Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/134/01. The contractual start date was in September 2014. The draft report began editorial review in March 2015 and was accepted for publication in July 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Craig Ramsay and Miriam Brazzelli declare grants from the UK Department of Health during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Sharma et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the health problem
Introduction
Surgical repair (herniorrhaphy) is undertaken in most people presenting with inguinal hernia in order to close the defect, alleviate symptoms of discomfort and prevent serious complications. Inguinal hernia repair is the most frequent and resource-consuming surgical intervention in the UK. 1,2 It is also the most common general surgical intervention performed in Europe1,3,4 and the USA. 5 Various surgical techniques and approaches are available for inguinal hernia. These can be classified in three main categories: open repair with suture (e.g. Bassini and Shouldice repair), open repair with mesh (e.g. Liechtenstein repair or preperitoneal repair) and laparoscopic repair with mesh [e.g. totally extraperitoneal (TEP) repair or transabdominal preperitoneal (TAPP) repair].
Open and laparoscopic ‘tension-free’ repairs, which are based on the use of ‘mesh’ (prosthetic and biological), are widely performed and considered superior to the traditional ‘tissue-suture’ repairs, such as the Bassini or Shouldice techniques. 6,7 Compared with the traditional sutured techniques, a reduction in the risk of recurrence between 50% and 75% has been demonstrated after mesh repair. 8,9 However, the laparoscopic mesh repair has a longer learning curve and higher resource cost compared with the open mesh repair. 2,7,10–12 Different open repairs with mesh, such as the Lichtenstein repair and the preperitoneal repair, have shown similar results and very low recurrence rates, ranging from 2% to 5%. 13,14 The preperitoneal mesh repair has demonstrated similar or better outcomes compared with the laparoscopic mesh repair. 15
Considering the low recurrence rate reported in the literature after surgical repair of inguinal hernia, the current key outcomes on which to measure the clinical success of hernia recovery include chronic pain, complications, time to return to normal activities and quality of life (QoL). 16,17 Published evidence assessing the effects of common mesh techniques (including open preperitoneal repair, Lichtenstein repair and laparoscopic repair) in lowering chronic pain and improving major clinical outcomes have produced inconsistent results. 3,15,18–20 The aim of this assessment was to evaluate the clinical effectiveness and cost-effectiveness of open preperitoneal repair compared with Lichtenstein repair in adults presenting with a clinically diagnosed primary unilateral inguinal hernia.
Aetiology, pathophysiology and clinical presentation
An inguinal hernia is a protrusion of the contents of the abdominal cavity through a defect in the inguinal canal. It manifests as a lump or swelling in the groin that may cause discomfort and pain, and impact on daily activities and ability to work. Unilateral hernias occur on one side of the lower abdominal wall, whereas bilateral hernias occur on both sides of the lower abdomen wall. Symptoms of inguinal hernia include swelling, pain or aching sensation in the groin, which develops gradually over time. Pain worsens with prolonged activities. 21 The bulge of the hernia increases in size with activities that cause intra-abdominal pressure, such as coughing, lifting or straining. Occasionally the hernia sac contents may get incarcerated causing obstruction or strangulation of the intestine, leading to ischaemia, necrosis and even perforation of the intestine. Rarely, inguinal hernias are asymptomatic.
Inguinal hernia is commonly diagnosed by clinical physical examination. Physical examination involves careful inspection of inguinal areas for bulges or impulses while the patient is standing and during a Valsalva manoeuvre (i.e. forceful attempted exhalation while keeping the mouth and nose closed). The sensitivity and specificity of physical examination for the diagnosis of inguinal hernia have been reported to be 75% and 96%, respectively. 22 In specific clinical situations such as recurrent hernia, hernia in female patients, surgical complications with chronic pain or uncertain aetiology, diagnosis of inguinal hernia can be improved by various imaging techniques (e.g. ultrasonography, magnetic resonance imaging or computerised tomography). 21 In particular, magnetic resonance imaging and ultrasonography have shown high sensitivity and specificity estimates (> 80% and > 90%, respectively) for the diagnosis of groin hernia. 22
The classification of hernia is a prerequisite to describe the anatomy or size of inguinal hernia and to choose the best management. 3,23 Many classifications of inguinal hernia have been proposed and they are all based on the presence of indirect hernia (occurs because of the natural weakness in the internal inguinal ring), direct hernia (caused by the weakness in the floor of inguinal canal) and femoral hernia (less common groin hernia occurring mostly in women). 23 Table 1 illustrates a number of different classifications currently available.
| Type of classification | Description |
|---|---|
| Gilbert | Based on anatomical and functional defects described during open (anterior) operation; includes five types [1, 2, 3 (indirect); and, 4 and 5 (direct)]. Modified by Rutkow and Robbins23 |
| Nyhus | Describes the status of the fascia transversalis in the posterior wall of the inguinal and femoral canal; includes four types [I, II, IIIb (indirect); IIIa, IV (direct); IIIc (femoral)] |
| Stoppa | Derived from the Nyhus classification, with special attention to the aggravating factors; includes four types [1, 2 (indirect); and, 3 and 4 (direct)] |
| Bendavid TSD | An elaborate and complex system with 20 different subtypes, including typing, staging and measuring the dimensions of the hernia to classify them [I (indirect); II and V (direct); and III and IV (femoral)] |
| Aachen (Schumpelick) | Based on the more traditional European anatomical classification (direct or indirect inguinal, and femoral) combined with measurement of the hernia orifice (< 1.5 cm, 1.5–3.0 cm, > 3.0 cm); recommended simple method |
| Corcione | Includes three types [1 (indirect); 2 (direct); and 3 (femoral)] |
| Cost | Includes three types each for indirect and direct hernias (1, 2 and 3) |
| Porrero | Includes five types [1, 2, 3 (indirect); and, 4 and 5 (direct)] |
| European Hernia Society | Modification of Aachen classification; clear description of combined or femoral hernias, primary or recurrent hernia, the largest diameter to be used for quantification of hernia orifice size as 1 (≤ 1 finger), 2 (1–2 fingers) and 3 (≥ 3 fingers) |
Epidemiology and prevalence
A large population-based prospective study conducted in the USA (National Health and Nutrition Examination Survey, 1971–5) reported a 20-year cumulative incidence of hospitalisation with inguinal hernia of 6.3%. 24 The condition is observed more frequently in males and incidence increases with age. 24 The lifetime chance of getting inguinal hernia is estimated to be 27% in men and 3% in women. 25 Most inguinal hernias are found in men because of the vulnerability of the male anatomy to the formation of hernias in this region. 26 The average age group for the manifestation of inguinal hernia has been reported to be 10 years older in women (60–79 years) than in men (50–69 years). 27
A register-based 5-year study conducted in Denmark found that 97% of all groin hernia repairs (n = 46,717) were inguinal hernias. 4 In the Netherlands, approximately 30,000 inguinal hernia repairs are carried out annually. In the USA, data from the National Centre for Health Statistics suggest that approximately 800,000 groin hernia repairs were performed in 2003, with over 90% of these surgeries performed on an outpatient basis. 5
Impact of health problem: significance for the NHS and burden of disease
Symptomatic patients often present with pain and discomfort. Patients may experience a localised pain or aching (burning or gurgling) sensation at the site of hernia defect or a heavy or dragging sensation in the groin. There are various factors that may contribute to pain, including stretching or tearing of the tissue around the hernia defect, prolonged activity or Valsalva manoeuvres. 21 A prospective study by Hair and colleagues,28 evaluated the association between hernia symptoms and time from hernia presentation in 699 patients. About 65% (457) of patients complained of pain and discomfort at the hernia site on presentation, with the cumulative probability of pain increasing to almost 90% at 10 years, whereas more than one-third of patients (267/699) were asymptomatic. Patients with inguinal hernia are at risk of bowel strangulation, which requires emergency resection. A retrospective study,29 reported a cumulative probability of strangulation for inguinal hernias of 2.8% at 3 months and 4.5% after 2 years.
Severe chronic pain, wound infection and recurrence are among the postoperative complications that are reported after inguinal hernia repair. 16,17 With incidence rates ranging from 10% to 54%, chronic pain is undoubtedly the dominant complication after inguinal hernia repair. 16,30,31 The reason for long-term postoperative pain is complex and often related to intraoperative nerve damage, which is often associated with technical aspects of the surgical procedures as well as with surgeon’s dexterity and expertise. 32 The position of the mesh is likely to be another crucial factor.
Management of condition and current service provision
Conservative management
Asymptomatic inguinal hernias, which do not cause symptoms, can be managed through watchful waiting. However, asymptomatic patients need to be monitored over time for occurrence of symptoms, especially those indicating strangulation or incarceration, which require immediate medical attention. 7,21
Trusses are often recommended for the temporary management of hernia while patients are waiting for an operation. A truss is a type of surgical appliance that provides support for the herniated area during daily and working activities. 3 However, the benefit achieved through the use of a truss is debatable, as up to 64% of the patients have declared that they find it uncomfortable. 33 Nevertheless, in the UK, 40,000 trusses are issued every year with the rate of supply being very high as compared with other industrialised countries. 3
Surgical management
Inguinal hernias are commonly repaired using surgery, where the abdominal bulge is pushed back into place and the weakness in the abdominal wall is strengthened. Most hernia repairs are undertaken as elective procedures. 34 Surgical procedures for inguinal hernia include open repair with suture (e.g. Shouldice or McVay), open repair with mesh (e.g. Liechtenstein repair or preperitoneal repair) and laparoscopic repair with mesh. 34
Open mesh repair is recommended for the management of primary unilateral inguinal hernia6,7 because of its low recurrence rates. Laparoscopic mesh repair is restricted to bilateral inguinal hernias, recurrent hernias, younger patients, patients with other chronic pain problems and those with severe groin pain. 3,6,35 When a mesh approach is not affordable or suitable (e.g. in older patients with significant comorbidity), a non-mesh repair is usually considered. 6,7
Current service cost
An increasing trend of primary inguinal hernia repairs performed as day-case procedures has been observed in England over the last decade. 2 In 2012/13, 41,384 out of 61,280 (68%) finished consultant episodes for primary inguinal hernia were performed as day-case procedures. 1 Based on national average reference costs, the costs for elective inpatient and day-case procedures are £2041 and £1471, respectively [see Appendix 1 for a derivation of the unit costs from Healthcare Resource Group (HRG) activity code FZ18]. The total annual cost to the NHS in England for primary hernia repair is estimated to be in the region of £114M per year, representing a substantial cost burden to the NHS. An implementation and uptake report completed by National Institute for Health and Care Excellence (NICE) in 201035 indicates an increasing proportion of procedures performed as laparoscopic repair (16% of all primary inguinal hernia repairs in 2008/9). Therefore, assuming a similar breakdown of elective inpatient and day-case procedures for laparoscopic repair, the total cost to the NHS of open mesh hernia repairs is likely to be around £95M per annum.
Earlier studies show that the most important cost parameters for economic evaluation of inguinal hernia include the time patients spent in the operating room and recovery room, and the length of overall hospital stay. 5 The resources required for open surgery are less than those required for laparoscopic surgery. 6,10,11,13 Although there is little evidence comparing the costs of different types of open mesh repair, it can be assumed that operative costs are similar owing to the fact that open mesh procedures are technically comparable. Recent studies indicate that because of the observed low recurrence rates, one of the most important components for total NHS cost is that related to the management of chronic pain after surgery. 36 Evidence shows that laparoscopic repair may reduce postoperative chronic pain, but with the trade-off of additional resources required to perform the surgical mesh procedure. 13 There is no evidence comparing the total costs (including surgical and postoperative costs) or cost-effectiveness of different open preperitoneal mesh repairs from the perspective of health-care providers in the UK.
Variation in services and/or uncertainty about best practice
The relevant mesh techniques commonly used for the treatment of primary inguinal hernia in the UK are open mesh repairs (e.g. Lichtenstein repair and preperitoneal repair) and laparoscopic mesh repairs (TAPP repairs and TEP repairs). The choice of open versus laparoscopic repair, as well as the choice of specific mesh material, is usually based on surgeons’ preference and patients’ characteristics. There is a considerable variation (more than a twofold variation) in the rate of inguinal hernia repair across the NHS. 2 Figure 1 illustrates the number of inguinal hernia repair procedures per 100,000 population per clinical commissioning group across England. Of the 67.2% of inguinal hernia repairs performed in 2011/12 as day cases, the rate varied from 32% to 100% across providers. Owing to the lack of a national audit and of an established follow-up system, and taking into consideration the current low recurrence rate after inguinal hernia repair, it is difficult to rule out with certainty which technique is best.
FIGURE 1.
National variation plot by clinical commissioning group for inguinal hernia repair (from 1 July 2013 to 30 June 2014) (Emma Fernandez, The Royal College of Surgeons of England, 2015, personal communication; permission gained from The Royal College of Surgeons of England for reproduction). The bubbles represent each clinical commissioning group and the size of the bubble represents the number of procedure undertaken.

Relevant national guidelines, including National Service Framework
The recent guidance from the British Hernia Society6 indicates that all adult inguinal hernias should be repaired using a flat mesh technique (or a non-mesh Shouldice technique, if experience is available). For the management of primary unilateral inguinal hernia, the British Hernia Society guidance suggests that an open technique under local anaesthesia should be regarded as an acceptable and cost-effective approach in suitable patients, that is those with significant comorbidity, those without other chronic pain problems and, in particular, older patients. A laparoscopic approach may be considered in bilateral inguinal hernias, groin hernias in women, younger patients, patients with other chronic pain problems or those with a severe groin pain even in the presence of a small hernia. The guidance concludes that at present there is conflicting information on whether or not laparoscopic repairs are better than open mesh repairs in terms of lowering the incidence and severity of pain. 6
Guidance from NICE on laparoscopic surgery for inguinal hernia repair suggests that laparoscopic repair should be considered one of the treatment options for inguinal hernia. 34 A shared decision-making model should be used for the choice of surgery by fully informing patients about the risks and benefits of open and laparoscopic repairs. Only trained and experienced surgeons should perform laparoscopic surgery for inguinal hernia repair. NICE guidance also provides recommendations for bilateral and recurrent hernias. 34
Description of interventions under assessment
The concept of ‘tension-free’ repair using a ‘mesh’ (prosthetic and biological) was introduced initially in the 1960s to overcome the drawbacks of tissue-suture techniques, which resulted in serious complications including ischaemia, pain, necrosis and recurrent hernia. A mesh technique repairs a defect in the posterior wall of the inguinal canal by blocking it with a plug or by placing the flat mesh prosthesis over the fascia transversalis to strengthen the inguinal wall. Meshes can be placed into the defect either anteriorly through open inguinal incision (i.e. Lichtenstein technique) or posteriorly in the peritoneal space through open (i.e. preperitoneal repair) or laparoscopic surgery.
Anterior Lichtenstein repair (open mesh)
Irving Lichtenstein developed the anterior open tension-free approach in 1984. 37 It is a very common and reproducible approach, and is relatively easy to perform. 7 The technique involves the placement of flat mesh (polypropylene) on top of the hernia defect through anterior dissection of the inguinal wall under local or general anaesthesia. Mesh is positioned between the internal and external oblique muscle and is sutured to the inguinal ligament such that there is adequate overlap of the posterior wall. At present, the Lichtenstein repair is considered the gold standard among open inguinal hernia procedures. Since its advent, the incidence of hernia recurrence has reduced up to 2%. 14
Different meshes and/or devices used for anterior open approach have been developed, including the mesh plug, the Prolene Hernia System (Ethicon, Somerville, NJ, USA) and the Hertra sutureless mesh (Herniamesh, Chivasso, Italy). Systematic reviews6,15,38,39 and clinical guidelines have assessed the effect of mesh plug repair and the Prolene Hernia System compared with Lichtenstein mesh repair. The Groin Hernia Guidelines published in 2013 included a meta-analysis of eight randomised controlled trials (RCTs), with a total of 2912 patients assessing the effects of mesh plug repair versus Lichtenstein repair. Meta-analyses results were similar with regard to postoperative complications and return to daily activities. 6 Similarly, a meta-analysis of 10 RCTs with a total of 2708 patients did not find significant differences in the number of recurrences between the Lichtenstein mesh repair, mesh plug repair and the Prolene Hernia System. 38 Another meta-analysis of six RCTs and a total of 1313 patients, which assessed the effects of the Prolene Hernia System versus the Lichtenstein repair, showed that the Prolene Hernia System was associated with a higher rate of perioperative complications. However, no significant differences were observed between the two techniques with regard to duration of operation, time to return to work, chronic groin pain or incidence of recurrences. 39 A more recent report commissioned by the US Agency for Healthcare Research and Quality (AHRQ), assessed the effectiveness of Lichtenstein open mesh with various mesh plug techniques. 15 Based on the findings of 21 studies (20 RCTs and one non-RCT), the report concluded that return to work was shorter after Lichtenstein mesh repair. No other significant differences were observed between the surgical procedures. In conclusion, current evidence seems to indicate that the standard Lichtenstein mesh repair performs better than mesh plug repairs and the Prolene Hernia System.
Posterior open repair (open preperitoneal mesh)
The open preperitoneal mesh approach involves incision of the abdominal wall and implantation of the mesh in the space between the peritoneum and the muscle layers. The mesh is held in place with intra-abdominal pressure and requires less or no fixation. Implantation of the mesh can be achieved through (1) a transinguinal method (e.g. Rives), (2) a small incision (2–3 cm) made in broad abdominal muscles [e.g. Kugel repair (Davol, Warwick, RI, USA)] or (3) a lower midline abdominal incision (e.g. Stoppa repair). 40 Open preperitoneal mesh repairs are mostly performed under general anaesthesia. The first open preperitoneal technique was reported by Stoppa in 1980 (i.e. Stoppa repair). 7 Since then a number of different techniques have been developed including the Kugel patch, the Nyhus repair, the Read–Rives repair and the transinguinal preperitoneal (TIPP) technique. There is a lack of robust evaluations comparing the clinical efficacy of each of these techniques. Kugel, using a specially designed hernia patch, observed only five recurrences out of 808 hernia repairs. 41 A retrospective study found similar results between the participants who underwent TIPP repair and those who underwent Lichtenstein mesh repair, with low incidence of chronic pain in both intervention groups. 11
The open preperitoneal technique with soft mesh has been reported to be a safe and potentially cost-effective approach with a short learning curve. 41–44 Irrespective of either open or laparoscopic techniques, the position of the mesh is considered an important factor in the interpretation of chronic pain because of the location of the nerves in the inguinal canal. Recent systematic reviews and meta-analyses, which assessed the effects of common open mesh techniques in lowering chronic pain and improving major clinical outcomes, have failed to provide definite conclusions. 15,18–20 A Cochrane review20 based on the findings of three RCTs showed some potential benefits of the open preperitoneal mesh repair compared with the Lichtenstein mesh repair in terms of incidence of acute and chronic pain and recurrence rate. However, the evidence base of this review was limited. A recent meta-analysis of 12 RCTs19 found that open preperitoneal mesh repair was associated with a lower risk of developing chronic groin pain than the Lichtenstein mesh repair, and the two techniques were comparable with regard to rate of recurrences and complications. It is worth noting that this meta-analysis did not focus exclusively on people with primary unilateral inguinal hernia but included people with recurrent and incarcerated hernias. Two further systematic reviews in the literature15,18 confirmed that various open repair procedures yielded similar results with further potential benefits for the open preperitoneal mesh techniques. The results of these systematic reviews were, however, inconclusive as both included trials assessing the Prolene Hernia System versus the Lichtenstein mesh repair.
Ralph Ger first introduced the use of a laparoscopic approach in 1982. 45 Laparoscopic repairs are minimally invasive and are performed under general anaesthesia. Small incisions are made for the insertion of the operating instruments and prosthetic mesh is placed to close the hernia defect. Mesh is placed in the preperitoneal plane by using one of the two approaches:
-
TAPP repair: the abdominal cavity is entered and a flap of the peritoneum is deflected to expose the preperitoneal plane. A mesh is inserted to cover the hernia defect in the inguinal region. The peritoneum is then closed over the mesh.
-
TEP repair: the mesh is inserted via the preperitoneal plane without entering the peritoneal cavity to cover hernia defects while remaining outside the peritoneum.
The effects of open versus laparoscopic techniques have been assessed in a considerable number of RCTs. A UK Health Technology Assessment (HTA) report published in 200513 identified 37 RCTs that compared open mesh repairs with laparoscopic mesh repairs. A more recent report by the AHRQ, published in 2012, assessed the effectiveness and adverse effects of various surgical interventions for inguinal hernia in both adults and children. 15 The report identified 123 RCTs, two clinical registries and 26 non-randomised studies published between January 1990 and November 2011. 15 Thirty-six of the included 123 RCTs compared open mesh repairs versus laparoscopic mesh repairs for primary inguinal hernia (indicating that the size of the evidence base for this comparison has not significantly changed since 2003), while 20 RCTs assessed various open mesh repairs (some of which no longer reflect the repairs commonly performed in clinical practice). Both these reports concluded that people who underwent laparoscopic mesh repair had faster return to normal activities, less chronic pain and numbness, and fewer postoperative complications (infection and haematoma), while those patients who underwent open mesh repair had lower rates of serious complications (especially visceral injuries). The AHRQ showed a lower risk of recurrence after open surgery (2.49%) than after laparoscopic surgery (4.46%) for the treatment of painful primary hernias in adults,15 while the UK HTA report observed similar recurrence rates between laparoscopic (2.47%, 26/1052) and open procedures (2.07%, 22/1062). 13 However, there is conflicting information on whether or not laparoscopic repair is better than open mesh repair in terms of lowering the incidence and severity of pain outcomes. 6,21,34 The uptake of laparoscopic technique by surgeons is very low (16% in the UK, ≈ 10% in the USA), probably owing to the complexity of the procedure, potential serious complications, long learning curve and high cost. 3,35
Current usage in the NHS
Inguinal hernia repair is the most common general surgical intervention performed in the UK. In England, 71,490 inguinal hernia procedures were carried out in 2012/13, with over 100,000 NHS bed-days of hospital resources utilised. 1,2 Of these procedures, 65,759 repairs (92%) were for the repair of primary hernias and 5731 repairs (8%) were for the repair of recurrent hernias. 1 Out of 65,759 procedures for primary inguinal hernia, 61,280 (93%) were procedures involving the use of a mesh. Of 71,427 admissions for unilateral or unspecified inguinal hernia, 6.8% (4867) were emergency admissions while almost 90% (64,017) were on a waiting list, with a mean waiting time of 62.5 days. In 86% of cases (61,169), primary repair of inguinal hernia was performed using mesh techniques (i.e. biological/prosthetic). The majority of inguinal hernia repairs were performed as day surgery procedures (> 80%) to overcome the demand of hospital bed requirement in the NHS. 2
The Lichtenstein open mesh repair is the most commonly performed procedure for hernia repair in the UK (performed by 96% of surgeons). 16 A NICE uptake report published in 201035 indicates that of all surgical repairs of inguinal hernia performed in 2008/9 in England, approximately 16% were performed using laparoscopic techniques. 35 In Scotland, the uptake of laparoscopic surgery in 2007/8 was lower, with only 13% of inguinal hernia repairs performed using a laparoscopic approach. 46
Chapter 2 Definition of the decision problem
Purpose of the decision to be made
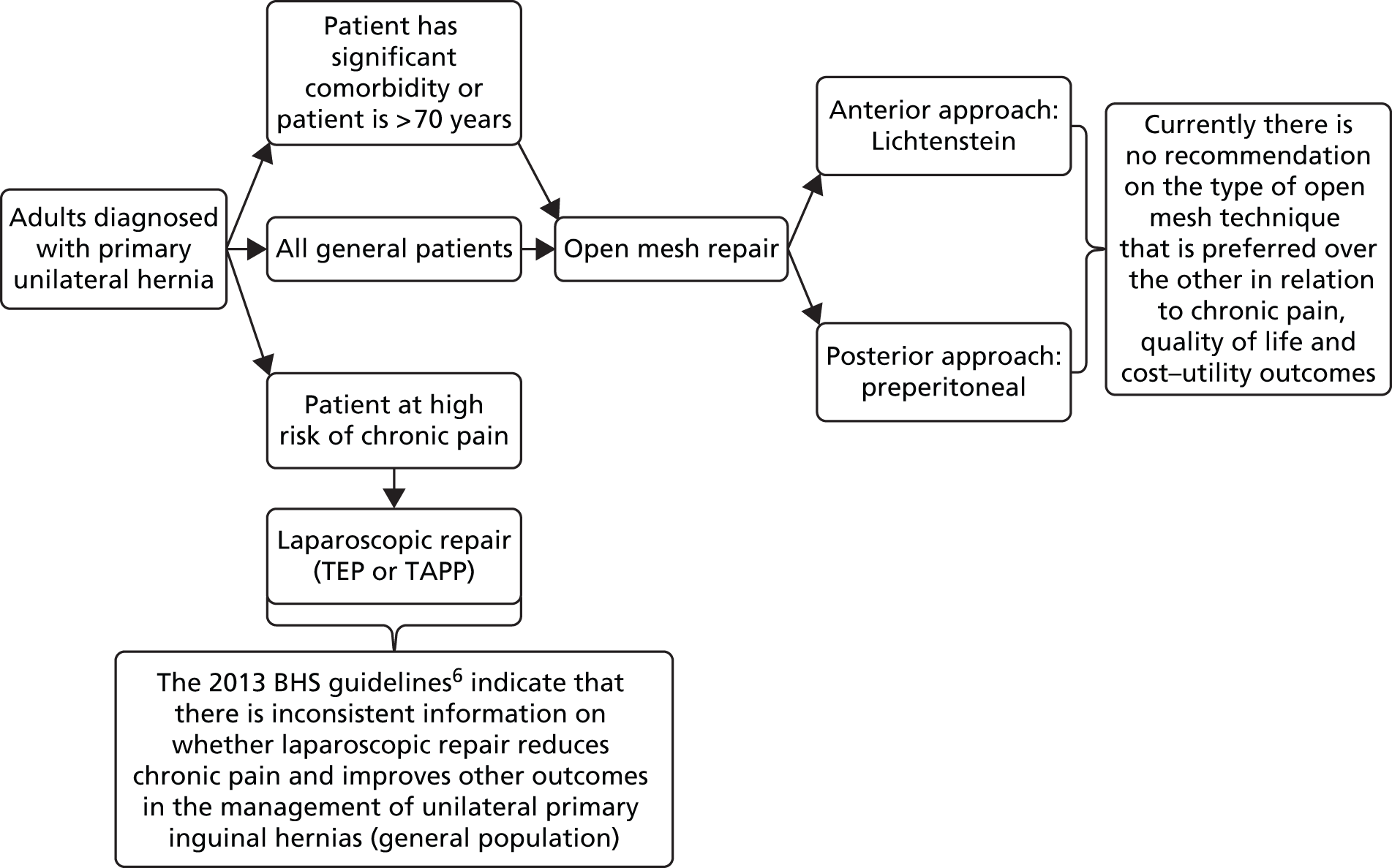
The general purpose of this assessment is to evaluate the current evidence on the effects of open mesh techniques for the treatment of unilateral, primary inguinal hernia. Figure 2 shows the care pathway for the management of adults diagnosed with primary unilateral inguinal hernia based on the current British Hernia Society guidelines. 6
FIGURE 2.
Framework of the care pathway for the management of patients diagnosed with primary unilateral inguinal hernia. BHS, British Hernia Society.
Population
The population considered for this assessment was adults presenting with a clinically diagnosed primary unilateral inguinal hernia who are operated in an elective setting. No relevant subgroups were identified. Recurrent or bilateral inguinal hernia will not be considered in this assessment.
Clinical diagnosis of inguinal hernia is usually established by physical examination. Imaging techniques such as ultrasonography, magnetic resonance imaging or computerised tomography may be used to confirm uncertain or challenging diagnoses. In particular, magnetic resonance imaging and ultrasonography are considered highly accurate techniques for the diagnosis of groin hernias. 22
Intervention
Surgical repair with ‘mesh’ is the recommended treatment for inguinal hernia. 2,7 Mesh repair methods include those involving mesh to strengthen the inguinal wall (e.g. Liechtenstein open mesh repair, open preperitoneal mesh repair and laparoscopic mesh repair). The Lichtenstein mesh repair is the most commonly performed procedure for hernia repair in the UK. 16 The laparoscopic mesh repair is technically more complex and requires longer operation time, special equipment and high surgeon’s experience and, therefore, is not routinely used in the UK. 46 The open preperitoneal mesh repair has shown similar or better outcomes compared with the laparoscopic approach. 15 Open preperitoneal techniques with soft mesh have been reported to be safer and potentially cost-effective with a short learning curve. 42–44 Published evidence comparing open preperitoneal mesh repair with Lichtenstein mesh repair with regard to relevant clinical outcomes, such as chronic pain and QoL, have produced conflicting and inconclusive results. 15,19,20
The following open mesh repairs of inguinal hernia are considered in this assessment:
-
Anterior Lichtenstein mesh repair: Lichtenstein mesh repair is a very common approach that involves the placement of flat mesh on top of the hernia defect through anterior dissection of the inguinal wall under local or general anaesthesia.
-
Open preperitoneal mesh repair: the open preperitoneal mesh approach involves incision of the abdominal wall and implantation of the mesh in the space between the peritoneum and the muscle layers. The mesh is held in place with intra-abdominal pressure and requires less or no fixation. Open preperitoneal repair can be performed using various methods including the Kugel patch, the Nyhus repair, the Read–Rives repair, the TIPP repair and the Stoppa repair.
Non-mesh techniques requiring suturing (e.g. Shouldice, Bassini, McVay, Maloney darn and plication darn techniques) will not be considered in this assessment as they have been proved to be inferior to current mesh techniques and hence no longer recommended. 7–9 Similarly, plug mesh repair and the Prolene Hernia System will not be included in this assessment as they have not demonstrated to be superior to the standard Lichtenstein method. 6,15,18,39 The effects of open versus laparoscopic mesh techniques have been assessed in a considerable number of RCTs. 13,15 In general, evidence has shown similar results with very low recurrence rates. 13–15,18 However, there is conflicting information on whether or not laparoscopic mesh repair is better than open mesh repair in terms of lowering the incidence and severity of pain. 2,21,34 Moreover, laparoscopic mesh repair is not commonly performed in the UK. Laparoscopic mesh techniques will not be considered in this assessment.
Overall aim and objectives of this assessment
This assessment will evaluate the current evidence for the clinical effectiveness and cost-effectiveness of open preperitoneal mesh repairs versus standard anterior Lichtenstein mesh repair, with particular attention to postoperative chronic pain.
The specific aims of this assessment will be the following:
-
systematically review the relative clinical effectiveness of surgical open preperitoneal mesh repairs compared with standard Lichtenstein mesh repair for the treatment of adults presenting with a clinically diagnosed primary unilateral inguinal hernia who are operated in an elective setting
-
systematically review existing economic evaluations on surgical open mesh techniques for the treatment of adults presenting with a clinically diagnosed primary unilateral inguinal hernia who are operated in an elective setting
-
develop a de novo economic model to assess the cost-effectiveness of surgical open mesh repairs for the treatment of adults presenting with a clinically diagnosed primary unilateral inguinal hernia who are operated in an elective setting.
Chapter 3 Clinical effectiveness of open mesh repairs
This chapter reports the assessment of clinical effectiveness of open mesh repairs for primary inguinal hernia. The methods were prespecified in a protocol (PROSPERO database CRD42014013510).
Methods for assessing the outcomes arising from the use of the intervention
We conducted an objective synthesis of the evidence for the clinical effectiveness of relevant open mesh surgical procedures for the repair of primary unilateral inguinal hernia. The evidence synthesis was carried out according to the general principles of the Centre for Reviews and Dissemination guidance for undertaking reviews in health care,47 the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions48 and the NICE Guide to the Methods of Technology Appraisal,49 and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. 50
Identification of studies
Comprehensive electronic searches were conducted to identify reports of published randomised trials. Highly sensitive search strategies were designed, including appropriate subject headings and text word terms, to combine the search facets for inguinal hernia repair, the surgical interventions under consideration and randomised trials. Final searches were carried out on 31 October and 1 November 2014 and were not restricted by year of publication or language. Full details of the search strategies are reported in Appendix 2. The databases searched were MEDLINE (1946 to October week 4 2014), MEDLINE in Process & Other Non-Indexed Citations (31 October 2014), EMBASE (1947 to 2014, week 44), Bioscience Information Service (BIOSIS; 1980 to 1981 November 2014), Science Citation Index (1980 to 1981 November 2014), Scopus Articles In Press (inception to 31 October 2014) and the Cochrane Central Register of Controlled Trials (inception to 2014). Evidence syntheses were sought by searching the Cochrane Database of Systematic Reviews (inception to 2014), Database of Abstracts of Review of Effectiveness (inception to 1 November 2014) and the HTA database (inception to 1 November 2014). Reference lists of all included studies were perused for further evidence. Members of our advisory group were contacted for details of additional reports.
Identification of other relevant information, including unpublished data
The World Health Organization International Clinical Trials Registry and ClinicalTrials.gov were searched on 1 November 2014 for evidence of ongoing studies. Websites of relevant professional groups and HTA organisations were also checked for additional reports (see Appendix 2).
Eligibility criteria
The studies fulfilling the following criteria were included in the assessment.
Population
Adults presenting with a clinically diagnosed primary unilateral inguinal hernia who are operated in any appropriate elective setting. Adults presenting with recurrent, bilateral or strangulated inguinal hernias and children (< 18 years old) were not deemed suitable for inclusion.
Interventions
Open preperitoneal mesh repairs and standard anterior Lichtenstein mesh repair. Open preperitoneal mesh repairs can be performed using various techniques including Kugel patch repair, Read–Rives repair, TIPP repair, Nyhus repair and Stoppa repair. The relative clinical effectiveness of any of these techniques compared with the standard Lichtenstein mesh repair was assessed.
Open non-mesh techniques (including Shouldice, Bassini, McVay, Maloney darn and plication darn techniques) and mesh repairs performed using both an anterior approach and a posterior approach (such as the Prolene Hernia System and the mesh plug repair) were not considered suitable for inclusion (see Chapter 1 for further information).
Outcomes
Studies providing data on any of the following outcomes (using any measure) were included:
-
patient-reported outcomes:
-
chronic pain (≥ 3 months after repair)
-
chronic numbness (≥ 3 months after repair)
-
acute pain (< 3 months after repair)
-
acute numbness (< 3 months after repair)
-
QoL.
-
As definitions of acute and chronic pain vary considerably among studies, we have also taken into consideration the specific definitions used by individual study investigators:
-
clinical and surgical outcomes:
-
mortality
-
complications (such as haematoma, seroma, wound/superficial infection, mesh/deep infection, vascular injury, visceral injury, port site hernia, other serious complications)
-
recurrence/reoperation rate
-
length of hospital stay (days)
-
time to return to normal activities (days).
-
Study design
Randomised controlled trials or quasi-RCTs assessing the clinical effectiveness of open preperitoneal mesh repairs compared with Lichtenstein mesh repair were considered for inclusion. There was no restriction on the publication status (published or unpublished), the year or the language in which trials were reported. Well-conducted systematic reviews were included as sources of relevant data.
Exclusion criteria
Studies not fulfilling the prespecified criteria and the following type of reports were excluded:
-
biological studies
-
editorials and opinions
-
case reports
-
conference abstracts.
Data extraction and management
Two reviewers (PS and MC) independently screened the titles and abstracts of all citations identified by the search strategies. Full-text copies of all potentially relevant studies were retrieved and assessed independently by the two reviewers for eligibility using a screening form developed for this purpose (see Appendix 3, Table A). Full-text copies of non-English-language studies deemed to be potentially relevant were translated before they were assessed for eligibility. Any disagreements during study selection were resolved by discussion or in consultation with a third reviewer (MB or IA).
A data extraction form was specifically designed and piloted for the purpose of this assessment (see Appendix 3, Table B). Two well-conducted RCTs were used to pilot the data extraction form. Detailed information on study design, characteristics of participants, settings, characteristics of interventions and outcome measures were recorded. Three reviewers carried out data extraction (PS, MC and NS); one reviewer completed the data extraction form for all selected studies and two other reviewers cross-checked the details extracted by the first reviewer. There were no disagreements between reviewers.
Further information from the included studies (methodological details or outcomes data) was requested by written correspondence to the original investigators (using open-ended questions and outcome tables for missing outcome data). Principal investigators of relevant ongoing trials were contacted to obtain unpublished data. Any relevant information retrieved in such a manner was included in the review. We contacted eight trial investigators and received further information from three of them. Where trials reported more than two study arms, only data from the relevant arms were extracted.
Where possible, means and standard deviations (SDs) were extracted for continuous data. When SD values were not available, we attempted to (1) calculate SD values using other reported values and (2) contact the original trial investigators for further details. Only when these attempts proved unfeasible or unsuccessful, values were imputed using the average of the SDs from other trials reporting the same outcomes.
Quality assessment strategy
The potential risk of bias for included studies was assessed by a single reviewer (PS or MC) and cross-checked by a second reviewer (PS or MC). Disagreements were resolved by consensus or arbitration with a third reviewer (MB). Studies were not included or excluded on the basis of their methodological quality. The Cochrane risk-of-bias tool was used to assess the risk of bias of all included RCTs48 (see Appendix 4). Critical judgements were made for all main domains: selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants), detection bias (blinding of outcome assessor), attrition bias (incomplete outcome data), reporting bias (free of selective reporting) and other sources of bias (e.g. bias related to the validity of the tools used for measuring outcomes and bias related to inappropriate source of funding). The domains ‘blinding of outcome assessment’ and ‘incomplete outcomes data’ were assessed for each outcome of interest. The blinding of personnel delivering the intervention was not considered relevant for this assessment, as in clinical practice it is not feasible to blind the surgeon who performs the operation.
Each included study was judged to be at ‘low risk of bias’, ‘high risk of bias ‘or ‘unclear risk of bias’ according to the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions. 48 Adequate sequence generation, allocation concealment, blinding of participants and blinding of outcome assessor were identified as key domains for the assessment of the risk of bias of the included trials. Studies were classified as follows: (1) high risk of bias, if one or more key domains were at high risk; (2) unclear risk of bias, if one or more key domains were judged to be at unclear risk; and (3) low risk of bias, if all key domains were judged to be at low risk.
