Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/116/85. The contractual start date was in May 2011. The draft report began editorial review in February 2014 and was accepted for publication in November 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Lecky et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Relevance of HITS-NS to the NHS
In 2007 the National Institute for Health and Care Excellence (NICE) Head Injury guideline update specified that all severely head-injured patients should be treated at or transferred to a specialist neuroscience centre (SNC). The bypass of non-specialist acute general hospital (NSAH) emergency departments (EDs) in order to allow brain-injured patients to have neurosurgical care at the earliest opportunity (early neurosurgery) was identified as an important research question. 1 The need to improve outcomes after traumatic brain injury (TBI) relates to its dominance as the major cause of death in children and young adults in the UK. After the injured patient reaches hospital alive, TBI results in 4000 deaths and 5000 lifelong disabilities annually in patients with a median age of 30 years. 2 This burden of morbidity profoundly changes families and relationships, through bereavement or coping with TBI-related physical, cognitive and emotional impairment. Each patient with TBI costs an average of £15,000 for acute NHS care, a figure which increases if the patient is admitted to a SNC. 3 Subsequent rehabilitation costs are also significant, but both are dwarfed by costs to society from premature death or lifelong dependency as a young adult. Observational evidence at the time of applying for funding in 2008 suggested that this NHS investment had failed to reduce case fatality from TBI over the 1994–2003 decade, with recent trials of neuroprotective agents failing to identify any single new effective therapy. 4
At the time of applying for funding, data from the Trauma Audit and Research Network (TARN) suggested that, nationally, one-third of patients with TBI were injured nearest to the ED of a SNC and would receive early neurosurgery. 4 The remaining two-thirds of patients were injured nearest to a NSAH, many undergoing later secondary transfer to SNCs, arriving between 5 and 7 hours later than if they had been transferred straight from the scene of injury. 5 Up to one-third were cared for entirely within acute hospitals. The reasons for restricted access to specialist care were found to be ambulance service (AS) pre-hospital protocols dictating that all injured patients are taken straight to the nearest ED and low numbers of critical care beds in SNCs causing requests for transfer from NSAH to be declined. There were perceived risks, however, in transporting patients past the nearest NSAH, which may delay stabilisation of the patients’ airway, breathing and circulation (ABC).
At the time of applying, we (the applicants) felt that using a randomised trial to determine whether or not early neurosurgery facilitated by direct transportation to a SNC is of benefit would provide an appropriate evidence base for any future reconfiguration of trauma services. However, we recognised that there were significant issues to consider in designing a successful randomised controlled trial (RCT) that recruits patients with TBI in the pre-hospital setting. These include time pressures and targets for ASs, the challenge of identification and recruitment, paramedic preferences for control or intervention pathways that may bias patient enrolment, capacity considerations in receiving SNCs and the views of patients, relatives and staff. These considerations necessitated the robust feasibility study provided in this report.
During the application and contracting phase of our study, the Department of Health (DH) commissioned, for the first time, formalised trauma systems in NHS England. These systems – which largely came into being in April 2012 when recruitment for Head Injury Transportation Straight to Neurosurgery (HITS-NS) commenced – are predicated on the majority of major trauma (most of which is characterised by the presence of TBI4) being identified in the pre-hospital environment by triage protocols – which are similar to the inclusion criteria for this study. Any patient who is identified as having potentially sustained major trauma (including TBI) is brought to a major trauma centre (MTC) as long as the time from leaving the scene is < 45 minutes – most MTCs have onsite neuroscience facilities so can be classified as interchangeable with SNC within our study. The challenges of implementing our study of bypass alongside this new trauma system model of bypass are described during the methods section below. The discussion of HITS-NS study results will delineate their relevance to this new NHS context alongside that of other literature on the new trauma systems.
Initial cost–benefit estimate for early neurosurgery
Early neurosurgery consists of interventions within 4 hours of injury to reduce intracranial pressure (ICP), which rises after TBI and, if unchecked, causes secondary brain injury and brain death. The interventions include evacuation of intracranial haematoma [subdural haematoma (SDH), extradural haematoma (EDH)], establishment of ICP monitoring and resulting surgical interventions, such as ventriculostomy and decompressive craniectomy. To justify the feasibility analysis, we hypothesised that, should early neurosurgery be shown in a full trial to be cost-effective at absolute 30-day mortality and 6-month severe disability reduction of 5%, the extra costs to the NHS (£17M per annum3) would be offset by the significant economic benefits of reduced morbidity in this young population, which can be estimated from a 5% reduction resulting in 450 fewer TBI deaths and severe disabilities per annum; the annual societal saving, given a conservative estimate of each TBI death or severe disability costing society £250,000 (J Nicholl, University of Sheffield, September 2011, personal communication), would be £117M (£100M after NHS costs) – this excludes the value of any legal settlements.
Benefits of specialist neuroscience centre care within 12 hours of traumatic brain injury
An analysis of data from the TARN examined the effect of care in neurosurgical centres on outcome after severe TBI [Glasgow Coma Scale (GCS) score of < 8 or intubated on arrival at hospital and subsequently shown to have a brain injury] in 6900 patients. 4 All patients in this study were taken by paramedics to the nearest accident and emergency (A&E) department, regardless of specialisation. Outcomes were compared between those who were transported directly to an ED at a SNC or transferred there after stabilisation at the nearest NSAH and those who received care solely in an acute hospital setting. After case-mix adjustment for age, injury severity score (ISS),6 presenting physiology [combined scoring of first ED (GCS), systolic blood pressure and respiratory rate] and propensity scoring, patients who were treated solely outside neurosurgical centres had double the odds of death of those treated in a SNC. This was true regardless of whether the TBI required formal craniotomy and evacuation of haematoma or was generalised swelling managed with ICP monitoring in neurointensive care. This analysis was unable to adjust for pupillary responses and was not ‘intention to treat’; however, it was felt to support the premise that care in a neurosurgical centre should be made available within 12 hours of severe TBI regardless of the need for formal neurosurgery. 4 This has been endorsed by NICE1 in the 2007 guideline and by the 2008 Society of British Neurosurgeons. 7 Within the latter, a case has been made for increasing the number of intensive care beds within SNCs to enable them to accept a higher proportion of the patients with TBI referred to them. However, even if this provision were made, the pre-trauma system patterns of care – highlighted in the recent National Confidential Enquiry into Patient Outcome and Death (NCEPOD) report and other publications – would not have allowed patients with TBI injured nearest a NSAH to receive early neurosurgery (within 4 hours of injury5,8).
UK pre-hospital care systems and incident postcode determine current traumatic brain injury patient access to early neurosurgery (within 4 hours of traumatic brain injury)
It is currently assumed that neurosurgery for severe TBI is time-critical because ICP rises as intracranial volume expands after TBI. Thus, the sooner any intracranial clot is evacuated, or other measures to lower the ICP are used, the lower the chance of irreversible brain damage occurring through (ischaemic) secondary brain injury. What is less clear is how quickly early neurosurgery needs to occur. Early studies prior to the advent of routine computed tomography (CT) scanning suggested clot evacuation should occur within 2 hours of coma in EDH, or 4 hours of injury for SDH. 9 These analyses of observational data have large effect sizes, making a trial apparently unethical. However, current NHS data show that the two-thirds of patients with TBI who sustain injury nearest a NSAH cannot receive neurosurgery within these time frames (i.e. early neurosurgery = within 4 hours of injury). The analysis showed that in 2005–7 patients with SDH/EDH who are taken first to a NSAH are transferred to the neurosurgical centre on average 5–7 hours after injury. 5 This is due to inherent delays of stabilisation for CT/transfer and referral/acceptance communications. The only means of achieving early neurosurgery is to take all patients with TBI from scene to the nearest SNC, which – excluding the 10% of the UK’s population who live in remote and rural areas – is commonly < 1 hour’s journey time from the scene of injury.
[For other forms of early neurosurgery a Cochrane Injuries Group systematic review found the evidence inconclusive as to whether or not interventions such as ICP monitoring, and the interventions that result from it (generally possible only in neurocentres), are of benefit at any time point. 10]
Systematic review of early neurosurgery
The real uncertainty, therefore, lies in whether or not pre-hospital care systems should be reconfigured to enable patients with TBI to receive early neurosurgery – the risk being that NSAH bypass delays ABC stabilisation, particularly haemorrhage control (relevant as one-third of patients with TBI will have significant extracranial injuries), and may result in the transportation of injured patients who are unconscious through non-TBI causes to the SNC when they would have possibly been better treated locally. A systematic review has been conducted in conjunction with the NICE Head Injury Guideline Development Group (GDG) to address this. There have been no trials, but two North American observational studies were relevant. The first was a cohort study that obtained data from the New York State Trauma Registry from 1996 to 1998. 11 The population were adults (> 13 years) with scene GCS score of < 14. A subgroup of 2763 head-injured patients from a data set of 5419 trauma patients was analysed. Group 1 (n = 2272, 82.2%) were transported to regional/area trauma centre. Group 2 (n = 491, 17.8%) were assessed via American Triage system (pre-hospital care) and referred directly to a non-trauma centre. Study limitations included retrospectively categorising patients as ‘head injured’ from data reported in the trauma registry, and no intention-to-treat analysis of non-TBI patients with GCS score of < 14. The results of this study showed that the mortality rate of immediate transfer to a neuroscience centre compared with a non-trauma centre were in favour of transfer to neuroscience centre (NC), with an odds ratio of 0.88 [95% confidence interval (CI) 0.64 to 1.22]. 11
The second study described a cohort of patients aged < 21 years, admitted to 1 of 90 paediatric hospitals or trauma centres. The cohort compared three branches defined by the site of intubation: field, trauma centre or non-trauma centre. 12 Taking data from the last two branches, risk stratification was performed; degree of head injury was measured using the New Injury Severity Score and Relative Head Injury Severity Scale. No significant differences were found between the two scales or the place of intubation. However, a correlation was drawn between severity of injury and increased likelihood of survival with direct transfer to a trauma centre. 12 With this study it is difficult to draw rational conclusions as to the benefits of direct transport of patients from the scene to either a neurosciences unit or general hospital, as there is doubt caused by retrospective definition of head injury and whether multiply injured patients were included. The other study showed that the mortality rate of immediate transfer to a neurosciences centre was more favourable (not significant). 11 From this review there is weak evidence for direct transport of head-injured patients from the scene to a neuroscience unit being beneficial. Neither study considered longer – post discharge – outcomes.
A simulation model showed improved survival and cost-effectiveness from directly transporting patients to a neurosciences hospital. However, parameters were based on expert judgement rather than clinical data. 13
Consequently, the NICE Head Injury 2007 GDG indicated that early versus delayed neurosurgery facilitated by direct SNC transport from scene is an important research question that requires better evidence than that provided from the observational data reviewed. 11 Subsequent to HITS-NS being funded, a further Health Technology Assessment (HTA)-funded systematic review of bypass in suspected TBI did not show convincing benefit; however, studies were often of a low quality and it was often questionable whether or not any bypass had occurred. 14
Other relevant evidence concerning timing of early airway, breathing and circulation control and outcome
Three other studies in relation to the timing of control of ABC in severe injury merit consideration alongside the findings from the review of early neurosurgery. First, a Cochrane review15 has examined the effects of early intubation for injured patients in coma. This relates to the HITS-NS study question, as the control (usual care) group get earlier advanced airway care when transported to the nearest NSAH. However, this systematic review indicates that this is not necessarily of importance in this patient group. 15 Second, a recently published uncontrolled analysis of Yorkshire ambulance data linked to patient records suggests a 1% increase in mortality for every additional 10-km distance to hospital for all ill and injured patients. The excess mortality could be attributed to delays in ABC stabilisation, but not without controlling for confounders, which was not possible in this data set. 16 Finally, modelling of unpublished Portuguese observational data suggests that early intubation was associated with greater mortality benefits than early neurosurgery for patients with TBI in coma; however, recording of timings in this analysis was suboptimal, as was the performance of the model for predicting survival. 17 A further important consideration is that TARN data suggest that only 80% of patients with apparent TBI [significant reductions in consciousness level (GCS score of < 13) at the scene] are subsequently shown to have brain injury as the explanation. Other causes include haemorrhage and alcohol. 18
Possibility of examining the effect of early neurosurgery through a cluster randomised pre-hospital trial
If the only way to determine the effects of early neurosurgery is through a randomised pre-hospital trial of bypassing NSAHs then feasibility is a major consideration. Conducting pre-hospital trials in the UK has proved problematic but not insurmountable. In the past there have been issues about compliance with randomisation in individual patients. The most successful method used to date internationally is time cluster allocation, used successfully in the PPOPs (Paramedic Practitioners for Older People) trial of paramedic practitioners within Sheffield (alternate day cluster randomisation of paramedic vs. paramedic practitioner). 19 In North America, alternate day time cluster allocation has been used with some success in two trials of fluid and airway therapy. 20,21 In both of these settings, no outcome clustering was observed. However, this approach is expensive, requiring constant ‘senior despatch paramedic’ presence to reinforce alternate day allocation. This reinforcement also increases scene time. Given recent NHS performance management of AS responses times, this approach is now not pragmatic. Alternate week allocations require less reinforcement but overwhelm neurosurgical centres for the ‘intervention’ 7 days, when all patients with TBI in their region would be transported directly to them. One possible solution to these difficulties could be a unit of service cluster: the ambulance station. All paramedics from the same station would practise consistently within the same arm of the trial for the duration of the study, thus requiring less reinforcement of trial randomisation. The potential pitfalls of this approach include clustering of outcomes, contamination of the control by intervention group and selective compliance by paramedics depending on preconceived ideas. However, if the regional trauma networks – established in the wake of health reforms proposed by Lord Darzi21,22 – are to function, then it is essential to provide robust evidence concerning the benefits of early neurosurgery, evidence that this feasibility study and any subsequent large-scale trial would hope to provide.
Systematic review of pre-hospital trauma trials
In order to put the findings of HITS-NS into context, a decision was made post funding – by the HITS investigators – to conduct this study in order to best understand the feasibility challenges that are inherent in conducting pre-hospital trauma trials. The review was conducted by Nathan Chapman, a student at the University of Sheffield, as part of his Bachelor of Medical Science dissertation, supervised by the HITS-NS chief investigator (FL). Nathan’s time was not funded by the HTA funding of HITS-NS.
The review is reproduced in Appendix 1.
Chapter 2 Study objectives
The a priori study research objectives as per the application were as follows.
HITS-NS will:
-
determine the feasibility of conducting a cluster randomised trial of early neurosurgery in patients with TBI
-
determine the acceptability of the intervention (early neurosurgery) and control (usual care) pathways to patients, families and staff
-
estimate the ‘magnitude of effect’ of early neurosurgery and other parameters required for sample size estimation, thereby enabling costing of a full study (given successful recruitment)
-
determine the accuracy with which paramedics identify isolated TBI at the incident scene (given successful recruitment)
-
estimate the cost per quality-adjusted life-year (QALY) of early neurosurgery compared with usual care based on currently available data (including data from this pilot) and the degree of uncertainty surrounding this estimate
-
determine the expected value of sample information (EVSI) from a fully powered cluster randomised trial of early neurosurgery in patients with TBI
-
identify the major barriers to conducting a cluster randomised trial of early neurosurgery in patients with TBI and the strategies to overcome them
-
contribute to the existing evidence about conducting randomised trials in pre-hospital care through identifying barriers and facilitators of successful strategies that are generic to pre-hospital trials.
The HITS-NS study has work streams A (feasibility pilot: objectives 1–4, 7 and 8) and B (objectives 5, 6 and 8), which ran concurrently from May 2011 until November 2013. The methods, results and discussion of each of these elements are described sequentially in Chapter 3.
Chapter 3 HITS-NS stream A: feasibility pilot cluster randomised trial of early neurosurgery
Stream A is a feasibility study of a cluster randomised trial of early neurosurgery in patients with signs of significant TBI and no other life-threatening injuries at the scene of the incident. Two alternative patient pathways were compared. The first was the current practice (at the time of application) of transporting all injured patients to the nearest ED. The intervention group were transported directly from the scene to the nearest neurosurgical centre, provided that this was a < 1-hour journey from the scene. The study took place within two UK ASs.
Methods
Cluster randomised design and cluster eligibility and randomisation
Consideration was given to number of possible designs for randomising patients to early neurosurgery facilitated by acute hospital bypass. After extensive consultation and previous peer review comments, the investigators identified unit of service cluster randomisation as the most efficient and effective design that would not hamper emergency services in the pursuit of time-based targets. This design would also not overwhelm local NCs. Eligible clusters were ambulance stations within the North East Ambulance Service (NEAS) or the Lancashire and South Cumbria division of the North West Ambulance Service (NWAS), which would regularly attend to patients meeting the eligibility criteria below. There were 74 eligible clusters in total within the two participating ASs. The ScHARR (School of Health and Related Research) trial statistician at the University of Sheffield randomised these ambulance stations to intervention (early neurosurgery) or control (usual care) in a 1 : 1 ratio, and stratified that randomisation using a matched pair design. Each matched pair of cluster ambulance stations was equivalent in terms of AS, distance from neurosurgical centre, distance from nearest acute hospital ED and number of full-time acute ambulance patient transportation vehicles. Each cluster ambulance station remained within its randomised trial arm for the 12 months’ duration of patient recruitment.
Eligibility criteria
These are articulated below for the individual patients recruited to the study from the grant application. These criteria underwent several modifications through substantial ethical amendments, which are consequently described later in the text (see Table 1). The final separate NEAS and NWAS protocols that were approved through substantial amendments – as a merged protocol [prepared at the request of the Data Monitoring and Ethics Committee (DMEC)] – are provided in Appendix 6.
Inclusion criteria
Patients injured nearest an acute general hospital ED (NSAH) but not more than a 1-hour land ambulance journey from a NC and thought to be aged ≥ 16 years, when assessed at scene by ambulance personnel, with both:
-
signs of significant TBI, such as a reduced consciousness level (GCS score of < 13) and external signs of head injury, and
-
no overt signs of ABC compromise.
Prospective exclusion criteria
Patients who fulfil any of the following criteria will be excluded; that is, those who:
-
are thought to be aged < 16 years
-
have been found by the treating paramedic not to have signs of TBI at the scene (i.e. full or only mildly impaired consciousness: GCS score of > 12)
-
have obvious life-threatening injuries affecting ABC:
-
A – partial or complete airway obstruction/contamination present after simple manoeuvres.
-
B – respiratory rate of < 12 or > 30 breaths per minute or sucking chest wound or signs of tension pneumothorax, such as absent air entry into a hemithorax with contralateral tracheal deviation.
-
C – significant external haemorrhage not easily controlled by pressure or amputation above the wrist or ankle or absence of radial pulse on palpation.
-
(Paramedics recognise these signs as part of their current scope of practice.)
-
-
are injured at ≥ 1 hour’s travelling time from a NC.
Retrospective exclusion criteria (stream A only)
Any surviving patient for whom consent has not been given for follow-up by the AS Mental Capacity Act (MCA) consultee, patient or relative.
Important changes from original eligibility criteria for patients
New trauma bypass protocols came into effect in NEAS and NWAS, just before the HITS-NS study commenced (Table 1). Therefore, the study eligibility criteria had to be amended to fit in with their bypass protocols if confusion and chaos in the ASs were to be avoided. Essentially, compared with the original study criteria, NWAS instituted a lower respiratory rate exclusion criteria (< 10 instead of < 12 breaths per minute) and NEAS allowed level 2 emergency medical technicians to assess patients for bypass and included patients with a higher GCS score (≤ 13 instead of ≤ 12).
| Protocol characteristic | Original | NWAS | NEAS |
|---|---|---|---|
| Assessed by | Attending paramedic | Attending paramedic | Attending paramedic or Level 2 EMT |
| Inclusion criteria | Patients injured nearest to an acute general hospital ED, but not more than 1 hour’s land ambulance journey from a NC, thought to be aged ≥ 16 years when assessed by ambulance personnel and with both: | ||
| Signs of significant TBI, such as a reduced consciousness level (GCS score of ≤ 12) and external signs of head injury | Signs of significant TBI, such as a reduced consciousness level (GCS score of ≤ 12) and external signs of head injury | Signs of significant TBI, such as a reduced consciousness level (GCS score of ≤ 13a) and external signs of head injury | |
| No overt signs of ABC compromise | |||
| Exclusion criteria | Thought to be aged < 16 years | ||
| No signs of signs of TBI identified at the scene (i.e. full or only mildly impaired consciousness (GCS score of ≥ 13) | No signs of signs of TBI identified at the scene (i.e. full or only mildly impaired consciousness (GCS score of ≥ 13) | No signs of signs of TBI identified at the scene (i.e. full or only mildly impaired consciousness (GCS score of ≥ 14a) | |
| Life-threatening injuries, affecting ABC, identified at scene: | |||
| A Partial or complete airway obstruction/contamination present after simple manoeuvres, or any patient who has been intubated or had a supraglottic device inserted at the scene of injury | |||
| B – Respiratory rate < 12 breaths or > 30 breaths per minute or sucking chest wound or signs of tension pneumothorax, such as absent air entry into a hemithorax with contralateral tracheal deviation | B – Respiratory rate < 10 breaths or > 30 breaths per minute or sucking chest wound or signs of tension pneumothorax such as absent air entry into a hemithorax with contralateral tracheal deviation | B – Respiratory rate < 12 breaths or > 30 breaths per minute or sucking chest wound or signs of tension pneumothorax such as absent air entry into a hemithorax with contralateral tracheal deviation | |
| C Significant external haemorrhage not easily controlled by pressure or amputation above the wrist or ankle or absence of radial pulse on palpation | |||
| Scene of injury > 1 hour’s ambulance journey time from a NC | |||
| Retrospective exclusion criteria | Any surviving patient for whom consent has not been given for follow-up by the AS MCA consultee, patient or relative | ||
Finally, the Trial Steering Group (TSG), prior to patient recruitment commencing, asked that the airway exclusion criteria be extended to include any patient for whom a supraglottic airway had been provided at the scene. These amendments to the original eligibility criteria were all approved in a series of substantial amendments by the study Research Ethics Committee (REC).
Patient identification
Eligible patients were identified both through NEAS and NWAS paramedics in the clusters making direct contact with the study research paramedics – when they had identified a patient as meeting the study inclusion criteria – and through daily screening of the AS patient report forms using electronic databases. The latter was highly resource intensive for research paramedics, as it entailed screening large numbers of patients daily who had been ‘trauma pre-alerts’ to receiving hospitals or for whom the word head injury had been written somewhere on the ambulance patient report form (PRF), and sometimes there were delays in the ambulance stations scanning the paper PRFs on to the service electronic data bases, which could result in delays in patient identification. Once eligible patients had been identified from the screening or direct contact, the research paramedic would contact the hospital in question to confirm the identification details (it is not uncommon for head injury patients with impaired consciousness levels to be solely ‘unknown males/females’ throughout their contact with the AS if there are no relatives/friends at the scene of injury) and determine the patient’s current location (inpatient/died/discharged/transferred to another hospital) in order to facilitate the approach for consent. In this way it was assumed that all eligible patients, whether they had been identified by the cluster trial paramedics or not, would be included in the trial. In the end it was the study research paramedics who determined finally whether or not the patient was eligible for recruitment. The screening standard operating procedure (SOP) developed by the Trial Management Group (TMG) and approved by TSG is given in Appendix 2.
Ethical basis and practicalities of obtaining informed consent from participants whenever possible or action for which fully informed consent was not possible
It was not possible to obtain informed consent for the trial from HITS-NS patients at the scene of injury. Patients did not have capacity owing to the HITS-NS inclusion criteria being a reduced consciousness level. The time frames involved did not allow patients’ next of kin, if available, or the nominated consultee for the AS, sufficient time for consideration. Consent for research in this situation is covered by the MCA Section 32. 9 Hence we obtained ethics approval to enrol patients into the trial at scene with later consent for follow-up and inclusion of data from the North Wales REC 10 (10/WNo03/30), which specialises in ethics approval for studies involving adults who lack capacity. Consent to follow-up and inclusion of data were obtained by the HITS-NS research paramedics – or occasionally by a Comprehensive Local Research Network (CLRN)-funded research nurse in one of the participating hospitals – from patients who recovered capacity. When capacity was not recovered, advice was sought from a consultee who was, in general, either the next of kin or the AS nominated consultee for the MCA as appropriate prior to hospital discharge.
It became clear early on in patient recruitment that significant numbers of study patients had relatively minor injuries and were being discharged from hospital within 24 hours of arrival before they could be approached face to face for consent by research paramedics. With the approval of a REC we amended the protocol and patient information sheet to allow these ‘early discharge’ patients to be written to for consent and to reply by sending back a slip in a stamped addressed envelope as agreement to discuss participation by telephone. The patient information sheet and consent form were also sent in the mailing. On receipt of the slip, the research paramedic telephoned the patient to explain the study and obtain consent, where appropriate; patients were encouraged to then sign and return the consent form. Patients were also able to return the consent form by post without discussion if they felt happy to do so. Further approval was gained to send text reminders when mobile phone details were available for patients to respond. At our request the REC was happy for us to retain anonymised data, including 30-day mortality on all eligible patients, counting those who did not respond to the postal invitations to participate further but had not explicitly refused consent. From the outset, we had REC approval to retain anonymised 30-day mortality data on all patients who died within a week of admission to hospital without approaching distressed relatives.
This proposed pathway for obtaining consent is consistent with the DH guidance in relation to research in the emergency setting and Good Clinical Practice in research on adults who lack capacity. 23 The consent SOP developed by the TMG and approved by TSG is given in Appendix 3.
Setting
The study was conducted in two regional AS NHS Trusts – the Lancashire and South Cumbria trauma network subdivision of the NWAS and the NEAS – covering three neuroscience hospital centres (SNCs) and 11 non-specialist ‘acute’ hospitals with type 1 (accept ‘999’ ambulances and supported by full resuscitation facilities) EDs (NSAHs).
The ASs, SNCs and NSAHs covered by the trial are shown below (Table 2). Each AS covers a mixed urban and rural population. TARN data suggest that the trauma cases, (and their outcomes) received by the hospitals in the proposed regions for this study are similar to those nationally represented on TARN. The participating ASs involved received 0.5 out of 3 million emergency ‘999’ ambulance calls in England (excluding London) in 2006–7 and generated similar performance data to that of the rest of England. 24 At the time of commencing recruitment, the London Ambulance Service was already operating early neurosurgery technology (no outcome data available) as part of their trauma system. Therefore, there was a lesser requirement for the trial population to be representative of that within London. The TMG felt that this evidence suggested that they were representative of the population to which early neurosurgery made available by NSAH bypass would apply.
| Trial NC | Trial acute hospitals | Ambulance service |
|---|---|---|
| RPH | Blackburn Royal Infirmary Blackpool Victoria Hospital Royal Lancaster Infirmary |
NWAS |
| RVI | North Tyneside General Hospital Queen Elizabeth Hospital (Gateshead) South Tyneside District Hospital Sunderland Royal Hospital Wansbeck General Hospital |
NEAS |
| JCUH | University Hospital of North Durham Darlington Memorial Hospital University Hospital of North Tees |
NEAS |
For the purposes of the study, the trial NCs were also identified as research sites, as patients frequently consented therein; the acute hosptials were designated as participant identification centres, where patients were followed up for consent and after discharge.
Interventions
The control and intervention patient pathways are described in Figure 1: within these, the following interventions are relevant.
FIGURE 1.
The HITS-NS feasibility pilot control and intervention patient pathways.

Time
The HITS-NS trial was not studying a new patient intervention; the new technology under scrutiny was the timing of neurosurgery in patients injured nearest an acute hospital ED compared with the time to any interventions that may be required to stabilise the injured patient’s ABC. Time zero was the time at which paramedics left the scene of the incident with the injured patient. The time frames were measured identically in all clusters/arms of the trial.
Neurosurgery
Neurosurgery included any craniotomy for evacuation of intracranial haematoma, debridement of open fractures and insertion of ICP monitor. Time to neurosurgery was from time zero to the time that the patient arrived in theatre, for whichever of these procedures came first. It was envisaged that this would occur early (within 4 hours of time zero) in patients presenting to the intervention clusters and later after secondary transfer in the control clusters, but was measured identically in both.
Airway, breathing and circulation stabilisation
The interventions that stabilise the injured patients’ ABC that fall outside the scope of paramedic practice include endotracheal intubation (ETI) facilitated by drugs, decompression of tension pneumothorax (if present) and surgery/interventional radiology to control internal haemorrhage, as dictated by the patient’s injuries and physiological status. It was envisaged that most HITS-NS patients would require ETI; the other interventions being less frequently required. The time to each of these interventions was to be recorded, the time to ABC stabilisation being from time zero to whichever ABC intervention procedure was first commenced. It was measured identically in all clusters/trial arms and it was thought likely, but not necessarily, to be a given that this would occur up to 30 minutes earlier from time zero in the control (usual care) group. Paramedics were trained to exclude patients with signs of imminently requiring these interventions from the study.
Study roll-out
Following the implementation of a range of set-up phase activities that needed to be completed before patient recruitment could begin and after delays by factors beyond the control of the chief investigator and trial manager, such as the signing of contracts and the recruitment of local study co-ordinators (the NEAS trial co-ordinator Graham McLelland started in post on 28 November 2011, and the NWAS trial co-ordinator Betty Pennington started in post on 13 February 2012), phased recruitment commenced in NEAS in January 2012 and in NWAS in April 2012.
The key set-up phase activities that required completion prior to the start of recruitment included:
-
research governance and information governance processes
-
obtaining REC approvals
-
finalising subcontracts with ASs and other partners
-
obtaining all site-specific information (SSI) approvals and NHS permissions including Caldicott approvals and letters of access for the two local trial co-ordinators and the trial manager
-
-
implementation of paramedic training
-
development of trial SOPs
-
preparation of numerous complex SOPs that were necessary for the conduct of the trial [e.g. participant recruitment, consent, data collection and management, participant follow-up, reporting of serious adverse events (SAEs), ‘stopping the trial’]
-
establishing processes for HITS-NS data collection, clinical record form (CRF) and data retrieval in collaboration with the TARN, who were to be responsible for assisting with trial data management and security
-
-
promoting the trial and raising awareness around trial recruitment
-
establishing links with colleagues within the trial neurocentres and trial acute hospitals, and raising awareness of the trial
-
issuing press releases and AS bulletins
-
displaying trial information posters in the NHS settings involved
-
creating a website.
-
Only once these activities had been launched and, where necessary, completed – including piloting of numerous and complex trial procedures, resolving a number of challenges impacting on progress – could recruitment commence.
Paramedic training
This was delivered to a large number of staff. In NWAS training was delivered to > 350 paramedics at 28 stations, and in NEAS to > 500 paramedics and advanced technicians at 46 stations. A paramedic training strategy was developed by the trial manager, Dr Wanda Russell, and was enhanced by additional material developed by the research paramedics (local trial co-ordinators) to ensure optimal delivery of the strategy within the two ASs. The content of the training covered the following dimensions.
The objectives were:
-
to introduce the HITS-NS trial
-
to inform paramedics of their role in HITS-NS trial
-
to identify sources of key information about the trial and further points of contact regarding any aspect of the conduct of the trial relevant to the role of the paramedic.
The structure of the training programme was as follows:
-
‘Why?’ The background to HITS-NS.
-
‘What?’ The research objectives of the trial.
-
‘How?’ The design of the trial – inclusion criteria, recruitment of patients, sample size, interventions, consent process and trial data.
-
‘Who?’ The roles and responsibilities of the chief investigator and the local principal investigators (PIs), research sites (ASs, neurocentres), participant information centres (PICs), research paramedics/local co-ordinators, trial manager, other collaborators and partners.
-
‘Where?’ Geographical locations.
-
‘When?’ Anticipated launch dates and timeline for the trial.
Initially, training in NEAS was delivered on a divisional basis, with the AS stations in the two NEAS divisions potentially involved in bypass to the James Cook University Hospital (JCUH) being targeted for training first. A more extensive training roll-out then followed; however, the roll-out of the new Major Trauma Bypass (MTB) protocol in NEAS required that HITS-NS training was redesigned to show how the HITS-NS trial would run alongside the MTB protocol, and a revised training package was delivered to all paramedics. Training was launched across all HITS-NS stations at the same time in NWAS, with training strategies, including face-to-face training, cascading down via team leaders, self-directed learning packs and online training via the HITS-NS website. Uptake of training among paramedics at all NEAS and NWAS ambulance stations was monitored and logged using processes appropriate to each region. This involved, for example, notification of completed training by individual paramedics via an e-mail sent to the local trial co-ordinator or by the return of a slip certifying completed training (see Appendix 4 for training materials).
Initially, recruitment in NEAS was launched using a phased approach, commencing in January 2012. There were 46 ambulance stations – with 23 randomised to the intervention arm and 23 to the control arm of the trial – involved in recruitment to HITS-NS, with caution initially being observed about allowing intervention stations to go live until a minimum of 70% paramedics at a given station had returned signed forms stating that they had completed their HITS-NS training. This resulted in a gradual roll-out of stations. However, from 2 April all 46 stations were formally launched alongside the launch of the new trauma bypass protocol in the NEAS region. The new trauma bypass roll-out involved a mail-out of the bypass protocol to all paramedic staff in NEAS, and mandatory HITS-NS training material for paramedics was included in this mail-out. Therefore, the NEAS approach to training sign-off was adopted in that the posting of the mail-out to individual paramedics provided HITS-NS training, which did not require a signed form to confirm completed training. This process, therefore, facilitated the launch of the trial across the region.
Similarly, in NWAS, an initial strategy required that a minimum of 70% of paramedics returned HITS-NS training confirmation to qualify individual stations as ready for launch. This was achieved with face-to-face training in the 28 cluster ambulance stations. However, as in NEAS, training was reinforced with the inclusion of a mandatory training pack in the roll-out of the new trauma bypass protocol, and from this point the launch of all stations was considered complete.
The trauma bypass protocols for NEAS and NWAS have had differences that have impacted on the inclusion/exclusion criteria for HITS-NS, necessitating protocol amendments to cater for both regions, which required submission of substantial amendments to the REC, with approvals being granted.
Promoting the trial and raising awareness around trial recruitment
Much work was done to raise awareness of the HITS-NS trial in the trial neurocentres and acute hospitals. Meetings were held with research nurses, consultants and research and development (R&D) staff at all three neurocentres (in NEAS and NWAS), at which staff from PICs also attended. The purpose of these meetings was to ensure that contacts in PICs and neurocentres could be established to assist in the process of tracking patients who were recruited into HITS-NS. The study was given good support and systems were put into place that allowed the local trial co-ordinators to make contact with designated individuals at PICs/neurocentres who were able to advise about the location and condition of HITS-NS patients. The research nurses received briefing about the consent-taking process in order to assist in this activity.
Trial site files, including delegation logs for completion, were established at all research sites and PICs.
Awareness of the HITS-NS trial was reinforced in the trial neurocentres and acute hospitals prior to the launch of the trauma bypass protocols, and meetings were held with clinical staff to discuss how HITS-NS could be conducted alongside the new trauma bypass protocols. 25 Close liaison continued with colleagues throughout the duration of the trial to ensure, in particular, that patient consenting processes and data collection ran smoothly.
The trial also received coverage by local press in the NEAS region and a press release was issued in NWAS.
A HITS-NS website was developed to inform the public of the trial and also to include comprehensive training reference material accessible to paramedics only (www.hits-ns.tarn.ac.uk).
Promotional materials – including small tins of a variety of sweets and pens, all with HITS-NS logos – were also distributed to paramedics in NWAS and NEAS.
Development of trial standard operating procedures
Standard operating procedures were successfully developed for the recruitment and consent processes, for data collection and management, for stopping the trial early and for the reporting of SAEs at the outset of the trial. These were subsequently reviewed and modified to include recommendations from the TMG and the TSG and based on observations and developments arising from piloting the procedures where relevant.
A comprehensive screening procedure for identifying potential HITS-NS patients and a screening log for identifying eligible patients were also developed as part of the recruitment SOP. The screening process for identifying potential HITS-NS patients also required close collaboration between the research paramedics and staff, in the informatics departments of NEAS and NWAS, to identify possible appropriate and efficient mechanisms involving data downloads of call data and electronic patient report forms. This particular activity required considerable planning and extensive testing (see Appendices 2 and 3).
In relation to data collection and management, the local trial co-ordinators both completed TARN data entry training, and meetings with TARN data co-ordinators took place both in the North East and the North West, as the TARN data co-ordinators were to assist in prioritising data entry on to the TARN database for HITS-NS patients. The data collection and management SOP included processes to allow the local trial co-ordinators to complete data entry for non-TARN patients using the TARN-based CRF, and also for non-TARN data variables for all patients. The trial manager worked with a TARN analyst to set up a data download system for HITS-NS patients, and the data entry system was also thoroughly tested prior to becoming operational (see Appendix 5).
Implementation challenges
It is important to note, given the feasibility nature of the trial, that there were a number of challenges to be managed during the roll-out (and subsequent implementation) of the study. The key challenges experienced can be summarised as follows.
-
Paramedic training for recruitment to HITS-NS:
-
This had been delivered to paramedic staff already pressured by a growing volume of new protocols and procedures in their work.
-
Slow uptake due to the dependency on goodwill among paramedics who are restricted by work rotas and unavailability of cover to allow for training during working hours.
-
Setting up effective systems to allow for monitoring training uptake was extremely difficult prior to the launch of mandatory training alongside the roll-out of the MTB protocols in NEAS and NWAS.
-
A confusion had arisen from misunderstood randomisation instructions from the trial statistician, which led to a number of ambulance stations being initially wrongly identified as intervention/control stations, with about 30 paramedics receiving the wrong training – this situation was promptly rectified as soon as this confusion came to light.
-
-
Challenges within the ASs:
-
The HITS-NS trial was conducted alongside a new MTB Protocol launched in April 2012 (which was applied to the whole trauma population attended by ASs). The protocols identify specific trauma patients outside HITS-NS inclusion criteria who were to bypass into the newly designated MTCs. In the study areas, the MTCs were the SNCs.
-
Previously limited involvement in complex research trials.
-
Wide geographical areas included in the study.
-
-
Diversity in research and governance procedures:
-
Individual organisations have different processes.
-
Time delays in obtaining research site approvals/letters of access for the research paramedic and participant identification centre permissions for the conduct of the trial.
-
-
Delays in staff recruitment.
Close collaboration with partners, and the continuous monitoring of these challenges by the research team and the TMG and steering group, allowed strategies and processes to be refined to ensure that the trial could continue in accordance with the study timeline and planned activities.
Main feasibility outcomes
In the application, the following separate stream A primary feasibility outcomes were identified as necessary to permit progression to a full trial application:
-
The actual recruitment rate compared with required recruitment rate to HITS NS for each AS. For the study to be considered feasible the monthly recruitment rate should be at least 50% of that required and increasing at 12 months. The required rate is determined by the power calculation as 700 over 12 months but 350 with the monthly rate increasing in both ASs was felt to be acceptable.
-
Rates of actual TBI in patients recruited to the trial. For further study to be considered feasible, this should exceed 80% in each AS and be equivalent between trial arms.
-
Rate of compliance with trial randomisation for each AS. For further study to be considered feasible the non-compliance rate should not exceed 10% (in each arm).
-
The degree of selection bias caused by non-compliance with HITS-NS randomisation for each AS. For further study to be considered feasible there should be no significant difference between the characteristics of patients in groups where randomisation is and is not complied with. This should be true overall and within each trial arm. These characteristics include absolute patient transportation times from the nearest NSAH and neurosurgical hospitals and the increase in transportation time involved in bypassing nearest NSAH. There should also be equivalence of factors determining survival and disability after TBI, including age, ISS and severity of TBI.
-
Rates of acceptability of control and intervention pathways to patients, staff and families. For a full trial to be feasible there should be no significant difference between trial arms. This was assessed by patient satisfaction questionnaires, logging of complaints and incident/SAE reporting.
-
Numbers of eligible patients with TBI on TARN database who presented to NEAS study hospitals during recruitment period and were not included in study. (This was added early in the study when the unanticipated case mix of mild TBI prevalence became apparent.)
The secondary outcomes are measures of patient morbidity and health-related quality of life, which would form primary outcomes in a full trial and will inform the effect size in a power calculation for a full trial. The secondary outcome data will also be fed into the EVSI (stream B) analysis. These include 30-day mortality and 6 months Extended Glasgow Outcome Scale (GOSE)26 and European Quality of Life-5 Dimensions (EQ-5D)27 values and responses to the patient satisfaction survey. A subset of patients consented to in-depth qualitative interviews about participating in research.
Randomisation and blinding
The process of cluster selection and randomisation by matched pairs has been described above (see Cluster randomised design and cluster eligibility and randomisation). Each ambulance station cluster remained within its original allocation for the 12-month duration of the feasibility pilot. As the study of early neurosurgery was being facilitated by hospital bypass it was ‘open label’ to a degree. Blinding was preserved where possible and is described below.
The practitioners involved in the patients’ care, including the paramedics attending the incident from control and intervention cluster ambulance stations, were fully aware of the patients’ trial status throughout the course of their treatment. It is possible that some of the ED, neurosurgical and intensive care unit (ICU) staff in both NCs and acute hospitals may have been aware, as they had been briefed about the study.
The patients themselves could be considered to have been initially ‘blinded’, as the majority had an impaired GCS score on initial recruitment, which would have rarely recovered by the time they reached hospital, and therefore they would not have been aware of their transportation destination. Patients who remained incapacitated up until their 6-month follow-up, or who died prior to the 30-day mortality recording, are likely to have remained unaware of their transportation or involvement in the trial and, therefore, continued to be ‘blinded’. Surviving patients who regained capacity would not have remained blinded in this sense, as they would have been approached for consent, or posted information regarding the trial, and would have been aware of whether or not they were taken to their nearest hospital.
The outcome recorder of 30-day mortality from the hospital or summary care record was blinded throughout the study. The outcome collator (research paramedic or TARN data co-ordinator), who records patients’ details, including 30-day mortality and baseline characteristics, on to the study database, was not blinded. They were aware of the patients’ pathways and identifiers as a result of the nature of the records they had to access (e.g. paramedic patient report form) to identify the relevant information.
Patients were recorded on the trial database by the outcome collators using a unique study number, for which the second digit was either ‘1’ or ‘2’ according to which trial arm they were in. Which trial arm was recorded as ‘1’ or ‘2’ was unknown to the trial statistician when they received the trial database, and therefore they were blinded when analysing the outcomes of the trial for the DMEC, but were unblinded for the analyses in this final report. However, the trial manager was responsible for checking that the labelling of patients into either ‘1’ or ‘2’ was consistent across both trial sites and, therefore, was not blinded.
Proposed sample size
We aimed to recruit 700 patients across the two ASs in the stream A feasibility pilot in 74 clusters.
This was based on our full trial power calculation, which for a 3-year full trial indicated that (3 × 1400) 4200 patients would have 80% power to detect an absolute 5% change in rate of poor outcome (two tailed for GOSE score of < 5) assuming a 30% (supported by TARN/CRASH28 data) event rate, and an intracluster correlation coefficient (ICC) of 0.02 between ambulance stations and 5% risk of type 1 error.
Assuming that the EVSI showed that a full trial was cost-effective, it would take place in 120 clusters in four ASs. The sample size target was half the required annual recruitment rate, as in the pilot we recruited from 50% of our clusters.
Data collection and follow-up
The baseline data that were collected to enable the description of the overall cohort included demographics, detailed cranial and extracranial injuries, on-scene physiology, transportation times, times to neurosurgical and other life-saving interventions, ISS,6 GCS score, systolic blood pressure, oxygen saturation and pupillary responses at scene. Length of stay data were collected on the first and subsequent hospital destinations. These data were collected by the HITS research paramedics and hospital TARN data co-ordinators using the TARN electronic data collection and reporting system [Electronic Data Collection & Reporting (EDCR); www.tarn.ac.uk]. TARN trained the trial staff in the use of the EDCR; some additional (non-TARN) data were collected on each patient to allow identification of to which cluster they had first presented and that all of the on-scene eligibility criteria applied. As per normal TARN procedures, all injuries were then coded using the Abbreviated Injury Scale (AIS)29 by TARN staff who are accredited in using the AIS 2005 dictionary.
All HITS-NS patients who survived to hospital discharge and had identifiable information were approached by research paramedics for consent for inclusion of data in the trial and follow-up at 6 months. Their details were checked against the summary of care record so that only patients who were still alive at 6 months were telephoned by either the HITS research paramedic or the trial manager for administering of the EQ-5D, GOSE interview and patient satisfaction questionnaire. Patients’ general practitioners (GPs) were also be informed of patient inclusion in the study. Through consultation with the Headway and the work of a University of Manchester year 4 medical student, Hannah Newcombe, the TMG developed a patient satisfaction questionnaire to determine acceptability of both trial arms (study objective 2). The follow-up SOP developed by the TMG and approved by the TSG is provided in Appendix 5.
The early interim analyses of HITS-NS data indicated a need to check the robustness of the screening procedures. Anonymised data from TARN TBI patients presenting to HITS-NS acute hospitals and transported by the NEAS during the study period were checked against anonymised NEAS records to ascertain reasons any for non-inclusion in HITS-NS. FL, as research director of TARN, has National Information Governance Board (NIGB) approval for research on anonymised records [ECC&–05(g)].
Patient and public involvement
Headway was closely involved in the design of the trial at application (FL presented at 2009–10 Salford and Trafford Headway meetings), including discussion about applying the Mental Health Act. Subsequently, Headway assisted in determining the content of the patient and consultee information sheets. As members of the TSG, Hugh Potter (HP) and Alastair White (AW), of Headway, represented the interests of patients and public during the conduct of the trial. HP and AW conducted regular discussions of trial progress with Headway members at regional Headway group meetings. Salford and Trafford Headway allowed some of their patient members to be interviewed about the appropriate design and timing of administration of the patient satisfaction questionnaire. Headway approved SOP guidance (see Appendix 3) for trial staff in establishing capacity to consent and continue with the trial.
Hugh Potter attended the HITS-NS collaborators meeting in November 2013, at which the study findings were presented to coapplicants and staff at the research sites. This collaborators meeting was also attended by Beryl Howgate (BH) from ‘Second Chance’, a head injury patient organisation affiliated with Headway. BH has worked with the chief investigator on other head injury projects. Both patient members supported and approved the analyses of HITS-NS and study findings as being important for improving the future care of patients with suspected significant TBI.
Research governance
Research governance was ensured at all stages of HITS-NS by REC approval (10/WNo03/30), registration of the trial with the CLRN injuries and emergencies portfolio, and R&D departments of all the NHS trusts involved as research sites or participant identification centres. The University of Manchester acted as HITS-NS sponsor and the trial adhered to International Committee on Harmonisation Good Clinical Practice regulations and the NHS Research Governance Framework 2005. Each amendment to the protocol was approved by a REC substantial amendment via the sponsor and all of trial sites. The reporting of the trial is consistent with CONSORT (Consolidated Standards of Reporting Trials) recommendations on the reporting of cluster randomised trials.
The HITS-NS TSG and DMEC (which reported to the TSG) each contained a majority of independent expertise, including patient and public involvement, and ensured effective trial management and conduct in accordance with these stipulations. These groups met regularly throughout the set-up and conduct of the trial; they approved the revised protocols, SOPs and interim 30-day outcome analyses to ensure that the interests of patients and the pursuit of high-quality data were paramount throughout the study.
Statistical analyses
The analyses are designed to address the outcomes of the feasibility arm of HITS-NS by:
-
Estimating the recruitment rate in control and intervention arms as a proportion of 350 with 95% confidence limits.
-
Estimating the rate of TBI in each study group; assuming that this will be 80%, the sample size of 350 per group could estimate this parameter with a standard error (SE) of 2.2%.
-
Estimating the rate of compliance with allocated treatment in each study group; assuming this could be 90%, the sample size of 350 per group could estimate this parameter with a SE of 1.6%.
-
Comparing patients in the control and intervention arms in terms of factors known to influence prognosis in severe TBI: age, GCS score, systolic blood pressure, oxygen saturation, type and severity of brain injury, overall ISS and pupillary responses; we will also compare times to neurosurgical centre and nearest hospital. These comparisons will be made with appropriate parametric and non-parametric tests to enable the detection of selection bias.
-
Estimating rates of acceptability of control and intervention patient pathways to patients and families using results from the questionnaire mailed at 6 months post inclusion.
-
Comparing 6-month GOSE (relative risk of poor outcome using a sliding dichotomy30 and EQ-5D scores [with 95% confidence limits or interquartile ranges (IQRs)] plus 30-day mortality rates (95% confidence limits in each arm), in the control and intervention groups, on an intention-to-treat basis. The study is not powered to detect differences that would be the purpose of a full trial; however, as our current effect size is an estimate, the data for this analysis were sent to the independent DMEC at (30-day mortality only) 6 and 9 months post start of recruitment.
The 95% CIs required were calculated by the Wilson procedure using an online resource. The CIs for the difference between two independent proportions in control and intervention were also calculated by the Wilson procedure, using a similar online resource. The Mann–Whitney U-tests were used to compare control and intervention groups in terms of ordinal variables (ISS, GCS, pupillary responses) or variables that are not normally distributed in adult trauma populations, such as age.
The merged study protocol is given in Appendix 6.
Stream A: feasibility study results
Clusters recruiting by ambulance station and study arm
Figures 2 and 3 describe the flow of patients through the study, and it is interesting and instructive to compare this with the study planned CONSORT diagram in Appendix 6 (the full protocol).
FIGURE 2.
Cluster randomisation within ASs.

