Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 13/111/01. The protocol was agreed in December 2013. The assessment report began editorial review in July 2014 and was accepted for publication in December 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Aileen Clarke is a member of the National Institute for Health Research Health Technology Assessment and Efficacy and Mechanism Evaluation Editorial Board and the Warwick Medical School receive payment for this work. Aileen Clarke and Sian Taylor-Phillips are partially supported by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care West Midlands at the University Hospitals Birmingham NHS Foundation Trust.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Freeman et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Overview
5-fluorouracil (5-FU) is a chemotherapy drug used to treat several cancers including those of the head and neck (H&N), pancreas, stomach and especially bowel (colorectal) cancer. 5-FU is usually given orally or by continuous intravenous (i.v.) infusion into the blood circulation and is often accompanied by additional chemotherapies. 5-FU is administered in a series of cycles usually over 3–6 months. 5-FU is cleared from patients’ blood at rates which vary between patients, and the dose that reaches cancer cells can therefore vary between individuals. As a result, some patients may receive doses which are too low to be fully effective, whereas others may experience toxicity because the circulating dose is too high.
The My5-FU test kit (Saladax Biomedical Inc., PA, USA; previously known as OnDose) is designed to measure the amount of 5-FU circulating in the blood using a small blood sample taken during the 5-FU infusion. Knowing the individual patient s level of 5-FU in the blood allows doctors to adjust the dose to be used at the next cycle of treatment so that it is more appropriate for that individual. The My5-FU assay can be used with patients who have various types of cancer. However, thus far most attention has been focussed on colorectal cancer (CRC), which is the third most common cancer in the UK, with around 40,000 new cases each year.
The current report was undertaken for the National Institute for Health and Care Excellence (NICE) Diagnostics Assessment Programme (DAP). We aimed to examine the clinical effectiveness and cost-effectiveness of 5-FU plasma monitoring with the My5-FU assay for guiding dose adjustment in patients receiving 5-FU chemotherapy by continuous infusion in the NHS in England and Wales.
Conditions and aetiologies
Therapeutic drug monitoring in cancer treatment aims to personalise chemotherapy to improve treatment efficacy, avoid severe toxicity and reduce health-care costs by using individual dosing schedules. It takes into account the interindividual variation in drug metabolism to bring drug exposure into the optimum therapeutic range. This is especially important for cytotoxic anticancer drugs which can have a narrow therapeutic window. 5-FU (or 5-fluoro-2,4-pyrimidinedione) is one of the most widely used cytotoxic drugs.
Descriptions of the health problem
The following sections will focus on the conditions of most relevance to the current report: CRC and H&N cancer. Additional, less detailed information will be provided for stomach cancer and pancreatic cancer, two other conditions which are also referred to in the report.
Colorectal cancer
Colorectal cancer is the third most common cancer in the Western world and is the second most common cancer-related cause of death in combined male and female populations in the UK. 1 In 2010, there were 15,708 deaths from bowel cancer in the UK (62% from colon cancer, 38% from rectal cancer, including the anus), with 8574 (55%) in men and 7134 (45%) in women. 2–4 Around half of people diagnosed with CRC survive for at least 5 years after diagnosis. 5
Aetiology, pathology and prognosis
Colorectal cancer (also known as large bowel cancer) can affect both males and females equally at any age; however, it is most common in people aged > 65 years. 6–8
Studies have reported that a diet high in fat (especially animal fat), red meats and low in fibre can be associated with CRC. Other possible causes include lack of exercise, smoking and alcohol. 8–10 Two inherited conditions, familial adenomatous polyposis and hereditary non-polyposis colon cancer, account for 1% and 5% of all CRC respectively. 11,12 Those with a history of inflammatory bowel disease has a six times greater risk of developing CRC than the general population. 13
The majority of CRCs (90%) are adenocarcinomas which originate from epithelial cells of the colorectal mucosa. 14 Adenomas or adenomatous polyps are benign in most cases, but around 10% of adenomas will change into cancer over time. 7,15 Tumours with a villous histology, larger in size and with severe dysplasia have a higher chance of converting to cancer and these are indicators for progression. 15,16
Spread of the disease and diagnosis determines the prognosis of patients. Around half of people diagnosed with CRC survive for at least 5 years after diagnosis. 5
In the UK there are inequalities in cancer survival following a diagnosis of CRC, in that patients who are more socioeconomically deprived are more likely to have both poorer cancer-specific and overall survival (OS). 17 Approximately 80% of patients with CRC undergo surgical treatment for the cancer with/without adjuvant radiotherapy or chemotherapy (including 5-FU). Recurrence has been reported in between 11% and 54% of patients. 7 More advanced cancers that have invaded other tissues or progressed to metastatic cancers tend to be treated with multiple chemotherapy drugs.
Advances in treatment and survival are likely to increase lifetime costs of managing CRC. 18 Cost-of-illness studies are key building blocks in economic evaluations of interventions and comparative effectiveness research. However, the methodological heterogeneity and lack of transparency of studies in this area have made it challenging to compare CRC costs between studies or over time. 18
Incidence and/or prevalence
In 2010 it was estimated that 42,747 cases of CRC were diagnosed in the UK of which 23,582 and 19,165 cases of CRC were diagnosed in men and women respectively. 19 Incidence rates of CRC have increased dramatically in both genders between 1999–2001 and 2008–10. Between 2001–3 and 2008–10, incidence rates increased by 6% in men and 7% in females. The incidence rate of CRC increases with increasing age (i.e. the highest rate is among those aged ≥ 85 years). 5 Around 73% of CRC cases diagnosed in the UK between 2008 and 2010 were among people aged > 65 years.
Significance for patients in terms of ill-health (burden of disease)
According to one study by Jayatilleke et al. ,20 CRC accounted for approximately 9% and 7% of all cancer disability-adjusted life-years in England and Wales among men and women respectively.
Significance for the NHS
In 2006, the NHS Bowel Cancer Screening Programme introduced faecal occult blood testing for both genders at age 60–69 years. The test is undertaken by taking small stool sample which is tested for the presence of blood. 21 The benefit of faecal occult blood testing in terms of reducing mortality was estimated from a systematic review of trials to be 16% and 23% for allocated and screened people respectively. 22 In addition, the test was cost-effective. 22
In addition, flexible sigmoidoscopy (NHS bowel scope screening) is a programme which has been introduced across England from 2013, for the prevention of CRC in high-risk patients by identifying and removing adenomatous polyps in the rectum and colon. This involves one-off flexible sigmoidoscopy around 55 years of age for both men and women. 23
Measurement of disease and/or response to treatment
In the UK, CRC causes around 16,000 deaths annually. The cancer mortality rates is 16,000 deaths over the time; however, it has been estimated that the overall 5-year relative survival is 50%. 24,25 A study by Coleman et al. reported that in the UK cancer survival rates are low in comparison with other Western countries. 26
Disease measurements are usually based on colonoscopy and histology for diagnosis and a range of other investigations including computerised tomography (CT) scans are undertaken for disease staging. Similarly, response to treatment is assessed by clinical consultation, with a range of tests including CT scans and regular serum antigen tests. Colonoscopy is also undertaken at annual and subsequent 5-yearly follow-up.
Diagnosis and management
The symptoms of CRC include rectal bleeding, a change in bowel habit (e.g. diarrhoea or loose stools), abdominal pain and weight loss. These symptoms become more prominent when the disease is in an advanced stage, although symptoms depend on location and size of the cancer. 27,28
Treatment options and prognosis depend on staging of the CRC. Staging is defined by how deeply the cancer has grown into the intestinal mucosa, whether or not it has spread to lymph nodes and other organs, and if the tumour node metastasis (TNM) classification system is most commonly used (see Box 1 and Table 1 with modified Dukes’ staging with 5-year survival). 29,30 Dukes’ classification of staging is:
-
Dukes’ A means the cancer is only in the innermost lining of the colon or rectum or slightly growing into the muscle layer
-
Dukes’ B means the cancer has grown through the muscle layer of the colon or rectum
-
Dukes’ C means the cancer has spread to at least one lymph node in the area close to the bowel
-
Dukes’ D means the cancer has spread elsewhere in the body such as the liver or lung.
TX: primary cannot be assessed.
T0: no evidence of primary carcinoma in situ (Tis) – intraepithelial or lamina propria only.
T1: invades submucosa.
T2: invades muscularis propria.
T3: invades subserosa or non-peritonealised pericolic tissues.
T4: directly invades other tissues and/or penetrates visceral peritoneum.
Lymph nodesNX: regional nodes cannot be assessed.
N0: no regional nodes involved.
N1: one to three regional nodes involved.
N2: four or more regional nodes involved.
MetastasisMX: distant metastasis cannot be assessed.
M0: no distant metastasis.
M1: distant metastasis present (may be transcoelomic spread).
Tis, tumour in situ.
| Stage (TNM status) | 5-year OS, % | Modified Dukes’ |
|---|---|---|
| Stage 0 (T in situ, N0, M0) | – | – |
| Stage I (T1, N0, M0) | 75 | A |
| Stage I (T2, N0, M0) | 57 | B1 |
| Stage II (T3, N0, M0) | – | B2 |
| Stage II (T4, N0, M0) | – | B3 |
| Stage III (T2, N1, M0/T2, N2, M0) | 35 | C1 |
| Stage III (T3, N1, M0/T3, N2, M0) | – | C2 |
| Stage III (T4, N1, M0) | – | C3 |
| Stage IV (any T, any N, M1) | 12 | D |
This brief account is based on NICE guideline CG1317 and advice of clinical experts.
Figures 1–3 summarise the clinical pathways for patients with CRC.
FIGURE 1.
Colorectal cancer diagnosis pathway. MRI, magnetic resonance imaging.
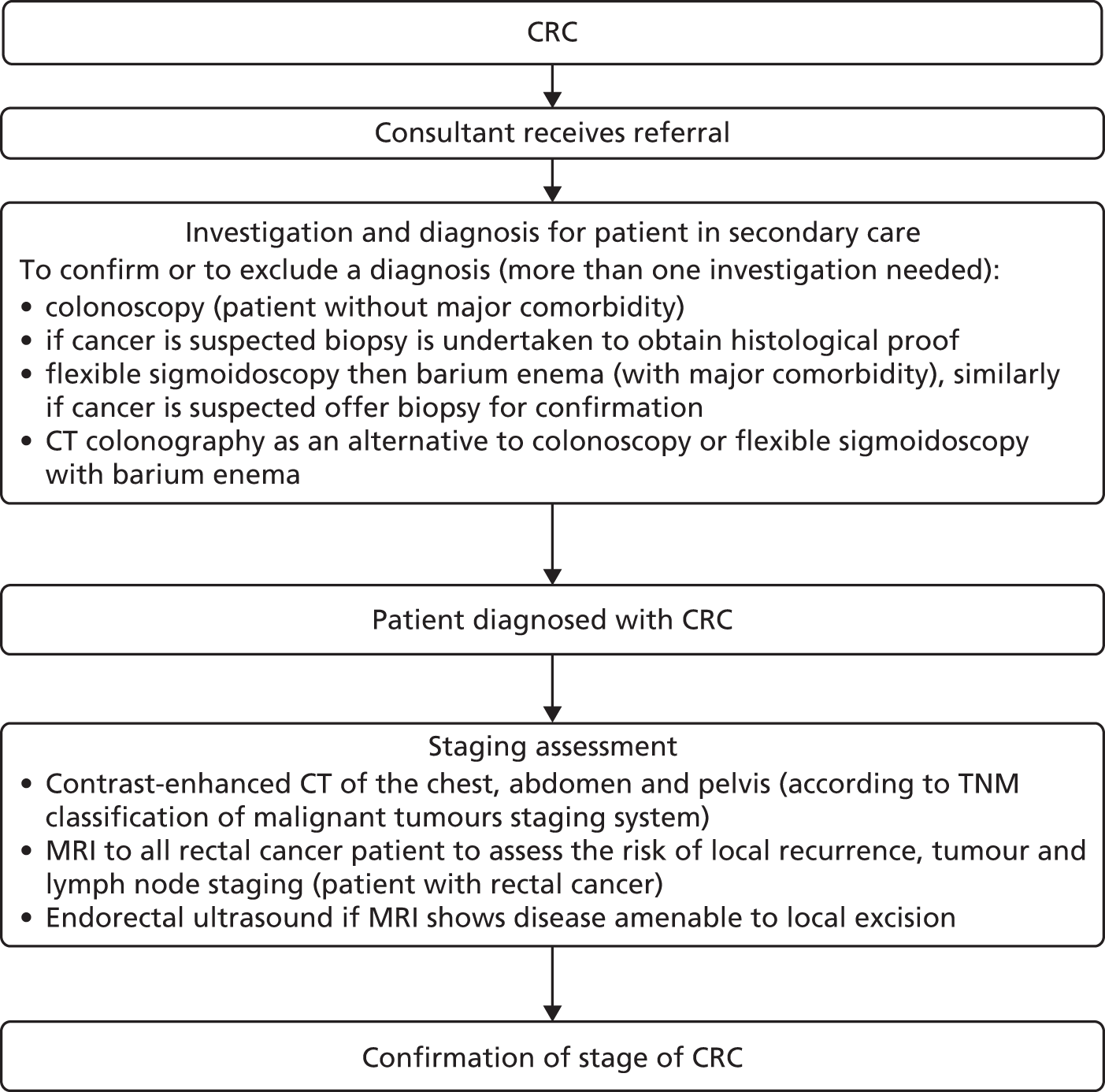
FIGURE 2.
Colorectal cancer management pathway. MRI, magnetic resonance imaging; PET-CT, positron emission tomography fused with computed tomography; SCPRT, short-course preoperative radiotherapy.
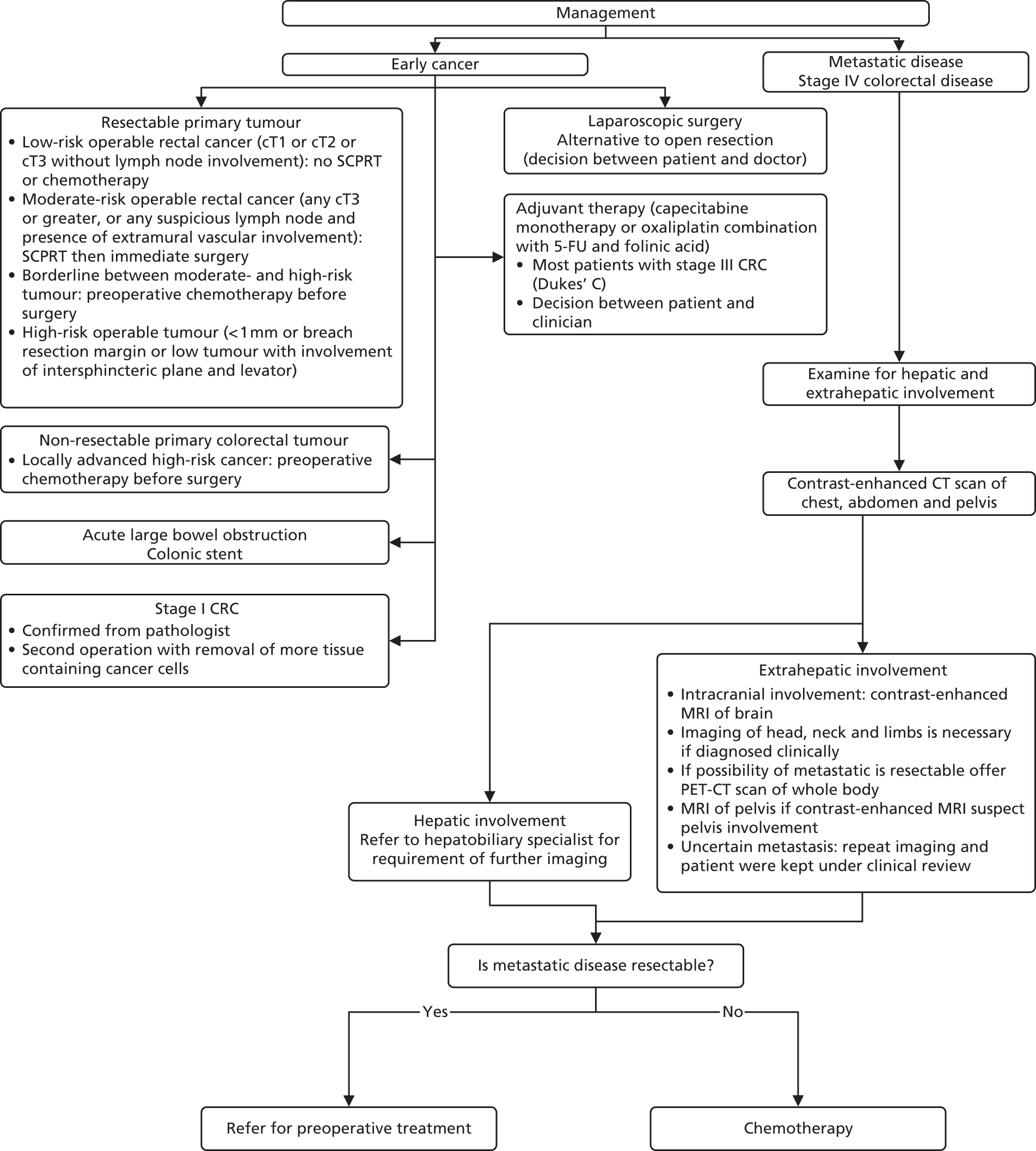
FIGURE 3.
Illustrative role of pharmacokinetic adjustment of 5-FU regimens in treatment of metastatic CRC in standard practice (in theory pharmacokinetic adjustment could be applied in any treatment regimen that includes 5FU). a, The FOLFOX4 regimen (oxaliplatin, leucovorin and 5-FU): oxaliplatin (85 mg/m2); leucovorin (200 mg/m2); 5-FU loading dose (400 mg/m2); i.v. bolus; then 5-FU (600 mg/m2) for a period of 22 hours. FOLFOX6 regimen (folinic acid, 5-FU and irinotecan): oxaliplatin (85–100 mg/m2); leucovorin (400 mg/m2); 5-FU loading dose (400 mg/m2); i.v. bolus; then 5-FU (2400–3000 mg/m2) for a period of 46 hours. BSA, body surface area; FOLFIRI, irinotecan in combination with 5-fluorouracil and folinic acid; FOLFOX, oxaliplatin in combination with 5-fluorouracil and folinic acid; FU, fluorouracil; FUFA, 5-FU + folinate; PK, pharmacokinetic.

There are various options for treatment of early-stage CRC including:
-
surgery (i.e. tumour resection if the tumour is resectable)
-
preoperative chemotherapy (this may be considered before surgery in patients with non-resectable primary colorectal tumours or borderline resectable tumours)
-
colonic stent in acute large bowel obstruction
-
further tumour resection in stage I CRC
-
laparoscopic surgery as an alternative surgery to open resection based on patient’s and doctor’s decision
-
adjuvant therapy: monotherapy capecitabine or a combination of oxaliplatin with 5-fluorouracil and folinic acid (FA) (FOLFOX) are recommended in most patients with stage III CRC based on patient’s and doctor’s decision.
According to NICE guideline CG1317 one of the following combinations of first- and second-line chemotherapies is used depending on side effects experienced and patient’s preferences:
-
FOLFOX as first-line treatment then single-agent irinotecan as second-line treatment; or
-
FOLFOX as first-line treatment then irinotecan in combination with 5-fluorouracil and folinic acid (FOLFIRI) as second-line treatment; or
-
oxaliplatin in combination with capecitabine (XELOX) as first-line treatment then FOLFIRI as second-line treatment.
In standard practice choice between 5-FU regimens, such as fluorouracil (FU) alone, FU + FA, FOLFOX (FOLFOX4 and FOLFOX6), is made with clinician’s advice. These regimens are administered for up to 12 cycles, one cycle every 2 weeks.
-
FU
-
FU + FA
-
FOLFOX4: oxaliplatin (85 mg/m2); FA (200 mg/m2); 5-FU loading dose (400 mg/m2); i.v. bolus; then 5-FU (600 mg/m2) administered via ambulatory for a period of 22 hours31
-
FOLFOX6: oxaliplatin (85–100 mg/m2); FA (400 mg/m2); 5-FU loading dose (400 mg/m2); i.v. bolus; then 5-FU (2400–3000 mg/m2) administered via ambulatory for a period of 46 hours. 31
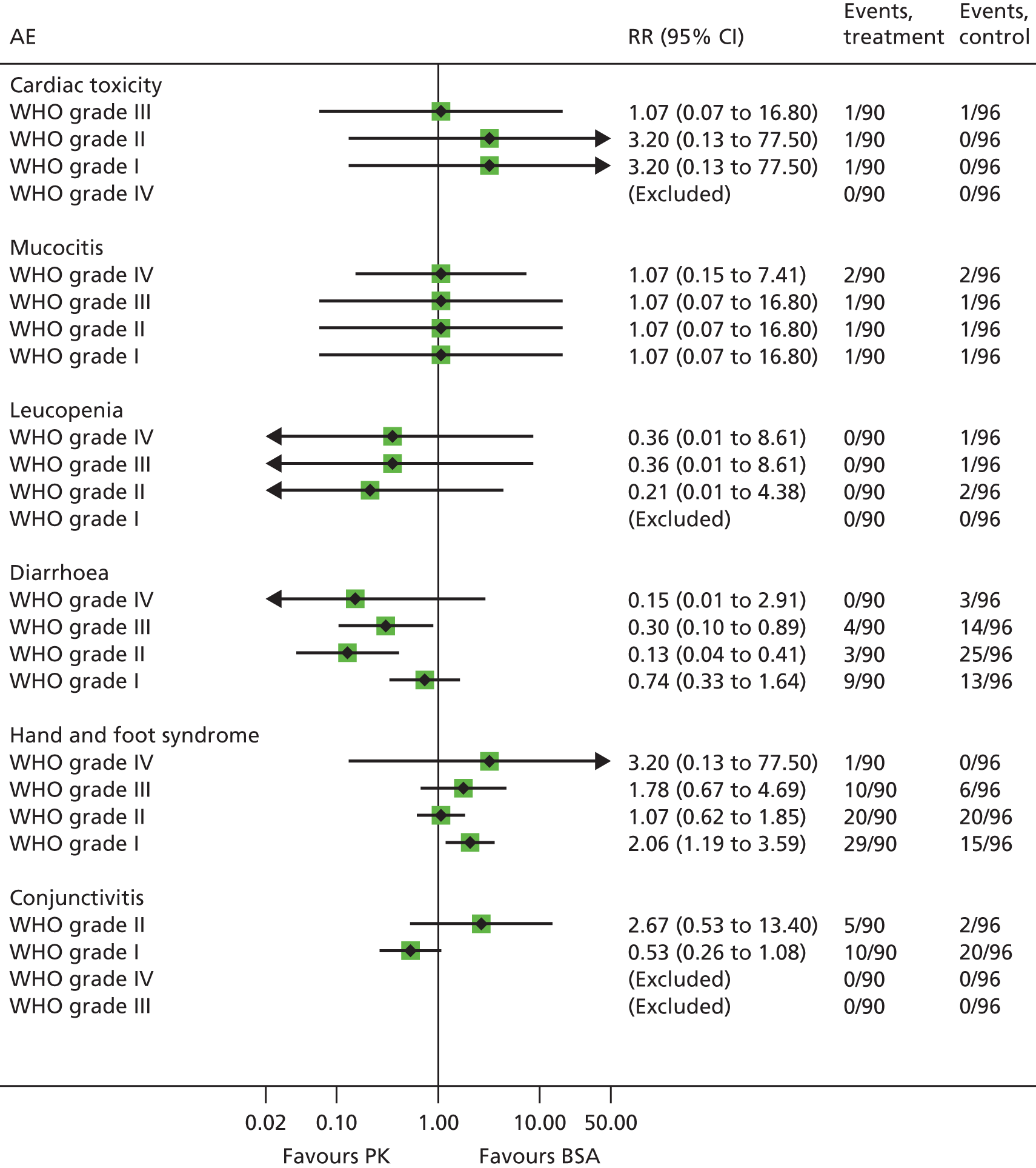
In standard practice the 5-FU dosage administered is based on patient body surface area (BSA). BSA is calculated using the Du Bois method:32 BSA (m2) = weight (kg) 0.425 × height (cm) 0.725 × 0.007184. Currently FOLFIRI and FOLFOX6 regimens recommend a 5-FU dose of 2400 mg/m2 administered by continuous infusion over 46 hours. It remains unclear how to dose cap, although dose capping is usually undertaken for large individuals because they may be overdosed using the BSA-based dosage and experience toxicity and adverse events (AEs). AEs range in severity and include diarrhoea, hand and foot syndrome, mucositis/stomatitis, neutropenia, anaemia, nausea/vomiting and cardiac toxicity. Dose capping is implemented at BSA > 2 m2 or > 2.25 m2. In practice, larger patients may be capped up to a BSA of 2.4 m2 (NICE committee assessment subgroup, 5 June 2014, personal communication). Dose may be reduced for patients judged at higher risk of toxicity (e.g. those heavily pre-treated with chemotherapy; those with poor performance status particularly 2 and above; those with impaired renal or hepatic function; and those with co-morbidities). In such instances dose of chemotherapy may be started low and cautiously increased while the patient is able to tolerate treatment.
It is well documented that the plasma concentrations of 5-FU vary greatly between individuals who have received ‘standard’ dosage calculated from their BSA. 33 In advanced CRC, treatment focuses on both length and palliation of symptoms (e.g. pain, obstruction). Individualised pharmacokinetic (PK) adjustment of 5-FU dosage, which tailors an individual’s dosage to achieve the required plasma 5-FU level, might optimise time without toxic effects, while not compromising therapeutic benefit. The potential position of PK dose adjustment in the clinical pathway is illustrated in Figure 3.
In PK-adjusted regimens when the dose at the first cycle is based on patient BSA, a steady state plasma sample is taken (e.g. after 40 hours of a 46-hour infusion). The plasma 5-FU estimate is used to calculate the PK ‘area under the curve’ (AUC = mg × hour/l; where mg/l is the steady state plasma 5-FU concentration and hour the total infusion time in hours). An algorithm that relates AUC to dose adjustment is then used to calculate the dosage required for the next cycle of treatment. 33
In both standard and PK regimes, if toxicity occurs, treatment is stopped and/or the dose is reduced after which treatment is resumed. If there is progression of the disease, it may be reasonable to switch treatment (e.g. from FOLFOX to FOLFIRI). If patients are tolerating treatment even after 12 cycles, the treatment is continued until progression, or at the discretion of the medical team, but should be reviewed every 12 weeks.
A recent UK randomised clinical trial has investigated if there is a clinical advantage from treatment holidays between successive 12-week cycles. 34 Figures 1–3 illustrate the CRC diagnosis pathway.
NICE CG1317 makes recommendations for diagnosis and management of CRC and for management of locally advanced and metastatic disease.
An economic evaluation was undertaken using a decision tree. The FOLFOX–irinotecan sequence was taken as a reference for comparisons. All the combinations except FOLFOX–FOLFIRI were found to be dominated by FOLFOX–irinotecan (i.e. the latter was less effective and more costly). The incremental cost-effectiveness ratio (ICER) of FOLFOX–FOLFIRI was found to be £109,604 per quality-adjusted life-year (QALY) gain. A sensitivity analysis was undertaken discounting the price of drug. The resulting ICER of FOLFOX–FOLFIRI was £47,801 per QALY gain. The probabilistic sensitivity analysis showed that three combination regimens, namely FOLFOX–irinotecan, FOLFOX–FOLFIRI and XELOX–FOLFIRI, had the highest probability of falling between £20,000 and £50,000 per QALY. Based on these findings, the Guideline Development Group made the following recommendation:
-
If there are no contraindications, then the three combination sequence namely FOLFOX–irinotecan, FOLFOX–FOLFIRI and XELOX–FOLFIRI should be considered as treatment options for treating patients with advanced and metastatic CRC (mCRC).
Head and neck cancer
Cancer of the H&N includes cancer of the mouth (i.e. oral cancer), throat and other rare cancers of the nose, sinuses, salivary glands and middle ear. Mouth cancer can be subdivided according to its location, such as lip cancer or cancer of the oral cavity. Similarly, throat cancer can be divided into nasopharyngeal cancer (the affected area is at the highest part of the throat behind the nose), oropharyngeal cancer (tonsils and the base of the tongue), cancer of larynx and thyroid cancer (thyroid gland). 35
The most common type of H&N cancer is squamous cell carcinoma (SCC), which comprises 90% of all H&N cancers. 36
Head and neck cancer begins with a non-invasive lesion in the squamous mucosa that lies in the inner part of the H&N (mouth, the nose and the throat). Following exposure to common carcinogens, a series of changes occurs (i.e. hyperplasia and dysplasia), this causes the cancer to finally become invasive. 36
The definitive cause for H&N cancer is still unknown; however, it has been thought that disease is associated with various factors. Cancer of the H&N is associated with risk factors such as active use tobacco and habitual drinking of alcohol. Dietary factors thought to be associated with increased risk include high intake of red meat, processed meat, fried food and poor diet. Other risk factors include a history of gastro-oesophageal reflux disease for laryngeal and pharyngeal cancer. 37 Human papillomavirus infection is also an important risk factor for some H&N cancer (oropharynx and oral cavity). 24,38
Aetiology, pathology and prognosis
Head and neck SCC is the sixth most common cancer and the one of the leading causes of cancer death in the world. 39 In 2011, around 49,260 new cases of H&N cancer were diagnosed in the USA and there were 11,480 cancer deaths in the same year. 40 In England and Wales, around 8100 new H&N cancer cases are diagnosed annually. 41 In the UK there were 6539 new cases of H&N cancer, 66% in male and 34% in female, in 2010. 42
Incidence and/or prevalence
The disease incidence increases with age. In the UK, 85% of cases are seen in people who are aged > 50 years. However, the incidence has been found to be increasing in younger men and women. 37 In the UK, in the period 2008–10, approximately 44% of oral cancers were diagnosed in both genders in people aged ≥ 65 years, and 50% were diagnosed in those aged between 45 and 64 years. 42
Significance for patients in terms of ill-health (burden of disease)
In England and Wales between 1995 and 1999 the age-adjusted mortality rate for oral cancer was 2.7 per 100,000 for males and 1.05 per 100,000 for females. Likewise, in Scotland, the age-adjusted mortality rate was 4.6 per 100,000 for males and 1.6 per 100,000 for females between years 1995 and 1999. In around 30–40% cases H&N SCCs present at an early stage which is potentially managed by surgery or adjuvant radiotherapy with an intention to cure the disease. In contrast, advanced diseases with unresectable H&N SCCs are treated by concurrent chemoradiotherapy as a palliative therapy mainly to improve survival. 43
Costs of treatment for (only surgical resection) and caring for H&N SCCs after surgery are substantial. Kim et al. 43 have reported the total cost of post-operative health-care utilisation over the 5-year follow-up. The cost was approximately £255.5M for 11,403 patients in the UK.
Measurement of disease and/or response to treatment
In the UK, about 7000 new cases of H&N cancer occur annually. At least 45% of cases survive ≥ 5 years. 44 Younger populations have better survival than older populations. 42 Within the UK there has been an increment of between 5% and 14% in 5-year survival for most cancers (e.g. oral cavity, oropharynx, nasopharynx and salivary glands). Epidemiological evidence that covered about 10% of the US population suggested an improvement in survival from 55% to 66% in people with H&N cancer between 1992–6 and 2002–6. 44
Similar to CRC cancer, disease progression and response to treatment are measured by a multidisciplinary team. After treatment, there are regular examinations in the first 2 years and routine follow-up after 5 years. The identification of recurrent tumours or new primary tumours are made by professionals during follow-up visits. Patients are also helped with complications of the disease and AEs due to treatment. Patients are also given help with functional and psychosocial problems. 45
Diagnosis and management
Signs and symptoms of H&N cancer depend on the location of the primary tumour and also on the extent of the disease. Common signs and symptoms of H&N cancer include hoarseness or change of voice, difficulty in swallowing, lump/swelling in the neck and non-healing mouth ulcers. 46
Tumour staging is necessary to determine the treatment and also to know the prognosis of the condition. Pathological or histological diagnosis is usually done according to the World Health Organization (WHO) classification from biopsy taken from surgery. Clinical staging of the H&N are done according to American Joint Committee on Cancer (AJCC) classification and TNM. The AJCC classification divides T4 tumours into two categories – T4a for moderately advanced cancer; and T4b for very advanced cancer. Stage IV cancers are divided into three categories: IVA, IVB and IVC. The latter indicates metastatic disease. The TNM classification of the Union International Contre Le Cancer (UICC, i.e. International Union Against Cancer) and AJCC are designed for staging/classifying SCC and minor salivary cancers47–49 (Tables 2 and 3).
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T3 | N0 | M0 |
| T1, T2, T3 | N1 | M0 | |
| Stage IVA | T1, T2, T3 | N2 | M0 |
| T4a | N0, N1, N2 | M0 | |
| Stage IVB | Tb | Any N | M0 |
| Any T | N3 | M0 | |
| Stage IVC | Any T | Any N | M1 |
| Primary tumour (T) | |||
|---|---|---|---|
| T1: tumour confined to the nasopharynx, or extends to oropharynx and/or nasal cavity without parapharyngeal extension T2: tumour with parapharyngeal extension T3: tumour involves bony structures of skull base and/or paranasal sinuses T4: tumour with intracranial extension and/or involvement of cranial nerves, hypopharynx, orbit, or with extension to the infratemporal fossa/masticator space |
|||
| Regional lymph nodes (N) | |||
| N1: unilateral metastasis in cervical lymph node(s), ≤ 6 cm in greatest dimension, above the supraclavicular fossa, and/or unilateral or bilateral, retropharyngeal lymph nodes, ≤ 6 cm, in greatest dimension N2: bilateral metastasis in cervical lymph node(s), ≤ 6 cm in greatest dimension, above the supraclavicular fossa N3: metastasis in a lymph node(s) > 6 cm and/or to supraclavicular fossa N3a: > 6 cm in dimension N3b: extension to the supraclavicular fossa |
|||
| Distant metastasis (M) | |||
| M0: no distant metastasis M1: distant metastasis |
|||
| Anatomic stage/prognostic groups | |||
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage II | T1 | N1 | M0 |
| T2 | N0 | M0 | |
| T2 | N1 | M0 | |
| Stage III | T1 | N2 | M0 |
| T2 | N2 | M0 | |
| T3 | N0 | M0 | |
| T3 | N1 | M0 | |
| T3 | N2 | M0 | |
| Stage IVA | T4 | N0 | |
| T4 | N1 | M0 | |
| T4 | N2 | M0 | |
| Stage IVB | Any T | N3 | M0 |
| Stage IVC | Any T | Any N | M1 |
According to Scottish Intercollegiate Guidelines Network guidelines,37 tumours are broadly subdivided into (a) early disease (stage I and II following the UICC/TNM classification of malignant tumour); and (b) locally advanced disease stages III and IV.
The management of H&N cancer falls into two broad categories: (1) management of early-stage cancer; and (2) management of locally advanced cancer (Figures 4 and 5).
FIGURE 4.
Head and neck diagnosis pathway. GP, general practice; MRI, magnetic resonance imaging; PET-CT, positron emission tomography fused with computed tomography.
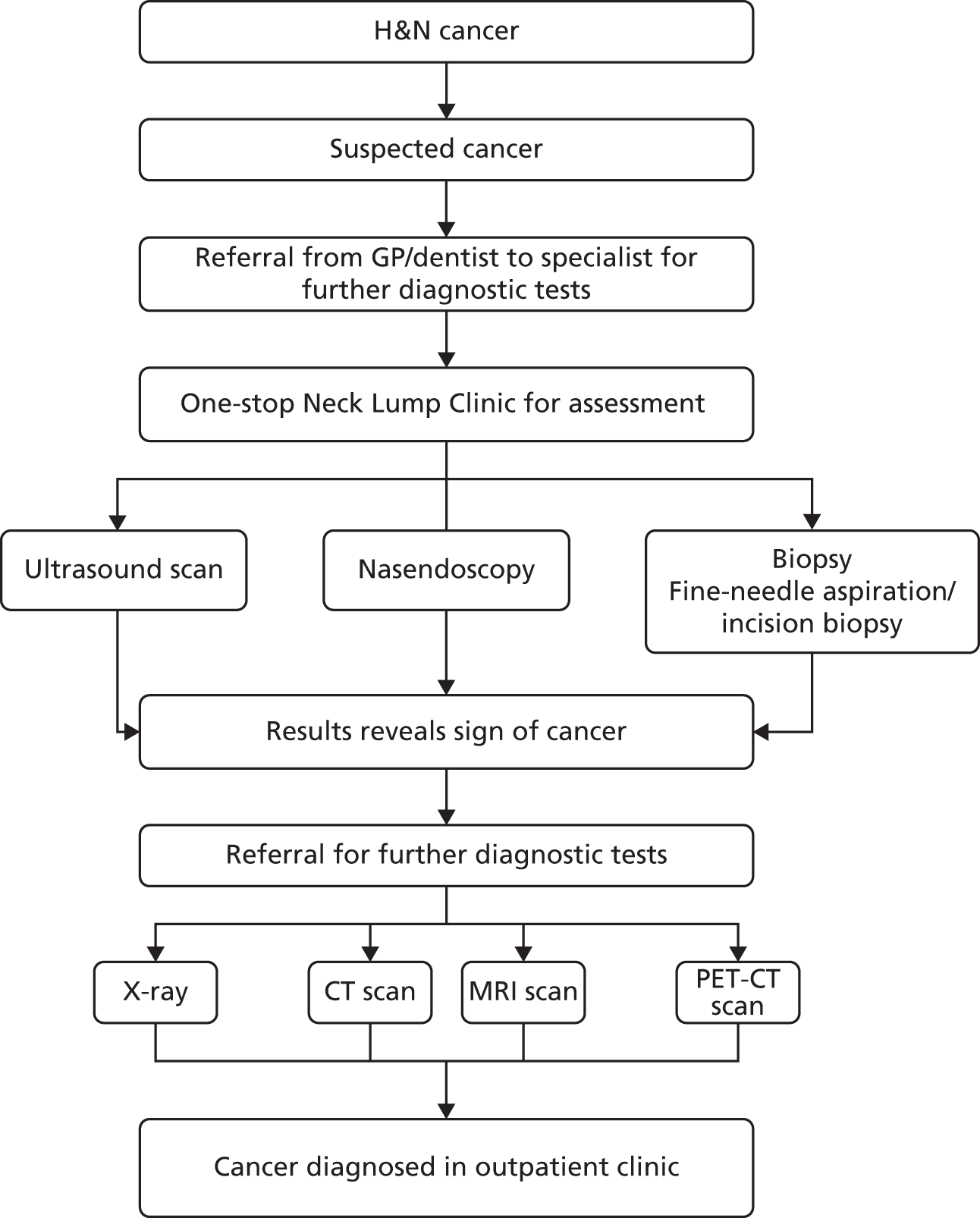
FIGURE 5.
Head and neck management pathway. a, Days 1, 22 and 43: i.v. cisplatin 100 mg/m2 + radiotherapy; b, day 1, i.v. docetaxel 75 mg/m2 + i.v. cisplatin 75 mg/m2 + days 1–5, 5-FU 750 mg/m2 continuous i.v. infusion. Repeat cycle every 3 weeks for four cycles. TPF, docetaxel, cisplatin, 5-fluorouracil.

Most (60–70%) patients present with locally advanced disease. The standard of care for this group is various combinations of surgery, radiotherapy and systemic treatments. Chemotherapy may be used prior to radiotherapy (induction) or combined with definitive or post-operative radiotherapy (synchronous).
Docetaxel, cisplatin, 5-fluorouracil (TPF) regimens are commonly used in the UK to treat locally advanced cancer (T3/4, N2/3). These regimens are also used as induction chemotherapy (prior to radiotherapy), for example to preserve the larynx, or in chemoradiation (concurrent radiation and cisplatin) followed by adjuvant chemotherapy (cisplatin + continuous infusion 5-FU) for nasopharynx cancer. Meta-analysis evidence supports the addition of docetaxel to cisplatin plus 5-FU doublet. 50
For nasopharyngeal cancer the use of neoadjuvant TPF rather than cisplatin and 5-fluorouracil (PF) is not as well established (clinical expert). The standard synchronous chemotherapy regimen (concurrent radiation + chemotherapy) is single-agent cisplatin 100 mg/m2, which is administered three weekly. 51 There are reports of severe side effects from 5-FU including diarrhoea; hand and foot syndrome; mucosities/stomatitis; neutropenia; anaemia; nausea and vomiting; and cardiotoxicity.
Stomach cancer
Stomach cancer refers to any malignant neoplasm occurring in the region between the gastro-oesophageal junction and the pylorus. 52 Stomach cancer also represents a major cause of cancer mortality worldwide. The most common cancer of the stomach is called adenocarcinoma. 53 This cancer starts in cells which line the innermost layer of the stomach, the mucosa. Stomach cancer spreads locally within the gastric wall and then to adjacent lymph nodes. 54 On reaching the serosa, it might spread into the peritoneal cavity, then distantly.
Aetiology, pathology and prognosis
The aetiology of gastric cancer is complex. More than 80% of new diagnoses are attributed to Helicobacter pylori (H. pylori) infection. 52 Lifestyle, diet, genetics, socioeconomic and a range of other factors appear to contribute to gastric carcinogenesis, despite a decline in the prevalence of H. pylori infection (a major cause of stomach cancer). 55,56
The incidence and mortality rates of stomach cancer appear to increase in socially and economically deprived groups. 57
The prognosis for patients with stomach cancer appears to depend on age, general health and how far the cancer has spread before it was diagnosed. No consensus has been reached on the best treatment option. 58
Incidence and/or prevalence
A total of 7610 new cases of stomach cancer were diagnosed in 2008 in the UK,59 with an estimated 5-year survival rate of 18%. 60 Currently, stomach cancer is the 15th most common cancer among adults in the UK. 59 In the UK approximately 13,400 people were still alive at the end of 2006, up to 10 years after being diagnosed with stomach cancer. 61 In the UK between 2009 and 2011, around 51% of cases of stomach cancer were diagnosed in people aged ≥ 75 years and stomach cancer incidence was strongly related to age, with the highest incidence rates in older men and women. 59 Overall, around 15% of people with stomach cancer will live at least 5 years after diagnosis and about 11% will live at least 10 years. Around 5000 people die from stomach cancer each year in the UK. 62
Significance for the NHS
Early-stage stomach cancer is often treated with surgery, with neo-adjuvant or adjuvant chemotherapy offered where appropriate. The main chemotherapy drugs used to treat stomach cancer include 5-FU, cisplatin and epirubicin. Advanced stomach cancer is treated with chemotherapy. NICE technology appraisal (TA) 19163 recommends capecitabine in conjunction with a platinum-based regimen for the treatment of inoperable advanced gastric cancer.
Measurement of disease and/or response to treatment
A 2013 Cochrane study-level meta-analysis, reviewing randomised controlled trials (RCTs) of post-surgical chemotherapy versus surgery alone for gastric cancer, reported a significant improvement in OS in 34 studies [hazard ratio (HR) 0.85, 95% confidence interval (CI) 0.80 to 0.90] and in disease-free survival in 15 studies (HR 0.79, 95% CI 0.72 to 0.87) as a result of adjuvant chemotherapy. 64 A recent meta-analysis concluded that D2 lymphadenectomy with spleen and pancreas preservation offers the most survival benefit for patients with gastric cancer when done safety. 65
Pancreatic cancer
Pancreatic cancer refers to a malignant epithelial neoplasm of the pancreas. Pancreatic cancer, sometimes referred to as pancreatic ductal adenocarcinoma, is the eighth and fifth leading cause of cancer-related deaths in the world and Europe respectively. 66,67 It has very few symptoms in its early stage so is often diagnosed when the disease is advanced. The primary symptoms of pancreatic cancer include weight loss, stomach pain and jaundice, and these symptoms are associated with a number of conditions. 62
Aetiology, pathology and prognosis
About 65% of pancreatic tumours starts in the head of the pancreas, 30% in the body and tail, and 5% can involve the whole pancreas. 68 The most common form of cancer occurs in the exocrine cells of the pancreas. These tumours account for over 95% of all pancreatic cancers.
Genetic factors, smoking and previous radiotherapy treatment for another cancer have been associated with an increased risk of developing pancreatic cancer. 69–71 Similarly, consumption of red and processed meat increased the risk of pancreatic cancer,72 and patients with chronic hepatitis B infection have an approximately 20–60% increased risk of pancreatic cancer. 73
Pancreatic cancer continues to be one of the most aggressive forms of tumour with a 5-year survival rate of less than 5% and a median survival of 6 months after diagnosis; as a result it has a poor prognosis of all solid tumours. 74,75
Incidence and/or prevalence
In the UK a total of 8085 people were newly diagnosed with pancreatic cancer in 2008. In 2009 a total of 8047 people died from this cancer. 76 In 2011 approximately 3600 men (2.6% of all newly-diagnosed male cancers) and 3700 women (2.7% of all newly-diagnosed female cancers) were diagnosed with pancreatic cancer in England. 2 Pancreatic cancer is more common in men than women but this has started to change.
Impact of health problem
Pancreatic cancer in England has a crude incidence rate of 13.6 per 100,000 population and similar rates are seen in both sexes. Survival is poor with 1-year relative survival estimates of around 19% for both sexes. In many patients, the clinical diagnosis is fairly straightforward, although there are no clear clinical features which identify a patient with curable form of pancreatic cancer. 77
Significance for the NHS
Currently, treatment focuses on palliative surgery to relieve symptoms, resectional surgery with intent to cure, and endoscopic or percutaneous biliary stenting to relieve jaundice. Chemotherapy and radiotherapy are often used, both as palliative treatments as well as in an adjuvant setting in conjunction with surgery. 78
The main chemotherapy drugs recommended to treat pancreatic cancer are 5-FU, gemcitabine and capecitabine. If surgery is possible, adjuvant treatment with 5-FU can reduce the risk of recurrence. NICE TA2579 recommends that gemcitabine may be considered as a treatment option for patients with advanced or metastatic adenocarcinoma of the pancreas and a Karnofsky Performance Status score ≥ 50, where first-line chemotherapy is to be used. The guidance also states that there was insufficient evidence to support the use of gemcitabine as a second-line treatment in patients with pancreatic adenocarcinoma.
Measurement of disease and/or response to treatment
The current management of pancreatic cancer is guided by tumour stage, comorbidities and performance status of the patients. In addition to gemcitabine and capecitabine FU, chemoradiation, and chemoradiation plus FU or gemcitabine are also used. 80 Surgical resection followed by a 6-month course of adjuvant gemcitabine-based chemotherapy is considered the standard care for early-stage disease. 81 Patients with metastatic disease can be considered for systemic palliative chemotherapy. In contrast, for patients with locally advanced disease without evidence of metastasis, optimal treatment remains unclear, with chemotherapy alone and chemoradiation both being an option for consideration. 82
Description of technology under assessment
5-fluorouracil
5-fluorouracil (5-FU or 5-fluoro-2,4-pyrimidinedione) is an antimetabolite of the pyrimidine analogue type, with a broad spectrum of activity against solid tumours (of the gastrointestinal tract, liver, pancreas, ovary, breast, brain, etc.), alone or in combination chemotherapy regimens. 83 5-FU has been used in daily clinical oncology practice for almost 50 years and continues to be the cornerstone of all major CRC treatment regimens for adjuvant therapy and for advanced metastatic disease. 84 The method of administration of 5-FU varies according to the type, location and stage of cancer, as well as the circumstances and preferences of the individual. 5-FU can be administered by infusion, injection, or orally as a pro-drug (e.g. capecitabine) and prescribed as either a single agent or in conjunction with other chemotherapy drugs.
Approximately ≥ 85% of administered 5-FU is inactivated and eliminated through the catabolic pathway; the remainder is metabolised through the anabolic pathway. 85 The enzyme dihydropyrimidine dehydrogenase (DPD) has a major role in clearance of 5-FU and the rate of clearance (inactivation) varies considerably from patient to patient. 5-FU chemotherapy typically lasts 3–6 months and usually for up to 12 cycles. Each cycle includes a period of 5-FU administration followed by a break to allow for recovery before the next cycle. Administration via continuous infusions usually lasts approximately 22–48 hours and requires patients to have a central venous access device such as a Hickman line or peripherally inserted central catheter line. Some patients have their 5-FU infusion via a portable pump which allows return to home during treatment.
When 5-FU was first developed in the USA, 5-FU monotherapy was usually administered via a bolus schedule; however, more recently these have been replaced by infusional regimens based on the work of de Gramont et al. 86,87
Intervention technology
My5-FU assay
The My5-FU assay is a nanoparticle immunoassay that measures levels of 5-FU in plasma samples. 88
As previously reported in the protocol to this work, the My5-FU assay is used with patients receiving 5-FU by continuous infusion to facilitate PK dose adjustment at the next cycle and drug monitoring to achieve an optimal plasma level of the drug. The assay uses two reagents: reagent 1 consists of a ‘5-FU conjugate’ which is a 5-FU-like molecule linked to a long spacer arm; reagent 2 consists of antibodies covalently bound to nanoparticles, these antibodies are able to bind either 5-FU or the 5-FU conjugate. When reagents 1 and 2 are mixed the nanoparticles aggregate together. In the presence of free 5-FU some of the antibodies bind 5-FU rather than 5-FU conjugate, the amount of aggregation of nanoparticles is reduced and this alters the light absorbing properties of the mixture (that is 5-FU and ‘5-FU conjugate’ compete for nanoparticle-bound antibodies). The light absorbance of the mixture is measured and can be compared against a calibrated standard curve in which light absorbance is compared with known concentrations of free 5-FU in the mixture. In short, photometric detection (changes in absorbance) of nanoparticle aggregation allows for determination of 5-FU concentration in plasma samples. 89 This assay can be performed on automated clinical chemistry analysers present in standard clinical laboratories. The assay requires a peripheral venous blood sample which is taken towards the end of each 5-FU infusion cycle using an ethylenediaminetetraacetic acid (EDTA) or a heparin tube. 90
Drug monitoring is potentially important for 5-FU because it has a narrow therapeutic index, with doses below the therapeutic window potentially limiting treatment efficacy and doses above the window more likely to cause side effects and toxicity. Commonly reported side effects of 5-FU chemotherapy include anaemia, thrombocytopenia, leucopenia, nausea/vomiting, diarrhoea, mucositis and hand and foot syndrome,91 all of which can be dose limiting when severe. Other consequences of 5-FU toxicity can include neuropathy, severe damage to organs, cardiotoxicity, neutropenia, sepsis and septic shock. 92 Patients with DPD deficiency are at significantly increased risk of developing severe and potentially fatal neutropenia, mucositis and diarrhoea when treated with 5-FU. 93,94
Results are reported in nanograms 5-FU/millilitre plasma and are converted to an AUC value by multiplying the concentration of 5-FU in a steady state by the time of the infusion (in hours). This is then compared with a pre-defined optimal therapeutic range and the results, reported as mg × hour/l, are used to guide the dose of 5-FU given in the next cycles. Outlier results > 50 mg × hour/l are assumed to indicate that the blood sample has been taken too close to the infusion port and these results are disregarded. The My5-FU assay has been validated against liquid chromatography-mass spectrometry (LC-MS)89,95 and high-performance liquid chromatography (HPLC) laboratory techniques commonly used in PK studies.
When using the My5-FU assay in clinical practice, the initial dose of 5-FU is based on a patient’s BSA. A blood sample is taken towards the end of the infusion cycle. For an infusion > 40 hours sampling is recommended at least 18 hours after starting infusion. 96 The sample should also be taken during a steady state period of the infusion which is usually about 4 hours before the end of the infusion using a non-battery operated device (which is commonly used in the UK). Depending on practice, it may require an additional visit by a district nurse or an additional outpatient attendance. Subsequent doses of 5-FU are calculated using the AUC result, according to a pre-determined dose adjustment algorithm. An example of a dose adjustment algorithm for patients with mCRC recommends an optimal therapeutic range of 20–30 mg × hour/l with adjustments of no more than 30% of the dose for each infusion. 96 Patients typically require three or four PK-directed dose adjustments to reach an optimal therapeutic range.
Dihydropyrimidine dehydrogenase is the rate-limiting enzyme involved in the catabolism of 5-FU. Up to 80–85% of an administered dose of 5-FU is broken down by this enzyme to inactive metabolites. DPD converts endogenous uracil into 5,6-dihydrouracil, and analogously, 5-FU into 5-fluoro-5,6-dihydrouracil. The presence of DPD deficiency results in a reduced ability to metabolise and clear 5-FU, and the half-life of the drug, which is normally approximately 10–15 minutes, can be markedly prolonged (to up to 159 minutes). 97–100 Response to 5-FU treatment is inconsistent with approximately 10–30% of patients displaying serious toxicity partly explained by reduced activity of DPD. 95
In the following section the key principles of the My5-FU assay procedure are provided; the majority of this information has been taken from the Saladax kit instructions. 90
Handling and storage instructions
Store reagents, calibrators and controls should be refrigerated at 2–8 °C (35–46 °F). Before use, the nanoparticle reagent (R2) should be mixed by gently inverting the R2 reagent vial three to five times, avoiding the formation of bubbles.
Sample collection
Plasma (EDTA or heparin) specimens may be used with the My5-FU assay. The sample is drawn towards the end of the infusion, preferably 2 hours before the end, ensuring that the pump still contains solution during the sample draw. The start time of continuous infusion and actual sampling time is recorded. A minimum of 2 ml of blood is collected into an EDTA or heparin tube. The blood sample is collected by venepuncture or through a peripheral i.v. line to avoid contamination by the infusing drug.
The sample stabiliser is available in Europe which negates the need for ice and immediate access to a centrifuge. The stabiliser maintains 5-FU levels in whole blood for up to 24 hours after collection.
Calibration
The My5-FU assay produces a calibration curve with a 0–1800 ng/ml range using the My5-FU calibrator kit. The minimum detectable concentration of 5-FU in plasma for the My5-FU assay is 52 ng/ml.
Quality control
The My5-FU control kit contains three levels of controls at low, medium and high concentrations of 5-FU. A laboratory should establish its own control ranges and frequency. At least two concentrations of quality control should be tested each day as patient samples are assayed and each time calibration is performed. It is important to reassess control targets and ranges following a change of reagent (kit) or control lot.
Limitations of the procedure
Performance characteristics for the My5-FU assay have not been established for body fluids other than human plasma containing EDTA or heparin.
High-performance liquid chromatography/liquid chromatography-mass spectrometry
During the last 40 years, several methods for the quantitation of 5-FU levels have been developed and evaluated, these include gas chromatography, tachophoresis, HPLC, or thin layer chromatography as separating modalities, and radioactivity, mass spectrometer (MS), fluorescence, ultraviolet absorption, or flame ionisation as detection techniques. 101 The majority of these assays have been useful in pre-clinical and clinical pharmacological studies. Drug monitoring combined with early detection of patients at risk enables timely dose adaptation and maintain drug concentrations within a therapeutic window; however, the most effective method to identify such patients is unclear. 102
High-performance LC-MS methodology comprises an HPLC column attached, via a suitable interface, to a MS and is capable of analysing a wide range of components. Compounds are separated on the relative interaction with the chemical coating of these particles and the solvent eluting through the column. Components eluting from the chromatographic column are introduced to the MS via a specialised interface. Two most common interfaces used for HPLC/MS are the electrospray ionisation and the atmospheric pressure chemical ionisation interfaces. 103 For more details on a HPLC method please refer to a paper by Gamelin et al. 104
A popular method involves LC-MS/MS. 105–107 Despite LC-MS/MS methods being found to be sensitive and robust, the instrumentation is not in standard use in routine clinical laboratories in the UK. For more details on a LC-MS/MS method please refer to a paper by Kosovec et al. 101
Current usage of the My5-FU assay in the NHS
The My5-FU assay is currently not in clinical use in the UK, other than for research purposes. Several ongoing clinical trials are taking place. As part of the current report a detailed consultation was made with the NICE committee assessment subgroup expert advisors and other clinical experts. The responses to a large range of questions relevant to this work have been used as part of the health economic modelling detailed in Chapter 6.
Comparators
Currently in most clinical practice in the UK the 5-FU dose administered is calculated according to patients BSA. As described in Diagnosis and management, BSA is calculated using the Du Bois method:32 BSA (m2) = weight (kg) 0.425 × height (cm) 0.725 × 0.007184. Currently, FOLFIRI and FOLFOX6 regimens recommend a 5-FU dose of 2500 mg/m2 administered by continuous infusion over 46 hours.
It is well documented that the plasma concentrations of 5-FU vary greatly between individuals who have received ‘standard’ dosage calculated from their BSA and this dose remains unadjusted at subsequent cycles unless the patient experiences sufficient toxic effects to mandate dose reduction. Such dose reductions are guided by clinical judgement. The dose is not increased above an evidence-based (trial) maximum dose even if there is no toxicity.
Associations have been reported between 5-FU plasma levels and the biological effects of 5-FU treatment, both in terms of toxicity and clinical efficacy. 108–111 Although this method is commonly used with many anticancer drugs, its use has been questioned112,113 and clinical investigations have also failed to show a association between 5-FU plasma clearance and BSA. 114
Pharmacokinetic-guided studies have identified an optimal target therapeutic range for 5-FU and have recommended dose adjustment algorithms to bring plasma concentrations into the optimal range. 84 However, 5-FU monitoring has not been widely used. Any advances in testing based on LC-MS/MS or nanoparticle antibody-based immunoassay, might facilitate monitoring of 5-FU in routine daily clinical practice. 101,102
Chapter 2 Definition of decision problem
The current report being undertaken for the NICE DAP examines the clinical effectiveness and cost-effectiveness of 5-FU plasma monitoring with the My5-FU assay for guiding dose adjustment in patients receiving 5-FU chemotherapy by continuous infusion. The report will allow NICE to make recommendations about how well the My5-FU assay works and whether or not the benefits are worth the cost of the tests for use in the NHS in England and Wales. The test allows a more tailored dosing of 5-FU which may lead to improved clinical outcomes and less side effects. The assessment will consider both clinical improvement in patients symptoms and the cost of the test used to measure the amount of 5-FU.
The decision question taken from the NICE scope for this project is:
What is the clinical and cost-effectiveness of the My5-FU assay for the PK dose adjustment of continuous infusion 5-FU chemotherapy?
Overall aim of the assessment
The overall aim of this report was to present the evidence on the clinical effectiveness and cost-effectiveness of the My5-FU assay for guiding dose adjustment in patients receiving 5-FU chemotherapy by continuous infusion.
Objectives
In the current report we:
-
(1) Provide a review of the studies which examine the accuracy of the My5-FU assay when tested against gold standard methods of estimation of 5-FU. HPLC and LC-MS are considered the gold standard for the purpose of assessing the accuracy of 5-FU plasma level measurements.
(2) Provide a review of the studies which have developed a treatment algorithm based on plasma 5-FU measures.
-
Systematically review the literature on the use of My5-FU to achieve adjusted dose regimen(s) to compare it with BSA-based dose estimation for patients receiving 5-FU administered by continuous infusion. Variations in current BSA-based dose regimens are considered where appropriate.
-
Systematically review the literature on the use of HPLC and/or LC-MS to achieve dose adjustment to compare it with BSA-based dose regimens for patients receiving 5-FU. This is undertaken for the purpose of performing a linked evidence analysis which incorporates estimates of comparability of assay performance [in terms of OS, progression-free survival (PFS) and AEs] of My5-FU relative to the gold standards (HPLC, LC-MS) as outlined in (a).
-
Provide an overview of systematic reviews of clinical outcomes in studies of 5-FU cancer therapies administered by continuous infusion in order to assess the generalisability of outcomes reported in the control arms of studies included in (b) and (c) above. Outcomes of interest include incidence of side effects and 5-FU toxicity; treatment response rates; PFS; OS; and health-related quality of life (HRQoL).
-
Identify evidence relevant to the costs of using My5-FU. Illustrative clinical pathways have been constructed; for this, we have used information provided by the manufacturer, advice from specialist committee members and other clinical experts, data collected from an identified UK clinical laboratory and analysis of the published literature. We have collected information on the following:
-
– cost of My5-FU testing
-
– cost of delivering 5-FU by infusion
-
– cost of side effects and 5-FU toxicity and their associated treatment or hospitalisation.
-
These will be considered from an NHS and Personal Social Services (PSS) perspective.
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Chapter 3 Clinical effectiveness methods
Identification and selection of studies
Search strategies for clinical effectiveness
Scoping searches were undertaken to inform the development of the search strategies and to assess the volume and type of literature relating to the assessment questions. An iterative procedure was used, with input from clinical advisors and the NICE Diagnostic Assessment Programme Manual. 115 One search strategy was developed for objectives A–C and another two were developed for objective D (see Searches for objective D). Search strategies are presented in Appendix 1.
Searches for objectives A–C
This search strategy focused on My5-FU/gold standard technologies, FU, PKs and dose adjustment, with a limit to English language. No study type or date limits were applied. This search strategy developed for EMBASE was adapted as appropriate for other databases. The bibliographic database searches were undertaken in January 2014. Other searches were undertaken between February and April 2014. All retrieved papers were screened for potential inclusion.
The search strategy comprised the following main elements:
-
searching of electronic bibliographic databases
-
contact with experts in the field
-
scrutiny of references of included studies
-
screening of manufacturers and other relevant organisations websites for relevant publications.
The following bibliographic databases were searched: MEDLINE; MEDLINE In-Process & Other Non-Indexed Citations; EMBASE; The Cochrane Library [including Cochrane Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, NHS Economic Evaluation Database and Health Technology Assessment (HTA) databases]; Science Citation Index and Conference Proceedings (Web of Science); National Institute for HTA programme; and PROSPERO (International Prospective Register of Systematic Reviews).
The following trial databases were also searched in April 2014: Current Controlled Trials; ClinicalTrials.gov; UK Clinical Research Network Portfolio Database; WHO International Clinical Trials Registry Platform.
The following specific conference proceedings, selected with input from clinical experts, were checked for the last 5 years. These websites accessed between 24 and 31 March 2014:
-
American Society of Clinical Oncology (main and American Society of Clinical Oncology – Gastrointestinal Cancer) – URL: http://meeting.ascopubs.org/site/misc/meetings_archive.xhtml
-
American Association for Cancer Research – URL: www.aacr.org/home/scientists/meetings--workshops/aacr-annual-meeting-2014/previous-annual-meetings.aspx
-
European Society for Medical Oncology Congress – URL: www.esmo.org/Conferences/Past-Conferences
-
European Cancer Organisation – URL: www.ecco-org.eu/Events/Past-conferences.aspx
-
World Congress of Gastrointestinal Cancer – URL: http://annonc.oxfordjournals.org/content/supplemental
The following websites were consulted via the internet between 24 and 31 March 2014:
-
Saladax – URL: www.saladax.com/
-
International Network of Agencies for Health Technology Assessment – URL: www.inahta.org/
-
The Association of Cancer Physicians – URL: www.cancerphysicians.org.uk/
-
Royal College of Physicians: Oncology – URL: www.rcplondon.ac.uk/specialty/medical-oncology
-
UK Oncology Nursing Society – URL: www.ukons.org/
-
American Society of Clinical Oncology – URL: www.asco.org/
-
Oncology Nursing Society – URL: www.ons.org/
-
European Society for Medical Oncology – URL: www.esmo.org/
-
European Oncology Nursing Society – URL: www.cancernurse.eu/
-
The Association of Coloproctology of Great Britain and Ireland – URL: www.acpgbi.org.uk/
-
British Society of Gastroenterology – URL: www.bsg.org.uk/
The reference lists of included studies and relevant review articles were checked. Identified references were downloaded into EndNote X7 software (Thomson Reuters, CA, USA).
Searches for objective D
Several UK guidelines and evidence updates based on systematic reviews were identified via searches7,37,116 or personal communication (NICE 2010 Head and Neck Cancer Annual Evidence Update) (Fran Wilkie, NICE, 23 April 2014, personal communication). Two search strategies were then developed focussing on finding systematic reviews on the use of FU in mCRC and H&N cancer (see Appendix 1). H&N cancer was not considered further in objective D. The searches were limited to English language and to articles published in or after 2011 (the year the searches were run for the NICE mCRC guideline7 and most recent H&N evidence update116). A focussed search filter for systematic reviews developed in house was used. This search filter was developed to miss less well-reported reviews (e.g. where the terms systematic or meta-analysis are not included in the title or abstract), but recent initiatives, such as Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), mean that this is less of a concern than in the past. 117 The search strategies developed for MEDLINE were adapted as appropriate for other databases. The searches were undertaken in April 2014.
The following bibliographic databases were searched: MEDLINE; MEDLINE In-Process & Other Non-Indexed Citations; The Cochrane Library (Cochrane Systematic Reviews, Database of Abstracts of Reviews of Effects and HTA databases)
The following website was consulted via the internet:
-
Saladax – URL: www.saladax.com/
Identified references were downloaded into EndNote X7 software.
The searches, inclusion and exclusion criteria for objective E will be considered separately in Chapter 5, Methods.
Inclusion and exclusion of relevant studies
Objective A: inclusion criteria
Population:
-
cancer patients (CRC, H&N, stomach, pancreatic) receiving 5-FU chemotherapy by continuous venous infusion.
Intervention:
-
PK monitoring using My5-FU.
Comparator:
-
HPLC, LC-MS/MS.
Outcome:
-
performance of My5-FU (e.g. correlation between My5-FU and ‘gold standard’).
Setting:
-
care services for cancer patients.
Objective A: exclusion criteria
Population:
-
animal studies
-
no patients, samples or cell lines only
-
patient group unclear
-
studies of cancer patients with cancers other than CRC, H&N, stomach, pancreatic
-
studies with < 80% of included cancers (CRC, H&N, pancreatic and gastric cancer).
Treatment:
-
treatment not containing 5-FU
-
non-included treatment (e.g. 5-FU + interferon alpha, chemotherapy + radiotherapy)
-
bolus only
-
oral 5-FU.
Intervention:
-
method for PK monitoring unclear
-
no PK monitoring
-
validation of other technology than My5-FU
-
tumour samples analysed.
Study type:
-
narrative reviews (but reference lists checked)
-
editorials/letters without original data
-
case studies
-
non-English-language papers.
Objectives B and C: inclusion criteria
Population:
-
cancer patients (CRC, H&N, stomach, pancreatic) receiving 5-FU chemotherapy by continuous venous infusion.
Intervention:
-
PK monitoring using HPLC or My5-FU.
Comparator:
-
BSA or no comparator.
Outcome:
-
intermediate measures for consideration:
-
proportion of patients with 5-FU plasma levels in the optimal target range
-
AUC measurements
-
incidence of over and underdosing
-
frequency of dose adjustment
-
test failure rates
-
-
clinical outcomes related to intermediate measures of 5-FU exposure:
-
treatment response rates
-
PFS
-
OS
-
HRQoL
-
incidence of side effects and 5-FU toxicity.
-
Setting:
-
care services for cancer patients.
Objectives B and C: exclusion criteria
Population:
-
animal studies
-
no patients; samples or cell lines only
-
patient group unclear
-
population with non-included cancers)
-
studies with < 80% of included cancers (CRC, H&N, pancreatic and gastric cancer).
Treatment:
-
not 5-FU
-
wrong treatment (e.g. 5-FU + interferon alpha, chemotherapy + radiotherapy)
-
bolus only
-
oral 5-FU.
Intervention:
-
method for PK monitoring unclear
-
no PK monitoring
-
tumour samples analysed.
Outcome:
-
AUC or 5-FU plasma concentration not related to outcomes.
Study type:
-
narrative reviews (but reference lists checked)
-
editorials/letters without original data
-
case studies
-
abstracts without dose adjustment following My5-FU measurement
-
Non-English-language papers.
Objective D: inclusion criteria
Population:
-
CRC patients receiving 5-FU chemotherapy by continuous venous infusion.
Intervention:
-
5-FU therapy as folinic acid and 5-fluorouracil (FUFOL) (Gamelin et al. 118) or FOLFOX6 (Capitain et al. 119) regimen.
Comparator:
-
none or any.
Outcome:
-
PFS, OS, AEs/toxicity.
Setting:
-
care services for cancer patients.
Study type:
-
systematic review or meta-analysis.
Objective D: exclusion criteria
Population:
-
cancers other than CRC.
Treatment:
-
treatment regimens other than FUFOL or FOLFOX6.
Study type:
-
non-English-language papers.
Review strategy
The general principles recommended in the PRISMA statement were used. 117 Records rejected at full-text stage and reasons for exclusion were documented. Two reviewers independently screened the titles and abstracts of all records identified by the searches and discrepancies were resolved through discussion. Disagreement was resolved by retrieval of the full publication and consensus agreement. Full copies of all studies deemed potentially relevant were obtained and two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus or discussion with a third reviewer.
Data extraction strategy
Data were extracted by one reviewer, using a piloted, data extraction form (see Appendices 2–4). A second reviewer checked the extracted data and any disagreements were resolved by consensus or discussion with a third reviewer. Further details about data extraction are provided for objective A(1) below.
Data extraction for objective A(1)
A data extraction sheet (see Appendix 2) was developed combining basic study information, results and fields from the data extraction sheets for the other objectives so these data can be linked. The key measure for whether or not My5-FU can be considered equivalent to LC-MS/MS and HPLC is if both the upper and lower limits of agreement [mean difference ± 2 standard deviation (s.d.)] on the Bland–Altman plot are sufficiently small that in the context of a cautious dose adjustment algorithm they could be considered of little clinical concern. Additionally, if the 95% CI of the mean difference (bias) does not intersect zero then an adjustment should be made when converting from one measuring instrument to the other. 120 We also extracted data on the regression between the index test and reference standard, but this can only give information on the correlation between the two measures, and is not informative to the question of whether or not the two measures can be considered equivalent. Significant correlation cannot be considered evidence for significant equivalence. 120
Quality assessment strategy
Adapting the revised quality assessment of diagnostic accuracy studies checklist for objective A(1)
Where appropriate, the quality of diagnostic accuracy studies was assessed using the revised quality assessment of diagnostic accuracy studies (QUADAS-2). 121 For reasons explained below, QUADAS-2 was adapted for objective A(1) (see Appendix 5).
The QUADAS-2 is a broad tool used to assess the quality of primary diagnostic accuracy studies. For this part of the review we were interested in analytic validity of the test only (i.e. its accuracy and reliability in measuring 5-FU plasma levels). Whether or not the test can accurately predict patients’ response to and side effects of treatment (its clinical validity) and be implemented to improve patient outcomes (its clinical utility) are considered in objectives B–D. We adapted the signalling questions in the QUADAS-2 tool for use with laboratory analytical studies. This was informed by the Analytic validity, Clinical validity, Clinical utility and Ethical guidance for assessing analytic validity for genetic tests. 122
In domain 1 (patient selection), one signalling question, ‘Was a case–control design avoided?’, was removed. In this measure of analytic validity the outcome of interest (5-FU plasma level) is continuous, and therefore by definition there were no cases or controls. The focus of concerns regarding applicability was adapted from relating entirely to patients to also including to plasma sample concentrations.
In domain 2 (index tests), the signalling question ‘Were the index test results interpreted without knowledge of the results of the reference standard?’ was removed because the index test is objective. The signalling question ‘If a threshold was used, was it pre-specified?‘ was removed as we were interested in agreement between two continuous measure without a threshold. An additional signalling question was added to account for the potential bias in under-reporting or not including failed tests: ‘Were the number of failed results and measurement repeats reported?’. Under applicability we added ‘Describe the preparation and storage of the sample before the index test was applied’ to check whether or not sample preparation was similar to potential NHS practice.
In domain 3 (reference standard), the signalling question ‘Were the reference standard results interpreted without knowledge of the results of the index test?’ was removed because the reference standard is objective.
In domain 4 (flow and timing), exclusions from the ‘2 × 2 table’ and ‘analysis’ were replaced with exclusions from the ‘Bland–Altman plot’ because there will be no thresholds used and therefore no 2 × 2 tables produced, and the outcome of most interest is the Bland–Altman plot (see Data extraction strategy). Additionally, ‘Did all patients receive a reference standard?’ was replaced with ‘Were both index test and reference standard conducted on all samples?’
Quality assessment strategy for objectives B and C
For objectives B and C, as a broad range of study designs were identified in the scoping searches, the use of a single checklist, in contrast to individual checklists for each study design, was considered appropriate. The Downs and Black checklist123 was therefore used to assess the quality of papers meeting the inclusion criteria (see Appendix 6). This 27-item checklist enabled an assessment of randomised and non-randomised studies and provides both an overall score for study quality and a profile of scores not only for the quality of reporting, internal validity (bias and confounding) and power, but also for external validity. However, as some questions were not appropriate for single-arm studies, the overall score was not considered useful or appropriate and was therefore not used. The results of the quality assessment provide an overall description of the quality of the included studies and provide a transparent method of recommendation for design of any future studies. Quality assessment was undertaken by one reviewer and checked by a second reviewer, any disagreements were resolved by a third reviewer through discussion.
Methods of analysis/synthesis
Diagnostic accuracy studies (My5-FU vs. high-performance liquid chromatography/liquid chromatography-mass spectrometry) [objective A(1)]
The My5-FU assay delivers an estimate of plasma 5-FU concentration. For a study population this may potentially allow discrimination of study populations into categories: overdosed, optimally dosed and underdosed. Where results from a gold standard were available, a 2 × 2 table was constructed allowing diagnostic accuracy to be estimated using standard statistics (e.g. sensitivity, specificity, positive and negative likelihood ratios, positive and negative predictive values).
Diagnostic accuracy studies (My5-FU vs. HPLC/LC-MS) are considered to be those where patient samples are assayed for 5-FU concentration but patient outcomes may not be reported. Those studies that aimed to test the internal and/or external validity of the My5-FU assay were identified and their findings were summarised and appraised. Studies that do not report test failure rates were noted; where available, test failure rates were tabulated.
Patient-based studies (objectives B and C)
Analyses was stratified according to cancer type, 5-FU delivery mode and cancer stage (e.g. metastatic).
Study, treatment, population and outcome characteristics were summarised and compared qualitatively and, where possible, quantitatively in text, graphically and in evidence tables. Pooling studies results by meta-analysis was considered. Where meta-analysis was considered unsuitable for some or all of the data identified (e.g. due to the heterogeneity and/or small numbers of studies), we employed a narrative synthesis. This involved the use of text and tables to summarise data allowing the reader to consider any outcomes in the light of differences in study designs and potential sources of bias for each of the studies being reviewed. Studies were organised by research objective addressed. A commentary on the major methodological problems or biases that affected the studies was included, together with a description of how this may have affected the individual study results.
For objectives B and C we aimed to identify studies which compared BSA-based dose regimens of 5-FU with continuous infusion in which measures of plasma 5-FU are not undertaken to inform dose changes with dose regimens in which dose adjustment is informed by the My5-FU assay results applied to a stated dose adjustment algorithm. These studies would best report the following outcomes: incidence and severity of side effects of 5-FU; OS; and PFS, as stated in the inclusion criteria. We considered using a linked evidence approach124 in which studies report dose adjustment informed by plasma 5-FU measured by other methods (e.g. HPLC, LC-MS); this required a narrative linking of evidence of comparable performance of My5-FU with such assay methods.
In studies where My5-FU had been used but there was no comparator arm, or the comparator arm was a convenience sample (retrospective/historical population), outcomes were listed and appraised. Outcomes reported for non-randomised comparator arms (i.e. historical controls) were assessed for their representativeness in the light of information gained from systematic reviews (objective D). Relevant clinical outcomes from single-arm studies were considered for pooling should they be reported in sufficient detail and be considered relevant to the objectives.
Time-to-event outcomes
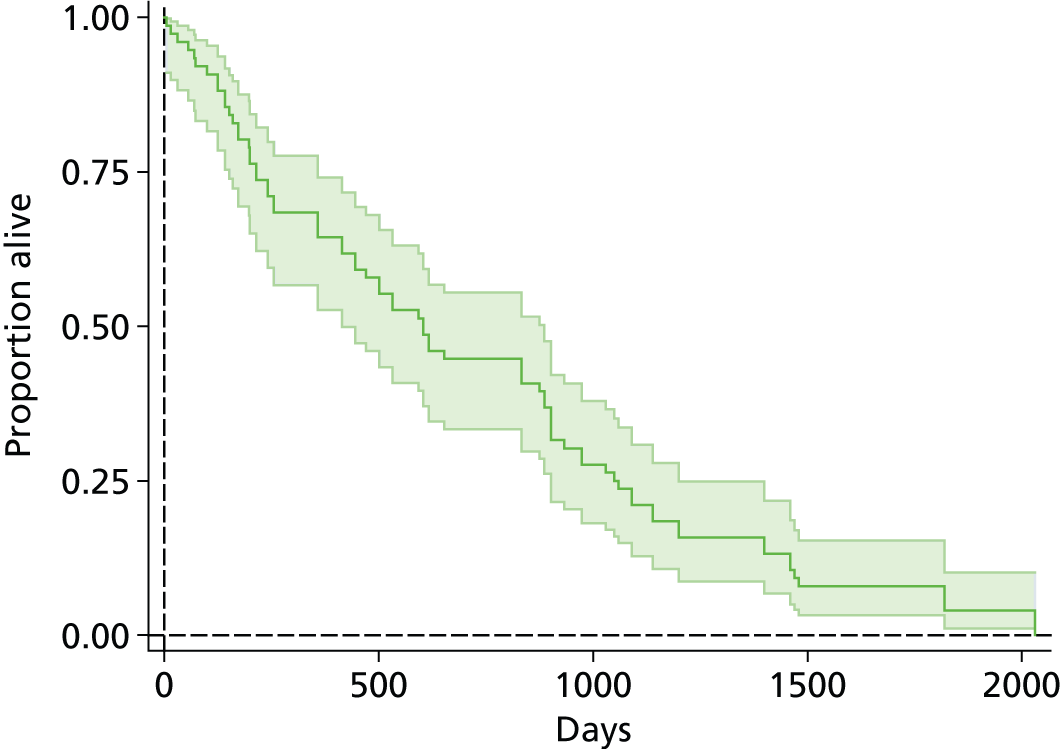
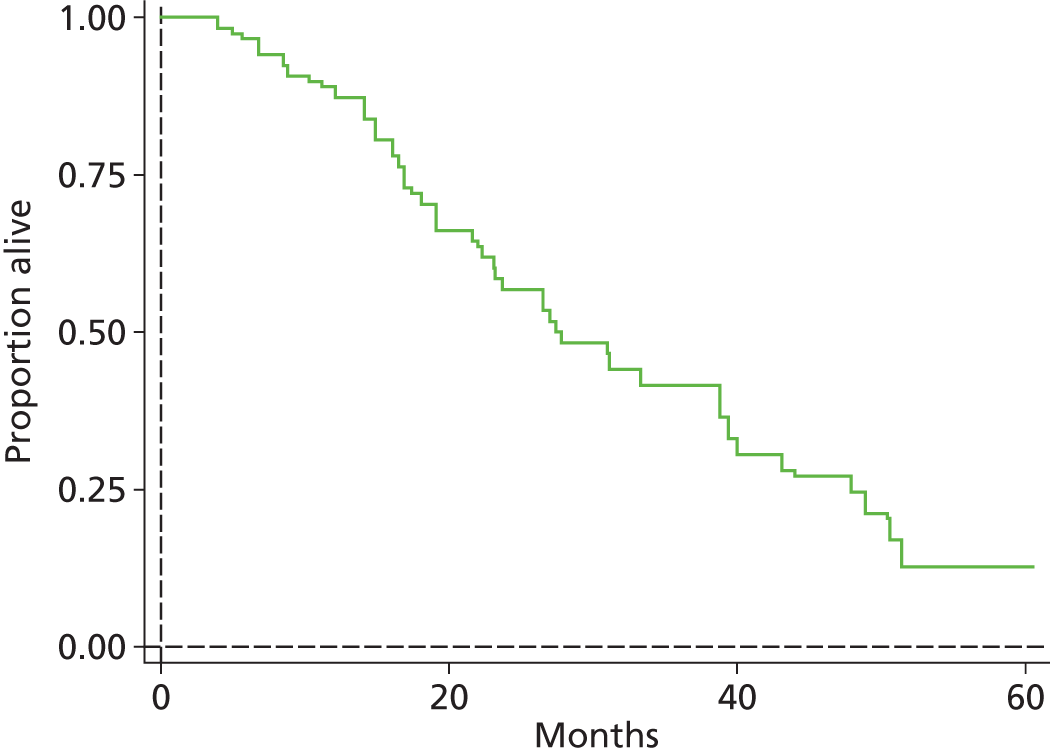
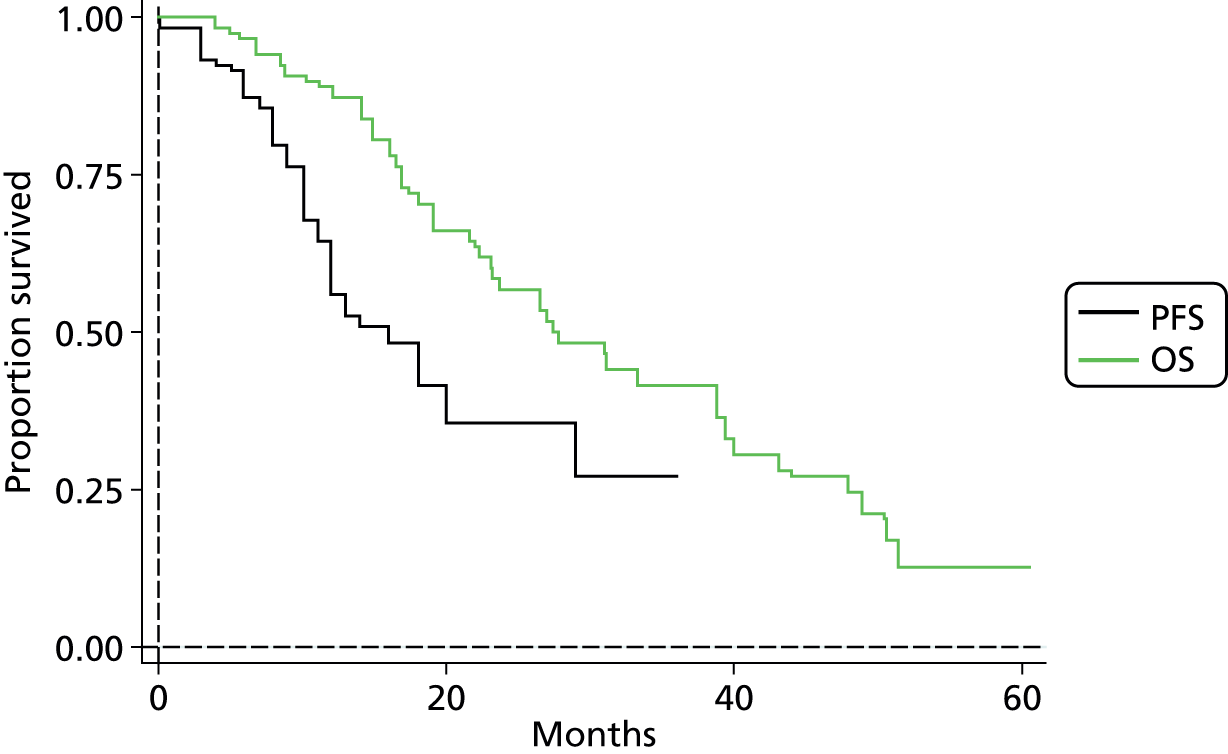
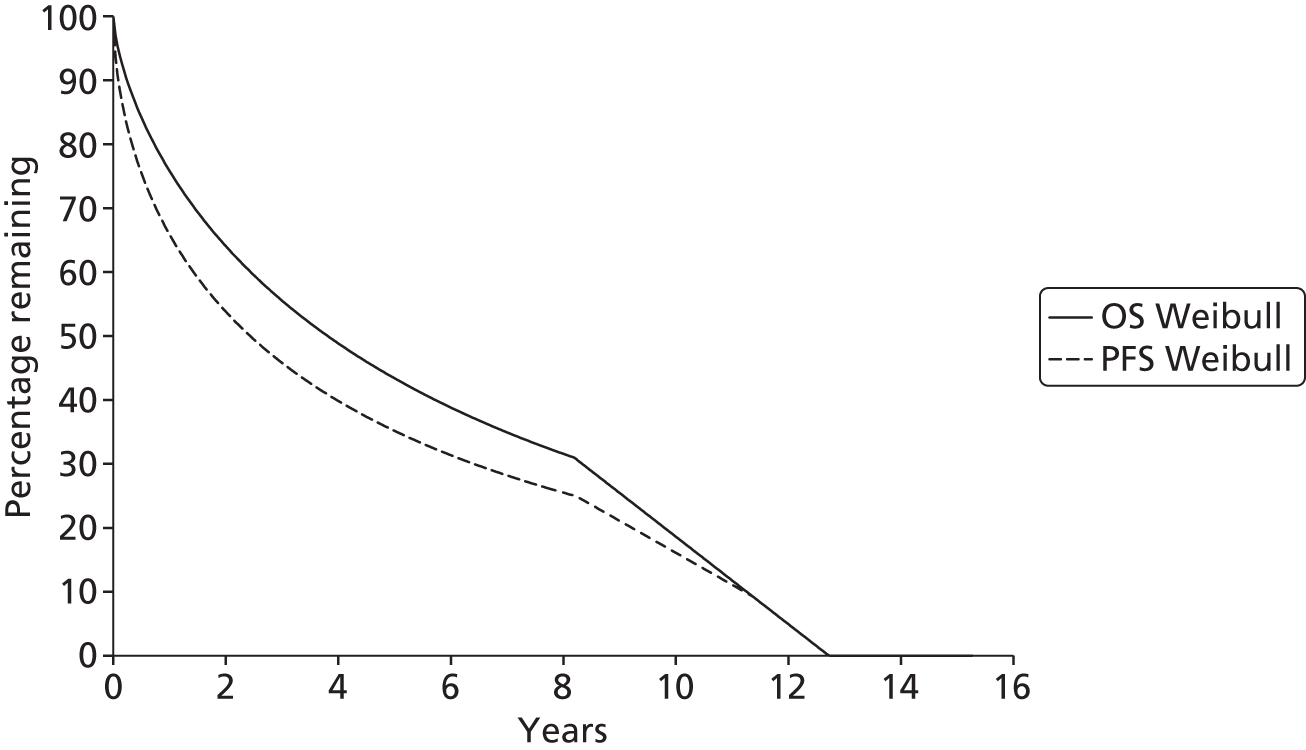
The protocol plan for the current report was to request individual patient data (IPD) from authors of important included papers, so as to inform parameterisation of OS, PFS and other relevant outcomes. In practice, efforts to obtain IPD were not successful. Therefore the method of Guyot et al. 125 was used for reconstruction of Kaplan–Meier plots and of IPD. For this the published Kaplan–Meier graphs were scanned using standard software (Digitizelt; Braunschweig, Germany). Reconstructed Kaplan–Meier plots were implemented from the IPD estimates using Stata version 11 software (StataCorp LP, College Station, TX, USA). As a visual test of faithful reconstruction the reconstructed plots were superimposed on the published originals (available on request from authors). Parametric fits using the estimated IPD were obtained for exponential, log-normal, Weibull, log-logistic and Gompertz distributions implemented with the ‘streg’ command with Stata version 11. Goodness of fit was judged visually and according to information criteria [Akaike information criterion (AIC), Bayesian information criterion (BIC)]. Simple least squares method, implemented with Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) with the solver add-in, was used to obtain parameters for distributions when only median survival values were available.
We requested IPD of key included papers from authors, to enable parameterisation of OS and PFS implemented using standard parametric distributions. Goodness of fit to the observed data was judged visually and according to information criteria (AIC, BIC). In the absence of IPD becoming available, we digitised published Kaplan–Meier (or competing risks) analyses using standard software (e.g. DigitizeIt software). The digitised product was used to construct curve fits using methods developed by Guyot et al. 125 or Hoyle and Henley. 126
Outcomes reported as proportions
Reported percentages were converted to the nearest whole number of patients and the 95% CIs around proportions were estimated using the binomial distribution. Relative risks and associated 95% CIs were estimated in Stata version 11 using the ‘metan’ package. Pooling of relative risks was not undertaken because of differences in treatments and populations between studies.
Indirect- and mixed-treatment comparison
The methods outlined in the protocol anticipated the existence of several RCTs or comparative studies that would be appropriate for formal meta-analysis or network meta-analysis (NMA); the evidence was insufficient to support this approach.
The authors of the NICE guideline for advanced CRC (CG131)7 undertook NMAs of OS and of PFS using RCT data for 5-FU treatment regimens, and this offered a potential template approach for the present project. The CG131 authors were constrained by the lack of full data and their analyses required assumptions of constant hazard (i.e. fitting of exponential survival curves) and of proportional hazards between treatments. CG1317 preceded publication of the Guyot et al. 125 procedure to estimate IPD from Kaplan–Meier plots. Our use of this method with available PK data revealed that the exponential distribution was the poorest performing of various parametric distributions tried in exploring reported Kaplan–Meier plots. We therefore considered the method described by Ouwens et al. 127 for NMA of Weibull parametric survival curves since this was reported to avoid proportional hazard assumptions. In practice, because of commercial considerations, the authors’ kept the published NMA code incomplete. There was insufficient time available to develop our own code and contact with the corresponding author failed to resolve the difficulty. A further problem confronting NMA approaches was the almost total lack of randomised evidence about PK dose adjustment and the heterogeneity of available studies. NMA was therefore not undertaken.
Face-to-face discussions and written questions
Information was extracted from face-to-face discussions and written questions undertaken with a relevant laboratory. Information was used within the model. Expert opinion from specialist committee members and other clinical experts was sought and appropriately accessed.
Chapter 4 Clinical effectiveness results
This chapter provides the search results for the clinical effectiveness assessment including results of:
-
objective A(1) which considers the accurate estimation of plasma 5-FU when using the My5-FU assay; and objective A(2) which considers available information about treatment algorithms based on 5-FU measures
-
objectives B and C which consider the evidence on PK dosing compared with traditional BSA-based dosing
-
objective D which examines the comparability of BSA comparators used in the PK comparison compared with the generality of BSA regimens.
Search results for objectives A–C
Figure 6 provides the PRISMA flow diagram for objectives A–C. A total of 3751 records were identified through electronic searches. One additional record was identified from other sources. The removal of duplicates left 2565 records to be screened, of which 2362 were excluded at title/abstract level as these were irrelevant. The remaining 203 records were examined for full text, of which 35 were included in the clinical effectiveness review (see Appendix 7). The included 35 references represent:
-
29 studies in 30 papers for objectives B and C,118,119,130–156 of which three studies addressed both objective A(2) and objectives B and C. 130–132
FIGURE 6.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram: My5-FU clinical effectiveness objectives A–C. a, Three studies addressed both objective A(2) and objectives B and C.

Full details on the reasons for excluding studies at full text can be found in Appendix 8.
Ten ongoing trials were identified by the manufacturer. The search of ongoing trials in ClinicalTrials.gov, Current Controlled Trials, UK Clinical Research Network Portfolio Database and WHO International Clinical Trials Registry Platform databases (carried out in April 2014) retrieved three of these, but we were unable to verify the remaining seven (see Appendix 9).
Objective A(1): review of studies examining the accuracy of the My5-FU assay when tested against gold standard methods
The first part of objective A [A(1)] aimed to provide a review of the studies that examine the accuracy of the My5-FU assay when tested against gold standard methods of estimation of 5-FU (HPLC and LC-MS will be considered the gold standard for the purpose of assessing the accuracy of 5-FU plasma level measurements). To achieve this we developed a data extraction template and adapted the QUADAS-2 checklist (see Appendices 2 and 5). Summaries of the data extraction and quality assessment for objective A(1) are in Tables 4 and 5. Completed data extraction and quality assessment forms are available from the authors on request.
| First author, year | Index test | Reference standard | Range of plasma concentrationsa (ng/ml) | Bland–Altman plot | |||
|---|---|---|---|---|---|---|---|
| Bias (95% CI) | Lower limit of agreement | Upper limit of agreement | Largest outliers | ||||
| Buchel, 201395 | My5-FU | LC-MS/MS | 93–17,881 | 7.0% (5.5% to 8.5%) | –18% | 30% | –50%;b 95%b |
| Beumer, 200989 | My5-FU | LC-MS/MS | 93–1774 | 23 ng/ml (NR) | NR | NR | –35%;b 52%b |
| Makihara, 2012129 | My5-FU | LC-MS/MS | 41–457 | NR | NR | NR | NR |
| Validation datac | My5-FU on 75 | LC-MS/MS | 100–1471 | 24.5 ng/ml | –285 to 171 ng/ml (approximately –25% to 70%b)d | ||
| Validation datac | My5-FU on 75 | HPLC | 100–1471 | 1.84 ng/ml | –80 to 137 ng/ml (approximately –30% to 35%b)d | ||
| Validation datac | My5-FU on 117 | HPLC | 100–1471 | 5.08 ng/ml | –80 to 150 ng/ml (approximately –30% to 35%b)d | ||
| Validation datac | HPLC | LC-MS/MS | 95–1370 | 22.7 ng/ml | –227 to 166 ng/ml (approximately –25% to 60%b) | ||
| First author, year | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Buchel, 201395 | High | Unclear | Low | Unclear | Low | High | Low |
| Beumer, 200989 | High | Unclear | Low | Unclear | Low | Low | Low |
| Makihara, 2012129 | Unclear | Unclear | Low | Unclear | Low | Unclear | Low |
| Validation dataa | Unclear | Unclear | Low | High | Low | Unclear | Low |
Two research papers89,95 and two abstracts,128,129 were found to include information relevant to whether or not My5-FU can be considered equivalent to LC-MS/MS and HPLC. However, one of the abstracts was considered part of the same study as one of the papers. 95,128 Therefore there were three unique studies. Three studies provided Bland–Altman plots, but only one95 provided the upper and lower limits of agreement and the 95%CI of the mean difference. Validation data provided by the manufacturer included three additional unpublished Bland–Altman plots (S. Salamone, Saladax Biomedical Inc., 2014, personal communication). These did not include upper and lower limits of agreement and there may have been some samples in common with one of the papers. 95
The risk of bias was difficult to judge due to incomplete reporting, particularly in the domain of flow and timing. There was a high risk of bias for patient selection for two out of the four studies,89,95 and it was unclear for the other two (S. Salamone, personal communication). 129 This was because there were no assurances given that inappropriate exclusions of patients or samples were made. This could have led to incomplete reporting of both failed samples and outliers, and lead to overly optimistic estimations of bias and limits of agreement. Validation data provided by the manufacturer were judged to have a high risk of bias for flow and timing. Overall, there were few concerns about applicability, with the tests considered generally representative of the tests of interest, the only potential concerns involved whether or not sample collection, preparation and storage matched the proposed NHS method.
Buchel et al. 95 took 197 samples from 32 gastrointestinal cancer patients in one Swiss hospital. They added 50 plasma samples from cancer patients of unspecified type provided by Saladax. It was not stated if there were any participants or plasma samples excluded from the analysis. They compared the measurements from the Saladax My5-FU immunoassay using the Roche Cobas Integra 800 analyser (Roche Diagnostics, Indianapolis, IN) (index test) to the reference standard LC-MS/MS. The range of concentrations for My5-FU was 93–17,881 ng/ml, and for LC-MS/MS was 102–18,590 ng/ml. Passing–Bablok regression showed strong correlation (Pearson’s R2 = 0.99) with slope 1.08 (95% CI 1.06 to 1.09) indicating that My5-FU measurements increase faster than LC-MS/MS measurements with increasing 5-FU concentrations. 157 The Bland–Altman plot did not appear to show any systematic pattern and therefore the coefficients are interpretable. There was a 7.0% bias (95% CI 5.5% to 8.5%) indicating that measurements using My5-FU assay were between 5.5% and 8.5% higher than when using LC-MS/MS. The lower and upper limits of agreement are shown on the graph at around –18% and 30%, although the exact figures are not given.
This indicates that the two measurement methods can only be considered equivalent if the overestimation of 5-FU concentration by 30% and the underestimation of 5-FU concentration by 18% by the My5-FU assay in comparison with LC-MS/MS are not considered clinically meaningful.
There are nine outliers from the 197 samples, including one outlier with over 90% bias. The authors do not propose an explanation for this. Such outliers merit investigation, in particular consideration of the potential impact on clinical care if such outlying measurements occur in practice. The outliers and upper and lower limits of agreement from the Bland–Altman plot are shown in Figure 7. This paper also presented data provided by Saladax giving the performance of the My5-FU assay using four different analysers over the smaller range (102–1560 ng/ml). The bias that was apparent using the Integra 800 analyser was just 1.4% (95% CI 0.2% to 2.6%) using the smaller subset of 50 samples. The authors propose that this is due to the much higher range of plasma concentrations they have used in the larger samples. This explanation appears reasonable as the regression results indicate that as the 5-FU plasma concentrations increase, the bias towards My5-FU giving higher estimates also increases. These much higher plasma concentrations may be unlikely in clinical practice. No lower and upper limits of agreement are presented for these analyses on the subset of 50 cases so it is difficult to draw any conclusions from them.
FIGURE 7.
Key elements of the Bland–Altman plot from Buchel et al. 95 Outliers are indicated by blue circles and upper and lower limits of agreement by the dashed lines. Values > 1800 ng/ml may be rare in clinical practice.

