Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/142/01. The contractual start date was in June 2012. The draft report began editorial review in August 2013 and was accepted for publication in August 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Madeleine Groom reports grants from Shire Pharmaceuticals and personal fees from Janssen Pharmaceuticals outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Hollis et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Clinical features
Tourette syndrome (TS) is a childhood-onset neurodevelopmental disorder characterised by multiple motor tics and one or more vocal/phonic tics, lasting longer than a year. 1,2 The Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria1 for TS are very similar to those in International Classification of Diseases, Tenth Edition. 2
The mean age at onset of TS is 7 years, with the onset of motor tics usually preceding vocal tics. Tics can be simple (e.g. blinking, eye rolling, nose twitching, head nodding, mouth pouting) or complex (e.g. touching, squatting, jumping, hopping). The onset of simple tics usually precedes more complex tics. Tics usually begin in the head and face, and blinking is often the first and most common tic. Simple vocal tics include sniffing, throat clearing, gulping, snorting and coughing. Complex vocal tics include barking, the making of animal noises and uttering strings of words. Other complex tic phenomena include echolalia (copying what other people say), echopraxia (copying what other people do) and palilalia (repeating the last word or part of sentence said by the individual). 3,4 Self-injurious behaviours5 and non-obscene socially inappropriate behaviours6 occur in one-third of TS patients. Tics typically increase in severity until mid-teens and then decline (but usually do not disappear) in late adolescence and early adult life. Tics follow a fluctuating (waxing and waning) course and may be exacerbated by stress, tiredness or boredom and improved by focused mental and physical activity. Tics are commonly preceded by an uncomfortable premonitory sensation or ‘urge’, either localised (around the area of the tic) or generalised (covering a wide area of the body), often described as an uncomfortable physical sensation and/or build up of tension, with the performance of the tic resulting in relief from the urge. Although involuntary, older children and young people may be able to briefly suppress or postpone a tic at the expense of increased feelings of tension and an urge to complete the tic and/or increased anxiety. 7,8
Coprolalia (inappropriate and involuntary swearing, which is often disguised by the patient) is uncommon, occurring in only 10–15% of patients, often starting at around 15 years of age. Many clinicians, and the wider public, are still under the misapprehension that coprolalia must be present in order to make the diagnosis, thereby leading to under-diagnosis. Instead of uttering a complete swear word, many people say only parts of the word (e.g. ‘Fu’, ‘Shi’, ‘Cu’), and disguise it (e.g. by coughing, saying something or covering their mouths). 9
Epidemiology and prevalence
Tourette syndrome occurs worldwide and in different cultures. Boys and men are more commonly affected, with the male-to-female ratio between three and four to one. Clinical characteristics are similar irrespective of the country of origin. It is often reported that within families, the affected males have predominant tic symptoms, whereas the females have obsessive–compulsive behaviours (OCBs). 10,11
Tourette syndrome was once considered to be very uncommon, but the worldwide prevalence in children and young people is now estimated to be around 1%. Good-quality epidemiological studies (involving direct assessment of the subjects) show remarkably consistent findings and suggest a prevalence of between 0.4% and 3.8% for young people between the ages of 5 and 18 years (studies undertaken in the UK, Europe, USA, South America, the Middle East, Oceana, Asia, North Africa). 10–12 The prevalence of TS is higher in those with learning difficulties, emotional and behavioural disorders or autism spectrum disorder (ASD). 10–12
Comorbidities and coexisting psychopathologies
Robertson9 suggested a differentiation between comorbid disorders and coexisting psychopathology. This was based on emerging clinical, epidemiological and aetiological data suggesting that some disorders have more in common with TS than others, particularly from a genetic perspective. Thus, Robertson9 suggested that OCB and obsessive–compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD) and ASD are comorbid with TS. It is important to distinguish OCB (also known as ‘compulsive tics’ that are common in TS), such as ‘evening-up behaviours’, compulsive touching and counting rituals, from symptoms more typical of OCD such as checking, washing and cleaning rituals. In TS, the performance of OCBs results in a relief from a premonitory ‘urge’ (similar to other tics), while in OCD performance of rituals results in temporary relief from anxiety linked to specific fears (e.g. contamination). For this reason, OCB associated with a premoniraty urge are regarded as a complex tic rather than a symptom of a separate disorder (OCD). Some refer to mild OCB as obsessive–compulsive symptoms (OCSs). 13
Prevalence of the comorbid disorders differ in clinical and community TS populations. In clinical TS samples, ADHD is present in about 60% of patients,7 while OCD occurs in just under 40%7,13 and OCBs/OCSs may occur in up to 90% of patients. 13 The prevalence of ASD in clinic patients with TS has not been widely studied, but in one large international multicentre study encompassing 7288 TS patients, the point prevalence of ASD was 4.6% (much higher than in the general population). 14
In community studies, around 90% of TS individuals have other comorbid diagnoses15–17 and in one community study, 36% of the individuals had three or more diagnoses. 15,16 In TS individuals, ADHD is the most common comorbid diagnosis occurring in 17.8% to 68%, then OCD in 10.9% to 20.5%, and “disruptive behaviours” in 10.9% to 16%. 18 TS occurs in between 6% and 11% of youngsters with ASD in community studies. 7,9
The common neurodevelopmental comorbidities (ADHD, OCB/OCD and ASD) not only occur much more than by chance,19 but the research data also suggest that they share a common genetic aetiology. 19,20 In contrast, while depression and depressive symptomatology are common in TS, the two disorders are not genetically related. 21 Thus, the coexistent psychopathologies (e.g. depression, social anxiety, substance misuse) may well be a consequence of having TS, rather than being an integral part of TS.
Thus, both in clinical populations as well as in the community, approximately only 10% of people with TS have solely tics or, another way of putting it is that 90% of individuals with TS, be they in the clinic or in the community, have other comorbid diagnoses or psychopathology.
Early research suggested that individuals with TS were at increased risk for depression, anxiety and hostility. 22 Thereafter, in several controlled studies in adults with TS, the patients were shown to have more depressive, anxious, obsessional symptomatology and hostility symptoms than age- and sex-matched healthy control subjects, and the subjects with TS were also shown to have significantly more personality disorders. 21,23–26 In further controlled studies, young people with TS have been shown to have more depressive and obsessional symptomatology than control subjects. 27,28
Robertson and Orth29 reviewed the literature of depressive symptoms and illness in people with TS in detail, showing that, in 16 uncontrolled studies in specialist centres that examined mood changes among 5409 TS patients, depressive symptomatology, dysthymia, mood swings and/or major depressive disorder (MDD) or depressive illness were found in between 13% and 76% of the patients . The main diagnosis was that of MDD. In addition, 13 controlled investigations found both young people and adults with TS (n = 741) to be significantly more depressed than age- and sex-matched healthy control subjects. 29
Since 2000, some research groups have separated TS individuals on the basis of clinical symptoms into subgroups, specifically separating those with and without ADHD, demonstrating significant differences. Thus, they have examined cohorts of children including children with TS only, and are comparing them with other groups such as TS plus ADHD, ADHD only and unaffected healthy controls. 27,30,31 These studies generally indicated that youngsters (aged 7–18 years) with TS only did not differ from unaffected controls on many ratings, including aggression, delinquency or conduct difficulties. In contrast, children with TS plus ADHD scored significantly above the unaffected controls and, similarly to those with ADHD only, on the indices of disruptive behaviours. Studies further showed that youngsters with TS plus ADHD showed more internalising behaviour problems and poorer social adaptation than children with TS only or controls. Of importance is that youngsters with TS only were not significantly different from unaffected controls on most measures of externalising behaviours and social adaptation, but did have more internalising symptoms. Similarly, in an adult TS population, those patients with TS only were not disadvantaged, whereas those with TS plus ADHD showed signs of social difficulties including drug and alcohol abuse and more aggression and more instances of breaking the law. 32 In summary, those individuals with TS only appear to be no different from healthy control subjects (apart from having more internalising problems) and have significantly fewer externalising problems and social adjustment difficulties than those with TS plus ADHD. This clearly has major management and prognostic implications.
After an initial publication on the reduced quality of life (QoL) in adult patients with TS,33 there have been over a dozen or so studies investigating QoL in young people with TS (review and references)7 and, more recently, a study by Cavanna et al. 34 The conclusions that can be drawn from these are that patients with TS have a reduced QoL when compared with healthy controls, but have a better QoL than patients with epilepsy or other psychiatric illnesses. The reduced QoL is generally associated with unemployment, underachievement, increased tic severity, the presence of OCB, ADHD, anxiety and depression. 7 It has also been shown that the OCD and ADHD have differential effects on the QoL. 35
The psychosocial aspects and adaptive functioning of people with TS have been addressed by both Robertson36 and Sukhodolsky et al. 37 There are numerous triggers which increase the tics and patients’ distress. In addition, patients with TS suffer as a consequence of their tics and associated comorbid conditions and coexistent psychopathologies. Sukhodolsky et al. 37 cited cases from their TS clinic (Yale Child Study Centre, CT, USA) and also reviewed studies not included/mentioned above as their brief was different. In essence, in their clinic, youngsters (aged 7–18 years) with TS had been teased because of their tics. In one controlled study, TS youngsters were rated by their peers as more withdrawn and less likeable than their peers. The severity of tics was not related with ratings of popularity, but was associated with ADHD. In a second controlled study,38 peer victimisation was compared between youngsters with chronic tic disorder (CTD) and type 1 diabetes mellitus and matched healthy controls: the youngsters with tics were classified as reporting clinically significant greater peer victimisation (27%) than for 9% of both youngsters with diabetes mellitus and healthy controls. 37
Lee et al. 39 showed that more ‘stress’ (e.g. parental burden, psychopathology) was reported in the parents of youngsters with TS, with one of the main stressors being child care difficulties. In addition, a correlation was found between parenting stress and child sex, age, school situation, disease severity, parent age and family income. Disease severity and family income were the variables with the greatest predictive power for parenting stress, accounting for 42% of the variance. 39 Cooper et al. 40 compared ‘caregiver burden’ (CGB) in parents of youngsters with TS with the parents of youngsters with asthma using standardised assessment schedules including the Child and Adolescent Impact Assessment. The TS parents were significantly more psychiatrically disturbed and had greater CGB than the parents of children with asthma. 40 Overall, studies have shown considerable parenting stress, CGB and psychopathology in the parents of youngsters with TS.
In summary, QoL in patients with TS is reduced, with children and young people with TS rated as less likeable by their peers and also suffering significant ‘peer-victimisation’; the adverse effects on parents of a child with TS are substantial.
The relationships between the comorbidities and psychopathology and psychosocial aspects and TS are complex and have been discussed fully elsewhere,7,9,36,41 and are summarised as follows: (1) OCB/OCD is generally accepted as an integral part of and genetically related to TS; (2) ADHD is common in TS and now recognised to be genetically related in some cases (a subgroup); and (3) ASD is common, sharing some genetic/neurodevelopmental underpinnings with TS.
In summary, the comorbidities of TS change with age as do some of the psychopathologies (e.g. depression worsens with age). This highlights the importance to address the TS phenotype and why the separation of the other disorders in TS is crucial. This also clearly has treatment implications.
The Tourette syndrome phenotype
Recent studies42–44 using quantitative methods, such as principal component factor analysis, hierarchical cluster analysis and latent class analysis have suggested that TS should no longer be considered a the unitary condition described in the International Classification of Diseases and Diagnostic and Statistical Manual of Mental Disorders classification systems. All studies also show that one type is ‘pure TS’, in other words, there is heterogeneity of symptomatic presentation within the diagnostic umbrella of TS. Both clinical and community studies have shown that as many as 90% of individuals with TS have comorbid conditions and/or coexistent psychopathology and this clearly has management and treatment choices and ramifications: treatment in TS should be symptom targeted in the patient, taking a holistic approach including the family. 7,9,45,46
Lifespan prognosis
It was initially thought that TS was lifelong with continuing severity, but then Erenberg et al. 47 first indicated that TS symptoms reduced with age. Leckman et al. 48 subsequently highlighted the natural course of the disorder, suggesting that the prognosis was better than previously thought, with the onset of TS at 5.6 years, the worst severity being at 10 years and the majority of symptoms disappearing/reducing in half of the patients by the age of 18 years. Coffey et al. 49 assessed youngsters (aged 6–17 years) with TS having a mean age of onset of 5.1 years. At baseline, 88% of subjects met threshold criteria for at least mild symptoms, but only 30% met criteria for impairment. At a 2-year follow-up, 82% of the subjects met criteria for tic persistence (no significant difference from baseline), but only 14% met criteria for TS-associated impairment, which was significant. Bloch et al. 50 more recently studied 46 TS children at follow-up after 7.6 years and reported 85% had a reduction in tics during adolescence, only increased tic severity in childhood was associated with increased tic severity at follow-up. The average age at worst tic severity was 10.6 years; however, worst ever OCD symptoms occurred approximately 2 years later than worst tic severity, and increased childhood intelligence quotient (IQ) was associated with increased OCD severity at follow-up. Thus, although the prognosis of TS is better than originally thought with regards to tic symptomatology, the course of associated psychopathology, such as OCD, may show greater persistence later on in the individual’s life. Finally, a follow-up study, using a rigorous design, reviewed TS patients’ (aged 8–14 years) assessments. 51 Patients (n = 56) were filmed for 5 minutes originally between 1978 and 1991 according to a strict protocol. Thirty-six of these patients aged > 20 years were contacted again and 31 were included into the follow-up video study. A blinded video-rater assessed the 62 tapes and rated five tic domains; the two videotapes were compared for each tic domain as well as a composite tic disability score. Results showed that 90% of the adults still had tics and many adults who had suggested that they were tic free were incorrect, as no fewer than 50% had objective evidence (on video) of tics. The mean tic disability score reduced significantly with age. All tic domains improved with age and there were significant improvements (p = 0.008) for motor tics. The improvements in tic disability were not related to medication, as only 13% of adults received medication for tics, compared with 81% of children. The authors concluded that although tics improve with time, most adults have persistent, but mild, tics. 51 Finally, in a recent study examining the effects of psychosocial stress predicting future symptoms in children with TS and/or OCD, advancing chronological age was robustly associated with reductions in tic severity. 52
The presence of comorbidities in a person with TS is associated with a worse prognosis. Rizzo et al. 53 investigated 100 TS patients who were assessed at onset and at then again at follow-up 10 years later to evaluate the severity of the tics, the presence of comorbidities and coexistent psychopathologies. In brief, they demonstrated that the comorbid conditions altered with increasing age and that those with the ‘Pure TS’ phenotype (38% at the original assessment) had the best long-term outcome. In contrast, those patients who presented at their original assessments with comorbidities (TS plus OCD, TS plus ADHD, TS plus ADHD plus OCD) had a poorer prognosis.
What is also important to understand is that in a person with TS who also has multiple comorbidities, the ASD typically begins first, then the ADHD, then the motor tics, then the vocal/phonic tics and then the OCB (which is usually present by the time of diagnosis), while the OCD usually develops later. The depression, anxiety, substance misuse and other coexistent psychopathologies usually emerge later and may well be a consequence as much of the comorbid disorders as of TS itself.
In summary, following onset of TS, tic symptomatology may get worse until early teens with subsequent improvement and although some tic symptoms usually remain into adult life, they usually do not impair the individual. Those with ‘TS-only’ (‘pure-tics’) have the best prognosis. The comorbidities (ASD, ADHD, OCD) and associated psychopathologies (e.g. depression) change with age and their impact many may well increase during adolescence.
Aetiological theories
The main aetiological candidates for TS include genetic influences, infections and perinatal difficulties. Originally, the aetiology of TS was considered to be psychological54,55 but, in the 1980s and 1990s, large pedigrees with multiple affected family members were documented with tics or obsessive–compulsive symptomatology, suggesting a familial pattern and possible genetic origin. Subsequent investigations employing complex segregation analysis indicated that TS was inherited, consistent with a single major gene and autosomal dominant transmission, but with incomplete penetrance. However, much of the genome was subsequently excluded. 7,9 The Tourette Syndrome Association International Consortium for Genetics56 using sib-pair analysis, undertook one of the first genome scans in TS, as well as the first Genome Wide Association Study (GWAS). 57 The genetic susceptibility in TS has been recently reviewed by Fernandez and State. 20 There are five essential findings/conclusions: (1) that the GWAS identified the COL27A1 gene as a possible contributing gene in a multiethnic cohort,58 but the finding requires replication, (2) some rare variant studies have identified a mutation to the SLITRK1 gene,59 but subsequent studies have been conflicting,60 (3) linkage analysis of an individual outlier TS family identified a deleterious mutation in the histidine decarboxylase gene suggesting that histaminergic neurotransmission may be involved in the pathophysiology of TS,61 (4) pathway analysis of rare copy number variants has proven useful,20 and (5) there seems to be a significant overlap of genes mapping within rare copy number variants in TS and those identified in ASD. 19,20
Neuroimmunological theories possibly operating via the process of molecular mimicry have become of interest in the aetiology of TS. Swedo et al. 62 described a group of 50 children with OCD and tic disorders, designated as Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection (PANDAS). The diagnostic criteria included presence of OCD and/or a tic disorder, pre-pubertal symptom onset (usually acute, dramatic), association with group A beta-haemolytic streptococcus (GABHS) infections, episodic course of symptom severity and association with neurological abnormalities. The relapsing, remitting course was associated with significant psychopathology including emotional liability, separation anxiety, night-time fears, bedtime rituals, cognitive deficits, oppositional behaviours and hyperactivity.
Other centres have found laboratory evidence of GABHS infections in some patients with TS, and/or documenting that some TS patients have increased antibasal ganglia antibodies in cohort studies63 and controlled studies. 63–69 As most researchers have findings that supported a role of GABHS and basal ganglia autoimmunity in a subgroup of TS patients, it was suggested that further research was needed to clarify the phenomenology associated with antibasal ganglia antibodies. 70 The majority of studies have indicated a role of GABHS infections in TS, but there is a dissenting view predominantly from one laboratory. 71 Clearly, streptococcus does not cause TS, but it may well be that individuals inherit a susceptibility to TS and to the way they react to some infections, including GABHS infections. The above theory has now expanded into broadly two theories: (1) a lowered immunity and (2) autoimmunity. 9
Murphy72 reviewed the literature on the role of infections in the aetiopathogenesis of tic disorders including TS and notes that many infectious agents have been suggested including not only GABHS but also Mycoplasma pneumoniae, other respiratory infections and, indeed, the common cold, have been linked to the exacerbation of both OCD and tic symptomatology. Lyme disease, a complex multisystemic illness, is the most common vector-borne disease in the USA and is caused by bacterial spirochete Borrelia. 73 Lyme disease has been reported as presenting as TS;74 in the UK,75 it is spread to humans by infected ticks and named after the city of Lyme in Connecticut. If the tick bites an infected animal, the tick becomes infected and can pass the disease to humans; most bites occur in late spring and early summer and it is estimated that there are 3000 new cases in UK per year. 76 Thus, there are more than one infectious agent thought to be involved in the aetiopathology of TS, none of which cause TS, but may trigger tics in an already vulnerable individual via mechanisms of lowered immunity or autoimmunity.
Leckman77 outlined the potential role of pre-natal and perinatal events in the pathogenesis of TS. The first study in the area was a controlled study78 embracing a large sample size and the use of obstetric hospital records, which demonstrated that the mothers of children with tics were 1.5 times as likely to have experienced a complication during pregnancy than the mothers of children who did not have tics. Two studies showed that among monozygotic twins discordant for TS, the twins with TS always had lower birthweights than their unaffected twins. Leckman’s own group demonstrated that the severity of maternal life stress during pregnancy, severe nausea and/or vomiting during the first trimester are risk factors for developing tic disorders. A second controlled study79 demonstrated that TS patients have had more pre-natal and perinatal difficulties than a control group. Several groups have now shown that maternal smoking of cigarettes during pregnancy can produce more severe TS in the child. 80 More recently, both smoking and psychosocial stressors during pregnancy have been implicated in altering the TS phenotype. 52 In addition to the factors mentioned above, other perinatal risk factors for TS in the child include older paternal age, more and earlier prenatal care visits, delivery complications, premature low birthweight children as well as those with low Apgar scores at 5 minutes after birth. 77,81 Maternal smoking during pregnancy and low birthweight are risk factors for the presence of comorbid ADHD. 81 Older paternal age, maternal use of caffeine, cigarettes or alcohol during pregnancy and forceps delivery are risk factors for the presence of comorbid OCD. 81 These perinatal factors appear to also non-specifically increase the risk of a range of neurodevelopmental disorders including TS, ADHD and ASD.
In summary, the aetiopathology of TS is more complex than previously recognised, with genetic risk potentially interacting with some infections and pre-natal and perinatal difficulties to affect the susceptibility to tics and phenotype of TS.
Cognitive functioning in Tourette syndrome
The neuropsychological assessment and functioning in TS has been recently reviewed by Murphy and Eddy. 82 The results of neuropsychological studies are, on the whole, inconsistent; more difficulties are demonstrated in child rather than adult populations; those with ‘Pure TS’ (tics only) show no evidence of global impairment of intellectual function and any difficulties (especially in adults) are mild and likely to consist of subtle decrements in attention or inhibitory processing; and patients with TS seem likely to have changes in social cognition. In addition, those with TS plus OCD may have a higher IQ, while those with TS plus ADHD have a lower IQ and many of the neuropsychological difficulties are associated with the presence of ADHD. 82 This ‘ADHD effect’ is also seen with more behavioural difficulties in both youngsters (aged 7–18 years)30 and adults32 and those with lower QoL, all of which have been mentioned above. 7,9,36
On the other hand, there may be some cognitive advantages in having TS. There has been some evidence that children with ‘pure TS’ (tics only) may have some enhanced aspects of executive function, possibly as a compensatory mechanism resulting from habitual tic suppression. 83 In addition, Jackson et al. 84 employed a manual switching task that created high levels of interhemispheric conflict in a study with those with ‘Pure TS’ and control subjects, and results indicated that the TS subjects showed faster reaction times than controls.
Neurobiology and neuroimaging
There have been many studies and reviews and these give evidence that those with TS may have the following abnormalities: (1) basal ganglia and corticostriatal thalamic circuitry dysfunction, (2) a reduction in caudate nucleus volume, with a negative correlation between caudate nucleus volume in childhood and tic severity in later in life, (3) thinning of the sensory–motor cortices in both children and adults with TS, with a negative correlation between thickness and orofacial tic severity, (4) adults with TS show cortical thinning and reduced grey matter in pre-frontal areas (suggesting a failure in neural compensation to control the tics into adulthood, (5) the supplementary motor area may be involved in the premonitory urges/premonitory symptoms as shown by functional magnetic resonance imaging, (6) white matter measurements have shown larger volumes and reduced fractional anisotropy in the corpus callosum in TS, (7) putative cognitive control networks in TS are functionally immature and anomalous, and (8) changes in the volume of the dorsolateral pre-frontal cortex. Apart from the suggestions given above, quite how the changes affect brain function are unclear. 85,86
Treatment interventions
The evidence for the clinical effectiveness of different interventions for the treatment of tics in children and young people with TS is the focus of this report and the results will be presented in detail in Chapter 2. Young people’s experiences and views on these interventions are presented in Chapter 3. In brief, the main types of interventions used are:
-
psychoeducation – the provision of accurate information about the diagnosis, course and treatment of TS provided to children and young people, their parents, teachers and other health-care professionals
-
behavioural interventions (see Chapter 2), which can be divided into:
-
specific interventions for tics such as habit reversal training (HRT) and exposure and response prevention (ERP)
-
other general behavioural/psychological interventions such as relaxation training, anger management training, individual and group counselling and psychotherapy and family therapy
-
-
medication and dietary interventions (see Chapter 2) – examples of medications commonly used for tics include noradrenergic agents [e.g. clonidine (Dixarit®, Boehringer Ingelheim)] and antipsychotics [e.g. risperidone (Risperdal®, Janssen), aripiprazole (Abilify®, Otsuka)]
-
physical treatments (see Chapter 2) – these are used rarely in children and young people except in exceptional circumstances. Examples include electrical and magnetic brain stimulation [repetitive transcranial magnetic stimulation (rTMS) and deep brain stimulation (DBS)], botulinum toxin, immunotherapy for PANDAS and acupuncture.
The guiding principle of the treatment and management of tics in children and young people with TS is to prevent, reverse or normalise the adverse effects of tics on self-esteem, physical health (e.g. pain, self-injury), learning (loss of concentration and distraction due to tics and their suppression, time off school), lifestyle (avoidance of normal physical and social activities) and relationships (victimisation and bullying, family stress and burden) rather than to eliminate tics, per se. Although tic reduction can contribute significantly to these outcomes, other supportive interventions including provision of information and psychosocial support to young people, families and schools that builds resilience, self-esteem and effective coping strategies are equally important.
Although psychoeducation should be offered in all cases following a diagnosis of TS, the decision to offer more targeted behavioural interventions and medication will be determined by various factors such as the severity and impairment associated with tics, the availability of trained (HRT/ERP) behavioural therapists and the treatment preferences and motivation of young people and their families. If comorbidities are present, relative impact of these different conditions on the child’s functioning will determine which condition (or group of symptoms) is given priority for treatment. Medication management of the common comorbidity of TS and ADHD is further complicated by the belief that psychostimulants used to treat ADHD can actually worsen or exacerbate tics.
Organisation of services and referral pathways for Tourette syndrome in the UK
In the UK, children and young people with TS currently receive services of varying type and quality, although there are few, if any, systematic data on current service provision. At present, there are four ‘tiers’ of health service provision from primary care to quaternary national services and children with tics are seen in all settings. There are several factors that might contribute to the variability and inconsistency of care received, including the nature of the condition itself, which sits at the interface between neurology/paediatric and mental health services. The impression from service users and clinicians is that if children and young people with TS access specialist (secondary) services, these may be either in paediatric or child and adolescent mental health services (CAMHS). Currently in the UK, the commissioning and delivery of paediatric services (primarily focused on physical health and development) and CAMHS are separate. These services often provided in separate locations and with different funding streams. This is not ideal for the child with TS, who may have need for input of skills from both mental health as well as paediatric services, depending on the reported difficulties and findings following an assessment.
Tourette syndrome is defined in terms of the tic disorder (see Clinical features), but many children with chronic motor and vocal tics may not come to the attention of services at all and, indeed, may not have any functional impairment. If tics are worrying, intrusive or disruptive, children with tics are seen initially by their general practitioner (GP) and may be subsequently referred to their community paediatrician, general paediatrician or local CAMHS. In some cases, the children may be referred on to a paediatric neurology services. Treatments that are then initiated by first-line clinicians (paediatrics or CAMHS) are variable, depending on their knowledge of tic disorders and the resources available to them. Access to behavioural therapy (e.g. HRT and/or ERP) outside specialist centres is very patchy and currently in the UK many children will not be offered these interventions if they are treated by local community paediatric services or local CAMHS. Consensus opinion in the management of tic disorders suggests that education for child, family and school should be the initial intervention, as this may be sufficient to allay fears and enable accommodation to the condition, if there is no functional impairment from the tics themselves. However, in practice, it appears that authoritative psychoeducation is patchy in its provision and the primary intervention from paediatricians is often a trial of medication for tics. Choice of first-line agent is also highly variable.
The initial assessment of a child with TS is a crucial part of the management and treatment. The range of needs that are identified at assessment should define the next steps in treatment and their prioritisation is a second reason why care pathways and treatments are so variable. For example, both clinical experience and several research studies34,35,87,88 show that the emotional and behavioural problems which often accompany TS (psychiatric comorbidities) are more likely to contribute to impaired QoL than the tics themselves. So, for many children, the identification and treatment of mental health needs such as ADHD and OCD, or behavioural problems such as oppositional defiant disorder are the priority for treatment. An additional comorbidity that is over-represented in children and young people with tics is ASD. If the initial assessment is carried out by a clinician without an adequate knowledge of screening for mental health problems, these comorbidities may be missed and the most important target for treatment may be bypassed.
Similarly, a substantial minority of children and young people with TS have either a specific or a global intellectual disability. 89 If this is not considered at the initial assessment it may be missed and often contributes significantly to a child’s impairment or distress if they have unidentified and unmet special educational needs. For this reason, a clinical or educational psychologist is likely to have an important role in the assessment of some children and young people with TS.
In summary, current service provision, referral pathways and treatments in the UK can be variable depending on both the configuration of services in the local catchment area of the child and availability of specific interventions (e.g. behavioural interventions). A possible depiction of current service provision and referral pathways is given in Figure 1.
FIGURE 1.
Current service provision and referral pathways in the UK for TS.
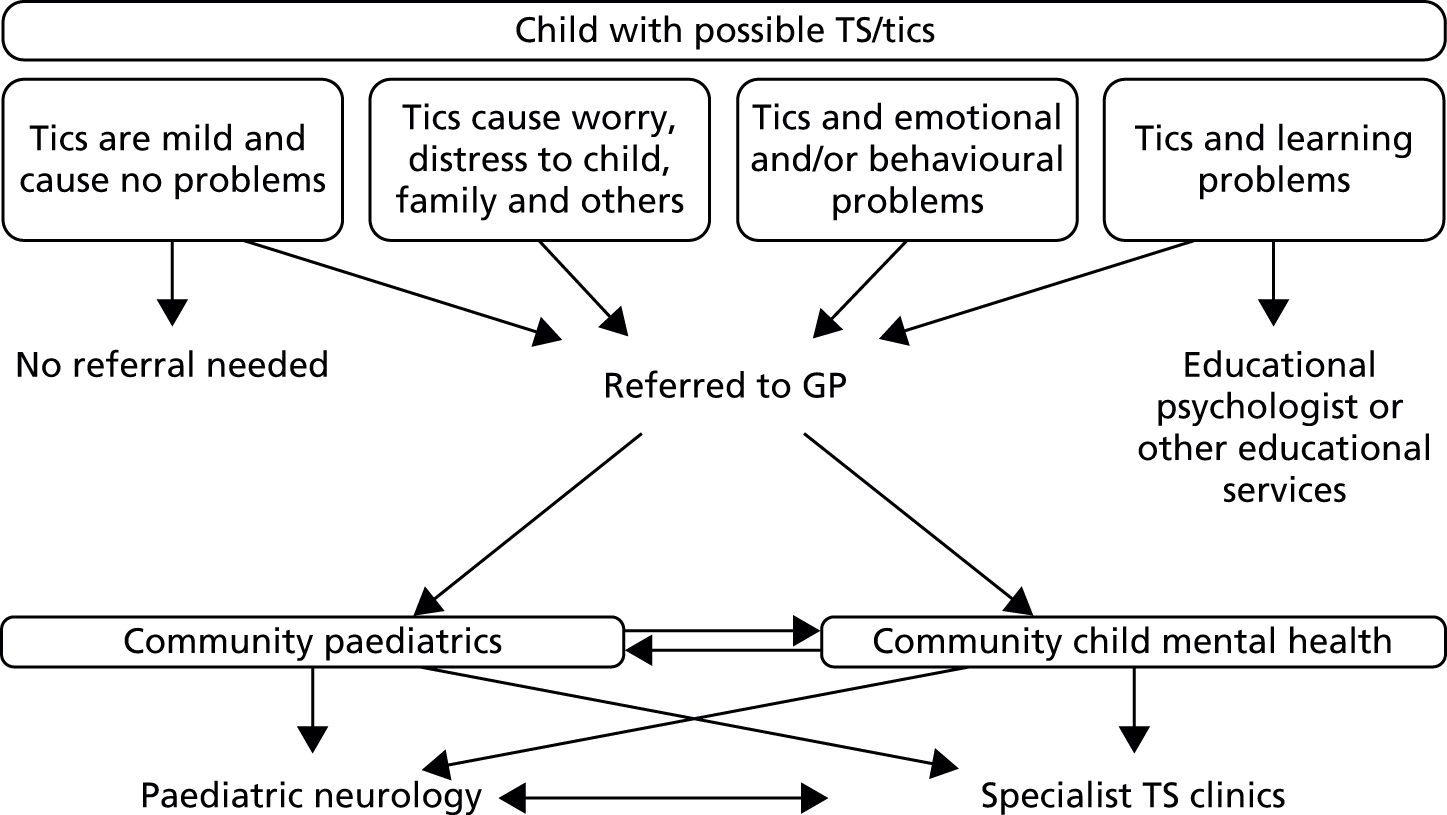
There are a wide range of services and treatments that might need to be deployed for children and young people with TS and one of the key aspects of successful management is to identify their individual needs and then attempt to find the appropriate intervention, which might be in a range of settings.
Assessment should include a careful history incorporating an account of the emergence and nature of tics and their impact, a medical and developmental history, a mental health assessment incorporating family and psychosocial history, and an account of school functioning. A full general physical examination and neurological assessment looking for unusual features and comorbid movements such as tremor, dystonia and chorea and associated dyskinesia should be performed. Cerebellar function examination and gait examination can be useful. Depending on findings, treatments offered currently in the UK may include:
-
for tics
-
psychoeducation
-
behavioural interventions (HRT or ERP)
-
school liaison
-
medication treatment (e.g. noradrenergic agents, antipsychotics)
-
physical treatments (e.g. rTMS, DBS)
-
-
for mental health problems, for example
-
medication and psychosocial management for ADHD [National Institute for Health and Care Excellence (NICE) guidelines]
-
cognitive–behavioural therapy +/– medication for OCD (NICE guidelines)
-
parent training and other interventions for oppositional defiant disorder/conduct disorder
-
behavioural intervention and medication for challenging behaviour associated with learning disability/ASD
-
treatments for depression, other anxiety disorders, etc.
-
-
for developmental/learning problems
-
psychometric/cognitive assessment
-
school liaison
-
special educational needs provision
-
assessment and management of social communication difficulties, that is ASD.
-
Overall aims and objectives of report
The overarching aims of the project are as follows.
-
To identify the most clinically effective interventions and treatment strategies for children and young people with TS. The primary objective is to answer the question: what interventions work for whom and when? Key clinical questions include the order in which drug and behavioural/psychological treatments should be offered and how they should be combined based on both clinical severity and comorbidity.
-
To elicit the views of young people with TS and their parents on their experiences of treatment and what outcomes they value most.
-
To produce recommendations for primary research that will fill gaps in the evidence and address key issues raised by this project.
The project presents an evidence synthesis from diverse sources that include:
-
A systematic review and meta-analysis (when applicable) of the clinical effectiveness of treatments for tics in children and young people.
-
A qualitative research study [Qualitative study of Experiences of Services and Treatment (QuEST)]. QuEST incorporates both a national survey of parents of children with TS and well as in-depth interviews with young people focusing on their experiences of treatment and identifying which outcomes matter most to them.
-
An expert project advisory group – the Tourette Expert Group (TEG). The TEG included experts in clinical practice, research and experience of care [service users/carers nominated by Tourettes Action (TA)]. The TEG defined the key review questions (see Chapter 2, Review questions) and synthesised diverse sources of evidence including the systematic review, review of grey literature, service user/carer survey and qualitative interviews with young people. The TEG also reviewed and agreed the recommendations for future primary research (see Chapter 3, Recommendations for further research).
Chapter 2 Systematic review of interventions for tics in children and adolescents with Tourette syndrome
Methods
The review was conducted according to the review protocol (see www.nets.nihr.ac.uk/) (PROSPERO registration number: CRD42012002059).
Review questions
Pharmacological interventions
-
For children and young people (< 18 years old) who have a clinical diagnosis of TS (including Tourette disorder and chronic motor tic disorder, or chronic vocal tic disorder), what are the benefits and potential harms associated with medication [including antipsychotic drugs, clonidine, tetrabenazine, fluoxetine (Prozac®, Lilly), clonazepam] and dietary interventions (including zinc, omega-3 fatty acids, caffeine) used for the treatment of tics, when compared with placebo or another intervention?
-
Is the clinical effectiveness of the treatment of tics with medication or diet moderated by the presence of a comorbid disorder (e.g. ADHD, OCD, ASD or learning disability), age or tic severity?
-
In those with a comorbid disorder, is the treatment of comorbidities (e.g. psychostimulants used for ADHD) associated with improvements or worsening of tic-related outcomes?
-
-
What are the views of patients and carers regarding the choice of medication and dietary interventions available (including benefits and adverse effects) and what outcomes are considered most important and meaningful?
Behavioural interventions
-
For children and young people (< 18 years old) who have a clinical diagnosis of TS (including Tourette disorder and chronic motor tic disorder, or chronic vocal tic disorder), what are the benefits and potential harms associated with psychological/behavioural and psychosocial interventions, when compared with treatment as usual (TAU), a control group or another intervention?
-
Is the clinical effectiveness of the treatment of tics with psychological/behavioural and psychosocial interventions moderated by the presence of a comorbid disorder (e.g. ADHD, OCD, ASD or learning disability), age, awareness of premonitory urges or tic severity?
-
Is combination treatment (medication plus therapy behavioural intervention) more effective than either treatment alone?
-
What is the most effective order of treatment (e.g. a behavioural intervention first followed by medication, or medication first, followed by a behavioural intervention as an adjunct)?
-
-
What are the views of patients and carers regarding the choice of psychological/behavioural and psychosocial interventions available and what outcomes are considered most important and meaningful?
-
What evidence is there that combining a behavioural intervention with medication increases engagement and adherence with treatment?
Physical interventions
-
For children and young people (< 18 years of age) who have a clinical diagnosis of TS (including Tourette disorder and chronic motor tic disorder, or chronic vocal tic disorder), what are the benefits and potential harms associated with physical interventions (including neurotherapeutic interventions, such as DBS, rTMS and other physical interventions such as acupuncture and botulinum toxin injection), when compared with TAU, a control group, or another intervention?
-
Is the clinical effectiveness of the treatment of tics with physical interventions moderated by the presence of a comorbid disorder (e.g. ADHD, OCD, ASD or learning disability), age or tic severity?
-
What are the views of patients and carers regarding the choice of physical interventions available and what outcomes are considered most important and meaningful?
Study selection
Search strategies
Searches for English-language and foreign-language studies were conducted in the following databases. Major bibliographic: EMBASE, MEDLINE, PREMEDLINE In-Process & Other Non-Indexed Citations and PsycINFO), The Cochrane Library, Cochrane Central Register of Controlled Trials (CENTRAL) – database of randomised controlled trials (RCTs) and other controlled trials, Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effectiveness (DARE) and Health Technology Assessment (HTA). Education databases: Australian Education Index (AEI), British Education Index (BREI) and Education Resources in Curriculum (ERIC). Social care databases: Applied Social Sciences Index and Abstracts (ASSIA), International Bibliography of Social Science (IBSS), Social Sciences Citation Index (SSCI), Social Services Abstracts (SSA), Sociological Abstracts and Web of Science. Grey literature databases: Health Management Information Consortium (HMIC), PsycBOOKS and PsycEXTRA; and additional sources: Allied and Complementary Medicine Database, BIOSIS Citation Index (BCI), Cumulative Index to Nursing and Allied Health Literature (CINAHL). Search terms comprised subject headings and text words for tic* and tourette* to identify populations with TS or with chronic phonic or motor tics (all referred to as TS in this review). Searches were limited to systematic reviews, RCTs and observational studies. For standard mainstream bibliographic databases, search terms for the population were combined with filters for each of the study designs under review. For searches generated in The Cochrane Library (CENTRAL, CDSR, DARE, HTA) and HMIC, search terms for the population were used without the appendage of a filter. Searches for RCTs and observational studies were generated from the inception of the databases to January 2013. Searches for systematic reviews were limited to research published from 1995 to January 2013. No language restrictions were applied at the searching stage. The search strategy is provided in Appendix 1.
Screening
Citations obtained from the searches were screened for inclusion using a two-stage process. Titles and abstracts were first screened to obtain potentially relevant studies. On further inspection of these studies, hard copies of potentially relevant studies were obtained. The review inclusion/exclusion criteria as specified in the protocol were applied in hard-copy screening to obtain the final list of included studies.
Inclusion/exclusion criteria
Participants
Studies of children and young people (< 18 years of age) with TS or CTD were included in the review. Studies in children were the basis for the review but mixed studies in adults and children, and adult studies, were considered to provide data in areas for which child studies were lacking or to corroborate findings in child studies.
Intervention
Included interventions were:
-
Any medication with marketing authorisation for any use in North America, Europe or Australasia or any dietary intervention.
-
Psychological/behavioural and psychosocial interventions including HRT/comprehensive behavioural intervention for tics (CBIT), ERP, counselling and supportive psychotherapy (SP), family intervention (including family therapy), psychoeducation, relaxation training, and self-hypnosis.
-
Physical interventions including neurotherapeutic interventions, such as transcranial rTMS, DBS and other physical interventions, such as acupuncture and botulinum toxin injection (botulinum toxin type A; Botox®, Allergan).
Control
Studies with any control group were eligible for the review including TAU, a waiting list control or another active intervention.
Outcomes
The critical outcome for the review was tic severity/frequency. The important, but non-critical, outcomes were QoL, educational attainment, psychological well-being and impact on peer relationships.
Study design
For the review questions concerning the clinical effectiveness and safety of treatments or moderators of treatment effect, the only eligible study designs were RCTs or controlled before-and-after studies. For review questions about patient views and barriers to treatment, any study design was eligible for inclusion.
Data abstraction
Data were abstracted by one reviewer for the outcomes specified and grouped under the headings of tics, impairment (personal and social), psychological well-being, overall clinical outcome, and safety (no studies reported controlled findings for QoL or educational attainment). When reported in studies, results for follow-up were extracted for all outcomes.
For some outcomes (e.g. tics), studies often reported numerous scales making the interpretation of findings difficult. For these outcomes, to avoid repetition and numerous statistical testing, this review limits reporting to one scale. Priority was given to the Yale Global Tic Severity Scale (YGTSS) because this was the most commonly reported scale in the included studies and is commonly used in clinical practice. For other outcomes, when sparse data were reported in studies, results for all scales were included in the review.
Quality assessment
The Cochrane risk of bias tool was used to assess the quality of intervention studies. 90 This tool assesses the risk of bias owing to methods of randomisation and allocation of the randomisation (selection bas), blinding of participants and providers to intervention allocation (performance bias), blinding of outcome assessors (detection bias), rates of dropout from the study and methods used to deal with missing data in the analysis (attrition bias) and the selective reporting of outcome data (selective reporting bias). For the purposes of this review, two additional aspects of study design were also considered: the suitability of the control group and, for crossover trials, the adequacy of the washout period between treatments.
Overall risk of bias for each study was based on an overall assessment of whether or not any biases in that study were likely to have had a significant impact on the findings, rather than a simple summation of the quality ratings. Any source of bias considered to be a particular risk for the overall findings was alone considered sufficient to determine that a study had a high overall risk of bias. Conversely, a study with a high number of potential sources of bias, if not considered likely to have had a serious potential impact on overall findings, may be classed as having a lower overall risk of bias.
The quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. 91 Within this approach, RCTs start as high-quality evidence, whereas observational studies start as low-quality evidence. For each outcome, quality may be reduced depending on five factors: risk of bias, inconsistency, indirectness, imprecision and publication bias. Studies may be down-graded by one or two levels for each of these elements depending on the seriousness of the problem. For the purposes of this review, each factor was evaluated using criteria provided in Table 1.
| Factor | Description | Criteria |
|---|---|---|
| Risk of bias | Limitations in the study design and implementation | Serious risks across most studies (that reported a particular outcome). The evaluation of risk of bias was made using the Cochrane risk of bias tool |
| Inconsistency | Unexplained variation between study results | Important heterogeneity (I2-value was statistically significant and greater than 50%) |
| Indirectness | How closely the outcome measures, interventions and participants match those of interest | For interventions in which child studies were not available and adult or mixed populations were used, these were considered as less applicable |
| Imprecision | Results are imprecise when studies include relatively few patients and few events and thus have wide CIs around the estimate of the effect | If either of the following two situations were met: (1) the optimal information size (for dichotomous outcomes, OIS = 300 events; for continuous outcomes, OIS = 400 participants) was not achieved, (2) the 95% CI around the pooled or best estimate of effect included both no effect and appreciable benefit or appreciable harm |
| Publication bias | Systematic underestimate or an overestimate of the underlying beneficial or harmful effect owing to the selective publication of studies | In no cases could the presence of publication bias be assessed (all meta-analyses contained small numbers of studies); therefore, for this review this was criteria not applied |
As child studies form the most applicable evidence for the review, when they were available, they formed the primary evidence base and GRADE was applied. Mixed and adult studies were used as supporting evidence. If child studies were not available, GRADE was applied to mixed or adult studies but these were down-graded for lower applicability.
Data analysis
For parallel trials, continuous outcomes were analysed as change from baseline in the intervention compared with the control group (i.e. change in intervention group mean score minus change in control group mean score). This information was directly reported by some parallel trails. For other trials, results were only presented as baseline and post treatment means and standard deviations (SDs) without an estimate of the precision of change scores. In some cases, p-values for the net effect were reported and, in these cases, p-values were used to calculate the standardised mean difference (SMD) and corresponding standard error.
When only baseline and post treatment means and SDs were reported, in order to estimate the precision of within-group changes, a correlation coefficient of 0.6 was assumed. This was selected after investigation of studies in which baseline, post treatment and change scores were all reported with precision measurements so that the correlation coefficient for outcomes could be determined. A range in correlation scores was obtained (median 0.60, range 0.30–0.89) and 0.6 was selected for the primary analysis. For crossover studies, outcomes were analysed as a comparison between post-treatment measures.
For continuous data, the SMD was used to judge the magnitude of effect, defined as small (–0.20), medium (–0.50) and large (–0.80). For dichotomous data, risk ratios (RRs) were calculated and the magnitude defined as small (< 2), medium (2–4) and large (> 4).
When possible, sensitivity analyses were used to test the robustness of findings for each intervention using the meta-analysis with the largest number of studies (sensitivity analyses for all interventions were not performed owing to the paucity of data). In the first set of sensitivity analyses, lower-quality studies and all crossover studies were removed. In the second set of sensitivity analyses, for calculating the precision of change scores, a correlation coefficient of 0.3 was used (rather than 0.6).
Data analysis was performed using the Cochrane Collaboration Review Manager software (RevMan version 5.2; The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) using a random effects model to pool results. Effect estimates were used to examine the magnitude of the effect and 95% confidence intervals (CIs) to examine precision of the estimate.
Tourette expert group
Tourette expert group membership consisted of professionals in psychiatry, paediatrics, neurology, clinical psychology, academic experts in psychiatry and psychology, and service user and carer representatives nominated by TA. The majority of TEG members were co-applicants on the HTA grant that funded this review. The review process was supported by staff from the National Collaborating Centre for Mental Health (NCCMH), who undertook the clinical literature searches, reviewed and presented the evidence to the Guideline Development Group, managed the process, and contributed to drafting the HTA report. Four TEG meetings were held between June 2012 and May 2013. At each meeting, all TEG members declared any potential conflicts of interest. During each day-long TEG meeting, in a plenary session, review questions and clinical evidence were reviewed and assessed, and research recommendations formulated.
Service users were invited to attend expert group meetings. One service user attended an expert group meeting. A dedicated time – ‘service user concerns’ – was allocated to giving the opportunity for the service user to express their thoughts on the progress and direction of the review.
Pharmacological interventions
Introduction
Although TS is now recognised as a biological disorder,9 no pharmacological treatment has yet been developed specifically to target tics. Instead, existing medications used as primary treatments for other conditions have been used [e.g. in the 1960s haloperidol (Haldol®, Janssen; an antipsychotic) used for schizophrenia was found to also have an effect on reducing tics when tested on people with TS]. 92
The beneficial antitic effects of haloperidol, a potent dopaminergic D2 receptor antagonist, suggested that dopaminergic system imbalances may be implicated in tics and TS. However, it is likely that noradrenergic, glutamatergic, [gamma-aminobutyric acid (GABA)]-ergic, serotonergic and cholinergic systems also play a role in tics and TS. 93
A broad range of medications, with different pharmacological targets and primary indications for other conditions, have been used to treat TS. These medications include antipsychotics (dopamine antagonists), noradrenergic agents, anticonvulsant drugs, monoamine oxidase inhibitors, 5-HT3 receptor antagonists (antiemetic and gastroprokinetic agents), dopamine receptor agonists (used to treat Parkinsonism), GABAB receptor agonists (skeletal muscle relaxants), tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs) and nicotinic acetylcholine receptor antagonists. Stimulant medication [methylphenidate (Ritalin®, Novartis Pharmaceuticals UK) and dexamphetamine] has also been used in the treatment of tics in association with ADHD.
In a recent prescribing survey among European expert clinicians,94 the most commonly used medications for tics were, in descending order, risperidone, clonidine, aripiprazole (Abilify®, Otsuka), pimozide (Orap®, Janssen), sulpiride (Dolmatil®, Sanofi-Aventis), tiapride (not available in the UK) and haloperidol. In the pharmacological treatment of tics in children and young people, newer antipsychotics (e.g. risperidone and aripiprazole) and noradrenergic agents (e.g. clonidine) have increasingly been favoured over the older antipsychotic drugs (e.g. pimozide, sulpiride and haloperidol).
There have been a number of reviews and clinical guidelines of pharmacological treatment for TS94–96 but no systematic review and quantitative meta-analyses of pharmacological treatments for tics have been conducted to date.
Antipsychotic drugs
Pharmacology and prescribing
Antipsychotic drugs are sometimes divided into ‘first’- and ‘second’-generation antipsychotics, although this really equates to older and newer antipsychotics. Both the older and newer antipsychotic drugs block dopamine receptors (act as dopamine antagonists), with the risk of inducing extrapyramidal symptoms (EPS) and adverse effects (e.g. dystonia, Parkinsonism and tardive dyskinesia). Other potential adverse effects of antipsychotics may include sedation, increased appetite, weight gain (with glucose and lipid dysregulation), hyperprolactinaemia, reduced seizure threshold and cardiac conduction changes (e.g. QT prolongation). Pimozide has been associated with sudden cardiac death (QT prolongation). Therefore, some antipsychotics require routine electrocardiogram (ECG) monitoring.
Risperidone (non-proprietary; Risperdal®, Janssen) is a second-generation antipsychotic licensed in the UK for a range of conditions, including the treatment of schizophrenia, manic episodes in bipolar disorder and persistent aggression in conduct disorder. It is the best studied antipsychotic drug for the treatment of TS97 and was the most common medication used for treatment of children with TS among clinical members of the European Society for the Study of Tourette Syndrome in a survey published in 2011. 94
Aripiprazole (Abilify®, Otsuka) is a second-generation antipsychotic licensed in the UK for the treatment of schizophrenia and the prevention and treatment of manic episodes in bipolar disorder. It is described as a partial dopamine agonist.
Olanzapine (non-proprietary; Zyprexia®, Lilly) is a second-generation antipsychotic licensed in the UK for the treatment of schizophrenia, the treatment of moderate-to-severe manic episodes and prevention of recurrence of manic episodes in patients with bipolar disorder.
Ziprasidone (Geodon/Zeldox®, Pfizer) is a second-generation antipsychotic approved by the US Food and Drug Administration (FDA) for the treatment of schizophrenia and acute manic or mixed episodes associated with bipolar disorder (with or without psychotic features), but is not currently licensed in the UK.
Haloperidol (non-proprietary; Dozic®, Rosemont; Haldol®, Janssen; Serenace®, TEVA UK) is a first-generation antipsychotic licensed in the UK for a range of conditions, most commonly used in the treatment of schizophrenia and psychosis. It is the only drug to have been granted a marketing authorisation (product license) for TS in most European countries. 97
Pimozide (Orap®, Janssen) is a first-generation antipsychotic licensed in the UK for the treatment and prevention of relapse in chronic schizophrenia and other psychoses.
Sulpiride (non-proprietary; Domatil®, Sanofi-Aventis; Sulpor®, Rosemont) is a first-generation antipsychotic licensed in the UK for the treatment of acute and chronic schizophrenia.
Included studies
Six studies presented data for the efficacy of antipsychotic medications compared with placebo [Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram, see Appendix 2]:
-
haloperidol versus placebo: one child98 and one mixed99 study
-
pimozide versus placebo: one child (same study as for haloperidol vs. placebo)98 and two mixed99,100 studies
-
risperidone versus placebo: one child101 and one mixed102 study
-
ziprasidone versus placebo: one child study. 103
Nine studies (head-to-head comparisons) compared the efficacy of one antipsychotic against another:
-
pimozide versus risperidone: one child104 and one mixed105 study
-
haloperidol versus pimozide: one child98 and two mixed99,106 studies
-
aripiprazole versus tiapride: one child study107
-
aripiprazole versus haloperidol: one child study108
-
aripiprazole versus pimozide: one child study109
-
olanzapine versus haloperidol: one child study. 110
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. Four studies compared haloperidol with pimozide and/or placebo. One crossover trial in 22 children compared haloperidol (mean dose of 3.5 mg/day) and pimozide (mean dose of 3.4 mg/day) with placebo for 6 weeks each. 98 A mixed parallel trial (crossover study but end of first-phase results used) in 57 participants compared the use of maximum dose of 10 mg/day haloperidol, maximum dose of 20 mg/day pimozide and placebo for 6 weeks. 99 In another mixed study, 20 participants underwent a crossover trial of maximum dose of 20 mg/day (10 mg/day for children aged ≤ 10 years) pimozide compared with placebo for 6 weeks each. 100 In another mixed crossover study, nine participants underwent treatment with maximum dose of 12 mg/day haloperidol and pimozide in a randomised order for 12 days each. 106
Two studies compared risperidone with placebo. One parallel trial in 26 children101 (a mixed study but the child data were presented separately) compared maximum dose of 3–4 mg/day risperidone with placebo for 8 weeks. A mixed parallel trial of 46 subjects compared the use of maximum dose of 6 mg/day risperidone with placebo for 8 weeks. 102
One parallel trial in 28 children compared the use of maximum dose of 20 mg/day ziprasidone103 with placebo for 8 weeks.
Two studies compared pimozide with risperidone. One study was a crossover trial in which 19 children underwent treatment with maximum dose of 4 mg/day risperidone and pimozide in a randomised order for 4 weeks each. 104 The other study was a mixed parallel trial in which 50 adults and children were randomised to either maximum dose of 6 mg/day risperidone or pimozide for 8 weeks. 105
One parallel study (quasi-randomised) in 200 children compared maximum dose of 15–25 mg/day aripiprazole with maximum dose of 300–500 mg/day tiapride for 12 weeks. 107
One parallel study (non-randomised) in 48 children compared maximum dose of 20 mg/day aripiprazole with maximum dose of 4.5 mg/day haloperidol for 8 weeks. 108
One controlled before-and-after study in 50 children investigated the comparative cardiovascular safety of a mean dose of 4.4 mg (SD 1.5 mg) twice a day of pimozide with a mean dose of 5.3 mg (SD 2.4 mg) twice a day of aripiprazole for 24 months. 109
One parallel RCT in 60 children compared maximum dose of 10 mg/day olanzapine with maximum dose of 12 mg/day haloperidol for 4 weeks. 110
In all studies, the use of other psychotropic drugs was prohibited and previous medications were stopped (with a washout period) before the start of the study.
Risk of bias
The crossover study of haloperidol, pimozide and placebo in children98 did not have clear methods for randomisation or sequence generation but participants, providers and outcome assessors were blind to treatment assignment. There was a 2-week washout period between treatments. The rate of dropout was relatively low and the last observation carried forward was used in the analysis. Overall, the study was considered to be at unclear risk of bias owing to possible cross-treatment contamination.
The mixed crossover study of haloperidol, pimozide and placebo99 had unclear methods of randomisation and the presence of allocation concealment and outcome assessor blinding was unclear. First-phase data were presented for this study and, for these results (used in this review), the risk of bias from cross-treatment contamination is avoided. Analysis was of available case data but dropout from the study was reasonably low (16%) and there may have been low risk of attrition bias. Overall, the study was considered to be at unclear risk of bias owing to unclear provider and outcome assessor blinding.
The mixed crossover study comparing pimozide with placebo100 had unclear methods of randomisation and the presence of allocation concealment and outcome assessor blinding was unclear. There was a 2-week washout period between treatment periods. Attrition from the study was reasonably low (17%). Overall, the study was considered to be at unclear risk of bias owing to unclear blinding of participants, providers and outcome assessors and unclear cross-treatment contamination.
The mixed study of haloperidol compared with pimozide,106 had unclear methods for randomisation and allocation concealment but there appears to have been blinding of participants, providers and outcome assessors. The between-intervention washout period was 6 days. The number of participants randomised in the study is not reported (only completers were analysed) and the risk of attrition bias is unclear. Overall, owing to the short between-treatment washout period and the unclear risk of attrition bias, the study was considered to be at unclear risk of bias.
The parallel trial of risperidone compared with placebo in children101 had unclear methods of randomisation and allocation concealment but participants, providers and outcome assessors were blind to treatment allocation. The rate of dropout was low and last observation forward was used in the analysis. Overall, the study was considered to be at low risk of bias.
The parallel study of ziprasidone compared with placebo in children103 had unclear methods of randomisation and allocation concealment. It is unclear whether or not participants, providers and outcome assessors were blind to treatment allocation. Dropout from the study was reasonably low and data from all participants with one post-baseline measurement were carried forward in the analysis. Overall, this study was considered to be at unclear risk of bias owing to unclear blinding of providers and outcome assessors.
The mixed parallel study of risperidone compared with placebo102 had unclear methods for randomisation and allocation concealment. It is stated that the drug was administered under double-blind conditions but there is no description of participant, provider or outcome assessor blinding. Analysis was by last observation carried forward for all participants with at least one post-baseline measurement and the risk of attrition bias may have been low. Overall, the study was considered to have unclear risk of bias owing to unclear patient, provider and outcome assessor blinding.
In the child crossover study of risperidone compared with pimozide,104 the method of randomisation, allocation concealment and blinding of outcome assessors was unclear. There was a 2-week washout period between interventions and it was unclear whether or not this was sufficient to avoid cross-treatment contamination. There was a relatively high rate of dropout and analysis was of available case data. Overall, the study was considered to be at high risk of bias owing to unclear risk of outcome assessor blinding, possible cross-intervention contamination and attrition bias.
In the mixed parallel study of risperidone compared with pimozide,105 a computer-generated code was used for randomisation but the presence of allocation concealment and blinding of participants, providers and outcome assessors was unclear. There was a low rate of dropout and intention to treat analysis was used. The study was considered to be at unclear risk of bias owing to unclear blinding of participants, providers and outcome assessors.
In the parallel trial of aripiprazole compared with tiapride in children,107 quasi-randomisation was used with participants assigned according to the order that the presented in clinic (odds aripiprazole, evens tiapride) although baseline tic severity was similar. The presence of allocation concealment or blinding or participants, providers or outcome assessors was unclear. The rate of dropout was low (2%) and although intention-to-treat analysis was not used, the risk of attrition bias was low. The study was considered to be at unclear risk of bias owing to unclear blinding of participants, providers and outcome assessors.
In the parallel study of aripiprazole compared with haloperidol in children,108 there was no randomisation and participants could choose which drug they received. There was no blinding of participants, providers and outcome assessors. The rate of dropout was moderate and different for aripiprazole (35%) and tiapride (16%). Analysis was of available cases and there was unclear risk of attrition bias. The study was considered to be at high risk of bias owing to lack of randomisation or blinding of participants, providers and outcome assessors and unclear risk of attrition bias.
In the parallel safety study of pimozide compared with aripiprazole in children,109 participants do not appear to have been randomised but the method of allocation is unclear. There was no blinding of participants, providers and outcome assessors and attrition from the study is not reported. Outcomes were objective (cardiovascular safety profiles) and the lack of blinding may not have been an important source of bias. However, owing to the lack of randomisation and baseline dissimilarity (baseline age 9 years vs. 13 years for pimozide and aripiprazole, respectively) the study was considered to be at unclear risk of bias.
In the study of olanzapine compared with haloperidol in children,110 participants were randomised according to their hospital admission number and a computer-generated randomisation code. There appears to have been allocation concealment and blinding of participants, providers and outcome assessors. The rate of attrition was apparently low (10%) but participants were also excluded from the study for poor compliance and serious adverse effects, but the numbers of these participants is not reported. The study was considered to be at unclear risk of bias owing to the possibility of attrition bias.
Clinical evidence for antipsychotic drugs
Child and mixed-study findings for each drug compared with placebo are presented and head-to-head comparisons (risperidone vs. pimozide and haloperidol vs. pimozide) are then presented. Findings for all antipsychotic drugs compared with placebo are then presented combined.
Haloperidol compared with placebo
Two studies, one crossover trial in children and one parallel trial in a mixed population of adults and children, investigated treatment with haloperidol compared with placebo.
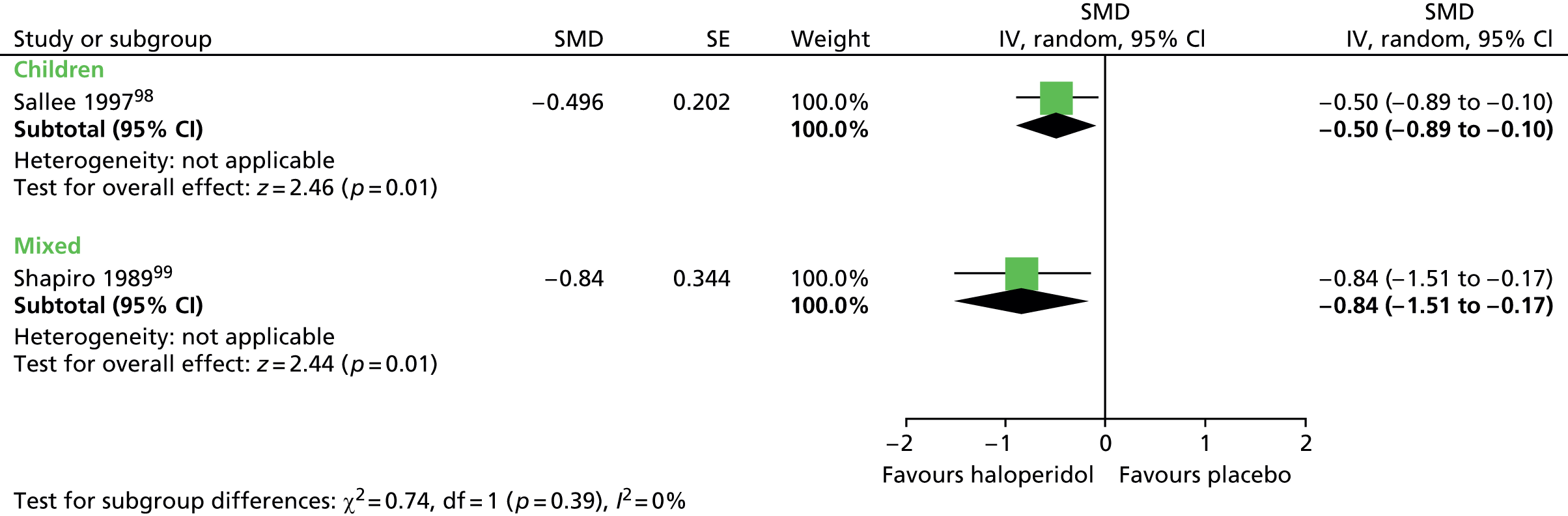
The child crossover study98 measured post-treatment tic severity with the TS global scale and the mixed parallel study99 used the Shapiro Tourette Syndrome Severity Scale (TSSS). Results from each study suggested a benefit for haloperidol compared with placebo on tic scores for children (SMD –0.50, 95% CI –0.89 to –0.10; n = 22) and the mixed group (SMD –0.84, 95% CI –1.51 to –0.17; n = 37) (Figure 2).
FIGURE 2.
Standardised post-treatment total tic scores for haloperidol compared with placebo in the child and mixed studies. df, degrees of freedom; IV, independent variable; SE, standard error.
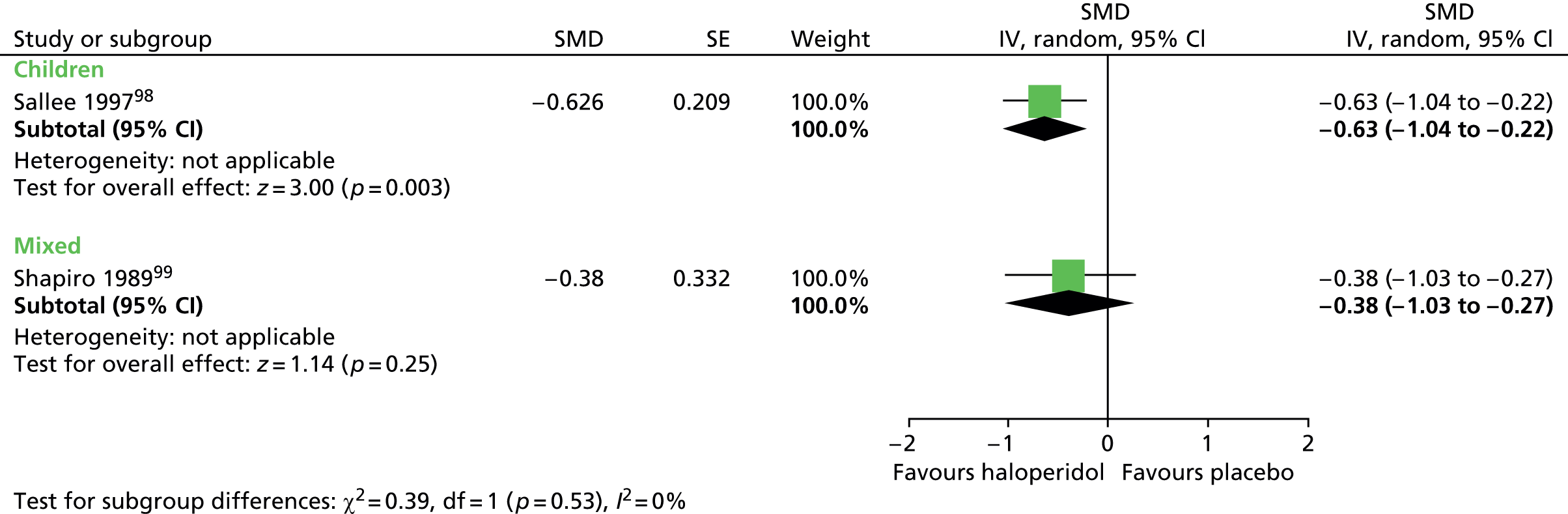
For motor tics alone, post-treatment motor tic scores from the child study suggested a benefit for haloperidol compared with placebo (SMD –0.63, 95% CI –1.04 to –0.22; n = 22) but the effect was inconclusive in the mixed study (SMD –0.38, 95% CI –1.03 to 0.27; n = 37) (Figure 3).
FIGURE 3.
Standardised motor tic scores for haloperidol compared with placebo in the child and mixed studies. df, degrees of freedom; IV, independent variable; SE, standard error.
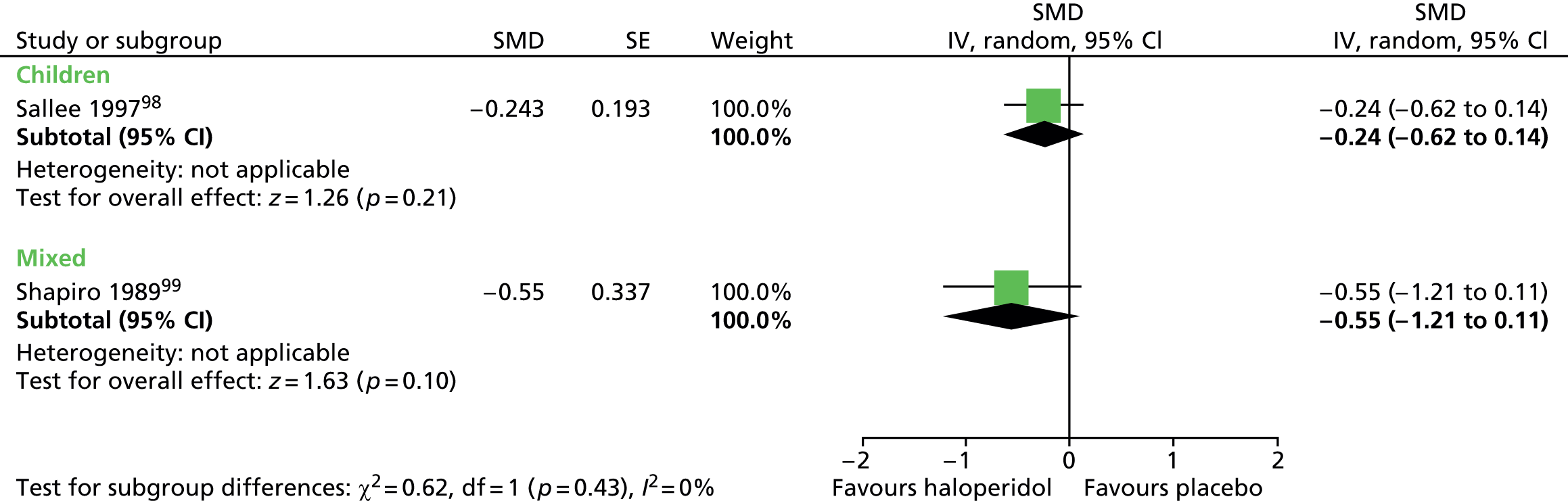
For vocal tics, the effects post treatment were inconclusive (Figure 4).
FIGURE 4.
Standardised post-treatment vocal tic score for haloperidol compared with placebo in the child and mixed studies. df, degrees of freedom; IV, independent variable; SE, standard error.
For haloperidol, one study (n = 22) in children measured global outcome with the TS global scale (contains components of tic frequency/severity and impairment),98 but the results were inconclusive (Figure 5).
FIGURE 5.
Standardised post-treatment TS global score for haloperidol compared with placebo in the child study. IV, independent variable; SE, standard error.
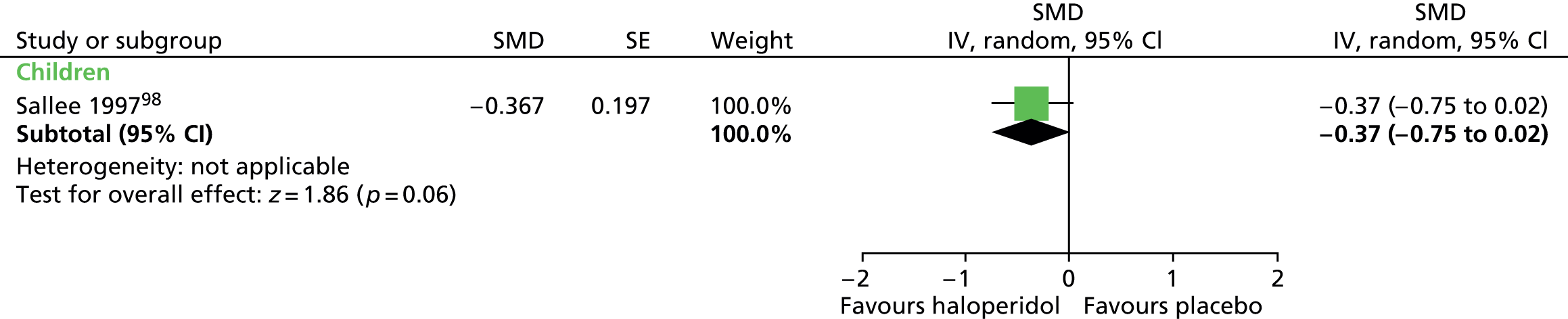
Impairment was also assessed with the Children’s Global Assessment Scale (C-GAS). Post treatment, the results favoured haloperidol compared with placebo (SMD –0.49, 95% CI –0.88 to –0.09; n = 22) (C-GAS higher scores show favourable effect, for consistency with other outcomes, scores reversed here) (Figure 6).
FIGURE 6.
Standardised post-treatment C-GAS score for haloperidol compared with placebo in the child study. IV, independent variable; SE, standard error.
No relevant studies reported this outcome.
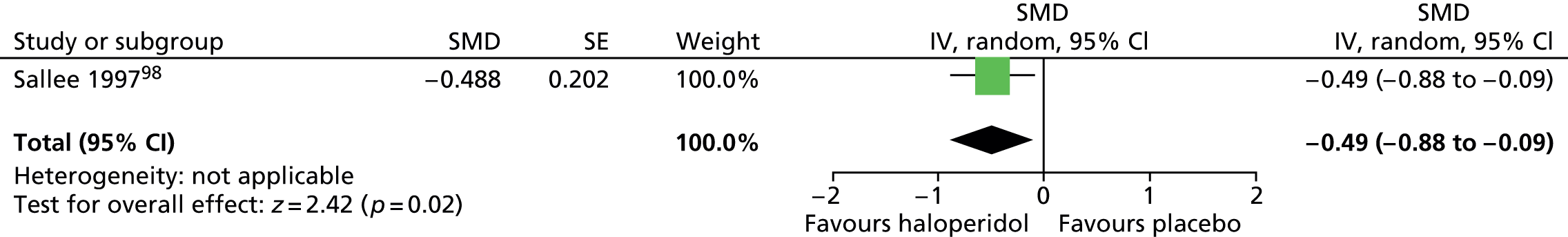
The child and mixed study assessed overall clinical outcome with the clinician-reported Clinical Global Impressions (CGI) Scale. In the child study, post-treatment CGI-Severity score suggested a benefit for haloperidol compared with placebo (SMD –1.23, 95% CI –1.73 to –0.74; n = 22) (Figure 7).
FIGURE 7.
Standardised post-treatment CGI-Severity score for haloperidol compared with placebo in the child study. IV, independent variable; SE, standard error.
In the mixed study, CGI scores suggest a benefit for haloperidol compared with placebo for clinician (SMD –0.80, 95% CI –1.47 to –0.13; n = 37) and patient rated scores (–0.87, 95% CI –1.55 to –0.19; n = 37) (Figure 8).
FIGURE 8.
Standardised post-treatment CGI score for haloperidol compared with placebo in the mixed study. IV, independent variable; ROB, risk of bias.