FIGURE 3.
Study CONSORT diagram. GOS, Glasgow Outcome Score. a, Median/IQR number of patients recruited by each cluster.

Using the matched pairs of randomised ambulance station clusters described above (see Cluster randomised design and cluster eligibility and randomisation) (37 matched pairs), eligible ambulance station clusters were randomised to either control or intervention. Forty-six clusters from NEAS and 28 from NWAS were randomised. No clusters were lost to follow-up. Of 46 NEAS clusters, 43 recruited patients, whereas fewer than half (13/28) of the NWAS clusters recruited patients.
In the intervention arm, therefore, 29 (78%) out of a possible 37 clusters recruited patients with a median cluster size of 6 patients (IQR 3–8 patients); in the control arm, 27 (73%) of 37 possible clusters recruited patients with a median cluster size of 3 patients (IQR 1–7 patients).
The matched pairing of clusters prior to randomisation was an attempt to secure equal numbers of patients in the two arms of the study, as the clusters were matched on numbers of ambulance vehicles at the station (‘busyness’) and distances from neuroscience and acute hospitals. However, as exact numbers of ‘HITS-NS eligible’ patients were not known prior to study commencement, this was clearly an estimate. In the end there was a preponderance of patients in the intervention arm (169) compared with those in the control arm (124), whereas, ideally, 146 patients would have been recruited into each arm of the study.
Screening and responses to consent requests in consort
Large numbers of patients (> 81,000 in both ASs; see Figure 2) were screened for eligibility to cover periods where the study co-ordinators were ‘off shift’ and, therefore, unable to take call and text alerts identifying study patients, or when paramedics had either not recognised or not had time to flag up a HITS-NS patient. As described in Chapter 3 (see Ethical basis and practicalities of obtaining informed consent from participants whenever possible or action for which fully informed consent was not possible) one of the unexpected observations early in the study was that a large numbers of eligible patients turned out not to have injuries that were serious enough to detain them in hospital for > 24 hours. This often meant that by the time the screening process had identified them as eligible and their location had been confirmed by the study co-ordinator, they had left hospital.
A substantial amendment to the original protocol (see Appendix 6) gave the investigators permission to approach patients by mailing the information sheets and consent forms (as personal approaches in hospital were not possible), followed by text reminders. The majority of patients did not respond to these mailings and texts; however, the REC allowed retention of anonymised data on these patients (non-responses to consent request) up to 30 days post injury. A small number of patients (six intervention and five control) declined to consent after face-to-face or mailed approaches; three intervention patients had no NHS number (and no registered GP), which made it impossible to meaningfully identify their 30-day mortality outcomes. Six patients (one intervention and five control) had no meaningful identifiable data on their ambulance PRF (not uncommon in unconscious patients arriving at the ED – often described as ‘unknown male’) and could not be further identified using ED records. One identified control patient was discharged early to ‘no fixed abode’ and therefore could not be approached.
Availability of patients for 30-day and 6-month follow-up in consort
As a consequence of the responses to consent requests, 159 out of 169 (94%) eligible intervention patients and 113 out of 124 (91%) patients had data that were sufficient to analyse their 30-day outcomes.
Twenty-four patients died early during the initial hospital admission. A priori REC approval to retain the anonymised data and 30-day outcomes of those who died prior to 7 days post admission without approaching distressed families for consent had been granted. Out of those who formally consented, some patients were not available on the telephone numbers supplied to provide 6-month outcome data. A minor REC amendment was granted, which allowed the outcome questionnaires to be mailed after no telephone contact had occurred: four intervention and two control patients responded to mailed 6-month outcome questionnaires, leaving (from 46 patients who formally consented) seven intervention patients and seven control patients who were not available for follow-up and were thought to be alive. Four patients (three intervention and one control) were known to have died between providing consent and approach for 6-month follow-up. At the time of writing this gave 33 out of 169 (20%) and 24 out of 124 (19%) of intervention and control patients, respectively, who had data that were sufficient to analyse their 6-month outcomes.
Recruitment period
The initial planned recruitment, as per the application, was to appoint the trial manager in July 2010 and commence recruitment in January 2011. In the end – owing to responding to HTA board questions, contract and subcontract negotiations and the time taken to recruit and train trial staff – the trial manager started in post in May 2011. Recruitment started in the NEAS in January 2012. The first 3 months of the study were spent training paramedics in the NEAS and piloting the study procedures of screening, identifying and locating patients and approaches for consent. Four study patients were recruited between 2 January 2012 and 31 March 2012. Recruitment started in full in the NEAS on 1 April 2012. It took slightly longer to get the NWAS study co-ordinator in post, so recruitment did not start in full until mid-April 2012. Recruitment ran in both ASs until 31 March 2013, as planned for the feasibility study. There was no withdrawal of clusters or research sites during this period, although one of the NHS trusts that acted as a PIC withdrew in mid-December 2012 (see Complaints and serious adverse events). This did not impact significantly on recruitment.
Table 3 compares the basic clinical characteristics of the control and intervention patients in an intention-to-treat analysis. The characteristics compared are those of which paramedics would have been aware at the scene of injury. In both groups, approximately two-thirds of patients were male, with the median age in the mid-forties.
| Patient characteristic | Intervention (n = 169) | Control (n = 124) | Difference (95% CI) |
|---|---|---|---|
| Percentage male (95% CI) | 169, 69.8% (62.6% to 76.4%) | 124, 66.1% (57.5% to 74.1%) | 3.6% (–7.1% to 14.5%) |
| Age in years, median (IQR) | 169, 44.6 (29.6 to 70.1) | 122, 48.8 (29.8 to 65.3) | –0.5 (–5.8 to 4.9) |
| Scene GCS score, median (IQR) | 169, 12 (8 to 13) | 124, 12 (8 to 13) | 0.02 (–0.80 to 0.84) |
| Percentage with normal pupillary response at scene (95% CI) | 115, 96.5% (91.4% to 98.6%) | 80, 95% (87.8% to 98.0%) | 1.5% (–4.3% to 7.4%) |
| Scene SBP in mmHg, median (IQR) | 148, 136 (122 to 152) | 109, 136 (121 to 151) | –0.12 (–6.20 to 6.04) |
| Scene % SaO2, median (IQR) | 154, 97% (95% to 98%) | 110, 97% (95% to 98%) | 0.17 (–0.81 to 1.14) |
| Scene % injured by RTC (95% CI) | 162, 7.4% (4.3% to 12.5%) | 114, 7.9% (4.3% to 14.3%) | –0.5% (–6.9% to 5.9%) |
| Scene % injured by low-energy fall (95% CI) | 162, 59.9% (52.2% to 67.1%) | 114, 59.6% (50.5% to 68.2%) | 0.2% (–11.5% to 12.0%) |
| Estimated time to nearest SNC in minutes, median (IQR) | 162, 26 (19.0 to 31.0) | 113, 28 (21.5 to 32.5) | –2.4 (–4.9 to 0.1) |
The median GCS score was ‘12’ in both trial arms, the median oxygen saturation was 97% and the median systolic blood pressure was 136 mmHg. In either group, the proportion of patients with normal pupillary responses was 95%. The proportion injured by road traffic collision (RTC) and low energy fall was similar in both groups, at 7–8% and 60%, respectively. The estimated distance to NC was similar (26 minutes vs. 28 minutes for control and intervention, respectively). The average difference with 95% confidence limits is given in the fourth column of the table and indicates that there were no significant differences between control and intervention groups in terms of the characteristics described in Table 3.
The left of each ‘control/intervention’ cell in Table 3 contains the number of patients in each group for which a measurement of the row specified variable was recorded. The following statements describe the circumstances surrounding missingness:
-
Two control patients who were subsequently not identified by hospital records had no age recorded on the ambulance PRF, although gender had been recorded.
-
Two intervention group patients did not have the exact GCS recorded on the PRF; it was merely recorded that the GCS score was < 13/14, thereby fulfilling the study inclusion criteria.
-
For patients in either group with missing physiological variables/mechanism of injury, these were not recorded on the PRF.
-
Similarly, the estimated time to nearest SNC could not be calculated as no incident postcode was recorded on the PRF for seven intervention patients and 11 control patients.
Table 4 presents an intention-to-treat comparison of the characteristics of patients in both groups in terms of factors that paramedics could not have known precisely of at the scene of injury. The time from leaving the scene to arrival at the first hospital was between 15 and 20 minutes in both groups. The median ISS was ‘1’ in both groups. The proportion of patients with significant extracranial injury was low in both groups, at 4% for intervention and control groups, respectively, as was the proportion of patients subsequently shown to have TBI in both groups (21.6% and 30.7%). Within the TBI subset of each trial arm, fewer than one-third of patients received any neurosurgical intervention in theatre (11.4% and 31.4%): 15 patients in total. A further 23 patients with TBI went to an ICU without neurosurgical interventions in theatre (10 intervention; 13 control). For these TBI ICU patients not requiring theatre, the ICU care was provided in the NCs in 86% of cases (20/23 patients). Four patients with TBI had ICP monitors inserted in intensive care; one enrolled patient, who did not have TBI but spontaneous subarachnoid haemorrhage, also had an ICP monitor inserted in the ICU.
| Patient characteristic | Intervention (n = 169) | Control (n = 124) | Difference (95% CI) |
|---|---|---|---|
| Time from leaving scene to hospital in minutes, median (IQR) | 145, 19 (12 to 25.5) | 102, 16 (8 to 25.3) | 1.37 (–1.13 to 3.87) |
| ISS, median (IQR) | 162, 1 (1 to 9) | 114, 1 (1 to 16) | –2.29 (–4.51 to –0.08) |
| Significant extracranial injury (%; 95% CI) | 162, 3.7% (1.7% to 7.8%) | 114, 4.4% (1.9%% to 9.9%) | –0.7% (–5.4% to 4.1%) |
| TBI (%; 95% CI) | 162, 21.6% (15.8% to 28.4%) | 114, 30.7% (22.8% to 39.6%) | –9.1% (–19.7% to 1.5%) |
| Percentage of those with TBI who had neurosurgery (%; 95% CI) | 35, 11.4% (3.7% to 25.3%) | 35, 31.4% (17.8% to 48.1%) | –20.0% (–38.6% to 1.4%) |
| ABC intervention within 6 hours of leaving scene (%; 95% CI) | 162, 13.6% (8.9% to 19.5%) | 113, 17.7% (11.5% to 25.6%) | –4.1% (–12.9% to 4.7%) |
| Transferred for further care (%; 95% CI) | 162, 4.9% (2.5% to 9.4%) | 114, 15.8% (10.2% to 23.6%) | –10.9% (–18.3% to –3.4%) |
| 30-day mortality (%; 95% CI) | 159, 9.4% (5.6% to 14.8%) | 113, 8.8% (4.6% to 15.2%) | 0.6% (–6.3% to 7.5%) |
Interventions directed at stabilising the ABC within 6 hours of leaving the scene were needed in fewer than one-fifth of patients in each trial arm (13.6% and 17.7%). As might be expected, a higher proportion of patients were transferred for further care in the control arm (15.8% vs. 4.9%). Transfers for further care occurred in the intervention arm owing to repatriation to NSAH (n = 4) when no TBI was present or to a SNC in three cases of non-compliance in patients with TBI. In this group of three, only one neurosurgical intervention of ICP monitoring occurred. The 30-day mortality was similar in both groups, being close to 9% (9.4% and 8.8%, respectively). There were no significant differences in the characteristics presented in Table 2 other than the injury severity being, on average, two points higher in the control group and – as expected – a higher rate of secondary transfer to neuroscience in this control group.
Time from leaving the scene to arriving in hospital was missing for 24 intervention and 22 control patients as a result of the time of leaving scene not being recorded on the ambulance PRF. There was little difference between the trial arms in this time interval but non-compliance in the intervention group will have influenced this finding.
Injury details (ISS, % extracranial injury, % TBI) were available for all 272 patient when 30-day mortality was available but were unable to be determined in patients who were not identified in hospital (n = 6: 1 intervention and 5 control) and in 4 out of 11 patients who had refused to consent (6 intervention and 5 control), although the patient information form had advised non-consenters that their anonymised injury details could still be retained. Study hospitals were at times reluctant to allow the study co-ordinators access to health records in these circumstances.
The availability of 30-day mortality outcomes has been described in the text following the CONSORT diagram (see Availability of patients for 30-day and 6-month follow-up in consort).
Feasibility outcomes for stream A: recruitment rate versus target
Figure 4 gives the recruitment rate as a percentage of target by month of study during the study recruitment period, by overall study recruitment rate and by recruitment rate in the two ASs. May 2012 to March 2013 is shown as the recruitment period, as the NWAS was not fully recruiting until the end of April 2012, when all the NWAS clusters were trained. The provisional power calculation for the full study suggested that 700 patients would be needed per annum from these research site ASs. However, a feasibility target of 50% (350 patients), with recruitment rising in both sites, was deemed by the TMG, in the grant application, to be an acceptable feasibility target. This gave a monthly recruitment target of 30 patients per month. Hence the 50% target is outlined in black in the figure.
FIGURE 4.
Recruitment rate vs. target for feasibility study.
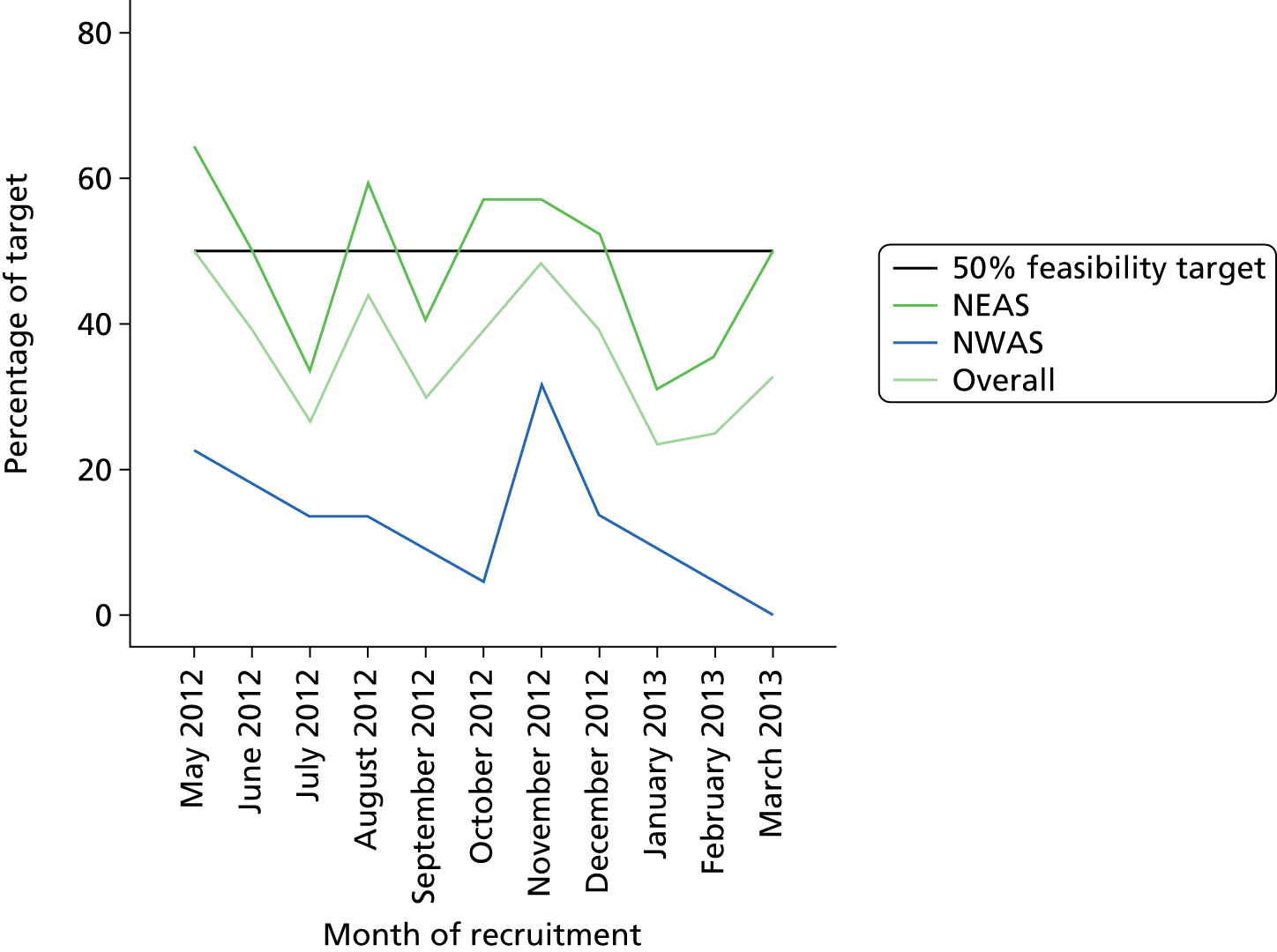
It had been anticipated by the TMG that (because the NWAS study catchment area was larger in terms of cluster numbers and population served) two-thirds of the recruitment would have occurred in NEAS and one-third would have occured in NWAS, giving a 20 : 10 NEAS/NWAS split in terms of patient numbers for monthly target recruitment of 50%. However, part of the target was that the recruitment rate would be increasing to > 50% towards the end of the feasibility period.
Figure 4 shows that the study was unable to recruit to the 50% target in the majority of months of the study, and that there was no increase in recruitment towards the end of the 12-month recruitment period (light green line). Although recruitment in NEAS was often close to the 50% target and sometimes exceeded it (dark green line), NWAS recruitment was usually about 20% of target (blue line). The higher GCS study inclusion criteria within NEAS need to be borne in mind when interpreting these findings.
Feasibility outcomes for stream A: proportion of study patients with traumatic brain injury on computed tomography head scan
In order to demonstrate the potential effect of early neurosurgery, the feasibility study target in the application was for 80% of enrolled study patients to be subsequently shown to have TBI on a CT scan. Figure 5 shows that the proportion of recruited patients with TBI – in patients whose injury details were known – fell far below this.
FIGURE 5.
Proportion of study patients with TBI vs. study target: all included patients with injury details.

A total of 25% (n = 70; 95% CI 21% to 31%) of 276 patients with known injury details were shown to have a TBI on CT head scan in the overall study sample. The proportion was similar to this in NEAS, at 21% (n = 52; 95% CI 16% to 26%) but significantly higher in NWAS at 55% (n = 18; 95% CI 40% to 70%). However, as mentioned previously, the GCS cut-off for study inclusion in NWAS was one point lower (< 13 vs. < 14) than in NEAS.
As mentioned previously (see Important changes from original eligibility criteria for patients) the application’s study GCS inclusion criteria in NEAS were by necessity changed from the original application (in order to make the study possible alongside the NEAS MTB criteria) from < 13 to < 14. Consequently, an a priori analysis of TBI prevalence, including only those patients who met the original GCS inclusion criteria (GCS score of < 13), was prespecified by the HITS-NS TMG. This is shown in Figure 6.
FIGURE 6.
Proportion of study patients with TBI vs. study target: all included patients with original inclusion criteria of GCS score of < 13.
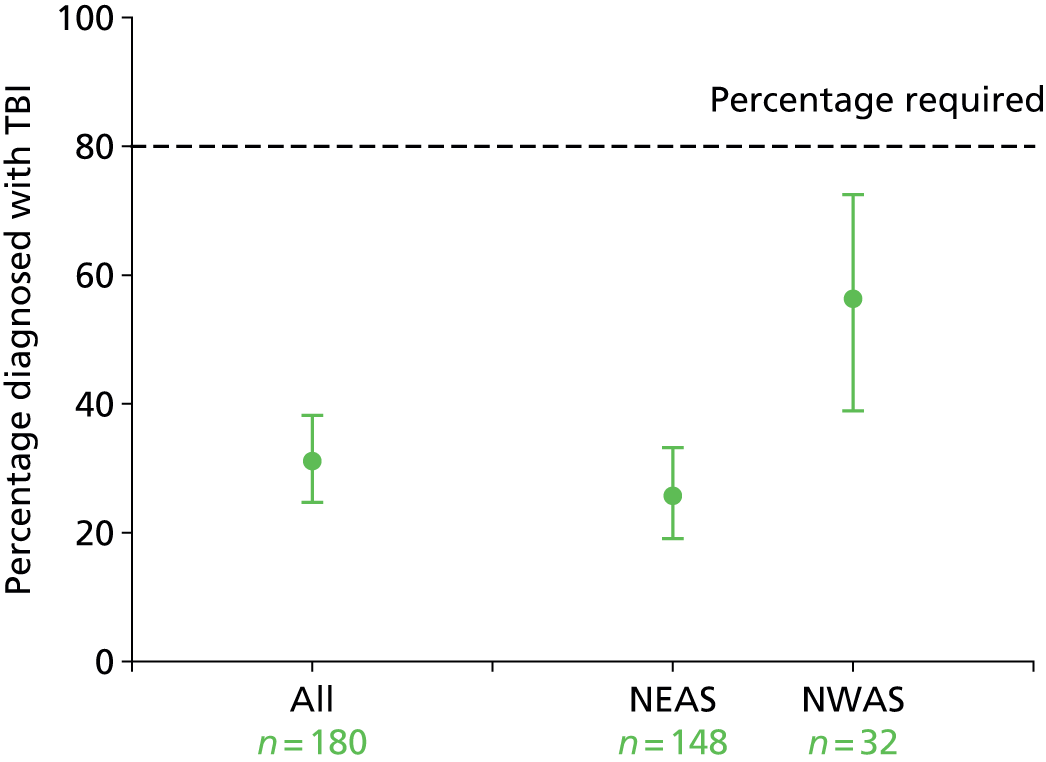
Figure 6 shows that, in the 180 patients who met the original inclusion criteria, the proportion of patients with TBI was 30% in the overall sample (n = 60; 95% CI 22% to 38%), with the proportion in NEAS being 28% (n = 42; 95% CI 19% to 36%). The NWAS results being guided by the original protocol are unchanged.
Feasibility outcomes for stream A: proportion of study patients when paramedics complied with study allocation
The prespecified rate of compliance with study allocation was 90% in order to demonstrate feasibility. Figure 7 shows that the percentage overall compliance rate was significantly lower than this, at 62% (183/293; 95% CI 57% to 67%). Compliance was significantly higher at 81% (100/124; 95% CI 72% to 91%) in the control group compared with 49% (83/169; 95% CI 41% to 57%) in the intervention group.
FIGURE 7.
Rate of compliance with study allocation overall and by study arm.
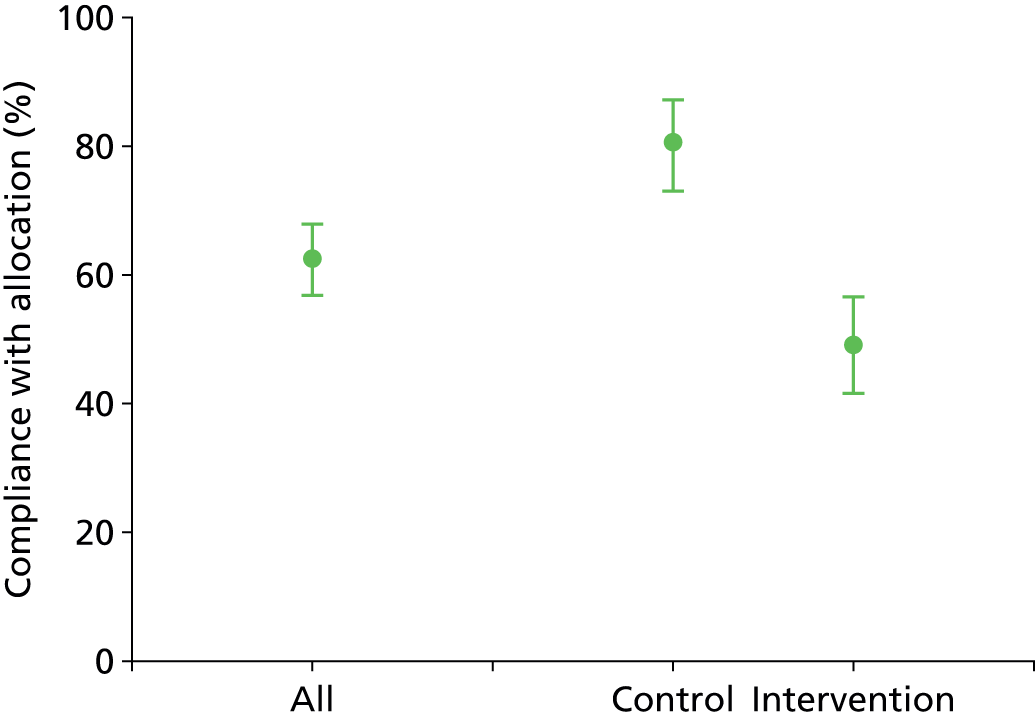
Figure 8 breaks this analysis down further by AS. Overall, the NWAS demonstrated excellent compliance with study allocation at 90% (33/37; 95% CI 78% to 95%), the rates being similar (at 91%) in the control group of 20 patients compared with the intervention group of 17 patients for whom compliance was 89%. The picture was somewhat different in the NEAS, with an overall compliance rate of 59% (150/256; 95% CI 50% to 66%), with a significant difference between the control group (for which compliance almost met the study target at 79% of 104 patients) compared with a much lower rate of 45% in the 152 intervention patients.
FIGURE 8.
Rate of compliance with study allocation by study arm and AS.

Feasibility outcomes for stream A: selection bias arising from non-compliance
Table 5 illustrates selection bias arising as a result of non-compliance with study allocation. For the factors of which the paramedics were aware, this occurred only with respect to estimated time to neuroscience centre (ETNC); in the control group, when compliance occurred, the ETNC was on average 2.6 minutes longer (95% CI 1.5 to 12 minutes) than when there was non-compliance; in the intervention group the converse occurred – with compliance the ETNC was 3.5 minutes (95% CI 0.6 to 6.3 minutes), on average, closer than when there was non-compliance.
| Patient characteristic | Complied | Not complied | Difference (95% CI) |
|---|---|---|---|
| Percentage male (95% CI) | |||
| Control | 100, 67.0% (57.4% to 75.7%) | 24, 62.5% (42.2% to 79.9%) | 4.5% (–17.0% to 26.0%) |
| Intervention | 83, 72.3% (62.0% to 81.1%) | 86, 67.4% (57.0% to 76.7%) | 4.9% (–9.0% to 18.7%) |
| Age, median (IQR) | |||
| Control | 98, 48.8 (29.0 to 70.0) | 24, 48.6 (31.6 to 62.5) | 2.8 (–7.5 to 12.9) |
| Intervention | 83, 44.3 (29.5 to 64.4) | 86, 45.4 (29.6 to 73.6) | –1.7 (–8.8 to 5.5) |
| GCS score, median (IQR) | |||
| Control | 100, 12 (8.3 to 13) | 24, 11 (7.3 to 13.8) | –0.2 (–1.6 to 1.3) |
| Intervention | 81, 12 (6 to 13) | 86, 12 (10 to 13) | –1.0 (–2.1 to 0.1) |
| Estimated time to nearest SNC (minutes), median (IQR) | |||
| Control | 89, 28 (22.5 to 35.5) | 24, 25 (17.3 to 29) | 2.6 (1.5 to 12.0) |
| Intervention | 81, 24 (18 to 29) | 81, 28 (20 to 33) | –3.5 (–6.3 to –0.6) |
Table 6 presents the same analysis in terms of factors of which paramedics could not have been directly aware but perhaps may have estimated at the scene of injury. This analysis indicates that in the intervention group compliance was more likely in patients with an ISS that was on average 5.2% higher (95% CI 2.8% to 7.7%) and a TBI prevalence that was on average 23.5% higher (95% CI 1.3% to 35.6%) than when non-compliance occurred. No such selection bias arose as a result of the small amounts of non-compliance in the control group or occurred in terms of subsequent mortality rate.
| Patient characteristic | Complied | Not complied | Difference (95% CI) |
|---|---|---|---|
| ISS, median (IQR) | |||
| Control | 90, 1 (1 to 16) | 24, 8.5 (1.8 to 20) | –4.6% (–9.3 to 0.07) |
| Intervention | 81, 1 (1 to 16.5) | 81, 1 (1 to 1) | 5.2% (2.8 to 7.7) |
| TBI, % (95% CI) | |||
| Control | 90, 26.7 (18.3 to 36.5) | 24, 45.8 (27.0 to 65.7) | –19.2 (–41.1 to 2.8) |
| Intervention | 81, 33.3 (23.7 to 44.1) | 81, 9.9 (4.7 to 17.9) | 23.5 (11.3 to 35.6) |
| 30-day mortality, % (95% CI) | |||
| Control | 100, 12.0 (6.7 to 19.5) | 24, 12.5 (3.3 to 30.4) | 0.1 (–11.9 to 13.2) |
| Intervention | 83, 9.6 (4.6 to 17.5) | 86, 5.8 (2.2 to 12.4) | –1.6 (–10.7 to 7.5) |
Prespecified analysis of robustness of screening
In May 2013, 2 weeks after the end of patient enrolment, a search of TARN submissions for TBIs revealed that 323 adults with TBIs presented in the catchment areas of the trial hospitals in the NEAS during the recruitment period and did not appear to have been entered into the study. A search for their ambulance patient report forms by the NEAS study co-ordinator – matching by anonymised information, such as age and date of injury – enabled exclusion of those who had one of the trial NCs as their nearest hospital to the scene of injury. This left 184 patients (Table 7) and revealed that only five could have been eligible for the study but were missed by screening. The reason for non-inclusion of the other patients was ineligibility due to too high a recorded scene GCS score (14 or 15) (n = 62), not having a head injury that was evident to the paramedics at the scene (n = 17), not having being transferred to hospital by a HITS-NS-trained land ambulance crew [helicopter = 18, transfer in = 24, Yorkshire Ambulance Service attended or not HITS-NS trained crew (n = 10) or not having used an ambulance to get to hospital (n = 23)].
| Reason why not enrolled in HITS-NS | n = 184 |
|---|---|
| GCS score of > 13 (higher than inclusion criteria) | 62, 23 transferred to neuroscience (details available for 16/23, 7/16 had neurosurgery and critical care, 3/16 critical care alone) |
| No pre-hospital record; therefore, probably not conveyed by ambulance | 23 |
| ABC unstable at scene/suspected polytrauma | 19 |
| Transfer in from non-trial hospital (mainly Carlisle) | 24 |
| Helicopter first attending | 18 |
| No external signs of head injury documented at scene | 17 |
| YAS attending AS | 5 |
| Non-trial NEAS crew/vehicle | 5 |
| Absconded from ambulance | 2 |
| Not picked up on screening | 5 |
| Sent to hospital by GP | 2 |
| Arrested at scene | 1 |
| Head injury sustained in hospital | 1 |
Patient satisfaction and nested qualitative cohort
The objectives were:
-
to determine the acceptability of the intervention (early neurosurgery) and control (usual care) pathways to patients, families and staff
-
to explore service providers’ views of their experiences of their involvement in the HITS-NS trial
-
to explore patients’ experiences of taking part in a feasibility study to compare different treatment pathways for patients with head injury.
Patient satisfaction
A patient satisfaction questionnaire was developed for use in the HITS-NS feasibility study to record patient satisfaction relating to the patients’ views of the time immediately after the initial incident and the arrival into hospital only. The initial design was created after completing a literature review on patient satisfaction to find out what components are important for determining patient satisfaction, as it has been shown that this can be used to evaluate the quality of care. The final version was developed after input from the HITS-NS TMG to get feedback and make improvements, and was then piloted with former brain-injured patients. The final version of the questionnaire was administered over the telephone during patient follow-up interviews, alongside administration of the EQ-5D and GOSE, in accordance with the 6-month follow-up SOP developed by the research team. The questionnaire explores 10 dimensions of patient satisfaction represented by statements, and patients were asked to indicate their extent of agreement with each statement by choosing a response from a 5-point Likert scale. The questionnaire proved to be straightforward in its administration and respondents seemed able to provide responses with ease.
Results
Patient follow-up was conducted between November 2012 and November 2013. Although the intention had been to conduct all follow-up interviews at approximately 6 months, this was not always possible because of a number of challenges in other areas of study activity (especially, for example, as a result of the workload challenges faced by the local study co-ordinator for NEAS), and also because of delays in receiving consent and the subsequent difficulties experienced in making contact with consented patients to complete the interviews.
A total of 28 patients and/or their carers took part in follow-up interviews at between 6 months and 17 months after recruitment into the study. Four additional patients who had consented to take part had died before their interviews could take place. Of the 28 patients who were followed up, 22 were able to give responses to the patient satisfaction questionnaire themselves, 3 were able to respond with assistance and additional comments provided by a carer, and for 3 the responses were given by a carer on the patient’s behalf. However, for one patient, who was in a nursing home, the carer was unable to provide answers to the satisfaction questionnaire, as he or she was unaware of the patient’s experiences during the period immediately following admission into hospital and a family member was unavailable to assist. The age range of respondents was between 20 and 92 years, and 19 of the respondents were female. Eleven (40%) of the patients had TBI.
Figure 9 shows the distribution of timings for the interviews.
FIGURE 9.
Timings of follow-up interviews.

Figure 10a–j shows the distributions of responses given to the 10 questions included in the questionnaire.
FIGURE 10a.
Responses to ‘the medical treatment I received was excellent’ (n = 27).

FIGURE 10b.
Responses to ‘I was satisfied with the first hospital where I was treated’ (n = 27).

FIGURE 10c.
Responses to ‘the surroundings and facilities at the hospital were excellent’ (n = 27).

FIGURE 10d.
Responses to ‘I felt well informed of my care throughout my stay in hospital’ (n = 26).
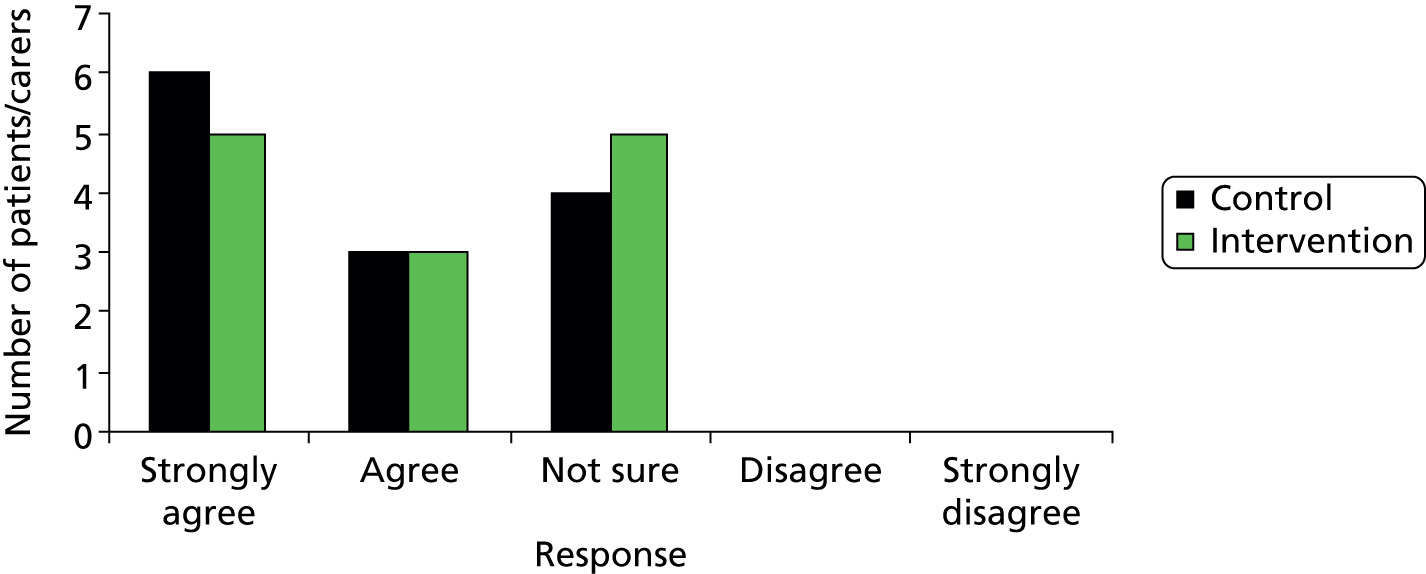
FIGURE 10e.
Responses to ‘the doctors and nurses were polite and friendly’ (n = 27).

FIGURE 10f.
Responses to ‘the doctors and nurses showed their concern for me’ (n = 27).

FIGURE 10g.
Responses to ‘I felt my care was explained to me clearly’ (n = 27).
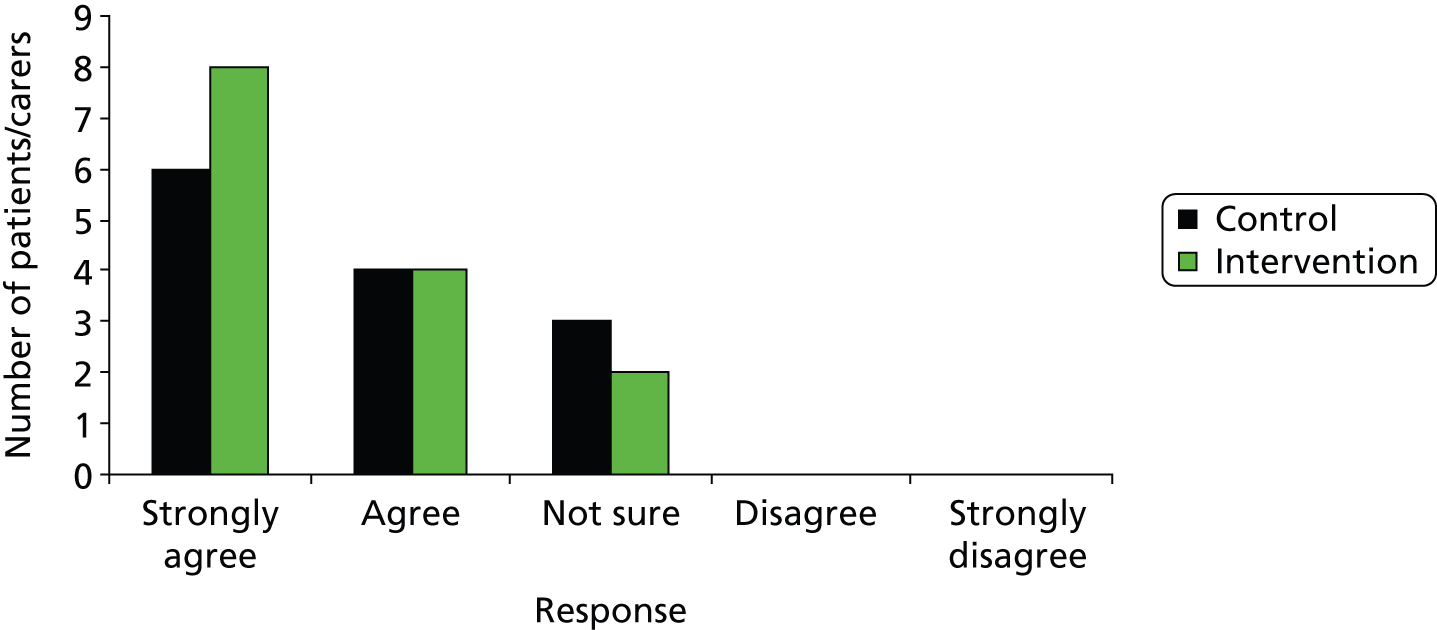
FIGURE 10h.
Responses to ‘the doctors and nurses answered all my questions’ (n = 27).

