Mean (SD) score | ||
|---|---|---|---|---|
| Intervention | Control | Difference between groups | ||
| van Os-Medendorp et al., 200789 | ACS QOL subscale | |||
| Baseline | 12.08 (5.00) (n = 29) | 12.56 (4.93) (n = 36) | ||
| 9 months | 13.10 (5.25) (n = 23) | 12.68 (4.58) (n = 30) | Stated NS | |
Disease severity outcomes
Disease severity was measured by two measures in the van Os-Medendorp and colleagues89 trial: frequency and intensity of itching and scratching. Little detail of the scales was provided except that the two measures were derived from four factors that had been recorded in diaries (frequency and intensity of itching, frequency and intensity of scratching), where severity ranged from 0 to 10 (lowest to highest severity) and the internal consistency was high. The authors subsequently converted these scores into a dichotomous measure with an arbitrary cut-off (high frequency defined as > 4 and high intensity defined as > 3) based on averages from the original scale. The paper reports analysis of only the high frequency between groups. Results are presented in Table 20 where it can be seen that at 9 months there were no statistically significant differences between the educational intervention and control group. No p-values or CIs were reported by the study authors and the numbers analysed were small, as there had been a high drop-out rate.
| Study | Measure, time point | n (%) | ||
|---|---|---|---|---|
| Intervention | Control | Difference between groups | ||
| van Os-Medendorp et al., 200789 | Patients with high frequency of itching and scratching | |||
| Baseline | 18 (72) (n = 25) | 21 (66) (n = 32) | ||
| 9 months | 12 (50) (n = 24) | 15 (52) (n = 29) | Stated NS | |
| Patients with high intensity of itching and scratching | ||||
| Baseline | 20 (80) (n = 25) | 25 (78) (n = 32) | ||
| 9 months | 12 (50) (n = 24) | 16 (55) (n = 29) | Stated NS | |
Other measured outcomes
van Os-Medendorp and colleagues89 measured general psychosocial morbidity using the Symptom Checklist-90. On this measure, lower scores reflect lower general psychosocial burden. Results can be seen in Table 21, where it is apparent that there were no statistically significant differences between those in the educational intervention and control groups.
| Study | Measure, time point | Score, mean (SD) | ||
|---|---|---|---|---|
| Intervention | Control | Difference between groups | ||
| van Os-Medendorp et al., 200789 | SCL-90 | |||
| Baseline | 146.30 (60.02) (n = 29) | 151.18 (52.60) (n = 36) | ||
| 9 months | 134.41 (47.68) (n = 23) | 159.81 (57.69) (n = 30) | Stated NS | |
Other outcomes measured in the van Os-Medendorp and colleagues89 trial included the Itching Cognitions Questionnaire (ICQ), other subscales of the ACS, medication use, and the proportion of patients visiting the dermatologist. The results of these various measures and indices are summarised narratively here (see the data extraction form in Appendix 4 for the full subscale results).
No statistically significant differences were seen between the educational intervention and control groups on the ICQ subscales of catastrophising and helpless coping, and problem-focused coping. Six subscales of the ACS were reported (skin-related psychosocial morbidity, social anxiety and avoidance, vicious circle of itching and scratching, helplessness, anxious depressive mood, and deficit in active coping). No statistically significant differences were seen on these scales between those receiving the educational intervention and those receiving the control intervention.
No statistically significant differences between groups were observed on the use of mild, moderately potent, potent, or very potent corticosteroids; systemic medication use; or itch-relieving medication use between groups (see Appendix 4). The proportion of participants visiting the dermatologist was statistically significantly lower in those in the educational intervention group initially (1–3 months), but by 7–9 months there were no statistically significant differences between the two groups.
For all of these analyses the numbers of participants in each group were small and it is unlikely that the analyses were powered to detect a difference between the groups.
Process evaluation findings
The van Os-Medendorp and colleagues89 trial did not report a process evaluation.
Summary of clinical effectiveness
Only seven RCTs, with adequately reported results, were identified that evaluated the effects of educational interventions that could improve HRQoL in people with chronic inflammatory skin conditions. Only one study74 explicitly stated in the primary publication that the intervention was aimed at improving HRQoL (but in one other87 the intervention for one of three age groups of participants in the study was based on an intervention of which part of the aim was to improve HRQoL78,93), and only three evaluated theory-based interventions. The current evidence base is mainly limited to small studies (four trials included between 40 to 64 participants; it was unclear if any of these were adequately powered, and one reported subgroup results only and two were pilot studies), of generally poor methodological quality, focusing on psoriasis, atopic dermatitis and acne, and studies including adults. Only two studies included children and adolescents and their parents or carers; these were the largest trials included in the review (992 and 148 randomised participants, respectively, with the smaller study being a pilot study).
The educational interventions have commonly been delivered face to face (either in a group or individually), as an adjunct to standard medical care, and have provided participants with information about their condition and methods for coping with stress or the negative effects of their disease, such as itch or appearance concerns. There have been some attempts to tailor interventions to individual needs or participants’ characteristics. The participants included in the trials generally had had their skin condition for a number of years and no studies were found that specifically targeted newly diagnosed patients. In two of the seven included studies, participants’ mean baseline HRQoL scores indicated that, generally, their disease had had a minimal impact on their HRQoL. Three studies had a follow-up of a reasonable duration to capture the clinical effects of the intervention (≥ 3 months post intervention). Adverse effects were evaluated in only one trial. The majority of the studies were considered to be of limited generalisability to UK clinical practice. Exceptions were the two large RCTs examining the effects of an online educational intervention (the SPaCE website) aimed at the carers of children with eczema in primary care and the group-based educational programme, delivered by a multidisciplinary team over 6 weeks, aimed at children and adolescents with atopic dermatitis and the children’s carers, which was delivered to patients recruited from secondary care. The UK trial by Ersser and colleagues86 of nurse-led education for psoriasis may also have some generalisability to patients seen in primary care whose disease has not extensively affected their HRQoL.
Three trials found statistically significant improvements in HRQoL following the educational interventions. Of these, two found benefits for adult patients with psoriasis at the end of the text message education intervention delivered over 12 weeks and the 3-month, group-based, educational programme intervention delivered by a multidisciplinary team compared with an unspecified control and usual medical therapy, respectively. The other one found benefits for parents of children with atopic eczema at 12 months’ follow-up (it was unclear if this follow-up time point was from baseline or the end of the intervention) from a 6-week group-based programme delivered by a multidisciplinary team compared with a no education control. All these trials also found improvements in patients’ disease severity at these time points. Only one of these psoriasis trials (of the group-based education) measured outcomes in the longer-term post intervention (specifically, 6 months after the end of the intervention) and it found that the improvements on only one of the two HRQoL measures used persisted over time, whereas improvements in disease severity were not maintained. In one of these studies, the same intervention was delivered to patients with psoriasis and patients with atopic dermatitis, but it did not improve HRQoL or disease severity in those with atopic dermatitis despite being effective for psoriasis, and the reasons for this are unclear. It could have been a chance finding, as the results were from potentially under-powered subgroup analyses, which were at unclear risk of bias because it was not stated if these were pre-specified. Of the trials reporting statistically significant improvements in HRQoL and disease severity, only one87 discussed how clinically meaningful the changes were to patients (suggesting that, on average, a meaningful improvement in disease severity had occurred in the children with atopic dermatitis in the trial of the multidisciplinary 6-week programme), but, generally, the clinical significance of the improvements found in these studies is unclear.
No effects of education on HRQoL or disease severity in comparison with usual care were found in studies of a one-off nurse-delivered educational session for adults with psoriasis, instructions on skin care and make-up use to women with acne, the SPaCE website (with or without HCP support) for carers of children with eczema or in a ‘Coping with itch’ programme for participants with a range of conditions delivered in a median of three sessions. It was unclear if any of these studies were adequately powered. However, two were pilot studies, so although they were possibly not powered adequately, they provided useful information about the feasibility and acceptability of the intervention to inform a more rigorous evaluation, as recommended by the MRC guidance on complex intervention development and evaluation. 65 Furthermore, in two studies, participants’ baseline HRQoL scores indicated that their disease had had minimal impact on their HRQOL, which may partly explain the lack of effects (e.g. no room for improvement). The ‘Coping with itch’ study had a high attrition rate, and as no process evaluation was included in the study, it was unclear why so many participants did not complete the study or what programme factors may have led to this.
The review findings also indicate that educational interventions may result in improvements in other outcomes, including decreases in itching catastrophisation, some improvements in coping with itch, reductions in depression and short-term reductions in consultations with HCPs. No impact on medication use was found. The one study that reported adverse effects found no negative impact of the intervention.
It is not possible to determine from the studies in this review the educational intervention components or characteristics that may contribute to improving patients’ and parents’ or carers’ HRQoL. Commonalities between effective interventions were delivery by a multidisciplinary team and delivery over a longer period than the interventions in the trials which did not find any statistically significant effects (ranging from 6 weeks to 3 months). However, given the limitations of the evidence, these commonalities should only be regarded as early indications of factors that may characterise effective interventions.
Overall, the current evidence base suggests that educational interventions including elements that could improve HRQoL do show some promise for improving HRQoL and disease severity in adults with psoriasis directly at the end of the intervention (with some indication that improvements in HRQoL may be maintained 6 months after the intervention, whereas improvements in disease severity may not be maintained), as well as for improving the HRQoL of parents of children with atopic dermatitis, and children and adolescents’ atopic dermatitis disease severity, in the longer term, up to 12 months. There was no evidence of effectiveness on HRQoL in adults with acne or atopic dermatitis or for an intervention aimed at improving itch in a mixed skin diseases population or for an online educational intervention (with or without HCP support) aimed at the carers of children with eczema. Based on the findings of two studies, there are indications that interventions aimed at mixed populations with different skin conditions may not be effective or may be more effective for one of the included patients groups than another. Characteristics of effective interventions may include intensive delivery over a period ranging from 6 weeks to 3 months (rather than shorter, less intensive interventions of one or a few sessions) and delivery by a multidisciplinary team, but, overall, at this stage, it is not possible to identify the intervention factors that may lead to improvements in HRQoL.
Ongoing studies
Six ongoing RCTs were identified that appear to meet the inclusion criteria for the systematic review (Table 22). Three of the RCTs are being conducted in Europe (excluding the UK), two in the USA and one in Japan. According to the study dates specified in trial registries, three of the six RCTs should have been completed, but we have classified these as ongoing trials given that their results have not yet been published.
| Title, trial number, funding and/or sponsor | Study design (country), study dates, estimated enrolment | Study aims | Population | Intervention(s)/comparator(s) | Outcomes |
|---|---|---|---|---|---|
| Group Eczema Education Visits: Impact on Patient and Family Quality of Life NCT01143012 Sponsor: Oregon Health and Science University |
RCT (USA) May 2010–August 2011 (final data collection) Estimated n = 60 (completed but not published) |
To examine if group education improves patients’ QoL and other outcomes, including health-care resource use and disease severity | Atopic dermatitis (children aged 2 months to 6 years and parents) |
|
Primary: HRQoL (CADIS) Secondary: disease severity (EASI); health-care resource use |
| A Psycho-educational Prevention for the Treatment of Atopic Dermatitis in Youth and Their Families NCT02067234 Sponsor: Seton Family of Hospitals |
RCT (USA) March 2014–December 2014 Estimated n = 50 |
To determine whether the inclusion of a parent/patient psychoeducational session in initial dermatology appointments with new paediatric atopic dermatitis patients affects (a) extent of medical follow-up, (b) patient’s QoL, and (c) parenting stress in comparison to treatment as usual | New paediatric patients (aged 2 months to 12 years) with atopic dermatitis |
|
Primary: disease severity (SCORAD) Secondary: HRQoL (Parenting Stress index, CDLQI, IDQoL index) Disease severity (PO-SCORAD) |
| A Randomised Clinical Trial on the Effect of Group Education on Patients With Occupational Hand Eczema (PREVEX)97 NCT01899287 Sponsor: University of Copenhagen; Copenhagen University Hospital |
RCT (Denmark) July 2012–December 2013 Estimated n = 742 (completed but not published) |
To evaluate the effect of group education on sick leave, HRQoL and disease severity among individuals with newly notified occupational hand eczema | Aged 18–65 years with self-reported occupational hand eczema |
|
Primary: total sickness absence; HRQoL (DLQI); subjective disease severity (photographic guide) Secondary: eczema-related sickness absence; several exploratory outcomes |
| A Prospective, Randomised, Controlled Study of the Impact of Education and Stress-reduction Techniques on Psoriasis and Eczema NCT02205593 Sponsor: University of Zurich |
RCT (Switzerland) July 2012–December 2015 Estimated n = 80 |
For patients to learn effective self-management strategies and attitude to one’s chronic skin disease and consequently improving QoL | Aged ≥ 18 years with chronic psoriasis or eczema |
|
Primary: HRQoL (DLQI, Skindex-29, SF-36, EQ-5D, EQ-5D VAS) Secondary: disease severity (EASI, PASI); depression (BDI) |
| The Effect of Patient Education in Initial Treatment of Children with Atopic Dermatitis by Paediatric Allergy Educator UMIN000012867 Sponsor: Tokyo Metropolitan Children’s Medical Centre |
RCT (Japan) April 2014–(end date not specified) Target n = 120 |
To test whether paediatric allergy educator leads the effectiveness of patient education at the stage of initial treatment for paediatric patients with atopic dermatitis | Children aged 1–10 years with atopic dermatitis |
|
Primary: disease severity (SCORAD) Secondary: family impact (DFI); HRQoL (QPCAD); skin care; medication use |
| The efficacy of a HRQoL intervention during 48 weeks of biologic treatment of patients with moderate to severe psoriasis98 Netherlands National Trial Register (NTR): NTR1364 Sponsor: Pfizer B.V., Rotterdam, the Netherlands |
RCT (the Netherlands) October 2008–December 2012 Target n = 200 (completed but not published) |
|
Patients aged ≥ 18 years with moderate or severe psoriasis who are receiving etanercept |
|
Primary: HRQoL (DLQI); doctor–patient communication (study-specific questionnaire) Secondary: health status (SF-36); disease severity (PASI, IGA, PGA); patient satisfaction (ad hoc questionnaire); intervention feasibility (ad hoc questionnaire) |
Three of the RCTs are being conducted with children with atopic dermatitis, although one also includes their parents; one RCT includes adults with psoriasis; one RCT includes adults with psoriasis or eczema; and one RCT includes adults with occupational hand eczema. The interventions are reported to varying levels of detail in trial registries, but appear to consist mostly of various types of group education. Each of the RCTs contains two arms, in which an educational intervention is compared with a comparator. In four RCTs, the interventions are being compared against no education, whereas in two RCTs the comparator is a different type of education. Four of the RCTs specified HRQoL as a primary outcome and two RCTs specified HRQoL as a secondary outcome. The target numbers of patients randomised ranges from 50 to 742. The specified completion dates of the RCTs range from August 2011 to December 2015, although no completion date is specified for the Japanese RCT (see Table 22).
Chapter 4 Economic analysis
The following section has two parts. First, the methods and findings of the systematic review of the cost-effectiveness of educational interventions as an adjunct to standard medical care in the treatment of patients with chronic inflammatory skin diseases is presented. The objective of this review is to provide useful information to inform the development of an economic model. The second part outlines a list of recommendations on the different aspects that should be considered in the development of any future health economic model to assess cost-effectiveness of educational interventions for chronic inflammatory skin conditions, based on the findings of the systematic review.
Systematic review of existing cost-effectiveness evidence
The methods of the systematic review are described above (see Inclusion and exclusion criteria), although the inclusion criteria were modified slightly from those used in the review of clinical effectiveness. First, the inclusion criteria for this review were not limited to studies where the effects of education on overall outcomes had to be isolated from effects of any non-educational intervention components that may also be present in the intervention. Second, studies that did not specifically aim to improve HRQoL were also included. The reason for incorporating broader criteria on HRQoL was because the reviewers anticipated fewer studies at the beginning of this review.
The studies are described in terms of their quality and generalisability to the UK and key issues arising from each of the studies are discussed.
Quantity and quality of published research
A total of 1394 citations were identified through the systematic searches. Following examination of titles and abstracts, 13 potentially relevant papers were retrieved for a more detailed inspection. Of these, 10 papers were excluded. The reasons for exclusion were: the nature of the intervention was not educational as defined within the scope of the project;99–102 the study design was inappropriate;75,93,103,104 the study was not published in English105,106 (see Appendix 7). Three studies were eligible for inclusion; one was based on adults with chronic pruritic skin diseases107 and the remaining two studies were on eczema in children. 108,109 In the study on pruritic skin disease, van Os-Medendorp and colleagues107 assessed health-care costs and costs associated with loss of work in patients with different chronic pruritic skin diseases (Table 23) enrolled in the nursing programme ‘Coping with itch’ compared with a control group of patients receiving usual dermatological care. Of the two eczema-based studies, Mason and colleagues108 conducted a cost analysis that examined the effectiveness of an educational support programme to increase emollient use and reduce atopic eczema symptoms in children, whereas Schuttelaar and colleagues109 conducted a cost-effectiveness analysis of care provided by nurse practitioners (NPs) versus dermatologists.
| Author | van Os-Medendorp et al., 2008107 | Mason et al., 2013108 | Schuttelaar et al., 2011109 |
|---|---|---|---|
| Publication year | 2008 | 2013 | 2011 |
| Country | The Netherlands | UK | The Netherlands |
| Study type | Economic evaluation based on the results of a RCT | Cost analysis based on a before-and-after study | Economic evaluation based on the results of a RCT |
| Perspective | Not reported | NHS | Societal |
| Study population | Adults with chronic pruritic skin diseases including eczema; atopic dermatitis; pruritus; prurigo; psoriasis; chronic urticaria; other skin disease including allergies, mycosis, fungoides, lichen ruber planus, Darier disease; unknown and non-skin diseases | Atopic eczema (children + carers) | Children with eczema |
| Intervention(s) | Intervention: nursing care according to the programme ‘Coping with itch’ consisting of educational and cognitive behavioural interventions. It was carried out in a specialised itch clinic run by dermatology nurses who provided individual sessions at the dermatology outpatient department (see also Clinical effectiveness, Results) Comparator: usual dermatologist care |
Educational support programme for parents and carers included an educational DVD, online daily diary and telephone helpline with dermatology nurses | Intervention: care provided in terms of education and coaching by a NP Comparator: conventional care by a dermatologist |
| Key outcome measure | Frequency of itching and scratching as recorded in patient diaries | Emollient use | Between-group differences in the QoL of the child between baseline and follow-up at 12 months measured by IDQoL and CDLQI |
| Currency base | EUR, currency year not reported | GBP, 2011 | EUR, 2008 |
| Time horizona | 9 months | 3 months | 1 year |
| Baseline cohort | Patients with chronic pruritic skin diseases aged 18 years or older, regardless of the underlying diagnosis, who visited dermatology outpatient departments | Eligible children were male and female, aged 3 months to 6 years, with mild to moderate atopic eczema; and using E45 cream (Reckitt Benkiser, Slough, UK) as their primary emollient | Patients aged ≤ 16 years with a diagnosis of eczema (‘atopic dermatitis’) |
| Funding source | Dutch College of Health Insurance (CVZ) | Reckitt Benkiser Healthcare | Health Care Efficiency Research Programme of the University Medical Centre Groningen, Groningen, the Netherlands |
The study by van Os-Medendorp and colleagues107 was included in the review of clinical effectiveness studies (discussed in Clinical effectiveness, Results), whereas the studies by Mason and colleagues108 and Schuttelaar and colleagues109 were excluded. This was due to the difference in HRQoL inclusion criteria between the two reviews as stated above (see Systematic review of existing cost-effectiveness evidence). In this context, the reviewers deemed the study by van Os-Medendorp and colleagues107 to be the most relevant of the three studies identified through the systematic searches, given that it also met the inclusion criteria for clinical effectiveness. As a result, this study107 is discussed in depth (see Description and results of the published economic evaluations); this is followed by discussion of the remaining two studies108,109 that met the inclusion criteria.
Characteristics of the three included studies107–109 are shown in Table 23 and discussed in more detail subsequently. The identification process of the included studies is shown in a PRISMA flow chart presented in Figure 2. Full data extraction forms for the included studies are included in Appendix 8.
FIGURE 2.
Flow chart of identification of studies for inclusion in the review of cost-effectiveness.

Critical appraisal of the studies
The included studies were assessed against a critical appraisal checklist (Table 24) to evaluate their quality and generalisability to the UK. The checklist was adapted by the review authors from a checklist by Drummond and colleagues. 110 More details of the studies are given below (see Description and results of the published economic evaluations).
| Item | van Os-Medendorp et al., 2008107 | Mason et al., 2013108 | Schuttelaar et al., 2011109 |
|---|---|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Yes | Yes | Yes |
| 2. Is the setting comparable to the UK? | No | Yes | No |
| 3. Is the analytical and modelling methodology appropriate? | ? | ? | ? |
| 4. Are all the relevant costs and consequences for each alternative identified? | Yes | ? | Yes |
| 5. Are the data inputs for the model described and justified? | Yes | ? | Yes |
| 6. Are health outcomes measured in QALYs? | No | No | No |
| 7. Is the time horizon considered appropriate? | Noa | Noa | Noa |
| 8. Are costs and outcomes discounted? | Nob | Nob | Nob |
| 9. Is an incremental analysis performed? | Yes | Yes | Yes |
| 10. Is uncertainty assessed? | Yes | ? | Yes |
All three studies clearly defined the decision problem and used a relevant intervention for the purpose of this review. The nature of the interventions and the comparators varied across the studies. The patient groups of interest were clearly stated in all the studies. Two studies108,109 stated the perspectives adopted. However, there remained uncertainty in the adopted analytical methodologies.
The studies did not incorporate a cost–utility analysis; two studies conducted cost-effectiveness analyses107,109 and the other performed a cost analysis. 108 This was considered as lacking usefulness from the UK NHS perspective, where a cost–utility analysis is generally deemed preferable for decision-making purposes.
Methods for estimating resource use and costs varied. van Os-Medendorp and colleagues107 provided adequate details of all the relevant elements that contributed to the overall costs of both the intervention and comparator strategies. Schuttelaar and colleagues109 described the resource used and costs in a detailed manner. Mason and colleagues,108 however, did not present a detailed overview of all the relevant cost components needed for an accurate estimation of total programme costs.
The studies used different measures to assess outcome. None of the studies reported a preference-based measure of health to estimate effectiveness in terms of QALYs. This is a potential limitation, as having a common numeraire such as QALYs would have facilitated comparison of the effectiveness across different interventions. The quality of the methodology adopted to estimate effectiveness was mixed. Within the two cost-effectiveness analyses conducted in the Netherlands,107,109 effectiveness in terms of HRQoL was assessed through condition-specific measures such as IDQoL and CDLQI109 and disease severity was measured by SCORAD. 109 Clinical outcomes such as frequency of itching and scratching were also estimated107 along with patient satisfaction. The UK-based cost-analysis,108 by contrast, used emollient use, severity of eczema, use of concurrent medication, parent measures and health-care contacts by patients to measure outcomes in the before-and-after study periods.
The economic evaluations conducted within the included studies did not involve extrapolating data beyond study durations. As a result, none of the studies applied discounting as the time horizons were 1 year or less.
Incremental analyses were conducted in all three studies. Uncertainties were assessed by bootstrapping methods in two studies;107,109 it is unclear if these analyses were conducted by Mason and colleagues. 108 Bootstrapping is a technique of resampling statistical data to derive estimates of summary statistics such as CIs and standard errors. The technique makes very limited assumptions about the probability distributions of the data, thereby limiting the introduction of data uncertainty, unlike in probabilistic sensitivity analysis (PSA) as PSA imposes additional assumptions on the real distribution of the data. 111,112 Therefore, even though the studies did not conduct the standard practice of PSA to assess uncertainty, the methods were still considered to be appropriate.
In summary, the included studies lacked detail on some aspects of methodology, making it difficult to assess the validity of the results. Therefore, there is uncertainty on the credibility of the study findings given the limitations outlined above. The study by Mason and colleagues108 is of most relevance to the UK as the health-care system in the study was the NHS.
Description and results of the published economic evaluations
van Os-Medendorp and colleagues
van Os-Medendorp and colleagues107 performed an economic evaluation based on a RCT conducted in the Netherlands that assessed the clinical effectiveness of the nursing programme ‘Coping with itch’ in patients aged ≥ 18 years with chronic pruritic skin diseases (also included in the clinical systematic review; see Clinical effectiveness, Results).
Analytical approach
Statistical analysis was conducted on trial-based outcomes to analyse cost-effectiveness of the intervention over a period of 9 months for 120 patients.
Assumptions
To estimate costs associated with days off work, the same value based on an overall mean hourly productivity cost for both men and women was applied. With respect to the frequency of itching and scratching measured through patient diaries, the authors converted the frequency of itching/scratching to a dichotomous measure where a high frequency was classed as > 4 and a low frequency as ≤ 4. The cut off score of 4 was used to discern a clinically relevant decrease after the intervention.
Estimation of effectiveness
Health benefits were expressed in terms of days with a low frequency of itching and scratching. These outcomes differed from those in the clinical effectiveness review as described in Clinical effectiveness, Results. Linear interpolation for the period between the measurements at baseline and 3 months and between baseline and 9 months was used to compute the number of days with a low frequency of itching and scratching. Data for HRQoL were not presented.
The mean scores of the frequency of itching and scratching declined in the intervention and control groups after 9 months [5.16 (SD 3.87) and 4.70 (SD 3.53), respectively].
Estimation of costs
Detailed information on the units of resource use across the different cost components was reported. The authors followed the guidelines for cost studies of the Dutch College of Health Insurance to assess the costs of visits to health-care workers, days off work and hospitalisations. Medication costs were listed from a Dutch website113 which represented the market price. The costing year was not reported.
Data on medical consumption were also collected; these included information on:
-
visits to the GP
-
visits to the dermatology outpatient department
-
visits to the other HCPs in or outside the hospital
-
days off work
-
hospitalisations; and
-
medication use.
Intervention costs comprised:
-
costs at the dermatology outpatient department, which included the costs associated with:
-
visits to the dermatologist
-
visits to the dermatologist nurse
-
ultraviolet therapy
-
visits to the medical social worker
-
-
costs of visits to the GP
-
costs of visits to other health-care providers
-
costs of medication
-
costs of hospitalisation; and
-
costs of days off work.
The study reported total costs for two time periods: 1–3 months and 1–9 months. The analysis indicated significantly higher costs associated with the visits to the dermatology nurse compared with usual care in the first 3 months (mean difference €107, 95% CI €77 to €137), whereas in the total study period of 9 months the mean difference was €198 (95% CI €131 to €265). This could potentially be due to a significantly larger number of consultations with the dermatology nurse by the intervention group during the first 3 months compared with the control group because of the nursing programme. No other differences were found between the two groups of patients.
Cost-effectiveness results
The cost-effectiveness results are presented in Table 25. The bootstrapped mean differences in total costs between the two groups were €794 (95% CI –€970 to €2693) and €582 (95% CI –€2730 to €3877) at 3 and 9 months, respectively. For the first 3 months, the incremental cost per day gained with a low frequency of itch was estimated at €129.91, which decreased to €16.60 after 9 months, thereby indicating that the benefits of the intervention in terms of days with little itch increased. The analysis showed that the intervention group experienced better outcomes in the longer term.
| Costs | Benefits (days with a low frequency of itching and scratching) | ICER | ||||
|---|---|---|---|---|---|---|
| Intervention | Control | Difference after bootstrap analyses (95% CI) | Intervention | Control | Difference after bootstrap analyses (95% CI) | |
| Months 1–3a | ||||||
| €2602.60 | €1808.00 | €793.80 (–€970.30 to €2692.70) | 34.3 | 28.4 | 6.1 (–15.7 to 27.8) | €129.90 |
| Months 1–9b | ||||||
| €5040.60 | €4476.70 | €582.00 (–€2730.00 to €3877.00) | 123.0 | 87.6 | 35.0 (–33.0 to 96.0) | €16.60 |
Sensitivity analysis
The bootstrap analysis indicated that patients in the intervention group might expect to gain 6 days (95% CI –16 to 28 days) with a low frequency of itching and scratching compared with the control group in the first 3 months and 35 days (95% CI –33 to 96 days) for the entire 9-month period. The authors concluded that there was 70% certainty that patients experienced benefits from the intervention, whereby in 14% of the simulations lower costs and favourable effects were observed. Furthermore, there was an 87% certainty of favourable results for the ‘Coping with itch’ intervention, with lower costs being observed in 31% of simulations. Scenario analysis was conducted to see the impact of a cut-off score of 3 or 5 for the frequency of itching and scratching on the overall cost-effectiveness results, which was found to be limited on the overall results.
Relevance in the context of economic modelling
Despite the limitations outlined above, the study was considered to be relevant to informing model parameters, should a de novo model be developed.
Mason and colleagues
Mason and colleagues108 reported on an educational support programme (ESP) that was provided for parents or carers of children aged 3 months to 6 years with mild to moderate atopic eczema in the UK. The programme included an educational DVD, diaries to record eczema condition, daily use of emollients, and telephone contact with dermatology nurses for regular and on-demand support. The study assessed the effectiveness of the ESP in increasing emollient use and reduction of atopic eczema symptoms in the children. The perspective adopted was that of the UK NHS.
Analytical approach
The study performed a before-and-after incremental within-study cost-analysis over a 12-week period on 135 British children.
Estimation of effectiveness
The support components within the intervention arm aimed to increase the use of emollient with a target of 250 g per week use per child. Over the study duration, emollient use increased by 87.6 g (95% CI 81.9 g to 119.5 g) on average, with 8.9% children receiving 250 g/week and 61.5% receiving 125 g/week at 12 weeks. In addition, there was also an increase in prescription of corticosteroids by 20.8% (95% CI 8.9% to 32.1%), although this was not planned as part of the educational support provided.
The POEM and Patient Eczema Severity Time (PEST) measured severity of eczema. Higher POEM and PEST scores indicate a negative impact from high disease severity. PEST scores are designed for parents to assess severity in young children who cannot vocalise for themselves. 108 The POEM score reduced significantly over the 3 months, on average by 5.38 (95% CI 4.36 to 6.41). This was a 47% reduction from the baseline score. The PEST also reduced on average by 0.61 (95% CI 0.47 to 0.75), with a reduction of 48% from the baseline score. These findings indicate a positive impact of the intervention. Furthermore, the number of nights per week on which family members experienced sleep disturbance was reduced during the study period by 1.27 nights (95% CI 0.85 nights to 1.68 nights). This was half the baseline level of disturbance. In addition, there was an improvement by 1.32 points (95% CI 1.16 to 1.48) in parental feelings of control of their child’s eczema, with 91.5% of parents reporting the highest level of control. Although the study outlined the statistical outcomes in detail, there was no indication if the results were clinically significant.
The evidence of effectiveness from the ESP should be treated with caution as it was not based on the evidence from a RCT or a study with a concurrent control group.
Estimation of costs
The cost analysis of the ESP was performed using nationally reported unit costs for 2011. The cost associated with the emollient [E45 cream (Reckitt Benkiser, Slough, UK) prescription-only medicine (POM) 500 g] was estimated at an average English Prescription Pricing Authority-reimbursement rate of £4.89; the cost associated with a GP visit was estimated at £36 per visit; and the cost of providing the ESP was estimated at £32 per child. The study did not report any details of the units of resource use. Owing to this lack of transparency, the results of the cost analysis should be interpreted with caution.
Cost analysis results
The overall cost of care was estimated as the sum total of costs associated with emollient use and GP visits.
Two methods were applied to estimate emollient use: ‘time-in-use’ (estimated time taken to use a 500-g pot of emollient) and diary method (programme diaries completed by the participating children). A statistically significant increase in the cost of emollient by £10 was observed using the diary method and £13 using the ‘time-in-use’ method. There was also a fall in GP visits by about 1 visit per child, on average, resulting in no overall or significant change in net cost, as the cost per GP visit was £36 compared with the cost of ESP, which was £32 per child. The results were found to be similar regardless of the method of estimating emollient use.
The mean cost of GP visits decreased from £68.53 in the pre-programme phase to £30.40 in the post-programme phase. The cost of emollient use, as well as that of care, estimated by the daily diary and ‘time-in-use’ methods increased in the post-programme phase. However, these increased costs were not significant, and, as a result, the authors concluded that the current programme, delivered at a distance using a specialised nurse, might be cost-neutral from a NHS perspective.
Sensitivity analysis
The study performed subgroup analysis in 117 patients by excluding those children who had visited an eczema specialist in the 3 months prior to joining the programme. The results of the analysis were similar to the base case.
Relevance in the context of economic modelling
Despite being based in the UK, the review team did not consider the study to provide any useful information in informing the development of an economic model, either structurally or in terms of model parameterisation. This conclusion was mainly driven by the limitations in non-reporting of disease progression, HRQoL parameters and very limited information on costs.
Schuttelaar and colleagues
Schuttelaar and colleagues109 reported a cost-effectiveness analysis based on a randomised, parallel-group study for patients aged ≤ 16 years with a diagnosis of eczema, described elsewhere. 11 The patients received either conventional care by a dermatologist or care by a nurse practitioner (NP). The study, based in the Netherlands, adopted a societal perspective. Care provided by the NP was considered as an educational intervention by the reviewers, because education and coaching by the NP was a part of the overall treatment.
Analytical approach
Statistical analysis was performed to assess cost-effectiveness of providing care by a NP compared with conventional care by a dermatologist for a 1-year period. Details of the design, inclusion criteria and sample size of the RCT were published elsewhere, reference provided. 11 Briefly, 160 participants were stratified by age and randomised to receive either conventional care from a dermatologist or care from a NP. The economic analysis was conducted on 147 patients.
Assumptions
Travel costs were estimated based on mean distance to hospital and cost per kilometre travelled. Costs associated with productivity losses were based on an overall mean hour productivity cost for both men and women.
Estimation of effectiveness
The HRQoL was assessed by the IDQoL and CDLQI (for descriptions of these measures, see Appendix 1). The IDQoL was completed by parents for children aged < 4 years. Those aged 4–16 years completed the illustrated version of the CDLQI. There were improvements in the mean changes of IDQoL scores in both the intervention and comparator groups from baseline to 12 months. The between-groups difference was –1.7 (95% CI –4.6 to 1.2; p-value = 0.26), which was not statistically significant. Similarly, there were significant improvements in the mean CDLQI scores in the two groups from baseline to 12 months. However, the between-group difference was not statistically significant at –0.7 (95% CI –3.3 to 1.7; p-value = 0.55).
The Client Satisfaction Questionnaire-8 (CSQ-8) was used to measure patient satisfaction at 12 months; this was completed by parents. The mean scores were higher in the NP group than the dermatologist group, with scores being 26.9 (95% CI 25.5 to 28.2) and 24.8 (95% CI 23.6 to 26.0), respectively. There was a significant difference in scores between groups, with a difference of –2.1 (95% CI –3.0 to –0.3; p-value < 0.02) favouring the NP group.
Estimation of costs
The study estimated societal costs by aggregating health-care costs (hospital costs and community costs), family costs and costs in other sectors. Resource-use data relating to visits to dermatologists and NPs, phone consultations, group education sessions, admission days and laboratory tests were collected at 4, 8 and 12 months. Other resource use relating to absence from work for visits to a dermatologist or NP, travelling expenses, out-of-pocket expenses, professional help at home and visits to the GP were registered in cost-diaries completed by parents. Volumes of medication used and refilled prescriptions were obtained from registration forms and medical records. Both hospital and community costs were reported. Resources were measured in natural units and valued using unit costs. The analysis did not include eczema-related costs, such as costs of visits to the allergologist or dietician.
The estimates for the units’ costs were based on the Dutch guideline process and prices were expressed for the year 2008. The study reported the methods applied to estimate costs associated with group education sessions, laboratory costs, medication costs, travel costs including that of parking, and costs attributable to productivity losses of parents as a result of visits to health-care providers. The mean annual societal cost per patient in the dermatological group was €1409 and in the NP group was €981, with a mean difference of –€428 (95% CI –€910 to €197). In children aged < 4 years, the annual societal cost was €1791 and €1186 in the dermatologist and NP groups, respectively. For those aged 4–16 years, the costs were €1039 and €778 in the dermatologist and NP groups, respectively.
The mean annual health-care costs were higher in children in the dermatologist group (€801) than those in the NP group (€658) with a mean difference of –€143 (95% CI –€544 to €299). Other costs, such as costs of community care, hospitalisation, outpatient visits, laboratory tests and medication, were higher in the dermatologist group than the NP group; however, costs associated with phone consultations and protective dressings were higher in the NP group. The mean annual family costs were twice as high in the dermatologist group (€608) than the NP group (€302) with a mean difference of –€306 (95% CI –€475 to –€16). Time costs and out-of-pocket expenses were also higher in the dermatologist group (€415 and €134, respectively) than the NP group (€178 and €83, respectively). By contrast, the mean annual costs for home help visits paid by the state in the Netherlands were higher in the NP group (€21) than the dermatologist group (€0.93).
Overall, the study presented comprehensive information on the costs and resource use during the study period in both the dermatologist and the NP groups. Detailed cost composition by disease severity levels was also presented. However, there appeared to be miscalculations of the aggregate costs by types, as the sum total of the individual costs within each of the different types of costs did not match the reported aggregate costs by a significant margin. Therefore, caution must be assumed when interpreting the cost analysis by disease severity level.
Cost-effectiveness results
The incremental cost-effectiveness ratios (ICERs) for IDQoL and CDLQI in the NP group compared with the dermatologist group were €925 and €751, respectively, which lies in the south-west quadrant of the cost-effectiveness plane, indicating lower costs and lower benefits. For the CSQ-8, the ICER was €251, which lies in the south-east quadrant, indicating lower costs and more effectiveness in the NP group. The results of the base-case analyses favoured care provided by an NP over that provided by a dermatologist in treating young children with eczema.
