Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 14/03/01. The protocol was agreed in April 2014. The assessment report began editorial review in November 2014 and was accepted for publication in March 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Nicholson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and definition of the decision problem
Brief description of the decision problem
There is no single definitive test for prostate cancer. In cases where prostate cancer could be the cause of presenting symptoms, the general practitioner (GP) carries out a number of tests. If, after carrying out this exploratory work, the GP feels that there is a risk of prostate cancer, then the patient will be referred to a hospital consultant to discuss the options for further tests.
The most commonly used test to detect prostate cancer is a transrectal ultrasonography (TRUS)-guided biopsy. However, this biopsy has a number of limitations. It can miss cancers altogether, it may identify small, low-risk cancers that do not need to be treated but the presence of which will cause anxiety, it is uncomfortable (sometimes painful) and there can be complications for the patient (including blood in semen and urine, rectal bleeding, voiding difficulties, and major and minor infections). 1 In some cases where prostate cancer has not been confirmed by the initial biopsy, a second biopsy may be recommended; however, there is no guarantee that the second biopsy will find cancers missed by the first biopsy and further biopsies may still be performed. Techniques such as magnetic resonance spectroscopy (MRS) and enhanced magnetic resonance imaging (MRI) have been introduced into diagnostic practice. Such techniques aid the localisation of prostate cancer abnormalities, thus improving the diagnostic performance of biopsies. However, MRS and MRI are not available in all hospitals.
The PROGENSA® prostate cancer antigen 3 (PCA3) assay (referred to as the PCA3 assay; Hologic Gen-Probe, Marlborough, MA, USA) and the Beckman Coulter Prostate Health Index (phi; Brea, CA, USA) are two new tests (a urine test and a blood test, respectively) that are designed to be used to help a clinician decide whether or not a repeat biopsy is necessary. The purpose of this assessment is to evaluate the clinical effectiveness and cost-effectiveness of these tests, in combination with existing tests, scans and clinical judgement, in the diagnosis of prostate cancer in men who are suspected of having malignant disease and in whom the results of an initial prostate biopsy were negative or equivocal. The perspective of this evaluation is the NHS in England and Wales.
This report contains reference to confidential information provided as part of the National Institute for Health and Care Excellence (NICE) appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Epidemiology of prostate cancer
The prostate is a gland that is part of the urinary and reproductive system of males. Women do not have a prostate gland. It is located in the pelvic region, beneath the bladder, and surrounds the upper part of the urethra, the tube that carries urine from the bladder through the penis. It has two functions: first, muscle fibres squeeze the urethra slightly and help control the flow of urine, and, second, the prostate is the site of production of fluids that are added to the seminal fluid (semen).
The prostate starts to develop before birth and grows rapidly during puberty, staying the same size or growing slowly in healthy adults. In a normal young adult male the gland is approximately 3 cm long and weighs approximately 20 g.
The prostate has three glandular regions, namely the peripheral zone, the central zone and the transition zone. 2 The vast majority of prostate cancers are adenocarcinomas (meaning that they originate from glandular epithelial cells). Up to 70% of cancers arise in the peripheral zone, 15–20% arise in the central zone and 10–15% arise in the transition zone. 3
The prognosis and natural history of prostate cancer vary depending on the extent of spread and the grade of cancer at diagnosis. The prognosis for men with disease localised to the prostate varies, and more aggressive changes on histopathology and higher prostate-specific antigen (PSA) levels are associated with a worse prognosis. 4 In the early stages, prostate cancer is localised to the prostate and its progression is driven by androgens. At this stage the disease may be cured with surgery or radiotherapy; alternatively, conservative management, that is active surveillance/watchful waiting, may be adopted. 5 Active surveillance involves regular tests to monitor the cancer. The tests are likely to vary by treatment centre but may include:
-
a PSA test every 3–6 months
-
a digital rectal examination (DRE) every 6–12 months
-
a biopsy about a year after diagnosis and every few years thereafter
-
a MRI scan if the patient’s PSA level and/or DRE result suggest the cancer is growing.
If the results of a test show that the cancer has grown, the patient will be offered curative treatment, for example surgery or radiotherapy. 6 Watchful waiting differs slightly from active surveillance. It is an approach that is generally suitable for men with other health problems who may be physically less able to cope with treatments or whose cancer may never cause major health problems during their lifetime. Active surveillance usually involves fewer tests, and these usually take place at the GP surgery rather than at a hospital. 6
Patients who have inoperable locally advanced or metastatic disease at diagnosis or who have inoperable recurrent disease are treated with androgen deprivation therapy. As the disease progresses, the tumour ceases to respond to androgen deprivation therapy, but may respond to antiandrogens and oestrogenic agents. 7 Most patients receive two or more hormonal therapies and are then offered chemotherapy. 8
Incidence
The most up-to-date figures (2011) indicate that prostate cancer is the most common cancer in men in the UK, accounting for 25% of all new cases of cancer in males. 9 In the same year, there were 35,567 new cases in England and 2346 new cases in Wales, giving a total of 37,913. 9 Age-standardised relative survival rates for prostate cancer in England during 2005–9 show that 93.5% of men with prostate cancer are expected to survive for at least 1 year, falling to 81.4% surviving 5 years or more. Survival rates in Wales are reported to be broadly similar to those in England. 10
Prostate cancer incidence is strongly related to age, with the highest incidence rates being in older men. In the UK between 2009 and 2011, an average of 36% of cases were diagnosed in men aged 75 years and over, and only 1% were diagnosed in the under-fifties. 9 There is also evidence of an inverse association between prostate cancer incidence and deprivation in England, with prostate cancer being one of the few cancers with incidence rates lower among more-deprived males. 9 England-wide data for 2006–10 show that European age-standardised incidence rates are 17% lower for men living in the most deprived areas than for those in the least deprived areas. 9 In addition, there are links between prostate cancer and ethnicity. Age-standardised rates for white men with prostate cancer range from 96.0 to 99.9 per 100,000. Rates for Asian men are significantly lower, ranging from 28.7 to 60.6 per 100,000, while the rates for black men are significantly higher, ranging from 120.8 to 247.9 per 100,000. 9
Mortality
Prostate cancer is the second most common cause of death due to cancer in men in England and Wales, second only to lung cancer. 11 Age-standardised mortality rates from prostate cancer declined by 13% between 2001 and 2012. 12 In 2012, there were 9133 from prostate cancer in England and 5556 deaths in Wales.
Quality of life of patients with prostate cancer
Glaser et al. 13 used a questionnaire survey to collect information about the quality of life (QoL) of patients with different types of cancer. Of the 1248 prostate cancer patients targeted, 866 (69.4%) returned completed questionnaires. The analysis indicated that patients who had surgery only (compared with radiotherapy and hormone treatment) had significantly higher QoL scores. The survey also revealed that:
-
38.5% reported some degree of urinary leakage
-
12.9% reported difficulty controlling their bowels
-
58.4% reported being unable to have an erection
-
11.0% reported significant difficulty in having or maintaining an erection.
The presence of urinary leakage was significantly associated with lower QoL scores, while erectile dysfunction and difficulty controlling bowels were not significantly associated with a reduction in QoL score.
Financial cost of prostate cancer
Biopsy cost
A study14 was carried out to assess the diagnostic accuracy and cost-effectiveness of MRS and enhanced MRI techniques to aid the localisation of prostate abnormalities in a population undergoing repeat biopsy. Following this approach, assuming that approximately 25% of cancers are detected by repeat TRUS-guided needle biopsy15 and that the cancer detection rate is approximately 25%,16,17 then, based on a figure of 37,913 cases of prostate cancer in England and Wales, it can be assumed that 38,000 repeat biopsies are undertaken. The 2012–13 NHS reference costs18 for the Healthcare Resource Group (HRG) of a needle biopsy of the prostate maps (LB27Z, outpatient procedure, urology) is £224, leading to a total cost to the NHS of approximately £8.5M in 2012–13. This figure should be considered as a lower limit for the cost of repeat biopsies, as it assumes that almost all men only receive a second biopsy and it takes little account of the cost of any subsequent biopsies.
First-year treatment cost
It has been estimated that the average first-year treatment cost per patient identified with prostate cancer is £2943.10 (2009 prices). 14 Inflating this cost to current prices (2012/13) results in a figure of £3167.72. 14 The number of cases in England and Wales in 2011 was 37,913, leading to an approximate first-year treatment cost of £120M. It should be noted that this is likely to be a conservative estimate, as the cost includes only active surveillance, radical prostatectomy and external beam radiation therapy. It does not include any other treatment costs, nor does it include any costs incurred by patients or the wider society. In addition, it is likely that this cost will rise even without any improvements in detection (and therefore incidence) because the population in the UK is ageing and, as the incidence of prostate cancer increases with age, it is likely that the number of cases of prostate cancer will increase over time. The number of patients treated and the cost of treatment are set to increase and this will lead to increased demand for resources (for example treatment facilities and trained specialists).
Current diagnostic practice
The recently updated NICE guideline,11 Prostate Cancer: Diagnosis and Treatment, CG175, summarises current best practice for the diagnosis and management of prostate cancer.
Decision to perform initial biopsy
According to the updated NICE guideline,11 men may initially present with clinical symptoms, such as difficulty with urination, or come to medical attention as the result of a raised PSA level. PSA is a protein produced in prostatic cell, which can be elevated in men with prostate cancer. However, it is also raised in other benign prostatic conditions, such as infections (prostatitis) and hypertrophy. A raised PSA is not, therefore, specific to the presence of cancer and not all men with prostate cancer have increased PSA levels. The decision whether or not to investigate for possible cancer is influenced by age as well as PSA level. Men in their fifties with PSA levels above 3 ng/ml are considered for further investigation, with threshold levels being 4 ng/ml for men in their sixties and 5 ng/ml for men in their seventies. 19 The updated NICE guideline11 recommends that the following factors should be taken into consideration when deciding to perform a biopsy: PSA level, DRE findings, comorbidities and individual risk factors such as increasing age, family history and Afro-Caribbean ethnicity. PSA level should not be used in isolation to guide clinician and patient decisions to biopsy.
Decision to perform a repeat biopsy
The NICE guideline11 reviewed evidence supporting the efficacy of various prognostic factors when used to determine the need for further investigation in men with a negative initial biopsy. The recommendations are as follows:
Recommendation 1: a core member of the urological cancer multidisciplinary team should review the risk factors of all men who have had a negative first prostate biopsy, and discuss with the man that the risk of prostate cancer is increased if any of the following risk factors is present:
-
the biopsy shows high-grade prostatic intraepithelial neoplasia (HGPIN)
-
the biopsy shows atypical small acinar proliferation (ASAP)
-
an abnormal DRE.
Recommendation 2: to consider multiparametric magnetic resonance imaging (mpMRI), using T2- and diffusion-weighted (DW) imaging, for men with a negative TRUS-guided 10- to 12-core biopsy, to determine whether or not another biopsy is needed.
Recommendation 3: do not offer another biopsy if the mpMRI, using T2-weighted and DW imaging, is negative, unless any of the risk factors listed in recommendation 1 are present.
However, in clinical practice, there may be considerable variation in the adherence to these recommendations.
Types of biopsy
Diagnosis usually relies on obtaining a biopsy for histopathological examination of prostate tissue. The prostate gland is situated deep in the pelvis and it is not easy to visualise. Needle biopsies of the prostate are obtained from the rectum under ultrasound control. The NICE guideline11 recommends that prostate biopsies should be carried out following the procedure advocated by the Prostate Cancer Risk Management Programme (2006), ‘undertaking a transrectal ultrasound (TRUS)[-]guided biopsy of the prostate’ (p. 123). 20 This Programme advises that ‘the prostate should be sampled through the rectum unless there is a specific condition that prevents this’ and also that ‘the scheme used at first biopsy should be a 10–12 core pattern that samples the mid-lobe peripheral zone and the lateral peripheral zone of the prostate only’ (section 11, Biopsy Scheme, p. 5).
In the UK NHS these initial TRUS biopsies are usually carried out under local anaesthetic as an outpatient or day-case procedure.
Transrectal ultrasonography biopsies are poor at accessing, and hence detecting, anterior, apical and central lesions. 21 Foci of cancerous cells may therefore be missed. If an initial biopsy fails to detect cancerous cells and the clinician still believes that cancer may be present, one or more repeat biopsies may be performed.
The second biopsy may be another standard TRUS biopsy with 10–12 cores. However, more often, an increased number of samples are taken. Men may prefer to have a general anaesthetic when undergoing a second biopsy, especially if they found the experience of their initial biopsy to be uncomfortable and/or distressing. The biopsy options include:
-
Saturation biopsy. A biopsy, which may be taken transrectally or transperineally, with an increased number of cores (minimum of 20).
-
Template biopsy. 25–40 biopsy cores are taken transperineally using a template or grid to access more areas of the prostate, including anterior and apical zones. In the UK, this procedure is usually performed under general anaesthetic.
-
Targeted biopsy. Information from a MRI is used to guide the biopsy to areas with disease (see Clinical assessment plus magnetic resonance imaging).
Prostate biopsies are recognised as being imperfect, and men with prostate cancer may have a negative prostate biopsy result. Prostate cancer detection rates vary by type of biopsy, number of cores taken and patient characteristics; published estimates are 14–22% for the initial biopsy, 10–28% for a second biopsy and 5–10% for a third biopsy. 17,22–24
Prostate biopsies are painful and associated with side effects. Relatively common minor complications include haematospermia, haematuria and rectal bleeding which subsides after intervention, while major complications, which are comparatively rare, include prostatitis, fever, sepsis, urinary retention, epididymitis and rectal bleeding for longer than 2 days. 1
Gleason score
A histopathologist reviews biopsy specimens. If cancerous cells are detected, the histopathology report includes the Gleason score;25 the Gleason score is a measure of the aggressiveness of the tumour. The Gleason score25 (range 2–10) describes the degree of abnormality of the tumour found in the biopsy. The higher the Gleason score, the more aggressive (and worse prognosis) the cancer.
The Gleason score25 is calculated by first assessing (using a microscope) the biopsy specimen for the degree of abnormality in the prostate tissue, which is categorised as one of five different Gleason patterns. Gleason pattern 1 is the most differentiated and therefore the most favourable, and pattern 5 is the most disrupted and aggressive. Pattern 3 is the most common. The Gleason score is obtained by adding together the number of the most widespread pattern (primary grade) and the number of the second most prevalent pattern (secondary grade). If a tumour has patterns 3 and 2, the score would be 5. If the tumour has only one pattern, or less than 5% of a secondary pattern, the single pattern is added to itself (e.g. 3 + 3 = 6). It is advised that the diagnosis of low-grade Gleason score 2–5 prostate carcinomas in the setting of needle biopsy should be made with extreme caution,25 as such a diagnosis on final radical prostatectomy is proved wrong most of the time. 26 Recent consensus is that diagnosed prostate cancer must have a minimum score of 6. 27,28 Cancers with a Gleason score higher than 7 are considered to be aggressive.
Other reported abnormalities
Apart from cancerous cells, other abnormalities which may be reported on histopathology reports include:
-
HGPIN. This is a premalignant change in glands which has been shown to be associated with increased risk of invasive cancer elsewhere in the prostate.
-
ASAP. Atypical changes are present in cells but the pathologist is uncertain of their significance.
Clinically insignificant prostate cancer
The prognosis and natural history of prostate cancer vary with the extent of spread and grade of cancer at diagnosis. Clinically insignificant prostate cancer can be defined as a cancer which will not affect the patient during the natural course of his lifetime, meaning that he is likely to die from other causes. 29 The detection of these potentially clinically insignificant cancers on either initial or second biopsy is an important issue and can lead to potentially invasive and unnecessary treatment as well as increased anxiety for men who live with a diagnosis of prostate cancer that may not affect their life expectancy.
There are a number of different definitions of the term ‘clinically insignificant prostate cancer’. The definitions are based on observed survival rates after radical prostatectomy. These pathology-based definitions require that the disease is restricted to the prostate, with a Gleason score of 6 or less. In addition, some definitions include limits on the total tumour volume and/or largest individual tumour volume. 30,31 However, in clinical practice the challenge is to correctly identify men with clinically insignificant disease before any treatment or surgery, that is at diagnosis. There are several systems for predicting the risk of localised prostate cancer progressing. 32–34 However, recent data have suggested that these tools may be inaccurate33 and the NICE guideline11 includes a research recommendation for further research in this area.
Comparators
There are two main comparator pathways for men suspected of having prostate cancer whose initial biopsy result was negative or equivocal, as shown in Box 1.
-
The use of established risk factors (including histopathology results of initial biopsy, PSA level and a DRE) to inform the decision to perform a second biopsy.
-
The use of established risk factors (including histopathology results of initial biopsy, PSA level and a DRE) followed by mpMRI to inform the decision to perform a second biopsy.
Clinical assessment
Clinicians and patients may consider a number of factors to help inform decisions whether or not a second (or subsequent) biopsy should be undertaken. These include:
-
DRE. This procedure involves a clinician inserting a finger into the patient’s rectum to feel the prostate. The purpose is to identify any hard or irregular areas and to estimate the size of the prostate. A prostate gland with hard bumpy areas may suggest prostate cancer.
-
PSA level. There are a number of different measures including:
-
Patient’s age. Prostate cancer is rare in men under the age of 50 years, and 86% of cases occur in men aged 65 years and over. 37
-
Family history. The family history of prostate cancer in first-degree relatives, such as father or brother, increases risk. 37
-
Nomograms. These are risk algorithms that combine multiple clinical and laboratory risk factors to create a cumulative risk score. Most nomograms aim to predict the likely course of a disease. However, some nomograms [e.g. risk calculator number four from the Prostate Cancer Research Foundation,38,39 the Prostate Cancer Prevention Trial (PCPT)40 and Montreal nomograms41] can predict the result of a biopsy in men suspected of having prostate cancer. It is not clear how often these tools are used to predict biopsy results in clinical practice, but they are used as a proxy for clinical decision-making in the research setting.
Clinical assessment plus magnetic resonance imaging
Clinical assessment may be combined with MRI when a repeat biopsy is being considered. MRI uses strong magnetic fields and radiowaves to form images of the body. 42 Standard anatomical imaging involves injection of a contrast agent and uses T2-weighted images to delineate the structures. The term mpMRI refers to the additional use of functional images including:
-
Magnetic resonance spectroscopy imaging (MRSI) – MRSI or metabolic imaging which measures the concentration of various substances or metabolites within the body.
-
Diffusion-weighted magnetic resonance imaging (DW-MRI) – DW-MRI is sensitive to the motion of water molecules in tissue and detects water.
-
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) – dynamic contrast-enhanced MRI injects a different contrast agent. The uptake and washout of this contrast agent is increased in prostate cancer.
Results from a MRI scan can be used to decide whether or not to perform a repeat biopsy and/or to guide and target the cores taken during the biopsy. The role of MRI varies depending on the MRI facilities and radiological expertise available throughout the NHS. The exact role of MRI in guiding biopsies varies. In cognitive targeting, knowledge of the MRI scan result guides the freehand targeting of suspicious areas and requires no additional equipment. In direct MRI-guided biopsy, the biopsy is performed within the MRI tube. However, in fusion targeting, software is used to combine a pre-acquired MRI-derived target with real-time TRUS imaging to guide the biopsy. 43,44
In current NHS practice, MRI may be prohibited for 6–12 weeks, or more, after a biopsy because of bleeding, as this can lead to imaging artefacts. This has important time implications for the diagnostic testing strategies involving MRI after a negative or equivocal initial biopsy and any subsequent treatment, and may lead to delays in investigation and treatment.
Clear definition of the interventions
PROGENSA prostate cancer antigen 3 assay
The PROGENSA PCA3 assay produced by Hologic Gen-Probe is an in vitro nucleic acid amplification test that is intended for the quantitative determination of PCA3 messenger ribonucleic acid (mRNA) in urine. The PCA3 gene (previously known as DD3) is overexpressed in prostate cancer cells and is, therefore, a potential biomarker for tumour cells. Prostatic cells are released into urine by prostatic massage, this leads to a general release of ribonucleic acid (RNA) and so the level of mRNA of another housekeeping gene is needed to correct for the overall level of prostatic cells in the urine. The gene which encodes PSA (KK3 gene) has been selected as the housekeeping gene, as its mRNA expression is relatively constant in normal prostate cells with only a weak downregulation of PSA gene expression in prostate cancer cells. The PCA3 score report is a ratio of the PCA3 mRNA copies/ml to PSA mRNA copies/ml multiplied by 1000. The score can be used as a continuous measure but studies45–48 have used threshold scores of 20, 25 or 35 to identify men who are at higher risk of an underlying cancer. The manufacturers of the PCA3 assay have recommended a threshold score of 25, with values 25 and higher suggesting the presence of cancer and values under 25 suggesting the absence of cancer. 49
The PCA3 assay requires 20–30 ml of first-catch urine after a DRE, which included a minimum of three strokes to each lobe of prostate. The manufacturers’ documents50,51 refer to the presence of prostatic cells in the urine and there is no literature to address whether the mRNA analysed in the urine samples is derived from prostatic cells or from prostatic secretions.
Urine must transferred within 4 hours to a transport specimen tube containing a urine transport medium that triggers lysis of prostatic cells and stabilises the RNA. The samples are then transferred to a laboratory within 5 days and are kept either at ambient temperature or frozen. Once at the laboratory, the samples can be kept for 14 days if stored at 2–8 °C, for 11 months if kept at –15 to –35 °C or for 36 months if kept below –65 °C. Samples may be subject to up to five freeze–thaw cycles. 51
The PCA3 assay should be used with the Hologic Gen-Probe Direct Tube Sampling 400, 800 and 1600 molecular laboratory systems (Hologic Gen-Probe, Marlborough, MA, USA). It is not compatible with other analysers. The PCA3 assay is indicated50 for use in conjunction with other patient information to inform the decision for repeat biopsy in men 50 years of age or older who have had one or more previous negative prostate biopsies and for whom a repeat biopsy would be recommended by a urologist based on current standard of care, before consideration of PCA3 assay results.
The PROGENSA PCA3 assay package insert51 states that:
PROGENSA PCA3 assay should not be used for patients who are taking medications known to affect serum PSA levels such as finasteride (Proscar, Propecia), dutasteride (Avodart), and anti-androgen therapy (e.g. Lupron). The effect of these medications on PCA3 gene expression has not yet been evaluated. 51
Certain therapeutic and diagnostic procedures, including prostatectomy, radiation and prostate biopsy may affect the viability of prostatic tissue and, subsequently, an individual’s PCA3 score. The effect of these procedures on assay performance has not yet been evaluated.
The assay has been granted US Food and Drug Administration (FDA) approval52 and a Conformité Européenne (CE) mark for use in the European Union.
Beckman Coulter Prostate Health Index
The phi has been developed by Beckman Coulter to combine several different components of PSA, with the aim of creating a sensitive index of risk of prostate cancer. Total PSA is measured in the bloodstream where it occurs, both unbound [free prostate-specific antigen (fPSA)] and bound to other proteins (such as proteases). There is some evidence that the proportion of PSA that occurs unbound (%fPSA) is lower in men with cancer. 53,54 fPSA has been shown to include several isoforms, including [–2]pro-prostate-specific antigen (p2PSA), which is associated with cancerous cells. phi is calculated using the equation (p2PSA/fPSA) × √tPSA;55,56 p2PSA is the unique component of phi.
According to the manufacturer, the phi test is designed for prostate cancer detection in men aged 50 years and older, with tPSA levels between 2 ng/ml and 10 ng/ml and DRE findings that are not suspicious for cancer. 57 The phi score is a continuous measure. The manufacturer, however, suggests using three categories: 0–20 (low risk); 21–39.9 (moderate risk); and 40 and above (high risk). The manufacturer states that estimates of the risk of cancer being detected at biopsy are 8.7% for men with a phi score in the low-risk category, 20.6% for men in the moderate-risk category and 43.8% for men in the high-risk category. 58
The phi score is not intended to be calculated using PSA or fPSA results from any other manufacturer’s assay and the phi assay is compatible only with Beckman Coulter Access instruments (Access2, DxI600, DxI800, DxC600i, DxC680i, DxC800i, DxC880i; Beckman Coulter Inc., Brea, CA, USA). All PSA assays may be standardised to either the Hybritech or the World Health Organization (WHO) calibration with an approximate 22% difference in reported PSA levels (lower for WHO calibration). 59 It is important to use either the Hybritech or the WHO calibration consistently for PSA, fPSA or p2PSA measurements used in the phi calculation and to not mix measurement calibration systems.
The p2PSA molecule is not stable in coagulated blood. The manufacturer’s draft pack insert57 states that ‘When left on a clotted sample at room temperature, the p2PSA concentration increases significantly after 3 hours, probably due to the degradation of other proPSA molecules’. However, the analyte is stable in serum at room temperature. Therefore, it is important that the serum sample is prepared (separated from the clot by centrifugation) within 3 hours of taking a blood sample. Blood taken for p2PSA specimens should be allowed to clot fully and the serum separated by centrifugation within 3 hours of collection. The serum can then be stored for 24 hours at 2–8 °C before assay or for up to 5 months at –20 °C or colder. Specimens requiring storage for longer than 5 months should be frozen at –70 °C.
Information provided by the manufacturer states that the effect of medication prescribed for benign prostate hyperplasia on the level of p2PSA is not known. 57 Specifically, the phi results cannot be interpreted in, and should not be offered to, patients receiving 5-α-reductase inhibitors medication.
The assay has been granted FDA approval60 and a CE mark for use in the European Union.
Implementing PROGENSA prostate cancer antigen 3 assay and the Prostate Health Index testing in the NHS
Various practical issues will need to be considered before/when introducing these tests into the NHS. These include acceptability of the tests to patients and the need for a DRE before a urine sample is voided for PCA3 analysis. The stability of samples and any processing required before transport to the laboratory may pose logistic challenges to health services. The requirement that blood samples for p2PSA assay must be centrifuged and separated within 3 hours may mean that the blood sample must be taken at a hospital with laboratory facilities on site.
Place of the intervention in the treatment pathways
The intervention pathways considered in this report are summarised in Box 2.
-
The use of the PCA3 score/the phi alongside established risk factors (including histopathology results, PSA level and a DRE) to inform the decision to perform a second biopsy.
-
The use of the PCA3 score/phi alongside established risk factors (including histopathology results, PSA level and a DRE) to inform the decision to perform a mpMRI before second biopsy. If the mpMRI is positive, a second biopsy would be performed.
-
The use of the PCA3 score/phi alongside established risk factors (including histopathology results, PSA level and a DRE) to inform the decision to perform a second biopsy in men who have had a negative mpMRI.
Outcome measures
The aim of this review is to assess the impact of the use of two new tests (PCA3 assay and phi) on the health and well-being of men undergoing investigation for suspected prostate cancer and who had a negative or equivocal initial prostate biopsy. Analytical validity outcomes, diagnostic process outcomes, clinical outcomes and patient-reported outcomes can be useful when considering the impact of using PCA3 scores and phi, and are listed in Box 3. Further details of commonly used outcome measures to assess diagnostic tests are described in Appendix 1.
Pre-analytic variability.
Analytical specificity.
Analytical sensitivity.
Accuracy.
Precision.
Diagnostic process outcomesClinical validity/diagnostic test accuracy outcomes.
Test failure rate.
Time to TP diagnosis.
Number of repeat biopsies required.
Grade and stage of cancers detected.
Clinical outcomesMorbidity and mortality from biopsies.
Morbidity and mortality from treatment of diagnosed cancer.
Adverse events from false test results including from treatment of clinically insignificant prostate cancer.
Health-related QoL.
Patient-reported outcomesPatient anxiety associated with undergoing a biopsy (initial and repeated biopsies), waiting for diagnosis and living with the diagnosis of a clinically insignificant prostate cancer.
Patient distress and sequelae associated with the detection of clinically insignificant prostate cancer.
TP, true positive.
Methodological challenges
The External Assessment Group (EAG)’s review of clinical effectiveness has been designed to assess the incremental gain associated with the use of the PCA3 score or the phi in addition to standard clinical assessment (with or without MRI). The following issues pose challenges to achieving this aim.
Lack of evidence
Lack of long-term evidence
Ideally, clinical utility would be assessed in ‘end-to-end’ or ‘test-to-treatment’ studies and it would be possible to follow men from early clinical investigation through to diagnosis, treatment and long-term follow-up for prostate cancer. Such end-to-end studies of clinical utility are often not available. Published studies of clinical validity frequently focus on the diagnostic process and assess the performance of the different tests. Thus, although available studies provide some information on the effectiveness of the intervention tests, data describing the long-term impact of using new tests are often scarce.
Lack of clinically relevant comparisons
Many clinical validity studies focus on the use of (1) a new test or (2) a new test that is a replacement for an existing test. However, usually the comparator and intervention pathways involve combining multiple tests.
Study measurements of clinical assessment
In clinical validity studies, the intervention and comparator test pathways are compared with the results from the reference standard (biopsy). To assess the accuracy of the comparator pathways, the biopsy results must be available for men who ‘test negative’ on the comparator (e.g. clinical assessment or clinical assessment + MRI) as well as those who ‘test negative’ for the intervention test (e.g. clinical assessment + PCA3 or clinical assessment + MRI + PCA3). This means that the study design must include some form of clinical assessment of the entire study population and report the biopsy results for all participants, including those who tested positive or negative on clinical assessment. Differences in the methods used for clinical assessment may make comparing results from different studies problematic.
Heterogeneity in study populations and between-study comparisons
The target population is all men with a negative or equivocal initial prostate biopsy. It is, therefore, important to assess whether or not the study populations in the included studies are representative of this target population. There is likely to be some selection bias in the published studies because referral, or patient acceptance of a biopsy, is expected to be related to PSA level and/or abnormal clinical results; this means that the study populations are likely to be made up of men who are considered to be at higher than average risk of cancer.
Differences in the patient populations of published studies are likely to lead to considerable heterogeneity in estimates of diagnostic test accuracy. Any between-study comparisons that assume that tests perform equally in different populations may, therefore, give misleading results. Combinations of tests used in sequence are rarely reported in the literature and the reconstruction of such test pathways by combining summary measures for the various components assumes not only that the summary measures are constant across populations but also that the tests are independent.
Potential sources of bias
Sampling bias
Study recruitment may be restricted to, for example, men in the PSA ‘grey zone’ (i.e. with a PSA of 4–10 ng/ml) or to men with abnormal DRE findings in addition to a negative or equivocal initial biopsy. This means that the range of clinical assessment variables is restricted in the study population and hence the observed diagnostic accuracy of these clinical variables will be reduced. This sampling bias affects the generalisability of study findings to the population of interest to this review (i.e. all men with a negative or equivocal first biopsy).
Verification bias
Studies that consist of opportunistic cohorts of patients presenting at referral centres will include biopsy results for men who have been referred for, and have accepted, a repeat biopsy. Acceptance of biopsy is likely to be related to PSA level or PCA3/phi score; it is more likely that men with higher PSA levels will accept a repeat biopsy. This leads to so-called differential verification bias, when the availability of the reference standard result is dependent on the result of the intervention or comparator test.
Imperfect reference standard
In clinical validity studies the diagnostic accuracy of a new or intervention test is assessed against a reference standard. The reference standard is the best test available, that is the current preferred method of diagnosing a disease. In the case of prostate cancer, the reference standard is a biopsy. The diagnostic capabilities of all new tests need to be compared against the diagnostic accuracy of a biopsy.
In prostate cancer the reference standard (biopsy) does not detect all cancers and is considered to be imperfect. Some men with a negative biopsy result do have an undetected cancer. These men are indicated by x and y in the Table 1.
| Test result | Biopsy results (standard biopsy) | |
|---|---|---|
| Prostate cancer | No prostate cancer | |
| Test positive | TP | FP (including x) |
| Test negative | FN | TN (including y) |
Different types of biopsy have different cancer detection rates and the sensitivity and specificity of the intervention pathways may therefore differ depending on the type of second biopsy that is carried out (see Types of biopsy). A different biopsy sampling scheme (such as saturation or extended) might mean that x and y are moved to the biopsy-positive column, as shown in Table 2.
| Test result | Biopsy results (saturation biopsy) | |
|---|---|---|
| Prostate cancer (positive) | No prostate cancer (negative) | |
| Test positive | TP + x | FP – x |
| Test negative | FN + y | TN – y |
The estimate of diagnostic accuracy for PCA3/phi scores will alter for different biopsy types if the proportion of x/false positive (FP) is not the same as the y/true negative (TN) value, that is if men in whom cancers were missed on a standard biopsy but would have been detected on a saturation biopsy are more likely or less likely than men with cancer detected on a standard biopsy to have raised PCA3/phi scores. This is plausible. For instance, a standard biopsy is more likely to detect widespread, rather than localised, cancers. If widespread cancers are also associated with higher PCA3/phi scores than localised disease, then a ‘better’ biopsy scheme which picks up more localised disease might reduce the diagnostic accuracy of the use of the PCA3 assay or phi.
Imaging used with biopsy: incorporation bias
A separate issue relating to biopsy is the type of imaging used. Using ultrasound or MRI to guide the biopsy in effect incorporates another test into the reference standard. Men with lesions detectable on ultrasound or MRI often have additional biopsy cores taken which have come from the area surrounding the identified lesions. This may well increase the chance of a positive biopsy result and so increase the observed diagnostic accuracy of MRI. However, this means that the type of reference standard used differs according to the MRI test result; if MRI is positive, more cores would be taken than if MRI was negative.
Chapter 2 Assessment of clinical effectiveness
Aims of the assessment of clinical effectiveness
Assessing the clinical effectiveness of the PCA3 assay and the phi in the diagnosis of prostate cancer involved three separate systematic reviews:
-
A review of the analytical validity of the intervention tests to assess how accurately the tests measure PCA3/phi level present in a sample. Analytical validity is the study of how well laboratory tests measure the substances they are intended to measure. As the p2PSA assay is the unique component of the phi, the analytical validity of the p2PSA assay was considered in this review. As the pre-analytical stability of samples may affect logistical issues concerning transport and storage before samples reach the laboratory for testing, this issue was also considered in the review.
-
A review of the clinical validity (diagnostic test accuracy) of comparator and intervention pathways to assess how the addition of the PCA3 assay or the phi might contribute to the diagnosis of prostate cancer.
-
A review of the clinical utility of the intervention test pathways to evaluate how the addition of the intervention tests might affect patient outcomes, including long-term outcomes such as mortality and morbidity from prostate cancer, and intermediate outcomes such as side effects from tests.
The methods used in each review followed the systematic review principles outlined in the Centre for Reviews and Dissemination guidance for undertaking reviews in health care,61 the NICE Diagnostics Assessment Programme Manual62 and publications from the Cochrane diagnostic test accuracy methods working group. 63 The review of analytical validity was informed by the principles outlined in the Agency for Healthcare Research and Quality methods guide64 and the Evaluation of Genomic Applications in Practice and Prevention initiative. 65
Analytical validity review
Search strategy: analytical validity review
Electronic databases
The following databases were searched on 28 April or 19 May 2014 for eligible studies:
-
MEDLINE
-
EMBASE
-
Cochrane Central Register of Controlled Trials
-
Health Technology Assessment (HTA) database
-
Cochrane Database of Systematic Reviews
-
Database of Abstracts of Reviews of Effectiveness
-
ISI Web of Science
-
Medion database for related diagnostic test accuracy reviews (www.mediondatabase.nl/)
-
Aggressive Research Intelligence Facility database (www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/databases/index.aspx)
-
PROSPERO systematic review register (www.crd.york.ac.uk/PROSPERO/).
No study design filters were applied and non-English-language reports were excluded. All databases were searched from 2000. The following types of report were excluded:
-
editorials, opinion pieces and correspondence on journal articles
-
conference abstracts.
Trial and research registers were searched on 24 July 2014 for ongoing trials and reviews including:
-
ClinicalTrials.gov (http://clinicaltrials.gov/)
-
metaRegister of Current Controlled Trials and International Standard Randomised Controlled Trial Number (ISRCTN) Register (www.controlled-trials.com/mrct/)
-
WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/).
Details of the search strategies used can be found in Appendix 2.
Study selection strategy: analytical validity review
Three reviewers (AN/AB/JH) independently screened all titles and abstracts identified via searching and obtained full-paper manuscripts that were considered relevant by any of the reviewers (stage 1). The relevance of each study was assessed (AN/AB/JH) in accordance with pre-specified inclusion criteria (stage 2). Studies that did not meet the criteria were excluded. Any discrepancies were resolved by consensus.
The analytical validity review focused on studies that addressed the ability of the intervention test to accurately and reliably measure the target analyte. Inclusion criteria are presented in Table 3.
| Item | Inclusion criteria |
|---|---|
| Patient population | All adult men |
| Intervention test | PCA3 assay or p2PSA or phi score |
| Outcomes |
|
| Study design | All study designs including collaborative studies, external proficiency testing, peer-reviewed repeatability studies, internal reports and manufacturer data |
Studies with precision or accuracy control data presented only as part of the methods section of a publication, in order to describe the test that was used, were not included in the review.
Data extraction and quality assessment strategy: analytical validity review
Data extraction and quality assessment were undertaken by two reviewers (AN/NF), with disagreements resolved by discussion. Data extraction included details of source population, number of samples, specific methods/platforms evaluated, number of positive samples and negative controls tested, as well as reported results. Quality assessment was informed by the checklist proposed by Teutsch et al. 65 and included the following:
-
quality of description of test undertaken
-
range of sample/study population tested representative of routine use
-
definition of correct answer
-
reporting of test failures.
A copy of the data extraction form used in the analytical validity review is included in Appendix 3.
Methods of data analysis/synthesis: analytical validity review
The design of the included studies and the types of outcomes reported were summarised in tabular form.
Results: analytical validity review
Search results
The results of the searches undertaken are summarised in Figure 1. A total of 2249 unique records were identified by database searching and via the use of additional resources (e.g. trial registers and backward citation searching). Of these, 2021 records were excluded at the title and abstract screening stage. Overall, 228 studies were reviewed in full text and six papers were considered to be relevant for inclusion in the analytical validity review.
FIGURE 1.
Flow chart of search results. ARIF, Aggressive Research Intelligence Facility database; WoS, Web of Science.

Six papers48,71–75 reporting on analytical validity or pre-analytical effects were identified from the electronic databases: three related to the PCA3 assay48,71,72 and three related to the p2PSA assay. 73–75 In addition, the Summary of Safety and Effectiveness Data (SSED) report to the US FDA for each test50,58 was obtained. The manufacturers included a pack insert for the PCA3 assay48 in their submission and the draft pack insert for the p2PSA assay was obtained from the FDA website. 57
Data from two of the identified studies73,74 for the p2PSA assay appear to be described in the SSED report58 but, as citations were not stated, it was not possible to confirm this potential double use of data; the results from different data sources have been reported separately in the review and potential overlaps have been highlighted. The draft pack insert for p2PSA57 does not appear to include any data that have not already been described in the SSED report58 and so full data extraction using the draft pack insert57 has not been undertaken; however, relevant data are reported in the study characteristics table for completeness.
A search of electronic trial databases found two trials76,77 that were potentially relevant to the review of analytical validity of the PCA3 assay: one76 was ongoing and the status of the other was unclear (Table 4). No relevant ongoing trials on phi or p2PSA were identified. 77
| Name | Details | Status | Registration number and URL |
|---|---|---|---|
| Comparing the Reliability of Expressed Prostatic Secretion (EPS) and Post Massage Urine (PMU) for the Prediction of Prostate Cancer Biopsy Outcome 76 | Randomised trial of expressed prostatic secretions vs. post-DRE urine in target population of 180 men undergoing first prostatic biopsy. Various biomarkers including PCA3 assessed in specimens | Ongoing | NCT01441687, http://clinicaltrials.gov/show/NCT01441687 (accessed 15 September 2014) |
| Pilot study: performance of the Progensa PCA3 test in post-oxytocin urine specimens 77 | To determine the yield of prostate cells and PCA3 score in the urine specimens from healthy male volunteers after oxytocin nasal spray, using a urine specimen with no manipulation as a reference method | Unclear | EUCTR2010-024649-61-NL, http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2010-024649-61-NL (accessed 15 September 2014) |
Characteristics and quality assessment of included studies: analytical validity review
The study characteristics and outcomes reported in the included studies are summarised in Appendix 4. Quality assessment of all studies using the Teutsch et al. checklist65 had been planned but, because of a lack of information in the included studies, it was not possible to use this checklist. 65 Instead, the EAG used a modified version of this checklist to assess the quality of the studies.
PROGENSA prostate cancer antigen 3 assay
All of the PCA3 studies48,50,51,71,72 were conducted in the USA and reported data for clinical validity and analytical validity. All studies48,50,51,71,72 measured precision. Four studies48,50,51,71 measured accuracy, but only the SSED report50 and pack insert51 reported on five or more different outcomes that were all relevant to analytical validity. Pre-analytical effects were considered by all but one study. 72 None of the studies compared the PCA3 assay with a ‘gold standard’, as such a reference test does not exist for analytical validity. However, the PCA3 assay analyte quantitation was compared with in vitro transcripts which had been value-assigned using ultra violet spectroscopy in three studies. 48,50,71 Four of the studies50,51,71,72 provided adequate descriptions of the test under study, that is reported specific methods/platforms evaluated and information on quality assurance measures. Although all studies used clinical samples, it was unclear whether or not the same population was used for both the analytical validity and clinical validity studies. Only in one study72 did it appear that specimens represented routinely analysed clinical specimens in all aspects (e.g. collection, transport, processing). None of the studies provided sample size/power calculations, and the number of samples analysed varied by outcome both within and across studies (see Appendix 4, Table 71).
[–2]pro-prostate-specific antigen assay
Studies of p2PSA were conducted in Germany73,75 and in the USA;74 one study58 described results from studies that had been conducted in both of these countries. The manufacturers have confirmed that the assay that was research use only used in the study by Stephan et al. 75 is the same assay that is now commercially available. All studies reported analytical sensitivity, specificity, accuracy, precision, linearity and range. Pre-analytical effects were considered only in the SSED report to the FDA58 and by Semjonow et al. 73 None of the studies compared the p2PSA test to a ‘gold standard’ as such a reference test does not exist for analytical validity. However, in the SSED report58 recovery (a measure of accuracy) used internal reference preparation of p2PSA. Stephan et al. 75 was the only study that did not adequately describe the test under study, that is the study did not report the specific methods/platforms evaluated or present sufficient information on quality assurance measures. As with PCA3 studies, precise details about the population from which the samples were derived was not provided. The number of samples varied by outcome within and across studies (see Appendix 4, Table 71). None of the studies provided sample size/power calculations. In all instances, it was unclear if the specimens represented routinely analysed clinical specimens in all aspects (e.g. collection, transport, processing).
Prostate cancer antigen 3 results: analytical validity review
Impact of digital rectal examination
Sokoll et al. ,48 in a sample of 179 patients, found that 74.4% of urine samples taken before a DRE were informative, compared with 95.5% of urine samples that were taken after a DRE. First-morning void urine samples (n = 56) had an informative rate of 80.4%. The number of strokes per lobe in the DRE did not affect the informative rate of tests (98.7% for three strokes and 94.4% for eight strokes per lobe). There were no significant differences in the reported PCA3 score for those tests which were informative, regardless of whether or not the men had a prior DRE.
Storage of unprocessed urine samples
The SSED report50 and pack insert51 both described the effect of time spent at 30 °C and 2–8 °C on urine samples before processing into the transport tubes. The SSED report50 included 12 specimens and the pack insert51 reports on 10 specimens, and it is not clear whether or not these are the same samples. At 30 °C, the PCA3 score showed a 5% drift over 4 hours and at 2–8 °C a 2% drift over 4 hours. Estimates of drift are not presented for more than 4 hours’ storage. 50
Storage of processed urine samples
In Groskopf et al. ,71 three previously frozen processed urine specimens were thawed and held at 4 °C or 30 °C for 14 days. Degradation of mRNA was noted from day 1 at 30 °C; the PCA3 score remained within 20% of initial value for 14 days. The SSED50 reported drift for 12 processed samples held for 6 days at varying ambient conditions between 30 °C and –70 °C and between 30 °C and 55 °C; both temperature ranges had a drift of 8%. The pack insert50 reported that 12 specimens were stable for 21 days at 4 °C, for 5 days at 30 °C and for 90 days between –20 °C and –70 °C; no raw data were presented.
Groskopf et al. 71 and the pack insert51 reported stable results in processed urine after five and six freeze–thaw cycles, respectively; neither of the studies presented raw data.
Analytical sensitivity
Limit of blank (LoB), limit of detection (LoD) and limit of quantitation (LoQ) were reported as shown in Table 5. The LoQ of both analytes (PCA3 and PSA) were the same as the corresponding LoD in Sokoll et al. 48 and in the SSED report. 50
| Study | Methods | Source | LoB (copies/ml) | LoD (copies/ml) | LoQ (copies/ml) |
|---|---|---|---|---|---|
| Sokoll 200848 | LoD – lowest measurable concentration of controls; LoB – 95th centile of zero calibrator; and LoQ – < 130% recovery and CV < 35% | PCA3 | 176 | 259 | 259 |
| PSA | 831 | 2338 | 2338 | ||
| SSED 201250 | Four blank female urine and four female urine spiked to calibrator 2 concentrations; LoD = LoB + 1.65 SD | PCA3 | 90 | 239 | 239 |
| PSA | 254 | 3338 | 3338 | ||
| Pack insert51 | Diluted in vitro transcripts. LoQ assessed according to Clinical & Laboratory Standards Institute EP17-A | PCA3 | NR | 80 | Calibrator 2 ≈ 750 |
| PSA | NR | 1438 | Calibrator 2 ≈ 7500 |
Analytical specificity
The assay did not detect unspliced PCA3 RNA. 50,51 No assay interference was recorded in the SSED report,50 with either 10 listed endogenous compounds or six micro-organisms; out of 27 exogenous compounds, only selenium and raw palmetto were reported to cause interference . 50 However, in the pack insert51 it was reported that none of the 35 therapeutic substances tested (which included selenium and raw palmetto) interfered with the assay. In addition, the SSED report50 states the effects of medications such as finasteride and dutasteride which are known to affect serum PSA levels were not evaluated. However, in the FDA pack insert51 (p. 34) but not in the SSED report, these two drugs are clearly listed among the therapeutic substances tested. Nevertheless, the pack insert51 states that ‘The PROGENSA PCA3 assay should not be used for patients who are taking medications known to affect serum PSA levels such as finasteride (Proscar, Propecia), dutasteride (Avodart), and anti-androgen therapy (Lupron). The effect of these medications on PCA3 gene expression has not yet been evaluated’ (p. 31). Urine samples from men after a prostatectomy and from female participants were below the assay limit for PCA3. 51,71 RNA from 10 tissue types throughout the male urogenital tract was tested, and only prostate tissue RNA was detected in the assay. 51 The SSED report50 included carryover studies with a 0% FP rate for negative samples interspersed with high-titre samples. 58
Accuracy
No gold standard is available, and without a gold standard that offers 100% specificity and 100% sensitivity it is difficult to confidently assess the accuracy of competing diagnostic strategies. Four studies48,50,51,71 assessed accuracy by calculating the percentage recovery of measured PCA3 or PSA RNA copies/ml compared with ultra violet-determined copies/ml of female urine samples spiked with varying concentration of in vitro transcripts or with control samples. Across four studies,48,50,51,71 accuracy varied from 90% to 118% for PCA3 and from 85% to 121% for PSA (Table 6).
| Study | Methods | Measurement | Minimum (%) | Maximum (%) |
|---|---|---|---|---|
| Sokoll 200848 | Three controls. Tested in two sites | PCA3 | 104.1 | 110.8 |
| PSA | 93.2 | 108.8 | ||
| SSED 201250 | Eight-member panel of female urine spiked with in vitro transcript | PCA3 | 90 | 118 |
| PSA | 85 | 121 | ||
| Pack insert51 | Eight-member panel of female urine spiked with in vitro transcript | PCA3 | 94 | 108 |
| PSA | 111 | 120 | ||
| Groskopf 200671 | Three controls | PCA3 | 102 | 109 |
| PSA | 94 | 111 |
Precision
Precision was assessed in four papers48,50,51,71 by including only within-laboratory variation (including intra-run and inter-run variance, reagent, observer) and in two papers50,71 by including both within- and between-laboratory variation. Multiple results were reported in some papers. 50,71 In six studies48,50,51,71 the within-laboratory total coefficient of variation (CV)% ranged from 4% to 27% for PCA3 and from 7% to 18.7% for PSA; in two studies50,51 the PCA3 score varied from 12% to 28% (Table 7).
| Study | Methods | Total CV%: maximum and minimum | ||
|---|---|---|---|---|
| PCA3 (range) | PSA (range) | PCA3 score = PCA3/PSA × 1000 (range) | ||
| SSED 201250 | Four control samples Maximum of 80 results each sample Variation: within-run, between-run, day |
5.2–18.3 | 9.5–18.7 | 14.0–20.7 |
| Pack insert51 | Three pooled samples and four control samples 36 results each sample Variation: within-run, between-run, operator, lot and equipment |
7–27 | 9–14 | 12–28 |
| Pack insert51 | Three control samples 80 results each sample Variation: within-run, between-run, day |
4–12 | 7–8 | Not reported |
| Sokoll 200848 | Three control samples 100 results each sample Two different sites Variation: within-run, between-run |
Within-run: 5.7–15.2 | Within-run: 10.8–11.6 | Not reported |
| Between-run: 6.1–18.6 | Between-run: 7.6–9.5 | Not reported | ||
| Groskopf 200671 | Three control samples 54 results each sample Variation: within-run, between-run |
6–19 | 8a | Not reported |
| Groskopf 200671 | Three patient samples 54 results each sample Variation: between-run |
Total CV% not reported | Total CV% not reported | Total CV% not reported |
| Between-run: 9–20 | Between-run: 10–11 | Between-run: 15–24 | ||
Within- and between-laboratory total CV% was reported by two studies48,50 and ranged from 5.9% to 17.2% for PCA3 and from 10.1% to 19.3% for PSA (Table 8). Only one study50 reported within- and between-laboratory total CV% for the PCA3 score, which ranged from 12.3% to 25.0%. Most variation occurs within laboratory, with assays on different sites adding little extra variability.
| Study | Methods | Total CV%: maximum and minimum | ||
|---|---|---|---|---|
| PCA3 | PSA | PCA3 score = PCA3/PSA × 1000 | ||
| SSED 201250 | Three control samples tested in three sites 360 results for each sample Variation: within-run, between-run, operator, lot and site |
6.8–17.2 | 10.5–19.3 | 12.3–25.0 |
| Sokoll 200848 | Three control samples 200 results each sample Variation: within-run, between-run, site |
5.9–17.1 | 10.1–11.5 | Not reported |
Shappell et al. 72 reported between-laboratory precision from 50 clinical samples sent to two different laboratories in terms of concordance of PCA3 scores. When the PCA3 score was divided into three categories (indeterminate, < 35, ≥ 35), results were concordant in 47 out of 50 samples (94%). Correlation was reported to be good for 48 informative samples (r = 0.85). When three outliers were omitted, this improved further (r = 0.96). The mean percentage difference in test values was 13.6% [standard deviation (SD) 42.5%].