Beumer et al. 89 describe part of the development and testing of the My5-FU assay with the corresponding author (a Saladax employee). They used 156 plasma samples from the blood of 156 H&N cancer and CRC patients. They compared the 5-FU personalised chemotherapy management assay using the Olympus AU400 analyser (Olympus Instruments, Irving, TX) as the index test against LC-MS/MS as the reference standard. They do not state whether or not there were any exclusions; however, there are 156 samples included in the regression analysis so there appear to be no exclusions in that part. The regression showed strong correlation (R2 = 0.97), with an intercept of 10.9 ng/ml and gradient of 1.035, indicating a trend towards the My5-FU assay estimating plasma levels as higher than LC-MS/MS, with this effect larger at higher concentrations. However, there are no CIs so we do not know if this is statistically significant. The Bland–Altman plot appears to show a positive bias with no visually detectable pattern of change with increasing average plasma concentration. No mean bias or limits of agreement are reported or shown in the plot and so it is difficult to reach conclusions about the levels of agreement between the two tests from this paper. However, from visual inspection there appears to be one measurement above 50% positive bias and 16 measurements between 25% and 50% positive bias. There appear to be no measurements above 50% negative bias, and three measurements between 25% and 50% negative bias.
The Makihara et al. 129 conference abstract does not include the key outcomes of interest from a Bland–Altman plot. However, in this sample of 50 CRC patients, although showing strong agreement between My5-FU and LC-MS (R2 = 0.8471), the agreement was weaker than in other studies. The reasons for this are unclear, but it merits consideration as there are few separate data sets on which the comparison between tests has been made.
Validation data provided by the manufacturer (S. Salamone, personal communication) includes a comparison between My5-FU using an AU400 analyser and LC-MS/MS 75 for 75 samples (although the methods section appears to only describe 56 samples). An unspecified number of outliers have been excluded. Deming regression shows a gradient of 1.005 that does not significantly differ from unity (95% CI 0.94 to 1.07) and a positive intercept of 22.7 ng/ml which is not significantly non-zero (95% CI –4.5 to 50.0). The Bland–Altman plot shows mean bias 24.5 ng/ml, CIs and upper and lower limits of agreement are not given, but values for bias range from –285 ng/ml to 171 ng/ml, although there will be more extreme values if outliers have been excluded. In percentage terms, the range is approximately –25% to 70%. There are also comparisons between the same My5-FU assay with HPLC as the reference standard, with mean bias just 1.84 ng/ml and outliers ranging from –80 to 137 ng/ml (approximately –30% to 35%). Comparison of the two reference standards used, HPLC and LC-MS/MS, is also made with mean bias 22.7 ng/ml and range –227 ng/ml to 166 ng/ml (approximately –25% to 60%) indicating that similar levels of variation in measurements appear to occur between HPLC and LC-MS/MS as do between My5-FU and LC-MS/MS.
Summary of objective A(1)
Although there is high correlation coefficient between My5-FU, HPLC and LC-MS/MS, the Bland–Altman plots show considerable variability. In the comparison of My5-FU with LC-MS/M,S even with additional outliers detailed as excluded, the validation data provided by the manufacturer shows outliers with a range of variation up to –285 ng/ml and 171 ng/ml (approximately –25% and 70%). Only one paper reported upper and lower limits of agreement. These were found to be –18% to 30%. These discrepancies between measurements need to be considered carefully from the point of view of clinical significance. If in the context of a cautious dose adjustment algorithm this range of values (–18% to 30%) can be considered of little clinical concern then My5-FU can be considered equivalent to LC-MS/MS, but with careful consideration of the clinical implications of outlier measurements beyond this range.
Buchel et al. 95 found that the mean difference between LC-MS/MS and My5-FU measurements was 7.0%, with upper and lower limits of agreement as –18% to 30%. This means that the s.d. of the differences was around 12%. Therefore, we would expect that 95% of the measurement differences between LC-MS/MS and My5-FU to lie between –18% and 30%. In addition, this paper has a positive bias (i.e. 7%), so My5-FU appears to systematically produce higher measurements than LC-MS/MS. The other papers do not share this same bias. It may be simply due to this paper taking measurements well above the clinical range, and these outliers beyond the clinical range are skewing the distribution. No other papers were found to calculate limits of agreement, but several papers reported plot distributions very similar to Buchel et al. 95 We did not find any data on test–retest reliability. Personal communication with a clinical advisor suggests that –18% to 30% may not be clinically significant in context of a cautious algorithm; therefore, we concluded that the tests may be equivalent, but remain cautious about outliers. The representativeness of the samples used in these studies is difficult to establish on the basis of the information provided.
We adapted the QUADAS-2 checklist121 for use in assessing the quality of laboratory measurements of analytic validity, and developed a data collection sheet with emphasis on the key outcomes of interest from the Bland–Altman plot. We systematically applied these to the research literature. The weaknesses of this analysis lies largely with the evidence base, with missing details of exclusions making interpretation difficult, and only one paper95 giving upper and lower limits of agreement on the Bland–Altman plot; however, it should be noted that these latter limits of agreement are reported for a range of plasma concentrations which are beyond the clinical range.
Overall, there is good correlation between My5-FU and the reference standard of HPLC or LC-MS/MS; however, significant variability remains and there was high risk of bias in included studies due to excluded samples. There may be substantial variability in 5-FU measurement of –18% and 30% between the two tests. It is unclear if this is clinically significant. Outliers show even greater variability.
Objective A(2)
This section provides an overview of published treatment algorithms for 5-FU dose adjustment in cancer patients. Dose adjustment during 5-FU treatment requires knowledge of the plasma concentration of 5-FU following the first cycle of treatment to calculate the AUC value, which is known to correlate better with outcomes than initial 5-FU dose. 96 Due to the non-linear PKs of 5-FU, an algorithm is then needed to calculated the new dose for the second 5-FU treatment cycle if the exposure is not within the desired range. Several algorithms have been reported in the literature for different treatment regimens which are described below. Complete data extraction forms for objective A(2) are available on request from the authors.
Gamelin et al. 130 developed a dose adaptation algorithm for the weekly 8-hour continuous infusion of 1000 mg/m2 5-FU plus 200 mg/m2 bolus FA. In a case series of 40 patients with advanced CRC the 5-FU dose was increased in 250 mg/m2 steps in all patients every 3–4 weeks up to 2000 mg/m2 or first signs of toxicity. 5-FU plasma concentrations were measured weekly and the dose adaptation algorithm was based on a regression analysis of the relationship between dose and plasma levels in two groups of patients achieving complete response (CR) or partial response (PR) versus patients with minimal response, stable disease (SD) or progressive disease (PD). The target concentration was set at 2000–3000 µg/l. The adjustment algorithm by Gamelin et al. 130 is shown in Table 6. This was adapted and used in subsequent studies by the French group (see Table 18). 118,133,134,138
| 5-FU plasma levels (µg/l) | 5-FU dose adjustment (% of previous dose) | In case of toxicity |
|---|---|---|
| < 500 | +50 | Grade II toxicity: 200-mg dose decrease Grade III toxicity: 1-week break then 300-mg dose decrease |
| 500–1000 | +40 | |
| 1000–1300 | +30 | |
| 1300–1500 | +20 | |
| 1500–1800 | +10 | |
| 1800–2000 | +5 | |
| 2000–3000 | No modification | |
| 3000–3200 | –5 | |
| 3200–3500 | –10 | |
| > 3500 | –30 |
Kaldate et al. 96 carried out a retrospective database analysis of PK data from a commercial laboratory setting to define a dose adjustment algorithm for the FOLFOX6 regimen (5-FU + FA + oxaliplatin) with or without bevacizumab. They used a simple regression analysis to model the change in AUC versus the change in dose in 187 CRC patients. The 187 patients produced a total of 307 cycle pair observations, i.e. AUC measurements from two consecutive infusion cycles that included a dose change. The difference observed in AUC for a consecutive cycle pair where a change in dose occurred was termed ‘change in AUC’ (mg × hour/l) and the corresponding dose change was termed ‘change in dose’ (mg/m2). The relationship between ‘change in AUC’ versus ‘change in dose’ was investigated using regression analysis. The regression model that described the relationship with an R2 = 0.51 was:
This relationship was used to establish a dose adjustment algorithm to be used as a practical tool in a clinical setting (Table 7). It is noteworthy that Kaldate et al. 96 used a revised optimal target AUC range of 20–30 mg × hour/l rather than the narrower target range of 20–24 mg × hour/l. The lower limit was accepted to be valid based on published efficacy data from Gamelin et al. 118,138 The upper limit, however, was thought to be too low as it was produced using outdated more toxic regimens. The longer infusion time of 46 hours and the combination with other therapies make more recent combination therapies such as FOLFOX6 less toxic and more tolerable. Therefore, the new target range is wider allowing greater 5-FU exposure. This algorithm has not been tested in a prospective study. However, Kaldate et al. 96 announced that the PROFUSE (PROspective 5-fluorouracil OnDoSe® Evaluation, NCT014623) study is under way in the USA to test the utility of this algorithm.
| AUC (mg × hour/l) from previous cycle | Change in dose (mg/m2) |
|---|---|
| ≥ 40 | ↑727 |
| 37–39 | ↑582 |
| 34–36 | ↑436 |
| 31–33 | ↑291 |
| 20–30 | No change needed |
| 17–19 | ↓291 |
| 14–16 | ↓436 |
| 11–13 | ↓582 |
| 8–10 | ↓727 |
Ychou et al. 131 investigated two different adaptation schedules for the bimonthly 5-fluorouracil + folinic acid (leucovorin) regimen (LV5FU2) (de Gramont86,87) regimen: FA (200 mg/m2 per day) by i.v. infusion over 2 hours, followed by a 5-FU bolus (400 mg/m2 per day), followed immediately after by continuous 5-FU infusion (600 mg/m2 per day) over 22 hours for 2 consecutive days (i.e. 2000 mg/m2 per cycle). A prospective cohort of 38 patients with advanced CRC was divided into two groups. Group A received progressive increase of 5-FU between 25% to 50% at every cycle (i.e. 150% maximum at cycle 6 in the absence of ≥ grade III toxicity). This steady increase was used to avoid severe toxicities early on. The insights from group A were then used to develop an adaptation algorithm for group B who received a dose increase at cycle 2, which could be extensive, according to the AUC value from cycle 1 in the absence of ≥ grade III toxicity. After cycle 2 the dose remained constant during subsequent cycles if toxicity grades remained < III. The algorithm is displayed in Table 8. The methods of how the algorithm was developed are unclear. This algorithm was used in subsequent studies by the same group. 136,147
| AUC in mg × hour/l × m2 | Dose increase, % |
|---|---|
| ≤ 5 | 150 |
| 5< AUC ≤ 10 | 100 |
| 10< AUC ≤ 15 | 50 |
| 15< AUC ≤ 20 | 25 |
| > 20 | No increase |
Santini et al. 132 studied dose adjustment in patients with H&N cancer using 5-FU (1000 mg/m2 × 24 hours) for 5 consecutive days (days 1–5) plus cisplatin (100 mg/m2) on day 0 as first-line chemotherapy treatment. 5-FU PK measurements were taken on day 3 to adjust the dose for the second half of treatment if required. They used a retrospective study group (n = 89) to establish the relationship between 5-FU exposure and toxicity in order to identify a threshold AUC0–3 value (15,000 ng/ml × hour) following 3 days of treatment that would be predictable of toxicity. In a prospective study of 81 patients, AUC0–3 values were determined to decide whether or not dose reduction was required for the second half of the cycle. It was estimated that a 30% reduction would lead to a subjective decrease in exposure if the AUC0–3 reached the threshold value of 15,000 ng/ml × hour. Furthermore, it was decided to stop treatment at an AUC0–3 value of ≥ 30,000 ng/ml × hour. Using these two relationships intermediate AUC0–3 values (between > 15,000 ng/ml × hour and < 30,000 ng/ml × hour) would require a dose decrease following a linear function between per cent of 5-FU dose and 5-FU AUC0–3. Table 9 illustrates the algorithm for four different AUC0–3 values. This algorithm was also used by the same group in a later study152 and was further developed to include dose increases for patients with an AUC1–2 days value lower than 5760 ng/ml × hour. 158
| AUC0–3 value (ng/ml × hour) | 5-FU dose adjustment (% of previous 5-FU dose) |
|---|---|
| < 15,000 | Same dose |
| 15,000 | 70 |
| 20,000 | 45 |
| ≥ 30,000 | Stop treatment |
Summary of objective A(2)
Dose adjustment algorithms have been developed by different groups for different regimens. They are based on the observed 5-FU exposure expressed as AUC values or the concentration of plasma 5-FU. 96,130–132
As other factors are involved in the modulation of 5-FU exposure it is advisable to consider additional parameters in the dose adjustment calculation such as genotype, phenotype, physiological, physiopathological and associated treatments. 159 Protocols have been developed known as the Onco Drug Personalised Medicine ProtocolTM which integrates these additional parameters to optimise previous dose adaptation algorithms. 119 These have been commercialised. Algorithms published for one treatment schedule cannot be extrapolated to other protocols but need to be adapted to different treatment schedules due to the non-linear nature of the 5-FU PKs. 159 Therefore, individual algorithms need to be developed for different regimens and adjusted if new combination therapies are developed.
Conclusions from objectives A(1) and A(2)
Equivalence of My5-FU with HPLC and LC-MS is reasonable; however, the evidence is based on studies which are at high risk of bias due to excluded samples and outliers. Studies developing algorithms have focused on one particular regimen and, therefore, algorithms might not be available for all 5-FU containing regimens. Algorithms cannot be simply transferred from one regimen to another but require adjustment to different or more recent regimens and commercialised algorithms may need to be purchased.
Objectives B and C: systematic review of the literature on the use of assays to achieve adjusted-dose regimens
The purpose of objectives B and C was to systematically review the literature on the use of My5-FU (objective B) and HPLC and/or LC-MS (objective C) to achieve adjusted-dose regimen(s) to compare with BSA-based dose estimation (in terms of OS, PFS and AEs) for patients receiving 5-FU administered by continuous infusion. Variations in current BSA-based dose regimens will be considered where appropriate.
The aim of 5-FU chemotherapies is to prolong life and delay disease progression while guarding against deterioration in life quality from toxic side effects. PK dose adjustment may be judged an advance on BSA dosing if it improves on these clinical outcomes in a cost-effective way. Therefore, the emphasis here is in studies that compare PFS, OS and toxic events for PK versus BSA treatments; these are crucial for an estimate of clinical effectiveness and for informing an economic model.
The following section provides an overview of the available evidence that was identified and eligible for inclusion to answer objectives B and C. It also provides justification for the evidence which was taken forward to the analysis and modelling stage. The 30 included references represented 29 studies. Of the 29 included studies, 24 were single-arm studies (i.e. studies that included either a BSA or a PK arm)130,131,133–154 and five were comparative studies. 118,119,132,155,156 Of these, three were in CRC118,134,155 and two in H&N cancer. 132,156 Comparative studies are defined as those in which patients who received a first-line PK-adjusted 5-FU dose regimen (the intervention arm) were compared with a similar group who received a BSA-based regimen (the control arm), the regimens being identical in all respects other than dose adjustment. It is important to note that two studies by Fety et al. (1998),156 (1994)158 both publish results from the same study [where Fety et al. (1994)158 presented preliminary results]. From here on the study is referred to as Fety et al. (1998). 156
This section provides an overview of the 24 single-arm studies, a summary which highlights our concerns with the quality of the studies and discusses evidence that can be taken forward to the analyses and cost-effectiveness modelling. This is followed by an overview of the comparative studies for CRC and H&N cancer separately, including a rational for the studies taken forward to inform the cost-effectiveness modelling. Finally, this section provides more detailed summaries of the studies which inform some of the model parameters.
Overview of single-arm studies
The 24 single-arm studies included 22 full papers130,131,133–143,146–154 and two abstracts144,145 (further details are provided in Appendix 10 and the full data extraction forms are available on request from the authors). The abstracts describe two investigations of dose adjustment using My5-FU in CRC patients. Of the 22 full papers, 16 investigated CRC patients,130,131,133–143,146–148 two used a mixed-patient population,149,150 three studied patients with H&N cancer151–153 and one study included only gastric cancer patients. 154 The major features of these studies are summarised in Table 10.
| First author, date, country | Study design | Cancer type | Regimen | 5-FU dose adjustment (algorithm)/PK method | n (cycles) | 5-FU-related AEs | Response rate (overall response) | OS (median OS); Kaplan–Meier | PFS (median PFS); Kaplan–Meier | Relationship between plasma level and outcome (positive, negative, neutral) |
|---|---|---|---|---|---|---|---|---|---|---|
| Boisdron-Celle, 2002, France133 | Prospective case series | Advanced CRC | 5-FU (8-hour infusion) + FA (+ oxaliplatin after progression) | Yes (Gamelin 1996130)/HPLC | 29 (122) | Yes counts (reported extensively but irregularities in numbers reported) | Yes (7/27, 25.9%) | No; no | No; no | Plasma level and toxicity (positive) |
| Capitain, 2008, France134 | Case series | Advanced CRC | 5-FU + FA (FUFOL 4 hours and modified de Gramont86,87) | Yes (Gamelin 1996130)/HPLC | 76 | Yes risk (time of assessment unclear) | Yes (25/76, 32.9%) | Yes (20 months); yes | Yes (100 days); no | No |
| Cattel, 2003, Italy135 | Prospective case series | mCRC (stage IV) | 5-FU (14 days) + oxaliplatin | No/HPLC | 13 | No | Yes (7/13, 54%) | Yes (9.6 months); no | Yes (7 months); no | No |
| Duffour, 2010, France136 | Retrospective database analysis | mCRC | de Gramont86,87 (LV5FU2) | Yes (Ychou 2003147)/HPLC | 103 | Yes risk | Yes (young group: 15/55, 27%; elderly group: 17/48, 35%) | Yes (young group: 18.7 months; elderly group: 13.4 months); no | No; no | AUC and severe toxicity (neutral) |
| Findlay, 1996, UK137 | Case series | CRC (not specified) | 5-FU (protracted; not specified) | No/HPLC | 19 | Yes risk (time of assessment unclear) | Yes (8/19, 42%) | No; no | No; no | Plasma level and response (neutral); plasma level and toxicity (positive) |
| Gamelin, 1996, France130 | Prospective case series (Phase II study) | mCRC | 5-FU (8-hour infusion) | No/liquid chromatography | 40 (2082) | Yes risk | Yes (18/40, 45%) | Yes (14 months); yes | Yes (unclear); yes | Plasma level and toxicity (positive), survival (neutral), response (positive) and quality of response (positive) |
| Gamelin, 1998, France138 | Prospective case series (multicentre Phase II study) | mCRC | 5-FU (8-hour infusion) | Yes (Gamelin 1996130)/liquid chromatography | 152 (4096) | Yes counts | Yes (66/117, 56.4%) | Yes (19 months); yes | Yes (11 months); yes | Plasma level and toxicity (positive) |
| Ho, 2011, China139 | Prospective case series | mCRC | 5-FU (48-hour infusion) + FA | No/HPLC | 16 (80) | Yes counts | Yes (3/16, 18.8%) | Yes (10.5 months); no | Yes (4.1 months); no | Plasma level and toxicity (neutral) |
| Jodrell, 2001, UK140 | Prospective case series and simulation study | CRC (relapsed or metastatic) | 5-FU (protracted 1–26 weeks) | No/HPLC | 61 | Yes risk (time of assessment unclear) | Yes (16/61, 26%) | Yes (11 months); no | No; no | Plasma level and toxicity/response (neutral) |
| Kline, 2011, USA141 | Case series | CRC (stages III and IV) | FOLFOX6 + avastatin; FOLFOX6; FOLFIRI; FOLXOX4 | Yes (NR, supplied by manufacturer)/My5-FU | 21 | No | No | No; no | No; no | NR |
| Metzger, 1994, France142 | Randomised triala | mCRC | 5-FU (5-day continuous infusion, flat or chronomodulated) + FA + oxaliplatin | No/HPLC | 9 | Yes risk (inconsistent grouping of toxicity grades) | No | No; no | No; no | AUC and stomatitis (unclear) |
| Milano, 1988, France143 | Prospective case series | Advanced CRC | 5-FU (5-day continuous infusion) | No/HPLC | 26 | Yes counts (grouping grades I + II and III + IV) | Yes (3/26, 12%) | No; no | No; no | AUC and toxicity (positive) |
| Patel, 2012, USA144 | Unclear (abstract only) | CRC (not specified) | mFOLFOX6 ± bevacizumab | Yes (NR)/My5-FU | 58 | Yes risk (grading tool unclear) | No | No; no | No; no | No |
| Patel, 2013, USA145 | Unclear (abstract only) | CRC (not specified) | mFOLFOX6 ± bevacizumab | Yes (NR)/My5-FU | 70 | Yes risk (grading tool unclear, total toxicities reported only) | No | No; no | No; no | No |
| Stremetzne, 1999, Germany146 | Randomised triala | mCRC (unresectable) | 5-FU (5-day continuous) + FA | No/reversed-phase ion-pair HPLC | 16 | Yes risk (time of assessment unclear) | Yes (0/16) | No; no | No; no | AUC and toxicity (neutral) |
| Ychou, 1999, France131 | Prospective case series | Advanced CRC | de Gramont86,87 (LV5FU2) | Yes (tested two different algorithms)/HPLC | 38 (204) | Yes risk and counts | Yes (unclear) | No; no | No; no | AUC and response (neutral) |
| Ychou, 2003, France147 | Prospective case series | mCRC | de Gramont86,87 (LV5FU2) | Yes (Ychou, 1999131)/HPLC | 53 (435) | Yes risk [grouping (1) cutaneous; (2) haematological III + IV; (3) digestive and mucositis III + IV] | Yes (19/52, 36.5%) | Yes (18.6 months); no | Yes (7 months); no | AUC and PFS (positive) |
| Yoshida, 1990, Japan148 | Prospective case series | Advanced colonic cancer | 5-FU | No/HPLC | 19 | Yes risk (time of assessment unclear, total toxicities reported only) | Yes (10/19, 53%) | No; no | No; no | AUC and toxicity (positive)/response (neutral) |
| Ciccolini, 2006, France149 | Prospective case series | Mixed-patient group: H&N cancer (46/80, 58%), CRC (30/80, 38%), breast cancer (4/80, 5%) | 5-FU + carboplatin; PF; FOLFIRI; FOLFOX | No/HPLC-UV | 80 | Yes risk (grades III and IV only) | No | No; no | No; no | Relationship between DPD activity and 5-FU plasma levels in patients with severe toxicities |
| Hendrayana, 2012, Germany150 | Prospective case series | Mixed-patient group (colon, gastric, rectal, H&N) | 5-FU plus one or more of the following: bevacizumab, cisplatin, folinate, irinotecan, oxaliplatin, carboplatin, cetuximab | No/My5-FU | 33 | Yes risk (time of assessment unclear, toxicity grades inconsistent) | No | No; no | No; no | Plasma level and toxicity (positive) |
| Etienne, 1994, France151 | Prospective cohort study | H&N (neo-adjuvant) | PF | Yes (NR)/HPLC | 68 (94) | Yes counts (haematological and mucositis grades III and IV only) | No | No; no | No; no | AUC and toxicity (positive) |
| Milano, 1994, France152 | Prospective case series | H&N | PF | Yes (Santini, 1989132)/HPLC | 186 | Yes counts (haematological and digestive grade II–IV only) | Yes (144/186, 77%) | Yes (33 months); yes | No; no | AUC and response/survival (positive) |
| Thyss, 1986, France153 | Prospective case series | Advanced H&N (no metastasis) | PF | No/HPLC | 29 (63) | Yes counts (total toxicities reported only) | Yes (24/25, 96%) | No; no | No; no | AUC and toxicity (positive), response (neutral) |
| Kim, 2000, Japan154 | Prospective case series (Phase II study) | Advanced gastric cancer | PF | No Liquid Chromatography | 36 | Yes risk | Yes (17/36, 47.2%) | Yes (8 months); no | No; no | Plasma level and response (neutral) |
Summary of single-arm studies
The single-arm studies included 11 PK131,133,134,136,138,141,144,145,147,151,152 and 13 BSA studies. 130,135,137,139,140,142,143,146,148–150,153,154 Study population (e.g. cancer stage) and treatment regimen varied considerably within the different cancer type studies. Considerable variation was identified for instance for the infusion time of 5-FU among CRC patients. The 11 PK studies used three different dose adjustment algorithms, while four studies141,144,145,151 did not specify the algorithm used. The majority of studies130,131,133–140,142,143,146–149,151–154 used HPLC as the method to determine 5-FU plasma concentration (n = 20).
Only four studies141,144,145,150 (in addition to Kline et al. 155 – see CRC comparative studies in Overview of comparative studies) were identified that used the My5-FU assay, of which one was an extended abstract150 and two were meeting abstracts144,145 confirming that, to date, My5-FU has not been investigated extensively.
Outcomes reported
Generally, the results confirm that higher levels of plasma 5-FU are related to improved outcome in terms of response, PFS and OS irrespective of the 5-FU regimen used. Furthermore, they appear to suggest that unfortunately the positive relationship between exposure to 5-FU and outcomes is stronger for AEs/toxicity than for response and survival. AEs were generally reported well; however, they were reported as risk (of experiencing at least one event) in 15 studies130,131,134,136,137,140,142,144–150,154 and as counts of events in seven studies. 133,138,139,143,151–153 Furthermore, studies that used different grading tools to grade severity of toxicity [WHO and National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) grouped grades of toxicity differently; some reported toxicity only for broad categories (i.e. haematological and digestive), others reported toxicities only as a total of all toxic events. Response rates varied hugely and median OSs from PK and BSA studies overlapped. Survival data in the form of Kaplan-Meier curves was only reported in three CRC studies130,134,138 and one H&N cancer study. 152
Quality concerns
Overall, the evidence from the single-arm studies is weak. Study conclusions were mainly based on small study populations. The majority of studies,130,131,133–135,137–141,143,147–150,152–154 were case series (18/24), which are generally of lower quality because selection bias cannot be assessed.
Evidence taken forward to the analyses and cost-effectiveness modelling
The emphasis here is in evidence that compares PFS, OS and toxic events for PK versus BSA treatments; these are crucial for an estimate of clinical effectiveness and for informing an economic model. Due to the heterogeneity among the studies, even within cancer types the studies do not lend themselves to pooling. Although 11 studies131,133,134,136,138,141,144,145,147,151,152 were identified that carried out dose adjustment, a comparison with the studies resembling the BSA arm is impossible due to the substantial differences in patient populations, treatment regimens and outcomes assessment (ways in which outcomes were graded, grouped and reported). Therefore, conclusions on the effectiveness of PK dosing cannot be inferred from the single-arm studies because of the lack of comparative evidence of PK versus BSA for any of the cancers. Survival data were reported for an obsolete H&N cancer treatment152 and for three CRC studies. 130,134,138 It was unclear whether the two studies by Gamelin et al. 130,138 used different populations, therefore only the later one was considered. Inferring Kaplan–Meier curves from single-reported medians would result in endless possibilities of different curves. Furthermore, it would be problematic to infer a curve from single-arm studies in the absence of complementary evidence from a comparative arm. Due to the clearly pronounced heterogeneity in reported medians in survival and the lack of CIs meant that no attempt was made at pooling values from single-arm studies. As a result, the usable evidence from this section comes from the two CRC studies which reported survival data in the form of Kaplan–Meier curves. 134,138 These supplemented the comparative CRC studies drawn from the following section on comparative studies and are considered further in Single-arm colorectal cancer studies taken forward in cost-effectiveness analysis. 134,138
Overview of comparative studies
Major features colorectal cancer studies
Three comparative CRC studies were identified. 118,134,155 These were published by Gamelin et al. ,118 Capitain et al. 119 and Kline et al. ;155 they were disparate with regard to design, population, treatments and reported outcomes. The major features of these studies are summarised in Table 11. Full data extraction forms are available from the authors on request.
| First author, date, country | Study design | Cancer type | Regimen | PK method | n | AEs | Response rate | OS | PFS |
|---|---|---|---|---|---|---|---|---|---|
| Gamelin, 2008, France118 | RCT | mCRC | FUFOL | HPLC | 104 PK; 104 BSA | Yes | Yes | Yes | No |
| Capitain, 2012, France119 | Retrospective with historical control | mCRC | FOLFOX6 | HPLC | 118 PK; 39 BSA | Yes | Yes | Yesa | Yesa |
| Kline, 2013, USA155 | Retrospective with two self-selected groups | Stage II/III CRC | FOLFOX6 or FOLFIRI | My5-FU | 19 PK; 16 BSA | Yes | No | No | Yes |
| Kline, 2013, USA155 | Retrospective with two self-selected groups | Stage IV CRC | FOLFOX6 or FOLFIRI | My5-FU | 19 PK; 30 BSA | Yes | No | No | Yes |
Summary of comparative colorectal cancer studies
Of three comparative studies identified, only Gamelin et al. 118 was a randomised trial, the other two119,155 were retrospective studies in which population balance between arms was reasonable on the variables reported. Capitain et al. 119 and Gamelin et al. 118 studied only mCRC patients while Kline et al. 155 included CRC patients of stages II–IV. The studies used different treatment regimens and different algorithms for 5-FU dose adjustment. None of the studies reported a complete set of outcomes in terms of AEs, survival and response.
Outcomes reported
Kaplan–Meier plots were reported variably by Kline et al. 155 for PFS; by Capitain et al. 119 for PFS and OS, but only for the PK arm; and by Gamelin et al. 118 for OS only. Response was reported by two of the three studies and AEs were reported as risks of experiencing at least one event.
Quality concerns
The only RCT118 did not report methods of randomisation and had perfectly balanced arms. In the other two studies,119,155 the absence of randomisation means that true comparability between groups is inevitably compromised. There is a further problem when patients are invited to self-select into PK or BSA dosing. See Appendix 11 for the Downs and Black123 quality assessment.
Evidence taken forward to the analyses and cost-effectiveness modelling
All three comparative CRC studies had useable information about PFS, OS and AEs/toxicity for the comparison of PK versus BSA treatment and are considered further in Comparative colorectal cancer studies taken forward in cost-effectiveness analysis.
Major features of head and neck studies
Two comparative H&N studies were identified. These were published by Fety et al. 156,158 and Santini et al. 132 These studies were disparate with regard to design, population, treatments and reported outcomes. The major features of these studies are summarised in Table 12. Full data extraction forms are available on request from the authors.
| First author, date, country | Study design | Cancer type | Regimen | PK method | n | AEs | Response rate | OS | PFS |
|---|---|---|---|---|---|---|---|---|---|
| Fety, 1998, France156 | Randomised prospective study | Advanced H&N cancer | PF (continuous 96 hours) | HPLC | 61 BSA; 61 PK | Yesa | Yes | No | No |
| Santini, 1989, France132 | Several (group 1, retrospective study; group 2, prospective study) | H&N cancer | PF (5 days 1000 mg/m2/24 hours) | HPLC | 89 group 1 (BSA); 81 group 2 (PK)b | Yesc | Yes | No | No |
Summary of comparative head and neck studies
Only Fety et al. 156 provided information in a randomised design, whereas Santini et al. 132 reported sequential cohorts of patients in whom dose modification was made based on 5-FU exposure. The studies used different treatment regimens and slightly different dose adjustment algorithms and did not report survival data.
Outcomes reported
Survival data for OS and PFS was not reported in either of the two studies. Information on response was provided and toxicity was reported as counts of toxic cycles.
Quality concerns
The only randomised evidence available for H&N cancer was hampered by mismatches between the description of methods undertaken and the reported results. Furthermore, since the patients with protocol violations were removed from the analysis and the induction therapy regimen used only two drugs, the generalisability to dose adjustment methods in current clinical practice remains questionable. See Appendix 11 for the Downs and Black123 quality assessment.
Evidence taken forward to the analyses and cost-effectiveness modelling
The studies by Santini et al. 132 and Fety et al. 156 date back to 1989 and 1998, respectively, and are the only comparative studies comparing BSA versus PK identified for H&N cancer. The two studies used regimens which are no longer in clinical use and did not provide estimates for OS and PFS. Fety et al. 156 provided some information on toxicity for the comparison of PK versus BSA dosing in H&N cancer patients and is therefore further considered in Comparative head and neck study taken forward in cost-effectiveness analysis. Further detail on the study by Santini et al. 132 is provided in Appendix 12.
Rationale for taking studies forward for synthesis and modelling of cost-effectiveness
For the assessment of the clinical effectiveness and cost-effectiveness of PK 5-FU dose adjustment, evidence is required for the comparison of BSA-based dosing and PK dosing in the same population (ideally randomised) receiving the same 5-FU regimen. The outcomes from this comparison that are needed for cost-effectiveness modelling are ideally: (1) IPD to produce Kaplan–Meier curves for OS and PFS to infer transition probabilities for model parameters; and (2) AEs reported as counts per unit time for PK versus BSA treatments. IPD is preferred because it gives the most reliable estimates of clinical effectiveness to inform an economic model.
Most included studies had a single-arm design (n = 24). They mainly reported data about PK adjustment and resulting plasma 5-FU levels, with inferences about DPD activity levels, and occasionally association between plasma 5-FU and incidence of AEs or toxicity. They could not provide between-group comparisons. The disparities between the single-arm studies in treatments, populations and modes of outcome reporting, precluded synthesis by combining studies. Key outcomes (OS and PFS) were rarely reported and AEs were reported inconsistently or not at all. Two single-arm studies115,138 provided survival data in the form of Kaplan–Meier curves; these are described below and have been included in the synthesis of evidence for CRC treatment (see Clinical effectiveness synthesis: an overview of pharmacokinetic- versus body surface area-based regimens to inform the cost-effectiveness analysis and evidence for objective D).
Three CRC comparative studies had useable information about PFS, OS and toxic events for PK versus BSA treatments. Two H&N cancer comparative studies132,156 were found, these did not report OS or PFS. One156 reported usable information on toxic events. Both H&N cancer studies employed chemotherapies no longer current in use. No comparative evidence was identified on gastric and pancreatic cancer patients.
In summary, the broad search strategy yielded a small volume of studies and these were of disappointingly weak study design; studies mostly failed to report outcomes important for estimating the clinical effectiveness or cost-effectiveness of PK versus BSA.
In the following section the two single-arm CRC studies (Capitain et al. 134 and Gamelin et al. 138), the three CRC comparative studies (Gamelin et al. ,118 Capitain et al. 119 and Kline et al. 155) and the one H&N comparative study (Fety et al. 156) are taken forward for synthesis are described in more detail.
Single-arm colorectal cancer studies taken forward in cost-effectiveness analysis
The two single-arm CRC studies134,138 are first described in terms of study design and quality, population, intervention and outcomes.
Study design and quality
Capitain et al. 134 carried out a case series of 76 patients treated with 5-FU for advanced CRC and most had not received previous 5-FU treatment. The study included two regimens weekly or every 2 weeks of folinic acid and FUFOL. PK dose adjustment was based on plasma 5-FU measurements determined by HPLC and dose adjustment followed Gamelin et al. ’s130 dose algorithm. The median follow-up was 3.5 years. The study aimed to determine simple genetic factors that may aid the tailoring of 5-FU administration in first-line chemotherapy of advanced CRC.
The study was a case series where it was impossible to assess whether or not the study population was representative of the population from which the participants were recruited. Information on recruitment was minimal. There were weaknesses in the clarity and presentation of data. OS and PFS were reported including a Kaplan–Meier plot for OS, but without numbers-at-risk tables. Although the study reported AEs as risks they were not reported separately for the two different regimens included (see table 4 of the publication). 134 The study lacks information on plasma measurements, frequency of dose adjustment and outcomes of dose adjustment for the purpose of this review. See Appendix 11 for the Downs and Black123 quality assessment.
The study by Gamelin et al. 138 is a prospective case series involving 152 patients with mCRC from nine different centres. The median length of follow-up was 3 years. 5-FU therapy with individual dose adjustment was investigated in terms of efficacy, tolerance and survival in mCRC patients. The primary and secondary efficacy end points were survival and response rate respectively. PK dose adjustment was based on plasma 5-FU measurements determined by liquid chromatography and dose adjustment followed Gamelin et al. ’s130 dose algorithm.
The study was a case series where again it was impossible to assess whether or not the study population was representative of the population from which the participants were recruited. Patients lost to follow-up were not accounted for in the analysis which was not stated to follow an intention-to-treat (ITT) analysis. The AE rates were reported by cycles (counts); however, the number of total cycles is unknown for the 3-month period. Response rates, OS and PFS were reported extensively, including duration of response and Kaplan-Meier curves but no numbers-at-risk tables. See Appendix 11 for the Downs and Black123 quality assessment.
Population
The reported demographic characteristics of patients are summarised in Table 13.
| Item | Capitain et al.,134 n (%) | Gamelin et al.,138 n (%) |
|---|---|---|
| Patient number | ||
| Total number | 76 | 152 |
| Sample attrition | 0 | 117 patients assessable for toxicity and response |
| Age (years) | ||
| Mean (s.d.) | NR | 62 |
| Median | 71.2 | NR |
| Range | 39–88 | 24–75 |
| Sex | ||
| Men | 46 (60.5) | 84 (55.3) |
| Women | 30 (39.5) | 68 (44.7) |
| Performance status | ||
| 0–1 | 71 (93.5) | 95 (62.5) |
| 2–3 | 5 (6.5) | 57 (37.5) |
| Previous 5-FU therapy | 13 (17) | 30 (19.7) |
| Metastatic sites | ||
| Liver | NR | 101 (66) |
| Lung | NR | 35 (23) |
| Lymph nodes | NR | 18 (12) |
| Others | NR | 47 (31) |
Intervention
The two studies investigated dose adjustment of different 5-FU treatment regimens which are detailed in Table 14.
| Item | Capitain et al.134 | Gamelin et al.138 | |
|---|---|---|---|
| Regimen | FUFOL 4 hours | Modified de Gramont86,87 | FUFOL weekly |
| 5-FU | 1200 mg/m2 weekly by 4-hour 5-FU continuous infusion | 2500 mg/m2 2-weekly by 46-hour continuous infusion | 1300 mg/m2 by 8-hour continuous infusion |
| FA | 100-mg/m2 bolus FA | 200-mg/m2 bolus FA with 400 mg/m2 5-FU bolus | 200 mg/m2 before and 4 hours after 5-FU infusion |
| Oxaliplatin/irinotecan | Treatment until progression then considering second-line therapy combining 5-FU with oxaliplatin or irinotecan | Treatment until progression then considering second-line therapy combining 5-FU with oxaliplatin or irinotecan | NA |
All patients outside the target plasma concentration of 2000–3000 µg/l138 or an AUC of 25 mg × hour/l134 received dose adjustment following a previously published algorithm130 (see Table 6). Patients with grade II toxicity received a dose reduction by 100 mg/m2,138 or by 10%. 134 In patients with grade III toxicity, treatment was interrupted and once toxicities were resolved, restarted with the dose reduced by 250 mg/m2,138 or by 25%. 134 Treatment was stopped for patients with grade IV toxicities.
Outcomes
Capitain et al. 134 reported that 9 out of the 76 patients were at high risk of 5-FU toxicity due to abnormally low 5-FU plasma clearance. Of these, three had known DPD polymorphisms. The objective response rate was 32.9%, with 6.6% of patients having CRs. Objective response rate was defined according to the Response Evaluation Criteria in Solid Tumours (RECIST) group. The median OS (Figure 8) and PFS were 20 months and 3.3 months respectively. AEs were reported as risks of having at least one toxic event. The most common side effects (all grades) were diarrhoea (22%), hand and foot syndrome (18%) and mucositis (7.5%). A total of 10.5% of all toxicities were grade III and IV, toxicities occurred in 10.5% of patients and nine patients were identified to be at high risk of toxicity due to low clearance of 5-FU. Certain genotypes were linked to toxicity and shorter OS. The authors presented genetic factors which warrant investigation in future clinical trials to determine patients at risk of 5-FU toxicity or resistance before treatment commences.
In Gamelin et al. 138 the mean 5-FU dose after 3 months of treatment was 1803 ± 386 (950–3695) mg/m2. After cycle 1 only 6 (4%) patients had 5-FU measures in the target range. Under- and overdosing occurred in 124 (82%) and 14 (9%) patients respectively. After dose adjustment the 5-FU target range was reached in 143 (94.1%) patients. OS for all patients was 19 months and PFS was 11 months (Figure 9). Overall response rate in patients with measurable disease was 66/117 (56.4%) of which 18 (15.4%) had CR (as according to RECIST). Duration of response from the start of treatment to the time of disease progression was 17 months for CR and 20 months for PR (range 1–36 months). The number of cycles required to reach optimal therapeutic levels was significantly associated with level of response (for CR + PR vs. SD + PD, p = 0.05; and for CR + PR + SD vs. PD, p = 0.029). The majority of toxic events (all grades) were diarrhoea (39%) and hand and foot syndrome (30%), which were reported as counts of events.
Conclusions from single-arm colorectal cancer studies taken forward in cost-effectiveness analysis
Capitain et al. 134 used dose adjustment to reach a pre-specified target AUC using a previously published algorithm for dose adjustment. In this study, the two regimens of treatment using PK monitoring were well tolerated in an elderly population with a median age of 71 years. Although the study reported AEs as cases, they were not reported separately for the two different regimens included. However, the focus of the study was the identification of single genetic factors predictive for toxicity and effectiveness and therefore linked genetic traits to response. The study lacks information on plasma measurements, frequency of dose adjustment and outcomes of dose adjustment for the purpose of this review.
Gamelin et al. 138 in his study used dose adjustment to reach a pre-specified target plasma concentration of 5-FU using a previously published algorithm for dose adjustment. The regimen of treatment using PK monitoring was well tolerated in a population with a mean age of 62 years. The study concluded that individual 5-FU dose adjustment with PK monitoring provided a high survival rate with good response and tolerance.
Comparative colorectal studies taken forward in cost-effectiveness analysis
The three comparative CRC studies (Gamelin et al. ,118 Capitain et al. 119 and Kline et al. 155) are first described in terms of study design and quality, population, intervention/comparator; then according to outcome results described in sequence according to importance (i.e. OS, PFS, AEs, dose adjustment and algorithms employed, and other minor outcomes reported). See Appendix 11 for the Downs and Black123 quality assessment of Gamelin et al. ,118 Capitain et al. 119 and Kline et al. 155
Study design and quality
Gamelin et al. 118 was a Phase III multicentre RCT which randomised 104 patients to a PK-adjusted FUFOL regimen and 104 patients to a BSA-based dose regimen. There were five centres all located in France. PK adjustment was achieved using HPLC-determined plasma 5-FU estimates coupled with a published dose adjustment algorithm. Median follow-up was 3 years. The pre-specified primary outcome was response rate according to RECIST criteria. Methods of randomisation and allocation concealment were not reported. There was no mention of stratification (e.g. according to performance status) or of the use of minimisation methods, yet arms were perfectly balanced for number of patients. Blinding to treatment was not possible; assessment of response rates was assessed by a panel of two independent radiologists and may have been blinded, but this was not specified. There was some mismatch between the description of methods undertaken and the reported results.
Capitain et al. 119 was a retrospective ‘proof-of-concept study’ comparing 118 patients who received a PK-directed dose-adjusted FOLFOX6 regimen with 39 patients who received a conventional BSA-directed FOLFOX6 regimen. PK adjustment was by HPLC-determined plasma 5-FU estimates coupled with a commercial adjustment protocol. The intervention patients came from eight centres and the comparator patients from two further and different centres. Median follow-up for PK patients was 1426 days (3.9 years; range 2.2–8.3 years) but was unreported for the BSA arm. The most important weakness in this study was that although the sampling frame for selecting the study populations was described, the proportion of eligible patients that was finally included was not reported; thus, the selection method was unclear. To what extent the study was prospective was unclear.
Kline et al. 155 was a small retrospective single-centre analysis of stage II/III and of stage IV CRC patients who self-selected for PK-adjusted (n = 38) or BSA-based (n = 46) FOLFOX6 or FOFIRI regimens. The numbers of patients receiving FOLFOX6 or FOFIRI were not reported. Median follow-up was 14 months (BSA) and 17 months (PK) for stage IV patients, and 23 months (BSA) and 16 months (PK) for stage II/III patients. Lack of randomisation was the major limitation. The allocation of treatments by patient self-selection increases the likelihood of allocation bias.
Populations
The reported demographic characteristics of patients are summarised in Table 15.
| Item | Gamelin et al.118 | Capitain et al.119 | Kline et al.155 | |||||
|---|---|---|---|---|---|---|---|---|
| Stage II/III | Stage IV | |||||||
| BSA | PK | BSA | PK | BSA | PK | BSA | PK | |
| Patients, n | 104 | 104 | 39 | 118 | 16 | 19 | 30 | 19 |
| Disease stage, % | ||||||||
| II | NR | NR | NR | NR | 19 | 5 | 0 | 0 |
| III | NR | NR | NR | NR | 81 | 95 | 0 | 0 |
| IV | NR | NR | NR | NR | 0 | 0 | 100 | 100 |
| Age (years) | ||||||||
| Mean (s.d.) | 71.2 (10.3) | 71.5 (9.5) | NR | NR | NR | NR | NR | NR |
| Median | NR | NR | 63 | 65 | 66 | 56 | 65 | 58 |
| Range | 50–85 | 52–84 | 32–80 | 35–81 | 19–77 | 32–78 | 46–76 | 41–81 |
| Sex, % | ||||||||
| Men | 62.5 | 58.7 | 62 | 59 | 68.7 | 52.6 | 60 | 63.1 |
| Women | 37.5 | 41.3 | 38 | 41 | 31.3 | 47.4 | 40 | 36.9 |
| Performance status, % | ||||||||
| 0 | 55 | 54 | NR | NR | NR | NR | NR | NR |
| 0 or 1 | 77 | 78 | NR | NR | NR | NR | ||
| 1 | 40 | 33 | NR | NR | NR | NR | NR | NR |
| 2 or 3 | 5 | 13 | 23 | 22 | NR | NR | NR | NR |
| Previous therapy, % | 15.4 | 10.6 | NR | NR | 88a | 95a | 27a | 37a |
| Metastatic sites, % | ||||||||
| Liver | 74b | 81b | 60c | 56c | NR | NR | NR | NR |
| Lung | 30b | 26b | 10c | 16c | NR | NR | NR | NR |
| Lymph nodes | 11b | 19b | 4.9 | 5.0 | NR | NR | NR | NR |
| Others | 9b | 15b | NR | NR | NR | NR | NR | NR |
| Different metastatic sites/patient, % | ||||||||
| 1 | 77 | 68 | 71 | 68 | NR | NR | 53 | 42 |
| ≥ 2 | NR | NR | NR | NR | NR | NR | 40 | 47 |
| 2 | 21 | 24 | 25 | 24 | NR | NR | NR | NR |
| 3 | 1 | 6 | 6 | 5 | NR | NR | NR | NR |
| 4 | 0 | 2 | NR | NR | NR | NR | NR | NR |
| Primary tumour site, % | ||||||||
| Colon | NR | NR | NR | NR | 75 | 90 | 53 | 79 |
| Rectosigmoid | NR | NR | NR | NR | 12.5 | 0 | 7 | 0 |
| Rectum | NR | NR | NR | NR | 12.5 | 10 | 33 | 21 |
Intervention/comparator
All three studies118,119,155 compared BSA-based dosing with PK dose adjustment based on steady state plasma 5-FU levels. Different treatment regimens were used (FUFOL, FOLFOX6, FOLFIRI); these are summarised in Table 16, which indicates the dose of 5-FU at the first cycle.
| Item | Gamelin et al.118 | Capitain et al.119 | Kline et al.155 |
|---|---|---|---|
| Regimen | FUFOL weekly | FOLFOX6 every 2 weeks | mFOLFOX6 or mFOLFIRI every 2 weeks |
| 5-FU | 5-FU dose of 1500 mg/m2 by 8-hour infusion | 5-FU 2500 mg/m2 by 46-hour infusion | 5-FU 2400 mg/m2 by infusion |
| FA | 200 mg/m2 up to a total weekly dose of 400 mg/m2 | 200-mg/m2 bolus with 10-minute push 400-mg/m2 5-FU | Details NR |
| Platin irinotecan | NA | Oxaliplatin 85 mg/m2 2-hour infusion every 2 weeks | Details NR |
Kline et al. 155 measured steady state plasma 5-FU with My5-FU, the other studies used HPLC. At the start of BSA-based therapy the same dose is applied for all patients, dose change only occurs when necessitated by toxicity and dose increases are not undertaken. Only Gamelin et al. 118 provided details of the PK dose adjustment algorithm; this is summarised in Table 17.
| In the absence of toxicity | In the presence of toxicity | ||
|---|---|---|---|
| FU plasma concentration (µg/l) | AUC (mg × hour/l) | FU dose adjustment (± % of previous dose) | |
| < 500 | < 4 | 70 | Grade II toxicity: dose decreased by 200 mg Grade III toxicity: 1-week break, then dose decreased by 300 mg |
| 500–1000 | 4 to < 8 | 50 | |
| 1000–1200 | 8 to < 10 | 40 | |
| 1200–1500 | 10 to < 12 | 3 | |
| 1500–1800 | 12 to < 15 | 20 | |
| 1800–2200 | 15 to < 18 | 10 | |
| 2200–2500 | 18 to < 20 | 5 | |
| 2500–3000 | 20 to < 24 | Unchanged | |
| 3000–3500 | 24 to < 28 | –5 | |
| 3500–3700 | 28 to < 31 | –10 | |
| > 3700 | > 31 | –15 | |
In the PK arm, Capitain et al. 119 adjusted dose according to an unreported commercial protocol; it is unclear if adjustments were guided by factors additional to plasma 5-FU. Kline et al. 155 used an algorithm supplied by the My5-FU manufacturer but details were not reported. In the PK arms both dose increases and decreases were implemented.
Outcomes: overall survival
Gamelin et al. 118 reported Kaplan–Meier analysis of OS; the plots appeared unusual implying that information for time of death was aggregated at spaced time intervals. The reconstructed Kaplan–Meier estimate using the method of Guyot et al. 125 is shown in Figure 10 and closely overlaps the published figure (available on request from authors).
Reported median survival was 16 and 22 months for BSA and PK arms, respectively, and the log-rank test for equivalence was p = 0.08. The median estimates appear sensitive to the long horizontal steps that occur at around 0.5 survival; it appears the 22 month estimate reported may be an overestimate.
Weibull, log-normal and log-logistic distributions provided satisfactory models for both arms (see Appendix 13). Cox proportional hazards regression for the reconstructed data provided a HR of 0.82618 (95% CI 0.6198087 to 1.101265). The log-rank test for equivalence provided a p-value of 0.18. A Weibull model assuming proportional hazards generated a HR of 0.829255.
Capitain et al. 119 reported 28 and 22 months median OS for PK and BSA arms respectively. No CIs were reported. A Kaplan–Meier plot for only the PK arm was published. The reconstructed plot (using the Guyot et al. 125 method) is shown in Figure 11 and closely overlaps the published figure (available on request from authors).
To investigate the reported OS difference between arms Weibull distributions were used with assumed proportional hazards (i.e. the PK shape parameter was retained for both arms); the median for the BSA arm was used to estimate the BSA scale parameter.
The resulting curves are shown in Figure 12(a). The HR for these was 0.586 (shape parameter 1.6691, scale parameters 0.002333 and 0.0039833 for PK and BSA respectively). The log-normal distribution also provided a good fit to the reconstructed PK OS data and was used to derive a log-normal estimate of BSA survival that satisfied the reported median of 22 months. Since the mu (µ) parameter defines the median, the PK sigma (σ) parameter was retained for the BSA arm and a new mu parameter found for BSA [here survival is given by: 1 – ɸ(ln(t) – µ)/σ; and median = exp(µ)]. The resulting curves are shown in Figure 12(b); this also shows the resulting HR which with this distribution is non-proportional between treatments. Table 18 summarises the model parameters and median and mean survival for the models.
FIGURE 12.
Overall survival modelled on Weibull and log-normal distributions (data from Capitain et al. 119).
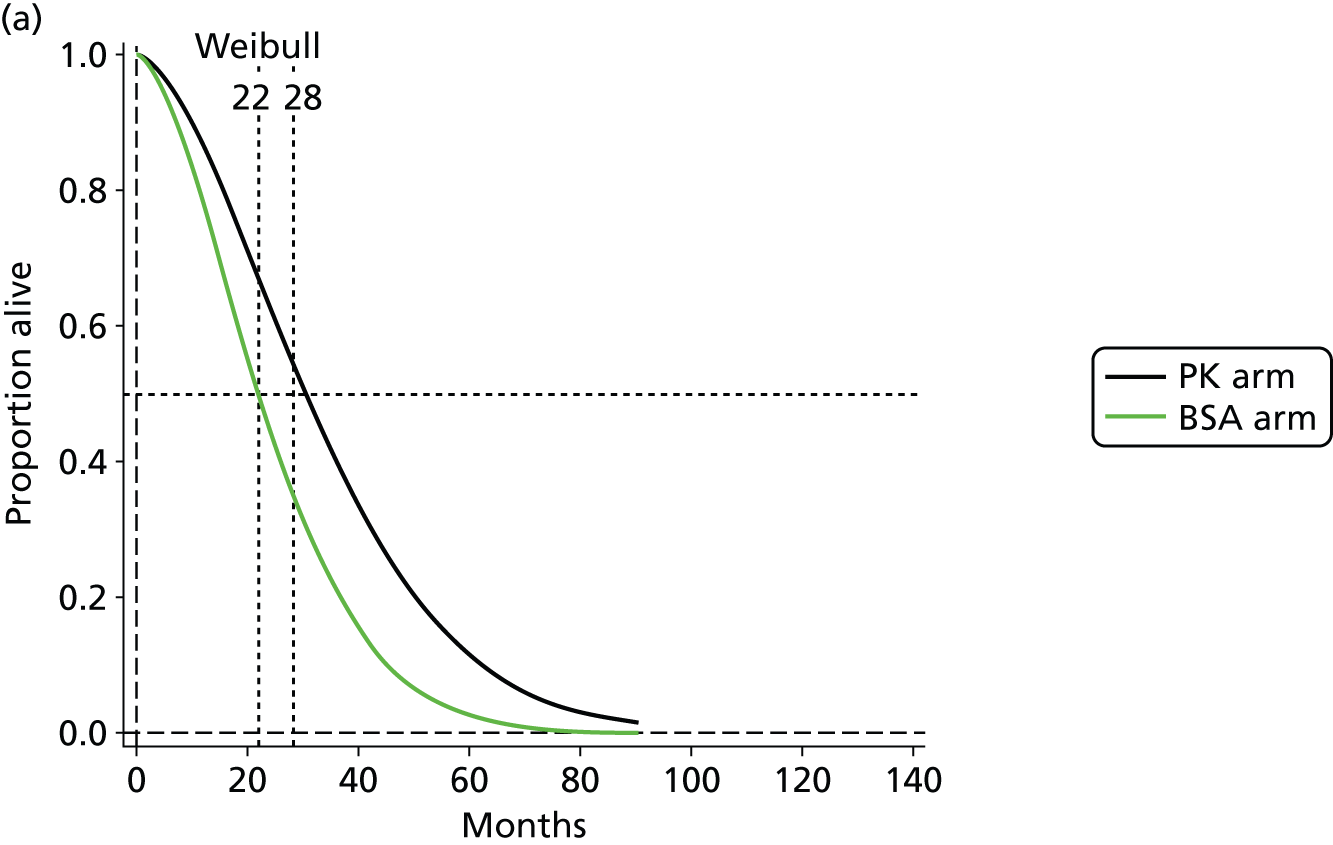
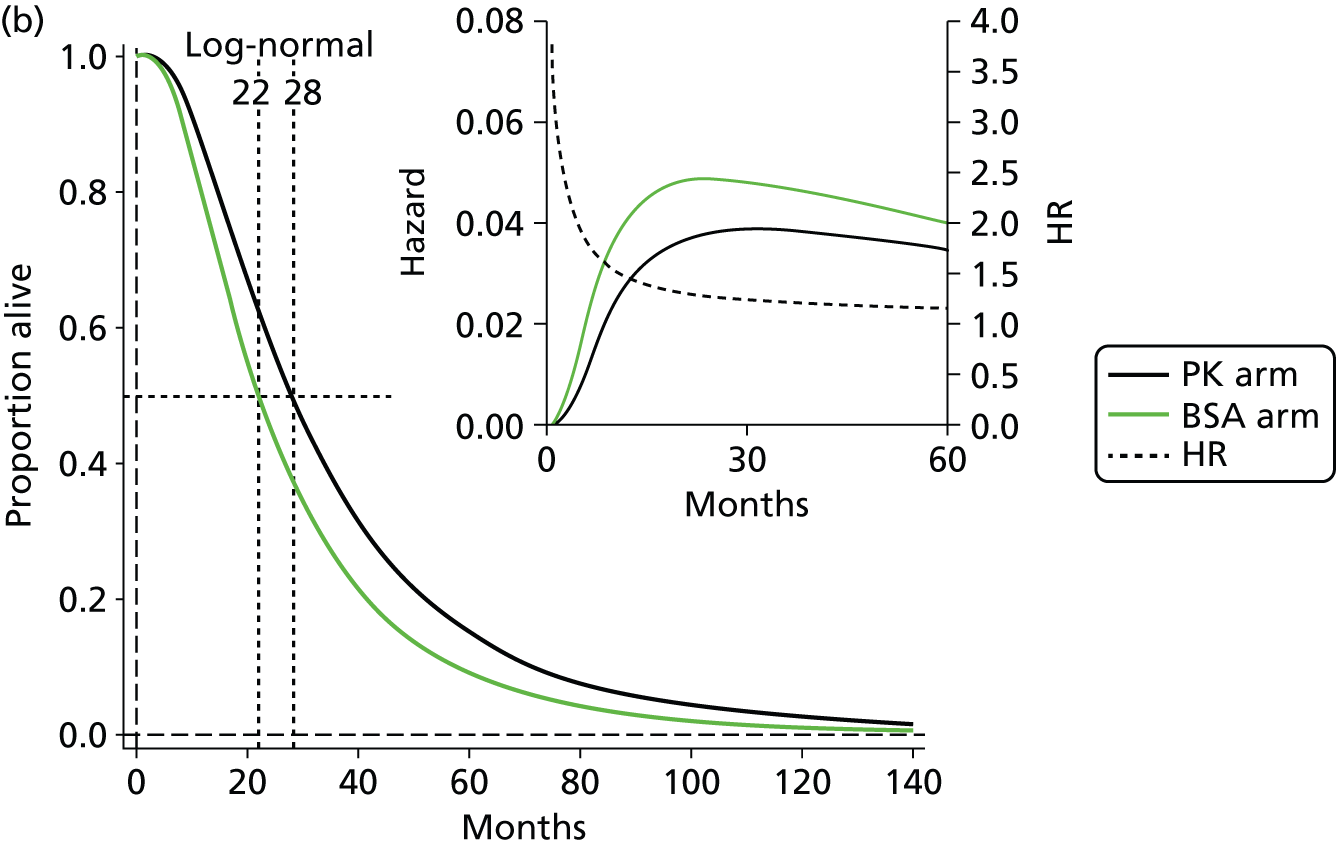
| Model | Lamda | Gamma | Median | Mean | Difference in means |
|---|---|---|---|---|---|
| Weibull PK | 0.0023328 | 1.669058 | 30.31 | 33.73 | |
| Weibull BSA | 0.00398331 | 1.669058 | 22 | 24.48 | 9.25 |
| Mu | Sigma | ||||
| Log-normal PK | 3.320461 | 0.745078 | 27.67 | 36.53 | 7.49 |
| Log-normal BSA | 3.091042 | 0.745078 | 22 | 27.7 |
The study by Kline et al. 155 did not report OS.
Outcomes: progression-free survival
Although the methods section in Gamelin et al. ’s study118 stated that PFS was analysed, no medians or Kaplan–Meier plots were presented. Requests to authors for IPD for PFS failed to illicit any data.
Values were reported for mean time spent in response categories (CR, PR, SD; as defined according to RECIST) and are summarised in Table 19. They infer a benefit from PK for PFS (i.e. the mean time for each response category prior to progression was greater for the PK arm than the BSA arm and involved a larger proportion of patients).
| Treatment response | Mean duration (months) | Number of patients | ||
|---|---|---|---|---|
| BSA arm | PK arm | BSA arm | PK arm | |
| CR | NRa | 10 | 1 | 6 |
| PR | 6.3 | 6.8 | 17 | 29 |
| SD | 5.7 | 7.6 | 30 | 26 |
If it assumed (scenario A) that each patient proceeds from each response category directly to the progressed state then the mean time to progression is 6.0 and 7.5 months in the BSA and PK arms respectively (we assume means, rather than medians were reported because the durations have reasonably normal distributions). An alternative (scenario B) may assume that if patients in each category proceed to the next category (e.g. CR to PR and then to SD) before reaching the progressive state and the mean durations are as reported, then mean time to progression is 8.27 and 12.48 months in the BSA and PK arms respectively.
Given a mean time to progression under the assumption of normal distribution of duration times for each response type, it is possible to calculate Weibull parameters for a parametric model of time to progression using the following relationship:
where λ and γ are scale and shape parameters, respectively, and Γ represents a gamma distribution. Using the above, and given the mean, there are many solutions for shape and scale parameters unless one is fixed. In order to obtain a single solution, the further assumption is required that the shape parameter for PFS is the same as that for OS (these values were 1.827686 for the PK arm and 1.54066 for the BSA arm). With these assumptions the Weibull parameters for PFS in the PK and BSA arms under scenarios A and B are as shown in Table 20.
| Treatment arm | A | B | ||
|---|---|---|---|---|
| λ | γ | λ | γ | |
| PK arm | 0.020467 | 1.82769 | 0.00798430047223383 | 1.82769 |
| BSA arm | 0.05378 | 1.54066 | 0.032798314 | 1.54066 |
Similarly for a log-normal distribution description of PFS the relationship between mean and median may be used: mean = median × exp([σ2]/2). This has several solutions unless either median or σ is fixed. In order to obtain a single solution the further assumption was made that the σ parameter was the same as that for OS for the corresponding study arm (these values were 0.648108 for the PK arm and 0.6944542 for the BSA arm). Median relates to µ parameter according to median = exp[(σ × (normsinv(0.5))) + (µ)]. Thus, the µ parameter for PFS can also be obtained. With these assumptions the log-normal parameters for PFS in the PK and BSA arms under scenarios A and B are as shown in Table 21. Figure 13 summarises the resulting models of PFS.
| Treatment arm | Scenario A | Scenario B | ||
|---|---|---|---|---|
| µ | σ | µ | σ | |
| PK arm | 1.799533 | 0.648108 | 2.314524667 | 0.648108 |
| BSA arm | 1.550626 | 0.6944542 | 1.871602091 | 0.6944542 |
FIGURE 13.
Progression-free survival for PK and BSA arms on Weibull (upper) and log-normal (lower) derived parameters under scenario A (figures a, c, e and g) and scenario B (figures b, d, f and h) (Gamelin et al. 118).

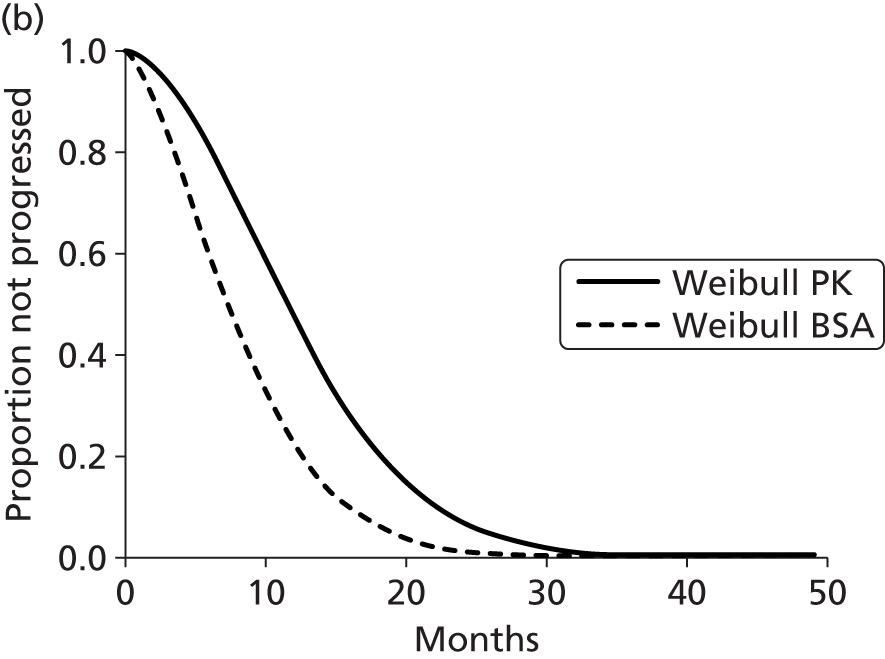
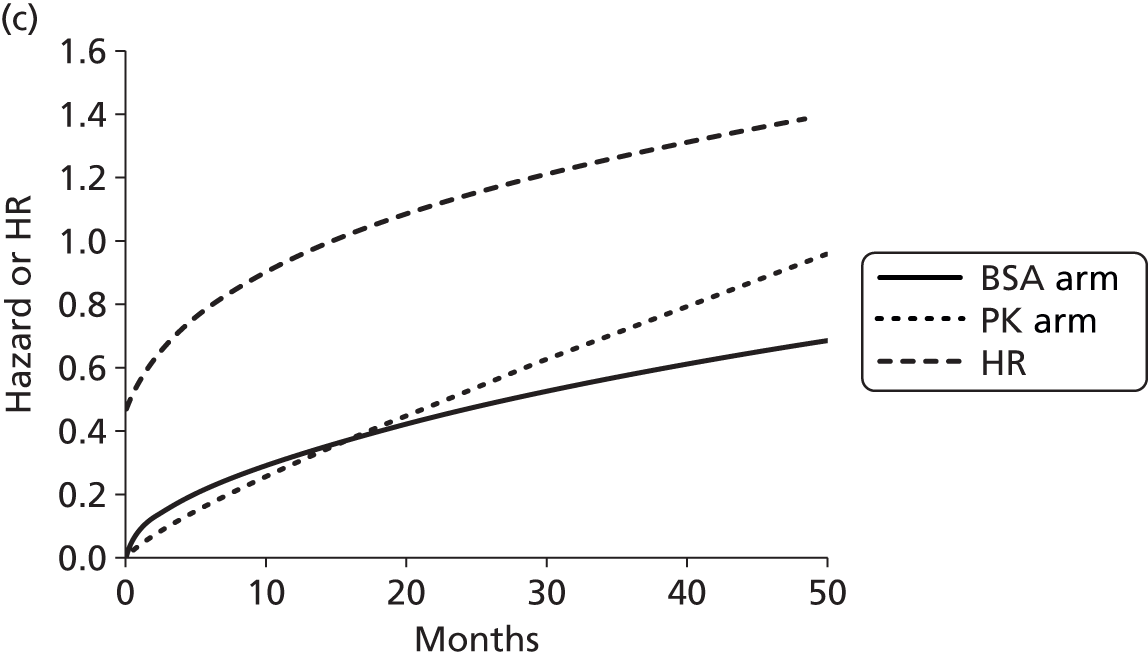

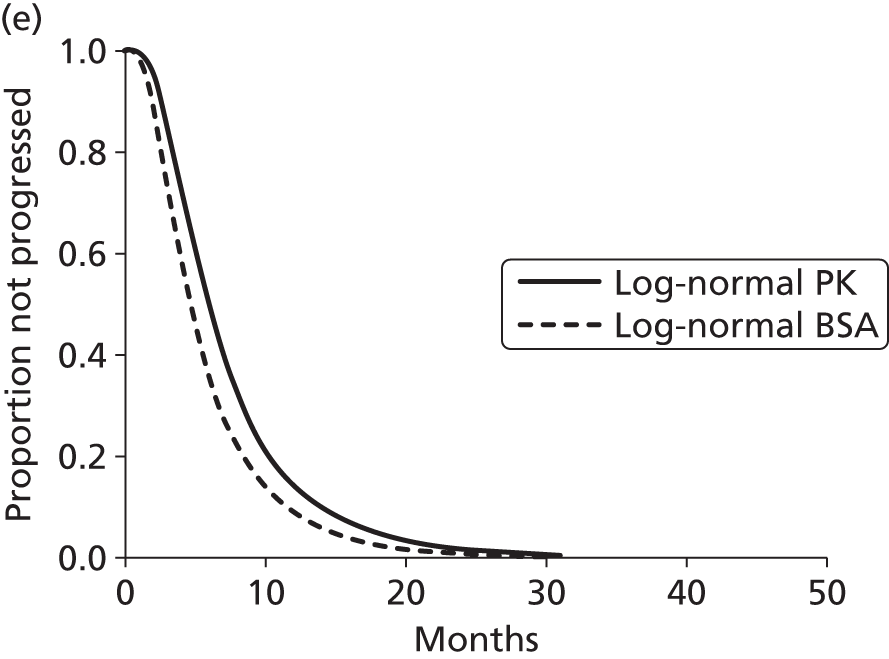

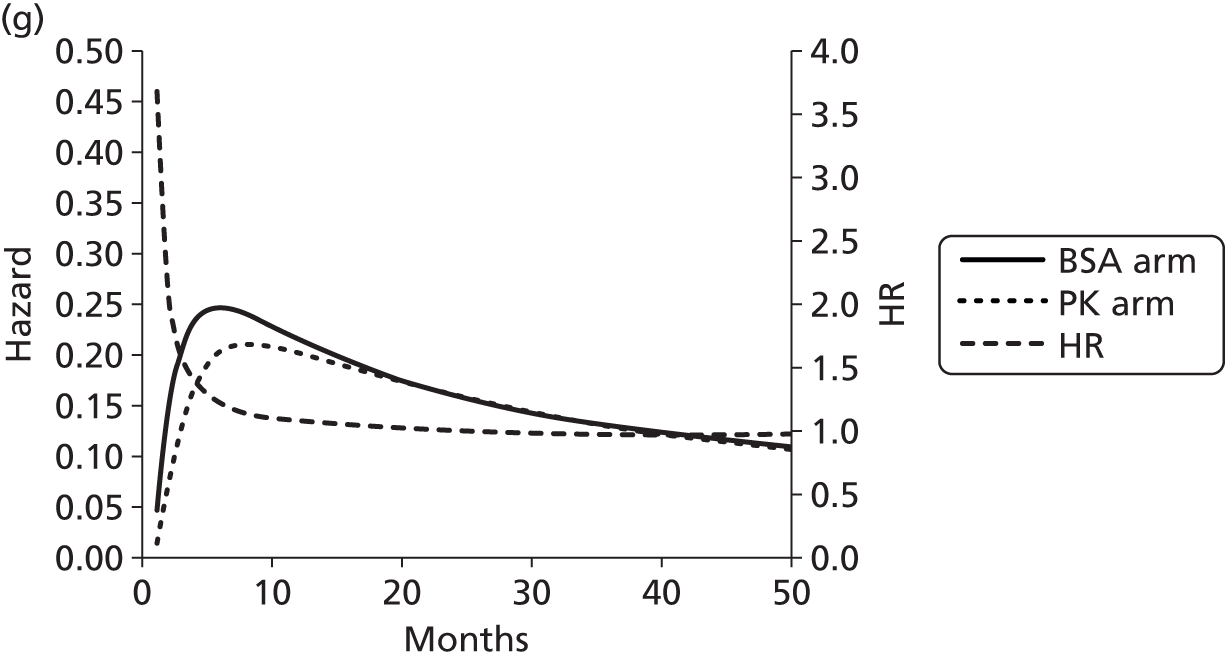
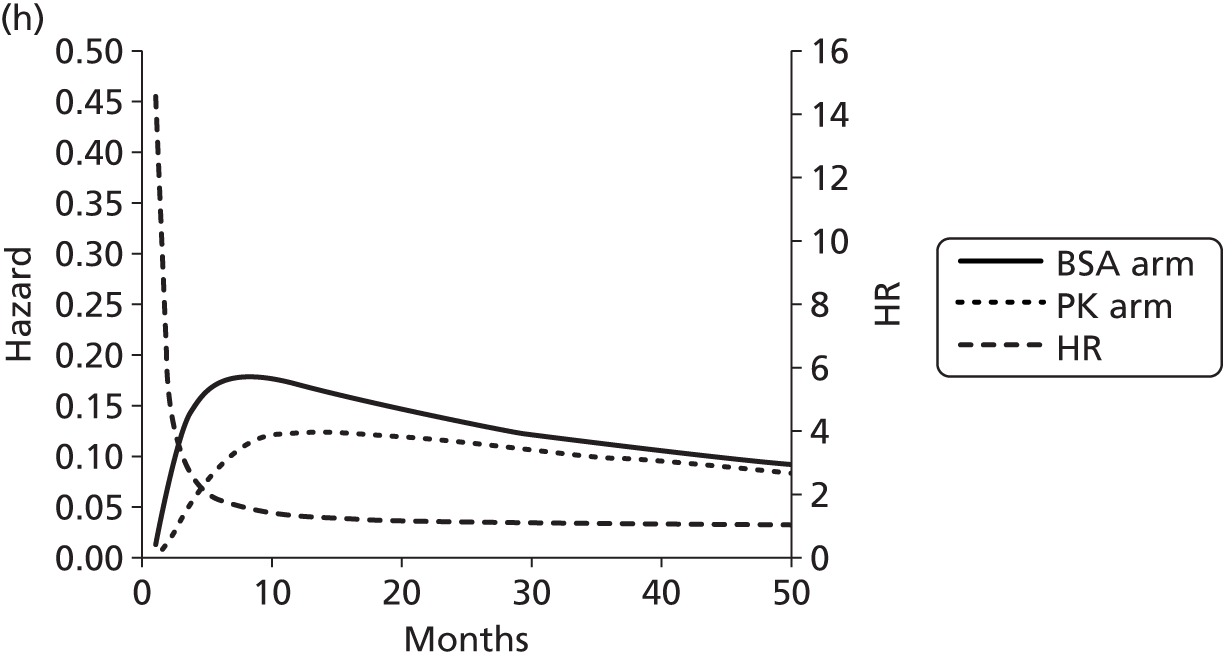
Capitain et al. 119 reported median PFS of 16 months and 10 months in PK and BSA arms respectively. CIs were not reported. A Kaplan–Meier plot was provided for only the PK arm. The reconstructed PK Kaplan–Meier (using the Guyot et al. 125 method) is shown in Figure 14 together with that for OS for the PK arm. Both plots closely overlap the published figures (available from authors on request).
Median PFS from the reconstructed PK arm plot was 16.0 (95% CI 12.0 to 20.0) months close to the reported value. Weibull, log-normal and log-logistic distributions provided moderately well-fitting models to reconstructed data (see Appendix 14). To obtain an estimate of PFS for the BSA arm the reported median of 10 months (based on 39 BSA arm patients) was used with the same procedure as described above for OS. For the Weibull model, proportional hazard was assumed (HR 0.4817). Table 22 summarises the model parameters for these models.
| Model | Lambda | Gamma | Median | Mean | Difference in means |
|---|---|---|---|---|---|
| Weibull PK | 0.0243758 | 1.136683 | 19.01 | 25.06 | |
| Weibull BSA | 0.050599 | 1.136683 | 10 | 13.18 | 11.88 |
| Mu | Sigma | ||||
| Log-normal PK | 2.878827 | 1.244953 | 17.79 | 38.62 | |
| Log-normal BSA | 2.302588 | 1.244953 | 10 | 21.70 | 16.92 |
| p-value | Gamma | ||||
| Log-logistic PK | 0.057355 | 0.638179 | 17.44 | 38.53 | |
| Log-logistic BSA | 0.1 | 0.638179 | 10 | 22.10 | 16.43 |
It is noticeable that the mean predicted with the log-normal and log-logistic models exceeds the means for OS shown in Table 22. This is probably due to the influence of the flatter part of the PFS Kaplan–Meier plot beyond 15 months. Figure 15 illustrates the modelled PFS. As log-normal and log-logistic models generate PFS means greater than OS means for the PK arm, they appear to be less appropriate than the Weibull. The availability of medians only for the BSA arm limits the reliability of the analysis.
FIGURE 15.
Progression-free survival modelled on Weibull and log-normal distributions (Capitain et al. 119).
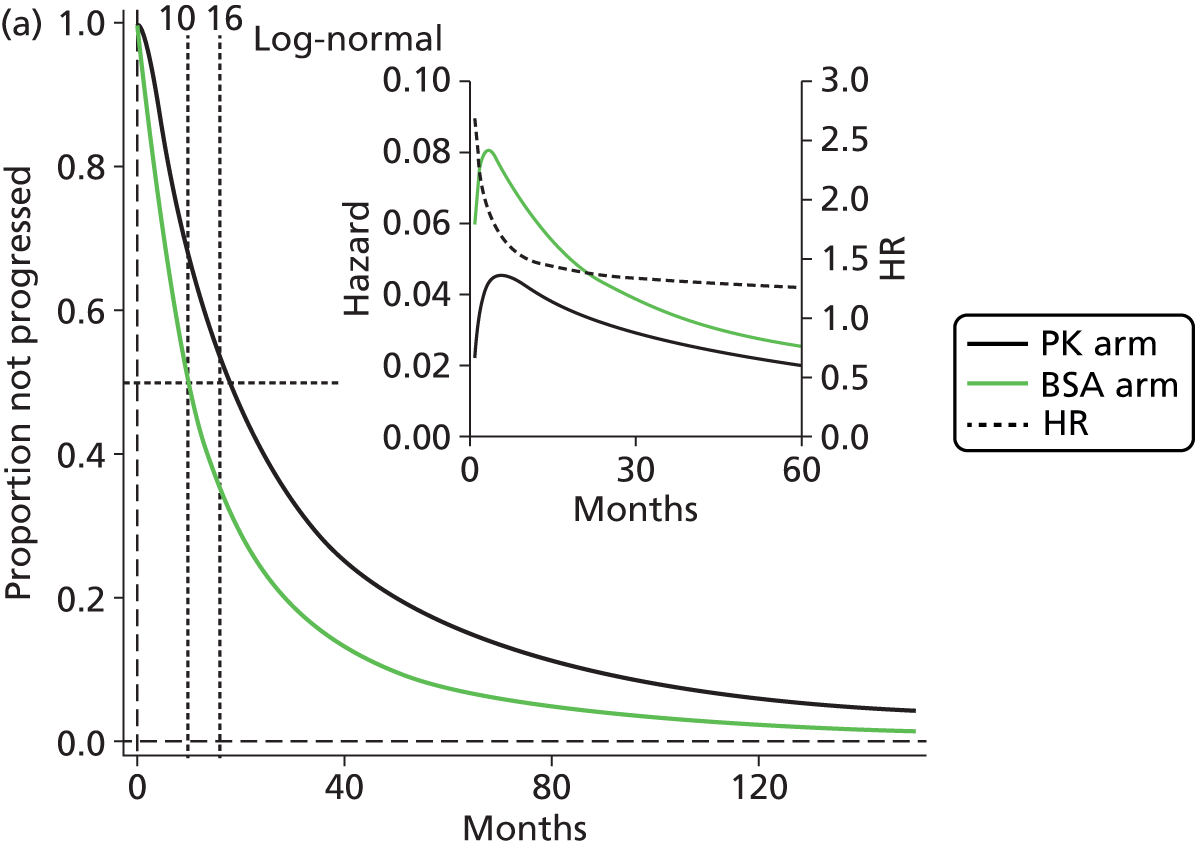
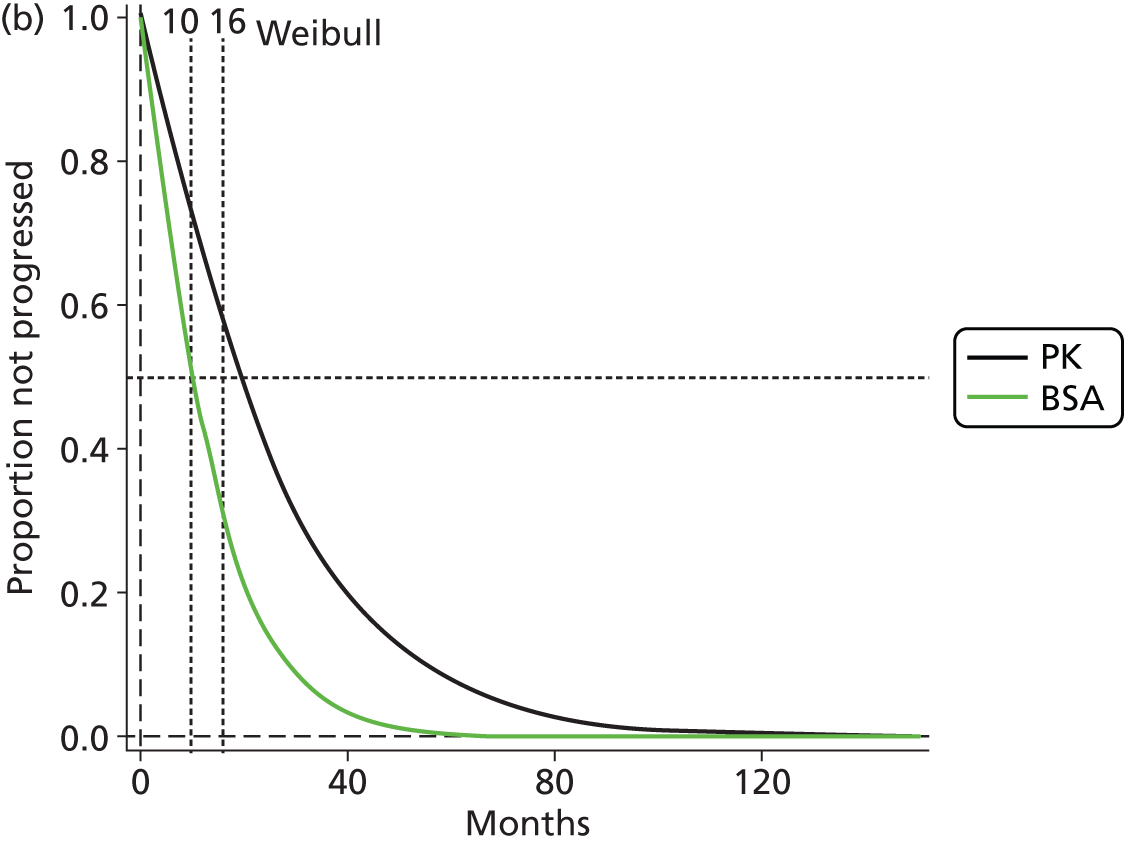
Kline et al. 155 presented Kaplan–Meier analyses of PFS for stage II/III and stage IV patients. Reconstructed plots with the method of Guyot et al. 125 were discrepant from the published graphs; therefore censoring (tick) and event data were extracted from the plots and used to generate the illustrative graph shown in Figure 16.
FIGURE 16.
Illustrative graph of PFS. (a) stage II/III (NPK = 16, NBSA = 19); and (b) stage IV (NPK = 19, NBSA = 30) (Klein et al. 155). NBSA, number of BSA regimen patients; NPK, number of PK regimen patients.

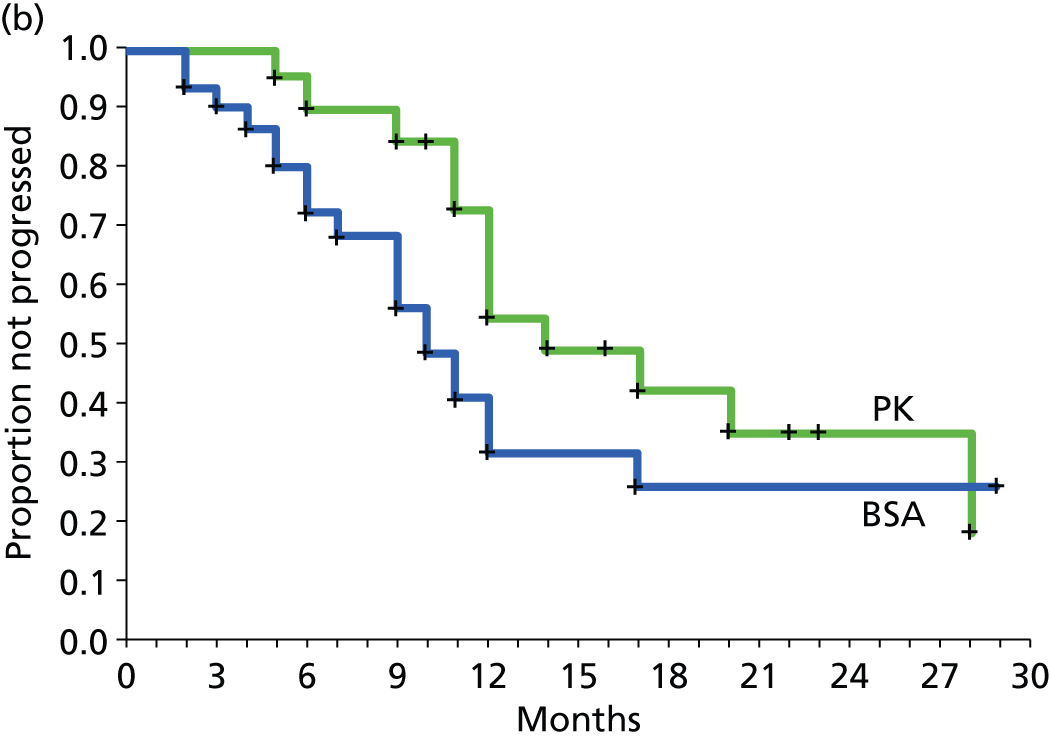
For stage IV the test for equivalence of BSA versus PK (log-rank test) yielded p = 0.16. Median PFS was reported to be 14 months and 10 months for PK and BSA groups, respectively, with median follow-up of 22 months and 20 months in BSA and PK groups respectively.
For stage II/III patients the reported log-rank test for equivalence yielded p = 0.0429, suggesting delayed progression for the PK group.
Outcomes: adverse events
Gamelin et al. 118 reported the percentage of patients that experienced six categories of AE categorised according to four WHO grades of severity. Results for diarrhoea, mucositis, hand and foot syndrome, leucopenia, cardiac toxicity and conjunctivitis at 3 months and at end of treatment showed little difference; it is therefore assumed the data represents the risk of a patient experiencing the event at least once. Patient risk of diarrhoea, of hand and foot syndrome and of conjunctivitis was higher than for the other AEs; the results in each arm at end of treatment are summarised in Table 23.
| AE | WHO grade | BSA (n = 96) | PK (n = 90) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Events, n | % | LCI, % | UCI, % | Events, n | % | LCI, % | UCI, % | ||
| Diarrhoea | IV | 3 | 3.1 | 0.6 | 8.9 | 0 | 0.0 | 0.0 | 4.0 |
| III | 14 | 14.6 | 8.2 | 23.3 | 4 | 4.4 | 1.2 | 11.0 | |
| II | 25 | 26.0 | 17.6 | 36.0 | 3 | 3.3 | 0.7 | 9.4 | |
| I | 13 | 13.5 | 7.4 | 22.0 | 9 | 10.0 | 4.7 | 18.1 | |
| Hand and foot syndrome | IV | 0 | 0.0 | 0.0 | 3.8 | 1 | 1.1 | 0.0 | 6.0 |
| III | 6 | 6.3 | 2.3 | 13.1 | 10 | 11.1 | 5.5 | 19.5 | |
| II | 20 | 20.8 | 13.2 | 30.3 | 20 | 22.2 | 14.1 | 32.2 | |
| I | 15 | 15.6 | 9.0 | 24.5 | 29 | 32.2 | 22.8 | 42.9 | |
| Conjunctivitis | IV | 0 | 0.0 | 0.0 | 3.8 | 0 | 0.0 | 0.0 | 4.0 |
| III | 0 | 0.0 | 0.0 | 3.8 | 0 | 0.0 | 0.0 | 4.0 | |
| II | 2 | 2.1 | 0.3 | 7.3 | 5 | 5.6 | 1.8 | 12.5 | |
| I | 20 | 20.8 | 13.2 | 30.3 | 10 | 11.1 | 5.5 | 19.5 | |
| Cardiac toxicity | IV | 0 | 0.0 | 0.0 | 3.8 | 0 | 0.0 | 0.0 | 4.0 |
| III | 1 | 1.0 | 0.0 | 5.7 | 1 | 1.1 | 0.0 | 6.0 | |
| II | 0 | 0.0 | 0.0 | 3.8 | 1 | 1.1 | 0.0 | 6.0 | |
| I | 0 | 0.0 | 0.0 | 3.8 | 1 | 1.1 | 0.0 | 6.0 | |
| Mucositis | IV | 2 | 2.1 | 0.3 | 7.3 | 2 | 2.2 | 0.3 | 7.8 |
| III | 1 | 1.0 | 0.0 | 5.7 | 1 | 1.1 | 0.0 | 6.0 | |
| II | 1 | 1.0 | 0.0 | 5.7 | 1 | 1.1 | 0.0 | 6.0 | |
| I | 1 | 1.0 | 0.0 | 5.7 | 1 | 1.1 | 0.0 | 6.0 | |
| Leucopenia | IV | 1 | 1.0 | 0.0 | 5.7 | 0 | 0.0 | 0.0 | 4.0 |
| III | 1 | 1.0 | 0.0 | 5.7 | 0 | 0.0 | 0.0 | 4.0 | |
| II | 2 | 2.1 | 0.3 | 7.3 | 0 | 0.0 | 0.0 | 4.0 | |
| I | 0 | 0.0 | 0.0 | 3.8 | 0 | 0.0 | 0.0 | 4.0 | |
The relative risk (PK vs. BSA) of AEs is summarised in Figure 17. The number of events was sufficiently small for cardiac toxicity and mucositis that any differences between treatments could be attributed to chance. For diarrhoea, and for leucopenia to a lesser extent, the PK regimen appeared to benefit patients. For hand and foot syndrome and for conjunctivitis risk was somewhat greater for patients receiving the PK regimen.
Capitain et al. 119 reported only four types of AE which fell within the National Cancer Institute’s (NCI’s) Common Terminology Criteria scale categories III or IV. 160 Surprisingly, hand and foot syndrome was not included. The results are summarised in Table 24.
| AE | Grade | BSA (n = 39) | PK (n = 118) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Events, n | % | LCI, % | UCI, % | Events, n | % | LCI, % | UCI, % | ||
| Diarrhoea | III/IV | 5 | 12 | 4.30 | 24.22 | 2 | 1.7 | 0.53 | 5.99 |
| Mucositis | III/IV | 6 | 15 | 5.86 | 27.43 | 1 | 0.8 | 0.02 | 3.08 |
| Thrombocytopenia | III/IV | 4 | 10 | 2.87 | 20.87 | 14 | 12 | 7.29 | 19.10 |
| Neutropenia | III/IV | 10 | 25 | 13.04 | 39.33 | 18 | 12.07 | 25.91 | 19.5 |
The relative risk (PK vs. BSA) of grade III/IV AEs is summarised in Figure 18. It appears PK reduces risk of diarrhoea and mucositis and also neutropenia (although the latter not significantly).
The result for diarrhoea is similar to that reported in Gamelin et al. 118 where the relative risk for grade III/IV combined was 0.251 (95% CI 0.088 to 0.718). Unlike Capitain et al. ,119 Gamelin et al. 118 reported a similar risk of mucositis for each arm.
Kline et al. 155 evaluated toxicity graded according to the NCI CTCAE version 4.0 scale. Side effects which were considered grade III using the NCI CTCAE scale or were deemed sufficiently serious by the physician to warrant a dose reduction were ‘designated as adverse effects’.
Among stage IV patients, 37% in both BSA and PK groups experienced grade III toxicity (Table 25). Among stage II/III patients grade III toxicity was more common among BSA patients than PK patients (69% vs. 32%; p = 0.0437 by Fisher’s exact test).
| Item | Stage IV CRC | Stage II/III CRC | ||
|---|---|---|---|---|
| BSA method | PK monitoring | BSA method | PK monitoring | |
| Patients with grade III toxicity, total n (%) | 11 (37) | 7 (37) | 11 (69) | 6 (32) |
| Dose-limiting toxicity, n (%) | ||||
| Diarrhoea | 4 (13) | 4 (21) | 8 (50) | 3 (16) |
| Nausea | 2 | 0 | 1 | 0 |
| Vomiting | 1 | 0 | 2 | 0 |
| Dehydrationa | 3 | 0 | 3 | 0 |
| Hand and foot syndrome | 2 | 0 | 0 | 1 |
| Dehydrationa | 1 | 2 | 0 | 1 |
| Mouth sore | 2 | 1 | ||
| Dysphagia | 2 | 0 | ||
| Decreased appetite | 0 | 1 | ||
| Malnutrition | 1 | 0 | 1 | 0 |
| Weight loss | 1 | 0 | ||
| Fatigue | 2 | 0 | ||
| Weakness | 1 | 0 | 1 | 0 |
| Syncope | 1 | 0 | 2 (12) | 3 (16) |
| Neutropenia | 3 (10) | 2 (10) | 8 (50) | 3 (16) |
Among all 19 CRC stage II/III patients receiving a BSA regimen, three experienced diarrhoea, vomiting or nausea, side effects associated more with 5-FU than the other components of chemotherapy; 8 of all the 16 CRC stage II/III patients who received PK regimens experienced these side effects (p = 0.0652; Fisher’s exact test). Among stage II/III patients, grade III toxicity was more common among BSA patients than PK patients (69% vs. 32%; p = 0.0437 by Fisher’s exact test). The incidence of different types of dose-limiting toxicities for both groups is listed in Table 25 according to stage of disease.
The number of treatment doses received before adverse side effects were observed was greater for PK-dose-adjusted patients than for BSA group patients; this applied for both stage II/III and stage IV patients. The results are summarised in Figure 19.
FIGURE 19.
Number of doses received before adverse side effects were observed. The horizontal line represents the median. Numbers of patients were 10, 6, 11 and 7 (Klein et al. 155).

Outcomes: response rates
Gamelin et al. 118 defined response rates as the primary outcome measure. Response rates are summarised in Table 26.
| Response | Number with response | |
|---|---|---|
| BSA arm (n = 104) | PK arm (n = 104) | |
| CR | 1 | 6 |
| PR | 17 | 29 |
| SD | 30 | 26 |
| PD | 48 | 39 |
More patients in the PK arm than the BSA arm experienced CR and PR and fewer experienced progression. The relative risk according to response type is summarised in Figure 20.
In the study by Capitain et al. ,119 patients’ therapeutic response was assessed according to RECIST 1.1 criteria. Reported results are summarised in Table 27.
| Response type | BSA arm (n = 39) at 3 months, n (%) with responsea | PK arm (n = 118) | |
|---|---|---|---|
| At 3 months, n (%) with response | At 6 months, n (%) with response | ||
| CR | 1 (2.6) | 3 (2.5) | 23 (20.3) |
| PR | 17 (44) | 80 (67.2) | 40 (35.4) |
| Overall responseb | 18 (46.6) | 83 (69.7) | 63 (55.7) |
| Disease controlc | 30 (77) | 104 (88.1) | 87 (77.0) |
| PD | 9 (23.0) | 14 (11.9) | 26 (23.0) |
At 3 months response rates were superior for the PK group. The relative risk (PK vs. BSA) of different response categories is shown in Figure 21.
FIGURE 21.
Relative risk (PK vs. BSA) of response according response type (Capitain et al. 119). A relative risk > 1 indicates a favourable result for PK except for ‘progression’ where a relative risk < 1 indicates a favourable result for PK. RR, relative risk.
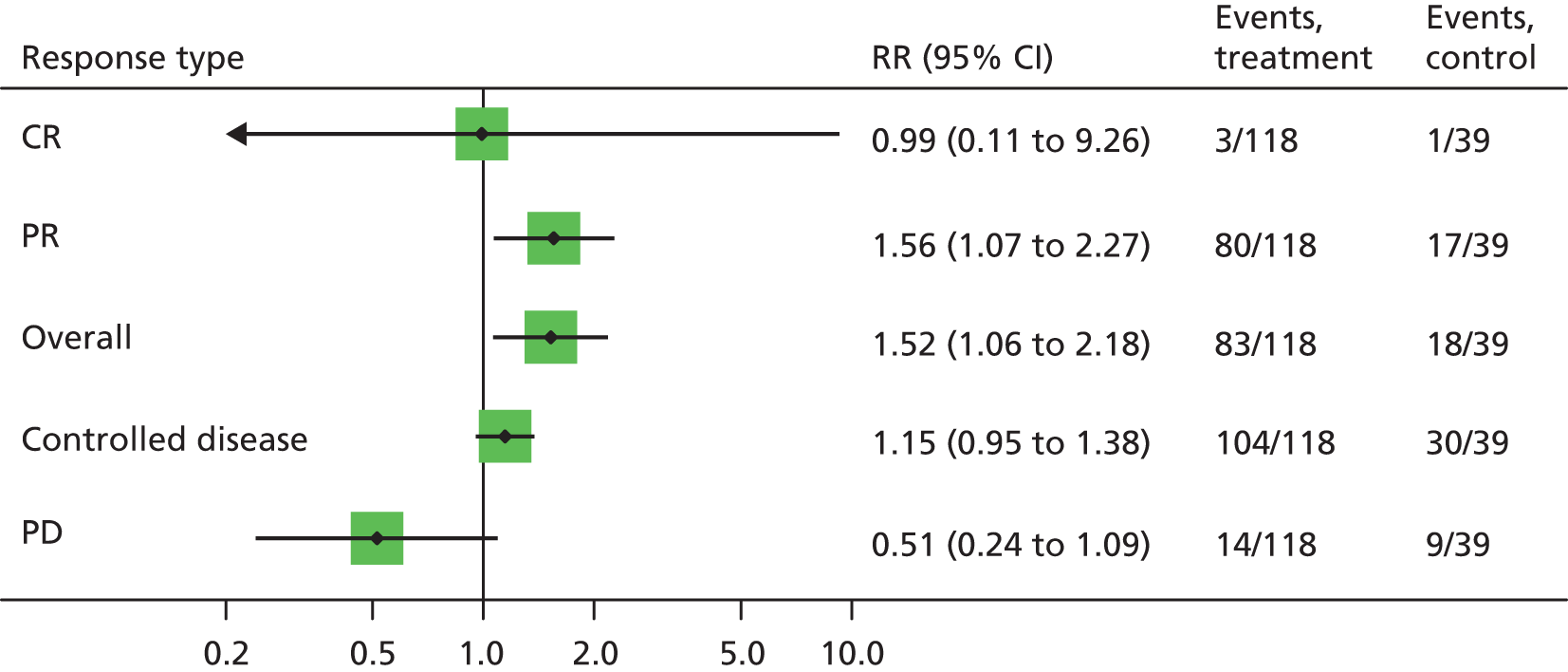
Response rates at 6 months for the PK arm, but not BSA arm, were also reported. These indicated that a CR was observed for 23 of 118 patients. At 3 and 6 months, 23% of both BSA and PK patients were classified as PD. This implies a difference of 3 months between arms when 77% remain un-progressed and this fits fairly well with the log-normal and Weibull models for PFS shown in Figure 15.
Kline et al. 155 did not report response rates.
Outcomes: dose adjustment and dose received
Gamelin et al. 118 reported that 94% of PK arm patients reached target range plasma 5-FU concentrations ( = 2500–3000 µg/l) in a mean of four treatment cycles. The dose received when in target range varied greatly between PK regimen patients (Figure 22). According to the algorithm (see Table 17), dose was adjusted in the PK arm if WHO grade II or III toxicities were experienced. Over the whole treatment period about 31% of PK arm patients had experienced grade II or III hand and foot syndrome (see Table 23). Consequently, it appears possible that an appreciable proportion of dose adjustments in the PK arm may not have been implemented in accordance with 5-FU AUC; this proportion was not reported.
FIGURE 22.
Weekly 5-FU dose received when in target range (PK group) and weekly dose received at 3 months (BSA-based regimen group); data read from graph and graph reconstructed (Gamelin et al. 118).
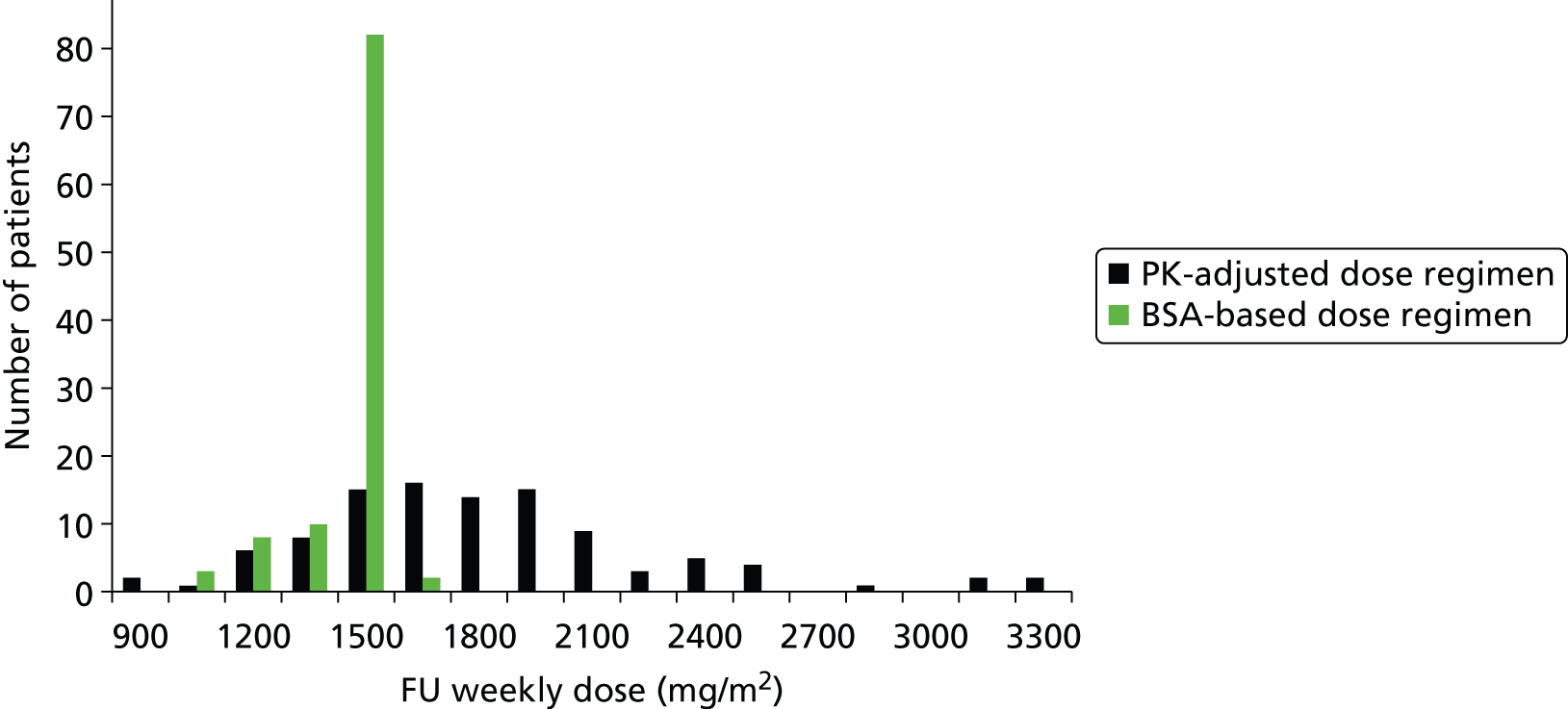
Most patients (≈ 85%) in the PK group had dose adjustment; the mean dose after 3 months of treatment was 1790 mg/m2 (range 765–3300 mg/m2). Only 4 of 49 BSA-based regimen patients whose plasma 5-FU concentration was measured were within target range.
Most PK patients had their dose increased to levels above the starting dose of 1500 mg/m2; indicating that without dose adjustment these patients’ steady state plasma 5-FU concentration was judged to have been less than that desirable for full effect on cancer cells. The implication of this is that in the BSA arm (where dose was retained at 1500 mg/m2 or was reduced because of toxicity), a substantial proportion of patients remained underdosed. According to the rationale of PK adjustment this might be expected to translate into reduced effectiveness in the BSA arm versus PK arm for outcomes such as PFS, OS and response rates. A smaller proportion of the PK group had dose reductions. Under the rational of PK adjustment this might be expected to translate into a more favourable toxicity profile for the PK arm than the BSA arm.
Gamelin et al. 118 reported that 5-FU plasma levels between 2.5 and 3 mg/l were correlated with grades I and II diarrhoea and grade I hand and foot syndrome. Grade III diarrhoea and hand and foot syndrome were reported to be associated with 5-FU plasma levels > 3 mg/l (3.5 mg/l and 3.9 mg/l, respectively; p = 0.02).
In the study by Capitain et al. 119 the PK group received a mean dose close to 2500 mg/m2 (mean 94.32% ± 13.7% of theoretical dose), by 3 months most were receiving an adjusted dose, and dose increases and reductions of 10% and 20% from 2500 mg/m2 were common. Thus, at 3 months, 56 of 118 patients received doses > 20% different from their starting dose. Dosage changes reported at 3 months for the PK group are summarised in Table 28. About 91% of PK patients required dose adjustment. About two-thirds of PK patients received dose increases and about 20% had their start dose reduced potentially translating into reduced toxicity compared with BSA patients.
| Dosage change from cycle 1 dosage | Number of patients | Mean % dose change (s.d.) | % range |
|---|---|---|---|
| > 10% increase | 75/118 | 20 (8) | 10–40 |
| > 20% increase | 42/118 | 26 (6) | 20–40 |
| > 10% decrease | 22/118 | 20 (9) | 10–40 |
| > 20% decrease | 14/118 | 26 (5.94) | 20–40 |
Within 3 months the 5-FU dose was decreased by 15% ± 4% (range 10–25%) due to grade III toxic adverse effects in 4 out of 39 patients in the BSA-based dosage group.
Kline et al. 155 did not report the proportion of patients that received dose adjustment. A graph was presented illustrating the distribution of doses at each successive cycle. The median dose (horizontal line) remained the same at 2400 mg/m2 across cycles irrespective of treatment regimen. Although BSA patients had dose reductions at increasing frequency with increasing cycles, in the PK group both dose increases and dose reductions were undertaken. Based on the published graph it appears that about 25–30% of 19 stage IV PK patients received dose increases by cycles 3 and 4. Some PK patients received dose reductions. Figure 23 summarises this for stage IV patients.
FIGURE 23.
Median dose and distribution of doses at successive cycles for stage IV patients. n = 30 and n = 19 for BSA and PK groups respectively (Klein et al. 155).