Method of analysis/synthesis
For binary outcomes, the Mantel–Haenszel approach was used to pool risk ratios (RRs) derived from each study. However, because some adverse outcomes were expected to be relatively uncommon for outcomes with rare events (i.e. < 5%), the Peto odds ratio (OR) approach was considered the most appropriate meta-analysis approach. For continuous outcomes, mean differences between groups were pooled using the inverse variance method. For time-to-event outcomes (recurrence and time to return to normal activities) we planned to pool hazard ratios if suitable data were available.
The statistical heterogeneity across studies was explored using the Chi-squared and I-squared statistics. The primary analysis used a random-effects model to calculate the pooled estimates of effect. This was not possible when the Peto approach was used for binary outcomes with rare events.
If a sufficient number of trials were available, sensitivity analyses restricted to trials at low risk of bias were planned.
Results of the evidence synthesis
Quantity and source of the evidence
The original primary searches and the subsequent updates retrieved a total of 1204 records. After reviewing all titles and abstracts, 1122 records were subsequently excluded because they were not relevant. Full-text copies of 82 potentially relevant reports were obtained and screened for inclusion. Six non-English full-text articles published in Dutch (n = 1), German (n = 1), Italian (n = 1), Spanish (n = 2) and Persian (n = 1) were identified and assessed. Colleagues from the School of Medicine and Dentistry, University of Aberdeen, who were native Dutch, German, Italian or Spanish speakers, translated five of these articles professionally. A full-text article, published in Persian, was initially translated using the Google (Google Inc., Mountain View, CA, USA) translator tool and subsequently checked by a colleague who could read Persian. After translation, only two non-English-language papers (published in Persian and Spanish) met the prespecified inclusion criteria and were included for further assessment.
Three systematic reviews of RCTs assessing open mesh repair versus Lichtenstein mesh repair were deemed suitable for inclusion (the Cochrane review by Willaert and colleagues,20 the meta-analysis by Sajid and colleagues,19 and the systematic review by Li and colleagues18). In line with the prespecified research protocol, these systematic reviews were used as a source of existing evidence but were not formally updated. Further methodological details and missing data from one included ongoing RCT51 were derived from the systematic review by Willaert and colleagues,20 which cited this RCT and was based on individual participant data, as we were not able to make contact with the principal investigator.
In total, 12 RCTs (11 RCTs published in 13 full-text papers and one ongoing RCT) met the inclusion criteria and were included for the clinical effectiveness assessment. 42,51–63 One of the included trials53 used a quasi-random method to allocate participants to interventions (i.e. order of admittance).
Figure 3 shows the flow diagram of the study selection process. Appendix 5 lists all the studies included in this assessment, together with the systematic reviews that were used as a source of relevant evidence.
FIGURE 3.
Flow diagram of the study selection process.

Appendix 6 lists the studies excluded after full-text scrutiny together with the reasons for their exclusion. Sixty-six studies were excluded because they failed to meet one or more of the specified inclusion criteria with regard to study design, participants, intervention or outcomes. In particular, 45 studies were excluded because they were non-RCTs, one study because patients did not present with primary inguinal hernia, 15 studies because they did not include a relevant comparison and five studies, published in non-English languages, because they could not be obtained.
Risk-of-bias assessment of included studies
Figure 4 illustrates the summary of the risk-of-bias assessment for all 12 included studies. 42,51–63 Risk of bias of individual studies is detailed in Appendix 7, Tables 30 and 31.
FIGURE 4.
Summary of risk-of-bias assessment of included 12 studies.
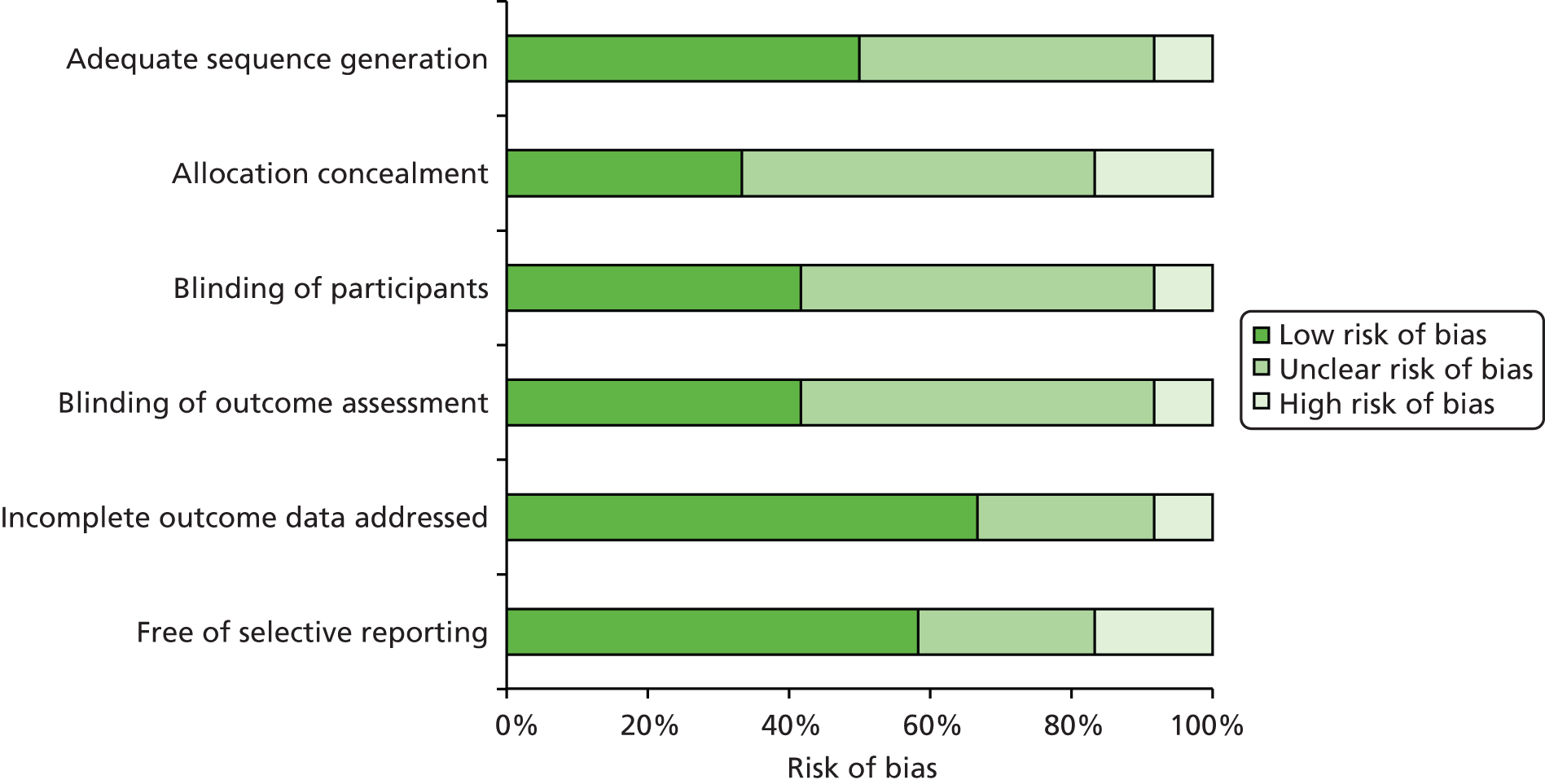
Generally, trials were at high or unclear risk of bias. Only two trials were judged to be at low risk of bias, with adequate sequence generation, allocation concealment, blinding of participants and blinding of outcome assessor. 52,56 Two trials were judged at high risk of selection bias because of the inadequate randomisation process,53,59 whereas one trial was judged at unclear risk of selection bias because not enough information was provided on allocation concealment. 61 One trial51 did not blind participants or outcome assessors, and was considered at high risk of performance and detection biases. Six of the included trials,54,55,57,58,60,63 failed to provide sufficient information to formulate a reliable judgement about risk of bias and no additional details were obtained from the corresponding authors.
Selection bias (adequate sequence generation/allocation concealment)
Of the 12 included trials, four reported adequate sequence generation and allocation concealment. 51,52,56,58 Randomisation was performed using a computer-generated list and allocation of participants was concealed by means of sequentially numbered, sealed opaque envelopes. Two trials used a computer-generated method59 and a random number table, respectively,61 but did not conceal participants’ allocation59 or failed to report information on concealment allocation. 61 Another trial had a high risk of selective enrolment of participants as randomisation was done according to order of admittance. 53 In the remaining trials, there was insufficient information on sequence generation and allocation to make a reliable judgement. 54,55,57,60,63
Performance and detection bias (blinding)
The blinding of personnel delivering the intervention was not assessed, as it is impossible to blind the surgeon performing the surgical operation. It was reported that participants were blinded to the specific type of surgical intervention in four trials. 52,56,59,61 Five trials reported that the main outcome assessor was blinded to the patients’ clinical reports and to the type of surgical procedure. 52,56,57,59,61
Attrition bias (incomplete outcome data)
Eight of the included trials were graded to be at low risk of attrition bias. 51,53,56–61 Missing data were adequately addressed in four trials53,56,58,59 and four other trials reported that there were no missing data. 51,57,60,61 One trial, conducted in Turkey, which did not address missing data adequately and had a 5% drop-out rate, was judged to be at high risk of attrition bias. 52 With regard to the remaining three trials,54,55,63 no information was provided on the number of participants enrolled, number of participants randomised or number of participants lost to follow-up.
Reporting bias (free of selective reporting)
In seven of the included trials, the outcomes assessed were either prespecified in a protocol or in the analysis section and there was no clear evidence of reporting bias. 51,52,56,58–61 Two other trials were judged to be at high risk of reporting bias as they failed to report all outcomes that were assessed. 53,63 Three other trials were graded to be at unclear risk of bias as they reported acute pain and/or patient satisfaction using a visual analogue scale (VAS), but did not report chronic pain. 54,55,57
Other sources of bias
One trial52 measured chronic pain using the Sheffield scale. We could not identify sufficient evidence to decide whether or not the Sheffield scale was a validated tool to measure pain in patients who underwent inguinal hernia repair. Therefore, this study was judged to be at unclear risk of other source of bias. None of the included trials was sponsored by industry. No other sources of bias were obvious in the published trials reports.
Study characteristics
Details of all included studies, including baseline characteristics of participants, description of surgical interventions (i.e. open preperitoneal mesh repair and Lichtenstein mesh repair) and clinical outcomes are tabulated in Appendix 8, Tables 32–34.
Included trials were conducted in Turkey (four studies),52–54,63 the Netherlands (two studies),56,59 Belgium (one study),51 Egypt (one study),55 Iran (one study),57 India (one study),60 the USA (one study)58 and Mexico (one study). 61 Four trials were sponsored by professional organisations. 51,55,56,61 In the remaining eight trials, the source of funding was not specified. 52–54,57–60,63
Among included studies, the lengths of follow-up ranged from 1 week63 to 110 months. 58 Only two trials had long follow-up assessments; the trial by Gunal and colleagues,54 conducted in Turkey, reported mean length of follow-up of 98 months and the trial by Muldoon and colleagues,58 conducted in the USA (224 participants in total), had a median length of follow-up of 82 months (range 24–110 months). In the remaining trials the mean length of follow-up was 17.3 months (range 0.25–54.5 months; median 12 months).
Participants
A total of 1568 participants with primary unilateral inguinal hernia were assessed among the 12 included trials. The characteristics of the participants’ hernia defects [classified according to either Nyhus classification or the American Society of Anaesthetists (ASA) grade] varied across trials. Four trials54–56,59 included low-risk participants such as those with ASA grade I–III or Nyhus type I–III defects, while two other trials57,58 included participants with large hernia defects (Nyhus type III–IV). The remaining trials did not specify the physical status of the hernia defect. 51–53,60,61,63
Table 2 summarises the baseline characteristics of all included trials and Appendix 8 describes the characteristics of each individual trial. The mean sample size among included trials was 130.7 ranging from 45 participants63 to 302 participants. 56 Apart from the TULIP (the Tilburg double-blind randomised controlled trial comparing inguinal hernia repair according to Lichtenstein with the TIPP technique) trial by Koning and colleagues,56 which was conducted in two large hospitals in the Netherlands, all trials were conducted in a single centre. The trial by Koning and colleagues,56 with a total of 302 participants, was also the largest included trial. The trial by Smolinski-Kurek,64 which originally planned to include 168 participants, reported only preliminary results based on a total of 90 participants. 61
The age of participants ranged from 18 to 85 years. Demographic characteristics of participants (age, sex and body mass index) were balanced between intervention groups (i.e. open preperitoneal mesh repair and Lichtenstein mesh repair).
| Baseline characteristics | Open preperitoneal | Lichtenstein | Total |
|---|---|---|---|
| Total randomised, N | 771 | 797 | 1568 |
| Total analysed, N | 743 | 775 | 1518 |
| Number lost to follow-up, n (%) | 28 (3.8) | 22 (3) | 50 (3.5) |
| Number of men,a n (%) | 698 (90.5) | 726 (91.1) | 1424 (90.8) |
| Range of mean age (years), n | 23.85–60.7 | 22.76–63.3 | 22.76–63.3 |
| Range of mean BMI (kg/m2) | 22.2–26.36 | 24.34–26.82 | 22.2–26.82 |
| Hernia typeb | N = 611 | N = 641 | N = 1252 |
| Indirect, n (%) | 382 (62.5) | 388 (60.4) | 770 (61.5) |
| Direct, n (%) | 176 (28.8) | 203 (31.6) | 379 (30.3) |
| Others,c n (%) | 53 (8.7) | 50 (8.0) | 103 (8.2) |
Hernia repair (open preperitoneal mesh repair and Lichtenstein mesh repair)
In all included trials, participants were randomised to either Lichtenstein mesh repair or open preperitoneal mesh repair. Appendix 8, Table 34 describes the details of these techniques including type of incision, type of mesh, mesh fixation methods, duration of operation and surgeon’s level of experience.
All 12 included trials referred to a ‘standard’ Lichtenstein repair even though the procedure has technically evolved since its first introduction in 1984. The type of mesh used to repair the hernia defect varied among trials. Eight of the included trials opted for a polypropylene mesh. 52,53,57–61,63 The trials by Koning and colleagues56 reported the use of a soft mesh, whereas Berrevoet51 used a lightweight mesh.
Various open preperitoneal mesh techniques were utilised in the included trials. Three of the most recent trials compared TIPP repair with Lichtenstein mesh repair. 51,56,60 Two of these trials56,51 used a polysoft mesh (soft mesh with memory ring), whereas the other trial used a polypropylene mesh. The other three trials used a Kugel approach. 52,53,59 Two of these trials used a double-layer mesh patch as originally described by Kugel53,59 while one trial52 used a modification of the original Kugel technique (i.e. a single-layer polypropylene mesh patch), with the intent to reduce the occurrence of a foreign body reaction. Another trial61 used an elliptical domed mesh preperitoneal technique with polypropylene mesh. Of the remaining five trials, two compared standard Read–Rives repair with Lichtenstein repair;57,58 two compared Nyhus repair with Lichtenstein repair;54,63 and one did not specify the type of open preperitoneal technique. 55
Among the 12 included trials, three had multiple arms and, alongside the Lichtenstein mesh repair and the open preperitoneal mesh repair, included additional comparisons with various laparoscopic techniques (TAPP and TEP repairs)54,55,63 or with the Bassini open non-mesh technique. 63 Only data from the comparisons relevant to the purpose of this assessment were considered suitable for inclusion.
Mean duration of open preperitoneal surgery ranged from 34.1 minutes (SD 9.9 minutes)56 to 59 minutes (SD 11 minutes)61 and that of Lichtenstein repair ranged from 34.2 minutes (SD 23.5 minutes)55 to 58 minutes (SD 10 minutes). 61 Six trials52–54,56,59,63 reported that the Lichtenstein mesh repair took longer to perform than the open preperitoneal mesh repair, while four trials reported that the open preperitoneal mesh repair (performed using either the TIPP technique,60 the Read–Rives technique57,58 or an unspecified preperitoneal technique55,61) required more time.
Assessment of outcomes and follow-up
Table 3 highlights the type of outcome measures that were assessed in the included trials. Pain was measured at different time points after surgery and during follow-up (see Appendix 8, Table 32). In total, seven trials assessed chronic pain (≥ 3 months),51,52,56,58–61 nine acute pain51,54–57,59–61,63 (< 3 months) and four chronic numbness56,58,59,61 (≥ 3 months).
| Outome reported | Study ID | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arslan et al. 201452 | Berrevoet51 | Dogru et al. 200653 | Gunal et al. 200754 | Hamza et al. 201055 | Koning et al. 201256 (Koning et al. 2013)42 | Moghaddam et al. 201157 | Muldoon et al. 200458 | Nienhuijs et al. 200759 (Staal et al. 2008)62 | Ray et al. 201460 | Smolinski-Kurek et al. 201261 | Vatansev et al. 200263 | |
| Chronic pain | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Chronic numbness | ✓ | ✓ | ✓ | ✓ | ||||||||
| Acute pain | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Recurrence/reoperation rate | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Length of hospital stay | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Time to return to normal activities | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Complications | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Mortality | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
The definition of pain and time points when pain was measured varied among trials (see Appendix 9, Tables 35–40). Four trials used the VAS score to measure chronic pain. 51,56,59,61 The VAS is a validated instrument for measuring pain on a scale ranging from 0 (no pain) to 10 (worst pain imaginable). Three of these four trials51,56,59 defined chronic pain as ‘any VAS score above zero which lasts for more than three months as defined by the International Association for the Study of Pain’ and reported chronic pain at 3 months,59 1 year56 and 2 years. 51 One trial61 defined chronic pain as ‘any VAS score between 3 and 10’ and reported chronic pain at 3 and 6 months. A different trial52 defined chronic pain as ‘a pain lasting for more than 6 months after surgery according to the Sheffield scale’. The Sheffield scale is a tool that measures pain using a score from 0 to 3, where 0 indicates no pain, 1 indicates no pain at rest but some pain during movement, 2 indicates temporary pain at rest and moderate pain during movement and 3 indicates constant pain at rest and severe pain during movement. The remaining two trials58,60 did not specify the instrument used to measure pain, but reported the number of participants suffering from ‘chronic pain’ at 6 months.
Of the nine trials that assessed acute pain51,54–57,59–61,63 (measured within 3 months after surgery), seven measured pain using the VAS score or reported the proportion of participants suffering from pain. 51,54–57,59,61 Follow-up measurements of acute pain varied among these seven trials (see Appendix 9, Tables 37–39). One trial assessed pain 1 month after surgery but did not specify how pain was measured60 and one trial reported postoperative pain levels by assessing the need for analgesia during the first 24 hours after surgery. 63
In five trials,51,52,56,59,61 chronic pain was the primary outcome. Four of these five trials52,56,59,61 were powered to detect differences in chronic postoperative pain.
Eleven of the 12 included trials51–61 assessed recurrences and complications,63 and five trials52,55–57,60 assessed length of hospital stay and time to return to normal activities.
None of the included trials specifically assessed QoL, but two of the trials reported health measures42 and patient satisfaction57 after open preperitoneal mesh repair and Lichtenstein mesh repair.
Results of the individual studies and data synthesis
The 12 trials identified included a total of 1568 participants; 771 randomised to open preperitoneal mesh repair and 797 to Lichtenstein mesh repair. Eleven trials with a total of 1523 participants provided suitable data for statistical analyses relevant to the comparisons and outcomes of interest. The main results are reported under two broad sections entitled Patient-reported outcomes and Clinical outcomes. See Tables 4–7 for the results and Appendix 9, Tables 35–44 for the results from individual studies.
Patient-reported outcomes
Table 4 displays the meta-analysis results for patient-reported outcomes (also see Figures 5–7).
| Outcomes | RR or WMD | 95% CI | p-value | Number of trials |
|---|---|---|---|---|
| Chronic pain | RR 0.50 | 0.20 to 1.27 | 0.15 | 7 |
| Chronic numbness | RR 0.48 | 0.15 to 1.56 | 0.23 | 4 |
| Acute pain | WMD –0.49 | –1.06 to 0.09 | 0.10 | 5 |
Chronic pain
Figure 5 shows the meta-analysis results for chronic pain. There was considerable variation in the definition of pain used (see Assessment of outcomes and follow-up) by the individual trial investigators and the reported rates of chronic pain (see Appendix 9, Table 35). With the exception Arslan and colleagues,52 who reported a high proportion of participants with chronic pain (90%) in both intervention groups, all the remaining trials reported relatively low rates of chronic pain.
FIGURE 5.
Open preperitoneal mesh repair vs. Lichtenstein mesh repair: chronic pain. df, degrees of freedom; M–H, Mantel–Haenszel.
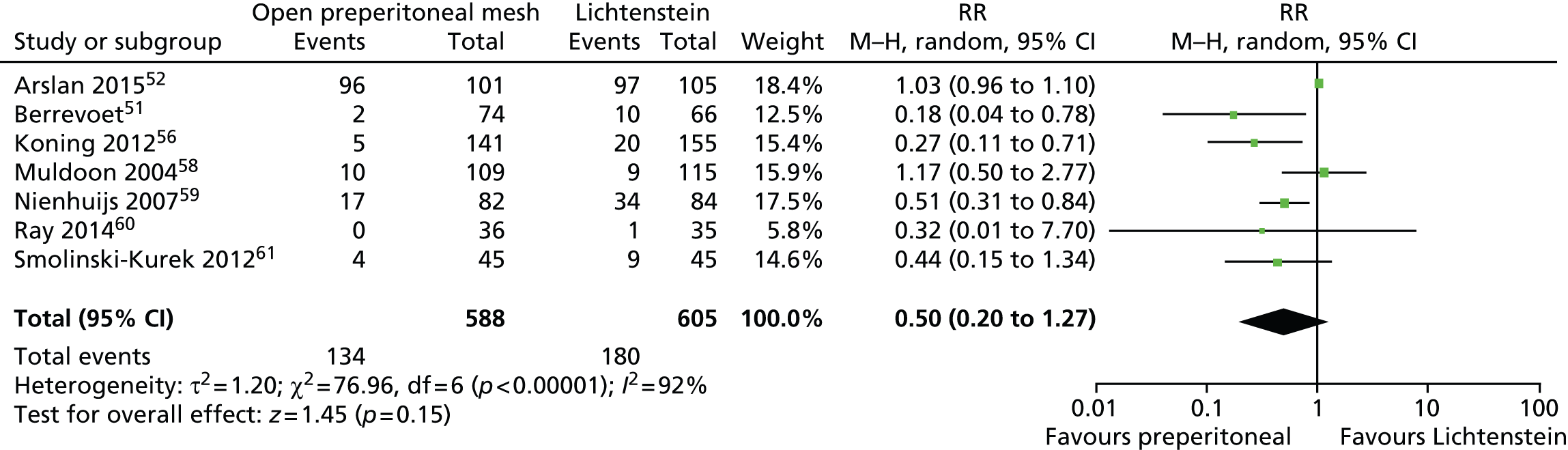
The random-effects meta-analysis showed a RR of 0.50, indicating a 50% reduction in the risk of chronic pain among participants who underwent open preperitoneal mesh repair compared with those who underwent Lichtenstein mesh repair. The difference between intervention groups was not statistically significant [95% confidence interval (CI) 0.20 to 1.27] and there was evidence of high statistical heterogeneity among trials (I2 = 92%). The observed variation in the rates of chronic pain between trials can be partly explained by the way trial investigators described chronic pain. ‘Chronic pain’ was (1) defined according to the definition of the International Association for the Study of Pain (IASP), including a VAS score above 0 which lasts for more than 3 months; (2) defined by a VAS score between 3 and 10; or (3) was not defined at all. Other possible sources of heterogeneity include the position of the mesh and the type of mesh fixation, the type of techniques used for inguinal hernia repair and the surgeon’s clinical expertise.
Additional chronic pain outcomes
Two trials also reported the proportion of participants with ‘activity-related pain’,56,58 and two other trials reported ‘mean pain scores’52,59 (see Appendix 9, Table 36).
However, owing to the observed heterogeneity between trials, data were not combined to provide a single estimate of effect. Koning and colleagues56 reported that significantly fewer patients in the open preperitoneal group experienced pain during activity compared with those in the Lichtenstein group (8.5% vs. 38.5%; p = 0.001). In contrast, the trial by Muldoon and colleagues,58 with a follow-up period of more than 2 years, showed that the proportion of patients who experienced pain during activity was slightly higher in the open preperitoneal group (9.2%) than in the Lichtenstein group (6.1%). Mean pain scores, measured using either the VAS score or the Sheffield scale, were lower in the open preperitoneal group than in the Lichtenstein group (mean VAS score, 0.4 vs. 0.9;59 mean Sheffield score, 1.12 vs. 1.34). 52
Chronic numbness
Four trials reported the proportions of participants with chronic numbness. 56,58,59,61 There was evidence of high heterogeneity (I2 = 91%). Two trials58,61 reported considerably higher rates of numbness following Lichtenstein mesh repair but the other two trials56,59 reported similar rates in each group. The random-effects meta-analysis did not provide evidence of statistically significant differences between the intervention groups (RR 0.48, 95% CI 0.15 to 1.56) (Figure 6). Potential sources of heterogeneity include the definition and measurements of numbness, the position of the mesh and the type of mesh fixation, the type of techniques used for inguinal hernia repair and the surgeon’s clinical expertise.
FIGURE 6.
Open preperitoneal mesh repair vs. Lichtenstein mesh repair: chronic numbness. df, degrees of freedom; M–H, Mantel–Haenszel.

Acute pain
Five trials54–57,59 reported mean VAS scores (Figure 7) and were included in a random-effects meta-analysis. The trial by Gunal and colleagues54 reported unrealistically high standard error (SE) values. It was assumed that these values indicated SDs rather than SEs, even though no confirmation from the authors was obtained. It is worth noting, however, that the Cochrane review by Willaert and colleagues,20 which included individual participant data, also interpreted these values as SDs. The trial by Moghaddam and colleagues57 did not report any measure of variability and, therefore, SD values were estimated using a simple average of the measures reported in the other trials (excluding the trial by Gunal and colleagues54).
FIGURE 7.
Open preperitoneal mesh repair vs. Lichtenstein mesh repair: acute pain. df, degrees of freedom; IV, instrumental variable.

The meta-analysis results showed no clear evidence of a difference between intervention groups (mean difference –0.49, 95% CI –1.06 to 0.09). Acute pain tended to be lower after open preperitoneal mesh repair, but moderate statistical heterogeneity was evident between trials (I2 = 53%).
Four trials51,57,60,61 reported the proportion of participants with acute pain assessed between 1 week and 1 month after surgery. In general, fewer participants suffered from acute pain after preperitoneal mesh repair than after Lichtenstein mesh repair at week 1 (62.2% vs. 84.4%),61 at week 2 (6.7% vs. 38.7%)51 and at 1 month (0–26.7% vs. 8.6–51.1%)57,60,61 (see Appendix 9, Table 38).
Two trials57,63 reported the postoperative need for analgesics. The use of analgesics during the first 24 hours after surgery was lower among participants who underwent Lichtenstein mesh repair than among those who underwent open preperitoneal mesh repair (see Appendix 9, Table 39).
Acute numbness
None of the included trials reported acute numbness.
Health status and patient satisfaction
The trial by Koning and colleagues42,56 examined the postoperative effects of the interventions using the Short Form questionnaire-36 items (SF-36) health survey. The SF-36 is a validated short questionnaire with 36 items that comprises eight domains: physical functioning (10 items); social functioning (two items); role limitations owing to physical problems (four items); role limitations owing to emotional problems (three items); mental health (five items); energy and vitality (four items); pain (two items); and general perception of health (five items). Scale scores are transformed to a scale from 0 to 100, where 0 indicates poorest health and 100 indicates best health. In the trial by Koning and colleagues,42,56 data on health status were prospectively collected after physical examination at scheduled follow-up visits. At 1 year, participants who underwent open preperitoneal mesh repair provided better mean responses to the physical functioning domain [94.9 (SD 12.0) vs. 91.4 (SD 14.9); p = 0.023] and to the pain domain [mean 91.6 (SD 16.4) vs. 85.5 (SD 17.0); p = 0.002] than those who underwent Lichtenstein mesh repair. For the remaining six domains no statistically significant differences were observed between intervention groups (Table 5).
| Health status domains | Open preperitoneal (n = 141) | Lichtenstein (n = 155) | Difference between groups (95% CI) | p-value |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| General health | 81.5 (18.0) | 82.5 (17.9) | –5.1 to 3.1 | 0.630 |
| Physical pain | 91.6 (16.4) | 85.5 (17.0) | 2.3 to 9.9 | 0.002 |
| Vitality | 77.6 (14.9) | 78.2 (15.1) | –4.1 to 2.7 | 0.696 |
| Mental health | 84.4 (14.7) | 86.5 (13.1) | –5.2 to 1.1 | 0.197 |
| Role emotional | 95.1 (18.5) | 93.9 (20.8) | –3.3 to 5.7 | 0.604 |
| Role physical | 93.5 (21.6) | 91.7 (22.1) | –3.2 to 6.8 | 0.474 |
| Social functioning | 94.1 (13.3) | 92.1 (15.4) | –1.3 to 5.3 | 0.230 |
| Physical functioning | 94.9 (12.0) | 91.4 (14.9) | 0.5 to 6.7 | 0.023 |
Moghaddam and colleagues57 measured patient satisfaction after surgery using a VAS score. A significantly higher mean score was observed among participants in the open preperitoneal group (9.6, SD 1.6) than among those in the Lichtenstein group (7.3, SD 3.1; p < 0.01).
Surrogate patient-reported outcome
The study by Staal and colleagues,62 which is a secondary report from the trial by Nienhuijs and colleagues,59 reported the Pain Disability Index (PDI) at 3 months. The PDI is a questionnaire that comprises seven subscales of activities: family and home duties (activities related to home and family); recreation (hobbies, sports and other leisure time activities); social functions (participation with friends and acquaintances other than family members); occupation (activities partly or directly related to working including housework or volunteering); sexual behaviour (frequency and quality of sex life); self-care (personal maintenance and independent daily living, such as bathing, dressing, etc.); and life-support functions (basic life-supporting behaviours, such as breathing, eating, sleeping, etc.). Impairment related to pain for each of the above items is rated on a 0 to 10 scale, where 0 indicates no impairment and 10 indicates maximum impairment. Scores for each of the seven subscales are summed to give a total PDI score (range 0–70). Nienhuijs and colleagues59 and Staal and colleagues62 reported that the mean PDI score was statistically significantly lower among participants in the open preperitoneal group (2.0, SD 6.2) than among those in the Lichtenstein group (4.1, SD 11.2; p = 0.006).
Clinical and surgical outcomes
Meta-analysis results for clinical and surgical outcomes are presented in below (see Figures 8–13). Table 6 shows the total number of early complications reported in the included studies while Table 7 summarises the meta-analyses results for the clinical and surgical outcomes.
| Study | Open preperitoneal | Lichtenstein | ||||
|---|---|---|---|---|---|---|
| Number of events (n) | Total (N) | % | Number of events (n) | Total (N) | % | |
| Arslan et al. 201452 | 28 | 101 | 27.7 | 22 | 105 | 20.9 |
| Berrevoet51 | 2 | 75 | 2.7 | 14 | 75 | 18.7 |
| Dogru et al. 200653 | 3 | 69 | 4.3 | 1 | 70 | 1.4 |
| Gunal et al. 200754 | 2 | 39 | 5.1 | 9 | 42 | 21.4 |
| aHamza et al. 201055 | 2 | 25 | – | 1 | 25 | – |
| Koning et al. 201256 | 9 | 141 | 6.4 | 29 | 155 | 18.7 |
| aMoghaddam et al. 201157 | 3 | 62 | – | 6 | 64 | – |
| aMuldoon et al. 200458 | 17 | 109 | – | 18 | 115 | – |
| Ray et al. 201460 | 1 | 36 | 2.8 | 1 | 35 | 2.9 |
| Smolinski-Kurek et al. 201261 | 9 | 45 | 20 | 8 | 45 | 17.8 |
| Outcomes | WMD or OR | 95% CI | p-value | Number of trials |
|---|---|---|---|---|
| Recurrence/reoperation | Peto OR 0.76 | 0.38 to 1.52 | 0.44 | 11 |
| Mortality | Peto OR 1.05 | 0.21 to 5.25 | 0.95 | 4 |
| Complications | ||||
| Wound infection | Peto OR 0.53 | 0.21 to 1.36 | 0.19 | 6 |
| Haematoma/seroma | Peto OR 1.29 | 0.75 to 2.20 | 0.35 | 8 |
| Urinary complications | Peto OR 0.87 | 0.37 to 2.07 | 0.76 | 3 |
| Time to return to normal activities | WMD –1.49 | –2.78 to –0.20 | 0.02 | 5 |
Mortality
Four trials reported mortality data. 53,56,57,59 There were only six deaths in total and this was balanced between the intervention groups. Three deaths occurred after open preperitoneal mesh repair and three after Lichtenstein mesh repair (Peto OR 1.05, 95% CI 0.21 to 5.25) (Figure 8).
FIGURE 8.
Open preperitoneal mesh repair vs. Lichtenstein mesh repair: mortality. df, degrees of freedom.

Complications
Eleven trials51–61 reported postoperative complications (see Appendix 9, Table 41). There was a considerable variation across trials in the way type of complications and time of assessments were reported. In many trials, complications were categorised as ‘early or ‘late’ by the trial investigators. When an adequate definition was not provided, any complication reported after 6 months was classified as ‘late’. Where time points were not specified, we consulted with our clinical experts to classify the complication as late or early. Complications, such as haematoma, seroma or urinary complications, were assumed to occur soon after surgery and were categorised as ‘early’ complications.
Ten trials51–58,60,61 reported data on early complications (see Table 6), including wound infection, haematoma, seroma, cord oedema, scrotal oedema and urinary complications (urinary retention/urinary tract infection). One trial59 reported overall complications data (haematoma, dysejaculation and infection) without separating the results for open preperitoneal group and Lichtenstein group. This trial, however, reported one participant with higher urinary frequency in the open preperitoneal group. While some trials reported each type of complication separately, others reported broad categories of complications. For three trials55,57,58 it was unclear whether complications were presented as number of people with any complication or as a total number of events. Owing to the lack of consistency among trials and taking into account the potential risk of double-counting events, a formal meta-analysis for early complications is not presented. Separate meta-analyses were, however, conducted for the following complication categories: wound infection, haematoma/seroma and urinary complications.
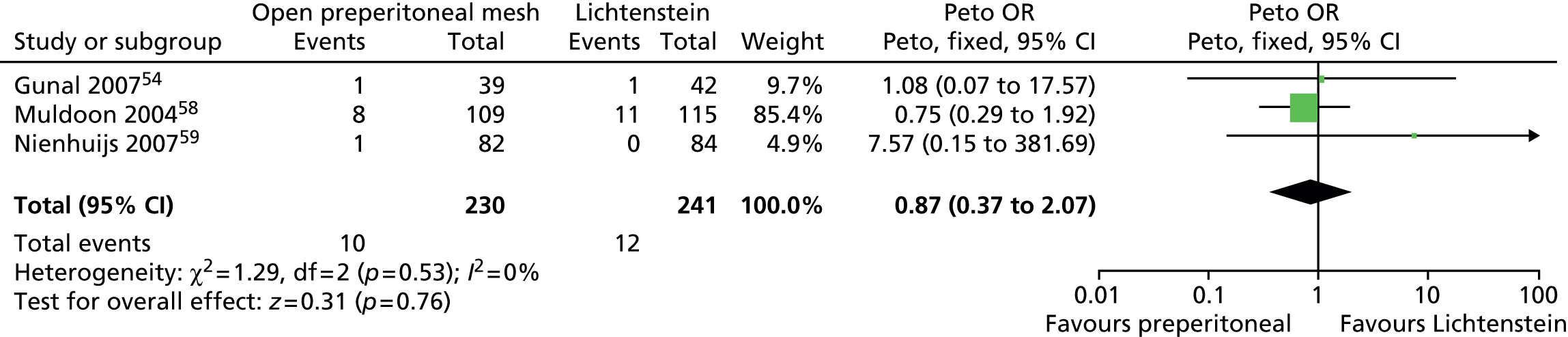
There was no clear evidence of differences between intervention groups for the incidence of wound infection (Peto OR 0.53, 95% CI 0.21 to 1.36), haematoma/seroma (Peto OR 1.29, 95% CI 0.75 to 2.20) or urinary complications (Peto OR 0.87, 95% CI 0.37 to 2.07) (Figures 9–11).
FIGURE 9.
Open preperitoneal mesh repair vs. Lichtenstein mesh repair: incidence of wound infection. df, degrees of freedom.

FIGURE 10.
Open preperitoneal mesh repair vs. Lichtenstein mesh repair: incidence of haematoma/seroma. df, degrees of freedom.

FIGURE 11.
Open preperitoneal mesh repair vs. Lichtenstein mesh repair: urinary complications. df, degrees of freedom.
Three trials reported information on late complications, including testicular atrophy58 and mesh infection/reaction. 51,53 Overall, events were balanced between intervention groups: one case of testicular atrophy and two cases of mesh infection/reaction were reported in the open preperitoneal groups while three cases of testicular atrophy were reported in the Lichtenstein groups. No trials reported any vascular/visceral injury, port site hernia or any other serious complications.
