Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 13/05/01. The protocol was agreed in October 2013. The assessment report began editorial review in June 2014 and was accepted for publication in May 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
David Lawrie was the principal investigator for Aberdeen for the multicentre Auxilium Study AUX-CC-867 trial (May 2013), which is now completed.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Brazzelli et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Introduction
Dupuytren’s disease is a benign, slowly progressive, fibroproliferative condition that affects the palmar and digital fascia in the hand (the ‘bands’ that anchor the skin of the palm). 1 The disease is common, costly and can impact on quality of life. 2–4 It is characterised by thickening of the palmar skin and by the formation of nodules, firm painless masses fixed to the skin and the deeper fascia. Nodules usually precede the development of fibrotic cords. As the disease progresses, cords gradually contract, leading to progressive flexion deformities in the fingers, particularly of the metacarpophalangeal (MCP) and/or proximal interphalangeal (PIP) joints. 5,6 Joint contractures and deformities are usually painless7 but are associated with considerable functional impairment which, in turn, impacts on activities of daily living and ability to work. 4
There is currently no cure for Dupuytren’s disease and the goal of treatment is to restore hand function. 7 Management of the disease is dependent on disease progression and degree of deformity, and not all people with Dupuytren’s disease seek or require treatment. 1,8 Surgery remains the treatment of choice for severe contractures and some cases of moderate symptoms. 1,8,9 Surgery, however, cannot be considered a definitive cure for Dupuytren’s disease, as contracture may recur in operated digits or in previously uninvolved areas of the hand. Moreover, perioperative and/or postoperative complications are relatively common after surgical treatments. 2,3,10 Recently, collagenase clostridium histolyticum (CCH) (Xiapex®, Pfizer Ltd) has been proposed as a non-operative, clinically viable alternative to surgery in some patients and has been licensed for the treatment of Dupuytren’s contracture (DC) in adults. 11,12
The aim of this appraisal is to assess the current evidence on the clinical effectiveness and cost-effectiveness of CCH as an alternative to surgery for treatment of adults presenting with DC with a palpable cord.
Aetiology, pathophysiology and clinical presentation
Although the aetiology of Dupuytren’s disease remains unknown,2 a clear genetic component13,14 involving autosomal dominant transmission with variable penetrance, has been established. 1,9,15
A number of risk factors have been reported to be associated with Dupuytren’s disease, such as alcohol consumption, smoking, diabetes, epilepsy, thyroid disorders and trauma, but none has been found to be definitively responsible for the condition. 16,17 A recent large cohort study conducted in France reported a significant dose–effect relationship between Dupuytren’s disease and age, diabetes, high alcohol consumption and long-term exposure to work-related vibration tools. 18 Smoking has also been associated with an increased risk of the disease, and the combination of smoking and high alcohol intake has been reported to increase the risk further. 19
The earliest signs of Dupuytren’s disease are changes in the skin, including loss of normal architecture and development of skin pits, caused by small vertical fibres (known as Grapow fibres) that connect the dermis to the palmar fascia. 1 The intermediate phase of the disease is characterised by an abnormal deposition of collagen. Initially, the collagen forms nodules (i.e. firm, painless masses fixed to the skin and deeper fascia) but it may thicken and lead to the formation of cords, which also adhere to the skin of the palm. 9,20 A cord may develop without prior formation of a nodule,9 although this remains contentious. 3 Further progression is marked by gradual contraction of cords, causing finger flexion deformities. 1,9 The MCP and PIP joints of the ring finger and the little finger are most commonly affected, but contractures may also occur in other fingers and the thumb. Nodules and cords are the defining features of DC (Figure 1). 9,21
FIGURE 1.
Hand affected by DC. Reproduced with permission from Donald Sammut, 2013 (www.donaldsammut.com).

When the cords contract, the condition is usually referred to as DC. Contractures of the MCP joints are caused by the pretendinous cord, which develops from the pretendinous band. Proximal interphalangeal joint contractures arise from the central, spiral or lateral cords. The lateral cord can also result in contracture of the distal interphalangeal joint. 9
The terms ‘Dupuytren’s disease’ and ‘Dupuytren’s contracture’ appear to be used interchangeably in the current literature even though some authors have stressed the importance of correct definitions. 16 In general, epidemiology studies tend to concentrate on Dupuytren’s disease, whereas experimental studies focus on DC.
Epidemiology and prognosis
Dupuytren’s disease occurs in all races, but is known to be more prevalent in Northern European Caucasians. The epidemiology of the disease has been extensively described and studies have been conducted in numerous countries around the world. Prevalence rates of Dupuytren’s disease vary widely according to age, population groups, geographical location and methods of data collection, with rates ranging from 0.2% to 56%. 22 In Europe, reports of prevalence range typically from 4% in the male population in England23 to 30% in the Norwegian population aged over 60 years,24 with the highest prevalence rate (56%) reported by a study conducted on a group of chronic epileptic patients in the UK. 25 DC has also been reported to be particularly prevalent in the north-east of Scotland. 25 Overall, approximately 2 million people in the UK are believed to suffer from Dupuytren’s disease. 1
The disease is more common in men than in women, with a ratio of approximately 6 : 1. 22 Men tend to present for treatment at a younger age (in their fifth decade) than women (in their sixth decade). Prevalence and incidence of the disease tend to increase with age and both sexes are affected in equal proportions after the age of 80 years. 26,27
The ring finger is most commonly affected. 28 Rates of bilateral disease vary between 17%29 and 59%28 with symmetrical involvement of fingers in many cases. 28 In unilateral disease, involvement of the right hand has been reported to be twice as likely as involvement of the left hand in a Norwegian population. 28 However, more recent studies in the UK and Germany have reported almost equal involvement of both hands. 30
Impact of health problem
Dupuytren’s disease impacts on patients’ quality of life and results in both psychosocial and physical consequences. 22 Increased finger flexion deformities have been associated with decreased hand function. 31 Impaired hand function can limit normal activities at home (e.g. washing and dressing), in the workplace (e.g. manual labour) and in recreational and social interactions (e.g. sports, shaking hands). 32,33
Although surgery is considered the mainstay of treatment for DCs, it does not guarantee success, and complications and recurrences are relatively common after surgery. 10 Surgery-related complications tend to increase according to the severity of the initial contracture and may be related to technical aspects of the surgical procedures (e.g. neurovascular injury, haematoma, infection) and to physiological characteristics of patients (e.g. stiffness, reflex symptomatic dystrophy). 9,29 A 20-year literature review reported complication rates for fasciectomy ranging from 4% to 39%, with a major complication rate of 16%. Pain and wound healing complications were more common after fasciectomy in people treated for primary disease, whereas in those with recurrent disease, necrosis and sensory abnormalities were more often reported. In studies directly comparing primary and recurrent disease, both digital nerve injuries and digital artery injuries were more common in patients with recurrent disease, but numbers were too small for drawing reliable conclusions. 10 A review of the literature that focused on the effects of fasciectomy and fasciotomy in European patients treated for Dupuytren’s disease showed that complications were experienced by approximately one-quarter of people undergoing fasciectomy and by one-fifth of those receiving fasciotomy. Overall, about 20% of patients who underwent fasciectomy or fasciotomy experienced a complication, including neurapraxia, nerve or arterial injury, infection, haematoma, pain and skin necrosis. 34
Recurrence of contracture following surgery is common and rates increase with severity of the initial contracture and with length of follow-up, regardless of the success of the surgery. 11,29 A systematic review of outcomes of surgery for primary disease reported recurrence rates of 0% to 71%, with follow-up ranging from 3 weeks to 13 years. A further review assessing the efficacy and safety of fasciectomy and fasciotomy in European patients with primary and recurrent disease showed an average recurrence rate of 39% after fasciectomy and 62% after fasciotomy at a median follow-up time of about 4 years. 34 In addition, a systematic review of surgical outcomes for DC found that recurrence rates for open partial fasciectomy (PF) ranged from 12% to 39% between 1.5 and 7 years and those for percutaneous needle fasciotomy (PNF) ranged from 50% to 58% between 3 and 5 years. 34 In the current literature, there is clear heterogeneity in recurrence rates reported after surgery.
Definitions of recurrence are not consistent across studies and some investigators do not define recurrence at all. It is, therefore, rather challenging to compare recurrence rates across studies with any degree of confidence. 35,36
Current service provision
Management of the disease
In the UK, the majority (76%) of DC diagnoses are made by the general practitioner (GP). Most patients (82%) are then referred by the GP, with around half (49%) of referrals being to a hand specialist. Post-operative management is carried out mainly by a physiotherapist (in 59% of cases) or the treating surgeon (in 25% of cases). 3
The British Society for Surgery of the Hand (BSSH) clinical classification of Dupuytren’s disease is displayed in Table 1.
| Classification of Dupuytren’s disease | Features |
|---|---|
| Mild | No functional problems No contracture Mild MCP joint contracture only (< 30°) |
| Moderate | Notable functional problems or moderate MCP joint contracture (30–60°) Moderate PIP joint contracture (< 30°) First web contracture |
| Severe | Severe contracture of both MCP joint (> 60°) and PIP joint (> 30°) |
Various algorithms for managing DC have been proposed in the literature. 1,2,8
Percutaneous needle fasciotomy is recommended, if appropriately trained, for the treatment of moderate MCP joint contracture. Although the marketing authorisation for CCH is ‘for the treatment of DC in adult patients with a palpable cord’, the BSSH tentatively recommends collagenase injections for adults with moderate contractures. 37 It is worth mentioning that this guidance was issued before collagenase was licensed for use in the UK. The BSSH has also recently withdrawn all previously published evidence-based guidelines with the intent to review them. No new updated information is currently available from the BSSH.
There is no established cure for Dupuytren’s disease and the therapeutic goal is to restore hand function. 6,38 Surgery has been the treatment of choice for severe contractures and for some people with moderate contractures. 1,2,9,32 There are no definitive indications for treatment but contraction of the MCP joint of > 30° is a commonly accepted indication. 9 Indications for surgery of PIP joints vary from contractures of 15°9 to any degree of contracture. 39 Either way, earlier intervention for PIP joints is common to guidance across the board. 3 In addition, the Hueston table top test40 is a simple assessment of the need for surgical intervention. Inability to place the hand flat on a table top indicates a positive test.
Various non-surgical interventions have been proposed for Dupuytren’s disease, including local vitamin E cream, local steroid injections, splinting and ultrasonic therapy, but these have been mostly abandoned because they are ineffective or inferior to surgery. 11,41 There is some evidence that radiation therapy may be effective in slowing down the progression of the disease without serious safety concerns. However, the use of radiation therapy in clinical practice in the UK is very limited and requires special arrangements for clinical governance, consent and audit or research. 42
Variation in services and/or uncertainty about best practice
There are currently no definitive guidelines regarding the type of surgery used to treat DC. Fasciectomy remains the preferred choice for the treatment of DC. However, none of the available surgical procedures has proved completely effective and complications and recurrences are common. The choice of surgery is influenced by a number of factors, including the age of patient, patient preference, comorbidities, severity of disease, as well as surgeon preference and expertise. 6,43 In 2008, a large survey was conducted among a sample of 687 orthopaedic/plastic surgeons across 12 European countries (including the UK) to estimate the geographical variation in the number of interventions for Dupuytren’s disease performed during the previous 12 months. Ninety five per cent of surgeons used fasciectomy, 70% used fasciotomy, 38% used dermofasciectomy and 35% used PNF. 43,44 In Europe, between 4% and 12% of patients with DC are treated with PNF, depending on the country.
Relevant national guidelines
Currently, there are two sets of National Institute for Health and Care Excellence (NICE) guidelines for the treatment of Dupuytren’s disease/contracture. The interventional procedure guidance 43 ‘Needle fasciotomy for Dupuytren’s contracture’ was published in February 2004. 45 The guideline states that needle fasciotomy, a procedure that can be repeated, offers a short-term reduction of contracture, with a recurrence rate of about 50% at 5 years. The guidance further indicates that people with less severe disease and/or MCP contracture are likely to benefit most from the procedure, although it is not clear whether such additional benefit is in terms of reduction of contracture or time to recurrence. Needle fasciotomy is reported to be less efficacious in the longer term than open surgery but is associated with lower morbidity and faster recovery times than open procedures. Complications of the procedure, including skin splitting, pain and nerve injury are acknowledged in the guideline, but with a rate of ≤ 1%. NICE conclude that needle fasciotomy is recommended especially for older people for whom major surgery is not an option.
The IPG368 NICE guideline was published in November 2010 and focuses on the use of radiation for early Dupuytren’s disease. 42 The guideline states that radiation offers correction of contraction, restoration of hand function and avoidance of future surgery but with a theoretical risk of radiation-induced cancer in the long term. The guideline concludes that the current efficacy and safety data for radiation therapy for early Dupuytren’s disease are limited and recommends the use of the procedure only with special arrangements for clinical governance, consent, and audit or research.
The BSSH guidelines classify Dupuytren’s disease as mild, moderate or severe. The guidelines recommend observation for mild disease, together with reassurance and a follow-up assessment between 6 months and 12 months. 37 Limited fasciectomy (LF) and PNF for MCP joints contracture (when the operating clinician has received appropriate training) are recommended for moderate disease, whereas fasciectomy (LF or dermofasciectomy) is recommended for severe disease. Collagenase injections are recommended only tentatively for moderate and severe disease owing to the lack of long-term safety data at the time of the recommendations.
Description of technologies under assessment
Collagenase clostridium histolyticum
Collagenase clostridium histolyticum is a novel non-surgical treatment which has a UK marketing authorisation for the treatment of DC in adults with a palpable cord. It is marketed in Europe as Xiapex® (Pfizer Ltd) and in the USA as Xiaflex® (Auxilium Pharmaceuticals, Inc.). 12 The collagenase preparation is a lyophilised product for parenteral administration which comprises two discrete types of collagenase in a defined mass ratio. These collagenases are representative of the two major collagenase classes (class I and II) produced by Clostridium histolyticum. Both collagenases effectively cleave interstitial collagen but at different sites on the molecule. Class I collagenase cleaves the terminal ends of the collagen and class II collagenase cleaves internal sections of collagenase. 11,46,47 According to the summary of product characteristics, it is administered by intralesional injection, with the recommended dose being 0.58 mg. 12
The cord is adjacent to the flexor tendons and precise depth of injection is of paramount importance11 to avoid injection of the flexor. 48 The injection is administered in a slow and steady fashion to prevent the liquid being forced through the cord and into the deep fat surrounding the flexor tendons. 11 Approximately 24 hours after injection, the treated joint is manipulated to allow finger extension and encourage rupture of the cord. 7 This finger extension procedure may require the use of a local anaesthetic. If a satisfactory response has not been achieved, the injection and finger extension procedure may be repeated after approximately 4 weeks. Up to three injections per cord are recommended, at approximately 4-week intervals. Only one cord may be treated at a time. Where there are multiple contractures, each cord must be treated sequentially. The current clinical experience is limited to up to three injections per cord and up to a maximum of eight injections in total. 12 The collagenase procedure is, in some respects, similar to PNF; both procedures are minimally invasive, performed as outpatient procedures and involve the use of a needle.
Surgical interventions for Dupuytren’s disease
The most common type of surgery in the UK and throughout Europe is fasciectomy, which is the excision of the fascia from the affected area. 6 Fasciectomy can be limited to the excision of the diseased fascia, with the dissection carried no further than the PIP joint (limited/partial fasciectomy) or can involve the removal of all the palmar fascia (total/radical fasciectomy). Longitudinal or transverse skin incisions can be used. Longitudinal incisions are commonly closed with a Z-plasty, whereas zigzag incisions are closed directly (Bruner technique). The procedure requires the use of a general or regional anaesthetic and can be carried out as day surgery or inpatient admission. 1 The overlying skin is normally preserved during the procedure but skin fixation to the cord can occur. 49 Dermofasciectomy involves excision of both the fascia and the overlying skin, with lost tissue being replaced by a full-thickness skin graft. 49–51 Fasciotomy involves the simple division of the affected cord, by means of open surgery or percutaneously. 1 In open fasciotomy, a scalpel is used to cut the cord. 34,52 By contrast, PNF is a less invasive technique where the cord is sectioned with a 25-gauge needle mounted on a syringe. 21 PNF is recommended by NICE IPG43 and is considered particularly suitable for older people who are unfit for, or may not tolerate, major surgery. 45 Amputation may be an option in severe cases with later presentation or recurrence of the disease. Hand therapy and the use of splints are often required after surgery in order to maximise and maintain reduction of the contraction and improvement in finger position.
In Europe, factors that influenced the surgeons’ decision to perform fasciectomy or dermofasciectomy were consistent across countries: contracture of MCP or PIP joints > 45°, recurrent contracture and high expectations of success. For PNF, defining factors were more variable but MCP flexion of < 20° was a crucial factor. 43
Identification of important subgroups
No subgroups were specified in the final scope for this appraisal. However, consideration of people with moderate contracture and with severe contracture as relevant subgroups may be informative, as these groups are likely to be offered different surgical treatments. In addition, it might be advantageous to consider treatment of MCP joints versus PIP joints as subgroups, as each type of joint responds differentially to treatment. 29
Current usage in the NHS
Data from Hospital Episode Statistics (HES) indicate that for the period 2012–13 (1 year), the number of consultant visits for ‘palmar fascial fibromatosis [i.e. Dupuytren disease]’ (code M72.0) was 18,247. 53 Of these, 18,222 (99%) were admissions and 14,436 (79%) involved men. The mean age of patients undergoing surgery was 65 years. During the same period, there were 655 outpatient visits for Dupuytren’s disease with three follow-up attendances for each first attendance. However, as reporting of primary diagnosis is optional in the HES outpatient data set, this figure may not capture what happens in clinical practice. 53 It is worth noting that the 2012–13 admissions for Dupuytren’s disease were higher than those reported between April 2003 and March 2008 [mean 12,901; standard deviation (SD) 330]. 6 This could be tentatively explained by a change in the way hospital data were collected and recorded after April 2008 and by a possible change in referral patterns. Moreover, it is worth noting that payment by results was implemented nationally in 2008–9, helping to drive improvements in clinical coding recording. 54 This has resulted in a subsequent increase in the number of secondary diagnosis and procedures.
With regard to the type of procedures performed for Dupuytren’s disease in England, 7810 palmar fasciectomies (code T52.1), 5292 digital fasciectomies (code T52.5), 762 dermofasciectomies (code T56.1) and 1262 divisions of palmar fascia (code T54.1) were carried out as in hospital procedures between April 2012 and March 2013. During the same period, a smaller number of procedures were performed on an outpatient basis (eight palmar fasciectomies, four digital fasciectomies, three dermofasciectomies and 38 divisions of palmar fascia). 53
Mean length of in-hospital stay was 0.8 days for palmar fasciectomy and 0.9 days for both digital fasciectomy and dermofasciectomy. Median length of stay was 1 day for all three procedures.
Use of CCH is still limited and, apart from clinical trials data, no audit databases are currently accessible.
Chapter 2 Definition of the decision problem
Decision problem
The purpose of this assessment is to evaluate the clinical effectiveness and cost-effectiveness of CCH compared with surgical interventions for treating DC with a palpable cord in adults.
The current clinical pathway for the treatment of DC is that recommended by the BSSH (see Chapter 1). This chapter considers the main components of the decision problem addressed by this appraisal. Specific information on the population, intervention, comparator and relevant outcomes considered for this assessment will also be provided in Chapter 3, Assessment of clinical effectiveness.
Population
The population considered for this assessment is adults (18 years of age and older) with DC with a palpable cord. CCH is licensed ‘for the treatment of DC in adult patients with a palpable cord.’ Nevertheless, BSSH current guidelines recommend reassurance/observation for mild cases and collagenase injections for adults with moderate or severe contractures.
Intervention: collagenase clostridium histolyticum
Collagenase clostridium histolyticum is the only pharmaceutical treatment with a UK marketing authorisation for adults with Dupuytren’s disease with a palpable cord. It is administered by injection at the recommended dose of 0.58 mg. Only one cord must be treated at a time. A finger extension procedure to rupture the affected cord is conducted 24 hours after the injection. This sequence can be repeated up to three times per cord at approximately 4-week intervals (Auxilium Pharmaceuticals, summary of product characteristics).
Comparator: surgery
The comparator interventions considered in this assessment are the current surgical treatments for DC, including – but not restricted to – fasciectomy, dermofasciectomy, needle fasciotomy and open fasciotomy.
Relevant outcomes
Main outcomes of interest are reduction of contracture, time to return to normal function, recurrence of contracture, adverse effects of treatment and health-related quality of life.
Overall aims and objectives of assessment
The aim of this assessment is to evaluate the clinical effectiveness and cost-effectiveness of CCH as an alternative to surgery for the treatment of adults with DC with a palpable cord.
To facilitate decision-making on the most appropriate treatment for people with DC with a palpable cord, the specific objectives of the assessment are to:
-
conduct a systematic review of the available evidence on the clinical effectiveness of CCH versus surgical interventions
-
conduct a systematic review of the available evidence on the cost-effectiveness of CCH versus surgical interventions
-
develop an economic model of the relative cost-effectiveness of CCH injections and surgical interventions
-
identify and prioritise future research.
Comments on the manufacturer’s definition of the decision problem
Table 2 illustrates the differences between the final scope issued by NICE and the decision problem addressed by the manufacturer of CCH.
| Element of scope to be addressed | Final scope issued by NICE | Decision problem addressed in the manufacturer’s submission |
|---|---|---|
| Population | Adults with DC with a palpable cord | Adults with moderate or severe DC and a palpable cord |
| Intervention | CCH | CCH |
| Comparator(s) | Surgical treatments including fasciectomy, open fasciotomy, needle fasciotomy | Fasciectomy |
| Outcomes | Reduction of contracture Time to return to normal function Recurrence of contracture Adverse effects of treatment Health-related quality of life |
Reduction of contracture Time to return to normal function Recurrence of contracture Adverse effects of treatment Health-related quality of life |
| Economic analysis | Incremental cost per QALY Time horizon should be sufficiently long to reflect differences in costs or outcomes between technologies being compared Costs will be considered from an NHS and PSS perspective |
Cost minimisation analysis 5 years Costs were considered from an NHS and PSS perspective |
In general, the methods used by the manufacturer to review the literature appear to have been appropriate.
Population
The manufacturer’s submission focused on adults with moderate or severe DC with a palpable cord. This is not in line with the final scope issued by NICE, which does not specify any severity of disease and considers suitable for inclusion ‘adults with Dupuytren’s contracture with a palpable cord’. However, as people with mild contracture would not be eligible for treatment with collagenase in any case (according to the BSSH guidelines), the manufacturer’s approach appears to be reasonable. Inclusion criteria for both CORD I55 and CORD II56 trials specify MCP joint contractures of 20–100° or PIP joints of 20–80°. The manufacturer’s submission reports 14/167 (8.4%) participants with mild disease in the subgroup of participants with two or fewer affected joints. The total number of participants with mild disease is unclear owing to total contracture index being the main reported measure of contracture.
Intervention
The intervention specified in the manufacturer’s submission (i.e. CCH) matches that of the NICE final scope.
Comparators
The NICE final scope states that the comparators are surgical treatments, including fasciectomy, open fasciotomy, needle fasciotomy. The manufacturer’s submission differs from the NICE final scope in that only fasciectomy was considered a suitable comparator. The manufacturer justifies this decision by arguing that fasciectomy is the main treatment recommended by the BSSH and is the most commonly used procedure in England. Furthermore, they maintain that the remaining specified treatments are either not recommended by the BSSH (i.e. open fasciotomy) or used in only a small proportion of patients with moderate to severe disease (i.e. PNF). The exclusion of PNF is questionable. PNF is still performed by some surgeons in some clinical departments in the NHS and is suitable for less severe disease and for elderly people who are unsuitable for, or may not tolerate, surgery. 45 In addition, among all surgical procedures, PNF shows more similarities with collagenase (both are minimally invasive procedures that do not require in-hospital stay).
Outcomes
The outcomes considered by the manufacturer were those specified in the NICE final scope (i.e. reduction of contracture, time to return to normal function, recurrence of contracture, adverse effects of treatment and health-related quality of life).
Chapter 3 Assessment of clinical effectiveness
The methods for this assessment were pre-specified in a research protocol (www.nice.org.uk/guidance/gid-tag364/documents/dupuytrens-contracture-collagenase-clostridium-histolyticum-final-protocol2).
Methods for reviewing effectiveness
Highly sensitive search strategies were designed to identify reports of clinical trials and cohort studies on the clinical effectiveness of CCH compared with surgery for the treatment of adults with DC. Appropriate subject headings and text word terms that reflected the clinical condition (Dupuytren’s disease/DC), relevant interventions and study design, were used. Searches were run from 1990 to October 2013 to reflect the introduction in clinical practice of CCH. All searches were subsequently updated in February 2014. Details of the searches are presented in Appendix 1.
The following main electronic databases were searched: MEDLINE (1990 to February Week 2 2014), MEDLINE In-Process & Other Non-Indexed Citations (25 February 2014), EMBASE (1990 to Week 8 2014), Science Citation Index (1990 to 21 February 2014), Biosis (1990 to 21 February 2014), Scopus (in-press articles February 2014) and the Cochrane Controlled Trials Register (Issue 1 2014). Reports of relevant evidence synthesis were also sought from the Cochrane Database of Systematic Reviews (Issue 1 2014) and from the Database of Abstracts of Reviews of Effects (February 2014). The World Health Organization International Clinical Trials Registry, Current Controlled Trials and ClinicalTrials.gov databases were searched for evidence of relevant ongoing studies.
Conference proceedings of the following organisations were screened for the period 2011–13: American Society for Surgery of the Hand, the Federation of the European Societies for Surgery of the Hand and the International Symposium on Dupuytren’s Disease. Websites of regulatory bodies and health technology assessment (HTA) agencies were checked for relevant unpublished reports, and websites of related non-profit organisations (i.e. the British Dupuytren’s Society, the International Dupuytren Society, The Dupuytren Foundation) and manufacturers were checked for further pertinent information and reports. Reference lists of all included studies were perused for additional reports.
Inclusion and exclusion criteria
Types of studies
We considered evidence from randomised controlled trials (RCTs), non-randomised comparative studies and observational studies. Head-to-head RCTs comparing CCH with surgery for the treatment of DC were considered the best source of evidence. In the absence of head-to-head RCTs, randomised trials comparing CCH with placebo, one type of surgical procedure with another type of surgical procedure, or surgery with a sham procedure were considered suitable for inclusion. Similarly, non-randomised comparative studies of collagenase injections versus surgical interventions were deemed suitable for inclusion. Observational studies for estimating durability of response and rates of adverse events and complications were included to complement the evidence available from RCTs and from non-randomised comparative studies. Case series that assessed the effects of CCH were included in the current assessment, whereas case series that focused on the effects of surgical procedures (some of which are quite dated) were retained for information only.
Relevant systematic reviews assessing CCH and/or surgical interventions were used as sources of relevant studies but not updated.
The following types of report were excluded:
-
narrative reviews, editorials and opinions
-
case reports
-
non-English-language reports for which a translation could not be organised.
Types of participants
The types of participants considered were adults (18 years of age and older) with DC with a palpable cord. As many studies did not provide a definition of DC and/or disease within their methods, any study population described as presenting with DC or Dupuytren’s disease was considered suitable for inclusion. In addition, even though the presence of a palpable cord was not consistently specified across included studies, it was assumed as being a distinctive symptom of the condition.
Intervention
The intervention considered was injectable CCH.
Comparator interventions
The comparator interventions assessed were current surgical treatments for DC, including – but not restricted to – fasciectomy, dermofasciectomy, PNF, open fasciotomy and amputation.
Types of outcomes
The following types of outcome measure were considered:
-
reduction of contracture
-
time to return to normal function
-
recurrence of contracture
-
adverse effects of treatment
-
health-related quality of life.
Data extraction strategy
Two reviewers (MC and CR) were involved in the screening process. Each reviewer screened half of the titles and abstracts identified by the search strategies. To ensure consistency, the first 20 citations were double-screened by both reviewers. Any disagreements were resolved by consensus. All potentially relevant reports were retrieved in full and assessed independently by the same two reviewers. Any disagreements were resolved by consensus or referred to a third author. A sample of the full-text screening form is shown in Appendix 2.
A data extraction form was developed and piloted for the purpose of this appraisal. From each included study, one reviewer (MC, CR or MB) collected information on journal name, publication year and status, study design, recruitment method, setting, characteristics of participants, characteristics of interventions and outcome measures. Data extraction was double-checked by a second reviewer with the exception of a Portuguese-language paper,57 which was not double-checked. Any disagreements were resolved by discussion or arbitration by a third author.
Critical appraisal strategy
The standard Cochrane risk of bias tool was used to assess the risk of bias in randomised trials. 58 Included RCTs were assessed using Cochrane’s tool for assessing risk of bias. A sample form is presented in Appendix 3. Two reviewers (CR and MC) independently assessed risk of bias within each included RCT, based on the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data and selective outcome reporting. Individual outcomes were categorised as being at high, low or unclear risk of bias. Any disagreements between reviewers were resolved by consensus.
An adapted version of the risk of bias tool recommended by the Cochrane Non-Randomised Studies Methods Group was used for non-randomised comparative studies (see Appendix 4). 58 The main confounders were identified a priori from data derived from the existing epidemiological literature and from consultation with the advisory panel convened for this assessment. A study was judged to be at high risk of bias if any of the identified confounders were imbalanced (e.g. type of joint, degree of baseline contracture).
Risk of bias of case series was assessed using a modified version of a 17-item checklist previously developed by our research team (see Appendix 5). The checklist was originally adapted from several sources and developed through a partnership with the Review Body for Interventional Procedures (ReBIP) for NICE. 59–62 The case series tool assessed the following domains: bias and generalisability, sample definition and selection, description of the intervention, outcome assessment, adequacy of follow-up and performance of statistical analyses. Individual items were rated as ‘yes’, ‘no’ or ‘unclear’. A rating of ‘yes’ indicated a low risk of bias.
Two reviewers (MC and CR) independently assessed the risk of bias of all included primary studies. Any disagreements or uncertainties were resolved by discussion between the two reviewers. A third reviewer (MB) acted as an arbitrator where consensus could not be reached.
Methods of data synthesis
Results of each included study were tabulated for all outcomes with means reported for continuous outcomes and proportions for dichotomous outcomes. Where the same outcome was assessed by more than one included study, a quantitative synthesis of results was carried out using Review Manager software (Version 5.2.; Copenhagen: The Nordic Cochrane Centre, Cochrane, 2012). Heterogeneity between studies was assessed by visual inspection of forest plots and from Mantel–Haenszel chi-squared and I2 statistics. Meta-analyses were carried out to estimate risk ratios (RRs) pooled across studies, with corresponding 95% confidence intervals (CIs). Where there was a large amount of heterogeneity between studies, random-effects models were applied using the inverse-variance method, otherwise fixed-effects models using the Mantel–Haenszel method were applied. Where SDs were not reported for a continuous outcome (and not subsequently supplied following a request to the authors), the values were imputed using data from other studies included in the meta-analysis, with this approach being tested in sensitivity analyses using differing values for the imputed SDs.
Quantity of research available
Number and type of studies included
The literature searches identified 720 potentially relevant citations and 502 conference proceedings. Eighteen ongoing trials were identified; the latest updates showed that two trials were not yet recruiting, one had finished recruiting but no results are available, 11 were still recruiting and four were ongoing but not recruiting. We selected and retrieved 187 reports for full-text assessment. Of these, 153 were subsequently excluded (Figure 2). We included a total of five RCTs (493 participants), published in six papers, comparing collagenase with placebo,55,56,63–66 three RCTs (334 participants), published in four papers, comparing various surgical procedures,67–70 two non-randomised studies (105 participants) comparing collagenase with surgery,71,72 five non-randomised studies (3571 participants), published in six papers, comparing various surgical procedures,44,57,73–76 and 15 collagenase case series (3154 participants) published in 16 papers. 48,75,77–90
FIGURE 2.
Flow diagram outlining the selection process.

Appendix 6 provides bibliographic details of all included and excluded studies.
Number and type of studies excluded
Forty-four reports were excluded because they failed to meet one or more of the pre-specified inclusion criteria with regard to type of study design, characteristics of participants, characteristics of intervention and comparators and outcomes measures.
Characteristics of the included studies
A summary of the baseline characteristics of included studies is reported in Table 3. An overview of the main characteristics of the included studies is presented in Table 4. Appendices 7 and 8 present the characteristics of the included collagenase and surgery studies, respectively.
| Characteristic | Collagenase studies | Surgery studies |
|---|---|---|
| Enrolled | ||
| RCT | 493 (n = 5) | 334 (n = 3) |
| NRS | 105 (n = 2) | 3571 (n = 4) |
| Case series | 3154 (n = 13) | N/A |
| Total | 3752 | 3665 |
| Analysed | ||
| RCT | 421 (n = 3) | 271 (n = 3) |
| NRS | 105 (n = 2) | 3538 (n = 5) |
| Case series | 3139 (n = 15) | N/A |
| Total | 3905 | 3809 |
| Age, median of means (range) | ||
| RCT | 63.6 (60.1–65.5) (n = 4) | 64.5 (60.2–65.7) (n = 3) |
| NRS | 66.5 (65–67) (n = 2) | 67 (61.9–70) (n = 4) |
| Case series | 65 (63–69) (n = 14) | N/A |
| Sex (% male), median of mean proportions (range) | ||
| RCT | 84 (71–87) (n = 4) | 81 (78–85) (n = 2) |
| NRS | 69.5 (62–92) (n = 2) | 87 (73–94) (n = 5) |
| Case series | 85 (65–100) (n = 12) | N/A |
| Baseline contracture | ||
| RCT | Median of means (range): MCP = 45 (44–51); PIP = 46 (43–53) (n = 3) | Median of means (range): MCP = 42 (21–44); PIP = 34 (34–59) (n = 2) |
| Total contracture index, collagenase group = 161.6 (149.1–174.1); placebo group = 149.7 (149.3–150.1) (n = 2) | ||
| NRS | Mean (range): collagenase group = 41.8 (40–43.5); surgery group = 39.2 (37–41.4) (n = 2) | NR |
| Case series | Median of means (range): MCP = 50.5 (41–64); PIP = 50.5 (39–83) (n = 6) | N/A |
| Study | Prospective/retrospective data collection | Geographical location | Intervention | Length of follow-up |
|---|---|---|---|---|
| Collagenase RCTs | ||||
| Badalamente et al., 200264 | Prospective | USA (1 site) | Collagenase (n = 25); placebo (n = 24) | 5 years |
| Badalamente and Hurst, 200563 | Prospective | USA (sites NR) | Collagenase (n = NR); placebo (n = NR); (total n = 35) | 1 year |
| Badalamente and Hurst, 200765 | Prospective | USA (sites NR) | Collagenase (n = 23); placebo (n = 12) | 1 year |
| Gilpin et al., 2010 (CORD II)56 | Prospective | Australia (5 sites) | Collagenase (n = 45); placebo (n = 21) | 90 days |
| Hurst et al., 2009 (CORD I)55 and Witthaut et al., 201166 | Prospective | USA (16 sites) | Collagenase (n = 204); placebo (n = 104) | 90 days |
| Collagenase versus surgery non-randomised comparative studies | ||||
| Naam 201371 | Retrospective | USA (1 site) | Collagenase (n = 25); fasciectomy (n = 21) | Mean 32 months; mean 39 months |
| Nydick et al., 201372 | Prospective | USA (1 site) | Collagenase (n = 29); PNF (n = 30) | 3–24 months; 3–28 months |
| Surgery RCTs | ||||
| Citron and Nunez 200567 | Prospective | UK (1 site) | Fasciectomy (Z-plasty) (n = 38); fasciectomy (modified Bruner approach) (n = 62) | Minimum 2 years |
| Ullah et al., 200968 | Prospective | UK (1 site) | Dermofasciectomy (n = 39); fasciectomy (Z-plasty) (n = 40) | 3 years |
| van Rijssen et al., 200669 van Rijssen et al., 201270 |
Prospective | Netherlands (1 site) | PNF (n = NR); LF (n = NR); (total randomised, n = 121) | 5 years |
| Surgery non-randomised comparative studies | ||||
| Bainbridge et al., 201273 Dias et al., 201344 |
Retrospective | Czech Republic, Denmark, Finland, France, Germany, Hungary, Italy, Netherlands, Poland, Spain, Sweden and the UK (12 sites) | PNF (n = 329); fasciotomy (n = 446); fasciectomy (n = 2311); dermofasciectomy (n = 200) | 1 year |
| Citron and Hearnden 200374 | Prospective | UK (1 site) | Fasciotomy (Z-plasty) (n = 15); fasciotomy (transverse incision) (n = 15) | Mean 2.2 years |
| Ribak et al., 201357 | NR | Brazil (1 site) | PNF (n = 16); open PF (n = 17) | 1 year |
| Skoff, 200475 | Prospective | USA (1 site) | Fasciectomy (synthesis technique) (n = 20); fasciectomy (open-palm technique) (n = 10) | Mean 2.7 years Mean 3.5 years |
| Toppi et al., 201476 | Retrospective | Australia (sites NR) | PNF (n = 73); open fasciectomy (n = 52) | Mean 2 years |
| Collagenase case series | ||||
| Badalamente and Hurst, 200048 | Prospective | USA (sites NR) | Collagenase (n = 34) | 2 years |
| Badalamente and Hurst, 201177 | Prospective | USA (17 sites) | Collagenase (n = 509) | 2 years |
| Coleman et al., 201278 | Prospective | Australia (1 site) | Collagenase (n = 12) | 30 days |
| Coleman et al., 201479 | Prospective | Australia (8 sites) | Collagenase (n = 60) | 60 days |
| Considine and Hirpara, 201380 | NR | NR | Collagenase (n = 10) | NR |
| Hayton et al., 201381 | Prospective | USA (30 sites) | Collagenase (n = 616) | NR |
| Kaplan et al., 201383 | Prospective | USA (sites NR) | Collagenase (n = 37) | 90 days |
| Martin-Ferrero et al., 201384 | Prospective | Spain (1 site) | Collagenase (n = 35) | 1 year |
| McMahon et al., 201385 | Retrospective | USA (1 site) | Collagenase (n = 48) | Mean 15 months |
| Peimer et al., 201386 | Retrospective | USA (10 sites) | Collagenase (n = 463) | NR |
| Peimer et al., 201387 (CORDLESS) Kaplan et al. 201282 |
Prospective | USA (sites NR) | Collagenase (n = 643) | 3 years |
| Skirven et al., 201388 | Prospective | USA (sites NR) | Collagenase (n = 21) | 4 weeks |
| Syed et al., 201391 | Prospective | UK (1 site) | Collagenase (n = 56) | 1 year |
| Watt et al., 201089 | Prospective | USA (1 site) | Collagenase (n = 8) | 8 years |
| Witthaut et al., 2013 (JOINT I/II)90 | Prospective | USA, Australia, UK, Switzerland, Sweden, Denmark, Finland (34 sites) | Collagenase (n = 587) | 9 months |
Study details
A total of eight RCTs were included in the review, seven full-text papers55,56,64,65,67–69 and one abstract. 63 Five RCTs compared collagenase with placebo55,56,63–65 and three RCTs focused on various surgical procedures for the treatment of DC. 67–69 All eight trials involved consecutive enrolment of participants and prospective data collection.
The two non-randomised studies comparing collagenase with surgery71,72 and the five non-randomised studies comparing various surgical procedures44,57,73–76 were published in full. Three studies collected data prospectively72,74,75 and three retrospectively. 71,73,76 One study did not report this information. 57
Of the 15 collagenase case series, 12 were published in full and three were published as abstracts. 77,80,83 One case series involved consecutive enrolment of study participants,83 two did not48,88 and the remaining 12 case series did not provide this information. 77–81,84–87,89–91
Four collagenase versus placebo RCTs were conducted in the USA,55,63–65 and one was conducted in Australia. 56 Two of the surgical RCTs took place in the UK67,68 and one in the Netherlands. 69 The two non-randomised studies assessing collagenase versus surgery were both conducted in the USA. 71,72 Of the surgery non-randomised studies, one was conducted in several countries,73 one in the UK,74 one in Brazil,57 one in the USA75 and one in Australia. 76 Nine of the collagenase case series were conducted in the USA48,77,81,83,85–89 two in Australia,78,79 one in the UK,91 one in Spain,84 one in several countries,90 and the remaining one did not report this information. 80
The RCTs comparing collagenase with placebo enrolled a total of 493 participants. 55,56,63–65 CORD I analysed 306 participants, CORD II analysed 66 participants and Badalamente et al. analysed 49 participants. 55,56,64 Badalamente and Hurst did not report the number of participants analysed. 63 The non-randomised studies comparing collagenase with surgery enrolled and analysed a total of 105 participants. 71,72 The three surgery RCTs enrolled a total of 334 participants and analysed 271 of them. 67–69 The five surgery non-randomised studies enrolled a total of 3571 participants and analysed 3538 of them. 57,73–76 The 15 collagenase case series enrolled a total of 3154 participants and analysed 3139 of them. However, some collagenase case series relied on the same patient cohorts81,87,90 and we could not establish with certainty whether there was duplication of data because of multiple publications.
Follow-up for collagenase RCTs ranged from 90 days for CORD I and CORD II55,56 to 5 years. 64 Follow-up for the non-randomised studies of collagenase versus surgery ranged from 3 months72 to a mean of 39 months. 71 Follow-up for the three RCTs on surgical interventions ranged from 6 weeks69 to 5 years. 70 Follow-up for non-randomised comparative studies assessing the effects of surgical procedures ranged from 1 year 57,73 to a mean of 3.5 years. 75
Of the collagenase versus placebo RCTs, CORD I and CORD II enrolled participants with primary occurrence of the disease as well as participants who were treated previously. 55,56 Three RCTs63–65 and the two non-randomised studies on collagenase versus surgery71,72 did not report this information. The three surgery RCTs focused on participants with primary occurrence of the disease. 67–69 Three of the surgery non-randomised comparative studies enrolled participants with primary disease,74–76 one reported treating both participants with primary disease and participants previously treated73 and one did not provide this information. 57 Eight of the collagenase case series involved both participants with primary disease and participants previously treated,77–81,84,88,90 one focused on participants with primary disease only,91 two involved previously treated participants87,89 and four did not report this information. 48,83,85,86
Participant details
With regard to the collagenase RCTs, mean age and sex of participants were reported across randomised groups or joint types, with the exception of one trial. 63 For one RCT,64 mean age for MCP joints was 65 years and for PIP joints was 64.3 years. Men comprised 86% of participants for MCP joints and 84.6% for PIP joints. In another RCT, the mean age of participants in the collagenase and placebo groups was 60.1 years and 63.8 years, respectively. The overall proportion of men in this study was 80%. 65 The CORD I trial involved 84% men in the collagenase group and 71% men in the placebo group. Mean age of participants was 62.3 years and 63.3 years in the two groups, respectively. 55 In the CORD II trial, mean age of participants in the collagenase and placebo groups was 63 years and 65.5 years, respectively. 56 The proportion of men was 87% in the collagenase group and 81% in the placebo group.
With regard to the two non-randomised studies comparing collagenase with surgery, one reported the mean age of participants in the collagenase and surgery groups as 65 years and 67 years, respectively, whereas the other reported 67 years and 66 years, respectively. 71,72 In the Naam study, more male participants received collagenase (92%) compared with those who underwent fasciectomy (62%). Similarly, Nydick et al. reported that the proportion of men who received collagenase was higher (86%) than those who underwent PNF (75%).
Median of mean ages for the surgery RCTs was 64.5 years (range 60.2–65.7 years). The mean proportion of men was 80% in one study and 82.5% in the other study. 67,68 The median of mean age in the non-randomised studies on surgery was 67 years (range 61.9–70 years). The mean proportion of men across the studies was 85%. Of the collagenase case series, all except one83 reported mean age of participants. The median of means was 65 years (range 63–69 years). The proportion of men was provided in all but three series. 80,83,89 Mean proportion across series was 85%.
Three of the collagenase RCTs reported mean baseline contracture for MCP and PIP joints separately, as 44° and 53°; 45° and 43°; 51° and 46°. 63–65 CORD I and CORD II reported mean total contracture index (i.e. the sum of all fixed-flexion contractures ≥ 20° caused by a Dupuytren’s cord) for collagenase and placebo groups; CORD I: 149.1° and 149.3° and CORD II: 174.1° and 150.1°. 55,56 The two collagenase versus surgery non-randomised studies reported mean baseline contracture of participants for collagenase and surgery as 43.5° and 41.4° for the collagenase group and the fasciectomy group, respectively;71 and as 40° and 37° for the collagenase group and the PNF group, respectively. 72 Three of the collagenase case series did not report baseline contracture. 77,83,87 Six cases series reported mean baseline contracture for MCP and PIP joints separately. The median of means was 50.5° (range 41–64°) for MCP joints and, similarly, 50.5° (range 39–83°) for PIP joints. McMahon et al. reported total mean baseline contracture of 48° and Syed et al. reported 41.8° for this index. 85,91 Coleman et al. reported five MCP joints ≤ 50°, three PIP joints ≤ 40° and four PIP joints ≥ 40°. 78 Hayton et al. reported baseline contracture for the two subgroups in terms of fixed flexion contracture: 48.2° and 49.7°. 81 Skirven et al. reported mean baseline passive PIP joint contracture of 56°. 88 Witthaut et al. reported mean baseline contracture of 132.5° for the JOINT I trial and 136.5° for the JOINT II trial. 90
Two of the three surgery RCTs reported mean baseline contracture for MCP and PIP joints separately. Ullah et al. reported MCP joint contracture of 21° and PIP joint contracture of 59°. 68 The study by van Rijssen et al. reported MCP joint contracture of 44° and PIP joint contracture of 34° in one intervention group, and MCP joint contracture of 42° and PIP joint contracture of 34° in the other group. 69 Three of the surgery non-randomised studies did not report baseline contracture. 57,73,76 Citron and Hearnden reported mean baseline contractures of 28° and 35° for the two intervention groups. 74 Skoff reported mean baseline MCP joint contracture of 57° and mean PIP joint contracture of 58° for participants in one intervention group, and 50° and 50° for MCP and PIP joint contracture, respectively, for the other treated group. 75
Risk of bias of the included studies
Randomised controlled trials
Collagenase versus placebo
The four collagenase RCTs published in full55,56,64,65 were assessed for risk of bias. Secondary reports63,66 were not assessed. CORD I and CORD II55,56 were judged as being at low risk of bias for both sequence generation and allocation concealment. The risk of bias was unclear in the remaining RCTs. All RCTs were judged as being at low risk of performance bias as all successfully blinded participants and, with the exception of the CORD II trial,56 the health-care providers. For all RCTs, the risk of detection bias was unclear as it was not possible to determine with certainty whether outcome assessors were blinded but all performed intention- to-treat analyses. All collagenase RCTs were sponsored by pharmaceutical companies involved in the development of collagenase (Advance Biofactures Corporation,64 Biospecifics Technologies Corporation65 and Auxilium Pharmaceuticals)55,56 and, therefore, were judged to be at high risk of ‘other bias.’
Surgery versus surgery
The three surgery RCTs,67–69 were assessed for risk of bias. The secondary report by van Rijssen et al. 70 was not assessed. Two studies67,69 were judged as being at low risk of bias for both sequence generation and allocation concealment. Only one of these studies67 blinded participants, but neither blinded health-care providers. In one trial,69 it was not possible to determine with certainty whether outcome assessors were blinded and, therefore, the risk of detection bias was judged as ‘unclear’. Two studies did not blind outcome assessors. 67,68 Two studies performed intention-to-treat analyses. 68,69 This was unclear in one trial. 67
Summaries of the risk-of-bias assessments for the RCTs comparing collagenase versus placebo and for those comparing different surgical interventions are presented in Figures 3 and 4, respectively. Summaries of the individual study level assessments are provided in Appendices 9 and 10 for collagenase and surgery studies, respectively.
FIGURE 3.
Summary risk-of-bias assessment for collagenase RCTs.

FIGURE 4.
Summary risk-of-bias assessment for surgery RCTs.