Figure 24 summarises the results for stage II/III patients. In the BSA group dose reductions and median dose decreased with increasing cycles. For the PK group the median dose remained unchanged and patients received dose increases and reductions in about equal proportions.
FIGURE 24.
Median dose and distribution of doses at successive cycles for stage II/III patients. n = 16 and n = 19 for BSA and PK groups respectively (Klein et al. 155).
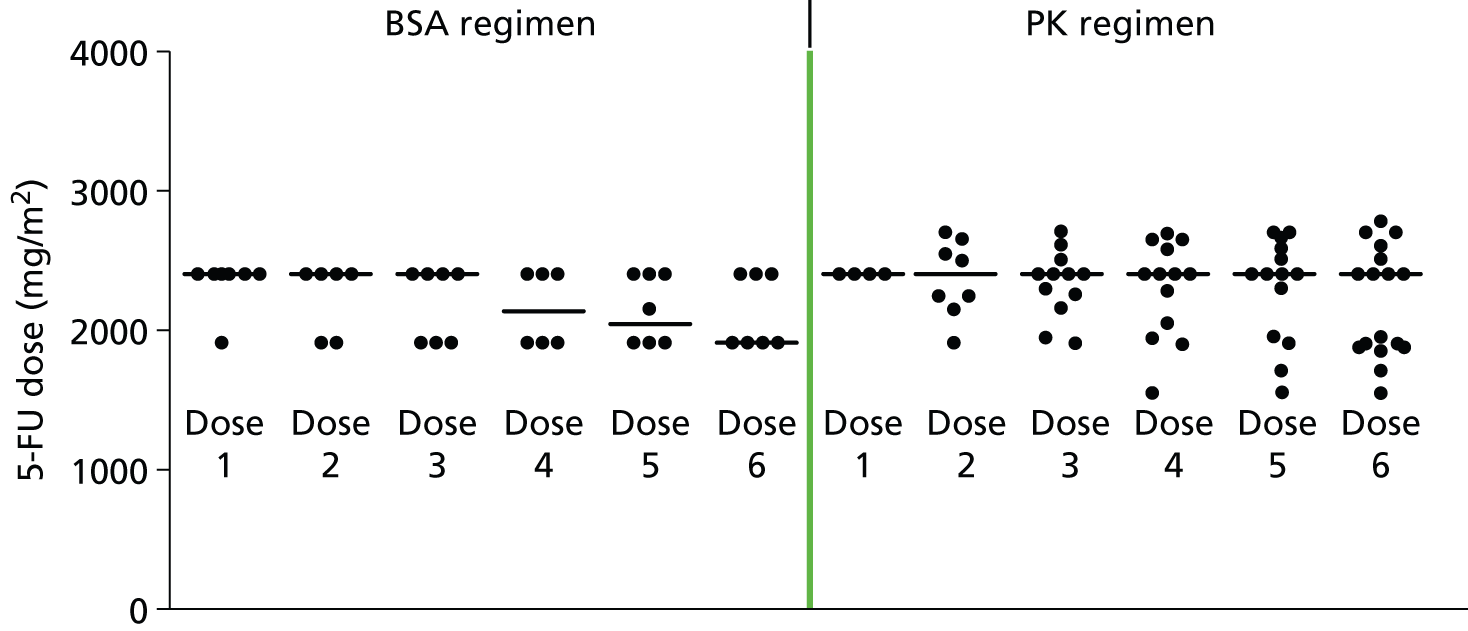
Outcomes: performance status
Gamelin et al. 118 reported the influence of treatment on patients’ performance status in terms of the percentage of patients whose status remained unchanged, worsened or improved relative to baseline. The results are summarised in Table 29. A few patients improved performance status, but for most performance status remained unchanged or worsened.
| Performance status | BSA arm, % | PK arm, % |
|---|---|---|
| Improved | 7.6 | 11.1 |
| Stable (no change) | 53.8 | 61.8 |
| Worsened | 37.6 | 26.9 |
Outcomes: plasma 5-fluorouracil clearance
Kline et al. 155 calculated 5-FU clearance by dividing the administered dose by the AUC measure of plasma 5-FU concentration (mg × hour/l). This estimate was interpreted as a measure of patients’ ability to metabolise 5-FU. The results indicated that clearance decreased as cycles of treatment accumulated, indicating a reducing ability to metabolise 5-FU. The results are summarised in Figure 25.
Outcomes: second-, third- and fourth-line treatments
Capitain et al. 119 was the only study that documented second-, third- and fourth-line treatments. The distribution of different types of post-first-line therapies was reported for the PK group only. These are summarised in Table 30. The proportion of patients that received second-, third- and fourth-line therapies gradually declined; the most common therapies used were FOLFIRI and targeted therapies.
| Type of chemotherapy | Line of therapy, n | All, n (%) | ||
|---|---|---|---|---|
| Second | Third | Fourth | ||
| FOLFIRI | 58 | 3 | 0 | 61 (51.47) |
| FOLFOX | 0 | 9 | 4 | 13 (11.0) |
| Targeted-therapy regimens | 27 | 29 | 6 | 62 (52.5) |
| i.v. 5-FU (FUFOLa or LV5FU2b regimen) | 8 | 2 | 4 | 14 (11.9) |
| Oral 5-FU prodrugs (capecitabine) | 6 | 5 | 4 | 15 (12.7) |
| Total number of treated patients, n (%) | 99 (83.9) | 48 (40.7) | 18 (15.3) | |
Conclusions from colorectal cancer comparative studies
Of the three studies identified, only Gamelin et al. 118 was a randomised trial, the other two119,155 were retrospective studies in which population balance between arms was reasonable on the variables reported. Nevertheless, in the absence of high-quality randomisation, true comparability between groups is inevitably compromised. There is a further problem when patients are invited to self-select into PK or BSA dosing, in that those who are either healthier or more unwell may self-select to undergo a new method of dose adjustment thus biasing the selection of the sample and reducing validity. It is interesting that in Kline et al. 155 the proportion of stage IV patients self-selecting for PK adjustment (19 of 49) was noticeably lower than among stage II/III patients (19 of 34).
Each study documented considerable interpatient variation in steady state plasma 5-FU concentration during continuous infusion. The use of dose adjustment algorithms based on these measures resulted in most patients in the PK arm requiring dose changes [in Capitain et al. 119 (91%) and Gamelin et al. 118 (85%)]; the proportion was not reported by Kline et al. ,155 but may have been less because of a wider target concentration range for steady state plasma 5-FU (algorithm unreported). In the studies by Capitain et al. 119 and Gamelin et al. 118 most patients required dose increases. This implied that in the comparator BSA group, patients would have remained underdosed according to the rationale for the PK intervention, which might result in less effective treatment for cancer cells in this group relative to the PK group, albeit potentially with fewer AEs.
Kaplan–Meier plots reported by Kline et al. 155 indicated that PK dose adjustment tended to delay disease progression (p = 0.16 and p = 0.043 for stage IV and stage II/III patients respectively). Capitain et al. 119 presented similar evidence, but only median time to progression was provided for the BSA group (medians were 16 months and 10 months for PK and comparator groups respectively). Gamelin et al. 118 did not present time to progression evidence; however, data on mean duration of CR and PR and of SD might possibly allow one to infer that PK dose adjustment prolonged the time to progression.
Gamelin et al. 118 reported Kaplan–Meier plots showing improvement in OS in the PK group relative to the BSA group (median survival 22 and 16 months respectively; p = 0.08). However, Capitain et al. 119 provided a Kaplan–Meier graph only for the PK group (median survival was 28 and 22 months for PK and comparator group respectively).
Capitain et al. 119 and Gamelin et al. 118 found that PK-based dose adjustment reduced the risk of grade III and grade IV diarrhoea [relative risk 0.013 (95% CI 0.03 to 0.65) and 0.251 (95% CI 0.088 to 0.718) respectively]. These studies indicated there may be reduced risk of mucositis and of neutropenia/leucopenia, but in Gamelin et al. 118 the risk of hand and foot syndrome increased. Kline et al. 155 reported that the risk of toxicities was not reduced with PK dose adjustment, but that on average toxicities were delayed and occurred later in the series of treatment cycles. It is difficult to identify reasons for these apparent differences. It would be important to report how AEs relate to plasma 5-FU concentrations. There was considerable dose variation in both arms, especially in the PK arm. However, in the PK arm the target plasma concentration was achieved in most patients for most cycles, it is therefore likely that the plasma 5-FU concentration was stable for these patients. For the BSA arms there was little reporting of plasma 5-FU levels.
Overall the evidence from the three studies118,119,155 tends to support the hypothesis of clinical benefit from PK adjustment; however, the evidence is not robust and is compromised by lack of randomisation in two of the studies. Furthermore, each study failed to present a complete set of comparative data for the major outcomes of clinical importance (i.e. PFS, OS and risk of treatment side effects), so that there appears to be appreciable risk of outcome reporting bias.
It should be emphasised that we failed to find any published randomised evidence about the effectiveness of PK-directed dose adjustment for any currently used 5-FU regimen for any cancer type.
Comparative head and neck study taken forward in cost-effectiveness analysis
The study by Fety et al. 156 is first described in terms of study design and quality, population, intervention and outcomes.
Study design and quality
This multicentre RCT156 (involving three centres) assigned 61 patients to a PK-adjusted 5-FU regimen and 61 patients to a standard-based dose regimen. PK dose adjustment was based on 5-FU measurements determined by HPLC and dose adjustment followed a modified algorithm by Santini et al. 132 Owing to 5-FU-related toxicity, three patients (5.2%) in the BSA arm and three patients in the PK arm (6.1%) left the study before completing chemotherapy. The length of follow-up was unclear. The primary end point was the incidence of haematological toxicity and the secondary end point was the equivalence of disease response.
Randomisation was stratified by centre (three centres were involved). Methods of allocation concealment were not reported. Blinding to treatment was not possible; assessment of response rates was assessed by a panel of two independent radiologists and may have been blinded, but this was not specified. There was some mismatch between the description of methods undertaken and the reported results. There were weaknesses in the clarity and presentation of data. It has been previously noted by other authors85 that the dose adjustment method in this study may have been too complicated, as the 12 protocol violations in the treatment arm (12/61 patients enrolled) were all related to 5-FU dose adjustment miscalculations. Furthermore, as the patients with protocol violations were removed from the analysis and the induction therapy regimen used only two drugs, the generalisability to dose adjustment methods in current clinical practice remains questionable. See Appendix 11 for the Downs and Black123 quality assessment checklist.
Population
The reported demographic characteristics are summarised in Table 31. Patients had advanced H&N cancer and most had not received previous chemotherapy.
| Item | Treatment arm | |
|---|---|---|
| BSA | PK | |
| Patient number, n/N (%) | ||
| Total number | 61/122 (50) | 61/122 (50) |
| Sample attrition/patients not evaluable | 4/61 (6.6) | 12/61 (19.7) |
| Age (years) | ||
| Mean (s.d.) | NR | NR |
| Median | 54 | 55 |
| Range | 29–72 | 36–69 |
| Sex, n/N (%) | ||
| Men | 52/57 (91.2) | 48/49 (98) |
| Women | 5/57 (8.8) | 1/49 (2) |
| Performance status, n/N (%) | ||
| 0 | 16/57 (28.1) | 11/49 (22.4) |
| 1 | 34/57 (59.6) | 35/49 (71.4) |
| 2 | 7/57 (12.3) | 3/49 (6.1) |
| 3 | 0 | 0 |
| 4 | 0 | 0 |
| Previous therapy, % | NR | NR |
| Metastatic sites, % | ||
| Liver | NA | NA |
| Lung | NA | NA |
| Lymph nodes | NA | NA |
| Others | NA | NA |
Intervention
Patients were assigned to receive three cycles of induction chemotherapy with cisplatin (100 mg/m2 on day 1) and 5-FU (96-hour continuous infusion), either at standard dose (BSA arm: 4 g/m2) or at a dose adjusted according to the 5-FU AUC (PK arm).
Outcomes
Quality of life (QoL), OS and PFS were not reported. AEs were reported per cycle (counts). Among the 122 patients randomly assigned to one of the two treatment arms, 16 patients (13%) were found to be ‘unevaluable’ for response and toxicity (four patients in the BSA arm, and 12 patients in the PK arm). Grade II and IV neutropenia and thrombopenia were reduced in the PK arm when compared with the BSA arm (7.6% vs. 17.5%; p = 0.013). Mucosity (grades II and IV) was only observed in the BSA arm (5.1%). There was no significant difference in the objective tumour response rate between both arms (77.2% in the BSA arm vs. 81.67% in the PK arm).
Conclusions from the head and neck study taken forward in cost-effectiveness analysis
The paper by Fety et al. 156 provides information in a randomised design on 5-FU dose adaptation according to PK parameters versus conventional dosing in patients with advanced H&N cancer. The overall 5-FU exposure in H&N cancer patients was significantly reduced in the dose adjustment arm compared with the fixed-dose arm. This resulted in reduced toxicity, but no improvement in clinical response. The impact on toxicity and efficacy suggests these patients might benefit from individual PK monitoring. The utility of monitoring 5-FU exposure to reduce toxicity was confirmed. It was noted that no link was found between PKs and mucositis. As for tumour response, no difference in 5-FU exposure was observed between patients who achieved a CR or PR and patients who had SD or progression. This finding was not consistent with previous studies,132,161 which reported that response and survival were significantly associated with high plasma concentrations in patients with H&N cancer. However, the findings from the study by Fety et al. 156,158 should be treated with caution as the methods and overall results were poorly presented.
Conclusions for objectives B and C
The evidence on PK versus BSA dosing in the treatment of CRC patients is weak in both quantity and quality. This holds to an even greater extent for H&N cancer. Evidence on My5-FU is sparse; we found only one study155 of clinical outcomes which compared BSA with PK dose adjustment after application of the My5-FU assay; this study155 was at risk of selection bias. Of the three CRC comparative studies identified,118,119,155 only one118 was a RCT and unfortunately this study used an unrepresentative 8-hour infusion regimen. Single-arm studies130,131,133–154 were heterogeneous, generally of poor design, and were severely limited in ability to deliver useful data for comparison of PK versus BSA dosing. We have been unable to identify any published randomised evidence about the effectiveness of PK-directed dose adjustment for any currently used 5-FU regimen for any cancer type. None of the studies we investigated, including the RCT, were of high quality, all had important drawbacks in design, methods and key outcome coverage; these factors limit their validity and generalisability. From these studies there is therefore little evidence that can be taken forward for the modelling of the cost-effectiveness of My5-FU dose adjustment versus BSA-based dose regimens.
Owing to the paucity of evidence from these studies we were concerned that their comparator BSA populations might be unrepresentative, potentially leading to a biased comparison of PK versus BSA. The apparent clinical benefits from PK dose adjustment in the key outcomes of PFS and OS and AEs could thus represent unrepresentative findings. In the next section we address this issue by comparing PK and BSA outcomes across multiple study arms by including studies retrieved for objective D which seeks to test if the outcomes reported for the control (BSA) arms of CRC studies included in sections B and C are generalisable. This section provides a synopsis of the available data that links the clinical effectiveness and cost-effectiveness elements of the report.
Clinical effectiveness synthesis: an overview of pharmacokinetic- versus body surface area-based regimens to inform the cost-effectiveness analysis and evidence for objective D
This section seeks to address objective D and present an overview of evidence assembled for objectives B–D to compare the clinical effectiveness of PK versus BSA in CRC.
The aim of objective D was to provide an overview of systematic review evidence about BSA-based 5-FU regimens in order to assess the generalisability of BSA results from the comparative studies included in the clinical effectiveness objectives B and C.
Search results for objective D
Figure 26 provides the PRISMA flow diagram for objective D. Electronic searches identified 67 records; an additional record was identified from other sources. After removal of 12 duplicates, 55 records were screened of which 50 were excluded as irrelevant at title/abstract level. Five records were examined at full text and one was included (NICE CG131, Colorectal Cancer: The Diagnosis and Management of Colorectal Cancer). 7 The reasons for the exclusion of the four studies are provided in Appendix 8. The focus of the following section will be on CG1317 and the included RCTs it reported.
FIGURE 26.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram: objective D.

Method of selection of randomised controlled trials from CG131
CG1317 used an extensive NMA of RCTs to compare many 5-FU treatments for CRC with focus on PFS and OS. Evidence was selected from 23 RCTs. Summary survival estimates (HRs) were obtained under assumptions of proportional hazards and of exponential distributions to model survival (these were mainly calculated from median survival values). Analysis of the comparative studies of PK versus BSA and from single-arm PK studies (objectives B and C) indicated that exponential distributions were the least appropriate of those tested for modelling survival data from the reconstructed Kaplan–Meier plots. This, together with a general lack of randomised evidence, meant that a NMA was not considered feasible. Instead, for the purposes of this section and for objective D, appropriate primary studies from the CG131 review were identified and PFS and OS Kaplan–Meier plots from these were reconstructed according to the method of Guyot et al. 125 These, supplemented with the evidence from studies in sections B and C, have been used to overview the PFS and OS evidence that can be utilised for a cost-effectiveness model. The criteria for selection of studies from CG1317 were the intervention or comparator were either the FOLFOX6 regimen or continuous infusion of a FUFOL regimen; and that the study report included a Kaplan–Meier analysis of OS and or PFS. All CG1317 study populations had advanced/mCRC and according to the CG131 authors all were well conducted RCTs.
The outcomes given emphasis are PFS, OS and AEs; this has been dictated by the relevance of these for the cost-effectiveness section (objective E) and by the limiting availability and quality of evidence for other outcomes. The limited evidence in CG1317 about AEs/toxicity is summarised at the end of this section.
Outcomes are considered in turn according to treatment regimens used in the comparative studies; that is FUFOL then FOLFOX6. In the comparative study of Kline et al.,155 patients received either FOLFOX6 or FOLFIRI, results were not separated according to regimen and proportions of patients receiving different treatments was not provided. It was considered impractical to search for published studies or reviews in which patient groups received a mixture of treatments; therefore this section only makes use of the Gamelin et al. 118 and Capitain et al. 119 comparative studies and the single-arm studies of Gamelin et al. 138 and Capitain et al. 134 from objectives B and C Owing to the uniqueness of FUFOL used in the Gamelin et al. studies,118,138 it was necessary to include CG1317 studies in which the duration of continuous infusion with FUFOL differed from that in the Gamelin et al. studies. 118,138
The studies considered from CG1317 were:
-
FUFOL regimen: Köhne et al. ,162,163 Seymour et al. 164 and Cunningham et al. 165
-
FOLFOX6 regimen: Seymour et al. ,164 Hochster et al. ,166 Ducreux et al. 167 and Tourningand et al. 168
In addition, the COIN trial as reported by Adams et al. 34 and Madi et al. 169 was indicated to us by clinical experts as a useful trial that included the FOLFOX6 regimen.
Overview of evidence assembled for objectives B–D to compare the clinical effectiveness of pharmacokinetic versus body surface area in colorectal cancer
5-Fuorouracil + folinic acid regimens: overall survival
Four CG1317 studies with usable Kaplan–Meier plots were identified in which patients with advanced mCRC received a BSA-based FUFOL regimen given by continuous infusion. 162,163,164,165 Reconstructed Kaplan–Meier plots are shown in Figure 27 together with the BSA arm for the Gamelin et al. 118 Note that infusion time used in Gamelin et al. 118 was different (shorter) than that of the other studies and that the plots are for single arms from different studies. The plots suggest that the control arm in Gamelin et al. 118 were not substantially different from the evidence available in the literature.
Comparison of overall survival for body surface area arms with that for pharmacokinetic arms
Three studies provided Kaplan–Meier plots for OS for patients who received continuous infusion FUFOL regimens with PK adjustment of dosage; each was a publication from the same French investigative group. 118,134,138 Figure 28(a) summarises the reconstructed Kaplan–Meier plots from these studies. Note that although the treatment regimens were the same in the Gamelin et al. studies,118,138 in Capitain et al. ’s study134 they involved a much longer infusion time. The plots generate very similar median survivals. When plotted together with the BSA arms [see Figure 28(b)] there appears to be a small gain in OS deriving from PK adjustment. It should be emphasised that except for the contribution from Gamelin et al. 118 (for both PK and BSA arms) this is a non-randomised comparison of arms from separate studies.
FIGURE 28.
Reconstructed OS Kaplan–Meier plots for PK regimens. (a) BSA regimens; and (b) both PK and BSA regimens.

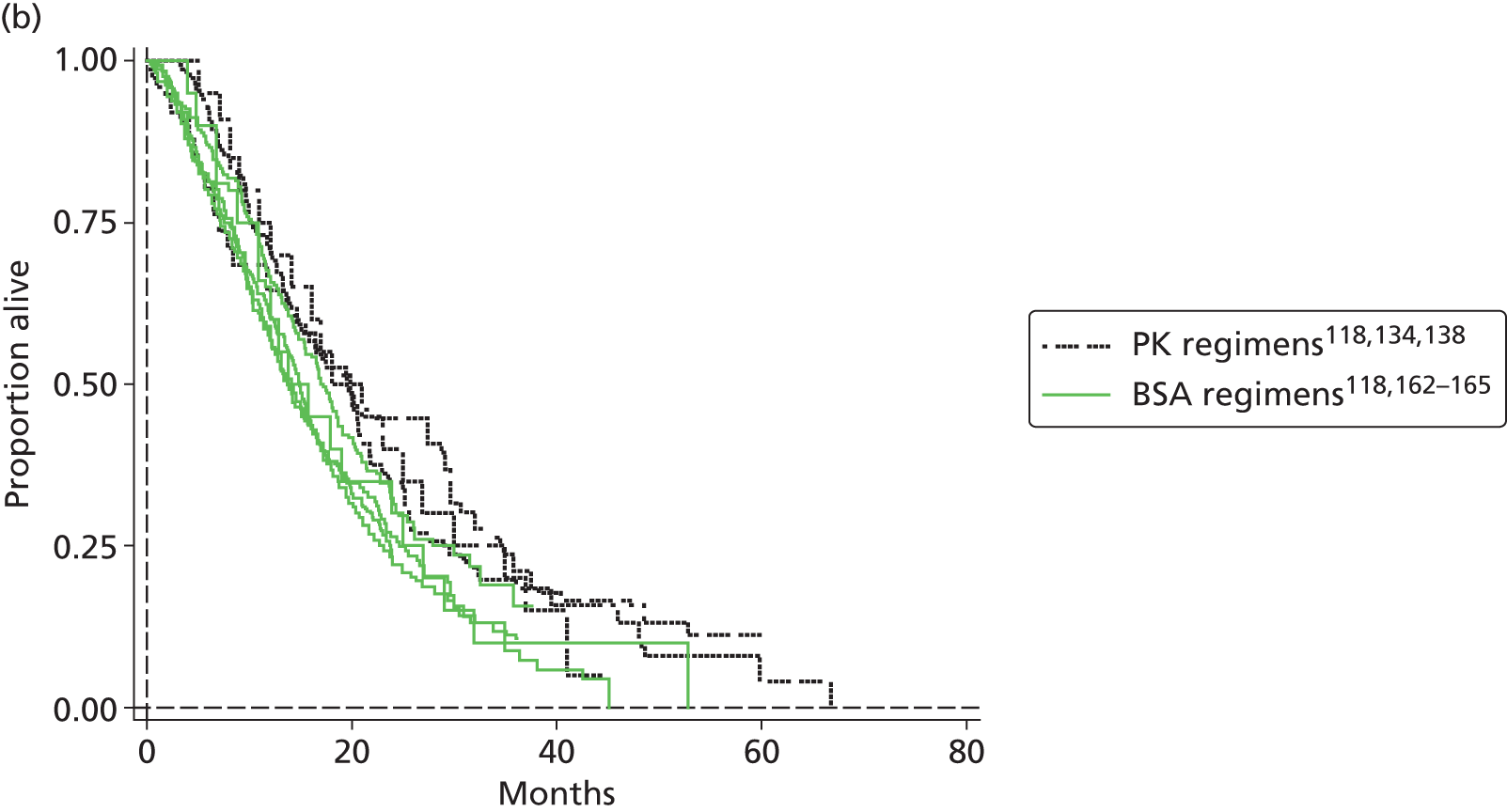
Baseline characteristics of the studies are summarised in Table 32.
| Item | FUFOL BSA arms | FUFOL PK arms | ||||||
|---|---|---|---|---|---|---|---|---|
| Gamelin et al.118 | Köhne et al.162 | Köhne et al.163 | Seymour et al.164 | Cunningham et al.165 | Gamelin et al.118 | Gamelin et al.138 | Capitain et al.134 | |
| Number | 104 | 164 | 216 | 710 | 363 | 104 | 152 | 76 |
| Age (years) | ||||||||
| Mean | 71.2 | NR | NR | NR | NR | 71.5 | 62 | NR |
| Median | NR | 62 | 60.5 | 63 | 62 | NR | NR | 71.2 |
| Range (IQR) | 50–85 | 23–76 | 24–80 | (56–69) | 29–81 | 52–84 | 24–75 | 39–88 |
| Sex, % | ||||||||
| Men | 62.5 | 62 | 61.1 | 70 | 61 | 58.7 | 55.3 | 60.5 |
| Women | 37.5 | 38 | 38.9 | 30 | 39 | 41.3 | 44.7 | 39.5 |
| Performance status, % | ||||||||
| 0 | 55 | 52 | 58.3 | 41 | 50 | 54 | 28.3 | 93.5a |
| 1 | 40 | 41 | 37.5 | 50 | 44 | 33 | 34.2 | |
| 2 or 3 | 5 | 7 | 4.2 | 9 | 6 | 13 | 37.5 | 6.5 |
| Previous therapy, % | 15.4 | 14 | 22.7 | NR | 26 | 10.6 | 19.7 | 17 |
| Metastatic sites, % | ||||||||
| Liver | 74b | NR | NR | 76b | 29b | 81b | 97b | 58b |
| Lung | 30b | NR | NR | 34b | 8b | 26b | 34b | 5.3b |
| Lymph nodes | 11b | NR | NR | 43b | 3b | 19b | 16b | 6.5b |
| Others | 9b | NR | NR | 27b | 13b | 15b | 13b | 6.5b |
If studies for each treatment arm are simply combined then Kaplan–Meier plots for each treatment appear as shown in Figure 29. It should be strongly cautioned that there are many caveats regarding the validity of this procedure, including the assumptions of similar treatments and similar populations. Furthermore, there is a lack of adjustment for potential patient- or study-level confounders. Parametric fits for these and for the individual studies are shown in Appendix 14.
FIGURE 29.
Kaplan–Meier plots resulting from combining OS data from studies: the plots and 95% CIs should be viewed with caution.
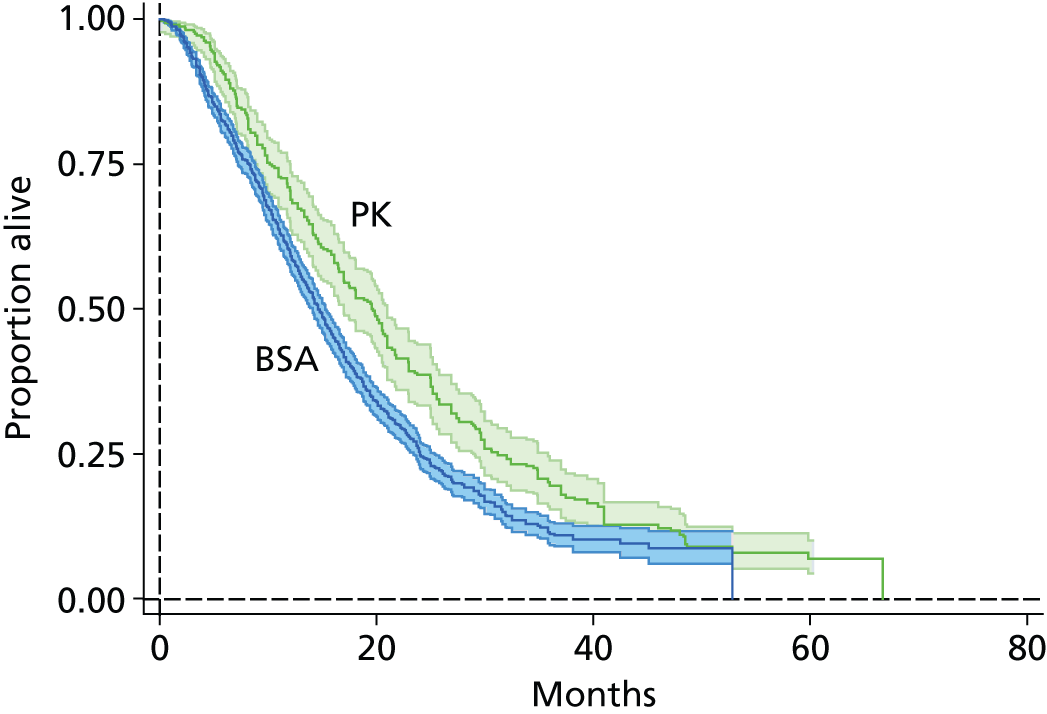
5-fluorouracil + folinic acid: progression-free survival
No PFS data was available for the BSA FUFOL regimen from the studies included in objectives B and C. Three CG1317 studies with usable Kaplan–Meier plots were identified162,163,165 (a further study by Giacchetti et al. 170 was excluded because the 5-day chrono-modulated continuous infusion employed was judged too dissimilar to the relevant regimen).
Figure 30(a) shows the reconstructed Kaplan–Meier plots for these studies. The plots are similar and generate medians that are very close to those in the published articles. Only one study138 from objectives B and C provided evidence about PFS of advanced CRC patients treated with a FUFOL PK regimen. When plotted with the CG1317 studies there is an apparent gain in PFS from the PK regimen. However, caution should be exercised as it should be born in mind that this evidence comes from single arms of independent studies. Table 33 summarises the baseline characteristics of the populations from these studies.
FIGURE 30.
Progression-free survival Kaplan–Meier plots for three BSA-based FUFOL studies (a) compared with that for one PK-based study (b).
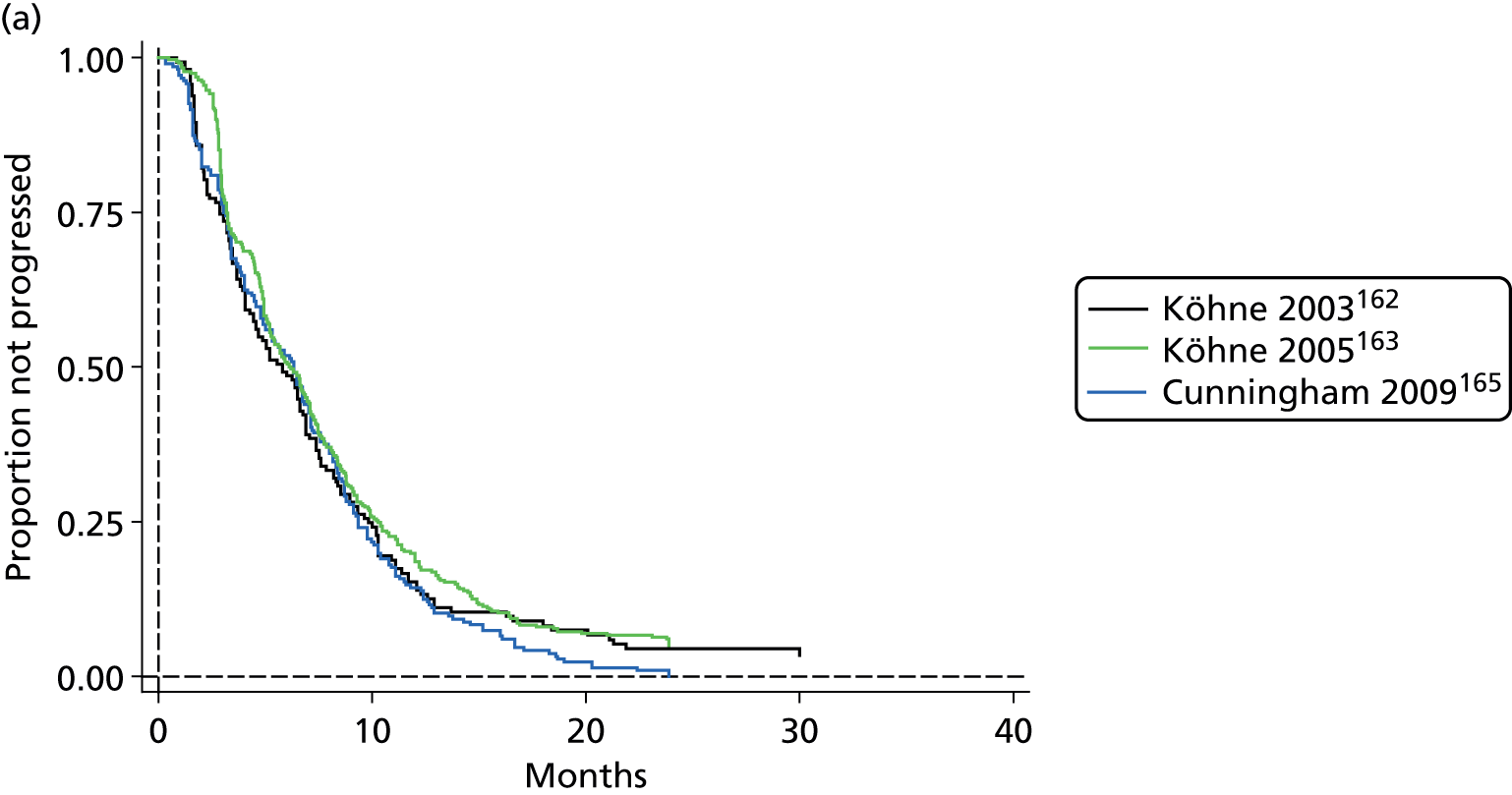
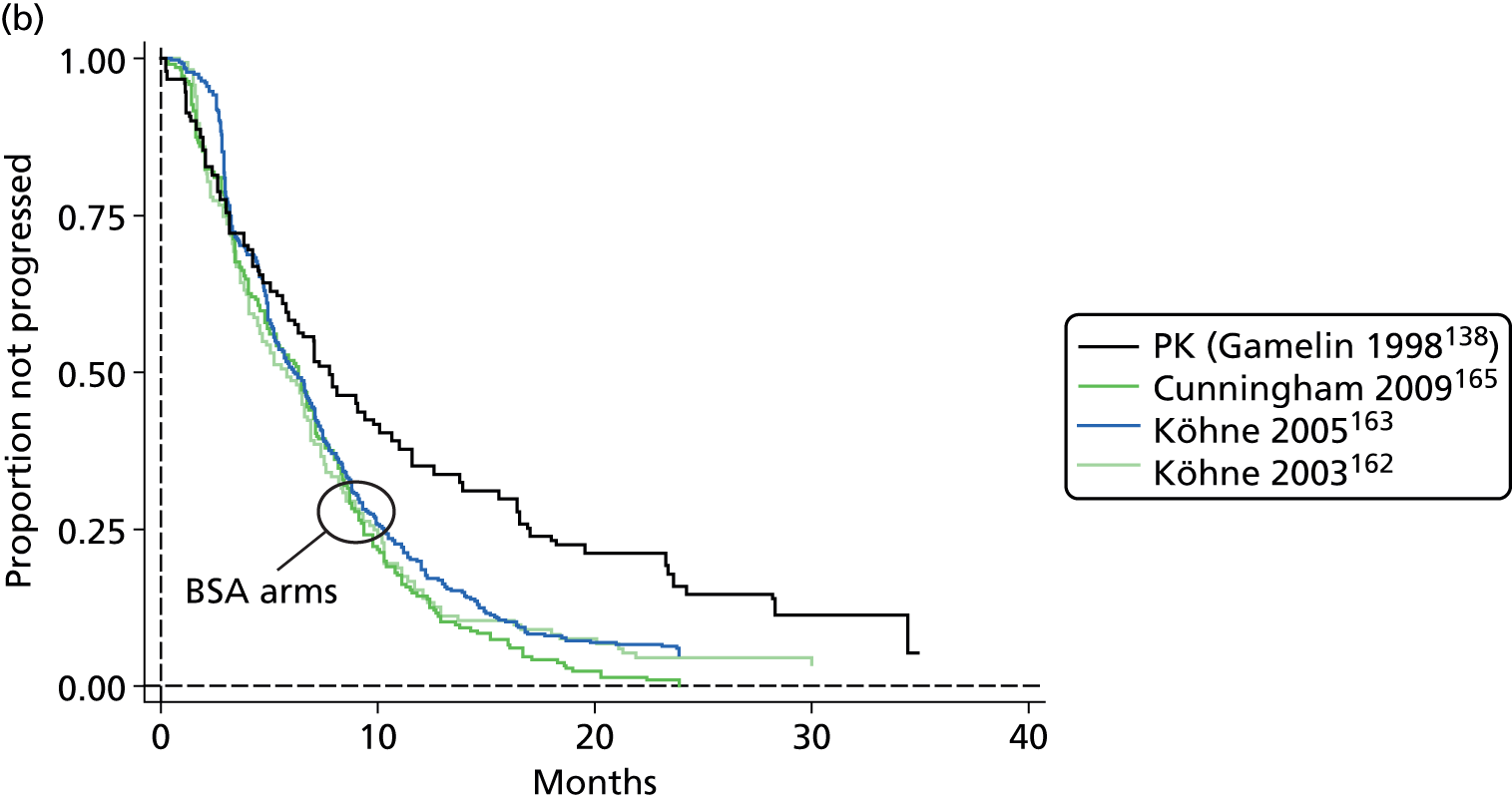
| Item | FUFOL BSA arms | FUFOL PK arms | ||
|---|---|---|---|---|
| Köhne et al.162 | Köhne et al.163 | Cunningham et al.165 | Gamelin et al.138 | |
| Number | 164 | 216 | 363 | 152 |
| Age (years) | ||||
| Mean | NR | NR | NR | 62 |
| Median | 62 | 60.5 | 62 | NR |
| Range | 23–76 | 24–80 | 29–81 | 24–75 |
| Sex, % | ||||
| Men | 62 | 61.1 | 61 | 55.3 |
| Women | 38 | 38.9 | 39 | 44.7 |
| Performance status, % | ||||
| 0 | 52 | 58.3 | 50 | 28.3 |
| 1 | 41 | 37.5 | 44 | 34.2 |
| 2 | 7 | 4.2 | 6 | 35.5 |
| 3 | 0 | 0 | 0 | 2 |
| Previous therapy, % | 14 | 22.7 | 26 | 19.7 |
| Metastatic sites, % | ||||
| Liver | NR | NR | 29a | 97a |
| Lung | NR | NR | 8a | 34a |
| Lymph nodes | NR | NR | 3a | 16a |
| Others | NR | NR | 13a | 13a |
| Number of metastatic sites (% of patients) | ||||
| 1 | 59 | 40.7 | 44 | 72.4 |
| 2 | 28 | 37.0 | 56 (≥ 2) | 20.4 |
| ≥ 3 | 9 (5 unknown) | 22.3 | 7.2 | |
| Number of metastases, n | ||||
| 1 | NR | NR | NR | 14 |
| 2 or 3 | NR | NR | NR | 22 |
| 3–10 | NR | NR | NR | 54 |
| > 10 | NR | NR | NR | 61 |
5-Fuorouracil + folinic acid regimens: difference between overall survival and progression-free survival under body surface area and pharmacokinetic regimens
Figure 31 summarises the apparent difference between OS and PFS under BSA- and PK-based FUFOL regimens based on the evidence described above.
FIGURE 31.
Kaplan–Meier plots illustrating the difference between OS and PFS for FUFOL regimens based on BSA dosage and PK-adjusted dosage. (a) BSA-based regimens; and (b) PK-based regimens.


FOLFOX6 regimens: comparison of body surface area and pharmacokinetic arms overall survival
Four CG1317 studies with usable Kaplan–Meier plots were identified in which patients with advanced mCRC received a BSA-based FOLFOX6 regimen;164,166–168 in addition, our clinical advisors pointed to the existence of the UK COIN trial. 34 Reconstructed Kaplan–Meier plots for these five studies are shown in Figure 32. The two UK studies (Seymour et al. 164 and COIN as reported in Madi et al. 169) provide very similar OS that is somewhat less than the other three European studies. 166–168 Also shown is the reconstructed Kaplan–Meier plot for the PK arm of the comparative study of Capitain et al. 119 Unfortunately, Capitain et al. 119 only provided the median OS for the BSA arm (22 months). This corresponds closely to the median for the three non-UK BSA arms.
FIGURE 32.
Reconstructed Kaplan–Meier plots of OS comparing PK and BSA regimens.
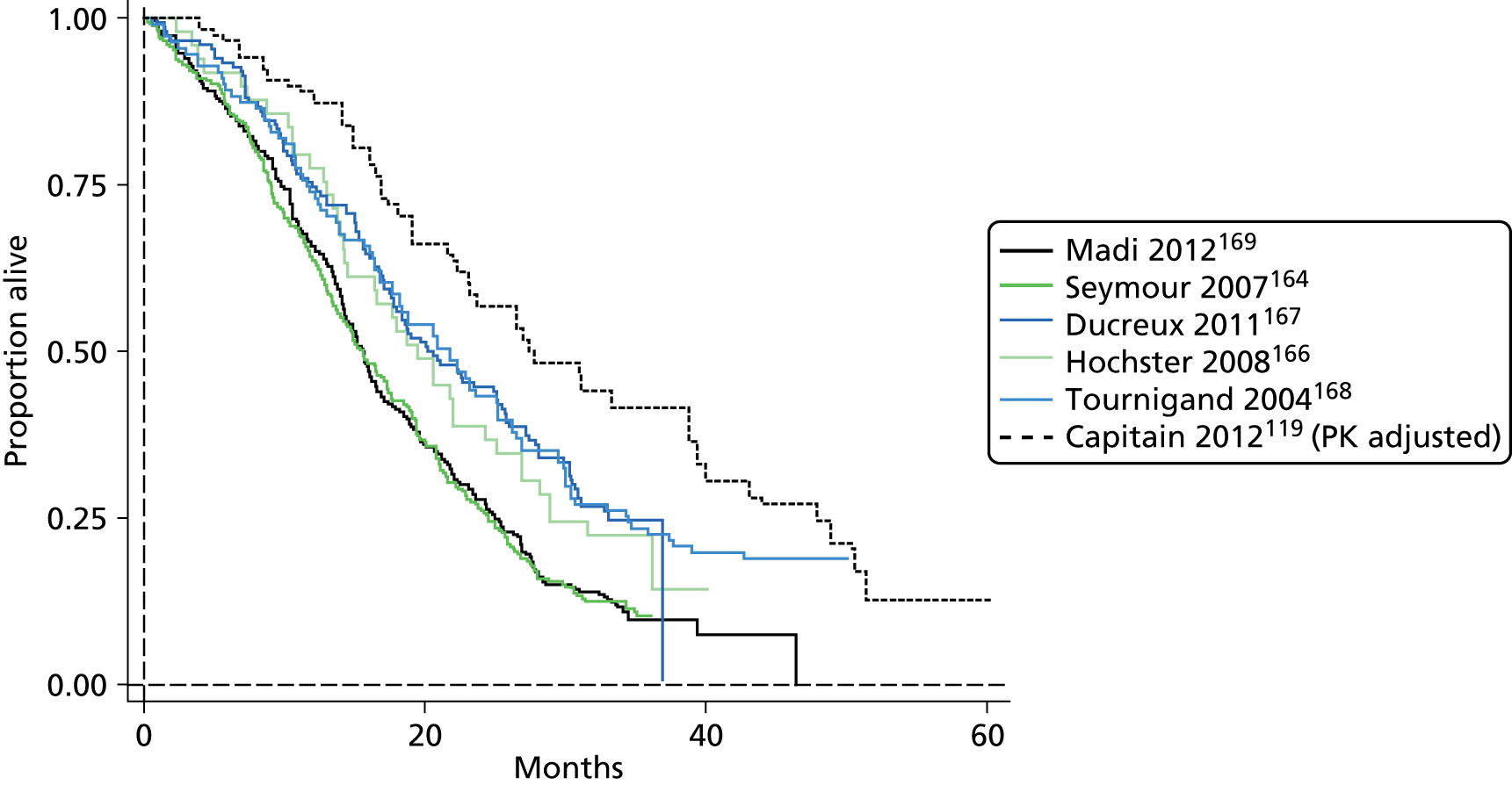
The difference between the PK and BSA plots implies an OS advantage from PK adjustment of dosage. It should be emphasised, however, that the plots are for single arms from different studies and do not represent a randomised comparison; baseline characteristics for these studies are summarised in Table 34.
| Item | BSA arms | PK arm | |||||
|---|---|---|---|---|---|---|---|
| Capitain et al.119 | Seymour et al.164 | COIN (Madi et al.169) | Hochster et al.166 | Ducreux et al.167 | Tournigand et al.168 | Capitain et al.119 | |
| Number | 39 | 357 | 266 | 49 | 150 | 111 | 118 |
| Age (years) | |||||||
| Mean (s.d.) | NR | NR | NR | NR | NR | NR | NR |
| Median | 63 | 64 | 63 | 62 | 64 | 65 | 65 |
| Range (IQR) | 32–80 | (56–69) | (57–69) | 35–79 | 42–84 | 40–75 | 35–81 |
| Sex, % | |||||||
| Men | 62 | 69 | 64 | 57 | 60 | 72 | 59 |
| Women | 38 | 31 | 36 | 43 | 40 | 28 | 41 |
| Performance status, % | |||||||
| 0 or 1 | 77 | 41 | NR | 100 | 93 | 94 | 78 |
| 2 or 3 | 23 | 58 | 8 | 0 | 7 | 6 | 22 |
| Previous therapy, % | NR | 26 | NR | Unclear | 19 | 21 | NR |
| Metastatic sites, % | |||||||
| Liver | 60a | 79a | 77a | 76a | NR | 80a | 56a |
| Lung | 10a | 39a | 43a | 47a | NR | 30a | 16a |
| Peritoneal or nodes | 4.9a | 49a | 56a | 55a | NR | 50a | 10a |
FOLFOX6 regimens: progression-free survival
Other than a reported median survival (10 months) without CIs no PFS data was available for the BSA FOLFOX6 regimen from the studies included in sections B and C. Two CG1317 studies with usable Kaplan–Meier plots were identified,167,168 in addition the COIN trial34 as reported by Madi et al. 169 was indicated to us by clinical experts. Figure 33 shows the reconstructed Kaplan–Meier estimates for these three trials and for the only available PFS data for FOLFOX6 with the PK-adjusted dosage (Capitain et al. 119). The median of 10 months for the BSA arm in Capitain et al. 119 is slightly greater than that for the three BSA plots (which were 9.3, 8.9 and 8.1 months in the reconstructed plots for Ducreux et al. ,167 COIN169 and Tournigand et al. 168 respectively).
FIGURE 33.
Progression-free survival Kaplan–Meier plots for three BSA-based FOLFOX6 studies compared with that for one PK-adjusted study.
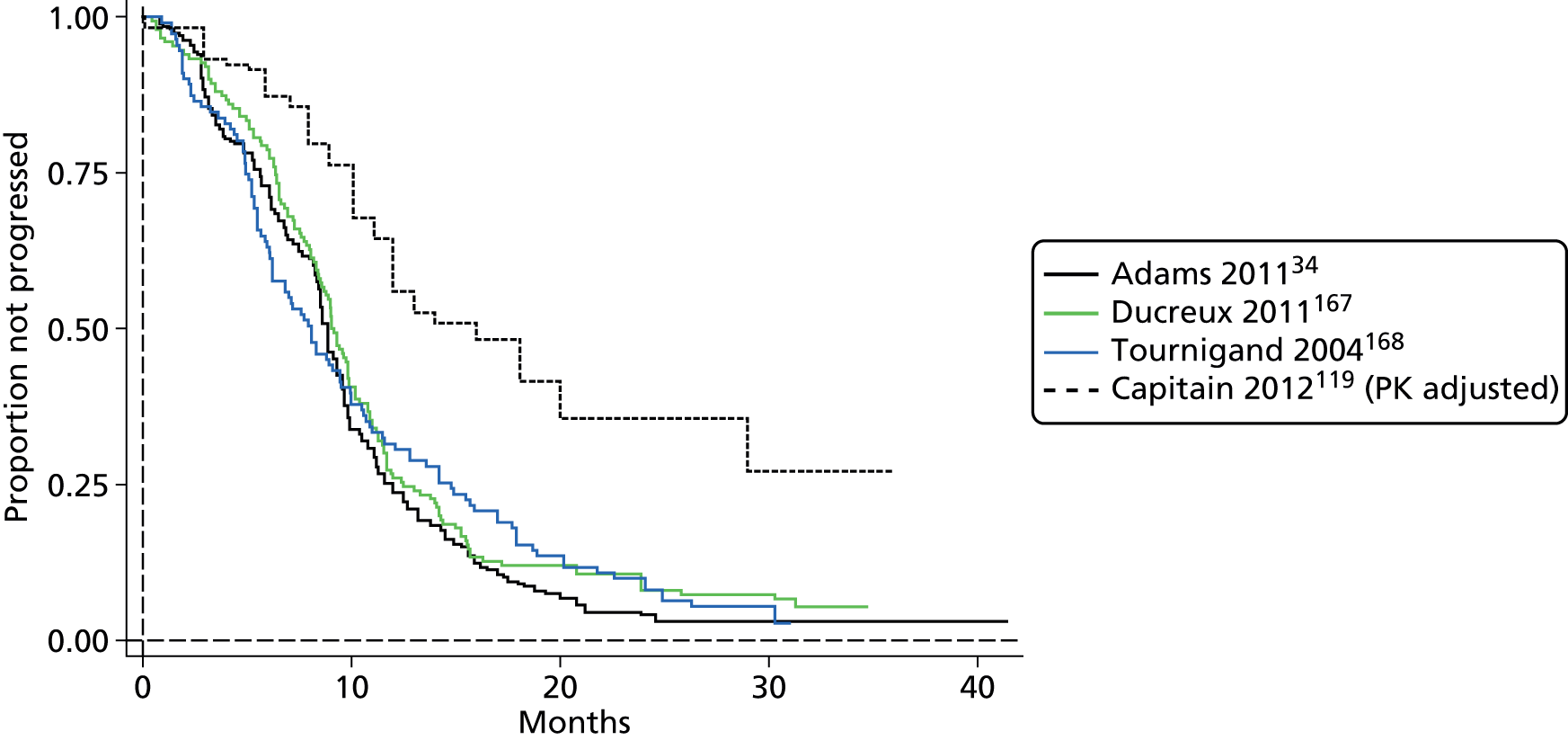
The difference between the PK and BSA plots implies a PFS advantage from PK adjustment of dosage. It should be noted that the plots are for single arms from different studies and do not represent a randomised comparison and that the PK evidence comes from a single study. Demographic characteristics of the studies are summarised in Table 35.
| Item | BSA arms | PK arm | ||
|---|---|---|---|---|
| COIN (Madi et al.169) | Ducreux et al.167 | Tournigand et al.168 | Capitain et al.119 | |
| Number | 266 | 150 | 111 | 118 |
| Age (years) | ||||
| Mean (s.d.) | NR | NR | NR | NR |
| Median | 63 | 64 | 65 | 65 |
| Range (IQR) | (57–69) | 42–84 | 40–75 | 35–81 |
| Sex, % | ||||
| Men | 64 | 60 | 72 | 59 |
| Women | 36 | 40 | 28 | 41 |
| Performance status, % | ||||
| 0 or 1 | NR | 93 | 94 | 78 |
| 2 or 3 | 8 | 7 | 6 | 22 |
| Previous therapy, % | NR | 19 | 21 | NR |
| Metastatic sites, % | ||||
| Liver | 77a | NR | 80a | 56a |
| Lung | 43a | NR | 30a | 16a |
| Peritoneal or nodes | 56a | NR | 50a | 10 |
FOLFOX6 regimens: difference between overall survival and progression-free survival under body surface are and pharmacokinetic regimens
Figure 34 summarises the apparent difference between OS and PFS under BSA- and PK-based FOLFOX6 regimens based on the evidence described above. The evidence for the PK-adjusted regimen comes from a single comparative study which did not provide Kaplan–Meier plots for the comparator BSA arm.
FIGURE 34.
Kaplan–Meier plots illustrating the difference between OS and PFS for FOLFOX6 regimens based on (a) BSA dosage and (b) PK-adjusted dosage.
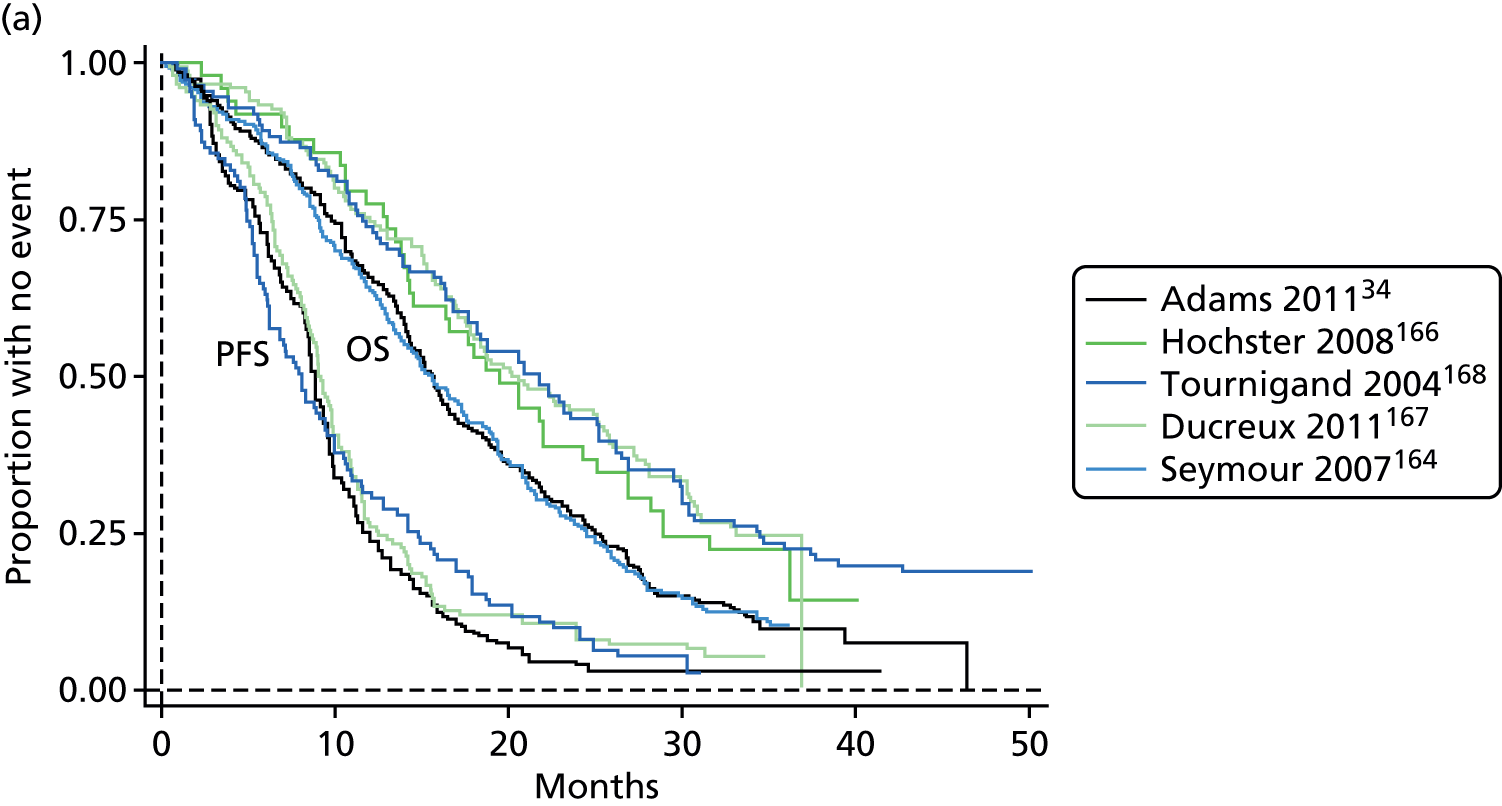

Adverse events/toxicity
The authors of NICE CG1317 commented that ‘mixed treatment methods were not applied to toxicity data as there was insufficient evidence to inform the analysis’. For the de novo economic model in CG131 (comparing different treatment strategies), the risk associated with only three potential toxicities were estimated: febrile neutropenia, grade III/IV diarrhoea and grade III/IV hand and foot syndrome. They were selected on the basis of data availability and their likely impact on QoL. CG1317 did not report any toxicity data for a first-line FUFOL regimen. Data for first-line FOLFOX regimens, taken from the appropriate arms of various studies, was presented and is summarised in Table 36, where data from the Capitain et al. 119 comparative study is added for comparison.
| Item | aCapitain et al.119 | bCG1317 | aCapitain et al.119 |
|---|---|---|---|
| Treatment | BSA: n = 39 | BSA: multiple studies | PK: n = 118 |
| AE | |||
| Diarrhoea grade III/IV | 12.0 (4.3 to 24.2) | 15.7 (10.7) | 1.7 (0.53 to 5.99) |
| Hand and foot syndrome grade III/IV | NR | 2.4 (2.7) | NR |
| Neutropeniac | 25 (13 to 39) | 6.2 (5.6) | 12.07 (19.50 to 25.91) |
The risk of diarrhoea in the Capitain et al. 119 BSA arm appeared similar to that estimated by authors of CG131. 7 It should be appreciated that CG1317 included FOLFOX4 as well as FOLFOX6 arms in their analysis. The difference between CG1317 and Capitain et al. 119 in the risk of neutropenia may be due to different definitions. The risk of hand and foot syndrome appears to be low, but was not reported by Capitain et al. 119
Conclusions from evidence for objective D and overview of pharmacokinetic versus body surface area
Rigorous assessment of the consistency of the PK comparative study results with the generality of the published literature was hampered by the existence of only a single randomised comparative study,118 the paucity of comparative studies (n = 3 for CRC118,119,154), their incomplete reporting of important outcomes, the use of an unrepresentative and now obsolete treatment regimen in the randomised CRC study,118 and the failure by authors to release requested IPD.
Guyot et al. ’s125 procedure for constructing an estimate of IPD from published Kaplan–Meier plots provided a platform for comparing survival outcomes and for exploring parametric models. This procedure is best served by existence of good-quality published Kaplan–Meier plots, risk table information at multiple time points of the plots and information about the total number of events. In nearly all the survival analyses undertaken for this section most of this information was absent; hence, although the reconstructed plots provide a substantially accurate representation of the published plots, the reconstructed IPD could not provide a true representation of censoring times so that parametric fits based on this data should be viewed with some caution.
The results for the BSA arms in Gamelin et al. 118 (OS) and in Capitain et al. 119 (median values only for OS and PFS) were compared with BSA arms in studies included in the NICE CG1317 systematic review of CRC. These were sufficiently similar to conclude that the comparison between PK and BSA regimens presented in the two CRC comparative studies was not biased by non-representative results for the BSA arms.
Nevertheless, it is important to appreciate that the comparison (PK vs. BSA) for survival outcomes is greatly weakened by the paucity of evidence for the PK arms. In the case of FOLFOX6, the PK evidence comes from a single non-randomised study which failed to provide full data for the comparator arm, and in the case of FUFOL the PK evidence for PFS was provided by a lone single-arm study in which no comparator data were presented. Thus, for objective D, an assessment of the effectiveness of PK for these outcomes relies on the dubious procedure of comparing various arms from different studies using ‘derived’ data.
There were similar difficulties in relating published toxicity data to PK comparative study estimates of the risk of 5-FU-induced toxicities. These stemmed from paucity of data, differing and selective reporting of toxicity outcomes, and the problems encountered by CG1317 authors in synthesising toxicity data from published studies. It is difficult to draw firm conclusions; however, with regards to serious diarrhoea both Gamelin et al. 118 and Capitain et al. 119 provide evidence supporting a beneficial effect of PK dose adjustment, and for Capitain et al. 119 the risk reported for the BSA arm appears to be in line with data from CG1317. 7 On the other hand, although risk of hand and foot syndrome appears low, it may be increased with a PK-based regimen.
Clinical effectiveness results informing the cost-effectiveness model
The clinical effectiveness review provided a range of reported34,118,119,130,134,138,162–168 and reconstructed estimates for the survival outcomes for BSA versus PK treatment in mCRC, and limited data on AEs from two CRC comparative studies118,119 and one H&N cancer RCT,156 which informed the cost-effectiveness analysis of PK 5-FU dosing compared with BSA dosing strategies. These are presented in Tables 37–40. Tables 38 and 39 give an overview of the reported median of overall or PFS (if reported) and the median of the reconstructed Kaplan–Meier plots, which were used in the modelling section. Please see Tables 44 and 45 in Chapter 5, The cost-effectiveness of pharmacokinetic dose adjustment using MY5-FU in metastatic colorectal cancer for the combination of these studies in the different base-case and scenario analyses undertaken.
| Study | BSA | PK | Concerns of quality/generalisability | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median OS (in months) | Median PFS (in months) | Median OS (in months) | Median PFS (in months) | ||||||
| Reported | Reconstructed | Reported | Reconstructed | Reported | Reconstructed | Reported | Reconstructed | ||
| Gamelin et al.118 | 16 | 16 | Mean response rate × time until progression | 7.2 | 22 | 18.1 | Mean response rate × time until progression | 11.5 | Obsolete FUFOL 8-hour regimen RCT but arms perfectly balanced (BSA n = 104; PK n = 104) Randomisation and allocation concealment methods not described No PFS reported |
| Gamelin et al.138 | NR | NA | NR | NA | 19 | 19.6 | 11 | 7.8 | Obsolete FUFOL 8-hour regimen Case series No ITT analysis Error in reported PFS as not corresponding with the published Kaplan–Meier plot |
| Pooled three BSA studies162,163,165 | NA | NA | NA | 6.2 | NA | NA | NA | NA | RCTs quality assessed see CG1317 |
| Pooled three PK studies118,134,138 | NA | NA | NA | NA | NA | 19.6 | NA | NA | In addition to concerns above: Capitain et al.119 Two different non-UK regimens Different infusion times to Gamelin et al.118,138 Case series Outcomes not reported separately for two different regimens |
| Pooled five BSA studies118,162–165 | NA | 14.6 | NA | NA | NA | NA | NA | NA | RCTs quality assessed see CG1317 |
| Study | BSA | PK | Concerns of quality/generalisability | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median OS (in months) | Median PFS (in months) | Median OS (in months) | Median PFS (in months) | ||||||
| Reported | Reconstructed | Reported | Reconstructed | Reported | Reconstructed | Reported | Reconstructed | ||
| Capitain et al.119 | 22 | Inferred from reported median assuming proportional hazard | 10 | Inferred from reported median assuming proportional hazard | 28 | 27.4 | 16 | 16 | Selection method unclear No randomisation Historic control Only median OS and PFS reported for BSA arm Commercial adjustment protocol (likely considered more than pure 5-FU levels) |
| Pooled five BSA studies164,166–169 | NA | 17 | NA | NA | NA | NA | NA | NA | RCTs quality assessed see CG1317 Adams et al.34 is a MRC trial with unclear risk of bias (formal quality appraisal available from authors on request) |
| Pooled three BSA studies167–169 | NA | NA | NA | 8.9 | NA | NA | NA | NA | RCTs quality assessed see CG1317 |
| Ducreux et al.167 | 20.5 | 20.2 | 9.3 | 9.0 | NA | NA | NA | NA | RCTs quality assessed see CG1317 |
| Tournigand et al.168 | 20.6 | 21.8 | 8.0 | 8.1 | NA | NA | NA | NA | RCTs quality assessed see CG1317 |
| Non-UK pooled three BSA OS studies166–168 or two BSA PFS167,168 studies | NA | 20.6 | NA | 8.98 | NA | NA | NA | NA | RCTs quality assessed see CG1317 Non-UK trials and UK trials differ in OS, applicability to UK is therefore questioned Comparative studies are non-UK studies |
| Study | Diarrhoea grade III/IV, n (%, 95% CI) | Nausea/ vomiting, n (%, 95% CI) | Hand and foot syndrome grade III/IV, n (%, 95% CI) | Mucositis grade III/IV, n (%, 95% CI) | Neutropenia, n (%, 95% CI) | Leucopenia grade III/IV, n (%, 95% CI) | Thrombocytopenia, n (%, 95% CI) | Concerns of quality/generalisability | |
|---|---|---|---|---|---|---|---|---|---|
| Capitain et al.119 | BSA (n = 39) | 5 (12.0, 4.3 to 24.2) | NR | NR | 6 (15.00, 5.86 to 27.43) | 10 (25, 13 to 39) | NR | 4 (10.00, 2.87 to 20.87) | As above in Table 38 Hand and foot syndrome not reported Grades III and IV toxicities only and grouped together |
| PK (n = 118) | 2 (1.70, 0.53 to 5.99) | NR | NR | 1 (0.80, 0.02 to 3.08) | 21 (18.0, 12.07 to 25.91) | NR | 14 (12.00, 7.29 to 19.10) | ||
| Gamelin et al.118 | BSA (n = 96) | Grade III: 14 (14.6, 8.2 to 23.3) Grade IV: 3 (3.1, 0.6 to 8.9) |
NR | Grade III: 6 (6.3, 2.3 to 13.1) Grade IV: 0 |
Grade III: 1 (1.0, 0.0 to 5.7) Grade IV: 2 (2.1, 0.3 to 7.3) |
NR | Grade III: 1 (1.0, 0.0 to 5.7) Grade IV: 1 (1.0, 0.0 to 5.7) |
NR | As above in Table 37 |
| PK (n = 90) | Grade III: 4 (4.4, 1.2 to 11.0) Grade IV: 0 |
NR | Grade III: 10 (11.1, 5.5 to 19.5) Grade IV: 1 (1.1, 0.0 to 6.0) |
Grade III: 1 (1.1, 0.0 to 6.0) Grade IV: 2 (2.2, 0.3 to 7.8) |
NR | Grade III: 0 (0.0, 0.0 to 4.0) Grade IV: 0 (0.0, 0.0 to 4.0) |
NR | ||
| Study | Digestive toxicity grade III/IV,% of cycles | Mucositis grade III/IV, % of cycles | Neutropenia/thrombocytopenia, % of cycles | Concerns of quality/generalisability | |
|---|---|---|---|---|---|
| Fety et al.156 | BSA (n = 61) | 17.8 | 5.1 | 5.2 | Toxicity reported by cycles rather than risk 16 patients (13%) were found to be ‘unevaluable’ for toxicity patients with protocol violations were removed |
| PK (n = 61) | 7.6 | 0.0 | 8.1 | ||
Chapter 5 Cost-effectiveness and health economics
Methods
Search strategy
A comprehensive search of the literature for published economic evaluations, utility studies and cost studies was performed. Several search strategies were required. Searches were undertaken in March and April 2014. Additional searches were undertaken to identify other relevant information to support the development of the economic model (e.g. past NICE assessments in mCRC).
Cost search 1: cost-effectiveness of pharmacokinetic dosing and 5-fluorouracil
The search strategy developed for objectives A–C of the clinical effectiveness review (for methods, see Chapter 3, Searches for objectives A–C and Appendix 2) was also used to identify any published cost-effectiveness studies. This was considered appropriate because no study type filters were applied. Full copies of all studies deemed potentially relevant by clinical effectiveness reviewers were obtained and assessed by a health economist for inclusion.
Cost search 2: adverse events associated with chemotherapy (all cancers) – quality of life
A series of search strategies was devised to update and expand the literature review of Shabaruddin et al. 171 The search strategies were developed iteratively and are provided in Appendix 1. Searches were undertaken in MEDLINE and EMBASE. All records were screened for inclusion by an information specialist and checked by a health economist. Full copies of all studies deemed potentially relevant were obtained and assessed by a health economist for inclusion.
Cost search 3: adverse events associated with chemotherapy (all cancers) – resource use
A scoping search was undertaken to look for existing reviews. Some reviews of interest were identified, but no relevant overarching review was found. Therefore, a search strategy was developed based on the strategies used for cost search 2. The search strategies were developed iteratively and are provided in Appendix 1. Searches were undertaken in MEDLINE. All records were screened for inclusion by an information specialist and checked by a health economist. Full copies of all studies deemed potentially relevant were obtained and assessed by a health economist for inclusion.
Cost search 4: metastatic colorectal cancer/head and neck cancer – quality of life; and cost search 5: metastatic colorectal cancer/head and neck cancer – resource use
Searches 4 and 5 were developed iteratively, with reference to the search strategies of several published systematic reviews. 172–176 Searches for resource use were limited to English, humans and the UK perspective (by the addition of several currency and location terms). No date limits were applied. Searches were undertaken in MEDLINE (see Appendix 1). All records were screened for inclusion by an information specialist and checked by a health economist. Full copies of all studies deemed potentially relevant were obtained and assessed by a health economist for inclusion.
Inclusion criteria for studies to address objective E
All study designs will be considered for inclusion. Studies will be included that provide information on the following:
-
cost of My5-FU testing
-
cost of delivering 5-FU by infusion
-
cost of side effects and 5-FU toxicity and their associated treatment or hospitalisation costs
-
additional costs associated with changes to continuous infusion protocols.
As no full-text economic evaluation studies were identified, no studies were assessed using the Consolidation Health Economic Evaluation Reporting Standards checklist. 177
Evaluation of costs, quality of life and cost-effectiveness
Model structure
Where data allowed, the preferred approach was to model the impact of PK dose adjustment using My5-FU assay compared with BSA dosing, using the clinical outcomes specified in the above and with a lifetime horizon. In the absence of such evidence, a linked evidence approach was adopted, linking My5-FU dose adjustment to other PK dose adjustment studies within the literature. It may have assumed equivalence between the My5-FU assay and other PK measures of plasma 5-FU (i.e. HPLC and LC-MS) if this appeared a reasonable assumption in the light of the clinical review. Model inputs may have utilised indirect treatment comparison results or NMA results to derive estimates of the clinical outcomes for the chemotherapy regimens relevant to current UK clinical practice. It was anticipated that this would be possible for mCRC, as outlined in more detail Chapter 4, Objective A(1): review of studies examining the accuracy of the My5-FU assay when tested against gold standard methods.
Although it was desirable to try to link evidence through to final survival outcomes, it should be recognised that due to data limitations this may be impossible for some cancers. Where this applied, the assessment still endeavoured to estimate the impact of My5-FU dose adjustment on test costs, treatment costs, side effect costs and the QoL impacts of side effects. This truncated analysis would be augmented by threshold analyses that estimate what, if any, additional impacts My5-FU would be required to have on PFS and/or OS for it to be cost-effective at conventional NICE willingness-to-pay (WTP) thresholds. The estimates of the additional survival were reported in terms of the absolute additional time required, with this being compared with estimates of the relevant current mean survival. It was also reported in the same metric as that used for the estimate of the impact of PK dose adjustment on OS in the mCRC modelling, in order to facilitate a comparison across clinical areas (e.g. as a relative risk or as a HR).
Necessary choices and definitions regarding the structure of the model depended on the findings from the literature review and consultation with clinical experts.
Issues relevant to analyses
During scoping, no end-to-end studies of the My5-FU assay were identified. Evidence was found relating to the validation of the My5-FU assay with LC-MS; the impact of 5-FU plasma levels on toxicity; PK variability of 5-FU when BSA dosing is used; and the impact of PK dose adjustment of 5-FU on survival. Studies comparing PK dosing with BSA dosing in mCRC were found that reported average 5-FU weekly doses, AE rates, PFS and OS with varying degrees of completeness. 118,119 These, together with other papers that may be identified during the literature searches, may provide sufficient information to enable estimation of the various clinical outcomes for mCRC. The papers’ authors will also be approached for the information about the outcomes that were ambiguously, partially or not reported for one or both arms.
Where clinical outcome estimates can be arrived at for My5-FU informed dose adjustment and BSA dosing, these will be the preferred basis of the modelling. The main model structure will be developed to favour these elements over those that may be drawn from a linked evidence approach or from expert opinion. This does not preclude more speculative model structures also being developed.
One-way sensitivity analyses will be performed for all key parameters and for parameters in the models which are based on expert opinion or lie within any more speculative linked evidence modelling. The appropriate model structure may also be subject to some uncertainty. Probabilistic modelling will be performed using parameter distributions instead of fixed values. It may be necessary to perform a number of probabilistic modelling exercises, given the uncertainty around parameter estimates that are based on expert opinion and the uncertainty around the most appropriate model structure.
Decision uncertainty regarding mutually exclusive alternatives will be reflected using cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs) or frontiers.
Longer-term costs and consequences will be discounted using the UK discount rates of 3.5% for both costs and effects.
Health outcomes
Utility values, based on literature or other sources, will be incorporated in the economic model. QALYs will be calculated from the economic modelling.
Costs
Data for the cost analyses will be drawn from routine NHS sources [e.g. NHS reference costs, Personal Social Services Research Unit (PSSRU), British National Formulary (BNF)], discussions with individual hospitals and with the manufacturer.
Costs for consideration will include:
-
cost of My5-FU testing
-
cost of delivering 5-FU by infusion
-
cost of side effects and 5-FU toxicity and their associated treatment or hospitalisation costs
-
additional costs associated with changes to continuous infusion protocols.
Other costs for consideration may include:
-
cost of second-line therapies
-
palliative care and end-of-life costs.
Cost and resource use
Resource use will be estimated in line with the DAP programme manual:
-
The perspective will be that of the NHS and PSS.
-
The cost of the My5-FU assay will be requested from the manufacturer on the basis of this being nationally and publicly available, with additional confirmation of this sought from a UK laboratory currently using My5-FU.
-
The base case will use list prices for the chemotherapy regimens, but as in the modelling for CG1317 the impact of discounted prices available to the NHS may also be explored.
-
The above two bullets may be augmented with advice from the UK NHS centre currently using the My5-FU assay and other bodies such as the NHS Purchasing and Supply Agency.
-
The effect of My5-FU on resource use in terms of physical units will be presented separately and then coupled with unit costs.
Results
Search results for objectives E
Figure 35 provides the PRISMA flow diagram for objectives E. A total of 4578 records were identified through electronic searches. Twelve additional records were identified from other sources. The removal of duplicates left 3614 records to be screened, of which 3514 were excluded at title/abstract level as these were irrelevant. The remaining 100 records were examined for full text, of which 54 were included in the cost-effectiveness review.
FIGURE 35.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram: My5-FU cost-effectiveness objective E.

Economics of My5-FU dose adjustment
Within the literature review, costs are given as stated in the literature, then uprated for inflation using the Hospital and Community Health Services Pay and Prices Index to 2012/13 prices which are reported in square brackets, for example [£37]. Where costs were reported in foreign currency, these are first converted at the then prevailing April exchange rate, and then uprated for inflation using the Hospital and Community Health Services Pay and Prices Index to 2012/13 prices. Where no year is given for prices, it is assumed to be the year of publication.
Literature review: economics
The literature review first reviews the only cost-effectiveness study of My5-FU for PK dose adjustment. A number of further literature reviews are undertaken and presented in full in the following appendices:
-
Appendix 15 , Previous National Institute for Health and Care Excellence metastatic colorectal cancer assessments and Department of Health report
-
Appendix 16 , Quality-of-life papers and metastatic colorectal cancer
-
Appendix 17 , Metastatic colorectal cancer UK resource use literature review
-
Appendix 18 , Previous National Institute for Health and Care Excellence assessments in head and neck cancer
-
Appendix 19 , Head and neck cancer: other quality of life literature review
-
Appendix 20 , Head and neck cancer: other UK resource use literature review
-
Appendix 21 , Adverse events and resource use
-
Appendix 22 , Literature review of quality of life and adverse events.
These are briefly summarised in what follows. Their contributions to the assumptions and parameter values for this assessment are highlighted but not reviewed in detail within the main body of the report.
Cost-effectiveness studies of pharmacokinetic 5-fluorouracil dosing in metastatic colorectal cancer
Becker et al. 178 (funded by Saladax) in an International Society for Pharmacoeconomics and Outcomes Research poster presentation summarising the results of a cost–utility modelling exercise that compared My5-FU dose adjustment with BSA dosing among patients with mCRC. This adopted a lifetime horizon, and discounted costs and benefits at 3.0%. A range of chemotherapy regimens were analysed, with My5-FU dose adjustment being compared with BSA dosing for:
-
FUFOL
-
FOLFOX4
-
FOLFOX6
-
FOLFIRI
-
FOLFOX6 + bevacizumab
-
FOLFIRI + bevacizumab.
The detail of the modelling as presented below is drawn from personal communication and an electronic copy of the model (Russell Becker, Russell Becker Consulting, Chicago, IL, USA, 2013, personal communication).
[Academic-in-confidence (AiC) information has been removed.]
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | |||
|---|---|---|---|---|---|---|
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
(AiC information has been removed.)
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | |||||
|---|---|---|---|---|---|---|---|---|---|
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | ||
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
(AiC information has been removed.)
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | |||||
|---|---|---|---|---|---|---|---|
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | ||
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
(AiC information has been removed.)
Previous National Institute for Health and Care Excellence assessments in metastatic colorectal cancer
There have been seven NICE assessments in mCRC: four multiple technology appraisals (MTAs) (TA93,30 TA118,179 TA242180 and TA61181), two single technology appraisals (STAs) (TA176182 and TA212183) and a CG (CG131). 7 Their methods and data sources are outlined in greater detail in Appendix 15. This can be further summarised, alongside further expert opinion and what it implies for the current assessment, as follows.
Chemotherapy administration costs in previous National Institute for Health and Care Excellence metastatic colorectal cancer assessments
For the chemotherapy administration costs the majority of assessments adopted a disaggregate approach, separately costing individual elements such as line insertion, pharmacy preparation, administration and line flushing. The most comprehensive source appears to be the costing of TA93,30 with these also being used for TA212. 183 CG1317 is a slight outlier in terms of only applying the NHS reference cost for the delivery of complex parenteral chemotherapy. The current assessment will adopt the approach of TA93. 30 It will also source some of the inputs, suitably uprated for inflation, such as the cost of pharmacy preparation from TA9330 and TA212. 183
Duration of treatment in previous National Institute for Health and Care Excellence metastatic colorectal cancer assessments
Assessments have varied between assuming only 12 weeks of treatment or treatment until progression or unacceptable toxicity. Most assumed treatment would be as per the relevant trials, which was in effect until progression or unacceptable toxicity.
However, this is complicated by UK clinical practice being now in part informed by the relatively recently reported COIN trial, as reported in Adams et al. 34 and Madi et al. 169 This assessed the impact of intermittent treatment compared with continuous treatment, with patients receiving oxaliplatin plus 5-FU and capecitabine plus 5-FU with some crossing over between the two treatments. Intermittent treatment during COIN consisted of an assessment at 12 weeks. Those with PD moved onto off-protocol treatment, but those with SD or responding disease entered a chemotherapy-free treatment period with ongoing monitoring. If PD was found during this ongoing monitoring, patients recommenced their original chemotherapy. This intermittent treatment continued until PD was observed while on therapy or the patient chose to stop.
Expert opinion suggests that a treatment break of between 6 and 12 cycles of FOLFOX would be usual UK practice, with the responses suggesting that 12 cycles would be the more common goal. Given 12 cycles, it is also suggested that very few patients would enter a second course of treatment: at most 20% and more probably < 10%.
In the light of this, the current assessment will assume for the base case that the goal of treatment is to achieve 12 cycles of treatment. Progression before this point will limit the number of patients that actually achieve this goal.
There remains uncertainty about the actual proportion of PFS that UK mCRC patients spend receiving first-line chemotherapy. The National Cancer Intelligence Network (NCIN) Systemic Anti-Cancer Therapy Dataset184 should in time be able provide information about the chemotherapy regimens being used by tumour type and stage. The NCIN Chemotherapy Intelligence Unit was approached by the External Assessment Group (EAG) about obtaining information about the dosing and durations of first-line therapy for mCRC. This was with a view to then obtaining information along the lines of the following five bullets, for example mCRC patients receiving FOLFOX:
-
the distribution between 5-FU doses during patients’ first cycles
-
the split between those receiving only one continuous course and those receiving sequenced courses with treatment holidays in between
-
the average course duration and standard error for those receiving only one continuous course
-
the average course duration and standard error for those receiving sequenced courses with treatment holidays; and
-
the average treatment holiday duration and standard error for those receiving sequenced courses with treatment holidays.
However, at present the NCIN Systemic Anti-Cancer Therapy Dataset184 is still in its infancy. Although some publications are available, the EAG was informed that the level of granularity and the completeness of the data are not sufficient to enable reliable information about the dosing and durations of first-line therapy for mCRC to be supplied. The Chemotherapy Intelligence Unit anticipates that the data will become sufficiently robust for analyses such as this in 2014/15.
Data from the COIN trial would have enabled the proportion of PFS spent on treatment to have been estimated. Unfortunately, despite the Trial Steering Committee agreeing to its release for this report, it was not possible to arrange an agreement with the University College London for this data to be released.
Modelling of progression-free survival and overall survival in previous National Institute for Health and Care Excellence metastatic colorectal cancer assessments
Buyse et al. 185 was quoted as concluding that tumour response is a weak predictor of overall response, summarising the results of the main systematic review of this. Pooling patient-level data from 3791 CRC patients enrolled in 25 RCTs suggested that only 38% (95% CI 9% to 69%) of the variation in OS was explained by variations in response rates. As a consequence, in common with the previous NICE assessments in mCRC the economics will restrict itself to modelling survival and PFS from parameterised curves reported in the clinical effectiveness section.
For the most part, parametric curves have been fitted to Kaplan–Meier curves. While not employing the method of Guyot et al. ,125 the Weibull was the most generally used, though CG1317 adopted the exponential. These curves have quite often been used to calculate the mean survival mathematically, rather than model it. Although compact, this method does mean that discounting was not applied. This was justified by the relatively short mean survival time, implying that discounting would have little effect on net quantities and the resulting cost-effectiveness estimates.
However, this is not obviously the case. For instance, the Guyot et al. 125 estimated OS Weibull for PK dosing as drawn from Capitain et al. 119 suggests a mean OS of 33.73 months. Applying the same shape parameter to the Capitain et al. 119 median OS of 22 months for BSA dosing suggests a mean OS of 24.48 months. A net gain from PK dosing of 9.25 months. But discounting at an annual rate of 3.5% reduces this net gain to 8.26 months: a reduction due to discounting of a little over 10%.
In the light of this, the current assessment will model the parameterised curves outlined within the clinical effectiveness section, with a 2-week cycle to reflect the duration of a cycle of FOLFOX. Continuous discounting, in the sense of each model cycle being associated with a unique discount factor to derive its present values, will be applied at an annual rate of 3.5% for both costs and benefits.
Quality-of-life values in previous National Institute for Health and Care Excellence metastatic colorectal cancer assessments
Previous NICE assessments in mCRC have typically applied two main QoL values: one for PFS and one for survival post progression. The literature underlying these estimates coupled with a systematic review of the literature in order to update these estimates is presented in Appendix 16. This can be briefly summarised as follows:
-
TA61181 performed a cost-minimisation analysis so there were no QoL estimates.
-
TA9330 drew a mean value of 0.76 from European Quality of Life-5 Dimensions (EQ-5D) data of the FOCUS trial (fluorouracil, oxaliplatin, irinotecan use and sequencing). 186
-
TA118179 drew a value for PFS of 0.80 from the Ramsey et al. ’s187 Health Utilities Index (HUI) study among 173 US CRC survivors. A multiplier of 0.75 for survival with progression (SWP) was informed by the standard gamble (SG) survey among 30 UK oncology nurses of the Petrou and Campbell study. 188
-
TA176182 drew a value of 0.79 for first-line treatment from EQ-5D data collected during the pivotal trial. Third-line therapy was assigned a value of 0.68 as drawn from Jonker et al. ,189 whereas second-line therapy was assigned a value at the mid-point of 0.73. SWP was assigned 75% of the value for first-line PFS.
-
CG1317 drew values of 0.51 for SD and 0.21 for PD from the Best et al. 190 time trade-off (TTO) study among 49 members of the US general public.
-
TA242180 used values of between 0.75 and 0.81 for PFS and between 0.69 and 0.79 for SWP. These were supplied within the manufacturer submission, these being based on a reanalysis of the data underlying the Mittman et al. 191 HUI study of a Canadian publicly funded trial of adding cetuximab to the treatment of 575 mCRC patients.
The values used for CG1317 are outliers compared with the values used in other NICE mCRC assessments. Note that Best et al. 190 also surveyed 49 colorectal patients who reported values of 0.46 and 0.38 for the health states of metastatic SD and metastatic PD.
The systematic literature review only identified an additional four references that had not been considered at some point within the previous NICE mCRC assessments. Färkkilä et al. 192 is probably the most interesting, having surveyed Finnish mCRC patients using the EQ-5D value using the UK social tariff. Those with advanced disease were divided into patients with mCRC who were still receiving oncological care (n = 110) and those only receiving palliative care (n = 41). The mean QoL values were 0.820 [standard error of measurement (SEM) 0.019] and 0.643 (SEM 0.049) respectively.
Shiroiwa et al. 193 undertook a TTO study among members of the Japanese general public for mCRC health states that resulted in estimates more in line with those of Best et al. :190 0.59 for treatment with XELOX and no AEs and 0.53 for treatment with FOLFOX and no AEs.
Wang et al. 194 analysed EQ-5D data from an open-label trial of panitumumab being added to best supportive care for chemotherapy-refractive mCRC patients. The valuation of EQ-5D data is not made clear, but QoL values for those without AEs were 0.768 in the panitumumab arm and 0.663 in the best supportive care arm.
There is also an argument that the last few months of survival may be at a somewhat reduced QoL. Odom et al. 195 analysed the EQ-5D values from a trial of panitumumab being added to best supportive care for chemotherapy refractive mCRC patients. Unfortunately this used a US valuation for the EQ-5D, but it suggested that there was a reasonably linear decline in QoL over time among patients at this stage of treatment.
The above suggests that both the results of Färkkilä et al. 192 and those used in the modelling of TA176182 and TA212183 are reasonable estimates. They both suggest a similar QoL decrement of around 0.18 for the move from PFS to PD, though it has to be recognised that the Färkkilä et al. 192 0.643 relates to mCRC patients receiving only palliative care. In the light of this, the current assessment will apply QoL values of 0.820 for PFS and 0.643 for SWP as drawn from Färkkilä et al. 192 The values of TA176182 and TA212183 will also be applied as a reasonable sensitivity analysis, whereas the general public values of Best et al. 190 will be applied as a further sensitivity analysis.
There is a need to model second-line treatment for a proportion of patients, and PFS from this line of treatment. One approach is to apply the same QoL value for PFS from second-line therapy as for PFS arising from first-line treatment. However, it can be argued that PFS from second-line therapy would tend to be at a lower QoL than that arising from first-line therapy.
Only the modelling of CG1317 applied QoL decrements for AEs. These were drawn from the Lloyd et al. 196 SG exercise among 100 members of the UK general public, though related to metastatic breast cancer.
Due to AEs possibly being more central to the current assessment than to the previous NICE assessments in mCRC, a systematic review of the QoL impacts of treatment-related AEs has been undertaken. This is presented in Appendix 22 and further summarised below in the section on AEs and QoL.
The overall QALY impact of AEs also depends on the duration of AEs. It had been hoped that data from the COIN trial would have informed this for grade III and grade IV events. Although the COIN Trial Steering Committee was willing to release patient-level data for this report, it proved impossible to arrange a data release agreement with the University College London. In the absence of other data, for the current assessment this has been drawn from expert opinion.
Adverse event costs in previous National Institute for Health and Care Excellence metastatic colorectal cancer assessments
In general, where AEs have been costed a single aggregate AEs cost has been applied within NICE mCRC assessments, though this is at time differentiated by treatment arm. This is usually not sufficiently disaggregated to be applied to particular AEs as required for the current assessment.
Although not entirely transparent, based on NHS reference costs TA176182 estimated a cost per admission for a grade I/II AE of £1050 [£1216] and for a grade III/IV AE of £1170 [£1354].
TA212183 provides a more disaggregate costing of AEs. However, the details of this are not presented and despite the Evidence Review Group (ERG) asking for further information, none was apparently forthcoming.
CG1317 costs grade III/IV diarrhoea at £388 [£420] based on the NHS reference cost FZ45C short stay NHS reference cost.
In the light of this, for the current assessment the costs of individual grade I/II and grade III/IV AEs will be based on:
-
the likelihood of hospitalisation
-
NHS reference costs for those hospitalised
-
medication costs for those not hospitalised.
The values for these are based on expert opinion as outlined in greater detail in the AEs sections below.
The cost-effectiveness of pharmacokinetic dose adjustment using My5-FU in metastatic colorectal cancer
The modelling approach
The model has been constructed along the lines of a cohort distributed between health states over a 20-year time horizon. Given the survival curves of the clinical effectiveness section, this is in effect a lifetime horizon. A 2-week cycle has been employed to be in line with the FOLFOX cycle length, with half cycle correction in order to align survival estimates with those of the clinical effectiveness section. First-line treatment is assumed to be FOLFOX6. The distribution between health states is determined by the parameterised curves, with the first-line OS curve and the first-line PFS curve determining the post progression from first-line therapy curve. A constant proportion of those progressing from first-line therapy go on to receive second-line therapy which is assumed to be FOLFIRI. This is also associated with a PFS curve, as drawn from Tournigand et al. 168
The main health states of the model in terms of PFS, SWP and death, and the possible movements between them are outlined below (Figure 36).
FIGURE 36.
Metastatic CRC model structure.

In terms of the model structure, for the base case it is assumed that the default is for patients to move from PFS into SWP and then on to death. Moving directly from PFS to death is the exception. This only applies when there is an adding up constraint (i.e. the incident number of deaths implied by the OS curve exceeds the number of patients in the SWP health state).
The cost and QALY impacts of AEs associated with first-line treatment, differentiated by a My5-FU dose adjustment arm and BSA dose adjustment arm, have been included within the modelled, as outlined in greater detail below.
First-line therapy: survival estimates
Capitain et al. 119 studied PK dose adjustment using FOLFOX6, which is directly relevant to current UK clinical practice. However, there are problems with the control arm. Not only was it not randomised and somewhat smaller than the PK dose adjustment arm, only the median OS and median PFS are reported for it.
Gamelin et al. 118 provides the clearest analysis of the possible difference in OS using PK dosing. However its relevance may be hampered by the regimen being of questionable relevance to current UK practice: FUFOL rather than FOLFOX6. There is also no reliable reporting of PFS, though some inference may be made from the reported durations of response.
The parameterised Weibull OS curves differ noticeably between Gamelin et al. 118 and Capitain et al. 119 Figure 37 shows the first 5 years.
In the light of this, two strands of analysis will be presented: one based on FOLFOX6 studies and one based on FUFOL studies. Within the strand based on FOLFOX6 studies a sensitivity analysis applying the HR for OS derived from Gamelin et al. 118 will be performed as a bridge. Note that within the strand based on FUFOL studies, owing to the regimens not being current standard UK practice, the drug costs will be based on FOLFOX6 for first line and FOLFIRI for second line.
The clinical effectiveness section concluded that an assumption of equivalence between My5-FU dose adjustment and PK dose adjustment by more traditional methods was justified reasonable assumption. As a consequence, the economic modelling will assume that the PK dosing curves of the literature are equally applicable to My5-FU dose adjustment. If this assumption is not valid, none of the results of the economic modelling hold.
FOLFOX studies analyses
The base case will apply the parameterised Weibull OS curve and overall PFS curve estimated from the PK dose adjustment arm of Capitain et al. 119 to the My5-FU arm. The Weibull OS curve and overall PFS curve for the BSA arm will be those estimated from the medians reported in Capitain et al. ,119 coupled with an assumption of them having the same shape parameter as the corresponding PK dose adjustment curve. This is reported in more detail in Tables 19 and 23.
Due to Capitain et al. 119 only reporting the median OS for the BSA arm, a scenario analysis will be presented that applies the proportionate hazard of OS derived from Gamelin et al. 118 of 0.829255, as reported in greater detail in Chapter 4, Outcomes: overall survival.
Due to the concerns around the size and derivation of the BSA control arm within Capitain et al. 119 and that only medians are reported, a range of scenario analyses that apply curves for the BSA arm derived from the wider literature will be presented. The studies underlying these estimates are reported in more detail in Chapter 4, FOLFOX6 regimens: comparison of body surface area and pharmacokinetic arms overall survival and Chapter 4, FOLFOX6 regimens: progression-free survival.
A scenario analysis will apply the Weibull OS curve estimated by combining the data from the five BSA studies [Seymour et al. ,164 Madi et al. 169 (COIN), Hochster et al. ,166 Ducreux et al. 167 and Tournigand et al. 168] and the PFS curve estimated by combining the data from the three BSA studies of FOLFOX [Madi et al. 169 (COIN), Ducreux et al. 167 and Tournigand et al. 168]. The curves of the base case will be retained for the My5-FU arm.
Two further scenario analyses will apply the Weibull OS curves and PFS curves estimated from (a) Ducreaux et al. 167 and (b) Tournigand et al. 168 The curves of the base case will be retained for the My5-FU arm.
The analysis of Chapter 4 suggests that the survival in the UK-based studies is somewhat worse than that of the other studies. This might imply that any analysis based on pooling data from single arms for the BSA arm that includes the UK studies may tend to bias the analysis in favour of My5-FU. A fairer comparison with the PK curves of Capitain et al. 119 might be to pool the data from single arms for the BSA arm, but excluding the UK studies. This will be undertaken as a final sensitivity analysis.
This leads to the following base-case analyses and scenario analyses (Table 44).
| Curve | Source | Scale | Shape | Mean (months) |
|---|---|---|---|---|
| Base case | ||||
| OS My5-FU | Capitain et al.119 | λ = 0.00233 | γ = 1.66906 | 33.76 |
| OS BSA | Capitain et al.119 from median | λ = 0.00398 | γ = 1.66906 | 24.49 |
| PFS My5-FU | Capitain et al.119 | λ = 0.02438 | γ = 1.13668 | 25.06 |
| PFS BSA | Capitain et al.119 from median | λ = 0.05060 | γ = 1.13668 | 13.19 |
| Scenario analysis 1 | ||||
| OS My5-FU | Capitain et al.119 | λ = 0.00233 | γ = 1.66906 | 33.76 |
| OS BSA | Gamelin et al.,118 0.829255 HR | – | – | 30.17 |
| PFS My5-FU | Capitain et al.119 | λ = 0.02438 | γ = 1.13668 | 25.06 |
| PFS BSA | Capitain et al.119 from median | λ = 0.05060 | γ = 1.13668 | 13.19 |
| Scenario analysis 2 | ||||
| OS My5-FU | Capitain et al.119 | λ = 0.00233 | γ = 1.66906 | 33.76 |
| OS BSA | Pooled five BSA studiesa | λ = 0.00942 | γ = 1.50343 | 20.09 |
| PFS My5-FU | Capitain et al.119 | λ = 0.02438 | γ = 1.13668 | 25.06 |
| PFS BSA | Pooled three BSA studiesb | λ = 0.03194 | γ = 1.40082 | 10.65 |
| Scenario analysis 3 | ||||
| OS My5-FU | Capitain et al.119 | λ = 0.00233 | γ = 1.66906 | 33.76 |
| OS BSA | Ducreux et al.167 | λ = 0.00183 | γ = 0.03058 | 21.91 |
| PFS My5-FU | Capitain et al.119 | λ = 0.02438 | γ = 1.13668 | 25.06 |
| PFS BSA | Ducreux et al.167 | λ = 1.96532 | γ = 1.38097 | 11.41 |
| Scenario analysis 4 | ||||
| OS My5-FU | Capitain et al.119 | λ = 0.00233 | γ = 1.66906 | 33.76 |
| OS BSA | Tournigand et al.168 | λ = 0.01411 | γ = 0.03568 | 28.19 |
| PFS My5-FU | Capitain et al.119 | λ = 0.02438 | γ = 1.13668 | 25.06 |
| PFS BSA | Tournigand et al.168 | λ = 1.24954 | γ = 1.35822 | 10.66 |
| Scenario analysis 5 | ||||
| OS My5-FU | Capitain et al.119 | λ = 0.00233 | γ = 1.66906 | 33.76 |
| OS BSA | Non-UK pooled three BSA studiesc | λ = 0.00570 | γ = 1.57760 | 23.75 |
| PFS My5-FU | Capitain et al.119 | λ = 0.02438 | γ = 1.13668 | 25.06 |
| PFS BSA | Pooled three BSA studies | λ = 0.03194 | γ = 1.40082 | 10.65 |
5-Fluorouracil + folinic acid studies analyses
Note that the following analyses still assume current UK practice in terms of the drug costs for first-line therapy being those of FOLFOX. The FUFOL study analyses apply the curves from the FUFOL studies, this being motivated, in part, by the only RCT of PK dosing being a FUFOL study.
Due to Gamelin et al. 118 not presenting the PFS curves, the base case will adopt the most conservative approach. It will apply the parameterised Weibull OS curves estimated from Gamelin et al. ,118 but it will assume equivalence between My5-FU and BSA dose adjustment for PFS, and apply the Weibull PFS curve estimated by combining the arms of the three main BSA studies. 167–169
Two scenario analyses will apply the Weibull PFS curves inferred from the durations of response reported in Gamelin et al. 118 as reported in more detail in Chapter 4, Outcomes: progression-free survival, while retaining the other curves of the base case.
A scenario analysis will apply the Weibull PFS curve estimated from Gamelin et al. 138 for the My5-FU arm, while retaining the other curves of the base case.
A scenario analysis will apply the Weibull OS curve estimated by pooling the results of Gamelin et al. 118,138 and Capitain et al. 134 in the My5-FU arm, while retaining the other curves of the base case.
A further scenario analysis will apply the Weibull OS curve estimated by pooling the results of Gamelin et al. 118,138 and Capitain et al. 134 in the My5-FU arm, the PFS curve estimated from Gamelin et al. 138 in the My5-FU arm, while retaining the curves of the base case for the BSA arm.
A final scenario analysis will apply the Weibull OS curve estimated by pooling the results of Gamelin et al. 118,138 and Capitain et al. 134 in the My5-FU arm, the PFS curve estimated from Gamelin et al. 138 in the My5-FU arm, the Weibull OS curve estimates from pooling five BSA studies,164,166–169 and the Weibull PFS curve estimates from pooling three BSA studies in the BSA arm. 167–169
This leads to the following base-case and scenario analyses (Table 45).
| Curve | Source | Scale | Shape | Mean (months) |
|---|---|---|---|---|
| Base case | ||||
| OS My5-FU | Gamelin et al.118 | λ = 0.00270 | γ = 1.82786 | 22.59 |
| OS BSA | Gamelin et al.118 | λ = 0.00865 | γ = 1.54066 | 19.65 |
| PFS My5-FU | Pooled three BSA studiesa | λ = 0.05541 | γ = 1.35834 | 7.71 |
| PFS BSA | Pooled three BSA studies | λ = 0.05541 | γ = 1.35834 | 7.71 |
| Scenario analysis 1 | ||||
| OS My5-FU | Gamelin et al.118 | λ = 0.00270 | γ = 1.82786 | 22.59 |
| OS BSA | Gamelin et al.118 | λ = 0.00865 | γ = 1.54066 | 19.65 |
| PFS My5-FU | Gamelin et al.:118 response duration A | λ = 0.02047 | γ = 1.82786 | 7.46 |
| PFS BSA | Gamelin et al.:118 response duration A | λ = 0.05378 | γ = 1.54066 | 6.00 |
| Scenario analysis 2 | ||||
| OS My5-FU | Gamelin et al.118 | λ = 0.00270 | γ = 1.82786 | 22.59 |
| OS BSA | Gamelin et al.118 | λ = 0.00865 | γ = 1.54066 | 19.65 |
| PFS My5-FU | Gamelin et al.:118 response duration B | λ = 0.00798 | γ = 1.82786 | 12.49 |
| PFS BSA | Gamelin et al.:118 response duration B | λ = 0.03280 | γ = 1.54066 | 8.27 |
| Scenario analysis 3 | ||||
| OS My5-FU | Gamelin et al.118 | λ = 0.00270 | γ = 1.82786 | 22.59 |
| OS BSA | Gamelin et al.118 | λ = 0.00865 | γ = 1.54066 | 19.65 |
| PFS My5-FU | Gamelin et al.138 | λ = 0.08197 | γ = 0.99089 | 12.54 |
| PFS BSA | Pooled three BSA studies | λ = 0.05541 | γ = 1.35834 | 7.71 |
| Scenario analysis 4 | ||||
| OS My5-FU | Pooled three PK studiesb | λ = 0.01089 | γ = 1.38189 | 24.05 |
| OS BSA | Gamelin et al.118 | λ = 0.00865 | γ = 1.54066 | 19.65 |
| PFS My5-FU | Pooled three BSA studies | λ = 0.05541 | γ = 1.35834 | 7.71 |
| PFS BSA | Pooled three BSA studies | λ = 0.05541 | γ = 1.35834 | 7.71 |
| Scenario analysis 5 | ||||
| OS My5-FU | Pooled three PK studies | λ = 0.01089 | γ = 1.38189 | 24.05 |
| OS BSA | Gamelin et al.118 | λ = 0.00865 | γ = 1.54066 | 19.65 |
| PFS My5-FU | Gamelin et al.138 | λ = 0.08197 | γ = 0.99089 | 12.54 |
| PFS BSA | Pooled three BSA studies | λ = 0.05541 | γ = 1.35834 | 7.71 |
| Scenario analysis 6 | ||||
| OS My5-FU | Pooled three PK studies | λ = 0.01089 | γ = 1.38189 | 24.05 |
| OS BSA | Pooled five BSA studiesc | λ = 0.00942 | γ = 1.50343 | 20.09 |
| PFS My5-FU | Gamelin et al.138 | λ = 0.08197 | γ = 0.99089 | 12.54 |
| PFS BSA | Pooled three BSA studies | λ = 0.05541 | γ = 1.35834 | 7.71 |
For the probabilistic modelling a Cholesky decomposition of the parameters variance-covariance matrix is applied.
Second-line therapy: survival estimates
The PFS among those receiving second-line chemotherapy is assumed to follow that of the second-line FOLFIRI of Tournigand et al. 168 (Figure 38). Analysis of this data suggests that both the log-normal and the log-logistic provide reasonable fits to this data (Table 46). However, there may be some concerns about extrapolating using these forms due to the relatively long tails they involve when extrapolating beyond the trial data. As a consequence, the base case will apply the Weibull parameterisation of the second-line FOLFIRI PFS curve, partly in order to increase consistency with the other curves that are being applied. The impact of this is likely to be slight, with all three parameterisations suggesting a mean PFS of around 0.34 years.
FIGURE 38.
Log-normal, log-logistic and Weibull for second-line FOLFIRI PFS (Tournigand et al. 168): over 50 2-week cycles.

| Functional form | Observation | Log-likelihood | df | AIC | BIC | |
|---|---|---|---|---|---|---|
| H0 | H1 | |||||
| Exponential | 111 | –135.781 | –135.781 | 1 | 273.562 | 276.271 |
| Weibull | 111 | –119.699 | –119.699 | 2 | 243.397 | 248.816 |
| Gompertz | 111 | – | –127.887 | 2 | 259.774 | 265.193 |
| Log-normal | 111 | – | –116.960 | 2 | 237.920 | 243.339 |
| Log-logistic | 111 | – | –116.364 | 2 | 236.727 | 242.146 |
Note that within this the duration, effect and cost of second-line therapy is treated as being independent of the duration, effect and cost of first-line therapy. This may not be accurate for even the deterministic analysis. If My5-FU increases PFS from first-line therapy compared with BSA dosing, this may also affect the duration, effect and cost of second-line therapy. The base-case assumption is that it does not. There is also no obvious data that would enable alternative assumptions about this to be parameterised.
Even with the base-case assumption that any increase in PFS from first-line therapy due to My5-FU dosing does not affect the duration, effect and cost of second-line therapy, a further problem may arise within the probabilistic modelling from modelling first-line effects and second-line effects independently. For some of the iterations of the probabilistic modelling the first-line PFS curve may be simulated as being quite close to the OS curve. This would squeeze the time spent in post first-line PFS, such that for some iterations there is insufficient time to accrue the simulated duration, effect and cost of second-line therapy. However, the mean second-line PFS of around 0.34 years is sufficiently short compared with the mean survival subsequent to first-line PFS for this not to be a concern.
Adverse events: rates
Adverse events rates are drawn from the key comparative papers. It should be borne in mind that these appear to report the proportion of patients experiencing events rather than the numbers of actual events. As a consequence, modelling underestimates the number of events to some degree, which could tend to bias the analysis.
As Capitain et al. 119 did not distinguish between grade III and grade IV AEs, where this distinction is required this balance will be drawn from the Gamelin et al. 118 data.
Progression-free survival and survival with progression quality of life
As previously reviewed in Quality-of-life values in previous National Institute for Health and Care Excellence metastatic colorectal cancer assessments, augmented with the results of the systematic review of Appendix 16, the results of Färkkilä et al. 192 and those used in the modelling of TA176182 and TA212183 are reasonable estimates. Both suggest a similar QoL decrement of around 0.18 for the move from PFS to PD, though it has to be recognised that the Färkkilä et al. 192 value of 0.643 relates to mCRC patients receiving only palliative care. In the light of this, the current assessment will apply QoL values of 0.820 for PFS and 0.643 for SWP as drawn from Färkkilä et al. 192 The values of 0.80 and 0.60 from TA176182 and TA212183 are also applied as a reasonable sensitivity analysis, whereas the general public values of 0.51 and 0.21 of Best et al. 190 are applied as a further sensitivity analysis.
Adverse events: quality of life
With the exception of CG131,7 the QoL impacts of AEs have not been separately modelled within previous NICE mCRC assessments. CG1317 included QALY decrements for AEs of 0.103 for grade III/IV diarrhoea, 0.150 for febrile neutropenia and 0.116 for hand and foot syndrome, as drawn from the Lloyd et al. 196 study of metastatic breast cancer.
In the light of this, a systematic literature review of the QoL impacts of chemotherapy-related AEs was undertaken, the results of this being reported in full in Appendix 22. This literature review updated and widened the literature review reported in Shabaruddin et al. 171 Based on just the values reported in this literature review, the following QoL decrements for grade III/IV AEs appear reasonable. Cardiac toxicity has not been further considered due to the only differences between the arms in Gamelin et al. 118 being for grade I/II cardiac AEs, these being asymptomatic (Table 47).
| Grade III/IV AE | QoL decrement | Source |
|---|---|---|
| Diarrhoea | 0.074 | Lloyd et al.,196 and informed by Boyd et al.197 and Shiroiwa et al.193 |
| Nausea/vomiting | 0.074 | |
| Mucositis | 0.074 | |
| Hand and foot syndrome | 0.085 | Lloyd et al.,196 and informed by Shiroiwa et al.193 |
| Leucopenia | 0.090 | Frederix et al.198 |
| Neutropenia | 0.073 | Informed by Tolley et al.,199 but conditioned by the relationship of the other Tolley et al.199 estimates with those of the broader literature |
| Febrile neutropenia | 0.112 | Lloyd et al.,196 and informed by Shiroiwa et al.193 |
| Thrombocytopenia | 0.081 | Swinburn et al.200 |
Due to the range of sources being drawn from for the above, clinical expert opinion was sought on the face validity of these estimates and their relative magnitudes. This can be summarised as viewing the QoL decrements for leucopenia, neutropenia and thrombocytopenia as too high. Apparently even for grade III/IV events, much of the leucopenia and neutropenia is asymptomatic. Similarly, thrombocytopenia was viewed as being unlikely to significantly affect QoL unless it led to bleeding. The probability of requiring platelet transfusion for thrombocytopenia was also viewed as being very small, suggesting that any QoL decrement associated with thrombocytopenia should perhaps be only applied to a small percentage of patients.
There was a suggestion that diarrhoea would have a larger QoL decrement than nausea/vomiting, whereas mucositis might have a smaller impact. Febrile neutropenia was agreed to have the largest QoL decrement.
The literature review identified the abstract by Boyd et al. 197 which reports the interim results from an analysis of the Medical Research Council (MRC)-funded Short Course Oncology Therapy (SCOT) trial201 of patients with fully resected stage III CRC or fully resected high-risk stage II disease. This presents the EQ-5D QoL decrements associated for a subset of both grade I/II and grade III/IV AEs. The interim analysis has been verbally presented in more detail by the author, with EQ-5D data being collected from 1292 patients at baseline, every cycle, 9, 12, 18, 24 months and annually thereafter. This data has been further analysed within a univariate analysis, with the following results (Kathleen Boyd, Glasgow University, 2013, personal communication) (Table 48).
| AE | No AE, n | Grade I/II | Grade III/IV | ||||
|---|---|---|---|---|---|---|---|
| n | QoL decrement | SEM | n | QoL decrement | SEM | ||
| Alopecia | 2082 | 246 | –0.0477 | 0.0317 | 8 | n.a. | n.a. |
| Anaemia | 2082 | 757 | –0.0202 | 0.0149 | 3 | n.a. | n.a. |
| Anorexia | 2083 | 315 | –0.0600 | 0.0209 | 22 | 0.1584 | 0.2033 |
| Constipation | 2084 | 512 | –0.0521 | 0.0166 | 11 | –0.1166 | 0.2033 |
| Diarrhoea | 2081 | 1190 | –0.0400 | 0.0125 | 94 | –0.0900 | 0.0379 |
| Fatigue | 2081 | 1826 | –0.0280 | 0.0103 | 60 | –0.0800 | 0.0615 |
| Hand and foot syndrome | 2082 | 383 | –0.0132 | 0.0268 | 21 | –0.3255 | 0.2035 |
| Mucositis clinical | 2082 | 181 | –0.0860 | 0.0320 | 60 | n.a. | n.a. |
| Mucositis functional | 2082 | 506 | –0.0525 | 0.0173 | 18 | –0.0375 | 0.1438 |
| Nausea | 2081 | 1117 | –0.0460 | 0.0123 | 29 | –0.1410 | 0.0769 |
| Neuropathy sensory | 2081 | 2220 | –0.0290 | 0.0096 | 33 | –0.1970 | 0.0910 |
| Neutropenia | 2081 | 305 | 0.0214 | 0.0245 | 85 | –0.0607 | 0.0457 |
| Photophobia | 2083 | 43 | 0.0103 | 0.0832 | 20 | 0.0000 | 0.0080 |
| Rash | 2082 | 161 | –0.0963 | 0.0309 | 15 | n.a. | n.a. |
| Taste alteration | 2082 | 723 | –0.0445 | 0.0157 | 20 | 0.1585 | 0.2030 |
| Thrombocytopenia | 2083 | 324 | –0.0309 | 0.0325 | 8 | n.a. | n.a. |
| Vomiting | 2081 | 257 | –0.0520 | 0.0224 | 22 | –0.0170 | 0.0910 |
| Watery eye | 2083 | 241 | –0.0638 | 0.0283 | 20 | n.a. | n.a. |
The ‘not available’ values for the grade III/IV events were due to insufficient numbers of events: none for alopecia and photophobia, one for rash and watery eye, two for anaemia and mucositis clinical and six for thrombocytopenia.
The univariate analysis regressed the AEs against QoL individually. A multivariate analysis was also undertaken in which all AEs were simultaneously regressed against QoL. The multivariate analysis led to inconclusive results, with the individual AEs being highly correlated with one another. Dropping some AEs from the multivariate analysis still led to inconclusive results. In the effective absence of multivariate results, the authors recommend using the results of the univariate analysis.
The multicollinearity between the AEs of Boyd et al. 197 would appear to raise the possibility that within the univariate analyses the estimated QoL decrements for an AE are picking up not only the impact of the AE under consideration, but also some of the impacts of the AEs with which it is highly correlated. As a consequence the univariate values should perhaps be treated with some caution, with there being the possibility of double counting the QoL impacts of AEs.
The Boyd et al. 197 data still appears to be the best data in terms of robustness and alignment with NICE methods, but Lloyd et al. 196 also seems a credible source and unaffected by the problems of multicollinearity. In the light of this, the QoL impacts of AEs have been calculated on the following basis.
For grade III/IV AEs:
-
diarrhoea taken directly from the SCOT trial201 univariate estimates
-
nausea/vomiting taken as the mean of the SCOT trial201 univariate estimates
-
hand and foot syndrome assumed to be greater than the diarrhoea decrement by the Best et al. 190 proportion
-
mucositis taken from the SCOT trial201 univariate estimates
-
neutropenia taken from the SCOT trial201 univariate estimates
-
leucopenia assumed to be as per neutropenia
-
thrombocytopenia taken from Swinburn et al. 200
However, in the light of expert opinion neutropenia, leucopenia and thrombocytopenia are assumed to have no QoL impact for the base case.
Table 49 includes QoL decrements for the grade I/II AEs. These are largely driven by the SCOT trial201 estimates, but as with the estimates for grade III/IV AEs they are also informed by the other estimates within the literature. For analyses based on the AE rates of Gamelin et al. 118 the QoL estimates for grade I/II AE rates were applied within sensitivity analyses. There remain concerns with the SCOT trial201 estimates for grade I/II AEs given the issues around multicollinearity.
Adverse event: durations
There is a paucity of data on the duration of AEs within the literature. The duration of AEs is as an important a driver of the QALY impact of AEs as the QoL decrements outlined above. As already noted, there is duration data for grade III and grade IV AEs within the COIN trial data set, but it was not possible to arrange an intellectual property agreement with the University College London.
Expert opinion suggests that the following may be reasonable (Table 50).
| AE | Grade I/II | Grade III/IV |
|---|---|---|
| Diarrhoea | 18 | 5 |
| Nausea/vomiting | 12 | 5 |
| Hand and foot syndrome | 15 | 5 |
| Mucositis | 12 | 3 |
| Neutropenia | – | 7 |
| Leucopenia | – | 5 |
| Thrombocytopenia | – | 3 |
The durations for grade III/IV events in Table 50 are broadly in line with those reported for the average length of stay (LOS) associated with AEs as reported by Twelves et al. 202 (and summarised in Appendix 21). Whether or not the AEs have completely resolved at discharge may be a moot point, but it might be anticipated that any remaining QoL impacts would be somewhat below their peak effect.
My5-FU costs
The costs (excluding value-added tax), volumes and shelf lives once opened for the My5-FU elements as supplied by Saladax are:
-
£60 per stabiliser kit and 20 stabilisers per kit
-
£835 per assay kit, 100 assays per kit and a shelf life of 30 days
-
£75 per quality control kit, sufficient for 40 quality control runs and a shelf life of 90 days
-
£200 per calibration kit and a shelf life of 90 days.
Laboratory staff timings are based on expert opinion (Andrew Teggert, South Tees Foundation Hospitals NHS Trust; Helen Haley, South Durham NHS, 2013, personal communication): 2 minutes of a band 3 for sample receipt and preparation, 2 minutes of a band 6 per quality control run and 2 minutes of a band 6 per calibration. These are costed using the 2013 NHS pay scale203 coupled with a percentage mark-up for overheads drawn from the 2013 PSSRU Unit Costs of Health and Social Care204 costing for a hospital pharmacist.
The staff costs incurred obtaining a blood sample are based on 30 minutes of health visitor face-to-face time with the patient (Delyth Mcentee, The Christie NHS Foundation Trust Hospital, 28 March 2014, personal communication), costed using the 2013 PSSRU Unit Costs of Health and Social Care. 204 An alternative would be to have the blood taken at an outpatient appointment. The 2012–13 NHS reference costs205 give the following: nurse-led outpatient WF01A 370 medical oncology, non-admitted face-to-face follow-up appointment £102.
The cost per completed My5-FU assay is sensitive to:
-
The number of assays per kit Saladax suggests that each assay kit contains 100 assays. The Middlesborough laboratory was achieving around 200 assays per assay kit on their platform (Andrew Teggert, Helen Haley, personal communication).
-
Whether assays are batched weekly or daily Each batch requires quality control which involves both staff time and three additional assays. Given the fortnightly cycle length for FOLFOX, Saladax argues that weekly batching would be clinically feasible. Weekly batching of samples would if anything be simpler to implement within the laboratory, provided that this met clinical needs (Andrew Teggert, Helen Haley, personal communication).
-
The annual laboratory throughput If throughput is low a proportion of the assay kit has to be thrown away due to the 30-day shelf life of the assay kit once opened. The following assumes that My5-FU is only used for treating mCRC patients. The patient numbers identified in TA118179 can be coupled with the proportions of mCRC patients receiving infusional 5-FU both first and second line. For the North Tees and Hartlepool NHS Trust population of 365,000 this suggests perhaps around an annual 30 mCRC patients receiving infusional 5-FU regimes at first-line and at second-line (note that this results in some inconsistency between the costing and the modelling, since due to the available clinical effectiveness evidence the modelling is only of PK dose adjustment of first-line therapy). Coupling these with the median number of cycles for first and second line, 12 and 7, respectively, as reported in Tournigand et al. 168 suggests around an annual 500 cycles of infusional 5-FU for mCRC patients. Expert opinion suggests that this approximately correct, though may be a little low (Nick Wadd, North Tees and Hartlepool Trust, 2013, personal communication). Saladax suggests around four My5-FU assays will be required per patient which, assuming the assays are repeated at switch to second-line FOLFIRI, would result in an annual throughput of around 250 My5-FU assays. More continuous monitoring would tend to raise this.
As shown in Table 51, at throughputs below an annual of 500, the cost per test is quite sensitive to the annual throughput. Once the annual throughput has risen to above 1000 the cost per test has largely stabilised (Figure 39).
| Annual | Weekly (£) | Daily (£) | ||
|---|---|---|---|---|
| 100/kit | 200/kit | 100/kit | 200/kit | |
| 100 | 86.77 | 68.39 | 180.03 | 120.35 |
| 200 | 67.47 | 56.19 | 114.73 | 82.17 |
| 300 | 61.03 | 52.12 | 92.96 | 69.44 |
| 400 | 57.82 | 50.09 | 82.08 | 63.08 |
| 600 | 54.60 | 48.05 | 71.19 | 56.71 |
| 800 | 52.99 | 47.03 | 65.75 | 53.53 |
| 1000 | 52.18 | 46.42 | 62.49 | 51.62 |
| 1200 | 51.72 | 46.02 | 60.31 | 50.36 |
| 1500 | 51.26 | 45.61 | 58.13 | 49.15 |
FIGURE 39.
Cost per completed My5-FU assay and annual throughput: by kit volume and batch frequency.