The SSED report50 also compared spiked female urine versus clinical samples (Table 9). Maximum variation in total CV% for PCA3, PSA and the PCA3 score appeared to be slightly less for clinical samples than for control samples. The sample precision in clinical samples was therefore reported to be comparable with that in control samples.
| Study | Methods | Total CV%: maximum and minimum | ||
|---|---|---|---|---|
| PCA3 | PSA | PCA3 score = PCA3/PSA × 1000 | ||
| SSED 201250 | Clinical specimens: 16 results for each specimen | 6.8–12.5 | 8.0–15.9 | 8.3–16.0 |
| Control specimens: 16 results for each specimen | 5.1–18.2 | 9.2–17.9 | 13.3–20.6 | |
Linearity
The SSED report50 assessed linearity using 11 samples of PCA3 and PSA in in vitro transcripts in processed female urine. Here the deviation from linearity for PCA3 was < 9% and for PSA deviation was < 7%. Linearity studies using 10 clinical specimens in specimen diluent or processed female urine were also reported. Deviation from linearity for PCA3 in specimen diluent and processed female urine was less than 6%. However, for PSA in specimen diluent, linearity was < 23% and in processed female urine deviation was < 30%. The higher than expected variance in PSA, although remaining within study acceptance criteria, may have been caused by variation in linearity panel preparation. The pack insert51 reported a direct proportional relationship between dilutions tested and analyte copies/ml.
[–2]pro-prostate-specific antigen/Prostate Health Index: analytical validity review
Stability of [–2]pro-prostate-specific antigen in blood and serum
Semjonow et al. 73 examined the stability of 22 clinical samples stored as clotted blood at 21 °C or as serum at 4 °C and 21 °C and then frozen at –20 °C or –70 °C. Percentage recovery of the samples over time from each baseline measurement was reported. The stability criterion used was that mean change in recovery did not exceed 10%.
In clotted blood, the mean recovery at 1 hour 9 minutes was 105.6% [95% confidence interval (CI) 103.2% to 107.9%], compared with 100% at the 37-minute baseline. At 3 hours 1 minute, the mean recovery was 112.7% (95% CI 109.7% to 115.6%). These data show that, by 3 hours after drawing the blood sample, the stability criterion is not met. No data are available for storage times between 1 hour 9 minutes and 3 hours 1 minute and so it is not clear precisely when the stability criterion is breached. These data are the basis for the recommendation stipulated in SSED,58 that is specimens should be spun and refrigerated within 3 hours. A regression equation extrapolated results to a baseline at 97% of time of specimen collection. The increase in value is considered to be because of proteolytic activity in the clot.
Samples in serum were within the stability criterion after 48 hours at either 4 °C or 21 °C and at least 12 months at –20 °C or –70 °C. Two freeze–thaw cycles did not result in < 10% variation compared with 21 °C.
Stability of reagents and calibration materials
The SSED report58 included data confirming stability of reagents and calibration products, both as sealed packs and once opened.
Thermal sensitivity of assay
The effect of change in ambient temperature (18 °C, 23 °C and 31 °C) on assay performance was investigated for three different analysers (Access 2, DxI 800 and DxI 600; Beckman Coulter Inc., Brea, CA, USA) and reported in the SSED. 58 Results were compared with results at the centre-point ambient temperature. A thermal effect was noted, with 1.84–2.82% change in p2pSA per 1 °C ambient temperature. This suggests a maximum of 16.9% variation in p2PSA result for ± 6 °C change in ambient temperature compared with temperature at which the calibration curve performed.
Analytical sensitivity
Limit of blank, LoD and LoQ were reported as shown in Table 10. The results reported in the SSED58 appear to be the same as the results reported in Sokoll et al. 74
| Study | Methods | p2PSA | ||
|---|---|---|---|---|
| LoB (pg/ml) | LoD (pg/ml) | LoQ (pg/ml) | ||
| SSED 201258 | LoB: 95th centile of zero analyte; LoD: LoB + 1.65 SD (SD from patient serum LoQ; dilutions of calibrators from LoD to 7 × LoD); LoQ: concentration with CV20% from quadratic model | 0.50 | 0.69 | 3.23 |
| Sokoll 201274 | Methods as for SSED. Appears to be same study results as in SSED | 0.50 | 0.70 | 3.23 |
| Stephan 200975 | LoD: repeat measurement of zero calibrator + 2 SD | Not reported | 2.27 | Not reported |
Analytical specificity
Potential interference with seven endogenous compounds was investigated by comparing test mean (with added compound) and control mean (without added compound) for three different concentrations of p2PSA. 74 Most recoveries were within 10% of the target 100%, with a mean of 93%, although the addition of 8.4 g/dl total protein reduced one recovery to 88.4%. The same seven compounds at the same concentrations were also reported to be analysed for interference. 58 The raw data (mean recoveries) were not reported, although a warning was given that protein levels greater than 8 g/dl may interfere with p2PSA measurement. It is unclear if the analyses reported in the SEED report58 are the same as those reported by Sokoll et al. 74 Forty-nine commonly encountered medications and therapeutic drugs were also tested and the SEED report58 concluded that they did not interfere with assay performance, although no raw data were presented.
Crossreactivity with other PSA isoforms, including α1-antichymotrypsin-PSA, benign PSA, fPSA, (–4) PSA and (–5/–7) PSA, was tested in three studies. 58,74,75 Minimal cross-reactivity was detected (recovered test dose < 5%58,74 or < 2.5%75 of expected dose).
Carryover was reported by only one study58 with no evidence of carryover from high concentration samples.
Accuracy
No gold standard is available and the reference material used is based on purified p2PSA. Accuracy was reported in three studies58,74,75 by calculating the per cent recovery of measured p2PSA pg/ml in male serum samples spiked with varying concentration of purified p2PSA (Table 11). The data reported in SSED58 and Sokoll et al. 74 appear to be from the same study.
Precision
Precision was assessed by including only within-laboratory variation (including intra-run and inter-run variance, reagent, observer) in three studies58,74,75 and by including both within- and between-laboratory variation in one study. 58
All studies reported CV% for p2PSA, but only the SSED report58 included CV% for phi. Within-laboratory precision as measured by total CV% varied from 2.91% to 13.05% for p2PSA and from 8.5% to 12.0% for phi (Table 12). Within- and between-laboratory precision for p2PSA was reported as being between 5.39% and 9.39% for p2PSA and between 4.9% and 7.3% for phi (Table 13). These maximum estimates are lower than those for within-laboratory only precision, and it is likely that this reflects the different populations used. There appears to be an overlap in data for p2PSA variability between Sokoll et al. 74 and the SSED report. 58 Sokoll et al. 74 reported within-laboratory precision data from four sites, but one of these had higher than expected variability and no total variance across all was reported in this paper (see Table 12). The variance for p2PSA across three sites reported in the SSED report58 may be from the three lower variance sites (see Table 13).
| Study | Methods | Total CV%: maximum and minimum | |
|---|---|---|---|
| p2PSA | phi | ||
| SSED 201258 | For p2PSA: three controls and six clinical samples Variation: within-run, between-run For phi: one control and four clinical samples Variation: within-run, between-run, day, lot, analyser |
2.94–10.83 | 8.5–12.0 |
| Sokoll 201274 | Three control and three clinical samples 80 runs each sample Variation: within-run, between-run, operator Four different sites reported separately – not combined. One site higher than expected variance |
2.91–13.05a (2.91–7.10, with high variance site excluded)a | Not reported |
| Stephan 200975 | Four control and/or three control and one pooled clinical sample Variation: within-run, between-run |
Total CV% not reported: within-run, 2.03–5.63; and between-run, 3.1–7.99 | Not reported |
| Study | Methods | Total CV%: maximum and minimum | |
|---|---|---|---|
| p2PSA | phi | ||
| SSED 201258 | For p2PSA: three control and three clinical samples 80 runs each sample Three sites combineda Variation: within-run, between-run, reagent lot, site For phi: 10 clinical samples. Variation: within-run, between-run, day, site |
5.53–9.39a | 4.9–7.3 |
Linearity
The SSED58 reported linearity studies using diluted known concentrations of p2PSA in 12 serum samples. Eleven out of the 12 samples had a slope of 1.0 ± 0.15. A linear range was confirmed to 4922 pg/ml. Sokoll et al. 74 assessed dilutions of three samples and Stephan et al. 75 assessed six samples; both confirmed a linear range to 5000 pg/ml and 4500 pg/ml, respectively. No hook effect to 15,000 pg/ml was found in the two studies. 58,74
Discussion: analytical validity review
To inform the assessment of the analytical validity of the two assays, the EAG has relied on data that have been published, primarily by the manufacturers, in the form of pack inserts and/or reports included in submissions for regulatory approval. The EAG could not reject the premise that, for some results, the same analytical validity data had been reported in multiple publications. The EAG considered that the analytical validity of both the PCA3 and the p2PSA assays had been comprehensively documented. The EAG identified several areas where further consideration of the data might be merited for both the PCA3 assay (e.g. precision, single threshold) and the phi (e.g. sample handling and thermal sensitivity).
PROGENSA prostate cancer antigen 3 assay
The analytical validity review has identified an important issue regarding the precision of the measurement of the PCA3 assay. Across the included studies, the CV% was estimated as being up to 25% for combined between- and within-laboratory variation and 28% for within-laboratory variation. Using a CV% of 25% means that, in a urine sample with a true PCA3 score of 25, the SD of the results obtained will be 6; this means that 67% of samples tested will have PCA3 scores between 19 and 31 and the remaining 33% will have PCA3 scores outside of this range. This uncertainty in the true PCA3 score is reflected in the SSED50 report, which includes the following guidance: ‘Due to normal assay variability, specimens with PCA3 Scores near the cut-off of 25 (i.e. 18 to 31) could yield a different overall interpretation of POSITIVE or NEGATIVE upon repeat testing. PCA3 Scores in the range from 18 to 31 should therefore be interpreted with caution’ (p. 6). The consequences of this imprecision for the use of the PCA3 assay in routine NHS clinical practice are unknown.
There are no concerns regarding the stability of samples during storage once the samples have been processed. However, urine samples need to be transferred into specialist transport tubes within 4 hours of the urine being voided.
None of the papers included in the analytical validity review explored whether or not genotype affected PCA3 scores. However, the authors of a recent publication78 have proposed that a single threshold for the PCA3 score may not be appropriate for all men and that multiple thresholds may be required, as the appropriate threshold may vary by genotype. This publication78 did not meet the inclusion criteria for the analytical validity review. However, in this genome-wide association study78 of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial population, two genotypes which were associated with PCA3 scores were identified. The study population included 278 subjects with prostate cancer detected on biopsy and 1371 without prostate cancer. The means of the PCA3 scores in the 1371 men with negative prostate biopsy varied from 13.35 to 20.76 depending on genotype. One of the genotypes (rs10992994 in the β-microseminoprotein gene) is a strong genetic marker for prostate cancer. 79,80 The authors calculated a personalised threshold score by adjusting the threshold of 35 by the relative genetic effect; the estimated personalised threshold scores varied between 24.9 and 60.6. Whether or not a single threshold for the PCA3 score is appropriate for all men with suspected prostate cancer is currently unknown.
[–2]pro-prostate-specific antigen/Prostate Health Index assay
Practical issues relating to the use of the p2PSA assay that may be important to consider are sample handling and thermal sensitivity. The draft pack insert57 states that blood should be centrifuged and serum separated within 3 hours of the blood sample being taken; this guidance is based on the work of Semjonow et al. 73 However, the data in this paper suggest that by 3 hours 95% of samples will have breached the stability criterion of a 10% increase in p2PSA level. As neither the manufacturer nor Semjonow et al. 73 present a rationale for the use of the 10% stability criterion or a time period of 3 hours, the consequences of breaching the 3-hour time period are not clear. In addition, whether or not sample handing can be carried out in routine clinical practice as per the instructions set out in the draft pack insert57 is not yet known; in particular, given the 3-hour time limit, only hospitals with on-site laboratory facilities may be able to offer this test.
Studies of the thermal sensitivity of the p2PSA assay indicated that there is a 16.9% variation in p2PSA result for a ± 6 °C change in ambient laboratory temperature. 58 However, the SSED58 report suggests that any differences in results because of temperature change would not affect clinical validity results.
Clinical validity review
Search strategy and study selection strategy: clinical validity review
The same search strategy and study selection strategy were used for the analytical validity and clinical validity reviews. Full details are presented in Search strategy: analytical validity review and Study selection strategy: analytical validity review.
Inclusion criteria: clinical validity review
Comparisons between the performance of the intervention tests (PCA3 assay and phi) and the comparison tests (clinical assessment and MRI) can be made using either data from studies carried out in the same study population (within-study or direct comparisons) or data from studies in which intervention and comparator tests are carried out in different populations (between-study or indirect comparisons). The preferred data for this review are derived from within-study comparisons of intervention and comparator test pathways.
Within-study (direct) comparisons
Owing to uncertainty about the diagnostic pathways used in NHS clinical practice and the limited availability of MRI facilities, the EAG initially included all studies with a direct comparison of the PCA3 assay or the phi with any one or more of following component comparator tests:
-
individual clinical risk factors such as age, a DRE
-
standard clinical judgement/nomograms
-
PSA levels
-
MRI results: T2-weighted magnetic resonance imaging (T2-MRI)/DW-MRI.
As the intervention tests (PCA3 assay or phi) can be used as replacement, add-on or triage tests to the comparator tests, studies that have directly compared the clinical validity of the PCA3 assay with the clinical validity of phi, with or without other comparators, were also included. The inclusion criteria used to select eligible within-study comparisons are presented in Table 14.
| Item | Inclusion criteria |
|---|---|
| Patient population | Men suspected of having prostate cancer who had had at least one negative or equivocal biopsy. The review was restricted to studies where at least six cores were taken in initial biopsy. Studies of men taking medications known to affect serum PSA levels such as finasteride (Proscar®, Merck Sharp & Dohme Ltd; Propecia®, Merck Sharp & Dohme Ltd), dutasteride (Avodart®, GSK), and anti-androgen therapy or leuprorelin (Lupron, Takeda-Abbott Pharmaceuticals) were excluded |
| Intervention | Diagnostic test or test pathway including PCA3 and/or phi |
| Comparator | Diagnostic test or test pathway without PCA3 or phi and including one or more of following comparator tests:
|
| Reference standard | Eligible studies compared the performance of comparator or intervention pathways to a histological analysis of prostatic tissue. This could have been obtained from a second prostatic biopsy or from a prostatectomy specimen. Biopsy must have taken place within 1 year of the intervention test Studies with all types of second biopsy were included:
|
| Outcomes | Studies reporting any of the following were included:
|
| Study design | Studies reporting within-study comparison of interventions/comparators:
|
Systematic reviews for use in between-study (indirect) comparisons
In the absence of any available within-study comparisons, the EAG would have considered carrying out between-study (indirect) comparisons of the intervention tests versus comparator tests. Given the probable large number of studies evaluating each of the intervention and comparator tests, estimates of the clinical validity of the intervention and comparator tests from good-quality systematic reviews with meta-analyses were sought to provide data for any between-study (indirect) comparisons undertaken. The inclusion criteria used to select eligible systematic reviews are presented in Table 14.
Data extraction strategy: clinical validity review
A paper-based data extraction form was created for the clinical validity review (see Appendix 3). These forms were revised after data had been extracted from three studies. Three reviewers (AN/JH/KD), who worked independently, extracted relevant data and the data extraction forms were cross-checked (AN/JH/KD). When more than one publication reported findings from a single study, a composite data form was created. In cases where reported data appeared to be missing or unclear, clarification was sought from study authors.
Within-study (direct) comparisons
Limited data [e.g. details relating to the comparator interventions, study population (including inclusion and exclusion criteria) and author conclusions] were extracted from studies that were eligible for inclusion but did not report data from a clinically relevant comparison, that is limited data were extracted from studies that reported the results of univariate PCA3 or univariate phi versus univariate PSA.
Complete data were extracted from all other eligible studies. Particular attention was paid to:
-
how the intervention and comparator tests were used (replacement, add-on, triage or not stated)
-
definition of positive biopsy, including grade and stage of tumour detected
-
threshold values used for intervention tests.
The available data on all reported clinical validity outcomes were recorded including:
-
2 × 2 tables of true positive (TP), FP, false negative (FN) and TN values
-
sensitivity, specificity, positive predictive value, negative predictive value, positive and negative likelihood ratios
-
area under the curve (AUC) and sensitivity and specificity values derived from receiver operator characteristics (ROC) curves
-
multivariate odds ratios (ORs) for logistic regression.
Outcomes were recorded for every reported:
-
threshold value
-
combinations or sequence of tests
-
grade of cancer.
Systematic reviews for use in between-study comparisons
Study extraction was limited to:
-
details relating to the comparator interventions
-
study population (including inclusion and exclusion criteria)
-
number of studies and participants included in meta-analyses
-
author conclusions.
Quality assessment strategy: clinical validity review
Quality assessment was not undertaken for studies which were eligible for inclusion but did not report data from a clinically relevant comparison. Quality assessment was not undertaken for systematic reviews for use in between-study comparisons as only the conclusions from these papers were included in the review.
The Quality Assessment of Diagnostic Accuracy Studies – version 2,81 a modified version of the Quality Assessment of Diagnostic Accuracy Studies tool,82,83 was used to assess the quality of included studies. This tool considers four domains: patient selection; index and comparator tests; reference standard; and flow and timing. These domains were assessed both for risk of bias (whether the conduct or design of the study led to a distortion of results) and for applicability issues (whether or not the study reflected the population and tests used in practice). The tool content was tailored to meet the requirements for this review and a copy of the tool is displayed in Appendix 3. The following issues were of particular importance to this review:
-
Patient selection: the extent to which the study population was pre-selected on variables such as PSA level, a DRE, ethnicity or family history. This is important as it affects both risk of bias for these variables and applicability.
-
Intervention test (PCA3 assay or phi): whether or not the tests were conducted and interpreted without knowledge of other comparators and of the reference standard; whether or not any lack of blinding posed an important risk of bias given the automated and objective nature of the test; whether or not thresholds used were determined in advance or selected to maximise the diagnostic power of the test; and whether or not the conduct of the test in the study was comparable to that used in standard clinical practice.
-
Comparator test [clinical and PSA (variables included in the multivariate analyses or nomogram were considered)]: whether or not the assessment was independent of, and blinded to, the results of the intervention tests, MRI and the reference standard; whether or not attempts to standardise assessment were carried out; whether or not methods used were a fair reflection of clinical practice.
-
Comparator test (MRI): whether or not a definition of abnormality was given and whether or not the radiologist interpreting the scan was blind to results of intervention tests, MRI and the reference standard.
-
Reference standard (biopsy): whether biopsy cores taken were standardised; or whether number and pattern of cores were affected by results such as clinical findings, TRUS result or MRI.
Methods of data analysis/synthesis: clinical validity review
Extracted data, grouped by type of outcome, were tabulated for each comparison. Measures of difference between the comparator test pathways were calculated for the following measures:
-
comparison of AUCs
-
sensitivity at set values of specificity
-
specificity at set values of sensitivity.
Odds ratios from multivariate logistic regression analyses were recorded as a measure of the independence of the effect of the intervention tests.
The following sensitivity analyses were considered:
-
type of second biopsy (saturation, template or guided)
-
threshold value used for intervention test
-
different risk groups (grades or stages) of tumour detected by the second biopsy.
Within-study comparisons: search results
The results of the searches undertaken are summarised in Figure 1. A total of 2249 unique records were identified by database searching and via the use of additional resources (e.g. trial registers and backward citation searching). Of these, 2021 records were excluded at the title and abstract screening stage because of ineligible study population (e.g. initial biopsy population only) or ineligible design. If the study population was unclear or a mixed biopsy population was reported in the abstract, the studies were retained and the full text obtained. Similarly, studies in which the design was unclear were retained. Studies were not excluded at this stage if comparators were not mentioned; comparisons with PSA and other clinical variables are not always highlighted in the abstract. Overall, 228 studies were reviewed in full text and 25 papers were considered to be eligible for inclusion in the review of clinical validity.
Clinical trials search results
A search of electronic trial databases found one ongoing randomised trial84 that was possibly relevant for the clinical validity review of the PCA3 assay; summary details of this trial are shown in Table 15. No relevant ongoing trials that included phi were identified.
| Name of trial | Details | Status | Registration number, URL |
|---|---|---|---|
| Medical Economics of Urinary PCA3 Test for Prostate Cancer Diagnosis84 | Randomised trial of men undergoing prostate biopsy. Intervention group will have PCA3 results available and control group will not. Outcomes include number of inappropriate biopsy, costs of management | Ongoing. Estimated completion 2021 | NCT01632930, http://clinicaltrials.gov/show/NCT01632930 (accessed 15 September 2014) |
Excluded studies
The studies excluded from the review at stage 2 (including any listed in the manufacturer submissions which were not eligible for inclusion in the review) are listed in Appendix 5 with the reasons for their exclusion. This list contains both excluded primary studies and excluded systematic reviews and meta-analysis papers. The most frequent reason for exclusion was ineligible or unclear study population.
Within-study comparison results: clinical validity review
A total of 25 papers45,46,85–106 met the inclusion criteria for the within-study comparisons. A total of 21 papers45,46,85,86,88–92,94–99,101–106 were identified which reported within-study comparisons between clinical assessment + PCA3 and/or clinical assessment + phi versus a comparator.
The results from 17 papers45,46,85,86,89–92,94,96,97,99,102–106 reporting 15 different study populations were included in the review; results from two study populations were published in two publications each (European cohort46,85 and the REDUCE trial86,105). Full data extraction and quality assessment were undertaken on these papers. Three other publications88,98,101 from the European cohort study and one additional publication by Pepe and Aragona95 were eligible for inclusion in the review but did not present additional study results and are included in the number of eligible studies (n = 21) for information only.
Four papers87,93,100,107 reported only univariate assessments of the PCA3 assay or phi versus univariate PSA and the limited data extracted from these studies are presented in Appendix 6.
The 17 included studies45,46,85,86,89–92,94,96,97,99,102–106 reported various comparisons of intervention and comparator tests and these are listed in Table 16.
| Number | Comparison | Studies reporting data on comparison |
|---|---|---|
| 1 | Clinical assessment vs. clinical assessment + PCA3 | European cohort46,85 |
| REDUCE placebo86,105 | ||
| Perdonà 201197 | ||
| Gittelman 201345 | ||
| Busetto 201390 | ||
| Scattoni 2013102 | ||
| Porgpiglia 201499 | ||
| Pepe 201396 | ||
| Goode 201391 | ||
| Wu 2012106 | ||
| Bollito 201289 | ||
| 2 | Clinical assessment vs. clinical assessment + phi | Stephan 2013104 |
| Lazzeri 201292 | ||
| Scattoni 2013102 | ||
| Porpiglia 201499 | ||
| 3 | Clinical assessment + MRI vs. clinical assessment + MRI + PCA3 | Busetto 201390 |
| Porpiglia 201499 | ||
| 4 | Clinical assessment + MRI vs. clinical assessment + MRI + phi | Porpiglia 201499 |
| 5 | Clinical assessment + PCA3 vs. clinical assessment + phi | Scattoni 2013102 |
| Porpiglia 201499 | ||
| 6 | Clinical assessment + MRI + PCA3 vs. clinical assessment + MRI + phi | Porpiglia 201499 |
| 7 | Clinical assessment vs. clinical assessment + PCA3 + phi | Scattoni 2013102 |
| Porpiglia 201499 | ||
| 8 | Clinical assessment + PCA3 vs. clinical assessment + MRI | Busetto 201390 |
| Porpiglia 201499 | ||
| Panebianco 201194 | ||
| 9 | Clinical assessment + PCA3 vs. clinical assessment + MRI + PCA3 | Sciarra 2012103 |
| 10 | Clinical assessment + phi vs. clinical assessment + MRI | Porpiglia 201499 |
Intervention pathways
Three intervention pathways are of interest to this review; these pathways are repeated here from Box 2.
-
The use of the PCA3 score/phi alongside established risk factors (including histopathology results, PSA level and a DRE) to inform the decision to perform a second biopsy.
-
The use of the PCA3 score/phi alongside established risk factors (including histopathology results, PSA level and a DRE) to inform the decision to perform mpMRI before second biopsy. If the mpMRI image is positive a second biopsy would be performed.
-
The use of the PCA3 score/phi alongside established risk factors (including histopathology results, PSA level and a DRE) to inform the decision to perform a second biopsy in men who have had a negative mpMRI.
Of the three intervention pathways described, data are available from the included studies to address only the first pathway. As the results of tests are most often presented as outputs from logistic regression models, it is not possible to determine from the data available whether or not carrying out the diagnostic tests in one order is better than carrying out the tests in a different order. Nor is it possible to determine whether diagnostic accuracy is improved if the PCA3 assay (or phi) test is carried out before or after a MRI. Therefore, there are no included studies which explicitly address the second or third pathways.
Within-study comparisons: baseline characteristics
The study characteristics of the 15 included study populations (17 papers) are summarised in Table 17. The EAG did not group the studies by PCA3 or phi as some studies included both the PCA3 assay and the phi, and often the tests were assessed using different combinations of tests and different criteria for assessment within a single publication.
| Study | Study design | Manufacturer funding/financial interest | n | Selection criteria | Age (years) | PSA (ng/ml) | Abnormal DRE (%) | Type of repeat biopsy (% positive) |
|---|---|---|---|---|---|---|---|---|
| European cohort (Ankerst 200885) | Prospective cohort; six centres in five European countries | Yes | 443 | Men scheduled for repeat biopsy with one or two previous negative biopsies (≥ 6 cores performed at ≥ 3 months prior to enrolment) | Median 66.0 (range 11–83) | Median 7.0 (range 0.3–85) | 18.7 | Minimum 10 cores from peripheral zone (27.8%) |
| European cohort (Haese 200846) | Yes | 463 | Mean 64.4 (SD 6.6) | Mean 8.9 (SD 7.6) | 19.0 | Minimum 10 cores from peripheral zone (27.6%) | ||
| REDUCE trial – placebo arm (Aubin 201086) | Prospective cohort of patients from placebo arm of REDUCE trial. International multicentre trial | Yes | 1072 | Selection to trial based on PSA level of 2.5–10 ng/ml < 60 years, 3–10 ng/ml > 60 years and a negative initial biopsy. Selection for this study depended on trial site being able to process urine sample for PCA3. Only routine scheduled biopsies used | NR | Range 0.30–33.9 | NR | 10-core TRUS (17.7%) |
| REDUCE trial – placebo arm (Tombal 2013105) | Yes | 1024 | Mean 65.5 (SD 6.0) | Mean 6.4 (SD 3.0) | 3.5 | 10-core TRUS (17.9%) | ||
| Perdonà 201197 | Prospective cohort; two centres in Italy | No | 84 | Men referred for prostate biopsy because of abnormal PSA and/or suspicious DRE. No PSA > 10 ng/ml | Median 66.0 (IQR 60–72)a | Median 6.7 (IQR 5.0–9.0)a | 22.5a | > 12-core TRUS. Median 12 core (IQR 12–16) (34.5%) |
| Panebianco 201194 | Prospective cohort; one centre in Italy | No | 41 | Men with first random TRUS-guided prostate biopsy negative for prostate adenocarcinoma or high-grade PIN; persistent elevated PSA levels (tPSA ≥ 4 ng/ml and < 10 ng/ml) and a negative DRE | Mean 60.3 (range 48–69) | Mean 6.37 (range 4–10) | 0 | 10-core TRUS + three additional cores if MRSI suspicious (68.3%) |
| Bollito 201289 | Prospective cohort; three centres in Italy | No | 509 | Men receiving PCA3 test and referred for repeat biopsy based on persistent PSA elevation. Men with a positive DRE or ASAP on initial biopsy excluded | Median 67 (range 42–89)a | Median 6.7 (range 2.5–48)a | 0 | 14–18 peripheral and transition zone core (24.2%) |
| Sciarra 2012103 | RCT in Italy. Men randomly assigned (1 : 1) to PCA3 only (arm A) or PCA3 plus MRI (arm B) before repeat biopsy | No | 168 | Men with first negative prostate biopsy to cancer and HGPIN, persistent tPSA > 4 ng/ml and a negative DRE | Given by study arm A/B. A: mean 63.2 (SD 7.1); B: mean 64.1 (SD 7.4) | A: mean 6.9 (SD 2.1); B: mean 7.1 (SD 3.5) | 0 | 10-core TRUS + MRI targeted in MRI arm if positive (32.7%) |
| Wu 2012106 | Retrospective cohort; one centre in USA | No | 103 | Indications for repeat prostate biopsy were based on a suspicious DRE, persistently elevated PSA, previous suspicious histology (such as HGPIN or ASAP) and/or patient preference | Mean 63.5 (SD 7.4) | Mean 11.0 (SD 5.5) | 13 | TRUS, at least 12 cores. Additional cores taken if TRUS abnormal (35.9%) |
| Busetto 201390 | Prospective cohort; one centre in Italy | No | 163 | Men with first random TRUS prostate biopsy negative for prostate carcinoma or high-grade PIN; persistent elevated PSA levels (tPSA ≥ 4 ng/ml and < 10 ng/ml) | Mean 66.4 (SD 5.3) | Mean 6.8 (SD 1.6) | 29.4 | 10-core TRUS + two extra cores if MRI abnormal (41.7%) |
| Gittelman 201345 | Prospective cohort in 14 centres in USA | Yes | 466 | Participants were men without prostate cancer and with one or more previous negative prostate biopsies who were recommended by their physician for repeat biopsy | Mean 67.0 (SD 8.1) | Mean 7.0 (SD 5.6) | 27.3 | TRUS, 12 cores or more (21.9%) |
| Goode 201391 | Retrospective cohort; one centre in USA | Not clear | 167 | Men with no known personal history of prostate cancer who underwent a prostate biopsy because of an elevated PSA level, abnormal DRE, or abnormal previous prostate biopsy PIN or ASAP | Median 66 (range 41–90)a | Mean 4.8 (range 0.1–54.2)a | NR | 12 cores (11.4%) |
| Pepe 2011,95 201396 | Retrospective cohort; one centre in Italy | Not clear | 100 | All men had negative family history, a negative DRE, PSA 4.1–10 ng/ml or 2.6–4 ng/ml. All Caucasian | Median 66 | Median 7.9 (range 3.7–10) | 100 | Transperineal saturation biopsy, median 30 (range 24–38) cores; US (28%) |
| Scattoni 2013102 | Prospective cohort; two centres in Italy | Yes | 95 | Indication for repeat biopsy, ASAP, plurifocal HGPIN, PSA 2–15 ng/ml and/or a positive DRE | Mean 67.7 (SD 7.3) | Mean 9.8 (SD 3.9) | 8.5a | Saturation TRUS, 14–24 cores, mean 18.7 (SD 3.2) cores (31.5%) |
| Porpiglia 201499 | Prospective cohort; one centre in Italy | No | 170 | Negative initial biopsy, 12 cores. Persistently elevated PSA levels, and/or a positive DRE | Median 65 (IQR 60–70) | Median 6.9 (IQR 5.2–9.8) | 7.6 | TRUS, 18/24 cores depending on prostate volume, blind to MRI results (30.6%) |
| Stephan 2013104 | Described as both case–control and cohort. Patients enrolled prospectively and retrospectively; four centres in France and Germany | Yes | 280 | Men scheduled for a repeated biopsy, tPSA level 1.6–8.0 ng/ml (WHO calibration) | Negative biopsy: median 61 (95% CI 59 to 63); positive biopsy: median 64 (95% CI 63 to 66) | Negative biopsy: median 5.2 (95% CI 4.9 to 5.6); positive biopsy: median 5.5 (95% CI 5.1 to 6.0) | Negative biopsy: 7; positive biopsy: 20 | TRUS, 10–22 cores (41%) |
| Lazzeri 201292 | Prospective cohort; one centre in Italy | Yes, analysers and reagents only | 222 | Men in whom a first biopsy was negative but in whom suspicion of prostate cancer persisted and who were scheduled for repeat biopsy in accordance with the European Association of Urology guidelines of increasing and/or persistently elevated PSA, a suspicious DRE, ASAP and HGPIN | Mean 63.9 (SD 7.1) | Median 7.6 (range 0.3–46.4) | 14 | 24-core saturation, TRUS. Median 20 (range 12–26) cores (31.9%) |
Study designs and populations
Fourteen studies45,46,85,86,89–92,94–97,99,102–106 were observational cohort studies and one was a randomised controlled trial (RCT). 103 Eleven studies were of a prospective cohort design,45,46,85,86,89,90,92,94,97,99,102,103 three studies91,95,96,106 were of a retrospective cohort design and one study104 was of mixed design. None of the studies was based in the UK; the relevance of the included study results to UK clinical practice is, therefore, uncertain.
In all but one trial, the study population was made up of men who had been referred for repeat prostatic biopsy for clinical indications; the exception was the REDUCE study,86,105 in which men were participants in the placebo arm of a clinical trial.
The criteria for referral for repeat biopsy were often unclear and differed across studies. Some studies were restricted to men with normal89,94,103 or abnormal DREs. 96 The terms ‘positive DRE’ and ‘negative DRE’ were often used, and we assumed that ‘positive’ meant abnormal and ‘negative’ meant normal. The percentage of men with abnormal DRE scores in the studies therefore varied from 0% to 100%. 47,96 The mean (or median) age of study populations was between 6094 and 67 years. 45,89,102 Mean or median PSA, when stated, ranged from 4.891 to 11.0 ng/ml. 106 Seven studies recruited only men with PSA scores within the grey zone of PSA (PSA levels between 4 and 10 ng/ml). 47,86,90,94,96,97,105,107 The prevalence of cancer detected on repeat biopsy varied from 11.4%76 to 68.3%. 94
Recruitment to the placebo arm of the REDUCE trial86,105 did not rely on referral for repeat biopsy. Men aged 50–75 years were recruited to the main REDUCE trial on the basis of increased PSA levels (2.5–10 ng/ml) and a negative initial biopsy. 86,105 Participants were then scheduled to receive a repeat biopsy at 2 and 4 years regardless of clinical indications. A subsample of these men, based on whether or not the trial centre was able to process urine samples for PCA3 testing, were included in this study. 86 However, this study excluded all biopsy results that were indicated by abnormal clinical assessment, such as rising PSA or an abnormal DRE, and used only the results from the biopsies that were mandated by the trial protocol. This study population is, in effect, the reverse selection of the clinically selected population seen in most studies and represents a low-risk population.
The details of the reference standards used in studies were poorly reported. An added complication was the fact that the number of biopsy cores taken often differed across patients within a study. Among cases for which details were provided, 10- or 12-core biopsies were the most common. Three studies96,99,102 exploring the efficacy of the PCA3 score and all four studies92,99,102,104 exploring the efficacy of phi used saturation biopsies or reported that the mean or median number of cores taken was 20 or more. The repeat biopsy was usually performed transrectally under ultrasonography guidance, taking 10–20 cores. Two studies described the repeat biopsy as saturation biopsies,96,102 and in one of these the biopsy route was transperineal96 and in the other transrectal. 102
Within-study comparisons: quality assessment
The results of the Quality Assessment of Diagnostic Accuracy Studies – version 2 assessment81 are presented in Figures 2 and 3, with the full assessments documented in Appendix 7.
FIGURE 2.
Risk of bias and applicability concerns summary: review authors’ judgements about each domain for each included study population. Empty cells shown where study did not report this comparison.
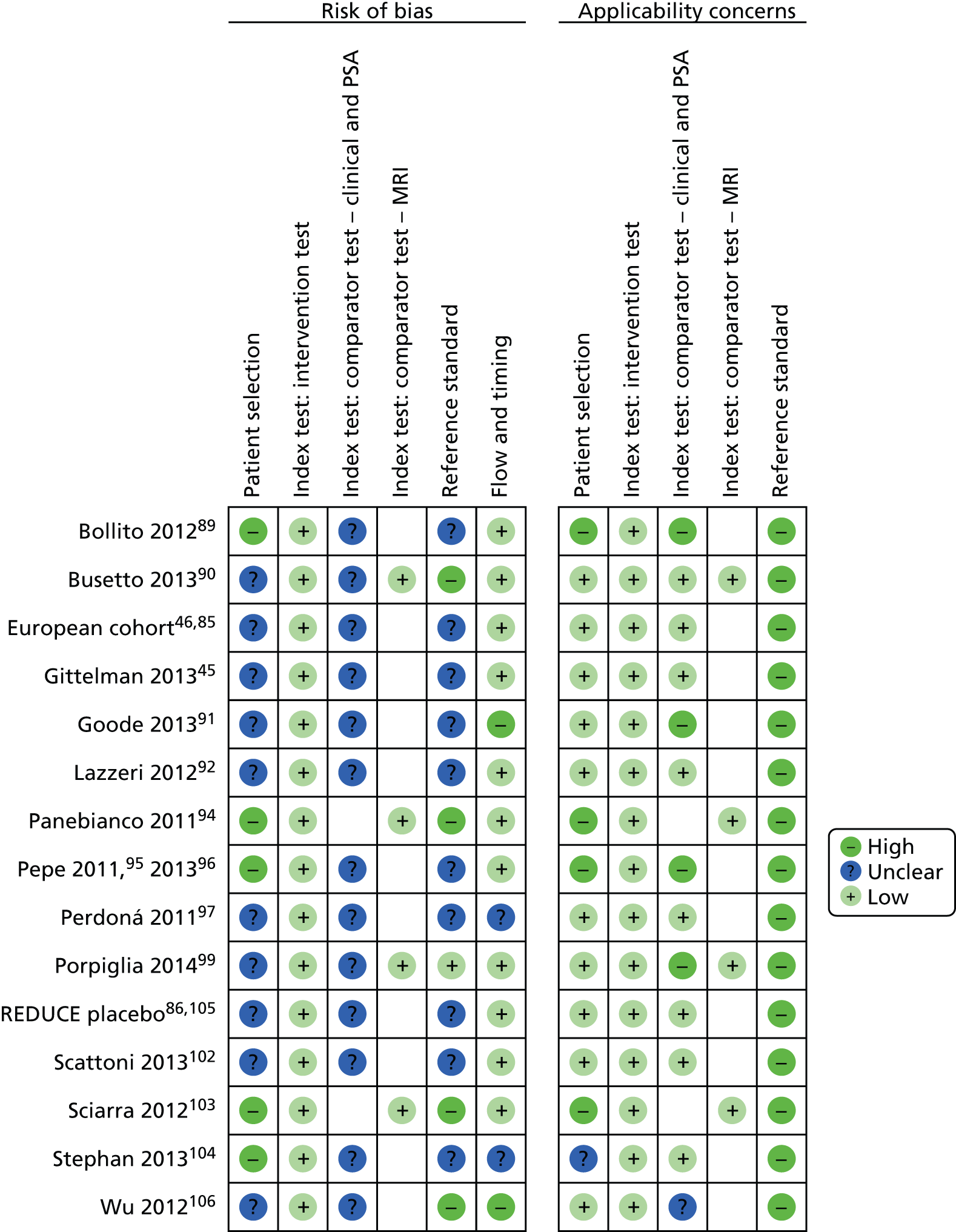
FIGURE 3.
Risk of bias and applicability concerns graph: review authors’ judgements about each domain presented as percentages of all study populations.
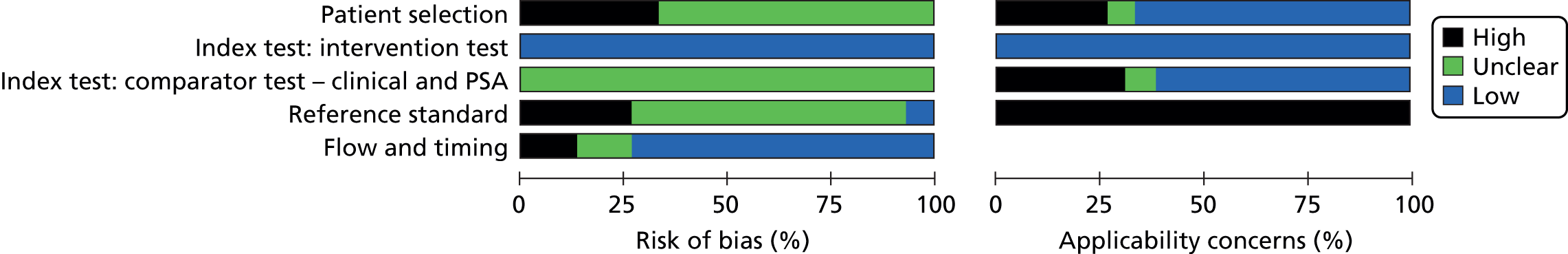
Patient selection
Risk of bias from patient selection was assessed as being unclear for 10 studies,45,46,85,86,90–92,97,99,102,105,106 as the type of clinical pre-selection operating was not explicit, and, therefore, it was impossible to assess how this might have biased the assessment of the clinical variables within the diagnostic models. Four studies89,94,96,103 were assessed as having a high risk of bias because of pre-selection on a DRE or other clinical variables, and these studies89,94,96,103 also had high concerns regarding applicability of patient selection. The study by Stephan et al. 104 was assessed as having a high risk of bias from patient selection as, in this mixed prospective and retrospective study, 29% of patients were excluded from the analyses because it was unclear whether the biopsy was initial or repeat.
Intervention tests
The majority of studies provided no details of whether the intervention tests (PCA3 assay or phi) were conducted with or without knowledge of other important considerations, for example results of comparator tests or the reference standard. Studies that did record this information included those by Gittelman et al. 45 and Goode et al. ,91 the REDUCE trial placebo arm86,105 and the study by Stephan et al. 104 However, given the objective nature of the intervention tests, all studies were assessed as having a low risk of bias. As all study authors reported using the PROGENSA PCA3 assay (or, in the case of four studies,85,86,105,106 this was confirmed by the manufacturer) and/or the Beckman Coulter assay systems, we had little concern regarding the applicability of the intervention tests.
Comparison tests
The risk of bias and concerns regarding applicability arising from the comparison tests were considered separately for clinical assessment, including PSA levels, and for MRI. Clinical assessment, including PSA levels, was often poorly described with no criteria given for an abnormal DRE. It was often not clear when the data used for clinical assessment had been recorded in relation to the timing of the biopsy or the intervention tests and if these had been collected or recorded by the analyst without knowledge of intervention tests or reference standard results. In multicentre studies there was no description of how clinical variables were standardised across centres. All studies were assessed as having an unclear risk of bias from the clinical comparator tests.
Concern regarding applicability of clinical assessment was assessed on the variables used in the multivariate models, algorithm or nomogram. Studies which pre-selected patients who had undergone DREs89,94,96,103 (which meant that a DRE was not included in the model) were marked as being of high concern, as this does not reflect clinical assessment in routine practice. Most studies, including studies of phi, included standard PSA measures such as tPSA or fPSA in the clinical comparator model and added p2PSA to the intervention test model. However, Porpiglia et al. 99 and Goode et al. 91 did not include PSA in the clinical comparator model, and so we had a high degree of concern about the applicability of these study results to UK clinical practice.
Four studies90,94,99,103 which compared the PCA3 assay or phi with MRI were all assessed as having a low risk of bias and concerns regarding applicability of the MRI were low. MRI was either performed before repeat biopsy90,94,103 or the radiologist was blinded to the biopsy result. 99 Diagnostic criteria were described in Porpiglia et al. 99 and Panebianco et al. 94 mpMRI with T2-imaging, DW imaging and DCE-MRI were performed in Porpiglia et al. 99 and mpMRI with T2-imaging, MRS, DW imaging and DCE-MRI were performed in the other studies. 90,94,103
Reference standard
The reference standard involves two procedures, both of which are prone to bias: the targeting and selection of the biopsy cores and the pathology reporting of the cores taken. In many studies,45,46,85,86,91,97,105,106 the reporting of the details of type and pattern of repeat biopsy performed was poor. In seven studies,46,85,89,96,97,102,104,106 the number of cores taken varied, indicating that the number and locations of cores taken were affected by clinical findings such as a DRE or TRUS. In two studies,92,97 although the methods specified a set number of cores to be taken, the number of cores actually taken was reported as a range. In addition to variation in the number and site of biopsy cores taken, pathology reporting is a potential source of bias. In three multicentre studies,45,46,85,104 pathology reporting was not centralised, with potential for differences in biopsy processing protocols and pathology reporting. By contrast, in the REDUCE study,86,105 all cores were processed at a single central laboratory. Four studies indicated that the pathologists were blinded to the clinical status of the patient,45,91,92,99 and one study reported blinding to MRI. 103 Owing to these uncertainties, eight studies45,46,85,86,89,96,97,102,104,105 were assessed as having an unclear risk of bias. If it was clear that additional cores were taken because of abnormalities identified on MRI90,94,103 or TRUS,106 the study was assessed as having a high risk of bias. The study by Porpiglia et al. 99 had a low risk of bias, as it was stated that the biopsies taken were not affected by MRI results or biomarker results and that a constant number of cores (depending on prostate volume) and pattern were taken.
In all studies, the applicability of the reference standard used was an area of high concern. Although the TRUS prostate biopsy is the usual method of diagnosing prostate cancer, this type of biopsy is inaccurate and often misses cancers.
Five studies had funding from, or financial links to, companies which produced the assays. 45,46,85,86,102,104,105 In another study, the manufacturer had supplied reagents. 92
Overall, the results of the quality assessment exercise revealed that none of the studies was free from the risk of bias. The main areas for concern were related to the applicability of the study populations, variation in clinical assessment and whether or not choice and use of the reference standard were linked to previous clinical results. None of the studies was carried out in the UK and so the results of the studies were not directly relevant to NHS decision-makers.
Within-study comparisons: outcome measures reported
Results were most frequently reported using multivariate logistic regression models using AUC statistics, ROC curves, multivariate ORs, and derived sensitivity and specificity values. In these logistic regression models a range of clinical variables were entered into the models separately and were sometimes formulated as a nomogram,85,91,97 sometimes using Bayesian methods. 85 These analyses relied on probabilities generated by the statistical model to classify patients as at risk or not, and generated ROC curves by varying the threshold probability. Intervention tests were added to the baseline clinical models either as a continuous variable or as a binary variable dichotomised at the reported threshold. Studies reported several models using intervention variables continuously and then dichotomously, or used different threshold values to create a dichotomous variable. Where appropriate, these models have been entered separately into the results tables. The EAG notes that only one study105 presented independent sensitivity and specificity estimates.
Two studies96,105 used the clinical variables that were incorporated into a decision tool that classified patients as test positive or negative rather than based on a continuous risk score. In the study by Tombal et al. ,105 ‘best clinical judgement’ was based on expert recommendations which had been formulated using a RAND/University of California, Los Angeles (UCLA) appropriateness model in a previous study. 108 Using variables of life expectancy, a DRE, prior biopsy, prostate volume and PSA score, recommendations for biopsy for each study participant (in the placebo arm of the REDUCE trial) were classified as appropriate or uncertain versus inappropriate. The PCA3 score was then incorporated into the decision tool, grouped into the following score levels: < 20, 20–34, 35–50 and > 50. As the decision tool combined all the variables and produced an overall assessment of test positive or not, conventional sensitivity and specificity were reported.
In Pepe and Aragona,96 a case-finding protocol was used to identify the study population and was tested within the population; results for the case-finding protocol were not included in the results as the case definition altered between analyses. Results for the PCPT risk calculator in this study population are included in results.
Six studies90,92,97,99,102,106 reported results using decision curve analysis. Decision curve analysis calculates the net benefit of a diagnostic model by subtracting the harm of unnecessary biopsies from the benefit of diagnosed cases of prostate cancer. Unlike the conventional trade-off between sensitivity and specificity, in decision curve analysis there is an attempt to weight the relative harms and benefits using the threshold probability of cancer at which the patient or clinician will opt for a biopsy. Further details describing this analysis method are summarised in Appendix 1. The net benefit of various diagnostic models was presented for threshold probability of cancer between 0% and 70%, with all studies reporting results between 10% and 40% threshold probability. The graphs of the decision curve analysis reported in the included studies are presented in Appendix 8.
Seven studies45,46,85,86,89,90,96,103,105 reported diagnostic accuracy results for the PCA3 assay for the detection of more aggressive cancers – usually based on a Gleason score of 7 or higher. In six studies,45,46,86,89,90,103,105 the authors employed univariate analyses and showed the ability of the PCA3 score to predict a Gleason score of 7 or higher. Only one study105 reported how the use of the PCA3 score in combination with clinical assessment contributed to the prediction of more aggressive cancers. Only one study92 considered the relationship between phi and the Gleason score. The results of these analyses are presented in Within-study comparisons: Gleason score.