No relevant randomised trials reported long-term outcomes.
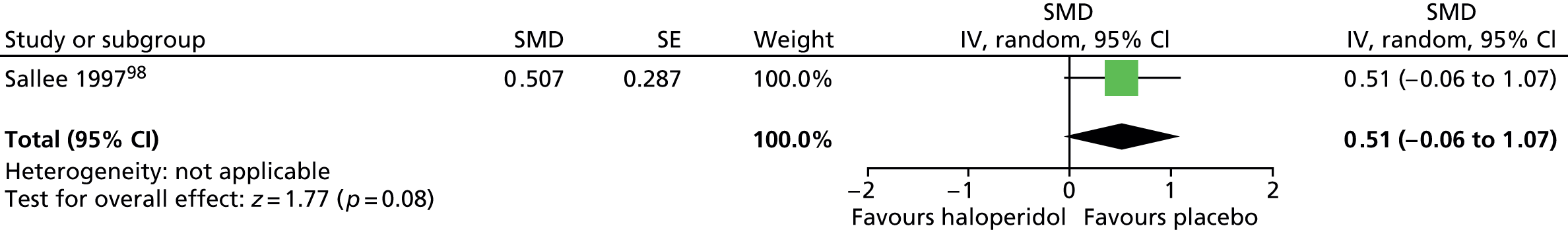
In the child study,89 it is reported that general adverse effects did not differ between treatment groups (p = 0.94). For EPS, because a carryover effect was detected in the whole study analysis, investigators analysed just the first-phase data for this outcome. There was no conclusive difference in Extrapyramidal Symptom Rating Scale (ESRS) score for haloperidol compared with placebo (SMD 0.51, 95% CI –0.06 to 1.07; n = 11) (Figure 9).
FIGURE 9.
Standardised post-treatment ESRS score for haloperidol compared with placebo in first phase of the child study. IV, independent variable; SE, standard error.
Abnormal involuntary movements, assessed by the Abnormal Involuntary Movement Scale (AIMS), were similar for haloperidol compared with placebo (SMD 0.11, 95% CI –0.27 to 0.48; n = 22) (Figure 10). There was no evidence of electrocardiovascular effects for haloperidol in this study and it was indistinguishable from placebo in its effects on heart rate, rhythm and waveform.
FIGURE 10.
Standardised post-treatment AIMS score for haloperidol compared with placebo in the child study. IV, independent variable; SE, standard error.
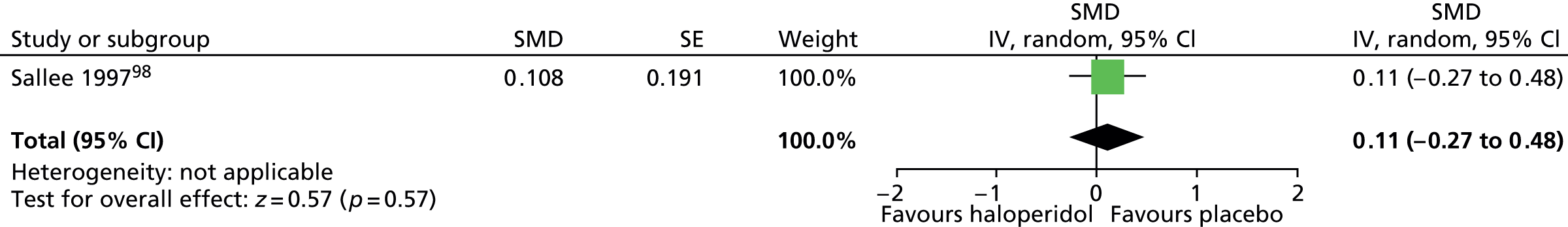
In the mixed study,99 based on the CGI score, there was evidence of increased adverse effects for haloperidol compared with placebo when rated by physicians (SMD 0.78, 95% CI 0.11 to 1.45; n = 37) and participants (SMD 0.75, 95% CI 0.08 to 1.42; n = 37) (Figure 11). In this study, for moderate or marked adverse effects, it is reported that there was no significant difference between haloperidol and placebo except for akinesia, and there were significantly more events in the haloperidol compared with the placebo group [haloperidol: 50% (9/18), placebo: 11% (2/19); p = 0.011].
FIGURE 11.
Standardised CGI adverse effects score assessed by physicians and patients in the mixed study for haloperidol compared with placebo. IV, independent variable; ROB, risk of bias.
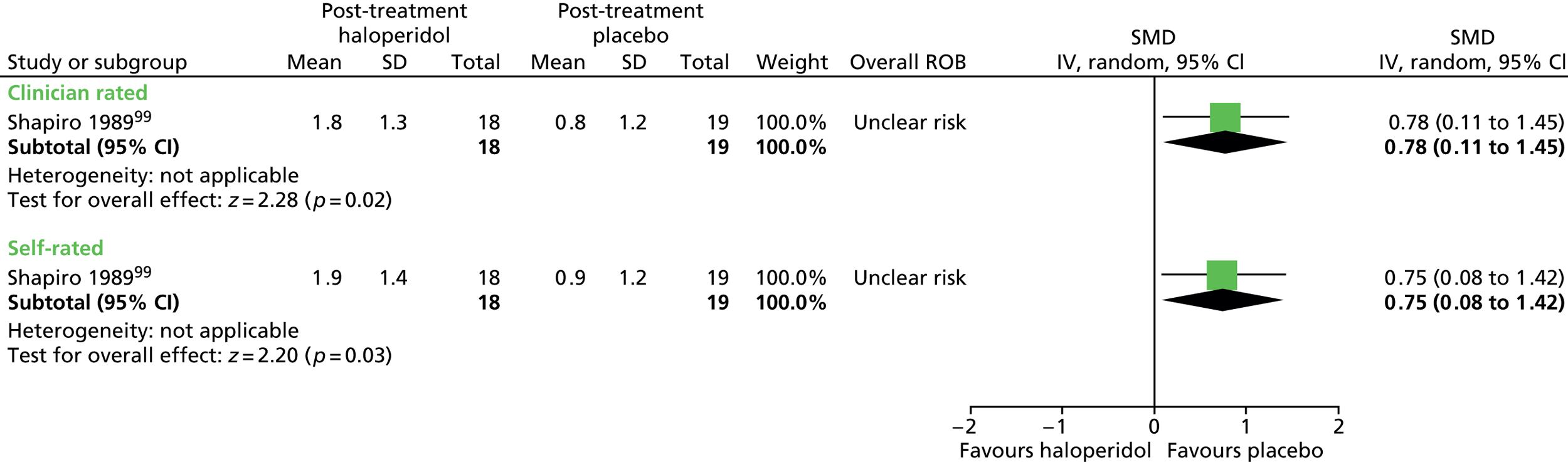
In the mixed study,99 investigators examined a large number of potentially moderating factors on outcomes of the CGI and Shapiro TSSS (52 factors examined, e.g. age, sex and history of tics). The only significant correlation was a negative correlation between age and CGI score [r = –0.56; p = 0.003, favoured younger age (age range of participants in the study was 8–65 years and was entered into the analysis as a continuous independent variable. The negative correlation of CGI score with age suggests that older participants in this age range (8–65 years) showed the least improvement with haloperidol)].
Pimozide compared with placebo
Tics
One child and two mixed studies measured intervention effects on tic scores for pimozide compared with placebo (Figure 12). The child study98 used the TS global scale tic score and the mixed studies used the Shapiro TSSS. 99,100 In the child study, results from the post-treatment TS global scale tic score suggested a benefit for pimozide compared with placebo (SMD –0.81, 95% CI –1.24 to –0.38; n = 22), but was inconclusive when results from the two mixed studies were pooled (SMD –0.75, 95% CI –1.95 to 0.46; n = 59). There was heterogeneity between the two mixed studies (I2 = 85).
FIGURE 12.
Standardised post-treatment total tic scores for pimozide compared with placebo in the child and mixed studies. df, degrees of freedom; ROB, risk of bias; SE, standard error.
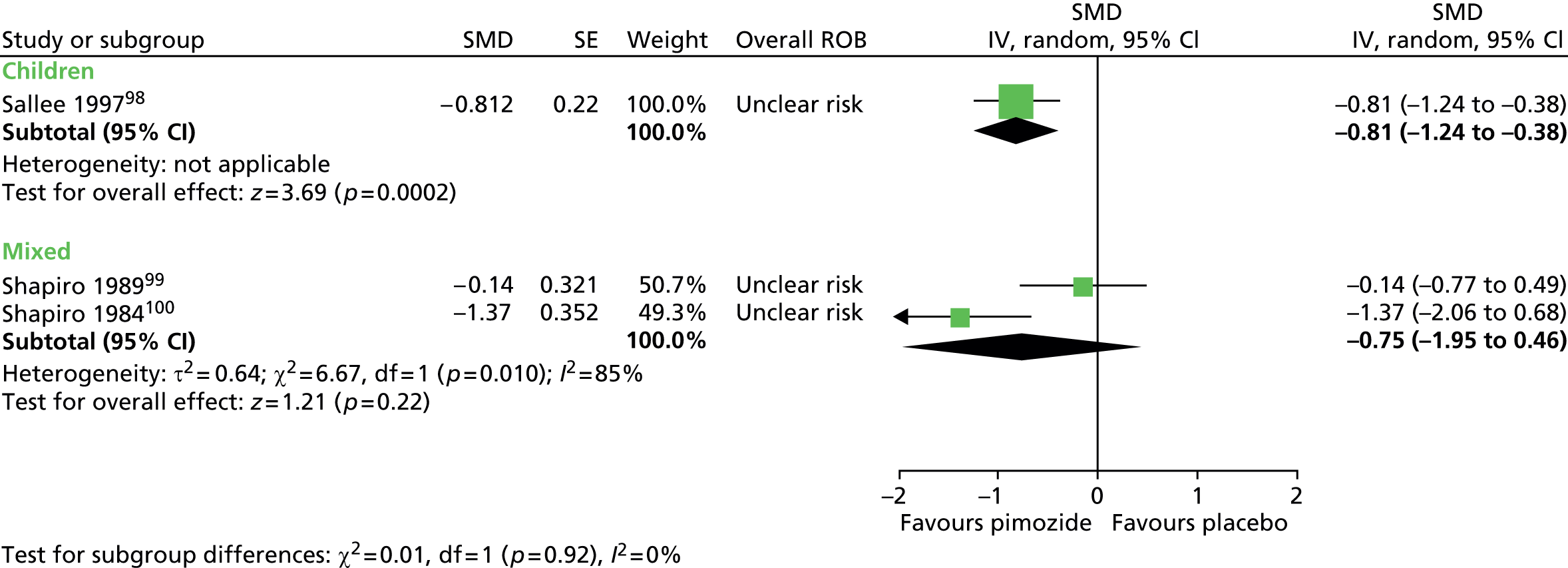
The studies presented data separately for motor (Figure 13) and vocal (Figure 14) tic components. The results for post-treatment motor tic score suggested a benefit for pimozide compared with placebo in children (SMD –0.75, 95% CI –1.17 to –0.32; n = 22) and the mixed group (SMD –0.94, 95% CI –1.76 to –0.12; n = 59). There was heterogeneity between the two mixed studies (I2 = 67%).
FIGURE 13.
Standardised post-treatment motor tic scores for pimozide compared with placebo in the child and mixed studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
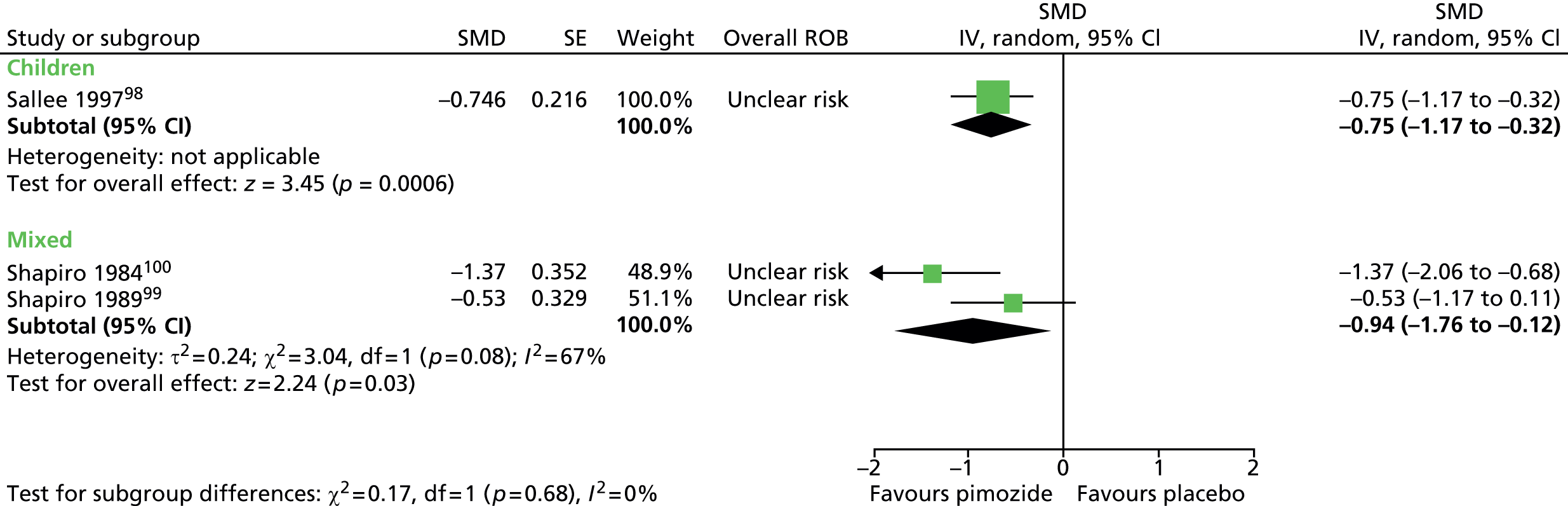
FIGURE 14.
Standardised post-treatment vocal tic score for pimozide compared with placebo in the child and mixed studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

The post-treatment vocal tic score suggested benefit for pimozide compared with placebo in children (SMD –0.66, 95% CI –1.07 to –0.25; n = 22), but the results were inconclusive when the two mixed studies were pooled (SMD –0.63, 95% CI –1.54 to 0.28; n = 59). There was heterogeneity between mixed studies (I2 = 75%).
One child study reported post-treatment results for global TS severity (contains tic and impairment components). 98 The results suggested a benefit for pimozide compared with placebo (SMD –0.65, 95% CI –1.06 to –0.23; n = 22) (Figure 15).
FIGURE 15.
Standardised post-treatment TS global score for pimozide compared with placebo in children. IV, independent variable; SE, standard error.
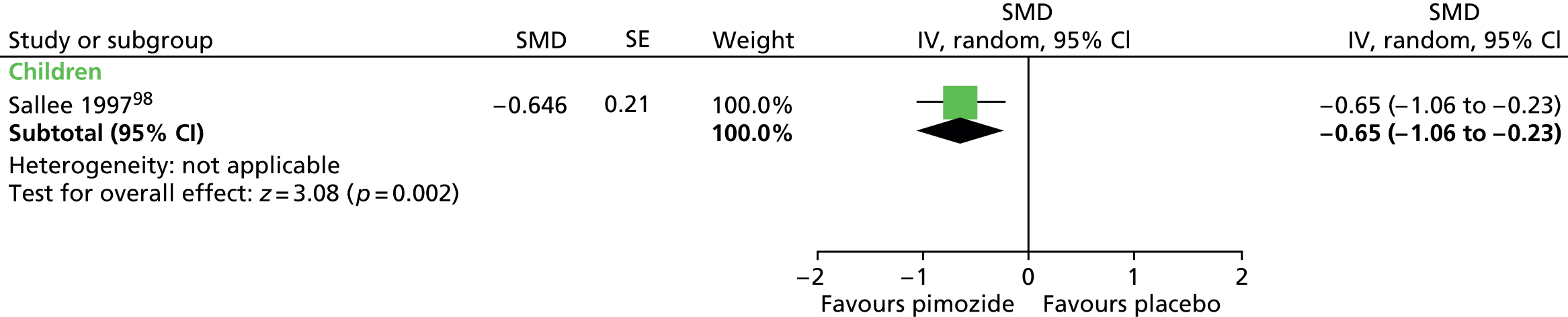
Impairment was also assessed with the C-GAS and post-treatment results suggested a benefit for pimozide compared with placebo (SMD –0.64, 95% CI –1.05 to –0.23; n = 22) (C-GAS higher scores show favourable effect, for consistency with other outcomes, scores reversed here) (Figure 16).
FIGURE 16.
Standardised post-treatment C-GAS score for pimozide compared with placebo in the child study. IV, independent variable; SE, standard error.
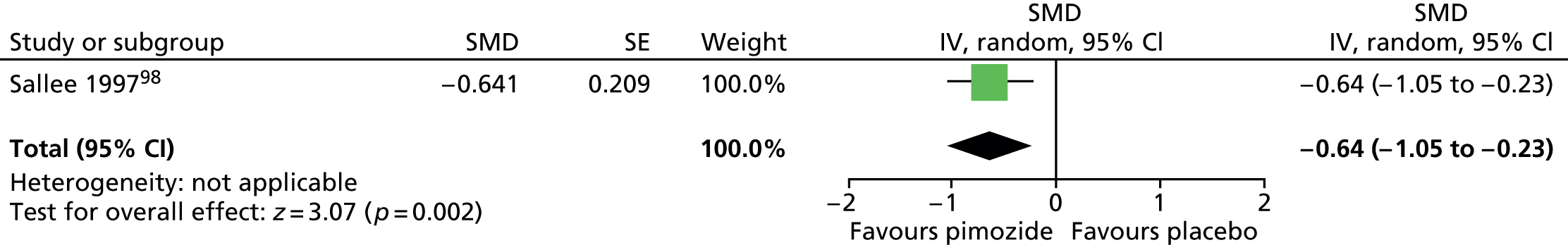
No relevant studies reported this outcome.
In the child study,98 post-treatment CGI-Severity score was measured. The results suggested a benefit for pimozide compared with placebo (SMD –1.23, 95% CI –1.73 to –0.74; n = 22) (Figure 17).
FIGURE 17.
Standardised post-treatment CGI-Severity score for children following treatment with pimozide compared with placebo in the child study. IV, independent variable; SE, standard error.

In one of the mixed studies,99 CGI score was assessed by clinicians and participants (unclear which type of CGI score this is, higher score appears to indicate better efficacy, axis have been reversed for clarity). Treatment with pimozide resulted in better CGI scores when assessed by clinicians (SMD –0.70, 95% CI –1.35 to –0.05; n = 39) and participants (SMD –0.68, 95% CI –1.33 to –0.04; n = 39) (Figure 18).
FIGURE 18.
Standardised CGI-Severity score for patients undergoing treatment with pimozide compared with placebo in the mixed study. IV, independent variable; ROB, risk of bias; SE, standard error.
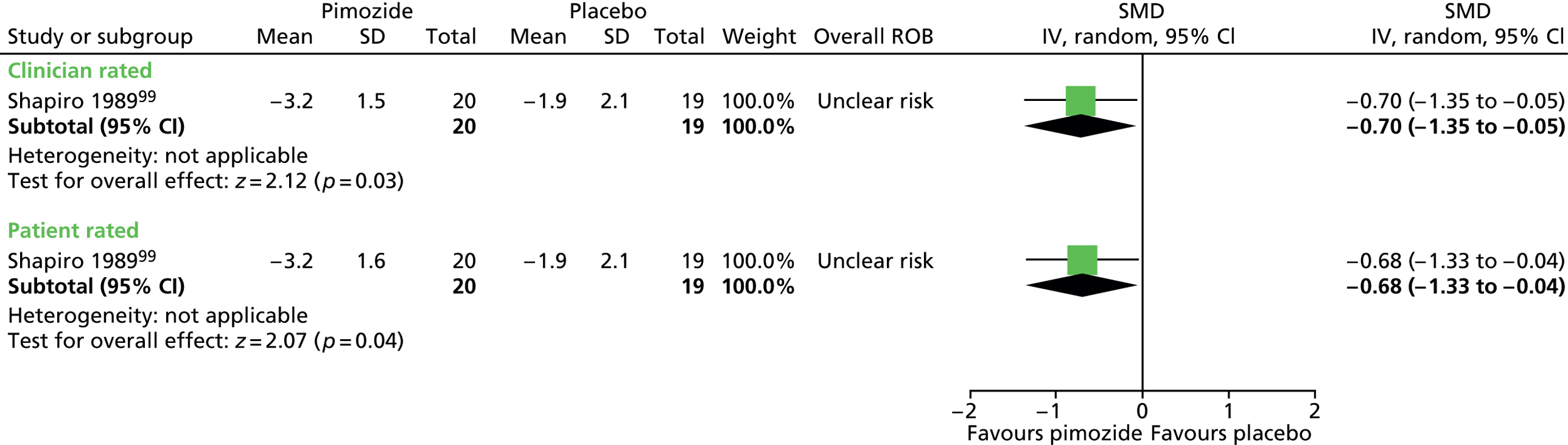
No relevant randomised trials reported long-term outcomes.
In the child study,98 it is reported that general adverse effects did not differ between treatment groups (p = 0.94). For EPS, because a carryover effect was detected in the whole study analysis, investigators analysed just the first-phase data for this outcome and ESRS scores were similar for pimozide compared with placebo (SMD 0.20, 95% CI –0.33 to 0.73; n = 11) (Figure 19).
FIGURE 19.
Standardised post-treatment ESRS score for pimozide compared with placebo in first phase of the child study. IV, independent variable; SE, standard error.

In this study, abnormal involuntary movements, assessed by the AIMS, were similar for pimozide compared with placebo (SMD 0.22, 95% CI –0.16 to 0.60; n = 22) (Figure 20). There was no evidence of electrocardiovascular effects for pimozide and it was indistinguishable from placebo in its effects on heart rate, rhythm and waveform.
FIGURE 20.
Standardised post-treatment AIMS score for pimozide compared with placebo in the child study. IV, independent variable; SE, standard error.

In the mixed study,99 for adverse effects rated on the CGI scale, there was no conclusive difference in adverse effect scores for pimozide compared with placebo when rated by physicians (SMD 0.44, 95% CI –0.19 to 1.08; n = 39) or participants (SMD 0.31, 95% CI –0.32 to 0.95; n = 39) (Figure 21). In this study, for moderate or marked adverse effects, it is reported that there was no significant difference in event rates for pimozide compared with placebo.
FIGURE 21.
Standardised CGI adverse effects scale for pimozide compared with placebo assessed by physicians and patients in the mixed study. IV, independent variable; ROB, risk of bias; SE, standard error.

In the safety study comparing pimozide with aripiprazole,109 there was no conclusive difference in heart rate for pimozide compared with aripiprazole [mean difference (MD) 4.90, 95% CI –1.16 to 10.96; n = 50], but there were greater reductions from baseline in systolic (MD –20.00, 95% CI –26.56 to –13.44; n = 50) and diastolic (MD –13.70, 95% CI –17.39 to –10.01; n = 50) blood pressure (BP) and greater increases in ECG-QT (MD 25.50, 95% CI 12.69 to 38.31; n = 50) and ECG-QTc (MD 19.10, 95% CI 10.07 to 28.31; n = 50) compared with aripiprazole.
In the mixed study,99 investigators examined a large number of potentially moderating factors on outcomes of the CGI and Shapiro TSSS (52 factors examined, e.g. age, sex and history of tics). The only significant correlation was a negative correlation between age and CGI score (r = –0.39; p = 0.04, favoured younger age).
Risperidone compared with placebo
One child101 and one mixed102 parallel trial compared the efficacy of risperidone with placebo.
Tics
Results from the YGTSS total tic score suggested a benefit for risperidone compared with placebo in children (SMD –1.10, 95% CI –1.94 to –0.26; n = 26) and in the mixed study in terms of Shapiro TSSS tic (SMD –0.62, 95% CI –1.21 to –0.03; n = 46) (Figure 22).
FIGURE 22.
Standardised change in total tic scores for risperidone compared with placebo in the child and mixed study. df, degrees of freedom; IV, independent variable; SE, standard error.

No relevant studies reported this outcome.
No relevant studies reported this outcome.
In both studies, the CGI scale was used to assess overall clinical change. In the child study, findings were dichotomised as the proportion of children who were much or very much improved compared with those who were not and, in the mixed study, findings were reported as the reduction in CGI-Severity score. A greater proportion of children were much or very much improved following treatment with risperidone compared with placebo (RR 0.27, 95% CI 0.10 to 0.73; n = 26) (Figure 23).
FIGURE 23.
Proportion of children CGI much or very much improved for risperidone compared with placebo in children (RR of not being much or very much improved). M–H, Mantel–Haenszel; ROB, risk of bias.

In the mixed study, the CGI-Severity score following treatment suggested a benefit for risperidone compared with placebo (SMD –0.66, 95% CI –1.26 to –0.07; n = 46) (Figure 24).
FIGURE 24.
Standardised change in CGI-Severity score for risperidone compared with placebo in the mixed study. IV, independent variable; ROB, risk of bias.
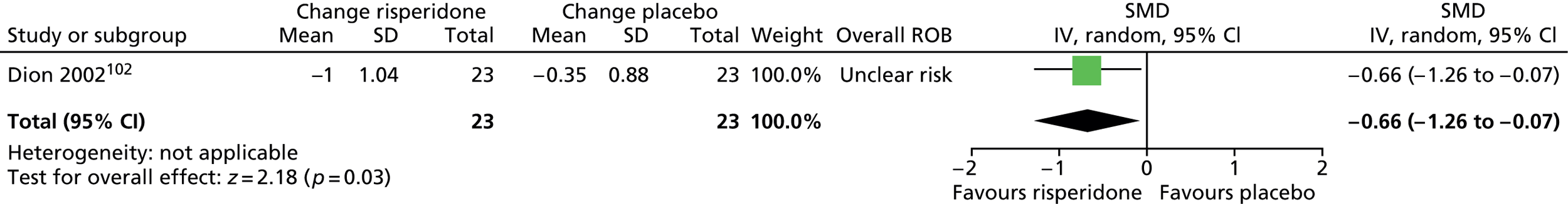
No relevant studies reported long-term outcomes.
Both studies recorded adverse effects [for child study, adverse effects reported for the whole study population (n = 34) and not the separate paediatric sample]. Common adverse effects reported in both studies are shown in Figure 25 and adverse effects reported in only one or other of the studies, and with an event rate of > 5, are shown in Figure 26.
FIGURE 25.
Adverse events reported in the child and mixed studies of risperidone compared with placebo. df, degrees of freedom; M–H, Mantel–Haenszel; ROB, risk of bias.
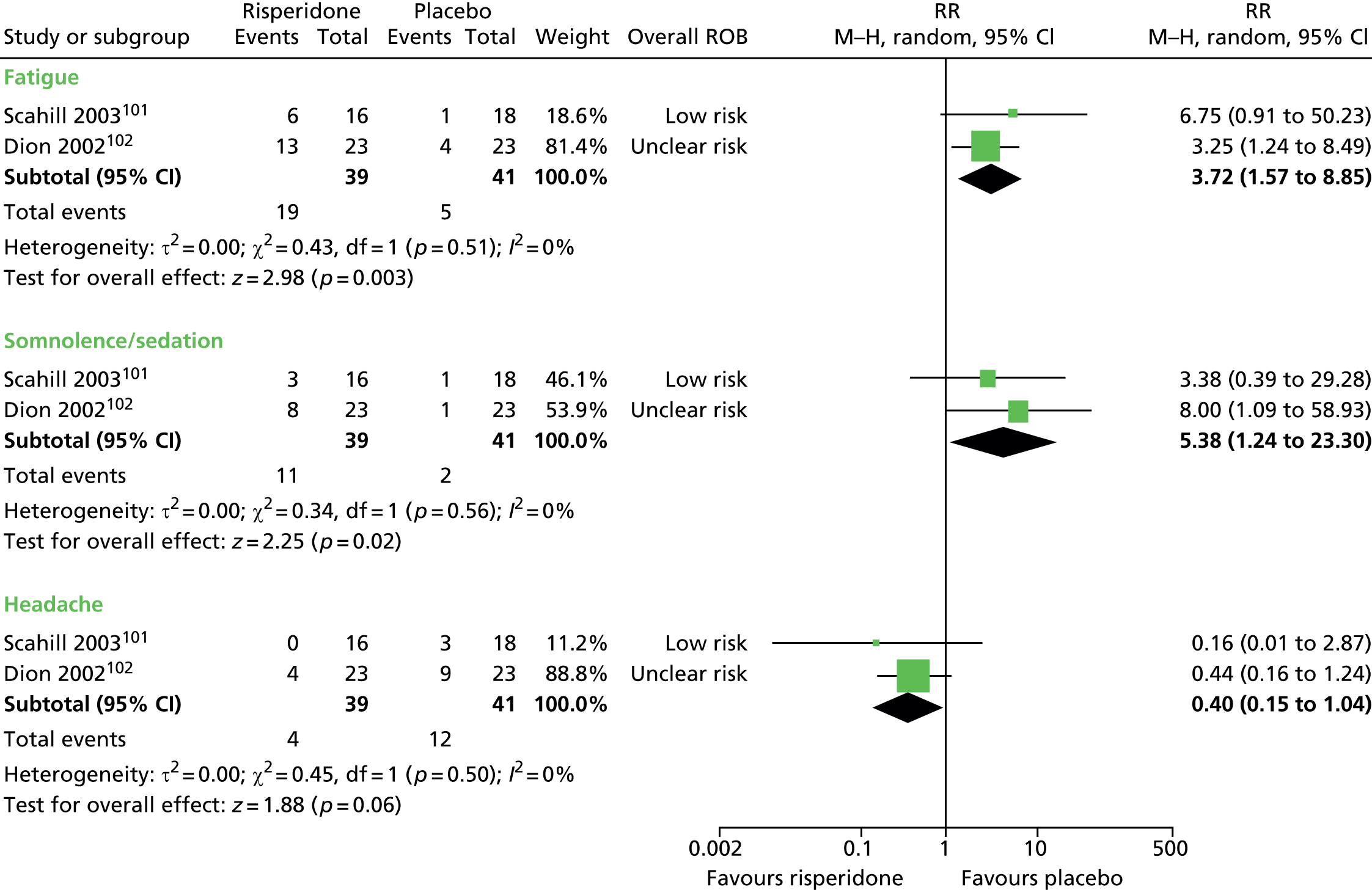
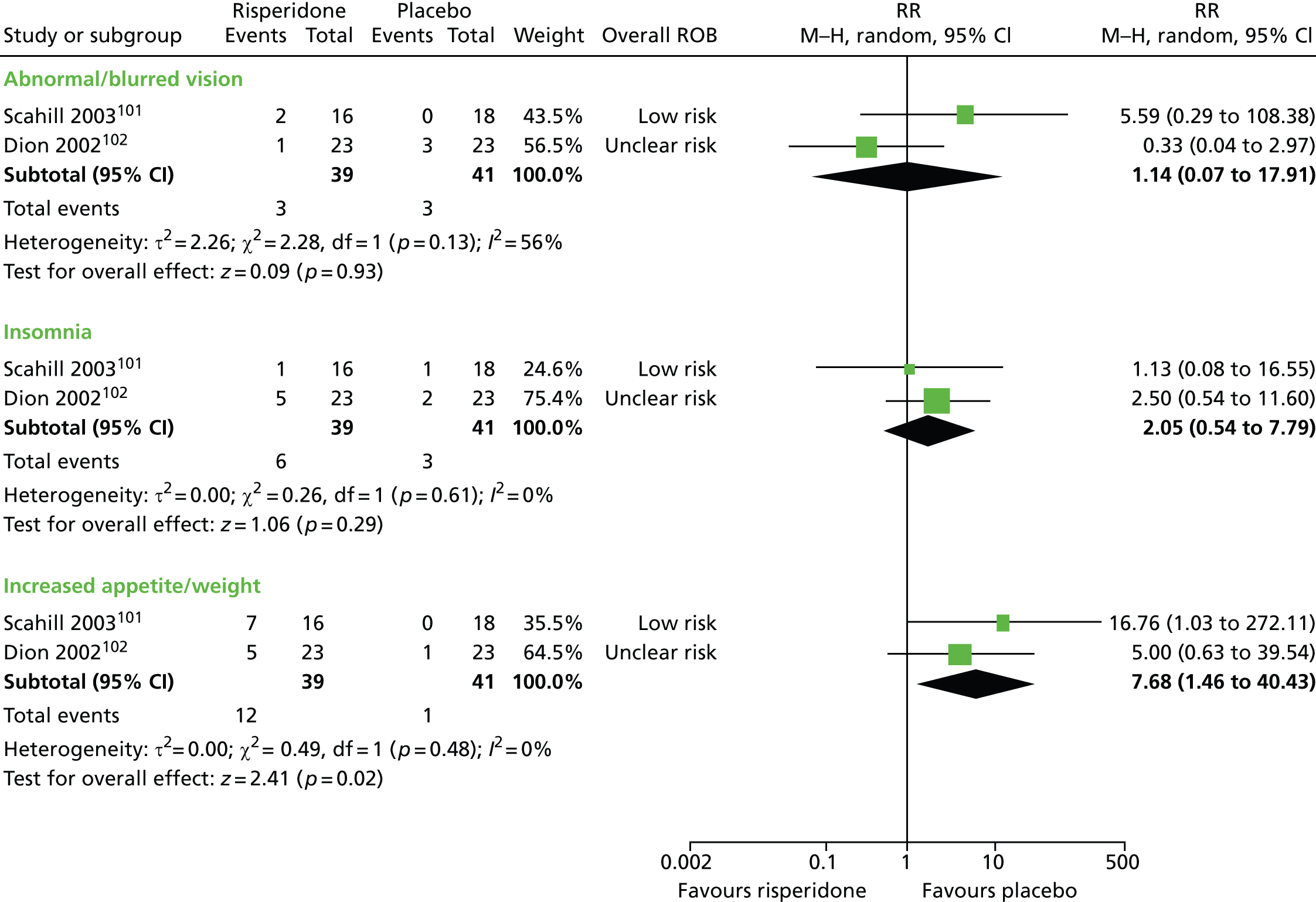
FIGURE 26.
Adverse events with more than five events reported in only the mixed study of risperidone compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.

For both studies combined, there were higher rates of fatigue (RR 3.72, 95% CI 1.57 to 8.85; n = 80), somnolence/sedation (RR 5.38, 95% CI 1.24 to 23.30; n = 80) and increased appetite/weight gain (RR 7.68, 95% CI 1.46 to 40.43; n = 80) but similar rates of headache (RR 0.40, 95% CI 0.15 to 1.04; n = 80) and abnormal vision (RR 1.14, 95% CI 0.07 to 17.91; n = 80) for risperidone compared with placebo.
For outcomes reported in only one of the studies with > 5 events (all from the mixed study), there was no conclusive difference in rates of dizziness (RR 1.50, 95% CI 0.64 to 3.53; n = 46), depression (RR 6.00, 95% CI 0.78 to 45.99; n = 46), increased saliva (RR 2.00, 95% CI 0.57 to 7.05; n = 46), tremor (RR 3.00, 95% CI 0.67 to 13.34; n = 46), dry mouth (RR 2.50, 95% CI 0.54 to 11.60; n = 46) or hypertonia (RR 1.33, 95% CI 0.34 to 5.30; n = 46).
In the study with the child subgroup,101 for the whole mixed study population there was an increase in weight of 2.8 kg in the risperidone group compared with no change in the placebo group (p = 0.0001). The study reported that a similar increase was observed in the paediatric sample alone. In this study, no EPS were reported and there were no abnormalities or clinically significant changes in laboratory values, cardiovascular indices or vital signs. Two children taking risperidone had acute social phobia and treatment was reduced in one child and discontinued in the other.
In the mixed study,102 the ESRS was used (Figure 27). The parkinsonism examination total score of the ESRS suggested a harm for risperidone compared with placebo (SMD 1.01, 95% CI 0.39 to 1.63; n = 46) but results from the dystonia subscale (SMD 0.00, 95% CI –0.58 to 0.58; n = 46) and the dyskinetic movements subscale (SMD –0.09, 95% CI –0.67 to 0.49; n = 46) were inconclusive.
FIGURE 27.
Standardised ESRS scores for risperidone compared with placebo in the mixed study. IV, independent variable; ROB, risk of bias.

Ziprasidone compared with placebo
One small parallel trial in children compared the efficacy of ziprasidone with placebo. 98
Tics
Tics were assessed with the YGTSS. The results suggested a benefit for ziprasidone when compared with placebo (SMD –1.10, 95% CI –1.93 to –0.27; n = 27) (Figure 28).
FIGURE 28.
Standardised post-treatment YGTSS total tic score for ziprasidone compared with placebo. IV, independent variable; ROB, risk of bias.

No results for impairment were reported separately but impairment was measured as part of the YGTSS global scale. There was a greater reduction from baseline in YGTSS global score for ziprasidone compared with placebo (SMD –1.02, 95% CI –1.84 to –0.20; n = 27) (Figure 29).
FIGURE 29.
Standardised post-treatment YGTSS global score for ziprasidone compared with placebo. IV, independent variable; ROB, risk of bias.

No relevant studies reported this outcome.
Overall clinical outcome was assessed using the CGI-Severity scale, but the results were not conclusive (SMD –0.71, 95% CI –1.50 to 0.09; n = 27) (Figure 30).
FIGURE 30.
Standardised change in CGI-Severity score for ziprasidone compared with placebo. IV, independent variable; ROB, risk of bias.

No relevant studies reported long-term outcomes.
There were a greater number of participants experiencing treatment-emergent adverse effects for ziprasidone compared with placebo (RR 1.68, 95% CI 1.05 to 2.70; n = 28) (Figure 31).
FIGURE 31.
Number of patients experiencing adverse events for ziprasidone compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.
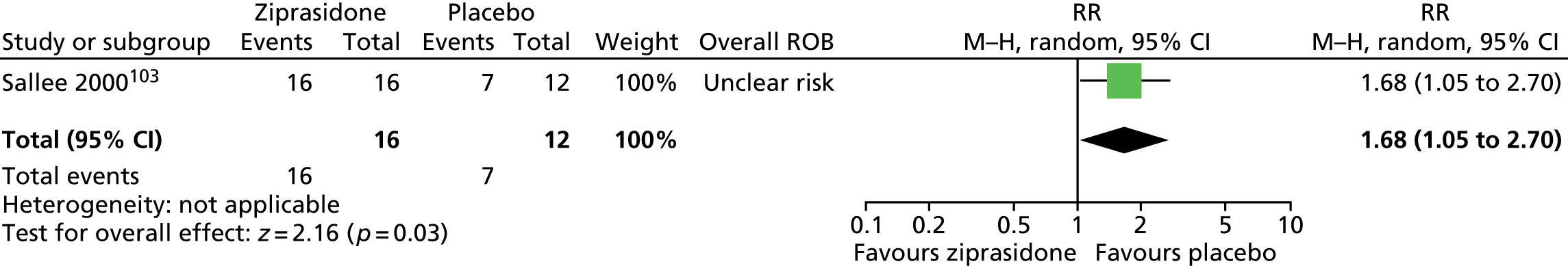
Most events were considered mild or moderate. One case of somnolence and one of akathisia were experienced in the ziprasidone group at maximum drug dosage (dosage was reduced but treatment did not have to be discontinued). The most common adverse effect was mild sedation (11 out of 16 in ziprasidone group compared with 5 out of 12 in the placebo group had increase in sedation score at some point, χ2 = 1.44; p = 0.21).
The increase in body weight was similar for the ziprasidone (+ 0.7 ± 1.5 kg) and placebo (+ 0.8 ± 2.3 kg) group (MD –0.1, 95% CI –1.59 to 1.39; n = 28) (Figure 32) and it was reported that there were no discernible patterns in abnormalities detected on laboratory tests and there were no between-group differences in vital signs, pulse rate, standing or sitting BP or ECG parameters.
FIGURE 32.
Change in body weight (kg) for ziprasidone compared with placebo. IV, independent variable; ROB, risk of bias.
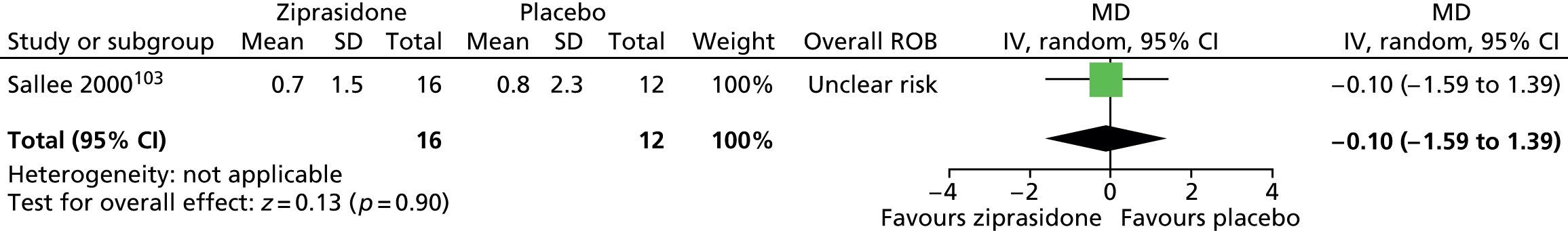
Risperidone compared with pimozide
One child crossover study104 and one mixed parallel study105 compared the efficacy of risperidone with pimozide.
Tics
In the child study,104 tic outcome was assessed with the YGTSS, whereas in the mixed study105 tic outcome was assessed with the Shapiro TSSS. Results were not conclusive (Figure 33).
FIGURE 33.
Standardised tic scores for treatment with risperidone compared with pimozide in the child and mixed studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
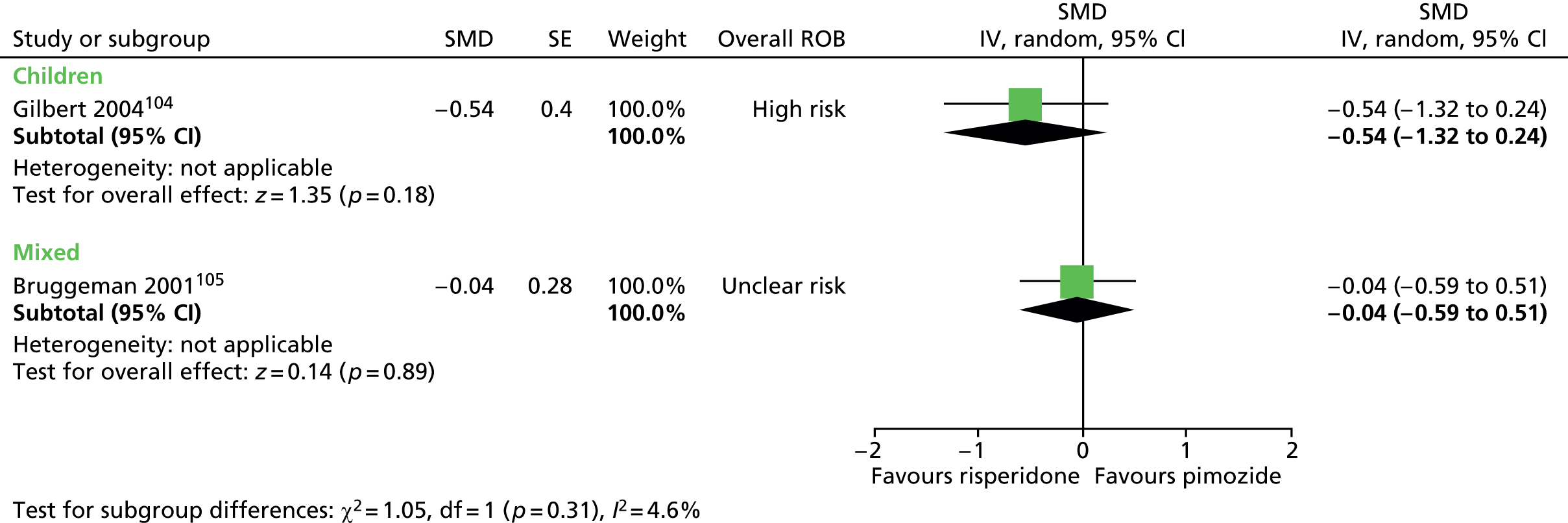
Within the mixed study, a subgroup analysis was conducted of participants aged < 18 years or ≥ 18 years. It was reported that, although efficacy tended to be better for both drugs in the younger age group, there was no significant difference for pimozide compared with risperidone for participants within either age group.
In the child study,104 impairment was measured with the YGTSS impairment scale. In the mixed study,105 impairment was measured with the Global Assessment of Functioning scale, which assesses changes in social and occupational functioning. Results from both the child study (n = 13) and the mixed study (n = 50) were not conclusive, although there was some evidence of a difference in the effect between the child and the mixed study (I2 = 72% for the subgroup difference) (Figure 34).
FIGURE 34.
Standardised impairment scores for treatment with pimozide compared with risperidone in the child and mixed studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
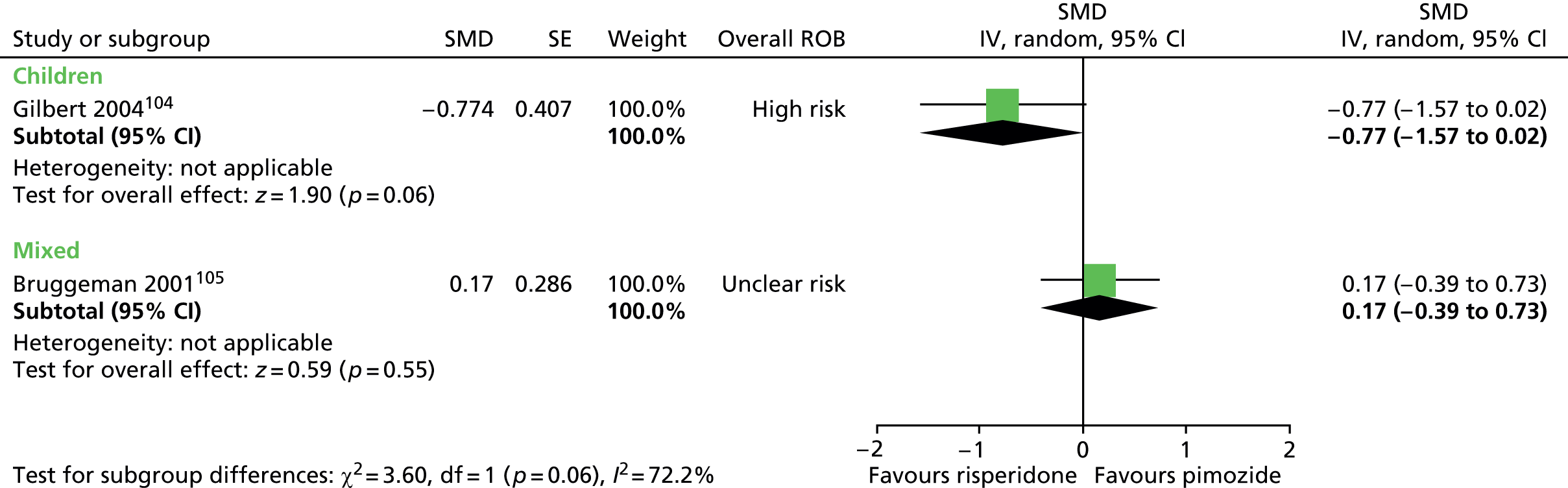
In the mixed study (n = 50),105 anxiety was assessed using the Hamilton Rating Scale for Anxiety, but the results were inconclusive (Figure 35).
FIGURE 35.
Standardised change from baseline Hamilton Rating Scale for Anxiety total score for risperidone compared with pimozide in the mixed study. IV, independent variable; SE, standard error.
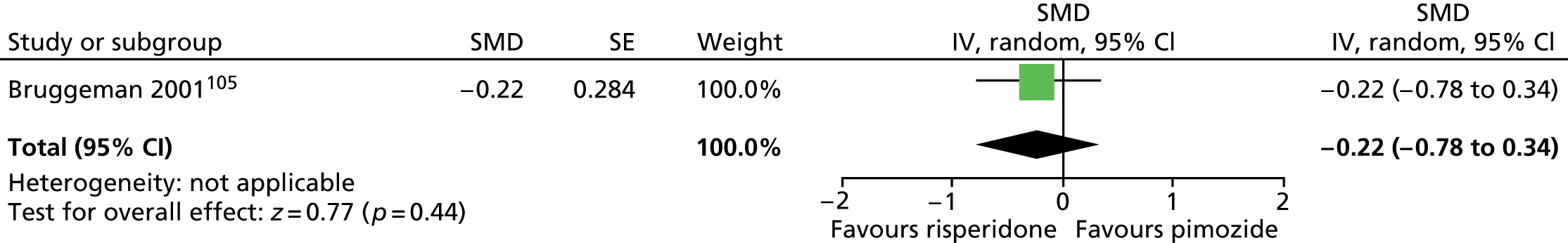
In the child study,104 overall clinical outcome was assessed with the Clinical Global Impressions – Improvement (CGI-I) scale and, in the mixed study105 outcome was assessed as the change from baseline on the CGI-Severity scale. In both the child study (n = 13) and the mixed study (n = 50) the results were inconclusive (Figure 36).
FIGURE 36.
Standardised CGI scores for treatment with risperidone compared with pimozide in the child and mixed studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
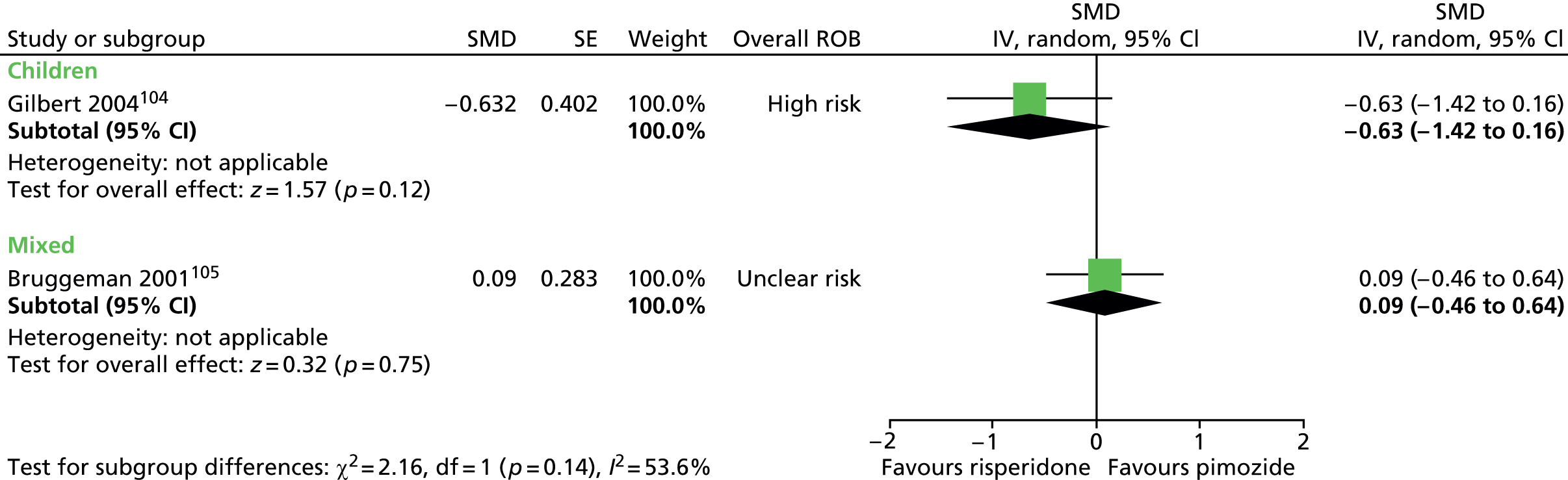
No relevant studies reported long-term outcomes.
In both studies, participants’ weight was measured before and after treatment. In both studies, there was a trend suggesting increased weight gain for risperidone compared with pimozide. The overall difference in weight gain for risperidone compared with pimozide was 0.77 kg (95% CI –0.18 to 1.73 kg; n = 80) (Figure 37).
FIGURE 37.
Weight gain (kg) following treatment with risperidone compared with pimozide in the child and mixed studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
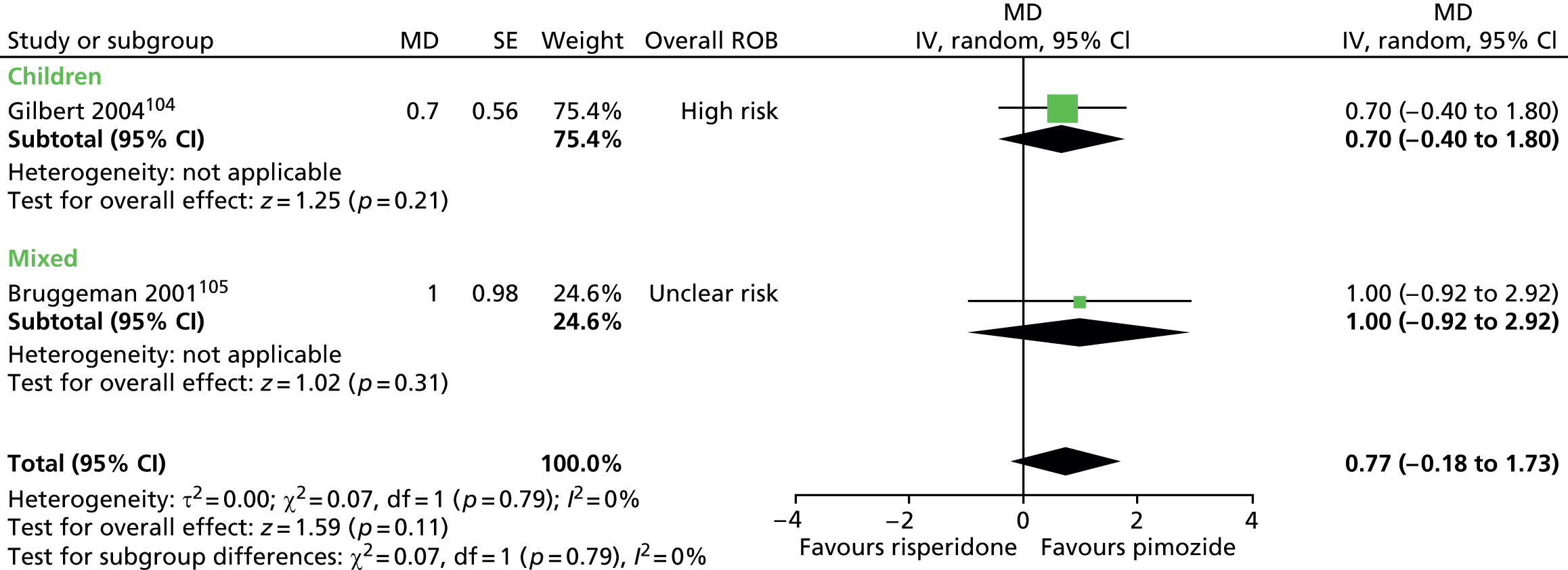
In the mixed study,105 participants were subgrouped by age and it is reported that weight gain during risperidone administration was greater for participants aged ≤ 18 years than for those > 18 years but, for pimozide, it was similar for participants aged ≤ 18 years and > 18 years (no data reported).
In both studies, the ESRS was used to assess any abnormal involuntary movements. There were similar post-treatment ESRS scores for risperidone compared with pimozide in the child study (SMD –0.05, 95% CI –0.77 to 0.66; n = 30) (Figure 38) and in the mixed study there was no change in the risperidone group and a reduction of 0.1 in the pimozide group (no precision reported).
FIGURE 38.
Standardised post-treatment ESRS score for risperidone compared with pimozide in the child study. IV, independent variable; ROB, risk of bias; SE, standard error.
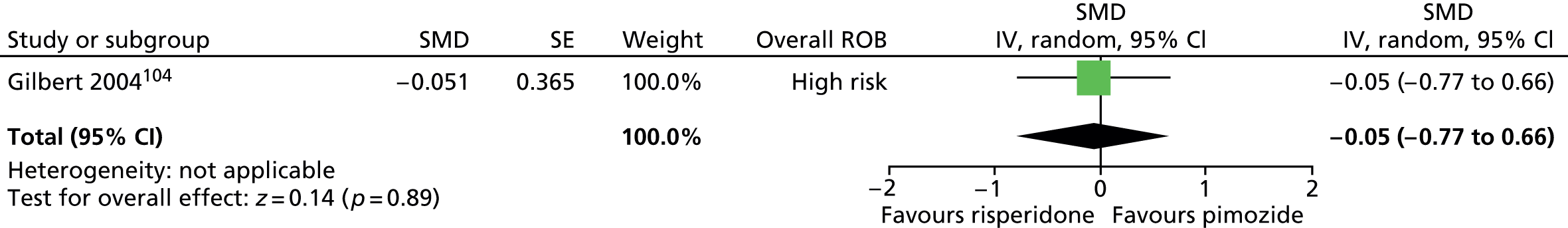
In the child study,104 the Side Effects Checklist was used and there were similar post-treatment scores for risperidone compared with pimozide (SMD –0.12, 95% CI –0.84 to 0.59; n = 30) (Figure 39).
FIGURE 39.
Standardised post-treatment Side Effects Checklist score for risperidone compared with pimozide in the child study. IV, independent variable; ROB, risk of bias; SE, standard error.
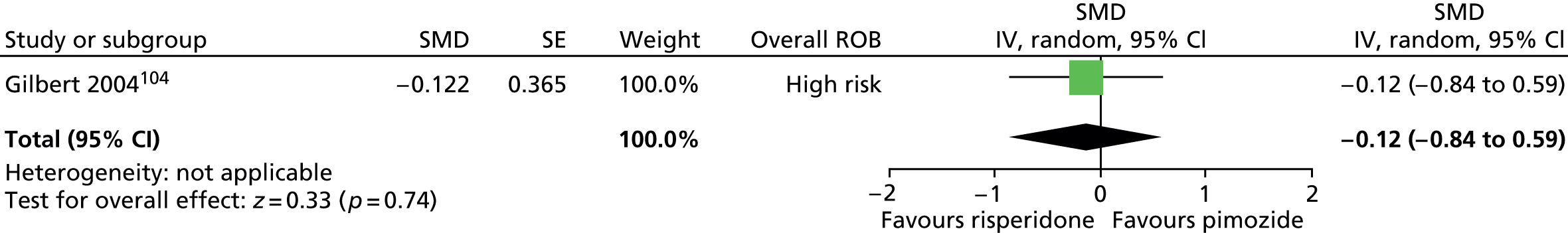
In the mixed study,105 adverse effect event rates were reported separately. There was a lower incidence of insomnia (RR 0.13, 95% CI 0.02 to 0.99; n = 50) but no conclusive difference in EPS-like adverse effects (RR 0.46, 95% CI 0.16 to 1.34; n = 50), or injuries (RR 0.15, 95% CI 0.02 to 1.19; n = 50) for risperidone compared with pimozide (Figure 40).
FIGURE 40.
Proportion of patients with EPS-like symptoms, insomnia and injuries for risperidone compared with pimozide in the mixed study. M–H, Mantel–Haenszel; ROB, risk of bias.
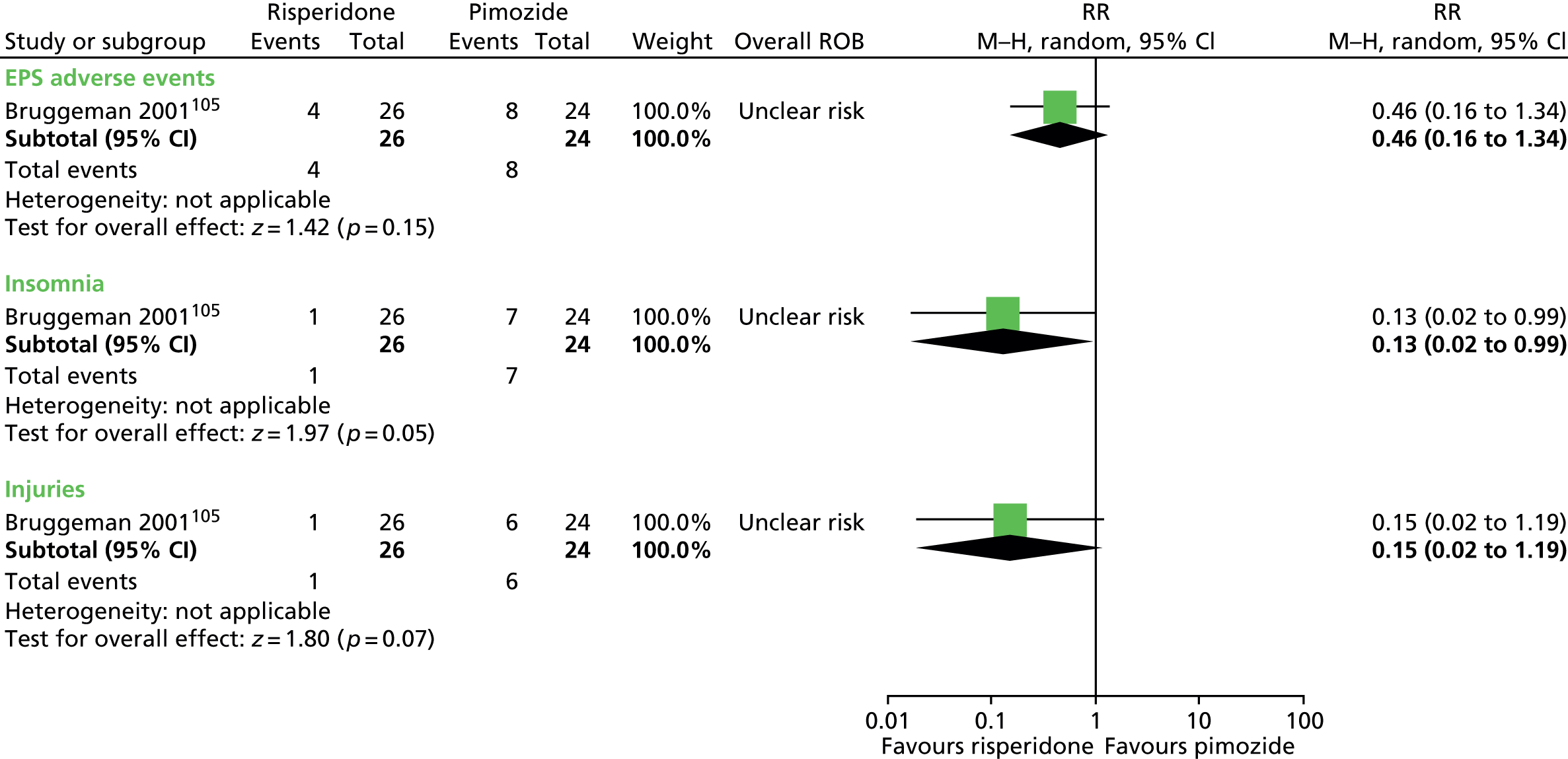
In both studies, cardiovascular measures were made and, for both studies, it was reported that there were no significant differences in any ECG parameters (including QTC) for pimozide compared with risperidone.
Haloperidol compared with pimozide
One child crossover trial98 and two mixed trials (one parallel99 and one crossover106) evaluated treatment with haloperidol compared with pimozide.
Tics
In the child study,98 tic outcome was assessed with the TS global scale. In one mixed study, they were assessed with the Shapiro TSSS99 and in the other as tic count per 5 minutes. 106 Results from both the child study (n = 22) and the mixed studies (n = 47) were inconclusive. There was no evidence of heterogeneity between the mixed studies (I2 = 0%) (Figure 41).
FIGURE 41.
Standardised tic scores for haloperidol compared with pimozide in the child and mixed studies. df, degrees of freedom; IV, independent variable; SE, standard error.
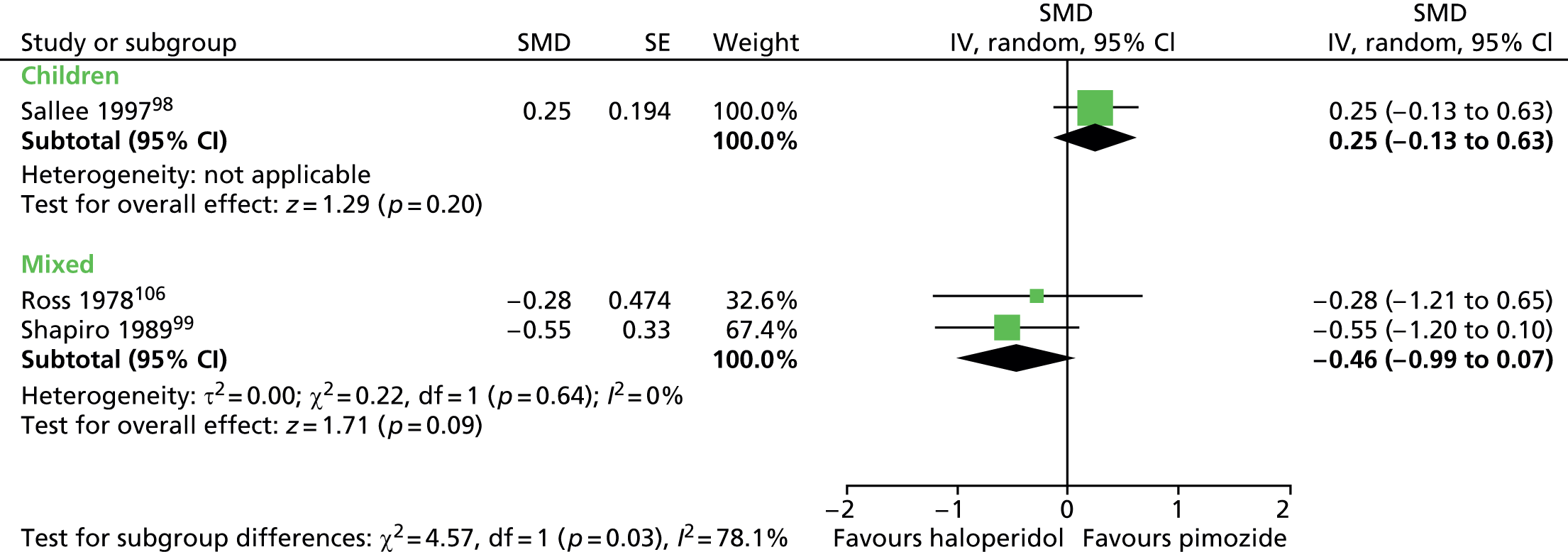
The child study98 and one of the mixed studies99 reported results for separate measures of motor and vocal tics, but results were inconclusive (Figure 42).
FIGURE 42.
Standardised post-treatment motor tic scores for haloperidol compared with pimozide in the child and mixed studies. df, degrees of freedom; IV, independent variable; SE, standard error.
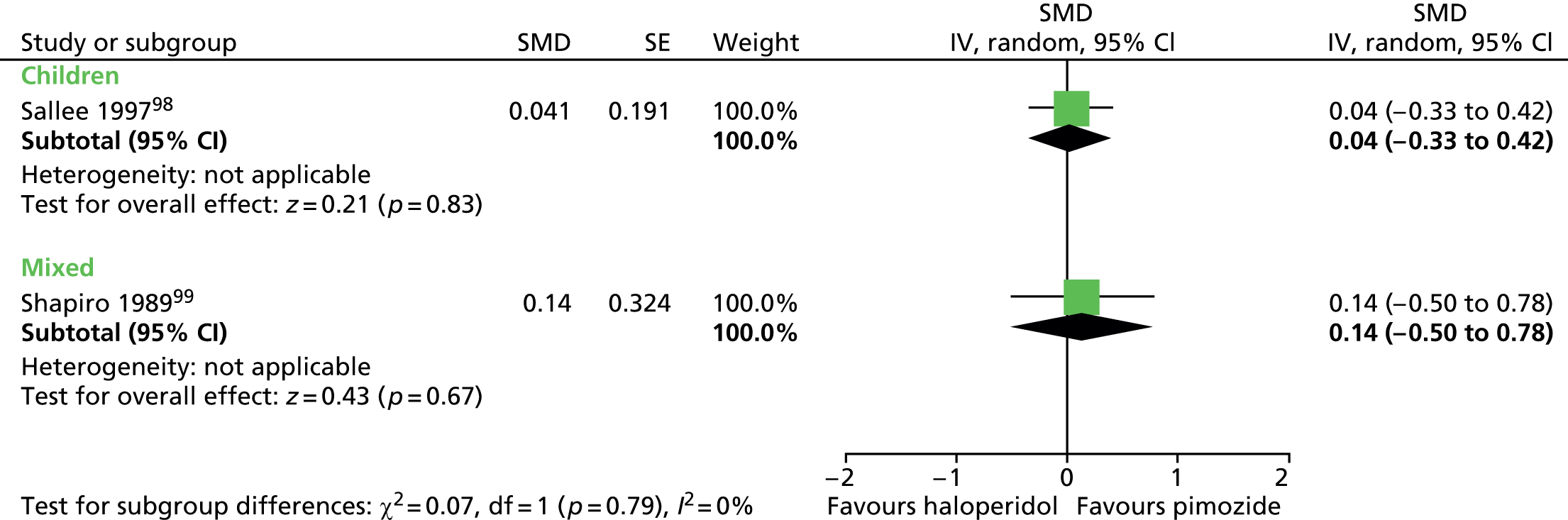
For vocal tics, results from post treatment were inconclusive for the child study (n = 22) and the mixed study (n = 38) (Figure 43).
FIGURE 43.
Standardised post-treatment vocal tic scores for haloperidol compared with pimozide in the child and mixed studies. df, degrees of freedom; IV, independent variable; SE, standard error.
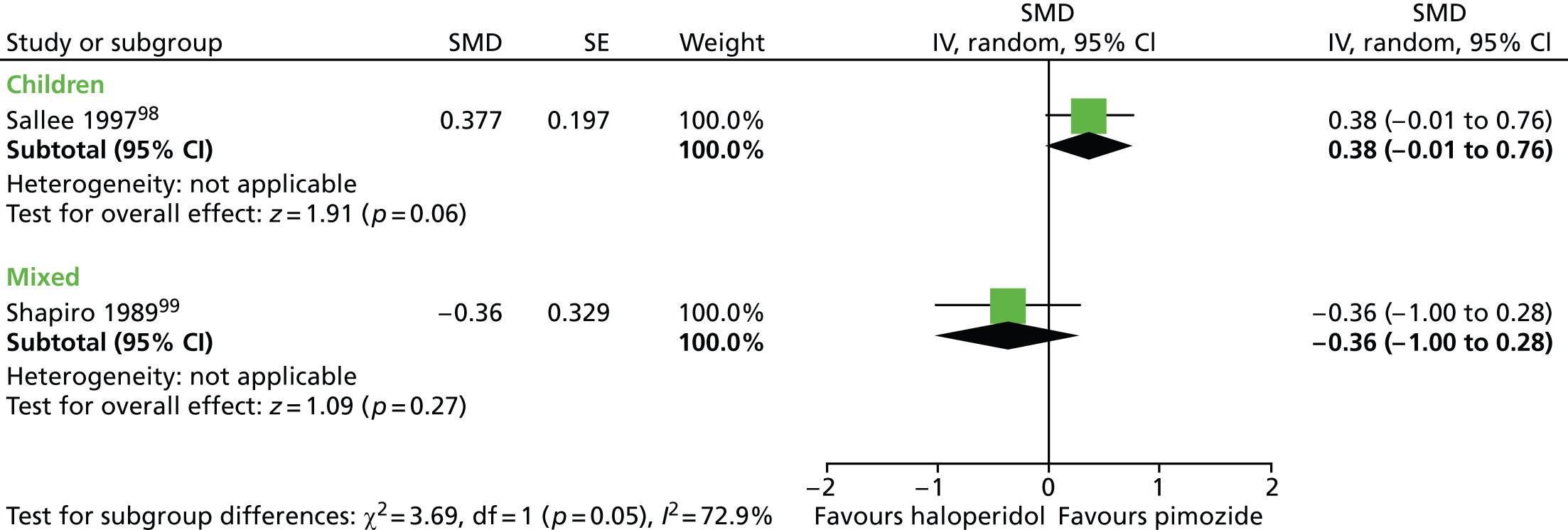
Only the child study assessed impairment and used the C-GAS. Post-treatment C-GAS score was similar for haloperidol compared with pimozide (SMD 0.14, 95% CI –0.24 to 0.52; n = 22) (Figure 44).
FIGURE 44.
Standardised post-treatment C-GAS score for haloperidol compared with placebo in the child study. IV, independent variable; SE, standard error.

No relevant studies reported this outcome.
The child98 and one of the mixed99 studies assessed overall clinical outcomes with the CGI scale. In the child study, post-treatment CGI-Severity score was similar for haloperidol and pimozide (SMD 0.00, 95% CI –0.37 to 0.37; n = 22) (Figure 45).
FIGURE 45.
Standardised post-treatment CGI-Severity score for haloperidol compared with pimozide in children. IV, independent variable; SE, standard error.
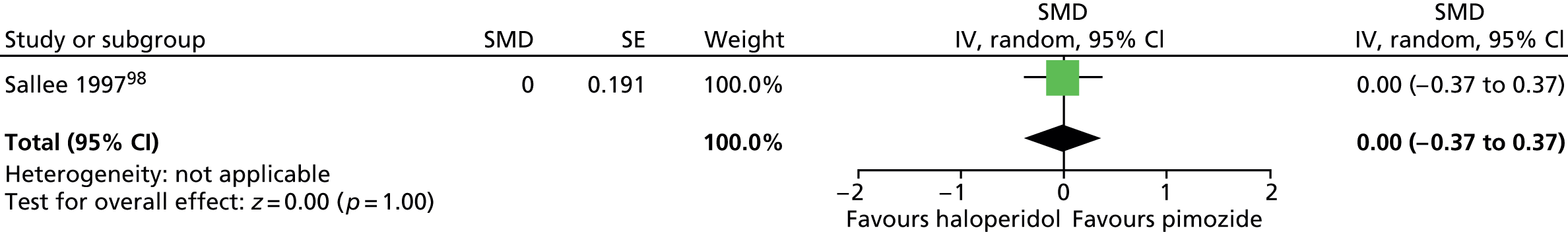
In the mixed study, the results were inconclusive for physician-rated CGI (SMD –0.13, –0.77 to 0.51; n = 38) and participant-rated CGI (SMD –0.19, 95% CI –0.83 to 0.44; n = 38) (Figure 46).
FIGURE 46.
Standardised post-treatment CGI score for haloperidol compared with pimozide in mixed parallel study. IV, independent variable; ROB, risk of bias.

No relevant studies reported long-term outcomes.
In the child study,98 it was reported that general adverse effects did not differ between treatment groups (p = 0.94). For EPS, because a carryover effect was detected in the whole study analysis, investigators analysed just the first-phase data for this outcome, but the results were inconclusive (Figure 47).
FIGURE 47.
Standardised post-treatment ESRS score for haloperidol compared with pimozide in the child study. IV, independent variable; SE, standard error.

For this study,98 post-treatment abnormal involuntary movements, assessed by the AIMS, were similar for haloperidol and pimozide (SMD –0.09, 95% CI –0.47 to 0.28; n = 22) (Figure 48).
FIGURE 48.
Standardised post-treatment AIMS score for haloperidol compared with pimozide in the child study. IV, independent variable; SE, standard error.
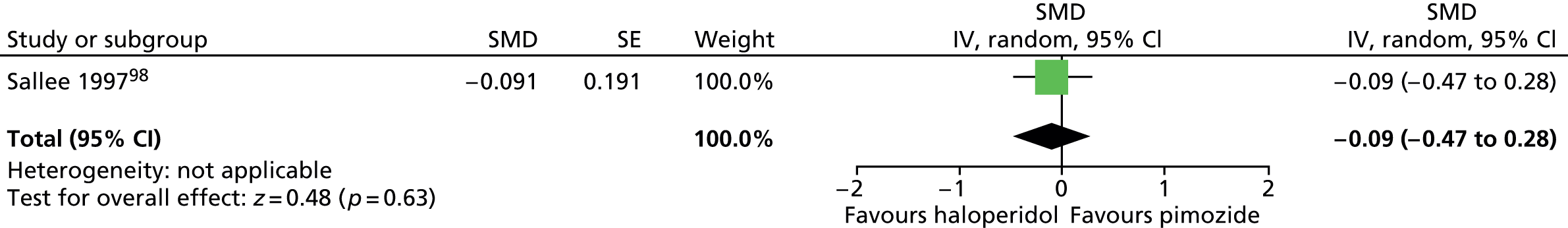
The number of children experiencing treatment-limiting adverse effects was greater for haloperidol compared with pimozide but not conclusively different (RR 3.00, 95% CI 0.94 to 9.62; n = 22) (Figure 49). It is reported that at least three haloperidol-treated participants developed treatment-emergent depression or anxiety and two participants experienced academic failure attributed to the effects of haloperidol. It is reported that most of the adverse effects caused by haloperidol were attributable to EPS and included akathisia (n = 2) and akinesia (n = 2). Two pimozide-treated participants experienced weight gain and one had treatment-emergent anxiety. It was reported that there was no evidence of electrocardiovascular effects for haloperidol or pimozide and both were indistinguishable from placebo in their effects on heart rate, rhythm and waveform.
FIGURE 49.
Proportion of children with treatment-limiting side effects for haloperidol compared with pimozide in the child study. M–H, Mantel–Haenszel; ROB, risk of bias.
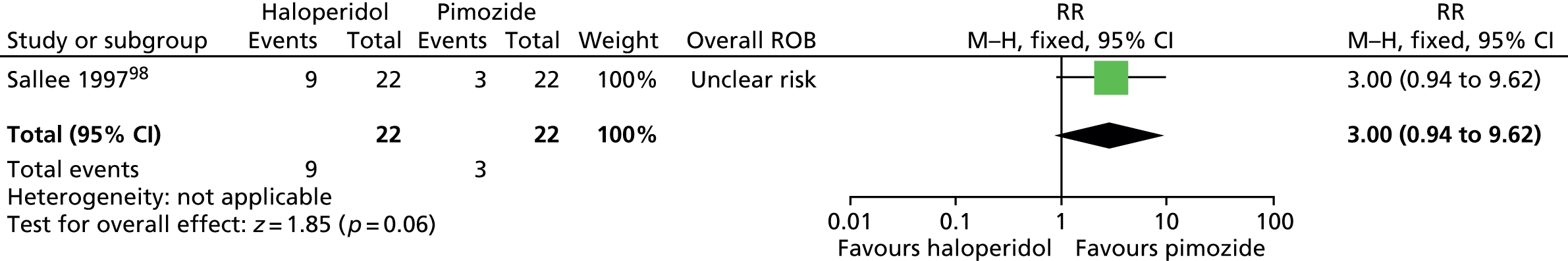
In one of the mixed studies,106 it was reported that both haloperidol and pimozide caused adverse effects including anticholinergic (dry mouth, blurred vision) and extrapyramidal (dystonic) reactions that were equally frequent, intense and short-lived for both drugs. The most common event (accounting for more than two-thirds of adverse effects) was lethargy or tiredness, but a very small sample size makes any comparison between groups difficult to interpret (Figure 50).
FIGURE 50.
Proportion of patients experiencing tiredness on more than one study day for haloperidol compared with pimozide in the mixed study. M–H, Mantel–Haenszel; ROB, risk of bias.

In the other mixed study,99 for adverse effects rated on the CGI scale, there was no conclusive difference in event rates for haloperidol compared with pimozide when rated by physicians (SMD 0.43, 95% CI –0.22 to 1.07; n = 38) or participants (SMD 0.44, 95% CI –0.21 to 1.08; n = 38) (Figure 51). In this study, it is reported that, for moderate or marked adverse effects, there were no significant differences for haloperidol compared with pimozide.
FIGURE 51.
Standardised CGI adverse effects scale for haloperidol compared with pimozide assessed by physicians and patients in the mixed study. ROB, risk of bias.

Aripiprazole compared with other antipsychotic drugs
One controlled before-and-after study compared aripiprazole to tiapride,107 one compared aripiprazole to haloperidol,108 and one compared aripiprazole to pimozide (safety study). 109
Tics
The study of aripiprazole compared with tiapride measured changes in tic severity/impairment with the YGTSS global tic scale and the study of aripiprazole compared with haloperidol measured changes in tics with the YGTSS total tic score. There was a similar reduction from baseline in global score for aripiprazole compared with tiapride (SMD –0.07, 95% CI –0.35 to 0.21; n = 195) and no conclusive difference in reduction from baseline tic score for aripiprazole when it was compared with haloperidol (SMD 0.53, 95% CI –0.07 to 1.13; n = 48). However, there was some evidence of a difference between studies (I2 = 68% for the subgroup difference), therefore, the two studies were not pooled (Figure 52).
FIGURE 52.
Standardised change in global/tic score for aripiprazole compared with other antipsychotics. df, degrees of freedom; ROB, risk of bias; IV, instrumental variable.
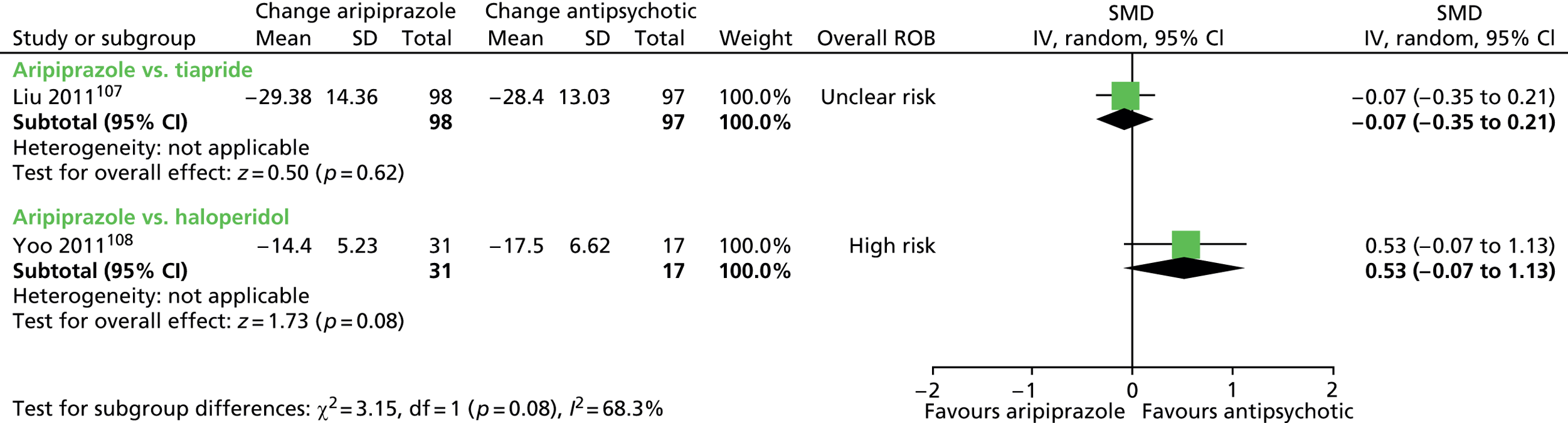
For motor tic score alone, there was a similar reduction from baseline for aripiprazole compared with tiapride (SMD –0.19, 95% CI –0.47 to 0.09; n = 195) and no conclusive difference compared with haloperidol (SMD 0.51, 95% CI –0.09 to 1.11; n = 48). However, there was some evidence of a difference between studies (I2 = 77% for the subgroup difference), therefore, the two studies were not pooled (Figure 53).
FIGURE 53.
Standardised change in motor tic score for aripiprazole compared with other antipsychotics. df, degrees of freedom; IV, independent variable; ROB, risk of bias.
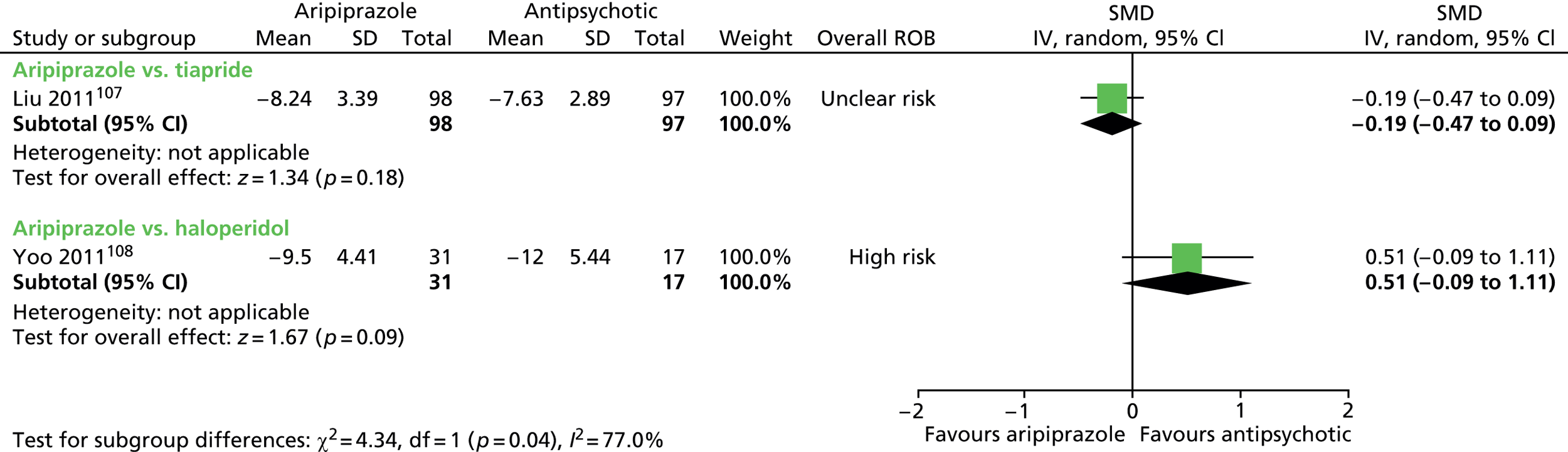
For vocal tic score, there was a similar reduction from baseline for aripiprazole compared with tiapride (SMD 0.02, 95% CI –0.26 to 0.30; n = 48) or haloperidol (SMD 0.03, 95% CI –0.56 to 0.63; n = 195). Overall, the change in vocal tic score was similar for aripiprazole compared with another antipsychotic drug (SMD 0.02, 95% CI –0.23 to 0.28; n = 243) (Figure 54).
FIGURE 54.
Standardised change in vocal tic score for aripiprazole compared with other antipsychotics. df, degrees of freedom; IV, independent variable; ROB, risk of bias.
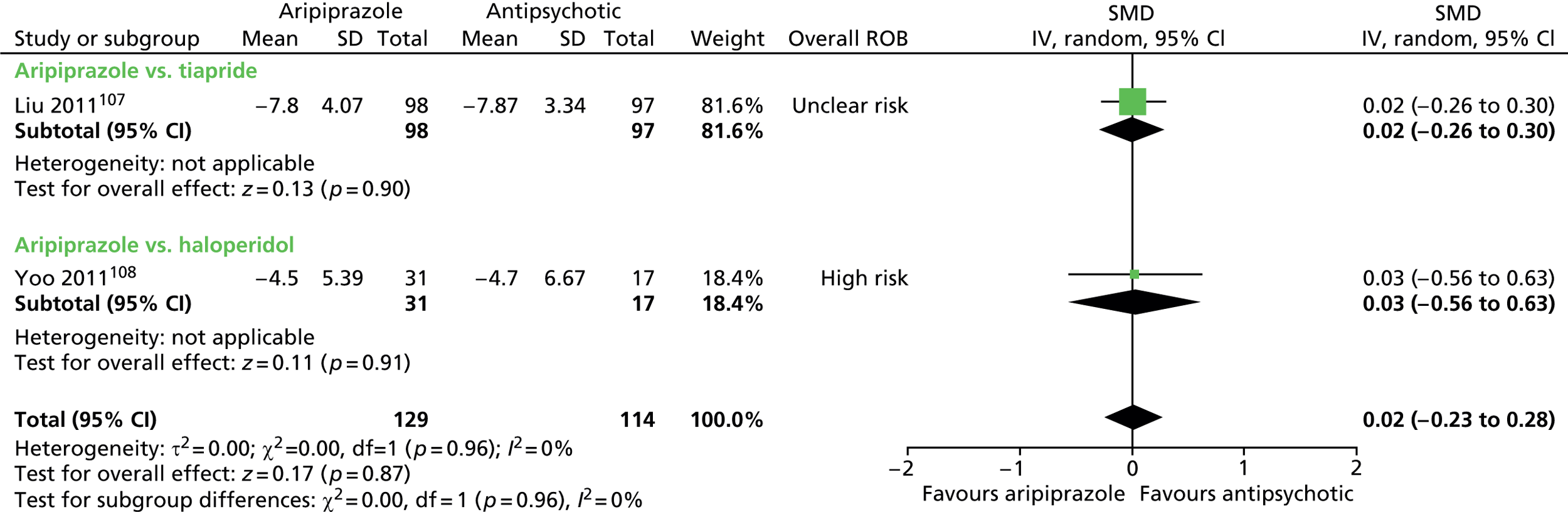
The study comparing aripiprazole with tiapride,107 measured impairment using the YGTSS impairment scale. There was little difference in change from baseline impairment score for aripiprazole compared with tiapride (SMD –0.04, 95% CI –0.32 to 0.24; n = 195) (Figure 55).
FIGURE 55.
Standardised change in impairment score for aripiprazole compared with tiapride. IV, independent variable; ROB, risk of bias.
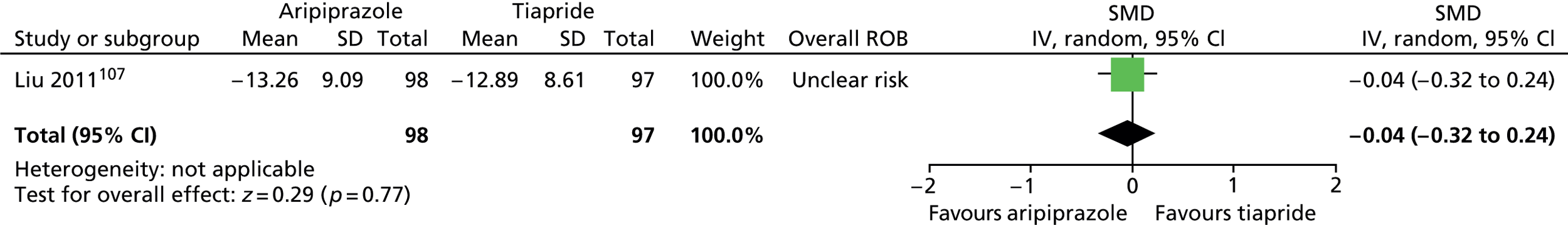
No relevant studies reported this outcome.
The study comparing aripiprazole with tiapride assessed the proportion of participants improved or markedly improved on the YGTSS and the study comparing aripiprazole with haloperidol assessed the proportion of participants much or very much improved on the CGI-I scale. There were similar proportions of participants much or very much improved for aripiprazole compared with tiapride (RR 1.01, 95% CI 0.80 to 1.27; n = 195) and compared with haloperidol (RR 1.21, 95% CI 0.76 to 1.91; n = 48). Overall, there were similar proportions of children much or very much improved for aripiprazole compared with other antipsychotic drugs (RR 1.05, 95% CI 0.85 to 1.29; n = 243) (Figure 56).
FIGURE 56.
Proportion of patients much or very much improved for aripiprazole compared with another antipsychotic. df, degrees of freedom; M–H, Mantel–Haenszel; ROB, risk of bias.