In the light of the above, the base case will assume an annual throughput of 300 with weekly batching and 100 assays per kit. This results in a cost per completed My5-FU assay of £61.03 as outlined in greater detail below (Table 52).
| Cost element | Cost (£) |
|---|---|
| Stabiliser | 3.00 |
| Staff input calibration | 0.05 |
| Staff input QC | 0.38 |
| Staff input initial handling | 1.30 |
| My5-FU assay | 17.78 |
| Calibration | 2.70 |
| QC | 0.33 |
| Total laboratory per test | 25.53 |
| Health visitor cost of taking sample | 35.50 |
| Total cost | 61.03 |
A sensitivity analysis that assumes 200 assays per assay kit will be performed, resulting in a cost per test of £52.12. Further sensitivity analyses of annual throughputs of 500 and 1000 were performed, as was sensitivity analyses that assume 200 assays per assay kit. For the 500 throughput this results in costs per test of £55.89 and £48.86, and for the 1000 throughput this results in costs per test of £52.18 and £46.42.
Note that within the above there is no allowance for the capital cost of the analyser. These typically have a daily capacity of between 1200 and 1800, though this may not be fully utilised. Service costs for this element are to some degree commercial in confidence. The underlying assumption is that the marginal analyser capital cost per additional assay is small to the point of insignificance. However, it has to be acknowledged that the analysers are expensive and a multiyear laboratory service contract may well stretch into seven figures.
My5-FU assays per patient
Academic in confidence information has been removed. Expert opinion suggests that testing would initially be until stabilisation had occurred, and then perhaps every third cycle or when there was unexpected toxicity.
Capitain et al. 119 report that 64% (n = 75/118) of patients in the PK dosing arm had increased their dose by at least 10% at 3 months, with a range of 10–40% and a mean increase of 20%. Thirty-six per cent (n = 42/118) increased their dose by at least 20%, among whom the range was 20–40% with a mean increase of 26%. Unfortunately, Capitain et al. 119 do not present any data on the number of adjustments that were required. The dose adjustment algorithm permits dose adjustments of as little as 5%, but adjustments may be somewhat larger than this, depending on how far from the target range the plasma concentration is.
Similarly, Capitain et al. 119 report that 19% (n = 22/118) had a dose reduction of at least 10%, with a range of 10–40% and a mean of 20%. Twelve per cent (n = 14/118) had a dose decrease of at least 20%, with a range of 20–40% and a mean of 26%.
In total, within the Capitain et al. 119 study the vast majority of patients in the PK dose adjustment arm had their dose adjusted: 82%. These patients would require a minimum of three My5-FU assays for stability; that is two consecutive results within the target range, to have been established.
Given an assumption that final bandings were all at 10% increments to the initial dose, this is consistent with 28% (n = 33/118) having a dose increase of 10%, 17% (n = 20/118) having a dose increase of 20%, 15% (n = 18/118) having a dose increase of 30% and 3% (n = 4/118) having a dose increase of 40%. For the proportion having their dose reduced, this is consistent with 7% (n = 8/118) having a dose reduction of 10%, 6% (n = 7/118) having a dose reduction of 20%, 5% (n = 6/118) having a dose reduction of 30% and 1% (n = 1/118) having a dose reduction of 40%.
The steps taken to get to these dose reductions is unknown. Some may have been in steps of 5%, some in steps > 10%. To some extent the likelihood of these different sized steps may be cancelled out by the larger number of patients only having a relatively small overall adjustment of 10% compared with the smaller number having a larger overall adjustment of 30% or 40%. With this in mind, as a working assumption, a common step of 10% can be assumed across all patients. In other words, those with:
-
0% adjustment would require two My5-FU assays
-
10% adjustment would require three My5-FU assays
-
20% adjustment would require four My5-FU assays
-
30% adjustment would require five My5-FU assays
-
40% adjustment would require six My5-FU assays.
Applying these suggests an average initial requirement of 3.23 My5-FU assays, broadly in line with the Saladax figure. If the adjustment to 10% is more usually done in 5% increments as may occur under the Gamelin et al. 118 dose adjustment algorithm, so resulting in a minimum requirement for four My5-FU assays for these patients, the average initial requirement rises to perhaps around 4.4 My5-FU assays. However, it should also be borne in mind that the minimum step in the Kaldate et al. 96 dose adjustment algorithm is around 10% of the initial dose.
The base case will assume an average initial requirement of 3.23 My5-FU assays per patient. Sensitivity analyses will vary this to (AiC information has been removed) and to 4.4 My5-FU assays per patient. The other extreme of assuming all adjustment occurs after the first test can also be assumed, capping the number of initial My5-FU assays per patient at a maximum of three.
Dose adjustment will also require further staff time to calculate the appropriate dose and communicate this to pharmacy. There is some uncertainty around what is reasonable to assume for this, but the base case will assume 10 minutes of consultant time per adjustment (Nick Wadd, personal communication). The base case applies this only in the My5-FU arm. It can be argued that it should also be applied to the 4% reported by Capitain et al. 119 who had their dose reduced in the BSA arm, though this would have little impact on results.
However, there remains some uncertainty as to how many further My5-FU assays would be required over the course of chemotherapy. Gamelin et al. 118 reported an average of four cycles to get the plasma concentration in range, though the relevance of this may be reduced by the initial 5-FU dose employed within Gamelin et al. 118 Kline et al. 155 also suggested that as the number of cycles increases the ability to metabolise 5-FU fall which may tend to increase plasma concentrations and so result in an ongoing need for testing using My5-FU.
Expert opinion suggests that unexpected toxicity could lead to more assays being used, or that there might be routine monitoring every third cycle post stabilisation. This will be explored as a sensitivity analysis.
Chemotherapy costs
The cost of line insertion is common to both arms and so has not been included.
Iveson et al. 206 estimate a cost for a disposable pump for 5-FU of £62 [£105], inclusive of all disposables and pharmacist time, though there is some ambiguity whether this is per cycle or per week within a 2-week cycle. This is broadly in line with the £35 [£38] per pump of TA212183 and the £37 [£39] per pump of Shabarrudin,207 when these are coupled with the £38 [£47] pharmacist time for the 5-FU infusion of TA9330 and TA118. 179 In the light of this, a cost per disposable pump of £39 will be applied (exclusive of pharmacist time).
Drug costs have been sourced from the Commercial Medicines Unit Electronic Market Information Tool (CMU eMIT) database208 in line with the 2013 NICE methods guide. 209 Note that though PK dosing results in 5-FU dose changes, the ingredient cost of the 5-FU is so small that the costs of the change in the 5-FU being administered has not been factored into the analysis.
Pharmacy preparation costs have been taken from TA93,30 uprated for inflation. A point worth bearing in mind is that some hospital pharmacies contract out the 5-FU preparation. This approach has not been taken into account in the analysis and it would be difficult to do so given considerations around commercial confidentiality.
The administration cost is based on the outpatient cost for a complex prolonged infusion: SBZ14Z (Helen Haley, personal communication).
Staff costs for flushing the line at the end of each cycle are based on 40 minutes of health visitor time face to face with the patient (Delyth Mcentee, personal communication), costed using the 2013 PSSRU Unit Costs of Health and Social Care. 204 Some areas recall patients for an outpatient appointment for termination of their cycle. As a consequence, the £102 for a nurse-led outpatient WF01A 370 medical oncology, non-admitted face-to-face follow-up appointment can be used for a sensitivity analysis.
This results in the following cost per cycle for FOLFOX and FOLFIRI (Table 53).
| Cost element | FOLFOX (£) | FOLFIRI (£) |
|---|---|---|
| Administration | 286.60 | 286.60 |
| Pharmacy | 189.06 | 189.06 |
| FA | 6.17 | 6.17 |
| Oxaliplatin | 18.12 | |
| Irinotecan | 29.02 | |
| 5-FU bolus | 1.27 | 1.27 |
| 5-FU infusion | 3.70 | 3.70 |
| Pump | 38.96 | 38.96 |
| Line flush | 40.67 | 40.67 |
| Total | 584.54 | 595.44 |
Other ongoing costs
Ongoing monthly costs have been drawn from Kerr et al. as reported in TA118:179 consultations £80 [£128], tests £65 [£103] and primary care costs £10 [£17].
Adverse events: resource use
The systematic literature review of AEs costs in sterling (as summarised in Appendix 21) found relatively little of use for current purposes.
Leese210 reviewed patient notes and estimated febrile neutropenia to cost £2445 [£4428] for patients with haematological disorders but no solid tumours. Leese et al. ’s study,211 which was based on expert opinion, suggested an estimate of £1542 [£2793] for patients with solid tumours.
Smith et al. 212 provide cost estimates for a number of grade III/IV AEs. The following estimates appear to be implied:
-
£1000 [£1532] for stomatitis
-
£1016 [£1557] for diarrhoea
-
£600 [£920] for hand and foot syndrome
-
£1100 [£1686] for nausea/vomiting
-
£200 [£307] for neutropenia
-
£780 [£1196] for sepsis/fever
-
£780 [£1196] for anaemiathrom/bocytopenia.
However, there is a lack of detail within the paper.
In the light of the above, AEs will be costed as either an inpatient episode or as a prescribed drug therapy.
The costs of AEs will be mainly driven by the proportion of AEs requiring hospitalisation. Expert opinion suggests that the following might be approximately reasonable (Table 54), though considerable uncertainty surrounds these estimates and there was a suggestion by one of the experts that the estimates may be on the high side. There was also disagreement on whether or not neutropenia would lead to admission, the following assuming that admission would only occur if it progressed to be febrile neutropenia.
| AE | Grade, % | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Diarrhoea | 0 | 5 | 50 | 100 |
| Vomiting/nausea | 0 | 5 | 50 | 100 |
| Mucositis | 0 | 5 | 50 | 100 |
| Hand and foot syndrome | 0 | 5 | 50 | 100 |
| Leucopenia | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 5 |
Adverse events: unit costs
The Healthcare Resource Groups (HRGs) for hospitalisations have been taken from the NHS reference cost grouper (Table 55). 213 CRC suggests a comorbidity and complication score of 2 leading to the following HRG and costs. The inpatient costs are taken from non-elective short stay and non-elective long stay as a weighted average of all admissions, and not limited to general medicine or oncology admissions.
| AE | HRG | Description | Mean (£) |
|---|---|---|---|
| Diarrhoea | FZ91M | Non-malignant gastrointestinal tract disorders, without interventions, with CC score 0–2 | 798 |
| Vomiting/nausea | FZ91M | Non-malignant gastrointestinal tract disorders, without interventions, with CC score 0–2 | 798 |
| Mucositis | CZ23Y | Major head, neck and ear disorders, without CC | 663 |
| CZ23X | Major head, neck and ear disorders, with intermediate CC | 781 | |
| Hand foot syndrome | JD07J | Skin disorders without interventions, with CC score 2–5 | 1102 |
| Leucopenia | SA35D | Agranulocytosis with CC score 2–4 | 1490 |
| Neutropenia | SA08J | Other haematological or splenic disorders, with CC score 0–2 | 921 |
| Thrombocytopenia | SA12J | Thrombocytopenia with CC score 2–4 | 1453 |
Those not treated as inpatients are assumed to be identified during routine follow-up and prescribed medication:
-
Diarrhoea: loperamide [BNF214 £1.74; CMU eMIT208 £0.28], codeine, antibiotics if felt to be infective.
-
Nausea/vomiting: domperidone [BNF214 £1.39; CMU eMIT208 £0.32], metoclopramide, ondansteron.
-
Mucositis: benzydamine [BNF214 £6.45], vaseline on lips.
-
Hand and foot syndrome: simple creams such as diprobase [BNF214 £6.32], topical antibacterial.
-
Thrombocytopenia: a small percentage of serious events may get platelet transfusion, with Varney et al. as reported in TA145215 suggesting a cost of £84.22 [£101.08].
Summary of main parameter input values to the base cases
The main parameter inputs that result from the above are summarised below (Tables 56–59).
| Curve | Value | Source |
|---|---|---|
| Clinical effectiveness: FOLFOX | ||
| PK λ OS | 0.00233 | Inferred from Captain et al.119 |
| PK γ OS | 1.66906 | Inferred from Captain et al.119 |
| BSA λ OS | 0.00398 | Inferred from Captain et al.119 |
| BSA γ OS | 1.66906 | Inferred from Captain et al.119 |
| PK λ PFS | 0.02438 | Inferred from Captain et al.119 |
| PK γ PFS | 1.13668 | Inferred from Captain et al.119 |
| BSA λ PFS | 0.05060 | Inferred from Captain et al.119 |
| BSA γ PFS | 1.13668 | Inferred from Captain et al.119 |
| Clinical effectiveness: FUFOL | ||
| PK λ OS | 0.00270 | Inferred from Gamelin et al.118 |
| PK γ OS | 1.82786 | Inferred from Gamelin et al.118 |
| BSA λ OS | 0.00865 | Inferred from Gamelin et al.118 |
| BSA γ OS | 1.54066 | Inferred from Gamelin et al.118 |
| PK λ PFS | 0.05541 | Inferred from pooled three studies162,163,165 |
| PK γ PFS | 1.35834 | Inferred from pooled three studies162,163,165 |
| BSA λ PFS | 0.05541 | Inferred from pooled three studies162,163,165 |
| BSA γ PFS | 1.35834 | Inferred from pooled three studies162,163,165 |
| AE rates | Grade, % | Source | |||
|---|---|---|---|---|---|
| III/IV | |||||
| FOLFOX | |||||
| Diarrhoea PK | 2 | Captain et al.119 | |||
| Neutropenia PK | 18 | Captain et al.119 | |||
| Mucositis PK | 1 | Captain et al.119 | |||
| Thrombocytopenia PK | 12 | Captain et al.119 | |||
| Diarrhoea BSA | 12 | Captain et al.119 | |||
| Neutropenia BSA | 25 | Captain et al.119 | |||
| Mucositis BSA | 15 | Captain et al.119 | |||
| Thrombocytopenia BSA | 10 | Captain et al.119 | |||
| I | II | III | IV | ||
| FUFOL | |||||
| Diarrhoea PK | 9 | 3 | 4 | 0 | Gamelin et al.118 |
| Mucositis PK | 2 | 1 | 1 | 1 | Gamelin et al.118 |
| Hand and foot syndrome PK | 30 | 21 | 10 | 1 | Gamelin et al.118 |
| Leucopenia PK | 0 | 0 | 0 | 0 | Gamelin et al.118 |
| Diarrhoea BSA | 14 | 28 | 15 | 3 | Gamelin et al.118 |
| Mucositis BSA | 2 | 1 | 1 | 1 | Gamelin et al.118 |
| Hand and foot syndrome BSA | 16 | 22 | 7 | 0 | Gamelin et al.118 |
| Leucopenia BSA | 0 | 2 | 1 | 1 | Gamelin et al.118 |
| QoL values | PFS | SWP | Source | ||
|---|---|---|---|---|---|
| Färkkilä et al.192 | 0.820 | 0.643 | |||
| TA176182 | 0.800 | 0.600 | |||
| Best et al.190 | 0.515 | 0.213 | |||
| AE QALYs | Grade I | Grade II | Grade III | Grade IV | |
| Diarrhoea | –0.0020 | –0.0020 | –0.0012 | –0.0012 | MRC SCOT trial201 + opinion |
| Nausea/vomiting | –0.0012 | –0.0012 | –0.0011 | –0.0011 | MRC SCOT trial201 + opinion |
| Hand and foot syndrome | –0.0005 | –0.0005 | –0.0014 | –0.0014 | MRC SCOT trial201 + opinion |
| Mucositis | –0.0017 | –0.0017 | –0.0003 | –0.0003 | MRC SCOT trial201 + opinion |
| Neutropenia | 0.0000 | 0.0000 | –0.0012 | –0.0012 | MRC SCOT trial201 + opinion |
| Leucopenia | 0.0000 | 0.0000 | –0.0008 | –0.0008 | MRC SCOT trial201 + opinion |
| Thrombocytopenia | 0.0000 | 0.0000 | –0.0007 | –0.0007 | MRC SCOT trial201 + opinion |
| Costs of My5-FU | Source | ||||
|---|---|---|---|---|---|
| Cost My5-FU, £ | 61.03 | Saladax plus NHS staffing costs | |||
| Number of My5-FU assays | 3.23 | Inferred from Captain et al.119 | |||
| Number dose adjustments | 1.58 | Inferred from Captain et al.119 | |||
| Cost dose adjustments (£) | 23.17 | 10 minutes consultant time | |||
| Chemotherapy cost (£) | |||||
| FOLFOX per cycle | 591.21 | CMU eMIT208 + NHS reference costs209 | |||
| FOLFIRI per cycle | 602.11 | CMU eMIT208 + NHS reference costs209 | |||
| AE cost (£) | Grade I | Grade II | Grade III | Grade IV | |
| Diarrhoea | 1.74 | 41.54 | 399.78 | 797.82 | CMU eMIT208 + NHS reference costs209 |
| Nausea/vomiting | 1.39 | 41.21 | 399.61 | 797.82 | CMU eMIT208 + NHS reference costs209 |
| Hand and foot syndrome | 6.32 | 61.09 | 554.07 | 1101.81 | CMU eMIT208 + NHS reference costs209 |
| Mucositis | 6.45 | 45.16 | 393.53 | 780.61 | CMU eMIT208 + NHS reference costs209 |
| Neutropenia | 0.00 | 0.00 | 0.00 | 0.00 | CMU eMIT208 + NHS reference costs209 |
| Leucopenia | 0.00 | 0.00 | 0.00 | 0.00 | CMU eMIT208 + NHS reference costs209 |
| Thrombocytopenia | 0.00 | 0.00 | 4.21 | 76.65 | CMU eMIT208 + NHS reference costs209 |
Results: FOLFOX studies analyses
For the FOLFOX studies base case, the following deterministic results apply for the two scenarios of treatment for 12 cycles and treatment until progression (Table 60).
| Item | My5-FU | BSA | Net |
|---|---|---|---|
| LYs | 2.63 | 1.95 | 0.69 |
| QALY | 2.07 | 1.47 | 0.60 |
| Costs (£) | |||
| My5-FU | 197 | 0 | 197 |
| Adjustment | 37 | 0 | 37 |
| FOLFOX | 6560 | 6092 | 467 |
| FOLFIRI | 2442 | 2578 | –136 |
| Monitoring | 7895 | 5847 | 2047 |
| AEs | 15 | 144 | –129 |
| Total | 17,145 | 14,663 | 2483 |
| ICER | 4148 | ||
Apparent in the above is that the second-line FOLFIRI provides a reasonably large cost offset: sufficient for two My5-FU test costs in the treatment for 12 cycles scenario. This appears to mainly arise due to the PFS curve for My5–FU crossing over, and so being assumed to follow, the OS curve. As second-line FOLFIRI is only administered on entering SWP after first-line FOLFOX the proportion of patients receiving second-line FOLFIRI within the My5-FU arm is not only later than in the BSA arm, it is also less. This may be more an artefact of the model structure than a reasonable assumption, and argues for a sensitivity analysis that excluded the impact of second-line FOLFIRI.
The probabilistic modelling of 10,000 iterations coincidentally results in the same central estimate of cost-effectiveness: £4148 per QALY. The probabilistic modelling results in the following scatterplot (Figure 40) and CEAC (Figure 41).
FIGURE 40.
FOLFOX base case: cost-effectiveness plane scatterplot of the results of each probabilistic sensitivity analysis iteration.
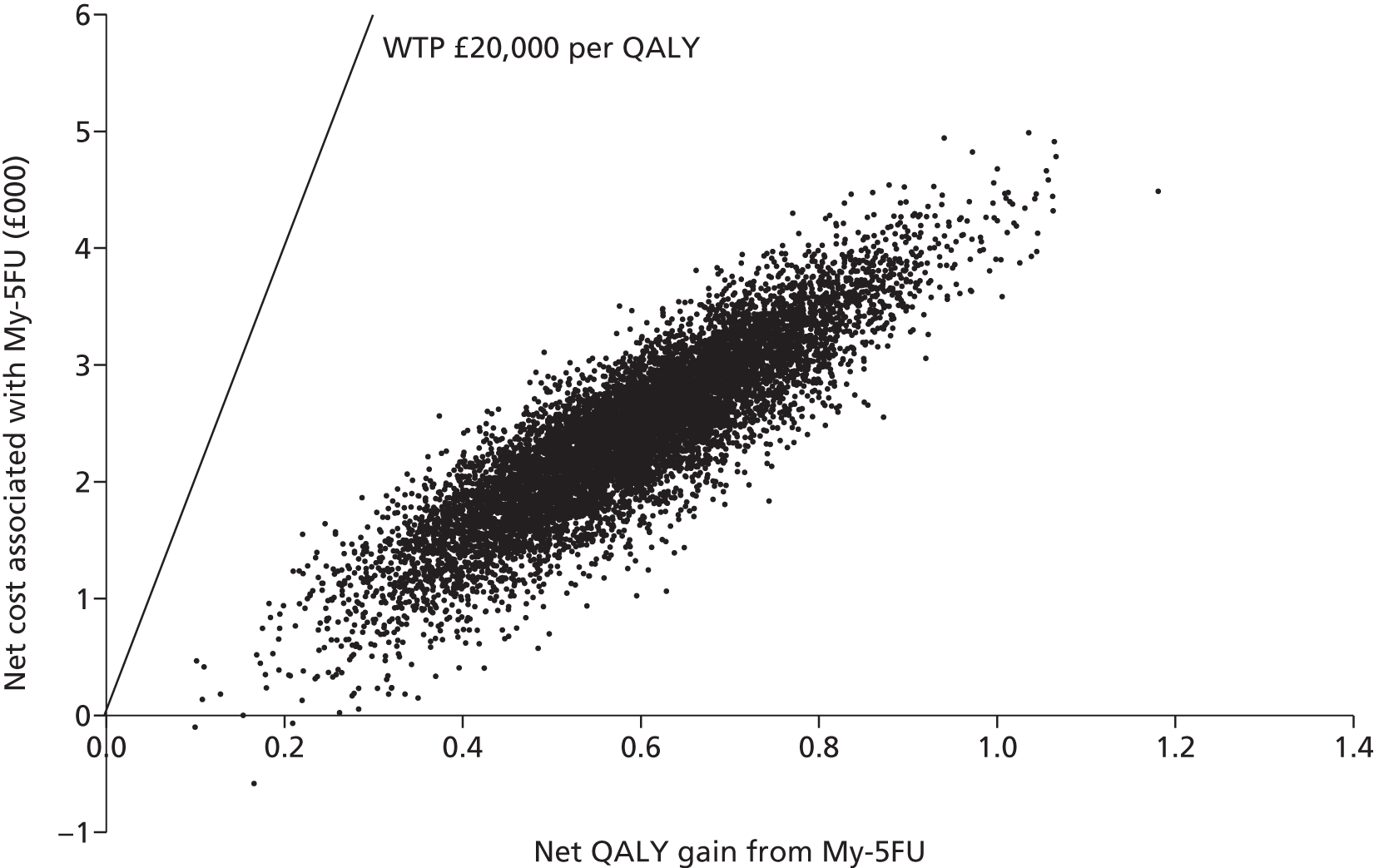
FIGURE 41.
FOLFOX base case: CEACs – probability that My5-FU dosing is cost-effective compared with BSA dosing.
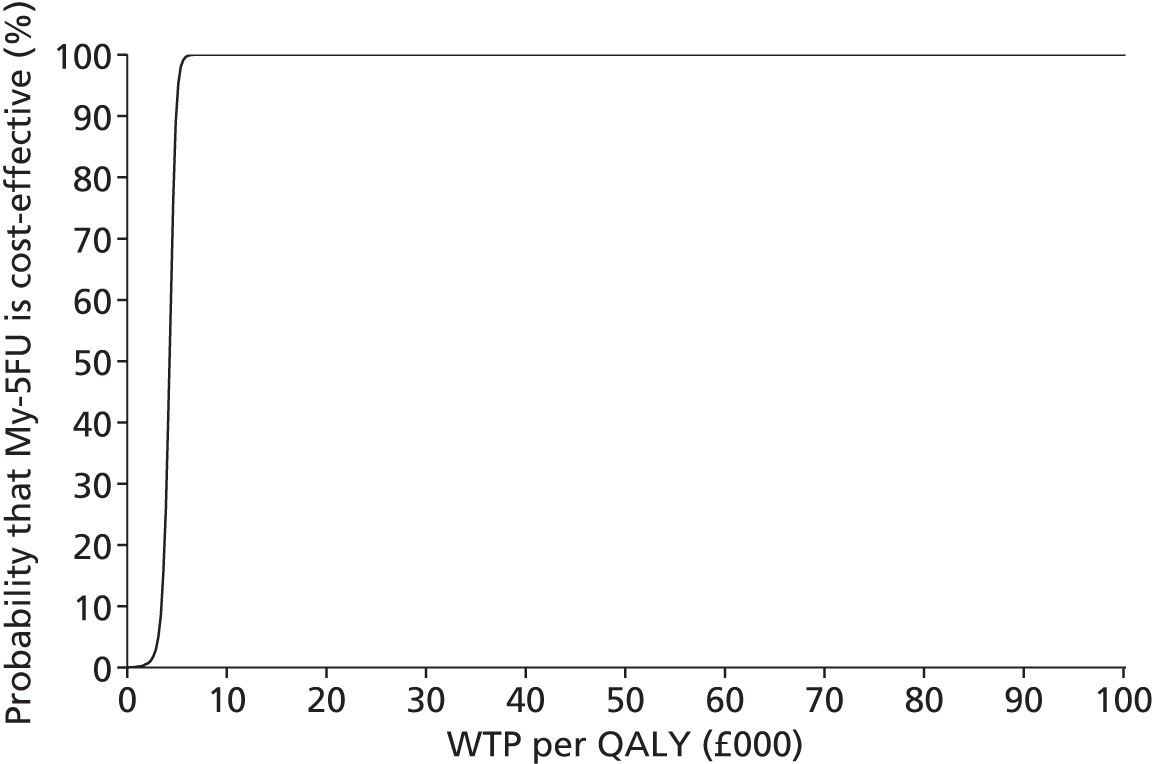
For the scenario analyses applying BSA curves from a range of studies within the literature, the following results apply (Table 61).
| Item | My5-FU | BSA | Net |
|---|---|---|---|
| Scenario analysis 1 | |||
| LY | 2.63 | 2.37 | 0.26 |
| QALY | 2.07 | 1.74 | 0.32 |
| Total | £17,145 | £15,933 | £1213 |
| ICER | £3740 | ||
| Scenario analysis 2 | |||
| LY | 2.63 | 1.61 | 1.03 |
| QALY | 2.07 | 1.22 | 0.85 |
| Total | £17,145 | £13,783 | £3362 |
| ICER | £3950 | ||
| Scenario analysis 3 | |||
| LY | 2.63 | 1.76 | 0.88 |
| QALY | 2.07 | 1.32 | 0.74 |
| Total | £17,145 | £14,281 | £2864 |
| ICER | £3850 | ||
| Scenario analysis 4 | |||
| LY | 2.63 | 2.20 | 0.44 |
| QALY | 2.07 | 1.60 | 0.47 |
| Total | £17,145 | £15,492 | £1653 |
| ICER | £3514 | ||
| Scenario analysis 5 | |||
| LY | 2.63 | 1.89 | 0.75 |
| QALY | 2.07 | 1.40 | 0.67 |
| Total | £17,145 | £14,624 | £2521 |
| ICER | £3762 | ||
The first scenario analysis of applying the HR from Gamelin et al. 118 to the Weibull for OS derived from the PK dosing arm of Capitain et al. ,119 in order to derive the Weibull for OS in the BSA dosing arm, improves the cost-effectiveness of My5-FU from the £4148 of the base case to £3740 per QALY.
This may initially seem a perverse result. The change increases discounted survival in the BSA arm to 2.37 years. As there is no change to the PFS curve in the BSA arm, all this survival is modelled as being experienced at the SWP QoL of 0.643, resulting in total QALYs in the BSA arm of 1.74: an increase of 0.27 over the base case. However, this additional survival involves an increase in ongoing treatment and monitoring costs from £5847 to £7117: an increase of £1269 compared with the base case. This can be interpreted as a cost per QALY of £4626. In other words, although there are additional ongoing costs from the increased survival in the BSA dosing arm there is no suggestion that the increase in survival is not cost-effective, even if it is only experienced at the SWP QoL.
The key point here is that £4626 per QALY for this additional survival in the BSA dosing arm lies above the base-case 4168 per QALY for My5-FU compared with BSA. As a consequence, the change improves the cost-effectiveness estimate for My5-FU compared with BSA. Had the cost-effectiveness for My5-FU compared with BSA dosing been somewhat higher than £4626 per QALY, applying the HR of Gamelin et al. 118 to derive the BSA OS curve would have somewhat worsened the cost-effectiveness estimate for My5-FU compared with BSA dosing.
This sensitivity analysis is also dependent on the model structure and it forcing the additional survival to be evaluated at the SWP QoL of 0.643. Had it been evaluated at the PFS QoL of 0.820, the cost-effectiveness of My5-FU would have worsened to £4876 per QALY.
Changing the source of the BSA PFS estimates and OS estimates as in scenario analysis 2, from those inferred from the Capitain et al. 119 medians, to those derived from pooling BSA arms within the literature, has relatively little impact on cost-effectiveness results. Similarly, changing the source to Ducreux et al. 167 improves the ICERs slightly further.
Changing the source of the BSA PFS estimates and OS estimates as in scenario analysis 4, from those inferred from the Capitain et al. 119 medians, to those derived from Tournigand et al. ,168 again, slightly improve the ICER but the effect is not dramatic.
The last scenario analysis explored the impact of applying the BSA OS curve derived from non-UK studies. The justification for this is that the UK studies may suggest a worse OS. In order to compare like with like, the estimates from Capitain et al. 119 for PK dosing can be compared with the estimates from the pooled non-UK studies for BSA dosing. The impact on results is again not large.
In short, if the HR for OS derived from Gamelin et al. 118 is applied, under a plausible scenario this may slightly worsen the cost-effectiveness estimate. However, in the main, applying the curves derived from single arms within the literature has relatively little impact on results.
Results: 5-fluorouracil + folinic acid studies analyses
For the FUFOL studies base case, the following deterministic results apply (Table 62).
| Item | My5-FU | BSA | Net |
|---|---|---|---|
| LYs | 1.81 | 1.57 | 0.23 |
| QALYs | 1.30 | 1.15 | 0.15 |
| Costs (£) | |||
| My5-FU | 197 | 0 | 197 |
| Adjustment | 37 | 0 | 37 |
| FOLFOX | 5751 | 5751 | 0 |
| FOLFIRI | 2619 | 2614 | 5 |
| Monitoring | 5433 | 4738 | 695 |
| AEs | 111 | 161 | –50 |
| Total | 14,147 | 13,264 | 883 |
| ICER | 5853 | ||
The probabilistic modelling of 10,000 iterations results in a very similar central estimate of cost-effectiveness: £5852 per QALY. Figure 42 (scatterplot) and Figure 43 (CEAC) show the probabilistic modelling results.
FIGURE 42.
5-fluorouracil + FA base case: cost-effectiveness plane scatterplot of the results of each probabilistic sensitivity analysis iteration.

FIGURE 43.
5-fluorouracil + FA base case: CEACs – probability that My5-FU doing is cost-effective compared with BSA dosing.

For the scenario analyses applying BSA curves from a range of studies within the literature, the following results apply (Table 63).
| Item | My5-FU | BSA | Net |
|---|---|---|---|
| Scenario analysis 1 | |||
| LY | 1.81 | 1.57 | 0.23 |
| QALY | 1.30 | 1.13 | 0.17 |
| Total | £14,540 | £13,059 | £1480 |
| ICER | £8615 | ||
| Scenario analysis 2 | |||
| LY | 1.81 | 1.57 | 0.23 |
| QALY | 1.37 | 1.16 | 0.21 |
| Total | £15,024 | £13,558 | £1466 |
| ICER | £6965 | ||
| Scenario analysis 3 | |||
| LY | 1.81 | 1.57 | 0.23 |
| QALY | 1.37 | 1.15 | 0.22 |
| Total | £14,127 | £13,264 | £862 |
| ICER | £3989 | ||
| Scenario analysis 4 | |||
| LY | 1.90 | 1.57 | 0.33 |
| QALY | 1.37 | 1.15 | 0.21 |
| Total | £14,427 | £13,264 | £1163 |
| ICER | £5459 | ||
| Scenario analysis 5 | |||
| LY | 1.90 | 1.57 | 0.33 |
| QALY | 1.43 | 1.15 | 0.28 |
| Total | £14,427 | £13,264 | £1163 |
| ICER | £4165 | ||
| Scenario analysis 6 | |||
| LY | 1.90 | 1.44 | 0.46 |
| QALY | 1.43 | 1.07 | 0.36 |
| Total | £14,427 | £12,864 | £1564 |
| ICER | £4291 | ||
For the first two scenario analyses that apply the PFS estimates derived from Gamelin et al. 134 response duration data, the cost-effectiveness of My5-FU is estimated to worsen compared with the base case. This appears to be due to a modelled increase in the proportion of patients receiving ongoing first-line FOLFOX6 tending to outweigh the QoL gains from an increased duration of PFS. This effect appears to work in reverse for the scenario analysis that applies the PFS curve of Gamelin et al. 134 in the My5-FU.
The remaining scenario analyses that pool results for a range of curves from a number of one-arm studies have only a limited impact on results.
Sensitivity analyses: FOLFOX studies
As previously discussed, sensitivity analyses around the cost per My5-FU assay can be conducted (Table 64). These assume annual throughputs of 500 and 1000, and also assume 200 assays per kit and, finally, the throughput and assays per kit of the base case but with daily batches.
| Sensitivity analysis | Δ QALY | Δ Cost (£) | ICER (£) |
|---|---|---|---|
| Base case | 0.599 | 2483 | 4148 |
| 500 throughput, 100 per kit | 0.599 | 2466 | 4120 |
| 1000 throughput, 100 per kit | 0.599 | 2454 | 4100 |
| 300 throughput, 200 per kit | 0.599 | 2454 | 4100 |
| 500 throughput, 200 per kit | 0.599 | 2443 | 4082 |
| 1000 throughput, 200 per kit | 0.599 | 2436 | 4069 |
| Daily My5-FU batches | 0.599 | 2586 | 4320 |
| 20% second-course FOLFOX6 | 0.599 | 3156 | 5272 |
| No second-line FOLFIRI | 0.600 | 2618 | 4363 |
| £3000 end-of-life cost | 0.599 | 2410 | 4026 |
| QoL: TA176182 | 0.589 | 2483 | 4214 |
| QoL: Best et al.190 | 0.413 | 2483 | 6016 |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| 4.4 My5-FU assays | 0.599 | 2554 | 4267 |
| Single adjustment/extra assay | 0.599 | 2465 | 4118 |
| Ongoing one-third My5-FU assays used | 0.599 | 2610 | 4361 |
| Outpatient visit for blood test | 0.599 | 2697 | 4506 |
| AEs from Gamelin et al.118 | 0.599 | 2562 | 4277 |
| Same OS and PFS | 0.000 | 104 | 435,819 |
Expert opinion suggests that between 10% and 20% of patients that started a 12-cycle course of FOLFOX6 might, after a treatment holiday, start another course of FOLFOX6. This can be explored by assuming that 40% of those remaining in PFS at the end of the first year start another course of FOLFOX6.
As also discussed above, a sensitivity analysis excluding second-line FOLFIRI appears justified due to the My5-FU PFS curve crossing and so being modelled as following the OS curve after a certain point.
The modelling has not considered end-of-life costs, as all patients will be modelled as incurring these costs. However, they will incur them at different times and discounting may have an effect. As a consequence, an admittedly arbitrary end-of-life cost of £3000 for each incident death can be modelled.
It is unclear whether or not there is sufficient health visitor capacity for blood samples to always be taken in the community. In the light of this, the cost of a nurse-led outpatient appointment can be assumed for the taking of the blood sample.
The alternative sources of QoL values for the main health states can also be explored.
The number of My5-FU assays required per patient for initial stabilisation can be raised to (AiC information has been removed) and 4.4. Based on expert opinion, it can also be assumed that there will be a need for ongoing monitoring using My5-FU subsequent to initial stabilisation in one-third of FOLFOX administrations.
Changing the source of AE estimates to be Gamelin et al. 118 can also be explored. The extent to which the OS estimates and the PFS estimates drive results can be explored by excluding them, leaving only the differences in AE costs and effects and the additional costs of My5-FU testing.
Cost-effectiveness estimates are relatively insensitive to the throughputs that are assumed, provided that weekly batching is possible. They are more sensitive to whether batching is weekly or daily, but again the difference is not dramatic.
Assuming that a proportion of patients remaining in PFS at 12 months would receive a second course of FOLFOX6 has a reasonable impact on the cost-effectiveness estimate, worsening it to £5272 per QALY.
Excluding second-line FOLFIRI has a relatively minor impact, as do end-of-life costs.
The source of the QoL values is rather more important, as might be anticipated. The values of TA176182 worsen the cost-effectiveness estimates to a degree. The values of Best et al. 190 have a rather larger impact as might be anticipated due to valuing the additional survival less highly.
Increasing the number of My5-FU assays to (AiC information has been removed) and to 4.4 for the initial stabilisation has only a limited impact. Assuming ongoing monitoring while on treatment has only a minor impact due to the base case assuming only 12 cycles of treatment.
Costing the taking of the blood sample at the outpatient visit rate has a reasonable impact, worsening the cost-effectiveness estimate to £4506 per QALY.
The source of AEs has only a limited impact.
Equalising OS and PFS between the arms shows the extent to which the cost-effectiveness of My5-FU rests on these. The costs effectiveness estimates increase dramatically, as would be expected. These cost-effectiveness estimates would further worsen if, after the initial stabilisation period, further ongoing monitoring with My5-FU was required.
Sensitivity analyses: 5-fluorouracil ± folinic acid studies
A similar set of sensitivity analyses to those presented above for the analyses based on FOLFOX studies can be undertaken for the analyses based on FUFOL studies, only the last changing the source of AE estimates to be Capitain et al. 119 (Table 65).
| Sensitivity analysis | Δ QALY | Δ Cost (£) | ICER (£) |
|---|---|---|---|
| Base case | 0.151 | 883 | 5853 |
| 500 throughput, 100 per kit | 0.151 | 866 | 5743 |
| 1000 throughput, 100 per kit | 0.151 | 854 | 5663 |
| 300 throughput, 200 per kit | 0.151 | 854 | 5662 |
| 500 throughput, 200 per kit | 0.151 | 844 | 5593 |
| 1000 throughput, 200 per kit | 0.151 | 836 | 5540 |
| Daily My5-FU batches | 0.151 | 986 | 6535 |
| 20% second-course FOLFOX6 | 0.151 | 883 | 5853 |
| No second-line FOLFIRI | 0.151 | 878 | 5820 |
| £3000 end-of-life cost | 0.151 | 859 | 5693 |
| QoL: TA176182 | 0.141 | 883 | 6270 |
| QoL: Best et al.190 | 0.051 | 883 | 17,485 |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| 4.4 My5-FU assays | 0.151 | 954 | 6324 |
| Single adjustment/extra assay | 0.151 | 865 | 5736 |
| Ongoing one-third My5-FU assays used | 0.151 | 990 | 6559 |
| Outpatient visit for blood test | 0.15 | 1098 | 7274 |
| AEs from Capitain et al.119 | 0.150 | 804 | 5344 |
| Same OS and PFS | 0.000 | 104 | 435,804 |
The pattern of results for the sensitivity analyses based on the FUFOL studies mirrors that of the sensitivity analyses based on the FOLFOX studies. The main sensitivity analysis of interest is the application of the Best et al. 190 QoL values, this worsening the cost-effectiveness estimate to £17,485 per QALY. If this is coupled with an outpatient visit being required for the taking of the blood sample the cost-effectiveness estimate would worsen further to £21,732 per QALY.
The cost-effectiveness of pharmacokinetic dose adjustment using My5-FU in head and neck cancer
Modelling approach
Blanchard et al. 50 undertook a meta-analysis of studies comparing induction chemotherapy using a taxane plus cisplatin and 5-FU (TPF) with induction chemotherapy using PF alone. They noted that the rates of radiotherapy and concomitant chemotherapy differed significantly following TPF induction and PF induction: 85% of patients received their planned radiotherapy, 49% received concomitant chemotherapy as planned and only 31% did not received any of the planned concomitant chemotherapy after TPF induction compared with 81%, 43% and 38% after PF induction. This may suggest that one of the main causes of the increased survival following TPF induction compared with PF induction was a better response to induction permitting more patients to undergo their planned chemo-radiotherapy.
This might suggest an approach of modelling an increase in response rate to induction therapy leading to an increase in the proportion of patients receiving chemo-radiotherapy as planned, with survival and PFS being modelled as a function of the proportion receiving chemo-radiotherapy. Fety et al. 156 provide response rates for PK dose adjustment of PF induction therapy compared with BSA adjustment of PF induction therapy. Blanchard et al. 50 go on to note that ‘No data on tumour response was collected’, but two of the papers underlying Blanchard et al. 50 do present some data on response rates. Hitt et al. 216 present both CR rates and PR rates. Pointreau et al. 217 present overall response rates. However, Posner et al. 218 and Vermorken et al. 219 do not present response rates.
As previously reviewed, Buyse et al. 185 in a meta-analysis of 25 RCTs raised concerns that response rates are quite poor predictors of OS within advanced CRC. The number of data points for a similar analysis based on Hitt et al. 216 and Pointreau et al. 217 for H&N cancer would be very considerably less than that available to Buyse et al. 185 Any resulting mapping from differences in induction chemotherapy response rates to differences in OS is likely to be questionable and subject to very high degree of uncertainty. Mapping from induction chemotherapy response rates to radiotherapy and concomitant chemotherapy treatment rates, and then on to survival would also seem to be questionable and subject to very high degree of uncertainty.
In the light of this, modelling survival in H&N cancer as a function of response rates to induction chemotherapy has not been explored further. However, the impact that response rates to induction chemotherapy might have on the rates of administration of subsequent chemo-radiotherapy is explored in the following section.
Given the relative paucity of data for H&N cancer, an exploratory analysis can be conducted that explores the cost and QALY impacts of the AEs that Fety et al. 156 report for PK dosing compared with BSA dosing for induction chemotherapy for locally advanced H&N cancer.
As requested during the NICE assessment subgroup, this can be coupled with a threshold analysis which examines what impact My5-FU monitoring would be required to have on OS for it to be cost-effective at conventional NICE thresholds. However, it should be borne in mind that induction chemotherapy and its effects are likely to work through somewhat different channels than FOLFOX in mCRC. If the main impact of induction chemotherapy is to permit more patients to undergo chemo-radiotherapy, an analysis of the required OS HR may be a rather poor guide as to the required effectiveness of My5-FU monitoring.
In the light of this, the modelling for locally advance H&N cancer will attempt to identify:
-
the PFS and OS of current therapy
-
AE rates, differentiated by arm
-
the QoL associated with advanced H&N cancer
-
the number of My5-FU assays that might be applied during induction therapy
-
the costs of induction therapy
-
the costs of subsequent chemo-radiotherapy
-
the proportion of patients going forward for chemo-radiotherapy, differentiated by arm
-
the ongoing costs having completed treatment.
This gives rise to the model structure presented in Figure 44.
FIGURE 44.
Model structure for locally advanced H&N cancer.