FIGURE 10i.
Responses to ‘the information sheets I received were useful and informative’ (n = 26).

FIGURE 10j.
Responses to ‘overall, I was satisfied with the care I received from doctors, nurses and paramedics’ (n = 27).
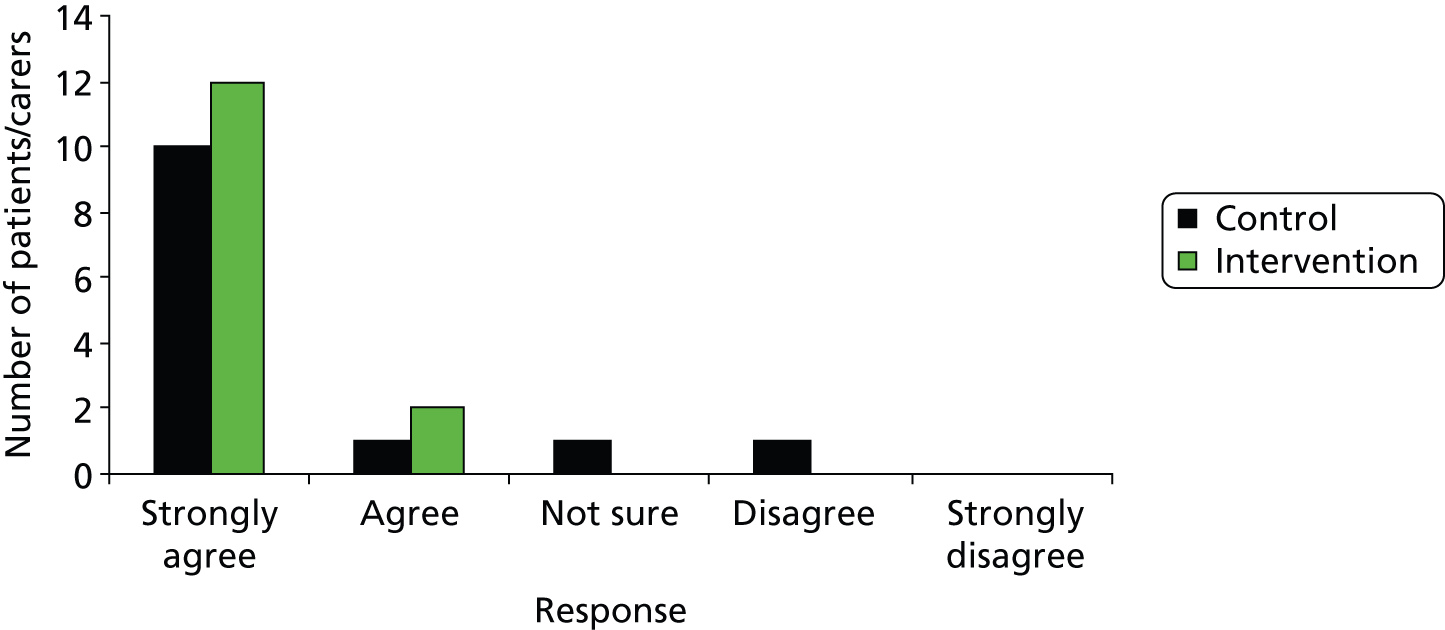
Nested qualitative cohort methods
This qualitative work was intended to explore the views of patients/carers and service providers: that is, paramedics who had been HITS-NS trained.
The original intention was to select a convenience sample of patients from those who had participated in HITS-NS and who had consented to remain in the trial for follow-up for inclusion in interviews. Criteria for selection included willingness to take part, mental capacity and confirmed diagnosis of presence/absence of TBI on recruitment to HITS-NS, GCS score at recruitment, trial arm, age and gender. Patients were to be selected to ensure as diverse a range as possible of criteria, including allocation to the pathways, being evaluated in the trial. Similarly, a convenience sample of carers willing to take part to represent patients with the same diverse range of criteria was to be selected. We had anticipated, based on our recruitment and consent figures, that it would be possible to select a minimum sample of 20 patients and carers to take part in this study, which would represent approximately 50% of the patients who had consented to take part in 6-month follow-up at the time of preparing the nested qualitative study proposal. However, owing to a poor response rate, all of the patients and carers who had indicated their willingness to take part in the qualitative study were included for interview.
The original intention was to select a convenience sample of paramedics from those responding to an invitation to take part in individual interviews, extended across all of the paramedics and advanced technicians (who number slightly over 850 staff) who had been involved in HITS-NS in NEAS and NWAS and who had received HITS-NS training. The sample drawn was to be based on a range of criteria, including geographical location, size of ambulance station where based, trial arm allocation of ambulance station where based, rank, gender and age. Owing to an extremely low rate of response (two in NEAS and zero in NWAS), a substantial amendment was submitted to the REC, who had originally given a favourable opinion for the qualitative study to request that invitations could be reissued for participation in a focus group, with payment offered for the time given to volunteering to take part. A favourable opinion was given to this amendment and invitations were reissued to take part in a focus group. This time all of the eight paramedics who responded in NEAS were included in one focus group. There were insufficient responses from NWAS paramedics to allow a focus group to take place on NWAS.
All of the participants included in the qualitative study had received participant information sheets and had consented to take part as part of the recruitment process. The interviews with patients/carers and the focus group involving the paramedics were arranged to take place at a time and venue acceptable to the participants.
Interviews and focus groups were chosen as the data-gathering methods of choice, as these methods would allow participants to think about and answer freely about their own experiences and perspectives. The interviews and the focus group were directed by a topic guide who covered the following dimensions:
-
patients’ and carers’ experiences of participating in HITS-NS
-
the reasons why patients consented to take part in a clinical trial of care pathways for brain-injured patients
-
patients’ experiences of their care pathway
-
service providers’ (i.e. paramedics) experiences of their involvement in the HITS-NS trial
-
service providers’ views of the acceptability and appropriateness of the two care pathways in the HITS-NS trial.
Owing to the time constraints regarding the conduct of the qualitative research, caused by the slow responses from participants to take part, interview and focus group data were recorded by taking field notes and content analysis was used to determine the key themes. 31
Results
The exploratory qualitative study was conducted between October 2013 and November 2013.
Nine patients (two intervention, seven control, five with TBI) took part in interviews. One additional patient, who had also consented to take part, died before the interview could take place. Six of the interviews were conducted face to face in the patients’ homes and three interviews were conducted over the telephone. Of those who took part in face-to-face interviews, four patients had carers/family members present during their interviews; however, all of these patients were able to respond fully for themselves and the findings represent the views of the patients only. One of the participants was the carer (wife) of a patient who was severely disabled as a result of head injury; however, the carer wished to take part and to give her views on her husband’s behalf. Four of the participants were female and five were male.
The paramedic focus group comprised eight paramedics who had all received HITS-NS training, of whom four had been involved with patient recruitment during the trial. The focus group took place at the NEAS headquarters and was facilitated by the trial manager, who was assisted by the local trial co-ordinator in the role of the main note-taker.
Results: patient interviews
Owing to the small sample size of participants, the main themes (identified as those representing the views of at least one-third of participants) to emerge in response to the key interview questions are presented in this report and are as follows (with illustrative quotes given).
-
For the future benefit of others.
-
Research provides the answers to determine appropriate care: ‘Only way we learn is by doing research.’
-
Altruism, and a sense that taking part in research is a valuable contribution: ‘Seemed like the right thing to do.’
-
Gratitude to the AS for its role in providing care and therefore wishing to be helpful in return: ‘Paramedics had been brilliant.’
-
Did not seem there would be any harm in taking part.
-
All research is important if it can help others.
-
Very important because of the severity of injury that other people may experience: ‘Some people may be more seriously injured than I was so that makes me realise how important it is.’
-
Different care pathways need to be tried and compared to understand what type of care is the most effective: ‘Need to try out different ways to see what works best.’
-
Three patients decided to take part on their own.
-
Three carers decided on their own but mentioned this to family members after their decision had been made.
-
Two patients discussed taking part and consenting with others.
-
None.
-
Did not know what would happen.
-
Partial understanding: ‘Information was a bit hazy.’
-
The researcher taking consent gave a clear explanation, although this may have lost clarity later on: ‘The nurse explained things clearly;’ ‘The lady paramedic explained it all very thoroughly, and I thought I understood it but afterwards I didn’t.’
-
The majority did not really understand.
-
None of the patients interviewed had knowledge of trial arms.
-
None of the patients expressed any concern about allocation; three patients said that they had been happy to be have been taken to a neurocentre, although one of these patients stated that she would not like to think she was using a bed in a neurocentre if someone more badly injured may have needed the bed more urgently.
-
Patients were all aware that they had contact details if they needed to ask any questions.
-
Majority said ‘yes’.
-
The majority at first could not think of any reason: ‘No idea.’ However, on reflection, a number of suggestions were made.
-
Concerns about how much time participating might take.
-
Did not want to relive a bad experience.
-
May be asked to do more, and then more . . .
-
Knowing that others may gain from the research.
-
Has found it helpful to talk.
-
None.
-
None.
Qualitative cohort summary
The majority of patients offered statements that demonstrated an altruistic attitude that influenced their willingness to take part in HITS-NS. There was also a consensus of opinion about the importance of research in generating information that could be useful to determine appropriate and ‘the best’ care for patients with head injury, and generally in the provision of medical care. There was a lack of understanding about the purpose of the trial and how it was being conducted but, once this was discussed, the participants remained happy about their participation. Despite a lack of understanding about the trial, most of the participants felt supported by the research team and had remained comfortable with their decision to be part of HITS-NS. The majority of participants also expressed interest in receiving information about the main findings from the trial.
Paramedic focus group
Four of the eight participating paramedics indicated that they had identified HITS-NS patients and all of the participants confirmed that they had received HITS-NS training in some form.
The questions explored and the key views given (with any direct quotations presented) are as follows.
Question: how important do people feel research into care pathways is within the context of traumatic brain injury?
-
Traumatic brain injury is really unrecognised, can be an occult condition that is difficult to detect, and research into getting the patient to the right treatment is therefore of value. The pathway is seen as important and paramedics need evidence to be able to say what is right or wrong.
-
Getting the patient to the right/appropriate care and appropriate doctor is important, as going to the wrong place can lead to delays in treatment and then further time loss due to secondary transfers (although this point was clarified with reference to unstable ABCs and the need to get to the nearest care).
-
The new protocol allows paramedics to bypass, it ‘covers your back’, whereas previously they would always have to go to the nearest casualty department.
-
There are cost implications in taking a patient to the incorrect destination and, even though these are not direct costs to NEAS, they need to be considered.
-
This research is important as it advocates standardising treatment for this group of patients.
-
Nurses have been using bundles of care for years and it feels like paramedics are significantly behind nurses, despite paramedics in some ways being ahead in the care that they can/could deliver. This consideration is important, as it shows that paramedics are aiming for the best possible care, which is a sign of progress for paramedicine as a profession.
-
Research that allows paramedics to make appropriate decisions would improve the professional standing of paramedics.
Question: your experiences of taking part in the trial?
-
Expectation of more in-depth training and a feeling of a lack of training; however, there was understanding regarding how it was delivered in terms of NEAS cost-effectiveness.
-
The involvement in innovative approaches is a positive experience but, unfortunately, owing to the lack of patients, the initial enthusiasm waned over time and the trial could have done with more refreshers and reminders.
-
There was confusion in the early days of the trial: ‘In hindsight I took one patient in to the local A&E under HITS-NS but maybe I shouldn’t have.’ The patient to whom the quotation refers had died, and this incident was referred to repeatedly throughout the focus group.
-
The timing with the MTB was unfortunate and caused confusion.
-
HITS-NS and the MTB ‘sort of merged into one’, despite the two separate protocols.
-
Some stations are involved in many trials so involvement in HITS-NS was nothing out of the ordinary.
-
Involvement in research was seen as ‘part of the job’.
-
There was awareness that staff in the more outlying stations would be interested in being involved in more research, and it was rewarding to be involved in a trial coming from a station that is not involved in a lot of research.
Question: was there an accurate understanding of the trial, both initially when training was received and, subsequently, as the trial progressed?
-
Face-to-face training rather than paper-based would have been better for making sure people had a grasp of what they should be doing and why, and the chance to ask questions would have been good. Physical training during which people can interact would have aided people in remembering aspects of their training.
-
Small groups similar to that attending the focus group would have been a better method of training, for example if delivered at a station level.
-
In some stations where there was little perception of involvement, the information was read once and then put away.
-
There was some confusion about whether the training followed the paramedic when they worked away from their base station, which was demonstrated by opposing views within the group.
-
The initial confusion was worsened by the launch of the MTB.
-
Despite the fairly clear protocol, there was felt to be room for individual interpretation of some elements.
Question: how easy was it to understand the information given about the trial?
-
The protocol was fairly straightforward.
-
Some patients could fall into grey areas, in which case the paramedic would call the hospital or the NC/MTC to confer.
-
The JRCALC (Joint Royal College Ambulance Liaison Committee) insert was easy to follow and the similar format to the MTB aided working with both.
-
HITS-NS and the MTB seemed to merge into one pathway. ‘It could come down to describing why you went/or wanted the crew backing you up to go to a specific hospital and justifying your decision to transport.’
-
There was awareness that some stations were experiencing some degree of difficulty regarding an understanding of the protocol.
-
Rapid response work across all areas so information about the trial could be confusing. A rapid responder would need to impress upon the crew backing them up the reason for his/her decision so that he/she can travel onward with the patient and proceed efficiently.
-
The trial did add some difficulty in some decisions that may have been more straightforward without knowledge of the trial. One of the participants said ‘the staff at my station didn’t feel included even though we were a control station’.
-
There was some frustration with the variety of local protocols.
-
There was a conflict between clinical decisions and the pathways. The pathways can introduce the element of doubt: ‘You are damned if you do and damned if you don’t.’
-
Protocols are seen as confusing and part of the old way of doing things. Pathways allow leeway in decision-making and clinical freedom. In order to utilise this there needs to be reasoning to support the actions taken.
-
‘We are professionals now, we have moved away from protocols.’
Question: views or concerns about their training?
-
The written material came thorough to staff and stations, but this might not have been the most effective method of training people.
-
Other research trials have emphasised more face-to-face and hands-on training successfully in NEAS, such as PILFAST (Paramedic Initiated Lisinopril For Acute Stroke Treatment), PARAMEDIC (Prehospital Randomised assessment of a Mechanical compression Device in Cardiac arrest) and the lactate monitor trial; therefore, face-to-face training should have been included.
-
Cascading training through a local contact may have been better than self-administered training material.
-
Perhaps a DVD (digital versatile disc) or a podcast may have been more effective; however, other options should have been used in addition to the paper-based training and the local contact.
-
Suggestions were offered that such training could be included in the ‘stat and man’ (statutory and mandatory) training and/or by e-learning.
-
Clinical team leaders could, and should, have a role in training if they are to be ‘clinical’ team leaders.
-
Although the training material was clear, there were some concerns about its application: ‘The information is pretty clear until you are faced with a real patient, then you need to think on your feet and make quick decisions, questions only occur once you are in the situation and it is impossible to anticipate all of these.’
-
All participants agreed that the points of contact were clear within the material.
-
It was felt that staff accepted their allocation to the trial arms without question and they did not give it any more thought.
Question: did paramedics feel that they received sufficient support from the research team?
-
Yes: ‘Support was available if needed at the end of the phone.’
Question: did paramedics access the HITS-NS website (which contained the training material)?
-
There was lack of awareness of the existence of a website among the majority of the group, and no one reported that they had accessed it.
-
One participant stated: ‘I knew about the website but have a biased view of the trial.’
Question: were there any challenges and/or benefits of being part of the trial?
-
The bypass procedure was seen to influence, both positively and negatively, the areas in which paramedic crews worked: ‘it means you can end up getting dragged out of your normal area which then means other crews get dragged in’ and ‘being involved can keep you closer to your normal patch if you are bypassing other hospitals to get closer to your local hospital’.
-
Any training is good, as it all counts towards continuing professional development (CPD), so research is a good thing.
-
Anything that enhances your skills is beneficial, and anything that allows for better patient treatment is beneficial.
Question: were there any operational issues?
-
The previously mentioned confusion between HITS-NS and the MTB was reiterated.
-
The geographical focus could cause issues for some crews; for example, crews in the middle of the patch could end up going to the Royal Victoria Infirmary in the north in the morning and down to the JCUH in the south in the afternoon: ‘It makes for long travels and a tiring day’.
-
The monetary justification for hospitals having bypass protocols and providing some or all facilities was raised.
-
It seemed to take some hospitals a little while to ‘get up to speed’: ‘You could take a HITS-NS patient in then have to explain HITS-NS to the receiving staff.’ However, this issue was then qualified as only happening early on in the trial.
Question: were there any ethics issues about the conduct of the trial?
-
Some respondents felt that there were no issues as ‘we are still doing the same as were doing before’ and because ‘the idea behind the trial made sense’.
-
There was some discussion on station as to how much the trial protocol took away the direction to go to an appropriate receiving hospital.
-
Questions were raised about which treatment is ‘right’, which have also been raised in other trials: ‘Why can’t we just give everyone what they need?’
-
It was felt that in some cases paramedics did what they thought was right for the patient and ignored the trial.
-
There was a belief that paramedics would generally assume that all of the required ethics approvals were in place.
-
Other trials (e.g. PILFAST) have been seen to have an obvious commercial interest; however, HITS-NS was viewed in a positive light, as there was no obvious benefit to anyone other than the patient.
-
With regard to ‘waived consent’ in the HITS-NS trial, in accordance with the MCA (2005), the respondents all agreed that they did not have any ethical issues with this, as they were used to implied consent.
An extra question was asked about paramedic compliance in the trial and what the reasons may have been for non-compliance
-
Poor documentation by paramedics was felt to be a key explanation.
-
There was a perception that in a stressful situation (i.e. when attending to a patient) it is easy to forget to write things down.
-
One participant stated that he believed he may have forgotten to document cases he thought of as eligible for inclusion in HITS-NS, and that additionally staff may have skim-read the protocol and so may not have picked up details such as writing ‘HITS-NS’ on the record pro forma, which was a required process.
-
There was agreement that the paramedics’ perceptions of the destination hospital may have affected their decision on what cases to take where.
Question: did paramedics find that there were any benefits or disadvantages from taking part?
-
Being involved in the trial was seen as being thought-provoking in terms of making decisions regarding patients’ care: ‘The trial made you think about where you were taking people. As a mentor it gave me something I could discuss with students.’
-
Potential improved patient outcomes, and therefore increased professional standing, was seen as a benefit of taking part in the trial.
-
The trial contributed to an enhanced knowledge base underpinning the paramedic profession: ‘Makes me feel more professional.’
-
It was thought that participating in research trials can help to change the sometimes negative perceptions of the paramedic profession held by other health-care staff; however, this view was challenged within the group: ‘It’s all about the improving the patient outcomes, I’m not bothered by how we are perceived by other people.’
-
Participation was overall viewed as a positive experience that enhanced the role of the paramedic: ‘It’s about delivering the right care, right place, right time message that is part of the NEAS corporate image.’
Question: were the paramedics glad that they took part in the trial?
-
The majority felt glad to have been part of HITS-NS for various reasons, but mainly because of potential benefits to patients and professional benefits: ‘The trial was a good thing, we need to start getting involved in things like this and getting patients to the right place’; ‘It’s nice to be looked at as part of a profession that is progressing’; ‘If trials like this will improve patient outcomes then I’m happy to be involved’.
-
There was also a view that being part of a trial is an accepted part of the paramedic’s role: ‘You don’t question it when a trial comes along, you just get on with it’; ‘There is a growing acceptance amongst staff that this is part of our role.’
Question: were there any questions the paramedics thought should have been asked, or any additional comments?
-
There was no real response from any participants when talking about research in general.
-
There were no specific answers in relation to the HITS-NS trial but there were a few views in relation to research within the AS in general:
-
‘There is a geographical focus on research around HQ, it would be nice to split the locations and research up a bit.’
-
‘More funding for research would be good. People value their time off so asking people to do anything in their own time such as this is always going to be difficult.’
-
‘Pre-trial focus groups like this with groups of paramedics would be a good idea in order to identify any issues.’
-
Summary paramedic focus groups
The themes that emerged from the focus group clearly demonstrated that paramedics considered there to be substantial value in participating in a trial such as HITS-NS from the perspective of determining the most appropriate care for patients, and also for professional development of individual paramedic staff and to promote the AS as an important clinical environment for undertaking research. The HITS-NS trial was well received in general, although one of the main areas of activity that precipitated suggestions for improvement was training. There were no other major issues relating to other aspects of the trial (such as ethics and consent) and its conduct.
Complaints and serious adverse events
There were no patient complaints about the conduct of the study from HITS-NS patients.
One non-study patient complained because he or she was written to in error by the NEAS study paramedic for consent when his or her address was erroneously supplied to the paramedic by the receiving hospital. The complaining patient had the same name as a HITS-NS patient. The chief investigator and the hospital trust in question carried out an investigation and responded in writing to the patient, who appeared satisfied that his or her personal information was safe and had not been disseminated to third parties without consent. To minimise any future similar errors, the need was emphasised to double check that the patient in question was the one admitted on the date in question with a head injury, when two patients with the same name were on the hospital database. There were no further episodes of this nature or patient complaints.
One of the trial acute hospitals – the Royal Lancaster Infirmary – withdrew its participation in the study at the end of December 2012, as it no longer felt able to resuscitate and investigate head injury patients with a reduced GCS score who were arriving by ambulance as a result of local trauma network reconfiguration. Screening of patients injured nearest that hospital continued in NWAS, and the trial co-ordinator felt that one possible patient attended by a control crew might have been eligible.
There were three SAEs during the conduct of the study. All of these occurred when NEAS intervention cluster paramedic crews bypassed patients who were ineligible as their respiratory rate was higher (> 30 breaths per minute) or lower (< 10 breaths per minute) than the range in the study eligibility criteria. All were investigated by the local PI, and it was deemed that no patient harm had resulted from bypassing these ineligible patients.
Long-term secondary stream A outcomes
As indicated in the CONSORT diagram, low numbers of enrolled patients responded to approaches to consent for study follow-up. The values below (Table 8) relate to patients who consented and were available for the 6-month follow-up interviews (13 control patients, 15 intervention patients) and those known to have died (18 intervention patients and 11 control patients were known to be deceased). There are no significant differences, but the low response rate – biased heavily towards those known to be deceased (n = 29) or with severe injury (n = 11) – prevents further meaningful comment.
| Study arm | Intervention n = 33 | Control n = 24 |
|---|---|---|
| Median GOSE | 1 (1–4) | 3 (1–5) |
| Median EQ-5D | 0 (0–80) | 25 (0–60) |
Stream A discussion
The ‘Head Injury Transportation Straight to Neurosurgery Trial – A feasibility study’ has established the following in relation to the stream A study objectives.
Objective 1: determine the feasibility of conducting a study of early neurosurgery using a cluster randomised trial of pre-hospital bypass
Stream A has shown that cluster randomised pre-hospital trials, with ambulance station as unit of cluster, can be used for health technology/complex intervention evaluation – compliance issues can be addressed if sufficient resources are dedicated to face-to-face training (see Feasibility outcomes for stream A: proportion of study patients where paramedics complied with study allocation).
However, it is not plausible to evaluate the impact of early neurosurgery using this design because of the unexpected case mix in the study cohort. The low rate (25%) of TBI and 7% rate of neurosurgery in included patients were unexpected but critical findings in determining feasibility. The identification of this case mix in those patients with head injury who are eligible for ‘bypass’ is an important new finding from the study. The effect of early neurosurgery is diluted in this cohort and a trial using these (or any prospective) inclusion criteria would be unfeasibly large (> 15,000 patients).
Objective 2: establish the acceptability of both pathways to patients, families and staff
Only a limited evaluation of this was possible because of the low rate of response to invitation to consent for follow-up after enrolment and the fact that patients who did consent to follow-up were (non-representative as they were) in the more severely injured group (who were in hospital for a sufficient time period to allow face-to-face consent). The qualitative work on a cohort of nine patients can be considered to be exploratory.
Notwithstanding this, there was no clear signal of patient/family preference for either pathway, with high levels of satisfaction expressed for either (see Patient satisfaction and nested qualitative cohort) and equal numbers of control and intervention patients having being interviewed.
The selection bias analysis suggests that paramedic preferences are influenced by proximity of hospital and perceptions about likely severity of injury (see Patient satisfaction and nested qualitative cohort).
Objective 3: estimate the magnitude of effect associated with early neurosurgery
It was not possible to do this given that the study recruited only 20 patients who required neurosurgery and most were in the control group.
Objective 4: determine accuracy of identification of isolated traumatic brain injury
In reality this was the rate of TBI in patients who met the study criteria, as paramedics were not given a ‘free hand’ with which to recruit. This lack of free hand reflects the pragmatic implementation of trauma triage tools within the new NHS England trauma systems. This rate of TBI varies between 23% and 55%, dependent on the GCS inclusion criteria and AS. The study had to vary the GCS inclusion criteria by AS in order to make the trial consistent with the varying MTB criteria that were introduced (during study recruitment) within both ASs in early 2012. Within the original study inclusion criteria of GCS score of < 13, the prevalence of TBI was 30%, with the observed values in the two ASs being 28% and 55% (see Feasibility outcomes for stream A: proportion of study patients with traumatic brain injury on computed tomography head scan).
Objective 7: identify major barriers to conducting a cluster randomised trial of early neurosurgery
The overwhelming major barrier is the low prevalence of TBI and need for neurosurgery (as described above) in those who are eligible for bypass according to current major trauma triage criteria that the HITS-NS study inclusion criteria typify.
The analysis of TBI prevalence (see Feasibility outcomes for stream A: proportion of study patients with traumatic brain injury on computed tomography head scan) indicates that, although the NEAS amended study inclusion criteria to the upper GCS score of < 14 (vs. < 13 in the original study protocol) recruited an additional 108 patients to the study, only an additional six (6%) had TBI, with one requiring neurosurgery but four requiring ICU.
With the TARN data analysis of TBIs, there were 62 patients who presented to HITS-NS NSAHs – in the NEAS area – during the study period, who had a scene GCS score recorded as 14 or 15 compared with 52 who met the study inclusion criteria of GCS score of < 14 (see Prespecified analysis of robustness of screening). Over this period, many thousands of patients with head injury and GCS scores of 14–15 presented to NEAS and did not subsequently have TBI (65,000 were screened by the HITS study co-ordinator), so although it would appear that the majority of patients with TBI occur in this high GCS cohort, the prevalence in this cohort of patients with a stable ABC is probably < 1%. It is not practical to bypass all of these patients to the ED of the NCs – without overwhelming them – for no clear patient benefit in the majority of cases.
A further barrier is that compliance was an issue in NEAS intervention clusters stations, but, in future, studies could be overcome by more resources for face-to-face training to achieve NWAS levels of compliance (where the lower numbers of clusters meant that face-to-face training was possible). The preference for face-to-face training was also confirmed by the paramedic focus group.
The low rate of response to invitation to consent – typical of mild head injury population in other studies – could be overcome by the ‘opt out of consent’ approach that we initially requested32 rather than ‘patients opt in’ which the REC insisted on. With an ‘opt-out’ approach, enrolled discharged patients are written to and informed that they will be telephoned to discuss the study unless they send back a slip or text back to say ‘do not contact me as I do not wish to hear more about the study’. The REC insisted that the slip read ‘yes please contact me’ instead, with no option to telephone non-respondents included. However, the REC was flexible in allowing us to record anonymised 30-day outcomes in this group, as long as the patients had been invited and had not refused to consent.
Objective 8: contribute to existing evidence about pre-hospital randomised trials
This has been a successful feasibility evaluation suggesting that the cluster randomised approach at ambulance station level works but that compliance needs face-to-face training.
Recruiting relevant patients with specific injuries has been identified in previous studies as a challenge. This is particularly the case in major trauma studies for two reasons. First, major civilian trauma is an ‘occult’ disease. The major vector is blunt trauma from road traffic collisions and falls, as opposed to more obvious wounding in civilian trauma. Second, as NHS ambulance patients’ records are often not computerised and difficult to link to hospital records (NHS numbers and reliable patient identifiers are often not available to crews during a brief interaction with an unconscious patient), it is hard to reliably predict the numbers of patients with given injuries who will be eligible for given ‘pre-hospital scene based’ study inclusion criteria. Hence there is a need for further pre-hospital major trauma studies to establish feasibility.
HITS-NS was viewed positively by paramedics and awakened enthusiasm for more pre-hospital research.
Limitations
The major limitation and barrier to a full trial is the low prevalence of patients with TBI/needing neurosurgery within the study cohort. This could not be predicted by existing evidence or done with database linkage, but is an important finding from the study. Prior to conducting the trial the investigators established that 80% of patients with ‘on-scene GCS score of < 13’ on the TARN database have a TBI. The investigators also established that the study inclusion criteria were consistent with the NICE high-risk criteria for identifying patients with head injury in EDs who require neurosurgery and, therefore, urgent CT scans. However, the TARN denominator is clearly very different from those with a GCS score of < 13 at scene because of the TARN eligibility criteria,18 which are as follows.
Trauma Audit and Research Network eligibility criteria
A patient of any age arrives at hospital alive after injury and at least one of the following subsequently occurs:
-
in-hospital death within 93 days during first admission
-
at least 3 days of inpatient care is required
-
level 2 or 3 care is required
-
hospital transfer for specialist care is required.
Most of the recruited HITS-NS study cohort did not meet TARN eligibility criteria, as none of these four criteria subsequently occurred. The predominant injury was mild head injury, with normal CT scan and discharge from hospital within 24 hours of presentation; this was true even in the subgroup with GCS score of < 13, in which > 65% had no brain injury (see Feasibility outcomes for stream A: proportion of study patients with traumatic brain injury on computed tomography head scan).
The NICE high-risk criteria33 for adult patients with head injury are any of:
-
GCS score of < 13 at any time
-
signs of open, depressed or basal skull fracture
-
focal neurological deficit
-
post-traumatic seizure
-
failure to reach GCS score of 15 within 2 hours of injury
-
more than one episode of vomiting post head injury.
The top two of these criteria were in the study eligibility criteria (see Eligibility criteria). Seizure as ‘unstable ABC’ would have been an exclusion criterion for which the patient would not have bypassed. Paramedics are not trained in formal neurological examination beyond the GCS, pupillary responses and FAST scores in stroke; the last two criteria require a period of observation post injury which is not applicable at the scene, so it was not possible to incorporate most of the NICE high-risk criteria into the inclusion criteria for HITS-NS.
The proportion of NICE high-risk patients with TBI/TBI requiring neurosurgery in the literature has not been well studied in the NHS, where studies have quoted rates of TBI for any of the NICE CT indications, which include medium-risk features such as age > 65 years with loss of consciousness or amnesia. 34 Rates of TBI for ‘NICE positive’ head injury patients vary between 10% and 30% but would be expected to be considerably higher in the ‘high-risk group’. Hence the TARN figure was adopted by investigators and 80% was the feasibility target.
The variation in rates of TBI by AS (even with GCS score of < 13 ; see Feasibility outcomes for stream A: proportion of study patients with traumatic brain injury on computed tomography head scan) can be seen as a limitation; however, head injury case mix presenting to EDs has been shown to vary quite significantly in other studies. 2,34 Both rates were too low to meet the feasibility target, particularly with regard to need for neurosurgery. The differences are likely to be caused by variation in the proportion of other patients who present with other causes of reduced GCS score in the context of a fall/assault with external signs of head injury. The major other causes of TBI are mainly intoxication or elderly ‘collapse’.
Compliance in the intervention clusters from the NEAS was limited and was a result of the same training resource (one study co-ordinator) being available to each AS for pragmatic reasons. In reality this made impossible the face-to-face training that achieved the almost-90% compliance in NWAS in NEAS – given the numbers of paramedics and large geographical area, the electronic media and cascade from team leaders were relied on instead. In the end, if rates of TBI had been higher, the investigators did not see this as a barrier to a full trial, as it could have been addressed by increasing resources for training therein.
The REC insistence on an active opt-in for being approached for telephone consent in patients with mild head injury who were discharged early limited meaningful follow-up of the study cohort beyond 30 days post injury. This has been highlighted in the literature as a persistent problem in this mild head injury group. 35 Again, the investigators feel that this could have been addressed in a full trial by persuading the REC with the pilot evidence to allow an opt-out approach that has been used successfully elsewhere. 32 With this approach the patient is written to and advised that a telephone call to discuss the study consent will follow unless a slip declining this is sent back within a fortnight; there have been no patient complaints using this approach.
The cluster randomised approach delivered balanced cohorts in terms of patient baseline characteristics. The median ISS was 2.5 higher in the control group; however, although statistically significant, on a median ISS of 1 this is not a clinically significant difference. 36 There was greater recruitment to the intervention cohort; however, it is unlikely that this reflects post randomisation bias, as the final arbiter of recruitment was the study co-ordinator rather than the scene paramedic. The analysis vis-à-vis the TARN database (see Prespecified analysis of robustness of screening) suggests that 90% of all eligible patients were approached for consent during the study period. It was difficult to predict exact recruitment rates to the study clusters prior to commencement from existing AS data and this is always likely to be the case as screening is very labour intensive (200 patients a day) and median numbers recruited per cluster over 12 months were in single figures (see Prespecified analysis of robustness of screening).
Relationship to other studies
The HITS-NS trial can be compared with other relevant literature in three respects: first, in terms of the feasibility of conducting pre-hospital controlled trials in general (as no trials of bypass have previously been conducted); second, with regard with to observational studies of trauma bypass; and, third, to the latest literature on trauma triage with reference to the sensitivity for detecting brain injury/need for neurosurgery.
The systematic review of pre-hospital trauma trials conducted from a feasibility perspective (see Appendix 1) highlights the same challenges that were encountered during the conduct of HITS-NS, supporting the feasibility approach particularly with regard to recruiting sufficient numbers of patients with the injury profile of interest over a given time. In blunt civilian trauma, the precise nature of a patient’s injuries are never ‘known’ with certainty at the scene – particularly when consciousness is impaired – and, in general, it is difficult for ambulance staff to collate sufficient patient identifiers at scene to permit anonymised data linkage (to hospital records with definitive injury details) to form the basis for feasibility using the pre-hospital population as a denominator.
Much of the literature around paramedic compliance was pessimistic, particularly from the NHS; however, the good rates of compliance with face-to-face HITS-NS training (≈90%), positively enforced by the paramedic focus group, suggest an approach that can work using a cluster randomised design. Training has been identified as key in other trauma trials in which compliance was high. 37
Follow-up rates and consent to follow-up, particularly when large numbers of patients with mild injuries are included, have been flagged as a challenge – as was the case for HITS-NS, although opt-out approaches to consent (which were not permitted by the HITS-NS REC) have worked elsewhere. 32
Two systematic reviews of trauma bypass have been conducted, one with a focus on head injury. These have been observational studies using hospitalised patients with TBI as a denominator rather than the population that the paramedic deals with at the scene. Hence they are of questionable external validity; furthermore, they tend to include patients whose nearest hospital was a NC and/or patients without any evidence of bypass having taken place and, therefore, lack internal validity. Notwithstanding this, the meta-analysis did not suggest benefit from ‘bypass’ in the TBI population. 14
The HITS-NS inclusion criteria – using the analysis in Prespecified analysis of robustness of screening – could be said to have detected up to 52 out of 114 (46%) patients with TBI presenting to non-specialist hospitals in the NEAS during the study period; the main reason for this ‘undertriage’ is the numbers of patients with a higher GCS score (14–15 in NEAS) at scene who are subsequently shown to have brain injury. This is particularly true in the elderly, in whom it is thought that cerebral atrophy allows early intracranial bleeding post head injury to not raise ICP and hence lower GCS. A recent study suggested that > 50% of all patients aged ≥ 65 years with TBI have a scene GCS recorded as 15. 38 Another group in which this is true is patients with extradural haematomas, who are, in general, young. In this group, there is rarely a primary brain injury but bleeding occurs in the vessels outside the brain within the skull, raising ICP and risking significant secondary brain injury if not treated. Many of these patients are observed post detection on CT brain scan, but in those who require operation the median GCS score on presentation to the ED is 14. 39
A further recent paper showed similar rates of undertriage or lack of sensitivity from the study inclusion criteria for patients on the TARN database with TBI; raising the GCS threshold increases sensitivity but reduces specificity, as the vast majority of head injury patients with GCS score of > 12 at scene do not have a brain injury. However, as this study used a pre-selected trauma registry rather than scene population the specificity is likely to have been markedly overestimated. 40 The lack of current trauma triage criteria, which are both sensitive and specific enough for optimum system functioning, has been highlighted elsewhere, but mainly with regard to all major trauma rather than head injury per se; however, in the NHS, 75% of all major trauma victims (defined as an ISS of > 15) have a brain injury. 18
It is known that the NICE high-risk criteria are 95% sensitive for detecting need for neurosurgery33 but, as indicated above, they are not applicable at the scene of injury, as they need observation for 2 hours post injury to be fully applied as well as practitioners who are trained in full neurological examination. Their specificity in this pre-hospital population is unknown.
Interpretation of findings
This is the first trial of pre-hospital bypass, highlighting the challenges of reliably diagnosing TBI and need for neurosurgery at the scene of injury.
As the Venn diagram in Figure 11 suggests, the main interpretation from the findings of HITS-NS is the difficulty in reliably identifying patients with TBI at the scene, as the majority will not be detected using current (GCS-based) triage criteria and some may indeed have no clear signs of head injury. Raising the GCS score criteria to > 13 is likely to result – given the findings of HITS-NS screening in the consort – in unsustainable levels of overtriage to the EDs of SNCs.
FIGURE 11.
Venn diagram of various head injury and TBI presentations to ASs at scene of injury.
Furthermore, there is no indication that many patients with TBI gain any benefit from being in SNCs. Within the study, 38 out of 70 patients with TBI required either neurosurgery or critical care but only 20 out of 70 required craniotomy or ICP monitoring in ICU. The evidence that the remaining 18 will have benefited from being in NCs is more open to question4,41 in terms of early-mortality reductions.
The current reconfiguration of trauma care in NHS England has switched resources from designated Trauma Units (non-specialist acute hospital in HITS-NS) to the MTCs, which, by and large, have on-site neuroscience or are linked closely to it. MTCs are equivalent to HITS-NS SNCs and the resource switch has occurred in the form of best practice tariff payments for MTCs for quality metrics being met. This has been done in anticipation that bypass would detect most serious injuries and that, therefore, the vast majority of early trauma resuscitation, diagnoses and management would occur in MTCs with an acceptable overtriage rate.
The results of HITS-NS call these assumptions into question, as it would appear that, for patients meeting the study inclusion criteria, the overtriage rate is close to 87% in terms of patients who could conceivably benefit from being in a NC, whereas the majority of patients with brain injury appear to have a GCS score of 14–15 at scene and will not be picked up by conventional trauma triage criteria. A proportion of these will require neurosurgery; indeed, this high GCS cohort may, even with bypass in place, represent the majority of patients within any trauma system that require neurosurgery. Therefore, robust secondary transfer procedures need to be in place in the new trauma systems.
As bypass is already implemented in NHS England, the results of HITS-NS may not be seen as relevant. The strength of evidence provided by this study – as it does not apply to all patients ‘being bypassed’ in the new trauma systems25 – is not enough to dismiss bypass, but the feasibility study and the health-economic analyses strongly suggest that further research is needed. A further trial may be cost-effective or it may be possible to conduct some observational analyses with comparative effectiveness modelling.
Implications for future clinical practice and research
-
Current trauma reconfiguration for head injury – for which bypass is now standard practice for patients meeting the HITS-NS inclusion criteria – has been implemented based on extremely limited research evidence and implicit expert opinion.
-
The low numbers of patients in the HITS cohort requiring neurosurgery plus transparent and explicit parameterisation of the economic model with expert opinion and cost data suggests that this decision may not be correct.
-
However, given that bypass is now standard care and there were many assumptions and areas of uncertainty in the parameterisation of the health-economic models, further challenging research is required to evaluate bypass. As trauma system reconfiguration has already occurred, further trials may be seen as disruptive and obtaining reliable follow-up is challenging, but comparative effectiveness research using registry data4 is a further option.
-
There is a definite need for a secondary transfer (NSAH to SNC) pathway to remain in place for ‘high-GCS score TBI’ presenters, which will not be picked up by triage criteria as eligible for bypass but may represent the majority of patients who require neurosurgery.
-
HITS-NS may have awakened an appetite for an evaluation of bypass in ASs.
-
There is currently insufficient evidence to inform the key trauma system questions:
-
Which patients with TBI definitely benefit from SNC care?
-
Which – if any – patients with TBI need to bypass to SNCs for early neurosurgery?
-
If ‘yes’ to the above, (how) can they be reliably identified in the pre-hospital environment?
-
Chapter 4 HITS-NS stream B: economic evaluation of management pathways for adult patients with stable suspected significant head injury
Introduction
We performed an economic evaluation using decision analysis modelling to examine alternative management pathways for adult patients with suspected significant TBI injured closest to a NSAH. The aim was to inform NHS decision-making about how best to organise trauma systems for head-injured patients. The evaluation addressed three related questions:
-
Given existing evidence, what is the most cost-effective management strategy?
-
What is the uncertainty surrounding this choice?
-
Is it cost-effective to perform further research to reduce this decision uncertainty?
This chapter presents the decision problem and reports the model structure and analytical methodology. Chapter 5 reports the cost–utility results of the decision analysis model, identifying the most cost-effective management pathway. Value of information analyses are additionally presented, evaluating whether or not further evidence may be indicated to help support the health technology adoption decision. Finally, the limitations of the analyses, the ramifications of findings in relation to future health policy and implications for the future research agenda are discussed in Chapter 6. Detailed information on the evidence and inputs used to parameterise the economic model are available from the authors.
Decision problem
Traumatic brain injury is a leading cause of death in young adults in the UK,42 and a significant cause of morbidity and socioeconomic costs. 43 Small absolute improvements in outcome accruing from optimisation of TBI management pathways could, therefore, potentially have a powerful impact on public health. Patients injured closest to a SNC will always be transported to that hospital for further management, and will, therefore, be unaffected by trauma service reconfiguration. Conversely, several alternative management pathways are possible for patients with suspected significant TBI who are injured closest to a NSAH.
Until 2012, patients with suspected significant head injury would initially be transported to their local non-specialist hospital for resuscitation and investigation, with subsequent ongoing management occurring in the NSAH, or in the regional SNC after secondary transfer. 4 More recently, services for patients with TBI in England have been reorganised, with the development of regional trauma networks and bypass of suspected significant head injuries to SNCs. 44 This treatment pathway could increase the numbers of patients treated in SNCs and will expedite definitive neuroscience care, but risks deterioration and secondary brain insults during prolonged primary transportation. Despite the investment of resources in reconfiguration, there is an absence of head-to-head clinical trials or economic evaluations to determine the cost-effectiveness of bypass compared with competing secondary transfer strategies. 17,45
Rationale for economic modelling
The HITS-NS pilot study examined a restricted subset of two potential options (pre-hospital triage and bypass vs. selective secondary transfer of patients with severe TBI), excluding potentially important strategies relevant to the NHS. As a pilot study, HITS-NS had a small sample size, underpowered for economic evaluation. Additionally, the study was unable to collect meaningful data on disability end points, longer-term resource use or quality of life, precluding a trial-based cost–utility evaluation.
We performed a literature review of previous economic models in adult head injury and failed to identify any previous economic evaluations appropriately investigating this decision problem. Two cost-effectiveness studies evaluating bypass in head injury were retrieved,1,13 but these did not examine competing strategies within a cost–utility framework or were not based on a systematic review of the literature, severely limiting their use to decision-makers. No further relevant health-economic studies were found in other literature searches during model parameterisation.
We therefore developed a mathematical decision analysis model, simulating the natural history of significant head injury and the impact of potential management pathways. Health-economic models allow abstraction of complex and uncertain real world phenomena into a manageable and transparent format to inform a decision problem. 46 This economic model, therefore, provided a framework to synthesise available data pertaining to all relevant patient management pathways calculate costs and benefits of alternative pathways identify the best management pathway given available evidence characterise the uncertainty inherent in this finding discern important determinants of cost-effectiveness and evaluate the need for further research in this area.
Model development
A critical source of an economic model’s validity and credibility is the model structure, comprising specification of included parameters and their mathematical and theoretical relationships. We followed expert technical guidance and developed conceptual models with relevant stakeholders at the beginning of the modelling project. 47,48 This approach aided understanding of the complexity of the decision problem, facilitated agreement on a proposed mathematical representation prior to model programming, and contributed to descriptive validity.
The HITS-NS model structuring process is summarised in Figure 12. Preliminary disease logic and treatment pathway conceptual models were developed by the primary modeller, informed by the HITS-NS study protocol, a detailed review of relevant head injury literature; a survey of head injury experts; and a literature review of existing adult head injury evaluations. Important features highlighted during this process included the potential differential quality of care between specialist and non-specialist hospitals, deterioration during primary transportation, and time from injury to neuroscience care. These preliminary problem-orientated conceptual models were then developed iteratively in a series of consensus meetings with clinical and modelling experts from the HITS-NS TMG, with subsequent formulation of a design-orientated conceptual model.
FIGURE 12.
Model structuring process.