No relevant studies reported long-term outcomes.
Adverse effects were measured in both studies and are shown in Figure 57 (for events occurring in at least two participants). There were no conclusive findings (all CIs included no effect).
FIGURE 57.
Proportion of children experiencing different adverse events for aripiprazole compared with another antipsychotic. df, degrees of freedom; M–H, Mantel–Haenszel; ROB, risk of bias.
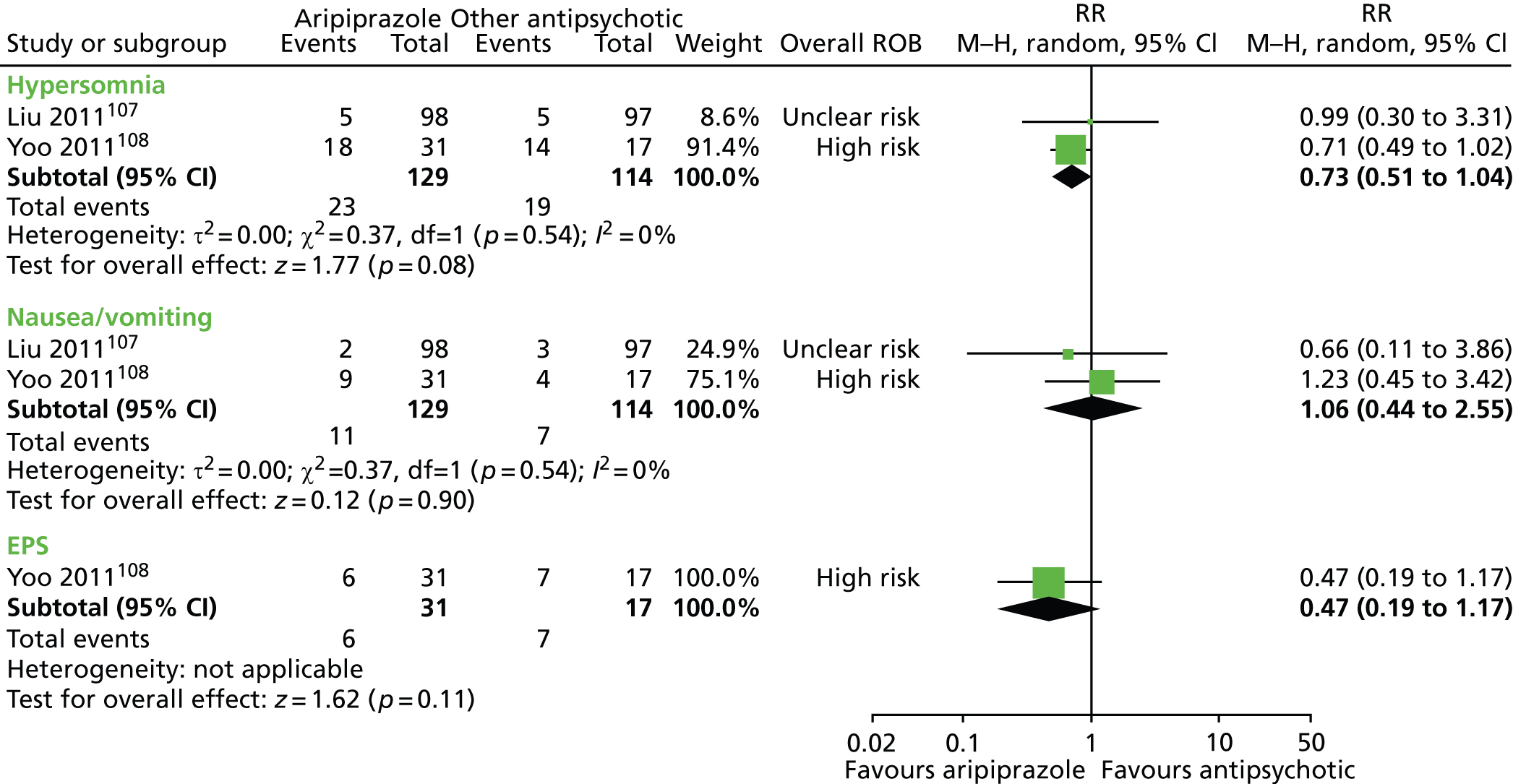

In the safety study comparing pimozide with aripiprazole,109 pimozide produced greater reductions from baseline in systolic BP (MD –20.00, 95% CI –26.56 to –13.44; n = 50) and diastolic BP (MD –13.70, 95% CI –17.39 to –10.01; n = 50), greater increases in ECG-QT (MD 25.50, 95% CI 12.69 to 38.31; n = 50) and ECG-QTc (MD 19.10, 95% CI 10.07 to 28.31; n = 50) compared with aripiprazole. There was no conclusive difference in heart rate for pimozide compared with aripiprazole (MD 4.90, 95% CI –1.16 to 10.96; n = 50).
Olanzapine versus haloperidol
In one study,110 olanzapine was compared with haloperidol.
Tics
Changes in tics were assessed with the YGTSS scale and there was no conclusive difference in reduction in total tic score for olanzapine compared with haloperidol (SMD –0.37, 95% CI –0.91 to 0.17; n = 54) (Figure 58).
FIGURE 58.
Standardised total tic score for olanzapine compared with haloperidol. IV, independent variable; ROB, risk of bias.

No findings for changes in impairment were reported by this study.
No relevant studies reported this outcome.
Overall clinical outcome was assessed with the CGI-Severity scale. The results suggested a benefit for olanzapine compared with haloperidol (SMD –1.39, 95% CI –1.99 to –0.79; n = 54) (Figure 59).
FIGURE 59.
Standardised change in CGI-Severity score for olanzapine compared with haloperidol. IV, independent variable; ROB, risk of bias.
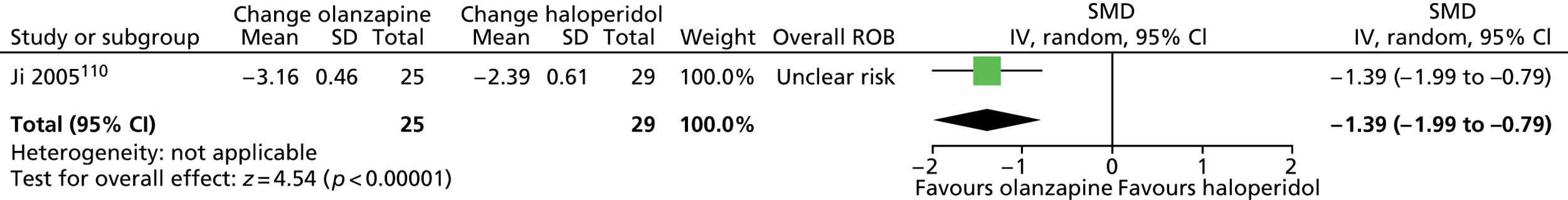
No relevant studies reported long-term outcomes.
This study measured adverse effects with the Subjective Treatment Emergent Symptom Scale (STESS). Lower adverse effect scores were reported for olanzapine compared with haloperidol (SMD –0.57, 95% CI –1.12 to –0.03) (Figure 60).
FIGURE 60.
Standardised STESS side effects score for olanzapine compared with haloperidol. IV, independent variable; ROB, risk of bias.
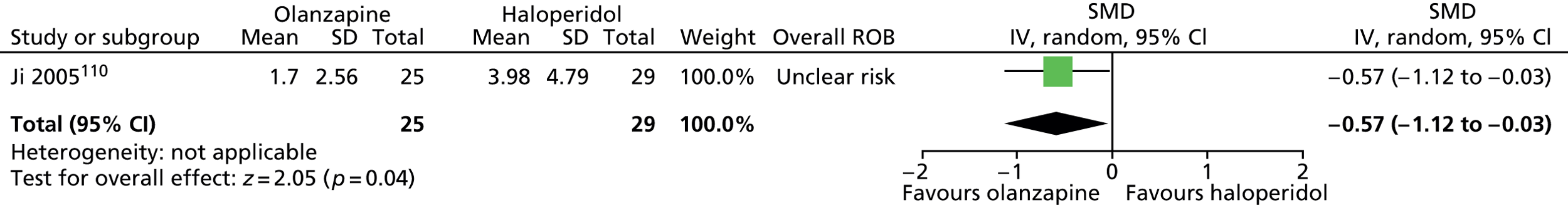
All antipsychotic drugs
Tics
As reported above, all studies of antipsychotic drugs measured changes in tic severity. In studies of children compared with placebo, all antipsychotic medications showed greater reductions in tic score as measured by the YGTSS total tic scale101,103 or the TS global tic subscale. 98 Overall, the SMD was –0.74 (95% CI –1.08 to –0.41; n = 75) (Figure 61), with no evidence of between drug differences (I2 = 0% for test of subgroup differences).
FIGURE 61.
Standardised post-treatment tic score for antipsychotics compared with placebo in studies of children. df, degrees of freedom; IV, independent variable; ROB, risk of bias.
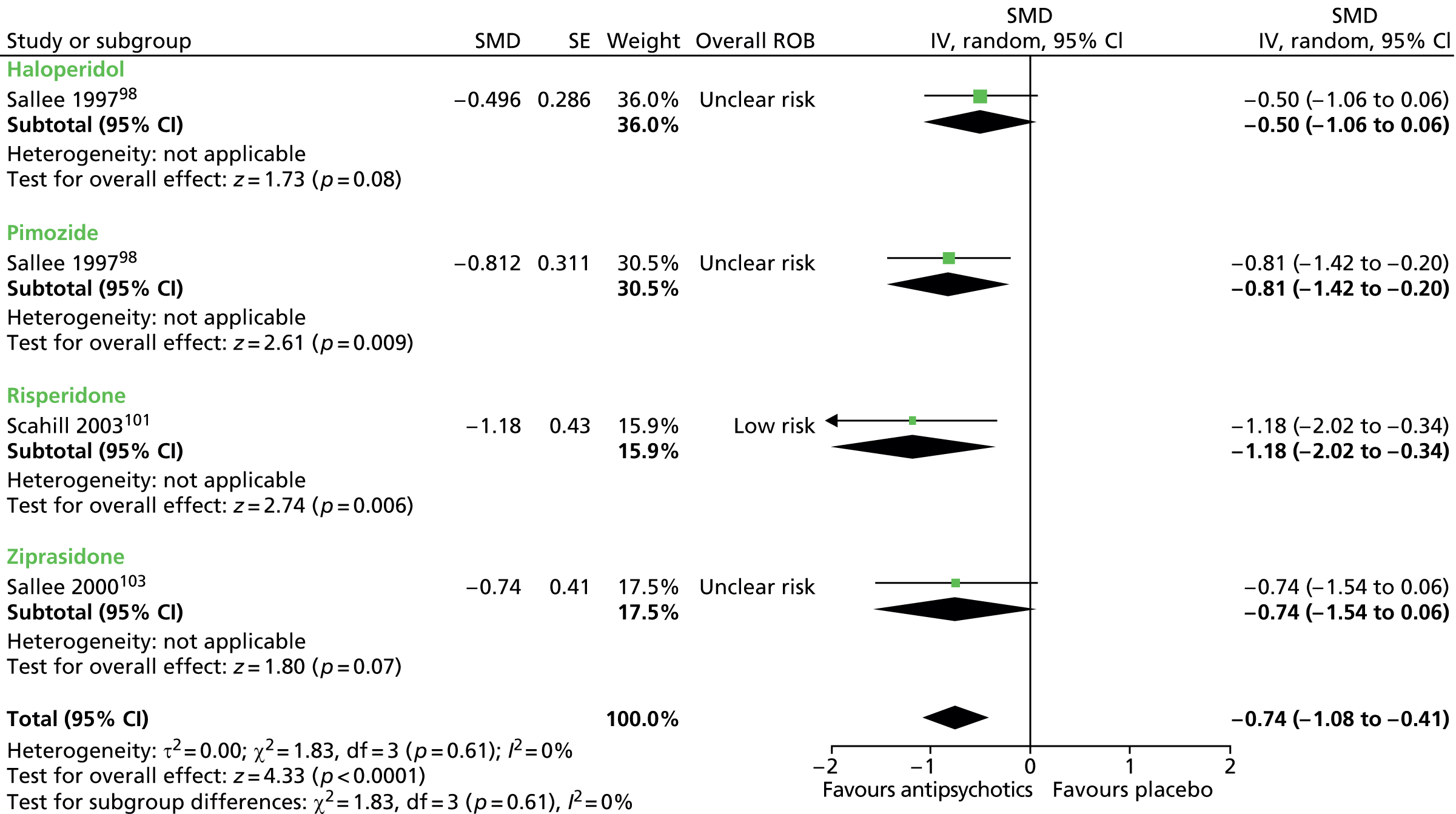
When mixed studies were included, the SMD was –0.75 (95% CI –1.01 to –0.49; n = 197) (Figure 62). Across all studies there was little evidence of between study variance (I2 = 12%) or between drug differences (I2 = 0% for subgroup differences).
FIGURE 62.
Standardised post-treatment total tic score for antipsychotics compared with placebo in the child and mixed studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

For motor score in the child and mixed studies, the SMD was –0.74 (95% CI –1.07 to –0.42; n = 99) (Figure 63). There was little evidence of a between drug difference (I2 for subgroup differences = 3.4%).
FIGURE 63.
Standardised post-treatment motor tic score for antipsychotics compared with placebo in the child and mixed studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

For vocal tics, the SMD for child and mixed studies combined was –0.56 (95% CI –0.89 to –0.23; n = 99) (Figure 64). There was little evidence of a between drug difference (I2 for subgroup differences = 0%).
FIGURE 64.
Standardised post-treatment vocal tic score for antipsychotics compared with placebo in the child and mixed studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
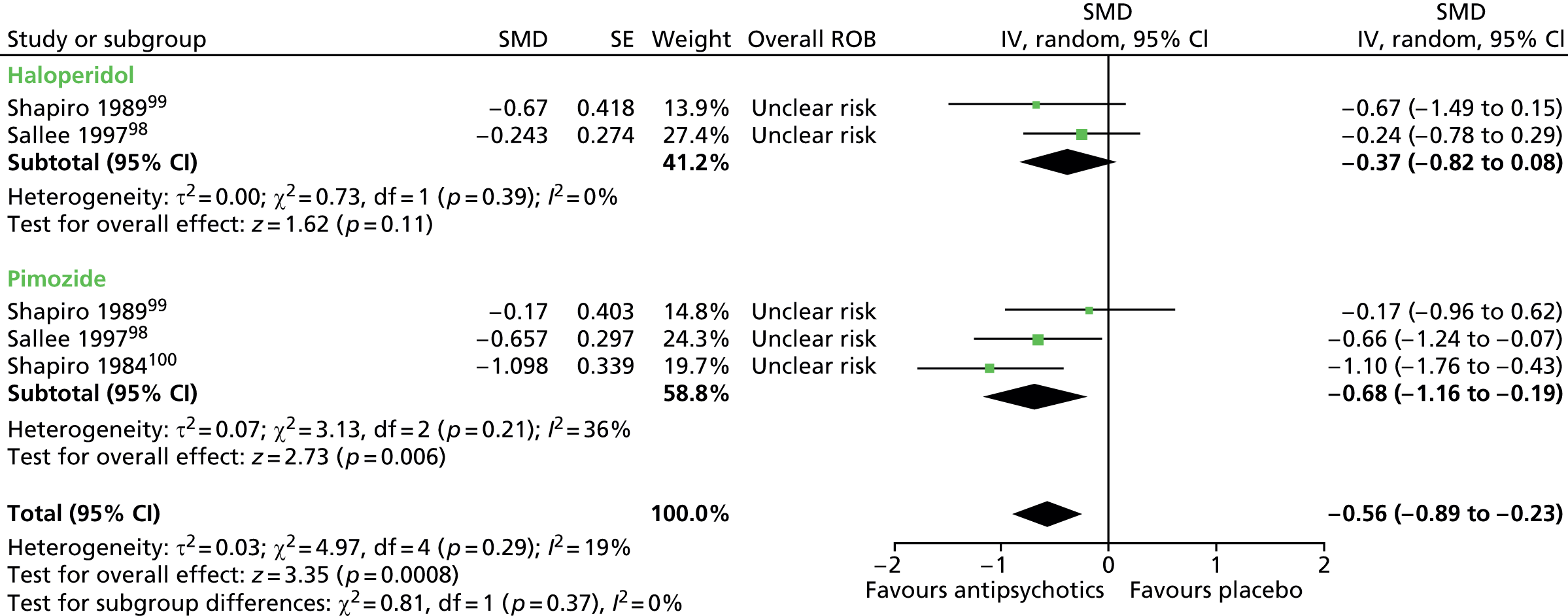
Two studies in children presented changes in global measures of TS severity (tic and impairment scores) measured with the YGTSS global scale103 or the TS global scale98 (Figure 65). No studies in mixed populations presented findings for intervention effects on global TS measures. Overall, the SMD was –0.51 (95% CI –0.86 to –0.15; n = 49), with no evidence of a between drug difference (I2 for subgroup differences = 0%).
FIGURE 65.
Standardised post-treatment global TS score for antipsychotics compared with placebo in child studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
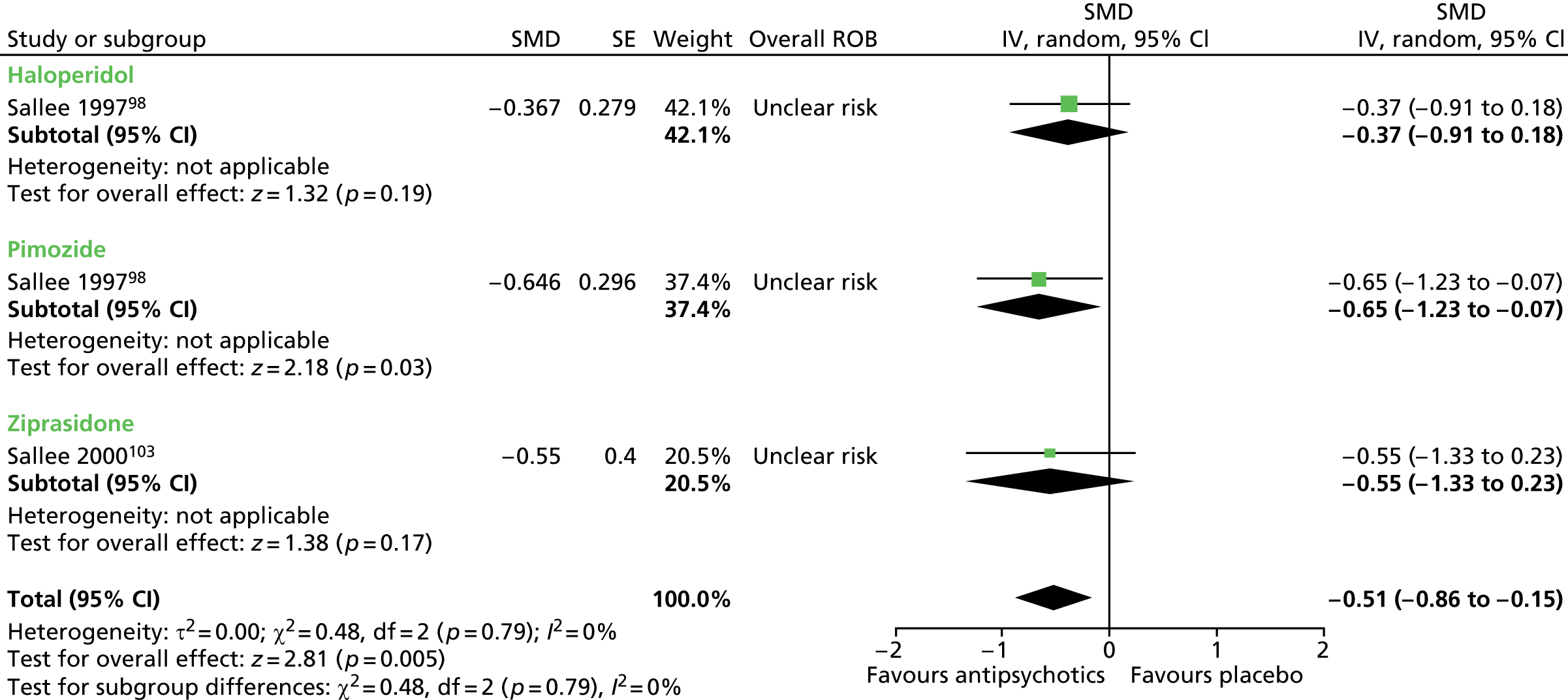
No relevant studies reported this outcome.
Overall clinical outcome was assessed with the CGI-Severity scale in two child studies. 98,103 Overall, there was a lower Clinical Global Impressions – Tic Severity (CGI-TS) score for antipsychotic drugs compared with placebo (SMD –1.01 95% CI –1.49 to –0.53; n = 49) (Figure 66), with little evidence of a between drug difference (I2 for subgroup differences = 23%).
FIGURE 66.
Standardised post-treatment CGI-Severity score for antipsychotics compared with placebo in child studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

No relevant studies reported long-term outcomes.
See separate safety sections for each drug compared with placebo or another drug above.
Clinical evidence summary for antipsychotic drugs
Haloperidol
In one crossover study with 22 children,98 there was low-quality evidence (see Appendix 4, Table 14) of a medium-sized effect in favour of haloperidol when compared with placebo in terms of tics measured at the end of 6 weeks of treatment. This was generally supported by evidence from global impairment and overall clinical outcome. The study was at risk of bias from cross-treatment contamination but the results were consistent with a parallel study in a mixed population of adults and children. 99 Both studies provided evidence of adverse effects with higher rates of EPS98 and adverse effects based on CGI. 99
Pimozide
In one study with 22 children98 (which also included a haloperidol arm), there was low-quality evidence (see Appendix 4, Table 15) of medium-to-large effects in favour of pimozide in terms of tics, impairment and overall clinical outcome at the end of 6 weeks of treatment. The study was at risk of bias from cross-treatment contamination but results were consistent with the overall effect in two mixed studies (one parallel, one crossover) of adults and children. 99,100 The studies were inconclusive as to the presence of adverse effects but pimozide tended to be associated with higher rates of EPS,98 abnormal involuntary movements98 and CGI-rated adverse effects. 99 In the safety study comparing pimozide with aripiprazole109 (graded as low-quality evidence), pimozide had greater increases in QT and QTc than aripiprazole. Pimozide is currently not regularly prescribed in the UK owing to concerns about its affect on increasing the QT interval111,112 and the potentially fatal interaction with other drugs and the current evidence on its safety may support caution over its use.
In the child study,98 when directly compared with haloperidol, there was low-quality evidence (see Appendix 4, Table 19) that pimozide had similar effects on tics, impairment and overall clinical outcome, but two mixed studies99,106 tended to favour haloperidol in terms of tics. For comparisons of adverse effects, findings were inconclusive but tended to favour pimozide for EPS,98 treatment-limiting adverse effects,98 CGI-rated adverse effects99 and tiredness. 106
Risperidone
In one study with 26 children,101 there was low-quality evidence (see Appendix 4, Table 16) of a large effect in favour of risperidone in terms of tics and overall clinical outcome after 8 weeks of treatment, and these results were supported by a mixed study in adults and children. 102 When both studies were combined, there was evidence of increased rates of fatigue, sedation and appetite/weight gain for those treated with risperidone.
From one study with 13 children,104 there was very low-quality evidence (see Appendix 4, Table 18) that was inconclusive with regards to the relative effect of risperidone compared with pimozide, but the direction of the effect favoured risperidone for tics, impairment and overall clinical outcome. These findings were not supported by a mixed study of adults and children105 that showed no difference in effects on tics, impairment or overall clinical outcome. Comparisons of adverse effects were inconclusive, but there tended to be greater weight gain,104,105 but less insomnia105 and physical injuries105 for risperidone compared with pimozide.
Ziprasidone
There was low-quality evidence (see Appendix 4, Table 17) from one study with 27 children103 of a large effect in favour of ziprasidone in terms of tics, global tic/impairment and overall clinical outcome after 8 weeks of treatment (there were no supporting mixed or adult studies). There were higher rates of adverse effects in the ziprasidone group, with sedation being the most common.
Aripiprazole
From one study with 195 children that compared aripiprazole with tiapride107 and one study in 48 children that compared aripiprazole with haloperidol,108 there was very low-quality evidence (see Appendix 4, Table 20) suggesting that aripiprazole was similar to other antipsychotic drugs in its effect on tics, impairment and overall clinical outcome. Regarding adverse effects, data indicate that aripiprazole produced fewer QT and BP problems than pimozide. In addition, although there was a tendency for there to be lower rates of hypersomnia, EPS and headaches in the aripiprazole group than other antipsychotic drugs, the results were inconclusive.
Olanzapine
From one study with 60 children,110 there was low-quality evidence (see Appendix 4, Table 21) that olanzapine produced similar efficacy as haloperidol for tics, greater efficacy for overall clinical outcome and lower rates of adverse effects.
All antipsychotic drugs
Given no apparent differences between antipsychotic drugs with regard to efficacy (I2 for subgroup differences = 0%), three studies in children (n = 75) were combined providing low-quality evidence (see Appendix 4, Table 22) of medium-to-large effects in terms of tics, impairment and overall clinical outcome. There were three mixed studies99,100,102 that, when added to the meta-analysis with the child studies, showed a similar overall effect size for tics.
Conclusion
Antipsychotic drugs are effective in the short term in reducing tics and tic-related impairments in children and young people with TS. There are few clear differences between antipsychotics in effectiveness. The side effect profile differs between antipsychotics, with weight gain, sedation, EPS and cardiac risks being particular concerns.
Noradrenergic agents
Pharmacology and prescribing
Noradrenergic agents are classified as either presynaptic alpha-2 adrenergic agonists (e.g. clonidine, guanfacine) or noradrenergic reuptake inhibitors [e.g. atomoxetine (Strattera®, Lilly)]. Clonidine and guanfacine were developed for use in higher doses as a treatment of hypertension and migraine, while atomoxetine was developed as a treatment for ADHD. Clonidine and guanfacine may also be used as a second-line treatment in ADHD.
The most common adverse effects of clonidine and guanfacine are sedation, bradycardia and postural hypotension. Atomoxetine may produce tachycardia, hypertension, nausea, reduced appetite and fatigue. More rarely, atomoxetine has also been associated with increased suicidal ideation, QTc prolongation and hepatic disturbance.
Clonidine (non-proprietary; Dixarit® and Catapres®, Boehringer Ingelheim) is an alpha-2-adrenergic agonist that inhibits noradrenergic activity and is licensed in the UK for the treatment of essential and secondary hypertension.
Guanfacine (Intuniv®, Shire) is a selective alpha-2-adrenergic agonist with a similar mode of action and clinical effects to clonidine. As with clonidine, guanfacine was developed originally for the treatment of hypertension, but is now also used in the USA for the treatment of ADHD (US FDA approval) and tics. Unlike clonidine, guanfacine does not have marketing authorisation for any treatment indication in the UK and most European countries.
Atomoxetine is a highly selective, non-stimulant, noradrenergic reuptake inhibitor, which selectively inhibits the presynaptic noradrenaline transporter. It is licensed in the UK for the treatment of ADHD.
Included studies
Nine studies in children and two mixed studies compared the efficacy of a noradrenergic agent with placebo (PRISMA diagram, Appendix 2):
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. One parallel trial in 136 children compared maximum dose of 0.6 mg/day clonidine, 60 mg/day methylphenidate and a combination of clonidine and methylphenidate with placebo for 16 weeks. 113 For one mixed crossover trial, data for a subset of 24 children were obtained from authors. 114 This study compared the use of 0.0075–0.015 mg/kg/day clonidine with placebo for 12 weeks each. One crossover trial in 37 children compared maximum dose of 0.2 mg/day clonidine (and maximum dose of 100 mg/day desipramine) with placebo for 6 weeks each. 115 One parallel trial in a mixed population of 47 adults and children compared maximum dose of 0.5 mg/day clonidine with placebo for 12 weeks. 116
Two child117,118 studies and one mixed119 study compared the use of clonidine patches with placebo patches. Both child studies were parallels trial of children given 1–2 mg/day clonidine patches (depending on body weight) over 4 weeks and one was in 437 children117 and the other in 76 children. 118 The mixed study was a crossover trial of 10 adults/children using maximum dose of 0.4 mg/day clonidine patches for 2 months.
Two parallel trials in children compared treatment with guanfacine and placebo. One compared the use of maximum dose of 4 mg/day guanfacine with placebo for 8 weeks in 34 children. 120 The other compared maximum dose of 2 mg/day guanfacine with placebo for 4 weeks in 24 children. 121
One parallel trial compared maximum dose of 1.5 mg/kg/day atomoxetine with placebo for 18 weeks in 148 children with ADHD and comorbid tic disorder. 122 In a subsequent publication,123 the subgroup of 117 children with TS (CTD patients excluded) were analysed.
Risk of bias
In one child study of clonidine compared with placebo,113 randomisation was computer generated and there is assurance of allocation concealment and blinding of participants, providers and outcome assessors. Dropout was relatively low (14%) and analysis was by intention to treat. Overall, this study was considered to be a low risk of bias.
In another child study of clonidine compared with placebo [mixed study but individual patient data (IPD) for children obtained],114 the method of randomisation and the presence of allocation concealment was unclear but there appears to have been blinding of participants and providers and outcome assessors. The presence of dropout from the study was unclear. The study was a crossover with 1-week washouts between treatments and this may have introduced bias from cross-treatment contamination. Overall, this study was considered to be at unclear risk of bias owing to possible cross-treatment contamination.
In another child study of clonidine compared with placebo,115 the method of randomisation and the presence of allocation concealment were unclear but there appears to have been blinding of participants, providers and outcome assessors. The study was a crossover with 1-week washouts between treatments and this may have introduced bias from cross-treatment contamination. The rate of dropout appears to have been low. Data were only reported for outcomes where findings were statistically significant and these outcomes were considered to be at risk from selective outcome reporting bias. Overall, this study was considered to be at high risk of bias owing to possible cross-treatment contamination and selective outcome reporting bias.
In a mixed study of clonidine compared with placebo,116 the method of randomisation and the presence of allocation concealment was unclear but there appears to have been blinding of participants, providers and outcome assessors. Attrition was higher in the placebo compared with the intervention group (17% vs. 4%). This may have led to more conservative estimates of efficacy but the risk of attrition bias was not considered to be high. Overall, this study was considered to be at low risk of bias.
For one child study of clonidine patch compared with placebo,117 randomisation was by layering segmenting randomisation of 800 random numbers but the presence of allocation concealment was unclear. There appears to have been blinding of participants and providers but blinding of outcome assessors was unclear. The rate of dropout was relatively low (13%) and the analysis was by intention to treat with last observation carried forward. The risk of selective outcome reporting bias was unclear (CGI score measured but results not reported). Overall, this study was considered to be at unclear risk of bias owing to unclear outcome assessment blinding.
For the other child study of clonidine patch compared with placebo,118 randomisation was by random number table and stratified by weight. The presence of allocation concealment and blinding of participants, providers and outcome assessors was unclear. Only participants completing the study were reported by the paper (stated in the exclusion criteria that participants unwilling/unable to take medication or be followed up were excluded) and the risk of attrition bias is unclear. Overall, this study was considered at high risk of bias owing to unclear blinding of participants, providers and outcome assessors and unclear risk of attrition bias.
In the mixed study of clonidine patch compared with placebo,119 the presence of randomisation, allocation concealment and patient, provider and outcome assessor blinding is unclear. Analysis was of available cases but the rate of dropout was low. There was a 2-week washout period between treatments and this may have introduced bias from cross-treatment contamination. Overall, this study was considered to be at high risk of bias owing to possible cross-treatment contamination and unclear randomisation and blinding of participants, providers and outcome assessors.
For one child study of guanfacine compared with placebo,120 the method of randomisation and the presence of allocation concealment was unclear but participants, providers and outcome assessors were blinded to treatment allocation. The rate of dropout from the study was unclear. Overall, this study was considered to be at low risk of bias.
For the other child study of guanfacine compared with placebo,121 the method of randomisation and the presence of allocation concealment was unclear but there appears to have been blinding of participants, providers and outcome assessors. The presence of dropout from the study was not reported. Overall, this study was considered to be at low risk of bias.
For the child study of atomoxetine compared with placebo,122 a computerised interactive voice response system was used for randomisation. All clinical trial materials were blinded when provided to the investigative site but there was no specific statement about blinding of outcome assessors. There was a high rate of dropout (30%) but an intention-to-treat analysis was used with last observation carried forward. Overall, this study was considered to have a low risk of bias.
Clinical evidence for noradrenergic agents
Clonidine compared with placebo
Two studies in children113,115 and two mixed studies114,116 in adults and children compared the efficacy of clonidine with placebo. For one of the mixed studies,114 IPD for children and adolescents in the study (24 children and adolescents from a total of 30 participants) were obtained. The other mixed study116 had participants with a mean age of 15.6 years (SD 10.4 years, range 7–48 years); it was assumed that the majority of participants were children and study data are combined with the other child studies in the meta-analysis.
The majority of studies reported motor and vocal tic scores separately (and not total tic scores). Three studies presented findings for motor tics and used the Tourette’s Syndrome Global Scale (TSGS) motor tic score,116 the YGTSS motor tic score113 and an assessment of motor tic severity (body areas and motor tic frequency also assessed). 114 Motor tic score favoured clonidine compared with placebo (SMD –0.55, 95% CI –0.90 to –0.20; n = 130) (Figure 67). There was little heterogeneity between studies (I2 = 14%; p = 0.31).
FIGURE 67.
Standardised motor tic scores for clonidine compared with placebo in predominantly child studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

For vocal tics, studies made assessments using the TSGS vocal tic scale,116 the YGTSS vocal tic scale113 and an assessment of vocal tic severity (vocal tic frequency also assessed)114 and favoured clonidine but there was no conclusive difference compared with placebo (SMD –0.30, 95% CI –0.62 to 0.01; n = 130) (Figure 68). There was no heterogeneity between studies (I2 = 0%).
FIGURE 68.
Standardised vocal tic scores for clonidine compared with placebo in predominantly child studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

In the other study,115 it is reported that there was no significant effect on tic suppression for the Shapiro TSSS, the Hopkins tic scale or a visual analogue scale.
Tic-related impairment was measured in two studies. In one, it was assessed as part of the TSGS with a measure of school and occupational function116 and, in the other, with the YGTSS impairment scale. 113 Impairment score favoured clonidine compared with placebo (SMD –0.54, 95% CI –0.93 to –0.16; n = 106) (Figure 69) and there was no heterogeneity between studies (I2 = 0%).
FIGURE 69.
Standardised impairment scores for clonidine compared with placebo in predominantly child studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
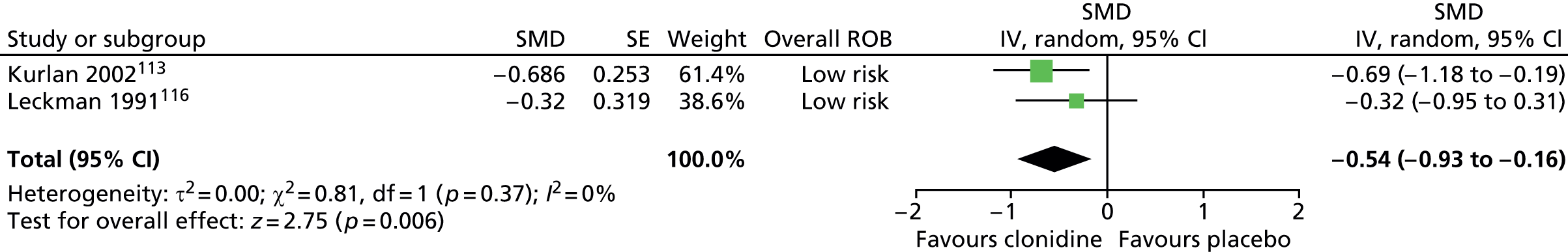
Both of these studies also measured changes in global TS scales (impairment and tic scores) and used the TSGS total score116 and the YGTSS global score. 113 Global TS favoured clonidine compared with placebo (SMD –0.71, 95% CI –1.10 to –0.31; n = 106) (Figure 70) and there was no heterogeneity between studies (I2 = 0%).
FIGURE 70.
Standardised change in global TS score for clonidine compared with placebo in predominantly child studies. df, degrees of freedom; IV, independent variable; SE, standard error.
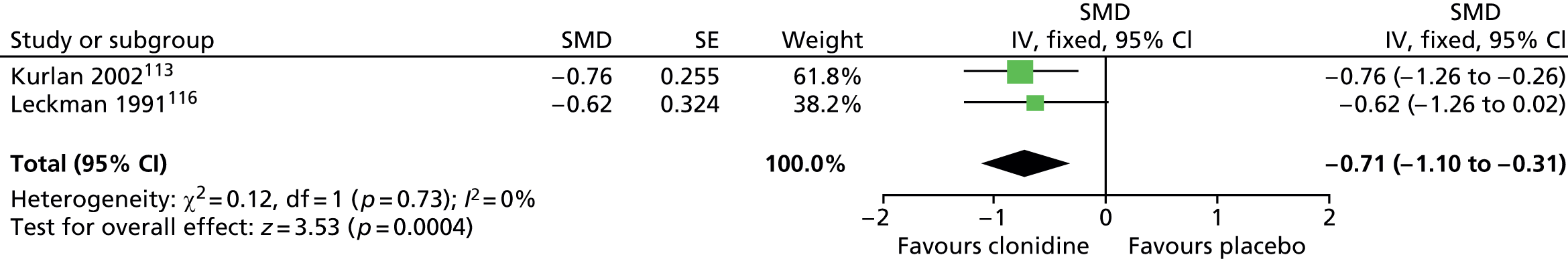
No relevant studies reported this outcome.
Two studies assessed overall clinical outcome and used the CGI scale. In one study,113 CGI outcome was graded by parents, teachers and investigators. A higher proportion of participants improved on the CGI for clonidine than placebo when rated by parents (RR 2.51, 95% CI 1.38 to 4.55; n = 66), teachers (RR 1.88, 95% CI 1.10 to 3.23; n = 66) and investigators (RR 2.41, 95% CI 1.32 to 4.39; n = 66) (Figure 71).
FIGURE 71.
Proportion of children improving on the CGI scale as rated by parents, teachers and investigators for clonidine compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.
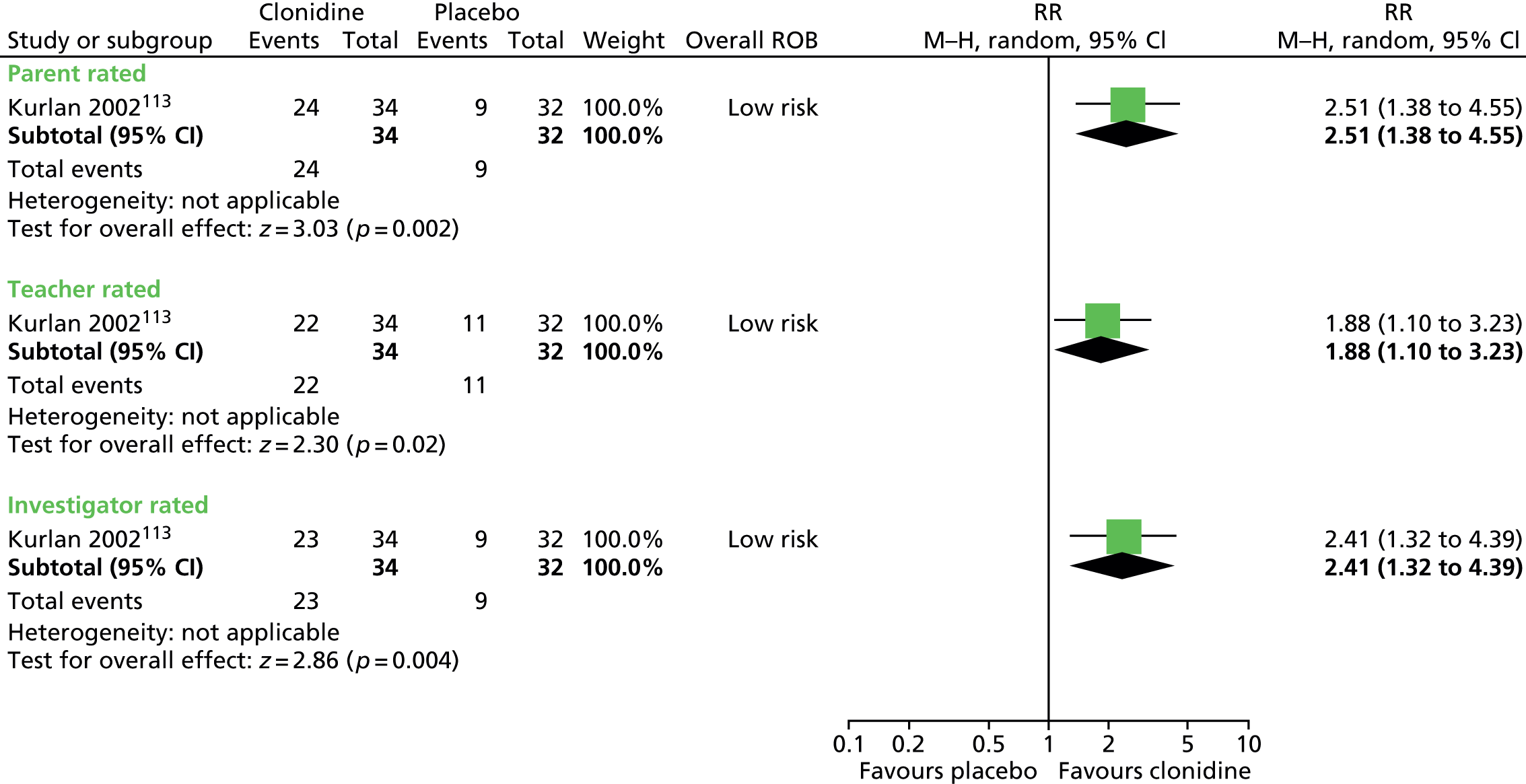
In the other study,116 reduction in CGI-TS severity score was greater for clonidine than placebo (SMD –1.04, 95% CI –1.71 to –0.38; n = 40) (Figure 72).
FIGURE 72.
Standardised change in CGI-TS score for clonidine compared with placebo. IV, independent variable; ROB, risk of bias.
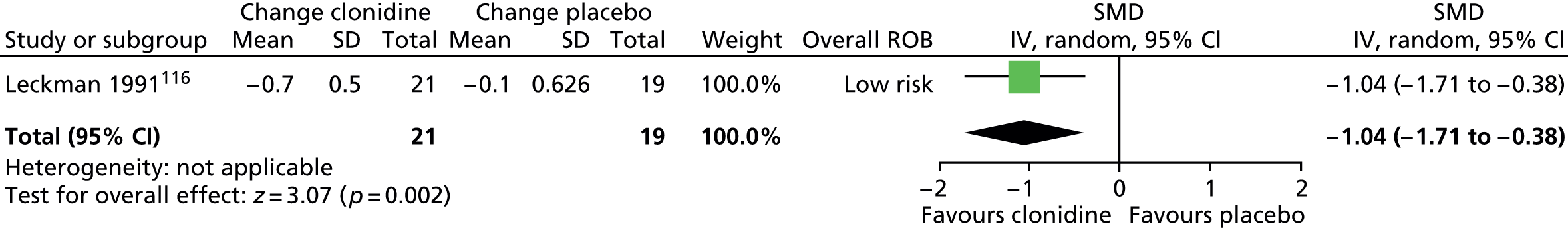
No relevant studies reported long-term outcomes.
In one study,114 it is reported that common adverse effects of clonidine were sedation (57%), dry mouth (37%) and restlessness (27%) and that these effects were not experienced to the same degree in the placebo group. In another study,115 a significantly higher proportion of children experienced adverse effects with clonidine than placebo (RR 1.87, 95% CI 1.24 to 2.81; n = 34) (Figure 73).
FIGURE 73.
Proportion of children with side effects for clonidine compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.
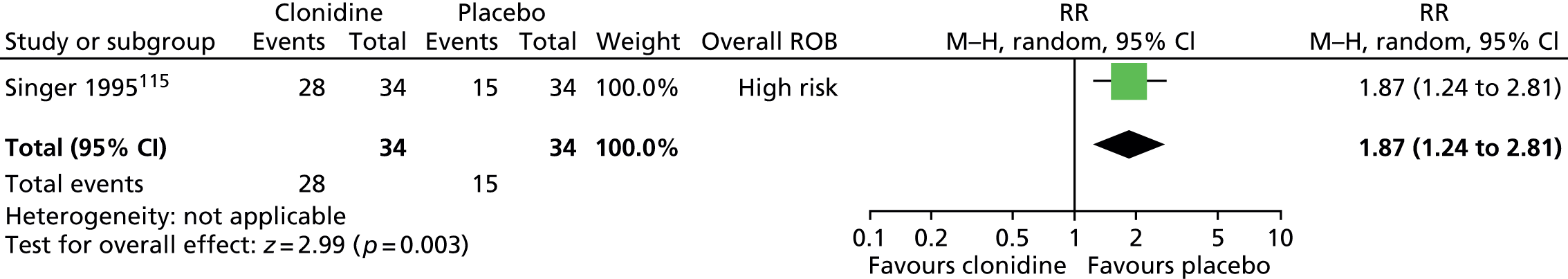
In another study,116 more participants in the clonidine compared with the placebo group experienced sedation/fatigue (RR 2.46, 95% CI 1.34 to 4.50; n = 40) but results were inconclusive for rates of dry mouth (RR 2.17, 95% CI 0.94 to 5.02; n = 40), faintness and/or dizziness (RR 2.04, 95% CI 0.75 to 5.54; n = 40) and irritability (RR 6.33, 95% CI 0.86 to 46.86; n = 40) (Figure 74).
FIGURE 74.
Proportion of patients experiencing side effects for clonidine compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.

Rates of sedation were also reported by another study,113 in which a greater proportion of participants experienced moderate or severe sedation for clonidine compared with placebo (RR 5.65, 95% CI 1.37 to 23.29; n = 40) (Figure 75).
FIGURE 75.
Proportion of participants experiencing moderate-to-severe sedation for clonidine compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.
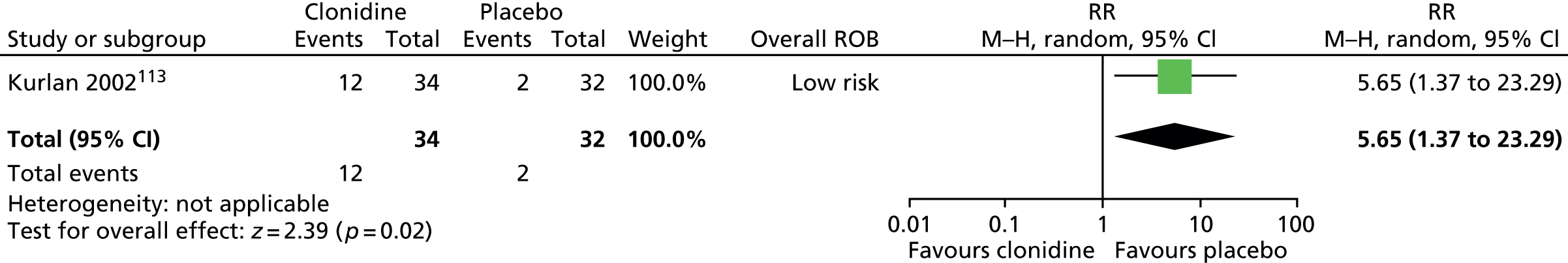
In one study,116 investigators conducted subgroup analysis and found no difference in treatment effectiveness for participants with and without ADHD and OCD, or those aged < 18 years or ≥ 18 years and it is reported that an analysis of variance showed no effect of baseline tic severity on treatment effect.
Guanfacine compared with placebo
Two parallel trials in children compared the efficacy of guanfacine with placebo. 120,121
Tics
Both studies used the YGTSS to assess changes in tics. Overall, reduction in YGTSS total tic score was greater for guanfacine compared with placebo (SMD –0.73, 95% CI –1.26 to –0.20; n = 58) (Figure 76).
FIGURE 76.
Standardised change in YGTSS total tic score for guanfacine compared with placebo. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

One of the studies presented findings for motor and vocal tics separately. 121 There was no conclusive difference in change from baseline tic scores for guanfacine compared with placebo for motor (SMD –0.64, 95% CI –1.46 to 0.18; n = 24) or vocal (SMD –0.70, 95% CI –1.53 to 0.12; n = 24) tics (Figures 77 and 78).
FIGURE 77.
Standardised change in YGTSS motor tic scale for guanfacine compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
FIGURE 78.
Standardised change in YGTSS vocal tic scale for guanfacine compared with placebo in the child study. IV, independent variable; ROB, risk of bias; SE, standard error.
One of the studies121 assessed changes in TS-related impairment with the YGTSS impairment scale. There was no conclusive difference in change from baseline impairment score for guanfacine compared with placebo (SMD 0.27, 95% CI –0.53 to 1.08; n = 24) (Figure 79) or for overall YGTSS global score (SMD –0.29, 95% CI –1.10 to 0.51; n = 24) (Figure 80).
FIGURE 79.
Standardised change in YGTSS impairment scale for guanfacine compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
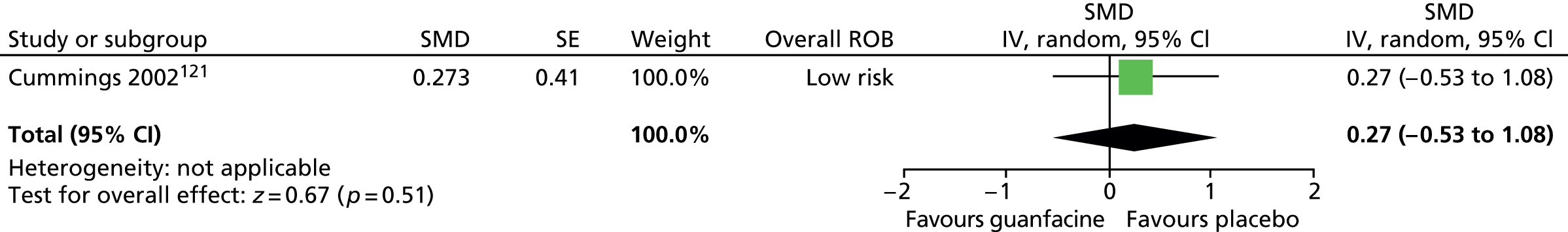
FIGURE 80.
Standardised change in YGTSS global scale for guanfacine compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
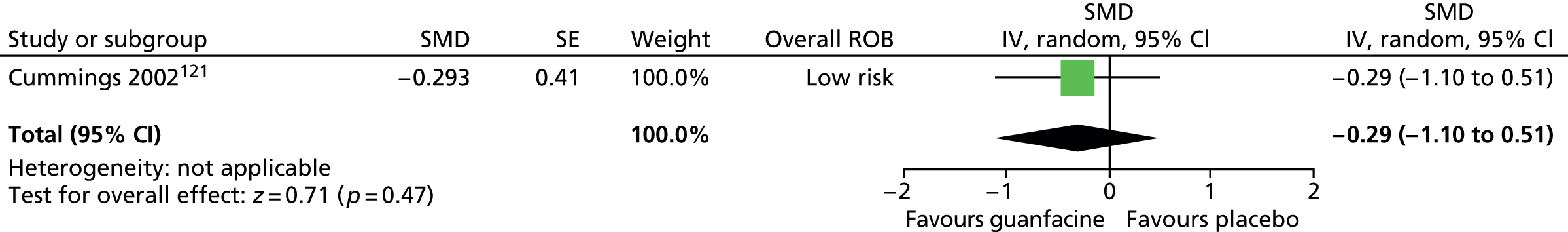
No relevant studies reported this outcome.
Overall clinical change was assessed by one study,120 but this was an assessment of changes in symptoms of ADHD (and not TS-related symptoms) and is, therefore, outside of the scope for this review.
No relevant studies reported long-term outcomes.
In one study121 it is reported that adverse effects for guanfacine were headache, flu-like symptoms and fatigue (n = 1), fatigue/sleepiness (n = 1) and bad dreams (n = 1) (rates for placebo not reported).
In the other study,120 it is reported that no serious adverse effects were observed and no participant showed a clinically meaningful change in cardiac conduction. The adverse effects reported included mild sedation (n = 6), sleep awakening (n = 3), dry mouth (n = 4), constipation (n = 2) and loss of appetite (n = 2), but rates of these events were not significantly more frequent than in the placebo group. It is reported that there were no significant changes in weight from baseline in either group and no difference between groups in weight change. It is reported that there were no significant differences in changes in BP and pulse for guanfacine compared with placebo.
Clonidine patch compared with placebo
Two studies in children117,118 and one mixed study119 compared the efficacy of clonidine patches with placebo patches. In one of the child studies,118 results were reported as percentage decreases and these data could therefore not be combined with the other studies in the meta-analysis.
Tics
Tics were assessed in the child study117 using the YGTSS and, in the mixed study,119 using the TSGS (Figure 81). In the child study, change from baseline tic score was similar for clonidine patch compared with placebo (SMD –0.10, 95% –0.32 to 0.12; n = 437) and, in the mixed study, there was no conclusive difference in post-treatment tic score for clonidine patch compared with placebo (SMD –0.36, 95% CI –0.97 to 0.24; n = 9).
FIGURE 81.
Standardised tic scores for clonidine patch compared with placebo for child and mixed studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
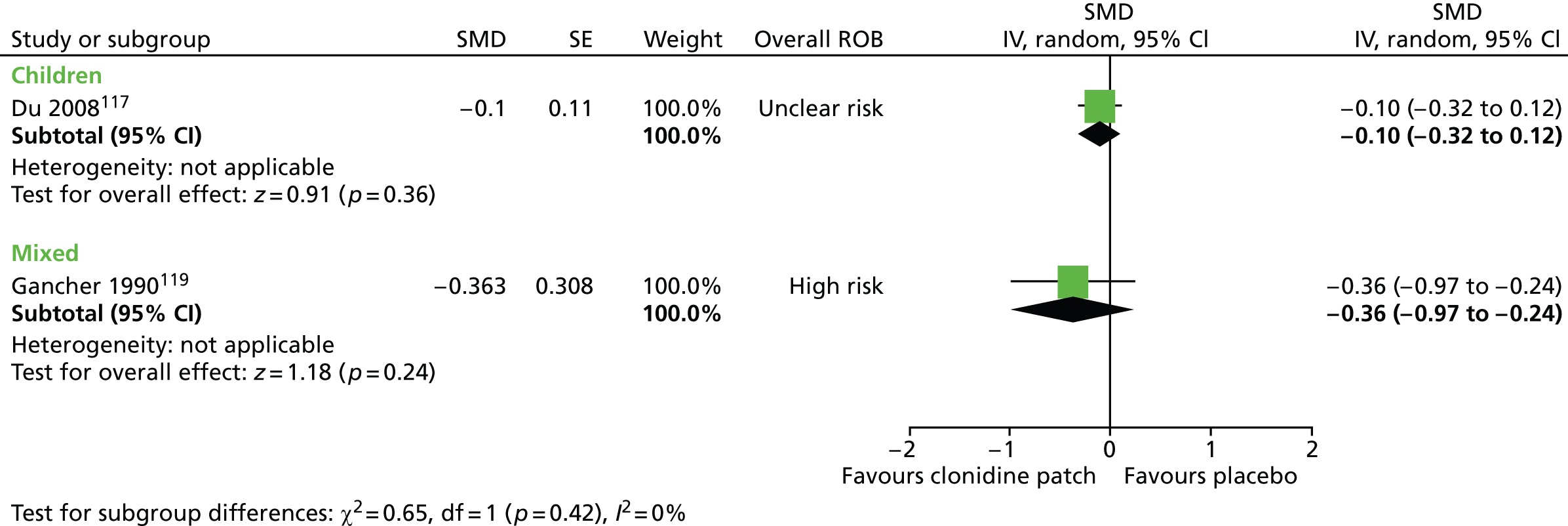
In the mixed study, motor and vocal tic elements were assessed separately using the Tourette Syndrome Symptom List (TSSL). Post-treatment score was lower for clonidine patch than placebo for complex motor score (SMD –1.17, 95% CI –1.93 to –0.41; n = 9), but there was no conclusive difference for simple motor (SMD –0.52, 95% CI –1.14 to 0.11; n = 9), simple phonic (SMD –0.62, 95% CI –1.25 to 0.02; n = 9), complex phonic (SMD –0.54, 95% CI –1.17 to 0.08; n = 9) and behavioural scores (SMD –0.28, 95% CI –0.88 to 0.31; n = 9) (Figure 82).
FIGURE 82.
Standardised post-treatment TSSL scores for clonidine patch compared with placebo in the mixed study. IV, independent variable; ROB, risk of bias; SE, standard error.

In the other child study,118 motor and vocal tics were assessed with the YGTSS scale. There were greater reductions for clonidine patch than placebo for motor [47.0% (SD 3.7%) vs. 12.1% (SD 11.4%)] and vocal [22.7% (SD 4.4%) vs. 2.8% (SD 6.3%)] tic score.
In one child study,118 impairment was assessed with the YGTSS impairment scale. There was a greater reduction in impairment score for clonidine patch than placebo [53.8% (SD 2.2%) vs. 12.0% (SD 5.9%)].
No relevant studies reported this outcome.
In the second child study,118 overall clinical change was assessed with the CGI-Severity scale. There was a greater reduction in CGI-Severity score for clonidine patch than placebo [40.2% (SD 2.4) vs. 17.0% (SD 4.1)].
Patient reports for long-term follow-up for the clonidine group are given by the mixed study. It is reported that, after an average of 13 months, six participants (three children and three adults) said that clonidine continued to be effective in controlling their tics.
In one study of children,117 it is reported that adverse effects occurred in eight children in the clonidine patch group (rashes n = 3, abnormal ECG n = 2, somnolence n = 1, light headedness n = 1, insomnia n = 1) and eight children in the placebo patch group (rashes n = 6, nausea n = 1, dry mouth, somnolence, light headedness and dizziness n = 1). In the mixed study, it is reported that adverse effects experienced were heartburn (clonidine n = 1, placebo n = 4), dose-dependent drowsiness (clonidine n = 7), dry mouth (clonidine n = 5, placebo n = 3) and erythema/dry skin (clonidine n = 4, placebo n = 2). In the other child study,118 the results for clonidine patch compared with placebo patch were inconclusive for itchy skin (RR 0.78, 95% CI 0.16 to 3.66; n = 76), dry mouth (RR 1.55, 95% CI 0.19 to 12.43; n = 76) and dizziness (RR 1.24, 95% CI 0.15 to 10.41; n = 76) (Figure 83).
FIGURE 83.
Proportion of patients with adverse events for clonidine patch compared with placebo patch. M–H, Mantel–Haenszel; ROB, risk of bias.
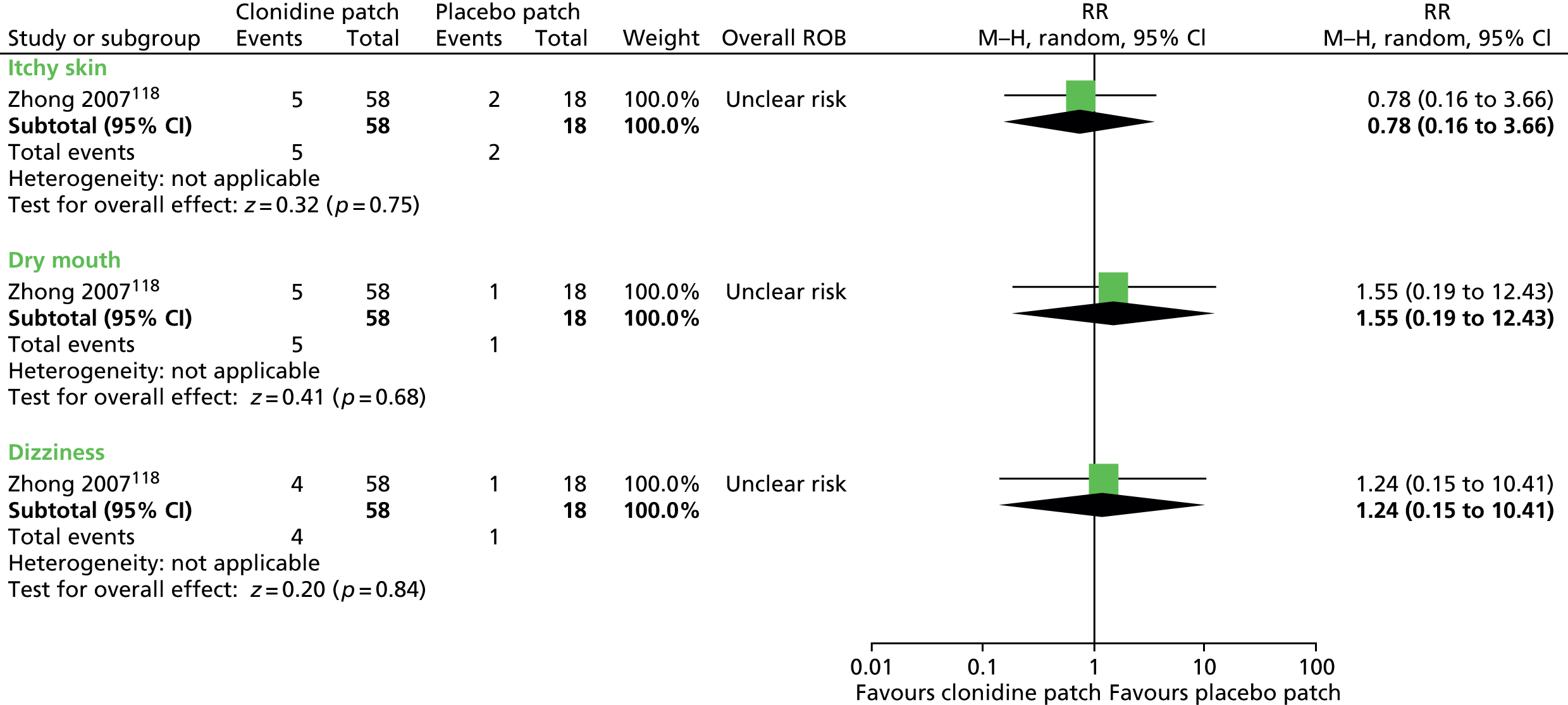
Atomoxetine compared with placebo
One parallel trial in children with TS and CTD compared the use of atomoxetine with placebo. 122 This study has since be published as a subgroup analysis of only children with TS (excluding those with CTD). 123 Results are presented below for the whole sample and the TS subsample.
Tics
Tic severity was measured using the YGTSS total tic scale (Figure 84). For children with TS or CTD, there was no conclusive reduction in YGTSS total tic score (SMD –0.32, 95% CI –0.65 to 0.01; n = 145) but for the subgroup of children with TS, YGTSS total tic score was reduced (SMD –0.40, 95% CI –0.77 to –0.03; n = 116) for atomoxetine compared with placebo.
FIGURE 84.
Standardised change in YGTSS total tic score for children with TS or CTD or with TS alone following treatment with atomoxetine compared with placebo. IV, independent variable; ROB, risk of bias.
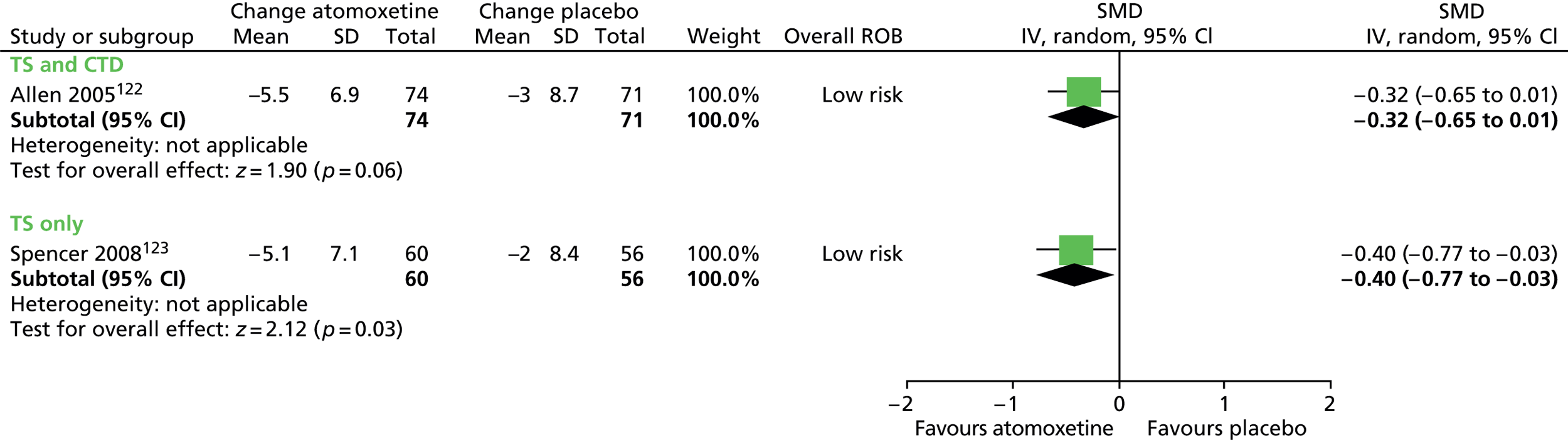
For the whole study sample, a greater proportion of children taking atomoxetine than placebo experienced a ≥ 25% reduction in YGTSS total tic score (RR 0.76, 95% CI 0.57 to 1.00; n = 145) (Figure 85) (RR is the RR of not having a 25% or more reduction).
FIGURE 85.
Proportion of children with TS or CTD showing a 25% or more reduction in YGTSS total tic score for atomoxetine compared with placebo (RR of not having a 25% or more reduction). M–H, Mantel–Haenszel; ROB, risk of bias.
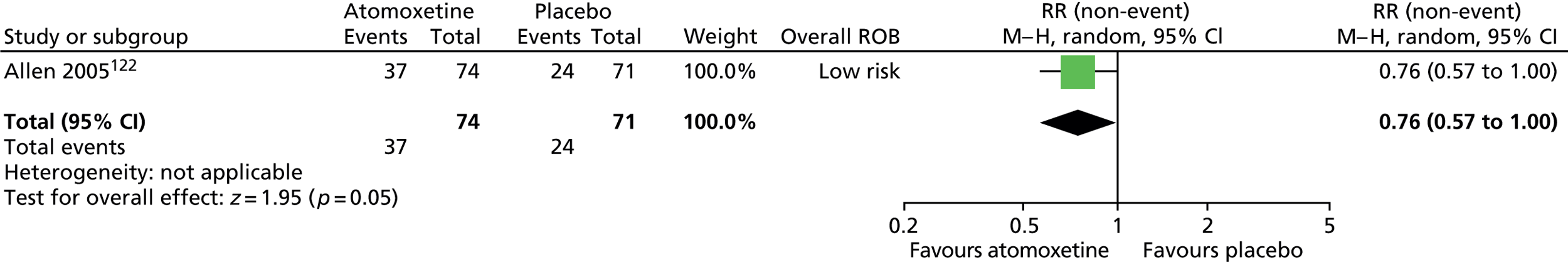
When motor and vocal tic scores were considered separately (Figures 86 and 87) for the whole TS/CTD population there was no conclusive difference for atomoxetine compared with placebo for motor tic score (SMD –0.30, 95% CI –0.62 to 0.03; n = 145) and vocal tic score (SMD –0.22, 95% CI –0.54 to 0.11; n = 145). For the subgroup with TS, the reduction was greater for atomoxetine compared with placebo for motor tic score (SMD –0.41, 95% CI –0.78 to –0.04; n = 116) but, for vocal tic score there was no conclusive difference (SMD –0.25, 95% CI –0.62 to 0.12; n = 116).
FIGURE 86.
Standardised change in YGTSS motor tic score for children with TS or CTD or with TS alone for atomoxetine compared with placebo. IV, independent variable; ROB, risk of bias.
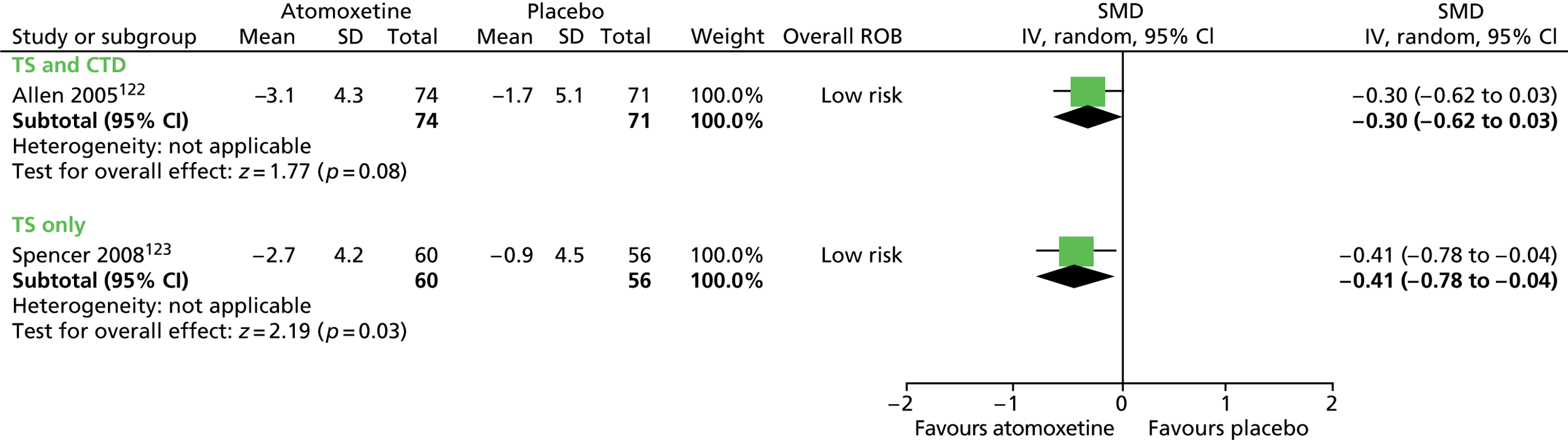
FIGURE 87.
Standardised change in YGTSS vocal tic score for children with TS or CTD or with TS alone for atomoxetine compared with placebo. IV, independent variable; ROB, risk of bias.

No findings for TS-related impairment were reported by these studies.
No relevant studies reported this outcome.
Overall clinical outcome was measured using the Clinical Global Impressions – severity of tics and other neurological symptoms (CGI-Tic/Neuro-S) scale, assessing TS and neurological symptoms. The CGI-Tic/Neuro-S score suggested a benefit for atomoxetine compared with placebo for children with TS or CTD (SMD –0.54, 95% CI –0.87 to –0.21; n = 145) and with TS alone (SMD –0.63, 95% CI –1.00 to –0.25; n = 116) (Figure 88).
FIGURE 88.
Standardised change in CGI score for children with TS or CTD or with TS alone for atomoxetine compared with placebo. IV, independent variable; ROB, risk of bias.

No relevant studies reported long-term outcomes.
The number of children experiencing different adverse effects for atomoxetine compared with placebo is reported for the whole trial population. 122 A greater number of children experienced decreased appetite (RR 5.68, 95% CI 1.32 to 24.52; n = 148) and nausea (RR 11.37, 95% CI 1.52 to 85.22; n = 148), but there were no conclusive differences in rates of vomiting (RR 1.89, 95% CI 0.75 to 4.78; n = 148), fatigue (RR 2.84, 95% CI 0.80 to 10.08; n = 148), cough (RR 0.42, 95% CI 0.14 to 1.31; n = 148), pharyngitis (RR 0.32, 95% CI 0.09 to 1.12; n = 148), diarrhoea (RR 0.36, 95% CI 0.10 to 1.29; n = 148), headaches (RR 1.08, 95% CI 0.57 to 2.05; n = 148) or upper abdominal pain (RR 0.74, 95% CI 0.29 to 1.87; n = 148) (Figure 89).
FIGURE 89.
Proportion of children experiencing adverse events for atomoxetine compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.