Non-randomised comparative studies
Collagenase versus surgery
We assessed two collagenase versus surgery non-randomised studies. 71,72 Both were judged as being at high risk of bias. Participants in both studies were allocated to treatments by patient or physician preference, and the majority of outcomes were at high risk of bias as a result of confounding and performance and detection biases, including the lack of blinding procedures. Both studies were either funded by Auxilium Pharmaceuticals71 or noted financial affiliations between study authors and the company. 72
Surgery versus surgery
We assessed five non-randomised studies on different surgical procedures. 57,73–76 All studies were judged as being at high or unclear risk of overall bias. Only two studies74,76 were judged to be at low risk of bias for sequence generation and allocation concealment. The majority of outcomes were also at either high or unclear risk for confounding and blinding. Studies were generally at lower risk for attrition bias and selective reporting. One study73 was funded by Pfizer. The remaining studies did not report information on sponsors.
Summaries of the risk-of-bias assessments are presented in Figures 5 and 6. The results of individual study level assessments for non-randomised comparative studies are provided in Appendices 11 and 12.
FIGURE 5.
Summary risk-of-bias assessment for non-randomised comparative studies on collagenase vs. surgery. HRQoL, health-related quality of life.

FIGURE 6.
Summary risk-of-bias assessment for non-randomised comparative studies on different surgical procedures. HRQoL, health-related quality of life.
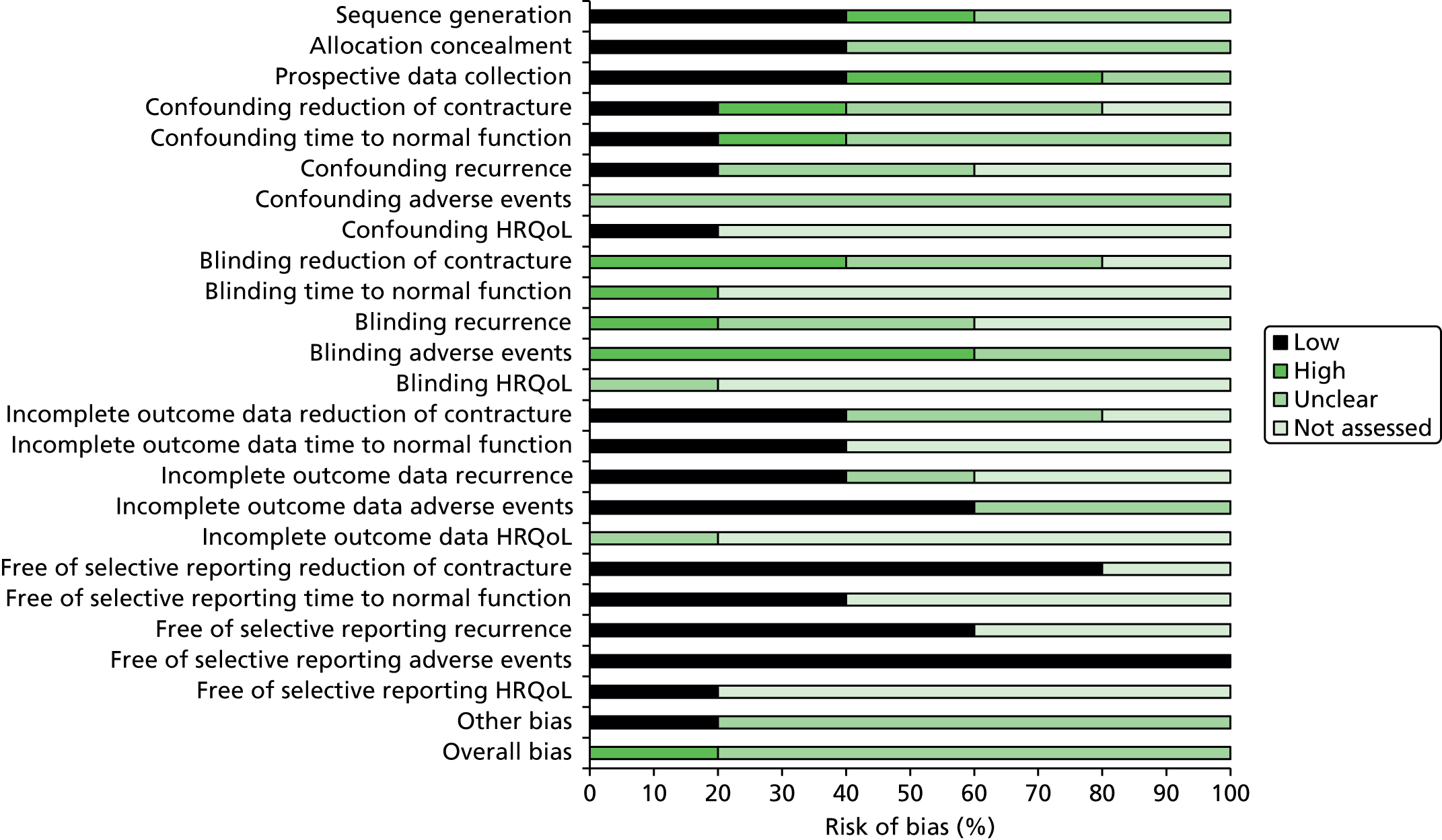
Case series studies
We assessed 11 collagenase case series. 48,78,79,84–91 Secondary reports81–83 and abstracts77,80 were not assessed. The majority of studies included a representative population, collected data prospectively, clearly defined the intervention, administered the collagenase injection in an appropriate setting by someone experienced at performing the procedure, and identified important prognostic factors. Three studies,78,90,91 however, included a mixed patient population (i.e. participants with primary disease and with disease recurrence). In five studies,48,85–87,89 it was unclear whether participants were at a similar point in their disease progression. In about half of the studies (54.6%), follow-up periods were not adequate (< 1 year) and the majority (72.7%) failed to provide information on the characteristics of participants who withdrew or did not complete follow-up. All studies were sponsored by Auxilium Pharmaceuticals48,78,79,84,86–91 or had a potential conflict of interest with the company. 85 A summary of the risk-of-bias assessment of the case series studies is presented in Figure 7 and the results of individual study level assessments are provided in Appendix 13.
FIGURE 7.
Summary risk-of-bias assessment for collagenase case series studies. N/A, not applicable.
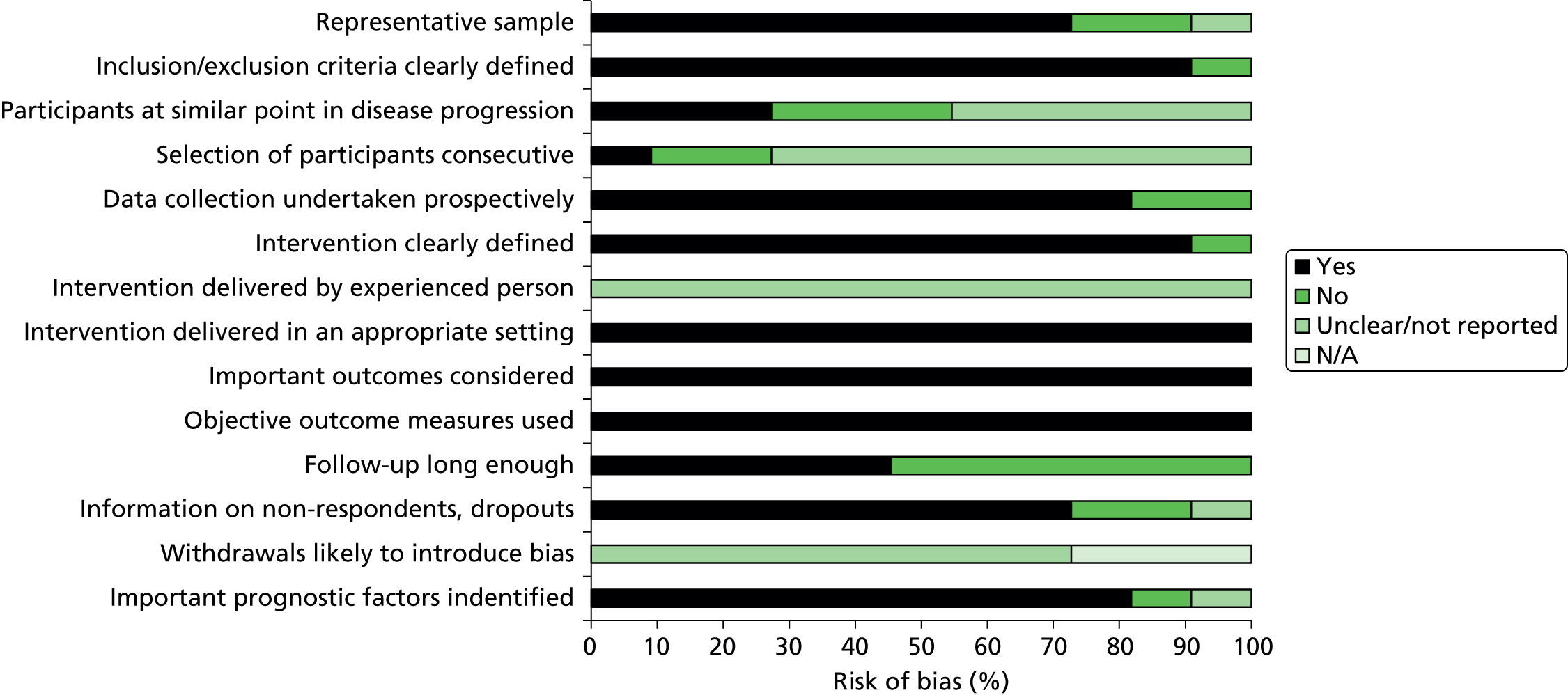
Summary of the risk of bias of the included studies
The quality of the included studies is variable. Inadequate reporting made it difficult to judge the risk of bias for many outcomes, for example, the quality of randomisation and allocation concealment of the RCTs. Although blinding procedures were used in most of the collagenase versus placebo RCTs, this was less evident in the surgery RCTs. The non-randomised studies were prone to confounding, often included a mixed patient population (participants with both primary disease and disease recurrence) and were of a shorter duration. Many of the collagenase studies were either funded by, or listed associations with, pharmaceutical companies involved in the manufacturing of the product and were, therefore, considered at potential risk of bias.
Assessment of clinical effectiveness
Meta-analyses of relevant clinical outcomes were performed, where appropriate. Fixed-effects models were used only where there were acceptable levels of homogeneity between the studies (as identified by visual inspection of forest plots and from Mantel–Haenszel chi-squared and I2 tests), otherwise random-effects models were preferred.
Overview of outcomes included in meta-analyses
Outcome measures included in the meta-analyses were those in which more than one trial reported the same outcome, with the same definition and the same outcome measure. The CORD I and CORD II studies tended to report identically defined outcomes and were included in all analyses. One study reported some outcomes consistent with the CORD I and CORD II studies and these were included in the appropriate meta-analyses. 65 The way outcomes were reported in two studies was not consistent with any other studies comparing collagenase and placebo and, therefore, data from these studies were not included in any meta-analyses. 63,64 Outcomes included in the meta-analysis were clinical end points (i.e. clinical success, clinical improvement, range of motion (ROM), change in contracture from baseline) and adverse events (i.e. proportion experiencing at least one adverse event, peripheral oedema, contusion, pain in extremity, injection site pain, injection site haemorrhage, injection site swelling, tenderness, pruritus, lymphadenopathy, axillary pain, injection site vesicles). Both CORD I and CORD II studies reported zero recurrence. 55,56 Badalamente et al. and Badalamente and Hurst both reported recurrence rates. 64,65 However, one of these studies did not report the criteria used to define recurrence64 and the other assessed recurrences over the entire period of the study, which included both a controlled phase and an open-label extension phase. 65 Therefore, a meta-analysis of recurrence rates was not feasible. No data were available for the outcomes time to return to normal function and health-related quality of life. In the meta-analyses reported below, a RR above 1 indicates greater efficacy of collagenase compared with placebo for the clinical outcomes and greater likelihood of adverse events following collagenase as opposed to placebo treatment.
Clinical success: all first joints
A total of 171/271 (63%) of all first joints treated with collagenase and 8/136 (6%) first joints treated with placebo achieved clinical success, defined as a reduction in contracture to 0° to 5° of normal, 30 days after the last injection (Figure 8). The difference between the groups was statistically significant (RR 10.21, 95% CI 5.29 to 19.69; p-value < 0.00001).
FIGURE 8.
Forest plot of proportion of all first joints achieving clinical success. df, degrees of freedom; M–H, Mantel–Haenszel.

Clinical success: first metacarpophalangeal joints
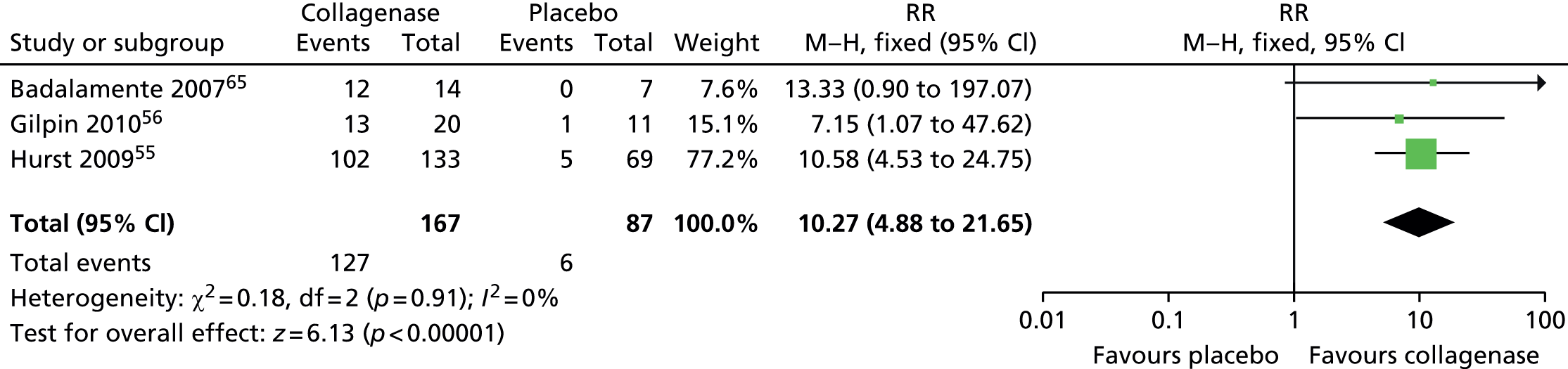
A total of 127/167 (76%) first MCP joints treated with collagenase achieved clinical success, compared with 6/87 (7%) joints treated with placebo (Figure 9). The difference between the groups was statistically significant (RR 10.27, 95% CI 4.88 to 21.65; p-value < 0.00001).
FIGURE 9.
Forest plot of proportion of first MCP joints achieving clinical success. df, degrees of freedom; M–H, Mantel–Haenszel.
Clinical success: first proximal interphalangeal joints
A total of 44 of 104 (42%) first PIP joints treated with collagenase and 2 of 49 (4%) joints treated with placebo achieved clinical success (Figure 10).The difference between the groups was statistically significant (RR 7.44, 95% CI 2.44 to 22.62; p-value = 0.0004).
FIGURE 10.
Forest plot of proportion of first PIP joints achieving clinical success. df, degrees of freedom; M–H, Mantel–Haenszel.

Clinical improvement: all first joints
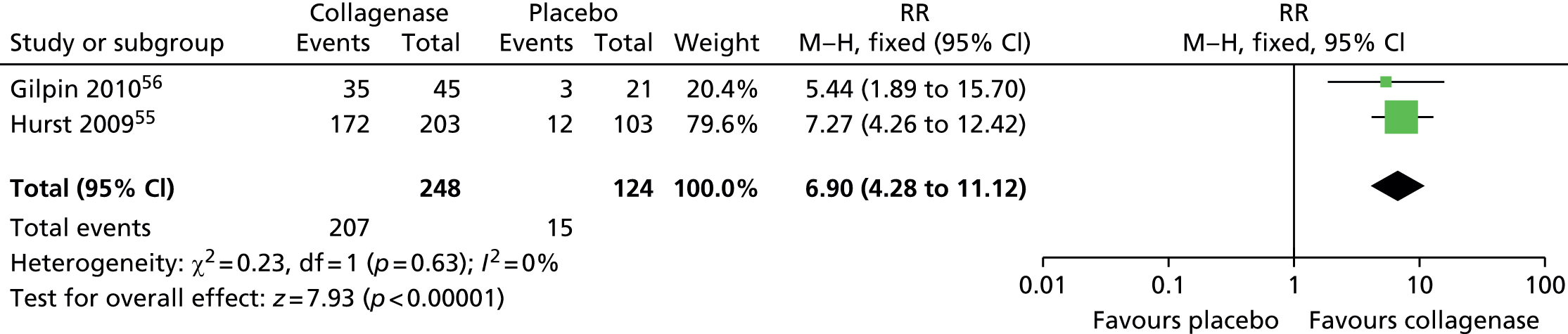
Significantly more first joints treated with collagenase (207/248; 83%) than with placebo (15/124; 12%) achieved clinical improvement (Figure 11), defined as a reduction in contracture of 50% or more from baseline 30 days after the last injection (RR 6.90, 95% CI 4.28 to 11.12; p-value < 0.00001).
FIGURE 11.
Forest plot of proportion of all first joints achieving clinical improvement. df, degrees of freedom; M–H, Mantel–Haenszel.
Clinical improvement: first metacarpophalangeal joints
A total of 144 of 153 (94%) first MCP joints treated with collagenase achieved clinical improvement, compared with 10 of 80 (13%) first joints treated with placebo (Figure 12). The difference between the groups was statistically significant (RR 7.54, 95% CI 4.21 to 13.49; p-value < 0.00001).
FIGURE 12.
Forest plot of proportion of first MCP joints achieving clinical improvement. df, degrees of freedom; M–H, Mantel–Haenszel.

Clinical improvement: first proximal interphalangeal joints
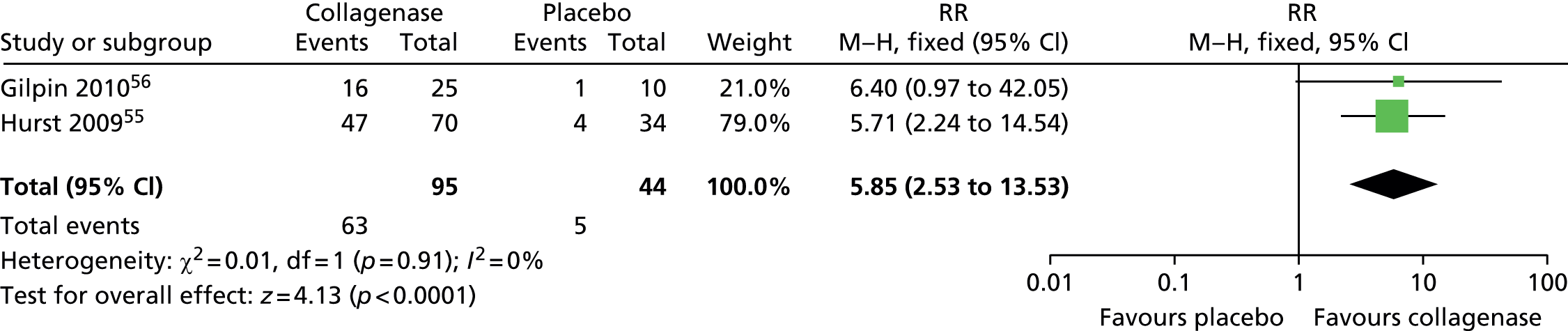
A total of 63 of 95 (66%) first PIP joints treated with collagenase achieved clinical improvement compared with 5 of 44 (11%) first PIP joints treated with placebo (Figure 13). The difference between the groups was statistically significant (RR 5.85, 95% CI = 2.53 to 13.53; p-value < 0.0001).
FIGURE 13.
Forest plot of proportion of first PIP joints achieving clinical improvement. df, degrees of freedom; M–H, Mantel–Haenszel.
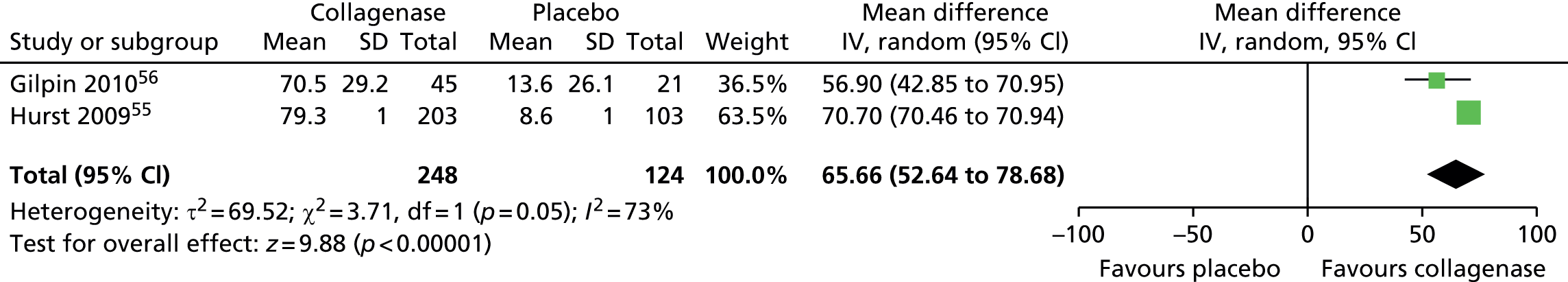
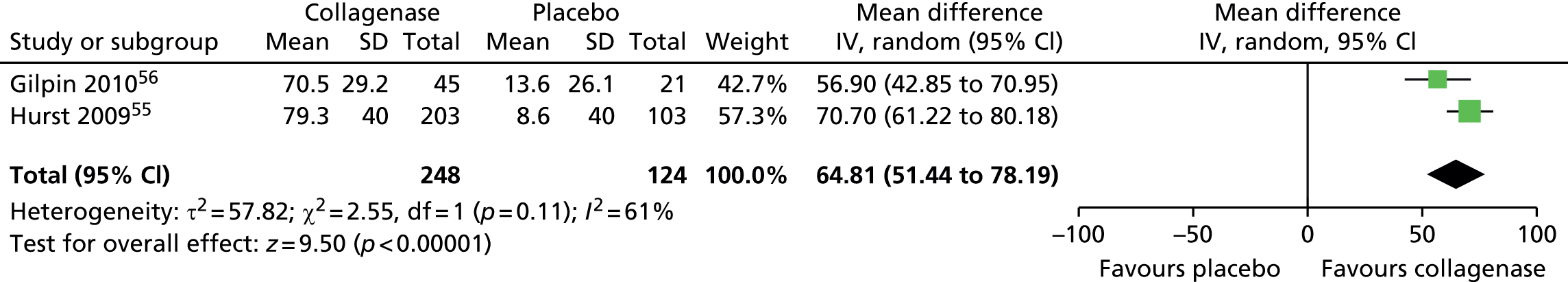
Mean change in range of motion from baseline for all first joints
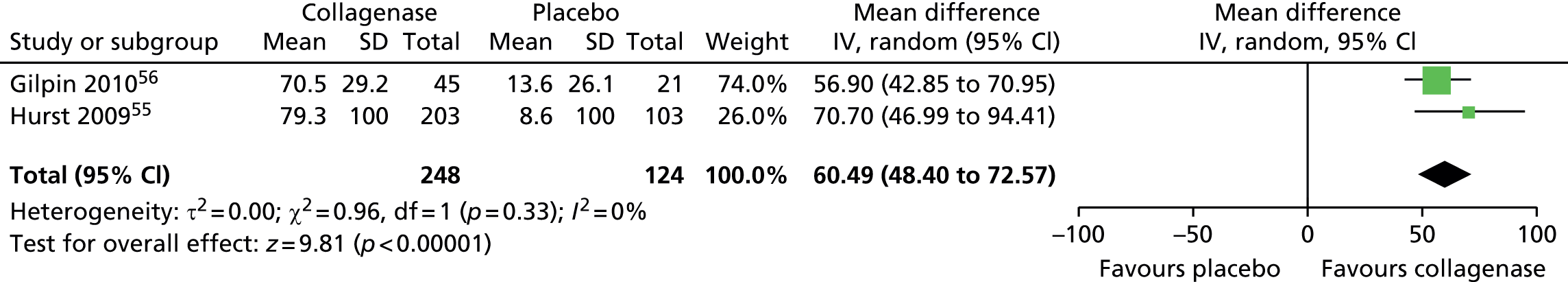
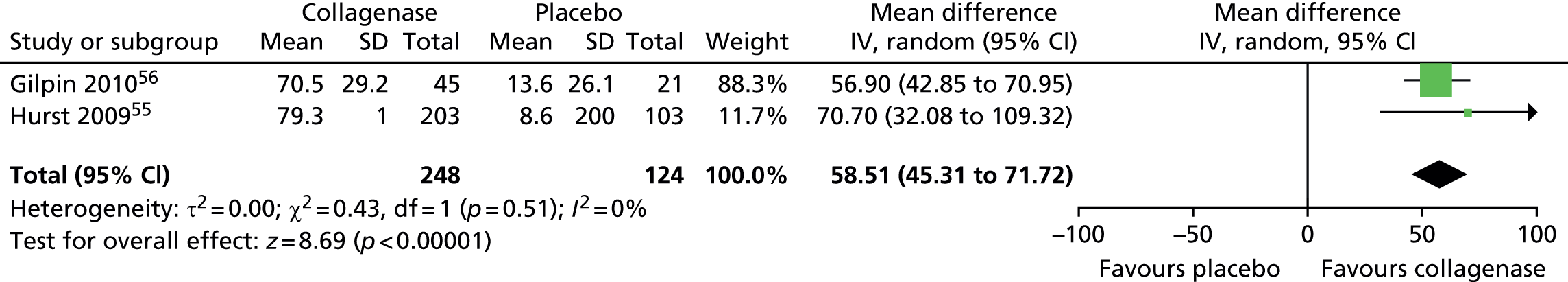
Standard deviation for ROM at 30 days after last injection was not reported in the CORD I trial and not available from the author;55 we used the corresponding SD from CORD II. 56 This approach is in line with the Cochrane Handbook recommendations. 58 In addition, we conducted sensitivity analyses in which we varied the SD for both groups with no change in the results of the analysis (see Appendix 14 for full details of sensitivity analyses). 55
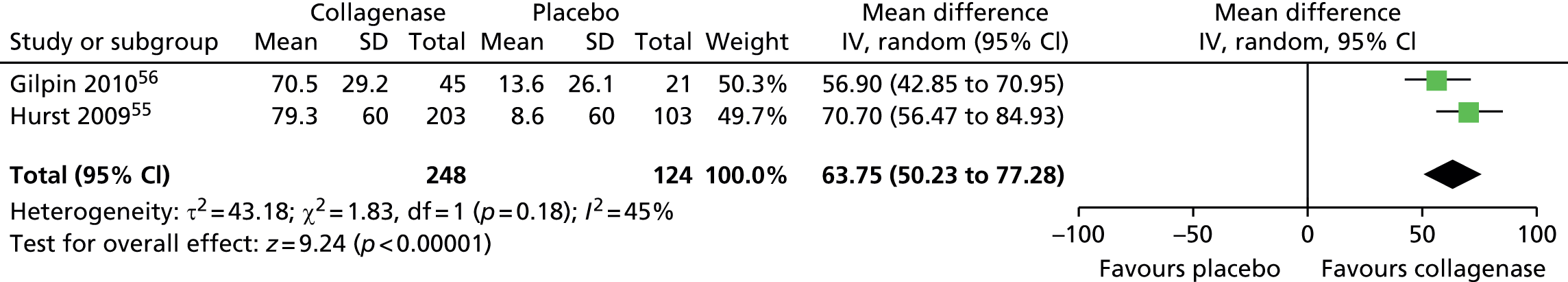
Figure 14 shows that the mean change in ROM from baseline was significantly greater for first joints treated with collagenase than for those treated with placebo (mean difference 31.84, 95% CI = 28.39 to 35.29; p-value < 0.00001).
FIGURE 14.
Forest plot of mean change in ROM from baseline for all first joints (using CORD II SD for both studies). df, degrees of freedom; IV, inverse variance; M–H, Mantel–Haenszel.
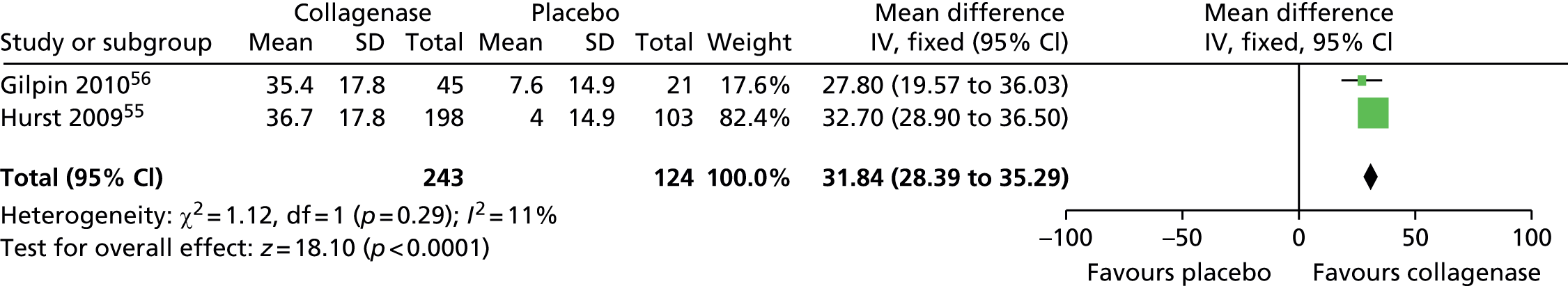
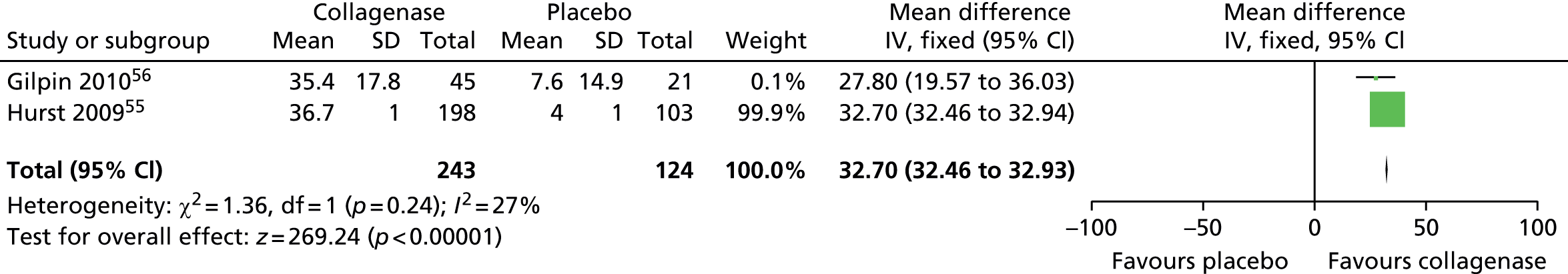
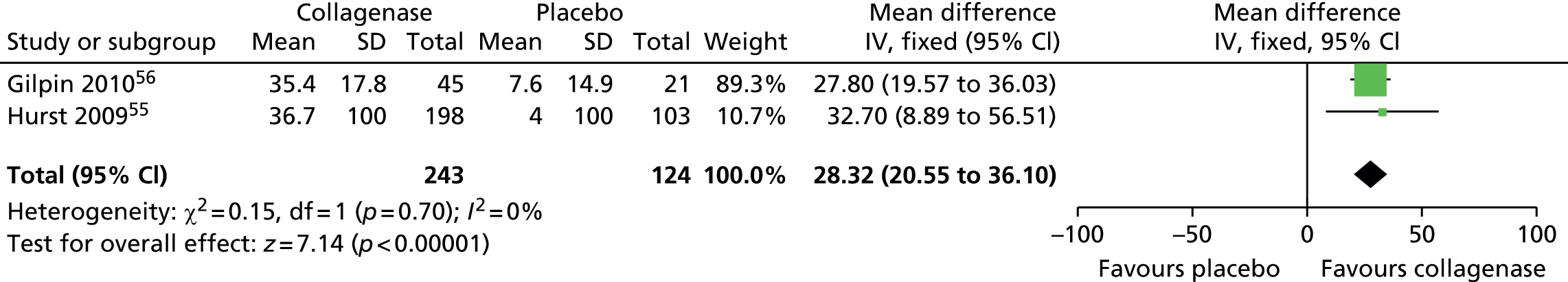
Mean change in first joint contracture from baseline
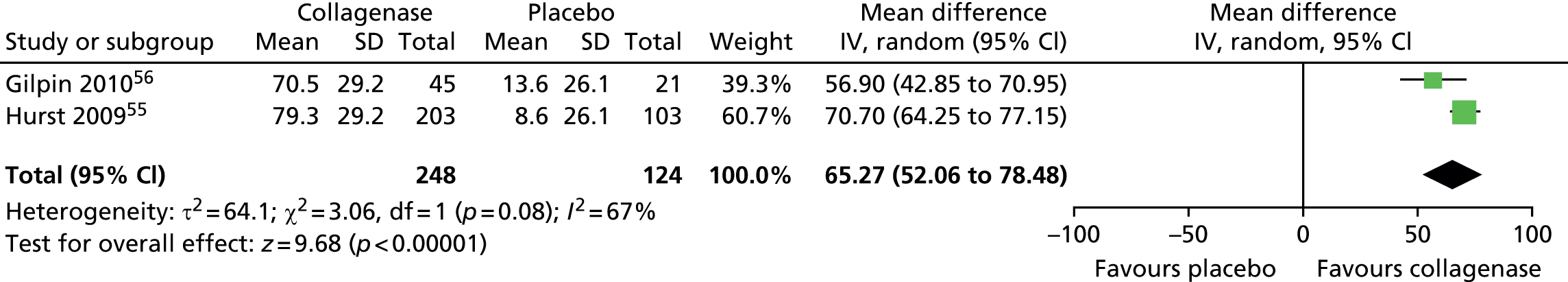
The CORD I trial did not report the SD for the mean change in contracture from baseline. Therefore, we used the corresponding value from the CORD II trial and performed sensitivity analyses, which showed no difference in results (see Appendix 14 for full details of sensitivity analyses). 56
Figure 15 shows that first joints treated with collagenase achieved a significantly greater change in contracture from baseline than joints treated with placebo (mean difference 65.27, 95% CI 52.06 to 78.48; p-value < 0.00001).
FIGURE 15.
Forest plot of mean change in first joint contracture from baseline (using CORD II SD for both studies). df, degrees of freedom; IV, inverse variance; M–H, Mantel–Haenszel.
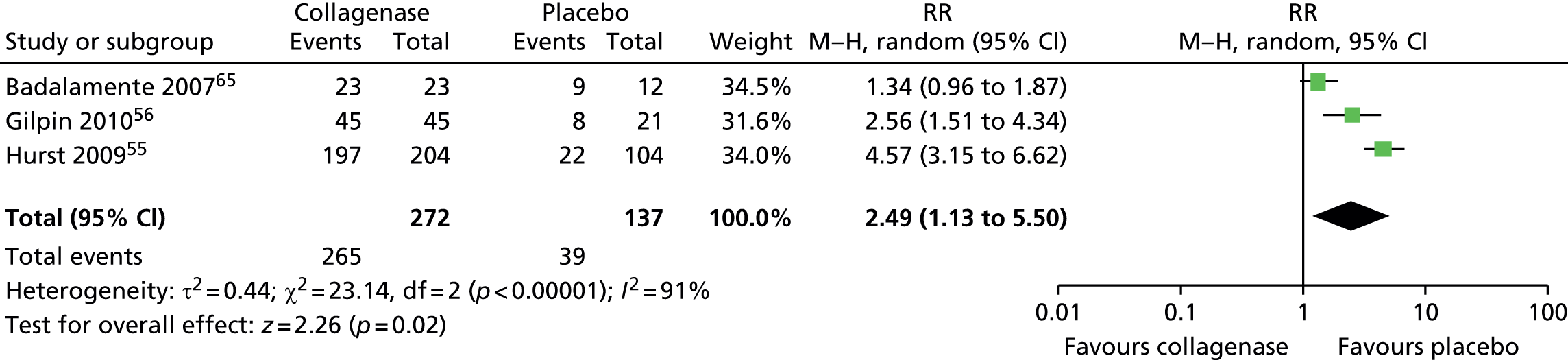
Adverse events: participants experiencing at least one adverse event
A total of 265 of 272 (97%) joints treated with collagenase experienced at least one adverse event compared with 39 of 137 (28%) joints treated with placebo (Figure 16). The difference between groups was significant (RR 2.49, 95% CI 1.13 to 5.50; p-value = 0.02).
FIGURE 16.
Forest plot of proportion of participants experiencing at least one adverse event. df, degrees of freedom; M–H, Mantel–Haenszel.
Adverse events: peripheral oedema
A total of 183 of 249 (73%) participants treated with collagenase and 6 of 125 (5%) of those treated with placebo experienced peripheral oedema (Figure 17). This difference between the groups was significant (RR 15.23, 95% CI 6.97 to 33.29; p-value < 0.00001).
FIGURE 17.
Forest plot of proportion of participants experiencing peripheral oedema. df, degrees of freedom; M–H, Mantel–Haenszel.

Adverse events: contusion
Figure 18 shows that significantly more participants treated with collagenase (137/249; 55%) experienced contusion than those (4/125; 3%) treated with the placebo (RR 14.09, 95% CI 4.20 to 47.30); p-value < 0.0001).
FIGURE 18.
Forest plot of proportion of participants experiencing contusion. df, degrees of freedom; IV, inverse variance; M–H, Mantel–Haenszel.

Adverse events: pain in extremity
A total of 88 of 249 (35%) participants treated with collagenase and 7 of 125 (6%) of those treated with placebo experienced pain in extremity (Figure 19). The difference between groups was statistically significant (RR 6.26, 95% CI 3.00 to 13.09; p-value < 0.00001).
FIGURE 19.
Forest plot of proportion of participants experiencing pain in extremity. df, degrees of freedom; M–H, Mantel–Haenszel.

Adverse events: injection site pain
A total of 106 of 272 (39%) participants treated with collagenase and 13 of 137 (9%) of those who received placebo suffered from injection site pain (Figure 20). The difference between groups was statistically significant (RR 3.49, 95% CI 1.48 to 8.27; p-value = 0.004).
FIGURE 20.
Forest plot of proportion of participants experiencing injection site pain. df, degrees of freedom; IV, inverse variance; M–H, Mantel–Haenszel.

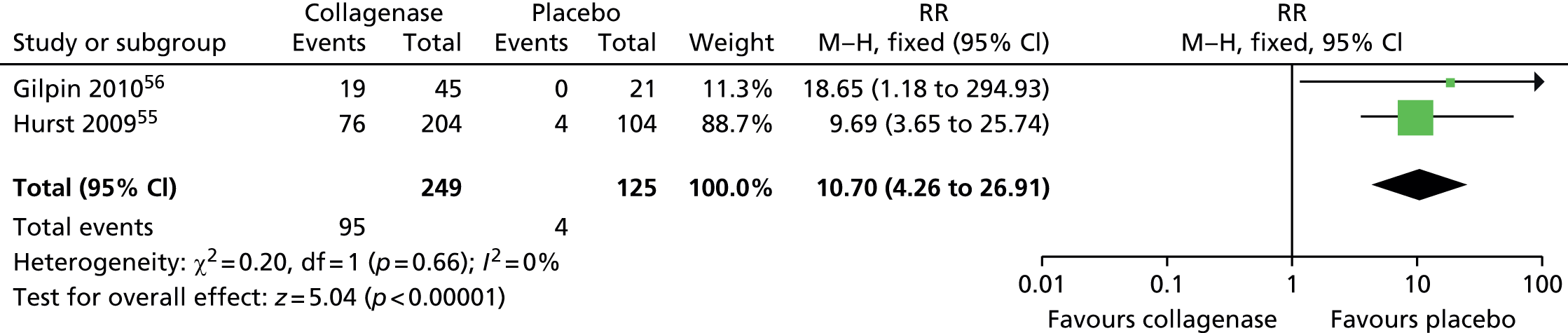
Adverse events: injection site haemorrhage
The risk of experiencing injection site haemorrhage was significantly higher for patients receiving collagenase than for patients receiving placebo (Figure 21). In total, 95/249 (38%) participants treated with collagenase and 4 of 125 (3%) of those who received placebo had an injection site haemorrhage (RR 10.70, 95% CI 4.26 to 26.91; p-value < 0.00001).
FIGURE 21.
Forest plot of proportion of participants experiencing injection site haemorrhage. df, degrees of freedom; M–H, Mantel–Haenszel.
Adverse events: injection site swelling
Figure 22 shows that the incidence of injection site swelling was significantly higher among participants treated with collagenase (59/249; 24%) than among those (7/125; 6%) treated with placebo (RR 4.18, 95% CI 1.98 to 8.81; p-value = 0.0002).
FIGURE 22.
Forest plot of proportion of participants experiencing injection site swelling. df, degrees of freedom; M–H, Mantel–Haenszel.
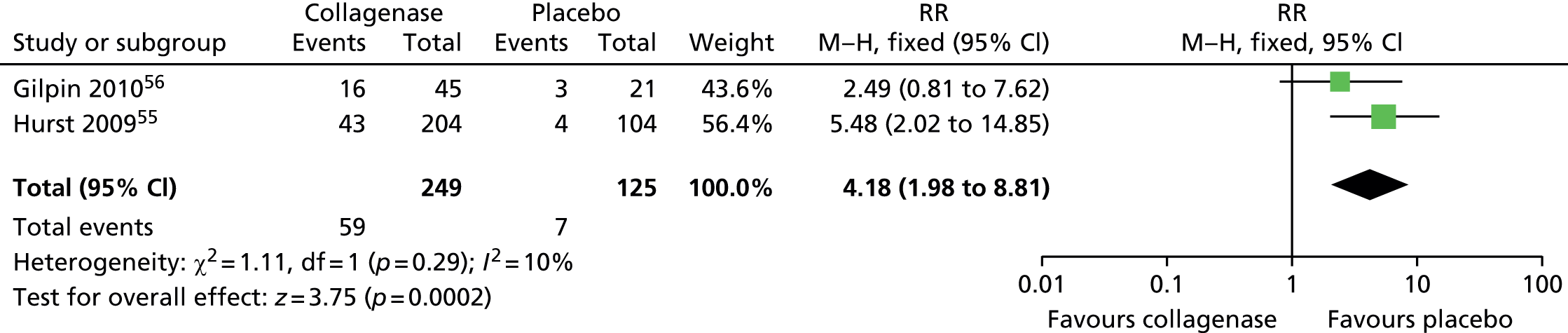
Adverse events: tenderness
Figure 23 demonstrates that the risk of experiencing tenderness was significantly greater among participants treated with collagenase (60/249; 24%) than among those (0/125; 0%) treated with placebo (RR 18.99, 95% CI 2.21 to 163.10; p-value = 0.007).
FIGURE 23.
Forest plot of proportion of participants experiencing tenderness. df, degrees of freedom; IV, inverse variance; M–H, Mantel–Haenszel.
It is worth noting that none of the participants treated with placebo subsequently experienced tenderness compared with almost one-quarter of those treated with collagenase.
Adverse events: pruritus
A total of 28 of 272 (10%) participants treated with collagenase and 1 of 137 (< 1%) treated with placebo experienced pruritus (Figure 24). The difference between groups was statistically significant (RR 7.35, 95% CI 1.77 to 30.43; p-value = 0.006).
FIGURE 24.
Forest plot of proportion of participants experiencing pruritus. df, degrees of freedom; M–H, Mantel–Haenszel.

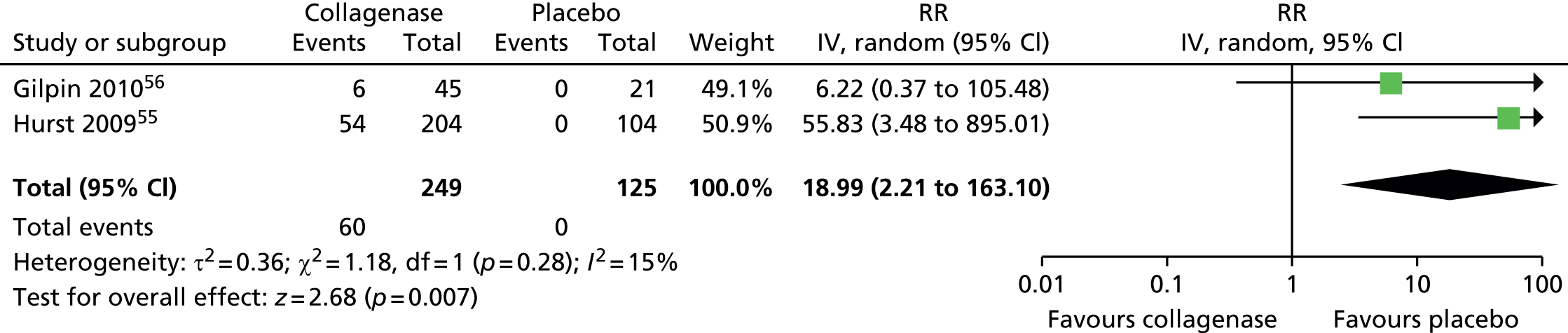
Adverse events: lymphadenopathy
The risk of lymphadenopathy was significantly higher after collagenase than after placebo (Figure 25). A total of 40 of 272 (15%) participants treated with collagenase and 0 of 137 (0%) of those treated with placebo experienced lymphadenopathy (RR 14.10, 95% CI 2.82 to 70.40; p-value = 0.001).
FIGURE 25.
Forest plot of proportion of participants experiencing lymphadenopathy. df, degrees of freedom; M–H, Mantel–Haenszel.
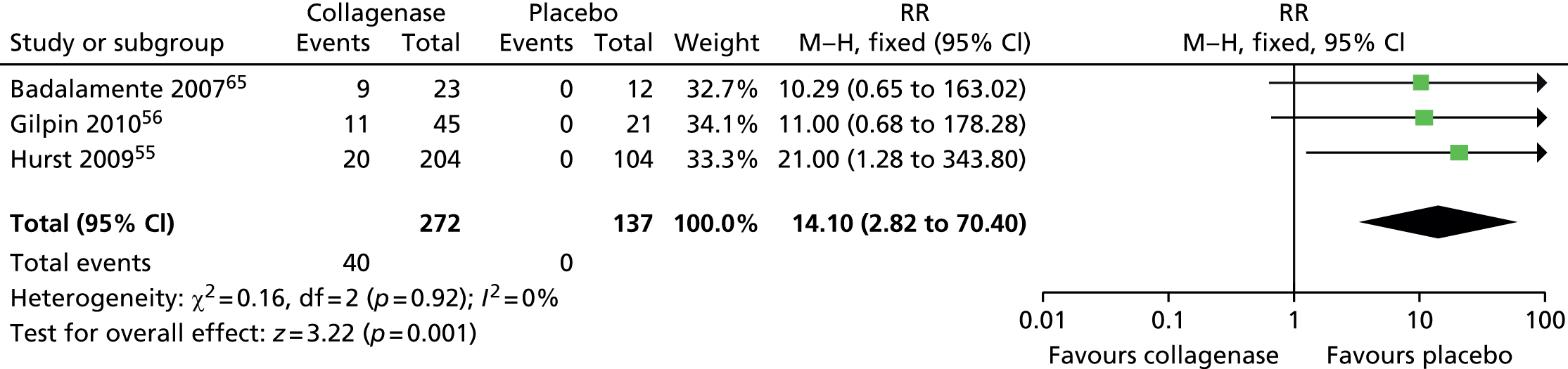
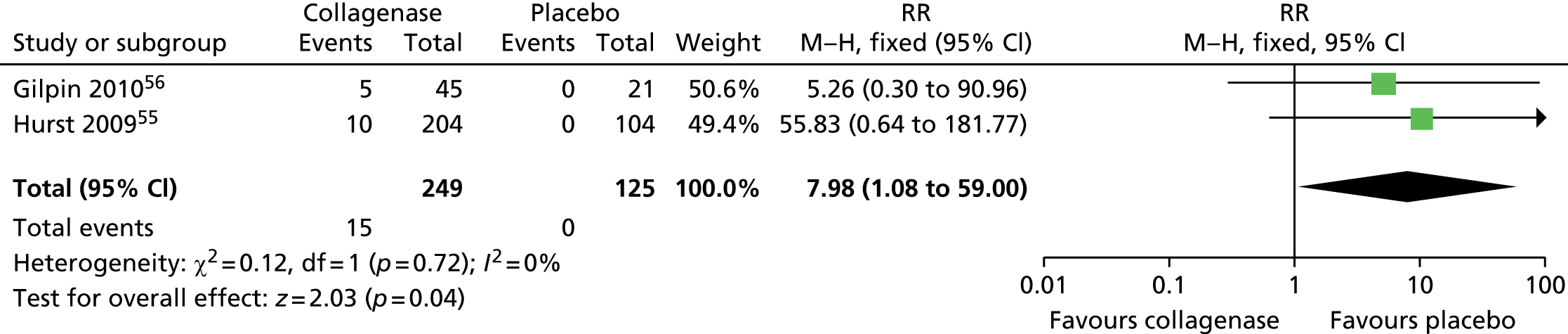
Adverse events: axillary pain
Few participants treated with collagenase (15/249; 6%) and none of those who received placebo (0/125; 0%) reported axillary pain (Figure 26). The difference between groups was statistically significant (RR 7.98, 95% CI 1.08 to 59.00; p-value = 0.04).
FIGURE 26.
Forest plot of proportion of participants experiencing axillary pain. df, degrees of freedom; M–H, Mantel–Haenszel.
Adverse events: injection site vesicles
The incidence of injection site vesicles was low and similar in both groups (Figure 27). Six of 249 (2%) participants treated with collagenase and 1/125 (< 1%) treated with placebo developed injection site vesicles (RR 2.16, 95% CI 0.37 to 12.55; p-value = 0.39).
FIGURE 27.
Forest plot of proportion of participants experiencing injection site vesicles. df, degrees of freedom; M–H, Mantel–Haenszel.