Model scope
The modelled population was congruent with the original HITS-NS inclusion criteria, comprising adult patients injured closest to a NSAH and attended by the AS, with suspected significant TBI, defined as external signs of head injury, pre-hospital GCS score of < 13 and stable pre-hospital cardiorespiratory physiology. The cohort was assigned a nominal age of 50 years, based on the mean age of the HITS-NS cohort (49.8 years).
Relevant management pathways were identified during the model structuring process. We considered any comparator that could be feasibly be implemented in the NHS. The following mutually exclusive management pathways, designated according to the treatment pathway for head injury patients requiring critical care, were included:
-
Pre-hospital triage with bypass – patients with suspected significant head injury are identified according to HITS-NS inclusion criteria. All patients should then be transported directly to the distant regional SNC, bypassing the local NSAH. However, paramedics are non-compliant with bypass in a proportion of patients informed by observed practice in the HITS-NS pilot study. Any patient with an acute neurosurgical lesion erroneously transported to a NSAH will undergo early secondary transfer to the regional SNC for urgent operative management. Patients with major extracranial injury or significant head injury requiring critical care taken to NSAH will undergo further triage by the regional specialist centre to determine early secondary transfer. This management pathway reflects the level of bypass implementation observed during the HITS-NS trial and may represent the real-world application of the HITS-NS intervention.
-
Selective secondary transfer – all patients are initially taken to the local NSAH. Any patient with an acute neurosurgical lesion undergoes early secondary transfer to the regional SNC. Patients with head injuries requiring critical care or major extracranial injury are triaged by the regional neurosurgical and trauma services, with selected patients also undergoing early secondary transfer. All other patients are managed within the NSAH or undergo later transfer to the specialist centre in the event of clinical complications. This strategy conforms to NHS practice prior to the introduction of regional trauma systems in 2012. 4
-
Routine secondary transfer – all patients are initially taken to the local NSAH. All patients with an acute neurosurgical lesion or head injury requiring critical care undergo routine early secondary transfer to the regional SNC. Patients with major extracranial injury are triaged by the regional trauma centre, with selected patients also undergoing secondary transfer. All other patients are managed within the NSAH, or undergo later transfer to the specialist centre. This strategy is recommended in the 2007 and 2014 NICE head injury guidelines. 1,33
-
No secondary transfer – all patients are initially taken to the local NSAH. Patients with an acute neurosurgical lesion undergo early secondary transfer to the regional SNC. Patients with major extracranial injury are triaged by the regional trauma centre, with selected patients also undergoing secondary transfer. All other patients (including moderate and severe head injury cases) are managed within the NSAH, or undergo later transfer to the specialist centre. This management strategy was historically implemented in the NHS and has been advocated by some head injury authorities. 49
The key difference between the competing secondary transfer strategies is the initial referral pattern for non-surgical patients with TBI requiring immediate critical care. This ranges from invariant transfer of all such patients in the routine transfer strategy, through triage by the regional neurosurgical unit in selective transfer, to management within the non-specialist hospital in the no-transfer strategy. Patients with mild TBI, acute neurosurgical lesions, requiring ward admission or sustaining major extracranial injury are managed identically between each alternative secondary transfer strategy.
Additionally, two further theoretical strategies were examined in sensitivity analyses to explore the maximal potential effects of bypass and illustrate the benefit of specialist care in trauma:
-
Pre-hospital triage with bypass (full compliance) – paramedics identify patients with suspected significant head injury who meet the HITS-NS triage criteria. All patients are then transported directly to the distant regional SNC, bypassing the local NSAH. This strategy represents full implementation of the HITS-NS intervention and is consistent with recommendations on pre-hospital trauma management in England since the introduction of major trauma systems in 2012. 50
-
No transfer (including patients with acute neurosurgical lesions and major extracranial injury) – all patients are initially taken to the local NSAH and remain there for further management, regardless of diagnosis, prognosis or complications. This ‘zero option’ could be compatible with the management of casualties in rural areas of the developing world. 51
Early secondary transfer is defined as the first inter-hospital transfer occurring within 12 hours of hospital presentation. This will therefore include all urgent transfer decisions made after patient stabilisation and investigation, including cases in which logistical constraints may delay immediate transportation.
It was assumed that these comparators were implemented in a geographical area and service configuration similar to those evaluated in the NEAS and NWAS during the HITS-NS study: that is, a NSAH serving a rural, semirural and small urban catchment area and a regional SNC, consisting of a neurosurgical department, ICU and supporting trauma specialties, located in the nearest city. In common with HITS-NS inclusion criteria, the model was restricted to patients within a 1-hour land ambulance journey of the SNC and excluded air ambulance transfers.
The model took the perspective of the NHS, and thus included direct medical costs and costs arising from personal and social services. As head injury may result in long-term disability, costs and consequences were studied over a lifetime horizon. Only the direct health effects of the competing strategies were considered.
Model structure
The theoretical basis for the model is that bypass may improve outcomes by expediting definitive care for patients with suspected significant TBI, but these benefits may be offset by a risk of pre-hospital deterioration and increased costs arising from more expensive specialist management. Explicit modelling of the effects of time to resuscitation and time to neurosurgery, using prognostic models developed in TARN data, was prespecified in the HITS-NS study protocol. However, during the model development process it was apparent that this was not a credible approach. Literature reviews revealed no convincing evidence of an association between time to treatment and outcome in head injury, with studies critically limited by an extremely high risk of confounding by indication. Furthermore, relevant disability end points are not available within the TARN registry,18 and attempts to impute outcomes from other data sets were not possible. Finally, when we examined survival using TARN data, there was no association observed between emergency services interval and 30-day mortality52 or time to NC care, although, in common with previously published studies in this area, interpretation of these findings is challenging because of a high risk of bias.
An analysis using existing data would therefore have had obvious results and scarce internal validity, and was consequently redundant. As conceptual models clearly defined a strong theoretical basis for potential differences in outcome between alternative management strategies, we therefore decided to over-ride this evidence in favour of the expert opinion of the clinical community. We subsequently implemented a hybrid cohort model focusing on differences in outcomes and costs for important patient subgroups. A decision tree delineated short-term costs and consequences, with a subsequent time-dependent Markov model extrapolating longer-term survival.
The decision tree modelled costs and consequences for the first year after injury. Each strategy was represented by a subtree of identical structure, as shown in Figure 13. The first chance node delineated the heterogeneous HITS-NS population into important subgroups with differing treatment pathways, costs and prognoses. Subgroups were mutually exclusive and defined retrospectively, based on the treatment patients received or needed to receive:
-
Acute neurosurgery – patients with acute expanding subdural haematomas, extradural haematomas, or other intracranial lesions requiring urgent neurosurgery.
-
Head injury requiring critical care – patients with moderate or severe TBI requiring admittance to high dependency or ICUs. These patients could also have sustained a concomitant major extracranial injury, but critical care was necessary to manage the head injury.
-
Head injury requiring admission – patients admitted to general hospital wards following a head injury.
-
Mild head injury – head injury patients discharged immediately from the ED or after overnight observation.
-
Major extracranial injury – patients with a mild or trivial head injury who have sustained a significant extracranial injury consistent with an Abbreviated Injury Scale score of ≥ 3.
FIGURE 13.
Short-term decision tree model. D, death; GR, good recovery; MD, moderate disability; S, survival; SD, severe disability.
Subsequent chance nodes modelled strategy-level outcomes, accounting for differences in costs and outcomes arising from different treatment pathways within each strategy. For subgroups considered to be homogeneous (mild head injury, head injury requiring ward admission and acute neurosurgery subgroups), the model’s mathematical formulation for the bypass strategy included the proportion of patients expected to be transported to either NSAHs or SNCs and their subsequent conditional outcomes. In these patient groups, compliance with bypass protocols was assumed to be unrelated to subsequent patient outcome. The remaining subgroups of head injury requiring critical care and major extracranial injury will consist of patients with a wide range of injury severities. Compliance with bypass protocols and secondary transfer decisions are therefore likely to be correlated with injury severity, and hence costs and outcomes. However, there is no evidence from the HITS-NS study or the literature to define this association accurately, and clinical experts could not confidently define the magnitude or direction of any relationship. We therefore elected to model strategy-level outcomes only, pooled across patient treatment pathways.
Head injury outcomes were defined by the basic Glasgow Outcome Scale (GOS), a validated 5-point ordinal scale measuring survival and disability after head injury. 53 In common with previous head injury studies, we omitted ‘persistent vegetative state’ due to diagnostic difficulties in defining this state, very low incidence and limited availability of evidence on this group of patients. 54 Outcomes for major extracranial injury were defined in terms of death or survival only, owing to the absence of a well-established validated disability scale and paucity of evidence on disability end points. Appropriate utility values were assigned to each short-term model end point.
Costs associated with patient transport, inpatient management and post-discharge care were assigned to each decision tree branch. Inpatient costs were averaged over all outcome categories, whereas post-discharge costs for the first year post injury were modelled for each specific GOS health state. Model inputs are described in detail in Chapter 5.
Differences between management options were accounted for by designating each chance node with a strategy-specific probability and by assigning differing costs and utility values to the terminal nodes of each individual subtree branch. Expected costs and QALYs for each strategy were subsequently calculated by summation of the terminal node values, weighted by the conditional branch probabilities. This model structure therefore captured relevant features of the service pathway conceptual model for included comparators. The effects of time to resuscitation, time to NC care and relative effectiveness between specialist and non-specialist hospital care were implicitly modelled through differential outcomes between management pathways.
The sum of GOS and mortality outcomes across patient subgroups gave the expected outcomes for each strategy at 1 year, and provided the starting values for the state transition model extrapolating long-term survival, quality of life and costs. A simple time-dependent Markov model was then implemented using alive (comprising the four GOS categories and survival from extracranial injury) and dead, as possible health states. Only one transition from alive to dead was possible, with patients assumed to remain at the same level of disability throughout their survival. As major trauma is a discrete, one-off insult, and subsequent inpatient management is time limited, there are no clinically plausible mechanisms for ongoing treatment effects on long-term life expectancy. We therefore assumed that competing management pathways provided no health benefits beyond the short-term model. A 1-year cycle length was modelled with transition probabilities derived as a function of the number of cycles that had elapsed since the start of the model, allowing the probability of death to increase as the cohort aged. Health-related quality of life naturally declines with age and the utility values associated with each GOS health state were therefore adjusted for age using a multiplicative model based on predicted UK EQ-5D tariff scores. 55 A half cycle correction was used to compensate for the timings of transitions, assuming that, on average, state transitions occurred half-way through the cycle. 56 The long-term Markov model is shown in Figure 14.
FIGURE 14.
Long-term Markov model.
Model analysis
Study design
We performed a cost–utility economic evaluation using a probabilistic decision analysis model to synthesise available evidence and compare alternative management strategies for patients with suspected TBI injured nearest to a NSAH. To maximise internal validity we followed expert recommendations, consensus modelling guidelines and NICE technical guidance. 57–60 Our base-case analysis followed NICE reference case methodology, taking the perspective of the UK NHS DH (the primary decision-maker) and employing a lifetime horizon.
Parameterisation
Details on the identification, appraisal and selection of evidence to determine model inputs can be obtained through contact with the authors. Each short-term model input was ideally informed by literature review or routine official data sources. When relevant and unbiased published evidence was unavailable, existing indirectly relevant but valid evidence was statistically adjusted to allow inclusion, HITS-NS pilot data were used or, finally, if no other valid data were available then expert opinion was formally elicited.
Each model input was assigned an average or most likely value, and a probability distribution representing a credible range and the relative likelihood of possible values for the uncertainty in this estimate was defined. Distributional choices were carefully chosen, based on theoretical considerations, logical constraint and the parameter estimation process. 56 When published estimates were used as model inputs, the method of moments was used to calculate appropriate distribution parameters. 61 As model inputs were derived from alternative sources, with no data available on the covariance structure, we were unable to account for any correlation between costs and outcomes. The mean values and distributions for each parameter are shown in Table 9.
| Model input | Deterministic value | Parameter type and distributional form | Distribution parameters for PSAa | Information source |
|---|---|---|---|---|
| Population subgroups | ||||
| Mild TBI | 0.57 | Proportion, Dirichlet | α1: 95 | HITS-NS pilot data |
| Acute neurosurgery | 0.06 | α2: 10 | ||
| TBI requiring critical care | 0.11 | α3: 19 | ||
| TBI requiring ward admission | 0.20 | α4: 33 | ||
| Major extracranial injury | 0.05 | α5: 9 | ||
| Compliance with bypassb | ||||
| Mild TBI | 0.35 | Proportion, beta | α: 22 β: 41 | HITS-NS pilot data |
| Acute neurosurgery | 0.83 | Proportion, beta | α: 2.5 β: 0.5 | |
| TBI requiring ward care | 0.57 | Proportion, beta | α: 12 β: 9 | |
| Mild TBI outcomes | ||||
| Dead | 0.01 | Proportion, Dirichlet | α1: 9 | OCTOPUS study62 |
| Severe disability | 0.05 | α2: 89 | ||
| Moderate disability | 0.06 | α3: 95 | ||
| Good recovery | 0.88 | α4: 1430 | ||
| Acute neurosurgery baseline outcomes (secondary transfer) | ||||
| Dead | 0.41 | Proportion, Dirichletc | α1: 36 | NHIR study63 |
| Severe disability | 0.13 | α2: 12 | ||
| Moderate disability | 0.22 | α3: 19 | ||
| Good recovery | 0.23 | α4: 20 | ||
| TBI requiring critical care baseline outcomes (selective transfer) | ||||
| Dead | 0.19 | Proportion, Dirichletc | α1: 149 | RAIN study41 |
| Severe disability | 0.22 | α2: 178 | ||
| Moderate disability | 0.31 | α3: 249 | ||
| Good recovery | 0.28 | α4: 219 | ||
| TBI requiring ward care baseline outcomes (selective transfer) | ||||
| Dead | 0.03 | Proportion, Dirichletc | α1: 3.5 | McCartan et al. 200764 |
| Severe disability | 0.02 | α2: 2.5 | ||
| Moderate disability | 0.06 | α3: 6 | ||
| Good recovery | 0.88 | α4: 84 | ||
| Survival | 0.96 | Proportion, beta | α: 1690, β: 46991 | TARN database18 |
| Relative effectiveness (vs. selective secondary transfer, proportional odds ratio for unfavourable outcome) | ||||
| Bypass: acute neurosurgery | 0.53 | Log-odds ratio, normal | µ: –0.68 SE: 0.34 | Expert opinion |
| Bypass: TBI requiring critical care | 1.00 | µ: 0.0 SE: 0.41 | ||
| Bypass: TBI requiring ward care | 0.98 | µ: –0.01 SE: 0.02 | ||
| Bypass: major extracranial injury | 0.80 | µ: –0.22 SE: 0.07 | Mullins et al. 199865 | |
| Routine transfer: TBI requiring critical care | 0.86 | µ: –0.15 SE: 0.12 | Expert opinion | |
| No transfer: TBI requiring critical care | 2.14 | µ: 0.76 SE: 0.35 | ||
| Inpatient costs, £ (vs. selective transfer, incremental mean cost) | ||||
| Bypass: mild TBI | 63 | Mean difference, normal | µ: 63.3 SE: 39.7 | HITS-NS pilot data |
| Bypass: acute neurosurgery | 32,044 | µ: 32,044 SE: 18,249 | ||
| Bypass: TBI requiring critical care | 6971 | µ: 6971 SE: 14,699 | ||
| Bypass: TBI requiring ward care | 2353 | µ: 2353 SE: 981 | ||
| Bypass: major extracranial injury | 5922 | µ: 5922 SE: 5283 | ||
| Routine transfer: TBI requiring critical care | 7500 | Mean difference, scaled beta | α: 3.66 β: 4.00 Max: 10,000 Min: 5000 | Expert opinion |
| No transfer: TBI requiring critical care | –7500 | α: 3.66 β: 4.00 Max: –10,000 Min: –5000 | ||
| Short-term post-discharge costs, £ (mean cost first year post injury) | ||||
| Dead | 0 | – | – | – |
| Severe disability | 58,292 | Mean costs, normal | µ: 58,292 SE: 3311 | Beecham et al. 200966 |
| Moderate disability | 29,507 | µ: 29,507 SE: 1577 | ||
| Good recovery | 413 | µ: 413 SE: 1.31 | ||
| Survival from extracranial injury | 7884 | µ: 7884 SE: 338 | HALO study67 | |
| Long-term post-discharge costs, £ (mean annual costs subsequent years) | ||||
| Dead | 0 | – | – | – |
| Severe disability | 12,500 | Mean cost, beta | α: 4.93 β: 6.14 | Expert opinion |
| Moderate disability | 1600 | Mean cost, log-normal | µ: 7.59 SD: 0.36 | |
| Good recovery | 24 | Mean cost, beta | α: 1.54 β: 5.81 | |
| Survival from extracranial injury | 413 | Mean cost, normal | µ: 7884 SE: 338 | HALO study67 |
| Health-state preference weightsd | ||||
| Dead | 0 | – | – | – |
| Severe disability | 0.15 | Mean utility decrement from perfect health, gamma | α: 164 β: 0.01 | Smits et al. 201068 |
| Moderate disability | 0.51 | α: 64 β: 0.01 | ||
| Good recovery | 0.88 | α: 6.8 β: 0.02 | ||
| Survival from extracranial injury | 0.67 | Mean utility, beta | α: 3022 β: 1475 | HALO study67 |
Costs and consequences
The consequences of alternative management strategies were measured in QALYs to allow comparison within and across different disease areas. 69 QALYs were calculated by multiplying survival duration with the appropriate utility value for the corresponding heath state.
Direct treatment and personal social services costs were included. The price base was assumed to be 2012, valuations were in UK pounds sterling and unit costs were considered to be time divisible. When unit costs were valued prior to 2012, the Bank of England’s Consumer Price Index data were used to inflate costs to current value70 in line with UK HTA guidelines costs, and QALYs were discounted at a 3.5% rate, reflecting NICE’s positive frame of time preference. 60,71–73
Primary analyses
Management options were compared in terms of incremental costs per additional QALYs gained [incremental cost-effectiveness ratio (ICER)] and net monetary benefit (NMB). We considered cost-effectiveness thresholds of between £10,000 and £50,000, based on NICE’s stated willingness to pay,60 theoretical estimates of λ72 and observed NICE adoption practices. 71 We specifically focused on values of λ of £20,000 and £30,000 as detailed in NICE HTA guidelines. 60
An initial base-case deterministic analysis, with parameters fixed at their mean or mostly likely values, estimated the mean expected costs and QALYs gained per patient for each management pathway. Management pathways were then compared according to established principles of strong and extended dominance. 56 In order to account for the uncertainty in model inputs, a base-case probabilistic sensitivity analysis (PSA) was conducted using Monte Carlo simulation to sample from input parameter distributions. Multiple model runs were performed, each with a random draw from every parameter distributions, thus evaluating the full range of cost-effectiveness results possible with current uncertainty. Mean ICERs, calculated from the average expected costs and effects over all model runs, were recomputed and compared with cost-effectiveness thresholds to inform adoption decisions. The number of PSA iterations to produce a stable mean estimate of incremental cost–utility was determined by visual inspections of mean cost per QALY plotted against the number of trial PSA simulations.
Mean NMB was also calculated for the defined values of λ, with the incremental difference subsequently calculated for each strategy relative to a baseline comparator of selective transfer. 95% confidence limits were computed using non-parametric bootstrapping, and the spectrum of potential cost-effectiveness results was demonstrated by taking the 2.5th and 97.5th percentiles of incremental NMB from the range of PSA simulations. A cost-effectiveness acceptability curve, plotting λ against the probability that each intervention was the most cost-effective, was additionally derived to summarise the uncertainty of PSA results. 74 In order to maximise health gains, adoption decisions should be based on expected NMB; however, in cases when the distribution of NMB is skewed, the intervention with the highest NMB may not have the highest probability of being cost-effective. We therefore also constructed a cost-effectiveness acceptability frontier identifying the probability that the technology with the highest expected NMB is cost-effective for a given λ. 75
Model uncertainty and sensitivity analyses
Uncertainty can arise in decision models because of variability, heterogeneity, parameter uncertainty and structural uncertainty. 56 Each of these factors needs to be addressed to explore important determinants of cost-effectiveness, afford decision-makers’ confidence in results, and to indicate the precision of cost-effectiveness estimates.
As a cohort model was implemented with examination of mean values, an examination of variability (or ‘first-order’ uncertainty) is extraneous. Furthermore, as head injury management pathways are population-level interventions, they will be implemented for any patients presenting with suspected significant head injury, making examination of heterogeneity superfluous. ‘Second-order’ parameter uncertainty, that is uncertainty surrounding the true value of a model input within the specified probability distribution, was fully explored in the PSA.
To examine ‘third-order’ parameter uncertainty (i.e. the correct statistical form has been specified for a probability distribution) we performed a number of univariate, threshold and scenario sensitivity analyses on parameter distributions that were thought to be important or uncertain. As influential relative effectiveness inputs were based on uncertain probability distributions elicited from expert opinion, and incremental costs were estimated from small HITS-NS samples, best- and worse-case analyses for parameters related to the pre-hospital triage and bypass strategy were initially performed. Model inputs were set to extreme favourable or unfavourable values and the model re-run to explore the most optimistic and pessimistic estimates for this strategy. An additional threshold analysis was implemented in which incremental costs, relative effectiveness and compliance were varied across their plausible range to determine the parameter levels at which the HITS-NS intervention may become cost-effective. Further univariate sensitivity analyses varied the individual probability distributions of certain model parameters, including population subgroups, baseline outcome probabilities, relative effectiveness, utilities, inpatient costs and post-discharge costs.
Structural uncertainty in methodological choices (varying the discount rates for costs and effects), choice of comparators (including theoretical bypass and no-transfer strategies) and perspective on outcomes (non-health effects of bypass) was also examined. Sensitivity analyses examining relative effectiveness and incremental costs were specified a priori. Other sensitivity analyses were informed by model structuring, evidence synthesis and emerging results. All sensitivity analyses treated unexamined model inputs probabilistically. The sensitivity analyses are summarised in Table 10.
| Sensitivity analysis | Description | Key parameters varied | Alternative specification of model inputa |
|---|---|---|---|
| Scenario analyses of parameter uncertainty | |||
| Bypass best-case scenario | Cost-effectiveness of bypass when parameters set to favourable, but still plausible values | Relative effectiveness and incremental costs:
|
0.25 and 0.75 quantiles of relevant parameter distributions |
| Bypass worst-case scenario | Cost-effectiveness of bypass when parameters set to unfavourable, but still plausible values | ||
| Threshold analysis | |||
| Bypass threshold analysis | Incremental costs and relative effectiveness parameters sequentially increased to identify parameter values at which bypass becomes cost-effective at λ = £20,000 and £30,000 | Relative effectiveness and incremental costs:
|
Increasingly favourable 0.05 quantiles of relevant parameter distributions |
| One-way sensitivity analyses of parameter uncertainty | |||
| Population subgroups | Alternative estimates for population subgroups | Dirichlet distribution for population subgroups | Estimates used from each trial region, Dirichlet distributions:
|
| Acute neurosurgery baseline outcomes | Alternative estimates for outcomes of patients requiring acute neurosurgery undergoing secondary transfer | Dirichlet distribution for acute neurosurgery (secondary transfer) | Dirichlet distribution derived from a published estimate:76 α1: 18 α2: 9 α3: 27 α4: 22 (posterior distribution after external bias adjustment) |
| Acute neurosurgery costs | Alternative estimates for incremental costs of patients requiring acute neurosurgery undergoing bypass | Normal distribution for mean incremental costs |
|
| Incremental inpatient costs for bypass | Alternative estimates for incremental inpatient costs associated with bypass | Normal distribution for mean incremental costs | Normal distributions elicited from clinical experts:
|
| Relative effectiveness for major extracranial injury patients | Alternative estimates for odds ratio for survival following major extracranial injury associated with bypass strategy | Normal distribution for log-odds ratio | Normal distribution elicited from clinical experts: µ: –0.18 SD: 0.05 |
| GOS utilities | Alternative estimates for utility values for GOS health states | GOS utility values | Alternative health states measured using scenarios and valued using the standard gamble:77 utility decrement from perfect health, gamma distributions:
|
| Post-discharge costs | Elicited long-term head injury costs evaluated | Post-discharge costs for GOS states | 0.25 quantiles of relevant distribution |
| One-way sensitivity analyses of structural uncertainty | |||
| Alternative comparators | Bypass with full compliance and no transfer (including no transfer of patients requiring acute neurosurgery) examined as potential strategies | Relative effectiveness and incremental costs for TBI bypass and no-transfer strategies modified | Normal distributions elicited from clinical experts for log proportional odds/odds ratios:
|
| Discount rate | Discount rates changed to explore different frames of time preference | Discount rate | Discount rate = 1.5% and discount rate = 6.0% |
| Proportional odds ratio | Assumption of proportional odds effect on relative outcomes removed | Relative effectiveness:
|
Odds ratio for unfavourable outcome applied, but the proportions of patients in each constituent GOS category of the dichotomised outcome group is equivalent to that observed in the baseline outcome strategy |
| Long-term survival | Increased mortality in survivors of TBI and major extracranial injury modelled | Time dependency in state transition model | Relative risk applied from literature applied to general population life tables78 |
| Consideration of non-health effects | Utility decrement applied to bypassed mild patients with TBI to account for inconvenience of unnecessary transport to a distant hospital | Mild TBI outcomes in bypass strategy | Utility decrement of 0.001 |
Model implementation
The decision analysis model was programmed in Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA) using Visual Basic macros. An Excel add-in was utilised to sample from Dirichlet distributions. 79 Bootstrapping to derive incremental NMB 95% CI, using bias-corrected CI and evaluating 3000 replications, was performed in Stata version 12.1 (StataCorp, College Station, TX, USA). Internal testing was performed throughout model development to ensure that mathematical calculations accurately represented model specifications and were correctly implemented in Visual Basic. 59 Debugging techniques included null and extreme input values, setting equal values across comparators, fixed distributions and code breaks with line-by-line checking of macros. The model was also independently verified by a second modeller.
Expected value of information analyses
Background
To avoid potential opportunity costs, health-care systems should typically choose interventions with the highest expected NMB, regardless of statistical uncertainty. 80,81 This approach will maximise health gains from available resources and result in correct decision-making based on current evidence. However, there is often considerable uncertainty in model inputs and the optimal management strategy could differ if the true parameter values were known with certainty. Any errors in the adoption decision will consequently result in forfeited health benefits and wasted resources.
Economic models use probability distributions to reflect the uncertainty of a decision problem and can simulate the entire spectrum of cost-effectiveness results that are possible given potential realisations of parameter values. The probability of making the wrong adoption decision and the resulting effects on costs and QALYs will therefore be available from implementing a PSA; the opportunity losses surrounding a decision can consequently be calculated for each patient. This value is termed the individual expected value of perfect information (EVPI); measuring the expected cost of current uncertainty for each patient by accounting for both the probability that a decision based on existing evidence is wrong and for the magnitude of the consequences of making the wrong decision. 82 A rational decision-maker should be willing to pay for additional perfect evidence up to this level of expected opportunity loss for the total population of patients who may benefit from additional research evidence over the lifespan of the technology, a value known as the population EVPI. 82
Population EVPI places an upper bound on any investment in future research, but does not indicate what type of evidence would be valuable. By focusing attention on eliminating the potential opportunity costs arising from uncertainty in particular model inputs, expected value of partial perfect information (EVPPI) can indicate research into which parameters would be most valuable. 59,83 Identification of variables for which precise estimates would be most valuable will indicate where research funds should be focused and may suggest appropriate research designs. For example, a large EVPPI value for an estimate of relative effectiveness would suggest that a future RCT is potentially useful, whereas a high EVPPI for the prevalence of a disease subgroup could indicate an epidemiological cohort study. Individual EVPPI, defined analogously to EVPI, is the expected NMB given perfect information about the parameter of interest minus the expected NMB with current information. Population EVPPI is similarly derived by multiplying the individual EVPPI by the population of patients that would potentially benefit from additional information. Extension of this technique to groups of parameters is possible using the same principles. 59
Comparing the costs of future research studies against population EVPI and EVPPI provides a necessary condition for future research: study costs must not exceed these values to be cost-effective. However, they do not provide a sufficient condition that any future research study will be helpful and cost-effective, and make the implausible assumption that all uncertainty can be removed from a parameter value. EVSI extends the value of information methodological framework to establish the expected value of conducting studies with different designs and sample sizes. 84,85 Individual EVSI is mathematically defined as the expected difference between the value of the optimal decision, based on a sample of data, informative for certain model parameters, minus the value of information based on existing evidence. 84 Population EVSI is computed equivalently to population EVPI and EVPPI, but should account for the fact that patients recruited into a study are ‘used up’ and cannot benefit from a study’s results, and that the length of a study may reduce the time period for which an intervention is applicable.
The expected benefits of a given study sample (the population EVSI) can be compared with the expected costs of collecting these data, with the difference denoting the expected net benefit of sampling (ENBS). ENBS measures the societal reward for conducting additional research, with ENBS > £0 demonstrating that the marginal benefits of gathering further evidence exceeds the marginal costs, and higher ENBS values representing more efficient study designs. Optimal study designs can then be identified by choosing the largest ENBS from different options with varying sample sizes, follow-up periods and study end points. 59
Expected value of information methodology
Individual EVPI was directly calculated directly from the model PSA output using the standard formula. 59
A state-of-the-art regression-based approach was used for calculation of EVSI and EVPPI. 86–88
Briefly, the method developed by Strong and Oakley86–88 rearranges the statistical formulas for computation of individual EVPPI and EVSI, reframing estimation into a regression problem. The expected NMB for each PSA simulation can be considered a function of the examined uncertain parameters in EVPPI, or simulated data in EVSI. The PSA output can therefore be treated as ‘noisy data’ through which this functional form can be characterised, thus allowing calculation of the individual EVPPI or EVSI at a particular willingness-to-pay threshold using fitted values from regression models. As NMB would not be expected to have a linear relationship with inputs of interest, a non-parametric regression method provides the flexibility to accurately model the correct shape of the association. The theoretical basis of this method is described in detail in the literature86–88 and examples of its use in HTAs are also available. 89,90 In common with previous applications of this method, generalised additive models were used for non-parametric regression,91 implemented in R (The R Foundation for Statistical Computing, Vienna, Austria) using the ‘mcgv package’ and a ‘n = 5000’ PSA sample. 92–94
Individual EVPPI was initially calculated for each model input separately. Groups of parameters were then chosen to match types of research studies that could be conducted in order to logically inform future research prioritisation. Study designs considered included observational studies examining the prevalence of different patient subgroups, utility values for GOS states post-discharge health-care costs, and an experimental study investigating the relative effectiveness of bypass.
The individual EVSI analysis examined the value of conducting a definitive HITS-NS trial, of varying size, examining relative effectiveness between bypass and selective transfer. Regression models in the EVSI analysis require a summary statistic to represent the information gained from additional research. For each patient subgroup the numbers of patients expected to have favourable (good recovery or moderate disability) and unfavourable outcome (death or severe disability) were simulated from the prior distribution in the PSA output, using a binominal distribution. This process accounted for the relative numbers of patients expected in each subgroup by multiplying the overall trial size by the relevant subgroup proportion. Log-odds ratios were subsequently calculated for each patient subgroup as the summary statistic. For small trials, subgroups with low prevalence could have very few patients and a continuity correction was applied, where necessary, to allow calculation of odds ratios. Log-odds ratios for each patient subgroup were included in the final regression model as linear predictors without interaction terms.
Similarity between patients treated by the same ambulance station in a cluster randomised trial could lead to correlated outcomes and between-cluster variability. Cluster sampling consequently provides less information than individually randomised trials. 95 Effective sample sizes used to calculate EVSI were therefore multiplied by the design effect to determine the actual required number of patients to account for the increase in variance resulting from the HITS–NS cluster design. 96 Standard formulas were used to calculate the design effect, using estimates of intracluster correlation coefficient and average cluster size. 97 As HITS-NS was pair-randomised it was not possible to deduce an intracluster coefficient directly from trial data, and a plausible value was taken from a previous pre-hospital trial in the UK. 98 Average cluster size was based on the recruitment observed in HITS-NS. Sample size requirements for a cluster trial are also influenced by variation in cluster size and method of randomisation. However, to simplify calculations we considered only simple randomisation, assuming equal cluster size.
Calculation of population values for each expected value of information metric requires assumptions about the incidence of suspected significant TBI with stable pre-hospital physiology, the predicted time for which pre-hospital triage and bypass is likely to be a viable management option and discount rate. Applicable incidence estimates were unavailable from the literature, and HITS-NS pilot data were used in conjunction with routine statistics to calculate a credible range of values. The total number of eligible trial patients recruited over 1 year in each region was divided by the total AS catchment area adult population, and then averaged across the two regions. Annual incidence of suspected significant TBI was then derived using estimates of the national adult population in England, using data from the 2011 Census. 70 The technology lifespan was arbitrarily defined as 10 years, based on previously published HTAs. 99 A standard discount rate of 3.5% was applied. 60 As the variables influencing the size of population that may benefit from future research are uncertain, we conducted two scenario sensitivity analyses to provide tenable upper and lower bounds. Disease incidence, technology lifespan and discount rate were set sequentially to plausible high and low values, informed by differential recruitment rates between the two HITS-NS trial regions and the literature. Table 11 summarises the values for population expected value of information calculations in base-case and sensitivity analyses.
| Population EVPI parameter | Base-case valuea | Sensitivity analysis | Information sources | |
|---|---|---|---|---|
| Optimistic | Pessimistic | |||
| Annual incidence of relevant patients | ||||
| Suspected significant patients with TBI presenting in 1 year | 197 | 164 | 33 | HITS-NS pilot data |
| Adult population in AS catchment area | 3.1 million | 1.8 million | 1.3 million | Office for National Statistics 2011 Census70 |
| Incidence of suspected significant TBI | 6.4 per 100,000 per year | 9.1 per 100,000 per year | 2.5 per 100,000 per year | Calculated from preceding data |
| Adult population of England | 36 million | – | – | Office for National Statistics 2011 Census70 |
| Total annual incidence of suspected significant TBI in England | 2290 | 3280 | 910 | Calculated from preceding data |
| Technology lifespan | ||||
| Time bypass is applicable, years | 10 | 13 | 8 | Previous HTAs |
| Positive time preference | ||||
| Discount rate, % | 3.5 | 1.5 | 6.0 | NICE HTA guidelines |
Calculation of population EVSI must also account for the fact that patients who are enrolled in a study will not benefit from the information generated by the research. Furthermore, as sample sizes increase, accordingly longer trials will reduce the length of time new information will be useful for. We therefore assumed that any trial could feasibly be conducted in up to 8 out of the 11 English NHS ASs, with each AS having 46 ambulance stations available for randomisation. Based on the expected incidence of suspected significant patients with TBI in England and average cluster size, we hypothesised an upper limit on patients who could be recruited in 1 year. Sample sizes exceeding this number would take additional years to enrol the necessary patients. It was also assumed that a further 2 years were required for analysis, reporting and dissemination of results and implementation of findings. Other scenarios were explored in optimistic and pessimistic sensitivity analyses and an analysis based on assumptions regarding a definitive trial detailed in the original HITS-NS protocol. Table 12 summarises the base-case population EVSI assumptions and presents alternative values used in sensitivity analyses.
| Assumptions in ENBS analysis | Base-case value | Sensitivity analysis | Envisaged HITS-NS triala | Information sources | |
|---|---|---|---|---|---|
| Optimistic | Pessimistic | ||||
| Design effect | |||||
| ICC | 0.02 | 0.01 | 0.04 | 0.02 | Snooks et al. 2010,98 Mason et al. 2007100 |
| Average cluster size | 3.0 | 3.6 | 2.5 | 3.01 | HITS-NS pilot data |
| Trial duration | |||||
| Number of ASs available for trial | 8 | 10 | 5 | 4 | DH |
| Available clusters per AS | 46 | 60 | 28 | 30 | HITS-NS pilot data, ambulance service annual accounts |
| Maximum recruitable patients in 1 year | 1380 | 2160 | 350 | 360 | Calculated from preceding data |
| Time for trial results to be analysed, reported, disseminated and implemented (years) | 2 | 1 | 3 | 2 | HITS-NS TMG opinion |
| Trial costs | |||||
| Cost per recruited patient per year, £ | 1000 | 500 | 2000 | Fixed and variable trial costsb | University of Sheffield Clinical Trials Research Unit, HITS-NS grant application |
| Additional study design assumptions | |||||
| Cluster randomised trial using simple randomisation | |||||
| Equal cluster size | |||||
| No loss to follow-up | |||||
| GOS measured as the primary end point Information pertaining to other model inputs, e.g. incremental costs not collected |
|||||
| Full compliance with management pathway in selective transfer arm | |||||
Population ENBS was calculated by subtracting the costs of performing a future trial for a given sample size from the population EVSI. The cost of running a proposed cluster RCT was assumed to be £1000 per recruit in the base-case analysis (Clinical Trials Unit, ScHARR, University of Sheffield, 8 November 2013, personal communication). Other eventualities, including an estimate examining fixed and variable costs based on the HITS-NS pilot study grant, were examined in sensitivity analyses (Table 13).
| Trial resource | Unit cost (£) | Horizon |
|---|---|---|
| Fixed costs | ||
| University estate costs | 43,000 | – |
| Other university indirect charges | 125,000 | – |
| Office consumables | 4000 | – |
| Project manager IT equipment | 1000 | – |
| Public Relations company to advertise trial | 7500 | – |
| Conference attendance to promote trial and disseminate results | 5000 | – |
| Data management and security | 15,000 | – |
| Variable costs | ||
| PI | 12,000 | Per year |
| Trial manager | 50,000 | Per year |
| Research paramedic | 42,000 | Per AS per year |
| Statistician | 4250 | Per year |
| Ambulance service advisor | 2500 | Per year |
| Other TMG advisors | 25,000 | Per year |
| Lease car | 14,000 | Per research paramedic per year |
| IT equipment | 850 | Per research paramedic |
| Public Relations company to advertise trial | 2500 | Per AS |
| Ambulance service training | 12,500 | Per AS |
| Ambulance service administrative support | 750 | Per AS per year |
| Trial manager travel and subsistence | 1000 | Per AS per year |
| Trial management and other meetings | 10,000 | Per year |
| Additional journey times for ambulances | 100 | Per patient in intervention arm |
| Accessing patient notes | 60 | Per patient |
| Patient leaflets, questionnaires and mailing costs | 20 | Per patient |
Economic evaluation of management pathways for adult patients with stable suspected significant head injury: results
Introduction
The results of the decision analysis model are presented in four ways. First, the mean lifetime costs and QALYs of alternative head injury management strategies are compared deterministically for the base-case model. Second, to account for parameter imprecision and the uncertainty of expert opinion informing model inputs, results are recalculated in a Monte Carlo PSA. Third, the influences of parameter uncertainty and methodological and structural assumptions inherent in the model’s design are explored in a series of sensitivity analyses. Finally, following this evaluation of the cost-effectiveness of competing management strategies, expected value of information techniques are used to examine the value of further research in this area.
Cost-effectiveness results
Deterministic base-case results
Table 14 presents the mean expected costs and QALYs accrued by each strategy over a lifetime horizon. Selective secondary transfer was less expensive than the competing management pathways but resulted in fewer QALYs than routine transfer or bypass strategies. The no-transfer strategy provided the fewest QALYs at the second highest cost.
| Treatment option | Expected QALYs gained | Expected cost (£) | Cost per QALY (£) | Average cost-effectiveness ratioa (£) | ICERb (£) |
|---|---|---|---|---|---|
| Selective transfer | 12.93 | 26,917 | 2081 | – | – |
| Routine transfer | 12.99 | 27,053 | 2082 | 2267 | 2217 |
| No transfer | 12.66 | 27,081 | 2140 | –607 | SD |
| Bypass | 13.07 | 29,221 | 2236 | 16,457 | 27,100 |
Average cost-effectiveness ratios for each strategy, compared with a baseline of selective secondary transfer, are shown on a cost-effectiveness plane in Figure 15. When choosing between different interventions, it is necessary to determine the extra cost incurred for each additional QALY gained when switching from one strategy to another. Average cost-effectiveness ratios ignore the alternative treatments available and calculation of cost-effectiveness ratios with the next-best alternative in a fully incremental analysis is required for a valid comparison. The no-transfer strategy, providing fewer QALYs at the second highest cost, was strongly dominated by other management options and, therefore, excluded from further consideration. Each option was then ranked in order of increasing effectiveness and an ICER calculated with the next most effective strategy. The final ICERs between selective secondary transfer and routine transfer and between routine transfer and bypass were £2217 and £27,100 respectively. Therefore, ignoring parameter uncertainty and assuming the model is valid, routine secondary transfer is the optimal strategy for management of patients with suspected significant head injury at the standard NHS willingness-to-pay thresholds of £20,000 but bypass would be considered cost-effective if the threshold was increased to £30,000.
FIGURE 15.
Cost-effectiveness plane presenting deterministic point estimates of average cost-effectiveness against a baseline of selective secondary transfer.
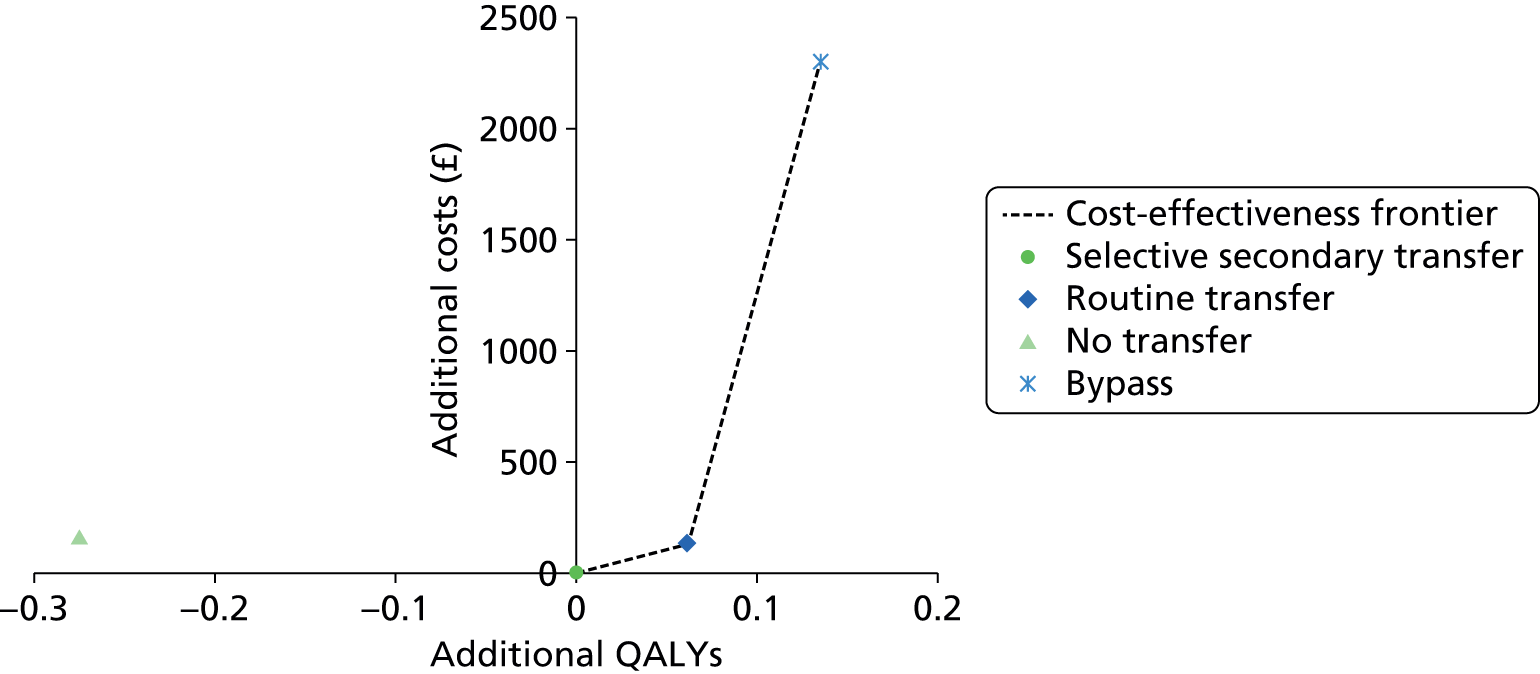
Probabilistic base-case results
Exploratory analyses indicated that 3000 PSA runs were sufficient to sample fully from parameter probability distributions and achieve stable estimates of NMB and incremental cost-effectiveness (Figure 16).
FIGURE 16.
Relationship between number of PSA simulations and stability of cost-effectiveness estimates. Little variation in NMB (λ = £20,000) is observed for the pre-hospital triage and bypass strategy at PSA with > 3000 simulations. A similar pattern was observed with other comparators.
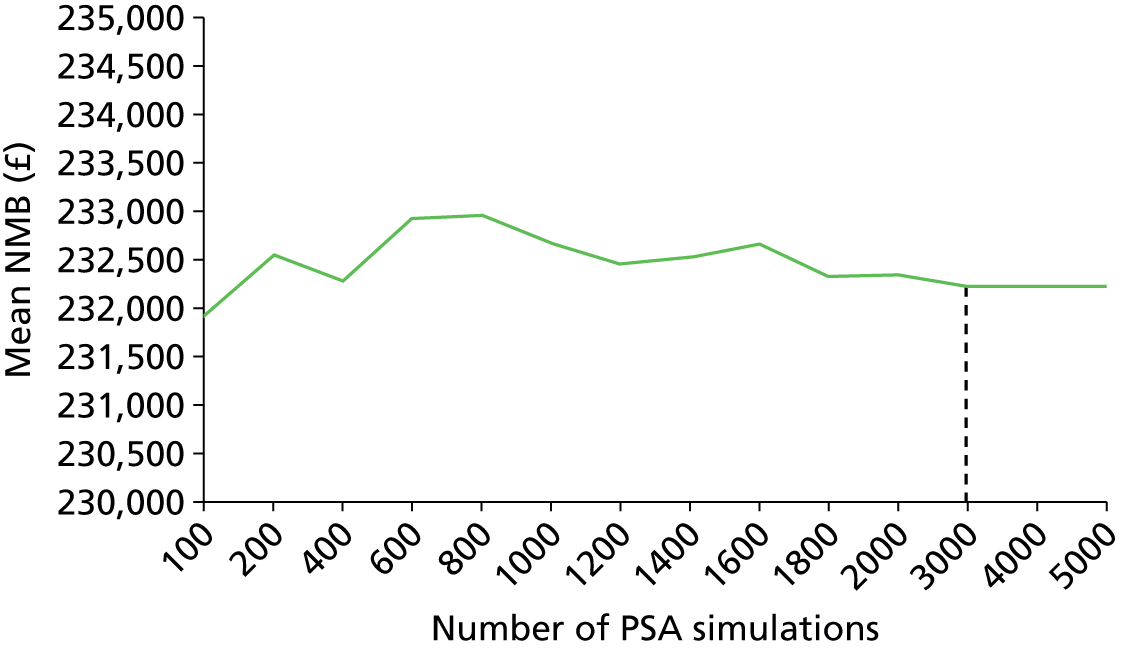
Incremental costs and QALYs from the PSA are shown in Figure 17 for each competing management pathway compared with a baseline strategy of selective secondary transfer. Each PSA simulation, representing a realisation of the joint distribution of possible model inputs, is depicted by a single point on the cost-effectiveness plane. It is apparent that there is a large degree of uncertainty in incremental costs and effects, reflected in the dispersal of PSA simulations and their location in different quadrants of the cost-effectiveness plane. The routine secondary transfer strategy offered more QALYs than selective secondary transfer in the majority of simulations, often at a lower cost. However, there were a relatively large number of realisations of parameter uncertainty where this option did not appear to be cost-effective at NICE’s stated willingness-to-pay threshold of £20,000, and a limited number of simulations for which selective transfer strongly dominated this comparator. The pre-hospital triage and bypass strategy demonstrated a wide spread of replications in all four quadrants of the cost-effectiveness plane, including the north-west (dominated) and south-east (dominating) quadrants. The no-transfer strategy was strongly dominated in virtually all simulations.
FIGURE 17.
Cost-effectiveness planes showing incremental costs and QALYs for each comparator compared with selective secondary transfer baseline (n = 2000 PSA simulations). Individual points depict a single PSA simulation. Solid line represents willingness-to-pay threshold of £20,000. Solid grey squares correspond to mean costs and QALYs. (a) Bypass vs. selective transfer; (b) routine transfer vs. selective transfer; and (c) no transfer vs. selective transfer.