Meta-analysis of noradrenergic agents
Studies in children (or predominantly children) comparing treatment with noradrenergic drugs (clonidine and guanfacine) compared with placebo were combined. The child study of transdermal clonidine was not included as the method of drug administration was different and likely to introduce clinical heterogeneity into the meta-analysis. The studies of atomoxetine were not included in the meta-analysis as this agent was considered by the TEG to be substantially different in its mode of action to clonidine and guanfacine.
Tics
Four studies presented data for tic score or global score and, in order to combine information across studies, these scales have been combined. 113,116,120,121 Studies used the YGTSS global score,116 the TS global score113 or the YGTSS total tic score. 120,121 Overall, the standardised tic/global score was –0.72 (95% CI –1.03 to –0.40; n = 164) (Figure 90), with no evidence of between-drug differences (I2 for subgroup differences = 0%).
FIGURE 90.
Standardised total tic/global scores for noradrenergic agents compared with placebo. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
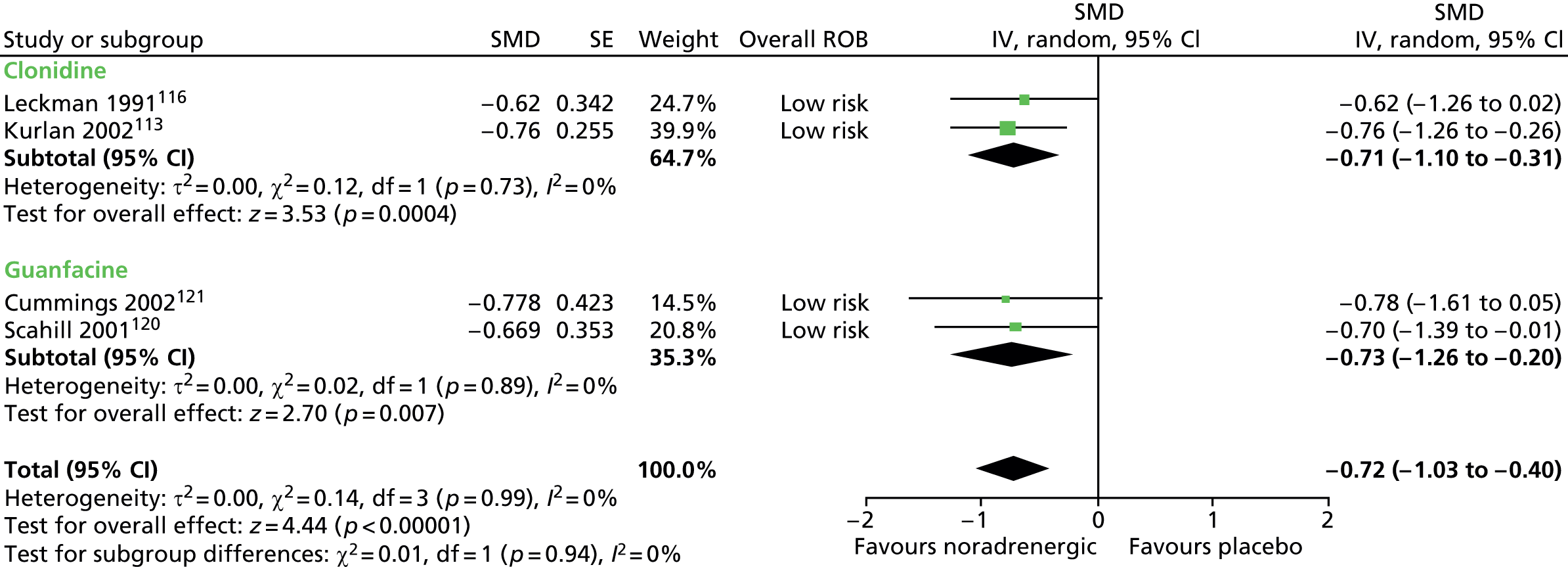
Three parallel studies reported changes in TS-related impairment using the YGTSS impairment score113,121 and the Shapiro TSSS school and occupational function score. 116 The SMD for impairment was –0.32 (95% CI –0.84 to 0.20; n = 130) (Figure 91), but there was some evidence of a between-subgroup difference (I2 for subgroup differences = 69%).
FIGURE 91.
Standardised impairment scores for noradrenergic agents compared with placebo. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

No relevant studies reported this outcome.
Only one parallel trial of clonidine measured changes in overall clinical outcome (CGI-Severity) and results are presented in the clonidine compared with placebo (see Clonidine versus placebo).
No relevant studies reported long-term outcomes.
See relevant sections for each drug above.
Clinical evidence summary for noradrenergic agents
Clonidine
There was moderate-quality evidence (see Appendix 4, Table 23) from two studies in children113,116 suggesting that clonidine produced a medium-sized to large-sized effect in terms of tic severity/impairment and overall clinical outcome, but was associated with increased rates of adverse effects,115 particularly sedation116 following 12–16 weeks of treatment.
Guanfacine
There was moderate-quality evidence (see Appendix 4, Table 24) from two studies in children (n = 58)120,121 that guanfacine, when compared with placebo, produced a large effect in tics following 4–8 weeks of treatment and did not appear to be associated with serious adverse effects.
Clonidine patch
In one study in 437 children,117 there was moderate-quality evidence (see Appendix 4, Table 25) that clonidine patch did not improve tics following 4 weeks of treatment. Another study in children,118 in which results were presented in terms of percentage changes (and, therefore, the two studies could not be combined), showed improvements in tics, impairment and overall clinical outcome, but this evidence was graded as very low quality and there is large uncertainty around these findings. Both studies showed similar rates of adverse effects for clonidine patch compared with a placebo patch.
Atomoxetine
There was moderate-quality evidence (see Appendix 4, Table 38) from one study with 145 children122 suggesting that atomoxetine gave a small/moderate improvement in tics and overall clinical outcome, but gave higher rates of decreased appetite and nausea following 18 weeks of treatment.
All noradrenergic agents
Overall, four studies in children113,116,120,121 (n = 164) provided moderate-quality evidence (see Appendix 4, Table 26) that noradrenergic agents (clonidine or guanfacine) improved tics by the end of 4–16 weeks of treatment. The adverse effect profile may differ for different noradrenergic agents.
Conclusion
Noradrenergic agents are effective in the short term in reducing tics and tic-related impairments in children and young people with TS. The most effective noradrenergic agents in reducing tics appear to be the alpha-2-noradrengic agonists, clonidine and guanfacine. Adverse effects of these agents are generally mild (e.g. sedation, dizziness, nausea) and differ between agents with guanfacine (not marketed in the UK) having the most favourable profile. Atomoxetine is effective in reducing tics in children and young people with comorbid TS and ADHD. There is no firm evidence that clonidine patches are effective in reducing tics and may be associated with adverse effects including local skin irritation.
Stimulant drugs
Pharmacology and prescribing
Central nervous system stimulant drugs are the first-line pharmacological treatment for ADHD. Similar to the use of atomoxetine, stimulants would only be considered in the treatment of comorbid ADHD and TS. Their mode of action is as indirect dopamine agonists, increasing dopamine activity in cortical and subcortical brain regions. While methylphenidate and dexmethylphenidate primarily act as noradrenaline-dopamine reuptake inhibitors, dexamphetamine also acts by releasing dopamine and noradrenaline from presynaptic storage vesicles, making dexamphetamine a more potent dopaminergic agonist than methylphenidate.
Methylphenidate (Ritalin®, Novartis Pharmaceuticals UK; and the modified-release preparations Concerta® XL, Janssen; EquasymXL®, Shire Pharmaceuticals Limited and; MedikinetXL®, Flynn) is licensed in the UK for ADHD.
Dexmethylphenidate (non-proprietary; Focalin®, Novartis; Attenade®, Celgene) is a noradrenaline-dopamine reuptake inhibitor and releasing agent, thereby classed as a stimulant. It is not licensed in the UK and is most commonly used for ADHD in the USA.
Included studies
Four studies in children investigated the use of stimulant medications compared with placebo in children with ADHD (see Appendix 2). 113,124–126 Studies were of methylphenidate and dexmethylphenidate (there were no included studies for dexamphetamine). The aim of these studies was to demonstrate that the use of stimulant drugs did not worsen tics and could be used to treat comorbid ADHD and TS without worsening of tics. For one study,126 the data could not be extracted. The three remaining studies involved the following comparisons:
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. One parallel113 and two crossover124,125 trials investigated treatment with methylphenidate compared with placebo. In the parallel trial, 136 children with ADHD and TS or with ADHD and CTD were randomised to a maximum dose of 60 mg/day methylphenidate, 0.6 mg/day clonidine, methylphenidate and clonidine, or placebo for 16 (methylphenidate) or 12 (other interventions) weeks. In one crossover trial,125 71 children with ADHD and TS or CTD (from two cohorts) underwent treatment with various doses of methylphenidate; 0.1 mg/kg/day, 0.3 mg/kg/day and 0.5 mg/kg/day (0.5 mg/kg/day data were used in the analysis in this review to match the dose used in the other methylphenidate study) and placebo in a randomised order for 2 weeks each. The other crossover trial was in 11 children124 and used the same study design (0.1, 0.3 and 0.5 mg/kg/day for 2 weeks in randomised order) (0.5 mg/kg/day data were used for this review).
One crossover study investigated treatment with immediate-release dexmethylphenidate compared with placebo in children with TS or CTD. 127 Experiments were conducted over 1-day periods with subjects having a mean dose of 7.5 mg dexmethylphenidate on one day and no medication on the other day in a randomised order.
Risk of bias
In the parallel trial of methylphenidate,113 randomisation was computer generated and stratified on centre and sexual maturity status. There was assurance of allocation concealment and of blinding of participants, providers and outcome assessors. Dropout was relatively low and analysis was by intention to treat and the risk of attrition bias was considered to be low. Overall, this study was considered to be at low risk of bias.
For one crossover trial of methylphenidate,125 the method of randomisation and presence of allocation concealment was unclear. There appears to have been blinding of participants, providers and outcome assessors. There was no washout between interventions and the study was considered to be at high risk of cross-intervention contamination. It was unclear how many participants dropped out of the study and how many were included in the analysis and the risk of attrition bias was unclear. Overall, this study was considered to be at high risk of bias.
For the other crossover trial of methylphenidate,124 the method of randomisation and presence of allocation concealment was unclear. There appears to have been blinding of participants, providers and outcome assessors. There was no washout between interventions and the study was considered to be at high risk of cross-intervention contamination. It was unclear how many participants dropped out of the study and how many were included in the analysis and the risk of attrition bias was unclear. Overall, this study was considered to be at high risk of bias.
In the crossover trial of immediate-release dexmethylphenidate,127 the method for randomisation and presence of allocation concealment and patient, provider and outcome assessor blinding were unclear. The control group had no treatment and this was not considered to be an adequate control. There was no washout period between the intervention and control days and the risk of cross-intervention contamination was considered to be high. Overall, the risk of bias in this study was considered to be high owing to the unsuitable control group, lack of washout between treatments and unclear blinding of participants, providers and outcome assessors.
Clinical evidence for stimulants
Methylphenidate and dexmethylphenidate
Three studies used the YGTSS scale113,125,127 and one used the global tic rating scale124 to assess tics. Findings were presented separately for motor and vocal tics. For the motor tic scale (Figure 92), reduction from baseline/post-treatment motor tic score was similar for methylphenidate compared with placebo (SMD –0.03, 95% CI –0.20 to 0.15; n = 151) and there was no heterogeneity between studies (I2 = 0%). For immediate-release dexmethylphenidate,127 post-treatment motor tic score was lower than the no treatment control (SMD –0.60, 95% CI –1.13 to –0.07; n = 10). For stimulant drugs combined, the SMD was –0.17 (95% CI –0.46 to 0.11; n = 161). A large proportion of the difference between drugs was not explained by random variation (I2 for subgroup differences = 76%).
FIGURE 92.
Standardised motor tic score for stimulant drugs compared with control in children with ADHD and tics. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
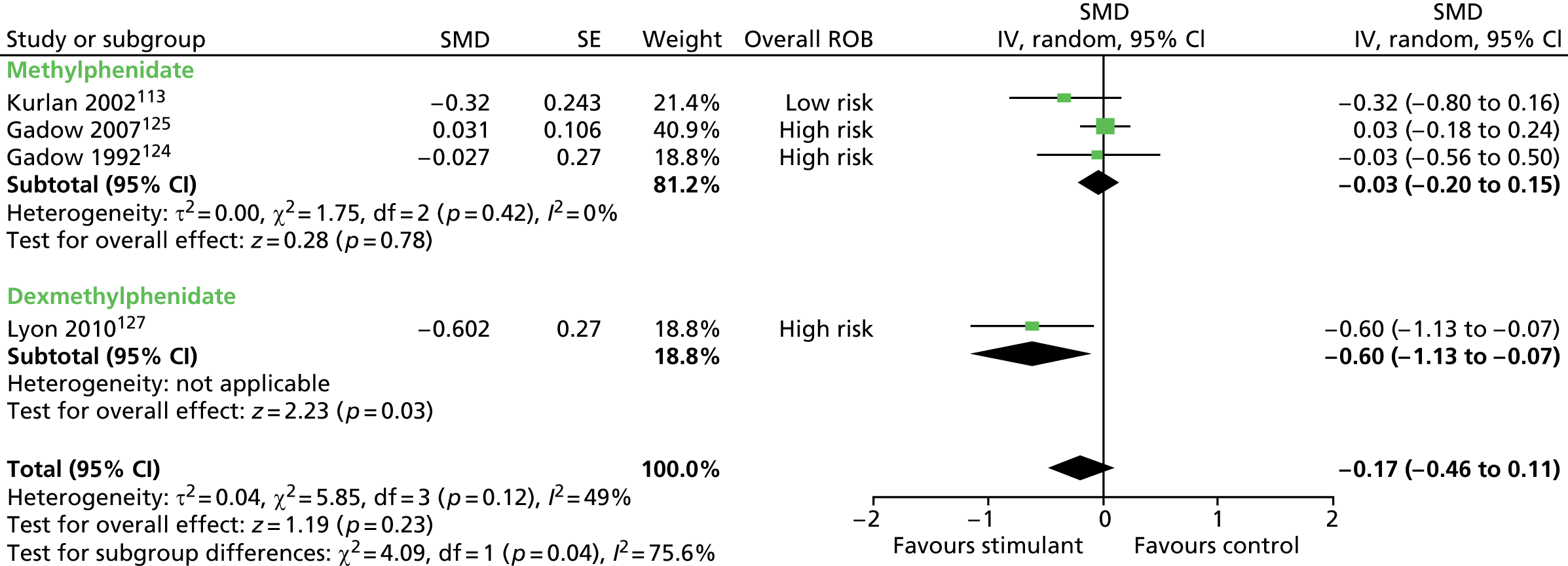
For the vocal tic scale (Figure 93) there was no conclusive difference in reduction from baseline/post-treatment vocal tic score for methylphenidate compared with placebo (SMD –0.28, 95% CI –0.73 to 0.16; n = 151). There was heterogeneity between studies (I2 = 71%). For immediate-release dexmethylphenidate, post-treatment vocal tic score was lower for dexmethylphenidate than no treatment (SMD –0.62, 95% CI –1.15 to –0.09; n = 10). For stimulant drugs considered as a whole, there was no conclusive difference in vocal tic score for stimulants compared with control (SMD –0.36, 95% CI –0.76 to 0.03; n = 150). All of the difference between drugs could be explained by random variation (I2 for subgroup differences = 0%).
FIGURE 93.
Standardised vocal tic score for stimulant drugs compared with control in children with ADHD and tics. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
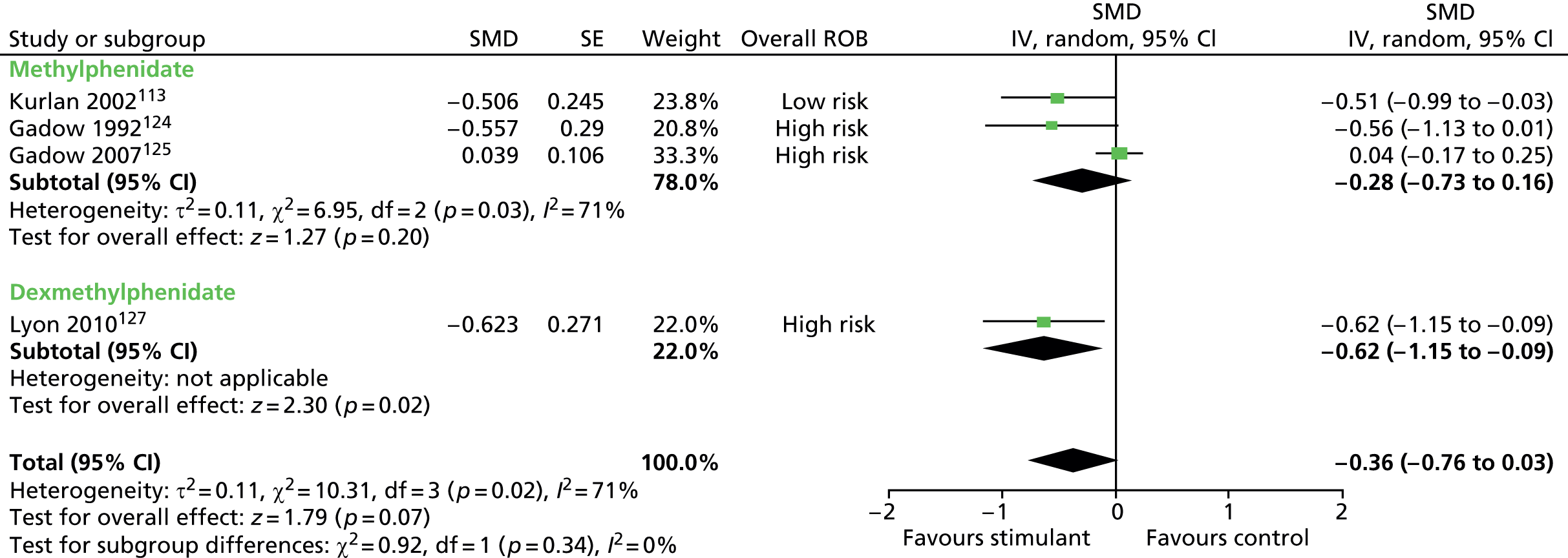
For the study where methylphenidate and clonidine were compared with placebo,113 there were greater reductions from baseline in motor (SMD –0.55, 95% CI –1.05 to –0.06; n = 65) and vocal (SMD –0.59, 95% CI –1.09 to –0.10; n = 65) tic scores for methylphenidate plus clonidine than placebo.
Three of the stimulant studies reported measures of impairment with the YGTSS impairment scale113,125,127 (Figure 94). For methylphenidate, there was no conclusive difference in reduction from baseline impairment score for methylphenidate compared with placebo (SMD –0.29, 95% CI –0.89 to 0.30; n = 140). There was heterogeneity between methylphenidate studies (I2 = 81%). For immediate-release dexmethylphenidate, there was no conclusive difference in impairment score compared with no treatment (SMD –0.41, 95% CI –1.30 to 0.47; n = 10). For stimulant drugs considered as a whole, the SMD was –0.30 (95% CI –0.76 to 0.15; n = 150) and there was no detectable difference between drugs (I2 for subgroup differences = 0%).
FIGURE 94.
Standardised YGTSS impairment score for stimulant drugs compared with a control in children with ADHD and tics. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
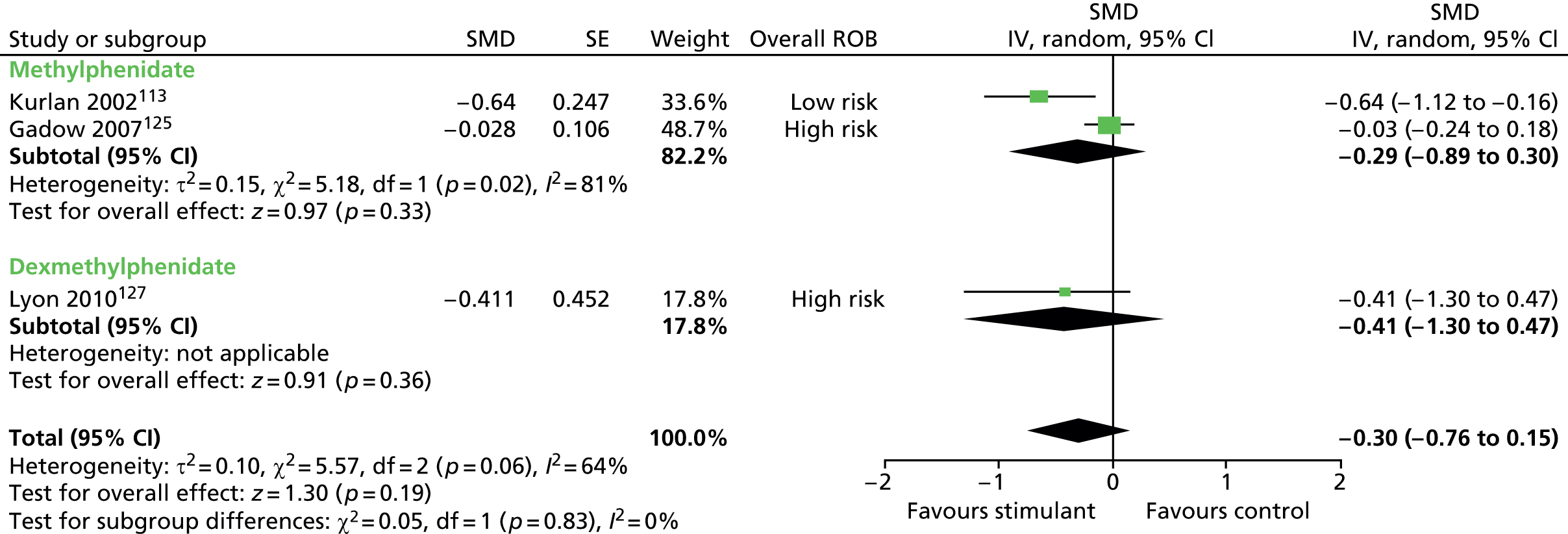
For the overall YGTSS global score (Figure 95), there was no conclusive difference in global score for methylphenidate compared with placebo (SMD –0.32, 95% CI –0.85 to 0.22; n = 140). There was heterogeneity between methylphenidate studies (I2 = 77%). For immediate-release dexmethylphenidate, there was no conclusive difference in global score for dexmethylphenidate compared with placebo (SMD –0.66, 95% CI –1.27 to 0.04; n = 10). When considered as a whole, the global score was SMD –0.40 (95% CI –0.84 to 0.04; n = 150) and all of the difference between drugs could be explained by random variation (I2 for subgroup differences = 0%).
FIGURE 95.
Standardised YGTSS global score for stimulant drugs compared with a control in children with ADHD and tics. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
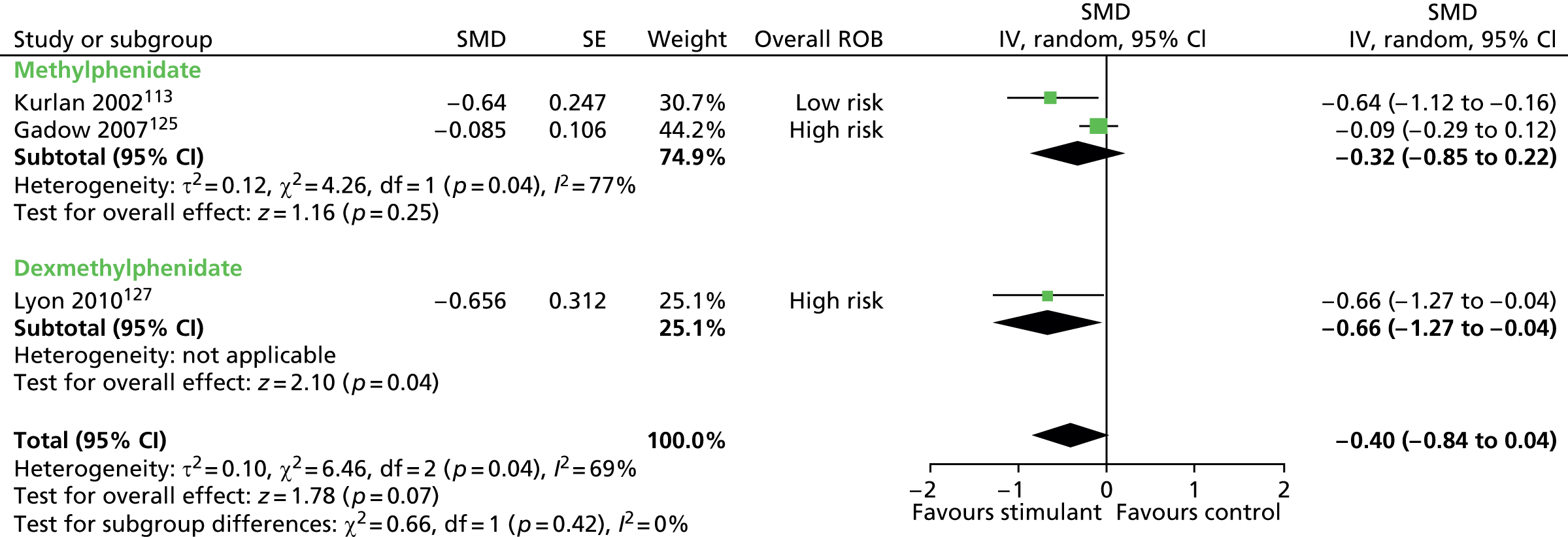
For the study in which methylphenidate and clonidine were compared with placebo,113 there were greater reductions from baseline in YGTSS impairment (SMD –0.66, 95% CI –1.16 to –0.16; n = 65) and global (SMD –0.77, 95% CI –1.27 to –0.26; n = 65) scores for methylphenidate plus clonidine than for placebo.
In one study of methylphenidate,125 assessment was made with the Stimulant Side Effects Checklist (SSEC) (Figure 96). For the SSEC mood index component, post-treatment teacher-rated score was lower for methylphenidate than for placebo (SMD –0.27, 95% CI –0.48 to –0.05; n = 71). For the parent-rated SSEC mood component, post-treatment score was similar for methylphenidate and placebo (SMD –0.10, 95% CI –0.31 to 0.10; n = 71).
FIGURE 96.
Standardised teacher and parent-rated SSEC mood index score for immediate-release methylphenidate compared with placebo in children with ADHD and tics. IV, independent variable; ROB, risk of bias; SE, standard error.
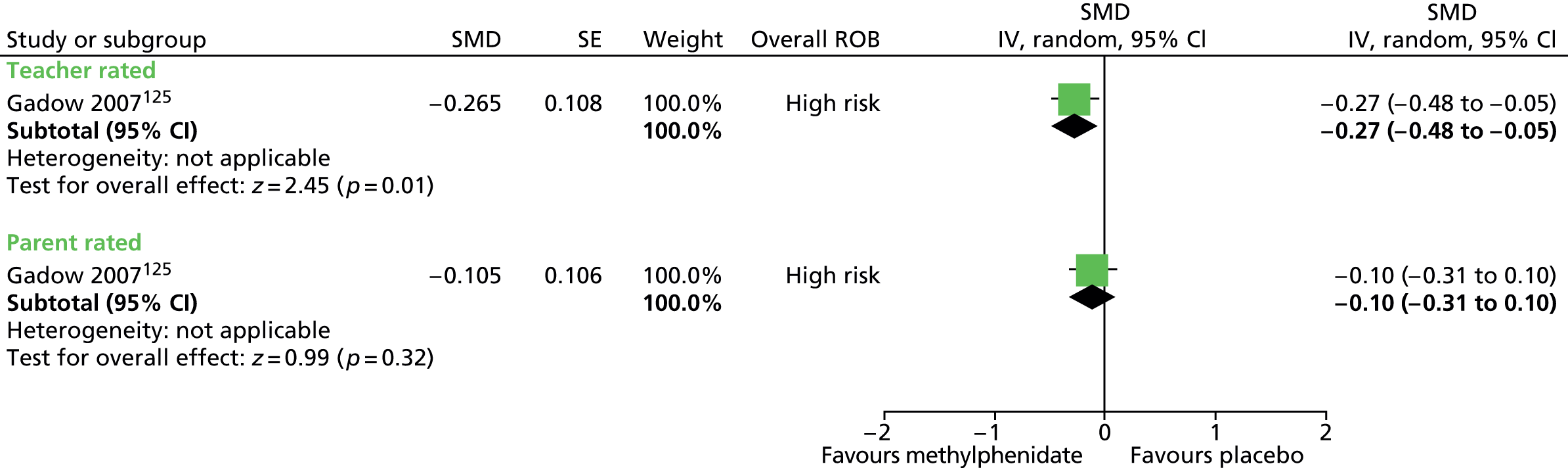
One study of methylphenidate measured overall clinical outcome using the CGI-I scale. 113 This assessment considered improvements in ADHD and tic symptoms separately. For CGI improvement in tics, a greater proportion of children were much or very much improved for methylphenidate compared with placebo when rated by parents (RR 0.64, 95% CI 0.42 to 0.96; n = 69) and teachers (RR 0.54, 95% CI 0.32 to 0.89; n = 69), but the difference was inconclusive when rated by investigators (RR 0.79, 95% CI 0.55 to 1.13; n = 69) (Figure 97) (the RR presented here is the RR of not being much or very much improved).
FIGURE 97.
Proportion of children much or very much improved on the CGI for methylphenidate compared with placebo in children with ADHD and tics (RRs of not being much or very much improved). M–H, Mantel–Haenszel; ROB, risk of bias.

For the study in which methylphenidate and clonidine were compared with placebo,113 a greater proportion of children were rated as much or very much improved by parents (RR 0.38, 95% CI 0.21 to 0.69; n = 65), teachers (RR 0.45, 95% CI 0.26 to 0.79; n = 65) and investigators (RR 0.45, 95% CI 0.27 to 0.75; n = 65) for methylphenidate plus clonidine than for placebo (the RR presented here is the RR of not being much or very much improved).
No relevant studies reported long-term outcomes.
The study of methylphenidate and clonidine reported that medications were well tolerated and that the methylphenidate alone group had lower rates of reported adverse effects than the clonidine, methylphenidate plus clonidine and placebo groups. Rates of moderate to severe sedation were similar for methylphenidate and placebo (RR 1.30, 95% CI 0.23 to 7.28; n = 69) (Figure 98).
FIGURE 98.
Proportion of patients experiencing moderate or severe sedation for methylphenidate compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.

There was also no conclusive difference when methylphenidate plus clonidine was compared with placebo (RR 3.03, 95% CI 0.68 to 13.55; n = 65). It was reported that, for all of the interventions, there was no overall evidence of cardiac toxicity by ECG monitoring. 113
In another study of methylphenidate,125 the SSEC was used to monitor adverse effects. In the somatic index of the SSEC (includes sleep and appetite problems, headache, upset stomach, dizziness), there was no difference in teacher-rated score for methylphenidate compared with placebo (SMD 0.09, 95% CI –0.12 to 0.30; n = 71) but, for parent-rated score, somatic index was higher for methylphenidate than for placebo (SMD 0.49, 95% CI 0.27 to 0.71; n = 71) (Figure 99).
FIGURE 99.
Stimulant Side Effects Checklist Somatic Index for immediate-release methylphenidate compared with placebo in children with ADHD and tics. IV, independent variable; ROB, risk of bias; SE, standard error.
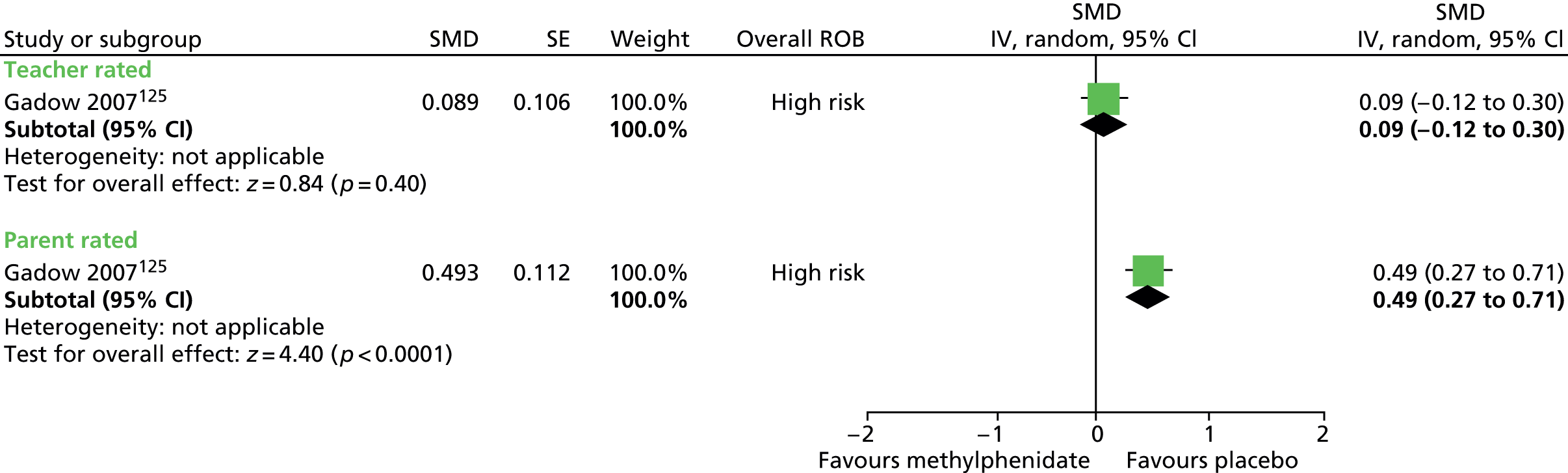
The study also reports that there were significant dose effects (doses of 0.1, 0.3 and 0.5 mg/kg were given) for heart rate, diastolic BP and weight loss. Findings for the 0.5 mg/kg dosage are compared here (Figure 100). Post-treatment measures were higher for methylphenidate compared with placebo for diastolic BP (MD 4.50, 95% CI 1.19 to 7.81; n = 71) and heart rate (MD 5.60, 95% CI 1.18 to 10.02; n = 71). There was no conclusive difference in post-treatment systolic BP (MD 5.30, 95% CI –0.16 to 10.76; n = 71) or weight gain for methylphenidate compared with placebo (MD –1.50, 95% CI –12.01 to 9.01; n = 71).
FIGURE 100.
Post-treatment cardiovascular and weight measurements for immediate-release methylphenidate compared with placebo in children with ADHD and tics. IV, independent variable; ROB, risk of bias.

For the study of immediate-release dexmethylphenidate,127 it is reported that participants tolerated the procedures well and that 7 out of 10 experienced at least one minor adverse effect during the study (no distinction made as to whether or not events occurred during the intervention or control days). The most common adverse effects considered to possibly be related to immediate-release dexmethylphenidate were drowsiness or sedation (20%) and stomach discomfort (20%).
In the study of immediate-release methylphenidate,125 participants were separated into two groups on the basis of their baseline YGTSS impairment score. The study reports that there were similar effects of the drug on tic and ADHD symptoms for groups with low or high baseline YGTSS impairment score.
Clinical evidence summary for stimulant drugs
Methylphenidate and dexmethylphenidate
Four studies (n = 161) provided very low-quality evidence (see Appendix 4, Tables 33–35) that the stimulant drugs methylphenidate113,124,125 and dexmethylphenidate127 do not increase motor or vocal tics, impairment or global tics/impairment following 0–16 weeks of treatment. One study of methylphenidate reported increased rates of diastolic BP, systolic BP and heart rate that were dose dependent,125 but other studies did not report adverse effects.
Conclusion
The available evidence suggests that stimulant drugs (methylphenidate and dexmethylphenidate) do not significantly increase or exacerbate tics when used in the short term to treat comorbid ADHD and TS. However, there are differences between stimulants in their effect on tics, with dexmethylphenidate (a stimulant not marketed in the UK) effective in reducing tics. Overall, the combination of methylphenidate and clonidine is well tolerated, with mild adverse effects (e.g. sedation) and no evidence of cardiac risk.
Anticonvulsant drugs
Pharmacology and prescribing
Levetiracetam (Keppra®, UCB Pharma) is licensed in the UK for monotherapy and adjunctive treatments of focal seizures, adjunctive therapy of myoclonic seizures and primary generalised tonic–clonic seizures.
Topiramate (Topamax®, Janssen) is a broad-spectrum antiepileptic drug licensed in the UK for monotherapy in adults, adolescents and children over 6 years of age with partial seizures with or without secondary generalised seizures, and primary generalised tonic–clonic seizures. It is also licenced for use in adults for the prophylaxis of migraine headache and is sometimes used in the management of headache, mood and behavioural disorders, pain and tremors. 128 Topiramate has been associated with significant adverse effects including myopia and close angle glaucoma, fatigue, weight loss and psychosis.
Included studies
Two child studies investigated the efficacy of treatment with levetiracetam and one mixed study investigated the efficacy of treatment with topiramate. 129,130
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. In one child crossover study,129 children received a maximum 30 mg/kg/day levetiracetam and placebo in a randomised order for 4 weeks each. In another child crossover study,130 children also received levetiracetam and placebo in a randomised order but only pooled intervention/control results appear to be presented and no efficacy data could be obtained.
One parallel mixed study investigated the use of topiramate compared with placebo. 131 In this study, 29 adults and children were randomised to maximum dose of 200 mg/day topiramate or placebo for 6 weeks. Because the mean age of this population was low (16.5 years, SD 9.9), it was assumed that the majority of the participants would be < 18 years and this study was combined with the child studies in the meta-analysis.
Risk of bias
For the study of levetiracetam with available data,129 quality assessment was conducted. Computer-generated randomisation codes were used but the presence of allocation concealment was unclear. There was assurance of blinding of participants, providers and outcome assessors. The risk of attrition bias was judged to be low because although analysis was of available cases, the rate of dropout was low. The study was a crossover trial with a 5–10 day period in which drugs were tapered between interventions and an additional 5-day washout period and the risk of bias from cross-intervention contamination was unclear. The overall risk of bias was considered to be unclear owing to the possibility of cross-intervention contamination.
For the study of topiramate,131 a computer-generated randomisation code was used but the presence of allocation concealment was unclear. There was assurance of blinding of participants, providers and outcome assessors. The risk of attrition bias was judged to be low because analysis was by intention to treat for subjects with post-baseline measurements. The overall risk of bias for the study was considered to be low.
Clinical evidence for anticonvulsant drugs
Levetiracetam and topiramate
In both studies, tics were assessed using the YGTSS total tic scale (Figure 101). In the child study of levetiracetam,129 change in total tic score was inconclusive for levetiracetam compared with placebo (SMD –0.23, 95% CI –0.85 to 0.39; n = 20). In the mixed study of topiramate,131 there was a greater reduction from baseline in total tic score for topiramate than for placebo (SMD –0.88, 95% CI –1.68 to –0.08; n = 27).
FIGURE 101.
Standardised YGTSS total tic score for anticonvulsants compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
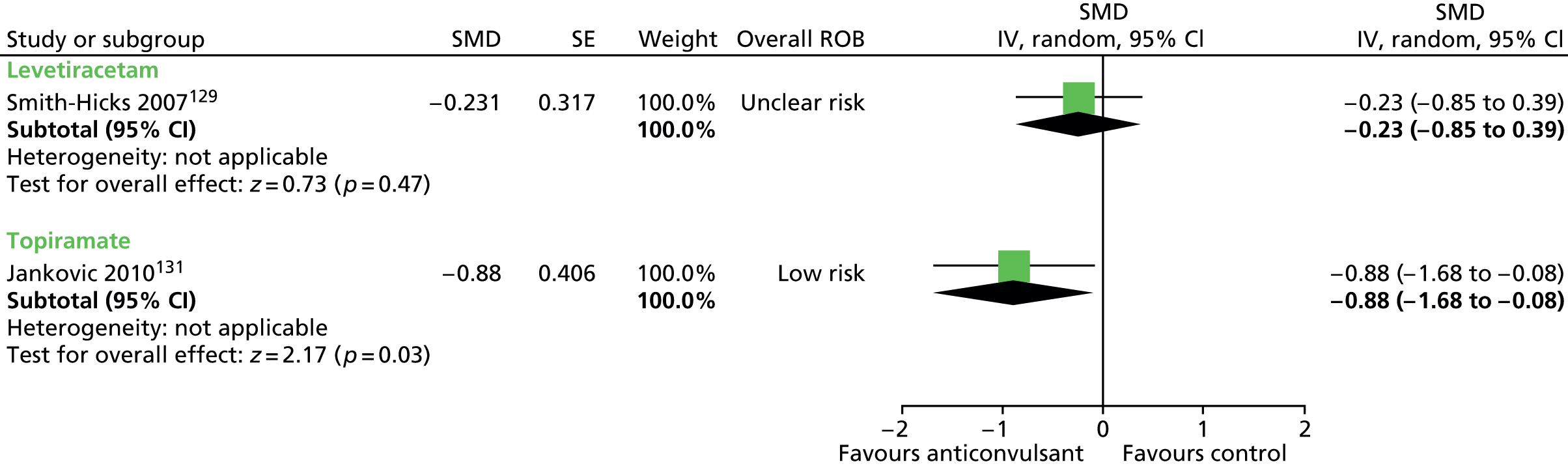
For the study of topiramate, YGTSS motor and vocal tic scale components were presented separately. Change from baseline suggested a benefit for topiramate compared with placebo for motor tic score (SMD –0.86, 95% CI –1.65 to –0.06; n = 27) (Figure 102), but was not conclusively different for vocal tic score (SMD –0.64, 95% CI –1.41 to 0.14; n = 27) (Figure 103).
FIGURE 102.
Standardised change from baseline YGTSS motor tic score for topiramate compared with placebo. IV, independent variable; ROB, risk of bias.
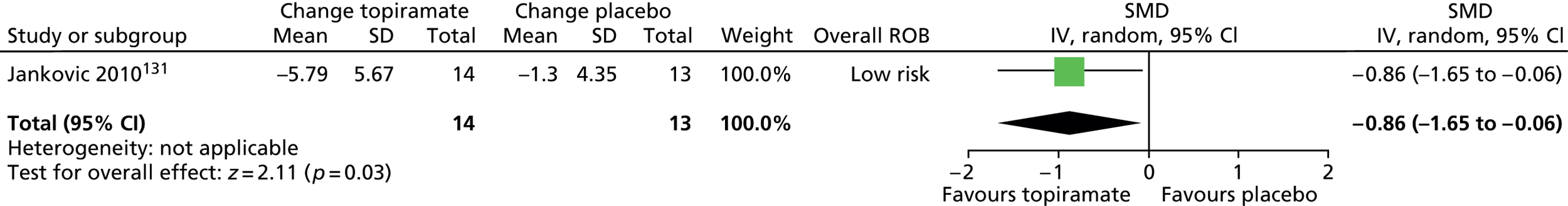
FIGURE 103.
Standardised change from baseline YGTSS vocal tic score for topiramate compared with placebo. IV, independent variable; ROB, risk of bias.
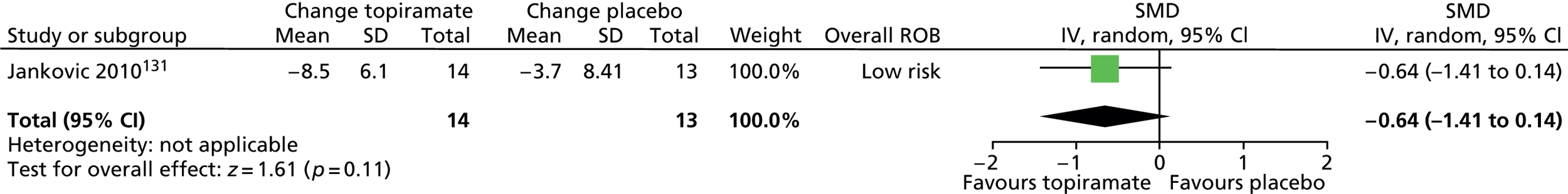
In both studies, the YGTSS global tic score was assessed (total tic score and impairment score) (Figure 104). Post-treatment global score was inconclusive for children treated with levetiracetam compared with placebo (SMD 0.09, 95% CI –0.53 to 0.70; n = 20), but change from baseline in YGTSS global score suggested a benefit for topiramate compared with placebo (SMD –1.19, 95% CI –2.02 to –0.36; n = 27).
FIGURE 104.
Standardised YGTSS global score for anticonvulsants compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
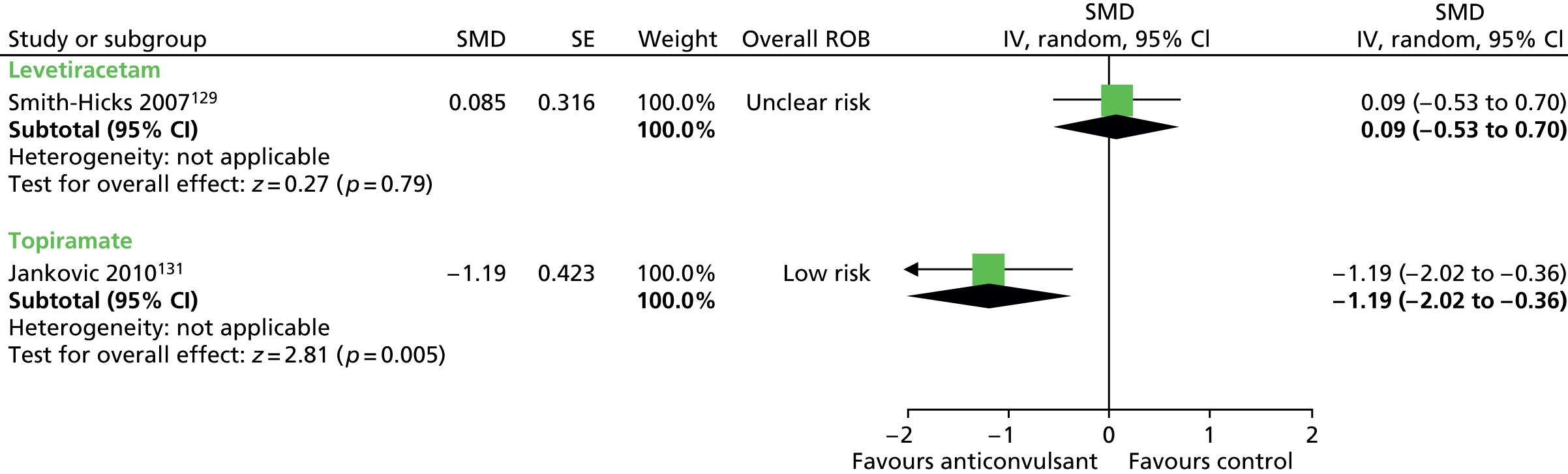
The study of levetiracetam assessed child anxiety using the Multidimensional Anxiety Scale for Children (MASC). Post-treatment anxiety score was inconclusive for children treated with levetiracetam compared with placebo (SMD –0.04, 95% CI –0.66 to 0.58; n = 20) (Figure 105).
FIGURE 105.
Standardised MASC score for levetiracetam compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
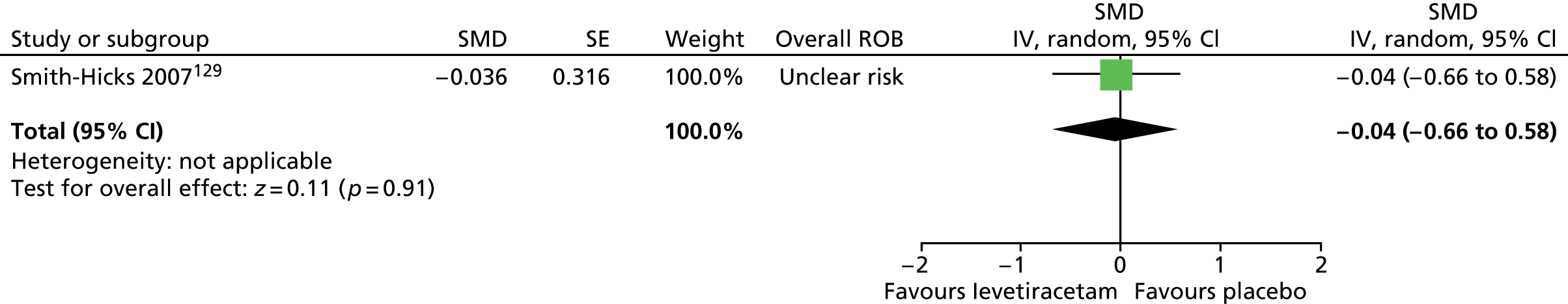
The study of levetiracetam assessed child depression using the Child Depression Inventory (CDI) score. Post-treatment depression score was inconclusive for children treated with levetiracetam compared with placebo (SMD 0.20, 95% CI –0.43 to 0.82; n = 20) (Figure 106).
FIGURE 106.
Standardised CDI score for levetiracetam compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
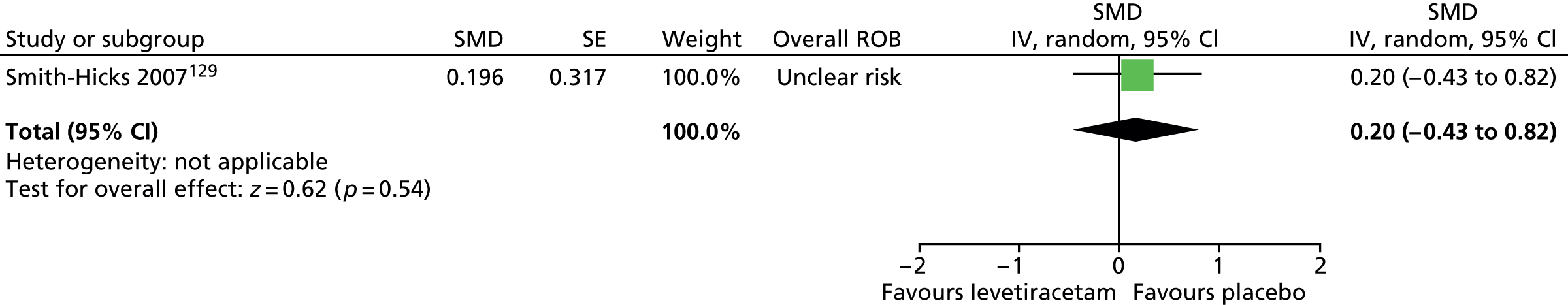
The study of levetiracetam assessed overall clinical outcome using the CGI scale. Post-treatment CGI-Severity score was inconclusive for children treated with levetiracetam compared with placebo (SMD 0.14, 95% CI –0.49 to 0.76; n = 20) (Figure 107).
FIGURE 107.
Standardised CGI score for levetiracetam compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
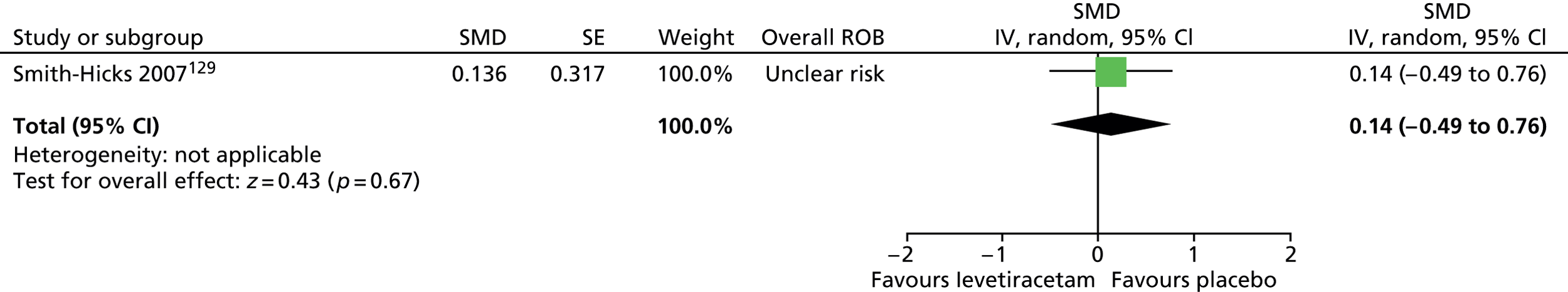
No relevant studies reported long-term outcomes.
In the study of levetiracetam, adverse effects reported during the levetiracetam phase were irritability that resolved with time, hyperkinesias during the initial dose escalation that resolved by the end of the second week, insomnia, sadness, tiredness, verbal aggression, reduced school participation, anxiousness and headache. During the placebo phase, there were complaints of headache, irritability, aggression, low frustration tolerance, insomnia, tiredness, sadness, worry, hyperkinesias, anxiousness and dry mouth (numbers not reported).
In the study of topiramate, the results were inconclusive for the proportion of participants experiencing at least one adverse effect for topiramate compared with placebo (RR 0.79, 95% CI 0.56 to 1.11; n = 29) (Figure 108). When considered separately, the results were inconclusive for the proportions of participants experiencing any adverse effect for topiramate compared with placebo (Figure 109). The mean change in weight from baseline was –2.1 kg in the topiramate compared with +1.9 kg in the placebo group (precision not reported).
FIGURE 108.
Proportion of patients experiencing more than one adverse event for topiramate compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.
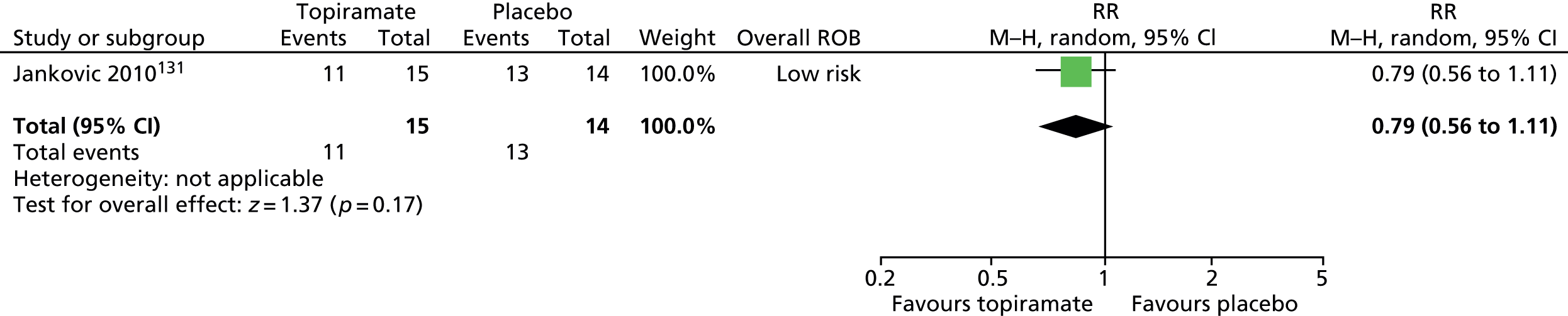
FIGURE 109.
Proportion of patients experiencing adverse events for topiramate compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.

Clinical evidence summary for anticonvulsant drugs
Levetiracetam
There was low-quality evidence (see Appendix 4, Table 31) from one study in 20 children129 that was inconclusive as to whether or not levetiracetam improved tics, global tic/impairment, symptoms of anxiety or depression, or overall clinical outcome following 4 weeks of treatment, but adverse effects were unclear.
Topiramate
One study with 27 children and adults131 provided low-quality evidence (see Appendix 4, Table 32) suggesting that topiramate produced a large effect in terms of improving tics and global tic/impairment following 6 weeks of treatment. There was no evidence of adverse effects.
Conclusion
There is no clear evidence that levetiracetam is an effective treatment for tics. Topiramate may be effective in the short-term treatment of tics in children and young people with TS. However, the evidence is drawn from a single small, mixed study and should be treated with caution. In addition, topiramate in wider use has been associated with significant adverse effects including myopia and close angle glaucoma, fatigue, weight loss and paraesthesia.
Monoamine oxidase B inhibitors
Pharmacology and prescribing
Monoamine oxidase B inhibitors act by inhibiting the action of monoamine oxidase in the metabolic pathway of dopamine. This causes dopamine metabolism to decrease and the levels of dopamine in the brain to increase. Use of these drugs is restricted because of serious dietary interactions that may cause hypertensive crises and psychiatric withdrawal symptoms including depression and psychosis.
Selegiline (Eldepryl®, Orion), is licensed in the UK for the treatment of Parkinson’s disease, or symptomatic parkinsonism.
Included studies
One study investigated the efficacy of treatment with selegiline (deprenyl) in children with ADHD and TS. 132
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. In a crossover study,132 24 children were treated with maximum dose of 5 mg selegiline twice a day and placebo for 8 weeks each in a randomised order. Data are presented as the MD of the improvement on selegiline compared with placebo (unlike most crossover studies, change and not post-treatment scores are presented).
Risk of bias
Randomisation was generated by a Fortran computer program (with stratification by centre) but the presence of allocation concealment and blinding of participants, providers and outcome assessors was unclear. It was a crossover trial with a 6-week washout period between interventions and this may have been sufficient to avoid cross-intervention contamination. The rate of dropout was reasonably high (37%). The number included in the analysis was unclear and the risk of attrition bias was considered to be unclear. Overall, this study was considered to be at unclear risk of bias owing to unclear participant, provider and outcome assessor blinding and possible attrition bias.
Clinical evidence for monoamine oxidase B inhibitors
Selegiline
Tics were assessed with the YGTSS tic scale. Results were inconclusive for selegiline compared with placebo for motor (SMD –0.64, 95% CI –1.37 to 0.09; n = 15) and vocal (SMD –0.30, 95% CI –1.02 to 0.41; n = 15) tics (Figure 110).
FIGURE 110.
Standardised YGTSS motor and vocal tic score for children treated with selegiline compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
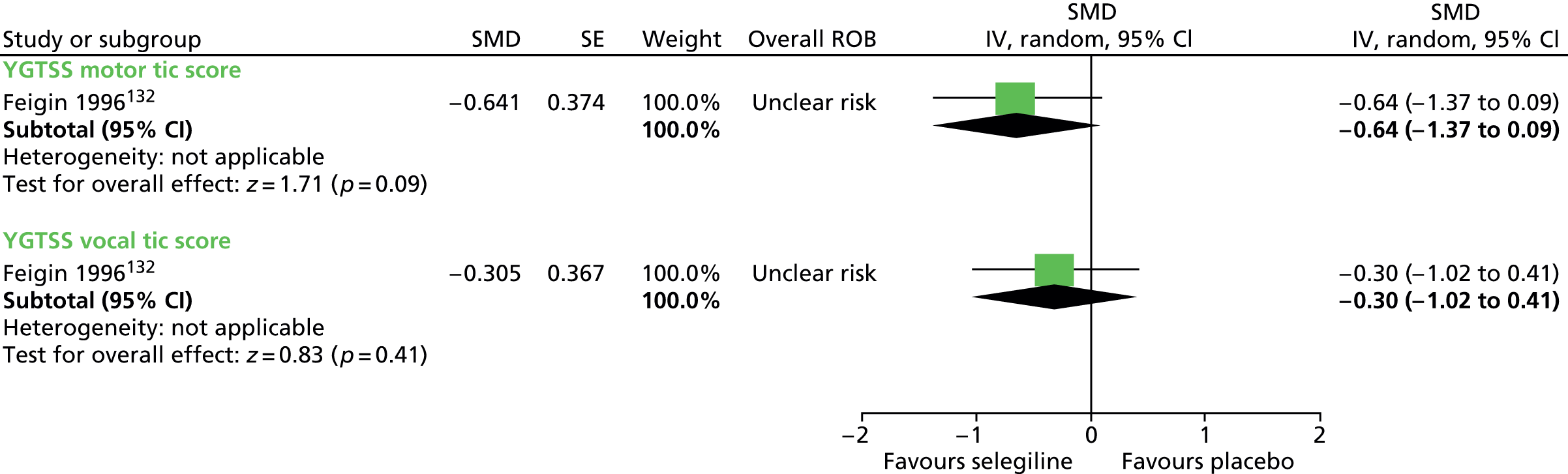
TS-related impairment was assessed with the YGTSS impairment scale and combined tic severity and impairment with YGTSS global scale. Reduction was greater following treatment with selegiline than for placebo for impairment score (SMD –0.75, 95% CI –1.49 to –0.01; n = 15) and for global score (SMD –0.72, 95% CI –1.45 to 0.02; n = 15) (Figure 111).
FIGURE 111.
Standardised YGTSS impairment and global score for children treated with selegiline compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.

No relevant studies reported this outcome.
No relevant studies reported this outcome.
No relevant studies reported long-term outcomes.
The occurrence of adverse effects is reported over the whole study period and it is not possible to distinguish events occurring during selegiline and placebo phases from one another. Events included rash (n = 1), nausea (n = 4), agitation (n = 4), irritability (n = 2), drowsiness (n = 5), headache (n = 4) and diarrhoea (n = 1) but it is reported that none of the adverse effects occurred more frequently during the selegiline compared with the placebo phase.
Clinical evidence summary for monoamine oxidase B inhibitors
Selegiline
One study in 15 children132 provided low-quality evidence (see Appendix 4, Table 39) that was inconclusive as to whether or not selegiline improved motor and vocal tics following 4 weeks of treatment, but the evidence suggested some benefit in terms of impairment. The relative occurrence of adverse effects was not reported.
Conclusion
There is little evidence that selegiline is an effective treatment for tics in children and young people with TS.
Dopamine receptor agonists
Pharmacology and prescribing
Dopamine receptor agonists are primarily used for treating Parkinson’s disease.
Pergolide (non-proprietary) was withdrawn by the US FDA in 2007 owing to two studies indicating that the drug could cause serious heart valve damage. 133,134 It is still used in the UK, mainly as an adjunctive treatment for Parkinson’s disease and for restless leg syndrome.
Pramipexole (Mirapexin®, Boehringer Ingelheim) is licensed in the UK for the treatment of the signs and symptoms of idiopathic Parkinson’s disease. At the time of this review, the US FDA has not withdrawn pramipexole from the US market but has issued a safety announcement, and is working with the manufacturer to further clarify the risk of heart failure.
Included studies
Three studies in children investigated the efficacy of dopamine agonists compared with placebo:
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. One pergolide study135 was a crossover trial where children received a maximum dose of 300 µg pergolide and placebo in a randomised order for 6 weeks each. The other study of pergolide136 was a parallel trial where 51 children were randomised 2 : 1 to receive 8 weeks of a maximum dose of 150 µg pergolide or placebo. The study of pramipexole137 was a parallel trial where 63 children were randomised to a maximum dose of 0.25 mg pramipexole twice a day or placebo for 8 weeks.
Risk of bias
In the crossover study of pergolide,135 randomisation was conducted by dice roll and therefore appears to have had poor allocation concealment. There was blinding of participants, providers and outcome assessors. There was only a 2-week washout period between treatment phases and the risk of cross-treatment contamination is unclear. Although analysis appears to be of available cases, there was a reasonably low rate of drop out (21%) and there may have been a low risk of attrition bias. Overall, this study was considered to be at unclear risk of bias owing to possible cross-treatment contamination.
In the parallel trial of pergolide,136 the method of randomisation and presence of allocation concealment were unclear. There was blinding of participants, providers and outcome assessors. Analysis was by last observation carried forward for participants with post-baseline measurements and the risk of attrition bias was considered to be low. Overall, this study was considered to be at low risk of bias.
In the parallel trial of pramipexole,137 the method of randomisation, presence of allocation concealment and blinding of participants, providers and outcome assessors were unclear. The rates of dropout from the study were not reported and the presence of attrition bias is unclear. Overall, this study was considered to be at unclear risk of bias owing to the unclear presence of patient, provider and outcome assessor blinding.
Clinical evidence
Pergolide and pramipexole
One study of pergolide136 and one of pramipexole137 measured effects on tics using the YGTSS total tic scale. Reduction from baseline tic score was greater but there was no conclusive difference for pergolide compared with placebo (SMD –0.59, 95% CI –1.21 to 0.02; n = 51). Reduction from baseline tic score was similar following treatment with pramipexole compared with placebo (SMD –0.00, 95% CI –0.53 to 0.53; n = 62) (Figure 112).
FIGURE 112.
Standardised change from baseline total tic score for dopamine agonists compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.

For all studies, the YGTSS global scale was used to assess tic outcome (Figure 113). Global score favoured pergolide in comparison with placebo (SMD –0.87, 95% CI –1.39 to –0.34; n = 70), with little heterogeneity between studies (I2 = 25%). Change from baseline global score was similar for pramipexole compared with placebo (SMD –0.01, 95% CI –0.54 to 0.52; n = 62).
FIGURE 113.
Standardised global tic score for dopamine agonists compared with placebo. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

One study of pergolide,136 reported effects on impairment using the YGTSS impairment score (Figure 114). Results were inconclusive for the reduction from baseline impairment score for pergolide compared with placebo (SMD –0.53, 95% CI –1.14 to 0.08; n = 51).
FIGURE 114.
Standardised change from baseline YGTSS impairment score for pergolide compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
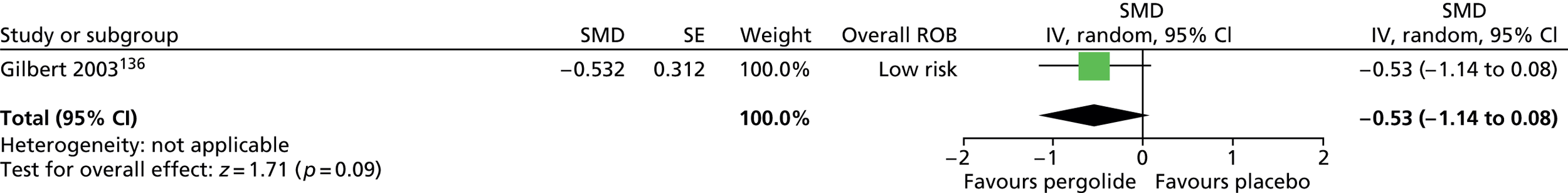
In the study of pramipexole,137 psychological well-being was measured using the Child Depression Inventory – short version (CDI-S) and the MASC. Results were inconclusive for the reduction from baseline in depression (SMD –0.44, 95% CI –0.99 to 0.11; n = 61) or anxiety scores (SMD –0.30, 95% CI –0.85 to 0.24; n = 61) for pramipexole compared with placebo (Figures 115 and 116).
FIGURE 115.
Standardised change from baseline in CDI-S score for pramipexole compared with placebo. IV, independent variable; ROB, risk of bias.

FIGURE 116.
Standardised change from baseline in MASC score for pramipexole compared with placebo. IV, independent variable; ROB, risk of bias.

In all studies, overall clinical outcome was measured using the CGI-Severity scale. In the pergolide studies,135,136 change from baseline/post-treatment CGI-Severity score was inconclusive for pergolide compared with placebo (SMD –0.28, 95% CI –0.78 to 0.23; n = 70) (Figure 117) and there was no heterogeneity between study results (in the crossover study, first-phase data were used for this outcome because of carry over effects).
FIGURE 117.
Standardised change from baseline/post-treatment CGI-Severity score for pergolide compared with placebo. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

In the study of pramipexole, results were inconclusive for the proportions of children who had an improved (RR 1.19, 95% CI 0.42 to 3.33; n = 62) or unchanged (RR 0.95, 95% CI 0.72 to 1.26; n = 62) CGI-Severity score for pramipexole compared with placebo (Figure 118).
FIGURE 118.
Proportion of children with improved and unchanged CGI-Severity scores for pramipexole compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.

No relevant studies reported long-term outcomes.
One study of pergolide135 and one of pramipexole137 reported the number of participants with one or more adverse effects (Figure 119). Results were inconclusive for the proportions of participants experienced an adverse effect for intervention compared with placebo in the study of pergolide (RR 0.88, 95% CI 0.40 to 1.93; n = 19) and in the study of pramipexole (RR 1.62, 95% CI 0.70 to 3.76; n = 62). In the pramipexole study, the most frequent adverse effects in the pramipexole group were headache (28%), nausea (19%), vomiting (12%), myalgia (9%) and fatigue (9%) (not reported for the placebo group).
FIGURE 119.
Proportion of patients with adverse events in studies of dopamine agonists compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.

Both studies135,136 of pergolide measured adverse effects using the STESS and patient-reported adverse effects were recorded. STESS scores were inconclusive for pergolide compared with placebo (SMD –0.05, 95% CI –0.49 to 0.38; n = 70) (Figure 120).
FIGURE 120.
Standardised STESS side effect score for pergolide compared with placebo. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

In one study of pergolide,136 results were inconclusive for the rates of gastrointestinal adverse effects (RR 0.98, 95% CI 0.68 to 1.42; n = 51), headaches (RR 0.74, 95% CI 0.43 to 1.29; n = 51), mild insomnia (RR 5.62, 95% CI 0.34 to 93.94; n = 51) and rashes (RR 1.67, 95% CI 0.20 to 13.70; n = 51) for pergolide compared with placebo (Figure 121).
FIGURE 121.
Proportion of patients experiencing various adverse events for pergolide compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.

In this study, ECG parameters were measured (Figure 122). Results were inconclusive for the changes from baseline for pergolide compared with placebo for PR interval (MD 9.90, 95% CI –5.27 to 25.07; n = 51), QT interval (MD 13.50, 95% CI –4.29 to 31.29; n = 51) and QRS width (MD 0.70, 95% CI –3.35 to 4.75; n = 51).
FIGURE 122.
Change (msec) from baseline in ECG parameters for pergolide compared with placebo. IV, independent variable; msec, milliseconds; ROB, risk of bias.
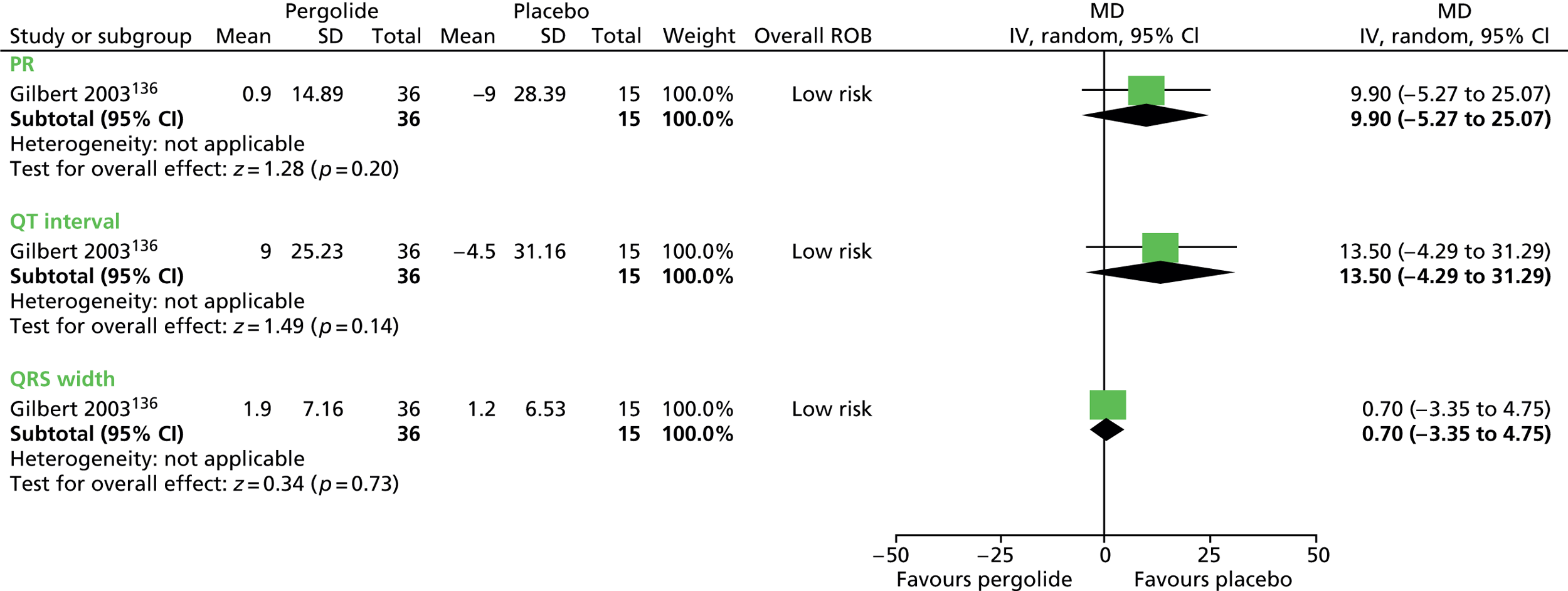
For the other pergolide study,135 adverse effects in the pergolide group were syncope, sedation, postural dizziness, dizziness, nausea, stomach ache and irritability and, in the placebo group, blurry vision, fever, hyperventilation, leg pain, nausea, rash, sedation, stomach ache, weight loss, diarrhoea and enuresis (numbers not reported).
Clinical evidence summary
Pergolide
There was low-quality evidence (see Appendix 4, Table 36) from two studies in children (n= 70)135,136 that pergolide produced medium-to-large effects in terms of tics and global tic/impairment, but overall clinical outcome was inconclusive.
Pramipexole
One study in 62 children137 provided low-quality evidence (see Appendix 4, Table 37) that pramipexole was unlikely to improve tics, global tic/impairment or overall clinical outcome (proportion of participants much or very much improved on the CGI scale) following 6 weeks of treatment. There was an increase in adverse effects, but the estimates were very imprecise.
Conclusion
Pergolide may be an effective agent for the reduction of tics in the short term in children and young people with TS, but effects on global outcomes are inconclusive. While there was no apparent increase in adverse effects in these studies, pergolide is now rarely considered for other neurological indications (Parkinson’s disease) in the UK owing to serious concerns over the risk of long-term fibrotic reactions (i.e. pulmonary and pericardial fibrosis). There is no clear evidence that pramipexole is an effective treatment for tics.
5HT3-receptor antagonists/antiemetic and gastroprokinetic agents
Pharmacology and prescribing
Metoclopramide (Maxolon®, AMCo) is a dopamine antagonist with a license in the UK to treat a number of conditions including the treatment of the nausea and vomiting associated with gastrointestinal disorders. Like other dopamine antagonists, metoclopramide antagonises dopamine D2 receptors within the striatum.
Ondansetron (Zofran®, GSK) is a selective 5-HT3 receptor antagonist licensed in the UK for the treatment of nausea and vomiting in relation to post-operative recovery and chemotherapy.
Included studies
One parallel trial investigated the efficacy of metoclopramide138 and one parallel trial investigated the efficacy of ondansetron. 139
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. One parallel trial in children compared metoclopramide with placebo. 138 Twenty-eight children with TS or CTD were randomised to maximum dose of 40 mg/day metoclopramide or to placebo for 8 weeks.
Another parallel trial in a mixed population of adults and children compared treatment with ondansetron with placebo. 139 The original study was of 30 adults and children, but the authors were able to supply IPD for 15 children who were randomised to 8 mg/day ondansetron or placebo for 3 weeks.
Risk of bias
Neither study provided information on the method of sequence generation, allocation concealment or statements about the presence of participant, provider or outcome assessor blinding. Neither study had a high rate of dropout and both were considered to be at low risk of attrition bias. Overall, owing to the unclear presence of blinding, both of these studies were considered to be at unclear risk of bias.
Clinical evidence for 5HT3-receptor antagonists/antiemetic and gastroprokinetic agents
Metoclopramide and ondansetron
Both studies measured tic changes using the YGTSS. For metoclopramide, reduction from baseline in total tic score was greater for metoclopramide than placebo (SMD –1.43, 95% CI –2.28 to –0.59; n = 27). For ondansetron, the reduction in total tic score was inconclusive for ondansetron compared with placebo (SMD –0.38, 95% CI –1.42 to 0.66; n = 15) (Figure 123).
FIGURE 123.
Standardised change from baseline YGTSS total tic score for children treated with antiemetic or gastroprokinetic agents compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
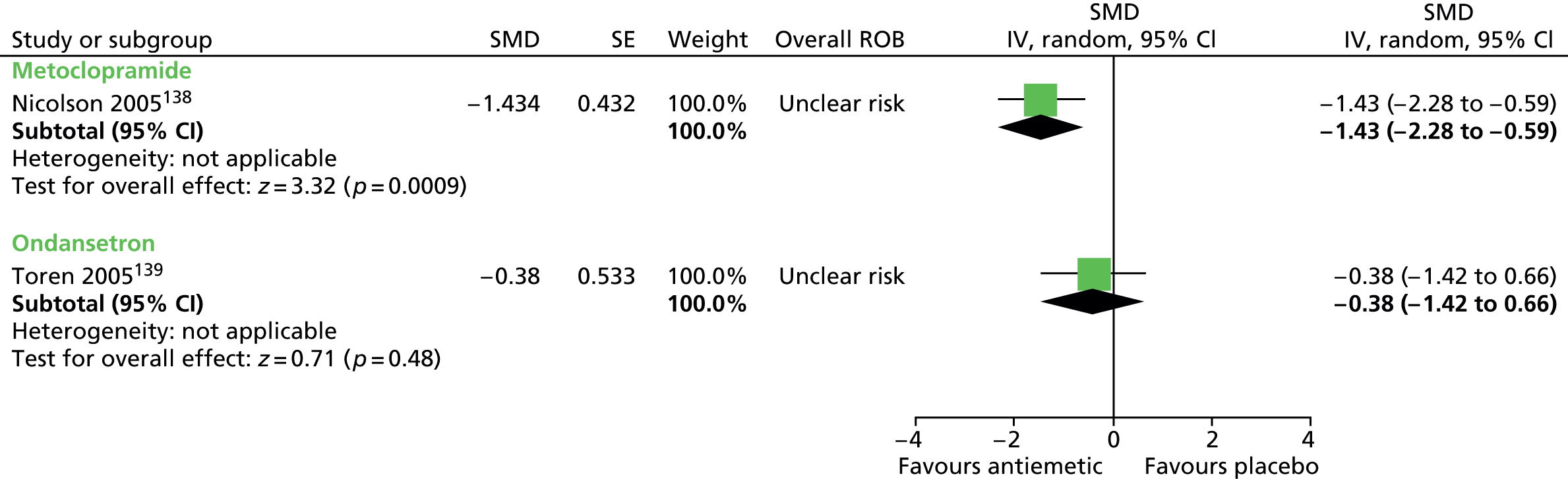
Both studies reported findings for motor and vocal tic components separately. For metoclopramide, there was a reduction in YGTSS motor tic score compared with placebo (SMD –1.44, 95% CI –2.28 to –0.59; n = 27). For ondansetron, the results were inconclusive when compared with placebo (SMD –0.14, 95% CI –1.18 to 0.89; n = 15) (Figure 124).
FIGURE 124.
Standardised change from baseline YGTSS motor tic score for children treated with an antiemetic or gastroprokinetic agent compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
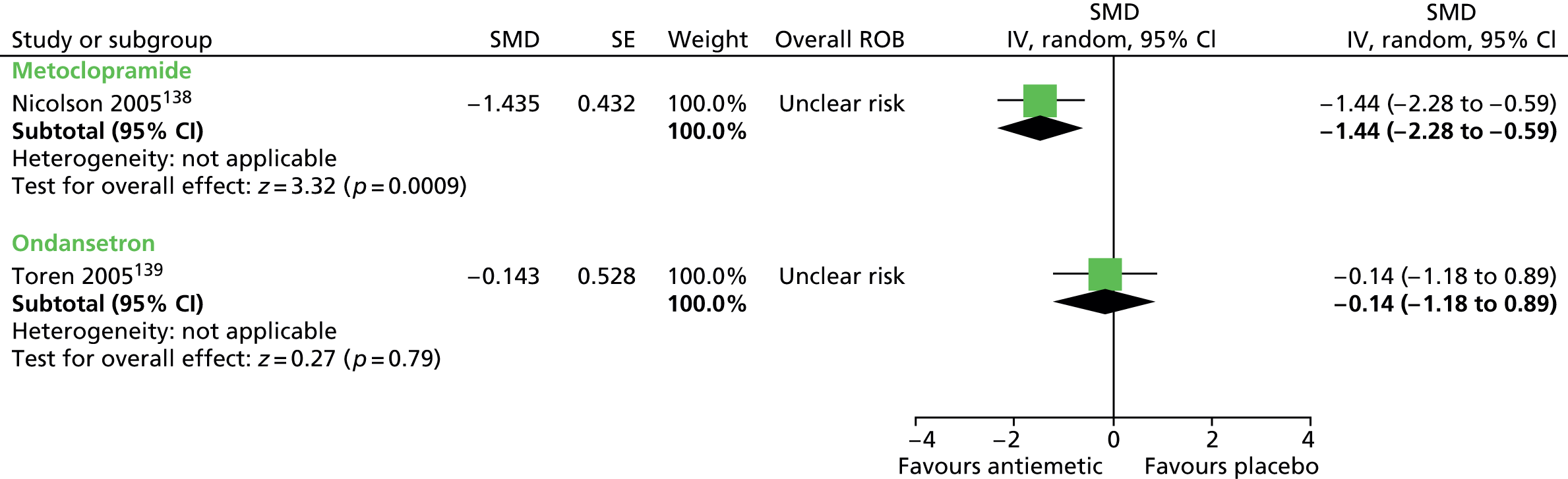
For vocal tics, there was a reduction in YGTSS vocal tic score for metoclopramide compared with placebo (SMD –0.79, 95% CI –1.58 to –0.01; n = 27), but findings were inconclusive for ondansetron compared with placebo (SMD –0.69, 95% CI –1.76 to 0.37; n = 15) (Figure 125).
FIGURE 125.
Standardised change from baseline YGTSS vocal tic score for children treated with an antiemetic or gastroprokinetic agent compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
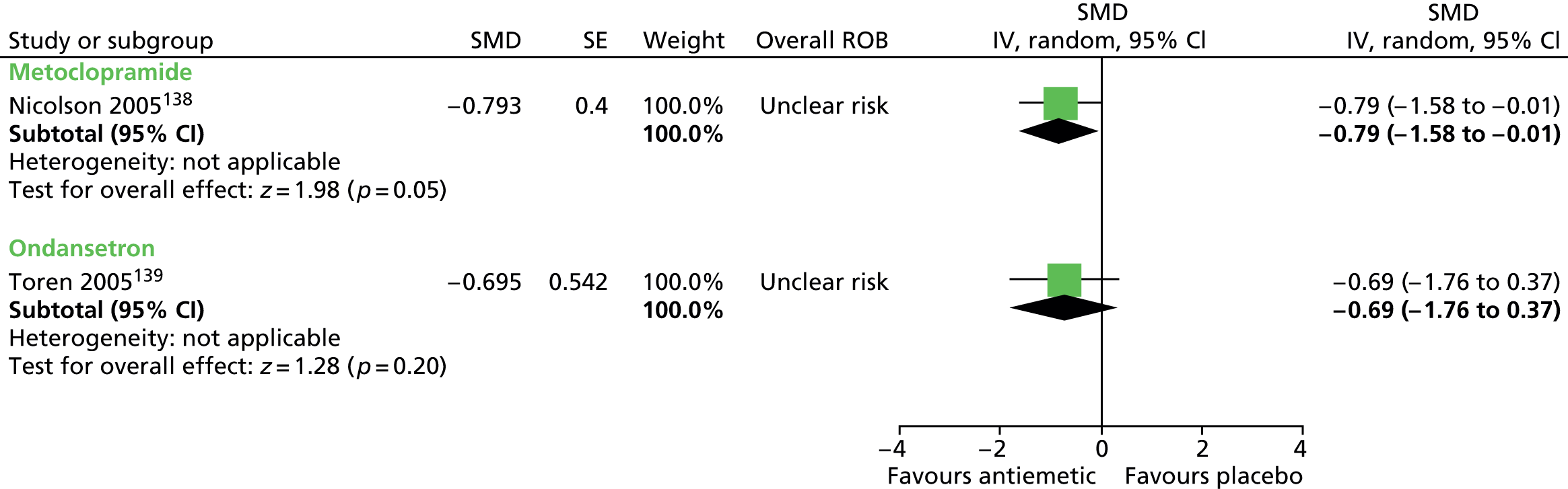
No measures of TS-related impairment were reported by these studies.
No relevant studies reported this outcome.
In both studies, overall clinical outcome was assessed using the CGI-Severity scale. CGI score suggested a benefit for metoclopramide compared with placebo (SMD –1.07, 95% CI –1.88 to –0.27; n = 27). For ondansetron compared with placebo, the results were inconclusive (SMD –0.50, 95% –1.55 to 0.55; n = 15) (Figure 126).
FIGURE 126.
Standardised change from baseline in CGI-Severity score for children treated with an antiemetic or gastroprokinetic agent compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
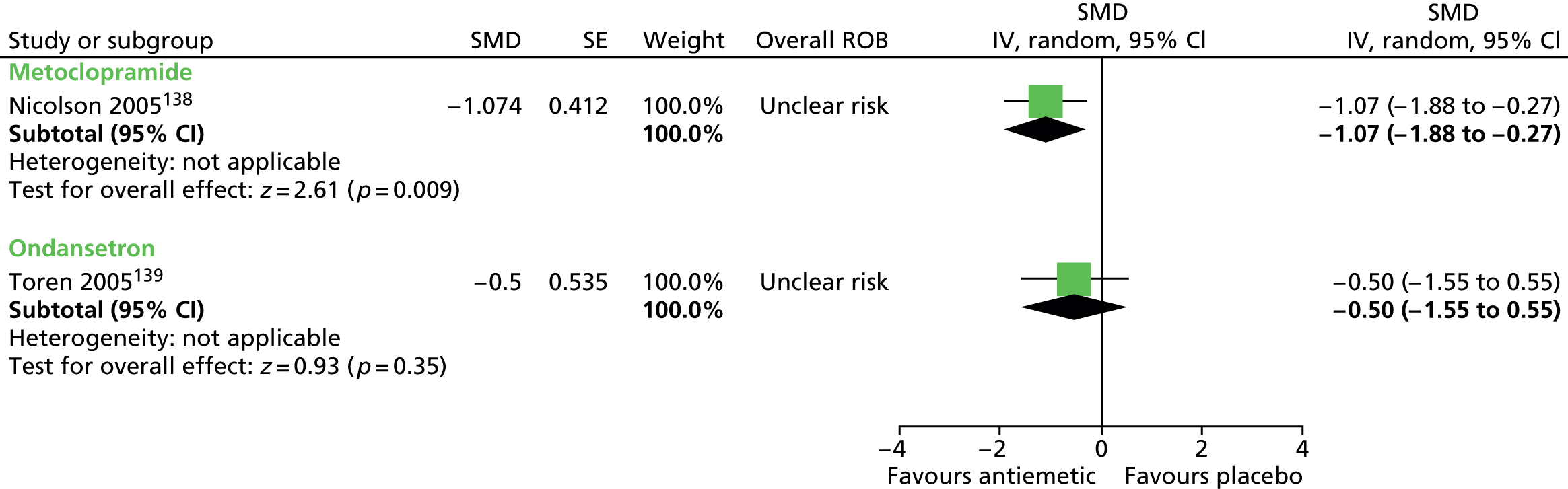
No relevant studies reported long-term outcomes.
In the study of metoclopramide,138 the occurrence of adverse effects is shown in Figure 127 (other adverse effects were measured but no events were reported). The results were inconclusive for rates of sedation (RR 2.79, 95% CI 0.33 to 23.52; n = 27), dysphoria (RR 0.93, 95% CI 0.06 to 13.37; n = 27) and increased appetite (RR 2.79, 95% CI 0.33 to 23.52; n = 27) for metoclopramide compared with placebo.
FIGURE 127.
Proportion of patients experiencing adverse events for metoclopramide compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.