Recurrence/reoperation
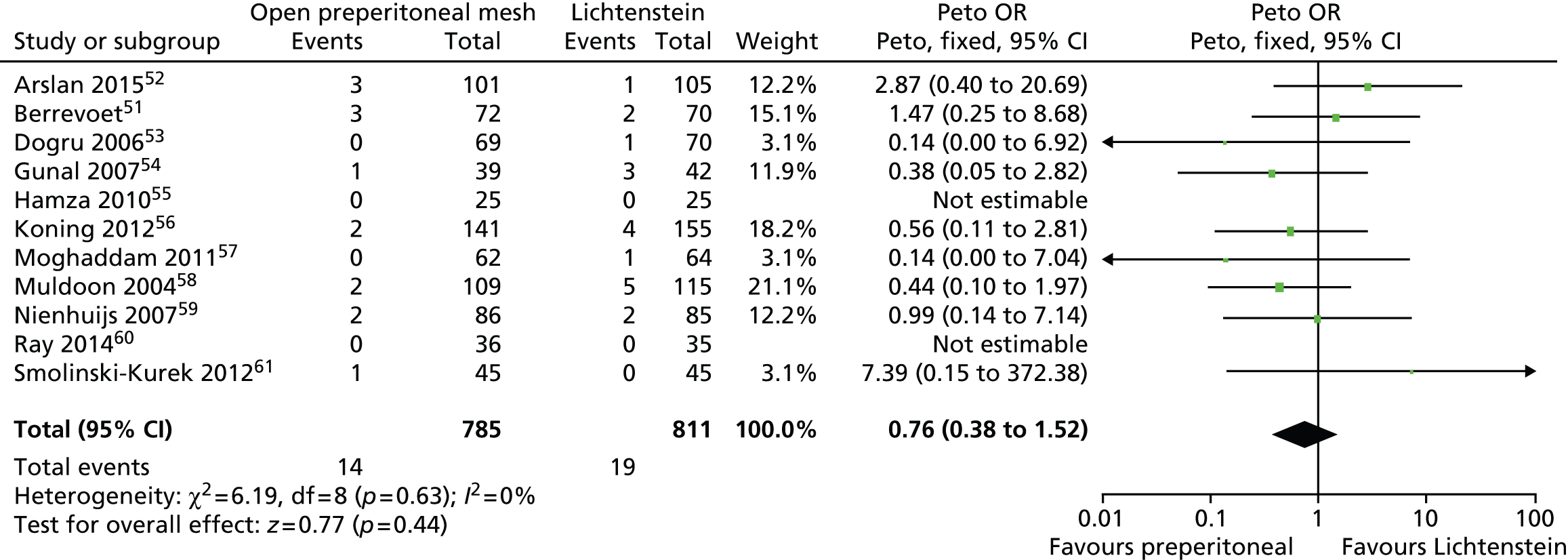
Information on recurrence or reoperation was available in 11 trials. 51–61 Rates of hernia recurrence were generally low. Overall, there were 14 recurrences among participants who underwent open preperitoneal mesh repair and 19 among those who underwent Lichtenstein mesh repair. There was no clear evidence of a difference between the two surgical procedures (Peto OR 0.76, 95% CI 0.38 to 1.52) (Figure 12).
FIGURE 12.
Open preperitoneal mesh repair vs. Lichtenstein mesh repair: rate of recurrence/reoperation. df, degrees of freedom.
Length of hospital stay
Owing to the observed heterogeneity between trial data, a statistical summary of length of hospital stay was deemed inappropriate. Some studies reported the mean number of days52,56,57,60 and others the proportion of patients with an overnight stay. 55,56 Appendix 9, Table 43 highlights the duration of hospital stay after surgery reported by five of the included trials. In each trial, no statistically significant differences were reported between intervention groups. Mean days of hospital stay ranged from 0.34 to 4.6 days in the preperitoneal groups and from 0.37 to 4.7 days in the Lichtenstein groups.
Time to return to normal activities
Five trials42,52,55–57 reported the time to return to work or to usual activities. There was considerable variation in the definition of the activities assessed but all of them included the word ‘work’ (see Appendix 9, Table 44). The SD values were not reported in one study;57 therefore, an average of the measures used in the other studies was imputed for the purposes of the meta-analysis.
Figure 13 shows the result of the random-effects meta-analysis. Overall, there was evidence that participants who underwent open preperitoneal mesh repair returned to normal activities around 1.5 days earlier than those who underwent Lichtenstein mesh repair (mean difference –1.49 days, 95% CI –2.78 to –0.20 days). There was evidence of statistical heterogeneity (I2 = 83%).
FIGURE 13.
Open preperitoneal mesh repair vs. Lichtenstein mesh repair: time to return to normal activities. df, degrees of freedom; IV, instrumental variable.
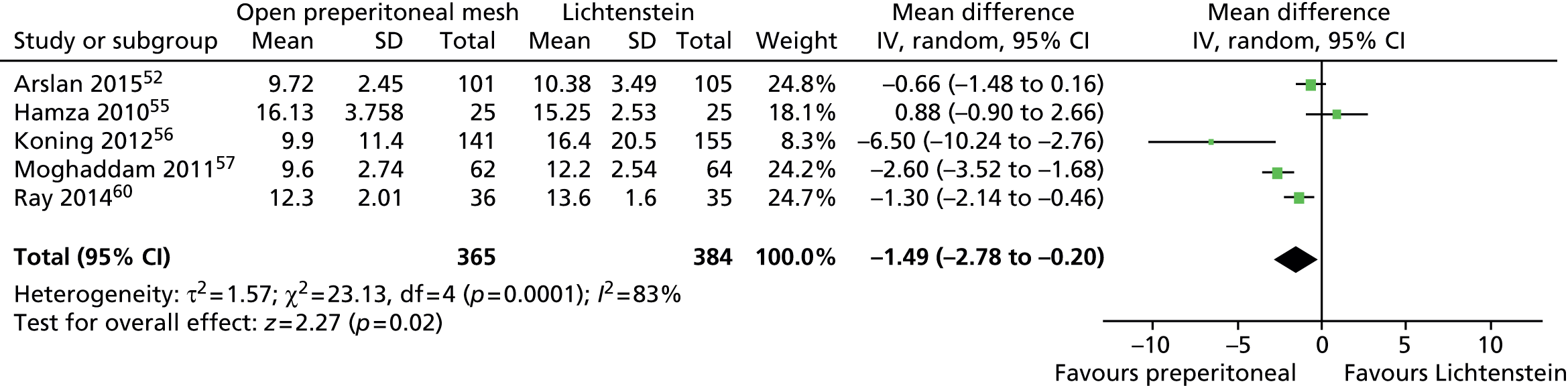
Summary of clinical effectiveness
The evidence base for this effectiveness review derives from 12 RCTs assessing open preperitoneal mesh repair versus Lichtenstein mesh repair for primary inguinal hernias. 42,51–63 Overall, the trials were at high or unclear risk of bias. The trials were generally small with a mean sample size of 130.7 participants (range 45–302 participants). Length of follow-up ranged from 1 week to 110 months, but the majority of trials were of relatively short duration (median 12 months). Trials included both low-risk (Nyhus I–III) and high-risk participants (Nyhus III and IV).
Eleven trials with a total of 1523 participants provided suitable data for further statistical analyses. Although formal meta-analyses were deemed appropriate for most outcomes, in most cases there was evidence of statistical heterogeneity between studies. Potential sources of clinical heterogeneity may include the definition and measurements of pain, length of follow-up, characteristics of the hernia defect, type of mesh and surgeon’s expertise.
There was evidence that patients who underwent open preperitoneal mesh repair returned to work or usual activities around 1.5 days earlier than those who underwent Lichtenstein mesh repair. Although, in general, patients randomised to open preperitoneal mesh repair showed lower incidence of pain and numbness, fewer recurrences and fewer complications than those randomised to Lichtenstein mesh repair, CIs for treatment effects were wide and most results were not statistically significant at the conventional 5% level.
Chapter 4 Assessment of cost-effectiveness
Review of cost-effectiveness studies
We conducted a systematic literature review to identify studies that reported an economic evaluation of open preperitoneal mesh repair in comparison with Lichtenstein mesh repair. The objective of the review was to summarise the economic evidence base and identify potential gaps in the literature.
Methods for review of cost-effectiveness studies
Search strategy
A review of existing economic evaluations assessing open preperitoneal mesh repairs versus standard Lichtenstein mesh repair was performed following similar objectives, clinical inclusion and exclusion criteria, and data extraction methods to those detailed in the clinical effectiveness review (see Chapter 3, Methods for assessing the outcomes arising from the use of the intervention). Comprehensive search strategies were designed to identify economic evaluations of inguinal hernia repair (see Appendix 2). Final searches were undertaken on 31 October and 1 November 2014. The following databases were searched: NHS Economic Evaluations Database (inception to 1 November 2014), HTA Database (inception to 1 November 2014), MEDLINE (1946 to October week 4 2014), MEDLINE In-Process & Other Non-Indexed Citations (31 October 2014), EMBASE (1947 to 2014, week 44) and Research Papers in Economics (inception to 1 November 2014). Websites of HTA organisations were consulted for additional reports. Reference lists of all included studies were scanned and appropriate experts were contacted for details of additional reports of cost-effectiveness.
Separate searches were undertaken on 31 October or 3 November 2014 to identify QoL studies (see Appendix 2). Databases searched were included MEDLINE (1946 to October, week 4 2014), MEDLINE In Process & Other Non-Index Citations (31 October 2014), EMBASE (1947 to 2014, week 44), Science Citation Index (1995 to 1993 November 2014) and the Cost-effectiveness Analysis Registry (3 November 2014).
A health economist (DB) screened the title and abstract of all citations identified by the search strategies for economic evaluations for inclusion. Full-text papers of potentially relevant studies were retrieved and formally assessed for inclusion. Any uncertainty regarding studies selection was discussed with a second health economist (RH) and the review team clinical expert (IA).
Inclusion and exclusion criteria
Only studies reporting a comparison between open preperitoneal mesh repair and Lichtenstein mesh repair were included. The criteria for studies inclusion were similar to those of the review of clinical effectiveness. In particular, studies had to (1) focus on people undergoing elective surgical repair for primary unilateral inguinal hernia and (2) present results in the form of cost minimisation analysis, cost-effectiveness analysis, cost–utility analysis or cost–benefit analysis. Formally, cost comparison studies were not included in the review but were retained as potentially useful information for populating resource use and costs in the economic model.
Economic evaluations in the form of both decision models and trial-based analyses were considered suitable for inclusion. Papers that reported decision models and/or utility values for hernia patients, but that did not meet the prespecified inclusion criteria were retained for informing the development of the de novo model structure and parameters.
Comparisons of different methods of open preperitoneal mesh repair (e.g. Kugel vs. TIPP), plug and patch repair, and non-mesh techniques were outside the scope of this assessment and were therefore excluded. Furthermore, studies that focused on a very specific technical aspect of surgical repairs, such as studies comparing the use of different types of mesh, methods of sealant and methods of anaesthesia, were not deemed suitable for inclusion.
Data extraction
Data were extracted from included studies using a prespecified data extraction form. A copy of the data extraction form is presented in Appendix 10. Where possible, data extracted included:
-
background information, such as research question, study design, intervention and comparator details
-
costing methodology, in particular the perspective, year, currency and the discount rate applied
-
characteristics of the study population (e.g. age, hernia type, setting, inclusion criteria, exclusion criteria)
-
methodology with a focus on statistical/regression methods for the analysis of costs, effectiveness and uncertainty
-
mean costs and outcomes, incremental costs and outcomes for differences between groups and incremental cost-effectiveness ratios (ICERs)
-
data on uncertainty, such as results of bootstrapped data and 95% CIs
-
study strengths and limitations as reported by the study authors
-
conclusions and suggestions for further research as reported by the study authors.
Quality assessment of included studies
Studies that reported economic evaluations alongside RCTs were quality assessed against the British Medical Journal checklist for referees of economic evaluations. 65 Where possible, results were assessed from the UK NHS perspective. However, as no decision modelling studies matching our inclusion criteria were identified, no studies were appraised against the NICE reference case. 49,63
Results of review of cost-effectiveness
Results of the cost-effectiveness searches
Our literature searches identified only one study assessing the cost-effectiveness of open preperitoneal mesh repair compared with Lichtenstein mesh repair that met our inclusion criteria. 66,67 We did not identify any decision modelling studies in the current literature. Two further Markov economic models13,36 that failed to meet our inclusion criteria were used to inform the development of the economic model structure.
Koning and colleagues,66,67 provide the only available evidence on the cost-effectiveness of open preperitoneal mesh repair versus Lichtenstein mesh repair for primary inguinal hernia. The study conducted a cost minimisation analysis alongside a RCT comparing the TIPP technique (randomised n = 143) with the Lichtenstein mesh repair (randomised n = 159) with 1-year follow-up. Costs were measured separately from a Dutch hospital and societal perspective. From a health services perspective, there was no significant difference in costs between TIPP and Lichtenstein mesh repair (mean difference –€13, 95% CI –€128 to €101). When a wider perspective of costs was adopted, cost savings in the TIPP group were statistically significant at the 5% level. From a societal perspective, TIPP was €1472 less costly than Lichtenstein mesh repair (95% CI –€2620 to –€325), indicating that incremental costs were heavily influenced by productivity gains generated by earlier return to work for non-retirees in the open TIPP repair group. The societal perspective analysis forms the main part of the authors’ conclusions.
Within the Koning and colleagues study,66,67 data were available on quality-adjusted life-weeks at 1-year follow-up and utilities were measured using the SF-36, converted to the Short Form questionnaire-6 Dimensions (SF-6D) measure of QoL, using published algorithms. 68 The authors found no difference in utility, based on the results from the SF-36. Mean quality-adjusted life-weeks difference for TIPP versus Lichtenstein mesh repair was 0.00983 (95% CI –1.01250 to 1.03217). They justified their decision not to conduct a cost–utility analysis on the basis of no difference in QoL between randomised trial arms.
If the data presented for QoL were combined with the reported cost data, TIPP would, on average, be less costly and more effective than Lichtenstein mesh repair and would thus be the most efficient, dominant treatment strategy. However, such results would be subject to a high degree of uncertainty. Furthermore, detailed sensitivity analyses and an extrapolation of results over a longer time horizon would be required before sound recommendations could be made on cost-effectiveness.
Quality assessment of the evidence
Quality assessment of the study by Koning and colleagues66,67 was performed according to the British Medical Journal guidelines for reviewers of economic evaluations. The study question was clear with justification for the approach used.
Quality of life measured over a period of only 1 year may be considered of insufficient duration to fully capture the impact, especially for those patients whose pain persists over time or who may suffer from longer-term recurrences. Responses to the SF-36 questionnaire were converted to the SF-6D preference-based measure of QoL and further adapted to derive utilities. 68 Although the SF-6D is a generic choice-based measure of QoL and is an accepted approach, it is not directly recommended for decision-making in a UK context, where the European Quality of Life-5 Dimensions-3 Level (EQ-5D-3L) is the preferred QoL instrument. 13,49 Furthermore, results are presented mainly from a societal perspective and, as such, are not directly transferable to a UK decision-making context where a health services perspective is normally adopted.
In general, the reporting of methods for the economic evaluation lacked details on analysis models used to estimate incremental costs and outcomes. There was little exploration of uncertainty in the study results, with the exception of some simple bootstrap analysis of incremental costs. The generalisability of results to inform UK policy-making regarding cost-effectiveness is likely to be limited because of the short time horizon and the differences between the methods used in the study and those typically used in a UK decision-making process. Nonetheless, the results of this study are promising for the potential cost savings, without any decrement in QoL associated with TIPP repair. In particular, earlier return to work and normal activities would be preferred from a patient and societal perspective if it could be achieved, especially if this also generated NHS cost savings in the longer term. Further research is needed to synthesise all the relevant outcomes of open preperitoneal mesh repair versus Lichtenstein mesh repair techniques for primary inguinal hernia. For this reason, we developed a de novo decision analysis model.
Economic analysis
Introduction
Owing to a paucity of evidence from the review of the literature, we developed a de novo economic model to assess the relative efficiency of open preperitoneal mesh repair versus Lichtenstein mesh repair for the treatment of primary inguinal hernia from a UK health services perspective. All methods of Lichtenstein mesh repair were considered equivalent for the purpose of the model. Similarly, all methods of open preperitoneal mesh repair (e.g. Kugel, TIPP) were assumed to be equivalent. The decision to group all open preperitoneal methods together was justified by the lack of available data from the systematic review of clinical evidence to populate a model based on specific repair techniques.
Methods
A probabilistic Markov model was developed by TreeAge ProTM 2014 software (TreeAge Software, Inc., Williamstown, MA, USA) to estimate expected values for costs from a NHS perspective and outcomes measured in terms of quality-adjusted life-years (QALYs) over a 25-year time horizon. The model was used to assess the relative efficiency of open preperitoneal mesh repair versus Lichtenstein mesh repair. Results were obtained with a Monte Carlo (probabilistic or second order) simulation of the developed Markov model with 100,000 iterations. The model parameters were drawn from appropriate distributions attached to baseline data, relative effect sizes, costs and utilities. Baseline results were presented as mean costs and QALYs from the iterations, and the simulation was used to present uncertainty in modelled outcomes.
Description of the model/model structure
Two key studies13,36 identified from the literature review of cost-effectiveness were consulted to assist with the development of the model structure. McCormack and colleagues13 assessed laparoscopic versus open repairs and found that recurrences and chronic pain were important drivers of cost-effectiveness. The base-case model file from this study was obtained from the authors. Achelrod and Stargardt36 developed a Markov cohort model with only a 1-year time horizon to compare heavy mesh techniques versus lightweight mesh techniques for the repair of inguinal hernia. The authors identified chronic pain as the most relevant factor contributing to cost-effectiveness and developed their model structure based exclusively on the development of chronic pain, for which an aggressive treatment protocol was used. We developed treatment pathways and appropriate health states after examining the above literature and after consultation with the members of the advisory group for this assessment, which consisted of hernia specialist surgeons, patient representatives and a general practitioner (GP). Revisions to the model structure were made according to the feedback received from the members of the advisory group.
Given the chronic nature of inguinal hernia and the potential for the development of chronic problems after surgery, a Markov state-transition model is regarded as the most appropriate method to estimate longer-term costs and health outcomes of the two index surgical procedures. The structure of a Markov model allows patients to move between defined mutually exclusive health states in a controlled manner over specified time periods. Patients after repair of primary inguinal hernia may enter one of the following mutually exclusive health states: (1) to recover from surgery with no further short-term consequences; (2) to develop short-run complications (within the first 3 months); (3) to develop chronic pain or numbness; (4) to have a recurrence; or (5) to die of natural causes. Transitions are allowed to occur over 5 years, where the best available data exist. Beyond this point, for the duration of the 25-year model time horizon, participants are assumed to be either well or to die of natural causes.
For ease of presentation, the model structure is presented in two diagrams. Figure 14 illustrates the transitions within the first model cycle and Figure 15 presents transitions for all remaining cycles of the model.
FIGURE 14.
Diagram of model structure (first 3-month model cycle).
FIGURE 15.
Diagram of model structure (longer-term health states). Note that the dotted arrows refer to tunnel states where patients may spend more than one cycle; for example, recovering from a recurrence or a late complication.

All patients enter the model at the ‘initial surgery’ health state, having a primary inguinal hernia repair (either open preperitoneal mesh repair or Lichtenstein mesh repair). Within the first 3-month model cycle, patients may recover (entering the ‘well’ health state), develop postoperative pain or numbness, incur other early complications (such as wound haematoma, seroma or urinary problems) or experience an early recurrence.
Transitions between states for the remainder of the model are dependent, in part, on a patient’s health state in the first cycle of the model. Early complications are assumed to be resolved within the cycle in which they occur. All patients having a recurrence in the first cycle (see Figure 14) enter the recurrence health state in Figure 15, where they join a separate model process. Patients with unresolved pain or numbness following the first model cycle progress to the chronic pain or the chronic numbness health states, respectively. All patients may recover, die, develop a recurrence or experience late complications at any point throughout the model. Patients may enter the death state because of operative mortality from initial surgery or because of all-cause mortality from any state in the model.
The next two sections present further details on the structural assumptions underpinning the economic model.
Pain and numbness
Neuropathic pain, which usually develops in the early stages post surgery, may progress over time and develop into chronic pain. 36 For the purposes of this model, we used the IASP definition of chronic pain of 3 months duration. 69 It is unlikely that chronic pain would develop in the absence of early postsurgery pain. 16 We, therefore, assumed that patients would not develop chronic pain in the model unless they suffered from pain in the first 3-month cycle. This is an approach adopted in other economic evaluations for hernia repair. 36,70 The implication of this assumption is that the proportion of the model patient cohort with chronic pain will always reduce over time. The members of the advisory group for this assessment, who suggested that a similar approach should be applied to the modelling of chronic numbness, have considered this assumption plausible. For each additional model cycle, a proportion of patients with chronic pain or numbness will have their symptoms resolved through treatment or they may continue to experience problems for another model cycle, up to a maximum of 5 years following surgery. Patients accrue further treatment costs and utility decrements for every additional cycle they spend in a chronic pain or chronic numbness health state. Linear interpolation is used for the proportion of patients progressing through pain or numbness between time points at which data are available.
Longer-term complications and recurrences
After progressing through the first model cycle of 3 months, with or without problems, patients may develop further ‘late’ complications or recurrences at any point up to 5 years after surgery. Late complications and recurrences are treated once they become symptomatic. There is no evidence that complications or recurrence over a longer period of time are directly related to outcomes in the first 3 months. Once a patient has developed complications, they are assumed to recover with treatment within one cycle. They then proceed to recover (‘well’ health state), die, develop a recurrence or experience further new complications in the future.
It is assumed that all recurrences will require further surgical interventions, after which a patient could recover or develop a second recurrence. Recurrence health states are assumed to last over two cycles (i.e. for 6 months). This is to allow adequate time for diagnosis of the recurrence, inclusion in a waiting list and time for convalescence post recurrence. Patients who survive and recover from a recurrence are assumed to be well, but have a probability of developing a second recurrence. The model care pathway for the second recurrence is similar to that of the first. A simplifying assumption that patients will not progress to develop pain, numbness or other complications post recurrence is based on the following considerations: (1) the probability of a recurrence is very low and hence further modelling beyond this point is unlikely to contribute significantly to overall costs or QALYs; (2) any problem post recurrence is likely to be a consequence of the surgical procedure used to repair the recurrence rather than a consequence of the index primary procedure; and (3) there is no robust evidence to inform RRs of such events. An alternative model structure where patients experience further problems, including pain, after a recurrence is explored by means of a sensitivity analysis.
Owing to limited longer-term data, no hernia-related problems are modelled after 5 years following surgery. Patients enter a stable phase where they remain well for the duration of the model, with a general population-based risk of all-cause mortality.
Model parameters
Baseline probabilities
In order to estimate reliable absolute differences in costs and outcomes between treatment strategies, it is important to ensure that relative effect sizes from the meta-analyses are applied to appropriate baseline data. Two sources of baseline data were used. Data on proportions of the modelled patient cohort with pain and numbness at 3 months, 1 year and 5 years were sourced from the Lichtenstein mesh repair arms of studies included in the systematic review of clinical evidence (see Chapter 3). For complications and recurrences, transition probabilities were sourced from the Lichtenstein mesh repair arm of studies included in the systematic review conducted by the AHRQ. 15
Pain and numbness
The decision to use baseline data for pain and numbness from selected studies within the systematic review of clinical evidence was motivated by a number of inter-related factors. First, there was significant variation in the way pain (different rating scales, pain affecting daily activities, pain requiring medication) and numbness (subjective, objective pinprick test) was measured across the hernia repair literature. If we chose to use the AHRQ systematic review evidence, combining studies using different measures, there would have been an increased risk of over- or underestimating the true baseline parameters, and hence, the absolute treatment effect, in the model. The second reason was that no single individual study measured chronic pain or numbness at all the time points of interest (3 months, 1 year and 5 years). Third, using the AHRQ review data may have compromised the face validity of the estimates of pain or numbness progression over time. According to expert opinion, to reflect clinical practice, the proportion of patients with chronic pain or numbness in the model should be reducing over time, as patients are usually treated and improve. The AHRQ review15 indicates that the proportion of patients with chronic numbness, for instance, is higher at 1 year than at 3 months. Furthermore, the proportion of patients with chronic pain at 5 years is no different from that at 1 year, which could be suggestive of no successful treatment. However, the variation in the definition of chronic pain and numbness should be taken into account. Using these data would result in a lack of face validity of the estimates obtained in the decision model.
We relied on studies included in our review of clinical effectiveness that shared more consistent outcome definitions and measurements. In particular, we used data from Berrevoet (ongoing trial)51 and Nienhuijs and colleagues59 as baseline for pain at 3 months, from Koning and colleagues42,56 for pain at 1 year and from Muldoon and colleagues58 for pain at 5 years (see Table 8). All these studies appeared to be reasonably comparable; they all measured chronic pain using the 10-point VAS scale and provided valid estimates over time. For the assessment of numbness, we used data from Koning and colleagues42,56 at 1 year and Muldoon and colleagues58 at 5 years. Data were also available from the study by Nienhuijs and colleagues59 that included subjective assessments at 3 months. However, the proportion of patients complaining of numbness at 3 months was substantially lower than that measured in the other studies. This is not surprising given the limited impact of numbness on patients’ QoL. The self-reported data on numbness could not be incorporated into the model because over time they were likely to generate a numbness profile that was incompatible with our clinical assumptions (i.e. incidence of numbness at 3 months would be lower than at 1 or 5 years after surgery). Thus, there was no appropriate data to determine the baseline proportion of the model patient cohort suffering from chronic numbness at 3 months after hernia repair. In agreement with the opinion of our advisory group clinical experts, we assumed that the proportion of patients with numbness at 3 months was equal to that reported in the study by Koning and colleagues42,56 at 1 year (Table 8). This represents a conservative, but clinically plausible, assumption at 3 months also taking into account the data available at later time points. Although there is a certain degree of uncertainty, this approach avoids overestimation of results.
| Variable | Point estimate | Alpha | Beta | Distribution applied | Source |
|---|---|---|---|---|---|
| P (pain 3 months) | 0.2933 | 44 | 106 | Beta | Berrevoet51 Nienhuijs et al. 200759 |
| P (pain 1 year) | 0.1290 | 20 | 135 | Beta | Koning et al. 201256 |
| P (pain 5 years) | 0.0609 | 7 | 108 | Beta | Muldoon et al. 200458 |
| P (numbness 3 months) | 0.5097 | 79 | 76 | Beta | Koning 2013 et al. (assume as 1 year)42,56 |
| P (numbness 1 year) | 0.5097 | 79 | 76 | Beta | Koning et al. 201256 |
| P (numbness 5 years) | 0.0957 | 11 | 104 | Beta | Muldoon et al. 200458 |
| P (recurrence 3 months) | 0.0060 | 6 | 991 | Beta | AHRQ review15 |
| P (recurrence 1 year)a | 0.0090 | 28 | 3083 | Beta | AHRQ review15 |
| P (recurrence 5 years)a | 0.0326 | 73 | 2167 | Beta | AHRQ review15 |
| P (complications 3 months) | 0.1620 | 716 | 3704 | Beta | AHRQ review15 |
| P (complications 1 year)a | 0.0999 | 201 | 1812 | Beta | AHRQ review15 |
| P (complications 5 years)a | 0.0634 | 97 | 1433 | Beta | AHRQ review15 |
| P (all-cause mortality)a | Age dependent | – | – | – | Constant age adjusted71 |
| P (operative mortality) | 0.0010 | – | – | – | Constant13 |
Complications and recurrences
Baseline data for the model were sourced from the AHRQ review15 by summing together the recurrences and complications from the Lichtenstein mesh repair arms of all included RCTs reporting data at 3 months, 1 year and 5 years. It was assumed that studies reporting beyond 5 years presented a reasonable proxy for 5-year data. These data allowed us to identify baseline probabilities of rare events. As a validity check, the baseline probabilities were compared with the data derived from our review of clinical evidence and found to be similar.
Mortality rates were sex and age specific, and based on national life tables for England and Wales. 71 An additional operative mortality risk of 0.1% was applied to all surgical procedures. 13
Table 8 reports baseline probability data incorporated within the model. Variability around these estimates was incorporated into the model by sampling from beta distributions, where the alpha parameter is the number of events of interest (e.g. chronic pain) in the Lichtenstein mesh repair arm of the relevant studies and the beta parameter is given as the total number of patients minus total number of patients with an event of interest.
Relative effect sizes
Absolute parameter values for open preperitoneal mesh repair were calculated by applying relative effect sizes (for open preperitoneal mesh repair vs. Lichtenstein mesh repair) to baseline probabilities of pain, numbness, complications and recurrences. All relative effect sizes are incorporated into the model as point estimates of RRs with 95% CIs, estimated using the Mantel–Haenszel random-effects method. There was insufficient information available from our review of clinical evidence to develop a RR of the majority of individual outcomes for all the time points of interest. We therefore assumed that the RR remained constant at all time points up to 5 years following surgery. However, data were available to split into early (up to 3 months) and late (beyond 3 months) complications (Table 9). Data for 3-month complications were used to construct log-normal distributions around the estimates. However, for late complications we simply used the point estimate of the RR of 1.1 from the meta-analysis, without sampling from the respective probability distribution. The assumption of a constant RR for late complications was made because of the rarity of the events, the high level of uncertainty (RR 1.1, 95% CI 0.08 to 14.79 for late complications) and the unlikely impact on cost-effectiveness results. In the absence of any data to suggest otherwise, the RR of having a second recurrence was assumed to be the same as having a first recurrence.
| Parameter | Mean | Lower value | Upper value | Distribution applied | Mean of logs | SE of logs | Notes/sources |
|---|---|---|---|---|---|---|---|
| RR chronic pain | 0.50 | 0.20 | 1.27 | Log-normal | –0.6931 | 0.4715 | Meta-analysis |
| RR chronic numbness | 0.48 | 0.15 | 1.56 | Log-normal | –0.734 | 0.5974 | Meta-analysis |
| RR early complications | 0.70 | 0.41 | 1.19 | Log-normal | –0.3567 | 0.2718 | Meta-analysis |
| RR late complications | 1.1 | – | – | – | – | – | Meta-analysisa |
| RR recurrence | 0.78 | 0.39 | 1.57 | Log-normal | –0.2485 | 0.3553 | Meta-analysis |
| RR second recurrence | 0.78 | 0.39 | 1.57 | Log-normal | –0.2485 | 0.3553 | Assumed equal to first recurrence |
| RR all-cause mortality | 1 | – | – | – | – | – | Assume constant |
| RR operative mortality | 1 | – | – | – | – | – | Assume constant |
Effect sizes for rare events are reported in Chapter 3 using Peto ORs. However, in order to apply risk estimates to baseline probabilities, the economic model uses RR estimates. The decision to use RRs, rather than Peto OR is unlikely to impact on cost-effectiveness outcomes because of the low incidence of recurrences and late complications.
We have assumed no difference in mortality risk between open preperitoneal mesh repair and Lichtenstein mesh repair strategies.
Table 9 details point estimates and 95% CIs of relative effect sizes used in the model. Uncertainty surrounding the point estimates was characterised using log-normal distributions. Data used to define the distributions are presented as mean and SEs of the log estimates.
Utilities and quality-adjusted life-years
Utility values for surgery (0.68), well (0.956), pain (0.836) and numbness (0.919) health states were sourced from data published in the UK Medical Research Council (MRC) hernia study,72 based on responses to the EQ-5D-3L and valued using UK general population tariffs estimated using the time trade-off technique. 73 No published data were available for the remaining health states, namely complications (early or late) or recurrences. Based on discussion with our clinical experts and on previous modelling conducted by McCormack and colleagues,13 for the base-case analysis we have applied the same utility weights to complication and recurrence health states and to chronic pain (0.836). For recurrences, this is likely to reflect the impact of suffering a recurrence event on QoL, where a patient might be expected to be in significant pain or discomfort. Utility weights for second recurrences were assumed equal to those of a first recurrence. For complications, this assumption may represent an overestimation of the utility decrement for minor complications. We therefore explore the use of a utility decrement for a milder health state (numbness) and apply this decrement to all complications as a sensitivity analysis.
Utility data applied to model health states were sourced from the MRC study. 72 Data for all individuals (both those receiving open and laparoscopic repair) in a health state (e.g. experiencing chronic pain) were used to inform the utility calculation. This could be argued to be a questionable approach given our research question focuses on different methods of open hernia repair. However, patients for whom utility data were available by trial arm for individual health states in the MRC study72 were sparse. Pooling the data across arms allowed for a larger sample and generated utility estimates with greater face validity for individual health states. It is unlikely that the utility of individual health states would be dependent on the index surgery; therefore, we have assumed that the utility estimates from the pooled data are applicable to the current model.
There were not enough data to stratify health states owing to severity of condition. All chronic pain patients receive the same utility, regardless of severity. The MRC study provides the best available utility data, based on EQ-5D-3L. The data and assumptions outlined above have been successfully used in previous cost–utility analyses and in decision modelling studies of hernia repair. 13,36 Nonetheless, alternative sources of utility data were available, and despite limitations, their incorporation into the economic model was explored by means of sensitivity analyses.
Table 10 reports the point estimate base-case utility, together with the attached beta distributions (and corresponding parameters). Utility weights reported below were further adjusted by the UK population norms for males and are age adjusted using published weights74 following recommended best practice. 75 Half-cycle corrections were applied to all utilities in the model and QALYs accruing into the future were discounted at 3.5% per annum in line with current NICE guidance. 49
| Utility values | Mean value | SE | Distribution | Alpha | Beta | Notes/sources13,72 |
|---|---|---|---|---|---|---|
| Initial surgery | 0.680 | 0.240 | Beta | 1.889 | 0.889 | MRC study72 (1 week, post operation) |
| Convalescence | 0.860 | 0.200 | Beta | 1.729 | 0.281 | MRC study72 (3 months post operation) |
| Pain | 0.836 | 0.021 | Beta | 259.071 | 50.823 | MRC study72 (long-term pain) |
| Numbness | 0.919 | 0.023 | Beta | 128.399 | 11.317 | MRC study72 (long-term numbness) |
| Recurrence | 0.836 | 0.021 | Beta | 259.071 | 50.823 | Assumed equal to long-term pain |
| Complications | 0.836 | 0.021 | Beta | 259.071 | 50.823 | Assumed equal to long-term pain |
| Well | 0.952 | 0.011 | Beta | 358.574 | 18.079 | MRC study72 |
Resource use and costs
Costs were calculated from the perspective of the NHS and reported in 2013/2014 UK pound sterling. Resource-use data were estimated using a combination of published literature and clinical expert opinion. Unit costs were taken from NHS reference costs for secondary care,76 the Personal and Social Services Research Unit77 unit costs for primary care and the British National Formulary for medication costs. 78 The main components of total cost in the economic model were the costs of surgery and the cost of treating chronic pain. All resource-use assumptions and costing assumptions were confirmed as clinically relevant in a UK setting by the clinical experts of our advisory group.
Cost of surgery
Costs of surgery have been calculated in the following way: activity level (finished consultant episodes) in all relevant subcodes (G, H, J and K) of HRG code FZ18 have been summed together and weighted by activity in each subgroup. The subgroups include those HRGs with complications and comorbidities with different levels of severity. Additionally, the data were based on completed episodes of care and were weighted by day-case/inpatient elective procedure. Non-elective procedures were beyond the scope of this assessment and, therefore, not included. It was assumed that activity was always based in a general surgery setting. All relevant open repairs for inguinal hernia, including the Lichtenstein approach and the open preperitoneal techniques, map to the same HRG base code and thus receive the same unit cost calculated using the weighted approach outlined above (£1640.29 per case). Full details of the calculation of surgical cost are presented in Appendix 1.
The decision to assign the same unit cost to both surgeries was confirmed by the clinical experts of our advisory group. This decision was based on the likely similarity of equipment and staff requirements, theatre time and time to discharge from hospital for both surgical procedures. Furthermore, data from the meta-analysis of hospital length of stay were supportive of equality in hospitalisation times across groups, mean difference in length of stay for open preperitoneal mesh repair versus Lichtenstein mesh repair was –0.03 (95% CI –0.18 to 0.12). Alternative costs of surgery were explored in sensitivity analysis.