Induction chemotherapy response rates and subsequent therapy rates
Blanchard et al. 50 did not meta-analyse response rates, noting that ‘No data on tumour response was collected’ but going on to note that ‘among patients who did start chemo-radiotherapy, there was no difference in compliance with concomitant chemotherapy (p = 0.51)’. The papers underlying Blanchard et al. 50 do report some data on response rates to induction chemotherapy and rates of subsequent therapy, as summarised below.
Hitt et al. 216 compared patients who received either cisplatin 100 mg/m2 on day 1 plus FU 1000 mg/m2 continuous infusion on days 1 through 5 (n = 193) or paclitaxel 175 mg/m2 on day 1, cisplatin 100 mg/m2 on day 2 plus FU 1000 mg/m2 continuous infusion on days 2 through 6 (n = 189). Patients with CR or PR of greater than 80% in primary tumour received additional CRT of cisplatin 100 mg/m2 on days 1, 22 and 43 plus 70 Gy. The following response rates to induction chemotherapy were observed (Table 66).
| Response type | CF (n = 193) | PCF (n = 189) | ||
|---|---|---|---|---|
| CR | 26 | 13.5% | 63 | 33.3% |
| PR | 105 | 54.4% | 89 | 47.1% |
| of which > 80% | 69 | 35.8% | 66 | 34.9% |
Hitt et al. 216 go on to note that 39.4% (n = 76) went on to receive per-protocol chemo-radiotherapy in the cisplatin and FU (CF) arm compared with 60.3% (n = 114) in the paclitaxel, cisplatin and FU (PCF) arm. Although a simplification, if it is assumed that all those achieving a CR to induction chemotherapy received subsequent chemo-radiotherapy, this would imply that 50 of the 69 partial responders with more than 80% response received it in the CF arm and 51 of the 66 in the PCF arm: reasonably constant proportions of 72.5% and 77.3% respectively.
Pointreau et al. 217 undertook a Phase III trial with the specific aim of larynx preservation in patients with invasive SCC. This compared three cycles of induction chemotherapy comparing TPF with PF. TPF consisted of docetaxel at 75 mg/m2 on day 1, cisplatin at 75 mg/m2 on day 1 and 5-FU at 750 mg/m2 by 24-hour continuous infusion for 5 days. PF consisted of cisplatin at 100 mg/m2 on day 1 and 5-FU at 1000 mg/m2 by 24-hour continuous infusion for 5 days. Patients whose cancer responded well with either CR of PR (Table 67) with normal larynx mobility were treated with radiotherapy, which could be augmented with chemotherapy. The specific treatment according to protocol was delivered to 90% of the TPF group with 63% receiving complete treatment without delay or dose reduction, compared with 80% and 32% of the PF group.
| Response type | PF (n = 103) | TPF (n = 110) | ||
|---|---|---|---|---|
| CR | 31 | 30.1% | 53 | 48.1% |
| PR | 30 | 29.1% | 42 | 38.2% |
Radiation therapy was performed in 76% (n = 84) of patients in the TPF group all of whom were responders. For the PF group 61% (n = 63) of patients received radiotherapy, though Pointreau et al. 217 note that 57 patients responded to induction therapy and six had refused surgery. This could be seen as suggesting that complete responders received radiotherapy, whereas perhaps between 70% and 80% of partial responders received radiotherapy.
Posner et al. 218 compared TPF (n = 255) with PF (n = 246). The docetaxel dose was 75 mg/m2 followed by cisplatin at 100 mg/m2 followed by 5-FU at 1000 mg/m2 per day as a continuous 24-hour infusion for 4 days. Those in the PF arm received cisplatin at 100 mg/m2 followed by 5-FU at 1000 mg/m2 per day as a continuous 24-hour infusion for 5 days. Three cycles of induction chemotherapy were administered.
Posner et al. 218 do not report response rates to induction therapy, only noting that 79% (n = 202) received radiotherapy in the TPF arm compared with 75% (n = 184) in the PF arm. PD is noted as one of a number of reasons for discontinuation of therapy, but the reporting of this does not appear to distinguish between PD during induction therapy from that during the entire course of therapy.
Vermorken et al. 219 compared TPF (n = 177) with PF (n = 181) where TPF involved docetaxel at 75 mg/m2 followed by cisplatin 75 mg/m2 on day 1 and 5-FU at 750 mg/m2 per day by continuous infusion for days 1–5. The PF regime increased the dose of cisplatin to 100 mg/m2 on day 1, and also involved 5-FU at 750 mg/m2 per day by continuous infusion for days 1–5. Up to four cycles were delivered. Patients without PD and without a number of AEs and adequate bone marrow function underwent radiotherapy.
More patients completed their induction chemotherapy in the TPF group (76%, n = 134) than in the PF group (66%, n = 119). However, rates of completion of radiotherapy were more similar between the groups: 73% (n = 129) for TPF and 66% (n = 120) for PF. Vermorken et al. 219 do not separately report induction chemotherapy response rates.
Although quite uncertain, the above could be taken as indicating that all complete responders to induction TPF will receive chemo-radiotherapy, whereas only 70% of partial responders to induction chemotherapy will receive chemo-radiotherapy. Fety et al. 156 report the following response rates which, if coupled with the assumptions about the rates of subsequent chemo-radiotherapy, imply the following (Table 68).
| Response type | BSA dosing | Subsequent CRT | PK dosing | Subsequent CRT |
|---|---|---|---|---|
| CR | 33% (19/57) | 33% | 29% (14/49) | 29% |
| PR | 44% (25/57) | 31% | 53% (26/49) | 37% |
| Total | 77% (44/57) | 64% | 82% (40/49) | 66% |
The relatively small differences in overall response rates could be taken as suggesting that while BSA dosing has a slightly lower overall response rate the differences in the proportions of patients receiving subsequent chemo-radiotherapy will be quite small: 2%. The extent of this difference may be slightly skewed in the above by the higher mean rate of CR in the BSA dosing arm. It may be more reasonable to assume equivalence in terms of CR and attribute any differences to changes in PR. If so, the additional 5% response rate in the PK dose adjustment arm could translate into perhaps an additional 3% receiving subsequent chemo-radiotherapy. While these differences seem slight, the chemo-radiotherapy costs per patient will be very much larger than the direct My5-FU tests costs.
The limited differences in overall response rates may also help illuminate how likely it is that the required threshold for the HR for OS and associated mean OS gain from My5-FU dose adjustment will be reached.
My5-FU administrations
Fety et al. 156 used a regimen in which 5-FU was administered over 96 hours, with the adjustment being based on the AUC during 0–48 hours. The AUC was measured during the first cycle. This led to any dose adjustment requirements for the following cycles, though the dose during the first cycle was only adjusted if the AUC during 0–48 hours was unusually high. The second cycle and the third cycle also had their AUC measured during 0–48 hours, with any required dose adjustment occurring at mid-cycle.
Table 69 shows the numbers of patients receiving treatment at each cycle reported by Fety et al. 156
| Arm | Cycle 1 | Cycle 2 | Cycle 3 |
|---|---|---|---|
| BSA | 57 (100%) | 52 (91%) | 49 (86%) |
| PK | 49 (100%) | 45 (92%) | 41 (84%) |
This suggests a total of 2.8 My5-FU assays over the three cycles.
Fety et al. 156 also report quite high dose adjustments during cycles 2 and 3. Within the BSA arm 3.9% received a 5-FU dose reduction during cycle 2 and 20.9% received a dose reduction during cycle 3. Within the PK arm 66.6% received a dose reduction during cycle 2 and 78.0% received a dose reduction during cycle 3. Also within the PK arm 8.8% received dose increases during cycle 2 and 4.8% received dose increases during cycle 3. These dose adjustments will be costed assuming an additional 10 minutes consultant time.
Progression-free survival and overall survival: body surface area dosing
Due to the Blanchard et al. 50 curves not being Kaplan–Meier plots, they have no steps and as a consequence are not suitable for the Guyot et al. 125 method. As a consequence, parameterised curves have been fitted to the Blanchard et al. 50 TPF OS and PFS curves using ordinary least squares (Figures 45 and 46).
The EAG statistical opinion suggests that the fitted curves are likely to extrapolate too high a long-term survival. In particular, the observed data appears to exhibit something of a downturn towards the end of both the OS curve and the PFS curve. For this reason the base case will apply a linear extrapolation using the last five values of the observed data. Applying this means that there are minimal differences between the parameterised curves up to this point (Figures 45–47). The Weibull will be applied.
Adverse events rates
The clinical effectiveness estimates of Fety et al. 156 are based on PK monitoring of the 5-FU dose within the context of PF induction chemotherapy: with dosing of 100 mg/m2 and a starting dose of 4000 mg/m2 for 5-FU, with the 5-FU being administered as a continuous infusion over 4 days. Current induction chemotherapy is 5-FU in combination with a taxane, either docetaxel or paclitaxel, in combination with cisplatin and 5-FU (TPF). Dosing would typically be 75 mg/m2, 75 mg/m2 and 750 mg/m2 per day for the 5-FU, with the 5-FU being administered for 4–5 days. In some instances, PF may also be given as induction chemotherapy: dosing would typically be 100 mg/m2 and 1000 mg/m2. Blanchard et al. 50 meta-analysed studies comparing TPF with PF.
Given the difference between current practice (TPF) and the regime of Fety et al. 156 (PF), the following assumes that the relative risks of grade III/IV AEs for PK dosing compared with BSA dosing reported by Fety et al. 156 for PF are equally applicable to TPF. Fety et al. 156 also only report the proportion of cycles at which the grade III/IV AEs of neutropenia/thrombocytopenia, mucositis and digestive tract were experienced. The relative risk for neutropenia/thrombocytopenia is applied equally to the baseline risks for neutropenia and thrombocytopenia for TPF induction chemotherapy as reported in Blanchard et al. 50 (and the papers underlying Blanchard et al. 50). Similarly, the relative risk of digestive tract toxicity is applied equally to the baseline risks for vomiting, nausea and diarrhoea as reported in Blanchard et al. 50 (and the papers underlying Blanchard et al. 50).
Fety et al. 156 report grade III/IV AEs as a proportion of the total number of cycles. This can be coupled with the data on the numbers of patients receiving treatment to suggest the number of events per patient presented in Table 70.
| AE | BSA | PK |
|---|---|---|
| Neutropenia/thrombocytopenia | 0.49 | 0.20 |
| Mucositis | 0.14 | 0.00 |
| Digestive tract | 0.14 | 0.22 |
The split between neutropenia and thrombocytopenia and the split of digestive tract AEs into diarrhoea and nausea/vomiting can be made in proportion to the rates reported in the studies underlying Blanchard et al. 50 (Table 71).
| AE | BSA | PK |
|---|---|---|
| Neutropenia | 0.47 | 0.20 |
| Thrombocytopenia | 0.02 | 0.01 |
| Mucositis | 0.14 | 0.00 |
| Diarrhoea | 0.06 | 0.10 |
| Nausea/vomiting | 0.08 | 0.13 |
As there is no split between grade III and grade IV AEs, for costing purposes the split that was used for the mCRC modelling has been applied.
The cost and QALY impacts estimated for each AE within the mCRC modelling have been reapplied.
Quality-of-life values
Appendix 18 summarises the QoL values used in previous NICE assessments of treatments for locally advanced H&N cancer. Both of these assessments were STAs and, as a consequence, the values used should be read with a degree of caution. TA145215 commissioned an EQ-5D study among oncology nurses which suggested a value of 0.659 for while on treatment and 0.129 for post treatment PD. TA172220 mapped patient-level European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ-C30) data onto EQ-5D scores using the algorithm developed by Kind. 221 This resulted in values of 0.65 for PFS under standard treatment and 0.52 for SWP.
The values from TA172220 appear to be more in line with the NICE methods guide. The values are also more in line with those used in the mCRC modelling, though being different diseases there is no particular reason that they should be entirely aligned. As a consequence, the modelling will apply QoL values of 0.65 for PFS and 0.52 for SWP. It will be further assume that those on treatment have the PFS QoL.
However, note that Appendix 19 summarises the broader QoL literature in H&N cancer. This suggests that remission or being recurrence free may be associated with a somewhat higher QoL. This again suggests that some scepticism should be applied to the following relatively crude modelling of H&N cancer, and that inferences made from the mCRC may not hold.
Cost of induction chemotherapy
Each cycle of induction chemotherapy is assumed to consist of docetaxel 75 mg/m2, cisplatin 75 mg/m2 and a daily total of 750 mg/m2 5-FU over 4 days. The 5-FU is assumed to be administered as two sequential 48-hour infusions using a balloon elastomer pump (Janice Szulc and James Cook, South Tees NHS Trust, 2013, personal communication). Table 72 shows the costs per cycle of induction chemotherapy.
| Induction chemotherapy | Cost (£) |
|---|---|
| Pharmacy | 189.06 |
| First administration | 238.39 |
| Subsequent administration | 255.06 |
| Docetaxel | 34.29 |
| Cisplatin | 20.50 |
| 5-FU | 3.70 |
| Elastomer pump | 38.96 |
| Line flush | 47.33 |
| Total per cycle | 827.29 |
| Total per course | 2481.86 |
In line with Fety et al. 156 and expert opinion it is assumed that three cycles of induction chemotherapy are planned, though some UK practise is apparently to aim for four cycles. Progression and deaths limit the number of induction chemotherapy cycles that are applied within the model to some degree.
Cost of chemo-radiotherapy
Chemo-radiotherapy is assumed to consist of cisplatin 100 mg/m2 at the start of weeks 1, 4 and 7 coupled with 70 Gy radiation therapy delivered in equal daily doses Monday to Friday for 7 weeks. The pharmacy and administration costs for chemotherapy are drawn from the same sources as the mCRC modelling with the cisplatin cost being drawn from the CMU eMIT database.
The costs of planning radiotherapy are based on a weighted average of the 2012–13 NHS references costs205 of outpatient planning codes SC40Z to SC52Z, excluding codes related to total body imaging SC42Z to SC44Z. Note that this assumes that there is only one preparation and imaging session per patient. These costs would increase substantially were it to be required (e.g. weekly). The weighted average outpatient cost of £552 only increases slightly to £557 if day cases are included.
The costs of administering radiotherapy are based on a weighted average of the 2012–13 NHS reference costs205 of outpatient delivery of a fraction of radiotherapy codes SC12Z to SC28Z, excluding the code SC25Z for a fraction of total body irradiation. This results in a cost per fraction of £117. Note that including day cases had little impact on the weighted average, only increasing it to £120.
This results in the following costs (Table 73).
| Radiotherapy | Cost (£) |
|---|---|
| Planning | 551.67 |
| Administration per fraction | 117.03 |
| Administration total | 4095.91 |
| Total per course radiotherapy | 4647.58 |
| Concurrent chemotherapy | |
| Pharmacy | 47.27 |
| Administration | 238.39 |
| Cisplatin | 27.18 |
| Total per administration | 312.83 |
| Total per course chemotherapy | 938.50 |
| Overall total per course | 5586.08 |
Note that it is assumed that once having started chemo-radiotherapy patients receive the full course. This is not entirely realistic, as some patients will die and some patients will cease therapy. In particular, it appears that a number of patients will cease chemotherapy while perhaps continuing with radiotherapy.
This assumption is made in order to avoid perhaps artificially differentiating the BSA arm from the My5-FU arm. The alternative of assuming that only those modelled as remaining in PFS as per the parameterised Weibulls would continue to receive chemo-radiotherapy could well be equally objectionable.
Costs of progression
TA145215 specifies a range of costs associated with treating patients who have progressed: nursing costs, salvage surgery, secondary radiotherapy and secondary systemic therapy. These are costed at an average of £1099 which when uprated to 2012–13 prices yields a cost of £1318.
Ongoing costs
TA145215 also specifies various frequencies of repeat follow-up visits depending on the length of time since treatment ranging from weekly up to 5 weeks from end of therapy, monthly thereafter for the first year, every 2 months for the second year and every 3 months thereafter. These have been costed using the 2012–13 NHS reference costs205 for medical oncology consultant-led follow-up appointment: £139.22.
Results
As outlined in Table 74, if there is no survival advantage associated with My5-FU there are only minimal QoL gains associated with its AE profile, though this does give rise to some small cost offsets. However, given the net costs, only a very small improvement in either PFS or OS is required to render My5-FU cost-effective.
| Item | HR PFS = 0.966, HR OS = 1.000 | HR PFS = 1.000, HR OS = 0.990 | ||||
|---|---|---|---|---|---|---|
| My5-FU | BSA | Net | My5-FU | BSA | Net | |
| QALYs | ||||||
| PFS | 2.454 | 2.386 | 0.068 | 2.387 | 2.386 | 0.001 |
| SWP | 0.297 | 0.351 | –0.054 | 0.366 | 0.351 | 0.015 |
| AEs | 0.000 | –0.001 | 0.000 | 0.000 | –0.001 | 0.000 |
| Total | 2.750 | 2.736 | 0.014 | 2.752 | 2.736 | 0.016 |
| Costs (£) | ||||||
| My5-FU | 168 | 0 | 168 | 168 | 0 | 168 |
| Dose adjustment | 37 | 6 | 31 | 37 | 6 | 31 |
| Induction | 2283 | 2283 | 0 | 2283 | 2283 | 0 |
| Chemo-radiotherapy | 5083 | 4916 | 167 | 5083 | 4916 | 167 |
| AEs | 102 | 149 | –47 | 102 | 149 | –47 |
| SWP | 1000 | 1034 | –34 | 1039 | 1034 | 5 |
| Ongoing | 5179 | 5179 | 0 | 5189 | 5179 | 10 |
| Total | 13,851 | 13,567 | 285 | 13,901 | 13,567 | 335 |
| ICER (£) | 20,586 | 20,601 | ||||
Sensitivity analyses
The base case assumes that constant proportions of patients receive induction chemotherapy and subsequent chemoradiotherapy, the latter being 3% higher in the My5-FU arm than in the BSA arm. An alternative assumption is to assume that only those modelled as being in PFS receive induction chemotherapy and chemoradiotherapy (Table 75). The alternative in the opposite direction is to equalise the proportions receiving subsequent chemotherapy.
| Item | HR PFS = 0.981, HR OS = 1.000 | HR PFS = 1.000, HR OS = 0.995 | ||||
|---|---|---|---|---|---|---|
| My5-FU | BSA | Net | My5-FU | BSA | Net | |
| QALYs | ||||||
| PFS | 2.424 | 2.386 | 0.038 | 2.386 | 2.386 | 0.000 |
| SWP | 0.321 | 0.351 | –0.030 | 0.358 | 0.351 | 0.008 |
| AEs | 0.000 | –0.001 | 0.000 | 0.000 | –0.001 | 0.000 |
| Total | 2.744 | 2.736 | 0.008 | 2.744 | 2.736 | 0.008 |
| Costs (£) | ||||||
| My5-FU | 174 | 0 | 174 | 173 | 0 | 173 |
| Dose adjustment | 37 | 6 | 31 | 37 | 6 | 31 |
| Induction | 2353 | 2350 | 2 | 2350 | 2350 | 0 |
| Chemo-radiotherapy | 4784 | 4770 | 14 | 4770 | 4770 | 0 |
| AEs | 102 | 149 | –47 | 102 | 149 | –47 |
| SWP | 1016 | 1034 | –18 | 1037 | 1034 | 3 |
| Ongoing | 5179 | 5179 | 0 | 5184 | 5179 | 5 |
| Total | 13,643 | 13,487 | 156 | 13,653 | 13,487 | 165 |
| ICER (£) | 19,909 | 20,064 | ||||
Assuming therapy to be equal to the proportion remaining progression free results in a smaller cost differential than assuming that 3% more patients would progress to chemo-radiotherapy in the My5-FU arm than in the BSA arm. As a consequence, the required treatment effect falls (Table 76).
| Item | HR PFS = 0.984, HR OS = 1.000 | HR PFS = 1.000, HR OS = 0.995 | ||||
|---|---|---|---|---|---|---|
| My5-FU | BSA | Net | My5-FU | BSA | Net | |
| QALYs | ||||||
| PFS | 2.418 | 2.386 | 0.032 | 2.386 | 2.386 | 0.000 |
| SWP | 0.325 | 0.351 | –0.025 | 0.358 | 0.351 | 0.008 |
| AEs | 0.000 | –0.001 | 0.000 | 0.000 | –0.001 | 0.000 |
| Total | 2.743 | 2.736 | 0.007 | 2.744 | 2.736 | 0.008 |
| Costs (£) | ||||||
| My5-FU | 168 | 0 | 168 | 168 | 0 | 168 |
| Dose adjustment | 37 | 6 | 31 | 37 | 6 | 31 |
| Induction | 2283 | 2283 | 0 | 2283 | 2283 | 0 |
| Chemo-radiotherapy | 4916 | 4916 | 0 | 4916 | 4916 | 0 |
| AEs | 102 | 149 | –47 | 102 | 149 | –47 |
| SWP | 1019 | 1034 | –15 | 1037 | 1034 | 3 |
| Ongoing | 5179 | 5179 | 0 | 5184 | 5179 | 5 |
| Total | 13,704 | 13,567 | 137 | 13,727 | 13,567 | 160 |
| ICER (£) | 20,740 | 19,463 | ||||
As would be anticipated, removing the 3% higher rate of chemo-radiotherapy in the My5-FU arm lessens the treatment effect that is required for My5-FU to be cost-effective at a WTP of £20,000 per QALY.
But in short, given the additional costs associated with My5-FU and the relatively long survival among locally advanced H&N cancer patients, only small treatment effects on PFS or OS are required for My5-FU to be cost-effective.
Discussion and conclusions
The costs of My5-FU testing
Based on an annual throughput of 300 and 100 assays being available per assay kit, the cost per My5-FU assay is around £61. This includes a staff cost of £35 for 30 minutes of health visitor time to take the blood sample. The cost per assay would rise further were My5-FU to require a dedicated outpatient appointment for the taking of the blood, this doubling the total cost per assay.
This does not include the costs of dose adjustment. Within the modelling, based on expert opinion, each dose adjustment associated with My5-FU has been assumed to involve a additional 10 minutes of consultant time.
All of the economic modelling depends on equivalence of My5-FU with HPLC and LC-MS being a reasonable assumption. If this is not a reasonable assumption, the cost-effectiveness estimates which are presented are not a reflection of the cost-effectiveness of My5-FU. If My5-FU has a worse performance than the other methods on which most of the clinical effectiveness evidence rests, the cost-effectiveness estimates that are presented will be systematically biased in favour of My5-FU. There is no obvious means of exploring this assumption or conducting sensitivity analyses around it.
Modelling the cost-effectiveness of My5-FU dose adjustment in metastatic colorectal cancer
The modelling is hampered by the trials that underline the estimates of OS and PFS treating patients until progression or unacceptable toxicity. Expert opinion suggests that current UK practice appears to have switched to 12 FOLFOX cycles followed by a treatment holiday, with only a minority of patients recommencing a second course of FOLFOX. Some centres may aim for six cycles of FOLFOX before taking a treatment holiday. Another key assumption is that the survival estimates are unaffected by the move to intermittent dosing and treatment holidays. This is supported by the results of the COIN trial.
The analysis of AEs suggests that given their short duration any differences between AEs rates for My5-FU compared with BSA dose adjustment will result in minimal QALY differences between the arms. The costs of hospitalisations for grade III/IV are more important, and provide some cost offset to the My5-FU assay costs. However, changes in the AE profile appear unlikely to be sufficient in themselves to render My5-FU cost-effective.
The analyses based on the FOLFOX studies are hampered by a lack of a proper BSA dosing control arm, with Capitain et al. 119 only reporting medians for the BSA dosing arm. Inferring the BSA dosing OS and PFS curves from the reported data suggests a gain of an additional OS of 0.770 years from PK dosing. This translates into an estimated gain of 0.599 QALYs. Given the additional survival, total routine ongoing monitoring and treatment costs are higher in the My5-FU arm. There are also additional costs of more FOLFOX treatments within the My5-FU arm of £467. This results in an additional total cost arising from My5-FU dose adjustment of £2483, and in turn into a cost-effectiveness estimate of £4148 per QALY.
The probabilistic modelling results in a similar central estimate with a reasonably tight CEAC.
The above estimates include a cost offset from reduced use of second-line FOLFIRI in the My5-FU arm. This appears to occur due to a quirk of the modelling, in that the My5-FU PFS curve touches the OS curve and is then assumed to follow the OS curve. As a consequence, a smaller proportion of patients in the My5-FU arm are modelled as receiving second-line FOLFIRI compared with the BSA arm. It is probably more sensible to exclude these cost offsets, but doing so only revises the cost-effectiveness estimate to £4363 per QALY.
These cost-effectiveness estimates are reasonably stable as the source of parameterised curves is varied. Although the net QALYs gained varies between the scenarios the net costs vary in a similar proportion, resulting in quite similar cost-effectiveness estimates. This arises mainly due to any changes in OS in the BSA dosing arm being associated with increased ongoing monitoring and treatment costs. When coupled with the additional survival being valued at the SWP QoL, this approximately results in the BSA cost-effectiveness point travelling along the line joining the old BSA point and the My5-FU point on the cost-effectiveness plane.
This stability of results also applies to the scenario analysis that derives the OS curve for My5-FU from Capitain et al. ,119 but derives the BSA OS curve by assuming that the HR for My5-FU compared with BSA dosing of 0.829255 derived from Gamelin et al. 118 applies. Gamelin et al. ,118 although a study of FUFOL, can be seen as the comparative study with the best randomisation.
Provided that the survival gain of the base case is a reasonable estimate, the cost-effectiveness of My5-FU is relatively insensitive to the laboratory throughput and varying the number of My5-FU assays per patient from the 3.23 of the base case to (AiC information has been removed) and to 4.4. If the estimated survival gain is too large, results are likely to be more sensitive to the laboratory throughput and assay numbers.
A sensitivity analysis that assumed around 10% of patients would commence a second course of 12 cycles of FOLFOX had a reasonable impact on the cost-effectiveness estimate, worsening it to £5272 per QALY.
The base case assumes that the blood sample for the My5-FU assay is taken in the community by a health visitor. Some expert opinion suggests that this may be difficult to source at times, leading to the blood test requiring a dedicated outpatient visit. If this is the case, the cost-effectiveness estimate worsens to £4506 per QALY.
Applying the QoL values of CG1317 rather than those of Färkkilä et al. 192 has a reasonable impact on the cost-effectiveness estimate, worsening it to £6016 per QALY.
However, these sensitivity analyses rely on My5-FU resulting in quite a large OS gain: 0.770 years. This estimate is based on Capitain et al. ,119 the BSA dosing arm of which was a historical control group that was somewhat smaller than the PK dose adjustment group. Also, only the median for the OS for the BSA dosing arm was reported. The OS in the BSA dosing arm was inferred from this relatively limited data, and as a consequence is subject to considerable uncertainty.
The cost-effectiveness modelling based on the FUFOL studies is motivated in part by these containing the main comparative study with reasonable randomisation: Gamelin et al. 118 In the light of UK practice typically being fortnightly cycles of FOLFOX, the costs of FOLFOX are retained for these analyses. They provide further scenario analyses around which parametric curves should be applied, and what additional survival gain might be anticipated.
The OS curves of Gamelin et al. 118 suggest a survival gain from My5-FU of 0.247 years. When this is coupled with a common PFS curve estimated from the three pooled studies,162,163,165 it results in a net gain of 0.151 QALYs. Due to the assumption of a common PFS curve, there are no additional FOLFOX treatment costs associated with My5-FU. As a consequence, the additional costs are largely limited to the costs of My5-FU, partly offset by AE costs, and the additional routine ongoing monitoring and treatment costs associated with the longer survival. These net additional costs of around £883 result in a cost-effectiveness estimate of around £5853 per QALY.
Changing the source of the parameterised curves for PFS to those inferred from the mean durations of response reported in Gamelin et al. 118 worsens the cost-effectiveness estimate. Depending on the method used to infer PFS, the cost-effectiveness estimate worsens to between £6965 per QALY and £8615 per QALY. This appears to be due to a relative increase in the costs of first-line treatment.
The FUFOL studies based cost-effectiveness analyses show similar sensitivities to univariate parameter changes as the FOLFOX studies based cost-effectiveness analyses. The only sensitivity analysis that differs is the application of the QoL values of CG1317 rather than those of Färkkilä et al. 192 This has a rather larger impact on the cost-effectiveness estimate based on the FUFOL studies, worsening it to £17,485 per QALY. If this is coupled with an outpatient visit being required for the taking of the blood sample the cost-effectiveness estimate would worsen further to £21,732 per QALY.
Modelling the cost-effectiveness of My5-FU in locally advance head and neck cancer
There is minimal clinical information to inform a cost-effectiveness analysis of My5-FU during induction chemotherapy for locally advance H&N cancer. What data there are is largely limited to that of Fety et al. 156 AE rates, response rates and dose adjustments are reported but there is no information about survival.
Progression-free survival and OS estimates are available for TPF induction therapy from the meta-analysis of Blanchard et al. 50 The modelling approach is to apply the costs of induction chemotherapy and subsequent chemo-radiotherapy, the costs and QoL impacts of AEs and the ongoing costs of monitoring. The modelling can then estimate what HR is required for either PFS or for OS for My5-FU to be cost-effective at a threshold of £20,000 per QALY.
The studies underlying Blanchard et al. 50 can also be read as suggesting that rates of subsequent chemo-radiotherapy tended to increase with response rates to a limited degree. An informal estimate of My5-FU resulting in an additional 3% of patients receiving subsequent chemo-radiotherapy has been included in the base-case modelling, though excluding this is explored in a sensitivity analysis.
The base-case results suggest that given the relatively long survival among patients with locally advanced H&N cancer compared with mCRC patients, the HRs required for My5-FU to be cost-effective in induction therapy for locally advance H&N cancer differ only slightly from unity. With a HR of 0.966 for PFS and no gain in OS there is an estimated net gain of 0.014 QALYs and an estimated cost increase of £285, resulting in a cost-effectiveness estimate of £20,586 per QALY. With a HR of 0.990 for OS and no gain in PFS there is an estimated gain of 0.016 QALYs and an estimated net cost of £335, resulting in a cost-effectiveness estimate of £20,601 per QALY.
Sensitivity analyses around the proportion of patients receiving subsequent chemo-radiotherapy suggest that a HR of around 0.980 for PFS or of around 0.995 for OS would be sufficient to justify the costs of My5-FU during induction therapy for locally advanced H&N cancer.
The EAG views these estimates as quite speculative. Although the estimates are considerably less than the HR for OS of 0.829255 derived for mCRC from Gamelin et al. ,118 they are also in an entirely different context: induction chemotherapy for locally advance H&N cancer rather than what could be described as palliative chemotherapy for mCRC. The OS estimates for the two groups of patients are also very different. Given an absolute cost difference, the longer the survival the smaller the relative treatment effect has to be to justify the additional cost. However, the required treatment effect also becomes more speculative, and difficult to practically identify and attribute.
Chapter 6 Discussion
In current clinical practice the dose of 5-FU-containing regimens given to cancer patients is based on the patient’s BSA, with downwards adjustment in case of severe toxicity. However, it has been suggested that about 40–50% of patients receiving 5-FU in this way may be underdosed. It has been hypothesised that dose adaptation might improve outcomes such as response rates and OS without increasing toxic side effects, by achieving optimal 5-FU exposure. The My5-FU assay in conjunction with dose adaptation algorithms offers a potential means to achieve more appropriate 5-FU exposure. In this assessment we investigated to what extent dose adjustment fulfils this aim of improved outcomes and if this approach is cost-effective.
Decision problem and objectives
Our overall objective was to undertake a clinical effectiveness and cost-effectiveness analysis of the PK dose adjustment of 5-FU in cancer patients treated with 5-FU containing chemotherapy regimens in the metastatic and adjuvant setting. We aimed to systematically review the literature on the accuracy of the My5-FU assay compared with gold standard methods (HPLC and LC-MS); the effectiveness of My5-FU PK dosing or of HPLC and/or LC-MS PK dosing compared with BSA dosing; and the generalisability of published My5-FU and PK studies. We also aimed to identify evidence relevant to the costs of using My5-FU and to develop a cost-effectiveness model.
Summary of methods and findings
Clinical effectiveness
We searched a number of databases including MEDLINE, EMBASE and the Science Citation Index. Two reviewers independently screened titles and abstracts and discrepancies were resolved through discussion. Quality assessment was undertaken. In the absence of IPD, we used the method of Guyot et al. 125 to reconstruct Kaplan–Meier plots for PFS and OS for comparison of BSA and PK dosing in two regimens.
We found 3751 records of which 35 papers (representing eight unique studies) were included. We found a high correlation coefficient reported between My5-FU, HPLC and LC-MS/MS but the Bland–Altman plots showed considerable variability. Personal communication with a clinical advisor suggested that within the context of a cautious dose adjustment algorithm the range of values (–18% to 30%) could be considered of little clinical concern; however, we remain cautious about outliers (Nick Wadd, North Tees and Hartlepool Trust, 2013, personal communication).
The evidence on PK versus BSA dosing in the treatment of CRC and H&N cancer patients is weak in both quantity and quality. Evidence for My5-FU was sparse with only one study of clinical outcomes which compared BSA with PK dose adjustment using the My5-FU assay and this study was at risk of selection bias. Of three CRC comparative studies118,119,155 identified, only one was a RCT118 but unfortunately it used an unrepresentative 8-hour infusion regimen. Single-arm studies were heterogeneous (see Table 10), of poor design, and severely limited in ability to deliver useful data for comparison of PK versus BSA dosing. There was no RCT evidence about the effectiveness of PK-directed dose adjustment for any currently used 5-FU regimen for any cancer type.
We reconstructed IPD of single arms from studies from a variety of sources and combined data to undertake a comparison of PK dosing with BSA. Overall, PK appeared to confer a benefit in both regimens for which any comparative data were available [median OSs were 19.6 (95% CI 17.0 to 21.0) months PK vs. 14.6 (95% CI 14.1 to 15.3) months BSA for FUFOL; and 27.4 (95% CI 23.2 to 38.8) months PK vs. 20.6 (95% CI 18.4 to 22.9) months BSA for FOLFOX6 in mCRC]. However, these apparent benefits should be viewed with extreme caution because of the quality of the evidence. For H&N cancer, only two studies132,156 both more than 15 years old were identified but they used regimens no longer in current use.
We found no useful evidence on stomach, pancreatic or any other cancers where 5-FU regimens are used and no evidence to allow meaningful analysis of the following subgroups:
-
people with DPD deficiency
-
people with impaired renal function
-
people with impaired liver function
-
people whose BSA is outside the standard range for dosing 5-FU
-
people with a less favourable performance status who may be undertreated in current practice.
We found the generalisability of the studies reporting PK versus BSA dosing to be acceptable.
Cost-effectiveness
A comprehensive search of the literature for published economic evaluations, utility studies and cost studies was performed. A total of 4578 records were identified through electronic searches of which 54 papers were included in the cost-effectiveness review.
For mCRC a de novo cost-effectiveness model was developed that compared dose adjustment using My5-FU with BSA dosing in line with the Diagnostic Assessment Report programme methods guide. This adopted a 20-year time horizon with a 2-week cycle to reflect the FOLFOX cycle length.
A bottom-up costing of the My5-FU assay was undertaken, with laboratory throughputs and staff timings being drawn from expert opinion. The costs of chemotherapy were also based on expert opinion coupled with drug costs from the CMU eMIT database, NHS reference costs and values from the literature. Other ongoing costs of treatment were drawn from a paper within the literature, as had typically been applied in previous modelling of mCRC for NICE.
Parameterised survival curves were drawn from the main comparative papers. As these did not use the My5-FU assay for dose adjustment, a key assumption was clinical equivalence between My5-FU and HLPC and LS-MS. Due to the different estimates of mean survival for BSA dosing from the main FOLFOX comparative study compared with the main FUFOL comparative study, these two strands were analysed and modelled separately. This is also partly motivated by the main FUFOL comparative study being the main paper with reasonable randomisation. 118,119 A range of scenario analyses and sensitivity analyses are presented for both strands. Both strands applied the FOLFOX chemotherapy costs due to FUFOL having been superseded.
Quality-of-life values for the base case were drawn from a recent paper192 within the literature that analysed the EQ-5D data of Finnish CRC patients using the UK social tariff, this separately reporting results for the subset of 110 patients with metastatic disease remaining on active treatment and for the subset of 41 patients with metastatic disease receiving only palliative care. Sensitivity analyses were undertaken using the QoL values used for the NICE CG for CRC. 7
The main comparative papers also provided estimates of AEs rates and their severities. The QoL impact of these was estimated using QoL values from the MRC SCOT trial201 coupled with additional values from the literature and expert opinion as to their likely duration. The costs of AEs were mainly based on expert opinion on the proportion of AEs requiring medication and the mean lengths of stay for those expected to receive inpatient care, coupled with drug tariff medication costs and NHS reference costs.
The deterministic base case for a FOLFOX regimen for mCRC given over 12 cycles resulted in an estimated life-year gain of 0.770 and a QALY gain of 0.599 at a net additional cost of £2483 for My5-FU dosing compared with BSA dosing. The additional direct costs of My5-FU made up only a small part of this additional cost, the majority being due to higher first-line treatment costs and routine ongoing monitoring and treatment costs. The ICER was £4148 per QALY. Probabilistic results were very similar as were deterministic results using a variety of different scenario analyses. For this analysis the CEAC showed My5-FU to be 100% likely to be cost-effective at a threshold of £20,000 per QALY.
The PFS curve touched and was then modelled as following the OS curve, which means that the costs of second-line therapy may have been underestimated in the My5-FU arm. Excluding these costs worsened the cost-effectiveness estimate to £4363 per QALY. Results were reasonably insensitive to the source of parameterised curves that was chosen.
For H&N cancer an exploratory analysis was undertaken which examined the possible drivers of cost-effectiveness and the survival HRs that might be required to render dose adjustment using My5-FU cost-effective at a WTP of £20,000 per QALY. For the BSA dosing arm parameterised OS and PFS curves were drawn from a systematic review and meta-analysis paper within the literature. AE rates for PK dose adjustment and for the BSA dosing arm were drawn from the main comparative paper. 118,119
Estimated cost increases associated with My5-FU were not large in the context of costs of current induction therapy followed by chemo-radiotherapy. Given the somewhat longer survival among patients with locally advanced H&N cancer compared with mCRC patients, HRs required to justify the additional cost at a WTP of £20,000 per QALY were not far from unity and a HR of 0.95 was modelled as being sufficient to justify the additional cost.
Strengths and limitations
We undertook rigorous and comprehensive systematic reviews for both clinical effectiveness and cost-effectiveness and we believe that we identified all relevant publications concerning the effectiveness of PK dose adjustment in the management of cancer patients treated with 5-FU containing chemotherapies. One of the main problems with this work is that the underlying evidence base for a ‘linked evidence’ approach is of concern. We found a high correlation between My5-FU, HPLC and LC-MS/MS but the Bland–Altman plots showed considerable variability. All of the economic modelling depends on equivalence of My5-FU with HPLC and LC-MS being a reasonable assumption. If this is not a reasonable assumption, the cost-effectiveness estimates which we have presented are not a reflection of the cost-effectiveness of My5-FU.
The evidence base for PK dose adjustment in both colorectal and H&N cancer is weak. None of the studies we investigated were of high quality; all had important drawbacks in design and methods which, coupled with patchy reporting of key outcomes, limits their validity and the generalisability of the findings. For example, we found no randomised evidence on the effect of PK dose adjustment for any currently used 5-FU regimen for any cancer type using either My5-FU or HPLC for dose adjustment.
Furthermore, in order to make best use of published studies we contacted authors in order to obtain IPD. However, we were unable to obtain IPD and, in the absence of IPD, we had to reconstruct survival data using the method of Guyot et al. 125 We consistently checked our findings against available empirical data, but, nevertheless, the process of construction is not as reliable as the use of the original IPD.
We combined reconstructed IPD of single arms from studies from a variety of sources to allow a comparison of PK dosing with BSA. It should be strongly cautioned that there are many caveats regarding the validity of this procedure including the assumptions of similar treatments and similar populations; furthermore, there is a lack of adjustment for potential patient or study level confounders.
Unfortunately, our work on the My5-FU assay for use in clinical practice with common UK regimens is therefore both indirect and based on non-randomised evidence, drawn together from a small number of non-UK PK versus BSA studies and complemented with data from BSA arms provided by a variety of RCTs which investigated various comparisons of 5-FU treatments, but which did not investigate PK adjustment. 118,119,134,138,155 The single randomised study118 used an out-of date regimen administered over an obsolete 8-hour infusion. In addition, we used our combined and reconstructed data in our estimations of cost-effectiveness. Where the evidence did not directly support a complete ‘end-to-end’ analysis from My5-FU through to OS, a linked evidence approach was undertaken, but as our results in relation to comparability of My5-FU and HPLC show there may be some concerns in relation to this approach.
As a consequence, the cost per QALY of My5-FU for mCRC estimate is subject to considerable structural uncertainty. The scenario and sensitivity analyses which were undertaken were broadly reassuring for the base-case analyses that they related to. However, neither these nor the probabilistic sensitivity analyses reflect or try to quantify the structural uncertainty around the cost-effectiveness estimates. All the cost-effectiveness results require an assumption that PK dose adjustment using My5-FU is clinically equivalent to PK dose adjustment using HPLC and LC-MS. All the cost-effectiveness results also require the application of the parameterised survival curves. The deficiencies of these are summarised in Chapter 3, and there is considerable uncertainty about their reliability as a guide to what will actually happen in practise.
For H&N cancer we were unable to find useful survival data and had to undertake analyses around possible HRs. Again, these methods are of concern, although the ratios generated in our calculation generate values which would be modelled as easily justifying the additional cost.
Practical considerations
Prerequisites for successful pharmacokinetic dose adjustment using My5-FU in clinical practice
Successful PK dose adjustment using My5-FU in clinical practice will depend on:
-
accurate estimation of plasma 5-FU
-
an appropriate algorithm for dose adaptation
-
an appropriate target plasma 5-Fu level (the target range).
In this section we therefore examine each of these practical issues for consideration in turn.
Accurate estimation of plasma 5-fluorouracil
No currently available RCT or comparative study used the My5-FU assay for dose adjustment of 5-FU containing chemotherapy regimens in the treatment of any cancer. As a result the current report has relied on comparisons with HPLC. Although there is a high correlation coefficient reported between My5-FU, HPLC and LC-MS/MS, the small amount of evidence available from the Bland–Altman plots showed considerable variability. If in the context of a cautious dose adjustment algorithm the range of values (–18% to 30%) found can be considered of little clinical concern then My5-FU may considered equivalent to HPLC. My5-FU has the advantage over HPLC in that it requires only a small amount of plasma, takes less time, is less expensive, requires less training and can be automated. 84
If PK is adopted for CRC in the UK then there will need to be an expansion of laboratories willing to undertake the assay procedure, and training will be needed for staff and nurses to collect samples correctly.
An appropriate algorithm for dose adaptation
No clinical study with known algorithm using My5-FU is currently available to assess the appropriateness of the algorithm best used with My5-FU in the UK.
Of studies using My5-FU for 5-FU monitoring Hendrayana et al. 150 did not perform dose adjustment, Saam et al. 222 did not bind physicians to adjust doses according to a specific algorithm and in Kline et al. 155 dose adjustment followed an unspecified algorithm supplied by the manufacturer (Myriad Genetic Laboratories). 155 This was unlikely to be the one published by the company96 as it used a target range of 20–24 mg × hour/l rather than Kaldate et al. ’s96 recommended 20–30 mg × hour/l.
Therefore, the evidence on algorithms also comes from an indirect comparison with HPLC studies. Ychou et al. 131,147 developed adaptation schedules for the bimonthly LV5FU2 (de Gramont86,87) regimen with dose increases up to an AUC of 20 mg × hour/l × m2. The methods of how the algorithm was developed are unclear. Gamelin et al. 118 used a dose adaptation algorithm developed for the weekly 8-hour continuous infusion of FUFOL with a target range of 20–24 mg × hour/l. The dose adaptation algorithm was based on a regression analysis of the relationship between dose and plasma levels in two groups of patients with different quality of response. 130 They reported differential median OS for the PK arm of 22 months compared with 16 months for the BSA arm. 118 Capitain et al. 119 used protocols based on the Gamelin et al. 118 algorithm modified for the FOLFOX regimen. These protocols have been commercialised and have not been published. Furthermore, the protocols may not be pure algorithms for 5-FU as they may include other tests/considerations such as DPD genotyping. It is unclear how such protocols would translate into English services. We therefore conclude that the only algorithms currently available which have been validated in CRC patients are based on regimens no longer in clinical practice in the UK or are unavailable in the public domain. It is unclear whether or not the survival gains can be generalised to other treatment regimens that may require alternative and as yet ill-defined adjustment algorithms. 84
In 2011, Saam et al. 222 reported US experience with My5-FU suggesting that physicians in practice made larger reductions than increases in 5-FU doses. While Gamelin et al. 118 used an algorithm that allowed 50–70% dose increases for some patients to reach the 5-FU target range; it appears that physicians not bound to an adaptation protocol generally increased doses by only 10–20%, illustrating a cautious attitude towards upwards dose adjustment. 222 It is important that dose increases are ruled by algorithms but they will also require clinical judgement. This might result in PK dose adjustment being less effective in clinical practice than in the research environment because different clinicians may apply dose increases more cautiously than in reported studies.
An appropriate target plasma 5-fluorouracil level (the target range)
Single-arm studies investigating PK dose adjustment in CRC patients used a target range of 20–24 mg × hour/l. This target range was established for the 8-hour FUFOL regimen130 and was subsequently used for most other regimens. 150,155,222 However, Kaldate et al. 96 developed a new algorithm and argued that newer extended infusion time regimens should use a wider target range with the upper limit increased to 30 mg × hour/l and are less toxic and. No study was identified that has made use of this new algorithm. Moreover, a study155 that was provided by an algorithm by the manufacturer Myriad Genetics Laboratories itself222 used the 20–24 mg × hour/l target range. This introduces some doubts as to the suitability of the Kaldate et al. 96 algorithm.
Chapter 7 Conclusions
With an ICER of £4148, PK dose adjustment using My5-FU appears to be cost-effective for 12 cycles of FOLFOX6 in the treatment of patients with mCRC. This is based on a survival benefit with questionable plausibility and substantial uncertainties due to limited available evidence. Evidence comes from out of date treatment regimens (e.g. 5-FU on its own in 8-hour rather than 46-hour infusions) and non-randomised comparative studies. In addition, there is uncertainty as to the direct applicability of results to UK clinical practice. Uncertainties remain as to how PK dose adjustment should be achieved and how much it will cost the NHS to implement the My-5-FU assay. Our work on H&N cancer suggests that PK dose adjustment is likely to be cost-effective at standard WTP thresholds. There was no evidence on 5-FU PK dose adjustment in comparison with BSA-based dosing for stomach or pancreatic cancer.
Recommendations for further research
We are conscious that improved data are becoming available with more information on current practice and experiences of CRC patients in terms of mechanisms of dosing and AEs, etc. (e.g. from the COIN trial). This will help in assessing cost-effectiveness of interventions to improve treatment and survival in CRC. However, given the poor quality of the clinical effectiveness and cost-effectiveness evidence available to us there are a number of research needs including (in priority order) a need for:
-
well-conducted RCTs of PK versus BSA dosing in
-
metastatic and adjuvant CRC
-
H&N cancer
-
other cancers where a 5-FU regimen is used
-
-
further in depth assessment of the comparability of different methods of current and any newly introduced PK dose adjustment
-
randomised assessment of different algorithms for adjusting 5-FU dosing
-
research on the QALY impact of AEs of 5-FU which would be of benefit in any further economic assessments.
Acknowledgements
The authors would like to thank Nick Wadd, Gireesh Kumaran, Joanne Lowe, Ann Cole, Anne-Marie Hunter, Andrew Teggert, Fatiha Shabaruddin, David Thomson, Nick Slevin, Michele Boisdron, Kathleen Mais, Linda Celle, Michael Chappell, Helen Haley, Janice Szulc, Kathleen Boyd and Eleanor Aynsley.
Contributions of authors
Paul Sutcliffe (Associate Professor) co-ordinated the review.
Karoline Freeman (Research Fellow), Martin Connock (Senior Research Fellow), Tara Gurung (Research Fellow), Sian Taylor-Phillips (Senior Research Fellow) and Paul Sutcliffe conducted the clinical effectiveness systematic review, this included screening and retrieving papers, assessing against the inclusion criteria, appraising the quality of papers and abstracting data from papers for synthesis.
Ewen Cummins (Health Economist) undertook the health economic work.
Martin Connock conducted the data analysis.
Rachel Court (Information Specialist) developed the search strategy and undertook searches.
Tara Gurung, Paul Sutcliffe and Aileen Clarke (Professor of Public Health) wrote the background section of the report.
Mark Saunders (Consultant Clinical Oncologist, The Christie NHS Foundation Trust) provided clinical comment and guidance.
Aileen Clarke provided project management, clinical and methodological input, and wrote the abstract and summary.
Karoline Freeman and Aileen Clarke wrote the discussion.
Mark Saunders provided clinical comment and guidance.
All authors were involved in writing draft and final versions of the report.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Mansouri D, McMillan DC, Grant Y, Crighton EM, Horgan PG. The impact of age, sex and socioeconomic deprivation on outcomes in a colorectal cancer screening programme. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0066063.
- Mortality Statistics: Deaths registered in 2010, England and Wales. London: ONS; 2011.
- General Register Office for Scotland . Deaths Time Series Data: Deaths, by Sex, Scotland, 1855 to 2012 2013. www.gro-scotland.gov.uk/statistics/theme/vital-events/deaths/time-series.html (accessed 9 November 2013).
- Registrar General Annual Report 2011. Belfast: NISRA; 2012.
- Cancer Research UK . Bowel Cancer Survival Statistics 2012. www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/survival/ (accessed 14 November 2013).
- The Royal Marsden . Colorectal Cancer n.d. www.royalmarsden.nhs.uk/cancer-information/types/pages/colorectal-cancer.aspx (accessed 1 April 2014).
- National Institute for Health and Care Excellence . Colorectal Cancer: The Diagnosis and Management of Colorectal Cancer 2011. www.nice.org.uk/guidance/CG131 (accessed 22 April 2014).
- American Cancer Society . Colorectal Cancer 2014. www.cancer.org/cancer/colonandrectumcancer/detailedguide/colorectal-cancer-key-statistics (accessed 1 May 2014).
- Potter JD. Nutrition and colorectal cancer. Cancer Causes Control 1996;7:127-46. http://dx.doi.org/10.1007/BF00115644.
- Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol 1998;148:761-74. http://dx.doi.org/10.1093/oxfordjournals.aje.a009697.
- Woolf CM. A genetic study of carcinoma of the large intestine. Am J Hum Genet 1958;10:42-7.
- Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. New Eng J Med 1994;331:1669-74. http://dx.doi.org/10.1056/NEJM199412223312501.
- Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res 2011;4:53-61.
- Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol 2012;3:153-73. http://dx.doi.org/10.3978/j.issn.2078-6891.2012.030.
- Cancer Research UK . Bowel Cancer Incidence Statistics: By Morphology 2009. www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/incidence/ (accessed 27 May 2014).
- Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 1975;36:2251-70.
- Hole DJ, McArdle CS. Impact of socioeconomic deprivation on outcome after surgery for colorectal cancer. Br J Surg 2002;89:586-90. http://dx.doi.org/10.1046/j.1365-2168.2002.02073.x.
- O Ceilleachair A, Hanly P, Skally M, O’Neill C, Fitzpatrick P, Kapur K, et al. Cost comparisons and methodological heterogeneity in cost-of-illness studies: the example of colorectal cancer. Med Care 2013;51:339-50. http://dx.doi.org/10.1097/MLR.0b013e3182726c13.
- World Cancer Research Fund . World Cancer Statistics: Bowel Cancer: UK. 2014 n.d. www.wcrf-uk.org/research/cancer_statistics/bowel_cancer_rates.php (accessed 1 April 2014).
- Jayatilleke N, Pashayan N, Powles JW. Burden of disease due to cancer in England and Wales. J Public Health (Oxf) 2012;34:287-95. http://dx.doi.org/10.1093/pubmed/fdr093.
- NHS Cancer Screening Programmes . Bowel Cancer Screening: The Facts 2011. www.cancerscreening.nhs.uk/bowel/publications/bowel-cancer-the-facts.pdf (accessed 1 April 2014).
- Towler B, Irwig L, Glasziou P, Kewenter J, Weller D, Silagy C. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, hemoccult. BMJ 1998;317:559-65. http://dx.doi.org/10.1136/bmj.317.7158.559.
- NHS Bowel Cancer Screening Programme . NHS Bowel Scope Screening: Information for Primary Care 2013. www.nwlh.nhs.uk/_assets/docs/general/GPSection/Bowel%20scope%20primary%20care%20factsheet%20FINAL%20v1%20Mar13.pdf (accessed 1 April 2014).
- National Cancer Intelligence Network . Colorectal Cancer Survival by Stage NCIN Data Briefing 2009. www.ncin.org.uk/publications/data_briefings/colorectal_cancer_survival_by_stage (accessed 1 April 2014).
- Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012;61:1439-46. http://dx.doi.org/10.1136/gutjnl-2011-300843.
- Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011;377:127-38. http://dx.doi.org/10.1016/S0140-6736(10)62231-3.
- Cappell MS. The pathophysiology, clinical presentation, and diagnosis of colon cancer and adenomatous polyps. Med Clin North Am 2005;89. http://dx.doi.org/10.1016/j.mcna.2004.08.011.
- Cancer Research UK . Bowel Cancer Symptoms 2014. www.cancerresearchuk.org/cancer-help/type/bowel-cancer/about/bowel-cancer-symptoms (accessed 1 April 2014).
- Patient.co.uk . Colorectal Cancer 2012. www.patient.co.uk/doctor/colorectal-cancer (accessed 1 March 2014).
- Hind D, Tappenden P, Tumur I, Eggington S, Sutcliffe P, Ryan A. The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12150.
- National Cancer Institute at the National Institutes of Health . Colon Cancer Treatment – Stage IV and Recurrent Colon Cancer Treatment n.d. www.cancer.gov/cancertopics/pdq/treatment/colon/HealthProfessional/page9 (accessed 1 February 2014).
- Wallington M. Variations in Body Surface Area of Patients Receiving Chemotherapy Treatment in England 2013. www.ncin.org.uk/view?rid=2293 (accessed 20 May 2014).
- Bertino J, Gamelin E, Milano G. Highlights from: 5-Fluorouracil drug management pharmacokinetics and pharmacogenomics workshop: Orlando, Florida; January 2007–5-Fluorouracil drug management: Pharmacokinetics and pharmacogenomics workshop meeting summary. Clin Colorectal Cancer 2007;6:407-22. http://dx.doi.org/10.1016/S1533-0028(11)70480-7.
- Adams RA, Meade AM, Seymour MT, Wilson RH, Madi A, Fisher D, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol 2011;12:642-53. http://dx.doi.org/10.1016/S1470-2045(11)70102-4.
- Macmillan Cancer Support . Head and Neck Cancers n.d. www.macmillan.org.uk/Cancerinformation/Cancertypes/Headneck/Headneckcancers.aspx (accessed 1 April 2014).
- Klein JD, Grandis JR. The molecular pathogenesis of head and neck cancer. Cancer Biol Ther 2010;9:1-7. http://dx.doi.org/10.4161/cbt.9.1.10905.
- Scottish Intercollegiate Guidelines Network . Diagnosis and Management of Head and Neck Cancer. A National Clinical Guideline 2006. www.sign.ac.uk/pdf/sign90.pdf (accessed 1 April 2014).
- Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772-83. http://dx.doi.org/10.1093/jnci/djg107.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. http://dx.doi.org/10.3322/canjclin.55.2.74.
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. http://dx.doi.org/10.3322/caac.20073.
- Healthcare Quality Improvement Partnership, Health and Social Care Information Centre, The British Association of Head & Neck Oncologists . National Head &Amp; Neck Cancer Audit 2011 2012. www.hscic.gov.uk/catalogue/PUB06288/clin-audi-supp-prog-head-neck-dahn-10-11-rep.pdf (accessed 1 April 2014).
- Cancer Research UK . Oral Cancer Incidence Statistics 2013. www.cancerresearchuk.org/cancer-info/cancerstats/types/oral/incidence/uk-oral-cancer-incidence-statistics (accessed 1 April 2014).
- Kim K, Amonkar MM, Hogberg D, Kasteng F. Economic burden of resected squamous cell carcinoma of the head and neck in an incident cohort of patients in the UK. Head Neck Oncol 2011;3. http://dx.doi.org/10.1186/1758-3284-3-47.
- Drugan C, Leary S, Mellor T, Bain C, Verne J, Ness A, et al. Head and neck cancer in the south west of England, Hampshire, and the Isle of Wight: trends in survival 1996–2008. Br J Oral Maxillofac Surg 2013;51:19-24. http://dx.doi.org/10.1016/j.bjoms.2012.02.013.
- National Institute for Health and Care Excellence . Guidance on Cancer Services: Improving Outcomes in Head and Neck Cancers 2004. www.nice.org.uk/guidance/csghn/evidence/improving-outcomes-in-head-and-neck-cancers-the-manual2 (accessed 1 April 2014).
- National Cancer Institute at the National Institutes of Health . Head and Neck Cancer n.d. www.cancer.gov/cancertopics/factsheet/Sites-Types/head-and-neck (accessed 1 March 2014).
- Patel SG, Shah JP. TNM staging of cancers of the head and neck: striving for uniformity among diversity. CA Cancer J Clin 2005;55:242-58.
- Gregoire V, Lefebvre JL, Licitra L, Felip E. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v184-6. http://dx.doi.org/10.1093/annonc/mdq185.
- Chan AT, Gregoire V, Lefebvre JL, Licitra L, Hui EP, Leung SF, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii83-5. http://dx.doi.org/10.1093/annonc/mds266.
- Blanchard P, Bourhis J, Lacas B, Posner MR, Vermorken JB, Hernandez JJC, et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol 2013;31:2854-60. http://dx.doi.org/10.1200/JCO.2012.47.7802.
- Head and Neck Cancers Treatment Regimens 2012. www.just.edu.jo/DIC/ClinicGuidlines/Head%20and%20Neck%20cancer%20treatment%20regimens.pdf (accessed 1 March 2014).
- Nagini S. Carcinoma of the stomach: a review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol 2012;4:156-69. http://dx.doi.org/10.4251/wjgo.v4.i7.156.
- Lewin K, Appelman HD. Tumors of the Esophagus and Stomach (Atlas of Tumor Pathology 3rd Series, Vol. 18). Washington, DC: Armed Forces Institute of Pathology; 1996.
- McLoughlin JM. Adenocarcinoma of the stomach: a review. Proceedings 2004;17:391-9.
- Vyse AJ, Gay NJ, Hesketh LM, Andrews NJ, Marshall B, Thomas HI, et al. The burden of Helicobacter pylori infection in England and Wales. Epidemiol Infect 2002;128:411-17. http://dx.doi.org/10.1017/s0950268802006970.
- Coupland VH, Allum W, Blazeby JM, Mendall MA, Hardwick RH, Linklater KM, et al. Incidence and survival of oesophageal and gastric cancer in England between 1998 and 2007, a population-based study. BMC Cancer 2012;12. http://dx.doi.org/10.1186/1471-2407-12-11.
- Quinn M, Wood H, Cooper N, Rowan S. SMPS No. 68: Cancer Atlas of the United Kingdom and Ireland 1991–2000. Basingstoke: Palgrave MacMillan; 2005.
- Miceli R, Tomasello G, Bregni G, Di Bartolomeo M, Pietrantonio F. Adjuvant chemotherapy for gastric cancer: current evidence and future challenges. World J Gastroenterol 2014;20:4516-25. http://dx.doi.org/10.3748/wjg.v20.i16.4516.
- Cancer Research UK . Stomach Cancer Incidence Statistics 2013. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/stomach-cancer/incidence (accessed 14 November 2013).
- Cancer Research UK . Stomach Cancer Survival Statistics 2012. www.cancerresearchuk.org/cancer-info/cancerstats/types/stomach/survival/ (accessed 14 November 2013).
- Cancer Network UK . One, Five and Ten Year Cancer Prevalence 2010. www.ncin.org.uk/view?rid=76 (accessed 5 May 2014).
- NHS Choices . Stomach Cancer 2014. www.nhs.uk/conditions/cancer-of-the-stomach/Pages/Introduction.aspx (accessed 2 May 2014).
- National Institute for Health and Care Excellence . Capecitabine for the Treatment of Advanced Gastric Cancer 2010. www.nice.org.uk/guidance/ta191/resources/guidance-capecitabine-for-the-treatment-of-advanced-gastric-cancer-pdf (accessed 12 May 2014).
- Diaz-Nieto R, Orti-Rodriguez R, Winslet M. Post-surgical chemotherapy versus surgery alone for resectable gastric cancer. Cochrane Database Syst Rev 2013;9. http://dx.doi.org/10.1002/14651858.cd008415.pub2.
- Jiang L, Yang KH, Guan QL, Zhao P, Chen Y, Tian JH. Survival and recurrence free benefits with different lymphadenectomy for resectable gastric cancer: a meta-analysis. J Surg Oncol 2013;107:807-14. http://dx.doi.org/10.1002/jso.23325.
- Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 2006;20:197-209. http://dx.doi.org/10.1016/j.bpg.2005.10.001.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. http://dx.doi.org/10.3322/CA.2007.0010.
- Pancreatic Cancer UK . Types of Pancreatic Cancer 2014. www.pancreaticcancer.org.uk/information-and-support/facts-about-pancreatic-cancer/types-of-pancreatic-cancer (accessed 6 May 2014).
- Shen M, Boffetta P, Olsen JH, Andersen A, Hemminki K, Pukkala E, et al. A pooled analysis of second primary pancreatic cancer. Am J Epidemiol 2006;163:502-11. http://dx.doi.org/10.1093/aje/kwj073.
- Permuth-Wey J, Egan KM. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer 2009;8:109-17. http://dx.doi.org/10.1007/s10689-008-9214-8.
- Jacobs EJ, Chanock SJ, Fuchs CS, Lacroix A, McWilliams RR, Steplowski E, et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Int J Cancer 2010;127:1421-8. http://dx.doi.org/10.1002/ijc.25148.
- Larsson SC, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br J Cancer 2012;106:603-7. http://dx.doi.org/10.1038/bjc.2011.585.
- Wang Y, Yang S, Song F, Cao S, Yin X, Xie J, et al. Hepatitis B virus status and the risk of pancreatic cancer: a meta-analysis. Eur J Cancer Prev 2013;22:328-34. http://dx.doi.org/10.1097/CEJ.0b013e32835b6a21.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. http://dx.doi.org/10.3322/caac.20107.
- Krejs GJ. Pancreatic cancer: epidemiology and risk factors. Dig Dis 2010;28:355-8. http://dx.doi.org/10.1159/000319414.
- Pancreatic Cancer Action . UK Pancreatic Cancer Statistics 2011. http://pancreaticcanceraction.org/wp-content/uploads/2010/05/UK-Pancreatic-Cancer-Statistics-2011.pdf (accessed 6 May 2014).
- NHS Commissioning Board . 2013/14/NHS/Standard/Contract/for/Cancer:/Pancreatic/(Adult) 2013. www.england.nhs.uk/wp-content/uploads/2013/06/a02-cncr-panc.pdf (accessed 12 May 2014).
- Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut 2005;54:v1-16. http://dx.doi.org/10.1136/gut.2004.057059.
- National Institute for Health and Care Excellence . Guidance on the Use of Gemcitabine for the Treatment of Pancreatic Cancer 2001. www.nice.org.uk/TA25 (accessed 22 April 2014).
- Liao WC, Chien KL, Lin YL, Wu MS, Lin JT, Wang HP, et al. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol 2013;14:1095-103. http://dx.doi.org/10.1016/S1470-2045(13)70388-7.
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 2010;7:163-72. http://dx.doi.org/10.1038/nrclinonc.2009.236.
- Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut 2013;62:317-26. http://dx.doi.org/10.1136/gutjnl-2012-303588.
- Arias J. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules 2008;13:2340-69. http://dx.doi.org/10.3390/molecules13102340.
- Saif MW, Choma A, Salamone SJ, Chu E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: A rational approach to improving therapeutic outcomes. J Natl Cancer Inst 2009;101:1543-52. http://dx.doi.org/10.1093/jnci/djp328.
- BlueCross BlueShield of Alabama . Laboratory Testing to Allow Area Under the Curve (AUC) Targeted 5-Fluorouracil (5-FU) Dosing for Patients Administered 5-FU for Cancer 2013. www.bcbsal.org/providers/policies/final/253.pdf (accessed 15 November 2013).
- de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 1997;15:808-15.
- de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938-47.
- Salamone SJ, Li Y, Courtney J, Harney R, Lundell G, Stocker D. 5-Fluorouracil determination in plasma by rapid nanoparticle immunoassay; highlights from: 5-Fluorouracil Drug Management Pharmacokinetics and Pharmacogenomics Workshop; Orlando, Florida; January 2007. Clin Colorectal Cancer 2007;6:419-20.
- Beumer JH, Boisdron-Celle M, Clarke W, Courtney JB, Egorin MJ, Gamelin E, et al. Multicenter evaluation of a novel nanoparticle immunoassay for 5-fluorouracil on the Olympus AU400 Analyzer. Ther Drug Monit 2009;31:688-94. http://dx.doi.org/10.1519/JSC.0b013e3181b866d0.
- Saladax Biomedical . 5-Fluorouracil (My5-FU™) Assay [package Insert] 2011. www.saladax.com/wp-content/uploads/2012/07/My5-FU-Assay-Package-Insert-rev06.pdf (accessed 22 April 2014).
- Piedbois P, Rougier P, Buyse M, Pignon J, Ryan L, . Meta-Analysis Group In Cancer . Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis Group In Cancer. J Clin Oncol 1998;16:301-8.
- Hale JP, Cohen DR, Maughan TS, Stephens RJ. Costs and consequences of different chemotherapy regimens in metastatic colorectal cancer. Br J Cancer 2002;86:1684-90. http://dx.doi.org/10.1038/sj.bjc.6600273.
- Lee A, Ezzeldin H, Fourie J, Diasio R. Dihydropyrimidine dehydrogenase deficiency: impact of pharmacogenetics on 5-fluorouracil therapy. Clin Adv Hematol Oncol 2004;2:527-32.
- Schwab M, Zanger UM, Marx C, Schaeffeler E, Klein K, Dippon J, et al. Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the German 5-FU Toxicity Study Group. J Clin Oncol 2008;26:2131-8. http://dx.doi.org/10.1200/JCO.2006.10.4182.
- Buchel B, Sistonen J, Joerger M, Aebi Y, Schurch S, Largiader CR. Comparative evaluation of the My5-FU immunoassay and LC-MS/MS in monitoring the 5-fluorouracil plasma levels in cancer patients. Clin Chem Lab Med 2013;51:1681-8. http://dx.doi.org/10.1515/cclm-2012-0641.
- Kaldate RR, Haregewoin A, Grier CE, Hamilton SA, McLeod HL. Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6. Oncologist 2012;17:296-302. http://dx.doi.org/10.1634/theoncologist.2011-0357.
- Harris BE, Song R, Soong SJ, Diasio RB. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res 1990;50:197-201.
- Di Paolo A, Danesi R, Falcone A, Cionini L, Vannozzi F, Masi G, et al. Relationship between 5-fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patients. Ann Oncol 2001;12:1301-6. http://dx.doi.org/10.1023/A:1012294617392.
- van Kuilenburg AB, Haasjes J, Richel DJ, Zoetekouw L, Van Lenthe H, De Abreu RA, et al. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res 2000;6:4705-12.
- Ezzeldin H, Diasio R. Dihydropyrimidine dehydrogenase deficiency, a pharmacogenetic syndrome associated with potentially life-threatening toxicity following 5-fluorouracil administration. Clin Colorectal Cancer 2004;4:181-9. http://dx.doi.org/10.3816/CCC.2004.n.018.
- Kosovec JE, Egorin MJ, Gjurich S, Beumer JH. Quantitation of 5-fluorouracil (5-FU) in human plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 2008;22:224-30. http://dx.doi.org/10.1002/rcm.3362.
- Ciccolini J, Mercier C, Blachon MF, Favre R, Durand A, Lacarelle B. A simple and rapid high-performance liquid chromatographic (HPLC) method for 5-fluorouracil (5-FU) assay in plasma and possible detection of patients with impaired dihydropyrimidine dehydrogenase (DPD) activity. J Clin Pharm Ther 2004;29:307-15. http://dx.doi.org/10.1111/j.1365-2710.2004.00569.x.
- Gates P. High Performance Liquid Chromatography Mass Spectrometry (HPLC MS) n.d. www.bris.ac.uk/nerclsmsf/techniques/hplcms.html (accessed 8 May 2014).
- Gamelin E, Boisdron-Celle M, Turcant A, Larra F, Allain P, Robert J. Rapid and sensitive high-performance liquid chromatographic analysis of halogenopyrimidines in plasma. J Chromatogr B Biomed Appl 1997;695:409-16. http://dx.doi.org/10.1016/S0378-4347(97)00211-9.
- Buchel B, Rhyn P, Schurch S, Buhr C, Amstutz U, Largiader CR. LC-MS/MS method for simultaneous analysis of uracil, 5,6-dihydrouracil, 5-fluorouracil and 5-fluoro-5,6-dihydrouracil in human plasma for therapeutic drug monitoring and toxicity prediction in cancer patients. Biomed Chromatogr 2013;27:7-16. http://dx.doi.org/10.1002/bmc.2741.
- Vainchtein LD, Rosing H, Schellens JH, Beijnen JH. A new, validated HPLC-MS/MS method for the simultaneous determination of the anti-cancer agent capecitabine and its metabolites: 5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, 5-fluorouracil and 5-fluorodihydrouracil, in human plasma. Biomed Chromatogr 2010;24:374-86. http://dx.doi.org/10.1002/bmc.1302.
- Peer CJ, McManus TJ, Hurwitz HI, Petros WP. Development and utilization of a combined LC-UV and LC-MS/MS method for the simultaneous analysis of tegafur and 5-fluorouracil in human plasma to support a phase I clinical study of oral UFT/leucovorin. J Chromatogr B Analyt Technol Biomed Life Sci 2012;898:32-7. http://dx.doi.org/10.1016/j.jchromb.2012.04.010.
- Hillcoat BL, McCulloch PB, Figueredo A. Levels of 5 fluorouracil (5FU) in plasma of patients treated by drug infusion. Proce Am Association Cancer Res 1976;17.
- Kirkwood JM, Ensminger W, Rosowsky A, Papathanasopoulos N, Frei E. Comparison of pharmacokinetics of 5-fluorouracil and 5-fluorouracil with concurrent thymidine infusions in a phase I trial. Cancer Res 1980;40:107-13.
- Au JLS, Rustum YM, Ledesma EJ. Clinical pharmacological studies of concurrent infusion of 5-fluorouracil and thymidine in treatment of colorectal carcinomas. Cancer Res 1982;42:2930-7.
- Van Groeningen CJ, Pinedo HM, Heddes J, Kok RM, De Jong APJM, Wattel E, et al. Pharmacokinetics of 5-fluorouracil assessed with a sensitive mass spectrometric method in patients on a dose escalation schedule. Cancer Res 1988;48:6956-61.
- Kaestner SA, Sewell GJ. Chemotherapy dosing part II: alternative approaches and future prospects. Clin Oncol 2007;19:99-107. http://dx.doi.org/10.1016/j.clon.2006.10.003.
- Lindman H, Astrom G, Ahlgren J, Villman K, Blomqvist C, Nygren P, et al. Individually tailored toxicity-based 5-fluorouracil, epirubicin and cyclophosphamide (FEC) therapy of metastatic breast cancer. Acta Oncologica 2007;46:165-71. http://dx.doi.org/10.1080/02841860600871087.
- Gamelin E, Boisdron-Celle M, Guerin-Meyer V, Delva R, Lortholary A, Genevieve F, et al. Correlation between uracil and dihydrouracil plasma ratio, fluorouracil (5-FU) pharmacokinetic parameters, and tolerance in patients with advanced colorectal cancer: A potential interest for predicting 5-FU toxicity and determining optimal 5-FU dosage. J Clin Oncol 1999;17:1105-10.
- National Institute for Health and Care Excellence . Diagnostics Assessment Programme Manual 2011. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-diagnostics-guidance/Diagnostics-assessment-programme-manual.pdf (accessed 19 November 2013).
- National Institute for Health and Care Excellence . Improving Outcomes in Head and Neck Cancers: Evidence Update May 2012 2012. http://arms.evidence.nhs.uk/resources/hub/719893/attachment (accessed 23 April 2014).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2535.
- Gamelin E, Delva R, Jacob J, Merrouche Y, Raoul JL, Pezet D, et al. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2099-105. http://dx.doi.org/10.1200/JCO.2007.13.3934.
- Capitain O, Asevoaia A, Boisdron-Celle M, Poirier AL, Morel A, Gamelin E. Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study. Clin Colorectal Cancer 2012;11:263-7. http://dx.doi.org/10.1016/j.clcc.2012.05.004.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. http://dx.doi.org/10.1016/S0140-6736(86)90837-8.
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Haddow JE, Palomaki GE, Khoury M, Little J, Burke W. Human Genome Epidemiology: A Scientific Foundation for Using Genetic Information to Improve Health and Prevent Disease. Oxford: Oxford University Press; 2003.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84. http://dx.doi.org/10.1136/jech.52.6.377.
- Merlin T, Lehman S, Hiller J, Ryan P. The ‘linked evidence approach’ to assess medical tests: a critical analysis. Int J Technol Assess Health Care 2013;29:343-50. http://dx.doi.org/10.1017/S0266462313000287.
- Guyot P, Ades A, Ouwens M, Welton N. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol 2012;12. http://dx.doi.org/10.1186/1471-2288-12-9.
- Hoyle M, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol 2011;11. http://dx.doi.org/10.1186/1471-2288-11-139.
- Ouwens MJNM, Philips Z, Jansen JP. Network meta-analysis of parametric survival curves. Res Synth Methods 2010;1:258-71. http://dx.doi.org/10.1002/jrsm.25.
- Buchel B, Sistonen J, Aebi Y, Largiader CR. Comparative evaluation of the My5-FU immunoassay and LC-MS/MS in the monitoring of 5-fluorouracil levels in cancer patients. Clin Chem Lab Med 2012;50:A169-70. http://dx.doi.org/10.1515/cclm-2012-0641.
- Makihara K, Mishima H, Azuma S, Matsuyama K, Komori K, Hasegawa H, et al. A pilot study of pharmacokinetically guided dose management of capecitabine in CRC patients. J Clin Oncol 2012;30.
- Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, Maillart PJ, Goudier MJ, Burtin PC, et al. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer 1996;77:441-51. http://dx.doi.org/10.1002/(SICI)1097-0142(19960201)77:3<441::AID-CNCR4>3.0.CO;2-N.
- Ychou M, Duffour J, Pinguet F, Kramar A, Joulia JM, Topart D, et al. Individual 5FU-dose adaptation schedule using bimonthly pharmacokinetically modulated LV5FU2 regimen: a feasibility study in patients with advanced colorectal cancer. Anticancer Res 1999;19:2229-35.
- Santini J, Milano G, Thyss A, Renee N, Viens P, Ayela P, et al. 5-FU therapeutic monitoring with dose adjustment leads to an improved therapeutic index in head and neck cancer. Br J Cancer 1989;59:287-90. http://dx.doi.org/10.1038/bjc.1989.59.
- Boisdron-Celle M, Craipeau M, Brienza S, Delva R, Guerin-Meyer V, Cvitkovic E, et al. Influence of oxaliplatin on 5-fluorouracil plasma clearance and clinical consequences. Cancer Chemother Pharmacol 2002;49:235-43. http://dx.doi.org/10.1007/s00280-001-0406-2.
- Capitain O, Boisdron-Celle M, Poirier AL, Abadie-Lacourtoisie S, Morel A, Gamelin E. The influence of fluorouracil outcome parameters on tolerance and efficacy in patients with advanced colorectal cancer. Pharmacogenomics J 2008;8:256-67. http://dx.doi.org/10.1038/sj.tpj.6500476.
- Cattel L, La Grotta G, Infante L, Passera R, Arpicco S, Brusa P, et al. Pharmacokinetic study of oxaliplatin iv chronomodulated infusion combined with 5-fluorouracil iv continuous infusion in the treatment of advanced colorectal cancer. Farmaco 2003;58:1333-8. http://dx.doi.org/10.1016/S0014-827X(03)00193-9.
- Duffour J, Roca L, Bressolle F, Abderrahim AG, Poujol S, Pinguet F, et al. Clinical impact of intesified 5-Fluorouracil-based chemotherapy using a prospective pharmacokinetically-guided dosing approach: comparative study in elderly and non-elderly patients with metastatic colorectal cancer. J Chemother 2010;22:179-85. http://dx.doi.org/10.1179/joc.2010.22.3.179.
- Findlay MPN, Raynaud F, Cunningham D, Iveson A, Collins DJ, Leach MO. Measurement of plasma 5-fluorouracil by high-performance liquid chromatography with comparison of results to tissue drug levels observed using in vivo 19F magnetic resonance spectroscopy in patients on a protracted venous infusion with or without interferon-alpha. Ann Oncol 1996;7:47-53. http://dx.doi.org/10.1093/oxfordjournals.annonc.a010476.
- Gamelin E, Boisdron-Celle M, Delva R, Regimbeau C, Cailleux PE, Alleaume C, et al. Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: Results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J Clin Oncol 1998;16:1470-8.
- Ho YF, Lu WC, Chen RRL, Cheng AL, Yeh KH. Phase I, pharmacokinetic, and bone marrow drug-level studies of trimonthly 48-h infusion of high-dose 5-fluorouracil and leucovorin in patients with metastatic colorectal cancers. Anti-Cancer Drugs 2011;22:290-8. http://dx.doi.org/10.1097/CAD.0b013e3283426112.
- Jodrell DI, Stewart M, Aird R, Knowles G, Bowman A, Wall L, et al. 5-Fluorouracil steady state pharmacokinetics and outcome in patients receiving protracted venous infusion for advanced colorectal cancer. Br J Cancer 2001;84:600-3. http://dx.doi.org/10.1054/bjoc.2000.1664.
- Kline CL, Sheikh HS, Scicchitano A, Gingrich R, Beachler C, Finnberg NK, et al. Preliminary observations indicate variable patterns of plasma 5-fluorouracil (5-FU) levels during dose optimization of infusional 5-FU in colorectal cancer patients. Cancer Biol Ther 2011;12:557-68. http://dx.doi.org/10.4161/cbt.12.7.18059.
- Metzger G, Massari C, Etienne MC, Comisso M, Brienza S, Touitou Y, et al. Spontaneous or imposed circadian changes in plasma concentrations of 5-fluorouracil coadministered with folinic acid and oxaliplatin: relationship with mucosal toxicity in patients with cancer. Clin Pharmacol Ther 1994;56:190-201. http://dx.doi.org/10.1038/clpt.1994.123.
- Milano G, Roman P, Khater R, Frenay M, Renee N, Namer M. Dose versus pharmacokinetics for predicting tolerance to 5-day continuous infusion of 5-FU. Int J Cancer 1988;41:537-41. http://dx.doi.org/10.1002/ijc.2910410411.
- Patel JN, O’Neil BH, McLeod HL, Sherrill GB, Olijade O, Inzerillo JJ, et al. Investigating the utilization of pharmacokinetic-guided fluorouracil in colorectal cancer. J Clin Oncol 2012;30. http://dx.doi.org/10.1634/theoncologist.2014-0132.
- Patel JN, Deal AM, O’Neil BH, Ibrahim J, Sherrill GB, Davies JM, et al. Application of pharmacokinetic (PK)-guided 5-fluorouracil (FU) in clinical practice. J Clin Oncol 2013;31.
- Stremetzne S, Streit M, Kreuser ED, Schunack W, Jaehde U. Pharmacokinetic and pharmacodynamic comparison of two doses of calcium folinate combined with continuous fluorouracil infusion in patients with advanced colorectal cancer. Pharm World Sci 1999;21:184-9. http://dx.doi.org/10.1023/A:1008671129128.
- Ychou M, Duffour J, Kramar A, Debrigode C, Gourgou S, Bressolle F, et al. Individual 5-FU dose adaptation in metastatic colorectal cancer: results of a phase II study using a bimonthly pharmacokinetically intensified LV5FU2 regimen. Cancer Chemother Pharmacol 2003;52:282-90. http://dx.doi.org/10.1007/s00280-003-0658-0.
- Yoshida T, Araki E, Iigo M, Fujii T, Shimada Y, Saito D, et al. Clinical significance of monitoring serum levels of 5-fluorouracil by continuous infusion in patients with advanced colonic cancer. Cancer Chemother Pharmacol 1990;26:352-4. http://dx.doi.org/10.1007/BF02897292.
- Ciccolini J, Mercier C, Evrard A, Dahan L, Boyer JC, Duffaud F, et al. A rapid and inexpensive method for anticipating severe toxicity to fluorouracil and fluorouracil-based chemotherapy. Ther Drug Monit 2006;28:678-85. http://dx.doi.org/10.1097/01.ftd.0000245771.82720.c7.
- Hendrayana T, Kurth V, Krolop L, Kenny P, Hilger RA, Schmidt-Wolf IGH, et al. Variability in fluorouracil exposure during continuous intravenous infusion. Int J Clin Pharmacol Ther 2012;50:82-4. http://dx.doi.org/10.5414/CPP50082.
- Etienne MC, Lagrange JL, Dassonville O, Fleming R, Thyss A, Renee N, et al. Population study of dihydropyrimidine dehydrogenase in cancer patients. J Clin Oncol 1994;12:2248-53.
- Milano G, Etienne MC, Renee N, Thyss A, Schneider M, Ramaioli A, et al. Relationship between fluorouracil systemic exposure and tumor response and patient survival. J Clin Oncol 1994;12:1291-5.
- Thyss A, Milano G, Renee N. Clinical pharmacokinetic study of 5-FU in continuous 5-day infusions for head and neck cancer. Cancer Chemother Pharmacol 1986;16:64-6. http://dx.doi.org/10.1007/bf00255288.
- Kim R, Nishimoto N, Inoue H, Yoshida K, Toge T. An analysis of the therapeutic efficacy of protracted infusion of low-dose 5-fluorouracil and cisplatin in advanced gastric cancer. J Infect Chemother 2000;6:222-8. http://dx.doi.org/10.1007/s101560070007.
- Kline CLB, Schiccitano A, Zhu J, Beachler C, Sheikh H, Harvey HA, et al. Personalized dosing via pharmacokinetic monitoring of 5-Fluorouracil (5-FU) may reduce toxicity in early or late stage colorectal cancer patients treated with infusional 5-FU-based chemotherapy regimens [published online ahead of print 20 November 2013]. Clin Colorectal Cancer 2013.
- Fety R, Rolland F, Barberi-Heyob M, Hardouin A, Campion L, Conroy T, et al. Clinical impact of pharmacokinetically-guided dose adaptation of 5-fluorouracil: results from a multicentric randomized trial in patients with locally advanced head and neck carcinomas. Clin Cancer Res 1998;4:2039-45.
- Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, part I. J Clin Chem Clin Biochem 1983;21:709-20. http://dx.doi.org/10.1515/cclm.1983.21.11.709.
- Fety R, Rolland F, Barberiheyob M, Merlin JL, Conroy T, Hardouin A, et al. Clinical randomized study of 5FU monitoring versus standard dose in patients with head and neck cancer: preliminary results. Anticancer Res 1994;14:2347-52.
- Boisdron-Celle M. Pharmacokinetic adaptation of 5-fluorouracil: where are we and where are we going?. Pharmacogenomics 2012;13:1437-9. http://dx.doi.org/10.2217/pgs.12.132.
- National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE): Version 4.03 2010. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed 11 June 2014).
- Vokes EE, Mick R, Kies MS, Dolan ME, Malone D, Athanasiadis I, et al. Pharmacodynamics of fluorouracil-based induction chemotherapy in advanced head and neck cancer. J Clin Oncol 1996;14:1663-71.
- Köhne CH, Wils J, Lorenz M, Schoffski P, Voigtmann R, Bokemeyer C, et al. Randomized phase III study of high-dose fluorouracil given as a weekly 24-hour infusion with or without leucovorin versus bolus fluorouracil plus leucovorin in advanced colorectal cancer: European Organization of Research and Treatment of Cancer Gastrointestinal Group Study 40952. J Clin Oncol 2003;21:3721-8. http://dx.doi.org/10.1200/JCO.2003.11.122.
- Köhne C-H, van Cutsem E, Wils J, Bokemeyer C, El-Serafi M, Lutz MP, et al. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J Clin Oncol 2005;23:4856-65. http://dx.doi.org/10.1200/JCO.2005.05.546.
- Seymour MT, Maughan TS, Ledermann JA, Topham C, James R, Gwyther SJ, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet 2007;370:143-52. http://dx.doi.org/10.1016/S0140-6736(07)61087-3.
- Cunningham D, Sirohi B, Pluzanska A, Utracka-Hutka B, Zaluski J, Glynne-Jones R, et al. Two different first-line 5-fluorouracil regimens with or without oxaliplatin in patients with metastatic colorectal cancer. Ann Oncol 2009;20:244-50. http://dx.doi.org/10.1093/annonc/mdn638.
- Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE study. J Clin Oncol 2008;26:3523-9. http://dx.doi.org/10.1200/JCO.2007.15.4138.
- Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer 2011;128:682-90. http://dx.doi.org/10.1002/ijc.25369.
- Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. http://dx.doi.org/10.1200/JCO.2004.05.113.
- Madi A, Fisher D, Wilson RH, Adams RA, Meade AM, Kenny SL, et al. Oxaliplatin/capecitabine vs oxaliplatin/infusional 5-FU in advanced colorectal cancer: the MRC COIN trial. Br J Cancer 2012;107:1037-43. http://dx.doi.org/10.1038/bjc.2012.384.
- Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil–leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 2000;18.
- Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics 2013;31:277-88. http://dx.doi.org/10.1007/s40273-013-0033-x.
- Tappenden P, Jones R, Paisley S, Carroll C. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11120.
- Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan RF, et al. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14320.
- Howes N, Lewis SJ, Thomas S. Immunonutrition for patients undergoing surgery for head and neck cancer (protocol). Cochrane Database Syst Rev 2014;3.
- Shivakumar T, Nair S, Gupta T, Kannan S. Concurrent chemoradiotherapy with weekly versus three-weekly cisplatin in locally advanced head and neck squamous cell carcinoma (protocol). Cochrane Database Syst Rev 2014;1.
- Sutcliffe P, Connock M, Shyangdan D, Court R, Ngianga-Bakwin K, Clarke A. A systematic review of evidence on malignant spinal metastases: natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17420.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care 2013;29:117-22. http://dx.doi.org/10.1017/S0266462313000160.
- Becker R, Hollenbeak CS, Choma A, Kenny P, Salamone SJ. Cost-effectiveness of pharmacokinetic dosing of 5-fluorouracil in metastatic colorectal cancer in the united kingdom. Value Health 2013;16. http://dx.doi.org/10.1016/j.jval.2013.03.680.
- National Institute for Health and Care Excellence . Bevacizumab and Cetuximab for the Treatment of Metastatic Colorectal Cancer 2007. http://guidance.nice.org.uk/TA118/Guidance/pdf/English (accessed 20 May 2014).
- National Institute for Health and Care Excellence . Cetuximab, Bevacizumab and Panitumumab for the Treatment of Metastatic Colorectal Cancer After First-Line Chemotherapy 2012. www.nice.org.uk/guidance/ta242 (accessed 20 May 2014).
- National Institute for Health and Care Excellence . Capecitabine and Tegafur Uracil for Metastatic Colorectal Cancer 2003. www.nice.org.uk/guidance/TA61 (accessed 22 April 2014).
- National Institute for Health and Care Excellence . Cetuximab for the First Line Treatment of Metastatic Colorectal Cancer 2009. www.nice.org.uk/guidance/TA176 (accessed 22 April 2014).
- National Institute for Health and Care Excellence . Bevacizumab in Combination With Oxaliplatin and Either Fluorouracil Plus Folinic Acid or Capecitabine for the Treatment of Metastatic Colorectal Cancer 2010. www.nice.org.uk/guidance/ta212 (accessed 20 May 2014).
- National Cancer Intelligence Network . Systemic Anti-Cancer Therapy (SACT) Dataset n.d. www.chemodataset.nhs.uk/ (accessed 20 May 2014).
- Buyse M, Thirion P, Carlson RW, Burzykowski T, Molenberghs G, Piedbois P. Relation between tumour response to first-line chemotherapy and survival in advanced colorectal cancer: a meta-analysis. Lancet 2000;356:373-8. http://dx.doi.org/10.1016/S0140-6736(00)02528-9.
- Manca A, Asseburg C, Bravo Vergel Y, Seymour MT, Meade A, Stephens R, et al. The cost-effectiveness of different chemotherapy strategies for patients with poor prognosis advanced colorectal cancer (MRC FOCUS). Value Health 2012;15:22-31. http://dx.doi.org/10.1016/j.jval.2011.07.008.
- Ramsey SD, Andersen MR, Etzioni R, Moinpour C, Peacock S, Potosky A, et al. Quality of life in survivors of colorectal carcinoma. Cancer 2000;88:1294-303. http://dx.doi.org/10.1002/(SICI)1097-0142(20000315)88:6<1294::AID-CNCR4>3.0.CO;2-M.
- Petrou S, Campbell N. Stabilisation in colorectal cancer. Int J Palliat Nurs 1997;3:275-80.
- Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. New Engl J Med 2007;357:2040-8. http://dx.doi.org/10.1056/NEJMoa071834.
- Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preference values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res 2010;19:391-400. http://dx.doi.org/10.1007/s11136-010-9589-5.
- Mittmann N, Au HJ, Tu D, O’Callaghan CJ, Isogai PK, Karapetis CS, et al. Prospective cost-effectiveness analysis of cetuximab in metastatic colorectal cancer: evaluation of National Cancer Institute of Canada Clinical Trials Group CO.17 trial. J Natl Cancer Inst 2009;101:1182-92. http://dx.doi.org/10.1093/jnci/djp232.
- Färkkilä N, Sintonen H, Saarto T, Järvinen H, Hänninen J, Taari K, et al. Health-related quality of life in colorectal cancer. Colorectal Dis 2013;15:e215-22. http://dx.doi.org/10.1111/codi.12143.
- Shiroiwa T, Fukuda T, Tsutani K. Health utility scores of colorectal cancer based on societal preference in Japan. Qual Life Res 2009;18:1095-103. http://dx.doi.org/10.1007/s11136-009-9513-z.
- Wang J, Zhao Z, Barber B, Sherrill B, Peeters M, Wiezorek J. A Q-TWiST analysis comparing panitumumab plus best supportive care (BSC) with BSC alone in patients with wild-type KRAS metastatic colorectal cancer. Br J Cancer 2011;104:1848-53. http://dx.doi.org/10.1038/bjc.2011.179.
- Odom D, Barber B, Bennett L, Peeters M, Zhao Z, Kaye J, et al. Health-related quality of life and colorectal cancer-specific symptoms in patients with chemotherapy-refractory metastatic disease treated with panitumumab. Int J Colorectal Dis 2011;26:173-81. http://dx.doi.org/10.1007/s00384-010-1112-5.
- Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer 2006;95:683-90. http://dx.doi.org/10.1038/sj.bjc.6603326.
- Boyd KA, Briggs AH, Paul J, Iveson T, Midgely R, Harkin A, et al. Analysis of adverse events and quality of life data for an economic evaluation of adjuvant chemotherapy in colorectal cancer: when can we stop collecting?. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-S1-A41.
- Frederix GWJ, Quadri N, Hovels AM, van de Wetering FT, Tamminga H, Schellens JHM, et al. Utility and work productivity data for economic evaluation of breast cancer therapies in the Netherlands and Sweden. Clin Ther 2013;35:e1-7. http://dx.doi.org/10.1016/j.clinthera.2013.03.009.
- Tolley K, Goad C, Yi Y, Maroudas P, Haiderali A, Thompson G. Utility elicitation study in the UK general public for late-stage chronic lymphocytic leukaemia. Eur J Health Econ 2013;14:749-59. http://dx.doi.org/10.1007/s10198-012-0419-2.
- Swinburn P, Wang J, Chandiwana D, Mansoor W, Lloyd A. Elicitation of health state utilities in neuroendocrine tumours. J Med Econ 2012;15:681-7. http://dx.doi.org/10.3111/13696998.2012.670175.
- EU Clinical Trials Register . Short Course Oncology Therapy – A Study of Adjuvant Chemotherapy in Colorectal Cancer n.d. www.clinicaltrialsregister.eu/ctr-search/trial/2007-003957-10/SE/ (accessed 29 September 2015).
- Twelves C, Boyer M, Findlay M, Cassidy J, Weitzel C, Barker C, et al. Capecitabine (xeloda) improves medical resource use compared with 5-fluorouracil plus leucovorin in a phase III trial conducted in patients with advanced colorectal carcinoma. Eur J Cancer 2001;37:597-604. http://dx.doi.org/10.1016/S0959-8049(00)00444-5.
- NHS Careers . Agenda for Change – Pay Rates 2014. www.nhscareers.nhs.uk/working-in-the-nhs/pay-and-benefits/agenda-for-change-pay-rates/ (accessed 20 May 2014).
- Personal Social Services Research Unit . Unit Costs of Health and Social Care 2013 2013. www.pssru.ac.uk/project-pages/unit-costs/2013/ (accessed 20 May 2014).
- Department of Health . NHS Reference Costs 2012 to 2013 2013. www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013 (accessed 21 May 2014).
- Iveson J, Hickish T, Schmitt C, Van Cutsem E. Irinotecan in second-line treatment of metastatic colorectal cancer: improved survival and cost-effect compared with infusional 5-FU. Eur J Cancer 1999;35:1796-804. http://dx.doi.org/10.1016/S0959-8049(99)00186-0.
- Shabaruddin FH. Economic Evaluation of the Ugt1a1 Pharmacogenetic Test. Manchester: University of Manchester; 2011.
- Department of Health . Commercial Medicines Unit (CMU) Electronic Market Information Tool (eMit) 2013. http://cmu.dh.gov.uk/electronic-market-information-tool-emit/ (accessed 21 May 2014).
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. http://publications.nice.org.uk/pmg9 (accessed 21 May 2014).
- Leese B. The costs of treating febrile neutropenia in six U.K. hospitals. Eur J Cancer 1993;29:S15-8. http://dx.doi.org/10.1016/0959-8049(93)90611-I.
- Leese B, Collin R, Clark DJ. The costs of treating febrile neutropenia in patients with malignant blood disorders. Pharmacoeconomics 1994;6:233-9. http://dx.doi.org/10.2165/00019053-199406030-00007.
- Smith DH, Adams JR, Johnston SR, Gordon A, Drummond MF, Bennett CL. A comparative economic analysis of pegylated liposomal doxorubicin versus topotecan in ovarian cancer in the USA and the UK. Ann Oncol 2002;13:1590-7. http://dx.doi.org/10.1093/annonc/mdf275.
- The National Casemix Office . HRG4+ 2013–14 Reference Costs Grouper 2014. www.hscic.gov.uk/casemix/costing (accessed 21 May 2014).
- British National Formulary 2014. www.bnf.org/bnf/index.htm (accessed 14 January 2014).
- National Institute for Health and Care Excellence . Cetuximab for the Treatment of Locally Advanced Squamous Cell Cancer of the Head and Neck 2008. www.nice.org.uk/guidance/ta145 (accessed 20 May 2014).
- Hitt R, Lopez-Pousa A, Martinez-Trufero J, Escrig V, Carles J, Rizo A, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 2005;23:836-45. http://dx.doi.org/10.1200/JCO.2004.00.1990.
- Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, Tortochaux J, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst 2009;101:498-506. http://dx.doi.org/10.1093/jnci/djp007.
- Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. New Engl J Med 2007;357:1705-15. http://dx.doi.org/10.1056/NEJMoa070956.
- Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. New Engl J Med 2007;357:1695-704. http://dx.doi.org/10.1056/NEJMoa071028.
- National Institute for Health and Care Excellence . Cetuximab for the Treatment of Recurrent and Or Metastatic Squamous Cell Cancer of the Head and Neck 2009. www.nice.org.uk/guidance/ta172 (accessed 20 May 2014).
- Kind P. Measuring the value of quality of life in cancer: an index based on EORTC QLQC-30. J Clin Oncol 2005;23.
- Saam J, Critchfield GC, Hamilton SA, Roa BB, Wenstrup RJ, Kaldate RR. Body surface area-based dosing of 5-fluoruracil results in extensive interindividual variability in 5-fluorouracil exposure in colorectal cancer patients on FOLFOX regimens. Clin Colorectal Cancer 2011;10:203-6. http://dx.doi.org/10.1016/j.clcc.2011.03.015.
- Kline CLB, El-Deiry WS. Personalizing colon cancer therapeutics: targeting old and new mechanisms of action. Pharmaceuticals 2013;6:988-1038. http://dx.doi.org/10.3390/ph6080988.
- Trueman P, Lowson K, Bending M, Ganderton M, Chaplin S, Wright D, et al. Bowel Cancer Services: Costs and Benefits: Final Report to the Department of Health 2007. www.sheffield.ac.uk/polopoly_fs/1.44050!/file/FinalBowelCancerReport---Apr07.pdf (accessed 29 May 2014).
- Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol 1999;94:1650-7. http://dx.doi.org/10.1111/j.1572-0241.1999.01157.x.
- Ko CY, Maggard M, Livingston EH. Evaluating health utility in patients with melanoma, breast cancer, colon cancer, and lung cancer: a nationwide, population-based assessment. J Surg Res 2003;114:1-5. http://dx.doi.org/10.1016/S0022-4804(03)00167-7.
- Beard SM, Holmes M, Price C, Majeed AW. Hepatic resection for colorectal liver metastases: a cost-effectiveness analysis. Ann Surg 2000;232:763-76. http://dx.doi.org/10.1097/00000658-200012000-00005.
- Cassidy J, Douillard JY, Twelves C, McKendrick JJ, Scheithauer W, Bustova I, et al. Pharmacoeconomic analysis of adjuvant oral capecitabine vs intravenous 5-FU/LV in Dukes’ C colon cancer: the X-ACT trial. Br J Cancer 2006;94:1122-9. http://dx.doi.org/10.1038/sj.bjc.6603059.
- Cunningham D, Falk S, Jackson D. Clinical and economic benefits of irinotecan in combination with 5-fluorouracil and folinic acid as first line treatment of metastatic colorectal cancer. Br J Cancer 2002;86:1677-83. http://dx.doi.org/10.1038/sj.bjc.6600204.
- Hoyle M, Peters J, Crathorne L, Jones-Hughes T, Cooper C, Napier M, et al. Cost-effectiveness of cetuximab, cetuximab plus irinotecan, and panitumumab for third and further lines of treatment for KRAS wild-type patients with metastatic colorectal cancer. Value Health 2013;16:288-96. http://dx.doi.org/10.1016/j.jval.2012.11.001.
- Starling N, Tilden D, White J, Cunningham D. Cost-effectiveness analysis of cetuximab/irinotecan vs active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Br J Cancer 2007;96:206-12. http://dx.doi.org/10.1038/sj.bjc.6603561.
- Hopper C, Niziol C, Sidhu M. The cost-effectiveness of Foscan mediated photodynamic therapy (Foscan-PDT) compared with extensive palliative surgery and palliative chemotherapy for patients with advanced head and neck cancer in the UK. Oral Oncol 2004;40:372-82. http://dx.doi.org/10.1016/j.oraloncology.2003.09.003.
- Baghust A, Barraza-Llorens M, Philips Z. Deriving a compound quality of life measure from the EORTC-QLQ-C30/LC13 instrument for use in economic evaluations of lung cancer clinical trials. Eur J Cancer 2001;37:1081-8. http://dx.doi.org/10.1016/S0959-8049(01)00078-8.
- Varney SJ, Guest JF. The annual cost of blood transfusions in the UK. Transfus Med 2003;13:205-18. http://dx.doi.org/10.1046/j.1365-3148.2003.00443.x.
- Gerson LB, Ullah N, Hastie T, Goldstein MK. Does cancer risk affect health-related quality of life in patients with Barrett’s esophagus?. Gastrointest Endosc 2007;65:16-25. http://dx.doi.org/10.1016/j.gie.2006.05.018.
- Llewellyn-Thomas HA, Sutherland HJ, Thiel EC. Do patients’ evaluations of a future health state change when they actually enter that state?. Med Care 1993;31:1002-12. http://dx.doi.org/10.1097/00005650-199311000-00003.
- McNamee P, Glendinning S, Shenfine J, Steen N, Griffin SM, Bond J. Chained time trade-off and standard gamble methods. Applications in oesophageal cancer. Eur J Health Econ 2004;5:81-6. http://dx.doi.org/10.1007/s10198-003-0217-y.
- Ringash J, Redelmeier DA, O’Sullivan B, Bezjak A. Quality of life and utility in irradiated laryngeal cancer patients. Int J Radiat Oncol Biol Phys 2000;47:875-81. http://dx.doi.org/10.1016/S0360-3016(00)00560-5.
- Rogers SN, Miller RD, Ali K, Minhas AB, Williams HF, Lowe D. Patients perceived health status following primary surgery for oral and oropharyngeal cancer. Int J Oral Maxillofac Surg 2006;35:913-19. http://dx.doi.org/10.1016/j.ijom.2006.07.017.
- Shenfine J, McNamee P, Steen N, Bond J, Griffin SM. A randomized controlled clinical trial of palliative therapies for patients with inoperable esophageal cancer. Am J Gastroenterol 2009;104:1674-85. http://dx.doi.org/10.1038/ajg.2009.155.
- Stalmeier PF, de Boer AG, Sprangers MA, de Haes HC, van Lanschot JJ. Validity of utilities of patients with esophageal cancer. Int J Technol Assess Health Care 2005;21:113-18. http://dx.doi.org/10.1017/S0266462305050142.
- Wildi SM, Cox MH, Clark LL, Turner R, Hawes RH, Hoffman BJ, et al. Assessment of health state utilities and quality of life in patients with malignant esophageal Dysphagia. Am J Gastroenterol 2004;99:1044-9. http://dx.doi.org/10.1111/j.1572-0241.2004.30166.x.
- Coyle D, Drummond MF. Costs of conventional radical radiotherapy versus continuous hyperfractionated accelerated radiotherapy (CHART) in the treatment of patients with head and neck cancer or carcinoma of the bronchus. Medical Research Council CHART Steering Committee. Clin Oncol (R Coll Radiol) 1997;9:313-21. http://dx.doi.org/10.1016/S0936-6555(05)80065-2.
- Farndon MA, Wayman J, Clague MB, Griffin SM. Cost-effectiveness in the management of patients with oesophageal cancer. Br J Surg 1998;85:1394-8. http://dx.doi.org/10.1046/j.1365-2168.1998.00916.x.
- Parthan A, Posner MR, Brammer C, Beltran P, Jansen JP. Cost utility of docetaxel as induction chemotherapy followed by chemoradiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck 2009;31:1255-62. http://dx.doi.org/10.1002/hed.21096.
- Buxton MJ, O’Brien BJ. Economic evaluation of ondansetron: preliminary analysis using clinical trial data prior to price setting. Br J Cancer Suppl 1992;19:S64-67.
- Flynn TN, Kelsey SM, Hazel DL, Guest JF. Cost effectiveness of amphotericin B plus G-CSF compared with amphotericin B monotherapy. Treatment of presumed deep-seated fungal infection in neutropenic patients in the UK. Pharmacoeconomics 1999;16:543-50. http://dx.doi.org/10.2165/00019053-199916050-00010.
- Schelenz S, Giles D, Abdallah S. Epidemiology, management and economic impact of febrile neutropenia in oncology patients receiving routine care at a regional UK cancer centre. Ann Oncol 2012;23:1889-93. http://dx.doi.org/10.1093/annonc/mdr520.
- Whyte S, Cooper KL, Stevenson MD, Madan J, Akehurst R. Cost-effectiveness of granulocyte colony-stimulating factor prophylaxis for febrile neutropenia in breast cancer in the United Kingdom. Value Health 2011;14:465-74. http://dx.doi.org/10.1016/j.jval.2010.10.037.
- Wolowacz SE, Cameron DA, Tate HC, Bagust A. Docetaxel in combination with doxorubicin and cyclophosphamide as adjuvant treatment for early node-positive breast cancer: a cost-effectiveness and cost–utility analysis. J Clin Oncol 2008;26:925-33. http://dx.doi.org/10.1200/JCO.2006.10.4190.
- Bennett L, Zhao Z, Barber B, Zhou X, Peeters M, Zhang J, et al. Health-related quality of life in patients with metastatic colorectal cancer treated with panitumumab in first- or second-line treatment. Br J Cancer 2011;105:1495-502. http://dx.doi.org/10.1038/bjc.2011.409.
- Beusterien KM, Szabo SM, Kotapati S, Mukherjee J, Hoos A, Hersey P, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer 2009;101:387-9. http://dx.doi.org/10.1038/sj.bjc.6605187.
- Beusterien KM, Davies J, Leach M, Meiklejohn D, Grinspan JL, O’Toole A, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes 2010;8. http://dx.doi.org/10.1186/1477-7525-8-50.
- Havrilesky LJ, Broadwater G, Davis DM, Nolte KC, Barnett JC, Myers ER, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol 2009;113:216-20. http://dx.doi.org/10.1016/j.ygyno.2008.12.026.
- Ossa DF, Briggs A, McIntosh E, Cowell W, Littlewood T, Sculpher M. Recombinant erythropoietin for chemotherapy-related anaemia: economic value and health-related quality-of-life assessment using direct utility elicitation and discrete choice experiment methods. Pharmacoeconomics 2007;25:223-37. http://dx.doi.org/10.2165/00019053-200725030-00005.
- Szabo SM, Dobson RL, Donato BMK, L’Italien G, Hotte SJ, Levy AR. The quality-of-life impact of head and neck cancer: preference values from the Canadian general public. Health Outcomes Res Med 2012;3:e11-23. http://dx.doi.org/10.1016/j.ehrm.2011.12.001.
- Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes 2008;6. http://dx.doi.org/10.1186/1477-7525-6-84.
- Tam VC, Ko YJ, Mittmann N, Cheung MC, Kumar K, Hassan S, et al. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Current Oncol 2013;20:e90-106. http://dx.doi.org/10.3747/co.20.1223.
- Franic DM, Pathak DS, Gafni A. Are health states ‘timeless’? A case study of an acute condition: post-chemotherapy nausea and vomiting. J Eval Clin Pract 2003;9:69-82. http://dx.doi.org/10.1046/j.1365-2753.2003.00381.x.
- Grunberg SM, Srivastava A, Grunberg KJ, Weeks J. Intensity of chemotherapy-induced emesis and overall survival as determinants of a global utility score. Support Care Cancer 2002;10:624-9. http://dx.doi.org/10.1007/s00520-002-0381-0.
- Hess LM, Malone DC, Reed PG, Skrepnek G, Weihs K. Preferences of patients and oncologists for advanced ovarian cancer treatment related health states. Health Outcomes Res Med 2010;1:51-9. http://dx.doi.org/10.1016/j.ehrm.2010.02.001.
- Hutton J, Brown R, Borowitz M, Abrams K, Rothman M, Shakespeare A. A new decision model for cost-utility comparisons of chemotherapy in recurrent metastatic breast cancer. Pharmacoeconomics 1996;9:8-22. http://dx.doi.org/10.2165/00019053-199600092-00004.
- Leung PP, Tannock IF, Oza AM, Puodziunas A, Dranitsaris G. Cost-utility analysis of chemotherapy using paclitaxel, docetaxel, or vinorelbine for patients with anthracycline-resistant breast cancer. J Clin Oncol 1999;17:3082-90.
- Tosh JC, Longworth LJ, George E. Utility values in National Institute for Health and Clinical Excellence (NICE) Technology Appraisals. Value Health 2011;14:102-9. http://dx.doi.org/10.1016/j.jval.2010.10.015.
- Brown RE, Hutton J, Burrell A. Cost effectiveness of treatment options in advanced breast cancer in the UK. Pharmacoeconomics 2001;19:1091-102. http://dx.doi.org/10.2165/00019053-200119110-00003.
- Grunberg SM, Weeks J, Fischer Magnan W, Herndon J, Naughton ML, Blackwell KL, et al. Determination of utility scores for control of chemotherapy-induced nausea or vomiting – CALGB 309801. J Support Oncol 2009;7:W17-22.
- Jewell EL, Smrtka M, Broadwater G, Valea F, Davis DM, Nolte KC, et al. Preference-based utility scores for adverse events associated with the treatment of gynecologic cancers. Int J Gynecol Cancer 2013;23:1158-66. http://dx.doi.org/10.1097/IGC.0b013e318299e2a6.
- Kuchuk I, Bouganim N, Beusterien K, Grinspan J, Vandermeer L, Gertler S, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Research and Treatment 2013;142:101-7. http://dx.doi.org/10.1007/s10549-013-2727-3.
- Lloyd A, van Hanswijck de Jonge P, Doyle S, Cornes P. Health state utility scores for cancer-related anemia through societal and patient valuations. Value Health 2008;11:1178-85. http://dx.doi.org/10.1111/j.1524-4733.2008.00394.x.
- Nguyen CT, Fu AZ, Gilligan TD, Wells BJ, Klein EA, Kattan MW, et al. Defining the optimal treatment for clinical stage I nonseminomatous germ cell testicular cancer using decision analysis. J Clin Oncol 2010;28:119-25. http://dx.doi.org/10.1200/JCO.2009.22.0400.
- Shih V, Chan A, Xie F, Ko Y. Health state utility assessment for breast cancer. Value Health Regional Issues 2012;1:93-7. http://dx.doi.org/10.1016/j.vhri.2012.03.009.
Appendix 1 Search strategies
Clinical effectiveness: objectives A–C
EMBASE Classic and EMBASE
1947 to week 1 2014 (Ovid).
Searched on 7 January 2014.
Search strategy
| 1 | (my5-fu* or My5-FU* or “my5 fu*” or “my 5fu*” or “my 5 fu*”).mp. | 6 |
| 2 | ondose.mp. | 6 |
| 3 | saladax.mp. | 10 |
| 4 | 1 or 2 or 3 | 18 |
| 5 | “myriad genetic*”.mp. | 125 |
| 6 | exp immunoassay/ | 389,934 |
| 7 | (immunoassay* or (immun* adj2 assay*)).mp. | 379,025 |
| 8 | 6 or 7 | 474,599 |
| 9 | high performance liquid chromatography/ | 197,019 |
| 10 | “high performance liquid chromatography”.tw. | 80,851 |
| 11 | HPLC.tw. | 130,008 |
| 12 | “high pressure liquid chromatography”.tw. | 11,005 |
| 13 | high speed liquid chromatography.tw. | 264 |
| 14 | 9 or 10 or 11 or 12 or 13 | 256,728 |
| 15 | liquid chromatography/ and mass spectrometry/ | 22,033 |
| 16 | Liquid chromatography-mass spectrometry.tw. | 9168 |
| 17 | LC?MS*.tw. | 866 |
| 18 | HPLC?MS.tw. | 42 |
| 19 | 15 or 16 or 17 or 18 | 27,415 |
| 20 | ((pharmacokinetic* or PK) adj2 (dosage* or dose* or dosing or adjust* or adapt* or monitor* or select* or calculat* or guided)).mp. | 29,883 |
| 21 | fluorouracil/pk | 2869 |
| 22 | fluorouracil/ | 105,631 |
| 23 | (fluorouracil* or 5-fluorouracil* or 5fluorouracil*).tw. | 37,057 |
| 24 | (5-fu* or 5fu* or fu).tw. | 31,818 |
| 25 | 22 or 23 or 24 | 118,732 |
| 26 | exp drug dose/ | 417,095 |
| 27 | drug monitoring/ or drug clearance/ | 79,769 |
| 28 | ((dose* or dosing or dosage* or fluorouracil* or 5-fluorouracil* or 5fluorouracil* or 5-fu* or 5fu*) adj2 (adjust* or adapt* or monitor* or select* or calculat* or intensi* or escalat* or modif* or reduc* or concentration* or level* or limit* or detect* or measur*)).tw. | 160,112 |
| 29 | ((drug* or blood or plasma) adj5 (monitor* or concentration* or level*) adj5 (fluorouracil* or 5-fluorouracil* or 5fluorouracil* or 5-fu* or 5fu* or fu)).tw. | 652 |
| 30 | (“optimal drug therapy” or (“optimal drug” adj (dosage* or dose* or dosing))).tw. | 338 |
| 31 | 26 or 27 or 28 or 29 or 30 | 604,858 |
| 32 | personalized medicine/ and exp chemotherapy/ | 865 |
| 33 | ((personal* or individual*) adj2 (chemotherap* or dosage* or dose* or dosing)).mp. | 10,000 |
| 34 | 32 or 33 | 10,652 |
| 35 | 31 or 34 | 611,253 |
| 36 | 5 and 25 | 5 |
| 37 | 5 and 35 | 5 |
| 38 | 36 or 37 | 8 |
| 39 | 8 and 25 and 35 | 251 |
| 40 | ((5-fu* or 5fu* or fu) adj “plasma assay*”).mp. | 2 |
| 41 | 21 and 35 | 1315 |
| 42 | 4 or 38 or 39 or 40 or 41 | 1565 |
| 43 | 14 and 25 | 1263 |
| 44 | 19 and 25 | 95 |
| 45 | 43 or 44 | 1331 |
| 46 | 35 and 45 | 496 |
| 47 | 20 and 25 | 319 |
| 48 | 42 or 46 or 47 | 2102 |
MEDLINE(R)
1946 to November week 3 2013 (Ovid).
Searched on 7 January 2014.
Search strategy
| 1 | (my5-fu* or My5-FU* or “my5 fu*” or “my 5fu*” or “my 5 fu*”).mp. | 0 |
| 2 | ondose.mp. | 2 |
| 3 | saladax.mp. | 1 |
| 4 | 1 or 2 or 3 | 3 |
| 5 | “myriad genetic*”.mp. | 92 |
| 6 | exp Immunoassay/ | 453,924 |
| 7 | (immunoassay* or (immun* adj2 assay*)).mp. | 248,384 |
| 8 | 6 or 7 | 527,480 |
| 9 | Chromatography, High Pressure Liquid/ | 155,449 |
| 10 | “high performance liquid chromatography”.tw. | 65,042 |
| 11 | HPLC.tw. | 91,531 |
| 12 | “high pressure liquid chromatography”.tw. | 9702 |
| 13 | high speed liquid chromatography.tw. | 156 |
| 14 | 9 or 10 or 11 or 12 or 13 | 209,442 |
| 15 | exp Chromatography, Liquid/ and exp Mass Spectrometry/ | 65,715 |
| 16 | Liquid chromatography-mass spectrometry.tw. | 7763 |
| 17 | LC?MS*.tw. | 365 |
| 18 | HPLC?MS.tw. | 5 |
| 19 | 15 or 16 or 17 or 18 | 68,737 |
| 20 | ((pharmacokinetic* or PK) adj2 (dosage* or dose* or dosing or adjust* or adapt* or monitor* or select* or calculat* or guided)).mp. | 6441 |
| 21 | exp Fluorouracil/pk | 1533 |
| 22 | exp Fluorouracil/ | 42,066 |
| 23 | (fluorouracil* or 5-fluorouracil* or 5fluorouracil*).tw. | 29,605 |
| 24 | (5-fu* or 5fu* or fu).tw. | 21,573 |
| 25 | 22 or 23 or 24 | 55,027 |
| 26 | Dose-response Relationship, Drug/ or Drug Dosage Calculations/ | 356,879 |
| 27 | Drug Monitoring/ or Metabolic Clearance Rate/ | 36,771 |
| 28 | ((dose* or dosing or dosage* or fluorouracil* or 5-fluorouracil* or 5fluorouracil* or 5-fu* or 5fu*) adj2 (adjust* or adapt* or monitor* or select* or calculat* or intensi* or escalat* or modif* or reduc* or concentration* or level* or limit* or detect* or measur*)).tw. | 111,057 |
| 29 | ((drug* or blood or plasma) adj5 (monitor* or concentration* or level*) adj5 (fluorouracil* or 5-fluorouracil* or 5fluorouracil* or 5-fu* or 5fu* or fu)).tw. | 549 |
| 30 | (“optimal drug therapy” or (“optimal drug” adj (dosage* or dose* or dosing))).tw. | 231 |
| 31 | 26 or 27 or 28 or 29 or 30 | 476,498 |
| 32 | Individualized Medicine/ | 4498 |
| 33 | ((personal* or individual*) adj2 (chemotherap* or dosage* or dose* or dosing)).mp. | 5704 |
| 34 | 32 or 33 | 10,111 |
| 35 | 31 or 34 | 484,057 |
| 36 | 5 and 25 | 3 |
| 37 | 5 and 35 | 4 |
| 38 | 36 or 37 | 5 |
| 39 | 8 and 25 and 35 | 172 |
| 40 | ((5-fu* or 5fu* or fu) adj “plasma assay*”).mp. | 1 |
| 41 | 21 and 35 | 773 |
| 42 | 4 or 38 or 39 or 40 or 41 | 942 |
| 43 | 14 and 25 | 857 |
| 44 | 19 and 25 | 138 |
| 45 | 43 or 44 | 904 |
| 46 | 35 and 45 | 319 |
| 47 | 20 and 25 | 92 |
| 48 | 42 or 46 or 47 | 1171 |
Medline In-Process & Other Non-Indexed Citations
7 January 2014 to week 3 November 2013 (Ovid).
Searched on 7 January 2014.
Search strategy
| 1 | (my5-fu* or My5-FU* or “my5 fu*” or “my 5fu*” or “my 5 fu*”).mp. | 1 |
| 2 | ondose.mp. | 0 |
| 3 | saladax.mp. | 1 |
| 4 | 1 or 2 or 3 | 1 |
| 5 | “myriad genetic*”.mp. | 11 |
| 6 | (immunoassay* or (immun* adj2 assay*)).mp. | 9114 |
| 7 | “high performance liquid chromatography”.tw. | 9130 |
| 8 | HPLC.tw. | 7931 |
| 9 | “high pressure liquid chromatography”.tw. | 470 |
| 10 | high speed liquid chromatography.tw. | 25 |
| 11 | 7 or 8 or 9 or 10 | 14,539 |
| 12 | Liquid chromatography-mass spectrometry.tw. | 872 |
| 13 | LC?MS*.tw. | 61 |
| 14 | HPLC?MS.tw. | 0 |
| 15 | 12 or 13 or 14 | 924 |
| 16 | ((pharmacokinetic* or PK) adj2 (dosage* or dose* or dosing or adjust* or adapt* or monitor* or select* or calculat* or guided)).mp. | 365 |
| 17 | (fluorouracil* or 5-fluorouracil* or 5fluorouracil*).tw. | 1413 |
| 18 | (5-fu* or 5fu* or fu).tw. | 1243 |
| 19 | 17 or 18 | 2089 |
| 20 | ((dose* or dosing or dosage* or fluorouracil* or 5-fluorouracil* or 5fluorouracil* or 5-fu* or 5fu*) adj2 (adjust* or adapt* or monitor* or select* or calculat* or intensi* or escalat* or modif* or reduc* or concentration* or level* or limit* or detect* or measur*)).tw. | 6026 |
| 21 | ((drug* or blood or plasma) adj5 (monitor* or concentration* or level*) adj5 (fluorouracil* or 5-fluorouracil* or 5fluorouracil* or 5-fu* or 5fu* or fu)).tw. | 19 |
| 22 | (“optimal drug therapy” or (“optimal drug” adj (dosage* or dose* or dosing))).tw. | 19 |
| 23 | 20 or 21 or 22 | 6051 |
| 24 | ((personal* or individual*) adj2 (chemotherap* or dosage* or dose* or dosing)).mp. | 306 |
| 25 | 23 or 24 | 6275 |
| 26 | 5 and 19 | 1 |
| 27 | 5 and 25 | 0 |
| 28 | 26 or 27 | 1 |
| 29 | 6 and 19 and 25 | 2 |
| 30 | ((5-fu* or 5fu* or fu) adj “plasma assay*”).mp. | 0 |
| 31 | 4 or 28 or 29 or 30 | 3 |
| 32 | 11 and 19 | 47 |
| 33 | 15 and 19 | 6 |
| 34 | 32 or 33 | 52 |
| 35 | 25 and 34 | 9 |
| 36 | 16 and 19 | 2 |
| 37 | 31 or 35 or 36 | 12 |
The Cochrane Library (Wiley)
Searched on 17 January 2014.
Search strategy
| #1 | (“my5-fu” or My5-FU* or “my5 fu” or “my 5fu” or “my 5 fu”):ti,ab,kw | 0 |
| #2 | ondose:ti,ab,kw | 0 |
| #3 | saladax:ti,ab,kw | 0 |
| #4 | #1 or #2 or #3 | 0 |
| #5 | (myriad next genetic*):ti,ab,kw | 2 |
| #6 | [mh immunoassay] | 4017 |
| #7 | (immunoassay* or (immun* near/2 assay*)):ti,ab,kw | 4787 |
| #8 | #6 or #7 | 6316 |
| #9 | [mh ^“Chromatography, High Pressure Liquid”] | 2376 |
| #10 | (“high performance liquid chromatography”):ti,ab,kw | 2356 |
| #11 | HPLC:ti,ab,kw | 2450 |
| #12 | (“high pressure liquid chromatography”):ti,ab,kw | 388 |
| #13 | (“high speed liquid chromatography”):ti,ab,kw | 1 |
| #14 | #9 or #10 or #11 or #12 or #13 | 5409 |
| #15 | [mh “Chromatography, Liquid”] | 2788 |
| #16 | [mh “Mass Spectrometry”] | 1052 |
| #17 | #15 and #16 | 581 |
| #18 | (“liquid chromatography-mass spectrometry”):ti,ab,kw | 157 |
| #19 | (“LC-MS” or LCMS* or “LC MS”):ti,ab,kw | 443 |
| #20 | (“HPLC-MS” or HPLCMS* or “HPLC MS”):ti,ab,kw | 105 |
| #21 | #18 or #19 or #20 | 651 |
| #22 | ((pharmacokinetic* or PK) near/2 (dosage* or dose* or dosing or adjust* or adapt* or monitor* or select* or calculat* or guided)):ti,ab,kw | 2463 |
| #23 | [mh Fluorouracil/PK] | 68 |
| #24 | [mh Fluorouracil] | 3825 |
| #25 | (fluorouracil* or 5-fluorouracil* or 5fluorouracil*):ti,ab,kw | 5908 |
| #26 | (“5 fu” or 5fu* or fu):ti,ab,kw | 2903 |
| #27 | #24 or #25 or #26 | 7097 |
| #28 | [mh “Dose-Response Relationship, Drug”] | 24,110 |
| #29 | [mh “Drug Dosage Calculations”] | 66 |
| #30 | [mh ^“Drug Monitoring”] | 1032 |
| #31 | [mh ^“Metabolic Clearance Rate”] | 1544 |
| #32 | ((dose* or dosing or dosage* or fluorouracil* or 5-fluorouracil* or 5fluorouracil* or “5-fu” or 5fu*) near/2 (adjust* or adapt* or monitor* or select* or calculat* or intensi* or escalat* or modif* or reduc* or concentration* or level* or limit* or detect* or measur*)):ti,ab,kw | 14,997 |
| #33 | ((drug* or blood or plasma) near/5 (monitor* or concentration* or level*) near/5 (fluorouracil* or 5-fluorouracil* or 5fluorouracil* or “5-fu” or 5fu* or fu)):ti,ab,kw | 47 |
| #34 | (“optimal drug therapy” or (“optimal drug” next (dosage* or dose* or dosing))):ti,ab,kw | 44 |
| #35 | #28 or #29 or #30 or #31 or #32 or #33 or #34 | 37,921 |
| #36 | [mh ^“Individualized Medicine”] | 78 |
| #37 | ((personal* or individual*) near/2 (chemotherap* or dosage* or dose* or dosing)):ti,ab,kw | 771 |
| #38 | #36 or #37 | 843 |
| #39 | #35 or #38 | 38,431 |
| #40 | #5 and #27 | 1 |
| #41 | #5 and #39 | 0 |
| #42 | #40 or #41 | 1 |
| #43 | #8 and #27 and #39 | 5 |
| #44 | ((“5-fu” or 5fu* or fu) next (plasma next assay*)):ti,ab,kw | 0 |
| #45 | #23 and #39 | 45 |
| #46 | #4 or #42 or #43 or #44 or #45 | 51 |
| #47 | #14 and #27 | 29 |
| #48 | #21 and #27 | 4 |
| #49 | #47 or #48 | 33 |
| #50 | #39 and #49 | 17 |
| #51 | #22 and #27 | 15 |
| #52 | #46 or #50 or #51 | 67 |
All results (67).
-
Cochrane Reviews (0).
-
Trials (65).
-
Methods Studies (0).
-
Technology Assessments (2).
-
Economic Evaluations (0).
-
Cochrane Groups (0).
Science Citation Index and Social Sciences Citation Index via Web of Science
Searched on 9 January 2014.
Search strategy
| #37 | #31 OR #35 OR #36 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
399 |
| #36 | #16 AND #19 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
156 |
| #35 | #25 AND #34 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
228 |
| #34 | #32 OR #33 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
731 |
| #33 | #15 AND #19 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
96 |
| #32 | #11 AND #19 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
680 |
| #31 | #4 OR #28 OR #29 OR #30 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
40 |
| #30 | TS=((5-fu* OR 5fu* OR fu) NEAR/1 (plasma NEAR/1 assay*)) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
2 |
| #29 | #6 AND #19 AND #25 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
34 |
| #28 | #26 OR #27 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
3 |
| #27 | #5 AND #25 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
2 |
| #26 | #5 AND #19 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
2 |
| # 25 | #23 OR #24 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
131,943 |
| #24 | TS=((personal* OR individual*) NEAR/2 (chemotherap* OR dosage* OR dose* OR dosing)) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
6959 |
| #23 | #20 OR #21 OR #22 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
127,286 |
| #22 | TS=(“optimal drug therapy” OR (“optimal drug” NEAR/1 (dosage* OR dose* OR dosing))) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
186 |
| #21 | TS=((drug* OR blood OR plasma) NEAR/5 (monitor* OR concentration* OR level*) NEAR/5 (fluorouracil* OR 5-fluorouracil* OR 5fluorouracil* OR 5-fu* OR 5fu* OR fu)) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
415 |
| #20 | TS=((dose* OR dosing OR dosage* OR fluorouracil* OR 5-fluorouracil* OR 5fluorouracil* OR 5-fu* OR 5fu*) NEAR/2 (adjust* OR adapt* OR monitor* OR select* OR calculat* OR intensi* OR escalat* OR modif* OR reduc* OR concentration* OR level* OR limit* OR detect* OR measur*)) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
127,025 |
| #19 | #17 OR #18 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
46,445 |
| #18 | TS=(5-fu* OR 5fu* OR fu) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
22,299 |
| #17 | TS=(fluorouracil* OR 5-fluorouracil* OR 5fluorouracil*) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
34,612 |
| #16 | TS=((pharmacokinetic* OR PK) NEAR/2 (dosage* OR dose* OR dosing OR adjust* OR adapt* OR monitor* OR select* OR calculat* OR guided)) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
11,242 |
| #15 | #12 OR #13 OR #14 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
38,936 |
| #14 | TS=HPLC$MS* Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
2 |
| #13 | TS=LC$MS* Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
44 |
| #12 | TS=(“liquid chromatography” NEAR/3 “mass spectrometry”) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
38,898 |
| #11 | #7 OR #8 OR #9 OR #10 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
185,457 |
| #10 | TS=“high speed liquid chromatography” Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
300 |
| #9 | TS=“high pressure liquid chromatography” Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
7726 |
| #8 | TS=HPLC Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
135,862 |
| #7 | TS=“high performance liquid chromatography” Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
79,350 |
| #6 | TS=(immunoassay* OR (immun* NEAR/2 assay*)) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
158,922 |
| #5 | TS=(myriad NEAR/1 genetic*) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
106 |
| #4 | #1 OR #2 OR #3 Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
4 |
| #3 | TS=saladax Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
2 |
| #2 | TS=ondose Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
2 |
| #1 | TS=((my5-fu*) OR (My5-FU*) OR “my5 fu” OR (my NEAR/1 5fu*) or “my 5 fu”) Databases=SCI-EXPANDED, CPCI-S Timespan=All years |
1 |
Clinical effectiveness: objective D
Metastatic colorectal cancer
Limited search below to publication year 2011 onwards to pick up records since the searches were run for CG131 (i.e. 25 February 2011). 7
Ovid MEDLINE(R)
1946 to week 2 April 2014.
Searched on 23 April 2014.
Search strategy
| 1 | exp Colorectal Neoplasms/ | 146,177 |
| 2 | ((colorect$ or colon$ or rectum$ or rectal$ or rectosigmoid$ or intestin$ or bowel) adj3 (metasta* or neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*)).tw. | 143,010 |
| 3 | 1 or 2 | 184,392 |
| 4 | Neoplasms/ | 268,877 |
| 5 | Carcinoma/ | 64,081 |
| 6 | Adenocarcinoma/ | 124,609 |
| 7 | 4 or 5 or 6 | 447,733 |
| 8 | Colonic Diseases/ | 13,498 |
| 9 | Rectal Diseases/ | 6964 |
| 10 | exp Colon/ | 53,847 |
| 11 | exp Rectum/ | 29,665 |
| 12 | 8 or 9 or 10 or 11 | 91,843 |
| 13 | 7 and 12 | 4451 |
| 14 | 3 or 13 | 185,328 |
| 15 | exp Neoplasm Metastasis/ | 153,885 |
| 16 | metasta*.mp. | 350,752 |
| 17 | 15 or 16 | 356,332 |
| 18 | 14 and 17 | 39,866 |
| 19 | exp Fluorouracil/ | 37,468 |
| 20 | (fluorouracil* or 5-fluorouracil* or 5fluorouracil*).tw. | 27,174 |
| 21 | (5-fu* or 5fu* or fu).tw. | 19,870 |
| 22 | 19 or 20 or 21 | 49,582 |
| 23 | 18 and 22 | 5623 |
| 24 | (metaanalys* or “meta analys*” or “meta-analys*”).tw. | 53,902 |
| 25 | “systematic* review*”.mp. | 46,337 |
| 26 | meta analysis.pt. | 46,905 |
| 27 | 24 or 25 or 26 | 97,421 |
| 28 | 23 and 27 | 94 |
| 29 | limit 23 to systematic reviews | 109 |
| 30 | 28 or 29 | 131 |
| 31 | limit 30 to yr=“2011 -Current” | 32 |
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations
22 April 2014.
Searched on 23 April 2014.
Search strategy
| 1 | ((colorect$ or colon$ or rectum$ or rectal$ or rectosigmoid$ or intestin$ or bowel) adj3 (metasta* or neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*)).tw. | 9793 |
| 2 | metasta*.mp. | 24,769 |
| 3 | 1 and 2 | 2470 |
| 4 | (fluorouracil* or 5-fluorouracil* or 5fluorouracil*).tw. | 1330 |
| 5 | (5-fu* or 5fu* or fu).tw. | 1231 |
| 6 | 4 or 5 | 2031 |
| 7 | (metaanalys* or “meta analys*” or “meta-analys*”).tw. | 7774 |
| 8 | “systematic* review*”.mp. | 8702 |
| 9 | 7 or 8 | 13,598 |
| 10 | 3 and 6 and 9 | 2 |
| 11 | 3 and 6 | 207 |
| 12 | limit 11 to (meta analysis or systematic reviews) | 5 |
| 13 | 10 or 12 | 5 |
| 14 | limit 13 to yr=“2011 -Current” | 3 |
The Cochrane Library (Wiley)
Searched on 23 April 2014.
Search strategy
| #1 | MeSH descriptor: [Colorectal Neoplasms] explode all trees | 5218 |
| #2 | ((colorect* or colon* or rectum* or rectal* or rectosigmoid* or intestin* or bowel) near/3 (metasta* or neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*)):ti,ab,kw | 8722 |
| #3 | #1 or #2 | 8841 |
| #4 | MeSH descriptor: [Neoplasms] this term only | 4747 |
| #5 | MeSH descriptor: [Carcinoma] this term only | 1002 |
| #6 | MeSH descriptor: [Adenocarcinoma] this term only | 2388 |
| #7 | #4 or #5 or #6 | 8049 |
| #8 | MeSH descriptor: [Colonic Diseases] this term only | 329 |
| #9 | MeSH descriptor: [Rectal Diseases] this term only | 232 |
| #10 | MeSH descriptor: [Colon] explode all trees | 1304 |
| #11 | MeSH descriptor: [Rectum] explode all trees | 1175 |
| #12 | #8 or #9 or #10 or #11 | 2521 |
| #13 | #7 and #12 | 70 |
| #14 | #3 or #13 | 8863 |
| #15 | MeSH descriptor: [Neoplasm Metastasis] explode all trees | 3741 |
| #16 | metasta*:ti,ab,kw | 13,237 |
| #17 | #15 or #16 | 13,319 |
| #18 | #14 and #17 | 2168 |
| #19 | MeSH descriptor: [Fluorouracil] explode all trees | 3873 |
| #20 | (fluorouracil* or 5-fluorouracil* or 5fluorouracil*):ti,ab,kw | 6164 |
| #21 | (5-fu* or 5fu* or fu):ti,ab,kw | 3150 |
| #22 | #19 or #20 or #21 | 7561 |
| #23 | #18 and #22 | 1057 |
| #24 | #23 Publication Date from 2011 to 2014 | 195 |
All results (195).
-
Cochrane Reviews (2).
-
Other Reviews (6).
-
Trials (181).
-
Methods Studies (0).
-
Technology Assessments (1).
-
Economic Evaluations (5).
-
Cochrane Groups (0).
Head and neck cancer
Scottish Intercollegiate Guidelines Network 90 guideline (2006):37 evidence identified for the guideline covers the period to the end of 2003.
National Institute for Health and Care Excellence evidence update (2010) (NICE, 23 April 2014, personal communication): searches cover 1 January 2004 to 30 June 2010.
National Institute for Health and Care Excellence evidence update (2012):116 searches cover 1 July 2010 to 12 December 2011.
Limited search below to publication year 2011 onwards to pick up records since the searches were run for NICE evidence update Improving Outcomes in Head and Neck Cancers: Evidence Update May 2012. 116
Ovid MEDLINE(R)
1946 to week 2 April 2014.
Searched on 23 April 2014.
Search strategy
| 1 | exp “Head and Neck Neoplasms”/ | 237,244 |
| 2 | (((head adj2 neck) or face or facial or oesophageal or esophageal or oesophagus or esophageal or esophagus or thyroid or salivary or paranasal or “aero digestive” or aerodigestive or aero-digestive or UADT or “nasal cavity” or larynx or laryngeal or glottis or glottic or “oral cavity” or ear or oropharynx or oropharyngeal or nasopharynx or nasopharyngeal or hypopharynx or hypopharyngeal or pharynx or pharyngeal or parapharyngeal or mouth) adj3 (metasta* or neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*)).tw. | 128,309 |
| 3 | 1 or 2 | 255,655 |
| 4 | exp Fluorouracil/ | 37,468 |
| 5 | (fluorouracil* or 5-fluorouracil* or 5fluorouracil*).tw. | 27,174 |
| 6 | (5-fu* or 5fu* or fu).tw. | 19,870 |
| 7 | 4 or 5 or 6 | 49,582 |
| 8 | 3 and 7 | 4957 |
| 9 | (metaanalys* or “meta analys*” or “meta-analys*”).tw. | 53,902 |
| 10 | “systematic* review*”.mp. | 46,337 |
| 11 | meta analysis.pt. | 46,905 |
| 12 | 9 or 10 or 11 | 97,421 |
| 13 | 8 and 12 | 62 |
| 14 | limit 8 to systematic reviews | 57 |
| 15 | 13 or 14 | 84 |
| 16 | limit 15 to yr=“2011 -Current” | 14 |
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations
22 April 2014.
Searched on 23 April 2014.
Search strategy
| 1 | (((head adj2 neck) or face or facial or oesophageal or esophageal or oesophagus or esophageal or esophagus or thyroid or salivary or paranasal or “aero digestive” or aerodigestive or aero-digestive or UADT or “nasal cavity” or larynx or laryngeal or glottis or glottic or “oral cavity” or ear or oropharynx or oropharyngeal or nasopharynx or nasopharyngeal or hypopharynx or hypopharyngeal or pharynx or pharyngeal or parapharyngeal or mouth) adj3 (metasta* or neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*)).tw. | 8487 |
| 2 | (fluorouracil* or 5-fluorouracil* or 5fluorouracil*).tw. | 1330 |
| 3 | (5-fu* or 5fu* or fu).tw. | 1231 |
| 4 | 2 or 3 | 2031 |
| 5 | 1 and 4 | 141 |
| 6 | (metaanalys* or “meta analys*” or “meta-analys*”).tw. | 7774 |
| 7 | “systematic* review*”.mp. | 8702 |
| 8 | 6 or 7 | 13,598 |
| 9 | 5 and 8 | 3 |
| 10 | limit 5 to (meta analysis or systematic reviews) | 2 |
| 11 | 9 or 10 | 3 |
| 12 | limit 11 to yr=“2011 -Current” | 3 |
The Cochrane Library (Wiley)
Searched on 23 April 2014.
Search strategy
| #1 | MeSH descriptor: [Head and Neck Neoplasms] explode all trees | 3908 |
| #2 | (((head near/2 neck) or face or facial or oesophageal or esophageal or oesophagus or esophageal or esophagus or thyroid or salivary or paranasal or “aero digestive” or aerodigestive or aero-digestive or UADT or “nasal cavity” or larynx or laryngeal or glottis or glottic or “oral cavity” or ear or oropharynx or oropharyngeal or nasopharynx or nasopharyngeal or hypopharynx or hypopharyngeal or pharynx or pharyngeal or parapharyngeal or mouth) near/3 (metasta* or neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*)):ti,ab,kw | 7750 |
| #3 | #1 or #2 | 7998 |
| #4 | MeSH descriptor: [Fluorouracil] explode all trees | 3873 |
| #5 | (fluorouracil* or 5-fluorouracil* or 5fluorouracil*):ti,ab,kw | 6164 |
| #6 | (5-fu* or 5fu* or fu):ti,ab,kw | 3150 |
| #7 | #4 or #5 or #6 | 7561 |
| #8 | #3 and #7 | 842 |
| #9 | #8 Publication Date from 2011 to 2014 | 158 |
All results (158).
-
Cochrane Reviews (0).
-
Other Reviews (4).
-
Trials (150).
-
Methods Studies (0).
-
Technology Assessments (2).
-
Economic Evaluations (2).
-
Cochrane Groups (0).
Cost-effectiveness: objective E
Cost search 1: cost effectiveness of pharmacokinetic dosing and 5-fluorouracil
Same as search strategies for clinical effectiveness: objectives A–C (see above).
Cost search 2: adverse events of chemotherapy – quality of life
Search 2a
Reran exactly the same search as Shabarrudin et al. ,171 limited to those added after their search was run (June 2011).
N.b. Their search was run in MEDLINE and EMBASE at the same time.
Ovid EMBASE
1980 to week 12 2014.
Ovid MEDLINE(R)
1946 to week 2 March 2014.
Searched on 26 March 2014.
Search strategy
| 1 | utility.af. | 249,345 |
| 2 | util$.af. | 1,267,175 |
| 3 | value$.af. | 3,027,887 |
| 4 | valuation$.af. | 9190 |
| 5 | 1 or 2 or 3 or 4 | 4,157,549 |
| 6 | time trade-off.af. | 1673 |
| 7 | TTO.af. | 1412 |
| 8 | (time adj2 trade adj2 off).af. | 1696 |
| 9 | time-trade-off.af. | 1673 |
| 10 | 6 or 7 or 8 or 9 | 2350 |
| 11 | person trade-off.af. | 95 |
| 12 | PTO.af. | 1150 |
| 13 | (person adj2 trade adj2 off).af. | 95 |
| 14 | person-trade-off.af. | 95 |
| 15 | 11 or 12 or 13 or 14 | 1205 |
| 16 | standard gamble.af. | 1403 |
| 17 | SG.af. | 24,199 |
| 18 | (standard adj2 gamble).af. | 1424 |
| 19 | 16 or 17 or 18 | 25,136 |
| 20 | visual analogue scale$.af. | 34,143 |
| 21 | VAS.af. | 71,777 |
| 22 | (visual adj2 analogue adj2 scale).af. | 30,245 |
| 23 | 20 or 21 or 22 | 90,806 |
| 24 | judgement$.af. | 24,572 |
| 25 | 10 or 15 or 19 or 24 | 52,652 |
| 26 | cancer.af. | 4,043,795 |
| 27 | chemotherapy.af. | 837,929 |
| 28 | cytotoxic.af. | 307,572 |
| 29 | exp cancer/ | 5,688,751 |
| 30 | 26 or 27 or 28 or 29 | 6,831,544 |
| 31 | 5 and 25 and 30 | 1537 |
| 32 | limit 31 to english language | 1350 |
| 33 | limit 32 to human (n.b. 602 from Medline, 589 from Embase) | 1191 |
| 34 | remove duplicates from 33 (n.b. 313 from Medline, 580 from Embase) | 893 |
| 35 | limit 34 to ed=20110601-20140326 [Limit not valid in Embase; records were retained] | 656 |
| 36 | limit 35 to dd=20110601-20140326 [Limit not valid in Ovid MEDLINE(R); records were retained] | 276 |
| 37 | limit 35 to em=201100-201412 [Limit not valid in Ovid MEDLINE(R); records were retained] | 269 |
| 38 | 36 or 37 | 285 |
| 39 | limit 38 to yr=“2011 -Current” (n.b. 124 from Medline, 186 from Embase – includes duplicates) | 261 |
Search 2b
Made improvements to search 2a (above) and only looked at any extras the improvements retrieved.
N.b. No date limit was applied and cancer and chemotherapy terms were combined with the boolean operator AND.
Ovid MEDLINE(R)
1946 to week 2 March 2014.
Searched on 26 March 2014.
Search strategy
| 1 | Health Status/ | 57,926 |
| 2 | exp “Quality of Life”/ | 114,259 |
| 3 | exp Quality-Adjusted Life Years/ | 6774 |
| 4 | (utilit* or disutilit* or (quality adj2 life) or QoL or hrql or hrqol or qaly* or health state* or health status).tw. | 265,920 |
| 5 | (value* or valuation*).tw. | 1,144,946 |
| 6 | 1 or 2 or 3 or 4 or 5 | 1,453,190 |
| 7 | ((standard adj2 gamble) or SG or (time adj2 trade adj2 off) or TTO or (person adj2 trade adj2 off) or PTO or (visual adj2 analog* adj2 scale*) or VAS or judgement*).tw. | 55,312 |
| 8 | chemotherap*.mp. | 305,412 |
| 9 | cytotoxi*.mp. | 215,983 |
| 10 | antineoplastic*.mp. | 350,668 |
| 11 | 8 or 9 or 10 | 655,061 |
| 12 | exp Neoplasms/ | 2,516,811 |
| 13 | (neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*).tw. | 2,052,611 |
| 14 | 12 or 13 | 2,906,356 |
| 15 | 6 and 7 and 11 and 14 | 228 |
| 16 | limit 15 to english language | 194 |
| 17 | Animals/ not Humans/ | 3,812,070 |
| 18 | 16 not 17 | 187 |
| 19 | Limit 18 to ed=20110601-20140326 | 38 |
| 20 | limit 19 to yr=“2011 -Current” | 35 |
| 21 | 18 not 19 | 149 |
EMBASE
1980 to week 12 2014.
Searched on 26 March 2014.
Search strategy
| 1 | exp health economics/ | 601,627 |
| 2 | exp health status/ | 133,750 |
| 3 | exp “quality of life”/ | 258,706 |
| 4 | exp quality adjusted life year/ | 11,658 |
| 5 | (utilit* or disutilit* or (quality adj2 life) or QoL or hrql or hrqol or qaly* or health state* or health status).tw. | 392,413 |
| 6 | (value* or valuation*).tw. | 1,470,360 |
| 7 | 2 or 3 or 4 or 5 or 6 | 1,981,954 |
| 8 | ((standard adj2 gamble) or SG or (time adj2 trade adj2 off) or TTO or (person adj2 trade adj2 off) or PTO or (visual adj2 analog* adj2 scale*) or VAS or judgement*).tw. | 81,071 |
| 9 | exp chemotherapy/ | 364,306 |
| 10 | chemotherap*.tw. | 357,534 |
| 11 | cytotoxi*.mp. | 309,321 |
| 12 | antineoplastic*.mp. | 300,253 |
| 13 | 9 or 10 or 11 or 12 | 949,783 |
| 14 | exp neoplasm/ | 3,171,940 |
| 15 | (neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*).tw. | 2,610,335 |
| 16 | 14 or 15 | 3,638,984 |
| 17 | 7 and 8 and 13 and 16 | 439 |
| 18 | limit 17 to english language | 392 |
| 19 | animal/ not human/ | 1,175,889 |
| 20 | 18 not 19 | 391 |
| 21 | limit 20 to dd=20110601-20140326 | 160 |
| 22 | limit 20 to em=201100-201412 | 157 |
| 23 | 21 or 22 | 171 |
| 24 | limit 23 to yr=“2011 -Current” | 153 |
| 25 | 20 not 23 | 220 |
Search 2c
Supplement the above searches 2a and 2b with one that includes generic instruments and AE terms.
Ovid MEDLINE(R)
1946 to week 4 March 2014.
Searched on 2 April 2014.
Search strategy
| 1 | (EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF6D or health utilities index or HUI).tw. | 15,952 |
| 2 | chemotherap*.mp. | 305,766 |
| 3 | cytotoxi*.mp. | 216,184 |
| 4 | exp “Antineoplastic Agents”/ | 817,215 |
| 5 | antineoplastic*.mp. | 351,082 |
| 6 | 2 or 3 or 4 or 5 | 1,110,394 |
| 7 | exp Neoplasms/ | 2,518,920 |
| 8 | (neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*).tw. | 2,054,720 |
| 9 | 7 or 8 | 2,908,935 |
| 10 | 1 and 6 and 9 | 228 |
| 11 | limit 10 to english language | 219 |
| 12 | Animals/ not Humans/ | 3,814,327 |
| 13 | 11 not 12 | 219 |
| 14 | Diarrhea/ | 38,106 |
| 15 | (diarrhoea or diarrhea).tw. | 66,815 |
| 16 | exp Thrombocytopenia/ | 38,591 |
| 17 | exp Leukopenia/ | 31,360 |
| 18 | (leukopenia* or leukocytopenia* or neutropenia* or thombocytopenia* or thrombopenia*).tw. | 33,614 |
| 19 | Mucositis/ | 782 |
| 20 | mucositis.tw. | 5932 |
| 21 | Stomatitis/ | 5131 |
| 22 | stomatitis.tw. | 11,197 |
| 23 | Hand-Foot Syndrome/ | 96 |
| 24 | Foot Dermatoses/ci [Chemically Induced] | 344 |
| 25 | Hand Dermatoses/ci [Chemically Induced] | 1282 |
| 26 | ((hand foot syndrome* or (acral erythema* or palmoplantar erythrodysesthesia*)) adj1 chemotherapy induced).tw. | 31 |
| 27 | exp Heart Diseases/ci [Chemically Induced] | 31,712 |
| 28 | ((cardiac* or heart*) adj1 (adverse or harm* or side-effect* or toxic* or complication*)).tw. | 10,899 |
| 29 | cardiotoxic*.tw. | 7809 |
| 30 | Nausea/ | 12,757 |
| 31 | Vomiting/ | 19,046 |
| 32 | (nause* or vomit* or emesis).tw. | 62,296 |
| 33 | 14 or 15 or 16 or 17 or 18 or 19 or 20 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 | 280,758 |
| 34 | 13 and 33 | 19 |
| 35 | exp “Drug-Related Side Effects and Adverse Reactions”/ | 87,091 |
| 36 | (risk* or safe or safety or adverse or undesirable effect* or harm* or pharmacovigilance or side-effect* or precaution* or warning* or contraindication* or contra-indication* or tolerability or toxic* or complication*).ti. | 592,138 |
| 37 | ae.fs. | 1,328,971 |
| 38 | 35 or 36 or 37 | 1,779,742 |
| 39 | 13 and 38 | 59 |
| 40 | 34 or 39 | 69 |
EMBASE
1980 to week 13 2014.
Searched on 2 April 2014.
Search strategy
| 1 | (EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF6D or health utilities index or HUI).tw. | 26,744 |
| 2 | “Short Form 36”/ | 11,771 |
| 3 | 1 or 2 | 31,039 |
| 4 | exp chemotherapy/ | 369,730 |
| 5 | chemotherap*.tw. | 362,434 |
| 6 | cytotoxi*.mp. | 312,608 |
| 7 | antineoplastic*.mp. | 302,930 |
| 8 | 4 or 5 or 6 or 7 | 960,409 |
| 9 | exp neoplasm/ | 3,205,221 |
| 10 | (neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*).tw. | 2,641,077 |
| 11 | 9 or 10 | 3,676,076 |
| 12 | 3 and 8 and 11 | 601 |
| 13 | limit 12 to english language | 575 |
| 14 | animal/ not human/ | 1,178,623 |
| 15 | 13 not 14 | 575 |
| 16 | diarrhea/ or acute diarrhea/ or chronic diarrhea/ | 156,385 |
| 17 | (diarrhoea or diarrhea).tw. | 89,281 |
| 18 | exp Thrombocytopenia/ | 113,656 |
| 19 | exp Leukopenia/ | 134,764 |
| 20 | (leukopenia* or leukocytopenia* or neutropenia* or thombocytopenia* or thrombopenia*).tw. | 48,087 |
| 21 | mucosa inflammation/ | 22,214 |
| 22 | mucositis.tw. | 9334 |
| 23 | stomatitis/ or oral mucositis/ | 19,653 |
| 24 | stomatitis.tw. | 12,143 |
| 25 | hand foot syndrome/ | 6869 |
| 26 | ((hand foot syndrome* or (acral erythema* or palmoplantar erythrodysesthesia*)) adj1 chemotherapy induced).tw. | 40 |
| 27 | cardiotoxicity/ | 31,377 |
| 28 | ((cardiac* or heart*) adj1 (adverse or harm* or side-effect* or toxic* or complication*)).tw. | 17,851 |
| 29 | cardiotoxic*.tw. | 10,452 |
| 30 | “chemotherapy induced nausea and vomiting”/ | 1065 |
| 31 | chemotherapy induced emesis/ | 5530 |
| 32 | (nause* or vomit* or emesis).tw. | 93,341 |
| 33 | 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 | 499,141 |
| 34 | 15 and 33 | 71 |
Totals for searches since June 2011:
Shabarrudin, et al. 171 search updated since June 2011 plus any extras retrieved by improved search and supplementary search since June 2011.
| MEDLINE | EMBASE | Total | Total after deduplication |
|---|---|---|---|
| Search 2a (line 39, just MEDLINE): 124 (run 26 March 2014) | Search 2a (line 39, just EMBASE): 186 (run 26 March 2014) | 310 | 255 |
| Extras to 2a (line 39) in MEDLINE found by search 2b (line 20): 31 (run 26 March 2014) | Extras to 2a (line 39) in EMBASE found by search 2b (line 24): 123 (run 26 March 2014) | 154 | 122 |
| Extras to 2a (line 39) and 2b (line 19) in MEDLINE found by search 2c (line 40): 20 (run 2 April 2014) | Extras to 2a (line 39) and 2b (line 24) in EMBASE found by search 2c (line 34): 32 (run 2 April 2014) | 52 | 45 |
Totals for searches pre June 2011:
Extras retrieved by improved search and supplementary search pre June 2011.
| MEDLINE | EMBASE | Total | Total after deduplication |
|---|---|---|---|
| (search 2b (line 21) NOT search 2a (line 33)): 84 (run 26 March 2014) | (search 2b (line 25) NOT search 2a (line 33)): 153 (run 26 March 2014) | 237 | 180 not sifted |
| (search 2c) NOT search 2a (line 33)): 44 (run 2 April 2014) | (search 2c) NOT search 2a (line 33)): 36 (run 2 April 2014) | 80 | 66 |
Cost search 3: adverse events of chemotherapy – resource use
Ovid MEDLINE(R)
1946 to week 3 March 2014.
Searched on 2 April 2014.
Search strategy
| 1 | chemotherap*.mp. | 305,766 |
| 2 | cytotoxi*.mp. | 216,184 |
| 3 | exp “Antineoplastic Agents”/ | 817,215 |
| 4 | antineoplastic*.mp. | 351,082 |
| 5 | 1 or 2 or 3 or 4 | 1,110,394 |
| 6 | exp Neoplasms/ | 2,518,920 |
| 7 | (neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*).tw. | 2,054,720 |
| 8 | 6 or 7 | 2,908,935 |
| 9 | exp Economics/ | 485,325 |
| 10 | exp “Costs and Cost Analysis”/ | 178,538 |
| 11 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 423,541 |
| 12 | (“resource use” or resource utili?ation).tw. | 8300 |
| 13 | 9 or 10 or 11 or 12 | 779,447 |
| 14 | 5 and 8 and 13 | 10,151 |
| 15 | limit 14 to english language | 9337 |
| 16 | Animals/ not Humans/ | 3,814,327 |
| 17 | 15 not 16 | 8673 |
| 18 | £.tw. | 1910 |
| 19 | (£ or pound* or UK or “United Kingdom” or GBP or sterling or “National Health Service” or NHS).tw. | 105,480 |
| 20 | 17 and 18 | 49 |
| 21 | 17 and 19 | 384 |
| 22 | Diarrhea/ | 38,106 |
| 23 | (diarrhoea or diarrhea).tw. | 66,815 |
| 24 | exp Thrombocytopenia/ | 38,591 |
| 25 | exp Leukopenia/ | 31,360 |
| 26 | (leukopenia* or leukocytopenia* or neutropenia* or thombocytopenia* or thrombopenia*).tw. | 33,614 |
| 27 | Mucositis/ | 782 |
| 28 | mucositis.tw. | 5932 |
| 29 | Stomatitis/ | 5131 |
| 30 | stomatitis.tw. | 11,197 |
| 31 | Hand-Foot Syndrome/ | 96 |
| 32 | Foot Dermatoses/ci [Chemically Induced] | 344 |
| 33 | Hand Dermatoses/ci [Chemically Induced] | 1282 |
| 34 | ((hand foot syndrome* or (acral erythema* or palmoplantar erythrodysesthesia*)) adj1 chemotherapy induced).tw. | 31 |
| 35 | exp Heart Diseases/ci [Chemically Induced] | 31,712 |
| 36 | ((cardiac* or heart*) adj1 (adverse or harm* or side-effect* or toxic* or complication*)).tw. | 10,899 |
| 37 | cardiotoxic*.tw. | 7809 |
| 38 | Nausea/ | 12,757 |
| 39 | Vomiting/ | 19,046 |
| 40 | (nause* or vomit* or emesis).tw. | 62,296 |
| 41 | 22 or 23 or 24 or 25 or 26 or 27 or 28 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 | 280,758 |
| 42 | 20 and 41 | 2 |
| 43 | 21 and 41 | 38 |
| 44 | 17 and 41 | 1074 |
Cost search 4: metastatic colorectal cancer/head and neck cancer – quality of life
Ovid MEDLINE(R)
1946 to week 4 March 2014.
Searched on 3 April 2014.
Search strategy
| 1 | exp Colorectal Neoplasms/ | 145,851 |
| 2 | ((colorect$ or colon$ or rectum$ or rectal$ or rectosigmoid$ or intestin$ or bowel) adj3 (metasta* or neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*)).tw. | 142,669 |
| 3 | 1 or 2 | 183,977 |
| 4 | Neoplasms/ | 268,242 |
| 5 | Carcinoma/ | 63,997 |
| 6 | Adenocarcinoma/ | 124,430 |
| 7 | 4 or 5 or 6 | 446,841 |
| 8 | Colonic Diseases/ | 13,485 |
| 9 | Rectal Diseases/ | 6962 |
| 10 | exp Colon/ | 53,746 |
| 11 | exp Rectum/ | 29,617 |
| 12 | 8 or 9 or 10 or 11 | 91,698 |
| 13 | 7 and 12 | 4442 |
| 14 | 3 or 13 | 184,912 |
| 15 | exp Neoplasm Metastasis/ | 153,603 |
| 16 | metasta*.mp. | 349,948 |
| 17 | 15 or 16 | 355,521 |
| 18 | 14 and 17 | 39,775 |
| 19 | exp “Head and Neck Neoplasms”/ | 236,849 |
| 20 | (((head adj2 neck) or face or facial or oesophageal or esophageal or oesophagus or esophageal or esophagus or thyroid or salivary or paranasal or “aero digestive” or aerodigestive or aero-digestive or UADT or “nasal cavity” or larynx or laryngeal or glottis or glottic or “oral cavity” or ear or oropharynx or oropharyngeal or nasopharynx or nasopharyngeal or hypopharynx or hypopharyngeal or pharynx or pharyngeal or parapharyngeal or mouth) adj3 (metasta* or neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*)).tw. | 128,028 |
| 21 | 19 or 20 | 255,226 |
| 22 | Health Status/ | 58,289 |
| 23 | exp “Quality of Life”/ | 115,314 |
| 24 | exp Quality-Adjusted Life Years/ | 6850 |
| 25 | (utilit* or disutilit* or (quality adj2 life) or QoL or hrql or hrqol or qaly* or health state* or health status).tw. | 267,987 |
| 26 | 22 or 23 or 24 or 25 | 344,340 |
| 27 | (standard gamble or SG or time trade off or TTO or visual analog* scale* or VAS or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF6D or health utilities index or HUI).tw. | 57,547 |
| 28 | 26 and 27 | 17,183 |
| 29 | 18 and 28 Colorectal Cancer AND metastases terms (may miss some) | 21 |
| 30 | 14 and 28 Colorectal Cancer | 139 |
| 31 | 21 and 28 Head and Neck Cancer | 159 |
| 32 | 30 or 31 Hardly any duplicates so worth keeping lists for each cancer separate to make sifting easier | 296 |
| 33 | limit 30 to english language Colorectal Cancer | 133 |
| 34 | limit 31 to english language Head and Neck Cancer | 144 |
| 35 | limit 29 to english language Colorectal Cancer AND metastases terms (may miss some) | 19 |
Cost search 5: metastatic colorectal cancer/head and neck cancer – resource use
Ovid MEDLINE(R)
1946 to week 4 March 2014.
Searched on 3 April 2014.
Search strategy
| 1 | exp Colorectal Neoplasms/ | 145,851 |
| 2 | ((colorect$ or colon$ or rectum$ or rectal$ or rectosigmoid$ or intestin$ or bowel) adj3 (metasta* or neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*)).tw. | 142,669 |
| 3 | 1 or 2 | 183,977 |
| 4 | Neoplasms/ | 268,242 |
| 5 | Carcinoma/ | 63,997 |
| 6 | Adenocarcinoma/ | 124,430 |
| 7 | 4 or 5 or 6 | 446,841 |
| 8 | Colonic Diseases/ | 13,485 |
| 9 | Rectal Diseases/ | 6962 |
| 10 | exp Colon/ | 53,746 |
| 11 | exp Rectum/ | 29,617 |
| 12 | 8 or 9 or 10 or 11 | 91,698 |
| 13 | 7 and 12 | 4442 |
| 14 | 3 or 13 | 184,912 |
| 15 | exp Neoplasm Metastasis/ | 153,603 |
| 16 | metasta*.mp. | 349,948 |
| 17 | 15 or 16 | 355,521 |
| 18 | 14 and 17 | 39,775 |
| 19 | exp “Head and Neck Neoplasms”/ | 236,849 |
| 20 | (((head adj2 neck) or face or facial or oesophageal or esophageal or oesophagus or esophageal or esophagus or thyroid or salivary or paranasal or “aero digestive” or aerodigestive or aero-digestive or UADT or “nasal cavity” or larynx or laryngeal or glottis or glottic or “oral cavity” or ear or oropharynx or oropharyngeal or nasopharynx or nasopharyngeal or hypopharynx or hypopharyngeal or pharynx or pharyngeal or parapharyngeal or mouth) adj3 (metasta* or neoplasm* or neoplasia or tumor* or tumour* or cancer* or carcinoma* or malignan* or adenocarcinoma*)).tw. | 128,028 |
| 21 | 19 or 20 | 255,226 |
| 22 | exp Economics/ | 487,398 |
| 23 | exp “Costs and Cost Analysis”/ | 179,191 |
| 24 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 425,718 |
| 25 | (“resource use” or resource utili?ation).tw. | 8363 |
| 26 | 22 or 23 or 24 or 25 | 783,201 |
| 27 | 14 and 26 | 5330 |
| 28 | 21 and 26 | 3771 |
| 29 | limit 27 to english language | 4814 |
| 30 | limit 28 to english language | 3271 |
| 31 | Animals/ not Humans/ | 3,826,890 |
| 32 | 29 not 31 | 4748 |
| 33 | 30 not 31 | 3237 |
| 34 | (£ or pound* or UK or “United Kingdom” or GBP or sterling or “National Health Service” or NHS).tw. | 106,315 |
| 35 | 32 and 34 Colorectal Cancer | 205 |
| 36 | 33 and 34 Head and Neck Cancer | 96 |
| 37 | 18 and 26 | 701 |
| 38 | limit 37 to english language | 602 |
| 39 | 38 not 31 | 591 |
| 40 | 34 and 39 Colorectal Cancer AND metastases terms (may miss some) | 42 |
Appendix 2 Sample data extraction form for objective A(1)
Name of first reviewer:
Name of second reviewer:
| Study details | ||
|---|---|---|
| Study ID (Reference Manager) | ||
| First author surname | ||
| Year of publication | ||
| Country | ||
| Declared Interests | ||
| Aim of the study | ||
| Selection and storage of patients/plasma samples | ||
| Description of method of selection | ||
| Description of method and duration of storage | ||
| Number of patients/healthy volunteers | ||
| Number of samples/patient | ||
| Total number of plasma samples | ||
| Age of participants | ||
| Gender of participants | ||
| Cancer patients or healthy volunteers | ||
| Type of cancer | ||
| Further details of cancer | ||
| Tests | Reference standard | Index test |
| Type of test (equipment) | ||
| Further description | ||
| Details of any repeat measurements to check (to check reliability, performance across different analysers or different laboratories) | ||
| Results | ||
| Range of concentrations for reference standard | ||
| Range of concentrations for index test | ||
| For correlation between reference standard and index test | ||
| Regression method | ||
| Linearity test/cusum test? | ||
| R2 (95% CI) | ||
| Slope (95% CI) | ||
| Intercept (95% CI) | ||
| From Bland–Altman plot | ||
| Per cent bias (95% CI) | ||
| Upper limit of agreement | ||
| Lower limit of agreement | ||
| Details of outliers | ||
| Visually is there a pattern between the mean value and the difference? (If no pattern then can statistics from Bland–Altman plot are interpretable) | ||
| Authors’ conclusion | ||
| Reviewer’s conclusion | ||
Appendix 3 Sample data extraction form for objective A(2) and single-arm studies from objectives B and C
Name of first reviewer:
Name of second reviewer:
| Study details | ||
|---|---|---|
| Study ID (Reference Manager) | ||
| First author surname | ||
| Year of publication | ||
| Country | ||
| Study design | ||
| Publication (full/abstract, etc.) | ||
| Study setting | ||
| Number of centres | ||
| Duration of study | ||
| Follow up period | ||
| Cancer type(s) | ||
| Funding | ||
| Aim of the study | ||
| Inclusion/exclusion criteria for patients | ||
| Inclusion criteria: | ||
| Exclusion criteria: | ||
| Participants (characteristics and numbers) | ||
| Item | ||
| Total number of participants | ||
| Sample attrition/drop out | ||
| Age | Mean SD | |
| Median (range) | ||
| Sex | Men | |
| Women | ||
| Cancer stage | I II III IV |
|
| Performance status | 0 1 2 3 4 |
|
| Number of Metastatic sites | 1 2 3 4 |
|
| Previous 5-FU treatment | ||
| Treatment | ||
| Item | ||
| Type of dose regimen used | ||
| Other interventions: yes/no | ||
| Cycle number | ||
| Outcomes reported | ||
| Primary outcome(s) | ||
| Secondary outcomes | ||
| Timing of assessments | ||
| PFS: yes/no | ||
| OS: yes/no | ||
| AE (toxicity): yes/no | ||
| HRQoL: yes/no; which measures used? | ||
| Response | ||
| Length of follow-up reported: yes/no | ||
| Proportion progressing to surgery: yes/no | ||
| Study end point | ||
| OS | ||
| Item | ||
| Median survival 95% CI | ||
| Kaplan–Meier plot: yes/no | ||
| Total events | ||
| Total censored | ||
| At risk table: yes/no | ||
| HR 95% CI | ||
| Log-rank test (p-value) | ||
| PFS | ||
| Item | ||
| Median survival 95% CI | ||
| Kaplan–Meier plot: yes/no | ||
| Total events | ||
| Total censored | ||
| At risk table: yes/no | ||
| HR 95% CI | ||
| Describe criteria for determining progression: | ||
| Incidence of AEs/side effects/toxicity – specify time/period = | ||
| Item (rename as appropriate) | p-value | |
| Diarrhoea grade (specify: WHO or NCI CTCAE, etc.) | ||
| I | ||
| II | ||
| III | ||
| IV | ||
| Nausea grade (specify: WHO or NCI CTCAE, etc.) | ||
| I | ||
| II | ||
| III | ||
| IV | ||
| Mucositis grade (specify: WHO or NCI CTCAE, etc.) | ||
| I | ||
| II | ||
| III | ||
| IV | ||
| Mouth sore grade (specify: WHO or NCI CTCAE, etc.) | ||
| I | ||
| II | ||
| III | ||
| IV | ||
| Hand and foot syndrome grade (specify: WHO or NCI CTCAE, etc.) | ||
| I | ||
| II | ||
| III | ||
| IV | ||
| Leucopenia grade (specify: WHO or NCI CTCAE, etc.) | ||
| I | ||
| II | ||
| III | ||
| IV | ||
| Neutropenia grade (specify: WHO or NCI CTCAE, etc.) | ||
| I | ||
| II | ||
| III | ||
| IV | ||
| Thrombocytopenia grade (specify: WHO or NCI CTCAE, etc.) | ||
| I | ||
| II | ||
| III | ||
| IV | ||
| Cardiac toxicity grade (specify: WHO or NCI CTCAE, etc.) | ||
| I | ||
| II | ||
| III | ||
| IV | ||
| Conjunctivitis grade (specify: WHO or NCI CTCAE, etc.) | ||
| I | ||
| II | ||
| III | ||
| IV | ||
| Other (specify) | ||
| Objective response rate [specify criteria (NR); specify time]: | ||
| Item | n (%) | |
| CR | ||
| PR | ||
| Overall response (CR + PR) | ||
| Disease control (SD) | ||
| Disease progression | ||
| Differential response | ||
| Duration of response (mean/median) | ||
| Objective response rate (CR and PR) of ITT patients (95% CI) | ||
| Disease control rate of ITT patients (95% CI) | ||
| 5-FU plasma concentration | ||
| Item | ||
| Method of 5-FU measurement | ||
| Frequency of 5-FU measures | ||
| Plasma concentration | ||
| Range | ||
| Mean | ||
| AUC | ||
| Range | ||
| Mean | ||
| Dose adjustment | ||
| Item | ||
| Dose adjustment: yes/no | ||
| Specify adjustment rule | ||
| Adjustment algorithm specified: yes/no | ||
| Algorithm provided: yes/no | ||
| Mean 5-FU dose (mg/m3/week) | ||
| Dose adjustment for toxicity rule | ||
| Dose adjustment for toxicity, n/N | ||
| Target AUC range | ||
| Proportion with 5-FU measures in target range | ||
| Incidence of over and under dosing (specify time) | ||
| Number of patients with dose adjustment | ||
| Frequency of dose adjustment (number adjustment/number of measures) | ||
| Test failure rates | ||
| Other | ||
| HRQoL | ||
| Item | ||
| Authors’ conclusion | ||
| Reviewer’s conclusion | ||
Supplementary information/data:
| Information on new algorithm | |
|---|---|
| Item | |
Appendix 4 Sample data extraction form for objectives B and C: primary comparative studies
Name of first reviewer:
Name of second reviewer:
| Study details | |||||
|---|---|---|---|---|---|
| Study ID (Reference Manager) | |||||
| First author surname | |||||
| Year of publication | |||||
| Country | |||||
| Study design | |||||
| Publication (full/abstract | |||||
| Study setting | |||||
| Number of centres (by arm) | |||||
| Duration of study | |||||
| Follow up period | |||||
| Cancer type(s) | |||||
| Funding | |||||
| Aim of the study | |||||
| Inclusion/exclusion criteria for patients | |||||
| Inclusion criteria: | |||||
| Exclusion criteria: | |||||
| Study flow (consort diagram) | |||||
| Item | BSA arm | PK arm | All | ||
| Screened | |||||
| Randomised/included | |||||
| Excluded | |||||
| Missing participants | |||||
| Withdrawals | |||||
| Participants (characteristics and numbers) | |||||
| Item | BSA arm, n (%) | PK arm, n (%) | All | ||
| Total number of participants | |||||
| Sample attrition/drop out | |||||
| Age | Mean SD Median (range) years |
||||
| Sex | Men Women |
||||
| Cancer stage | |||||
| I II III IV |
|||||
| Performance status | |||||
| 0–1 2–3 4 |
|||||
| Number of metastatic sites | |||||
| 1 2 3 |
|||||
| Treatment | |||||
| Item | BSA arm | PK arm | |||
| Type of dose regimen used | |||||
| Other interventions: yes/no | |||||
| How many cycles | |||||
| Outcomes reported | |||||
| Primary outcome(s) | |||||
| Secondary outcomes | |||||
| Timing of assessments | |||||
| PFS: yes/no | |||||
| OS: yes/no | |||||
| AE (toxicity): yes/no | |||||
| HRQoL: yes/no; which measures used? | |||||
| Length of follow-up reported: yes/no | |||||
| Proportion progressing to surgery: yes/no | |||||
| Study end point | |||||
| OS | |||||
| Item | BSA arm | PK arm | All | ||
| Median survival 95% CI, months | |||||
| Kaplan–Meier plot: yes/no | |||||
| Total events | |||||
| Total censored | |||||
| At risk table: yes/no | |||||
| HR 95% CI | |||||
| Log-rank test (p-value) | |||||
| PFS | |||||
| Item | BSA arm | PK arm | p-value | ||
| Median survival 95% CI | |||||
| Kaplan–Meier plot: yes/no | |||||
| Total events | |||||
| Total censored | |||||
| At risk table: yes/no | |||||
| HR 95% CI | |||||
| Incidence of AEs/side effects/toxicity – specify time/period = | |||||
| Item (rename as appropriate) | BSA arm n/N (%) | PK arm n/N (%) | p-value | ||
| Diarrhoea grade (WHO) | |||||
| I | |||||
| II | |||||
| III-IV | |||||
| Nausea grade (specify: WHO or NCI CTCAE) | |||||
| I | |||||
| II | |||||
| III | |||||
| IV | |||||
| Vomiting grade (specify: WHO or NCI CTCAE) | |||||
| I | |||||
| II | |||||
| III | |||||
| IV | |||||
| Mucositis grade (specify: NCI CTCAE) | |||||
| I | |||||
| II | |||||
| III–IV | |||||
| Mouth sore grade (specify: WHO or NCI CTCAE) | |||||
| I | |||||
| II | |||||
| III | |||||
| IV | |||||
| Hand and foot syndrome grade (specify: WHO or NCI CTCAE) | |||||
| I | |||||
| II | |||||
| III | |||||
| IV | |||||
| Leucopenia grade (specify: WHO or NCI CTCAE) | |||||
| I | |||||
| II | |||||
| III | |||||
| IV | |||||
| Neutropenia grade (specify: NCI CTCAE) | |||||
| I | |||||
| II | |||||
| III–IV | |||||
| Thrombocytopenia grade (specify: NCI CTCAE) | |||||
| I | |||||
| II | |||||
| III-IV | |||||
| IV | |||||
| Cardiac toxicity grade (specify: WHO or NCI CTCAE) | |||||
| I | |||||
| II | |||||
| III | |||||
| IV | |||||
| Conjunctivitis grade (specify: WHO or NCI CTCAE) | |||||
| I | |||||
| II | |||||
| III | |||||
| IV | |||||
| Other (specify) | |||||
| Neuropathy | |||||
| Severe damage to organ | |||||
| Sepsis | |||||
| Septic shock | |||||
| Objective response rate (specify criteria RECIST; specify time, 3 months and 6 months) | |||||
| Item | BSA arm n/N (%) | PK arm n/N (%) | p-value | ||
| CR | |||||
| PR | |||||
| Overall response (CR + PR) | |||||
| Disease control | |||||
| Disease progression | |||||
| Duration of response (mean/median) | |||||
| Dose adjustment | |||||
| Item (please define if necessary) | BSA arm | PK arm | |||
| Specify adjustment rule | |||||
| Method of 5-FU measurement | |||||
| Frequency of 5-FU measures | |||||
| Adjustment algorithm specified: yes/no | |||||
| Algorithm provided: yes/no | |||||
| Mean 5-FU dose (mg/m3/week) (SD) | |||||
| Proportion (%) of patients reached target range | |||||
| Dose adjustment for toxicity, n/N | |||||
| Proportion with 5-FU measures levels in target range | |||||
| Incidence of over and under dosing (please specify) | |||||
| Frequency of dose adjustment (number of adjustment/number of measures) | |||||
| Test failure rates | |||||
| Other | |||||
| HRQoL | |||||
| Item | |||||
| Authors’ conclusion | |||||
| Reviewer’s conclusion | |||||
Appendix 5 Sample quality assessment form for objective A(1): revised quality assessment of diagnostic accuracy studies tool with index questions adapted to the review
Name of first reviewer:
Name of second reviewer:
Phase 1: state the review question
| Patients (setting, intended use of index test, presentation, prior testing): |
| Index test(s): |
| Reference standard: |
Phase 2: draw a flow diagram for the primary study
Phase 3: risk of bias and applicability judgements
The QUADAS-2 is structured so that four key domains are each rated in terms of the risk of bias and the concern regarding applicability to the review question (as stated in phase 1). Each key domain has a set of signalling questions to help reach the judgements regarding bias and applicability.
Domain 1: patient selection
| A. Risk of bias |
|---|
| Describe methods of patient selection: |
| Was a consecutive or random sample of patients enrolled? |
| Did the study avoid inappropriate exclusions? |
| Could the selection of patients have introduced bias? |
| Risk: |
| B. Concerns regarding applicability |
| Describe included patients (prior testing, presentation, intended use of index test and setting): Range of plasma concentrations: |
| Is there concern that the included patients or range of plasma concentrations do not match the review question? |
| Concern: |
Domain 2: index test(s)
| A. Risk of bias |
|---|
| Describe the index test and how it was conducted and interpreted: |
| Were the number of failed results and measurement repeats reported? |
| Could the conduct or interpretation of the index test have introduced bias? |
| Risk: |
| B. Concerns regarding applicability |
| Describe the preparation and storage of the sample before the index test was applied: |
| Is there concern that the index test, its conduct, or interpretation differ from the review question? |
| Concern: |
Domain 3: reference standard
| A. Risk of bias |
|---|
| Describe the reference standard and how it was conducted and interpreted: |
| Is the reference standard likely to correctly classify the target condition? |
| Could the reference standard, its conduct, or its interpretation have introduced bias? |
| Risk: |
| B. Concerns regarding applicability |
| Is there concern that the target condition as defined by the reference standard does not match the review question? |
| Concern: |
Domain 4: flow and timing
| A. Risk of bias |
|---|
| Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the Bland–Altman plot: |
| Describe the time interval and any interventions between index test(s) and reference standard: |
| Was there an appropriate interval between index test(s) and reference standard? |
| Were both index test and reference standard conducted on all samples? |
| Did patients receive the same reference standard? |
| Were all patients included in the Bland–Altman plot? |
| Could the patient flow have introduced bias? |
| Risk: |
Appendix 6 Sample quality assessment forms for objectives B and C
First author (year) study ID:
Name of first reviewer:
Name of second reviewer:
| Reporting | Rating |
|---|---|
| 1. Is the hypothesis/aim/objective of the study clearly described? (Yes/no) | |
| 2. Are the main outcomes to be measured clearly described in the Introduction or Methods section? (Yes/no) If the main outcomes are first mentioned in the Results section, the question should be answered ‘no’ |
|
| 3. Are the characteristics of the patients included in the study clearly described? (Yes/no) In cohort studies and trials, inclusion and/or exclusion criteria should be given. In case–control studies, a case-definition and the source for controls should be given |
|
| 4. Are the interventions of interest clearly described? (Yes/no) Treatments and placebo (where relevant) that are to be compared should be clearly described |
|
| 5. Are the distributions of principal confounders in each group of subjects to be compared clearly described? (Yes/partially/no) A list of principal confounders is provided |
|
| 6. Are the main findings of the study clearly described? (Yes/no) Simple outcome data (including denominators and numerators) should be reported for all major findings so that the reader can check the major analyses and conclusions. (This question does not cover statistical tests which are considered below) |
|
| 7. Does the study provide estimates of the random variability in the data for the main outcomes? (Yes/no) In non-normally distributed data the interquartile range of results should be reported. In normally distributed data the standard error, s.d. or CIs should be reported. If the distribution of the data is not described, it must be assumed that the estimates used were appropriate and the question should be answered ‘yes’ |
|
| 8. Have all important AEs that may be a consequence of the intervention been reported? (Yes/no) This should be answered ‘yes’ if the study demonstrates that there was a comprehensive attempt to measure AEs. (A list of possible AEs is provided) |
|
| 9. Have the characteristics of patients lost to follow-up been described? (Yes/no) This should be answered ‘yes’ where there were no losses to follow-up or where losses to follow-up were so small that findings would be unaffected by their inclusion. This should be answered ‘no’ where a study does not report the number of patients lost to follow-up |
|
| 10. Have actual probability values been reported (e.g. 0.035 rather than < 0.05) for the main outcomes except where the probability value is less than 0.001? (Yes/no) | |
| External validity | Rating |
| 11. Were the subjects asked to participate in the study representative of the entire population from which they were recruited? (Yes/no/unable to determine) The study must identify the source population for patients and describe how the patients were selected. Patients would be representative if they comprised the entire source population, an unselected sample of consecutive patients, or a random sample. Random sampling is only feasible where a list of all members of the relevant |
|
| 12. Were those subjects who were prepared to participate representative of the entire population from which they were recruited? (Yes/no/unable to determine) The proportion of those asked who agreed should be stated. Validation that the sample was representative would include demonstrating that the distribution of the main confounding factors was the same in the study sample and the source population |
|
| 13. Were the staff, places, and facilities where the patients were treated, representative of the treatment the majority of patients receive? (Yes/no/unable to determine) For the question to be answered ‘yes’ the study should demonstrate that the intervention was representative of that in use in the source population. The question should be answered ‘no’ if, for example, the intervention was undertaken in a specialist centre unrepresentative of the hospitals most of the source population would attend |
|
| Internal validity: bias | Rating |
| 14. Was an attempt made to blind study subjects to the intervention they have received? (Yes/no/unable to determine) For studies where the patients would have no way of knowing which intervention they received, this should be answered ‘yes’ |
|
| 15. Was an attempt made to blind those measuring the main outcomes of the intervention? (Yes/no/unable to determine) | |
| 16. If any of the results of the study were based on ‘data dredging’, was this made clear? (Yes/no/unable to determine) Any analyses that had not been planned at the outset of the study should be clearly indicated. If no retrospective unplanned subgroup analyses were reported, then answer ‘yes’ |
|
| 17. In trials and cohort studies, do the analyses adjust for different lengths of follow-up of patients, or in case–control studies, is the time period between the intervention and outcome the same for cases and controls? (Yes/no/unable to determine) Where follow-up was the same for all study patients the answer should ‘yes’. If different lengths of follow-up were adjusted for by, for example, survival analysis the answer should be ‘yes’. Studies where differences in follow-up are ignored should be answered ‘no’ |
|
| 18. Were the statistical tests used to assess the main outcomes appropriate? (Yes/no/unable to determine) The statistical techniques used must be appropriate to the data. For example nonparametric methods should be used for small sample sizes. Where little statistical analysis has been undertaken but where there is no evidence of bias, the question should be answered ‘yes’. If the distribution of the data (normal or not) is not described it must be assumed that the estimates used were appropriate and the question should be answered ‘yes’ |
|
| 19. Was compliance with the intervention/s reliable? (Yes/no/unable to determine) Where there was non-compliance with the allocated treatment or where there was contamination of one group, the question should be answered ‘no’. For studies where the effect of any misclassification was likely to bias any association to the null, the question should be answered ‘yes’ |
|
| 20. Were the main outcome measures used accurate valid and reliable? (Yes/no/unable to determine) For studies where the outcome measures are clearly described, the question should be answered ‘yes’. For studies which refer to other work or that demonstrates the outcome measures are accurate, the question should be answered as ‘yes’ |
|
| Internal validity: confounding (selection bias) | Rating |
| 21. Were the patients in different intervention groups (trials and cohort studies) or were the cases and controls (case–control studies) recruited from the same population? (Yes/no/unable to determine) For example, patients for all comparison groups should be selected from the same hospital. The question should be answered ‘unable to determine’ for cohort and case–control studies where there is no information concerning the source of patients included in the study |
|
| 22. Were study subjects in different intervention groups (trials and cohort studies) or were the cases and controls (case–control studies) recruited over the same period of time? (Yes/no/unable to determine) For a study which does not specify the time period over which patients were recruited, the question should be answered as ‘unable to determine’ |
|
| 23. Were the subjects randomised to intervention groups? (Yes/no/unable to determine) Studies which state that subjects were randomised should be answered ‘yes’ except where method of randomisation would not ensure random allocation. For example alternate allocation would score ‘no’ because it is predictable |
|
| 24. Was the randomised intervention assignment concealed from both patients and health care staff until recruitment was complete and irrevocable? (Yes/no/unable to determine) All non-randomised studies should be answered ‘no’. If assignment was concealed from patients but not from staff, it should be answered ‘no’ |
|
| 25. Was there adequate adjustment for confounding in the analyses from which the main findings were drawn? (Yes/no/unable to determine) This question should be answered ‘no’ for trials if the main conclusions of the study were based on analyses of treatment rather than ITT; the distribution of known confounders in the different treatment groups was not described; or the distribution of known confounders differed between the treatment groups but was not taken into account in the analyses. In non-randomised studies if the effect of the main confounders was not investigated or confounding was demonstrated but no adjustment was made in the final analyses the question should be answered as ‘no’ |
|
| 26. Were losses of patients to follow-up taken into account? (Yes/no/unable to determine) If the numbers of patients lost to follow-up are not reported, the question should be answered as ‘unable to determine’. If the proportion lost to follow-up was too small to affect the main findings, the question should be answered ‘yes’ |
|
| Power | Rating |
| 27. Did the study have sufficient power to detect a clinically important effect where the probability value for a difference being due to chance is less than 5%? (Yes/no/unable to determine) |
Appendix 7 Included studies for clinical effectiveness objectives
| Objective A |
|---|
| 1. Beumer JH, Boisdron-Celle M, Clarke W, Courtney JB, Egorin MJ, Gamelin E, et al. Multicenter evaluation of a novel nanoparticle immunoassay for 5-fluorouracil on the olympus AU400 analyzer. Ther Drug Monit 2009;31:688–94 |
| 2. Buchel B, Sistonen J, Aebi Y, Largiader CR. Comparative evaluation of the My5-FU immunoassay and LC-MS/MS in the monitoring of 5-fluorouracil levels in cancer patients. Clin Chem Lab Med 2012;50:A169–70 |
| 3. Buchel B, Sistonen J, Joerger M, Aebi Y, Schurch S, Largiader CR. Comparative evaluation of the My5-FU immunoassay and LC-MS/MS in monitoring the 5-fluorouracil plasma levels in cancer patients. Clin Chem Lab Med 2013;51:1681–8 |
| 4. Kaldate RR, Haregewoin A, Grier CE, Hamilton SA, McLeod HL. Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6. Oncologist 2012;17:296–302 |
| 5. Makihara K, Mishima H, Azuma S, Matsuyama K, Komori K, Hasegawa H, et al. A pilot study of pharmacokinetically guided dose management of capecitabine in CRC patients. J Clin Oncol 2012;1 |
| Objectives B and C |
| 6. Boisdron-Celle M, Craipeau M, Brienza S, Delva R, Guerin-Meyer V, Cvitkovic E, et al. Influence of oxaliplatin on 5-fluorouracil plasma clearance and clinical consequences. Cancer Chemother Pharmacol 2002;49:235–43 |
| 7. Capitain O, Asevoaia A, Boisdron-Celle M, Poirier AL, Morel A, Gamelin E. Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study. Clin Colorectal Cancer 2012;11:263–7 |
| 8. Capitain O, Boisdron-Celle M, Poirier AL, Abadie-Lacourtoiseie S, Morel A, Gamelin E. The influence of fluorouracil outcome parameters on tolerance and efficacy in patients with advanced colorectal cancer. Pharmacogenomics J 2008;8:256–67 |
| 9. Cattel L, La Grotta G, Infante L, Passera R, Arpicco S, Brusa P, et al. Pharmacokinetic study of oxaliplatin iv chronomodulated infusion combined with 5-fluorouracil iv continuous infusion in the treatment of advanced colorectal cancer. Farmaco 2003;58:1333–8 |
| 10. Ciccolini J, Mercier C, Evrard A, Dahan L, Boyer JC, Duffaud F, et al. A rapid and inexpensive method for anticipating severe toxicity to fluorouracil and fluorouracil-based chemotherapy. Ther Drug Monit 2006;28:678–85 |
| 11. Duffour J, Roca L, Bressolle F, Abderrahim AG, Poujol S, Pinguet F, et al. Clinical impact of intesified 5-Fluorouracil-based chemotherapy using a prospective pharmacokinetically-guided dosing approach: comparative study in elderly and non-elderly patients with metastatic colorectal cancer. J Chemother 2010;22:179–85 |
| 12. Etienne MC, Lagrange JL, Dassonville O, Fleming R, Thyss A, Renee N, et al. Population study of dihydropyrimidine dehydrogenase in cancer patients. J Clin Oncol 1994;12:2248–53 |
| 13. Fety R, Rolland F, Barberi-Heyob M, Hardouin A, Campion L, Conroy T, et al. Clinical impact of pharmacokinetically-guided dose adaptation of 5- fluorouracil: Results from a multicentric randomized trial in patients with locally advanced head and neck carcinomas. Clin Cancer Res 1998;4:2039–45 |
| 14. Fety R, Rolland F, Barberiheyob M, Merlin JL, Conroy T, Hardouin A, et al. Clinical randomized study of 5FU monitoring versus standard-dose in patients with head and neck-cancer – preliminary-results. Anticancer Res 1994;14:2347–52 |
| 15. Findlay MPN, Raynaud F, Cunningham D, Iveson A, Collins DJ, Leach MO. Measurement of plasma 5-fluorouracil by high-performance liquid chromatography with comparison of results to tissue drug levels observed using in vivo 19F magnetic resonance spectroscopy in patients on a protracted venous infusion with or without interferon-alpha. Ann Oncol 1996;7:47–53 |
| 16. Gamelin E, Boisdron-Celle M, Delva R, Regimbeau C, Cailleux PE, Alleaume C, et al. Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J Clin Oncol 1998;16:1470–8 |
| 17. Gamelin E, Delva R, Jacob J, Merrouche Y, Raoul JL, Pezet D, et al. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2099–105 |
| 18. Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, Maillart PJ, Goudier MJ, Burtin PC, et al. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer 1996;77:441–51 |
| 19. Hendrayana T, Kurth V, Krolop L, Kenny P, Hilger RA, Schmidt-Wolf IGH, et al. Variability in fluorouracil exposure during continuous intravenous infusion. Int J Clin Pharmacol Ther 2012;50:82–4 |
| 20. Ho YF, Lu WC, Chen RRL, Cheng AL, Yeh KH. Phase I, pharmacokinetic, and bone marrow drug-level studies of trimonthly 48-h infusion of high-dose 5-fluorouracil and leucovorin in patients with metastatic colorectal cancers. Anti-Cancer Drugs 2011;22:290–8 |
| 21. Jodrell DI, Stewart M, Aird R, Knowles G, Bowman A, Wall L, et al. 5-Fluorouracil steady state pharmacokinetics and outcome in patients receiving protracted venous infusion for advanced colorectal cancer. Br J Cancer 2001;84:600–3 |
| 22. Kim R, Nishimoto N, Inoue H, Yoshida K, Toge T. An analysis of the therapeutic efficacy of protracted infusion of low-dose 5-fluorouracil and cisplatin in advanced gastric cancer. J Infect Chemother 2000;6:222–8 |
| 23. Kline CL, Sheikh HS, Scicchitano A, Gingrich R, Beachler C, Finnberg NK, et al. Preliminary observations indicate variable patterns of plasma 5-fluorouracil (5-FU) levels during dose optimization of infusional 5-FU in colorectal cancer patients. Cancer Biol Ther 2011;12:557–68 |
| 24. Kline CLB, Schiccitano A, Zhu J, Beachler C, Sheikh H, Harvey HA, et al. Personalized dosing via pharmacokinetic monitoring of 5-Fluorouracil (5-FU) may reduce toxicity in early or late stage colorectal cancer patients treated with infusional 5-FU-based chemotherapy regimens [published online ahead of print 20 November 2013]. Clin Colorectal Cancer 2013 |
| 25. Metzger G, Massari C, Etienne MC, Comisso M, Brienza S, Touitou Y, et al. Spontaneous or imposed circadian changes in plasma concentrations of 5-fluorouracil coadministered with folinic acid and oxaliplatin: relationship with mucosal toxicity in patients with cancer. Clin Pharmacol Ther 1994;56:190–201 |
| 26. Milano G, Etienne MC, Renee N, Thyss A, Schneider M, Ramaioli A, et al. Relationship between fluorouracil systemic exposure and tumor response and patient survival. J Clin Oncol 1994;12:1291–5 |
| 27. Milano G, Roman P, Khater R, Frenay M, Renee N, Namer M. Dose versus pharmacokinetics for predicting tolerance to 5-day continuous infusion of 5-FU. Int J Cancer 1988;41:537–41 |
| 28. Patel JN, Deal AM, O’Neil BH, Ibrahim J, Sherrill GB, Davies JM, et al. Application of pharmacokinetic (PK)-guided 5-fluorouracil (FU) in clinical practice. J Clin Oncol 2013;1 |
| 29. Patel JN, O’Neil BH, McLeod HL, Sherrill GB, Olijade O, Inzerillo JJ, et al. Investigating the utilization of pharmacokinetic-guided fluorouracil in colorectal cancer. J Clin Oncol 2012;1 |
| 30. Santini J, Milano G, Thyss A, Renee N, Viens P, Ayela P, et al. 5-FU therapeutic monitoring with dose adjustment leads to an improved therapeutic index in head and neck cancer. Br J Cancer 1989;59:287–90 |
| 31. Stremetzne S, Streit M, Kreuser ED, Schunack W, Jaehde U. Pharmacokinetic and revueodynamics comparison of two doses of calcium folinate combined with continuous fluorouracil infusion in patients with advanced colorectal cancer. Pharm World Sci 1999;21:184–9 |
| 32. Thyss A, Milano G, Renee N. Clinical pharmacokinetic study of 5-FU in continuous 5-day infusions for head and neck cancer. Cancer Chemother Pharmacol 1986;16:64–6 |
| 33. Ychou M, Duffour J, Kramar A, Debrigode C, Gourgou S, Bressolle F, et al. Individual 5-FU dose adaptation in metastatic colorectal cancer: results of a phase II study using a bimonthly pharmacokinetically intensified LV5FU2 regimen. Cancer Chemother Pharmacol 2003;52:282–90 |
| 34. Ychou M, Duffour J, Pinguet F, Kramar A, Joulia JM, Topart D, et al. Individual 5FU-dose adaptation schedule using bimonthly pharmacokinetically modulated LV5FU2 regimen: a feasibility study in patients with advanced colorectal cancer. Anticancer Res 1999;19:2229–35 |
| 35. Yoshida T, Araki E, Iigo M, Fujii T, Shimada Y, Saito D, et al. Clinical significance of monitoring serum levels of 5-fluorouracil by continuous infusion in patients with advanced colonic cancer. Cancer Chemother Pharmacol 1990;26:352–4 |
Appendix 8 Excluded studies with reasons
Excluded studies for objectives A–C
| Citation | Reason for exclusion |
|---|---|
| 1. Ackland SP, Garg MB, Dunstan RH. Simultaneous determination of dihydrofluorouracil and 5-fluorouracil in plasma by high-performance liquid chromatography. Anal Biochem 1997;246:79–85 | Wrong population |
| 2. Adjei AA, Reid JM, Diasio RB, Sloan JA, Smith DA, Rubin J, et al. Comparative pharmacokinetic study of continuous venous infusion fluorouracil and oral fluorouracil with eniluracil in patients with advanced solid tumors. J Clin Oncol 2002;20:1683–91 | Population: < 80% included cancers |
| 3. Akiyama S, Nakayama H, Takami H, Gotoh H, Gotoh Y. Pharmacodynamic study of the Saltz regimen for metastatic colorectal cancer in a hemodialyzed patient. Chemotherapy 2007;53:418–21 | Treatment: bolus |
| 4. Allegra CJ. Dihydropyrimidine dehydrogenase activity: prognostic partner of 5-fluorouracil? Clin Cancer Res 1999;5:1947–9 | Editorial |
| 5. Anderson LW, Parker RJ, Collins JM, Ahlgren JD, Wilkinson D, Strong JM. Gas chromatographic-mass spectrometric method for routine monitoring of 5-fluorouracil in plasma of patients receiving low-level protracted infusions. J Chromatogr Biomed Appl 1992;581:195–201 | Technology: GC-MS |
| 6. Au JLS, Rustum YM, Ledesma EJ. Clinical pharmacological studies of concurrent infusion of 5-fluorouracil and thymidine in treatment of colorectal carcinomas. Cancer Res 1982;42:2930–7 | Wrong treatment |
| 7. Aubert C, Sommadossi JP, Coassolo P, Cano JP, Rigault JP. Quantitative analysis of 5-fluorouracil and 5,6-dihydrofluorouracil in plasma by gas chromatography mass spectrometry. Biomed Mass Spectrom 1982;9:336–9 | No patients, samples only |
| 8. Azzopardi N, Lecomte T, Ternant D, Boisdron-Celle M, Piller F, Morel A, et al. Cetuximab pharmacokinetics influences progression-free survival of metastatic colorectal cancer patients. Clin Cancer Res 2011;17:6329–37 | Not 5-FU |
| 9. Bailey H, Wilding G, Tutsch KD, Arzoomanian RZ, Alberti D, Tombes MB, et al. A phase I trial of 5-fluorouracil, leucovorin, and dipyridamole given by concurrent 120-h continuous infusions. Cancer Chemother Pharmacol 1992;30:297–302 | Population: < 80% included cancers |
| 10. Baker SD, Verweij J, Rowinsky EK, Donehower RC, Schellens JHM, Grochow LB, et al. Role of body surface area in dosing of investigational anticancer agents in adults, 1991–2001. J Natl Cancer Inst 2002;94:1883–8 | Patient group unclear |
| 11. Bamias A, Syrigos K, Fountzilas G, Tzamakou E, Soulti K, Karavasilis V, et al. Intensified bimonthly cisplatin with bolus 5-fluorouracil, continuous 5-fluorouracil and high-dose leucovorin (LV5FU2) in patients with advanced gastrointestinal carcinomas: a phase I dose-finding and pharmacokinetic study. Am J Clin Oncol 2004;27:465–71 | Population: < 80% included cancers |
| 12. Barberi-Heyob M, Merlin JL, Weber B. Analysis of 5-fluorouracil in plasma and urine by high-performance liquid chromatography. J Chromatogr Biomed Appl 1992;581:281–6 | Wrong population |
| 13. Ben Fredj R, et al. The dihydrouracil/uracil ratio in plasma, clinical and genetic analysis for screening of dihydropyrimidine dehydrogenase deficiency in colorectal cancer patients treated with 5-Fluorouracil. Pathologie Biologie 2009;57:470–6 | AUC or 5-FU plasma concentration not related to outcomes |
| 14. Beneton M, Chapet S, Blasco H, Giraudeau B, Boisdron-Celle M, Deporte-Fety R, et al. Relationship between 5-fluorouracil exposure and outcome in patients receiving continuous venous infusion with or without concomitant radiotherapy. Br J Clin Pharmacol 2007;64:613–21 | Population: < 80% included cancers |
| 15. Bergh J. Is pharmacokinetically guided chemotherapy dosage a better way forward? Ann Oncol 2002;13:343–4 | Editorial |
| 16. Bertino J, Gamelin E, Milano G. Highlights from: 5-Fluorouracil drug management pharmacokinetics and pharmacogenomics workshop: Orlando, Florida; January 2007 – 5-Fluorouracil drug management: pharmacokinetics and pharmacogenomics workshop meeting summary. Clin Colorectal Cancer 2007;6:407–22 | Meeting highlights |
| 17. Beumer JH, Parise RA, Newman EM, Doroshow JH, Synold TW, Lenz HJ, et al. Concentrations of the DNA methyltransferase inhibitor 5-fluoro-2-deoxycytidine (FdCyd) and its cytotoxic metabolites in plasma of patients treated with FdCyd and tetrahydrouridine (THU). Cancer Chemother Pharmacol 2008;62:363–8 | Not 5-FU |
| 18. Biffi M, Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Coinu A, et al. 5-FU Monitoring in clinical practice: pharmacokinetic variability. Eur J Cancer 2013;49:S173–4 | Abstract only: no dose adjustment – excluded |
| 19. Blaschke M, Cameron S, Blumberg J, Wegner U, Ramadori G. Measurements of 5-FU levels in plasma of patients with gastrointestinal cancer. J Clin Oncol 2012;1 | Population: < 80% included cancers |
| 20. Blaschke M, Cameron S, Emami K, Blumberg J, Wegner U, Nischwitz M, et al. Measurement of 5-FU plasma levels in patients with advanced cancer: Correct approach to practical procedures is essential. Int J Clin Pharmacol Ther 2011;49:83–5 | AUC not related to outcomes |
| 21. Blaschke M, Cameron S, Goeschen C, Ramadori G. 5-FU schedules, serum 5-FU levels and their relationship to therapy response and toxicity in patients with gastrointestinal cancer. Int J Clin Pharmacol Ther 2013;51:56–8 | Population: lack of information on patient population; patient population unclear |
| 22. Bocci G, Barbara C, Vannozzi F, Di Paolo A, Melosi A, Barsanti G, et al. A pharmacokinetic-based test to prevent severe 5-fluorouracil toxicity. Clin Pharmacol Ther 2006;80:384–95 | Treatment: bolus |
| 23. Bocci G, Di Paolo A, Barbara C, Masi G, Fornaro L, Loupakis F, et al. Pharmacokinetics, a main actor in a many-sided approach to severe 5-FU toxicity prediction. Br J Clin Pharmacol 2009;67:132–4 | Letter |
| 24. Bocci G, et al. Severe 5-Fluorouracil toxicity associated with a marked alteration of pharmacokinetics of 5-Fluorouracil and its catabolite 5-fluoro-5,6-dihydrouracil: a case report. Eur J Clin Pharmacol 2002;58:593–5 | Treatment: bolus |
| 25. Boisdron-Celle M, Boulanger N, Gamelin E. Monitoring pharmacocinetique. [French.] Bulletin du cancer 2000;87:86–92 | Narrative review |
| 26. Boisdron-Celle M, Le Guellec C. Therapeutic drug monitoring of 5-fluorouracil after its administration in high-dose protocols. Niveau de revue du suivi therapeutique pharmacologique du 5-fluorouracile au decours de son administration dans le traitement des cancers des voies aerodigestives superieures et du cancer colorectal. [French.] Therapie 2010;65:171–6 | Non-English |
| 27. Boisdron-Celle M. Pharmacokinetic adaptation of 5-fluorouracil: where are we and where are we going? Pharmacogenomics 2012;13:1437–9 | Editorial |
| 28. Boisdron-Celle M, et al. 5-Fluorouracil-related severe toxicity: a comparison of different methods for the pretherapeutic detection of dihydropyrimidine dehydrogenase deficiency. Cancer Letters 2007;249:271–82 | 5-FU plasma concentration not related to outcomes |
| 29. Boisdron-Celle M, et al. Prevention of 5-FU-induced toxicities using pretherapeutic DPD deficiency screening: medical and economic assessment of a multiparametric approach. J Clin Oncol 2013;1 | AUC or 5-FU plasma concentration not related to outcomes |
| 30. Boisdron-Celle M, et al. Severe fluoropyrimidines toxicities: screen effectively for DPD deficiencies. Fundam Clin Pharmacol 2013;27:39–40 | Abstract without dose adjustment following My5-FU measurement |
| 31. Borner MM, Schoffski P, De Wit R, Caponigro F, Comella G, Sulkes A, et al. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer 2002;38:349–58 | Treatment: bolus |
| 32. Bressolle F, Joulia JM, Pinguet F, Ychou M, Astre C, Duffour J, et al. Circadian rhythm of 5-fluorouracil population pharmacokinetics in patients with metastatic colorectal cancer. Cancer Chemother Pharmacol 1999;44:295–302 | 5-FU plasma concentration not related to outcomes |
| 33. Buchel B, Rhyn P, Schurch S, Buhr C, Amstutz U, Largiader CR. LC-MS/MS method for simultaneous analysis of uracil, 5,6-dihydrouracil, 5-fluorouracil and 5-fluoro-5,6-dihydrouracil in human plasma for therapeutic drug monitoring and toxicity prediction in cancer patients. Biomed Chromatogr 2013;27:7–16 | Accuracy of other method than My5-FU |
| 34. Buckpitt AR, Longo NS, Londer H, Boyd MR. Assay of 5-fluorouracil (FU) and 5-fluorodeoxyuridine (FUDR) in plasma at low nanogram level using high-pressure liquid-chromatography (HPLC). Proc Am Association Cancer Res 1978;19:231 | No patients, samples only |
| 35. Cai X, Xue P, Song WF, Hu J, Gu HL, Yang HY, et al. Role of pharmacokinetic monitoring of serum fluorouracil concentration in patients with local advanced and metastatic colorectal cancer and further improving efficacy of fluorouracil-based chemotherapy. [Chinese.] Chinese J Oncol 2012;34:39–43 | Non-English |
| 36. Cai X, Xue P, Song WF, Hu J, Gu HL, Yang HY, et al. The role of pharmacokinetic monitoring of fluorouracil in improvement of efficacy and reduction of adverse reactions for patients with advanced gastric cancer. [Chinese.] Tumor 2011;31:930–6 | Non-English |
| 37. Chan R, Kerr DJ. Can we individualise chemotherapy for colorectal cancer? Ann Oncol 2004;15:996–9 | Editorial |
| 38. Cho HK, Lee ES, Lee JW, Park JK, Kang JH, Lee KS, et al. Clinical pharmacokinetics of oxaliplatin and 5-fluorouracil administered in combination with leucovorin in Korean patients with advanced colorectal cancer. J Cancer Res Clin Oncol 2006;132:320–6 | 5-FU plasma concentration not related to outcomes |
| 39. Cho H, et al. Combined 5-FU and CDDP in a gastric cancer patient undergoing hemodialysis--pharmacokinetics of 5-FU and CDDP. [Japanese.] Gan to kagaku ryoho Cancer Chemother 2000;27:2135–8 | Case study |
| 40. Christophidis N, Mihaly G, Vajda F, Louis W. Comparison of liquid- and gas–liquid chromatographic assays of 5-fluorouracil in plasma. Clin Chem 1979;25:83–6 | Treatment: bolus |
| 41. Ciccolini J, Mercier C, Blachon MF, Favre R, Durand A, Lacarelle B. A simple and rapid high-performance liquid chromatographic (HPLC) method for 5-fluorouracil (5-FU) assay in plasma and possible detection of patients with impaired dihydropyrimidine dehydrogenase (DPD) activity. J Clin Pharm Ther 2004;29:307–15 | Patient group unclear |
| 42. Climente-Marti M, Merino-Sanjuan M, Almenar-Cubells D, Jimenez-Torres NV. A Bayesian method for predicting 5-fluorouracil pharmacokinetic parameters following short-term infusion in patients with colorectal cancer. J Pharm Sci 2003;92:1155–65 | 5-FU plasma concentration not linked to outcomes |
| 43. Czejka MJ, Jager W, Schuller J, Fogl U, Schernthaner G. Pharmacokinetic aspects of the combination of interferon-alpha-2b and folic acid with fluorouracil. [Pharmakokinetische aspekte der kombination von interferon-alpha-2b und folinsaure mit fluorouracil.] [German.] Arzneimittel-Forschung/Drug Research 1991;41:860–3 | Treatment: bolus |
| 44. Czejka MJ, Schuller J, Jager W, Fogl U, Weiss C. Influence of different doses of interferon-alpha-2b on the blood plasma levels of 5-fluorouracil. Eur J Drug Metabol Pharm 1993;18:247–50 | Population: < 80% included cancers |
| 45. Daher GC, et al. The role of dihydropyrimidine dehydrogenase (DPD) and thymidine phosphorylase (dThdPase) in the circadian variation of plasma drug levels of 5-Fluorouracil (FUra) and 5-fluorodeoxyuridine (FdUrd) following infusion of FUra or FdUrd. Ann Rev Chronopharmacol 1990;7:227–30 | Not 5-FU |
| 46. Danhauser LL, Freimann JH Jr, Gilchrist TL, Gutterman JU, Hunter CY, Yeomans AC, et al. Phase I and plasma pharmacokinetic study of infusional fluorouracil combined with recombinant interferon alfa-2b in patients with advanced cancer. J Clin Oncol 1993;11:751–61 | Wrong population |
| 47. De Forni M, Gualano V, Canal P, Martel P, Izar-Soum F, Chevreau C, et al. Continuous infusion of 5-fluorouracil by double route (intravenous and intraperitoneal) with modulation by folinic acid: clinical and pharmacokinetic, phase I study in patients with intra-abdominal malignancies. [5-fluorouracile en perfusion continue double voie (intraveineuse et intraperitoneale) module par l’acide folinique: etude clinique de phase i et pharmacocinetique chez des patients porteurs de cancers a evolution intra-abdominale.] [French.] Bulletin du Cancer 1993;80:408–17 | Population: < 80% included cancers |
| 48. de Mattos AC, Khalil NM, Mainardes RM. Development and validation of an HPLC method for the determination of fluorouracil in polymeric nanoparticles. Brazilian J Pharm Sci 2013;49:117–26 | No patients |
| 49. DeGregorio MW, Holleran WM, Benz CC, Cadman EC. Quantitative analysis of 5-fluorouracil in human serum by high-performance liquid chromatography. Analytical Letters 1985;18:51–5 | No patients, samples only |
| 50. Di Paolo A, Danesi R, Ciofi L, Vannozzi F, Bocci G, Lastella M, et al. Improved analysis of 5-fluorouracil and 5,6-dihydro-5-fluorouracil by HPLC with diode array detection for determination of cellular dihydropyrimidine dehydrogenase activity and pharmacokinetic profiling. Ther Drug Monit 2005;27:362–8 | Treatment: bolus |
| 51. Di Paolo A, et al. Relationship between 5-Fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patients. Ann Oncol 2001;12:1301–6 | Treatment: bolus |
| 52. Di Paolo A, et al. Relationship between plasma concentrations of 5-Fluorouracil and 5-fluoro-5,6-dihydrouracil and toxicity of 5-Fluorouracil infusions in cancer patients. Ther Drug Monit 2002;24:588–93 | Wrong treatment: bolus |
| 53. Dong QM, Huand SH, Zheng WH, He YJ. Clinical significance of 5-FU concentration at steady state in colorectal cancer detected by HPLC method. [Chinese.] Chinese J Cancer Prev Treat 2010;17:1476–8, 81 | Non-English |
| 54. Dong QM, et al. Relationship of serum level of dihydropyrimidine dehydrogenase and serum concentration of 5-Fluorouracil to treatment response and adverse events in colorectal cancer patients. [Chinese.] Chinese J Cancer 2005;24:483–7 | Non-English |
| 55. Escoriaza J, Aldaz A, Calvo E, Giraldez J. Simple and sensitive determination of 5-fluorouracil in plasma by high-performance liquid chromatography: application to clinical pharmacokinetic studies. J Chromatogr B Biomed Sci Appl 1999;736:97–102 | No patients, samples only |
| 56. Etienne MC, Chatelut E, Pivot X, Lavit M, Pujol A, Canal P, et al. Co-variables influencing 5-fluorouracil clearance during continuous venous infusion. A NONMEM analysis. Eur J Cancer 1998;34:92–7 | Population: < 80% included cancers |
| 57. Feng WY, Cai S, Shen JW. Determination of 5-fluorouracil in human plasma by high performance liquid chromatography. [Chinese.] Chinese Pharm J 2003;38:289–90 | No patients, samples only |
| 58. Fleming RA, Milano G, Thyss A, Etienne MC, Renee N, Schneider M, et al. Correlation between dihydropyrimidine dehydrogenase activity in peripheral mononuclear cells and systemic clearance of fluorouracil in cancer patients. Cancer Res 1992;52:2899–902 | 5-FU plasma concentrations not related to outcomes |
| 59. Fleming RA, Milano GA, Etienne MC, Renee N, Thyss A, Schneider M, et al. No effect of dose, hepatic function, or nutritional status on 5-FU clearance following continuous (5-day), 5-FU infusion. Br J Cancer 1992;66:668–72 | AUC not related to outcomes |
| 60. Fleming RA, Milano GA, Gaspard MH, Bargnoux PJ, Thyss A, Plagne R, et al. Dihydropyrimidine dehydrogenase activity in cancer patients. Eur J Cancer 1993;29:740–4 | Excluded: case study of two patients |
| 61. Fleming GF, et al. Phase I and pharmacokinetic study of 24-hour infusion 5-Fluorouracil and leucovorin in patients with organ dysfunction. Ann Oncol 2003;14:1142–7 | Population: < 80% included cancers |
| 62. Gamelin E, Boisdron-Celle M, Turcant A, Larra F, Allain P, Robert J. Rapid and sensitive high-performance liquid chromatographic analysis of halogenopyrimidines in plasma. J Chromatogr B Biomed Sci Appl 1997;695:409–16 | No patients, samples only |
| 63. Gamelin E, Boisdron-Celle M. Dose monitoring of 5-fluorouracil in patients with colorectal or head and neck cancer-status of the art. Crit Rev Oncol Hematol 1999;30:71–9 | Narrative review |
| 64. Gamelin E, Boisdron-Celle M. Individual dose adjustment in cancer chemotherapy. [L’adaptation individuelle de posologie en chimiotherapie anticancereuse.] [French.] Revue de Medecine Interne 1996;17:529–33 | Editorial |
| 65. Gamelin E, Gamelin L, Larra F, Turcant A, Alain P, Maillart P, et al. Acute cardiac toxicity of 5-fluorouracil: pharmacokinetic correlation. [Toxicite cardiaque aigue du 5-fluorouracile: correlation pharmacocinetique.] [French.] Bulletin du cancer 1991;78:1147–53 | Non-English |
| 66. Gamelin E, Jacob J, Danquechin Dorval EM, Pezet D, Delva R, Raoul JL, et al. Multicentric randomized trial comparing in weekly treatment of advanced colorectal cancer (CRC) intensified 5 fluorouracil and folinic acid (FA) with 5 fu pharmacokinetic monitoring to a constant dose calculated with body surface area. 1998. In Proceedings of the Annual Meeting of the American Society for Clinical Oncology. Abstract #1039 | Abstract only: not My5-FU |
| 67. Gamelin E, Metges J, Adenis A, Raoul J, Lam Y, Lecomte T, et al. Dose intensity and tolerance improvement of cetuximab, irinotecan, 5-Fu and folinic acid in patients with metastatic CRC; a pharmacokinetic and pharmacogenetic approach. Ann Oncol 2008;19:viii,131 | Abstract only: not My5-FU |
| 68. Gamelin E, et al. Correlation between uracil and dihydrouracil plasma ratio, fluorouracil (5-FU) pharmacokinetic parameters, and tolerance in patients with advanced colorectal cancer: a potential interest for predicting 5-FU toxicity and determining optimal 5-FU dosage. J Clin Oncol 1999;17:1105–10 | 5-FU plasma concentration not related to outcomes |
| 69. Garg MB, Lincz LF, Adler K, Scorgie FE, Ackland SP, Sakoff JA. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: a multivariate analysis. Br J Cancer 2012;107:1525–33 | Treatment: bolus |
| 70. Gonzalez MS, Rebollo J, Escudero V, Valenzuela B, Nalda R, Duart M, et al. Pharmacokinetic (PK) guide for dose adjustments in cancer patients (PTS) treated with 5-fluoruracil (5-FU) infusions. Preliminary results. J Clin Oncol 2008;26 | Abstract only: patient group unknown, not My5-FU |
| 71. Grem JL, Quinn M, Ismail AS, Takimoto CH, Lush R, Liewehr DJ, et al. Pharmacokinetics and revueodynamics effects of 5-fluorouracil given as a one-hour intravenous infusion. Cancer Chemother Pharmacol 2001;47:117–25 | Wrong treatment |
| 72. Grim J, Hroch M, Chladek J, Slanar O, Petera J, Martinkova J. PK/PD of 5-fluorouracil during neoadjuvant chemoradiotherapy in locally advanced rectal carcinoma. [Farmakokinetika, ucinek a tolerabilita 5-fluorouracilu behem predoperacni chemoradioterapie lokalne pokrocileho karcinomu rekta.] [Czech.] Onkologie 2012;6:214–19 | Wrong treatment |
| 73. Gudauskas G, Goldie JH. Pharmacokinetics of high-dose continuous – 5-fluorouracil infusions. Proc Am Association Cancer Res 1978;19:364 | Patient group unclear |
| 74. Guo XD, Harold N, Wasif Saif M, Schuler B, Szabo E, Hamilton JM, et al. Pharmacokinetic and revueodynamics effects of oral eniluracil, fluorouracil and leucovorin given on a weekly schedule. Cancer Chemother Pharmacol. 2003;52:79–85 | Treatment: oral |
| 75. Gusella M, Frigo AC, Bolzonella C, Marinelli R, Barile C, Bononi A, et al. Predictors of survival and toxicity in patients on adjuvant therapy with 5-fluorouracil for colorectal cancer. Br J Cancer 2009;100:1549–57 | Treatment: bolus |
| 76. Hara Y, Kono A, Tanaka M. Measurement of 5′-deoxy-5-fluorouridine (5′-DFUR) by high-performance liquid chromatography and studies on pharmacokinetics of 5′-DFUR and 5-fluorouracil by oral and intravenous administration. [Japanese.] Gan to kagaku ryoho. Cancer Chemother 1984;11:2261–6 | 5-FU concentration not related to outcomes |
| 77. Haregewoin A, Hamilton SA, Grier CE, Kaldate RR. BSA dosing and suboptimal 5-FU exposure among colorectal cancer patients of varying gender and age. J Clin Oncol 2012;1 | Abstract only: no dose adjustment |
| 78. Haregewoin A, Kaldate RR, Hamilton SA, Saam JR, Wenstrup RJ. Modeling 5-FU AUC–dose relationship to develop a PK dosing algorithm. J Clin Oncol 2011;1 | Abstract only: no dose adjustment |
| 79. Harris BE, Song R, Soong SJ, Diasio RB. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res 1990;50:197–201 | 5-FU plasma concentration not related to outcomes |
| 80. Hayes Inc. Pharmacokinetically Guided Dose Adjustment of 5-fluorouracil (5-FU). Lansdale, PA: Hayes Inc.; 2012. | Not available: too expensive to buy |
| 81. Hayes Inc. TheraGuide 5-FU (Myriad Genetic Laboratories Inc.) for Predicting Toxicity to 5-Fluorouracil (5-FU)/Capecitabine-Based Chemotherapy. Lansdale, PA: Hayes Inc.; 2009 | Not available: too expensive to buy |
| 82. Hendrayana T, Kenny P, Hilger RA, Schmidt-Wolf I, Ko YD, Jaehde U. Variability of systemic fluorouracil (5-FU) exposure during continuous infusion: there is a need for TDM. Ther Drug Monit 2011;33:548 | Patient group unclear |
| 83. Hilger RA, Koehler J, Kalkavan H, Richly H, Hoffmann AC, Heinrichs D, et al. Interpatient pharmacokinetic variability of 5-FU within metastatic and adjuvant colon cancer patients: first results from the West German Cancer Center. Onkologie 2010;33:173 | Abstract only: no dose adjustment |
| 84. Hillcoat BL, McCulloch PB, Figueredo A. Levels of 5 fluorouracil (5FU) in plasma of patients treated by drug infusion. Proc Am Association Cancer Res 1976;17 | Patient group unclear |
| 85. Ho DH, Pazdur R, Covington W, Brown N, Huo YY, Lassere Y, et al. Comparison of 5-fluorouracil pharmacokinetics in patients receiving continuous 5-fluorouracil infusion and oral uracil plus N1-(2-tetrahydrofuryl)–5-fluorouracil. Clin Cancer Res 1998;4:2085–8 | 5-FU plasma concentration not related to outcomes |
| 86. House LK, Ramirez J, Ratain MJ. Simultaneous determination of 5-fluorouracil and uracil by high-performance liquid chromatography using four serial columns. J Chromatogr B 1998;720:245–50 | No patients, samples only |
| 87. Jiang H, Lu J, Jiang J, Hu P. Important role of the dihydrouracil/uracil ratio in marked interpatient variations of fluoropyrimidine pharmacokinetics and pharmacodynamics. J Clin Pharmacol 2004;44:1260–72 | Population: < 80% included cancers |
| 88. Jones RA, Buckpitt AR, Londer HH, Myers CE, Chabner BA, Boyd MR. Potential clinical applications of a new method for quantitation of plasma levels of 5-fluorouracil and 5-fluorodeoxyuridine. Bulletin du Cancer 1979;66: 75–8 | Treatment: bolus |
| 89. Joulia JM, Pinguet F, Grosse PY, Astre C, Bressolle F. Determination of 5-fluorouracil and its main metabolites in plasma by high-performance liquid chromatography. J Chromatogr B Biomed Appl 1997;692:427–35 | No patients, samples only |
| 90. Joulia JM, Pinguet F, Ychou M, Duffour J, Astre C, Bressolle F. Plasma and salivary pharmacokinetics of 5-fluorouracil (5-FU) in patients with metastatic colorectal cancer receiving 5-FU bolus plus continuous infusion with high-dose folinic acid. Eur J Cancer 1999;35:296–301 | 5-FU plasma concentration not related to outcomes |
| 91. Joulia JM, Pinguet F, Ychou M, Duffour J, Topart D, Grosse PY, et al. Pharmacokinetics of 5-fluorouracil (5-FUra) in patients with metastatic colorectal cancer receiving 5-FUra bolus plus continuous infusion with high dose folinic acid (LV5FU2). Anticancer Res 1997;17:2727–30 | 5-FU plasma concentration not related to outcomes |
| 92. Kim R, Tanabe K, Inoue H, Toge T. Mechanism(s) of antitumor action in protracted infusion of low dose 5-fluorouracil and cisplatin in gastric carcinoma. Int J Oncol 2002;20:549–55 | No patients, samples only |
| 93. Kobayashi K, Ratain MJ. Individualizing dosing of cancer chemotherapy. Semin Oncol 1993;20:30–42 | Narrative review |
| 94. Kohne CH, Hiddemann W, Schuller J, Weiss J, Lohrmann HP, Schmitz- Hubner U, et al. Failure of orally administered dipyridamole to enhance the antineoplastic activity of fluorouracil in combination with leucovorin in patients with advanced colorectal cancer: a prospective randomized trial. J Clin Oncol 1995;13:1201–8 | Treatment: bolus |
| 95. Kojima T, Suzumura K, Kanemitsu T, Miyashita A, Inamura Y, Owa Y, et al. Concentrations of 5-fluorouracil (5-FU) in serum and tissues at venous injection of tegafur or 5-FU–clinical study on colorectal cancer. [Japanese.] Gan to kagaku ryoho. Cancer Chemother 1998;25:547–51 | Wrong treatment |
| 96. Konings IRHM, Sleijfer S, Mathijssen RHJ, De Bruijn P, Ghobadi Moghaddam-Helmantel IM, Van Dam LM, et al. Increasing tumoral 5-fluorouracil concentrations during a 5-day continuous infusion: a microdialysis study. Cancer Chemother Pharmacol 2011;67:1055–62 | Wrong population |
| 97. Kosovec JE, Egorin MJ, Gjurich S, Beumer JH. Quantitation of 5-fluorouracil (5-FU) in human plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 2008;22:224–30 | No patients, samples only |
| 98. Kreuser ED, Hilgenfeld RU, Matthias M, Hoksch B, Boewer C, Oldenkott B, et al. A phase I trial of interferon alpha-2b with folinic acid and 5-fluorouracil administered by 4-hour infusion in metastatic colorectal carcinoma. Semin Oncol 1992;19:197–203 | Wrong treatment |
| 99. Kwiatkowski F, Chevrier R, De Renzis JP, Charrier S, Cure H, Bargnoux PJ, et al. 5-Fluorouracile chrono-pharmacokinetic in metastatic colorectal cancer treatment and metabolic response index. [Chrono-pharmacocinetique du 5-fluoro-uracile dans le traitement des cancers colorectaux metastatiques et indicateurs de reponse metabolique.] [French.] Journal de Pharmacie Clinique 1999;18:138–43 | Non-English |
| 100. LaCreta FP, Williams WM. High-performance liquid chromatographic analysis of fluoropyrimidine nucleosides and fluorouracil in plasma. J Chromatogr B Biomed Appl 1987;414:197–201 | Animal study |
| 101. Lamezec B, Alleaume C, Gamelin E, Goudier MJ, Cailleux PE, DanquechinDorval E, et al. Long-term weekly treatment of advanced colorectal cancer (CRC) with fluorouracil (5-FU) and leucovorin (LV): 5 year-results of a multicentric phase II trial of 5-FU pharmacokinetic monitoring in 152 patients. Eur J Cancer 1997;33:726 | Abstract only: not My5-FU |
| 102. Link KH, Kreuser ED, Safi F, Ullrich J, Schalhorn A, Schmoll E, et al. The status of 5-FU and folinic acid (FA, Rescuvolin) in the treatment concept of nonresectable colorectal liver metastases. A comparison of 5-FU/FA i.a. vs. 5-FU/FA i.v. vs. 5-FUDR i.a. vs. 5-FUDR i.a. + i.v. in an observation study. [Die intraarterielle chemotherapie mit 5-FU und folinsaure (FA, rescuvolin) im therapiekonzept bei nicht resektablen kolorektalen lebermetastasen. Ein vergeich von 5-FU/FA i.v. vs. 5-FUDR i.a. oder 5-FUDR i.a. + i.v.] [German.] Tumor Diagnostik und Therapie 1993;14:224–31 | No PK monitoring |
| 103. Lokich J. Pharmacokinetic modulation of 5-fluorouracil: emulating continuous infusion? Cancer Invest 1999;17:543–4 | Editorial |
| 104. MacMillan WE, Wolberg WH, Welling PG. Pharmacokinetics of fluorouracil in humans. Cancer Res 1978;38:3479–82 | Treatment: bolus |
| 105. Malothu N, Veldandi UK, Yellu NR, Yadala N, Devarakonda RK. Population pharmacokinetics of 5-flouro uracil in Indian cancer patient population. Asian J Pharm Clin Res 2010;3:197–200 | 5-FU plasma concentration not related to outcomes |
| 106. Mani S, Rudin CM, Kunkel K, Holmlund JT, Geary RS, Kindler HL, et al. Phase I clinical and pharmacokinetic study of protein kinase C-alpha antisense oligonucleotide ISIS 3521 administered in combination with 5-fluorouracil and leucovorin in patients with advanced cancer. Clin Cancer Res 2002;8:1042–8 | Population: < 80% included cancers |
| 107. Maring JG, Schouten L, Greijdanus B, De Vries EGE, Uges DRA. A simple and sensitive fully validated HPLC-UV method for the determination of 5-fluorouracil and its metabolite 5,6-dihydrofluorouracil in plasma. Ther Drug Monit 2005;27:25–30 | Treatment: bolus |
| 108. Marsh S, Van Rooij T. Challenges of incorporating pharmacogenomics into clinical practice. Gastroint Cancer Res 2009;3:206–7 | Editorial |
| 109. Martens-Lobenhoffer J, Fuhlroth J, Ridwelski K. Influence of the administration of amifostine on the pharmacokinetics of 5-fluorouracil in patients with metastatic colorectal carcinoma. Int J Clin Pharmacol Ther 2000;38:41–4 | AUC not related to outcomes |
| 110. Matsuo T, Nishizuka SS, Ishida K, Endo F, Katagiri H, Kume K, et al. Evaluation of chemosensitivity prediction using quantitative dose–response curve classification for highly advanced/relapsed gastric cancer. World J Surg Oncol 2013;11 | Tumour samples analysed |
| 111. Meadows LM, Walther P, Ozer H. Alpha-interferon and 5-fluorouracil: possible mechanisms of antitumor action. Semin Oncol 1991;18:71–6 | Narrative review |
| 112. Mercier C, Yang C, Dahan L, Ciccolini J, Bagarry D, Seitz JF, et al. 5-fluorouracil in head and neck cancer patients: a population pharmacokinetics study. J Clin Oncol 2010;1 | Abstract only: not 5-FU measured |
| 113. Milano G, Thyss A, Santini J, Frenay M, Francois E, Schneider M, et al. Salivary passage of 5-fluorouracil during continuous infusion. Cancer Chemother Pharmacol 1989;24:197–9 | Population: < 80% included cancers |
| 114. Milano G, et al. Influence of sex and age on fluorouracil clearance. J Clin Oncol 1992;10:1171–5 | AUC or 5-FU plasma concentration not related to outcomes |
| 115. Miyauchi M, Yamamoto N, Matsumoto M, Shishikura T, Hyakutake K. Comparative clinical study on 5-FU concentrations for oral HCFU and i.v. 5-FU. [Japanese.] Gan to kagaku ryoho. Cancer Chemother 2000;27:1011–14 | AUC not related to outcomes |
| 116. Mross K, Buchert M, Fasol U, Jaehde U, Kanefendt F, Strumberg D, et al. A preliminary report of a Phase II study of folinic acid, 5-fluorouracil, irinotecan (FOLFIRI) plus sunitinib with toxicity, efficacy, pharmacokinetics, biomarker, imaging data in patients with colorectal cancer with liver metastases as 1st line treatment – a study of the CESAR central revueo society for anticancer drug research – EWIV. Int J Clin Pharmacol Ther 2011;49:96–8 | Technology: imaging |
| 117. Mueller F, Buchel B, Koberle D, Schurch S, Pfister B, Krahenbuhl S, et al. Gender-specific elimination of continuous-infusional 5-fluorouracil in patients with gastrointestinal malignancies: Results from a prospective population pharmacokinetic study. Cancer Chemother Pharmacol 2013;71:361–70 | AUC not related to outcomes |
| 118. Muneoka K, Shirai Y, Sasaki M, Kanda J, Wakai T, Asakura T, et al. Pharmacokinetic monitoring of 5-fluorouracil may improve the clinical benefit with an individualized regimen-a case report. [Japanese.] Gan to Kagaku Ryoho. J Cancer Chemother 2009;36:131–4 | Case report |
| 119. Nakatsu T, Yokoyama I, Tsuyuki K, Soh Y, Hanai G, Matsumoto H, et al. [Clinical reevaluation of continuous intravenous infusion of 5-fluorouracil–plasma concentrations and clinical dose by continuous intravenous and 60-min infusions.] [Japanese.] Gan to Kagaku Ryoho. J Cancer Chemother 1990;17:253–8 | Non-English |
| 120. Nassim MA, Shirazi FH, Cripps CM, Veerasinghan S, Molepo MJ, Obrocea M, et al. An HPLC method for the measurement of 5-fluorouracil in human plasma with a low detection limit and a high extraction yield. Int J Mol Med 2002;10:513–16 | No patients, samples only |
| 121. Peng RJ, Dong QM, Shi YX, Cao Y, Zhou ZM, Yuan ZY, et al. Correlative analysis between serum dihydropyrimidine dehydrogenase, activity, concentration of 5-fluorouracil and adverse events in the treatment of advanced gastric cancer patients. [Chinese.] Chinese J Cancer 2006;25:1039–43 | Non-English |
| 122. Pittman KB, Perren T, Ward U, Primrose J, Slevin M, Patel N, et al. Pharmacokinetics of 5-fluorouracil in colorectal cancer patients receiving interferon. Ann Oncol 1993;4:515–16 | Treatment: bolus |
| 123. Ploylearmsaeng SA, Fuhr U, Jetter A. How may anticancer chemotherapy with fluorouracil be individualised? Clin Pharmacokinetics 2006;45:567–92 | Narrative review |
| 124. Port RE, Daniel B, Ding RW, Herrmann R. Relative importance of dose, body surface area, sex, and age for 5-fluorouracil clearance. Oncology 1991;48:277–81 | Treatment: bolus |
| 125. Port RE, Edler L, Herrmann R, Feldmann U. Pharmacokinetics of 5-fluorouracil after short systemic infusion: plasma level at the end of the distribution phase as an indicator of the total area under the plasma concentration-time curve. Ther Drug Monit 1991;13:96–102 | Treatment: bolus |
| 126. Porta-Oltra B, Perez-Ruixo JJ, Climenti-Marti M, Merino-Sanjuan M, Almenar-Cubells D, Jimenez-Torres NV. Population pharmacokinetics of 5-fluorouracil in colorectal cancer patients. J Oncol Pharm Pract 2004;10:155–67 | Treatment: bolus |
| 127. Quebbeman EJ, Hoffman NE, Hamid AAR, Ausman RK. An HPLC method for measuring 5-fluorouracil in plasma. J Liquid Chromatogr 1984;7:1489–94 | Patient group unclear |
| 128. Rebollo J, Valenzuela B, Duart-Duart M, Escudero-Ortiz V, Gonzalez MS, Brugarolas A. Use of therapeutic drug monitoring of cancer chemotherapy to modify initial per-protocol doses. J Clin Oncol 2010;1 | Abstract only: method for PK monitoring unclear |
| 129. Remick SC, Grem JL, Fischer PH, Tutsch KD, Alberti DB, Nieting LM, et al. Phase I trial of 5-fluorouracil and dipyridamole administered by seventy-two-hour concurrent continuous infusion. Cancer Res 1990;50:2667–72 | Population: < 80% included cancers |
| 130. Saam J, Critchfield GC, Hamilton SA, Roa BB, Wenstrup RJ, Kaldate RR. Body surface area-based dosing of 5-fluoruracil results in extensive interindividual variability in 5-fluorouracil exposure in colorectal cancer patients on FOLFOX regimens. Clin Colorectal Cancer 2011;10:203–6 | AUC not related to outcomes |
| 131. Sadee W, Finn C, Schwandt HJ. 5 Fluorouracil (5 FU) pharmacokinetics following various routes of administration. Proc Am Association Cancer Res 1975;16:745 | Method for PK monitoring unclear |
| 132. Saif MW, Choma A, Salamone SJ, Chu E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J Nat Cancer Inst 2009;101:1543–52 | Narrative review |
| 133. Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf 2009;8:191–202 | Narrative review |
| 134. Salamone SJ, Benfield CN, Courtney JB, Harney RL, Kozo DR, Li Y, et al. Rapid 5-fluorouracil plasma quantification by immunoassay: validation with FOLFOX6 clinical samples. J Clin Oncol 2011;1 | Intervention unclear |
| 135. Salamone SJ, Courtney JB, Cline DJ, Harney RL, Lundell GD, Galloway K. 5-Fluoruracil plasma determination: automated immuoasay for general chemistry analyzers. Asia-Pacific J Clin Oncol 2009;5:A199 | Intervention unclear |
| 136. Schaaf LJ, Ferry DG, Hung CT. Analysis of 5-deoxy-5-fluorouridine and 5-fluorouracil in human plasma and urine by high-performance liquid chromatography. J Chromatogr Biomed Appl 1985;342:303–13 | No patients, samples only |
| 137. Schuller J, Czejka MJ, Jager W, Bosse C, Fogl U. Comparative bioavailability of fluorouracil and its prodrug, ftorafur, following intra-arterial, intravenous and preoral administration. [Vergleichende bioverfugbarkeit von fluorouracil und seinem prodrug ftorafur nach intraarterieller, intravenoser und peroraler verabreichung.] [German.] Die Pharmazie 1991;46:587–8 | Treatment: bolus |
| 138. Serdar MA, et al. Determination of 5-Fluorouracil and dihydrofluorouracil levels by using a liquid chromatography-tandem mass spectrometry method for evaluation of dihydropyrimidine dehydrogenase enzyme activity. Cancer Chemother Pharmacol 2011;68:525–9 | No patients, samples only |
| 139. Sharma S, Abhyankar V, Burgess RE, Infante J, Trowbridge RC, Tarazi J, et al. A phase I study of axitinib (AG-013736) in combination with bevacizumab plus chemotherapy or chemotherapy alone in patients with metastatic colorectal cancer and other solid tumors. Ann Oncol 2010;21:297–304 | AUC not related to outcomes |
| 140. Siegel-Lakhai WS, Beijnen JH, Vervenne WL, Boot H, Keessen M, Versola M, et al. Phase I pharmacokinetic study of the safety and tolerability of lapatinib (GW572016) in combination with oxaliplatin/fluorouracil/leucovorin (FOLFOX4) in patients with solid tumors. Clinical Cancer Res 2007;13:4495–502 | Population: < 80% included cancers |
| 141. Sparano JA, Wadler S, Diasio RB, Zhang R, Lu Z, Schwartz EL, et al. Phase I trial of low-dose, prolonged continuous infusion fluorouracil plus interferon-alfa: Evidence for enhanced fluorouracil toxicity without pharmacokinetic perturbation. J Clin Oncol 1993;11:1609–17 | Population: < 80% included cancers |
| 142. Stein TA, Burns GP, Bailey B, Citron ML. 5-Fluorouracil pharmacokinetics in patients with metastatic colorectal carcinoma after high-dose leucovorin. Cancer Invest 1994;12:375–8 | Treatment: bolus |
| 143. Stetson PL, Shukla UA, Ensminger WD. Sensitive high-performance liquid chromatographic method for the determination of 5-fluorouracil in plasma. J Chromatogr Biomed Appl 1985;344:385–90 | No patients, samples only |
| 144. Stoffregen C, Zurborn KH, Boehme V, Schmid A, Lorenz G, Arendt T, et al. Weekly high-dose 5-fluorouracil 24-hour infusion and intermediate-dose folinic acid bolus in metastatic colorectal cancer. Onkologie 1996;19:410–14 | 5-FU plasma concentration not related to outcomes |
| 145. Sugiyama E, Kaniwa N, Kim SR, Hasegawa R, Saito Y, Ueno H, et al. Population pharmacokinetics of gemcitabine and its metabolite in Japanese cancer patients: impact of genetic polymorphisms. Clin Pharmacokinetics 2010;49:549–58 | Wrong treatment |
| 146. Takimoto CH, Yee LK, Venzon DJ, Schuler B, Grollman F, Chabuk C, et al. High inter- and intrapatient variation in 5-fluorouracil plasma concentrations during a prolonged drug infusion. Clin Cancer Res 1999;5:1347–52 | AUC not related to outcomes |
| 147. Teh LK, et al. Potential of dihydropyrimidine dehydrogenase genotypes in personalizing 5-Fluorouracil therapy among colorectal cancer patients. Ther Drug Monit 2013;35:624–30 | AUC or 5-FU plasma concentration not related to outcomes |
| 148. Terret C, Erdociain E, Guimbaud R, Boisdron-Celle M, McLeod HL, Fety-Deporte R, et al. Dose and time dependencies of 5-fluorouracil pharmacokinetics. Clin Pharm Ther 2000;68:270–9 | 5-FU plasma concentration not related to outcomes |
| 149. Trump DL, Egorin MJ, Forrest A, Willson JKV, Remick S, Tutsch KD. Pharmacokinetic and revueodynamics analysis of fluorouracil during 72-hour continuous infusion with and without dipyridamole. J Clin Oncol 1991;9:2027–35 | Excluded: patient group unknown |
| 150. Tsume Y, Provoda CJ, Amidon GL. The achievement of mass balance by simultaneous quantification of floxuridine prodrug, floxuridine, 5-fluorouracil, 5-dihydrouracil, alpha-fluoro-beta-ureidopropionate, alpha-fluoro-beta-alanine using LC-MS. J Chromatogr B 2011;879:915–20 | No patients, cell lines |
| 151. Van Kuilenburg ABP, Maring JG. Evaluation of 5-fluorouracil pharmacokinetic models and therapeutic drug monitoring in cancer patients. Pharmacogenomics 2013;14:799–811 | Narrative review |
| 152. Van Kuilenburg ABP, Van Lenthe H, Maring JG, Van Gennip AH. Determination of 5-fluorouracil in plasma with HPLC-tandem mass spectrometry. Nederlands Tijdschrift voor Klinische Chemie en Laboratoriumgeneeskunde 2006;31:218–19 | Treatment: bolus |
| 153. Van Kuilenburg ABP, Van Lenthe H, Maring JG, Van Gennip AH. Determination of 5-fluorouracil in plasma with HPLC-tandem mass spectrometry. Nucleosides Nucleotides Nucleic Acids 2006;25:1257–60 | Treatment: bolus |
| 154. Van Kuilenburg ABP, et al. Evaluation of 5-Fluorouracil pharmacokinetics in cancer patients with a c.19051G>A mutation in DPYD by means of a Bayesian limited sampling strategy. Clin Pharmacokinetics 2012;51:163–74 | Treatment: bolus |
| 155. Vokes EE, Mick R, Kies MS, Dolan ME, Malone D, Athanasiadis I, et al. Pharmacodynamics of fluorouracil-based induction chemotherapy in advanced head and neck cancer. J Clin Oncol 1996;14:1663–71 | Treatment combination with interferon |
| 156. Watayo Y, et al. Drug monitoring during FOLFOX6 therapy in a rectal cancer patient on chronic hemodialysis. Japanese J Clin Oncol 2010;40:360–4 | Case study |
| 157. Wattanatorn W, McLeod HL, Cassidy J, Kendle KE. High-performance liquid chromatographic assay of 5-fluorouracil in human erythrocytes, plasma and whole blood. J Chromatogr B Biomed Appl 1997;692:233–7 | No PK monitoring |
| 158. Wattanatorn W, McLeod HL, Macklon F, Reid M, Kendle KE, Cassidy J. Comparison of 5-fluorouracil pharmacokinetics in whole blood, plasma, and red blood cells in patients with colorectal cancer. Pharmacotherapy 1997;17:881–6 | 5-FU plasma concentration not related to outcomes |
| 159. Wihlm J, Leveque D, Velten M, Klein T. Pharmacokinetic monitoring with dosage adjustment of 5 fluorouracil administered by continuous infusion. [Surveillance pharmacocinetique avec adaptation de posologie du 5-fluorouracile administre en perfusion continue.] [French.] Bulletin du cancer 1993;80:439–45 | Non-English |
| 160. Wilbur BJ, De Gregorio MW, Benz CC. Quantitation of purines and pyrimidines in human serum by high-performance liquid chromatography. Analytical Letters 1985;18:315–21 | No patients, samples only |
| 161. Woloch C, et al. Population pharmacokinetic analysis of 5-FU and 5-FDHU in colorectal cancer patients: search for Biomarkers associated with gastro-intestinal toxicity. Curr Top Med Chem 2012;12:1713 19 | Treatment: bolus |
| 162. Wright MA, Morrison G, Lin P, Leonard GD, Nguyen D, Guo X, et al. A phase I pharmacologic and pharmacogenetic trial of sequential 24-hour infusion of irinotecan followed by leucovorin and a 48-hour infusion of fluorouracil in adult patients with solid tumors. Clin Cancer Res 2005;11:4144–50 | Population: < 80% included cancers |
| 163. Wrightson WR, Myers SR, Galandiuk S. HPLC analysis of 5-FU and FdUMP in tissue and serum. Biochem Biophys Res Commun 1995;216:808–13 | Animal study |
| 164. Yu GS, He YJ, Liao H, Li S. Relationship of plasma concentration of 5-fluorouracil with toxicity and response in patients with nasopharyngeal carcinoma. [Chinese.] Chinese J Cancer 2003;22:1349–51 | Non-English |
| 165. Zhou ZW, Wang GQ, Wan DS, Lu ZH, Chen YB, Li S, et al. The dihydrouracil/uracil ratios in plasma and toxicities of 5-fluorouracil-based adjuvant chemotherapy in colorectal cancer patients. Chemotherapy 2007;53:127–31 | Treatment: bolus |
| 166. Zhou ZW, Wang GQ, Wan DS, Pan ZZ, Li S, Chen G, et al. Relationship between dihydropyrimidine dehydrogenase (DPD) activity and toxicity of 5-FU-based adjuvant chemotherapy in colorectal cancer patients. [Chinese.] Chinese J Cancer 2004;23:1512–6 | Treatment: bolus |
| 167. Zhu L, Shen GJ, Ding SQ, Hua X. Determination of 5-fluorouracil in 5-fluorouracil injection and human serum by HPLC. J Food Drug Anal 2012;20:947–50, 86 | No patients, samples only |
| 168. Zufia L, Egues A, Aldaz A. Validation of an LC/UV method based on accuracy profiles for daily 5-fluorouracil dose adjustment in cancer patients. Ther Drug Monit 2013;35:727 | Accuracy of other method than My5-FU |
Excluded studies for objective D
| Citation | Reason for exclusion |
|---|---|
| 1. Asseburg C, Frank M, Kohne CH, Hartmann JT, Griebsch I, Mohr A, et al. Cost-effectiveness of targeted therapy with cetuximab in patients with K-ras wild-type colorectal cancer presenting with initially unresectable metastases limited to the liver in a German setting. Clin Ther 2011;33:482–97 | Population KRAS WT only |
| 2. Cassidy J, Saltz L, Twelves C, Van Cutsem E, Hoff P, Kang Y, et al. Efficacy of capecitabine versus 5-fluorouracil in colorectal and gastric cancers: a meta-analysis of individual data from 6171 patients. Ann Oncol 2011;22:2604–9 | Mix of 5-FU therapies vs. capecitabine |
| 3. Ling W, Fan J, Ma Y, Ma Y, Wang H. Capecitabine-based chemotherapy for metastatic colorectal cancer. J Cancer Res Clin Oncol 2011;137:927–38 | Mix of 5-FU therapies vs. capecitabine |
| 4. Zhang C, Gu H, Zhu D, Li Y, Zhu P, Wang Y, Wang J. Capecitabine plus oxaliplatin compared with 5-fluorouracil plus oxaliplatin in metastatic colorectal cancer: meta-analysis of randomized controlled trials. Oncol Letters 2012;3:831–8 | Mix of 5-FU therapies vs. capecitabine |
Appendix 9 Clinical trials identified from ClinicalTrials.gov, Current Controlled Trials, UK Clinical Research Network portfolio databases and Saladax
Ongoing
Retrospective Evaluation of 5-FU Exposure Optimisation in CRC Patients (5-FU RECORD). NCT02055560. URL: http://clinicaltrials.gov/show/NCT02055560
A Single Arm Study in Metastatic Colorectal Cancer Patients Treated with Pharmacokinetically (PK) Dose Adjusted Weekly or Biweekly 5-Fluorouracil (5-FU) Regimes – C-5FU-TDM (CESARC-II-009). EUCTR2011–003553–26-DE. URL: www.clinicaltrialsregister.eu/ctr-search/search?query=2011-003553-26
The Optimisation of 5-Fluorouracil Dose by Pharmacokinetic Monitoring in Asian Patients With Advanced Stage Cancer. NCT00943137. URL: http://clinicaltrials.gov/show/NCT00943137
Terminated
Study Comparing Optimised 5-FU Dosing and Standard Dosing in Metastatic Colorectal Cancer Patients Treated With mFOLFOX6 (PROFUSE). NCT01468623. URL: http://clinicaltrials.gov/show/NCT01468623
Appendix 10 Narrative overview of single-arm studies and included abstracts
Single-arm studies for clinical effectiveness review by cancer type
Colorectal cancer
Boisdron-Celle et al. 133 investigated in a case series the influence of oxaliplatin on plasma 5-FU levels by adding it to weekly FUFOL treatment in patients with 5-FU refractory advanced CRC. The main finding was that while (1) oxaliplatin reduces 5-FU plasma clearance and (2) higher plasma concentrations of 5-FU are related to toxicity, oxaliplatin does not seem to potentiate 5-FU toxicity. 5-FU-related toxicities were presented and positively linked to 5-FU plasma concentrations. However, the credibility of the conclusion and extent of toxicity reported is questionable due to considerable discrepancies found in the numbers presented in the paper.
Cattel et al. 135 studied the PK behaviour of oxaliplatin in combination with 5-FU in stage IV CRC patients in a prospective case series. The regimen was unusual containing of 14-day continuous 5-FU without FA and chronomodulated oxaliplatin at three different doses. Omission of FA did not affect the combination effect of oxaliplatin and 5-FU and PK behaviour was unchanged of both drugs. The focus of this study was on oxaliplatin rather than 5-FU therefore the two cases of neurotoxicity were related to oxaliplatin which prompted the dose reduction of oxaliplatin. The regimen is unusual and therefore the results are of limited interest.
Duffour et al. 136 compared, in a retrospective database analysis, the clinical outcomes of PK dose adjustment in two different age groups (age < 65 years or ≥ 65 years) of mCRC patients and concluded that age does not affect the PK behaviour of 5-FU and does not limit intensified chemotherapy. This study included data from two other included studies (Ychou et al. 131,147) and did not specify how many patients had what dose increase. The first two cycles AUC values from the low toxic (grades 0–II) and severe toxic (≥ grade III) groups did not seem to suggest a significant association between AUC and severe toxicity. However, a significant association seems to be existent between cycle number (< 6/≥ 6) and severe toxicity (p = 0.0005).
Findley et al. 137 measured plasma 5-FU levels and 5-FU levels in liver tissue. They investigated, using a case series, the metabolism of 5-FU with and without interferon-α in patients with CRC. They concluded that protracted venous infusion may result in greater interpatient variation of plasma 5-FU levels and addition of interferon-α to 5-FU increases plasma 5-FU levels. Levels of 5-FU correlated with treatment toxicity but not with antitumour activity. However, protracted venous infusion was not specified (i.e. it was unclear how long patients had received 5-FU for and when plasma measurements were taken). The study population was very small and poorly characterised because they were merged from two different studies, therefore it is likely to be retrospective. The grading tool used for toxicity and response was not reported. The link between plasma concentration and outcomes is unlikely to be useful since an arbitrary split of the data was used to compare the data on response/toxicity between two sets of participants with different plasma concentrations (> 5 nM and < 5 nM).
Gamelin et al. 130 compared the relationship between 5-FU dose intensity and therapeutic response in patients with advanced CRC in a prospective case series and found a wide variation of 5-FU metabolism, whatever the dose, and a clear relationship between 5-FU levels and toxicity and efficacy. This is a thorough study, reporting the relationship between 5-FU plasma levels and outcomes extensively in order to produce a dose adjustment algorithm that is based on previous 5-FU dose and 5-FU plasma levels. The dose adaptation algorithm is based on a regression analysis of the relationship between dose and plasma levels in two groups of patients achieving CR or PR versus minimal response, SD or PD.
Ho et al. 139 carried out a prospective case series to determine the maximum tolerated dose and dose-limiting toxicity of FUFOL with modified trimonthly 48-hour continuous infusion of high-dose 5-FU/FA (HDFL48) in patients with mCRC. They recommended a 5-FU dose for future trials of 3500 mg/m2/48 hours/week with a fixed dose of FA of 300 mg/m2/48 hours/week. This study used an unusual HDFL48 regimen. In contradiction to other studies, toxicities did not seem to correlate with high plasma concentrations.
Jodrell et al. 140 assessed the variability in 5-FU concentrations at steady state during protracted venous infusion of 5-FU in CRC patients and attempted to correlate response and toxicity with the 5-FU steady state concentrations in a prospective case series. Due to the lack of a relationship between mean 5-FU plasma concentration and outcomes, the study discouraged the use of 5-FU measurements to individualise dosing in patients with protracted venous infusion 5-FU for advanced CRC. The lack of a correlation between plasma concentrations and outcomes is inconsistent with other studies; however, the dose regimen is unusual. Outcomes were reported with varying rigour.
Kline et al. 141 carried out a case series to identify novel and heterogeneous patterns in 5 FU levels, at baseline and during dose adjustment of 5 FU infusions in CRC patients. Based on the findings they call for further studies to investigate physiological and or genetic differences underlying heterogeneity in 5-FU levels during dose optimisation. This paper and the comparative study by Kline and El-Deiry223 are the only two full papers that used My5-FU for dose adjustment. The paper describes AUCs and adjustments well, but does not report overall results needed for data extraction such as mean 5-FU dose and frequency of dose adjustment. Furthermore, the paper fails to specify the adjustment rules for toxicity and AUC values. The results are not linked with any patient outcomes which is why the results are of limited value.
Metzger et al. 142 analysed, in a randomised trial of nine patients, the circadian change kinetics of anticancer drugs infused at a constant rate versus circadian rate and concluded that patients with circadian rhythms in 5-FU concentrations were sensitive to 5-FU-related toxicity. Chronomodulated 5-FU exposure may permit dose escalation. However, the patient numbers were too small to allow generalisation of conclusions. AEs were reported inconsistently and only stomatitis results were linked to AUC values in all nine patients.
Milano et al. 143 carried out a prospective case series to compare drug dose and individual PK data for their respective ability to predict cycle tolerance for the 5-day continuous infusion schedule in advanced CRC. They reported an AUC threshold of 30,000 ng/ml × hour that can be used to predict toxicity using the 5-day continuous infusion schedule of 5-FU.
Stremetzne et al. 146 compared PKs/pharmacodynamics of two doses of calcium folinate in a randomised trial with random allocation to two different calcium folate concentrations and did not find a significant effect of folinate dose or the day of treatment on PKs of 5-FU in advanced CRC patients. The study aimed to evaluate two different doses of folinate, therefore the results are of limited usefulness. High incidence of mucositis might be related to high-dose folinate and less dependent on 5-FU levels.
Ychou et al. 131 applied the concept of 5-FU dose adaptation, using PK parameters, in a prospective case series to the bimonthly LV5FU2 schedule in advanced CRC and established a dose adaptation strategy with a control for toxicity. The adjustment algorithm was solely based on AUC value from first cycle (and toxicity < grade III). The relationship between plasma 5-FU and response is minimal and not significant. Toxicities were grouped in three categories: digestive, hematologic and cutaneous, and only reported for grades III and IV.
Ychou et al. 147 determined the efficacy and tolerability of the pharmacokinetically-adjusted LV5FU2 regimen in the treatment of mCRCs in a prospective case series and concluded that the promising results should be confirmed in a subsequent Phase III trial. Patients who did not attain an AUC of at least 15 mg × hour/l × m2 in cycle 2 had an unfavourable PFS as compared with the other patients. This was a reasonable thorough trial that reports AEs extensively. However, Kaplan–Meier survival functions were not included with the survival data and the relationship between AUC values and outcomes were not reported extensively.
Yoshida et al. 148 aimed to clarify in a prospective case series whether the dose of 5-FU is related to tumour response and/or toxicity in advanced CRC patients. They cautioned that increased serum concentrations do not always provide therapeutic benefits to patients receiving continuous infusion of 5-FU. However, the author’s conclusion is based on a small study sample of 19 patients. They found a significant correlation between plasma concentration/AUC and toxicity but not response. The results presented for AEs and response were not useful as toxicity was not reported by type of toxicity and non-responders were not divided into SD and PD.
Mixed-patient group
Ciccolini et al. 149 aimed to validate a simple and rapid method to determine the DPD status of cancer patients presenting with severe toxicities following 5-FU treatment in a prospective case series. They concluded that systematic detection of DPD-deficient patients prior to 5-FU administration is warranted. The results seem to suggest that DPD activity and plasma 5-FU concentration will not reliably identify all toxic cases; however, plasma concentration was only measured in six patients with severe toxicity (grades III and IV only) and, therefore, the conclusions are weak.
Hendrayana et al. 150 explored prospectively in a case series of cancer patients the feasibility of measuring plasma concentrations of 5-FU using My5-FU in routine clinical practice and concluded that (1) BSA dosing results in a large variability in 5-FU exposure and fails to achieve the target exposure in more than 90% of patients and (2) dose adjustment might reduce incidence of diarrhoea and mucositis. This useful study was presented as an extended abstract only and lacks detail on patient characteristics and shows inconsistency in detail of reporting of AEs.
Head and neck cancer
Etienne et al. 151 evaluated in a prospective cohort study the incidence of complete and partial DPD deficiency in patients with H&N cancer and concluded that DPD deficiency is rare and that knowledge of DPD status before chemotherapy might not improve the 5-FU dose adaptation strategy, but might be suggestive of reducing the starting dose in these patients to avoid severe toxicities. The study is of limited interest to the study objective because of the focus on DPD deficiency. Dose adjustment rules and algorithms were not reported either for plasma concentrations or for toxicity. Toxicities were only reported as mucositis or hematologic toxicities. Toxicity values for toxic and non-toxic values were suggestive of a positive association between AUC and toxicity.
Milano et al. 152 analysed the link between systemic exposure of 5-FU and tumour response and OS in patients with H&N cancer in a prospective case series. They established a positive relationship between plasma exposure and outcomes in terms of response and OS, and are supportive of individual 5-FU dose adaptation based on PKs. This is a useful study reporting OS with a Kaplan–Meier function. An AUC threshold of 29,000 ng/ml × hour was reported for response. However, responses were grouped differently than in other studies and details on AEs were insufficient (i.e. reported as haematological and digestive).
Thyss et al. 153 compared patient response and toxicity with individual total 5-FU exposure during treatment with PF in patients with H&N cancer. This prospective case series demonstrated a close relationship between elevated 5-FU AUC values and the frequency of cycles in which signs of toxicity were observed. The study presented an AUC threshold of 30,000 ng/ml × hour to be predictive for toxicity. The reporting of AEs was not useful for the review because they were not reported by type of toxicity. AUC values for cycles were only presented in graphical form and there was no mean AUC per cycle reported.
Gastric cancer
Kim et al. 154 analysed, in a case series, the clinical efficacy of a protracted infusion of low-dose 5-FU in combination with cisplatin in the treatment of gastric cancer patients. They considered this to be a useful regimen for patients with advanced gastric cancer pointing out the high response rate and low toxicity and positive results in terms of OS. The lack of a relationship between plasma concentration and response was based on seven patients; however, details of how the seven patients were selected was not reported. This study considered QoL in terms of improvement in performance status and improved oral intake both showing a positive trend following treatment.
Included abstracts for the clinical effectiveness review investigating dose adjustment following My5-FU measurement
Patel et al.144
The aim of this study was to investigate the application and feasibility of PK-guided FU with 58 patients with CRC from six academic and community sites and provide an assessment of toxicity. The study design was unclear. Patients received mFOLFOX6 (5-FU 2400 mg/m2 over 46 hours every 2 weeks) with or without bevacizumab. An algorithm to target an AUC of 20–25 mg × hour/l was used to adjust 5-FU doses for cycles 2–4. My5-FU was used to determine the AUCs and peripheral blood was obtained 2–46 hours after the beginning of infusion. This study found the mean AUC post cycle 1 in 39 patients was 19.8 ± 6.3 mg × hour/l with 18% over the AUC target, 31% within, and 51% under. According to cycle 1 findings, the mean dose to achieve AUC 20–25 mg × hour/l was estimated to be 2505 ± 304 mg/m2. Nineteen patients were not included due to logistical problems and three hospitalisations following serious AEs occurred (two at AUCs > 30). Table 77 shows the most common AEs was fatigue and diarrhoea for all patients, with neutropenia being the most common AE for grade III/IV. In conclusion, there was significant heterogeneity noted in 5-FU AUC with BSA-based dosing, with the majority of patients below the 20–25 mg × hour//l AUC threshold.
| AE | All grades, n (%) | Grade III/IV, n (%) |
|---|---|---|
| Diarrhoea | 21 (38) | 4 (7) |
| Fatigue | 24 (44) | 2 (4) |
| Mucositis/stomatitis | 11 (20) | 2 (4) |
| Neutropenia | 19 (35) | 15 (27) |
Patel et al.145
In this second study by Patel et al. ,145 the aim was to investigate the application of PK-guided 5-FU in clinical practice. The study design was unclear. Seventy CRC patients from six academic and community sites received mFOLFOX6 (5-FU 2400 mg/m2 over 46 hours every 2 weeks) with or without bevacizumab. An algorithm to target an AUC of 20–25 mg × hour/l was used to adjust 5-FU doses for cycles 2–4. My5-FU was used to determine the AUCs and peripheral blood was obtained 2–44 hours after the beginning of infusion. The primary outcome was the percentage of patients within target AUC by cycle 4 and the secondary outcome was toxicity rates compared with historical data. The percentage of patients within target AUC post cycle 1 and cycle 4 was 30% (95% CI 18% to 43%) and 46% (95% CI 32% to 61%) respectively (odds ratio = 2.16; p = 0.05). For each subsequent cycle, the likelihood of a patients being within target range increased by 28% (p = 0.04). See Table 78 for details of patients below, within and above the target AUC at cycles 1–4. The median dose needed to achieve target AUC at C4 was 2580 mg/m2. There were fewer grade III/IV mucositis and diarrhoea seen compared with historical data (3% vs. 15% and 6% vs. 12%, respectively), but no difference in grade III/IV neutropenia (27% vs. 33%) compared with historical data. Nine patients were not included due to sampling/processing errors. Overall PK-guided 5-FU resulted in more patients achieving the targeted AUC and fewer patients underdosed at C4 compared with C1.
| AUC mg × hour/l | C1 (n = 57), n (%) | C2 (n = 57), n (%) | C3 (n = 53), n (%) | C4 (n = 52), n (%) |
|---|---|---|---|---|
| < 20 | 30 (53) | 24 (42) | 25 (47) | 17 (33) |
| 20–25 | 17 (30) | 21 (37) | 21 (40) | 24 (46) |
| > 25 | 10 (17) | 12 (21) | 7 (13) | 11 (21) |
Summary
In summary these two studies by Patel et al. 144,145 demonstrate that individualisation of FU dosing in the frontline, in both community and academic settings, appears to be achievable for the treatment of CRC. Due to a paucity of data on PK-guided FU dosing in the clinical setting, a large prospective clinical trial is needed to define the clinical utility of PK-guided FU and more specifically to confirm the promising findings reported to date about dose adjustment following My5-FU measurement with CRC patients.
Appendix 11 Formal quality assessment using an adapted Downs and Black assessment tool123
Table 79 is a summary of the quality assessment for the three CRC comparative studies,118,119,155 the two CRC single-arm studies134,138 and two H&N cancer comparative studies. 132,156,158 All the studies described the main outcomes to be measured clearly in the introduction or methods and used appropriate statistical tests to assess the main outcomes. In contrast, none of the studies provided sufficient information on the staff, places and facilities where the patients were treated to allow assessment of whether or not the patients and their treatments were representative of underlying populations. Furthermore, none of the studies made an attempt to blind those measuring the main outcomes of the intervention. Overall the quality of these studies varied.
| First author, year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Q17 | Q18 | Q19 | Q20 | Q21 | Q22 | Q23 | Q24 | Q25 | Q26 | Q27 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRC comparative studies | |||||||||||||||||||||||||||
| Capitain, 2012119 | Y | Y | N | Y | P | Y | Y | Y | Y | N | UD | UD | UD | NA | N | Y | Y | Y | Y | Y | N | UD | N | N | N | Y | UD |
| Gamelin, 2008118 | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | UD | UD | UD | N | N | Y | Y | Y | Y | Y | Y | Y | Y | UD | Y | Y | Y |
| Kline, 2013155 | Y | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | UD | NA | N | Y | Y | Y | Y | Y | Y | Y | N | N | UD | UD | UD |
| CRC single-arm studies | |||||||||||||||||||||||||||
| Capitain, 2008134 | Y | Y | Y | N | Y | N | Y | Y | Y | Y | UD | UD | UD | NA | N | Y | Y | Y | Y | Y | UD | NA | NA | NA | N | Y | UD |
| Gamelin, 1998138 | Y | Y | Y | Y | P | Y | Ya | Y | N | Y | UD | UD | UD | NA | N | Y | UD | Y | UD | Y | UD | NA | NA | NA | N | N | UD |
| H&N cancer comparative studies | |||||||||||||||||||||||||||
| Fety, 1998,156 1994158 | Y | Y | Y | Y | P | Y | Y | Y | Y | Y | UD | UD | UD | N | N | Y | N | Y | Y | N | Y | Y | Y | UD | N | Y | Y |
| Santini, 1989132 | N | Y | N | Y | N | N | Y | N | Y | N | UD | UD | UD | NA | N | N | UD | Y | Y | N | Y | Y | N | N | Y | Y | UD |
| Overall ratings | |||||||||||||||||||||||||||
| Y | 6 | 7 | 5 | 6 | 3 | 4 | 5 | 6 | 5 | 5 | 1 | 1 | 0 | 0 | 0 | 6 | 4 | 7 | 6 | 5 | 4 | 4 | 2 | 0 | 2 | 5 | 2 |
| N | 1 | 0 | 2 | 1 | 1 | 3 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 2 | 7 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 3 | 3 | 4 | 1 | 0 |
| UD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 6 | 7 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 5 |
| NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 0 |
| P | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Appendix 12 Summary of two comparative head and neck cancer studies
Fety et al.156
Study design
This multicentre RCT assigned 61 patients to a PK-adjusted 5-FU regimen and 61 patients to a standard dose regimen. Patients were assigned to receive induction chemotherapy with cisplatin (100 mg/m2 on day 1) and 5-FU (96-hour continuous infusion), either at standard dose (BSA arm; 4 g/m2) or at a dose adjusted according to the 5-FU AUC (AUC0–48 hours; PK arm). Due to 5-FU-related toxicity, three patients (5.2%) in the BSA arm and three patients in the PK arm (6.1%) left the study before completing chemotherapy. The length of follow-up was unclear. The primary end point was the incidence of haematological toxicity and the secondary end point was the equivalence of disease response.
Study quality
Randomisation was stratified by centre (three centres were involved). Methods of allocation concealment were not reported. Blinding to treatment was not possible; assessment of response rates was assessed by a panel of two independent radiologists and may have been blinded, but this was not specified. There was some mismatch between the description of methods undertaken and the reported results. There were weaknesses in the clarity and presentation of data. QoL, OS and performance status were not reported. AE were reported per cycle (counts). It has been previously noted by other authors85 that the dose adjustment method in this study may have been too complicated, as the 12 protocol violations in the treatment arm (12/61 patients enrolled) were all related to 5-FU dose adjustment miscalculations. Furthermore, as the patients with protocol violations were removed from the analysis and the induction therapy regimen used only two drugs, the generalisability to dose adjustment methods in current clinical practice remains questionable. For formal quality assessment see Appendix 11.
Population
The reported demographic characteristics are summarised in Table 80. Patients had advanced H&N cancer and most had not received previous chemotherapy. Among the 122 patients randomly assigned to one of the two treatment arms, 16 patients (13%) were found to be unevaluable for response and toxicity (4 patients in the BSA arm and 12 patients in the PK arm).
| Item | Treatment arm | |
|---|---|---|
| BSA | PK | |
| Patient number, n/N (%) | ||
| Total number | 61/122 (50) | 61/122 (50) |
| Sample attrition/patients not evaluable | 4/61 (6.6) | 12/61 (19.7) |
| Age (years) | ||
| Mean (s.d.) | NR | NR |
| Median | 54 | 55 |
| Range | 29–72 | 36–69 |
| Sex, n/N (%) | ||
| Men | 52/57 (91.2) | 48/49 (98) |
| Women | 5/57 (8.8) | 1/49 (2) |
| Performance status, n/N (%) | ||
| 0 | 16/57 (28.1) | 11/49 (22.4) |
| 1 | 34/57 (59.6) | 35/49 (71.4) |
| 2 | 7/57 (12.3) | 3/49 (6.1) |
| 3 | 0 | 0 |
| 4 | 0 | 0 |
| Previous therapy, % | NR | NR |
| Metastatic sites, % | ||
| Liver | NA | NA |
| Lung | NA | NA |
| Lymph nodes | NA | NA |
| Others | NA | NA |
Summary
The paper by Fety et al. 156,158 provides useful information in a randomised design on 5-FU dose adaptation according to PK parameters versus conventional dosing in patients with advanced H&N cancer. The overall 5-FU exposure in H&N cancer patients was significantly reduced in the dose adjustment arm compared with the fixed-dose arm. This resulted in reduced toxicity, but no improvement in clinical response. The impact on toxicity and efficacy suggests these patients might benefit from individual PK monitoring. The utility of monitoring 5-FU exposure to reduce toxicity was confirmed. It was noted that no link was found between PKs and mucositis. As for tumour response, no difference in 5-FU exposure was observed between patients who achieved a CR or PR and patients who had SD or progression. This finding was not consistent with previous studies132,161 which reported that response and survival were significantly associated with high plasma concentrations in patients with H&N cancer. However, the findings from the study by Fety et al. 156,158 should be treated with caution as the methods and overall results were poorly presented. Fety et al. 156,158 also reported that the costs associated with toxicity were considerably reduced for patients receiving 5-FU by a dose managed approach (US$6803) when compared with those treated with standard 5-FU dosing (at US$21,758 this represents approximately 70% reduction in medical costs).
Santini et al.132
Study design
This study involved several study designs at one centre in France. Group 1 (89 patients, 228 cycles) corresponded to a retrospective study during which 5-FU blood concentrations were measured for each individual cycle of 77 patients (177 cycles), which allowed comparison of the distribution of AUC values in relation to response and tolerance to treatment. Group 2 (81 patients, 249 cycles) corresponded to patients entered into a prospective study based on initial data for group 1. For all patients and all cycles of group 2 the AUC0–3 days value was used to determine the extent of reduction of the 5-FU dose for the second half of the cycle. Treatment involved the following. Day 0: 6-hour hydration with 5% dextrose (2 l), sodium chloride (6 gl−1) and potassium chloride (3 gl−1), followed by cis-diamminedichloroplatinum (100 mg m−1), 1 mg minute−1 i.v. in normal saline (0.5 l) with 1.6% mannitol (0.25 l), and then 5% dextrose (1 l), sodium chloride (6 gl−1) and potassium chloride (3 gl−1). Days 1–5: 5-FU 1000 mg m−2 24-hours−1 by continuous i.v. infusion with a controlled flow pump. The scheduled protocol called for three courses per patient every 3 weeks. 5-FU PK measurements were taken on day 3 to adjust the dose for the second half of treatment if required. Median follow-up was unclear. The pre-specified primary outcome was unclear although treatment response and toxicity rates were reported.
Study quality
This was a non-randomised study. Inclusion and exclusion criteria were not reported. There was a lack of useful information in the paper due to the poor reporting of both methods and results. Blinding to treatment was not possible. Response was evaluated by the same physician 10 days after completion of the last chemotherapy course, and although they may have been blinded, this was not specified. For formal quality assessment see Appendix 11.
Population
The reported demographic characteristics for each group are poorly presented. The overall mean age was 61 years (range 36–82 years) with 145 males and 25 females participating.
Summary
Santini et al. 132 reported sequential cohorts of patients in whom dose modification was made based on 5-FU exposure. AUC levels > 30 mg × hour/l were associated with the development of toxicity. There was a statistical difference in CR rates between groups 1 and 2 and a statistically significant reduction was observed in the incidence of toxic cycles. However, this was a non-randomised study and differences in the tumour stage of patients among the two cohorts may have explained some of the differences in outcomes reported.
Appendix 13 Gamelin 2008 overall survival parametric models
Pharmacokinetic: information criteria
| Model | Observations | ll(model) | df | AIC | BIC |
|---|---|---|---|---|---|
| Exponential | 100 | –120.817 | 1 | 243.6345 | 246.2397 |
| Weibull | 100 | –100.258 | 2 | 204.5161 | 209.7264 |
| Gompertz | 100 | –104.723 | 2 | 213.4463 | 218.6567 |
| Log-normal | 100 | –99.5674 | 2 | 203.1349 | 208.3452 |
| Log-logistic | 100 | –102.125 | 2 | 208.2502 | 213.4606 |
Body surface area: information criteria
| Model | Observations | ll(model) | df | AIC | BIC |
|---|---|---|---|---|---|
| Exponential | 103 | –126.959 | 1 | 255.9181 | 258.5528 |
| Weibull | 103 | –113.273 | 2 | 230.5459 | 235.8154 |
| Gompertz | 103 | –119.726 | 2 | 243.4518 | 248.7212 |
| Log-normal | 103 | –108.594 | 2 | 221.1878 | 226.4572 |
| Log-logistic | 103 | –111.199 | 2 | 226.3971 | 231.6665 |
Pharmacokinetic: model parameters
| Treatment and outcome | Log-normal | Weibull | Log-logistica | |||
|---|---|---|---|---|---|---|
| Mu | Sigma | Lambda | Gamma | p-value | Gamma | |
| PK OS | 2.937171 | 0.648108 | 0.002698 | 1.827858 | 0.052014 | 2.588279 |
| BSA OS | 2.737613 | 0.694454 | 0.008654 | 1.540663 | 0.06445 | 2.457767 |
Appendix 14 Parametric fits to reconstructed Kaplan–Meier estimates
Fluorouracil and folinic acid regimens
Gamelin 2008118