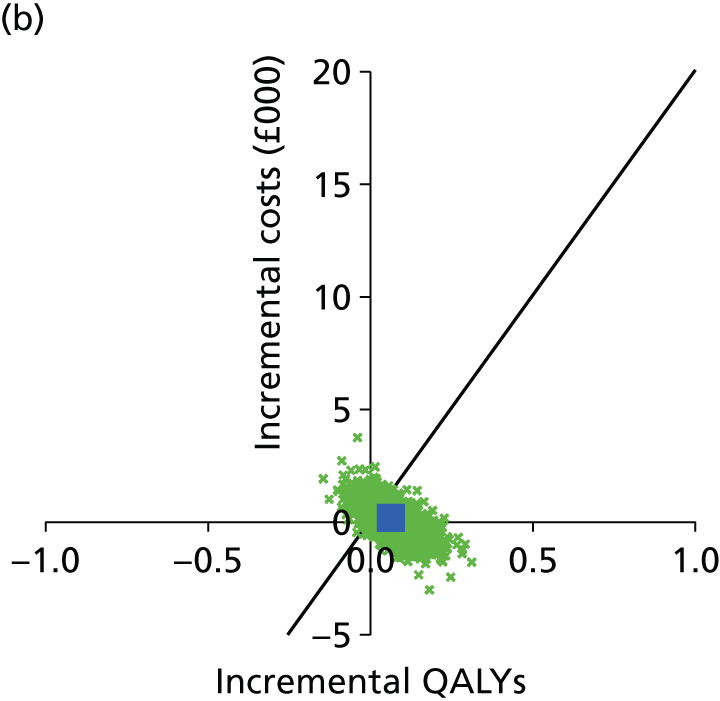

In calculation of mean ICERs, averaging incremental costs and QALYs over all PSA simulations, the no-transfer strategy was again strongly dominated. The ICERs between selective secondary transfer and routine transfer and between routine transfer and bypass were £2260 and £27,157 respectively. The cost-effectiveness plane and ICERs have limited utility in analysing PSA in the case of multiple comparators, as correlation between different strategies within simulations cannot be easily represented. We therefore present results using the NMB framework, which transforms cost-effectiveness results to a linear scale [NMB = (QALYS × λ) – costs]. If the aim of decision-making is to maximise health benefits within a fixed budget then the option with the highest expected NMB for a given λ is typically the most cost-effective comparator and should be chosen, regardless of statistical significance.
Table 15 summarises the mean expected costs and QALYs accrued by each strategy, and the corresponding mean expected NMB, for each management strategy. Routine secondary transfer demonstrated the highest incremental NMB compared to selective transfer at NICE’s stated willingness-to-pay threshold of λ = £20,000 (£1090, 95% CI £1034 to £1145), with pre-hospital triage and bypass (£588, 95% CI £403 to £757) showing lower, but relatively comparable, values. The no-transfer strategy demonstrated substantially lower incremental NMB (–£5065, 95% CI –£5475 to –£5280). At λ = £30,000, NICE’s upper limit for potential cost-effectiveness, bypass had the highest mean incremental NMB (£1903, 95% CI £1662 to £2135), marginally larger than routine transfer (£1694, 95% CI £1627 to £1778). The no-transfer strategy again demonstrated notably lower incremental NMB (–£7718, 95% CI –£16,845 to –£742).
| Strategy | Mean cost (£) | Mean QALYs | Mean NMB (£) | Mean incremental NMBa (95% CI) (£) | 2.5th and 97.5th percentiles PSA NMBs (£) | Probability most cost-effective | Error |
|---|---|---|---|---|---|---|---|
| λ = £20,000 | |||||||
| Bypass | 29,086 | 13.06 | 232,189 | 588 (403 to 757) | (–8406 to 10,619) | 0.42 | 0.58 |
| Selective transfer | 27,044 | 12.93 | 231,601 | 0 | – | 0.10 | 0.90 |
| Routine transfer | 27,183 | 12.99 | 232,691 | 1090 (1662 to 2135) | (–1879 to 4315) | 0.46 | 0.54 |
| No transfer | 26,805 | 12.66 | 226,459 | –5380 (–5475 to –5280) | (–11,230 to –495) | 0.01 | 0.99 |
| λ = £30,000 | |||||||
| Bypass | 29,086 | 13.06 | 362,827 | 1903 (1662 to 2135) | –10,172 to 15,248 | 0.48 | 0.52 |
| Selective transfer | 27,044 | 12.93 | 360,923 | 0 | – | 0.07 | 0.93 |
| Routine transfer | 27,183 | 12.99 | 362,627 | 1704 (1627 to 1778) | –2221 to 6019 | 0.44 | 0.56 |
| No transfer | 26,805 | 12.66 | 352,853 | –8070 (–8216 to –7924) | –16,845 to –742 | 0.01 | 0.99 |
The cost-effectiveness acceptability curves for each comparator are shown in Figure 18. Between willingness-to-pay thresholds of £20,000 and £30,000 there is considerable uncertainty over which comparator is most likely to be cost-effective. At λ = £20,000, routine secondary transfer had the highest probability (45%) of cost-effectiveness – slightly higher than pre-hospital triage and bypass (43%). Selective transfer (11%) also had a meaningful likelihood of being the most cost-effective at this level of willingness to pay. At λ = £30,000 there was a slightly higher probability that pre-hospital triage and bypass was the most cost-effective strategy compared with routine transfer (49% vs. 43%). At this threshold there was again a much lower, but still appreciable, chance that the selective transfer comparator (7%) was the most cost-effective. The no-transfer strategy had zero, or negligible, probability of being cost-effective at all, but the lowest levels of λ (λ = £0, probability cost-effective = 51% to λ = £20,000, probability cost-effective = 1%).
FIGURE 18.
Cost-effectiveness acceptability curves for each management strategy for a range of willingness-to-pay thresholds (λ).
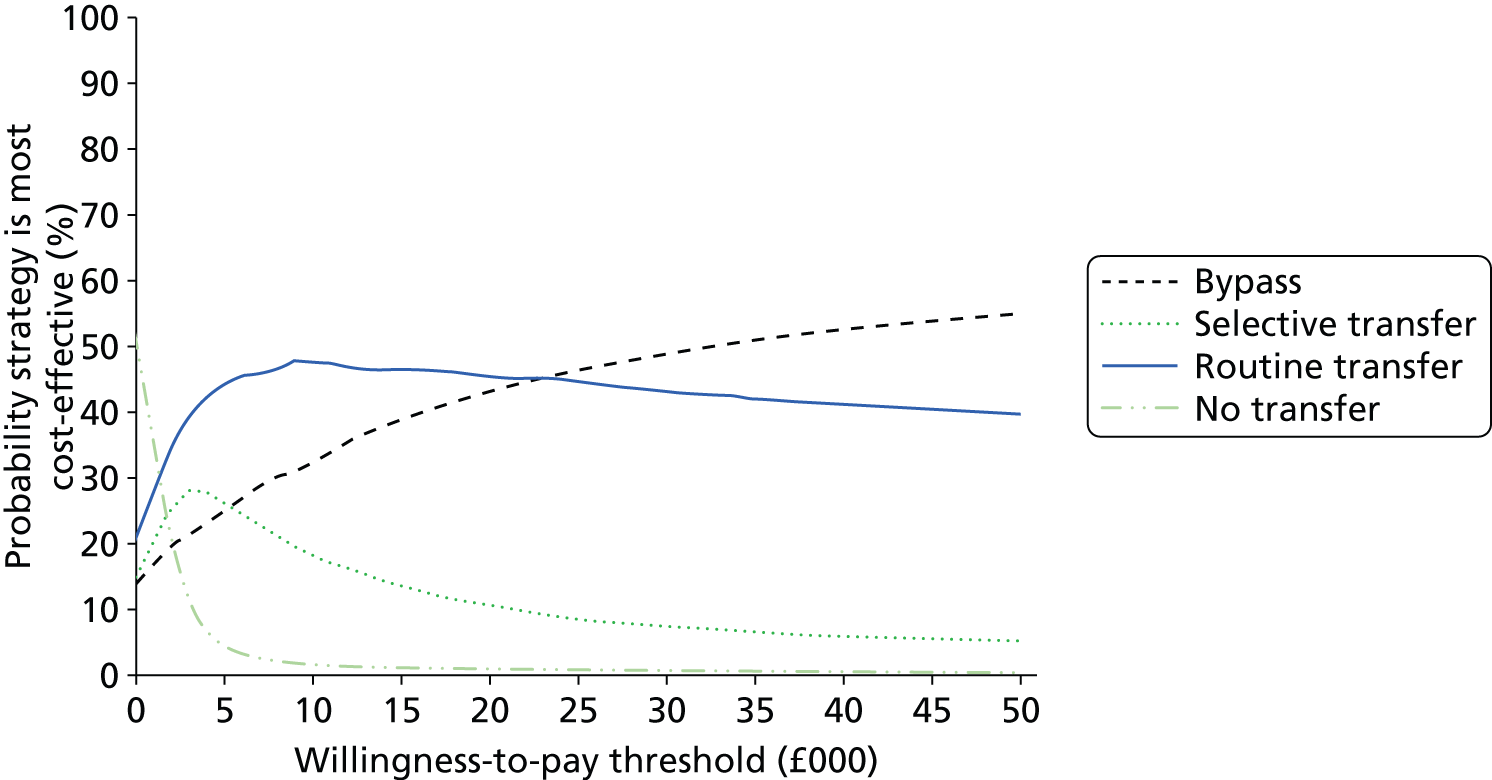
Cost-effectiveness acceptability curves evaluate decision uncertainty but should not be used to determine the optimal decision. If net benefit has a skewed distribution it is possible that the management option with the greatest expected NMB may not have the highest probability of being cost-effective. We therefore present a cost-effectiveness acceptability frontier in Figure 19 which demonstrates the decision uncertainty surrounding the optimal management strategy. At very low willingness-to-pay thresholds, no transfer (λ = £0, 52% probability cost-effective) and selective secondary transfer (λ = £1000 to £2000, 20–25% probability cost-effective) are the optimal strategies. At more plausible levels of λ, routine secondary transfer provided the greatest expected NMB (λ = £3000 to £27,000, probability of cost-effectiveness = 39–44%). Above λ = £27,000 pre-hospital triage with bypass provided the greatest NMB with an increasing probability of cost-effectiveness (from 48% at λ = £28,000 to 55% at λ = £50,000).
FIGURE 19.
Cost-effectiveness acceptability frontier showing the probability that the strategy with the highest mean NMB is cost-effective.
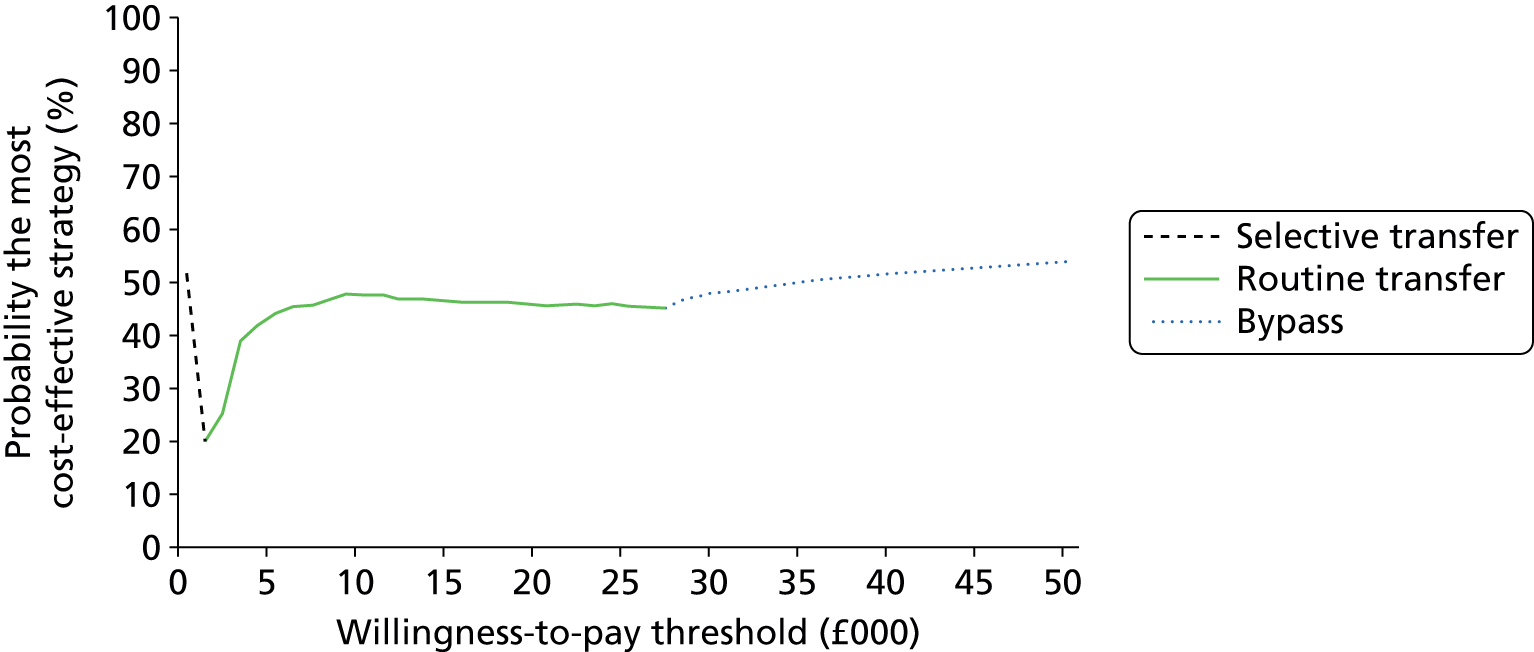
Sensitivity analysis results
Initial sensitivity analyses explored the influence of model inputs relating to inpatient costs and relative effectiveness for pre-hospital triage and bypass in a scenario analysis. The results of fixing relevant parameters to the 25th or 75th quantile of their probability distributions are presented in Table 16. In a best-case scenario for pre-hospital triage and bypass the base-case adoption decision at λ = £20,000 would be reversed, with bypass providing an incremental NMB of £7530 with 100% probability of cost-effectiveness (compared with routine transfer NMB = £1033, 0% probability of cost-effectiveness).
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Bypass: best case | Bypass | 25,996 | 13.28 | 239,530 | 7530 | 1.00 | 0.00 | 372,293 | 10,733 | 1.00 | 0.00 |
| Selective transfer | 27,119 | 12.96 | 232,000 | 0 | 0.00 | 1.00 | 361,560 | 0 | 0.00 | 1.00 | |
| Routine transfer | 27,270 | 13.02 | 233,033 | 1033 | 0.00 | 1.00 | 363,185 | 1625 | 0.00 | 1.00 | |
| No transfer | 26,876 | 12.68 | 226,818 | –5182 | 0.00 | 1.00 | 353,664 | –7896 | 0.00 | 1.00 | |
| Bypass: worst case | Bypass (observed) | 31,862 | 12.94 | 226,962 | –4744 | 0.00 | 1.00 | 356,374 | –4656 | 0.00 | 1.00 |
| Selective transfer | 26,942 | 12.93 | 231,706 | 0 | 0.22 | 0.78 | 361,030 | 0 | 0.20 | 0.80 | |
| Routine transfer | 27,094 | 12.99 | 232,746 | 1040 | 0.77 | 0.23 | 362,666 | 1636 | 0.79 | 0.21 | |
| No transfer | 26,714 | 12.66 | 226,484 | –5222 | 0.01 | 0.99 | 353,083 | –7947 | 0.01 | 0.99 | |
Conversely, in the contingency that bypass parameters were set to more unfavourable but still plausible values, pre-hospital triage and bypass was not cost-effective, even at extremely high willingness-to-pay thresholds. At both λ = £20,000 and λ = £30,000 routine secondary transfer was the optimal strategy. The sensitivity of cost-effectiveness results to parameterisation of the pre-hospital triage and bypass strategy highlights the large degree of parameter uncertainty in the decision analysis model.
Opposite cost-effectiveness results were observed when probability distributions for bypass costs and effects were fixed at favourable or unfavourable values. A threshold sensitivity analysis was therefore performed to establish the parameter values that would be necessary for bypass to be the optimal management strategy at NICE’s willingness-to-pay threshold of £20,000. Relevant model inputs were initially set at their median values and then fixed sequentially at more favourable vigintiles of their probability density distributions. Thus, relative effectiveness of bypass strategies progressively improved, while associated costs gradually decreased. All other model inputs were examined probabilistically under base-case assumptions. This analysis allows decision-makers to weigh their beliefs on the likelihood of model inputs required for bypass to become the optimal strategy. Tables 17 and 18 summarise the results of the threshold analysis. Fixing relevant model inputs at only a small deviation of 0.05 quantiles from the median value resulted in pre-hospital triage and bypass becoming the favoured option at λ = £20,000.
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Bypass: threshold analysis | Bypass | 28,458 | 13.14 | 234,419 | 2174 | 0.75 | 0.25 | 365,857 | 4100 | 0.85 | 0.16 |
| Selective transfer | 26,779 | 12.95 | 232,245 | 0 | 0.00 | 1.00 | 361,757 | 0 | 0.00 | 1.00 | |
| Routine transfer | 26,927 | 13.01 | 233,334 | 1089 | 0.22 | 0.78 | 363,465 | 1708 | 0.15 | 0.85 | |
| No transfer | 26,510 | 12.68 | 227,103 | –5142 | 0.00 | 1.00 | 353,909 | –7848 | 0.00 | 1.00 | |
| Patient subgroup | Parameter (bypass vs. selective transfer) | Distribution (mean, SE/SD) | Deterministic value at 55th quantile (odds ratio for unfavourable outcome) |
|---|---|---|---|
| Mild TBI | Incremental cost | Normal (63, 40) | £58.30 |
| TBI requiring critical care | Relative effectiveness: log-odds ratio | Normal (0.00, 0.56) | –0.07 (0.93) |
| Incremental cost | Normal (6970, 14,699) | £5123 | |
| Acute neurosurgery | Relative effectiveness: log-odds ratio | Normal (–0.68, 0.34) | –0.72 (0.48) |
| Incremental cost | Normal (32,044, 18,249) | £29,751 | |
| TBI requiring ward care | Relative effectiveness: log-odds ratio | Normal (–0.02, 0.02) | –0.02 (0.98) |
| Incremental cost | Normal (2353, 981) | £2229 | |
| Major extracranial injury | Relative effectiveness: log-odds ratio | Normal (–0.22, 0.66) | –0.23 (0.79) |
| Incremental cost | Normal (2922, 5283) | £2258 |
Uncertainty in model inputs was further explored in a number of one-way sensitivity analyses examining parameters elicited from expert opinion, potentially susceptible to bias or considered to be influential in determining cost-effectiveness. Alternative specifications of inputs were assessed by varying parameterisation of distributions or fixing distributions at defined quantiles. All other parameters were treated probabilistically. The robustness and key determinants of the cost-effectiveness results were therefore thoroughly investigated. The findings of these sensitivity analyses are detailed in Appendix 7 and summarised in Table 19 and Figure 20.
| Sensitivity analysis | Description | Strategy with highest NMB at λ = £20,000 | Probability that most cost-effective strategy | Strategy with highest NMB at λ = £30,000 | Probability that most cost-effective strategy |
|---|---|---|---|---|---|
| Parameter uncertainty | |||||
| Bypass: best-case scenario | Bypass costs and effects set to most favourable plausible quartile | Bypass | 1.00 | Bypass | 1.00 |
| Bypass: worst-case scenario | Bypass costs and effects set to least favourable plausible quartile | Routine transfer | 0.77 | Routine transfer | 0.79 |
| Bypass: threshold analysis (55th quantile of distribution) | Bypass costs and effects increased gradually from median values to level at which bypass has highest NMB | Bypass | 0.75 | Bypass | 0.85 |
| Utilities: scenarios/standard gamble | Alternative source of utility estimates used, based on case scenarios and valued by standard gamble by health professionals (Aoki 1998101) | Routine transfer | 0.43 | Bypass | 0.48 |
| Acute neurosurgery baseline outcomes: improved outcomes | Alternative estimate used for acute neurosurgery baseline outcome (Taussky 2008,102 external bias adjusted) | Routine | 0.44 | Bypass | 0.52 |
| Neurosurgery costs: equal inpatient costs | Incremental inpatient costs for acute neurosurgery assumed to be equal | Bypass | 0.62 | Bypass | 0.65 |
| Neurosurgery costs: based on NHIR | Incremental costs for acute neurosurgery based on costs regression from NHIR data (Fuller 201445) | Bypass | 0.58 | Bypass | 0.62 |
| Incremental inpatient costs: expert opinion | Incremental inpatient costs for bypass elicited from expert opinion | Bypass | 0.60 | Bypass | 0.61 |
| Relative effectiveness of bypass in major extracranial injury: elicited | Odds ratio for survival following major extracranial injury associated with bypass elicited from clinical experts | Routine transfer | 0.46 | Bypass | 0.48 |
| Patient subgroups: NEAS | Distribution of patient subgroups based on estimates from NEAS HITS-NS data | Routine transfer | 0.45 | Routine transfer | 0.44 |
| Patient subgroups: NWAS | Distribution of patient subgroups based on estimates from NWAS HITS-NS data | Bypass | 0.61 | Bypass | 0.70 |
| Post-discharge costs: 2.5th quantile | Post-discharge costs set to their 2.5th quantile | Routine transfer | 0.43 | Bypass | 0.48 |
| Decreased life expectancy | Life expectancy for TBI and extracranial injury survivors assumed to be reduced (McMillan et al. 201178) | Bypass | 0.51 | Bypass | 0.56 |
| Structural uncertainty | |||||
| Relative effectiveness: no proportional odds assumption | Odds ratio applied to favourable/unfavourable GOS outcomes. Proportions within each dichotomised group equal to that found in the baseline population | Routine transfer | 0.38 | Bypass | 0.55 |
| Discount rate: 1.5% | Discount rate reduced to 1.5% | Bypass | 0.55 | Bypass | 0.59 |
| Discount rate: 6.0% | Discount rate reduced to 6.0% | Routine transfer | 0.45 | Bypass | 0.48 |
| Alternative comparators: bypass (full compliance) | Pre-hospital and triage management pathway replaced by a theoretical strategy with full compliance | Bypass (full compliance) | 0.49 | Bypass (full compliance) | 0.58 |
| Considering non-health effects of bypass | Utility decrement applied to bypassed patients with mild TBI to reflect the inconvenience of being taken to a distant hospital | Routine transfer | 0.46 | Routine transfer | 0.44 |
FIGURE 20.
Mean incremental NMB for competing strategies in sensitivity analyses examining parameter and structural uncertainty.

Adoption decisions at NICE willingness-to-pay thresholds were highly sensitive to modification of model inputs in these analyses, emphasising marked second- and third-order parameter uncertainty. At λ = £20,000 the base-case adoption decision was transformed, with bypass now identified as the optimal strategy, when alternative estimates were used for incremental inpatient costs or decreased life expectancy following trauma. The case mix of suspected significant patients with TBI was also highly influential in determining cost-effectiveness. When NEAS estimates for population subgroups were used, demonstrating a relatively lower proportion of significant patients with TBI, routine transfer was the most cost-effective option at both λ = £20,000 and λ = £30,000. Implementing NWAS estimates, with higher prevalence of more seriously injured patients, suggested that bypass was optimal at these thresholds. Conversely, using alternative estimates for post-discharge costs, acute neurosurgery baseline outcomes, GOS health state–utility values, or the relative effectiveness of bypass in patients with major extracranial injury did not materially change cost-effectiveness results.
The importance of structural uncertainty was exposed in further sensitivity analyses. A discount rate of 1.5% resulted in bypass having the highest mean NMB at NICE thresholds, whereas considering non-health effects of bypass (applying a small utility decrement for unnecessary bypass in mild TBI cases) resulted in the opposing finding that routine transfer was cost-effective at both λ = £20,000 and λ = £30,000. A theoretical variant of the bypass strategy suggested that if maximal compliance with pre-hospital triage was possible this management could potentially be more cost-effective than routine transfer at λ = £20,000 (Table 20). A no-transfer ‘zero’ option, through which all patients were admitted to non-specialist hospitals and remained there regardless of injury severity, unsurprisingly resulted in extremely unfavourable mean incremental NMB (see Appendix 7, Table 38). Relaxing the proportional odds assumptions for calculation of relative effectiveness or increasing the discount rate to 6.0% did not alter the base-case results.
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Alternative comparators | Bypass | 29,591 | 13.12 | 232,889 | 1466 | 0.49 | 0.51 | 364,129 | 3432 | 0.58 | 0.42 |
| Bypass: 100% compliance | Selective transfer | 27,126 | 12.93 | 231,423 | 0 | 0.09 | 0.91 | 360,697 | 0 | 0.06 | 0.94 |
| No transfer | Routine transfer | 27,265 | 12.99 | 232,543 | 1120 | 0.40 | 0.60 | 362,447 | 1750 | 0.35 | 0.65 |
| No neurosurgical transfers | No transfer | 30,333 | 12.55 | 220,607 | –10,816 | 0.00 | 1.00 | 346,076 | –14,621 | 0.00 | 1.00 |
Expected value of information results
Expected value of perfect information
Reflecting the uncertainty in costs and effects, together with the large potential opportunity losses from making the incorrect adoption decision, individual EVPI was substantial at NICE willingness-to-pay thresholds: £1807 at λ = £20,000 and £2594 at λ = £30,000. Given the relatively large annual population with suspected significant head injury and the long time period over which pre-hospital triage and bypass is likely to be applicable, population EVPI was also correspondingly large in the base-case analysis: £35,589,500 at λ = £20,000, and £51,080,000 at λ = £30,000. These figures represent the maximum that the NHS should be willing to invest in future research to eliminate uncertainty about which management strategy to implement; assuming an infinitely sized study, evaluation of all model inputs and the economic model is correctly specified. Figure 21 displays the base-case population EVPI at relevant willingness-to-pay thresholds. Two inflections in the slope of the EVPI curve are visible, at λ = £2000 and λ = £28,000, corresponding to threshold values at which the comparator with highest expected NMB transitions between selective transfer/routine transfer and routine transfer/bypass. Above λ = £28,000 the EVPI continues to rise as the consequences of decision errors are valued more highly and the probability of erroneously adopting bypass remains high. Estimates for population EVPI remained substantial in pessimistic and optimistic scenario sensitivity analyses, varying from £10.9M to £70.7M at λ = £20,000, and £15.6M to £101.3M at λ = £30,000 (Table 21). Individual and population EVPI values for a wider range of willingness-to-pay thresholds are presented in Appendix 7.
FIGURE 21.
Population EVPI for the base-case probabilistic model.
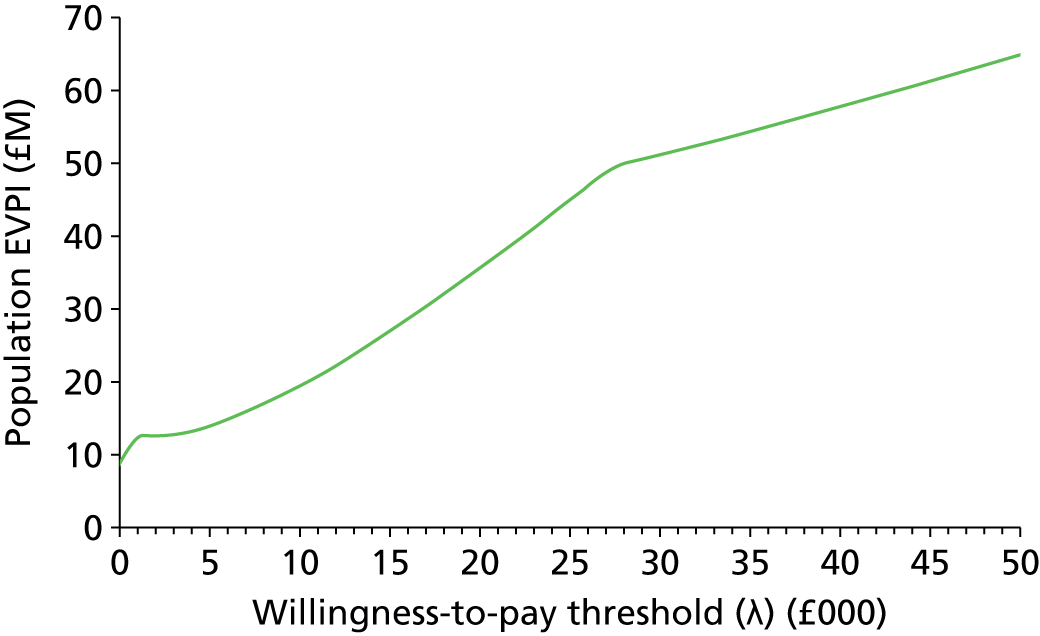
| Assumption basis | Suspected significant TBI incidence per 100,00 per year | Lifespan of bypass | Discount rate (%) | Population benefiting from future research | Individual EVPI (£) (λ = £20,000) | Population EVPI (£) (λ = £20,000) | Individual EVPI (£) (λ = £30,000) | Population EVPI (£) (λ = £30,000) |
|---|---|---|---|---|---|---|---|---|
| Base case | 6.4 | 10 | 3.5 | 19,692 | 1807 | 35,589,500 | 2594 | 51,080,000 |
| Optimistic scenario | 9.0 | 13 | 1.5 | 39,057 | 1807 | 70,675,000 | 2594 | 101,313,000 |
| Pessimistic scenario | 2.5 | 8 | 6.0 | 6015 | 1807 | 10,885,000 | 2594 | 15,604,000 |
Expected value of partial perfect information
The individual EVPPI and concomitant population EVPPI for each model parameter are detailed in Appendix 7 for a relevant range of willingness-to-pay thresholds. The key model parameters for which there is highest additional value in future research are relative outcomes and incremental inpatients costs, between selective transfer and bypass strategies, particularly in patients requiring acute neurosurgery and critical care. The upper limit for returns to research on these individual parameters ranged from £5.1M to £26.0M under base-case assumptions and λ = £20,000.
Five categories of parameters (case mix of suspected significant patients with TBI, long-term costs, utility values for GOS health states and incremental costs and effects between selective transfer and bypass strategies) were considered in further base-case partial EVPPI analyses, reflecting potential future research designs. At NICE cost-effectiveness thresholds all of these groups of parameters demonstrated substantial population EVPPI values, indicating that further research could have a meaningful impact on reducing the overall decision uncertainty. The relative effectiveness of bypass compared with selective transfer was the most important determinant of decision uncertainty, and a future randomised trial providing perfect information on this model input would be cost-effective if research costs were < £27.6M (λ = £20,000) or £44.3M (λ = £30,000). A trial investigating the incremental cost difference between bypass and selective transfer strategies also demonstrated high population EVPPI values of £11.2M and £15.7M, respectively. The EVPPI estimates for TBI case mix, GOS utilities and long-term costs were notably lower (£282,000–864,000 at λ = £20,000) but these parameters would still provide valuable information and could be investigated in epidemiological studies with significantly less investment of cost and time. Table 22 and Figure 22 report the EVPPI estimates for groups of model parameters under base-case assumptions over a range of relevant estimates for λ. EVPPI results for these groups of parameters, under optimistic and pessimistic scenarios for the numbers of patients who may gain from future research, are shown in Appendix 7.
| Model parameter | Individual EVPPI (£) [λ = (£)] | Population EVPPI (£) [λ = (£)] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | 0 | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Patient subgroups | 0 | 0 | 44 | 317 | 204 | 158 | 413 | 0 | 864,271 | 6,236,989 | 4,026,985 | 3,105,055 |
| TBI utilities | 0 | 0 | 14 | 113 | 33 | 18 | 0 | 3753 | 281,606 | 2,229,370 | 651,365 | 361,439 |
| Post-discharge costs | 45 | 0 | 19 | 138 | 26 | 13 | 885,345 | 5607 | 378,766 | 2,718,970 | 516,704 | 247,208 |
| Bypass: relative effectivenessa | 21 | 556 | 1403 | 2249 | 2541 | 2848 | 409,743 | 10,953,314 | 27,626,808 | 44,283,564 | 50,038,304 | 56,092,483 |
| Bypass: inpatient costsa | 119 | 333 | 571 | 795 | 515 | 320 | 2,339,102 | 6,552,338 | 11,237,468 | 15,664,084 | 10,137,674 | 6,296,215 |
FIGURE 22.
Population EVPPI for groups of model parameters at different willingness-to-pay thresholds under base-case assumptions.

Expected value of sample information
Expected value of sample information and ENBS of a future definitive HITS-NS trial were considered in base-case and scenario analyses, making optimistic and pessimistic assumptions about the size of the future population who could benefit from the data collection. The characteristics of the theoretical trial are summarised in Chapter 4. As sample size increases, simulated estimates of relative effectiveness will become more precise until uncertainty is completely removed at infinite sample sizes. Individual EVSI will therefore asymptotically approach the individual EVPPI value for bypass relative effectiveness parameters at very high sample sizes (£1403 at λ = £20,000, £2249 at £30,000), as shown in Figure 23. An analogous pattern is observed for population EVSI, assuming that the disease incidence and the ability to recruit patients far exceed sample size.
FIGURE 23.
Individual EVSI for a definitive HITS-NS cluster randomised trial at NICE cost-effectiveness thresholds. WTP, willingness to pay.

As the number of study participants increases, the number of ambulance stations and ASs required to recruit the necessary number of patients will expand, determined by the average cluster size and number of ambulance stations available for randomisation in each AS. At higher levels the sample size will exceed the maximum number of patients recruitable per year, extending the length of the trial, curtailing the time that the study findings are useful and consequently reducing the size of the future population that may benefit from the trial. Additionally, patients enrolled in a study will not be able to benefit from the information generated, as they will have already received treatment. In actuality, population EVSI therefore falls at larger trial sizes, as shown in Figure 24 (calculated under base-case assumptions). Inflection points in the population EVSI curve denote sample sizes requiring trials with additional years of recruitment for the necessary number of patients.
FIGURE 24.
Population EVSI for a definitive HITS-NS cluster randomised trial at NICE cost-effectiveness thresholds accounting for prolonged trial duration at large sample sizes (base-case assumptions). WTP, willingness to pay.