Costs of treating hernia related problems
The number of required consultation visits with clinicians, GPs, outpatient clinics and pain management clinics was based on the available literature and clinical expert opinion. Costs of an outpatient procedure were calculated based on the weighted average of consultant-led and non-consultant-led outpatient consultations for general surgery from Hospital Episode Statistics (HES) data. 1 Unit costs were applied using national average reference costs. 76 GP visit costs were based on Personal Social Service Research Unit cost data for a GP consultation lasting 11.7 minutes. 77 Costs of professional consultations and treatment were summed for all health states and converted to per-cycle costs for use in the economic model. Full details of all resources for chronic pain treatment, recurrences and complications are summarised in Tables 11 and 12 and unit costs are presented in Table 13.
| Component of treatment | Model year (i.e. time after surgery) | Frequency/duration of treatment | Proportion of patients with chronic pain having this treatment | Notes/sources |
|---|---|---|---|---|
| Contact with health professionals | ||||
| GP visits | Years 1–5 | Monthly | 100.00% | Assumption |
| Outpatient consultation | Years 1–5 | Two per year | 100.00% | Assumption |
| Pain clinic (multidiscipline) | Year 1 | None | – | Assumption |
| Years 2–5 | Four per year | 10.00% | Assumption | |
| Proportions of chronic pain patients requiring active treatments | ||||
| Oral analgesics [e.g. ibuprofen (non-proprietary)] | Years 1–5 | Ongoing | 45.00% | Lau et al.79 (45% of chronic pain patients requiring any analgesia at 1 year, assumed applicable from 1–5 years also) |
| Percentage of total chronic pain patients requiring further treatment | – | – | 32.50% | Based on Aroori and Spence.80 13/18 chronic pain patients did not respond to oral analgesia and required further treatment |
| Details of active treatmentsa | ||||
| Amitriptyline (Triptafen®, AMCo) 50 mg per day (57.1%) | Year 1 | None | – | Assumption |
| Year 2 | 4.5 monthsb | 9.28% | Calculation from Achelrod and Stargardt36 | |
| Year 3 | 4.5 months | 9.28% | Calculation from: Achelrod and Stargardt36 | |
| Years 4 and 5 | None | – | Assumption | |
| Injection of levobupivacaine (Chirocaine®, AbbVie) and methylprednisolone (Depo-Medrone®, Pharmacia) (11.9%) | Year 1 | None | – | – |
| Year 2 | Once | 1.93% | Calculation from Achelrod and Stargardt36 | |
| Year 3 | Once | 1.93% | Calculation from Achelrod and Stargardt36 | |
| Years 4 and 5 | None | – | – | |
| Injection of Chirocaine, Depo-Medrone and amitriptyline, 50 mg per day (28.6%) | Year 1 | None | – | – |
| Year 2 | One injection/4.5 months | 4.65% | Calculation from Achelrod and Stargardt36 | |
| Year 3 | One injection/4.5 months | 4.65% | Calculation from Achelrod and Stargardt36 | |
| Years 4 and 5 | None | – | – | |
| Return for chronic pain surgery (2.4%) | Year 1 | None | – | Assumption |
| Year 2 | Once only | 0.39% | Calculation from Achelrod and Stargardt36 | |
| Year 3 | Once only | 0.39% | Calculation from Achelrod and Stargardt36 | |
| Years 4 and 5 | None | – | Assumption | |
| Complication | Percentage of modelled complications | Treatment | Percentage receiving | Frequency | Source/notes |
|---|---|---|---|---|---|
| Early complications (occurring within 3 months of surgery) | |||||
| Wound infection | Systematic review (LR): 23% | GP visits | 100% | Weekly for 1 month | Assumption |
| Antibiotics | 100% | One course | Amoxicillin 500 mg | ||
| Outpatient | 50% | One visit | Assumption | ||
| Haematoma/seroma | Systematic review (LR): 37% | GP visits | 100% | Weekly for 1 month | Assumption |
| Antibiotics | 50% | One course | Amoxicillin 500 mg | ||
| Outpatient | 100% | One visit | Assumption | ||
| Urinary complicationsa | Systematic review (LR): 24% | GP visits | 100% | Weekly for 1 month | Assumption |
| Antibiotics | 100% | One course | Amoxicillin 500 mg | ||
| Outpatient | None | None | Assumption | ||
| Cord/scrotal oedema | Systematic review (LR): 16% | GP visits | 100% | Weekly for 1 month | Assumption |
| Antibiotics | 100% | One course | Amoxicillin 500 mg | ||
| Outpatient | None | None | Assumption | ||
| Late complications (beyond 3 months), weighted based on serious (such as testicular atrophy, mesh reaction, mesh infection) and minor (such as those outlined in early complications above) | |||||
| Complications similar to those identified in first 3 months | Calculation from AHRQ review15 97.3% of all late complications | – | – | – | Average of all early complications outlined above |
| – | – | – | |||
| – | – | – | |||
| Testicular atrophy/mesh infection/mesh reaction | Calculation from AHRQ review15: 2.7% of all late complications | GP visits | 100% | Once | Clinical expert opinion |
| Outpatients | 50% | Twice | Clinical expert opinion | ||
| Surgery | 50% | Once | Clinical expert opinion | ||
| Resource use applied to other health states | |||||
| Numbness | 100% of numbness | GP visit | 100% | Annually | Assumption |
| Recurrence | 100% of recurrences | GP visit | 100% | Three monthly for 1 year | Assumption |
| Outpatients | 100% | Twice | Koning et al.42 | ||
| Surgery | 100% | Once | Koning et al.42 | ||
| Cost element | Value (£) | Unit | Distribution | Values for distributiona | Source |
|---|---|---|---|---|---|
| Unit costs of surgical procedure | |||||
| Inpatient hernia procedure | 1716.52 | Per procedure | Gamma | Alpha: 17.4681 | NHS reference costs (2013/14)76 |
| Beta: 98.2661 | |||||
| Unit cost of health-care professionals | |||||
| Outpatient appointment | 115.84 | Per consultation | Gamma | Alpha: 11.206 | NHS reference costs (2013/14)76 |
| Beta: 10.3373 | |||||
| GP visit | 45.00 | Per consultation | – | Assume constant | PSSRU, 201477 |
| Unit costs for chronic pain treatments | |||||
| Amitriptyline, 50 mg | 4.95 | Per course of treatment (4.5 months) | – | Assume constant | Based on cost of £0.93 per 28-tablet pack, five packs required78 |
| Injection of Chirocaine and Depo-Medrone | 5.06 | Per treatment | – | Assume constant | 10-ml ampoule of Chirocaine plus 1-ml vial of Depo-medrone78 |
| Injection of Chirocaine and Depo-Medrone and amitriptyline, 50 mg per day | 10.01 | Per treatment | – | Assume constant | Sum of amitriptyline and injection78 |
| Pain management clinicb | 167.20 | First attendance | Gamma | Alpha: 5.7469 | NHS reference costs (2013/14)76 |
| Beta: 29.0942 | |||||
| 133.72 | Follow up | Gamma | Alpha: 4.184 | ||
| Beta: 31.9597 | |||||
| Pain surgeryc | 824 | Day-case complex pain procedure | Gamma | Alpha: 4.0598 | NHS reference costs (2013/14)76 |
| Beta: 202.9665 | |||||
| Oral analgesics (e.g. ibuprofen)d | 11.64 | Cost per cycle | – | Assume constant | BNF78 |
| Treatment for other complications | |||||
| Amoxicillin (non-proprietary) 500 mge | 1.54 | Per course | – | Assume constant | BNF78 |
| Surgery for testicular atrophy/mesh infection | 1716.52 | Per procedure | Gamma | Alpha: 17.4681 | Assume as recurrence surgery, NHS reference costs (2013/14)76 |
| Beta: 98.2661 | |||||
Cost of chronic pain treatment
Other than the cost of surgery, treatment of chronic pain is the main driver of resource use. Despite a lack of clear guidelines on the appropriate treatment approach for chronic pain following hernia repair,79 the consensus among the review team and the advisory group for this assessment appeared to favour an initial conservative treatment approach before moving to more aggressive drug therapy, nerve blocks and, in the most extreme cases, surgery. Chronic pain costs were calculated based on a number of conservative assumptions informed by clinical expert opinion and, where possible, UK data.
All patients with chronic pain were assumed to have fixed regular contact with health professionals (both outpatient and primary care) over 5 years following surgery. Frequency of contact was based on advice from our clinical experts and agreed among the advisory group for this assessment. All patients had contact with their GP and outpatient consultations. In addition, 10% of patients with chronic pain were treated in a multidisciplinary pain clinic, with one treatment per 3-month cycle. Treatment in a pain management clinic would only occur following the first year in chronic pain. As all patients were assumed to have regular contact with health professionals, no additional consultations were required to administer pain therapy.
Not all patients were assumed to receive identical active treatments. Of patients with chronic pain, 45% were assumed to require ongoing oral analgesia, such as ibuprofen, for the duration of their chronic pain. 80 Of those requiring simple oral analgesia, 72% (32.5% of all patients with chronic pain) were modelled to require further treatment81 including stronger pharmacotherapy with amitriptyline, intramuscular injections and nerve block, with only the most extreme cases requiring surgery. Treatment details were based on data reported in a UK study. 81
As an example of the calculation of the number of patients requiring different treatments, we assumed that if 100 patients present with chronic pain, 45 will take regular ibuprofen,80 33 of whom will require additional treatment. 81 Of those 33 requiring additional treatment, 18.84 will require amitriptyline (57.1%), 3.93 (11.9%) will require nerve block injections of Chirocaine and Depo-Medrone, 9.44 (28.6%) will require combination treatment but only 0.79 (2.4%) will require further surgery. 36 It was assumed that the costs of such treatment would occur beyond 1 year (given the conservative approach outlined), were split evenly between years 2 and 3, and patients would receive one treatment course only. This conservative approach departs from that adopted by Achelrod and Stargardt,36 where patients received a more aggressive treatment approach, which appeared to include repeat treatments in each model cycle for patients whose pain remained unresolved.
All costs were converted to per-cycle unit costs of treating chronic pain. The average cost of chronic pain treatment per 3-month cycle was estimated as £198.16, £213.43, £213.43, £211.53 and £211.53 for years 1–5, respectively. Per-cycle cost in years 4 and 5 reduced slightly, which reflects the exhaustion of the main treatment phase and the movement to a maintenance treatment phase for the majority of chronic pain patients. The majority of chronic pain costs were attributable to consultations with health professionals as opposed to drug costs, which are comparatively less expensive. Full details of all resource use for chronic pain treatment are summarised in Table 11.
Unit costs are presented in Table 13. We conducted sensitivity analyses to explore the use of no treatment and aggressive treatment approaches for managing chronic pain.
Costs of recurrences and complications (including numbness)
For early complications, data from the systematic review of clinical evidence (see Chapter 3) were used to distinguish between early complications as follows: wound infections, haematoma and seroma, urinary problems and oedema (each of which required slightly different treatments). It was assumed that patients with numbness would only require one GP visit per month because of a minimal impact on QoL. Based on details of complications reported within the AHRQ review,15 complications occurring beyond 3 months post surgery were classed into major (such as testicular atrophy or mesh infection, 2.7%) and minor (such as haematoma and seroma, similar to early complications outlined above, 97.3%).
Resource use for recurrence treatment was taken from the study by Koning and colleagues42 and was based on two outpatient consultation visits (one to diagnose the recurrence and one to follow-up the patient after surgery) and the operative procedure to correct the recurrence, which was assumed to be similar to the initial procedure in terms of costs to the health services. See Table 12 for details.
All resource-use data to estimate costs of complications, numbness and recurrence were informed and validated as reflective of UK clinical practice by both the clinical experts and the patient representatives of our advisory group (see Table 12).
No resource use or costs were included in the model for those patients who died from natural causes. Similarly, no costs were applied to the ‘well’ health state as the British hernia treatment guidelines6 state that no routine follow-up of hernia surgery is required, unless patients present with complications after surgery (e.g. pain, numbness).
Costs parameters used in the model were assumed to follow a gamma distribution. Unit costs, together with alpha and beta parameters (where appropriate), are presented in Table 13, in accordance with the specifications outlined by the TreeAge software. All costs used in the model were discounted by 3.5% per annum, in line with current best practice guidance. 49
Combining costs and outcomes: assessment of cost-effectiveness
The results of the base-case analysis are presented for men aged 58 years. This is based on the mean age for hernia repair across all classification of interventions and procedures codes (from the Office of Population Censuses and Surveys) for primary repair of inguinal hernia taken from the 2012/13 HES data1 projected over a 25-year time horizon. For the economic analysis, the different outcomes from the systematic review of clinical evidence and the modelling of costs, resource use and QALYs were combined into a single measure of relative efficiency, measured using the ICER. The ICER presents results in terms of incremental cost per QALY gained by adopting open preperitoneal mesh repair over Lichtenstein mesh repair. The base-case model results were calculated using a probabilistic analysis using second order Monte Carlo simulation with 100,000 repetitions. Results from the CEAs were based on mean estimates of baseline probabilities, relative effect sizes, utilities and costs drawn from the sampling distributions outlined in Tables 8–10 and 13, respectively. Expected values of total costs and QALYs were estimated based on sampled data and used to calculate incremental costs, incremental QALYs and ICERs for open preperitoneal mesh repair versus Lichtenstein mesh repair.
In terms of decision rules, the most common approach is to identify the intervention providing additional gain in outcomes (QALYs) and to use the ICER to identify the cost of that marginal gain in benefit. Traditionally, NICE deem interventions to be cost-effective if the cost of an additional QALY gain is less than £20,000. 49
Interventions that are less costly and generate greater QALYs than a comparator are the dominant treatment and offer an even stronger case for cost-effectiveness.
Results from the probabilistic analysis are presented using cost-effectiveness acceptability curves (CEACs), and scatterplots with results from the simulations are plotted on the cost-effectiveness plane. Scatterplots are useful to illustrate the uncertainty in the respective quadrants of the cost-effectiveness plane and are particularly useful in this analysis where the CEACs show a high probability of cost-effectiveness for one option over another. CEACs and scatterplots are both used to illustrate the uncertainty in cost-effectiveness outcomes caused by the combined statistical variability in the models parameter estimates. They show the likelihood of a treatment strategy for open preperitoneal mesh repair and Lichtenstein mesh repair being cost-effective at various threshold values of society’s willingness to pay (WTP) for a QALY gained. Further to the illustrations of cost-effectiveness, all results are also presented using numerical estimates of cost-effectiveness at various thresholds of WTP for a QALY gained (£0, £10,000, £20,000, £30,000 and £50,000) for the base case and for all sensitivity analyses undertaken.
Assessment of uncertainty (sensitivity analysis)
The sensitivity analyses focused on varying parameters and assumptions, which were potentially influential on the overall outcomes of costs, QALYs or cost-effectiveness. Sensitivity analyses were split into those focusing on baseline parameters, RR parameters, utilities, costs, methodological uncertainties and structural assumptions. For all analyses, results were presented as for the base-case analysis, varying one single parameter at a time (one-way sensitivity analyses). For inter-related parameters, such as chronic pain cost and RR of chronic pain, two-way sensitivity analyses were conducted. Threshold analyses were used to determine the difference in surgery costs that would be required in order to change cost-effectiveness conclusions.
Changes to baseline model parameters
For the base-case analysis, data on chronic pain and numbness were derived from the systematic review of clinical evidence, while data on recurrences and complications were derived from the AHRQ review. 15 Data were uncertain, particularly in relation to the progression of chronic pain over time. A number of sensitivity analyses were carried out (Table 14). The first explored the impact of using AHRQ data, while the second explored the impact of using data from our systematic review of clinical evidence (see Chapter 3) for all baseline data. The advantages and disadvantages of alternative sources have been addressed (see Model parameters, baseline probabilities). In all cases, data are sourced from the Lichtenstein mesh repair arms of the included RCTs.
| Key variable/health state | Base-case assumption/value | Alternative assumption/value | Justification for choice of analysis |
|---|---|---|---|
| All baseline probabilities | Our review for pain and numbness/AHRQ review for recurrence and complications15 | Our review for all parameters | The data included from the clinical effectiveness review provide the most consistent definitions and are most applicable to the research question |
| All baseline probabilities | Our review for pain and numbness/AHRQ review for recurrence and complications15 | AHRQ review for all parameters15 | Data from the AHRQ review15 are based on a larger sample from which to pool event data. They may also be more generalisable to a wider group of hernia patients |
| Baseline proportion with chronic pain or numbness at 1 year and 5 years | Resolution of pain and numbness follows linear interpolation between 3 months, 1 year and 5 years post surgery | Vary between all pain resolved by 1 year and no pain resolved at 5 years | The progression of pain and numbness over time is uncertain, with no evidence from included studies detailing treatment to resolve pain over time. These analyses estimate the impact of extreme but plausible upper and lower bounds of pain probabilities |
Differences across studies at different time points contributed to increase the uncertainty related to the progression of pain over time. The studies in our systematic review did not present details of a treatment protocol for patients with chronic pain. It was, therefore, unclear whether the baseline data referred to patients managed conservatively, aggressively or not treated at all. Thus, base-case data may have over- or underestimated the true pain profile over time. Similar concerns exist for numbness, although the impact on cost-effectiveness would be less given the lower utility decrement and lower treatment costs. We therefore explored two extreme analyses for both pain and numbness, assuming that (1) the baseline probability at 3 months does not fall over time (i.e. no one with chronic pain/numbness is cured over the first 5 years) and (2) the proportion with chronic pain/numbness at 1 and 5 years is 0 (i.e. all problems resolved by 1 year after surgery).
Changes to risk ratio parameters
The results of the base-case economic analysis were strongly influenced by the chosen RR data. As outlined in Chapter 3, all the point estimates from the RR data tended to favour the open preperitoneal mesh repair approach. However, results lacked statistical significance based on random-effects meta-analyses, therefore, some uncertainty remained. The two most likely parameters to impact on cost-effectiveness (e.g. those that generate the highest treatment cost and greatest loss of utility) are chronic pain and recurrences. We explored a potentially worst-case scenario analysis against open preperitoneal mesh repair, by combining the upper ends of the CIs for the RR of chronic pain (1.27) and recurrence (1.57). Although highly unlikely, the analysis serves to explore a plausible combination of effect sizes which could be seen as being most unfavourable, illustrating a lower bound on the probability of cost-effectiveness of open preperitoneal mesh repair. A two-way sensitivity analysis combining RRs of pain and recurrence is also presented to demonstrate the relative impact of these key parameters on the optimal treatment strategy. Two-way sensitivity analyses were based on identifying the treatment option with the greatest net benefit at a WTP of £20,000 for a QALY gained. Table 15 outlines the sensitivity analyses considered for RR parameters.
| Key variable/health state | Base-case assumption/value | Alternative assumption/value | Justification for choice of analysis |
|---|---|---|---|
| Relative effect sizes for chronic pain and recurrence | Base-case analysis used mean values sampled from distributions around relative effect sizes | Sensitivity analysis assumed point estimates at upper end of CIs for chronic pain (1.27) and recurrence (1.57) | RRs were uncertain and lacking statistical significance. Chronic pain and recurrence may impact costs and QoL. A worst-case scenario (for open preperitoneal mesh repair) was considered |
| Relative effect sizes of chronic pain and recurrence | Base-case analysis used mean values sampled from distributions around relative effect sizes | Two-way sensitivity analysis explored the combinations of RRs for chronic pain and recurrence | Chronic pain and recurrence were two important model variables; therefore, this analysis illustrates the combined uncertainty in the modelled estimates as well as the relative impact of each parameter on cost-effectiveness outcomes |
Changes to utility parameters
Utilities for surgery, ‘well’, chronic pain and numbness health states were available from the UK MRC hernia trial72 and were used in the base-case model. However, uncertainty surrounded the most appropriate utility weights to choose for complications and recurrences. For the base-case analysis, it was assumed, as in McCormack and colleagues,13 that the utility of a recurrence was equivalent to the utility of chronic pain (0.836). For the purposes of the current model, we made a similar assumption for complications. However, the impact of complications on QoL may be minimal, especially for minor complications identified in the systematic review of clinical evidence (e.g. urinary infection or minor haematoma). We also conducted a sensitivity analysis to explore the use of the numbness utility applied to all complications (0.919) (as this may represent a plausible estimate, especially for less severe complications).
Patient-reported outcome measures (PROMs), based on EQ-5D (European Quality of Life-5 Dimensions) data, were available for groin hernia repair in England. 82 Data were available in aggregate form for wound complications (grouping numbness and pain), reoperation and readmission. However, it was not possible to disentangle the utility weights for numbness and pain. It was also unclear which readmissions were for a recurrence or which were for other complications. However, by assuming that the majority of readmissions were for hernia recurrence, utility data for readmissions provided a plausible alternative estimate for recurrence utility, which could be explored within the model. Data were based on the full data release from April 2012 to March 2013,82 in which records are reported 4 months following all types of hernia surgery. The mean estimated utility for recurrence using PROMs data was 0.858 (SD 0.2083). These estimates were based on 3393 completed EQ-5D-3L profiles for readmissions out of 22,314 total records.
Alternative sources of utility data were available, although their appropriateness for use in the current decision model is questionable. QALY data from Koning and colleagues42,67 were available for TIPP repair (0.8588) versus Lichtenstein mesh repair (0.8566) at 1-year follow-up, based on SF-36 responses, converted to SF-6D utilities. 68 The data were not health-state specific; they were based on a measurement inconsistent with current recommended practice for UK-based analyses49 and, therefore, were of potentially limited value for the economic model. Nonetheless, the data were based on preference-based QoL measures and presented a plausible alternative. Therefore, we conducted sensitivity analyses to explore their use within the first year of the model analysis (adjusted to a 3-month cycle length). Data included beyond 1 year were the same as those used in the base-case analysis.
Data were available from the MRC study72 for a convalescence period at 3 months following surgery, but were not used in the base-case analysis, owing to the potential for counterintuitive results attaching greater weight to the numbness health state than 3-month surgical convalescence. The base-case analysis assumed that patients who were well 3 months following surgery had the same QoL as all patients in the ‘well’ health state (0.952). However, as a sensitivity analysis, we applied the convalescence utility (0.86) to all patients at the end of the first 3-month cycle. Although the base-case analysis may have overestimated the assumed time to recovery from surgery, the sensitivity analysis may underestimate the impact of chronic pain or recurrence within the first 3-month cycle. Summary details of all sensitivity analyses on utilities are presented in Table 16.
| Key variable/health state | Base-case assumption/value | Alternative assumption/value | Justification for choice of analysis |
|---|---|---|---|
| Utility of complications | Assume a utility weight equivalent to chronic pain (0.836)13 | Assume lower impact on QoL, equivalent to numbness (0.919) | No data were available for utilities for complications. The base case assumed an impact on QoL similar to chronic pain. This may have overestimated the utility impact of minor complications, and so the utility for numbness (0.919) offers a plausible alternative, especially for less serious complications |
| Utility of recurrence | Assume equal to chronic pain | PROMs data for readmission82 | Data from the PROMs data set in England were used, based on the assumption that readmissions were a valid proxy for recurrence utility82 |
| All utilities at 1 year post surgery | Health-state dependent | Based on trial analysis at 1 year42,67 | Koning et al.42 compared the utility of TIPP with Lichtenstein repair at 1 year post surgery, although were not health-state specific. They were included as a potentially feasible alternative to explore differences between treatments 1 year following surgery42,67 |
| Utility of surgical recovery | Health-state dependent | 3-month convalescence from the MRC study72 | The convalescence utility data represent actual EQ-5D data at 3 months following surgery for all patients, but were not health-state dependent |
Changes to cost parameters
Open preperitoneal mesh repair and Lichtenstein mesh repair require similar staff and theatre resource use, equipment and time. Both procedures map to the same HRG and so received equal surgical costs for the base-case analysis. However, the base case negates the fact that open preperitoneal mesh repair is not routinely used in the UK. If the open preperitoneal mesh repair approach were to be recommended as standard UK practice, surgeons would face a learning curve while they become skilled in the technique. There may be an initial increase in operative times and/or supervisory requirement for training clinicians in the early post recommendation phase. There may be also differences in the percentage of procedures performed under general or local anaesthesia, which are not captured in HRG codes. We, therefore, conducted a sensitivity analysis where open preperitoneal mesh repair was 20% more costly than Lichtenstein mesh repair. We also explored an analysis where Lichtenstein mesh repair was 20% more costly. A threshold analysis is presented to determine the additional surgical cost that would be required for open preperitoneal mesh repair in order to render Lichtenstein mesh repair the preferable option.
The base-case model assumed a conservative treatment approach for chronic pain. A sensitivity analysis explored a more aggressive approach, as outlined in the study by Achelrod and Stargardt,36 costing £855.98 per cycle and a ‘no treatment’ approach where the cost per cycle was assumed to be nil. Two-way sensitivity analyses investigated the impact various combinations of risk and cost of treating chronic pain on the optimal treatment strategy. Table 17 summarises the sensitivity analyses on cost data.
| Key variable/health state | Base-case assumption/value | Alternative assumption/value | Justification for choice of analysis |
|---|---|---|---|
| Cost of surgery | Equal costs for open preperitoneal mesh repair and Lichtenstein mesh repair = £1716.52 | Analysis 1: increase cost of open preperitoneal mesh repair by 20% Analysis 2: increase cost of Lichtenstein mesh repair by 20% |
If recommended as routine practice, open preperitoneal mesh repair may incur additional costs when surgeons complete a learning curve. Additional costs of 20% assumed a reasonable, maximum, short-term resource implication. A comparable analysis was undertaken for Lichtenstein mesh repair |
| Cost of chronic pain treatment | Conservative treatment cost of £198.00–212.00 per cycle | Analysis 1: £0.00 per cycle (no treatment) Analysis 2: £855.98 per cycle36 (aggressive treatment) |
Uncertainty in the appropriate treatment protocol for chronic pain and the availability of a wide variety of choices of approach are incorporated within a widely varying analysis of no treatment, conservative treatment (base case) and aggressive treatment approaches36 |
| RR of chronic pain and costs of treatment | RR chronic pain (from meta-analysis). Treatment costs for pain as above | Two-way analysis:
|
There were no data linking chronic pain treatment to health outcomes from the review. The impact of each parameter on cost-effectiveness was linked to the value of the other. Both parameters are varied simultaneously to identify the impact of various combined estimates on the optimal treatment strategy |
Structural uncertainty
The base-case model assumed that patients having a recurrence would transition into a separate model process whereby they run the risk of having a second recurrence, being well for the remainder of the model time horizon or dying from natural causes. There were insufficient data to model pain, numbness or other complications following the recurrent surgery. However, a sensitivity analysis tested the impact of allowing all patients having a recurrence to develop pain, other complications, or numbness with the same likelihood as those having an index surgery.
Other methodological uncertainty
Base-case results were analysed using a probabilistic model from which values for RRs were sampled from a log-normal distribution. However, the mean of a log-normal distribution calculated using the standard approach would not return the original point estimate of the RR from the meta-analysis. This reflects that the standard reporting for RRs is the modal value on the RR scale, as opposed to the mean. As a sensitivity analysis and validation check on the results, we incorporated the deterministic value of the RR point directly from the meta-analysis.
Standard methodological practice recommends that discount rates are tested within sensitivity analysis for both cost and QALY outcomes. We specifically tested the impact of varying the base-case discount rate of 3.5% per annum for costs and QALYs between 0% and 6%. Alternative discount rates were applied jointly to costs and QALYs in the sensitivity analysis.
The base-case analysis modelled utilities to account for age- and sex-adjusted UK population norms. 73 This reflects that as a patient grows older, their utility falls over time. A sensitivity analysis was conducted where the population norm adjustment was not carried out and data reported in the UK MRC study were incorporated directly into the model. 13,72
The most accurate data to populate model parameters were available at 1 year following surgery, with data becoming comparatively scarcer for longer follow-up periods, with no accurate data available beyond 5 years. Three alternative model time horizons were explored: short (1 year, from which the best trial data exist); medium (5 years, for which some data exist); and longer term (25 years, as in the base-case analysis). All methodological sensitivity analyses are summarised in Table 18.
| Key variable/health state | Base-case assumption/value | Alternative assumption/value | Justification for choice of analysis |
|---|---|---|---|
| All RR parameters | Mean estimates from log-normal distributions | Point estimates from the meta-analyses | Estimates of RR sampled from log-normal distributions do not directly replicate point estimates from meta-analyses. Sensitivity analysis explored directly incorporating meta-analysis results in the economic model |
| Discount rate costs and QALYs | 3.5% | 0–6% | Methodological uncertainty varied following NICE guidance49 |
| All utility weights | Adjustment for population norms | No adjustment for population norms | Analysis conducted to test the impact of using health-state specific utility data directly as reported in the MRC trial without further adjustment for UK population norms13,72 |
| Model time horizon | 25 years | 1 year 5 years |
To test the impact of varying the time horizon depending on the quality of data available to populate the model |
Subgroup analyses
There was a lack of available data to populate the economic model for individual subgroups and, therefore, no formal subgroup analyses were possible. However, a separate analysis is presented allowing the model to run, assuming a starting age of 40 years old and a starting age of 70 years old. It is worth noting that the only parameters to vary within these analyses were those relating to age-adjusted population utility and all-cause mortality. As such, the subgroup analyses should be considered exploratory in nature.
Results of cost-effectiveness analysis
Base-case cost-effectiveness results
Results for the base-case probabilistic analysis for the comparison of open preperitoneal mesh repair with Lichtenstein mesh repair are presented in Table 19. We have followed best practice guidelines for economic evaluation and presented results in terms of a probabilistic analysis. 83 The results of the economic model, based on expected values of costs and QALYs show that the open preperitoneal mesh repair improves health outcomes measured as QALYs. The additional QALYs can be achieved while also saving NHS costs. Open preperitoneal mesh repair is thus the dominant treatment strategy, with a very high probability of cost-effectiveness at all usual threshold values of WTP for a QALY gain in the UK,49 including if decision-makers were not willing to pay any additional cost for health gains. The strength of the base-case conclusions in favour of open preperitoneal mesh repair is driven by a reduction in hernia-related problems after surgery. Patients receiving the open preperitoneal mesh repair are, on average, less likely to experience chronic pain, recurrence, chronic numbness or early complications. There is greater uncertainty surrounding late complications, although events are extremely rare. Overall cost savings to the NHS are achievable by the reduction in requirement for costly treatments post surgery, especially those for chronic pain, which can be achieved without any additional surgical cost.
| Surgical strategy | Costs (£) | Difference in costs (£) | QALYs | Difference in QALYs | ICER (£/QALY) | Probability of cost-effectiveness at threshold values of WTP per QALY gained (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Lichtenstein | 2292 | – | 10.677 | – | – | 2 | 1 | 1 | 1 | 2 |
| Open preperitoneal | 2036 | –256 | 10.718 | +0.041 | Dominant | 98 | 99 | 99 | 99 | 98 |
Uncertainty in the modelled results is illustrated in a number of ways, based on the output from the probabilistic analysis, namely: (1) data that reports the likelihood of cost-effectiveness at each threshold value (£0, £10,000, £20,000, £30,000 and £50,000) (see Table 19); (2) as CEACs (Figure 16); and (3) as scatterplots of incremental costs and outcomes, presented on the cost-effectiveness plane (Figure 17).
FIGURE 16.
Base-case CEAC.

FIGURE 17.
Scatterplot of the cost-effectiveness plane.

The results clearly show that, based on our model base-case assumptions, open preperitoneal mesh repair is highly cost-effective, with < 2% chance of Lichtenstein mesh repair being the preferred treatment strategy, regardless of WTP for a QALY. The scatterplot visually represents the likelihood of cost-effectiveness at a typical threshold value of WTP for a QALY of £20,000. 49 The majority of simulations (97%) fall within the south-east quadrant of the cost-effectiveness plane, indicating the strength of the base-case dominance conclusions for open preperitoneal mesh repair.
Results of sensitivity analyses
The probabilistic analysis captures the uncertainty in data inputs and simultaneously varies all parameters to which distributions are attached. It is therefore a good representation of overall sampling uncertainty. The analysis, however, does not account for methodological or structural uncertainty in the model, nor does it identify the individual parameters to which the model outcomes are most sensitive. We therefore undertook a range of one- and two-way sensitivity analyses of parameter values and methodological and structural assumptions used in the base-case model. See Tables 14–18 for details of the analyses carried out and see Tables 20–26 for the results from these analyses for baseline parameters, RRs, utilities, cost, structural assumptions, methodological uncertainty and exploratory subgroup analyses, respectively. All sensitivity analyses are presented as probabilistic estimates of expected values for costs, QALYs and ICERs, together with the probability of cost-effectiveness, as for the base-case analysis.
Changes to baseline parameters
Table 20 shows the results of changes to the baseline model data. A higher probability of chronic pain for Lichtenstein mesh repair increases the incremental cost savings and QALY gains for open preperitoneal mesh repair. A more extreme scenario analysis, where no pain remains beyond 1 year, substantially reduces the magnitude of cost saving and QALY gains for open preperitoneal mesh repair. The magnitude of impact from similar assumptions regarding numbness on incremental costs and QALYs was much smaller, owing to much lower treatment costs and a less negative impact on QoL compared with pain. For all analyses around baseline parameters, open preperitoneal mesh repair remains dominant and highly likely to be cost-effective (> 95% probability at a WTP of £20,000 per QALY gained).
| Surgical strategy | Costs (£) | Difference in costs (£) | QALYs | Difference in QALYs | ICER (£/QALY) | Probability of cost-effectiveness at threshold values of WTP per QALY gained (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Populate all baseline parameters using data from systematic review (see Chapter 3) | ||||||||||
| Lichtenstein | 2297 | – | 10.678 | – | – | 1 | 1 | 1 | 1 | 1 |
| Open preperitoneal | 2032 | –265 | 10.720 | +0.042 | Dominant | 99 | 99 | 99 | 99 | 99 |
| Populate all baseline parameters using data from the AHRQ review15 (Lichtenstein arm of all included studies) | ||||||||||
| Lichtenstein | 2506 | – | 10.657 | – | – | 1 | 0 | 0 | 0 | 0 |
| Open preperitoneal | 2100 | –407 | 10.712 | +0.055 | Dominant | 99 | 100 | 100 | 100 | 100 |
| Baseline probability of numbness at 1 and 5 years = 0 (no chronic numbness persisting beyond the first year) | ||||||||||
| Lichtenstein | 2261 | – | 10.709 | – | – | 2 | 2 | 2 | 3 | 3 |
| Open preperitoneal | 2023 | –239 | 10.731 | +0.022 | Dominant | 98 | 98 | 98 | 97 | 97 |
| All those with numbness at 3 months also have numbness at 1 and 5 years (assumes treatment protocol is never successful) | ||||||||||
| Lichtenstein | 2316 | – | 10.656 | – | – | 1 | 1 | 2 | 2 | 3 |
| Open preperitoneal | 2040 | –275 | 10.715 | +0.059 | Dominant | 99 | 99 | 98 | 98 | 97 |
| Baseline probability of pain at 1 and 5 years = 0 (no chronic pain persisting beyond the first year) | ||||||||||
| Lichtenstein | 1950 | – | 10.714 | – | – | 2 | 3 | 4 | 5 | 6 |
| Open preperitoneal | 1854 | –96 | 10.737 | +0.023 | Dominant | 98 | 97 | 96 | 95 | 94 |
| All those with pain at 3 months, also have pain at 1 and 5 years (assumes treatment protocol is never successful) | ||||||||||
| Lichtenstein | 2978 | – | 10.604 | – | – | 1 | 1 | 1 | 1 | 1 |
| Open preperitoneal | 2168 | –810 | 10.704 | +0.100 | Dominant | 99 | 99 | 99 | 99 | 99 |
Changes to relative effect sizes
Base-case model results are informed by random-effects meta-analyses. The use of fixed-effects meta-analyses generates relative effect sizes with greater statistical precision. As effect sizes favour open preperitoneal mesh repair, the use of fixed-effects meta-analysis would further improve the cost-effectiveness case for open preperitoneal mesh repair. Table 21 presents results of further sensitivity analyses conducted around relative effect sizes used in the model. A plausible but worst-case scenario for open preperitoneal mesh repair is created by combining the upper ends of the 95% CIs for two of the main model result drivers, namely chronic pain (1.27) and recurrence (1.57). In this analysis, open preperitoneal mesh repair remains more effective (QALYs gained), but is also more costly, with an ICER of £15,109 per QALY gained. Open preperitoneal mesh repair is thus potentially cost-effective. However, great uncertainty exists with a probability of 49% and 53% of cost-effectiveness at £20,000 and £30,000, respectively.
| Surgical Strategy | Costs (£) | Difference in costs (£) | QALYs | Difference in QALYs | ICER (£/QALY) | Probability of cost-effectiveness at threshold values of WTP per QALY gained (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Lichtenstein | 2292 | – | 10.677 | – | – | 97 | 63 | 51 | 47 | 43 |
| Open preperitoneal | 2411 | +119 | 10.685 | +0.008 | 15,109 | 3 | 37 | 49 | 53 | 57 |
Overall, the analyses presented in Table 21 show that the model is sensitive to extreme assumptions regarding the RRs of pain and recurrence. Noting this sensitivity, we conducted a two-way analysis of alternative combinations of RR for chronic pain and recurrence (Figure 18).
FIGURE 18.
Two-way sensitivity analysis comparing RRs of chronic pain with RRs of recurrence. LR, Lichtenstein repair; OPP, open preperitoneal.

Figure 18 shows that the model conclusions are much more sensitive to the RR of chronic pain than they are to the RR of recurrence. This is driven by comparatively fewer recurrence events compared with chronic pain. No plausible values of recurrence would change the cost-effectiveness conclusions alone, however, when combined with RRs of chronic pain above 1.25, greater uncertainty exists. However, given that the upper end of the CI for RR of chronic pain is 1.27, it is unlikely that feasible combinations of risk of recurrence and pain would change cost-effectiveness conclusions.
Changes to utilities
None of the sensitivity analyses conducted around utilities in the model changes the dominance conclusion. There is some variation in the magnitude of incremental QALYs gained, however, in all cases, open preperitoneal mesh repair has a > 98% probability of cost-effectiveness at all plausible thresholds of WTP for a QALY. Imputing a utility value from PROMs data for reoperations (0.858) as a proxy for recurrence, this generates similar utilities to our base-case assumption, where a value of 0.836 was assumed. This suggests broad external validity of the assumption that impact of QoL may be broadly similar for recurrence and chronic pain. Similarly, data from Koning and colleagues42,67 for QALYs at 1 year following surgery (TIPP vs. Lichtenstein mesh repair) do not change overall cost-effectiveness conclusions. However, the estimates substantially reduce incremental QALY gains for open preperitoneal mesh repair (0.029) compared with the base case (0.041). The analysis adds a degree of uncertainty, based on no difference in utility at 1-year follow-up between TIPP and Lichtenstein mesh repair. However, even in an analysis where QALYs are assumed equal, open preperitoneal mesh repair still generates NHS cost savings and so would be the preferred treatment strategy from a cost-minimisation point of view. Results of sensitivity analyses around utility data are presented in Table 22.
| Surgical strategy | Costs (£) | Difference in costs (£) | QALYs | Difference in QALYs | ICER (£/QALY) | Probability of cost-effectiveness at threshold values of WTP per QALY gained (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Assume a higher utility weight for all complications (0.919, mild complication) | ||||||||||
| Lichtenstein | 2292 | – | 10.680 | – | – | 2 | 1 | 1 | 1 | 2 |
| Open preperitoneal | 2036 | –256 | 10.721 | +0.041 | Dominant | 98 | 99 | 99 | 99 | 98 |
| Using data from Koning et al.42 as a sensitivity analysis for TIPP and Lichtenstein at 1 year | ||||||||||
| Lichtenstein | 2292 | – | 10.806 | – | – | 2 | 1 | 1 | 2 | 2 |
| Open preperitoneal | 2036 | –256 | 10.826 | +0.029 | Dominant | 98 | 99 | 99 | 98 | 98 |
| Using best publicly available PROMs data to derive a utility weight for recurrence | ||||||||||
| Lichtenstein | 2292 | – | 10.678 | – | – | 2 | 1 | 1 | 1 | 2 |
| Open preperitoneal | 2036 | –256 | 10.718 | +0.041 | Dominant | 98 | 99 | 99 | 99 | 98 |
| Utility of surgical recovery: convalescence from UK MRC study at 3 months72 | ||||||||||
| Lichtenstein | 2292 | – | 10.676 | – | – | 2 | 1 | 1 | 2 | 2 |
| Open preperitoneal | 2036 | –256 | 10.714 | +0.038 | Dominant | 98 | 99 | 99 | 98 | 98 |
Changes in costs
Changes to cost data used in the model have a proportionally greater impact on results than changes in utility data. A number of assumptions have been made regarding costs, each of which is tested in Table 23.
| Surgical strategy | Costs (£) | Difference in costs (£) | QALYs | Difference in costs QALYs | ICER (£/QALY) | Probability of cost-effectiveness at threshold values of WTP per QALY gained (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| More heavily treated chronic pain.36 Cost = £855.98 per cycle | ||||||||||
| Lichtenstein | 3681 | – | 10.677 | – | – | 3 | 2 | 2 | 1 | 1 |
| Open preperitoneal | 2783 | –897 | 10.718 | +0.041 | Dominant | 97 | 98 | 98 | 99 | 99 |
| No treatment for chronic pain (assume cost per cycle = £0). Also assumes chronic pain resolves by itself over a period of time | ||||||||||
| Lichtenstein | 1825 | – | 10.677 | – | – | 4 | 1 | 2 | 2 | 2 |
| Open preperitoneal | 1785 | –41 | 10.718 | +0.041 | Dominant | 96 | 99 | 98 | 98 | 98 |
| Assume that cost of surgery is 20% higher for Lichtenstein mesh repair vs. open preperitoneal mesh repair (cost Lichtenstein mesh repair = £1968) | ||||||||||
| Lichtenstein | 2620 | – | 10.677 | – | – | 0 | 0 | 0 | 0 | 1 |
| Open preperitoneal | 2036 | –584 | 10.718 | +0.041 | Dominant | 100 | 100 | 100 | 100 | 99 |
| Assume that cost of surgery is 20% higher for open preperitoneal mesh repair vs. Lichtenstein mesh repair (cost of open preperitoneal mesh repair = £1968) | ||||||||||
| Lichtenstein | 2292 | – | 10.677 | – | – | 71 | 12 | 6 | 4 | 3 |
| Open preperitoneal | 2364 | +72 | 10.718 | +0.041 | £1,778 | 29 | 88 | 94 | 96 | 97 |
The model is most sensitive to the assumed equal costs for both index surgical procedures. A scenario where open preperitoneal mesh repair is 20% more costly than Lichtenstein mesh repair generates more uncertainty in the modelled estimates of cost-effectiveness, especially at lower threshold values of WTP for a QALY gained. Trade-offs will occur when additional surgical cost of open preperitoneal mesh repair is compared with cost savings attributable to a reduction in future hernia-related problems such as chronic pain. However, in the modelled scenario, open preperitoneal mesh repair remains the most cost-effective treatment strategy, with an ICER of £1778 and a probability of cost-effectiveness of 94% at a threshold value of WTP for a QALY gained of £20,000. Noting the sensitivity of the modelled estimates of cost-effectiveness to the cost of surgery, a threshold analysis was conducted, indicating that the surgical cost for open preperitoneal mesh repair would need to be £2601 (59% greater than Lichtenstein mesh repair) to change cost-effectiveness conclusions from a net monetary benefit point of view with a threshold value of WTP of £20,000 per QALY gained. Such a scenario is unlikely, given similar equipment, resource use, staff time, theatre time and time to discharge for both groups.