Within-study comparisons: definition of clinical assessment
There was considerable variation in the definition of clinical assessment used in the included studies. Three studies used the PCPT nomogram,85,96,97 which includes age, ethnicity, a DRE, prostate volume and PSA family history. One study97 used the Chun nomogram,109 which includes age, ethnicity, PSA, a DRE previous biopsies and prostate volume. In other studies, the base model of a series of logistic regression models was taken to represent clinical assessment. For one study99 this included only age and a DRE, and for another89 age and PSA alone, but for others a wider range of clinical risk factors were included, such as PSA, prostate volume, family history and ethinicity. 45,46,85,86,91,92,97,102,104,105 Studies of phi differed according to whether or not tPSA and/or fPSA were included in the clinical assessment definition. Porpiglia et al. 99 did not include PSA in the definition of clinical assessment, whereas other studies92,102,104 included PSA.
Tombal et al. 105 used a clinical decision algorithm which had been developed using RAND/UCLA appropriateness methods and by consulting 12 European urologists. This included measures of life expectancy, a DRE and previous biopsy prostate volume. Pepe and Aragona96 used a case-finding protocol to identify their study population and also included this measure in some analyses, but these results are not included in this review because of differences in definition.
Within-study comparisons: study results
The order in which the results of the comparisons (Tables 18–27) are presented reflects the relevance of the results to health-care professionals who are likely to use the tests in routine clinical practice in the NHS. The EAG considers the four most clinically relevant comparisons to be:
-
clinical assessment versus clinical assessment + PCA3
-
clinical assessment versus clinical assessment + phi
-
clinical assessment + MRI versus clinical assessment + MRI + PCA3
-
clinical assessment + MRI versus clinical assessment + MRI + phi.
Comparison 1: clinical assessment versus clinical assessment + PROGENSA prostate cancer antigen 3 assay
| AUC | ||||||
|---|---|---|---|---|---|---|
| Study | Clinical assessment | Clinical assessment + PCA3 | Difference and p-value if given | |||
| Variables included | Result | Threshold | Result | |||
| aEuropean cohort (Haese 2008)46 | Age, prostate volume, DRE, tPSA and %fPSA | 0.67 | Continuous | 0.71 | +0.04; p < 0.001 | |
| aEuropean cohort (Ankerst 2008)85 | PCPT nomogram: family history, number of previous biopsies, DRE and PSA | 0.65 (95% CI 0.59 to 0.71) | Continuous | 0.70 (95% CI 0.64 to 0.75) | +0.04; p < 0.05 | |
| REDUCE placebo (Aubin 2010)86 | Age, family history, prostate volume, PSA and %fPSA | 0.72 (95% CI 0.68 to 0.76) | Continuous | 0.75 (95% CI 0.71 to 0.79) | +0.04; p = 0.0009 | |
| Scattoni 2013102 | Age, DRE, prostate volume, tPSA and fPSA | 0.75 (95% CI 0.64 to 0.87) | Continuous | 0.76 (95% CI 0.64 to 0.88) | +0.01; p = 0.719 | |
| Busetto 201390 | Age, DRE and PSA | 0.55 (95% CI 0.46 to 0.64) | Continuous | 0.74 (95% CI 0.66 to 0.82) | +0.19; p = 0.0002 | |
| Porpiglia 201499 | Age and DRE | 0.62 (95% CI 0.53 to 0.72) | Continuous | 0.69 (95% CI 0.60 to 0.78) | +0.06 | |
| Gittelman 201345 | Age, family history, race, number of previous biopsies and DRE | 0.65 | 25 | 0.74 | +0.09 (95% CI 0.04 to 0.14); p = 0.0007 | |
| REDUCE placebo (Aubin 2010)86 | Age, family history, prostate volume, PSA and %fPSA | 0.72 (95% CI 0.68 to 0.76) | 35 | 0.74 (95% CI 0.70 to 0.78) | +0.02; p = 0.0558 | |
| Goode 201391 | Age, DRE, prostate volume, race and family history | – | Unclear | 0.61 | – | |
| Perdonà 201197 | PCPT nomogram: age, race, PSA, family history, DRE and previous biopsies | – | Continuous | 0.74 (95% CI 0.63 to 0.83) | – | |
| Perdonà 201197 | Chun nomogram: age, PSA, DRE, previous biopsies and prostate volume | – | Continuous | 0.74 (95% CI 0.64 to 0.83) | – | |
| Multivariate OR for PCA3 | ||||||
| Study | Clinical assessment | Clinical assessment + PCA3 | ||||
| Variables included | Threshold | Result | ||||
| REDUCE placebo (Aubin 2010)86 | Age, family history, prostate volume, tPSA and %fPSA | Continuous | 1.02 (95% CI 1.01 to 1.02) | |||
| Wu 2012106 | DRE, TRUS, PSA and PSAD | Continuous | 1.02 (95% CI 1.00 to 1.03) | |||
| Gittelman 201345 | Age, family history, race, number of previous biopsies, DRE and tPSA | 25 | 4.56 (95% CI 2.65 to 7.84) | |||
| Porpiglia 201499 | Age and DRE | Unclear | 3.88 (95% CI 1.27 to 12.95) | |||
| REDUCE placebo (Aubin 2010)86 | Age, family history, prostate volume, tPSA and %fPSA | 35 | 2.65 (95% CI 1.86 to 3.79) | |||
| Bollito 201289 | Age, PSA and %fPSA | 39 | 9.44 (95% CI 5.15 to 17.31) | |||
| Bollito 201289 | Age, PSA and %fPSA | 50 | 9.29 (95% CI 5.11 to 16.89) | |||
| Sensitivity and specificity | ||||||
| Study | Clinical assessment | Clinical assessment + PCA3 | ||||
| Variables included | Sensitivity (%) | Specificity (%) | Threshold | Sensitivity (%) | Specificity (%) | |
| REDUCE placebo (Tombal 2013)105 | Best clinical judgement (life expectancy, DRE, prior biopsy, prostate volume and PSA): all cancers | 75 (95% CI 68 to 81) | 26 (95% CI 23 to 30) | Grouped: < 20, 20–34, 35–50, > 50 | 66 (95% CI 58 to 72) | 71 (95% CI 67 to 74) |
| REDUCE placebo (Tombal 2013)105 | Best clinical judgement: Gleason score of ≥ seven | 75 (95% CI 61 to 85) | 26 (95% CI 23 to 29) | Grouped: < 20, 20–34, 35–50, > 50 | 85 (95% CI 73 to 93) | 67 (95% CI 64 to 70) |
| Derived sensitivity and specificity at various risk thresholds | ||||||
| Study | Clinical assessment | Clinical assessment + PCA3 | ||||
| Variables included | Sensitivity (%) | Specificity (%) | Threshold | Sensitivity (%) | Specificity (%) | |
| Pepe 201396 | PCPT nomogram: age, race, family history, PSA, DRE and prior biopsy. 25% risk threshold | 100 | 1 | PCPT + continuous PCA3. 25% risk threshold | 100 | 8 |
| Pepe 201396 | PCPT nomogram: age, race, family history, PSA, DRE and prior biopsy. 40% risk threshold | 75 | 26 | PCPT + continuous PCA3. 40% risk threshold | 85.8 | 25 |
| Derived sensitivity: for various set specificity levels | ||||||
| Study | Clinical assessment | Clinical assessment + PCA3 | Difference (%) and p-value if given | |||
| Variables included | Result (%) | Threshold | Result (%) | |||
| 80% specificity | ||||||
| European Cohort (Ankerst 2008)85 | PCPT nomogram: age, family history, number of previous biopsies, DRE and PSA | 43.9 | Continuous | 46.3 | +2.4 | |
| Porpiglia 201499 | Age and DRE | 48.0 | Continuous | 38.5 | –9.5 | |
| 90% specificity | ||||||
| European Cohort (Ankerst 2008)85 | PCPT nomogram: age, family history, number of previous biopsies, DRE and PSA | 24.4 | Continuous | 28.5 | +4.1 | |
| Porpiglia 201499 | Age and DRE | 23.0 | Continuous | 26.9 | +3.9 | |
| 95% specificity | ||||||
| European Cohort (Ankerst 2008)85 | Age, family history, number of previous biopsies, DRE and PSA | 11.4 | Continuous | 17.1 | +5.7 | |
| Porpiglia 201499 | Age and DRE | 17.3 | 32.5 | 19.2 | +1.9 | |
| Derived specificity: for various set sensitivity levels | ||||||
| Study | Clinical assessment | Clinical assessment + PCA3 | Difference (%) and p-value if given | |||
| Variables included | Result (%) | Threshold | Result (%) | |||
| 80% sensitivity | ||||||
| Scattoni 2013102 | Age, DRE, tPSA, fPSA and prostate volume | 49 | Continuous | 47 | –2 | |
| Porpiglia 201499 | Age and DRE | 27.1 | Continuous | 37.3 | +10.2 | |
| 90% sensitivity | ||||||
| Scattoni 2013102 | Age, DRE, tPSA, fPSA and prostate volume | 35 | Continuous | 25 | –10 | |
| Gittelman 201345 | Age, family history, race, number of previous biopsies and DRE | 18.9 (95% CI 10.3 to 36.9) | 25 | 41.5 (95% CI 32.5 to 49.9) | +22.6 (90% CI 9.0 to 33.1) | |
| Porpiglia 201499 | Age and DRE | 12.7 | Continuous | 11.0 | –1.7 | |
| 95% sensitivity | ||||||
| Porpiglia 201499 | Age and DRE | 0.8 | Continuous | 8.5 | +7.7 | |
| AUC | |||||
|---|---|---|---|---|---|
| Study | Clinical assessment | Clinical assessment + phi | Difference and p-value if given | ||
| Variables included | Result | Threshold | Result | ||
| Stephan 2013104 | Age, DRE, prostate volume, tPSA and %fPSA | 0.74 (95% CI 0.67 to 0.80) | Continuous | 0.80 (95% CI 0.74 to 0.85) | +0.06 |
| Lazzeri 201292 | DRE, prostate volume, tPSA, %fPSA and PSAD | 0.68 (95% CI 0.60 to 0.74) | Continuous | 0.78 (95% CI 0.71 to 0.84) | +0.10 |
| Scattoni 2013102 | Age, DRE, tPSA, fPSA and prostate volume | 0.75 (95% CI 0.64 to 0.87) | Continuous | 0.81 (95% CI 0.70 to 0.92) | +0.06; p = 0.137 |
| Porpiglia 201499 | Age and DRE | 0.62 (95% CI 0.53 to 0.72) | Continuous | 0.65 (95% CI 0.55 to 0.74) | +0.02 |
| Multivariate OR for phi | |||||
| Study | Clinical assessment | Clinical assessment + phi | |||
| Variables included | Threshold | Result | |||
| Lazzeri 201292 | DRE, prostate volume, tPSA, %fPSA and PSAD | Continuous | 1.05 (95% CI 1.02 to 1.07) | ||
| Porpiglia 201499 | Age and DRE | Unclear | 3.52 (95% CI 1.04 to 14.14) | ||
| Derived sensitivity: for various set specificity levels | |||||
| Study | Clinical assessment | Clinical assessment + phi | Difference (%) and p-value if given | ||
| Variables included | Result (%) | Threshold | Result (%) | ||
| 80% specificity | |||||
| Porpiglia 201499 | Age and DRE | 48.0 | Continuous | 42.3 | –5.7 |
| 90% specificity | |||||
| Porpiglia 201499 | Age and DRE | 23.0 | Continuous | 25 | +2.0 |
| 95% specificity | |||||
| Porpiglia 201499 | Age and DRE | 17.3 | Continuous | 19.2 | +1.9 |
| Derived specificity: for various set sensitivity levels | |||||
| Study | Clinical assessment | Clinical assessment + phi | Difference (%) and p-value if given | ||
| Variables included | Result (%) | Threshold | Result (%) | ||
| 80% sensitivity | |||||
| Scattoni 2013102 | Age, DRE, tPSA, fPSA and prostate volume | 49 | Continuous | 66 | +17 |
| Porpiglia 201499 | Age and DRE | 27.1 | Continuous | 24.6 | –2.5 |
| 90% sensitivity | |||||
| Scattoni 2013102 | Age, DRE, tPSA, fPSA and prostate volume | 35 | Continuous | 37 | +2 |
| Porpiglia 201499 | Age and DRE | 12.7 | Continuous | 2.5 | –10.2 |
| 95% sensitivity | |||||
| Porpiglia 201499 | Age and DRE | 0.8 | Continuous | 1.7 | +0.9 |
| AUC | |||||
|---|---|---|---|---|---|
| Study | Clinical assessment + MRI | Clinical assessment + MRI + PCA3 | Difference and p-value if given | ||
| MRI type and biopsy | Result | Threshold | Result | ||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 0.94 (95% CI 0.90 to 0.98) | Continuous | 0.93 (95% CI 0.89 to 0.98) | –0.04 |
| Busetto 201390 | T2, MRS, DCE-MRI and DWI. MRI-targeted biopsy | 0.78 (0.71 to 0.85) | Continuous | 0.81 (0.74 to 0.87) | +0.03 |
| Multivariate ORs for PCA3 | |||||
| Study | Clinical assessment + MRI | Clinical assessment + MRI + PCA3 | |||
| MRI type and biopsy | Result | Threshold | Result | ||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 99.52 (95% CI 34.00 to 365.17) | PCA3 – unclear | 1.85 (95% CI 0.26 to 9.90) | |
| MRI | 94.55 (95% CI 32.14 to 346.54) | ||||
| Derived sensitivity: for various set specificity levels | |||||
| Study | Clinical assessment + MRI | Clinical assessment + MRI + PCA3 | Difference (%) and p-value if given | ||
| MRI type and biopsy | Result (%) | Threshold | Result (%) | ||
| 80% specificity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 94.2 | Continuous | 94.2 | 0 |
| 90% specificity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 90.4 | Continuous | 90.7 | +0.3 |
| 95% specificity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 55.8 | Continuous | 55.8 | 0 |
| Derived specificity: for various set sensitivity levels | |||||
| Study | Clinical assessment + MRI | Clinical assessment + MRI + PCA3 | Difference (%) and p-value if given | ||
| MRI type and biopsy | Result (%) | Threshold | Result (%) | ||
| 80% sensitivity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 93.2 | Continuous | 93.2 | 0 |
| 90% sensitivity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 89.0 | Continuous | 89.8 | +0.8 |
| 95% sensitivity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 64.4 | Continuous | 58.5 | –5.9 |
| AUC | |||||
|---|---|---|---|---|---|
| Study | Clinical assessment + MRI | Clinical assessment + MRI + phi | Difference and p-value if given | ||
| MRI type and biopsy | Result | Threshold | Result | ||
| Porpiglia 201499 | Age, DRE, T2, DWI and DCE-MRI. No targeted biopsy | 0.94 (95% CI 0.90 to 0.98) | Continuous | 0.94 (95% CI 0.90 to 0.98) | 0 |
| Multivariate ORs for phi | |||||
| Study | Clinical assessment + MRI | Clinical assessment + MRI + phi | |||
| MRI type and biopsy | Result | Threshold | Result | ||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 99.52 (95% CI 34.00 to 365.17) | phi – unclear | 0.76 (95% CI 0.17 to 4.40) | |
| MRI | 103.47 (95% CI 34.49 to 387.45) | ||||
| Derived sensitivity: for various set specificity levels | |||||
| Study | Clinical assessment + MRI | Clinical assessment + MRI + phi | Difference (%) and p-value if given | ||
| MRI type and biopsy | Result (%) | Threshold | Result (%) | ||
| 80% specificity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 94.2 | Continuous | 94.2 | 0 |
| 90% specificity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 90.4 | Continuous | 90.7 | +0.3 |
| 95% specificity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 55.8 | Continuous | 55.8 | 0 |
| Derived specificity: for various set sensitivity levels | |||||
| Study | Clinical assessment + MRI | Clinical assessment + MRI + phi | Difference (%) and p-value if given | ||
| MRI type and biopsy | Result (%) | Threshold | Result (%) | ||
| 80% sensitivity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 93.2 | Continuous | 93.2 | 0 |
| 90% sensitivity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 89.0 | Continuous | 89.8 | +0.8 |
| 95% sensitivity | |||||
| Porpiglia 201499 | T2, DWI and DCE-MRI. No targeted biopsy | 64.4 | Continuous | 65.3 | +0.9 |
| AUC | |||||
|---|---|---|---|---|---|
| Study | Clinical assessment + PCA3 | Clinical assessment + phi | Difference and p-value if given | ||
| Threshold | Result | Threshold | Result | ||
| Porpiglia 201499 | Continuous | 0.69 (95% CI 0.60 to 0.78) | Continuous | 0.65 (95% CI 0.55 to 0.74) | –0.04 |
| Scattoni 2013102 | Continuous | 0.76 (95% CI 0.64 to 0.88) | Continuous | 0.81 (95% CI 0.70 to 0.92) | +0.05 |
| Multivariate ORs for PCA3/phi | |||||
| Study | Clinical assessment + PCA3 | Clinical assessment + phi | |||
| Threshold | Result | Threshold | Result | ||
| Porpiglia 201499 | Unclear | 3.88 (95% CI 1.28 to 12.95) | Unclear | 3.52 (95% CI 1.04 to 14.14) | |
| Derived sensitivity: for various set specificity levels | |||||
| Study | Clinical assessment + PCA3 | Clinical assessment + phi | Difference (%) and p-value if given | ||
| Threshold | Result (%) | Threshold | Result (%) | ||
| 80% specificity | |||||
| Porpiglia 201499 | Continuous | 38.5 | Continuous | 42.3 | +3.8 |
| 90% specificity | |||||
| Porpiglia 201499 | Continuous | 26.9 | Continuous | 25 | –1.9 |
| 95% specificity | |||||
| Porpiglia 201499 | Continuous | 19.2 | Continuous | 19.2 | 0 |
| Derived specificity: for various set sensitivity levels | |||||
| Study | Clinical assessment + PCA3 | Clinical assessment + phi | Difference and p-value if given | ||
| Threshold | Result (%) | Threshold | Result (%) | ||
| 80% sensitivity | |||||
| Scattoni 2013102 | Continuous | 47 | Continuous | 66 | +19 |
| Porpiglia 201499 | Continuous | 37.3 | Continuous | 24.6 | –12.7 |
| 90% sensitivity | |||||
| Scattoni 2013102 | Continuous | 25 | Continuous | 37 | +12 |
| Porpiglia 201499 | Continuous | 11 | Continuous | 2.5 | –8.5 |
| 95% sensitivity | |||||
| Porpiglia 201499 | Continuous | 8.5 | Continuous | 1.7 | –6.8 |
| AUC | |||||
|---|---|---|---|---|---|
| Study | Clinical assessment + MRI + PCA3 | Clinical assessment + MRI + phi | Difference | ||
| Threshold | Result | Threshold | Result | ||
| Porpiglia 201499 | Continuous | 0.93 (95% CI 0.89 to 0.98) | Continuous | 0.94 (95% CI 0.90 to 0.98) | +0.01 |
| Multivariate ORs for PCA3/phi | |||||
| Study | Clinical assessment + MRI + PCA3 | Clinical assessment + MRI + phi | |||
| Threshold | Result | Threshold | Result | ||
| Porpiglia 201499 | PCA3 – unclear | 1.85 (95% CI 0.26 to 9.90) | phi – unclear | 0.76 (95% CI 0.17 to 4.40) | |
| MRI | 94.55 (95% CI 32.14 to 346.54) | MRI | 103.47 (95% CI 34.49 to 387.45) | ||
| Derived sensitivity: for various set specificity levels | |||||
| Study | Clinical assessment + MRI + PCA3 | Clinical assessment + MRI + phi | Difference and p-value if given | ||
| Threshold | Result (%) | Threshold | Result (%) | ||
| 80% specificity | |||||
| Porpiglia 201499 | Continuous | 94.2 | Continuous | 94.2 | 0 |
| 90% specificity | |||||
| Porpiglia 201499 | Continuous | 90.7 | Continuous | 90.7 | 0 |
| 95% specificity | |||||
| Porpiglia 201499 | Continuous | 55.8 | Continuous | 55.8 | 0 |
| Derived specificity: for various set sensitivity levels | |||||
| Study | Clinical assessment + MRI + PCA3 | Clinical assessment + MRI + phi | Difference and p-value if given | ||
| Threshold | Result (%) | Threshold | Result (%) | ||
| 80% sensitivity | |||||
| Porpiglia 201499 | Continuous | 93.2 | Continuous | 93.2 | 0 |
| 90% sensitivity | |||||
| Porpiglia 201499 | Continuous | 89.8 | Continuous | 89.8 | 0 |
| 95% sensitivity | |||||
| Porpiglia 201499 | Continuous | 58.5 | Continuous | 65.3 | +6.8 |
| AUC | |||||
|---|---|---|---|---|---|
| Study | Clinical | Clinical + PCA3 + phi | |||
| Variables included | Result | Threshold | Result | Difference and p-value if given | |
| Scattoni 2013102 | Age, DRE, tPSA, fPSA and prostate volume | 0.75 (95% CI 0.64 to 0.87) | Continuous | 0.81 (95% CI 0.70 to 0.92) | +0.06; p = 0.17 |
| Porpiglia 201499 | Age and DRE | 0.62 (95% CI 0.53 to 0.72) | Continuous | 0.69 (95% CI 0.60 to 0.78) | +0.07 |
| Multivariate ORs for PCA3 and phi | |||||
| Study | Clinical | Clinical + PCA3 + phi | |||
| Variables included | Threshold | Result | |||
| Porpiglia 201499 | Age and DRE | PCA3 – unclear | 3.87 (95% CI 1.25 to 13.23) | ||
| phi – unclear | 3.44 (95% CI 1.01 to 13.87) | ||||
| Derived sensitivity: for various set specificity levels | |||||
| Study | Clinical | Clinical + PCA3 + phi | Difference (%) and p-value if given | ||
| Variables included | Result (%) | Threshold | Result (%) | ||
| 80% specificity | |||||
| Porpiglia 201499 | Age and DRE | 48.0 | Continuous | 51.9 | +3.9 |
| 90% specificity | |||||
| Porpiglia 201499 | Age and DRE | 23.0 | Continuous | 26.9 | +3.9 |
| 95% specificity | |||||
| Porpiglia 201499 | Age and DRE | 17.3 | Continuous | 19.2 | +1.9 |
| Derived specificity: for various set sensitivity levels | |||||
| Study | Clinical | Clinical + PCA3 + phi | Difference (%) and p-value if given | ||
| Variables included | Result (%) | Threshold | Result (%) | ||
| 80% sensitivity | |||||
| Scattoni 2013102 | Age, DRE, tPSA, fPSA and prostate volume | 49 | Continuous | 49 | 0 |
| Porpiglia 201499 | Age and DRE | 27.1 | Continuous | 39.8 | +12.7 |
| 90% sensitivity | |||||
| Scattoni 2013102 | Age, DRE, tPSA, fPSA and prostate volume | 35 | Continuous | 33 | –2 |
| Porpiglia 201499 | Age and DRE | 12.7 | Continuous | 22.9 | +10.2 |
| 95% sensitivity | |||||
| Porpiglia 201499 | Age and DRE | 0.8 | Continuous | 7.6 | 6.8 |
| AUC | |||||
|---|---|---|---|---|---|
| Study | Clinical + PCA3 | Clinical + MRI | Difference and p-value if given | ||
| Threshold | Result | MRI type and biopsy | Result | ||
| Porpiglia 201499 | Continuous | 0.69 (95% CI 0.60 to 0.78) | T2, DWI and DCE-MRI. No targeted biopsy | 0.94 (95% CI 0.90 to 0.98) | +0.25 |
| Busetto 201390 | Continuous | 0.74 (0.66 to 0.82) | T2, MRS, DCE-MRI and DWI. MRI-targeted biopsy | 0.78 (0.71 to 0.85) | +0.04 |
| aPanebianco 201194 | 35 | 0.76 (0.60 to 0.88) | T2, MRS, DCE-MRI and DWI. MRI-targeted biopsy | 0.86 (0.73 to 0.95) | +0.10 |
| Multivariate OR | |||||
| Study | Clinical + PCA3 | Clinical + MRI | |||
| Threshold | Result | MRI type and biopsy | Result | ||
| Porpiglia 201499 | Unclear | 3.88 (95% CI 1.27 to 12.95) | T2, DWI and DCE-MRI. No targeted biopsy | 99.52 (95% CI 34.00 to 363.17) | |
| Derived sensitivity | |||||
| Study | Clinical + PCA3 | Clinical + MRI | Difference (%) and p-value if given | ||
| Threshold | Result (%) | MRI type and biopsy | Result (%) | ||
| 80% specificity | |||||
| Porpiglia 201499 | Continuous | 38.5 | T2, DWI and DCE-MRI. No targeted biopsy | 94.2 | +55.7 |
| 90% specificity | |||||
| Porpiglia 201499 | Continuous | 26.9 | T2, DWI and DCE-MRI. No targeted biopsy | 90.4 | +63.5 |
| 95% specificity | |||||
| Porpiglia 201499 | Continuous | 19.2 | T2, DWI and DCE-MRI. No targeted biopsy | 55.8 | +36.6 |
| Derived specificity | |||||
| Study | Clinical + PCA3 | Clinical + MRI | Difference (%) and p-value if given | ||
| Threshold | Result (%) | MRI type and biopsy | Result (%) | ||
| 80% sensitivity | |||||
| Porpiglia 201499 | Continuous | 37.3 | T2, DWI and DCE-MRI. No targeted biopsy | 93.2 | +55.9 |
| 90% sensitivity | |||||
| Porpiglia 201499 | Continuous | 11 | T2, DWI and DCE-MRI. No targeted biopsy | 89.0 | +78.0 |
| 95% sensitivity | |||||
| Porpiglia 201499 | Continuous | 8.5 | T2, DWI and DCE-MRI. No targeted biopsy | 64.4 | +55.9 |
| AUC | |||||
|---|---|---|---|---|---|
| Study | Clinical + PCA3 | Clinical + MRI + PCA3 | Difference and p-value if given | ||
| MRI type and biopsy | Result | Threshold | Result | ||
| aSciarra 2012103 | T2, MRS, DCE-MRI and DWI. MRI targeted biopsy | 0.83 (95% CI 0.73 to 0.90) | 35 | 0.86 (95% CI 0.76 to 0.92) | +0.03; p < 0.001 |
| AUC | |||||
|---|---|---|---|---|---|
| Study | Clinical + phi | Clinical + MRI | Difference and p-values if given | ||
| Threshold | Result | MRI type and biopsy | Result | ||
| Porpiglia 201499 | Continuous | 0.65 (95% CI 0.55 to 0.74) | T2, DWI and DCE-MRI. No targeted biopsy | 0.94 (95% CI 0.90 to 0.98) | +0.29 |
| Multivariate ORs | |||||
| Study | Clinical + phi | Clinical + MRI | |||
| Threshold | Result | MRI type and biopsy | Result | ||
| Porpiglia 201499 | Unclear | 3.52 (95% CI 1.04 to 14.14) | T2, DWI and DCE-MRI. No targeted biopsy | 99.52 (95% CI 34.00 to 363.17) | |
| Derived sensitivity – for various set specificity levels | |||||
| Study | Clinical + phi | Clinical + MRI | Difference (%) and p-value if given | ||
| Threshold | Result (%) | MRI type and biopsy | Result (%) | ||
| 80% specificity | |||||
| Porpiglia 201499 | Continuous | 42.3 | T2, DWI and DCE-MRI. No targeted biopsy | 94.2 | +51.9 |
| 90% specificity | |||||
| Porpiglia 201499 | Continuous | 25.0 | T2, DWI and DCE-MRI. No targeted biopsy | 90.4 | +65.4 |
| 95% specificity | |||||
| Porpiglia 201499 | Continuous | 19.2 | T2, DWI and DCE-MRI. No targeted biopsy | 55.8 | +36.6 |
| Derived specificity – for various set sensitivity levels | |||||
| Study | Clinical + phi | Clinical + MRI | Difference (%) and p-value if given | ||
| Threshold | Result (%) | MRI type and biopsy | Result (%) | ||
| 80% sensitivity | |||||
| Porpiglia 201499 | Continuous | 24.6 | T2, DWI and DCE-MRI. No targeted biopsy | 93.2 | +68.6 |
| 90% sensitivity | |||||
| Porpiglia 201499 | Continuous | 2.5 | T2, DWI and DCE-MRI. No targeted biopsy | 89.0 | +86.5 |
| 95% sensitivity | |||||
| Porpiglia 201499 | Continuous | 1.7 | T2, DWI and DCE-MRI. No targeted biopsy | 64.4 | +62.7 |
Area under the curve
Eight AUC results were reported from six study populations45,46,85,86,90,99,102 for the comparison of clinical assessment versus clinical assessment + PCA3; one study86 reported the results from two models, one using the PCA3 score as a continuous variable and one employing a threshold value of 35. Results from the same study population were reported in two separate papers. 46,85 The studies showed an increase in discrimination of between 1% and 19% when the PCA3 score was added to the clinical assessment model, either as a continuous or binary variable.
In addition, two studies91,97 reported AUC results only for models of clinical assessment + PCA3, and these results were similar to the AUC results reported in other studies; Goode et al. 91 reported an AUC of 0.61 for a multivariate logistic regression model and Perdonà et al. 97 reported an AUC of 0.74 for the Chun nomogram and an AUC of 0.74 for the PCPT nomogram.
Multivariate odds ratios for PROGENSA prostate cancer antigen 3 assay
Five studies45,86,89,99,106 reported seven multivariate ORs for clinical assessment + PCA3. Four studies45,86,89,99 presented statistically significant results (ORs were above 1 and CIs did not include 1). One study had an OR above 1 with a CI that included 1. 106 Haese et al. 46 reported that the multivariate OR for the PCA3 score was significant (p = 0.006) in the model but did not report the effect size. These results are consistent with the AUC results and indicate that the addition of the PCA3 score to the clinical assessment model increases discrimination. Two studies86,106 reported ORs for PCA3 using the PCA3 score as a continuous variable; in the remaining studies45,86,89,99 various different thresholds were used to divide the PCA3 scores into a dichotomous variable.
Sensitivity and specificity
Only one study105 presented independent sensitivity and specificity estimates. In this study, the addition of PCA3 scores to best clinical judgement reduced sensitivity from 75% to 66% and increased specificity from 26% to 71%. In this population (prevalence of all cancers = 17.9%) adding the PCA3 score to clinical assessment meant that 18 cancers would have been missed and 371 biopsies would have been avoided compared with clinical assessment alone. However, when the analyses were repeated for cancers with a Gleason score of 7 or higher (prevalence = 5.4%), the addition of PCA3 the score increased sensitivity from 75% to 85% and specificity from 26% to 67%, meaning that six more cancers would have been detected and 395 biopsies would have been avoided compared with clinical assessment alone.
Derived sensitivity and specificity
Pepe and Aragona96 reported sensitivity and specificity for various risk thresholds in the logistic regression model. At a 25% risk threshold, models of the PCPT nomogram alone and of the PCPT + PCA3 had 100% sensitivity and low specificity (1% and 8%, respectively). Using a 40% risk threshold, the model with PCPT alone had 75% sensitivity and 26% specificity, whereas PCPT + PCA3 had 85.8% sensitivity and 25% specificity. This study population comprised Caucasian men with an abnormal DRE and negative family history; the diagnostic power of the PCPT was therefore likely to be reduced.
Two studies85,99 reported derived sensitivity values for specificity levels set at 80%, 90% and 95%. At 90% and 95% specificity, both studies show an improvement in sensitivity when the PCA3 score is added to clinical assessment. However, the derived sensitivity results for 80% specificity are conflicting: Porpiglia et al. 99 shows a 9.5% decrease, whereas Ankerst et al. 85 shows a 2.4% increase in discrimination.
Three studies45,99,102 reported derived specificity for sensitivity set at 80%, 90% or 95%. The results are conflicting. When sensitivity is set at 80% or 90%, Scattoni et al. 102 shows that derived specificity decreases when PCA3 score is added to clinical assessment. Gittelman et al. 45 reports increased derived specificity when sensitivity is set at 90%, when the PCA3 score is added to clinical assessment specificity increases from 18.9% to 41.5%. Porpiglia et al. 99 reports that adding the PCA3 score to clinical assessment increases derived specificity when sensitivity is set at 80% and 95% and reduces derived specificity when sensitivity is set at 90%.
Decision curve analysis
Three studies90,99,102 presented decision curve analyses comparing net benefit for clinical assessment and for clinical assessment + PCA3. The results are presented graphically with no statistical significance testing. The graphs are included in Appendix 7. Visual review of the published graphs in Busetto et al. 90 and Porpiglia et al. 99 suggest that no benefit is gained from adding the PCA3 score to clinical assessment at a threshold probability between 10% and 20%. Net benefit was greater for the model including the PCA3 score in Busetto et al. 90 from 25% to 50% threshold; in Porpiglia et al. 99 the increase in net benefit for the model including the PCA3 score appears only between 20% and 35%, and then the curves are similar. In Scattoni et al. 102 net benefit was reduced when the PCA3 score was added to the clinical assessment at a threshold probability between 10% and 40%. At 40% the curves then reversed with increased net benefit associated with the clinical assessment + PCA3 model from 50% to 90% threshold probability.
Comparison 2: clinical assessment versus clinical assessment + Prostate Health Index
Area under the curve
Four studies92,99,102,104 reported AUC for the comparisons of clinical assessment versus clinical assessment plus phi. All studies showed an increase in discrimination of between 2% and 10% when phi was added to the clinical assessment model as a continuous variable.
Multivariate odds ratios for PROGENSA prostate cancer antigen 3 assay
Two studies92,99 reported multivariate ORs for phi. Both studies presented statistically significant results indicating that an increase in phi score was associated with an increased probability of cancer on biopsy (ORs were above 1 and CIs did not include 1). These results are consistent with the AUC results and indicate that the addition of phi to the clinical assessment model increases discrimination.
Derived sensitivity and specificity
One study99 reported derived sensitivity values for 80%, 90% and 95% specificity. The results were mixed. Adding phi to clinical assessment is associated with either a small increase (2% at 90% and 95% specificity) or a decrease (–5.7% at 80% specificity) in derived sensitivity. Two studies99,102 reported derived specificity for 80% and 90% sensitivity. Scattoni et al. 102 showed that adding phi to clinical assessment increased derived specificity at 80% and 90% sensitivity by 17% and 2%, respectively. Porpiglia et al. 99 showed that adding phi to clinical assessment reduced derived specificity by –2.5% and –10.2% at 80% and 90% sensitivity, respectively; at 95% sensitivity, adding phi to clinical assessment increased derived specificity by 0.9%.
Decision curve analysis
Three studies92,99,102 presented decision curve analyses comparing net benefit for clinical assessment and clinical assessment + phi. Lazzeri92 showed that net benefit was greater for the clinical assessment model at threshold probabilities from 20% to 25% and showed that the clinical assessment + phi model had a greater net benefit at threshold probabilities between 25% and 40%. Scattoni et al. 102 showed increased net benefit for the clinical assessment + phi model at threshold probabilities from 10% to 50%. Porpiglia et al. 99 demonstrated that estimates of net benefit for both models were similar at threshold probabilities between 10% and 70%.
Comparison 3: clinical assessment + magnetic resonance imaging versus clinical assessment + magnetic resonance imaging + PROGENSA prostate cancer antigen 3 assay
Area under the curve
Two studies90,99 investigated the addition of the PCA3 score to a diagnostic model which comprised clinical assessment + MRI. Adding PCA3 score to clinical assessment + MRI had very little effect on the size of the AUC reported. Porpiglia et al. 99 found a slight decrease (–1%) in AUC and Busetto et al. 90 reported a slight increase (3%) in AUC. Only small changes in AUC were expected as models of clinical assessment + MRI give very high estimates of AUC and so adding to these models is not likely to generate substantial gains or losses.
Multivariate odds ratios
Multivariate ORs for clinical assessment + MRI versus clinical assessment + MRI + PCA3 were reported in one study. 99 In the model containing both MRI and PCA3 score, the OR for MRI was much larger (OR 94.55, 95% CI 32.14 to 346.54) than that for PCA3 score (OR1.85, 95% CI 0.26 to 9.90); in this model, the OR for PCA3 score was not statistically significant.
Derived sensitivity and specificity
At 80%, 90% and 95% specificity, Porpiglia et al. 99 reported minimal changes in derived sensitivity for clinical assessment + MRI compared with clinical assessment + MRI + PCA3; derived sensitivity increased by 0%, 0.3% and 0%, respectively.
At 80% and 90% sensitivity, Porpiglia et al. 99 reported minimal changes in derived specificity for clinical assessment + MRI compared with clinical assessment + MRI + PCA3; derived specificity increased by 0%, and 0.8%, respectively. At 95% sensitivity, Porpiglia et al. 99 reported a change in derived specificity of –5.9% when PCA3 score was added to clinical assessment + MRI.
Comparison 4: clinical assessment + magnetic resonance imaging versus clinical assessment + magnetic resonance imaging + Prostate Health Index
Area under the curve
One study99 reported the results of a head-to-head comparison of clinical assessment + MRI versus clinical assessment + MRI + phi. Porpiglia et al. 99 demonstrated that the addition of phi to a model comprising clinical assessment + MRI had no effect on the size of the AUC.
Multivariate odds ratios
Multivariate ORs for clinical assessment + MRI + phi compared with clinical assessment + MRI were reported in one study. 99 In the model containing both MRI and phi, the OR for MRI was larger (OR 103.45, 95% CI 34.49 to 387.45) than the OR for phi (OR 0.76, 95% CI 0.17 to 4.40). In this model, the OR for phi was not statistically significant.
Derived sensitivity and specificity
At 80%, 90% and 95% specificity, Porpiglia et al. 99 reported minimal change in derived sensitivity for clinical assessment + MRI + phi compared with clinical assessment + MRI; derived sensitivity increased by 0%, 0.3% and 0%, respectively.
At 80%, 90% and 95% sensitivity, Porpiglia et al. 99 reported minimal change in derived specificity for clinical assessment + MRI compared with clinical assessment + PCA3; derived specificity increased by 0%, 0.8% and 0.9%, respectively. The addition of phi to diagnostic models incorporating clinical assessment + MRI had a negligible effect on outcome measures.
Decision curve analyses
The decision curve analysis graphs in the study by Porpiglia et al. 99 demonstrate that the addition of phi does not improve diagnostic accuracy when added to clinical assessment + MRI at threshold probabilities between 10% and 60%.
Comparison 5: clinical assessment + PROGENSA prostate cancer antigen 3 assay versus clinical assessment + Prostate Health Index
Area under the curve
Two studies99,102 reported the results of a head-to-head comparison of clinical assessment + PCA3 and clinical assessment + phi. The AUC results of the two studies99,102 were conflicting. Porpiglia et al. 99 reported a 4% decrease in AUC with the use of clinical assessment + phi compared with clinical assessment + PCA3. In contrast, Scattoni et al. 102 demonstrated a 5% increase in AUC with the use of clinical assessment + phi compared with clinical assessment + PCA3.
Multivariate odds ratios
Multivariate ORs for phi and PCA3 scores in separate models were reported in one study. 99 Both statistically significant ORs indicated an increased risk of cancer on biopsy for increases in phi or PCA3 score, with CIs that did not cross 1.
Derived sensitivity and specificity
One study99 showed derived sensitivity for specificity set at 80%, 90% and 95%. At 80% specificity, derived sensitivity was 3.8% higher when using clinical assessment + phi compared with clinical assessment + PCA3. At 90% specificity, derived sensitivity was 1.9% lower when using clinical assessment + phi compared with clinical assessment + PCA3. At 95% specificity, derived sensitivity was the same for both models.
Two studies99,102 reported derived specificity for sensitivity set at 80% and 90%. Scattoni et al. 102 found higher derived specificity for clinical assessment + phi compared with clinical assessment plus PCA3 score for sensitivity set at 80% and 90%. In contrast, Porpiglia et al. 99 reported higher derived specificity for clinical assessment + PCA3 compared with clinical assessment + phi for sensitivity set at 80% and 90%. At 95% sensitivity, Porpiglia et al. 99 showed higher derived specificity for clinical assessment + PCA3 compared with clinical assessment + phi.
Decision curve analysis
The decision curve analyses results reflect the derived sensitivity and specificity results for clinical assessment + PCA3 and clinical assessment + phi. Porpiglia et al. 99 shows a larger net benefit for clinical assessment + PCA3 than for clinical assessment + phi between 15% and 35% threshold probability of cancer. In contrast, Scattoni et al. 102 found that clinical assessment + phi had greater net benefit than clinical assessment + PCA3 at threshold probabilities between 10% and 45%.
Comparison 6: clinical assessment + magnetic resonance imaging + PROGENSA prostate cancer antigen 3 assay versus clinical assessment + magnetic resonance imaging + Prostate Health Index
Area under the curve
Porpiglia et al. 99 reported the results of a head-to-head comparison of clinical assessment + MRI + PCA3 with clinical assessment + MRI + phi. Porpiglia et al. 99 demonstrated that using phi instead of PCA3 score alongside clinical assessment + MRI led to a 1% increase in the AUC.
Multivariate odds ratio
The multivariate OR results from the study by Porpiglia et al. 99 confirm that MRI remains a significant predictor of biopsy outcome when used in addition clinical assessment + PCA3 or clinical assessment + phi, but neither PCA3 score nor phi is a significant predictor in these models.
Derived sensitivity and specificity
Data from the study by Porpiglia et al. 99 suggest that, at 80%, 90% and 95% specificity, derived sensitivity values for clinical assessment + MRI + PCA3 are identical to the derived sensitivity values for clinical assessment + MRI + phi.
Data from the study by Porpiglia et al. 99 suggest that, at 80% and 90% specificity, derived sensitivity values for clinical assessment + MRI + PCA3 are identical to the derived sensitivity values for clinical assessment + MRI + phi. At 95% sensitivity, use of clinical assessment + MRI + phi leads to a 6.8% gain in derived specificity over clinical assessment + MRI + PCA3.
Decision curve analysis
The decision curve analysis graphs for all models containing MRI overlapped at threshold probabilities 10% and 60%, which means that there is no additional increase in net benefit from adding either PCA3 score or phi to clinical assessment + MRI.
Comparison 7: clinical assessment versus clinical assessment + PROGENSA prostate cancer antigen 3 assay + Prostate Health Index
Area under the curve
The effect of adding both PCA3 score and phi to clinical assessment was assessed in two studies;99,102 both studies reported a 6–7% increase in AUC.
Multivariate odds ratios
Multivariate ORs for phi and PCA3 score used together were reported in one study. 99 Both ORs were statistically significant and indicated an increased risk of cancer on biopsy for increases in phi or PCA3 score, with CIs that did not cross 1.
Derived sensitivity and specificity
When adding PCA3 score and phi to clinical assessment, Porpiglia et al. 99 demonstrated small improvements (1.9–3.9%) in derived sensitivity when specificity was set at 80%, 90% and 95%. When sensitivity was set at 80% and 90%, the addition of the PCA3 score and phi to clinical assessment increased derived specificity by 12.7% and 10.2%, respectively; at 95% sensitivity there was a 6.8% increase in derived specificity.
In the study by Scattoni et al. ,102 the addition of PCA3 score and phi to clinical assessment led to no change in derived specificity at 80% sensitivity and a very small decrease (–2%) in derived specificity at 90% sensitivity.
Comparison 8: clinical assessment + PROGENSA prostate cancer antigen 3 assay versus clinical assessment + magnetic resonance imaging
Area under the curve
Three studies90,94,99 reported AUC results for the head-to-head comparison of clinical assessment + PCA3 and clinical assessment + MRI. All of the studies90,94,99 demonstrated an increase in AUC for clinical assessment + MRI compared with clinical assessment + PCA3; Porpiglia et al. 99 showed an increase of 25% and Busetto et al. 90 showed an increase of 4%. In Panebianco et al. ,94 the AUC for clinical assessment + PCA3, for a threshold of 35, was estimated to be 0.76, and the AUC for clinical assessment + MRI was estimated to be 0.86. The data showed an increase in the AUC of 10%; however, it is not clear from the data presented in the study to what extent clinical assessment had been undertaken.
Multivariate odds ratios
Multivariate ORs for PCA3 score and for MRI were reported in one study. 99 The OR for clinical assessment + MRI was high (OR 99.52, 95% CI 34.00 to 363.17) compared with the OR for clinical assessment + PCA3 (OR 3.88, 95% CI 1.27 to 12.95); both ORs were statistically significant. The data indicate that a positive MRI is a better predictor of cancer detected on biopsy than a raised PCA3 score.
Derived sensitivity and specificity
At 80%, 90% and 95% specificity, Porpiglia et al. 99 reported substantial increases in derived sensitivity for clinical assessment + MRI compared with clinical assessment + PCA3; derived sensitivity increased by 55.7%, 63.8% and 36.6%, respectively.
At 80%, 90% and 95% sensitivity Porpiglia et al. 99 reported substantial increases in derived specificity for clinical assessment + MRI compared with clinical assessment + PCA3; derived specificity increased by 55.9%, 78% and 55.9%, respectively.
Comparison 9: clinical assessment + PROGENSA prostate cancer antigen 3 assay versus clinical assessment + magnetic resonance imaging + PROGENSA prostate cancer antigen 3 assay
Area under the curve
The RCT reported in Sciarra et al. 103 randomised participants to PCA3 score alone or PCA3 + MRI. It is not clear from the published paper to what extent clinical assessment was included in any of the analyses. This study demonstrated that the addition of MRI to clinical assessment + PCA3 improved discrimination, as the reported AUC increased by 3 percentage points from 0.83 in the clinical assessment + MRI group to 0.86 in the clinical assessment + MRI + PCA3 group (p < 0.001).
Comparison 10: clinical assessment + Prostate Health Index versus clinical assessment + magnetic resonance imaging
Area under the curve
One study99 reported the results of a head-to-head comparison of clinical assessment + phi versus clinical assessment + MRI. Porpiglia et al. 99 demonstrated a 29% gain in AUC when clinical assessment + MRI was used instead of clinical assessment + phi.
Multivariate odds ratios
Multivariate ORs for clinical assessment + phi and for clinical assessment + MRI were reported for separate models in one study. 99 The OR for MRI in the clinical assessment + MRI model was much larger (OR 99.52, 95% CI 34.00 to 363.17) than that for phi in the clinical assessment + phi model (OR 3.52, 95% CI 1.04 to 14.14).
Derived sensitivity and specificity
Porpiglia et al. 99 showed that large differences in derived sensitivity and derived specificity were achieved when using clinical assessment + MRI compared with clinical assessment + phi.
At 80%, 90% and 95% specificity, Porpiglia et al. 99 reported substantial increases in derived sensitivity for clinical assessment + MRI compared with clinical assessment + phi; derived sensitivity increased by 51.9%, 65.4% and 36.6%, respectively.
At 80%, 90% and 95% sensitivity, Porpiglia et al. 99 reported substantial increases in derived specificity for clinical assessment + MRI compared with clinical assessment + PCA3; derived specificity increased by 68.6%, 86.5% and 62.7%, respectively.
Decision curve analysis
The decision curve analysis graphs in the study by Porpiglia et al. 99 showed a sustained increase in net benefit for clinical assessment + MRI compared with clinical assessment + phi at threshold probabilities between 10% and 60%.
Within-study comparisons: additional data analyses
As the included studies were heterogeneous in many ways (e.g. study population, outcomes reported, threshold used and type of analysis), it was not appropriate, from a clinical or statistical perspective, to carry out a meta-analysis of sensitivity or specificity.
There were insufficient data reported in the included studies in any one subgroup for any of the sensitivity analyses that were considered, as listed in Methods of data analysis/synthesis: clinical validity review, to be undertaken.
Within-study comparisons: Gleason score
Seven studies45,46,86,89,90,92,103,105 reported diagnostic accuracy results for PCA3 score for the detection of more aggressive cancers, usually based on a Gleason score of ≥ 7. In six studies45,46,86,89,90,103 the authors employed univariate analyses and showed the ability of PCA3 score to predict a Gleason score of ≥ 7.
Two studies46,86 reported median PCA3 scores for detected cancers with Gleason score of > or < 7. Both found that the PCA3 scores were higher in cancers with higher Gleason scores. In Haese et al. ,46 the median PCA3 scores were 28.1 for cancers with a Gleason score of < 7 and 45.3 for cancers with a Gleason score of ≥ 7 (p = 0.04). In Aubin et al. ,86 the corresponding median PCA3 scores were 31.8 and 49.5, respectively (p = 0.002). In addition, Busetto et al. 90 reported a statistically significant association (p < 0.001, χ2 = 71.27) between the Gleason score and PCA3 score. Hease et al. 46 also reported significant differences in the median PCA3 scores for clinical stage T1c cancers compared with T2 cancers (26.8 vs. 61.7; p = 0.005) and for indolent cancers (defined as clinical stage T1c, PSA density < 0.15, Gleason score of ≤ 6 and percentage of positive cores ≤ 33%) versus significant cancers (21.4 vs. 42.1; p = 0.006).
Gittelman et al. 45 reported the sensitivity and specificity and the AUC for PCA3 using a score of 25 as the threshold for the detection of all cancers, of cancers with a Gleason score of ≥ 7 and of significant cancers (defined as clinical stage T2 or above, PSA density > 0.15, Gleason score of ≥ 7 and three or more cores positive for cancer). The AUC values reported were 0.707 for all cancers, 0.638 for cancers with a Gleason score of ≥ 7 and 0.689 for significant cancers. The sensitivity values were 77.5 (95% CI 68.4 to 84.5), 76.5 (95% CI 60.0 to 87.6) and 78.9 (95% CI 68.5 to 86.6), respectively, and specificity values were 57.1 (95% CI 52.0 to 62.1), 51.6 (95% CI 46.9 to 56.3) and 55.1 (95% CI 50.2 to 60.0), respectively. There was no evidence that the sensitivity or specificity of the PCA3 assay varied between the groups. Sciarra et al. 103 also reported that there was no statistically significant difference in the predictive accuracy of PCA3 score for cancers with a Gleason score of ≤ 7 or less (3 + 4) and cancers with a Gleason score of ≥ 7 (4 + 3) (p = 0.089).