Serious adverse events
Four serious adverse events were reported among participants treated with collagenase: one case of complex regional pain syndrome and two cases of tendon rupture in CORD I,55 and one case of flexion pulley rupture in CORD II. 56
Appendix 15 presents a summary of outcomes, including adverse events, for all included collagenase studies.
Summary
Evidence for the assessment of the effects and safety of collagenase injections for the treatment of DC was derived primarily from two RCTs, CORD I and CORD II, with a total of 374 participants. 55,56 Data from a third RCT with a total of 35 participants65 were included in the analyses, where possible. Table 5 presents an overview of the meta-analyses findings.
| Event | RR (95% CI) | Test for overall effect (z-value; p-value) |
|---|---|---|
| Clinical success: all first jointsa | 10.21 (5.29 to 19.69) | 6.93; < 0.00001 |
| Clinical success: first MCP jointsa | 10.27 (4.88 to 21.65) | 6.13; < 0.00001 |
| Clinical success: first PIP jointsa | 7.44 (2.44 to 22.62) | 3.53; 0.0004 |
| Clinical improvement: all first joints | 6.90 (4.28 to 11.12) | 7.93; < 0.00001 |
| Clinical improvement: first MCP joints | 7.54 (4.21 to 13.49) | 6.81; < 0.00001 |
| Clinical improvement: first PIP joints | 5.85 (2.53 to 13.53) | 4.13; < 0.0001 |
| Mean change in ROM from baseline: all first joints | 31.84 (28.39 to 35.29) | 18.10; < 0.00001 |
| Mean change in contracture from baseline: All first joints | 65.27 (52.06 to 78.48) | 9.68; < 0.00001 |
| Adverse events | ||
| One or more adverse eventa | 2.49 (1.13 to 5.50) | 2.26; 0.02 |
| Peripheral oedema | 15.23 (6.97 to 33.29) | 6.83; < 0.00001 |
| Contusion | 14.09 (4.20 to 47.30) | 4.28; < 0.0001 |
| Pain in extremity | 6.26 (3.00 to 13.09) | 4.88; < 0.00001 |
| Injection site paina | 3.49 (1.48 to 8.27) | 2.85; 0.004 |
| Injection site haemorrhage | 10.70 (4.26 to 26.91) | 5.04; < 0.00001 |
| Injection site swelling | 4.18 (1.98 to 8.81) | 3.75; 0.0002 |
| Tenderness | 18.99 (2.21 to 163.10) | 2.68; 0.007 |
| Pruritusa | 7.35 (1.77 to 30.43) | 2.75; 0.006 |
| Lymphadenopathya | 14.10 (2.82 to 70.40) | 3.22; 0.001 |
| Axillary pain | 7.98 (1.08 to 59.00) | 2.03; 0.04 |
| Injection site vesicles | 2.16 (0.37 to 12.55) | 0.86; 0.39 |
Joints treated with collagenase were significantly more likely to achieve clinical success (i.e. a reduction of contracture to < 5° within 30 days after the last injection) and clinical improvement (i.e. reduction in contracture of 50% or more from baseline) than those treated with placebo, with MCP joints more likely to improve than PIP joints. Similarly, significantly more joints treated with collagenase showed a change in contracture and an increase in ROM from baseline than those treated with placebo. Nevertheless, joints treated with collagenase were significantly more likely to experience at least one treatment-related adverse event and a significantly higher number of adverse events than those treated with placebo, with the exception of injection site vesicles, which had an overall low incidence. Adverse events were generally mild or moderate, with the exception of four serious adverse events (one case of complex regional pain syndrome, two cases of tendon rupture and one case of flexion pulley rupture). The two largest trials (CORD I and CORD II)55,56 reported that none of the participants experienced a recurrence during the 90-day study period.
Summary of adverse events in collagenase case series studies
Two flexor tendon ruptures were reported in the 15 collagenase case series studies: Coleman et al. 79 reported a flexor tendon rupture 4 months after treatment and McMahon et al. 85 reported a flexor tendon rupture in a ‘small finger’ PIP joint. In addition, Coleman et al. reported a pulley rupture and a participant with hand pain requiring hospitalisation. 79 No other studies reported any treatment-related serious adverse events. Peimer et al. reported no new long-term or serious adverse events in the 3-year follow-up of the CORDLESS study. 87 Overall, 30% of CORDLESS participants experienced 370 mild or moderate adverse events.. Two studies did not report adverse events. 77,89 Another study reported that minor events but no major adverse events occurred, even though full details were not provided. 48 Five studies reported that all participants78,79 or the majority of participants (98%,81 87%,91 97%90 experienced at least one adverse event. Contusion was a frequently experienced adverse event, ranging from 60%90 to 100% of participants. 78 Reported rates of lymphadenopathy were generally low, ranging from < 1%86 to 7%90 of participants. Frequencies of other adverse events were more variable. Pruritus, for example, ranged from < 1%86 to 42% of participants. 78 Reported rates of peripheral oedema ranged between < 1%86 and 100% of participants. 78 Skin-related adverse events were also common. Skin tears ranged from < 1%86 to 85% of participants;80 injection site pain from 25%78 to 42%. 90
In summary, two collagenase case series reported a total of four serious adverse events: two flexor tendon ruptures, one pulley rupture and one episode of pain requiring hospitalisation. Mild or moderate adverse events were common, with some studies reporting that all participants experienced at least one adverse event. Common adverse events were contusion, peripheral oedema and skin-related events.
Overview of success and recurrence rates in all included studies
Table 6 shows the proportion of joints with a reduction of initial contracture and the proportion of those that experienced a recurrence for both RCTs and non-randomised comparative studies. A reliable comparison of findings among different procedures for the treatment of DC was hampered by major inconsistencies in reporting. Studies varied considerably in comparator procedures, duration and follow-up assessments and definition of end points (i.e. clinical success, clinical improvement and recurrence).
| Study | Outcome measure (for reduction of contracture) | Proportion (%) of participants achieving outcome measure | Recurrence rate (%) | Follow-up | ||
|---|---|---|---|---|---|---|
| MCP joints | PIP joints | MCP joints | PIP joints | |||
| Collagenase RCTs | ||||||
| Badalamente et al., 200264 | Reduction of contracture to 0–5° of normal (within 1 month) | 78 | 71 | 22 | 57 | 5 years |
| Badalamente and Hurst, 200563 | Joints achieving full finger extension (0°) | 92 | 75 | NR | NR | 1 year |
| Badalamente and Hurst, 200765 | Reduction in deformity to within 0–5° of normal within 30 days of last injection (up to three injections) | 86 | 100 | 3 (from both controlled and open-label phases) | 13 (from both controlled and open-label phases) | 1 year |
| Gilpin et al., 2010 (CORD II)56 | Reduction in deformity to within 0–5° of normal within 30 days of last injection (up to three injections) | 65 | 28 | 0 | 0 | 90 days |
| Hurst et al., 2009 (CORD I);55 (Witthaut et al., 2011)66 | Reduction in deformity to within 0–5° of normal within 30 days of last injection (up to three injections) | 76.7 | 40 | 0 | 0 | 90 days |
| Collagenase versus surgery non-randomised comparative studies | ||||||
| Naam 201371 | ||||||
| Collagenase | Mean change in degrees of contracture from baseline | –39.9 (SD 21.9) | –12.5 (SD 24.7) | 0 | 0 | Mean 32 months |
| Fasciectomy | –37.7 (SD 15.4) | –21.8 (SD 15.3) | 0 | 0 | Mean 39 months | |
| Nydick et al., 201372 | ||||||
| Collagenase | Mean reduction of contracture to 0–5° of normal (within 1 month) | 64 | 42 | NR | NR | 3–24 months |
| PNF | 81 | 50 | NR | NR | 3–28 months | |
| Surgery RCTs | ||||||
| Citron and Nunez 200567 | ||||||
| Fasciectomy (Z-plasty) | NR | NR | 18 | Minimum 2 years | ||
| Fasciectomy (modified Bruner technique) | NR | NR | 32 | |||
| Ullah et al., 200968 | ||||||
| Fasciectomy (Z-plasty) | Mean degree to which corrected | 0 | 6.3 (SEM 1.2) | 13 | 3 years | |
| Dermofasciectomy | 0 | 6.1 (SEM 1.1) | 15 | |||
| van Rijssen et al., 200669 (van Rijssen et al., 201270) | ||||||
| PNF | % of joints successfully treated (total passive extension deficit of 0–5°) | 55 | 26 | PNF: 84.9% of total hands treated LF: 20.9% of total hands treated |
6 weeks/5 years for recurrence | |
| LF | 94 | 47 | 21.8% of successfully treated joints 5.3% of successfully treated joints |
23.5% of successfully treated joints 5.3% of successfully treated joints |
||
| Surgery non-randomised comparative studies | ||||||
| Bainbridge et al., 201273 (Dias et al., 201344) | ||||||
| PNF/fasciotomy/ fasciectomy/dermofasciectomy/amputation | % participants with optimal Tubiana stage after surgery lower than pre-surgery | 96% | NR | 1 year | ||
| Citron and Hearnden 200374 | ||||||
| Fasciotomy (direct closure) | NR | N/A | 50 | N/A | Mean 2.2 years | |
| Fasciotomy (Z-plasty) | NR | N/A | 15 | N/A | ||
| Ribak et al., 201357 | ||||||
| PF | Proportion of participants with reduction of contracture | 23.8 | NR | NR | 1 year | |
| PNF | 33.3 | NR | NR | |||
| Skoff 200475 | ||||||
| Fasciectomy (synthesis technique) | Change in contracture from baseline to post-treatment, mean | 57–0° | 58–10° | 0 | Mean 2.7 years | |
| Fasciectomy (open-palm technique) | 50–0° | 40–6° | 50 | Mean 3.5 years | ||
| Toppi et al., 201476 | ||||||
| PNF | Immediate full correction reported | 36 | NR | NR | Mean 2 years | |
| Open fasciectomy | 33 | NR | NR | |||
Two non-randomised studies compared collagenase with surgery: fasciectomy in one case71 and PNF in the other. 72 In one study, the mean post-injection contracture among 25 patients treated with collagenase was 3.6° for MCP joints and 17.5° for PIP joints compared with 3.7° and 8.1°, respectively, among 21 patients who underwent fasciectomy. 71 Patients returned to normal activities, on average, 1.9 days after collagenase compared with 37.4 days after fasciectomy. In the study by Nydick et al. , clinical success (reduction of contracture within 0° to 5° of normal for both MCP and PIP joints) was achieved by 67% (35/50) of patients who underwent PNF and by 56% (19/34) of those treated with collagenase. 72 More people treated with collagenase experienced mild complications, such as oedema, ecchymosis, pruritus or lymphadenopathy, than those treated with PNF. By contrast, skin tears were observed more often in participants treated with PNF. It is worth noting that both studies were retrospective, of small sample size and at high risk of bias (e.g. no attempt to blind outcomes measurements; prognostic factors not compared at baseline; analyses not adjusted to take into account baseline differences).
Overall, comparative studies that assessed the effects of different surgical procedures showed greater clinical success for MCP joints compared with PIP joints, with slightly higher success rates for fasciectomy than fasciotomy. Recurrence rates ranged from 13% for fasciectomy to 85% for PNF among RCTs assessing different surgical interventions. It is worth noting that the 85% recurrence rate was reported by the study70 that assessed the effects and complications of PNF compared with LF. The van Rijssen et al. study defined recurrence as an increase of total passive extension deficit of at least 30° compared with the 6-week postoperative values in all treated hands. 70 However, if the definition of the CORD trials was applied (i.e. return of contracture of at least 20° in successfully treated joints), the 5-year recurrence rate for PNF would be 21.8% for MCP joints and 23.5% for PIP joints (see Table 6). van Rijssen et al. reported also the participants’ choice of treatment for recurrent disease. Four of nine participants who initially underwent LF chose to have their recurrence treated with PNF. Six participants (five with recurrence and one with extension) chose not to be treated. Twenty-six of 45 participants who experienced a recurrence after PNF chose to undergo a second treatment with PNF while seven participants chose LF and 12 did not opt for further treatment. Recurrence rates ranged from 0% to 50% for fasciectomy and 15% to 50% for open fasciotomy among non-randomised comparative studies, albeit various definitions of recurrence were used. Mild adverse events such as oedema, ecchymosis, skin tears, swelling, infection, haematoma, pain and delayed wound healing were reported more often after fasciectomy than PNF, fasciotomy or dermofasciectomy. 92
Appendix 16 presents a summary of the adverse events reported in the randomised and non-randomised comparative studies that assessed various surgical interventions for DC.
Table 7 presents the proportion of joints with a reduction of contracture and those with recurrences in the collagenase case series. Overall, mean baseline degree of contracture was greater for MCP joints than for PIP joints. In general, contractures tended to reduce with collagenase, with greater reductions observed in MCP joints than in PIP joints. Recurrence rates ranged from 0% at 1 year to 27% at 3 years for MCP joints and from 0% at 1 year to 56% at 3 years for PIP joints. Watt 2010 provided the longest published follow-up assessment after collagenase treatment in a series of eight patients (six MCP joints and two PIP joints). 89 Recurrence rates ranged from 0% (1 year) to 67% (8 years) for MCP joints and from 0% (1 year) to 100% (8 years) for PIP joints.
| Study | Reduction of contracture | Recurrence rate (%) | Follow-up | ||
|---|---|---|---|---|---|
| MCP joints | PIP joints | MCP joints | PIP joints | ||
| Collagenase case series | |||||
| Badalamente and Hurst, 200048 | 30/34 (88%) corrected to 0–5° of normal | 4/9 (44%) corrected to 0–5° of normal | 9 | 0 | 2 years |
| Badalamente and Hurst, 201177 | NR | NR | 13% | 34% | 2 years |
| Coleman et al., 201278 | After period 1 (single-dose CCH) – mean change in contracture from baseline | NR | NR | 30 days (period 1) | |
| 29 (SD 20.7) | 30.7 (SD 21.1) | ||||
| Coleman et al., 201479 | % change from baseline = mean 86 (SD 25) | % change from baseline = mean 66 (SD 28) | NR | NR | 60 days |
| Considine and Hirpara, 201380 | Change from mean 58.6° at baseline to mean 4.2° post treatment | Change from mean 39° at baseline to mean 9° post treatment | NR | NR | NR |
| Hayton et al., 201381 | NR | 28% PIP joint contractures spontaneously corrected after first CCH injection for MCP joint deformity | NR | NR | NR |
| Kaplan et al., 201383 | > 50% reduction 30 days after CCH injection (manipulation at 1 day) | NR | N/A | 90 days | |
| 92% | N/A | ||||
| Martin-Ferrero et al., 201384 | Change from 64° at baseline to 4° at 6 months | Change from 83.3° at baseline to 15° at 6 months | 0 | 0 | 1 year |
| McMahon et al., 201385 | Change from mean (SD) 51° (20) at baseline to 9° (15) at latest follow-up | Change from mean (SD) 39° (23) at baseline to 29° (20) at latest follow-up | 24 | 39 | Mean 15 months |
| Peimer et al., 201387 (CORDLESS); (Kaplan 2012)82 | Mean fixed flexion contracture at 3 years | 27 | 56 | 3 years | |
| Recurrent joints = 33; non-recurrent joints = 3 | Recurrent joints = 37; non-recurrent joints = 8 | ||||
| CORDLESS 5-year findings (from manufacturer’s submission)a | % participants with contracture of 0° to 5° at 30 days after last injection: | 46.7 | 5 years | ||
| 51% | 14.4% | ||||
| Peimer et al., 201386 | Full release of cord | NR | |||
| 75% | 48% | NR | NR | ||
| Skirven et al., 201388 | N/A | Change from mean 56° (range 40–80°) at baseline to mean 7° (range 0–35°) at 4 weeks | N/A | NR | 4 weeks |
| Syed et al., 201391 | Change from mean 41.8° (SD 9.7°) to mean 1.9° (SD 8.1°) | 0 | 0 | 1 year | |
| Watt et al., 201089 | Change from mean 57° at baseline to mean 22° at 8 years | Change from mean 45° at baseline to mean 60° at 8 years | 67 | 100 | 8 years |
| Witthaut et al., 201390 | Reduction in deformity to within 0–5° of normal within 30 days of last injection (up to three injections) | 4 | 9 months | ||
| 70% | 37% | ||||
CORDLESS study: findings at the 5-year follow-up
The manufacturer’s submission provides 5-year data for the CORDLESS study, an open-label study evaluating participants who were enrolled in previous collagenase clinical trials. Forty-seven per cent of the previously successfully treated joints (291/623) recurred and 16.9% (105/623) had further medical or surgical interventions by 5 years.
Comparison of surgical findings with existing literature reviews
Reliable comparisons of outcomes of different procedures for the treatment of DC are not straightforward owing to large inconsistencies in the way study outcomes are defined and reported. There are no standard methods for assessing the severity of contracture, defining complications and recurrences or determining the length of follow-up assessments. Study investigators tend to define criteria subjectively, hampering the possibility of any valid comparison of data. We considered findings from relevant reviews of the literature assessing the efficacy and/or safety of surgical procedures for DCs. These reviews were essentially narrative in nature. For each review, an overview of the main results is reported below.
Becker and Davis35 conducted a systematic review to evaluate the evidence on the effects of surgical treatments for primary Dupuytren’s disease. They identified 69 articles published up to 2009 (57 retrospective case series, seven prospective case series, two prospective comparative studies and three RCTs). Recurrence rates ranged from 0% to 71%. These rates are consistent with those reported in the studies included in this assessment, with the exception of the recurrence rate for PNF (85%) reported by van Rijssen et al. at 5 years,70 which was published after the Becker and Davis review. The authors pointed out the inconsistency in the definitions of ‘recurrence’ across their included studies, with some study investigators failing to provide a definition at all. Five of the six studies (randomised and non-randomised) that compared two surgical procedures were included in this assessment. 67–69,74,75 The remaining study did not meet our eligibility criteria (it was published prior to 1990). 93
Forty-three of the 69 studies included in the Becker and Davis review35 reported rates for specific complications: haematoma was reported in 35% of papers, nerve injury in 39%, complex regional pain syndrome in 32% and infection in 25%. These rates appear higher than those reported in our included studies. The study by van Rijssen et al. 69 did not observe any infection, haematoma or digital nerve injury among participants treated with PNF and few events (1/57 hands for each type of complication) among participants treated with LF. However, the authors reported only those participants who required treatment for specified complications and not all participants who experienced a complication. Citron and Nunez reported a total rate of 5% for digital nerve injury after fasciectomy,67 which is again substantially lower than the rate reported in Becker and Davis’ review. These differences may be explained by a number of factors, including differences in the comparator treatments, length of follow-up and definitions of complications and recurrences, making any comparison of data across studies challenging.
Brandt’s review evaluated evidence for surgical and non-surgical treatments for DC. 94 Level of evidence varied from high-quality RCTs to case-series and expert opinion. The author included three RCTs, one comparative study and three case series. The three RCTs68,69,74 and the non-randomised comparative study75 were also included in this assessment and therefore confirmed our findings. With regard to the case series, the author reported the findings of Beyermann et al. ,95 who suggested that people with severe PIP contractures not corrected by fasciectomy would benefit from capsuloligamentous release in which the flexor tendon sheath is cut, followed by sequential incision of further ligaments until the contracture is straightened; Roush and Stern,96 who retrospectively assessed three different procedures for recurrent severe DC; and Watson,97 who observed no recurrences at 2 years in a series of nine fasciotomies and suggested that this was probably a result of the release of skin tension.
Chen et al. ’s systematic review98 compared recurrence and complication rates following open PF, PNF and collagenase for DC. With regard to surgical interventions, they included six studies on PF (two RCTs and four retrospective case series) and three studies on PNF (one RCT and two retrospective case series). Recurrence rates after PF ranged from 0% to 39% (between 1.5 and 7.3 years) and are broadly in line with those of this assessment (from 0% at mean 39 months to 23% at 3.5 years). Recurrence rates after PNF ranged from 50% to 58% (between 6 weeks and 5 years). Only one RCT in our assessment, by van Rijssen et al. ,70 assessed recurrences after PNF and reported a rate of 85% at 5 years. It is worth noting that the only PNF clinical trial included in the Chen et al. review was van Rijssen et al. 69 (an earlier version of the 2012 publication), which did not provide numerical results for recurrences. The conclusion of the authors, that PNF had a higher recurrence rate than PF, is in accordance with the findings of this assessment.
The pattern of complications reported by Chen et al. 98 was rather distinctive, with nerve injury more often observed after PF than PNF (this information was not reported for collagenase studies). However, it is worth noting that complex regional pain syndrome was reported after PF, PNF and collagenase.
Crean et al. 34 conducted a structured review to assess the efficacy and safety of fasciectomy and fasciotomy for DC in cohorts of European patients. They included a total of 46 studies (published in 48 papers) describing a total of 57 treatment groups. The majority of the treatment groups (50/57) involved fasciectomies: 66% were LFs, 14% were dermofasciectomies, 6% were total fasciectomies and 12% involved mixed procedures. Seven treatment arms featured fasciotomies, of which 87% were PNF.
The majority of patients treated with fasciectomy (61–97%) achieved a ‘complete release of the contracture’ at up to 6 months after operation. In general, LF produced larger improvements for MCP joints (mean improvement 94%) than for PIP joints (mean improvement 66%). Similarly, MCP joints treated with fasciotomy showed a larger improvement (mean 80%) than PIP joints (mean 49%), and most of the fingers (median 73%) were fully corrected. Recurrence of disease occurred in 30% of patients treated with LF (mean time to recurrence 3.3–4.4 years), in 10% of those treated with dermofasciectomy (mean follow-up 3.5 years) and in 58% of those treated with PNF. Overall, these findings are consistent with those reported in this assessment (see Table 6).
Approximately one-quarter of fasciectomy patients and one-fifth of fasciotomy patients experienced an adverse event or complication. More patients treated with LF or dermofasciectomy experienced neurapraxia than those who underwent total fasciectomy or fasciotomy. Nearly half of people treated with dermofasciectomy suffered from a nerve injury.
Denkler’s review of the literature10 assessed the intraoperative and postoperative complications associated with fasciectomy for Dupuytren’s disease. They included 41 studies and summarised results separately for primary disease, recurrent disease and mixed disease. Complications in people with primary disease included digital nerve injury (0–7.7%), digital artery injury (0–2.6%), wound healing complications (0–86%), incisional scar pain (17.4%), dysaesthesia/paraesthesia (13.5%), hypoesthesia (10.1%) and flare reaction (2.1–51.5%), reflex sympathetic dystrophy (0–69.2%), infection (0–8.6%) and haematoma (0–13%). Complications in people with recurrent disease were reported in two small studies. Most common complications were hyperaesthesia (20%), hypoesthesia (15.8%) and necrosis (11.1%). For mixed populations (primary and recurrent disease combined), relevant complications were digital artery injuries (mean 3.6%, range 0.6–7.8%) digital nerve injuries (mean 3.6%, range 0.6–7.8%), tendon injuries (mean 0.2%), stiffness (mean 15.4%, range 1.6–51.5%), hypoesthesia (mean 14%), scar hypertrophy (mean 10%), haematoma (mean 1.8%, range 1.3–2.9%) and delayed wound healing complications (mean 1.2%). In our assessment, the most common complications reported in a large-scale comparative study, by Bainbridge et al. ,73 which featured fasciectomy and involved a mixed population, were haematoma (10%) and delayed wound healing (7%). In general, complications associated with fasciectomy are varied and with broad ranges of incidence. Differences in the rates of reported complications could be explained by the manner in which complications were assessed in the original studies (e.g. per finger, per hand, per patient), time of follow-up, and characteristics of patient population (e.g. age, severity of the disease).
Salhi et al. ’s review99 focused on the effects of PNF for the treatment of Dupuytren’s disease. The authors included eight cohort studies and one randomised trial. Surgical techniques and characteristics of patients varied across studies, as did the definition of recurrence and the threshold at which the decision to re-operate was taken. Overall, recurrences and reinterventions (mainly fasciectomy) were common, especially at longer follow-ups. Complication rates were generally low. The main reported complications were skin tears (27%), digital nerve paraesthesia (4.6%) and hypoesthesia (5.5%). Only two cases of serious complications were observed: complex regional pain syndrome (one case) and partial flexor tendon injury (one case). The van Rijssen et al. study69 was also included in this assessment. It was reported by van Rijssen et al. that both MCP (67%) and PIP (34%) joints showed improved extension at 1 week after treatment, with further improvement for MCP joints (75%), but not for PIP joints, at 6 weeks. The reported recurrence rate was 65% at 32 months. The subsequent van Rijssen et al. trial70 reported an overall recurrence rate of 85% at 5 years. These results are consistent with the overall findings of the Salhi review. The high recurrence rate reported by van Rijssen et al. may be explained by the longer follow-up. 70
Azzopardi and Boyce11 conducted a narrative review on surgical and non-surgical procedures for Dupuytren’s disease but failed to specify their inclusion criteria and did not provide details of the databases searched. The authors summarised the findings of three RCTs, four comparative studies and a previously published systematic review. 34 Five studies (including three RCTs) were also considered in this assessment67–69,74,75 and the remaining two studies were case series published before 1990. The authors concluded that the approaches used to close the wound may be an independent risk factor for recurrence, with direct closure of incision having higher recurrence rates than those performed by means of Bruner, Z-plasty and V-Y plasty closure techniques. It is worth noting that the Citron and Hearnden study,74 which prospectively compared direct closure of transverse palmar incision with a Z-plasty closure, reported recurrence rates of 50% and 15%, respectively, at a mean of 2.2 years after open fasciotomy.
In brief, many of the studies included in the published reviews were identified and considered in this assessment. Our findings are broadly consistent with those reported in the published literature. MCP joints tend to be more receptive to treatment than PIP joints, but recurrences are common. All surgical procedures carry the risk of adverse events, although most are minor or moderate. Inclusion criteria and demographic characteristics of patients were poorly or not reported in all reviews detailed above. Two reviews reported age and sex of included participants. Chen et al. 98 reported an age range of 55–65 years and a proportion of male participants ranging from 78% to 91% across studies. Crean et al. 34 reported a mean age of 60 years (range 48–74 years) and male to female ratio of 6 : 1. These characteristics are in line with those of the participants included in this assessment. Only two reviews attempted to assess the quality of included studies: Brandt94 used the Critical Appraisal Skills Program criteria and the American Society of Plastic Surgeons evidence rating scales; Chen et al. 98 adopted a hierarchical system (not validated) to grade the level of evidence across studies (from 1 = high-quality RCT or prospective study to 5 = expert opinion). Of the reviews that assessed rates of recurrence, none reported the definition of recurrence or the threshold at which re-operation was considered.
Table 8 presents a summary of the main reviews’ findings, where available.
| Outcomes | Current assessment | Becker and Davis, 201035 | Brandt 201094 | Chen et al., 201198 | Crean et al., 201134 | Denkler, 201010 | Salhi et al., 201199 | Azzopardi and Boyce, 201211 |
|---|---|---|---|---|---|---|---|---|
| Fasciectomy | NR | NR | Open PF | NR | NR | |||
| Reduction of contracture | 24–33% | NR | NR | NR | 61–97% had 100% correction; mean improvement in contracture angle = 58–79% | NR | NR | NR |
| Recurrence | 0–50% | NR | NR | 12–39% (at mean 1.5–7.3 years) | Mean 39% (median 4y) | NR | NR | NR |
| Adverse events | NR | NR | NR | 14–67% | 20% | Overall: 3.6–39.1% Major: 15.7% |
NR | NR |
| Fasciotomy | NR | NR | PNF | NR | PNF | NR | ||
| Reduction of contracture | 33–81% | NR | NR | NR | Mean improvement in contracture angle = 46–88% | NR | NR | NR |
| Recurrence | 15–85% | NR | NR | 50–58% (at mean 3–5 years) | Mean 62% (median 4 years) | NR | NR | NR |
| Adverse events | NR | NR | NR | NR | 20% | NR | NR | NR |
| Other | NR | Surgery: recurrence rates = 0–71% | NR | NR | N | NR | NR | NR |
Chapter 4 Assessment of cost-effectiveness
This chapter focuses on the cost-effectiveness of CCH versus alternative surgical treatments in adults with DC with a palpable cord. For comments on, and a critique of, the economic evaluation submitted to NICE by the manufacturer, see below (Comments on the economic evaluation submitted to NICE by the manufacturer); the Assessment of cost-effectiveness section independently assesses the cost-effectiveness evidence and includes a review of the cost-effectiveness literature and a de novo model of patients presenting with DC who are being considered as candidates for surgical intervention of CCH injections; the Results and Discussion sections present the discussion and conclusions of the economic analysis.
Comments on the economic evaluation submitted to the National Institute for Health and Care Excellence by the manufacturer
Cost-effectiveness evidence
The manufacturer conducted two literature reviews. The first was a pragmatic literature review to identify previous economic evaluations of CCH. They identified only two cost-effectiveness papers from the pragmatic literature search. The searches for economic evaluations were undertaken in PubMed and in the National Health Service Economic Evaluation Database (NHS EED). No further details, in terms of search methods, key words or dates, were provided. The second was a systematic literature review conducted initially in 2011 and subsequently updated in August 2013 (see section 5.2 of the submission). This review had two primary objectives: (1) to assess the short- and long-term efficacy and safety of treatments for DC; and (2) to determine the possibility of performing an indirect comparison between injectable CCH and surgical interventions. The review did not identify any studies drawing a comparison between injectable CCH and surgical interventions. It was, therefore, impossible for the manufacturer to compare directly current therapies. Thus, they opted to conduct a cost-minimisation analysis rather than a cost–utility analysis.
The two papers identified in the pragmatic literature search were authored by Chen et al. 100 and Baltzer and Binhammer101 and were published in 2011 and 2013, respectively. We also identified these studies as part of our systematic literature review of economic evidence. Further details of these studies are provided (see Systematic review of existing cost-effectiveness evidence). In brief, Chen et al. developed an expected-value decision-analysis model, with a 20-year time horizon, to examine the cost-effectiveness of open PF, PNF and CCH injection compared with no treatment in a US setting. 100 A societal perspective was adopted. Quality-of-life (utility) weights were estimated from a utility survey constructed by the authors and specific to people with DC. Baltzer and Binhammer assessed the cost-effectiveness of percutaneous needle aponeurotomy (PNA) versus PF versus injectable CCH in a Canadian setting. 101 A 15-year time horizon, expected-value decision-analysis model was developed for patients undergoing primary treatment for DC affecting a single finger. The analysis was conducted from a societal perspective and utility weights were derived from previously published literature. 100 Both Chen et al. and Baltzer and Binhammer identified PNF as the most cost-effective treatment strategy. Overall, similar conclusions were reported in both studies, in that a non-surgical approach to DC is preferred and the cost of CCH needs to reduce significantly if it is to offer a cost-effective alternative to surgery.
As highlighted by the manufacturer in their submission, these studies display a number of limitations. First, data were derived from different studies; however, no adjustment was conducted to account for the heterogeneity across studies, in particular for the differences related to patient characteristics. Second, a standard gamble approach to derive utility weights may not fully capture the utility implications, especially if respondents were not willing to trade an additional risk of death. Furthermore, these values were applied for the rest of the time horizon within the models. However, as DC is not life-threatening, it may be challenging to capture accurately differences in quality of life associated with different health states using this method of estimation (i.e. respondents may have found the choice task difficult as it requires them to weigh up life lived in a chronic health state with certainty against a risky alternative of either death or return to full health).
Critique of the manufacturer’s economic evaluation
The manufacturers submitted a de novo cost-minimisation analysis comparing CCH and fasciectomy. The adopted perspective of the analysis was direct NHS and Personal Social Services (PSS) costs. A 5-year time horizon was adopted, with costs discounted at 3.5% per annum. This analysis was conducted for patients suffering from moderate or severe disease with two or fewer joints affected in the same hand. The rationale for choosing this subset of patients was that CCH is more likely to be administered to patients with fewer joints affected. Nevertheless, for all patients within the CORD I and CORD II trials, the mean number of joints affected per patient treated with CCH was 3 and 3.4, respectively. Furthermore, the manufacturer’s analyses were restricted to fasciectomy, as PNF was not judged to be a suitable comparator. Table 9 shows the findings of the comparison between the manufacturer’s economic submission and the NICE reference case. 102
| Element of health technology assessment | Reference case | Does the de novo economic evaluation match the reference case |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | No, the NICE final scope stated that a cost–utility analysis should be conducted |
| Comparator(s) | As listed in the scope developed by NICE | No, the NICE final scope specified any surgical treatments including the minimally invasive PNF |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, carers | No/not applicable, the manufacturer conducted a cost-minimisation analysis only |
| Perspective on costs | NHS and PSS | Yes |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | No/not applicable, a cost-minimisation analysis was conducted |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | 5 years |
| Synthesis of evidence on health effects | Based on systematic review | No |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of health-related quality of life in adults | No |
| Source of data for measurement of health-related quality of life | Reported directly by patients and/or carers | No |
| Source of preference data for valuation of changes in health-related quality of life | Representative sample of the UK population | No |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | QALYs were not included in the analysis |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | Yes |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | Yes, costs are discounted at an annual of 3.5%. There was no assessment of health effects within the model |
Model structure
The manufacturer’s model structure adopts an annual cycle length. There are two phases in the model structure: initial treatment and recurrent disease. The patient enters the model and is treated with either CCH or fasciectomy (Figure 28). If two joints are affected in the CCH arm, a first joint is selected and treatment begins on this joint. Fasciectomy occurs in the initial treatment only, as a number of joints can be treated at one time. In the CCH arm, patients could receive a maximum of three cycles of treatment. If, after either strategy, the treatment was unsuccessful, it was considered a treatment failure and the patient accumulated no further costs. This is a potentially problematic assumption if the success rates between treatment options vary, and if a patient would go on to have further treatment if initial treatment failed. There is evidence that patients do indeed receive second-line treatment (Mr David Lawrie, Woodend Hospital, 2014, personal communication), and the model we have developed for this assessment takes this into account. If the strategies were successful, patients entered a treatment success health state where they incurred a probability of recurrence. Success rates were assumed equivalent for both strategies. This is a further problematic assumption; there is no evidence from non-inferiority RCTs to support the assumption of equivalence and, therefore, the method of cost-minimisation analysis is not supported by existing evidence. Moreover, the model could potentially bias the results favouring the strategy with a higher proportion of treatment failures. If the patient experienced recurrence, they were eligible for further treatment either in the form of fasciectomy or CCH, with the manufacturer assuming an equal distribution of treatments following recurrence between the two arms.
FIGURE 28.
The model structure. Source: manufacturer’s submission.

Population
Patient characteristics are drawn from CORD I and CORD II, two prospective, randomised, placebo-controlled trials with 90-day double-blind follow-up. CORD I enrolled 308 patients with a mean total contracture index of 149.1 in the CCH arm and 149.3 in the placebo arm. The CORD II study enrolled 66 patients with a mean total contracture index of 174.7 in the CCH arm and 150.1 in the placebo arm. The primary outcome for both studies was reduction of contracture to 0–5°, 30 days after the last injection was administered. The manufacturer’s model included patients with moderate (37.91%) and severe (62.09%) disease, with a maximum of two joints affected in the same hand. This is a subgroup of patients included in the CORD I and CORD II studies.
Intervention and comparators
The manufacturer’s submission focused exclusively on fasciectomy as the relevant comparator, deviating from the NICE final scope. The BSSH recommend PNF as an alternative to surgery for patients with moderate disease. 37 Advice from our clinical expert indicates that needle fasciotomy is an appropriate starting point for the treatment of Dupuytren’s disease and is currently used in clinical practice in the UK (Mr David Lawrie, personal communication). The economic analyses identified by the manufacturer in their literature review, have, despite their methodological limitations, identified PNF as the most cost-effective treatment strategy. Considering that the manufacturer’s analysis focused on patients with both moderate and severe diseases, PNF might have been an appropriate comparator.
Resource identification, measurement and valuation
The costs included in the manufacturer’s submission are provided in Tables 10 and 11. The model includes 1.6 injections of CCH per affected joint. This is the mean number of injections administered per joint from the post hoc analysis of the CORD I and CORD II studies and assumes that 1.4 joints are treated. Physiotherapy costs have not been included in the cost calculation of CCH. One session of physiotherapy is usually required following treatment with CCH. The manufacturer has also costed a total of 9.4 hand therapy appointments after fasciectomy. This appears to be an overestimation. Advice from our clinical expert indicates that the average number of follow-up appointments after fasciectomy is five. Table 10 provides a summary of the CCH costs used in the manufacturer’s submission. Table 11 shows the comparable costs used for the assessment of fasciectomy.
| Category | Description | Reference cost code | Value, £ | Justification |
|---|---|---|---|---|
| Administration visit | Visit at which CCH is administered | HB56C (outpatient procedure, trauma and orthopaedics) | 225 | CCH is administered in an outpatient setting and is a minor procedure |
| CCH | Cost per 0.9-mg vial | N/A | 650 | Manufacturer submission |
| Finger manipulation | Additional visit following CCH administration at which the finger is manipulated | 110 (Consultant-led: follow-up attendance non-admitted face to face, trauma and orthopaedics) | 102 | Finger manipulation may take place during a consultant visit |
| Splint | The cost of the splint provided post finger manipulation | N/A | 10 | Based on internet search of average hand splint costs |
| Category | Description | Reference cost code(s) | Value | Justification |
|---|---|---|---|---|
| Palmar day-case cost | The cost of undergoing palmar fasciectomy as a day-case | HB53Z, HB52C, HB52B (day-case) | 1757, 1975 1863 | Gerber showed that the OPCS code for palmar fasciectomy (T521) contained the three NHS reference cost codes given. In the model an average was used |
| Palmar inpatient cost | The cost of undergoing palmar fasciectomy as an inpatient | HB53Z, HB52C, HB52B (elective inpatient) | 2553, 3060, 3240 | Gerber showed that the OPCS code for palmar fasciectomy (T521) contained the three NHS reference cost codes given. In the model an average was used |
| Digital day-case cost | The cost of undergoing digital fasciectomy as a day-case | HB51Z, HB99Z (day-case) | 1917, 1400 | Gerber showed that the OPCS code for digital fasciectomy (T525) contained the two codes given. In the model an average was used |
| Digital inpatient cost | The cost of undergoing digital fasciectomy as an inpatient | HB51Z, HB99Z (elective inpatient) | 2997, 3532 | Gerber showed that the OPCS code for digital fasciectomy (T525) contained the two codes given. In the model an average was used |
| Follow-up visit | Post-surgery follow-up visit | 110 (follow-up attendance) | 102 | DC is a musculoskeletal disease but is not life-threatening |
| Hand therapy cost | Post-surgery hand rehabilitation | WF01 A (physiotherapy) (outpatient) WF01 A (occupational therapy) (outpatient) | 40.46 | Hand therapy may be administered by a physiotherapist or an occupational therapist |
Perspective, time horizon and discounting
For costs, the perspective adopted was that of the NHS and PSS. As the chosen analysis was a cost-minimisation, health benefits were not included. The time horizon was 5 years, which mirrors the length of follow-up of the CORDLESS study. Costs were discounted at an annual rate of 3.5%.
Treatment effectiveness
The overall success rate (63.9%) was assumed equivalent between the CCH and fasciectomy arms. Patients who experienced a successful treatment entered a successfully treated health state, where they incurred a probability of recurrence. Patients who experienced a treatment failure dropped out of the model and incurred no further costs. Essentially, this means that patients successfully treated could, in theory, accumulate higher costs than those for whom treatment failed, and it is questionable whether this may reflect current clinical practice. The majority of recurrences were treated with fasciectomy (61.9%) and the rest (38.1%) with CCH. The distribution of treatments following recurrence was the same for both arms (CCH and fasciectomy).
The total recurrence costs over a 5-year time frame equated to £102. To achieve this figure, the manufacturer multiplied the success rate by the recurrence rate by the proportion treated for recurrent disease. This figure was subsequently multiplied by the unit cost of the procedure. There is a potential risk of double counting in this calculation (i.e. multiplication of the recurrence rate by the percentage of patients treated for a recurrence), which may underestimate the number of patients who are retreated.
Results
The base-case results (Table 12) identify CCH as cost saving by an amount of £943, compared with fasciectomy. The base-case analysis was based on an average of 1.4 joints treated. An identical cost of recurrence was applied for both treatments and thus the main driver in the observed difference in cost was the cost of surgery.
| CCH, £ | Fasciectomy, £ | Difference | ||
|---|---|---|---|---|
| Total | 1841 | Total | 2784 | 943 |
| Initial Tx cost | 1739 | Initial Tx cost | 2682 | 943 |
| Outpatient visits | 756 | Surgery cost | 2200 | |
| Drug cost | 969 | Surgeon follow-up cost | 102 | |
| Splint cost | 14 | Hand therapist cost | 380 | |
| Total recurrence cost | 102 | Total recurrence cost | 102 | 0 |
| Year 2 | 40 | Year 2 | 40 | |
| Year 3 | 36 | Year 3 | 36 | |
| Year 4 | 16 | Year 4 | 16 | |
| Year 5 | 9 | Year 5 | 9 | |
Sensitivity analysis
The manufacturer undertook a univariate sensitivity analysis to illustrate the impact on results of altering key model parameters. The number of CCH injections administered was the largest overall driver in the cost difference. However, when the maximum number of injections was administered, CCH remained cost-saving. The second largest driver of the cost difference was the proportion of patients receiving fasciectomy as inpatients; in the base-case analysis, 37.8% of patients were assumed to be inpatients. This assumption was taken from Gerber et al. ,6 who found that inpatient admission rates decreased from 58% in 2003–4 to 38% in 2007–8. The manufacturer demonstrated that the more patients treated in an outpatient setting, the less the cost saving experienced when comparing fasciectomy with CCH. Nonetheless, in the scenario in which 0% of patients received fasciectomy in an inpatient setting, CCH treatment still remained cost saving relative to fasciectomy. The manufacturer also included a scenario in which waste was included in the analysis; this occurs when a vial is opened but not entirely used and so must be discarded. Finally, a scenario was included in which the impact of 0% of patients receiving hand therapy was assessed. In all scenarios presented in sensitivity analyses, the manufacturer found CCH to be cost saving (Figure 29).
FIGURE 29.
Tornado diagram showing cost impact of varying model assumptions. Source: manufacturer’s submission. Ba, base; Hi, high; Lo, low.