Other than the assumption of equal surgery costs, the magnitude of incremental costs is primarily driven by the costs attached to chronic pain treatment. An aggressive treatment scenario, with very high costs, improved the case of cost-effectiveness for open preperitoneal mesh repair, as comparatively fewer patients need to incur such costs under that treatment strategy. A scenario where no chronic pain is treated but utility decrements remain constant is the least favourable for open preperitoneal mesh repair. Although the cost attached to chronic pain has a large impact on incremental costs, in all cases, open preperitoneal mesh repair remains dominant and has a high probability of cost-effectiveness (> 98% at a WTP of £20,000 per QALY gained).
The results presented throughout this section confirm the robustness of the model conclusions for a range of parameter estimates. However, while they do not alter conclusions, analyses that vary RRs within plausible ranges and those that simultaneously vary the cost of treatment, especially chronic pain, appear to have the greatest impact on the strength of cost-effectiveness outcomes. Figure 19, therefore, presents a two-way sensitivity analysis illustrating the preferred strategy following a net benefit approach for alternative combinations of treatment cost per cycle and RR of pain for open preperitoneal mesh repair compared with Lichtenstein mesh repair.
FIGURE 19.
Two-way sensitivity analysis comparing per cycle treatment cost and RR of developing chronic pain in open preperitoneal mesh repair and Lichtenstein mesh repair. LR, Lichtenstein repair; OPP, open preperitoneal.

Higher treatment costs for chronic pain favour open preperitoneal mesh repair; however, RRs of chronic pain would need to be > 1.2 before any treatment cost for chronic pain would alter cost-effectiveness conclusions. Figure 19 illustrates some uncertainty but indicates that only in highly unlikely scenarios could Lichtenstein mesh repair be considered cost-effective.
Changes in structural and methodological assumptions
A range of additional sensitivity analyses explored the impact of structural and methodological assumptions on cost-effectiveness with results presented in Tables 24 and 25, respectively. Cost-effectiveness results were robust to changes in the model structure. In particular, changing the way in which recurrence was modelled, allowing patients to re-enter pain and complications health states post recurrence, did not alter cost-effectiveness results. From a methodological point of view, results were robust to changes in the discount rate applied, adjustments for population-based utility norms and alternative model time horizons. Estimates of RRs sampled from log-normal distributions in the probabilistic analysis are not equivalent to estimates generated directly from the meta-analysis. Base-case estimates from the probabilistic simulations were marginally higher (less favourable to open preperitoneal mesh repair) than the reported meta-analysis outcomes. Therefore, a sensitivity analysis where RRs were incorporated directly from the meta-analysis without drawing from the log-normal distributions actually improves the case of cost-effectiveness for open preperitoneal mesh repair. None of the structural or methodological sensitivity analyses had any material impact on incremental costs, incremental outcomes or on the likelihood of open preperitoneal mesh repair being the most cost-effective strategy.
| Surgical strategy | Costs (£) | Difference in costs (£) | QALYs | Difference QALYs | ICER (£/QALY) | Probability of cost-effectiveness at threshold values of WTP per QALY gained (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Lichtenstein | 2295 | – | 10.677 | – | – | 2 | 1 | 1 | 1 | 2 |
| Open preperitoneal | 2035 | –260 | 10.718 | +0.041 | Dominant | 98 | 99 | 99 | 99 | 98 |
| Surgical strategy | Costs (£) | Difference in costs (£) | QALYs | Difference in QALYs | ICER (£/QALY) | Probability of cost-effectiveness at threshold values of WTP per QALY gained (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Discount rate 0% applied to costs and QALYs | ||||||||||
| Lichtenstein | 2324 | – | 15.058 | – | – | 2 | 1 | 1 | 1 | 2 |
| Open preperitoneal | 2055 | –269 | 15.101 | +0.043 | Dominant | 98 | 99 | 99 | 99 | 98 |
| Discount rate 6% applied to costs and QALYs | ||||||||||
| Lichtenstein | 2271 | – | 8.678 | – | – | 2 | 1 | 1 | 1 | 2 |
| Open preperitoneal | 2024 | –247 | 8.717 | +0.039 | Dominant | 98 | 99 | 99 | 99 | 98 |
| No adjustment for population utility tariffs | ||||||||||
| Lichtenstein | 2292 | – | 13.784 | – | – | 2 | 1 | 1 | 2 | 2 |
| Open preperitoneal | 2036 | –256 | 13.836 | +0.052 | Dominant | 98 | 99 | 99 | 98 | 98 |
| Model time horizon: 1 year | ||||||||||
| Lichtenstein | 1908 | – | 0.685 | – | – | 2 | 1 | 1 | 1 | 1 |
| Open preperitoneal | 1810 | –99 | 0.697 | +0.012 | Dominant | 98 | 99 | 99 | 99 | 99 |
| Model time horizon: 5 years | ||||||||||
| Lichtenstein | 2278 | – | 3.293 | – | – | 1 | 1 | 1 | 1 | 2 |
| Open preperitoneal | 2028 | –251 | 3.333 | +0.040 | Dominant | 99 | 99 | 99 | 99 | 98 |
| Using mean point estimates from meta-analysis, not based on log-normal transformed estimates | ||||||||||
| Lichtenstein | 2292 | – | 10.677 | – | – | 0 | 0 | 0 | 0 | 0 |
| Open preperitoneal | 2019 | –272 | 10.717 | +0.039 | Dominant | 100 | 100 | 100 | 100 | 100 |
Subgroup analyses
No detailed subgroup analyses were conducted owing to a lack of data availability. However, analyses were conducted starting the model at age 40 years or at age 80 years (Table 26). The results were not sensitive to the model start age, although it should be noted that the only parameters to vary in these analyses were all-cause mortality and population norm utility weights. There were no data available to update RR or baseline parameters by the age of the model cohort.
| Surgical strategy | Costs (£) | Difference in costs (£) | QALYs | Difference in QALYs | ICER (£/QALY) | Probability of cost-effectiveness at threshold values of WTP per QALY gained (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Model start age: 40 years (time horizon 40 years) | ||||||||||
| Lichtenstein | 2298 | 15.988 | – | – | 2 | 1 | 1 | 2 | 2 | |
| Open preperitoneal | 2040 | –258 | 16.036 | +0.048 | Dominant | 98 | 99 | 99 | 98 | 98 |
| Model start age: 70 years (time horizon 10 years) | ||||||||||
| Lichtenstein | 2274 | – | 5.425 | – | – | 2 | 1 | 1 | 1 | 2 |
| Open preperitoneal | 2026 | –249 | 5.464 | +0.039 | Dominant | 98 | 99 | 99 | 99 | 98 |
Summary of cost-effectiveness
Overall, the results indicate that surgical treatment of primary inguinal hernia repair using open preperitoneal mesh repair surgery is likely to be a highly cost-effective use of NHS resources compared with the standard Lichtenstein mesh repair. Open preperitoneal mesh repair reduces the risk of patients experiencing a range of postsurgical problems, including early complications, recurrences and the development of chronic pain or numbness. Fewer problematic health issues leads to 0.041 more QALYs for patients having the open preperitoneal mesh repair. Open preperitoneal mesh repair and Lichtenstein mesh repair techniques are assumed to have equal costs. Therefore, because of the cost savings achieved from treating fewer episodes of complications, chronic problems and recurrence the open preperitoneal mesh repair also saves costs from a NHS perspective. Overall cost savings estimated in the base-case analysis were £256 per surgical repair treated by open preperitoneal mesh repair instead of Lichtenstein mesh repair.
Cost savings to the NHS combined with improved health outcomes in terms of QALYs gained mean that open preperitoneal mesh repair is a dominant treatment strategy when compared with Lichtenstein mesh repair. For the base-case analysis open preperitoneal mesh repair was highly likely to be cost-effective (> 98% probability) at a WTP of £20,000 per QALY gained. For a range of sensitivity analyses considered, the results remained robust, with open preperitoneal mesh repair being less costly while also generating greater QALY gains than Lichtenstein mesh repair. The probability of cost-effectiveness across the majority of sensitivity analyses remained in excess of 95% at a WTP threshold of £20,000 per QALY gained.
In all but two extreme-case analyses, the conclusion of dominance remained unbroken. The first assumption, that both the RR of chronic pain and recurrence were both assumed equal to the upper end of their 95% CIs, generates an ICER of £15,109 per QALY gained, but is surrounded by a high degree of uncertainty. Such a scenario is, however, highly unlikely but even in this case the point estimate of the ICER remains favourable to open preperitoneal mesh repair. The second analysis, where the cost of open preperitoneal mesh repair is assumed to be 20% greater than Lichtenstein mesh repair generates an ICER of £1778 per QALY gained. This scenario would be plausible should open preperitoneal mesh repair be rolled out as standard practice across the UK. However, additional costs, if any, would only be attributable for a short time period while surgeons became familiar with the technique and progressed along their learning curves. Beyond this point, there is no evidence to suggest any additional costs for open preperitoneal mesh repair compared with Lichtenstein mesh repair. Even when the cost of surgery is increased by 20%, open preperitoneal mesh repair is highly cost-effective, with a probability of cost-effectiveness of 94% at a WTP of £20,000 per QALY gained. The cost of surgery would need to be 59% greater than Lichtenstein mesh repair to change cost-effectiveness conclusions.
Although there are some uncertainties related to the most appropriate data to populate the model (especially around the baseline probabilities of pain and numbness, and appropriate utility weights for recurrence and complications health states), the results remain robust to plausible variation in these parameters, and we can conclude that the open preperitoneal mesh repair is potentially an efficient approach for the treatment of primary inguinal hernia relative to the standard Lichtenstein mesh repair as it is likely to generate greater QALYs and save resources for the NHS.
Further work is required to determine the most cost-effective method to conduct an open preperitoneal mesh repair (e.g. TIPP repair, Kugel repair). Data were not available to explore this research question in our model. Furthermore, work is required to determine if the improvements in chronic pain achieved from open preperitoneal mesh repair would offer a cost-effective alternative to laparoscopic surgery, which has also been shown to reduce pain, but at higher costs relative to Lichtenstein mesh repair. 13,70 Further decision-analysis modelling is required to explore this issue.
Chapter 5 Discussion
Statement of principal findings
This assessment provides the most up-to-date evidence base on the effects of open preperitoneal mesh repair compared with Lichtenstein mesh repair for the treatment of primary inguinal hernia (12 RCTs42,51–63 with a total of 1568 participants). Most of the included trials, however, were at high or unclear risk of bias and meta-analyses results showed statistical heterogeneity for most of the outcomes assessed. Overall, the findings of this assessment, although associated with some uncertainty, indicate that the open preperitoneal mesh repair may be a safe, efficacious and potentially less costly alternative to the standard Lichtenstein mesh repair.
Clinical effectiveness
There is evidence that participants who underwent open preperitoneal mesh repair returned to work or usual activities around 1.5 days earlier than those in who underwent Lichtenstein mesh repair. In general, participants randomised to open preperitoneal mesh repair tended to have a lower incidence of pain and numbness, fewer recurrences and fewer complications than those randomised to Lichtenstein mesh repair. However, CIs for treatment effects were wide and most of our meta-analyses results were not statistically significant at the conventional 5% level.
The advent of tension-free mesh repairs has reduced considerably the incidence of recurrence, and as such, other postoperative complications, including chronic pain, have gained importance in the assessment of surgical approaches for inguinal hernia. Chronic pain after inguinal repair is common. Evidence suggests that approximately 20% of patients suffer from chronic pain28,31,84 and this has a substantial impact on patients’ daily activities, such as walking, working and sleeping, as well as on personal relationships and social interactions. 28
Our meta-analysis, which included data from six published trials52,56,58–61 and one unpublished trial,51 showed a 50% reduction in the risk of chronic pain after open preperitoneal mesh repair (RR 0.50, 95% CI 0.20 to 1.27), but failed to demonstrate a statistically significant difference between the two surgical approaches. This finding is in line with previously published systematic reviews. 18–20 A meta-analysis of 12 RCTs, with a total of 1437 participants published by Sajid and colleagues19 in 2013, showed that the risk of developing chronic groin pain was reduced after open preperitoneal mesh repair (RR 0.48, 95% CI 0.26 to 0.89; p < 0.02). This meta-analysis, however, assessed specifically the effects of TIPP versus Lichtenstein mesh repair and did not focus exclusively on participants with primary unilateral inguinal hernias but included bilateral, recurrent or incarcerated inguinal hernias. Similarly, a meta-analysis by Li and colleagues18 published in 2012, which included 10 RCTs and two comparative studies, concluded that the ‘open preperitoneal approach is a feasible alternative to the Lichtenstein procedure with similar complication rates’. Li and colleagues18 included, however, the Prolene Hernia System as a relevant comparator. On the other hand, a Cochrane review by Willaert and colleagues20 published in 2012, comparing open preperitoneal techniques versus Lichtenstein mesh repair for elective inguinal hernia, failed to provide firm conclusions owing to the of dearth of suitable RCTs (only three RCTs were included) and the variation in the outcome data across trials. 20
Incidence of numbness was not consistently assessed in the included trials perhaps because among all the possible postoperative complications, numbness is not considered as relevant as chronic pain. Only four trials56,58,59,61 contributed to our meta-analysis and rates varied between trials. Overall, we did not find a statistically significant difference between the two surgical approaches (RR 0.48, 95% CI 0.15 to 1.56). Our results are consistent with those of Li and colleagues18 who did not find any significant difference in postoperative numbness between open preperitoneal mesh repair and standard Lichtenstein mesh repair (RR 0.50, 95% CI 0.18 to 1.43).
Return to work or usual activities tended to be earlier following open preperitoneal mesh repair than following standard Lichtenstein mesh repair and it was found to be statistically significant (mean difference –1.49 days, 95% CI –2.78 to –0.20 days; p = 0.02). Some systematic reviews18–20 did not assess ‘time to return to normal activities’, which is an important measure for establishing patients’ recovery after hernia surgery and for assessing the cost-effectiveness of different surgical strategies from a wider patient and societal perspective.
We did not observe any statistically significant differences between the open preperitoneal approach repair and the Lichtenstein approach in terms of number of deaths, recurrences, complications (i.e. wound infection, haematoma/seroma or urinary problems) and length of hospital stay. In particular, the risk of developing recurrences and complications observed in our analyses is consistent with that of other systematic reviews in the literature. 19,20 It is also worth observing that while in the past recurrence of inguinal hernia was an important postoperative complication, after the advent of the tension-free mesh technique the risk of recurrence has decreased considerably and the current incidence rates are as low as 1–4%. 14,85
Cost-effectiveness
The probabilistic Markov cohort model developed as part of this assessment is the first attempt within the literature to synthesise systematically evidence on costs and outcomes of open preperitoneal mesh repair versus Lichtenstein mesh repair for the treatment of primary inguinal hernia. It is currently the most complete evidence base on cost-effectiveness in a UK context. Results of a cost-minimisation analysis from a Dutch societal perspective, over a 1-year time horizon, conducted alongside a RCT are available in the literature. 67 However, the follow-up period of this cost-minimisation analysis is insufficient to capture all costs and benefits of different surgical approaches and it is uncertain whether or not the findings may apply reliably to a UK setting.
The results of our economic evaluation were informed by data from the systematic review of clinical effectiveness. On average, patients who underwent open preperitoneal mesh repair were less likely to experience chronic pain, chronic numbness, early postoperative complications and recurrences. Fewer postoperative problems resulted in an average of 0.041 QALYs gained over the 25-year horizon. As both procedures require similar resource use (e.g. staff, equipment, mesh, time in theatre and time to discharge), we assumed equal costs in the model. Fewer postoperative problems resulted in fewer treatment requirements and a reduced NHS burden of treating complications such as chronic pain. We found that because of similar surgical costs and reduced costs in the follow-up period, the open preperitoneal mesh repair was £256 less costly per case completed compared with the Lichtenstein mesh repair using base-case model assumptions.
By generating greater QALYs and potentially saving NHS resources, the open preperitoneal mesh repair appears to be the dominant, most efficient treatment option for inguinal hernia with a high probability of cost-effectiveness (> 98%) at the typical threshold values of WTP for a QALY recommended by NICE. 49 Furthermore, from a wider societal perspective, lower incidence of chronic pain and numbness and earlier return to work or to normal activities would probably be preferred from a patient and family perspective with fewer days of lost wages and potentially lower costs of self-medication for the management of chronic pain after surgery. Earlier return to work would also be preferable from an employer point of view, as fewer days of absence would reduce productivity losses.
Despite some differences, our results are broadly consistent with those of Koning and colleagues67 who found that TIPP was significantly less costly from a societal perspective than the Lichtenstein mesh repair. Although the direction of effect was similar to that of our results, from a health-care perspective, their results were less convincing over a 1-year time period. Our results present a stronger case for open preperitoneal mesh repair owing to the longer time horizon in which the full costs of treating long-term complications such as chronic pain, numbness and recurrences become apparent.
The results of the CEA were robust to a range of different model input data, especially where alternative choices of data were available for baseline probabilities and utilities. Results were more sensitive to estimates of cost and especially the cost of treatment for chronic pain. Different estimates of treatment resource use and cost such as the modelled conservative base-case assumptions, aggressive treatment assumptions,36 or no treatment at all generated substantial differences in incremental costs (and hence estimates of potential cost savings to the NHS). However, in all cases the conclusion of dominance remained unbroken.
Similarly to the findings reported by Achelrod and Stargardt,36 we found chronic pain to be an important driver of cost-effectiveness outcomes. Achelrod and Stargardt modelled a decision to perform laparoscopic hernia repair using heavy- versus lightweight mesh and used similar model input data to those in our model (especially around utility weights). 36 They modelled the development of chronic pain over a 1-year time horizon, finding a strong impact of pain on cost-effectiveness. McCormack and colleagues who modelled a decision to perform hernia repairs using laparoscopic or open mesh procedures also drew similar conclusions. 13 McCormack and colleagues found recurrence to be an important factor. While our model includes a health state for recurrence, variation in recurrence data within the model had minimal impact on overall results, owing to the current low recurrence rate after hernia repairs. 7,36,86 Our findings are consistent with a number of existing studies, all of which find chronic pain to be an important driver of outcomes following primary inguinal hernia repair. 15,17,20
In our model, in all but two extreme scenario analyses, the conclusion of dominance remained unbroken. The first scenario analysis, where both the RR of chronic pain and the risk of recurrence were assumed equal to the upper end of their 95% CI, generated an ICER of £15,109 per QALY gained. However, the results were surrounded by considerable uncertainty. Despite the low likelihood of such an extreme scenario analysis, the point estimate of the ICER remains favourable to the open preperitoneal mesh repair. The second scenario analysis, where the surgical cost of open preperitoneal mesh repair was assumed to be 20% greater than that of the Lichtenstein mesh repair, generated an ICER of £1778 per QALY gained. This scenario can be regarded as plausible, especially in an early post recommendation phase when surgeons would be required to progress along a learning curve, which may generate short-run additional costs. However, even when cost of surgery is increased by 20%, the open preperitoneal approach remains likely to be cost-effective with a probability of cost-effectiveness of 94% at a WTP of £20,000 per QALY gained. The cost of open preperitoneal surgery would need to be 59% greater than that of the Lichtenstein surgery to change the overall cost-effectiveness conclusions.
The lack of baseline, relative effect size or utility data for hernia repair in different age groups hampered the possibility to estimate cost-effectiveness for different age subgroups reliably. However, there was no obvious reason to believe that costs of the two surgical procedures would differ by age, and utility values were adjusted to reflect population norms in the base-case analysis. Furthermore, there were no data available to model the cost-effectiveness of the different techniques used to perform open preperitoneal mesh repairs.
Uncertainties from the assessment
Clinical effectiveness
This assessment has been conducted according to current methodological standards and is the most up to date and complete evidence base assessing the effects of open preperitoneal mesh repair versus Lichtenstein repair for the treatment of primary inguinal hernia. We need to acknowledge, however, some potential limitations.
The meta-analyses results demonstrated evidence of statistical heterogeneity between included trials. For binary outcomes we used a random-effects model as the primary meta-analysis method, except when events were rare, when we used the recommended Peto approach. Although we observed a trend in favour of the open preperitoneal approach, CIs for treatment effects were wide and most results were not statistically significant at the conventional 5% level. Varying differences in the way outcomes were assessed and reported were observed across trials. In particular, considerable variation existed among included trials with regard to: (1) the definition of chronic pain and the use of measurement scales after surgery; (2) the definition of ‘work and normal activities’; (3) the time of follow-up assessments and study duration; (4) characteristics of the hernia defect (high-risk and low-risk hernias); (5) type of open preperitoneal techniques and type of mesh (soft mesh, mesh with memory ring, single layer, double layer); and (6) surgeon’s expertise.
The assessment of pain (acute and chronic) is quite challenging as ‘pain’ is essentially a subjective outcome and the tools for its measurement are variable and not straightforward. The majority of included studies used a VAS, which is considered a valid tool for measuring pain after inguinal hernia repair (IASP). However, the VAS has the limitation to measure pain only as one-dimension score, while pain is a more complex phenomenon. Moreover, the exact significance of scores higher than 0 may be difficult to capture or interpret. The observed variation in the rates of chronic pain between trials can be partly explained by the way trial investigators described chronic pain. Chronic pain was (1) defined according to the definition of the IASP including a VAS score above 0 which lasts for more than 3 months, (2) defined as a VAS score between 3 and 1061 or (3) not defined at all. Moreover, as chronic pain may be triggered by the position of the mesh and the type of mesh fixation, different type of techniques used for inguinal hernia repair as well as the surgeon’s expertise may have contributed to the observed differences between trials. Two trials,56,59 both conducted in the Netherlands, reported an unusual high incidence of chronic pain (13% and 40%, respectively) and persistent numbness (51% and 25%, respectively) after Lichtenstein repair. It is possible, but we have no information to confirm this, that the pain was the result of nerve damage, which is more likely to occur after Lichtenstein repair than after open preperitoneal repair.
Time of follow-up assessments varied among included trials, which made it challenging to combine outcome data reliably. Furthermore, we could not assess long-term complications and recurrences as the majority of trials were of relatively short duration (mean 17 months).
We were not able to quantify the impact of the potential risk of bias on the observed estimates of effects. Overall, only two trials were judged at low risk of bias,52,56 whereas remaining trials were judged at high or unclear risk of bias because of inadequate randomisation methods, blinding procedures (lack of participants and/or outcome assessors blinding) or reporting. We contacted the authors of trials who did not report important methodological details but did not receive any further information.
The open preperitoneal approach (e.g. Kugel, TIPP) may require more cranial incision than the Lichtenstein approach and outcome assessors may recognise the shape of the performed incision even when they are blinded to the surgical methods. However, the main outcome of interest for this assessment, ‘chronic pain’, was self-reported by participants and it is unlikely they were able to identify the type of hernia repair from the surgical incision. Therefore, two trials52,59 that blinded participants but reported a difference in the shape of the surgical incisions were judged to be at low risk of bias of detection bias.
As all included trials were conducted outside the UK, there is some uncertainty on whether or not the results of this assessment are applicable to a UK setting.
Cost-effectiveness
The cost-effectiveness results were broadly robust to a range of sensitivity analyses undertaken, and there is a high degree of confidence that the open preperitoneal approach is cost-effective compared with the Lichtenstein approach. Although, we used the best available evidence to populate our economic model, our results are subject to a number of assumptions and uncertainties. Most of these uncertainties impact on the magnitude of cost savings or QALY gains for open preperitoneal repair, however, do not change the overall conclusions and recommendations.
There were no data available to explore the cost-effectiveness of different types of open preperitoneal techniques (e.g. Kugel, TIPP repair). While we can conclude, with a high degree of confidence, that the open preperitoneal approach is cost-effective for the treatment of inguinal hernia, we are not in the position to make any claims on the most appropriate or preferred open preperitoneal technique. Different techniques may contribute to substantial differences in outcomes and costs. The economic model failed to account for such differences between techniques.
With regard to the surgical cost of the two interventions, we could not identify secondary procedure codes from HRG data to develop individual costs for the open preperitoneal approach compared with the Lichtenstein approach. All procedures mapped to the same code and were, therefore, assumed to have equal cost in the economic model. While the assumption of similar treatment costs would remain the same, the value of these costs might not truly represent opportunity costs of the procedures, especially given that procedure codes may include the higher cost of laparoscopic procedures. Although the model may overestimate the cost of open hernia repairs, the error would have no impact on the marginal analysis because of the assumption of costs equivalence across all open mesh techniques. Data related to time to discharge from hospital show no difference between procedures, and clinical expert opinion indicates almost identical surgical procedures. The main difference between surgical approaches appears to be the exact placement of the mesh. Thus, it is likely that the performed sensitivity analyses account for any differences in the costs of performing the surgical procedures in clinical practice.
Another factor that might contribute to surgical costs in the UK is the current limited clinical expertise in performing the open preperitoneal mesh repair. The majority of UK surgeons (96%) prefer the standard Lichtenstein approach. 16 Consequently, if the open preperitoneal mesh repair were to be recommended, there would be significant training costs to take into consideration as surgeons would be expected to progress along a learning curve until they acquire expertise and confidence with the new technique. In the short term, this may add an additional cost burden to the NHS for the open preperitoneal approach. However, it is likely that any additional cost would be offset by a reduction in the costs of treating postoperative complications, such as chronic pain and recurrences.
A number of assumptions were made regarding the structure and the relevant data to populate the economic model. We assumed that a patient could have a maximum of two hernia recurrences before they entered either a ‘well’ health state or died of natural causes. This assumption mirrors that made previously by McCormack and colleagues. 13 We also assumed that no pain or numbness occurred after a recurrence as the modelling of such health states was beyond the scope of this assessment, given that the differences would probably be because of the recurrence operation and not to the index procedure. Sensitivity analyses, which explored the impact of imputing similar rates of pain and numbness as for the primary procedure, did not alter our conclusions.
We further assumed that recurrence procedures would be carried out using the same technique as for the index procedure. This assumption had a limited impact on cost-effectiveness results, given that all procedures were assumed to generate the same surgical cost. Again, modelling the decision on the most appropriate procedure to treat recurrences was beyond the scope of the review.
A further concern relates to the quantity and quality of data available to populate the economic model, especially for chronic pain and numbness after hernia repair. The model was developed to detail the progression of pain or numbness over time. However, data were not available from included trials to populate the model at all the time points of interest (3 months, 1 year and 5 years after surgery). The heterogeneity observed between trials adds uncertainty to the baseline data used in the model. This is particularly problematic for pain and numbness that were measured inconsistently across trials. Nevertheless, in all cases we have managed to apply baseline data for a single treatment strategy, namely the Lichtenstein repair arm of appropriate RCTs. To limit the heterogeneity impact as much as possible, where possible, we have selected trials that reported similar measures of pain over time.
None of the included trials from which baseline data were sourced, detailed the treatment strategy used to manage chronic pain. It was therefore unclear whether pain reduction over time was owing to any treatment at all, to self-medication, to conservative pharmacotherapy or to more aggressive treatment approaches (see Achelrod and Stargardt,36 for example). The incremental costs (i.e. the magnitude of cost saving to the NHS) predicted by the economic model are highly sensitive to the cost of treatment for chronic pain. There is a lack of evidence in the literature to identify appropriate treatment strategies for chronic pain after hernia repair. However, it is reasonable to assume an initial conservative treatment approach that may move towards a more aggressive treatment, if chronic pain persists over time. Uncertainties in the preferred treatment approach used in UK clinical practice together with a lack of data to link treatment to pain outcomes weaken the economic model projections of the magnitude of incremental costs and outcomes. It is reasonable to assume, for example, that minor pain would require no treatment, whereas more serious pain (e.g. a VAS score of 7 and above) would require a more aggressive approach. The lack of appropriate data to stratify pain according to severity is an important limitation, which may add uncertainty to the modelled chronic pain costs and outcomes. The base-case economic model follows a conservative treatment approach as this approach is more likely to reflect UK clinical practice and less likely to overstate cost-effectiveness findings. However, alternative treatment strategies, from minor or no treatment to more aggressive approaches, may also be applied. We used evidence from the available literature36 to identify a more aggressive treatment approach and thus range the cost of treatment from no treatment (assumed cost per cycle = £0) to aggressive (cost per cycle = £855.98). The variation in the costs applied allows for a range of plausible treatment approaches for chronic pain. The assumed modelled cost of chronic pain does not impact on the probability of cost-effectiveness, but the magnitude of cost savings to the NHS varies substantially.
Quality-adjusted life-years calculated for the base-case economic analysis were based on utility weights calculated from a single UK study,27 which raises some concerns with regard to generalisability. Furthermore, for health states where data from the UK MRC study were not available, we assumed that early postoperative complications and recurrences would have an impact similar to that of chronic pain in the base-case analysis. This assumption generated some uncertainty in QALY estimates. We explored alternative data sources for incidence of recurrence, using assumptions about reported PROMs data, and imputed a less severe health-state utility of numbness for all complications as sensitivity analyses. While different utility weights for the model generated different QALY estimates, none altered the cost-effectiveness conclusions.
Chapter 6 Conclusions
Implications for health care
Open mesh repair, and in particular Lichtenstein mesh repair, is the standard treatment for inguinal hernia in the UK. The results of this assessment, although associated with some uncertainty, suggest that open preperitoneal mesh repair may be a safe and efficacious alternative to Lichtenstein mesh repair with similar recurrence and complication rates, potentially lower incidence of postoperative pain and a significantly earlier return to work and to usual daily activities.
NHS budgetary impact
At present, the majority of open mesh repairs for inguinal hernia performed in the UK are based on the standard Lichtenstein approach with very few, if any, based on the open preperitoneal approach. The results of our assessment and our economic evaluation, however, showed better patient outcomes after open preperitoneal mesh repair over the longer term and hence potential cost savings. The budgetary impact of increasing the use of the open preperitoneal approach in the UK clinical setting is dependent on the clinical effectiveness of the open preperitoneal mesh approach compared with the Lichtenstein mesh approach as well as on the number of open mesh procedures for primary inguinal hernias performed annually and the likely proportion of procedures that might be conducted using the preperitoneal approach. As such, the budgetary impact projections should be interpreted as explanatory. The cost savings to the NHS are heavily dependent on the willingness of surgeons to change current routine practice and their confidence in the open preperitoneal mesh repair. Data from the HES show that out of 65,759 primary inguinal hernia repairs carried out in England during the period 2012/13, 61,280 (93%) were open procedures based on the use of prosthetic materials (e.g. mesh). 1 The 2010 NICE implementation uptake report35 on laparoscopic surgery for inguinal hernia repair indicated that 16.36% of all primary repairs were performed using laparoscopy. Based on the 2012/13 hospital activity level and assuming that the rates of laparoscopic hernia repair remain unchanged from the 2010 NICE uptake report, we can reasonably infer that 10,758 primary mesh procedures were likely to be conducted using a laparoscopic approach, while the remaining 50,522 were likely to be conducted using an open mesh approach (probably the Lichtenstein approach, as nearly all open mesh repairs in the UK are performed using the standard Lichtenstein technique). 16
Figure 20 outlines the projected annual cost savings to the NHS for various uptake rates of open preperitoneal mesh repair. The projected cost savings are informed by the base-case economic model projections of average cost savings of £256 for every case completed as open preperitoneal mesh repair rather than Lichtenstein mesh repair.
FIGURE 20.
Annual impact on NHS England budgets of performing open procedures using open preperitoneal technique.
If all cases were to be transferred to the open preperitoneal approach, the cost savings to the NHS in England alone would be substantial, amounting to a maximum of £12.93M per year. It is worth noting that these estimates are based on projections from the economic model, which is informed by an uncertain evidence base. Further work would be required to re-estimate budgetary impacts once more conclusive data on clinical effectiveness are available.
In Scotland, 8068 inguinal hernia repairs were completed in 2012/13. 87 Assuming that 92% of hernia repairs were for a primary hernia (as in England),1 7423 repairs were performed for the treatment of primary hernias. In Scotland, the proportion of completed laparoscopic surgeries appears to be slightly lower than in England. Data from 2007/8 indicate that only 13% of all inguinal hernia repairs were completed using laparoscopic surgery. 46 Therefore, considering the assumptions outlined above, the estimated number of procedures to which the economic model result applies is 6374 per annum. Figure 21 reproduces a similar assessment of budget impact for NHS Scotland.
FIGURE 21.
Annual impact on NHS Scotland budgets of performing open procedures using open preperitoneal technique.
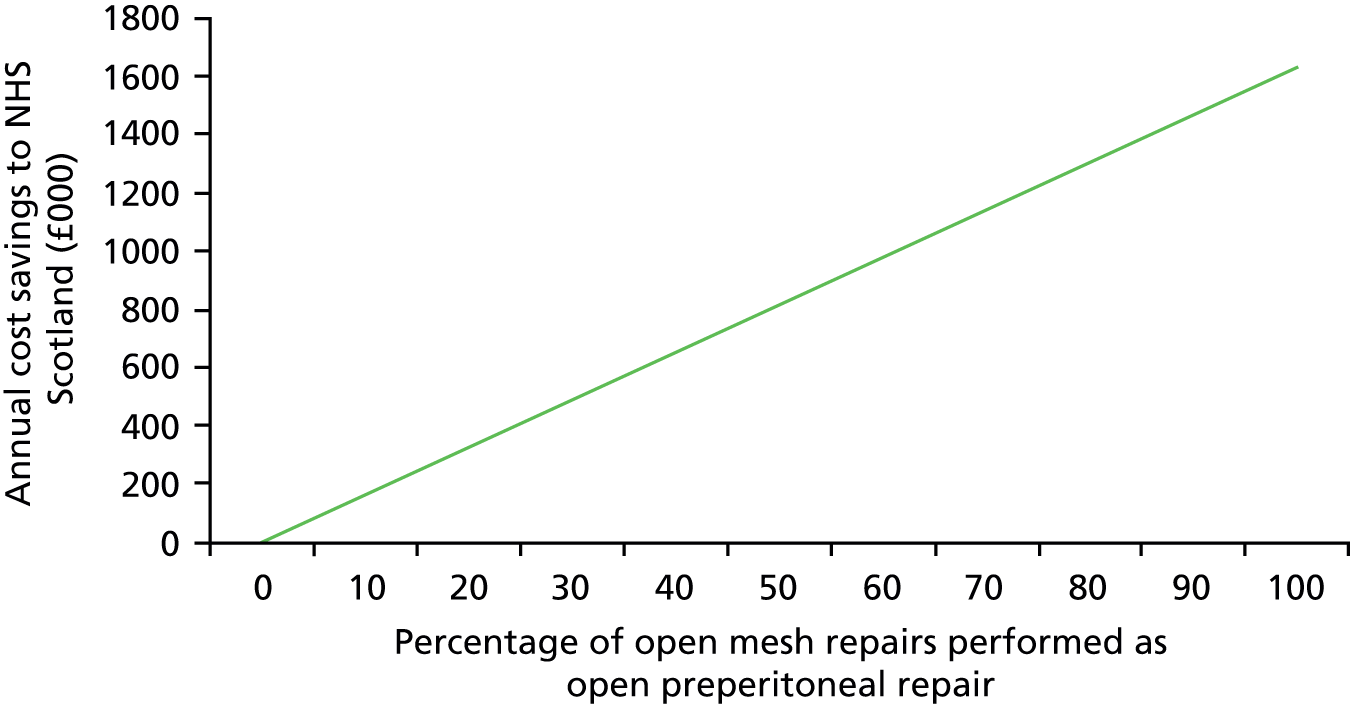
As the magnitude of cost savings are sensitive to a number of factors outlined throughout our economic evaluation, the estimated cost savings to the NHS should be considered in light of the uncertainty around some parameters in the economic model. Factors such as additional training costs of moving to the open preperitoneal approach could reduce the magnitude of NHS cost savings in the early post recommendation phase. The true cost savings will depend in part on how easily change of routine practice could be achieved and how quickly surgeons could progress along a learning curve. It is likely that any additional costs for open preperitoneal mesh repair owing to development of the surgeon’s skills and training would be short term and would be more than offset by the projected cost savings over the longer period. It is likely that any change in practice would take place over an extended period of time and any additional training costs would be expected to be staggered over time.
There is no guidance regarding the preferred method for open preperitoneal mesh repair. Owing to a lack of suitable data, the systematic review of clinical evidence and the economic model assessed different open preperitoneal mesh techniques together as one single analysis. It is feasible to assume that different approaches may generate different cost estimates and further research is required to identify the most efficient open preperitoneal mesh technique in order to optimise the use of current NHS resources.