Sensitivity analysis
For the ICER based on IDQoL score, the cost-effectiveness plane showed that 51% of the cost–effect pairs were plotted in the south-west quadrant, indicating lower costs and less effectiveness in the NP group. For the ICER based on CDLQI, the cost-effectiveness plane showed that 59% of the cost–effect pairs were plotted in the south-west quadrant, indicating lower costs and less effectiveness in the NP group. For the ICER based on CSQ-8, 92% of the plots fell in the south-east quadrant, which meant that the NP care dominated the dermatologist group as it was more effective and less expensive. Therefore, the authors concluded that NP-led care is a cost-effective option when compared with care provided by the dermatologists, as the associated costs were lower than those provided by the dermatologists with comparable effectiveness.
Relevance in the context of economic modelling
With regard to model parameterisation, the study provides detailed information on condition-specific HRQoL as well as resource use and costs, which could be used to inform an economic model.
Summary of the published cost-effectiveness studies
-
Two cost-effectiveness studies based in the Netherlands and one cost-analysis conducted in the UK were described in our review of cost-effectiveness studies.
-
The nature of the interventions and comparators varied in each of the three included studies.
-
Statistical analyses were performed on study data in all three studies and bootstrapping was conducted in two studies to check robustness of the base-case results. None of the studies extrapolated data beyond study durations and there was no information on disease progression. Omission of long-term results from extrapolation makes it difficult to draw conclusions from the reported results.
-
Although the included interventions measured the impact on the QoL of patients with chronic inflammatory skin diseases, none reported HRQoL in terms of QALYs. The study by Schuttelaar and colleagues109 used IDQoL and CDLQI to measure HRQoL.
-
The studies, in general, provided detailed information on the resources used and unit costs. Two of the three included studies could be used to inform model parameters in a de novo cost-effectiveness model.
-
The populations of interest across the included studies were children and adolescents in two studies and in adults in one study.
-
The time horizon of the analyses ranged from 3 months to 1 year, which is considered inadequate for an analysis of chronic inflammatory skin conditions.
-
The cost analysis by Mason and colleagues108 is of most relevance to the UK as the health-care system in the study was the NHS and the study was conducted from UK perspective. However, omission of reporting details of data inputs in terms of QALYs and costs reduced transparency, thereby making it difficult to draw conclusions from the results of the analysis.
Owing to limitations in the evidence base, a de novo economic model was not developed as part of this project. Instead, we discuss recommendations for data that could aid the development of any future economic evaluation in this area.
Future health economic evaluations
This section aims to identify, discuss and address the issues in the current evidence base for educational interventions in chronic skin inflammatory diseases that have emerged in the systematic review of cost-effectiveness studies discussed (see Systematic review of existing cost-effectiveness evidence). The limitations of the current evidence base are discussed first, followed by a list of recommendations and reporting guidelines to be considered for future health-economic analyses of educational interventions in such conditions.
Limitation of current evidence base
The systematic review highlighted the following gaps in existing evidence of literature.
Limited information on educational intervention
The literature identified in the systematic review indicates that there is no clear definition of an educational intervention. The scope of such an intervention could vary considerably with respect to the type and level of the intervention needed at different points in a clinical pathway to have an impact on overall QoL. The broad and diverse nature of such an intervention, reflected in the studies included in the review, means that it is difficult to compare the findings of the studies owing to their wide variability. In addition, limited literature in this domain further confines the comparison and interpretation of the study findings.
Poor effectiveness data and lack of relevant health-related quality-of life measures
As observed in the systematic review, no consistent measure was used to estimate effectiveness of educational interventions. Only one study109 used validated condition-specific measures to estimate effectiveness data. None of the included studies reported any information on HRQoL measure to express outcome in terms of QALYs, which is the main outcome measure in the decision-making context of the UK NHS.
The primary outcome measures reported in the three included studies were discussed above (see Systematic review of existing cost-effectiveness evidence) and the tools used to measure these outcomes are presented in Table 26. The studies reported a number of outcomes, including frequency of itching and scratching, emollient use, disease severity, use of concurrent medication, condition-specific health outcomes and patient satisfaction. The tools used to measure and report the health outcomes varied, with variations in the dimensions of health accounted for in each of the instruments. This heterogeneity in the reported outcomes and the tools used to measure health benefits has restricted the degree of comparability of the results across the studies. In addition, this also raises questions relating to the clinically relevant differences across different instruments.
| Study | Instrument used to measure health outcomes |
|---|---|
| Mason et al., 2013108 |
|
| Schuttelaar et al., 2011109 |
|
| van Os-Medendorp et al., 2008107 |
|
Lack of information on relevant costs and resource use
In general terms, all three studies included in the review provided a detailed overview of the resources used and/or the cost components. However, the nature of the resource use/costs varied. This was potentially driven by the difference in the nature and settings of the intervention.
The resources used and/or costs reported across the three included studies were reviewed to explore the breadth of the various costs and resource utilisation covered in the economic evaluations in chronic inflammatory skin diseases. Studies that did not meet one or more of the inclusion criteria in the screening stage were also reviewed to provide a broad overview of the range of resources and/or costs used in the economic evaluation of such interventions (see Other studies). Table 27 reports the resources used and/or costs reported within each of the studies.
| Resource use | Study | ||||||
|---|---|---|---|---|---|---|---|
| Mason et al., 2013108 | van Os-Medendorp et al., 2008107 | Schuttelaar et al., 2011109 | Hartman et al., 200299 | Parsi et al., 2012101 | Kernick et al., 2000100 | van Gils et al., 2013102 | |
| Interventional | |||||||
| Programme | |||||||
| Raising awareness | |||||||
| Improving knowledge | ✓ group education was provided | ||||||
| Impacting behavioural change | |||||||
| Guidance on use of treatments | |||||||
| Equipment | |||||||
| Using booklets | |||||||
| Face-to-face sessions | |||||||
| Lectures | |||||||
| Workshop | |||||||
| Online [including audiovisual slideshows delivered through, e.g. Microsoft PowerPoint (Microsoft Corporation, Redmond, WA, USA] | |||||||
| Electronic media (such as computer, laptop, CD, DVD) | ✓ | ||||||
| Programme training provided to | |||||||
| Staff | ✓ nurse training; hospital dermatology | ||||||
| Patient group | |||||||
| Carer | |||||||
| Follow-up services | |||||||
| Comment | Reported an overall programme cost but no information on resources used for the programme implementation | ||||||
| Service use | |||||||
| Primary care | |||||||
| GP | ✓ reported only total mean costs | ||||||
| Number of outpatient-visits | ✓ | ✓ | ✓ | ||||
| Phone consultations/assistance | |||||||
| Number of home-visits | ✓ | ||||||
| Nurse | |||||||
| Number of outpatient-visits | ✓ | ||||||
| Phone consultations/assistance | ✓ | ||||||
| Number of home-visits | ✓ | ||||||
| Medication | |||||||
| Oral medication | ✓ provided by nurse | ||||||
| Prescription pharmaceuticals | ✓ | ✓ this includes repeat prescription by GPs, dermatologists and other HCPs | ✓ provided by nurse | ||||
| Visits to alternative medicine provider | ✓ | ||||||
| Comment | It was not clearly stated if GP visits were home-based or outpatient. Depending on the nature of the programme, it was assumed that the visits were home-based | Reported annual cost of dermatology clinic and annual potential in GP consultations | |||||
| Secondary care | |||||||
| Hospitalisation | |||||||
| Number of outpatient-visits | |||||||
| Number of hospital admission days | ✓ | ✓ | ✓ | ||||
| A&E care | |||||||
| Number of day care | ✓ | ||||||
| Nursing time | ✓ | ||||||
| Equipment | |||||||
| Hospital equipment fee (computer, camera, etc.) | |||||||
| Clinic fee | ✓ | ||||||
| Facility fee | ✓ | ||||||
| Laboratory tests | ✓ | ||||||
| Other | ✓ includes emollients, bandages, dressings | ||||||
| Community set-up | |||||||
| Number of visits by community worker | |||||||
| Number of training sessions (if applicable) | |||||||
| Comment | Reported number of visits to the hospital (physician, nurse emergency room, laboratory, transport ambulance | Reported annual cost of dermatology clinic | |||||
| Tertiary care | |||||||
| Consultations | ✓ did not provide the type of consultations | ||||||
| Number of specialist consultations | ✓ did not provide the type of consultations | ✓ | |||||
| Number of physiotherapy consultations | |||||||
| Number of visits to dermatologist | ✓ also reported number of telephonic consultations with the dermatologists | ✓ | ✓ | ||||
| Number of visits to dermatology nurse | ✓ | ||||||
| Number of visit to medical social worker | ✓ | ||||||
| Number of visit to other health-care provider | |||||||
| Number of visits to occupational physician | ✓ | ||||||
| Number of visits to homeopath | ✓ | ||||||
| Number of visits to clinical occupational physician | ✓ | ||||||
| Number of visits to paramedical services | ✓ | ||||||
| Number of visits to psychosocial worker (psychologist or psychiatrist, social worker, ambulatory psychosocial treatment) | ✓ | ✓ | |||||
| Alternative therapies | |||||||
| Number of acupuncture sessions | ✓ | ||||||
| Number of light therapy sessions | ✓ | ✓ | |||||
| Number of ultraviolet therapy sessions | |||||||
| Comment | In addition, reported cost of Internist and insurance physician | ||||||
| Non-service use | |||||||
| Patient and/or family time | ✓ | ✓ includes both professional and family/spouse home help | ✓ | ||||
| Days off work/productivity loss | ✓ | ✓ includes absenteeism | ✓ | ✓ | |||
| Travel expenses | ✓ | ✓ | |||||
| Other family costs | ✓ includes bath oil, out of pocket costs, home help visits | ||||||
| Comment | |||||||
| Overall comments | The study did not report resources used but mean costs | The study reported costs; not resources used | The study reported costs; not resources used | The study reported costs; not resources used | |||
The studies reported a range of resources used. Except for Schuttelaar and colleagues,109 none of the studies provided any information on resource use associated with the intervention. Only two studies107,109 presented detailed information regarding service use and only van Os-Medendorp and colleagues107 provided information on the types ands physical units of resources used. This was considered a limitation, as the studies did not meet the requirement of reporting both physical units, as well as costs, as advocated by Drummond and colleagues. 110
Limited information on disease pathway
Across all the three studies included in the systematic review (see Systematic review of existing cost-effectiveness evidence), statistical analyses were conducted based on trial data where the time-period of the analyses ranged from 3 months to 1 year. There was no information about patient progression through disease pathways among those suffering from different chronic inflammatory conditions. As a result, it is difficult to conceptualise a comprehensive disease progression encompassing all the pathways associated with different chronic inflammatory skin conditions as a whole.
Other studies
This section summarises four additional studies that were identified through the systematic searches. These studies were excluded from the review at the full paper screening stage because they did not meet the inclusion criteria owing to the nature of the interventions (for details, see Appendix 7). Nonetheless, they report useful information with respect to resource utilisation and QoL measures that could be used to inform the list of recommendations to aid the development of future economic analysis. A brief overview of these studies is outlined below for context.
Hartman and colleagues99
This study conducted a cost-effectiveness analysis of moderate to severe psoriasis patients receiving dithranol short contact therapy in a care instruction programme (short contact therapy) with ultraviolet B phototherapy (UVB) and inpatient dithranol treatment (inpatient treatment). The study was based in the Netherlands and the analysis was conducted from a societal perspective. Both medical and non-medical costs were included. Disease severity was quantified with the PASI and the area of involved skin. Clinical effectiveness was measured in terms of the clinical response rate and the number of clearance days. The study concluded that short contact treatment with dithranol in a care instruction programme was an attractive alternative. However, given the associated higher costs this strategy was not a first choice when compared with UVB.
Parsi and colleagues101
A cost-effectiveness analysis was conducted to assess conventional in-office care with a patient-centred, online model for follow-up treatment of patients with psoriasis. The setting of the analysis was the USA and the analysis adopted a societal perspective. The study accounted for both medical and non-medical costs. The DLQI was used to measure outcome and the scores were mapped to the EQ-5D to obtain utility-based HRQoL scores. The results of the study indicated no significant difference in the mean change in DLQI scores between the two strategies, or the mean improvement in quality-adjusted life expectancy. However, costs associated with online visits during follow-up psoriasis care were 1.7 times lower than the cost associated with in-office visits. Based on their findings, the authors concluded the patient-centred online care model to be cost-saving, as both the strategies were similar in terms of effectiveness.
Kernick and colleagues100
Kernick and colleagues100 conducted a cost–consequence analysis on patients with psoriasis or eczema to assess whether a primary care dermatology liaison nurse should be introduced by the NHS in the UK. The analysis was conducted based on a limited economic perspective and, therefore, included the costs associated with nurse and GP care only. DLQI was the primary outcome measure. There was no significant improvement in DLQI scores between the intervention and control groups. The authors concluded that there were difficulties involved in attaining relevant information necessary to assist decisions on resource allocation in primary care. It was acknowledged that large multicentred trials could not answer all the questions and, therefore, the authors concluded that local resource decisions should be based on satisfactory partial evidence-yielding solutions rather than optimum decisions taken on the basis of no evidence.
van Gils and colleagues102
This analysis, based on a Dutch setting, was conducted to examine the cost-effectiveness of an integrated care (IC) programme compared with a usual care programme for patients with moderate to severe chronic hand dermatitis. A societal perspective was adopted in collecting cost data. The difference in clinical severity of hand dermatitis, measured with the Hand Eczema Severity Index (HECSI), was the primary outcome measure. The EQ-5D measure was used to estimate QALYs. On the basis of the findings, the study found the IC programme to be neither cost-effective nor clinically effective compared with usual care after a follow-up of 12 months.
Recommendations for the future
As has already been outlined, with the complexity involved in defining and implementing educational interventions, there has been considerable variation in the nature and type of services provided by them. Although some guidelines exist on the management of inflammatory skin diseases in the UK, no guideline has specified best practice for implementing educational interventions. In light of this wide heterogeneity and evidence gap, we list the following recommendations to address the limitations discussed earlier (see Limitation of current evidence base).
Characteristics of the intervention
One of the fundamental bases for developing an economic model is to have clear boundaries for the characteristics of the intervention in question. This would facilitate two objectives: first, to follow a systematic approach in understanding the key components of the intervention and, second, to enable feasibility to compare and contrast the findings of different studies for the same intervention. It is, therefore, important to understand the components of the intervention, its target at-risk groups; the characteristics of the target population with respect to their physical and/or emotional needs, age, sex and ethnicity, and the characteristics of the settings of the intervention, such as where and how it is delivered and if the programme is delivered as a single initiative or incorporated within the existing health and social services. 114,115
The nature and setting of the educational interventions can vary widely and can be categorised in a number of ways according to: (1) the theoretical approach employed including educational, behavioural, and/or psychological; (2) who provides the education (e.g. self-help, nurse, dermatologist, multiprofessional group, support group); (3) to whom it is delivered (e.g. patient or carer; individual or group); (4) where the education takes place (e.g. at home or in a clinic); (5) how the education is delivered (e.g. using booklets, face-to-face sessions, lectures, workshops, or the internet); (6) the intensity of education (number, duration and frequency of sessions); and (7) the duration of follow-up. 2,36,55,70 It is important to consider these different aspects when defining an educational intervention.
Methodological approach
One of the key features of the existing evidence that has come to light through the findings of the systematic review is the methodological approaches adopted to assess the cost-effectiveness of educational interventions. Statistical analyses conducted over time, ranging from 3 months to 1 year were performed in the three studies included in the systematic review (see Systematic review of existing cost-effectiveness evidence). However, it is unclear what evidence is needed to account for behaviour change in primary studies in order to assess the persistence of effect of an intervention beyond the trial duration.
Resource use/cost
The Drummond checklist for economic evaluation110 points out that all the important and relevant costs of each alternative in question should be identified and measured in appropriate physical units. To do so in educational interventions for chronic inflammatory skin diseases, the resources used can be grouped under three categories for providing a comprehensive resource use framework:
-
interventional
-
service use
-
non-service use.
Interventional and service use groups include resources that have a direct impact on government budget or decision-making bodies. For example, decisions regarding allocation of resources used in the programme delivery, the budget for equipment used to deliver the programme, such as books, online tools, resources required during follow-up services, staff training along with services received in the primary, secondary and tertiary care from the health-care providers are directly influenced by government budgets or that of the health service provider. The primary care setting includes use of outpatient services by the GPs, nurses and alternative medical provider, whereas secondary care includes resources used in inpatient settings. Tertiary care, however, includes all resource use associated with specialised consultations.
Resources within non-service use that mainly consists of indirect costs have a wider societal impact. Within this category, costs associated with productivity loss, travel expenses, family costs, costs associated with patient and/or family time can be included. Such classifications aim to provide a reference base of the resources that should ideally be considered and reported in economic evaluations of such complex multidimensional educational interventions.
Table 28 outlines the items of resource use that should be considered while conducting economic evaluations of educational interventions in chronic inflammatory skin diseases. Table 27 presents a detailed overview of the resource use items from the three included and four additional studies and shows the variability in reporting and gaps in resource coverage. Of the seven studies tabulated, five included information on non-service use. 99,101,102,107,109 These findings indicate that there is no clear pattern of the resources accounted for in the economic analyses. Such inconsistencies in accounting and reporting of key input parameters across the studies limit their comparability. 114
| Interventional | Service use | Non-service use |
|---|---|---|
|
|
|
Health outcomes
Preference-based generic measures of health include multidimensional questionnaires that include questions on a person’s mental, physical, social and functional dimensions to assess overall QoL which enable comparisons across different health conditions. Disease-specific measures of health, however, are more sensitive to change in QoL specific to the conditions. 116 Because of the advantages of both disease-specific and generic HRQoL measures, the majority of current research endorse the combined use of the two measures to assess QoL. 116
The findings of the systematic review (see Systematic review of existing cost-effectiveness evidence) have indicated wide variability in reported health outcomes. To address this issue, the common health outcome measure of QALYs could be obtained from the reported health outcomes in the primary studies through process mapping the reported outcomes to the associated dimensions of a preference-based measure of health, such as EQ-5D. The process mapping would need to relate the outcomes to both direct and indirect effects that a reported health condition might impact upon, as the two effects might not be mutually exclusive. 114 For example, a person experiencing itching and scratching might, in turn, suffer from psychological symptoms (such as depression) and difficulties in mobility as a result of pain and/or discomfort. In this case, if the outcome measure considers only pain and/or discomfort, and does not account for the person’s mental state, then such a measure will reflect only a partial effect on the overall health state of the individual. 114 Another way is to conduct mapping from condition-specific measures of health in skin diseases such as IDQoL to preference-based measures, as done by Parsi and colleagues. 101
None of the three studies included in the review assessed causal questions such as why and how educational interventions could be expected to positively influence the overall health of the patients with chronic inflammatory skin diseases. 114,117 One way to explore this could be by segregating the contribution of each element in the educational intervention to the overall health outcome. This, however, could be challenging given the complex nature of such an intervention by definition and the possibility of intricate links between direct and indirect impacts of the health conditions with the dimensions of a preference-based measure of health. 114 The studies included in the review used condition-specific instruments which could possibly be explained by the assumption that generic measures may be insensitive. One disadvantage of this approach, as has been implicit in the discussion above, is that these instruments limit the generalisability and comparability of the study findings. Therefore, future studies should:
-
use a generic, preference-based measure to assess QoL; or
-
choose a condition-specific measure that can map to a preference-based measure; or
-
use both, test the sensitivity of the preference-based measure and develop (then publish) the mapping as a basis for future research.
Summary
-
There are a number of gaps in the current economic evidence of educational interventions in improving QoL of patients with chronic inflammatory skin conditions, as identified through the systematic review. These include limited information on the type of intervention and clinical pathways of the different conditions and poor-quality data on economic costs and QoL.
-
Based on the gaps identified, it is recommended that future studies consider a wide range of aspects (see Recommendations for the future) to define an educational intervention, use appropriate methodological approaches, consider a broad range of intersectoral costs and resource use, and use either a generic preference-based measure of QoL to assess effectiveness or condition-specific measures that could be used to map to a preference-based measure to assess cost-effectiveness in the UK context.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
Seven RCTs74,76,85–89 met the inclusion criteria for the systematic review of the clinical effectiveness of educational interventions that aim to, or could, improve HRQoL in people with chronic inflammatory skin conditions. Of these, two focused on adult patients with psoriasis,85,86 two focused on children (and adolescents in one study87) with eczema or atopic dermatitis and their carers,74,87 and one focused on women with acne aged over 16 years. 88 The remaining two included adult patients with mixed skin conditions, with one focusing on patients with atopic dermatitis or psoriasis76 and reporting subgroup results for each condition, and the other focusing on adults with chronic pruritic skin diseases. 89 In six RCTs,74,76,85,86,88,89 the educational interventions were delivered as an adjunct to usual care, and it was unclear if this was the case in the remaining trial of group education for children with atopic dermatitis and their carers. 87 The quality of the reporting and methodology of the included trials was judged to be generally poor. The studies were considered to have some generalisability to UK clinical practice, including both studies of education for children with atopic dermatitis and/or their carers. 74,87 Overall, there was heterogeneity in the types of interventions evaluated and patient populations included, precluding meta-analysis and allowing for a narrative synthesis of findings only.
Three of the seven included RCTs found statistically significant greater improvements in HRQoL among patients taking part in the educational interventions than patients in the control group comparisons following the interventions. 76,85,87 Of these, two studies76,85 found evidence of efficacy among adults with psoriasis and one study87 found evidence of efficacy among the carers of children with atopic dermatitis. Among adults with psoriasis, once-daily educational text messages delivered over 12 weeks85 and an intensive group-based educational programme delivered by a multidisciplinary team twice a week over 3 months76 significantly improved HRQoL by the end of the interventions in comparison to an unspecified control condition and medical therapy alone, respectively. Of these psoriasis studies, the one study utilising a longer-term follow-up period76 found that improvements in one of the two HRQoL measures used were maintained 6 months after the intensive educational programme. Among parents of children with atopic dermatitis taking part in a group-based education programme delivered by a multidisciplinary team over 6 weeks,87 there were significant improvements in their HRQoL at 12 months (it was unclear if this was from baseline or the intervention end) compared with a no education control. In the three studies, greater improvements in patients’ disease severity in the educational intervention groups compared with the control groups were also found at these time points, with the exception that the patients with psoriasis who took part in the intensive educational programme76 did not maintain the improvements seen at the end of the intervention by 6 months post intervention.
Only the authors of the study of the educational intervention for children with atopic dermatitis and their parents87 suggested that a clinically meaningful improvement in disease severity had occurred at 12 months. The authors of the other studies finding statistically significant effects did not measure or discuss how clinically meaningful changes in outcomes were. The reviewers note that the minimally clinically important difference has been defined for some of the measures used in the three trials finding statistically significant effects (e.g. the DLQI, PASI and SCORAD – see Appendix 1), and recommend that future trials report how clinically meaningful changes in outcomes are (see Suggested research priorities).
No effects of education on HRQoL or disease severity in comparison with usual care were found in studies of a one-off nurse-delivered educational session in primary care for adults with psoriasis (a pilot study),86 instructions on skin care and make-up use provided by a dermatologist to women with acne,88 the SPaCE website (with or without HCP support) for carers of children with eczema (a pilot study)74 or in a ‘Coping with itch’ programme, incorporating educational and cognitive behavioural interventions, for adults with chronic pruritic skin diseases. 89 Overall, no effective interventions were found for acne or atopic dermatitis in adults.
Commonalities between the effective interventions were delivery by a multidisciplinary team and delivery over a longer period (ranging from 6 weeks to 3 months) than the non-effective interventions. However, this was inferred by the reviewers and not tested in any way and it is not possible to say with confidence from the current evidence whether particular elements of educational interventions and how they are delivered may be associated with improvement in HRQoL.
Cost-effectiveness
Three studies were included in the cost-effectiveness review. Two of these were economic evaluations based on controlled trials conducted in the Netherlands and the remaining study was a cost-effectiveness analysis conducted in the UK. The study populations in two of the three included studies had atopic eczema. One of these studies108 included children with the condition and their carers, whereas the other study109 focused on children alone. Adults with chronic pruritic skin diseases, including a range of skin conditions, were studied in the remaining study, which was the only trial meeting the inclusion criteria for the cost-effectiveness review that was also included in the review of clinical effectiveness. The nature of the interventions and comparators varied in each of the three included studies. The interventions provided nursing care consisting of educational and cognitive behavioural interventions carried out in a specialised itch clinic (the ‘Coping with itch’ programme), an educational support programme for parents and carers, and support in terms of education and coaching by a HCP. None of the studies reported HRQoL in terms of QALYs and only one study measured HRQoL, using the IDQoL and CDLQI. Omission of reporting such a QoL measure limits study comparability. Furthermore, none of the studies reported any adverse events or comorbidities. None of the studies conducted their analyses beyond 1 year. As a result, it is difficult to draw conclusions on the long-term effects from the reported results. Overall, based on the findings of the review of the cost-effectiveness studies, it is unclear if educational interventions are cost-effective in the treatment of patients of chronic inflammatory skin diseases from the perspective of the UK NHS.
Other relevant factors
Gaps in the clinical effectiveness evidence base
The findings of this review highlight a number of gaps in the current clinical effectiveness evidence base. Given how few studies were identified, there is, overall, a lack of evidence for the effectiveness of educational interventions that aim to or could improve HRQoL across all chronic inflammatory skin conditions, although there are some indications of promise of effectiveness in adults with psoriasis and children with atopic dermatitis and their carers. In particular, no studies focused on some of the rarer chronic inflammatory skin conditions, such as lichen planus, lichen sclerosus and hidradenitis suppurativa, so the effectiveness of educational interventions aimed at HRQoL in these populations is unknown. Only two studies74,87 of children (one of which also included adolescents87) were included in the review, and both focused on atopic dermatitis and eczema, with one providing education to older children, in addition to their carers, and adolescents. It may be important for future studies to focus on age-appropriate education for children and young people themselves, particularly as children grow older and transition to taking on more responsibility for disease management from their carers. Such early intervention, for example in eczema or atopic dermatitis, may help improve prognosis and prevent further damage to the skin. There were no studies including children or adolescents affected by other conditions, including acne, which is common in adolescents, with around 50% of 14- to 16-year-olds being affected. 25 However, this may be a hard-to-reach group, as acne may commonly be treated with OTC medications.
Part of the remit of this review was to include studies focusing on carers of people with chronic inflammatory skin conditions. Two of the included studies74,87 assessed education for carers of children with atopic dermatitis or eczema. However, we identified no studies that considered carers of adults with disabilities or other conditions who may also be involved in supporting patients’ self-care.
None of the included studies focused exclusively on patients newly diagnosed with a chronic inflammatory skin condition, which may be a group with particular needs. Indeed, the process evaluation included in the trial by Santer and colleagues,74 which examined the SPaCE website for carers of children with eczema, found a minority of carers did not perceive value from education at this stage in their child’s disease and commented that it had previously been more of a need than it was at the time of the intervention.
In terms of study design and outcome measurement, only the acne trial of instructions in make-up use and skin care88 measured adverse events, and this showed no negative impact of the intervention in a small sample of 25 participants in intervention arm. It may be desirable for studies to include measures of adverse effects or any undesirable or unintended effects of treatment, both of the education and any medical therapy included in either the intervention or comparator arms. There was also a lack of longer-term follow-up data, with only three of the seven trials76,87,89 measuring outcomes at or beyond 3 months post intervention (the review Advisory Group suggested ≥ 3 months would be the minimum follow-up time necessary in studies to measure the effect and durability of patient benefits from an intervention). Only one study compared two different kinds of educational intervention (the SPaCE website with or without HCP support for carers of children with eczema). 74 Comparisons of different approaches would be useful for identifying which intervention factors may be associated with effectiveness.
Target groups for education
It is not clear from the studies included in this review or the NICE guidance5,7 which groups of patients may particularly benefit from education and, more specifically, education aimed at improving HRQoL. NICE guidance5,7 suggests education should be provided at all stages of care and be reiterated in each medical consultation, and from this point of view it could be considered an ongoing need for all patients. However, the place for more structured and planned education in the clinical pathway, such as the more intensive group-based education delivered in some of the studies included in this review, is not clear. These interventions are likely to be costly in terms of resource use and may therefore need to be targeted at particular groups. In all the effective interventions found in this review, the patients targeted and included had more severe psoriasis and atopic dermatitis at baseline than those who took part in the non-effective interventions, providing some indication that those with more severe disease may benefit the most; however, owing to the low number of studies included and their poor quality, this conclusion is tentative. However, HRQoL has not always been found to correlate with disease severity,41–43 so other factors could be considered in how interventions are targeted. For example, the literature has identified that experiencing psychological distress (e.g. depression)38,44 is associated with poor HRQoL, and a subgroup of patients with psoriasis who experience a high psychosocial burden from their disease and who may require additional support has been identified. 86 One consideration is whether patients experiencing psychological distress may particularly benefit from educational interventions aimed at improving HRQoL and which include elements that help patients cope with and address these feelings. Another consideration is whether education may be best targeted at patients who have recently been diagnosed with a condition (when HRQoL scores may not necessarily show a negative impact of the condition) to help patients develop effective self-care behaviours and strategies for enhancing their HRQoL which they can then endeavour to maintain in the future. As well as benefiting patients, this may help prevent costly referrals to secondary care and decrease health-care resource costs for these conditions.
A related issue is whether education should be targeted generically, that is, the same intervention should be used for patients with a range of skin conditions or whether it should be targeted at particular conditions. The studies included in this review indicate that a ‘one-size-fits-all’ model may not be the best approach. Two studies included patients with a mixture of skin conditions and one of these found no benefits for a ‘Coping with itch’ programme for patients with pruritic skin diseases,89 whereas the other found that an intensive group-based programme delivered over 3 months was effective in improving HRQoL in patients with psoriasis, but not those with atopic dermatitis. 76 Given that conditions differ in their disease processes and, potentially the factors that may negatively affect HRQoL, it may be better to target specific conditions, although more generic approaches that focus on particular symptoms or issues common to a range of conditions could be considered.
Quality of reporting and methodology
Generally, the quality of reporting and methodology of the included RCTs was judged to be poor. Three studies (reported in four publications78,91–93), around one-third of the relevant evidence base, met our initial inclusion criteria for the review, but at data extraction it was found that the studies did not provide sufficient adequate information about the results to be included in the review and they were, therefore, excluded. This, along with the poor quality of the included studies, indicates a need for better reporting in this research area.
Reporting of the interventions was generally good, but reporting of the intervention aims, theoretical basis, where the intervention was delivered, any tailoring to participants’ needs, sensitivity to participants’ characteristics, and any ongoing support provided post intervention could be improved, as these elements were less commonly reported in the included studies. Overall, this suggests that more attention needs to be given to reporting and theorising about intervention aims, underpinning theories and hypothesised mechanisms of change, and ensuring (and reporting) that interventions are sensitive to the target groups’ needs and characteristics.
Most of the analyses, with the exception of those of the 3 month to 7 years age group in the trial by Staab and colleagues of group education for children and their carers,87 were potentially underpowered. However, three of these included studies were pilot studies,74,85,86 and, therefore, it is not essential that they are adequately powered, as they will provide information that can inform a power calculation for a larger, more rigorous trial and, as outlined in the MRC guidance on the development and evaluation of complex interventions,65 such pilot studies are an important part of intervention development in offering insight into the feasibility and patient acceptability of an intervention.
Intervention development
As outlined in the Background section of this report, the MRC guidance on the development and evaluation of complex interventions65 underscores that intervention development work is crucial. In line with the MRC guidance, three of the seven included studies reported the underpinning rationales and theoretical bases to the interventions,74,86,87 with two of these, both UK studies,74,86 additionally using qualitative research with patients and/or HCPs to develop the intervention. Two of these studies, both investigating eczema or atopic dermatitis in children, aimed to improve HRQoL through promoting better disease management by the use of behaviour-change techniques in the intervention. 74,87 The other study aimed to improve patients’ self-management through incorporation of techniques addressing constructs from social cognitive theory (e.g. self-efficacy). 86 There is a need for studies to specify more carefully how the interventions may bring about changes in HRQoL and other outcomes, and to make the aims of, and rationale behind, the intervention explicit when reporting findings. Overall, there is a need to clarify in the literature what factors may need to be targeted in an educational intervention to improve HRQoL. Although improving self-management of disease has been put forward as a hypothesised mechanism and may give patients or carers a greater sense of control over the disease (which in itself could be beneficial for HRQoL33), as mentioned above, HRQoL does not always correlate with disease severity. This suggests that target factors other than disease severity that are associated with poor HRQoL in chronic inflammatory skin conditions, such as psychological distress33,39 or feelings of stigmatisation owing to changes in skin appearance,39 may need to be considered as possible mechanisms of change. Intervention developers and investigators could also give consideration to how more informal aspects of educational intervention, such as social contact with other people with or caring for someone with the same condition during educational sessions, may also enhance HRQoL.
There were some reported attempts in the interventions in the included studies to tailor interventions to individual needs and design interventions to be sensitive to participants’ characteristics. However, more attention could be given to these aspects of intervention. Ensuring that interventions are sensitive to participants’ needs may be particularly important when interventions include participants with English as an additional language or people with low levels of literacy. Little consideration seemed to be given in the interventions to the characteristics of the intervention provider, in terms of ensuring they had adequate training for the role or how similar they were in characteristics such as social and ethnic background to the participants, which are also important considerations in intervention delivery. 66,67,69
The MRC guidance on the development and evaluation of complex interventions65 recommends that pilot studies are undertaken to inform further development and evaluation. Three of the seven included studies were pilot studies,85–87 and provided some information on possible effectiveness and the feasibility and acceptability of the interventions. For example, one indicated that daily text message education over 12 weeks reminding patients with psoriasis to use their medications and providing them with general educational statements, used as an adjunct to usual medical care, may result in improvements in HRQoL and disease severity at the end of the intervention compared with an unspecified control condition. 85 The remaining pilot studies, both including patients with more mild eczema74 and psoriasis86 recruited from primary care in the UK, did not find evidence of effectiveness. However, both found that, on the whole, the interventions (a one-off nurse-delivered educational session for patients with psoriasis, supplemented by a follow-up phone call,86 and the SPaCE website for carers of children with eczema74) were feasible and generally perceived as useful by the patients, although a DVD and HCP support provided in each of these interventions, respectively, were perceived as less useful than other aspects, suggesting that inclusion of these approaches may not be needed in follow-on RCTs.
Three studies – all the pilot studies74,85,86 – included process measures. There is a need for more studies to include process evaluations, as recommended by the MRC guidance on complex interventions,65 to identify the contextual factors in intervention delivery which may help explain why an intervention is found to be effective or not. Such process evaluations may offer insight into the best way such interventions could be delivered to patients and carers. For example, if evening meetings may be more suitable for those who work, if allowing patients to bring along relatives or friends for support might encourage better attendance or impact effectiveness, if having easily accessible additional support outside the sessions (e.g. from a specialist nurse) to ask questions patients or carers forgot to ask or were reluctant to ask in the more formal educational session or medical consultation may be beneficial, and the best location for the intervention to be delivered (e.g. at local hospital venues).
Intervention developers could also draw on evidence about what has worked in educational interventions for other long-term conditions that require long-term self-management, such as diabetes, when designing interventions. For example, the Diabetes Education and Self Management for ONgoing and Diagnosed (DESMOND) programme and the Diabetes X-PERT programme for people with diabetes in the UK have been criticised for not adequately addressing the needs of people in particular communities,118 and this points to the need for programmes to be sensitive to the local context. Involvement of patients, family members or carers, general practitioners and Clinical Commissioning Groups could be useful for determining these needs. Additionally, involvement of a lay educator in delivering the DESMOND programme alongside a HCP has been found to be successful,119 and this is an approach that could be considered in educational interventions for chronic inflammatory skin conditions (e.g. patients, family members or carers could be involved as educators). This may also have implications for the cost-effectiveness of interventions, as those delivered with lay person involvement may be less costly than those delivered solely by HCPs.
Educational intervention studies that did not measure health-related quality of life
At the full-text screening stage of the review, we excluded 28 references because the studies either did not report a HRQoL outcome or use a validated HRQoL measure. This was the first criterion studies were screened against at this stage and so not all of the references excluded for this reason were studies of educational interventions (as it was not always clear at the title and abstract screening stage that the studies were about education). Of the 28 references excluded for this reason, 18 were studies of educational interventions for chronic inflammatory skin conditions. It is a notable finding that so many of these studies either did not measure HRQoL or did not use a validated measure. This suggests that studies of educational interventions for these conditions in general may need to give more attention to HRQoL outcomes and, when HRQoL is measured, ensure that validated measures are used.
Strengths and limitations of the assessment
Strengths
-
Thorough searches of a range of literature databases were conducted for both the clinical and cost-effectiveness reviews, including the Global Resource for Eczema Trials (GREAT) database which contains all atopic dermatitis RCTs and systematic reviews. Additionally, the reference lists of included studies and relevant systematic reviews were searched and experts were asked about studies of which they were aware. This means that the review is unlikely to have missed relevant studies.
-
The systematic reviews were conducted in line with good practice principles of conducting systematic reviews in health care. 120
-
The review methods were set out in a protocol prior to the start of the review and published on the PROSPERO website (PROSPERO reference number: CRD42014007426).
-
Both the development of the protocol and the project were informed by an Advisory Group including clinicians, researchers in the field and patient representatives.