Expected net benefit of sampling
The ENBS for a definitive HITS-NS trial with different samples sizes under base-case assumptions is shown in Table 23 and in Figures 25 and 26 for λ = £20,000 and λ = £30,000, respectively. As relative effectiveness between bypass and selective transfer is very uncertain, and a key determinant of cost-effectiveness, even small trials providing relatively imprecise effect estimates would have a substantial impact on reducing decision uncertainty and therefore have positive ENBS. In the base case, ENBS was maximal with a trial of 1040 patients (520 randomised per arm) recruited across 347 ambulance stations in eight ASs over 1 year (with a further 2 years for trial analysis, reporting and dissemination): ENBS £11.0M at λ = £20,000; ENBS £19.6M at λ = £30,000.
| Sample size | Number of clusters | Number of ASs | Total trial duration (years)a | Trial costs (£) | λ = £20,000 | λ = £30,000 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Individual EVSI (£) | Population EVSI (£) | ENBS (£) | Individual EVSI (£) | Population EVSI (£) | ENBS (£) | |||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 104 | 35 | 1 | 3 | 104,000 | 240 | 3,134,385 | 3,030,385 | 644 | 8,412,535 | 8,308,535 |
| 208 | 69 | 2 | 3 | 208,000 | 420 | 5,481,025 | 5,273,025 | 915 | 11,947,407 | 11,739,407 |
| 260 | 87 | 2 | 3 | 260,000 | 535 | 6,986,810 | 6,726,810 | 990 | 12,928,756 | 12,668,756 |
| 520 | 173 | 4 | 3 | 520,000 | 741 | 9,682,274 | 9,162,274 | 1310 | 17,108,245 | 16,588,245 |
| 624 | 208 | 5 | 3 | 624,000 | 837 | 10,934,908 | 10,310,908 | 1338 | 17,471,204 | 16,847,204 |
| 832 | 277 | 6 | 3 | 832,000 | 882 | 11,512,147 | 10,680,147 | 1510 | 19,714,717 | 18,882,717 |
| 1040 | 347 | 8 | 3 | 1,040,000 | 919 | 12,006,060 | 10,966,060 | 1583 | 20,669,797 | 19,629,797 |
| 1248 | 368 | 8 | 4 | 1,248,000 | 976 | 10,727,439 | 9,479,439 | 1694 | 18,623,531 | 17,375,531 |
| 1664 | 368 | 8 | 4 | 1,664,000 | 1032 | 11,349,568 | 9,685,568 | 1791 | 19,686,516 | 18,022,516 |
| 2080 | 368 | 8 | 4 | 2,080,000 | 1104 | 12,136,788 | 10,056,788 | 1869 | 20,549,294 | 18,469,294 |
| 3120 | 368 | 8 | 5 | 3,120,000 | 1174 | 10,566,146 | 7,446,146 | 1975 | 17,776,096 | 14,656,096 |
| 4160 | 368 | 8 | 6 | 4,160,000 | 1228 | 8,687,271 | 4,527,271 | 2001 | 14,156,672 | 9,996,672 |
| 5200 | 368 | 8 | 7 | 5,200,000 | 1264 | 8,941,976 | 3,741,976 | 2067 | 14,621,142 | 9,421,142 |
| 8320 | 368 | 8 | 10 | 8,320,000 | 1315 | 2,208,126 | –6,111,874 | 2130 | 3,575,748 | –4,744,252 |
| 10,400 | 368 | 8 | 12 | 10,400,000 | 1337 | 2,244,217 | –8,155,783 | 2151 | 3,610,050 | –6,789,950 |
FIGURE 25.
Expected net benefit of sampling for a definitive HITS-NS cluster randomised trial at λ = £20,000 (base-case assumptions).
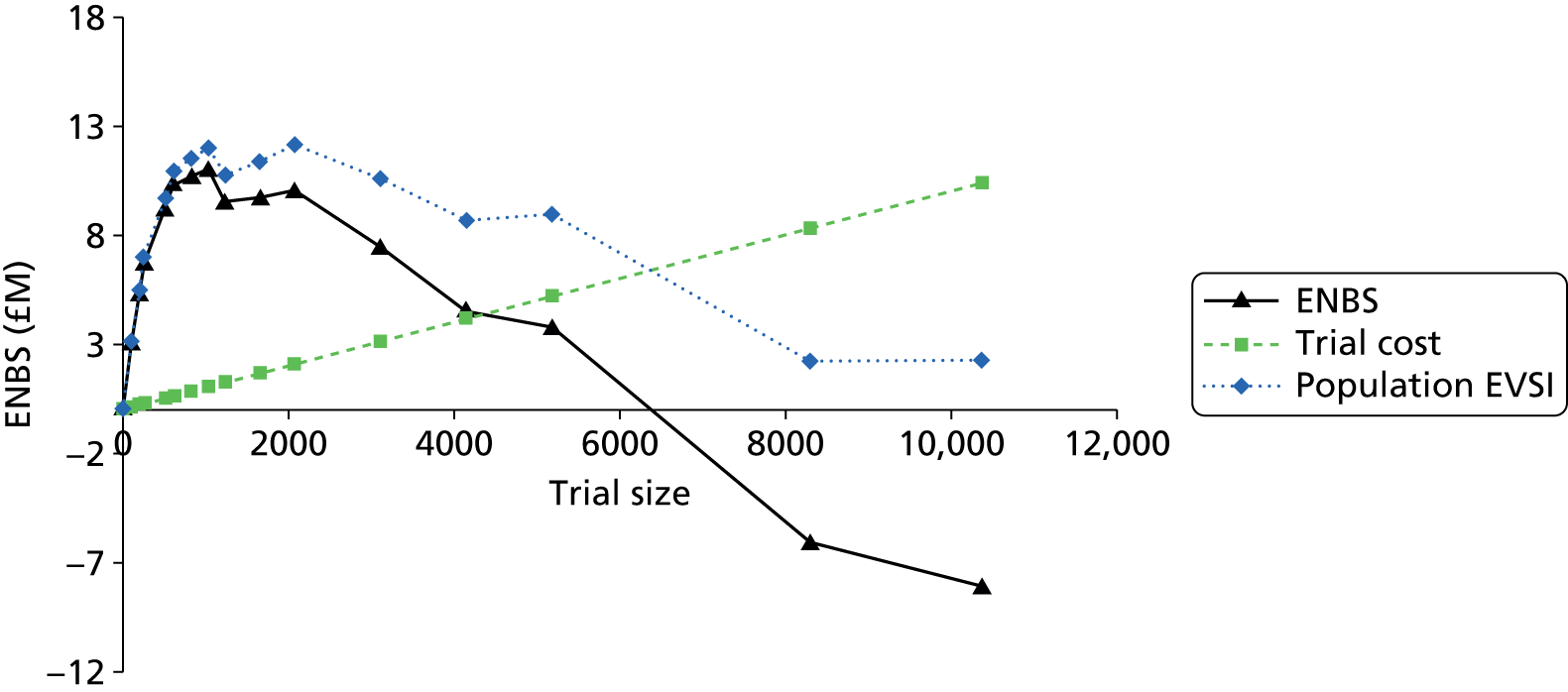
FIGURE 26.
Expected net benefit of sampling for a definitive HITS-NS cluster randomised trial at λ = £30,000 (base-case assumptions).