Bollito et al. 89 and Haese et al. 46 report the numbers of ‘missed’ cancers that would have been missed using PCA3 screening alone and would have had a Gleason score of ≥ 7. In Haese et al. ,46 using a PCA3 score of 20 as the threshold for detection of cancer, 35 out of 128 cancers would have been missed, and 12 of these 35 missed cancers would have had a Gleason score of ≥ 7. Using a PCA3 score of 35 as the threshold for the detection of cancer, 68 out of 128 cancers would have been missed, and 27 of these 68 cancers would have had a Gleason score of ≥ 7. In Bollito et al. ,89 using a PCA3 score of 39 for the threshold for detection of cancer, 22 out of 281 cancers would have been missed and none of these would have had a Gleason score of > 7 (4 + 3). Using a threshold of 50, 29 out of 281 cancers would have been missed and 5 of these 29 would have had a Gleason score of > 7 (4 + 3).
Only one study105 reported how the use of the PCA3 score in combination with clinical assessment contributed to the prediction of more aggressive cancers. It found that PCA3 score had higher sensitivity and specificity for the detection of cancers with a Gleason score of ≥ 7 than for the detection of all cancers. This study105 presented independent sensitivity and specificity estimates for all cancers and demonstrated that the addition of the PCA3 score to best clinical judgement reduced sensitivity from 75% to 66% and increased specificity from 26% to 71%. In this population (prevalence of all cancer = 17.9%), adding the PCA3 score to clinical assessment meant that 18 cancers would have been missed and 371 biopsies would have been avoided compared with clinical assessment alone. However, when the analyses were repeated for cancers with a Gleason score of ≥ 7 (prevalence = 5.4%), the addition of the PCA3 score increased sensitivity from 75% to 85% and specificity from 26% to 67%, meaning that six more cancers would have been detected and 395 biopsies would have been avoided compared with clinical assessment alone.
Only one study92 considered the relationship between phi and the Gleason score. The authors found a significant correlation, with increased phi score being associated with a higher Gleason score (Spearman’s rho 0.299; p = 0.013). It is not clear from the published paper92 whether these findings are for all biopsies or for repeat biopsy only.
Between-study comparisons: search results
Six papers1,15,66,67,70,110 reporting five systematic reviews and meta-analyses were identified which met the inclusion criteria. As data from within-study comparisons were available, these reviews are summarised for completeness in Table 28. The EAG notes that none of these reviews considers clinically relevant comparisons.
| Study | Tests reviewed | Comparator | Number of studies (patients) | Inclusion criteria for studies included in review | Author conclusions |
|---|---|---|---|---|---|
| Luo 2014110 | PCA3 | None | 11 (3373) | Population consisted of adult men who had undergone a repeat biopsy for PCa. The intervention must have consisted of a quantitative determination of PCA3 gene expression in urine samples by molecular biology methods. The prostate biopsy was the gold standard with which to assess the technique. The results had to include the specific values of the diagnostic tests, such as sensitivity, specificity, positive predictive value, negative predictive value and receiver operating characteristic curves, which must have been calculated using TPs, FPs, FNs and TNs | PCA3 can be used for repeat biopsy of the prostate to improve accuracy of PCa detection. Unnecessary biopsies can be avoided by using a PCa cut-off score of 20 |
| Bradley 201466,67 | PCA3 | Clinical nomograms; PSA | 7 (2586) | Appropriate study design; study subjects at increased prostate cancer risk based on increased tPSA and/or an abnormal DRE; and reported results for PCA3, comparator test(s) and prostate biopsy. Studies of initial and repeat population included and repeat studies reported separately | Seven matched studies addressed diagnostic accuracy for PCA3 in populations where all men were having a repeat biopsy. Five studies reported on PCA3 and tPSA, four on %fPSA, one on PSA velocity and two on externally validated nomograms. However, the numbers of comparisons possible for each of these matched analyses remained small. For example, one of three tPSA studies providing AUC data restricted recruitment to men with tPSA levels in the ‘grey zone’. No studies addressed other comparators or outcomes. Strength of evidence was insufficient for all comparisons |
| Mowatt 201314 | MRS; dynamic contrast-enhanced MRI; DW-MRI | T2-MRI; TRUS prostate biopsy | 51 (10,264) | The population considered will be men with suspected prostate cancer and elevated PSA levels up to 20 ng/ml but previously negative biopsy | MRS had higher sensitivity and specificity than T2-MRI. Relative cost-effectiveness of alternative strategies was sensitive to key parameters/assumptions. Under certain circumstances, T2-MRI may be cost-effective compared with systematic TRUS. If MRS and DW-MRI can be shown to have high sensitivity for detecting moderate-/high-risk cancer, while negating patients with no cancer/low-risk disease to undergo biopsy, their use could represent a cost-effective approach to diagnosis. However, owing to the relative paucity of reliable data, further studies are required. In particular, prospective studies are required in men with suspected PCa and elevated PSA levels but previously negative biopsy, comparing the utility of the individual and combined components of a multiparametric magnetic resonance approach (MRS, DCE-MRI and DW-MRI) with both a magnetic resonance-guided/-directed biopsy session and an extended 14-core TRUS-guided biopsy scheme against a reference standard of histopathological assessment of biopsied tissue obtained via saturation biopsy, template biopsy or prostatectomy specimens |
| Zhang 201470 | MRI | Subgroup analysis of MRI vs. MRS vs. MRI + MRSI | 14 (698) | First, only prospective studies with patients having MRI and prior negative prostate biopsies and persistently elevated PSA levels were selected. Second, a histopathological analysis was used as the reference standard. Third, sufficient data were reported to construct 2 × 2 contingency tables consisting of the TP, FP, FN and TN values. Fourth, 10 or more patients had to be included | A limited number of studies suggest that the value of MRI to target prostate cancer in patients with previous negative biopsies and elevated PSA levels appears significant. MRI combined with MRSI is particularly accurate. Further studies are necessary to confirm the eventual role of DW-MRI in this field |
| Eichler 20051 | Systematic prostate biopsy | None | 11 (1071) | Prospective studies were included that compared the cancer yield of a systematic prostate biopsy scheme (index test) with a systematic reference test. Sufficient information had to be available to construct a 2 × 2 table. Excluded were studies that did not compare the index test with the reference test in the same population, non-systematic biopsy schemes (e.g. lesion-directed biopsies), and computer simulation studies. Included participants were men of all age groups with suspected prostate cancer scheduled for a prostate biopsy. Men with already proven prostate cancer were excluded. Studies of initial and repeat population included and repeat studies reported separately when possible | Schemes which apply additional laterally directed cores showed a higher cancer yield. It still has to be demonstrated that extended biopsy schemes with a higher cancer yield do lead to a survival benefit as a result of early cancer detection. The influence first vs. repeated biopsies on the pooled results could not be assessed systematically |
Between-study comparisons: summary of systematic reviews
Two reviews66,67,110 assessed the clinical validity of using the PCA3 score to predict prostate cancer. Luo et al. 110 considered a repeat biopsy population and included studies without a comparator. Luo et al. 110 concluded that use of the PCA3 score improved the accuracy of prostate cancer detection and the authors claimed that unnecessary biopsies could be avoided using a PCA3 threshold of 20%. The Bradley et al. 66,67 review restricted inclusion to studies which compared the PCA3 score with a comparator of either clinical assessment or PSA and concluded that, although the PCA3 score appeared to be more discriminatory for detecting prostate cancer than tPSA, the strength of evidence was low. Bradley et al. 66,67 included initial and repeat biopsy study populations in the full review; the repeat biopsy results were reported separately.
Two reviews14,70 assessed the clinical validity of MRI in the detection of prostate cancer. The review by Mowatt et al. 14 was the most comprehensive, including DW-MRI, dynamic contrast-enhanced MRI and MRS, compared with Zhang et al. ,70 which included MRI and MRS. The number of studies and patients included differed significantly across the reviews; Mowatt et al. 14 included 51 studies with 10,264 patients, and Zhang70 included 14 studies with 698 patients. Zhang et al. 70 concluded that there was some evidence for the effectiveness of MRI in detecting prostate cancer and Mowatt et al. 14 concluded that MRS had higher sensitivity and specificity than T2-MRI. The authors of both reviews highlighted the lack of reliable data and the need for further research.
The review by Eichler et al. 1 assessed the effectiveness of various systematic prostate biopsy schemes to diagnose prostate cancer; entry was not restricted to studies with a comparator. This review1 of 11 studies and 1071 patients included initial and repeat biopsy study populations. Results were reported separately for the repeat biopsy population for some biopsy schemes only. The authors concluded that schemes which apply additional laterally directed cores showed a higher cancer yield and conclude that the impact this has on patient survival has yet to be determined.
Two systematic reviews assessed the clinical validity of PCA3 scores in the diagnosis of prostate cancer,66,67,110 two described the effectiveness of MRI-guided biopsies70,15 and one investigated the effect of systematic protocols for prostate biopsies. 1 No reviews were identified that assessed the effectiveness of phi in a repeat biopsy population.
Discussion of results: clinical validity review
Quality assessment
Generalisability of study population
The clinical validity review addressed the potential use of the PCA3 assay and the phi, in combination with clinical assessment, to assess the need for a second biopsy in men suspected of having prostate cancer whose initial biopsy result was negative or equivocal. This population can be considered to be made up of three different groups of men: first, men who have signs which are strongly suggestive of prostate cancer (such as abnormal DRE findings and/or abnormal histopathology) and who would be referred for second biopsy by most, if not all, clinicians; second, men who no longer display signs of prostate cancer, for example men whose PSA levels have returned to normal and who have no other evident risk factors – many clinicians would not refer this group of men for a second biopsy; and, third, men who fall between these two positions, that is men who have some signs of prostate cancer. In the case of this last group of men, referral to second biopsy may vary between clinicians. The use of PCA3 scores and phi may contribute to the diagnostic process in all three of these populations by providing clinicians with an additional source of clinical information which they can consider before the decision regarding referral to a second biopsy is made.
All but two78,79 of the populations described in the included studies comprise men who were referred for a second biopsy because, following a negative initial biopsy result, clinicians still suspected that malignant prostate cancer was present. In these cases, the effect of adding the PCA3 assay or phi to clinical assessment was tested in populations of men whose clinician had already made the decision that a second biopsy was necessary. The study entry criteria differ across the included studies, suggesting that the disease characteristics of the study populations may be heterogeneous. Therefore, some of these study populations include patients for whom the reason for referral for a second biopsy is clear, while in other studies the reason is unclear.
The two papers78,79 in which the populations had not been referred for a second biopsy report diagnostic accuracy outcomes for participants in the placebo arm of the REDUCE trial. Participants in this trial constitute a low-risk population of men who do not exhibit any clinical signs to suggest that a second biopsy might be appropriate.
These differences in patient selection criteria mean that it may not make clinical sense to apply the results of this review, without clearly stated caveats, to all men with negative or equivocal biopsy results. Furthermore, none of the included studies was conducted in the UK.
Variation in clinical assessment
An additional issue that makes it difficult to draw firm conclusions from the data is that the representation of clinical assessment varies in the included studies. Although clinical assessment is not standardised in practice, it is difficult to meaningfully compare the results of studies which have markedly different representations of clinical assessment. For example, it may be inappropriate to compare the results of clinical assessment (a DRE, age) + phi versus clinical assessment (a DRE, age, family history, prostate volume, ethnicity and PSA level) + PCA3.
Reference standard
How the reference standard was used was unclearly reported in some of the studies, and this is an indication of a poor-quality study. As the choice and conduct of the reference standard may be affected by the results of the comparator or intervention test, studies that stated explicitly that the MRI results influenced the choice of reference standard were assessed as having a high risk of bias. Finally, the reference standard (prostate biopsy) is an imperfect diagnostic tool as it does not detect all cancers. Without a gold standard that offers 100% specificity and 100% sensitivity, it is difficult to confidently assess the accuracy of competing diagnostic strategies. The applicability of all of the included studies was, therefore, assessed as being of high concern and so the quality of the studies was inevitably low.
Summary of key findings
Seventeen45,46,85,86,89–92,94,96,97,99,102–106 relevant studies of within-study comparisons were identified for inclusion in this review of the clinical effectiveness of the PCA3 assay and the phi in combination with existing tests, scans and clinical judgement in the diagnosis of prostate cancer in men who are suspected of having malignant disease and in whom the results of an initial prostate biopsy were negative or equivocal. Data were available from the included studies to compare 10 distinct sets of comparisons. The following four comparisons are most relevant to NHS clinicians:
-
clinical assessment versus clinical assessment + PCA3
-
clinical assessment versus clinical assessment + phi
-
clinical assessment + MRI versus clinical assessment + MRI + PCA3
-
clinical assessment + MRI versus clinical assessment + MRI + phi.
Addition of PROGENSA prostate cancer antigen 3 assay score to clinical assessment
Study findings varied depending on the outcome metric used in the analysis.
Eight45,46,85,86,90,99,102 efficacy comparisons of clinical assessment versus clinical assessment + PCA3 using AUC data demonstrated that the addition of PCA3 score to clinical assessment led to improvements (1–19%) in diagnostic accuracy. Two studies45,86 used the PCA3 score as a dichotomous variable using thresholds of 25 and 35; the remainder used PCA3 score as a continuous variable. The representation of clinical assessment varied across the studies.
Seven45,86,89,99,106 efficacy comparisons using multivariate ORs also showed that the addition of PCA3 score to clinical assessment increased diagnostic accuracy compared with clinical assessment alone; six of the seven ORs for PCA3 score were statistically significant and one OR was borderline. Two studies86,106 reported ORs for unit increase in PCA3 score, four studies45,86,89 used the PCA3 score as a dichotomous variable (25, 35, 39 and 50), and in one study99 the threshold used was unclear. The representation of clinical assessment varied across the studies.
Diagnostic performance was assessed in terms of sensitivity and specificity in only one study. 105 The study by Tombal et al. 105 showed that the addition of PCA3 score to clinical assessment led to a small decrease in sensitivity (from 75% to 66%) but led to a marked increase in specificity (from 26% to 71%).
Studies45,99,102 which fixed sensitivity at 80% or 90% and derived specificity from logistic regression models also reported mixed results. The results from studies90,99,102 that assessed efficacy using decision curve analyses were also mixed, with no clear benefit associated with adding PCA3 score to clinical assessment; increased net benefit was shown in two studies90,99 when the risk threshold was set at 25%. The implications of adding the PCA3 score to clinical assessment are not clear and it is not possible to identify a single-threshold value for use in a clinical setting.
Addition of Prostate Health Index to clinical assessment
Four studies92,99,102,104 compared clinical assessment versus clinical assessment plus phi, and demonstrated higher AUC estimates (2–6%) when phi was included. All of the studies used the phi result as a continuous variable. The representation of clinical assessment varied across studies. Two studies92,99 reported multivariate ORs for clinical assessment + phi and the results indicated that the addition of phi to clinical assessment led to a small improvement in diagnostic accuracy (OR > 1). No studies reported sensitivity and/or specificity and the studies92,99,102 reporting results for derived sensitivity and specificity or decision curve analysis have conflicting results. The implications of adding phi to clinical assessment are not clear and it is not possible to identify threshold values for use in a clinical setting.
The addition of PCA3 score to clinical assessment + MRI to the diagnostic process did not have a noticeable impact on discrimination.
Addition of PROGENSA prostate cancer antigen 3 assay score to clinical assessment + magnetic resonance imaging
Two studies90,99 assessed the incremental gain in diagnostic accuracy resulting from adding PCA3 score to clinical assessment + MRI using AUC estimates; the addition of PCA3 score in both studies had negligible impact. Both studies used PCA3 score as a continuous variable. The study by Busetto et al. 90 employed a MRI-targeted biopsy, whereas the study by Porpiglia et al. 99 did not. The OR for PCA3 score when added to clinical assessment + MRI was not statistically significant.
No studies reported sensitivity or specificity. Porpiglia et al. 99 reported results at set levels of specificity and showed that adding PCA3 score had minimal effect on derived sensitivity; Porpiglia et al. 99 also demonstrated that adding PCA3 score to clinical assessment + MRI at set levels of sensitivity had minimal effect on derived specificity (–5.9% to 0.8%). In these two studies,90,99 the results of decision curve analyses showed that the addition of the PCA3 score to clinical assessment + MRI did not improve diagnostic accuracy when added to clinical assessment + MRI at threshold probabilities between 10% and 50%.
Addition of Prostate Health Index to clinical assessment + magnetic resonance imaging
Only the study by Porpiglia et al. 99 assessed the gain associated with adding phi to clinical assessment + MRI. Adding phi to clinical assessment + MRI had no effect on AUC. The OR for phi when added to clinical assessment + MRI was not statistically significant. At set levels of sensitivity and specificity, the addition of phi had a minor effect on derived specificity and sensitivity. In this study,90,99 the results of decision curve analyses showed that the addition of phi to clinical assessment + MRI did not improve diagnostic accuracy at threshold probabilities between 10% and 60%.
The addition of phi to clinical assessment + MRI to the diagnostic process did not have a noticeable impact on discrimination.
Systematic reviews
None of the systematic reviews identified for inclusion in the clinical validity review included comparisons that assessed the addition of the PCA3 assay or phi to clinical assessment with or without MRI.
Clinical utility review
The planned methods for the clinical utility review were as described in the protocol. 111 No studies were identified for inclusion in the clinical utility review and therefore no results can be reported.
Chapter 3 Assessment of cost-effectiveness
There are two distinct elements to this section on cost-effectiveness. First, the methods and results of a literature search for economic evidence are presented. Second, the EAG’s independent de novo economic model is described alongside comprehensive interpretation of the results generated by the model.
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Systematic review of existing cost-effectiveness evidence
Search strategy
Full details of the main search strategy conducted by the EAG are presented in Chapter 2, Search strategy: analytical validity review. The EAG did not use specific economics-related search terms in the main strategy, as all of the potential references were scanned for studies containing economic evidence.
Inclusion and exclusion criteria
Three reviewers (AN, AB and JH) independently screened all titles and abstracts identified via searching and set aside the subset of records with the term ‘cost’ or ‘economic’ included in the title or abstract (stage 1). At stage 2, two reviewers (AB and SB) independently screened the titles and abstracts of the records that were potentially relevant to the cost-effectiveness review. Full-paper manuscripts of any titles and abstracts that were considered relevant by either reviewer were obtained. The relevance of each study was then assessed (AB and SB) in accordance with the criteria set out in Table 29. Studies that did not meet the criteria were excluded. Any discrepancies were resolved by consensus and, where necessary, a third reviewer was consulted.
| Item | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Intervention or comparator | PCA3, phi | – |
| Study design | Full economic evaluation | Methodological paper, lettera or abstractb |
| Perspective | UK or European perspective | Non-European perspective |
| Population | Men suspected of having prostate cancer who had had at least one negative or equivocal biopsy | Screening population |
Data extraction and quality assessment strategy
The EAG planned to extract data relating to both study design and quality by two reviewers (AB and SB) into an Excel spreadsheet (Excel Software, Henderson, NV, USA). The EAG planned to quality assess all economic evaluations identified for inclusion in the review according to the Drummond and Jefferson112 10-point checklist.
Results: quantity and quality of research available
After deduplication, the 2249 remaining titles and abstracts (when available) were screened for inclusion at stage 1. Of these, 2146 references were immediately excluded because they did not include the term ‘cost’ or ‘economic’ in either the title or the abstract. The remaining 103 records were assessed for eligibility and 99 were excluded because they did not include the relevant comparators or did not consider an eligible study population. Full texts were obtained for four references. 113–116 However, none of the four references met the study inclusion criteria and they were, therefore, excluded from the systematic review. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for the cost-effectiveness review is shown in Figure 4.
FIGURE 4.
The PRISMA flow diagram for the systematic review of cost-effectiveness evidence. ARIF, Aggressive Research Intelligence Facility database; WoS, Web of Science.
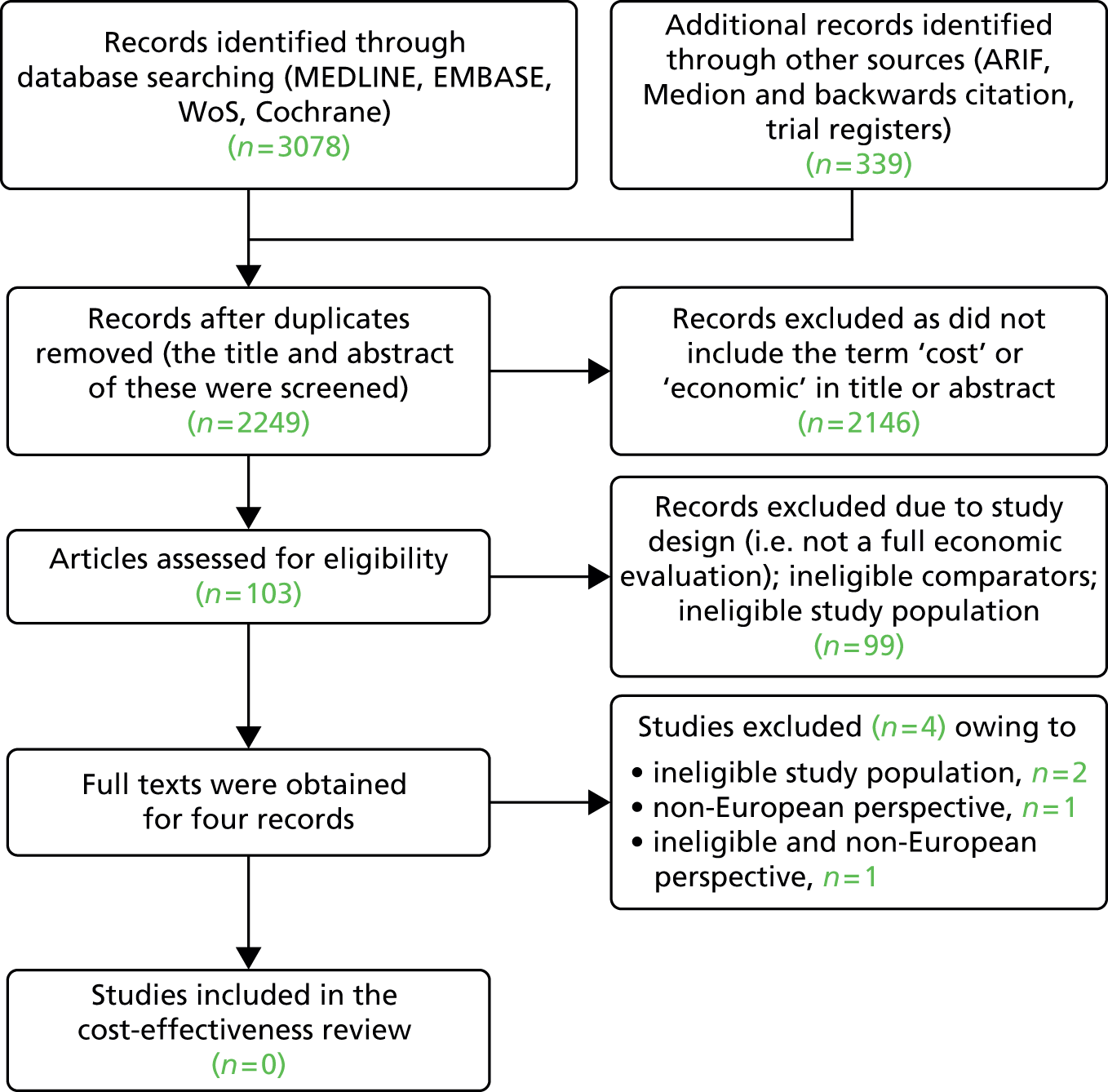
The search carried out by the EAG identified the two studies114,116 (one considering the PCA3 assay and the other phi) that had been summarised in the NICE scope. 49 One of these studies116 focused on a screening population and was carried out from a US health-care perspective. The second study was carried out in France, but only 21.1% of the population had had a prior negative biopsy. 114 Both of these studies were, therefore, excluded from the EAG’s review. The two further studies (both abstracts) identified by the EAG’s search were a study that focused on patients with a prior negative biopsy that was carried out from a US perspective115 and a study that considered a screening population. 113 Both of these studies were, therefore, also excluded from the EAG’s review.
Details of the four studies identified by the EAG search and the reasons for their exclusion from the review are provided in Table 30.
| Study | Title | Reason for exclusion |
|---|---|---|
| Excluded studies | ||
| Heijnsdijk 2012113 | The cost-effectiveness of prostate cancer detection using Beckman Coulter Prostate Health Index | A screening population |
| Malavaud 2013114 | Impact of adoption of a decision algorithm including PCA3 for repeat biopsy on the cost for prostate cancer in France | Only 13.2% of the population had had one negative biopsy (an additional 7.9% had had ≥ 2 negative biopsies) |
| Nepple 2012115 | Cost-analysis of PCA3 vs. PSA in the detection of prostate cancer in men with a prior negative biopsy | Non-European perspective. Additionally, not a full economic evaluation |
| Nichol 2011116 | Cost-effectiveness of prostate health index for prostate cancer detection | A screening population |
Conclusions of the External Assessment Group cost-effectiveness literature review
The EAG did not identify any published papers that met the inclusion criteria for the review.
Independent economic assessment
Approach to modelling
The search for economic literature did not identify any studies that evaluated the cost-effectiveness (from a UK NHS perspective) of PCA3 assay or phi, in combination with existing tests, scans and clinical judgement, in the diagnosis of prostate cancer in men suspected of having malignant disease in whom the results of an initial prostate biopsy were negative or equivocal. A de novo economic analysis was therefore undertaken by the EAG.
Modelling effectiveness
A number of different measures are used by researchers to show the relative efficacy of different diagnostic strategies being considered in this assessment (including sensitivity, specificity, AUC, multivariate ORs and decision curve analyses results). Of these measures, those that are the most readily useable in an economic model are sensitivity and specificity. This is because, in combination, these metrics allow a simple comparison to be made of the number of cancers that are correctly identified and the number of unnecessary biopsies that are undertaken when using competing diagnostic strategies.
The differences in benefits and costs arising from the diagnostic strategies can, therefore, be separated into the benefits and costs arising from differences in:
-
undetected, untreated cancers (the higher the sensitivity, the higher the rate of detected cancer)
-
unnecessary repeat biopsies for patients without cancer (the higher the specificity, the lower the rate of unnecessary biopsies for those without cancer).
Only one of the studies included in the review of clinical validity reported sensitivity and specificity estimates; it was more common for the studies to report derived sensitivity and derived specificity values for a range of different intervention and comparator diagnostic strategies. It was, therefore, not possible for the EAG to undertake a meta-analysis of sensitivity and specificity across trials as the data were unavailable, as explained in Chapter 2, Within-study comparisons: additional data analyses.
In the review of clinical validity, the included studies present sensitivity and specificity results either for specific test thresholds or, more often, as estimates that are derived from logistic regression models.
The EAG’s de novo economic model uses the derived specificities for stated sensitivity levels. The questions that this approach address are ‘Given a desired cancer detection rate for the target population, what proportion of the population would need a second biopsy?’ and ‘What proportion of these second biopsies would be unnecessary?’.
The EAG acknowledges that this approach to modelling may not precisely reflect clinical practice in the NHS in England and Wales. As stated in Appendix 1, derived sensitivity or derived specificity estimates are calculated from ROC curves, and it is often not possible to associate a stated sensitivity/specificity combination with a particular threshold of the intervention test.
As sensitivity and specificity are required to populate the model, the EAG also considered using sensitivity values for the tests that were based on the estimated cancer rates associated with the different threshold levels recommended by the manufacturers. However, to translate these values into estimates of sensitivity and specificity, several other pieces of information would be required:
-
the proportion of patients that would be at each threshold
-
how clinical assessment of other patient information (PSA level, DRE findings, etc.) would influence whether or not a biopsy would be recommended at different thresholds
-
the proportion of patients who, on being recommended to receive a second biopsy, choose to receive one.
As this information is not readily available, the values for the above would have had to be assumed to generate sensitivity–specificity combinations. The EAG considered that such an approach would generate considerable uncertainty and that a more robust approach would be to focus on the available evidence on derived sensitivity–specificity combinations that is underpinned by findings from clinical studies, if not from clinical practice.
The use of derived specificity at stated sensitivity levels allows a fair comparison to be made between different testing strategies. Using this approach, the percentage of cancers that are detected is always the same regardless of the strategy chosen, but the number of biopsies required to detect these cancers differs. This simplifies the decision problem, negating issues such as which test threshold values to use in the model and how test results interplay with patient and clinician risk preferences.
As the percentage of detected underlying cancers is the same for all diagnostic strategies in the EAG model, the proportion of patients with treated and untreated cancers is also the same for all diagnostic strategies. Consequently, patient benefits and costs from cancer detection and treatment are the same for all diagnostic strategies. Therefore, as specificity levels for a given level of sensitivity differ across the comparator diagnostic strategies, the differences in patients’ benefits and costs between strategies are driven only by the difference in unnecessary biopsies carried out on patients without cancer. Although there is some evidence that biopsies may be linked to increased mortality in the short term, this is as yet unproven. 117 The EAG model, therefore, only considers the short-term impact of a biopsy on QoL and associated complications.
Population
As stated in Chapter 2, Within-study comparisons: baseline characteristics, the populations described in the studies included in the clinical validity review are mainly made up of men who have been referred for a second biopsy because, following a negative initial biopsy result, clinicians still suspect that malignant prostate cancer is present. Data from one study population were reported in two publications that included men in a clinical trial who were scheduled for a second biopsy without obvious clinical signs of prostate cancer and a negative or equivocal initial biopsy. However, data from these two studies78,79 did not provide evidence on sensitivity and specificity in a form that could be incorporated into the economic model and so the model population comprises those for whom a suspicion of cancer remains despite negative or equivocal results following their initial biopsy.
In the EAG model, the assumed prevalence of undetected cancer after initial biopsy is 24%. This is based on a study of cancer detection rates using a saturation biopsy17 for a cohort of patients with a previous negative or equivocal biopsy result but persistently elevated PSA levels (> 4 ng/ml) and/or an abnormal DRE.
Comparators
Clinical validity data were available for the following diagnostic strategies and these are the diagnostic strategies that have, therefore, been included in the economic model:
-
clinical assessment
-
clinical assessment + PCA3
-
clinical assessment + phi
-
clinical assessment + PCA3 + phi
-
clinical assessment + mpMRI
-
clinical assessment + mpMRI + PCA3
-
clinical assessment + mpMRI + phi
-
clinical assessment + mpMRI + PCA3 + phi.
Model structure
A schematic of the diagnostic strategy used in the model is shown in Figure 5. Following an initial negative biopsy, clinical assessment alone, or results from an alternative diagnostic strategy are used by the clinician to decide whether or not to recommend a second biopsy.
FIGURE 5.
Model pathway. –ve, negative; +ve, positive.

As part of the development of the NICE clinical guideline CG175,11 an economic model was produced which explored the use of mpMRI before TRUS-guided prostate biopsy in men with suspected prostate cancer. Following the approach taken in the CG175 MRI model,11 the EAG has assumed that all patients who are recommended for a second biopsy choose to have a biopsy and all those for whom a second biopsy is not recommended do not demand one. Patients having a biopsy may experience a short-term deterioration in QoL; in addition, biopsies may result in complications.
In the CG17511 MRI model, patients whose second biopsy results are negative or equivocal do not immediately have a third biopsy; instead they enter a PSA monitoring phase. When choosing the most appropriate monitoring and future biopsy strategy to employ, the EAG considered the PSA monitoring strategy used in the CG17511 MRI model and also drew on the content of a recently published HTA report by Mowatt et al. ,14 which included a model that explored the cost-effectiveness of mpMRI to aid the localisation of prostate abnormalities for biopsy; the authors of CG17511 also drew on data reported in the HTA report.
The CG17511 MRI model, which assessed the use of TRUS biopsy with or without mpMRI, included the following assumptions:
-
If a first TRUS biopsy is negative, 50% of patients are offered, and accept, a second TRUS biopsy, which is undertaken 3 months after the first biopsy.
-
None of the patients in whom the first biopsy, a mpMRI-targeted-biopsy, is negative is offered a second biopsy.
-
All patients in whom a first mpMRI-targeted biopsy is negative or in whom a first or second TRUS biopsy is negative (if a second biopsy is undertaken) are assumed to remain at risk of cancer and their PSA level is monitored. It is assumed that after 1 year, 2 years and 3 years, respectively, 25%, 50% and 100% of these patients are sent for a second investigation. Thus, after 3 years, all patients will have had two investigations. The second investigation is either a repeat TRUS biopsy or mpMRI followed by a biopsy (if the mpMRI indicates that a biopsy should be carried out).
-
Patients with negative findings after a second investigation continue to have their PSA level monitored but, after 1 year, 2 years and 3 years, 25%, 50% and 100% of these patients, respectively, are sent for a saturation biopsy.
Under these assumptions, all patients with cancer have a correct diagnosis after 6 years, with the majority of those whose cancer was originally missed having a correct diagnosis after 3 years.
The population in the Mowatt et al. 14 model comprised patients who had already been selected to undergo a second biopsy. The Mowatt et al. 14 model includes the following assumptions:
-
Following a second biopsy, patients who are classified as having no cancer have their PSA level monitored every 6 months for a year. Those with undetected cancer are assumed to have a rising PSA level at the end of the year, whereas the PSA level of those without cancer is assumed to be stable.
-
Patients with an elevated PSA level are offered a saturation biopsy and 90% agree to undergo this procedure.
The PSA monitoring assumption used in the CG17511 MRI model is that every man shown to be cancer free at the time of his initial biopsy, and who is not sent for a second biopsy, requires PSA monitoring and goes on to have one, possibly two, further biopsies over the next 3–6 years. Those in whom the results of a second biopsy are negative or equivocal do not enter PSA monitoring and so do not incur PSA monitoring costs or have the potential to undergo a third biopsy. This means that, at best, the comparator diagnostic strategies can achieve a reduction in the number of second biopsies at the expense of up to 6 years of PSA monitoring and at least one further biopsy. In the EAG model, such an assumption would result in the optimal strategy being to immediately carry out a further biopsy on everyone whose initial biopsy was negative or equivocal and undertake no additional PSA monitoring.
In the base case, the EAG has, therefore, adopted the assumption used in the Mowatt et al. 14 model, that is that patients with undiagnosed cancer, whether or not they have undergone a second biopsy, will continue to have elevated PSA levels. In addition, the EAG has assumed that 25% of men without cancer will also continue to have a rising PSA level and that, at 1, 2 and 3 years, 25%, 50% and 100% of patients, respectively, with a rising PSA level will have a saturation biopsy. The EAG has included sensitivity analyses to explore the impact of 0%, 25%, 50% and 75% of men with a negative second biopsy entering PSA monitoring.
In addition, the following two scenario analyses have been undertaken by the EAGA:
-
the monitoring and second biopsy strategy used in the CG17511 MRI model
-
the monitoring strategy used in the Mowatt et al. model. 14
Time horizon
The NICE reference case118 states that the time horizon of economic models should be:
Long enough to reflect all important differences in costs or outcomes between the technologies being compared.
p. 57118
In the EAG economic assessment, the differences in costs and outcome are limited to:
-
the differences in costs and complication-related outcomes from the additional biopsies indicated by the testing strategies
-
the costs and outcomes of any monitoring of patients who are either indicated as negative for cancer by the testing strategy or who have a negative repeat biopsy.
As a PSA monitoring strategy can run for several years, the time horizon of the model is limited to the time that patients spend within any such strategy. The monitoring strategy is independent of the diagnostic strategies assessed in the model, so unless there is a lifetime PSA monitoring strategy the model does not require a lifetime horizon. In the base case, the PSA monitoring strategy runs for 3 years so the time horizon is also 3 years. The time horizons for the scenario analyses exploring the impact of the PSA monitoring strategies used in the CG17511 MRI model and the Mowatt et al. 14 model are 6 years and 1 year, respectively.
There is currently no unequivocal evidence that the biopsy procedure increases mortality. In the EAG model, the proportion of cancers identified and treated is assumed to be identical regardless of testing strategy. Thus, overall mortality rates will also be identical across testing strategies and so were not included in the model. Mortality could influence costs during the monitoring phase, but Bill-Axelson et al. ,119 who collected data on 348 patients with localised cancer being monitored by watchful waiting, found very low mortality rates over 3 years (under 5%). As almost all of the population in the EAG model who enter PSA monitoring do not have prostate cancer, and those who do are picked up and treated in a relatively short period of time, mortality rates would be even lower for patients in the EAG model than the levels reported by Bill-Axelson et al. 119 Given this, the impact on cost from introducing mortality into the model would be negligible and so has been excluded.
In addition, the following two scenario analyses were undertaken by the EAG:
-
the monitoring and second biopsy strategy used in the CG17511 MRI model
-
the monitoring strategy used in the Mowatt et al. model. 14
These two scenarios can be considered to represent the ‘least costly’ (Mowatt et al. 14) and ‘most costly’ (CG175 MRI model11) PSA monitoring scenarios.
A sensitivity analysis which involved varying the percentage of patients without cancer who had persistently elevated PSA levels, and so would require re-biopsy while under PSA monitoring, was considered. However, the Mowatt et al. 14 scenario considers the case that no men without cancer continued to have an elevated PSA level and the CG17511 MRI model scenario considered the case where all men without cancer continued to have an elevated PSA level. These two scenarios represent the two extremes of the percentage of men with persistently elevated PSA levels. Thus, the EAG felt that the inclusion of a sensitivity analysis varying the percentage of patients without cancer who had a persistently elevated PSA level would be uninformative and so such an analysis was not undertaken.
Model parameters
Clinical effectiveness
The clinical effectiveness estimates for different diagnostic testing strategies have been taken from the available published clinical evidence (see Chapter 2). As it has not been possible to carry out between-trial analysis and pool effectiveness data, the data in each study have been considered independently. Three studies provide information on derived specificity at differing sensitivity levels: Porpiglia et al. ,99 Scattoni et al. 102 and Gittelman et al. 45 Of these, Porpiglia et al. 99 provide data for all of the diagnostic testing strategies considered by the other two studies102,45 plus additional strategies that include mpMRI. Therefore, results from Porpiglia et al. 99 have been used in the base case, while data from the other two studies102,45 have been used in scenario analyses to explore the effect that different levels of effectiveness (and elements of clinical assessment) might have on conclusions.
Clinical validity data reported by Tombal et al. 105 were considered for incorporation into the model. However, while sensitivity and specificity values are reported they are only reported for a specific PCA3 threshold value. Reported results, therefore, do not allow comparisons of specificity rates (at stated sensitivity levels) for the PCA3 assay against alternative strategies and so have not been used in the model.
Clinical advice to the EAG is that it is very difficult to pinpoint a precise sensitivity estimate that most clinicians use in clinical practice. Furthermore, clinical decisions regarding biopsy referral are made with sensitivity implicitly in mind but not explicitly stated. Choosing a sensitivity level to use in the EAG base case was necessarily arbitrary; 90% sensitivity was chosen as it is the middle estimate of the three levels of sensitivity data that were provided in the study reported by Porpiglia et al. 99 The impact of using sensitivity levels of 80% and 95% was explored in scenario analyses. Only the Gittelman et al. 45 paper included data on the variance, or range, of the derived specificity estimates and, therefore, it has not been possible to vary these values in the probabilistic sensitivity analysis.
Studies reporting clinical validity data use a biopsy as the reference standard despite biopsies not being 100% sensitive or specific. To check the impact that this assumption has on model findings, sensitivity analyses were undertaken in which the proportion of cancers detected at biopsy were set at 50% and 100%.
As stated in CG175,11 mpMRI-targeted biopsy has greater sensitivity and specificity than TRUS biopsy alone. However, in the Porpiglia et al. study,99 which includes data on the sensitivity and specificity of mpMRI in combination with PCA3 assay or phi, the urologists were blinded to the mpMRI results before a biopsy was taken. Although mpMRI can influence biopsy sensitivity and specificity, the EAG has assumed that a biopsy after mpMRI does not influence the final diagnostic accuracy of the second biopsy. This assumption will put downwards pressure on the efficacy of mpMRI, but the decision question is ultimately about the addition of PCA3 assay or phi with or without MRI. Thus, this assumption will influence only the comparison of mpMRI with strategies without mpMRI, biasing the findings against mpMRI.
The sensitivity/derived specificity values used for the different diagnostic strategies are presented in Table 31.
| Study | Sensitivity (%) | Derived specificity |
|---|---|---|
| Clinical assessment | ||
| Porpiglia 201499 | 80 | 27.1 |
| 90 | 12.7 | |
| 95 | 0.8 | |
| Scattoni 2013102 | 80 | 49.0% |
| 90 | 35.0% | |
| Gittelman 201345 | 90 | 18.9% |
| Clinical assessment + PCA3 | ||
| Porpiglia 201499 | 80 | 37.3 |
| 90 | 11.0 | |
| 95 | 8.5 | |
| Scattoni 2013102 | 80 | 47.0% |
| 90 | 25.0% | |
| Gittelman 201345 | 90 | 41.5% |
| Clinical assessment + phi | ||
| Porpiglia 201499 | 80 | 24.6 |
| 90 | 2.5 | |
| 95 | 1.7 | |
| Scattoni 2013102 | 80 | 66.0% |
| 90 | 37.0% | |
| Clinical assessment + phi + PCA3 | ||
| Porpiglia 201499 | 80 | 39.8 |
| 90 | 22.9 | |
| 95 | 7.6 | |
| Scattoni 2013102 | 80 | 49.0% |
| 90 | 33.0% | |
| Clinical assessment + mpMRI | ||
| Porpiglia 201499 | 80 | 93.2 |
| 90 | 89.0 | |
| 95 | 64.4 | |
| Clinical assessment + mpMRI + PCA3 | ||
| Porpiglia 201499 | 80 | 93.2 |
| 90 | 89.8 | |
| 95 | 58.5 | |
| Clinical assessment + mpMRI + phi | ||
| Porpiglia 201499 | 80 | 93.2 |
| 90 | 89.8 | |
| 95 | 65.3 | |
| Clinical assessment + mpMRI + phi + PCA3 | ||
| Porpiglia 201499 | 80 | 93.2 |
| 90 | 89.8 | |
| 95 | 56.8 | |
Biopsy complications
The CG17511 MRI model provides a detailed description of biopsy complication rates identified by a literature review. As CG17511 was published in the same year as the EAG model was constructed, it was postulated that it might be appropriate to use the complication rates used in CG17511 in the EAG model. Citation searches were carried on the relevant studies. 120,121 These searches failed to identify more up-to-date rates and so the complication rates used in the CG17511 MRI model were also used in the EAG model.
Biopsy complication rates are shown in Table 32. The costs associated with biopsy complications that are included in the model should be considered as conservative, as literature searches failed to identify any published studies reporting the costs associated with sepsis or antibiotic resistance. To establish whether or not this omission could affect findings, a sensitivity analysis was undertaken in which all complication costs were increased by 100%.
| Event | Probability (95% CI) | Distribution for PSA | Source |
|---|---|---|---|
| Biopsy complication | 0.117 (0.100 to 0.137) | Beta distribution: alpha 134; beta 1013 | Rosario 2012121 |
| Probability of hospital admission given biopsy complication | 0.112 (0.069 to 0.176) | Beta distribution: alpha 15; beta 119 | Rosario 2012121 |
| Reasons for hospital admission | |||
| Urinary infection | 0.716 (0.675 to 0.738) | Dirichlet distribution: alpha 556 | Nam 2010120 |
| Urinary bleeding | 0.194 (0.166 to 0.221) | Dirichlet distribution: alpha 151 | Nam 2010120 |
| Urinary obstruction | 0.090 (0.081 to 0.124) | Dirichlet distribution: alpha 79 | Nam 2010120 |
| Biopsy-related consultation after complication | 0.888 (0.824 to 0.931) | Beta distribution: alpha 119; beta 15 | Rosario 2012121 |
| Location of consultation | |||
| GP | 0.773 (0.690 to 0.839) | Dirichlet distribution: alpha 92 | Rosario 2012121 |
| Urology department nurse | 0.118 (0.071 to 0.138) | Dirichlet distribution: alpha 14 | Rosario 2012121 |
| Other – NHS Direct | 0.109 (0.065 to 0.178) | Dirichlet distribution: alpha 13 | Rosario 2012121 |
Values for the upper and lower CIs have been used to model pessimistic and optimistic resource use scenarios.
Cost year
Unless otherwise stated, the costs are in 2014 GBP.
Cost of clinical assessment
The diagnostic strategies included in the EAG model comprise one or more of four separate components: clinical assessment, phi, PCA3 assay and mpMRI. While the nature of clinical assessment varies between studies, within studies it is the same for all participants. As the model does not pool data but looks at evidence from studies individually, and clinical assessment is required in all diagnostic strategies, there is no requirement to model the cost of clinical assessment, as it will make no difference to costs between strategies.
Cost of PROGENSA prostate cancer antigen 3 assay
The PCA3 assay costs provided by the manufacturer have been calculated by applying UK costs to resource use obtained from a US study. (Commercial-in-confidence information has been removed.) The estimated cost of the PCA3 testing kit was given as £164.67 including value-added tax (VAT) and (commercial-in-confidence information has been removed). This higher cost of £175.11 has been used in a scenario analysis.
The cost of the PCA3 assay has not been varied in the probabilistic sensitivity analysis.
Cost of Prostate Health Index
The manufacturer provided the cost of a single phi test. This was £89.83 including VAT. (Commercial-in-confidence information has been removed.) Deterministic sensitivity analysis has been used to explore the impact of the number of tests being 50% lower and 50% higher than this figure, effectively changing the cost of the test by ± 50%.
With no evidence available on the distribution of tests conducted in a year, the cost of phi testing has not been varied in the probabilistic sensitivity analysis.
Cost of multiparametric magnetic resonance imaging
The CG17511 report provided detailed costings of mpMRI and these costings were used in the EAG model, updated where necessary with up-to-date unit or NHS Reference Costs (2012/13). 18
The unit costs for staff time and equipment costs used in the CG17511 MRI model were taken directly from Mowatt et al. 14 However, staffing costs, which were based on bottom-up calculations of staff time, were increased in the CG17511 MRI model, as the NICE Guidelines Development Group considered that they were an underestimate. Resource use and costs of mpMRI are provided in Table 33.
| Resource | Time per patient (minutes) | Cost per hour (£) | Total cost (£) |
|---|---|---|---|
| Radiographer 1 | 43.33 | 48.33 | 34.91 |
| Radiographer 2 | 43.33 | 50.00 | 36.11 |
| Radiologist – consultant | 45.00 | 162.00 | 121.50 |
| Equipment cost per patient | – | – | 88.42 |
| Administration and consumable cost | – | – | 34.62 |
| Total mpMRI cost | – | – | 315.56 |
As no measures of dispersion were available on the cost per hour, or time, per patient no sensitivity analyses were undertaken around this cost.
Costs of biopsy
Biopsy costs are dependent on the type of biopsy undertaken. In the base case, the EAG has assumed that the second biopsy will be a TRUS biopsy carried out as an outpatient appointment.
The assumption used in the CG17511 MRI model is that mpMRI results influence whether a TRUS or a transperineal biopsy is performed. Incorporating this assumption into the EAG model is difficult as it contradicts the model assumption that mpMRI does not influence the nature, or accuracy, of the second biopsy, an assumption made because the evidence99 from which efficacy data were drawn explicitly blinded clinicians performing the biopsy to the mpMRI results. Therefore, while in the base case the model assumption is that all patients have a TRUS biopsy as an outpatient procedure, a scenario analysis has explored the impact on results of a situation in which 50% of second biopsies are transperineal biopsies carried out as day-case procedures.
The CG17511 MRI model uses NHS reference costs as the basis for costing the different biopsy procedures. The clinical experts advising on the CG17511 model considered that NHS reference costs did not take adequate account of pathology costs and, therefore, that they underestimate the true cost of biopsy. The developers of the CG17511 MRI model therefore increased the cost of the HRG for histopathology by adding an estimate provided by a NHS Pathology Department in Bristol. A saturation biopsy was assumed to have a higher cost than a routine biopsy, as the former procedure generates a greater number of cores for analysis. The biopsy costs used in the CG17511 MRI model were based on NHS reference costs from 2011/12. These have been updated to 2012/13 prices by the EAG (Table 34).
| Cost element | Cost (£) | Probabilistic sensitivity analysis distribution | Source |
|---|---|---|---|
| TRUS (standard) biopsy | |||
| Outpatient | 224 | Log-normal | Department of Health 2013,18 NHS reference cost LB27Za in outpatient procedures – urology |
| Histopathology | 112.79 | NCCC 201411 | |
| Total | 336.79 | – | |
| Transperineal (standard) biopsy | |||
| Day case | 595 | Log-normal | Department of Health 2013,18 NHS reference cost LB27Za in day-case procedures – urology |
| Histopathology | 112.79 | NCCC 201411 | |
| Total | 707.79 | – | |
| Saturation biopsy | |||
| Day case | 595 | Log-normal | Department of Health 2013,18 NHS reference cost LB27Za in day-case procedures – urology |
| Histopathology | 281.97 | NCCC 201411 | |
| Total | 876.97 | – | |
Costs of biopsy complications
In line with the CG17511 MRI model, the model developed by the EAG uses the costs of biopsy complications reported by Mowatt et al. 14 (updated to 2012/13 prices). However, some HRG codes have changed slightly since the Mowatt et al. 14 model was developed; where appropriate, the HRG codes that appear most relevant to the codes reported by Mowatt et al. 14 have been used in the EAG model. Biopsy complication costs are presented in Table 35.
| Event | Cost (£) | PSA distribution | Source |
|---|---|---|---|
| Hospital stay | |||
| Urinary tract infection | 445 | Log-normal | Department of Health 2013.18 HRG LA04Q (Inpatient short-stay general medicine) kidney or urinary tract infections, without interventions, with CC score of 4–7 |
| Urinary bleeding | 483 | Log-normal | Department of Health 2013.18 HRG LB18Z (Inpatient short-stay urology). Attention to suprapubic catheter |
| Urinary obstruction | 1504 | Log-normal | Department of Health 2013.18 HRG LB09D (Inpatient short-stay urology) intermediate endoscopic ureter procedures, 19 years and over; HRG LB15E (Inpatient short-stay urology) minor bladder procedures, 19 years and over plus cost of catheter bags at £19.08 |
| Consultation | |||
| GP | 45 | Not varied | Curtis 2013.122 11.7-minute consultation with qualification costs |
| Urology department nurse | 78 | Log-normal | NICE CG175 201311 |
| Other – NHS Direct | £20 | Not varied | Mowatt 201314 |
The costs associated with biopsy complications that are included in the model should be considered as conservative, as literature searches failed to identify any published studies reporting the costs associated with sepsis or antibiotic resistance. To establish if this omission could affect findings, a sensitivity analysis was undertaken in which all complication costs were increased by 100%.