-
The use of a taxonomy of intervention elements69 during data extraction to ensure that detailed information about the content and characteristics of the educational interventions was included in the review and to help evaluate the completeness of reporting of the intervention characteristics in the included studies.
-
The systematic reviews of both the clinical effectiveness and cost-effectiveness studies have been carried out independently of any vested interest and the results of both the reviews are presented in a consistent and transparent manner.
Limitations
-
Owing to the nature of the intervention and to many of the studies retrieved for full-text screening not explicitly reporting the aims of the interventions, the reviewers found it challenging to infer if the interventions were aimed at improving HRQoL or could improve HRQoL, in accordance with the review inclusion criteria. Therefore, this was sometimes a judgement call and there was some disagreement between pairs of reviewers, which was resolved through arbitration by a third reviewer. To resolve these disagreements, reviewers considered whether the interventions included elements that targeted compliance with therapy or patients’ ability to cope with the negative effects of the chronic skin disease, as per the examples given in our a priori inclusion criteria. As mentioned above, this highlights that what defines such interventions generally needs more consideration and theorising in the literature. We consider our approach satisfactory, given that intervention aims were not well reported and given the lack of definition of these kinds of interventions in the literature.
-
Meta-analysis could not be conducted owing to heterogeneity of studies and interventions and the limited evidence-base for each skin condition.
-
The review was limited to English-language studies only.
-
When determining if a HRQoL or patient-reported outcome measure was validated, the reviewers relied on statements in the included publications that these were validated and, if this was not reported, they then checked the general literature to see if a measure met at least one validation criteria. Therefore, some measures included were more validated than others. However, the studies used a range of commonly used and well validated measures in this area, such as the DLQI and PASI, so this is likely to be only a minor issue in the review.
-
Outcomes for the end of intervention and longest follow-up period only were data extracted in this review, owing to limited resources and time and consideration that the longest follow-up time point would be the most informative. Given the episodic nature of skin diseases and impact of seasonal changes, however, it may have been useful to data extract interim time points too.
-
The main focus of this review was on the effect of educational interventions that aim to, or could, improve HRQoL. As such, the data presented on other outcomes from education, such as improved disease severity, are drawn only from studies of this particular type of education and, therefore, do not represent all data on how these outcomes may change following patient education in general for chronic inflammatory skin conditions.
-
Three studies of educational interventions that aimed to or could improve HRQoL were excluded from the review for not reporting results in sufficient detail to be informative. Owing to time and resource limitations for the review, we were unable to contact study authors to request them to provide the missing information.
-
Length of follow-up of the studies included in the cost-effectiveness review was inadequate to assess the long-term costs and outcomes of educational interventions in patients with chronic inflammatory skin diseases.
-
There were a number of limitations in the evidence base for the cost-effectiveness of educational interventions, including limited information, lack of relevant HRQoL measures and information on costs and resources and, as such, we were able only to make recommendations for future economic evaluations rather than undertake a de novo economic model.
This review builds on previous systematic reviews of RCTs of educational interventions for chronic inflammatory skin conditions55,71,121 through its focus on those interventions that specifically aim to improve HRQoL or those that include aspects which could improve HRQoL. In line with the other reviews of educational interventions in general, we found that some studies showed statistically significant positive impacts on HRQoL and other outcomes, whereas some did not. Therefore, even when considering only interventions that focus on some way on HRQoL, it is still uncertain from the evidence if educational interventions for people with chronic inflammatory skin conditions can improve HRQoL. Ersser and colleagues71 suggested from a review of educational interventions in children with atopic dermatitis that nurse-led or multidisciplinary interventions may be the most effective in improving outcomes. In our review, we similarly found that delivery by a multidisciplinary team was a commonality between the effective interventions in comparison with those that were not effective.
Uncertainties
There is overall uncertainty about the effectiveness of educational interventions aimed at improving HRQoL in all chronic inflammatory skin conditions, particularly over whether beneficial effects are maintained in the longer term. This is due to the limitations of the evidence base (i.e. studies are generally small, of a poor quality, likely to be underpowered, lack long-term follow-up and there is a lack of consistent evidence for a positive effect on HRQoL). The characteristics and content of educational interventions that may be associated with improvements in HRQoL remain uncertain. The effectiveness of such interventions in rarer chronic inflammatory skin diseases, such as lichen planus, lichen sclerosus and hidradenitis suppurativa, is unknown, as no evidence is available. There are no indications from the evidence reviewed or current clinical guidelines about the best place for such interventions in the clinical pathways for chronic inflammatory skin conditions, about which patients may benefit the most from such interventions, or the settings in which they should be implemented.
Chapter 6 Conclusions
Implications for service provision
There is overall uncertainty over whether educational interventions that include components that could improve HRQoL are effective in improving HRQoL and other outcomes among people with chronic inflammatory skin conditions. However, there are some indications of effectiveness in patients with psoriasis and children and adolescents with atopic dermatitis and their carers. Among patients with psoriasis, when used as an adjunct to usual care, text message education improved patients’ HRQoL in the short term at the intervention end compared with an unspecified control condition (based on one RCT), and intensive group-based education delivered over 3 months by a multidisciplinary team improved HRQoL at the intervention end and possibly up to 6 months post intervention compared with medical therapy alone (based on one RCT). Among carers of children with moderate to severe atopic dermatitis, a 6-week, group-based, education programme delivered by a multidisciplinary team may improve their HRQoL in the long term (i.e. up to 12 months), in comparison with no education (based on one large RCT). These interventions also had positive impacts on patients’ disease severity, compared with usual care or the control conditions, with the authors of the trial of education for atopic dermatitis in children suggesting that the average improvements in disease severity found were clinically significant. Other than in this trial, it is unclear how clinically meaningful these improvements are, as authors of these trials did not comment on this. No effective interventions have yet been evaluated for adults with atopic dermatitis or acne, and no evidence exists for rarer conditions or acne in young people.
The best approach to delivering these kinds of interventions is yet to be established and there is much uncertainty around this, but face-to-face, group, sessions or delivery by text messages may be effective. There are some early indications that delivery over a long period (ranging from 6 weeks to 3 months) and delivery by a multidisciplinary team may be associated with positive outcomes. Other than these structural elements, it is not clear what intervention content may be associated with improvements in HRQoL.
Owing to the limitations of the included studies, there is uncertainty over whether educational interventions aimed at improving QoL are cost-effective in the treatment of chronic inflammatory skin diseases.
Suggested research priorities
There is a need for high-quality, adequately powered RCTs in all chronic inflammatory skin conditions, including rarer conditions, in adults, children and adolescents and carers. These should evaluate theory-based interventions that are sensitive to patients’ needs and characteristics, measure adverse events or undesirable effects of both the educational intervention and treatment it is supplementing, and include an adequate long-term follow-up (of at least 3 months post intervention – as recommended by the review Advisory Group – but preferably 12 months or more as these are long-term conditions which require the maintenance of self-care behaviours, disease control and positive HRQoL over time). Ideally, such RCTs should include an economic evaluation, ITT analyses and a process evaluation, define and measure clinically meaningful changes in outcomes, and follow good reporting standards (e.g. the Consolidated Standards of Reporting Trials statement). Ideally, process evaluations should be included and it would be useful to include measures of hypothesised mechanisms and carry out mediation analyses to explore theoretical explanations about how such interventions may work.
Prior to carrying out rigorous RCTs, in line with the MRC guidance on complex intervention development and evaluation, careful intervention development work should be carried out. This could include reviews of existing evidence and theory to define more accurately the theoretical basis of such interventions and what content should be included to help improve HRQoL in chronic inflammatory skin conditions, supplemented by qualitative work with relevant stakeholders, such as patients and HCPs, where needed. For example, investigators could carry out reviews of the factors associated with HRQoL in specific skin conditions to help inform the factors that could be targeted in the intervention to improve HRQoL. Our brief literature review (see Impact of the disease) suggests that psychological distress and stress, along with symptoms, such as itch, and concerns about appearance may negatively impact patients’ HRQoL. 33,39,40 Additionally, the authors of some of the included studies hypothesised that the effects of education on HRQoL may be mediated by improved disease self-management. It could be argued that improved patient activation from education might also increase HRQoL, as it has been found to be associated with improvements in HRQoL in patients in general. 64 These are among the target factors that could be identified in reviews, qualitative work and in consultation with stakeholders, and which then could be considered in the design of future interventions. In particular, our review suggests that there is a paucity of studies of educational interventions that include components addressing the psychosocial issues experienced by people with chronic inflammatory skin conditions, so this may be a useful focus in future educational interventions. Given the paucity of current evidence for this kind of intervention in chronic inflammatory skin diseases, investigators could also draw on evidence about what has been successful in educational interventions for improving HRQoL in other long-term conditions where patients need to self-manage their condition (e.g. diabetes and asthma) when designing interventions. Pilot studies should also be conducted to inform the development of the intervention, its feasibility and acceptability to patients.
RCTs examining the effectiveness of different approaches to intervention delivery would be useful, such as comparing face-to-face sessions or online interventions, or comparing the effectiveness of shorter educational sessions with more intensive programmes or comparing delivery by a multidisciplinary team with delivery by other kinds of intervention providers (e.g. a specialist nurse).
Future studies of educational interventions aimed at improving HRQoL could include measurements of, and clearly report the resources and costs used to deliver, the intervention, to help inform future economic evaluations. It would also be ideal if future economic evaluations used a generic, preference-based measure to assess HRQoL or a disease-specific one that could then be mapped to a preference-based measure.
Acknowledgements
We would like to thank members of our Advisory Group who provided expert advice and comments on the protocol and/or a draft of this report:
-
Professor Jennifer Cleland, Division of Medical and Dental Education, University of Aberdeen
-
Mr Raymond David Edwards, Psoriasis Association
-
Professor Steven Ersser, Dean and Professor of Nursing and Dermatology Care, Faculty of Health and Social Care, University of Hull
-
Professor Eugene Healy, University of Southampton/Southampton University Hospitals NHS Trust
-
Ms Karina Jackson, Consultant Nurse, St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust
-
Dr Matthew Ridd, Clinical Lecturer, Centre for Academic Primary Care, University of Bristol
-
Ms Amanda Roberts, Nottingham Support Group for Carers of Children with Eczema
-
Dr Andrew R Thompson, Reader in Clinical Psychology, Department of Psychology, University of Sheffield.
We are also grateful to Ms Karen Welch, Information Specialist, Southampton Health Technology Assessments Centre (SHTAC), University of Southampton, for generating and running the literature searches and Dr Jonathan Shepherd, Principal Research Fellow, SHTAC, University of Southampton, for reviewing a draft of this report.
Contribution of authors
Karen Pickett (Research Fellow) developed the research protocol, contributed to the background section, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted and edited the final report.
Emma Loveman (Senior Research Fellow) developed the research protocol, contributed to the background section, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted and edited the final report, project managed the study and acted as guarantor for the project.
Neelam Kalita (Research Fellow) contributed to the background section, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence and drafted the report.
Geoff K Frampton (Senior Research Fellow) developed the research protocol, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence and drafted the report.
Jeremy Jones (Principal Research Fellow) assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence and drafted the report.
Publication
Education to improve quality of life of people with chronic inflammatory skin conditions: a systematic review of the evidence. Br J Dermatol. Submitted for publication.
Data sharing statement
All available data are included in the appendices to this report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Schofield J, Grindlay D, Williams H. Skin Conditions in the UK: A Health Care Needs Assessment. Nottingham: Centre of Evidence Based Dermatology, University of Nottingham; 2009.
- Ersser SJ, Latter S, Sibley A, Satherley PA, Welbourne S. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst Rev 2007;3. http://dx.doi.org/10.1002/14651858.CD004054.pub2.
- British Association of Dermatologists . Eczema n.d. www.bad.org.uk/for-the-public/patient-information-leaflets/atopic-eczema/?showmore=1&returnlink=http%3a%2f%2fwww.bad.org.uk%2ffor-the-public%2fpatient-information-leaflets#.Vh3uPf-FO70 (accessed 7 June 2013).
- Burr ML, Dunstan FDJ, Hand S, Ingram JR, Jones KP. The natural history of eczema from birth to adult life: a cohort study. Br J Dermatol 2013;168:1339-42. http://dx.doi.org/10.1111/bjd.12216.
- Atopic Eczema in Children. Management of Atopic Eczema in Children from Birth up to the Age of 12 Years. NICE Clinical Guideline. London: Royal College of Obstetricians and Gynaecologists; 2007.
- British Association of Dermatologists . Psoriasis – An Overview n.d. www.bad.org.uk/for-the-public/patient-information-leaflets/psoriasis---an-overview/?showmore=1&returnlink=http%3a%2f%2fwww.bad.org.uk%2ffor-the-public%2fpatient-information-leaflets#.Vh3uwf-FO70 (accessed 7 June 2013).
- Psoriasis. The Assessment and Management of Psoriasis. NICE Clinical Guideline 153. London: NICE; 2012.
- Nestle FO, Kaplan DH, Barker J. Mechanisms of Disease: Psoriasis. N Engl J Med 2009;361:496-509. http://dx.doi.org/10.1056/NEJMra0804595.
- Purdy S, de Berker D. Clinical review: Acne. BMJ 2006;333:949-53. http://dx.doi.org/10.1136/bmj.38987.606701.80.
- National Institute for Health and Care Excellence . NHS Choices: Acne 2014. www.nhs.uk/conditions/acne/pages/introduction.aspx (accessed 18 February 2014).
- Schuttelaar MLA, Vermeulen KM, Drukker N, Coenraads PJ. A randomized controlled trial in children with eczema: nurse practitioner vs. dermatologist. Br J Dermatol 2010;162:162-70. http://dx.doi.org/10.1111/j.1365-2133.2009.09502.x.
- Kay J, Gawkrodger DJ, Mortimer MJ, Jaron AG. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol 1994;30:35-9. http://dx.doi.org/10.1016/S0190-9622(94)70004-4.
- Emerson RM, William HC A, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. Br J Dermatol 1998;139:73-6. http://dx.doi.org/10.1046/j.1365-2133.1998.02316.x.
- Kurukulaaratchy R, Fenn M, Matthews S, Hasan Arshad S. The prevalence, characteristics of and early life risk factors for eczema in 10-year-old children. Pediatric Allergy Immunol 2003;14:178-83. http://dx.doi.org/10.1034/j.1399-3038.2003.00036.x.
- Wadonda-Kabondo N, Sterne JA, Golding J, Kennedy CT, Archer CB, Dunnill MG, et al. A prospective study of the prevalence and incidence of atopic dermatitis in children aged 0–42 months. Br J Dermatol 2003;149:1023-8. http://dx.doi.org/10.1111/j.1365-2133.2003.05605.x.
- Deckers IA, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0039803.
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377-85. http://dx.doi.org/10.1038/jid.2012.339.
- Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol 2005;141:1537-41. http://dx.doi.org/10.1001/archderm.141.12.1537.
- Seminara NM, Abuabara K, Shin DB, Langan SM, Kimmel SE, Margolis D, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol 2011;163:602-9. http://dx.doi.org/10.1111/j.1365-2133.2010.10134.x.
- Danielsen K, Olsen AO, Wilsgaard T, Furberg AS. Is the prevalence of psoriasis increasing? A 30-year follow-up of a population-based cohort. Br J Dermatol 2013;168:1303-10. http://dx.doi.org/10.1111/bjd.12230.
- Huerta C, Rivero E, Garcia Rodriguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol 2007;143:1559-65. http://dx.doi.org/10.1001/archderm.143.12.1559.
- Khalid JM, Globe G, Fox KM, Chau D, Maguire A, Chiou CF. Treatment and referral patterns for psoriasis in United Kingdom primary care: a retrospective cohort study. BMC Dermatol 2013;13. http://dx.doi.org/10.1186/1471-5945-13-9.
- Robeva R, Assyov Y, Tomova A, Kumanov P. Acne vulgaris is associated with intensive pubertal development and altitude of residence – a cross-sectional population-based study on 6,200 boys. Eur J Pediatr 2013;172:465-71. http://dx.doi.org/10.1007/s00431-012-1907-1.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol 2013;168:474-85. http://dx.doi.org/10.1111/bjd.12149.
- Acne: Prevalence, Symptoms and Diagnosis. London: The Dairy Council; n.d.
- Spoendlin J, Voegel JJ, Jick SS, Meier CR. A study on the epidemiology of rosacea in the UK. Br J Dermatol 2012;167:598-605. http://dx.doi.org/10.1111/j.1365-2133.2012.11037.x.
- Patient . Hidradenitis Suppurativa 2014. www.patient.co.uk/doctor/hidradenitis-suppurativa-pro (accessed 2 July 2014).
- Oakley A, DermNet NZ. Cutaneous Lupus Erythematosus 2014. http://dermnetnz.org/immune/cutaneous-lupus.html (accessed 15 August 2014).
- Neill SM, Tatnall FM, Cox NH. Guidelines for the managment of lichen sclerosus. Br J Dermatol 2002;147:640-9. http://dx.doi.org/10.1046/j.1365-2133.2002.05012.x.
- British Association of Dermatologists . Seborrhoeic Dermatitis 2012. www.bad.org.uk/for-the-public/patient-information-leaflets/seborrhoeic-dermatitis (accessed 15 August 2014).
- Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol 2005;22:192-9. http://dx.doi.org/10.1111/j.1525-1470.2005.22303.x.
- Evers AWM, Lu Y, Duller P, Van Der Valk PGM, Kraaimaat FW, van de Kerkhof PCM. Common burden of chronic skin diseases? Contributers to psychological distress in adults with psoriasis and atopic dermatitis. Br J Dermatol 2005;152:1175-281. http://dx.doi.org/10.1111/j.1365-2133.2005.06565.x.
- Bhosle MJ, Kulkarni A, Feldman SR, Balkrishnan R. Quality of life in patients with psoriasis. Health Qual Life Outcomes 2006;4. http://dx.doi.org/10.1186/1477-7525-4-35.
- Schmid-Ott G, Steen T. International Encyclopedia of Rehabilitation. Buffalo, NY: The State University of New York; 2010.
- Richards HL, Fortune DG, Griffiths CEM, Main CJ. The contributions of perceptions of stigmatisation to disability in patients with psoriasis. J Psychosomatic Res 2013;50:11-5. http://dx.doi.org/10.1016/S0022-3999(00)00210-5.
- Fordham B, Griffiths CEM, Bundy C. Can stress reduction interventions improve psoriasis? A review. Psychology Health Med 2013;18:501-14.
- Devrimci-Ozguven H, Kundakci N, Kumbaser H, Boyvat A. The depression, anxiety, life satisfaction and affective expression levels in psoriasis patients. J Eur Acad Dermatol Venereol 2000;14:267-71. http://dx.doi.org/10.1046/j.1468-3083.2000.00085.x.
- Kohli Kurd S, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 2010;146:891-5. http://dx.doi.org/10.1001/archdermatol.2010.186.
- Wittkowski A, Richards HL, Griffiths CEM, Main CJ. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J Psychosomat Med 2004;57:195-200. http://dx.doi.org/10.1016/S0022-3999(03)00572-5.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol 2004;150:284-90. http://dx.doi.org/10.1111/j.1365-2133.2004.05776.x.
- Kierbert G, Sorenson SV, Revicki D, Fagan SC, Doyle JJ, Cohen J, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol 2002;41:151-8. http://dx.doi.org/10.1046/j.1365-4362.2002.01436.x.
- de Jager MEA, van de Kerkhof PCM, de Jong EMGJ, Seyger MMB. A cross-sectional study using the Children’s Dermatology Life Quality Index (CDLQI) in childhood psoriasis: negative effect on quality of life and moderate correlation of CDLQI with severity scores. Br J Dermatol 2010;163:1099-101. http://dx.doi.org/10.1111/j.1365-2133.2010.09993.x.
- Sampogna F, Sera F, Abeni D. IDI Multipurpose psoriasis research on vital experiences investigators. Measures of clinical severity, quality of life, and psychological distress in patients with psoriasis: a cluster analysis. J Invest Dermatol 2004;122:602-7. http://dx.doi.org/10.1046/j.0022-202X.2003.09101.x.
- Arck P, Paus R. From the brain-skin connection: the neuroendocrine-immune misalliance of stress and itch. Neuroimmunomodulation 2006;13:347-56. http://dx.doi.org/10.1159/000104863.
- Feldman SR, Horn EJ, Balkrishnan R, Basra MK, Finlay AY, McCoy D, et al. Psoriasis: improving adherence to topical therapy. J Am Acad Dermatol 2008;59:1009-16. http://dx.doi.org/10.1016/j.jaad.2008.08.028.
- Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol 2003;56:395-407. http://dx.doi.org/10.1016/S0895-4356(03)00044-1.
- Bowling A. Measuring Disease. Buckingham: Open University Press; 1995.
- Fox-Rusby J, Cairns J, Fox-Rushby J, Cairns J. Economic Evaluation. Maidenhead: Open University Press; 2005.
- Dermatology Life Quality Index: Information & Conditions Concerning Use. Cardiff: Section of Dermatology, Department of Dermatology & Wound Healing, School of Medicine, Cardiff University; 2014.
- Tareen RS, Greydanus DE, Jafferany M. Pediatric Psychodermatology: A Clinical Manual of Child and Adolescent Psychocutaneous Disorders. Berlin: Walter de Gruyter & Co.; 2012.
- Prescribing and Primary Care, Health and Social Care Information Centre . Prescriptions Dispensed in the Community: England 2003–13 n.d. www.hscic.gov.uk/catalogue/PUB14414 (accessed 11 June 2014).
- Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA 2002;288:2469-75. http://dx.doi.org/10.1001/jama.288.19.2469.
- Griesbach D, Taylor A. Educational Interventions to Prevent Hepatitis C: A Review of the Literature and Expert Opinion. Glasgow: NHS Scotland; 2009.
- Management of Atopic Eczema in Primary Care: A National Clinical Guideline. Edinburgh: SIGN; 2011.
- De Bes J, Legierse CM, Prinsen CAC, De Korte J. Patient education in chronic skin diseases: a systematic review. Acta Derm Venereol 2011;91:12-7. http://dx.doi.org/10.2340/00015555-1022.
- National Institute for Health and Care Excellence . Atopic Eczema in Children: NICE Quality Standard (QS44) 2014.
- The James Lind Alliance: Eczema Priority Setting Partnership. Southampton: The James Lind Alliance; 2014.
- Psoriasis Assessment and Management of Psoriasis. Clinical Guideline Methods, Evidence and Recommendations. London: Royal College of Physicians; 2012.
- Lapsley P. The double benefits of educational programmes for patients with eczema. BMJ 2006;332. http://dx.doi.org/10.1136/bmj.332.7547.936.
- Hamilton Larsen M, Birger Hagen K, Krogstad A-L, Aas E, Klopstad Wahl A. Limited evidence of the effects of patient education and self-management interventions in psoriasis patients: a systematic review. Patient Educ Counsel 2014;94:158-69. http://dx.doi.org/10.1016/j.pec.2013.10.005.
- Rasmussen GS, Terkildsen Maindal H, Lomborg K. Self-management in daily life with psoriasis: an integrative review of patient needs for structured education. Nurs Res Prac 2012. http://dx.doi.org/10.1155/2012/890860.
- Feldman S, Behnam SM, Behnam SE, Koo JYM. Involving the patient: impact of inflammatory skin disease and patient-focused care. J Am Acad Dermatol 2005;53. http://dx.doi.org/10.1016/j.jaad.2005.04.033.
- Hibbard JH, Mahoney ER, Stock R, Tusler M. Do increases in patient activation result in improved self-management behaviours?. Health Serv Res 2007;42:1443-63. http://dx.doi.org/10.1111/j.1475-6773.2006.00669.x.
- Magnezi R, Glasser S, Shaley H, Sheiber A, Reuveni H. Patient activation, depression and quality of life. Patient Educ Counsel 2014;94:432-7. http://dx.doi.org/10.1016/j.pec.2013.10.015.
- Developing and Evaluating Complex Interventions: New Guidance. London: MRC; 2008.
- Barbarot S, Bernier C, Deleuran M, De Raeve L, Eichenfield L, El Hachem M, et al. Therapeutic patient education in children with atopic dermatitis: position paper on objectives and recommendations. Pediatr Dermatol 2013;30:199-206. http://dx.doi.org/10.1111/pde.12045.
- Hong J, Nguyen TV, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: Part II: Patient education. J Am Acad Dermatol 2013;68:364.e1-364.e10. http://dx.doi.org/10.1016/j.jaad.2012.10.060.
- Kreuter MW, Sugg Skinner C. Tailoring: what’s in a name?. Health Educ Res 2000;15:1-4. http://dx.doi.org/10.1093/her/15.1.1.
- Schulz R, Czaja SJ, McKay JR, Ory MG, Belle SH. Taxonomy (ITAX): describing essential features of interventions (HMC). Am J Health Behav 2010;34:811-21.
- Lavda AC, Webb TL, Thompson AR. A meta-analysis of the effectiveness of psychological interventions for adults with skin condirtions. Br J Dermatol 2012;167:970-9. http://dx.doi.org/10.1111/j.1365-2133.2012.11183.x.
- Ersser SJ, Cowdell F, Latter S, Gardiner E, Flohr C, Thompson AR, et al. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst Rev 2014;1. http://dx.doi.org/10.1002/14651858.cd004054.pub3.
- Jackson K, Ersser SJ, Dennis H, Farasat H, More A. The Eczema Education Programme: intervention development and model feasibility. J Eur Acad Dermatol Venereol 2013;28:949-56. http://dx.doi.org/10.1111/jdv.12221.
- Ersser SJ, Farasat H, Jackson K, Dennis H, Sheppard Z, More A. A service evaluation of the Eczema Education Programme: an analysis of child, parent and service impact outcomes. Br J Dermatol 2013;169:629-36. http://dx.doi.org/10.1111/bjd.12414.
- Santer M, Muller I, Yardley L, Burgess H, Selinger H, Stuart BL, et al. Supporting self-care for families of children with eczema with a web-based intervention plus health care professional support: pilot randomized controlled trial. J Med Internet Res 2014;16. http://dx.doi.org/10.2196/jmir.3035.
- van der Meer EW, Boot CR, Jungbauer FH, van der Klink JJ, Rustemeyer T, Coenraads PJ, et al. Hands4U: a multifaceted strategy to implement guideline-based recommendations to prevent hand eczema in health care workers: design of a randomised controlled trial and (cost) effectiveness evaluation. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-669.
- Bostoen J, Bracke S, De KS, Lambert J. Br J Dermatol 2012;167:1025-31. http://dx.doi.org/10.1111/j.1365-2133.2012.11113.x.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. http://dx.doi.org/10.1037/0278-6133.27.3.379.
- Wenninger K, Kehrt R, den Uv, Lehmann C, Binder C, Wahn U, et al. Structured parent education in the management of childhood atopic dermatitis: The Berlin model. Patient Educ Counsel 2000;40:253-61. http://dx.doi.org/10.1016/S0738-3991(99)00074-9.
- Bandura A. Self-efficacy: towards a unifying theory of behavioral change. Psycholog Rev 1977;84:191-215. http://dx.doi.org/10.1037/0033-295X.84.2.191.
- Bandura A. Self-Efficacy: the Exercise of Control. New York, NY: W.H. Freeman and Company; 1997.
- Bewley A, Burrage DM, Ersser SJ, Hansen EM, Ward C. Identifying individual psychosocial and adherence support needs in patients with psoriasis: a multinational two-stage qualitative and quantitative study. J Eur Acad Dermatol Venereol 2013;28:763-70. http://dx.doi.org/10.1111/jdv.12174.
- Lavada AC, Thompson AR. Psychosocial impact of skin conditions: interventions for nurses. Dermatolog Nurs 2010;9:38-41.
- Higgins J. P., Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Balato N, Megna M, Di CL, Balato A, Ayala F. Educational and motivational support service: a pilot study for mobile-phone-based interventions in patients with psoriasis. Br J Dermatol 2013;168:201-5. http://dx.doi.org/10.1111/j.1365-2133.2012.11205.x.
- Ersser SJ, Cowdell FC, Nicholls PG, Latter SM, Healy E. A pilot randomized controlled trial to examine the feasibility and efficacy of an educational nursing intervention to improve self-management practices in patients with mild-moderate psoriasis. J Eur Acad Dermatol Venereol 2012;26:738-45. http://dx.doi.org/10.1111/j.1468-3083.2011.04158.x.
- Staab D, Diepgen TL, Fartasch M, Kupfer J, Lob-Corzilius T, Ring J, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ 2006;332:933-8. http://dx.doi.org/10.1136/bmj.332.7547.933.
- Matsuoka Y, Yoneda K, Sadahira C, Katsuura J, Moriue T, Kubota Y. Effects of skin care and makeup under instructions from dermatologists on the quality of life of female patients with acne vulgaris. J Dermatol 2006;33:745-52. http://dx.doi.org/10.1111/j.1346-8138.2006.00174.x.
- van Os-Medendorp H, Ros WJ, Eland-de Kok PC, Kennedy C, Thio BH, van der Schuur-van der Zande A, et al. Effectiveness of the nursing programme ‘Coping with itch’: a randomized controlled study in adults with chronic pruritic skin disease. Br J Dermatol 2007;156:1235-44. http://dx.doi.org/10.1111/j.1365-2133.2007.07919.x.
- Pagliarello C, Calza A, Armani E, di PC, Tabolli S. Effectiveness of an empowerment-based intervention for psoriasis among patients attending a medical spa. Eur J Dermatol 2011;21:62-6. http://dx.doi.org/10.1684/ejd.2010.1197.
- Jaspers JP, Span L, Molier L, Coenraads PJ. A multimodal education and treatment program for young adults with atopic dermatitis: a randomized controlled trial. Dermatol Psychosom 2000;1:148-53. http://dx.doi.org/10.1159/000057970.
- Lora V, Gisondi P, Calza A, Zanoni M, Girolomoni G. Efficacy of a single educative intervention in patients with chronic plaque psoriasis. Dermatology 2009;219:316-21. http://dx.doi.org/10.1159/000250826.
- Staab D, von RU, Kehrt R, Erhart M, Wenninger K, Kamtsiuris P, et al. Evaluation of a parental training program for the management of childhood atopic dermatitis. Pediatr Allergy Immunol 2002;13:84-90. http://dx.doi.org/10.1034/j.1399-3038.2002.01005.x.
- Lambert J, Bostoen J, Geusens B, Bourgois J, Boone J, De SD, et al. A novel multidisciplinary educational programme for patients with chronic skin diseases: Ghent pilot project and first results. Arch Dermatolog Res 2011;303:57-63. http://dx.doi.org/10.1007/s00403-010-1082-z.
- Basra MKA, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008;159:997-1035. http://dx.doi.org/10.1111/j.1365-2133.2008.08832.x.
- Dermatology Life Quality Index: Information and Conditions Concerning Use. Cardiff: Cardiff University; 2014.
- Fisker MH, Agner T, Lindschou J, Bonde JP, Ibler KS, Gluud C, et al. Protocol for a randomised trial on the effect of group education on skin-protective behaviour versus treatment as usual among individuals with newly notified occupational hand eczema – the Prevention of Hand Eczema (PREVEX) Trial. BMC Dermatol 2013;13. http://dx.doi.org/10.1186/1471-5945-13-16.
- Prinsen CA, Spuls PI, Sprangers MA, de Rie MA, Legierse CM, de KJ. The efficacy of a health-related quality-of-life intervention during 48 weeks of biologic treatment of patients with moderate to severe psoriasis: study protocol for a multicenter randomized controlled trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-236.
- Hartman M, Prins M, Swinkels OQ, Severens JL, De BT, Van Der Wilt GJ, et al. Cost-effectiveness analysis of a psoriasis care instruction programme with dithranol compared with UVB phototherapy and inpatient dithranol treatment. Br J Dermatol 2002;147:538-44. http://dx.doi.org/10.1046/j.1365-2133.2002.04920.x.
- Kernick D, Cox A, Powell R, Reinhold D, Sawkins J, Warin A. A cost consequence study of the impact of a dermatology-trained practice nurse on the quality of life of primary care patients with eczema and psoriasis. Br J Gen Prac 2000;50:555-8.
- Parsi K, Chambers CJ, Armstrong AW. Cost-effectiveness analysis of a patient-centered care model for management of psoriasis. J Am Acad Dermatol 2012;66:563-70. http://dx.doi.org/10.1016/j.jaad.2011.02.022.
- van Gils RF, Bosmans JE, Boot CRL, Rustemeyer T, van MW, Van Der Valk PGM, et al. Economic evaluation of an integrated care programme for patients with hand dermatitis. Contact Dermatitis 2013;69:144-52. http://dx.doi.org/10.1111/cod.12095.
- Bathe A, Matterne U, Dewald M, Grande T, Weisshaar E. Educational multidisciplinary training programme for patients with chronic pruritus. Acta Derma Venereol 2009;89:498-501. http://dx.doi.org/10.2340/00015555-0684.
- Beikert FC, Langenbruch AK, Radtke MA, Augustin M. Willingness to pay and quality of life in patients with rosacea. J Eur Acad Dermatol Venereol 2013;27:734-8. http://dx.doi.org/10.1111/j.1468-3083.2012.04549.x.
- Evaluation of the Combination Day- and Home Treatment of Psoriasis – Primary Research. Diemen, The Netherlands: Health Care Technology Assessment Program; 2000.
- An Economic Evaluation Comparing Usual Care with Internet-Guided Monitoring and Self-Management Training by a Nurse Practitioner. The Hague, The Netherlands: The Netherlands Organisation for Health Research and Development (ZonMw); 2000.
- van Os-Medendorp H, Guikers CL, Eland-de Kok PC, Ros WJ, Bruijnzeel-Koomen CA, Buskens E. Costs and cost-effectiveness of the nursing programme ‘Coping with itch’ for patients with chronic pruritic skin disease. Br J Dermatol 2008;158:1013-21. http://dx.doi.org/10.1111/j.1365-2133.2008.08477.x.
- Mason JM, Carr J, Buckley C, Hewitt S, Berry P, Taylor J, et al. Improved emollient use reduces atopic eczema symptoms and is cost neutral in infants: before-and-after evaluation of a multifaceted educational support programme. BMC Dermatol 2013;13. http://dx.doi.org/10.1186/1471-5945-13-7.
- Schuttelaar ML, Vermeulen KM, Coenraads PJ. Costs and cost-effectiveness analysis of treatment in children with eczema by nurse practitioner vs. dermatologist: results of a randomized, controlled trial and a review of international costs. Br J Dermatol 2011;165:600-11. http://dx.doi.org/10.1111/j.1365-2133.2011.10470.x.
- Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Glick HA, Oshi JA, Sonnad SS, Olsky D. Economic Evaluation in Clinical Trials (Handbooks for Health Economic Evaluation. Oxford: Oxford University Press; 2007.
- Kirkwood BR, Sterne JAC. Medical Statistics. Malden, MA, Oxford, Carlton, VIC: Blackwell Science Ltd; 2003.
- Zorginstituut Nederland . Medicijnkosten n.d. www.medicijnkosten.nl/ (accessed 17 June 2014).
- Kalita N. Developing and Applying a Framework for the Economic Evaluation of Home-Based Nurse-Led Health Promotion Programmes for Older People 2011.
- Tappenden P, Campbell F, Rawdin R, Wong R, Kalita N. The clinical effectiveness and cost-effectiveness of home-based, nurse-led health promotion for older people: a systematic review. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16200.
- Augustin M, Langenbruch AK, Herberger K, Baade K, Goepel L, Blome C. Quality of life measurement in chronic wounds and inflammatory skin diseases: definitions, standards and instruments. Wound Med 2014;5:29-38. http://dx.doi.org/10.1016/j.wndm.2014.04.008.
- Markle-Reid M, Browne G, Weir R, Gafni A, Roberts J, Henderson SR. The effectiveness and efficiency of home-based nursing health promotion for older people: a review of the literature. Med Care Res Rev 2006;63:531-69. http://dx.doi.org/10.1177/1077558706290941.
- Loveman E, Frampton GK, Clegg AJ. The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12090.
- Carey ME, Madalia PK, Daly H, . Increasing capacity to deliver diabetes self-management education: results of the DESMOND lay educator non-randomized controlled equivalence trial. Diabetic Med 2014;31:1431-8. http://dx.doi.org/10.1111/dme.12483.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: York Publishing Services Ltd, CRD; 2009.
- Larsen MH, Hagen KB, Krogstad AL, Aas E, Wahl AK. Limited evidence of the effects of patient education and self-management interventions in psoriasis patients: a systematic review. Patient Educ Counsel 2014;94:158-69. http://dx.doi.org/10.1016/j.pec.2013.10.005.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol 2001;144:104-10. http://dx.doi.org/10.1046/j.1365-2133.2001.03960.x.
- Lewis V, Finlay A. 10 years experience of the Dermatology Life Quality Index (DLQI). J Invest Dermatol Symposium Proc 2004;9:169-80. http://dx.doi.org/10.1111/j.1087-0024.2004.09113.x.
- Nijsten T. Dermatology life quality index: time to move forward. J Invest Dermatol 2012;132:11-3. http://dx.doi.org/10.1038/jid.2011.354.
- Basra MK, Gada V, Ungaro S, Finlay AY, Salek SM. Infants’ Dermatitis Quality of Life Index: a decade of experience of validation and clinical application. Br J Dermatol 2013;169:760-8. http://dx.doi.org/10.1111/bjd.12563.
- Children’s Dermatology Life Quality Index (CDLQI): Information & Conditions Concerning Use. Cardiff: Section of Dermatology, Department of Dermatology & Wound Healing. School of Medicine, Cardiff University; 2014.
- Salek MS, Jung S, Brincat-Ruffini LA, MacFarlane L, Lewis-Jones MS, Basra MK, et al. Clinical experience and psychometric properties of the Children’s Dermatology Life Quality Index (CDLQI), 1995–2012. Br J Dermatol 2013;169:734-59. http://dx.doi.org/10.1111/bjd.12437.