Uncertainties related to the manufacturer’s submission
There are three main limitations in the case submitted by the manufacturer. First, there is an assumption that the success and recurrence rates between the treatment strategies are equivalent. However, equivalence, defined usually in terms of non-inferiority, has yet to be conclusively demonstrated (e.g. no RCTs comparing CCH vs. different forms of surgery have been conducted). Thus, at present, a cost-minimisation analysis may not be a valid approach to estimate cost-effectiveness, as it is entirely possible that the assumption of equivalence is not valid and that recurrence and success rates vary between CCH and fasciectomy.
Second, PNF is a viable alternative to both fasciectomy and CCH for patients with moderate disease. By excluding this relevant comparator from the cost-minimisation analysis, the manufacturer has deviated from the NICE final scope. This omission is far from negligible, as PNF is potentially a less expensive treatment option6 in terms of quantity and type of resources used at the point of delivery.
Third, there are concerns relating to some of the costing assumptions made by the manufacturer, namely (1) including 9.4 follow-up appointments for hand therapy following fasciectomy but 0 following the CCH treatment strategy; (2) assuming treatment failures would not incur any further costs; and (3) the possibility that recurrence costs may have been underestimated.
Assessment of cost-effectiveness
This section presents (1) the results of a systematic review of the cost-effectiveness evidence; and (2) the structure and results of a de novo, independent economic model developed for the purpose of this assessment.
Systematic review of existing cost-effectiveness evidence
In addition to the literature review and cost-minimisation analysis prepared by the manufacturer, we conducted a comprehensive, structured review of the literature to identify relevant cost-effectiveness studies comparing the different interventions for DC.
A formal systematic review of existing economic evaluations relating to CCH injections or surgical treatments for adults with DC was performed. Highly sensitive search strategies were designed using appropriate combinations of both controlled vocabulary and text terms. The following databases were searched without language restrictions from 1995 onwards: NHS EED, HTA Database, MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Science Citation Index and the Health Management Information Consortium (HMIC) database. Two previous cost-effectiveness studies were retrieved and were appraised against the British Medical Journal checklist for referees of economic analyses. The main findings of these studies are summarised in a narrative way and tabulated for comparison.
Chen et al.100
This study in the USA constructed an expected-value decision-analysis model, with a 20-year time horizon, to examine the cost-effectiveness of open PF, PNA and CCH injection versus no treatment. The model assumed an average age of presentation for treatment of 63 years. Utility scores were obtained for patients suffering from DC in the small and ring fingers of the dominant hand. It is not explicitly stated whether patients were receiving primary or secondary treatment, although we can assume from model input parameters incorporated that it was the patients’ first line of treatment. A societal perspective was adopted and quality-adjusted life-year (QALY) (utility) weights were estimated from a utility survey (standard gamble) constructed by the authors, specific to people living with DC. The results illustrated that adopting a willingness-to-pay (WTP) threshold of US$50,000 per QALY gained, open PF was not cost-effective; PNA was cost-effective only when the success rates were high or when it was performed in an outpatient setting; and CCH was considered cost-effective only if the cost of a course of treatment significantly reduces. The authors reported that at current market price (US$5400 per average course of treatment), the cost per QALY gained of CCH was US$166,268. However, if the price reduced to US$945 for the entire series of treatment, this strategy was found to produce a favourable cost per QALY (US$49,995). A key strength of this study is that utility weights were directly elicited, using a standard gamble method, from the general population and were therefore specific to the decision model. However, it is not clear that all other model parameters (e.g. recurrence rates) incorporated in the model were exclusive of the patient subgroup experiencing DC in the small and ring finger of the dominant hand.
Baltzer and Binhammer101
This study in Canada assessed the cost-effectiveness of PNA versus injectable CCH versus PF. A 15-year time horizon, expected-value decision-analysis model was constructed for patients undergoing primary treatment for DC affecting a single finger. The analysis was conducted from a societal perspective and utility weights were derived from previously published literature (see Chen et al. 100). Results indicated that PF was dominated, as it was both more costly and less effective than the other treatment strategies. PNA was found to be the least costly option; injectable CCH produced an incremental cost-effectiveness ratio (ICER) of CA$284,383 per QALY gained, over PNA. The authors concluded that PNA was the preferred option to treat DC affecting a single finger. It was highlighted that if the current market price of CCH reduced significantly, it could offer a cost-effective option. A number of one-way sensitivity analyses were conducted, which illustrated the most sensitive variables in the model. This model was analysed for patients receiving treatment on a single finger and, therefore, the results may not be generalisable to patients with multiple fingers affected.
Summary of findings from identified studies
This literature review demonstrates that the potential cost-effectiveness of CCH is largely dependent on the cost of a series of CCH treatment. Chen et al. 100 stated that the entire CCH injection series of three injections would need to reduce to US$945 to offer a favourable cost per QALY. Baltzer and Binhammer101 drew a similar conclusion, although the price of CCH would need to be further reduced to CA$875 for a series of treatment to be considered cost-effective. It should be noted that a substantial variation in the cost per QALY of CCH was reported by both studies. Baltzer and Binhammer101 reported including patient-incurred costs in their analysis, whereas Chen et al. did not. 100 Furthermore, it is not clear if Chen et al. included the cost of treatment after recurrence or complications in their analysis. 100 Both studies appear to have derived the QALY values by simple multiplication of the health-state values derived by the time perspective of the model, with no further adjustment for probability of further treatment following recurrences and failures and subsequent incorporation of quality-of-life impacts (and changes in cost).
This approach does not fully account for changes in health states, which would be expected to occur over the time frame adopted. Also, the standard gamble method may not be able to account fully for the effect of differences in health state on utility, if respondents were not prepared to accept an additional risk of death in order to receive a greater probability of living in better health states.
Information regarding the Chen et al. 100 model is not fully reported in the paper and so it is difficult to understand properly the model structure and pathways. Baltzer and Binhammer illustrated the algorithm of the decision-analysis and discussed their model structure in more detail. 101 A summary of probabilities for the failure of treatment strategies and the complication development were tabulated. The main contrast between these studies is the manner in which the cost-effectiveness analysis was conducted: Chen et al. compared all treatment strategies to a status quo (no treatment), whereas Baltzer and Binhammer ranked strategies in order of increasing costs, with the least costly strategy becoming the first comparator in their analysis. 100,101
Furthermore, both studies incorporated different model time-frames. However, as a common starting age of 63 years was assumed in both models, with an average life-expectancy of 78 years, differing model time horizons should not account for any differences experienced in results. It is also important to note that, although univariate sensitivity analyses were conducted for both studies, neither study conducted a probabilistic sensitivity analysis to test the robustness of results to simultaneous changes in model parameters. It is widely accepted that to address uncertainty accurately within a model, both deterministic and probabilistic sensitivity analyses should be conducted. 102
Overall, similar conclusions were reported as both studies found that, firstly, a non-surgical approach to Dupuytren’s is preferred and, secondly, the cost of CCH needs to significantly reduce if it is to offer a cost-effective alternative to treatment. However, given the specificity of these studies to a US and Canadian health system in terms of quality-of-life valuation and costing technique, it is not clear if these results can be generalised to a UK setting. Table 13 provides a tabulated summary of the retrieved studies from the review. Our team’s searches did not identify any further cost-effectiveness studies in addition to those described in the manufacturer’s submission.
| Author | Country | Study design | Cohort | Intervention/comparators | Study perspective | Time frame | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Chen et al., 2011100 | USA | Cost–utility analysis: expected-value decision analysis | Cohort aged 63 years | Compared traditional PNF, PNA and CCH injection vs. no treatment | Societal | 20 years | Open PF cost $820,114 per QALY gained | Needle aponeurotomy is cost-effective if the success rate is high |
| Needle aponeurotomy cost $96,474 per QALY gained | Open PF is not cost-effective | |||||||
| Injectable CCH cost $166,268 per QALY gained | CCH could only be cost-effective if it costs significantly less | |||||||
| Baltzer and Binhammer, 2013101 | Canada | Cost–utility analysis: expected-value decision analysis | Males aged 63 years undergoing primary treatment in a single finger | PNA vs. PF vs. injectable CCH | Societal | 15 years | Injectable CCH cost $284,383 per QALY gained over PNA | PNA is the preferred option in terms of cost-effectiveness |
| PF is not cost-effective | ||||||||
| The PF strategy was dominated, more costly and less effective | CCH could only be deemed cost-effective if the price of treatment significantly reduces |
Economic assessment
Given the limitations of existing evidence to suggest equal effectiveness and, following the NICE scope and protocol, we developed a de novo decision analytical model to assess the cost–utility of CCH compared with PNF or LF in adults with DC.
Methods
A cost–utility Markov model was developed using TreeAge Pro (TreeAge Software, Inc., Williamstown, MA, USA, 2014). The alternative pathways were embedded in the Markov model simulating the downstream impact of treatment on recurrence and health-related quality of life. A Markov model considers patients in a discrete health state and allows the consequences of treatment strategies in terms of recurrence rates, health-related quality of life and costs to be captured for a particular patient population, over the adopted time horizon.
Costs incorporated in the model included those associated with treatment, complications and further treatment following a possible recurrence. Health-state utilities associated with pre and post treatment were incorporated in the model to calculate QALYs. Strategies were ranked incrementally in terms of their costs and ICER was calculated, applying a ceiling ratio of £20,000 per QALY. 102 Uncertainty was assessed by conducting deterministic and probabilistic sensitivity analyses. For the latter, the strategy with the highest net monetary benefit (NMB) was identified using the formula:
where NMB is the NMB of the strategy, E is the mean effect (in terms of QALYs), rc represents the decision-makers’ maximum WTP for a QALY and C is the mean cost of the strategy. A value of £20,000 was applied for rc.
Relevant patient population
The modelled cohort consisted of patients presenting with moderate or severe DC. Focus was on moderate and severe disease, as no surgical trials were identified that considered patients with mild disease only. Furthermore, BSSH guidelines do not recommend treatment for mild disease. 37 Although future updated guidelines are not expected to deviate from current recommendations not to provide treatment for mild disease, there is uncertainty regarding the extent to which these guidelines are implemented in clinical practice across the UK. For example, at the scoping workshop, consultees stated that CCH may be used to treat mild contracture in some cases. Thus, the decision problem is examining the value of CCH among patients who are considered suitable candidates for surgery. For the purposes of the model, the definition of patients who are eligible for surgery is derived from that of van Rijssen et al. ,70 who define the surgical cohort as ‘patients with total passive extension deficit of at least 30 degrees in any ray, the existence of a clearly defined palmar cord, and willingness to be considered for surgery’. The model is built on three key studies of effectiveness, described earlier in the systematic review, which identified one suitable head-to-head trial of LF versus PNF,70 in addition to the two placebo controlled randomised trials of CCH (CORD I and CORD II). 56,63–65 The mean age of the patients within these studies was 63 years, with a sex distribution of 84% male and 16% female; this was, therefore, the starting age and sex distribution of the modelled cohort. Within these trial populations, the mean number of joints affected per patient is three;55,70 therefore, our modelled cohort was assumed to have three joints affected. The base-case analysis was conducted using recurrence rates obtained from the literature. We carried out several analyses varying these recurrence rates to test the impact on cost-effectiveness. Analyses were also conducted where we varied the number of CCH injections required to complete a treatment course. In the base-case analysis, the number of injections required was 1.6 per joint; the impact of varying this to a lower estimate of 1 was also assessed. Further sensitivity analyses regarding the number of joints affected (1 or 2) was also conducted.
Care pathways
The care pathways were determined based on expert opinion and availability of data. Figure 30 shows the graphical representation of the model structure. For the purposes of the economic model, the representative cohort is patients with moderate or severe DC who are considered suitable for surgical treatment with LF, PNF, or CCH.
FIGURE 30.
Schematic representation of the economic model structure.

Following treatment with one of these procedures, patients can either experience treatment-related adverse events or no treatment-related adverse events. It was assumed that all patients would recover from any treatment-related complications experienced. Subsequently, patients entered either a treatment success or treatment failure health state. Treatment success and treatment failure rates were assumed to be equivalent for patients who did or did not experience complications. Patients who entered the treatment success health state incurred a risk of further disease recurrence. In the case of recurrence, patients could proceed with further treatment or, alternatively, could remain within the recurrence state, where they accumulated a lower health-related quality-of-life value. Patients who did not experience recurrence remained in the treatment success health state, in which they incurred no costs and accumulated a higher quality-of-life value. Finally, patients who do not achieve treatment success (i.e. have a treatment failure) could either transit to receive further treatment or remain in that health state, accumulating a lower health-related quality-of-life value.
Strategies to be evaluated
The strategies chosen for evaluation were selected based on the availability of data from the systematic review. It was not possible to obtain data for all strategies of clinical interest (e.g. for some patients with particular characteristics, dermofasciectomy may be considered a treatment option). Thus, the economic analysis focused on comparing CCH, PNF and LF. An important limitation of the available clinical effectiveness and cost-effectiveness literature is that no direct comparisons between CCH and any of the surgical treatments have been performed using a RCT study design, and no reliable indirect comparisons can be made owing to the lack of a common comparator. There are also differences in the patient characteristics between the surgical randomised studies and the CCH versus placebo trials and, additionally, probable unobservable differences in patient treatment history and study centres in terms of surgeon quality. The economic model is built from a naive indirect comparison and, as such, represents a departure from the NICE reference case. 102 The outputs from the model should, therefore, be considered cautiously.
Decision analytical model
The pathways were embedded in a Markov model developed to simulate the progression of treated DC. Thirteen events and states were used to model the care pathways: (1) initial treatment; (2) treatment success; (3) treatment failure; (4) recurrence; (5) second-line treatment; (6) treatment success following second-line treatment; (7) treatment failure following second-line treatment; (8) recurrence following second-line treatment; (9) third-line treatment; (10) treatment success following third-line treatment; (11) treatment failure following third-line treatment; (12) recurrence following third-line treatment; and (13) treatment complications. Patients who achieved a treatment success were modelled to progress towards a recurrent state based on observed recurrence rates from the literature. In the first cycle of the model, all patients were modelled to undergo treatment consisting of CCH, PNF or LF. Patients were then modelled to transit to a treatment success or treatment failure state for the subsequent model cycle. All patients faced a risk of recurrence, modelled based on rates observed for recurrence following the appropriate treatment strategy received. Each cycle length was 6 months and included a half cycle correction, such that probabilities of recurrence and costs of treatment and utility values were expressed within this cycle length. The age and sex-specific risk of death from all causes was also incorporated in the model based on interim UK life tables. 103
Complications of treatment
The occurrence of treatment complications that resulted in further treatment and/or quality-of-life impact was included in the model. The incidence of adverse events post surgical treatment was determined from the study by Dias et al. ;44 these were categorised into minor and major adverse events. This study explored the surgical management of DC in 12 European countries, including the UK, using a surgeon survey and patient chart review. More than 3500 patient charts were reviewed and, therefore, this is largely representative of all complications following the surgical interventions of interest. Complications reported were both intra- and post-operative, by procedure and type of complication. No deaths from treatment or treatment-related complications have been reported in the trials. Although minor adverse events were reported, they were transient in nature and did not lead to any additional, long-term costs or utility decrements and were, therefore, excluded from the model. The resultant probabilities of experiencing a treatment complication that resulted in further treatment are provided in Table 14.
Treatment success and failure
Treatment success was defined as a correction in contracture to within 0–5° of full extension. van Rijssen et al. 70 reported the percentage of successfully treated joints 6 weeks post treatment for both PNF- and LF-treated joints. This was reported as the success rate for MCP and PIP joints. Owing to limited data availability, we were unable to structure the model in such a way as to incorporate the different types of joints. We therefore took the mean of the reported success rate and applied it in the model. Treatment success following CCH was taken from the CORD I and CORD II trials and from the Badalamente and Hurst trial,55,56,65 and generated the meta-analysis result reported in Chapter 3, which was then applied in the model. Treatment success rates used to populate the model are shown in Table 15.
| Procedure | Probability | Distribution for PSA | Source |
|---|---|---|---|
| Beta | |||
| CCH | |||
| Treatment success | 0.63 | Alpha: 171 | CORD I, CORD II and Badalamente and Hurst and Badalamente et al.56,63–65 |
| Beta: 100 | |||
| Treatment failure | 0.37 | CORD I, CORD II, Badalamente and Hurst and Badalamente et al.56,63–65 | |
| PNF | |||
| Treatment success | 0.41 | Alpha: 68 | van Rijssen et al.70 |
| Beta: 99 | |||
| Treatment failure | 0.59 | van Rijssen et al.70 | |
| LF | |||
| Treatment success | 0.71 | Alpha: 89 | van Rijssen et al.70 |
| Beta: 36 | |||
| Treatment failure | 0.29 | van Rijssen et al.70 | |
Recurrence
After first-line treatment, patients who achieved a treatment success incurred a risk of recurrence. Recurrence in the CORD I and CORD II trials and in the CORDLESS observational study was defined as a return of contracture of at least 20° in a joint that was successfully treated. 56,63–65,87 The manufacturer’s submission and van Rijssen et al. 70 reported the 5-year recurrence rates of the included treatment strategies. The van Rijssen et al. primary end point was recurrence defined as an extension deficit of > 30°; however, they also reported the recurrence rate defined as a return in contracture of at least 20°, making the recurrence rates between the chosen studies comparable. 70 These were converted into constant, 6-monthly probabilities of recurrence and incorporated in the model (Table 16). As data on recurrence were only available until year 5, we assumed that for the base-case analysis, recurrence could only occur within the first 5 years post treatment. We assessed the impact of this assumption through a deterministic sensitivity analysis where the constant 6-month probability was extrapolated for the full model time horizon.
Retreatment for recurrence and treatment failure
The study by van Rijssen et al. reported the probability of further treatment following a recurrence. 70 Owing to a lack of available data, for the CCH and PNF strategies, we assumed that the probability of further treatment following a treatment failure after CCH and PNF was the same as that following a recurrence for CCH and PNF. Thus, patients who underwent further treatment following a recurrence after CCH or PNF were as likely to undergo further treatment following a treatment failure. We had no data for the probability of receiving PNF following CCH, and so we assumed that patients who opted for CCH would have been as likely to undergo PNF, had it been an option. Thus, patients who underwent further treatment had an equivalent probability of receiving either PNF or CCH. Further treatment options following PNF were based on clinical opinion, with patients modelled to receive LF (50%), PNF (40%) or CCH (10%). Patients experiencing treatment failure following LF (either on first-, second- or third-line treatment) did not receive any further treatment and thus entered a treatment failure health state (Mr David Lawrie, personal communication). Patients who experienced a recurrence following a successful treatment with LF could not receive any of the minimally invasive techniques and were therefore modelled to receive second- or third-line LF as appropriate (Mr David Lawrie, personal communication).
No evidence was identified on which procedure patients would choose following a second treatment failure or recurrence. For the base-case analysis, we therefore assumed that patients who proceeded for further (i.e. third-line) treatment following a second treatment failure (for CCH and PNF only) or recurrence would receive LF; this assumption was based on advice from our clinical expert. A sensitivity analysis was conducted in which all patients were modelled to undergo further treatment to test the effect on cost-effectiveness.
Table 17 reports the probabilities incorporated in the model of patients who received further treatment and those who did not. If patients failed after LF for any of the treatment strategies, they did not proceed for further treatment. Instead they entered a semi-absorbing state where they continued to accumulate a lower quality-of-life value for the remainder of the model. In the manufacturer’s model, 83% of patients did not undergo any further treatment. It is unclear whether this value is protocol driven and reflects current clinical practice; furthermore, it is much higher than the value reported by van Rijssen et al. 70 Therefore, for the base-case analysis, we assumed that the same proportion of patients who opted for further treatment in the LF strategy would also choose further treatment in the CCH arm. This assumption was made, as it was deemed to provide a fair balance between the manufacturer’s model where 83% did not undergo further treatment and the reported percentage of patients in the PNF strategy who did not undergo further treatment (27%) in the van Rijssen et al. study. 70 A sensitivity analysis was conducted to test the impact of this assumption, where the same proportion of patients in the CCH arm proceeded for further treatment as those in the PNF arm. Figure 31 outlines the treatment pathway used in the model for patients initially receiving either CCH or PNF. Figure 32 provides the pathway for patients initially receiving LF.
FIGURE 31.
Treatment pathway of patients with initial treatment of CCH or PNF.
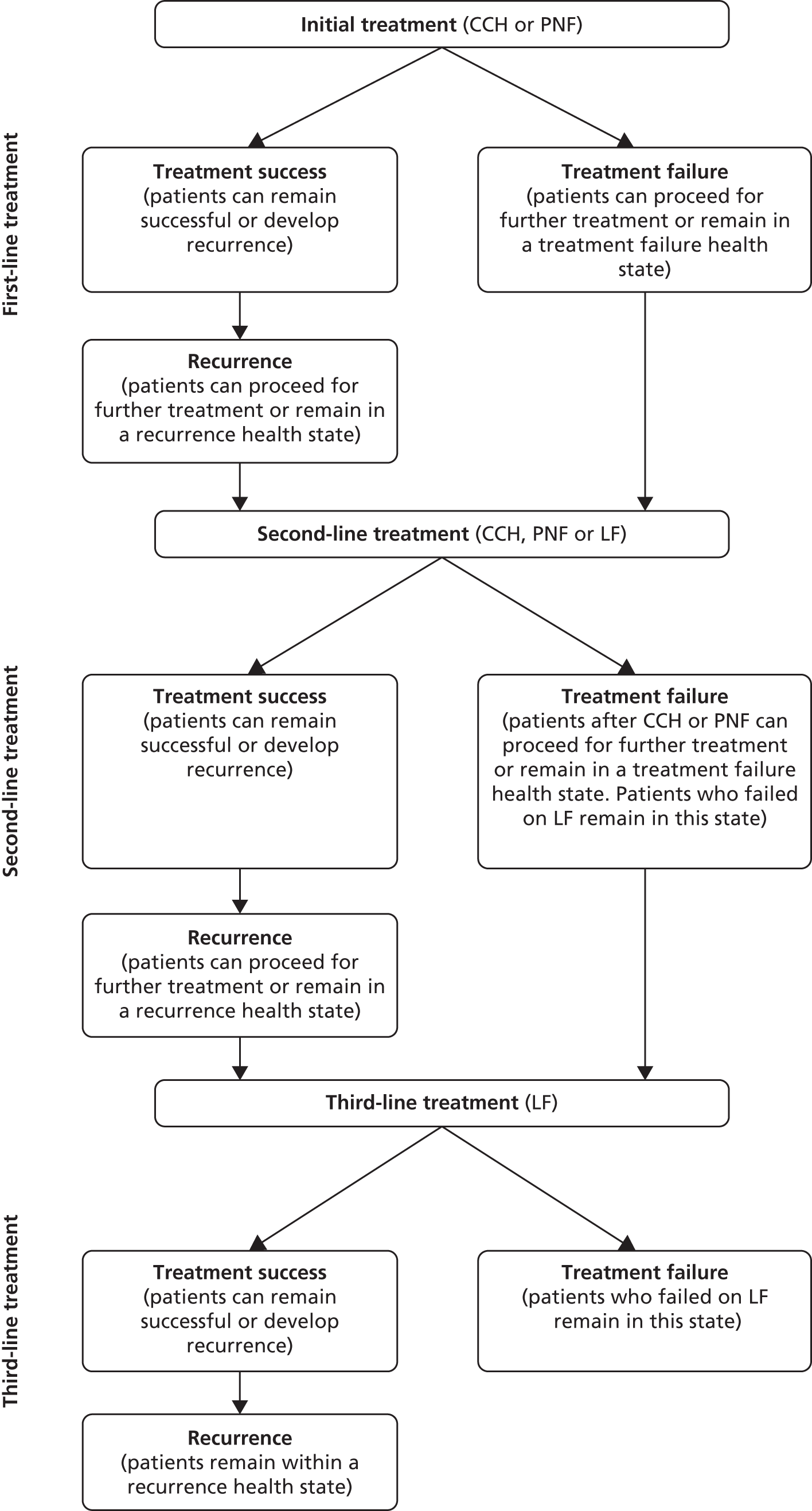
FIGURE 32.
Pathway of patients with initial treatment of LF.
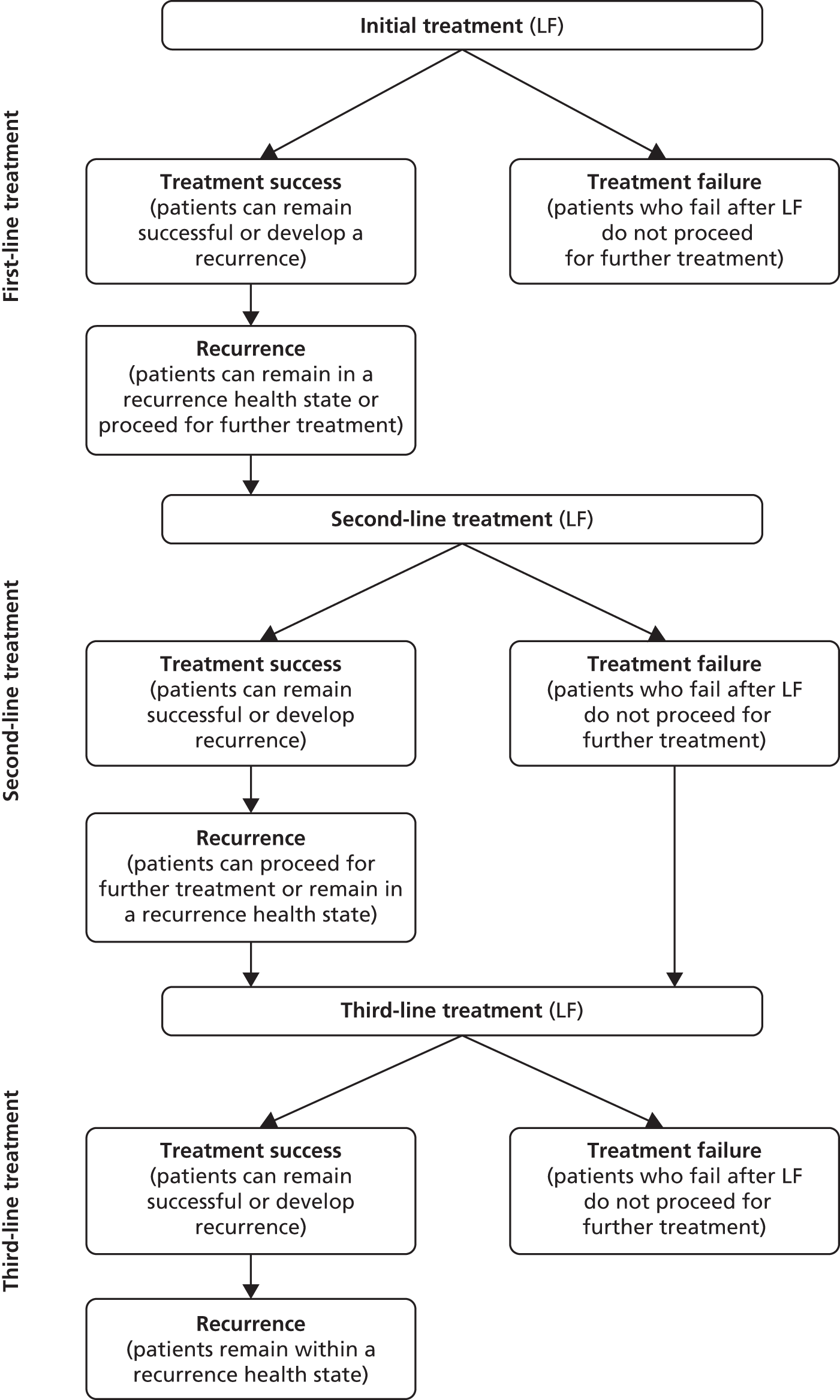
| Procedure | Probability of further treatment | Distribution for PSA | Source |
|---|---|---|---|
| Beta | |||
| CCH | |||
| No further treatment | 0.60 | Alpha: 6 | Assumption: equivalent value as van Rijssen LF70 |
| Beta: 4 | |||
| Further treatment | 0.40 | Assumption: equivalent value as van Rijssen LF70 | |
| Second-line treatment for CCH | |||
| CCH | 0.19 | Manufacturer’s submission and assumption | |
| PNF | 0.19 | Manufacturer’s submission and assumption | |
| LFa | 0.62 | Manufacturer’s submission | |
| Third-line treatment for CCH | |||
| CCH | 0 | Manufacturer’s submission and assumption | |
| PNF | 0 | Manufacturer’s submission and assumption | |
| LFa | 1 | Manufacturer’s submission | |
| PNF | |||
| No further treatment | 0.27 | Alpha: 12 | van Rijssen et al., 201270 |
| Beta: 33 | |||
| Further treatment | 0.73 | van Rijssen et al., 201270 | |
| Second-line treatment for PNF | |||
| CCH | 0.10 | Clinical opinion | |
| PNF | 0.40 | Clinical opinion | |
| LFa | 0.50 | Clinical opinion | |
| Third-line treatment for PNF | |||
| CCH | 0 | Clinical opinion | |
| PNF | 0 | Clinical opinion | |
| LFa | 1 | Clinical opinion | |
| LFa | |||
| No further treatment | 0.60 | Alpha: 6 | van Rijssen et al., 201270 |
| Beta: 4 | |||
| Further treatment | 0.40 | van Rijssen et al., 201270 | |
| Second- and third-line recurrence treatment for CCH | |||
| CCH | 0 | Clinical opinion | |
| PNF | 0 | Clinical opinion | |
| LFa | 1 | Clinical opinion | |
Resource use and unit cost estimation
Costing was conducted for the financial year 2012/13 and unit costs were acquired where possible from national sources including NHS reference costs,104 Unit Costs of Health and Social Care105 and British National Formulary (BNF). 106
Collagenase clostridium histolyticum
The cost of the administration procedure of CCH was taken from the NHS reference costs104 using the appropriate health-care resource group (HB56C), assuming it was performed in an outpatient setting (£225). One injection of CCH can be administered at any one time. The cost of a single injection applied in the model was £650. 106 For each injection of CCH, an additional appointment is required to have the cord manipulated under local anaesthetic, and the patient receives a splint. We assumed that each joint would require one splint. This process is repeated until the final course of CCH has been completed or the patient achieves clinical success (a maximum of three injections is allowed per joint). For the base-case analysis, 1.6 injections per joint were costed, assuming no vial sharing. Given that there are three joints to be treated, this leads to a total number of consultant outpatient appointments of 9.6 (1.6 × 3 injection appointments + 1.6 × 3 finger manipulation appointments) over each 6-month cycle. Furthermore, the cost of a session of physiotherapy was costed for each procedure (one injection was assumed to be one procedure), based on clinical opinion (Mr David Lawrie, personal communication).
The reason for the difference in collagenase initial treatment costs between our model and that of the manufacturer is that the manufacturer has limited their analysis to a subgroup of the population. Also, the manufacturer assumes that vial sharing occurs. Their initial treatment costs of £1739 have been derived by costing treatment for a mean of 1.445 joints, using a mean of 1.6 injections per joint. This comprises administration costs of £969 (0.58/0.9 × £650 vial price × 1.6 injections × 1.445 joints) and outpatient visit costs of £756 ((1.6 × £225 (injection visit) + 1.6 × £102 (finger manipulation visit)) × 1.445 joints. A further £14 is added for the splint. Our model is based on no vial sharing, the treatment of three joints and 1.6 injections per joint, based on the means reported in CORD I.
Percutaneous needle fasciotomy
Percutaneous needle fasciotomy involves inserting a sharp blade or fine needle to divide the cord. PNF is a minimally invasive procedure and was assumed to be conducted under local anaesthetic in an outpatient setting, where the patient is also fitted with a splint. The appropriate NHS reference cost was applied for an outpatient procedure (£225). 104 We assumed that patients receive one session of physiotherapy after PNF (Table 18).
| Procedure | Unit cost, £ | Assumption | Lower/higher estimates, £ (distribution) | Source |
|---|---|---|---|---|
| CCHa | ||||
| Cost of injection | 650 | Cost of 0.9-mg vial | BNF, 2013106 | |
| Administration visit | 225 | Outpatient procedure: Minor Hand Procedures for Non-Trauma, Category 1, without CC. HB56C (trauma and orthopaedics) | 201/247 (alpha: 42.96; beta: 5.24) | NHS Reference Costs, 2013104 |
| Finger extension examination | 102 | Consultant-led follow-up attendance appointment: (trauma and orthopaedics: 110) | 78/121 (alpha: 10.10; beta: 10.10) | NHS Reference Costs, 2013104 |
| Splint | 10 | Manufacturer’s submission | ||
| Physiotherapy appointment | 19.74 | Applied for all patients receiving CCH. Hospital physiotherapist (£36 per hour), assuming 32.9 minutes per surgery consultation | PSSRU, 2013105 | |
| PNF | ||||
| Administration visit | 225 | Outpatient procedure: minor hand procedures for non-trauma, Category 1, without CC. HB56C (trauma and orthopaedics) | 201/247 (alpha: 42.96; beta: 5.24) | NHS Reference Costs, 2013104 |
| Splint | 10 | Manufacturer’s submission | ||
| Physiotherapy appointment | 19.74 | Applied for all patients receiving CCH. Hospital physiotherapist (£36 per hour), assuming 32.9 minutes per surgery consultation | PSSRU, 2013105 | |
| LF | ||||
| Cost of procedure | 2089 | Weighted average of day-case and inpatient procedures (HB53Z, HB52B, HB52C, HB51Z, HB99Z) | 1598/2450 (alpha: 10.79; beta: 193.52) | NHS Reference Costs, 2013104 |
| Follow-up visit | 102 | Consultant-led follow-up attendance appointment: trauma and orthopaedics: 110 | 78/121 (alpha: 10.10; beta: 10.10) | NHS Reference Costs, 2013104 |
| Physiotherapy appointment | 19.74 | Applied for all patients receiving CCH. Hospital physiotherapist (£36 per hour), assuming 32.9 minutes per surgery consultation | PSSRU, 2013105 | |
Limited fasciectomy
Limited fasciectomy is performed under general or regional anaesthetic. The weighted average cost of performing LF as a day-case (74%) and inpatient (26%) procedure was calculated from the activity reported in the NHS Reference Costs and applied in the model (£2089). 104 The cost of a consultant-led follow-up attendance was also costed (see Table 18). Furthermore, five sessions of physiotherapy were applied (Mr David Lawrie, personal communication).
Costs of treatment adverse events
Adverse events associated with PNF and LF treatments were reported by Dias et al. 44 These complications were categorised into intra- and post-operative. The clinician adviser within our team identified five adverse events that would have an impact on quality of life and would require further treatment: nerve damage, arterial damage, tendon damage, bleeding, ischaemia. For the identified major adverse events, a weighted average of the NHS Reference cost of the category 1 and category 2 intermediate hand procedures for trauma was applied (Table 19). All procedures were assumed to be conducted as day-case procedures.
| Description | Unit cost | Code | Lower/higher estimates (distribution) | |
|---|---|---|---|---|
| Intermediate hand procedures for trauma, Category 2 | £1900 | HRG HA53Z | Department of Health, 2013104 | |
| Intermediate hand procedures for trauma, Category 1 | £1690 | HRG HA54Z | Department of Health, 2013104 | |
| Weighted average | £1824 | £1390/£2184 (Alpha: 9.45; Beta: 192.94) |
Health measurement and valuation
The systematic review of economic evidence revealed that no preference-weighted quality-of-life values have been reported from patients with DC. However, a recently published study by Gu et al. elicited health-state utilities for DC from the general population using a discrete choice experiment (DCE). 107 At present, this is the only study that reports preference-weighted quality-of-life values and we incorporate these values into the model. In terms of the validity of the methods employed, this study adopted best practice to identify appropriate attributes and levels, via a focus group conducted in Scotland, and to employ appropriate, standard methods of design (orthogonal DCE fractional factorial design) and data analysis (conditional logistic modelling). Tests of rationality were also employed. The expected association between greater problems with contracture and reduction in health-state values was observed. There is no evidence, therefore, to suggest that the values are biased in any way through use of inappropriate methods.
In this study, health-state preferences were elicited via an internet survey from 1745 respondents resident in the UK. This study sample appeared to be relatively representative of the UK population. Participants were shown 10 pairs of hand profiles with both hands affected by DC and were required to identify a preferable hand. The eight joints (PIP and MCP joints of the index, middle, ring and little fingers) were each categorised for three different degrees of contracture (0°, 45°, 90°) and correspond to the Tubiana scale. The Tubiana scale is a staging system for DC which measures total flexion deformity for the PIP and MCP joints. The authors employed an anchoring method to restrict participants’ preferences onto the traditional 0–1 utility scale. Hands that were unaffected by DC were assigned a perfect utility score of 1, whereas the utility value for a hand that exhibited the worst possible DC (i.e. 90° of contracture in all eight joints) was derived by asking participants what levels of the European Quality of Life-5 Dimensions (EQ-5D) 5 levels of severity (EQ-5D-5L) profile would be most likely to be affected by living with this hand. Conditional logistic logit models were employed to estimate indirect utility estimates, which were subsequently rescaled to the anchor points on the EQ-5D-5L. The utility estimates were therefore dependent on degree of contracture, joint type and the finger in which the contracture occurred.
The van Rijssen et al. 70 and CORD I55 studies did not report which finger was affected by DC. Although mean baseline contracture and mean post-treatment contracture were reported in van Rijssen et al. ,70 CORD I55 reported the mean baseline total contracture index (this is the sum of fixed-flexion contractures in all 16 joints measured at screening). Both studies reported a mean of three joints affected per patient. We calculated the utility estimate from information on baseline patient characteristics in both studies. In both studies, this resulted in the average patient beginning at Tubiana stage 3 prior to treatment (> 90° but < 135°contracture of PIP and MCP joints). As the definition of treatment successes is to have a correction of contracture to within 0–5° of full extension, we assumed treatment successes would move from stage 3 to stage 0, whereas treatment failures would remain at Tubiana stage 3. Patients experiencing recurrence would move from stage 0 to stage 1 (45° or less contracture, but greater than 0); however, patients who received further treatment were assumed to move to stage 3 in the cycle prior to receiving treatment. This was to reflect that patients who proceed for further treatment following a recurrence are most likely to be clinically worse than patients who do not undergo any further treatment. We conducted a sensitivity analysis to assess the impact of patients who were clinically defined as a treatment failure achieving some quality-of-life improvement and, therefore, moving from stage 3 to stage 2.
To calculate health-state values, we calculated the pre- and post-treatment utility estimates for each finger individually and then applied a weighted estimate of mean utility at baseline and post treatment. The pre- and post-treatment utility values were estimated from the equation specified by Gu et al. :107
where U(handi) = the pre- or post-treatment value for handi, V (handi) = indirect utility weight for handi from the Conditional Logistic model estimate, V(best) = indirect utility weight for a hand with no contracture problems, V (anchor) = indirect utility weight associated with the worst (anchor) hand and EQ-5Dmean = the mean value of the EQ-5D-5L in the worst hand. To take a worked example of how a value is derived, consider the baseline patient, with Tubiana stage 3. First, we take the coefficient estimates reported in the study by Gu et al. 107 (supplementary article data, appendix B1) and reproduced below (Table 20). Then, to account for the mean patient at baseline, who has three joints affected, the indirect utility weights for Tubiana stage 3 are multiplied by 3 to give V baseline (with values for 1 and 2 used for the 1 joint and 2 joint models respectively, reported in the sensitivity analyses). U baseline is then derived from the formula in Equation 2, where V(best) = 0, V (anchor) = –23.5298 and EQ-5D mean = 0.4909.
| The Tubiana stage model | V(handi) | V baseline | U baseline | |
|---|---|---|---|---|
| Index | 0 | 0 | ||
| 1 | –3.47746 | |||
| 2 | –4.41626 | |||
| 3 | –3.35616 | –10.068468 | 0.782154669 | |
| 4 | –9.92425 | |||
| Middle | 0 | 0 | ||
| 1 | –1.56919 | |||
| 2 | –0.53417 | |||
| 3 | –1.948 | –5.843988 | 0.873557179 | |
| 4 | –3.37811 | |||
| Ring | 0 | 0 | ||
| 1 | –1.13854 | |||
| 2 | –2.54806 | |||
| 3 | –3.79923 | –11.397693 | 0.753395035 | |
| 4 | –5.00851 | |||
| Little | 0 | 0 | ||
| 1 | –1.74886 | |||
| 2 | –2.39839 | |||
| 3 | –3.5804 | –10.741212 | 0.767598916 | |
| 4 | –5.21892 | |||
| Weighted Utility | 0.776268113 | |||
This weighted utility estimate of 0.776268113 is then derived by multiplying the four calculated utility baseline estimates reported above by the probability of that finger being affected. Dias and Braybrooke29 reported the patterns of disease in the form of digit affected based on a large audit of the outcomes of surgery for DC, thereby enabling a calculation of this probability (Table 21). Values for Tubiana stages 0, 1 and 2 are calculated following the same procedure as outlined in the worked example. Table 22 presents the health-state utility values for each Tubiana stage as incorporated in the model.
| Affected finger | N | Probability | Source |
|---|---|---|---|
| Index | 92 | 0.050605 | Dias and Braybrooke, 200629 |
| Middle | 216 | 0.118812 | Dias and Braybrooke, 200629 |
| Ring | 596 | 0.327833 | Dias and Braybrooke, 200629 |
| Little | 914 | 0.50275 | Dias and Braybrooke, 200629 |
| Health-state utility | Utility value | Assumed health state | Distribution for PSA | Source |
|---|---|---|---|---|
| Tubiana | Beta | |||
| Stage 0 | 1 | Success | Beta Alpha: 23.51 Beta: 0 |
Gu et al., 2013107 |
| Stage 1 | 0.965 | Recurrence (if not followed by further treatment) | Beta Alpha: 80.39 Beta: 2.91 |
Gu et al., 2013107 |
| Stage 2 | 0.899 | Treatment failure in sensitivity analysis | Used only in deterministic analysis | Gu et al., 2013107 |
| Stage 3 | 0.776 | Baseline, base-case treatment failure and recurrence if followed by further treatment | Beta Alpha: 336.27 Beta: 96.92 |
Gu et al., 2013107 |
Utility values for treatment complications
The clinician from our team identified major adverse events that would have an impact on quality of life (Mr David Lawrie, personal communication). No data on the utilities from adverse events following treatment for DC have been published. Thus, we assumed that patients experiencing a complication would receive a utility decrement of –0.0615; this is half a decrement on the European Quality of Life-5 Dimensions-3 levels (of severity) instrument (EQ-5D-3L) of a move from no pain or discomfort to some pain or discomfort. The utility decrement was applied for a time period of 6 weeks, which is the median referral to treatment waiting time for trauma and orthopaedics. After the 6 weeks it was assumed that patients would return to the appropriate utility value.
Comparison with previously published utility estimates
Utility estimates from the Gu et al. 107 study were included in this model as it was conducted in the UK and included a total of 1745 participants. Furthermore, the values are scaled in such a way that they have been estimated relative to an EQ-5D state. In comparison, the only other health-state valuation was reported by Chen et al. ,100 who constructed a utility survey specific to their study in a US setting and surveyed a total of 50 participants. They reported much higher utility values, ranging from 0.971 to 0.994, than those included in our model. The high utility values could be attributable to the standard gamble method of utility elicitation. This approach requires participants to choose between two alternatives. The first alternative is a treatment with two outcomes: (1) the patient is cured or; (2) the patient dies. Chen et al. 100 offered participants, in a hypothetical exercise, the opportunity to take a pill, which would lead to an immediate return to full health or death. The second alternative is to live in a particular chronic state over a number of years. In this case, participants could live the rest of their life with DC. However, as DC is not a life-threatening disease, participants may have been averse to choosing an alternative with a sudden risk of death, and this may explain the high utility values.
Discount rate (costs and benefits)
Costs and benefits (QALYs) were discounted at the NICE recommended rate of 3.5% per annum. We assessed the impact of discounting costs and benefits at a rate of 0% and 6% and also of discounting benefits at a rate of 1.5% while maintaining a discount rate of 3.5% for costs.
Summary of key assumptions
-
The modelled patient cohort had three joints affected by DC.
-
1.6 CCH injections were required to treat each joint in separate visits to hospital outpatient clinics.
-
Equivalent recurrence rates were applied to first-, second- and third-line treatment.
-
Equivalent treatment success and treatment failure rates were applied to first-, second- and third-line treatment.
-
Patients who failed after LF did not proceed for any further treatment.
-
The proportion of patients proceeding for further treatment in the CCH strategy was assumed to be the same as LF.
-
All patients received LF for third-line treatment.
Time horizon
The analysis proceeded over a 37-year (i.e. lifetime) time horizon, following the cohort from age 63 to 100 years.
Deterministic sensitivity analysis
The process of populating the model required a number of parameter and structural assumptions. To assess the sensitivity of the base-case results to these assumptions, several deterministic sensitivity analyses were conducted. Sensitivity analyses were conducted on the success rates of treatment strategies, varying utility values, varying the cost of treatments, assuming patients had a lower number of mean joints affected at baseline and altering the discount rate. Where a strategy is dominated, either extendedly or absolutely, the reporting ICER is against the next less costly, non-dominated strategy. As PNF is the least costly option in all scenarios presented in the deterministic analysis, it automatically becomes the comparator. However, as PNF may not be available everywhere, the ICER is also reported separately for strategies that are extendedly dominated. The following is the list of the deterministic analyses that were undertaken:
-
number of injections to achieve a clinical success reduced to 1 for CCH
-
applied a 100% success rate to CCH
-
applied an equivalent success rate for CCH and LF (71%)
-
upper limit of the CI for the success rate for collagenase, with all other parameters the same as the base case
-
varied the success rate of CCH, holding all other parameters constant, so that the cost per QALY was under the threshold of £20,000 (79%)
-
applied a constant lifetime recurrence rate
-
lower limit of the CI for the recurrence rate for collagenase, with all other parameters the same as the base case
-
all treatments have the same recurrence rate, with all other parameters the same as the base case
-
both the upper limit of the CI for the success rate for collagenase and equal recurrence rates for all three treatments
-
assumed that all patients would proceed for further treatment following a recurrence
-
further treatment options for second-line PNF based on the van Rijssen trial (21% receive LF; 79% receive PNF)
-
CCH is not an option for second-line treatment after PNF (50% receive LF; 50% receive PNF)
-
applied a utility decrement for half a model cycle, post LF, to capture the potential recovery time following a surgical procedure
-
applied a baseline utility decrement for patients undergoing LF to assess the potential that patients may be feeling anxious prior to receiving a surgical procedure
-
applied a stage-2 utility value for treatment failures to reflect that even though patients may not have achieved clinical success, they may have gained some improvement in quality of life
-
applied a lower cost estimate for treatment strategies (CCH: £4584; PNF: £231; and LF: £1775)
-
applied a higher cost estimate for treatment strategies (CCH: £5011; PNF: £277; and LF: £2670)
-
assumed patients had a mean of 1 joint affected, with adjusted utility values to reflect the earlier success in the cycle as well as improved baseline utility
-
assumed patients had a mean of 1 joint affected, with adjusted utility values to reflect the earlier success and increased the recurrence rate for PNF to be equivalent to CCH
-
assumed patients had a mean of 1 joint affected, with adjusted utility values to reflect the earlier success with an equal proportion of patients receiving further treatment for PNF and CCH
-
assumed patients had a mean of 1 joint affected, with adjusted utility values to reflect the earlier success with an equal proportion of patients receiving further treatment for PNF and CCH and increased the recurrence rate for PNF to be equivalent to CCH
-
assumed patients had a mean of 2 joints affected, with adjusted utility values to reflect the earlier success in the cycle as well as improve baseline utility
-
assumed patients had a mean of 2 joints affected, with adjusted utility values to reflect the earlier success and increased the recurrence rate for PNF to be equivalent to CCH
-
assumed patients had a mean of 2 joints affected, with adjusted utility values to reflect the earlier success with an equal proportion of patients receiving further treatment for PNF and CCH
-
assumed patients had a mean of 2 joints affected, with adjusted utility values to reflect the earlier success with an equal proportion of patients receiving further treatment for PNF and CCH and increased the recurrence rate for PNF to be equivalent to CCH
-
discounting benefits at a rate of 1.5% per annum while maintaining a discount rate of 3.5% for costs
-
no discounting for costs and benefits
-
discounting costs and benefits at an annual rate of 6%.
Probabilistic sensitivity analysis
In order to characterise the uncertainty surrounding the selection of the optimal strategy, the model was analysed probabilistically. An appropriate distribution was assigned to hospital-based procedure unit costs, recurrence rates, treatment success rates, complication rates, probability of further treatment and utility values to reflect the uncertainty surrounding them. The probabilistic analysis proceeded by randomly selecting a value from the assigned distribution for the model parameters and recomputing the model results. This process was repeated 1000 times. To illustrate the uncertainty surrounding the estimates of cost-effectiveness, cost-effectiveness acceptability curves (CEACs) were generated using these 1000 estimates, using the NMB approach. CEACs demonstrate the probability of an intervention being cost-effective at different ceiling ratios of decision-makers’ WTP per QALY.
Results
Mean costs, mean effects and incremental analysis
Table 23 presents the mean costs, mean QALYs and incremental cost per QALY gained associated with each strategy. Figure 33 presents the findings of cost per QALY gained graphically on the cost-effectiveness frontier. Strategies that do not fall on the line (the cost-effectiveness frontier) are strategies that are absolutely dominated (more costly and less effective than other strategies) and, therefore, they do not have the potential to be considered cost-effective. Strategies that fall on the line represent strategies that have the potential to be cost-effective, dependent on decision-makers’ WTP per QALY gained.
| Strategy | Cost, £ | Incremental cost, £ | QALYs | Incremental QALYs | ICER, £ |
|---|---|---|---|---|---|
| PNF | 1212 | – | 10.332 | – | – |
| LF | 2410 | 1199 | 10.442 | 0.11 | 10,871 |
| Collagnase | 5342 | 2931 | 10.36 | –0.082 | Dominated |
FIGURE 33.
Cost-effectiveness frontier based on cost per QALY.
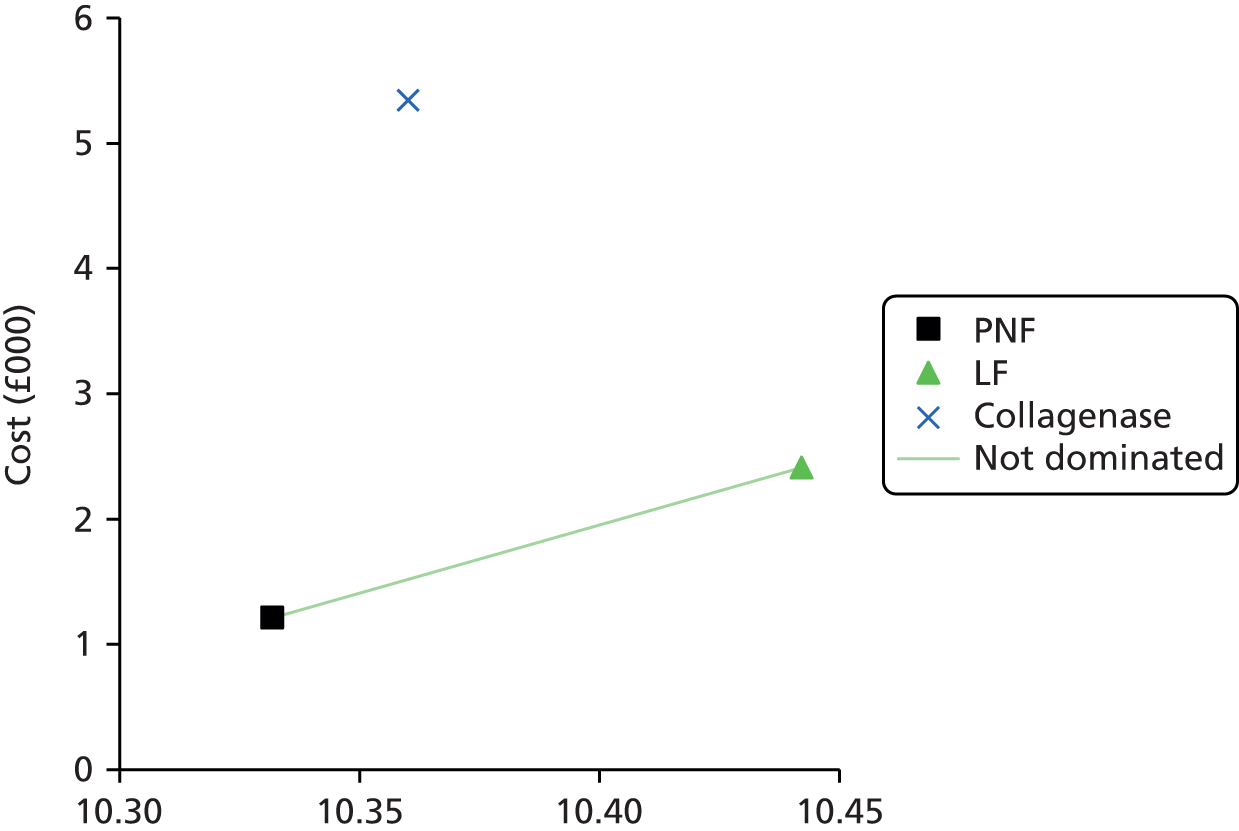
The base-case results show PNF to be the least costly option, followed by LF and CCH. LF produced an increase in QALYs compared with both PNF and CCH. CCH was dominated by LF as it was more costly and less effective. The ICER for LF versus PNF was £10,871 per QALY gained (LF was more costly and more effective than PNF). Therefore, applying a ceiling WTP threshold of £20,000, LF was the favourable option from a cost-effectiveness perspective.
Deterministic sensitivity analysis
Table 24 shows the cost per QALY findings to the deterministic sensitivity analysis alterations to the findings of the base-case analysis. These analyses demonstrate that CCH has the most favourable cost-effectiveness only when it can achieve a success rate of at least 77%. There are some scenarios to which the cost per QALY estimate is sensitive for PNF. PNF becomes the preferred option in terms of cost-effectiveness when all patients proceed for further treatment and when the mean number of joints affected was assumed to be 1.
In the majority of scenarios presented in the deterministic analysis, LF remains the preferred option from a cost-effectiveness perspective.
| Strategy | Cost, £ | Incremental Cost, £ | QALYs | Incremental QALYs | ICER (not reporting ICER for extendedly dominated strategies), £ | ICER (reporting ICER for extendedly dominated strategies), £ |
|---|---|---|---|---|---|---|
| Number of injections to achieve a clinical success reduced to 1 for CCH | ||||||
| PNF | 1183 | – | 10.332 | – | – | – |
| LF | 2410 | 1227 | 10.442 | 0.11 | 11,130 | – |
| Collagenase | 3520 | 1110 | 10.36 | –0.082 | Dominated | – |
| Applied a 100% success rate to CCH | ||||||
| PNF | 1202 | – | 10.358 | – | – | – |
| LF | 2410 | 1208 | 10.442 | 0.084 | Extendedly dominated | – |
| Collagenase | 5148 | 3946 | 10.979 | 0.621 | 6353 | 5099 |
| Applied an equivalent success rate for CCH and LF (71%) | ||||||
| PNF | 1210 | – | 10.338 | – | – | – |
| LF | 2410 | 1201 | 10.442 | 0.105 | 11,486 | – |
| Collagenase | 5299 | 2889 | 10.496 | 0.054 | 53,961 | – |
| Upper limit of the CI for the success rate for collagenase, with all other parameters the same as the base case | ||||||
| PNF | 1210 | – | 10.336 | – | – | – |
| LF | 2410 | 1200 | 10.442 | 0.106 | 11,326 | – |
| Collagenase | 5310 | 2900 | 10.462 | 0.02 | 146,909 | – |
| Varied success rate of CCH, holding all other parameters constant, so that it became cost–effective (77%) | ||||||
| PNF | 1208 | – | 10.342 | – | – | – |
| LF | 2410 | 1202 | 10.442 | 0.1 | 11,994 | – |
| Collagenase | 5267 | 2857 | 10.597 | 0.155 | 18,482 | – |
| Applied a constant lifetime recurrence rate | ||||||
| PNF | 1351 | – | 10.284 | – | – | – |
| LF | 2450 | 1099 | 10.427 | 0.143 | 7696 | – |
| Collagenase | 5474 | 3024 | 10.3 | –0.127 | Dominated | – |
| Lower limit of the CI for the recurrence rate for collagenase, with all other parameters the same as the base case | ||||||
| PNF | 1211 | – | 10.332 | – | – | – |
| LF | 2410 | 1199 | 10.442 | 0.11 | 10,927 | – |
| Collagenase | 5322 | 2912 | 10.372 | –0.07 | Dominated | – |
| All treatments have the same recurrence rate, with all other parameters the same as the base case | ||||||
| PNF | 1425 | – | 10.221 | – | – | – |
| LF | 2674 | 1249 | 10.301 | 0.08 | 15,560 | – |
| Collagenase | 5382 | 2708 | 10.337 | 0.036 | 75,452 | – |
| Both the upper limit of the CI for the success rate for collagenase and equal recurrence rates for all three treatments | ||||||
| PNF | 1423 | – | 10.226 | – | – | – |
| LF | 2674 | 1250 | 10.301 | 0.075 | 16,591 | – |
| Collagenase | 5348 | 2674 | 10.44 | 0.139 | 19,214 | – |
| Assumed that all patients would proceed for further treatment following a recurrence | ||||||
| PNF | 1733 | – | 10.642 | – | – | – |
| LF | 2456 | 723 | 10.435 | –0.207 | Dominated | – |
| Collagenase | 6293 | 4559 | 10.696 | 0.054 | 85,200 | – |
| Further treatment options for second-line PNF based on Van Rijssen trial (21% receive LF; 79% receive PNF) | ||||||
| PNF | 1030 | – | 10.319 | – | – | – |
| LF | 2410 | 1381 | 10.442 | 0.123 | 11,250 | – |
| Collagenase | 5342 | 2931 | 10.36 | –0.082 | Dominated | – |
| CCH is not an option for second-line treatment after PNF (50% receive LF; 50% receive PNF) | ||||||
| PNF | 1174 | – | 10.331 | – | – | – |
| LF | 2410 | 1,237 | 10.442 | 0.111 | 11,138 | – |
| Collagenase | 5342 | 2931 | 10.36 | –0.082 | Dominated | – |
| Applied a utility decrement for half a model cycle, post LF, to capture the potential recovery time following a surgical procedure | ||||||
| PNF | 1212 | – | 10.327 | – | – | – |
| LF | 2410 | 1199 | 10.427 | 0.1 | 12,002 | – |
| Collagenase | 5342 | 2931 | 10.357 | –0.069 | Dominated | – |
| Applied a baseline utility decrement for patients undergoing LF to assess the potential that patients may be feeling anxious prior to receiving a surgical procedure | ||||||
| PNF | 1212 | – | 10.262 | – | – | – |
| LF | 2410 | 1199 | 10.334 | 0.072 | 16,725 | – |
| Collagenase | 5342 | 2931 | 10.322 | –0.012 | Dominated | – |
| Applied a stage-2 utility value for treatment failures to reflect that even though patients may not have achieved clinical success, they may have gained some improvement in quality of life | ||||||
| PNF | 1212 | – | 10.75 | – | – | – |
| LF | 2410 | 1199 | 10.838 | 0.088 | 13,678 | – |
| Collagenase | 5342 | 2931 | 10.756 | –0.081 | Dominated | – |
| Applied a lower cost estimate for treatment strategies (CCH: £4584; PNF: £231; and LF: £1775) | ||||||
| PNF | 1004 | – | 10.332 | – | – | – |
| LF | 1889 | 885 | 10.442 | 0.11 | 8025 | – |
| Collagenase | 5012 | 3123 | 10.36 | –0.082 | Dominated | – |
| Applied a higher cost estimate for treatment strategies (CCH: £5011; PNF: £277; and limited LF: £2670) | ||||||
| PNF | 1370 | – | 10.332 | – | – | – |
| LF | 2795 | 1425 | 10.442 | 0.11 | 12,921 | – |
| Collagenase | 5613 | 2818 | 10.36 | –0.082 | Dominated | – |
| Assumed patients had a mean of 1 joint affected, with adjusted utility values to reflect the earlier success in the cycle as well as improved baseline utility | ||||||
| PNF | 1212 | – | 10.926 | – | – | – |
| Collagenase | 2133 | 921 | 10.907 | –0.019 | Dominated | – |
| LF | 2410 | 1199 | 10.96 | 0.034 | 35,752 | – |
| Assumed patients had a mean of 1 joint affected, with adjusted utility values to reflect the earlier success and increased the recurrence rate for PNF to be equivalent to CCH | ||||||
| PNF | 1364 | – | 10.895 | – | – | – |
| Collagenase | 2139 | 775 | 10.906 | 0.011 | Extendedly dominated | – |
| LF | 2410 | 1046 | 10.96 | 0.064 | 16,316 | 5019 |
| Assumed patients had a mean of 1 joint affected, with adjusted utility values to reflect the earlier success with an equal proportion of patients receiving further treatment for PNF and CCH | ||||||
| PNF | 1234 | – | 10.929 | – | – | – |
| LF | 2410 | 1177 | 10.96 | 0.03 | Extendedly dominated | – |
| Collagenase | 2607 | 1373 | 10.983 | 0.054 | 25,607 | 9381 |
| Assumed patients had a mean of 1 joint affected, with adjusted utility values to reflect the earlier success with an equal proportion of patients receiving further treatment for PNF and CCH and increased the recurrence rate for PNF to be equivalent to CCH | ||||||
| PNF | 1389 | – | 10.899 | – | – | – |
| LF | 2410 | 1021 | 10.96 | 0.061 | Extendedly dominated | – |
| Collagenase | 2619 | 1230 | 10.981 | 0.082 | 15,006 | 9952 |
| Assumed patients had a mean of 2 joints affected, with adjusted utility values to reflect the earlier success in the cycle as well as improved baseline utility | ||||||
| PNF | 1212 | – | 10.647 | – | – | – |
| LF | 2410 | 1199 | 10.703 | 0.056 | 21,515 | – |
| Collagenase | 3738 | 1327 | 10.662 | –0.041 | Dominated | – |
| Assumed patients had a mean of 2 joints affected, with adjusted utility values to reflect the earlier success and increased the recurrence rate for PNF to be equivalent to CCH | ||||||
| PNF | 1364 | – | 10.597 | – | – | – |
| LF | 2410 | 1046 | 10.703 | 0.105 | 9931 | – |
| Collagenase | 3744 | 1334 | 10.66 | –0.043 | Dominated | – |
| Assumed patients had a mean of 2 joints affected, with adjusted utility values to reflect the earlier success with an equal proportion of patients receiving further treatment for PNF and CCH | ||||||
| PNF | 1234 | – | 10.652 | – | – | – |
| LF | 2410 | 1177 | 10.703 | 0.051 | Extendedly dominated | – |
| Collagenase | 4212 | 2978 | 10.782 | 0.13 | 22,917 | 26,677 |
| Assumed patients had a mean of 2 joints affected, with adjusted utility values to reflect the earlier success with an equal proportion of patients receiving further treatment for PNF and CCH and increased the recurrence rate for PNF to be equivalent to CCH | ||||||
| PNF | 1389 | – | 10.603 | – | – | – |
| LF | 2410 | 1021 | 10.703 | 0.1 | 10,250 | – |
| Collagenase | 4224 | 1814 | 10.779 | 0.076 | 23,759 | – |
| Discounting benefits at a rate of 1.5% per annum while maintaining a discount rate of 3.5% for costs | ||||||
| PNF | 1212 | – | 12.122 | – | – | – |
| LF | 2410 | 1199 | 12.24 | 0.118 | 10,114 | – |
| Collagenase | 5342 | 2931 | 12.144 | –0.096 | Dominated | – |
| No discounting for costs and benefits | ||||||
| PNF | 1237 | – | 13.834 | – | – | – |
| LF | 2413 | 1177 | 13.96 | 0.126 | 9321 | – |
| Collagenase | 5368 | 2954 | 13.851 | –0.109 | Dominated | – |
| Discounting costs and benefits at an annual rate of 6% | ||||||
| PNF | 1196 | – | 8.659 | – | – | – |
| LF | 2408 | 1212 | 8.762 | 0.102 | 11,845 | – |
| Collagenase | 5325 | 2917 | 8.693 | –0.069 | Dominated | – |
Probabilistic sensitivity analysis
To assess the impact of combined uncertainty surrounding all model parameters and inputs, appropriate distributions were assigned and randomly sampled for each 1000 iterations of the base-case analysis. Owing to the limited data available, distributions were applied to hospital-based procedure unit costs, recurrence rates, treatment success rates, complication rates, probability of further treatment and utility values. These results were used to estimate the probability of each treatment strategy being preferred in terms of cost-effectiveness for different value decision-makers’ WTP for a QALY (Table 25). The resultant CEAC is illustrated in Figure 34. Figure 35 and Table 24 show that when applying a WTP threshold of £10,000 per QALY, PNF has a 53.2% probability of being cost-effective. However, at a WTP threshold of ≈ £14,000, the CEACs for PNF and LF cross. Implementing a WTP threshold of £20,000 per QALY, LF has a 64.3% probability of being considered cost-effective, followed by PNF (35.5%) and CCH (0.2%). Figure 35 shows the empirical estimate of the joint distribution of incremental costs and effects for PNF, CCH and LF. This figure clearly illustrates that CCH is dominated, in that it is more costly and less effective than LF, and PNF is the preferred option up until decision-makers’ WTP per QALY gained exceeds ≈ £14,000.
| Strategy | Probability of cost effectiveness at society’s threshold value of WTP | |||
|---|---|---|---|---|
| £10,000 | £20,000 | £30,000 | £50,000 | |
| CCH | 0.0% | 0.2% | 2.8% | 6.8% |
| PNF | 53.2% | 35.5% | 25.8% | 21.1% |
| LF | 46.8% | 64.3% | 71.4% | 71.1% |
FIGURE 34.
Cost-effectiveness acceptability curve.
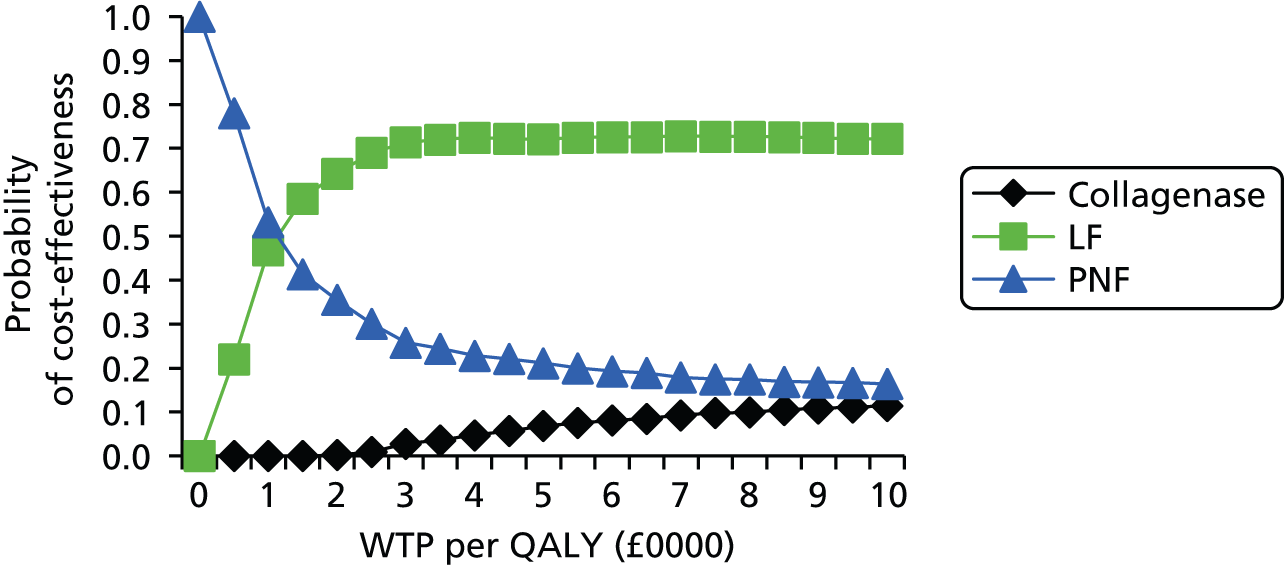
FIGURE 35.
Incremental cost-effectiveness scatterplot.