Previous NICE guidance34 recommends the use of laparoscopic repairs, even though more costly, because of a lower incidence of chronic pain and an earlier return to normal activities compared with open mesh repair. The open preperitoneal mesh repair allows patients to return to work and normal activities earlier and potentially improves pain outcomes. Further research is required to determine any potential trade-offs between the laparoscopic approach and the open preperitoneal approach and establish if cost savings could be enhanced further without adverse consequences for patients.
Impact on patients and their families
Patients having an open preperitoneal mesh repair returned to normal activities (including work) around 1.5 days earlier than those in the Lichtenstein mesh repair groups (mean difference –1.49 days, 95% CI –2.78 to –0.20 days). The earlier resumption of daily and working activities is likely to be of benefit to patients, their families (with a potentially reduced burden of care) and to the society more generally. We also observed a trend towards a lower incidence of postoperative pain, recurrences and complications even though results were not statistically significant at the conventional 5% level of confidence. These findings may translate into reduced requirements for oral analgesics in the immediate postoperative period. With early return to normal activities patients reduce their time off work after hernia surgery. Therefore, the open preperitoneal mesh repair can be associated with a reduction in lost wages as well as a reduction in lost productivity because of absence from work.
Hospital Episode Statistics show that 34,147 out of 61,280 (56%) of all primary inguinal hernia repairs using mesh were performed in people of working age (age 18–64 years). 1 Applying this information to the estimated number of total open repairs outlined above, 28,292 people treated with open mesh repair would be of working age in England. Applying a standard average hourly unit tariff for working time in the UK of £12.80 per hour88 to the estimated difference in return to normal activities, the average cost saving from reduction in lost wages is £19.07 per additional open mesh repair conducted using an open preperitoneal approach (95% CI £2.56 to £35.58). Figure 22 presents the cost savings from a reduction in lost wages in England per annum for different implementation rates of open preperitoneal mesh repair. If all repairs were performed using the open preperitoneal approach instead of the Lichtenstein approach, the estimated cost savings from a reduction in lost wages in England is £539,528 annually (95% CI £72,428 to £1,006,629). Figure 23 shows the projected cost savings from a reduction in lost wages for different uptake rates in Scotland.
FIGURE 22.
Annual impact on reduction in lost wages from open preperitoneal mesh repair in England.
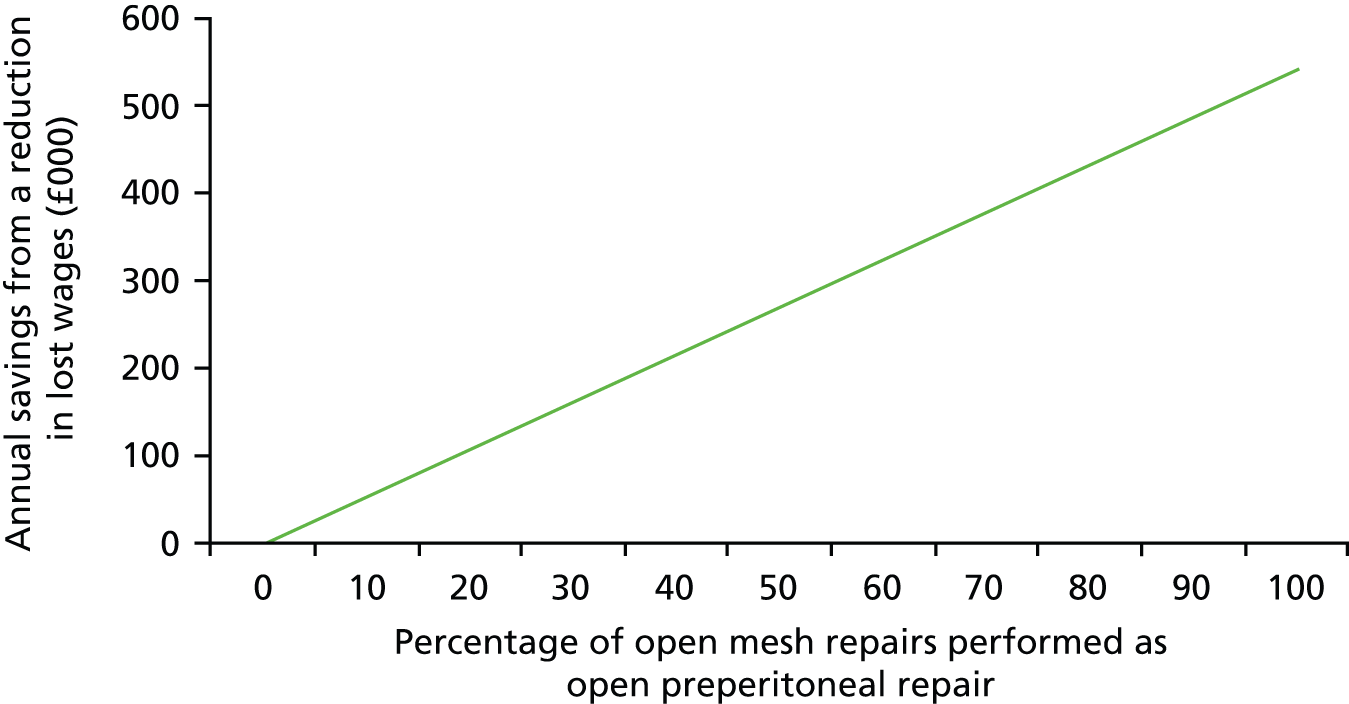
FIGURE 23.
Annual impact on reduction in lost wages from open preperitoneal mesh repair in Scotland.
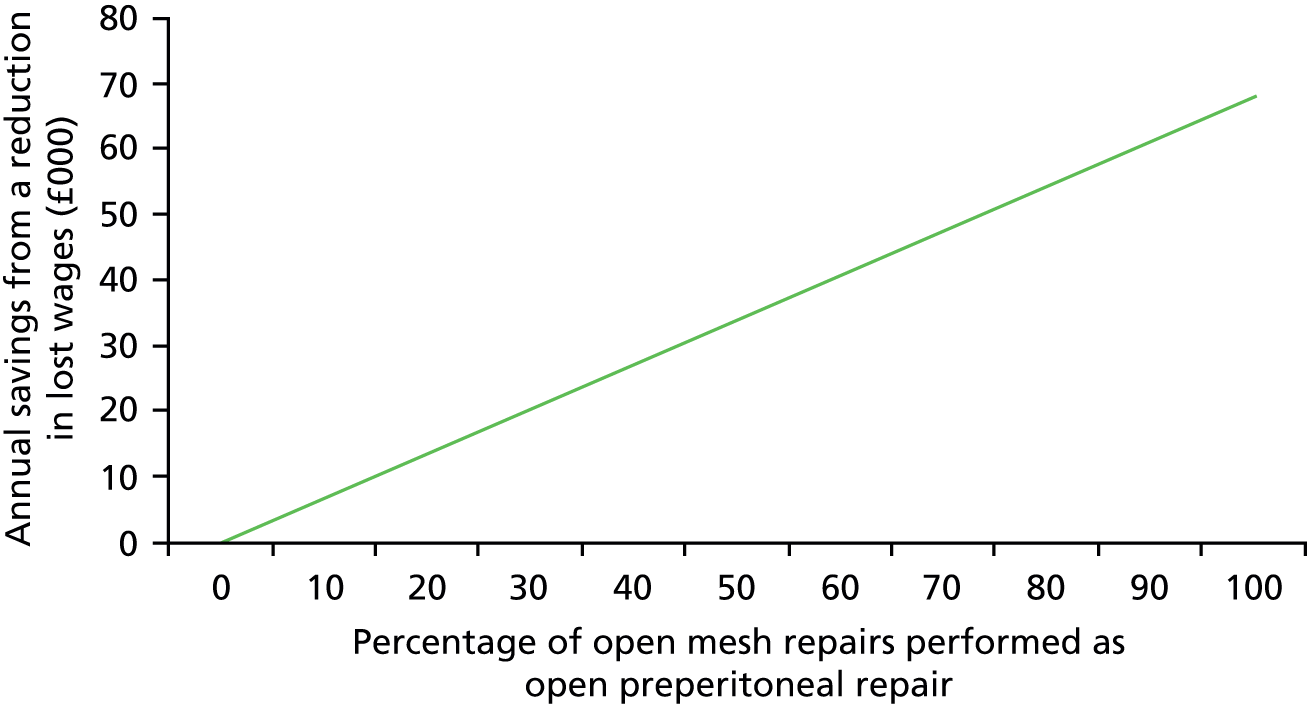
If all repairs were performed using the open preperitoneal approach instead of the Lichtenstein approach, estimated cost savings from a reduction in lost wages in Scotland is £68,061 annually (95% CI £9137 to £126,985).
Recommendations for research
The main gap in the current evidence is the limited evidence on the long-term effects of open preperitoneal mesh versus Lichtenstein mesh repair, especially in the UK setting. For this assessment we identified RCTs of relatively short duration conducted outside the UK.
A large, good quality, clinical trial needs to be undertaken to compare the effects and costs of open preperitoneal mesh versus Lichtenstein mesh repair in people presenting with primary unilateral inguinal hernia. Ideally, such a trial would be multicentre, have a long-term follow-up, would include relevant outcome measures, such as postoperative pain, recurrences, complications and QoL measures, a clear definition of measures (e.g. chronic pain) and a full economic evaluation.
Areas in which further research would also be important are the following:
-
research based on well-designed clinical trials to determine the most effective open preperitoneal repair technique (e.g. Kugel patch, Nyhus repair, Read–Rives repair, TIPP repair) in terms of both clinical efficacy and cost-effectiveness
-
research to identify longer-term resource use for people undergoing inguinal hernia repair in order to develop more robust cost estimates for the UK (especially for the treatment of chronic pain).
Acknowledgements
The authors are grateful to Lara Kemp for her secretarial support; Yuen Soon, Ian Beckingham and Ewan Paterson for providing clinical guidance as members of the Advisory Group for this assessment; David Stephen and Mohammad Moonis for sharing their experience as patient representatives; Marjon van der Pol and Imran Omar for their assistance with the translation of non-English publications; Sergio Solorio (Mexican Social security institute, Mexico) and Shaban Mehrvarz (Baqiyatallah University of Medical Sciences, Iran) for kindly providing additional trials details; and Luke Vale (Newcastle University) for providing access to a previously conducted decision model for hernia repair.
Source of funding
This report was commissioned by the National Institute for Health Research HTA programme as project number 13/134/01. The Health Services Research Unit and Health Economics Research Unit are core-funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates.
Contribution of authors
Pawana Sharma (Research Fellow) led the day-to-day running of the assessment, reviewed the evidence on the clinical effectiveness of the surgical interventions and drafted the first version of this report.
Dwayne Boyers (Research Fellow) conducted the economic evaluation.
Neil Scott (Medical Statistician) contributed to data extraction, interpretation of results and conducted all statistical analyses.
Rodolfo Hernández (Research Fellow) conducted the economic evaluation.
Cynthia Fraser (Senior Information Officer) was responsible for running the literature searches, obtaining full-text papers and compiling the reference list of this report.
Moira Cruickshank (Research Fellow) contributed to studies selection, quality assessment and data extraction.
Irfan Ahmed (Consultant Surgeon) provided expert advice on the clinical aspects of the surgical procedures and commented on the draft version of this report.
Craig Ramsay (Health Care Assessment Programme Director) jointly co-ordinated the assessment and commented on the draft version of this report.
Miriam Brazzelli (Senior Research Fellow) oversaw and co-ordinated all aspects of the assessment, led and co-ordinated the expert advisory group participation, interpreted data and revised the draft version of this report.
All authors approved the final version of this report.
Data sharing statement
Technical appendices are available from the corresponding author and all other relevant data are provided within the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Health and Social Care Information Centre . Hospital Episode Statistics, Admitted Patient Care – England, 2012–13; Main Operations, 4 Character Tables 2013. www.hscic.gov.uk/catalogue/PUB12566/hosp-epis-stat-admi-proc-2012–13-tab.xlsx (accessed February 2015).
- Commissioning Guide 2013. Groin Hernia. London: Association of Surgeons of Great Britain and Ireland; 2013.
- Kingsnorth A, LeBlanc K. Hernias: inguinal and incisional. Lancet 2003;362:1561-71. http://dx.doi.org/10.1016/S0140-6736(03)14746-0.
- Burcharth J. The epidemiology and risk factors for recurrence after inguinal hernia surgery. Dan Med J 2014;61.
- Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am 2003;83:1045-51. http://dx.doi.org/10.1016/S0039-6109(03)00132-4.
- Sanders DL, Kurzer M. British Hernia Society Groin Hernia Guidelines. London: Association of Surgeons of Great Britain and Ireland; 2013.
- Simons MP, Aufenacker T, Bay-Nielsen M, Bouillot JL, Campanelli G, Conze J, et al. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia 2009;13:343-40. http://dx.doi.org/10.1007/s10029-009-0529-7.
- Grant AM. Open mesh versus non-mesh repair of groin hernia: meta-analysis of randomised trials based on individual patient data [corrected]. Hernia 2002;6:130-6. http://dx.doi.org/10.1007/s10029-002-0073-1.
- Scott NW, McCormack K, Go PMNY, Ross SJ, Grant AM. EU Hernia Trialists Collaboration . Open mesh versus non-mesh for groin hernia repair. Cochrane Database System Rev 2001;3.
- Butler RE, Burke R, Schneider JJ, Brar H, Lucha PA. The economic impact of laparoscopic inguinal hernia repair: results of a double-blinded, prospective, randomized trial. Surg Endosc 2007;21:387-90. http://dx.doi.org/10.1007/s00464-006-9123-6.
- Koning GG, Koole D, de Jongh MA, de Schipper JP, Verhofstad MH, Oostvogel HJ, et al. The transinguinal preperitoneal hernia correction vs Lichtenstein’s technique; is TIPP top?. Hernia 2011;15:19-22. http://dx.doi.org/10.1007/s10029-010-0744-2.
- Kingsnorth A. Inguinal hernia – laparoscopic or open repair? The case for open repair. Ann R Coll Surg Engl 2005;87:59-60.
- McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al. Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9140.
- McCormack K, Scott NW, Go PM, Ross S, Grant AM. Laparoscopic techniques versus open techniques for inguinal hernia repair. Cochrane Database System Rev 2003;1.
- Treadwell J, Tipton K, Oyesanmi O, Sun F, Schoelles K. Surgical Options for Inguinal Hernia: Comparative Effectiveness Review No 70. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
- Jenkins JT, O’Dwyer PJ. Inguinal hernias. BMJ 2008;336:269-72. http://dx.doi.org/10.1136/bmj.39450.428275.AD.
- Kehlet H, Bay-Nielsen M, Danish Hernia Database Collaboration. Nationwide quality improvement of groin hernia repair from the Danish Hernia Database of 87,840 patients from 1998 to 2005. Hernia 2008;12:1-7. http://dx.doi.org/10.1007/s10029-007-0285-5.
- Li J, Ji Z, Cheng T. Comparison of open preperitoneal and Lichtenstein repair for inguinal hernia repair: a meta-analysis of randomized controlled trials. Am J Surg 2012;204:769-78. http://dx.doi.org/10.1016/j.amjsurg.2012.02.010.
- Sajid MS, Craciunas L, Singh KK, Sains P, Baig MK. Open transinguinal preperitoneal mesh repair of inguinal hernia: a targeted systematic review and meta-analysis of published randomized controlled trials. Gastroenterol Rep 2013;1:127-37. http://dx.doi.org/10.1093/gastro/got002.
- Willaert W, De BD, Rogiers X, Troisi R, Berrevoet F. Open preperitoneal techniques versus Lichtenstein repair for elective inguinal hernias. Cochrane Database System Rev 2012;7. http://dx.doi.org/10.1002/14651858.cd008034.pub2.
- LeBlanc KE, LeBlanc LL, LeBlanc KA. Inguinal hernias: diagnosis and management. Am Fam Physician 2013;87:844-8.
- van den Berg JC, de Valois JC, Go PM, Rosenbusch G. Detection of groin hernia with physical examination, ultrasound, and MRI compared with laparoscopic findings. Invest Radiol 1999;34:739-43. http://dx.doi.org/10.1097/00004424-199912000-00002.
- Miserez M, Alexandre JH, Campanelli G, Corcione F, Cuccurullo D, Pascual MH, et al. The European Hernia Society groin hernia classification: simple and easy to remember. Hernia 2007;11:113-16. http://dx.doi.org/10.1007/s10029-007-0198-3.
- Ruhl CE, Everhart JE. Risk factors for inguinal hernia among adults in the US population. Am J Epidemiol 2007;165:1154-61. http://dx.doi.org/10.1093/aje/kwm011.
- Primatesta P, Goldacre MJ. Inguinal hernia repair: incidence of elective and emergency surgery, readmission and mortality. Int J Epidemiol 1996;25:835-9. http://dx.doi.org/10.1093/ije/25.4.835.
- McIntosh A, Hutchinson A, Roberts A, Withers H. Evidence-based management of groin hernia in primary care – a systematic review. Fam Pract 2000;17:442-7. http://dx.doi.org/10.1093/fampra/17.5.442.
- Kark AE, Kurzer M. Groin hernias in women. Hernia 2008;12:267-70. http://dx.doi.org/10.1007/s10029-007-0330-4.
- Hair A, Duffy K, McLean J, Taylor S, Smith H, Walker A, et al. Groin hernia repair in Scotland. Br J Surg 2000;87:1722-6. http://dx.doi.org/10.1046/j.1365-2168.2000.01598.x.
- Gallegos NC, Dawson J, Jarvis M, Hobsley M. Risk of strangulation in groin hernias. Br J Surg 1991;78:1171-3. http://dx.doi.org/10.1002/bjs.1800781007.
- Franneby U, Sandblom G, Nordin P, Nyren O, Gunnarsson U. Risk factors for long-term pain after hernia surgery. Ann Surg 2006;244:212-19. http://dx.doi.org/10.1097/01.sla.0000218081.53940.01.
- Poobalan AS, Bruce J, Smith WC, King PM, Krukowski ZH, Chambers WA. A review of chronic pain after inguinal herniorrhaphy. Clin J Pain 2003;19:48-54. http://dx.doi.org/10.1097/00002508-200301000-00006.
- Kurzer M, Kark A, Selouk S, Belsham P. Open mesh repair of incisional hernia using a sublay technique: long-term follow-up. World J Surg 2008;32:31-6. http://dx.doi.org/10.1007/s00268-007-9118-z.
- Law NW, Trapnell JE. Does a truss benefit a patient with inguinal hernia?. BMJ 1992;304. http://dx.doi.org/10.1136/bmj.304.6834.1092.
- Laparoscopic Surgery for Inguinal Hernia Repair TA83. London: NICE; 2004.
- NICE Implementation Uptake Report: Laparoscopic Surgery for Inguinal Hernia Repair. London: NICE; 2010.
- Achelrod D, Stargardt T. Cost-utility analysis comparing heavy-weight and light-weight mesh in laparoscopic surgery for unilateral inguinal hernias. Appl Health Econ Health Policy 2014;12:151-63. http://dx.doi.org/10.1007/s40258-014-0082-0.
- Lichtenstein IL, Shulman AG. Ambulatory outpatient hernia surgery. Including a new concept, introducing tension-free repair. Int Surg 1986;71:1-4.
- Zhao G, Gao P, Ma B, Tian J, Yang K. Open mesh techniques for inguinal hernia repair: a meta-analysis of randomized controlled trials. Ann Surg 2009;250:35-42. http://dx.doi.org/10.1097/SLA.0b013e3181ad63cc.
- Sanjay P, Watt DG, Ogston SA, Alijani A, Windsor JA. Meta-analysis of Prolene Hernia System mesh versus Lichtenstein mesh in open inguinal hernia repair. Surg 2012;10:283-9. http://dx.doi.org/10.1016/j.surge.2012.06.001.
- Amid PK. Groin hernia repair: open techniques. World J Surg 2005;29:1046-51. http://dx.doi.org/10.1007/s00268-005-7967-x.
- Kugel RD. Minimally invasive, nonlaparoscopic, preperitoneal, and sutureless, inguinal herniorrhaphy. Am J Surg 1999;178:298-302. http://dx.doi.org/10.1016/S0002-9610(99)00181-6.
- Koning GG, de VJ, Borm GF, Koeslag L, Vriens PW, van Laarhoven CJ. Health status one year after transinguinal preperitoneal inguinal hernia repair and Lichtenstein’s method: an analysis alongside a randomized clinical study. Hernia 2013;17:299-306. http://dx.doi.org/10.1007/s10029-012-0963-9.
- Pelissier EP. Inguinal hernia: preperitoneal placement of a memory-ring patch by anterior approach. Preliminary experience. Hernia 2006;10:248-52. http://dx.doi.org/10.1007/s10029-006-0079-1.
- Schumpelick V, Conze J, Klinge U. Preperitoneal mesh-plasty in incisional hernia repair. A comparative retrospective study of 272 operated incisional hernias. Chirurg 1996;67:1028-35. http://dx.doi.org/10.1007/s001040050099.
- Ger R. Laparoscopic hernia surgery: from birth to adolescence. Hernia 2003;7:110-13. http://dx.doi.org/10.1007/s10029-003-0140-2.
- Stevenson AD, Nixon SJ, Paterson-Brown S. Variation of laparoscopic hernia repair in Scotland: a postcode lottery?. Surg 2010;8:140-3. http://dx.doi.org/10.1016/j.surge.2009.11.001.
- Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care. York: Centre for Reviews and Dissemination; 2009.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Oxford: The Cochrane Collaboration; 2011.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123-30.
- Berrevoet F. Minimally Invasive Open Preperitoneal Surgery With Polysoft Mesh Versus a Classic Open Surgery With Light Weight Mesh n.d. https://clinicaltrials.gov/ct2/show/NCT00323674?term=NCT00323674&rank=1 (accessed February 2015).
- Arslan K, Erenoglu B, Turan E, Koksal H, Dogru O. Minimally invasive preperitoneal single-layer mesh repair versus standard Lichtenstein hernia repair for inguinal hernia: a prospective randomized trial. Hernia 2015;19:373-81. http://dx.doi.org/10.1007/s10029-014-1306-9.
- Dogru O, Girgin M, Bulbuller N, Cetinkaya Z, Aygen E, Camci C. Comparison of Kugel and Lichtenstein operations for inguinal hernia repair: results of a prospective randomized study. World J Surg 2006;30:346-50. http://dx.doi.org/10.1007/s00268-005-0408-z.
- Gunal O, Ozer S, Gurleyik E, Bahcebasi T. Does the approach to the groin make a difference in hernia repair?. Hernia 2007;11:429-34. http://dx.doi.org/10.1007/s10029-007-0252-1.
- Hamza Y, Gabr E, Hammadi H, Khalil R. Four-arm randomized trial comparing laparoscopic and open hernia repairs. Int J Surg 2010;8:25-8. http://dx.doi.org/10.1016/j.ijsu.2009.09.010.
- Koning GG, Keus F, Koeslag L, Cheung CL, Avci M, van Laarhoven CJ, et al. Randomized clinical trial of chronic pain after the transinguinal preperitoneal technique compared with Lichtenstein’s method for inguinal hernia repair. Br J Surg 2012;99:1365-73. http://dx.doi.org/10.1002/bjs.8862.
- Moghaddam JA, Mehrvarz S, Mohebbi HA, Panahie F. Comparison of ‘Read–Rives’ and ‘Lichtenstein’ repair for treatment of unilateral inguinal hernia. Koomesh 2011;13:57-61.
- Muldoon RL, Marchant K, Johnson DD, Yoder GG, Read RC, Hauer-Jensen M. Lichtenstein vs anterior preperitoneal prosthetic mesh placement in open inguinal hernia repair: a prospective, randomized trial. Hernia 2004;8:98-103. http://dx.doi.org/10.1007/s10029-003-0174-5.
- Nienhuijs S, Staal E, Keemers-Gels M, Rosman C, Strobbe L. Pain after open preperitoneal repair versus Lichtenstein repair: a randomized trial. World J Surg 2007;31:1751-7. http://dx.doi.org/10.1007/s00268-007-9090-7.
- Ray R, Kar M, Mukhopadhyay M. Transinguinal preperitoneal technique of inguinal hernioplasty – a better alternative to lichtenstein procedure. J Clin Diag Res 2014;8:NC01-3. http://dx.doi.org/10.7860/jcdr/2014/7632.4320.
- Smolinski-Kurek RL, Gonzalez JL, Hernandez-Gonzalez MA, Meza SS. Comparison of a dome-shaped elyptical mesh (DSEM) technique with the Lichtenstein technique to diminish post-surgical pain in open hernioplasty. Preliminary results. Cirujano Gen 2012;34:9-17.
- Staal E, Nienhuijs SW, Keemers-Gels ME, Rosman C, Strobbe LJ. The impact of pain on daily activities following open mesh inguinal hernia repair. Hernia 2008;12:153-7. http://dx.doi.org/10.1007/s10029-007-0297-1.
- Vatansev C, Belviranli M, Aksoy F, Tuncer S, Sahin M, Karahan O. The effects of different hernia repair methods on postoperative pain medication and CRP levels. Surg Laparosc Endosc Percutan Tech 2002;12:243-6. http://dx.doi.org/10.1097/00129689-200208000-00008.
- Smolinski-Kurek RL. Comparison Between Elliptical Domed Mesh Technique and Lichtenstein Technique in Inguinal Hernia Clinical Trials 2011. https://clinicaltrials.gov/ct2/show/NCT01348477 (accessed February 2015).
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Koning GG, de Schipper HJ, Oostvogel HJ, Verhofstad MH, Gerritsen PG, van Laarhoven KC, et al. The Tilburg double blind randomised controlled trial comparing inguinal hernia repair according to Lichtenstein and the transinguinal preperitoneal technique. Trials 2009;10. http://dx.doi.org/10.1186/1745-6215-10-89.
- Koning GG, Adang EM, Stalmeier PF, Keus F, Vriens PW, van Laarhoven CJ. TIPP and Lichtenstein modalities for inguinal hernia repair: a cost minimisation analysis alongside a randomised trial. Eur J Health Econ 2013;14:1027-34. http://dx.doi.org/10.1007/s10198-012-0453-0.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. http://dx.doi.org/10.1016/S0167-6296(01)00130-8.
- Merskey H, Bogduk N. Task Force on Taxonomy of the IASP. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions Of Pain Terms. Seattle, WA: IASP Press; 1994.
- Vale L, Grant A, McCormack K, Scott NW. EU Hernia Trialists Collaboration . Cost-effectiveness of alternative methods of surgical repair of inguinal hernia. Int J Technol Assess Health Care 2004;20:192-200. http://dx.doi.org/10.1017/S0266462304000972.
- UK Office for National Statistics . Mortality Statistics: Deaths Registered in England and Wales (Series DR) 2011. www.ons.gov.uk/ons/taxonomy/index.html?nscl=Mortality+Rates (accessed February 2015).
- Grant AM, Scott NW, O’Dwyer PJ. MRC Laparoscopic Groin Hernia Trial Group . Five-year follow-up of a randomized trial to assess pain and numbness after laparoscopic or open repair of groin hernia. Br J Surg 2004;91:1570-4. http://dx.doi.org/10.1002/bjs.4799.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D CHE Discussion Paper 172. University of York: Centre for Health Economics; 1999.
- Ara R, Wailoo A. NICE DSU Technical Support Document 12: The Use of Health State Utility Values in Decision Models 2011. www.nicedsu.org.uk/TSD12%20Utilities%20in%20modelling%20FINAL.pdf (accessed February 2015).
- NHS Reference Costs 2013–14. London: DoH; 2015.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Medicines Complete . British National Formulary 2015. www.medicinescomplete.com/mc/bnf/current/index.htm (accessed February 2015).
- Lau H, Patil NG, Yuen WK. Day-case endoscopic totally extraperitoneal inguinal hernioplasty versus open Lichtenstein hernioplasty for unilateral primary inguinal hernia in males: a randomized trial. Surg Endosc 2006;20:76-81. http://dx.doi.org/10.1007/s00464-005-0203-9.
- Aroori S, Spence RA. Chronic pain after hernia surgery – an informed consent issue. Ulster Med J 2007;76:136-40.
- Martin CW. Treatment of chronic pain post inguinal hernia repair. Vancouver, BC: WorkSafeBC; 2012.
- Health and Social Care Information Service . Finalised Patient Reported Outcome Measures (PROMs) in England – April 2012 to March 2013 2014. www.hscic.gov.uk/article/2021/Website-Search?productid=15139&q=Finalised+Patient+Reported+Outcome+Measures+(PROMs)+in+England+%e2%80%93+April+2012+to+March+2013+%e2%80%93+August+2014+Release&sort=Relevance&size=10&page=1&area=both#top (accessed February 2015).
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Bay-Nielsen M, Perkins FM, Kehlet H, Danish HD. Pain and functional impairment 1 year after inguinal herniorrhaphy: a nationwide questionnaire study. Ann Surg 2001;233:1-7. http://dx.doi.org/10.1097/00000658-200101000-00001.
- Olmi S, Erba L, Magnone S, Bertolini A, Mastropasqua E, Perego P, et al. Prospective study of laparoscopic treatment of incisional hernia by means of the use of composite mesh: indications, complications, mesh fixation materials and results. Chir Ital 2005;57:709-16.
- Gilbert AI, Young J, Graham MF, Divilio LT, Patel B. Combined anterior and posterior inguinal hernia repair: intermediate recurrence rates with three groups of surgeons. Hernia 2004;8:203-7. http://dx.doi.org/10.1007/s10029-004-0238-1.
- ISD Scotland . Hospital Care Data Tables 2014. www.isdscotland.org/Health-Topics/Hospital-Care/Publications/data-tables.asp?id=1343#1343 (accessed February 2015).
- UK Office for National Statistics . EARN01 Average Weekly Earnings 2015. www.ons.gov.uk/ons/rel/lms/labour-market-statistics/february-2015/table-earn01.xls (accessed February 2015).
Appendix 1 Derivation of the cost of surgery used in the economic model
| Currency code | Currency description | Service code | Service description | FCEs | Weight | National average unit cost (£) | Weighted by activity national average unit cost (£) |
|---|---|---|---|---|---|---|---|
| FZ18G | Inguinal, umbilical or femoral hernia procedures, 19 years and over with CC score of > 6 | 100 | General surgery | 115 | 0.007 | 3391 | 24.09 |
| FZ18H | Inguinal, umbilical or femoral hernia procedures, 19 years and over with CC score of 3–5 | 100 | General surgery | 1393 | 0.086 | 2299 | 197.80 |
| FZ18J | Inguinal, umbilical or femoral hernia procedures, 19 years and over with CC score of 1–2 | 100 | General surgery | 5329 | 0.329 | 2105 | 6192.83 |
| FZ18K | Inguinal, umbilical or femoral hernia procedures, 19 years and over with CC score of 0 | 100 | General surgery | 9354 | 0.578 | 1949 | 1125.99 |
| Total | 16,191 | 1 | 2040.70 |
| Currency code | Currency description | Service code | Service description | FCEs | Weight | National average unit cost (£) | Weighted by activity national average unit cost (£) |
|---|---|---|---|---|---|---|---|
| FZ18G | Inguinal, umbilical or femoral hernia procedures, ≥ 19 years with CC score of > 6 | 100 | General surgery | 18 | 0.0005 | 1500 | 0.70 |
| FZ18H | Inguinal, umbilical or femoral hernia procedures, ≥ 19 with CC score of 3–5 | 100 | General surgery | 596 | 0.0156 | 1464 | 22.78 |
| FZ18J | Inguinal, umbilical or femoral hernia procedures, ≥ 19 with CC score of 1–2 | 100 | General surgery | 5246 | 0.1369 | 1503 | 205.82 |
| FZ18K | Inguinal, umbilical or femoral hernia procedures, ≥ 19 years with CC score of 0 | 100 | General surgery | 32,448 | 0.8470 | 1466 | 1241.75 |
| Total | 38,308 | 1 | 1471.05 |
| Procedures | n | Weight (%) | Average cost (£) | Lower quartile cost (£) | Upper quartile cost (£) |
|---|---|---|---|---|---|
| Elective inpatients | 16,191 | 29.71 | 2040.70 | 1652.53 | 2285.55 |
| Day-case procedure | 38,308 | 70.29 | 1471.05 | 1189.39 | 1635.92 |
| Total procedures | 54,499 | 100 | |||
| Total cost for model | 1640.29 | 1326.98 | 1828.91 |
Appendix 2 Literature search strategies
Open mesh repairs for inguinal hernia: clinical effectiveness
EMBASE Classic and EMBASE
Date range searched: from 1947 to 2014, week 44.
Ovid MEDLINE
Date range searched: from 1946 to October 2014, week 4.
Ovid MEDLINE In-Process & Other Non-Indexed Citations
Date range searched: from 31 October 2014.
Ovid multifile search. URL: https://shibboleth.ovid.com/
Date of search: 31 October 2014.
Search strategy
-
hernia,inguinal/su use mesz
-
inguinal hernia/su use emcz
-
hernia, inguinal/ use mesz
-
inguinal hernia/ use emcz
-
(inguinal or groin).tw.
-
hernioplast$.tw.
-
herniorrhaph$.tw.
-
herniorrhaphy/
-
(hernia adj3 repair$).tw
-
(3 or 4 or 5) and (6 or 7 or 8 or 9)
-
1 or 2 or 10
-
lichtenstein.tw.
-
(kugel or stoppa or nyhus or read-rives).tw.
-
(open adj3 mesh).tw.
-
(pre -peritoneal or preperitoneal).tw.
-
or/12-15
-
exp clinical trial/ use emcz
-
randomized controlled trial.pt.
-
controlled clinical trial.pt
-
randomization/ use emcz
-
randomi?ed.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
or/17-26
-
exp animals/ not humans/
-
nonhuman/ not human/
-
27 not (28 or 29)
-
11 and 16 and 30
-
remove duplicates from 31
Science Citation Index
Date range searched: from 1980 to 1 November 2014.
Bioscience Information Service (BIOSIS)
Date range searched: from 1980 to 1 November 2014.
ISI Web of Knowledge
Date of search: 1 November 2014.
Search strategy
# 1 TS=(inguinal NEAR/3 hernia*)
# 2 TS=(groin NEAR/3 hernia*)
# 3 # 4 TS=(hernia NEAR/3 repair*)
# 5 (#1 OR #2) AND (#3 OR #4)
# 6 TS=(Lichtenstein OR kugel OR stoppa OR nyhus OR read-rives)
# 7 TS=(open NEAR/3 mesh)
# 8 TS=(pre-peritoneal or preperitoneal)
# 9 #5 AND (#6 OR #7 OR #8)
# 10 TS=randomized
# 11 TS=randomised
# 12 TS=randomly
# 13 TS=trial*
# 14 #9 AND (#10 or #11 OR #12 OR #13)
TS=(hernioplast* OR herniorrhaph* )
Scopus
Date range searched: 31 October 2014.
Date of search: 31 October 2014.
Search strategy
#1 inguinal or groin
#2 Lichtenstein OR kugel OR stoppa OR nyhus OR read-rives)
#3 open mesh
#4 (pre-peritoneal or preperitoneal)
#5 #1 and (#2 or #3 or #4) [restricted to articles ahead of print]
The Cochrane Library [Cochrane Central Register of Controlled Trials (Issue 10 2014), Cochrane Database of Systematic Reviews (Issue 11, 2014)]
URL: www3.interscience.wiley.com/
Date of search: 31 October 2014.
Search strategy
#1MeSH descriptor: [Hernia, Inguinal] this term only
#2 (inguinal or groin) (Word variations have been searched)
#3 MeSH descriptor: [Herniorrhaphy] this term only
#4 hernioplast* (Word variations have been searched)
#5 herniorrhaph* (Word variations have been searched)
#6 (hernia near/3 repair*) (Word variations have been searched)
#7 (#1 or #2) and (#3 or #4 or #5 or #6)
#8 MeSH descriptor: [Hernia, Inguinal] explode all trees and with qualifier(s): [Surgery - SU] #9 #7 or #8
#10 “Lichtenstein” (Word variations have been searched)
#11 (kugel or stoppa or nyhus or read-rives) (Word variations have been searched)
#12 open near/3 mesh (Word variations have been searched)
#13 (pre-peritoneal or preperitoneal) (Word variations have been searched)
#14#10 or #11 or #12 or #13
#15#9 and #14
HTA database/Database of Abstracts of Reviews of Effects
Centre for Reviews and Dissemination
URL: http://nhscrd.york.ac.uk/welcome.htm
Date of search: 1 November 2014.
Search strategy
-
MeSH DESCRIPTOR Hernia, Inguinal WITH QUALIFIER SU
-
MeSH DESCRIPTOR Hernia, Inguinal EXPLODE ALL TREES
-
(inguinal or groin)
-
MeSH DESCRIPTOR Herniorrhaphy
-
(hernioplast*) OR (herniorrhaph*) OR ((hernia repair*))
-
#4 OR #5
-
#2 OR #3
-
#6 AND #7
-
#1 OR #8
-
0 (lichtenstein or kugel) OR (stoppa or nyhus or read-rives) OR (preperitoneal or preperitoneal)
-
1 (open mesh)
-
2 #10 OR #11
-
3 #9 AND #12
ClinicalTrials.gov
URL: http://clinicaltrials.gov/ct/gui/c/r
Date of search: 1 November 2014.
Search strategy
Condition=hernia, inguinal
Interventions=lichtenstein
International Clinical Trials Registry Platform (ICTRP)
World Health Organization
Date of search: 1 November 2014.
Search strategy
Condition=inguinal hernia
Intervention=lichtenstein
Open mesh repairs for inguinal hernia: economic evaluations
Ovid multifile search. URL: https://shibboleth.ovid.com/
EMBASE Classic and EMBASE
Date range searched: from 1947 to 2014, week 44.
Ovid MEDLINE
Date range searched: from 1946 to October, 2014 week 4.
Ovid MEDLINE In-Process & Other Non-Indexed Citations
Date range searched: from 31 October 2014.
Date of search: 31 October 2014.
Search strategy
-
hernia,inguinal/su use mesz
-
inguinal hernia/su use emcz
-
hernia, inguinal/ use mesz
-
inguinal hernia/ use emcz
-
(inguinal or groin).tw.
-
hernioplast$.tw.