- Holme SA, Man I, Sharpe JL, Dykes PJ, Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol 2003;148:285-90. http://dx.doi.org/10.1046/j.1365-2133.2003.05157.x.
- Kondo-Endo K, Ohashi Y, Nakagawa H, Katsunuma T, Ohya Y, Kamibeppu K, et al. Development and validation of a questionnaire measuring quality of life in primary caregivers of children with atopic dermatitis (QPCAD). Br J Dermatol 2009;161:617-25. http://dx.doi.org/10.1111/j.1365-2133.2009.09177.x.
- Chren M, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol 1996;107:707-13. http://dx.doi.org/10.1111/1523-1747.ep12365600.
- Chren MM. The Skindex Instruments to measure the effects of skin disease on quality of life. Dermatol Clin 2012;30:231-6. http://dx.doi.org/10.1016/j.det.2011.11.003.
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rhuem Dis 2005;64:ii65-8. http://dx.doi.org/10.1136/ard.2004.031237.
- DermNet NZ. PASI Score 2014. www.dermnetnz.org/scaly/pasi.html (accessed 13 August 2014).
- Henseler T, Schmitt-Rau K. A comparison between BSA, PASI, PLASI and SAPASI as measures of disease severity and improvement by therapy in patients with psoriasis. Int J Dermatol 2008;47:1019-23. http://dx.doi.org/10.1111/j.1365-4632.2008.03753.x.
- DermNet NZ. SCORAD 2014. http://dermnetnz.org/dermatitis/scorad.html (accessed 13 August 2014).
- Oranje AP, Glazenburg EJ, Wolkerstorfer A, Waard-van der Spek FB. Practical issues on intrepretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol 2007;157:645-8. http://dx.doi.org/10.1111/j.1365-2133.2007.08112.x.
- Schram ME, Spuls PhI, Leeflang MMG, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012;67:99-106. http://dx.doi.org/10.1111/j.1398-9995.2011.02719.x.
Appendix 1 Common health-related quality-of-life and disease severity measures
Health-related quality-of-life measures
Dermatological Life Quality Index
The DLQI was developed as a dermatology-specific QoL measure because generic HRQoL instruments, for example the SF-36, were not specific enough to capture aspects of QoL related to dermatological conditions. The DLQI is widely used in clinical practice and clinical trials across the world, and has been particularly used in psoriasis, atopic dermatitis and acne. 122,123
The questionnaire is used in adults aged over 16 years. The DLQI consists of 10 questions and provides a total HRQoL score, as well as scores for six aspects of QOL: symptoms and feelings; daily activities; leisure; work and school; personal relationships; and treatment. Question responses range from ‘not at all’, ‘a little’, ‘a lot’ to ‘very much’. The DLQI score is calculated by adding the scores together, with a maximum score of 30 and a minimum score of 0. A higher score reflects a worse HRQoL. A minimally clinically important difference on the DLQI is generally defined as a score change of at least four points, with studies estimating that this ranges from 2.2 to 6.9 across specific inflammatory skin conditions. 49 The time frame of DLQI is one week (i.e. it focuses on the QoL in the previous week because it is easy to recall accurately). 49 Lewis and Finlay123 reported that DLQI has been validated against a number of other dermatology and health measures.
An advantage of the DLQI is that it is simple and quick to complete. Furthermore, there is a very high success rate of accurate completion of the measure. 123 The DLQI is accepted by dermatology professionals, researchers and regulatory authorities as a standard by which HRQoL can be reliably measured. Despite being a valid and reliable measure, there are a few limitations. There is a focus on physical limitations as a result of the skin condition and few items address the possible implications to psychological health. Evidence suggests that the measure is, therefore, most valid in assessing HRQoL in those with more severe disease. 124 The DLQI is also affected by some bias in terms of factors such as age, sex, diagnosis and nationality ,which can affect responses on more than half of the questions. Therefore, caution in the interpretation is recommended when using the measure in a heterogeneous patient population, for example in international studies, as these factors may impact on the scores seen across patients. 124
The Infants’ Dermatitis Quality of Life Index
The IDQoL Index questionnaire is designed to assess QoL in infants with atopic dermatitis aged between 0 and 3 years. This widely used questionnaire is completed by the children’s parents or their carers. It has wide international use, having been translated into 21 languages. 125
The questionnaire consists of two main domains: dermatitis severity and life quality index. Within the life quality index, there are 10 questions relating to: itching and scratching; mood of the child; how long it takes for the child to sleep; has the eczema interfered with their playing; swimming or participation in other family activities; problems during meal times; problems caused by treatment; level of comfort while dressing or undressing the child; and problems during bath times. Each question is scored between 0 and 3 and these are then totalled to generate the IDQoL score, which therefore ranges from 0 to 30. Higher scores reflect greater impact. 125
Basra and colleagues reported that, despite available evidence across 11 countries on IDQoL sensitivity to change, there is currently no means by which to estimate what score change is clinically meaningful. 125
The IDQoL measure has several positive aspects of psychometric performance. The measure has shown high specificity, thereby indicating that it can distinguish between infants with disease and infants without disease well. As it is simple to use it has been considered to be the ‘gold standard’ to measure HRQoL in infants with atopic dermatitis. Some validation factors have not been fully researched however, such as factor analysis, and the test–retest reliability and internal consistency across the different adaptations of the measure. 125
Children’s Dermatology Life Quality Index
The CDLQI questionnaire was designed as a tool to allow assessment of QoL in children aged 4 to 16 years with skin conditions. 126,127 It is a simple questionnaire, which is self-explanatory and very quick to complete in 1 or 2 minutes. Children are asked to fill it with the help of their parents or guardian. 126 This questionnaire has been a widely used tool and is available in 44 languages. It has been applied in a range of different skin conditions. It is available in two versions: text only and text with cartoons. 127
There are six headings in CDLQI covering 10 questions. 126 These are:
-
symptoms and feelings (Questions 1 and 2)
-
leisure (Questions 4, 5 and 6)
-
school or holidays (Question 7)
-
personal relationships (Questions 3 and 8)
-
sleep (Question 9)
-
treatment (Question 10).
Each question is scored on a scale of 0–3, which ranges from ‘Very much’; ‘Quite a lot’; ‘Only a little’; ‘Not at all’; ‘Question unanswered’; and ‘Prevented school’ for Question 7. A score of 3 is assigned to a question if it is scored ‘very much’ or ‘prevented school’ for Question 7. ‘Quite a lot’ is assigned a score of 2; ‘Only a little’ is assigned a score of 1; and for questions answered as ‘not at all’ or unanswered is given a score of 0. 126
The CDLQI score is calculated when each score is added together, with a range of 0 to 30. Higher scores represent greater impairment with suggested severity bandings as follows:126
-
Score 0–1: No effect on child’s life.
-
Score 2–6: Small effect.
-
Score 7–12: Moderate effect.
-
Score 13–18: Very large effect.
-
Score 19–30: Extremely large effect.
In addition, the overall CDLQI score can be expressed as a percentage of the maximum possible score. 126
Holme and colleagues128 conducted a study to validate the cartoon-based questionnaire and suggested that it was equivalent to the written questionnaire (which was previously validated). The authors point out that the cartoon version was beneficial because it was easier for children to use and both the children and their parents felt that it was the preferred method.
Salek and colleagues127 tested internal consistency, test–retest reliability and responsiveness to change and concluded that the measure met these criteria well. The minimally clinically important difference in those with psoriasis has been suggested to be 2.5; however, this was based on results of one study only and the authors suggest that further research is warranted. The CDLQI is for use in children aged from 4 to 16 years, which may limit the validity of the measure, as it is likely that adolescents’ responses to the disease will differ from those of very young children. 127
Quality of life in Primary Caregivers of children with Atopic Dermatitis
The QPCAD is a self-reported questionnaire developed to evaluate the QoL of primary caregivers of a child with atopic dermatitis. The questionnaire was developed in Japan. 129
The QPCAD includes 19 items within the following four categories: ‘exhaustion’ (seven items), ‘worry about atopic dermatitis’ (six items), ‘family cooperation’ (three items), and ‘achievement’ (three items). Responses to these items are made on a five-point scale. The overall score is calculated by summing all scores, with a higher score indicating a worse health state. The questionnaire only takes into account the QoL of the carer(s) in the past week. 129
The QPCAD is simple and quick to complete and it addresses both positive and negative aspects of life in children with atopic dermatitis (there are six items relating to positive influences out of the total of 19 items). 129 Kondo-Endo and colleagues129 reported that the QPCAD is internally consistent, has good retest reliability and also has reasonable validity. With respect to responsiveness, the study stated that QPCAD was better in detecting severity change compared with the other well-being instruments. However, it was pointed out that there was a need for further study to examine the responsiveness of the subscales to improvements in disease severity over longer periods. 129 Some limitations to this study are that it was based in a Japanese setting, data were based on caregivers of patients with mild to modest disease, and participants were a relatively homogenous group. 129
Skindex
Skindex is a self-report questionnaire with 61 items in eight scales: cognitive effects; social effects; depression; fear; embarrassment; anger; physical discomfort; and physical limitations. The item responses range from 0 to 100, with 0 indicating no effect and 100 indicating maximum effect. 130
Skindex scales have been shown to have good internal reliability, to be reproducible and to be valid. 130 In the analysis by Chren and colleagues,130 the measure also demonstrated responsiveness to clinical change and demonstrated both content validity and construct validity. However, the scores were found to be inconsistent with judgements made by physicians about the severity of the condition.
The earlier version of Skindex was refined into a 29-item Skindex-29, which comprised three domains, namely: symptoms (constituting 7 items), emotions (constituting 10 items) and functioning (constituting 12 items). Like the previous version, all the item responses were converted to a linear scale, ranging from 0 to 100. 131 This version was further refined to develop a shorter version comprising 16 items (Skindex-16) across three domains: symptoms (4 items), emotions (7 items) and functioning (5 items). Like the two parent measures, in this version, responses are summed across the three domains and scores range from 0 (for no effect) to 100 (maximum effect). Based on analyses performed over 500 patients, the authors assessed Skindex-16 to be reliable, valid and responsive to clinical change. 131
Both Skindex-29 and Skindex-16 have advantages and the choice between the two instruments depends on the nature of the research question. 131 For instance, Skindex-26 could be better suited to examine and understand the impacts of a condition on QoL owing to its comprehensive nature, whereas Skindex-16 addresses many other relevant aspects of skin diseases that are not covered by Skindex-29. Despite the advantages, there is room for further exploration and interpretation in future research. 131
Other measures
The studies included in the review of clinical effectiveness also included other HRQoL measures, apart from the ones discussed above. These are:
-
ACS, reported by van Os-Medendorp and colleagues,89 using one sub-scale
-
German questionnaire ‘Quality of life in parents of children with atopic dermatitis’, reported by Staab and colleagues87
-
PDI, reported by Bostoen and colleagues76
-
QoLIAD, reported by Bostoen and colleagues76
-
WHOQOL-26, reported by Matsuoka and colleagues. 88
Disease severity measures
Psoriasis
Psoriasis Area Severity Index
The PASI is the gold-standard measure used by doctors and nurses to assess the severity of psoriasis or the progress of patients receiving treatment for the condition. It is a measure of the average redness, thickness and scaliness of the lesions. Each criterion is graded 0–4, and weighted by the area of involvement. 132
The questionnaire consists of four sections, covering the head, arms, trunk and legs. Within each of these four areas, the percentage of area of skin involved is estimated and then transformed into a grade from 0 to 6:132
-
grade: 0; 0% of involved area
-
grade: 1; < 10% of involved area
-
grade: 2; 10–29% of involved area
-
grade: 3; 30–49% of involved area
-
grade: 4; 50–69% of involved area
-
grade: 5; 70–89% of involved area
-
grade: 6; 90–100% of involved area.
Disease severity is assessed by three clinical signs: erythema (redness), induration (thickness) and desquamation (scaling). Each of these signs is graded on a scale of 0–4, where 0 represents none; 1 represents mild; 2 represents moderate; 3 represents severe and 4 represents maximum severity. 133
Psoriasis Area Severity Index is measured by adding the three severity parameters weighted by the area of involvement. It provides a total score of disease severity, taking into account both coverage and intensity. 134 A clinically meaningful change in clinical trials is a 75% improvement, although there is also evidence of a 50% improvement being clinically meaningful. 132
One of the key advantages of PASI is that it is widely used and has been shown to correlate with QoL. It has been accepted as a valid measure of disease severity by approving agencies. However, it has a few limitations: it has poor sensitivity to change when there is only a small area of involvement, despite being the most widely used instrument for assessing severe psoriasis; there have been a few validation studies; and the construct validity, face validity and sensitivity to change are not well characterised. 132
Self-Administered Psoriasis Area Severity Index
The SAPASI is a structured instrument that allows patients to assess accurately the severity of their psoriasis. As a patient-administered measure, the SAPASI requires the patient to shade on an anatomic sketch, which is then assigned values from an investigator, with a value of 0–6. The scoring is similar to that used in the PASI as described above. When used as a measure of treatment effectiveness, the patient is required to grade a sketch of a typical psoriasis plaque and grade the colour, thickness and scaling. There tool also uses visual analogue scales (VASs) which describe the strength of colour, thickness and scaling. The scoring range of SAPASI is 0–72. 134
Two studies tested the validity of SAPASI. 132,134 In their comparison study, Henseler and colleagues134 found a good correlation between the PASI and the SAPASI. This could be a potential advantage of the instrument in research, as the SAPASI can be used in situations where the investigator is unable to see the patient. The study also noted that there was good regression between the two instruments, which suggests that there is equivalent value between both tools. 134 However, one limitation of SAPASI as outlined by Feldman and Krueger132 was that, despite the high correlation between the PASI and SAPASI across a broad range of severity tested, for any given PASI score there was a wide range of corresponding SAPASI scores. This indicates that the SAPASI score may not accurately determine the severity of psoriasis in some individual patients. 132
SCORing Atopic Dermatitis
The SCORAD can be used to assess the extent and severity of eczema or atopic dermatitis and can be used as a marker of treatment effectiveness. The extent of the disease is estimated as a percentage of the whole body by applying the proportions shown below for different sections of the body affected in an individual and these are then summed up to a maximum of 100 (classed as ‘A’):135
-
head and neck: 9%
-
upper limbs: 9% each
-
lower limbs: 18% each
-
anterior trunk: 18%
-
back: 18%
-
genitals: 1%.
Intensity of the disease is assessed based on redness, swelling, oozing/crusting, scratch marks, skin thickening and dryness. These are rated as: none (0); mild (1); moderate (2); or severe (3) and, when added, give a maximum score of 18 to give ‘B’. 135
Finally, patients or representatives are asked to rate on a VAS their subjective symptoms, such as itch and sleeplessness, using a score of 0 to 10 (where 0 means none and 10 is the worst imaginable symptom). The scores are summed to give ‘C’ (maximum 20). 135
Total SCORAD for that individual is then estimated as: A/5 + 7B/2 + C.
The SCORAD combines objective assessments of the extent and intensity of disease made by investigators with subjective patient or carer ratings of severity (subjective ratings are specifically made for pruritus and sleep loss over the past 3 days). The objective SCORAD is also available, which does not include the subjective ratings. On the SCORAD, a higher score indicates more severe disease. 136 A minimal clinically important difference on the SCORAD is defined as a score change of 8.7 points (8.2 points for the objective SCORAD). 137
Appendix 2 Search strategy
MEDLINE search strategies for clinical effectiveness are shown here. These were adapted for other databases and the cost effectiveness searches (and are available on request).
-
exp acneiform eruptions/ or exp dermatitis/ or exp dermatomyositis/ or exp facial dermatoses/ or hand dermatoses/ or leg dermatoses/ or exp lupus erythematosus, cutaneous/ or nephrogenic fibrosing dermopathy/ or prurigo/ or exp pruritus/ or exp rosacea/ or exp skin diseases, eczematous/ or psoriasis/ or exp skin diseases, bacterial/ (159,684)
-
(eczema* or dermatitis or dermato* or acne or psoriasis or pruritus or prurigo or erythem* or rosacea or rozacea or “cutaneous lupus erythematosus” or “lichen sclerosus” or “lichen planus” or “hidradenitis suppurativa”).tw. (214,088)
-
(chronic* and inflam* and skin).tw. (5447)
-
1 or 2 or 3 (289,612)
-
Patient Education as Topic/ (68,477)
-
exp Health Education/ (132,310)
-
Health Promotion/ (50,756)
-
Health Behavior/ (31,842)
-
Life Style/ (41,162)
-
Health Knowledge, Attitudes, Practice/ (68,962)
-
Self Care/ (22,573)
-
"Continuity of Patient Care"/ (13,997)
-
consumer participation/ or patient participation/ (30,827)
-
Telemedicine/ or Teledermatology/ (10,676)
-
Webcasts/ (291)
-
Internet/ (46,581)
-
Cellular Phone/ or Telephone/ (12,862)
-
Counseling/ (27,044)
-
Behavior Therapy/ (22,902)
-
Physician-Patient Relations/ or Nurse-Patient Relations/ (87,702)
-
exp Mind-Body Therapies/ (40,139)
-
((educat* or train or learn* or teach* or instruct* or knowledge or support*) adj3 (patient* or self* or program* or model* or system*1 or intervention*)).tw. (158,193)
-
(patient* adj3 information*).tw. (23,746)
-
((program* or intervention* or instruction* or teach* or learn* or educat* or plan* or strategy or strategies) adj10 (literature or handout* or leaflet* or inform* or video* or audiovisual or “AV” or internet or web or website* or telecare or telemedicine or teledermatology or telephone or phone or mobile or teleconferenc* or telehealth or transtelephonic* or podcast or broadcast*)).tw. (106,105)
-
(self* adj3 (care or monitor* or help or management)).tw. (25,940)
-
exp Psychotherapy/ (146,141)
-
psychotherap*.tw. (29,205)
-
(psychosomatic and therap*).tw. (1513)
-
Cognitive Therapy/ (14,733)
-
Family Therapy/ (7499)
-
(“health promotion” or “health education” or “patient education” or “patient information”).tw. (49,573)
-
(educat* and intervention*).tw. (41,292)
-
(intervention* adj3 (group* or study or studies or trial*)).tw. (50,074)
-
(“e-consult*” or “e-health” or “e-learn*”).tw. (1790)
-
(“patient teaching” or “patient training” or “patient learning”).tw. (862)
-
or/5-35 (873,959)
-
4 and 36 (6230)
-
(psychosocial* and (intervention* or educat*)).tw. (16,709)
-
(psychological* and (intervention* or educat*)).tw. (23,824)
-
(psychosomatic* and (intervention* or educat*)).tw. (821)
-
Stress, Psychological/ed, pc, th [Education, Prevention & Control, Therapy] (9164)
-
or/38-41 (46,080)
-
4 and 42 (428)
-
37 or 43 (6430)
-
exp Randomized Controlled Trial/ (359,500)
-
Randomized Controlled Trials as Topic/ (88,564)
-
randomized controlled trial.pt. (359,493)
-
controlled clinical trial.pt. (86,909)
-
Controlled Clinical Trial/ (86,909)
-
placebos/ (31,924)
-
random allocation/ (78,664)
-
Double-Blind Method/ (122,243)
-
Single-Blind Method/ (18,296)
-
(random* adj2 allocat*).tw. (19,059)
-
placebo*.tw. (145,886)
-
((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. (119,800)
-
crossover studies/ (32,896)
-
(crossover* or (cross adj over*)).tw. (54,490)
-
Research Design/ (75,676)
-
((random* or control* or compar*) adj5 (trial* or stud*)).tw. (750,980)
-
Clinical Trials as Topic/ (166,646)
-
Comparative Study/ (1,647,117)
-
or/45-62 (2,601,095)
-
44 and 63 (1222)
-
limit 64 to english language (1116)
Appendix 3 Quality assessment criteria
| Criteria | Judgement of risk of biasa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | ||
| Allocation concealment (selection bias) | ||
| Blinding of participants and personnel (performance bias) | ||
| Blinding of outcome assessment (detection bias) | ||
| Incomplete outcome data (attrition bias) | ||
| Selective reporting (reporting bias) | ||
| Other bias |
Appendix 4 Data extraction tables: clinical effectiveness
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author and year: Balato et al., 201385 Study ID: 51 Source: published Country/location: Italy Setting: home Trial design: RCT Includes process evaluation: yes Number of study centres: 1 (assumed) Funding: none Conflicts of interest: none Trial/study number: not reported Study dates: September 2011–not stated Was the educational intervention an adjunct to standard medical care? Yes |
Treatment intervention Overview: text message education Intervention aims: not stated explicitly but implicit from the paper that aim was to use text messaging to improve treatment adherence and patient outcomes including HRQoL Where delivered: via mobile phone Self-help, individual- and/or group-based? (state group size): individual-based intervention Mode: text messaging Materials: text messages Provider: not stated who the ‘investigators’ were but physicians enrolled participants Duration and intensity: 1 text message per day for a period of 12 weeks Scripting (level of detail guiding interaction between interventionist and participants): general educational statements and reminders sent in a random order with four educational and three reminders sent each week. Details of the types of information provided in the paper but no details of the coverage of these (i.e. if all were eventually sent to each participant) Sensitivity to participant characteristics: text messages were created using simple language Interventionist characteristics and training: no details Content and topics: summary – covered frequently asked questions about psoriasis drugs (e.g. administration and adverse effects) and general recommendations to take care of overall health. All text messages between one and three sentences. Educational topics included daily care statements (e.g. use moisturisers, wear light clothes), healthy lifestyle statements (e.g. avoid smoking, pay attention to your diet), prompts about the use of treatments (e.g. do not abuse steroids, common side effects of certain drugs) and one statement about the psychosocial effects of psoriasis (e.g. do not feel ashamed or guilty, psoriasis is not contagious). Reminders reinforced many of the same principles Tailoring: does not appear to be tailored Ongoing support: none reported Theory: none reported Control intervention: Description: no details of the control intervention Duration and intensity: not reported |
Skin condition: plaque psoriasis Diagnostic criteria: not reported Specify if patients, parents and/or carers: patients Patient general age group (specify if children, young adults and/or adults): adults Stated target group: no details How recruited: consecutive patients Eligibility criteria: aged between 18 and 65 years, current systemic and topical treatment, PASI between 5 and 15, owner of a mobile phone capable of receiving text messages and the ability to use it. The presence of comorbidities was not an exclusion criteria Numbers involved (randomised/allocated): total 40; intervention: 20; control: 20 Numbers (%) completing, attrition and reasons: attrition: intervention group = 0; control group = 0 Reasons: not applicable Completing: 100% Sample cross-overs: none Baseline characteristics Comorbidities, n (%) as reported in paper is percentage of those with any comorbidity, reviewer also calculated % of total group [%]: Hypertension Intervention: 3/6 (50) [15]; Control: 4/7 (57) [20] Dysplipidemia Intervention: 2/6 (33) [10]; Control: 2/7 (28.5) [10] Type 2 diabetes Intervention: 1/6 (17) [5]; Control: 1/7 (14.5) [5] Co-medications/interventions: Intervention group: acitretin 2 (10%); biologics 10 (50%); ciclosporin 3 (15%); methotrexate 5 (25%) Control group: acitretin 3 (15%); biologics 11 (55%); ciclosporin 2 (10%); methotrexate 4 (20%) Duration of disease, mean (SD): Intervention 10.7 (5.3) years Control 12.1 (5.8) years Sex (M/F) n/N: Intervention 10/10 Control 12/8 Average age: mean (SD) age, years Intervention 38.4 (9.5); Control 39.3 (10.2) Ethnic groups: not reported Socioeconomic characteristics: Education, n (%) Intervention: middle school 5 (25); high school 12 (60); college graduate 3 (15) Control: middle school 6 (30); high school 10 (50); some college 1 (5); college graduate 3 (15) Currently employed, n (%) Intervention: 16 (80) Control 15 (75) |
Primary outcomes: not reported as primary or secondary outcomes. PASI, PGA, BSA, SAPASI; HRQoL (DLQI) Other outcomes: evaluation of patient–physician relationship; treatment adherence Secondary outcomes: adverse events: not reported Process evaluation measures: usability and satisfaction with the text messaging education Individual preferred learning style addressed? No Any sub groups: none reported How outcomes assessed? DLQI, SAPASI and adherence are self-report; PASI, BSA and PGA are clinician reported but not clear who assessed. Unclear for the patient–physician relationship. DLQI, PASI and SAPASI details of measure not stated; patient–physician relationship was a scale of 0–10; treatment adherence through a multiple-choice question about how often they forgot to use products/medications in days per week in the past week. Also a 7-day calendar marking days when they were adherent as outlined in a cited reference. States the PASI, BSA and PGA were used to support the results of the self-reported adherence Normal range(s) for outcomes/clinically meaningful improvement defined: not reported Validated? Yes for all measures except patient–physician relationship and adherence (therefore not data extracted) Timing of outcomes same for both groups: yes Length of follow-up: 12 weeks |
| Methods | |||
|---|---|---|---|
| Statistical analysis, including how missing data dealt with: minimal details about statistical approaches used, data presented as means (SDs). No missing data Power calculation: not reported. Described as a pilot study Study adequately powered? Unclear ITT used? No missing data or drop outs so ITT (although not described as such by authors) Groups comparable at baseline? Yes Subgroup analyses: none reported Process evaluation methods (if relevant): in the treatment group only, usability and satisfaction with the text messaging intervention were assessed using a series of questions |
|||
| Outcome evaluation results | |||
| HRQoL Outcomes | Intervention (n = 20) | Control (n = 20) | p-value/CIs |
| DLQI, mean (SD) baseline | 7.9 (3.2) | 7 (3) | |
| DLQI, mean 12 weeks | 4.2 | 5.8 | p < 0.05 |
| Comments: 12 weeks scores estimated from figure by reviewer | |||
| Other relevant outcomes | Intervention (n = 20) | Control (n = 20) | p-value/CIs |
| PASI, mean (SD) baseline | 10.64 (4.2) | 10.13 (4.7) | |
| PASI, mean 12 weeks | 5.8 | 6.8 | p < 0.05 |
| SAPASI, mean (SD) baseline | 11 (6.6) | 10.90 (5.9) | |
| SAPASI, mean, 12 weeks | 5.9 | 8 | p < 0.05 |
| BSA, mean (SD) baseline | 16 (7.5) | 14.2 (8) | |
| BSA, mean 12 weeks | 5.8 | 8 | p < 0.05 |
| PGA, mean (SD) baseline | 2.6 (1.04) | 2.3 (1.3) | |
| PGA score | 0.7 | 1.5 | p < 0.05 |
| Comments: all 12 weeks’ data estimated from figure by reviewer Treatment adherence scores and patient–physician relationship scores not data extracted as not validated measures |
|||
| Adverse events | Intervention | Control | p-value/CIs |
| Comments: | |||
| Subgroup analysis results | |||
| Comments: no subgroups reported | |||
| Outcome | Intervention | Control | p-value/CI |
| Comments: | |||
| Process evaluation results | |||
| Usability and satisfaction with the text messaging education: 85% found text messaging useful 75% would recommend to a friend 75% would like to continue using the text messaging 15% would be willing to pay a small fee for the service |
|||
| Comments: | |||
| Generalisability: Italian population all with plaque psoriasis of moderate-to-large effect based on baseline PASI scores (reviewer observation). Participants on a range of different treatments, unclear how generalisable these are to UK standards of care Other: no details of the control group so unclear whether they were seen as usual care or not seen at all |
|||
Quality criteria (Cochrane ‘risk of bias’ tool) randomised controlled trials
| Criteria | Judgement of risk of biasa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Low risk | Allocation by a computer-generated random number list |
| Allocation concealment (selection bias) | Unclear risk | States group assignment was stored electronically and that investigators performing randomisation had no contact with participants, but no other details |
| Blinding of participants and personnel (performance bias) | High risk | Physicians were blinded to group assignment until the end of the study. No details of blinding of participants, which would be difficult to do |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | All outcomes stated were reported |
| Other bias | Low risk |
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author and year: Bostoen et al., 201276 Study ID: 84 Country/location: Belgium Setting: unclear, assume outpatient Trial design: RCT Includes process evaluation: no Number of study centres: unclear, assume one Funding: grants from various pharmaceutical companies Conflicts of interest: states none Trial/study number: NCT01077882 Study dates: February 2010–11 Was the educational intervention an adjunct to standard medical care? Yes |
Treatment intervention Overview: educational programme for patients with psoriasis and atopic dermatitis Intervention aim(s): not explicitly stated. Study aimed to examine if the educational intervention ‘added value to medical therapy’ (p. 1025) and examine the effects on disease severity and QoL Where delivered: not reported Self-help, individual- and/or group-based? (State group size): group-based intervention. Group sizes differed and were 14, 23 and 13 Mode: face-to-face workshop Materials: secondary publication99 of a before-and-after study. Used the same intervention notes as the syllabus that was offered to participants Provider: dermatologist, dermatology nurse, pharmacists, dietician, training expert, psychiatrist, psychologist, philosopher, and a sports, yoga and mindfulness teacher Duration and intensity: 3-month programme, with two, 2-hour sessions a week Scripting (level of detail guiding interaction between interventionist and participants): secondary publication99 refers to a syllabus Sensitivity to participant characteristics: not reported Interventionist characteristics and training: none reported apart from job title noted above Content and topics: information on specific skin disease, and skin care sessions, healthy lifestyle (diet, sleep hygiene, smoking, substance abuse) and stress-reducing techniques (physical training, psychodermatology, practical philosophy and mindfulness), feedback sessions. Further details of the intervention provided in a separate publication99 Tailoring: not reported Ongoing support: not reported Theory: not reported All patients continued with medical therapy Control intervention Description: received medical therapy alone Duration and intensity: no details |
Skin condition: psoriasis or atopic dermatitis Diagnostic criteria: not reported except that diagnosis was checked by a dermatologist at study entry Specify if patients, parents and/or carers: patients Patient general age group (specify if children, young adults and/or adults): adults Stated target group: adults with psoriasis or atopic dermatitis How recruited: recruited from Ghent University Hospital, patient advocacy groups and dermatologists Eligibility criteria: aged 18 years or older, psoriasis or atopic dermatitis Excluded if other severe illnesses, psychiatric disorders or cognitive disorders Numbers involved (randomised/allocated): Total: 50 (29 psoriasis; 21 AD) Intervention (intervention): 25 (15 (60%) psoriasis, 10 (40%) AD) Control (control): 25 (14 (56%) psoriasis, 11 (44%) AD) Numbers (%) completing, attrition and reasons (by study end, reports rates for each follow-up period but not data extracted): Attrition: N (%) 13 (26%) [intervention 8 (32%); control 4 (16%)] In addition, one participant in the intervention group was excluded from the analysis Reasons: range of reasons provided but not linked to number of individuals dropping out for each reason. These included lack of time, too intensive programme and moving house in the intervention group and worsening of disease and loss of motivation in the control group Completers: N (%) [intervention: 16 (64%); control 21 (84%)] Sample cross-overs: none reported Baseline characteristics Co-morbidities: not reported Co-medications/interventions, n: Topical therapies Intervention: 17 (psoriasis 9, AD 8); control: 21 (psoriasis 12, AD 9) Systemic therapies Intervention: 0; control: 1 (psoriasis 1) Combination Intervention: 4 (psoriasis 2, AD 2); control: 3 (psoriasis 1, AD 2) None Intervention: 4 (psoriasis 4); control: 0 Total Intervention: 25 (psoriasis 15, AD 10); control: 25 (psoriasis 14, AD 11) Duration of disease, mean (SD) years: Intervention: 18.9 (11.0); control: 20.1 (11.4) Disease severity: see below Sex (M/F),%: Intervention: 48/52; control: 48/52 Average age, mean (SD) years: Intervention: 38.5 (12.3); control: 40.6 (12.2) Ethnic groups: not reported Socioeconomic characteristics: Education: low/medium/high (%) Intervention: 4/22/74; control: 4/52/44 |
Primary outcomes: disease severity (PASI; SCORAD, EASI). HRQoL (DLQI; Skindex-29; PDI; QoLIAD) Secondary outcomes: depression (BDI); lifestyle (set of questions un-validated) Stress (EPC) Medical consumption Cost-effectiveness (states used costs of medical consumption and EQ-5D for health outcomes to assess cost per EQ-5D gain in Euros) Adverse events: not reported Process evaluation measures: none reported Individual preferred learning style addressed? No How outcomes assessed?: Severity measures assessed by two clinicians, having been trained at the start of the trial, level of agreement was good (intraclass correlation coefficient 0.86 (95% CI 0.72 to 0.94) for PASI, 0.89 (95% CI 0.74 to 0.96) for SCORAD, 0.92 (95% CI 0.82 to 0.97) for EASI) Normal range(s) for outcomes/clinically meaningful improvement defined: yes for BDI and EPC and DLQI Validated? Severity, HRQoL measures, BDI and EPC validated Lifestyle questionnaire not validated and not data extracted Timing of outcomes same for both groups: yes Length of follow-up: 9 months |
| Methods | |||
|---|---|---|---|
| Statistical analysis, including how missing data dealt with: reports how data were analysed including mixed modelling to identify differences in time between the intervention and control groups for each outcome variable Power calculation: power calculation showed that 34 patients were required. There was an 80% probability that the study would detect a treatment difference at a two-sided 0.05 significance level if the mean difference between treatments is 2 with a SD of 2 Study adequately powered? Yes for total sample; however, analyses presented are essentially subgroup analyses and unclear if the sample is adequately powered ITT used? No details reported; paper does suggest that those dropping out were included in the analysis with the exception of one participant in the intervention group who was excluded from the analysis (p. 1027). However, the reporting of the results suggests that different numbers may have been analysed at each follow-up point because the baseline scores are different in each case Groups comparable at baseline? Not reported but appear to be well matched Subgroups analyses: all analyses were subgroup analyses of psoriasis and atopic dermatitis patients Process evaluation methods (if relevant): none reported |