Costs of prostate-specific antigen monitoring
In CG17511 and in Mowatt et al. 14 PSA monitoring was assumed to occur twice a year and to be carried out by a GP practice nurse. A targeted literature search was undertaken by the EAG to identify any additional information that could indicate alternative PSA monitoring strategies, but no information was found that invalidated this assumption.
The cost, estimated to be £19.60, is based on a PSA test cost provided by Newcastle upon Tyne Hospitals NHS Foundation Trust (reported in CG17511) and the cost of a consultation with a practice nurse reported by Curtis. 122
Utility values
The only utility values required in the model are the disutilities associated with a biopsy. A targeted search of the literature was undertaken but no primary studies collecting disutility values specifically associated with prostate biopsy were identified. Neither the CG17511 MRI model nor the Mowatt et al. 14 model considers any disutility from the actual biopsy. Both studies include a discussion of the impact of including the disutility associated with urinary incontinence, should this occur as a biopsy complication or as a result of treatment; however, it is not clear how this is applied, as urinary tract infection, urinary bleeding and urinary obstruction do not necessarily lead to urinary incontinence.
The literature search identified one study (Heijnsdijk et al. 117) that investigated the utility values associated with a PSA screening programme. This study reported a utility decrement of 0.1 that lasted 3 weeks following a biopsy. This utility value was taken from an earlier study123 that focused on breast cancer biopsy and the duration of decrement was an assumption based on clinical opinion. Although this utility value is not ideal, in the absence of any other evidence, it has been incorporated into the EAG base-case model as a quality-adjusted life-year (QALY) loss of 0.0058 from a prostate biopsy.
It is not clear if the decrement used in the Heijnsdilk et al. 117 study is an average value that includes disutility as a result of complications of biopsy [including those listed in the table presenting the costs of biopsy complications (see Table 35) and additional complications such as sepsis]. In the absence of evidence to the contrary, the EAG has assumed that there was no additional QALY loss as a result of biopsy-associated complications. This assumption favours less specific strategies and therefore it is likely that it would bias results against the proposed tests.
Heijnsdilk et al. 117 also report lower and upper bounds of biopsy-related disutility of 0.06 and 0.13, respectively (QALY losses of 0.00346 and 0.0075, respectively), based on minimum and maximum values reported in the breast biopsy study. 123 As no measure of dispersion of disutility was provided in the published paper beyond these minimum and maximum values, the disutility has not been varied in the probabilistic sensitivity analysis. However, as suggested by the NICE Decision Support Unit report,124 the uncertainty has been explored using sensitivity analysis.
Discount rates
Both costs and benefits have been discounted at 3.5% per annum, as suggested in the NICE guide to the methods of technology appraisal. 118 A scenario analysis exploring the impact of a discount rates of 0% and 5% per annum was considered. However, as the model was based on a decision tree with linear transition through a pathway, changing the discount rate would change the costs and QALYs in each arm by exactly the same proportion and so leave the incremental cost-effectiveness ratio per QALY gained unchanged.
Uncertainty
Uncertainty in parameter values and the impact this could have on results have been explored both through the scenario analyses and sensitivity analyses previously described and also through probabilistic sensitivity analysis, varying those parameters for which probability distributions could be derived from, or were provided in, the literature. Probabilistic sensitivity analysis results have been presented as cost-effectiveness acceptability curves (CEACs) where different willingness-to-pay thresholds for a QALY are used to show which strategy is likely to have the largest net benefit for that threshold.
Interpreting results
Incremental cost-effectiveness ratios
The results of cost-effectiveness analysis are presented as incremental cost-effectiveness ratios per QALY gained. These are calculated by dividing the difference in costs associated with two alternative strategies by the difference in QALYs:
Where more than two strategies are being compared, the incremental cost-effectiveness ratio is calculated according to the following process:
-
The strategies are ranked in terms of cost, from least to most expensive.
-
If a strategy is more expensive and less effective than the preceding strategy it is said to be ‘dominated’ and is excluded from further analysis.
-
Incremental cost-effectiveness ratios are then calculated for each strategy compared with the next most expensive non-dominated option. If the incremental cost-effectiveness ratio for a strategy is higher than that of the next most effective strategy, then it is ruled out by ‘extended dominance’.
-
Incremental cost-effectiveness ratios are recalculated excluding any strategy subject to dominance or extended dominance.
-
The non-dominated strategies form an ‘efficiency frontier’ of strategies that are cost-effective and can then be judged against the value of an incremental cost-effectiveness ratio that is generally considered cost-effective by NICE, that is £20,000–30,000 per QALY gained.
Base-case results
The model was executed with a hypothetical cohort of 1000 patients. The results throughout this section are the values generated from the model for this cohort.
Total number of biopsies
The different number of biopsies under each diagnostic strategy drives the different patient outcomes in the model. In the base case, the total number of biopsies is split into second biopsies recommended by the testing strategy and biopsies undertaken during PSA monitoring (Table 36).
| Strategy | Second biopsies | Biopsies while under PSA monitoring | Total biopsies |
|---|---|---|---|
| Clinical assessment | 879 | 220 | 1099 |
| Clinical assessment + phi | 957 | 220 | 1177 |
| Clinical assessment + PCA3 | 892 | 220 | 1112 |
| Clinical assessment + phi + PCA3 | 802 | 220 | 1022 |
| Clinical assessment + mpMRI | 300 | 220 | 520 |
| Clinical assessment + phi + mpMRI | 294 | 220 | 514 |
| Clinical assessment + PCA3 + mpMRI | 294 | 220 | 514 |
| Clinical assessment + phi + PCA3 + mpMRI | 294 | 220 | 514 |
Under the base-case PSA monitoring scenario, all patients without a second biopsy, or with a negative second biopsy, enter PSA monitoring. The total number of these patients is the same regardless of the strategy; therefore, the number of patients undergoing a repeat biopsy during PSA testing is independent of the strategy chosen and is always the same.
Mean costs and benefits
Costs and QALYs generated using the base-case parameter values are shown in Table 37.
| Strategy | Test costs (£) | Biopsy costs (£) | Biopsy complication costs (£) | PSA monitoring costs (£) | Total costs (£) | Total QALY loss |
|---|---|---|---|---|---|---|
| Clinical assessment | 0 | 481,088 | 15,168 | 83,007 | 579,264 | 6.29 |
| Clinical assessment + phi | 89,830 | 507,196 | 16,079 | 83,007 | 696,113 | 6.74 |
| Clinical assessment + PCA3 | 164,670 | 485,440 | 15,320 | 83,007 | 748,437 | 6.36 |
| Clinical assessment + phi + PCA3 | 254,500 | 454,980 | 14,257 | 83,007 | 806,745 | 5.84 |
| Clinical assessment + mpMRI | 315,560 | 285,791 | 8355 | 83,007 | 692,713 | 2.94 |
| Clinical assessment + phi + mpMRI | 405,390 | 283,743 | 8284 | 83,007 | 780,424 | 2.91 |
| Clinical assessment + PCA3 + mpMRI | 480,230 | 283,743 | 8284 | 83,007 | 855,264 | 2.91 |
| Clinical assessment + phi + PCA3 + mpMRI | 570,060 | 283,743 | 8284 | 83,007 | 945,094 | 2.91 |
Incremental analysis
The incremental results from the base-case analysis are presented in Table 38.
| Strategy | Discounted costs | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio (£) |
|---|---|---|---|---|---|
| Clinical assessment | 579,264 | –6.29 | – | – | – |
| Clinical assessment + mpMRI | 692,713 | –2.94 | 113,449 | 3.35 | 33,911 |
| Clinical assessment + phi | 696,113 | –6.74 | 3399 | –3.79 | Dominated |
| Clinical assessment + PCA3 | 748,437 | –6.36 | 55,724 | –3.42 | Dominated |
| Clinical assessment + phi + mpMRI | 780,424 | –2.91 | 87,711 | 0.04 | 2,500,530 |
| Clinical assessment + phi + PCA3 | 806,745 | –5.84 | 26,321 | –2.93 | Dominated |
| Clinical assessment + PCA3 + mpMRI | 855,264 | –2.91 | 74,840 | 0 | Dominated |
| Clinical assessment + phi + PCA3 + mpMRI | 945,094 | –2.91 | 164,670 | 0 | Dominated |
Summary of base-case results
The incremental analysis shows that the testing strategies that lie on the efficiency frontier are clinical assessment + mpMRI and clinical assessment + phi + mpMRI. However, the incremental cost-effectiveness ratio per QALY gained for both strategies exceeds the £20,000–30,000 threshold that NICE generally considers cost-effective.
Scenario analysis
Full results are presented in tables for each of the scenario analyses that alter (from the base case) the number of biopsies undertaken.
Results are not shown for the scenario in which 50% of patients who have mpMRI have a transperineal rather than a TRUS biopsy. This is because this scenario will result in an increase in the cost of mpMRI but will not alter effectiveness. The consequence of this is that the incremental cost-effectiveness ratio per QALY gained will be greater than in the base case. As the base-case incremental cost-effectiveness ratio per QALY gained is already above the £20,000–30,000 threshold that NICE generally considers cost-effective, results from this scenario would be uninformative.
Varying derived sensitivity
The total numbers of biopsies performed if sensitivity is set at 80% or 95%, using data from the Porpiglia et al. study,99 are shown in Table 39.
| Strategy | 80% sensitivity | 95% sensitivity | ||||
|---|---|---|---|---|---|---|
| Second biopsies | Biopsies while under PSA monitoring | Total biopsies | Second biopsies | Biopsies while under PSA monitoring | Total biopsies | |
| Clinical assessment | 746 | 250 | 996 | 982 | 205 | 1187 |
| Clinical assessment + phi | 765 | 250 | 1015 | 975 | 205 | 1180 |
| Clinical assessment + PCA3 | 669 | 250 | 919 | 923 | 205 | 1128 |
| Clinical assessment + phi + PCA3 | 650 | 250 | 900 | 930 | 205 | 1135 |
| Clinical assessment + mpMRI | 244 | 250 | 494 | 499 | 205 | 704 |
| Clinical assessment + phi + mpMRI | 244 | 250 | 494 | 492 | 205 | 697 |
| Clinical assessment + PCA3 + mpMRI | 244 | 250 | 494 | 543 | 205 | 748 |
| Clinical assessment + phi + PCA3 + mpMRI | 244 | 250 | 494 | 556 | 205 | 761 |
Mean costs and benefits
Costs and QALYs generated by varying sensitivity values are shown in Tables 40 and 41.
| Strategy | Test costs (£) | Biopsy costs (£) | Biopsy complication costs (£) | PSA monitoring costs (£) | Total costs (£) | Total QALY loss |
|---|---|---|---|---|---|---|
| Clinical assessment | 0 | 461,359 | 14,260 | 84,902 | 560,521 | 5.69 |
| Clinical assessment + phi | 89,830 | 467,758 | 14,483 | 84,902 | 656,974 | 5.80 |
| Clinical assessment + PCA3 | 164,670 | 435,251 | 13,349 | 84,902 | 698,173 | 5.24 |
| Clinical assessment + phi + PCA3 | 254,500 | 428,852 | 13,126 | 84,902 | 781,380 | 5.13 |
| Clinical assessment + mpMRI | 315,560 | 292,169 | 8358 | 84,902 | 700,990 | 2.79 |
| Clinical assessment + phi + mpMRI | 405,390 | 292,169 | 8358 | 84,902 | 790,820 | 2.79 |
| Clinical assessment + PCA3 + mpMRI | 480,230 | 292,169 | 8358 | 84,902 | 865,660 | 2.79 |
| Clinical assessment + phi + PCA3 + mpMRI | 570,060 | 292,169 | 8358 | 84,902 | 955,490 | 2.79 |
| Strategy | Test costs (£) | Biopsy costs (£) | Biopsy complication costs (£) | PSA monitoring costs (£) | Total costs (£) | Total QALY loss |
|---|---|---|---|---|---|---|
| Clinical assessment | 0 | 502,983 | 16,042 | 82,060 | 601,085 | 6.80 |
| Clinical assessment + phi | 89,830 | 500,679 | 15,962 | 82,060 | 688,531 | 6.76 |
| Clinical assessment + PCA3 | 164,670 | 483,274 | 15,354 | 82,060 | 745,358 | 6.46 |
| Clinical assessment + phi + PCA3 | 254,500 | 485,578 | 15,435 | 82,060 | 837,572 | 6.50 |
| Clinical assessment + mpMRI | 315,560 | 340,192 | 10,363 | 82,060 | 748,175 | 4.01 |
| Clinical assessment + phi + mpMRI | 405,390 | 337,889 | 10,283 | 82,060 | 835,621 | 3.97 |
| Clinical assessment + PCA3 + mpMRI | 480,230 | 355,294 | 10,890 | 82,060 | 928,474 | 4.27 |
| Clinical assessment + phi + PCA3 + mpMRI | 570,060 | 359,645 | 11,042 | 82,060 | 1,022,807 | 4.34 |
Incremental analysis
The incremental results from varying the sensitivity level are presented in Tables 42 and 43.
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio (£) |
|---|---|---|---|---|---|
| Clinical assessment | 560,521 | –5.69 | – | – | – |
| Clinical assessment and phi | 656,974 | –5.80 | 96,452 | –0.11 | Dominated |
| Clinical assessment + PCA3 | 698,173 | –5.24 | 137,651 | 0.45 | Extendedly dominated |
| Clinical assessment + mpMRI | 700,990 | –2.79 | 140,468 | 2.90 | 48,467 |
| Clinical assessment + phi + PCA3 | 781,380 | –5.13 | 80,390 | –2.341 | Dominated |
| Clinical assessment + phi + mpMRI | 790,820 | –2.79 | 89,830 | 0 | Dominated |
| Clinical assessment + PCA3 + mpMRI | 865,660 | –2.79 | 164,670 | 0 | Dominated |
| Clinical assessment + phi + PCA3 + mpMRI | 955,490 | –2.79 | 254,500 | 0 | Dominated |
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio (£) |
|---|---|---|---|---|---|
| Clinical assessment | 601,085 | –6.80 | – | – | – |
| Clinical assessment and phi | 688,531 | –6.76 | 87,446 | 0.04 | Extendedly dominated |
| Clinical assessment + PCA3 | 745,358 | –6.46 | 144,274 | 0.34 | Extendedly dominated |
| Clinical assessment + mpMRI | 748,175 | –4.01 | 147,090 | 2.79 | 52,747 |
| Clinical assessment + phi + mpMRI | 835,621 | –3.97 | 87,446 | 0.04 | 2,215,980 |
| Clinical assessment + phi + PCA3 | 837,572 | –6.50 | 1951 | –2.53 | Dominated |
| Clinical assessment + PCA3 + mpMRI | 928,474 | –4.27 | 92,852 | –0.30 | Dominated |
| Clinical assessment + phi + PCA3 + mpMRI | 1,022,807 | –4.34 | 187,186 | –0.37 | Dominated |
Different prostate-specific antigen monitoring assumptions
The total numbers of biopsies performed when the PSA monitoring strategies assumed in CG17511 and Mowatt et al. 14 are adopted are shown in Table 44.
| Strategy | CG17511 | Mowatt et al.14 | ||||
|---|---|---|---|---|---|---|
| Second biopsies | Biopsies while under PSA monitoring | Total biopsies | Second biopsies | Biopsies while under PSA monitoring | Total biopsies | |
| Clinical assessment | 879 | 241 | 1122 | 879 | 22 | 901 |
| Clinical assessment + phi | 957 | 86 | 1043 | 957 | 22 | 979 |
| Clinical assessment + PCA3 | 892 | 215 | 1107 | 892 | 22 | 914 |
| Clinical assessment + phi + PCA3 | 802 | 396 | 1198 | 802 | 22 | 824 |
| Clinical assessment + mpMRI | 300 | 1401 | 1701 | 300 | 22 | 322 |
| Clinical assessment + phi + mpMRI | 294 | 1413 | 1707 | 294 | 22 | 316 |
| Clinical assessment + PCA3 + mpMRI | 294 | 1413 | 1707 | 294 | 22 | 316 |
| Clinical assessment + phi + PCA3 + mpMRI | 294 | 1413 | 1707 | 294 | 22 | 317 |
Mean costs and benefits
Costs and QALYs generated when the PSA monitoring strategies assumed in CG17511 and Mowatt et al. 14 are adopted are shown in Tables 45 and 46, respectively.
| Strategy | Test costs (£) | Biopsy costs (£) | Biopsy complication costs (£) | PSA monitoring costs (£) | Total costs (£) | Total QALY loss |
|---|---|---|---|---|---|---|
| Clinical assessment | 0 | 448,471 | 15,423 | 20,511 | 484,405 | 6.35 |
| Clinical assessment + phi | 89,830 | 381,638 | 13,093 | 5693 | 490,254 | 5.99 |
| Clinical assessment + PCA3 | 164,670 | 437,332 | 15,034 | 18,042 | 635,078 | 6.29 |
| Clinical assessment + phi + PCA3 | 254,500 | 515,304 | 17,752 | 35,329 | 822,886 | 6.72 |
| Clinical assessment + mpMRI | 315,560 | 948,410 | 32,851 | 131,354 | 1,428,175 | 9.11 |
| Clinical assessment + phi + mpMRI | 405,390 | 953,652 | 33,034 | 132,516 | 1,524,592 | 9.14 |
| Clinical assessment + PCA3 + mpMRI | 480,230 | 953,652 | 33,034 | 132,516 | 1,599,432 | 9.14 |
| Clinical assessment + phi + PCA3 + mpMRI | 570,060 | 953,652 | 33,034 | 132,516 | 1,689,262 | 9.14 |
| Strategy | Test costs (£) | Biopsy costs (£) | Biopsy complication costs (£) | PSA monitoring costs (£) | Total costs (£) | Total QALY loss |
|---|---|---|---|---|---|---|
| Clinical assessment | 0 | 315,143 | 10,828 | 30,733 | 356,703 | 5.20 |
| Clinical assessment + phi | 89,830 | 341,251 | 11,739 | 30,733 | 473,552 | 5.65 |
| Clinical assessment + PCA3 | 164,670 | 319,494 | 10,980 | 30,733 | 525,877 | 5.27 |
| Clinical assessment + phi + PCA3 | 254,500 | 289,035 | 9917 | 30,733 | 584,185 | 4.75 |
| Clinical assessment + mpMRI | 315,560 | 119,845 | 4015 | 30,733 | 470,153 | 1.85 |
| Clinical assessment + phi + mpMRI | 405,390 | 117,797 | 3944 | 30,733 | 557,864 | 1.82 |
| Clinical assessment + PCA3 + mpMRI | 480,230 | 117,797 | 3944 | 30,733 | 632,704 | 1.82 |
| Clinical assessment + phi + PCA3 + mpMRI | 570,060 | 117,797 | 3944 | 30,733 | 722,534 | 1.82 |
Incremental analysis
The incremental results when the PSA monitoring strategies assumed in CG17511 and Mowatt et al. 14 are adopted are presented in Tables 47 and 48, respectively.
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio (£) |
|---|---|---|---|---|---|
| Clinical assessment | 484,405 | –6.35 | – | – | – |
| Clinical assessment + phi | 490,254 | –5.99 | 5849 | 0.37 | 15,898 |
| Clinical assessment + PCA3 | 635,078 | –6.29 | 144,824 | –0.31 | Dominated |
| Clinical assessment + phi + PCA3 | 822,886 | –6.72 | 332,632 | –0.74 | Dominated |
| Clinical assessment + mpMRI | 1,428,175 | –9.11 | 937,921 | –3.12 | Dominated |
| Clinical assessment + phi + mpMRI | 1,524,592 | –9.14 | 1,034,338 | –3.15 | Dominated |
| Clinical assessment + PCA3 + mpMRI | 1,599,432 | –9.14 | 1,109,178 | –3.15 | Dominated |
| Clinical assessment + phi + PCA3 + mpMRI | 1,689,262 | –9.14 | 1,199,008 | –3.15 | Dominated |
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio (£) |
|---|---|---|---|---|---|
| Clinical assessment | 356,703 | –5.20 | – | – | – |
| Clinical assessment + mpMRI | 470,153 | –1.85 | 113,449 | 3.35 | 33,911a |
| Clinical assessment + phi | 473,552 | –5.65 | 3399 | –3.79 | Dominated |
| Clinical assessment + PCA3 | 525,877 | –5.27 | 55,724 | –3.42 | Dominated |
| Clinical assessment + phi + mpMRI | 557,864 | –1.82 | 87,711 | 0.03 | 2,500,530a |
| Clinical assessment + phi + PCA3 | 584,185 | –4.75 | 26,321 | –2.933 | Dominated |
| Clinical assessment + PCA3 + mpMRI | 632,704 | –1.82 | 74,840 | 0 | Dominated |
| Clinical assessment + phi + PCA3 + mpMRI | 722,534 | –1.82 | 164,670 | 0 | Dominated |
As the Mowatt et al. 14 PSA monitoring scenario results in an identical reduction in biopsy numbers (and therefore cost and QALY loss) across the testing strategies, this scenario does not affect the base-case incremental costs and QALYs between strategies. Therefore, the resultant incremental cost-effectiveness ratios per QALY gained for this scenario are the same as those for the base case.
Alternative effectiveness data sources
The total number of biopsies performed if the derived sensitivity values presented in Scattoni et al. 102 (80% and 90%) or Gittelman et al. 45 (90%) are employed in the model are shown in Tables 49 and 50, respectively.
| Strategy | 80% sensitivity | 90% sensitivity | ||||
|---|---|---|---|---|---|---|
| Second biopsies | Biopsies while under PSA monitoring | Total biopsies | Second biopsies | Biopsies while under PSA monitoring | Total biopsies | |
| Clinical assessment | 580 | 250 | 830 | 710 | 220 | 930 |
| Clinical assessment + PCA3 | 450 | 250 | 700 | 695 | 220 | 915 |
| Clinical assessment + phi | 595 | 250 | 845 | 786 | 220 | 1006 |
| Clinical assessment + phi + PCA3 | 580 | 250 | 830 | 725 | 220 | 945 |
| Strategy | Second biopsies | Biopsies while under PSA monitoring | Total biopsies |
|---|---|---|---|
| Clinical assessment | 832 | 220 | 1052 |
| Clinical assessment + PCA3 | 661 | 220 | 881 |
Mean costs and benefits
Costs and QALYs generated if the derived sensitivity values presented in Scattoni et al. 102 (80% and 90%) or Gittelman et al. 45 (90%) are employed in the model are shown in Tables 51–53, respectively.
| Strategy | Test costs (£) | Biopsy costs (£) | Biopsy complication costs (£) | PSA monitoring costs (£) | Total costs (£) | Total QALY loss |
|---|---|---|---|---|---|---|
| Clinical assessment | 0 | 405,304 | 12,304 | 84,902 | 502,511 | 4.73 |
| Clinical assessment + PCA3 | 89,830 | 361,791 | 10,786 | 84,902 | 547,309 | 3.98 |
| Clinical assessment + phi | 164,670 | 410,423 | 12,483 | 84,902 | 672,478 | 4.81 |
| Clinical assessment + phi + PCA3 | 254,500 | 405,304 | 12,304 | 84,902 | 757,011 | 4.73 |
| Strategy | Test costs (£) | Biopsy costs (£) | Biopsy complication costs (£) | PSA monitoring costs (£) | Total costs | Total QALY loss |
|---|---|---|---|---|---|---|
| Clinical assessment | 0 | 424,009 | 13,177 | 83,007 | 520,194 | 5.31 |
| Clinical assessment + PCA3 | 89,830 | 418,890 | 12,998 | 83,007 | 604,726 | 5.22 |
| Clinical assessment + phi | 164,670 | 449,605 | 14,070 | 83,007 | 711,352 | 5.75 |
| Clinical assessment + phi + PCA3 | 254,500 | 429,128 | 13,356 | 83,007 | 779,991 | 5.40 |
| Strategy | Test costs (£) | Biopsy costs (£) | Biopsy complication costs (£) | PSA monitoring costs (£) | Total costs (£) | Total QALY loss |
|---|---|---|---|---|---|---|
| Clinical assessment | 0 | 465,219 | 14,615 | 83,007 | 562,841 | 6.02 |
| Clinical assessment + PCA3 | 164,670 | 407,372 | 12,597 | 83,007 | 667,646 | 5.03 |
Incremental analysis
The incremental results if the derived sensitivity values presented in Scattoni et al. 102 (80% and 90%) or Gittelman et al. 45 (90%) are employed in the model are shown in Tables 54–56, respectively.
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio |
|---|---|---|---|---|---|
| Clinical assessment | 502,511 | –4.73 | – | – | – |
| Clinical assessment + PCA3 | 547,309 | –3.98 | 44,799 | 0.75 | 59,732 |
| Clinical assessment + phi | 672,478 | –4.81 | 169,968 | –0.44 | Dominated |
| Clinical assessment + phi + PCA3 | 757,011 | –4.73 | 254,500 | –0.09 | Dominated |
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio |
|---|---|---|---|---|---|
| Clinical assessment | 520,194 | –5.31 | – | – | – |
| Clinical assessment + PCA3 | 604,726 | –5.22 | 84,532 | 0.09 | 963,964 |
| Clinical assessment + phi | 711,352 | –5.75 | 106,627 | –0.53 | Dominated |
| Clinical assessment + phi + PCA3 | 779,991 | –5.40 | 175,266 | –0.18 | Dominated |
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio |
|---|---|---|---|---|---|
| Clinical assessment | 562,841 | –6.02 | – | – | – |
| Clinical assessment + PCA3 | 667,646 | –5.03 | 104,805 | 0.99 | 105,765 |
Deterministic sensitivity analysis
Some of the parameters varied in the deterministic sensitivity analyses could only increase the incremental cost-effectiveness ratios per QALY gained for any of the diagnostic strategies compared with clinical assessment alone. As the incremental cost-effectiveness ratios per QALY gained in the base case are already above the threshold (£20,000 to £30,000) generally considered cost-effective by NICE, the results of these analyses are not shown. For this reason, results from the following sensitivity analysis have been excluded:
-
increasing the cost of PCA3 assay or phi
-
lower bound of biopsy complication rates
-
QALY loss from biopsy reduced by 50%.
Where a sensitivity analysis does not change the number of biopsies, only the incremental analysis is shown. Where biopsy numbers are also changed, full results are provided.
Upper bound of biopsy complication rates
Table 57 shows the incremental analysis if the upper bound of complication rates from Table 32 are used.
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio (£) |
|---|---|---|---|---|---|
| Clinical assessment | 591,003 | –6.29 | – | – | – |
| Clinical assessment + mpMRI | 698,401 | –2.94 | 107,397 | 3.35 | 32,102 |
| Clinical assessment + phi | 708,661 | –6.74 | 10,260 | –3.79 | Dominated |
| Clinical assessment + PCA3 | 760,311 | –6.36 | 61,911 | –3.42 | Dominated |
| Clinical assessment + phi + mpMRI | 786,048 | –2.91 | 87,647 | 0.035 | 2,498,721 |
| Clinical assessment + phi + PCA3 | 817,676 | –5.84 | 31,627 | –2.933 | Dominated |
| Clinical assessment + PCA3 + mpMRI | 860,888 | –2.91 | 74,840 | 0 | Dominated |
| Clinical assessment + phi + PCA3 + mpMRI | 950,718 | –2.91 | 164,670 | 0 | Dominated |
Lower price of Prostate Health Index
Table 58 shows the incremental analysis if the cost of phi test decreased by 50% (i.e. £44.92 as opposed to £89.83).
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio (£) |
|---|---|---|---|---|---|
| Clinical assessment | 579,264 | –6.32 | – | – | – |
| Clinical assessment + phi | 651,203 | –6.77 | 71,939 | –0.45 | Dominated |
| Clinical assessment + mpMRI | 692,713 | –2.96 | 113,449 | 3.36 | 33,732 |
| Clinical assessment + phi + mpMRI | 735,514 | –2.93 | 42,801 | 0.04 | 1,213,727 |
| Clinical assessment + PCA3 | 748,437 | –6.40 | 12,923 | –3.47 | Dominated |
| Clinical assessment + phi + PCA3 | 761,835 | –5.87 | 26,321 | –2.91 | Dominated |
| Clinical assessment + PCA3 + mpMRI | 855,264 | –2.93 | 119,750 | 0 | Dominated |
| Clinical assessment + phi + PCA3 + mpMRI | 900,184 | –2.93 | 164,670 | 0 | Dominated |
Quality-adjusted life-year loss from biopsy
Table 59 shows the incremental analysis if the QALY loss from biopsy was at the upper bound suggested in the literature117 (i.e. 0.0075 as opposed to 0.0058).
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio (£) |
|---|---|---|---|---|---|
| Clinical assessment | 579,264 | –8.18 | – | – | – |
| Clinical assessment + mpMRI | 692,713 | –3.83 | 113,449 | 4.35 | 26,086 |
| Clinical assessment + phi | 696,113 | –8.76 | 3399 | –4.93 | Dominated |
| Clinical assessment + PCA3 | 748,437 | –8.27 | 55,724 | –4.44 | Dominated |
| Clinical assessment + phi + mpMRI | 780,424 | –3.78 | 87,711 | 0.05 | 1,923,484 |
| Clinical assessment + phi + PCA3 | 806,745 | –7.60 | 26,321 | –3.82 | Dominated |
| Clinical assessment + PCA3 + mpMRI | 855,264 | –3.78 | 74,840 | 0 | Dominated |
| Clinical assessment + phi + PCA3 + mpMRI | 945,094 | –3.78 | 164,670 | 0 | Dominated |
One hundred per cent increase in biopsy complication costs
Table 60 shows the incremental analysis if the costs of biopsy complications are increased by 100%.
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio |
|---|---|---|---|---|---|
| Clinical assessment | 592,073 | –6.29 | – | – | – |
| Clinical assessment + mpMRI | 698,710 | –2.94 | 106,637 | 3.35 | 31,875 |
| Clinical assessment + phi | 709,833 | –6.74 | 11,123 | –3.79 | Dominated |
| Clinical assessment + PCA3 | 761,398 | –6.36 | 62,688 | –3.42 | Dominated |
| Clinical assessment + phi + mpMRI | 786,350 | –2.91 | 87,639 | 0.035 | 2,498,493 |
| Clinical assessment + phi + PCA3 | 818,644 | –5.84 | 32,294 | –2.933 | Dominated |
| Clinical assessment + PCA3 + mpMRI | 861,190 | –2.91 | 74,840 | 0 | Dominated |
| Clinical assessment + phi + PCA3 + mpMRI | 951,020 | –2.91 | 164,670 | 0 | Dominated |
Fifty per cent of cancers are missed on second biopsy
Table 61 shows the incremental analysis if the sensitivity of second biopsy indicated by a testing strategy is 50% rather than 100% (as assumed in the base case).
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio |
|---|---|---|---|---|---|
| Clinical assessment | 704,213 | –7.04 | – | – | – |
| Clinical assessment + mpMRI | 817,663 | –3.69 | 113,449 | 3.35 | 33,911 |
| Clinical assessment + phi | 821,062 | –7.48 | 3399 | –3.79 | Dominated |
| Clinical assessment + PCA3 | 873,386 | –7.11 | 55,724 | –3.42 | Dominated |
| Clinical assessment + phi + mpMRI | 905,374 | –3.66 | 87,711 | 0.04 | 2,500,530 |
| Clinical assessment + phi + PCA3 | 931,694 | –6.59 | 26,321 | –2.93 | Dominated |
| Clinical assessment + PCA3 + mpMRI | 980,214 | –3.66 | 74,840 | 0 | Dominated |
| Clinical assessment + phi + PCA3 + mpMRI | 1,070,044 | –3.66 | 164,670 | 0 | Dominated |
As was the case with the Mowatt et al. 14 PSA monitoring scenario, reducing the sensitivity of biopsy by 50% changes biopsy numbers by the same amount for all strategies. Thus, it alters overall costs for strategies, but incremental costs and incremental cost-effectiveness ratios remain the same as in the base case.
Variation in the proportion of patients with negative second biopsies entering prostate-specific antigen monitoring
Reducing the percentage of patients with negative second biopsies entering PSA monitoring should favour those testing strategies with lower specificity. This is because such testing strategies result in more second biopsies and under this sensitivity analysis fewer patients receive PSA monitoring. Therefore, the results of only the extreme end of the sensitivity analysis are shown, that is where 0% of patients with negative second biopsies enter PSA monitoring. The different numbers of biopsies associated with each diagnostic strategy, assuming 0% of patients with negative second biopsy enter PSA monitoring, are shown in Table 62.
| Strategy | Second biopsies | Biopsies while under PSA monitoring | Total biopsies |
|---|---|---|---|
| Clinical assessment | 879 | 54 | 934 |
| Clinical assessment + phi | 957 | 35 | 992 |
| Clinical assessment + PCA3 | 892 | 51 | 943 |
| Clinical assessment + phi + PCA3 | 802 | 74 | 875 |
| Clinical assessment + mpMRI | 300 | 199 | 499 |
| Clinical assessment + phi + mpMRI | 294 | 201 | 494 |
| Clinical assessment + PCA3 + mpMRI | 294 | 201 | 494 |
| Clinical assessment + phi + PCA3 + mpMRI | 294 | 201 | 494 |
This sensitivity analysis shows that testing strategies with higher specificity result in more biopsies being undertaken during PSA monitoring than testing strategies with lower specificity.
Mean costs and benefits
Costs and QALYs generated by each diagnostic strategy, assuming that 0% of patients with negative second biopsy enter PSA monitoring, are shown in Table 63.
| Strategy | Test costs (£) | Biopsy costs (£) | Biopsy complication costs (£) | PSA monitoring costs (£) | Total costs (£) | Total QALY loss |
|---|---|---|---|---|---|---|
| Clinical assessment | 0 | 341,691 | 11,522 | 12,196 | 365,410 | 5.37 |
| Clinical assessment + phi | 89,830 | 351,512 | 12,007 | 3923 | 457,272 | 5.71 |
| Clinical assessment + PCA3 | 164,670 | 343,328 | 11,603 | 10,817 | 530,418 | 5.43 |
| Clinical assessment + phi + PCA3 | 254,500 | 331,870 | 11,038 | 20,470 | 617,877 | 5.03 |
| Clinical assessment + mpMRI | 315,560 | 268,226 | 7896 | 74,085 | 665,767 | 2.83 |
| Clinical assessment + phi + mpMRI | 405,390 | 267,456 | 7858 | 74,734 | 755,438 | 2.80 |
| Clinical assessment + PCA3 + mpMRI | 480,230 | 267,456 | 7858 | 74,734 | 830,278 | 2.80 |
| Clinical assessment + phi + PCA3 + mpMRI | 570,060 | 267,456 | 7858 | 74,734 | 920,108 | 2.80 |
Incremental analysis
The incremental results, assuming that 0% of men with a negative result from a second biopsy enter PSA monitoring, are shown in Table 64.
| Strategy | Discounted costs (£) | Discounted QALYs | Incremental costs (£) | Incremental QALYs | Incremental cost-effectiveness ratio (£) |
|---|---|---|---|---|---|
| Clinical assessment | 365,410 | 5.37 | – | – | – |
| Clinical assessment + phi | 457,272 | 5.71 | 91,862 | –0.34 | Dominated |
| Clinical assessment + PCA3 | 530,418 | 5.43 | 165,009 | –0.06 | Dominated |
| Clinical assessment + phi + PCA3 | 617,877 | 5.03 | 252,468 | 0.34 | Extendedly dominated |
| Clinical assessment + mpMRI | 665,767 | 2.83 | 300,358 | 2.54 | 118,066 |
| Clinical assessment + phi + mpMRI | 755,438 | 2.80 | 89,671 | 0.03 | 3,361,804 |
| Clinical assessment + PCA3 + mpMRI | 830,278 | 2.80 | 74,840 | 0 | Dominated |
| Clinical assessment + phi + PCA3 + mpMRI | 920,108 | 2.80 | 164,670 | 0 | Dominated |
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was undertaken using (1) the base-case evidence and assumptions and (2), individually, the alternative evidence sources and sensitivity rates. The probabilistic analysis was undertaken by running 1000 iterations of the model, with each iteration choosing a value at random for each variable in the model, where applicable, from the distributions shown in Model parameters.
Base-case analysis
The CEAC for the base-case analysis is shown in Figure 6. It demonstrates that the most cost-effective strategy at £20,000 per QALY gained is clinical assessment alone in 100% of model iterations. At about £33,500 per QALY gained approximately half of the iterations suggest that clinical assessment alone is the most cost-effective strategy, whereas the remaining iterations suggest that it is clinical assessment + mpMRI that is the most cost-effective strategy. At a threshold of £37,000 per QALY gained, all iterations suggest that clinical assessment + mpMRI dominates all other strategies.
FIGURE 6.
Cost-effectiveness acceptability curve (base case).

Figures 7 and 8 show the CEACs for the base case using 80% and 95% sensitivity estimates, respectively. As with sensitivity at 90%, both CEACs show that no testing strategy other than clinical assessment was cost-effective in any model iterations at threshold values below £30,000 per QALY gained.
FIGURE 7.
Cost-effectiveness acceptability curve (base case: 80% sensitivity).

FIGURE 8.
Cost-effectiveness acceptability curve (base case: 95% sensitivity).
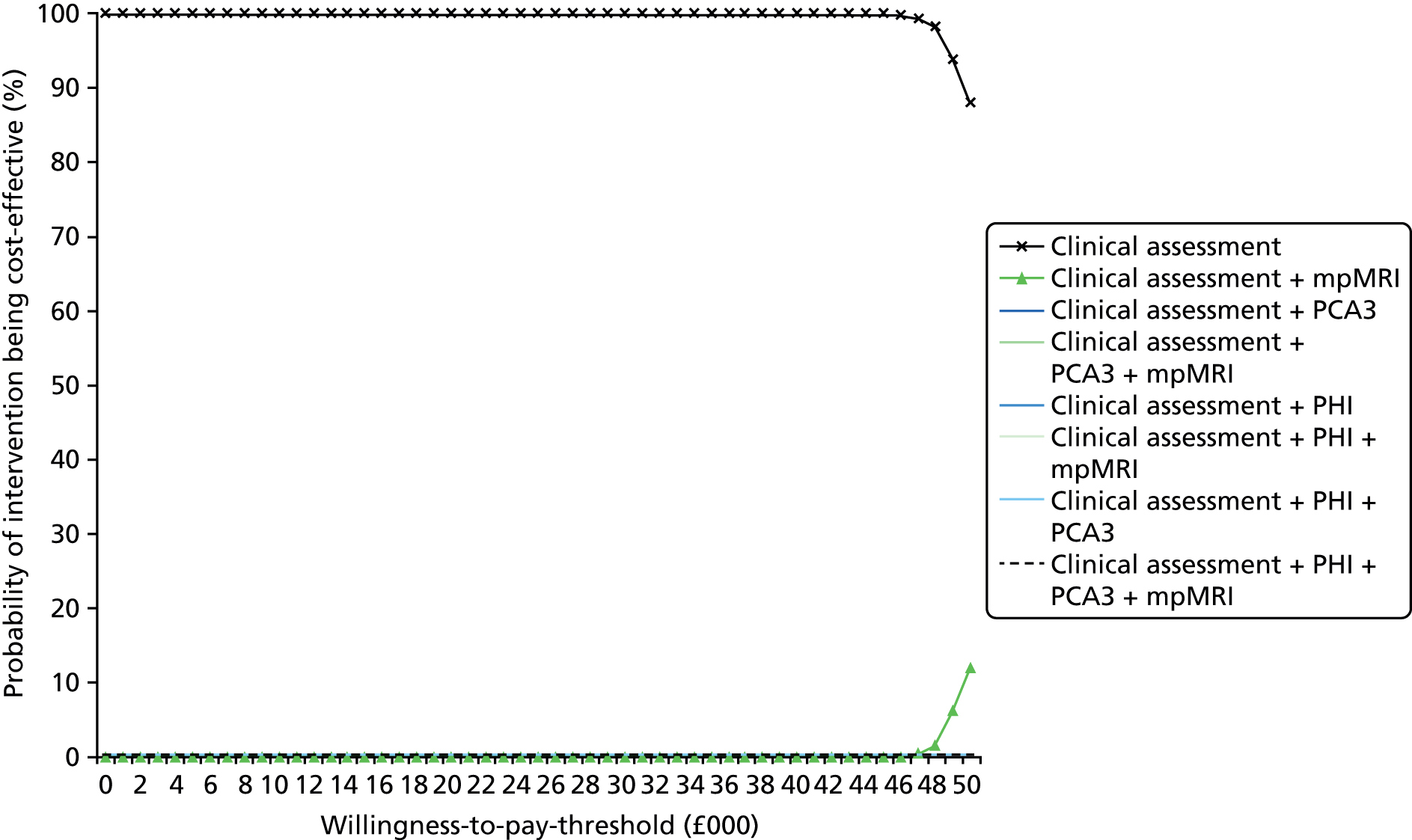
Alternative effectiveness data sources
Cost-effectiveness acceptability curves using alternative effectiveness data reported by Scattoni et al. 102 and Gittelman et al. 45 are shown in Figures 9–11. All three CEACs show that there were no model iterations in which the PCA3 assay or phi was cost-effective compared with clinical assessment alone at threshold values at, or below, £30,000 per QALY gained.
Summary of scenario analyses, deterministic sensitivity analyses and probabilistic sensitivity analyses
Other than the case in which the PSA monitoring strategy employed in the CG17511 MRI model is used, the incremental cost-effectiveness ratios that were generated to test model uncertainty were all above £20,000 per QALY gained. The probabilistic sensitivity analyses, in particular, confirm that alternative testing strategies using any test in addition to clinical assessment are not cost-effective, although it should be noted that QALY loss associated with a biopsy was not varied in the probabilistic analyses.
Changing the desired sensitivity level does alter the efficiency frontier. However, the frontier does not change such that the inclusion of PCA3 or phi has a favourable incremental cost-effectiveness ratio. The change in frontier occurs because at different sensitivity levels the specificity of phi also changes. At 80% sensitivity, phi adds nothing to sensitivity if added into a strategy of clinical assessment and mpMRI and is therefore dominated by the strategy that excludes it. However, at 90% and 95% sensitivity, the inclusion of phi does improve specificity slightly when added to clinical assessment and mpMRI, and so is on the efficiency frontier, albeit at a high incremental cost-effectiveness ratio.
Discussion of the External Assessment Group model results
The de novo economic model, both in the base case and across an extensive range of scenarios and sensitivity analyses, shows that neither the PCA3 assay nor the phi is likely to be cost-effective when identifying patients for second biopsy over clinical assessment alone or over clinical assessment + MRI.
The only time that one of the tests appears to be cost-effective is when the PSA monitoring strategy used in the CG17511 MRI model is employed. This approach leads to phi testing having an incremental cost-effectiveness ratio below £20,000 per QALY gained; the EAG cautions that this is a somewhat misleading finding. The testing strategy in that scenario, as stated in the methodology, favours the strategies that have lower specificity. Thus, as phi testing has the lowest specificity (90% sensitivity in the base case), it generates the most QALYs. This finding, unless the PSA monitoring strategy used in the CG17511 MRI model does accurately reflect routine clinical practice, should not be given any weight.
The base-case results show that the biggest reduction in the number of second biopsies performed comes from the use of mpMRI. In combination with PCA3 score, a further 2% reduction in second biopsies can be achieved. As, at 90% sensitivity, the PCA3 assay and the phi have a lower specificity than clinical assessment alone, model results show that their use leads to more biopsies being undertaken than when clinical assessment alone is employed. The lower specificity of these two tests can be interpreted as meaning that, to achieve 90% sensitivity, thresholds for the tests have to be set very low.
The use of the PCA3 assay or phi would appear to create more uncertainty in the decision-making process than use of clinical assessment alone with the result that, even though no more patients in total would actually have cancer, more patients would be identified as potentially having cancer than if clinicians had simply relied on their assessment of other clinical parameters.
Several caveats need to be considered when interpreting the cost-effectiveness results generated by the EAG model.
The modelling provides strong evidence that if mpMRI is undertaken then adding the PCA3 assay or phi to the diagnostic strategy is not cost-effective. The results from the model also suggest that mpMRI is not cost-effective compared with clinical assessment alone. However, in the analysis, the EAG assumed that mpMRI did not alter the sensitivity of the biopsy itself as the study99 from which the data were taken blinded the clinicians taking the biopsy to the mpMRI results. This is, however, a conservative assumption, and there is evidence in CG17511 that this is not the case and that biopsy is more accurate after mpMRI and as such the cost-effectiveness of mpMRI has probably been underestimated by the EAG model.
In any case, the focus of this assessment and the EAG model is on the PCA3 assay and the phi and whether or not these are clinically effective and/or cost-effective in combination with or without mpMRI rather than on whether or not mpMRI alone should be used. The EAG model found that adding PCA3 score or phi into a testing strategy with mpMRI was highly cost-ineffective. This finding will not change if the model has underestimated the sensitivity of mpMRI.
The mpMRI modelling undertaken in the CG17511 and Mowatt et al. 14 attempted to address a different decision problem regarding the use of different forms of mpMRI, including T2-MRI, to inform the location of a second biopsy rather than to inform whether or not a second biopsy should be undertaken. Therefore, although much of the biopsy cost and complication information contained within these models are transferable to the EAG model presented here, the results are not directly relevant to the decision problem addressed by the EAG model.
It is noted that CG17511 and Mowatt et al. 14 reported conflicting findings on the cost-effectiveness of MRI to inform biopsy; CG17511 reported that mpMRI was not cost-effective, whereas Mowatt et al. 14 reported that there was a favourable incremental cost-effectiveness ratio for T2-MRI over saturation TRUS biopsy. Both studies14,49 reported that more evidence was required on the effectiveness of MRI and that the level of available evidence may influence the results found.
The EAG analysis is built on the assumption that the same level of sensitivity can be achieved for all strategies with the only difference being the number of biopsies that need to be performed for this to be achieved. While allowing simple comparison between strategies, this assumption may be difficult to achieve in clinical practice. However, the clinical validity evidence shows that the clinician’s decision regarding whether or not a patient is referred for a second biopsy is unlikely to be made simply on the results of a single test but rather on the test result in combination with assessment of a range of other patient characteristics and biological parameters.
The published clinical validity studies also show that the sensitivity and specificity of clinical assessment can vary quite markedly between studies, presumably because of the parameters that were incorporated into the assessment. This variation may influence the sensitivity and specificity of individual tests in combination with clinical assessment between studies; it also shows that different sensitivity–specificity combinations can be achieved depending on how the clinical assessment is undertaken. This may have implications for the EAG cost-effectiveness results.
The base-case results in the EAG model are reliant on data from the Porpiglia et al. study. 99 The EAG had no quality concerns about how the statistical analysis in the study99 had been performed; however, the clinical assessment used in that study involved only DREs and age. This approach does not include assessment of PSA level and may not reflect clinical practice in the NHS or, indeed, anywhere outside the study setting.
Given these limitations, the EAG considered that the fairest way to compare the testing strategies was to analyse the individual tests from the perspective of which testing strategy required the fewest biopsies to identify a given percentage of cancers and then to explore how the different clinical assessments undertaken in different studies affect the results through scenario analyses. The results of the scenario analyses clearly show that different clinical effectiveness evidence did not affect the conclusions suggested by the base case.
Related to this point is the level of sensitivity that the EAG chose to incorporate into the model. As desired sensitivity rises, the specificity of the PCA3 assay and the phi falls. At 90% sensitivity and higher, as previously stated, there is evidence that the use of the PCA3 assay or phi can actually reduce specificity compared with clinical assessment alone. However, at lower sensitivities, the specificities of the PCA3 assay and the phi are higher than clinical assessment. It may be that, at sensitivities below 80%, either test may be cost-effective. However, the EAG was not able to explore this, as there was insufficient evidence to incorporate such scenarios into the model.
Varying the number of biopsies, the cost of a biopsy and utility loss from a biopsy for each testing strategy will affect cost-effectiveness results. Although there is evidence, which has been used in the model, on the number and cost of prostate biopsy, the EAG did not find a utility value in the literature specifically associated with prostate biopsy and so a disutility value associated with breast biopsy was used. This could have implications for findings if, as a result of future research, the disutility from prostate biopsy is demonstrated to be more severe and/or longer lasting than disutility from a breast biopsy.
There may also be disutilities resulting from the stress arising from the testing strategy itself, such as waiting for MRI or the patient being told that they have a probability of having cancer but are not being recommended for a second biopsy. With no evidence on the magnitude of such disutilities, these factors have not been included in the analysis.
For men who are more likely to experience adverse events because of biopsy or who suffer from marked anxiety about having a second biopsy, it is possible that utility gains and averted costs from avoided biopsy may be higher than for the average man used in the EAG analysis. In this case, mpMRI may well be cost-effective. When mpMRI is not available, then, unless a lower test sensitivity of 80% is thought desirable for a patient and his clinician, the potential QALY gain would have to be significant for either the PCA3 assay or the phi to be cost-effective.
It should be noted that sensitivity analysis was used to explore the impact of a significantly larger disutility associated with a biopsy and that this did not change the EAG conclusions regarding cost-effectiveness.