-
herniorrhaph$.tw.
-
herniorrhaphy/
-
(hernia adj3 repair$).tw
-
(3 or 4 or 5) and (6 or 7 or 8 or 9)
-
1 or 2 or 10
-
lichtenstein.tw
-
(kugel or stoppa or nyhus or read-rives).tw
-
(open adj3 mesh).tw
-
(pre -peritoneal or preperitoneal).tw
-
or/12-15
-
10 and 16
-
exp “costs and cost analysis”/ use mesz
-
exp economic evaluation/ use emcz
-
economics/
-
health economics/ use emcz
-
exp economics,hospital/ use mesz
-
exp economics,medical/ use mesz
-
economics,pharmaceutical/ use mesz
-
exp budgets/
-
exp models, economic/ use mesz
-
exp decision theory/
-
monte carlo method/
-
markov chains/
-
exp technology assessment, biomedical/
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab
-
economics model$.tw.
-
(economic$ or pharmacoeconomic$).tw
-
(price or prices or pricing).tw
-
(value adj1 money).tw
-
markov$.tw
-
monte carlo.tw.
-
(decision$ adj2 (tree? or analy$ or model$)).tw
-
or/18-39
-
17 and 40
-
remove duplicates from 41)
NHS Economic Evaluations Database/HTA database
Centre for Reviews and Dissemination
URL: http://nhscrd.york.ac.uk/welcome.htm
Date of search: 1 November 2014.
Search strategy
-
MeSH DESCRIPTOR Hernia, Inguinal WITH QUALIFIER SU
-
MeSH DESCRIPTOR Hernia, Inguinal EXPLODE ALL TREES
-
(inguinal or groin)
-
MeSH DESCRIPTOR Herniorrhaphy
-
(hernioplast*) OR (herniorrhaph*) OR ((hernia repair*))
-
#4 OR #5
-
#2 OR #3
-
#6 AND #7
-
#1 OR #8
-
(lichtenstein or kugel) OR (stoppa or nyhus or read-rives) OR (preperitoneal or preperitoneal)
-
(open mesh)
-
#10 OR #11
-
#9 AND #12
Ideas
Research Papers in Economics (RePEc)
Date of search: 1 November 2014.
Search strategy
Inginual hernia or groin hernia.
Open mesh repairs for inguinal hernia: quality of life and utilities
Ovid multifile search. URL: https://shibboleth.ovid.com/
EMBASE Classic and EMBASE
Date range searched: from 1947 to 2014, week 44.
Ovid MEDLINE
Date range searched: 1946 to October 2014, week 4.
Ovid MEDLINE In-Process & Other Non-Indexed Citations
Date range searched: from 31 October 2014.
Date of search: 31 October 2014.
Search strategy
-
hernia,inguinal/su use mesz
-
inguinal hernia/su use emcz
-
hernia, inguinal/ use mesz
-
inguinal hernia/ use emcz
-
(inguinal or groin).tw.
-
hernioplast$.tw
-
herniorrhaph$.tw.
-
herniorrhaphy/
-
(hernia adj3 repair$).tw.
-
(3 or 4 or 5) and (6 or 7 or 8 or 9)
-
1 or 2 or 10
-
lichtenstein.tw.
-
(kugel or stoppa or nyhus or read-rives).tw.
-
(open adj3 mesh).tw.
-
(pre -peritoneal or preperitoneal).tw.
-
or/12-15
-
10 and 16
-
quality of life/
-
quality adjusted life year/
-
“Value of Life”/ use mesz
-
health status indicators/ use mesz
-
health status/ use emcz
-
sickness impact profile/ use mesz
-
disability evaluation/ use mesz
-
disability/ use emcz
-
activities of daily living/ use mesz
-
exp daily life activity/ use emcz
-
cost utility analysis/ use emcz
-
rating scale/
-
questionnaires/
-
(quality adj1 life).tw.
-
quality adjusted life.tw.
-
disability adjusted life.tw.
-
(qaly? or qald? or qale? or qtime? or daly?).tw.
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(hql or hqol or h qol or hrqol or hr qol).tw.
-
health$ year$ equivalent$.tw.
-
(hui or hui1 or hui2 or hui3).tw.
-
(health adj3 (utilit$ or disutili$)).tw
-
(health adj3 (state or status)).tw.
-
(sf36 or sf 36 or short form 36 or shortform 36).tw.
-
(sf6 or sf 6 or short form 6 or shortform 6).tw.
-
(sf12 or sf 12 or short form 12 or shortform 12).tw.
-
(sf16 or sf 16 or short form 16 or shortform 16).tw
-
(sf20 or sf 20 or short form 20 or shortform 20).tw.
-
willingness to pay.tw.
-
standard gamble.tw.
-
trade off.tw.
-
conjoint analys?s.tw.
-
discrete choice.tw.
-
or/18-52
-
(case report or editorial or letter).pt.
-
case report/
-
53 not (54 or 55)
-
17 and 56
-
remove duplicates from 57
Science Citation Index
Date range searched: from 1995 to 3 November 2014.
ISI Web of Knowledge
Date of search: 3 November 2014.
Search strategy
# 1 TS=(inguinal NEAR/3 hernia*)
# 2 TS=(groin NEAR/3 hernia*)
# 3 TS=(hernioplast* OR herniorrhaph* )
# 4 TS=(hernia NEAR/3 repair*)
# 5 (#1 OR #2) AND (#3 OR #4)
# 6 TS=(Lichtenstein OR kugel OR stoppa OR nyhus OR read-rives)
# 7 TS=(open NEAR/3 mesh)
# 8 TS=(pre-peritoneal or preperitoneal)
# 9 #5 AND (#6 OR #7 OR #8)
#10 TS=quality of life
#11 TS=(utility or utilities)
#12 TS=quality adjusted life year*
#13 TS=disutilit*
#14 TS= (sf36 or sf 36 or short form 36 or shortform 36)
# 15 TS=(euroqol or euro qol or eq5d or eq 5d)
#16 TS=discrete choice.
#17 TS=conjoint analys*
#18 TS=trade off.
#19 TS=standard gamble
#20 TS=willingness to pay.
#21 #20 OR#19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10
#22 #21 AND #9
Cost-effectiveness Analysis Registry
URL: https://research.tufts-nemc.org/cear4/default.asp
Date of search: 3 November 2014.
Search strategy
Hernia.
Websites consulted
AHRQ. URL: www.ahrq.gov/
Association of Surgeons of Great Britain and Ireland. URL: www.asgbi.org.uk/
Australian Safety and Efficacy Register of New Interventional Procedures. URL: www.surgeons.org/for-health-professionals/audits-and-surgical-research/asernip-s
Belgian Health Care Knowledge Centre. URL: https://kce.fgov.be/
British Hernia Society. URL: www.britishherniasociety.org/
Canadian Agency for Drugs and Technologies in Health. URL: www.cadth.ca/
European Hernia Society. URL: www.europeanherniasociety.eu/home.html
French National Authority for Health. URL: www.has-sante.fr/
Health Information & Quality Authority. URL: www.hiqa.ie/
Institute for Clinical and Economic Review. URL: www.icer-review.org/
Institute for Quality and Efficiency in Health Care. URL: www.iqwig.de/
Medical Services Advisory Committee, Australia. URL: www.msac.gov.au/
National Institute for Health and Care Excellence. URL: www.nice.org.uk/
NHS Quality Improvement Scotland. URL: www.healthcareimprovementscotland.org/
Appendix 3 Data extraction
Appendix 4 Cochrane’s tool for assessing risk of bias
| Domain | Support for judgement | Review authors’ judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether or not it should produce comparable groups | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
| Performance bias | ||
| Blinding of participants and personnel. Assessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether or not the intended blinding was effective | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
| Detection bias | ||
| Blinding of outcome assessment. Assessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether or not the intended blinding was effective | Detection bias due to knowledge of the allocated interventions by outcome assessors |
| Attrition bias | ||
| Incomplete outcome data. Assessments should be made for each main outcome (or class of outcomes) | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether or not attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions where reported and any re-inclusions in analyses performed by the review authors | Attrition bias due to amount, nature or handling of incomplete outcome data |
| Reporting bias | ||
| Selective reporting | State how the possibility of selective outcome reporting was examined by the review authors, and what was found | Reporting bias due to selective outcome reporting |
| Other bias | ||
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were prespecified in the review’s protocol, responses should be provided for each question/entry | Bias due to problems not covered elsewhere in the table |
Appendix 5 Included primary studies, relevant ongoing studies and systematic reviews
List of included studies
Arslan 2014
Arslan K, Erenoglu B, Turan E, Koksal H, Dogru O. Minimally invasive preperitoneal single-layer mesh repair versus standard Lichtenstein hernia repair for inguinal hernia: a prospective randomized trial. Hernia 2015;19:373–81.
Berrevoet (unpublished ongoing study)
Minimally invasive open preperitoneal surgery with polysoft mesh versus a classic open surgery with light weight mesh. Clinical trails.gov identifier NCT00323674.
Dogru 2006
Dogru O, Girgin M, Bulbuller N, Cetinkaya Z, Aygen E, Camci C. Comparison of Kugel and Lichtenstein operations for inguinal hernia repair: results of a prospective randomized study. World J Surg 2006;30:346–50.
Gunal 2007
Gunal O, Ozer S, Gurleyik E, Bahcebasi T. Does the approach to the groin make a difference in hernia repair? Hernia 2007;11:429–34.
Hamza 2010
Hamza Y, Gabr E, Hammadi H, Khalil R. Four-arm randomized trial comparing laparoscopic and open hernia repairs. Int J Surg 2010;8:25–8.
Koning 2012
Koning GG, Keus F, Koeslag L, Cheung CL, Avci M, van Laarhoven CJ, et al. Randomized clinical trial of chronic pain after the transinguinal preperitoneal technique compared with Lichtenstein’s method for inguinal hernia repair. Br J Surg 2012;99:1365–73.
Koning 2013 (secondary report)
Koning GG, de Vries J, Borm GF, Koeslag L, Vriens PW, van Laarhoven CJ. Health status one year after Transinguinal preperitoneal inguinal hernia repair and Lichtenstein’s method: an analysis alongside a randomized clinical study. Hernia 2013;17:299–306.
Moghaddam 2011
Moghaddam JA, Mehrvarz S, Mohebbi HA, Panahie F. Comparison of Read–Rives and Lichtenstein’ repair for treatment of unilateral inguinal hernia. Koomesh 2011;13:57–61.
Muldoon 2004
Muldoon RL, Marchant K, Johnson DD, Yoder GG, Read RC, Hauer-Jensen M. Lichtenstein vs anterior preperitoneal prosthetic mesh placement in open inguinal hernia repair: a prospective, randomized trial. Hernia 2004;8:98–103.
Nienhuijs 2007
Nienhuijs S, Staal E, Keemers-Gels M, Rosman C, Strobbe L. Pain after open preperitoneal repair versus Lichtenstein repair: a randomized trial. World J Surg 2007;31:1751–7.
Staal 2008 (secondary report)
Staal E, Nienhuijs SW, Keemers-Gels ME, Rosman C, Strobbe LJ. The impact of pain on daily activities following open mesh inguinal hernia repair. Hernia 2008;12:153–7.
Ray 2014
Ray R, Kar M, Mukhopadhyay M. Transinguinal preperitoneal technique of inguinal hernioplasty – a better alternative to Lichtenstein procedure. J Clin Diag Res 2014;8:NC01–3.
Smolinski-Kurek 2012
Smolinski-Kurek RL, Gonzalez JL, Hernandez-Gonzalez MA, Meza SS. Comparison of a dome-shaped elliptical mesh (DSEM) technique with the Lichtenstein technique to diminish post-surgical pain in open hernioplasty. Preliminary results. Cir Gen 2012;34:9–17.
Vatansev 2002
Vatansev C, Belviranli M, Aksoy F, Tuncer S, Sahin M, Karahan O. The effects of different hernia repair methods on postoperative pain medication and CRP levels. Surg Laparosc Endosc Percutan Tech 2002;12:243–6.
Included systematic reviews
Li 2012
Li J, Ji Z, Cheng T. Comparison of open preperitoneal and Lichtenstein repair for inguinal hernia repair: a meta-analysis of randomized controlled trials. Am J Surg 2012;204:769–78.
Sajid 2013
Sajid MS, Craciunas L, Singh KK, Sains P, Baig MK. Open transinguinal preperitoneal mesh repair of inguinal hernia: a targeted systematic review and meta-analysis of published randomized controlled trials. Gastroenterol Rep 2013;1:127–37.
Willaert 2012
Willaert W, De Bacquer D, Rogiers X, Troisi R, Berrevoet F. Open preperitoneal techniques versus Lichtenstein repair for elective inguinal hernias. Cochrane Database Syst Rev 2012;7:CD008034.
Appendix 6 List of excluded studies with rationale
Non-randomised controlled trial (n = 45)
Aasvang E, Kehlet H. Chronic postoperative pain: the case of inguinal herniorrhaphy. Br J Anaesth 2005;95:69–76.
Aldridge AJ, Nehra D. Mesh compared with non-mesh methods of open groin hernia repair: systematic review of randomized controlled trials and Laparoscopic compared with open methods of groin hernia repair: systematic review of randomized controlled trials. Br J Surg 2001;88:471.
Almanza JM, Yazde Y, Almanza AA. [Surgical treatment of hernias and eventrations using the open tension-free mesh path.] Prensa Med Argent 2001;88:578–84.
Andresen K, Burcharth J, Rosenberg J. Lichtenstein versus Onstep for inguinal hernia repair: protocol for a double-blinded Lichtenstein trial. Dan Med J 2013;60:A4729.
Beets GL. Randomized clinical trial on chronic pain after the transinguinal preperitoneal technique compared with Lichtenstein’s method for inguinal hernia repair. Br J Surg 2012;99:1365–73.
Cheek CM, Black NA, Devlin HB, Kingsnorth AN, Taylor RS, Watkin DF. Groin hernia surgery: a systematic review. Ann R Coll Surg Engl 1998;80:S1–80.
da Costa PM. Complications and recurrences after different types of hernia repair: how to deal with it? Acta Chir Belg 2009;109:36–41.
Dasari B, Grant L, Irwin T. Immediate and long-term outcomes of Lichtenstein and Kugel patch operations for inguinal hernia repair. Ulster Med J 2009;78:115–18.
De Jonge PVH, Lloyd A, Horsfall L, Tan R, O’Dwyer PJ. The measurement of chronic pain and health-related quality of life following inguinal hernia repair: a review of the literature. Hernia 2008;12:561–9.
Erhan Y, Erhan E, Aydede H, Mercan M, Tok D. Chronic pain after Lichtenstein and preperitoneal (posterior) hernia repair. Can J Surg 2008;51:383–7.
EU Hernia Trialist Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg 2002;235:322–32.
Ferzli GS, Edwards ED, Khoury GE. Chronic pain after inguinal herniorrhaphy. J Am Coll Surg 2007;205:333–41.
Franneby U, Sandblom G, Nyren O, Nordin P, Gunnarsson U. Self-reported adverse events after groin hernia repair, a study based on a national register. Value Health 2008;11:927–32.
Frisen A, Starck J, Smeds S, Nystrom PO, Kald A. Analysis of outcome of Lichtenstein groin hernia repair by surgeons-in-training versus a specialized surgeon. Hernia 2011;15:281–8.
George SM Jr., Mangiante EC, Voeller GR, Britt LG. Preperitoneal herniorrhaphy for the acutely incarcerated groin hernia. Am Surg 1991;57:139–41.
Janu PG, Sellers KD, Mangiante EC. Mesh inguinal herniorrhaphy: a ten-year review. Am Surg 1997;63:1065–9.
Kawji R, Feichter A, Fuchsjager N, Kux M. Postoperative pain and return to activity after five different types of inguinal herniorrhaphy. Hernia 1999;3:31–5.
Koning GG, Koole D, de Jongh MA, de Schipper JP, Verhofstad MH, Oostvogel HJ, et al. The transinguinal preperitoneal hernia correction vs Lichtenstein’s technique; is TIPP top? Hernia 2011;15:19–22.
Kovachev LS. Possibilities of preperitoneal approach methods in the treatment of groin hernias. Preperitoneal approach methods. Int Surg 1991;76:154–8.
Kurzer M, Belsham PA, Kark AE. The Lichtenstein repair. Surg Clin North Am 1998;78:1025–46.
Lange JF, Lange MM, Voropai DA, van Tilburg MW, Pierie JP, Ploeg RJ, et al. Trans rectus sheath extra-peritoneal procedure (TREPP) for inguinal hernia: the first 1,000 patients. World J Surg 2014;38:1922–8.
Lerut J, Foxius A, Collard A. Evaluation criteria of inguinal hernia repair. Acta Chir Belg 1998;98:127–31.
Loos MJA, Roumen RMH, Scheltinga MRM. Classifying postherniorrhaphy pain syndromes following elective inguinal hernia repair. World J Surg 2007;31:1760–7.
Lourenco A, da Costa RS. The ONSTEP inguinal hernia repair technique: initial clinical experience of 693 patients, in two institutions. Hernia 2013;17:357–64.
Lundstrom KJ, Sandblom G, Smedberg S, Nordin P. Risk factors for complications in groin hernia surgery a national register study. Ann Surg 2012;255:784–8.
Margoles JS, Braun RA. Preperitoneal versus classical hernioplasty. Am J Surg 1971;121:641–3.
Matthews RD, Neumayer L. Inguinal hernia in the 21st century: an evidence-based review. Curr Probl Surg 2008;441:261–321.
Mattioli F, Puglisi M, Priora F, Millo F, Bottaro P. [Treatment of inguinal hernia: a prospective study comparing Bassini’s procedure, the inguinal preperitoneal prosthesis and the Lichtenstein technique.] Chir Ital 2002;54:317–21.
Miserez M, Peeters E, Aufenacker T, Bouillot JL, Campanelli G, Conze J, et al. Update with level 1 studies of the European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia 2014;18:151–63.
Nienhuijs S, Staal E, Strobbe L, Rosman C, Groenewoud H, Bleichrodt R. Chronic pain after mesh repair of inguinal hernia: a systematic review. Am J Surg 2007;194:394–400.
Poobalan AS, Bruce J, Cairns W, Smith S, King PM, Krukowski ZH, et al. A review of chronic pain after inguinal herniorrhaphy. Clin J Pain 2003;19:48–54.
Prins MW, Voropai DA, van Laarhoven CJ, Akkersdijk WL. [The transrectus sheath preperitoneal procedure: a safe, effective and cheap surgical approach to inguinal hernia?] Ned Tijdschr Geneeskd 2013;157:A6048.
Prins MW, Koning GG, Keus EF, Vriens PW, Mollen RM, Akkersdijk WL, et al. Study protocol for a randomized controlled trial for anterior inguinal hernia repair: transrectus sheath preperitoneal mesh repair compared to transinguinal preperitoneal procedure. Trials 2013;14:65.
Quyn AJ, Weatherhead KM, Daniel T. Chronic pain after open inguinal hernia surgery: suture fixation versus self-adhesive mesh repair. Langenbecks Arch Surg 2012;397:1215–18.
Reinpold WMJ, Nehls J, Eggert A. Nerve management and chronic pain after open inguinal hernia repair: a prospective two phase study. Ann Surg 2011;254:163–8.
Richards SK, Earnshaw JJ. Management of primary and recurrent inguinal hernia by surgeons from the South West of England. Ann R Coll Surg Engl 2003;85:402–4.
Sanders DL, Waydia S. A systematic review of Lichtenstein control trials assessing mesh fixation in open inguinal hernia repair. Hernia 2014;18:165–76.
Schmedt CG, Sauerland S, Bittner R. Comparison of endoscopic procedures vs Lichtenstein and other open mesh techniques for inguinal hernia repair: a meta-analysis of randomized controlled trials. Surg Endosc 2005;19:188–99.
Simons MP, Aufenacker T, Bay-Nielsen M, Bouillot JL, Campanelli G, Conze J, et al. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia 2009;13:343–403.
Slater GH, Hopkins G, Bailey M. Mesh compared with non-mesh methods of open groin hernia repair: systematic review of randomized controlled trials and laparoscopic compared with open methods of groin hernia repair: systematic review of randomized controlled trials. Br J Surg 2001;88:470–1.
Slim K, Voyles CR. Meta-analysis of laparoscopic inguinal hernia repair favors open hernia repair with preperitoneal mesh prosthesis. Am J Surg 2003;185:1.
Tamme C, Kockerling F. Surgical treatment of primary inguinal hernias. Chirurg 2004;75:315–16.
Wijesuriya LI. Mesh compared with non-mesh methods of open groin hernia repair: systematic review of randomized controlled trials and laparoscopic compared with open methods of groin hernia repair: systematic review of randomized controlled trials. Br J Surg 2001;88:471.
Zhao G, Gao P, Ma B, Tian J, Yang K. Open mesh techniques for inguinal hernia repair: a meta-analysis of randomized controlled trials. Ann Surg 2009;250:35–42.
Zhou X. Comparison of the posterior approach and anterior approach for a Kugel repair of treatment of inguinal hernias. Surg Today 2013;43:403–7.
Participants (not primary inguinal hernia) (n = 1)
Karatepe O, Adas G, Battal M, Gulcicek OB, Polat Y, Altiok M, et al. The comparison of preperitoneal and Lichtenstein repair for incarcerated groin hernias: a randomised controlled trial. Int J Surg 2008;6:189–92.
Surgical procedure (not Lichtenstein vs. open preperitoneal) (n = 15)
Callesen T, Bech K, Andersen J, Nielsen R, Roikjaer O, Kehlet H. Pain after primary inguinal herniorrhaphy: influence of surgical technique. J Am Coll Surg 1999;188:355–9.
Coskun F, Ozmen MM, Moran M, Ozozan O. New technique for inguinal hernia repair. Hernia 2005;9:32–6.
Friis E, Lindahl F. The tension-free hernioplasty in a randomized trial. Am J Surg 1996;172:315–19.
Gondal SH, Anwer T, Bhatti AA. A comparative study between Lichenstein and sutureless inguinal mesh hernioplasty. Pak J Med Health Sci 2013;7:940–4.
Gonullu NN, Cubukcu A, Alponat A. Comparison of local and general anesthesia in tension-free (Lichtenstein) hernioplasty: a prospective randomized trial. Hernia 2002;6:29–32.
Kingsnorth AN, Porter CS, Bennett DH, Walker AJ, Hyland ME, Sodergren S. Lichtenstein patch or Perfix plug-and-patch in inguinal hernia: a prospective double-blind randomized controlled trial of short-term outcome. Surgery 2000;127:276–83.
Kingsnorth AN, Hyland ME, Porter CA, Sodergren S. Prospective double-blind randomized study comparing Perfix plug-and-patch with Lichtenstein patch in inguinal hernia repair: one year quality of life results. Hernia 2000;4:255–8.
Kingsnorth AN, Bennett D, Walker A, Porter C. Randomized double blind study to compare the short term outcome of the Lichtenstein operation with the Perfix plug in inguinal hernioplasty. Br J Surg 1999;86:20.
Langeveld HR, Klitsie P, Smedinga H, Eker H, Van’t Riet M, Weidema W, et al. Prognostic value of age for chronic postoperative inguinal pain. Surg Endosc Other Intervent Tech 2014;28:S44.
Lionetti R, Neola B, Dilillo S, Bruzzese D, Ferulano GP. Sutureless hernioplasty with light-weight mesh and fibrin glue versus Lichtenstein procedure: a comparison of outcomes focusing on chronic postoperative pain. Hernia 2012;16:127–31.
Payne JH, Grininger LM, Izawa M, Lindahl PJ, Podoll EF. A randomized prospective comparison between a laparoscopic, preperitoneal, and anterior ‘tension free’ repair of inguinal hernia with mesh, part I: early results abstract. Surg Laparosc Endosc 1994;4:471–2.
Pielacinski K, Szczepanik AB, Wroblewski T. Effect of mesh type, surgeon and selected patients’ characteristics on the treatment of inguinal hernia with the Lichtenstein technique. Randomized trial. Wideochirurg Tech Maloinwaz 2013;8:99–106.
Ripetti V, La Vaccara V, Greco S, Bono F, Valeri S, Coppola R. Randomised trial comparing Lichtenstein vs Trabucco vs Valenti techniques in inguinal hernia repair. Hernia 2014;18:205–12.
Sanders DL, Samarakoon DH, Ganshirt SW, Porter CS, Kingsnorth AN. A two-centre blinded randomised control study comparing the Lichtenstein patch, Perfix plug and ProLoop plug in the repair of primary inguinal hernia. Hernia 2009;13:499–503.
Sezer HK, Celikkol G. Lichtenstein versus Lichtenstein plus plug in open hernia repair. Eur Surg Res 2014;52:204.
Foreign-language paper: unobtainable (n = 5)
Mitura K, Romanczuk M. [Comparison between two methods of inguinal hernia surgery – Lichtenstein and Desarda.] Pol Merkuriusz Lek 2008;24:392–5.
Slim K, Flament B, Begin GF. [Which surgical procedure to choose for inguinal hernia repair?] Ann Chir 2003;128:323–5.
Weber G, Csontos Z, Horvath OP. [Hernia surgery in Hungary today – effect of the Lichtenstein study.] Magyar Sebeszet 2006;59:405–10.
Wojcik B, Majewski WD. [Does inguinal hernia repair influence on quality of life of elderly males?] Ann Acad Med Stetin 2007;53:74–81.
Zhou J-P, Liu Q. [Tension-free hernioplasty for groin hernia in adult: a meta-analysis.] Chin J Evid Based Med 2005;5:310–13.
Appendix 7 Detailed risk-of-bias assessment results
| Risk-of-bias domain | Arslan et al. 201552 | Berrevoet51 | Dogru et al. 200653 | Gunal et al. 200754 | Hamza et al. 201055 | Koning et al. 201256 | Moghaddam et al. 201157 | Muldoon et al. 200458 | Nienhuijs et al. 200759 | Ray et al. 201460 | Smolinski-Kurek et al. 201261 | Vatansev et al. 200263 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Random sequence generation | Low | Low | High | Unclear | Unclear | Low | Unclear | Low | Low | Unclear | Low | Unclear |
| Allocation concealment | Low | Low | High | Unclear | Unclear | Low | Unclear | Low | High | Unclear | Unclear | Unclear |
| Blinding of participants | Low | High | Unclear | Unclear | Low | Low | Unclear | Unclear | Low | Unclear | Low | Unclear |
| Blinding of outcome assessment | Low | High | Unclear | Unclear | Low | Low | Low | Unclear | Low | Unclear | Low | Unclear |
| Chronic pain | Low | High | Not studied | Not studied | Not studied | Low | Not studied | Unclear | Low | Unclear | Low | Not studied |
| Acute pain | Not studied | High | Not studied | Unclear | Low | Low | Low | Not studied | Low | Unclear | Low | Unclear |
| Numbness | Not studied | Not studied | Not studied | Not studied | Not studied | Low | Not studied | Unclear | Low | Not studied | Low | Not studied |
| Recurrence | Low | High | Unclear | Unclear | Low | Low | Low | Unclear | Low | Unclear | Low | Not studied |
| Mortality | Low | Not studied | Unclear | Not studied | Not studied | Low | Not studied | Unclear | Low | Unclear | NR | Not studied |
| Complications | Low | High | Unclear | Unclear | Low | Low | Low | Unclear | Low | Unclear | Low | Not studied |
| Time to return to normal activities | Low | Not studied | Not studied | Not studied | Low | Low | Low | Not studied | Not studied | Unclear | Not studied | Not studied |
| Hospital stay | Low | Not studied | Not studied | Not studied | Low | Low | Unclear | Not studied | Not studied | Unclear | Not studied | Not studied |
| Incomplete outcome data | High | Low | Low | Unclear | Unclear | Low | Low | Low | Low | Low | Low | Unclear |
| Chronic pain | High | Low | Not studied | Not studied | Not studied | Low | Not studied | Low | Low | Low | Low | Not studied |
| Acute pain | Not studied | Not available | Not studied | Unclear | Unclear | Low | Low | Not studied | Low | Low | Low | Unclear |
| Numbness | Not studied | Not studied | Not studied | Not studied | Not studied | Low | Not studied | Low | Low | Not studied | Low | Not studied |
| Recurrence | High | Low | Low | Unclear | Unclear | Low | Low | Low | Low | Low | Low | Not studied |
| Mortality | High | Not studied | Low | Not studied | Not studied | Low | Low | Low | Low | Low | Not studied | Not studied |
| Complications | High | Low | Low | Unclear | Unclear | Low | Low | Low | Low | Low | Low | Not studied |
| Time to return to normal activities | High | Not studied | Not studied | Not studied | Unclear | Low | Low | Not studied | Not studied | Low | Not studied | Not studied |
| Hospital stay | High | Not studied | Not studied | Not studied | Unclear | Unclear | Low | Not studied | Not studied | Low | Not studied | Not studied |
| Selective reporting | Low | Low | High | Unclear | Unclear | Low | Unclear | Low | Low | Low | Low | High |
| Other source of bias | Unclear | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low |
| Overalla | Low | High | High | Unclear | Unclear | Low | Unclear | Unclear | High | Unclear | Unclear | Unclear |
| Study ID | Method of randomisation | Concealment of allocation | Blinding of participants | Blinding of outcome assessor | Loss to follow-up |
|---|---|---|---|---|---|
| Arslan et al. 201552 | Computer generated | Sealed opaque envelope | Blinded to the surgical method | Blinded | All reported outcomes: Kugel: 8.1% (9/110); Lichtenstein: 4.5% (5/110); and reasons for missing data: NR |
| aBerrevoet51 | Computer generated | Secretary look at the computer-generated list and ordered the next treatment on the list | No blinding | No blinding | No loss to follow-up |
| Dogru et al. 200653 | According to order of admittance | According to order of admittance | NR | NR | All reported outcomes: Kugel: 1/70; Lichtenstein: 0/70 |
| Gunal et al. 200754 | NR | NR | NR | NR | NR |
| Hamza et al. 201055 | Random number allocation | Random number allocation | Blinded to the surgical method | Blinded | NR |
| Koning et al. 201256 | Computer-generated list | Sealed opaque envelope | Blinded to the surgical method | Blinded | Early complications: no loss to follow-up |
| All other reported outcomes: TIPP, 1.4% (2/143); Lichtenstein, 2.5% (4/159) (deaths and loss to follow-up in second visit) | |||||
| Moghaddam et al. 201157 | NR | NR | NR | Blinded | All outcomes: no loss to follow-up |
| Muldoon et al. 200458 | Computer-generated list | Sequentially numbered, sealed opaque envelope | NR | NR | All reported outcomes: Read–Rives, 9.9% (12/121); Lichtenstein, 8.7% (11/126) (deaths or lost to follow-up in next visit) |
| Nienhuijs et al. 200759,62 | Computer-generated list | Consecutive order | Blinded to the surgical method | Blinded | Chronic pain: Lichtenstein, 1.2% (1/85); Kugel, 3.5% (3/85) (recurrence repaired or mesh removal) |
| Acute pain: Lichtenstein, 7% (6/86); Kugel, 5.8% (5/86) (incomplete diary or death) | |||||
| Recurrence: Lichtenstein, 1.2% (1/86); Kugel, 2.3% (2/86) owing to death or mesh removal | |||||
| Ray et al. 201460 | NR | NR | NR | NR | All outcomes: no loss to follow-up |
| Smolinski-Kurek et al. 201261 | Table of random numbers | NR | Blinded to the surgical method | Blinded | All outcomes: no loss to follow-up |
| Vatansev et al. 200263 | Via patient names in sealed envelopes | Via patient names in sealed envelopes | NR | NR | NR |
Appendix 8 Characteristics tables
| Study ID, country | Surgical setting | Inclusion/exclusion criteria | Interventions | Outcomes reported | Source of funding |
|---|---|---|---|---|---|
| Arslan et al. 2015,52 Turkey | One hospital (General Surgery Clinic of Konya Training and Research Hospital, Turkey) |
|
Lichtenstein and modified Kugel repair | Primary outcomes: hernia recurrence and chronic pain defined as a pain lasting > 6 months after surgery (Sheffield scale 0–3; cut-off 0) Secondary outcomes: early and late complications, duration of surgery, recovery time to return to work Mode of assessment: outcomes including hospital stay, early postoperative complications, recovery time to return to work and recurrence were recorded on preprepared forms during follow-up (physical examination) Timing: patients were evaluated at 10 and 30 days and 6, 12 and 24 months after the surgery |
NR |
| Berrevoet,51 Belgium | One hospital (University Hospital Ghent) |
|
TIPP and Lichtenstein | Outcomes: percentage of relapse, postoperative pain assessment (VAS 0–10; cut-off 0), duration to full recovery (able to do all activities) Mode of assessment: NR Timing: patients evaluated at day 0, 3 weeks, 3 months, 1 year and 3 years |
University Hospital Ghent |
| Dogru et al. 2006,53 Turkey | One hospital (Surgical department of Medical School of Firat University) |
|
Lichtenstein and Kugel | Outcomes: duration of operation, surgical findings, postoperative complications Mode of assessment: NR Timing: every 6 months for first 2 years and then annually |
NR |
| Gunal et al.54 2007, Turkey | One hospital |
|
Lichtenstein vs. Nyhus vs. TAPP repair vs. TEP repair | Outcomes: inflammatory response, pain (VAS), perioperative/postoperative complications Mode of assessment: NR Timing: NR |
NR |
| Hamza et al. 2010,55 Egypt | One hospital (department of surgery of the Alexandria Main University Hospital) |
|
Open preperitoneal vs. Lichtenstein vs. TAPP repair vs. TEP repair | Outcomes: postoperative pain (VAS), postoperative hospital stay, time to resume domestic activities and time to return to work, complications Mode of assessment: NR Timing: clinic visits at 2, 12 and 24 weeks |
University of Alexandria |
| Koning et al. 201256 TULIP study (Koning et al. 2013, QoL study),42 Netherlands | Two large hospitals (St Elisabeth Hospital Tilburg and TweeSteden Hospital Tilburg/Waalwijk) |
|
TIPP and Lichtenstein | Primary outcome: chronic pain at 1 year (proportion) (VAS 0–10; cut-off 0) Secondary outcomes: minor/early complications, duration of operation, length of hospital stay, time to return to usual daily activities, recurrence and numbness Mode of assessment: patients kept a VAS pain diary for the first 14 days after surgery. The pinprick test on the operated side was used to assess numbness in the dermatomes related to the inguinal nerves. A figure of dermatomes was used for anatomical orientation Timing: physical examination at 14 days, 3 months and 1 year |
No industry funding |
| Moghaddam et al. 2011,57 Iran | One hospital |
|
Read–Rives and Lichtenstein | Outcomes: duration of surgery, length of stay, amount of analgesics in the postoperative period and complications of surgery (infection, haematoma and pain), time to return to work, early recurrences, patient satisfaction using VAS scale Mode of assessment: clinical examination and direct examination were done; at follow-up telephone interview was done, recurrences and complications were recorded in the forms. Patient satisfaction measured using VAS Timing: NR |
NR |
| Muldoon et al. 2004,58 USA | One hospital (Central Arkansas Veterans Healthcare System) |
|
Lichtenstein and Read–Rives preperitoneal repair | Outcomes: early postoperative complications (scrotal haematoma, wound haematoma or infection, urinary retention or infection), hernia recurrence, groin discomfort, numbness, testicular atrophy, pain on exertion Pain measure: NR Mode of assessment: early complications were recorded, outcomes including recurrence, pain, etc., were assessed at follow-up; pain scores were obtained along with activity level and its relation to surgery Timing: patients were followed-up at 2, 6, 12 and 26 weeks, and then annually for at least 2 years |
NR |
| Nienhuijs et al. 2007,59,62 Netherlands | One regional teaching hospital (Canisius-Wilhelmina Hospital) |
|
Kugel and Lichtenstein | Primary outcome: chronic pain at 3 months (VAS: a score above 0 at this time point is regarded as chronic pain as defined by the IASP) Secondary outcomes: pain scores and number of analgesics consumed during the first 2 weeks, chronic pain descriptions, complications, duration of operation, recurrence, numbness Participants were also assessed using the PDI score Mode of assessment: patient completed VAS pain score, PDI and a questionnaire about the use of analgesia and reasons for their consumption preoperatively and postoperatively for first two weeks and then at 3-months follow-up visit. Timing: physical and neurological exam at 3 months |