|||
| Outcome evaluation results | |||
| Total group, psoriasis and AD combined | |||
| HRQoL outcomes | Intervention (n = 25) | Control (n = 25) | p-value/CI |
| DLQI, mean (SD) baseline | 9.7 (6.0) | 7.5 (5.0) | |
| Skindex-29 total, mean (SD) baseline | 45.5 (16.1) | 43.3 (17.7) | |
| Skindex-29 symptoms, mean (SD) baseline | 58.1 (15.4) | 55.8 (18.4) | |
| Skindex-29 emotions, mean (SD) baseline | 48.9 (19.6) | 49.0 (22.7) | |
| Skindex-29 functioning, mean (SD) baseline | 35.2 (20.4) | 30.8 (21.4) | |
| BDI, mean (SD) baseline | 11.3 (8.2) | 8.4 (6.5) | |
| PDI, mean (SD) baseline | 9.0 (6.8) | 7.6 (7.8) | |
| Comments: paper does not specify which outcomes were reported for the total group and which for the two subgroups, so reviewer has assumed generic instruments at baseline are reporting the total group. However, these measures are also presented for baselines within the two disease subgroups (see below) and there are no end-point measurements for these in the total group DLQI, Skindex-29, PDI, QoLIAD: higher scores indicate a greater negative impact of the skin disease on QoL. In another publication, the same authors state that the minimal clinical important difference of the DLQI is reported to be between 2.2 and 6.9 depending on the skin disease BDI: self-administered questionnaire with 21 questions. Patients categorised as minimal (score 0–9), mild (score 10–18), moderate (score 19–29) or severe (score 30–36) depression Paper states the EPC was applied to 27 participants but it is not clear which group these participants were allocated to. Reports that there were no significant differences between groups during the study, no further details Paper states EQ-5D values were not significantly better than in the control group at 6 months. Cost–utility analysis taking into account programme cost per patient and medical resource use per individual patient did not show cost-effectiveness at 6 months |
|||
| Psoriasis subgroup | |||
| Other relevant outcomes | Intervention (n = unclear) | Control (n = unclear) | p-value/CI |
| DLQI, mean (95% CI) baseline | 8.4 (5.6 to 11.2)a | 6.6 (3.9 to 9.3)b | |
| DLQI, mean (95% CI) 3 months | 4.4 (1.3 to 7.4) | 6.4 (3.6 to 9.2) | p-value = 0.019 |
| DLQI, mean (95% CI) 9 months | 4.0 (0.6 to 7.4) | 5.8 (2.9 to 8.8) | p-value = 1.00 |
| PASI, mean (SD) baseline | 8.4 (CI 6.0 to 10.8)c | 7.1 (CI 4.8 to 9.4)d | |
| PASI, mean (95% CI) 3 months | 6.8 (4.3 to 9.3) or 6.5 (3.6 to 9.4) or 6.5 (3.3 to 9.8) |
8.1 (5.8 to 10.4) or 8.1 (5.6 to 10.7) or 8.1 (5.3 to 10.9) |
p-value = 0.036 |
| PASI, mean (95% CI) 9 months | 7.0 (3.8 to 10.3) | 7.0 (3.8 to 10.3) | p-value = 0.116 |
| PDI, mean (SD) [95% CI] baseline | 9.0 (6.8) [5.0 to 13.0]e | 7.6 (7.8) [3.8 to 11.5]f | |
| PDI, mean (95% CI) 3 months | 4.3 (0.1 to 8.4) or 4.5 (0.1 to 9.0) or 4.5 (–0.1 to 9.1) |
6.7 (2.9 to 10.6) or 6.7 (2.7 to 10.7) or 6.7 (2.6 to 10.8) |
p-value = 0.015 |
| PDI, mean (95% CI) 9 months | 4.9 (0.3 to 9.5) | 7.4 (3.3 to 11.6) | p-value = 0.021 |
| Skindex-29g | No data | No data | Not significant |
| BDI, mean (95% CI) baseline | 12.3 (8.3 to 16.4) | 7.4 (3.5 to 11.3) | |
| BDI, mean (95% CI) 3 months | 10.5 (6.1 to 14.9) | 6.3 (2.3 to 10.3) | p-value < 0.05 |
| BDI, mean (95% CI) 9 months | 6.1 (1.7 to 10.5) | 7.3 (3.2 to 11.3) | p-value = 0.029 |
| Comments: 3-month and 9-month outcomes extracted – 3 month relates to the end of the intervention, 9 months was the longest period of follow-up. Also reports 6-month data, not extracted | |||
| Medical therapy use | Intervention (n = 14) | Control (n = 14) | |
|---|---|---|---|
| At 3 months Topical Systemic Combination None Total |
5 – 2 2 9 |
12 – – 1 13 |
|
| At 9 months Topical Systemic Combination None Total |
3 – 2 3 8 |
10 1 1 1 13 |
|
| Comments: paper states no major differences between groups (could not test for significant differences) | |||
| AD subgroup | |||
| HRQoL | Intervention (n = 10) | Control (n = 11) | |
| QoLIAD, mean (SD) baseline | 9.1 (5.6) | 9.6 (6.1) | |
| Disease severity | |||
| SCORAD, mean (SD) baseline | 38.9 (18.0) | 38.8 (15.5) | |
| EASI, mean (SD) baseline | 11.9 (10.9) | 10.4 (8.1) | |
| Comments: paper states no significant differences between intervention and control groups for EASI, SCORAD, DLQI, Skindex-29 or QoLIAD. No data presented Paper also reports no statistically significant differences on the BDI but no data presented |
|||
| Medical therapy use | Intervention (n = 10) | Control (n = 11) | |
| For baseline see above At 3 months Topical Systemic Combination None Total |
7 – 1 1 9 |
8 – 2 – 10 |
|
| At 9 months Topical Systemic Combination None Total |
7 – 1 – 8 |
7 – 1 – 8 |
|
| Comments: Paper states no significant differences between groups |
|||
| Adverse events | Intervention | Control | p-value/CI |
| Comments: | |||
| Process evaluation results | |||
| Not reported | |||
| Comments: | |||
| General comments | |||
| Generalisability: psoriasis patients classified on average as mild at baseline, atopic dermatitis moderate Other: patients recruited from three different sources, a university hospital, patient advocacy groups and peripheral dermatologists. There were three runs of the educational programme, one in spring 2010, one in autumn 2010 and one in spring 2011 – unclear how uniform the delivery of these was between the three groups |
|||
Quality criteria (Cochrane ‘risk of bias’ tool) randomised controlled trials
| Criteria | Judgement of risk of biasa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Low | Randomised on 1 : 1 basis using computer-generated randomisation list, allocation was indicated and stratified by diagnosis using a block size of two |
| Allocation concealment (selection bias) | Unclear | Sequentially numbered envelopes were used to assign patients to the two study groups, does not state is opaque envelopes |
| Blinding of participants and personnel (performance bias) | Unclear | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear | Clinicians performing the assessments of disease severity were blinded to randomisation, unclear who assessed other outcomes |
| Incomplete outcome data (attrition bias) | High risk | Numbers and general reasons provided but high drop-out in intervention group, no ITT analysis and there are different data reported for many outcomes for the same time points. Also one in intervention group excluded from analysis owing to extreme stress at work |
| Selective reporting (reporting bias) | Unclear risk | Paper appears to report all outcomes but in many cases no data were presented and the reviewer has to rely on statements which state there were no significant differences |
| Other bias | Unclear | All results are essentially subgroup analyses and unclear if powered adequately |
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author and year: Ersser et al., 201186 Study ID: 135 Country/location: UK Setting: primary care Trial design: cluster RCT (pilot) Includes process evaluation: yes Number of study centres: 8 (health centres) Funding: Psoriasis Association, UK Conflicts of interest: none Trial/study number: not reported Study dates: June–September 2009 Was the educational intervention an adjunct to standard medical care? Yes |
Treatment intervention Overview: a theory-based self-management educational intervention for individuals with psoriasis. Patients also received usual care (only mentioned in abstract) Intervention aim(s): to support self-management in psoriasis Where delivered: not reported Self-help, individual- and/or group-based? (State group size): group-based face-to-face session (maximum of 9 participants), with supporting information and follow-up telephone consultation Mode: face to face, written and audio-visual materials, individual telephone consultation Materials: supporting written and audio-visual materials provided; DVD and workbook, including relaxation material Provider: nurse-led group sessions, with supporting materials provided (see Materials) Duration and intensity: group session: one-off 2-hour session Telephone consultation: one-off 20-minute session Scripting (level of detail guiding interaction between interventionist and participants): in delivering the intervention, the nurse was expected to follow the intervention protocol, but with flexibility for individualisation. Outline script was used for follow-up telephone consultation Sensitivity to participant characteristics: not reported, but intervention allowed flexibility for individualisation Interventionist characteristics and training: nurse attended training on self-efficacy-based education Content and topics: practical element (no details provided), individual action planning, stress reduction (through provision of relaxation materials), feedback on action plans (through telephone consultation) Tailoring: intervention included individual action planning Ongoing support: nurse provided one 20 minute follow-up telephone consultation one month after the group session (based on outline script) Theory: based on social learning theory – specifically self-efficacy theory. Intervention incorporated elements designed to address the four sources of self-efficacy (mastery, verbal persuasion, vicarious experience, emotional regulation). Intervention was also informed by the findings of previous qualitative research into the self-management needs of individuals with psoriasis Control intervention Description: continued with their usual treatment (topical therapies only), with access to primary care as needed Duration and intensity: not reported |
Skin condition: psoriasis Diagnostic criteria: not reported Specify if patients, parents and/or carers: patients Patient general age group (specify if children, young adults and/or adults): adults Stated target group: individuals with mild-to-moderate plaque psoriasis How recruited: from primary care, with CLNR support Eligibility criteria: aged ≥ 18 years; mild to moderate plaque psoriasis (defined as patients using only topical therapies and having had no secondary care contact in preceding 3 months) Numbers involved (randomised/allocated): total: 64 Intervention: 28; control (control): 36 Numbers (%) completing, attrition and reasons: Attrition: 5 (8%) [intervention 2 (7%); control (8%)] Reasons: all lost to follow-up Completers: 59 (92%) [intervention 26 (93%); control 33 (92%)] (All percentages calculated by reviewer.) Sample cross-overs: none Baseline characteristics Comorbidities: not reported Comedications/interventions (n): Current topical therapies: None: intervention 2; control 2 Emollients only: intervention 6; control 2 GP prescribed active therapies: intervention 20; control 32 Mean duration of disease: Intervention 22.68 (SD 17.99) years; control 24.17 (SD 18.63) years Disease severity: Described as mild-moderate Sex (M/F): Intervention 8 (29%)/20 (71%); control 20 (55%)/16 (45%) Mean age: Intervention 56.86 (SD 12.67) years; control 59.03 (SD 13.53) years Ethnic groups: not reported Socioeconomic characteristics: not reported |
Primary outcomes: HRQoL (DLQI) Secondary outcomes: disease severity (PASI) Adverse events: not reported Process evaluation measures: participant questionnaire assessing perceived value, accessibility and extent to which intervention met individual needs (operationalised as a single rating of usefulness for each intervention delivery mode) (see Methods and Results below) Individual preferred learning style addressed? No How outcomes assessed? DLQI (designed as self-report; not reported if used as a self-report measure in this study). PASI (unclear if self-report or scored by assessors) Normal range(s) for outcomes/clinically meaningful improvement defined: yes (in discussion) PASI score ranges: < 7 mild 7–12 moderate > 12 severe Clinically meaningful improvement: Typically defined as PASI 75 if used as a primary outcome in a trial (defined as a 75% reduction in PASI at the end of the intervention), but some also regard PASI 50 (i.e. 50% reduction) to be a clinically meaningful improvement (though this is opinion and has not been formally defined) Validated? Yes (for both DLQI and PASI) Timing of outcomes same for both groups: yes Length of follow-up: 6 weeks |
| Methods | |||
|---|---|---|---|
| Statistical analysis, including how missing data dealt with: unpaired t-tests used to assess differences in changes from pre–post intervention scores between the intervention and control groups. Multilevel modelling also used to take into account the cluster RCT design, but not reported as results were similar to those of the t-test. No imputation of missing data for those lost to follow-up – those lost to follow-up were excluded from the analysis as they had incomplete information Power calculation: not reported Study adequately powered? Pilot study to test feasibility of intervention, not expected to have enough power to detect statistically significant differences ITT used? No, completers only Groups comparable at baseline? Yes, on all baseline characteristics reported (only sex, age, duration and current topical therapies reported), except sex (proportionally more women in the intervention than the control group; p-value = 0.031) Subgroups analyses: post hoc subgroup analyses conducted for both outcomes for patients with more severe disease and worse HRQoL at baseline (participants with DLQI or PASI scores of > 6) – not data extracted Process evaluation methods (if relevant): assessment of the feasibility of the intervention (participant feedback); participant completion of a questionnaire post intervention assessing perceived value, accessibility and extent to which intervention met individual needs (rated on a scale of 1 (not useful at all) to 10 (very useful); score interpretation: 1–3 = not useful, 4–7 = moderately useful, 8–10 = very useful); participants’ views of the intervention obtained through written feedback at their 6-week follow-up visit; research team also assessed intervention practicality and value. Feedback analysed through qualitative data analysis and descriptive statistics |
|||
| Outcome evaluation results | |||
| HRQoL Outcomes | Intervention (n = 26) | Control (n = 33) | p-value/CI |
| DLQI, mean (SD) | |||
| baseline | 4.86 (5.14) | 4.18 (3.19) | |
| at 6 weeks | 4.58 (5.05) | 3.70 (3.71) | |
| change from baseline | 0.28 (2.16)a | 0.48 (3.02)a | p = 0.772 (95% CI: –1.20 to 1.61) |
| Comments: DLQI scores can range from 0–30, a higher score indicates worse HRQoL | |||
| Other relevant outcomes | Intervention (n = 26) | Control (n = 33) | p-value (95% CI) |
| PASI, mean (SD) | |||
| baseline | 2.34 (2.66) | 3.22 (2.26) | |
| at 6 weeks | 1.78 (1.62) | 2.82 (2.20) | |
| change from baseline | 0.56 (1.42)b | 0.40 (1.06)b | 0.619 (–0.81 to 0.49) |
| Comments: PASI scores can range from 0–72, a higher score indicates more severe disease | |||
| Adverse events | Intervention | Control | p-value/CI |
| Not reported | |||
| Comments: | |||
| Subgroup analysis results: | |||
| Subgroup: no pre-specified subgroup analyses reported | |||
| Outcome | Intervention | Control | p-value/CI |
| Comments: | |||
| Process evaluation results | |||
| Patient perspective: Qualitative data – participant feedback: Participants found the intervention practical (not too time-consuming), convenient (because it was partly home-based), and felt it provided follow-up care Qualitative data on how useful participants found each intervention component and their perceptions on the difference it has made to how they manage their psoriasis are presented (not data extracted; see Table 4) Quantitative data: Rated usefulness of intervention components, % (n = 26) (Data were derived from the questionnaire where participants rated the usefulness of the intervention) Group learning: 3.8 not useful; 30.8 moderately useful; 65.4 very useful; 0 no response DVD: 3.8 not useful; 26.9 moderately useful; 26.9 very useful; 42.3 no response Workbook: 3.8 not useful; 38.5 moderately useful; 57.7 very useful; 0 no response Telephone consultation: 7.7 not useful; 30.8 moderately useful; 53.8 very useful; 7.7 no response Educational intervention attendance (n/N): Group-learning session: 26/28 Watched DVD: 15/28 (others had technical difficulties, did not like DVD format and/or found workbook more convenient to intermittently consult) Research team perspective: noted that smaller group learning sessions resulted in more interaction between participants |
|||
| Comments: | |||
| General comments | |||
| Generalisability: patients’ baseline HRQoL and disease severity scores were very low, suggesting many participants had only mild disease and that their HRQoL had not been extensively affected by their disease. This may have impacted outcomes, e.g. less room for improvement, participants’ need for support and education may have been less than for people more severely affected by their disease | |||
Quality criteria (Cochrane ‘risk of bias’ tool) randomised controlled trials
| Criteria | Judgement of risk of biasa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Low risk | ‘Randomisation was performed by the flip of a coin by a colleague independent of the study’ (p. 740) |
| Allocation concealment (selection bias) | Unclear risk | Randomisation of clusters performed independently of the study team, but unclear how allocation of clusters to intervention or control group was concealed from participants |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information about blinding provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information about blinding or who conducted the PASI assessment provided |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention and control groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section of the paper were reported. Paper does not report if a protocol is available |
| Other bias | Unclear risk | A statistically significant higher proportion of participants in the intervention than control group were women; unclear whether this might bias outcomes |
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author and year: Matsuoka et al., 200688 Study ID: 543 Country/location: Kagawa, Japan Setting: Outpatient clinic Trial design: RCT Includes process evaluation: no Number of study centres: 1 Funding: not reported, but sample cosmetics prescriptions provided by a pharmaceutical company Conflicts of interest: stated no relevant financial interests Trial/study number: not reported Study dates: April 2004–November 2005 Was the educational intervention an adjunct to standard medical care? Yes |
Treatment intervention Overview: women with acne vulgaris were instructed on skin care and how to use make-up by a dermatologist. The women also received acne treatment (topical therapy and/or oral medication) Intervention aim(s): not explicitly stated, but part of the aim of the study was to examine if instructions in make-up use from a dermatologist could affect female acne patients’ QoL Where delivered: outpatient clinic based in a university dermatology department Self-help, individual- and/or group-based? (State group size): not reported Mode: face to face, with supporting videotape instructions and detailed leaflets/prescriptions Materials: videotaped instructions; leaflets and make-up prescriptions with more detailed instructions; sample cosmetics provided Provider: dermatologist Duration and intensity: not reported Scripting (level of detail guiding interaction between interventionist and participants): not reported Sensitivity to participant characteristics: not reported Interventionist characteristics and training: not reported Content and topics: summary – patients received instructions on use of skin care and make-up products. Instructions included general skin care and how to use ‘point make-up’ (e.g. eyeliner and lipstick) Tailoring: not reported Ongoing support: not reported Theory: not reported Control intervention Description: acne treatment (topical and/or oral medication), with no specific instructions from a dermatologist. Patients were told to use cosmetics in the same way they usually do Duration and intensity: not reported |
Skin condition: acne vulgaris Diagnostic criteria: not reported Specify if patients, parents and/or carers: patients Patient general age group (specify if children, young adults and/or adults): adults (age > 16 years) Stated target group: women How recruited: not reported Eligibility criteria: pregnant or lactating women were excluded Numbers involved (randomised/allocated): Total: 50 Intervention (intervention): 25 Control (control): 25 Numbers (%) completing, attrition and reasons: Attrition: none Reasons: not applicable Completers: 50 (100%) [intervention 25 (100%); control 25 (100%)] Sample cross-overs: not reported Baseline characteristics Comorbidities: Complications (%): Intervention: yes/no: 28/72 Control: yes/no: 24/76 Comedications/interventions: not reported. Study is Japanese; assumed predominantly Japanese patients Duration of disease: intervention 7 (SD 4) years; control 4 (SD 4) years Disease severity: data provided on number and proportion of patients in each of eight different severity categories for both right and left side of face; not data extracted Sex (M/F) (n): 0/50 Average age: intervention 24 years (SD 3 years) years; control 25 years (SD 5 years) Ethnic groups: not reported Socioeconomic characteristics: not reported |
Primary outcomes: outcomes not specified as either primary or secondary Outcomes measured: acne severity (Plewig and Kligman’s grade method) HRQoL (WHOQOL-26 and DLQI) Secondary outcomes: see above Adverse events: dermatologists evaluated safety in both groups Process evaluation measures: not applicable Individual preferred learning style addressed? No How outcomes assessed? WHOQOL-26 and DLQI both self-report Dermatologist rated disease severity using the Plewig and Kligman’s grade method although reference provided Normal range(s) for outcomes/clinically meaningful improvement defined: WHOQOL-26: Authors cite a normative total mean score of 3.33 (SD 0.49) in healthy Japanese women aged 20–29 (based on Nakane et al., 1999) Validated? Yes (for WHOQOL-26 and DLQI) Unclear for Plewig and Kligman’s grade method Timing of outcomes same for both groups: yes Length of follow-up: 4 weeks |
| Methods | |||
|---|---|---|---|
| Statistical analysis, including how missing data dealt with: authors detail statistical tests used to analyse HRQoL data. Alpha level was not adjusted for multiple comparisons. A p-value of < 0.05 was considered to be statistically significant Power calculation: not reported Study adequately powered? Unclear ITT used? Yes – not explicitly stated, but all randomised patients were included in the analyses Groups comparable at baseline? Yes – no statistically significant differences between groups on age, duration of disease, complications, severity of disease, HRQoL or medication use (although data not reported) Subgroups analyses: none reported Process evaluation methods (if relevant): no process evaluation |
|||
| Outcome evaluation results | |||
| HRQoL outcomes | Cosmetic instructions (n = 25) | No instructions control (n = 25) | p-value/CI |
| WHOQOL-26, mean (SD) | |||
| Physical domain | |||
| Baseline | 3.23 (0.62) | 3.45 (0.55) | Not reported |
| 4 weeks after | 3.35 (0.46) | 3.54 (0.45) | Not reported |
| Psychological domain | |||
| Baseline | 3.09 (0.69) | 3.21 (0.62) | Not reported |
| 4 weeks after | 3.26 (0.59)* | 3.29 (0.60) | Not reported |
| Social relationships | |||
| Baseline | 3.65 (0.55) | 3.68 (0.50) | Not reported |
| 4 weeks after | 3.65 (0.49) | 3.64 (0.61) | Not reported |
| Environment domain | |||
| Baseline | 3.35 (0.53) | 3.42 (0.49) | Not reported |
| 4 weeks after | 3.42 (0.51) | 3.45 (0.49) | Not reported |
| Overall QoL | |||
| Baseline | 3.04 (0.88) | 2.78 (0.76) | NS |
| 4 weeks after | 3.38 (0.77)** | 3.18 (0.72)** | NS |
| Total mean score | |||
| Baseline | 3.27 (0.54) | 3.36 (0.44) | Not reported |
| 4 weeks after | 3.39 (0.45) | 3.44 (0.46) | Not reported |
| DLQI, mean (SD) | |||
| Symptoms, feelings | |||
| Baseline | 3.16 (1.49) | 2.60 (1.58) | NS |
| 4 weeks after | 1.52 (1.08)*** | 1.52 (1.16)*** | NS |
| Daily activities | |||
| Baseline | 1.76 (1.81) | 1.20 (1.73) | NS |
| 4 weeks after | 0.56 (0.77)** | 0.64 (1.25)** | NS |
| Leisure | |||
| Baseline | 1.20 (0.96) | 1.12 (1.48) | NS |
| 4 weeks after | 0.72 (0.84)* | 0.52 (1.08)** | NS |
| Work/school | |||
| Baseline | 0.68 (0.69) | 0.48 (0.67) | NS |
| 4 weeks after | 0.48 (0.51) | 0.20 (0.65)** | Statistically significant difference |
| Personal relationships | |||
| Baseline | 0.68 (0.90) | 0.48 (1.19) | NS |
| 4 weeks after | 0.24 (0.44)** | 0.16 (0.55)* | NS |
| Discomfort of treatment | |||
| Baseline | 0.76 (0.83) | 0.40 (0.65) | NS |
| 4 weeks after | 0.36 (0.49)** | 0.20 (0.50) | NS |
| Total mean score | |||
| Baseline | 8.24 (5.06) | 6.24 (6.06) | NS |
| 4 weeks after | 3.88 (2.79)*** | 3.24 (4.36)*** | NS |
| Comments: p-values only reported for within-group changes from baseline. WHOQOL-26 analyses: no between-group p-values reported, except that it is stated that the QOL score was not statistically significant between groups either before or after the intervention. DLQI analyses: statistically significant differences between-groups reported narratively only; p-values not provided Statistically significant within-group changes: *p < 0.05, **p < 0.01, ***p < 0.001 Interpretation of WHOQOL-26 score: a lower score indicates more disability. Interpretation of DLQI: a higher score indicates more disability |
|||
| Other relevant outcomes | Cosmetic instructions (n = 25) | No instructions control (n = 25) | p-value (CI) |
| Degree of improvement in acne severity on right side of face at 4 weeks, n (%) | |||
| Markedly improved | 5 (20) | 8 (32) | 0.62 |
| Improved | 10 (40) | 8 (32) | |
| Unchanged | 10 (40) | 9 (36) | |
| Exacerbated | 0 (0) | 0 (0) | |
| Degree of improvement in acne severity on left side of face at 4 weeks, n (%) | |||
| Markedly improved | 9 (36) | 7 (28) | 0.83 |
| Improved | 8 (32) | 9 (36) | |
| Unchanged | 8 (32) | 9 (36) | |
| Exacerbated | 0 (0) | 0 (0) | |
| Comments: | |||
| Adverse events | Cosmetic instructions (n = 25) | No instructions control (n = 25) | p-value/CI |
| Reported side effects of cosmetic and conventional medicine use | None | None | |
| Comments: | |||
| Subgroup analysis results: | |||
| Comments: no subgroups reported | |||
| Outcome | Cosmetic instructions | No instructions control | p-value/CI |
| None | |||
| Comments: | |||
| Process evaluation results | |||
| No process evaluation | |||
| Comments: | |||
| General comments | |||
| Generalisability: small-scale study conducted in one study centre in Japan examining the impact of instructions in make-up and cosmetic use in women, so results are likely to be of limited generalisability Other: none |
|||
Quality criteria (Cochrane ‘risk of bias’ tool) randomised controlled trials
| Criteria | Judgement of risk of biasa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Low risk | All randomised patients were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | All outcomes specified in the methods section of the paper are reported in the results, but p-values for between-group differences are only reported for one domain of the WHOQOL-26 measure |
| Other bias | Low risk |
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| First author and Year: Santer et al., 201474 Study ID: 42 (update searches) Country/location: UK (South West England) Setting: participants recruited from primary care and intervention delivered online Trial design: RCT (pilot) Includes process evaluation: yes (includes measures that could be regarded as a process evaluation; not described as a process evaluation by authors) Number of study centres: recruitment from 31 general practices, but one centre for evaluation Funding: National Institute for Health research (NIHR) Research for Patient Benefit programme (ref. number PB-PG-0110–20243) Conflicts of interest: none declared Trial/study number: ISRCTN 98560867 Study dates: not reported Was the educational intervention an adjunct to standard medical care? Yes |
Treatment intervention 1: website only + usual care Overview: online intervention delivered through the SPaCE website (see ‘usual care alone’ description below for what usual care consisted of) Intervention aim(s): aimed to improve carers’ management of their child’s eczema by increasing regular use of emollients. Ultimate aim of intervention was to improve HRQoL through enhancing carers’ management of the condition Where delivered: internet (website) Self-help, individual- and/or group-based? (State group size): individual-based Mode: online intervention Materials: delivered through a website. Some modules on the website contained videos (e.g. demonstrating techniques for emollient application) and print sheets of information. Both of these could also be accessed from a menu bar at the bottom of the website. 2-week challenge involved SMS text alerts Provider: medical experts developed the website; website delivered through LifeGuide software. HCPs (practice nurses, a health-care assistant and a GP) provided support to participants in one group Duration and intensity: two 20-minute compulsory modules, and then participants could complete other modules of their choice from a selection of 14, watch videos, download print sheets and take part in a 2-week challenge (see below) involving SMS alerts Scripting (level of detail guiding interaction between interventionist and participants): delivery of website intervention the same for all participants, except that participants could choose optional modules after completing the compulsory ones Sensitivity to participant characteristics: not reported Interventionist characteristics and training: HCPs received minimal training (one hour to familiarise themselves with the website) Content and topics: first stage of intervention: participants completed two compulsory modules: ‘What is eczema?’ and ‘Emollient moisturisers’. Then 14 other modules were available to complete, which covered ‘common concerns of carers of children with eczema’ (p. 3) including: diet and allergy; topical steroids, talking to your GP, starting school, sleep problems, bath time, washing clothes, eczema in the winter, eczema in the summer, swimming, going on holiday, avoiding stress for parents, involving your child in treatment, managing scratching (extracted from screenshot of SPaCE website in publication). Participants could access videos and print sheets on website, covering, for example, use of emollients, how to bath their child, action plan to use during GP consultation, and details on how to manage eczema to pass to relatives, school or nursery. Intervention strategies used are detailed in table 1 of the publication, but not data extracted. Participants could take part in a 2-week challenge involving SMS text alerts for setting goals, monitoring and rehearsing behaviours Tailoring: intervention was partly tailored – after completing the two compulsory modules, participants could choose to complete other optional modules that they were interested in (from a menu of 14 modules) Ongoing support: not reported (assume none) Theory: theory-based: used PRECEDE–PROCEED model to develop the intervention. The intervention incorporated 20 of the 26 behaviour-change techniques listed in Abraham and Michie’s taxonomy of behaviour-change techniques.83 Full list of exact intervention techniques used is provided in table 1 of the publication, but not data extracted. The development of the modules about ‘common concerns of carers of children with eczema’ (p. 3) were informed by qualitative interviews and input from patient support groups. Intervention also informed by ‘evidence-based patient information leaflets’ (p. 1), think-aloud interviews with users of a draft version of the website and feedback from other parents and HCPs Treatment intervention 2: website + plus HCP support + usual care Website intervention: same as above HCP support: one-off 20-minute appointment with HCP to promote engagement with the website intervention and to go through the 2 compulsory modules and 2-week challenge with participants if they had not already completed them/if participants had completed them, HCP helped them choose other modules to work through together. HCP varied across the general practices taking part: practice nurse in 11 practices, health-care assistant in 1 practice and GP in 1 practice. HCPs were not dermatology trained, except for one. All spent one hour familiarising themselves with the website (see ‘usual care alone’ description below for what usual care consisted of) Control intervention: usual care alone Description: usual care alone – participants consulted with GPs or attended secondary care dermatology appointments as needed. Most participants were being managed in primary care Duration and intensity: N/A |
Skin condition: eczema Diagnostic criteria: GP diagnosis of eczema Specify if patients, parents and/or carers: parents/carers and patients Patient general age group (specify if children, young adults and/or adults): children (aged ≤ 5 years) Stated target group: carers of children aged ≤ 5 years with mild to moderate eczema How recruited: primary care (31 general practices) Eligibility criteria Inclusion criteria: Parent/carer of child aged ≤ 5 years with GP diagnosis of eczema and who had received a prescription for eczema in past year Exclusion criteria: child aged older than 5 years, severe mental distress, experienced recent bereavement, unwillingness to be involved in research, parent could not give informed consent, English language skills not advanced enough to use website or complete study measures Numbers involved (randomised/allocated): Total: 148 (with useable data; 1 additional participant was randomised but technical difficulties meant no useable baseline data, no details about which condition they were randomised to) Intervention 1 (intervention 1 – website): 46 Intervention 2 (intervention 2 – website + HCP): 51 Control (control – usual care): 51 Numbers (%) completing, attrition and reasons: Attrition: 5 (3%) [website: 2 (4%); website + HCP: 1 (2%); usual care: 2 (4%)]. (Ns and %s calculated by reviewer) Reasons: not reported (but states that these participants did not complete primary outcome measures) Completers: 143 (97%) [website: 44 (96%); website + HCP: 50 (98%); usual care: 49 (96%)] (Total N and all % calculated by reviewer) Sample cross-overs: not reported Baseline characteristics Comorbidities: not reported Comedications/interventions: not reported Duration of disease: not reported Disease severity: see ‘results’ below for continuous POEM score at baseline Sex (M/F) of carer, n (%): Website: 2 (4)/44 (96) Website + HCP: 1 (2)/50 (98) Usual care: 1 (2)/50 (98) Average age: age of carer: detailed information provided across six categories for each of the three groups; not data extracted. The majority of participants (72–84%) across each arm were aged between 26 and 40 Age of child: detailed information provided across six categories for each of the three groups; not data extracted Ethnic groups: not reported Socioeconomic characteristics: age carer left education: detailed information provided across four categories for each of the three groups; not data extracted. Across study arms, between 12–16% left school between the ages of 15 and 16 |
Primary outcomes: disease severity (POEM; measures eczema symptoms over past week) Secondary outcomes: HRQoL (DFI, measures impact on family’s QoL) Other outcomes (not specified if primary or secondary): HRQoL (IDQoL, measures HRQoL in children aged ≤ 4 years; CDLQI, measures HRQoL in children aged ≥ 5 years) Adherence (self-report measure of emollient use) Adherence to intervention (PETS) Attitudes (measure not stated) Participants’ perceptions about if they understand and can manage treatment better (Patient Enablement Instrument) Adverse events: not reported Process evaluation measures: interviews with/feedback from participants and HCP (see Methods below) Intervention use (amount of time website used; completion of core modules; website visits; video use, took part in 2-week challenge SMS text alerts; uptake of HCP appointment) Individual preferred learning style addressed? No How outcomes assessed? POEM, use emollients (adherence) and Patient Enablement Instrument: self-reported, completed by carers online. No details in publication about who completed the DFI, IDQoL, CDLQI, PETS and attitudes measure Normal range(s) for outcomes/clinically meaningful improvement defined: yes, for POEM: authors state change in score of ≥ 2 in primary care is clinically significant. Authors also provide score classifications: 0–2 = clear/almost clear, 3–7 = mild, 8–16 = moderate, 17–24 = severe, 25–28 = very severe Validated? POEM, DFI, IDQoL and CDLQI: yes, authors provide evidence of validation/state measures are validated PETS and Patient Enablement Instrument: yes, wider literature confirms these are validated measures No details provided in publication about if the measures of self-reported emollient use and attitudes were validated Timing of outcomes same for both groups: yes Length of follow-up: 3 months (from baseline) |
| Methods | ||||
|---|---|---|---|---|