Differential results for subgroups according to patients with moderate and severe disease
Although few data were available from the identified studies to conduct subgroup analyses, the manufacturer’s submission presented the CCH treatment success rates and the mean number of joints affected for both patients with moderate and severe disease. Thus, two additional subgroup analyses, according to the severity of disease, were performed:
-
Population of patients with moderate disease and up to two affected joints. Patients with moderate disease were assumed to have a mean of 1.47 joints affected, with a mean of 1.6 CCH injections required per joint. CCH had a success rate of 80.7%. The cost of initial CCH treatment was £2359 (consisting of treatment of 1.47 joints), while the cost of CCH treatment per joint recurrence was £1605.
-
Population of patients with severe disease and up to two affected joints. Patients with severe disease were assumed to have a mean of 1.43 affected joints, with a mean of 1.6 CCH injections required per joint. The success rate of CCH was 53.7%. The cost of initial CCH treatment was £2295 (consisting of treatment of 1.43 joints), whereas the cost of CCH treatment per joint recurrence was £1605.
As fewer joints than the original base case were affected, utility values were revised. Expert opinion was used to identify five possible configurations of contracture for moderate and severe disease to derive appropriate utility values (Tables 26 and 27). For each configuration, a utility weight was calculated using the equations by Gu et al. for the dominant and non-dominant hands. An assumption was made as to the proportion of patients suffering Dupuytren’s disease in the dominant (67%) and non-dominant hand (33%). 107
| Health state | Utility value |
|---|---|
| < 75 years | |
| Treatment success | 0.780 |
| Baseline, treatment failure and recurrence | 0.743 |
| ≥ 75 years | |
| Treatment success | 0.730 |
| Baseline, treatment failure and recurrence | 0.695 |
| Health state | Utility value |
|---|---|
| < 75 years | |
| Treatment success | 0.780 |
| Baseline, treatment failure and recurrence | 0.725 |
| ≥ 75 years | |
| Treatment success | 0.730 |
| Baseline, treatment failure and recurrence | 0.678 |
A series of deterministic sensitivity analyses were conducted to test the sensitivity of the base-case results to the changes in key parameter values. Results of the additional analyses are presented separately for the moderate disease (Table 28) and the severe disease (Table 29) subgroups.
-
Base case: a mean of 1.47 affected joints; 1.6 CCH injections required; success rate of 80.7% for CCH; cost of initial CCH treatment £2359 and recurrent CCH treatment £1605.
-
The upper limit of the 95% CI surrounding the success rate for CCH (82.1%).
-
Constant lifetime recurrence, with recurrence rates the same as the original base case.
-
A 6-month probability of recurrence of 0.061 for all three treatments (based on the CORDLESS 5-year results) with recurrence occurring only in the first 5 years of the model.
-
Utility values calculated using the Assessment Group’s original method (i.e. condition specific with a utility of 1 for treatment success). Utility values: baseline, treatment failure = 0.950; treatment success = 1; recurrence = 0.965.
| Strategy | Cost, £ | Incremental cost, £ | QALYs | Incremental QALYs | ICER (not reporting ICER for extendedly dominated strategies), £ | ICER (reporting ICER for extendedly dominated strategies) |
|---|---|---|---|---|---|---|
| Base case | ||||||
| PNF | 1207 | – | 8.458 | – | – | – |
| LF | 2410 | 1203 | 8.481 | 0.023 | 51,731 | – |
| Collagenase | 2793 | 383 | 8.457 | –0.024 | Dominated | – |
| The upper limit of the 95% CI surrounding the success rate for CCH (82.1%) | ||||||
| PNF | 1207 | – | 8.458 | – | – | – |
| LF | 2410 | 1203 | 8.481 | 0.023 | 52,009 | – |
| Collagenase | 2786 | 375 | 8.46 | –0.021 | Dominated | – |
| Constant lifetime recurrence, with recurrence rates the same as the original base case | ||||||
| PNF | 1348 | – | 8.441 | – | – | – |
| LF | 2450 | 1103 | 8.473 | 0.032 | 34,291 | – |
| Collagenase | 2956 | 506 | 8.418 | –0.055 | Dominated | – |
| A 6-month probability of recurrence of 0.061 for all three treatments (based on the CORDLESS 5-year results) with recurrence occurring only in the first 5 years of the model | ||||||
| PNF | 1420 | – | 8.407 | – | – | – |
| LF | 2674 | 1254 | 8.403 | –0.004 | Dominated | – |
| Collagenase | 2827 | 1407 | 8.446 | 0.039 | 36,462 | – |
| Utility values calculated using the Assessment Group’s original method (i.e. condition-specific with a utility of 1 for treatment success) | ||||||
| PNF | 1207 | – | 11.018 | – | – | – |
| LF | 2410 | 1203 | 11.046 | 0.028 | 42,718 | – |
| Collagenase | 2793 | 383 | 11.042 | –0.004 | Dominated | – |
A probabilistic sensitivity analysis was conducted. A gamma distribution was assigned to hospital-based procedure unit costs and beta distributions were applied to recurrence rates, treatment success rates, complication rates, probability of further treatment and utility values to reflect the uncertainty surrounding them.
Figure 36 shows the CEAC for patients with moderate disease and Figure 37 shows the incremental cost-effectiveness scatterplot for patients with moderate disease. Figure 37 illustrates that, implementing a WTP threshold of £20,000 per QALY gained, PNF has a 40.6% probability of being cost-effective, followed by LF (39.7%) and CCH (19.7%).
FIGURE 36.
Cost-effectiveness acceptability curve: moderate disease.
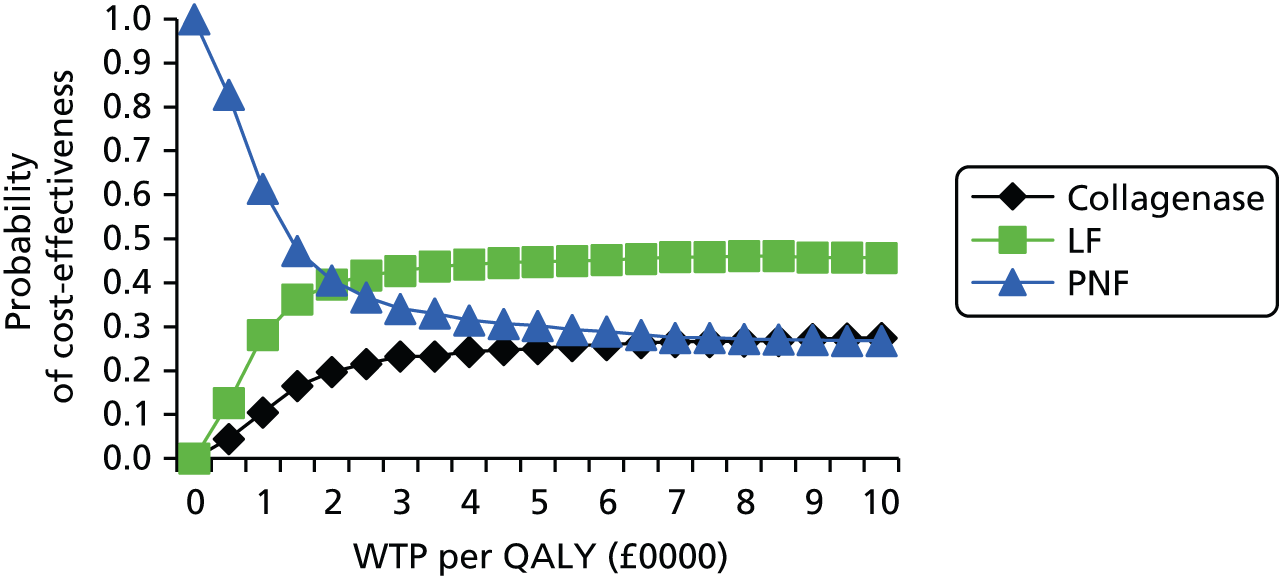
FIGURE 37.
Incremental cost-effectiveness scatterplot: moderate disease.

Severe disease
Table 29 shows the same types of cost-effectiveness analyses presented in Table 28 for a population of patients with severe disease and up to two affected joints.
-
Base case: 1.43 affected joints; 1.6 injections of CCH required; 53.7% success rate of CCH; initial CCH treatment £2,359 and £1,605 for recurrent CCH treatment.
-
The upper limit of the 95% CI surrounding the success rate for CCH (82.1%).
-
Constant lifetime recurrence, with recurrence rates the same as the original base case.
-
A 6-month probability of recurrence of 0.061 for all three treatments (based on the CORDLESS 5-year results) with recurrence occurring only in the first 5 years of the model.
-
Utility values calculated using the Assessment Group’s original method (i.e. condition-specific with a utility of 1 for treatment success). Utility values: baseline and treatment failure = 0.925; treatment success = 1; and recurrence 0.965.
| Strategy | Cost, £ | Incremental cost, £ | QALYs | Incremental QALYs | ICER (not reporting ICER for extendedly dominated strategies), £ | ICER (reporting ICER for extendedly dominated strategies) |
|---|---|---|---|---|---|---|
| Base case | ||||||
| PNF | 1214 | – | 8.378 | – | – | – |
| LF | 2410 | 1196 | 8.416 | 0.038 | 31,461 | – |
| Collagenase | 2872 | 462 | 8.3 | –0.116 | Dominated | – |
| The upper limit of the 95% CI surrounding the success rate for CCH (54.7%) | ||||||
| PNF | 1214 | – | 8.378 | – | 0 | – |
| LF | 2410 | 1196 | 8.416 | 0.038 | 31,571 | – |
| Collagenase | 2867 | 457 | 8.303 | –0.113 | Dominated | – |
| Constant lifetime recurrence, with recurrence rates the same as the original base case | ||||||
| PNF | 1353 | – | 8.353 | – | – | – |
| LF | 2450 | 1097 | 8.404 | 0.051 | 21,625 | – |
| Collagenase | 2989 | 539 | 8.259 | –0.145 | Dominated | – |
| A 6-month probability of recurrence of 0.061 for all three treatments (based on the CORDLESS 5-year results) with recurrence occurring only in the first 5 years of the model | ||||||
| PNF | 1428 | – | 8.302 | – | – | – |
| LF | 2674 | 1246 | 8.301 | –0.002 | Dominated | – |
| Collagenase | 2917 | 1489 | 8.277 | –0.025 | Dominated | – |
| Utility values calculated using the Assessment Group’s original method (i.e. condition specific with a utility of 1 for treatment success) | ||||||
| PNF | 1214 | – | 10.916 | – | – | – |
| LF | 2410 | 1196 | 10.96 | 0.044 | 27,221 | – |
| Collagenase | 2872 | 462 | 10.85 | –0.11 | Dominated | – |
A probabilistic sensitivity analysis was also conducted for the severe group. A gamma distribution was assigned to hospital-based procedure unit costs and beta distributions were applied to recurrence rates, treatment success rates, complication rates, probability of further treatment and utility values to reflect the uncertainty surrounding them.
Figure 38 shows the CEAC for patients with severe disease and Figure 39 shows the incremental cost-effectiveness scatterplot for patients with severe disease. Figure 39 shows that, applying a WTP ratio of £20,000 per QALY gained, LF has a 45.1% probability of being considered cost-effective, followed by PNF (30.8%) and CCH (24.1%).
FIGURE 38.
Cost-effectiveness acceptability curve: severe disease.
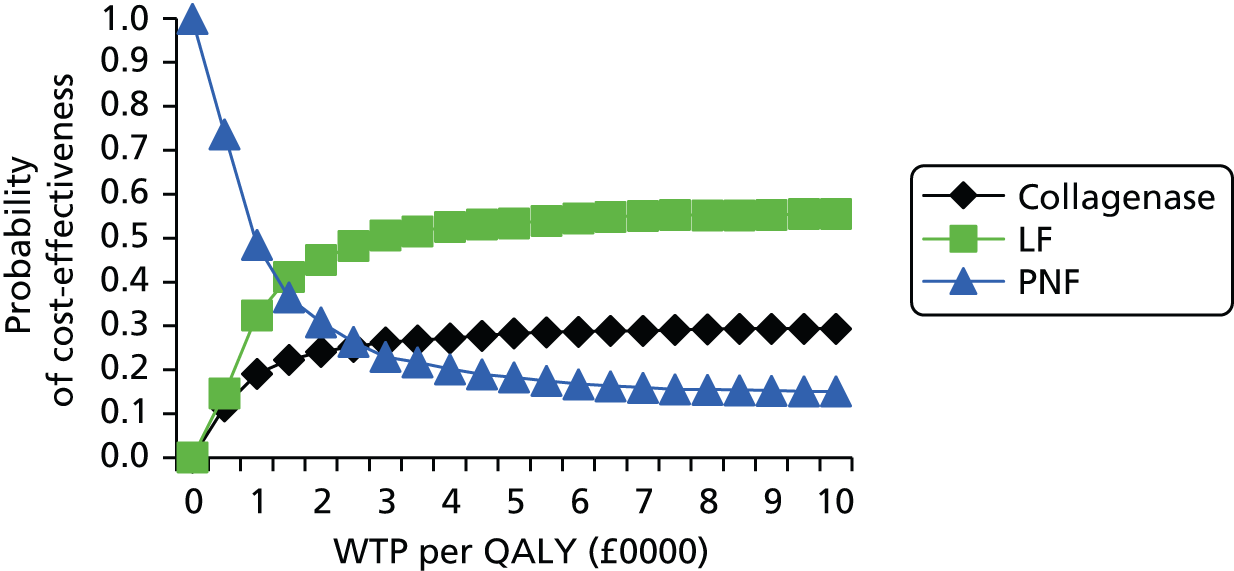
FIGURE 39.
Incremental cost-effectiveness scatterplot: severe disease.