NR |
| Ray et al. 2014,60 India | One hospital |
|
TIPP and Lichtenstein | Outcomes: operative time, duration of hospital stay, return to work, complication, chronic pain and recurrences Mode of assessment: NR Timing: NR |
None |
| Nienhuijs et al. 2007,59 Mexico | One hospital (Mexican Social Security Institute of the city of Leon) |
|
Elliptical domed-mesh technique (preperitoneal) and Lichtenstein | Primary outcomes: acute and chronic pain, (at 1, 3 and 6 months) (VAS 0–10; cut-off 3) Secondary outcomes: dysaesthesia at 1, 3 and 6 months, recurrence, complications Mode of assessment: physical assessment by third-year student. Check-up includes VAS, number of painkillers used from the day of surgery or last evaluation, wounds complications and diagnosis of dysaesthesia using inguinal map dermatomes Timing: at day 7 and at 1, 3 and 6 months |
Instituto Mexicano del Seguro Social |
| Vatansev et al. 200263 Turkey | One university hospital |
|
Lichtenstein vs. Nyhus vs. TEP repair vs. Bassini | Outcomes: postoperative pain levels (by measuring the need of analgesia via patient-controlled analgesia during the 24 hours after surgery), inflammatory mediators (C-reactive protein) Mode of assessment: NR Timing: during 24 hours after surgery |
NR |
| Study ID | Liechtenstein/open preperitoneal | Follow-up, months | |||||
|---|---|---|---|---|---|---|---|
| Randomised, n | Analysed, n | Male, n | Age, years [mean (SD or range)] | BMI, kg/m2 [mean (SD)] | Hernia type, n | ||
| Arslan et al. 201552 | 110/110 | 105/101 | 110/110 | 45.3 (SD 15.7)/49.3 (SD 15.5) | 26.82 (3.34)/26.36 (3.46) | Indirect: 81/79 Direct: 19/11 Pantaloon: 5/11 |
30 (range 24–37) |
| Berrevoet51 | 75/75 | 75/75 | 142 | Range 18–65 | NR | NR | > 3 |
| Dogru et al. 200653 | 70/70 | 70/69 | 67/67 | 51.1 (SD 16.2)/50.1 (SD 16.4) | NR | Indirect: 43/48 Direct: 27/21 |
Mean 54.5 (range 24–56) |
| Gunal et al. 200754 | 42/39 (plus 39 to TAPP repair; 40 to TEP repair) | 42/39 | NR | 22.76 (SEM 0.3)/23.85 (SEM 0.49) | NR | NR | Mean 99 |
| Hamza et al. 201055 | 25/25 (plus 25 to TAPP repair and 25 to TEP repair) | 25/25 | 25/25 | 35.12 (SD 10.11)/35.67 (12.965) | 24.34 (14.22)/22.2 (1.568) | Indirect: 100% | 3 |
| Koning et al. 201256 | 159/143 | 155/141 | 153/135 | 56.5 (SD 13.2)/57.0 (SD 12.1) | 25.4 (2.9)/25.1 (2.8) | Indirect: 104/96 Direct: 42/38 Pantaloon: 12/8 Not classified: 1/1 |
12 |
| Moghaddam et al. 201157 | 64/62 | 64/62 | 63/60 | 55.6 (SD NR)/58.8 (SD NR) | NR | Indirect: 22/18 Direct: 35/33 Both: 7/11 |
Mean 12 (range 8–20) |
| Muldoon et al. 200458 | 126/121 | 115/109 | 126/121 | 63.3 (range 18–85)/60.7 (range 26–86) | NR | Indirect: 41/44 Direct: 55/48 Pantaloon: 17/13 Unclassified: 2/4 |
Median 82 (range 24–110) |
| Nienhuijs et al. 200759 | 86/86 | 84/82 | 85/85 | 54.4 (SD 13.6)/55.6 (SD 15.8) | 25.4 (2.7)/25.1 (2.9) | NR | 3 |
| Ray et al. 201460 | 35/36 | 35/36 | 35/36 | 48.3 (range 22–68) | NR | Indirect: 27/25 Direct: 7/10 Pantaloon: 1/1 |
Median 24 (range 6–36) |
| Smolinski-Kurek et al. 201261 | 45/45 | 45/45 | 38/38 | 39 (SD 14)/41 (SD 14) | NR | Indirect: 28/31 Direct: 13/11 Pantaloon: 2/2Femoral: 1/1 |
> 6 months |
| Vatansev et al. 200263 | NR | 24/21 | 22/18 | 53.2 (SD 12.6), range 22–83/mean 50.7 (SD 15.3) range 18–85 | NR | Indirect: 17/16 Direct: 5/4 Femoral: 2/1 |
1 week |
| Study ID | Description of intervention | Open preperitoneal | Lichtenstein |
|---|---|---|---|
| Arslan et al. 201552 | Type of open preperitoneal/Lichtenstein | Modified Kugel: a single-layer polypropylene mesh patch was placed in the preperitoneal space with a technique similar to the one described by Kugel et al.41 (which used a double-layered polypropylene mesh with a memory ring placed in the preperitoneal space through a small incision) | Classical technique |
| Type of incision | A transverse incision of 4 cm was made, two-thirds medial and one-third lateral, corresponding to a position above the internal ring | An 8-cm oblique skin incision was made medially down to and through the external inguinal ring | |
| Type of mesh used | A 15 × 15-cm monofilament 38 g/m2 polypropylene [Supromesh (Sayin Tip, Istanbul, Turkey)]. This patch was clipped in oval shape with dimensions of 14 × 9 cm and four arrays of memory recoil rings [Monofilament synthetic polydioxanone, absorbable (Pedesente®, Dogsan, Ankara, Turkey)]. A 2 × 2-cm polypropylene pocket was created on the mesh to allow entry of the pulp of the index finger | A 12 × 8-cm polypropylene mesh (same polypropylene mesh used for preperitoneal without memory recoil rings) was trimmed to a foot-like shape to fit the inguinal floor | |
| Mesh fixation techniques | Mesh is not fixed except for a single suture through the mesh while closing the fascia transversalis. Following layers are closed in their anatomical order | The mesh was sutured to the Poupart’s ligament using polypropylene continuous 2/0 sutures. An absorbable 2/0 suture or skin stapler was used to close following layers | |
| Surgeon’s experience | All surgeries were performed by two experienced surgeons | ||
| Duration of operation, mean (SD) | Mean 38.2 minutes (SD 7.4 minutes) | Mean 40.3 minutes (SD 6.6 minutes); p = 0.031 | |
| Berrevoet51 | Type of open preperitoneal/Lichtenstein | Transinguinal preperitoneal technique with polysoft mesh | Modified Lichtenstein |
| Type of incision | Not available | Not available | |
| Type of mesh used | Polysoft mesh | Lightweight mesh | |
| Mesh fixation techniques | Not available | Not available | |
| Surgeon’s experience | Not available | Not available | |
| Duration of operation, mean (SD), minutes | Not available | Not available | |
| Dogru et al. 200653 | Type of open preperitoneal/Lichtenstein | Kugel mesh was introduced through the abdominal wall incision into peritoneum space to cover Hesselbach’s triangle, the internal inguinal ring and the femoral ring, and also to cover the obturator foramen | Standard technique |
| Type of incision | A 3- to 4-cm incision was made one-third lateral and two-thirds medial | NR | |
| Type of mesh used | A special mesh (Kugel’s Patch; Surgical Sense, Arlington, TX, USA) as described by Kugel | Polypropylene meshes (6 × 11 cm) (Prolene; Ethicon, Brussels, Belgium). The size of the mesh was modified for each patient in accordance with their anatomic variance | |
| Mesh fixation techniques | NR | NR | |
| Surgeon’s experience | Patients were treated similarly by surgeons who were experienced in both techniques in an effort to avoid the bias of a learning period | ||
| Duration of operation | Mean 45.36 minutes (SD 6.2 minutes) | Mean 47.06 minutes (SD 7.5 minutes), p = 0.352 | |
| Gunal et al. 200754 | Type of open preperitoneal/Lichtenstein | Nyhus repair was performed by emplacing a Prolene mesh to the posterior aspect of the inguinal defect | A Lichtenstein tension-free hernia repair was accomplished by emplacing a Prolene mesh to the anterior aspect of the posterior wall |
| Type of incision | NR | NR | |
| Type of mesh used | A 6 × 12-cm Prolene mesh | A 6 × 12-cm Prolene mesh | |
| Mesh fixation techniques | NR | NR | |
| Surgeon’s experience | All operations were performed under general anaesthesia by two consultant general surgeons who were highly experienced in open and laparoscopic hernia surgery | ||
| Duration of operation | Mean 36.54 minutes (SEM 1.55 minutes) | Mean 39.64 minutes (SEM 1.28 minutes) | |
| Hamza et al. 201055 | Type of open preperitoneal/Lichtenstein | NR | NR |
| Type of incision | NR | NR | |
| Type of mesh used | NR | NR | |
| Mesh fixation techniques | NR | NR | |
| Surgeon’s experience | All operations performed by a consultant surgeon | ||
| Duration of operation | Mean 54.5 minutes (SD 13.2 minutes) ‘significant difference’ | Mean 34.21 minutes (SD 23.5 minutes) | |
| Koning et al. 201256 | Type of open preperitoneal/Lichtenstein | TIPP technique: hernia sac was reduced into the preperitoneal space; the preperitoneal space was bluntly dissected with a finger | Current standard technique |
| Type of incision | 2-cm skin incisions was made | 2-cm skin incisions was made | |
| Type of mesh used | 16 × 9.5-cm soft mesh with memory ring [PolysoftTM (BARD, Benelux, Belgium)] | 6 × 13.7-cm soft mesh [SoftMesh (BARD, Benelux, Belgium)] | |
| Mesh fixation techniques | A 3/0 Prolene [Ethicon (Johnson & Johnson, Somerville, NJ, USA] suture was used for fixation of the mesh. The inguinal canal was closed with 3/0 Vicryl [Ethicon (Johnson & Johnson, Somerville, NJ)]. Scarpa’s fascia was closed with one stitch of 3/0 Vicryl. The skin was closed intracutaneously with 3/0 Vicryl Rapide [Ethicon, Johnson & Johnson, Somerville, NJ, USA) in both techniques | ||
| Surgeon’s experience | Dedicated hernia surgeons or supervised surgical junior doctors. The operating teams (resident supervised by surgeon or surgeon assisted by resident) were similar in both groups (and equally divided; ratio 50 : 50). Surgeons were experienced in both procedures | ||
| Duration of operation | Mean 34.1 minutes (SD 9.9 minutes), p < 0.001 | Mean 39.9 minutes (SD 12.0 minutes) | |
| Moghaddam et al. 201157 | Type of open preperitoneal/Lichtenstein | Read–Rives technique | Lichtenstein standard |
| Type of incision | Classic inguinal incision in both techniques | ||
| Type of mesh used | A 16 × 10-cm or 11 × 15-cm propylene mesh | A 16 × 10-cm or 11 × 15-cm propylene mesh | |
| Mesh fixation techniques | A nylon suture 20 and pinned with cooper ligament | Silk suture 20 used and nylon mesh fixation used 20 | |
| Surgeon’s experience | Two different experienced surgeons | ||
| Duration of operation | Mean 47.0 minutes (SD 1.9 minutes) | Mean 46.8 minutes (SD 8.8 minutes) ‘difference not significant’ | |
| Muldoon et al. 200458 | Type of open preperitoneal/Lichtenstein | Read–Rives: a mesh was placed in the preperitoneal position and secured | Standard technique: mesh was placed into the groin and secured and then was split to recreate the internal ring. The two tails of the mesh were crossed, sutured together and attached to the inguinal ligament, lateral to the cord |
| Type of incision | A standard oblique groin incision was made in both techniques | ||
| Type of mesh used | A 12 × 6-cm piece of polypropylene mesh | A 7.5 × 15-cm polypropylene mesh | |
| Mesh fixation techniques | Mesh was secured with three sutures at the pubic tubercle, Cooper’s ligament, and the psoas muscle laterally | Mesh was secured to the lateral border of the rectus sheath and the inguinal ligament, using a running 20 Prolene suture | |
| Surgeon’s experience | NR | NR | |
| Duration of operation | The Read–Rives repair took 9 minutes longer to perform than the Lichtenstein repair (p = 0.04) (values NR) | Mean 49.5 minutes (SD 11.5 minutes), p < 0.0061 | |
| Nienhuijs et al. 200759 | Type of open preperitoneal/Lichtenstein | Kugel approach as described by Kugel and colleagues41; with a transverse opening in the anterior layer (creates pocket to facilitate positioning) | Standard technique |
| Type of incision | NR | NR | |
| Type of mesh used | An 11 × 14-cm Kugel mesh, medium oval size [consist of a double layer of monofilament polypropylene and a memory ring (allows the patch to maintain its shape during placement in the preperitoneal space)] | A 6 × 11-cm polypropylene mesh (Prolene; Ethicon) was trimmed to fit the inguinal floor | |
| Mesh fixation techniques | The mesh was sutured to the ligament of Poupart with a non-absorbable suture and secured cranially using an absorbable suture. In both techniques, the skin was closed with a subcuticular absorbable suture | ||
| Surgeon’s experience | Performed by staff surgeons as well as surgeons in training | ||
| Duration of operation | Mean 41 minutes (p < 0.001) | Mean 54 minutes | |
| Ray et al. 201460 | Type of open preperitoneal/Lichtenstein | Transinguinal preperitoneal technique: mesh is trimmed in a semicircular fashion to prevent trauma to the bladder neck. The mesh is placed in the preperitoneal space and anchored to the Cooper’s ligament | Standard technique |
| Type of incision | Similar incision was made in both techniques | ||
| Type of mesh used | A 15 × 15-cm polypropylene mesh, cut into a dimension of 15 × 12 cm, the inferior medial angle of the mesh is trimmed in a semicircular fashion | NR | |
| Mesh fixation techniques | Mesh was anchored to the Cooper’s ligament with a single 20 interrupted Prolene suture | NR | |
| Surgeon’s experience | NR | NR | |
| Duration of operation | Mean 49.5 minutes (SD 11.5 minutes) | Mean 37.9 minutes (SD 13.7 minutes) | |
| Smolinski-Kurek et al. 201261 | Type of open preperitoneal/Lichtenstein | Preperitoneal elliptical dome mesh technique | Standard technique |
| Type of incision | 3–4 cm of the deep inguinal ring | NR | |
| Type of mesh used | Polypropylene mesh cut in an elliptical dome shape | Heavy polypropylene mesh | |
| Mesh fixation techniques | Mesh was fixed using two stitches | NR | |
| Surgeon’s experience | Two certified surgeons with similar opportunity to conduct surgery | ||
| Duration of operation | Mean 59 minutes (SD 11 minutes), difference not significant | Mean 58 minutes (SD 10 minutes) | |
| Vatansev et al. 200263 | Type of open preperitoneal/Lichtenstein | Nyhus: after the usual steps involved in the operation were taken, polypropylene mesh was used for the reinforcement of the preperitoneal area | After the usual steps involved in the operation were taken, polypropylene mesh was used for the reinforcement of the posterior wall of the inguinal canal |
| Type of incision | NR | NR | |
| Type of mesh used | Polypropylene mesh | Polypropylene mesh | |
| Mesh fixation techniques | NR | NR | |
| Surgeon’s experience | NR | NR | |
| Duration of operation | Mean 51.9 minutes (SD 6.5 minutes), range 49–68 minutes | Mean 54.7 minutes (SD 7.2 minutes), range 50–74 minutes | |
Appendix 9 Results of the individual study
| Study ID | Outcome measure | Follow-up time point | Open preperitoneal | Lichtenstein | p-value | ||
|---|---|---|---|---|---|---|---|
| n (%) | Total | n (%) | Total | ||||
| Arslan et al. 201552 | Patients with ‘chronic pain’. Chronic pain defined as a pain lasting more than 6 months after surgery according to the Sheffield pain score | Between 6 and 24 months | 96 | 101 | 97 | 105 | NR |
| Berrevoet51 | Patient reporting ‘chronic pain’. Pain measured using 0–10 VAS pain score. The cut-off value for pain score was 0 (chronic pain at 3 months or in later follow-up time) | After 3 months | 2 | 74 | 10 | 66 | Not available |
| Koning et al.42,56 | Patients with ‘continuous chronic pain’. Pain measured using 0–10 VAS. The cut-off value for pain score was 0 | 1 year | 5 (3.5) | 141 | 20 (12.9) | 155 | 0.004 |
| Patients with VAS score 1–3 (mild pain) | 1 year | 0 | 141 | 0 | 155 | NR | |
| Patients with VAS score 4–6 (moderate) | 1 year | 5 (3.5) | 141 | 18 (11.6) | 155 | NR | |
| Patients with VAS score 7–10 (severe) | 1 year | 0 | 141 | 2 (1.3) | 155 | NR | |
| Muldoon et al. 200458 | Groin discomfort. Pain measure NR in the published paper. Based on information from published Cochrane review,20 which included this trial. Pain was measured using 0–10 VAS pain score | Between 24 and 110 months | 10 | 109 | 9 | 115 | NR |
| Nienhuijs et al. 200759 (Staal et al. 2008)62 | Patient reporting ‘chronic pain’. Pain measured using 0–10 VAS. The cut-off value for pain score was 0 | 3 months | 17 (20.7) | 82 | 34 (40.5) | 84 | 0.007 |
| Ray et al. 201460 | Patients with ‘chronic pain’. Pain measure NR | 6 months | 0 | 36 | 1 | 35 | NR |
| 1 year | 0 | 36 | 0 | 35 | NR | ||
| aSmolinski-Kurek et al. 201261 | Patients with ‘persistent pain’. Pain measured using VAS scale 0–10. Any pain between VAS 3–10 was defined as presence of pain and 0–2 as absence of pain | 3 months | 4 | 45 | 9 | 45 | 0.13 |
| 6 months | 1 | 45 | 4 | 45 | 0.15 | ||
| Study ID | Outcome measure | Follow-up time point | Open preperitoneal | Lichtenstein | p-value | ||
|---|---|---|---|---|---|---|---|
| Values | Total | Values | Total | ||||
| Koning et al. 201242,56 | Activity-related paina (VAS), n (%) | 1 year | 12 (8.5) | 141 | 60 (38.7) | 155 | 0.001 |
| Muldoon et al. 200458 | Pain on exertion,b n (%) | Between 24 and 110 months | 10 (9.2) | 109 | 7 (6.1) | 115 | NR |
| Testicular pain,b n (%) | Between 24 and 110 months | 1 (≈ 1) | 109 | 2 (1.7) | 115 | NR | |
| Nienhuijs et al. 200759,62 | VAS pain score, mean (SD) | 3 months | 0.4 (0.7) | 82 | 0.9 (1.6) | 84 | 0.026 |
| Arslan et al. 201552 | Sheffield pain score, mean (SD) | 6 months | 1.12 (0.79) | 101 | 1.34 (0.92) | 105 | 0.070 |
| 12 months | 0.94 (0.69) | 101 | 1.09 (0.79) | 105 | 0.160 | ||
| 24 months | 0.66 (0.67) | 101 | 0.87 (71) | 105 | 0.032 | ||
| Study ID | Measures/unit | Follow-up time point | Open preperitoneal | Lichtenstein | p-value | Definition/additional information | ||
|---|---|---|---|---|---|---|---|---|
| Total analysed | Values | Total analysed | Values | |||||
| Gunal et al. 200754 | VAS pain score, mean (SD) | 6 hours post operation | 39 | 6 (1.4) | 42 | 7.3 (1.6) | 0.001 Bonferroni test | The VAS scores decreased significantly in each group 48 hours post operatively (p < 0.05) when compared with the score at 6 hours Assume SEM as SD |
| 48 hours post operation | 39 | 3.7 (1) | 42 | 4.8 (1.4) | 0.001 Bonferroni test | |||
| Hamza et al. 201055 | VAS pain score, mean (SD) | 6 hours post operation | 25 | 7.067 (1.831) | 25 | 6.5 (3.5) | NS | |
| 48 hours post operation | 25 | 4.933 (1.624) | 25 | 4.63 (2.22) | NS | |||
| Koning et al. 201256 (Koning et al. 2013)42 | VAS pain score, mean (SD) | 1 day post operation | 141 | 5.1 (4.63) | 155 | 5.1 (5.48) | NS | The pain diary after surgery showed no differences in VAS scores in the first 14 days after TIPP and Lichtenstein repair |
| Moghaddam et al. 201157 | VAS pain score, mean (SD) | First 24 hours post operation | 62 | 4.58 (2.62) | 64 | 4.64 (3.59) | NS | SD imputed from other studies excluding Gunal et al. 200754 |
| Nienhuijs et al. 200759 (Staal et al. 2008)62 | VAS pain score, mean (SD) | 14 days post operation | 81 | 2.0 (1.4) | 80 | 2.6 (1.8) | 0.104 | On the days that the patients did not consume any analgesics (1176 days), the mean VAS pain score was 1.4 |
| Study ID | Outcome measure/unit | Open preperitoneal | Lichtenstein | p-value | ||
|---|---|---|---|---|---|---|
| Total | n (%) | Total | n (%) | |||
| Smolinski-Kurek et al. 201261 | Pain at 1 week after surgery (VAS) | 45 | 28 (62.2) | 45 | 38 (84.4) | 0.01 |
| Berrevoet51 | Pain at 2 weeks after surgery (VAS) | 75 | 5 (6.7) | 75 | 29 (38.7) | Chi-squared Fisher’s exact test < 0.001 |
| Smolinski-Kurek et al. 201261 | Pain at 1 month after surgery (VAS) | 45 | 12 (26.7) | 45 | 23 (51.1) | 0.13 |
| Ray et al. 201460 | Pain at 1 month after surgery | 36 | 0 | 35 | 3 (8.6) | Fisher’s exact test 0.12 |
| Moghaddam et al. 201157 | Local tenderness at 1 month after surgery | 62 | 5 (8.1) | 64 | 20 (31.2) | < 0.01 |
| Study ID | Outcome measure/unit | Time point/follow-up at analysis | Open preperitoneal | Lichtenstein | p-value | ||
|---|---|---|---|---|---|---|---|
| Total | Mean (SD) | Total | Mean (SD) | ||||
| Moghaddam et al. 201157 | Postoperative need for Pethidine, mg | First 24 hours post operation | 62 | 37.1 (22.5) | 64 | 34.4 (38.8) | NS |
| Vatansev et al. 200263 | Postoperative need for Pethidine, mg | First 24 hours post operation | 21 | 382.9 (189.1) | 24 | 253.9 (129.23) | < 0.001 |
| Study ID | Specify measures | Follow-up time point | Open preperitoneal | Lichtenstein | p-value | ||
|---|---|---|---|---|---|---|---|
| n (%) | Total | n (%) | Total | ||||
| Koning et al. 201256 (Koning et al. 201342) | Patients with persisting numbness. The pinprick test on the operated side was used to assess numbness in the dermatomes related to the inguinal nerves. A figure of dermatomes was used for anatomical orientation | 1 year | 15 (10.6) | 141 | 79 (51.0) | 155 | < 0.001 |
| Nienhuijs et al. 200759 (Staal et al. 200859) | Numbness (measure NR) | 3 months | 3 | 82 | 22 | 84 | < 0.001 |
| Cutaneous sensory changes | 3 months | 6 | 82 | 24 | 84 | NR | |
| Muldoon et al. 200458 | Numbness (measure NR) | 2 years | 13 | 109 | 11 | 115 | NR |
| Smolinski-Kurek et al. 201261 | Anaesthesia and hypothesia (numbness). A figure of dermatomes was used for anatomical orientation | 3 months | 11 | 22 | 9 | 24 | NR |
| Study ID | Follow-up time point | Type of complications | Open preperitoneal | Lichtenstein | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number/events | Total | % | Number/events | Total | % | ||||
| Koning et al. 201256 (Koning et al. 201342) | Within first year after hernia repair | Patients with minor early complications (minor complications were not critical for decision-making according to GRADE) | 9 | 141 | 6.4 | 29 | 155 | 18.7 | 0.003 |
| Superficial wound infection | 2 | 141 | 1.4 | 4 | 155 | 2.6 | 0.478 | ||
| Other minor complications (such as urinary retention, urinary tract infection, nausea, headache, haematoma without intervention | 7 | 141 | 5 | 25 | 155 | 16.1 | 0.002 | ||
| Conversion of TIPP to Lichtenstein technique or vice versa | 0 | 141 | 0 | 0 | 155 | 0 | NR | ||
| Nienhuijs et al. 200759 (Staal et al. 200862) | 3 months | Haematoma | Total = 14/166 | ||||||
| Infection | Total = 5/166 | ||||||||
| Dysejaculation | Total = 1/166 | ||||||||
| Higher urinary frequency | 1 | 82 | 1.2 | 0 | 84 | 0 | NR | ||
| Muldoon et al. 200458 | Between 2 and 26 weeks | Early complications | |||||||
| Scrotal haematoma | 4 | 109 | 3.7 | 4 | 115 | 3.5 | NR | ||
| Wound haematoma | 5 | 109 | 4.6 | 3 | 115 | 2.6 | NR | ||
| Wound infection | 0 | 109 | 0 | 0 | 115 | 0 | NR | ||
| Urinary retention | 7 | 109 | 6.4 | 9 | 115 | 7.8 | NR | ||
| Urinary tract infection | 1 | 109 | 0.9 | 2 | 115 | 1.7 | NR | ||
| Median 82 (range 24–110) months | Late complications | ||||||||
| Testicular atrophy | 1 | 109 | 0.9 | 3 | 115 | 2.6 | NR | ||
| Late infection | 0 | 109 | 0 | 0 | 115 | 0 | NR | ||
| Ray et al. 201460 | Perioperative | ||||||||
| Injury to peritoneum | 3 | 36 | 8.5 | 0 | 35 | 0 | NR | ||
| Injury to vessels | 1 | 36 | 2.7 | 0 | 35 | 0 | NR | ||
| Immediate post operation | Wound seroma | 0 | 36 | 0 | 2 | 35 | 5.7 | NR | |
| At 1 month | Wound induration | 0 | 36 | 0 | 6 | 35 | 17.1 | NR | |
| At 6 months | Wound induration | 1 | 36 | 2.8 | 3 | 35 | 8.6 | NR | |
| At 1 year | Wound induration | 0 | 36 | 0 | 0 | 35 | 0 | NR | |
| Immediate post operation | Scrotal collection | 1 | 36 | 2.7 | 1 | 35 | 2.7 | NR | |
| At 1 month | Scrotal collection | 1 | 36 | 2.8 | 1 | 35 | 2.8 | NR | |
| At 6 months | Scrotal collection | 0 | 36 | 0 | 0 | 35 | 0 | NR | |
| Dogru et al. 200653 | Unclear (range of follow-up 24–56 months) | Cord oedema | 1 | 69 | 1.4 | 1 | 70 | 1.4 | 0.74 |
| Haematoma | 1 | 69 | 1.4 | 0 | 70 | 0 | 0.49 | ||
| Seroma | 1 | 69 | 1.4 | 0 | 70 | 0 | 0.49 | ||
| Infection | 1 | 69 | 1.4 | 0 | 70 | 0 | 0.49 | ||
| Mesh reaction | 1 | 69 | 1.4 | 0 | 70 | 0 | 0.49 | ||
| Total | 5 | 69 | 7.2 | 1 | 70 | 1.4 | 0.21 | ||
| Gunal et al. 200754 | Perioperative complications | 7 | 39 | 17.9 | 10 | 42 | 23.8 | NR | |
| Inferior epigastric vessel bleeds | 7 | 39 | 17.9 | 4 | 42 | 9.5 | NR | ||
| Pampiniform plexus bleeds | 0 | 39 | 0 | 2 | 42 | 4.8 | NR | ||
| Ilioinguinal nerve injury | 0 | 39 | 0 | 3 | 42 | 7.1 | NR | ||
| Ductus deference injury | 0 | 39 | 0 | 1 | 42 | 2.4 | NR | ||
| Unclear (mean 97 months for Lichtenstein) and 99 months for Nyhus | Postoperative complications | 2 | 39 | 5.1 | 9 | 42 | 21.4 | NR | |
| Scrotal oedema | 0 | 39 | 0 | 7 | 42 | 16.7 | NR | ||
| Urinary retention | 1 | 39 | 2.6 | 1 | 42 | 2.4 | NR | ||
| Haematoma in penis | 0 | 39 | 0 | 1 | 42 | 2.4 | NR | ||
| Incisional haematoma | 1 | 39 | 2.6 | 0 | 42 | 0 | NR | ||
| Hamza et al. 201055 | Unclear (range of follow-up 2–24 weeks) | Scrotal haematoma | 1 | 25 | 4 | 0 | 25 | 0 | NR |
| Wound infection | 1 | 25 | 4 | 1 | 25 | 4 | NR | ||
| Conversion | 0 | 25 | 0 | 0 | 25 | 0 | NR | ||
| Serious visceral complications | 0 | 25 | 0 | 0 | 25 | 0 | NR | ||
| Moghaddam et al. 201157 | Unclear (8–20 months) | Wound infection | 1 | 62 | 1.6 | 3 | 64 | 4.7 | NS |
| Haematoma or seroma | 2 | 62 | 3.2 | 3 | 64 | 4.7 | NS | ||
| Berrevoet51 | Not available | Early complications (within 30 days) | 2 | 75 | 2.7 | 14 | 75 | 18.7 | NR |
| Mesh infection (more than 30 days) | 0 | 72 | 0 | 0 | 72 | 0 | NR | ||
| Arslan et al. 201552 | Unclear (follow-up visits at 10 and 30 days and 6, 12 and 24 months after the surgery) | Total (day 10) | 28 | 101 | 27.7 | 22 | 105 | 20.9 | 0.099 |
| Cord oedema | 11 | 101 | 10.9 | 8 | 105 | 7.6 | 0.284 | ||
| Haematoma | 3 | 101 | 3.0 | 5 | 105 | 4.8 | 0.382 | ||
| Pseudohernia (seroma) | 6 | 101 | 5.9 | 0 | 105 | 0 | 0.013 | ||
| Wound infection | 2 | 101 | 2.0 | 4 | 105 | 3.8 | 0.36 | ||
| Scrotal oedema at day 10 | 8 | 101 | 7.9 | 5 | 105 | 4.8 | 0.26 | ||
| Scrotal oedema at day 30 | 3 | 101 | 3.0 | 4 | 105 | 3.8 | 0.522 | ||
| Scrotal oedema at month 6 | 0 | 101 | 0 | 0 | 105 | 0 | NR | ||
| Smolinski-Kurek et al. 201261 | Unclear (follow-up visits at 1, 3 and 6 months) | Total | 9 | 45 | 20 | 8 | 45 | 17.8 | 0.78 |
| Seroma | 3 | 45 | 6.7 | 1 | 45 | 2.2 | NR | ||
| Haematoma | 4 | 45 | 8.9 | 6 | 45 | 13.3 | NR | ||
| Cord oedema | 2 | 45 | 4.4 | 1 | 45 | 2.2 | NR | ||
| Surgical wound infection | 0 | 45 | 0 | 0 | 45 | 0 | Not applicable | ||
| Study ID | Follow-up time point at analysis | Outcomes as reported | Open preperitoneal | Lichtenstein | p-value | Additional information | ||
|---|---|---|---|---|---|---|---|---|
| n | Total | n | Total | |||||
| Koning et al. 201256 (Koning et al. 201342) | 1 year | Recurrence/reoperation | 2 | 141 | 4 | 155 | 0.478 | |
| Nienhuijs et al. 200759 (Staal et al. 200862) | 3 months | Recurrence | 2 | 86 | 2 | 85 | NR | Ultrasound test/physical examination |
| Swelling/bulging but no recurrence | 5 | 86 | 12 | 85 | NR | Ultrasound test/physical examination | ||
| Muldoon et al. 200458 | Between 2 and 26 weeks | Early re-operation | 1 | 109 | 0 | 115 | NR | Reoperation was required owing to femoral nerve injury (add the recurrence in total: five vs. two) |
| Lichtenstein between 18–53 months; open preperitoneal at 2 months | Recurrence | 1 | 109 | 5 | 115 | NR | ||
| Ray et al. 201460 | 1 month | Recurrence | 0 | 36 | 0 | 35 | NR | |
| 6 months | Recurrence | 0 | 36 | 0 | 35 | NR | ||
| 1 year | Recurrence | 0 | 36 | 0 | 35 | NR | ||
| Dogru et al. 200653 | 24–56 months | Recurrence | 0 | 69 | 1 | 70 | 0.34 | |
| Gunal et al. 200754 | Mean 97 months (Lichtenstein) and 99 months (Nyhus) | Recurrence | 1 | 39 | 3 | 42 | NR | |
| Hamza et al. 201055 | 2–24 weeks | Recurrence | 0 | 25 | 0 | 25 | NR | |
| Berrevoet51 | < 30 days | Early recurrence | 0 | 75 | 0 | 75 | NR | |
| > 30 days | Late recurrence | 3 | 72 | 2 | 70 | NR | ||
| Moghaddam et al. 201157 | 8–20 months | Recurrence | 0 | 62 | 1 | 64 | NS | |
| Arslan et al. 201552 | 1 year | Recurrence | 3 | 101 | 1 | 105 | 0.296 | |
| Smolinski-Kurek et al. 201261 | 3 months | Recurrence/reoperation | 1 | 45 | 0 | 45 | NR | |
| Study ID | Unit of measure | Open preperitoneal | Lichtenstein | p-value | ||
|---|---|---|---|---|---|---|
| Values | Total | Values | Total | |||
| Arslan et al. 201552 | Mean (SD), days | 1.39 (0.87) | 101 | 1.25 (0.49) | 105 | 0.133 |
| Koning et al. 201256 | Mean (SD), days | 0.34 (0.27) | 141 | 0.37 (0.21) | 155 | 0.151 |
| Moghaddam et al. 201157 | Mean (SD), days | 2.4 (0.8) | 62 | 2.7 (0.9) | 64 | NS |
| Ray et al. 200460 | Mean (SD), days | 4.6 (1.23) | 36 | 4.65 (1.39) | 35 | NS |
| Koning et al. 201256 | Number of patients with 1-night stay after surgery (%) | 12 (8.5) | 141 | 16 (10.3) | 155 | 0.646 |
| Hamza et al. 201055 | Number of patients with 1-day stay (%) | 22 (88) | 25 | 21 (84) | 25 | NS |
| Number of patients with 2-day stay (%) | 3 (12) | 25 | 3 (12) | 25 | NS | |
| Number of patients with > 2-day stay (%) | 0 (0) | 25 | 1 (4) | 25 | NS | |
| Study ID | Open preperitoneal, mean (SD), days | Lichtenstein, mean (SD) days | p-value | Definition of normal activities | ||
|---|---|---|---|---|---|---|
| Values | Total | Values | Total | |||
| Koning et al. 201256 (Koning et al. 201342) | 9.9 (11.4) | 141 | 16.4 (20.5) | 155 | 0.001 | Normal activities included work, sport and gardening |
| Ray et al. 200460 | 12.3 (2.01) | 36 | 13.6 (1.6) | 35 | 0.036 | Time to return to sedentary work |
| Hamza et al. 201055 | Domestic 12.27 (3.535) | 25 | Domestic 12.11 (4.23) | 25 | NS | Domestic activities included going to the toilet, showering, self-dressing and driving |
| Work 16.13 (3.758) | 25 | Work 15.25 (2.53) | 25 | NS | ||
| Moghaddam et al. 201157 | 9.6 (range 7–15) | 62 | 12.2 (range 7–20) | 64 | NS | Time to return to work (impute SD) |
| Arslan et al. 201552 | 9.72 (2.45) | 101 | 10.38 (3.49) | 105 | 0.121 | Time to return to work |
Appendix 10 Data extraction form for cost-effectiveness review
| Reviewer ID | ||||
|---|---|---|---|---|
| Date | ||||
| Administration details | ||||
| Study ID | ||||
| Publication status | ||||
| Study objective/research question | ||||
| Study details (COMPLETE ONLY IF ECONOMIC EVALUATION ALONGSIDE RCT) | ||||
| Study design (RCT/cohort comparison/other) | ||||
| Country | ||||
| Surgical setting | ||||
| Study duration | ||||
| Type of economic evaluation (CEA/CMA/CUA/CBA/CCA) | ||||
| Eligibility criteria for the study | ||||
| Inclusion criteria | ||||
| Exclusion criteria | ||||
| Modelling information (COMPLETE ONLY IF ECONOMIC EVALUATION as part of decision–analysis model) | ||||
| Model type (Markov cohort, microsimulation etc.) | ||||
| Size of modelled cohort | ||||
| Model time horizon | ||||
| Health states modelled (narrative of model structure and methods) | ||||
| Country | ||||
| Surgical setting | ||||
| Type of economic evaluation (CEA/CMA/CUA/CBA/CCA) | ||||
| Other information on modelling methods | ||||
| Details of model cohort (if applicable) | ||||
| Age of modelled cohort | ||||
| Gender information | ||||
| Type of inguinal hernia | ||||
| Other info on model cohort | ||||
| Interventions and comparators | ||||
| Comparisons (intervention vs. comparator) | ||||
| Details of the surgical procedure of intervention? | ||||
| Details of the surgical procedure of comparator? | ||||
| Description of follow-up after surgery (state time points) | ||||
| Primary economic outcomes reported | ||||
| Secondary economic outcomes reported | ||||
| Methods – costs | ||||
| Analysis method (e.g. ITT, PPA) | ||||
| Study perspective | ||||
| Currency and costing year | ||||
| Discount rate (costs – if applicable) | ||||
| What costs were included? (e.g. intervention/follow-up, etc.) | ||||
| Analysis methods of costs (e.g. regression/other) | ||||
| Methods – outcomes | ||||
| Clinical outcomes measured | ||||
| Economic evaluation outcomes measured | ||||
| Discount rate (outcomes – if applicable) | ||||
| Analysis method for outcomes | ||||
| Methods – cost-effectiveness | ||||
| Cost-effectiveness reported as: | ||||
| Sensitivity analyses undertaken | ||||
| Bootstrapping | ||||
| Distributions applied to costs and outcomes | ||||
| Uncertainty reported as: | ||||
| Patient baseline characteristics | ||||
| Total | Lichtenstein | OPP | Difference | |
| Total patients, n | ||||
| Age (years) (mean/median, SD/range) | ||||
| Gender (M/F), n (%) | ||||
| Type of inguinal hernia | ||||
| Direct, n (%) | ||||
| Indirect, n (%) | ||||
| Pantaloon, n (%) | ||||
| Unclassified, n (%) | ||||
| Results | ||||
| Primary analysis | Unit | Lichtenstein | OPP | Difference/incremental; mean (95% CI) |
| Costs | ||||
| Outcomes | ||||
| Cost-effectiveness | ||||
| Secondary analysis – provide details | Unit | Lichtenstein | OPP | Difference/incremental; mean (95% CI) |
| Costs | ||||
| Outcomes | ||||
| Cost-effectiveness | ||||
| Sensitivity analysis – provide details | Unit | Lichtenstein | OPP | Difference/incremental; mean (95% CI) |
| Costs | ||||
| Outcomes | ||||
| Cost-effectiveness | ||||
| Results of bootstrapping/CEACs/scatterplots | Uncertainty presented as: | P (Lichtenstein – C/E)a | P (OPP – C/E)b | |
| Costs | ||||
| Outcomes | ||||
| Cost-effectiveness | ||||
| Discussions | ||||
| Study strengths | ||||
| Study weaknesses | ||||
| Conclusions | ||||
| Base-case conclusions | ||||
| Sensitivity analysis conclusions | ||||
| Subgroup analysis conclusions | ||||
| PSA conclusions | ||||
| Other details | ||||
| Recommendations for future research | ||||
| Other (specify) | ||||
| Other (specify) | ||||
| Other relevant info for de novo model | ||||
| Other (specify) | ||||
| Other (specify) | ||||
| Other (specify) | ||||
List of abbreviations
- AHRQ
- Agency for Healthcare Research and Quality
- ASA
- American Society of Anaesthetists
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions-3 Level
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- IASP
- International Association for the Study of Pain
- ICER
- incremental cost-effectiveness ratio
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PDI
- Pain Disability Index
- PROM
- patient-reported outcome measure
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- risk ratio
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- TAPP
- transabdominal preperitoneal
- TEP
- totally extraperitoneal
- TIPP
- transinguinal preperitoneal
- VAS
- visual analogue scale
- WTP
- willingness to pay