| Statistical analysis, including how missing data dealt with: methods of statistical analysis reported Power calculation: none reported, but study was intended to be a pilot RCT Study adequately powered? Unclear ITT used? States yes – but no details provided about how missing data were imputed. States that ‘follow-up questions not asked by phone received response rates below 60%; therefore they will not be presented here’ (p. 5); it is unclear to which questions this relates. Also, Ns reported for outcomes are not the ITT population Groups comparable at baseline? No comment provided on this by authors. Reviewer notes that POEM baseline scores were slightly higher in the website and website + HCP groups than the usual care group Subgroups analyses: subgroup analysis conducted of participants with a higher baseline eczema severity score (≥ 5), but not clear if pre-specified or post-hoc, so results are not data extracted below Process evaluation methods (if relevant): interviews with HCPs, covering their experiences of the study and details about the appointments they had with carers (e.g. use of the website during the appointment, any difficulties, perceived usefulness of the appointments with carers). Feedback interviews with 26 carers (plus e-mail feedback from one carer). Qualitative data were analysed thematically. Quantitative data: see ‘Process evaluation methods’ above for details about quantitative measures used |
||||
| Outcome evaluation results | ||||
| HRQoL outcomes | Website (n = 44) | Website + HCP (n = 50) | Usual care alone (n = 49) | p-value/CI |
| DFI score, mean (SD) | ||||
| Baseline | 5.3 (5.3) | 6.4 (5.6) | 5.2 (5.9) | Not reported |
| 3 months | 4.0 (4.2) | 5.9 (5.3) | 4.4 (5.5) | Not reported |
| Comments: DFI total score can range from 0–30, with a higher score showing worse HRQoL. Authors suggest that because baseline scores were low, follow-up scores represent floor effects. Results for IDQoL and CDLQI measures not reported | ||||
| Other relevant outcomes | Website (n = 44) | Website + HCP (n = 50) | Usual care alone (n = 49) | p-value/CI |
| POEM score (disease severity), mean (SD) | ||||
| Baseline | 10.3 (7.0) | 9.4 (6.2) | 7.47 (6.2) | Not reported |
| 3 months | 7.6 (6.1) | 8.7 (7.0) | 7.1 (6.6) | Not reported |
| Clinically significant change in POEM score between baseline and 3 months, n/N (%) | 23/42 (55) | 18/47 (38) | 16/49 (33) | p-value = 0.09 |
| Comments: POEM score can range from 0–28, with a higher score representing more severe disease. Clinically significant change in POEM score in a primary care context is a change of ≥ 2. Mean change in POEM score between baseline and 3 months and associated mean difference and 95% CIs also reported for the website groups combined and compared with usual care, but not data extracted | ||||
| Adverse events | Intervention | Control | p-value/CI | |
| Not reported | ||||
| Subgroup analysis results: | ||||
| Comments: no subgroups reported | ||||
| Outcome | Intervention | Control | p-value/CI | |
| No pre-specified analyses reported | ||||
| Process evaluation results | ||||
| Quantitative data Parents/carers Intervention use, measured by time spent on website (minutes), median (IQR): Website only: 34 (20–50) Website + HCP: 45 (26–70) Completion of core modules, n/N (%): Website only: 38/44 (86) Website + HCP: 37/49 (76) Three or more website visits, n/N (%): Website only: 16/44 (36) Website + HCP: 29/49 (59) Watched ≥ one video, n/N (%): Website only: 16/44 (36) Website + HCP: 17/49 (35) Took part in 2-week challenge SMS text alerts, n/N (%): Website only: 18/44 (41) Website + HCP: 18/49 (37) Notes: not the ITT populations. Results also reported for website groups combined, but not data extracted Authors report that there were no differences between web and web + HCP group in any aspect of intervention use (does not appear to have been statistically tested) Uptake of HCP appointment (in web + HCP group), n/N (%): 23/50 (46). Reasons for non-uptake, n: 12 declined appointment; 9 could not be contacted; 6 did not attend Qualitative data HCP Feedback from interviews: feedback included being pleased to provide a self-care support role, but apprehensions because they did not view themselves as eczema specialists. There were some concerns that they had received only minimal training for the role. On the whole, reported that the consultations were useful and worked well. Perceived that some carers found the intervention more useful than others and that some did not need the support of the HCP Carers Feedback on website: most feedback that the website was useful and easy to use. Only five of the 26 participants interviewed reported that they did not find it useful (reasons: had previously more need for such support than now, and child’s eczema was mild) Feedback on HCP support: variation in the perceived value of this. Reasons for perceiving it as not useful: did not feel they needed help in looking after their child’s eczema and confidence in ability to source information from the internet. Reasons for finding it useful: helped them engage more with the website, increased confidence for consulting with HCPs in the future, used it as an opportunity to discuss other health problems, had opportunity to obtain emollient samples (not part of the intervention) and felt more comfortable consulting with HCP than using website |
||||
| Comments: | ||||
| General comments | ||||
| Generalisability: good generalisability to patients managed in primary care the UK, as participants were recruited from 31 general and authors ensured that participants were recruited from a range of socioeconomic areas Other: authors do not provide details about any inter-centre variability in the HCP support component of the intervention |
||||
Quality criteria (Cochrane ‘risk of bias’ tool) randomised controlled trials
| Criteria | Judgement of risk of biasa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Low risk | Computer randomisation carried out via LifeGuide software, allocating 1 : 1 : 1 |
| Allocation concealment (selection bias) | Low risk | Central (web-based) allocation used |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) | Unclear risk | States ITT analysis used, but Ns reported for outcomes are not the ITT population. Attrition rates are small and similar across groups, but reasons for attrition are unclear. Additionally, one participant was randomised, but due to technical difficulties did not provide useable baseline data and thus was not included in the analysis, and it is unclear which group they were randomised to and whether they took part in the intervention |
| Selective reporting (reporting bias) | High risk | Results for IDQoL and CDLQI measures of HRQoL not reported. Results are also not reported for a number of other measures used (specifically: self-report measure of emollient use; PETS, attitudes measure, Patient Enablement Instrument) |
| Other bias | Unclear risk | Baseline imbalances in disease severity across groups, with both website groups having slightly more severe disease at baseline. Unclear if this is accounted for in the results. Also reports statistical analyses inconsistently (e.g. combined website groups versus usual care, not individual website groups versus usual care for mean change in the POEM score, which has therefore not been data extracted) |
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| First author and Year: Staab et al., 200687 Study ID: 575 Country/location: Germany Setting: not reported Trial design: RCT Includes process evaluation: no Number of study centres: seven hospitals Funding: German Federal Ministry of Health and Social Services (grant Number 01GL0010) Conflicts of interest: none Trial/study number: not reported Study dates: not reported Was the educational intervention an adjunct to standard medical care? Unclear – treatment with topical therapy or special diets was not included in the intervention and remained the responsibility of the patients’ doctor |
Treatment intervention Overview: group-based educational programme, with different educational sessions for a) parents of children aged 3 months to 7 years, b) children aged 8–12 years and their parents and c) adolescents with atopic dermatitis aged 13–18 years (parents optional for selected sessions) Intervention aim(s): not reported in primary publication, but the intervention for children aged 3 months to 7 years was based on one reported in Staab et al.93 and Wenninger et al.,78 the aim of which was improve parents’ ability to manage their child’s disease and thus improve disease course and the family’s HRQoL Where delivered: not reported Self-help, individual- and/or group-based? (State group size): group-based (5–8 participants) Mode: face-to-face group sessions Materials: handouts for participants with summary points and timetable for the sessions Provider: multiprofessional team of dermatologists or paediatricians, psychologists or dieticians Duration and intensity: six weekly 2-hour sessions Scripting (level of detail guiding interaction between interventionist and participants): a manual specified content Sensitivity to participant characteristics: Different groups for parents and children of different age groups Interventionist characteristics and training: all professionals had undergone a 40-hour training programme to qualify as trainers Content and topics: summary – intervention for parents of 3-month to 7-year-olds was based on the one reported in Staab et al.93 and Wenninger et al.78 Across the three groups, the educational sessions covered the following issues: medical (information about atopic dermatitis, understanding triggers, skin care, symptom treatment, unconventional therapies), nutritional (general child nutrition, food allergies, types of diet) and psychological (relaxation techniques, managing scratching and itching, sleep, coping, self-management plan, difficulties encountered in transferring skills to participants’ lives). Participants were encouraged to share experiences and to put new skills into practice Tailoring: not reported Ongoing support: not reported Theory: social cognitive theory – see Staab 801 and Wenniger 2863 data extraction Control intervention Description: no education control Duration and intensity: not reported |
Skin condition: atopic dermatitis Diagnostic criteria: diagnosis of atopic dermatitis was made by dermatologists or paediatricians Specify if patients, parents and/or carers: Patients and parents Patient general age group (specify if children, young adults and/or adults): Children and adolescents Stated target group: parents of children aged 3 months to 7 years or 8–12 years, children aged 8–12 years and adolescents aged 13–18 years (parents of adolescents optional for selected sessions) with moderate to severe atopic dermatitis How recruited: recruited consecutively from three children’s hospitals, three specialist dermatology hospitals and one department of psychosomatic medicine Eligibility criteria: Inclusion criteria: atopic dermatitis diagnosis based on criteria of Hanifin and Rajka; eczema for ≥ 3 months; eczema severity of ≥ 20 points on the atopic dermatitis scale. Exclusion criteria: presence of any other physical or psychiatric conditions that require treatment Numbers involved (randomised/allocated): Total: 992 Intervention: 496 Control: 496 Numbers (%) completing, attrition and reasons: Attrition: 169 (17%) [intervention 50 (10%); control 119 (24%)] Reasons (n): Died: intervention 0; control 1 Lost interest: intervention 7; control 45 No sufficient response: intervention 34; control 51 Other reasons: intervention 9; control 22 Discontinued intervention: intervention 0; control 0 Completers (n): 823 (intervention 446; control 377) Sample cross-overs: none Baseline characteristics Comorbidities: not reported Comedications/interventions: not reported Duration of disease: not reported Disease severity: (see baseline values in results) Sex (M/F), %: 3 months to 7 years age group: intervention 52/48; control 52/48 8–12 years age group: intervention 40/60; control 48/52 13–18 years age group: intervention 41/59; control 36/64 Mean age (SD), years: 3 months to 7 years age group: intervention 2.4 (1.8); control 2.4 (1.9) 8–12 years age group: intervention 9.5 (1.6); 9.5 (1.5) 13–18 years age group: intervention 14.9 (1.7); 14.8 (1.7) Ethnic groups: not reported Socioeconomic characteristics: not reported |
Primary outcomes: eczema severity (SCORAD index) Parents’ QoL – parents of children aged ≤ 13 years (German questionnaire: ‘Quality of life in parents of children with atopic dermatitis’) Secondary outcomes: subjective severity score (‘skin detective’) Itching behaviour (JUCKKI for 8–12 year olds and JUCKKU for 13- to 18-year-olds) Adverse events: not reported Process evaluation measures: not applicable Individual preferred learning style addressed? No How outcomes assessed? The SCORAD was scored by study investigators Normal range(s) for outcomes/clinically meaningful improvement defined: no Validated? Yes – all validated Timing of outcomes same for both groups: yes Length of follow-up: 12 months |
| Methods | |||
|---|---|---|---|
| Statistical analysis, including how missing data dealt with: authors report how data were statistically analysed. Covariance analysis used for patient reported outcomes, but not stated what the covariates were (i.e. whether adjusted for some or all baseline variables) Power calculation: yes – power calculation was based on the eczema severity outcome (atopic dermatitis total score) (the primary outcome). Based on an anticipated effect size was d = 0.40 with an alpha level of 0.05 and assuming a 20% loss to follow-up, it was estimated that 125 participants would be needed per group to provide a power of 80% Study adequately powered? Adequately powered for the 3 months to 7 years age group; may not be adequately powered for the 8–12 years and 13–18 years age groups (sample sizes smaller than the 125 participants needed per group) ITT used? Number Only completers included in analysis Groups comparable at baseline? Yes – no statistically significant differences in eczema severity or any other outcome measures at baseline. Mean age and sex similar between groups Subgroups analyses: none, but data were analysed separately for the 3 months to 7 years, 8–12 years and 13–18 years age groups. So essentially all were subgroups Process evaluation methods (if relevant): no process evaluation |
|||
| Outcome evaluation results | |||
| HRQoL outcomes: 3 months to 7 years age group | Educational programme (n = 274) | No education (n = 244) | Difference (95% CI; p-value) in change between baseline and 12 months between groups |
| Parental QoL | |||
| Psychosomatic well-being | |||
| Baseline, mean (SD) | 29.3 (7.6) | 29.1 (7.7) | |
| 12 months, mean (SD) | 33.7 (7.0) | 32.1 (7.1) | |
| Mean difference/change in score (95% CI) | 4.4 (3.6 to 5.2) | 3.1 (2.2 to 3.9) | 1.4 (0.2 to 2.5; 0.0040) |
| Effects on social life | |||
| Baseline, mean (SD) | 24.9 (4.0) | 24.5 (4.4) | |
| 12 months, mean (SD) | 26.7 (3.4) | 25.5 (4.1) | |
| Mean difference/change in score (95% CI) | 1.8 (1.4 to 2.3) | 1.0 (0.6 to 1.5) | 0.8 (0.2 to 1.4; < 0.0001) |
| Confidence in medical treatment | |||
| Baseline, mean (SD) | 16.0 (4.0) | 15.8 (4.4) | |
| 12 months, mean (SD) | 20.0 (3.5) | 17.8 (4.2) | |
| Mean difference/change in score (95% CI) | 4.0 (3.5 to 4.5) | 1.9 (1.4 to 2.4) | 2.1 (1.4 to 2.8; < 0.0001) |
| Emotional coping | |||
| Baseline, mean (SD) | 13.7 (3.2) | 14.2 (3.4) | |
| 12 months, mean (SD) | 16.8 (2.9) | 15.4 (3.2) | |
| Mean difference/change in score (95% CI) | 3.1 (2.7 to 3.5) | 1.1 (0.7 to 1.6) | 1.9 (1.3 to 2.5; < 0.0001) |
| Acceptance of disease | |||
| Baseline, mean (SD) | 7.1 (1.9) | 7.0 (1.9) | |
| 12 months, mean (SD) | 8.2 (1.7) | 7.5 (1.8) | |
| Mean difference/change in score (95% CI) | 1.1 (0.8 to 1.3) | 0.5 (0.3 to 0.8) | 0.6 (0.2 to 0.9; < 0.0001) |
| HRQoL outcomes – 8 – 12 years age group | Educational programme (n = 102) | No education (n = 83) | Difference (95% CI, p-value) in change between baseline and 12 months between groups |
| Parental QoL | |||
| Psychosomatic well-being | |||
| Baseline, mean (SD) | 31.5 (7.9) | 31.2 (6.1) | |
| 12 months, mean (SD) | 34.7 (6.0) | 33.8 (7.0) | |
| Mean difference/change in score (95% CI) | 3.2 (1.9 to 4.5) | 2.6 (1.4 to 3.8) | 0.6 (–1.2 to 2.4; 0.360) |
| Effects on social life | |||
| Baseline, mean (SD) | 25.8 (4.2) | 26.3 (4.0) | |
| 12 months, mean (SD) | 27.0 (3.8) | 27.2 (3.5) | |
| Mean difference/change in score (95% CI) | 1.1 (0.4 to 1.8) | 0.9 (0.2 to 1.6) | 0.2 (–0.8 to 1.2; 0.940) |
| Confidence in medical treatment | |||
| Baseline, mean (SD) | 17.0 (4.0) | 17.4 (3.9) | |
| 12 months, mean (SD) | 20.1 (3.2) | 17.5 (4.4) | |
| Mean difference/change in score (95% CI) | 3.1 (2.2 to 3.9) | 0.1 (–0.7 to 1.0) | 2.9 (1.7 to 4.1; < 0.0001) |
| Emotional coping | |||
| Baseline, mean (SD) | 13.7 (3.3) | 14.7 (3.2) | |
| 12 months, mean (SD) | 16.4 (2.8) | 15.6 (3.4) | |
| Mean difference/change in score (95% CI) | 2.7 (2.0 to 3.4) | 0.9 (0.2 to 1.6) | 1.8 (0.9 to 2.8; 0.002) |
| Acceptance of disease | |||
| Baseline, mean (SD) | 7.3 (1.9) | 7.4 (1.7) | |
| 12 months, mean (SD) | 8.1 (1.5) | 7.7 (1.8) | |
| Mean difference/change in score (95% CI) | 0.8 (0.4 to 1.2) | 0.2 (–0.2 to 0.6) | 0.6 (0 to 1.2; 0.031) |
| Comments: outcome data also collected at 6 months, but not reported in the paper 26 item questionnaire with five subscales as demonstrated above. No details of scoring provided, reference provided for validation is in German |
|||
| Other relevant outcomes – 3 months to 7 years age group | Educational programme (n = 274) | No education (n = 244) | Difference (95% CI; p-value) in change between baseline and 12 months between groups |
| SCORAD: total severity score | |||
| Baseline, mean (SD) | 41.1 (16.6) | 40.6 (15.2) | |
| 12 months, mean (SD) | 23.7 (16.7) | 28.4 (16.5) | |
| Mean difference/change in score (95% CI) | –17.5 (–19.6 to –15.3) | –12.2 (–14.3 to –10.1) | –5.2 (–8.2 to –2.2; 0.0002) |
| SCORAD: objective severity score | |||
| Baseline, mean (SD) | 32.5 (14.3) | 31.4 (13.0) | |
| 12 months, mean (SD) | 19.5 (13.9) | 22.6 (13.4) | |
| Mean difference/change in score (95% CI) | –13.0 (–14.8 to –11.2) | –8.7 (–10.5 to –7.0) | –4.2 (–6.8 to –1.7; 0.0009) |
| Subjective severity | |||
| Baseline, mean (SD) | 8.3 (3.8) | 8.3 (3.8) | |
| 12 months, mean (SD) | 4.8 (3.4) | 6.1 (3.6) | |
| Mean difference/change in score (95% CI) | –3.3 (–3.9 to –2.8) | –2.2 (–2.7 to –1.6) | –1.1 (–1.9 to –0.3; < 0.001) |
| Other relevant outcomes: 8- to 12-years age group | Educational programme (n = 102) | No education (n = 83) | Difference (95% CI, p-value) in change between baseline and 12 months between groups |
| SCORAD: total severity score | |||
| Baseline, mean (SD) | 41.8 (16.6) | 40.4 (15.1) | |
| 12 months, mean (SD) | 25.8 (17.7) | 32.6 (16.5) | |
| Mean difference/change in score (95% CI) | –16.0 (–20.0 to –12.0) | –7.8 (–11.4 to –4.3) | –8.2 (–13.6 to –2.8; 0.003) |
| SCORAD: objective severity score | |||
| Baseline, mean (SD) | 34.0 (14.1) | 32.5 (13.1) | |
| 12 months, mean (SD) | 21.7 (15.1) | 26.9 (14.2) | |
| Mean difference/change in score (95% CI) | –12.3 (–15.6 to –8.9) | –5.6 (–8.7 to –2.5) | –6.7 (–11.2 to –2.1; 0.005) |
| Subjective severity | |||
| Baseline, mean (SD) | 8.5 (3.9) | 8.6 (3.5) | |
| 12 months, mean (SD) | 4.9 (2.9) | 7.0 (3.8) | |
| Mean difference/change in score (95% CI) | –3.7 (–4.6 to –2.7) | –1.6 (–2.5 to –0.7) | –2.1 (–3.4 to –0.8; < 0.001) |
| Itching behaviour: catastrophisisationa | |||
| Baseline, mean (SD) | 13.6 (8.5) | 13.6 (8.2) | |
| 12 months, mean (SD) | 6.6 (6.5) | 11.8 (8.6) | |
| Mean difference/change in score (95% CI) | –7.0 (–8.9 to –5.1) | –1.8 (–3.5 to –0.2) | –5.2 (–7.7 to –2.7; < 0.0001) |
| Itching behaviour: coping | |||
| Baseline, mean (SD) | 7.7 (5.1) | 7.6 (4.6) | |
| 12 months, mean (SD) | 8.8 (5.4) | 7.2 (5.0) | |
| Mean difference/change in score (95% CI) | 1.0 (–0.3 to 2.3) | –0.4 (–1.6 to 0.8) | 1.5 (–0.3 to 3.2; 0.047) |
| Other relevant outcomes: 13–18 years age group | Educational programme (n = 70) | No education (n = 50) | Difference (95% CI, p-value) in change between baseline and 12 months between groups |
| SCORAD: total severity score | |||
| Baseline, mean (SD) | 43.1 (14.7) | 40.4 (13.9) | |
| 12 months, mean (SD) | 23.4 (12.6) | 35.2 (15.2) | |
| Mean difference/change in score (95% CI) | –19.7 (–23.7 to –15.7) | –5.2 (–10.5 to 0.1) | –14.5 (–21.2 to –7.9; < 0.0001) |
| SCORAD: objective severity score | |||
| Baseline, mean (SD) | 34.4 (12.4) | 33.4 (12.0) | |
| 12 months, mean (SD) | 19.5 (11.1) | 28.3 (12.0) | |
| Mean difference/change in score (95% CI) | –15.0 (–18.4 to –11.6) | –5.1 (–9.5 to –0.6) | –9.9 (–15.5 to –4.3; < 0.0001) |
| Subjective severity | |||
| Baseline, mean (SD) | 8.9 (3.2) | 8.8 (3.5) | |
| 12 months, mean (SD) | 5.8 (3.4) | 8.1 (4.0) | |
| Mean difference/change in score (95% CI) | –3.1 (–4.1 to –2.2) | –1.0 (–2.1 to 0.1) | –2.1 (–3.5 to –0.7; < 0.0002) |
| Itching behaviour: catastrophisisationa | |||
| Baseline, mean (SD) | 16.6 (7.9) | 16.9 (8.6) | |
| 12 months, mean (SD) | 9.8 (8.1) | 14.9 (9.0) | |
| Mean difference/change in score (95% CI) | –6.8 (–8.6 to –5.0) | –2.0 (–3.9 to –0.2) | –4.7 (–7.3 to –2.2; 0.0002) |
| Itching behaviour: coping | |||
| Baseline, mean (SD) | 15.4 (7.8) | 14.0 (7.0) | |
| 12 months, mean (SD) | 15.2 (8.2) | 14.5 (7.0) | |
| Mean difference/change in score (95% CI) | –0.2 (–1.9 to 1.5) | 0.4 (–1.2 to 2.1) | –0.6 (–3.0 to 1.7; 0.875) |
| Comments: outcome data also collected at 6 months, but not reported in the paper | |||
| Adverse events | Educational programme | No education | p-value/CI |
| Not reported | |||
| Comments: | |||
| Subgroup analysis results: | |||
| Comments: no subgroups reported | |||
| Outcome | Educational programme | No education | p-value/CI |
| None | |||
| Comments: | |||
| Process evaluation results | |||
| No process evaluation | |||
| Comments: | |||
| General comments | |||
| Generalisability: large-scale study across seven study centres in Germany, conducted with a wide range of children age groups. Therefore the study is likely to have good generalisation to the German context. Atopic dermatitis was moderate to severe Other: differences between groups on parental HRQoL appear to be small – unclear whether the statistically significant changes are clinically meaningful |
|||
Quality criteria (Cochrane ‘risk of bias’ tool) randomised controlled trials
| Criteria | Judgement of risk of biasa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Low risk | Computer-generated random numbers were used |
| Allocation concealment (selection bias) | Unclear risk | ‘The randomisation code was concealed in closed envelopes from those entering patients into the study.’ Randomisation was carried out by an independent study centre, but unclear if the envelopes containing the randomisation code were opaque or sequentially numbered to adequately conceal allocation from those entering patients into the study |
| Blinding of participants and personnel (performance bias) | High risk | Participants and trainers were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) | High risk | Participants were not blinded to treatment allocation. Unclear if the investigators who rated eczema severity were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | High risk | A higher proportion of patients in the no education group (24%) than in the education intervention group (10%) were lost to follow-up, mainly due to no response or lost interest, which may have impacted outcomes. Missing data were not imputed and ITT analysis was not conducted |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were measured at 6 and 12 months, but only 12-month results are reported |
| Other bias | Low risk |
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author and year: van Os-Medendorp et al., 200789 Study ID: 505 (some data from a secondary publication 466) Country/location: Netherlands Setting: secondary care Trial design: RCT Includes process evaluation: no Number of study centres: not reported (recruitment was from four centres) Funding: Dutch College of Health Insurance (CVZ) Conflicts of interest: stated none declared Trial/study number: not reported Study dates: not reported Was the educational intervention an adjunct to standard medical care? Yes |
Treatment intervention ‘Coping with itch’ Overview: individual sessions with dermatology nurse, in addition to usual medical treatment by dermatologist. Includes educational and cognitive behavioural interventions such as individual patient education, awareness training and habit reversal, relaxation exercises and psychosocial support given according to a nursing care plan. Referral to other members of outpatient dermatology multidisciplinary team (social workers, psychologists and dermatologists) if needed Intervention aim(s): to reduce itch and to help patients cope with itch Where delivered: specialised itch clinic in dermatology outpatient department Self-help, individual- and/or group-based? (State group size): individual-based Mode: face to face Materials: not reported Provider: dermatology nurse Duration and intensity: patients visited the itch clinic a mean of 2.9 times (median 3, range 1–6); duration of sessions not reported Scripting (level of detail guiding interaction between interventionist and participants): not reported Sensitivity to participant characteristics: not reported Interventionist characteristics and training: not reported Content and topics: education about: itch causes, consequences and treatment; patient advocacy groups; avoiding triggers; diet; interventions to relieve itching and scratching and their consequences. Cognitive behavioural therapy including diary-based awareness training, habit reversal to reduce scratching, and relaxation. Based on an initial itch medical history assessment taken by the nurse and structured according to an individual-based nursing care plan Tailoring: nursing care plan for intervention was structured according to patient’s individual needs Ongoing support: individual counselling and ‘support’ (not defined) provided as required (no details given) Theory: not reported Control intervention Description: normal care, which consists of outpatient consultations with a dermatologist involving diagnosis and therapeutic intervention such as the use of emollients and topical steroids Duration and intensity: not reported |
Skin condition: chronic pruritic skin disease (regardless of specific diagnosis) Diagnostic criteria: not reported Specify if patients, parents and/or carers: patients Patient general age group (specify if children, young adults and/or adults): adults (aged 18 years or older) Stated target group: adults with chronic pruritic skin disease How recruited: by dermatologists from four dermatology outpatient departments (no further details reported) Eligibility criteria: not reported Numbers involved (stated as the numbers included – unclear if this means the number randomised): Total:120 Intervention (intervention): 63 Control: 57 Numbers (%) completing, attrition and reasons (percentages calculated by reviewer):a Attrition: 9 months: 69 (58) [intervention 40 (63); control 29 (51)] Reasons: did not return baseline and/or 3-month and/or 9-month follow-up patient-reported outcome measures; in control group 4 patients received the intervention and were excluded Completers: (9 months): 51 (43) [intervention 23 (37); control 28 (49)] Sample cross-overs: four patients in control group received the intervention and were excluded from the analysis Baseline characteristicsb Comorbidities: not reported Comedications/interventions: Not reported for baseline Duration of disease and itch, years, mean (SD):d Disease duration: intervention 14.6 (14.4); control 17.4 (18.3) Itch duration: intervention: 12.3 (13.8); control 12.3 (16.6) Disease severity: not reported, other than patients with high frequency or high intensity of itching/scratching (see outcomes section below) Diagnosis of skin disease, n (%)d,e Eczema: intervention 10 (34); control 10 (29) AD: intervention 5 (17); control 3 (9) Pruritus: intervention 4 (14); control 5 (15) Prurigo: intervention 1 (3); control 2 (6) Psoriasis: intervention 4 (14); control 3 (8) Chronic urticaria: intervention: 2 (7); control 2 (6) Other: intervention 2 (7); control 6 (18) Unknown: intervention 1 (3); control 1 (3) Non-skin disease: intervention 0 (0); control 2 (6) Sex M/F, n (%):d intervention: 13 (45)/16 (55) control: 10 (28)/26 (72) Mean (SD) [range] age, years:d intervention: 57.0 (17.3) [25.5–86.8] control: 55.7 (17.2) [23.8–82.8] Ethnic groups: not reported Socioeconomic characteristics: Education level: low/medium/high, n (%)d,f intervention (n = 28): 13 (46)/6 (21)/9 (32) control (n = 35): 18 (51)/11 (31)/6 (17) |
Primary outcomes: disease severity (patient-reported frequency and intensity of itching and scratching); itch-related coping (ICQ); skin-related psychosocial morbidity including QoL (ACS); general psychosocial morbidity (SCL-90) Secondary outcomes (assessed by monthly telephone interviews): Health resource use (number of dermatologist visits); medication and ointment use Adverse events: not reported Process evaluation measures: no process evaluation Individual preferred learning style addressed? No How outcomes assessed?: By patient self-report:
Validated? Stated that the ICQ, ACS and SCL-90 are validated instruments. Self-reported itching and scratching also validated (internal consistency reported) Timing of outcomes same for both groups: yes Length of follow-up: longest follow-up time point: 9 months after start of programme (same as programme duration) |
| Methods |
|---|
| Statistical analysis, including how missing data dealt with: analysis methods are reported. Missing data were not included in analyses. Note that to analyse the use of medications and the number of visits to the dermatologist, data for months 1–3 of the study period were used in lieu of baseline data (no explanation reported) and data for months 7–9 were provided as the follow-up data. Reported results are those with a ‘trend to significance’, defined as p-value < 0.10 Power calculation: not reported Study adequately powered? Not reported [note high rates of attrition (> 50%) at 9 months] ITT used? No. Attrition was not accounted for in analyses Groups comparable at baseline? Yes (stated that no significant differences were found between the characteristics of the intervention group and the control group) Subgroups analyses: none reported Process evaluation methods (if relevant): none reported |
| Outcome evaluation results | |||
|---|---|---|---|
| HRQoL outcomes, mean (SD) score (3-month outcomes also reported but not extracted here) | ‘Coping with itch’ group Baseline n = 29 9 months n = 23 |
Control group Baseline n = 36 9 months n = 30 (unless stated) |
p-value |
| ACS subscale: impact on QoL | |||
| Baseline | 12.08 (5.00) | 12.56 (4.93) | Stated NS |
| 9 months | 13.10 (5.25) | 12.68 (4.58) | Stated NS |
| Other outcomes, mean (SD) score (3-month outcomes also reported but not extracted here) | |||
| ICQ subscale: catastrophising and helpless coping | |||
| Baseline | 22.47 (11.44) | 23.06 (8.88) | Stated NS |
| 9 months | 17.23 (10.42) | 20.19 (10.18) [n = 28] | Stated NS |
| ICQ subscale: problem-focused coping | |||
| Baseline | 18.95 (8.79) | 21.24 (6.68) | Stated NS |
| 9 months | 19.39 (9.47) | 20.79 (7.06) [n = 28] | Stated NS |
| ACS skin-related psychosocial morbidity | |||
| Baseline | 138.39 (38.00) | 141.26 (37.60) | Stated NS |
| 9 months | 134.79 (42.69) | 134.01 (40.85) | Stated NS |
| ACS subscale: social anxiety and avoidance | |||
| Baseline | 34.62 (14.49) | 36.09 (14.19) | Stated NS |
| 9 months | 34.80 (15.99) | 35.46 (13.97) | Stated NS |
| ACS subscale: vicious circle of itching and scratching | |||
| Baseline | 28.41 (7.61) | 30.20 (7.07) | Stated NS |
| 9 months | 26.07 (8.17) | 26.70 (8.00) | Stated NS |
| ACS subscale: helplessness | |||
| Baseline | 26.02 (8.29) | 26.01 (7.19) | Stated NS |
| 9 months | 26.61 (7.87) | 24.70 (8.27) | Stated NS |
| ACS subscale: anxious depressive mood | |||
| Baseline | 24.18 (8.74) | 24.58 (8.67) | Stated NS |
| 9 months | 23.30 (8.79) | 23.97 (8.89) | Stated NS |
| ACS subscale: deficit in active coping | |||
| Baseline | 13.07 (3.36) | 11.82 (3.24) | Stated NS |
| 9 months | 10.91 (3.01) | 10.50 (3.27) | Stated NS |
| SCL-90 general psychosocial morbidity | |||
| Baseline | 146.30 (60.02) | 151.18 (52.60 | Stated NS |
| 9 months | 134.41 (47.68) | 159.81 (57.69) | Stated NS |
| Comments: lower ICQ scores reflect better itch-related coping (or less itch-related catastrophising); lower ACS scores reflect lower skin-related psychosocial morbidity; lower SCL-90 scores reflect lower general psychosocial morbidity burden | |||
| Patients with high frequency or high intensity of itching and scratching, n (%) | ‘Coping with itch’ group Baseline n = 25 9 months n = 24 |
Control group Baseline n = 32 9 months n = 29 |
p-value |
|---|---|---|---|
| High frequency | |||
| Baseline | 18 (72) | 21 (66) | Stated NS |
| 9 months | 12 (50) | 15 (52) | Stated NSa |
| High intensity | |||
| Baseline | 20 (80) | 25 (78) | Stated NS |
| 9 months | 12 (50) | 16 (55) | Stated NS |
| Comments: continuous scale for frequency and intensity of itching/scratching was validated (internal consistency reported) but authors subsequently converted it to a dichotomous measure with arbitrary cut-off (high frequency defined as > 4 and high intensity defined as > 3 based on average from an original continuous itching scale of 0 (none) to 10 (unbearable) and a continuous scratching scale of 0 (none) to 10 (bloody skin) Data for 3 months also reported but not extracted |
|||
| Medication use, n (%) | ‘Coping with itch’ group Months 1–3 n = 29 Months 7–9 n = 27 |
Control group Months 1–3 n = 35 Months 7–9 n = 31 |
p-value |
|---|---|---|---|
| Topical corticosteroids: mild | |||
| Months 1–3 | 3 (10) | 0 (0) | p = 0.053 |
| Months 7–9 | 2 (7) | 2 (6) | Stated NS |
| Topical corticosteroids: moderately potent | |||
| Months 1–3 | 5 (17) | 12 (34) | Stated NS |
| Months 7–9 | 6 (22) | 7 (23) | Stated NS |
| Topical corticosteroids: potent | |||
| Months 1–3 | 9 (31) | 11 (31) | Stated NS |
| Months 7–9 | 5 (18) | 7 (23) | Stated NS |
| Topical corticosteroids: very potent | |||
| Months 1–3 | 0 (0) | 3 (9) | Stated NS |
| Months 7–9 | 3 (11) | 2 (6) | Stated NS |
| Systemic medication (ciclosporin, acitretin, prednisone, etc.) | |||
| Months 1–3 | 4 (14) | 3 (9) | Stated NS |
| Months 7–9 | 4 (15) | 3 (10) | Stated NS |
| Itch-relieving medication (hydroxyzine, cetirizine, etc.) | |||
| Months 1–3 | 12 (41) | 17 (49) | Stated NS |
| Months 7–9 | 13 (48) | 13 (42) | Stated NS |
| Proportion of patients visiting the dermatologist, n (%) | ‘Coping with itch’ group Months 1–3 n = 29 Months 7–9 n = 27 |
Control group Months 1–3 n = 35 Months 7–9 n = 31 |
p-value |
| Months 1–3 | 17 (59) | 30 (86) | p = 0.015 |
| Months 7–9 | 17 (63) | 18 (58) | Stated NS |
| Comments: cost-effectiveness paper107 also reports the following outcomes as numbers of visits or events (mean and SD reported but not extracted here): phone and face-to-face visits to the dermatologist; dermatology repeat prescriptions; ultraviolet therapy visits; visits to the dermatology nurse; visits to the dermatology social worker; visits to the GP; repeat prescriptions from the GP; repeat prescriptions from other HCPs; visits to hospital (any department except dermatology); use of paramedical services; visits to a psychosocial worker; home visits by a nurse; visits to an alternative medicine provider; days off work; days of hospitalisation. At 9 months of follow-up the difference between ‘Coping with itch’ and control groups was not significant (at α = 0.05) (95% CIs for the differences included zero). An exception is that there were significantly more visits to the dermatology nurse in the intervention group than the control group: intervention mean (SD) = 3.1 (2.0); control mean (SD) = 0.5 (0.7); difference = 2.7; 95% CI 1.8 to 3.6107 | |||
| Adverse events | ‘Coping with itch’ group | Control group | p-value |
| Comments: no adverse events reported | |||
| Subgroup analysis results: | |||
| Comments: no subgroups reported | |||
| Process evaluation results | |||
| Comments: no process evaluations reported | |||
| General comments | |||
| Generalisability: adult population, but generalisability unclear as ethnicity, body weight/BMI and comorbidities not reported; range of skin diseases covered in population so unclear whether results applicable to specific skin diseases | |||
Quality criteria (Cochrane ‘risk of bias’ tool) randomised controlled trials
| Criteria | Judgement of risk of biasa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | High rate of attrition in both study groups, with attrition excluded from analyses (non-ITT). Slightly higher attrition rate in control than intervention, especially early in period (9% difference after 3 months). Partly accounted for by four patients in control group who elected to receive ‘Coping with itch’ intervention and were excluded from analyses. Primary reason for attrition was failure to provide patient-reported outcome measures for baseline and follow-up assessments. Sample sizes are difficult to follow and are different for patient-reported outcomes and medication use; and data on attrition were different in the publications89,107 |
| Selective reporting (reporting bias) | Unclear risk | No protocol reported. All outcomes stated in the methods are reported in the results. However, arbitrary thresholds were used for defining high frequency and high intensity of itching and scratching (i.e. continuous measure converted to dichotomous with arbitrary cut-off). Outcome timing for medication use appears arbitrary without explanation (months 1–3 were used in lieu of baseline and months 7–9 in lieu of 9-month follow-up) |
| Other bias | Low risk | No additional risk identified |
Appendix 5 List of excluded studies with rationale
Below is a list of the publications excluded at the full-text screening stage of the clinical effectiveness review and reasons for exclusion.