Gamelin 2008118

Gamelin 1998138

Gamelin 1998138
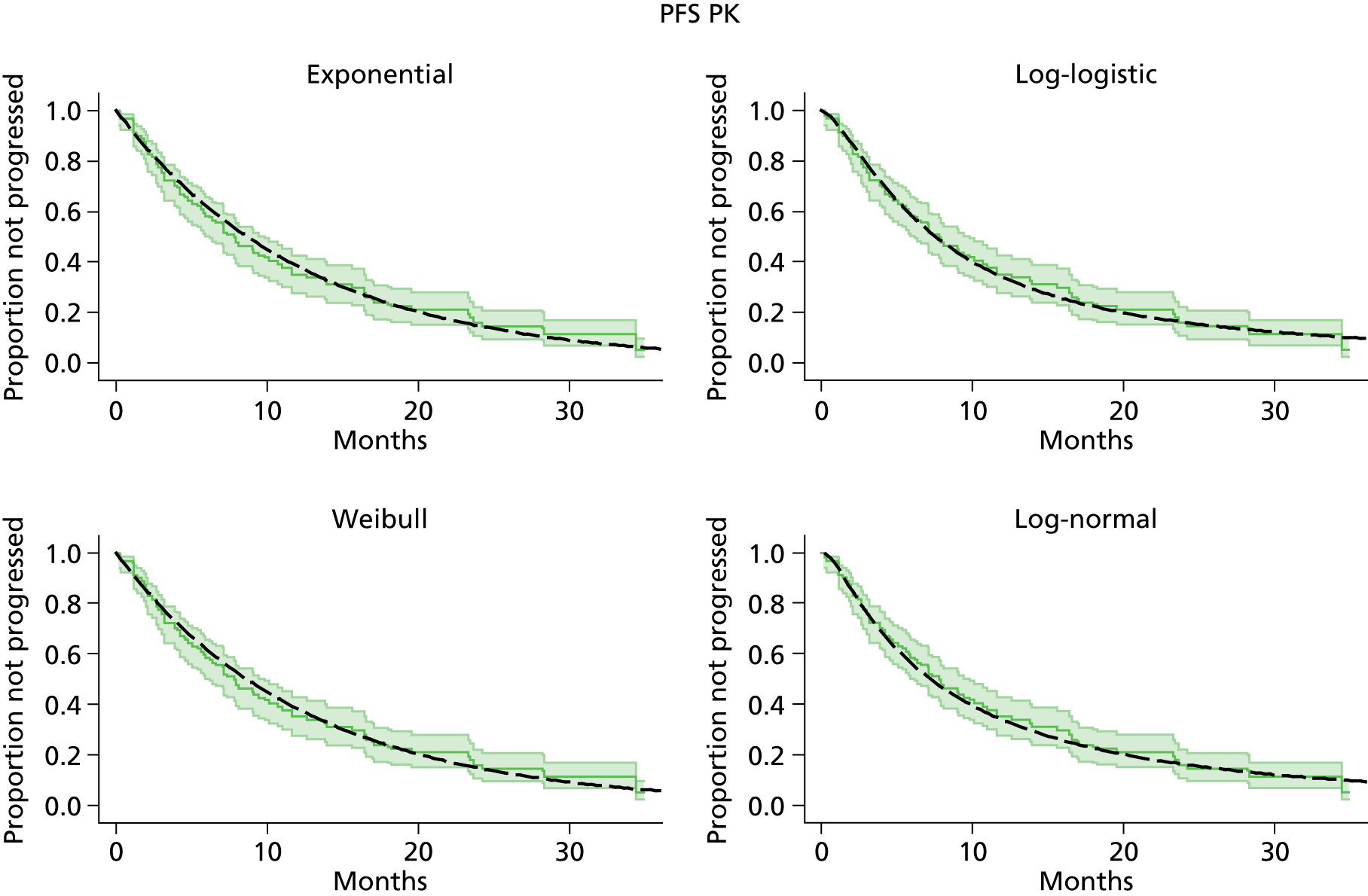
Capitain 2008134
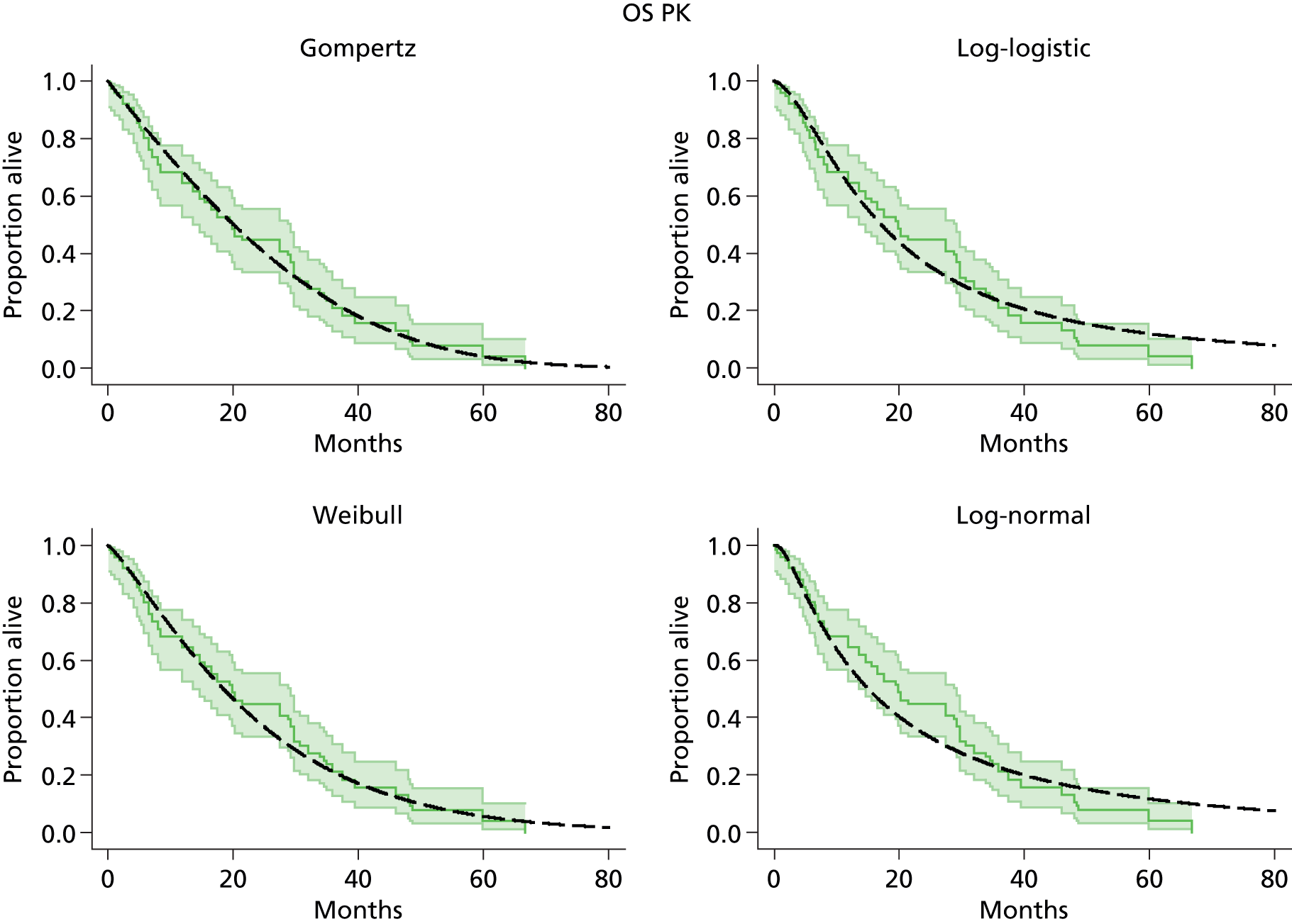
Köhne 2003162