Weight gain (kg) was inconclusive for metoclopramide compared with placebo (MD 0.50, 95% CI –0.75 to 1.75; n = 27) (Figure 128) and it is reported that there was no difference in changes in any cardiac conduction parameters (PR, QRS and QTc) or liver function measures for children treated with metoclopramide compared with placebo.
FIGURE 128.
Weight gain (kg) for children for metoclopramide compared with placebo. IV, independent variable; ROB, risk of bias.

In the study of ondansetron,139 in the whole mixed study sample, one patient from the ondansetron and one from the placebo group experienced mild and transient abdominal pain. It is reported that adverse effects also included gastrointestinal complaints leading to drop-out of one patient in the ondansetron group.
For the study of metoclopramide, no investigation into moderating factors was conducted. In the study of ondansetron,139 in the whole mixed study population (not the child subset used for efficacy results in this review), investigators found that there was no significant difference in response to ondansetron for participants aged ≤ 18 years compared with those aged > 18 years, for those with OCD compared with those without OCD and for those with ADHD compared with those without ADHD.
Clinical evidence summary for 5HT3-receptor antagonists/antiemetic and gastroprokinetic agents
Metoclopramide
There was low-quality evidence (see Appendix 4, Table 29) from one study with 27 children138 suggesting that metoclopramide produced a large effect in terms of tics and overall clinical outcome following 8 weeks of treatment, by estimates of adverse effects were very imprecise.
Ondansetron
One study in 15 children139 provided very low-quality evidence (see Appendix 4, Table 30) that was inconclusive as to whether or not ondansetron improves tics and overall clinical outcome following 3 weeks of treatment. There was no apparent increase in adverse effects, although the study was too small to detect relative differences.
Conclusion
Metoclopramide may be effective in the short-term treatment of tics in children and young people with TS. However, this conclusion must be treated with caution as the evidence is drawn from a single small study. 138 There is no clear evidence that ondansetron is an effective treatment for tics.
Gamma-aminobutyric acidB receptor agonists/skeletal muscle relaxants
Pharmacology and prescribing
The GABAB receptor agonists act as muscle relaxants on the central nervous system and are usually prescribed for chronic severe spasticity.
Baclofen (Lioresal®, Novartis) binds to GABAB receptors and inhibits the release of the excitatory neurotransmitters glutamate and aspartate. It is licensed in the UK for the relief of spasticity of voluntary muscle resulting from disorders such as multiple sclerosis.
Included studies
One study in children investigated treatment with baclofen compared with placebo. 140
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. In one crossover study,140 10 children with TS were randomised to receive a maximum dose of 60 mg/day baclofen and placebo in a randomised order for 4 weeks each.
Risk of bias
Methods for sequence generation and allocation concealment were unclear but participants, their parents, providers and outcome assessors were blind to order of allocation. There was a short washout period (2–3 weeks including drug tapering) and there was considered to be high risk of cross-treatment contamination. Analysis was of only participants completing the study but attrition was reasonably low (10%). Overall, this study was considered to be at unclear risk of bias owing to possible cross-treatment contamination.
Clinical evidence for gamma-aminobutyric acidB receptor agonists/skeletal muscle relaxants
Baclofen
The effect on tics was assessed using the YGTSS total tic scale. The results were inconclusive for baclofen compared with placebo (SMD –0.54, 95% CI –1.50 to 0.42; n = 9) (Figure 129).
FIGURE 129.
Standardised YGTSS total tic score for baclofen compared with placebo. IV, independent variable; SE, standard error.
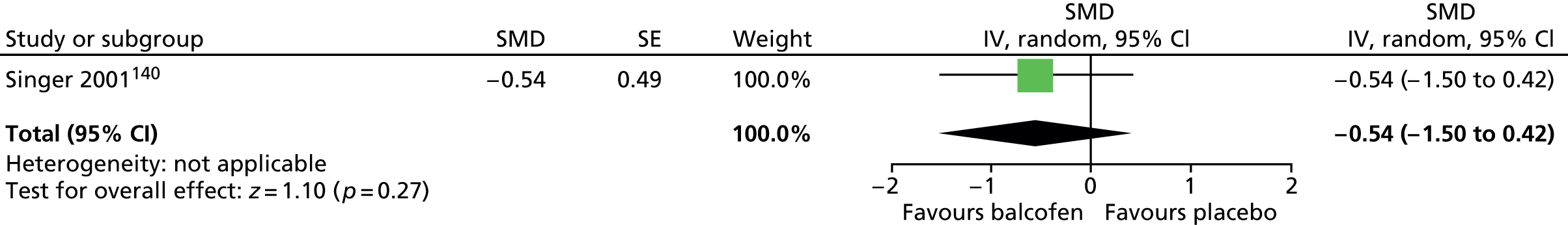
Impairment was assessed with the YGTSS impairment scale. The results favoured baclofen compared with placebo for impairment score (SMD –1.38, 95% CI –2.46 to –0.30; n = 9) (Figure 130) and overall global score (SMD –0.95, 95% CI –1.93 to 0.02; n = 9) (Figure 131).
FIGURE 130.
Standardised YGTSS total impairment score for baclofen compared with placebo. IV, independent variable; SE, standard error.

FIGURE 131.
Standardised YGTSS global score for baclofen compared with placebo. IV, independent variable; SE, standard error.
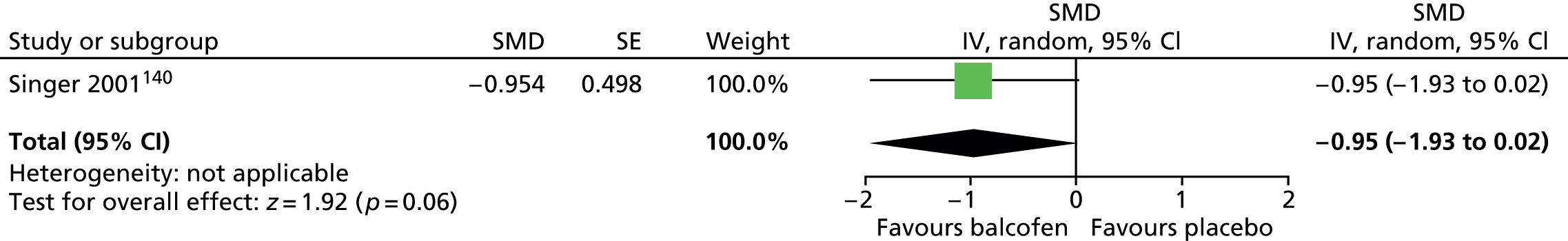
No relevant studies reported this outcome.
Overall clinical outcome was assessed with the CGI-Severity score and favoured baclofen compared with placebo (SMD –1.05, 95% CI –2.03 to –0.07; n = 9) (Figure 132).
FIGURE 132.
Standardised CGI-Severity score for baclofen compared with placebo. IV, independent variable; SE, standard error.
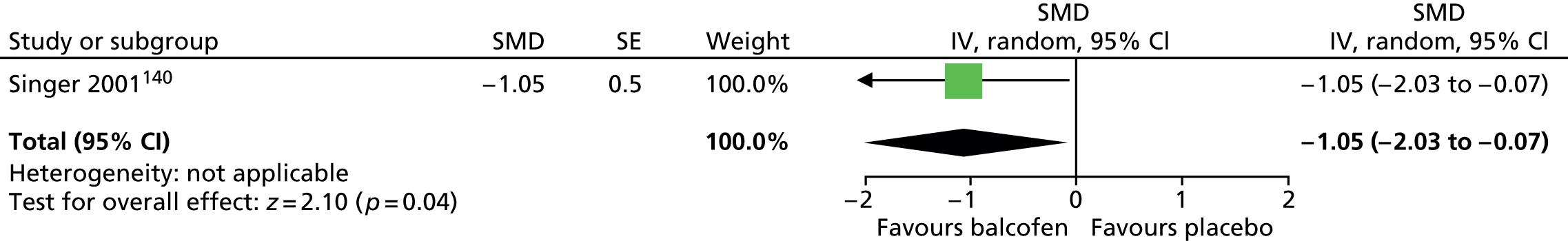
No relevant studies reported long-term outcomes.
Reported rates of abdominal pain, nausea, anxiety and headache were the same for participants when taking baclofen and placebo (one patient for each) and rates of constipation (one vs. two), palpitations (zero vs. one) and social phobia (zero vs. one) were similar.
Clinical evidence summary for gamma-aminobutyric acidB receptor agonists/skeletal muscle relaxants
Baclofen
There was very low-quality evidence (see Appendix 4, Table 41) from one study140 with nine children that baclofen improved impairment, tics and overall clinical outcome following 4 weeks of treatment. There was no reported increase in rates of adverse effects, although the study was too small to detect relative differences.
Conclusion
There is no clear evidence that baclofen is an effective treatment for tics in children and young people with TS.
Tricyclic antidepressants
Pharmacology and prescribing
Tricyclic antidepressants are one of the oldest classes of antidepressants and are also used for the management of panic and other anxiety disorders. They inhibit the reuptake of noradrenaline and serotonin. TCAs have largely been replaced by SSRIs because of the greater cardiac risk in overdose with TCAs.
Desipramine is a noradrenergic TCA (with pharmacological similarities to atomoxetine) that has been trialled in children with TS and comorbid ADHD, with the aim to reduce both tics and ADHD symptoms. 141
The license for desipramine was withdrawn in the UK in the late 1980s following reports of sudden unexplained deaths in children. Desipramine is still available in some countries including the USA.
Included studies
Two studies in children investigated treatment with desipramine compared with placebo. 115,141
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. In both studies, children with concurrent tic disorder and ADHD were treated. One study was a crossover, in which 37 children received 100 mg/day desipramine, 0.2 mg/day clonidine and placebo in a randomised order for 6 weeks each. 115 In the other study, children were randomised in parallel to 3.5 mg/kg/day desipramine or placebo for 6 weeks. 141
Risk of bias
In the crossover trial,115 the methods for randomisation and allocation concealment were not reported but there appears to have been blinding of participants, providers and outcome assessors. There was only a 1-week washout period between treatments and there may have been a high risk of cross-intervention contamination. There appears to have been a low rate of dropout and the risk of attrition bias may be low. Only data for outcomes with statistically significant findings were reported and, for these outcomes, there is considered to be a high risk of selective outcome reporting bias. Overall, the study was considered to be at high risk of bias owing to potential cross-treatment contamination and selective outcome reporting bias.
In the parallel trial,141 randomisation was conducted stratified for pre/post adolescence and sex but the method used was not stated. Allocation concealment was ensured through the use of sealed envelopes and participants, providers and outcome assessors appear to have been blinded to intervention allocation. The rate of dropout was relatively low (8%). Overall, this study was considered to be at low risk of bias.
Clinical evidence for tricyclic antidepressants
Desipramine
The parallel trial141 measured tics with the YGTSS total tic scale and the crossover trial115 used the YGTSS, Hopkins scale, Shapiro TSSS and a linear analogue scale completed by parents. In the parallel trial, the proportion of participants with ≥ 30% reductions in the YGTSS was greater for children taking desipramine compared with placebo for the total (RR 0.47, 95% CI 0.27 to 0.81; n = 39), motor (RR 0.59, 95% CI 0.35 to 1.00; n = 39) and vocal tic scores (RR 0.47, 95% CI 0.27 to 0.81; n = 39) (RRs of not having a ≥ 30% reduction) (Figure 133).
FIGURE 133.
Proportion of children with ≥ 30% reductions in YGTSS total, motor and vocal tic scores for desipramine compared with placebo (RRs are the relative risk of not having a ≥ 30% reduction). M–H, Mantel–Haenszel; ROB, risk of bias.

In the crossover trial,115 there was no significant differences in post-treatment measures for the YGTSS (conducted in 23 of 34 participants), or in motor or vocal measures on the Hopkins scale or the Shapiro TSSS (data are not presented in the publication), but for a parent-completed linear analogue scale measuring changes in tic severity and frequency, there was a greater change score for desipramine than for placebo.
In the parallel trial,141 the YGTSS global scale was used (measure of tic severity and impairment). There was a greater reduction from baseline in global score for desipramine than for placebo (SMD –1.15, 95% CI –1.83 to –0.46; n = 39) (Figure 134).
FIGURE 134.
Standardised change in YGTSS global scale score for desipramine compared with placebo. IV, independent variable; SE, standard error.
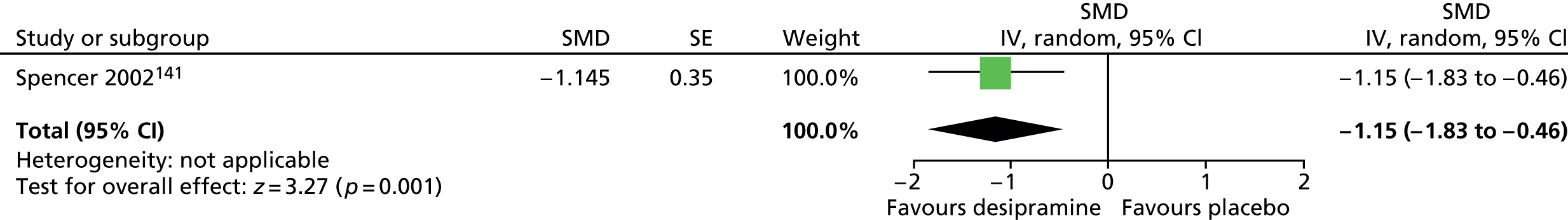
For this study, the proportion of participants with ≥ 30% reductions in global score was greater for children taking desipramine than for placebo (RR 0.39, 95% CI 0.21 to 0.70; n = 39) (Figure 135) (the RR presented here is the RR of not having a ≥ 30% reduction).
FIGURE 135.
Proportion of children with ≥ 30% reductions in YGTSS global score for desipramine compared with placebo (RRs are the relative risk of not having a ≥ 30% reduction). M–H, Mantel–Haenszel; ROB, risk of bias.
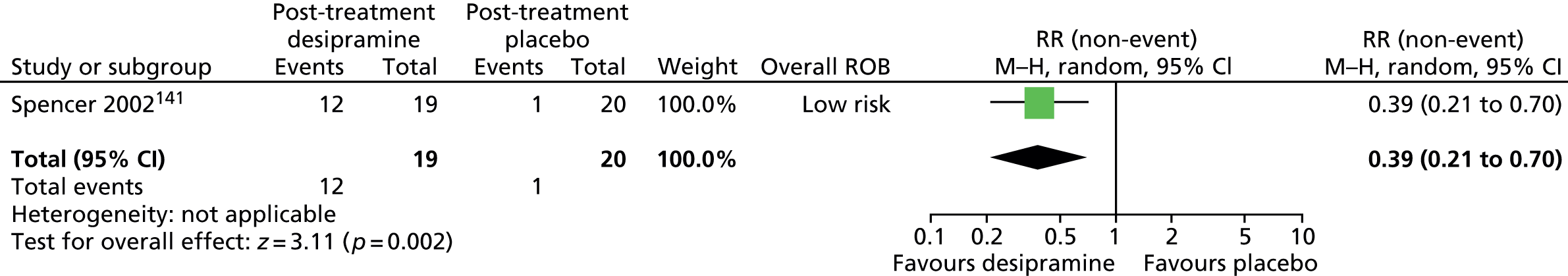
No relevant studies reported this outcome.
The parallel trial141 reports the proportion of children that were much or very much improved on the CGI scale. A higher proportion of participants were CGI much or very much improved for desipramine than placebo (RR 0.44, 95% CI 0.26 to 0.76; n = 39) (the RR presented here is the RR of not being much or very much improved) (Figure 136).
FIGURE 136.
Proportion of children much or very much improved in the CGI-I scale for desipramine compared with placebo (the RR presented here is the RR of not being much or very much improved). M–H, Mantel–Haenszel; ROB, risk of bias.
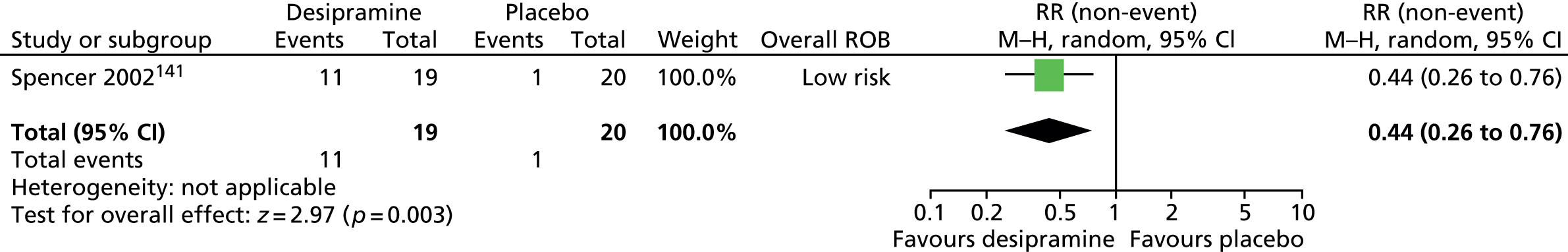
No relevant studies reported long-term outcomes.
In the crossover trial,115 a higher proportion of participants reported one or more drug-related adverse effect while taking desipramine than when taking the placebo (RR 1.73, 95% CI 1.14 to 2.64; n = 34) (Figure 137).
FIGURE 137.
Proportion of children with adverse events for desipramine compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.
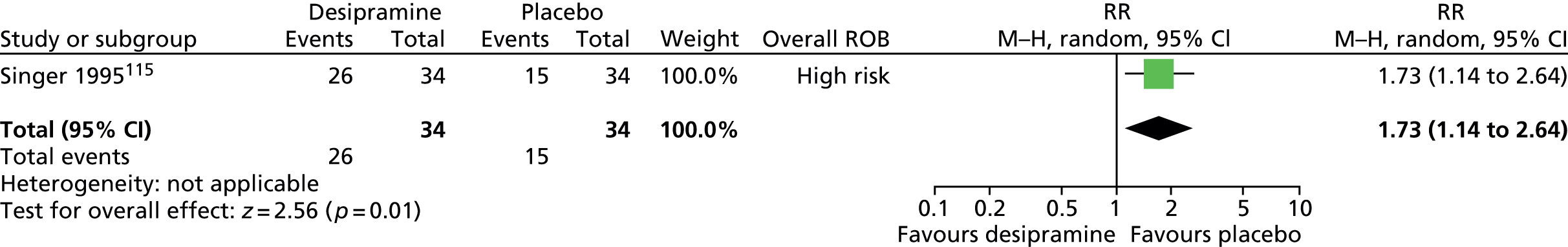
In the parallel trial,141 adverse effects occurring in greater than two children are shown in Figure 138. Findings were inconclusive for decreased appetite (RR 11.55, 95% CI 0.68 to 195.63; n = 39) and difficulty sleeping (RR 4.21, 95% CI 0.52 to 34.36; n = 39) for desipramine compared with placebo.
FIGURE 138.
Proportion of children with adverse events occurring in more than two participants for desipramine compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.

Clinical evidence summary for tricyclic antidepressants
Desipramine
There was low-quality evidence (see Appendix 4, Table 28) from one study with 39 children141 that desipramine produced a large effect in terms of tics (proportion of children with ≥ 30% reduction in tics), global tic/impairment and overall clinical outcome (CGI much or very much improved) following 6 weeks of treatment. In two studies,115,141 adverse effects were reported and there was evidence of overall increased rates of adverse effects and a tendency to higher rates of decreased appetite and difficulty sleeping.
Conclusion
Desipramine may be effective in the short-term treatment of tics in children and young people with comorbid TS and ADHD; however, this conclusion must be treated with caution as the evidence is drawn from a single small study. 141 The clinical indication (i.e. treatment of comorbid TS plus ADHD), mechanism of action and clinical effectiveness of desipramine appears broadly similar to atomoxetine. However, the less favourable adverse effect profile of desipramine (in particular, reports of sudden unexplained deaths in children) resulted in its withdrawal from the UK.
Selective serotonin reuptake inhibitors
Pharmacology and prescribing
Selective serotonin reuptake inhibitors selectively inhibit the reuptake of serotonin (5-hydroxytryptamine), so that the effects of serotonin are prolonged. They are commonly used to treat depression, anxiety and OCD.
Fluoxetine (non-proprietary; Prozac®, Lilly) is licensed in the UK for the treatment of MDD, OCD and bulimia nervosa. It is the most common first-line medication prescribed for comorbid OCD/OCSs in children with TS. 142
Included studies
Two studies, one in children143 and one in a mixed population144 of adults and children, investigated treatment with fluoxetine compared with placebo.
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. One study was a parallel trial in 11 children with TS of maximum dose of 40 mg/day fluoxetine compared with placebo for 4 months. 143 The other was a crossover trial in a mixed population of 14 adults and children with TS of fixed dose of 20 mg/day fluoxetine compared with placebo for 8 weeks. 144 Half of the participants in this study were children and results were, therefore, combined with the child study. This study is a crossover trial but presents data at the end of the first phase of the study and the first-phase data have been used in the analysis for this review.
Risk of bias
In the study of fluoxetine compared with placebo in children,143 the method of randomisation and presence of allocation concealment were unclear. The study is described as double blind but there is no description of patient, provider or outcome assessor blinding. Analysis was of available case data but, as dropout was moderate and similar in both groups, the risk of attrition bias may have been low. Overall, the study was considered to have unclear risk of bias owing to unclear patient, provider and outcome assessor blinding.
In the mixed study of fluoxetine compared with placebo,144 the method of randomisation and presence of allocation concealment were unclear. There appears to have been blinding of participants, providers and outcome assessors. Analysis was of available case data but, as dropout was moderate and similar in both groups, the risk of attrition bias may have been low. Although this was a crossover trial, first-phase data were available and this avoided bias from cross-treatment contamination. Overall, the study was considered to have a low risk of bias.
Clinical evidence for selective serotonin reuptake inhibitors
Fluoxetine
Tics were assessed in one study using the YGTSS total tic scale144 and in the other using the Unified Tic Rating Scale (UTRS) and the Goetz scale. 143 Using the Goetz simple motor tics for the study with multiple tic measures,143 there was no conclusive difference in tic score for fluoxetine compared with placebo (SMD –0.41, 95% CI –1.23 to 0.42; n = 21) (Figure 139), with no important heterogeneity between studies (I2 = 6%).
FIGURE 139.
Standardised tic scores for fluoxetine compared with placebo in predominantly child studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

No relevant studies reported TS-related impairment.
No relevant studies reported this outcome.
No relevant studies reported this outcome.
No relevant studies reported long-term outcomes.
In the study of children,143 it is reported that adverse effects were mild and reported by two children taking fluoxetine (hypomanic behaviour, irritability, fatigue and agitation) and three children taking placebo (fatigue, irritability, hypomanic behaviour and diarrhoea). In the mixed study,144 outcomes with events for greater than two participants are shown in Figure 140.
FIGURE 140.
Adverse events experienced by more than two patients for fluoxetine compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.

There was no conclusive difference in motor restlessness (RR 3.50, 95% CI 0.91 to 13.53; n = 12), insomnia (RR 2.50, 95% CI 0.60 to 10.46; n = 12), decreased appetite (RR 7.00, 95% CI 0.40 to 122.44; n = 12) and diarrhoea (RR 3.00, 95% CI 0.36 to 24.92; n = 12) for fluoxetine compared with placebo. In this study, it is reported that none of the participants had clinically significant changes in vital signs or laboratory measures.
Clinical evidence summary for selective serotonin reuptake inhibitors
Fluoxetine
One study in 11 children143 provided low-quality evidence (see Appendix 4, Table 27) that was inconclusive as to whether or not fluoxetine gives a clinically significant improvement in tics after 4 months of treatment. Evidence from a mixed study showed fluoxetine to have a similar efficacy to placebo. The child study reported no difference in adverse effects, but the mixed study reported higher rates of motor restlessness, insomnia, decreased appetite and diarrhoea for fluoxetine than for placebo.
Conclusion
There is no clear evidence that fluoxetine is effective in reducing tics in children and young people with comorbid TS and OCD/OCSs. However, fluoxetine when used to treat comorbid OCD/OCSs does not appear to result in tic worsening or exacerbation.
Nicotinic acetylcholine receptor antagonists
Pharmacology and prescribing
Mecamylamine (Inversine®, Targacept Inc.) has mainly been used as an antihypertensive, but is not licensed in the UK. At lower doses, it can be used to act as a selective nicotinic receptor antagonist and has been suggested as a potentially useful treatment for TS adjunctive to an antipsychotic drug. 145,146
Included studies
One study compared treatment with mecamylamine with placebo. 146
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. Sixty-one children with TS were randomised to receive maximum dose of 7.5 mg/day mecamylamine or placebo for 8 weeks. In order to be included in the study, behavioural and emotional TS symptoms had to be rated by the parent as more disturbing than the tics themselves.
Risk of bias
Methods for sequence generation and allocation concealment are unclear and no details are given of patient, provider or outcome assessor blinding. There was a high rate of dropout from the study (41% of those on mecamylamine and 34% of those on placebo) and, although analysis was by last observation carried forward for those with at least 3 weeks of data (86% of those on mecamylamine and 78% of those on placebo), the risk of attrition bias was unclear. Overall, this study was considered to be at unclear risk of bias owing to unclear participant, provider and outcome assessor blinding and possible risk of attrition bias.
Clinical evidence for nicotinic acetylcholine receptor antagonists
Mecamylamine
The YGTSS scale was used to assess tic changes but, for most results, the precision of study findings was not reported. It is reported there was no significant difference in baseline or end point measures for mecamylamine compared with placebo (Bonferroni adjustment; p < 0.0025 needed for statistical significance) for the YGTSS total tic (MD of group changes 7.6, favours placebo) or vocal tic scores (MD of group changes 2.6, favours placebo). YGTSS motor tic score was significantly different at baseline (baseline MD 2.5; p = 0.02) and end point (post-treatment MD 4.2; p < 0.002) but it is reported that the difference in group changes was not significant (MD of group changes 1.7, favours placebo).
It is reported that there was no difference in baseline or end point YGTSS impairment score for mecamylamine compared with placebo (MD of group changes 2.3, favours placebo).
For the anxiety rating in the Conners’ Parent Rating Scale, it is reported that there was no significant difference for mecamylamine compared with placebo for baseline or post-treatment scores (MD of group changes –0.9, favours mecamylamine).
The CGI-I was used to assess overall clinical outcome. It is reported that there was no significant difference in clinician-rated CGI-I score for mecamylamine compared with placebo (MD –2.2, favours mecamylamine).
No relevant studies reported long-term outcomes.
It is reported that adverse effects occurred more frequently in children taking mecamylamine than those taking placebo. Adverse effects occurring in twice as many children for mecamylamine than placebo were reported and there were no conclusive differences (Figure 141).
FIGURE 141.
Proportion of children experiencing adverse events for events where occurred greater than twice as often in the mecamylamine compared with the placebo group. M–H, Mantel–Haenszel; ROB, risk of bias.
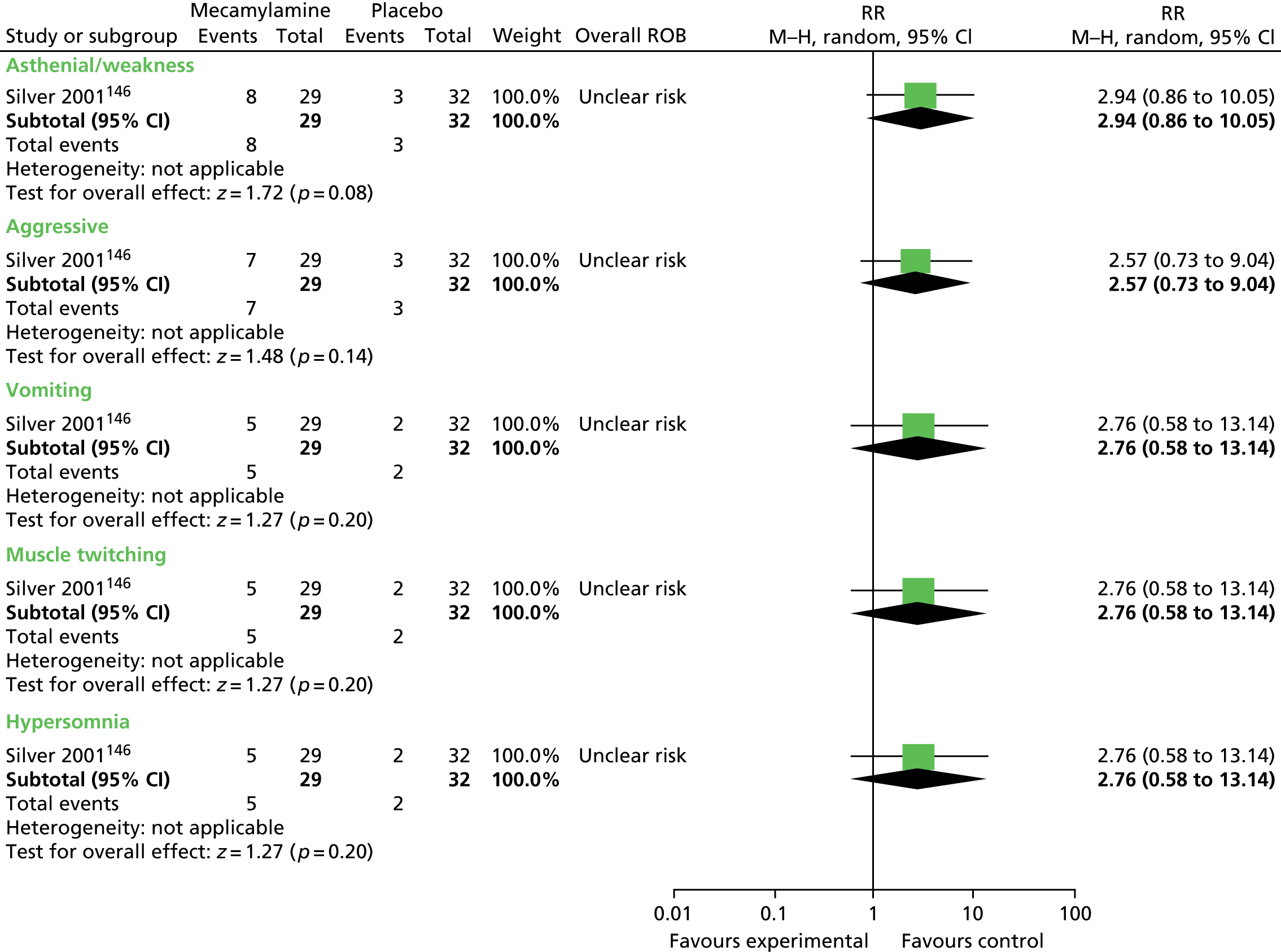

There were no between-group differences or changes in sitting or standing systolic or diastolic BP. When a cut-off of 90/60 mmHg was used to define hypertension, there was no conclusive difference in incidence for mecamylamine compared with placebo (RR 9.00, 95% CI 0.51 to 158.85; n = 50) (Figure 142).
FIGURE 142.
Proportion of children experiencing 90/60 BP for mecamylamine compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.
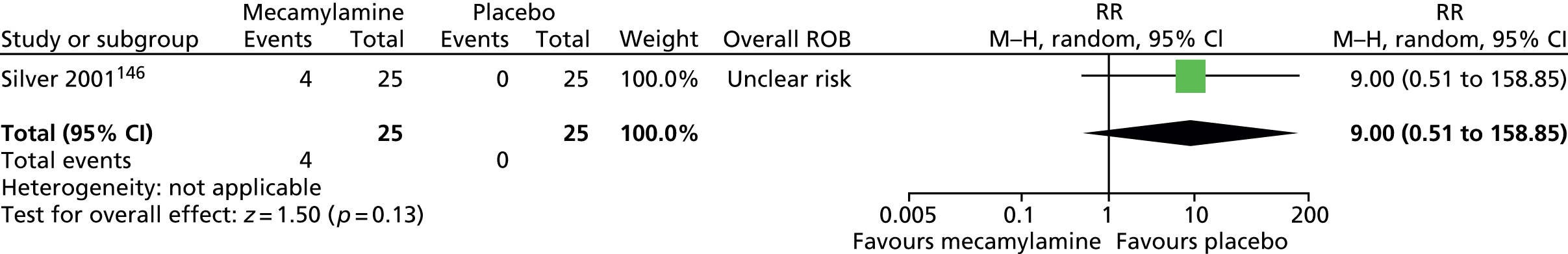
Results suggested heart rate measured at the end of the first week was higher for mecamylamine than placebo (MD 8.50, 95% CI 2.22 to 14.78; n = 61) (Figure 143), but not at other time points. It was reported that there were no clinically significant group differences or abnormalities in complete blood cell count, blood chemistry or ECGs.
FIGURE 143.
Heart rate after one week of treatment for mecamylamine compared with placebo. IV, independent variable; ROB, risk of bias.
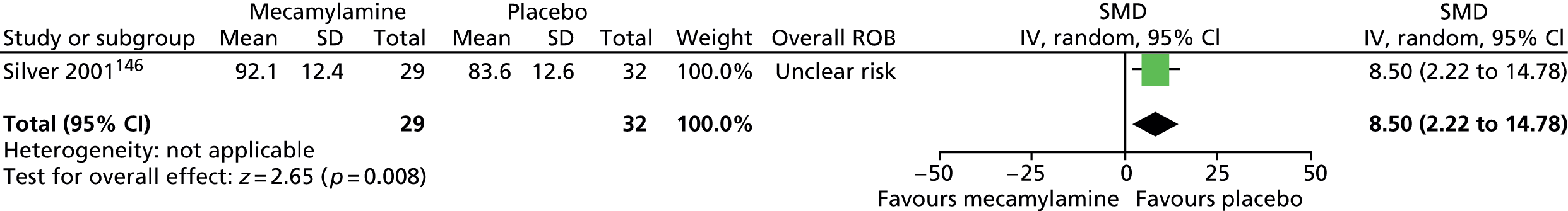
Investigators conducted a post hoc subgroup analysis of participants whose TS was defined as moderate or severe [Tourette’s Disorder Scale – Clinician Rated (TODS-CR) score of ≥ 60, n = 32]. More children with moderate or severe TS taking mecamylamine compared with placebo had a ≥ 4-point reduction on the TODS-CR ‘sudden mood changes’ score (8/17 vs. 1/15) and the TODS-CR ‘depressed or uninterested in most things’ score (5/17 vs. 2/15). However, the opposite pattern was observed for children with baseline TODS-CR scores of < 60.
Clinical evidence summary for nicotinic acetylcholine receptor antagonists
Mecamylamine
One study (n = 61)146 provided low-quality evidence (see Appendix 4, Table 40) that mecamylamine does not improve tics, impairment or overall clinical outcome but may increase the risk of adverse effects.
Conclusion
There is no clear evidence that mecamylamine is an effective treatment for tics in children and young people with TS.
Transdermal nicotine
Pharmacology and prescribing
Nicotine transdermal patches (Nicotinell®, Novartis) are licensed in the UK to relieve and/or prevent craving and nicotine withdrawal symptoms associated with tobacco dependence.
Included studies
Two studies in children investigated the efficacy of transdermal nicotine for the treatment of tics. 147,148
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. One study was a parallel trial where children treated with haloperidol (optimum dose titrated over 2 weeks before entering the trial) were randomised to nicotine patches (7 mg/day) or placebo for 19 days. 147 The dosage of haloperidol was reduced to 50% after 5 days and outcomes assessed 3 hours after patch application and at 5, 19 and 33 days. The other study was a crossover trial in which children being treated with an antipsychotic drug (96% of children) were given a single dose of transdermal nicotine (7 mg) or placebo in a randomised order and measurements made 7 days after treatment. 148
Risk of bias
For the parallel trial,147 randomisation was conducted using a random number generator but the presence of allocation concealment was unclear. There appears to have been blinding of providers and outcome assessors. Blinding of participants was attempted but it is unclear as to whether or not differences in the smell and adverse effects associated with nicotine resulted in loss of patient blinding. Analysis was of available case data. Dropout was moderate (26%) and the risk of attrition bias was considered to be unclear. Overall, this study was considered to be at unclear risk of bias owing to unclear risks of attrition bias and unclear patient blinding.
For the crossover trial,148 the method for randomisation and presence of allocation concealment was unclear. There appears to have been blinding of participants and outcome assessors but blinding of providers was unclear. An 8-week washout period was used and this may have been long enough to avoid cross-treatment contamination. Analysis was by available case. Dropout was high (39%) and the risk of attrition bias was considered to be high. The presence of selective outcome reporting was unclear as some outcomes (e.g. YGTSS) appear to be listed in the methods section as outcome measures, but are not reported in the results. Overall, this study was considered to be at unclear risk of bias owing to risk of attrition bias and possible selective outcome reporting.
Clinical evidence for transdermal nicotine
Nicotine transdermal patches
In the parallel trial,147 tics were assessed with the YGTSS and, in the crossover trial,148 the Tourette Syndrome Symptom List – Child’s report (TSSL-C) was used. Studies showed similar motor (SMD –0.03, 95% CI –0.49 to 0.43; n = 70) (Figure 144) and vocal tic scores (SMD 0.34, 95% CI –0.37 to 1.05; n = 70) (Figure 145) for transdermal nicotine plus antipsychotic drug compared with placebo plus antipsychotic drug (using the simple motor and vocal scores from the study using the TSSL-C). There was moderate heterogeneity between studies for motor tic score (I2 = 40%) and large heterogeneity between studies for vocal tic score (I2 = 73%).
FIGURE 144.
Standardised motor tic score for transdermal nicotine compared with placebo. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
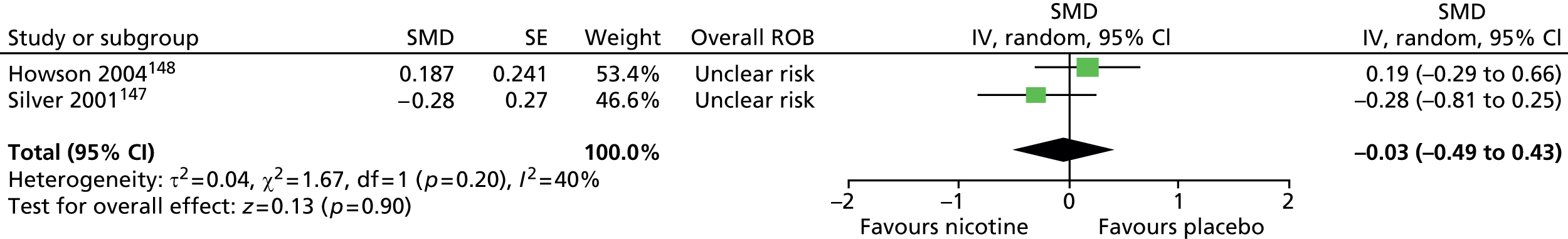
FIGURE 145.
Standardised vocal tic score for transdermal nicotine compared with placebo. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
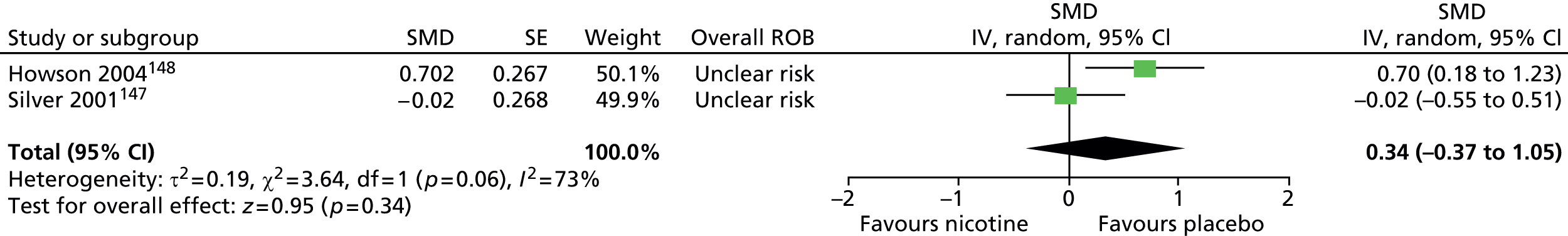
In the parallel trial147 impairment was assessed with the YGTSS impairment scale and, in the crossover study,148 impairment was assessed with the Conners’ Global Index social problems score. There was no conclusive difference in impairment/social problems score for nicotine plus antipsychotic drug compared with placebo plus antipsychotic drug (SMD –0.30, 95% CI –0.75 to 0.15; n = 70) (Figure 146). There was some heterogeneity between studies (I2 = 38%).
FIGURE 146.
Standardised impairment/social problems score for transdermal nicotine compared with placebo. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.
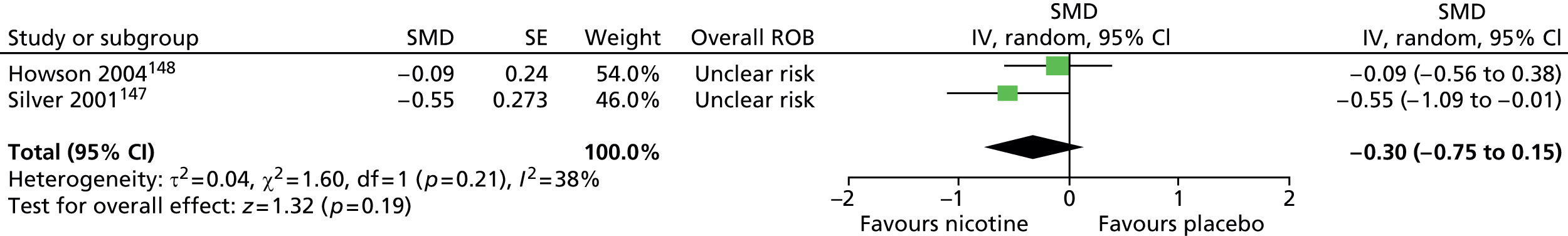
The parallel trial reported global tic outcome with the YGTSS global score and there was no conclusive difference in change from baseline score for transdermal nicotine plus antipsychotic drug compared with placebo plus antipsychotic drug (SMD –0.43, 95% CI –0.96 to 0.10; n = 56) (Figure 147).
FIGURE 147.
Standardised change from baseline in YGTSS global score for transdermal nicotine compared with placebo. IV, independent variable; ROB, risk of bias.

The crossover study used the Conners’ Parent Rating Scale. 148 There were similar post-treatment scores for anxiousness/shyness (SMD –0.08, 95% CI –0.55 to 0.39; n = 14) and emotional liability for transdermal nicotine compared with placebo (SMD –0.29, 95% CI –0.77 to 0.19; n = 14) (Figure 148).
FIGURE 148.
Standardised Conners’ Parent Rating Scale Anxiousness/shyness and Emotional liability scores for transdermal nicotine compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
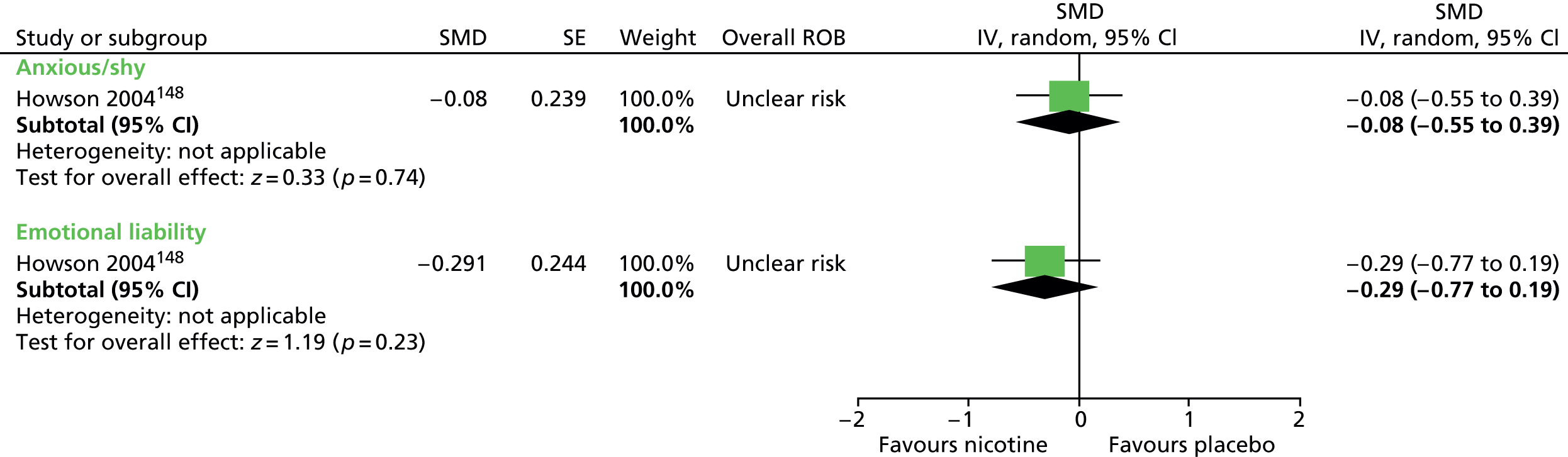
The parallel study147 reported changes in the global improvement scale as assessed by clinicians and parents. Global improvement ratings were better for transdermal nicotine than for placebo when assessed by parents (SMD –0.79, 95% CI –1.34 to –0.25; n = 56) and clinicians (SMD –0.53, 95% CI –1.06 to 0.01; n = 56) (Figure 149).
FIGURE 149.
Standardised clinician and parent-rated global improvement scale ratings for nicotine compared with placebo. IV, independent variable; ROB, risk of bias.
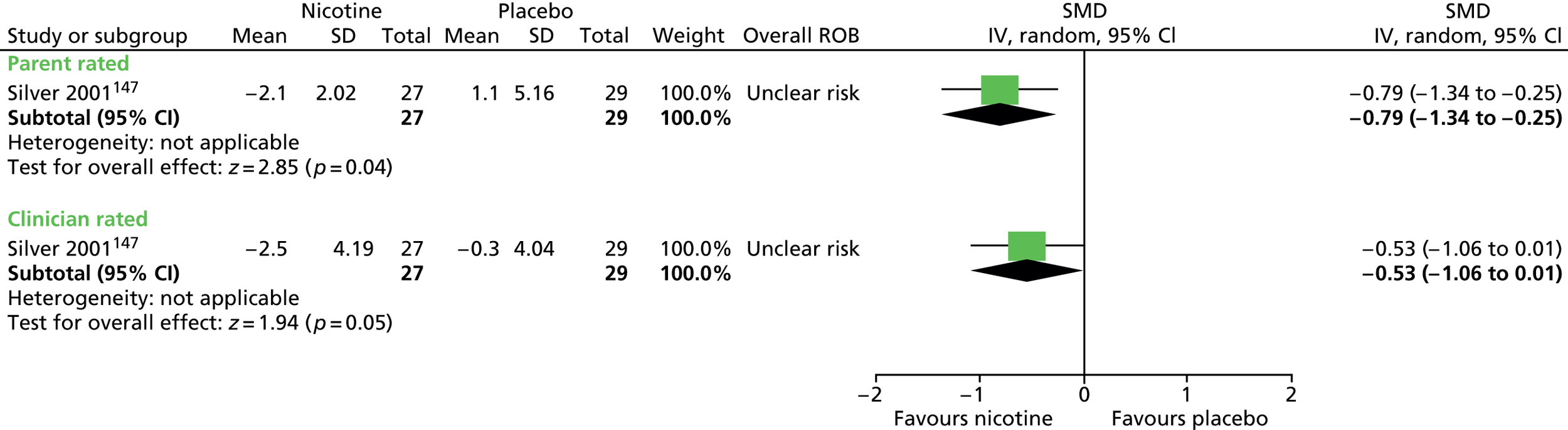
No relevant studies reported long-term outcomes.
Adverse effects were reported by the parallel trial. 147 For events occurring in more than 30% of children, there were more instances of nausea (RR 4.17, 95% CI 1.95 to 8.89; n = 70), vomiting (RR 4.67, 95% CI 1.47 to 14.82; n = 70), and a similar incidence for transdermal nicotine compared with placebo of itching at the site of the patch (RR 1.33, 95% CI 0.83 to 2.15; n = 70) and headache (RR 1.21, 95% CI 0.71 to 2.06; n = 70) (Figure 150).
FIGURE 150.
Adverse events occurring in ≥ 30% of participants for nicotine compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.
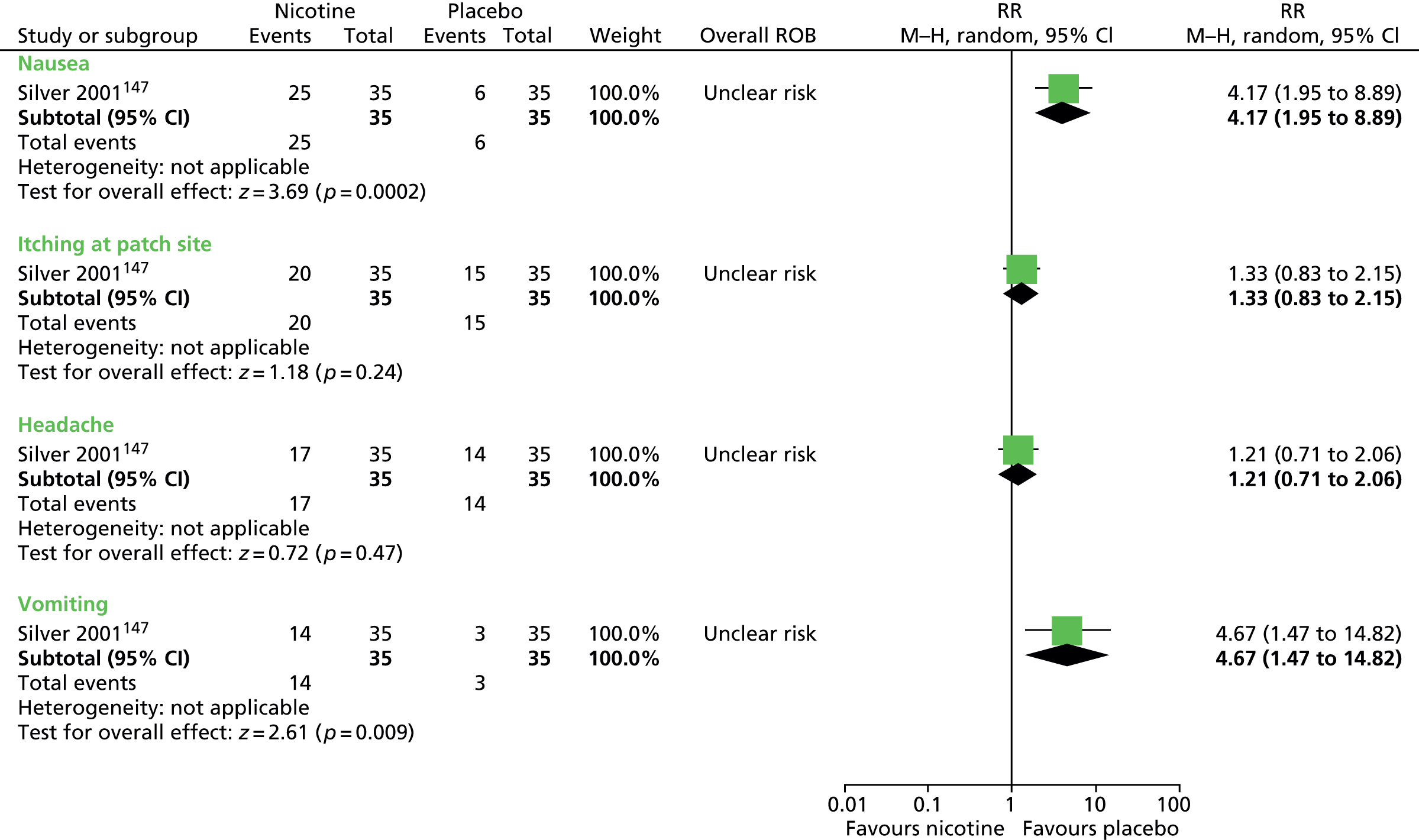
Clinical evidence summary for transdermal nicotine
Two studies in children (n = 70)147,148 provided low-quality evidence (see Appendix 4, Table 42) that transdermal nicotine did not improve motor or vocal tics by the end of 1–3 weeks of treatment. The evidence was inconclusive as to its effects on impairment, although overall clinical outcome was improved. There were increased rates of nausea and vomiting.
Conclusion
There is no clear evidence that transdermal nicotine is an effective treatment for tics in children and young people with TS.
Omega-3 fatty acids
Pharmacology and prescribing
Long-chain polyunsaturated fatty acids are used for many purposes including development of nerve cells and membranes. Omega-3 and omega-6 polyunsaturated fatty acids differ in their chemical structure and potentially their physiological effects. Omega-3 fatty acids (fish oils) have been advocated for ameliorating the symptoms and preventing a range of behavioural and psychiatric conditions.
Included studies
One study in children compared omega-3 fatty acids with placebo. 149
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. Thirty-three children with TS were randomised to receive maximum dose of 6000 mg/day omega-3 fatty acids or olive oil (placebo) capsules for 20 weeks. 149
Risk of bias
In this study, the methods for randomisation and allocation concealment are unclear. Fish oil and placebo were administered in capsules scented with vanilla and, despite this attempt to blind study participants, the continuation of patient blinding is unclear. Dosage was titrated on the basis of efficacy and it is unclear whether or not blinding was maintained throughout this process. It was unclear whether or not there was blinded outcome assessment. Analysis was by intention to treat and the risk of attrition bias appears to be low. Measures of depression and anxiety were measured at baseline and follow-up, but the results are not reported and the risk of outcome reporting bias is unclear. Overall, this study was considered to be at unclear risk of bias owing to unclear patient blinding.
Clinical evidence for omega-3 fatty acids
Tics
Changes in tics were assessed with the YGTSS total tic scale. Reduction from baseline total tic scores was inconclusive for omega-3 fatty acids compared with placebo (SMD –0.24, 95% CI –0.92 to 0.45; n = 33) (Figure 151). There was no conclusive difference in the proportion of children with ≥ 30% reductions in total tic score for omega-3 fatty acids compared with placebo (RR 0.75, 95% CI 0.40 to 1.42; n = 33) (Figure 152) (the RR presented here is the RR of not having a ≥ 30% reduction in tic score).
FIGURE 151.
Standardised change from baseline YGTSS total tic score for children treated with omega-3 fatty acids compared with placebo. FA, fatty acid; IV, independent variable; ROB, risk of bias.

FIGURE 152.
Proportion of children treated with ≥ 30% reductions in YGTSS total tic score for omega-3 fatty acids compared with placebo (RR is relative risk of not having a ≥ 30% reduction). FA, fatty acid; M–H, Mantel–Haenszel; ROB, risk of bias.
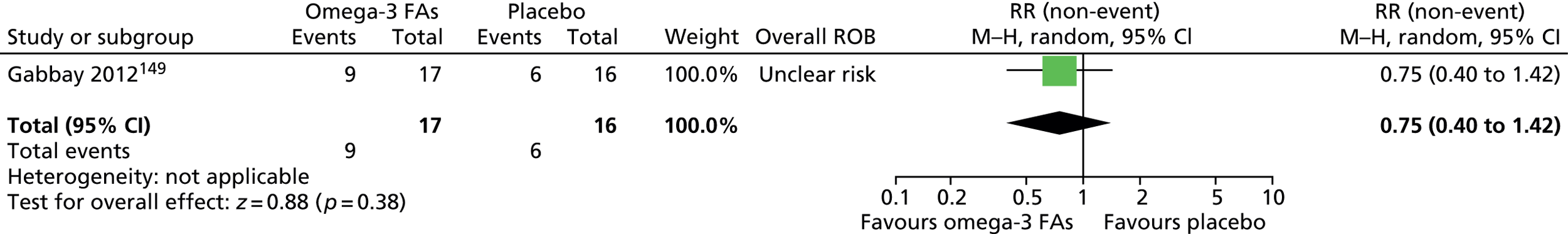
Impairment was measured using the YGTSS impairment scale. Reduction from baseline impairment score was greater for omega-3 fatty acids than placebo (SMD –0.76, 95% CI –1.47 to –0.05; n = 33) (Figure 153). For the overall YGTSS global score, there was no conclusive difference in reduction from baseline global score for omega-3 fatty acids compared with placebo (SMD –0.67, 95% CI –1.38 to 0.03; n = 33) (Figure 154).
FIGURE 153.
Standardised change from baseline YGTSS impairment score for children treated with omega-3 fatty acids compared with placebo. FA, fatty acid; IV, independent variable; ROB, risk of bias.

FIGURE 154.
Standardised change from baseline YGTSS global score for children treated with omega-3 fatty acids compared with placebo. FA, fatty acid; IV, independent variable; ROB, risk of bias.

There was no conclusive difference in the proportion of children with ≥ 30% reductions for omega-3 fatty acids compared with placebo for YGTSS impairment score (RR 0.55, 95% CI 0.29 to 1.04; n = 33) (Figure 155) and YGTSS global score (RR 0.68, 95% CI 0.37 to 1.25; n = 33) (Figure 156) (RRs presented are the relative risk of not having a ≥ 30% reduction).
FIGURE 155.
Proportion of children with ≥ 30% reductions in YGTSS impairment score for omeg-3 fatty acids compared with placebo (RR is relative risk of not having a ≥ 30% reduction). FA, fatty acid; M–H, Mantel–Haenszel; ROB, risk of bias.

FIGURE 156.
Proportion of children with ≥ 30% reductions in YGTSS global score for omega-3 fatty acids compared with placebo (RR is relative risk of not having a ≥ 30% reduction). FA, fatty acid; M–H, Mantel–Haenszel.

Depression and anxiety scores were assessed at baseline and end point using the Children’s Depression Rating Scale-Revised and the MASC. It was reported that there was no difference in change from baseline scores for omega-3 fatty acids compared with placebo.
At each visit, the CGI-I was used to assess overall clinical outcome but no results were presented in the included study.
No relevant studies reported long-term outcomes.
It is reported that there were no significant differences in adverse effects for omega-3 fatty acids compared with placebo. The most frequently reported treatment-related events (reported to be tolerable and self-limited) in the omega-3 fatty acids group were headache (n = 4), nausea/stomach ache (n = 4) and diarrhoea/loose stool (n = 2). One subject in the omega-3 fatty acids group experienced several nosebleeds and bruised easily and one subject in the placebo group had elevated clotting times but all follow-up laboratory test results were within normal limits.
Clinical evidence summary for omega-3 fatty acids
One study in 33 children,149 provided low-quality evidence (see Appendix 4, Table 43) that was inconclusive as to whether or not omega-3 fatty acids improved tics, but a medium-sized improvement in impairment was observed and there was no increase in adverse effects compared with the control (olive oil) following 20 weeks of treatment.
Conclusion
There is no clear evidence that omega-3 fatty acids are an effective treatment for tics in children and young people with TS.
Head-to-head comparisons between different classes of drugs
Included studies
Five studies made comparisons between drugs in different classes:
-
risperidone (antipsychotic) compared with clonidine (noradrenergic agent)150
-
sulpiride (antipsychotic) compared with fluvoxamine (antidepressant)151
-
clonidine (noradrenergic agent) compared with levetiracetam (anticonvulsant)152
-
clonidine (noradrenergic agent) compared with desipramine (TCA)115
-
haloperidol (antipsychotic) compared with clonidine patch (noradrenergic agent). 153
Study characteristics
Study characteristics are shown in Appendix 3, Table 9. A parallel trial in children compared risperidone with clonidine. 150 Twenty-one children were randomised to maximum dose of 0.06 mg/kg/day risperidone or maximum dose of 0.005 mg/kg/day clonidine for 8 weeks.
A mixed parallel trial of adults and children with TS and comorbid OCD compared the efficacy of maximum dose of 300 mg/day fluvoxamine with maximum dose of 1 g/day sulpiride for 6 weeks. 151
A mixed crossover study of adults and children compared clonidine with levetiracetam. 152 Ten participants were allocated to maximum dose of 0.4 mg/day clonidine and maximum dose of 2500 mg/day levetiracetam in a randomised order for 6 weeks each.
A crossover study in children compared treatment with clonidine, desipramine and placebo. 115 Thirty-four children were treated with maximum dose of 0.2 mg/day clonidine, maximum dose of 100 mg/day desipramine and placebo in a randomised order for 6 weeks each.
A parallel controlled before-and-after study in children compared clonidine patch with haloperidol. 153 One hundred and nineteen children were treated with clonidine patches giving doses of 1–2 mg/week (depending on body weight) or with maximum dose of 2 mg/day haloperidol for 4 weeks.
Risk of bias
In the parallel study of risperidone versus clonidine,150 the method of randomisation and presence of allocation concealment was unclear. Participants and providers appear to have been blind to intervention allocation but the presence of outcome assessor blinding is unclear. The rate of dropout was low and there may have been a low risk of attrition bias. Overall, this study was considered to have an unclear risk of bias owing to unclear outcome assessor blinding.
In the crossover study of fluvoxamine compared with sulpiride,151 the randomisation method is unclear and there is no evidence of allocation concealment. There appears to have been blinding of participants, providers and outcome assessors but the small between-drug washout period (2 weeks) may have introduced cross-intervention contamination. The rate of dropout was reasonably high (5/11 participants, 45%) and, although last observation carried forward was used for the analysis, the risk of attrition bias is unclear. Overall, this study was considered to be at unclear risk of bias owing to the small between-intervention washout period and the unclear risk of attrition bias.
In the crossover study of levetiracetam compared with clonidine,152 the sequence was generated using a computer program but the presence of allocation concealment is unclear. Participants, providers and outcome assessors appear to have been blind to treatment allocation sequence. There was a 2-week washout period between interventions and there was considered to be an unclear risk of cross-treatment contamination. The rate of dropout was low and the risk of attrition bias was considered to be low. Overall, owing to possible cross-treatment contamination, this study was considered to be at unclear risk of bias.
In the crossover study of clonidine compared with desipramine,115 the method for randomisation and presence of allocation concealment are unclear but there appears to be blinding of participants, providers and outcome assessors. There was a 1-week washout period between treatments and there was considered to be an unclear risk of cross-treatment contamination. It is reported that 3 out of 37 (8%) participants dropped out of the study and the risk of attrition bias appears to be low. Results are presented for significant findings only. For non-significant results, no data were presented and the study was considered to be at high risk of selective outcome reporting bias. Overall, this study was considered to be at high risk of bias owing to selective outcome reporting bias and possible cross-treatment contamination.
In the controlled before-and-after study of haloperidol compared with clonidine,153 the method used for the allocation of participants to intervention groups and the presence of allocation concealment was unclear and there was unclear blinding of participants, providers and outcome assessors. Rates of attrition were not reported. One outcome (CGI-Severity scale) was measured but the results are not reported. This study was considered to be at high risk of bias owing to the absence of randomisation, unclear patient, provider and outcome assessor blinding and unclear risk of attrition and selective reporting bias.
Clinical evidence
Risperidone compared with clonidine
In the parallel trial of risperidone compared with clonidine in children,150 tics and impairment were assessed with the YGTSS global scale. Results for reduction in baseline global score were similar for risperidone compared with clonidine (SMD 0.19, 95% CI –0.68 to 1.05; n = 21) (Figure 157) as were the proportions of participants with a ≥ 30% reduction in YGTSS global score for risperidone compared with clonidine (RR 0.89, 95% CI 0.35 to 2.24; n = 21) (Figure 158).
FIGURE 157.
Standardised change from baseline YGTSS global score for risperidone compared with clonidine. IV, independent variable; ROB, risk of bias.

FIGURE 158.
Proportion of patients with a ≥ 30% reduction in YGTSS global score for risperidone compared with clonidine. M–H, Mantel–Haenszel; ROB, risk of bias.
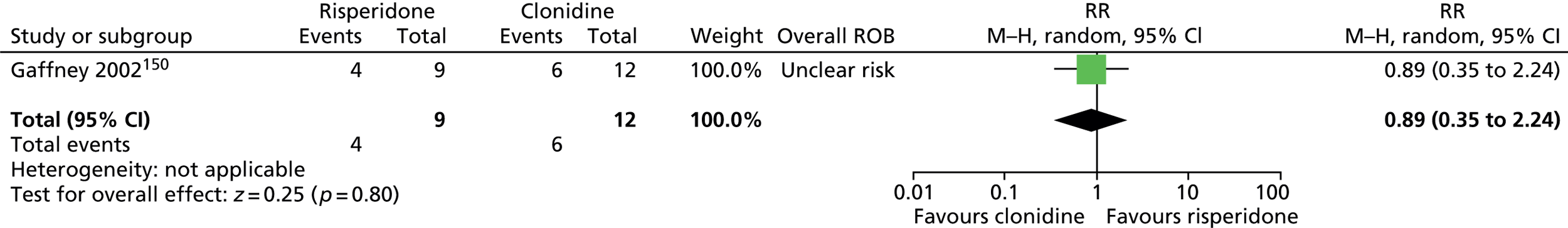
No separate impairment score was presented but the YGTSS global score (results reported above) contains a component of impairment (50% of total score).
The Hamilton Rating Scale for Depression (HAM-D) was used to assess changes in depression. Results were inconclusive for changes from baseline in HAM-D score for risperidone compared with clonidine (SMD 0.03, 95% CI –0.83 to 0.89; n = 21) (Figure 159).
FIGURE 159.
Standardised change from baseline HAM-D score for risperidone compared with clonidine. IV, independent variable; ROB, risk of bias.

Overall clinical outcome was assessed using the CGI-Severity scale. There was no conclusive difference in change from baseline CGI-Severity score for risperidone compared with clonidine (SMD 0.65, 95% CI –0.24 to 1.54; n = 21) (Figure 160).
FIGURE 160.
Standardised change from baseline CGI-Severity score for risperidone compared with clonidine in parallel child study. IV, independent variable.
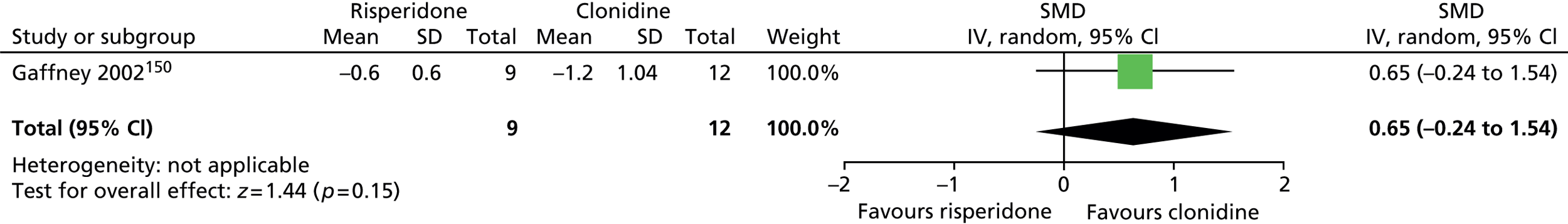
No relevant studies reported long-term outcomes.
For adverse effects, results were inconclusive for risperidone compared with clonidine (RR 0.57, 95% CI 0.20 to 1.62; n = 21) (Figure 161). The most common adverse effects with clonidine were sedation (n = 5), dizziness (n = 2), stiffness (n = 1) and dry mouth (n = 1) and, in the risperidone group, they were sedation (n = 1), dizziness (n = 1) and stiffness (n = 2).
FIGURE 161.
Proportion of children experiencing clinically significant adverse events for risperidone compared with clonidine. M–H, Mantel–Haenszel; ROB, risk of bias.

There was no conclusive difference in weight gain (kg) for risperidone compared with clonidine (MD 2.00, 95% CI –1.66 to 5.66; n = 21) (Figure 162). It was reported that there were no significant differences in changes in systolic or diastolic BP or pulse and no apparent pattern in laboratory test abnormalities for risperidone compared with clonidine and no significant changes in ECG in either group.
FIGURE 162.
Weight gain (kg) for children taking risperidone compared with clonidine. IV, independent variable; ROB, risk of bias.
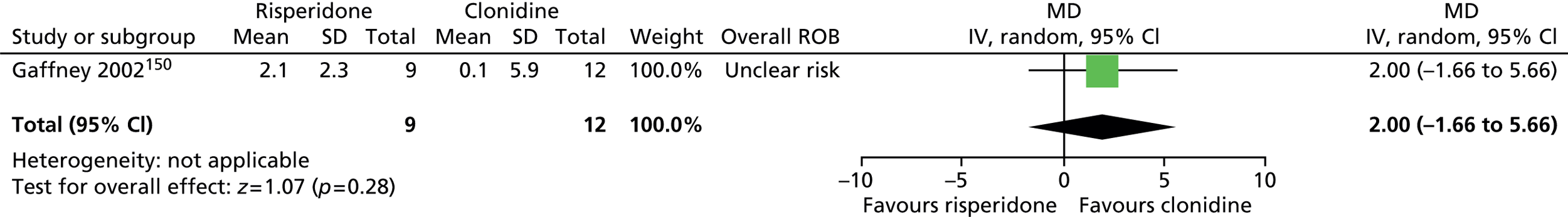
It was reported that improvement in tic symptoms was consistent across the range of ages and did not differ significantly between male and female participants and that the degree of improvement in tic symptoms was not significantly related to the baseline severity of ADHD or OCD symptoms. 150
Fluvoxamine versus sulpiride
In the crossover study of fluvoxamine compared with sulpiride,151 the YGTSS global scale was used to measure changes in tics and impairment. Results were inconclusive for post-treatment global score for fluvoxamine compared with sulpiride (SMD 0.23, 95% CI –0.30 to 0.77; n = 11) (Figure 163).
FIGURE 163.
Standardised post-treatment YGTSS global score for patients following treatment with fluvoxamine compared with sulpiride. IV, independent variable; ROB, risk of bias; SE, standard error.

Impairment was rated as part of the YGTSS global score but no separate impairment scores were reported.
No scales were used to measure outcomes of psychological well-being but it is reported that two participants dropped out owing to severe depression while in the sulpiride phase of the trial.
No relevant studies reported this outcome.
No relevant studies reported long-term outcomes.
Adverse effects reported during fluvoxamine compared with sulpiride treatment are shown in Figure 164. For outcomes with greater than two adverse effects, there was no conclusive difference in rates of mild-to-moderate depression (RR 0.14, 95% CI 0.01 to 2.48; n = 11), mild and transient nausea (RR 9.00, 95% CI 0.54 to 149.50; n = 11) and akathisia (RR 1.00, 95% CI 0.17 to 5.89; n = 11).
FIGURE 164.
Proportion of patients experiencing adverse events following treatment with fluvoxamine compared with sulpiride. M–H, Mantel–Haenszel; ROB, risk of bias.

Levetiracetam compared with clonidine
In the mixed crossover study of levetiracetam compared with clonidine,152 the effect on tics was assessed using the YGTSS total tic scale. Post-treatment total tic score was inconclusive for levetiracetam compared with clonidine (SMD –0.22, 95% CI –0.78 to 0.34; n = 10) (Figure 165).
FIGURE 165.
Standardised post-treatment YGTSS total tic score for clonidine compared with levetiracetam. IV, independent variable; ROB, risk of bias.

No separate results for impairment are presented but results for the YGTSS global scale (combination of tic and impairment score) were given. Results for post-treatment global score were inconclusive for levetiracetam compared with clonidine (SMD –0.33, 95% CI –0.90 to 0.24; n = 10) (Figure 166).
FIGURE 166.
Standardised post-treatment YGTSS global score for clonidine compared with levetiracetam. IV, independent variable; ROB, risk of bias; SE, standard error.
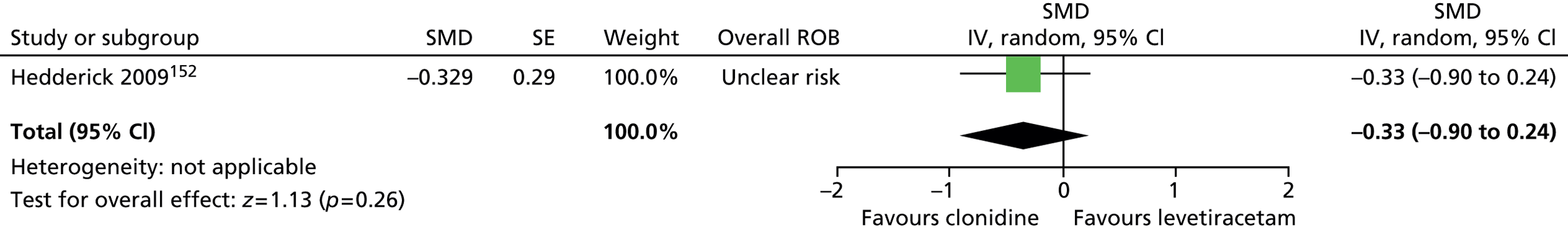
Anxiety was measured using the MASC. Post-treatment anxiety results were inconclusive for levetiracetam compared with clonidine (SMD –0.16, 95% CI –0.72 to 0.40; n = 10) (Figure 167).
FIGURE 167.
Standardised post-treatment MASC score for levetiracetam compared with clonidine. IV, independent variable; ROB, risk of bias; SE, standard error.
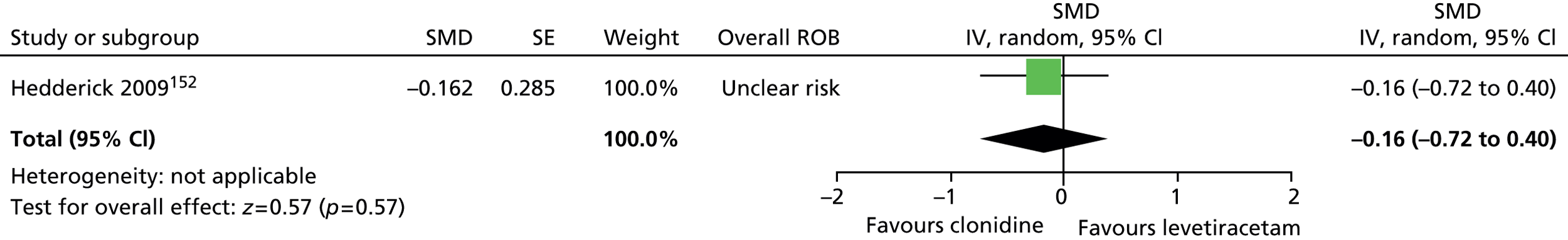
Depression was measured using the CDI-S. Post-treatment depression results were inconclusive for levetiracetam compared with clonidine (SMD 0.08, 95% CI –0.48 to 0.63; n = 10) (Figure 168).
FIGURE 168.
Standardised post-treatment CDI-S score for levetiracetam compared with clonidine. IV, independent variable; ROB, risk of bias; SE, standard error.