A targeted literature review failed to identify evidence (in a form that could be used in the model) on the costs associated with sepsis or antibiotic resistance resulting from a biopsy and so, in line with the CG17511 MRI model, the impact of this complication was not included in any analyses. However, sensitivity analysis shows that even if complication costs had been underestimated by 100% it would make minimal difference to results. The exclusion of sepsis as a potential complication is, therefore, deemed unlikely to change the conclusions that can be drawn from the model results.
There was limited information in the literature to describe the characteristics of PSA monitoring strategies currently employed in clinical practice. The cost-effectiveness results of the scenarios that were explored suggest that, unless PSA monitoring is akin to that used in the CG17511 MRI model, the PSA monitoring strategy employed is unlikely to influence results.
One area that could impact on the cost-effectiveness results that could not be explored because of a lack of evidence was whether or not the cancers identified under different strategies differed in levels of aggressiveness. If the PCA3 assay or phi has higher sensitivity for detecting aggressive cancer than clinical assessment alone, it may be that these tests are cost-effective options. Unfortunately, the studies that provided data that could be included in the model did not report this type of result.
Chapter 4 Discussion
Statement of principal findings
Ten documents48,50,51,57,58,71–75 were included in the EAG review of analytical validity. The EAG concluded that the analytical validity of the PCA3 and p2PSA assays had been comprehensively documented. The EAG identified some important issues relating to the precision of PCA3 assay measurements. Issues were also highlighted in relation to the use of the p2PSA assay, namely sample handling and the thermal stability of samples.
The review of clinical validity data included results from 15 study populations from 17 publications. 45,46,85,86,89–92,94,96,97,99,102–106 More clinical validity data were available to assess the impact of adding the PCA3 assay to clinical assessment than were available to assess the impact of adding phi to clinical assessment. Although the addition of the PCA3 assay and the phi to clinical assessment improved measures of overall diagnostic accuracy, there was no consistent evidence of an improvement in derived sensitivity or derived specificity. The EAG concluded that it was not possible to identify a threshold for the PCA3 score or phi result for clinical use. Similarly, when MRI is carried out alongside clinical assessment, there is no evidence of any benefit associated with the addition either the PCA3 assay or the phi.
The EAG did not identify any studies for inclusion in the review of cost-effectiveness. The results from EAG base-case analyses involving either the PCA3 assay or the phi are unambiguous. The threshold below which NICE generally considers an intervention to be cost-effective (an incremental cost-effectiveness ratio of £20,000–30,000 per QALY gained) is clearly exceeded in all analyses (the lowest incremental cost-effectiveness ratio is over £2M per QALY gained). The probabilistic sensitivity analysis results, the deterministic sensitivity analysis results and results from the scenario analyses demonstrate that this finding is robust to variations in the magnitude of key parameters.
Comparison of results with other published studies
No systematic reviews of clinically relevant comparisons describing the addition of the PCA3 assay or the phi to clinical assessment, with or without MRI, were identified. One previous review110 of the use of the PCA3 assay in a repeat biopsy population concluded that it was effective in improving accuracy, but the review did not consider its use in combination with other diagnostic tests. A major review66,67 of the PCA3 assay compared with PSA in a combined population of initial and repeat biopsies concluded that the PCA3 assay improved accuracy but the strength of the evidence reviewed was considered to be low. The reviews by Bradley et al. 66,67 did not consider the use of PCA3 score in combination with PSA or clinical variables. No systematic reviews of phi in a repeat biopsy population were identified.
No relevant cost-effectiveness studies or reviews which included the PCA3 assay or phi were identified.
Strengths and limitations of the assessment
Strengths of analysis
The review of analytical validity has highlighted some important issues concerning the precision of PCA3 assay measurements and the requirements for storage and stability samples for phi.
From a clinical perspective, the key strength of this review is the restriction to those studies reporting the incremental effect of the addition of the PCA3 assay or the phi in combination with existing tests, scans and clinical judgement, in the diagnosis of prostate cancer in men who are suspected of having malignant disease and in whom the results of an initial prostate biopsy were negative or equivocal. This restriction was introduced as the issue of importance to clinical decision-makers is the impact of adding the PCA3 assay or phi to tests currently carried out in routine clinical practice, rather than the theoretical efficacy as reflected in any assessment of the use of the PCA3 assay or phi as stand-alone diagnostic tests. Other authors have noted this important issue125,126 and it is expected that future studies will focus on assessing the most clinically relevant comparisons, that is an approach which will involve considering combinations of tests.
The clinical validity review has reported results for a wide range of outcome measures from 10 different clinical comparisons. The EAG has made best use of all of the available published data and highlighted the comparisons that are most likely to be clinically relevant to clinicians working in the NHS in England and Wales.
A key strength of the EAG economic evaluation is that the de novo model provides a flexible framework that allows the comparison of many different diagnostic strategies. It is based on the best available clinical validity evidence (identified through the systematic review) and captures the trade-off between high upfront costs of diagnostic tests and the reduction in subsequent biopsies that they may offer. The model design captures all of the main factors that are relevant to the decision problem. It is user friendly and calculations are transparent. Furthermore, the model can easily be updated to incorporate new clinical validity evidence as it becomes available.
Limitations of the analysis
Predominance of one study
Of the 10 clinically relevant comparisons described in the 17 papers,45,46,85,86,89–92,94,96,97,99,102–106 data from the study by Porpiglia et al. 99 are used in nine comparisons. Data from Scattoni et al. 102 are used in four comparisons and the remaining 13 populations provide data for a single comparison. Clearly, relying heavily on the study by Porpiglia et al. 99 is a limitation of both the clinical validity review and the results of the economic modelling undertaken by the EAG. The EAG acknowledges that clinical assessment in this study is not representative of clinical practice in the NHS and that MRI results were not considered before the biopsy because of the design of the study protocol. However, the EAG considers the Porpiglia et al. study99 to be the only published study that reports data that could be used to inform the de novo economic model.
Clinical relevance of reported outcome measures
Only one of the included studies105 reported sensitivity and specificity estimates and the EAG considers this to be a substantial weakness in the available data. Logistic regression models offer the potential to study multiple tests. However, although outcome measures such as AUC and multivariate diagnostic ORs may show improved accuracy, they lack clinical relevance as it is not clear whether improvements in overall accuracy are because of improved sensitivity or improved specificity. 127 Derived sensitivity and derived specificity values offer clearer advice but it is not possible to identify individual threshold values of the risk factors included in the model which are associated with a particular achieved level of sensitivity or specificity.
Decision curve analysis results were reported in several publications90,92,97,99,102,106 included in the review of clinical validity. Neither the PCA3 assay nor phi when added to clinical assessment (or clinical assessment plus MRI) showed increased net benefit below a threshold risk of approximately 15% (i.e. if the assessed risk from the model is below 15% then the addition of a new test does help decision-making).
The EAG noted the following limitations in the use of decision curve methodology as used in the included studies:
-
None of the reviewed studies used the option of adding a variable for the harm associated with a test (i.e. complications).
-
The reviewed studies weighted the benefit of diagnosed cases as 1 but it is not clear whether or not this approach is appropriate when considering the identification of clinically insignificant cancers.
-
The method does not consider the harm arising from missing cancers.
Clinical assessment
The process of clinical decision-making is difficult to capture, standardise and evaluate within a study population. In the reviewed studies, descriptions of clinical assessment varied widely. When definitions of clinical assessment are unclear or are very different across studies, it may not be clinically meaningful to compare results. In two studies85,97 previously validated nomograms were used to reflect clinical assessment. Another study105 used a clinical decision algorithm that had been developed in conjunction with 12 European urologists. The EAG considers that this type of decision tool may be the best representation of clinical assessment in the included studies.
The EAG notes that the inclusion of PSA in logistic regression models used to assess the efficacy of phi is inconsistent and gives rise to concerns about the validity of the model results as the phi result already includes a measure of PSA.
Use of different thresholds across the studies
The manufacturer of the PCA3 assay proposed a threshold value of 25 to differentiate between the presence and absence of prostate cancer. However, results using this threshold value were reported in only one study. 45 Other studies used 35,86 3989 and 5089 or used the PCA3 score as a continuous variable. 46,86,90,97,99,102
The manufacturer of phi proposed using three groups: low risk of cancer (score of 0 to 20.9), moderate risk of cancer (score of 21 to 39.9) and high risk of cancer (score of 40 and above). However, the four studies90,97,100,103 that used phi used the phi test results as a continuous variable.
It was difficult for the EAG to draw conclusions from the limited data available, as the included studies used a range of threshold values for the PCA3 assay and none of the studies used the phi tests results recommended by the manufacturer.
Confidence intervals and statistical significance of clinical validity results
Many of the reported results for the clinical validity outcomes do not include either standard errors or CIs. It has, therefore, not been possible for the EAG to assess whether or not the differences between groups were statistically significant. Values for derived sensitivity and derived specificity reported in Porpiglia et al. 99 were similar for several models that involved different combinations of diagnostic tests. This may have been because there were small numbers of participants above or below the required threshold associated with a given level of sensitivity and specificity.
Lack of generalisable clinical validity data to inform the economic model
As is the case with all economic models, the results are limited by the generalisability of the available evidence data used to populate the model. In the study99 used to inform the base-case analysis in the EAG model, the analysis undertaken is appropriate; however, there are differences in clinical practice between the study99 and the NHS in England and Wales. To explore the impact that these differences may have had on the incremental cost-effectiveness ratios, data from other studies with alternative clinical assessments were modelled. The EAG is confident that using alternative assumptions did not change the model findings regarding the probable cost-effectiveness of adding the PCA3 assay or phi into a testing strategy.
Limited incorporation of utility values
Although the model attempted to capture all the important clinical and cost events, it was not possible to capture and/or value all the key factors that might influence cost-effectiveness. The main area where information is lacking is in relation to utility decrements associated with prostate biopsies. It was necessary to use a proxy value, based on the findings from a study117 that focused on breast cancer biopsy, to represent pain and short-term complications. Any utility decrements associated with anxiety prior to a biopsy were omitted from the model because of lack of information. Inclusion of specific utility values would require a study that assessed utility across the testing process and would need to take into account anxiety not just from a second biopsy or waiting for mpMRI, but also from any change in anxiety associated with the change in risk information that different testing strategies may offer patients.
Uncertainties
Owing to the lack of published literature, the EAG assessment was unable to address three important clinical issues outlined in the final scope,49 namely detection of clinically insignificant cancer, optimal order of the tests and the effect of using different forms of reference standard (biopsy). Other relevant uncertainties are also discussed.
Issues identified in the final scope
Detection of clinically insignificant cancers
The management of men who have been diagnosed with prostate cancer varies depending on the grade and extent of the cancer at diagnosis. Clinically insignificant cancers are monitored with active surveillance or watchful waiting. Many clinicians are concerned that, rather than improve health, increased diagnosis of clinically insignificant cancers may lead to an increase in morbidity because of anxiety.
One aim of the clinical validity review was to assess the ability of the PCA3 assay and the phi to improve the detection of more aggressive cancers. A lack of evidence meant that it was not possible for the EAG to draw any conclusions about the impact of the PCA3 assay or phi on the detection of clinically insignificant cancers.
Evidence for the relationship between the aggressiveness of tumours detected at prostate biopsy and the PCA3 assay or the phi has been reported in previous reviews. 128,129 These reviews128,129 were not restricted to studies of repeat biopsy populations and do not consider the intervention test results used in combination with other diagnostic tests. Filella et al. 128 in a narrative review, highlighted inconsistencies in the evidence linking higher PCA3 scores to various markers of tumour aggressiveness. Wang et al. ,129 in a meta-analysis of four studies, found that the AUC of phi for discriminating cancers with a Gleason score of above or below 7 was 0.67 (95% CI 0.57 to 0.77), with a sensitivity of 90% (95% CI 87% to 92%) and a specificity of 17% (95% CI 14% to 19%). The authors of the review129 comment on the need for further research.
If the PCA3 assay or phi has higher sensitivity for detecting aggressive cancer than clinical assessment alone (or clinical assessment plus MRI), the use of these tests may be cost-effective. Unfortunately, the studies that provided data that could be included in the EAG model did not report this type of result.
Order of tests
In the included studies, the results of tests were often presented as outputs from logistic regression models with all tests entered into one model. This approach meant that it was not possible to determine from the available data whether or not carrying out the diagnostic tests in one order was better than carrying out the tests in a different order. For example, it was not possible to determine whether or not diagnostic accuracy was improved if the PCA3 assay (or phi) was carried out before or after MRI. However, in clinical practice the order of the tests is important and has substantial cost and benefit implication, as the costs of MRI are higher than either the PCA3 assay or the phi and the order of the tests may result in different sensitivity and specificity estimates. There is no clinical, and therefore no economic, evidence on using the PCA3 assay or the phi to indicate whether or not mpMRI should be performed before a second biopsy. However, the economic model results provide evidence that if mpMRI is performed then the added information from the PCA3 assay or phi is minimal and incorporation of either into a testing strategy that will include mpMRI will, therefore, not be cost-effective.
Effect of different type of reference standard (prostate biopsy)
It has been shown that, in a given population, biopsy schemes that take a large number of cores spread widely across the prostate, such as saturation schemes, result in a higher prevalence of detected cancer than schemes that involve taking only a few cores. 1 An important clinical point is, therefore, to question if any incremental gain associated with the addition of the PCA3 assay or the phi to clinical assessment would vary depending on the biopsy scheme used to confirm the presence or absence of cancer. As discussed in Chapter 1, Potential sources of bias, any advantage gained by adding the PCA3 assay, or the phi, to clinical assessment could be reduced if a more extensive biopsy scheme were used. The EAG planned to assess this issue in the review of clinical validity by comparing results in studies which used different types of reference standard; however, the details of reference standards used in studies were poorly reported. An added complication was the fact that the number of biopsy cores taken often differed between patients within a single study. Where details were provided, 10- or 12-core biopsies were the most common.
Other relevant uncertainties
Extent to which the model reflects NHS clinical practice
The EAG economic model addresses clear questions:
Given a desired cancer detection rate for the target population, what proportion of the population would need a second biopsy and what proportion of these second biopsies would be necessary?
However, the extent to which these questions reflect clinical practice is unclear. It is hypothesised that clinicians are more likely to think in terms of individual patients rather than in terms of desired cancer detection rates for the whole population of men suspected of having prostate cancer.
The actual achieved sensitivity and specificity of incorporating the PCA3 assay and the phi into a diagnostic strategy are unknown. The clinical evidence available does not address all of the factors that influence diagnostic practice, such as patient preferences for second biopsies given previous biopsy experience and increased/decreased anxiety levels resulting from the findings of additional tests that place patients in different risk categories. Ideally the cost-effectiveness model would be populated using ‘real-world’ data. In the absence of real-world data the EAG model has been constructed in such a way as to allow the tests to be compared fairly but at arbitrary levels of sensitivity.
Prostate-specific antigen monitoring strategy after a negative or equivocal biopsy
A key area of uncertainty is related to the best representation of the PSA monitoring strategy within the economic model. No published information was found by the EAG that described NHS monitoring practice in England and Wales. As a consequence, this parameter was varied in the sensitivity analyses and the incremental cost-effectiveness ratio per QALY gained fell below £20,000 only if the PSA monitoring regime employed in the CG17511 MRI model was used. In the PSA monitoring strategy described in CG175,11 all men with negative or equivocal results from an initial biopsy go on to receive at least one further biopsy and up to 6 years of PSA monitoring. When used in the EAG model, this assumption would mean that the optimal strategy would be to immediately carry out a further biopsy on everyone shown to be negative or equivocal on the initial biopsy and undertake no PSA monitoring.
Unclear clinical priorities
Improvements in diagnostic test accuracy are often a balance between a gain in sensitivity at the expense of lower specificity, or vice versa. Clinical priorities determine whether sensitivity or specificity is the most important outcome in any particular diagnostic situation. To understand the clinical implications of the findings of the clinical validity review, and to inform the design of the economic model, the EAG surveyed a convenience sample of five clinicians. The clinicians were asked whether, for men undergoing repeat prostatic biopsy, sensitivity or specificity was the most important and whether it was possible to identify a minimum level of sensitivity or specificity that that a test should achieve. Disparate views were expressed, with some clinicians favouring high sensitivity so that all cancers were identified, while others expressed a desire for a test that only identified the more aggressive cancers. No minimum level of sensitivity or specificity was suggested. The EAG, therefore, took the pragmatic approach of using sensitivity estimates of 85%, 90% and 95% in the economic model.
Unclear target population
The precise target patient population for the new tests is also unclear. As discussed in Chapter 2, Quality assessment, men with a negative result following an initial biopsy can be categorised into three groups: those with clear risk factors for a repeat biopsy (at high assessed risk), those with no remaining risk factors (at low assessed risk) and those where clinicians are uncertain (intermediate assessed risk). Most of the eligible studies included only men who had been referred for a repeat biopsy and had, therefore, presumably been assessed as at high or intermediate risk. It is not clear whether or not clinicians would wish to use the PCA3 assay and/or phi in all men, including those currently assessed as at low risk.
False-negative results
The impact of a FN result at repeat biopsy on the length of time to final diagnosis (and the impact that that delay might have on disease progression) is also an issue. However, recent data suggest that risk reductions associated with radical treatment for low-risk patients (and even moderate-risk patients) may be small and insignificant. 130 If this is the case, it might undermine the cost-effectiveness of strategies that increase cancer detection rates and costs over standard practice, unless those strategies are able to discriminate by grade of tumour. Furthermore, there appears to be no published information on the rate of FPs and overtreatment, although the EAG modelling approach means that these would be the same for all testing strategies and therefore should not impact on results.
Equality and diversity
The incidence of aggressive prostate cancer is greater in people with obesity, which can lead to the positive predictive value of a DRE being higher; DRE can be more difficult to perform in people with obesity. 131 The economic results rely on the results from clinical studies in which a DRE is part of a clinical assessment. It may be that cost-effectiveness results for the PCA3 assay and the phi differ depending on whether or not clinical assessment includes a DRE; this should be considered against the possibility that for some people a DRE may not be possible or may be more difficult to undertake.
Chapter 5 Conclusions
The main findings of the EAG assessment of using the PCA3 assay and the phi in combination with existing tests, scans and clinical judgement in the diagnosis of prostate cancer in men who are suspected of having malignant disease and in whom the results of an initial prostate biopsy were negative or equivocal, are presented in Table 65.
| Clinical comparison | EAG clinical conclusions | Base-case EAG economic results |
|---|---|---|
| Clinical assessment vs. clinical assessment + PCA3 | The implications of adding the PCA3 assay to clinical assessment are not clear and it is not possible to identify a single-threshold value for use in a clinical setting | Clinical assessment dominates clinical assessment + PCA3; clinical assessment costs less and generates more QALYs than clinical assessment + PCA3 |
| Clinical assessment vs. clinical assessment + phi | The implications of adding phi to clinical assessment are not clear and it is not possible to identify threshold values for use in a clinical setting | Clinical assessment dominates clinical assessment + phi; clinical assessment costs less and generates more QALYs than clinical assessment + phi |
| Clinical assessment + MRI vs. clinical assessment + MRI + PCA3 | The addition of the PCA3 assay to clinical assessment + MRI does not have a noticeable impact on discrimination | Clinical assessment + MRI costs less but is less effective than clinical assessment + MRI + PCA3; the incremental cost-effectiveness ratio per QALY gained for clinical assessment + MRI + PCA3 is £5,418,366 compared with clinical assessment + MRI |
| Clinical assessment + MRI vs. clinical assessment + MRI + phi | The addition of phi to clinical assessment + MRI does not have a noticeable impact on discrimination | Clinical assessment + MRI costs less but is less effective than clinical assessment + MRI + phi; the incremental cost-effectiveness ratio per QALY gained for clinical assessment + MRI + phi is £2,500,530 compared with clinical assessment + MRI |
Implications for service provision
Several findings from the analytical validity review may affect the successful implementation of the assays in the NHS.
The PROGENSA prostate cancer antigen 3 assay
The patient must undergo a DRE before giving a urine sample for the PCA3 assay and the voided urine sample needs to be transferred to specialist transport tubes within 4 hours. It is likely that these requirements will pose little challenge within a secondary care setting; however, implementation within a primary care setting may require some staff training.
The published precision estimates for the PCA3 assay raise concerns about the interpretation and use of the PCA3 score in clinical practice for detecting men with prostate cancer.
Prostate Health Index
The analytical review highlighted concerns about sample handling. Blood samples for the p2PSA assay need to centrifuged and the serum separated within 3 hours. The rationale for this 3-hour limit is unclear, but the current recommendation of 3 hours may pose challenges to implementing the test throughout the NHS. It is not clear if blood samples taken in a primary care setting could be routinely transported to a laboratory and processed as required within 3 hours.
Suggested research priorities
The clinical validity review has been limited by a lack of data directly addressing the dilemmas that clinicians and patients face when deciding whether or not to continue investigations after the results of an initial prostate biopsy are negative or equivocal. Longitudinal end-to-end studies following men from initial investigation through to diagnosis and treatment of prostate cancer are required. Ideally, these studies would be RCTs with men allocated to different diagnostic test pathways after an initial negative or equivocal biopsy. A RCT design would be required to address the following issues:
-
Minimisation of sampling and verification bias. By recruiting all men with negative results following an initial biopsy into a trial population, the contribution of the intervention tests to diagnosis can be assessed in men with all levels of risk and so the role of sampling and verification bias will be minimised.
-
Standardisation of clinical assessment. Within RCT protocols, the measurement of risk factors such as age, a DRE and family history should be standardised and this will enable results from different studies to be compared.
-
QoL. It would be beneficial to include measurement of health-related QoL into future RCTs assessing the accuracy of alternative approaches to diagnosis.
The EAG is aware that it may be many years before any reliable data are available from RCTs. Descriptive data from observational cohorts following men over several years from initial referral onwards could address some unanswered issues including:
-
Patient-reported outcomes. Available studies focus on clinical validity outcomes and do not report the morbidity associated with biopsy, either in the short or long term. These studies should also include men who do not receive a repeat biopsy, as the impact of continued monitoring and uncertainty in this group is not known. In particular, the disutility associated with undergoing a biopsy should be captured. It would also be helpful to gain an understanding of the level of anxiety and depression that results from waiting for mpMRI or biopsy as well as that resulting from receiving equivocal results from following these procedures.
-
Number of repeat biopsies. Longitudinal observational studies would also document how many men required multiple (more than two) biopsies in order to establish or exclude the presence of prostatic cancer.
A recent paper78 raised the possibility that PCA3 scores may vary with genotype. Further research may be required on genotype variation in PCA3 scores and the implications that this variation has for setting appropriate PCA3 score thresholds to indicate increased risk of prostate cancer.
Acknowledgements
Dr Isabel Syndikus, Consultant Clinical Oncologist, The Clatterbridge Cancer Centre NHS Foundation Trust, Wirral, UK.
Dr Graham Scotland, Senior Research Fellow, University of Aberdeen, Aberdeen, UK.
Professor Chris Hyde, Professor of Public Health and Clinical Epidemiology, University of Exeter, Exeter, UK.
Dr Ana Alfirevic, Senior Lecturer, The Wolfson Centre for Personalised Medicine, Department of Molecular and Clinical Pharmacology, University of Liverpool, Liverpool, UK.
Contributions of authors
Amanda Nicholson: project lead and review of clinical evidence.
James Mahon: development of the de novo economic model.
Angela Boland: support of review process (clinical and economics).
Sophie Beale: support of review process (clinical and economics).
Kerry Dwan: clinical quality assessment, data extraction and statistical advisor.
Nigel Fleeman: review of analytical validity.
Juliet Hockenhull: literature selection and data extraction.
Yenal Dundar: literature searching.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Eichler K, Wilby J, Hempel S, Myers L, Kleijnen J. Diagnostic Value of Systematic Prostate Biopsy Methods in the Investigation for Prostate Cancer: A Systematic Review. York: Centre for Reviews and Dissemination; 2005.
- McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol 1988;12:897-906. http://dx.doi.org/10.1097/00000478-198812000-00001.
- McNeal JE. Normal histology of the prostate. Am J Surg Pathol 1988;12:619-33. http://dx.doi.org/10.1097/00000478-198808000-00003.
- American Cancer Society . How Is Prostate Cancer Staged? 2013. www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-staging (accessed March 2014).
- Heidenreich A, Abrahamsson PA, Artibani W, Catto J, Montorsi F, Van Poppel H, et al. Early detection of prostate cancer: European Association of Urology recommendation. Eur Urol 2013;64:347-54. http://dx.doi.org/10.1016/j.eururo.2013.06.051.
- Prostate Cancer UK . Prostate Cancer Treatment 2014. http://prostatecanceruk.org/information/prostate-cancer/treatment (accessed 15 September 2014).
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev 2004;25:276-308. http://dx.doi.org/10.1210/er.2002-0032.
- Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS. Secondary hormonal therapy for advanced prostate cancer. J Urol 2006;175:27-34. http://dx.doi.org/10.1016/S0022-5347(05)00034-0.
- Cancer Research UK . Prostate Cancer Incidence Statistics 2014. www.cancerresearchuk.org/cancer-info/cancerstats/types/prostate/incidence/ (accessed 15 September 2014).
- Cancer Research UK . Prostate Cancer Survival Statistics 2014. www.cancerresearchuk.org/cancer-info/cancerstats/types/prostate/survival/ (accessed 15 September 2014).
- Prostate Cancer: Diagnosis and Treatment. Cardiff: National Collaborating Centre for Cancer; 2014.
- Cancer Research UK . Prostate Cancer Mortality Statistics 2014. www.cancerresearchuk.org/cancer-info/cancerstats/types/prostate/mortality/ (accessed 15 September 2014).
- Glaser A, Fraser L, Corner J, Feltbower R, Morris E, Hartwell G, et al. Patient reported outcomes of cancer survivors in England 1–5 years after diagnosis: a cross-setional survey. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002317.
- Mowatt G, Scotland G, Boachie C, Cruickshank M, Ford JA, Fraser C, et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: a systematic review and economic evaluation. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17200.
- Zaytoun OM, Jones JS. Prostate cancer detection after a negative prostate biopsy: lessons learnt in the Cleveland Clinic experience. Int J Urol 2011;18:557-68. http://dx.doi.org/10.1111/j.1442-2042.2011.02798.x.
- Kirby R, Fitzpatrick JM. Optimising repeat prostate biopsy decisions and procedures. BJU Int 2011;109:1750-4. http://dx.doi.org/10.1111/j.1464-410X.2011.10809.x.
- Scattoni V, Raber M, Capitanio U, Abdollah F, Roscigno M, Angiolilli D, et al. The optimal rebiopsy prostatic scheme depends on patient clinical characteristics: results of a recursive partitioning analysis based on a 24-core systematic scheme. Eur Urol 2011;60:834-41. http://dx.doi.org/10.1016/j.eururo.2011.07.036.
- National Schedule of Reference Costs: The Main Schedule. London: DH; 2013.
- Burford DC, Kirby M, Austoker J. Prostate Cancer Risk Management Programme. Information for Primary Care; PSA Testing in Asymptomatic Men. Sheffield: NHS Cancer Screening Programmes; 2010.
- Prostate Cancer Risk Management Programme. Undertaking a Transrectal Ultrasound Guided Biopsy of the Prostate. Sheffield: NHS Cancer Screening Programmes; 2006.
- Norberg M, Egevad L, Holmberg L, Sparen P, Norlen BJ, Busch C. The sextant protocol for ultrasound-guided core biopsies of the prostate underestimates the presence of cancer. Urology 1997;50:562-6. http://dx.doi.org/10.1016/S0090-4295(97)00306-3.
- Djavan B, Waldert M, Zlotta A, Dobronski P, Seitz C, Remzi M, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol 2001;166:856-60. http://dx.doi.org/10.1016/S0022-5347(05)65851-X.
- Lujan M, Paez A, Santonja C, Pascual T, Fernandez I, Berenguer A. Prostate cancer detection and tumor characteristics in men with multiple biopsy sessions. Prostate Cancer Prostatic Dis 2004;7:238-42. http://dx.doi.org/10.1038/sj.pcan.4500730.
- Mian BM, Naya Y, Okihara K, Vakar-Lopez F, Troncoso P, Babaian RJ. Predictors of cancer in repeat extended multisite prostate biopsy in men with previous negative extended multisite biopsy. Urology 2002;60:836-40. http://dx.doi.org/10.1016/S0090-4295(02)01950-7.
- Shah RB. Current perspectives on the Gleason grading of prostate cancer. Arch Pathol Lab Med 2009;133:1810-16. http://dx.doi.org/10.1043/1543-2165-133.11.1810.
- Epstein JI. Gleason score 2–4 adenocarcinoma of the prostate on needle biopsy: a diagnosis that should not be made. Am J Surg Pathol 2000;24:477-8. http://dx.doi.org/10.1097/00000478-200004000-00001.
- Towards a Consensus Protocol on Prostate Biopsies: Indications, Techniques and Assessment. London: Royal College of Pathologists; 2003.
- Epstein JI, Allsbrook WC, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 2005;29:1228-42. http://dx.doi.org/10.1097/01.pas.0000173646.99337.b1.
- Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-101. http://dx.doi.org/10.1001/jama.293.17.2095.
- Stamey TA, Freiha FS, McNeal JE, Redwine EA, Whittemore AS, Schmid HP. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 1993;71:933-8. http://dx.doi.org/10.1002/1097-0142(19930201)71:3+<933::AID-CNCR2820711408>3.0.CO;2-L.
- Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RCN, Hoedemaeker RF, van Leenders GJLH, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol 2011;185:121-5. http://dx.doi.org/10.1016/j.juro.2010.08.082.
- Rodrigues G, Warde P, Pickles T, Crook J, Brundage M, Souhami L, et al. Pre-treatment risk stratification of prostate cancer patients: a critical review. Can Urol Assoc J 2012;6:121-7. http://dx.doi.org/10.5489/cuaj.11085.
- Shaw GL, Thomas BC, Dawson SN, Srivastava G, Vowler SL, Gnanapragasam VJ, et al. Identification of pathologically insignificant prostate cancer is not accurate in unscreened men. Br J Cancer n.d.;110:2405-11. http://dx.doi.org/10.1038/bjc.2014.192.
- D’Amico AV, Whittington R, Malkowicz S, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969-74. http://dx.doi.org/10.1001/jama.280.11.969.
- Benson MC, Whang IS, Pantuck A, Ring K, Kaplan SA, Olsson CA, et al. Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol 1992;147:815-16.
- Thanigasalam R, Mancuso P, Tsao K, Rashid P. Prostate-specific antigen velocity (PSAV): a practical role for PSA?. ANZ J Surg 2009;79:703-6. http://dx.doi.org/10.1111/j.1445-2197.2009.05055.x.
- Patel AR, Klein EA. Risk factors for prostate cancer. Nat Clin Pract Urol 2009;6:87-95. http://dx.doi.org/10.1038/ncpuro1290.
- SWOP – The Prostate Cancer Research Foundation, Rotterdam . Risk Calculators n.d. www.prostatecancer-riskcalculator.com/seven-prostate-cancer-risk-calculators (accessed April 2014).
- van Vugt HA, Roobol MJ, Kranse R, Maattanen L, Finne P, Hugosson J, et al. Prediction of prostate cancer in unscreened men: external validation of a risk calculator. Eur J Cancer 2011;47:903-9. http://dx.doi.org/10.1016/j.ejca.2010.11.012.
- Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006;98:529-34. http://dx.doi.org/10.1093/jnci/djj131.
- Karakiewicz PI, Benayoun S, Kattan MW, Perrotte P, Valiquette L, Scardino PT, et al. Development and validation of a nomogram predicting the outcome of prostate biopsy based on patient age, digital rectal examination and serum prostate specific antigen. J Urol 2005;173:1930-4. http://dx.doi.org/10.1097/01.ju.0000158039.94467.5d.
- Multi-Parametric Prostate Cancer Staging Exam. San Francisco, CA: University of California; 2014.
- Le JD, Huang J, Marks LS. Targeted prostate biopsy: value of multiparametric magnetic resonance imaging in detection of localized cancer. Asian J Androl 2014;16:522-9. http://dx.doi.org/10.4103/1008-682X.122864.
- Marks L, Young S, Natarajan S. MRI–ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol 2013;23:43-50. http://dx.doi.org/10.1097/MOU.0b013e32835ad3ee.
- Gittelman MC, Hertzman B, Bailen J, Williams T, Koziol I, Henderson RJ, et al. PCA3 molecular urine test as a predictor of repeat prostate biopsy outcome in men with previous negative biopsies: a prospective multicenter clinical study. J Urol 2013;190:64-9. http://dx.doi.org/10.1016/j.juro.2013.02.018.
- Haese A, de la Taille A, van Poppel H, Marberger M, Stenzl A, Mulders PF, et al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol 2008;54:1081-8. http://dx.doi.org/10.1016/j.eururo.2008.06.071.
- Pepe P, Fraggetta F, Galia A, Skonieczny G, Aragona F. PCA3 score and prostate cancer diagnosis at repeated saturation biopsy. Which cut-off: 20 or 35?. Int Braz J Urol 2012;38:489-95. http://dx.doi.org/10.1590/S1677-55382012000400008.
- Sokoll LJ, Ellis W, Lange P, Noteboom J, Elliott DJ, Deras IL, et al. A multicenter evaluation of the PCA3 molecular urine test: pre-analytical effects, analytical performance, and diagnostic accuracy. Clin Chim Acta 2008;389:1-6. http://dx.doi.org/10.1016/j.cca.2007.11.003.
- Diagnosis and Monitoring of Prostate Cancer: Progensa PCA3 Assay and Prostate Health Index (PHI): Final Scope. London: National Institute for Health and Care Excellence; 2014.
- Summary of Safety and Effectiveness Data. PMA P100033: PROGENSA PCA3 Assay. Silverspring, MD: Food and Drug Administration; 2012.
- Gen-Probe . Pack Insert for PROGENSA PCA3 Assay n.d.
- Food and Drug Administration . PMA Approval. PROGENSA PCA3 Assay 2012. www.accessdata.fda.gov/cdrh_docs/pdf10/p100033a.pdf (accessed October 2014).
- Lee R, Localio AR, Armstrong K, Malkowicz SB, Schwartz JS, Free PSA. Study Group. A meta-analysis of the performance characteristics of the free prostate-specific antigen test. Urology 2006;67:762-8. http://dx.doi.org/10.1016/j.urology.2005.10.052.
- Roddam AW, Duffy MJ, Hamdy FC, Ward AM, Patnick J, Price CP, et al. Use of prostate-specific antigen (PSA) isoforms for the detection of prostate cancer in men with a PSA level of 2–10 ng/ml: systematic review and meta-analysis. Eur Urol 2005;48:386-99. http://dx.doi.org/10.1016/j.eururo.2005.04.015.
- Jansen FH, van Schaik RH, Kurstjens J, Horninger W, Klocker H, Bektic J, et al. Prostate-specific antigen (PSA) isoform p2PSA in combination with total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur Urol 2010;57:921-7. http://dx.doi.org/10.1016/j.eururo.2010.02.003.
- Le BV, Griffin CR, Loeb S, Carvalhal GF, Kan D, Baumann NA, et al. [–2]Proenzyme prostate specific antigen is more accurate than total and free prostate specific antigen in differentiating prostate cancer from benign disease in a prospective prostate cancer screening study. J Urol 2010;183:1355-9. http://dx.doi.org/10.1016/j.juro.2009.12.056.
- Beckman Coulter . Draft Directional Insert. Access Immunoassay Systems. Hybritech p2PSA 2011. www.accessdata.fda.gov/cdrh_docs/pdf9/P090026c.pdf (accessed 21 August 2014).
- Summary of Safety and Effectiveness Data. PMA P090026. Quantitative test for determination of [–2]proPSA levels. Silverspring, MD: Food and Drug Administration; 2012.
- Vignati G, Giovanelli L. Standardization of PSA measures: a reappraisal and an experience with WHO calibration of Beckman Coulter Access Hybritech total and free PSA. Int J Biol Markers 2007;22:295-301.
- Food and Drug Administration . PMA Approval. Access Hybritech p2PSA on Access Immunoassay Systems 2012. www.accessdata.fda.gov/cdrh_docs/pdf9/p090026a.pdf (accessed October 2014).
- Systematic reviews: CRD’s Guidance on Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination; 2009.
- Diagnostics Assessment Programme Manual. London: National Institute for Health and Care Excellence; 2011.
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y, Deeks JJ, et al. Cochrane Handbook for Systematic Reviews of Diagnostic test Accuracy (Version 10). Cochrane; 2010.
- Jonas DE, Wilt TJ, Taylor BC, Wilkins TM, Matchar DB. Chapter 11: challenges in and principles for conducting systematic reviews of genetic tests used as predictive indicators. J Gen Intern Med 2012;27:S83-93. http://dx.doi.org/10.1007/s11606-011-1898-z.
- Teutsch SM, Bradley LA, Palomaki GE, Haddow JE, Piper M, Calonge N, et al. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: methods of the EGAPP Working Group. Genet Med 2009;11:3-14. http://dx.doi.org/10.1097/GIM.0b013e318184137c.
- Bradley LA, Palomaki GE, Gutman S, Samson D, Aronson N. PCA3 Testing for the Diagnosis and Management of Prostate Cancer. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013.
- Bradley LA, Palomaki GE, Gutman S, Samson D, Aronson N. Comparative effectiveness review: prostate cancer antigen 3 testing for the diagnosis and management of prostate cancer. J Urol 2013;190:389-98. http://dx.doi.org/10.1016/j.juro.2013.02.005.
- Bruzzese D, Mazzarella C, Ferro M, Perdona S, Chiodini P, Perruolo G, et al. Prostate health index vs. percent free prostate-specific antigen for prostate cancer detection in men with ‘gray’ prostate-specific antigen levels at first biopsy: systematic review and meta-analysis. Transl Res 2014;14:441-51. http://dx.doi.org/10.1016/j.trsl.2014.06.006.
- Filella X, Gimenez N. Evaluation of [–2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med 2013;51:729-39. http://dx.doi.org/10.1515/cclm-2012-0410.
- Zhang ZX, Yang J, Zhang CZ, Li KA, Quan QM, Wang XF, et al. The value of magnetic resonance imaging in the detection of prostate cancer in patients with previous negative biopsies and elevated prostate-specific antigen levels: a meta-analysis. Acad Radiol 2014;21:578-89. http://dx.doi.org/10.1016/j.acra.2014.01.004.
- Groskopf J, Aubin SM, Deras IL, Blase A, Bodrug S, Clark C, et al. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem 2006;52:1089-95. http://dx.doi.org/10.1373/clinchem.2005.063289.
- Shappell SB, Fulmer J, Arguello D, Wright BS, Oppenheimer JR, Putzi MJ. PCA3 urine mRNA testing for prostate carcinoma: patterns of use by community urologists and assay performance in reference laboratory setting. Urology 2009;73:363-8. http://dx.doi.org/10.1016/j.urology.2008.08.459.
- Semjonow A, Kopke T, Eltze E, Pepping-Schefers B, Burgel H, Darte C. Pre-analytical in-vitro stability of –2 proPSA in blood and serum. Clin Biochem 2010;43:926-8. http://dx.doi.org/10.1016/j.clinbiochem.2010.04.062.
- Sokoll LJ, Chan DW, Klee GG, Roberts WL, van Schaik RH, Arockiasamy DA, et al. Multi-center analytical performance evaluation of the Access Hybritech p2PSA immunoassay. Clin Chim Acta 2012;413:1279-83. http://dx.doi.org/10.1016/j.cca.2012.04.015.
- Stephan C, Kahrs AM, Cammann H, Lein M, Schrader M, Deger S, et al. A –2 proPSA-based artificial neural network significantly improves differentiation between prostate cancer and benign prostatic diseases. INC 2009;69:198-207. http://dx.doi.org/10.1002/pros.20872.
- Clinicaltrials.gov . NCT01441687. Comparing the Reliability of Expressed Prostatic Secretion (EPS) and Post Massage Urine (PMU) for the Prediction of Prostate Cancer Biopsy Outcome n.d. http://clinicaltrials.gov/show/NCT01441687 (accessed 30 September 2014).
- Pilot Study: Performance of the Progensa PCA3 Test in Post-oxytocin Urine Specimens. Geneva: WHO; n.d.
- Chen Z, Sun J, Kim ST, Groskopf J, Feng J, Isaacs WB, et al. Genome-wide association study identifies genetic determinants of urine PCA3 levels in men. Neoplasia 2013;15:448-53. http://dx.doi.org/10.1593/neo.122144.
- Kote-Jarai Z, Leongamornlert D, Tymrakiewicz M, Field H, Guy M, Al Olama AA, et al. Mutation analysis of the MSMB gene in familial prostate cancer. BJC 2010;102:414-18. http://dx.doi.org/10.1038/sj.bjc.6605485.
- Lou H, Yeager M, Li H, Bosquet JG, Hayes RB, Orr N, et al. Fine mapping and functional analysis of a common variant in MSMB on chromosome 10q11.2 associated with prostate cancer susceptibility. Proc Natl Acad Sci USA 2009;106:7933-8. http://dx.doi.org/10.1073/pnas.0902104106.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Reitsma JB, Rutjes AWS, Whiting P, Vlassov VV, Leeflang MMG, Deeks JJ, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, Version 100. n.d.
- Whiting PF, Westwood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 2006;6. http://dx.doi.org/10.1186/1471-2288-6-9.
- ClinicalTrials.gov . NCT01632930. Medical Economics of Urinary PCA3 Test for Prostate Cancer Diagnosis n.d. http://clinicaltrials.gov/show/NCT01632930 (accessed 30 September 2014).
- Ankerst DP, Groskopf J, Day JR, Blase A, Rittenhouse H, Pollock BH, et al. Predicting prostate cancer risk through incorporation of prostate cancer gene 3. [Erratum published in J Urol 2009;181:1507.]. J Urol 2008;180:1303-8. http://dx.doi.org/10.1016/j.juro.2008.06.038.
- Aubin SM, Reid J, Sarno MJ, Blase A, Aussie J, Rittenhouse H, et al. PCA3 molecular urine test for predicting repeat prostate biopsy outcome in populations at risk: validation in the placebo arm of the dutasteride REDUCE trial. J Urol 2010;184:1947-52. http://dx.doi.org/10.1016/j.juro.2010.06.098.
- Auprich M, Augustin H, Budaus L, Kluth L, Mannweiler S, Shariat SF, et al. A comparative performance analysis of total prostate-specific antigen, percentage free prostate-specific antigen, prostate-specific antigen velocity and urinary prostate cancer gene 3 in the first, second and third repeat prostate biopsy. BJU Int 2012;109:1627-35. http://dx.doi.org/10.1111/j.1464-410X.2011.10584.x.
- Auprich M, Haese A, Walz J, Pummer K, de la Taille A, Graefen M, et al. External validation of urinary PCA3-based nomograms to individually predict prostate biopsy outcome. Eur Urol 2010;58:727-32. http://dx.doi.org/10.1016/j.eururo.2010.06.038.
- Bollito E, De Luca S, Cicilano M, Passera R, Grande S, Maccagnano C, et al. Prostate Cancer Gene 3 urine assay cutoff in diagnosis of prostate cancer a validation study on an italian patient population undergoing first and repeat biopsy. Anal Quant Cytol Histol 2012;34:96-104.
- Busetto GM, De Berardinis E, Sciarra A, Panebianco V, Giovannone R, Rosato S, et al. Prostate Cancer Gene 3 and multiparametric magnetic resonance can reduce unnecessary biopsies: decision curve analysis to evaluate predictive models. Urology 2013;82:1355-62. http://dx.doi.org/10.1016/j.urology.2013.06.078.
- Goode RR, Marshall SJ, Duff M, Chevli E, Chevli KK. Use of PCA3 in detecting prostate cancer in initial and repeat prostate biopsy patients. INC 2013;73:48-53. http://dx.doi.org/10.1002/pros.22538.
- Lazzeri M, Briganti A, Scattoni V, Lughezzani G, Larcher A, Gadda GM, et al. Serum Index Test percent –2 proPSA and Prostate Health Index are more accurate than prostate specific antigen and percent fPSA in predicting a positive repeat prostate biopsy. J Urol 2012;188:1137-43. http://dx.doi.org/10.1016/j.juro.2012.06.017.
- Marks LS, Fradet Y, Deras IL, Blase A, Mathis J, Aubin SM, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology 2007;69:532-5. http://dx.doi.org/10.1016/j.urology.2006.12.014.
- Panebianco V, Sciarra A, De Berardinis E, Busetto GM, Lisi D, Buonocore V, et al. PCA3 urinary test versus 1H-MRSI and DCEMR in the detection of prostate cancer foci in patients with biochemical alterations. Anticancer Res 2011;31:1399-405.
- Pepe P, Aragona F. PCA3 score vs. PSA free/total accuracy in prostate cancer diagnosis at repeat saturation biopsy. Anticancer Res 2011;31:4445-9.
- Pepe P, Aragona F. Prostate cancer detection rate at repeat saturation biopsy: PCPT risk calculator versus PCA3 score versus case-finding protocol. Can J Urol 2013;20:6620-4.
- Perdonà S, Cavadas V, Di Lorenzo G, Damiano R, Chiappetta G, Del Prete P, et al. Prostate cancer detection in the ‘grey area’ of prostate-specific antigen below 10 ng/ml: head-to-head comparison of the updated PCPT calculator and Chun’s nomogram, two risk estimators incorporating prostate cancer antigen 3. Eur Urol 2011;59:81-7. http://dx.doi.org/10.1016/j.eururo.2010.09.036.
- Ploussard G, Haese A, Van Poppel H, Marberger M, Stenzl A, Mulders PF, et al. The prostate cancer gene 3 (PCA3) urine test in men with previous negative biopsies: does free-to-total prostate-specific antigen ratio influence the performance of the PCA3 score in predicting positive biopsies?. BJU Int 2010;106:1143-7. http://dx.doi.org/10.1111/j.1464-410X.2010.09286.x.
- Porpiglia F, Russo F, Manfredi M, Mele F, Fiori C, Bollito E, et al. The roles of multiparametric magnetic resonance imaging, PCA3 and Prostate Health Index – which is the best predictor of prostate cancer after a negative biopsy?. J Urol n.d.:60-6. http://dx.doi.org/10.1016/j.juro.2014.01.030.
- Ramos CG, Valdevenito R, Vergara I, Anabalon P, Sanchez C, Fulla J. PCA3 sensitivity and specificity for prostate cancer detection in patients with abnormal PSA and/or suspicious digital rectal examination. First Latin American experience. Urologic Oncol-Semin ORI n.d.;31:1522-6. http://dx.doi.org/10.1016/j.urolonc.2012.05.002.
- Remzi M, Haese A, van Poppel H, de la Taille A, Stenzl A, Hennenlotter J, et al. Follow-up of men with an elevated PCA3 score and a negative biopsy: does an elevated PCA3 score indeed predict the presence of prostate cancer?. BJU Intern 2010;106:1138-42. http://dx.doi.org/10.1111/j.1464-410X.2010.09330.x.
- Scattoni V, Lazzeri M, Lughezzani G, De Luca S, Passera R, Bollito E, et al. Head-to-head comparison of Prostate Health Index and urinary PCA3 for predicting cancer at initial or repeat biopsy. J Urol 2013;190:496-501. http://dx.doi.org/10.1016/j.juro.2013.02.3184.
- Sciarra A, Panebianco V, Cattarino S, Busetto GM, Berardinis E, Ciccariello M, et al. Multiparametric magnetic resonance imaging of the prostate can improve the predictive value of the urinary prostate cancer antigen 3 test in patients with elevated prostate-specific antigen levels and a previous negative biopsy. BJU Intern 2012;110:1661-5. http://dx.doi.org/10.1111/j.1464-410X.2012.11146.x.
- Stephan C, Vincendeau S, Houlgatte A, Cammann H, Jung K, Semjonow A. Multicenter evaluation of [–2]proprostate-specific antigen and the prostate health index for detecting prostate cancer. Clin Chem n.d.;59:306-14. http://dx.doi.org/10.1373/clinchem.2012.195784.
- Tombal B, Andriole GL, Taille A, Gontero P, Haese A, Remzi M, et al. Clinical judgment versus biomarker prostate cancer gene 3: which is best when determining the need for repeat prostate biopsy?. Urology 2013;81:998-1004. http://dx.doi.org/10.1016/j.urology.2012.11.069.
- Wu AK, Reese AC, Cooperberg MR, Sadetsky N, Shinohara K. Utility of PCA3 in patients undergoing repeat biopsy for prostate cancer. Prostate Cancer Prostatic Dis 2012;15:100-5. http://dx.doi.org/10.1038/pcan.2011.52.
- Stephan C, Jung K, Semjonow A, Schulze-Forster K, Cammann H, Hu X, et al. Comparative assessment of urinary prostate cancer antigen 3 and TMPRSS2:ERG gene fusion with the serum [–2]proprostate-specific antigen-based prostate health index for detection of prostate cancer. Clin Chem 2013;59:280-8. http://dx.doi.org/10.1373/clinchem.2012.195560.
- Tombal B, Ameye F, de la Taille A, de Reijke T, Gontero P, Haese A, et al. Biopsy and treatment decisions in the initial management of prostate cancer and the role of PCA3; a systematic analysis of expert opinion. World J Urol 2012;30:251-6. http://dx.doi.org/10.1007/s00345-011-0721-0.
- Chun FK, de la Taille A, van Poppel H, Marberger M, Stenzi A, Mulders PF, et al. Prostate cancer gene 3 (PCA3): development and internal validation of a novel biopsy nomogram. Eur Urol 2009;56:659-68. http://dx.doi.org/10.1016/j.eururo.2009.03.029.
- Luo Y, Gou X, Huang P, Mou C. The PCA3 test for guiding repeat biopsy of prostate cancer and its cut-off score: a systematic review and meta-analysis. Asian J Androl 2014;16:487-92. http://dx.doi.org/10.4103/1008-682X.125390.