The optimal trial design was sensitive to varying assumptions about trial characteristics, disease incidence, technology lifespan and patient recruitment limits. Taking an optimistic view of these factors, the most cost-effective trial design at NICE thresholds would be achieved with a trial of 2052 patients recruited from 10 ASs, in 480 ambulance stations and lasting a total of 2 years. Taking pessimistic assumptions resulted in much lower ENBS, but a future trial was still shown to be cost-effective, with the most favourable trial recruiting 636 patients (5 ASs, 140 clusters, 5 years in total) at both λ = £20,000 and λ = £30,000. The study characteristics for a definitive trial envisaged in the original HITS-NS pilot study protocol resulted in a very similar optimum design of 624 patients (4 ASs, 120 clusters, 4 years in total). Interestingly, careful enumeration of fixed and variable trial expenditure, informed by HITS-NS pilot study funding, gave very similar trial costs to the base-case estimate using a marginal per patient estimate. Table 24 summarises the properties of the optimal trials in these different scenarios, with further detail provided on ENBS and trial designs provided in Appendix 7.
| Assumption basis | Total sample size | Number of clusters | Number of ASs | Trial duration (years) |
|---|---|---|---|---|
| Base case | 1040 | 347 | 8 | 3 |
| Optimistic scenario | 2052 | 480 | 10 | 2 |
| Pessimistic scenario | 636 | 140 | 5 | 5 |
| Planned definitive HITS-NS trial | 624 | 120 | 4 | 4 |
Economic evaluation of management pathways for adult patients with stable suspected significant head injury: discussion
Summary of results
-
Objectives 5 and 6 of the HITS-NS study were addressed in the stream B economic evaluation:
-
– determine the cost per QALY of early neurosurgery and the degree of uncertainty surrounding this estimate
-
– calculate the EVSI of a fully powered HITS-NS trial.
-
-
The base-case probabilistic analysis suggests that routine transfer (transport to the local non-specialist hospital and routine secondary transfer of all patients with acute expanding intracranial haematomas or TBI requiring critical care to regional NCs) may provide the optimal management strategy at a willingness-to-pay threshold of £20,000 (mean ICER £2260). At a higher threshold of £30,000, bypass was the most cost-effective option (mean ICER £27,157). At both thresholds there was considerable decision uncertainty, with a high probability of erroneously adopting a suboptimal strategy (54% and 52%, respectively).
-
Sensitivity analyses demonstrate that this result is critically dependent on parameterisation of incremental costs and relative treatment effects for routine transfer and bypass strategies. These model inputs are predominantly based on unadjusted estimates from HITS-NS data or are informed by expert opinion unsupported by empirical evidence and are therefore at risk of systematic error. Plausible alternative assumptions on incremental costs and effects, life expectancy following injury and discounting rates all resulted in reversal of the adoption decision at λ = £20,000, with bypass identified as the optimal strategy. Base-case results should, therefore, be interpreted with extreme caution.
-
The considerable decision uncertainty and important public health burden of TBI is reflected in the large population EVPI. At λ = £20,000, further research up to a value of £35.6M may be indicated to minimise opportunity costs from making the wrong adoption decision for patients with suspected significant TBI.
-
EVPPI analyses demonstrate that future research would have high value in comparing costs and relative effectiveness between bypass and selective secondary transfer: that is, a definitive HITS-NS trial-based economic evaluation.
-
If feasible, EVSI results suggest that a definitive HITS-NS trial examining comparative effectiveness is potentially cost-effective. Maximal ENBS (£11.M) would be achieved with a trial of 520 patients per arm, randomised across 347 ambulance stations in eight ASs and taking 3 years. At higher sample sizes ENBS falls as recruitment costs increase, and trial duration lengthens secondary to the relatively low incidence of suspected significant TBI and the finite number of ambulance stations available for randomisation.
-
EVI analyses are predicated on the parameterisation of the base-case model and assumptions regarding incidence of suspected significant TBI, technology lifespan and cluster trial characteristics. As these factors are highly uncertain, results should be viewed as exploratory.
Limitations
This economic evaluation followed consensus modelling guidelines and has a number of strengths. 57–60 A formal model structuring process was implemented to derive a valid and clinically convincing model structure. Comprehensive systematic evidence searches ensured that the model was populated with valid evidence where possible. Elicitation of expert opinion, informed using the established SHELF framework103 ensured transparency in model inputs when empirical data was lacking. Decision uncertainty was explored extensively in sensitivity analyses and the potential benefit of future research was evaluated in state-of-the-art EVI analyses. However, there are limitations in the model design and parameterisation which could challenge the internal validity and generalisability of results.
First, the model had a limited scope and several potentially important aspects of managing patients with suspected significant TBI were not examined. The HITS-NS cohort included an appreciable minority (approximately 5%) of non-patients with TBI with a major medical diagnosis, for example non-traumatic subarachnoid haemorrhage or sepsis. As a result of heterogeneity and lack of empirical evidence, we did not evaluate this patient subgroup. If there are important differences in costs and effects arising from bypassing such cases, exclusion of these patients could bias results.
Introduction of bypass or routine transfer strategies could incur expenditure relating to changes in patient treatment pathways, such as staff training or administrative support. Given the lack of information available on the extent of these costs and expert opinion that start-up costs are likely to be insignificant, we did not model this factor. However, if reconfiguration costs are substantial, the reported cost-effectiveness of bypass will be overestimated.
The economic model assumes that specialist neuroscience care is scalable with infinite capacity. Surveys of SNC capacity suggest that many units are operating close to their maximum volume,104,105 and admitting increasing numbers of patients for neurocritical care in routine transfer or bypass strategies may not be possible. The potential impact of overcrowding in EDs or ICUs arising from treatment of additional patients, possibly unlikely to benefit from specialist care, has also not been examined. Similarly, capacity constraints arising from difficulties in repatriating bypassed patients to local hospitals after a period of specialist care were not considered. The complexity of representing these real-world phenomena precluded modelling but, reassuringly, expert opinion suggested that they are unlikely to be important.
Traumatic brain injury frequently results in chronic disability, leading to productivity losses and a long-term informal care burden for family members. 106 Our narrow perspective, including only direct medical and personal social services costs, will therefore substantially underestimate the societal costs of competing strategies. As the proportion of patients with long-term disability was comparable between bypass and selective/routine transfer strategies, it could be argued that exclusion of these costs is unlikely to qualitatively change the results of the base-case model. Similarly, only direct health effects were assessed, but a sensitivity analysis, including a small utility decrement for patients with mild TBI undergoing unnecessary transport to SNCs, illustrated the potential for a marked decrease in the cost-effectiveness of bypass if such factors are important. However, qualitative interviews with HITS-NS participants and relatives did not reveal any serious concerns regarding non-health-related consequences arising from the bypass strategy.
Furthermore, examination of aero-medical transfers, urban environments and suspected patients with TBI with unstable pre-hospital physiology were outside the remit of the economic evaluation. The model also consciously focused on simulating management within the NHS. Any generalisation of results to other populations beyond the HITS-NS setting and inclusion criteria should therefore be circumspect.
Second, model structuring was restricted by the limited availability of evidence on TBI epidemiology, hospital costs, treatment effectiveness and pathophysiology. Theoretical disease logic and treatment pathway conceptual models emphasised the importance of time to resuscitation and neurosurgery on outcome, but we were unable to represent these factors directly. The final model, comprising strategy level estimates of costs and consequences for relevant patient subgroups, provided a pragmatic structure indirectly accounting for these factors and retaining clinical credibility. However, the ability to examine the influence of different geographical settings, triage compliance and secondary transfer rates was curtailed.
No information was available on the covariance between inpatient costs and short-term outcomes, and our treatment of these variables as independent in the PSA is a further limitation of the model structure. Expert opinion was highly uncertain as to the magnitude and direction of any correlation. Given the large degree of decision uncertainty at NICE willingness-to-pay thresholds, small differences in expected costs and QALYs arising from incorrect specification of this relationship could potentially influence the choice of optimal management strategy.
Within each modelled patient subgroup there will be a considerable diversity of patients with differing characteristics and prognoses. Applying a cohort methodology, with consequent use of mean values, impeded an examination of uncertainty due to heterogeneity. However, competing management strategies are service-level interventions and hence would be applied to the entire population presenting with HITS-NS inclusion criteria. Exploration of heterogeneity, for example the cost-effectiveness in different age groups, may therefore be less relevant.
Third, and most importantly, there was a very limited evidence base available to parameterise model inputs. No level 1 RCT data were identified and systematic reviews highlighted the dearth of valid observational evidence. The shortage of applicable information was compounded by the unique HITS-NS inclusion criteria, which, to our knowledge, have not been previously studied, and the lack of 6-month follow-up data available from the pilot study. To ensure a believable and accurate model, we therefore prescribed a minimum quality standard for including evidence, eliciting expert opinion, when necessary, in preference to ‘cherry-picking’ data or using inappropriate studies at high risk of bias. Moreover, in the event of valid evidence that was not directly applicable because of differing study populations, we transparently modified published estimates using formal ‘external bias adjustment’ techniques. 107 The base-case model consequently provides a framework to accurately represent current beliefs on competing management strategies and synthesise relatively unbiased evidence.
Although inclusion of expert opinion allows full representation of the uncertainty associated with the decision problem, it is possible that elicited probability distributions do not accurately reflect true parameter values. Beliefs about pivotal relative effectiveness estimates were characterised from intensive care consultants working in a NC. These clinicians may not have full insight into outcomes in patients treated in non-specialist centres and could have imperfect knowledge on the effects of operative management of expanding intracranial haematomas compared with neurosurgeons. Strategy-level estimates of outcomes are also dependent on weighing several other competing factors including pre-hospital deterioration, compliance with bypass and secondary transfer rates. It is debatable whether or not clinicians can accurately integrate all of these considerations into credible effect estimates. Further challenges to elicitation include well-recognised difficulties in understanding probability distributions and odds ratios103,108 and cognitive biases inherent in the elicitation process. 103 To maximise the validity of expert opinion we followed an established elicitation framework, used natural frequencies to derive effect estimates and conducted detailed briefing and training exercises. 103 Other, less critical model inputs were elicited and reviewed within the TMG, and this lack of independence could be considered a limitation with the potential for introducing subjectivity into the analysis.
In the absence of any relevant external studies, HITS-NS pilot study data were used to inform incremental cost differences between selective secondary transfer and bypass. The low sample size available resulted in very imprecise results and prevented regression modelling to adjust for case mix differences in all but the mild TBI subgroup. Although patient characteristics were broadly similar between study groups, findings could potentially be biased secondary to confounding. Additionally, complete case analyses were performed and, if cost data were missing at random, or missing not at random, selection bias may have arisen. 109 Missing data levels were low and hence unlikely to influence results, but ideally a principled statistical method for imputing missing data, such as multiple imputation,110 would have been used. Resource use was valued deterministically using NHS reference costs averaged over NHS hospitals, and limited data was available on management intensity for patients admitted to critical care. It is therefore possible that cost differences between specialist and non-specialist hospital care were not fully delineated.
Other notable weaknesses in model parameterisation include GOS utility values (risk of selection bias and valuation in a non-UK population), relative effectiveness data for patients with major extracranial injury (estimate from non-contemporaneous study with likelihood of subsequent improvements in trauma outcomes) and post-discharge costs (empirical evidence source largely based on expert opinion). All of these model inputs were assessed as borderline with respect to the minimum quality standards required for model inclusion and were therefore subjected to sensitivity analyses to illustrate the potential impact on results from alternative assumptions.
In common with established modelling practice, we used an informal Bayesian approach to fitting parameter distributions, based on the evidence available. 56 In practice this will lead to very similar distributions to an analysis using uninformative prior distributions. However, given the paucity of available evidence and low sample sizes informing some model inputs, a formal Bayesian synthesis using subjective priors may have been more appropriate to account for existing beliefs about suspected significant TBI management.
Fourth, an inability to fully evaluate the model is a further study weakness. Detailed model verification was performed and descriptive validity has been expounded in the foregoing discussion. However, the inclusion of theoretical interventions and reliance on expert opinion for parameterisation restricted the possibilities for internal or external model validation, with little opportunity to check that model results match actual management strategy outcomes. To our knowledge there are no other ongoing or planned studies examining this population or decision problem, making prospective validation unlikely. However, the model output appears to have face validity and is not inconsistent with costs and outcomes reported in other broadly related studies. 3,41,111
Finally, there are a number of potential limitations that could affect expected value of information results. These analyses are premised on the implemented decision analysis model and are therefore subject to identical biases arising from model inputs and structure described previously. Additionally, population-level results are heavily dependent on assumptions regarding the lifespan of bypass as a relevant health technology and the unknown true incidence of suspected significant TBI in the UK. Incidence rate estimates differed widely between the two trial regions and, although different scenarios were explored in sensitivity analyses, the actual value of future research is consequently uncertain.
When computing EVSI we calculated trial sample sizes based on an individually randomised trial, inflated by a design effect determined by assumptions on intracluster correlation coefficient and average cluster size. Generating simulated trial results using an explicit multilevel statistical model to directly account for clustering may have offered a more theoretically sound approach, but is likely to be an academic distinction with minimal influence on results. Owing to the paired cluster randomisation method used in HITS-NS, we were unable to calculate an ICC directly from pilot study data, and we therefore had to use plausible estimates from other non-TBI pre-hospital trials. Several real-world aspects of trials, such as restricted randomisation, loss to follow-up, cluster dropout, unequal cluster sizes and non-compliance were also not accounted for, but are unlikely to materially change the findings. Although not a prespecified objective, ideally we would have also examined the EVSI of including incremental costs in a trial-based economic evaluation or calculated ENBS for a trial comparing bypass with routine transfer. Unfortunately, the computational challenges were significant and implementing these analyses was beyond the resource and time constraints of the current study.
Interpretation of findings
The main determinants of cost-effectiveness in the decision analysis model were incremental costs and effects for patients with TBI requiring acute neurosurgery or critical care. Model inputs for these variables were very uncertain and small changes in their values resulted in conflicting adoption decisions. Previous observational studies, consistent with cost data in HITS-NS, suggest large incremental differences in costs between management in specialist and non-specialist centres. 3,41 Disparities of this magnitude are less likely to be explained by confounding, suggesting that our parameterisation may indeed reflect reality. However, ultimately, evidence from a well-conducted randomised trial is necessary to provide a definitive estimate.
The incremental cost difference in patients with TBI requiring critical care may be intuitively explained by more aggressive management, with longer ICU stays and higher rates of neurosurgical interventions in specialist centres. 41,112 However, the reasons for the higher costs observed in the HITS-NS study between bypassed patients with acute neurosurgical lesions compared with those undergoing secondary transfer are much less clear. The finding could be explained by the play of chance arising from the small pilot study sample. Outliers with high treatment costs, possibly secondary to management of associated extracranial injuries, will have much greater influence in such a small sample. Confounding arising from crude analyses may also be responsible. Alternatively, there may be a true difference in costs possibly explained by the much worse prognosis expected in patients undergoing delayed neurosurgery, which increases early mortality and reduces treatment costs. Interestingly, the HITS-NS results were replicated to some extent in adjusted analyses studying similar patients from the Nottingham Head Injury Register (NHIR), lending credence to these findings.
The result from the base-case economic model that bypass may not be cost-effective for suspected patients with TBI at the standard NICE cost-effectiveness threshold may be unexpected, given the strong support for introducing regional trauma networks from professional bodies and expert opinion leaders. 113,114 The conceptual models of pathophysiology and treatment pathways elucidate the potential benefits and hazards of bypassing patients with TBI, and indicate the manifold factors that will interact to determine strategy level cost-effectiveness. Considering this complexity, the plethora of low-quality and poorly applicable evidence that has been cited in support of bypass and lack of familiarity with heath-care costs, it may be unsurprising that subjective expert opinion will differ from an objective and careful dissection of the decision problem. Contrarily, proponents of bypass could conjecture that nuanced clinical opinion based on extensive experience will be superior to an economic model ignoring many subtleties of trauma management.
Expert beliefs on the comparative effectiveness of bypass for patients with TBI requiring critical care were very cautious, indicating a wide range of possible effect estimates consistent with either a beneficial or a harmful effect. This may suggest that the support for regional trauma networks and bypass of general major trauma patients may not extend to patients with TBI, with the potential for deterioration and secondary brain injury.
Expected net benefit of sampling analyses suggest that a future definitive bypass trial would, if feasible, be cost-effective to reduce decision uncertainty, even in conservative sensitivity analyses. Regardless of model limitations, this finding is unsurprising as elicited expert opinion was very uncertain on the effectiveness of alternative management strategies and empirical evidence was entirely lacking. The major areas of uncertainty pertained to relative effectiveness in patients with TBI requiring acute neurosurgery or critical care, and a relatively small trial would provide substantial information on their posterior distributions and impact adoption decisions. As the EVPPI for these parameters is very high, but the prevalence of relevant patient subgroups in the HITS-NS population is low, EVSI continued to increase at very large sample sizes. However, because of a reduction in the number of patients who may benefit from the trial, and time period for which study results would be useful, as study duration increased, ENBS falls at higher sample sizes.
Relationship to previous studies
No previous economic evaluations have been conducted that specifically investigated pre-hospital triage and bypass in patients with suspected significant TBI and stable pre-hospital physiology. However, a number of previous studies have studied patients with TBI using simulation techniques, or have examined bypass in major trauma patients. Dissimilar populations, lack of valid model parameterisation and non-cost–utility approaches limit the inferences that can be drawn from comparisons with these studies.
Stevenson et al. 13 preformed a simulation study examining bypass of patients with TBI in a UK setting. The model, almost entirely based on informal expert opinion, estimated an additional six survivors per million total population per year if a bypass strategy was introduced. This finding is consistent with the increased number of surviving patients observed with bypass within the HITS-NS model. Unfortunately, further insights are not possible, as costs, disability and triage of undifferentiated patients were not considered.
The 2007 NICE head injury guidelines included a cost–utility study examining bypass of patients with TBI compared to a routine transfer strategy1 using a similar decision tree approach to the HITS-NS model. A base-case ICER of –£26,340 in the south-east quadrant of the cost-effectiveness plane was reported, with bypass strongly dominating the secondary transfer approach. This result was consistent in conservative sensitivity analyses and the conspicuous discrepancy with findings from the current model deserves close scrutiny. A clear difference in the NICE model, generating the extremely favourable ICER for bypass, is an assumption that ED and inpatient costs do not differ between each treatment pathway. In common with the Stevenson study,13 the model also only included patients with severe head injury, rather than the wider spectrum of TBI and non-patients with TBI to which bypass will apply. Moreover, the rationale for parameterisation of the model is not transparent, with very heavy reliance on subjective, informal expert opinion and inclusion of evidence rejected from the HITS-NS model because of extremely high risk of bias.
Nicholl et al. 67 investigated the cost-effectiveness of introducing regional trauma networks in England for major trauma patients using a very simple decision analysis model, reporting an ICER of £1262 and an 80% probability of cost-effectiveness if the cost of implementing a fully effective bypass system was < £34M. 67 The authors highlight the limited evidence available, necessitating a number of ‘heroic assumptions’. These simplifications are not necessarily a problem, as the purpose of a model is to usefully inform a decision question rather than replicate real life. However, the postulation that acute care costs are the same before or after the introduction of trauma networks appears untenable. Furthermore, the similarity between average major trauma patients and the specific HITS-NS population is uncertain, making external validity questionable.
Other patient-level health-economic studies have examined the cost-effectiveness of trauma centre care using observational data. 115–117 These studies compared patients treated in trauma centres (bypassed, directly admitted and transferred in) to untransferred patients cared for in non-specialist hospitals. As they do not examine counterfactual outcomes for patients treated with or without bypass, they have little relevance to the HITS-NS decision problem. Overall, in common with other many other areas of TBI research, there is little cost-effectiveness literature available to inform the HITS-NS economic model.
Implications for clinical practice
Since the inception of the HITS-NS study, trauma care in the NHS has been reconfigured, with the introduction of regional trauma networks. 44,118 Pre-hospital triage with bypass of patients meeting HITS-NS inclusion criteria has now surpassed selective transfer as conventional practice. The relevance of these results to fully implemented trauma systems therefore requires careful consideration.
Above the conventional willingness-to-pay threshold of £20,000, NICE judgements about the acceptability of a health technology will depend on the relative degree of cost-effectiveness, the nature of the intervention and disease and any wider societal costs and benefits. Bypass demonstrated an ICER within NICE’s stated range of borderline cost-effectiveness and results were highly uncertain, even without considering the model limitations or absence of robust evidence. The introduction of trauma networks could be considered an innovative health technology addressing a previously disadvantaged population of trauma patients8 and improved outcomes after head injury could have a major societal impact through increased productivity and a reduced burden on families. It could therefore be contended that in this context the rational course of action would be to avoid any risks and costs from further reorganisation, and persist with the bypass-adoption decision. Decision-makers may also find alternative sensitivity analyses, for which bypass had a favourable ICER of < £20,000, to be more believable than the assumptions inherent in the base-case analysis.
Furthermore, routine transfer could be viewed as a theoretical intervention, unlikely to ever be implemented in the NHS. From this perspective bypass would be the optimal strategy, providing noticeably higher expected net benefit than selective secondary transfer (mean PSA ICER £15,526). However, recent studies have highlighted that a very high proportion of non-surgical patients with severe TBI are now transferred for specialist care [83% in HITS-NS, 73% in RAIN (Risk Adjustment In Neurocritical care)], suggesting that there may be little difference between selective and routine transfer strategies in practice. 41 Likewise, as routine transfer is the standard of care currently recommended in NICE head injury guidelines, excluding it from deliberation seems unreasonable.
Conversely, if the assumptions of the base-case model are considered convincing to decision-makers, it could be asserted that bypass protocols should be modified to exclude patients with suspected significant TBI and stable pre-hospital physiology, although, based on a transparent and thorough exposition of the available evidence, there are several rebuttals to this position. The accuracy and reliability of pre-hospital triage is poorly understood and HITS-NS data suggest that a meaningful number of non-TBI major trauma patients would consequently not be bypassed to trauma centres. Administering multiple triage rules simultaneously or further complicating existing major trauma triage instruments may not be practicable in the pressured pre-hospital environment. Given the financial and administrative investment in trauma system reconfiguration, there is also likely to be sizable clinical and public opposition to further re-organisations of care based on cost-effectiveness results with a high risk of error.
Notwithstanding its potential lack of cost-effectiveness, the adoption of bypass may also be fundamentally irreversible. Ongoing regionalisation of hospital services may result in closure of non-specialist hospitals or degradation in the skills required to manage patients with moderate and severe TBI. 119,120 This may prevent reintroduction of secondary transfer strategies and could have important implications for the substantial numbers of patients with significant TBI who present with GCS levels higher than triage rule inclusion cut points. Recent analysis of TARN registry data and the analysis in stream A, suggests that appreciable numbers of patients with TBI ultimately requiring critical care or neurosurgery will continue to be transported to NSAHs. 40
Implications for future research
The extensive literature reviews conducted for the HITS-NS model demonstrate that the evidence base supporting bypass in TBI is extremely tenuous. As noted by Nicholl et al. 67 in a related economic evaluation ‘it is remarkable how poor the design of relevant studies has been’. Although there are possible theoretical benefits from achieving earlier definitive care through bypass,113 there are potential hazards from prolonged primary transport of patients with TBI, and it could be posited that proof of concept for bypass has not yet been unequivocally proven. This viewpoint would support further research to reduce the probability that an incorrect adoption decision has been implemented, with the concomitant opportunity costs for suspected significant patients with TBI.
However, despite a theoretical demonstration that both necessary (EVPPI exceeds the costs of research) and sufficient (ENBS shows that marginal benefits of sampling exceed the marginal costs) conditions for future research are met, a definitive bypass trial is unlikely to be feasible. Principally, the key HITS-NS feasibility objectives of treatment compliance and incidence of significant TBI were not met. Moreover, prevailing ‘opt-in’ consent requirements would prevent any meaningful follow-up data. Trauma systems may also be a fait accompli, with clinical opinion resisting further experimental research in this area. It is also possible that the relevance of a comparison between bypass and selective transfer will continue to recede over the duration of any trial, secondary to continued regionalisation of emergency and trauma services. 121
Alternative non-randomised research designs to investigate the effectiveness of bypass are likely to be at very high risk of bias. Future cohort or case–control studies of neuroscience care compared with non-neuroscience care are critically limited by confounding, cannot provide valid evidence of comparative effectiveness and would add nothing to the existing weak evidence base. An interrupted time series study, examining outcomes before and after implementation of trauma systems, is a more promising design. However, this would require access to disability outcome information on the entire spectrum of patients to which bypass technology would apply, including patients with mild TBI, and data not available from current routine data collection sources such as Hospital Episode Statistics (HES) or TARN. 18,122 Case submissions to TARN are now linked to best-practice tariff payments to SNCs, incentivising data collection and potentially resulting in differential enrolment of patients between specialist and non-specialist centres. Additionally, the significant numbers of unmatched submissions resulting from interhospital transfers and discrepancy between TARN and HES data suggest the further potential for irresolvable selection bias.
In the contingency that bypass is thought to be cost-effective, its introduction is considered irreversible or collecting further valid evidence on its effectiveness is impossible; however, there are other areas in which future research may be beneficial. Current major trauma triage rules are primarily based on expert opinion, and results from HITS-NS and other recent studies suggest that they may have suboptimal accuracy for identifying patients with TBI requiring specialist care. 40 A cohort study designed to measure the sensitivity and specificity of major trauma triage rules would further examine this hypothesis. If corroborated, future research in this area could include derivation, validation and impact studies to improve over and undertriage rates.
Population EVPPI analyses identified several other groups of variables with a high upper bound on the returns to future research. Cohort studies investigating the incidence of relevant patient subgroups, utility values for GOS health states and post-discharge costs are likely to be cost-effective in reducing uncertainty within the decision analysis model. In contrast with a trial examining comparative effectiveness, such studies would be of shorter duration and require fewer resources. Lack of information on these parameters has been a weakness in previous TBI health-economic models and additional evidence may have considerable value in future HTAs (external to the bypass decision problem).
Acknowledgements
Contributions of authors
Fiona Lecky, Steve Goodacre, Suzanne Mason, Tim Coats, Kevin Mackway-Jones, Andrew Curran, Kyee Han and Gordon Fuller are emergency physicians with expertise in pre-hospital/trauma research.
Fiona Lecky was the HITS-NS lead applicant/chief investigator.
Fiona Lecky led in the conduct of stream A, the cluster randomised trial, chaired the TMG, cosupervised the conduct of stream B with Steve Goodacre and wrote the summaries and HITS-NS stream A report.
Gordon Fuller conducted the HITS-NS stream B literature reviews, economic modelling and write-up, also supervised by Mark Strong, and Matt Stevenson, who are health service researchers with expertise in health-economics decision-analysis modelling.
Gordon Fuller is also supported by a NIHR Doctoral Research Fellowship.
Graham McClelland and Elspeth Pennington are paramedics who were employed full-time during recruitment and co-ordinated HITS-NS stream A in NEAS/NWAS (paramedic training, study roll-out, patient screening, identification, consent and data collection), supervised by Wanda Russell (the HITS-NS trial manager), Sonia Byers and Mary Peters (the AS R&D leads) and Kyee Han and Kevin Mackway-Jones (the AS medical directors).
Andrew Curran and Kyee Han were PIs at two (Royal Preston, James Cook) of the three NCs; Damien Holliman, a neurosurgeon, was PI at the third (Royal Victoria Infirmary) NC in Newcastle.
Wanda Russell managed all aspects of HITS-NS stream A, wrote interim HTA reports and also conducted, analysed and wrote the report sections on patient follow-up interviews, the nested qualitative cohort study and paramedic focus group. Nathan Chapman, a University of Sheffield medical student, studied the conduct of HITS-NS as his BMedSci intercalation and took part in the TMG; his systematic review is in Appendix 1.
Jennifer Freeman is an experienced statistician who conducted the cluster randomisation, stream A analyses in the report and interim analyses for the DMEC.
All of the aforementioned, along with Jane Shewan, the Yorkshire Ambulance Service R&D director, took part in the TMG, apart from Mark Strong and Matt Stevenson. The majority were coapplicants on the original 2008 application.
Hugh Potter, a personal injury solicitor and secretary of Salford and Trafford Headway, was an applicant and took part in the TSG, leading on patient and public involvement in the review of progress, information sheets and satisfaction questionnaires, and representing Headway.
All authors have viewed and contributed to the content of this report.
Assistance
The authors would also like to acknowledge the help and support of the following:
-
Professor Jonathan Benger, who chaired the HITS-NS Trial Steering Group, and the independent members Alastair White and Mike Page
-
Professor Helen Snooks, who chaired the HITS-NS Data Monitoring and Ethics Committee, and the independent members Professor Peter Hutchinson, Dr Simon Gates and Dr Rod Mackenzie
-
all the staff in NEAS, NWAS, the Royal Victoria Infirmary Newcastle, JCUH Middlesbrough, the Royal Preston Hospital, HITS-NS eight acute hospital trusts, and Lancashire and South Cumbria, Durham and Tees Valley and Northumberland Tyne and Wear CLRNs, who helped in making this challenging study possible
-
Laura White, Tom Jenks and Thomas Stacey from TARN for their expertise and support with data collection, coding and management
-
Antoinette Edwards from TARN for creating and developing the HITS-NS website
-
Joanne Casson for assistance in report preparation and administration
-
Hannah Newcombe and members of Salford and Trafford Headway for developing the HITS-NS patient satisfaction questionnaire
-
staff from the University of Manchester, Greater Manchester CLRN and the North Wales REC NW10, who assisted with research governance/study amendments.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Head Injury: Triage, Assessment, Investigation and Early Management of Head Injury in Infants, Children and Adults. London: NICE; 2007.
- Hassan Z, Smith M, Littlewood S, Bouamra O, Hughes D, Biggin C, et al. Head injuries: a study evaluating the impact of the National Institute of Clinical Excellence (NICE) Head Injury Guidelines. Emerg Med J 2005;22:845-9. http://dx.doi.org/10.1136/emj.2004.021717.
- Morris S, Ridley S, Lecky FE, Munro, V, Christensen MC. Determinants of hospital costs associated with traumatic brain injury in England and Wales. Anaesthesia 2008;63:499-508. http://dx.doi.org/10.1111/j.1365-2044.2007.05432.x.
- Patel H, Bouamra O, King AT, Woodford M, Yates DW, Lecky FE. Trends in head injury outcome 1989–03 and the effect of neurosurgical care. Lancet 2005;366:1538-44. http://dx.doi.org/10.1016/S0140-6736(05)67626-X.
- Leech P, Childs C, Evans J, Johnston N, Protheroe R, King A. Transfer times for patients with extradural and subdural haematomas to neurosurgery in Greater Manchester. Br J Neurosurg 2007;21:11-5. http://dx.doi.org/10.1080/02688690701210562.
- Baker SP, O’Neill B, Haddon W, Long WB. The Injury Severity Score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 1974;14:187-96. http://dx.doi.org/10.1097/00005373-197403000-00001.
- Mendelow AD, Timothy J, Steers AJW, Lecky F, Yates D, Bouamra O, et al. Management of patients with head injury: a viewpoint from the Society of British Neurological Surgeons (SBNS) and the Trauma and Audit Research Network (TARN). Lancet 2008;372:685-7. http://dx.doi.org/10.1016/S0140-6736(08)61280-5.
- Trauma: Who Cares? A Report of the National Confidential Enquiry into Patient Outcome and Death. London: NCEPOD; 2007.
- Mendelow AD, Karmi MZ, Paul KS, Fuller GA, Gillingham FJ. Extradural haematoma: effect of delayed treatment. BMJ 1979;1:1240-2. http://dx.doi.org/10.1136/bmj.1.6173.1240.
- Forsyth RJ, Rodriguez B. Routine intracranial pressure monitoring in acute coma. Cochrane Database Syst Rev 2001;3. http://dx.doi.org/10.1002/14651858.cd002043.
- Hannan EL, Farrell L, Bessey PQ, Cayten CG, Cooper A, Mottley L. Accounting for intubation status in predicting mortality for victims of motor vehicle crashes. J Trauma 2000;48:76-81. http://dx.doi.org/10.1097/00005373-200001000-00013.
- DiRusso SM, Sullivan T, Risucci D, Nealon P, Slim M. Intubation of paediatric trauma patients in the field: predictor of negative outcome despite risk stratification. J Trauma 2005;59:84-90. http://dx.doi.org/10.1097/01.TA.0000171462.28379.F3.
- Stevenson MD, Oakley P, Beard SM, Brennan A, Cook AL. Triaging patients with serious head injury; results of a simulation evaluating strategies to bypass hospitals without neurosurgical facilities. Injury 2001;32:267-74. http://dx.doi.org/10.1016/S0020-1383(00)00191-1.
- Pickering A, Cooper K, Harnan S, Holmes M, Sutton A, Mason S, et al. Comparison of Direct Transfer to Specialist Care Centres with Delivery to the Nearest Hospital: A Systematic Review and Economic Evaluation. NIHR Service Delivery and Organisation programme; 2014.
- Lecky F, Bryden D, Little R, Tong N, Moulton C. Emergency intubation for acutely ill and injured patients. Cochrane Database Syst Rev 2008;2. http://dx.doi.org/10.1002/14651858.cd001429.pub2.
- Nicholl J, West J, Goodacre S, Turner J. The relationship between distance to hospital and patient mortality in emergencies: an observational study. Emerg Med J 2007;24:665-8. http://dx.doi.org/10.1136/emj.2007.047654.
- Gomes E, Araújo R, Carneiro A, Dias C, Costa-Pereira A, Lecky FE. The importance of pre-trauma centre treatment of life-threatening events on the mortality of patients transferred with severe trauma. Resuscitation 2010;81:440-5. http://dx.doi.org/10.1016/j.resuscitation.2009.12.014.
- Trauma Audit & Research Network (TARN) . Website for the Publication of the Standards of Care across England and Wales n.d. www.tarn.ac.uk/ (accessed 6 May 2013).
- Dixon S, Mason S, Knowles E, Colwell B, Wardrope J, Gorringe R. Cost-effectiveness of a paramedic practitioner scheme (PPOPS) for elderly patients. Emerg Med J 2009;26:446-51. http://dx.doi.org/10.1136/emj.2008.061424.
- Bickell WH, Wall MJ, Pepe PE, Martin RR, Ginger VF, Allen MK, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med 1994;331:1021-5. http://dx.doi.org/10.1056/NEJM199410273311701.
- Gausche M, Lewis RJ, Stratton SJ, Haynes BE, Gunter CS, Goodrich SM, et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. JAMA 2000;9:783-90. http://dx.doi.org/10.1001/jama.283.6.783.
- A High Quality Workforce: NHS Next Stage Review. London: Department of Health; 2008.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Health and Social Care Information Centre . Ambulance Services, England – 2006-07 n.d. www.hscic.gov.uk/searchcatalogue?productid=162&q=title%3a%22Ambulance+Services%2c+England%22&sort=Relevance&size=10&page=1#top (accessed 5 October 2013).
- Cheung R, Ardolino A, Lawrence T, Bouamra O, Lecky F, Berry K, et al. The accuracy of existing prehospital triage tools for injured children in England: an analysis using trauma registry data. Emerg Med J 2013;30:476-9. http://dx.doi.org/10.1136/emermed-2012-201324.
- Wilson JT, Pettigrew LE, Teasdale GM. Emotional and cognitive consequences of head injury in relation to the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry 2000;69:204-9. http://dx.doi.org/10.1136/jnnp.69.2.204.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. CRASH Triallists. Effect of intravenous corticosteroids on death within 14 days in 10,008 adults with clinically significant head injury (MRC CRASH Trial): randomised placebo-controlled trial. Lancet 2004;364:1321-8. http://dx.doi.org/10.1016/S0140-6736(04)17188-2.
- Abbreviated Injury Scale (AIS) 2005 Revision, Update 1998. Des Plaines, IL: Association for the Advancement of Automotive Medicine; 2005.
- Murray GD, Butcher I, McHugh GS, Lu J, Mushkudiani NA, Maas AI, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007;24:329-37. http://dx.doi.org/10.1089/neu.2006.0035.
- Robson C. Real World Research. Chichester: John Wiley and Sons; 2011.
- Pickering A, Grundy K, Clarke A, Townend W. A cohort study of outcomes following head injury among children and young adults in full-time education. Emerg Med J 2012;29:451-4. http://dx.doi.org/10.1136/emj.2010.094755.
- Head Injury: Triage, Assessment, Investigation and Early Management of Head Injury in Infants, Children and Adults. London: NICE; 2013.
- Mooney JS, Hassan Z, Thomas M, Smith M, Lecky F, Yates A, et al. Emergency head injury imaging: Implementing NICE 2007 in a tertiary neurosciences centre and a busy district general hospital. Emerg Med J 2011;28:778-82. http://dx.doi.org/10.1136/emj.2009.083360.
- Yuh EL, Mukherjee P, Lingsma HF, Yue JK, Ferguson AR, Gordon WA, et al. TRACK-TBI Investigators . Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol 2013;73:224-35. http://dx.doi.org/10.1002/ana.23783.
- Bouamra O, Wrotchford A, Hollis S, Vail A, Woodford M, Lecky F. A new approach to outcome prediction in trauma: a comparison with the TRISS model. J Trauma 2006;61:701-10. http://dx.doi.org/10.1097/01.ta.0000197175.91116.10.
- Bernard SA, Nguyen V, Cameron P, Masci K, Fitzgerald M, Cooper DJ, et al. Prehospital rapid sequence intubation improves functional outcome for patients with severe traumatic brain injury. Ann Surg 2010;252:959-65. http://dx.doi.org/10.1097/SLA.0b013e3181efc15f.
- Caterino JM, Raubenolt A, Cudnik MT. Modification of Glasgow Coma Scale criteria for injured elders. Acad Emerg Med 2011;18:1014-21. http://dx.doi.org/10.1111/j.1553-2712.2011.01164.x.
- Ruff LM, Mendelow AD, Lecky FE. Improving mortality after extradural haematoma in England and Wales. Br J Neurosurg 2013;27:19-23. http://dx.doi.org/10.3109/02688697.2012.709555.
- Fuller G, Lawrence T, Woodford M, Lecky F. The accuracy of alternative triage rules for identification of significant traumatic brain injury: a diagnostic cohort study. Emerg Med J 2014;31:914-19. http://dx.doi.org/10.1136/emermed-2013-202575.
- Harrison DA, Prabhu G, Grieve R, Harvey SE, Sadique MZ, Gomes M, et al. Risk Adjustment In Neurocritical care (RAIN) – prospective validation of risk prediction models for adult patients with acute traumatic brain injury to use to evaluate the optimum location and comparative costs of neurocritical care: a cohort study. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17230.
- Kay A, Teasdale G. Head injury in the United Kingdom. World J Surg 2001;25:1210-20. http://dx.doi.org/10.1007/s00268-001-0084-6.
- Finfer S, Cohen J. Severe traumatic brain injury. Resuscitation 2001;48:77-90. http://dx.doi.org/10.1016/S0300-9572(00)00321-X.
- Vondy A, Willett K. Trauma care in England: London’s trauma system goes live. Emerg Med J 2011;28. http://dx.doi.org/10.1136/emj.2010.101022.
- Fuller G, Pallot D, Coats T, Lecky F. The effectiveness of specialist neuroscience care in severe traumatic brain injury: a systematic review. Br J Neurosurg 2014;28:452-60. http://dx.doi.org/10.3109/02688697.2013.865708.
- Petrou S, Gray A. Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d1766.
- Kaltenthaler E, Tappenden P, Paisley S, Squires H. NICE DSU Technical Support Document 13: Identifying and Reviewing Evidence to Inform the Conceptualisation and Population of Cost-Effectiveness Models 2011. www.nicedsu.org.uk (accessed 12 September 2010).
- Robinson S. Conceptual modelling for simulation Part I: definition and requirements. J Operational Res Soc 2008;59:278-90. http://dx.doi.org/10.1057/palgrave.jors.2602368.
- Petsas A, Waldmann C. Where should patients with severe traumatic brain injury be managed?: Patients can be safely managed in a non-specialist center. J Neurosurg Anesthesiol 2010;22:354-6. http://dx.doi.org/10.1097/ANA.0b013e3181f0da95.
- Major Trauma Care in England. London: NAO; 2010.
- Ganapathy K. Telemedicine and neurosciences in developing countries. Surg Neurol 2002;58:388-94. http://dx.doi.org/10.1016/S0090-3019(02)00924-2.
- Fuller G, Lawrence T, Woodford M, Coats T, Lecky F. Emergency medical services interval and mortality in significant head injury: a retrospective cohort study. Eur J Emerg Med 2015;22:42-8. http://dx.doi.org/10.1097/MEJ.0000000000000117.
- Teasdale GM, Pettigrew L, Wilson L, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J Neurotrauma 1998;15:587-97. http://dx.doi.org/10.1089/neu.1998.15.587.
- Shiel A, Gelling L, Wilson B, Coleman M, Pickard JD. Difficulties in diagnosing the vegetative state. Br J Neurosurg 2004;18:5-7. http://dx.doi.org/10.1080/02688690410001660625.
- Ara R, Wailoo A. Using health state utility values in models exploring the cost-effectiveness of health technologies. Value Health 2012;15:971-4. http://dx.doi.org/10.1016/j.jval.2012.05.003.
- Briggs A, Claxton K, Sculpher MD. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Bennett C, Manuel DG. Reporting guidelines for modelling studies. BMC Med Res Methodol 2012;12. http://dx.doi.org/10.1186/1471-2288-12-168.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics 2006;24:355-71. http://dx.doi.org/10.2165/00019053-200624040-00006.
- Chilcott J, Tappenden P, Rawdin A, Johnson M, Kaltenthaler E, Paisley S, et al. Avoiding and identifying errors in health technology assessment models: qualitative study and methodological review. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14250.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Hall AR. Generalized Method of Moments (Advanced Texts in Econometrics). Oxford: Oxford University Press; 2005.
- af Geijerstam JL, Oredsson S, Britton M. OCTOPUS Study Investigators . Medical outcome after immediate computed tomography or admission for observation in patients with mild head injury: randomised controlled trial. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38918.669317.4F.
- Fuller G, Pattani H, Yeoman P. The Nottingham Head Injury Register: a survey of 1,276 adult cases of moderate and severe traumatic brain injury in a british neurosurgery centre. J Intens Care Soc 2011;12:1-7. http://dx.doi.org/10.1177/175114371101200108.
- McCartan DP, Fleming FJ, Motherway C, Grace PA. Management and outcome in patients following head injury admitted to an Irish Regional Hospital. Brain Inj 2008;22:305-12. http://dx.doi.org/10.1080/02699050801995124.
- Mullins RJ, Mann NC, Hedges JR, Worrall W, Jurkovich GJ. Preferential benefit of implementation of a statewide trauma system in one of two adjacent states. J Trauma 1998;44:609-16. http://dx.doi.org/10.1097/00005373-199804000-00009.
- Beecham J, Perkins M, Snell T, Knapp M. Treatment paths and costs for young adults with acquired brain injury in the United Kingdom. Brain Inj 2009;23:30-8. http://dx.doi.org/10.1080/02699050802590338.
- Nicholl J, Young T, Pickering A, Turner J, Goodacre S. The Cost-Effectiveness of Regional Networks for Major Trauma in England. Sheffield: ScHARR, University of Sheffield; 2011.
- Smits M, Dippel DW, Nederkoorn PJ, Dekker HM, Vos PE, Kool DR, et al. Minor head injury: CT-based strategies for management: a cost-effectiveness analysis. Radiology 2010;254:532-40. http://dx.doi.org/10.1148/radiol.2541081672.
- Torrance GW, Feeny D. Utilities and quality-adjusted life-years. Int J Technol Assess Health Care 1989;5:559-75. http://dx.doi.org/10.1017/S0266462300008461.
- Office for National Statistics (ONS) n.d. www.ons.gov.uk/ons/taxonomy/index/html/?nsd=economy (accessed 2 January 2014).
- Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ 2004;13:437-52. http://dx.doi.org/10.1002/hec.864.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the Estimation of the NICE Cost-effectiveness Threshold. York: Centre for Health Economics, University of York; 2013.
- Culyer A, McCabe C, Briggs A, Claxton K, Buxton M, Akehurst R, et al. Searching for a threshold, not setting one: the role of the National Institute for Health and Clinical Excellence. J Health Serv Res Policy 2007;12:56-8. http://dx.doi.org/10.1258/135581907779497567.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97. http://dx.doi.org/10.1111/j.1524-4733.2008.00358.x.
- Taussky P, Widmer HR, Takala J, Fandino J. Outcome after acute traumatic subdural and epidural haematoma in Switzerland: a single-centre experience. Swiss Med Wkly 2008;138:281-5. http://dx.doi.org/2008/19/smw-12056.
- Aoki N, Kitahara T, Fukui T, Beck JR, Soma K, Yamamoto W, et al. Management of unruptured intracranial aneurysm in Japan: a Markovian decision analysis with utility measurements based on the Glasgow Outcome Scale. Med Decis Making 1998;18:357-64. http://dx.doi.org/10.1177/0272989X9801800401.
- McMillan TM, Teasdale GM, Weir CJ, Stewart E. Death after head injury: the 13 year outcome of a case control study. J Neurol Neurosurg Psychiatry 2011;82:931-5. http://dx.doi.org/10.1136/jnnp.2010.222232.
- Centre for Bayesian Statistics in Health Economics (CHEBS), School of Health and Related Research, University of Sheffield . Excel Macros for Sampling from Multivariate Distributions Archive n.d. www.shef.ac.uk/chebs/software (accessed 2 January 2014).
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:S68-80. http://dx.doi.org/10.1177/0272989X9801800209.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64. http://dx.doi.org/10.1016/S0167-6296(98)00039-3.
- Eckermann S, Willan AR. Expected value of information and decision making in HTA. Health Econ 2007;16:195-209. http://dx.doi.org/10.1002/hec.1161.
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24. http://dx.doi.org/10.1002/(SICI)1099-1050(199611)5:6<513::AID-HEC237>3.0.CO;2-9.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27. http://dx.doi.org/10.1177/0272989X04263162.
- Brennan A, Kharroubi S, O’Hagan A, Chilcott J. Calculating partial expected value of perfect information via Monte Carlo sampling algorithms. Med Decis Making 2007;27:448-70. http://dx.doi.org/10.1177/0272989X07302555.
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making 2014;34:311-26. http://dx.doi.org/10.1177/0272989X13505910.
- Strong M, Oakley J, Brennan A. Fast efficient computation of expected value of sample information from a probabilistic sensitivity analysis sample: a non-parametric regression approach. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-S1-O25.
- Strong M, Oakley J. An Efficient Method for Computing the Expected Value of Sample Information. A Non-Parametric Regression Approach. Sheffield: ScHARR, University of Sheffield; 2013.
- Holmes M, Rathbone J, Littlewood C, Rawdin A, Stevenson M, Stevens J, et al. Routine echocardiography in the management of stroke and transient ischaemic attack: a systematic review and economic evaluation. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18160.
- Leaviss J, Sullivan W, Ren S, Everson-Hock E, Stevenson M, Stevens JW, et al. What is the clinical effectiveness and cost-effectiveness of cytisine compared with varenicline for smoking cessation? A systematic review and economic evaluation. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18330.
- Hastie T, Tibshirani R. Generalized additive models for medical research. Stat Methods Med Res 1995;4:87-96. http://dx.doi.org/10.1177/096228029500400302.
- Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: Chapman and Hall/CRC; 2006.
- R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2013.
- DeBoor C. Practical Guide to Splines. New York, NY: Springer; 1974.
- Donner A, Klar N. Pitfalls of and controversies in cluster randomization trials. Am J Public Health 2004;94:416-22. http://dx.doi.org/10.2105/AJPH.94.3.416.
- Donner A, Klar N. Design and Analysis of Cluster Randomised Trials in Health Research. London: Arnold; 2000.
- Hayes R, Moulton L. Cluster Randomised Trials. Boca Raton, FL: Chapman and Hall; 2009.
- Snooks H, Cheung W, Close J, Dale J, Gaze S, Humphreys I. Support and Assessment for Fall Emergency Referrals (SAFER 1) trial protocol. Computerised on-scene decision support for emergency ambulance staff to assess and plan care for older people who have fallen: evaluation of costs and benefits using a pragmatic cluster randomised trial. BMC Emerg Med 2010;10. http://dx.doi.org/10.1186/1471-227X-10-2.
- Soares MO, Welton NJ, Harrison DA, Peura P, Shankar-Hari M, Harvey SE, et al. An evaluation of the feasibility, cost and value of information of a multicentre randomised controlled trial of intravenous immunoglobulin for sepsis (severe sepsis and septic shock): incorporating a systematic review, meta-analysis and value of information analysis. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16070.
- Mason S, Knowles E, Colwell B, Dixon S, Wardrope J, Gorringe R, et al. Effectiveness of paramedic practitioners in attending 999 calls from elderly people in the community: cluster randomised controlled trial. BMJ 2007;335:919-24. http://dx.doi.org/10.1136/bmj.39343.649097.55.
- Aoki N, Kitahara T, Fukui T, Beck JR, Soma K, Yamamoto W, et al. Management of unruptured intracranial aneurysm in Japan: a Markovian decision analysis with utility measurements based on the Glasgow Outcome Scale. Med Decis Making 1998;18:357-64. http://dx.doi.org/10.1177/0272989X9801800401.
- Taussky P, Widmer HR, Takala J, Fandino J. Outcome after acute traumatic subdural and epidural haematoma in Switzerland: a single-centre experience. Swiss Med Wkly 2008;138:281-5. http://dx.doi.org/2008/19/smw-12056.
- O’Hagan A, Buck CE, Daneshkhah A, Eiser JR, Garthwaite PH, Jenkinson DJ, et al. Uncertain Judgements Eliciting Experts’ Probabilities. Chichester: John Wiley and Sons Ltd; 2006.
- Neuroanaesthesia Society of Great Britain and Ireland (NASGBI) . Neurocritical Care Capacity and Demand: A Report from the Neurocritical Care Stakeholder Group n.d. www.nasgbi.org.uk/resources/1/y/neurocriticalcaresnapshotauditfinal.doc (accessed 21 December 2013).
- Crimmins D, Palmer J. Snapshot view of emergency neurosurgical head injury care in Great Britain and Ireland. J Neurol Neurosurg Psychiatry 2000;68:8-13. http://dx.doi.org/10.1136/jnnp.68.1.8.
- Maas A, Stocchetti N. Moderate and severe traumatic brain injury in adults. Lancet Neurol 2008;7:728-41. http://dx.doi.org/10.1016/S1474-4422(08)70164-9.
- Turner RM, Spiegelhalter DJ, Smith GC, Thompson SG. Bias modelling in evidence synthesis. J R Stat Soc Ser A Stat Soc 2009;172:21-47. http://dx.doi.org/10.1111/j.1467-985X.2008.00547.x.
- Cummings P. The relative merits of risk ratios and odds ratios. Arch Pediatr Adolesc Med 2009;163:438-45. http://dx.doi.org/10.1001/archpediatrics.2009.31.
- Allison PD. Missing Data. Thousand Oaks, CA: Sage Publications; 2001.
- Schafer J. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3-15. http://dx.doi.org/10.1191/096228099671525676.
- McGarry LJ, Thompson D, Millham FH, Cowell L, Snyder PJ, Lenderking WR, et al. Outcomes and costs of acute treatment of traumatic brain injury. J Trauma 2002;53:1152-9. http://dx.doi.org/10.1097/00005373-200212000-00020.
- Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL. Early management of severe traumatic brain injury. Lancet 2012;380:1088-98. http://dx.doi.org/10.1016/S0140-6736(12)60864-2.
- Sleat G, Willett K. Evolution of trauma care in the UK: current developments and future expectations. Injury 2011;42:838-40. http://dx.doi.org/10.1016/j.injury.2011.05.014.
- Trauma Care in Scotland. Edinburgh: Royal College of Surgeons of Edinburgh; 2012.
- MacKenzie EJ, Weir S, Rivara FP, Jurkovich GJ, Nathens A, Wang W, et al. The value of trauma center care. J Trauma 2010;69:1-10. http://dx.doi.org/10.1097/TA.0b013e3181e03a21.
- Durham R, Pracht E, Orban B, Lottenberg L, Tepas J, Flint L. Evaluation of a mature trauma system. Ann Surg 2006;243:775-85. http://dx.doi.org/10.1097/01.sla.0000219644.52926.f1.
- Séguin J, Gaber BG, Coyle D, Hébert PC. An economic evaluation of trauma care in a Canadian lead trauma hospital. J Trauma 1999;47:S99-103. http://dx.doi.org/10.1097/00005373-199909001-00022.
- Lendrum RA, Lockey DJ. Trauma system development. Anaesthesia 2013;68:30-9. http://dx.doi.org/10.1111/anae.12049.
- A Framework for Action. London: The Stationery Office; 2007.
- Our NHS, Our Future. London: The Stationery Office; 2007.
- Meadow C, Rattenberry W, Waldmann C. Centralisation of specialist critical care services. JICS 2011;12:88-9. http://dx.doi.org/10.1177/175114371101200202.
- Department of Health . Hospital Episode Statistics n.d. www.hesonline.nhs.uk (accessed 22 November 2013).
- Greenhalgh T. How To Read a Paper: The Basics of Evidenced-Based Medicine. Chichester: Wiley-Blackwell; 2010.
- Callaham M. Quantifying the scanty science of prehospital emergency care. Ann Emerg Med 1997;30:785-90. http://dx.doi.org/10.1016/S0196-0644(97)70049-0.
- Schmidt TA, Nelson M, Daya M, DeIorio NM, Griffiths D, Rosteck P. Emergency medical service providers’ attitudes and experiences regarding enrolling patients in clinical research trials. Prehosp Emerg Care 2009;13:160-8. http://dx.doi.org/10.1080/10903120802708852.
- Wang HE, Yealy DM. Out-of-hospital clinical trials: challenges in advancing the evidence base. Ann Emerg Med 2011;57:232-3. http://dx.doi.org/10.1016/j.annemergmed.2010.11.034.
- Building the Evidence Base in Pre-Hospital Urgent and Emergency Care. London: Department of Health; 2010.
- Morrison LJ, Baker AJ, Rhind SG, Kiss A, MacDonald RD, Schwartz B, et al. The Toronto prehospital hypertonic resuscitation – head injury and multiorgan dysfunction trial: feasibility study of a randomized controlled trial. J Crit Care 2011;26:363-72. http://dx.doi.org/10.1016/j.jcrc.2010.08.021.
- Hargreaves K, Goodacre S, Mortimer P. Paramedic perceptions of the feasibility and practicalities of prehospital clinical trials: a questionnaire survey. Emerg Med J 2014;31:499-504. http://dx.doi.org/10.1136/emermed-2013-202346.
- Burges Watson DL, Sanoff R, Mackintosh JE, Saver JL, Ford GA, Price C, et al. Evidence from the scene: paramedic perspectives on involvement in out-of-hospital research. Ann Emerg Med 2012;60:641-50. http://dx.doi.org/10.1016/j.annemergmed.2011.12.002.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2700.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. http://dx.doi.org/10.1111/j. 2002.384.doc.x.
- Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D. A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma. Health Technol Assess 2000;4.
- Woollard M, Whitfield R, Smith K, Jones T, Thomas G, Hinton C. Less IS less: a randomised controlled trial comparing cautious and rapid nalbuphine dosing regimens. Emerg Med J 2004;21:362-4. http://dx.doi.org/10.1136/emj.2004.014324.
- Snooks H, Foster T, Nicholl J. Results of an evaluation of the effectiveness of triage and direct transportation to minor injuries units by ambulance crews. Emerg Med J 2004;21:105-11. http://dx.doi.org/10.1136/emj.2003.009050.
- Bulger EM, May S, Brasel KJ, Schreiber M, Kerby JD, Tisherman SA, et al. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. J Am Med Assoc 2010;304:1455-64. http://dx.doi.org/10.1001/jama.2010.1405.
- Bulger EM, Jurkovich GJ, Nathens AB, Copass MK, Hanson S, Cooper C, et al. Hypertonic resuscitation of hypovolemic shock after blunt trauma: a randomized controlled trial. Arch Surg 2008;143:139-48. http://dx.doi.org/10.1001/archsurg.2007.41.
- Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, Hoyt DB, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg 2009;208:1-13. http://dx.doi.org/10.1016/j.jamcollsurg.2008.09.023.
- Bulger EM, May S, Kerby JD, Emerson S, Stiell IG, Schreiber MA, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg 2011;253:431-41. http://dx.doi.org/10.1097/SLA.0b013e3181fcdb22.
- Baker AJ, Rhind SG, Morrison LJ, Black S, Crnko NT, Shek PN, et al. Resuscitation with hypertonic saline-dextran reduces serum biomarker levels and correlates with outcome in severe traumatic brain injury patients. J Neurotrauma 2009;26:1227-40. http://dx.doi.org/10.1089/neu.2008.0868.
- Cooper DJ, Myles PS, McDermott FT, Murray LJ, Laidlaw J, Cooper G, et al. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. J Am Med Assoc 2004;291:1350-7. http://dx.doi.org/10.1001/jama.291.11.1350.
- Jennings PA, Cameron P, Bernard S, Walker T, Jolley D, Fitzgerald M, et al. Morphine and ketamine is superior to morphine alone for out-of-hospital trauma analgesia: a randomized controlled trial. Ann Emerg Med 2012;59:497-503. http://dx.doi.org/10.1016/j.annemergmed.2011.11.012.
- Schiferer A, Gore C, Gorove L, Lang T, Steinlechner B, Zimpfer M, et al. A randomised controlled trial of femoral nerve blockade administered preclinically for pain relief in femoral trauma. Anesth Analg 2007;105:1852-4. http://dx.doi.org/10.1213/01.ane.0000287676.39323.9e.
- Kober A, Fleischackl R, Scheck T, Lieba F, Strasser H, Friedmann A, et al. A randomized controlled trial of oxygen for reducing nausea and vomiting during emergency transport of patients older than 60 years with minor trauma. Mayo Clin Proc 2002;77:35-8. http://dx.doi.org/10.4065/77.1.35.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. CONSORT 2010 statement: extension to cluster randomised trials. BMJ 2012;345:19-22. http://dx.doi.org/10.1136/bmj.e5661.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT Statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. http://dx.doi.org/10.7326/0003-4819-148-4-200802190-00008.
- Benger JR, Voss S, Coates D, Greenwood R, Nolan J, Rawstorne S, et al. Randomised comparison of the effectiveness of the laryngeal mask airway supreme, i-gel and current practice in the initial airway management of prehospital cardiac arrest (REVIVE-Airways): a feasibility study research protocol. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002467.
- NHS Reference Costs 2007/2008. London: Department of Health; 2008.
Appendix 1 Systematic review of pre-hospital controlled trials in trauma patients
Chapman N (Lecky FE supervisor), as part of dissertation for B Med Sci, University of Sheffield, 2013.
Introduction
Controlled trials are used to investigate the efficacy of health-care interventions. They involve procedures, such as randomisation, to ensure that the groups of patients involved are, on average, identical apart from the interventions received. Thus, when the groups are followed up after a pre-specified time period, and assessed in terms of pre-specified outcomes, any differences observed should be attributable to the intervention(s) under investigation and not any other factors. 123 Therefore, it is accepted that controlled trials provide the most robust level of evidence for assessing health-care interventions. 124
Most research concerning health-care interventions is undertaken in a hospital setting. 125 In 1988, while many hundreds of in-hospital controlled trials had been conducted regarding interventions for emergency medical conditions, a MEDLINE search identified only 54 randomised controlled trials conducted in the out-of-hospital environment. 124 While the numbers of trials has increased since this analysis, an evidence gap still persists,126 and the research used to support even the most standard practices employed by the emergency medical system (EMS), is limited. 125
The reason for this dearth of pre-hospital research is that it is a complex area that presents immense challenges to researchers. 127 The most common issues of difficulty arise in regards to recruitment, paramedic participation and compliance and data collection. 128
Studies have been conducted regarding paramedic perceptions of controlled pre-hospital trials in order to elucidate some of the barriers and facilitators to their successful conduct. 129 The main issues that have arisen include that research is not their responsibility,129 that it limits their autonomy,130 that they do not have time to recruit participants129 and that, because of the often incapacitated nature of the patients involved, it is unethical. 125
Pre-hospital research involving EMS and paramedics, has been identified in the UK as a priority for strengthening the practice of care in this area. 127,130 Therefore, in order to have suitable comparisons by which to judge the successes and failures of the HITS-NS trial, a systemic review was undertaken of recently conducted pre-hospital controlled trials regarding interventions for patients with traumatic injuries.
Methods
Reference was made to the PRISMA (Preferred Reporting Items Systematic Reviews and Meta-Analyses) statement in guiding the conduct and reporting of this review. 131 Complete methods are given in Appendix E and are briefly summarised here. Three databases (MEDLINE, Cumulative Index to Nursing and Allied Health Literature and The Cochrane Library) were searched using terms related to ‘pre-hospital’, ‘trauma’ and ‘controlled trials’. Reference searching, along with contact with an experienced pre-hospital researcher to identify grey literature (see Appendix E for Table 26 and Contact with Dr Janette Turner), was also employed. A date limit of 15 years was set. This was chosen pragmatically, to try to maintain ecological validity with current ambulance service practice, while including all relevant trials.
Eligibility criteria, including that trials had been conducted in the pre-hospital environment, with over 50% of patients having encountered pre-hospital care as a result of traumatic injuries, were chosen to try to identify a homogenous group of trials, focused on the pre-hospital care of those with traumatic injuries by land-based ambulances services. The trials finally selected for inclusion in the report were reviewed in terms of five criteria: recruitment, proportion of relevant participant, compliance with trial protocols, selection bias created by non-compliance and acceptability of trials and treatments to patients, their families, and staff. These have been highlighted in previous reports as being key to the successful conduct of controlled trials132 and of pre-hospital trials in particular. 128
Results
A PRISMA diagram demonstrating the number of articles rejected at each stage of the review process, with reasons, is shown in Figure 27. For the purposes of analysis, and to maintain relevance with the HITS-NS trial, the 15 trials identified were divided based on location (NHS, UK/outside NHS, UK), type of trauma (head-injury/general trauma) and type of intervention under investigation. Four pre-hospital controlled-trials investigating general trauma were conducted in the UK NHS. 100,133–135 No pre-hospital trials concerning head-injury, conducted in the UK, could be identified. The remaining 11 studies were conducted in the USA,136–139 Canada,128,140 Australia30,141,142 and Austria. 143,144 A table (Table 25) summarising the appraisal of the four trials conducted in the UK NHS, upon which the discussion will focus, is presented below (the complete appraisal tables can be found in Appendix E (see Critical Appraisal tables).
FIGURE 27.
A PRISMA flow diagram for a systematic review to investigate the barriers to successful completion of pre-hospital controlled trials regarding interventions for patients with traumatic injuries. CINAHL, Cumulative Index to Nursing and Allied Health Literature.
| Appraisal | Trials | |||
|---|---|---|---|---|
| Fluids | Analgesia | Patient pathway | ||
| Turner et al.133 | Woollard et al.134 | Snooks et al.135 | Mason et al.100 | |
| Study purpose | To assess the effects of two pre-hospital fluid protocols: fluids administered to all qualifying trauma patients vs. fluids withheld until arrival at hospital | To assess which of two pre-hospital analgesia dosing regimens with nalbuphine produced the greatest analgesic effect | To assess the impact of transportation of selected patients to a MIU compared with standard practice of transporting all patients to the ED | To evaluate the possible benefits of paramedic practitioners assessing and treating older people with minor injuries or illnesses in the community |
| Study design | Cluster controlled trial; paramedics (n = 401) randomised to one of the two treatment protocols and then crossed over half-way through trial | Randomised controlled trial; patients randomised, by the opening of an opaque envelope, into either a rapid or slow dosing regimen | Cluster controlled trial; ambulance crews (n = 55) transported patients to participating MIUs during randomly selected weeks, totalling 6 months; all other weeks transferred to the ED | Cluster controlled trial; weeks (n = 56) were randomised so patients either received the paramedic practitioner service (n = 30 weeks) or standard care (n = 26 weeks) |
| Recruitment | 1583 patients required; 1309 recruited over 17 months (53.4% IG, 46.6% CG); 64% useable response rate to questionnaires | 152 patients required; 175 patients were randomised (49.1% IG; 50.9% CG); 37.7% of patients missing in-hospital data | Patients required not stated; 834 patients recruited over 1 year (49.0% IG, 51.0% CG); 59.8% useable response rate to questionnaires | 2200 patients required; 3018 recruited over 1 year (51.3% IG, 48.7% CG); 64.6% useable response rate to questionnaires |
| Relevant participants | Not applicable | Not reported | Seven patients (0.9%) taken to MIU were subsequently transferred to ED as injuries more severe than initially identified | Not reported |
| Compliance | Poor; 69.1% of Protocol A patients and 20.2% of Protocol B patients received the incorrect treatments; very small difference between the two groups in terms of those who received fluids (30.9% vs. 20.2%) | Poor; paramedics failed to give the maximum dose of nalbuphine available to patients who continued to report significant pain following their first dose | Poor; 90.0% of intervention group and 23.1% of control group taken to incorrect destination; very small difference in proportion of patients taken to MIU during the intervention and control weeks (10.0% vs. 8.7%) | Excellent; 191% compliance reported |
| Selection bias | Not analysed | Reasons for non-compliance could not be accounted for by on-scene or transportation time or initial pain score | The main influence on patient destination was distance to facility, along with distance to nearest other facility and presence of head injury | None |
| Acceptability | Not reported | Not investigated; however, reasons for non-compliance could not be accounted for by on scene or transportation time or initial pain score and the authors concluded that it implied refusal to administer full dose to those in continued pain | Acceptability of the trial protocols was very poor; perceived barriers to MIU use by crews included: distance to MIU compared to ED, opening times of MIU and underlying medical condition | Patients treated during the paramedic practitioner intervention weeks were more likely to be very satisfied with their care (relative risk 1.16, 95% CI 1.09 to 1.23; p < 0.001) |
Discussion
Pre-hospital controlled trials conducted in the UK NHS
Study purpose and design
Three of the four trials used a cluster randomised design. In such trials, rather than randomising each individual patient, another unit (such as practitioner or week) is randomised. 145 This is favoured in many non-pharmacological trials, in which switching back and forth between one or more treatments would be impractical. The dangers of this approach include clustering of effects and contamination between groups. 146
In the two trials investigating alternative patient pathways, by Snooks et al. 135 and Mason et al. ,100 week was used as the unit of cluster so that on some weeks the experimental intervention was given, and on others the control. In Turner et al.’s133 study of two fluid protocols, the unit of cluster was the paramedic. A cross-over design was used: half-way through the trial those administering protocol A (fluid for all trauma patients) switched to B (no fluids), and vice versa. 133
Recruitment
All but one of the trials (Turner et al. 133), recruited an adequate number of patients. However, issues arose regarding patient response to follow-up questionnaires, which was less than 65% in all cases. 100,133,135 This is despite the fact that in one study some patients were sent consent forms with their questionnaires, rather than before, as with the other studies. 133 Reasons for the low response rate were not elucidated, but it meant that a potentially unrepresentative sample of responses may have been collected (e.g. patients in better health who were able to respond). 123
Relevance of participants recruited
This factor is related to the capacity of pre-hospital triage criteria to correctly identify injuries which cannot be fully diagnosed in the pre-hospital environment (e.g. TBI). Therefore, it is only relevant to those studies which aimed to recruit patients whose injuries could not be fully diagnosed in the pre-hospital environment.
In the study by Snooks et al. 135 seven patients (0.9%) diagnosed by paramedics as having a minor injury and taken to the Minor Injuries Unit were subsequently transferred to the ED, as their injuries were in fact more severe. 135 This demonstrates that pre-hospital triage criteria may not always correctly identify the injuries, nor the severity of those injuries, that a patient has sustained.
Compliance
Excepting the study by Mason et al. ,100 compliance with study protocols was poor. In the studies by Turner et al. 133 and Snooks et al. 135 the difference between those who did and did not receive the experimental intervention in the control and intervention groups was only 10.7% and 1.3%, respectively (i.e. nearly as many patients in the intervention group received the control intervention, as in the control group). In fact, in these studies, the study protocol had no significant effect on the intervention given. 133,135
The 191% compliance found in the study by Mason et al. 100 can be explained by the fact that, according to the weeks of randomisation, paramedic practitioners were either available or not available during the study period. This eliminated the chance of non-compliance. 100
Selection bias due to non-compliance
In the study by Snooks et al. 135 it was identified that paramedics were primarily choosing the patient destination (Minor Injuries Unit vs. ED) based on distance from the incident to those facilities. Therefore, if patients were injured nearest to a MIU, they were more likely to be taken there, regardless of the week of randomisation. 135 However, selection bias was not investigated in this or any of the other studies. 135
Acceptability to patients, families and staff
None of the studies appraised the views of the paramedics involved in the study, except that by Snooks et al. 135 Semistructured interviews were conducted with 15 of the 55 ambulance crews. Perceived barriers to Minor Injuries Unit use by crews included distance to the units compared with the ED, opening times and uncertainty regarding willingness to accept patients. 135 In preparing for the trial, pre-trial training was given to all 55 participating crews, including the necessity for intervention and control groups. The investigators conclude that, for pre-hospital research, ‘the lower the reliance on participants to decide when to apply protocols, the more robust the study will be’. 135
Additionally, reasons for lack of paramedic compliance with controlled trials in general have been explored by two recent qualitative studies in the UK. Both of these identified additional reasons for potential non-compliance. 129,130 The first employed a questionnaire study in the Yorkshire Ambulance Service. 129 A response rate of only 32% (n = 187) was achieved, itself suggesting a limited engagement with research. The barriers to trial participation included the perception that use of evidence in the pre-hospital setting is limited, that research is not the responsibility of paramedics, that it is impractical and that there is not enough time for the recruitment process. It appeared that a cluster randomisation approach was preferred, but even this raised issues regarding treatment delays and the ethics of pre-consent randomisation of patients. 129
The second study, involving paramedics who had taken part in a pre-hospital investigation regarding treatments for stroke, had similar findings. 130 Concerns were raised that patients would not receive the best care, that the process of randomisation would limit their clinical decision-making and that research protocols would increase the time necessary to assess and treat patients before transportation. 130
Therefore, it is clear that, in the UK at least, there are issues regarding the conduct of research by paramedics which go beyond any single trial. The issues of compliance encountered by the studies under appraisal may relate to some of the more general barriers identified in these two qualitative studies.
Pre-hospital controlled trials conducted outside the UK NHS
Study purpose and design
The remaining 11 studies (Appendix E) were all randomised controlled trials (i.e. patients randomised directly). Five investigated interventions in head-injury, four of which fluid concerned administration128,136,140,141 and one pre-hospital intubation. 37 The final six investigated interventions in general trauma: three fluid administration,137–139 one oxygen144 and two analgesia. 142,143
Recruitment
In three of the trials concerning head injury,30,136,141 recruitment took place for over 3 years. In the study by Cooper et al. ,141 set in Melbourne, Australia (a region with more than 4 million inhabitants) it took 3 years and 4 months to recruit the 262 adult patients with a GCS score of ≤ 8 and hypotension. In the study by Bernard et al. ,37 set in Victoria, Australia (again, a region with more than 4 million inhabitants), it took 4 years to recruit 312 adult patients with a GCS score of ≤ 9.
Trials recruiting patients with all traumatic injuries (e.g. head, long bone, abdominal) were able to recruit the same number of patients as these, but over a shorter duration. For example, Bulger et al. recruited 209 adult blunt trauma patients with hypotension, but in half the time of the other two studies (2 years) and in a region with one-eighth of the inhabitants (Seattle, USA, population 621,000). 137 Therefore, it could be that, as the eligibility criteria for entry into a trial become more specific, the recruitment rate will fall.
As in the studies conducted in the UK, loss to follow-up was an issue. In one head-injury study, loss to follow-up at 6 months was 42.9%. 128 In the head-injury studies, reasons identified for high losses to follow-up included being unable to consent patients because of minimal injury with rapid discharge from hospital136 and changes in patient location. 128
Relevance of participants recruited
In the trial investigating pre-hospital intubation for head-injury, of the 312 patients randomised, six (1.9%) had diagnoses other than TBI (e.g. spontaneous intracranial haemorrhage) and 22 (7.1%) only had a minor head injury and it was assumed that intoxication was the reason for the initial degree of coma (GCS score of ≤ 9). 37 As mentioned above, this demonstrates that pre-hospital triage criteria may not always correctly identify the injuries a patient has sustained, leading to patients not relevant to the study being recruited.
Compliance
In the two trials investigating interventions performed by a specialist (intensive care paramedic intubation of TBI patients37 and pre-hospital doctor administration of pain relief143), the compliance was 191%. These were both ‘open-label’ studies, in which the specialists were fully aware of the treatments they were administering. It is possible that their high compliance levels can be explained by intensive pre-trial training (the intensive care paramedics required a 16-hour training programme before acceptance onto the trial) and better comprehension of the necessity for a robust, well-conducted study. 37,143
Those trials involving fluid administration, including four of the head-injury trials, to which paramedics were blinded (using identical, randomised fluid bags) and therefore had no control over which interventions they were giving, had compliance levels of over 90%. 139,141 In these cases the paramedics were unaware of which treatments they were administering and were therefore unable to decide to break treatment protocol. 137
However, an open-label study involving fluid administration, which investigated the effect of pre-hospital administration of a haemoglobin-based oxygen carrier (PolyHeme) compared to standard fluids, had a compliance level of 80.5% in the control group. 138 The investigators identified that the control group patients who received PolyHeme had higher injury severity scores, lower pre-randomisation blood pressure and lower GCS scores. This suggests that paramedics were breaking the protocol for control patients who they felt were in a more severe condition. 138 Therefore, it appears that Snooks et al. 135 could be correct in their assertion that where paramedics are aware of which interventions are being given, compliance levels will be low, and therefore that only double-blind pre-hospital controlled trials or those involving highly trained specialists will achieve above 90% levels of compliance.
Selection bias owing to non-compliance
Selection bias was poorly investigated and reported across all studies. The one exception to this was the PolyHeme trial, which identified that paramedics were selecting patients with more severe injuries from the control group to receive the experimental intervention. 138
Acceptability to patients, families and staff
None of the studies conducted outside the UK NHS assessed the acceptability of the interventions under investigation to patients, families and staff. However, one survey study, conducted in the USA, has served to evaluate paramedics’ attitudes and experiences in enrolling critically injured trauma patients under federal rules for exemption from informed consent. 125 This study found that while over 90% of responders (n = 787) agreed that ‘research in EMS care is important’, nearly 40% felt that they should retain a personal right to refuse to enrol patients and over 50% felt that the autonomy of research subjects is above the interests of the community. In light of these findings, some researchers have suggested that paramedics should be allowed to ‘opt out’ of research to help increase compliance. 125,147 As in the previous studies, this involves issues of paramedic autonomy and research ethics.
Barriers identified
The four pre-hospital trials conducted in the UK NHS demonstrated that all five of the issues addressed by this review may present as barriers to the successful completion of studies. While recruitment numbers were generally good, responses to 6-month follow-up questionnaires were poor. Pre-hospital triage criteria may not always correctly identify the injuries a patient has sustained, which can lead to irrelevant patients being recruited into a study. Compliance was poor in trials in which paramedics were aware of which group their patients were randomised to. Whether non-compliance resulted in selection bias was poorly investigated. Acceptability to staff was also poorly investigated.
Pre-hospital trials conducted outside of the UK also have much to teach investigators operating within the NHS. Response to 6-month follow-up questionnaires was a particular issue in some head-injuries studies. Reasons identified for this included rapid discharge from hospital. Another issue of specific consequence to the head-injury studies was recruitment of relevant patients. In some cases, patients with a head-injury and a GCS suggesting severe TBI were actually intoxicated or had a non-traumatic intracerebral haemorrhage. High levels of compliance were only achieved where specialist paramedics, who had undertaken extra training to be eligible to take part in the trial, were involved or paramedics had no knowledge of which treatment they were administering. Barriers to paramedic involvement in controlled trials, specifically where incapacitated patients were being recruited, were identified as paramedic and patient autonomy, the perception that research was unnecessary and the issue of lack of time.
APPENDIX E: systematic review of pre-hospital controlled trials investigating interventions for patients with traumatic injuries
Methods
Search terms (Table 26) were developed with reference to the US National Library of Medicine’s ‘Medical Subject Headings’ database and through contact with a researcher with extensive experience of both conducting and appraising pre-hospital controlled trials (see Contact with Dr Janette Turner, University of Sheffield, below). Search terms of more than one word were enclosed in quotation marks, and where more than one word ending was possible (e.g. injury, injuries, injured) a truncation symbol (*) was used.
| Key concept | Pre-hospital | Controlled Trial | Trauma |
|---|---|---|---|
| Search terms | Pre-hospital | Controlled trial | Trauma |
| Out of hospital | Wound | ||
| Ambulance | Injur* | ||
| Paramedic | |||
| EMS | |||
| EMS |
Three databases were used to conduct the search: MEDLINE (via the OVID Technologies interface: http://gateway.ovid.com), CINAHL (via the EBSCOhost interface: http://ebscohost.com/academic) and the Cochrane Library (www.thecochranelibrary.com). These were chosen based on the scope of each database covering all the journals it was thought that pre-hospital controlled trials would be published in, as advised by the two above-named researchers. As well as these databases, reference searching of articles identified was also employed, along with communication with above-named authors for any trials that had been missed or any ‘grey literature’ (studies conducted and completed but unpublished).
The MEDLINE database search was conducted on the 24 March 2013 and that of the CINAHL and Cochrane Databases was conducted on 25 March 2013. An ‘autoalert’ search was set up on the MEDLINE database so that any trials which were published following the initial search, up to and including 30 June 2013, could be identified and included. Only the MEDLINE search will be described here.
Each search term was searched for individually in both the ‘title’ and ‘abstract’ fields, using the OVID ‘search fields’ function. Following this, search terms from the same key concept were combined with ‘or’ and the search terms from different key concepts were combined with ‘and’. This gave the following search strategy:
(pre-hospital or ‘out of hospital’ or ambulance or paramedic or ‘EMS’ or EMS) and
‘controlled trial’ and
(trauma or wound or injur*)
A date limit of 15 years was set. This was chosen pragmatically, based on advice from Professor Lecky (see also Contact with Dr Janette Turner, below), to try to maintain ecological validity with current ambulance practice. A date limit of less than 15 years would have missed important pre-hospital trials conducted in the UK. A date limit of greater than 15 years would mean that practices and availability of resources may have been very different from those today, making associations between older and contemporary trials meaningless.
Following the conduct of the above searches, eligibility criteria (Box 1) were applied to the papers identified. These were chosen so that the scope of the trials included in the review would be focused on the pre-hospital care of those with traumatic injuries by land-based ambulance services. It was predicted that this would give a homogenous group of studies from which meaningful comparisons and conclusions could be drawn. The eligibility criteria were first applied to the titles of the papers, then the abstracts and finally the full reports.
Conducted, at least partly, in the pre-hospital environment.
Controlled trial (randomised or cluster).
Over 50% of participants had traumatic injuries.
Involved, at least partly, land-based ambulance services (i.e. no helicopter-only studies).
Actual report of trial conduct, not just protocol (although protocols identified were used to find the actual report of the trial if already published).
The trials finally selected for inclusion in the report were reviewed in terms of five criteria (Table 27) highlighted in previous reports as being key to the success of the conduct of controlled trials,132 and of pre-hospital trials in particular. 128 As this review was concerned with the success of the conduct of the trials, rather than with the efficacy of the health-care interventions they investigated, no assessment, in terms of the success of the interventions under investigation, was made. All trials were reviewed by the author using the criteria specified in Table 4 and the preliminary results were reviewed by Professor Lecky in terms of adequacy and consistency.
| Criteria | Explanation |
|---|---|
| Recruitment | The actual recruitment achieved during the trial compared to the recruitment numbers specified by any power calculations undertaken, both overall and in different arms of the trial |
| Proportion of relevant participants | If participants were recruited based on assessment undertaken in the pre-hospital environment (e.g. GCS) designed to identify injuries which cannot be diagnosed before admission to hospital (e.g. TBI), what was the proportion of relevant participants entered into the study (e.g. TBI) vs. non-relevant (e.g. intoxicated) |
| Compliance with trial protocols | What was the proportion of compliance by paramedics and other pre-hospital health-care personnel with the study protocols |
| Selection bias created by non-compliance | Where non-compliance existed, was it identified whether this led to patients with similar characteristics (e.g. male, elderly) not being recruited into the study |
| Acceptability | What was the level of acceptability of control and experimental interventions to patients, families and staff |
Contact with Dr Janette Turner, University of Sheffield
from: Nathan Chapman
to: Janette Kay Turner
date: Fri, Mar 29, 2013 at 2:00 PM
subject: Re: Student of Fiona Lecky – request for advice regarding lit review of pre-hospital RCTs
Hi Janette, I hope you’re well. I have now completed the literature search for my systematic review of pre-hospital controlled trials. I refined my research question following a preliminary review of the literature, and decided to focus on pre-hospital controlled trials regarding trauma published in the last 15-years. The 15-year limit was chosen pragmatically to include important pre-hospital trials conducted in the UK in the late 90s early 00s, but not to go so far back as to lose relevance with up-to-date pre-hospital practice. I searched for the following terms in titles or abstracts:
-
(pre-hospital or pre-hospital or ‘out of hospital’ or ambulance or paramedic or ‘EMSs’ or EMS) and (trauma* or wound* or injury or injuries) and ‘controlled trial’
The eligibility criteria were as follows:
-
Conducted, at least in part) in the pre-hospital environment
-
Controlled trial (randomised or cluster)
-
At least 50% of participants suffering trauma
-
Involved, at least in part, land based ambulance services (i.e. not helicopter EMS only studies)
-
Actual report of trial conduct and results (not just trial protocol)
I also kept any systematic reviews and protocols I identified during the search to identify furthers controlled trials. I identified 15 articles in medline, an additional 2 in Cochrane and no additional articles in EMBASE. Then, using the systematic review and protocols I had found, as well as the document you sent me, I identified 2 further articles, giving me a total of 19. I was wondering if when you have time you could have a quick look over this list and let me know if you think I have missed anything important? Please only do this if you have time though, I know you must be busy. Best wishes, Nathan.
from: Janette Kay Turner
to: Nathan Chapman
date: Fri, Apr 5, 2013 at 4:28 PM
subject: Re: Student of Fiona Lecky – request for advice regarding lit review of pre-hospital RCTs
Hi Nathan I’ve had a scan and i can’t think of anything to be added now you have narrowed it down to trauma. There were a few I thought of but when I checked they were old studies outside your 15-year period. Your search strategy is good and by looking and checking the systematic reviews you should have captured everything. Bit of a sad scenario isn’t it! Let me know if you need any more help. Janette
Critical appraisal tables
Pre-hospital controlled trials conducted in the UK NHS from 1998 to 2013
| Author/publication year/study title | Study description and feasibility assessment | Feasibility findings |
|---|---|---|
| Turner (2000)133 A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias due to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
| Author/publication year/study title | Study description and feasibility assessment | Feasibility findings |
|---|---|---|
| Woollard (2004)134 Less IS less: a randomised controlled trial comparing cautious and rapid nalbuphine dosing regimens | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias due to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
| Author/publication year/study title | Study description and feasibility assessment | Feasibility findings |
|---|---|---|
| Snooks (2004)135 Results of an evaluation of the effectiveness of triage and direct transportation to minor injuries units by ambulance crews | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias due to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
|
| Mason (2007)100 Effectiveness of paramedic practitioners in attending 999 calls from elderly people in the community: cluster randomised controlled trial | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias related to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
Pre-hospital controlled trials conducted outside the UK NHS from 1998 to 2013
| Trial | Feasibility outcomes appraisal | |
|---|---|---|
| Outcome | Appraisal | |
| Cooper (2004)141 Prehospital hypertonic saline resuscitation of patients with hypotension and severe TBI: a randomized controlled trial | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias due to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
|
| Baker (2009)140 Resuscitation with hypertonic saline-dextran reduces serum biomarker levels and correlates with outcome in severe TBI patients | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias related to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
|
| Bulger (2010)136 Out-of-hospital hypertonic resuscitation following severe TBI: a randomized controlled trial | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias due to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
|
| Morrison (2011)128 The Toronto prehospital hypertonic resuscitation-head injury and multiorgan dysfunction trial: feasibility study of a randomized controlled trial | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias due to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
|
| Author/publication year/study title | Study description and feasibility assessment | Feasibility findings |
|---|---|---|
| Bernard (2010)37 Prehospital rapid sequence intubation improves functional outcome for patients with severe TBI: a randomized controlled trial | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias due to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
| Author/publication year/study title | Study description and feasibility assessment | Feasibility findings |
|---|---|---|
| Bulger (2008)137 Hypertonic resuscitation of hypovolemic shock after blunt trauma: a randomized controlled trial | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias due to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
|
| Moore et al. (2009)138 Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicentre trial | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias related to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
|
| Bulger (2011)139 Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias related to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
| Author/publication year/study title | Study description and feasibility assessment | Feasibility findings |
|---|---|---|
| Kober (2002)144 A randomised controlled trial of oxygen for reducing nausea and vomiting during emergency transport of patients older than 60 years with minor trauma | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias related to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
| Author/publication year/study title | Study description and feasibility assessment | Feasibility findings |
|---|---|---|
| Schiferer (2007)143 A randomized controlled trial of femoral nerve blockade administered preclinically for pain relief in femoral trauma | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias related to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
|
| Jennings (2012)142 Morphine and ketamine is superior to morphine alone for out-of-hospital trauma analgesia: a randomized controlled trial | Study design and outcomes |
|
| Actual vs. required recruitment |
|
|
| Proportion of relevant participants |
|
|
| Compliance with trial protocols |
|
|
| Selection bias due to non-compliance |
|
|
| Acceptability |
|
|
| Barriers identified |
|
Appendix 2 HITS-NS recruitment standard operating procedure (v2)
Appendix 3 HITS-NS consent process
Appendix 4 HITS-NS promotional and training materials
Appendix 5 Further trials standard operating procedures
Appendix 6 HITS-NS study NEAS/NWAS protocols v5/v61
Appendix 7 Chapter 4 (health-economic) literature reviews and additional analyses
Cost-effectiveness model sensitivity analyses
Parameter uncertainty sensitivity analyses
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Bypass: best case | Bypass | 25,996 | 13.28 | 239,530 | 7530 | 1.00 | 0.00 | 372,293 | 10,733 | 1.00 | 0.00 |
| Selective transfer | 27,119 | 12.96 | 232,000 | 0 | 0.00 | 1.00 | 361,560 | 0 | 0.00 | 1.00 | |
| Routine transfer | 27,270 | 13.02 | 233,033 | 1033 | 0.00 | 1.00 | 363,185 | 1625 | 0.00 | 1.00 | |
| No transfer | 26,876 | 12.68 | 226,818 | –5182 | 0.00 | 1.00 | 353,664 | –7896 | 0.00 | 1.00 | |
| Bypass: worst case | Bypass (observed) | 31,862 | 12.94 | 226,962 | –4744 | 0.00 | 1.00 | 356,374 | –4656 | 0.00 | 1.00 |
| Selective transfer | 26,942 | 12.93 | 231,706 | 0 | 0.22 | 0.78 | 361,030 | 0 | 0.20 | 0.80 | |
| Routine transfer | 27,094 | 12.99 | 232,746 | 1040 | 0.77 | 0.23 | 362,666 | 1636 | 0.79 | 0.21 | |
| No transfer | 26,714 | 12.66 | 226,484 | –5222 | 0.01 | 0.99 | 353,083 | –7947 | 0.01 | 0.99 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Bypass: threshold analysis | Bypass | 28,458 | 13.14 | 234,419 | 2174 | 0.75 | 0.25 | 365,857 | 4100 | 0.85 | 0.16 |
| Selective transfer | 26,779 | 12.95 | 232,245 | 0 | 0.00 | 1.00 | 361,757 | 0 | 0.00 | 1.00 | |
| Routine transfer | 26,927 | 13.01 | 233,334 | 1089 | 0.22 | 0.78 | 363,465 | 1708 | 0.15 | 0.85 | |
| No transfer | 26,510 | 12.68 | 227,103 | –5142 | 0.00 | 1.00 | 353,909 | –7848 | 0.00 | 1.00 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Utility estimates (Aoki et al.77) | Bypass | 29,045 | 12.74 | 225,709 | 433 | 0.43 | 0.57 | 353,086 | 1670 | 0.48 | 0.52 |
| Selective transfer | 27,005 | 12.61 | 225,275 | 0 | 0.12 | 0.88 | 351,416 | 0 | 0.09 | 0.91 | |
| Routine transfer | 27,160 | 12.67 | 226,287 | 1012 | 0.43 | 0.57 | 353,011 | 1595 | 0.41 | 0.59 | |
| No transfer | 26,774 | 12.32 | 219,718 | –5557 | 0.01 | 0.99 | 342,964 | –8452 | 0.01 | 0.99 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Alternative acute neurosurgery baseline outcomes (Taussky et al.76) | Bypass | 29,621 | 13.02 | 230,684 | 750 | 0.45 | 0.55 | 360,837 | 2256 | 0.52 | 0.48 |
| Selective transfer | 27,361 | 12.86 | 229,934 | 0 | 0.09 | 0.91 | 358,581 | 0 | 0.07 | 0.93 | |
| Routine transfer | 27,492 | 12.93 | 231,011 | 1077 | 0.44 | 0.56 | 360,262 | 1681 | 0.4 | 0.6 | |
| No transfer | 27,105 | 12.59 | 224,769 | –5165 | 0.01 | 0.99 | 350,707 | –7874 | 0.01 | 0.99 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Neurosurgery (transfer costs only) | Bypass | 27,322 | 13.09 | 234,520 | 2957 | 0.62 | 0.38 | 365,441 | 4575 | 0.65 | 0.35 |
| Selective transfer | 27,043 | 12.93 | 231,563 | 0 | 0.05 | 0.95 | 360,866 | 0 | 0.04 | 0.96 | |
| Routine transfer | 27,170 | 12.99 | 232,693 | 1130 | 0.31 | 0.69 | 362,624 | 1758 | 0.31 | 0.69 | |
| No transfer | 26,856 | 12.66 | 226,342 | –5221 | 0.01 | 0.99 | 352,940 | –7926 | 0 | 1 | |
| Neurosurgery (NHIR incremental costs) | Bypass | 27,865 | 13.1 | 234,225 | 2417 | 0.58 | 0.42 | 365,270 | 4046 | 0.62 | 0.38 |
| Selective transfer | 27,024 | 12.94 | 231,808 | 0 | 0.07 | 0.93 | 361,224 | 0 | 0.06 | 0.94 | |
| Routine transfer | 27,173 | 13 | 232,855 | 1047 | 0.33 | 0.67 | 362,868 | 1644 | 0.32 | 0.68 | |
| No transfer | 26,779 | 12.68 | 226,728 | –5080 | 0.01 | 0.99 | 353,481 | –7743 | 0.01 | 0.99 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Mean incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Mean incremental NMB (£) | Probability cost-effective | Error | ||||
| Bypass incremental costs (expert opinion) | Bypass | 27,175 | 13.06 | 234,065 | 2611 | 0.60 | 0.40 | 364,684 | 3926 | 0.61 | 0.39 |
| Selective transfer | 27,156 | 12.93 | 231,453 | 0 | 0.06 | 0.94 | 360,758 | 0 | 0.05 | 0.95 | |
| Routine transfer | 27,278 | 12.99 | 232,579 | 1126 | 0.34 | 0.66 | 362,508 | 1749 | 0.34 | 0.66 | |
| No transfer | 26,920 | 12.66 | 226,261 | –5192 | 0.01 | 0.99 | 352,852 | –7906 | 0.01 | 0.99 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Mean incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Mean incremental NMB (£) | Probability cost-effective | Error | ||||
| Elicited or for extracranial injury | Bypass | 28,906 | 13.09 | 232,902 | 651 | 0.42 | 0.58 | 363,805 | 2008 | 0.48 | 0.52 |
| Selective transfer | 26,843 | 12.95 | 232,251 | 0 | 0.10 | 0.90 | 361,798 | 0 | 0.07 | 0.93 | |
| Routine transfer | 26,984 | 13.02 | 233,320 | 1069 | 0.46 | 0.54 | 363,472 | 1674 | 0.44 | 0.56 | |
| No transfer | 26,583 | 12.68 | 226,994 | –5257 | 0.01 | 0.99 | 353,782 | –8015 | 0.01 | 0.99 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Population subgroups: NEAS estimate | Bypass | 27,762 | 13.25 | 237,291 | 567 | 0.43 | 0.57 | 369,818 | 1557 | 0.47 | 0.53 |
| Selective transfer | 26,351 | 13.15 | 236,724 | 0 | 0.1 | 0.9 | 368,261 | 0 | 0.08 | 0.92 | |
| Routine transfer | 26,527 | 13.22 | 237,778 | 1054 | 0.45 | 0.55 | 369,930 | 1669 | 0.44 | 0.56 | |
| No transfer | 26,127 | 12.89 | 231,585 | –5139 | 0.01 | 0.99 | 360,441 | –7820 | 0.01 | 0.99 | |
| Population subgroups: NWAS estimate | Bypass | 36,373 | 12.29 | 209,535 | 4315 | 0.61 | 0.39 | 332,454 | 9147 | 0.7 | 0.3 |
| Selective secondary transfer | 30,954 | 11.81 | 205,220 | 0 | 0.06 | 0.94 | 323,307 | 0 | 0.04 | 0.96 | |
| Routine transfer | 30,843 | 11.88 | 206,688 | 1468 | 0.3 | 0.7 | 325,453 | 2146 | 0.26 | 0.74 | |
| No transfer | 30,699 | 11.5 | 199,237 | –5983 | 0 | 1 | 314,205 | –9102 | 0 | 1 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Post-discharge costsa | Bypass | 14,892 | 13.07 | 246,533 | 324 | 0.43 | 0.57 | 377,246 | 1666 | 0.48 | 0.52 |
| Selective transfer | 12,532 | 12.94 | 246,209 | 0 | 0.12 | 0.88 | 375,580 | 0 | 0.09 | 0.91 | |
| Routine transfer | 12,935 | 13.00 | 247,008 | 799 | 0.43 | 0.57 | 376,980 | 1400 | 0.41 | 0.59 | |
| No transfer | 11,701 | 12.67 | 241,612 | –4598 | 0.01 | 0.99 | 368,268 | –7312 | 0.01 | 0.99 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Decreased life expectancy | Bypass | 25,693 | 11.49 | 204,187 | 1372 | 0.51 | 0.49 | 319,127 | 2794 | 0.56 | 0.44 |
| Selective transfer | 24,222 | 11.35 | 202,815 | 0 | 0.08 | 0.92 | 316,333 | 0 | 0.06 | 0.94 | |
| Routine transfer | 24,398 | 11.41 | 203,742 | 927 | 0.39 | 0.61 | 317,812 | 1479 | 0.37 | 0.63 | |
| No transfer | 23,913 | 11.12 | 198,491 | –4324 | 0.01 | 0.99 | 309,692 | –6641 | 0.01 | 0.99 | |
Structural sensitivity analyses
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Modifying the proportional odds assumptiona | Bypass | 29,027 | 13.05 | 231,986 | 648 | 0.48 | 0.52 | 362,493 | 1975 | 0.55 | 0.45 |
| Selective transfer | 27,020 | 12.92 | 231,338 | 0 | 0.12 | 0.88 | 360,517 | 0 | 0.09 | 0.91 | |
| Routine transfer | 27,044 | 12.96 | 232,107 | 769 | 0.38 | 0.62 | 361,683 | 1166 | 0.36 | 0.64 | |
| No transfer | 28,366 | 12.68 | 225,294 | –6044 | 0.01 | 0.99 | 352,123 | –8394 | 0.01 | 1.00 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Discount rate 1.5% | Bypass (observed) | 35,088 | 17.06 | 306,172 | 2476 | 0.55 | 0.45 | 476,803 | 4561 | 0.59 | 0.41 |
| Selective transfer | 33,397 | 16.85 | 303,696 | 0 | 0.06 | 0.94 | 472,242 | 0 | 0.05 | 0.95 | |
| Routine transfer | 33,416 | 16.93 | 305,275 | 1579 | 0.37 | 0.63 | 474,620 | 2378 | 0.36 | 0.64 | |
| No transfer | 33,368 | 16.50 | 296,614 | –7082 | 0.01 | 0.99 | 461,606 | –10,636 | 0.00 | 1.00 | |
| Discount rate 6.0% | Bypass | 24,231 | 9.97 | 175,161 | 283 | 0.42 | 0.58 | 274,857 | 1513 | 0.48 | 0.52 |
| Selective transfer | 22,054 | 9.85 | 174,878 | 0 | 0.12 | 0.88 | 273,344 | 0 | 0.09 | 0.91 | |
| Routine transfer | 22,270 | 9.89 | 175,614 | 736 | 0.45 | 0.56 | 274,557 | 1213 | 0.42 | 0.58 | |
| No transfer | 21,659 | 9.64 | 171,195 | –3683 | 0.01 | 0.99 | 267,622 | –5722 | 0.01 | 0.99 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Alternative comparators | Bypass | 29,591 | 13.12 | 232,889 | 1466 | 0.49 | 0.51 | 364,129 | 3432 | 0.58 | 0.42 |
| Bypass: 100% compliance | Selective transfer | 27,126 | 12.93 | 231,423 | 0 | 0.09 | 0.91 | 360,697 | 0 | 0.06 | 0.94 |
| No transfer: no neurosurgical transfers | Routine transfer | 27,265 | 12.99 | 232,543 | 1120 | 0.40 | 0.60 | 362,447 | 1750 | 0.35 | 0.65 |
| No transfer | 30,333 | 12.55 | 220,607 | –10,816 | 0.00 | 1.00 | 346,076 | –14,621 | 0.00 | 1.00 | |
| Sensitivity analysis | Strategy | Mean cost (£) | Mean QALY | λ = £20,000 | λ = £30,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | Mean NMB (£) | Incremental NMB (£) | Probability cost-effective | Error | ||||
| Utility decrement for bypassing mild TBI cases | Bypass | 28,934 | 13.04 | 231,955 | 509 | 0.42 | 0.58 | 362,399 | 1664 | 0.48 | 0.52 |
| Selective transfer | 27,133 | 12.93 | 231,446 | 0 | 0.10 | 0.90 | 360,735 | 0 | 0.07 | 0.93 | |
| Routine transfer | 27,259 | 12.99 | 232,557 | 1111 | 0.46 | 0.54 | 362,465 | 1730 | 0.44 | 0.56 | |
| No transfer | 26,904 | 12.65 | 226,188 | –5257 | 0.01 | 0.99 | 352,735 | –8001 | 0.01 | 0.99 | |
Expected value of perfect information
| Assumption | Individual EVPI (£) (λ =) | Population EVPI (£) (λ =) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £40,000 | £50,000 | £0 | £10,000 | £20,000 | £30,000 | £40,000 | £50,000 | |
| Base case | 445 | 986 | 1807 | 2594 | 2927 | 3292 | 8,756,236 | 19,416,946 | 35,589,507 | 51,079,936 | 57,648,281 | 64,824,398 |
| Optimistic scenario | 445 | 986 | 1807 | 2594 | 2927 | 3292 | 17,366,923 | 38,511,136 | 70,587,431 | 101,310,801 | 114,338,310 | 128,571,260 |
| Pessimistic scenario | 445 | 986 | 1807 | 2594 | 2927 | 3292 | 2,674,605 | 5,930,934 | 10,870,866 | 15,602,439 | 17,608,750 | 19,800,705 |
Expected value of partial perfect information
| Parameter category | Relevant model inputs | Input type | Population EVPPI (£) at λ = | |||||
|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £40,000 | £50,000 | |||
| Population subgroups | Mild TBI | Proportion | 0 | 0 | 0 | 1 | 0 | 0 |
| Acute neurosurgery | Proportion | 0 | 0 | 18 | 260 | 150 | 106 | |
| Head injury requiring critical care | Proportion | 0 | 0 | 0 | 172 | 64 | 29 | |
| Head injury requiring ward care | Proportion | 0 | 0 | 0 | 28 | 0 | 0 | |
| Major extracranial injury | Proportion | 0 | 0 | 3 | 42 | 0 | 0 | |
| Relative effectiveness outcomes (vs. selective transfer) | Acute neurosurgery: SNC care | Proportional odds ratio | 0 | 20 | 302 | 728 | 654 | 623 |
| TBI requiring critical care: bypass | Proportional odds ratio | 15 | 511 | 1322 | 2133 | 2388 | 2664 | |
| TBI requiring critical care: routine transfer | Proportional odds ratio | 39 | 16 | 250 | 616 | 499 | 435 | |
| TBI requiring critical care: no transfer | Proportional odds ratio | 0 | 25 | 21 | 41 | 13 | 11 | |
| TBI requiring ward care: bypass | Proportional odds ratio | 0 | 0 | 1 | 32 | 0 | 0 | |
| Major extracranial injury: bypass | Odds ratio | 0 | 0 | 0 | 4 | 0 | 0 | |
| Inpatient costs (vs. selective transfer) | Mild TBI: bypass | Incremental cost | 0 | 0 | 0 | 104 | 33 | 28 |
| Acute neurosurgery: SNC care | Incremental cost | 3 | 40 | 161 | 340 | 114 | 27 | |
| TBI requiring critical care: bypass | Incremental cost | 74 | 251 | 472 | 694 | 424 | 241 | |
| TBI requiring critical care: routine transfer | Incremental cost | 0 | 0 | 0 | 1 | 0 | 0 | |
| TBI requiring critical care: no transfer | Incremental cost | 1 | 0 | 0 | 0 | 0 | 0 | |
| TBI requiring ward care: bypass | Incremental cost | 0 | 0 | 0 | 36 | 0 | 0 | |
| Major extracranial injury: bypass | Incremental cost | 0 | 0 | 2 | 78 | 1 | 0 | |
| First-year post-discharge costs | GOS severe disability | Mean cost | 0 | 0 | 0 | 18 | 0 | 0 |
| GOS moderate disability | Mean cost | 0 | 0 | 0 | 1 | 0 | 0 | |
| GOS good recovery | Mean cost | 0 | 0 | 1 | 4 | 0 | 0 | |
| EC injury survivors | Mean cost | 0 | 0 | 0 | 0 | 0 | 0 | |
| Long-term costs | GOS severe disability | Mean cost | 43 | 0 | 0 | 2 | 0 | 0 |
| GOS moderate disability | Mean cost | 0 | 0 | 0 | 2 | 0 | 0 | |
| GOS good recovery | Mean cost | 0 | 0 | 8 | 73 | 0 | 0 | |
| EC injury survivors | Mean cost | 0 | 0 | 0 | 22 | 0 | 0 | |
| Utilities | GOS severe disability | Mean utility | 0 | 0 | 0 | 31 | 0 | 0 |
| GOS moderate disability | Mean utility | 0 | 0 | 0 | 1 | 0 | 0 | |
| GOS good recovery | Mean utility | 0 | 0 | 0 | 40 | 0 | 0 | |
| EC injury survivors | Mean utility | 0 | 0 | 0 | 8 | 0 | 0 | |
| Parameter category | Relevant model inputs | Input type | Population EVPPI (£) at λ = | |||||
|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £40,000 | £50,000 | |||
| Population subgroups | Mild TBI | Proportion | 0 | 0 | 0 | 20,744 | 0 | 0 |
| Acute neurosurgery | Proportion | 0 | 0 | 347,385 | 5,125,420 | 2,945,377 | 2,094,278 | |
| Head injury requiring critical care | Proportion | 4551 | 0 | 78 | 3,395,577 | 1,265,634 | 573,440 | |
| Head injury requiring ward care | Proportion | 0 | 0 | 0 | 545,185 | 0 | 0 | |
| Major extracranial injury | Proportion | 0 | 7741 | 52,908 | 820,492 | 0 | 0 | |
| Relative effectiveness outcomes (vs. selective transfer) | Acute neurosurgery: SNC care | Proportional odds ratio | 0 | 387,949 | 5,950,242 | 14,341,036 | 12,887,713 | 12,273,609 |
| TBI requiring critical care: bypass | Proportional odds ratio | 291,628 | 10,053,152 | 26,030,719 | 42,001,498 | 47,032,039 | 52,453,233 | |
| TBI requiring critical care: routine transfer | Proportional odds ratio | 770,554 | 320,195 | 4,918,315 | 12,131,555 | 9,828,163 | 8,565,781 | |
| TBI requiring critical care: no transfer | Proportional odds ratio | 0 | 496,243 | 407,628 | 808,166 | 254,226 | 212,872 | |
| TBI requiring ward care: bypass | Proportional odds ratio | 0 | 0 | 13,981 | 622,469 | 1969 | 0 | |
| Major extracranial injury: bypass | Odds ratio | 0 | 0 | 0 | 70,892 | 0 | 0 | |
| Inpatient costs (vs. selective transfer) | Mild TBI: bypass | Incremental cost | 0 | 0 | 0 | 2,042,851 | 643,573 | 553,572 |
| Acute neurosurgery: SNC care | Incremental cost | 58,426 | 784,175 | 3,173,639 | 6,698,113 | 2,252,809 | 525,606 | |
| TBI requiring critical care: Bypass | Incremental cost | 1,465,843 | 4,941,885 | 9,303,495 | 13,673,458 | 8,343,931 | 4,750,486 | |
| TBI requiring critical care: routine transfer | Incremental cost | 0 | 0 | 0 | 10,129 | 0 | 0 | |
| TBI requiring critical care: no transfer | Incremental cost | 12,809 | 0 | 0 | 0 | 0 | 0 | |
| TBI requiring ward care: bypass | Incremental cost | 0 | 0 | 0 | 709,539 | 807 | 0 | |
| Major extracranial injury: bypass | Incremental cost | 0 | 0 | 45,775 | 1,539,086 | 12,438 | 0 | |
| First-year post-discharge costs | GOS severe disability | Mean cost | 0 | 0 | 0 | 363,534 | 0 | 0 |
| GOS moderate disability | Mean cost | 4 | 0 | 0 | 28,111 | 0 | 0 | |
| GOS good recovery | Mean cost | 0 | 0 | 15,928 | 69,894 | 0 | 0 | |
| EC injury survivors | Mean cost | 0 | 0 | 0 | 0 | 0 | 0 | |
| Long-term costs | GOS severe disability | Mean cost | 839,124 | 0 | 0 | 44,478 | 0 | 0 |
| GOS moderate disability | Mean cost | 51 | 0 | 0 | 47,796 | 0 | 0 | |
| GOS good recovery | Mean cost | 0 | 0 | 152,706 | 1,440,957 | 0 | 0 | |
| EC injury survivors | Mean cost | 0 | 0 | 0 | 437,994 | 0 | 0 | |
| Utilities | GOS severe disability | Mean utility | 0 | 0 | 0 | 608,237 | 0 | 0 |
| GOS moderate disability | Mean utility | 0 | 0 | 0 | 11,555 | 0 | 0 | |
| GOS good recovery | Mean utility | 0 | 0 | 0 | 787,818 | 0 | 0 | |
| EC injury survivors | Mean utility | 0 | 0 | 0 | 153,447 | 0 | 0 | |
| Model parameter | Optimistic scenario | Pessimistic scenario | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population EVPPI (λ = ) | Population EVPPI (λ =) | |||||||||||
| £0 | £10,000 | £20,000 | £30,000 | £40,000 | £50,000 | £0 | £10,000 | £20,000 | £30,000 | £40,000 | £50,000 | |
| Patient subgroups | 818 | 0 | 1,714,159 | 12,370,184 | 7,986,953 | 6,158,436 | 126 | 0 | 264,005 | 1,905,187 | 1,230,106 | 948,488 |
| TBI utilities | 0 | 7443 | 558,526 | 4,421,639 | 1,291,890 | 716,864 | 0 | 1146 | 86,021 | 680,996 | 198,970 | 110,407 |
| Post-discharge costs | 1,755,955 | 11,120 | 751,228 | 5,392,692 | 1,024,809 | 490,302 | 270,443 | 1713 | 115,700 | 830,553 | 157,835 | 75,514 |
| Bypass: relative effectivenessa | 812,668 | 21,724,345 | 54,793,856 | 87,830,169 | 99,243,879 | 111,251,485 | 125,163 | 3,345,863 | 8,439,046 | 13,527,116 | 15,284,993 | 17,134,338 |
| Bypass: inpatient costsa | 4,639,278 | 12,995,632 | 22,287,924 | 31,067,488 | 20,106,639 | 12,487,650 | 714,516 | 2,001,515 | 3,432,663 | 4,784,843 | 3,096,713 | 1,923,279 |
Expected net benefit of sampling
Expected net benefit of sampling under optimistic assumptions for recruitment and technology lifespan
| Sample size | Number of clusters | Number of ASs | Total trial duration (years)a | Trial costs (£) | λ = £20,000 | λ = £30,000 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Individual EVSI (£) | Population EVSI (£) | ENBS (£) | Individual EVSI (£) | Population EVSI (£) | ENBS (£) | |||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 103 | 29 | 1 | 2 | 51,300 | 240 | 7,811,733 | 7,760,433 | 644 | 20,966,309 | 20,915,009 |
| 205 | 57 | 1 | 2 | 102,600 | 420 | 13,660,193 | 13,557,593 | 915 | 29,776,163 | 29,673,563 |
| 257 | 71 | 1 | 2 | 128,250 | 535 | 17,413,016 | 17,284,766 | 990 | 32,221,950 | 32,093,700 |
| 513 | 143 | 2 | 2 | 256,500 | 741 | 24,130,840 | 23,874,340 | 1310 | 42,638,364 | 42,381,864 |
| 616 | 171 | 3 | 2 | 307,800 | 837 | 27,252,741 | 26,944,941 | 1338 | 43,542,955 | 43,235,155 |
| 821 | 228 | 4 | 2 | 410,400 | 882 | 28,691,377 | 28,280,977 | 1510 | 49,134,396 | 48,723,996 |
| 1026 | 285 | 5 | 2 | 513,000 | 919 | 29,922,343 | 29,409,343 | 1583 | 51,514,712 | 51,001,712 |
| 1231 | 342 | 6 | 2 | 615,600 | 976 | 31,753,108 | 31,137,508 | 1694 | 55,125,459 | 54,509,859 |
| 1642 | 456 | 8 | 2 | 820,800 | 1032 | 33,594,604 | 32,773,804 | 1791 | 58,271,886 | 57,451,086 |
| 2052 | 480 | 10 | 2 | 1,026,000 | 1104 | 35,924,767 | 34,898,767 | 1869 | 60,825,699 | 59,799,699 |
| 3078 | 480 | 10 | 3 | 1,539,000 | 1174 | 34,465,433 | 32,926,433 | 1975 | 57,983,380 | 56,444,380 |
| 4104 | 480 | 10 | 3 | 2,052,000 | 1228 | 36,051,502 | 33,999,502 | 2001 | 58,749,093 | 56,697,093 |
| 5130 | 480 | 10 | 4 | 2,565,000 | 1264 | 33,144,144 | 30,579,144 | 2067 | 54,194,420 | 51,629,420 |
| 8208 | 480 | 10 | 5 | 4,104,000 | 1315 | 30,432,323 | 26,328,323 | 2130 | 49,280,849 | 45,176,849 |
| 10,260 | 480 | 10 | 6 | 5,130,000 | 1337 | 26,859,074 | 21,729,074 | 2151 | 43,205,532 | 38,075,532 |
FIGURE 28.
Expected net benefit of sampling for a definitive HITS-NS cluster randomised trial at λ = £20,000 (optimistic assumptions).