Overall clinical outcome was assessed using the CGI-Severity scale. Post-treatment CGI-Severity results were inconclusive for levetiracetam compared with clonidine (SMD –0.16, 95% CI –0.72 to 0.40; n = 10) (Figure 169).
FIGURE 169.
Standardised post-treatment CGI-Severity score for levetiracetam compared with clonidine. IV, independent variable; ROB, risk of bias; SE, standard error.
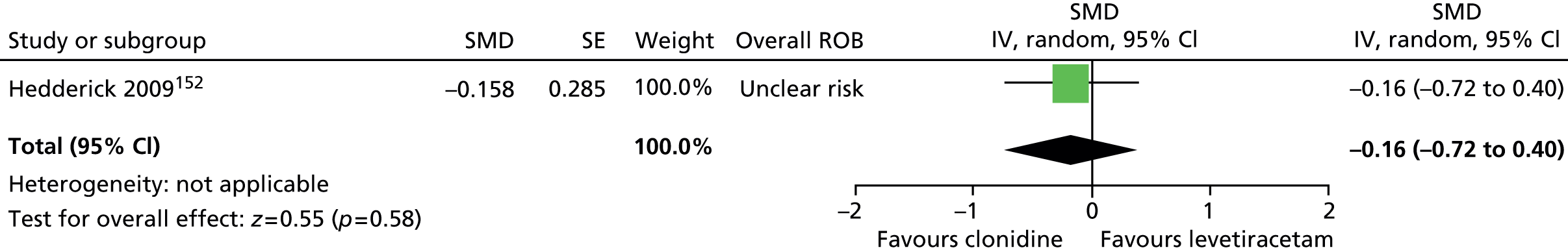
No relevant studies reported long-term outcomes.
The proportion of participants with different adverse effects for outcomes with more than four events is shown in Figure 170. There was no conclusive difference in rates of irritability (RR 1.33, 95% CI 0.40 to 4.49; n = 10), anxiousness (RR 0.75, 95% CI 0.22 to 2.52; n = 10), aggression (RR 0.67, 95% CI 0.14 to 3.17; n = 10) or tiredness/sleepiness (RR 0.40, 95% CI 0.10 to 1.60; n = 10) for levetiracetam compared with clonidine.
FIGURE 170.
Adverse events occurring in more than four patients during treatment with levetiracetam compared with clonidine. M–H, Mantel–Haenszel; ROB, risk of bias.
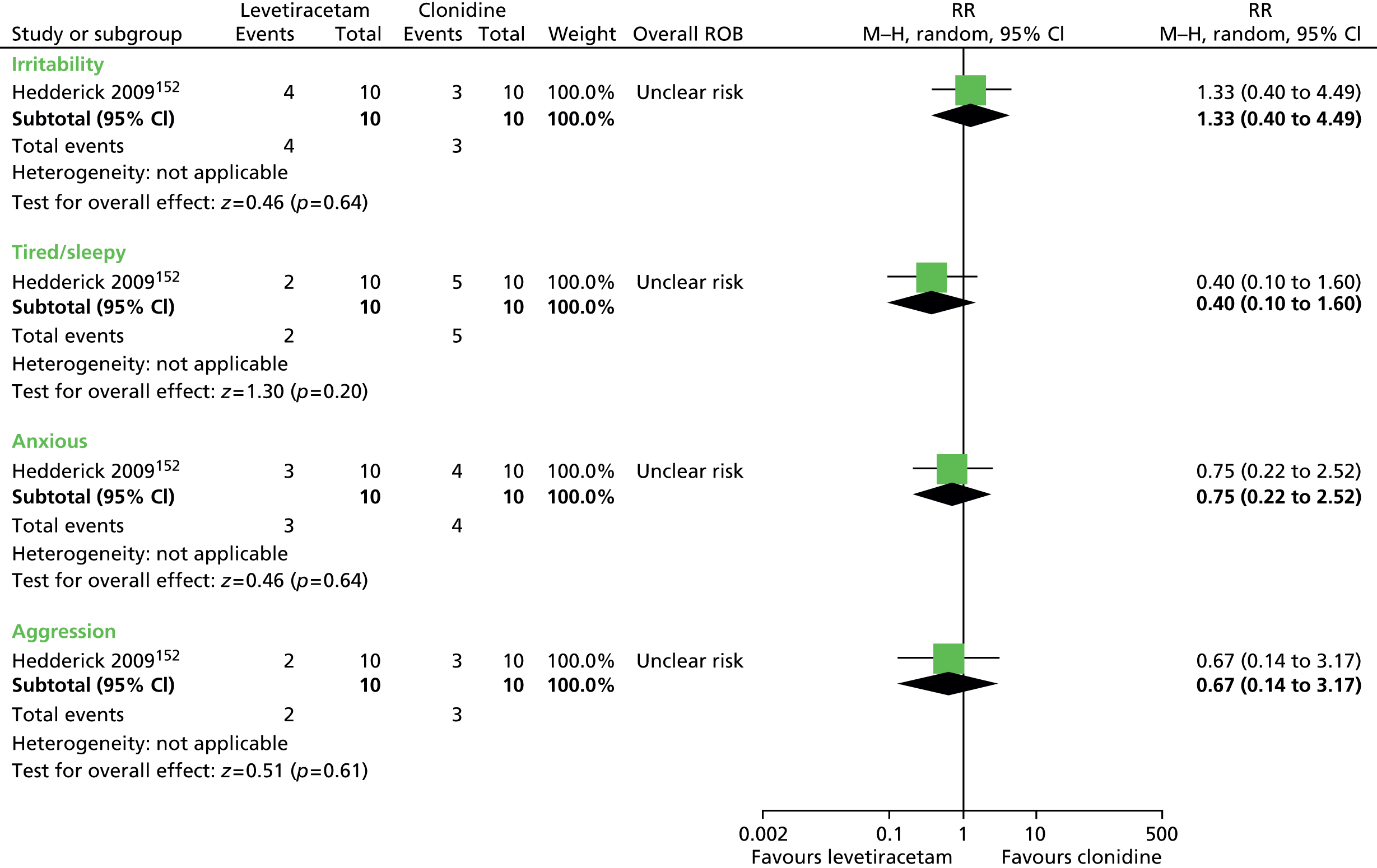
Clonidine compared with desipramine
For the crossover study of clonidine compared with desipramine,115 it was reported that there was no significant motor or vocal tic suppression for clonidine or desipramine compared with placebo when assessed with the Shapiro TSSS or the Hopkins scale or YGTSS scale. There was a significant effect on the parent linear analogue scale and results for this outcome are reported. Post-treatment parent linear analogue score favoured desipramine compared with clonidine (SMD 2.12, 1.58 to 2.66; n = 34) (Figure 171).
FIGURE 171.
Standardised post-treatment parent linear analogue scale for clonidine compared with desipramine. IV, independent variable; ROB, risk of bias; SE, standard error.
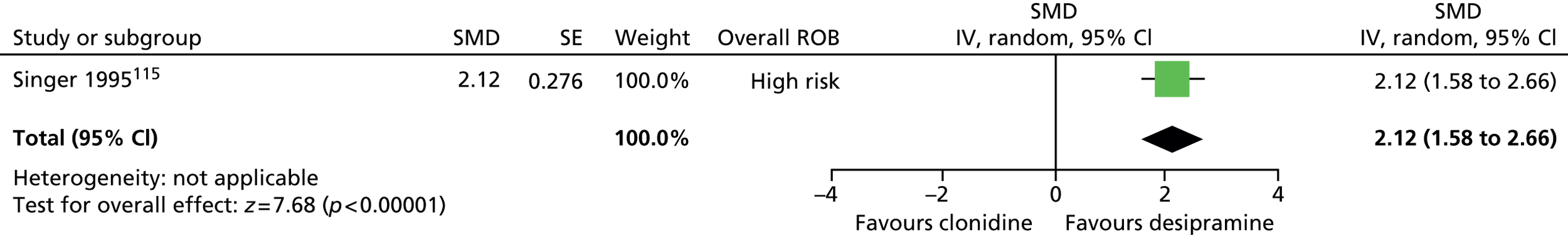
No relevant studies reported TS-related impairment.
Results for the whole sample are not reported, but for boys aged > 12 years, post-treatment anxious teacher subscale score was lower for desipramine than clonidine (SMD 0.40, 95% CI 0.09 to 0.71; n = 34) (Figure 172).
FIGURE 172.
Standardised post-treatment Anxious Teacher subscale (boys aged > 12 years) for clonidine compared with desipramine. IV, independent variable; ROB, risk of bias; SE, standard error.
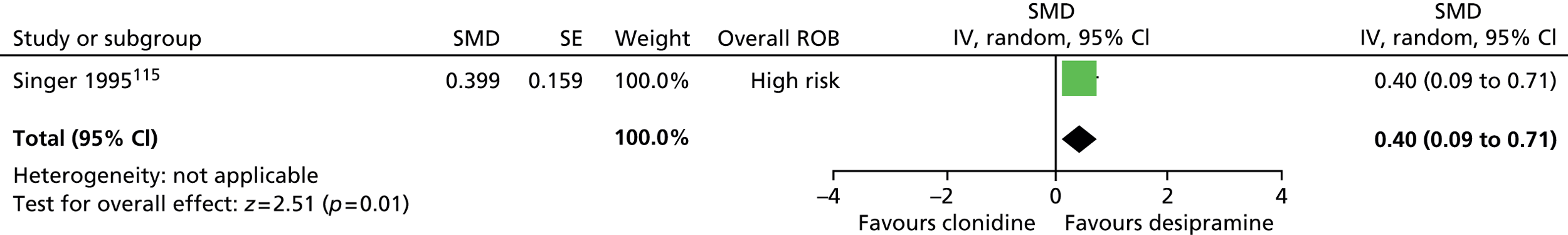
No relevant studies reported this outcome.
No relevant studies reported long-term outcomes.
The number of adverse effects were similar for clonidine compared with desipramine (RR 1.08, 95% CI 0.84 to 1.37; n = 34) (Figure 173).
FIGURE 173.
Proportion of children with adverse events for clonidine compared with desipramine. M–H, Mantel–Haenszel; ROB, risk of bias.

Transdermal clonidine compared with haloperidol
In the study of transdermal clonidine compared with haloperidol,153 tics/impairment was measured with a Chinese version of the YGTSS global scale. There was a greater reduction from baseline in global score for clonidine patch than haloperidol (SMD –0.38, 95% CI –0.75 to –0.02; n = 119) (Figure 174) and a possible greater reduction for clonidine patch than haloperidol in the proportion of children with ≥ 50% reduction in global score (RR 0.55, 95% CI 0.29 to 1.05; n = 119) (the RR presented here is the RR of not achieving ≥ 50% reduction) (Figure 175).
FIGURE 174.
Standardised change from baseline YGTSS global score for clonidine patch compared with haloperidol. IV, independent variable; ROB, risk of bias.

FIGURE 175.
Proportion of children with ≥ 50% reductions in global score for clonidine patch compared with haloperidol (the RR presented here is the RR of not achieving ≥ 50% reduction). M–H, Mantel–Haenszel; ROB, risk of bias.

No relevant studies reported TS-related impairment.
No relevant studies reported this outcome.
No relevant studies reported this outcome.
No relevant studies reported long-term outcomes.
There was no conclusive difference in rates of low BP, muscle tension (RR 0.17, 95% CI 0.01 to 3.40; n = 119) or fatigue (RR 0.09, 95% CI 0.01 to 1.68; n = 119) for clonidine patch compared with haloperidol (Figure 176).
FIGURE 176.
Proportion of children reporting adverse events reported for clonidine patch compared with haloperidol. M–H, Mantel–Haenszel; ROB, risk of bias.
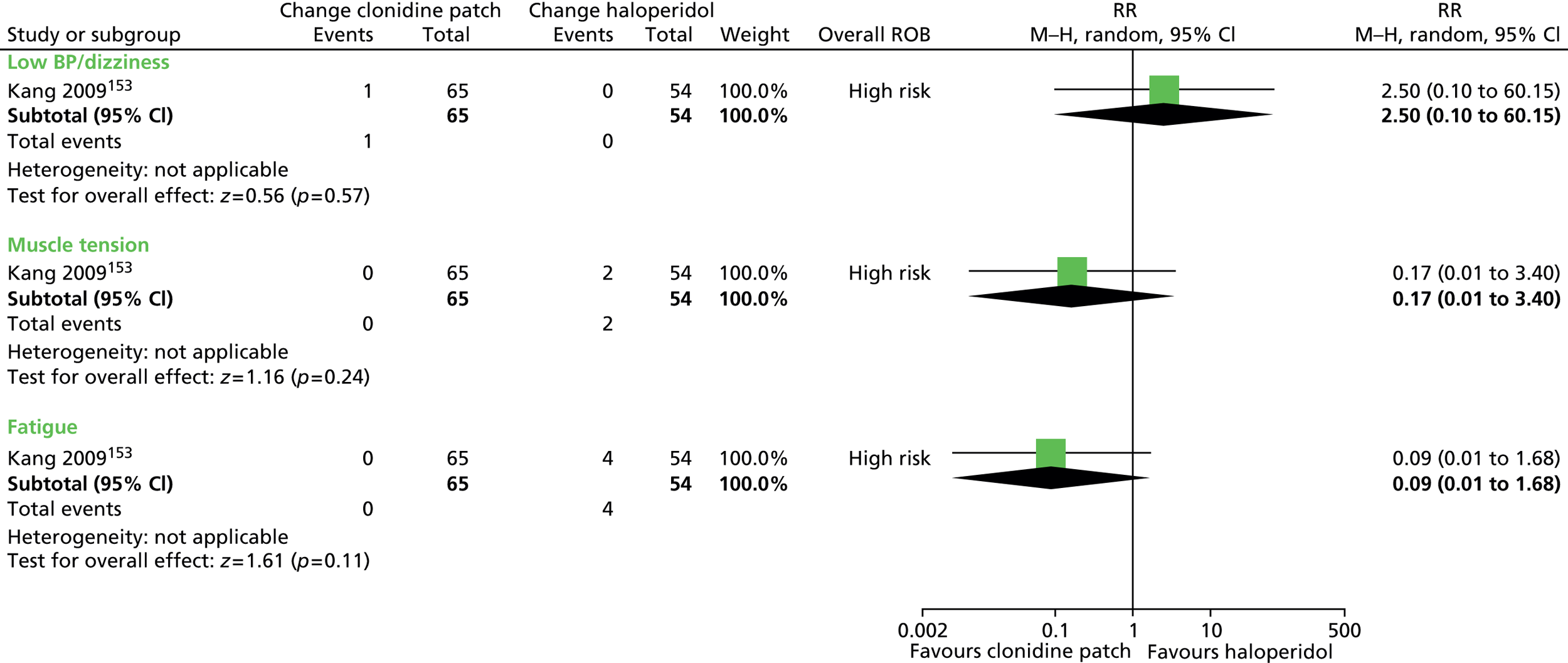
Clinical evidence summary
Five studies made comparisons between drugs of different classes:
-
risperidone compared with clonidine150
-
sulpiride compared with fluvoxamine151
-
clonidine compared with levetiracetam152
-
clonidine compared with desipramine115
-
haloperidol compared with clonidine patch. 153
The evidence for all studies was graded as low or very low quality (see Appendix 4, Tables 44–48). All studies showed inconclusive results.
Conclusion
It is difficult to draw firm conclusions as it is not clear whether or not these studies were adequately powered to test non-inferiority between different drug classes. The most important findings to consider involve comparisons between drugs that have established benefits over placebo. The comparison between risperidone and clonidine suggests that there are unlikely to be clinically important differences between antipsychotic drugs and noradrenergic agents in reducing tics. However, there is evidence that noradrenergic agents may have better global outcomes and a more favourable adverse effect profile than antipsychotics.
Overall conclusions from pharmacological intervention trials
Antipsychotics and noradrenergic agents are the only classes of drug with clear RCT evidence of short-term clinical effectiveness for treating tics in children and young people with TS. The size of effect on tic reduction for antipsychotics and noradrenergic agents is moderate to large and is likely to be clinically meaningful. Among noradrenergic agents, clonidine and guanfacine have the best evidence for clinical effectiveness; however, only clonidine is available and licensed in the UK. Overall, head-to-head studies suggest that there are unlikely to be important clinical differences in tic reduction among antipsychotics and between antipsychotics and noradrenergic agents. Antipsychotics are known to produce a range of adverse effects including weight gain, metabolic and cardiac conduction disturbances and EPS. Furthermore, the pattern of adverse effects differs between antipsychotics. Clonidine and guanfacine have a similar adverse effect profile that includes dose-dependent sedation, bradycardia and hypotension. Although most studies have not been designed to be adequately powered to address moderating effects, there is no clear evidence that the effectiveness of antipsychotics or noradrenergic agents is moderated by either tic severity or comorbidity.
Topiramate, pergolide, metoclopramide and desipramine are other agents with RCT evidence that suggests they may be effective in reducing tics. However, the known adverse effect profiles of these drugs, balanced against relatively weak evidence of benefits, means that these agents are unlikely to be considered clinically useful for the treatment of tics in children and young people with TS. Among these drugs, desipramine has been withdrawn from use in the UK and pergolide is rarely used owing to concerns over the risk of pulmonary and pericardial fibrosis.
A number of other agents are used primarily to treat comorbid TS and ADHD (e.g. stimulants and atomoxetine) and comorbid TS and OCD/OCSs (e.g. fluoxetine). There is clear RCT evidence that, in the short term, neither stimulants nor fluoxetine significantly exacerbates or worsens tics and in fact, atomoxetine may reduce tics.
Finally, the following agents have been subjected to RCTs and have been shown not to be clinical effective for treating tics: levetiracetam, selegiline, pramipexole, mecamylamine, ondansetron, baclofen, omega-3 fatty acids and transdermal nicotine patches.
In summary, the balance of clinical benefits to harm favours noradrenergic agents (e.g. clonidine) as first-line drug treatments for tics, with antipsychotics (e.g. risperidone or possibly aripiprazole) reserved for treatment of tics when clonidine is either ineffective or poorly tolerated. When treating comorbid conditions (e.g. TS plus ADHD, and TS plus OCD/OCSs) there is no evidence that first, the presence of comorbidity moderates the effects antitic treatment and second, that drug treatments for comorbid ADHD (e.g. with stimulants or atomoxetine) or comorbid OCD/OCSs (e.g. with fluoxetine) worsens or exacerbates tics.
Behavioural interventions
Introduction
Behavioural interventions aim to improve symptoms through sessions of counselling with a therapist. These interventions have included HRT, the CBIT, ERP, negative (massed) practice, relaxation therapy, anger control (AC) training and parent training. There have been a number of reviews and clinical guidelines of behavioural therapies, but no quantitative meta-analyses of treatment outcomes appear to have been conducted to date.
Habit reversal training
Habit reversal training was originally based on Azrin and Nunn’s (1973) behavioural model in which tics were assumed to result from repetition of a normal, or trauma-induced, habit that has been reinforced by repetition over time. 154 It is proposed that tics occur in response to a premonitory urge and, by associating another competing response with that urge, tics can be inhibited. HRT aims to equip patients with the knowledge, skills and strategies to avoid performing tics and includes components of awareness training, competing response practice, habit control motivation and generalisation training. 154
-
Awareness training aims to make the patient conscious of their tics and the accompanying premonitory urges (uncomfortable sensory experiences that precede many tics), by describing them to the therapist in great detail and noticing each instance of urges and tics and situations in which they are likely to occur. 154
-
Competing response training involves learning competing motor and/or vocal responses which can be prompted by tic urges, in order to physically impede tics until the urge subsides154 and a number of established responses have been developed for use. 155 These actions are designed to be inconspicuous, able to be maintained for several minutes and act to strengthen motor or vocal responses antagonistic, or different, to those involved in tics. 154
-
Habit control motivation aims to motivate patients to tic control through reviewing inconveniences and embarrassments related to tics, praising successful control, involving family and friends and prompting patients to maintain their practice.
-
Generalisation training aims to help patients contextualise their preventative actions in everyday situations. This involves imagining common tic-eliciting situations and performing the competing movement in response the urge to tic. 154
Comprehensive behavioural intervention for tics
Comprehensive behavioural intervention for tics is a behavioural intervention, with habit reversal as its primary component. 156 CBIT is similar to HRT but contains additional elements of relaxation training and training to identify and modulate situations that sustain or worsen tics. 156 Another difference between HRT as it was initially introduced by Azrin and Nunn154 and the current more widely used CBIT programme is that the competing response developed is now not necessarily an action to strengthen muscles incompatible with tic expression. It is proposed that other responses act as activities that the individual implements as they habituate to the premonitory urge and subsequently avoid tics. Evidence of this is given because an implemented competing response may not always be physiologically incompatible to the identified tic. The evidence for the link between premonitory urge and tic inhibition is inconclusive157 but it is currently the rationale for CBIT. Current guidelines recommend HRT/CBIT for use in children and adults. 158,159
Exposure and response prevention
Exposure and response prevention is based on the theory that tics are performed in order to dispel the premonitory urge sensation that precede them. 160 ERP aims to habituate patients to these sensations so that their effect is reduced and patients can avoid performing the tic. ERP involves exposing patients to tic eliciting prompts and sensations and encouraging them to resist their tics. 160 Unlike in HRT/CBIT, no competing response is used, but patients are repeatedly encouraged by their therapist to try harder to resist their tics. 160 Tic suppression training is first given without tic elicitation and then, in subsequent sessions, patients are encouraged to concentrate on pre-tic sensations to induce the urge to tic. 160 Social support, relaxation and contingency management are also components included in the ERP programme. 158
Current guidelines recommend the use of ERP in adults and children. 158,159
Negative (massed) practice
With a similar underlying model to that of habit reversal, negative, or massed, practice is based on the concept that tics are initiated by some kind of trauma and, with reinforcement through association with fear cessation, they develop as learned habitual behaviours. 161 The rationale for negative practice is that tics are behaviours that have reached their maximum habit strength and, by building an opposing habit of not performing the tic, they can be avoided. 161 In negative practice, patients are instructed to repeatedly perform their tics for a specified period of time (e.g. 30 minutes) and, when the drive to tic dissipates, they rest. A reduction in the drive to tic is consequently associated with not performing it and, with repeated negative practice, a habit of not performing tics is developed. 161 Currently, guidelines do not recommend the use of negative practice. 158,159
Relaxation therapy
Relaxation training has been studied for the treatment of tic disorders but mostly in combination with other behavioural interventions162 and CBIT and ERP identify relaxation training as an adjunct treatment. Components of relaxation training sessions may include review of the rationale behind relaxation practice of progressive muscular relaxation and therapist-lead relaxation exercises. 162
In one current guideline, relaxation therapy is recommended as a second-line behavioural therapy158 but, in another, no recommendation regarding relaxation training is made. 159
Anger control training
Anger control training has been developed for use in children. It is based on CBT and has been conducted in a variety of settings such as schools and outpatient and inpatient facilities. 163 In recent guidelines, no recommendation regarding AC training is made. 158,159
Parent training
Parent training programmes have frequently been used for parents of children with ADHD and one study has evaluated the use of the approach in the treatment of children with tic disorders. 164 Components of treatment may include training for effective child behaviour management, classroom behaviour modification and academic interventions and special educational placement with family therapy in problem solving and communication skills and the co-ordination of school resources. 165 Parent training was not assessed in recent guidelines158,159 and no recommendation regarding its use for children with tic disorders has been made.
Included studies
The search obtained seven studies of behavioural interventions for children and young people with TS (see Appendix 3, Table 10). The search also obtained four behavioural intervention studies in adult or mixed populations that were considered as supporting evidence.
The types of studies are shown below:
-
HRT compared with waiting list control (one child study169)
-
HRT compared with negative practice (one mixed study170)
-
HRT compared with ERP (one child study171)
-
Videoconference CBIT compared with face-to-face CBIT (one child study172)
-
Relaxation training compared with minimal therapy (one child study173)
-
AC training compared with TAU (one child study174)
-
Parent training compared with TAU (one child study164).
Study characteristics for behavioural intervention studies are given in Appendix 3, Table 10.
Habit reversal training/comprehensive behavioural intervention for tics
Habit reversal training/comprehensive behavioural intervention for tics compared with control
Study characteristics
Five RCTs compared HRT/CBIT with a control group (see Appendix 3, Table 10). Two of the studies investigated CBIT156,166 and three investigated HRT. 167–169 Two studies were conducted in child populations156,169 and three in adults. 166–168 In four studies, HRT or CBIT was compared with SP156,166–168 and in the other, HRT was compared with a waiting list control group. 169
The four studies that compared HRT or CBIT with SP used similar methods. Treatment duration varied between studies and lasted for 10 weeks in two studies156,166 and 20 weeks in the other two studies. 167,168 Outcome was assessed post intervention and, in three studies,156,166,168 mid-way through treatment. The study that used a waiting list as the control group169 delivered an average of 20 sessions of HRT over 8–11 months but the waiting list control was only for 3 months (in this review, outcome assessment at 3 months is used for both study arms so that intervention/control findings are compared at the same time point). In these studies, 25–53% of participants were on other medications. In studies for which the type of current medication was reported,156,166,168 there were most commonly antipsychotics, noradrenergic agents (clonidine or guanfacine) or SSRIs.
All HRT/CBIT studies reported that the primary elements of the behavioural intervention were tic awareness training, self-monitoring, relaxation training, competing response training and contingency management and as the components of these interventions appeared to be similar, the studies were combined in the meta-analysis.
Risk of bias
For studies of HRT/CBIT, three reported their method of randomisation156,166,169 but none of the studies had clear evidence of allocation concealment. All studies used SP as the control group except for one,169 in which a waiting list control was used. SP was considered to be a suitable control as it avoided some forms of performance bias (general effect of the therapy process) but the waiting list control group was not considered a suitable control. Because of the person-delivered nature of behavioural intervention studies, none of the studies could blind participants and providers to intervention allocation.
Only two of the studies stated that outcome assessors were blinded to randomisation allocation156,166 and for the remaining studies, the risk of detection bias was unclear. Four of the studies were considered to have reasonably addressed missing outcome data. In these studies, dropout was relatively low and data for participants with at least one post-baseline measurement,156,166 or post 8-week data,167,168 were carried forward for the analysis. In the other study, there was considered to be some potential risk of bias as a high proportion of participants dropped out of the study and only available data were used in the analysis. 169 It is unclear whether or not the participants dropping out were adults or children (only the child data were used for this review) and the risk of attrition bias was, therefore, unclear.
Overall, one of the child156 and one of the adult166 studies were considered to be of low risk of bias and the remaining child169 and two adult167,168 studies were considered to be of high or unclear risk of bias, primarily owing to the use of a waiting list control group in the child study and to the uncertainty around blinding of outcome assessors in the two adult studies.
Clinical evidence for habit reversal training/comprehensive behavioural intervention for tics compared with control
As HRT and CBIT appear to have had very similar training components, for this analysis these studies have been combined. The HRT/CBIT compared with SP studies used the YGTSS to measure tic severity after 10156,166 or 20167,168 weeks of intervention, while the study of HRT compared with a waiting list control group169 measured tic frequency using 10-minute video records after 12 weeks of intervention. The reduction from baseline in total tic severity/frequency was greater for HRT/CBIT than for control in child (SMD –0.64, 95% CI –0.99 to –0.29; n = 133) and adult studies (SMD –1.23, 95% CI –2.06 to –0.39; n = 172) (Figure 177). There was no heterogeneity between studies of children (I2 = 0%) but significant heterogeneity between adult studies (I2 = 77%). There was little evidence of a difference between child and adult studies (I2 = 38.5% for subgroup differences).
FIGURE 177.
Standardised change from baseline in tic score for HRT/CBIT compared with control in child and adult studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias.

For studies measuring YGTSS total tic score, the magnitude of effect in terms of the YGTSS is shown in Figure 178. The mean change from baseline in YGTSS total tic rating for HRT/CBIT compared with SP was –4.1 (95% CI –6.31 to –1.8; n = 126) in children and –7.29 (95% CI –13.4 to –2.79; n = 172) in adults.
FIGURE 178.
Standardised change from baseline in YGTSS total tic rating for HRT/CBIT compared with SP in child and adult studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias.
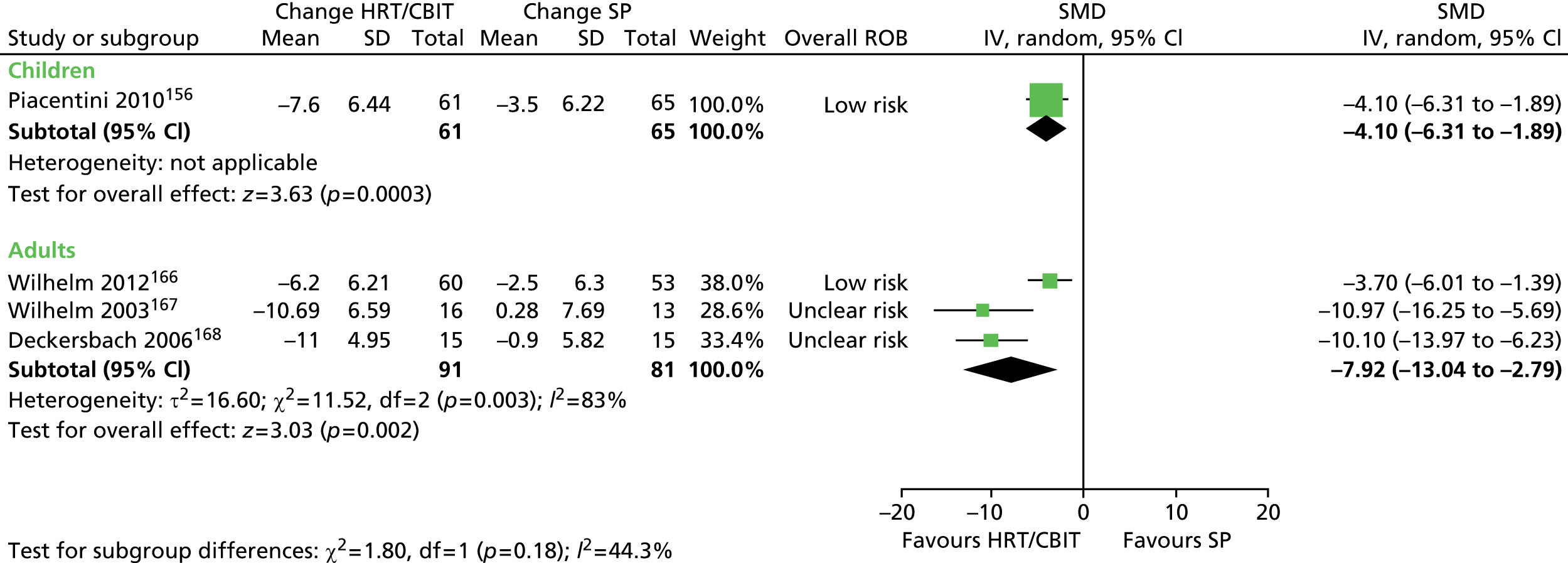
One child156 and one adult166 CBIT study presented separate data for YGTSS motor and vocal tic ratings. Mean reduction from baseline in motor tic rating suggested a benefit for CBIT compared with SP in the child (SMD –0.45, 95% CI –0.81 to –0.10; n = 126) and adult studies (SMD –0.62, 95% CI –1.00 to –0.24; n = 113) (Figure 179).
FIGURE 179.
Standardised change from baseline for CBIT compared with SP for YGTSS motor tic rating in children and adults. df, degrees of freedom; IV, independent variable; ROB, risk of bias.
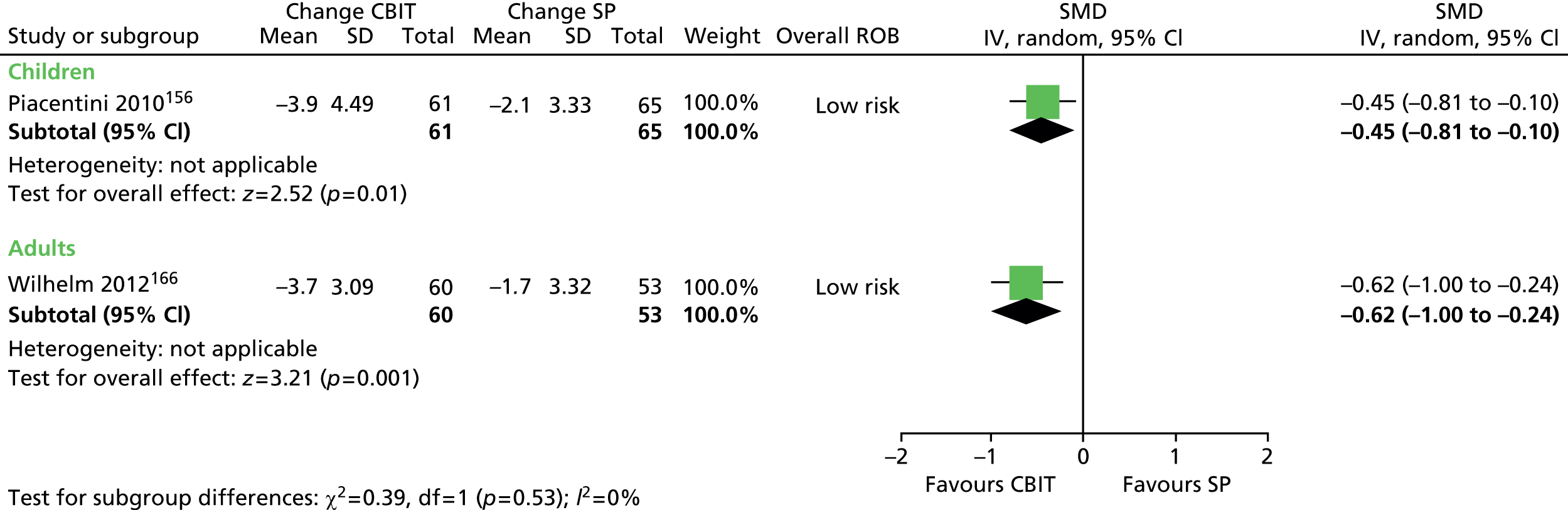
For vocal tic rating, results suggested a benefit for CBIT compared with SP in the child (SMD –0.54, 95% CI –0.9 to –0.19; n = 126) and adult study (SMD –0.41, 95% CI –0.78 to –0.03; n = 113) (Figure 180).
FIGURE 180.
Standardised change from baseline for CBIT compared with SP for YGTSS vocal tic score in children and adults. df, degrees of freedom; IV, independent variable; ROB, risk of bias.

One child156 and two adult166,167 studies presented impairment results in terms of YGTSS impairment score (Figure 181). The change from baseline YGTSS impairment score favoured HRT/CBIT compared with SP in the child study (SMD –0.51, 95% CI –0.87 to –0.16; n = 126) and in the adult study (SMD –0.50, 95% CI –0.86 to –0.15; n = 142).
FIGURE 181.
Standardised change from baseline YGTSS impairment score for HRT/CBIT compared with SP in child and adult studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias.
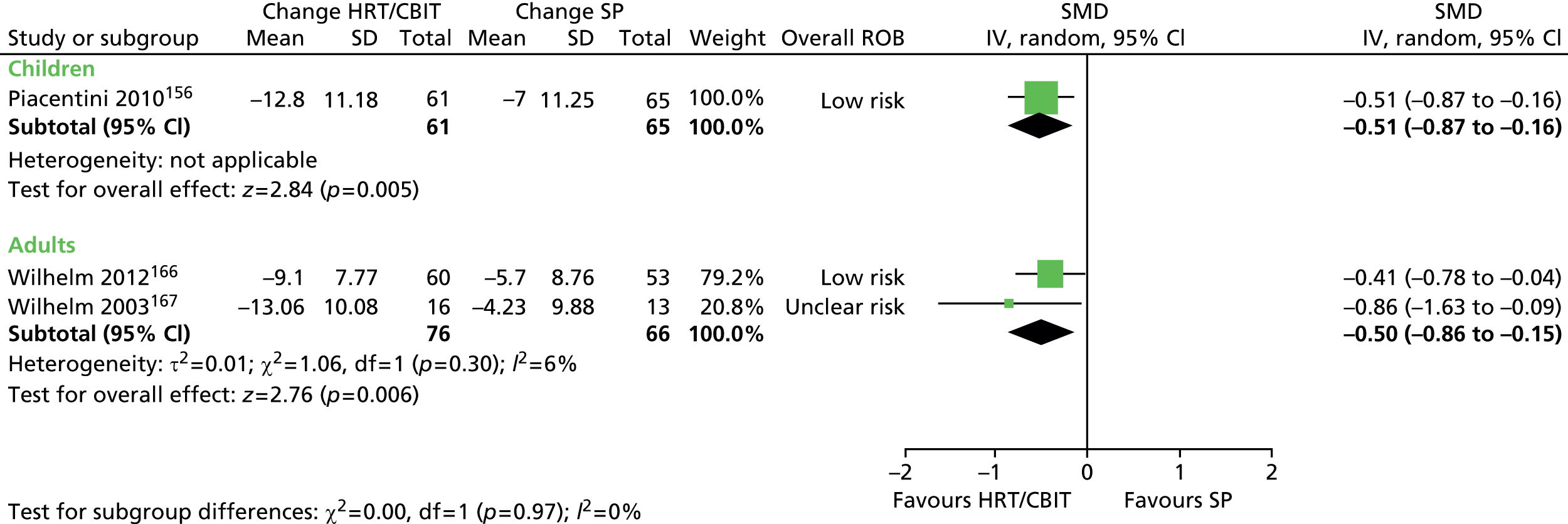
In one study of children,156 impairments in functioning within the family and school environment were assessed using the Family Assessment Measure-III. In one study of children,156 impairments in functioning within the family and school environment were assessed using the Family Assessment Measure-III (short form), Caregiver Strain Questionnaire, Social Adjustment Scale Self-Report and subscales of the Child Behaviour Checklist (CBCL). 175 There was no evidence of benefit for CBIT compared with SP for changes from baseline in the Family Assessment Measure for children (SMD –0.02, 95% CI –0.37 to 0.33; n = 126) or adults (SMD 0.16, 95% CI –0.19 to 0.51; n = 126), or for the Caregiver Strain Questionnaire (SMD –0.02, 95% CI –0.37 to 0.33; n = 126), Social Adjustment Scale Self-Report – family (SMD –0.15, 95% CI –0.50 to 0.20; n = 126), friends (SMD –0.08, 95% CI –0.43 to 0.27; n = 126) or school functioning measures (SMD 0.10, 95% CI –0.25 to 0.45; n = 126), or the CBCL activities (SMD 0.17, 95% CI –0.18 to 0.52; n = 126), social (SMD 0.08, 95% CI –0.27 to 0.43; n = 126), school (SMD –0.05, 95% CI –0.40 to 0.30; n = 126) or total competency (SMD 0.04, 95% CI –0.31 to 0.39; n = 126) subscales.
In one study of children,156 results for the Screen for Child Anxiety Related Emotional Disorders completed by children and their parents are reported in a later publication. 175 There were similar changes in anxiety scores from baseline to follow-up for CBIT compared with SP as assessed by children (SMD –0.10, 95% CI –0.45 to 0.25; n = 126) and their parents (SMD –0.15, 95% CI –0.50 to 0.20; n = 126) (Figure 182).
FIGURE 182.
Standardised change from baseline Screen for Child Anxiety Related Emotional Disorders rating for CBIT compared with SP as rated by children and parents. df, degrees of freedom; IV, independent variable; ROB, risk of bias.

One study in children175 and one in adults168 report changes in patient depression. The child study used the CDI and the adult study used the Beck Depression Inventory. There were similar changes in depression scores for HRT/CBIT compared with SP in the study of children (SMD –0.22, 95% CI –0.57 to 0.13; n = 126) and adults (SMD 0.01, 95% CI –0.70 to 0.73; n = 30) (Figure 183).
FIGURE 183.
Standardised change from baseline in depression rating for HRT/CBIT compared with SP in child and adult studies. df, degrees of freedom; IV, independent variable; ROB, risk of bias.
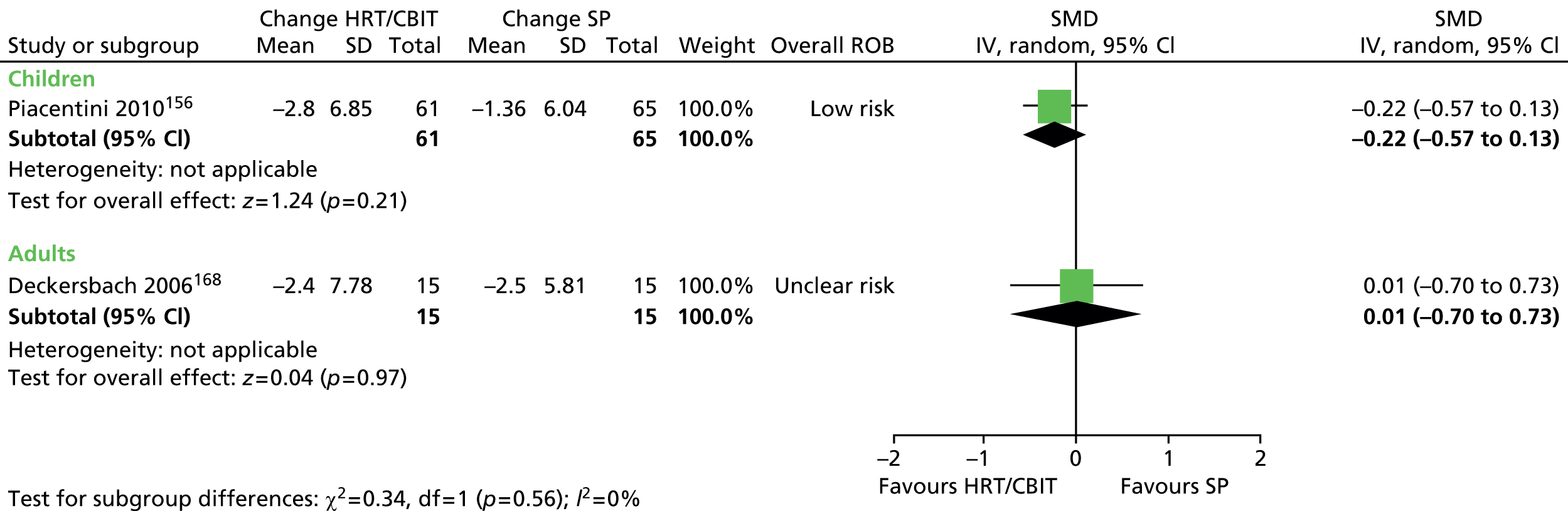
One study in children156 and two in adults166,168 reported the proportions of participants showing improvements on the CGI-I scale. A greater number of participants were much or very much improved for HRT/CBIT compared with SP for the child (RR 2.84, 95% CI 1.62 to 4.99; n = 126) and adult (RR 5.39, 95% CI 2.42 to 11.9; n = 152) studies (Figure 184). There was no important heterogeneity between the two adult studies.
FIGURE 184.
Proportion of patients rated as much or very much improved on the CGI-I scale for HRT/CBIT compared with SP in child and adult studies. df, degrees of freedom; M–H, Mantel–Haenszel; ROB, risk of bias.
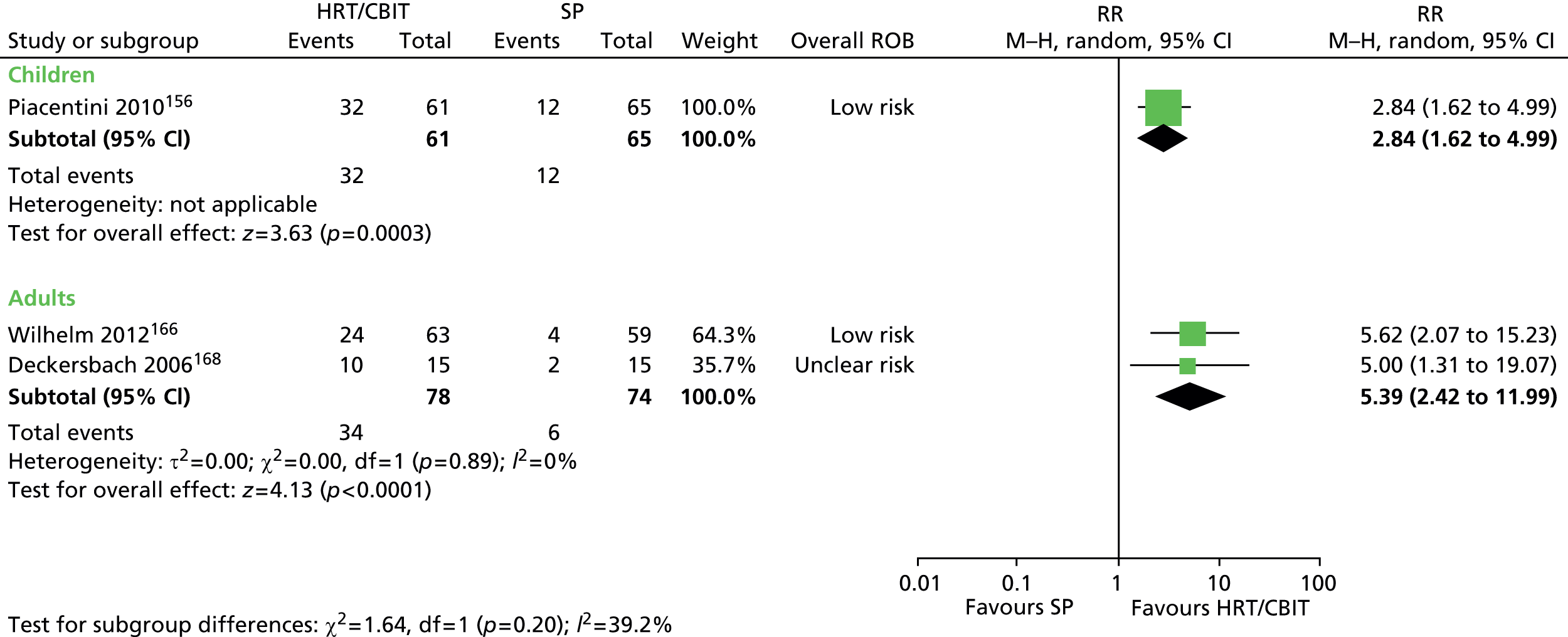
One study presented continuous data for the CGI-I scale (1 = very much improved, 7 = very much worse). 167 After the 20-week intervention, mean CGI-I score was better for HRT than SP (SMD –1.32, 95% CI –2.18 to –0.46; n = 27) (Figure 185).
FIGURE 185.
Standardised CGI-I score for HRT compared with SP (1 = very much improved, 7 = very much worse) in adults. IV, independent variable; ROB, risk of bias.

No studies in children have reported long-term outcomes but two of the adult studies reported YGTSS total tic rating167,168 and one study reported YGTSS impairment rating167 at 10 months. Results were analysed including only participants retained in the study at 10 months but, by this time, approximately one-third of participants had dropped out. Treatment effect size remained similar to post-treatment effects for both outcomes. HRT showed improvement from baseline compared with SP for YGTSS total tic score (SMD –1.11, 95% CI –1.80 to –0.42; n = 39) (Figure 186).
FIGURE 186.
Standardised change in YGTSS total tic score from baseline to 10-month follow-up for HRT compared with SP in adults. df, degrees of freedom; IV, independent variable; ROB, risk of bias.

However, for YGTSS impairment score, there was no conclusive difference in change from baseline for HRT compared with SP (SMD –0.42, 95% CI –1.29 to 0.45; n = 21) (Figure 187).
FIGURE 187.
Standardised change in YGTSS impairment rating from baseline to 10-month follow-up for HRT compared with SP in adults. IV, independent variable; ROB, risk of bias.
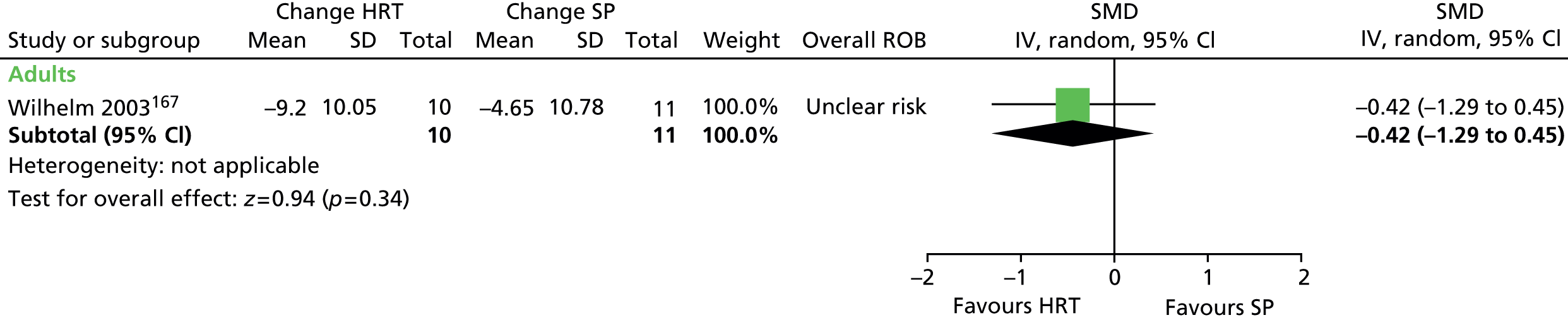
Adverse effects are reported in one child156 and one adult study. 166 There were no significant differences in event rates apart from a higher incidence of falls/athletic injuries for children undergoing SP compared with CBIT (19 vs. 7; p = 0.02) and a higher incidence of irritability in adults undergoing SP compared with CBIT (6 vs. 0; p = 0.01).
Moderators of effectiveness
One adult study conducted a regression analysis to examine the relationship between baseline tic severity and response to treatment. 168 There was no significant correlation between baseline YGTSS total tic severity score and post-treatment response (r = 0.17; p = 0.55).
One adult study conducted a regression analysis to examine the relationship between age at tic onset and response to treatment. 168 There was no significant correlation between age at onset and post-treatment response (r = 0.15; p = 0.60).
There are no published RCTs investigating the effects of comorbidity on the outcome of behavioural interventions in children or adults with tic disorders.
There are no published RCTs investigating the effects of medication in addition to behavioural therapy in children or adults with tic disorders.
One study investigated whether or not CBIT was as effective when delivered via videoconference when compared with face-to-face delivery. 172 Twenty children were randomly assigned to receive eight sessions of CBIT over 10 weeks with a therapist via teleconference or with traditional face-to-face interaction. The primary components of the CBIT intervention in both modes of delivery were psychoeducation, HRT, function-based assessment and intervention and relaxation training. Each week, a new tic was targeted and children were encouraged to practice therapeutic activities every day.
In the quality assessment of this study, methods for randomisation and allocation concealment were unclear. It was not possible to blind study participants or providers delivering interventions and, although both were delivered with a similar time frame and intensity, the risk of performance bias is unclear. Outcome assessors were blinded to intervention allocation. Two participants dropped out of the face-to-face group during the 10-week intervention and one dropped out after the post-test assessment (during follow-up). None dropped out of the videoconference group and the risk of attrition bias is unclear (analysis was of available case data). Overall, this study was considered to be at low risk of bias.
This study reports changes in tics in terms of the YGTSS total tic score and overall clinical improvement with the CGI scale. Results were inconclusive for changes from baseline in total tic score (SMD –0.18, 95% CI –1.11 to 0.75; n = 18) (Figure 188) and numbers of children with CGI scored as improved or very much improved (RR 1.07, 95% CI 0.64 to 1.77; n = 18) (Figure 189) for videoconference compared with face-to-face delivery.
FIGURE 188.
Standardised change from baseline to post-intervention in YGTSS total tic rating for videoconference CBIT compared with face-to-face CBIT. IV, independent variable; ROB, risk of bias.
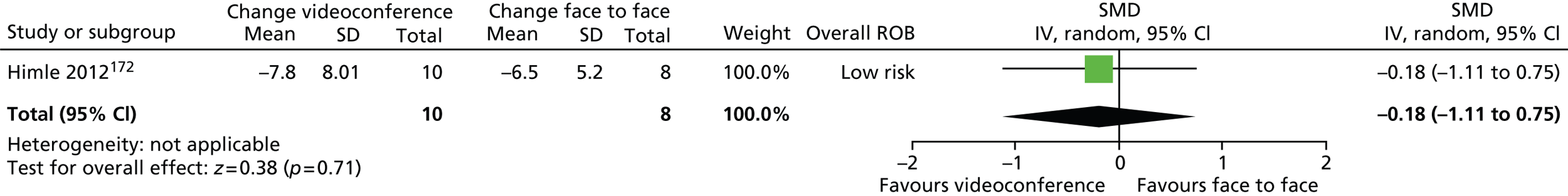
FIGURE 189.
Proportion of children rated as much or very much improved on the CGI-I scale for videoconference CBIT compared with face-to-face CBIT. M–H, Mantel–Haenszel; ROB, risk of bias.
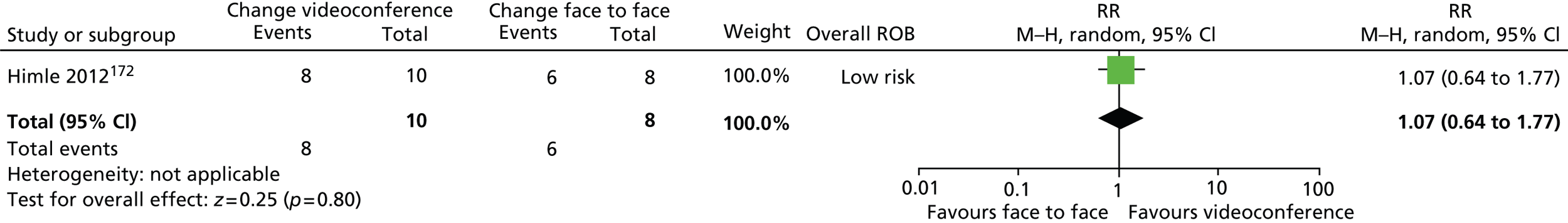
At the 4-month follow-up, the same pattern was observed. For videoconference compared with face-to-face delivery, results were inconclusive for changes from baseline in total tic score (SMD –0.32, 95% CI –1.32 to 0.67; n = 16) (Figure 190) and for proportions of children who were CGI-I much or very much improved (RR 1.30, 95% CI 0.46 to 3.65; n = 16) (Figure 191).
FIGURE 190.
Standardised change from baseline to 4-month follow-up in YGTSS total tic rating for videoconference CBIT compared with face-to-face CBIT. IV, independent variable; ROB, risk of bias.

FIGURE 191.
Proportion of children rated as much or very much improved on the CGI-I scale for videoconference CBIT compared with face-to-face CBIT at 4-month follow-up. M–H, Mantel–Haenszel; ROB, risk of bias.
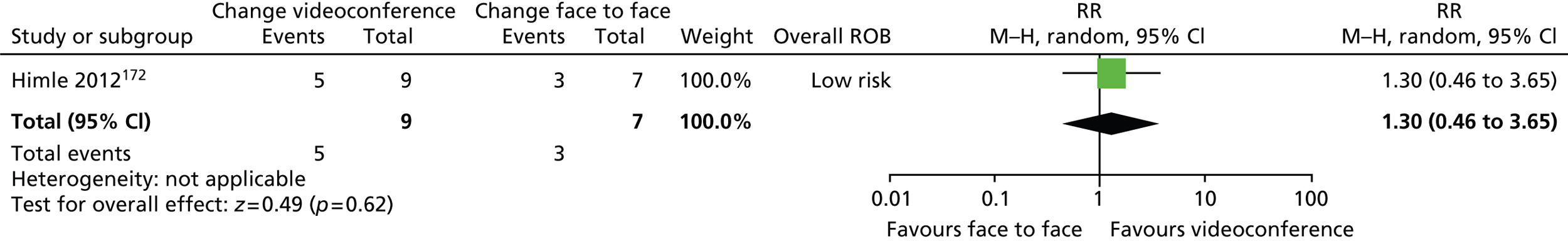
HRT/CBIT studies varied in length and some measured outcomes at different time points during the intervention. One child156 and one adult166 10-week CBIT study also measured outcomes at 5 weeks and one adult168 20-week HRT intervention also measured outcome at 10 weeks.
For both 10-week intervention studies,156,166 the effect on YGTSS total tic score in favour of CBIT was smaller at 5 weeks (SMD –0.32, 95% CI –0.64 to 0.00; n = 239; p = 0.08) compared with the final 10-week assessment (SMD –0.62, 95% CI –0.88 to –0.36; n = 239) (Figure 192) (for this comparison, child and adult study data were combined).
FIGURE 192.
Standardised change from baseline in YGTSS total tic score in child and adult studies with outcome measured at 5 and 10 weeks for CBIT compared with SP. df, degrees of freedom; IV, independent variable.

In the 20-week adult study,168 findings were similar at both 10 and 20 weeks (Figure 193). There was an advantage of HRT over SP at 10 (SMD –1.53, 95% CI –2.35 to –0.70; n = 30) and 20 (SMD –1.82, 95% CI –2.69 to –0.95; n = 30) weeks.
FIGURE 193.
Standardised change from baseline in YGTSS total tic score in adult study with outcome measured at 10 and 20 weeks for HRT compared with SP. df, degrees of freedom; IV, independent variable; ROB, risk of bias.

Habit reversal training versus negative (massed) practice
Study characteristics
One mixed study of 22 adults and children (aged 11–62 years) measured changes in the number of tics per day following HRT compared with negative practice therapy. 170 In this study, HRT was delivered in one or two sessions of around 2.5 hours. The primary HRT component was considered to be competing response training, but the treatment also included a review of inconveniences caused by tics, identification of people or situations associated with tics, awareness training and relaxation training. Methods were to be practised and utilised at home between sessions. After the training sessions, telephone contact was maintained, with reducing contact over time.
For negative practice, in a 2.5-hour session, participants reviewed the difficulties caused by tics, the origins of their habits and previous treatments. They were given written instructions for the negative practice technique and discussed the underlying rationale. They were to purposefully perform tics for 30 second periods with rests over 1 hour, saying to themselves ‘this is what I’m supposed not to do’. They were to continue the practice each day until their tics subsided and to restart if tics returned. The study does not report whether or not telephone contact was maintained for participants undergoing treatment with negative practice. For HRT and negative practice, outcome was assessed after 4 weeks.
Risk of bias
The method of randomisation was stated but the study was unclear in its use of allocation concealment. Providers of interventions could not be blinded to treatment allocation and, although this could not be avoided, the risk of performance bias is unclear. Participants were asked to estimate their own frequency of tics and there was, therefore, no blinded outcome assessment. At 4 weeks, there was no attrition from the study and the risk of attrition bias at this time point was low. Overall, the study was considered to be at unclear risk of bias owing to the lack of patient, provider and outcome assessor blinding.
Clinical evidence for habit reversal training compared with negative (massed) practice
Tic severity was assessed by counts of tic frequency per day by participants. At baseline, median tic frequency was 425 (range 50–5000) and 450 (range 6–9000) per day in the HRT and negative practice groups, respectively. At 4 weeks, estimated tics per day reduced by 92% in the HRT group (size of reduction not reported for negative practice group) and tic reduction was greater for HRT than for negative practice (SMD –1.74, 95% CI –2.72 to –0.76; n = 22) (Figure 194).
FIGURE 194.
Standardised change from baseline for number of tics per day for HRT compared with negative practice. IV, independent variable; NP, negative practice; ROB, risk of bias.

At the end of the intervention, more participants in the HRT group had tic counts of < 1 per day compared with participants in the negative practice group (RR 0.21, 95% CI 0.06 to 0.77; n = 22) (Figure 195).
FIGURE 195.
Proportion of patients with < 1 tic per day in patients for negative practice compared with HRT. M–H, Mantel–Haenszel; NP, negative practice; ROB, risk of bias.

No relevant studies reported TS-related impairment.
No relevant studies reported this outcome.
No relevant studies reported this outcome.
Long-term outcome was reported for HRT at 18 months but not for negative practice. At 18 months, one-half of the HRT participants (5/10) remained in the study. For these participants, the intervention-associated reduction in tics was maintained and was 97% lower than at baseline.
Habit reversal training compared with exposure and response prevention
Study characteristics
One mixed study of children and adults compared 10 weeks of HRT with 12 weeks of ERP. 171 The authors provided IPD and results could therefore be calculated for the child subset (25 out of 43 participants).
In this study, participants were randomised to 10 weekly treatment sessions of HRT or 12 sessions of ERP (two training and 10 treatment sessions). HRT consisted of awareness and competing response training, in which participants learnt to detect and describe each tic and then to apply a competing response for 1 minute when a tic was about to occur. In the ERP training sessions, participants were trained to suppress their tics for increasing lengths of time. In the next 10 ERP treatment sessions, participants were to apply the response prevention technique over 2 hours and, during these sessions, tics were provoked by asking participants to concentrate on the sensory experiences of their tics and to take tic-eliciting objects into the sessions. For both interventions, participants were encouraged to practice exercises at home.
Risk of bias
For this study, the method of randomisation and presence of allocation concealment was unclear. Providers of interventions could not be blinded to treatment allocation and although this could not be avoided, the risk of performance bias is unclear. Outcome assessors were blind to intervention allocation so there was a low risk of detection bias. The risk of attrition bias was low because the rate of dropout of children was relatively small (4% at mid-point, 20% at end point) and, in the analysis of the subgroup of child data, intention to treat by last observation carried forward was used. Overall, the study was rated as low risk of bias but, as this review does not utilise the whole randomised sample of participants (only the subset of child data), some caution may be necessary when interpreting the findings.
Clinical evidence for habit reversal training/comprehensive behavioural intervention for tics
As this review171 does not utilise the whole randomised sample of participants, the subset of child data used was checked for baseline similarity. There were no significant differences at baseline in age, sex, age at onset, duration of disease or use of other medications. However, baseline measure of YGTSS total tic score approached being significantly different for the different intervention groups [mean HRT 21.9 (SD 7.0), ERP 17.9 (SD 4.1); p = 0.08].
The Dutch version of the YGTSS was used to assess patient outcome. This is similar to the standard YGTSS (motor and tic severity are scored out of 25 each and total tic severity is the sum of both) except that impairment is scored out of 5 (50 for the standard YGTSS) to give a total score out of 55 (100 for the standard YGTSS). There was no conclusive difference in change from baseline total tic score for ERP compared with HRT (SMD –0.43, 95% CI –1.23 to 0.37; n = 25) (Figure 196).
FIGURE 196.
Standardised change from baseline in YGTSS total tic score for ERP compared with HRT. IV, independent variable; ROB, risk of bias.
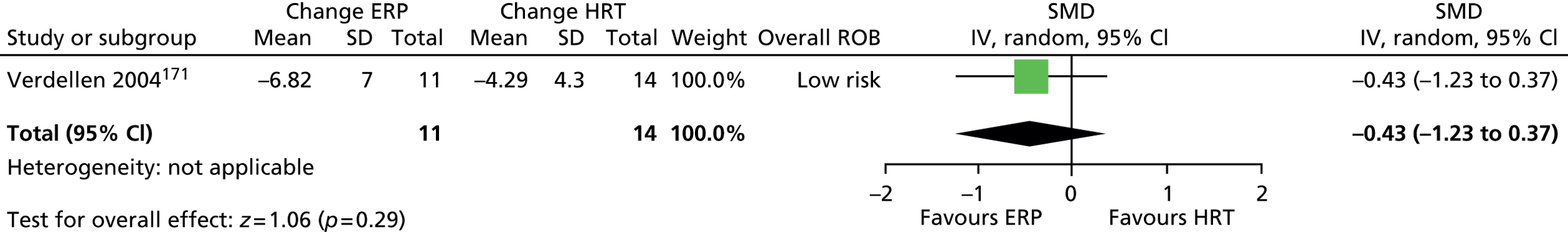
For vocal tic score, there was a greater reduction from baseline for ERP compared with HRT but the difference was not conclusive (SMD –0.73, 95% CI –1.55 to 0.09; n = 25) (Figure 197).
FIGURE 197.
Standardised change from baseline in YGTSS vocal tic score for ERP compared with HRT. IV, independent variable; ROB, risk of bias.

For motor tic score, there were similar changes from baseline for ERP compared with HRT (SMD 0.08, 95% CI –0.71 to 0.87; n = 25) (Figure 198).
FIGURE 198.
Standardised change from baseline in YGTSS motor tic score for ERP compared with HRT. IV, independent variable; ROB, risk of bias.
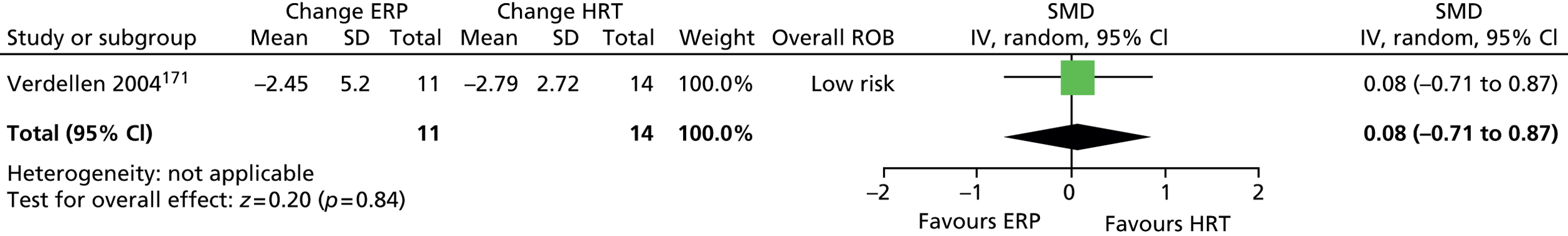
The proportion of children showing ≥ 30% reductions in total tic score was compared. There were similar numbers of children showing ≥ 30% reductions in YGTSS total tic score for ERP compared with HRT (RR 1.48, 95% CI 0.70 to 3.15; n = 25) (Figure 199).
FIGURE 199.
Proportion of children with ≥ 30% reduction in YGTSS total tic score for ERP compared with HRT. M–H, Mantel–Haenszel; ROB, risk of bias.

Impairment was rated with the Dutch version of the YGTSS (scored out of five) and there were similar reductions from baseline in impairment score for ERP compared with HRT (SMD 0.04, 95% CI –0.75 to 0.83; n = 25) (Figure 200).
FIGURE 200.
Standardised change from baseline in Dutch YGTSS impairment score for ERP compared with HRT. IV, independent variable; ROB, risk of bias.

No relevant studies reported this outcome.
No relevant studies reported this outcome.
Follow-up was conducted 3 months after the intervention period. However, for over half of the participants (58%), this was assessed after they had crossed over and undergone the other treatment arm (HRT or ERP). The interpretation of these longer-term findings is therefore unclear.
No results for adverse effects were reported for this study.
IPD were available for this study but, owing to the small sample size (n = 25), investigation into moderating factors was not considered appropriate.
Clinical evidence summary for habit reversal training/comprehensive behavioural intervention for tics
There was moderate-quality evidence (see Appendix 4, Table 49) from one study in 126 children,156 suggesting that CBIT produced a medium-sized effect in terms of tics and impairment following a 10-week intervention. These favourable effects are supported by findings in three studies of adults (n = 172). 166–168 The intervention did not produce improvements in measures of social functioning and psychological well-being (anxiety and depression) in comparison with SP.
The optimum length of intervention is currently unclear but the child study156 provided moderate-quality evidence that after 5 weeks of treatment there was a small-sized to medium-sized effect on tics and there was no statistical difference between results after 5 and 10 weeks of intervention (I2 for subgroup difference = 0%; p = 0.51). This was not consistent with a study in adults,166 for which intervention at 10 weeks, but not 5 weeks, was effective.
One study in 22 adults and children of negative practice compared with HRT170 provided very low-quality evidence (see Appendix 4, Table 49) that HRT was more effective than negative practice in treating tic disorders.
One study in 18 children172 provided low-quality evidence (see Appendix 4, Table 49) for the impact of delivering CBIT by videoconference compared with face to face. There were similar changes in tics and overall clinical change for CBIT when delivered by videoconference compared with face-to-face therapy (the size of changes in both groups were similar to those observed in Piacentini et al. 156) and comparable efficacy was maintained at the 4-month follow-up.
One mixed study in adults and children compared HRT with ERP. 171 For the subsample of 25 children, the study provided low-quality evidence (see Appendix 4, Table 49) that was inconclusive with regard to whether or not ERP and HRT were similar in their efficacy.
Conclusion
There is clear RCT evidence that HRT/CBIT is an effective treatment for tics in children and young people with TS. In head-to-head studies, HRT appears more effective than negative massed practice but there is no evidence of meaningful differences in clinical effectiveness between HRT and ERP. There is no evidence that HRT/CBIT is effective in reducing associated symptoms of anxiety and depression in TS. In children and young people the optimal duration of treatment appears to be between 5 and 10 sessions, with the suggestion that remotely delivered CBIT (video consultation) may be as effective as face-to-face therapy. There is currently no evidence available regarding the potential moderating effects of comorbidity and medication on HRT/CBIT.
Relaxation training
Relaxation training compared with minimal therapy
Study characteristics
One study of 23 children compared relaxation training with a control group of minimal therapy. 173 Relaxation training consisted of six weekly 1-hour sessions covering awareness training, diaphragmatic breathing, behavioural relaxation training, applied relaxation techniques and electromyographic biofeedback. Children were expected to spend 25 minutes per day on exercises including self-monitoring of tics, practising relaxation exercises with an audiotape guide and reading on topics related to breathing, relaxation and biofeedback. Minimal therapy was also conducted in six 1-hour sessions. Sessions covered awareness training and quiet time training, in which children listened to tapes of music or environmental sounds. Participants in this group were also expected to spend 25 minutes per day on exercises. These included daily self-monitoring and practice logs. Regardless of their assigned treatment group, participants and their families were given the rationale for their treatment and encouraged with positive expectations of improvement in tics.
Risk of bias
For this study, the method for randomisation is reported but the presence of allocation concealment is unclear. Minimal therapy was considered to be a suitable control group and attempts were made to ensure that participants in each group had the same expectations for improvements from therapy. Participants and providers could not be blinded to treatment allocation and the risk of patient or provider performance bias is unclear. Outcome assessors were blind to treatment allocation. Sixteen out of 23 randomised children completed the study. Only data from those completing were analysed and, therefore, there was considered to be a risk of attrition bias. Overall, largely due to the high rate of attrition, this study was considered to have an unclear risk of bias.
Clinical evidence for relaxation training compared with minimal therapy
Changes in tics were assessed with the YGTSS and results reported as the proportion of children improving/staying the same/getting worse. 173 For the global score, the evidence was inconclusive regarding the proportion of children improving following relaxation training compared with minimal therapy (RR 0.43, 95% CI 0.06 to 3.28; n = 16) (Figure 201) (the RR presented here is the RR of not improving).
FIGURE 201.
Proportion of children with improved YGTSS global score for relaxation training compared with minimal therapy (the RR presented here is the RR of not improving). M–H, Mantel–Haenszel; ROB, risk of bias.
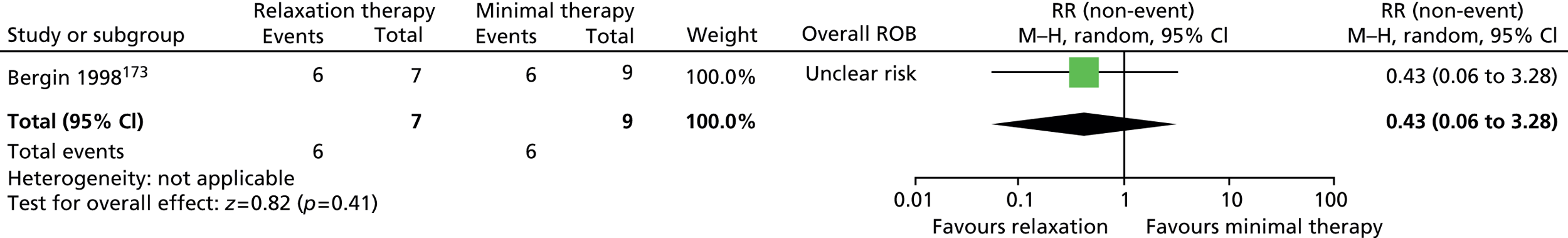
No separate results for patient impairment were reported but the YGTSS global score (reported above) includes an impairment rating that constitutes half of the score. 173
No relevant studies reported this outcome.
No relevant studies reported this outcome.
Longer-term outcome was assessed 3 months after baseline measurements (about 9 weeks post intervention). 173 For the YGTSS global score, the evidence was inconclusive for the proportions of children who were improved for relaxation therapy compared with minimal therapy (RR 0.64, 95% CI 0.16 to 2.56; n = 16) (Figure 202) (the RR presented here is the RR of not improving).
FIGURE 202.
Proportion of children with improved in YGTSS global score for relaxation training compared with minimal therapy at 9 weeks’ follow-up (the RR presented here is the RR of not improving). M–H, Mantel–Haenszel; ROB, risk of bias.
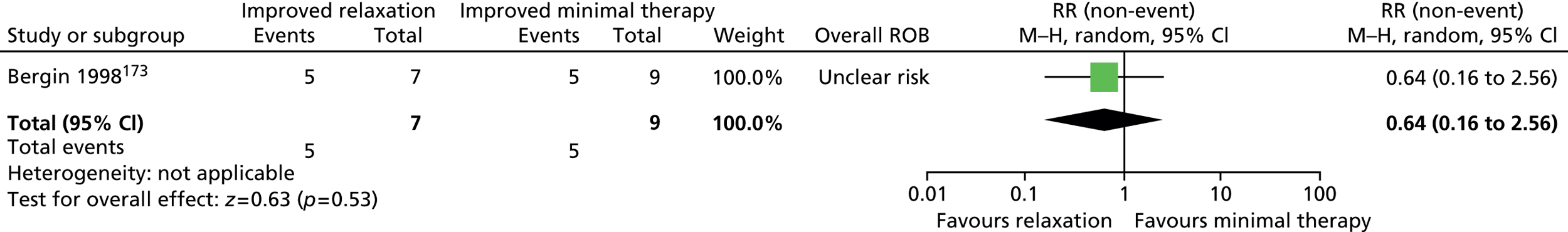
No relevant studies reported this outcome.
Investigators tested whether or not the ability of children to learn relaxation techniques affected the rate of improvement in measures of the CBCL (no comparison with YGTSS). 173 When children across both treatment groups with improved behavioural relaxation scores (measure of ability to relax) (n = 8) were compared with those with unchanged behavioural relaxation scores (n = 6), there was no significant difference in change from baseline scores for any of the elements of the CBCL.
Clinical evidence summary: relaxation training
There was very low-quality evidence (see Appendix 4, Table 53) from one study in 16 children173 that was inconclusive with regard to the effect of relaxation therapy compared with minimal therapy.
Conclusion
There is no clear evidence that relaxation therapy, when delivered in isolation, is an effective treatment for tics in children and young people with TS.
Anger control training
Anger control training compared with treatment as usual
Study characteristics
One study in 26 young people with both TS and disruptive behaviour compared the efficacy of AC training with TAU174 The young people were randomised to attending 10 weekly 1-hour sessions of AC training, or to continue with their usual treatment. AC training consisted of 10 1-hour sessions including, in sessions 1–3, education about anger triggers, experience and expression followed by practice of common arousal management skills such as deep breathing, muscle relaxation and positive imagery; in sessions 4–6, cognitive restructuring and practising problem solving skills; and, in sessions 7–9, behavioural practice of skills for preventing or resolving potentially anger-provoking situations with friends, siblings, parents and teachers. Children allocated to TAU, as well as those allocated to AC training, continued to see their treating clinicians and were treated according to usual practice, commonly including patient education, clinical monitoring, medication management and school consultations as needed.
Risk of bias
The method for randomisation was stated for this study but the presence of allocation concealment was unclear. The control group, TAU, was not considered to be suitable as it did not include elements of the intervention that were not specific to AC training (e.g. time spent in counselling, attention given, etc.) and was likely to have led to some performance bias. Participants and providers could not be blinded to treatment allocation and, although this could not be avoided, the further risk of performance bias from the actions/perceptions of participants or providers is unclear. Outcome was assessed by an evaluator who was blind to treatment allocation. It is stated that this evaluator conducted measurement of the CGI-I scale rating and it seems likely that this was also the case for the measurement of the YGTSS. However, for outcomes assessed by parents (CBCL, Family Assessment Device, etc.) who were not blind to treatment allocation, outcome assessment was not blind to treatment allocation. It was reported that there were no dropouts from the study and the risk of attrition bias may be low. Overall, largely due to the unsuitability of the control group and no blinded outcome assessment for parent-rated outcomes, this study was considered to have an unclear risk of bias.
Clinical evidence for anger control training
Changes in tics were assessed with the YGTSS total tic score. There was no conclusive difference in reduction from baseline total tic score for AC training compared with TAU (SMD –0.58, 95% CI –1.37 to 0.20; n = 26) (Figure 203). 174
FIGURE 203.
Standardised change from baseline in YGTSS total tic score for AC training compared with TAU. IV, independent variable; SE, standard error.

The CBCL was used to measure intervention effects on behavioural change and the total competence component measures competence in social, school and community activity. CBCL total competence score showed greater improvement in the AC training compared with the TAU group (SMD –1.18, 95% CI –2.01 to –0.35; n = 26) (Figure 204).
FIGURE 204.
Standardised change from baseline in CBCL total competence score for AC training compared with TAU. IV, independent variable; SE, standard error.

The Family Assessment Device general functioning scale was rated by parents to assess family functioning. There were similar changes from baseline in family functioning for the AC than for the TAU group (SMD –0.42, 95% CI –1.20 to 0.35; n = 26) (Figure 205).
FIGURE 205.
Standardised change from baseline in Family Assessment Device general functioning score for AC training compared with TAU; IV, independent variable; SE, standard error.

No relevant studies reported this outcome.
Overall clinical outcome (based on improvements in all types of behaviour and not specific to improvements in TS) was assessed using the CGI-I scale. A greater proportion of children were rated much or very much improved following AC training compared with TAU (RR 0.36, 95% CI 0.16 to 0.85; n = 26) (Figure 206) (the RR presented here is the RR of not being much nor very much improved).
FIGURE 206.
Proportion of children assessed as much or very much improved on the CGI-I scale for AC training compared with TAU (the RR presented here is the RR of not being much or very much improved). M–H, Mantel–Haenszel; ROB, risk of bias.

At 3 months post intervention, follow-up findings were reported but only for the group that underwent AC training (n = 13). Eight of these children were still rated as much or very much improved on the CGI-I scale.
No relevant studies reported this outcome.
Clinical evidence summary for anger control training
One study in 26 young people with TS and disruptive behaviour174 provided low-quality (for tics) or very low-quality evidence (for other outcomes) (see Appendix 4, Table 54) for the efficacy of AC training compared with TAU. Findings favoured AC training for tics, total competence (CBCL total competence score) and overall clinical outcome (CGI much or very much improved).
Conclusion
Anger control training may be an effective treatment for improving overall outcomes and tics in children and young people with comorbid TS and disruptive behavioural disorders. However, the low quality of evidence means that caution must be applied to this finding.
Parent training
Parent training compared with treatment as usual
Study characteristics
One RCT evaluated parent training in children with both tic disorders and disruptive behaviour. 164 Twenty-four children with TS or CTD and moderate-to-severe disruptive behaviour were randomised to a structured 10-session programme for parent management in addition to their usual treatment, or to TAU.
Parent training consisted of 10 sessions in which skills were taught through the use of modelling, role play and corrective feedback. Training aimed to improve parental competence in dealing with their child’s behavioural problems, increase caregivers’ understanding of the origins of non-compliant and defiant behaviour, improve the child’s compliance with parental instructions and decrease family conflict. Core skills that were taught included providing positive reinforcement for appropriate behaviour, communicating directions effectively and being consistent with consequences for disruptive behaviour, as well as techniques of positive attending, selective ignoring, token economies and timeout. Assignments to implement skills at home were given after each session.
Treatment as usual was maintained for children in both groups, in which they continued to be treated according to usual practice, commonly including patient education, clinical monitoring, medication management and school consultations as needed. Study participants were also allowed to receive other community clinical services, such as child individual psychotherapy and school-based mental health services, but were asked not to initiate new treatments, or stop existing treatments, during the course of the study.
Risk of bias
The method for randomisation and the presence of allocation concealment was unclear. The control group (TAU) was not considered to be suitable as it did not include elements of the intervention not specific to parent training (e.g. time spent in counselling, attention given, etc.) and was likely to have caused aspects of performance bias. Participants and providers could not be blinded to treatment allocation and although this could not be avoided, the further risk of performance bias from the actions/perceptions of participants or providers is unclear. It is unclear whether or not outcome assessors of all measures were blind to treatment allocation (it is only stated that assessment of CGI-I was blinded). Of 24 randomised children, 23 completed the study and, although data analysis was of available cases, the risk of attrition bias was considered to be low. Overall, largely due to the unsuitability of the control group and unclear blinded outcome assessment, this study was considered to have an unclear risk of bias.
Clinical evidence for parent training versus treatment as usual
Changes in tics were assessed with the YGTSS total tic score. There were similar changes from baseline in total tic score for parent training, compared with TAU (SMD 0.29, 95% CI –0.53 to 1.12; n = 23) (Figure 207).
FIGURE 207.
Standardised change from baseline in YGTSS total tic score for parent training compared with TAU. IV, independent variable; SE; standard error.
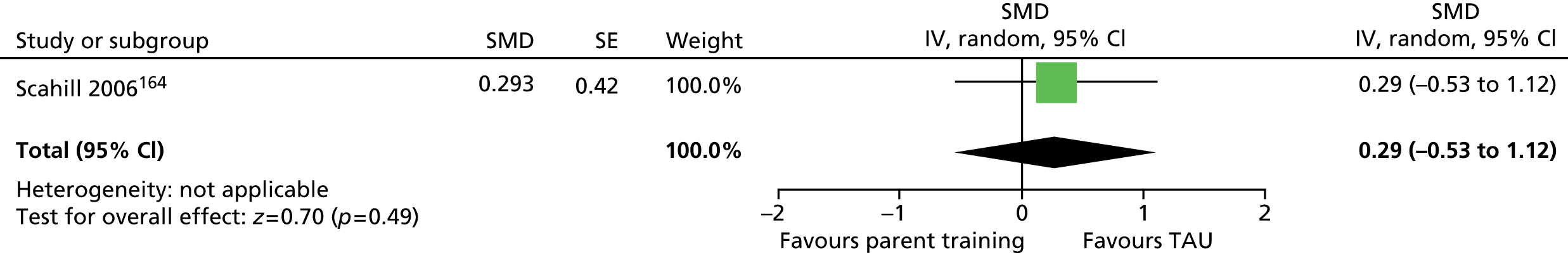
No results for patient impairment were reported for this study but the family impact of the tic disorder was assessed using the Parenting Stress Index-Short Form. There were similar changes in parenting stress scores for parent training compared with TAU (SMD –0.12, 95% CI –0.94 to 0.70; n = 23) (Figure 208).
FIGURE 208.
Standardised change from baseline in Parenting Stress Index for parent training compared with TAU. IV, independent variable; SE; standard error.
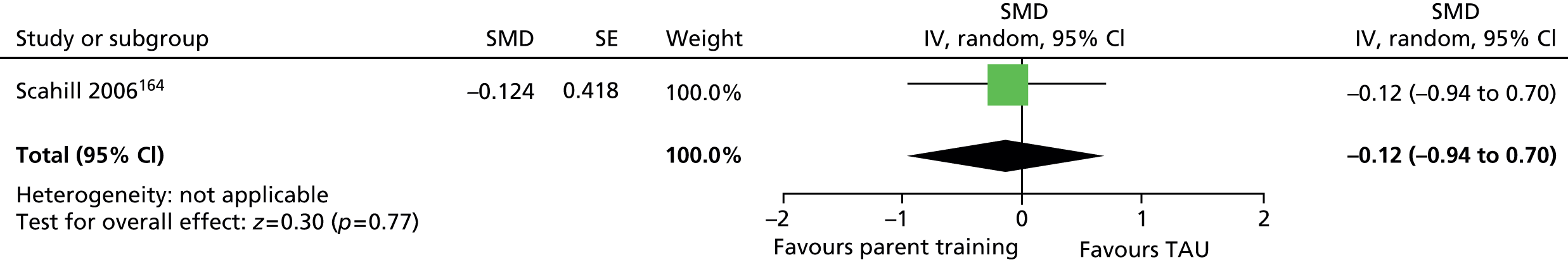
No relevant studies reported this outcome.
Overall clinical outcome (based on improvements in all types of behaviour and not specific to improvements in TS) was assessed using the CGI-I scale. A greater number of children were CGI much or very much improved following parent training compared with TAU (RR 0.44, 95% CI 0.19 to 0.99; n = 23) (Figure 209) (the RR presented here is the RR of not being much or very much improved).
FIGURE 209.
Proportion of children assessed as much or very much improved on the CGI-I scale for parent training compared with TAU (the RR presented here is the RR of not being much or very much improved). M–H, Mantel–Haenszel; ROB, risk of bias.