| Publication | Exclusion reason |
|---|---|
| Armstrong AW, Kim RH, Idriss NZ, Larsen LN, Lio PA. Online video improves clinical outcomes in adults with atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol 2011;64:502–7 | Did not measure HRQoL, using a validated measure |
| Basak PY, Ozturk M, Baysal V. Assessment of information and education about topical corticosteroids in dermatology outpatient departments: experience from Turkey. J Eur Acad Dermatol Venereol 2003;17:652–8 | Did not measure HRQoL, using a validated measure |
| Bauer A, Kelterer D, Bartsch R, Schlegel A, Pearson J, Stadeler M, et al. Prevention of hand dermatitis in bakers’ apprentices: different efficacy of skin protection measures and UVB hardening. Int Arch Occup Environ Health 2002;75:491–9 | Did not measure HRQoL, using a validated measure |
| Bauer A, Kelterer D, Bartsch R, Pearson J, Stadeler M, Kleesz P, et al. Skin protection in bakers’ apprentices. Contact Dermatitis 2002;46:81–5 | Did not measure HRQoL, using a validated measure |
| Bostoen J, Geusens B, Lambert J, Bracke S, Dekeyser S. An educational program for patients with psoriasis and atopic dermatitis: A prospective randomized, controlled trial. J Am Acad Dermatol 2012;66(Suppl. 1):AB84 | Abstract or conference presentation with insufficient information |
| Bostoen J, Geusens B, Bracke S, Dekeyser S, Lambert J. Follow-up on the effect of a patient educational programme: Early results of a prospective randomized controlled trial in psoriasis and atopic dermatitis. Br J Dermatol 2011;165:e34–5 | Abstract or conference presentation with insufficient information |
| Broberg A, Kalimo K, Lindblad B, Swanbeck G. Parental education in the treatment of childhood atopic eczema. Acta Dermato-Venereologica 1990;70:495–9 | Did not measure HRQoL, using a validated measure |
| Chinn DJ, Poyner T, Sibley G. Randomized controlled trial of a single dermatology nurse consultation in primary care on the quality of life of children with atopic eczema. Br J Dermatol 2002;146:432–9 | Not an educational intervention focused on improving HRQoL |
| Ehlers A, Stangier U, Gieler U. Treatment of atopic dermatitis: a comparison of psychological and dermatological approaches to relapse prevention. J Consult Clin Psychol 1995;63:624–35 | Did not measure HRQoL, using a validated measure |
| Evers AWM, Duller P, de Jong EMGJ, Otero ME, Verhaak CM, Van Der Valk PGM, et al. Effectiveness of a multidisciplinary itch-coping training programme in adults with atopic dermatitis. Acta Derm Venereol 2009;89:57–63 | Educational and other aspects inseparable |
| Feldman SR, Vanarthos J, Fleischer AB, Jr. The readability of patient education materials designed for patients with psoriasis. J Am Acad Dermatol 1994;30:284–6 | Did not measure HRQoL, using a validated measure |
| Fisker MH, Agner T, Lindschou J, Bonde JP, Ibler KS, Gluud C, et al. Protocol for a randomised trial on the effect of group education on skin-protective behaviour versus treatment as usual among individuals with newly notified occupational hand eczema – the Prevention of Hand Eczema (PREVEX) Trial. BMC Dermatol 2013;13:16 | Not a RCT or CCT |
| Flanders PA, McNamara JR. Enhancing acne medication compliance: a comparison of strategies. Behav Res Ther 1985;23:225–7 | Did not measure HRQoL, using a validated measure |
| Flyvholm MA, Mygind K, Sell L, Jensen A, Jepsen KF. A randomised controlled intervention study on prevention of work related skin problems among gut cleaners in swine slaughterhouses. Occup Environ Med 2005;62:642–9 | Did not measure HRQoL, using a validated measure |
| Fortune DG, Richards HL, Kirby B, Bowcock S, Main CJ, Griffiths CE. A cognitive-behavioural symptom management programme as an adjunct in psoriasis therapy. Br J Dermatol 2002;146:458–65 | Did not measure HRQoL, using a validated measure |
| Fortune DG, Richards HL, Griffiths CE, Main CJ. Targeting cognitive-behaviour therapy to patients’ implicit model of psoriasis: results from a patient preference controlled trial. Br J Clin Psychol 2004;43:1–82 | Did not measure HRQoL, using a validated measure |
| Fukuie T, Nomura I, Narita M, Suzuki T, Tajima I, Natsume O, et al. A randomized, open-label, parallel group study to evaluate the efficacy and safety of proactive management in pediatric subjects with moderate to severe atopic dermatitis. J Allergy Clin Immunol 2013;131:AB101 | Did not measure HRQoL, using a validated measure |
| Futamura M, Masuko I, Hayashi K, Ohya Y, Ito K. Effects of a short-term parental education program on childhood atopic dermatitis: a randomized controlled trial. Pediatr Dermatol 2013;30:438–43 | Not an educational intervention focused on improving HRQoL |
| Gradwell C, Thomas KS, English JS, Williams HC. A randomized controlled trial of nurse follow-up clinics: do they help patients and do they free up consultants’ time? Br J Dermatol 2002;147:513–17 | Not an educational intervention focused on improving HRQoL |
| Grillo M, Gassner L, Marshman G, Dunn S, Hudson P. Pediatric atopic eczema: the impact of an educational intervention. Pediatr Dermatol 2006;23:428–36 | Not an educational intervention focused on improving HRQoL |
| Habib S, Morrissey S. Stress management for atopic dermatitis. Behav Change 1999;16:226–36 | Did not measure HRQoL, using a validated measure |
| Hampel P, Rudolph H, Petermann F, Stachow R. Stress management training for children and adolescents with atopic dermatitis during inpatient rehabilitation. Dermatol Psychosomat 2001;2:116–22 | Did not measure HRQoL, using a validated measure |
| Huang C, Yan S, Ren J, Xiang L, Hu Y, Kang K, et al. A quantitative assessment of the effects of formal sun protection education on photosensitive patients. Photodermatol Photoimmunol Photomed 2013;29:261–5 | Not an educational intervention focused on improving HRQoL |
| Hudson P, Grillo M. Atopic eczema management: What do patients value in eczema education workshops? Allergy 2011;66(Suppl. 94):619–20 | Did not measure HRQoL, using a validated measure |
| Ibler K, Agner T, Jemec G. Hand eczema in healthcare workers: Results of a randomised trial. Contact Dermatitis 2012;66(Suppl. s2):26 | Abstract or conference presentation with insufficient information |
| Ibler KS, Jemec GB, Diepgen TL, Gluud C, Lindschou HJ, Winkel P, et al. Skin care education and individual counselling versus treatment as usual in healthcare workers with hand eczema: randomised clinical trial. BMJ 2012;345:e7822 | Not an educational intervention focused on improving HRQoL and/or educational and other aspects inseparable |
| Ibler KS, Agner T, Hansen JL, Gluud C. The Hand Eczema Trial (HET): Design of a randomised clinical trial of the effect of classification and individual counselling versus no intervention among health-care workers with hand eczema. BMC Dermatol 2010;10:8 | Not an educational intervention focused on improving HRQoL |
| Jaspers JP, Span L, Molier L, Coenraads PJ. A multimodal education and treatment program for young adults with atopic dermatitis: a randomized controlled trial. Dermatol Psychosom 2000;1:148–53 | Insufficient reporting of results in paper |
| Koch PE, Ryder HF, Dziura J, Njike V, Antaya RJ. Educating adolescents about acne vulgaris: a comparison of written handouts with audio-visual computerized presentations. Arch Dermatol 2008;144:208–14 | Did not measure HRQoL, using a validated measure |
| Kupfer J, Gieler U, Diepgen TL, Fartasch M, Lob-Corzilius T, Ring J, et al. Structured education program improves the coping with atopic dermatitis in children and their parents-a multicenter, randomized controlled trial. J Psychosom Res 2010;68:353–8 | Did not measure HRQoL, using a validated measure |
| Lambert J, Bostoen J, Geusens B, Bourgois J, Boone J, De SD, et al. A novel multidisciplinary educational programme for patients with chronic skin diseases: Ghent pilot project and first results. Arch Dermatolog Res 2011;303:57–63 | Not a RCT or CCT |
| Lora V, Gisondi P, Calza A, Zanoni M, Girolomoni G. Efficacy of a single educative intervention in patients with chronic plaque psoriasis. Dermatology 2009;219:316–21 | Insufficient reporting of results in paper |
| Macedo O. ‘Got sick, got a gift’: A new tool for optimising adherence to acne therapy. J Am Acad Dermatol 2011;64(Suppl. 1):AB13 | Did not measure HRQoL, using a validated measure |
| Masuko I, Futamura M, Hahashi K, Ito K, Ohya Y. A randomized evaluator-blinded trial of behavioral modification program for mothers of children with atopic dermatitis. J Allergy Clin Immunol 2009;123:S47 | Abstract or conference presentation with insufficient information |
| Mollerup A, Veien NK, Johansen JD. The effectiveness of tailored nurse-led counselling in hand eczema. Contact Dermatitis 2014;Conference: 31 | Abstract or conference presentation with insufficient information |
| Moore EJ, Williams A, Manias E, Varigos G, Donath S. Eczema workshops reduce severity of childhood atopic eczema. Australas J Dermatol 2009;50:100–6 | Did not measure HRQoL, using a validated measure |
| Mork NJ, Austad J, Brolund L. An open, parallel groups, study of the importance of thoroughness of application in the treatment of psoriasis with a dithranol cream (Micanol). Acta Derm Venereol Suppl 1992;172:23–4 | Did not measure HRQoL, using a validated measure |
| Noren P, Melin L. The effect of combined topical steroids and habit-reversal treatment in patients with atopic dermatitis. Br J Dermatol 1989;121:359–66 | Did not measure HRQoL, using a validated measure |
| Rosenkranz MA, Davidson RJ, Maccoon DG, Sheridan JF, Kalin NH, Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav Immun 2013;27:174–84 | Did not measure HRQoL, using a validated measure |
| Rothman AI, Byrne N, Schacter RK, Rosenberg L, Mitchell D. An educational program for psoriatics: an evaluation. Eval Health Prof 1980;3:191–203 | Did not measure HRQoL, using a validated measure |
| Schulte MB, Cormane RH, van DE, Wuite J. Group therapy of psoriasis. Duo formula group treatment (DFGT) as an example. J Am Acad Dermatol 1985;12:61–6 | Not a RCT or CCT |
| Schuttelaar ML, Vermeulen KM, Drukker N, Coenraads PJ. A randomized controlled trial in children with eczema: nurse practitioner vs. dermatologist. Br J Dermatol 2010;1:162–70 | Educational and other aspects inseparable |
| Shaw M, Morrell DS, Goldsmith LA. A study of targeted enhanced patient care for pediatric atopic dermatitis (STEP PAD). Pediatr Dermatol 2008;25:19–24 | Not an educational intervention focused on improving HRQoL |
| Shi VY, Nanda S, Lee K, Armstrong AW, Lio PA. Improving patient education with an eczema action plan: a randomized controlled trial. JAMA Dermatol 2013;149:481–3 | Did not measure HRQoL, using a validated measure |
| Staab D, von RU, Kehrt R, Erhart M, Wenninger K, Kamtsiuris P, et al. Evaluation of a parental training program for the management of childhood atopic dermatitis. Pediatr Allergy Immunol 2002;13:84–90 | Insufficient reporting of results in paper |
| Tabolli S, Pagliarello C, Sampogna F, di PC, Abeni D. Evaluation of the impact of writing exercises and educational interventions on quality of life in patients with psoriasis. Value Health 2011;14:A509–10 | Educational and other aspects inseparable |
| Tabolli S, Naldi L, Pagliarello C, Sampogna F, di PC, Spagnoli A, et al. Evaluation of the impact of writing exercises interventions on quality of life in patients with psoriasis undergoing systemic treatments. Br J Dermatol 2012;167:1254–64 | Educational and other aspects inseparable |
| van Os-Medendorp H, Guikers CL, Eland-de Kok PC, Ros WJ, Bruijnzeel-Koomen CA, Buskens E. Costs and cost-effectiveness of the nursing programme ‘Coping with itch’ for patients with chronic pruritic skin disease. Br J Dermatol 2008;158:1013–21 | Did not measure HRQoL, using a validated measure |
| van Os-Medendorp H, Koffijberg H, Eland-de Kok PC, van der Zalm A, de Bruin-Weller MS, Pasmans SG, et al. E-health in caring for patients with atopic dermatitis: a randomized controlled cost-effectiveness study of internet-guided monitoring and online self-management training. Br J Dermatol 2012;166:1060–8 | Not an educational intervention focused on improving HRQoL |
| Wang A, Armstrong A, Schupp C, Wu J. Randomized controlled trial examining effectiveness of a novel personalized interactive health care education tool for acne patients (MyPACE). J Am Acad Dermatol 2013;68(Suppl. 1):AB18 | Abstract or conference presentation with insufficient information |
| Wang AS, Wu J, Foolad N, Armstrong AW. Developing a novel personalized interactive health care education system (MyPACE) for acne patients. J Invest Dermatol 2012;132:S87 | Did not measure HRQoL, using a validated measure |
| Weber MB, Fontes Neto PT, Prati C, Soirefman M, Mazzotti NG, Barzenski B, et al. Improvement of pruritus and quality of life of children with atopic dermatitis and their families after joining support groups. J Eur Acad Dermatol Venereol 2008;22:992–7 | Not an educational intervention focused on improving HRQoL |
| Wenninger K, Kehrt R, den Uv, Lehmann C, Binder C, Wahn U, et al. Structured parent education in the management of childhood atopic dermatitis: The Berlin model. Patient Educ Counsel 2000;40:253–61. | Insufficient reporting of results in paper |
| West C, Narahari S, O’Neill J, Davis S, Huynh M, Clark A, et al. Adherence to adalimumab in patients with moderate to severe psoriasis. Dermatol Online J 2013;19:18182 | Did not measure HRQoL, using a validated measure |
| Williams HC. Educational programmes for young people with eczema. BMJ 2006;332:923–4 | Did not measure HRQoL, using a validated measure |
Appendix 6 Full details of the structure and content of the educational interventions
| Study | Balato et al., 201385 | Ersser et al., 201186 | Bostoen et al., 201276 | Santer et al., 201474 | Staab et al., 200687 (further information provided by Wenninger et al.78) | Matsuoka et al., 200688 | van Os-Medendorp et al., 200789 |
|---|---|---|---|---|---|---|---|
| Skin condition(s), population | Psoriasis, patients (adults) | Psoriasis, patients (adults) | Psoriasis or atopic dermatitis, patients (adults) | Eczema (children aged ≤ 5 years) and parents/carers | Atopic dermatitis, patients (children and adolescents) and parents | Acne, patients (adults; women aged > 16 years) | Chronic pruritic skin diseases, patients (adults) |
| Overview | Text message education | A theory-based self-management educational intervention for individuals with psoriasis. Patients also received usual care (only mentioned in abstract) | Educational programme for patients with psoriasis and atopic dermatitis. All patients continued with medical therapy | Two educational intervention groups:
|
Group-based educational programme, with different educational sessions for (a) parents of children aged 3 months to 7 years, (b) children aged 8–12 years and their parents and (c) adolescents with atopic dermatitis aged 13–19 years (parents optional for selected sessions) | Women with acne vulgaris were instructed on skin care and how to use make-up by a dermatologist. The women also received acne treatment (topical therapy and/or oral medication) | Individual sessions with dermatology nurse, in addition to usual medical treatment by dermatologist. Includes educational and cognitive behavioural interventions such as individual patient education, awareness training and habit reversal, and relaxation exercises and psychosocial support given according to a nursing care plan. Referral to other members of outpatient dermatology multidisciplinary team (social workers, psychologists and dermatologists) if needed |
| Stated target group | No details | Individuals with mild-to-moderate plaque psoriasis | Adults with psoriasis or atopic dermatitis | Carers of children aged ≤ 5 years with mild to moderate eczema | Parents of children aged 3 months to 7 years or 8–12 years, children aged 8–12 years and adolescents aged 13–18 years (parents of adolescents optional for selected sessions) with moderate to severe atopic dermatitis | Women | Adults with chronic pruritic skin disease |
| Adjunct to standard medical care? | Yes | Yes | Yes | Yes | Unclear – treatment with topical therapy or special diets was not included in the intervention and remained the responsibility of the patients’ doctor | Yes | Yes |
| Intervention aim(s) | Not stated explicitly but implicit from the paper that was to use text messaging to improve treatment adherence and patient outcomes including HRQoL | To support self-management in psoriasis | Not explicitly stated. Study aimed to examine if the educational intervention ‘added value to medical therapy’ (p. 1025) and examine the effects on disease severity and QoL | Aimed to improve carers’ management of their child’s eczema by increasing regular use of emollients. Ultimate aim of intervention was to improve HRQoL through enhancing carers’ management of the condition | Not reported in primary publication, but the intervention for children aged 3 months to 7 years was based on one reported in Staab et al.93 and Wenninger et al.,78 the aim of which was improve parents’ ability to manage their child’s disease and thus improve disease course and families’ QoL | Not explicitly stated, but part of the aim of the study was to examine if instructions in make-up use from a dermatologist could affect female acne patients’ QoL | To reduce itch and to help patients cope with itch |
| Where delivered | Via mobile phone | Not reported | Not reported |
|
Not reported | Outpatient clinic based in a university dermatology department | Specialised itch clinic in dermatology outpatient department |
| Self-help, individual- and/or group-based? | Individual-based | Group-based face-to-face session (maximum of nine participants), with supporting information and follow-up telephone consultation | Group-based intervention. Group sizes differed and were 14, 23 and 13 | Individual-based | Group-based (5–8 participants) | Not reported | Individual-based |
| Mode | Text messaging | Face to face, written and audio-visual materials, individual telephone consultation | Face-to-face workshop | 1. Online intervention 2. Online intervention, plus face-to-face appointment with HCP | Face-to-face group sessions | Face to face, with supporting videotape instructions and detailed leaflets/prescriptions | Face to face |
| Materials | Text messaging | Supporting written and audio-visual materials provided; DVD and workbook, including relaxation material | Secondary publication99 of a before-and-after study using the same intervention notes that the syllabus was offered to participants | Delivered through a website. Some modules on the website contained videos, e.g. demonstrating techniques for emollient application, and print sheets of information. Both of these could also be accessed from a menu bar at the bottom of the website. 2-week challenge involved SMS text alerts | Handouts for participants with summary points and timetable for the sessions | Videotaped instructions; leaflets and make-up prescriptions with more detailed instructions; sample cosmetics provided | Not reported |
| Provider | Not stated who the ‘investigators’ were but physicians enrolled participants | Nurse-led group sessions, with supporting materials provided (see Materials) | Dermatologist, dermatology nurse, pharmacists, dietician, training expert, psychiatrist, psychologist, philosopher, and a sports, yoga and mindfulness teacher |
|
Multiprofessional team of dermatologists or paediatricians, psychologists or dieticians | Dermatologist | Dermatology nurse |
| Duration and intensity | 1 text message per day for a period of 12 weeks | Group session: one-off 2-hour session Telephone consultation: one-off 20-minute session |
3 months programme, with two, 2-hour sessions a week |
|
Six weekly 2-hour sessions | Not reported | Patients visited the itch clinic a mean of 2.9 times (median 3 times, range 1–6 times); duration of sessions not reported |
| Scripting (level of detail guiding interaction between interventionist and participants) | General educational statements and reminders sent in a random order with four educational and three reminders sent each week. Details of the types of information provided in the paper but no details of the coverage of these (i.e. if all were eventually sent to each participant) | In delivering the intervention, the nurse was expected to follow the intervention protocol, but with flexibility for individualisation. Outline script was used for follow-up telephone consultation | Secondary publication99 refers to a syllabus |
|
A manual specified content | Not reported | Not reported |
| Sensitivity to participant characteristics | Text messages were created using simple language | Not reported, but intervention allowed flexibility for individualisation | Not reported | Not reported | Different groups for parents and children of different age groups | Not reported | Not reported |
| Individual preferred learning style addressed? | No | No | No | No | No | No | No |
| Interventionist characteristics and training | No details | Nurse attended training on self-efficacy-based education | None reported apart from job title noted above | HCPs received minimal training (one hour to familiarise themselves with the website) | All professionals had undergone a 40-hour training programme to qualify as trainers | Not reported | Not reported |
| Content and topics | Summary: covered frequently asked questions about psoriasis drugs (e.g. administration and adverse effects) and general recommendations to take care of overall health. All text messages between 1 and 3 sentences. Educational topics included daily care statements (e.g. use moisturisers, wear light clothes), healthy lifestyle statements (e.g. avoid smoking, pay attention to your diet), prompts about the use of treatments (e.g. do not abuse steroids, common side effects of certain drugs) and one statement about the psychosocial effects of psoriasis (e.g. do not feel ashamed or guilty, psoriasis is not contagious). Reminders reinforced many of the same principles | Practical element (no details provided), individual action planning, stress reduction (through provision of relaxation materials), feedback on action plans (through telephone consultation) | Information on specific skin disease, and skin care sessions, healthy lifestyle (diet, sleep hygiene, smoking, substance abuse) and stress-reducing techniques (physical training, psychodermatology, practical philosophy and mindfulness), feedback sessions. Further details of the intervention provided in a separate publication99 |
|
Summary: intervention for parents of 3-month to 7-year-olds was based on the one reported in Staab et al.87 and Wenninger et al.78 Across the three groups, the educational sessions covered the following issues: medical (information about atopic dermatitis, understanding triggers, skin care, symptom treatment, unconventional therapies), nutritional (general child nutrition, food allergies, types of diet) and psychological (relaxation techniques, managing scratching and itching, sleep, coping, self-management plan, difficulties encountered in transferring skills to participants’ lives) Participants were encouraged to share experiences and to put new skills into practice | Summary: patients received instructions on use of skin care and make-up products. Instructions included general skin care and how to use ‘point make-up’ (e.g. eyeliner and lipstick) | Education about: itch causes, consequences and treatment; patient advocacy groups, avoiding triggers, diet; interventions to relieve itching and scratching and their consequences, cognitive behavioural therapy including diary-based awareness training, habit reversal to reduce scratching, and relaxation. Based on an initial itch medical history assessment taken by the nurse and structured according to an individual-based nursing care plan |
| Tailoring | Does not appear to be tailored | Intervention included individual action planning | Not reported | Intervention was partly tailored: after completing the two compulsory modules, participants could choose to complete other optional modules that they were interested in (from a menu of 14 modules) | Not reported | Not reported | Nursing care plan for intervention was structured according to patient’s individual needs |
| Ongoing support | None reported | Nurse provided one 20-minute follow-up telephone consultation one month after the group session (based on outline script) | Not reported | Not reported | Not reported | Not reported | Individual counselling and ‘support’ (not defined) provided as required (no details given) |
| Theory | None reported | Based on Social Learning Theory – specifically self-efficacy theory. Intervention incorporated elements designed to address the four sources of self-efficacy (mastery, verbal persuasion, vicarious experience, emotional regulation). Intervention was also informed by the findings of previous qualitative research into the self-management needs of individuals with psoriasis | Not reported | Theory-based: used PRECEDE–PROCEED model to develop the intervention. The intervention incorporated 20 of the 26 behaviour-change techniques listed in Abraham and Michie’s taxonomy of behaviour change techniques.82 Full list of exact intervention techniques used is provided in table 1 of the publication, but not data extracted. The development of the modules about ‘common concerns of carers of children with eczema’ (p. 3) were informed by qualitative interviews and input from patient support groups. Intervention also informed by ‘evidence-based patient information leaflets’ (Santer et al., 2014,74 p. 1), think-aloud interviews with users of a draft version of the website and feedback from other parents and HCPs | Social cognitive theory | Not reported | Not reported |
Appendix 7 Excluded cost-effectiveness studies
| Excluded study | Reason for exclusion |
|---|---|
| Beikert FC, Langenbruch AK, Radtke MA, Augustin M. Willingness to pay and quality of life in patients with rosacea. J Eur Acad Dermatol Venereol 2013;27:734–8 | Inappropriate study design |
| van der Meer EW, Boot CR, Jungbauer FH, van der Klink JJ, Rustemeyer T, Coenraads PJ, et al. Hands4U: a multifaceted strategy to implement guideline-based recommendations to prevent hand eczema in health care workers: design of a randomised controlled trial and (cost) effectiveness evaluation. BMC Public Health 2011;11:669 | Ongoing trial |
| Bathe A, Matterne U, Dewald M, Grande T, Weisshaar E. Educational multidisciplinary training programme for patients with chronic pruritus. Acta Derm Venereol 2009;89:498–501 | Inappropriate study design |
| Hartman M, Prins M, Swinkels OQ, Severens JL, De BT, Van Der Wilt GJ, et al. Cost-effectiveness analysis of a psoriasis care instruction programme with dithranol compared with UVB phototherapy and inpatient dithranol treatment. Br J Dermatol 2002;147:538–44 | Intervention was not educational |
| Staab D, von RU, Kehrt R, Erhart M, Wenninger K, Kamtsiuris P, et al. Evaluation of a parental training program for the management of childhood atopic dermatitis. Pediatr Allergy Immunol 2002;13:84–90 | Inappropriate study design |
| van Gils RF, Bosmans JE, Boot CRL, Rustemeyer T, van MW, Van Der Valk PGM, et al. Economic evaluation of an integrated care programme for patients with hand dermatitis. Contact Dermatitis 2013;69:144–52 | Not clear if the intervention was educational |
| Parsi K, Chambers CJ, Armstrong AW. Cost-effectiveness analysis of a patient-centered care model for management of psoriasis. J Am Acad Dermatol 2012;66:563–70 | Intervention was not educational |
| Healthcare Insurance Board/College voor Zorgverzekeringen. Evaluation of the Combination Day- and Home Treatment of Psoriasis – Primary Research. Healthcare Insurance Board/College voor Zorgverzekeringen (CVZ); 2000 | Not in the English language |
| The Netherlands Organisation for Health Research and Development. E-health in Caring for Patients With Atopic Dermatitis. An Economic Evaluation Comparing Usual Care with Internet-Guided Monitoring and Self-Management Training by a Nurse Practitioner. The Netherlands Organisation for Health Research and Development (ZonMw). 2000. URL: www.zonmw.nl (accessed 19 October 2015) | Not in the English language |
| Kernick D, Cox A, Powell R, Reinhold D, Sawkins J, Warin A. A cost consequence study of the impact of a dermatology-trained practice nurse on the quality of life of primary care patients with eczema and psoriasis. Br J Gen Prac 2000;50:555–8 | Not clear if the intervention was educational |
Appendix 8 Data extractions from systematic review of cost-effectiveness
| 1 | Study | Mason, 2013108 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Research question | To examine the effectiveness of a multifaceted educational support programme to increase emollient use and reduce atopic eczema symptoms in children | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | Country/setting | The UK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | Funding source | Reckitt Benkiser Healthcare | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | Analysis type | Cost analysis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | Study type | Before-and-after study | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | Perspective | NHS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | Time horizon | Trial duration: 3 months | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | Analysis method | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| If estimations are based on a model, state the model assumptions where relevant | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | Discounting (rate) | None | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | Costing year, currency | 2011, £ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | Population | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Definition of condition | Atopic eczema | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Characteristics of baseline cohort/risk factors | Eligible children were male and female aged 3 months to 6 years, with mild to moderate atopic eczema; and using E45 Cream as their primary emollient | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | Intervention(s), comparator(s) | A multifaceted educational support programme was evaluated as a method of increasing emollient use and reducing atopic eczema in children. Support provided for parents and carers included an educational DVD, online daily diary and telephone helpline with dermatology nurses | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 | Outcome measure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The outcomes were collected as part of the before-and-after study in which a purpose-designed multifaceted educational support programme (ESP) was provided Emollient use was estimated through telephonic questionnaire; severity of eczema was captured using the Patient-Oriented Eczema Measure (POEM) and Patient Eczema Severity Time (PEST). Health-care contacts were determined via recorded number of GP and dermatology specialist visits; parent measures were captured by telephone questionnaire and concurrent medication use was recorded through telephone surveys |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | Health benefits | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Not reported but appears to be based on improved emollient uptake, reduced eczema severity, reduced sleep disturbance and improved parent control | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severity of eczema was captured using POEM and PEST. No single instrument was used to measure health benefits; different aspects of health benefits were measured separately | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 | Costs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intervention cost | The cost of providing the ESP programme was estimated to be £32 per child based on a resource analysis of providing the service | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indirect Costs | Not stated | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 | Results Health economic outcomes were expressed in terms of costs, presented in the table below Costs (£, 2011)Pre-programmeaProgrammebChangec,dMean(SD)Mean(SD)Mean95% CInpProgramme32–32–––Emolliente9.29(9.30)19.57(12.03)10.28(7.93 to 12.64)1320.001Emollientf9.72(9.69)22.64(12.21)12.91(10.72 to 15.10)1150.001GP visits68.53(76.70)30.40(31.67)–38.13(–52.58 to –23.68)1350.001Overall coste78.29(79.34)82.66(34.16)4.37(–10.55 to 19.30)1320.62Overall costsf80.32(82.48)84.37(31.29)4.06(–12.00 to 20.11)1150.56aEstimated for the 12 weeks preceding intervention, using 2-week baseline data and 12-week recall (GP visits).b12 weeks while receiving the patient support programme.cDifference in costs in the two periods (negative denotes a reduction).dEstimated by bootstrapping with 1000 replications.eEmollient use costed using the daily diary method.fEmollient use costed using the estimated time taken to use a 500-g pot of emollient. Clinical outcomes of relevance (as included within the sensitivity analysis) are extracted in the table below OutcomesBaselinea9–12 weeksMean changebMeanSDMeanSDMean95% CIcnp-value3Emollient use (g/week)dDaily diary–167.8109.587.681.9 to 119.51320.001Time to use79.279.2197.4106.5110.094.6 to 131.31150.001Severity scoresPOEMe11.346.274.855.04–5.38–6.41 to –4.361350.001PESTf2.260.811.530.68–0.61–0.75 to –0.471350.001aWeeks –2 to 0, except GP visits which included the previous 12 weeks to week 0.bThe mean change is the average of the programme period scores minus the baseline period score in subjects with complete data. It is to be noted that data for week 1–4 weeks and 5–8 weeks were not extracted.cEstimated by bootstrapping with 1000 replications.dEmollient use was estimated using 2 methods: time to use a reported weight of emollient (500 g) and a daily diary record of counts of pumps of emollient used by weight; the latter method was not available for the baseline period.ePOEM score from 0 to 28, including seven signs of eczema at four levels of frequency in the past week at the end of each period.fPEST score, the child’s unhappiness with eczema: score 1 (not at all) to 5 (very unhappy), daily diary score averaged over each period. |
Costs (£, 2011) | Pre-programmea | Programmeb | Changec,d | Mean | (SD) | Mean | (SD) | Mean | 95% CI | n | p | Programme | 32 | – | 32 | – | – | – | Emolliente | 9.29 | (9.30) | 19.57 | (12.03) | 10.28 | (7.93 to 12.64) | 132 | 0.001 | Emollientf | 9.72 | (9.69) | 22.64 | (12.21) | 12.91 | (10.72 to 15.10) | 115 | 0.001 | GP visits | 68.53 | (76.70) | 30.40 | (31.67) | –38.13 | (–52.58 to –23.68) | 135 | 0.001 | Overall coste | 78.29 | (79.34) | 82.66 | (34.16) | 4.37 | (–10.55 to 19.30) | 132 | 0.62 | Overall costsf | 80.32 | (82.48) | 84.37 | (31.29) | 4.06 | (–12.00 to 20.11) | 115 | 0.56 | aEstimated for the 12 weeks preceding intervention, using 2-week baseline data and 12-week recall (GP visits).b12 weeks while receiving the patient support programme.cDifference in costs in the two periods (negative denotes a reduction).dEstimated by bootstrapping with 1000 replications.eEmollient use costed using the daily diary method.fEmollient use costed using the estimated time taken to use a 500-g pot of emollient. | Outcomes | Baselinea | 9–12 weeks | Mean changeb | Mean | SD | Mean | SD | Mean | 95% CIc | n | p-value3 | Emollient use (g/week)d | Daily diary | – | 167.8 | 109.5 | 87.6 | 81.9 to 119.5 | 132 | 0.001 | Time to use | 79.2 | 79.2 | 197.4 | 106.5 | 110.0 | 94.6 to 131.3 | 115 | 0.001 | Severity scores | POEMe | 11.34 | 6.27 | 4.85 | 5.04 | –5.38 | –6.41 to –4.36 | 135 | 0.001 | PESTf | 2.26 | 0.81 | 1.53 | 0.68 | –0.61 | –0.75 to –0.47 | 135 | 0.001 | aWeeks –2 to 0, except GP visits which included the previous 12 weeks to week 0.bThe mean change is the average of the programme period scores minus the baseline period score in subjects with complete data. It is to be noted that data for week 1–4 weeks and 5–8 weeks were not extracted.cEstimated by bootstrapping with 1000 replications.dEmollient use was estimated using 2 methods: time to use a reported weight of emollient (500 g) and a daily diary record of counts of pumps of emollient used by weight; the latter method was not available for the baseline period.ePOEM score from 0 to 28, including seven signs of eczema at four levels of frequency in the past week at the end of each period.fPEST score, the child’s unhappiness with eczema: score 1 (not at all) to 5 (very unhappy), daily diary score averaged over each period. | ||||||||||||||||||||||||||||||||||||||||||||||
| Costs (£, 2011) | Pre-programmea | Programmeb | Changec,d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean | (SD) | Mean | (SD) | Mean | 95% CI | n | p | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Programme | 32 | – | 32 | – | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emolliente | 9.29 | (9.30) | 19.57 | (12.03) | 10.28 | (7.93 to 12.64) | 132 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emollientf | 9.72 | (9.69) | 22.64 | (12.21) | 12.91 | (10.72 to 15.10) | 115 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GP visits | 68.53 | (76.70) | 30.40 | (31.67) | –38.13 | (–52.58 to –23.68) | 135 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall coste | 78.29 | (79.34) | 82.66 | (34.16) | 4.37 | (–10.55 to 19.30) | 132 | 0.62 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall costsf | 80.32 | (82.48) | 84.37 | (31.29) | 4.06 | (–12.00 to 20.11) | 115 | 0.56 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aEstimated for the 12 weeks preceding intervention, using 2-week baseline data and 12-week recall (GP visits).b12 weeks while receiving the patient support programme.cDifference in costs in the two periods (negative denotes a reduction).dEstimated by bootstrapping with 1000 replications.eEmollient use costed using the daily diary method.fEmollient use costed using the estimated time taken to use a 500-g pot of emollient. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcomes | Baselinea | 9–12 weeks | Mean changeb | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean | SD | Mean | SD | Mean | 95% CIc | n | p-value3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emollient use (g/week)d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daily diary | – | 167.8 | 109.5 | 87.6 | 81.9 to 119.5 | 132 | 0.001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Time to use | 79.2 | 79.2 | 197.4 | 106.5 | 110.0 | 94.6 to 131.3 | 115 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severity scores | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| POEMe | 11.34 | 6.27 | 4.85 | 5.04 | –5.38 | –6.41 to –4.36 | 135 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PESTf | 2.26 | 0.81 | 1.53 | 0.68 | –0.61 | –0.75 to –0.47 | 135 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aWeeks –2 to 0, except GP visits which included the previous 12 weeks to week 0.bThe mean change is the average of the programme period scores minus the baseline period score in subjects with complete data. It is to be noted that data for week 1–4 weeks and 5–8 weeks were not extracted.cEstimated by bootstrapping with 1000 replications.dEmollient use was estimated using 2 methods: time to use a reported weight of emollient (500 g) and a daily diary record of counts of pumps of emollient used by weight; the latter method was not available for the baseline period.ePOEM score from 0 to 28, including seven signs of eczema at four levels of frequency in the past week at the end of each period.fPEST score, the child’s unhappiness with eczema: score 1 (not at all) to 5 (very unhappy), daily diary score averaged over each period. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 | Sensitivity analysis Bootstrapping method was adopted with 1000 replications. Analyses were repeated in 117 children, excluding 18 children who had visited an eczema specialist in the 3 months prior to joining the programme. Results of the analyses are presented in the table below Sensitivity analysis: excluding children who had recently seen a specialist Pre-programmeaProgrammebChangec,dMeanSDMeanSDMean95% CInp-valueEmollient use (g/week)Daily diary69.749.5170.399.0100.681.9 to 119.51140.001Time to use72.249.6184.397.6112.294.6 to 131.3990.001Severity scoresPOEM11.036.495.834.67–5.20–6.39 to –4.341170.001PEST2.260.821.660.62–0.60–0.74 to –0.471170.001Costs (£, 2011)Programme32–32–––Emolliente8.185.8019.9811.6111.819.81 to 14.011140.001Emollientf8.475.8221.6311.4613.1610.94 to 15.39990.001GP visits63.2363.8030.4630.86–32.77–45.77 to –19.771170.001Overall coste71.8166.3783.2533.7511.44–1.99 to 24.861140.123Overall costsf72.1168.2483.4533.7511.35–3.14 to 25.83990.239aEstimated for the 12 weeks preceding intervention, using 2 week baseline data and 12 week recall (GP visits).b12 weeks while receiving the patient support programme.cDifference in costs in the two periods (negative denotes a reduction).dEstimated by bootstrapping with 1000 replications.eEmollient use costed using the daily diary method.fEmollient use costed using the estimated time taken to use a 500-g pot of emollient. |
Pre-programmea | Programmeb | Changec,d | Mean | SD | Mean | SD | Mean | 95% CI | n | p-value | Emollient use (g/week) | Daily diary | 69.7 | 49.5 | 170.3 | 99.0 | 100.6 | 81.9 to 119.5 | 114 | 0.001 | Time to use | 72.2 | 49.6 | 184.3 | 97.6 | 112.2 | 94.6 to 131.3 | 99 | 0.001 | Severity scores | POEM | 11.03 | 6.49 | 5.83 | 4.67 | –5.20 | –6.39 to –4.34 | 117 | 0.001 | PEST | 2.26 | 0.82 | 1.66 | 0.62 | –0.60 | –0.74 to –0.47 | 117 | 0.001 | Costs (£, 2011) | Programme | 32 | – | 32 | – | – | – | Emolliente | 8.18 | 5.80 | 19.98 | 11.61 | 11.81 | 9.81 to 14.01 | 114 | 0.001 | Emollientf | 8.47 | 5.82 | 21.63 | 11.46 | 13.16 | 10.94 to 15.39 | 99 | 0.001 | GP visits | 63.23 | 63.80 | 30.46 | 30.86 | –32.77 | –45.77 to –19.77 | 117 | 0.001 | Overall coste | 71.81 | 66.37 | 83.25 | 33.75 | 11.44 | –1.99 to 24.86 | 114 | 0.123 | Overall costsf | 72.11 | 68.24 | 83.45 | 33.75 | 11.35 | –3.14 to 25.83 | 99 | 0.239 | aEstimated for the 12 weeks preceding intervention, using 2 week baseline data and 12 week recall (GP visits).b12 weeks while receiving the patient support programme.cDifference in costs in the two periods (negative denotes a reduction).dEstimated by bootstrapping with 1000 replications.eEmollient use costed using the daily diary method.fEmollient use costed using the estimated time taken to use a 500-g pot of emollient. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pre-programmea | Programmeb | Changec,d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean | SD | Mean | SD | Mean | 95% CI | n | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emollient use (g/week) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daily diary | 69.7 | 49.5 | 170.3 | 99.0 | 100.6 | 81.9 to 119.5 | 114 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Time to use | 72.2 | 49.6 | 184.3 | 97.6 | 112.2 | 94.6 to 131.3 | 99 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severity scores | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| POEM | 11.03 | 6.49 | 5.83 | 4.67 | –5.20 | –6.39 to –4.34 | 117 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PEST | 2.26 | 0.82 | 1.66 | 0.62 | –0.60 | –0.74 to –0.47 | 117 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs (£, 2011) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Programme | 32 | – | 32 | – | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emolliente | 8.18 | 5.80 | 19.98 | 11.61 | 11.81 | 9.81 to 14.01 | 114 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emollientf | 8.47 | 5.82 | 21.63 | 11.46 | 13.16 | 10.94 to 15.39 | 99 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GP visits | 63.23 | 63.80 | 30.46 | 30.86 | –32.77 | –45.77 to –19.77 | 117 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall coste | 71.81 | 66.37 | 83.25 | 33.75 | 11.44 | –1.99 to 24.86 | 114 | 0.123 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall costsf | 72.11 | 68.24 | 83.45 | 33.75 | 11.35 | –3.14 to 25.83 | 99 | 0.239 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aEstimated for the 12 weeks preceding intervention, using 2 week baseline data and 12 week recall (GP visits).b12 weeks while receiving the patient support programme.cDifference in costs in the two periods (negative denotes a reduction).dEstimated by bootstrapping with 1000 replications.eEmollient use costed using the daily diary method.fEmollient use costed using the estimated time taken to use a 500-g pot of emollient. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 | Author’s conclusions | Based on their findings, the authors concluded that, although a community-based multi-faceted education support programme did not increase cost, it helped increase the use of emollient. This further helped in reducing symptoms associated with atopic eczema as well as contacts with the GPs. It was also observed that such a programme could benefit the families and carers of children with the condition as the children experienced improved sleep patterns which gave the parents and carers greater feeling of control. The authors, however, recommended further evaluation to assess the effectiveness of PEST in helping parents and children to monitor and manage eczema | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20 | Reviewer’s comments | This study is relevant for the purpose of this review as it was based in the UK. However, from an economic perspective, there were a few limitations. First, a cost-effectiveness analysis was not conducted. Also, the sources of the cost inputs and detailed composition of costs parameters used in the analysis were not reported. Second, the analysis was not conducted over a long period of time but for 3 months as the duration of the trial. Although sensitivity analyses were conducted to check the robustness of the base case results, probabilistic distributions were not assigned to the parameters, rather bootstrapping method was adopted to avoid parametric assumptions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Systematic review quality-assessment checklist for economic evaluations and notes for completion
Critical appraisal checklist for economic evaluations (based on Drummond et al. , 2005110)
| Item | Y/N/? |
|---|---|
| 1.Is the decision problem (including interventions compared and patient group) relevant to the UK? | Yes |
| 2.Is the setting comparable to the UK? | Yes |
| 3.Is the analytical and modelling methodology appropriate? | ? |
| 4.Are all the relevant costs and consequences for each alternative identified? | ? |
| 5.Are the data inputs for the model described and justified? | ? |
| 6.Are health outcomes measured in QALYs? | No |
| 7.Is the time horizon considered appropriate? | No |
| 8.Are costs and outcomes discounted? | Noa |
| 9.Is an incremental analysis performed? | Yes |
| 10.Is uncertainty assessed? | ? |
| 1 | Study | Schuttelaar, 2011109 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Research question | To determine costs and cost-effectiveness of care provided by NPs vs. dermatologists and to compare the results with those in studies from other countries | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | Country/setting | The Netherlands | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | Funding source | Health Care Efficiency Research Programme of the University Medical Centre Groningen, Groningen, the Netherlands | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | Analysis type | CEA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | Study type | Economic evaluation based on the results of a RCT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | Perspective | Societal | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | Time horizon | Trial duration: 1 year | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | Analysis method | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | Discounting (rate) | None | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | Costing year, currency | 2008, € | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | Population | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Childhood eczema (mild, moderate and severe) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients aged ≤ 16 years with a diagnosis of eczema (‘atopic dermatitis’) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | Intervention(s), comparator(s) | Intervention: care by a NP Comparator: conventional care by a dermatologist |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 | Outcome measure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Between-group differences in the QoL of the child between baseline and follow-up at 12 months | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The outcome measure were collected as primary end point of the RCT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean difference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | Health benefits | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Scores assigned to individual health dimensions as well as patient satisfaction within the instruments were added to obtain an overall score | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For children aged < 4 years, IDQoL was completed by their parents. Those aged 4–16 years completed CDLQI. For the cost-effectiveness analysis, the severity of eczema was measured by the mean objective SCORAD Patient satisfaction at 12 months was measured by CSQ-8 which were completed by the parents |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 | Costs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intervention cost Types of costUnit price (£)Health-care costs hospitalVisits dermatologista1.66 per minuteVisit NPb0.52 per minutePhone consultations dermatologistc8.30 per minutePhone consultations NPd5.20 per minutePrescriptionsDiverseLaboratory testsDiverseAdmission day512 per dayGroup education session by the NPDiverseHealth-care costs communityVisits GP21.70 per visitPrescriptionsVariableCosts in other sectorsHome help visits32.97 per visitaFirst visit 20 minutes, follow-up visits 10 minutes.bFirst visit NP 30 minutes, follow-up visits 20 minutes.cPhone consultations 5 minutes.dPhone consultations NP 10 minutes. Estimates of unit costs were based on the Dutch guideline prices. Costs of medications were based on the listed prices, including value added tax, obtained from the website of the Dutch Health Insurance Board (www.fk.cvz.nl) |
Types of cost | Unit price (£) | Health-care costs hospital | Visits dermatologista | 1.66 per minute | Visit NPb | 0.52 per minute | Phone consultations dermatologistc | 8.30 per minute | Phone consultations NPd | 5.20 per minute | Prescriptions | Diverse | Laboratory tests | Diverse | Admission day | 512 per day | Group education session by the NP | Diverse | Health-care costs community | Visits GP | 21.70 per visit | Prescriptions | Variable | Costs in other sectors | Home help visits | 32.97 per visit | aFirst visit 20 minutes, follow-up visits 10 minutes.bFirst visit NP 30 minutes, follow-up visits 20 minutes.cPhone consultations 5 minutes.dPhone consultations NP 10 minutes. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Types of cost | Unit price (£) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health-care costs hospital | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Visits dermatologista | 1.66 per minute | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Visit NPb | 0.52 per minute | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phone consultations dermatologistc | 8.30 per minute | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phone consultations NPd | 5.20 per minute | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prescriptions | Diverse | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory tests | Diverse | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Admission day | 512 per day | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group education session by the NP | Diverse | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health-care costs community | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Visits GP | 21.70 per visit | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prescriptions | Variable | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs in other sectors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Home help visits | 32.97 per visit | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aFirst visit 20 minutes, follow-up visits 10 minutes.bFirst visit NP 30 minutes, follow-up visits 20 minutes.cPhone consultations 5 minutes.dPhone consultations NP 10 minutes. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indirect costs Indirect costs were not reported explicitly in the study but based on the types of costs included; the reviewer regarded family costs as indirect costsTypes of costsUnit prices (£)Family Costs Absence from work37.23 per hour Travelling expensesa6.72/4.04 per visit Out-of-pocketVariableaTravelling expenses determined as standard price for private car/public transport based on mean distance to hospital. |
Types of costs | Unit prices (£) | Family Costs | Absence from work | 37.23 per hour | Travelling expensesa | 6.72/4.04 per visit | Out-of-pocket | Variable | aTravelling expenses determined as standard price for private car/public transport based on mean distance to hospital. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Types of costs | Unit prices (£) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Family Costs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Absence from work | 37.23 per hour | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Travelling expensesa | 6.72/4.04 per visit | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Out-of-pocket | Variable | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aTravelling expenses determined as standard price for private car/public transport based on mean distance to hospital. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 | Results Overall results
Children aged < 4 yearsChildren aged 4–16 yearsDermatologist group33.4 (19.3)35.4 (17.3)NP group33.4 (15.6)29.9 (16.0)
Clinical effectiveness MeasureBaseline (SD) [95% CI]12 months (SD) [95% CI]Mean change (SD) [95% CI]IDQOLDermatologist group11.6 (SD 8.1) [9.0 to 14.2]5.6 (SD 3.9) [4.3 to 7.0]–6.5 (SD 6.6) [–14.2 to –8.9]NP group10.7 (SD 4.9) [9.1 to 12.3]5.7 (SD 5.4) [ 4.0 to 7.5]–4.9 (SD 5.5) [ –6.8 to –3.0]CDLQIDermatologist group12.1 (SD 6.3) [9.9 to 14.2]5.6 (SD 4.2) [4.2 to 7.1]–5.9 (SD 6.0) [–8.0 to –3.9]NP group10.0 (SD 4.4) [8.5 to 11.4]4.9 (SD 3.5) [3.7 to 6.1]–5.2 (SD 4.0) [6.6 to –3.8]CSQ-8Dermatologist group–24.8 (SD 4.3) [23.6 to 26.0]–NP group–26.9 (SD 4.9) [25.5 to 28.2]–Costs
NP (n = 76)Dermatologist (n = 71)Difference (95% CI)aHealth-care costs hospital632 (1198)771 (1590)–139 (–520 to 291)Health-care costs community26 (39)30 (59)–4 (–17 to 12)Family costs302 (511)608 (1018)–306 (–475 to –16)Costs other sectors21 (182)0.93 (7.83)20 (–3 to 59)Total costs981 (1339)1409 (2289)–428 (–910 to 197)aNegative cost differences represent lower cost in the NP arm.