Discussion
The results of the deterministic economic modelling suggest that, when considering QALYs as the unit of outcome in patients with three joints affected, LF is likely to be the most cost-effective option in comparison with PNF and CCH. The ICER for LF in many of the scenarios remained stable and is, therefore, reasonably robust, with an ICER under the threshold of £20,000 per QALY gained. As demonstrated by the sensitivity analyses, the cost-effectiveness results are primarily driven by treatment effectiveness. LF appears to provide the most favourable estimates of cost-effectiveness, owing to more favourable success rates and recurrence rates compared with PNF and CCH. The model results are also driven by the incremental costs; as well as the incremental costs of first-line treatment, which are lowest in PNF and highest for collagenase. Higher failure and a higher recurrence will have subsequent knock-on effects on costs, through more patients progressing for further second- and third-line treatments. However, the success rates and recurrence rates for LF are sufficient to offset the extra ‘up-front’ surgery costs of fasciectomy relative to the lower ‘up-front’ costs of PNF.
The deterministic sensitivity analysis shows the conditions under which the incremental cost per QALY estimates for CCH may start to become favourable. The modelling suggests that the success rate of CCH would need to increase from 63% to 77% if it were to offer a cost-effective alternative using base-case assumptions, compared with PNF and LF. This is due to the higher initial treatment cost and higher recurrence rate. Treatment costs can, however, be reduced through patient selection (i.e. where one joint is affected, costs are lower compared with LF).
In the subgroup analyses, for patients with moderate (1.47 joints affected) and severe (1.43 joints affected) disease, the results of the base-case deterministic analyses suggest that PNF is the preferable option in terms of cost-effectiveness. CCH remained dominated in the majority of scenarios and did not produce an ICER under the threshold of £20,000 in any of the analyses.
Summary of key results
The findings of the economic modelling suggest that, for patients with two or three joints affected, when considering QALYs as the unit of outcome, LF is likely to be the most cost-effective in comparison with PNF and CCH. The base-case estimate for LF, relative to PNF, is £10,871 per QALY gained. LF costs less and produces more QALYs than CCH. Based on the probabilistic sensitivity analysis, LF had a 64.3% chance of being cost-effective at a ceiling WTP ratio of £20,000 per QALY.
These estimates were generally found to be robust to uncertainty surrounding various model parameter inputs and assumptions, as illustrated by the deterministic sensitivity analysis.
The base-case finding that LF is the preferred option is driven by a number of factors: (1) greater QALY gains versus PNF and CCH; (2) lower cost compared with CCH; (3) lower clinical recurrence rate compared with PNF and CCH; and (4) higher treatment success rate compared with PNF and CCH.
Generalisability of results
Given that there are currently no head-to-head RCTs evaluating the strategies of interest, the effectiveness parameters within the model are largely populated by two studies from the USA and the Netherlands. This leads to a potential problem of transferability of effectiveness results to the UK, as surgeon quality and thresholds for surgery may not be the same in the UK. Furthermore, it is difficult to draw a definitive conclusion when the modelled patient population is not homogenous.
Attempts were made to identify EQ-5D data from UK cohorts for patients suffering from DC, but no data were identified. The modelling therefore relied on utility estimates derived from a recently published DCE study (deviation in part from the NICE reference case). At present, this is the only study that reports preference-weighted quality-of-life values in a UK setting.
Cost estimates were based on resource use in the UK according to expert opinion and national average unit costs were applied to resource-use estimates where possible.
Strengths and limitations
Attempts have been made to use the best available evidence for the economic model. The model does provide a flexible framework that can be easily updated to incorporate new evidence as it becomes available. This study has also identified gaps in the current literature and can identify the most important parameters that should be included in any future trials.
A major limitation is that the economic model is built from a naive indirect comparison and, as such, represents a departure from the NICE reference case. 102 The modelling was hampered by a dearth of suitable data and, as a result, a number of assumptions are inherent within the model. At present, there are no head-to-head RCTs or high-quality comparative studies comparing the relevant treatment strategies. This can be problematic as it introduces heterogeneity into the model if the patients’ characteristics differ between treatment arms of the individual studies. Moreover, no studies with sufficient follow-up to track patients post DC treatment were available. Therefore, uncertainty remains regarding the applicability of many parameter model inputs. This emphasises the importance of further research to be conducted in the UK.
Conclusions
Overall, the naive indirect comparison, the level of uncertainty surrounding the model inputs and the large number of assumptions used makes it challenging to draw definitive conclusions of the cost-effectiveness of CCH, PNF and LF for the treatment of adults with DC. However, our modelling does suggest that if success and recurrence rates included in the model are reflective of those in the UK general population, then LF is likely to offer the most cost-effective approach, for patients with two or three joints affected. The subgroup probabilistic sensitivity analyses indicate that for patients with moderate disease and fewer joints affected, PNF is the preferred treatment option, whereas for patients with severe disease and fewer joints affected, LF has the highest probability of being cost-effective (45.1%). Future research should comprise a well-designed RCT, which would enable the generation of comparable estimates, in the form of recurrence rates, success and failure rates, complications and a measurement of utility from the EQ-5D, across the treatment strategies of interest.
Chapter 5 Discussion
Statement of principal findings
Principal findings: clinical effectiveness
No head-to-head randomised trials of collagenase versus surgery were identified in the current literature. For the purpose of this assessment we considered evidence from:
-
five RCTs comparing collagenase with placebo (493 participants)
-
fifteen case series assessing the effects and safety of collagenase (3154 participants)
-
two non-randomised comparative studies of collagenase versus surgery (105 participants)
-
three RCTs comparing different surgical procedures (334 participants) and
-
five non-randomised comparative studies assessing different surgical procedures (3571 participants).
Of the five RCTs comparing collagenase with placebo, three55,56,65 provided outcome measures that could be assessed in meta-analyses. Only one trial complied with the time horizon of 12 months’ follow-up recommended by the European Medicines Agency for efficacy trials, whereas both the CORD I and CORD II trials had a double-blind, randomised phase of 90 days and a subsequent open-label phase of 9 months.
-
Primary MCP joints and PIP joints treated with collagenase were significantly more likely to achieve clinical success (i.e. reduction of contracture to 0–5° of normal 30 days after last injection) or clinical improvement (i.e. reduction in contracture of at least 50% 30 days after last injection) than those treated with placebo, with a greater reduction observed in MCP joints than in PIP joints.
-
Significantly more participants treated with collagenase experienced adverse events (i.e. peripheral oedema, contusion, pain in extremity, injection site pain, injection site haemorrhage, injection site swelling, tenderness, pruritus, lymphadenopathy, axillary pain), with the exception of injection site vesicles, where the risk was low and similar for both treatment groups. In addition, four serious adverse events were reported among participants treated with collagenase (one case of complex regional pain syndrome and two cases of tendon rupture in CORD I55 and one case of flexion pulley rupture in CORD II56). It is worth also noting that two collagenase case series79,85 reported a total of four serious adverse events after collagenase injections: two flexor tendon ruptures, one pulley rupture and one episode of pain requiring hospitalisation.
-
No recurrences were observed in the CORD I55 and CORD II56 trials during the study period (90 days). Badalamente and Hurst5 reported that five joints (four PIP joints and one MCP joint) had a recurrence over the 24-month follow-up period (12-month controlled trial followed by an open-label phase of 12 months).
Rates of recurrence for collagenase were derived from the Badalamente et al. RCT64 and single-arm observational studies (collagenase case series):
-
In general, recurrence rates for MCP joints varied from 0% at 1 year to 27% at 3 years, whereas those for PIP joints varied from 0% at 1 year to 56% at 3 years.
-
The manufacturer provided 5-year data for the CORDLESS study that included patients from previous collagenase cohorts (e.g. CORD I, CORD II, JOINT I, JOINT II). The rate of recurrence for successfully treated joints at 5 years was 46.7%.
-
One small study reported the results of eight patients treated with collagenase (six treated for MCP joint contracture and two for PIP joint contracture) who completed the 8-year assessment, the longest follow-up reported in the literature. The observed recurrence rate was 67% (4/6) for MCP joints and 100% (2/2) for PIP joints.
The two small, non-randomised studies, at high risk of bias, that compared collagenase with fasciectomy71 and PNF72 showed that:
-
MCP joints treated with collagenase had a similar reduction in contracture from baseline than those treated with fasciectomy (mean post treatment: 3.6° vs. 3.7°). However, PIP joints treated with fasciectomy had a greater reduction in contracture (mean post treatment: 8.1°) from baseline than those treated with collagenase (mean post treatment: 17.5°). 71
-
A greater proportion of both MCP and PIP joints treated with PNF showed reduction in contracture (67%) than collagenase-treated joints (56%). 72
-
No recurrences were observed during the study period (mean follow-up was 6 months in one study and 32–39 months in the other study).
Owing to the small sample size and the high risk of bias of these two non-randomised comparative studies, it is not possible to draw any firm conclusions on the relative efficacy of collagenase versus either fasciectomy or PNF.
The RCTs and non-randomised comparative studies assessing different surgical techniques tended towards greater clinical success for MCP joints than PIP joints, with slightly higher success rates for fasciectomy than fasciotomy.
Randomised controlled trials assessing different surgical interventions reported rates of recurrence that ranged from 13% for fasciectomy at 3 years to 85% for PNF at 5 years. It is worth noting that the 85% recurrence rate was reported by the van Rijssen et al. study70 that assessed the effects and complications of PNF compared with fasciectomy. van Rijssen et al. defined recurrence as an increase of total passive extension deficit of at least 30° compared with the 6-week post-operative values in all treated hands. 70 However, if the definition of the CORD trials was applied (i.e. return of contracture of at least 20° in successfully treated joints), the 5-year recurrence rate for PNF would be 21.8% for MCP joints and 23.5% for PIP joints (see Table 6), considerably lower than that reported for collagenase. Recurrence rates in non-randomised comparative studies ranged from 0% to 50% for fasciectomy at around 3 years and from 15% to 50% for open fasciotomy at around 2 years. Mild adverse events such as oedema, ecchymosis, skin tears, swelling, infection, haematoma, pain and delayed wound healing were reported more often after fasciectomy than PNF, fasciotomy or dermofasciectomy.
The inconsistencies in the definitions and measurement of treatment efficacy, characteristics of participants, and choice of comparators across studies made any comparison of data challenging. Given that no common comparator treatment was identified between the various comparative studies, it was impossible to conduct an indirect meta-analysis as recommended by the NICE methods guide.
In brief, collagenase proved to be superior to placebo in short-term RCTs, with better results for MCP than PIP joints. The long-term effects of collagenase are yet to be determined. Similarly, the treatment of recurrences after collagenase injections needs to be clarified. It is presently unclear whether collagenase can be used as a second-line treatment for DC. Further surgery (i.e. LF) after collagenase could be potentially more difficult than standard surgical revision98 (Mr David Lawrie, personal communication). Observational data indicated a recurrence rate of 47% at 5 years, which is not dissimilar to, and even higher than, the recurrence rates of LF and PNF reported in some cohorts in the literature. However, collagenase has not yet been compared with any surgical interventions in RCTs or high-quality comparative studies.
Principal findings: cost-effectiveness
The manufacturer submitted an economic evaluation, which suggested that collagenase was cost-effective. However, our economic modelling found that under base-case assumptions, LF is the preferable option from a cost-effectiveness perspective. This estimate was generally found to be robust to varying key model parameter inputs and assumptions. These findings stem from the higher success rate and lower recurrence rate compared with collagenase and PNF. However, the deterministic sensitivity analyses show that PNF may be a cost-effective alternative to LF when the proportion of patients proceeding for further treatment are varied. PNF is the most cost-effective option when all patients proceeded for further treatment following a recurrence. The modelling suggests that the success rate of collagenase would need to increase from 63% at base case to 77% if it were to offer a cost-effective alternative to PNF or LF. This is due to the higher initial treatment cost and higher recurrence rate. Treatment costs can, however, be reduced through patient selection (i.e. where one joint is affected, costs are lower compared with LF, although this alone would not be sufficient to make collagenase less costly than PNF and so it remains dominated in this analysis).
A probabilistic sensitivity analysis was conducted to assess the impact of combined uncertainty surrounding certain model parameters and inputs. Owing to the limited data available, distributions were applied to hospital-based procedure unit costs, recurrence rates, treatment success rates, complication rates, probability of further treatment and utility values and were randomly sampled for 1000 iterations of the base-case analysis. The probabilistic sensitivity analysis suggest that at a WTP for a QALY gained of £20,000, the chance of collagenase, PNF and LF being the most cost-effective treatment strategy is 0.2%, 35.5% and 64.3%, respectively. The case for cost-effectiveness of LF increases to 71.4% and 72.1% at threshold values of WTP of £30,000 and £50,000.
The subgroup analyses indicate that collagenase is dominated in the majority of scenarios and did not produce an ICER under the threshold of £20,000 per QALY. The results from the probabilistic sensitivity analyses show that for patients with moderate disease and with fewer joints affected, PNF may offer a cost-effective treatment option, whereas for patients with severe disease, LF has the highest probability of being cost-effective.
Strengths and limitations of the assessment
A key strength of this assessment is that the systematic reviews of clinical and economic evidence have been conducted by an independent research team to high methodological standards. Exhaustive systematic searches of the major electronic databases were performed and we are quite confident that we have not missed any relevant reports on collagenase. All potentially relevant studies were retrieved and reviewed for eligibility. The risk of bias of all identified evidence, including non-randomised comparative studies and case series, was assessed using the best available tools. Data were extracted using standard forms by two independent reviewers. Despite these efforts, there is still a possibility that some relevant evidence could have been missed, although such omissions are likely to be minimal.
Limitations of the review of clinical effectiveness
The main limitation of this assessment was the lack of comparative evidence on collagenase versus surgery and the small evidence base for estimating the effects of specific surgical procedures (i.e. fasciectomy and PNF). In particular, the review of clinical effectiveness was hampered by (1) the lack of randomised trials or high-quality comparative studies assessing collagenase versus surgery; (2) the use of observational studies for estimating rates of recurrence and complications after collagenase treatment; and (3) the major inconsistencies in the way efficacy was measured and reported across studies.
The substantial differences across studies in terms of characteristics of patient population (e.g. age, sex, severity of disease), measures of efficacy (e.g. changes in degree contracture, passive or active extension deficit, total passive extension deficit, percentage of improvement), population denominator (e.g. by patient, by hand, by finger, by joint), definition of recurrence, and length of follow-up inevitably limited the comparability of evidence.
Strengths and limitations of the economic evaluation
Attempts were made to use the best available evidence to populate the economic model. The model provided a flexible framework that can be updated to incorporate new evidence as it becomes available. This study has also highlighted gaps in the current literature and identified the most important parameters that should be included in any future trials. The conduct of a wide range of sensitivity analysis helped to offset some of the uncertainty and to identify the most important drivers of cost-effectiveness.
There were a number of limitations associated with the estimates used to populate the model parameters, and the results should be interpreted with caution. A major limitation was that the economic model was built from a naive indirect comparison of data and, as such, represents a departure from the NICE reference case. The modelling was hampered by a dearth of suitable data and, as a result, many untestable assumptions are built within the model. At present, there are no RCTs or high-quality comparative studies comparing the relevant treatment strategies. This can be problematic as it introduces heterogeneity into the model if the patients’ characteristics differ between treatment arms of the individual studies. Moreover, no studies with sufficient follow-up to track patients post DC treatment were available. Therefore, uncertainty remains regarding the appropriateness of many parameter model inputs. This emphasises the importance of further research to be conducted in the UK.
Uncertainties
Clinical effectiveness
The comparative efficacy of collagenase versus any surgical procedures has yet to be demonstrated. Evidence on long-term effectiveness, recurrence rate and risk of complications is still lacking. Second-line treatments after unsuccessful collagenase injections are yet to be defined.
There was considerable variation in measurements of contractures, population denominators and definitions of success and recurrence across included studies.
At present, there is little consistency in the way outcomes for Dupuytren’s disease are measured and reported. In particular, there are no standard methods or consensus for assessing the degree of contracture, defining recurrence and determining the length of follow-up. Moreover, indications for specific surgical procedures (e.g. PNF) and for second-line treatments after disease recurrence (e.g. collagenase, PNF) are not clearly defined.
Cost-effectiveness
There was substantial uncertainty surrounding the values for many of the variables in the model, and so the estimated ICERs should be interpreted with caution. Estimates of utilities for health states in the model were indirectly derived from a recently published DCE rather than directly measured from a preference-weighted quality-of-life instrument. Thus, the extent to which changes in quality of life have been adequately captured, in the sense of them representing valid estimates of change on the usual 0 = dead, 1 = full health scale, is unknown. Direct measurement of health-related quality of life using a generic preference-based instrument, such as the EQ-5D, the Short Form questionnaire-12 items or the Health Utilities Index-3, would more accurately capture the impact of improvement in joint contracture that is offered by all treatments. Although the deterministic and probabilistic analyses address some of these uncertainties, there is an underlying weakness in the clinical effectiveness evidence base that cannot be dealt with through sensitivity analysis, no matter how extensively this is conducted.
Overall, the naive indirect comparison, the level of uncertainty surrounding the model inputs and the large number of assumptions used makes it challenging to draw definitive conclusions on the cost-effectiveness of collagenase relative to PNF and LF for the treatment of adults with DC. However, our modelling does suggest that if the parameter estimates are broadly reflective of those likely to apply in UK clinical practice, then LF is likely to offer the most cost-effective approach for DC patients with more than one joint affected.
Chapter 6 Conclusions
Implications for service provision
The manufacturer’s submission suggested that collagenase was cost-effective. However, we found no evidence to suggest that collagenase is a cost-effective use of NHS resources for the treatment of DC in a patient population with moderate to severe disease who would otherwise be considered for surgery.
Suggested research priorities
Further research on treatments for DC would benefit from a consensus on measurement of contractures, unit of measurement (e.g. joints) and definitions of success and recurrence.
Large, well-designed, RCTs need to be undertaken to compare the efficacy and safety of collagenase with surgical interventions, especially PNF and LF, in people with DC. Ideally, such clinical trials would include a clear and agreed definition of recurrence, objective measurements of outcomes, longer follow-up assessments and quality-of-life measurements. For consistency with previously published studies, a reasonable definition of recurrence is that reported in the CORD I, CORD II and van Rijssen et al. clinical trials: a return in contracture of at least 20° in successfully treated joints. 55,56,70 The longest follow-up reported in current clinical studies is 5 years. Given that the estimated mean age of patients is 63 years, it would be helpful if future trials could follow patients for at least 5 years and, ideally, for a longer period, such as 10 years. This would enable a comparison of longer-term recurrence rates between treatments and would be useful to capture second- and third-line treatment options following initial treatment failures or recurrences. It would also enable a prospective collection of patient-specific health-care resource use and preference-based health status (utility) data.
Acknowledgements
The authors would like to thank Lara Kemp for her secretarial support.
Source of funding
This report was commissioned by the National Institute for Health Research (NIHR) HTA programme as project number 13/05/01. The Health Services Research Unit and Health Economics Research Unit are core-funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates. The views and opinions expressed are those of the authors and do not necessarily reflect those of the funders.
Contributions of authors
Miriam Brazzelli (Senior Research Fellow) oversaw and co-ordinated all aspects of the assessment, led the expert Advisory Group participation and contributed to the review of clinical effectiveness.
Moira Cruickshank and Clare Robertson (Research Fellows) led the day-to-day running of the assessment and undertook the review of clinical effectiveness.
Emma Tassie (Research Assistant) undertook the health economic review and conducted the economic evaluation with supervision from Paul McNamee (Professor of Health Economics).
Andrew Elders (Statistician) provided statistical advice.
Cynthia Fraser (Senior Information Officer) developed and ran the search strategies and was responsible for obtaining full-text papers and compiling the reference list of the report.
Rodolfo Hernandez (Research Fellow) contributed to the checking of the economic model.
David Lawrie (Clinical Senior Lecturer) provided expert advice on the clinical aspects and management of the disease.
Craig Ramsay (Health Care Assessment Programme Director) jointly co-ordinated the assessment and commented on the draft version of the report.
All authors assisted in preparing the final version of the report and commenting on early drafts.
Rider on responsibility for report
The views expressed in this report are those of the authors and not necessarily those of the Chief Scientist Office of the Scottish Government Health Directorates or of the National Institute for Health Research Health Technology Assessment programme. Any errors are the responsibility of the authors.
Data sharing statement
Technical appendices are available from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Townley WA, Baker R, Sheppard N, Grobbelaar AO. Dupuytren’s contracture unfolded. BMJ 2006;332:397-400. http://dx.doi.org/10.1136/bmj.332.7538.397.
- Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nature Rev Rheumatol 2010;6:715-26. http://dx.doi.org/10.1038/nrrheum.2010.180.
- van Dijk D, Finigan P, Gerber RA, Szczypa PP, Werker PM. Recognition, diagnosis and referral of patients with Dupuytren’s disease: a review of current concepts for general practitioners in Europe. Curr Med Res Opin 2013;29:269-77. http://dx.doi.org/10.1185/03007995.2013.766163.
- Wilburn J, McKenna SP, Perry-Hinsley D, Bayat A. The impact of dupuytren disease on patient activity and quality of life. J Hand Surg 2013;38:1209-14. http://dx.doi.org/10.1016/j.jhsa.2013.03.036.
- Luck CV. Dupuytren’s contracture; a new concept of the pathogenesis correlated with surgical management. J Bone Joint Surg Am 1959;41:635-64.
- Gerber RA, Perry R, Thompson R, Bainbridge C. Dupuytren’s contracture: a retrospective database analysis to assess clinical management and costs in England. BMC Musculoskelet Disord 2011;12. http://dx.doi.org/10.1186/1471-2474-12-73.
- Hentz VR. Collagenase injections for treatment of Dupuytren disease. Hand Clin 2014;30:25-32. http://dx.doi.org/10.1016/j.hcl.2013.08.016.
- Bayat A, McGrouther DA. Management of Dupuytren’s disease – clear advice for an elusive condition. Ann R Coll Surg Engl 2006;88:3-8. http://dx.doi.org/10.1308/003588406X83104.
- Rayan GM. Dupuytren disease: anatomy, pathology, presentation, and treatment. J Bone Joint Surg Am 2007;89:190-8.
- Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty 2010;10.
- Azzopardi E, Boyce DE. Clostridium histolyticum collagenase in the treatment of Dupuytren’s contracture. Br J Hosp Med 2012;73:432-6. http://dx.doi.org/10.12968/hmed.2012.73.8.432.
- Assessment Report: Xiapex Procedure No. EMEA/H/C/2048. London: European Medicines Agency; 2011.
- Dolmans GH, Werker PM, Hennies HC, Furniss D, Festen EA, Franke L, et al. Wnt signaling and Dupuytren’s disease. N Engl J Med 2011;365:307-17. http://dx.doi.org/10.1056/NEJMoa1101029.
- Gudmundsson KG, Jonsson T, Arngrimsson R. Guillaume Dupuytren and finger contractures. Lancet 2003;362:165-8. http://dx.doi.org/10.1016/S0140-6736(03)13871-8.
- Hu FZ, Nystrom A, Ahmed A, Palmquist M, Dopico R, Mossberg I, et al. Mapping of an autosomal dominant gene for Dupuytren’s contracture to chromosome 16q in a Swedish family. Clin Genet 2005;68:424-9. http://dx.doi.org/10.1111/j.1399-0004.2005.00504.x.
- Picardo NE, Khan WS. Advances in the understanding of the aetiology of Dupuytren’s disease. Surgeon 2012;10:151-8. http://dx.doi.org/10.1016/j.surge.2012.01.004.
- Thoma A, Kaur MN, Ignacy TA, Levis C, Martin S, Duku E, et al. Psychometric properties of health-related quality of life instruments in patients undergoing palmar fasciectomy for dupuytren’s disease: a prospective study. Hand 2014;9:166-74. http://dx.doi.org/10.1007/s11552-013-9597-3.
- Descatha A, Bodin J, Ha C, Goubault P, Lebreton M, Chastang JF, et al. Heavy manual work, exposure to vibration and Dupuytren’s disease? Results of a surveillance program for musculoskeletal disorders. Occup Environ Med 2012;69:296-9. http://dx.doi.org/10.1136/oemed-2011-100319.
- Godtfredsen NS, Lucht H, Prescott E, Sorensen TI, Gronbaek M. A prospective study linked both alcohol and tobacco to Dupuytren’s disease. J Clin Epidemiol 2004;57:858-63. http://dx.doi.org/10.1016/j.jclinepi.2003.11.015.
- Shaw RB, Chong AK, Zhang A, Hentz VR, Chang J. Dupuytren’s disease: history, diagnosis, and treatment. Plast Reconst Surg 2007;120:44-54e. http://dx.doi.org/10.1097/01.prs.0000278455.63546.03.
- McFarlane RM. Patterns of the diseased fascia in the fingers in Dupuytren’s contracture. Displacement of the neurovascular bundle. Plast Reconstr Surg 1974;54:31-44. http://dx.doi.org/10.1097/00006534-197407000-00004.
- Hindocha S, McGrouther DA, Bayat A. Epidemiological evaluation of Dupuytren’s disease incidence and prevalence rates in relation to etiology. Hand 2009;4:256-69. http://dx.doi.org/10.1007/s11552-008-9160-9.
- Early PF. Population studies in Dupuytren’s contracture. J Bone Joint Surg 1962;44:602-13.
- Burge P. Genetics of Dupuytren’s disease. Hand Clin 1999;15:63-71.
- Lennox IA, Murali SR, Porter R. A study of the repeatability of the diagnosis of Dupuytren’s contracture and its prevalence in the grampian region. J Hand Surg Br 1993;18:258-61. http://dx.doi.org/10.1016/0266-7681(93)90124-X.
- Ross DC. Epidemiology of Dupuytren’s disease. Hand Clin 1999;15:53-62.
- Anthony SG, Lozano-Calderon SA, Simmons BP, Jupiter JB. Gender ratio of Dupuytren’s disease in the modern U.S. population. Hand 2008;3:87-90. http://dx.doi.org/10.1007/s11552-007-9076-9.
- Mikkelsen OA. Dupuytren’s disease – a study of the pattern of distribution and stage of contracture of the hand. Hand 1976;8:265-71. http://dx.doi.org/10.1016/0072-968X(76)90013-9.
- Dias JJ, Braybrooke J. Dupuytren’s contracture: an audit of the outcomes of surgery. J Hand Surg 2006;31:514-21. http://dx.doi.org/10.1016/j.jhsb.2006.05.005.
- Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital – a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord 2007;8. http://dx.doi.org/10.1186/1471-2474-8-60.
- Sinha R, Cresswell TR, Mason R, Chakrabarti I. Functional benefit of Dupuytren’s surgery. J Hand Surg Br 2002;27:378-81. http://dx.doi.org/10.1054/jhsb.2002.0776.
- Bayat A, Cunliffe EJ, McGrouther DA. Assessment of clinical severity in Dupuytren’s disease. Br J Hosp Med 2007;68:604-9. http://dx.doi.org/10.12968/hmed.2007.68.11.27683.
- Engstrand C, Boren L, Liedberg GM. Evaluation of activity limitation and digital extension in Dupuytren’s contracture three months after fasciectomy and hand therapy interventions. J Hand Ther 2009;22:21-6. http://dx.doi.org/10.1016/j.jht.2008.08.003.
- Crean SM, Gerber RA, Le Graverand MP, Boyd DM, Cappelleri JC. The efficacy and safety of fasciectomy and fasciotomy for Dupuytren’s contracture in European patients: a structured review of published studies. J Hand Surg Eur 2011;36:396-407. http://dx.doi.org/10.1177/1753193410397971.
- Becker GW, Davis TR. The outcome of surgical treatments for primary Dupuytren’s disease – a systematic review. J Hand Surg Eur 2010;35:623-6. http://dx.doi.org/10.1177/1753193410376286.
- Kan HJ, Verrijp FW, Huisstede BMA, Hovius SER, Van Nieuwenhoven CA, Selles RW. The consequences of different definitions for recurrence of Dupuytren’s disease. J Plast Reconstruct Aesthetic Surg 2013;66:95-103. http://dx.doi.org/10.1016/j.bjps.2012.08.019.
- BSSH Evidence for Surgical Treatment 1 Dupuytren’s Disease. London: BSSH; 2010.
- Diaz R, Curtin C. Needle aponeurotomy for the treatment of dupuytren’s disease. Hand Clin 2014;30:33-8. http://dx.doi.org/10.1016/j.hcl.2013.09.005.
- Smith AC. Diagnosis and indications for surgical treatment. Hand Clin 1991;7:635-42.
- Hueston JT. The table top test. Hand 1982;14:100-3. http://dx.doi.org/10.1016/S0072-968X(82)80053-3.
- Hurst LC, Badalamente MA. Nonoperative treatment of Dupuytren’s disease. Hand Clin 1999;15:97-107.
- Radiation Therapy for Early Dupuytren’s Disease IPG368. London: National Institute for Health and Clinical Excellence; 2010.
- Dahlin LB, Bainbridge C, Leclercq C, Gerber RA, Guerin D, Cappelleri JC, et al. Dupuytren’s disease presentation, referral pathways and resource utilisation in Europe: regional analysis of a surgeon survey and patient chart review. Int J Clin Pract 2013;67:261-70. http://dx.doi.org/10.1111/ijcp.12099.
- Dias J, Bainbridge C, Leclercq C, Gerber RA, Guerin D, Cappelleri JC, et al. Surgical management of Dupuytren’s contracture in Europe: regional analysis of a surgeon survey and patient chart review. Int J Clin Pract 2013;67:271-81. http://dx.doi.org/10.1111/ijcp.12106.
- Needle Fasciotomy for Dupuytren’s Contracture IPG43. London: National Institute for Clinical Excellence; 2004.
- French MF, Mookhtiar KA, Van Wart HE. Limited proteolysis of type I collagen at hyperreactive sites by class I and II Clostridium histolyticum collagenases: complementary digestion patterns. Biochemistry (Mosc) 1987;26:681-7. http://dx.doi.org/10.1021/bi00377a004.
- Thomas A, Bayat A. The emerging role of Clostridium histolyticum collagenase in the treatment of Dupuytren disease. Ther Clin Risk Manag 2010;6:557-72. http://dx.doi.org/10.2147/TCRM.S8591.
- Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am 2000;25:629-36. http://dx.doi.org/10.1053/jhsu.2000.6918.
- Searle AE, Logan AM. A mid-term review of the results of dermofasciectomy for Dupuytren’s disease. Ann Chir Main Memb Super 1992;11:375-80. http://dx.doi.org/10.1016/S0753-9053(05)80273-X.
- Hueston JT. Limited fasciectomy for Dupuytren’s contracture. Plast Reconstr Surg Transplant Bull 1961;27:569-85. http://dx.doi.org/10.1097/00006534-196106000-00001.
- Hall PN, Fitzgerald A, Sterne GD, Logan AM. Skin replacement in Dupuytren’s disease. J Hand Surg Br 1997;22:193-7. http://dx.doi.org/10.1016/S0266-7681(97)80061-7.
- Rowley DI, Couch M, Chesney RB, Norris SH. Assessment of percutaneous fasciotomy in the management of Dupuytren’s contracture. J Hand Surg Br 1984;9:163-4. http://dx.doi.org/10.1016/S0266-7681(84)80019-4.
- Hospital Episode Statistics, Admitted Patient Care – England, 2012–13 Diagnosis. Leeds: Health and Social Care Information Centre; 2013.
- A Simple Guide to Payment by Results. London: Department of Health; 2012.
- Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FTD, Meals RA, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med 2009;361:968-79. http://dx.doi.org/10.1056/NEJMoa0810866.
- Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N. Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am 2010;35:2027-38. http://dx.doi.org/10.1016/j.jhsa.2010.08.007.
- Ribak S, Borkowski J, Amaral RP, Massato A, Ávila I, de Andrade D. Dupuytren contracture: comparative study between parcial fasciotomy and percutaneous fasciotomy. Braz J Orthop 2013;48:545-53.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011.
- Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York; 2009.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84. http://dx.doi.org/10.1136/jech.52.6.377.
- Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235-41. http://dx.doi.org/10.1016/S0895-4356(98)00131-0.
- Jackson R, Ameratunga S, Broad J, Connor J, Lethaby A, Robb G, et al. The GATE frame: critical appraisal with pictures. Evid Based Med 2006;11:35-8. http://dx.doi.org/10.1136/ebm.11.2.35.
- Badalamente M, Hurst L. Nonsurgical treatment of Dupuytren’s disease. J Investig Med 2005;53.
- Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am 2002;27:788-98. http://dx.doi.org/10.1053/jhsu.2002.35299.
- Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am 2007;32:767-74. http://dx.doi.org/10.1016/j.jhsa.2007.04.002.
- Witthaut J, Bushmakin AG, Gerber RA, Cappelleri JC, Le Graverand-Gastineau MP. Determining clinically important changes in range of motion in patients with Dupuytren’s Contracture: secondary analysis of the randomized, double-blind, placebo-controlled CORD I study. Clin Drug Invest 2011;31:791-8. http://dx.doi.org/10.1007/BF03256918.
- Citron ND, Nunez V. Recurrence after surgery for Dupuytren’s disease: a randomized trial of two skin incisions. J Hand Surg Br 2005;30:563-6. http://dx.doi.org/10.1016/j.jhsb.2005.07.001.
- Ullah AS, Dias JJ, Bhowal B. Does a ‘firebreak’ full-thickness skin graft prevent recurrence after surgery for Dupuytren’s contracture?: a prospective, randomised trial. J Bone Joint Surg Br 2009;91:374-8. http://dx.doi.org/10.1302/0301-620X.91B3.21054.
- van Rijssen AL, Gerbrandy FS, ter Linden H, Klip H, Werker PM. A comparison of the direct outcomes of percutaneous needle fasciotomy and limited fasciectomy for Dupuytren’s disease: a 6-week follow-up study. J Hand Surg Am 2006;31:717-25. http://dx.doi.org/10.1016/j.jhsa.2006.02.021.
- van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg 2012;129:469-77. http://dx.doi.org/10.1097/PRS.0b013e31823aea95.
- Naam NH. Functional outcome of collagenase injections compared with fasciectomy in treatment of Dupuytren’s contracture. Hand 2013;8:410-16. http://dx.doi.org/10.1007/s11552-013-9540-7.
- Nydick JA, Olliff BW, Garcia MJ, Hess AV, Stone JD. A comparison of percutaneous needle fasciotomy and collagenase injection for Dupuytren disease. J Hand Surg Am 2013;38:2377-80. http://dx.doi.org/10.1016/j.jhsa.2013.08.096.
- Bainbridge C, Dahlin LB, Szczypa PP, Cappelleri JC, Guerin D, Gerber RA. Current trends in the surgical management of Dupuytren’s disease in Europe: an analysis of patient charts. Eur Orthop Traumatol 2012;3:31-4. http://dx.doi.org/10.1007/s12570-012-0092-z.
- Citron N, Hearnden A. Skin tension in the aetiology of Dupuytren’s disease; a prospective trial. J Hand Surg Br 2003;28:528-30. http://dx.doi.org/10.1016/S0266-7681(03)00221-3.
- Skoff HD. The surgical treatment of Dupuytren’s contracture: a synthesis of techniques. Plast Reconstr Surg 2004;113:540-4. http://dx.doi.org/10.1097/01.PRS.0000101054.80392.88.
- Toppi JT, Trompf L, Smoll NR, Lim V, Smith K, Findlay MW, et al. Dupuytren’s contracture: an analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy [published online ahead of print ]. ANZ J Surg 2015;85:639-43. http://dx.doi.org/10.1111/ans.12513.
- Badalamente MA, Hurst LC. Longer-term follow-up of recurrence after collagenase injection for dupuytren’s contracture level 1 evidence. J Hand Surg Am 2011;36. http://dx.doi.org/10.1016/S0363-5023(11)60018-X.
- Coleman S, Gilpin D, Tursi J, Kaufman G, Jones N, Cohen B. Multiple concurrent collagenase clostridium histolyticum injections to Dupuytren’s cords: an exploratory study. BMC Musculoskelet Disord 2012;13. http://dx.doi.org/10.1186/1471-2474-13-61.
- Coleman S, Gilpin D, Kaplan FT, Houston A, Kaufman GJ, Cohen BM, et al. Efficacy and safety of concurrent collagenase clostridium histolyticum injections for multiple Dupuytren contractures. J Hand Surg Am 2014;39:57-64. http://dx.doi.org/10.1016/j.jhsa.2013.10.002.
- Considine S, Hirpara KM. Early results of the use of collagenase in the treatment of Dupuytren’s contracture. Ir J Med Sci 2013;182:S52-S53. http://dx.doi.org/10.1007/s11845-014-1111-6.
- Hayton MJ, Bayat A, Chapman DS, Gerber RA, Szczypa PP. Isolated and spontaneous correction of proximal interphalangeal joint contractures in Dupuytren’s disease: an exploratory analysis of the efficacy and safety of collagenase clostridium histolyticum. Clin Drug Invest 2013;33:905-12. http://dx.doi.org/10.1007/s40261-013-0139-0.
- Kaplan FTD, Hentz VR, Tursi J, Kaufman G, Smith T. Patterns of recurrent contracture in patients with Dupuytren’s disease 3 years following successful treatment with collagenase clostridium histolyticum: Level 4 evidence. J Hand Surg 2012;37. http://dx.doi.org/10.1016/S0363-5023(12)60019-7.
- Kaplan FTD, Badalamente M, Hurst L, Merrell GA, Pahk R. Delayed manipulation following clostridial collagenase histolyticum injection for dupuytren contracture. J Hand Surg 2013;38:e52-e53. http://dx.doi.org/10.1016/j.jhsa.2013.08.084.
- Martin-Ferrero MA, Simon-Perez C, Rodriguez-Mateos JI, Garcia-Medrano B, Hernandez-Ramajo R, Brotat-Garcia M. Treatment of Dupuytren’s disease using collagenase from Clostridium histolyticum. Rev Esp Cirug Ortop Traumatol 2013;57:398-402. http://dx.doi.org/10.1016/j.recot.2013.07.005.
- McMahon HA, Bachoura A, Jacoby SM, Zelouf DS, Culp RW, Osterman AL. Examining the efficacy and maintenance of contracture correction after collagenase clostridium histolyticum treatment for Dupuytren’s disease. Hand 2013;8:261-6. http://dx.doi.org/10.1007/s11552-013-9524-7.
- Peimer CA, Skodny P, Mackowiak JI. Collagenase clostridium histolyticum for dupuytren-contracture: patterns of use and effectiveness in clinical practice. J Hand Surg Am 2013;38:2370-6. http://dx.doi.org/10.1016/j.jhsa.2013.08.114.
- Peimer CA, Blazar P, Coleman S, Kaplan FT, Smith T, Tursi JP, et al. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS study): 3-year data. J Hand Surg Am 2013;38:12-2. http://dx.doi.org/10.1016/j.jhsa.2012.09.028.
- Skirven TM, Bachoura A, Jacoby SM, Culp RW, Osterman AL. The effect of a therapy protocol for increasing correction of severely contracted proximal interphalangeal joints caused by dupuytren disease and treated with collagenase injection. J Hand Surg Am 2013;38:684-9. http://dx.doi.org/10.1016/j.jhsa.2013.01.038.
- Watt AJ, Curtin CM, Hentz VR. Collagenase injection as nonsurgical treatment of Dupuytren’s disease: 8-year follow-up. J Hand Surg Am 2010;35:534-9. http://dx.doi.org/10.1016/j.jhsa.2010.01.003.
- Witthaut J, Jones G, Skrepnik N, Kushner H, Houston A, Lindau TR. Efficacy and safety of collagenase clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am 2013;38:2-11. http://dx.doi.org/10.1016/j.jhsa.2012.10.008.
- Syed A, Mcfarlane J, Chester T, Powers D, Sibly F, Talbot-Smith A. Clinical efficacy and cost-effectiveness of Clostridium histolyticum collagenase injections in a subpopulation of Dupuytren’s contracture patients. Eur Orthop Traumatol 2013;5:311-16. http://dx.doi.org/10.1007/s12570-013-0237-8.
- Bainbridge C, Gerber RA, Szczypa PP, Smith T, Kushner H, Cohen B, et al. Efficacy of collagenase in patients who did and did not have previous hand surgery for Dupuytren’s contracture. J Plast Surg Hand Surg 2012;46:177-83. http://dx.doi.org/10.3109/2000656X.2012.683795.
- Matton G, Beck F. Our experience with 186 operated Dupuytren hands. Comparison of two techniques. Acta Orthop Belg 1982;48:775-93.
- Brandt KE. An evidence-based approach to Dupuytren’s contracture. Plast Reconstr Surg 2010;126:2210-5. http://dx.doi.org/10.1097/PRS.0b013e3181f83129.
- Beyermann K, Prommersberger KJ, Jacobs C, Lanz UB. Severe contracture of the proximal interphalangeal joint in Dupuytren’s disease: does capsuloligamentous release improve outcome?. J Hand Surg Br 2004;29:240-3. http://dx.doi.org/10.1016/j.jhsb.2004.02.002.
- Roush TF, Stern PJ. Results following surgery for recurrent Dupuytren’s disease. J Hand Surg Am 2000;25:291-6. http://dx.doi.org/10.1053/jhsu.2000.jhsu25a0291.
- Watson JD. Fasciotomy and Z-plasty in the management of Dupuytren’s contracture. Br J Plast Surg 1984;37:27-30. http://dx.doi.org/10.1016/0007-1226(84)90036-5.
- Chen NC, Srinivasan RC, Shauver MJ, Chung KC. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand 2011;6:250-5. http://dx.doi.org/10.1007/s11552-011-9326-8.
- Salhi S, Cardin-Langlois E, Luc M. Percutaneous fasciotomy for the treatment of Dupuytren’s disease-a systematic review. Hand 2011;6:349-55. http://dx.doi.org/10.1007/s11552-011-9355-3.
- Chen NC, Shauver MJ, Chung KC. Cost-effectiveness of open partial fasciectomy, needle aponeurotomy, and collagenase injection for dupuytren contracture. J Hand Surg Am 2011;36:1826-34. http://dx.doi.org/10.1016/j.jhsa.2011.08.004.
- Baltzer H, Binhammer PA. Cost-effectiveness in the management of Dupuytren’s contracture. A Canadian cost–utility analysis of current and future management strategies. Bone Joint J 2013;95:1094-100. http://dx.doi.org/10.1302/0301-620X.95B8.31822.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Interim Life Tables, England and Wales, 2010–2012. London: ONS; 2013.
- NHS Reference Costs 2012–13. London: Department of Health; 2013.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- British National Formulary. London: BMA and RPS; 2013.
- Gu NY, Botteman MF, Gerber RA, Ji X, Postema R, Wan Y, et al. Eliciting health state utilities for Dupuytren’s contracture using a discrete choice experiment. Acta Orthopaedica 2013;84:571-8. http://dx.doi.org/10.3109/17453674.2013.865097.
Appendix 1 Search strategies
Collagenase for Dupuytren’s contracture clinical effectiveness
EMBASE
Date searched from: 1990 to 2014 Week 8.
Ovid MEDLINE
Date searched from: 1990 to February Week 2 2014.
Ovid MEDLINE In-Process & Other Non-Indexed Citations
Date searched from: 25 February 2014.
OVID multifile search URL: https://shibboleth.ovid.com/
-
Dupuytren Contracture/
-
dupuytren$.tw.
-
(palm$ adj3 fibromatosis).tw.
-
or/1-3
-
Microbial Collagenase/ use mesz
-
clostridiopeptidase A/ use oemez
-
(collagenase or xiapex or xiaflex).tw.
-
or/5-7
-
Dupuytren Contracture/su
-
(fasciectom$ or fasciotom$).tw.
-
(dermatofasciectom$ or dermofasciectom$ or dermo fasciectom$).tw
-
aponeurotom$.tw.
-
((open or closed) adj2 palm technique?).tw.
-
or/9-13
-
4 and (8 or 14)
-
exp clinical trial/ use oemez
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomization/ use oemez
-
randomi?ed.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab
-
groups.ab
-
or/16-25
-
comparative study/ use mesz
-
follow-up studies/ use mesz
-
time factors/ use mesz
-
Treatment outcome/ use oemez
-
major clinical study/ use oemez
-
controlled study/ use oemez
-
(chang$ or evaluat$ or reviewed or baseline).tw.
-
(prospective$ or retrospective$).tw.
-
(cohort$ or series).tw.
-
(compare$ or compara$).tw.
-
or/27-36
-
15 and (26 or 37)
-
exp animals/ not humans/
-
nonhuman/ not human
-
38 not (39 or 40)
-
case report/ use oemez
-
case reports.pt.
-
41 not (42 or 43)
-
remove duplicates from 44
Science Citation Index
Date searched from: 1990–27 February 2014.
Bioscience Information Service (BIOSIS)
Date searched from: 1990–27 February 2014.
Conference Proceedings Citation Index – Science
Date searched from: 2012–27 February 2014.
ISI Web of Knowledge
# 1 TS=dupuytren*
# 2 TS=(palm* NEAR/3 fibromatosis).
# 3 #1 OR #2
# 4 TS=(collagenase or xiapex ot xiaflex)
# 5 TS=(fasciectom* or fasciotom*).# 6 TS=(dermatofasciectom* or dermofasciectom* or dermo fasciectom*).
# 7 TS=aponeurotom*
# 8 TS= ((open or closed) NEAR/2 "palm technique*")
# 9 #8 OR #7 OR #6 OR #5 OR #4
#10 #9 AND #3
The Cochrane Library Issue 1 20134 [Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment Database]
URL: www3.interscience.wiley.com/
ID Search Hits
#1 MeSH descriptor: [Dupuytren Contracture] this term only
#2 dupuytren*:ti,ab,kw (Word variations have been searched)
#3 (palm* near/3 fibromatosis) .ti,ab,kw
#4 #1 or #2 or #3
#5 MeSH descriptor: [Microbial Collagenase] this term only
#6 (collagenase or xiapex or xiaflex) .ti,ab,kw
#7 (fasciectom* or fasciotom*) .ti,ab,kw
#8 (dermatofasciectom* or dermofasciectom* or dermo fasciectom*) .ti,ab,kw
#9 (aponeurotom* or ((open or closed) near/2 palm technique*)
#10 #5 or #6 or #7 or #8 or #9
#11 #4 and #10
Scopus
Date searched: 26 February 2014.
Dupuytren* [Articles In Process]
Health Technology Assessment/Database of Abstracts of Reviews of Effects
Date searched: February 2014.
Centre for Reviews and Dissemination
URL: http://nhscrd.york.ac.uk/welcome.htm
-
MeSH DESCRIPTOR Dupuytren Contracture
-
(dupuytren)
-
#1 OR #2
Additional Conference Proceedings
2013 ASSH Annual Meeting Abstracts 68th Annual Meeting, San Francisco, CA, USA.
2012 ASSH Annual Meeting Abstracts 67th Annual Meeting, Chicago, IL, USA.
2011 ASSH Annual Meeting Abstracts 66th Annual Meeting, Las Vegas, NV, USA.
FESSH 2013 29 May 2013–1 June 2013 Antalya, Turkey.
FESSH 2012, 21–23 June 2012, Antwerp, Belgium.
EUROHAND 2011, Combined XVI FESSH Congress and X EFSHT Congress, Oslo, Norway, 26–28 May 2011.
Clinical Trials
Date searched: February 2014.
URL: http://clinicaltrials.gov/ct/gui/c/r
Topic= Dupuytren Contracture
International Clinical Trials Registry Platform (ICTRP)
Date searched: June 2013.
Collagenase for Dupuytren’s contracture cost effectiveness
EMBASE
Date searched from: 1990 to 2014 Week 8.
Ovid MEDLINE(R)
Date searched from: 1990 to February Week 2 2014.
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations
Date searched: 25 February 2014.
OVID multifile search URL: https://shibboleth.ovid.com/
-
Dupuytren Contracture/
-
dupuytren$.tw.
-
(palm$ adj3 fibromatosis).tw
-
or/1-3
-
exp “costs and cost analysis”/ use mesz
-
exp economic evaluation/ use emez
-
economics/
-
health economics/ use emez
-
exp economics,hospital/ use mesz
-
exp economics,medical/ use mesz
-
economics,pharmaceutical/ use mesz
-
exp budgets/
-
exp models, economic/ use mesz
-
exp decision theory/
-
monte carlo method/
-
markov chains/
-
exp technology assessment, biomedical/
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab.
-
economics model$.tw.
-
(economic$ or pharmacoeconomic$).tw.
-
(price or prices or pricing).tw.
-
(value adj1 money).tw.
-
markov$.tw.
-
monte carlo.tw.
-
(decision$ adj2 (tree? or analy$ or model$)).tw.
-
or/5-26
-
4 and 27
-
remove duplicates from 28
Database: Health Management Information Consortium (HMIC)
Date searched from: 1979 to February 2014.
URL: https://auth.athensams.net/
-
dupuytrens contracture/
-
dupuytren$.tw.
-
(palm$ adj3 fibromatosis).tw.
-
1 or 2 or 3
Science Citation Index
Date searched from: 1990–27 February 2014.
ISI Web of Knowledge
# 1 TS=dupuytren*
# 2 TS=cost*
# 3 TS=(decision* N/2 (tree* or analy* or model))
# 4 TS=(markov or monte carlo)
# 5 TS=economic*
# 6 #5 OR #4 OR #3 OR #2
# 7 #6 AND #1
NHS Economic Evaluation Database (EED)
Seached from: February 2014.
Centre for Reviews and Dissemination
URL: http://nhscrd.york.ac.uk/welcome.htm
-
MeSH DESCRIPTOR Dupuytren Contracture
-
(dupuytren)
-
#1 OR #2
RePEc (Research Papers in Economics). URL: http://repec.org/
Dupuytren
Collagenase for Dupuytren’s contracture quality of life
EMBASE
Date searched from: 1990 to 2014 Week 8.
Ovid MEDLINE(R)
Date searched from: 1990 to February Week 2 2014.
Ovid MEDLINE In-Process & Other Non-Indexed Citations
Date seached: 25 February 2014.
OVID multifile search: URL: https://shibboleth.ovid.com/
-
Dupuytren Contracture/
-
dupuytren$.tw.
-
(palm$ adj3 fibromatosis).tw.
-
or/1-3
-
quality of life/
-
quality adjusted life year/
-
“Value of Life”/ use mesz
-
health status indicators/ use mesz
-
health status/ use emez
-
sickness impact profile/ use mesz
-
disability evaluation/ use mesz
-
disability/ use emez
-
activities of daily living/ use mesz
-
exp daily life activity/ use emez
-
cost utility analysis/ use emez
-
rating scale/
-
questionnaires/
-
(quality adj1 life).tw.
-
quality adjusted life.tw.
-
disability adjusted life.tw.
-
(qaly? or qald? or qale? or qtime? or daly?).tw
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(hql or hqol or h qol or hrqol or hr qol).tw.
-
(hye or hyes).tw
-
health$ year$ equivalent$.tw.
-
(hui or hui1 or hui2 or hui3).tw
-
(health adj3 (utilit$ or disutili$)).tw.
-
(health adj3 (state or status)).tw.
-
(sf36 or sf 36 or short form 36 or shortform 36).tw
-
(sf6 or sf 6 or short form 6 or shortform 6).tw.
-
(sf12 or sf 12 or short form 12 or shortform 12).tw.
-
(sf16 or sf 16 or short form 16 or shortform 16).tw.
-
(sf20 or sf 20 or short form 20 or shortform 20).tw.
-
willingness to pay.tw.
-
standard gamble.tw
-
trade off.tw.
-
conjoint analys?s.tw.
-
discrete choice.tw.
-
or/5-38
-
(case report or editorial or letter).pt.
-
case report/
-
4 and 39
-
42 not (40 or 41)
-
remove duplicates from
Science Citation Index (1990–27 February 2014)
ISI Web of Knowledge
# 1 TS=dupuytren*
# 2 TS=quality of life
# 3 TS=(euroqol or euro qol or eq5d or eq 5d)
# 4 TS=(utility or utilities)
# 5 TS=quality adjusted life year*
# 6 TS= (sf36 or sf 36 or short form 36 or shortform 36)
# 7 TS=disutilit*
# 8 TS=discrete choice.
# 9 TS=conjoint analys*
# 10 TS=trade off.
# 11 TS=standard gamble
# 12 TS=willingness to pay.
# 13 #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2
# 14 #13 AND #1
Cost-effectiveness Analysis Registry (CEA)
Date searched: February 2014.
URL https://research.tufts-nemc.org/cear4/default.asp
Dupuytren
Websites consulted
Agency for Healthcare Research and Quality, URL: www.ahrq.gov/
All Wales Medicine Strategy Group, URL: www.awmsg.org/
American Society for Surgery of the Hand, URL: www.assh.org/
Belgian Health Care Knowledge Centre (KCE), URL: https://kce.fgov.be/
British Dupuytren’s Society, URL: http://dupuytrens-society.org.uk/
Canadian Agency for Drugs and Technologies in Health, URL: www.cadth.ca/
European Medicine Agency, URL: www.ema.europa.eu/ema/
Federation of European Societies for Surgery of the Hand, URL: www.fessh2014.com/
French National Authority for Health (HAS), URL: www.has-sante.fr/
Health Information and Quality Authority, URL: www.hiqa.ie/
Institute for Clinical and Economic Review, URL: www.icer-review.org/
Institute for Quality and Efficiency in Health Care, URL: www.iqwig.de/
International Dupuytren Society, URL: www.dupuytren-online.info/
International Symposium on Dupuytren’s Disease, URL: http://dupuytrensymposium.com/
Medicines and Healthcare Products Regulatory Agency, URL: www.mhra.gov.uk/
Medical Services Advisory Committee, Australia, URL: www.msac.gov.au/
National Institute for Health and Care Excellence, URL: www.nice.org.uk/
NHS Quality Improvement Scotland, URL: www.healthcareimprovementscotland.org/
Scottish Medicines Consortium, URL: www.scottishmedicines.org.uk/
US Food and Drug Administration, URL: www.fda.gov/default.htm
Appendix 2 Screening form for full-text papers
Appendix 3 Risk-of-bias checklist: randomised controlled trials
| Randomised studies | ||
|---|---|---|
| Domain | Details | Low/high/unclear risk of bias |
| Adequate sequence generation? | ||
| Allocation concealment? | ||
| Blinding? | ||
| Incomplete outcome data addressed? | ||
| Free of selective reporting? | ||
| Other sources of bias: | ||
| Sample size calculation | ||
| Conflict of interest/industry funding | ||
Appendix 4 Risk-of-bias checklist: non-randomised comparative studies
| Domain | Details | High/low/unclear risk of bias |
|---|---|---|
| Was clear information provided on the way in which groups/cohorts were recruited? | ||
| How were participants allocated to groups? | ||
| Which parts of the study were prospective? | ||
| Identification of participants? | ||
| Assessment of baseline? | ||
| Allocation to intervention? | ||
| Assessment of outcomes? | ||
| Generation of hypotheses? | ||
| On what variables was comparability between groups assessed? | ||
| Baseline assessment of outcome variables? | ||
| Potential confounders? |
| Did the study…… | |
|---|---|
| … restrict participant selection so that all groups had the same value for the named confounder? | |
| … demonstrate balance between groups for the confounder? | |
| … match on the confounder? | |
| … adjust for the confounder in statistical analyses to quantify the effect size? |
| Outcome | Confounders | Confounders balanced? | Statistical adjustment Y/N | Outcome judgement (high/low/unclear) |
|---|---|---|---|---|
| Reduction of contracture | Type of joint MCP/PIP | |||
| Baseline contracture | ||||
| Type of previous treatment | ||||
| Time to normal function | Type of joint MCP/PIP | |||
| Baseline contracture | ||||
| Age | ||||
| Recurrence | Age | |||
| Sex | ||||
| Type of joint MCP/PIP | ||||
| Type of previous treatment | ||||
| Family history of disease | ||||
| Which finger affected | ||||
| Duration of disease | ||||
| Knuckle pads | ||||
| Alcohol intake | ||||
| Ectopic fibromatosis | ||||
| Ledderhose disease | ||||
| Adverse effects | Type of joint MCP/PIP | |||
| Age | ||||
| Health-related quality of life | Age |
| Outcome | Risk-of-bias domain | Y/N | Risk of bias (high/low/unclear) |
|---|---|---|---|
| Reduction of contracture | Blinding participants | ||
| Blinding health-care providers | |||
| Blinding outcome assessors | |||
| Time to normal function | Blinding participants | ||
| Blinding health-care providers | |||
| Blinding outcome assessors | |||
| Recurrence | Blinding participants | ||
| Blinding health-care providers | |||
| Blinding outcome assessors | |||
| Adverse events | Blinding participants | ||
| Blinding health-care providers | |||
| Blinding outcome assessors | |||
| Health-related quality of life | Blinding participants | ||
| Blinding health-care providers | |||
| Blinding outcome assessors |
| Outcome | Risk of bias (high/low/unclear) |
|---|---|
| Reduction of contracture | |
| Time to normal function | |
| Recurrence | |
| Adverse events | |
| Health-related quality of life |
| Outcome | Risk of bias (high/low/unclear) |
|---|---|
| Reduction of contracture | |
| Time to normal function | |
| Recurrence | |
| Adverse events | |
| Health-related quality of life |
| Study level | Risk of bias (high/low/unclear) |
|---|---|
| Free of other bias? | |
| A priori protocol | |
| A priori analysis plan |
Appendix 5 Risk-of-bias checklist: case series
Checklist for quality assessment of non-randomised studies (comparative and cohort studies)
Version 1, August 2012
Assessor initial: Date evaluated:
Study ID:
| Criteria | Yes | No | Unclear | Comments |
|---|---|---|---|---|
| 1. Were participants a representative sample selected from a relevant patient population (e.g. randomly selected from those seeking treatment despite age, duration of disease, primary or secondary disease and severity of disease)? | ||||
| 2. Were the inclusion/exclusion criteria of participants clearly described? | ||||
| 3. Were participants entering the study at a similar point in their disease progression (i.e. severity of disease)? | ||||
| 4. Was selection of patients consecutive? | ||||
| 5. Was data collection undertaken prospectively? | ||||
| 6. Were the groups comparable on demographic characteristics and clinical features? | ||||
| 7. Was the intervention (and comparison) clearly defined? | ||||
| 8. Was the intervention undertaken by someone experienced at performing the procedure?a | ||||
| 9. Were the staff, place and facilities where the patients were treated appropriate for performing the procedure (e.g. access to back-up facilities in hospital or special clinic)? | ||||
| 10. Were any of the important outcomes considered (i.e. on clinical effectiveness, cost-effectiveness, or learning curves)? | ||||
| 11. Were objective (valid and reliable) outcome measures used, including satisfaction scale? | ||||
| 12. Was the assessment of main outcomes blind? | ||||
| 13. Was follow-up long enough (≥ 1 year) to detect important effects on outcomes of interest? | ||||
| 14. Was information provided on non-respondents, dropouts?b | ||||
| 15. Were the characteristics of withdrawals/dropouts similar to those that completed the study and therefore unlikely to cause bias?c | ||||
| 16. Was length of follow-up similar between comparison groups | ||||
| 17. Were the important prognostic factors identified (e.g. age, duration of disease, disease severity)?d | ||||
| 18. Were the analyses adjusted for confounding factors? |
Appendix 6 List of included and excluded studies
Included studies
Badalamente and Hurst48
-
Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am 2000;25:629–36.
Badalamente et al.64
-
Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am 2002;27:788–98.
Badalamente and Hurst63
-
Badalamente M, Hurst L. Nonsurgical treatment of Dupuytren’s disease. J Investig Med 2005;53:S394.
Badalamente and Hurst65
-
Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am 2007;32:767–74.
Badalamente and Hurst77
-
Badalamente MA, Hurst LC. Longer-term follow-up of recurrence after collagenase injection for Dupuytren’s contracture level 1 evidence. J Hand Surgery Am 2011;36(Suppl. 1):14.
Bainbridge et al.73
-
Bainbridge C, Dahlin LB, Szczypa PP, Cappelleri JC, Guerin D, Gerber RA. Current trends in the surgical management of Dupuytren’s disease in Europe: an analysis of patient charts. Eur Orthop Traumatol 2012;3:31–41.
-
Dias JJ, Singh HP, Ullah A, Bhowal B, Thompson JR. Patterns of recontracture after surgical correction of Dupuytren disease. J Hand Surg 2013;38:1987–93.
Citron and Hearnden74
-
Citron N, Hearnden A. Skin tension in the aetiology of Dupuytren’s disease; a prospective trial. J Hand Surg Br 2003;28:528–30.
Citron and Nunez67
-
Citron ND, Nunez V. Recurrence after surgery for Dupuytren’s disease: a randomized trial of two skin incisions. J Hand Surg Br 2005;30:563–6.
Coleman et al.78
-
Coleman S, Gilpin D, Tursi J, Kaufman G, Jones N, Cohen B. Multiple concurrent collagenase clostridium histolyticum injections to Dupuytren’s cords: an exploratory study. BMC Musculoskelet Disord 2012;13:61.
Coleman et al.79
-
Coleman S, Gilpin D, Kaplan FT, Houston A, Kaufman GJ, Cohen BM, et al. Efficacy and safety of concurrent collagenase clostridium histolyticum injections for multiple Dupuytren contractures. J Hand Surg Am 2014;39:57–64.
Considine and Hirpara80
-
Considine S, Hirpara KM. Early results of the use of collagenase in the treatment of Dupuytren’s contracture. Ir J Med Sci 2013;182(Suppl. 2):52–3.
Gilpin et al.56
-
Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N. Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am 2010;35:2027–38.
Hayton et al.81
-
Hayton MJ, Bayat A, Chapman DS, Gerber RA, Szczypa PP. Isolated and spontaneous correction of proximal interphalangeal joint contractures in Dupuytren’s disease: an exploratory analysis of the efficacy and safety of collagenase clostridium histolyticum. Clin Drug Invest 2013;33:905–12.
Hurst et al.55
-
Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FTD, Meals RA, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med 2009;361:968–79.
-
Witthaut J, Bushmakin AG, Gerber RA, Cappelleri JC, Le Graverand-Gastineau MP. Determining clinically important changes in range of motion in patients with Dupuytren’s Contracture: secondary analysis of the randomized, double-blind, placebo-controlled CORD I study. Clin Drug Invest 2011;31:791–8.
Kaplan et al.83
-
Kaplan FTD, Badalamente M, Hurst L, Merrell GA, Pahk R. Delayed manipulation following clostridial collagenase histolyticum injection for dupuytren contracture. J Hand Surg Am 2013;38(Suppl. 10):e52–3.
Martin-Ferrero et al.84
-
Martin-Ferrero MA, Simon-Perez C, Rodriguez-Mateos JI, Garcia-Medrano B, Hernandez-Ramajo R, Brotat-Garcia M. [Treatment of Dupuytren’s disease using collagenase from Clostridium histolyticum.] Rev Esp Cir Ortop Traumatol 2013;57:398–402.
McMahon et al.85
-
McMahon HA, Bachoura A, Jacoby SM, Zelouf DS, Culp RW, Osterman AL. Examining the efficacy and maintenance of contracture correction after collagenase clostridium histolyticum treatment for Dupuytren’s disease. Hand 2013;8:261–6.
Naam71
-
Naam NH. Functional outcome of collagenase injections compared with fasciectomy in treatment of Dupuytren’s contracture. Hand 2013;8:410–16.
Nydick et al.72
-
Nydick JA, Olliff BW, Garcia MJ, Hess AV, Stone JD. A comparison of percutaneous needle fasciotomy and collagenase injection for Dupuytren disease. J Hand Surg Am 2013;38:2377–80.
Peimer et al.86
-
Peimer CA, Skodny P, Mackowiak JI. Collagenase clostridium histolyticum for Dupuytren-contracture: patterns of use and effectiveness in clinical practice. J Hand Surg Am 2013;38:2370–6.
Peimer et al.87
-
Peimer CA, Blazar P, Coleman S, Kaplan FT, Smith T, Tursi JP, et al. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS study): 3-year data. J Hand Surg Am 2013;38:12–22.
-
Kaplan FTD, Hentz VR, Tursi J, Kaufman G, Smith T. Patterns of recurrent contracture in patients with Dupuytren’s disease 3 years following successful treatment with collagenase clostridium histolyticum: Level 4 evidence. J Hand Surg Am 2012;37(Suppl. 1):14.
Ribak et al.57
-
Ribak S, Borkowski J, Amaral RP, Massato A, Ávila I, de Andrade D. Dupuytren contracture: comparative study between partial fasciotomy and percutaneous fasciotomy. J Braz Orthoped 2013;48:545–53.
Skirven et al.88
-
Skirven TM, Bachoura A, Jacoby SM, Culp RW, Osterman AL. The effect of a therapy protocol for increasing correction of severely contracted proximal interphalangeal joints caused by dupuytren disease and treated with collagenase injection. J Hand Surg Am 2013;38:684–9.
Skoff75
-
Skoff HD. The surgical treatment of Dupuytren’s contracture: a synthesis of techniques. Plast Reconstr Surg 2004;113:540–4.
Syed et al.91
-
Syed A, Mcfarlane J, Chester T, Powers D, Sibly F, Talbot-Smith A. Clinical efficacy and cost-effectiveness of Clostridium histolyticum collagenase injections in a subpopulation of Dupuytren’s contracture patients. Eur Orthop Traumatol 2013;5:311–16.
Toppi et al.76
-
Toppi JT, Trompf L, Smoll NR, Lim V, Smith K, Findlay MW, et al. Dupuytren’s contracture: an analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy [published online ahead of print]. ANZ J Surg 2014.
Ullah et al.68
-
Ullah AS, Dias JJ, Bhowal B. Does a ‘firebreak’ full-thickness skin graft prevent recurrence after surgery for Dupuytren’s contracture?: a prospective, randomised trial. J Bone Joint Surg Br 2009;91:374–8.
van Rijssen et al.69,70
-
van Rijssen AL, Gerbrandy FS, ter Linden H, Klip H, Werker PM. A comparison of the direct outcomes of percutaneous needle fasciotomy and limited fasciectomy for Dupuytren’s disease: a 6-week follow-up study. J Hand Surg Am 2006;31:717–25.