FIGURE 29.
Expected net benefit of sampling for a definitive HITS-NS cluster randomised trial at λ = £30,000 (optimistic assumptions).

Expected net benefit of sampling under pessimistic assumptions for recruitment and technology lifespan
| Sample size | Number of clusters | Number of ASs | Total trial duration (years)a | Trial costs (£) | λ = £20,000 | λ = £30,000 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Individual EVSI (£) | Population EVSI (£) | ENBS (£) | Individual EVSI (£) | Population EVSI (£) | ENBS (£) | |||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 106 | 42 | 2 | 4 | 212,000 | 240 | 638,166 | 426,166 | 644 | 1,712,807 | 1,500,807 |
| 212 | 85 | 3 | 4 | 424,000 | 420 | 1,115,946 | 691,946 | 915 | 2,432,513 | 2,008,513 |
| 265 | 106 | 4 | 4 | 530,000 | 535 | 1,422,527 | 892,527 | 990 | 2,632,318 | 2,102,318 |
| 530 | 140 | 5 | 5 | 1,060,000 | 741 | 1,434,622 | 374,622 | 1310 | 2,534,928 | 1,474,928 |
| 636 | 140 | 5 | 5 | 1,272,000 | 837 | 1,620,225 | 348,225 | 1338 | 2,588,707 | 1,316,707 |
| 848 | 140 | 5 | 6 | 1,696,000 | 882 | 1,103,736 | –592,264 | 1510 | 1,890,164 | 194,164 |
| 1060 | 140 | 5 | 7 | 2,120,000 | 919 | 558,782 | –1,561,218 | 1583 | 962,006 | –1,157,994 |
| 1272 | 140 | 5 | 7 | 2,544,000 | 976 | 592,970 | –1,951,030 | 1694 | 1,029,435 | –1,514,565 |
| 1696 | 140 | 5 | 8 | 3,392,000 | 1032 | 0 | –3,392,000 | 1791 | 0 | –3,392,000 |
| 2120 | 140 | 5 | 10 | 4,240,000 | 1104 | 0 | –4,240,000 | 1869 | 0 | –4,240,000 |
| 3180 | 140 | 5 | 13 | 6,360,000 | 1174 | 0 | –6,360,000 | 1975 | 0 | –6,360,000 |
| 4240 | 140 | 5 | 16 | 8,480,000 | 1228 | 0 | –8,480,000 | 2001 | 0 | –8,480,000 |
| 5300 | 140 | 5 | 19 | 10,600,000 | 1264 | 0 | –10,600,000 | 2067 | 0 | –10,600,000 |
| 8480 | 140 | 5 | 28 | 16,960,000 | 1315 | 0 | –16,960,000 | 2130 | 0 | –16,960,000 |
| 10,600 | 140 | 5 | 34 | 21,200,000 | 1337 | 0 | –21,200,000 | 2151 | 0 | –21,200,000 |
| Sample size | Number of clusters | Number of ASs | Total trial duration (years)a | Trial costs | λ = £20,000 | λ = £30,000 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Individual EVSI (£) | Population EVSI (£) | ENBS (£) | Individual EVSI (£) | Population EVSI (£) | ENBS (£) | |||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 104 | 35 | 1 | 3 | 707,792 | 240 | 3,134,385 | 2,426,592 | 644 | 8,412,535 | 7,704,743 |
| 208 | 69 | 2 | 3 | 897,835 | 420 | 5,481,025 | 4,583,190 | 915 | 11,947,407 | 11,049,573 |
| 260 | 87 | 3 | 3 | 978,146 | 535 | 6,986,810 | 6,008,664 | 990 | 12,928,756 | 11,950,610 |
| 520 | 120 | 4 | 4 | 1,454,508 | 741 | 8,152,339 | 6,697,831 | 1310 | 14,404,903 | 12,950,395 |
| 624 | 120 | 4 | 4 | 1,468,028 | 837 | 9,207,039 | 7,739,011 | 1338 | 14,710,509 | 13,242,481 |
| 832 | 120 | 4 | 5 | 1,775,818 | 882 | 7,935,501 | 6,159,683 | 1510 | 13,589,659 | 11,813,841 |
| 1040 | 120 | 4 | 5 | 1,802,858 | 919 | 8,275,963 | 6,473,105 | 1583 | 14,248,010 | 12,445,152 |
| 1248 | 120 | 4 | 6 | 2,110,648 | 976 | 6,902,976 | 4,792,328 | 1694 | 11,984,014 | 9,873,366 |
| 1664 | 120 | 4 | 7 | 2,445,478 | 1032 | 5,382,213 | 2,936,735 | 1791 | 9,335,775 | 6,890,297 |
| 2080 | 120 | 4 | 8 | 2,780,308 | 1104 | 3,770,655 | 990,347 | 1869 | 6,384,251 | 3,603,943 |
| 3120 | 120 | 4 | 11 | 3,757,758 | 1174 | 0 | –3,757,758 | 1975 | 0 | –3,757,758 |
| 4160 | 120 | 4 | 14 | 4,735,208 | 1228 | 0 | –4,735,208 | 2001 | 0 | –4,735,208 |
| 5200 | 120 | 4 | 17 | 5,712,658 | 1264 | 0 | –5,712,658 | 2067 | 0 | –5,712,658 |
| 8320 | 120 | 4 | 26 | 8,645,008 | 1315 | 0 | –8,645,008 | 2130 | 0 | –8,645,008 |
| 10,400 | 120 | 4 | 31 | 10,319,158 | 1337 | 0 | –10,319,158 | 2151 | 0 | –10,319,158 |
FIGURE 30.
Expected net benefit of sampling for a definitive HITS-NS cluster randomised trial at λ = £20,000 (pessimistic assumptions).
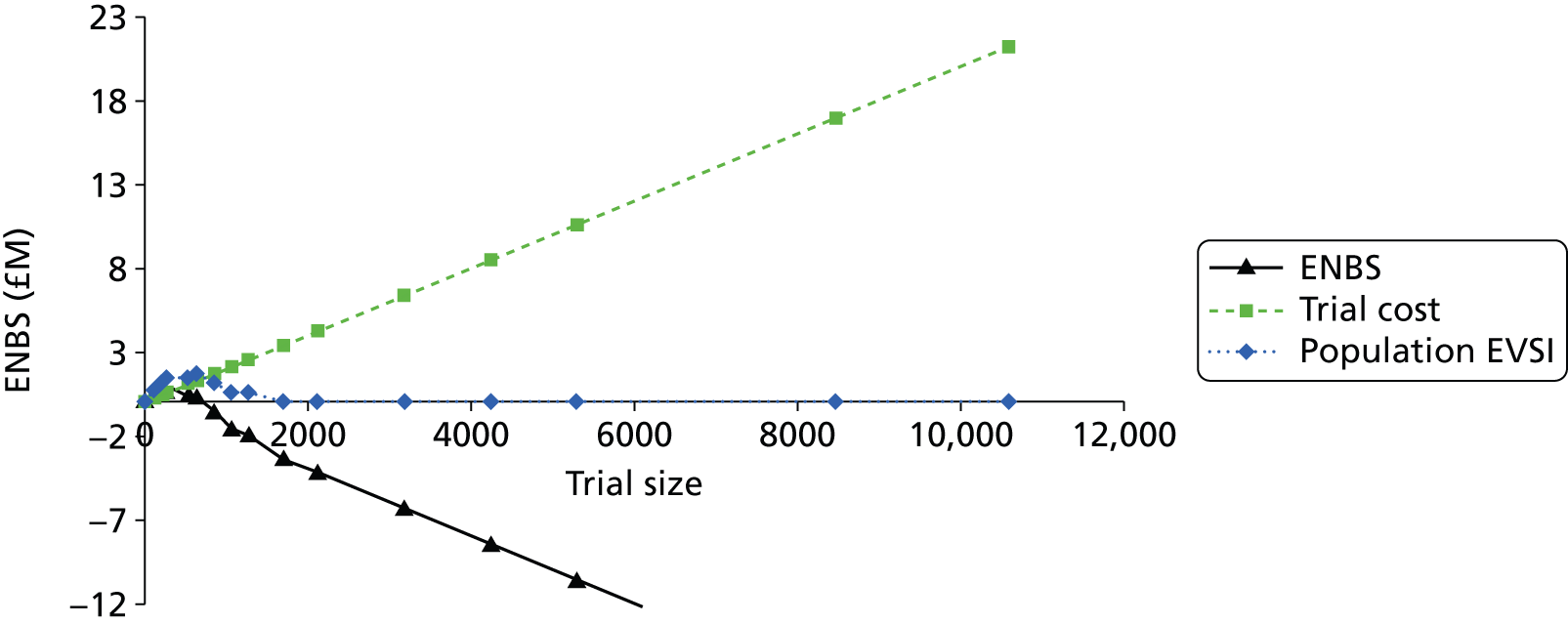
FIGURE 31.
Expected net benefit of sampling for a definitive HITS-NS cluster randomised trial at λ = £30,000 (pessimistic assumptions). Expected net benefit of sampling under assumptions consistent with initially envisaged definitive HITS-NS trial.

FIGURE 32.
Expected net benefit of sampling for a definitive HITS-NS cluster randomised trial at λ = £20,000 (originally planned definitive HITS-NS trial assumptions).
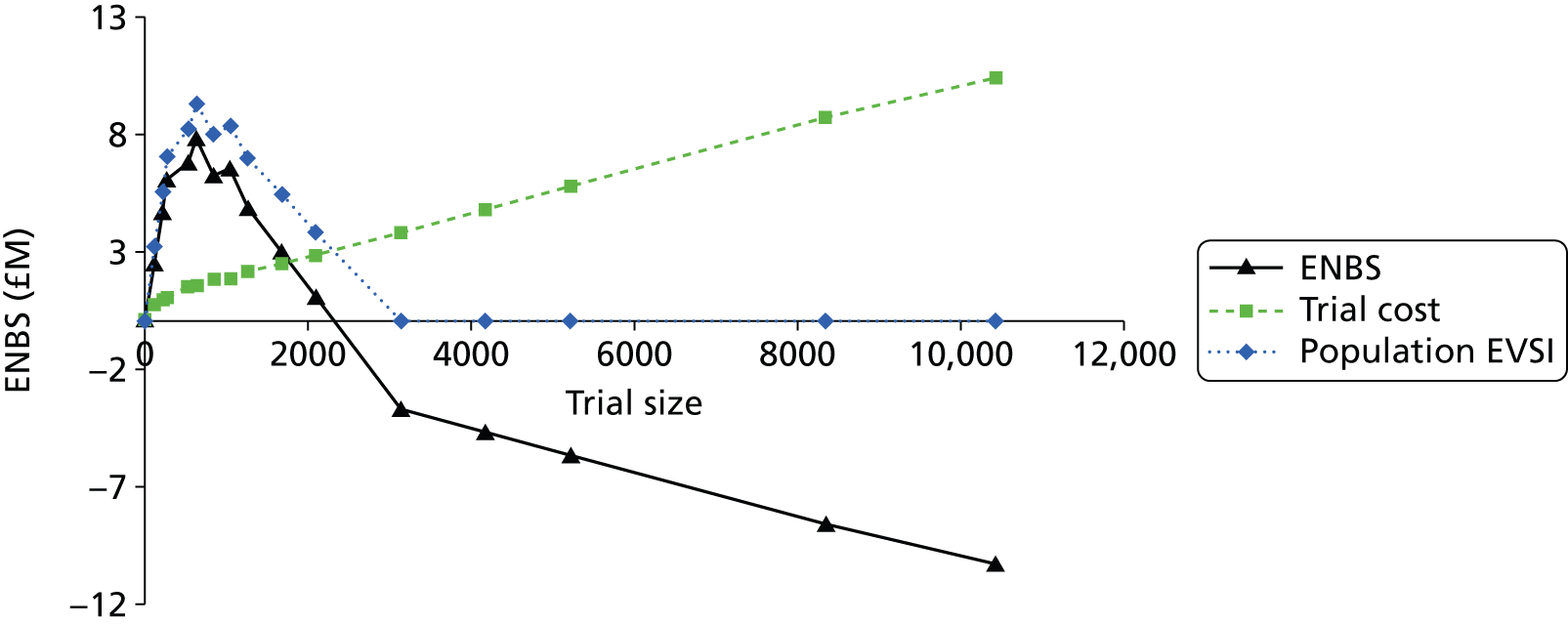
FIGURE 33.
Expected net benefit of sampling for a definitive HITS-NS cluster randomised trial at λ = £30,000 (originally planned definitive HITS-NS trial assumptions).

List of abbreviations
- A&E
- accident and emergency
- ABC
- airway, breathing and circulation
- AIS
- Abbreviated Injury Scale
- AS
- ambulance service
- CI
- confidence interval
- CLRN
- Comprehensive Local Research Network
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- clinical record form
- CT
- computed tomography brain scan
- DH
- Department of Health
- DMEC
- Data Monitoring and Ethics Committee
- ED
- emergency department
- EDCR
- Electronic Data Collection & Reporting
- EDH
- extradural haematoma
- ENBS
- expected net benefit of sampling
- EQ-5D
- European Quality of Life-5 Dimensions
- ETI
- endotracheal intubation
- ETNC
- estimated time to neuroscience centre
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partially perfect information
- EVSI
- expected value of sample information
- GCS
- Glasgow Coma Scale
- GDG
- Guideline Development Group
- GOS
- Glasgow Outcome Score
- GOSE
- Extended Glasgow Outcome Scale
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HITS-NS
- Head Injury Transportation Straight to Neurosurgery
- HTA
- Health Technology Assessment
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ICP
- intracranial pressure
- ICU
- intensive care unit
- IQR
- interquartile range
- ISS
- injury severity score
- JCUH
- James Cook University Hospital
- MCA
- Mental Capacity Act
- MTB
- Major Trauma Bypass
- MTC
- major trauma centre
- NC
- neuroscience centre
- NEAS
- North East Ambulance Service
- NHIR
- Nottingham Head Injury Register
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- NSAH
- non-specialist acute hospital
- NWAS
- North West Ambulance Service
- PI
- principal investigator
- PIC
- participant information centre
- PRF
- patient report form
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RTC
- road traffic collision
- SAE
- serious adverse event
- ScHARR
- School of Health and Related Research
- SD
- standard deviation
- SDH
- subdural haematoma
- SE
- standard error
- SNC
- specialist neuroscience centre
- SOP
- standard operating procedure
- SSI
- site-specific information
- TARN
- Trauma Audit and Research Network
- TBI
- traumatic brain injury
- TMG
- Trial Management Group
- TSG
- Trial Steering Group