No relevant studies reported long-term outcomes.
No relevant studies reported this outcome.
Clinical evidence summary for parent training
One study of 23 children164 provided very low-quality evidence (see Appendix 4, Table 55) for the use of parent training compared with TAU. There was no clear difference in overall tic/impairment outcome, but parent training improved overall clinical outcome. The aim of this intervention primarily seems to have been to address behavioural issues and overall clinical outcome related to changes in behaviour as well as TS symptoms.
Conclusion
Although parent training may improve general behavioural outcomes, there is no clear evidence that parent training is an effective treatment for tics in children and young people with TS.
Overall conclusions from behavioural intervention trials
There is clear evidence that HRT/CBIT produces improvements in tics that may be clinically meaningful. Shorter intervention than the standard 10-session CBIT course may be effective but the optimum length of treatment is currently unclear. There is some evidence that delivering HRT/CBIT via video consultation (telemedicine) may be as effective as face-to-face therapy; however, further research is required before firm conclusions can be drawn. There is no evidence that the effects of HRT/CBIT are moderated by tic severity. However, there remain important gaps in the evidence concerning the benefits of behavioural interventions in the presence of comorbid conditions and the effects of combining behavioural and drug interventions for tic disorders. There is little evidence for the effect of behavioural interventions on QoL and longer-term outcomes.
There are no RCTs of negative massed practice or ERP compared with control interventions. However, head-to-head comparisons suggest that HRT is a more effective intervention than negative practice, while HRT and ERP may be equally effective interventions for tics.
Currently, there is no clear evidence to suggest that relaxation therapy in isolation is an effective treatment for tics. AC training may be a useful intervention for young people with tics and comorbid disruptive behaviour and behaviour problems may be improved by parent training, although there is no evidence that parent training is an effective treatment for tics.
Overall, the quality of evidence for behavioural interventions other than HRT/CBIT is low and, therefore, conclusions drawn from this evidence must be treated with caution. In summary, future research will need to consider how behavioural interventions are best combined with medication, the potential for alternatives to face-to-face delivery of therapy and whether or not the effects of behavioural interventions are sustained over time.
Physical interventions
Introduction
A number of physical interventions have been used to treat TS, including DBS, rTMS, intravenous (i.v.) immunoglobulin, botulinum toxin and acupuncture.
Deep brain stimulation
Deep brain stimulation is an invasive procedure that has been used to treat movement disorders such as Parkinson’s disease, essential tremor and dystonia. 176 It has also, more recently, been used to treat psychiatric disorders such as resistant depression and OCD. 177 DBS aims to correct disorders by sending electrical impulses to the brain to modulate brain activity in specific regions. Surgery is performed to make a small hole in the skull through which an electrode is positioned on the brain using imaging techniques, stereotactic atlases and microelectrode recordings. 178 A lead connects the electrode to a pulse generator inserted under the skin in the chest or abdomen. The generator transmits tiny electric pulses to the electrode resulting in stimulation of the surrounding brain. This stimulation interrupts the brain signals that are thought to be responsible for the unwanted behaviour. The generator is programmed by a transdermal unit that can be adjusted to vary settings and optimise therapy. 178
Deep brain stimulation has been applied for the treatment of TS in place of ablative neurosurgery. It was first used in 1997179 and it has been estimated that around 100 DBS operations have been conducted to date. 180 DBS has predominantly been performed in adults – the youngest patients treated for TS being 16 years of age. 181 DBS has been applied to different areas of the basal ganglia and nearby structures, but the optimum target has been a source of controversy. It appears that, although many different targets may be used,180 different patients may benefit from stimulation of different areas of the brain. 181 There is little reported on adverse effects177 but surgery-associated morbidity (e.g. haemorrhage, infection and fracture) for all indications has been estimated at 3–4% of patients. 178 There is little information on other adverse effects affecting cognition and emotional well-being and the long-term impacts are currently unknown.
Current guidelines recommend DBS for use in patients with:
-
chronic and severe TS (YGTSS total tic score of > 35)
-
severe functional impairment
-
previous failed treatment with conventional TS medications and previous consideration/treatment with behavioural therapy
-
stable, optimised, treatment of comorbidities
-
no medical, physical or social contraindications for DBS. 159,177,182,183
Although DBS has been performed in TS patients < 18 years of age,181 TS guidelines only recommend its use in adults. There is some variation in the lowest recommended age for DBS; patients > 25 years,182 > 20 years177 and > 18 years. 183
Repetitive transcranial magnetic stimulation
Transcranial magnetic stimulation is a non-invasive method of brain stimulation in which an electric coil is used to induce a magnetic field over the scalp. This induces circular electrical (eddy) currents in the brain that in turn cause stimulation of neurons and muscle activity. 184 Repeated stimulation with short bursts of high-frequency stimulation has been shown to be effective in inducing long-term changes in the excitability of synapses of the motor system in animal studies and rTMS has been applied for the treatment of movement disorders in humans. 184 Very high intensity rTMS can induce seizures and guidelines recommend limits on the maximum stimulation intensity. 184 The intensity of stimulation is often determined relative to a person’s resting motor threshold (minimum intensity needed to cause a response in the target muscle)184 reducing intensity settings to safe limits. The use of rTMS has mainly been focused on application to Parkinson’s disease but it has also been used for dystonia and TS. 184
Repetitive transcranial magnetic stimulation is considered to be safe159,184 and suitable for use in children. 185 Current guidelines do not recommend the use of rTMS for treatment of children with TS but only for use in the context of research studies. 159
Intravenous immunoglobulin
Intravenous immunoglobulin been proposed for the treatment of children with PANDAS. For this population, where infection is believed to trigger an autoimmune reaction that precedes the onset of symptoms, i.v. immunoglobulin may be administered to remove the infection with the aim of subsequently reducing TS-like or OCD-like symptoms. It has been proposed that infection may be implicated more widely in childhood-onset TS and OCD186 and intervention with immune modulating agents in a more general tic disorder/OCD population may be effective. 187 Owing to the risks associated with treatment, this approach is not currently recommended and its use is limited to children with associated evidence of severe encephalopathy or evidence of immune mediated disorder, such as positive antineuronal antibodies. 188
Botulinum toxin
Botulinum toxin (Botox®, Allergan) is an agent produced by the bacteria Clostridium botulinum. It has the ability to impede nerve function, resulting in reduced contraction of muscle fibres189 and has been used to induce temporary muscle weakness for the treatment of movement disorders. 190 Injections of botulinum toxin have been used to treat motor tics in people with TS, particularly those with a singular, problematic tic, or tics in a singular muscle group. 190
Botulinum toxin has been considered reasonably safe but risks may be associated with errors in dosing or injection site189 and injection into some areas may be particularly vulnerable (e.g. laryngeal and soft-palette botulinum toxin can lead to complications in swallowing). It is considered to be effective for the treatment of some movement disorders and has been recommended as a possible treatment for tics;191,192 however, recent TS guidelines give no recommendations on its use. 159,183
Acupuncture
Acupuncture is an ancient form of treatment that originated in China. Very thin metal needles are inserted into specific points on the surface of the body with the aim of improving health and well-being. 193 There is no consensus on the proposed mechanism of action. Although some propose biological mechanisms, Chinese tradition claims a non-biological role, in which acupuncture replenishes a person’s vital essence by restoration of the balance between Yin and Yang. 193 Acupuncture is used to treat a wide variety of physical and mental conditions; however, acupuncture was not assessed for recommendation in recent TS guidelines. 159,183
Included studies
The search obtained nine RCTs of physical treatments; one in children and eight studies in adults or mixed adult and child populations that were considered as supporting evidence. The types of studies are shown below:
-
rTMS compared with sham therapy (three adult studies)197–199
-
i.v. immunoglobulin compared with i.v. saline (one mixed study)187
-
botulinum toxin compared with placebo (one mixed study)200
-
acupuncture and Chinese herbs compared with haloperidol and artane (one child study). 201
In separate sections below, studies for each intervention are presented.
Deep brain stimulation
Study characteristics
No studies of DBS in children were identified. Three studies of DBS in adults were obtained and these are presented as supporting evidence (see Appendix 3, Table 11). 194–196 Participants included in these studies had severe TS with typical YGTSS total tic scores of around 40 and the majority were on medication at the time of treatment. All these studies were randomised controlled crossover trials, in which participants received both DBS and sham (control) treatments and the order of treatments was randomised.
In a 3-month study,194 participants underwent two study phases: DBS of the centromedian nucleus–substantia periventricularis–nucleus ventro-oralis internus crosspoint in the thalamus and sham treatment. In a 2-month study,195 participants underwent four study phases in a randomised crossover (n of one study) design: (i) DBS treatment with thalamic stimulation; (ii) DBS treatment with pallidal stimulation; (iii) DBS treatment with thalamic plus pallidal stimulation; and (iv) sham treatment. Each study phase was compared with YGTSS pre-operative assessment. In a 4-week randomised crossover double-bind study,196 participants underwent four study phases lasting 1 week each: (i) DBS of the thalamus on the left side; (ii) DBS of the thalamus on the right side; (iii) DBS of the thalamus on the right plus left sides; and (iv) sham treatment. Each study phase was compared with YGTSS pre-operative assessment.
Risk of bias
None of the studies reported the method of randomisation or had clear evidence of allocation concealment. In one study, owing to ethical constraints, participants appear to have been allowed to switch their order of treatments. 194 This resulted in five out of six participants undergoing the DBS before the sham treatment and only one out of six undergoing sham before DBS. The sham treatment in all studies was considered to be an appropriate control group.
Only one of the studies appeared to have conducted the study with both the participants and providers blind to the order of DBS and sham treatments. 196 In one study, participants, but not providers, appear to have been blinded195 and, in the final study194 it appears that neither participants nor providers were blind to intervention order. In one of the studies in which DBS and sham treatments were each given for a 1-week period,196 there was no washout between treatments and the risk of bias owing to cross-intervention contamination was considered high. In the other two studies, the intervention periods were reasonably long (2 months195 and 3 months194) and although there was no washout period between treatments, there may have been enough time for effects from the previous period to washout by the end of the next period. For these studies, the risk of bias owing to contamination was considered unclear.
All studies used blinded outcome assessment. The impact of dropout from some studies was unclear. In the 3-month intervention study,194 a high proportion of participants did not undergo treatment for the full treatment period. Data from the last observation were carried forward for the analysis but the risk of bias from this assumption is unclear. In the 2-month study,195 only data from participants undergoing both DBS and sham treatments were presented and it was unclear whether or not there were any dropouts. In the 1-week study,196 it is reported that all participants completed both treatments and there were no dropouts during the crossover intervention period.
Overall, one of the studies195 was considered to have an unclear risk of bias owing to the possibility of cross-treatment contamination. The remaining two studies were considered to be at high risk of bias owing to imbalance of allocation groups194 and a high risk of cross-treatment contamination. 196
Clinical evidence for deep brain stimulation
Tics
All three studies compared DBS with sham treatment and measured tic severity with the YGTSS total tic scale. However, meta-analysis was not possible and very small sample sizes (≤ 6 participants) in these studies makes any comparison between groups unreliable (all comparisons were inconclusive).
Safety
In these studies, safety outcomes are largely reported uncontrolled. That is, adverse effects are discussed in relation to treatments but no distinction is made between events owing to intervention compared with sham phases. Most adverse effects appear to be owing to the associated surgery, rather than the action of DBS.
In the study for which various DBS treatments were given over 1-week periods,196 one participant had an episode of acute psychosis at the end of the crossover phase but this was attributed to life stress and a personal and family history of psychiatric illness. No other adverse effects were reported in the study.
In the study for which participants were treated with 2 months of different DBS protocols,195 thalamic stimulation caused transient (few minutes) ‘cheiro-oral or arm parenthesis’ and pallidal stimulation caused lethargy for 3–4 days (number of participants undergoing adverse effects not reported). With increasing pallidal stimulation intensity, two participants reported having nausea and vertigo and one patient reported having anxiety. In addition, one patient reported a libido decrease when under thalamic stimulation.
In the study in which participants underwent 3 months of DBS and sham treatment, three people had adverse effects that were related to their surgery. 194 One participant had a small parenchymal haemorrhage deep at the tip of the left electrode that resulted in vertical gaze palsy. This resolved after 6 months but persistent subjective slowing of vertical fixation and pursuit on stimulation remained and led to the patient choosing to have the stimulator switched off. Another participant developed a staphylococcus aureus infection in the infraclavicular region. Another participant had symptoms including lethargy, binge eating, dysarthria, apathy, gait disturbances and frequent falls but switching off the simulator did not affect these symptoms. One year after surgery, all participants reported substantial restriction in their daily activities because of a lack of energy. Three years after surgery, one patient developed severe multidirectional nystagmus when the stimulation was turned off. When interviewed, all participants reported visual disturbances that varied from blurred vision to fixation problems but no objective abnormalities could be detected by an optometrist and neuro-ophthalmologist. Participants were tested for vestibular and oculomotor function and all showed no signs of central pathology except for one patient who showed impaired vertical gaze with normal vertical vestibular ocular reflexes, indicating a supranuclear deficit.
Clinical evidence summary for deep brain stimulation
Three small crossover RCTs (n = 3, 5 and 6)194–196 of DBS compared with sham treatment in adults with severe TS provided very low-quality evidence that could not be meaningfully interpreted.
Conclusion
It is unknown if DBS is an effective treatment in highly selected adults with severe TS. There is uncertainty regarding the most effective neuroanatomical targets for DBS. Various adverse effects have been reported, generally as a consequence of surgery rather than brain stimulation. There is no evidence available regarding the clinical effectiveness and safety of DBS in children and young people with TS.
Repetitive transcranial magnetic stimulation
Study characteristics
No studies of rTMS in children were identified. Three studies of rTMS in adults were obtained and these are presented as supporting evidence (see Appendix 3, Table 11). 197–199 All of these studies were crossover trials in which participants received both rTMS and sham treatment and the order of treatments was randomised. Participants included in these studies had moderate-to-severe TS. In one study,197 baseline YGTSS total tic score was 23 and in the other two studies, baseline YGTSS global scores (baseline total tic score not reported) were 70198 and 57. 199 Most participants were on medication at the time of treatment.
In one study,198 participants underwent five treatment phases on five consecutive days with no washout between treatments: (i) rTMS of the pre-frontal cortex at high frequency; (ii) rTMS of the pre-frontal cortex at at low frequency; (iii) rTMS of the motor cortex at high frequency; (iv) rTMS of the motor cortex at low frequency; and (v) sham treatment. All phases were compared with pre-treatment baseline. In another study,199 participants underwent treatment on two consecutive days for each of three treatment phases with a 2-week washout period between phases: (i) rTMS of the motor cortex; (ii) rTMS of the pre-motor cortex; and (iii) sham treatment. All phases were compared with pre-treatment baseline. In another study,197 participants underwent treatment on two consecutive days for each of three treatment phases with a 4-week washout period between phases: (i) rTMS of the pre-motor cortex on the left-hand side; (ii) rTMS of the pre-motor cortex on the left plus right-hand side; and (iii) sham treatment. All phases were compared with pre-treatment baseline.
Risk of bias
None of the studies reported the method of randomisation or had clear evidence of allocation concealment. The sham treatment in all studies was considered to be an appropriate control group. In all of the studies, the participants but not the providers appeared to be blinded to the order of rTMS and sham treatments. Two of the studies used washout periods between treatments197,199 but one study did not. 198 In one study, the outcome assessor was blinded to treatment order. 198 Another study also had blinded outcome assessment as outcomes were patient-rated and participants were blind to treatment order. 199 In the other study, outcomes were measured by investigators but the presence of blinded outcome assessment was not reported. 197 For all studies, it was unclear whether or not there was dropout from the studies because only data from participants undergoing both rTMS and sham treatments were presented.
Overall, one of the studies199 was considered to have an unclear risk of bias from contamination and the remaining two studies were considered to be at high risk of bias owing to contamination from inadequate washout198 and unclear risk of contamination and unclear blinded outcome assessment. 197
Clinical evidence for repetitive transcranial magnetic stimulation
Tics
Two studies used the YGTSS and reported data for results for rTMS applied at different treatment sites. 197,198 However, meta-analysis was not possible and very small sample sizes (≤ 16 participants) in these studies makes any comparison between groups unreliable (all comparisons were inconclusive).
Safety
In the rTMS study with five consecutive days of successive treatments, there were no significant differences in ratings for pain and discomfort between treatments. 198 Three headaches were reported (study does not report under which rTMS/sham treatment they occurred). In one participant, high frequency motor cortex stimulation resulted in an increase in excitability that was manifested by a progressive increase in evoked twitch and motor evoked potential amplitude. In this study, there is no comparative (rTMS compared with sham treatment) safety analysis but there was no significant change in the AIMS from baseline (mean 13.9, SD 8.8) to the end of the treatment period (mean 9.6, SD 6.8; p = 0.11). The mean motor threshold in participants did not significantly change (p = 0.12) from baseline (51.9, SD 11.4) to the end of the treatment period (50.6, SD 10.3).
The study that administered each treatment for two 20-minute sessions on two consecutive days (with a 2-week washout between treatments),199 reported that rTMS was well tolerated. One participant reported a mild headache following pre-motor rTMS and two participants reported excessive tiredness after both pre-motor and motor rTMS that lasted for about 1 day. As with the first study, the active motor threshold did not significantly change after any of the rTMS treatments.
In the study for which rTMS treatments were administered on two consecutive days (with a 4-week washout between treatments),197 findings related to adverse effects were not reported.
Clinical evidence summary for repetitive transcranial magnetic stimulation
Three small crossover RCTs (n = 5, 8 and 16)197–199 of rTMS at different treatment sites compared with sham treatment in adults with severe TS provided very low-quality evidence that could not be meaningfully interpreted.
Conclusion
It is unknown if rTMS is an effective treatment for tics in adults with severe TS. No evidence is available for the use of rTMS in children and young people with TS.
Intravenous immunoglobulin versus placebo
Study characteristics
One parallel trial investigated the efficacy of i.v. immunoglobulin in 29 children and adults with TS (90%) or CTD (10%) (see Appendix 3, Table 11). 187 The study used i.v. albumin as the control group and measured outcome at various time points from 2 to 14 weeks post intervention.
Risk of bias
The method of randomisation was not reported and there was no statement about allocation concealment. The study used a suitable control group (i.v. albumin) that allowed participants and providers to be blinded to treatment allocation and there was blinding of outcome assessors. Only data from participants completing treatment were analysed but the dropout rate was low (3%). Overall, the study was considered to have a low risk of bias.
Clinical evidence for intravenous immunoglobulin
Tics
The YGTSS total tic scale was used to assess tic severity at 2, 4, 6, 10 and 14 weeks. 187 There was variation in differences in change from baseline tic score for i.v. immunoglobulin compared with placebo at different time points (Figure 210). At 14 weeks, there was no conclusive difference in change from baseline tic score for i.v. immunoglobulin compared with placebo (SMD –0.51, 95% CI –1.25 to 0.23; n = 29).
FIGURE 210.
Standardised change from baseline YGTSS total tic score for i.v. immunoglobulin compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.

Impairment
No relevant studies reported TS-related impairment.
Psychological well-being
No relevant studies reported this outcome.
Overall clinical outcome
This study reported the proportion of participants much or very much improved on the CGI-I scale. The proportion of participants who were CGI much or very much improved for i.v. immunoglobulin compared with placebo varied at the different time points assessed (Figure 211). At 14 weeks post treatment, there was no conclusive difference in the proportion of participants who were much or very much improved for i.v. immunoglobulin compared with placebo (RR 0.77, 95% CI 0.54 to 1.09; n = 29) (the RR presented here is the RR of not being much or very much improved).
FIGURE 211.
Proportion of patients rated as much or very much improved on the TS-CGI scale for i.v. immunoglobulin compared with placebo (the RR presented here is the RR of not being much or very much improved). IVIG, i.v. immunoglobin; M–H, Mantel–Haenszel; ROB, risk of bias.

Long-term outcome
No relevant studies reported long-term outcomes.
Safety
This study compared the number of participants with adverse effects for i.v. immunoglobulin compared with i.v. saline. There was an increased rate of adverse effects for participants undergoing i.v. immunoglobulin compared with i.v. saline (RR 3.48, 95% CI 1.49 to 8.16; n = 29) (Figure 212). Adverse effects included headache (n = 11), nausea (n = 7), chills (n = 6), fever (n = 5), vomiting (n = 4) and dizziness (n = 3).
FIGURE 212.
Proportion of patients with adverse events for i.v. immunoglobulin compared with placebo. IVIG, i.v. immunoglobin; M–H, Mantel–Haenszel; ROB, risk of bias.
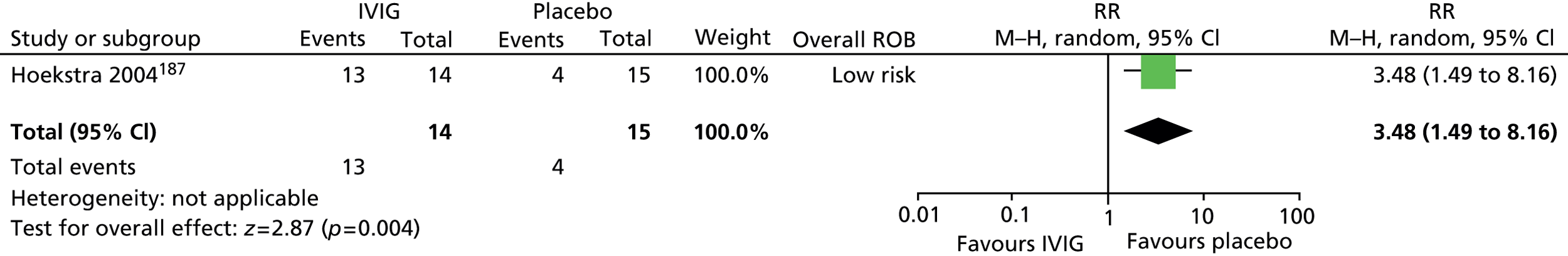
Clinical evidence summary for intravenous immunoglobulin
One parallel RCT (n = 29) in adults and children with tic disorders187 provided low-quality evidence (see Appendix 4, Table 56) for the efficacy of i.v. immunoglobulin. i.v. immunoglobulin was ineffective for the treatment of tic disorders and overall clinical outcome and was associated with an increased risk of adverse effects.
Conclusion
There is no clear evidence that i.v. immunoglobulin is an effective treatment for tics in children or adults with TS.
Botulinum toxin compared with placebo
Study characteristics
One crossover study compared the efficacy of botulinum toxin with placebo in a mixed population of 18 adults and children (age range 15–55 years, median 31.5 years) (see Appendix 3, Table 11). 200 Participants had TS (78%) or CTD (22%) of moderate severity (median YGTSS motor tic score 9, range ≈5–14). Participants were injected with botulinum toxin and placebo (substance not reported) in the area of the body affected by motor tics in a randomised order. Outcome was assessed after 2 weeks and there was an 8-week (or more) washout period between treatments.
Risk of bias
An adequate method of sequence generation was reported and there appears to have been allocation concealment of the randomisation code. The nature of the placebo was unclear but, as for the intervention, it was given as an i.v. injection and was, therefore, considered to be a suitable control. Participants, providers and outcome assessors were blinded to treatment order but, owing to the paralyses associated with botulinum toxin injections, maintenance of blinding appears unlikely. Although analysis was done only of participants completing the study, there was a low rate of dropout and bias from attrition may have been low. The primary outcome measure was reduction in treated tics. A motor tic for each patient was selected for treatment and botulinum/placebo injections were made into the relevant area of the body. The use of this outcome makes overall assessment of the efficacy of botulinum toxin on all tics difficult. In some cases, the effect on other tics was also reported but this was not done consistently across outcomes and the study was judged to be at risk of selective reporting bias. The overall risk of bias for this study was considered to be unclear owing to unclear patient, provider and outcome assessor blinding and possible selective outcome reporting bias.
Clinical evidence for botulinum toxin
Findings for this study are presented as net changes from baseline (baseline measured just before the intervention or control treatment). Because net change scores only are presented, the data for this study are not analysed as for other cross-sectional studies in this review (comparison of end points) but data are reported for net changes from pre-treatment to post treatment.
Tics
Measures of YGTSS motor tic score were reported, but only for treated tics. Three aspects of the YGTSS motor tic scale were reported (Figure 213). The data were inconclusive for reductions from baseline for botulinum toxin compared with placebo for motor tic frequency (SMD –0.14, 95% CI –0.80 to 0.51; n = 18) and interference (SMD –0.05, 95% CI –0.70 to 0.61; n = 18) but botulinum toxin was associated with greater reduction in the intensity of the treated tic compared with placebo (SMD –0.91, 95% CI –1.59 to –0.22; n = 18).
FIGURE 213.
Standardised change in YGTSS motor tic frequency, intensity and interference for treated tics for botulinum toxin compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
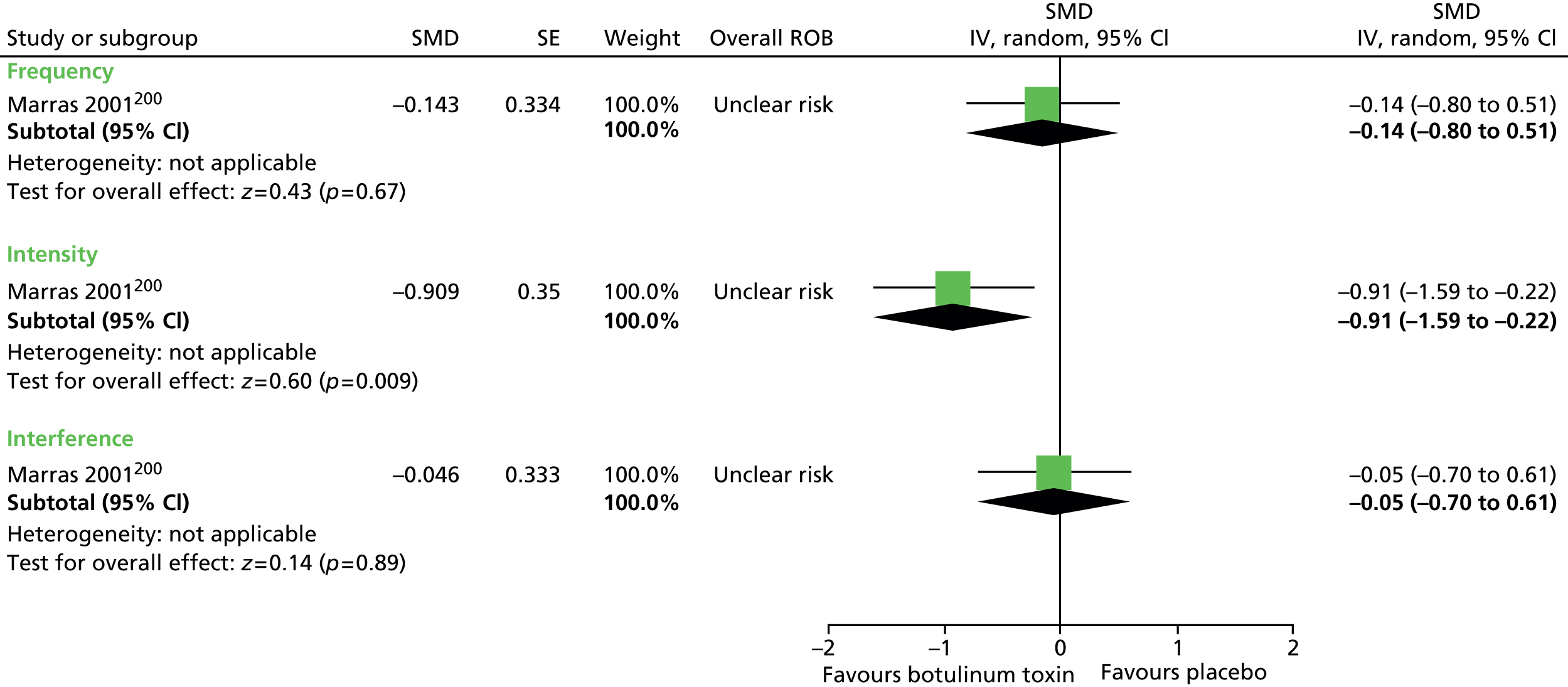
Overall tic score (both treated and untreated tics) were reported for the Shapiro TSSS. Results were inconclusive for reductions from baseline in overall tic score for botulinum toxin compared with placebo (SMD 0.02, 95% CI –0.63 to 0.67; n = 18) (Figure 214).
FIGURE 214.
Standardised change from baseline in Shapiro TSSS for all tics for botulinum toxin compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.
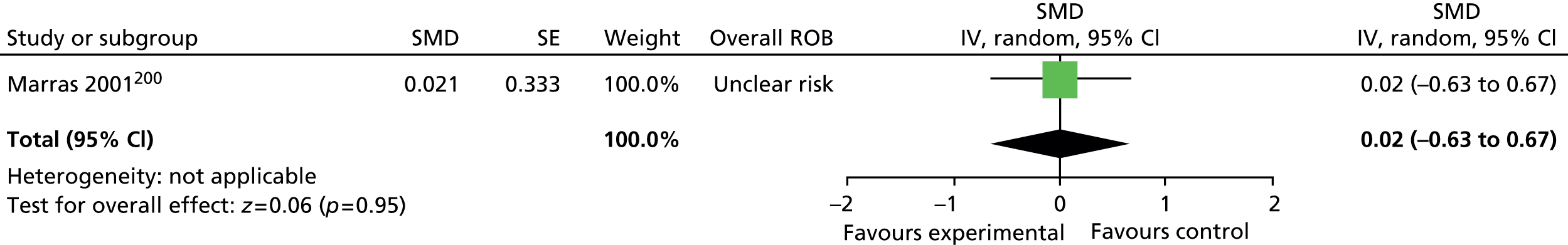
Impairment
No relevant studies reported TS-related impairment.
Psychological well-being
No relevant studies reported this outcome.
Overall clinical outcome
The authors asked patients to rule if their tics were ‘better’, ‘unchanged’ or ‘worse’. The authors called this outcome variable “patients’ global impressions of change”. There was no conclusive difference in the proportion of participants feeling better (RR 0.82, 95% CI 0.63 to 1.08; n = 18) (the RR presented here is the RR of not feeling better), unchanged or worse for botulinum toxin compared with placebo (Figure 215).
FIGURE 215.
Proportion of patients feeling better, unchanged or worse for botulinum toxin compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.
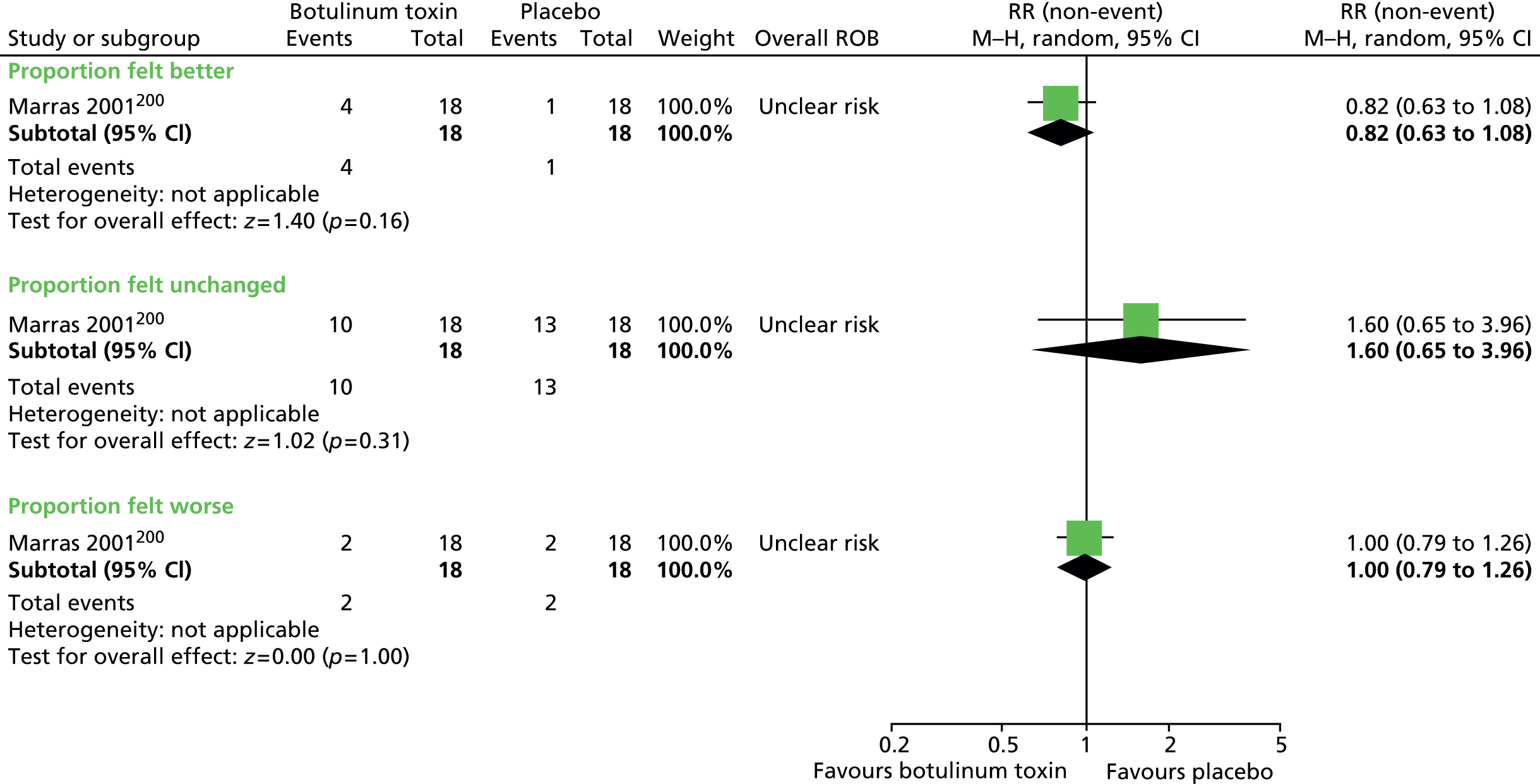
Long-term outcome
No relevant studies reported long-term outcomes.
Safety
Pain score for the treated tic area (scale not reported) was greater for botulinum toxin than for placebo, but the effect was inconclusive (SMD 0.52, 95% CI –0.15 to 1.18; n = 18) (Figure 216).
FIGURE 216.
Standardised pain score for treated tics for botulinum toxin compared with placebo. IV, independent variable; ROB, risk of bias; SE, standard error.

There was no conclusive difference for other adverse effects (Figure 217), but there was increased weakness in the injected muscle (RR 6.00, 95% CI 1.56 to 23.07; n = 18) for botulinum toxin than for placebo.
FIGURE 217.
Proportion of patients experiencing adverse events for botulinum toxin compared with placebo injections. M–H, Mantel–Haenszel; ROB, risk of bias.


Clinical evidence summary for botulinum toxin
One crossover RCT (n = 18) in children and adults with tic disorders200 provided very low-quality evidence (see Appendix 4, Table 57) for the efficacy of botulinum toxin. When the effect on all tics was considered (not just treated tics), there was no improvement for botulinum toxin compared with placebo. For the treated motor tic, there was no difference in YGTSS motor tic frequency or interference but there was a large improvement in tic intensity and there was a tendency to greater patient-reported improvement. Botulinum toxin was associated with increased rates of adverse effects compared with placebo, particularly an increased likelihood of muscle weakness in the injected area and there was a tendency towards increased pain score.
Conclusion
Botulinum toxin may decrease the intensity of tics in the local area treated but there is no evidence of reduced tic frequency or improvements in untreated tics. Adverse effects, including reports of local muscle weakness and pain, may reduce the acceptability of this intervention.
Acupuncture and Chinese herbs
Study characteristics
One randomised parallel study in 60 children (4–20 years) with TS investigated the combined use of acupuncture and Chinese herbs compared with a combination of haloperidol and artane (trihexyphenidyl hydrochloride) (see Appendix 3, Table 11). 201 Children were randomised to receive 20 half-hour sessions of acupuncture and Chinese herbs (dosage not reported) or to haloperidol at a maximum dose of 1.5–8.0 mg/day with artane of the same dosage given to alleviate adverse effects (length of intervention period not reported).
Risk of bias
The study reports no methods for random sequence generation or allocation concealment and there is no statement about blinding of participants, providers or outcome assessors. It is unclear whether or not the control group, haloperidol and artane, is suitable. Some participants in the study were young children (minimum age 4 years) and maximum doses of 1.5–8.0 mg/day were used (starting dose for 4 year olds 0.5 mg/day, for 5 year olds 1 mg twice a day). Artane of the same dosage was given to decrease adverse effects but it is unclear if this type of treatment strategy would be beneficial and if this control group provides a reasonable group for comparison. Attrition from the study is not reported and it is unclear whether or not there were any dropouts. For outcome measurements, only the YGTSS score is reported and it is unclear whether or not this was selectively reported in favour of other measurements if they were made. Overall, this study was considered to be at high risk of bias.
Clinical evidence for acupuncture and Chinese herbs
Tics
Within-person changes in tics/impairment were presented as the proportion of children with ≥ 30% and ≥ 60% reductions from baseline in YGTSS global score. There was a greater proportion of children showing ≥ 60% reductions from baseline for acupuncture and Chinese herbs compared with haloperidol and artane (RR 0.62, 95% CI 0.39 to 0.99; n = 60) (Figure 218) (RRs are for relative risk of not achieving 30 or 60% reductions from baseline) but no conclusive difference in the proportion of children showing ≥ 30% reductions (RR 0.29, 95% CI 0.06 to 1.26; n = 60).
FIGURE 218.
Proportion of children with ≥ 30% and ≥ 60% reductions from baseline in YGTSS global score for acupuncture and Chinese herbs compared with haloperidol and artane (the RR presented here is the RR of not achieving 30 or 60% reductions from baseline). M–H, Mantel–Haenszel; ROB, risk of bias.
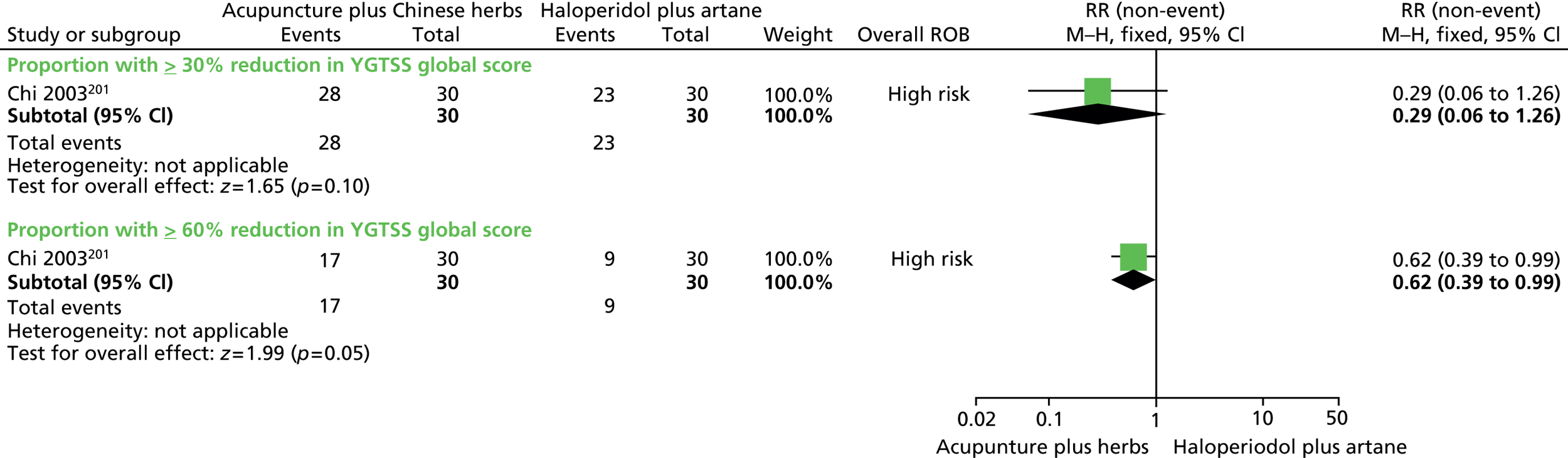
Impairment
Impairment score was reported as part of the YGTSS global score as described under Tics above.
Psychological well-being
No relevant studies reported this outcome.
Overall clinical outcome
No relevant studies reported this outcome.
Long-term outcome
No relevant studies reported long-term outcomes.
Safety
No relevant studies reported this outcome.
Clinical evidence summary for acupuncture and Chinese herbs
One parallel RCT (n = 60) in children with TS201 provided very low-quality evidence (see Appendix 4, Table 58) for the efficacy of a combination of acupuncture and Chinese herbs compared with a combination of haloperidol and artane (described as western medicine). The study showed some evidence of a benefit of acupuncture and Chinese herbs compared with haloperidol and artane for the treatment of tics.
Conclusion
Acupuncture may be an effective treatment for tics in children and young people with TS. However, the limitations in the quality of the available evidence and lack of information on adverse effects do not allow firm conclusions to be drawn.
Overall conclusions from physical intervention trials
It is unknown if DBS is a useful treatment for selected adults with severe TS. Future parallel trials may provide better evidence on which to base judgements. Studies in children have yet to be conducted, but the invasive nature of treatment is likely to make DBS an unacceptable intervention for children and young people with TS except in very exceptional circumstances.
Currently, there is no clear evidence that rTMS is an effective treatment for tics and the predominance of crossover trials with the potential for carry-over effects makes it difficult to drawn any firm conclusions. Future parallel trials may provide better evidence on which to base judgements. Although rTMS is a less invasive a procedure than DBS, there remains uncertainty over its safety and suitability for use in children and young people.
Overall, there is no clear evidence that i.v. immunoglobulin is an effective treatment for tics in children and young people with TS.
Botulinum toxin may reduce intensity of treated tics; however, the low quality of evidence makes it difficult to draw firm conclusions regarding benefits. Furthermore, reports of adverse effects mean that botulinum toxin may not be a suitable treatment for tics in children and young people with TS.
The evidence of benefit for acupuncture and Chinese herbs is of extremely low quality and, therefore, there remains uncertainty about the effectiveness of this intervention.
In summary, there is currently no evidence to suggest that the physical interventions reviewed (DBS, rTMS, i.v. immunoglobulin, botulinum toxin and acupuncture) are sufficiently effective and safe to be considered as treatments for tics in children and young people with TS.
Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection
Introduction
Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection describes disorders that have been identified in pre-pubescent children who are thought to have developed OCD, a tic disorder, or both, that is associated with a group A streptococcal infection. PANDAS has been defined by temporal association (infection precedes symptoms), dramatic symptom onset, infection-related exacerbations and concurrent neurological abnormalities during exacerbations. 202 However, the concept of PANDAS remains controversial. 203
The PANDAS population does not fall under the population set out in the review protocol (children and young people with TS or CTD) as some PANDAS-defined children do not experience tics (only OCD symptoms). The proposed cause and aetiology of illness is also different from that of childhood tic disorders and this population would not necessarily be included in this review. However, the question of whether or not supposed PANDAS can be successfully treated was considered to be a clinically relevant and important question and these studies have therefore been included in the review. Their findings are not applicable to the general child tic disorder population and vice versa.
Included studies
The studies identified use different intervention strategies but have the same underlying principle of intervention: treatment of streptococcal infection and or the removal of streptococcus A immunoglobulin that is hypothesised to bring about PANDAS symptoms. Three studies in children were identified:
Intravenous immunoglobulin and plasma exchange versus placebo
Study characteristics
One study investigated the efficacy of i.v. immunoglobulin and plasma exchange in children with PANDAS (see Appendix 3, Table 12). 204 Twenty-nine children defined as having PANDAS [tic disorder, OCD (or both) onset before puberty, history of sudden onset or episodic course, evidence of association with streptococcal infection] and with a current severe exacerbation were randomised to i.v. immunoglobulin, plasma exchange or i.v. saline (placebo). i.v. immunoglobulin was given at 1 g/kg body weight and administered on two consecutive days. i.v. saline was also administered on two consecutive days with a similar volume to that as for i.v. immunoglobulin. Children undergoing i.v. immunoglobulin or i.v. saline were given diphenhydramine and paracetamol to alleviate adverse effects of treatment. Children undergoing plasma exchange had treatment consisting of five to six procedures conducted over 10–12 days (treatment on alternative days) with one plasma volume (45 ml/kg body weight) exchanged during each procedure. Children in all treatment arms were followed up at 1 month. After this point, children originally allocated to the placebo group were started on i.v. saline or plasma exchange and, together with children originally allocated to those groups, they were followed up at 1 year.
Risk of bias
The method of randomisation was reported but there was no clear evidence of allocation concealment and groups were markedly different at baseline, with mean tic scores of 6.8 (SD 9.2), 11.0 (SD 9.5) and 21.7 (SD 14.7) for i.v. immunoglobulin, placebo and plasma exchange, respectively. There was a suitable control group for comparison with i.v. immunoglobulin (i.v. saline) but this was not a suitable control for the plasma exchange intervention as this has a different mode of administration and, for this treatment arm, participants and providers could not be blinded to treatment allocation. It was unclear if outcome assessment was blinded for i.v. immunoglobulin or plasma exchange groups. Both studies analysed only data from participants who had completed the treatments but the dropout rate was low (3%).
Overall, the study results were considered to be at high risk of bias for i.v. immunoglobulin owing to imbalances at baseline and unclear blinded outcome assessment and to be at particularly high risk of bias for plasma exchange owing to baseline imbalances, lack of patient and provider blinding and unclear blinded outcome assessment.
Clinical evidence for intravenous immunoglobulin and plasma exchange
Tics
The effect of i.v. immunoglobulin and plasma exchange on the severity of tics was measured using the UTRS. 204 For i.v. immunoglobulin, there was the same change from baseline tic score for i.v. immunoglobulin compared with i.v. saline (SMD 0.00, 95% CI –0.90 to 0.90; n = 19). For plasma exchange, there was a greater but inconclusive reduction from baseline for plasma exchange compared with i.v. saline (SMD –0.88, 95% CI –1.81 to 0.05; n = 20) (Figure 219).
FIGURE 219.
Standardised change from baseline TS unified rating scale score for children undergoing i.v. immunoglobulin and plasma exchange compared with control (i.v. saline). IV, independent variable; ROB, risk of bias.
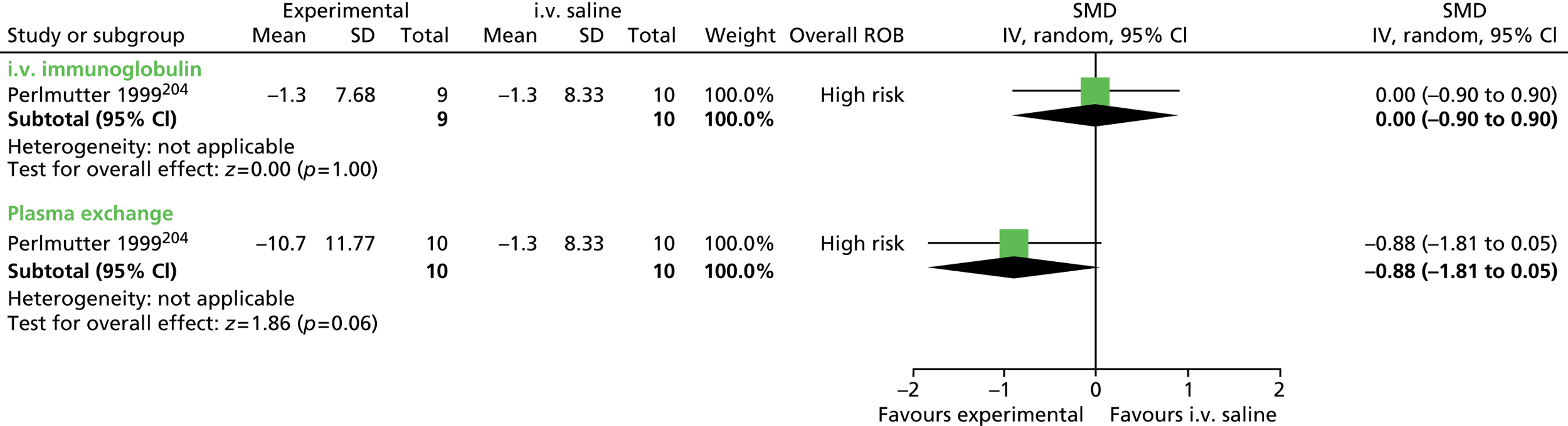
Impairment
The study measured changes in impairment using the National Institute for Mental Health (NIMH) global impairment scale. Compared with those undergoing i.v. saline, there were greater reductions in impairment score for participants undergoing i.v. immunoglobulin (SMD –1.87, CI –3.00 to –0.75; n = 19) and plasma exchange (SMD –1.42, 95% CI –2.42 to –0.41; n = 20) (Figure 220).
FIGURE 220.
Standardised change from baseline NIMH global impairment score for children undergoing i.v. immunoglobulin or plasma exchange compared with control (i.v. saline). IV, independent variable; ROB, risk of bias.
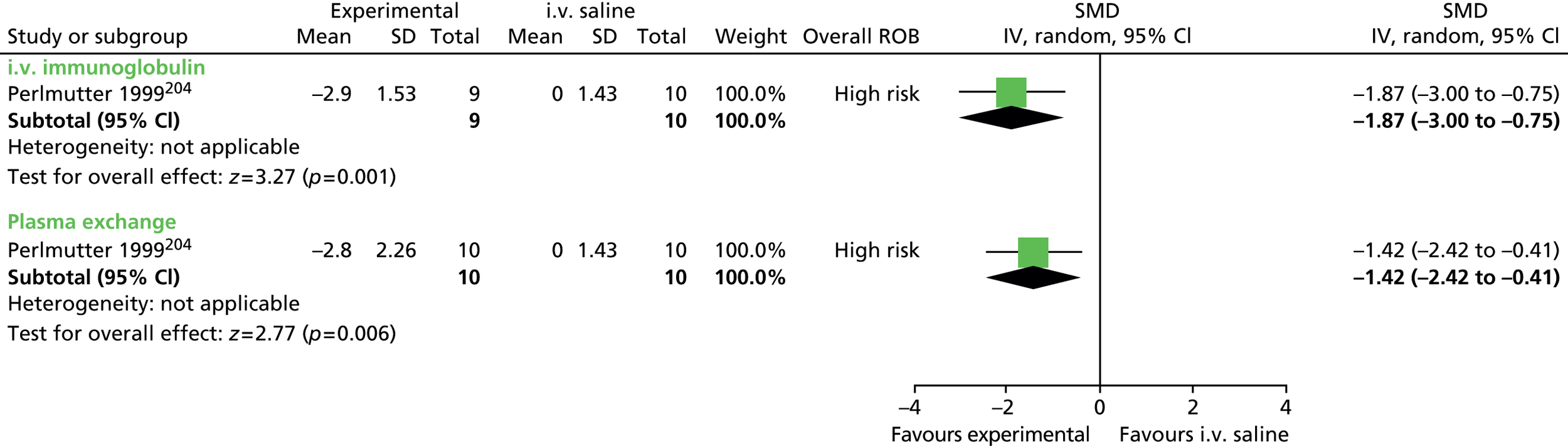
Emotional well-being
Changes in anxiety were measured using the NIMH anxiety scale. Compared with i.v. saline, there were greater reductions in anxiety score for i.v. immunoglobulin (SMD –1.03, CI –2.00 to –0.05; n = 19) and plasma exchange (SMD –1.24, 95% CI –2.21 to –0.26; n = 20) (Figure 221).
FIGURE 221.
Standardised change from baseline NIMH anxiety score for children undergoing i.v. immunoglobulin and plasma exchange compared with control (i.v. saline). IV, independent variable; ROB, risk of bias.
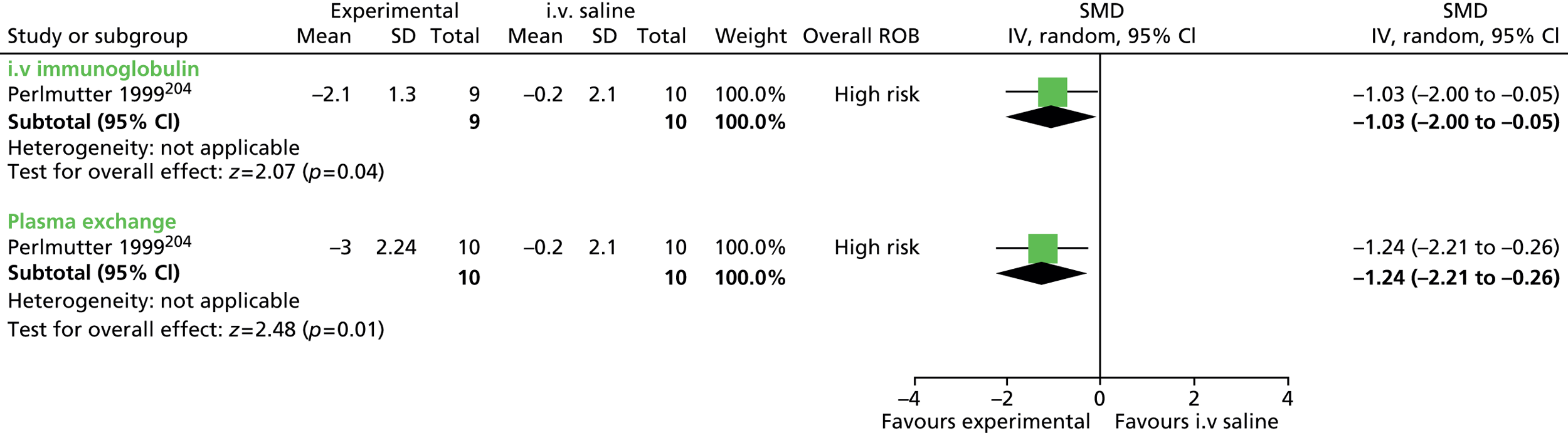
Changes in depression were reported using the NIMH depression scale. Plasma exchange showed an improvement in depression score than for i.v. saline (SMD –1.06, 95% CI –2.00 to –0.11; n = 20) (Figure 222), but i.v. immunoglobulin was not conclusively different (SMD –0.64, CI –1.57 to 0.29; n = 19).
FIGURE 222.
Standardised change from baseline NIMH depression score for children undergoing i.v. immunoglobulin or plasma exchange compared with control (i.v. saline). IV, independent variable; ROB, risk of bias.

Overall clinical outcome
Overall clinical outcome was measured with the CGI scale and results are presented as improvement scores (1 = very much improved, 7 = very much worse). CGI-I score was better for i.v. immunoglobulin (SMD –1.86, 95% CI –2.98 to –0.74; n = 19) and plasma exchange (SMD –2.38, 95% CI –3.58 to –1.18; n = 20) than for i.v. saline (Figure 223).
FIGURE 223.
Standardised CGI-I rating for children undergoing i.v. immunoglobulin or plasma exchange compared with control (i.v. saline). IV, independent variable; ROB, risk of bias.
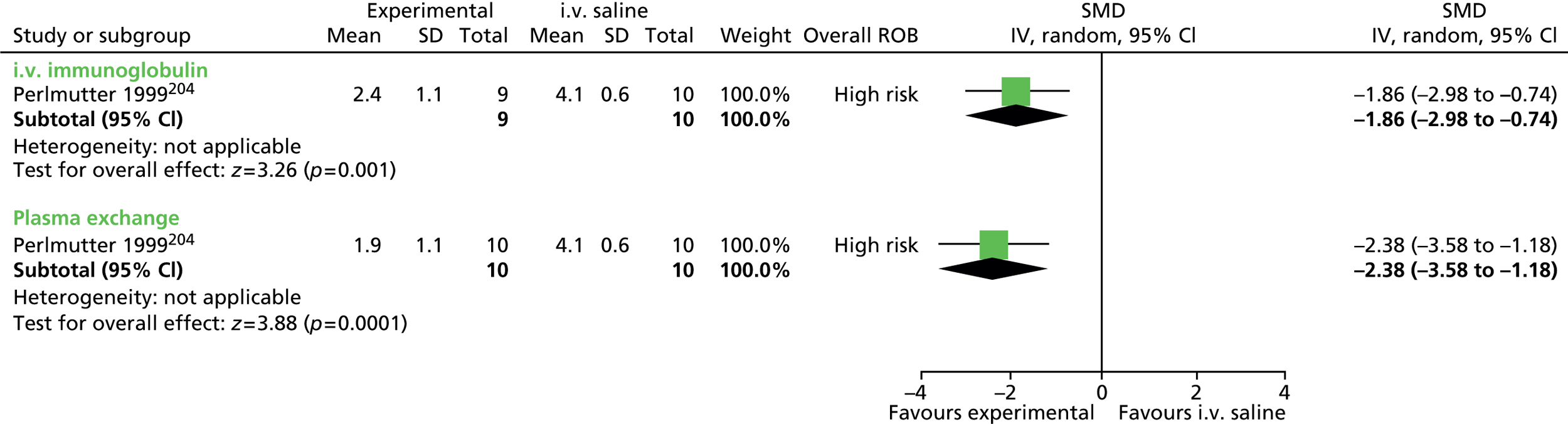
Long-term outcome
Children were followed up at 1 year but, following the controlled trial period, blinding was broken and children who had received placebo were offered treatment with an intervention. At 1 year, mean scores were similar to post-treatment levels for the UTRS for i.v. immunoglobulin [5.5 (SD 7.7) and 5.8 (SD 8.7) for 1 month and 1 year, respectively] and plasma exchange [11.0 (SD 9.2) and 8.9 (SD 9.6) for 1 month and 1 year, respectively]. The mean CGI-I score was also similar to post-treatment scores for i.v. immunoglobulin [2.4 (SD 1.1) and 2.3 (SD 1.1) for 1 month and 1 year, respectively] and plasma exchange [1.9 (SD 1.1) and 1.75 (SD 0.9) for 1 month and 1 year, respectively].
Safety
The numbers of children experiencing adverse effects for the active intervention compared with i.v. saline were compared. There were higher rates of adverse effects but the difference was not conclusive for children undergoing i.v. immunoglobulin (RR 3.33, 95% CI 0.89 to 12.51; n = 19) or plasma exchange (RR 3.50, 95% CI 0.95 to 12.90; n = 20) than for i.v. saline (Figure 224). Adverse effects in the i.v. immunoglobulin group included nausea and vomiting (n = 5), mild to moderately severe headache (n = 3), and low-grade fever (n = 4). In the plasma exchange group, adverse effects included pallor, dizziness and nausea (n = 7), vomiting (n = 2) and anxiousness (n = 3).
FIGURE 224.
Proportion of children experiencing adverse events for i.v. immunoglobulin and plasma exchange compared with control (i.v. saline). M–H, Mantel–Haenszel; ROB, risk of bias.

Penicillin versus placebo
Study characteristics
One crossover study compared penicillin prophylaxis with placebo in children assessed as having PANDAS. 205 Thirty-seven children were assigned to receive 4 months of penicillin (250 mg tablets twice a day) and 4 months of placebo (tablets twice daily) in a randomised order.
Risk of bias
For this study, no methods of sequence generation or allocation concealment were reported, although baseline characteristics for children randomised to each intervention order were similar. Participants were blinded to treatment order but blinding of treatment providers was unclear. There was no statement about blinding of outcome assessors and the risk of assessment bias is unclear. There was no washout period between treatments and there may have been some risk of cross-treatment contamination. Analysis was restricted to children completing the study but there was a low rate of dropout and the risk of attrition bias may be low. Overall, this study was considered to be at unclear risk of bias owing to the presence of unclear blinded outcome assessment.
Clinical evidence for penicillin
Tics
Tics were assessed with the YGTSS. There was no conclusive difference in changes in total tic score for penicillin compared with placebo (SMD –0.26, 95% CI –0.72 to 0.21; n = 37) (Figure 225).
FIGURE 225.
Standardised post-treatment YGTSS total tic score for penicillin compared with placebo. IV, independent variable; SE, standard error.

Scores were also similar when motor (SMD –0.14, 95% CI –0.61 to 0.32; n = 37) and vocal (SMD –0.23, 95% CI –0.69 to 0.24; n = 37) tic scores were considered separately (Figures 226 and 227).
FIGURE 226.
Standardised post-treatment YGTSS motor tic score for penicillin compared with placebo. IV, independent variable; SE, standard error.
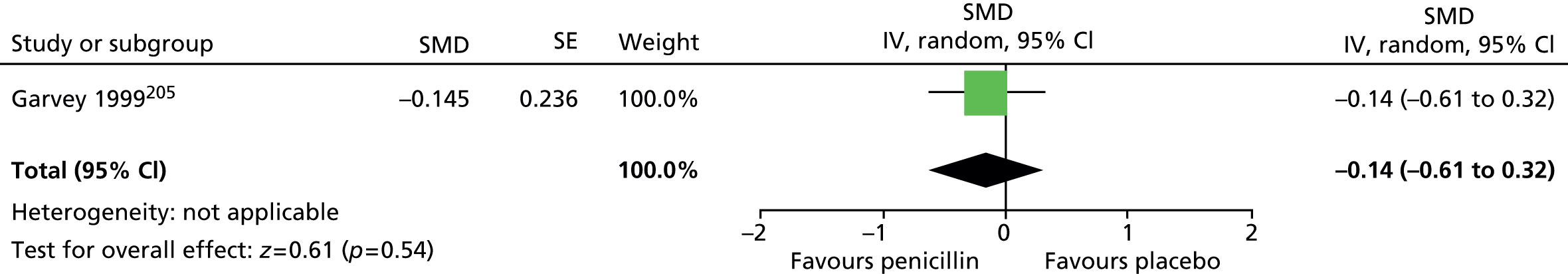
FIGURE 227.
Standardised post-treatment YGTSS vocal tic score for penicillin compared with placebo. IV, independent variable; SE, standard error.
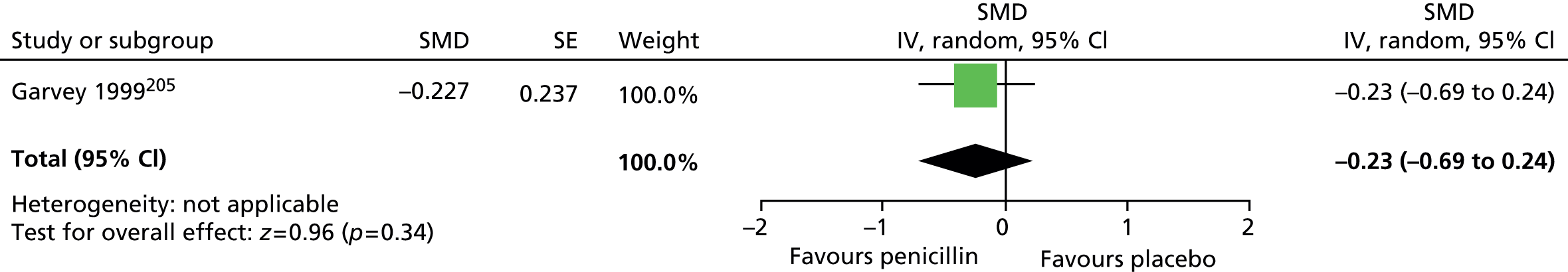
Rates of streptococcal infections were not conclusively different for the placebo than for the penicillin group (RR 0.70, 95% CI 0.43 to 1.15; n = 37) (Figure 228), as were rates of neuropsychiatric exacerbations (RR 1.00, 95% CI 0.64 to 1.57; n = 37) (Figure 229).
FIGURE 228.
Rate of streptococcal infection penicillin compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.

FIGURE 229.
Rate of neuropsychiatric exacerbations for penicillin compared with placebo. M–H, Mantel–Haenszel; ROB, risk of bias.
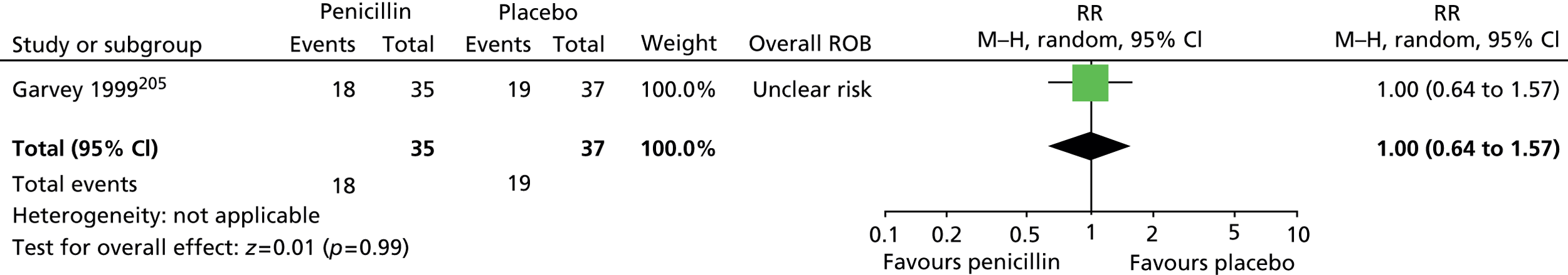
Impairment
Impairment was measured using the C-GAS. Post-treatment impairment score was inconclusive following penicillin compared with placebo (SMD –0.20, 95% CI –0.66 to 0.27; n = 37) (Figure 230).
FIGURE 230.
Standardised post-treatment C-GAS scores for penicillin compared with placebo. IV, independent variable; SE, standard error.

Psychological well-being
Anxiety was measured using the NIMH anxiety scale. Anxiety scores favoured penicillin over placebo (SMD –0.52, 95% CI –0.99 to –0.05; n = 37) (Figure 231).
FIGURE 231.
Standardised post-treatment NIMH anxiety scores for penicillin compared with placebo. IV, independent variable; SE, standard error.

Depression was measured using the NIMH depression scale. Depression scores favoured penicillin over placebo (SMD –0.62, 95% CI –1.10 to –0.15; n = 37) (Figure 232).
FIGURE 232.
Standardised post-treatment NIMH depression scores for penicillin compared with placebo. IV, independent variable; SE, standard error.
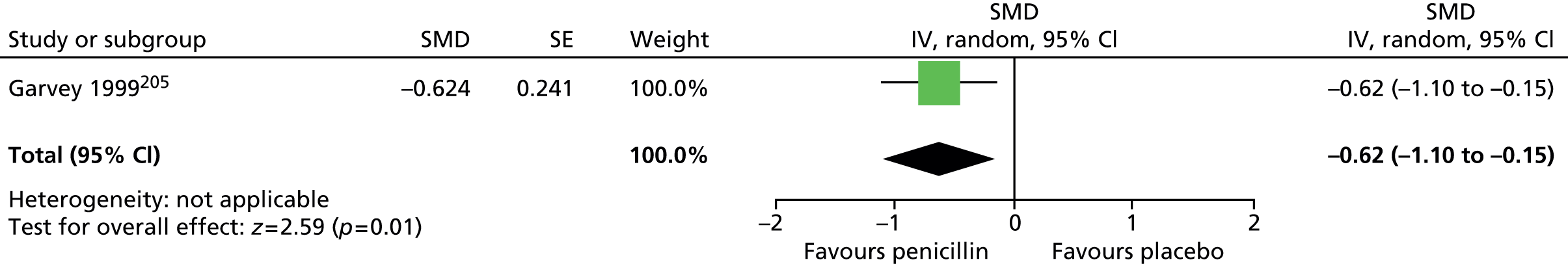
Overall clinical outcome
Overall clinical outcome was assessed with the CGI-I. CGI-I score was similar for overall global improvement (SMD –0.07, 95% CI –0.53 to 0.39; n = 37) (Figure 233) and for CGI TS-specific improvement score (SMD –0.01, 95% CI –0.47 to 0.45; n = 37) for penicillin compared with placebo (Figure 234).
FIGURE 233.
Standardised post-treatment CGI-I score for children following 4 months of treatment with penicillin compared with placebo. IV, independent variable; SE, standard error.

FIGURE 234.
Standardised post-treatment CGI-TS improvement score for penicillin compared with placebo. IV, independent variable; SE, standard error.
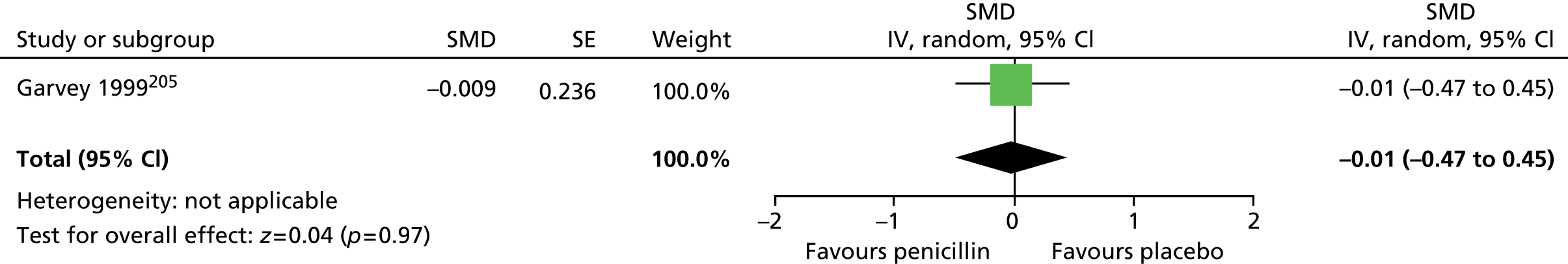
Long-term outcome
No relevant studies reported long-term outcomes.
Safety
No relevant studies reported this outcome.
Penicillin compared with azithromycin
Study characteristics
One study compared the efficacy of penicillin with azithromycin in children assessed as having PANDAS (see Appendix 3, Table 12). 206 Children were randomised to 12 months of penicillin or azithromycin and tablets were taken twice on one day a week (placebo tablets taken on the other days). Children were aged from 5–10 years and comorbid disease included ADHD (26%) and OCD (69%).
Risk of bias
For this study, methods of sequence generation and allocation concealment were not reported. Participants and providers appear to have been blinded throughout the study period but blinding of the outcome assessors is unclear. Although analysis was done only of children completing the study, there was a low rate of dropout and bias from attrition may have been low. During the intervention period, the YGTSS and the Yale–Brown Obsessive–Compulsive Scale were used by investigators to monitor participants. These results are not reported in the publication and the study was therefore considered at high risk of selective outcome reporting bias. Overall, this study was considered to be at high risk of bias owing to unclear blinded outcome assessment and potential selective outcome reporting bias.
Clinical evidence for penicillin compared with azithromycin
Unlike for the analysis of other parallel-group trials in this review (for which change from baseline is assessed), for this study, because the event rates at follow-up were very small compared with the between-group differences at baseline, results are analysed as differences at end point.
Tics
No measurement of tic outcomes were reported but investigators measured the number of neuropsychiatric symptom exacerbations. These were defined as an exacerbation of tic or OCD symptoms and were based on child/parent reports obtained at the end of the year. There was no conclusive difference in rates of reported exacerbations for the penicillin compared with the azithromycin group (SMD –0.77, 95% CI –1.63 to 0.08; n = 23) (Figure 235).
FIGURE 235.
Standardised mean number of post-treatment neuropsychiatric symptom exacerbations for penicillin compared with azithromycin. IV, independent variable; ROB, risk of bias.