- Clinical and Cost Effectiveness of the PROGENSA PCA3 Assay and the Prostate Health Index in the Diagnosis of Prostate Cancer: A Systematic Review and Economic Evaluation [Protocol]. Liverpool: Liverpool Reviews and Implementation Group; 2014.
- Drummond M, Jefferson T. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ economic evaluation working party. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Heijnsdijk E, Huang J, Denham D, De Koning H. The cost-effectiveness of prostate cancer detection using Beckman Coulter Prostate Health Index. Eur Urol Suppl 2012;11. http://dx.doi.org/10.1016/S1569-9056(12)60257-7.
- Malavaud B, Cussenot O, Mottet N, Rozet F, Ruffion A, Smets L. Impact of adoption of a decision algorithm including PCA3 for repeat biopsy on the costs for prostate cancer diagnosis in France. J Med Econ 2013;16:358-63. http://dx.doi.org/10.3111/13696998.2012.757552.
- Nepple K, Strope S, Kibel A, Sandhu G, Weigand L, Kymes S. Cost-analysis of PCA3 versus PSA in the detection of prostate cancer in men with a prior negative biopsy. J Urol 2012;187. http://dx.doi.org/10.1016/j.juro.2012.02.184.
- Nichol M, Wu J, Huang J, Denham D, Frencher S, Jacobsen S. Cost-effectiveness of prostate health index for prostate cancer detection. BJU Int 2011;110:353-62. http://dx.doi.org/10.1111/j.1464-410X.2011.10751.x.
- Heijnsdijk EA, Wever EM, Auvinen A, Hugosson J, Ciatto S, Nelen V, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med 2012;367:595-60. http://dx.doi.org/10.1056/NEJMoa1201637.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark J, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2011;364:1708-17. http://dx.doi.org/10.1056/NEJMoa1011967.
- Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH. Increasing hospital admission rates for urological complications after transrectal utlrasound guided prostate biopsy. J Urol 2010;183:963-8. http://dx.doi.org/10.1016/j.juro.2009.11.043.
- Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, Goodwin L, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.d7894.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- de Haes JC, de Koning HJ, van Oortmarssen GJ, van Agt HM, de Bruyn AE, van Der Maas PJ. The impact of a breast cancer screening programme on quality-adjusted life-years. Int J Cancer 1991;49:538-44. http://dx.doi.org/10.1002/ijc.2910490411.
- Ara R, Wailoo AJ. The Use of Health State Utility Values in Decision Models 2011.
- Vickers AJ. Counterpoint: Prostate-specific antigen velocity is not of value for early detection of cancer. J Natl Compr Canc Netw 2013;11:286-90.
- Vickers AJ, Savage C, O’Brien MF, Lilja H. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol 2009;27:398-403. http://dx.doi.org/10.1200/JCO.2008.18.1685.
- Vickers AJ, Cronin AM. Everything you always wanted to know about evaluating prediction models (but were too afraid to ask). Urology 2010;76:1298-301. http://dx.doi.org/10.1016/j.urology.2010.06.019.
- Filella X, Foj L, Mila M, Auge JM, Molina R, Jimenez W. PCA3 in the detection and management of early prostate cancer. Tumour Biol 2013;34:1337-47. http://dx.doi.org/10.1007/s13277-013-0739-6.
- Wang W, Wang M, Wang L, Adams TS, Tian Y, Xu J. Diagnostic ability of %p2PSA and prostate health index for aggressive prostate cancer: a meta-analysis. Sci Rep 2014;4. http://dx.doi.org/10.1038/srep05012.
- Wilt TJ. The Prostate Cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy with watchful waiting for men with clinically localized prostate cancer. J Natl Cancer Inst Monogr 2012;2012:184-90. http://dx.doi.org/10.1093/jncimonographs/lgs041.
- Chu DI, De Nunzio C, Gerber L, Thomas JA, Calloway EE, Albisinni S, et al. Predictive value of digital rectal examination for prostate cancer detection is modified by obesity. Prostate Cancer Prostatic Dis 2011;14:346-53. http://dx.doi.org/10.1038/pcan.2011.31.
- Saah AJ, Hoover DR. ‘Sensitivity’ and ‘specificity’ reconsidered: the meaning of these terms in analytical and diagnostic settings. Ann Intern Med 1997;126:91-4. http://dx.doi.org/10.7326/0003-4819-126-1-199701010-00026.
- Betz JM, Brown PN, Roman MC. Accuracy, precision, and reliability of chemical measurements in natural products research. Fitoterapia 2011;82:44-52. http://dx.doi.org/10.1016/j.fitote.2010.09.011.
- Labnetwork . Method Validation n.d. www.labnetwork.org/en/chemical-lab-recent/111-method-validation (accessed 1 September 2014).
- Hayen A, Macaskill P, Irwig L, Bossuyt P. Appropriate statistical methods are required to assess diagnostic tests for replacement, add-on, and triage. J Clin Epidemiol 2010;63:883-91. http://dx.doi.org/10.1016/j.jclinepi.2009.08.024.
- Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565-74. http://dx.doi.org/10.1177/0272989X06295361.
- Steyerberg EW, Vickers AJ. Decision curve analysis: a discussion. Med Decis Making 2008;28:146-9. http://dx.doi.org/10.1177/0272989X07312725.
Appendix 1 Outcome measures
Analytical validity
Analytical validity can be subdivided into the following components:
-
Pre-analytical variability refers to the extent to which factors such as sampling methods, transport, storage and temperature of the samples before they are analysed affect the results of the assay. Pre-analytical variables considered can also include age, ethnicity and genotype, which affect the normal ranges of the results.
-
Analytical specificity refers to the ability of an assay to measure a particular substance, rather than others, in a sample. 132 It is tested by examining the crossover reaction with other substances and drugs.
-
Analytical sensitivity represents the smallest amount of substance in a sample that can accurately be measured by an assay. 132 It is usually measured by:
-
LoQ which is the lowest amount of analyte in a sample that can be reliably detected and at which the total error meets the pre-specified requirement for accuracy. 48
-
LoB which is the highest measurement that is likely to be observed for a blank sample. 48
-
LoD which may be defined as LoB plus 1.65 SD. 74
-
-
Accuracy is a measure of the closeness of the experimental value to the actual amount of the substance in the matrix. 133 Accuracy often depends on what is used as the true value and whether or not there is a gold standard available. Accuracy is typically assessed by spiked recovery studies in which the amount of a target compound is determined as a percentage of the theoretical amount present in the matrix.
-
Precision measures how close individual measurements of a sample are to each other. 133 Precision is often measured using the CV, which is the SD of the repeated measurement divided by the mean value expressed as a percentage. Precision is subdivided into various components:
-
Repeatability is a measure of the within-laboratory uncertainty. It can be divided into within- and between-run variability.
-
Intermediate precision is a measure of the ruggedness of the method, that is reliability when performed in different environments. Demonstration of intermediate precision requires that the method be run on multiple days by different analysts and on different instruments. Robustness is the capacity of a method to remain unaffected by small deliberate variations in method parameters. The robustness of a method is evaluated by varying method parameters. 134
-
Clinical validity
In clinical validity studies the diagnostic accuracy of a new or intervention test is assessed against a reference standard. The reference standard is the best test available, that is the current preferred method of diagnosing a disease. In the case of prostate cancer the reference standard is a biopsy. All new tests need to be compared against the diagnostic accuracy of a biopsy.
Measures for assessing a single test against a reference standard
The classic presentation of the results of a clinical validity study is the so-called 2 × 2 table as shown in Table 66.
| Test result | Biopsy results (reference standard) | |
|---|---|---|
| Prostate cancer | No prostate cancer | |
| New test positive | a | b |
| New test negative | c | d |
The number entered into cell ‘a’ is the number of patients for whom the new test correctly diagnoses prostate cancer (as determined by the reference standard, in this case a biopsy). For these people, the new test is positive as is the reference standard: these are the TPs.
The number entered into cell ‘b’ is the number of patients for whom the new test is positive (i.e. indicates the presence of prostate cancer) but who do not, according to the reference standard (biopsy), have prostate cancer. The new test has incorrectly diagnosed prostate cancer: these are FPs.
The number entered into cell ‘c’ is the number of patients who are identified through the reference standard (biopsy) as having prostate cancer but for whom the new test gave negative results. The new test has incorrectly labelled the patient as having prostate cancer: these are FNs.
The number in cell ‘d’ is the number of patients who do not, according to the reference standard (biopsy), have prostate cancer and who are also shown by the new test to be free from disease: these are TNs.
The numbers displayed in a 2 × 2 table are used to generate other summary measures. These are set out in Table 67.
| Term | Formula | Notes |
|---|---|---|
| Sensitivity | a/(a + c) | Proportion of those who actually have disease who are correctly identified with positive test results. TP rate. High sensitivity = few FNs |
| Specificity | d/(b + d) | Proportion of those who do not actually have the disease who are correctly identified with negative test results. 1 – FP rate. High specificity = few FPs |
| Positive predictive value | a/(a + b) | The proportion of those with positive test results who actually have the disease |
| Negative predictive value | d/(c + d) | The proportion of those with negative test results who do not have the disease |
In an ideal world, a test would be 100% sensitive and 100% specific. However, in reality there is often a trade-off between the two, with tests that have high sensitivity also having low specificity and vice versa.
The use of a 2 × 2 tables requires that the test results are dichotomous, that is can be divided into two groups: test positive and test negative. If the actual test results are continuous variables, similar to PCA3 or phi scores, this means that a threshold (or a cut-off point) needs to be selected to divide the results into positive and negative groups.
Differences in means or medians
Another way of comparing the results of continuous variables is to compare the means or medians of test results between biopsy-positive and -negative men. The difference can be compared using analysis of variance.
Receiver operating characteristic curve
When an intervention test has a range of possible thresholds which could be used to divide results into test positive and test negative, the relationship between the threshold used and the performance of the test can be examined in a ROC curve. This is a graphical plot of the sensitivity (TP rate) against 1 – specificity or the FP rate for each threshold; examples of a ROC curve are shown in Figure 12 with the associated distribution of the intervention tests in diseased and non-diseased populations. An ideal test would have a point in the top-left corner, with 100% specificity and 100% sensitivity.
FIGURE 12.
Examples of a ROC curve (image reproduced with permission from Chapter 10 of the Cochrane DTA handbook63). For explanatory text describing the ROC curves, please see Chapter 10 of the Cochrane handbook (p. 13).
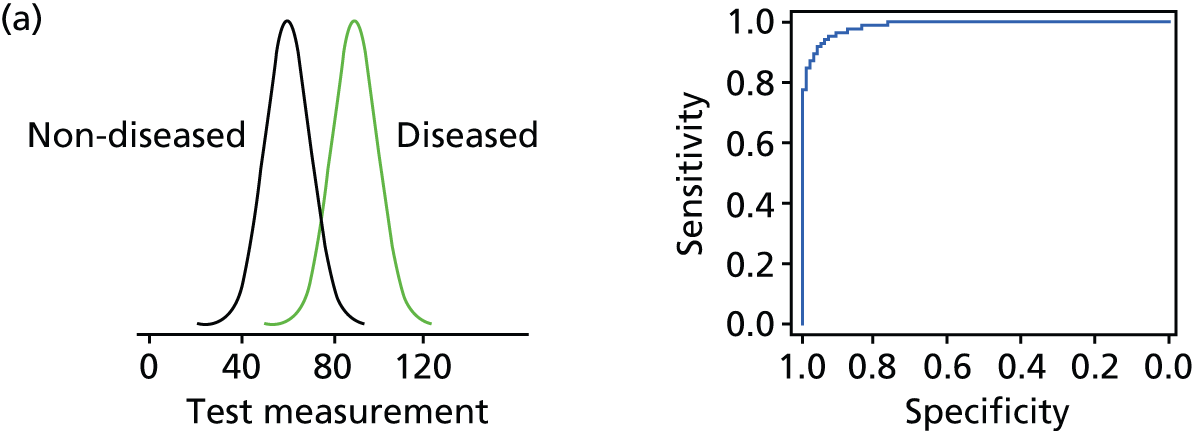

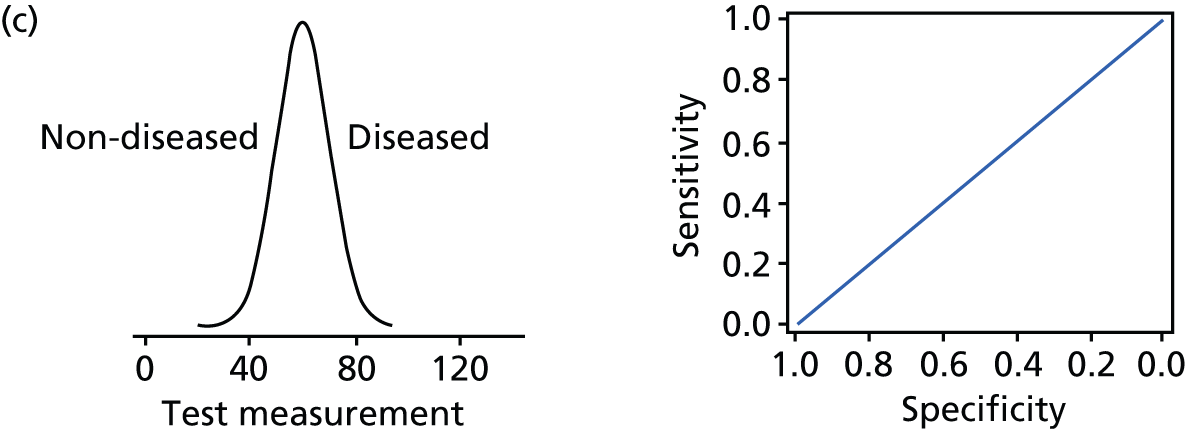
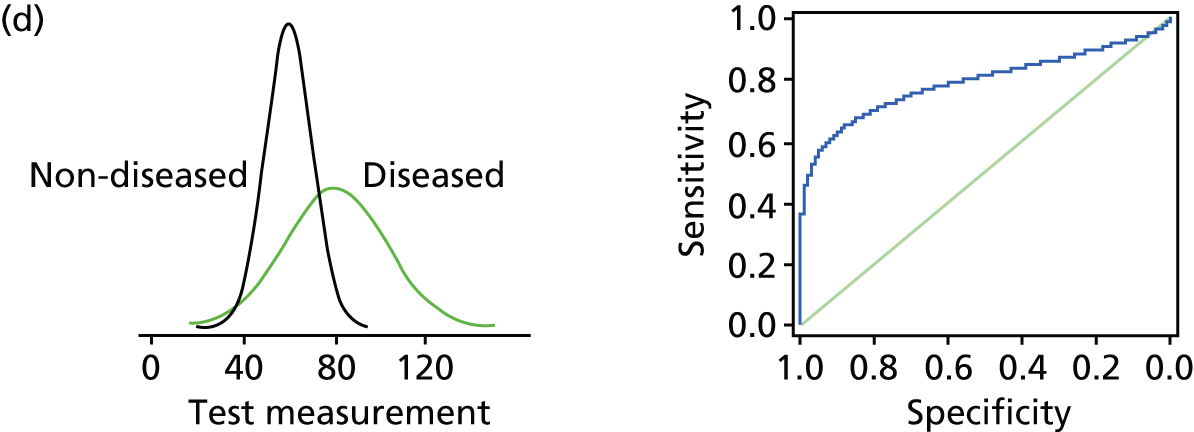
The ROC curve can be used to assess the degree to which sensitivity changes at different levels of specificity or vice versa. Some studies report AUC as a proportion of the total area of the graph. This is a measure of the predictive accuracy or discrimination of the diagnostic test, that is the ability of the test to discriminate between those who have (or will develop) prostate cancer from those who do not have (or will not develop) prostate cancer. The AUC can also be expressed as the probability that someone with the disease will have a higher test result than someone without the disease. It is also referred to as the c-statistic. An AUC of 1.0 indicates a perfect test, and an AUC of 0.5 (the diagonal line) indicates that the test is no better than chance (i.e. 50% probability) in predicting whether or not the disease is present. An AUC of 0.5–0.7 is considered as poor discrimination, 0.7–0.8 acceptable discrimination, 0.8–0.9 excellent discrimination and above 0.9 exceptional discrimination.
Measures for assessing multiple tests against a reference standard
The measures discussed in Measures for assessing a single test against a reference standard can be used to compare a single intervention test with a reference standard. However, in clinical practice, test pathways often involve a series of tests used together. It is possible to combine 2 × 2 tables for a sequence of different tests if it is clear how the tests are used135 (e.g. in parallel or in sequence), but results are rarely reported in this way. If the results of various tests are combined into an algorithm or decision tool within a study, data can be presented as a single test and analysed using sensitivity and specificity. However, when results are presented in this way, it can be unclear how each variable is used within the decision tool.
Most often, combinations of diagnostic tests are analysed using logistic regression models.
Logistic regression models
Logistic regression is a statistical method for analysing a data set in which there are one or more independent variables that determine an outcome. The outcome is measured with a dichotomous variable. A dichotomous variable is one with only two possible outcomes.
The goal of logistic regression is to find the best-fitting (yet biologically reasonable) model to describe the relationship between the dichotomous characteristic of interest (dependent variable = response or outcome variable) and a set of independent (predictor or explanatory) variables. In diagnostic logistic regression models, the outcome is the presence or absence of disease. In prostate cancer biopsy models, the outcome is presence or absence of prostate cancer and the independent variables are the intervention tests such as PCA3 score, age and/or PSA level. The independent variables may be used as dichotomous, continuous or categorical variables.
An OR is the outcome measure reported from the logistic regression model. An OR is a way of quantifying how strongly the outcome is associated with each of the variables used in the model, such as PSA level. The OR in a diagnostic logistic regression model (also called the diagnostic OR) is, for example, the odds that an individual with a ‘raised PSA level’ has prostate cancer relative to the odds that an individual without a ‘raised PSA level’ has prostate cancer. If the OR is greater than 1, then having ‘prostate cancer’ is considered to be ‘associated’ with having a ‘raised PSA level’, meaning that having a ‘raised PSA level’ raises (relative to not having a ‘raised PSA level’) the odds of having ‘prostate cancer’. The OR demonstrates only an association between the two variables; causality has not been shown. When multiple variables are entered into a logistic regression model, the OR of each variable is adjusted to take account of the effects of other variables.
Receiver operating characteristics curves and derived sensitivity, and derived specificity from logistic regression models
The output from diagnostic logistic regression models can be used to generate ROC curves. These analyses rely on the predicted probability risk of having the outcome (in this case, prostate cancer) generated by the statistical model for each participant. By selecting a threshold probability risk of, say, 0.3, the participants can be classified as test positive or test negative depending on whether or not their predicted probability from the model is above or below 0.3. By varying the threshold predicted probability, ROC curves can be generated. The performance of different diagnostic models can be compared using the AUC. The AUC gives a measure of predictive accuracy, but is not very meaningful in clinical practice.
Receiver operating characteristics curves can also be used to derive sensitivity and specificity for alternative diagnostic models. For a set level, of for example 90% sensitivity, the specificity of various models can be calculated along with the associated threshold for predicted probability of a positive biopsy that has been used to generate these levels of sensitivity and specificity. However, the threshold predicted probability does not have relevance clinically and cannot be used to identify the threshold of an intervention test used in clinical practice above which patients should be recommended for biopsy.
Estimates of derived sensitivity and specificity from logistic regression models are more useful clinically than AUC estimates, as improvements in sensitivity or specificity can be described in terms of numbers of missed cancers or avoided biopsies. However, these derived sensitivity or specificity estimates are derived from ROC curves generated from logistic regression models and it is often not possible to associate the demonstrated improvement in sensitivity or specificity with the use of a particular threshold of the intervention test.
Decision curve analysis
Decision curve analysis is designed to present more clinically useful results when comparing diagnostic strategies. 136 The method calculates the net benefit of a diagnostic model by subtracting the harm of unnecessary biopsies from the benefit of diagnosed cases of prostate cancer. Unlike the conventional trade-off between sensitivity and specificity, in decision curve analysis there is an attempt to weight the relative harms and benefits using the threshold probability of cancer at which the patient or clinician will opt for a biopsy. For instance, when a clinician recommends a biopsy for any patient with a 10% or higher risk of cancer, which suggests that that the risk associated with an unnecessary biopsy is weighted less than the risk associated with an unnecessary biopsy when a 50% or higher risk of cancer is required before a biopsy is offered. The results are presented as graphs of net benefit over the range of probability risk stated to be clinically important, that is in which patients or clinicians might be uncertain whether or not to biopsy. This clinically important range of probability risk is typically from 10% to 40% for repeat prostatic biopsy. In the decision curve analysis graph, as well as displaying curves for each included model, there are two references lines shown: one for treating/biopsying no patients and one for biopsying all patients. The percentage reduction in biopsies for each diagnostic model compared with the biopsy-all strategy is another way of presenting the results. When interpreting results an emphasis is placed on whether or not the model adds any information to decision-making over the indicated range of probability. 137 The results do not present statistical significance tests and no methods of comparing or pooling the results across different studies are available.
Appendix 2 Literature search strategies
MEDLINE (via Ovid) and OLDMEDLINE (via Ovid)
Date range: 1946 to present with daily update.
Search name: PCA3_analytic.
Date run: 28 April 2014.
Search strategy
-
exp prostatic neoplasms/ (91,977)
-
(prostat* adj3 (cancer or carcinoma* or neoplasm* or malignant* or tumor* or tumour*)).tw. (84,448)
-
or/1-2 (104,576)
-
(Prostat* adj2 cancer* adj2 (antigen* or gene*) adj2 “3”).tw. (107)
-
(PCA3 or PCA-3 or “PCA 3”).tw. (275)
-
uPM3.tw. (7)
-
7(“differential display code 3 antigen” or DD3).tw. (80)
-
progensa.tw. (26)
-
or/4-8 (356)
-
prostate health index.tw. (30)
-
Beckman Coulter.tw. (515)
-
(proPSA or p2proPSA).tw. (70)
-
or/10-12 (582)
-
or/9,13 (928)
-
3 and 14 (355)
-
exp animals/ not humans/ (3,930,803)
-
nonhuman/ not human/ (0)
-
or/16-17 (3,930,803)
-
15 not 18 (354)
-
limit 19 to yr=2000-2014 (344)
-
Accuracy/ (106)
-
exp Diagnostic Errors/ (93,798)
-
exp “Sensitivity and Specificity”/ (411,853)
-
exp “reproducibility of results”/ (270,891)
-
analytic validity.mp. (47)
-
(repeatability or reproducibility).mp. (300,172)
-
or/21-26 (674,714)
-
14 and 27 (410)
-
28 not 19 (236)
-
or/9-10,12 (432)
-
30 and 27 162)
-
31 not 19 (4)
MEDLINE (via Ovid) and OLDMEDLINE (via Ovid)
Date range: 1946 to present with daily update.
Search name: PCA3_comparator.
Date run: 28 April 2014.
Search strategy
-
exp Magnetic Resonance Spectroscopy/ (177,430)
-
magnetic resonance imaging/ or exp diffusion magnetic resonance imaging/ (289,134)
-
magnetic resonance imag$.tw. (128,216)
-
magnetic resonance spectroscop*.tw. (15,525)
-
mrs.tw. (10,926)
-
(dynamic contrast enhanced adj3 (MRI or magnetic)).tw. (2026)
-
dce-mri.tw. (1185)
-
(diffusion weight$ adj3 (MRI or magnetic)).tw. (3682)
-
dw-mri.tw. (425)
-
((multi-parametric or multiparametric or mp) adj (MRI or magnetic)).tw. (294)
-
or/1-10 (498,078)
-
exp Prostate/ah, pa, us [Anatomy & Histology, Pathology, Ultrasonography] (11,845)
-
(transrectal adj (biops* or ultrasound or ultrason*)).tw. (5135)
-
trus.tw. (1664)
-
exp Biopsy, Needle/ (52,622)
-
(biopsy or biopsies or pathol* or histopathol*).tw. (821,981)
-
or/12-16 (854,276)
-
exp Prostate-Specific Antigen/ (18,924)
-
psa.tw. (20,988)
-
prostat* specific antigen*.tw. (18,277)
-
or/18-20 (31,453)
-
exp nomograms/(1280)
-
nomogram*.tw. (4429)
-
(neural adj2 network).tw. (12,074)
-
or/22-24 (16,748)
-
exp prostatic neoplasms/ (91,977)
-
(prostat* adj3 (cancer or carcinoma* or neoplasm* or malignant* or tumor* or tumour*)).tw.(84,448)
-
or/26-27 (104,576)
-
or/11,17,21,25 (1,336,436)
-
28 and 29 (38,357)
-
exp meta-analysis/ (47,236)
-
exp Meta-Analysis as Topic/ (13,686)
-
Meta-analys*.mp. or (meta adj analys*).ti,ab. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] (74,826)
-
meta-regress*.mp. or (meta adj regress*).ti,ab. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] (2040)
-
meta analysis.pt. (47,236)
-
systematic review.ti. (26,922)
-
or/31-36 (90,728)
-
30 and 37 (262)
MEDLINE (via Ovid) and OLDMEDLINE (via Ovid)
Date range: 1946 to present with daily update.
Search name: PCA3_analytic.
Date run: 28 April 2014.
Search strategy
-
exp prostatic neoplasms/ (91,977)
-
(prostat* adj3 (cancer or carcinoma* or neoplasm* or malignant* or tumor* or tumour*)).tw. (84,448)
-
or/1-2 (104,576)
-
(Prostat* adj2 cancer* adj2 (antigen* or gene*) adj2 “3”).tw. (107)
-
(PCA3 or PCA-3 or “PCA 3”).tw. (275)
-
uPM3.tw. (7)
-
(“differential display code 3 antigen” or DD3).tw. (80)
-
progensa.tw. (26)
-
or/4-8 (356)
-
prostate health index.tw. (30)
-
Beckman Coulter.tw. (515)
-
(proPSA or p2proPSA).tw. (70)
-
or/10-12 (582)
-
or/9,13 (928)
-
3 and 14 (355)
-
exp animals/ not humans/ (3,930,803)
-
nonhuman/ not human/ (0)
-
or/16-17 (3,930,803)
-
15 not 18 (354)
-
limit 19 to yr=2000-2014 (344)
-
Accuracy/ (106)
-
exp Diagnostic Errors/ (93,798)
-
exp “Sensitivity and Specificity”/ (411,853)
-
exp “reproducibility of results”/ (270,891)
-
analytic validity.mp. (47)
-
(repeatability or reproducibility).mp. (300,172)
-
or/21-26 (674,714)
-
14 and 27 (410)
-
28 not 19 (236)
-
or/9-10,12 (432)
-
30 and 27 (162)
-
31 not 19 (4)
MEDLINE (via Ovid) and OLDMEDLINE (via Ovid)
Date range: 1946 to present with daily update.
Search name: PCA3_comparator.
Date run: 28 April 2014.
Search strategy
-
exp Magnetic Resonance Spectroscopy/ (177,430)
-
magnetic resonance imaging/ or exp diffusion magnetic resonance imaging/ (289,134)
-
magnetic resonance imag$.tw. (128,216)
-
magnetic resonance spectroscop*.tw. (15,525)
-
mrs.tw. (10,926)
-
(dynamic contrast enhanced adj3 (MRI or magnetic)).tw. (2026)
-
dce-mri.tw. (1185)
-
(diffusion weight$ adj3 (MRI or magnetic)).tw. (3682)
-
dw-mri.tw. (425)
-
((multi-parametric or multiparametric or mp) adj (MRI or magnetic)).tw. (294)
-
or/1-10 (498,078)
-
exp Prostate/ah, pa, us [Anatomy & Histology, Pathology, Ultrasonography] (11,845)
-
(transrectal adj (biops* or ultrasound or ultrason*)).tw. (5135)
-
trus.tw. (1664)
-
exp Biopsy, Needle/ (52,622)
-
(biopsy or biopsies or pathol* or histopathol*).tw. (821,981)
-
or/12-16 (854,276)
-
exp Prostate-Specific Antigen/ (18,924)
-
psa.tw. (20,988)
-
prostat* specific antigen*.tw. (18,277)
-
or/18-20 (31,453)
-
exp nomograms/ (1280)
-
nomogram*.tw. (4429)
-
(neural adj2 network).tw. (12,074)
-
or/22-24 (16,748)
-
exp prostatic neoplasms/ (91,977)
-
(prostat* adj3 (cancer or carcinoma* or neoplasm* or malignant* or tumor* or tumour*)).tw. (84,448)
-
or/26-27 (104,576)
-
or/11,17,21,25 (1,336,436)
-
28 and 29 (38,357)
-
exp meta-analysis/ (47,236)
-
exp Meta-Analysis as Topic/ (13,686)
-
Meta-analys*.mp. or (meta adj analys*).ti,ab. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] (74,826)
-
meta-regress*.mp. or (meta adj regress*).ti,ab. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] (2040)
-
meta analysis.pt. (47,236)
-
systematic review.ti. (26,922)
-
or/31-36 (90,728)
-
30 and 37 (262)
The Cochrane Library
Date range: start date to April 2014.
Search name: PCA3_studies.
Date run: 28 April 2014.
Search strategy
#1 MeSH descriptor: [Prostatic Neoplasms] explode all trees (3325)
#2 prostat* near/3 (cancer or carcinoma* or neoplasm* or malignant* or tumor* or tumour*) (5935)
#3 #1 or #2 (5935)
#4 Prostat* near/2 cancer* near/2 (antigen* or gene*) near/2 “3” (15)
#5 PCA3 or PCA-3 or “PCA 3” (26)
#6 uPM3 (0)
#7 “differential display code 3 antigen” or DD3 (3)
#8 progensa (6)
#9 [or #4-#8] (30)
#10 “prostate health index” (5)
#11 Beckman Coulter (20)
#12 proPSA or p2proPSA (3)
#13 [or #10-#12] (24)
#14 (#9 or #13) and #3 (28)
#15 #14 Publication Date from 2000 to 2014 (28)
EMBASE
Date range: 1980 to week 20 2014.
Date run: 19 May 2014.
Search strategy
-
exp prostate cancer/ (127,704)
-
(prostat* adj3 (cancer or carcinoma* or neoplasm* or malignant* or tumor* or tumour*)).tw. (119,035)
-
or/1-2 (153,396)
-
(prostate cancer* adj2 (antigen* or gene*) adj2 “3”).tw. (204)
-
(PCA3 or PCA-3 or “PCA 3”).tw. (600)
-
uPM3.tw. (11)
-
(“differential display code 3 antigen” or DD3).tw. (123)
-
progensa.tw. (81)
-
or/4-8 (732)
-
prostate health index.tw. (135)
-
Beckman Coulter.tw. (1719)
-
(proPSA or p2proPSA).tw. (182)
-
or/10-12 (1845)
-
9 or 13 (2543)
-
3 and 14 (794)
-
animal/ or animal experiment/ (3,209,456)
-
exp human/ or human experiment/ (14,662,346)
-
16 not (16 and 17) (2,687,279)
-
15 not 18 (793)
-
limit 19 to yr=“2000 - 2014” (781)
EMBASE
Date range: 1974 to 16 May 2014.
Date run: 19 May 2014.
Search strategy
-
exp nuclear magnetic resonance spectroscopy/ (92,226)
-
exp nuclear magnetic resonance imaging/ or exp diffusion weighted imaging/ (528,862)
-
magnetic resonance imag*.tw. (163,725)
-
magnetic resonance spectroscop*.tw. (18,845)
-
mrs.tw. (17,870)
-
(dynamic contrast enhanced adj3 (MRI or magnetic)).tw. (2790)
-
dce-mri.tw. (1936)
-
(diffusion weight* adj3 (MRI or magnetic)).tw. (5296)
-
dw-mri.tw. (749)
-
((multi-parametric or multiparametric or mp) adj (MRI or magnetic)).tw. (688)
-
or/1-10 (650,710)
-
exp prostate/ (38,897)
-
(transrectal adj (biops* or ultrasound or ultrason*)).tw. (7484)
-
trus.tw. (3448)
-
exp needle biopsy/ (34,761)
-
(biopsy or biopsies or pathol* or histopathol*).tw. (1,149,785)
-
or/12-16 (1,197,743)
-
exp prostate specific antigen/ (35,908)
-
psa.tw. (37,014)
-
prostat* specific antigen*.tw. (23,016)
-
or/18-20 (56,049)
-
exp nomogram/ (4169)
-
nomogram*.tw. (6493)
-
(neural adj2 network).tw. (17,045)
-
or/22-24 (24,162)
-
exp prostate tumor/ (154,040)
-
(prostat* adj3 (cancer or carcinoma* or neoplasm* or malignant* or tumor* or tumour*)).tw. (120,976)
-
26 or 27 (164,596)
-
11 or 17 or 21 or 25 (1,817,863)
-
28 and 29 (68,602)
-
exp meta analysis/ (78,651)
-
exp Meta-Analysis as Topic/ (13,178)
-
Meta-analys*.mp. or (meta adj analys*).ti,ab. (118,781)
-
meta-regress*.mp. or (meta adj regress*).ti,ab. (2908)
-
systematic review*.ti,ab. (64,099)
-
or/31-35 (158,492)
-
30 and 36 (674)
Appendix 3 Data extraction forms
| Number | Item | Comment |
|---|---|---|
| Miscellaneous | ||
| 1 | Publication type (e.g. full report, abstract, letter, unpublished) | |
| 2 | Other reports from same study population? | |
| 3 | Funding | |
| 4 | Conflict of interest? | |
| 5 | Notes | |
| Test and study population | ||
| 6 | Test name (e.g. PCA3/phi/p2PSA) | |
| 7 | Details of test platform/methods evaluated | |
| 8 | Country and setting | |
| 9 | Number of participants | |
| 10 | Age of participants | |
| 11 | Ethnicity of participants | |
| 12 | % of participants with prostatic disease | |
| 13 | Pre-analytical variables studied (age, DRE, genetic or ethnicity) | |
| 14 | Number of centres/labs tested | |
| 15 | Number of samples tested | |
| 16 | Timing and locations of repeat assays | |
| 17 | Standard/control sample used | |
| 18 | Other notes about how test conducted and/or data collected (e.g. likely to reflect how samples collected in clinical practice?) | |
| Results | ||
| 19 | Test failure rate | |
| 20 | Analytical sensitivity (e.g. LoB, LoD or LoQ) | |
| 21 | Analytical specificity (e.g. crossreactivity and carry over) | |
| 22 | Accuracy (e.g. comparison to a ‘gold standard’ reference test and recovery) | |
| 23 | Linearity and range | |
| 24 | Precision (reproducibility and %CV) | |
| 25 | Other | |
| Quality assessment | ||
| 26 | Adequate descriptions of test under evaluation (reports specific methods/platforms evaluated, quality assurance measures, e.g. control samples; see responses to 6 to 18 above) | |
| 27 | Comparison to a ‘gold standard’ reference test? | |
| 28 | Specimens represent routinely analysed clinical specimens in all aspects (e.g., collection, transport, processing; see response to 18 above) | |
| 29 | Relevant outcomes to assess analytical validity adequately addressed? (see responses to 19 to 25 above) | |
| 30 | Sample size/power calculations addressed? | |
| Study details | Description/location in text |
|---|---|
| Date form completed (dd/mm/yyyy) | |
| Name/ID of person extracting data | |
| Record number, author, year (ID for this paper/abstract/report) | |
| Name of study | |
| Other reports from same study population | |
| Publication type (e.g. full report, abstract or letter) | |
| Funding/conflicts of interest |
| Study design | Description/location in text | |
|---|---|---|
| Aim of study | ||
| Design (e.g. cohort, cross-sectional, case–control, randomised) | ||
| Number of centres | ||
| Country, type of hospital | ||
| Method/s of recruitment of participants (e.g. consecutive, random sample, retrospective selection) | ||
| Informed consent obtained | ||
| Ethical approval needed/obtained for study | ||
| Method of allocation to test pathway if not all participants received both tests | ||
| Start date | ||
| End date | ||
| Total study duration | ||
| Participants | Description/location in text | |
| Total number in study | ||
| Number with previous negative biopsy (use this number for results) | ||
Inclusion criteria include:
|
||
| Exclusion criteria | ||
| Age | ||
| Race/ethnicity | ||
| PSA mean | ||
| Other (DRE +ve, family history, # previous biopsy, imaging abnormalities, HGPIN, etc.) | ||
| Baseline imbalances if not all participants received both tests | ||
Details of first negative biopsy taken:
|
||
| Intervention test group: repeat if needed | Description as stated in report/paper | |
| Test name (e.g. PCA3/phi/p2PSA) | ||
| Details of urine/blood sample collection | ||
| Details of test platform used | ||
| Details of a DRE and test collection | ||
| Number (%) informative test | ||
| Threshold values used | ||
| Was threshold pre-specified in Methods? | ||
| Timing of test in relation to initial biopsy | ||
| Timing of test in relation to repeat biopsy | ||
| Was assessor blinded to other study results? | ||
| Comparator tests reported | Tick if included | Details |
| PSA | □ | |
| MRI | □ | |
| Nomograms | □ | |
| Clinical risk factors (e.g. age, a DRE, etc.) – please list | □ | |
| Other – please list | □ | |
| Comparator test: PSA | Description as stated in report/paper. Location in text | |
| Test name (e.g. tPSA/fPSA etc.) | ||
| Number of participants test collected from | ||
| Threshold values used | ||
| Was threshold pre-specified in methods? | ||
| Timing of test in relation to initial biopsy | ||
| Timing of test in relation to repeat biopsy | ||
| Was assessor blinded to other study results? | ||
| Comparator test: MRI | Description as stated in report/paper. Location in text | |
| Type of MRI name (e.g. T2, DW, DCE-MRI) | ||
| Details of MRI used | ||
| Number of participants received MRI | ||
| Number (%) informative results | ||
| Who did assessment/interpretation of MRI? | ||
| Definition of abnormality? | ||
| Was definition pre-specified in methods? | ||
| Was assessor blinded to other results? | ||
| Timing of MRI in relation to initial biopsy | ||
| Timing of MRI in relation to PCA3/phi | ||
| Timing of MRI in relation to repeat biopsy | ||
| Comparator test: nomograms/clinical risk factors | Description as stated in report/paper. Location in text | |
| Number of participants with nomogram/clinical assessment results | ||
| Name of nomograms used | ||
| Reference for nomogram | ||
| Data incorporated in nomogram or clinical risk factors used | ||
| Threshold values used for nomogram | ||
| Was threshold/abnormal definition pre-specified in methods? | ||
| Timing of data collection in relation to initial biopsy | ||
| Timing of data collection in relation to PCA3/phi | ||
| Timing of data collection in relation to repeat biopsy | ||
| Blinding of clinical assessment to other results? | ||
| Reference standard | Description as stated in report/paper | |
Type of repeat biopsy:
|
||
| Number of cores taken | ||
| Timing of biopsy | ||
| Definition of positive biopsy. HGPIN/ASAP included? | ||
| Number (%) positive | ||
| Other end points reported (Gleason score, % cores positive) |
||
| Histopathology procedures and expertise | ||
| Use of ultrasound for guiding biopsy | ||
| Use of MRI-targeting technology | ||
Results of intervention/comparator test known to:
|
||
| Please draw up a flow chart of number of participants completing study | ||
| Reference standard | Test pathway 1 | Test pathway 2 | Test pathway 3 | Test pathway 4 | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCA3 | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Cut-off/continuous | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Cut-off/continuous | ||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical risk factors/nomogram | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Results | ||||||||||||||||||||||||||||||||||||||||||||||||
| N | ||||||||||||||||||||||||||||||||||||||||||||||||
| Means/medians (SD/IQR), units | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| 2 × 2 table – TP, TN, FN, FP | □ | Bx+Bx–Test+Test– | Bx+ | Bx– | Test+ | Test– | □ | Bx+Bx–Test+Test– | Bx+ | Bx– | Test+ | Test– | □ | Bx+Bx–Test+Test– | Bx+ | Bx– | Test+ | Test– | □ | Bx+Bx–Test+Test– | Bx+ | Bx– | Test+ | Test– | ||||||||||||||||||||||||
| Bx+ | Bx– | |||||||||||||||||||||||||||||||||||||||||||||||
| Test+ | ||||||||||||||||||||||||||||||||||||||||||||||||
| Test– | ||||||||||||||||||||||||||||||||||||||||||||||||
| Bx+ | Bx– | |||||||||||||||||||||||||||||||||||||||||||||||
| Test+ | ||||||||||||||||||||||||||||||||||||||||||||||||
| Test– | ||||||||||||||||||||||||||||||||||||||||||||||||
| Bx+ | Bx– | |||||||||||||||||||||||||||||||||||||||||||||||
| Test+ | ||||||||||||||||||||||||||||||||||||||||||||||||
| Test– | ||||||||||||||||||||||||||||||||||||||||||||||||
| Bx+ | Bx– | |||||||||||||||||||||||||||||||||||||||||||||||
| Test+ | ||||||||||||||||||||||||||||||||||||||||||||||||
| Test– | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sensitivity, specificity, LRs | Sensitivity: | Sensitivity: | Sensitivity: | Sensitivity: | ||||||||||||||||||||||||||||||||||||||||||||
| Specificity: | Specificity: | Specificity: | Specificity: | |||||||||||||||||||||||||||||||||||||||||||||
| ROC curves – graph | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Area under curve (95% CI) | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Derived sensitivity and specificity from curves | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Univariate ORs | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | ||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| Multivariate ORs | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | ||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| Definition of high grade cancer | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference standard | ||||||||||||||||||||||||||||||||||||||||||||||||
| Test pathway 1 | Test pathway 2 | Test pathway 3 | Test pathway 4 | |||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Cut-off/continuous | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Cut-off/continuous | ||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical risk factors/nomogram | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Results | ||||||||||||||||||||||||||||||||||||||||||||||||
| N | ||||||||||||||||||||||||||||||||||||||||||||||||
| Means/medians (SD/IQR), units | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| 2 × 2 table – TP, TN, FN, FP | □ | Bx+Bx–Test+Test– | Bx+ | Bx– | Test+ | Test– | □ | Bx+Bx–Test+Test– | Bx+ | Bx– | Test+ | Test– | □ | Bx+Bx–Test+Test– | Bx+ | Bx– | Test+ | Test– | □ | Bx+Bx–Test+Test– | Bx+ | Bx– | Test+ | Test– | ||||||||||||||||||||||||
| Bx+ | Bx– | |||||||||||||||||||||||||||||||||||||||||||||||
| Test+ | ||||||||||||||||||||||||||||||||||||||||||||||||
| Test– | ||||||||||||||||||||||||||||||||||||||||||||||||
| Bx+ | Bx– | |||||||||||||||||||||||||||||||||||||||||||||||
| Test+ | ||||||||||||||||||||||||||||||||||||||||||||||||
| Test– | ||||||||||||||||||||||||||||||||||||||||||||||||
| Bx+ | Bx– | |||||||||||||||||||||||||||||||||||||||||||||||
| Test+ | ||||||||||||||||||||||||||||||||||||||||||||||||
| Test– | ||||||||||||||||||||||||||||||||||||||||||||||||
| Bx+ | Bx– | |||||||||||||||||||||||||||||||||||||||||||||||
| Test+ | ||||||||||||||||||||||||||||||||||||||||||||||||
| Test– | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sensitivity, specificity, LRs | Sensitivity: | Sensitivity: | Sensitivity: | Sensitivity: | ||||||||||||||||||||||||||||||||||||||||||||
| Specificity: | Specificity: | Specificity: | Specificity: | |||||||||||||||||||||||||||||||||||||||||||||
| ROC curves – graph | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Area under curve (95% CI) | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Derived sensitivity and specificity from curves | □ | □ | □ | □ | ||||||||||||||||||||||||||||||||||||||||||||
| Univariate ORs | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | ||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| Multivariate ORs | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | □ | PCA3phiPSAclinMRI | PCA3 | phi | PSA | clin | MRI | ||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| PCA3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| phi | ||||||||||||||||||||||||||||||||||||||||||||||||
| PSA | ||||||||||||||||||||||||||||||||||||||||||||||||
| clin | ||||||||||||||||||||||||||||||||||||||||||||||||
| MRI | ||||||||||||||||||||||||||||||||||||||||||||||||
| Patient selection | |
|---|---|
| A. Risk of bias | Risk assessed as low/high/unclear |
| Was a consecutive or random sample of patients enrolled? | |
| Was a case–control design avoided? | |
| Did the study avoid inappropriate exclusions? | |
| Were men selected into study on basis of cancer risk such as on PSA range, a DRE, MRI, etc. | |
| Could the selection of patients have introduced bias? | |
| Comments: | |
| B. Concerns regarding applicability | Concerns assessed as low/high/unclear |
| Was risk of underlying risk of cancer in men in study population representative? | |
| Are there concerns that the included patients and setting do not match the review question? | |
| Comments: | |
| Intervention test | |
| A. Risk of bias | Risk assessed as low/high/unclear |
| Were the intervention test results interpreted without knowledge of the results of the reference standard? | |
| If a threshold was used, was it prespecified? | |
| Were the intervention test results interpreted without knowledge of the results of the comparator tests? | |
| Could the conduct or interpretation of the intervention test have introduced bias? | |
| Comments: | |
| B. Concerns regarding applicability | Concerns assessed as low/high/unclear |
| Are there concerns that the intervention test, its conduct or its interpretation differs from the review question? | |
| Comments: | |
| Comparator test – clinical and PSA | |
| A. Risk of bias | Risk assessed as low/high/unclear |
| Were the comparator test results interpreted without knowledge of the results of the reference standard? | |
| If a threshold was used, was it prespecified? | |
| Were the comparator test results interpreted without knowledge of the results of the comparator tests? | |
| Could the conduct or interpretation of the comparator test have introduced bias? | |
| Comments: | |
| B. Concerns regarding applicability | Concerns assessed as low/high/unclear |
| Are there concerns that the comparator test, its conduct, or interpretation differs from the review question? | |
| Comments: | |
| Comparator test – MRI | |
| A. Risk of bias | Risk assessed as low/high/unclear |
| Were the comparator test results interpreted without knowledge of the results of the reference standard? | |
| If a threshold was used, was it prespecified? | |
| Were the comparator test results interpreted without knowledge of the results of the comparator tests? | |