-
van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg 2012;129:469–77.
Watt et al.89
-
Watt AJ, Curtin CM, Hentz VR. Collagenase injection as nonsurgical treatment of Dupuytren’s disease: 8-year follow-up. J Hand Surg Am 2010;35:534–9.
Witthaut et al.90
-
Witthaut J, Jones G, Skrepnik N, Kushner H, Houston A, Lindau TR. Efficacy and safety of collagenase clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am 2013;38:2–11.
Excluded studies
Ineligible study design (n = 34)
Anonymous. Collagenase clostridium histolyticum. Short-term benefit in Dupuytren’s contracture, unknown long-term risks. Prescrire Int 2011;20:285–7.
Bainbridge C, Gerber RA, Szczypa PP, Smith T, Kushner H, Cohen B, et al. Efficacy of collagenase in patients who did and did not have previous hand surgery for Dupuytren’s contracture. J Plast Surg Hand Surg 2012;46:177–83.
Ball C, Pratt AL, Nanchahal J. Optimal functional outcome measures for assessing treatment for Dupuytren’s disease: a systematic review and recommendations for future practice. BMC Musculoskel Disord 2013;14:131.
Boyer MI, Gelberman RH. Complications of the operative treatment of Dupuytren’s disease. Hand Clin 1999;15:161–6.
Breed CM, Smith PJ. A comparison of methods of treatment of pip joint contractures in Dupuytren’s disease. J Hand Surg Br 1996;21B:246–51.
Brenner P. Dupuytren’s disease of ring and little finger. Orthop Traumatol 2002;10:138–58.
Chen W, Zhou H, Pan ZJ, Chen JS, Wang L. The role of skin and subcutaneous tissues in Dupuytren’s contracture: an electron microscopic observation. Orthopaed Audio-Synopsis Contin Med Educ 2009;1:216–21.
Chick LR, Lister GD. Surgical alternatives in Dupuytren’s contracture. Hand Clin 1991;7:715–9.
Crean SM, Gerber RA, Le Graverand MP, Boyd DM, Cappelleri JC. The efficacy and safety of fasciectomy and fasciotomy for Dupuytren’s contracture in European patients: a structured review of published studies. J Hand Surg Eur 2011;36:396–407.
Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty 2010;10:e15.
Denkler K. Collagenase for recurrent Dupuytren contracture with skin grafts. J Hand Surg Am 2013;38:1264.
Diaz R, Curtin C. Needle aponeurotomy for the treatment of Dupuytren’s disease. Hand Clin 2014;30:33–8.
Friedrich JB. Discussion: extensive percutaneous aponeurotomy and lipografting: a new treatment for Dupuytren disease. Plast Reconstr Surg 2011;128:229–30.
Hallock GG. Skin laceration as a serious adverse sequela of injectable collagenase for Dupuytren contracture. Plastic Reconstruct Surg 2012;129:205–6e.
Hentz VR. Collagenase injections for treatment of Dupuytren disease. Hand Clin 2014;30:25–32.
Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FTD, Meals RA, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med 2009;361:968–79.
Jerosch-Herold C, Shepstone L, Chojnowski AJ, Larson D, Barrett E, Vaughan SP. Night-time splinting after fasciectomy or dermo-fasciectomy for Dupuytren’s contracture: a pragmatic, multi-centre, randomised controlled trial. BMC Musculoskel Dis 2011;12:136.
Kan HJ, Verrijp FW, Huisstede BMA, Hovius SER, Van Nieuwenhoven CA, Selles RW. The consequences of different definitions for recurrence of Dupuytren’s disease. J Plast Reconstruct Aest Surg 2013;66:95–103.
Ketchum LD. The use of the full thickness skin graft in Dupuytren’s contracture. Hand Clin 1991;7:731–41.
Knobloch K, Kuehn M, Vogt PM. Focused extracorporeal shockwave therapy in Dupuytren’s disease – a hypothesis. Med Hypotheses 2011;76:635–7.
Larocerie-Salgado J, Davidson J. Nonoperative treatment of PIPJ flexion contractures associated with Dupuytren’s disease. J Hand Surg Eur 2012;37:722–7.
Lubahn JD. Open-palm technique and soft-tissue coverage in Dupuytren’s disease. Hand Clin 1999;15:127.
McFarlane RM. Severe contractures of the proximal interphalangeal joint in Dupuytren’s disease: combined fasciectomy with capsuloligamentous release versus fasciectomy alone – discussion. Plast Reconstr Surg 1996;97:567.
McKenna SP, Wilburn JN, Crawford SR, Perry-Hinsley D, Bayat A. Dupuytren’s disease and quality of life. Wound Repair Regenerat 2012;20:A31.
Raina S, Jaryal A. Dupuytren’s contracture. Indian J Med Res 2012;136:502.
Raven RB, III, Kushner H, Nguyen D, Naam N, Curtin C. Analysis of efficacy and safety of treatment with collagenase clostridium histolyticum among subgroups of patients with dupuytren contracture. Ann Plast Surg 2013:73:286–90.
Salhi S, Cardin-Langlois E, Luc M. Percutaneous fasciotomy for the treatment of Dupuytren’s disease - a systematic review. Hand 2011;6:349–55.
Stanbury SJ, Hammert WC. Dupuytren Contracture. J Hand Surg Am 2011;36:2038–40.
Townley WA, Baker R, Sheppard N, Grobbelaar AO. Dupuytren’s contracture unfolded. Br Med J 2006;332:397–400.
Tropet Y, Deck D, Vichard P. Dupuytren’s contracture of the little finger. [French]. Ann Chir Main Memb Super 1994;13:101–6.
Varian JPW, Hueston JT. Occurrence of Dupuytren’s disease beneath a full thickness skin graft: a semantic reappraisal. Ann Chir Main Memb Super 1990;9:376–8.
Wegman T. Treatment of Dupuytren’s disease with collagenase. Official Gazette of the United States Patent and Trademark Office Patents 1996;1193:3493.
Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg 1999;24:456–9.
Wilbrand S, Ekbom A, Gerdin B. Dupuytren’s contracture and sarcoma. J Hand Surg 2002;27:50–2.
No relevant outcomes (n = 6)
Badalamente MA, Hurst LC, Ward A, Parker T, Liu J, Tatka J. Histology of cords from Dupuytren’s patients: a comparison after collagenase injection/fasciectomy, primary fasciectomy and repeat fasciectomy: not a clinical study. J Hand Surg Am 2012;37:08.
De Salas-Cansado M, Ruiz-Antoran MB, Ramirez E, Abad SF. Healthcare resource utilization and associated costs with surgical treatment of Dupuytren disease in Spain. Basic Clin Pharmacol Toxicol 2001;109(Suppl. 3):33.
De Salas-Cansado M, Ruiz-Antoran MB, Ramirez E, Abad SF. Healthcare resources utilization and associated costs with surgical treatment of Dupuytren’s disease in Spain. Basic Clin Pharmacol Toxicol 2011;109:37.
Messore L, De DC, Battaglini G, Pizza G, Novelli A, Franculli F. Dupuytren disease. Remarks in surgical technique. [Italian]. Minerva Orthop Traumatol 1998;49:165–8.
Skodny P, Mackowiak JI, Peimer C. Efficacy and effectiveness of collagenase clostridium histolyticum for Dupuytren’s contracture. Value Health 2011;14:A302.
Starkweather KD, Lattuga S, Hurst LC, Badalamente MA, Guilak F, Sampson SP, et al. Collagenase in the treatment of Dupuytren’s disease: an in vitro study. J Hand Surg Am 1996;21:490–5.
No translation (n = 2)
Eisenschenk A. Collagenase therapy for Dupuytren. Unfallchirurg 2011;114:965.
Anonymous. Dupuytren needle fasciotomy for primary treatment of Dupuytren’s contracture. Unfallchirurg 2012;115:765.
Pre-1990 (n = 1)
Norotte G, Apoil A, Travers V. A ten years follow-up of the results of surgery for Dupuytren’s disease. A study of fifty-eight cases. Ann Chir Main 1988;7:277–81.
Unable to obtain (n = 1)
Donato De FA, Pardini Junior AG, Tufi Neder FA. Dupuytren’s contracture: treatment using the open palm technique. [Portuguese]. J Braz Orthoped 1997;32:301–4.
Appendix 7 Study characteristics of collagenase studies
| Study details | Participant characteristics | Intervention characteristics | Outcomes summary |
|---|---|---|---|
| RCTs | |||
| First author, year: Badalamente 200264 Secondary reports: Language: English Publication type: full text Number of centres: 1 Setting: University Health Science Centre Country: USA Start/end dates: NR Prospective/retrospective data collection: prospective Study design: RCT, Phase II Randomisation method: NR Length of follow-up: 5 years (mean for MCP joints = 4 years; PIP joints = 3.8 years) Source of funding: supported by grants from the US Food and Drug Administration (FD-R- 001437), the National Institutes of Health (General Clinical Research Centre Grant [M01RR1071002]) and the Advance Biofactures Corporation, Lynbrook, NY, USA (A subsidiary of BioSpecifics Technologies, a biopharmaceutical company that has developed injectable collagenase for 12 clinical indications to date) |
Enrolled: A: 25; B: 24 Randomised: A: 25; B: 24 Analysed: A: 25; B: 24 Consecutive: yes Age (years), mean: MCP 65; PIP 64.3 Sex, n (%): MCP: M 31 (86), F 5 (14); PIP: M 11 (84.6), F 2 (15.4) Primary/previously treated patients: NR Baseline contracture: mean (SD) degrees MCP joints: 44° (17.4°); PIP joints 53° (18.7°) Inclusion criteria: patients with either MCP joint contractures or those with PIP joint contractures (only) entered the study Exclusion criteria: NR |
A: collagenase injections at a single dose of 10,000 U. Total volume used was 0.25 ml for MCP joints and 0.20 ml for PIP joints. The results of the first injection were evaluated for 30 days, at days 1, 7, 14, and 30. If the finger contracture did not respond to 0–5° of normal extension (0°) there was a retreatment option for a potential four additional injections using 10,000 U of collagenase on an open-label basis. Subsequent retreatments using 10,000 U of collagenase were based on patient’s response to treatment with clinical success being defined as to within 0–5° of normal extension (0°). If the initial target joint was successfully treated, and if patients presented with other involved joint contractures, these were treated on an open-label basis by using 10,000 U of collagenase. The total maximum dose that a patient could receive was 50,000 U of collagenase with an interval of 4–6 weeks between each retreatment injection. On day 1 after injection a therapist fitted the patients with a night extension splint that was to be worn for 4 months. Patients were also instructed by the therapist to do extension exercises at home. Daily vitamin E massage for 4 months was also suggested to keep the treatment area soft and pliable. Serial follow-up examinations occurred on days 7 and 14 and at months 1, 2, 3, 6, 9 and 12 B: placebo – consisted of sterile normal saline containing 2 mmol/l calcium chloride |
Reduction of contracture to within 5°, adverse events, ROM, grip strength |
| First author, year: Badalamente 200563 Secondary reports: Language: English Publication type: abstract Number of centres: NR Setting: NR Country: USA Start/end dates: NR Prospective/retrospective data collection: prospective Study design: RCT, Phase III (followed by open-label extension) Randomisation method: NR Length of follow-up: mean 125 days for MCP joints/107 days for PIP joints Source of funding: FDA (Grant 001437), the NIH (GCRC-M01RR10710) and the Biospecifics Technologies Corp. |
Enrolled: 35 Randomised: NR Analysed: NR Consecutive: yes Age (years): NR Sex n (%): NR Primary/previously treated patients: NR Baseline contracture: mean MCP joints: 45°; PIP joints 43° Inclusion criteria: NR Exclusion criteria: NR |
A: collagenase injections, 0.58 mg (0.25 ml for MCP joints and 0.20 ml for PIP joints). Maximum three injections B: saline placebo, maximum three injections |
Reduction of contracture, adverse events |
| First author, year: Badalamente 200765 Secondary reports: Language: English Publication type: full text Number of centres: NR Setting: NR Country: USA Start/end dates: NR Prospective/retrospective data collection: prospective Study design: RCT, Phase III (followed by open-label extension) Randomisation method: joints were randomised in a 2 : 1 ratio to receive 10,000 U of collagenase Length of follow-up: up to 12 months Source of funding: FDA (Grant 001437), the NIH (GCRC-M01RR10710) and the Biospecifics Technologies Corp. |
Enrolled: A: 23 B: 12 Randomised: A: 23 B: 12 Analysed: NR Consecutive: yes Age (years), mean (SD): A: 60.1 (7.6); B: 63.8 (10.0) Sex, n (%): A + B: M 28 (80); F 7 (20) Primary/previously treated patients: NR Baseline contracture: mean (SD): MCP joints: 51° (12°); PIP joints 46° (14°) Inclusion criteria: age ≥ 18 years; fixed flexion deformity of ≥ 20° of the MCP or PIP joints in at least one finger Exclusion criteria: NR |
A: collagenase injections, 0.58 mg (0.25 ml for MCP joints and 0.20 ml for PIP joints). Maximum three injections. All patients were splinted at night for an interval of 4 months after the last injection was administered B: saline placebo, maximum 3 injections |
Reduction of contracture, adverse events |
| First author, year: Gilpin 201056 (CORD II) Secondary reports: Language: English Publication type: full text Number of centres: 5 Setting: multiple hospital sites Country: Australia Start/end dates: August 2007 to September 2008 Prospective/retrospective data collection: prospective Study design: RCT, Phase III (with open-label extension) Randomisation method: randomisation achieved with the use of a computer-generated permuted block design (block size of 6) within each baseline severity level for each study site. Patients were randomly assigned to receive collagenase or placebo in the double-blind phase in a 2 : 1 ratio, to maximise the number of patients who could benefit with active treatment versus placebo Length of follow-up: double-blind phase: 90 days Open-label extension: 12 months Source of funding: Auxilium Pharmaceuticals |
Enrolled: A: 45; B: 21 Randomised: A: 45; B: 21 Analysed: A: 45; B: 21 Consecutive: yes Age (years), mean (SD): A: 63 (7.8); B: 65.5 (11.1) Sex, n (%): A: M 39 (86.7); F 6 (13.3); B: M 17 (81); F 4 (19) Primary/previously treated patients: both Baseline contracture: mean (SD) total contracture index A: 174.7° (107.2°); B: 150.1° (84°) Inclusion criteria: good health, aged ≥ 18 years; MCP joint contracture ≥ 20° and ≤ 100°; PIP joint contracture ≥ 20° and ≤ 80° in at least one digit (not the thumb); inability to simultaneously place the affected finger and palm flat on a table. Women were required to be postmenopausal or using contraception. Patients with recurrent disease were eligible for study participation if other eligibility criteria were met Exclusion criteria: breastfeeding or pregnancy; bleeding disorder; recent stroke; previous treatment of the primary joint within 90 days of first dose of study drug; collagenase treatment or treatment with any investigational drug within 30 days of first dose of study drug; use of a tetracycline derivative within 14 days of first dose of study drug; anticoagulant within 7 days of first dose of study drug (with the exception of low-dose aspirin); allergy to collagenase; and chronic muscular, neurological, or neuromuscular disorders affecting the hands |
A: collagenase injection (0.58 mg per injection). When needed, a standardised finger extension procedure was implemented up to three times the day after injection to facilitate cord disruption. After the finger extension procedure, patients were instructed to wear night splints for up to 4 months, perform at-home finger flexion and extension exercises, and return to normal daily activities. Each affected cord could undergo a maximum of three treatment cycles in 30-day intervals, and each patient could receive a maximum of eight treatment cycles during the 12-month study. During the 90-day double-blind phase, if the primary joint (first treated joint) met the primary end point in fewer than three treatment cycles, a second joint could be treated. If the first and second joints met the primary end point with one treatment cycle each, a third joint could be treated. During the 9-month open-label phase, treatment was at the discretion of the investigator. Patients from the double-blind phase who still required collagenase treatment could receive up to five additional collagenase injections in the open-label phase. These included patients who received placebo treatment, patients who did not achieve clinical success with fewer than three collagenase injections, and patients who had other Dupuytren’s cords that were not injected with collagenase B: placebo (lyophilised Tris and sucrose only) and treated as above |
Reduction of contracture, recurrence, adverse events |
| First author, year: Hurst 200955 (CORD I) Secondary reports: Witthaut 201166 Language: English Publication type: full text Number of centres: 16 Setting: Multiple hospital sites Country: USA Start/end dates: August 2007 to October 2008 Prospective/retrospective data collection: prospective Study design: RCT, Phase III (with open-label extension) Randomisation method: patients were randomised with a centralised interactive voice response system to active treatment or placebo with a ratio of 2 : 1 in favour of the active treatment within each joint type/baseline severity strata. Equal allocation to treatment groups was deemed unnecessary because of the anticipated high rate of clinical success. Randomisation was achieved with the use of a permuted-block design (block size of 6) with random assignment within each stratum for each study site Length of follow-up: 30 days Source of funding: Auxilium Pharmaceuticals |
Enrolled: A: 204; B:104 Randomised: A: 204; B:104 Analysed: A: 203; B 103 Consecutive: yes Age (years), mean (SD): A: 62.3 (9.7); B: 63.3 (9.1) Sex, n (%): A: M 171 (83.8%), F 33 (16.2%); B: M 74 (71.2%), F 30 (28.8%) Primary/previously treated patients: both Baseline contracture: mean (SD) total contracture index A: 149.1° (127.6°); B: 149.3°(111.4°) Inclusion criteria: aged > 18 years; diagnosis of DC, with a fixed flexion deformity of at least one finger, other than the thumb, that had a contracture at least 20°, but not greater than 100°, for MCP (80° for PIP) joints, caused by a palpable cord that had never been treated with collagenase; a positive ‘table top test,’ defined as the inability to simultaneously place the affected finger(s) and palm flat against a table top; judged to be in good health Women included in the study were postmenopausal or used contraception Exclusion criteria: breastfeeding or pregnancy, a bleeding disorder, a recent stroke, previous treatment of the primary joint within 90 days before the beginning of the study, collagenase treatment or treatment with any investigational drug within 30 days before the beginning of the study, the use of a tetracycline derivative within 14 days before the beginning of the study, the use of an anticoagulant within 7 days before the beginning of the study, an allergy to collagenase and a chronic muscular, neurological, or neuromuscular disorder affecting the hands |
A: Collagenase clostridium histolyticum (0.58 mg per injection) was reconstituted in 0.25 ml of sterile diluent (for MCP joints) or 0.20 ml of sterile diluent (for PIP joints) and injected directly into the affected cords. If needed, the joints were then manipulated up to three times with the use of a standardised procedure the day after injection in an effort to rupture the cords. Patients were given a splint to wear nightly for up to 4 months B: Placebo (10 mM Tris per 60 mM sucrose reconstituted in diluent) was administered in a similar manner. Subjects could have received up to three injections of placebo into the cord of the affected hand. Each injection was separated by at least 30 days. Individual cords may have received up to a maximum of three injections |
Reduction of contracture, ROM, recurrence, adverse events |
| Non-randomised comparative studies | |||
| First author, year: Naam 201371 Secondary reports: Language: English Publication type: full text Number of centres: 1 Setting: Hospital surgery department Country: USA Start/end dates: NR Prospective/retrospective data collection: retrospective Study design: non-randomised, comparative, patient chart-review Length of follow-up: 2 years Source of funding: Auxilium Pharmaceuticals |
Enrolled: A: 25; B 21 Analysed: A: 25; B 21 Consecutive: NR Age (years), mean (range): A: 65 (42–83); 67 (39–84) Sex, n (%): A: M 23 (92), F 2 (8); M 13 (62), F 8 (38) Primary/previously treated patients: NR Baseline contracture: mean degrees A: 43.5°; B: 41.4° Inclusion criteria: DC with a fixed-flexion deformity > 20 and < 100°, measured by finger goniometry for MCP joints and > 20 and < 80° for PIP joints in at least one finger other than the thumb caused by a palpable cord Exclusion criteria: NR |
A: injection of 0.58 mg dose of collagenase – 0.25 ml for MCP joints; 0.02 ml for PIP joints. Approximately 24 hours post-injection, a finger-extension procedure was conducted to facilitate cord disruption. Finger-extension consisted of manipulation of the treated finger, recommended no more than three times regardless of whether or not the cord ruptured. Hand therapists were involved in fitting all patients with a night splint to be worn for 3 months following each injection and finger extension procedure B: open fasciectomy with multiple Z-plasties was performed under axillary block anaesthesia. Multiple Z-plasties were performed at the level of the distal palmar crease, proximal digital crease and PIP joint flexion crease. The hand was splinted with full extension of the digit and slight extension of the wrist. Postoperatively, the splint was removed in 2–3 days and the patient was started on AROM exercises. The patient continued to wear a night splint for 3 months following the surgery |
Reduction of contracture, ROM, adverse events, time to normal function, health-related quality of life |
| First author, year: Nydick 201372 Secondary reports: Language: English Publication type: full text Number of centres: 1 Setting: orthopaedic institute Country: USA Start/end dates: NR Prospective/retrospective data collection: prospective Study design: non-randomised, comparative, patient chart-review Length of follow-up: mean 6 months Source of funding: NR |
Enrolled: A: 29; B: 30 Analysed: A: 29; B: 30 Consecutive: NR Age (years), mean (SD): A: 67 (10); B: 66 (10) Sex, n (%): A: M 25 (86), F 4 (14); B: M 23 (77), F 7 (23) Primary/previously treated patients: NR Baseline contracture: mean (SD): A: 40° (12°); B: 37° (20°) Inclusion criteria: NR Exclusion criteria: NR |
A: Collagenase injection: 0.25 ml for MCP joint contracture and 0.20 ml for PIP joint contracture. Patients returned the next day for manipulation of the contracted digit after local anaesthetic block. Repeat collagenase injections were offered if the desired reduction of contracture was not met at 4 weeks. There was no requirement to continue treatment until a specific contracture correction was achieved as described in the Collagenase Option for the Reduction of Dupuytren (CORD) studies B: PNF: The hand was sterilely prepped and injected with subdermal lidocaine without adrenaline. Palpable and visible cords were released with 25- and 22-gauge needles. Patients actively flexed and extended the fingers to ensure the flexor tendons were not penetrated. After needle release along multiple sites of the cord, the finger was manipulated with gentle, progressive extension force. A digital block was performed before manipulation |
Reduction of contracture, recurrence, adverse events |
| Case series studies | |||
| First author, year: Badalamente 200048 Secondary reports: Language: English Publication type: full text Number of centres: NR Setting: orthopaedic department Country: USA Start/end dates: Prospective/retrospective data collection: prospective Study design: case series Length of follow-up: mean MCP joints: 20 months; PIP joints: 14.1 months Source of funding: NR |
Enrolled: 35 Analysed: 34 Consecutive: no Age (years), mean (SD): 64.8 (11.0) Sex, n (%): M 32 (91.4), F 3 (8.6) Primary/previously treated patients: NR Baseline contracture: mean (SD) 49 (11); MCP 42 (13); PIP 52 (16) Inclusion criteria: Dupuytren’s patients Exclusion criteria: NR |
A: The first study patient received a 300-U collagenase injection into the cord, that was causing MCP joint contracture. This failed to cause cord rupture and a dose escalation protocol was then used. The next 5 patients received 600, 1200, 2400, 4800 and 9600 units of collagenase, respectively, injected into the cord that was causing contracture of the MCP joints. One patient who had no benefit in the dose escalation study entered the following phase of the study. The remaining 29 patients, had collagenase injections at a dose level of 10,000 U (delivered in 0.25 ml for MCP joints and 0.20 ml for PIP joints) followed by a 10- to 12-hour period of hand immobilisation in a soft bulky gauze dressing. After this period there was no further immobilisation. If cord rupture did not occur on the day after the injection, the patients were instructed to apply extension force themselves. On the day after the injection the patients were fitted with a night extension splint that was worn for 4 months. All patients were instructed to do extension exercises at home. Daily vitamin E massage for 4 months was suggested | Reduction of contracture, recurrence, adverse events, time to correction |
| First author, year: Badalamente 201177 (see entry for Hurst 2009 for details of the multicentre primary RCT) Secondary reports: Language: English Publication type: abstract Number of centres: 17 Setting: Multiple hospital sites Country: USA Start/end dates: NR Prospective/retrospective data collection: prospective Study design: case series (follow-up of collagenase-treated patients only from one single-centre and one multicentre Phase III, double-blind, placebo controlled trials) Length of follow-up: 3–5 years (mean 3.9 years) Source of funding: Biospecifics Technologies Corp. and Auxilium Pharmaceuticals Inc. |
Enrolled: 509 Analysed: 509 Consecutive: NR Age (years), mean: single centre 61; multicentre 65 Sex, n (%): M 428 (84), F 81 (16) Primary/previously treated patients: Both Baseline contracture: NR Inclusion criteria: NR Exclusion criteria: NR |
A: Collagenase clostridium histolyticum, 0.58 mg per injection, (0.25 ml MCP joints or 0.20 ml PIP joints) | Recurrence |
| First author, year: Coleman 201278 Secondary reports: Language: English Publication type: full text Number of centres: 1 Setting: hand and upper limb clinic Country: Australia Start/end dates: NR Prospective/retrospective data collection: prospective Study design: case series Length of follow-up: 30 or 90 days Source of funding: Auxilium Pharmaceuticals |
Enrolled: 12 Analysed: 12 Consecutive: NR Age (years), mean (SD): 63.7 (5.5) Sex, n (%): M 11 (92), F 1 (8) Primary/previously treated patients: both Baseline contracture: MCP joints ≤ 50° n = 5; PIP joints ≤ 40° n = 3, ≥ 40° n = 4 Inclusion criteria: general good health, age ≥ 18 and ≤ 70 years, diagnosis of DC with at least three fixed-flexion contractures, caused by palpable cords, that were ≥ 20 in PIP and/or MCP joints in fingers (not the thumbs), unable to simultaneously place the affected finger(s) and palm flat against a table top Exclusion criteria: previous treatment of the selected joints within 90 days of first dose of study drug; chronic muscular, neurological, or neuromuscular disorders affecting the hands; known allergy to collagenase; collagenase treatment or treatment with any investigational drug within 30 days of first dose of study drug; anticoagulant within 7 days of first dose of study drug (with the exception of low-dose aspirin or NSAIDs); breastfeeding or pregnancy; and known history of stroke, bleeding disorder, or other medical condition that in the opinion of the investigator would compromise the subjects’ safety or the study objectives |
A: single injection of collagenase (0.58 mg; volume of 0.25 ml for MCP joints and 0.20 ml for PIP joints) into a single cord. A finger extension procedure was conducted within 24 hours post-dosing. Patients were bandaged for the first few days following the finger extension procedure and then were fitted for a splint to be worn each night for up to 4 months The same participants entered a second treatment period 30 days later, where two different affected joints on the same hand were treated during the same visit (i.e. patients received two concurrent 0.58-mg CCH doses) |
Reduction of contracture, adverse events |
| First author, year: Coleman 201479 Secondary reports: Language: English Publication type: full text Number of centres: 8 Setting: hand and upper limb clinic Country: Australia Start/end dates: September 2011 to February 2012 Prospective/retrospective data collection: prospective Study design: case series Length of follow-up: 60 days Source of funding: Auxilium Pharmaceuticals |
Enrolled: 60 Analysed: 60 Consecutive: NR Age (years), mean (SD): 64 (11) Sex, n (%): M 51 (85), F 9 (15) Primary/previously treated patients: both Baseline contracture: all MCP joints (n = 75): 41 (17); all PIP joints (n = 45): 49 (19) Inclusion criteria: 18 years or older, diagnosis of Dupuytren disease with at least two flexion contractures on the same hand that were 20° or greater in MCP and/or PIP joints in fingers (not thumbs) caused by palpable cords suitable for treatment, and a positive table top test Exclusion criteria: |
A: on day 1, finger goniometry was performed on joints to be treated and was followed by two concurrent injections of 0.58 mg CCH into cords affecting two joints in the same hand. A finger extension procedure was performed approximately 24 hours after administration of CCH in patients who did not have spontaneous disruption of the cord. Patients were fitted with an orthosis to be worn at night for up to 4 months and were instructed on how to perform a series of finger flexion–extension exercises at home. Follow-up visits to assess efficacy and safety occurred on days 8, 30 and 60. On completion of the day-60 visit, patients who required additional treatment in the treated hand could receive up to three additional injections of CCH (given as single injections 30 days apart). Patients could receive up to a total of five injections, and individual cords could receive up to a total of three injections | Change in joint contracture and ROM at 30 days; patient ratings of treatment satisfaction; physician ratings of improvement; clinical success rates (i.e. flexion contracture ≤ 5°) |
| First author, year: Considine 201380 Secondary reports: Language: English Publication type: abstract Number of centres: NR Setting: outpatient clinic Country: NR Start/end dates: NR Prospective/retrospective data collection: NR Study design: case series Length of follow-up: mean 4 days Source of funding: NR |
Enrolled: 10 Analysed: 10 Consecutive: NR Age (years), mean: 66 Sex: NR Primary/previously treated patients: both Baseline contracture: mean MCP joints: 58.6°; PIP joints 39° Inclusion criteria: patients waiting for fasciectomy for DC Exclusion criteria: NR |
A: collagenase injection and subsequent Dupuytren’s release performed in outpatient clinic. No anaesthetic was used. Patients were dressed with a bulky bandage and seen in the outpatients 48 hours later. Regional anaesthesia was administered using 10 ml of 0.5% bupivacaine hydrochloride (Marcaine, Hospira) to block the median and ulnar nerves at the wrist. An extension procedure was performed, and if skin tears developed the patients were dressed appropriately. Therapy was performed as for patients that had undergone Dupuytren’s fasciectomy | Reduction of contracture, adverse events |
| First author, year: Hayton 201381 Secondary reports: Language: English Publication type: full text Number of centres: 30 (16 CORD, 14 JOINT) Setting: multiple hospital sites Country: USA Start/end dates: NR Prospective/retrospective data collection: prospective Study design: case series Length of follow-up: NR Source of funding: Auxilium, Pfizer |
Enrolled: 616 Randomised: N/A Analysed: 616 Consecutive: NR Age (years), mean (SD): 63 (10) Sex, n (%): M 508 (83), F 108 (17) Primary/previously treated patients: both Baseline contracture: FFC, mean (SD), °: Group A: 48.2 (20.2) Group B: 49.7 (18.5) Inclusion criteria: ≥ 1 PIP joint contracture at study entry and received ≥ 1 CCH injection during the study Exclusion criteria: NR |
A: For this secondary analysis, two patient subgroups were analysed to evaluate the direct and indirect effects of CCH on PIP joint contracture | Reduction of contracture, improvement in contracture, change in fixed-flexion contracture and ROM, adverse events |
| First author, year: Kaplan 201383 Secondary reports: Language: English Publication type: abstract Number of centres: NR Setting: NR Country: USA Start/end dates: NR Prospective/retrospective data collection: prospective Study design: RCT (treated as case series data for the purpose of this review) Length of follow-up: 90 days Source of funding: Auxilium Pharmaceuticals |
Enrolled: NR Randomised: NR Analysed: 37 Consecutive: Yes Age (years): NR Sex: NR Primary/previously treated patients: NR Baseline contracture: NR Inclusion criteria: DC involving the MCP joint > 20° caused by a palpable cord Exclusion criteria: NR |
A: all patients received 1 dose of collagenase (0.58 mg) and were observed for 90 days. Manipulation for cord rupture was performed at 1 (GROUP 1), 2 (GROUP 2) or 4 (GROUP 3) days after injection | Reduction of contracture, adverse events |
| First author, year: Martin-Ferrero 201384 Secondary reports: Language: English Publication type: full text Number of centres: 1 Setting: hospital outpatient clinic Country: Spain Start/end dates: 2011 to 2013 Prospective/retrospective data collection: prospective Study design: case series Length of follow-up: 1 year Source of funding: NR |
Enrolled: 35 Analysed: 35 Consecutive: NR Age (years): 68 (range 45–89) Sex: M 35/35 (100%) Primary/previously treated patients: both Baseline contracture: mean, MCP 64°; PIP 83° Inclusion criteria: adults over 60 years (except where patient requested this treatment), Dupuytren’s disease, with a palpable band in at least 1 finger, excluding the thumb, and contraction of at least 20–90° at the MCP and 80° at the PIP Exclusion criteria: patients with haemorrhagic disorders or recent stroke, with other neuromuscular hand disorders, patients who had received treatment including surgery for DC in the previous 90 days, allergy to collagenase or excipients, use of doxycycline in the previous 14 days and anticoagulant drugs in the previous 7 days |
A: collagenase was administered by local injection directly into the palpable band, taking into account the specific doses necessary of both collagenase and solvent, depending on the joints to be treated according to the recommendations of the product. EMLA (numbing cream) (AstraZeneca) was topically administered in the injection area, half an hour earlier in an outpatient surgery room. After the injection, a compression bandage was applied and patients recommended to avoid movement. Extension of the finger and breakage of the band took place after 24 hours in an outpatient operating room, with local anaesthesia or sedation and subsequent compressive bandaging of the hand. Follow-up consultations were at 1 week, 2 weeks, 1 month, 3 months, 6 months and 1 year | Local complications, decrease of joint contracture, increase of range of motion |
| First author, year: McMahon 201385 Secondary reports: Language: English Publication type: full text Number of centres: 1 Setting: hand, wrist, arm, elbow and shoulder centre Country: USA Start/end dates: June 2010 to June 2012 Prospective/retrospective data collection: retrospective chart review with prospective patient recall Study design: case series (chart review) Length of follow-up: mean 15 months Source of funding: NR |
Enrolled: 102 Analysed: 48 Consecutive: NR Age (years), mean: 66 Sex, n (%): M 31 (65), F 17 (35) Primary/previously treated patients: NR Baseline contracture: mean (SD), 48° (21°) Inclusion criteria: at least 18 years of age at time of injection and minimum of 6 months between injection and research query Exclusion criteria: patients who were enrolled in a separate study that involved a regimented hand therapy programme for severe PIP joint contractures were excluded |
A: CCH injection administered by one of seven different board-certified hand surgeons and 24 digital manipulation and cord rupture 24 hours later. Digital block with 1–2% lidocaine hydrochloride (Xylocaine, AstraZeneca) was used just prior to digit manipulation to obtain pain relief. Patients then referred to a hand therapist, who fitted them with a customised thermoplastic dorsal- or volar-based orthosis and provided home therapy exercise education. All patients who met the inclusion criteria were then asked to return to the clinic to have their finger contractures measured. One observer collected the degree of passive contracture using a finger goniometer | Reduction of contracture, recurrence, adverse events, health-related quality of life, additional interventions required, patient satisfaction |
| First author, year: Peimer 201386 Secondary reports: Language: English Publication type: full text Number of centres: 10 Setting: community and academic centres Country: USA Start/end dates: January to February 2011 Prospective/retrospective data collection: retrospective Study design: case series (chart review) Length of follow-up: NR Source of funding: Auxilium Pharmaceuticals |
Enrolled: 501 Analysed: 463 Consecutive: NR Age (years), mean (SD), range: 65.5 (10.1), 23–89 Sex, n (%): M 343 (74), F 120 (26) Primary/previously treated patients: NR Baseline contracture: mean (SD) = total: 49° (21°); MCP joints: 44° (20°); PIP joints 57° (21°) Inclusion criteria: non-pregnant adult patients whose first collagenase injection was administered after 2 February 2010, the date of FDA approval, but before 31 December 2010 Exclusion criteria: NR |
A: collagenase injection | Reduction of contracture, ROM, adverse events |
| First author, year: Peimer 201387 (CORDLESS) Secondary reports: Kaplan 2012 Language: English Publication type: full text Number of centres: NR Setting: NR Country: USA Start/end dates: NR Prospective/retrospective data collection: prospective Study design: case series [long-term follow-up of patients who received 1 or more CCH injections in their study of origin (i.e. JOINT I, JOINT II, CORD I, CORD I extension, or CORD II) and who had 1 or more post-treatment assessments] Length of follow-up: 3 years (interim data) Source of funding: Auxilium Pharmaceuticals |
Enrolled: 643 (from 950 enrolled in the original studies) Analysed: 643 Consecutive: NR Age (years), mean (SD): 66 (9.4) Sex, n (%): M 542 (84), F 101 (16) Primary/previously treated: all previously treated Baseline contracture: NR Inclusion criteria: participation in any of the previous studies: two 9-month, open-label CCH trials (JOINT I and JOINT II); two 12-month, double-blind trials (CORD I and CORD II); and 1 open-label extension (CORD I extension). For inclusion in the previous study, see entry for Hurst 2009 above Exclusion criteria: see entry for Hurst 2009 above |
A: a single injection of 0.58 mg of CCH into the cord followed by a finger extension the next day, with 30 days of follow-up. An individual cord could receive a maximum of three CCH injection cycles to achieve the primary end point of success (FFC ≤ 5°), but if patients had multiple fingers involved, they could not receive more than eight total injections CORDLESS patients are being observed once per calendar year for 4 years (year 2–5 after the first injection) with 6 or more months between consecutive visits |
Recurrence, adverse events, worsening of contracture, progression |
| First author, year: Skirven 201388 Secondary reports: Language: English Publication type: full text Number of centres: NR Setting: NR Country: USA Start/end dates: NR Prospective/retrospective data collection: prospective Study design: case series Length of follow-up: 4 weeks Source of funding: Auxilium Pharmaceuticals |
Enrolled: 21 Analysed: 21 Consecutive: no Age (years), mean (range): 63 (37–80) Sex, n (%): M 19 (90), F 2 (10) Primary/previously treated patients: both Baseline contracture: mean (range) passive PIP joint contracture: 56° (40–80°) Inclusion criteria: PIP joints contracted at least 40° Exclusion criteria: any patient with a passive contracture of < 40° was excluded |
A: collagenase injection followed by digital manipulation to rupture the cord. 1 week following manipulation, a custom-fabricated, finger-based cylinder PIP joint orthosis in maximum extension was made and provided for daytime use for 4–6 weeks. This cylinder orthosis was used continuously during the day and removed only for exercises and hygiene. The hand-based extension orthosis was continued at night for 6 months following the injection | Reduction of contracture, adverse events |
| First author, year: Syed 201391 Secondary reports: Language: English Publication type: full text Number of centres: 1 Setting: trauma and orthopaedic department Country: UK Start/end dates: NR Prospective/retrospective data collection: prospective Study design: case series Length of follow-up: 12 months Source of funding: NR |
Enrolled: NR Analysed: 56 Consecutive: NR Age (years), mean: 65 Sex, n (%): M 48 (86), F 8 (16) Primary/previously treated patients: primary only Baseline contracture: mean (SD) 41.8° (9.7°) Inclusion criteria: Aged ≥ 18 years, in good general health, a single or spiral palpable cord AND involvement of a single MCP joint only AND moderate contracture severity (i.e. 30–60°) Exclusion criteria: NR |
A: collagenase injection (dose of 0.58 mg per injection). When the patient returned for the manipulation, an injection of 10 ml of 0.5% Marcaine was infiltrated around the CCH injection site 10 minutes prior to a controlled manual passive manipulation of the finger to full extension. No repeat collagenase injections or manipulations were performed | Reduction of contracture, recurrence, adverse events, health-related quality of life |
| First author, year: Watt 201089 Secondary reports: Badalamente 200264 Language: English Publication type: full text Number of centres: 1 Setting: medical centre Country: USA Start/end dates: 1999 to 2000 (treatment dates) Prospective/retrospective data collection: prospective Study design: case series Length of follow-up: 8 years Source of funding: Auxilium Pharmaceuticals |
Enrolled: 23 Analysed: 8 Consecutive: NR Age (years), mean: 69 (range 52–86); MCP mean 67 (range 52–70); PIP mean 82 (range 77–86) Sex: NR Primary/previously treated patients: all previously treated Baseline contracture: MCP 57 (range 30–76); PIP 45 (range 35–75) Inclusion criteria: participated in collagenase arm of the Phase II RCT Exclusion criteria: participants who did not receive collagenase |
A: review of original records and abstraction of the initial degree of contracture, response to injection and dose response to injection. Participants returned for 8-year follow-up examination, which was performed by an independent examiner who had not been involved in the Phase II clinical trial. The examiner was blinded to the original location of injection. Goniometric measurements were obtained at both the MCP and PIP joint level of the index through little fingers of both hands and were expressed as degree of extensor lag. Subjects then completed a Dupuytren’s disease questionnaire which confirmed basic clinical history, including age at diagnosis, family history and prior treatment, including surgical fasciectomy, PNA and therapy. Participants answered questions related to disease recurrence and progression and were asked to judge the overall clinical success of the collagenase injection | Average contracture, recurrence |
| First author, year: Witthaut 201390 (JOINT I, JOINT II) Secondary reports: some participants also participated in the Peimer 201387 CORDLESS study Language: English Publication type: full text Number of centres: 34 Setting: Multiple hospital departments Country: USA (JOINT I); Australia, UK, Switzerland, Sweden, Denmark and Finland (JOINT II) Start/end dates: September 2007 to December 2008 Prospective/retrospective data collection: prospective Study design: case series Length of follow-up: 9 months Source of funding: Auxilium Pharmaceuticals |
Enrolled: 587 Analysed: 587 Consecutive: NR Age (years), mean (SD), range: JOINT I: 64.7 (9.9), 39–87; JOINT II: 63.2 (9.6), 35–86 Sex, n (%): JOINT I: M 164 (82), F 37 (18); JOINT II: M 334 (87), F 52 (13) Primary/previously treated patients: both Baseline contracture: mean (SD) JOINT I: 132.5 (109.8); JOINT II: 136.5 (104.2) Inclusion criteria: aged ≥ 18 years, DC with a fixed-flexion deformity ≥ 20° and ≤ 100° (MCP joints) and ≥ 20° and ≤ 80° (PIP joints) in at least one finger other than the thumb that was caused by a palpable cord Exclusion criteria: previous treatment, including surgery, for DC in the past 90 days; other muscular, neurological, or neuromuscular disorders affecting the hands; allergic to collagenase; pregnancy; history of stroke or bleeding or recent anticoagulant use; received doxycycline in the past 14 days |
A: patients could receive up to five collagenase injections (five treatment cycles) with a maximum of three per cord, separated by at least 30 days. Only one cord could be injected within a given treatment cycle. The investigator prioritised the joints to be treated. A treatment cycle consisted of one 0.58-mg injection (0.25 ml MCP joints; 0.20 ml PIP joints. Patients underwent a standardised finger extension procedure to facilitate cord disruption on day 1 if a spontaneous disruption had not occurred. Patients were instructed to wear a splint at night for up to 4 months but otherwise to return to normal activities and perform finger flexion-extension exercises at home. The decision to reinject a cord that did not achieve correction to within 0–5° of normal was subject to patient or physician preference at day 30 | Reduction of contracture, ROM, adverse events |
Appendix 8 Study characteristics of surgery studies
| Study details | Participant characteristics | Intervention characteristics | Outcomes summary |
|---|---|---|---|
| RCTs | |||
| First author, year: Citron 200567 Secondary reports: none Language: English Publication type: full text Number of centres: 1 Setting: hospital outpatient clinic Country: UK Start/end dates: February 1998 to August 2002 Prospective/retrospective data collection: prospective Study design: RCT Randomisation method: 100 numbers under 10 (0–9) were generated by a random number generator by an independent statistician and written on small cards. These were placed in plain opaque envelopes and shuffled. The envelopes were then marked 1 to 100 and drawn in sequence just before surgery. The envelope was either opened by the operating surgeon himself or by a member of the nursing staff in the operating theatre where the envelopes were held. Patients with even numbers (including 0) had a longitudinal incision, subsequently closed with Z-plasties and those with odd numbers had a modified Bruner incision closed with multiple ‘Y-V plasties’ Length of follow-up: NR Source of funding: NR |
Enrolled: A + B: 100 Randomised: A: 38; B: 62 Analysed: A: 33; B: 46 Consecutive: yes Age (years), mean (SD): A: 65 (10); B: 65 (10) Sex, n (%): analysed participants A: M 27 (81.8), F 6 (18.2); B: M 36 (78.3), F 10 (21.7) Primary/previously treated patients: primary only Baseline contracture: mean A: 57°; B: 56° Inclusion criteria: Dupuytren’s disease in one ray only and any degree of resultant contracture Exclusion criteria: recurrent disease |
A: fasciectomy + Z-plasty B: fasciectomy through a modified Bruner incision |
Recurrence, extension, adverse events |
| First author, year: Ullah 200968 Secondary reports: Language: English Publication type: full text Number of centres: 1 Setting: NR Country: UK Start/end dates: NR Prospective/retrospective data collection: prospective Study design: RCT Randomisation method: random numbers were held in sealed envelopes and the theatre sister was responsible for selecting and noting the consecutive numbers. An even number was drawn for 40 patients (43 hands, 46 fingers) and, in these, the Z-plasty was closed. An odd number was drawn for 39 patients (41 hands, 44 fingers) and, in these, the palmar skin was excised and a full-thickness skin graft taken from the elbow flexion crease or inner side of the arm Length of follow-up: 36 months Source of funding: NR |
Enrolled: A + B: 113 Randomised: A + B: 82 Analysed: A:39; B: 40 Consecutive: yes Age (years), mean (SEM): A: 60.2 (2.0); B: 65.7 (1.5) Sex, n (%): A: M 31 (79.5), F 8 (20.5); B: M 34 (85), F 6 (15) Primary/previously treated patients: primary only? Baseline contracture: mean (SEM) A + B, MCP: 21° (3°); PIP: 59° (2°) Inclusion criteria: contracture > 30° of the PIP joint, including those with several affected fingers in the same hand Exclusion criteria: previous surgery on the affected hand; receiving anti-coagulation treatment; unable to complete questionnaires; unable/unwilling to give consent, unable to attend follow-up; unfit for operation |
A: dermofasciectomy. The finger was explored through a longitudinal incision. All fibrous bands and nodules were identified and recorded before their excision. After correction of the contracture a Z-plasty was performed. Careful haemostasis was achieved after release of the tourniquet and it was confirmed that the skin could be closed without tension. The palmar skin was excised and a full-thickness skin graft taken from the elbow flexion crease or inner side of the arm. After fenestration, the graft was secured using 5–0 Vicryl sutures to cover the defect (figure 2). A compressive dressing with a palmar plaster-of-Paris slab was applied. The hand was elevated in a sling overnight and the patients then discharged B: fasciectomy. As above without the skin graft (Z-plasty closed) |
Correction of contracture, recurrence, adverse events, duration of surgery, time to recurrence |
| First author, year: van Rijssen 200669 Secondary reports: van Rijssen 201270 Language: English Publication type: full text Number of centres: 1 Setting: outpatient clinic (A); surgical theatre (B) Country: the Netherlands Start/end dates: August 2002 to January 2005 Prospective/retrospective data collection: prospective Study design: RCT Randomisation method: patients were asked to pull a numbered envelope out of a box that had been prepared at the start of the study and that contained a note reading either ‘Limited Fasciectomy’ or ‘Percutaneous Needle Fasciotomy.’ This determined which treatment each patient would receive Length of follow-up: 6 weeks (2006), 5 years (2012) Source of funding: NR |
Enrolled: A + B: 121 Randomised: A + B: 121 Analysed: A: 57; B: 56 Consecutive: Yes Age (years), mean: A: 64; B: 64 Sex, n (%): A: M 49, F 9; B: M 45, F 10 Primary/previously treated patients: primary only Baseline contracture: mean, A: MCP 44°; PIP 34°; B: MCP 42°; PIP 34° Inclusion criteria: flexion contracture of at least 30° in the MCP, PIP or DIP joints; a clearly defined pathologic cord in the palmar fascia; and willingness to participate in the trial Exclusion criteria: patients with postsurgical recurrence or extension of the disease, unable to stop anticoagulants, generally unfit to have surgery and unwilling to participate in this study or had a specific treatment wish |
A: PNF. The cord responsible for the flexion contracture was sectioned at as many levels as possible in the palm and fingers, depending on the extent and location of the disease, with a 25-gauge needle mounted on a syringe. If fatty tissue was present between the cord and the skin, fasciotomy in the distal part of the palm was performed with extra care taken to avoid a potential spiral nerve. After division of the cord the affected finger was extended passively to pull the ends of the sectioned cord apart and to obtain maximal release of the contractures. A small dressing was applied thereafter for 24 hours. Patients were encouraged to start practising flexion and extension of the fingers immediately after treatment. No formal hand therapy was initiated B: LF. In the palm a transverse incision was performed with a longitudinal proximal extension over the cords and a distal extension towards the second and fourth web spaces. In the digits a Bruner-type incision was used. After mobilisation of the skin flaps all pathologic cords were excised under loupe magnification. In the palm the transverse palmar ligament was left intact. Care was taken to try to preserve all digital nerves and arteries. Adversely inflicted damage to these structures was repaired with standard microsurgical techniques. The skin was closed after transposition as necessary. In case there was a shortage of skin in the palm the transverse incision was left open. A light compressive bandage was applied and left in place for 1 week. Patients were encouraged to start practising flexion and extension of the fingers immediately after surgery, as soon as anaesthesia had resolved. Hand therapy was not standard but was used only as needed. The stitches were removed after a minimum of 10 days |
Reduction of contracture, reduction of total passive extension deficit, recurrence, extension, adverse events, health-related quality of life |
| Non-randomised comparative studies | |||
| First author, year: Bainbridge 201273 Secondary reports: Dias 201344 Language: English Publication type: full text Number of centres: 12 Setting: NR Country: Czech Republic, Denmark, Finland, France, Germany, Hungary, Italy, Netherlands, Poland, Spain, Sweden, UK Start/end dates: data collection period November 2009 to January 2010 Prospective/retrospective data collection: retrospective Study design: medical chart review Length of follow-up: 1 year Source of funding: Pfizer Ltd |
Enrolled: A: 329; B: 446; C: 2311; D: 200; E: 34 Analysed: A: 329; B: 446; C: 2311; D: 200; E: 34 Consecutive: yes Age (years), mean (SD): A + B + C + D + E: 61.9 (10.2) Sex, n (%): A + B + C + D + E: M 2734 (81), F 623 (19) Primary/previously treated patients: both Baseline contracture: NR Inclusion criteria: diagnosed with Dupuytren’s disease and undergone a surgical procedure between September–December 2008. The surgical procedure must have been performed by an orthopaedic or plastic surgeon (hand surgeons included) Exclusion criteria: none |
A: PNF (needle fasciotomy/aponeurotomy) B: fasciotomy (subcutaneous and open) All fasciotomies were usually performed under local anaesthesia C: fasciectomy – limited, local, partial, regional, selective, segmental, subtotal, total. Performed under general anaesthesia or nerve block D: dermofasciectomy E: amputation |
Reduction of contracture, adverse events |
| First author, year: Citron 200374 Secondary reports: Language: English Publication type: full text Number of centres: 1 Setting: outpatient department Country: UK Start/end dates: 1996 to 2000 Prospective/retrospective data collection: prospective Study design: non-randomised comparative study Length of follow-up: mean 2.2 years (range, 2.0–3.5) Source of funding: NR |
Enrolled: NR Analysed: A: 15; B: 15 Consecutive: yes Age (years), mean: A: 66; B: 67 Sex, n (%): A: M 11 (73.3), F 4 26.7); B: M 13 (86.7), F 2 (13.3) Primary/previously treated patients: primary only Baseline contracture: mean A: 28°; B: 35° Inclusion criteria: DC of a single ray confined to the palm and affecting only the MCP joint, a single cord of Dupuytren’s tissue, no previous surgery for Dupuytren’s disease in that ray, agreement to surgery and consent to participate in the trial Exclusion criteria: NR |
A: fasciotomy + Z-Plasty. Fasciotomy through a longitudinal incision in which the cord was merely divided transversely and not excised. The skin was separated from the cord and the skin alone closed with a single Z-plasty B: fasciotomy: fasciotomy of the cord performed through a transverse palmar incision. The longitudinal fibres were dissected from the skin proximally then distally and divided under direct vision. The finger was straightened and the cord was noted to separate as the digit extended. The skin was then closed over the gap with interrupted sutures |
Post-operative deformity, recurrence |
| First author, year: Ribak 201357 Secondary reports: Language: Portuguese Publication type: full text Number of centres: 1 Setting: NR Country: Brazil Start/end dates: NR Prospective/retrospective data collection: NR Study design: non-randomised comparative study Length of follow-up: 12 months Source of funding: NR |
Enrolled: A: 16; B: 17 Analysed: A: 16; B: 17 Consecutive: NR Age (years): NR Sex, n (%): A: M 14 (88), F 2 (12); B: M 16 (94) F 1 (6) Primary/previously treated patients: NR Baseline contracture: NR Inclusion criteria: patients with a diagnosis of DC for whom surgery was considered Exclusion criteria: previous surgery |
A: PNF B: PF |
Reduction of contracture, adverse events, time to return to work |
| First author, year: Skoff 200475 Secondary reports: Language: English Publication type: full text Number of centres: 1 Setting: NR Country: USA Start/end dates: prospective/retrospective data collection: prospective Study design: non-randomised comparative study Length of follow-up: average follow-up was 3.5 years (range, 3.1–4.0 years) for the open palm group and 2.7 years (range, 2.0–3.0 years) for the synthesis group Source of funding: NR |
Enrolled: A: 20; B: 10 Analysed: A: 20; B: 10 Consecutive: yes Age (years), mean: A: 70; B: 67 Sex, n (%): A: M 18 (90), F 2 (10); B: M 9 (90), F 1 (10) Primary/previously treated patients: primary only Baseline contracture: mean, A: MCP: 57°, PIP 58°; B: MCP 50°, PIP 50° Inclusion criteria: primary cases of Dupuytren’s, failure of the ‘flat table’ test, MCP joint contracture of at least 30° and PIP joint contracture of at least 10° Exclusion criteria: NR |
A: synthesis surgical technique: An ulnar midlateral incision is performed from the distal interphalangeal joint crease of the affected digit to the distal palm flexion crease. This incision is connected to a transverse palmar incision to form an ‘L’ and create a full-thickness radially based flap. A selective fasciectomy is performed. After release, the radially based full-thickness flap is advanced distally to close the incision of the extended digit, producing an elliptical palmar defect. The defect is left open. An identically sized full-thickness skin graft is then harvested from the hypothenar eminence and refrigerated in normal saline-bacitracin solution at 34°F. After 4 days of extension splinting, maximum tolerable ROM is obtained in the office. The palmar defect is cleaned of haematoma and the stored full-thickness skin graft is applied to the palm. A bolster-type compression dressing is applied and full-time extension splinting is reintroduced. 1 week later, the extension splint is removed, but maintained during sleep. Daily wound care and ROM exercises are initiated B: open-palm technique: A Bruner zigzag incision is made in the affected digit. A transverse incision is made in the palm. A selective fasciectomy is performed as previously described. After release, the digital incision is closed; the palmar incision is left open. After 4 days of extension splinting, maximum tolerable ROM is obtained in the office and daily dressing changes are initiated. Motion exercises are conducted by day and extension splinting is maintained during sleep |
Reduction of contracture, recurrence, adverse events, health-related quality of life, healing time |
| First author, year: Toppi 201476 Secondary reports: Language: English Publication type: full text Number of centres: NR Setting: NR Country: Australia Start/end dates: treatment January 2003 to June 2011. Recruitment July to August 2011 Prospective/retrospective data collection: retrospective Study design: non-randomised comparative – retrospective cohort study Length of follow-up: mean 2 years Source of funding: NR |
Enrolled: 188 Analysed: A: 73; B: 52 Consecutive: NR Age (years), mean (SD): A: 64 (9.8); B: 68 (8.9) Sex n (%): A: M 60 (82), F 13 (19); B: M 40 (77), F 12 (23) Primary/previously treated patients: primary only Baseline contracture: NR Inclusion criteria: patients who underwent either PNF or open fasciotomy were eligible if this was the first treatment for the given contracture Exclusion criteria: patients with recurrent or severe disease (i.e. those who required skin grafting or amputation) were excluded |
A: 19-gauge hypodermic needle was used to divide cords at various levels. Starting distally and working proximally, pathological cords were divided while keeping the finger in passive extension, allowing progressive straightening of the finger with each cord division. A light dressing and splint were applied postoperatively | Post-operative change in flexion deformity, complication rates, patient satisfaction |
Appendix 9 Risk-of-bias assessment for individual collagenase randomised controlled trials
| Domain (type of bias assessed) | Badalamente 200264 | Badalamente 200765 | Hurst 200955 | Gilpin 201056 |
|---|---|---|---|---|
| Sequence generation (selection bias) | ? | ? | ✓ | ✓ |
| Allocation concealment (selection bias) | ? | ? | ✓ | ✓ |
| Blinding of participants (performance bias) | ✓ | ✓ | ✓ | ✓ |
| Blinding of health-care providers (performance bias) | ✓ | ✓ | ✓ | ? |
| Blinding of outcome assessment (detection bias) | ? | ? | ? | ? |
| Groups treated identically (performance bias) | ✓ | ✓ | ✓ | ✓ |
| Incomplete outcome data (attrition bias) | ? | ✓ | ✓ | ✓ |
| Intention to treat (attrition bias) | ✓ | ✓ | ✓ | ✓ |
| Selective reporting (reporting bias) | ✓ | ✗ | ✓ | ✓ |
| Other bias | ✗ | ✗ | ✗ | ✗ |
Appendix 10 Risk-of-bias assessment for individual randomised controlled trials on surgical procedures
| Domain (type of bias assessed) | Citron and Nunez, 200567 | Ullah et al, 200968 | van Rijssen et al., 200669 |
|---|---|---|---|
| Sequence generation (selection bias) | ✓ | ? | ✓ |
| Allocation concealment (selection bias) | ? | ? | ✓ |
| Blinding of participants (performance bias) | ✓ | ? | ✗ |
| Blinding of health-care providers (performance bias) | ✗ | ✗ | ✗ |
| Blinding of outcome assessment (detection bias) | ✗ | ✗ | ? |
| Groups treated identically (performance bias) | ✓ | ✓ | ✓ |
| Incomplete outcome data (attrition bias) | ? | ✓ | ✓ |
| Intention to treat (attrition bias) | ? | ✓ | ✓ |
| Selective reporting (reporting bias) | ✓ | ✗ | ✓ |
| Other bias | ? | ✓ | ? |
Appendix 11 Risk-of-bias assessment for non-randomised comparative studies on collagenase versus surgery
| Domain | Outcome | Naam 201371 | Nydick et al., 201372 |
|---|---|---|---|
| Sequence generation | ✗ | ✗ | |
| Allocation concealment | ✗ | ✗ | |
| Prospective data collectiona | ✗ | ✓ | |
| Confounding | Reduction of contracture | ✗ | ✗ |
| Time to normal function | ✗ | N/A | |
| Recurrence | ✗ | N/A | |
| Adverse events | ✗ | ✗ | |
| Health-related quality of life | ✗ | N/A | |
| Blinding | Reduction of contracture | ✗ | ✗ |
| Time to normal function | ✗ | N/A | |
| Recurrence | ✗ | N/A | |
| Adverse events | ✗ | ✗ | |
| Health-related quality of life | ✗ | N/A | |
| Incomplete outcome data | Reduction of contracture | ✓ | ✓ |
| Time to normal function | ✓ | N/A | |
| Recurrence | ✓ | N/A | |
| Adverse events | ✓ | ✓ | |
| Health-related quality of life | ✗ | N/A | |
| Free of selective reporting | Reduction of contracture | ✓ | ✓ |
| Time to normal function | ✓ | N/A | |
| Recurrence | ✓ | N/A | |
| Adverse events | ✓ | ✓ | |
| Health-related quality of life | ✓ | N/A | |
| Other bias | ? | ✓ | |
| Overall risk of bias | ✗ | ✗ | |
Appendix 12 Risk-of-bias assessment for non-randomised comparative studies on surgical procedures
| Domain | Outcome | Bainbridge et al., 201273 | Citron and Hearnden 200374 | Ribak et al., 201357 | Skoff 200475 | Toppi et al., 201476 |
|---|---|---|---|---|---|---|
| Sequence generation | ? | ✓ | ? | ✗ | ✓ | |
| Allocation concealment | ? | ✓ | ? | ? | ✓ | |
| Prospective data collectiona | ✗ | ✓ | ? | ✓ | ✗ | |
| Confounding | Reduction of contracture | ✗ | N/A | ✓ | ? | ? |
| Time to normal function | ✗ | N/A | ✓ | N/A | N/A | |
| Recurrence | N/A | ✓ | ? | ? | N/A | |
| Adverse events | ? | ? | ? | ? | ? | |
| Health-related quality of life | N/A | N/A | N/A | ✓ | N/A | |
| Blinding | Reduction of contracture | ✗ | N/A | ? | ? | ✗ |
| Time to normal function | ✗ | N/A | N/A | N/A | N/A | |
| Recurrence | N/A | ✗ | ? | ? | N/A | |
| Adverse events | ✗ | ✗ | ? | ? | ✗ | |
| Health-related quality of life | N/A | N/A | N/A | ? | N/A | |
| Incomplete outcome data | Reduction of contracture | ✓ | N/A | ✓ | ? | ? |
| Time to normal function | ✓ | N/A | ✓ | N/A | N/A | |
| Recurrence | N/A | ✓ | ✓ | ? | N/A | |
| Adverse events | ✓ | ✓ | ✓ | ? | ? | |
| Health-related quality of life | N/A | N/A | N/A | ? | N/A | |
| Free of selective reporting | Reduction of contracture | ✓ | N/A | ✓ | ✓ | ✓ |
| Time to normal function | ✓ | N/A | ✓ | N/A | N/A | |
| Recurrence | N/A | ✓ | ✓ | ✓ | N/A | |
| Adverse events | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Health-related quality of life | N/A | N/A | N/A | ✓ | N/A | |
| Other bias | ? | ✓ | ? | ? | ? | |
| Overall risk of bias | ✗ | ✗ | ? | ? | ? | |
Appendix 13 Results of the quality assessment for individual case series on collagenase
| Domain | Badalamente and Hurst, 200048 | Coleman et al., 201278 | Coleman et al., 201479 | Martin-Ferrero et al., 201384 | McMahon et al., 201385 | Peimer et al., 201387 (CORDLESS) | Peimer et al., 201386 | Skirven et al., 201388 | Syed et al., 201391 | Watt et al., 201089 | Witthaut et al., 201390 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Representative sample | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ | ✗ | ✓ |
| Inclusion/exclusion criteria clearly defined | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Participants at similar point in disease progression | ? | ✓ | ✗ | ✗ | ? | ? | ? | ✗ | ✓ | ? | ✓ |
| Selection of participants consecutive | ✗ | ? | ? | ? | ? | ? | ? | ✗ | ? | ✓ | ? |
| Data collection undertaken prospectively | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Intervention clearly defined | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Intervention delivered by an experienced person | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Intervention delivered in an appropriate setting | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Important outcomes considered | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Objective outcome measures used | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Follow-up long enough | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ |
| Information on non-respondents, dropouts | ✓ | ✓ | ✓ | ? | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Withdrawals likely to introduce bias | ? | N/A | N/A | ? | ? | ? | ? | N/A | ? | ? | ? |
| Important prognostic factors identified | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✓ |
Appendix 14 Sensitivity analyses: replacing missing standard deviation in the meta-analyses assessing change in range of motion and change in contracture
Change in range of motion
FIGURE 45.
Change in ROM (SD for Hurst et al. 55 = high collagenase SD, low placebo SD). IV, inverse variance.