Köhne 2005163

Seymour 2007164
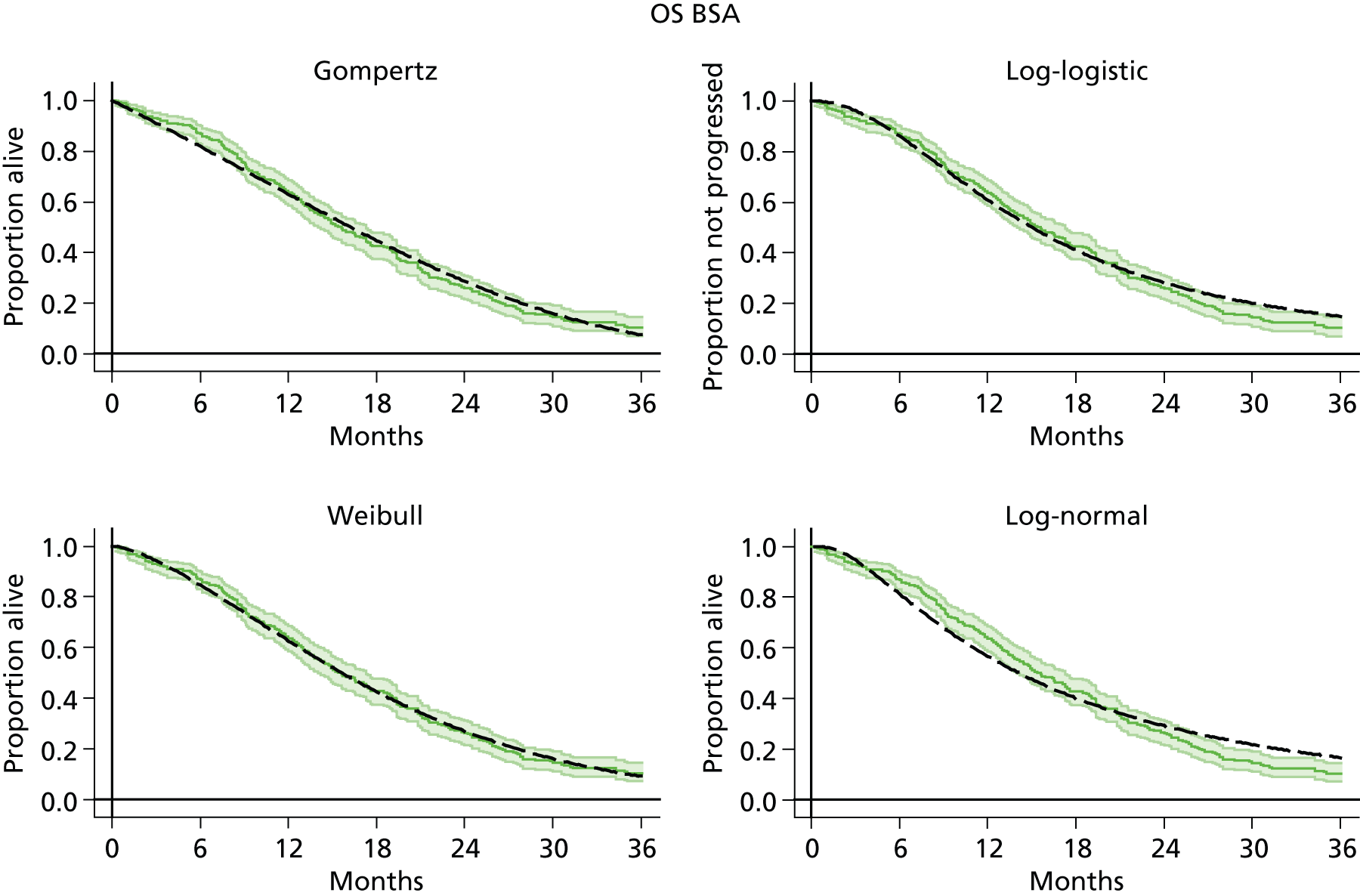
Cunningham 2009165
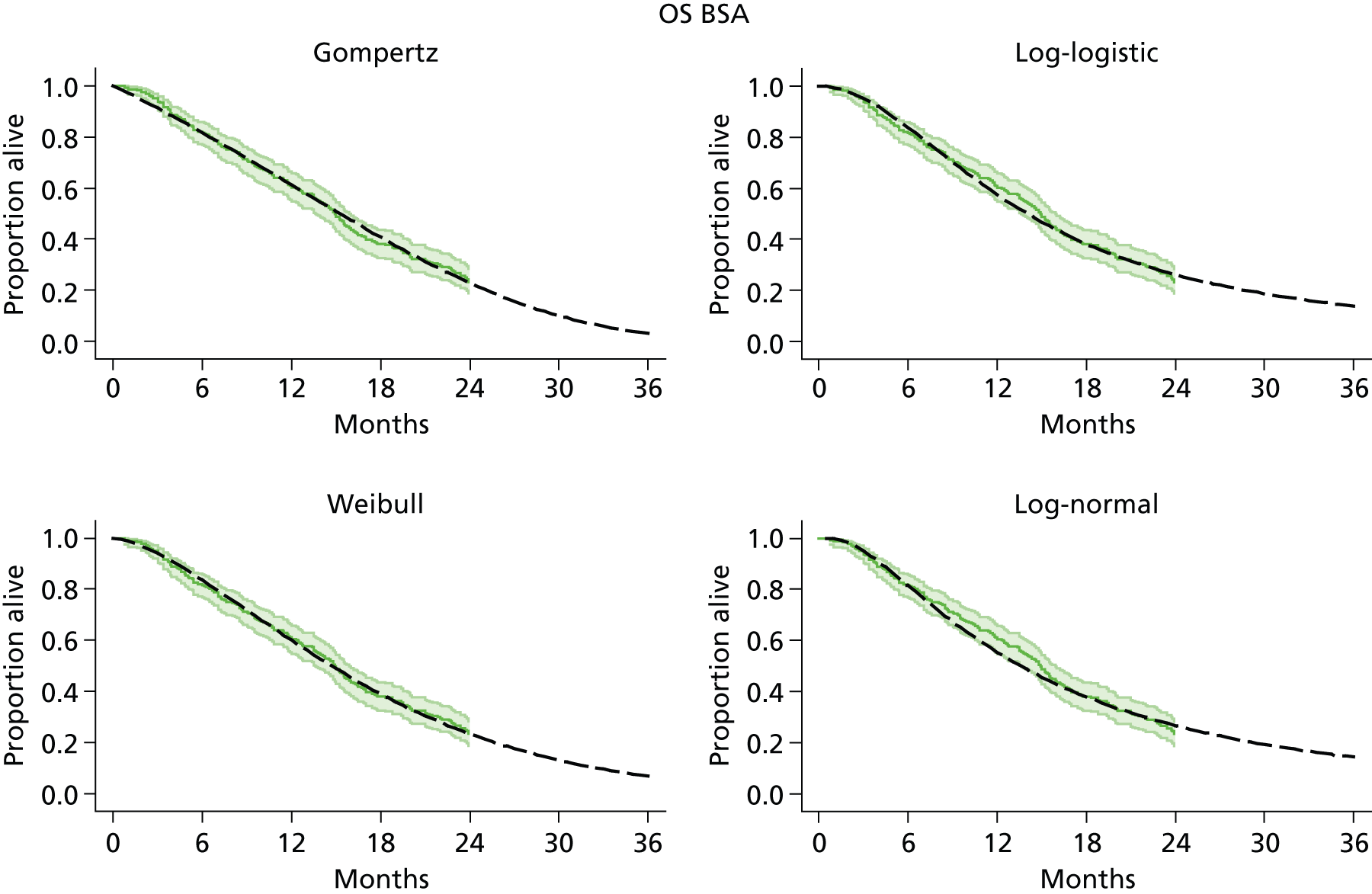
Köhne 2005163
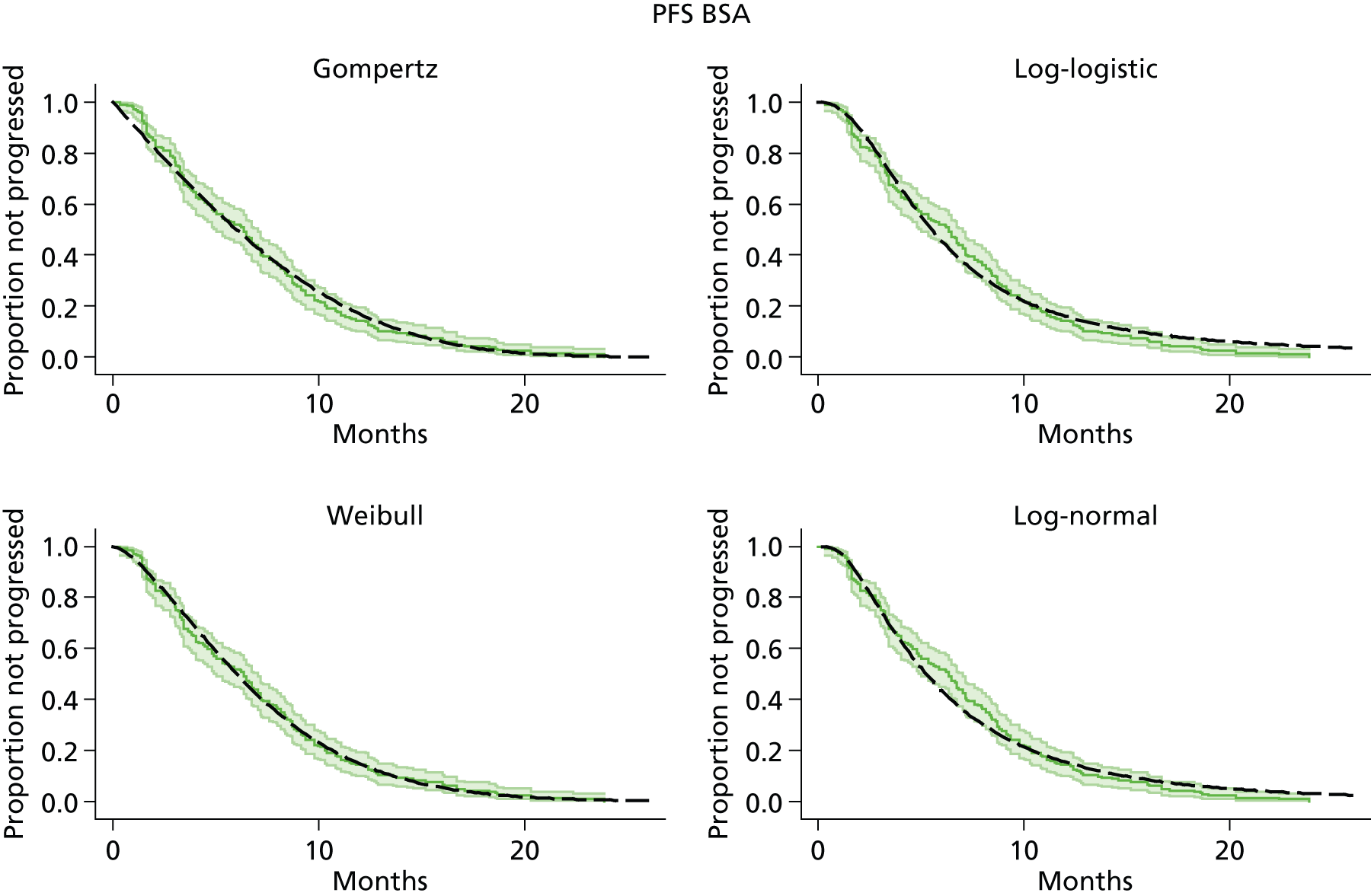
Köhne 2003162

Cunningham 2009165
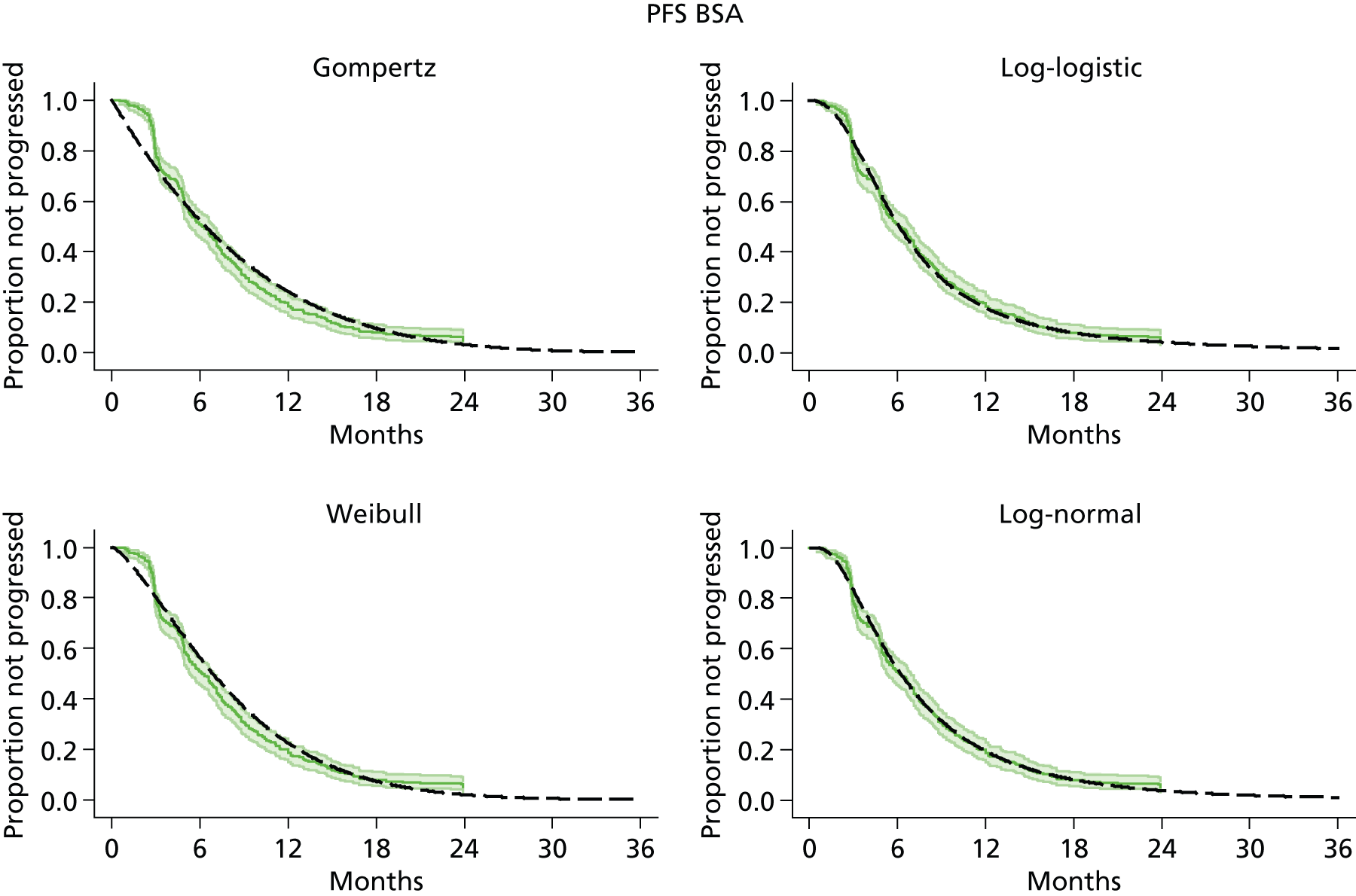
FOLFOX6 regimen
Capitain 2012119

Capitain 2012119
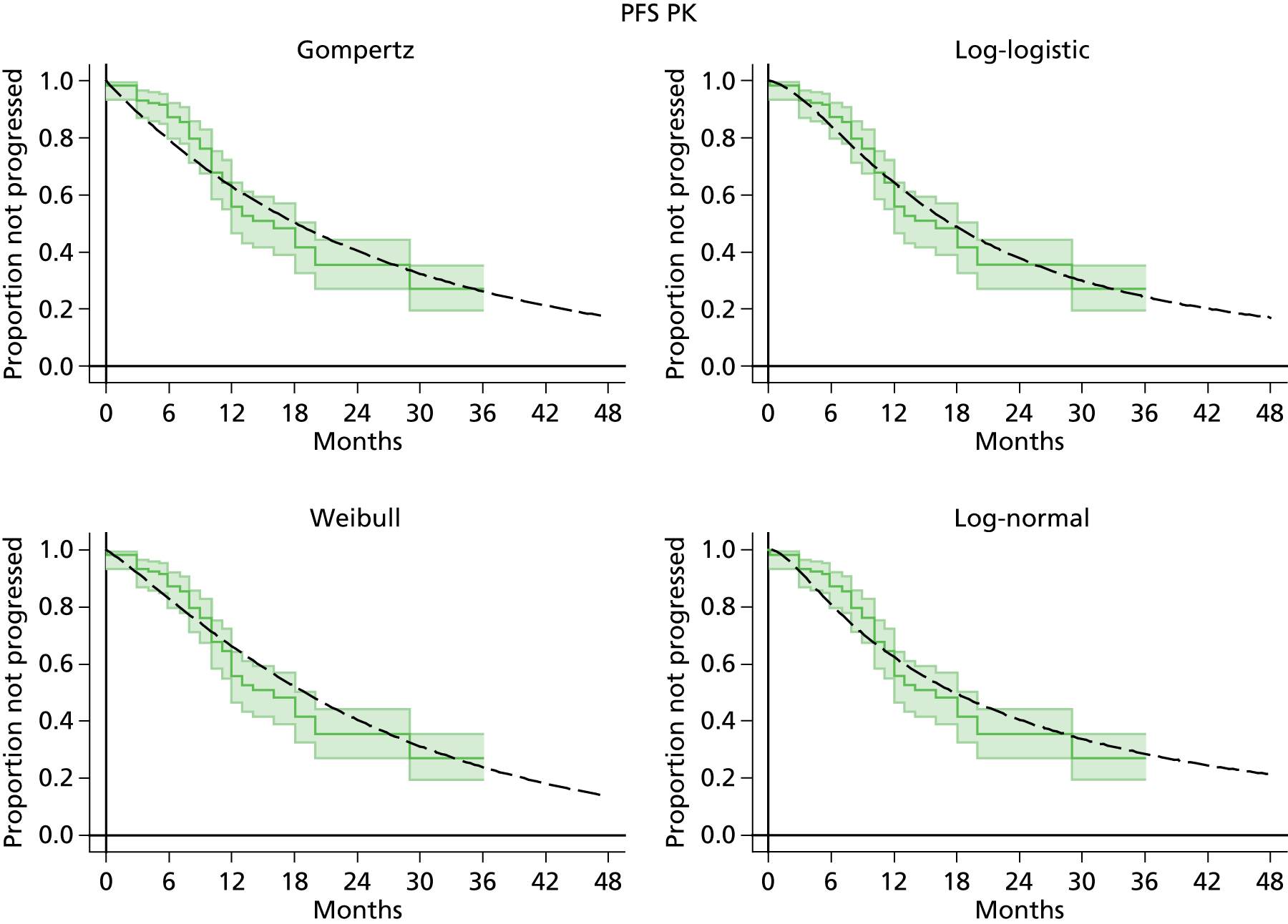
Seymour 2007164
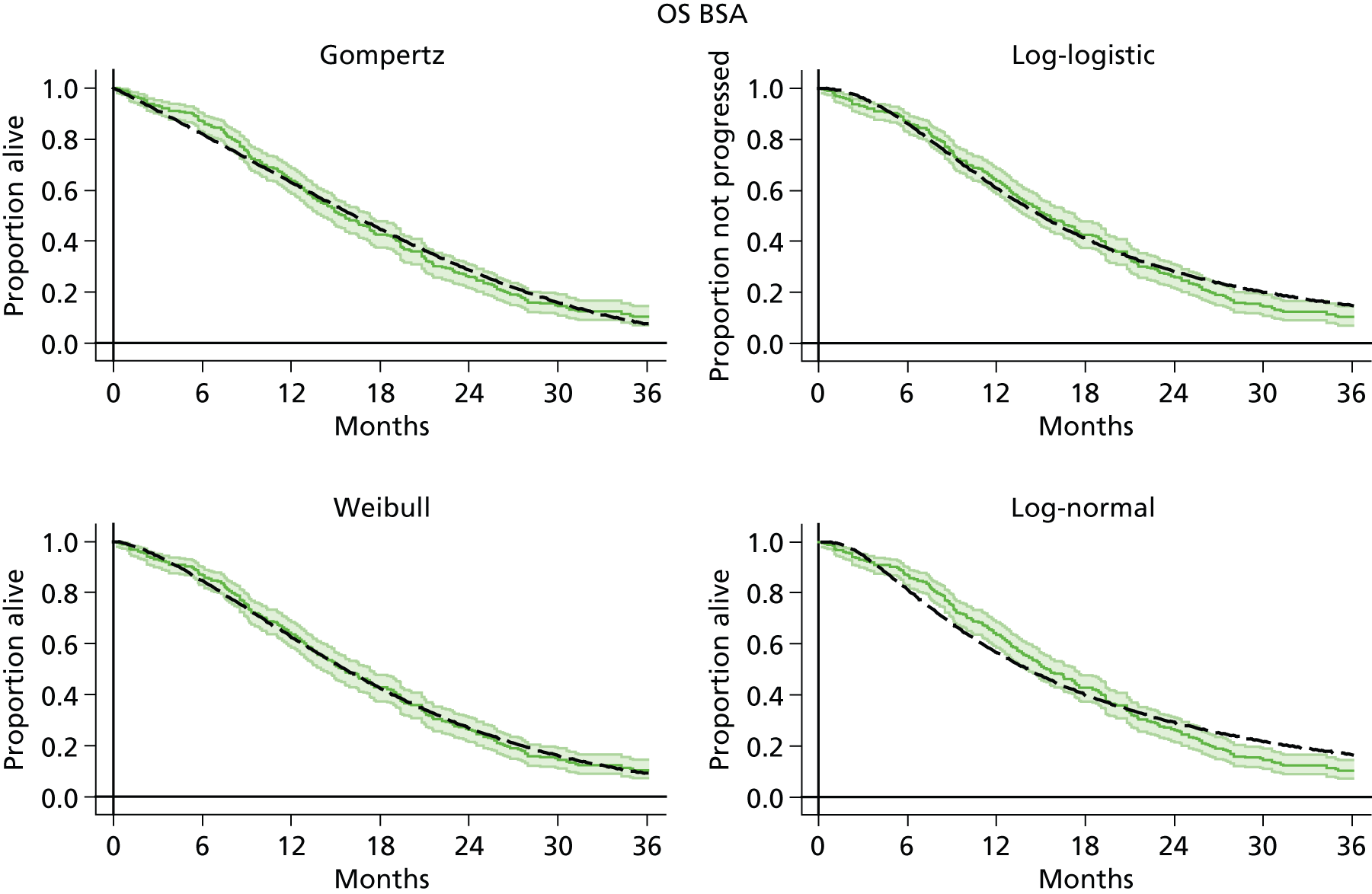
Hochster 2008166

Ducreux 2011167
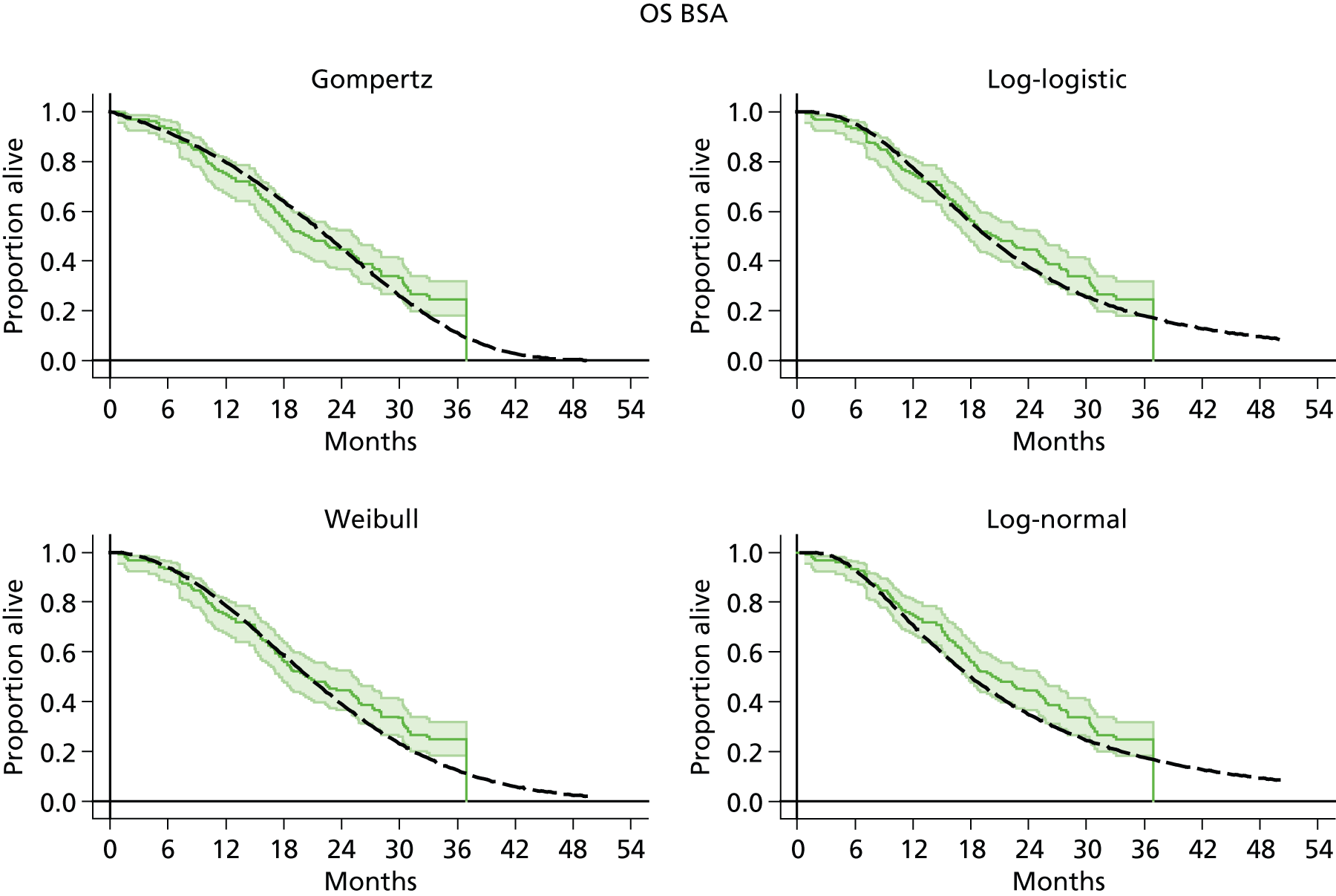
Tournigand 2004168 (FOLFOX first line, FOLFIRI second line)

Madi 2012169 (COIN)

Tournigand 2004168

Madi 2012169 (COIN)
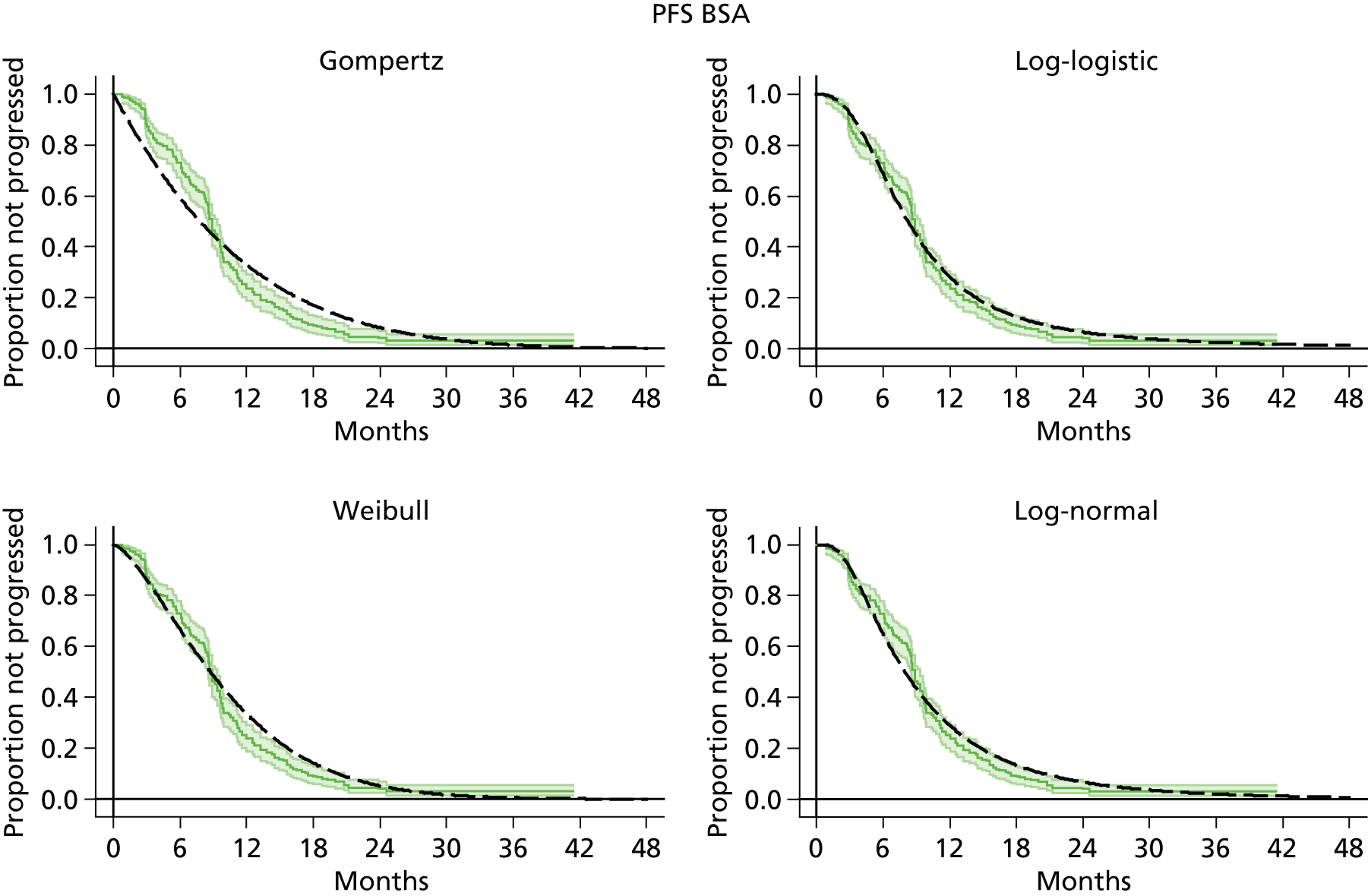
Ducreux 2011167
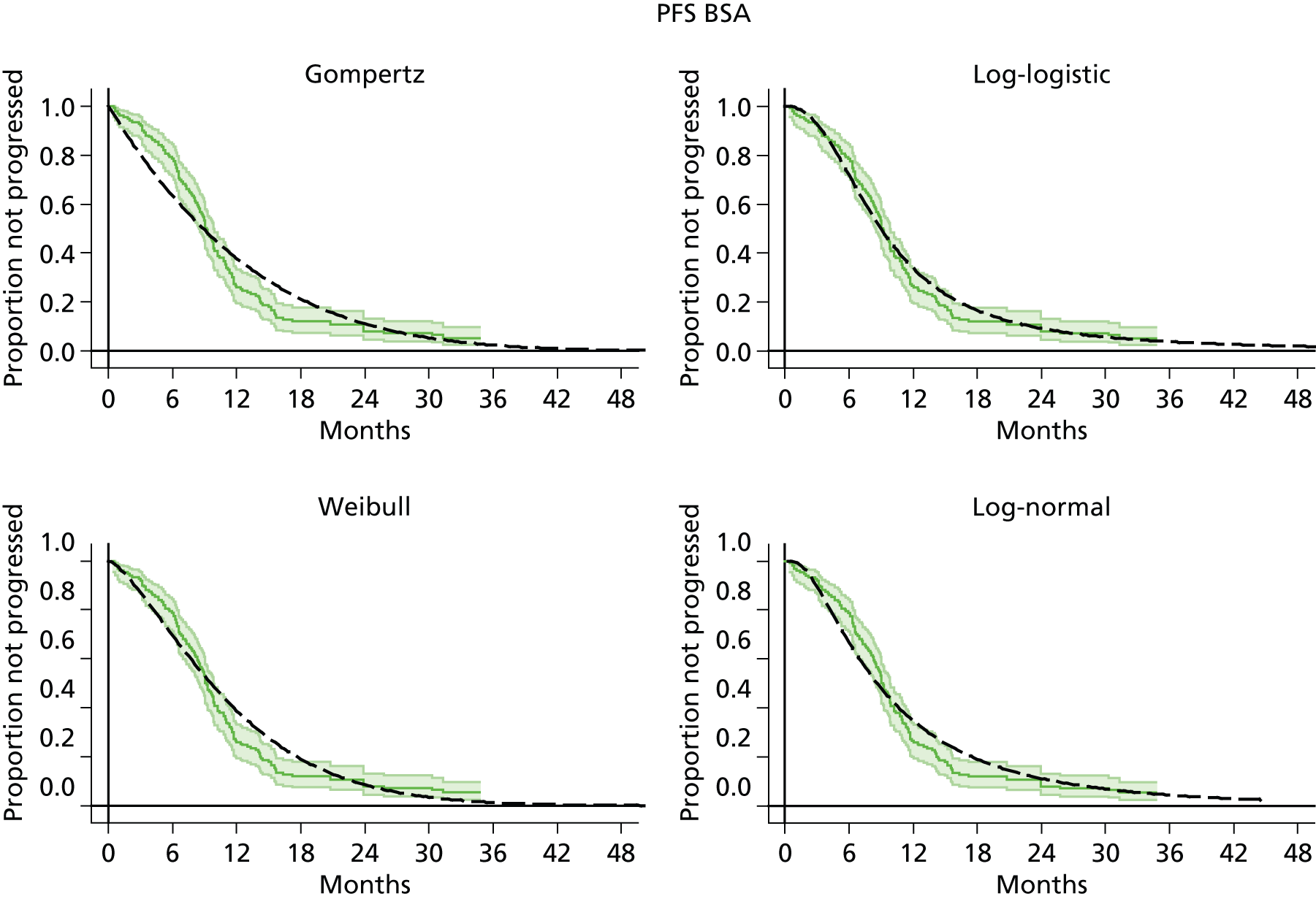
Combined fluorouracil and folinic acid studies
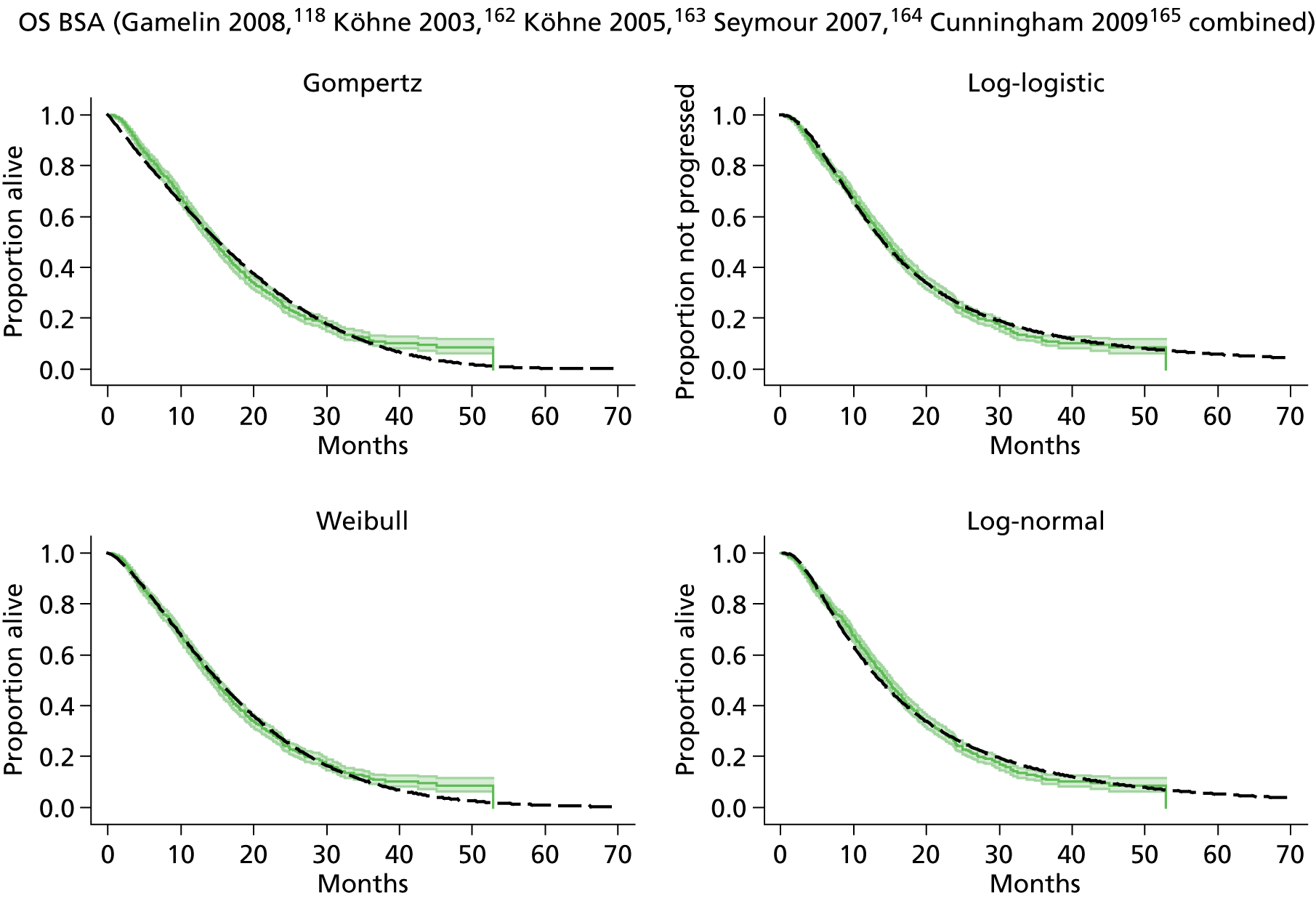

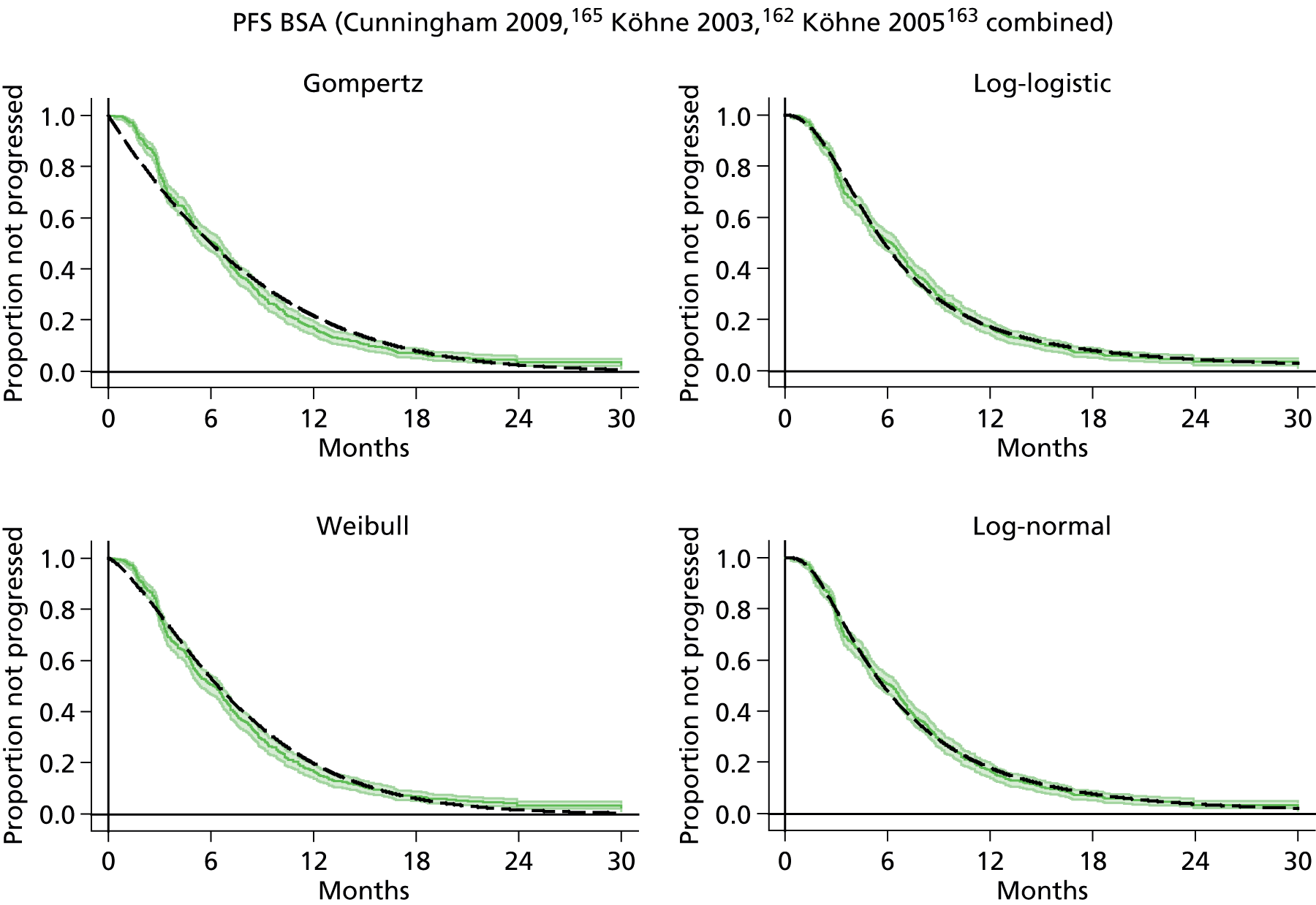
Combined FOLFOX6 studies
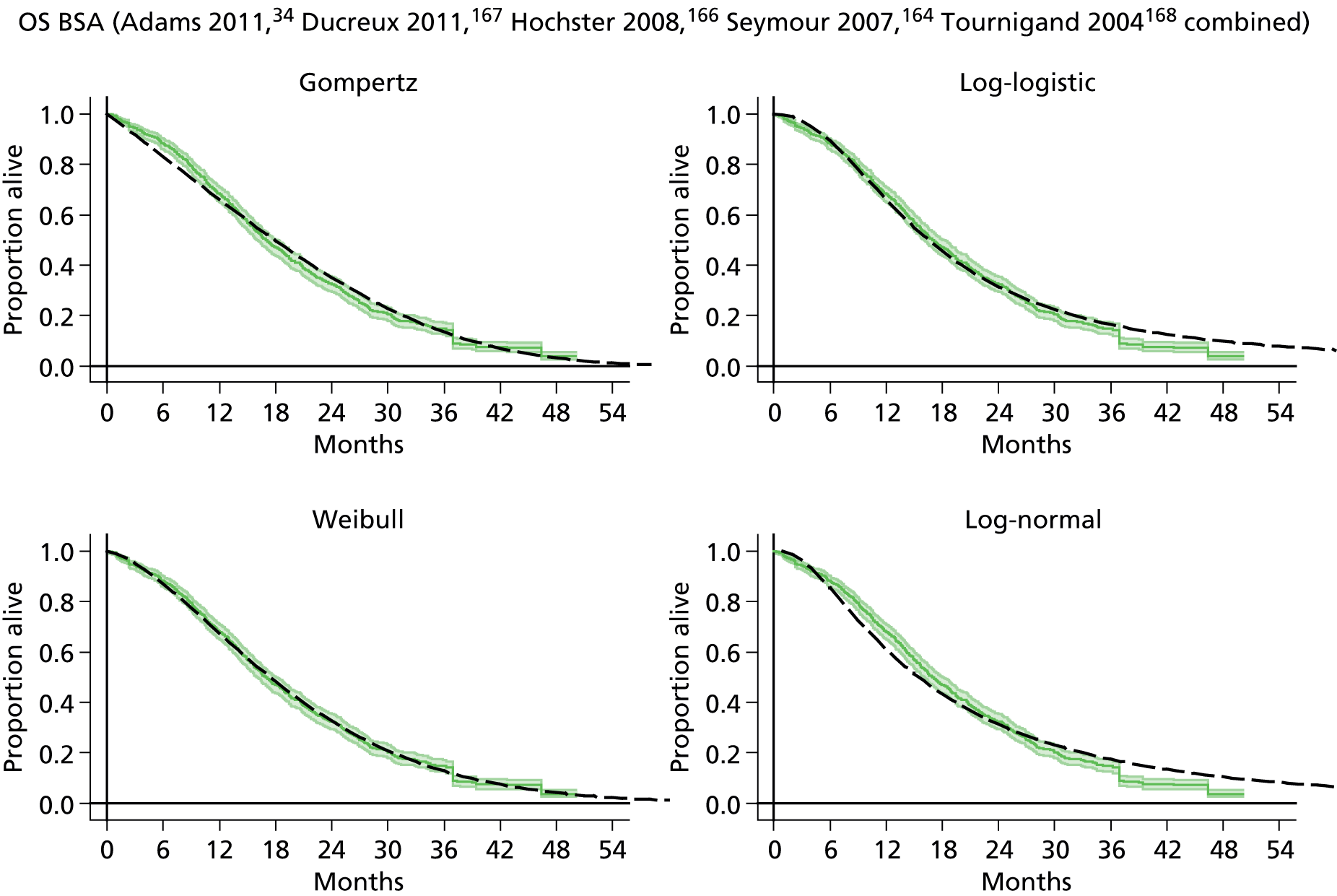