| Could the conduct or interpretation of the comparator test have introduced bias? | |
| Comments: | |
| B. Concerns regarding applicability | Concerns assessed as low/high/unclear |
| Are there concerns that the comparator test, its conduct or its interpretation differ from the review question? | |
| Comments: | |
| Reference standard | |
| A. Risk of bias | Risk assessed as low/high/unclear |
| Is the reference standard likely to correctly classify the target condition? | |
| Was the reference standard performed and results interpreted without knowledge of the results of the intervention tests? | |
| Was the reference standard performed and results interpreted without knowledge of the results of the comparator tests? | |
| Were the same number and pattern of cores taken in all participants? | |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | |
| Comments: | |
| B. Concerns regarding applicability | Concerns assessed as low/high/unclear |
| Are there concerns that the target condition as defined by the reference standard does not match the question? | |
| Comments: | |
| Flow and timing | |
| A. Risk of bias | Risk assessed as low/high/unclear |
| Was there an appropriate interval between intervention test and reference standard? | |
| Could the patient flow have introduced bias? | |
| Comments: | |
| Summary | |
| Key conclusions of study authors | |
| Comments of review authors | |
| Action/queries/further investigation needed | |
Appendix 4 Study characteristics of included studies for analytical validity review
| Study | Samples used | Outcomes reported | |||||
|---|---|---|---|---|---|---|---|
| Pre-analytical variables | Analytical sensitivity | Analytical specificity | Accuracy | Precision | Linearity and range | ||
| PCA3 studies | |||||||
| FDA SSED report50 | Controls based on in vitro transcripts, clinical samples | Temperature stability of 12 clinical urine samples before and after processing, reagents, urine transport kit | LoB, LoD, LoQ using control samples | Unspliced transcript, interfering substances, carry-over | Recovery of female urine spiked with in vitro transcripts | Within-laboratory repeatability of control samples and patient samples; between-laboratory reproducibility of control samples | Linearity using both control and clinical samples |
| Pack insert51 | Controls based on in vitro transcripts, clinical samples | Temperature stability of 10 clinical urine samples before and after processing | LoD, LoQ using control samples | Unspliced transcript, urine from post-prostatectomy patients, tissue specificity, interfering substances | Recovery of female urine spiked with in vitro transcripts | Within-laboratory repeatability of control samples; between-laboratory reproducibility of control and pooled clinical samples | Linearity using both control material diluted in female urine and diluent |
| Groskopf 200671 | Controls based on transcripts in detergent solution, clinical samples or ‘previously characterised pooled processed urine specimens’ | Temperature stability of three clinical urine samples after processing | NR | Urine from post-prostatectomy patients, urine from female patients | Recovery of three control samples | Within-laboratory repeatability of three control and three pooled urine samples | NR |
| Sokoll 200848 | Clinical samples, control samples based on in vitro transcripts | Informative rate for 179 clinical samples taken with or without a DRE and varying the strokes/lobe | LoB, LoD, LoQ using control samples | NR | Recovery of three control samples in two different sites | Within-laboratory repeatability of three control; between-laboratory reproducibility of three control | NR |
| Shappell 200972 | Clinical samples | NR | NR | NR | NR | Between-laboratory reproducibility of 50 clinical samples | NR |
| p2PSA or phi studies | |||||||
| FDA SSED58 Draft Pack insert57 | Patient samples (unspecified source), control samples based on internal reference preparation of p2PSA | Temperature stability of samples (same as in Semjonow et al.73), thermal sensitivity of assay; stability of reagents, calibrator and controls | LoB, LoD, LoQ using zero analyte and calibration samples (same as in Sokoll et al.74) | Interfering substances, cross-reactivity with other forms PSA (same as in Sokoll et al.74), carryover | Recovery of six spiked samples, (same as in Sokoll et al.74) | p2PSA: within-laboratory repeatability of three control and six patient samples; between-laboratory reproducibility of three control and three patient samples (same as in Sokoll et al.74) | Linearity of 12 unspecified samples. Hook effect examined |
| phi score: within-laboratory repeatability of one control and four patient samples; between-laboratory reproducibility of 10 patient samples | |||||||
| Stephan 200975 | Control materials, spiked patient serum, in-house serum pool | NR | LoD, based on zero calibrator | Cross-reactivity with other forms PSA | Recovery of six spiked samples | p2PSA: within-laboratory repeatability of four control or three control and one pooled clinical sample; inter-assay precision of sample from serum pool and control samples | Linearity of six spiked samples |
| Semjonow 201073 | 22 clinical samples from volunteers | Temperature stability of: clotted samples at 21 °C; serum at 4 °C, 21 °C, –20 °C and –70 °C freeze–thaw cycles | NR | NR | NR | NR | NR |
| Sokoll 201274 | Control samples, patient samples | NR | LoB, LoD, LoQ using zero analyte | Interfering substances, cross-reactivity with other forms PSA | Recovery of six spiked samples | p2PSA: within-laboratory repeatability of three control and three patient samples. Reported separately for four different laboratories | Linearity of three serum samples. Hook effect examined |
Appendix 5 Tables of excluded studies
| PCA3 studies excluded | Reason for exclusion |
|---|---|
| Chevli KK, Duff Ml, Walter P, Yu C, Capuder B, Elshafeli A, et al. Urinary PCA3 as a predictor for prostate cancer in a cohort of 3073 men undergoing initial prostate biopsy. J Urol 2014;191:1743–8 | Initial biopsy population and abstract |
| Crawford ED, Rove KO, Trabulsi EJ, Qian J, Drewnowska KP, Kaminetsky JC, et al. Diagnostic performance of PCA3 to detect prostate cancer in men with increased prostate specific antigen: a prospective study of 1,962 cases. J Urol 2012;188:1726–31 | Initial biopsy population |
| Day JR, Jones LA, Meyer SE, Hodge PN, Aussie J, Saltzstein DR, et al. Urinary PCA3 and TMPRSS2:ERG help predict biopsy outcome prior to initial prostate biopsy using a risk group analysis. 28th Annual EAU Congress, Milan, Italy, 15–19 March 2013. Eur Urol Suppl 2013;12:e1045 | Initial biopsy population and abstract |
| de la Taille A, Irani J, Graefen M, Chun F, de Reijke T, et al. Clinical evaluation of the PCA3 assay in guiding initial biopsy decisions. J Urol 2011;185:2119–25 | Initial biopsy population |
| Deras IL, Aubin SMJ, Blase A, Day JR, Koo S, Partin AW, et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol 2008;179:1587–92 | PSA/PCA3 only. Single study |
| Kella N, Day JR, Jones LA, Meyer SE, Hodge PN, Aussie J, et al. Urinary PCA3 and TMPRSS2: ERG help predict biopsy outcome prior to initial prostate biopsy using a risk group analysis. Annual Meeting of the American Urological Association, San Diego, CA, 4–8 May 2013 | Initial biopsy population and abstract |
| Ochiai A, Okihara K, Kamoi K, Iwata T, Kawauchi A, Miki T, et al. Prostate cancer gene 3 urine assay for prostate cancer in Japanese men undergoing prostate biopsy. Int J Urol 2011;18:200–5 | Mixed population |
| Roobol MJ, Schröder FH, van Leeuwen P, Wolters T, van den Bergh RC, van Leenders GJ, et al. Performance of the prostate cancer antigen 3 (PCA3) gene and prostate-specific antigen in prescreened men: exploring the value of PCA3 for a first-line diagnostic test. Eur Urol 2010;58:475–81 | Not all repeat biopsies |
| Wei J, Sanda M, Thompson I, Partin A, Feng Z, Sokoll L, et al. The NCI Early Detection Research Network (EDRN) Urinary PCA3 Validation Trial. Annual Meeting of the American Urological Association, Atlanta, Georgia, 19–23 May 2012 | Abstract only |
| Aubin SMJ, Reid J, Sarno MJ, Blase A, Aussie J, Rittenhouse H, et al. PCA3 molecular urine test for predicting repeat prostate biopsy outcome in populations at risk: validation in the placebo arm of the dutasteride REDUCE trial. J Urol 2010;184:1947–52 | Ineligible population |
| Deras IL, Aubin SMJ, Blase A, Day JR, Koo S, Partin AW, et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol 2008;179:1587–92 | Mixed biopsy population |
| Tombal B, Ameye F, de la Taille A, de Reijke T, Gontero P, Haese A, et al. Biopsy and treatment decisions in the initial management of prostate cancer and the role of PCA3; a systematic analysis of expert opinion. World J Urol 2012;30:251–6 | Expert opinion of PCA3 impact on repeat biopsy decision |
| Wei J, Sanda M, Thompson I, Partin A, Feng Z, Sokoll L, et al. The NCI Early Detection Research Network (EDRN) Urinary PCA3 Validation Trial. Annual Meeting of the American Urological Association Atlanta, Georgia, 19–23 May 2012 | Abstract only |
| Auprich M, Chun FKH, Ward JF, Pummer K, Babaian R, Augustin H, et al. Critical assessment of pre-operative urinary prostate cancer antigen 3 on the accuracy of prostate cancer staging. Eur Urol 2011;59:96–105 | Indolent and aggressive prostate cancers following diagnosis |
| Lin DW, Newcomb LF, Brown EC, Brooks JD, Carroll PR, Feng Z, et al. Urinary TMPRSS2: ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the Canary Prostate Active Surveillance Study. Clin Cancer Res 2013;19:2442–50 | Indolent and aggressive prostate cancers following diagnosis |
| Nakanishi H, Groskopf J, Fritche HA, Bhadkamkar V, Blase A, Kumar SV, et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol 2008;179:1804–10 | Unclear population |
| Ploussard G, Durand X, Xylinas E, Moutereau S, Radulescu C, Forgue A, et al. PCA3 score accurately predicts tumor volume and might help in selecting prostate cancer patients for active surveillance. Eur Urol 2011;59:422–9 | Indolent and aggressive prostate cancers following diagnosis |
| van Poppel H, Haese A, Graefen M, de la Taille A, Irani J, de Reijke T, et al. The relationship between Prostate CAncer gene 3 (PCA3) and prostate cancer significance. BJU Int 2011;109:360–6 | Results not reported separately for repeat |
| Vlaeminck-Guillem V, Devonec M, Colombel M, Rodriguez-Lafrasse C, Decaussin-Petrucci M, Ruffion A. Urinary PCA3 Score predicts prostate cancer multifocality. J Urol 2011;185:1234–9 | Indolent and aggressive prostate cancers following diagnosis |
| Whitman EJ, Groskopf J, Ali A, Chen Y, Blase A, Furusato B, et al. PCA3 score before radical prostatectomy predicts extracapsular extension and tumor volume. J Urol 2008;180:1975–9 | Indolent and aggressive prostate cancers following diagnosis |
| Marks LS, Bostwick DG. Prostate cancer specificity of PCA3 gene testing: examples from clinical practice. Rev Urol 2008;10:175–81 | Review without meta-analysis |
| Schilling D, de Reijke T, Tombal B, de la Taille A, Hennenlotter J, Stenzl A. The Prostate Cancer gene 3 assay: indications for use in clinical practice. BJU Int 2009;105:452–5 | Non-systematic review |
| Schilling D, Hennenlotter J, Munz M, Bökeler U, Sievert KD, Stenzl A. Interpretation of the prostate cancer gene 3 in reference to the individual clinical background: implications for daily practice. Urol Int 2010;85:159–65 | Unclear population and study design – only some biopsied |
| Wang R, Chinnaiyan AM, Dunn RL, Wojno KJ, Wei JT. Rational approach to implementation of prostate cancer antigen 3 into clinical care. Cancer 2009;115:3879–86 | Repeat results not reported separately |
| phi studies excluded | Reason for exclusion |
| Rhodes T, Jacobson DJ, McGree MS, St Sauver JL, Sarma AV, Girman CJ, et al. Distribution and associations of [–2]proenzyme-prostate specific antigen in community dwelling black and white men. J Urol 2012;187:92–6 | Ineligible design |
| Nichol MB, Wu J, An JJ, Huang J, Denham D, Frencher S, et al. Budget impact analysis of a new prostate cancer risk index for prostate cancer detection. Prostate Cancer Prostatic Dis 2011;14:253–61 | Ineligible design |
| Eichholz A, McCarthy F, Nening D, Thomas K, Howlett T, Iqbal J, et al. Prostate Health Index (phi) as a novel biomarker in active surveillance of prostate cancer (PCa). J Clin Oncol 2014;32:81 | Abstract only |
| Boegemann M, Vincendeau S, Stephan C, Houlgatte A, Krabbe LM, Blanchet J-S, et al. The effect of [–2]proPSA and prostate health index (phi) on the accuracy of the prediction of initial and repeat prostate biopsies compared to tPSA and percent fPSA in young men (age 65 or younger). J Clin Oncol 2014;32:Abstract 171 | Abstract only |
| Lughezzani G, Lazzeri M, Haese A, McNicholas T, de la Tailee A, Buffi NM, et al. Multicenter European external validation of a prostate health index-based nomogram for predicting prostate cancer at extended biopsy. Eur Urol 2014;66:906–12 | Initial biopsy population |
| Lippi G, Aloe R, Cervellin G. p2PSA but not total and free PSA increases after myocardial infarction: results of a preliminary investigation. Int J Cardiol 2011;153:119 | Letter RE: MI |
| Guazzoni G, Lazzeri M, Buffi NM, Abrate A, Mistretta FA, Hurle R, et al. Preoperative prostate-specific antigen isoform p2PSA and its derivatives, percent p2PSA and prostate health index, predict pathologic outcomes in patients undergoing radical prostatectomy for prostate cancer. Eur Urol 2012;61:455–66 | Men diagnosed with clinically localised PCa |
| Filella X, Gimenez N. Evaluation of [–2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med 2013;51:729–39 | Not a repeat biopsy population |
| Lazzeri M, Haese A, Abrate A, de la Taille, Redorta JP, NcNicholas T, et al. Clinical performance of serum prostate-specific antigen isoform [–2]proPSA (p2PSA) and its derivatives, per cent p2PSA and the prostate health index (PHI), in men with a family history of prostate cancer: results from a multicentre European study, the PROMEtheuS project. BJU Int 2013;112:313–21 | Not a repeat biopsy population |
| Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, et al. A multicenter study of [–2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol 2011;185:1650–5 | Not a repeat biopsy population |
| Jansen FH, van Schaik RH, Kurstjens J, Horninger W, Klocker H, Bektic J, et al. Prostate-specific antigen (PSA) isoform p2PSA in combination with total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur Urol 2010;57:921–7 | Not a repeat biopsy population |
| Ito K, Miyakubo M, Sekine Y, Koike H, Matsui H, Shibata Y, et al. Diagnostic significance of [–2]pro-PSA and prostate dimension-adjusted PSA-related indices in men with total PSA in the 2.0–10.0 ng/ml range. World J Urol 2013:31:305–11 | Not a repeat biopsy population |
| Ferro M, Bruzzese D, Perdona S, Mazzarella C, Marino A, Sorrentino A, et al. Predicting prostate biopsy outcome: prostate health index (phi) and prostate cancer antigen 3 (PCA3) are useful biomarkers. Clin Chim Acta 2012;413:1274–8 | Not repeat biopsy population |
| Heidegger I, Klocker H, Steiner E, Skradski V, Ladurner M, Pichler R, et al. [–2]proPSA is an early marker for prostate cancer aggressiveness. Prostate Cancer Prostatic Dis 2014;17:70–4 | Not repeat biopsy population |
| Ferro M, Bruzzese D, Perdona S, Mazzarella C, Marino A, Sorrentino A, et al. Prostate Health Index (Phi) and Prostate Cancer Antigen 3 (PCA3) significantly improve prostate cancer detection at initial biopsy in a total PSA range of 2–10 ng/ml. PLOS ONE 2013;8:e67687 | Not repeat biopsy population |
| Lazzeri M, Haese A, de la Taille A, Palou Rodorta J, McNicholas T, Lughezzani G, et al. Serum isoform [–2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2–10 ng/ml: a multicentric European study. Eur Urol 2013:63:986–94 | Not repeat biopsy population |
| Perdona S, Bruzzese D, Ferro M, Autorino R, Marino A, Mazzarella C, et al. Prostate health index (phi) and prostate cancer antigen 3 (PCA3) significantly improve diagnostic accuracy in patients undergoing prostate biopsy. Prostate 2013;73:227–35 | Not repeat biopsy population |
| Ng CF, Chiu PK, Lam NY, Lam HC, Lee KW, Hou SS, et al. The Prostate Health Index in predicting initial prostate biopsy outcomes in Asian men with prostate-specific antigen levels of 4–10 ng/ml. Int Urol Nephrol 2014;46:711–17 | Not repeat biopsy population |
| Isharwal S, Makarov DV, Sokoll LJ, Landis P, Marlow C, Epstien JI, et al. ProPSA and diagnostic biopsy tissue DNA content combination improves accuracy to predict need for prostate cancer treatment among men enrolled in an active surveillance program. Urology 2011;77:e761–6 | Patients with low-risk cancer |
| Tosoian JJ, Loeb S, Feng Z, Isharwal S, Landis P, Elliot DJ, et al. Association of [–2]proPSA with biopsy reclassification during active surveillance for prostate cancer. J Urol 2012;188:1131–6 | Patients with low-risk cancer |
| Hirama H, Sugimoto M, Ito K, Shiraishi T, Kakehi Y. The impact of baseline [–2]proPSA-related indices on the prediction of pathological reclassification at 1 year during active surveillance for low-risk prostate cancer: the Japanese multicenter study cohort. J Cancer Res Clin Oncol 2014;140:257–63 | Patients with low-risk cancer |
| Reference | Reason for exclusion |
|---|---|
| Filella X, Gimenez N. Evaluation of [–2] proPSA and prostate health index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med 2013;51:729–39 | Initial biopsies only |
| Wang W, Wang M, Wang L, Adams TS, Tian Y, Xu J. Diagnostic ability of percent p2PSA and prostate health index for aggressive prostate cancer: a meta-analysis. Sci Rep 2014;4:5012 | Mixed or unclear biopsy population |
| Luo Y, Gou X, Huang P, Mou C. Prostate cancer antigen 3 test for prostate biopsy decision: a systematic review and meta analysis. Chin Med J 2014;127:1768–74 | Mixed or unclear biopsy population |
| Bruzzese D, Mazzarella C, Ferro M, Perdona S, Chiodini P, Perruolo G, et al. Prostate health index vs. percent free prostate-specific antigen for prostate cancer detection in men with ‘gray’ prostate-specific antigen levels at first biopsy: systematic review and meta-analysis. Transl Res 2014;164:444–51 | Initial biopsies only |
| Harvey P, Basuita A, Endersby D, Curtis B, Lacovidou A, Walker M. A systematic review of the diagnostic accuracy of prostate specific antigen. BMC Urol 2009;9:14 | Unclear initial or repeat biopsy |
| Lawrentschuk N, Fleshner N. The role of magnetic resonance imaging in targeting prostate cancer in patients with previous negative biopsies and elevated prostate-specific antigen levels (Structured abstract). BJU Int 2009;103:730–3 | No meta-analysis, no reference standard |
| Overduin CG, Futterer JJ, Barentsz JO. MRI-guided biopsy for prostate cancer detection: a systematic review of current clinical results. Curr Urol Rep 2013;14:209–13 | No meta-analysis, no reference standard |
| de Rooij M, Hamoen EH, Futterer JJ, Barentsz JO, Rovers MM. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. Am J Roentgenol 2014;202:343–51 | Unclear initial or repeat biopsy |
| Nelson AW, Harvey RC, Parker RA, Kastner C, Doble A, Gnanapragasam VJ. Repeat prostate biopsy strategies after initial negative biopsy: meta-regression comparing cancer detection of transperineal, transrectal saturation and MRI guided biopsy. PLOS ONE 2013;8:e57480 | No meta-analysis, no reference standard |
Appendix 6 Within-study comparisons reporting univariate prostate cancer antigen 3 or Prostate Health Index scores only
| Study name | Inclusion/exclusion criteria | Repeat biopsies (type, number of positive/total sample (%) | Comparisons reported | Author conclusions |
|---|---|---|---|---|
| Marks 200793 | Consecutive men with serum PSA levels of 2.5 ng/ml or greater who had a history of at least one negative biopsy, documented by the study site investigator and who had been scheduled for a follow-up biopsy | 12 cores peripheral; 60/226 (22.6%) | Univariate only. PCA3 continuously and PSA continuously | In men undergoing repeat prostate biopsy to rule out cancer, the urinary PCA3 score was superior to serum PSA determination for predicting the biopsy outcome. The high specificity and informative rate suggest that the PCA3 assay could have an important role in prostate cancer diagnosis |
| Auprich 201287 | Previously biopsy with 8 or 10 cores, aged ≤ 70 years, a suspicious DRE and/or persistently raised age-specific tPSA thresholds (2.5–6.5 ng/ml) and/or suspicious prior histology (ASAPs ≥ 2 cores affected by HGPIN), but no patient with a tPSA levels of > 50 ng/ml | 12 or 24 TRUS; specific sampling of anterior/transition zone; 44/127 (34.6%). Note that the first/second/third repeat biopsy reported separately | Univariate only. PCA3, PSA, PSA velocity and %fPSA | The findings of the present study promote the concept that the number of previous repeat biopsy sessions strongly influences the performance characteristics of biopsy risk factors. tPSA was no significant risk factor in the entire analysis. By contrast, %fPSA performed best at second and third and higher repeat biopsies. PSAV’s diagnostic potential was reserved to patients at second and third and higher repeat biopsies. Finally, PCA3 demonstrated the highest diagnostic accuracy and potential to reduce unnecessary biopsies at first repeat biopsy. However, this advantage dissipated at second and third and higher repeat biopsies |
| Ramos 2013100 | Indication of transrectal prostate biopsy, either for elevated PSA and/or a suspicious DRE | ≥ 12 core TRUS, at least two cores per sextant; 9/15 (60%) | Univariate analysis reported a PCA3 score of > 35 and a PSA level of > 4 | This is the first report in Latin America on the use of PCA3 in diagnosing PCa. Our results are comparable to those reported in other populations in the literature, demonstrating the reproducibility of the test. PCA3 score was highly specific and we specially recommend its use in patients with persistent elevated PSA and prior negative biopsies |
| Stephan 2013107 | Men scheduled for prostate biopsy owing to a suspicious DRE, suspicious transrectal ultrasonography findings, or increased PSA concentration or PSA velocity. Study exclusion criteria included urinary infections, medications (androgen or 5-α-reductase inhibitors) or interventions that could alter PSA concentrations | 10–22 cores; 40/110 (36.7%) | Univariate analysis of PCA3, phi and PSA %fPSA. Multivariate model with PCA3, phi and T2:ERG | PCA3 and phi were superior to the other evaluated parameters but their combination gave only moderate enhancements in diagnostic accuracy for PCa at first or repeat prostate biopsy |
Appendix 7 Full results of quality assessment exercise
The outputs in this appendix are from RevMan: Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
Appendix 8 Graphs of decision curve analysis results
FIGURE 13.
Decision curve analysis graph from Busetto et al. 90 The dark green line indicates the base clinical model (age, digital rectal examination, and prostate-specific antigen); the light blue line indicates the base clinical model plus prostate cancer gene 3 (PCA3) assay; the dark blue line indicates the base clinical model plus multiparametric magnetic resonance imaging (mMRI); and the light green line indicates the base clinical model plus PCA3 and mMRI. [Reprinted from Urology, vol. 82, Busetto GM, De Berardinis E, Sciarra A, Panebianco V, Giovannone R, Rosato S, D’Errigo P, Di Silverio F, Gentile V, Salciccia S, Prostate cancer gene 3 and multiparametric magnetic resonance can reduce unnecessary biopsies: decision curve analysis to evaluate predictive models, pp. 1355–1362, © 2013, with permission from Elsevier.]
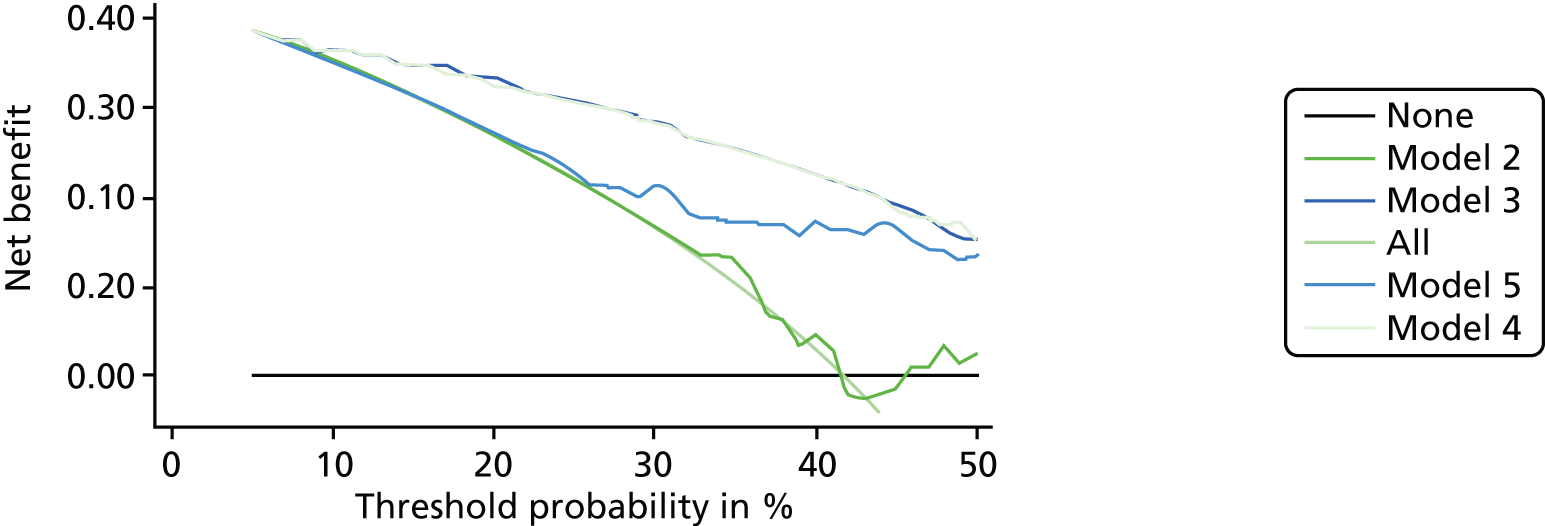
FIGURE 14.
Decision curve analysis graph from Porpiglia et al. 99 DCAs of effect of various models on PCa detection. Threshold probability to undergo biopsy is reported vs. net benefit. Broken black line represents assumption that all patients will harbour PCa (biopsy all patients). Horizontal line represents assumption that no patients will harbour PCa (biopsy no patients). [Reprinted from The Journal of Urology, vol. 192, Porpiglia F, Russo F, Manfredi M, Mele F, Fiori C, Bollito E, Papotti M, Molineris I, Passera R, Regge D, The roles of multiparametric magnetic resonance imaging, PCA3 and prostate health index – which is the best predictor of prostate cancer after a negative biopsy?, pp. 60–6, © 2014, with permission from Elsevier.] DCA, decision curve analysis.

FIGURE 15.
Decision curve analysis graph from Perdonà et al. 97 [Reprinted from European Urology, vol. 59, Perdonà S, Cavadas V, Di Lorenzo G, Damiano R, Chiappetta G, Del Prete P, Franco R, Azzarito G, Scala S, Arra C, De Sio M, Autorino R, Prostate cancer detection in the ‘grey area’ of prostate-specific antigen below 10 ng/ml: head-to-head comparison of the updated PCPT calculator and Chun’s nomogram, two risk estimators incorporating prostate cancer antigen 3, pp. 81–7, © 2011, with permission from Elsevier.]
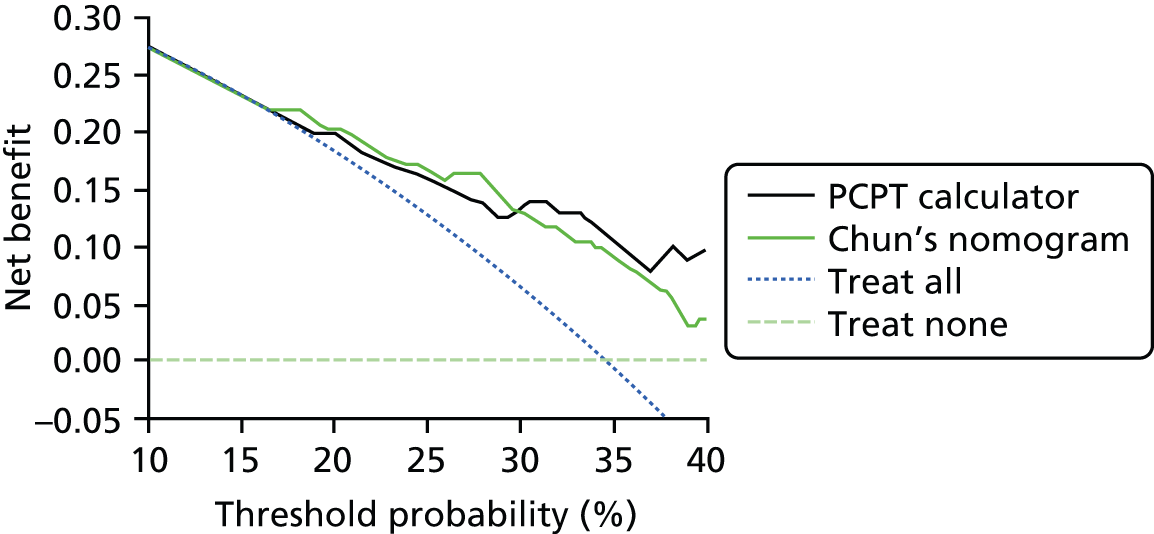
FIGURE 16.
Decision curve analysis graph from Scattoni et al. 102 DCA of PCa diagnosis at biopsy shows net benefit of BMM 1 (black curve) consisting of age, PSA, per cent fPSA, DRE and prostate volume, and net benefit of adding PCA3 model 2 (dark green curve), PHI model 3 (light green curve) or PCA3 plus PHI model 4 (light blue curve) to BMM. Dark blue curve represents assumption that all patients will harbour PCa and be treated. Horizontal green curve represents assumption that no patient will harbour PCa and none will be treated. A, initial biopsy. B, repeat biopsy. [Reprinted from The Journal of Urology, vol. 190, Scattoni V, Lazzeri M, Lughezzani G, De Luca S, Passera R, Bollito E, Randone D, Abdollah F, Capitanio U, Larcher A, Lista G, Maria Gadda G, Bini V, Montorsi F, et al. , Head-to-head comparison of prostate health index and urinary PCA3 for predicting cancer at initial or repeat biopsy, pp. 496–501, © 2013, with permission from Elsevier.] BBM, base multivariate model; fPSA, free PSA.
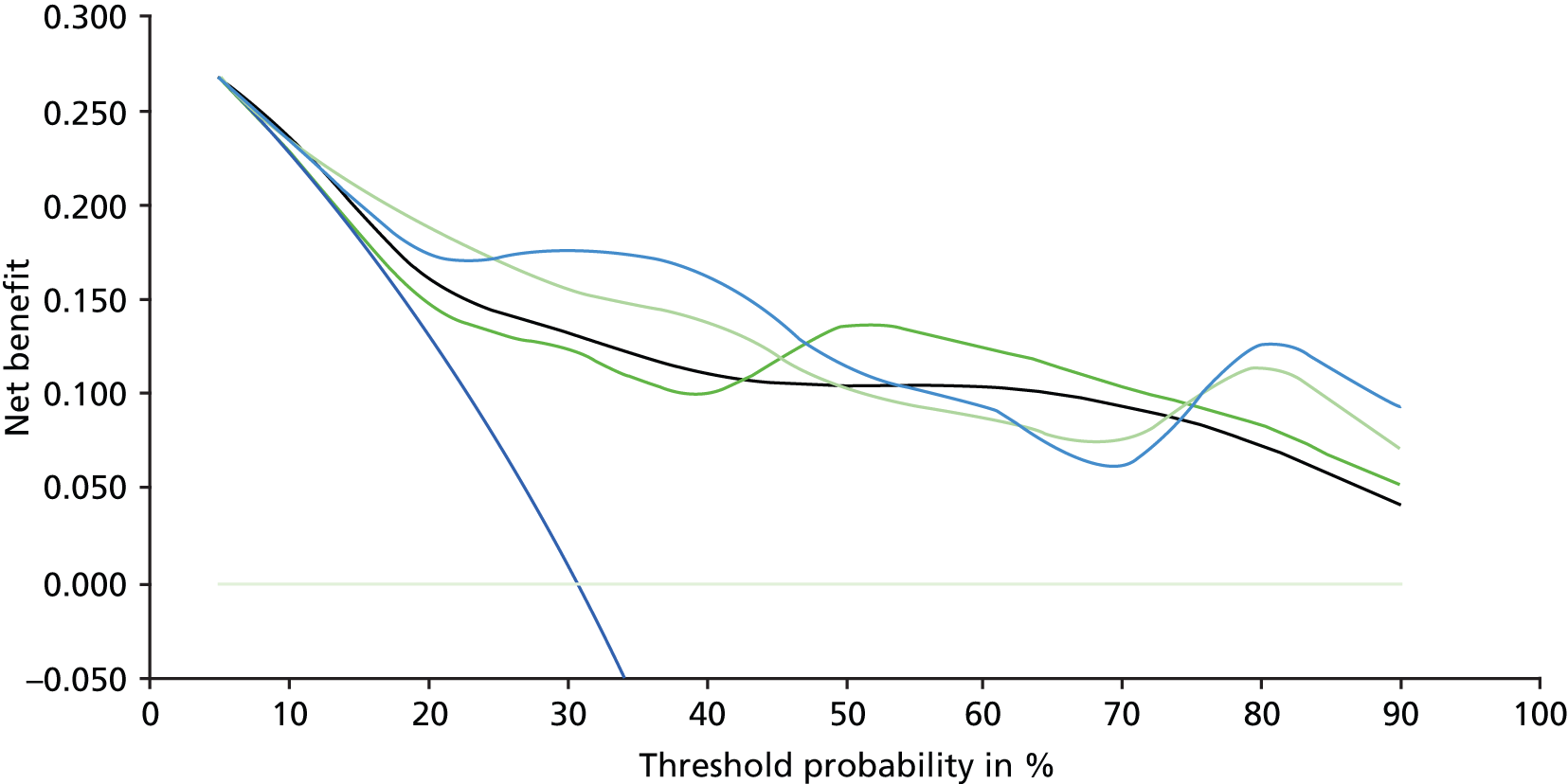
FIGURE 17.
Decision curve analysis graph from Lazzeri et al. 92 DCA for models shown in Table 3. Models 3 (base plus %fPSA and p2PSA) 4 (base plus %fPSA and %p2PSA) and 5 (base plus %fPSA and phi) resulted in greater net benefit in PCa probability threshold range from 25% to 40% compared with model 1 (base) and model 2 (base plus %fPSA), which are perfectly superimposed. (Reprinted from The Journal of Urology, vol. 188, Lazzeri M, Briganti A, Scattoni V, Lughezzani G, Larcher A, Maria Gadda G, Lista G, Cestari A, Buffi N, Bini V, Freschi M, Rigatti P, Montorsi F, Guazzoni G, Serum Index Test %[-2]proPSA and Prostate Health Index are more accurate than prostate specific antigen and %fPSA in predicting a positive repeat prostate biopsy, pp. 1137–43, © 2012, with permission from Elsevier.) DCA, decision curve analysis.
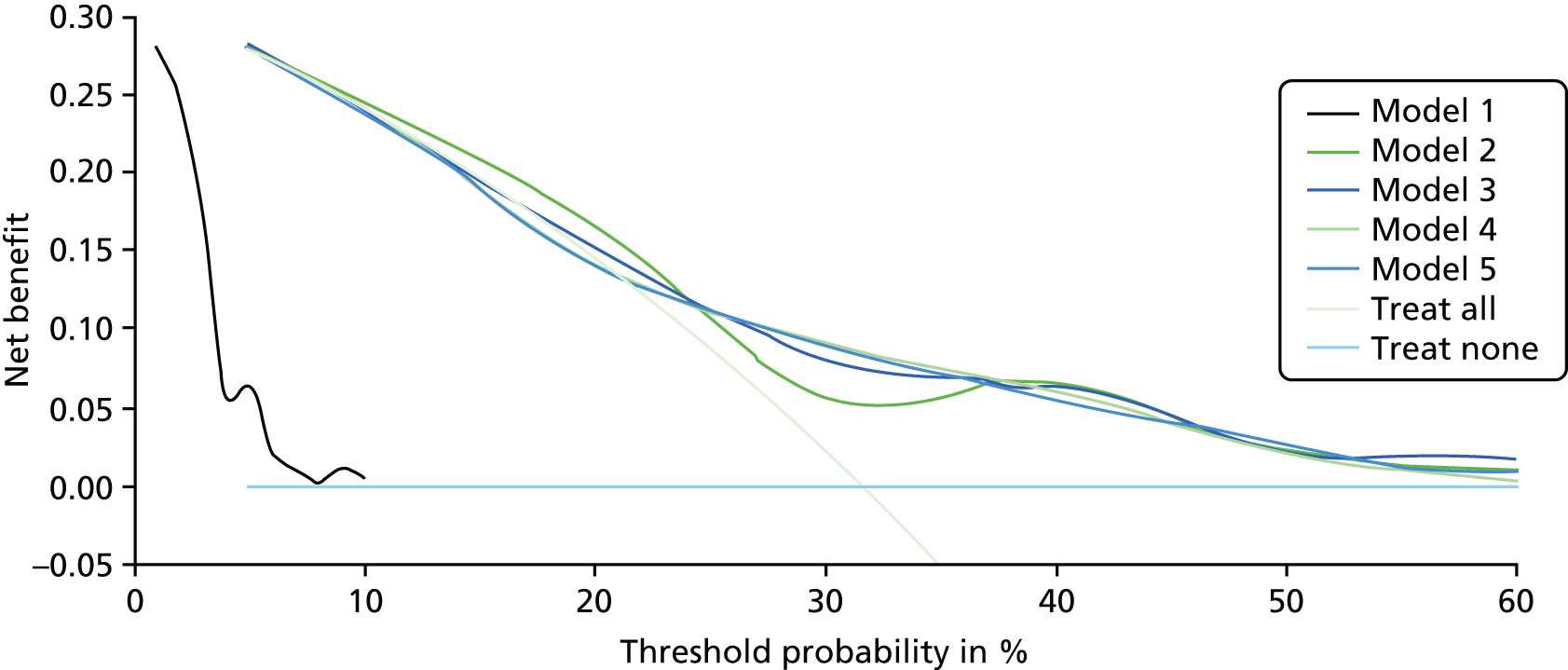
FIGURE 18.
Decision curve analysis graph from Wu et al. 106 The ‘all’ line shows the net benefit if all patients were taken for repeat prostate biopsy. The ‘none’ line shows the net benefit if no patients were taken for repeat prostate biopsy. As the threshold probability increases, the net benefit for each nomogram decreases. DRE, digital rectal examination; PCA3, prostate cancer antigen 3; PSAD, PSA density; TRUS, transrectal ultrasound. (Adapted by permission from Macmillan Publishers Ltd: Prostate Cancer and Prostatic Diseases Wu AK, Reese AC, Cooperberg MR, Sadetsky N, Shinohara K. Utility of PCA3 in patients undergoing repeat biopsy for prostate cancer. Prostate Cancer Prostatic Dis 2012;15:100–5, © 2012.)
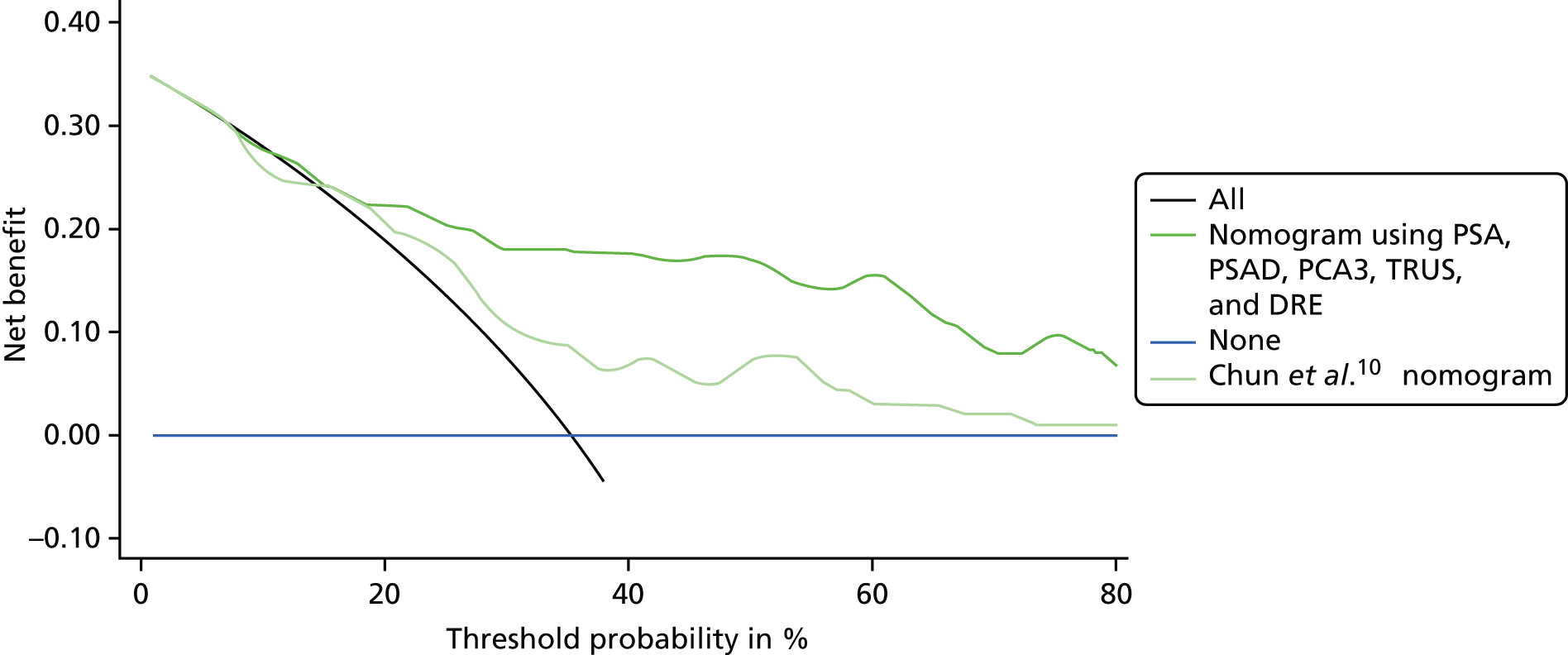
Glossary
- Accuracy
- A measure of the closeness of the experimental value to the actual amount of the substance in the matrix.
- Active surveillance
- A form of monitoring patients with slow-growing prostate cancer. It differs from watchful waiting in that if the patient needs treatment the aim of the treatment will be curative, it is suitable for some men with cancer that is contained in the prostate (i.e. localised) and it usually involves more regular hospital tests such as biopsies and magnetic resonance imaging.
- Analytical sensitivity
- A measure that represents the smallest amount of substance in a sample that can accurately be measured by an assay.
- Analytical specificity
- The ability of an assay to measure a particular substance, rather than others, in a sample.
- Area under the curve
- A measure of the diagnostic accuracy of a technology. The measure is based on the geometric inspection of a receiver operating characteristics curve. A receiver operating characteristics curve is a plot of the true-positive rate against the false-positive rate at different threshold settings. A technology with perfect diagnostic accuracy will have an area under the curve of 1, a technology which is no better than chance will have an area under the curve of 0.5 and a technology which miscategorises on every occasion will have an area under the curve of zero.
- Atypical small acinar proliferation
- A collection of small prostatic glands, identified on prostate biopsy, whose significance is uncertain and cannot be determined to be benign or malignant.
- Benign prostatic hyperplasia
- A common urological condition caused by the non-cancerous enlargement of the prostate gland in ageing men. Urinating symptoms can occur as the prostate enlarges.
- Clinically significant/insignificant prostate cancer
- Prostate cancer that is unlikely to result in death. A cancer is said to be clinically significant if it is likely to be the cause of death.
- Clinical utility
- A measure (preferably in a quantitative form) of the extent to which diagnostic testing improves health outcomes relative to the current best alternative, which could be some other form of testing or no testing at all.
- Clinical validity
- The predictive value of a test for a given clinical outcome, for example the likelihood that cancer will develop in someone with a positive test.
- Core
- Sample of material taken from the prostate during a biopsy.
- Cost-effectiveness acceptability curve
- A curve that shows, for a range of maximum amounts of money, how much a decision-maker might be willing to pay for a particular unit change in outcome and the probability that (given the available data) one intervention is cost-effective compared with the alternative(s).
- Cut-off
- See Threshold (clinical) and Threshold (economics).
- Decision curve analysis
- A graphical analysis showing the net benefit of various diagnostic models which take account of the benefit of diagnosed cases and harms of unnecessary biopsies.
- Derived sensitivity
- Sensitivity estimates derived from a receiver operating characteristics curve rather than from a 2 × 2 table.
- Derived specificity
- Specificity estimates derived from a receiver operating characteristics curve rather than from a 2 × 2 table.
- Diagnostic accuracy
- The effectiveness of a diagnostic test to correctly categorise patients as either ‘positive’ or ‘negative’ for the presence of a disease. There are several ways this can be expressed, for example the area under the curve or as sensitivity and specificity.
- Diagnostic odds ratio
- The ratio of the odds of a positive intervention test in those with the disease to the odds of a positive intervention test in those without the disease.
- Direct head-to-head study
- A study in which participants receive both intervention and comparator tests, and the tests are therefore evaluated in the same population (also called a within-study comparison).
- Discounting
- A method used to adjust the value of costs and outcomes which occur in different time periods into a common time period, usually the present.
- End-to-end study
- A study following participants from early clinical investigation and the decision to have a repeat biopsy through to diagnosis, treatment and long-term follow-up for prostate cancer (same as Test-to-treatment study).
- External Assessment Group
- An independent group of researchers commissioned to review the evidence on a group of diagnostic technologies. The Diagnostics Assessment Committee bases its discussions on the diagnostic assessment report produced by the External Assessment Group.
- False negative
- In the case of prostate cancer, a negative intervention test in a man in who is found on biopsy to have prostate cancer.
- False positive
- In the case of prostate cancer, a positive intervention test in man who is found on biopsy not to have prostate cancer.
- Forest plot
- A graphical display designed to illustrate the relative strength of treatment effects in multiple quantitative scientific studies addressing the same question.
- Gleason score
- A scoring system used to help evaluate the prognosis of men with prostate cancer. A score is given based on the cancer’s microscopic appearance. Gleason scores range from 2 to 10; the higher the Gleason score, the more aggressive the cancer.
- Healthcare Resource Group
- A grouping that consists of patient events that have been judged to consume a similar level of resource.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Indirect (between-study) comparison
- An analysis comparing the performance of intervention and comparator tests using data from studies in which tests are evaluated in different study populations (also called a between-study comparison).
- Intervention test
- The diagnostic test which is being evaluated.
- Likelihood ratio
- A description of how much more likely it is that a person with a disease than one without that disease will have a particular test result.
- Logistic regression models
- A statistical method for analysing a data set in which there are one or more independent variables that determine an outcome. The outcome is measured with a dichotomous variable (i.e. one with only two possible outcomes).
- Negative predictive value
- The proportion of patients with negative test results who do not have the disease. The probability that a patient who is test negative on an intervention does not have prostate cancer detected on biopsy.
- Nomogram
- Risk algorithms that combine multiple clinical and laboratory risk factors to create a cumulative risk score. Most nomograms aim to predict the probable course of a disease; however, some nomograms aim to predict the result of a biopsy in men suspected of having prostate cancer.
- Positive predictive value
- The proportion of patients with positive test results who actually have the disease. The probability that a patient who tests positive on an intervention test has prostate cancer detected at biopsy.
- Precision
- The extent to which individual measurements of a sample are close to each other.
- Probabilistic sensitivity analysis
- A way to quantify the level of confidence that a decision-maker has in the conclusions of an economic evaluation.
- Prostate biopsy
- A procedure in which small, hollow needle core samples are removed from a man’s prostate gland to be examined microscopically for the presence of cancer.
- Prostate-specific antigen
- An enzyme secreted by the epithelial cells of the prostate gland. It is present in small quantities in the serum of men with healthy prostates, but the level of prostate-specific antigen is often elevated in the presence of prostate cancer or other prostate disorders.
- Quality-adjusted life-years
- An index of survival that is adjusted to account for the patient’s quality of life which incorporates changes in both quantity (longevity/mortality) and quality (morbidity, psychological, functional, social and other factors) of life. They are used to measure benefits in cost–utility analysis. The number of quality-adjusted life-years gained is the mean number of quality-adjusted life-years associated with one intervention minus the mean number of quality-adjusted life-years associated with an alternative intervention.
- Quality of life
- A concept incorporating all the factors that might impact on an individual’s life, including factors such as the absence of disease or infirmity as well as other factors which might affect physical, mental and social well-being.
- Radical prostatectomy
- The surgical removal of all of the prostate gland.
- Receiver operating characteristics curve
- A plot of the true-positive rate against the false-positive rate of a test at different threshold settings.
- Reference standard
- A diagnostic test used to estimate the sensitivity and specificity of another diagnostic test, known as an index test. The reference standard is assumed to have perfect sensitivity and specificity; thus, when both tests categorise something differently, the reference standard test categorisation is assumed to be correct (either true negative or true positive).
- Saturation biopsy
- A type of biopsy that may be carried out transrectally or transperineally. A minimum of 20 cores are taken. This procedure may be carried out under general anaesthetic, particularly if a man has found the experience of a previous biopsy to be uncomfortable and/or distressing.
- Sensitivity
- The proportion of those who actually have the disease and who are correctly identified with positive test results, that is the proportion of men with prostate cancer at biopsy who are identified by the intervention test (also called the True-positive rate).
- Sensitivity analysis
- In health economics, the study of how the uncertainty in the magnitude of the output from the cost-effectiveness model (the incremental cost-effectiveness ratio per quality-adjusted life-year gained) can be apportioned to different sources of uncertainty in model inputs.
- Specificity
- The proportion of those who do not have the disease who are correctly identified as having a negative test result, that is the proportion of men without prostate cancer at biopsy who are test negative on the intervention test (also called the True-negative rate).
- Template biopsy
- A type of biopsy that involves taking 25–40 cores transperineally. A template or grid is used.
- Test-to-treatment study
- See End-to-end study.
- Threshold (clinical)
- A value, within a range of values, used to categorise observations into one of two mutually exclusive groups. For example, guidelines suggest that the decision whether or not to investigate for possible prostate cancer is influenced by prostate-specific antigen level, with a threshold of above 3 ng/ml used for men in their fifties, 4 ng/ml for men in their sixties and 5 ng/ml for men in their seventies.
- Threshold (economics)
- The amount of variation needed in the parameter values of a model to achieve a specified outcome. In the context of cost-effectiveness analysis in the UK NHS, this specified outcome is usually the cost-effectiveness threshold of £20,000–30,000 per additional quality-adjusted life-year gained.
- True negative
- In the case of prostate cancer, a negative intervention test in a man who does not, in fact, have prostate cancer.
- True positive
- In the case of prostate cancer, a positive intervention test in a man who does, in fact, have prostate cancer.
- Utility
- A measure of the strength of an individual’s preference for a specific health state in relation to alternative health states. The utility scale assigns numerical values on a scale from 0 (death) to 1 (optimal or ‘perfect’ health). Health states can be considered worse than death and thus have a negative value.
- Watchful waiting
- A form of cancer monitoring. It differs from active surveillance in that, if treatment is needed, its aim will be to control rather than cure the cancer. It is generally suitable for men with concomitant health problems who may be less able to cope with treatment or whose cancer may never cause a problem during their lifetime; it usually involves fewer tests and these usually take place at the general practitioner’s surgery rather than at the hospital.
List of abbreviations
- ASAP
- atypical small acinar proliferation
- AUC
- area under the curve
- CE
- Conformité Européenne
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CV
- coefficient of variation
- DCE-MRI
- dynamic contrast-enhanced magnetic resonance imaging
- DRE
- digital rectal examination
- DW
- diffusion weighted
- DW-MRI
- diffusion-weighted magnetic resonance imaging
- EAG
- External Assessment Group
- FDA
- Food and Drug Administration
- FN
- false negative
- FP
- false positive
- fPSA
- free prostate-specific antigen
- GP
- general practitioner
- HGPIN
- high-grade prostatic intraepithelial neoplasia
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- LoB
- limit of blank
- LoD
- limit of detection
- LoQ
- limit of quantitation
- mpMRI
- multiparametric magnetic resonance imaging
- MRI
- magnetic resonance imaging
- mRNA
- messenger ribonucleic acid
- MRS
- magnetic resonance spectroscopy
- MRSI
- magnetic resonance spectroscopy imaging
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- p2PSA
- [–2]pro-prostate-specific antigen
- PCA3
- PROSTATE cancer antigen 3
- PCPT
- Prostate Cancer Prevention Trial
- phi
- Prostate Health Index
- PSA
- prostate-specific antigen
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REDUCE
- Reduction by Dutasteride of Prostate Cancer Events trial
- RNA
- ribonucleic acid
- ROC
- receiver operating characteristics
- SD
- standard deviation
- SSED
- Summary of Safety and Effectiveness Data
- T2-MRI
- T2-weighted magnetic resonance imaging
- TN
- true negative
- TP
- true positive
- tPSA
- total prostate-specific antigen
- TRUS
- transrectal ultrasonography
- WHO
- World Health Organization
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed commercial-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence data removed and replaced by the statement ‘commercial-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.