FIGURE 46.
Change in ROM (SD for Hurst et al. 55 = low collagenase SD, high placebo SD). IV, inverse variance.
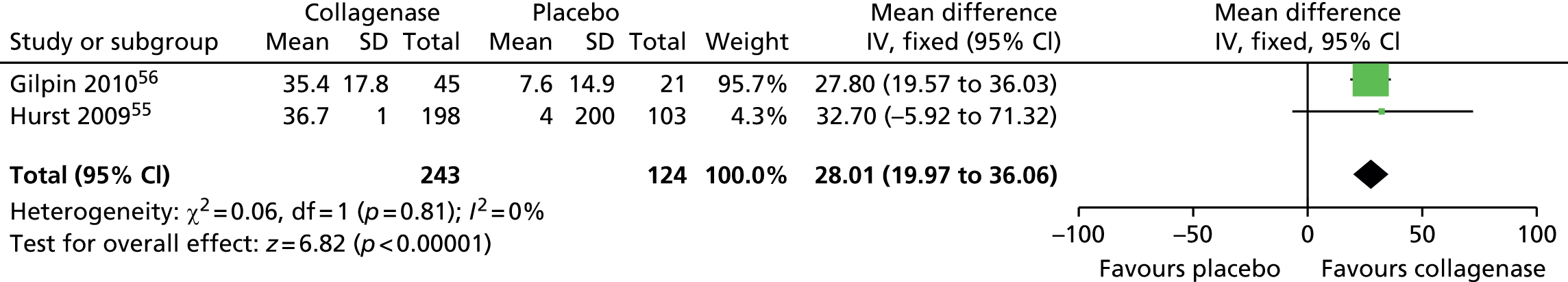
Appendix 15 Characteristics and results of collagenase studies
| Study details | Baseline | Outcomes | |||||
|---|---|---|---|---|---|---|---|
| Study/location/dates | Study design (intervention/comparator)/length of follow-up | Number of participants (% men)/number of hands, etc. | Degree of baseline contracture | Reduction of contracture | Recurrence of contracture/definition | Adverse events | Secondary outcomes |
| RCTs | |||||||
| Badalamente et al., 200264 USA Study dates: NR |
RCT (CCH vs. placebo)/ total = 5 years (MCP = mean 4 years, PIP = mean 3.8 years) | n = 49 (86% men)/49 hands, 49 fingers | Mean: MCP joints = 44° (SD 17.4); PIP joints = 53° (SD 18.7) | Reduction of contracture to 0–5° of normal, n: Within 7 days: MCP joints CCH = 9/18 (50%) Placebo = 2/18 (11%) p-value = 0.001 Within 1 month: MCP joints CCH = 14/18 (78%) Placebo = 2/18 (11%) p-value = 0.001 Within 1 month: PIP joints CCH = 5/7 (71%) Placebo = 0/6 (0%) p-value NR |
4/18 (22%) MCP joints 4/7 (57%) PIP joints Recurrence definition = NR |
Three skin tears on cord rupture. Around one-third had an elbow and/or axillary lymphadenopathy. Other adverse events included pain on cord rupture, pain to pressure at the injection site, dorsal and volar oedema, minimal haematoma and occasional limited ecchymosis | ROM (% change from baseline): CCH improved significantly (p-value = 0.001) compared with placebo Grip strength: CCH = 35.41 kg, placebo = 32.89 kg, NS |
| Badalamente and Hurst, 200563 USA Study dates: NR |
RCT (CCH vs. placebo)/ 12 months (mean 125 days for MCP joints/107 days for PIP joints) | n = 35 (% men NR)/NR | Mean: MCP joints = 45°; PIP joints = 43° | % of joints achieving full finger extension (0°) after CCH injection: MCP = 92%; PIP = 75% | NR | Included pain associated with cord rupture, hand oedema and palmar ecchymosis | NR |
| Badalamente and Hurst, 200765 USA Study dates: NR |
RCT (CCH vs. placebo)/up to 24 months | n = 35 (80% men)/26 digits, 45 joints | Mean: MCP joints = 51° (SD 12°); PIP joints = 46° (SD 14°) | Number of participants achieving reduction in deformity to within 0–5° of normal within 30 days of last injection: CCH = 21/23 (91%); placebo = 0/12 (0%); MCP joints = 12/14 (86%); PIP joints = 9/9 (100%) | 1 MCP, 4 PIP (from both blinded and open-label phases) Recurrence defined as ‘return of contracture (≥ 20°) in successfully treated joints’ |
After first injection, %: oedema = 100 CCH/8 placebo injection site pain = 100 CCH/50 placebo Ecchymosis = 43 CCH/0 placebo Pruritus = 4 CCH/0 placebo Skin laceration = 13 CCH/0 placebo Lymphadenopathy = 39 CCH/0 placebo Blood blister = 39 CCH/0 placebo |
NR |
| Gilpin et al., 201056 (CORD II) Australia August 2007 to September 2008 |
RCT (CCH vs. placebo)/ 90 days | n = 66 (85% men)/NR | Total contracture index, mean: CCH = 174.7 (SD 107.2) Placebo = 150.1 (SD 84) p-value = 0.36 |
Reduction of contracture to 0–5° of normal 30 days after last injection: All primary joints CCH = 20/45 (44%) Placebo = 1/21 (5%) MCP joints CCH = 13/20 (65%) Placebo = 1/11 (9.1%) PIP joints CCH = 7/25 (28%) Placebo = 0/10 (0%) |
Zero recurrences Recurrence defined as ‘an increase in joint contracture to 20° or greater in the presence of a palpable cord at any time during the study in joints that attained a reduction in contracture to 0–5° of normal’ |
Severe treatment-related adverse events: n = 1 flexion pulley rupture (CCH group), %: ≥ 1 adverse event = 100 CCH/38 placebo Peripheral oedema = 78 CCH/10 placebo Contusion = 73 CCH/10 placebo Injection site haemorrhage = 42 CCH/0 placebo Injection site pain = 38 CCH/10 placebo Pain in extremity = 49 CCH/10 placebo Tenderness = 13 CCH/0 placebo Injection site swelling = 36 CCH/14 placebo Pruritus = 11 CCH/0 placebo Lymphadenopathy = 24 CCH/0 placebo Axillary pain = 11 CCH/0 placebo Injection site vesicles = 4 CCH/0 placebo |
Clinical improvement, n (%): CCH = 35 (78%); placebo = 3 (14%); p-value < 0.001 Mean change in contracture from baseline, %: CCH = 70.5% (SD 29.2); placebo = 13.6% (SD 26.1); p-value < 0.001 Mean change in ROM from baseline, °: CCH = 35.4 (SD 17.8); placebo = 7.6 (SD 14.9); p-value < 0.001 Median time to reach primary end point (CCH joints) = 57 days |
| Hurst et al., 200955 (CORD I) USA August 2007 to October 2008 |
RCT (CCH vs. placebo)/ 90 days | n = 308 (80% men)/308 joints | Total contracture index, mean: CCH = 149.1 (SD 127.6); placebo = 149.3 (SD 111.4) |
Reduction of contracture to 0–5° of full extension 30 days after last injection: CCH = 130/203 (64%); placebo = 7/103 (6.8%) |
Zero recurrences (in any CCH-treated primary joint that had reached the primary end point) Recurrence defined as ‘an increase in joint contracture to 20° or more in the presence of a palpable cord at any time during the study’ |
Severe adverse events, n (%) (from CORD I supplementary appendix): ≥ 1 severe treatment-related adverse event = 20 (9.8) CCH/2(1.9) placebo (placebo events were unrelated to study drug) CCH adverse events related to study drug, n (%): Tendon rupture = 1 (0.5) (two ruptures) CRPS = 1 (0.5) Peripheral oedema = 4 (2) Injection site pain = 4 (2) Upper-extremity pain = 4 (2) Injection site haemorrhage = 3 (1.5) Contusion = 2 (1) Tenderness = 2 (1) Chest wall pain = 1 (0.5) Ecchymosis = 1 (0.5) Injection site cellulitis = 1 (0.5) Skin laceration = 1 (0.5) Other adverse events, %: ≥ 1 adverse event = 97 CCH/21 placebo ≥ 1 severe adverse event = 10 CCH/2 placebo Peripheral oedema = 73 CCH/4 placebo Contusion = 51 CCH/2 placebo Injection site haemorrhage = 37 CCH/4 placebo Injection site pain = 32 CCH/5 placebo Upper-extremity pain = 31 CCH/3 placebo Tenderness = 27 CCH/0 placebo Ecchymosis = 25 CCH/1 placebo Injection site swelling = 21 CCH/4 placebo Pruritus = 11 CCH/1 placebo Skin laceration = 11 CCH/0 placebo Lymph node enlargement = 10 CCH/0 placebo Lymphadenopathy = 10 CCH/0 placebo Erythma = 6 CCH/0 placebo Blister = 5 CCH/0 placebo Injection site pruritus = 5 CCH/0 placebo Axillary pain = 5 CCH/0 placebo Arthralgia = 3 CCH/0 placebo Inflammation = 4 CCH/0 placebo Blood blister = 3 CCH/0 placebo Joint swelling = 3 CCH/0 placebo Headache = 3 CCH/1 placebo Swelling = 3 CCH/0 placebo Injection site vesicles = 2 CCH/1 placebo |
30 days after last injection (all p-values < 0.001): Clinical improvement, n (%): All joints CCH = 172 (85%); placebo = 12 (12%) MCP joints CCH = 125 (94%); placebo = 8 (12%) PIP joints CCH = 47 (67%); placebo = 4 (12%) Change in contracture from baseline (%): All joints CCH = –79.3; placebo = –8.6 MCP joints CCH = –87.1; placebo = –7.2 PIP joints CCH = –64.5; placebo = –11.4 Mean change in ROM from baseline, °: All joints CCH = 36.7; placebo = 4.0 MCP joints CCH = 40.6; placebo = 3.7 PIP joints CCH = 29.0; PIP = 4.7 Median time to reach primary end point (CCH joints) = 56 days |
| Witthaut et al., 201166 (secondary analysis of CORD I data) USA August 2007 to October 2008 |
RCT (CCH vs. placebo)/ 90 days | n = 308 (80% men)/299 joints | Total contracture index, mean: CCH = 149.1 (SD 127.6); placebo = 149.3 (SD 111.4) | Mean increase in ROM from baseline to day 30, %: CCH = 36.7; placebo = 4.0 | NR | NR | Day 30, mean (SD) improvement in contracture: participants’ ratings: 77%; physicians ratings: 55% very much improved, 35% much improved, 10% minimally improved, 0% no change |
| Non-randomised comparative studies | |||||||
| Naam 201371 USA Study dates: NR |
Single-centre, retrospective, observational, longitudinal chart review (CCH vs. fasciectomy)/ CCH = 32mo; Fasciectomy = 39mo | n = 46 (78% men)/84 joints | MCP joints CCH = 43.5°; fasciectomy = 41.4°, p-value = 0.76 PIP joints CCH = 30°; fasciectomy = 29.9°, p-value = 0.99 |
Mean decrease in contracture from baseline, degrees MCP joints CCH = –39.9 (SD 21.9); fasciectomy = –37.7 (SD 15.4), p-value = 0.73 PIP joints CCH = –12.5 (SD 24.7); fasciectomy = –21.8 (SD 15.3), p-value = 0.45 |
Zero recurrences Recurrence defined as ‘an increase of ≥ 20° from the point of correction as measured at the 2-year visit’ |
CCH, n: Bruising at the injection site = 25 Transiently enlarged axillary lymph node = 1 Fasciectomy, n: Transient paraesthesia of the radial side of the index finger = 1 |
Mean increase in ROM from baseline: MCP joints CCH = 40.7° (SD 22°); fasciectomy = 40.6° (SD 17.9°); p-value = 0.99 PIP joints CCH = 15° (SD 28.3°); fasciectomy = 27.8° (SD 14.6°); p-value = 0.29 DASH scores, mean (higher scores represent greater disability): 1 month CCH = 3 (range 1–34); fasciectomy = 38 (range 30–60); p-value = 0.002 2 months CCH = 3 (range 0–30); fasciectomy = 25 (range 15–45); p-value = 0.007 3 months CCH = 2 (range 0–25); fasciectomy = 15 (range 5–45); p-value = 0.02 1 year CCH = 2 (range 0–25); fasciectomy = 4 (range 2–25); p-value = 0.5 2 years CCH = 3 (range 0–30); fasciectomy = 3 (range 0–35); p-value = 0.6 |
| Nydick et al., 201372 USA Study dates: NR |
Retrospective non-randomised comparative study (CCH vs. PNF)/mean, months: CCH = 6 (range 3–24); PNF = 6 (range 3–28) | n = 59 (81% men)/ NR | Mean MCP joints CCH = 40 (SD 12); PNF = 37 (SD 20) PIP joints CCH = 50 (SD 57); PNF = 41 (SD 33) |
Mean reduction of contracture MCP joints CCH = 30°; PNF = 32°, p-value = 0.67 PIP joints CCH = 24°; PNF = 21°, p-value = 0.84 |
NR | %: oedema = 100 CCH/17 PNF (p-value < 0.01); ecchymosis = 97 CCH/10 PNF (p-value < 0.01); pruritus = 24 CCH/0 PNF (p-value < 0.01); skin tear = 34 CCH/50 PNF (p-value = 0.29); lymphadenopathy = 21 CCH/0 PNF (p-value < 0.01) | Clinical success, no of joints (%): MCP joints CCH = 14 (64%); PNF = 26 (81%); p-value = 0.37 PIP joints CCH = 5 (42%); PNF = 9 (50%), p-value = 0.72 |
| Collagenase case series | |||||||
| Badalamente and Hurst, 200048 USA Study dates: NR |
Prospective case series/ 2 years | n = 35 (91% men)(n = 6 on dose escalation study; n = 29 on 10,000 U study)/dose escalation study: 6 MCP joints 10,000 U study: 34 MCP joints, 9 PIP joints, 1 thumb cord |
Dose escalation study: mean 49 (SD 11) 10,000 U study: MCP joints Mean 42 (SD 13) PIP joints Mean 52 (SD 16) |
Dose escalation study: CCH had no effect in reducing MCP contractures 10,000 U study: MCP joints 30/34 (88%) corrected to 0–5° of normal PIP joints 4/9 (44%) corrected to 0–5° of normal |
10,000 U study: 9% MCP and 0% PIP joint recurrences Recurrence definition = NR |
No major adverse reactions. Minor local adverse reactions included tenderness to pressure at the injection site with minimal palmar, and sometimes dorsal, oedema and minimal haematoma. Six patients with only PIP joint contractures experienced ulnar border forearm tenderness with elbow and axilla lymphadenopathy after injection | Number of injections required, n: 15 patients required repeat injections: 6 patients received two injections, 5 received three injections, 2 received four injections, 1 received five injections and 1 received six injections Time to cord rupture/joint correction: MCP cord rupture was achieved at day 1 in 15 cases, at day 7 in 13 cases, and at day 14 in 6 cases. Correction of PIP joints occurred within the first 2 weeks of injection. Two joint corrections were also achieved simultaneously in 11 fingers |
| Badalamente and Hurst, 201177 (follow-up of CORD I?) USA Study dates: NR |
Prospective case series/ 2 years | n = 509 (84% men)/ 672 joints (476 MCP, 196 PIP) | NR | NR | Single site study (n = 35): MCP joints = 4/28 (14%) PIP joints = 6/26 (23%) Overall = 18.5% Multicentre study (n = 474): MCP joints = 61/448 (13%) PIP joints = 58/170 (34%) Overall = 19.3% Recurrence defined as ‘20 degree contracture’ |
NR | NR |
| Coleman et al. 201278 Australia Study dates: NR |
Prospective case series/ 90 days | n = 12 (92% men)/36 hands, 36 joints | Period 1 MCP joints ≤ 50° n = 5 PIP joints ≤ 40° n = 3 > 40° n = 4 Period 2 MCP joints ≤ 50° n = 15 > 50° n = 2 PIP joints ≤ 40° n = 5 > 40° n = 2 |
Period 1 Mean change in contracture from baseline, °: MCP joints = 29 (SD 20.7) PIP joints = 30.7 (SD 21.1) Mean change in ROM from baseline, °: MCP joints = 29 (SD 21) PIP joints = 32.1 (SD 24.3) Period 2 Mean change in contracture from baseline, °: MCP joints = 30.3 (SD 10.9) PIP joints = 22.1 (SD 4.9) Mean change in ROM from baseline: MCP joints = 30° (SD 10.8°) PIP joints = 17.1° (SD 2.7°) |
NR | Period 1, %: ≥ 1 adverse event= 100 Lymphadenopathy = 50 Injection site discomfort = 0 Injection site pain = 25 Peripheral oedema = 100 Contusion = 100 Skin laceration = 17 Pain in extremity = 92 Paraesthesia = 8 Blood blister = 0 Pruritus = 42 Skin discolouration = 0 Period 2, %: ≥ 1 adverse event = 100 Lymphadenopathy = 58 Injection site discomfort = 8 Injection site pain = 42 Peripheral oedema = 100 Contusion = 100 Skin laceration = 17 Pain in extremity = 92 Paraesthesia = 0 Blood blister = 17 Pruritus = 83 Skin discolouration = 8 |
Period 1, n: 9 ‘very satisfied’, n = 3 patients ‘quite satisfied’ with treatment Period 2, n: 12 ‘very satisfied’ with treatment |
| Coleman et al. 201479 USA, Australia September 2011 to February 2012 |
Prospective case series/60 days | n = 60 (85% men)/ 120 joints | Mean (SD), °: MCP joints = 41 (17); PIP joints = 48 (19) | Mean (SD) contracture at day 30, °: MCP = 8 (14) PIP = 19 (18) % change, mean (SD): MCP = 86 (25) PIP = 66 (28) |
NR | % of total (N = 60) ≥ 1 adverse event = 100 Pain in extremity = 83 Peripheral oedema = 82 Contusion = 80 Lymphadenopathy = 37 Pruritus = 33 Skin laceration = 25 Injection site haematoma = 18 Injection site swelling = 13 Blood blister = 12 Ecchymosis = 5 |
Mean (SD) ROM at 30 days, °: MCP joints = 82 (15) PIP joints = 78 (20) Mean (SD) change in ROM, °: MCP joints = 32 (14) PIP joints = 27 (13) Patient ratings of treatment satisfaction, n (%): Very satisfied = 36 (60) Quite satisfied = 17 (28) Neither satisfied nor dissatisfied = 5 (8) Quite dissatisfied = 1 (2) Very dissatisfied = 1 (2) Physician ratings of improvement, n (%): Very much improved = 33 (55) Much improved = 22 (37) Minimally improved = 4 (7) No change = 1 (2) Minimally worse = 0 Much worse = 0 Very much worse = 0 |
| Considine and Hirpara 201380 Location: NR Study dates: NR |
Prospective case series/mean 4 days | n = 10 (% men NR)/13 fingers | Mean: MCP joints = 58.6°; PIP joints = 39° | Mean post-treatment contracture: MCP = 4.23°; PIP = 9° |
NR | Post-injection bruising = 13/13 fingers Skin tears = 11/13 fingers Transient paraesthesia = 1/10 participants |
NR |
| Hayton et al. 201381 USA Study dates: NR |
Case series/ NR | n = 616 (83% men)/ 577 joints (201 MCP, 376 PIP) | Mean pre-treatment FFC: Group A = 48.2° (20.2); Group B = 49.7° (18.5) | PIP joint contractures spontaneously correcting after first CCH injection for MCP joint deformity = 28% | NR | % Oedema peripheral = 81 Injection site pain = 39 Injection site haemorrhage = 38 Tenderness = 29 Injection site swelling = 28 Contusion = 65 Pain in extremity = 43 Pruritus = 15 Ecchymosis = 14 Skin laceration = 13 Blood blister = 11 Lymphadenopathy = 11 |
Mean change in FFC: After first injection: Group A = 66% Group B = 55% After last injection: Group A = 77% Group B = 62% Mean change in ROM, °: After first injection: Group A = 27.8 (SD 19.7) Group B = 24.4 (SD 18.1) After last injection: Group A = 34.3 (SD 19.8) Group B = 29.1 (SD 20) |
| Kaplan et al. 201282 (CORDLESS?) Location: NR Study dates: NR |
Retrospective case series/3 years | n = 643 (84% men)/ 1080 joints (648 MCP, 432 PIP) | Mean pre-treatment FFC: Recurrent MCP joints = 37° Non-recurrent MCP joints = 38° Recurrent PIP joints = 40° Non-recurrent PIP joints = 35° |
Mean FFC, °: Post-treatment Recurrent MCP joints = 2° Non-recurrent MCP joints = 1° Recurrent PIP joints = 3° Non-recurrent PIP joints = 2° 1 year Recurrent MCP joints = 6° Non-recurrent MCP joints = 2° Recurrent PIP joints = 12° Non-recurrent PIP joints = 3° 2 years Recurrent MCP joints = 23° Non-recurrent MCP joints = 2° Recurrent PIP joints = 29° Non-recurrent PIP joints = 6° 3 years Recurrent MCP joints = 33° Non-recurrent MCP joints = 3° Recurrent PIP joints = 37° Non-recurrent PIP joints = 8° |
185/572 joints (96/417 MCP, 89/155 PIP) Recurrence defined as ‘ = 20° increase in contracture in the presence of a palpable cord in a joint previously successfully corrected (reduction of contracture to 0°–5°) or patient underwent further medical/surgical intervention’ |
No new long-term adverse events or serious adverse events | NR |
| Kaplan et al. 201383 Location: NR Study dates: NR |
Prospective case series/ 90 days |
n = 37 (% men NR)/NR | > 20° (MCP joints only) | > 50% reduction in contracture at 30 days: Manipulation at 1 day = 92% 2 days = 91% 4 days = 85% p-value = 0.83 |
NR | n: Swelling = 18 Bruising = 14 Pain = 5 Blood blister = 3 1 each of: Armpit pain Armpit bruising Hand bruising Itching Lymphadenopathy Numbness Oedema Itching in armpit Palm pain Skin tear |
Reduction in contracture to ≤ 5° maintained to 90 days: Manipulation at 1 day = 91% 2 days = 82% 4 days = 83% p-value = 0.81 |
| Martin-Ferrero et al. 201384 Spain 2011 to 2013 |
Prospective case series/ 1 year | n = 35 (100% men)/35 palpable bands | MCP joints = 64°; PIP joints = 83.3° | 2 weeks: MCP joints = 8° PIP joints = 20° 2 months: MCP joints = 4° PIP joints = 15° 3 months: MCP joints = 4° PIP joints = 15° 6 months: MCP joints = 4° PIP joints = 15° |
Zero recurrences Recurrence definition = NR |
n: Skin dehiscence during finger stretching = 9 Axillary lymphadenopathy = 2 Axillary pain with no palpable lymphadenopathy = 1 Increase in transaminases without clinical consequences = 2 |
Number requiring physiotherapy treatment = 3 |
| McMahon et al. 201385 USA June 2010 to June 2012 |
Retrospective case series/mean 15 months | n = 48 (65% men)/ 53 digits, 64 joints | Mean 48° (SD 21) | Mean (SD): MCP contracture at cord rupture = 4 (8) MCP contracture at latest follow-up = 9 (15) PIP contracture at cord rupture = 14 (14) PIP contracture at latest follow-up = 29 (20) The differences in means were statistically significant at all time points (p-value < 0.05) |
MCP joints = 24% PIP joints = 39% Recurrence defined as ‘a 20° or greater increase in contracture relative to the most corrected measurement achieved at cord rupture’ |
Flexor tendon rupture = 1/102 (1%) patients Ecchymosis = 88% joints Localised or palmar oedema = 39% joints Skin tear following manipulation = 17% joints Swelling or tenderness of axillary lymph nodes = 6% joints |
Mean (range) DASH score at latest follow-up = 3 (0–18) Additional interventions required: CCH injection = 10/102 eligible patients Surgical release = 5/102 patients Patient satisfaction = mean 8.5 (range 1–10) |
| Peimer et al. 201387 (CORDLESS) USA Study dates: NR |
Case series/ 3 years | n = 643 (84% men)/1080 joints (648 MCP, 432 PIP) | NR | 623/1080 joints had been successfully treated (i.e. 0° to 5° contracture) in the original study | 217/623 successfully treated joints (121/451 MCP, 96/172 PIP) at 3 years recurred Recurrence defined as ‘an increase in joint contracture 20° or greater in the presence of a palpable cord, or as the need for the joint to have further medical or surgical intervention (which could include CCH injection) to correct new or worsening contracture’ |
193/643 patients had 370 adverse events | Measurably improved (partially corrected) joints meeting criteria for lack of durability of response by year 3 = 150/301 Worsening (i.e. increase in joint contracture ≥ 20°, or further medical/surgical intervention) of contracture in successfully treated and measurably improved (partially corrected) joints = 353/924 Progression in joints not effectively treated = 59/156 |
| Peimer 201386 USA January 2011 to February 2011 |
Retrospective case series/NR | n = 463 (74% men)/ 629 joints | Mean: Total = 49° (SD 21°) MCP joints = 44° (SD 20°) PIP joints = 57° (SD 21°) |
Post-treatment contracture, mean: Total = 12° (SD 17°) MCP joints = 8° (SD 13°) PIP joints = 19° (SD 20°) |
NR | Peripheral oedema = 4 Skin laceration = 2 1 each of: Blood blister Epicondylitis Erythema Injection site vesicles Limb immobilisation Lymphadenitis Lymphadenopathy Petechiae Pruritus |
Injections per joint, mean = 1.08 (95% CI 1.05 to 1.11) Injections per finger, mean = 1.2 (SD 0.5) Days between first and second injection (n = 42 joints), mean = 61 (SD 49) Cord release, n: full release: All joints = 284 (67%) MCP joints = 222 (75%) PIP joints = 62 (48%) partial release: All joints = 131 (31%) MCP joints = 68 (23%) PIP joints = 63 (49%) no release: All joints = 9 (2%) MCP joints = 5 (2%) PIP joints = 4 (3%) |
| Skirven 201388 USA Study dates: NR |
Prospective case series/ 4 weeks | n = 21 (90% men)/ 22 fingers, 22 joints | Mean passive PIP joint contracture: Total = 56° (range 40–80) Primary disease group = 53° (SD 10°) Recurrent disease group = 58° (SD 12°) |
At 4 weeks, mean, °: Total = 7 (range 0–35) Primary disease group = 8 (SD 11) Recurrent disease group = 6 (SD 7) p = 0.575 |
NR | n: Digital swelling and ecchymosis = 18 Skin tears = 4 Skin blistering = 2 Localised pruritus = 1 |
Mean joint contracture, °: At cord rupture = 22 (range 0–22) At 1 week after cord rupture and therapy = 12 (range 0–36) |
| Syed et al. 201391 UK Study dates: NR |
Prospective case series/12 months | n = 56 (86% men)/ 56 hands | Contracture angle: Mean = 41.8 (SD 9.7) | Contracture angle, mean: 1 month = –0.9 (SD 8.1) 3 months = 1.4 (SD 7.5) 6 months = 0.4 (SD 2.4) 12 months = 1.0 (SD 1.9) |
NR | n: Skin tear = 4 Skin tear and bruising = 11 Bruising = 13 Bruising and diarrhoea = 1 Bruising and axillary discomfort = 2 Axillary swelling = 8 Axillary swelling and skin tear = 3 Axillary swelling and fever = 1 Axillary swelling, fever and axillary discomfort = 2 Discomfort at injection site = 1 Blister = 3 |
URAM score, mean (higher scores represent poorer functional outcome): 1 month = 1.9 (SD 3.3) 3 months = 0.6 (SD 1.6) 6 months = 0.8 (SD 2.8) 12 months = 0.3 (SD 0.5) |
| Watt et al. 201089 USA 1999 to 2000 (treatment dates) |
Case series/ 8 years | n = 8 (% men NR)/ 8 joints | Mean: MCP joints = 57° (range 30–76°); PIP joints = 45° (range 35–55°) | Mean, °: 1 week: MCP joints = 9 (range 0–35) PIP joints = 8 (range 0–15) 6 months: MCP joints = 8 (range 0–35) PIP joints = 13 (range 0–25) 1 year: MCP joints = 11 (range 0–35) PIP joints = 15 (range 0–30) 8 years: MCP joints = 22 (0–55) PIP joints = 60 (range 50–70) |
MCP joints = 4/6 PIP joints = 2/2 Recurrence defined as ‘any increase in the degree of contracture of the injected joint compared with maximal extension achieved after extension’ |
NR | Patients subjectively rated overall clinical success at 60% (range 0–100%) and 7/8 patients would have further collagenase injection for recurrent or progressive disease |
| Witthaut et al. 201390 USA (JOINT I); Australia, UK, Switzerland, Sweden, Denmark and Finland (JOINT II) September 2007 to December 2008 |
Prospective case series/ 9 months | n = 587 (85% men)/ 879 joints | Total contracture index, mean: JOINT I = 132.5 (SD 109.8) JOINT II = 136.5 (SD 104.2) Total = 135.1 (SD 106.0) |
Proportion of joints achieving a reduction in contracture to within 0–5° of normal within 30 days of last injection: Total = 497/879 (57%), MCP joints = 369/531 (70%), PIP joints = 128/348 (37%) |
19/497 joints achieving clinical success recurred Recurrence defined as ‘when joint contracture increased to ≥ 20° with palpable cord in patients who had previously achieved clinical success’ |
n: ≥ 1 treatment-related adverse event = 567 (97%) Peripheral oedema = 439 (75%) Contusion = 350 (60%) Injection site pain = 248 (42%) Pain in extremity = 224 (38%) Injection site haemorrhage = 216 (37%) Injection site swelling = 156 (27%) Tenderness = 146 (25%) Pruritus = 59 (10%) Skin laceration = 55 (9%) Blood blister = 48 (8%) Haematoma = 45 (8%) Axillary pain = 45 (8%) Lymphadenopathy = 42 (7%) Ecchymosis = 41 (7%) Injection site vesicles = 34 (6%) |
Mean change in contracture from baseline, all joints, %: JOINT I = 66.8 (SD 41) JOINT II = 75.4 (SD32) Total = 72.6 (35) Clinical improvement, all joints, n: JOINT I = 209 (72%) JOINT II = 463 (79%) Total = 672 (76%) ROM, mean change from baseline, all joints, %: JOINT I = 28.2 (SD 20) JOINT II = 30.6 (SD 17) Total = 29.8 (SD 18) Mean change in contracture from baseline, MCP joints, % JOINT I = 81.7 (SD 28) JOINT II = 85.2 (SD 23) Total = 84 (SD 25) Clinical improvement, MCP joints, n: JOINT I = 161 (86%) JOINT II = 309 (90%) Total = 470 (89%) ROM, mean change from baseline, MCP joints, %: JOINT I = 33.3 (SD 17) JOINT II = 32.9 (SD 16) Total = 33 (SD 17) Mean change in contracture from baseline, PIP joints, %: JOINT I = 40 (SD 47) JOINT II = 61.6 (SD 37) Total = 55.2 (SD41) Clinical improvement, PIP joints, n: JOINT I = 48 (47%) JOINT II = 154 (63%) Total = 202 (58%) ROM, mean change from baseline, PIP joints, %: JOINT I = 18.9 (SD 22) JOINT II = 27.5 (SD 19) Total = 25 (SD 20) |
Appendix 16 Adverse events reported in surgery trials
| Complication/adverse event | Median (range), % | ||||
|---|---|---|---|---|---|
| All fasciectomies | LF | Dermofasciectomy | Total fasciectomy | Fasciotomy (including PNF) | |
| Neurapraxia (n = 1)68 | 50a | NR | 90a,b | NR | NR |
| Nerve injury (n = 4)67,69,73,75 | 1 (0–2) | NR | 0.05b | NR | 0.05 (0–0.05) |
| Artery injury (n = 1)73 | 1b | NR | 3b | NR | 1b |
| Infection (n = 5)68,69,73,75,76 | 2 (0–18) | NR | 11 (4–18) | NR | 1.5 (0–3) |
| Haematoma (n = 2)70,73 | 6 (2–10) | NR | NR | NR | 2.5 (0–5) |
| Complex regional pain syndrome (n = 5)57,67–69,75 | 4 (0–13) | NR | 3b | NR | 2 (0–4) |
| Skin (n = 4)57,68,69,75 | 3 (3–23) | NR | 10b | NR | 26 (4–48) |
| Pain (n = 1)73 | 6b | NR | 14b | NR | 2 (PNF)b/7 (fasciotomy)b |
List of abbreviations
- BNF
- British National Formulary
- BSSH
- British Society for Surgery of the Hand
- CCH
- collagenase clostridium histolyticum
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- DC
- Dupuytren’s contracture
- DCE
- discrete choice experiment
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions 3 levels (of severity)
- EQ-5D-5L
- European Quality of Life-5 Dimensions 5 levels (of severity)
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- LF
- limited fasciectomy
- MCP
- metacarpophalangeal
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- PF
- partial fasciectomy
- PIP
- proximal interphalangeal
- PNA
- percutaneous needle aponeurotomy
- PNF
- percutaneous needle fasciotomy
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- ROM
- range of motion
- RR
- risk ratio
- SD
- standard deviation
- WTP
- willingness to pay