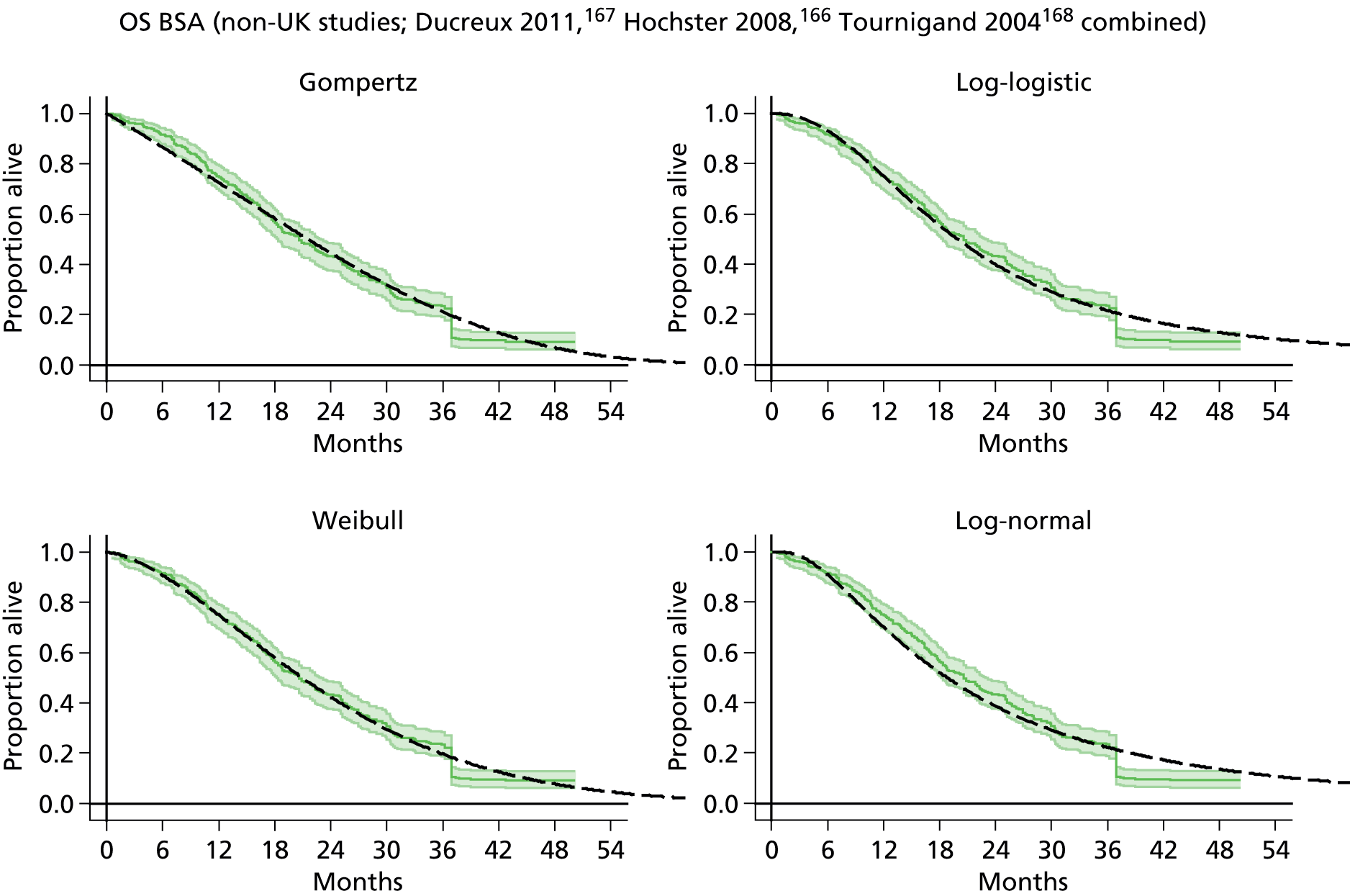
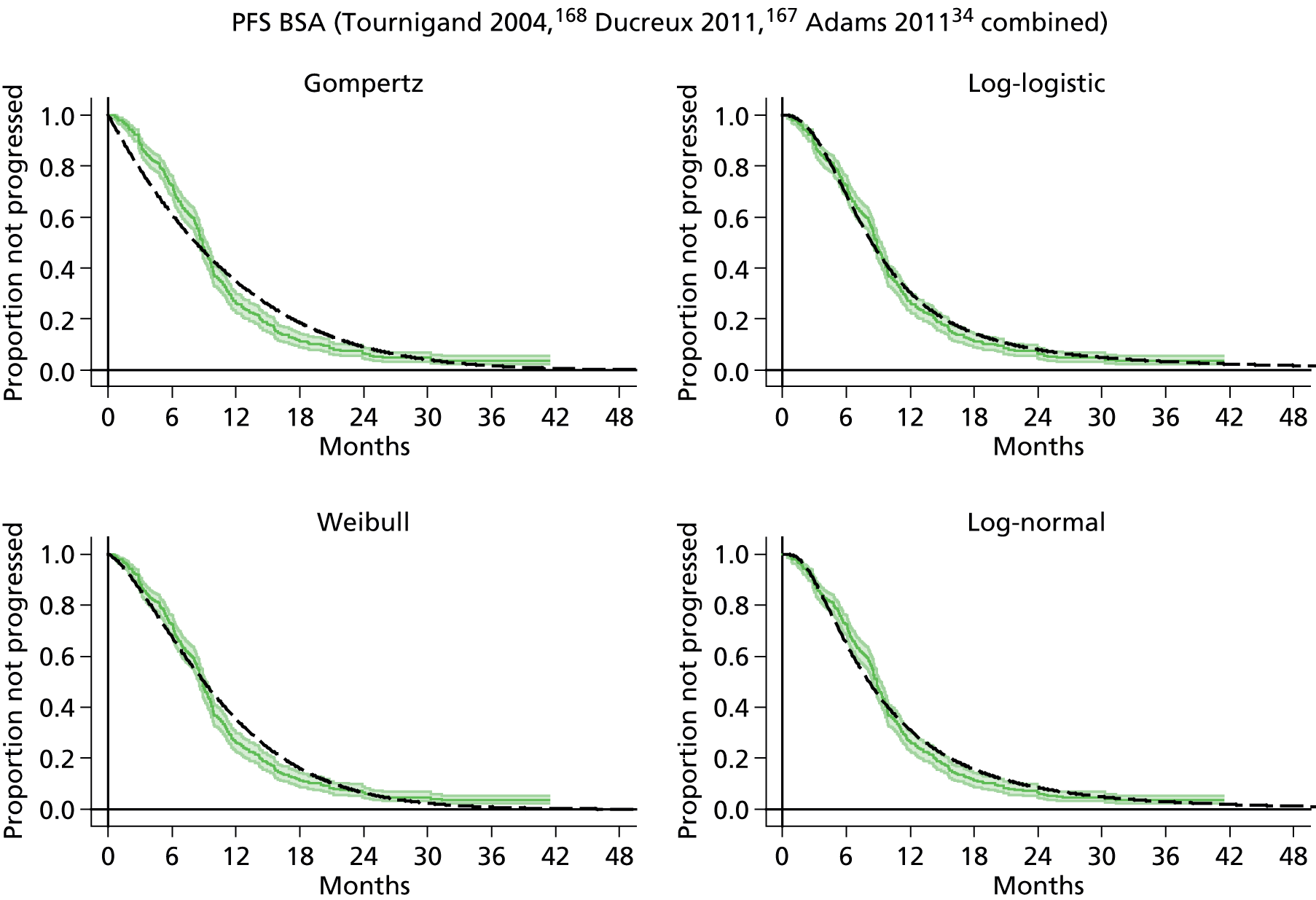
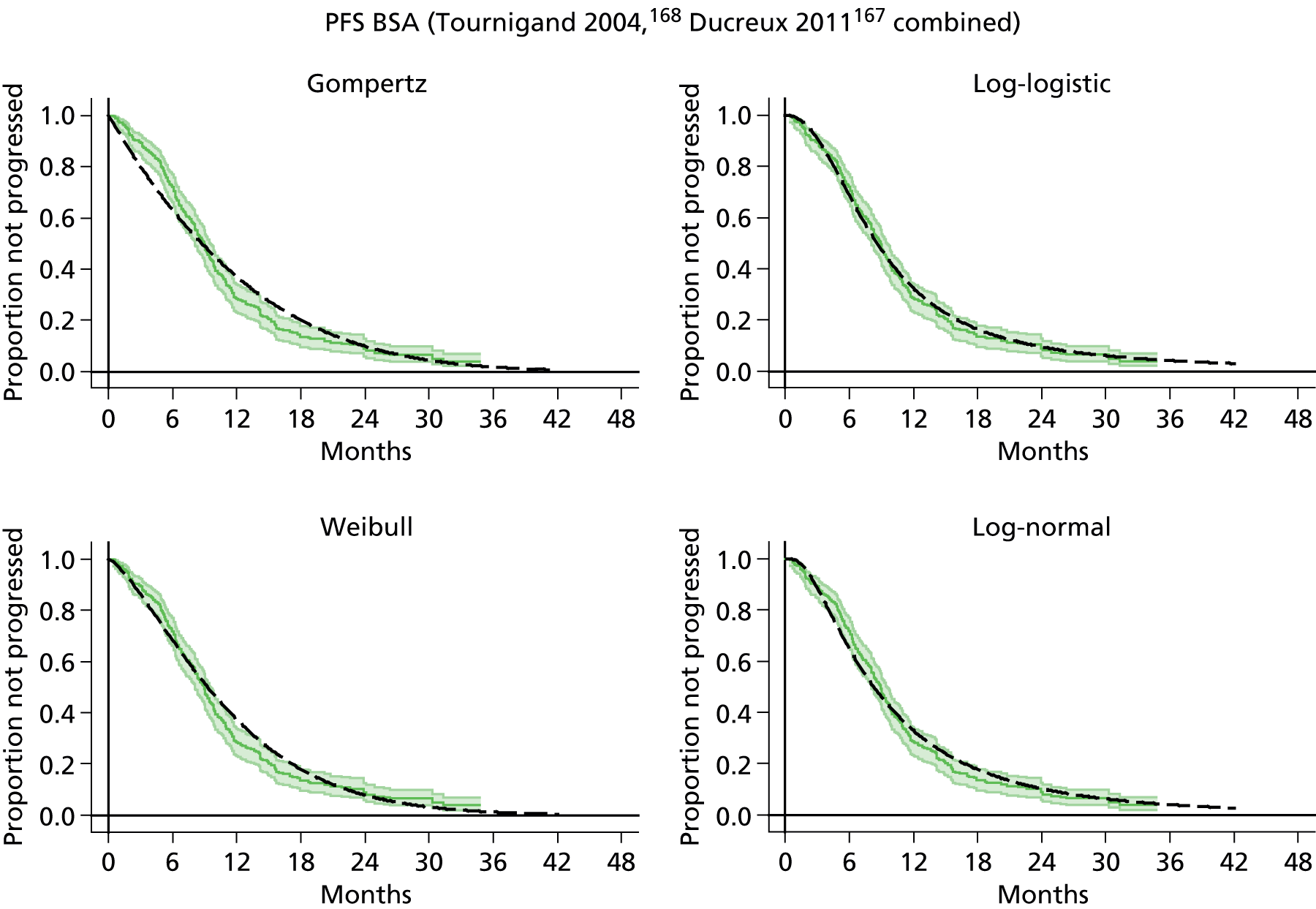
Weibull and log-normal model parameters for individual studies and combinations of studies
| Treatment and study | Log-normal | Log-normal | Weibull | Weibull |
|---|---|---|---|---|
| Mu | Sigma | Lambda | Gamma | |
| FOLFOX6: OS PK | ||||
| Capitain 2012119 | 3.320461 | 0.7450776 | 0.0023328 | 1.669058 |
| FOLFOX6: OS BSA | ||||
| Ducreux 2011167 | 2.890615 | 0.7452636 | 0.0018259 | 1.965318 |
| Tournigand 2004168 | 3.018546 | 1.041158 | 0.0141104 | 1.249536 |
| Madi 2012169 (COIN) | 2.640272 | 0.8339556 | 0.0091487 | 1.557174 |
| Hochster 2008166 | 2.932136 | 0.7831604 | 0.0049491 | 1.635496 |
| Seymour 2007164 | 2.649206 | 0.9678668 | 0.0116269 | 1.486356 |
| Combined | 2.739721 | 0.8999154 | 0.0094214 | 1.503426 |
| Non-UK combined | 2.933888 | 0.850545475 | 0.0056984 | 1.5776 |
| UK only combined | 2.64068 | 0.9050935 | 0.0104068 | 1.520104 |
| Treatment and study | Log-normal | Log-normal | Weibull | Weibull |
|---|---|---|---|---|
| Mu | Sigma | Lambda | Gamma | |
| FOLFOX6: PFS PK | ||||
| Capitain 2012119 | 2.878827 | 1.244953 | 0.0243758 | 1.136683 |
| FOLFOX6: PFS BSA | ||||
| Madi 2012169 (COIN) | 2.07606 | 0.736317 | 0.0310386 | 1.434147 |
| Ducreux 2011167 | 2.156429 | 0.838911 | 0.0305782 | 1.380975 |
| Tournigand 2004168 | 2.059369 | 0.834821 | 0.0356843 | 1.358216 |
| Combined | 2.095161 | 0.788257 | 0.0319368 | 1.400817 |
| Non-UK combined | 2.1151 | 0.838378 | 0.03271 | 1.370594 |
| Treatment and study | Log-normal | Log-normal | Weibull | Weibull |
|---|---|---|---|---|
| Mu | Sigma | Lambda | Gamma | |
| FUFOL: OS PK | ||||
| Gamelin 2008118 | 2.937171 | 0.6481075 | 0.0026976 | 1.827858 |
| Gamelin 1998138 | 2.936224 | 0.8194655 | 0.0135412 | 1.29762 |
| Capitain 2008134 | 2.710242 | 1.157483 | 0.0205514 | 1.20758 |
| Combined | 2.88818 | 0.8788956 | 0.0108903 | 1.381893 |
| FUFOL: OS BSA | ||||
| Gamelin 2008118 | 2.737613 | 0.694454 | 0.0086544 | 1.540663 |
| Cunningham 2009165 | 2.608949 | 0.913819 | 0.0119712 | 1.510307 |
| Köhne 2003162 | 2.582476 | 0.90816 | 0.0154912 | 1.407952 |
| Köhne 2005163 | 2.77669 | 0.999576 | 0.0132561 | 1.373619 |
| Seymour 2007164 | 2.557337 | 0.914055 | 0.0178338 | 1.374292 |
| Combined | 2.613328 | 0.915224 | 0.0155657 | 1.396487 |
| Cunningham 2009165 | 2.608949 | 0.913819 | 0.0119712 | 1.510307 |
| Gamelin 2008118 | 2.737613 | 0.694454 | 0.0086544 | 1.540663 |
| Giacchetti 2000170 | 3.022251 | 1.047264 | 0.0149354 | 1.223547 |
| Köhne 2003162 | 2.582476 | 0.90816 | 0.0154912 | 1.407952 |
| Köhne 2005163 | 2.77669 | 0.999576 | 0.0132561 | 1.373619 |
| Seymour 2007164 | 2.557337 | 0.914055 | 0.0178338 | 1.374292 |
| Combined | 2.64079 | 0.930805 | 0.0169611 | 1.350418 |
| Treatment and study | Log-normal | Log-normal | Weibull | Weibull |
|---|---|---|---|---|
| Mu | Sigma | Lambda | Gamma | |
| FUFOL: PFS PK | ||||
| Gamelin 1998138 | 1.975084 | 1.220492 | 0.0819713 | 0.9908913 |
| FUFOL: PFS BSA | ||||
| Cunningham 2009165 | 1.82961 | 0.759104 | 0.0485681 | 1.378005 |
| Giacchetti 2000170 | 1.762772 | 0.92053 | 0.0771431 | 1.151069 |
| Köhne 2005163 | 1.657345 | 0.813518 | 0.0499585 | 1.467809 |
| Köhne 2003162 | 1.692872 | 0.840886 | 0.0748098 | 1.233541 |
| Combined | 1.751379 | 0.814365 | 0.0591133 | 1.319453 |
| Cunningham 2009165 | 1.82961 | 0.759104 | 0.0485681 | 1.378005 |
| Köhne 2005163 | 1.657345 | 0.813518 | 0.0499585 | 1.467809 |
| Köhne 2003162 | 1.692872 | 0.840886 | 0.0748098 | 1.233541 |
| Combined | 1.74937 | 0.797924 | 0.0554057 | 1.358335 |
Appendix 15 Previous National Institute for Health and Care Excellence metastatic colorectal cancer assessments and Department of Health report
There have been a number of NICE mCRC assessments:
-
TA61: MTA of capecitabine and tegafur with uracil for mCRC – 2002. 181
-
TA93: MTA of irinotecan, oxaliplatin and raltitrexed for mCRC – 2005. 30
-
TA118: MTA of bevacizumab and cetuximab for mCRC – 2006. 179
-
TA176: STA of cetuximab for mCRC – 2008. 182
-
TA212: STA of bevacizumab for mCRC – 2009. 183
-
CG131: CG for diagnosis and management of colorectal cancer – 2011. 7
-
TA242: MTA of cetuximab, bevacizumab and panitumumab for second-line treatment of mCRC – 2012. 180
The approach of these assessments and the sources of their inputs are reviewed below, grouped under the following headings:
-
the approach to estimating chemotherapy administration costs for infusion regimes
-
the approach to modelling the duration of treatment
-
the approach to modelling survival
-
QoL values for disease states and AEs
-
the costs of AEs.
The QoL summary is supplemented by a brief review of the relevant findings of the Trueman et al. 224 bowel cancer services report to the Department of Health, which also conducted a systematic literature review.
Chemotherapy administration costs in National Institute for Health and Care Excellence assessments for metastatic colorectal cancer
This section focuses on the chemotherapy administration costs for infusion regimes similar to FOLFOX within NICE assessments for mCRC.
TA61: multiple technology appraisal of capecitabine and tegafur with uracil for metastatic colorectal cancer – 2002181
Administration costs were divided into those only incurred at the start of treatment and those incurred each cycle.
One-off costs relevant to the current assessment were the line insertion costs, which for the modified de Gramont86,87 regime were costed at £265 [£371] based on Iveson et al. 206 Outpatient costs were taken from The Christie NHS Foundation Trust Hospital: £150 [£210] with chemotherapy administration and £80 [£112] without chemotherapy administration.
Per cycle costs included inpatient and outpatient hospital visits, preparatory drugs, community nurse infusion administration and home visits, infusion pumps, pharmacy preparation and materials. Patients on the modified de Gramont86,87 regime were assumed to require one outpatient visit to a cancer ward plus two community nurse home visits to disconnect and maintain their infusion lines. The administration cost per 28 days was estimated to be £650 [£910] for the modified de Gramont regime86,87 or £325 [£455] per 2-week cycle.
TA93: multiple technology appraisal of irinotecan, oxaliplatin and raltitrexed for metastatic colorectal cancer – 200530
A cost per line insertion of £440 [£595] was drawn from Boland et al. (2003). A cost of £62 [£99] for disposable infusion pumps was taken from Iveson et al. 206 Inpatient and outpatient costs were taken from the PSSRU unit costs of health care (1999): £356 [£571] per medical oncology inpatient day and £109 [£175] per medical oncology outpatient visit. These were uplifted to 2004 costs using health service inflation indices.
Pharmacy costs of £23 [£30] per simple i.v. infusion and £38 [£49] per complex i.v. infusion were drawn from expert opinion from The Christie NHS Foundation Trust Hospital, Manchester. For the modified de Gramont regime,86,87 the £38 [£49] cost was applied to each of the 5-FU bolus, the 5-FU infusion and the FA infusion resulting in a total pharmacy cost of £114 [£147]. Adding oxaliplatin to this increase the pharmacy cost by a further £38 [£49] to £152 [£195]. FOLFOX6 was assumed to have the same £152 [£195] pharmacy cost.
The modified de Gramont,86,87 oxaliplatin plus the modified de Gramont,86,87 FOLFOX6, irinotecan plus the modified de Gramont,86,87 and FOLFIRI were all assumed to require 2 inpatient days if receiving it as inpatients, and one outpatient appointment if receiving it as an outpatient. The proportions receiving their treatment as inpatients and outpatients were based on data from the Aventis submission. This summarised data on 163 UK patients in ‘previous chemotherapy trials’: 15 (21%) of 71 modified de Gramont86,87 patients receiving inpatient administration; 7 (25%) of 28 irinotecan patients receiving inpatient administration; 3 (7%) of 41 FOLFOX6 patients receiving inpatient administration; and 4 (17%) of 23 FOLFIRI patients receiving inpatient administration. Subsequent text suggests that this sample was constructed from the FOCUS trial and the Tournigand et al. trial, though the assessment report notes that ‘no information was available concerning how this sample of patients was constructed’.
Additional costs for diagnostic tests of £64.55 [£103.44] were drawn from the Kerr and O’Connor (1999) study of raltitrexed and 5-FU/FA, as were monthly primary care costs of £10.42 [£16.70]. The cost per cycle for clinical consultations of £79.81 [£127.90] was drawn from Iveson et al. 206
TA118: multiple technology appraisal of bevacizumab and cetuximab for metastatic colorectal cancer – 2006179
The administration costs were the same as those used for TA93. 30 The only difference was to assume that no patients had their chemotherapy administered as inpatients due to this increasingly being the case.
TA176: single technology appraisal of cetuximab for metastatic colorectal cancer – 2008182
Administration costs were drawn from the NHS 2006 tariff: £123 for a single outpatient infusion based on HRG 370F Outpatient Adult Follow-Up Attendance Medical Oncology [Attendance without treatment]: Face to Face; and, for a day case chemotherapy administration £277 based on HRG F98 Day Case Chemotherapy with Digestive System Primary Diagnosis.
TA212: single technology appraisal of bevacizumab for metastatic colorectal cancer – 2009183
The administration costs were based on the pharmacy costs of Tappenden et al. 172 plus NHS reference costs for administration: for the first day of a cycle £317 [£357] based on SB14Z Deliver Complex Chemotherapy Including Prolonged Infusional Treatment At First Attendance and for the second day of a cycle £227 [£255] based on SB15Z Deliver Subsequent Elements Of A Chemotherapy Cycle. Inpatient administration was costed at £1052 [£1183] based on PA44Z: Elective inpatient stay for Neoplasm Diagnoses.
The cost of the elastomer pump was estimated to be £35 [£38] based on the 48-hour pump supplied by Baxter Healthcare. This was assumed to be in addition to the pharmacy on-costs and to the NHS reference costs.
An hour of district nurse time to flush the infusion line at the end of each cycle was also included.
CG131: clinical guideline for diagnosis and management of colorectal cancer – 20117
The 2008–9 NHS reference cost of £335 [£363] for SB13Z for the outpatient delivery of more complex parenteral chemotherapy was applied for each FOLFOX administration and for each FOLFIRI administration.
TA242: multiple technology appraisal of cetuximab, bevacizumab and panitumumab for second-line treatment of metastatic colorectal cancer – 2012180
The assessment group assumed a £227 [£246] cost in 2008–9 prices for i.v. infusion of cetuximab monotherapy and panitumumab, drawn from the 2008–9 NHS reference costs for outpatients HRG SB15Z: Deliver Subsequent Elements Of A Chemotherapy Cycle. This was inflated by an annual 4%, based on the Hospital and Community Health Services Pay and Prices Index, to give a cost per administration of £255 [£261].
For the additional administration of irinotecan half of the £255 [£261]; that is £128 [£131], was added to the £255 [£261] administration cost of cetuximab.
Pharmacy preparation time for each infusion was estimated to cost £15 [£15] for all drugs.
Note that the costs of medical management for PD were taken from Remak and Brazil (2004), a study among breast cancer patients.
The modelling of the duration of treatment in National Institute for Health and Care Excellence assessments for metastatic colorectal cancer
TA61: multiple technology appraisal of capecitabine and tegafur with uracil for metastatic colorectal cancer – 2002181
The assessment report noted that there was no consistent UK policy about whether treatment should continue to progression or stopped after a fixed period of time. For the modelling it was assumed that patients would be treated for 12 weeks, and that this had no detrimental effect on survival despite the relevant RCTs treating patients until progression.
A scenario analysis of treating patients until progression was also undertaken. The assessment report noted that a proportion of patients would probably recommence treatment, with the mean treatment duration probably lying between the two extremes that were modelled.
TA93: multiple technology appraisal of irinotecan, oxaliplatin and raltitrexed for metastatic colorectal cancer – 200530
The mean number of treatment cycles for first- and second-line therapies within the Tournigand et al. 168 trial was available to the assessment group from a personal communication from A. Gramont.
TA118: multiple technology appraisal of bevacizumab and cetuximab for metastatic colorectal cancer – 2006179
For the bevacizumab modelling, the mean number of doses of the first-line therapies was taken from the trial data reported in the Roche submission to NICE. Similarly, for the cetuximab modelling the mean number of vials administered was taken from the BOND trial as reported in the Merck submission to NICE.
Table 6 of the assessment group report notes that bevacizumab was administered in repeat 8-week cycles. Although not specifying treatment duration for trial AVF2107g, trials AVF0780g and AVF2192g both continued treatment to progression, or 48 weeks for AVF0780g and 96 weeks for AVF2192g. Table 18 of the assessment group report does not specify treatment cessation rules for the BOND trial, but other cetuximab trials appear to be until progression or unacceptable toxicity.
TA176: single technology appraisal of cetuximab for metastatic colorectal cancer – 2008182
The number of vials used were apparently derived from data for the relevant subset of the CRYSTAL and OPUS trials.
TA212: single technology appraisal of bevacizumab for metastatic colorectal cancer – 2009183
The monthly drug costs were based on the per cycle cost multiplied by the mean number of cycles per month that were observed in the pivotal NO16966 trial: 1.83 for 5-FU-based regimes and 1.31 for capecitabine-based regimes. Kaplan–Meier curves for time-to-treatment cessation were used to model the time on treatment, though note that these are marked as commercial in confidence.
CG131: clinical guideline for diagnosis and management of colorectal cancer – 20117
The numbers of treatment cycles were drawn from the literature. For first-line treatments, a mean of 8.99 cycles for FOLFOX and a mean of 7.89 cycles for FOLFIRI were applied. For those receiving second-line therapies a mean of 7.13 cycles for FOLFOX and 6.00 cycles for FOLFIRI were applied.
TA242: multiple technology appraisal of cetuximab, bevacizumab and panitumumab for second-line treatment of metastatic colorectal cancer – 2012180
For cetuximab plus best supportive care and cetuximab plus irinotecan, patients were assumed to remain on treatment until disease progression. For panitumumab plus BSC a mean of 20 weeks treatment (i.e. 10 doses), was drawn from data reported by Amado et al. (2008). These data also suggested a PFS of 4.0 months when the assessment group s indirect comparison increased this to 5.1 months. The number of doses of panitumumab was proportionately increased to 12.7 doses.
The approach to modelling survival in National Institute for Health and Care Excellence assessments for metastatic colorectal cancer
TA61: multiple technology appraisal of capecitabine and tegafur with uracil for metastatic colorectal cancer – 2002181
Overall mean survival was based on Twelves (2002) with the additional assumption of equivalence between the Mayo regime and the modified de Gramont regime. 86,87 The AUC resulted in an estimated mean survival of 15.1 months. What was applied for PFS is less clear, though there is reference to a median of 4.7 months within the assessment report.
TA93: multiple technology appraisal of irinotecan, oxaliplatin and raltitrexed for metastatic colorectal cancer – 200530
Parametric Weibull curves were fitted to Kaplan–Meier OS curves and PFS curves of the FOCUS trial. The modified de Gramont86,87 plus irinotecan arm was used as the baseline, with the log-rank HRs for the other arms being estimated. Log-rank HRs were also estimated for the first-line FOLFIRI, second-line FOLFOX6 arm and the first-line FOLFOX6, second-line FOLFIRI arm of the Tournigand et al. 168 study.
Table 66 on page 91 of Hind et al. 30 reports mean OS estimates of 2.28 years for the first-line FOLFIRI, second-line FOLFOX6 arm and 2.15 years for the first-line FOLFOX6, second-line FOLFIRI arm of the Tournigand et al. 168 study.
TA118: multiple technology appraisal of bevacizumab and cetuximab for metastatic colorectal cancer – 2006179
Two models were developed to estimate the cost-effectiveness of bevacizumab:
-
being added to irinotecan plus 5-FU/FA;
-
being added to 5-FU/FA.
Note that these also allowed for second- and third-line therapies. For both OS and PFS, parametric Weibull curves were fitted to digitised Kaplan–Meier curves as reported in Hurwitz et al. (2004) and Kabbinavar et al. (2005). (Note that the section header suggested that this only applied to the non-bevacizumab containing regimes, but figures 13 and 14 suggest that this applied to both arms for the relevant comparison.) This resulted in estimates for the mean PFS, including PFS from second- and third-line therapies, as 1.27 years for bevacizumab plus irinotecan plus 5-FU/FA compared with 0.97 years for irinotecan plus 5-FU/FA, and 1.16 years for bevacizumab plus 5-FU/FA compared with 0.83 years for 5-FU/FA. Mean OS estimates were 1.98 years for bevacizumab plus irinotecan plus 5-FU/FA compared with 1.57 years for irinotecan plus 5-FU/FA, and 1.59 years for bevacizumab plus 5-FU/FA compared with 1.41 years for 5-FU/FA.
One model was developed to estimate the cost-effectiveness of cetuximab in combination with irinotecan compared with active supportive care as second-line therapy among those epidermal growth factor receptor-expressing mCRC patients who had failed on irinotecan containing chemotherapy. This was hampered by there being no direct comparative evidence on survival and QoL. Although there was evidence on tumour response rates, the assessment group report notes that ‘the impact of cetuximab treatment on HRQoL and OS remains unquantified’. Due to this the primary analysis sought to identify the threshold for the additional OS that would render cetuximab cost-effective.
For the modelling of the cost-effectiveness of adding cetuximab to irinotecan, parametric Weibull curves were fitted to Kaplan–Meier OS curves whereas the PFS was estimated ‘using the empirical Kaplan Meier progression free survival curve’ as reported in the BOND trial. The mean OS estimate for cetuximab plus irinotecan was 0.79 years.
A secondary analysis undertook a systematic review of OS from active best supportive care. The mean OS estimates for active best supportive care were 0.60 years based on Cunningham et al. (1999), 0.67 years based on Rao et al. (2004) and 0.77 years based on Barni et al. (1995).
TA176: single technology appraisal of cetuximab for metastatic colorectal cancer – 2008182
The manufacturer submission estimates the effectiveness of the first-line therapies through parameterised survival curves being fitted to trial data for death before progression and PFS. A log-normal curve for death before progression and a Weibull curve for PFS was fitted for the CRYSTAL trial analysis of cetuximab added to FOLFIRI, while log-normal curves were fitted to both death before progression and PFS for the OPUS trial analysis of cetuximab added to FOLFOX4. Second-line time to progression was based on a log-normal fit to data from Tournigand et al. ,168 third-line time to progression was based on a log-logistic fit to data within Jonker et al. 189 Resection was also incorporated within the model, with an associated survival rate and PFS estimated from the data of Adam et al. (2004). However, it is not immediately clear how OS has been modelled.
Cetuximab plus FOLFIRI was estimated by the manufacturer to result in OS of 2.28 years compared with 1.92 years for FOLFIRI. Cetuximab plus FOLFOX4 was estimated by the manufacturer to result in OS of 1.89 years compared with 1.41 years for FOLFOX4.
TA212: single technology appraisal of bevacizumab for metastatic colorectal cancer – 2009183
The cost-effectiveness of bevacizumab added to FOLFOX and added to XELOX was modelled. Parametric curves for PFS, the exponential, and for OS, the Weibull, were fitted to the Kaplan–Meier data of the pivotal NO16966 trial. (Based on table 23 of the manufacturer submission. Subsequent text suggests that Weibulls may have been used for PFS.) Patients were assumed to follow the Kaplan–Meier curves until the median of the relevant curve had been reached, and then to follow the relevant parametric curve.
The estimates of mean PFS and OS are marked as commercial in confidence.
CG131: clinical guideline for diagnosis and management of colorectal cancer – 20117
Parameterised exponential curves were fitted to data drawn from the literature, the exponential form being adopted in large part due to only median survival data being reported for a number of studies. This resulted in mean PFS estimates of 11.8 months for first-line FOLFOX and 10.9 months for first-line FOLFIRI. The mean PFS estimate for second-line FOLFOX was 3.6 months, compared with 6.1 months for FOLFIRI. Note that based on the literature, only around 60% of patients were assumed to received second-line treatment. The mean OS estimates for FOLFOX followed by FOLFIRI was 29.9 months, compared with 31.2 months for FOLFIRI followed by FOLFOX.
TA242: multiple technology appraisal of cetuximab, bevacizumab and panitumumab for second-line treatment of metastatic colorectal cancer – 2012180
Due to a lack of data, the assessment group modelled cost-effectiveness among patients with Kirsten Rat Sarcoma-Wild Type (KRAS-WT) status for third-line treatment or beyond.
Progression-free survival for BSC was modelled using a parameterised Weibull curve fitted to the monthly data points of the relevant Kaplan–Meier curve reported within Karapetis et al. (2008) to permit estimation of the Weibull shape. This was linked to the mean PFS reported in the Merck submission of 2.72 months in order to estimate the scale parameter. The same approach was used to parameterise the Weibull curve for OS, drawing on the mean OS reported by Merck of 6.2 months.
A similar approach was used for cetuximab plus BSC, based on means of 4.78 months for PFS and 10.0 months for OS as reported by Merck.
An indirect comparison for panitumumab plus BSC is undertaken by first characterising the BSC and panitumumab plus BSC AUCs from the relevant trial, based on monthly data points from the Kaplan–Meier. This resulted in a mean PFS of 2.2 months for BSC and 4.0 months for panitumumab plus BSC. The Bucher method was then used in conjunction with the 2.7 months BSC survival estimate from the Merck trial to yield a mean PFS estimate of 5.1 months for panitumumab plus BSC. This was then used to fir a parameterised Weibull curve.
A similar method was used to parameterise a Weibull for OS for panitumumab plus BSC, though for this Weibull curves were fitted to the 4 weekly Kaplan–Meier data. This resulted in an OS estimate of 9.9 months for panitumumab plus BSC and of 9.4 months for BSC. A further adjustment was made to these figures for cross-over within the trial. The Bucher method was then used to provide an estimate of a mean of 8.5 months OS for panitumumab plus BSC.
The PFS for cetuximab plus irinotecan is based on the median survival estimate, adjusted for KRAS-WT status. The same shape parameter as for cetuximab plus BSC is assumed, to enable characterisation of the scale parameter and the parameterised Weibull curve. A similar approach was adopted for OS. This resulted in a mean OS estimate of 16.6 months for cetuximab plus irinotecan.
Quality-of-life values used in previous National Institute for Health and Care Excellence metastatic colorectal cancer assessments
TA61: multiple technology appraisal of capecitabine and tegafur with uracil for metastatic colorectal cancer – 2002181
A cost minimisation analysis was performed due to a lack of convincing evidence of a survival difference between the oral treatments and infusional regimes used in the UK. As a consequence, HRQoL estimates were not required.
TA93: multiple technology appraisal of irinotecan, oxaliplatin and raltitrexed for metastatic colorectal cancer – 200530
European Quality of Life-5 Dimensions data from the MRC-sponsored FOCUS trial is applied. As outlined in more detail in figure 12 on page 68 of the Hind et al. 30 HTA monograph, after a slight improvement between baseline and 8 weeks, the average QoL values applied remain reasonably steady over the period from 8 weeks to 48 weeks. HRQoL values for the various regimens of the FOCUS trial vary between 0.72 and 0.80. The mean HRQoL values across the various regimens of the FOCUS trial of 0.76 was assumed to apply to first-line FOLFOX6 followed by second-line FOLFIRI and for first-line FOLFIRI followed by FOLFOX6.
However, Hind et al. 30 caution that:
. . . it should be noted that . . . these data had not been subject to full checking and validation, nor had the data been adjusted for the effects of either informative or uninformative censoring within the trial. Consequently, the resulting cost-utility estimates . . . should be interpreted with caution. The QoL impacts of adverse events were not separately modelled.
TA118: multiple technology appraisal of bevacizumab and cetuximab for metastatic colorectal cancer – 2006179
For PFS a HRQoL estimate of 0.80 was drawn from the Ramsay et al. (2000) study of QoL among CRC survivors. For with progression survival a relative risk of 0.75 was applied to the PFS value, resulting in a HRQoL estimate of 0.80 × 0.75 = 0.60. The 0.75 was informed by the estimates of Petrou and Campbelll. 188
The QoL impacts of AEs were not separately modelled.
TA176: single technology appraisal of cetuximab for metastatic colorectal cancer – 2008182
The Merck submission for TA176182 calculated a HRQoL value for PFS under first-line therapy based on EQ-5D responses by UK patients collected during one of the pivotal trial. EQ-5D was collected at baseline, 8, 16, 24, 32 and 48 weeks.
The submission is slightly ambiguous, but appears to suggest that the EQ-5D may only have been administered among the UK patient subset. The full analysis set consisted of 1217 patients, whereas the KRAS-WT subset Merck stated as being the relevant population subset for the assessment, consisted of 348 patients.
Thirty-nine UK patients completed the EQ-5D at baseline, 22 patients in the cetuximab plus FOLFIRI arm and 17 patients in the FOLFIRI arm. The numbers of EQ-5D responses at 8, 16, 24, 32 and 48 weeks were 26, 22, 16, 12 and 7 respectively.
The Merck submission for TA176182 performed an odd averaging exercise that resulted in a HRQoL estimate of 0.777. A more standard weighted averaging would have resulted in a slightly higher HRQoL estimate of 0.790. This estimate may also have been slightly skewed by the one response at week 48 in the FOLFIRI arm resulting in a HRQoL of 0.090 for that patient. Arbitrarily, excluding this patient results in a slightly higher weighted average for a HRQoL of 0.796.
The weighted average across the 74 respondents in the cetuximab plus FOLFIRI arm was 0.790. Across the 46 respondents in the FOLFIRI arm it was also 0.790. Excluding the one response at week 48 in the FOLFIRI arm with a QoL of 0.090 results in a weighted average of 0.805.
The Merck submission also modelled progression to second- and third-line therapy. For third-line therapy a HRQoL estimate of 0.68 was drawn from the Jonker et al. 189 study of cetuximab for CRC. For second-line therapy an average of the 0.77 first-line estimate and 0.68 third-line estimate resulted in a HRQoL estimate of 0.73.
The QoL impacts of AEs were not separately modelled.
TA212: single technology appraisal of bevacizumab for metastatic colorectal cancer – 2009183
Quality-of-life values were apparently mainly drawn from TA176 cetuximab for mCRC182 as summarised above: 0.77 for first-line PFS on treatment, 0.73 for second-line PFS and 0.67 for survival post progression. An additional state of first-line PFS post treatment was attributed a HRQoL estimate of 0.79. This appears to have been based on the 0.77 for first-line PFS post treatment coupled with an adjustment due to expert opinion:
It was deemed that utility values in the PFS post treatment health state would be higher than that of patients receiving first-line treatment given that patients disease is stable at this point and that they would no longer be experiencing the adverse effects of chemotherapy treatment.
The QoL impacts of AEs were not separately modelled.
CG131: clinical guideline for diagnosis and management of colorectal cancer – 20117
Quality-of-life values of 0.510 for SD and 0.210 for PD were drawn from the Best et al. 190 study of stage III colon cancer.
Quality-adjusted life-year decrements for AEs of 0.103 for grade III/IV diarrhoea, 0.150 for febrile neutropenia and 0.116 for hand and foot syndrome were drawn from the Lloyd et al. 196 study of metastatic breast cancer.
TA242: multiple technology appraisal of cetuximab, bevacizumab and panitumumab for second-line treatment of metastatic colorectal cancer – 2012180
The QoL values applied by the EAG were based on those supplied in the Merck submission to the assessment, with some further adjustments by the EAG. The data underlying these estimates has been published in the Mittman et al. 191 reporting of the main clinical trial, this reporting HUI version 3 scores valued using the HUI version 3 tariff of the Canadian general population sample. The reanalysis by Merck was necessary to align the estimates with the progression-free and post-progression health states of the model, rather than being averages at specific time points. This resulted in HRQoL estimates of 0.81 for PFS and 0.79 for SWP in the cetuximab plus best supportive care arm, and of 0.75 for PFS and 0.69 for SWP in the best supportive care arm.
The EAG noted that these may be overestimates due to patients who were less well-being less likely to complete the questionnaires. The EAG also noted that it may overestimate the impact of cetuximab being added to best supportive care due to the trial not being blinded.
The EAG revised a number of values in the light of expert opinion and parsimony with other values. PFS had HRQoL values of 0.75 for best supportive care, 0.81 for cetuximab plus best supportive care, 0.75 for cetuximab plus irinotecan and 0.87 for panitumumab plus best supportive care. Post-progression survival was estimated to have a HRQoL of 0.69.
The QoL impacts of AEs were not separately modelled.
Trueman et al.:224 bowel cancer services report to the Department of Health
In brief, a systematic review for QoL data was undertaken for the Department of Health report. This identified the following possible sources: Ness et al. ,225 Ko et al. ,226 Ramsey et al. ,187 Petrou and Campbell,188 the MRC FOCUS trial, and the Merck MABEL trial. These sources have all been identified within the above NICE assessments.
Adverse event costs used in previous National Institute for Health and Care Excellence metastatic colorectal cancer assessments
TA61: multiple technology appraisal of capecitabine and tegafur with uracil for metastatic colorectal cancer – 2002181
The report notes that the toxicity profiles were similar, though capecitabine might have a slightly better profile than the Mayo regime with the exception of hand and foot syndrome. The detail of the costings is not presented. The average 28-day AE cost is estimated to be £131 [£183] for capecitabine, £170 [£238] for Mayo, £29 [£41] for the modified de Gramont86,87 regimen and £22 [£31] for the inpatient de Gramont86,87 regimen.
TA93: multiple technology appraisal of irinotecan, oxaliplatin and raltitrexed for metastatic colorectal cancer – 200530
Adverse event costs were split into hospitalisation costs and drug costs. For the base case, the distribution of hospitalisations across specialities was drawn from the Schmitt et al. (1999) study of irinotecan with 5-FU in patients with mCRC after 5-FU failure. This was coupled with PSSRU unit cost data and an assumption of a mean LOS of 1 day per month. This resulted in an estimate of a monthly hospitalisation cost due to AEs of £258 [£413].
Drug costs for AEs were estimated at £9.74 [£15.61] per month, based on data from the Kerr et al. (1999) study of raltitrexed plus the Mayo regime in advanced CRC.
These costs were uplifted for inflation using the Health Service Inflation Indices and applied to all regimes.
TA118: multiple technology appraisal of bevacizumab and cetuximab for metastatic colorectal cancer – 2006179
Adverse event costs were split into hospitalisation costs and drug costs, with the costings relying on the same sources as TA93. 30
The monthly costs of hospitalisations of £258 [£413] and AEs of £9.74 [£15.61] per month were increased by a factor of 1.13 for bevacizumab, based on the relative risk reported in the Roche submission.
These costs were uplifted for inflation using the Health Service Inflation Indices.
TA176: single technology appraisal of cetuximab for metastatic colorectal cancer – 2008182
Adverse events treated as outpatients are ‘estimated from the UK NHS national tariff’: £191 [£221] for a second-line outpatient visit for an AE, £162187 for an outpatient visit for a grade III/IV AE and £166 [£192] for an outpatient visit for a serious AE. (Note that these values are taken from table H28 of the Merck submission, which is not entirely in line with the values reported in table H30 of the Merck submission.). Quite how these events were distinguished from one another is not clear.
Adverse events treated as inpatients are ‘based on the mapping of the types of AEs onto the UK HRGs that can likely be assigned to them’: £1050 [£1216] for inpatient treatment of a non-serious AE and £1170 [£1354] for inpatient treatment of a serious AE.
Unfortunately, the details of the calculations are in an appendix to the Merck submission which is not publicly available. It is also not clear what estimates were applied for the balances between outpatient treatment and inpatient treatment for the various AEs.
TA212: single technology appraisal of bevacizumab for metastatic colorectal cancer – 2009183
The following treatment costs (Table 81) were applied to grade III/IV AEs within the Roche submission. 183
| AE | Unit cost (£) | Reference/comment | |
|---|---|---|---|
| Cardiac disorders | £1201 | [£1300] | Reference costs 2006/7 |
| Diarrhoea | £237 | [£257] | LRiG 2006 erlotinib |
| Febrile neutropenia | £1575 | [£1705] | Reference costs 2006/7 |
| Hypertension | £200 | [£217] | Palmer 2004 |
| Infections (excluding febrile neutropenia) | £1077 | [£1166] | Reference costs 2006/7 |
| Neurotoxicity | £18 | [£19] | LRiG 2006 erlotinib |
| Neutropenia/granulocytopenia | £140 | [£152] | LRiG 2006 erlotinib |
| Hand and foot syndrome | £137 | [£148] | York CRD, September 2004 |
| Stomatitis | £819 | [£887] | Capri et al. 2003 |
| Venous thromboembolism | £741 | [£802] | Reference costs 2006/7 |
| Vomiting/nausea | £240 | [£260] | Reference costs 2006/7 |
Only the calculation of the reference cost-related AEs was documented within the submission, with the apparent additional assumption that all would be treated as inpatients. Those related to Palmer 2004, LRIG 2006 Erlotinib, York Centre for Reviews and Dissemination 2004, September 2004 and Capri et al. 2003 were not documented, and the full citations were not supplied in the submission’s list of references. The ERG noted in its report that it asked for details of the procedure/treatment/drugs which were included in these costs, but that none were provided by Roche.
CG131: clinical guideline for diagnosis and management of colorectal cancer – 20117
The cost of £388 [£420] for grade III/IV diarrhoea was based on the NHS reference cost FZ45C: Short Stay Non-Elective Inpatient: Non-Malignant Large Intestinal Disorders. The cost of £6278 [£6797] for febrile neutropenia was based on the PbR tariff PA45Z: Febrile Neutropenia with Malignancy. The cost of hand and foot syndrome was assumed to be zero due to it typically being treated through treatment cessation and/or dose reduction.
TA242: multiple technology appraisal of cetuximab, bevacizumab and panitumumab for second-line treatment of metastatic colorectal cancer – 2012180
The assessment group based their estimates of the costs of treating AEs on the Merck submission. Costs for AEs of £2760 [£2824] for best supportive care, £3671 [£3757] for cetuximab plus best supportive care, £880 [£901] for panitumumab plus best supportive care, and £3671[£3757] for cetuximab plus irinotecan were estimated by Merck. The EAG revised the £880 [£901] for panitumumab plus best supportive care upwards to be equal to the £2760 [£2824] for best supportive care. This was in the light of the estimates for the rates of grade III/IV AEs typically being higher for panitumumab plus best supportive care than for best supportive care.
The EAG considered that Merck had performed an extensive analysis of these costs based on their main trial, noted that they had found no logical flaw in the calculations and that the AE costs are very small compared with other costs.
Appendix 16 Quality-of-life papers and metastatic colorectal cancer
Literature within previous metastatic colorectal cancer National Institute for Health and Care Excellence assessments
Best et al. 190 undertook a TTO study among 49 Californian CRC patients and 49 members of the general US public to estimate QoL values for stage III CRC. The method of recruitment from the general public is not clear, but efforts were apparently made to recruit respondents who were similar to the patient group. The mean age of patients was 60 years, and the mean age of respondents from the general public was 61 years. Seven health states were involved:
-
adjuvant chemotherapy, no neuropathy
-
adjuvant chemotherapy, moderate neuropathy
-
adjuvant chemotherapy, severe neuropathy
-
remission
-
metastatic SD
-
metastatic PD
-
death.
Neuropathy was included due to it being a potential side effect of oxaliplatin. The health state vignettes included descriptions of the rates and severity of both diarrhoea and fatigue, with some health states also including descriptions of vomiting and loss of weight. Both adjuvant chemotherapy and metastatic disease were associated with i.v. treatment at hospital for a few hours, one or two times a fortnight. Each respondent only rated four of the above health states: remission, two of the adjuvant health states and one metastatic health state, plus their own health.
Seven patients and 16 members of the general public stated a preference of zero year of perfect health to spending the rest of their life in at least one of the chronic health states. This covered 51 TTO values: 19 for metastatic PD, 5 for metastatic SD, 11 for adjuvant chemotherapy with severe neuropathy and the other 16 spread over the other four health states. As far as can be gleaned from the paper, these 51 zero TTO values were included in the statistical analysis.
Results were presented as the raw averages and also as averages adjusted for age, education and current health status. Table 82 reports the raw mean TTO values, the adjusted mean TTO values, for the adjusted TTO values compared with the remission health state, and the standard error of the means of the coefficients.
| Health state | Patient respondents | General public respondents | ||||||
|---|---|---|---|---|---|---|---|---|
| Raw | Adjusted | Coefficient | SEM | Raw | Adjusted | Coefficient | SEM | |
| Remission | 0.87 | 0.83 | 0.83 | 0.82 | ||||
| Adjuvant, no neuropathy | 0.67 | 0.61 | –0.221 | 0.063 | 0.62 | 0.60 | –0.223 | 0.054 |
| Adjuvant, mild neuropathy | 0.65 | 0.61 | –0.224 | 0.075 | 0.52 | 0.51 | –0.310 | 0.060 |
| Adjuvant, moderate neuropathy | 0.55 | 0.53 | –0.309 | 0.075 | 0.48 | 0.46 | –0.362 | 0.056 |
| Adjuvant, severe neuropathy | 0.48 | 0.48 | –0.352 | 0.073 | 0.35 | 0.34 | –0.475 | 0.060 |
| Metastatic, stable | 0.46 | 0.40 | –0.433 | 0.076 | 0.54 | 0.51 | –0.305 | 0.055 |
| Metastatic, progressive | 0.38 | 0.37 | –0.464 | 0.074 | 0.21 | 0.21 | –0.607 | 0.058 |
The authors noted a potential limitation in that their elicitation method did not allow for states worse than death, so potentially biasing their estimates upwards. This could also be the reason behind the quite high number of zero-rated TTO values within the analysis. However, there would also seem to be some concerns around the zero-rated TTO values, particularly for the 16 responses spread over health states other than adjuvant chemotherapy with severe neuropathy or metastatic disease. It should also be noted that within the vignettes neuropathy was not time delimited so was assumed to last for the entire duration of the health state.
Ramsey et al. 187 undertook a survey using the Functional Assessment of Cancer-Colorectal and the HUI version 3 questionnaires among 173 adult US patients who had survived for a minimum of 1 year since being diagnosed with CRC. Suitable respondents were identified from the NCI’s cancer surveillance system of western Washington State, stratified by stage. A first phase recruited 74 respondents for face-to-face interviews (from a pool of 450 who were contacted), to design a self-administered survey. A second phase recruited a further 193 respondents, of whom 98 completed the self-administered survey by post. Table 83 shows the mean HUI scores (s.d.).
| Stage | Time since diagnosis (months), mean (s.d.) | ||||
|---|---|---|---|---|---|
| 13–24 | 25–36 | 37–60 | > 60 | Mean | |
| I | 0.72 (0.27) | 0.89 (0.11) | 0.90 (0.06) | 0.89 (0.05) | 0.84 (0.17) |
| II | 0.85 (0.15) | 0.87 (0.13) | 0.79 (0.18) | 0.91 (0.11) | 0.86 (0.14) |
| III | 0.82 (0.15) | 0.95 (n = 1) | 0.79 (0.25) | 0.92 (0.05) | 0.85 (0.14) |
| IV | 0.95 (n = 1) | 0.92 (0.04) | 0.76 (0.11) | 0.84 (0.13) | 0.84 (0.12) |
| Mean | 0.80 (0.20) | 0.88 (0.12) | 0.84 (0.14) | 0.90 (0.09) | 0.85 (0.15) |
Respondents were also further split by whether they survived for the year subsequent to being surveyed or died during it. Of the survivors only 7% were in stage IV when surveyed, whereas those who died were reasonably equally split across the four stages when surveyed. Among the 161 survivors the mean HUI was 0.85, whereas among the 10 who died it was 0.65 (p = 0.002). Ramsey et al. 187 conclude that the time since diagnosis is a predictor of HRQoL, the longer the survival since diagnosis the higher HRQoL tends to be among those surviving.
Petrou and Campbell188 surveyed 30 UK oncology nurses using the SG. (Note, this article was sponsored by an educational grant from Rhône-Poulenc Rorer, which apparently became a part of Sanofi-Aventis. The article also includes text boxes referring to irinotecan, which is manufactured by Pfizer.) This valued PR, SD and PD with the median reported HRQoL values for these being 1.000, 0.950 and 0.575 respectively. Grade III/IV AEs were also rated. Hand and foot syndrome and an episode of febrile neutropenia were reported as not having a significant QoL impact, with a detriment of between 0.200 and 0.250. Diarrhoea was rated as having a moderate impact on QoL, with a detriment of between 0.325 and 0.500. Nausea/vomiting was seen as more serious, with a detriment of between 0.550 and 0.625, whereas mucositis was rated as having a detriment of between 0.575 and 0.625. Petrou and Campbell188 noted in the discussion that the QoL detriments from the grade III/IV AEs did not take into account any difference in the likely durations of the AEs. Their main conclusion was that there was little evidence for a major difference in HRQoL between PR and the SD, but clear evidence for a preference for these over the PD health state.
Ness et al. 225 identified 166 US patients who had previously had colorectal adenoma removed, identified using the endoscopy clinical database of the Indiana University Medical Centre. Some could not be contacted, refused to be interviewed or did not attend, leaving 90 respondents of whom nine were excluded. The mean respondent age was 54 years, with respondents being equally split between men and women.
Seven health states were developed based on the stage of CRC, method of treatment, presence of serious side effects and presence of ostomy. Health state vignettes were developed, splitting effects into the short term of 18 months and the long term of 30 years, though terminal health states were limited to 18 months. These were valued using the SG, which resulted in the following mean HRQoL values. Due to the two stage III colon cancer health states never being compared directly and the difference between them not being statistically significant (p = 0.093) these were combined. This also applied to the two stage IV metastatic health states (p = 0.595) (Table 84).
| Health state | n | Mean (95% CI) | |
|---|---|---|---|
| Stage I: rectal or stage I/II colon, resected | 81 | 0.74 (0.69 to 0.78) | 0.74 (0.69 to 0.78) |
| Stage III: colon, resection + chemotherapy, no significant AEs | 40 | 0.70 (0.63 to 0.77) | 0.67 (0.62 to 0.72) |
| Stage III: colon, resection + chemotherapy, significant AEs | 41 | 0.63 (0.56 to 0.70) | |
| Stage II/III: rectal, resection/chemotherapy/radiotherapy | 81 | 0.59 (0.54 to 0.64) | 0.59 (0.54 to 0.69) |
| Stage II/III: rectal, resection/chemotherapy/radiotherapy, with ostomy | 81 | 0.50 (0.44 to 0.56) | 0.50 (0.44 to 0.56) |
| Stage IV: metastatic/unresectable no ostomy | 41 | 0.24 (0.16 to 0.32) | 0.25 (0.20 to 0.31) |
| Stage IV: metastatic/unresectable with ostomy | 40 | 0.27 (0.18 to 0.36) | |
Ko et al. 226 analysed the US nationwide 1998 National Health Interview Survey database, extracting all patients identified with melanoma, breast, colon or lung cancer. These patients had had the Health and Activities Limitation Index administered, for which there is a standard scoring algorithm for conversion to a single utility index. A total of 169 colon cancer patients were identified with a mean age 61 years and a broad balance between men and women. There were 32 patients within 1 year of diagnosis and had a mean utility of 0.67 (s.d. 0.21), 80 patients were between 1 and 5 years from diagnosis and had a mean utility of 0.68 (s.d. 0.22) and 80 patients were > 5 years since diagnosis and had a mean utility of 0.71 (s.d. 0.25).
Mittmann et al. 191 in a cost-effectiveness study of adding cetuximab to best supportive care for the treatment of mCRC analysed HUI version 3 QoL data from a publicly-funded Canadian RCT. A total of 575 patients were recruited to the trial, with HUI data being collected at baseline, 4, 8, 16 and 24 weeks. The HRQoL values resulting from this are listed in Table 85.
| Time point | Cetuximab + BSC | BSC | ||||
|---|---|---|---|---|---|---|
| n | Mean | s.d. | n | Mean | s.d. | |
| Baseline | 263 | 0.72 | 0.23 | 260 | 0.71 | 0.24 |
| Week 4 | 220 | 0.73 | 0.26 | 184 | 0.68 | 0.26 |
| Week 8 | 190 | 0.73 | 0.24 | 149 | 0.66 | 0.28 |
| Week 16 | 119 | 0.73 | 0.24 | 72 | 0.63 | 0.30 |
| Week 24 | 82 | 0.77 | 0.22 | 36 | 0.70 | 0.24 |
CG131 adverse events
Lloyd et al. 196 (employed by the United Biosource Corporation, also co-authored by J Watkins, an employee of Eli-Lilly and Company Ltd.) surveyed 100 members of the UK general public of average age 40 years and equally split between men and women. They assessed the HRQoL of various health states associated with metastatic breast cancer using the SG, with 969 observations being analysed. The method of recruitment of respondents is not clear. Respondents were not told that the health states were related to metastatic breast cancer. A mixed model, where the HRQoL was equal to the exponent of the sum of the relevant coefficients divided by one plus the exponent of the sum of the relevant coefficients, was applied.
Health state vignettes were developed from a rapid literature review, expert opinion and a focus group with oncology nurses. A further content validation study of the health states was conducted by interviewing three clinical oncologists. The health states were designed to describe a 3-week period. Two base health state vignettes were developed: one for responding metastatic breast cancer and one for stable metastatic breast cancer.
The parameter estimates of the mixed model for treatment response, treatment progression and all the AEs were all significant at the 1% level. They resulted in the following HRQoL value for the base state of SD with no toxicity, and HRQoL increments and decrements (Table 86).
| Health state | HRQoL |
|---|---|
| Stable with no toxicity | 0.715 |
| Treatment response | +0.075 |
| Disease progression | –0.272 |
| Febrile neutropenia | –0.150 |
| Diarrhoea and vomiting | –0.103 |
| Hand and foot syndrome | –0.116 |
| Stomatitis | –0.151 |
| Fatigue | –0.115 |
| Hair loss | –0.114 |
Metastatic colorectal cancer quality of life literature review update
Färkkilä et al. 192 reviewed the EQ-5D data of 580 Finnish CRC patients, a proportion of whom had mCRC disease. All patients with CRC treated at the Helsinki University Central Hospital were invited to participate by post, with non-responders receiving one reminder. The EQ-5D was scored using the UK social tariff. Local disease patients were divided into those in primary treatment, deemed to be within 6 months of diagnosis; those in rehabilitation, being 6–18 months from diagnosis; and those in remission, being > 18 months from diagnosis. How reasonable these categories are is open to question, particularly given the possibility of survivor bias. Advanced CRC patients were divided into the metastatic who were still receiving oncological care and those who were receiving only palliative care. This resulted in the following EQ-5D QoL estimates (Table 87).
| Health state | n | EQ-5D | SEM | 95% CI |
|---|---|---|---|---|
| Local disease | ||||
| Primary treatment | 61 | 0.760 | 0.030 | 0.699 to 0.823 |
| Rehabilitation | 79 | 0.835 | 0.023 | 0.777 to 0.881 |
| Remission | 217 | 0.850 | 0.014 | 0.828 to 0.882 |
| Advanced disease | ||||
| Metastatic disease | 110 | 0.820 | 0.019 | 0.783 to 0.858 |
| Palliative care | 41 | 0.643 | 0.049 | 0.546 to 0.747 |
Odom et al. 195 (funded by Amgen) analysed EQ-5D data from a Phase III trial of panitumumab plus best supportive care versus best supportive care among chemotherapy-refractive mCRC patients (n = 463). EQ-5D data were measured at baseline and monthly thereafter until disease progression. Unfortunately, this was evaluated using a US valuation of EQ-5D health states. Baseline EQ-5D scores were 0.72 (s.d. 0.24) in the panitumumab arm (n = 188) and 0.68 (s.d. 0.25) in the best supportive care arm (n = 175). The changes from baseline appear to be reasonably linear, with the change at 17 weeks in the best supportive care arm being around –0.5 with a 95% CI of around –0.3 to –0.7.
Shiroiwa et al. ,193 in what appears to be a paper prior to that reported in greater detail in the literature review of AEs QoL impact, undertook a TTO study among members of the Japanese general public. This assessed the QoL of hypothetical mCRC patients undergoing chemotherapy and with a range of grade III/IV AEs. The number of respondents surveyed was considerably smaller than that of the paper reported in the literature review of AEs QoL impact. This resulted in the following QoL values being reported (Table 88). Given the different QoL scores for being treated with XELOX and with FOLFOX, it appears that the QoL values for the AEs were estimated in isolation from these.
| Health state | n | QoL | 95% CI |
|---|---|---|---|
| XELOX no AE | 191 | 0.59 | 0.55 to 0.64 |
| FOLFOX no AE | 183 | 0.53 | 0.49 to 0.57 |
| Grade III/IV AEs | |||
| Febrile neutropenia | 175 | 0.39 | 0.36 to 0.42 |
| Nausea/vomiting | 192 | 0.38 | 0.35 to 0.42 |
| Diarrhoea | 188 | 0.42 | 0.39 to 0.45 |
| Hand and foot syndrome | 174 | 0.39 | 0.36 to 0.42 |
| Fatigue | 185 | 0.45 | 0.41 to 0.48 |
| Peripheral neuropathy | 176 | 0.45 | 0.41 to 0.48 |
| Stomatitis | 202 | 0.42 | 0.39 to 0.45 |
Wang et al. 194 (funded by Amgen) undertook a Q-TWiST analysis of data from a Phase III open-label trial comparing panitumumab plus best supportive care against best supportive care among patients with chemotherapy-refractive mCRC. Survival in both arms was partitioned into time without symptoms of disease of a grade III/IV toxicity, time with toxicity, and relapse or disease progression. The duration of grade III/IV toxicities was recorded within the trial, with the mean duration of toxicity across all patients being 3.47 weeks in the panitumumab arm and 1.09 weeks in the best supportive care arm. EQ-5D data were collected during the trial, but unfortunately the paper does not make clear the valuation method. It resulted in the following QoL values (Table 89).
| Health state | Panitumumab (n = 124) | BSC (n = 119) | ||
|---|---|---|---|---|
| n | EQ-5D | n | EQ-5D | |
| No symptoms or toxicity | 104 | 0.768 | 103 | 0.663 |
| Toxicity: grade III/IV AE | 37 | 0.601 | 13 | 0.441 |
| Relapse/progression | 68 | 0.632 | 63 | 0.641 |
Appendix 17 Metastatic colorectal cancer UK resource use literature review
Beard et al. 227 undertook a cost-effectiveness of resection for liver metastases compared with standard nonsurgical chemotherapy among mCRC patients. A total of 100 liver resections performed at the Royal Hallamshire Hospital between 1997 and 1999 were analysed. A mean operating time of 3.5 hours was recorded, coupled with a mean LOS of 10.3 days. The average cost per resection was estimated to be £6742 [£10,334]. The average cost of chemotherapy was estimated as £2223 [£3408] per month.
Cassidy et al. 228 undertook a cost-effectiveness analysis of capecitabine compared with FUFOL based on the Xeloda in Adjuvant Colon Cancer Therapy trial. The drug use for AEs was recorded, with the text noting capecitabine reducing the use of fluconazole for stomatis, 5-HT3 antagonists for nausea/vomiting and cytokines for vomiting. However, individual costs per event are unfortunately not given. Note that they also drew QoL values of 0.86 for SD, 0.86 for when undergoing chemotherapy and 0.59 for PD from Ramsey et al. 187
Cunningham et al. 229 undertook a cost-effectiveness analysis of adding irinotecan for 5-FU/FA among mCRC patients. A cost per disposable pump inclusive of disposables and pharmacist time of £62 [£87] was estimated. Total AEs costs in the FOLFIRI arm were estimated to be £1480 [£2072] compared with £1147 [£1606] in the 5-FU/FA arm. Unfortunately, no breakdown of costs by AE was given.
Hale et al. 92 undertook an analysis of the costs of the de Gramont, the Lokich and raltitrexed chemotherapy among a subsample of 68 patients taking part in an MRC-funded chemothrerapy trial among 905 mCRC patients. Costs were not sufficiently disaggregated to be useful for current purposes, with the possible exception of primary care costs. The 12-week costs of care for the de Gramont, Lokich and raltitrexed were £2672 [£4282], £983 [£1575] and £1305 [£2091] for chemotherapy, £1699 [£2722], £666 [£1067] and £814 [£1304] for hospitalisations and £114 [£182], £126 [£201]202 and £152 [£244] for primary care. Societal costs of £914 [£1465], £762 [£1221] and £404 [£647] were also estimated, these being mainly composed of carer time.
Hoyle et al. 230 undertook a cost-effectiveness analysis of cetuximab in conjunction compared with among other things panitumumab. Little by way of resource use is provided, other than to note that PFS was assumed to require a consultant visit every 2 weeks.
Iveson et al. 206 analysed the data from a clinical trial of irinotecan versus infusional 5-FU to estimate their relative cost-effectiveness. Line insertion was estimated to cost £250 [£423], whereas disposable pumps inclusive of pharmacist time was costed at £62 [£105]. Unplanned hospitalisations were recorded during the trial and were costed using 1996/7 NHS reference costs. Total costs appear to relate to the patient survival though this is not entirely clear: a median of 10.8 months in the irinotecan arm and 8.5 months across the 5-FU arms. Hospitalisations were costed at £2810 [£4763] for the irinotecan arm and £3416 [£5788] for the 5-FU arms. Hospital consultations were costed at £357 [£605] and £393 [£666], general practice (GP) visits £23 [£39] and £13 [£22], district nurse visits £39 [£65] and £23 [£39] and tests £19 [£32] and £8 [£13].
Starling et al. 231 undertook a cost-effectiveness analysis of cetuximab/irinotecan compared with best supportive care for the treatment of mCRC among patients having failed previous chemotherapy. The data were apparently trial based, with the data for best supportive care coming from 43 eligible patients who were not enrolled due to recruitment having been completed. Ignoring the chemotherapy drug and administration costs for cetuximab/irinotecan, these patients incurred an additional £59.70 [£69.09] per week treatment costs while on chemotherapy. The average weekly cost when not on therapy was £50.00 [£57.87], the same as that for other costs incurred in the best supportive care arm whether receiving chemotherapy or not. Of the 31% of best supportive care patients who received additional palliative chemotherapy, the mean drug cost was £5327 [£6165] and the mean administration cost £1482 [£1715]. Startling et al. 231 note that the mean survival in the best supportive care arm was 5.2 months but it is unclear what proportion of this time the best supportive care patients received palliative chemotherapy.
Appendix 18 Previous National Institute for Health and Care Excellence assessments in head and neck cancer
As in Appendix 15, the following reviews the previous NICE assessments in H&N cancer, examining:
-
the approach to estimating chemotherapy administration costs for infusion regimes
-
the approach to modelling the duration of treatment
-
the approach to modelling survival
-
QoL values for disease states and AEs
-
the costs of AEs.
Chemotherapy administration costs for infusion regimes
TA145: cetuximab for locally advanced squamous head and neck cancer215
The i.v. infusion of cetuximab was apparently costed as a medical oncology outpatient visit using NHS reference costs: £178.66 for the initial visit and £124.66 thereafter. The ERG noted the i.v. infusion administration cost, but did not particularly comment on it.
TA172: cetuximab for recurrent and/or metastatic squamous head and neck cancer220
A weighted average of inpatient and outpatient administration was drawn from Hopper et al. 232 These were costed using the NHS 2007–8 tariff for X99OST: Solid Tumour Cancer Chemotherapy: All Drugs at £296.00, and the NHS reference costs 2004 for a medical oncology outpatient visit of £124.66.
The approach to modelling the duration of treatment
TA145: cetuximab for locally advanced squamous head and neck cancer215
The cost of radiotherapy and cetuximab was drawn from IPD in the pivotal trial. Three radiotherapy regimens were possible, with cetuximab being added to these. The maximum duration of treatment was 8 weeks.
TA172: cetuximab for recurrent and/or metastatic squamous head and neck cancer220
The cost of cetuximab was drawn from IPD in the pivotal trial. The pivotal trial permitted up to six 21-day cycles of therapy. Some of the regimen drugs could be withdrawn if not tolerated. Study treatment was discontinued early if there was unacceptable toxicity or PD.
The approach to modelling survival
TA145: cetuximab for locally advanced squamous head and neck cancer215
For those deemed to be cured, UK life tables coupled with a proportionate hazard of 2.786 were used to model mortality. For the remainder, PFS and OS was modelled by fitting log-normal parametric curves to the Kaplan–Meier data.
The ERG comments on the modelling of survival appeared to mainly be with its treatment in the probabilistic modelling, the large uncertainty around the extrapolated survival not be reflected within this and the probabilistic results.
TA172: cetuximab for recurrent and/or metastatic squamous head and neck cancer220
Parameterised Weibull curves were fitted to the trial Kaplan–Meier data for PFS and OS.
Quality-of-life values for disease states and adverse events
TA145: cetuximab for locally advanced squamous head and neck cancer215
The manufacturer commissioned a utility valuation study from M-TAG Ltd. This aimed to estimate utility values for a series of health states describing a range of side effects and post-treatment outcomes among patients with locally advanced squamous cell H&N cancer. A literature search identified how the AE profiles changed with the addition of cetuximab to radiotherapy. This informed the choice of AEs that should be included in the study:
-
stomatis/mucus membrane disorders
-
nausea/vomiting
-
haematological toxicities
-
rash/acne
-
late onset peripheral neuropathy
-
late onset ototoxicity.
Seven health states were used to describe different toxicity grades, based on the NCI Common Toxicity Criteria. Two further health states described the late toxicities of peripheral neuropathy and ototoxicity, and a further two health states described the final outcomes of treatment success and treatment failure.
A total of 50 UK oncology nurses were recruited for the study, as it was thought unethical to recruit patients. Nurses were screened before being accepted into the study in order to ensure they were familiar with the area, having:
-
a minimum of 2 years working as an oncology nurse
-
a minimum of 11 patients in clinic with locally advanced squamous cell H&N cancer in the last 3 months; and,
-
experience in treating patients with radiotherapy, chemotherapy or concomitant chemoradiation therapy.
They rated the 11 health states using the EQ-5D and the EQ-5D visual analogue scale (VAS), and also ranked the various health states from 1 to 11. This resulted in the following utility estimates (Table 90).
| State | Description | Mean (s.d.) | ||
|---|---|---|---|---|
| EQ-5D | EQ-5D VAS | Rank | ||
| A | On treatment, range of AEs ≤ 1 | 0.659 (0.131) | 73.5 (17.14) | 2.6 (1.57) |
| B | A plus mucositis grade III/IV | 0.062 (0.299) | 23.5 (17.17) | 8.8 (1.85) |
| C | A plus mucositis grade II | 0.608 (0.310) | 52.3 (16.55) | 5 (1.52) |
| D | A plus nausea grade III/IV | 0.108 (0.350) | 30.7 (16.72) | 8 (1.71) |
| E | A plus nausea grade II | 0.573 (0.247) | 55.1 (17.37) | 4.6 (1.56) |
| F | A plus acne/rash grade III or IV | 0.226 (0.404) | 40.2 (20.11) | 7.3 (1.7) |
| G | A plus haematological grade IV | 0.101 (0.392) | 30.7 (19.17) | 8.2 (1.96) |
| H | Post-treatment peripheral neuropathy | 0.473 (0.266) | 57 (14.43) | 4.9 (2.10) |
| I | Post-treatment ototoxicity | 0.657 (0.239) | 60.9 (17.63) | 4.2 (2.38) |
| J | Post-treatment loco regional control | 0.862 (0.019) | 82.6 (15.23) | 1.8 (1.94) |
| K | Post-treatment PDa | 0.129 (0.266) | 10.8 (11.81) | 10.5 (1.13) |
These values needed to be coupled with the mean times in the health states. For treatment duration as defined by the acute phase this was differentiated by arm. For the AEs for health states B through to G, these were calculated based on the average time spent with the AE pooled across the arms. To do this, health states B–G were ordered according to the ranking of the utility study. For patients experiencing more than one of the health states B–G, the QALY impact of the AEs was determined by the mean EQ-5D utility for the worst health state experienced multiplied by the mean duration of the AE during the pivotal trial. The submission is not quite clear about how the mean durations of the AEs were calculated, but it appears to be across all events observed during the trial without any similar ranking for multiple events being applied. The ERG report, in its assessment of the costing of AEs, further suggests that overlapping AEs were ignored in the calculation of the mean durations of AEs (Table 91).
| State | Description | EQ-5D | Duration (days) | QALY decrement | |
|---|---|---|---|---|---|
| Mean | Decrement | ||||
| A | On treatment, range of AEs ≤ 1 | 0.659 | |||
| B | A plus mucositis grade III/IV | 0.062 | 0.597 | 55.43 | 0.0907 |
| C | A plus mucositis grade II | 0.608 | 0.051 | 34.46 | 0.0048 |
| D | A plus nausea grade III/IV | 0.108 | 0.551 | 13.14 | 0.0198 |
| E | A plus nausea grade II | 0.573 | 0.086 | 29.82 | 0.0070 |
| F | A plus acne/rash grade III or IV | 0.226 | 0.433 | 72.92 | 0.0865 |
| G | A plus haematological grade IV | 0.101 | 0.558 | 44.32 | 0.0678 |
The ERG noted that given the absence of other studies for locally advanced SCC of the H&N, the nurses were reasonable patient proxies given their experience. The ERG also noted that not taking into account multiple AEs could have tended to lessen the estimated impact of AEs within the modelling, though it is not clear whether or not this is a criticism of the estimated utilities and QALY losses for the given health states per se. The ERG also noted that censoring could have tended to reduce the estimated duration of AEs and so their estimated QALY impact.
TA172: cetuximab for recurrent and/or metastatic squamous head and neck cancer220
Individual patient EORTC QLQ-C30 data were mapped onto EQ-5D scores using the algorithm developed by Kind221 study among pancreatic cancer patients where:
HRQoL = 0.633 + 0.047 × Q29 –0.124 × Q3 –0.167 × Q5 – 0.102 × Q20 – 0.082 × Q26
This resulted in HRQoL values of 0.69 for stable/response with cetuximab, 0.65 for stable/response with standard treatment and 0.52 for PD. Unfortunately, the detail of this is given in an appendix to the submission that is not publicly available. QoL values for AEs were not separately calculated.
The ERG noted the uncertainty inherent in the mapping function, and the lack of any statistically significant difference for health states between the arms. The ERG also noted that AEs had not been explicitly considered, and also that these would not have been captured within the mapping function of Kind221 It felt that some elements of the mapping function for EORTC QLQ-C30 in lung cancer of Baghust et al. 233 could have proxied for those elements not within Kind. 221
The cost of adverse events
TA145: cetuximab for locally advanced squamous head and neck cancer215
Some AEs were grouped into a single category for costing purposes: mucositis/stomatitis/dysphagia, acne/rash and nausea/vomiting. An expert panel was convened to estimate the proportion of AEs that would result in a hospital admission, and the medication that would be administered for both those who were and were not admitted. NHS reference costs were applied to the proportion that were assumed to be admitted, these costs being assumed to cover all relevant procedures. Thrombocytopenia was associated with the cost of a platelet transfusion, the estimate for this being drawn from Varney and Guest234 Medication costs were conditioned by the duration of events as estimated from trial data, and described in greater detail in Quality-of-life values for disease states and adverse events. No primary care costs were included presumably due to it being assumed that ongoing routine hospital follow-up identified and prescribed medication for the AEs.
An ERG expert suggested that the grouping of AEs for costing purposes into mucositis/stomatitis/dysphagia, acne/rash and nausea/vomiting was reasonable. The ERG also noted the possibility of bias arising from censored data and questioned the elimination of overlapping AEs from the analysis in order to estimate the mean durations of individual AEs.
TA172: cetuximab for recurrent and/or metastatic squamous head and neck cancer220
The AE costs as estimated in TA145215 were applied (Table 92).
| AE | Grade | Medication | Cost (£) | Admitted, % | HRG for non-elective inpatient | Cost (£) | Total (£) |
|---|---|---|---|---|---|---|---|
| Mucositis/stomatitis/dysphagia | II | Antifungal mouth rinse | 4.01 | 5 | C37 – complex major head, neck or ear diagnoses no complication | 1818 | 95 |
| Mucositis/stomatitis/dysphagia | III | Antifungal mouth rinse | 4.01 | 10 | C36 – complex major head, neck or ear diagnoses with complication | 3036 | 308 |
| Mucositis/stomatitis/dysphagia | IV | NA as 100% admitted | 100 | C36 – complex major head, neck or ear diagnoses with complication | 3036 | 3036 | |
| Nausea/vomiting | II | Antiemetics | 4.86 | 10 | F47 – general abdominal disorders no complication | 702 | 75 |
| Nausea/vomiting | III | Antiemetics | 4.86 | 30 | F46 – general abdominal disorders with complication | 1099 | 335 |
| Nausea/vomiting | IV | NA as 100% admitted | 100 | F46 – general abdominal disorders with complication | 1099 | 1099 | |
| Weight loss | III or IV | None | 0 | NA | NA | 0 | |
| Dry mouth | III or IV | None | 0 | NA | NA | 0 | |
| Fatigue | III or IV | None | 0 | NA | NA | 0 | |
| Dehydration | III or IV | NA as 100% admitted | 100 | K09 – disorders of nutrition | 1519 | 1519 | |
| Acne/rash | III or IV | Topical/oral antibacterial | 21.24 | 0 | NA | NA | 42 |
| Thrombocytopenia | III or IV | Platelet transfusion | 84.22 | 0 | NA | NA | 84 |
| Febrile neutropenia | III or IV | NA as 100% admitted | 100 | P23 – blood cell disorders | 1337 | 1337 | |
| Leucopenia | III or IV | None | 0 | NA | NA | 0 | |
| Anaemia | III or IV | NA as 100% admitted | 100 | S06 – red blood cell disorders without complication | 930 | 930 | |
| Fever/infection | III or IV | Antipyretic | 50 | P05 – major infections | 2207 | 1103 |
Appendix 19 Head and neck cancer: other quality of life literature review
Gerson et al. 235 surveyed 130 US patients with Barrett’s oesophagus using the TTO. Few of the health states were relevant to the current assessment. However, patients were asked to value oesophageal cancer using the TTO and reported a mean value of 0.67 (s.d. 0.19).
Llewellyn-Thomas et al. 236 surveyed 66 US patients with cancer of the larynx using the TTO prior to a 4-week course of radiotherapy. The majority were stage I/II with only one-third being stage III/IV. Three hypothetical health states were developed as outlined in Table 93.
| Severity | Mouth/throat pain | Usual activities | Talking |
|---|---|---|---|
| Low | None | Enough energy | As usual |
| Moderate | Moderate | Fatigue reduced | Minimally |
| Severe | Severe | Fatigue stopped | None |
Subsequent to treatment, patients were asked to rate their own health state as being closest to either low, moderate or severe and again asked to rate the health states using the TTO. This resulted in the following mean TTO values and s.d. values, where pre is pre-treatment and post is post treatment (Table 94).
| Outcome group | Outcome group post treatment | Pooled | ||
|---|---|---|---|---|
| Mild (n = 24) (s.d.) | Moderate (n = 36) (s.d.) | Severe (n = 6) (s.d.) | ||
| Mild | ||||
| Pre treatment | 0.721 (0.262) | 0.750 (0.199) | 0.750 (0.241) | 0.739 |
| Post treatment | 0.735 (0.235) | 0.757 (0.190) | 0.866 (0.075) | 0.759 |
| Moderate | ||||
| Pre treatment | 0.629 (0.269) | 0.644 (0.229) | 0.700 (0.270) | 0.644 |
| Post treatment | 0.571 (0.264) | 0.667 (0.218) | 0.758 (0.150) | 0.640 |
| Severe | ||||
| Pre treatment | 0.352 (0.283) | 0.344 (0.253) | 0.233 (0.227) | 0.337 |
| Post treatment | 0.429 (0.292) | 0.381 (0.267) | 0.408 (0.390) | 0.401 |
Somewhat to the authors’ surprise, they concluded that patients’ valuations of health states remained reasonably consistent through time.
McNamee et al. 237 surveyed 56 UK patients who had received curative treatment for oesophageal cancer. These were surveyed using both the SG and the TTO, with 28 patients being randomly allocated to each assessment mechanism. Five health states were developed, as outlined in Table 95.
| Health state | Solid foods | Usual eating | Usual activities | Symptoms |
|---|---|---|---|---|
| 1 | Fine | Usual | No problems | One of pain, short breath |
| 2 | Difficult | Less | Some | One or more of pain, short breath, vomiting |
| 3 | None | A lot less | Frequent problems | Two or more of pain, short breath, vomiting, sore muscles, taste loss, bad breath |
| 4 | Liquid diet | Little | Lot of problems | Three or more of pain, short breath, vomiting, sore muscles, taste loss, bad breath |
| 5 | – | None | None | Four or more of pain, short breath, vomiting, sore muscles, taste loss, bad breath plus others |
McNamee et al. 237 also defined three possible treatments, with patients experiencing frequent problems carrying out their usual activities across all three treatments. The first involved one trip to hospital, a stay of 2 nights and moderate pain for a few days after treatment; the second involved one trip to the hospital without admission and having moderate pain for 2 weeks after treatment; and, the third involved making two or three trips to hospital with each involving a night’s stay and each having mild pain for a few days after each treatment.
The QoL values that resulted are presented in Table 96.
| Health state | TTO, mean (95% CI) | SG, mean (95% CI) |
|---|---|---|
| 1 | 0.66 (0.50 to 0.81) | 0.78 (0.66 to 0.89) |
| 2 | 0.45 (0.31 to 0.60) | 0.49 (0.35 to 0.63) |
| 3 | 0.35 (0.21 to 0.50) | 0.27 (0.15 to 0.40) |
| 4 | 0.25 (0.13 to 0.38) | 0.20 (0.08 to 0.31) |
| 5 | 0.08 (0.00 to 0.17) | 0.08 (0.00 to 0.17) |
| Treatment 1 | 0.64 (0.50 to 0.78) | 0.60 (0.47 to 0.74) |
| Treatment 2 | 0.54 (0.40 to 0.69) | 0.61 (0.48 to 0.74) |
| Treatment 3 | 0.62 (0.50 to 0.76) | 0.59 (0.45 to 0.74) |
Although there were some differences in the mean QoL values reported using the SG compared with the TTO, none were statistically significant.
Ringash et al. 238 surveys 114 Canadian larynx cancer patients who had been treated with radiotherapy with the previous 6 months using the TTO. Most (83%) were stage I/II. Of the 114 patents, 2 did not complete the TTO. A further 18 patients were excluded for not preferring perfect health. Among the remaining 84 patients the mean QoL was estimated to be 0.878, with a s.d. of 0.174.
Rogers et al. 239 surveyed 348 US oral/oropharyngeal cancer patients who had previously been treated by primary surgery using the EQ-5D. A total of 224 patients returned evaluable forms, with the EQ-5D being evaluated using the UK social tariff. The mean QoL was 0.75, with a standard error of 0.02.
Shenfine et al. 240 apparently applied the EQ-5D among 215 UK patients with inoperable oesophageal cancer during a trial of palliative therapies. There is mention of the UK social tariff, but the reported EQ-5D values range between 6.82 and 8.04 so appear to be of little use.
Stalmeier et al. 241 surveyed 45 Dutch oesophageal patients 6 months after surgery for their tumour using both the VAS and the SG. No details of the stage of patients are given within the paper. Seven health states were developed, all reflecting differing degree of recovery after surgery for oesophageal cancer (Table 97).
| Health state | Eating | Weight loss | Usual activities | Tired | Walking | Pain | Other |
|---|---|---|---|---|---|---|---|
| 2: recurrence-free at home | Small meals | – | – | A little | – | – | – |
| 3: recovery at home | Difficult | Some | Not much | Yes | – | Slight | – |
| 4: in hospital | – | – | – | – | No | – | Dependent |
| 5: in hospital with pneumonia | – | – | – | – | – | – | Dependent |
| 6: recurrence in neoesophagus | Difficult | Not hungry | – | Yes | – | – | Depressed |
| 7: skeletal metastases | – | Not hungry | – | Yes | – | Yes | Depressed |
| 8: unresectable primary tumour | Difficult | Not hungry | – | Yes | – | – | Depressed |
This resulted in the following utility values (Table 98).
| Health state | Rank | VAS | SG | ||
|---|---|---|---|---|---|
| Mean | Mean | s.d. | Mean | s.d. | |
| 1: own health | 2.30 | 0.77 | 0.14 | 0.97 | 0.06 |
| 2: recurrence-free | 2.98 | 0.77 | 0.11 | 0.96 | 0.07 |
| 3: recovery at home | 4.47 | 0.55 | 0.19 | 0.92 | 0.15 |
| 4: in hospital | 4.60 | 0.54 | 0.17 | 0.90 | 0.15 |
| 5: in hospital with pneumonia | 5.84 | 0.39 | 0.16 | 0.82 | 0.25 |
| 6: recurrence in neoesophagus | 7.60 | 0.18 | 0.13 | 0.41 | 0.31 |
| 7: skeletal metastases | 8.22 | 0.16 | 0.13 | 0.35 | 0.30 |
| 8: unresectable primary tumour | 8.33 | 0.11 | 0.10 | 0.34 | 0.31 |
Wildi et al. 242 surveyed 50 US patients with oesophegal cancer using the TTO, the VAS and the EQ-5D. It appears that the EQ-5D was converted to utilities using the US mapping. All patients were staged using spiral CT scans and endoscopic ultrasound. The following HRQoL values by the Surveillance, Epidemiology and End Results Program of the NCI were derived (Table 99).
| Stage | n | VAS | TTO | EQ-5D | |||
|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | ||
| 0 | 3 | 0.83 | 0.18 | 0.99 | 0.00 | 0.93 | 0.12 |
| 1 | 11 | 0.56 | 0.14 | 0.80 | 0.30 | 0.60 | 0.29 |
| 2 | 24 | 0.57 | 0.19 | 0.54 | 0.39 | 0.71 | 0.21 |
| 3 | 12 | 0.58 | 0.21 | 0.52 | 0.31 | 0.69 | 0.35 |
Appendix 20 Head and neck cancer: other UK resource use literature review
Coyle and Drummond243 undertook a costing analysis of data from two distinct but concurrent trials comparing conventional radiotherapy with continuous hyperfractionated accelerated radiotherapy (CHART). These trials recruited patients from 10 UK centres and three European centres, with 212 H&N cancer patients receiving conventional radiotherapy and 314 H&N cancer patients receiving CHART. The H&N cancer patients were roughly equally split between TI/II and TIII/IV, but the majority (68%) were N0. A societal costing perspective was adopted, though the non-NHS PSS costs appear to be limited to patient travel costs and MacMillan nurse support.
For the H&N cancer patients conventional treatment was given 5 days per week to the large volume (44 Gy in 22 fractions) and then to the small volume (22 Gy in 11 fractions) resulting in a treatment over 33 days or 6.5 weeks. CHART saw radiotherapy given three times on each of 12 consecutive days, including the weekend, with an interval of at least 6 hours between each administration. The large volume received a total dose of 37.5 Gy in 25 fractions and the small volume 16.5 Gy in 11 fractions. This resulted in the following resource use estimates (Table 100).
| Resource use | CHART, n (s.d.) | Conventional, n (s.d.) |
|---|---|---|
| Hospital days | ||
| Ward | 20.6 (15.4) | 12.3 (19.7) |
| Hostel | 2.3 (5.0) | 1.6 (7.0) |
| Total | 22.8 (13.9) | 13.8 (20.2) |
| Radiotherapy treatments | ||
| Before normal working hours | 6.8 (4.6) | 0.0 (0.0) |
| During normal working hours | 13.2 (5.0) | 33.0 (0.3) |
| After normal working hours | 9.8 (1.4) | 0.0 (0.1) |
| Weekends | 6.1 (1.1) | 0.0 (0.2) |
| Total | 35.8 (2.3) | 33.0 (0.3) |
| Hospital outpatient appointments | 1.7 (1.4) | 1.5 (1.3) |
| GP consultations | 1.5 (1.8) | 1.3 (2.9) |
| Other community service consultations | 4.9 (14.4) | 5.4 (21.9) |
| Miles travelled for treatment | 42.6 (52.3) | 795.1 (801.0) |
Which in turn resulted in the following cost estimates (Table 101).
| Arm | Radiotherapy | Other hospital | Total hospital | Community | Patient | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHART | £1171 | [£2120] | £2153 | [£3898] | £3325 | [£6017] | £84 | [£152] | £6 | [£11] |
| s.d. | £407 | [£736] | £1492 | [£2701] | £1633 | [£2956] | £196 | [£354] | £12 | [£22] |
| Conventional | £587 | [£1062] | £1557 | [£2818] | £2144 | [£3881] | £93 | [£169] | £85 | [£154] |
| s.d. | £120 | [£217] | £2045 | [£3702] | £2041 | [£3695] | £395 | [£714] | £129 | [£233] |
The large s.d. for the societal elements suggest highly skewed data.
Farndon et al. 244 report the median NHS costs of treatment per month of survival over a 3-year period among 132 UK oesophageal cancer patients, mainly seeking to compare resection with palliation costs. After 3 years around 36% of the resection patients survived, compared with none in the palliation group. Few details of the costing are presented, with the median costs being estimates £8070 [£13,444] for resection compared with palliation costs of radiotherapy of £4720 [£7863], brachytherapy of £1790 [£2982], laser of £3540 [£5897], intubation of £2450 [£4081] and no treatment of £1390 [£2315]. After the initial treatment costs, the costs per remaining month of survival were more similar being £457 [£761] for resection compared with between £342 [£569] and £1125 [£1874] for palliation.
Hopper et al. 230 (funded by biolitec Pharma Ltd), in a cost-effectiveness analysis of Foscan photodynamic therapy compared with palliative chemotherapy for advanced H&N cancer, estimated a cost for four cycles of palliative chemotherapy of £9924 [£13,425].
Kim et al. 43 undertook a retrospective data analysis of the inpatient and outpatient records of 11,403 UK patients with resected SCC of the H&N, accessed through the Health Episode Statistics. Among survivors the mean costs per year rapidly declined after the first year and continued to decline thereafter as shown in Table 102.
| Cost | Year 1 (n = 11,403) (£) | Year 2 (n = 9697) (£) | Year 3 (n = 8433) (£) | Year 4 (n = 7774) (£) | Year 5 (n = 7399) (£) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Second surgery | 208 | [217] | 45 | [47] | 27 | [28] | 29 | [30] | 19 | [20] |
| Reconstructive surgery | 2275 | [2377] | 85 | [89] | 49 | [51] | 55 | [57] | 38 | [40] |
| Radiotherapy | 187 | [195] | 4 | [4] | 6 | [6] | 1 | [1] | 2 | [2] |
| Chemotherapy | 67 | [70] | 17 | [18] | 10 | [10] | 11 | [11] | 8 | [8] |
| AE | 144 | [150] | 23 | [24] | 13 | [14] | 7 | [7] | 5 | [5] |
| Inpatient stay | 16,448 | [17,185] | 1050 | [1097] | 536 | [560] | 378 | [395] | 230 | [240] |
| Total inpatient | 19,330 | [20,196] | 1224 | [1279] | 641 | [670] | 482 | [504] | 302 | [316] |
| Outpatient visits | 414 | [433] | 249 | [260] | 198 | [207] | 163 | [170] | 143 | [149] |
| Radiotherapy | 34 | [36] | 4 | [4] | 7 | [7] | 5 | [5] | 8 | [8] |
| Chemotherapy | 1 | [1] | 0 | [0] | 1 | [1] | 3 | [3] | 3 | [3] |
| Total outpatient | 448 | [468] | 254 | [265] | 206 | [215] | 172 | [180] | 153 | [160] |
| Total cost | 19,778 | [20,664] | 1477 | [1543] | 847 | [885] | 653 | [682] | 455 | [475] |
Parthan et al. 245 (funded by Sanofi-Aventis), in a cost-effectiveness analysis of docetaxel for induction chemotherapy prior to chemo-radiotherapy among patients with locally advances SCC of the H&N, provide resource use estimates for both the induction phase and the chemo-radiotherapy phase, coupled with resource use for surgery and for long-term follow-up to death (Table 103).
| Resource use | Induction, mean (95% CI) | Chemoradiation, mean (95% CI) | Surgery, mean (95% CI) | Follow-up, mean (95% CI) |
|---|---|---|---|---|
| Number of 3-week cycles | 3.00 | 2.00 | 1.00 | – |
| Inpatient days (medical oncology) | 4.00 (3.20 to 4.80) | 6.00 (4.80 to 7.20) | – | – |
| Surgery | – | – | 1.00 | – |
| ICU stay (days) | – | 4.00 (3.20 to .80) | 1.00 (0.80 to 1.20) | – |
| Inpatient (surgical) | – | – | 20.00 (16.00 to 24.00) | – |
| Consultant oncologist (hours) | 1.00 (0.80 to 1.20) | – | – | – |
| Chemotherapy nurse (hours) | 5.00 (4.00 to 6.00) | 2.00 (1.60 to 2.40) | – | – |
| Clinical nurse specialist (hours) | 0.50 (0.40 to 0.60) | 0.50 (0.40 to 0.60) | 3.30 (2.64 to 3.96) | 0.35 (0.28 to 0.42) |
| Radiologist (hours) | – | 0.50 (0.40 to 0.60) | – | – |
| Blood test (test) | 1.00 (0.80 to 1.20) | 1.00 (0.80 to 1.20) | 1.00 (0.80 to 1.20) | – |
| Biochemistry (test) | 1.00 (0.80 to 1.20) | 1.00 (0.80 to 1.20) | – | – |
| CT scan (test) | 0.50 (0.40 to 0.60) | 1.00 (0.80 to 1.20) | – | – |
| Endoscopy (test) | 0.50 (0.40 to 0.60) | 1.00 (0.80 to 1.20) | – | – |
| Dietitian (hours) | 1.25 (1.00 to 1.50) | 2.00 (1.60 to 2.40) | 6.00 (4.80 to 7.20) | 0.43 (0.34 to 0.52) |
| Speech therapy | 1.75 (1.40 to 2.10) | 0.50 (0.40 to 0.60) | 2.50 (2.00 to 3.00) | 0.69 (0.55 to 0.83) |
| Surgery-related outpatient | – | – | 1.00 (0.80 to 1.20) | – |
An estimate for palliative chemotherapy of £11,058 [£11,973] is also given.
Appendix 21 Adverse events and resource use
Buxton and O’Brien246 (funded by Glaxo holdings), in an economic evaluation of ondansteron, note that ‘No empirical data exists on the costs of emetic episodes’. As a consequence, they estimate a cost per ‘significant’ episode of £30 [£58] based on 2 hours of nursing time, 20 minutes of junior doctor time and a 10% probability of requiring a day’s stay, plus some additional disposables. This appears to be a cost estimate for an inpatient.
Flynn et al. 247 (funded by Amgen) analysed trial data from 29 patients receiving granulocyte colony-stimulating factor (G-CSF) plus amphotericin B and 30 patients receiving amphotericin B for neutropenia with suspected deep-seated fungal infection. Case note review provide resource use estimates, an average LOS of 9.4 days for G-CSF patients and 14.6 days for non-G-CSF patients being recorded. Inpatient days were costed at £273 [£437], which with other costs resulted in a total costs of £11,247 [£18,023] for G-CSF patients and of £14,317 [£22,943] for non-G-CSF patients.
Leese et al. 211 (funded by Amgen Roche) analysed the cost of treating febrile neutropenia with malignant blood disorders. Febrile neutropenia is defined as a neutrophil count of less than 1.5 × 109/l, and Leese et al. 211 note that the standard treatment is hospitalisation and antibiotic therapy. Leese et al. 211 collected data from patients admitted to a district general hospital with febrile neutropenia, or who developed febrile neutropenia while receiving inpatient chemotherapy. Only patients with haematological disorders were included: there were no patients with solid tumours. Patients were required to have a neutrophil count of < 1.5 × 109/l and sustained pyrexial illness in excess of 37.5 °C. Forty-six episodes of febrile neutropenia were recorded among 27 patients. The average LOS was 17 days. There was also some use of an intensive care unit which further increased costs. Mean test costs were £400 [£725], mean drug costs were £631 [£1143], mean inpatient costs were £1413 [£2559], resulting in a total mean cost of £2445 [£4428].
Leese210 surveyed six oncologists from six English hospitals to estimate the cost of treating febrile neutropenia in patients with solid tumours. Mean LOSs of 6.3 days in routine care were estimated, with 2.2% of patients requiring critical care of 5.3 days, with costs ranging £640–1317 and a mean cost of £960 [£1738]. Drug and pharmacy costs ranged between £161 [£292] and £489 [£886], averaging £297 [£538]. Diagnostic tests added a further £210 [£380]. This resulted in a total cost estimate ranging between £1049 and £1993, with a mean of £1542 [£2793].
Schlenz et al. 248 undertook a prospective observational study at the regional cancer centre of the Norfolk and Norwich University Hospital. All adult patients admitted with febrile neutropenia during 2007 were identified. A cost per patient was estimated based on their LOS and their probable use of antibiotics and G-CSF based on defined daily doses. Thirty-two patients were identified, all of whom had had prior chemotherapy, with seven patients receiving G-CSF. Unfortunately, average LOSs are not given, but an average hospitalisation cost of £2159 [£2499] was estimated. Antibiotics added a further £194 [£225] to take the total to £2353 [£2723], while among those receiving G-CSF this added a further £189 to take the total to £2542 [£2942].
Twelves et al. 202 present a range of resource use data from a Phase III trial of capecitabine versus 5-FU/FA for advanced or mCRC. Patients were recruited from 59 countries which may lessen the relevance of the resource use data to the UK setting, hospital LOSs for AEs are reported in (Table 104). The Twelves et al. 202 data also suggests that multiple hospitalisations for AEs did not occur.
| AE | Capecitabine (n = 297) | 5-FU/FA (n = 299) | Pooled (n = 596) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Admissions | Total days | Mean LOS | Patients | Admissions | Total days | Mean LOS | Patients | Admissions | Total days | Mean LOS | |
| Dehydration | 5 | 5 | 50 | 10.0 | 0 | 0 | 0 | – | 5 | 5 | 50 | 10.0 |
| Diarrhoea | 13 | 13 | 112 | 8.6 | 14 | 14 | 106 | 7.6 | 27 | 27 | 218 | 8.1 |
| Hand and foot syndrome | 2 | 2 | 3 | 1.5 | 0 | 0 | 0 | – | 2 | 2 | 3 | 1.5 |
| Infection/sepsis | 1 | 1 | 11 | 11.0 | 10 | 10 | 106 | 10.6 | 11 | 11 | 117 | 10.6 |
| Neutropenia | 1 | 1 | 5 | 5.0 | 2 | 2 | 19 | 9.5 | 3 | 3 | 24 | 8.0 |
| Stomatitis | 1 | 1 | 32 | 32.0 | 11 | 11 | 129 | 11.7 | 12 | 12 | 161 | 13.4 |
| Vomiting | 1 | 1 | 4 | 4.0 | 1 | 1 | 5 | 5.0 | 2 | 2 | 9 | 4.5 |
Whyte et al. 249 (funded by Amgen) developed a cost-effectiveness model of the use of G-CSF for the prophylaxis of febrile neutropenia in breast cancer. One-off investigations were costed at £47.86 [£55.39] with additional daily investigations of £9.27 [£10.73], while the average duration of hospitalisation for febrile neutropenia was 8 (s.d. 0.2041) days.
Wolowacz et al. 250 (funded by Sanofi-Aventis) in a cost-effectiveness analysis of docetaxel for early node-positive breast cancer drew a range of costs for grade III/IV AEs from clinical opinion and the literature. Clinical opinion suggests costs per episode of stomatis of £390 [£439]. Other costs drawn from Smith et al. 212 and Twelves et al. 202 were £1965 [£2210] for anaemia, £2527 [£2842] for diarrhoea and £2209 [£2485] for vomiting.
Smith et al. 212 (funded by Schering Plough) undertook a cost minimisation analysis of pegylated liposomal doxorubicin for ovarian cancer, based on data from a RCT coupled with UK unit costs. Of the European patients analysed, 49% were from the UK. Rates of grade III/IV AEs are given in figure 1 of the paper, which, when coupled with the data in table 3 of the paper and an assumption of the trial being randomised on a 1 : 1 basis between the arms, suggests estimates of £1000 [£1532] for stomatitis, £1016 [£1557] for diarrhoea, £600 [£920] for hand and foot syndrome, £1100 [£1686] for nausea/vomiting, £200 [£307] for neutropenia, £780 [£1196] for sepsis/fever and £780 [£1196] for anaemia/thrombocytopenia. This assumes that the same cost is applied per AE regardless of arm. It also places greater weight on the arm in which more events occur in an attempt to reduce rounding errors from what are in some case quite rare events. No real details of the calculations underlying these estimates are given within the paper.
Appendix 22 Literature review of quality of life and adverse events
Summary
This literature review updates and expands the literature review of Shabaruddin et al. 171,207 A summary of the main results of interest to the My5-FU Diagnostic Assessment Report is reported in Tables 105 and 106, followed by a more detailed presentation of the results from the identified papers. Note that of the papers identified within the literature review of Shabaruddin et al. ,171,207 seven (Franic et al. ,259 Grunberg et al. ,260 Hess et al. ,261 Hutton et al. ,262 Leung et al. ,263 Ness et al. 225 and Tosh et al. 264) have not been summarised here due to either repetition of previous work, insufficient detail or AE categories too broad to be useful for assigning utility decrements to individual AEs. An additional 10 papers identified through the update and expansion of the literature review of Shabaruddin et al. 171,207 are also summarised. This summary covers the main AEs reported in the mCRC papers, coupled with the comparative BSA versus PK dosing papers.
| Paper | Bennett251 | Bennett251 | Beuerstein252 | Beuerstein252 | Beuerstein253 | Boyd197 | Havrilesky254 | Havrilesky254 | Ossa255 | Szabo256 | TA145215 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2011 | 2011 | 2009 | 2009 | 2010 | 2011 | 2009 | 2009 | 2007 | 2012 | 2008 |
| Country | – | – | UK | Australia | UK | UK | USA | USA | UK | Canada | UK |
| Cancer | mCRC | mCRC | Advanced melanoma | Advanced melanoma | CLL | CRC | Ovarian | Ovarian | Anaemia | H&N | H&N |
| Qualifier | First line | Second line | – | – | – | – | – | – | – | – | – |
| n patients | 656 | 530 | – | – | – | NR | 13 | – | – | – | – |
| n nurses | – | – | – | – | – | – | – | – | – | – | 50 |
| n clinicians | – | – | – | – | – | – | – | – | – | – | – |
| n public | – | – | 63 | 77 | 93 | – | – | 37 | 110 | 106 | – |
| TTO | – | – | TTO | TTO | – | – | TTO | TTO | TTO | – | – |
| SG | – | – | – | – | SG | – | – | – | – | SG | – |
| EQ-5D | EQ-5D | EQ-5D | – | – | – | EQ-5D | – | – | – | – | EQ-5D |
| Industry funded | Yes | Yes | Yes | Yes | Yes | – | – | – | Yes | Yes | Yes |
| Grade I/II | |||||||||||
| Diarrhoea | – | – | 0.06 | 0.11 | 0.08 | 0.04 | – | – | – | – | – |
| Nausea | – | – | 0.07 | 0.12 | 0.05 | 0.05 | 0.35 | 0.24 | – | – | 0.086 |
| Vomiting | – | – | 0.07 | 0.12 | 0.05 | 0.05 | 0.35 | 0.24 | – | – | – |
| Mucositis | – | – | – | – | – | – | – | – | – | – | 0.051 |
| Stomatitis | – | – | 0.10 | 0.14 | – | – | 0.12 | 0.09 | – | – | – |
| Hand and foot syndrome | 0.042 | 0.077 | 0.03 | 0.08 | – | – | – | – | – | 0.050 | – |
| Leucopenia | – | – | – | – | – | – | – | – | – | – | – |
| Fatigue | – | – | – | – | – | 0.02 | – | – | – | – | – |
| Anaemia | – | – | – | – | – | – | – | – | 0.08 | – | – |
| Neutropenia | – | – | – | – | – | – | – | – | – | – | – |
| Febrile neutropenia | – | – | 0.09 | 0.13 | – | – | – | – | – | – | – |
| Infection/sepsis | – | – | – | – | – | – | – | – | – | – | – |
| Haematological | – | – | – | – | – | – | – | – | – | – | – |
| Cardiac | – | – | – | – | – | – | – | – | – | – | – |
| Alopecia | – | – | 0.03 | 0.03 | – | – | 0.10 | 0.16 | – | – | – |
| Paper | Bennett251 | Bennett251 | Beuerstein253 | Boyd197 | Frederix198 | Frederix198 | Havrilesky254 | Havrilesky254 | Lloyd196 | Lloyd196 | Nafees257 | Ossa255 | Shiroiwa193 | Shiroiwa193 | Swinburn200 | Szabo256 | Tolley199 | Tam258 | TA145215 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2011 | 2011 | 2010 | 2011 | 2013 | 2013 | 2009 | 2009 | 2006 | 2006 | 2008 | 2007 | 2009 | 2009 | 2012 | 2012 | 2013 | 2013 | 2008 |
| Country | – | – | UK | UK | Sweden | Netherlands | USA | USA | UK | UK | UK | UK | Japan | Japan | UK | Canada | UK | Canada | UK |
| Cancer | mCRC | mCRC | CLL | CRC | Advanced breast | Advanced breast | Ovarian | Ovarian | Metastatic breast | Metastatic breast | NSCLC | Anaemia | mCRC | mCRC | Neuroendocrine | H&N | CLL | Metastatic pancreatic | H&N |
| Qualifier | First line | Second line | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| n patients | 656 | 530 | – | NR | – | – | 13 | – | – | – | – | – | – | – | – | – | – | – | – |
| n nurses | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 50 |
| n clinicians | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 60 | – |
| n public | – | – | 93 | – | 100 | 100 | – | 37 | 100 | 100 | 100 | 110 | 1582 | 1582 | 100 | 106 | 100 | – | – |
| TTO | – | – | – | – | TTO | TTO | TTO | TTO | – | – | – | TTO | TTO | – | TTO | – | – | – | – |
| SG | – | – | SG | – | – | – | – | – | SG | SGa | SG | – | – | SG | – | SG | TTO | – | – |
| EQ-5D | EQ-5D | EQ-5D | – | EQ-5D | – | – | – | – | – | – | – | – | – | – | – | – | – | EQ-5D | EQ-5D |
| Industry funded | Yes | Yes | Yes | – | – | – | – | – | Yes | Yes | Yes | Yes | – | – | Yes | Yes | Yes | – | Yes |
| Grade III/IV | |||||||||||||||||||
| Diarrhoea | – | – | – | 0.09 | 0.29 | 0.19 | – | – | 0.103 | 0.074 | 0.047 | – | 0.054 | 0.056 | 0.171 | – | – | 0.212 | |
| Nausea | – | – | – | 0.14 | – | – | 0.40 | 0.37 | – | – | 0.048 | – | 0.090 | 0.090 | 0.061 | 0.100 | – | 0.194 | 0.551 |
| Vomiting | – | – | – | – | – | – | 0.40 | 0.37 | 0.103 | 0.074 | 0.048 | – | 0.090 | 0.090 | 0.061 | 0.100 | – | 0.194 | |
| Mucositis | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0.100 | – | – | 0.597 |
| Stomatitis | – | – | – | – | – | – | – | – | 0.151 | 0.113 | – | – | 0.055 | 0.034 | – | 0.100 | – | 0.441 | – |
| Hand and foot syndrome | 0.017 | 0.056 | – | – | 0.23 | 0.15 | – | – | 0.116 | 0.085 | 0.032 | – | 0.084 | 0.084 | 0.188 | 0.120 | – | 0.311 | 0.433 |
| Leucopenia | – | – | – | – | 0.23 | 0.09 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Thrombocytopenia | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0.081 | – | 0.108 | – | – |
| Fatigue | – | – | – | – | 0.17 | 0.13 | 0.34 | 0.42 | 0.115 | 0.084 | 0.073 | – | 0.027 | 0.046 | – | – | – | 0.473 | – |
| Anaemia | – | – | 0.09 | – | 0.12 | 0.10 | – | – | – | – | – | 0.38 | – | – | – | 0.060 | – | – | – |
| Neutropenia | – | – | – | – | – | – | 0.30 | 0.36 | – | – | 0.090 | – | – | – | – | – | 0.163 | – | – |
| Febrile neutropenia | – | – | – | – | – | – | 0.46 | 0.44 | 0.150 | 0.112 | 0.090 | – | 0.082 | 0.042 | – | – | – | 0.131 | – |
| Infection/sepsis | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0.195 | – | – |
| Haematological | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0.070 | – | – | 0.558 |
Methods and results of individual papers
Bennett et al. 251 (supported by Amgen) analysed EQ-5D data from a panitumumab mCRC trial among the KRAS-WT subset. EQ-5D data from 576 of 656 first-line patients and from 530 of 597 second-line patients were analysed, and were valued using the UK social tariff. Around 95% of first-line patients and of second-line patients were of European Cooperative Oncology Group performance score 0–1. The mean baseline QoL for first-line line patients was 0.778 (0.247, n = 284) in the panitumumab + FOLFOX4 arm and 0.756 (0.244, n = 292) in the FOLFOX arm. The mean baseline QoL for second-line patients was 0.769 (0.230, n = 263) in the panitumab + FOLFIRI arm and 0.762 (0.252, n = 267) in the FOLFIRI arm. In a further analysis the QoL decrements associated with grade II and grade III+ skin toxicities were estimated for first- and for second-line patients. These estimates do not appear to have controlled for the impact of other comorbidities, which if correlated with skin toxicities could bias the analysis (Table 107).
| Grade | First-line patients | Second-line patients |
|---|---|---|
| II | 0.042 (95% CI –0.012 to 0.095) | 0.077 (95% CI 0.014 to 0.140) |
| III+ | 0.017 (95% CI –0.038 to 0.071) | 0.056 (95% CI –0.003 to 0.116) |
Best et al. 190 surveyed 49 CRC patients and 49 members of the US general public using TTO, to elicit QoL values for stage III CRC and the QoL decrements associated with mild, moderate and severe neuropathy. The raw TTO mean scores were reported, alongside mean scores adjusted for education and current health for a typical 60 year old (Table 108).
| Health state | Patients | Public | ||||||
|---|---|---|---|---|---|---|---|---|
| Raw | Adjusted | Adjusted decrement | Raw | Adjusted | Adjusted decrement | |||
| Mean | Mean | Mean | SEM | Mean | Mean | Mean | SEM | |
| Remission | 0.87 | 0.83 | 0.83 | 0.82 | ||||
| Adjuvant, no AE | 0.67 | 0.61 | –0.221 | 0.063 | 0.62 | 0.60 | –0.223 | 0.054 |
| with mild neuropathy | 0.65 | 0.61 | –0.224 | 0.075 | 0.52 | 0.51 | –0.310 | 0.060 |
| with moderate neuropathy | 0.55 | 0.53 | –0.309 | 0.075 | 0.48 | 0.46 | –0.362 | 0.056 |
| with severe neuropathy | 0.48 | 0.48 | –0.352 | 0.073 | 0.35 | 0.34 | –0.475 | 0.060 |
| Metastatic, stable | 0.46 | 0.40 | –0.433 | 0.076 | 0.54 | 0.51 | –0.305 | 0.055 |
| Metastatic, progressive | 0.38 | 0.37 | –0.464 | 0.074 | 0.21 | 0.21 | –0.607 | 0.058 |
Beuerstein et al. 252 (funded by Bristol-Myers Squibb) surveyed 63 members of the UK general public and 77 members of the Australian general public using the SG to elicit QoL values for advanced melanoma and the side effects of treatment. Health state vignettes were developed using the NCI CTCAE definitions (Table 109).
| Health state | All | Australia | UK | |||
|---|---|---|---|---|---|---|
| Mean | s.e. | Mean | s.e. | Mean | s.e. | |
| Clinical response states | ||||||
| PR | 0.88 | 0.01 | 0.91 | 0.01 | 0.85 | 0.02 |
| SD | 0.80 | 0.01 | 0.83 | 0.01 | 0.77 | 0.02 |
| PD | 0.52 | 0.02 | 0.47 | 0.03 | 0.59 | 0.02 |
| Best supportive care | 0.52 | 0.02 | 0.46 | 0.03 | 0.59 | 0.02 |
| Utility decrement for grade I/II AEs | ||||||
| Hair loss | –0.03 | 0.01 | –0.03 | 0.01 | –0.03 | 0.01 |
| Skin reaction | –0.06 | 0.01 | –0.08 | 0.01 | –0.03 | 0.01 |
| Diarrhoea | –0.09 | 0.01 | –0.11 | 0.01 | –0.06 | 0.01 |
| Nausea/vomiting | –0.10 | 0.01 | –0.12 | 0.01 | –0.07 | 0.01 |
| Flu-like syndrome | –0.11 | 0.01 | –0.13 | 0.01 | –0.09 | 0.01 |
| Stomatitis | –0.13 | 0.01 | –0.14 | 0.01 | –0.10 | 0.02 |
| Symptomatic melanoma | –0.16 | 0.01 | –0.20 | 0.02 | –0.11 | 0.02 |
| Utility decrements for grade III/IV AEs | ||||||
| Day case/outpatient for grade III/IV AE | –0.13 | 0.01 | –0.14 | 0.01 | –0.11 | 0.02 |
| 2–5 day/inpatient for grade III/IV AE | –0.17 | 0.01 | –0.20 | 0.02 | –0.13 | 0.02 |
Beuerstein et al. 253 (funded by Napp Pharmaceuticals Group Ltd) surveyed 93 members of the UK general public using the SG to elicit QoL values for chronic lymphocytic leukaemia. Four respondents were excluded due to illogical responses. Within the health state vignettes, the AE elements were added to the base health state of ‘no change’ (Table 110).
| Health state | Mean | s.d. | 95% CI | Decrement | ||
|---|---|---|---|---|---|---|
| Lower | Upper | Mean | s.d. | |||
| CR | 0.91 | 0.11 | 0.88 | 0.93 | – | – |
| PR | 0.84 | 0.14 | 0.81 | 0.87 | – | – |
| No change | 0.78 | 0.14 | 0.75 | 0.82 | – | – |
| and nausea grade I/II | 0.73 | 0.17 | 0.69 | 0.76 | –0.05 | 0.02 |
| and nausea/vomiting grade I/II | 0.73 | 0.16 | 0.69 | 0.76 | –0.05 | 0.02 |
| Second-line treatment | 0.71 | 0.17 | 0.68 | 0.75 | – | – |
| and diarrhoea grade I/II | 0.70 | 0.19 | 0.66 | 0.74 | –0.08 | 0.02 |
| and anaemia grade III/IV | 0.69 | 0.18 | 0.65 | 0.72 | –0.09 | 0.02 |
| PD | 0.68 | 0.20 | 0.64 | 0.72 | – | – |
| and pyrexia grade III/IV | 0.67 | 0.17 | 0.63 | 0.70 | –0.11 | 0.02 |
| Third-line treatment | 0.65 | 0.22 | 0.60 | 0.69 | – | – |
| and pneumonia grade III/IV | 0.58 | 0.19 | 0.54 | 0.62 | –0.20 | 0.02 |
Boyd et al. 197 report interim results from an analysis of the MRC SCOT trial201 of patients with fully resected stage III CRC or full resected high-risk stage II disease. Limited data are presented but the QoL impact of a number of grade I/II and grade III/IV AEs measured by the EQ-5D is summarised in Table 111. This seems likely to have used the UK social tariff. No measures of uncertainty around the central estimates were presented.
| AE | Grade I/II | Grade III/IV |
|---|---|---|
| Diarrhoea | –0.04 | –0.09 |
| Fatigue | –0.02 | – |
| Nausea | –0.05 | –0.14 |
| Neuropathy sensory | –0.02 | –0.19 |
| Vomiting | –0.05 | – |
Brown et al. 265 (funded by Aventis) surveyed 30 UK oncology nurses using the SG for a cost-effectiveness study of docetaxel for advanced breast cancer. No further details are provided in the paper (Table 112).
| Health state | Mean | s.d. | Decrement |
|---|---|---|---|
| Start of second-line therapy | 0.64 | 0.15 | – |
| PR/CR | 0.84 | 0.12 | – |
| with peripheral neuropathy | 0.62 | 0.16 | 0.22 |
| with severe oedema | 0.78 | 0.15 | 0.06 |
| with severe skin condition | 0.56 | – | 0.28 |
| SD | 0.62 | 0.22 | – |
| PD | 0.33 | 0.24 | – |
| Terminal disease | 0.13 | 0.12 | – |
| Infection without hospitalisation | 0.48 | – | – |
| Febrile neutropenia and hospitalised | 0.24 | 0.12 | – |
Frederix et al. 198 recruited 100 members of the Swedish general public and 100 members of the Dutch general public for a TTO study. Within their results the authors noted the differences between the Swedish and Dutch responses, but for reasons that are unclear the age profiles of the two samples were noticeably different: Swedish respondents were typically aged > 50 years while Dutch respondents were typically aged < 50 years and very much younger. All Swedish respondents were female, while only 50% of Dutch respondents were female. Health state vignettes for human epidermal growth factor receptor-2 plus advanced breast cancer were developed for SD and for PD, and for a range of grade III/IV AEs. Frederix et al. 198 are not explicit about the health state vignettes and, in particular, whether AEs are in conjunction with SD or with PD. In the light of the values reported, to calculate the decrements associated with the AEs it has been assumed that AEs are in conjunction with SD (Table 113).
| Health state | Swedish (n = 100) | Dutch (n = 100) | ||||
|---|---|---|---|---|---|---|
| Mean | s.d. | Decrement | Mean | s.d. | Decrement | |
| SD | 0.81 | 0.23 | 0.69 | 0.25 | ||
| Diarrhoea | 0.52 | 0.31 | 0.29 | 0.50 | 0.25 | 0.19 |
| Fatigue | 0.64 | 0.30 | 0.17 | 0.56 | 0.27 | 0.13 |
| Anaemia | 0.69 | 0.29 | 0.12 | 0.59 | 0.26 | 0.10 |
| Leucopenia | 0.58 | 0.31 | 0.23 | 0.60 | 0.26 | 0.09 |
| Anorexia | 0.56 | 0.30 | 0.25 | 0.66 | 0.24 | 0.03 |
| Skin rash | 0.58 | 0.31 | 0.23 | 0.54 | 0.27 | 0.15 |
| Decrease in LVEF | 0.54 | 0.29 | 0.27 | 0.47 | 0.25 | 0.22 |
| PD | 0.61 | 0.34 | 0.20 | 0.49 | 0.31 | 0.20 |
Grunberg et al. 266 surveyed 96 US patients receiving chemotherapy for either breast cancer or lung cancer, using the SG. Six health states were constructed: perfect health; no nausea or vomiting per cycle; limited vomiting of three episodes per cycle; limited nausea of 3 days of nausea per cycle; limited nausea and vomiting of 3 days of nausea and three episodes of vomiting per cycle; and, continuous nausea and vomiting. Perfect health was anchored at 1.00, with the mean for continuous nausea and vomiting being rated at zero. The intermediate health states varied with 0.59 for no nausea and vomiting to 0.51 for limited nausea.
Havrilesky et al. 254 surveyed 13 ovarian cancer patients and 37 female members of the US general public to estimate QoL values for ovarian cancer. Health state vignettes based on the NCI CTCAE were drawn up, and subsequently amended by a focus group of clinicians. The paper reports a range of QoL values for ovarian cancer states. It then separately reports a range of QoL values for AEs, graphing these against perfect health. Although unclear from the text, in the light of the values reported it appears that the AEs may have been in effect added to the state of perfect health. The decrements reported in Tables 114 and 115 for the individual AEs are calculated on this basis.
| Health state | Mean | s.d. |
|---|---|---|
| Ovarian cancer: clinical remission | 0.83 | 0.25 |
| Early ovarian cancer: newly diagnosed | 0.81 | 0.26 |
| Newly diagnosed ovarian cancer: chemotherapy/grade I/II toxicity | 0.60 | 0.31 |
| Recurrent ovarian cancer: responding to chemotherapy/grade III/IV toxicity | 0.61 | 0.24 |
| Recurrent ovarian cancer: responding to chemotherapy/grade I/II toxicity | 0.50 | 0.34 |
| Advanced ovarian cancer: newly diagnosed | 0.55 | 0.29 |
| Newly diagnosed ovarian cancer: chemotherapy/grade III/IV toxicity | 0.49 | 0.36 |
| Recurrent ovarian cancer: progressive/grade III/IV toxicity | 0.47 | 0.34 |
| Recurrent ovarian cancer: progressive/grade I/II toxicity | 0.40 | 0.33 |
| End-stage ovarian cancer | 0.16 | 0.25 |
| AE | Patients | Public | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | s.d. | Decrement | n | Mean | s.d. | Decrement | |
| Alopecia: grade II | 12 | 0.90 | 0.15 | 0.10 | 14 | 0.84 | 0.29 | 0.16 |
| Peripheral neuropathy: grade I/II | 13 | 0.95 | 0.04 | 0.05 | 15 | 0.81 | 0.29 | 0.19 |
| Stomatitis: grade II | 13 | 0.88 | 0.14 | 0.12 | 14 | 0.91 | 0.08 | 0.09 |
| Myalgia/pain: grade I/II | 13 | 0.86 | 0.15 | 0.14 | 15 | 0.89 | 0.12 | 0.11 |
| Nausea/vomiting: grade I/II | 12 | 0.65 | 0.38 | 0.35 | 15 | 0.76 | 0.28 | 0.24 |
| Myalgia/pain: grade III/IV | 13 | 0.72 | 0.30 | 0.28 | 15 | 0.46 | 0.39 | 0.54 |
| Neutropenia: grade IV | 13 | 0.70 | 0.30 | 0.30 | 16 | 0.64 | 0.36 | 0.36 |
| Peripheral neuropathy: grade III/IV | 13 | 0.73 | 0.27 | 0.27 | 14 | 0.65 | 0.31 | 0.35 |
| Nausea/vomiting: grade III/IV | 13 | 0.60 | 0.40 | 0.40 | 16 | 0.63 | 0.30 | 0.37 |
| Fatigue grade III/IV | 13 | 0.66 | 0.35 | 0.34 | 13 | 0.58 | 0.33 | 0.42 |
| Febrile neutropenia | 13 | 0.54 | 0.33 | 0.46 | 15 | 0.56 | 0.34 | 0.44 |
Jewell et al. 267 in a US study recruited 15 cervical cancer survivors and 45 women without a cancer diagnosis and undertook a TTO exercise to estimate the QoL living with a range of grade III/IV AEs. Health state vignettes were developed using the NCI CTCAE, which were subsequently reviewed by clinical experts. Unfortunately, Jewell et al. 267 did not estimate a utility for a baseline health state to which the AEs were added to, meaning that utility decrements associated with the AEs cannot be identified (Table 116).
| AE | All | Patients | Volunteers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | s.d. | n | Mean | Median | n | Mean | Median | |
| Infection | 0.92 | 1.00 | 0.18 | 13 | 0.86 | 0.93 | 23 | 0.96 | 1.00 |
| Pyelonephritis | 0.87 | 1.00 | 0.25 | 13 | 0.91 | 1.00 | 24 | 0.85 | 1.00 |
| Thrombosis | 0.87 | 0.97 | 0.25 | 13 | 0.87 | 0.97 | 24 | 0.87 | 0.97 |
| Vaginal stenosis | 0.86 | 0.97 | 0.23 | 13 | 0.88 | 0.90 | 24 | 0.84 | 0.97 |
| Neutropenia | 0.86 | 0.97 | 0.26 | 13 | 0.83 | 0.87 | 24 | 0.88 | 1.00 |
| Lymphedema | 0.84 | 0.95 | 0.26 | 13 | 0.88 | 0.93 | 23 | 0.81 | 0.97 |
| Bladder dysfunction | 0.83 | 0.93 | 0.28 | 13 | 0.86 | 0.93 | 23 | 0.81 | 0.93 |
| Radiation cystitis | 0.86 | 0.93 | 0.21 | 13 | 0.80 | 0.90 | 24 | 0.69 | 0.85 |
| Anaemia | 0.83 | 0.93 | 0.28 | 13 | 0.84 | 0.93 | 24 | 0.83 | 0.97 |
| Genitourinary fistula | 0.76 | 0.90 | 0.31 | 13 | 0.84 | 0.90 | 24 | 0.72 | 0.89 |
| Bowel obstruction | 0.79 | 0.89 | 0.29 | 13 | 0.77 | 0.83 | 23 | 0.80 | 0.93 |
| Hydroureter | 0.75 | 0.87 | 0.30 | 13 | 0.76 | 0.87 | 24 | 0.75 | 0.87 |
| Radiation proctitis | 0.72 | 0.87 | 0.32 | 13 | 0.80 | 0.90 | 24 | 0.69 | 0.85 |
| Genital-intestinal fistula | 0.66 | 0.83 | 0.31 | 13 | 0.75 | 0.87 | 24 | 0.61 | 0.67 |
Kuchuk et al. 268 (funded by Eisai Pharmaceuticals) in a Canadian study used the SG among 102 women with breast cancer who were undergoing chemotherapy. Health state vignettes for the AEs were based on the CTC grading criteria and patients’ own descriptions. Of the 102 women, only 69 responses were analysed due to a variety of problems such as illogical responses with the others. Unfortunately, as with Jewell et al. ,267 Kuchuk et al. 268 did not estimate a utility for a baseline health state to which the AEs were added to, meaning that utility decrements associated with the AEs cannot be identified. However, the additional decrement associated with a grade III/IV event compared with a grade I/II event can be calculated (Table 117).
| AE | Grade I/II | Grade III/IV | ||
|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | |
| Diarrhoea | 0.760 | 0.168 | 0.677 | 0.221 |
| Hand and foot syndrome | 0.754 | 0.167 | 0.700 | 0.189 |
| Mucositis/stomatis | 0.747 | 0.179 | 0.739 | 0.179 |
| Nausea | 0.730 | 0.130 | 0.621 | 0.222 |
| Neuropathy (sensory) | 0.725 | 0.189 | 0.694 | 0.191 |
| Neuropathy (motor) | 0.715 | 0.145 | 0.725 | 0.151 |
| Fatigue | 0.719 | 0.214 | 0.717 | 0.181 |
| Myalgia | 0.715 | 0.145 | 0.704 | 0.138 |
| Alopecia | 0.716 | 0.225 | – | – |
Lloyd et al. 196 (funded by Eli Lilly) surveyed 100 members of the UK general public using the SG to estimate QoL values for metastatic breast cancer health states and grade III/IV AEs. Health state vignettes were developed through a rapid literature review coupled with expert opinion. The mean values were reported, together with the coefficients of a mixed-model analysis. All coefficients were significant with the exception of the intercept. Note that there is not an immediate read across from the TTO utilities and decrements and the mixed model coefficients because utilities from the mixed model are derived according to exp(sum_coefs)/(1 + exp(sum_coefs)) (Table 118).
| Coefficient for: | Mean | Mixed model | |
|---|---|---|---|
| TTO | Coefficient | s.e. | |
| Intercept | – | 0.0089 | 0.3196 |
| Age | – | 0.0239 | 0.0069 |
| SD with no toxicity | 0.715 | – | – |
| Treatment response | +0.075 | 0.4063 | 0.0552 |
| Disease progression | –0.272 | –1.1477 | 0.1031 |
| Febrile neutropenia | –0.15 | –0.6603 | 0.0850 |
| Diarrhoea and vomiting | –0.103 | –0.4629 | 0.0993 |
| Hand and foot syndrome | –0.116 | –0.5184 | 0.0993 |
| Stomatitis | –0.151 | –0.6634 | 0.0993 |
| Fatigue | –0.115 | –0.5142 | 0.0993 |
| Hair loss | –0.114 | –0.5086 | 0.0993 |
Lloyd et al. 269 (funded by Ortho Biotec) surveyed 26 oncology patients and 83 members of the UK general public using the TTO to derive QoL values for different severities of anaemia (Table 119). Slightly unusually, anaemia was defined by haemoglobin levels with seven different haemoglobin bands being evaluated. Trial data were used to map between the haemoglobin bands and Functional Assessment of Cancer Therapy-Anaemia (FACT-An) responses. The FACT-An responses that showed little difference between haemoglobin bands were discarded. The tables of the paper report the ‘95% CI’ but include only one value for this. As a consequence, it is unclear quite what this is: the s.d. or 1.96 × s.d.?
| Haemoglobin (g/dl) | Public | Patients | ||
|---|---|---|---|---|
| Mean | ‘95% CI’ | Mean | ‘95% CI’ | |
| 7.0–8.0 | 0.583 | 0.067 | 0.297 | 0.127 |
| 8.0–9.0 | 0.608 | 0.064 | 0.360 | 0.126 |
| 9.0–10.0 | 0.640 | 0.060 | 0.408 | 0.125 |
| 10.0–10.5 | 0.642 | 0.062 | 0.446 | 0.122 |
| 10.5–11.0 | 0.661 | 0.061 | 0.454 | 0.111 |
| 11.0–12.0 | 0.703 | 0.056 | 0.545 | 0.105 |
| 12.0+ | 0.708 | 0.057 | 0.611 | 0.112 |
Nafees et al. 257 (funded by Eli Lilly) surveyed 100 members of the UK general public using the SG to estimate QoL values for metastatic non-small cell lung cancer and grade III/IV AE. Health state vignettes were developed through a rapid literature review coupled with expert opinion. The resulting utilities were analysed using a fixed-effect repeated measure model, resulting in the coefficients listed in Table 120.
| Coefficient for: | Coefficient | SEM |
|---|---|---|
| Intercept (SD) | 0.65320 | 0.02223 |
| Progressive | –0.17980 | 0.02169 |
| Response | 0.01930 | 0.00656 |
| Neutropenia | –0.08973 | 0.01543 |
| Febrile neutropenia | –0.09002 | 0.01633 |
| Fatigue | –0.07346 | 0.01849 |
| Nausea and vomiting | –0.04802 | 0.01618 |
| Diarrhoea | –0.04680 | 0.01553 |
| Hair loss | –0.04495 | 0.01482 |
| Rash | –0.03248 | 0.01171 |
Nguyen et al. 270 surveyed 24 members of the US general public using the SG to estimate QoL values for testicular cancer. The development of the health state vignettes used for this is not described in the paper, and it is unclear what severity of AEs was involved. As a consequence, the resulting utility values are of questionable value for health economic modelling (Table 121).
| Health state | Mean | SEM |
|---|---|---|
| Untreated cancer | 0.92 | 0.03 |
| Peripheral neuropathy | 0.94 | 0.02 |
| Ototoxicity | 0.96 | 0.02 |
| Cardiovascular disease | 0.91 | 0.02 |
| Secondary malignant neoplasm | 0.77 | 0.05 |
| Small bowel obstruction | 0.94 | 0.02 |
| Infertility | 0.98 | 0.01 |
Ossa et al. 255 (funded by Roche) surveyed 110 members of the UK general public using the TTO to estimate the QoL impact of chemotherap-induced anaemia, within the context of a cost-effectiveness analysis of recombinant erythropoietin. Health states vignettes for no, mild, moderate and severe chemotherapy-induced anaemia were developed, based on the elements of the FACT-An questionnaire supplemented with information from the general literature. Three oncologists and six cancer patients subsequently reviewed the vignettes. Results were derived from 106 respondents (Table 122).
| Anaemia type | Mean | SEM | Decrement |
|---|---|---|---|
| No anaemia | 0.86 | 0.014 | |
| Mild anaemia | 0.78 | 0.016 | 0.08 |
| Moderate anaemia | 0.61 | 0.020 | 0.25 |
| Severe anaemia | 0.48 | 0.020 | 0.38 |
Shih et al. 271 applied the SG among 20 Singaporean oncology nurses with a minimum of 2 years’ experience to estimates the QoL among breast cancer patients undergoing hormonal therapies (Table 123).
| Health state | Mean | Decrement | Median |
|---|---|---|---|
| No recurrence and no side effect | 0.678 | – | 0.775 |
| with hip fracture | 0.504 | 0.174 | 0.475 |
| with wrist fracture | 0.533 | 0.145 | 0.500 |
| with spine fracture | 0.458 | 0.220 | 0.463 |
| with vaginal bleeding | 0.554 | 0.124 | 0.500 |
| with deep-vein thrombosis | 0.515 | 0.163 | 0.475 |
| with pulmonary embolism | 0.463 | 0.215 | 0.475 |
| with cataract | 0.519 | 0.159 | 0.475 |
| with ischaemic cerebrovascular events | 0.408 | 0.270 | 0.425 |
| with common side effects – musculoskeletal disorder | 0.510 | 0.168 | 0.500 |
| with common side effects – hot flushes | 0.588 | 0.090 | 0.550 |
| with endometrial cancer | 0.501 | 0.177 | 0.475 |
| New contralateral breast cancer | 0.443 | – | 0.425 |
| Locoregional recurrence and no side effects | 0.473 | – | 0.438 |
| with side effects – general | 0.438 | 0.035 | 0.425 |
| Distant recurrence and no side effects | 0.470 | – | 0.450 |
| with side effects – chemotherapy | 0.458 | 0.012 | 0.413 |
| with side effects – hormonal therapy | 0.445 | 0.025 | 0.413 |
Shiroiwa et al. 193 surveyed 1582 members of the Japanese general public using both the TTO and the SG to estimate the QoL associated with mCRC and grade III/IV events. The development of health state vignettes was based on the literature, expert opinion and the NCI CTCAE. Respondents were recruited through a large online panel of the Japanese public, with respondents also completing the questionnaire online. Statistical analysis computed the mean utility decrements associated with receiving FOLFOX compared with receiving XELOX; experiencing an AE compared with not experiencing an AE; and receiving chemotherapy compared with having completed chemotherapy. The mean estimates coupled with their 95% confidence limits are presented in Table 124.
| Health state | SG | TTO | ||||
|---|---|---|---|---|---|---|
| Mean | CI low | CI high | Mean | CI low | CI high | |
| Analysis 1 | ||||||
| Chemotherapy | 0.0535 | 0.0087 | 0.0983 | 0.0636 | 0.0187 | 0.1084 |
| Stoma | 0.0926 | 0.0484 | 0.1369 | 0.1099 | 0.0655 | 0.1543 |
| Analysis 2 | ||||||
| Febrile neutropenia | 0.0424 | 0.0008 | 0.0841 | 0.0816 | 0.0425 | 0.1208 |
| Nausea/vomiting | 0.0898 | 0.0479 | 0.1316 | 0.0898 | 0.0505 | 0.1292 |
| Diarrhoea | 0.0558 | 0.0134 | 0.0981 | 0.0538 | 0.0139 | 0.0936 |
| Hand and foot syndrome | 0.0841 | 0.0423 | 0.1258 | 0.0839 | 0.0446 | 0.1231 |
| Fatigue | 0.0464 | 0.0047 | 0.0882 | 0.0269 | –0.0124 | 0.0662 |
| Peripheral neuropathy | 0.0345 | –0.0080 | 0.0770 | 0.0257 | –0.0143 | 0.0656 |
| Stomatitis | 0.0341 | –0.0078 | 0.0760 | 0.0552 | 0.0157 | 0.0946 |
| Stoma | 0.0463 | 0.0255 | 0.0671 | 0.0404 | 0.0209 | 0.0600 |
| Analysis 3 | ||||||
| Chemotherapy | 0.0603 | 0.0121 | 0.1085 | 0.0661 | 0.0175 | 0.1146 |
| Stoma | 0.2063 | 0.1581 | 0.2545 | 0.1281 | 0.0795 | 0.1767 |
Swinburn et al. 200 (funded by Novartis) used TTO among 100 members of the UK general public to estimate QoL values for health states associated with neuroendocrine tumours. Health state vignettes were developed based on the literature, clinician and patient interviews and five pilot interviews to check the appropriateness of the descriptions. The text is ambiguous and it may be that only diarrhoea was explicitly at grade III/IV, but it seems likely that all AEs were grade III/IV (Table 125).
| Health state | Mean | s.d. | 95% CI | Decrement |
|---|---|---|---|---|
| Stable no AE | 0.771 | 0.20 | 0.731 to 0.810 | – |
| with diarrhoea | 0.600 | 0.25 | 0.546 to 0.645 | 0.171 |
| with hand and foot syndrome | 0.583 | 0.23 | 0.538 to 0.627 | 0.188 |
| with hyperglycaemia | 0.781 | 0.19 | 0.743 to 0.818 | –0.010 |
| with nausea/vomiting | 0.710 | 0.21 | 0.668 to 0.752 | 0.061 |
| with pneumonitis | 0.612 | 0.26 | 0.561 to 0.662 | 0.159 |
| with rash | 0.623 | 0.23 | 0.578 to 0.668 | 0.148 |
| with stomatis | 0.557 | 0.24 | 0.509 to 0.604 | 0.214 |
| with thrombocytopenia | 0.690 | 0.24 | 0.643 to 0.737 | 0.081 |
| Progressive | 0.612 | 0.24 | 0.564 to 0.659 | – |
Szabo et al. 256 (funded by Bristol-Myers Squibb), in a Canadian study, applied the SG among 106 members of the Canadian general public to estimate QoL values for H&N cancer. Health state vignettes were drawn up based on a literature search, supplemented by European Organisation for Research and Treatment of Cancer (EORTEC) survey data from a Phase III trial. A total of 101 responses were used for the statistical analysis. This undertook a mixed regression analysis with random intercepts to allow for individuals contributing multiple responses (Table 126).
| Coefficient for: | Coefficient | SEM |
|---|---|---|
| Intercept | 0.610 | 0.070 |
| Age | 0.000 | 0.001 |
| Sex | ||
| Male | – | – |
| Female | –0.080 | 0.043 |
| Stage | ||
| Locoregional | – | – |
| Metastatic | –0.110 | 0.013 |
| Recurrent | –0.050 | 0.013 |
| Type | ||
| Non-laryngeal | – | – |
| Laryngeal | 0.000 | 0.009 |
| Post progression | –0.280 | 0.013 |
| Skin reactions grade I/II | –0.050 | 0.021 |
| Grade III/IV AEs | ||
| Haematological | –0.070 | 0.021 |
| Anaemia | –0.060 | 0.021 |
| Nausea/vomiting | –0.100 | 0.021 |
| Mucositis/stomatitis | –0.100 | 0.021 |
| Peripheral neuropathy | –0.090 | 0.021 |
| Anorexia/weight loss | –0.090 | 0.021 |
| Skin reactions | –0.120 | 0.021 |
| Hospitalisation due to toxicity | –0.160 | 0.021 |
| Treatment cessation due to toxicity | –0.060 | 0.021 |
Tolley et al. 199 (funded by GlaxoSmithKline) apply the TTO among 100 members of the UK general public to elicit QoL values for late stage chronic lymphocytic leukaemia. A range of health state vignettes were developed using the literature and expert clinical opinion, the aim being to reflect an average patient of 70 years of age. AE descriptions were guided the NCI CTCAE criteria. An anchor state representative of a patient that has received two prior lines of therapy and is about to start another was developed: night sweats, being very tired all the time, weight loss and loss of appetite, swollen glands, chest infections and sore throat, and can walk short distances. From the health state vignettes it appears that the AEs were grade III/IV, though this is not unambiguous for neutropenia (Table 127).
| Health state | TTO | s.d. | 95% CI | Decrement |
|---|---|---|---|---|
| Anchor state | 0.549 | 0.231 | 0.506 to 0.592 | – |
| PFS responder | 0.671 | 0.236a | 0.627 to 0.715 | – |
| with AE: thrombocytopenia | 0.563 | 0.108 | 0.516 to 0.610 | –0.108 |
| with AE: neutropenia, no infection | 0.508 | 0.163a | 0.464 to 0.551 | –0.163 |
| with AE: severe infection | 0.476 | 0.195a | 0.432 to 0.519 | –0.195 |
| PFS non-responder | 0.394 | 0.219a | 0.353 to 0.435 | – |
| with AE: severe infection | 0.333 | 0.061a | 0.294 to 0.372 | –0.061 |
| Disease progression | 0.214 | 0.18a | 0.180 to 0.247 | – |
Tam et al. 258 in a cost-effectiveness study of therapies for metastatic pancreatic cancer surveyed 60 Canadian oncologists using the EQ-5D. Health state vignettes were developed for a hypothetical 60-year-old man with metastatic pancreatic cancer. The QoL values were derived from the 33 respondents, though the mapping used for estimating these from the EQ-5D data is not clear. From the health state descriptors is appears that the AE health states are broadly supplemental to the SD health state (Table 128).
| Health state | Mean | s.d. | Decrement |
|---|---|---|---|
| SD | 0.720 | 0.185 | – |
| SD with grade III/IV | |||
| nausea and vomiting | 0.526 | 0.235 | 0.194 |
| diarrhoea | 0.508 | 0.207 | 0.212 |
| stomatitis | 0.279 | 0.231 | 0.441 |
| febrile neutropenia | 0.589 | 0.171 | 0.131 |
| fatigue | 0.247 | 0.239 | 0.473 |
| rash | 0.626 | 0.166 | 0.094 |
| hand and foot syndrome | 0.409 | 0.210 | 0.311 |
| neuropathy | 0.494 | 0.177 | 0.226 |
| Supportive care | 0.136 | 0.184 | 0.584 |
Although not peer reviewed and officially published the manufacturer submission for TA145:215 cetuximab for locally advanced squamous H&N cancer included a utility elicitation study commissioned from M-TAG Ltd. A literature search identified the main AEs associated with H&N cancer. Seven health states based on the NCI CTCAE were developed and rated using the EQ-5D by 50 UK oncology nurses of a minimum of 2 years’ experience (Table 129).
| Health state | Mean | s.d. | Decrement |
|---|---|---|---|
| On treatment, range of AEs ≤ 1 | 0.659 | 0.131 | – |
| plus mucositis grade III/IV | 0.062 | 0.299 | 0.597 |
| plus mucositis grade II | 0.608 | 0.310 | 0.051 |
| plus nausea grade III/IV | 0.108 | 0.350 | 0.551 |
| plus nausea grade II | 0.573 | 0.247 | 0.086 |
| plus acne/rash grade III/IV | 0.226 | 0.404 | 0.433 |
| plus haematological grade IV | 0.101 | 0.392 | 0.558 |
| Post-treatment peripheral neuropathy | 0.473 | 0.266 | – |
| Post-treatment ototoxicity | 0.657 | 0.239 | – |
| Post-treatment locoregional control | 0.862 | 0.019 | – |
| Post-treatment PD | 0.129 | 0.266 | – |
Glossary
- FOLFIRI
- Irinotecan (180 mg/m2 intravenous over 90 minutes) concurrently with folinic acid [400 mg/m2 (or 2 x 250 mg/m2) intravenous over 120 minutes], followed by fluorouracil (400–500 mg/m2 intravenous bolus) then fluorouracil (2400–3000 mg/m2 intravenous infusion over 46 hours). The dosages shown may vary from cycle to cycle, and the duration of fluorouracil infusion may vary (e.g. 22 hours) depending on the particular regimen employed.
- FOLFOX4
- Day 1: oxaliplatin (85 mg/m2 intravenous infusion) and leucovorin (200 mg/m2 intravenous infusion) both given over 120 minutes, followed by 5-FU (400 mg/m2 intravenous bolus) given over 2–4 minutes, followed by 5-FU (600 mg/m2 intravenous infusion) as a 22-hour continuous infusion.Day 2: Leucovorin (200 mg/m2 intravenous infusion) over 120 minutes, followed by 5-FU (400 mg/m2 intravenous bolus) given over 2–4 minutes, followed by 5-FU (600 mg/m2 intravenous infusion) as a 22-hour continuous infusion; the duration of fluorouracil infusion may vary (e.g. 46 hours) depending on the particular regimen employed.
- FOLFOX6
- Days 1–2: oxaliplatin (100 mg/m2 intravenous infusion) given as a 120-minute intravenous infusion, concurrent with leucovorin (400 mg/m2 or 200 mg/m2 intravenous infusion), followed by 5-FU (400 mg/m2 intravenous bolus), followed by 46-hour 5-FU infusion (2400–3000 mg/m2); the duration of fluorouracil infusion may vary (e.g. 22 hours) depending on the particular regimen employed.
- TPF
- Docetaxel (75 mg/m2 intravenous infusion) on day 1, cisplatin (75 mg/m2 intravenous infusion) on day 1, 5-fluorouracil (750 mg/m2/day) as continuous intravenous infusion over 4 days.
- Xelox
- Oxaliplatin (130 mg/m2) day 1 as intravenous infusion + capecitabine (1000 mg/m2) twice daily as oral tablets on days 1–14.
List of abbreviations
- 5-FU
- 5-fluorouracil
- AE
- adverse event
- AiC
- academic-in-confidence
- AIC
- Akaike information criterion
- AJCC
- American Joint Committee on Cancer
- AUC
- area under the curve
- BIC
- Bayesian information criterion
- BNF
- British National Formulary
- BSA
- body surface area
- CEAC
- cost-effectiveness acceptability curve
- CF
- cisplatin and fluorouracil
- CG
- clinical guideline
- CHART
- continuous hyperfractionated accelerated radiotherapy
- CI
- confidence interval
- CMU eMIT
- Commercial Medicines Unit Electronic Market Information Tool
- CR
- complete response
- CRC
- colorectal cancer
- CT
- computerised tomography
- DAP
- Diagnostics Assessment Programme
- DPD
- dihydropyrimidine dehydrogenase
- EAG
- External Assessment Group
- EDTA
- ethylenediaminetetraacetic acid
- EORTC QLQ-C30
- European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire
- EQ-5D
- European Quality of Life-5 Dimensions
- ERG
- Evidence Review Group
- FA
- folinic acid
- FACT-An
- Functional Assessment of Cancer Therapy-Anaemia
- FOCUS
- fluorouracil, oxaliplatin, irinotecan use and sequencing
- FOLFIRI
- irinotecan in combination with 5-fluorouracil and folinic acid
- FOLFOX
- oxaliplatin in combination with 5-fluorouracil and folinic acid
- FU
- fluorouracil
- FUFOL
- folinic acid and 5-fluorouracil
- G-CSF
- granulocyte colony-stimulating factor
- GP
- general practice
- H&N
- head and neck
- HDFL48
- 48-hour continuous infusion of high-dose 5-FU/FA
- HPLC
- high-performance liquid chromatography
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- HUI
- Health Utilities Index
- ICER
- incremental cost-effectiveness ratio
- IPD
- individual patient data
- ITT
- intention to treat
- i.v.
- intravenous
- KRAS-WT
- Kirsten Rat Sarcoma-Wild Type
- LC-MS
- liquid chromatography-mass spectrometry
- LOS
- length of stay
- LV5FU2
- 5-fluorouracil + folinic acid (leucovorin) regimen
- mCRC
- metastatic colorectal cancer
- MRC
- Medical Research Council
- MS
- mass spectrometer
- MTA
- multiple technology appraisal
- NCI
- National Cancer Institute
- NCI CTCAE
- National Cancer Institute Common Terminology Criteria for Adverse Events
- NCIN
- National Cancer Intelligence Network
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- OS
- overall survival
- PCF
- paclitaxel, cisplatin and fluorouracil
- PD
- progressive disease
- PF
- cisplatin and 5-fluorouracil
- PFS
- progression-free survival
- PK
- pharmacokinetic
- PR
- partial response
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QUADAS-2
- revised quality assessment of diagnostic accuracy studies
- RCT
- randomised controlled trial
- RECIST
- Response Evaluation Criteria in Solid Tumours
- SCC
- squamous cell carcinoma
- SCOT
- Short Course Oncology Therapy
- s.d.
- standard deviation
- SD
- stable disease
- SEM
- standard error of measurement
- SG
- standard gamble
- STA
- single technology appraisal
- SWP
- survival with progression
- TA
- technology appraisal
- TNM
- tumour node metastasis
- TPF
- docetaxel, cisplatin, 5-fluorouracil
- TTO
- time trade-off
- UICC
- Union International Contre Le Cancer/International Union Against Cancer
- VAS
- visual analogue scale
- WHO
- World Health Organization
- WTP
- willingness to pay
- XELOX
- oxaliplatin in combination with capecitabine
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed academic-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of academic-in-confidence data removed and replaced by the statement ‘academic-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk. The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.