Note: the individual costs within each sub-group do not add to the total costs reported
The point estimate for ICER was €925 implying that one point less improvement in IDQOL in the NP group compared to the dermatologist group at 1 year would save €925 Bootstrapping results showed a 95% CI of –€5748 to €6667 for the ICER
|
Children aged < 4 years | Children aged 4–16 years | Dermatologist group | 33.4 (19.3) | 35.4 (17.3) | NP group | 33.4 (15.6) | 29.9 (16.0) | Measure | Baseline (SD) [95% CI] | 12 months (SD) [95% CI] | Mean change (SD) [95% CI] | IDQOL | Dermatologist group | 11.6 (SD 8.1) [9.0 to 14.2] | 5.6 (SD 3.9) [4.3 to 7.0] | –6.5 (SD 6.6) [–14.2 to –8.9] | NP group | 10.7 (SD 4.9) [9.1 to 12.3] | 5.7 (SD 5.4) [ 4.0 to 7.5] | –4.9 (SD 5.5) [ –6.8 to –3.0] | CDLQI | Dermatologist group | 12.1 (SD 6.3) [9.9 to 14.2] | 5.6 (SD 4.2) [4.2 to 7.1] | –5.9 (SD 6.0) [–8.0 to –3.9] | NP group | 10.0 (SD 4.4) [8.5 to 11.4] | 4.9 (SD 3.5) [3.7 to 6.1] | –5.2 (SD 4.0) [6.6 to –3.8] | CSQ-8 | Dermatologist group | – | 24.8 (SD 4.3) [23.6 to 26.0] | – | NP group | – | 26.9 (SD 4.9) [25.5 to 28.2] | – | NP (n = 76) | Dermatologist (n = 71) | Difference (95% CI)a | Health-care costs hospital | 632 (1198) | 771 (1590) | –139 (–520 to 291) | Health-care costs community | 26 (39) | 30 (59) | –4 (–17 to 12) | Family costs | 302 (511) | 608 (1018) | –306 (–475 to –16) | Costs other sectors | 21 (182) | 0.93 (7.83) | 20 (–3 to 59) | Total costs | 981 (1339) | 1409 (2289) | –428 (–910 to 197) | aNegative cost differences represent lower cost in the NP arm. | NP (n = 76) | Dermatologist (n = 71) | Difference (95% CI)a | Health-care costs hospital | Outpatient visits | 272 (143) | 422 (238) | –150 (–194 to –75) | Phone consultations | 7.22 (9.23) | 3.63 (7.72) | 3.59 (0.91 to 5.93) | Oral medication | 14 (36) | 19 (30) | –5 (–14 to 6) | Ointment active ingredients | 69 (80) | 87 (113) | –18 (–42 to 15) | Emollients | 17 (19) | 17 (22) | 0 (–6.48 to 5.92) | Bandages, dressings | 47 (69) | 26 (60) | 21 (2 to 40) | Laboratory tests | 9 (33) | 17 (40) | –8 (–17 to 4) | Hospital admission days | 179 (1133) | 163 (1376) | 16 (–334 to 380) | Group education, NP | 4.63 (7.91) | – | 4.63 (2.65 to 5.89) | Health-care cost community | General practitioner | 10 (28) | 18 (36) | –8 (–15 to 3) | Oral medicationb | 3.06 (13.42) | 2.81 (10.30) | 0.25 (–2.97 to 4.05) | Ointment with active ingredientsc | 6.54 (21.09) | 7.14 (40.20) | –0.86 (–10.43 to 7.62) | Emollients | 3.20 (5.79) | 1.45 (3.93) | 1.75 (0.28 to 3.08) | Protective dressingsd | 2.64 (11.07) | 0 (0) | 2.64 (0.48 to 4.88) | Total health-care costs | 658 (1213) | 801 (1607) | –143 (–544 to 299) | Family costs | Time costse | 178 (357) | 415 (735) | –237 (–360 to –37) | Travelling expenses | 20 (18) | 30 (26) | –10 (–13 to –1) | Bath oil | 21 (20) | 23 (26) | –2 (–8 to 6) | Out-of-pocketf | 83 (370) | 134 (684) | –51 (–221 to 97) | Costs other sectors | Home-help visits | 21 (182) | 0.93 (7.83) | 20 (–3 to 59) | aNegative cost differences represent lower costs in the NP arm.bAntibiotics, antihistamines.cSteroids, calcineurin inhibitors, tar.dBandages, garments and gloves.eTime missed in paid work and days missed in non-working activities of the parents.fSelf-medication, alternative practitioner, carpet changes, nutrition. | NP | Dermatologist | Mild eczema (n = 12) | Moderate eczema (n = 44) | Severe eczema (n = 20) | Mild eczema (n = 13) | Moderate eczema (n = 31) | Severe eczema (n = 29) | Health-care costs hospital | Outpatient visits | 178 (85) | 270 (147) | 340 (143) | 257 (152) | 404 (206) | 521 (260) | Phone consultations | 1.21 (1.99) | 6.55 (8.13) | 13 (12) | 3.93 (9.49) | 2.04 (6.33) | 5.17 (8.05) | Oral medication | 0 (0) | 15 (44) | 20 (26) | 16 (33) | 12 (21) | 28 (35) | Ointment active ingredients | 35 (50) | 71 (88) | 85 (72) | 26 (24) | 83 (122) | 118 (118) | Emollients | 18 (18) | 18 (21) | 13 (17) | 18 (12) | 16 (17) | 19 (30) | Bandages, dressings | 15 (29) | 45 (73) | 73 (70) | 16 (57) | 17 (49) | 40 (71) | Laboratory tests | 11 (34) | 12 (39) | 0.45 (2.0) | 7.62 (20.74) | 19 (33) | 19 (53) | Hospital admission days | 0 (0) | 0(0) | 6.80 (2.71) | 0 (0) | 0 (0) | 429 (2239) | Group education, NP | 4.4 (9.52) | 5.84 (8.48) | 2.10 (4.61) | – | – | – | Total | 287 (141) | 450 (280) | 1237 (2222) | 386 (149) | 567 (284) | 1192 (2528) | Health-care cost community | General practitioner | 11 (25) | 13 (33) | 3.3 (10.6) | 13 (24) | 11 (22) | 25 (47) | Oral medicationa | 0 (0) | 4.9 (17) | 0.9 (3.9) | 8.90 (17.40) | 1.69 (8.93) | 1.27 (6.00) | Ointment with active ingredientsb | 1.23 (3.4) | 3.16 (10.6) | 17 (36) | 8.30 (21.80) | 2.20 (6.68) | 12 (62) | Emollients | 3.97 (6.5) | 3.50 (6.3) | 2.1 (3.89) | 2.75 (6.25) | 1.17 (2.76) | 1.18 (3.72) | Protective dressingsc | 3.24 (8.60) | 1.15 (7.74) | 5.47 (16.90) | 0 (0) | 0 (0) | 0 (0) | Total | 19 (27) | 26 (40) | 30 (43) | 35 (57) | 17 (25) | 42 (82) | Total health-care costs | 307 (146) | 476 (275) | 1267 (2254) | 420 (144) | 584 (295) | 1234 (2553) | Family costs | Time costsd | 31 (72) | 153 (291) | 320 (520) | 315 (428) | 256 (396) | 645 (1048) | Travelling expenses | 13 (16) | 20 (17) | 26 (20) | 17 (13) | 25 (20) | 42 (32) | Bath oil | 17 (63) | 301 (560) | 440 (516) | 25 (50) | 22 (17) | 23 (22) | Out-of-pockete | 15 (28) | 110 (432) | 64 (113) | 33 (37) | 233 (1023) | 69 (201) | Total | 77 (63) | 301 (560) | 440 (516) | 366 (440) | 522 (1068) | 761 (1141) | Costs other sectors | Home-help visits | 0(0) | 36 (239) | 0 (0) | 0(0) | 0 (0) | 2.13 | Total costs all categories | 384 (128) | 814 (707) | 1707 (2256) | 811 (518) | 1128 (1100) | 2022 (3452) | aAntibiotics, antihistamines.bSteroids, calcineurin inhibitors, tar.cBandages, garments and gloves.dTime missed in paid work and days missed in non-working activities of the parents.eSelf-medication alternative practitioner, carpet changes, nutrition. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Children aged < 4 years | Children aged 4–16 years | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dermatologist group | 33.4 (19.3) | 35.4 (17.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NP group | 33.4 (15.6) | 29.9 (16.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Measure | Baseline (SD) [95% CI] | 12 months (SD) [95% CI] | Mean change (SD) [95% CI] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IDQOL | Dermatologist group | 11.6 (SD 8.1) [9.0 to 14.2] | 5.6 (SD 3.9) [4.3 to 7.0] | –6.5 (SD 6.6) [–14.2 to –8.9] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NP group | 10.7 (SD 4.9) [9.1 to 12.3] | 5.7 (SD 5.4) [ 4.0 to 7.5] | –4.9 (SD 5.5) [ –6.8 to –3.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CDLQI | Dermatologist group | 12.1 (SD 6.3) [9.9 to 14.2] | 5.6 (SD 4.2) [4.2 to 7.1] | –5.9 (SD 6.0) [–8.0 to –3.9] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NP group | 10.0 (SD 4.4) [8.5 to 11.4] | 4.9 (SD 3.5) [3.7 to 6.1] | –5.2 (SD 4.0) [6.6 to –3.8] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CSQ-8 | Dermatologist group | – | 24.8 (SD 4.3) [23.6 to 26.0] | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NP group | – | 26.9 (SD 4.9) [25.5 to 28.2] | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NP (n = 76) | Dermatologist (n = 71) | Difference (95% CI)a | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health-care costs hospital | 632 (1198) | 771 (1590) | –139 (–520 to 291) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health-care costs community | 26 (39) | 30 (59) | –4 (–17 to 12) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Family costs | 302 (511) | 608 (1018) | –306 (–475 to –16) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs other sectors | 21 (182) | 0.93 (7.83) | 20 (–3 to 59) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total costs | 981 (1339) | 1409 (2289) | –428 (–910 to 197) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aNegative cost differences represent lower cost in the NP arm. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NP (n = 76) | Dermatologist (n = 71) | Difference (95% CI)a | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health-care costs hospital | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outpatient visits | 272 (143) | 422 (238) | –150 (–194 to –75) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phone consultations | 7.22 (9.23) | 3.63 (7.72) | 3.59 (0.91 to 5.93) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oral medication | 14 (36) | 19 (30) | –5 (–14 to 6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ointment active ingredients | 69 (80) | 87 (113) | –18 (–42 to 15) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emollients | 17 (19) | 17 (22) | 0 (–6.48 to 5.92) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bandages, dressings | 47 (69) | 26 (60) | 21 (2 to 40) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory tests | 9 (33) | 17 (40) | –8 (–17 to 4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hospital admission days | 179 (1133) | 163 (1376) | 16 (–334 to 380) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group education, NP | 4.63 (7.91) | – | 4.63 (2.65 to 5.89) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health-care cost community | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General practitioner | 10 (28) | 18 (36) | –8 (–15 to 3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oral medicationb | 3.06 (13.42) | 2.81 (10.30) | 0.25 (–2.97 to 4.05) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ointment with active ingredientsc | 6.54 (21.09) | 7.14 (40.20) | –0.86 (–10.43 to 7.62) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emollients | 3.20 (5.79) | 1.45 (3.93) | 1.75 (0.28 to 3.08) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Protective dressingsd | 2.64 (11.07) | 0 (0) | 2.64 (0.48 to 4.88) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total health-care costs | 658 (1213) | 801 (1607) | –143 (–544 to 299) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Family costs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Time costse | 178 (357) | 415 (735) | –237 (–360 to –37) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Travelling expenses | 20 (18) | 30 (26) | –10 (–13 to –1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bath oil | 21 (20) | 23 (26) | –2 (–8 to 6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Out-of-pocketf | 83 (370) | 134 (684) | –51 (–221 to 97) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs other sectors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Home-help visits | 21 (182) | 0.93 (7.83) | 20 (–3 to 59) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aNegative cost differences represent lower costs in the NP arm.bAntibiotics, antihistamines.cSteroids, calcineurin inhibitors, tar.dBandages, garments and gloves.eTime missed in paid work and days missed in non-working activities of the parents.fSelf-medication, alternative practitioner, carpet changes, nutrition. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NP | Dermatologist | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mild eczema (n = 12) | Moderate eczema (n = 44) | Severe eczema (n = 20) | Mild eczema (n = 13) | Moderate eczema (n = 31) | Severe eczema (n = 29) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health-care costs hospital | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outpatient visits | 178 (85) | 270 (147) | 340 (143) | 257 (152) | 404 (206) | 521 (260) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phone consultations | 1.21 (1.99) | 6.55 (8.13) | 13 (12) | 3.93 (9.49) | 2.04 (6.33) | 5.17 (8.05) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oral medication | 0 (0) | 15 (44) | 20 (26) | 16 (33) | 12 (21) | 28 (35) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ointment active ingredients | 35 (50) | 71 (88) | 85 (72) | 26 (24) | 83 (122) | 118 (118) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emollients | 18 (18) | 18 (21) | 13 (17) | 18 (12) | 16 (17) | 19 (30) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bandages, dressings | 15 (29) | 45 (73) | 73 (70) | 16 (57) | 17 (49) | 40 (71) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory tests | 11 (34) | 12 (39) | 0.45 (2.0) | 7.62 (20.74) | 19 (33) | 19 (53) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hospital admission days | 0 (0) | 0(0) | 6.80 (2.71) | 0 (0) | 0 (0) | 429 (2239) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group education, NP | 4.4 (9.52) | 5.84 (8.48) | 2.10 (4.61) | – | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 287 (141) | 450 (280) | 1237 (2222) | 386 (149) | 567 (284) | 1192 (2528) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health-care cost community | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General practitioner | 11 (25) | 13 (33) | 3.3 (10.6) | 13 (24) | 11 (22) | 25 (47) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oral medicationa | 0 (0) | 4.9 (17) | 0.9 (3.9) | 8.90 (17.40) | 1.69 (8.93) | 1.27 (6.00) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ointment with active ingredientsb | 1.23 (3.4) | 3.16 (10.6) | 17 (36) | 8.30 (21.80) | 2.20 (6.68) | 12 (62) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emollients | 3.97 (6.5) | 3.50 (6.3) | 2.1 (3.89) | 2.75 (6.25) | 1.17 (2.76) | 1.18 (3.72) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Protective dressingsc | 3.24 (8.60) | 1.15 (7.74) | 5.47 (16.90) | 0 (0) | 0 (0) | 0 (0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 19 (27) | 26 (40) | 30 (43) | 35 (57) | 17 (25) | 42 (82) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total health-care costs | 307 (146) | 476 (275) | 1267 (2254) | 420 (144) | 584 (295) | 1234 (2553) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Family costs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Time costsd | 31 (72) | 153 (291) | 320 (520) | 315 (428) | 256 (396) | 645 (1048) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Travelling expenses | 13 (16) | 20 (17) | 26 (20) | 17 (13) | 25 (20) | 42 (32) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bath oil | 17 (63) | 301 (560) | 440 (516) | 25 (50) | 22 (17) | 23 (22) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Out-of-pockete | 15 (28) | 110 (432) | 64 (113) | 33 (37) | 233 (1023) | 69 (201) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 77 (63) | 301 (560) | 440 (516) | 366 (440) | 522 (1068) | 761 (1141) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs other sectors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Home-help visits | 0(0) | 36 (239) | 0 (0) | 0(0) | 0 (0) | 2.13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total costs all categories | 384 (128) | 814 (707) | 1707 (2256) | 811 (518) | 1128 (1100) | 2022 (3452) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aAntibiotics, antihistamines.bSteroids, calcineurin inhibitors, tar.cBandages, garments and gloves.dTime missed in paid work and days missed in non-working activities of the parents.eSelf-medication alternative practitioner, carpet changes, nutrition. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 |
Sensitivity analysis
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 | Author’s conclusions | The authors concluded that care by NPs was cost-saving as well as cost-effective compared with care by dermatologists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20 | Reviewer’s comments | The analysis was well-conducted; detailed descriptions of the model parameters were presented. Uncertainty was accounted for; but probabilistic sensitivity analysis was not conducted. However, the findings of the analyses did not match exactly with the study conclusion. This is considered to be a limitation of the study. Secondly, the different sub-group of costs reported across the different levels of disease severity did not match the sum total of the individual costs components within the sub group | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Systematic review quality-assessment checklist for economic evaluations and notes for completion
Critical appraisal checklist for economic evaluations (based on Drummond et al. , 2005110)
| Item | Y/N? |
|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Yes |
| 2. Is the setting comparable to the UK? | No |
| 3. Is the analytical and modelling methodology appropriate? | ? |
| 4. Are all the relevant costs and consequences for each alternative identified? | Yes |
| 5. Are the data inputs for the model described and justified? | Yes |
| 6. Are health outcomes measured in QALYs? | No |
| 7. Is the time horizon considered appropriate? | Noa |
| 8. Are costs and outcomes discounted? | N/Ab |
| 9. Is an incremental analysis performed? | Yes |
| 10. Is uncertainty assessed? | Yes |
| 1 | Study | van Os-Medendorp, 2008107 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Research question | To assess medical consumption, health-care costs and costs due to loss of work in patients with chronic pruritic skin diseases enrolled in the nursing programme ‘Coping with itch’ compared with a control group of patients receiving the usual dermatological care | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | Country/setting | The Netherlands | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | Funding source | Dutch College of Health Insurance (CVZ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | Analysis type | CEA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | Study type | Economic evaluation based on the results of a RCT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | Perspective | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | Time horizon | Trial duration: 9 months | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | Analysis method | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | Discounting (rate) | None | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | Costing year, currency | Not stated, € | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | Population | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Patients with chronic pruritic skin diseases | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients with chronic pruritic skin diseases aged 18 years or older, regardless of the underlying diagnosis who visited dermatology outpatient department of the UMC of Utrecht, Leiden or Rotterdam or the dermatology outpatient department of the Meander Medical Centre in Amersfoort, the Netherlands | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | Intervention(s), comparator(s) | Intervention: usual medical care from a dermatologist as well as nursing care according to the programme ‘Coping with itch’. The programme was carried out in a specialised itch clinic run by dermatology nurses who provided individual sessions at the dermatology outpatient department, while medical treatment by the dermatologists continued as usual. The programme consisted of educational and cognitive behavioural interventions, such as individual patient education, awareness training and habit reversal, and relaxation exercises Comparator: usual dermatologist care, comprising diagnostic tests and subsequent therapeutic intervention, such as the use of emollients and topical steroids |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 | Outcome measure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Frequency of itching and scratching | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Data for the primary outcome was recorded in diaries by patients in the RCT that assessed the clinical effectiveness of the nursing programme ‘Coping with itch’ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean difference collected from the RCT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | Health benefits | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Factor analysis was applied to the data on the number of times patients felt itchy or scratched to reduce to one factor: the factor of itching/scratching which was converted to a dichotomous measure: high (> 4) and low (≤ 4) frequency of itching/scratching Health benefits were expressed in terms of days with a low frequency of itching and scratching |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| None used | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 | Costs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intervention cost All the costs are expressed in €Months 1–3Months 1–9Intervention (n = 22)Comparator (n = 26)Difference (95% CI)Intervention (n = 25)Comparator (n = 31)Difference (95% CI)Costs at the dermatology outpatient department, mean (± SD)Costs of visit to the dermatologist212.7 (± 220.8)303.7 (± 548.9)–91.0 (–325.3 to 143.3)742.0 (± 507.0)770.7 (± 980.1)–28.7 (–495.0 to 437.6)Costs of visit to the dermatology nurse133.3 (± 64.2)26.3 (± 40.8)107.1 (77.2 to 137.0)232.3 (± 145.1)34.2 (± 52.3)198.2 (131.1 to 265.2)Costs of ultraviolet therapy47.2 (± 103.3)22.4 (± 72.4)24.8 (–24.5 to 74.1)52.6 (± 106.6)56.2 (± 122.7)–3.7 (–71.1 to 63.7)Costs of visits to a medical social worker9.2 (± 45.8)2.5 (± 13.7)6.7 (–10.6 to 24.0)10.4 (± 48.8)2.9 (± 14.6)7.5 (–12.8 to 27.7)Total costs at the dermatology outpatient department402.3 (± 263.3)354.8 (± 554.6)47.5 (–194.5 to 289.6)1037.3 (± 614.9)864.0 (± 1019.3)173.2 (–327.4 to 673.9)Costs of visits to the GP40.0 (± 37.1)42.1 (± 39.1)–2.0 (–22.6 to 18.6)72.1 (± 56.0)119.9 (± 93.2)–39.8 (–83.9 to 4.3)Costs of visits to other HCPs238.2 (± 252.9)217.6 (± 296.5)20.6 (–129.2 to 170.4)668.1 (± 813.2)521.0 (± 496.8)147.1 (–237.9 to 532.1)Costs of medication243.5 (± 459.8)129.4 (± 167.4)114.1 (–64.3 to 292.5)559.5 (± 630.3)426.6 (± 486.9)132.9 (–191.9 to 457.6)Costs of days off work1320.8 (± 3601.4)343.0 (± 1271.6)977.8 (–568.0 to 2523.6)1946.2 (± 4852.0)1302.3 (± 4285.1)643.8 (–2010.8 to 3298.5)Costs of hospitalisations357.7 (± 1271.0)721.2 (± 2585.3)–363.5 (–1498.0 to 771.0)757.6 (± 1866.2)1250.8 (± 3617.5)–493.2 (–2213.3 to 1226.9)Total costs2602.6 (± 3841.7)1808.0 (± 2949.2)794.5 (–1024.4 to 2603.5)5040.6 (± 5442.4)4476.7 (± 5822.9)564.0 (–2731.9 to 3859.9) Costs of visits to health-care workers, costs of days off work and hospitalisations were based on the guidelines for cost studies of the Dutch College of Health Insurance (CVZ), whereas medication costs were obtained from the Dutch website: www.medicijnkosten.nl |
Months 1–3 | Months 1–9 | Intervention (n = 22) | Comparator (n = 26) | Difference (95% CI) | Intervention (n = 25) | Comparator (n = 31) | Difference (95% CI) | Costs at the dermatology outpatient department, mean (± SD) |
|
212.7 (± 220.8) | 303.7 (± 548.9) | –91.0 (–325.3 to 143.3) | 742.0 (± 507.0) | 770.7 (± 980.1) | –28.7 (–495.0 to 437.6) |
|
133.3 (± 64.2) | 26.3 (± 40.8) | 107.1 (77.2 to 137.0) | 232.3 (± 145.1) | 34.2 (± 52.3) | 198.2 (131.1 to 265.2) |
|
47.2 (± 103.3) | 22.4 (± 72.4) | 24.8 (–24.5 to 74.1) | 52.6 (± 106.6) | 56.2 (± 122.7) | –3.7 (–71.1 to 63.7) |
|
9.2 (± 45.8) | 2.5 (± 13.7) | 6.7 (–10.6 to 24.0) | 10.4 (± 48.8) | 2.9 (± 14.6) | 7.5 (–12.8 to 27.7) |
|
402.3 (± 263.3) | 354.8 (± 554.6) | 47.5 (–194.5 to 289.6) | 1037.3 (± 614.9) | 864.0 (± 1019.3) | 173.2 (–327.4 to 673.9) | Costs of visits to the GP | 40.0 (± 37.1) | 42.1 (± 39.1) | –2.0 (–22.6 to 18.6) | 72.1 (± 56.0) | 119.9 (± 93.2) | –39.8 (–83.9 to 4.3) | Costs of visits to other HCPs | 238.2 (± 252.9) | 217.6 (± 296.5) | 20.6 (–129.2 to 170.4) | 668.1 (± 813.2) | 521.0 (± 496.8) | 147.1 (–237.9 to 532.1) | Costs of medication | 243.5 (± 459.8) | 129.4 (± 167.4) | 114.1 (–64.3 to 292.5) | 559.5 (± 630.3) | 426.6 (± 486.9) | 132.9 (–191.9 to 457.6) | Costs of days off work | 1320.8 (± 3601.4) | 343.0 (± 1271.6) | 977.8 (–568.0 to 2523.6) | 1946.2 (± 4852.0) | 1302.3 (± 4285.1) | 643.8 (–2010.8 to 3298.5) | Costs of hospitalisations | 357.7 (± 1271.0) | 721.2 (± 2585.3) | –363.5 (–1498.0 to 771.0) | 757.6 (± 1866.2) | 1250.8 (± 3617.5) | –493.2 (–2213.3 to 1226.9) | Total costs | 2602.6 (± 3841.7) | 1808.0 (± 2949.2) | 794.5 (–1024.4 to 2603.5) | 5040.6 (± 5442.4) | 4476.7 (± 5822.9) | 564.0 (–2731.9 to 3859.9) | ||||||||||||
| Months 1–3 | Months 1–9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intervention (n = 22) | Comparator (n = 26) | Difference (95% CI) | Intervention (n = 25) | Comparator (n = 31) | Difference (95% CI) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs at the dermatology outpatient department, mean (± SD) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
212.7 (± 220.8) | 303.7 (± 548.9) | –91.0 (–325.3 to 143.3) | 742.0 (± 507.0) | 770.7 (± 980.1) | –28.7 (–495.0 to 437.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
133.3 (± 64.2) | 26.3 (± 40.8) | 107.1 (77.2 to 137.0) | 232.3 (± 145.1) | 34.2 (± 52.3) | 198.2 (131.1 to 265.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
47.2 (± 103.3) | 22.4 (± 72.4) | 24.8 (–24.5 to 74.1) | 52.6 (± 106.6) | 56.2 (± 122.7) | –3.7 (–71.1 to 63.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
9.2 (± 45.8) | 2.5 (± 13.7) | 6.7 (–10.6 to 24.0) | 10.4 (± 48.8) | 2.9 (± 14.6) | 7.5 (–12.8 to 27.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
402.3 (± 263.3) | 354.8 (± 554.6) | 47.5 (–194.5 to 289.6) | 1037.3 (± 614.9) | 864.0 (± 1019.3) | 173.2 (–327.4 to 673.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs of visits to the GP | 40.0 (± 37.1) | 42.1 (± 39.1) | –2.0 (–22.6 to 18.6) | 72.1 (± 56.0) | 119.9 (± 93.2) | –39.8 (–83.9 to 4.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs of visits to other HCPs | 238.2 (± 252.9) | 217.6 (± 296.5) | 20.6 (–129.2 to 170.4) | 668.1 (± 813.2) | 521.0 (± 496.8) | 147.1 (–237.9 to 532.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs of medication | 243.5 (± 459.8) | 129.4 (± 167.4) | 114.1 (–64.3 to 292.5) | 559.5 (± 630.3) | 426.6 (± 486.9) | 132.9 (–191.9 to 457.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs of days off work | 1320.8 (± 3601.4) | 343.0 (± 1271.6) | 977.8 (–568.0 to 2523.6) | 1946.2 (± 4852.0) | 1302.3 (± 4285.1) | 643.8 (–2010.8 to 3298.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs of hospitalisations | 357.7 (± 1271.0) | 721.2 (± 2585.3) | –363.5 (–1498.0 to 771.0) | 757.6 (± 1866.2) | 1250.8 (± 3617.5) | –493.2 (–2213.3 to 1226.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total costs | 2602.6 (± 3841.7) | 1808.0 (± 2949.2) | 794.5 (–1024.4 to 2603.5) | 5040.6 (± 5442.4) | 4476.7 (± 5822.9) | 564.0 (–2731.9 to 3859.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indirect costs To estimate the costs of days off work, an overall mean hourly productivity cost of €34.98 was applied for both men and women |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 | ResultsCostsBenefits (days with a low frequency of itching and scratching)Cost-effectiveness ratioInterventionControlDifference after bootstrap analyses (95% CI)InterventionControlDifference after bootstrap analyses (95% CI)Months 1–3a €2602.6€1808.0€793.8 (–970.3 to 2692.7)34.328.46.1 (–15.7 to 27.8)€129.9Months 1–9b €5040.6€4476.7€582.0 (–2730.0 to 3877.0)123.087.635.0 (–33.0 to 96.0)€16.6aIntervention group n = 25, control group n = 31.bIntervention group n = 22, control group n = 27. | Costs | Benefits (days with a low frequency of itching and scratching) | Cost-effectiveness ratio | Intervention | Control | Difference after bootstrap analyses (95% CI) | Intervention | Control | Difference after bootstrap analyses (95% CI) | Months 1–3a | €2602.6 | €1808.0 | €793.8 (–970.3 to 2692.7) | 34.3 | 28.4 | 6.1 (–15.7 to 27.8) | €129.9 | Months 1–9b | €5040.6 | €4476.7 | €582.0 (–2730.0 to 3877.0) | 123.0 | 87.6 | 35.0 (–33.0 to 96.0) | €16.6 | aIntervention group n = 25, control group n = 31.bIntervention group n = 22, control group n = 27. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Costs | Benefits (days with a low frequency of itching and scratching) | Cost-effectiveness ratio | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intervention | Control | Difference after bootstrap analyses (95% CI) | Intervention | Control | Difference after bootstrap analyses (95% CI) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Months 1–3a | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| €2602.6 | €1808.0 | €793.8 (–970.3 to 2692.7) | 34.3 | 28.4 | 6.1 (–15.7 to 27.8) | €129.9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Months 1–9b | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| €5040.6 | €4476.7 | €582.0 (–2730.0 to 3877.0) | 123.0 | 87.6 | 35.0 (–33.0 to 96.0) | €16.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aIntervention group n = 25, control group n = 31.bIntervention group n = 22, control group n = 27. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 | Sensitivity analysis The authors assessed the implications of variation in cut-off score of the frequency of itching and scratching on the cost-effectiveness results. Using a cut-off score of 3 or 5, 61% of patients experienced benefits from the intervention, and 12% had lower costs after 3 months. After 9 months, 63% and 78% of patients experienced benefits using a cut-off score of 3 or 5, respectively and 22% and 28% of patients, respectively, had lower costs |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 | Author’s conclusions | It was observed that, although the first 3 months of the programme ‘Coping with itch’ incurred most of the expenses, the benefits (in terms of days with little itch) accrued from the programme persisted and increased beyond this period. Based on this observation, the authors concluded that the ‘Coping with itch’ programme led to a more favourable ICER | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20 | Reviewer’s comments | Overall, the analysis was well conducted. The results of the analysis were in line with study conclusions. Sources of model parameters were well-defined. Price year was not reported. Although the study accounted for uncertainty through bootstrapping, probabilistic sensitivity analysis was not conducted. Only two sets of one-way sensitivity analyses were conducted. The analysis accounted for a time horizon of 9 months; however a longer time horizon would have been appropriate. Discounting of costs and benefits were not performed which was considered appropriate given the time horizon of the model was < 1 year | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Systematic review quality-assessment checklist for economic evaluations and notes for completion
Critical appraisal checklist for economic evaluations (based on Drummond et al. , 2005110)
| Item | Y/N? |
|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Yes |
| 2. Is the setting comparable to the UK? | No |
| 3. Is the analytical and modelling methodology appropriate? | ? |
| 4. Are all the relevant costs and consequences for each alternative identified? | Yes |
| 5. Are the data inputs for the model described and justified? | Yes |
| 6. Are health outcomes measured in QALYs? | No |
| 7. Is the time horizon considered appropriate? | No |
| 8. Are costs and outcomes discounted? | Noa |
| 9. Is an incremental analysis performed? | Yes |
| 10. Is uncertainty assessed? | Yesb |
List of abbreviations
- ACS
- Adjustment to Chronic Skin Diseases Questionnaire
- BDI
- Beck Depression Inventory
- BSA
- body surface area
- CCT
- controlled clinical trial
- CDLQI
- Children’s Dermatology Life Quality Index
- CI
- confidence interval
- CSQ-8
- Client Satisfaction Questionnaire-8
- DESMOND
- Diabetes Education and Self Management for ONgoing and Diagnosed
- DFI
- Dermatitis Family Impact
- DLQI
- Dermatology Life Quality Index
- DVD
- digital versatile disc
- EASI
- Eczema Area and Severity Index
- EQ-5D™
- European Quality of Life-5 Dimensions
- ESP
- educational support programme
- HCP
- health-care professional
- HRQoL
- health-related quality of life
- IC
- integrated care
- ICER
- incremental cost-effectiveness ratio
- ICQ
- Itching Cognitions Questionnaire
- IDQoL
- Infants’ Dermatitis Quality of Life Index
- ITT
- intention to treat
- JUCKKI
- Juckreiz-Kognitions-Fragebogen Kinder
- JUCKKU
- Juckreiz-Kognitions-Fragebogen Jugendliche
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NP
- nurse practitioner
- OTC
- over the counter
- PASI
- Psoriasis Area Severity Index
- PDI
- Psoriasis Disability Index
- PETS
- Problematic Experiences of Therapy Scale
- PGA
- physician’s global assessment
- POEM
- Patient-Oriented Eczema Measure
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QoLIAD
- Quality of Life Index for Atopic Dermatitis
- QPCAD
- Quality of life in Primary Caregivers of children with Atopic Dermatitis
- RCT
- randomised controlled trial
- SAPASI
- Self-Administered Psoriasis Area Severity Index
- SCORAD
- SCORing Atopic Dermatitis
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SIGN
- Scottish Intercollegiate Guidelines Network
- SMS
- short message service
- SPaCE
- Supporting Parents and Carers of Children with Eczema
- VAS
- visual analogue scale
- WHOQOL-26
- World Health Organization Quality of Life-26 items