The number of streptococcal infections over the study year was measured. There was no conclusive difference in the mean number of streptococcal infections per child in the year of study for those treated with penicillin compared with azithromycin (SMD 0.00, 95% CI –0.82 to 0.82; n = 23) (Figure 236).
FIGURE 236.
Standardised mean number of post-treatment streptococcal infections for penicillin compared with azithromycin. IV, independent variable; ROB, risk of bias.
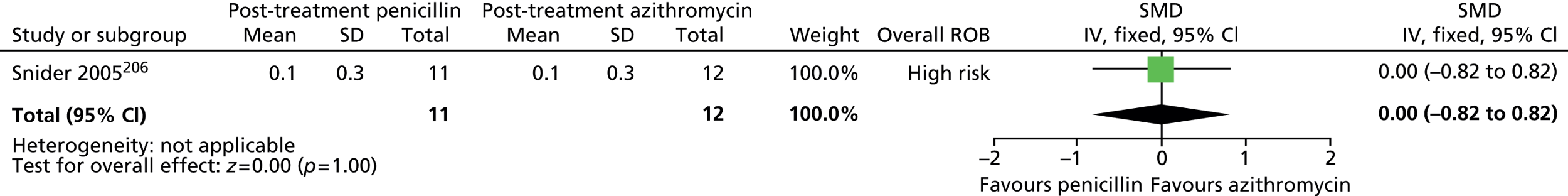
Impairment
No relevant studies reported TS-related impairment.
Psychological well-being
No relevant studies reported this outcome.
Overall clinical outcome
No relevant studies reported this outcome.
Long-term outcome
No relevant studies reported long-term outcomes.
Safety
No relevant studies reported this outcome.
Summary of Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection trials
Intravenous immunoglobulin
One study in 19 children defined as having PANDAS204 provided very low-quality evidence (see Appendix 4, Table 59) of no conclusive benefit of i.v. immunoglobulin compared with i.v. saline on tics, but there was a large effect in terms of impairment, symptoms of anxiety and depression and improved overall clinical outcome. There was a tendency towards increased rates of adverse effects of treatment.
Plasma exchange
One study in 20 children defined as having PANDAS204 provided very low-quality evidence (see Appendix 4, Table 60) of possible benefits for plasma exchange compared with i.v. saline on tic symptoms, impairment, symptoms of anxiety and depression and overall clinical outcome. Plasma exchange was associated with increased rates of adverse effects.
Penicillin
One study investigated the use of penicillin compared with placebo to treat 72 children defined as having PANDAS. 205 The study provided low-quality evidence (see Appendix 4, Table 61) of no effect of penicillin on neuropsychological exacerbations, tics or symptoms of anxiety and depression. No findings for adverse effects were reported by this study.
One study compared the use of penicillin with azithromycin for treating 23 children defined as having PANDAS. 206 The study provided low-quality evidence (see Appendix 4, Table 62) of no conclusive difference in the rates of streptococcal infections, but there was lower rates of neuropsychological exacerbations for penicillin.
Overall conclusions from Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection trials
There is no clear evidence that i.v. immunoglobulin is an effective treatment of tics in children and young people identified with PANDAS. Plasma exchange may be an effective treatment of tics in children and young people identified with PANDAS. However, the low-quality of the available evidence means that there is a high degree of uncertainty with respect to the benefits and harms of plasma exchange in this population. Finally, there is no clear evidence that penicillin is an effective treatment for tics in children and young people with PANDAS.
Chapter 3 Qualitative Study of Experiences of Services and Treatments
Very little is known about the views of young people with TS and their parents on their experiences of treatment and what outcomes they value most. The QuEST aims to capture qualitative and descriptive data on young people’s experiences of treatment and to evaluate the outcomes that are important to parents and young people. Three sources of information will be used: (1) systematic review of qualitative and descriptive studies examining experience of treatment; (2) survey of parents of children and young people with TS; and (3) in-depth interviews of children and young people with TS. A systematic review of qualitative and descriptive studies examining health professionals’ parents’ and children’s experience of treatment for TS will summarise and synthesise the extent of existing knowledge. As we know that few studies have specifically explored perceived clinical effectiveness of treatment, we will collect primary data from parents of children with TS using an online survey.
A survey methodology was considered appropriate and pragmatic to collect data from a large sample of parents of children with a wide range of ages. Parents of children with chronic conditions are key drivers of adherence to treatment for children with chronic conditions207 and are able to give a longitudinal perspective. A combination of structured questions and text boxes gave flexibility and allowed us to probe important issues in more depth while retaining the advantages of an online survey.
The views of children aged 10–17 years were explored using in-depth interviews.
Semistructured interviews were felt to be most appropriate method to capture the views of young people because, apart from enhancing the richness of data, we could be sure that the information was from the young person himself or herself and that communication was not constrained by their level of literacy. Interviews have the flexibility to prompt and encourage responses when participants may lack confidence or are reflecting on more challenging issues.
The results are presented separately for each study and the findings are synthesised in the discussion to address the issues of experience of access to care and the process of diagnosis, experiences of treatment and treatment expectations.
Systematic review of qualitative studies
Methods
Search strategy
In addition to the searches undertaken for intervention studies, additional searching was generated for qualitative studies and survey literature in the following databases: major bibliographic – EMBASE, MEDLINE, PREMEDLINE In-Process & Other Non-Indexed Citations and PsycINFO; education databases – AEI, BREI and ERIC; social care databases – ASSIA, IBSS, SSCI, SSA, Sociological Abstracts and Web of Science; grey literature databases – HMIC, PsycBOOKS, PsycEXTRA; and additional sources – BCI, CINAHL. Search terms comprised text word and subject headings for tic* and tourette* to identify populations with TS or with chronic phonic or motor tics (all referred to as TS in this review). Searches were limited to qualitative systematic reviews, qualitative primary studies and survey literature and generated from 1995 to January 2013. No language restrictions were applied at the searching stage. Search results for the intervention searches were also screened for any literature relevant to the review. The search strategy is provided in Appendix 1.
Selection of studies
Study selection consisted, first, of screening the titles and abstracts of all citations obtained from the searches. For articles that appeared relevant on screening, or for articles that had insufficient information in the title or abstract to determine their relevance, full-paper copies were obtained. These papers were then screened thoroughly, using pre-defined inclusion/exclusion criteria to determine the final set of included studies.
Inclusion/exclusion criteria
Full-paper articles were screened for inclusion by one reviewer, with reference to a second reviewer in cases of uncertainty around their eligibility. The selection criteria used for inclusion of studies into this part of the review were based on the study population and outcomes.
Population
Included studies were to have been conducted in populations of children/young people with TS or their carers or in health professionals with experience of treating TS.
Outcomes
Included studies were to have examined the views of patients or carers about one or more of the interventions (medication or diet, behavioural/psychological or psychosocial, physical) or to provide information on patient/carer/health professional experiences of health-care services for TS. Studies of the following were considered for inclusion:
-
attitudes, or perceived barriers, towards the use of interventions
-
experiences of access to care
-
experiences of assessment and diagnosis
-
experiences of treatment services and community care
-
interventions for improving service user experience.
Excluded studies
Studies of non-health-care experiences of TS patients were excluded. For example, studies on the experiences of bullying or alienation, school performance or self-esteem of children with TS were excluded. Studies of patient anxiety and stress were also excluded unless these emotions were in relation to, or as a result of, their care or treatments. Although the review included studies of patient access to care, it did not aim to review current treatment practices and studies of intervention usage, such as the prevalence of different medication use, were excluded from the review. In addition, although studies of interventions aimed at improving care for TS were eligible for inclusion, descriptions of current care projects, with no assessment of change in patient experience, were not.
Data synthesis
As there was only one qualitative study identified208 and surveys presented different types of outcomes, neither formal qualitative or quantitative synthesis was attempted. However, results are presented under theme headings, to group information from different studies on similar outcomes or themes. For the qualitative study, relevant themes highlighted by study authors are presented. Related participant quotations are presented to illustrate the themes but no exploration or synthesis of the original quotes from the primary study was performed.
Results
The searches identified 6345 citations (5434 from the RCT/systematic review/observational study and grey literature search and 911 from the qualitative/survey search). On title and abstract screening, 14 were selected and, on full-paper review, four studies were considered as relevant to some aspect of the review and included. Ten studies were excluded on full-paper review and details of these are given in Appendix 6.
Included studies
The characteristics of the four included studies are given in Appendix 3, Table 13. Three were surveys of TS patients and their carers and one was a qualitative study of health professionals and TS patients and their relatives. Two studies208,209 included information on patient/parent experiences of the diagnostic process, one on parents’ concerns about the adverse effects of antipsychotic drugs210 and one on parents’ perceived barriers to their child undergoing treatment for TS and also, specifically, to undergoing behavioural therapy. 211
Mol Debes et al. ,209 examined the TS diagnostic process by conducting a structured interview on 314 children with TS and their parents attending a TS clinic in Denmark. The survey included questions about the professionals consulted prior to diagnosis and the professional who made the final TS diagnosis.
Rivera-Navarro et al. 208 also investigated the adequacy of TS diagnosis in a qualitative study with five focus groups: (1) five doctors, (2) six young people with TS, (3) six adults with TS, (4) six parents of young people with TS and (5) six parents of adults with TS. Focus groups used an initiating question but, after that, they were unstructured and topics were guided by the experiences of participants. For doctors, the initiating question related to their perception of the main problems encountered in TS while for patients, it related to the support provided by health professionals and, for relatives, it related to their opinion of the Spanish social and medical system.
Kompoliti et al. 210 surveyed 100 consecutive TS adult patients and the parents of TS child patients attending a TS tertiary referral clinic in the USA. The study aimed to assess the use of antipsychotic drugs for TS and the level of awareness and concern about their adverse effects. For the measurement of relative concern, subjects were given a list of nine adverse effects and were asked to rate their concern from 0 to 10 (0, no concern; 10, extremely concerned).
Woods et al. 211 conducted an internet survey of adults and parents of young people with TS in the USA. This was part of a larger survey212 that assessed the impact of TS on patients, but the current study specifically reports the results for questions on treatment utilisation, barriers to care and factors affecting the use of behavioural therapy. For the assessment of barriers to care, participants (465 adults and 487 children) were provided with a list of different statements to mark as yes or no according to whether or not they considered that item to be a barrier. Additionally, adults or parents of children who had not undergone behavioural therapy for their TS (327 children and 385 adults) were asked to provide reasons for this and these reasons were grouped by the investigators into common areas.
Evidence summary
Difficulty in Tourette Syndrome diagnosis/health professional knowledge
In Mol Debes et al. ’s survey of TS diagnosis in children,209 participants reported that the number of professionals they had consulted before diagnosis was between 0 and 16. The majority saw two professionals prior to consulting the professional who diagnosed TS and these were mostly GPs (77%), psychologists (47%) and paediatricians (31%). They were also diagnosed by psychiatrists (12%), ophthalmologists (11%), ear/nose/throat physicians (10%) and professionals from other disciplines, such as allergists and speech and language therapists (31%). When a diagnosis was made, in most cases it had been done by a child neurologist (76%) and, less frequently, diagnosis was made by a paediatrician (16%), psychiatrist (5%) or another type of physician (4%). The median time from the first appearance of symptoms until diagnosis was 5.5 years (range 0–16.7 years) and from the onset of tics until diagnosis was 2.8 years (range 0–12.3 years).
Difficulties in diagnosis was also identified as a theme in the focus groups in Rivera-Navarro’s qualitative study of doctors, patients and parents of TS patients. 208 Doctors said that diagnosis was difficult owing to the complex symptoms and confusion with other psychological disorders such as hyperactivity, depression, anxiety and behavioural disorders:
I suppose sometimes TS symptoms stay hidden because of hyperactivity, mobility, or other problems such as behavioural disorders, until there is finally a professional who is able to determine what is really happening to that person.
Doctor (quoted in Rivera-Navarro et al.,208 p. 18)
The theme was also identified in the TS patient focus groups in which patients (children and young people with TS) related that poor symptom interpretation by doctors caused mistakes in diagnosis. Patients also thought that lack of doctors’ knowledge about TS was a problem:
Because they have never given me a diagnosis or explained exactly what it was . . . I have visited several doctors but they did not diagnose TS, perhaps by the lack of knowledge of physicians.
Adult patient (quoted in Rivera-Navarro et al.,208 p. 18)
Focus groups in the parents of TS patients in this study, similarly, identified a lack of doctors’ knowledge as a problem in recognising symptoms and diagnosing TS. This perception was also identified in the survey of Woods et al. ,211 in which the barrier to treatment of ‘finding a provider who understood tics’ was agreed with by 42% of parents of TS children.
In the qualitative study by Rivera-Navarro et al. ,208 a problem identified by parents of TS children was that doctors implied that they were overoccupied by their child’s behaviour and exaggerated their symptoms:
I have not had a bad experience with physicians it is only that I think they did not know it . . . I have suffered from the lack of credibility as a mother and from accusations because they told me I was extremely obsessed with my son.
Mother of teenage patient (quoted in Rivera-Navarro et al.,208 p. 19)
Barriers to seeking treatment
Fear of stigmatisation
In the study of Rivera-Navarro et al. ,208 the focus group of doctors identified the problem of parents’ fears of associated stigmatisation if their child was diagnosed with TS. This was thought, in some cases, to delay or inhibit them going to the doctors:
I think that many families stopped visiting doctors because they were afraid of the fact that TS is an inherited disease.
Doctor (quoted in Rivera-Navarro et al.,208 p. 19)
In this study, patients and parent focus groups also agreed about the stigma caused by identifying TS as a mental disorder. However, in the survey of Woods et al. ,211 a relatively small proportion of parents of TS children (5%) agreed with the barrier to treatment of ‘Don’t want to be labelled or have a diagnosis’.
Concerns about drug adverse effects
In the parents of TS children surveyed by Woods et al. ,211 the barrier to treatment most commonly agreed with was ‘worry about the adverse effects of medication’ (43% of parents). In the survey by Kompoliti et al. 210 of adults with TS and parents of children with TS, from the list of antipsychotic drug adverse effects that participants were given, participants identified that they were most concerned with the risk of seizures, tardive dyskinesia, disturbances of thinking and emotion and cardiac disturbances and the median concern rating for each of these was 10 (extreme concern). Other adverse effects of concern were gastrointestinal (median 9), movement disorders (median 8), sexual function (median 7) and body image (median 6). In this study, patients who had taken antipsychotic drugs in the past (45%), or their parents, were less concerned about adverse effects than those who had not (χ2 test; p = 0.004). In addition, those who claimed to know nothing about neuroleptics were more concerned (Mann–Whitney U-test; p = 0.002) and the well-informed were less concerned (Mann–Whitney U-test; p = 0.027) about adverse effects than other participants.
Concerns about negative impacts of behavioural therapy
In the survey by Woods et al. ,211 for those who had never undergone behavioural therapy, when asked their reasons for not receiving therapy, participants identified that they were worried that tic suppression would cause a later ‘rebound’ effect, when tics became worse (14%), cause other tics to start (12%), make other activities such as schoolwork more difficult (15%) and that tics would get worse if they were focused on (12%).
Other barriers
Other barriers to general treatment and, specifically, to treatment with behavioural therapy were identified in the survey of Woods et al. 211 General barriers, proposed by investigators and agreed with by participants, were the financial cost (41%), the time needed to receive treatment (26%) and not having a treatment specialist in a close locality (33%). Some patients/parents indicated that they did not feel the need for treatment as tics did not interfere with life (32.4%) or they considered that they had never been very bad (35.2%).
For behavioural therapy, regarding children who had never undergone that type of treatment, when asked to give the reasons as to why not the most commonly identified were that patients/parents had not heard of behavioural therapy (21%) and they did not know where to go to receive it (28%). Some participants did not feel that behavioural therapy was necessarily the right approach and worried that behavioural issues would interfere with therapy (12%) or that it would not treat the underlying problem (5%). As for barriers to general treatment, participants identified that there were not behavioural therapy services in a close locality (13%) and that it was too difficult to travel the distance for these services (7%).
Online survey of parents of children with Tourette syndrome and other tic disorders
[Reproduced from © 2015 Cuenca et al. ;215 licensee BioMed Central. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.]
Methods
Participants
Participants were parents or carers of children and young people with TS or any tic disorder. Exclusion criterion was children or young people aged > 17 years.
Measures
The QuEST survey was designed to gather information about parents’ experiences of treatment for their children’s tics. Survey questions were developed by consulting the existing literature (e.g. Roessner et al. ;94 Verdellen et al. 158), including the systematic review (see Systematic review of qualitative studies) and in consultation with the TEG, which included 11 clinicians working with this population, academics and two service users. A survey draft was presented to and discussed with the TEG, which provided suggestions of question presentation, wording and inclusion or removal of items. After revisions were made, the survey was placed in SurveyMonkey® (CA, USA; www.surveymonkey.com) and three parents recruited through TA completed the survey to ensure that questions were clear and comprehensible. Parents’ comments and suggestions were integrated into the survey and a final version was prepared and approved by all the authors.
The survey included forced choice tick box responses (e.g. yes/no), rating scales, ranking scales and text boxes for open comments. Areas covered were (1) clinical characteristics of the child and demographic characteristics of both the child and the parent or carer, (2) psychoeducation (i.e. information about tics to parents and carers, and information about tic management to the child’s school), (3) behavioural interventions for tics, (4) medication for tics, (5) other treatments used for tics (i.e. alternative/complementary therapies), and (6) desired outcomes of treatment for tics. Skip patterns were used to move participants past questions that were not applicable.
For the child’s clinical characteristics, parents and carers were asked to report the child’s age of tic onset, diagnosis of tic disorders and diagnosis of coexisting conditions. Open questions probed responses to access to treatment and diagnosis of TS. To assess the child’s tic-related impairment, parents and carers were asked to rate the impact of tics on their child’s self-esteem, social relationships and academic performance using the overall impairment scale of the YTGSS,213 adapted for online presentation. Specifically, parents and carers were asked to rate the impact of tics over the previous week using a 0- (‘none’) to 5- (‘severe’) point scale with slightly modified descriptors for ease of comprehension. Descriptors that made reference to the impact of tics in occupational settings (e.g. job functioning or job performance) were not included.
To assess whether or not the young person had ever received a behavioural intervention for tics, participants were presented with a brief description of the CBIT that was developed based on the therapist guide for behavioural intervention for children and adults. 214 The description included components of habit reversal and function-based intervention (i.e. contingency management). Relaxation training was excluded from the description on the advice of the expert panel to avoid confusing participants whose child had used relaxation training in isolation as a complementary therapy (i.e. not as part of a comprehensive behavioural intervention). The description read:
Here we would like to ask you about behaviour treatment for tics. Behaviour treatment for tics may include one or both of the following: (please read these treatment descriptions before answering the question below)
the child is taught how to identify and change or avoid situations or triggers that make tics more likely to happen – for example, some places, people or thoughts. (health care professionals may call this treatment function-based intervention or contingency management)
the child is taught how to recognise the urges or feelings that can precede tics, and practises responding to these with a competing or opposite response—such as briefly tensing some muscles or breathing deeply so that the tics cannot occur. (health care professionals may call this treatment HRT)
Has your child ever received any of the treatments described above?
The QuEST survey assessed parents’ views on treatment (10 drugs were listed for tics). The target drugs [clonidine, risperidone, aripiprazole, sulpiride, haloperidol, olanzapine (Zyprexia®, Lilly), quetiapine (Seroquel®, AstraZeneca) pimozide (Orap®, Janssen), clonazepam (Rivotril®, Roche) and lorazepam (non-proprietary)] were selected based on a 2011 survey of choice of medication for tics among members of the European Society for the Study of Tourette Syndrome94 and expert panel reports of everyday practice in the UK. Parents were also prompted for additional drugs used in the treatment of tics.
Participants were presented a list of treatments other than medication and behavioural interventions (e.g. yoga, fish oils, massage, relaxation training and aromatherapy) and were asked to select all the therapies that the child had tried with the intention of helping with his or her tics.
To examine what outcomes of treatment are hoped for, parents were presented with two open-ended questions: (1) ‘What would you most hope a treatment for tics would do for your child?’ and (2) ‘Are there any other benefits from treatment that you would like to see’. In addition, parents were asked to rank, from most important to least important, seven preselected outcomes of treatment for tics (e.g. ‘School is better able to manage your child’s tics’, ‘Your child gets on better with friends’, ‘Your child is better able to cope with his or her tics’).
Procedure
Participants were recruited through study adverts that were posted on the UK TA website (www.tourettes-action.org.uk) and on its electronic newsletter. In addition, invitation e-mails that included a link to the survey were sent by TA to its members and study leaflets were distributed in TA events such as conferences and group meetings. The survey was available for six consecutive months, from December 2012 to May 2013. Participants gave online consent to participate and the survey was anonymous. After completing the survey, participants’ responses were automatically stored in the research team’s SurveyMonkey account, which required a password to be accessed. Participants were then were directed to a different web page (created with SurveyMonkey) that invited them to take part in the second part of the QuEST. The study was approved by the Medical School Ethics Committee of the University of Nottingham.
Statistical analysis
The percentages, means and SDs reported were calculated based on the number of responses to each item and exclude missing values.
Results
Demographic and clinical characteristics
A total of 358 respondents consented to participate in the survey, of whom 297 answered at least one question concerning treatment utilisation, which was the minimum response criterion for inclusion. Two entries were identified as repeat respondents based on their responses to demographic questions and were removed from the data analysis, resulting in 295 participants with usable data.
Of the 295 participants, 276 (93.6%) reported that their child had received a diagnosis of TS, eight (2.7%) reported a diagnosis of one or more tic disorders (i.e. chronic motor tic disorder, chronic vocal tic disorder or transient tic disorder) and 11 (3.7%) did not report a diagnosis of TS or tic disorder or had missing values on these items. All 11 reported age of onset for tics and were included in the survey. The majority of participants were biological mothers (92.2%) and the mean age of the sample was 44.0 (SD = 6.3). The sample was predominantly married (78.2%) and 40% were educated to graduate level (see Appendix 9).
Young people’s demographic and clinical characteristics as reported by parents and carers are presented in Appendix 9. Young people’s mean age was 12.4 years (SD = 3.0 years; range 5–17 years), 79.3% were males and the mean age of tic onset was 5.9 years (SD = 2.8 years; range 1–17 years). The mean age at diagnosis of TS was 9.1 years (SD = 2.7 years; range 3–17 years). On average, parents and carers reported that in the last 7 days, their child’s tic-related impairment was mild.
Access to care
Open text boxes probed parents’ perceptions of access to care and the process of receiving a diagnosis. Of the 295 participants, 195 made codeable comments. The pathway into treatment for tics was frustrating and difficult for many parents as testified by 100 free text comments.
We went to see a Doctor twice and was told it was a habit. It took a year before a Doctor referred our son to a consultant Paediatrician.
Parent ID 125
It was very difficult to get someone to believe my son had a problem.
Parent ID 49
At first our concerns were not taking seriously, and we were advised to go away and explore self-help therapies such as ‘tapping’. We wouldn’t have been asking for help if we could have managed the problem our selves!
Parent ID 266
Getting referred for specialist treatment and access to appropriate treatment was slow and difficult for many unless parents opted for a private referral. GP knowledge or attitudes were explicitly blamed by 31 parents.
We went to a GP at our local practice three times within the first year and were told it was a phase and nothing to worry about – despite the fact that the tics (vocal and motor) were frequent and having a pronounced impact on my son’s school and home life.
Parent ID 70
It took 18 months before my child was seen and several visits to GP.
Parent ID 9
I started with my GP as I didn’t know where else to go, when I said I thought he had TS he laughed saying I don’t think so. I left feeling stupid, but had to go back as things got worse and insisted he refer me somewhere, which he did; to CAMH. I was told I had to wait a year before they could assess the situation. They only started seeing him regularly because I phoned in tears as he was suicidal.
Parent ID 5
GP dismissed our concerns. Told us to ignore them (tics) and not to focus on them. We had already monitored the tics ourselves for six months and so didn’t want to wait any longer. Used private medical insurance to see consultant, then by chance someone we knew mentioned X Hospital. We managed to get a referral there to their Tourette’s Clinic.
Parent ID 61
In response to an open question about the receiving a diagnosis of TS, 39 parents commented on a lack of support or inadequate information. Specifically in relation to receiving a diagnosis of TS, qualitative data from the survey suggested that many parents felt unsupported and would have liked more information.
I was surprised at the absolute lack of information received from them about the condition following their diagnosis, and total lack of support for child and family in dealing with Tourettes. Most of our subsequent knowledge of Tourettes and dealing with this has come from Tourettes Action.
Parent ID 76
I had already guessed my child had Tourettes from research I had done on the internet, but when he was diagnosed I was expecting to be referred to experts who could help and give us advice, we weren’t offered any support or advice, not even a leaflet!
Parent ID 143
We were told by our GP he would grow out of this habit and we were given antihistamines.
Parent ID 27
GPs show no knowledge or awareness of Tics and have told me to offer reward charts to stop the ‘behaviour’! It was purely my husband researching our son’s movements on the internet that led us to realising that he suffers with tics/tourettes.
Parent ID 203
Primary care physicians are not well educated re tourettes and services available. I feel especially qualified to make this statement being a GP myself. Often children are referred to local community paeds who often have insufficient knowledge to manage this condition. We were also referred to neurology who again were not best placed to manage all the psychosocial implications but who fortunately directed us to child and adolescent specialist psychiatry services at [X].
Parent ID 34
There were also other barriers to treatment and many parents struggled to get paediatric services to take the child’s difficulties seriously.
Hard work! Tests for epilepsy inconclusive, so ‘off you go’ was the attitude from the paediatrician. Was a battle to get to see the correct person and took nearly 18 months to do.
Parent ID 77
A substantial number of comments referred (n = 49) to the difficulty of getting a diagnosis.
Neurology gave us the diagnosis of chronic motor tic disorder. I feel there is a lot of stigma around the use of the term Tourette’s and subsequent fear of using the term-something the media does not help. Child and adolescent psychiatry were the first service to use the term.
Parent ID 34
Frustrating for us as parents that it took so long. Once diagnosed everything made sense and we felt that we could help our son.
Parent ID 90
Many parents felt they had to pressurise services to make a diagnosis, resorting to strategies such as filming the child’s tics.
We had to demand to our GP to send him to a hospital referral but I myself had a video of him with his tics and he was diagnosed straight away.
Parent ID 27
Lack of support for diagnosis
Once a diagnosis had been made, many parents (n = 45) commented in the lack of support to help them cope with the diagnosis.
They never at any point sat us down and explained a diagnosis, they just dropped it into a casual conversation one day as if I knew. If you have cancer or something a doctor sits with you and explains everything, with mental health it seems different. WHY?
Parent ID 5
We also felt lost and scared. Like being sent home to get on with something we didn’t really know how to get on with.
Parent ID 11
For many parents the diagnosis did not seem to provide a pathway to treatment.
Very direct, told us the diagnosis whilst my son was in the room. He was very upset once we got outside. Told us that he may or may not grow out of it, that his Tourettes was mild (did not seem that way to us) and that there was no treatment unless it got worse.
Parent ID 46
Psychoeducation
More than two-thirds (68.5%, n = 202) of parents and carers reported that a health-care professional had given them information about tics. If participants reported that they received information about tics, they were asked how helpful they perceived the information to be, which service provided the information and how was the information provided. Among those parents and carers who received information about tics, most (157/202; 77.7%) considered that the information was somewhat helpful or very helpful. Less than one-third (21.3%, n = 43) were neutral and only 1% (n = 2) considered that the information made things worse. Parents also reported receiving information about tics through different services, of which the two most commonly reported were CAMHS (58.7%, n = 118) and paediatric services (37.3%, n = 75). Fewer than 10% of parents received information from an adult neurology service (6.5%, n = 13) or from private practice and only 3.5% (n = 7) received information from a GP. The most frequently reported way of receiving the information was through a conversation with the health-care professional (82.1%, n = 165) and about half (52.2%, n = 105) of participants received the information in written form and 37.8% (n = 76) were directed to a website.
Only seven parents (3.5%) reported that they had received information about tics from GP. Seventy-seven participants gave open comments about the information they had received. Many parents (n = 26) felt that they had had to search out information for themselves, often on the web or through TA.
No info as such just directed to websites that we have already found ourselves.
Parent ID 96
My research has been more enlightening than anything I’ve learned through my son’s doctors.
Parent ID 172
Most helpful was being directed to the Tourette Action.
Parent ID 105
Comments suggested there was a gap in information provision at the primary care level.
Two years after the diagnosis, I don’t always feel that I have somebody to go to for an update/review. Our GP is unashamedly unfamiliar with the syndrome.
Parent ID 31
When information had been given, particularly from specialist services, parents appreciated its value.
No real information given to us about tics until we were referred to the consultant specialist team . . . approx. 6 months after our son’s initial diagnosis. Once we were with the specialist team, we were given lots of information, both practical and information based. Our son’s habit reversal therapist also helped with explaining about tics.
Parent ID 160
Information about tic management to the child’s school
From the survey participants, just over one-third of parents/carers (111/291; 38.1%) reported that a health-care professional ever contacted their child’s school to give information about managing tics at school. Among those parents/carers whose child’s school was contacted, most of them (77/111; 70.0%) reported that the information given to the school was somewhat helpful or very helpful; 22.7% (25/111) felt that the information was neither helpful nor unhelpful and 7.3% (8/111) reported that the information made things worse.
Of the parents/carers who reported that their child’s school was contacted, 60 (54.1%) provided comments elaborating on the information that their child’s school received. An inductive content analysis of these comments revealed that parents/carers felt that the information provided by the health-care professional helped others at school understand the condition better.
she [health care professional] visited the school and spoke to the class and made the children aware of my child’s disorder as it was quite severe and he was the only one in the school with the condition. It helped the children to understand and they accepted it fully.
Parent ID 99
Helped the teachers to understand the condition. believe it or not, some teachers have got no idea what it involves.
Parent ID 109
However, parents/carers also felt that schools were not receptive to the information provided by the health-care professional.
Our healthcare professional has tried her best to explain to my son school about his condition but it’s the school that hasn’t been that interested and not really taken much notice.
Parent ID 120
The school didn’t take any notice and used their own methods . . . They wouldn’t adapt to meet my child’s needs.
Parent ID 94
Furthermore, parents/carers felt that schools have difficulties disseminating the information among the teachers.
The teachers change so often at his school that many are still unaware of the issues.
Parent ID 25
The school had no knowledge of how to help kids with tic disorders or TS but were willing although as they passed through the school years each teacher got less info and seemed to have no handover so we had to raise the issue ourselves.
Parent ID 210
There is evidence from the survey that informed schools also have a part to play in improving access to care for TS. When asked about accessing treatment for tics many people gave examples of how the process had been difficult. Of the 29 parents who gave neutral or positive responses about accessing care for TS, seven parents specifically mentioned the role of the school suggesting that better informed schools could reduce barriers to care.
The school where my child went had also had a massive impact with this as they were noticing unusual behaviours in my child. This is what really stemmed it off!
Parent ID 26
Our school referred us to the local paediatrician, was very thorough and have been well supported.
Parent ID 192
It seems that for many parents, GPs are the gatekeepers to services and children are often referred back to primary care following diagnosis.
Other treatments
As shown in Table 2, more than three-quarters of participants (78.5%, n = 219) reported that the child had tried at least one other (alternative/complementary) treatment to help with tics. Of these other treatments, the three most commonly reported were relaxation training (39.8%, n = 111), exercise (34.1%, n = 95) and fish oils (30.5%, n = 85). Between 20% and 25% of parents reported that their child had tried individual counselling, diet and massage; whereas fewer than 10% of parents reported that their child has tried therapies such as yoga, homeopathy and aromatherapy. Of the other treatments, the two that were least frequently reported were dental therapies (2.2%, n = 6) and neurolinguistic programming (1.1%, n = 3).
| Other treatment | n | % |
|---|---|---|
| One or more other treatments | 219 | 78.5 |
| Relaxation training | 111 | 39.8 |
| Exercise | 95 | 34.1 |
| Fish oils (omega-3 fatty acids) | 85 | 30.5 |
| Individual counselling | 69 | 24.7 |
| Diet | 63 | 22.6 |
| Massage | 58 | 20.8 |
| Martial arts | 40 | 14.3 |
| Yoga | 19 | 6.8 |
| Homeopathy | 18 | 6.5 |
| Aromatherapy | 17 | 6.1 |
| Hypnotherapy | 12 | 4.3 |
| Dental therapies | 6 | 2.2 |
| Neurolinguistic programming | 3 | 1.1 |
| Other | 28 | 10.0 |
Medication for tics
More than half (54.7%, n = 202) of parents and carers reported that their child has taken medication for tics. The three most frequently reported drugs for tics were risperidone (27.7%, n = 77), clonidine (26.3%, n = 73) and aripiprazole (19.9%, n = 55).
For each target drug, the survey included questions to assess (1) perceived helpfulness (‘How helpful has this medicine been for your child’s tics?’, scored on a 5-point Likert scale from –2 (unhelpful tics got a lot worse) to 2 (very helpful) and (2) adverse effects [‘Has this medicine had unwanted effects [side effects] on your child?’, scored on a 4-point scale from 0 (none) to 3 (severe)]. The doses of each target drug were not known and as a consequence it is not possible to determine to what extent dosage influenced participants’ reports of helpfulness and adverse effects.
Table 3 presents how parents and carers perceived the helpfulness and adverse effects of medication for tics. Mean helpfulness scores ranged between –0.2 and 1.0, indicating that parents and carers felt that drugs were neither helpful nor unhelpful for tics or somewhat helpful. The drug with the highest mean helpfulness score was aripiprazole (mean = 1.0), indicating that, on average, participants perceived that this drug was somewhat helpful for their child’s tics. To calculate the proportion of participants who considered that a drug was helpful, for each drug the number of participants who reported that it was ‘somewhat helpful’ or ‘very helpful’ were counted. As Table 3 shows, the proportion of participants who considered that a drug was helpful ranged between 15.4% (for sulpiride) and 69.1% (for aripiprazole). For most of the drugs examined, fewer than 50% of parents and carers felt that the drug was helpful for their child’s tics. An exception to this was aripiprazole; of the 55 parents who reported that their child took this drug, almost 70% considered that aripiprazole was somewhat helpful or very helpful for the child’s tics. Of the five parents who reported that their child took lorazepam, three (60%) of them considered that lorazepam was somewhat helpful or very helpful.
| Helpfulness | Adverse effects | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean helpfulness (–2 to 2 scale) | Proportion who found it helpful for child’s tics | Mean adverse effects (0 to 3 scale) | Proportion who reported moderate or severe adverse effects | |||||||
| Treatment | N | % | Mean | SD | n | % | Mean | SD | n | % |
| Medication | ||||||||||
| Risperidone | 77 | 27.7 | 0.1 | 1.3, n = 76 | 30 | 39.5 | 1.9 | 1.1, n = 76 | 48 | 63.2 |
| Clonidine | 73 | 26.3 | 0.1 | 1.1, n = 73 | 30 | 41.1 | 1.5 | 1.1, n = 73 | 34 | 46.6 |
| Aripiprazole | 55 | 19.9 | 1.0 | 1.0, n = 55 | 38 | 69.1 | 0.9 | 1.1, n = 55 | 14 | 25.5 |
| Haloperidol | 24 | 8.7 | –0.1 | 1.2, n = 24 | 6 | 25.0 | 1.9 | 1.3, n = 24 | 17 | 70.8 |
| Sulpiride | 13 | 4.7 | –0.2 | 1.0, n = 13 | 2 | 15.4 | 1.3 | 1.4, n = 13 | 6 | 46.2 |
| Pimozide | 7 | 2.5 | 0.3 | 1.3, n = 7 | 3 | 42.9 | 1.7 | 1.4, n = 7 | 4 | 57.1 |
| Clonazepam | 7 | 2.5 | –0.1 | 1.5, n = 7 | 3 | 42.9 | 2.0 | 1.4, n = 7 | 5 | 71.4 |
| Lorazepam | 5 | 1.8 | 0.2 | 1.3, n = 5 | 3 | 60.0 | 1.6 | 1.5, n = 5 | 3 | 60.0 |
| Behavioural interventions for tics | 74 | 25.9 | 0.4 | 1.0, n = 70 | 34 | 48.6 | 0.4 | 0.8, n = 70 | 8 | 11.4 |
Mean adverse effects scores ranged from 0.9 to 2.0, indicating that, in general, parents and carers perceived that medication for tics had mild-to-moderate adverse effects. The drug with the lowest mean adverse effect score was aripiprazole (mean = 0.9), indicating that, on average, participants felt that this drug had mild adverse effects. Table 3 shows that the proportion of participants who reported moderate or severe adverse effects ranged from 25.5% (for aripiprazole) to 70.8% (for haloperidol). For most of the drugs examined, more than 45% of participants reported that the drug had moderate-to-severe adverse effects. For aripiprazole, however, of the 55 parents who reported that their child took this drug about 25% of them felt that the drug had moderate-to-severe adverse effects.
For each medication examined, the survey also included an open-ended question that probed respondents’ views on the target drugs (i.e. ‘Are there any comments you would like to make about this medicine?’). Examination of parents’ responses to each of these questions revealed that between 14.3% and 60% of parents commented on some aspect of drug adverse effects. Although parents mentioned a wide variety of adverse effects, those frequently described were sleepiness, tiredness or drowsiness, and weight gain. Table 4 shows the number of parents who commented on adverse effects, frequently described adverse effects and example comments.
| Medication | Number received medication | Parents commenting on adverse effects | Frequently described adverse effectsa (number of parents describing adverse effect, % among those who received medication) | Example comment | |
|---|---|---|---|---|---|
| n | % | ||||
| Risperidone | 77 | 32 | 41.6 | Weight gain/increase of appetite (9, 11.7) | He only took it for 3 weeks as he developed a monstrous appetite and gained rapid weight . . . |
| Other (21, 27.3) | My son became more agitated and impulsive on this medicine. It was a nightmare! | ||||
| Clonidine | 73 | 17 | 23.3 | Depression (5, 6.8) | Our son became depressed on clonidine – extremely weepy . . . |
| Sleepy/tired/drowsy (5, 6.8) | He was very sleepy on this medicine and it didn’t help | ||||
| Other (10, 13.7) | Make his blood pressure very low and was told by hospital doctor to stop taking it | ||||
| Aripiprazole | 55 | 14 | 25.5 | Sleepy/tired/drowsy (4, 7.3) | He has been on this med for approx. 16 months. Was very sleepy to begin with |
| Weight gain/increase of appetite (4, 7.3) | Have to watch what he eats, makes him hungry . . . | ||||
| Other (e.g. nausea, akathisia/tremor) (8, 14.5) | . . . she had very bad nausea and unable to eat in the beginning but that has now gone sometimes she gets a bit shaky when taking it mainly shaky hands . . . | ||||
| Haloperidol | 24 | 12 | 50.0 | Sleepy/tired/drowsy (n = 5, 20.8) | . . . is very sleepy at times, he often falls asleep at school |
| Other (e.g. dystonia) (6, 25.0) | Only took this medication for a couple of days. Caused breathing problems and muscle spasms | ||||
| Sulpiride | 13 | 5 | 38.5 | Sleepy/tired/drowsy (2, 15.4) | Became easily fatigued when he first started taking medication . . . |
| Weight gain/increase of appetite (2, 15.4) | . . . she put on weight . . . | ||||
| Other (3, 23.1) | Took it at age 6. Only took it for 10 days. Vomited every day he took it . . . | ||||
| Pimozide | 7 | 1 | 14.3 | Mixed (weight gain, insomnia) | . . . caused weight gain and insomnia and tiredness in the day unable to get up in the morning also caused dizziness |
| Clonazepam | 7 | 2 | 28.6 | Mixed | Only took on one occasion but had such a horrible almost hallucinogenic effect we didn’t use again |
| Lorazepam | 5 | 3 | 60.0 | Sleepy/tired/drowsy (2, 40.0) | Too sleepy unable to think |
| Other (2, 40.0) | Caused increase in anxiety . . . | ||||
Behavioural intervention for tics
About one-quarter of participants (25.9%, n = 74) reported that their child had received a behavioural intervention for tics. Participants reported that behavioural interventions for tics were mostly delivered by CAMHS (71.4%, 50/70), followed by paediatric services (15.7%, n = 11), private practice (7.1%, n = 5) and adult neurology (4.3%, n = 3). Among participants who reported that their child had received a behavioural intervention for tics, 52.2% (36/69) reported that the child received fewer than five sessions, 23.2% (n = 16) reported receiving between 5 and 10 sessions, and 24.6% (n = 17) received more than 10 sessions. As shown in Table 3, participants who reported that their child received a behavioural intervention (n = 74) felt, on average, that it was neither helpful nor unhelpful for tics (mean = 0.4). Of those participants who reported that their child had received a behavioural intervention, 48.6% (40/74) reported that the behavioural intervention was ‘somewhat helpful’ or ‘very helpful’ for the child’s tics. Examination of mean helpfulness scores by number of sessions received showed that participants who received more than 10 sessions had slightly higher scores (n = 17; mean = 0.8, SD = 0.6) than participants who received 5–10 (n = 16; mean = 0.3, SD = 1.4) or < 5 sessions (n = 36; mean = 0.3, SD = 1.0), although there were no significant differences between the groups with statistical significance threshold of p < 0.05.
Regarding adverse effects, participants felt that behavioural interventions had none or mild adverse effects (mean = 0.4). Given that adverse effects are often not reported for behavioural intervention trials, it is interesting to note that eight participants (8/70; 11.4%) reported that behavioural interventions had moderate or severe adverse effects, such as worsening of tics attributed to treatment exercises.
Desired outcomes of treatment
Participants were asked to rank seven preselected outcomes of treatment for tics, with 1 being the most important and 7 being the least important. As shown in Table 5, reduction in severity and frequency of the child’s tics was ranked as most important (i.e. received the lowest mean score). The child being better able to cope with tics was ranked as second in importance and the school being better able to manage the child’s tics was ranked as third in importance. Of note, the child can take part in more leisure activities was ranked as least important.
| Desired outcome | Mean | SD, n | Importance of outcome: mode ranked score |
|---|---|---|---|
| Reduction in severity and frequency of your child’s tics | 1.8 | 1.3, 253 | 1 |
| Your child is better able to cope with his or her tics | 1.9 | 1.0, 252 | 2 |
| School is better able to manage your child’s tics | 4.3 | 1.4, 253 | 3 |
| Your child gets on better with friends | 4.4 | 1.4, 253 | 5 |
| Your child gets on better at school | 4.7 | 1.4, 253 | 6 |
| Your family is better able to cope with your child’s tics | 5.0 | 1.6, 253 | 7 |
| Your child can take part in more leisure activities | 6.0 | 1.3, 252 | 7 |
Parents were also asked to describe in free text their desired outcomes of treatment for tics. The two questions presented read: ‘What would you most hope a treatment for tics would do for your child?’ and ‘Are there any other benefits from treatment that you would like to see?’. A total of 247 parents answered at least one of these questions and parents’ responses were examined for commonalities and coded/divided into nine categories. Some parents described more than one desired outcome of treatment for tics. Table 6 shows the nine categories, the number and percentage of parents who endorse each category and typical responses.
| Category | n parents who endorsed each category | % (overall sample) | Example comment |
|---|---|---|---|
| Reduce or stop tics | 121 | 41.0 | Bring them to a tolerable level or even stop them |
| Ideally I would like a treatment that would eliminate his tics with no side effects | |||
| Manage or reduce negative emotions associated with tics | 64 | 21.7 | Reduce feelings of anxiety and frustration |
| A feeling of being less anxious, so as to not worry about everything . . . | |||
| Child controls/manages tics | 57 | 19.3 | For him to be able to control them at his instigation |
| Give him control over them and be able to substitute one for a less noticeable/harmful type | |||
| Increase self-esteem and confidence | 46 | 15.6 | Help him overcome his lack of self-confidence |
| Boost his self-esteem, make him feel ‘normal’ and not as inadequate in social situations | |||
| Increase knowledge and acceptance of condition | 35 | 11.9 | Just give us a better understanding of why they happen and how to deal with them |
| A place where doctors send children with tics where the practitioner understands what you are describing and can give you a straight answer would be nice | |||
| Enhance general QoL | 18 | 6.1 | Raising of quality of life through an ability to spend time on other activities when time lots of time is spent ticcing . . . |
| Social integration | 16 | 5.4 | . . . being able to have a good social life without being self-conscious about his condition |
| Reduce physical pain associated with tics | 16 | 5.4 | Reduce the pain caused by constant tics and jerks |
| Other | 43 | 14.6 |
Although the most common desired outcome of treatments were stopping or reducing the child’s tics (41.0%), many identified emotionally focused outcomes such as reducing anxiety or increasing self-esteem. Reducing or managing negative emotions such as anxiety was the second most frequently reported desired outcome of treatment for tics, accounting for almost 22% of responses. Yet, participants also described more behaviourally oriented outcomes such as managing or controlling tics, which were reported by a notable portion of participants (19.3%). Although less frequently reported, about 12% of participants described desiring outcomes that increase knowledge and understanding of TS and tics.
Psychoeducation by type of treatment
To examine delivery of psychoeducation and use of other treatments beyond medication and behavioural intervention, the sample was divided in three groups: (1) medication only, (2) medication and behavioural intervention and (3) behavioural intervention only. The medication only group consisted of participants who had only received medication for tics. The medication and behavioural intervention group consisted of participants who had received medication and a behavioural intervention for tics, whereas the behaviour only group consisted of participants who had only received a behavioural intervention. Table 7 shows delivery of psychoeducation and use of other treatments as a function of type of main treatment (medication and/or behavioural) received for tics.
| Treatment | N | % | YGTSS tic-related impairment score (0 to 5 scale) | Information about tics to parent/carer | Information about tic management to child’s school | One or more other treatments | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | % | n | % | n | % | |||
| No medication or behavioural intervention | 105 | 37.8 | 1.9 | 1.2, n = 105 | 56 | 53.3 | 27 | 25.7 | 68 | 64.8 |
| Medication only | 103 | 37.1 | 2.6 | 1.6, n = 101 | 75 | 72.8 | 43 | 41.7 | 83 | 80.6 |
| Medication and behavioural intervention | 49 | 17.6 | 2.6 | 1.5, n = 49 | 43 | 87.8 | 27 | 55.1 | 46 | 93.9 |
| Behavioural intervention only | 21 | 7.6 | 2.0 | 1.2, n = 20 | 19 | 90.5 | 8 | 38.1 | 21 | 100 |
Although most participants across the different treatment groups reported that a health professional gave them information about tics, the group with the lowest proportion of participants who received tic-related information was the medication only group (72.8%). Among participants whose child had received medication and/or behavioural intervention, provision of tic-related information to schools was consistently less common than provision of information to parents and carers. Just over half (55.1%) of participants whose child had received medication and/or behavioural intervention reported that a health professional provided information to their child’s school. The behavioural intervention only group had the lowest proportion of participants who reported that the child’s school received tic-related information (38.1%).
In-depth qualitative interviews with young people with Tourette syndrome
[Reproduced from © 2015 Cuenca et al. ;215 licensee BioMed Central. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.]
Methods
Participants
Participants were children and young people with TS between 10 and 17 years of age. Participants were recruited via the QuEST online survey by asking parents who completed the survey if their child would be interested in taking part in an interview that explored young people’s views on treatments for tics. In addition, recruitment was conducted through study announcements that were posted on the TA website and social media, and study leaflets that were handed out at information sharing and social events organised by TA. The target sample size was flexible and aimed to include up to 50 young people depending on the number of participants needed to achieve saturation of themes. This sample size was considered feasible within the study period and sufficient to ensure participation of young people with different treatment and service experiences. As the systematic review has identified barriers to treatment for tics among young people with TS, participants who were in the process of receiving treatment or who have not received treatment at the time of the interview were included in the analysis. This allowed exploring young people’s experiences of access to care and perceived barriers towards the use of different treatments for tics.
Interview schedule
The interviews aimed to explore young people’s thoughts and experiences concerning services and treatments for tics. Interviews were conducted by telephone or, when practical, face to face. The interview schedule, which was developed by members of the research team and the TEG, explored three main areas: (1) experiences concerning tics and TS, including factors that are perceived as improving or worsening tics and aspects of tics that are considered problematic, (2) experiences of services and treatments for tics, including positive and negative experiences, as well as views on information about tics, medication for tics and behavioural interventions for tics, and (3) desired outcomes of treatment.
For young people who reported not having received medication and/or behavioural intervention for tics, the interview schedule included a short description of each of these treatments (see Appendix 8) that were read aloud to the participant to explore his or her views. As with the QuEST survey, the description of behavioural interventions for tics excluded the relaxation training component to avoid confusion with relaxation exercises delivered in isolation and not part of a more comprehensive behavioural intervention.
Procedure
After parents and carers completed the QuEST survey, they were automatically directed to a different website (developed with the software SurveyMonkey) that presented information about the interviews with young people. This website explained that the interviews were conducted with young people with TS and asked parents and carers if they would be interested in their child taking part in the study. Parents and carers who reported being interested in the study were then asked for their contact details (e.g. e-mail address and telephone number) so that a member of the research team could contact them to provide more information about the study. Parents’ and carers’ contact details were kept confidential and separate from their QuEST survey responses.
The study announcements and leaflets used to advertise the study included the web address of a website similar to the one described above, but with the option for young people between 16 and 17 years of age to provide their contact details. In addition, this website included information for young people below 16 years of age on how their parents and carers could contact the researchers in case they were interested in taking part in the study.
Parents and young people between 16 and 17 years of age who provided their contact details were then contacted to discuss the study in more detail and to answer any further questions. They were then sent the information sheet in print, a consent form and a freepost envelope to facilitate the return of the consent form. Interviews were carried out at least 1 week after the initial contact was made.
On the day of the interview, parents and carers of young people aged 10–15 years were asked to provide verbal informed consent, which was digitally recorded with their permission. In addition, after explaining and answering any questions about the study, their children were asked to provide verbal consent to take part in the study and this was digitally recorded. Young people aged 16–17 years provided verbal informed consent on the day of the interview.
After completing the interview and with the participant’s permission, parents were asked follow-up questions about the child’s treatment if the young person had been unable to recall specific information (e.g. drug dose). Interviews were digitally recorded and transcribed verbatim following completion. Any personal identifiers were removed from the transcript. The study was approved by the Medical School Ethics Committee of the University of Nottingham.
Analysis
The interviews were analysed using thematic analysis and the development of themes was conducted as described by Boyatzis. 216 The purpose of the analysis was to explore young people’s needs and perceptions of treatments for tics. Thematic analysis was chosen because it is a flexible method that results in a detailed description and organisation of the data;217 allowing full exploration of young people’s needs and perceptions of treatments for tics without the constraints that other theoretically bounded methods impose. Theme analysis encourages maximum variation sampling whereby the population of interest is represented by a heterogeneous sample and thus tends to require a relatively large number of participants in order to ensure saturation of themes. 216 We wanted to capture views of young people from early adolescence to adulthood and with a range of tic severity. A method such as interpretative phenomenological analysis, which obtains very dense experiential data from small homogenous samples, would not have been sufficiently flexible to capture this range. To become familiar with the data, the interview transcripts were read repeatedly and ideas about important features or segments of the data were noted down to develop initial codes. These codes helped to organise the data into more meaningful groups and they were reviewed, combined or discarded to identify potential themes. The themes were developed inductively and based on the raw data because past research has not examined experiences of treatments for tics among young people with TS. This inductive approach is considered appropriate when previous research findings do not allow developing theory driven or research driven themes. 216 Each thematic code was labelled using five elements: (1) a label or name, (2) a definition of what the theme concerned, (3) a description of how to know when a theme occurred, (4) a description of any qualifications or exclusion and (5) examples, including positive and negative examples. 216 This allowed examining the reliability of the themes.
Results
A total of 40 interviews were conducted (38 by telephone, two face to face) (see Appendix 7). The data were reviewed to determine that saturation of themes had been achieved. Young people’s demographic characteristics are presented in Table 8.
| Characteristic | n | % | Median | Range |
|---|---|---|---|---|
| Age (years) | 13.0 | 10–17 | ||
| Sex | ||||
| Male | 31 | 77.5 | ||
| Female | 9 | 22.5 | ||
| Ethnicity | ||||
| White British | 34 | 85.0 | ||
| Mixed/multiple ethnic groups | 6 | 15.0 | ||
| Co-occurring conditions as reported by child’s parent (may be more than one) | 18 | 45.0 | ||
| ADHD | 8 | 20.0 | ||
| OCD | 7 | 17.5 | ||
| ASD | 4 | 10.0 | ||
| Anxiety | 4 | 10.0 | ||
| Other | 4 | 10.0 |
Eight main themes were identified, of which seven had subthemes. To examine the trustworthiness of the themes and subthemes, a code book was prepared which included for each theme and subtheme: (1) a descriptive label, (2) a definition of the theme, (3) pointers to look for when identifying the theme, (4) any examples of aspects which would exclude an extract from the theme and (5) an illustrative sample extract. 216 An experienced qualitative researcher who was not involved in the study used the code book to recode 26 unlabelled extracts of text. Her ratings were compared with the original coding conducted by the study research fellow (JC) and agreement was excellent (24/26, 92.3%).
Theme 1: need for access to informed and expert care
This theme describes young people’s perceptions about the importance of receiving care from health professionals who were knowledgeable about the condition including the challenges of accessing expert care and the consequences of receiving ill-informed care.
Young people perceived that some health professionals have limited or no knowledge of TS and tics, as well as about treatments or interventions for tics. Furthermore, some health professionals were perceived as unsupportive or not understanding of the condition. There was a feeling they lacked adequate training in providing treatment for tics. Young people often described health professionals as having little knowledge about their condition, both when seeking medical help for tics and while receiving treatment. Young people remembered visiting different services to receive treatment for tics, often finding that knowledge of TS and tics was limited.
. . . most of the places we have been to about my Tourettes like it seems like no one actually knows about it, like we know more than them . . . when we go there they usually ask us about it more than we ask them.
How does that make you feel?
Ermm quite annoyed . . . because like we have been asked to go there by someone saying they know a lot about it but they barely know anything.
Another participant described their experience of treatment:
. . . I saw him [a healthcare professional] for two years and on the second year he told me to do habit reversal and then habit reversal didn’t work so we didn’t know if that was cos he had given us the wrong information or what, so we decided to go elsewhere and then we thought we would give him a second chance cos he was just in training for the habit helping at the time. All that he said was sort of out of a text book or something that he thought might have helped.
YP ID 39
Some young people described wanting to receive more information about TS and tics, as well as information about their treatment.
I would like to know what the Clonidine does, simply because the yeah, the doctor that prescribed it said and I quote ‘I am not really sure about this, so I can’t really say for definite that it is Tourettes’. So I don’t really kind of trust what he says.
YP ID 2
This subtheme describes how young people perceive that there have been difficulties or delays in receiving specialist care. It can also include perceptions that the information received about the condition did not meet expectations, or was not perceived as relevant or sufficient to understand more about TS or tics.
. . . After I got diagnosed 2 years after that, that’s when I started to get information about it. . . . I would have liked it to have been just more of a frequent thing when I actually needed the help more. Some sort of like guidance on things I could do, instead of just finding out that I had to deal with it all on my own.
YP ID 32
This subtheme captures how young people perceive benefits to be treated by health professionals who are knowledgeable and was represented in one-quarter of the interviews. Perceived benefits included feeling understood and more confident, as well as feeling that health professionals can provide useful information about TS.
. . . I can talk to my parents about anything but she [health professional] was just like another person, who, I don’t know but just who knows a bit more about Tourettes you know what I mean. So she knows she has some knowledge of Tourettes so she knows what message could help me . . .
YP ID 9
. . . he [health professional] has had a few, not interview, sessions with me, and talked to me about how, he has tried to understand, he is verbally very good he has explained to me about what he knows about Tourettes and he and another member he explained what’s happening and from that he suggested ways of being able to not necessarily prevent the twitches but to limit them. Which I did find very helpful.
YP ID 38
. . . I think the psychologist that I was talking to was very understanding about my Tourettes and it kind of reassured me that there are people that do understand it and didn’t make me feel so bad about it really.
YP ID 13
Theme 2: need to manage emotional responses associated with tics
This theme captures young people’s experience of negative emotions in relation to tics, such as self-reported anxiety, worries, anger or frustration, and young people’s attempts and interest to manage or reduce these emotions.
Young people spoke about experiencing negative emotions associated with tics. These emotions were described variously as ‘worries’, ‘anxiety’ and ‘stress’. These emotions were considered to worsen tics, which in turn contributed to experiencing further negative emotions.
. . . it makes me worried and then because I am worried I do it [tic] more and then because I am doing it more and people are looking I do it even more than that.
YP ID 19
. . . at one point they [tics] just kind of like fade off but then the next point you think it’s all gone and you have anxiety levels build up and it starts all over again.
YP ID 21
. . . I try to not do them [tics] but I find it a bit hard when we are doing something and I am nervous like when we are doing something and I am a bit nervous about it, it is a bit worse.
YP ID 34
For some young people, the subjective emotional responses associated with tics were sometimes considered more troublesome than actual tics, and they tried different practices to help them manage their emotions and to reduce their tics.
. . . I am fine with the tics it is more the stuff that comes along with the tics like the emotion that’s worse I find.
YP ID 14
. . . I have got some relaxation CD’S, they seem to make it better, yeah and when mum comes into me cos I am twitching really bad. Mum comes in to calm me down and that makes me feel a lot better. . . . She [mum] like just like talks to me and tries to make me think about something else and just calms me down.
YP ID 19
When asked about what they would hope a treatment for tics to achieve, some young people spoke about having a treatment or intervention that could help them feel calmer.
. . . it (treatment) could make you feel more relaxed so then, then you wouldn’t be well you would be less stressed so then I wouldn’t have done them [tics] as much.
YP ID 18
Say some day I had an important exam I could just like have some of that treatment or that medication or something and then that could like calm me down, it would be like a way to get me more focused for something like if I had an exam.
YP ID 32
Theme 3: the importance of gaining a sense of control over Tourette syndrome
This theme captures how young people feel they have little or no control over their tics and how they feel they would like to have or learn to have control over tics. It also includes attempts to control tics, as well as interest in treatment for tics that is perceived as helping to gain control over tics.
Young people described feeling little or no control over their tics as if TS had its own will or personality or as being something foreign or alien to them.
. . . one of the things about tics is it is something that your body is doing that you don’t really have any control of but it is kind of a major physical thing. [Pause] cos a lot of your body functions you don’t have any control over like digestion and stuff like that but with this yeah it is something you feel you should have control over but your body is not letting you control and being able to control it is a good thing I think.
YP ID 16
. . . sometimes I lose like all control and my Tourettes takes over and does what it wants to do.
YP ID 13
Young people considered that gaining a sense of control over them was a desired outcome of treatment. This sense of control was described as an ability to stop the tics when the young people wanted to and as a general sense of having control over oneself.
To almost be able to control my tics so like I could hold them in but then like let some of them out at different times.
YP ID 40
Just personally, really, to know that I can be able to control myself, as well as you know be able to be a normal person.
YP ID 32
In relation to this, young people spoke about behavioural interventions as something that could help them gain a sense of control over tics.
I just like to feel that I am in control of it myself. Rather than something else doing it for me, which is why, I like habit reversal because I like to be in control.
YP ID 5
It [behavioural intervention for tics] sounds good because it is a different way of trying to stop the tics. Trying to do something else to resist the kind of urge to do it. I personally quite like that idea more because you, the person who has tics has to do something to try and stop it and it’s really ermm well it is a way of doing something else . . .
YP ID 34
Theme 4: desire for a treatment to stop or reduce tics and the urge to tic
Not surprisingly, wanting an intervention that would stop or reduce their tics, as well the urge to tic, was a strong theme that emerged from the data.
This subtheme reflects how young people would like their tics to be stopped, cured, taken away or eliminated, as well as how they would like their tics to be reduced or ameliorated.
So something that could reduce or eliminate tics, even if it is only at night or in certain situations or things like that, that would probably be my ideal treatment.
YP ID 29
The urge to tic was perceived as uncomfortable and reducing the urge to tic was seen as an important outcome of treatment.
. . . I would want the urge to twitch to be reduced. Also if my Tourettes could go overall that would be fantastic, I don’t know how realistic that is, but I would certainly want the urge to be less.
YP ID 38
Theme 5: concerns and limitations about taking medication for tics
This theme describes young people’s negative perceptions of medication for tics, based on their direct experience with medication or on their understanding of this treatment. Perceived adverse effects of medication for tics were frequently mentioned by young people.
In this subtheme, the young person who has not taken medication perceives potential difficulties or drawbacks about taking medication for tics, including adverse effects, difficulties to remember to take the drug, the need to take medication for a prolonged period of time and bad taste of medication. There is a general perception that medicines cannot be good and some young people described an interest in other forms of treatment.
. . . they [drugs] do have side effects and they make you drowsy and stuff like that and maybe you feel weird when you have that drowsiness.
YP ID 25
I wouldn’t want to be taking that much drugs . . . it can’t really be good for you.
YP ID 16
. . . I would probably prefer to learn to suppress them [tics] almost, I don’t really want to be taking tablets . . . I just wouldn’t like to take them everyday almost and like with most tablets if you suddenly stop them as well you can get ill from them and that.
YP ID 40
This subtheme captures young people’s actual experience of taking medication for tics, often the feeling that medication caused adverse effects. These effects, which were associated with different types of medication, included drowsiness, tiredness, self-reported depression, nightmares, weight gain and a sense of not being oneself.
I used to get really hungry so I put on loads of weight or I used to get tired. . . . It was horrible because I could just go to sleep in the middle of the day in class and that, and I put on quite a bit of weight as well.
YP ID 8
For some young people, adverse effects were the main reason to stop taking medication, or for changing to a different one, even if they perceived an improvement in tics.
. . . I was happy that my tics weren’t as bad when I was on them [clonidine tablets] but I just wasn’t happy about being so depressed all the time.
And you said that you stopped taking it, why did you stop?
Just because it wasn’t just making me depressed it was also giving me really scary dreams.
Young people described seeking a balance between the adverse effects associated with certain drugs and the positive benefits of tic reduction. When seeking this balance, young people spoke about seeking advice from their parents and health professionals.
. . . I found that [aripiprazole] gave me the kind of dizzy feeling so I always got light headed when I stood up and stuff . . . I was very tired as well. So we have decided with the doctor that I would do one [5 milligram tablet of aripiprazole] in the morning and one [5 milligram tablet of aripiprazole] in the evening and we kept that going for a bit but then eventually when the dizziness wasn’t going I decided that I would go back down to one tablet but have a few more tics but I didn’t mind.
YP ID 12
. . . when we are making decisions about Tourettes I usually talk it over with my mum and dad first because obviously I don’t know everything that is best for me. So if I am planning on reducing a medication or increasing it or changing it or if I think I have got a new Tourette or tic I always tell my parents and see what they think of it and everything just to make sure it’s not something I didn’t think it was.
YP ID 39
This subtheme captures how young people feel that medication left the tics unchanged, or had a positive effect on tics for only a limited period of time. It can also include young people’s perception that medication worsened tics.
. . . it [aripiprazole] wasn’t really doing much I stopped taking it and they said if you feel like you want to start taking it again you can but then I didn’t.
YPar ID 18
I was on a medication called risperidone for a while, which was helpful to start off with, it certainly had a noticeable effect but once I had got the level, my risperidone level sort of steady after about a year or after about 6 months it stopped being so effective so I went off it . . .
YP ID 29
Theme 6: positive experiences of medication for tics
This theme describes how young people felt that medication for tics can help them to reduce their tics and to have better control over them.
. . . it [risperidone] has helped me, it hasn’t completely like stop them (tics) but it has helped me. Before I was on the tablets I was ticcing really, really loads and the medication has controlled them a better, so I don’t really tic that much anymore.
YP ID 27
For some young people medication for tics allowed them to feel less self-conscious about their tics and to dissimulate them better when in public.
It [clonidine] makes them better . . . sort of makes them easier to control . . . they just don’t sort of happen as much . . . It’s good because it is easier and I don’t have to feel at all self-conscious at school.
YP ID 24
[On aripiprazole] Basically ermm I probably say I still do it at school but I can easily make it look like I am just looking at something cos all it is really is a tensing of the muscle in the neck. It is not doing the movement it is just straining a muscle to get rid of a burning feeling. That’s it, I just look around and that’s what I do really, just make it look like nothing.
YP ID 22
Theme 7: behavioural intervention for tics is a ‘natural’ intervention that could be incorporated into daily life
This theme captures positive perceptions of behavioural intervention for tics either from direct experience or based on their understanding of what the treatment involves. The perception that it is ‘natural’ and fits with their existing strategies used to manage tics.
In response to a description of behavioural interventions, young people perceive that such an intervention involves doing something that they have tried before and that makes sense to them. However, of the 40 participants, 33 young people had not received a behavioural intervention. Of the 33 young people who had not received a behavioural intervention, 14 endorsed this subtheme, while a small number (n = 4) perceived both potential benefits and difficulties with this intervention.
Young people’s responses to the description of behavioural interventions suggested that many perceived that this treatment was similar to some of the behavioural strategies they have previously used when attempting to reduce or manage their tics. As such, behavioural interventions for tics were described as ‘natural’ with few or no adverse effects that, if learned adequately, could be incorporated into daily life.
I don’t really think it will be difficult. I think it will be like something I have done in the past, I think getting myself out of the habit of it could slow the process down a bit . . .
YP ID 32
That sounds like it makes sense, like it doesn’t sound like a magical cure, it sounds natural and that it would eventually work.
YP ID 22
It is good because it is behaviour it is not like taking a pill or doing something to you like making you dizzy or changing your mind set it is just to do with your behaviour.
Participant ID 25
Although young people who received a behavioural intervention for tics described engaging in a process that took time, the process was perceived as being positive. They describe from experience how they are able to practice exercises or techniques and how a behavioural intervention is generally a helpful approach. Seven participants received some form of behavioural intervention and some of them (n = 3) described both positive and negative experiences related to this treatment. Of the seven participants who received a behavioural intervention, five endorsed this subtheme.
So it wasn’t anything at all troubling about it [behavioural intervention for tics], it was not anything physically painful, it wasn’t anything mentally stressing, I didn’t find it stressing what so ever.
Participant ID 38
. . . it took me a while but now it works more. Like I don’t think about it. I don’t have to think oh I must bite my lip I just do it without thinking, but at the time I was just like oh this isn’t going to work but it got better.
Participant ID 5
Theme 8: limitations of behavioural interventions for the treatment of tics
This theme captures how young people perceive potential limitations or difficulties in practising a behavioural intervention or in the limited helpfulness of this treatment. Other direct experiences of behavioural interventions suggest they could be unhelpful or difficult.
When asked about their views on the description of behavioural interventions for tics, some young people also perceived that this treatment might require considerable effort and support from others. Young people also perceived that a behavioural intervention could be a slow process that involved several sessions with a health professional, and some of them anticipated difficulties remembering to practice treatment exercises. Thirteen of the 33 young people who had not received a behavioural intervention endorsed this subtheme.
It [behavioural intervention] requires a lot of proactivity on the person’s behalf so they need to make sure that they have got the support around them, that they have got a doctor that understands this therapy, which might be easier said than done. It is not easy to think about what you are doing and to change your behaviour, you know it is not an easy thing.
Participant ID 4
Well, [pause] I don’t mind going once but I probably wouldn’t want to go loads of times because when I get home . . . I would probably want to do something else like watch some telly or play with my mates. I don’t mind going once and then trying it out, but if it is going loads of times I probably wouldn’t want to do that.
Participant ID 20
Some young people felt that a behavioural intervention for tics would not be helpful for ‘major’ or strong tics, and one young person perceived that having to think about tics while receiving this treatment could worsen his tics.
Just having to think about it, you know when someone says this is what you think about when you have a tic, and then it makes you think about it more, so then you tic more.
Participant ID 8
Those young people who had received a behavioural intervention sometimes felt that it had not helped. Of the seven young people who had received behavioural intervention, five endorsed this subtheme. They described difficulties identifying and executing competing or antagonistic responses for motor or vocal tics, and one young person remembered developing a tic from a competing response.
. . . I thought habit reversal did work for a while and I thought yeah it is helping a lot and I am quite enjoying this, you know cos it is getting a lot better and it is helping my Tourettes go away. A week after I started to see improvements I started to do the habit that I was reversing.
Participant ID 39
When she [health professional] was telling me of the ways of how I could transfer my tics from like being a big massive leg flick to just like scrunching my hand up or something, I just couldn’t do it, I didn’t know how.
Participant ID 6
Qualitative evidence summary
Evidence summary from the systematic review of qualitative studies
The main themes emerging from the systematic review concerned access to care, lack of knowledge among health professionals regarding TS and difficulties and delays in obtaining a diagnosis.
Difficulties in accessing care
The qualitative review identified a number of barriers to treatment for tics among parents of young people with TS, such as difficulties finding well-informed treatment providers and concerns about the adverse effects of treatment, including medication and behavioural intervention. Some health professionals reported concerns that stigmatisation of the TS diagnosis may act as barrier to treatment among parents of young people with TS. However, there was little evidence from parents’ reports that they had not sought treatment because of concerns about being labelled or having a TS diagnosis.
Difficulties and delays in obtaining a Tourette syndrome diagnosis
The qualitative review suggested problems regarding the diagnosis of TS, including delays in receiving a diagnosis and perceived lack of knowledge of health professionals in recognising symptoms. Mol Debes et al. 209 identified delays in receiving a diagnosis of TS among young people, with a median of 2.8 years from age of tic onset until diagnosis.
Medications for tics
There was evidence from the qualitative review that concerns about adverse effects affects medication adherence. The survey conducted by Woods et al. 211 found that 43% of parents reported that an important reason for their child not receiving treatment were worries about the adverse effects of medication.
Evidence summary from the Qualitative study of Experiences of Services and Treatment survey of parents and carers of young people with Tourette syndrome
The key topic areas that emerged from the results of the survey are as follows.
Difficulties accessing care
The survey results identified that many parents and carers of young people with TS experienced problems accessing specialist care, including delays in being referred to specialist treatment and perceived a lack of knowledge and understanding from health professionals, particularly at primary care level.
Difficulties and delays in obtaining the diagnosis of Tourette syndrome
In the QuEST survey, the mean age at onset of tics was 5.9 years and the mean age of diagnosis was 9.1 years, giving an average delay of just over 3 years between symptom onset and diagnosis. The survey results showed that parents and carers of young people with TS experienced difficulties in accessing a TS diagnosis, inadequate information was given at diagnosis and lack of support from health professionals after a diagnosis was given.
Importance of providing information to parents and schools
The survey results supported the importance of providing comprehensive information about TS and tics to parents and carers of young people and to schools. More than two-thirds (68.5%) of parents and carers reported that a health-care professional had given them information about tics. Of those who received information, over three-quarters (77.7%) considered that the information was somewhat helpful or very helpful. The majority of parents who received information received this from CAMHS (58.7%) and paediatric services (37.3%). Rarely was information on TS provided to parents by primary care (3.5%).
Only just over one-third of parents/carers (38.1%) reported that a health-care professional had given information about TS and managing tics to their child’s school. When schools were given information, the majority of parents (70%) felt that this had been either helpful or very helpful. Parents commented that information given to schools had increased teachers’ knowledge and understanding of TS and about management strategies they could employ. In addition, this information was felt also to help teachers to better recognise tics and potentially access help for other children and young people with tics.
Parents’ experiences of medication for tics
Just over half of the parents (54.7%) reported that their child had received medication for tics. The most commonly prescribed drugs were two antipsychotics [risperidone (27.7%) and aripiprazole (19.9%)] and the noradrenergic agent clonidine (26.3%). The helpfulness (reported as somewhat helpful/very helpful) of medications varied, being greatest for aripiprazole (69.1%), intermediate for risperidone (39.5%) and clonidine (41.1%) and least for the older antipsychotics including haloperidol (25.0%) and sulpiride (15.4%). Adverse effects were also reported for all medications, with the fewest reports of moderate/severe adverse effects for aripiprazole (25.5%), intermediate for clonidine (46.6%) and greatest for risperidone (63.2%) and haloperidol (70.8%). Specific adverse effects were reported by parents for different drugs: risperidone – increased appetite, weight gain; clonidine – tiredness/fatigue, depression; and aripiprazole – tiredness/fatigue, weight gain, nausea, tremor/shakiness. However, the survey was not able to assess the prescribed dosage of particular medications and this limits the interpretation of the parents’ reports of both helpfulness and adverse effects.
Parents’ experiences of behavioural intervention for tics
Just over one-quarter of parents (25.9%) reported that their child had received a behavioural intervention for tics. Just under half of parents (48.6%) reported that their child’s behavioural intervention had been helpful (i.e. somewhat helpful/very helpful). Interestingly, adverse effects of behavioural interventions were reported by just over 10% of parents. Behavioural interventions were most commonly delivered by CAMHS (71.4%), followed by paediatric services (15.7%). Just over half (52.2%) of parents reported that their child had received fewer than five sessions, with just under one-quarter (23.2%) receiving between 5 and 10 sessions, and a similar proportion (24.6%) receiving more than 10 sessions.
Parents’ experiences of other treatments
A total of 78.5% of parents reported that their child had tried at least one additional treatment approach other than medication or a behavioural intervention (i.e. HRT/CBIT). The three most commonly reported were relaxation training (delivered in isolation from a comprehensive behavioural intervention, 39.8%), exercise (34.1%) and fish oils (30.5%).
Parents’ desired outcomes of treatment
For parents and carers of young people with TS, there was clear evidence that the most important outcome was reduction in their child’s tics and their child gaining greater control over their tics. Parents also mentioned the importance of managing their child’s anxiety and emotions associated with tics and increasing their child’s self-esteem. Interestingly, other broader outcomes such as the effect of tics on their child’s friendships, school functioning, leisure activities and family functioning were rated as significantly less important than tic reduction.
Evidence summary from in-depth interviews with young people with Tourette syndrome
The following key themes emerged from the interviews.
Perceived limited knowledge and understanding of TS by health professionals
Young people with TS described limited knowledge and understanding of TS among the health professionals they encounter. This theme was associated with difficulties accessing expert care, receiving insufficient information about the condition and problems trusting the adequacy of the treatment that was offered. When young people described being treated by knowledgeable health professionals, they felt understood, more confident and perceived that the advice given by health professionals was helpful.
Young people’s direct experience of medication for tics
Just over half (55%) of young people interviewed had received medication for tics. Among those that had received medication, there was a range of views expressed regarding both experiences of benefits (e.g. tic reduction and greater control over tics) as well as adverse effects. These adverse effects, which were associated with different types of medication, included drowsiness, tiredness, self-reported depression, nightmares, weight gain and a sense of not being oneself.
Young people’s perceived potential difficulties of medication for tics
Among those young people who had not taken medication for tics, the qualitative interviews identified concerns about taking medication, including adverse effects, difficulties remembering to take medication and a general perception that drugs cannot be ‘good’ and are not an appropriate way of managing behaviour.
Young people’s direct experience with behavioural interventions for tics
Less than one-fifth (17.5%) of young people interviewed had received a behavioural intervention for tics. Among this group with direct experience of a behavioural intervention, there were mixed views. Some young people felt they were able to practice the techniques learned and spoke about experiencing more control over their tics, and that the behavioural intervention built on previous strategies developed by the young people themselves to manage their tics. However, others found it difficult to practice behavioural strategies and one young person described a competing response that developed into a tic.
Young people’s perceived potential benefits and limitations of behavioural interventions for tics
The majority of young people interviewed had not received a behavioural intervention and generally held positive views about this type of approach. Behavioural intervention was perceived as being ‘natural’ and easy to engage with. However, some young people expressed reservations concerning behavioural intervention including the perception that behavioural intervention could be cumbersome and may not be effective with more severe tics.
Young people’s desired outcomes of treatment for tics
For young people, the most important outcomes of treatment were reducing or stopping tics and urges to tic, being able to manage emotions associated with tics (e.g. self-reported anxiety, stress and worries) and of gaining a sense of control over tics.
Discussion
Overall, the results of the part 2 study (QuEST) provide a unique insight into the perspectives of young people with TS, and their parents, concerning their experiences of care and treatment. The three components of QuEST (systematic review of qualitative studies, national online survey of parents, and in-depth interviews with young people) produced converging findings. The results are discussed below with recommendations for practice and further research.
Access to care and knowledge of Tourette syndrome among health professionals
All three components of QuEST highlighted difficulties and delays in both obtaining an initial referral to a specialist from primary care and then receiving a timely diagnosis and relevant information, appropriate treatment and follow-up once referred. The average delay of 3 years between onset of tics and diagnosis illustrates this problem. Some young people and parents reported feeling abandoned after receiving a diagnosis and were returned, in their view, prematurely to primary care without further follow-up and support.
Tourette syndrome is a condition that does not fit easily within the traditional partition of physical health and mental health services. We found that children and young people receiving care from a range of services including CAMHS, paediatrics, neurology, private practice and primary care. TS is often referred to as a ‘neurological disorder’ with the assumption that it will be managed within paediatric/neurology services. However, skilled management of disabling associated symptoms (e.g. anxiety, OCSs), common comorbidities (e.g. ADHD, OCD, ASD) and the provision of behavioural interventions typically falls within the remit of CAMHS. Clearly, a more integrated approach is required with the provision of information, diagnostic and evidence-based interventions for TS available from a single specialist service in a local area.
Recommendations
Those working in primary care should be aware of the prevalence and clinical features of children and young people presenting with tics and suspected TS. Local care pathways for children with suspected TS need to be established to accelerate access to expert assessment and diagnosis. It is important that health commissioners recognise that skilled assessment and treatment of TS should be provided by specialist CAMHS. Further health services research is required to identify barriers to care and unmet need for services for young people with TS and to develop targeted interventions to improve referral practice and reduce the delay between onset of tics and diagnosis.
Psychoeducation and information on Tourette syndrome
Young people and their parents valued the provision of clear information on TS and its treatment. While information on TS and its treatment was offered to the majority of parents, this was not universal, with up to 25% not receiving information from their clinical service.
There is a significant unmet need for provision of information on TS and its management designed for schools.
Recommendations
Information in various formats [written, digital versatile disc (DVD), internet and mobile applications (‘apps’)] should be made available for young people, parents and teachers. Research is required to investigate the impact of providing structured information on treatment adherence/engagement, self-efficacy and management of TS in schools.
Recognition of the role of anxiety and emotional symptoms in Tourette syndrome
Both young people and their parents consistently raised the issue of anxiety as both a cause and associated feature of TS. Young people described both anticipatory anxiety associated with tics (e.g. fears of ticcing in front of friends or in public places), anxious and tense feelings associated with ‘urges’ and tic suppression and the impact of extraneous stressors (e.g. exams) that exacerbate tics. Young people felt that interventions that targeted and reduced anxiety were particularly important.
Recommendations
Assessment of anxiety symptoms should be incorporated into routine assessments and outcome measures. The role of behavioural interventions and medication in reducing anxiety should be the subject of future research.
Medication for tics
Young people requested better understanding/information about how drugs work and their adverse effects. Findings from the systematic review of qualitative research suggested that lack of accurate information about medication and adverse effects was associated with less acceptance and uptake of medication, potentially leading to poorer outcomes. From the perspective of parents, aripiprazole was viewed as most helpful with least adverse effects. Regarding adverse effects associated with other drugs, clonidine was intermediate with the adverse effect most commonly reported with risperidone and the older antipsychotic drugs.
Recommendations
Better information should be provided with respect to both the rationale for using medication to treat tics and potential adverse effects. Given the positive perception of aripiprazole, RCTs are needed to evaluate clinical effectiveness and safety and the impact of dosage on outcome.
Behavioural interventions for tics
Access to behavioural interventions for young people with TS is clearly limited. Only one-quarter of young people in the online survey and less than one-fifth of young people interviewed had experience of a behavioural intervention. All of those young people had received medication in addition to behavioural interventions. Parents perceived that the helpfulness of behavioural interventions was similar to medication such as clonidine but with fewer adverse effects. The perception of behavioural interventions was generally positive, particularly among those young people that had not received this treatment. A particular attraction of HRT/CBIT for young people appeared to be that it involved learning techniques that built on their own efforts to manage tics and could then be practised so they became automatic responses. However, more than half of parents indicated that their child received fewer than five sessions of a behavioural intervention, which is below the recommended number of eight or more sessions for CBIT. 214 Overall, the limited access to behavioural interventions and the suggestion that young people may receive a shorter course of therapy than provided in clinical trials indicates that different forms of delivery, including greater use of technology, may be required.
Recommendations
Increased access to evidence-based behavioural interventions for tics is required for young people with TS. Future research should explore the potential for delivering behavioural interventions (e.g. HRT/CBIT) via video consultation and developing computerised interactive treatment HRT/CBIT packages that could widen access to behavioural interventions. The acceptability, feasibility and efficacy of alternative modes of delivery for behavioural interventions should be investigated through RCTs.
Important outcomes of treatment from the perspective of young people with Tourette syndrome and their parents
Both young people and their parents placed greatest importance on tic reduction and gaining control over tics. This concurs with the primary clinical trial outcomes that focus on tic reduction using scales such as the YGTSS. However, young people also indicated that reduction in anxiety and stress was also important and should be considered as a relevant outcome.
Recommendation
Existing outcome scales such as the YGTSS that focus on tics are clearly relevant from the perspective of young people and their parents. However, measuring reductions in anxiety and stress should also be considered as a relevant outcome of treatment and these measures incorporated into clinical trials.
Strengths and limitations
An anonymous online survey gave parents the opportunity to describe both positive and negative perceptions of treatment for tics. Internet access has increased rapidly and the Office for National Statistics has reported that in 2013, 83% of households had internet access. Text boxes provided the opportunity to express views with more breadth and depth and a large number of parents took advantage of this option. Parents were recruited through TA membership and through the TA website, which may have biased the sample towards those who are better informed, more concerned or more proactive in seeking information which is a limitation. A further limitation is that we cannot estimate the response rate of the survey as we do not know how many people accessing the TS website would be eligible for the survey. However, the sample size is a strength meeting the target recruitment.
The use of in-depth, semistructured interviews to explore young people’s views was a strength. The majority of interviews were conducted by telephone and it has been suggested that telephone interviews may be shorter and contain proportionately more prompting by the interviewer than face-to-face interviews. 218 However, telephone interviews provide more geographic heterogeneity and allow for a more inclusive sampling strategy. Young people are arguably comfortable using phone technology and the medium can give an enhanced sense of anonymity. Some young people chose to be interviewed with their parent present, which might have influenced responses; however, young people seemed to talk openly and to consult the parent only when they were unable to remember details of treatment, such as a drug name or the time when they received an intervention. The failure to include health professionals in the study could be considered a limitation but the views of clinicians were represented by the TEG. Furthermore, the focus of the research was to explore the experiences and perceptions of young people with TS and parents.
Conclusion
The perspective of young people with TS and their parents on their experience of treatment and care had previously received little attention. Therefore, these findings should lay the foundations for future research and contribute to the development of patient-centred treatment guideline.
Overall discussion
This report combined a systematic review and meta-analysis of interventions for TS with a qualitative study of young people with TS and their parents regarding their experiences and perceptions of care and treatment.
To our knowledge, this is the first comprehensive systematic review and meta-analysis of pharmacological, behavioural and physical interventions for children and young people with TS. It is also the first qualitative study involving in-depth interviews with young people with TS exploring their experiences of care and treatment.
Detailed discussion of the results from the part 1 and part 2 studies has followed the individual sections above, therefore, the aim of this overall discussion is to draw together and integrate the main findings from both studies and discuss implications for practice and future research.
Key findings
Pharmacological interventions
Qualitative study of Experiences of Services and Treatment national survey and qualitative study
-
The online national survey found that just over half of young people with TS had received medication for tics. The most commonly used drugs were risperidone, clonidine and aripiprazole.
-
Young people and parents reported that medication could be helpful in reducing tics but frequently expressed concerns about adverse effects and lack of provision of relevant information explaining the rationale for using medication for tics and possible adverse effects.
-
Of the medications surveyed, aripiprazole was perceived by parents of young people with TS as being most helpful with least troublesome adverse effects.
Systematic review and meta-analysis
-
Antipsychotics and noradrenergic agents are the only classes of drug with clear RCT evidence suggesting short-term clinical effectiveness for treating tics in children and young people with TS. The size of effect on tic reduction for antipsychotics and noradrenergic agents is moderate to large and is likely to be clinically meaningful.
-
There are no RCTs of aripiprazole compared with placebo. However, head-to-head comparisons suggest that aripiprazole may be equally effective as other antipsychotics for tics. The main differences between antipsychotic drugs concerns their adverse effect profiles.
-
Among noradrenergic agents, clonidine and guanfacine (not available in UK) have the best evidence for clinical effectiveness. However, there are unlikely to be important clinical differences in tic reduction among antipsychotics and between antipsychotics and noradrenergic agents. There is no clear evidence that the clinical effectiveness of antipsychotics or noradrenergic agents is moderated by either tic severity or comorbidity.
-
Topiramate, pergolide, metoclopramide and desipramine are other agents with RCT evidence that suggests they may be effective in reducing tics. However, the known adverse effect profiles of these drugs, balanced against relatively weak evidence of benefits, means that these agents are unlikely to be considered clinically useful for the treatment of tics in children and young people with TS.
-
There is clear RCT evidence that, in the short term, neither stimulants and atomoxetine (used to treat comorbid TS and ADHD) nor fluoxetine (used to treat comorbid TS and OCD) significantly exacerbate or worsen tics and atomoxetine may reduce tics.
-
A number of other agents were reviewed and were found unlikely to be clinically effective for treating tics: levetiracetam, selegiline, pramipexole, mecamylamine, ondansetron, baclofen, omega-3 fatty acids and transdermal nicotine patches.
Behavioural interventions
Qualitative study of Experiences of Services and Treatment national survey and qualitative study
-
The online national survey found that about one-quarter of young people with TS had received a behavioural intervention (broadly conforming to HRT/CBIT) for tics. Behavioural interventions were almost always delivered together with medication.
-
Young people with TS and parents reported that behavioural interventions (HRT/CBIT) could be helpful in reducing tics and adverse effects were rarely reported. Young people reported that they valued the opportunity to learn behavioural techniques that helped them control their tics and build on strategies that they had developed themselves. However, some young people found these approaches could be difficult to use and were not always helpful.
Systematic review and meta-analysis
-
There is clear evidence that HRT/CBIT produces improvements in tics that may be clinically meaningful. There is no evidence that the effects of HRT/CBIT are moderated by tic severity.
-
There is some preliminary evidence that delivering HRT/CBIT remotely via video consultation (telemedicine) may be as effective as face-to-face therapy.
-
There are no RCTs of negative massed practice or ERP compared with control interventions. However, head-to-head comparisons suggest that HRT is a more effective intervention than negative practice, while HRT and ERP may be equally effective interventions for tics. The quality of evidence for interventions other than HRT/CBIT is low and so conclusions drawn from this evidence should be treated with caution.
-
There is no clear evidence to suggest that relaxation therapy in isolation is an effective treatment for tics. AC training may be a useful intervention for young people with tics and comorbid disruptive behaviour and behaviour problems may be improved by parent training, although there is no evidence that parent training is an effective treatment for tics.
Physical interventions and Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection
Systematic review and meta-analysis
-
There is no evidence to suggest that the physical interventions reviewed – DBS, rTMS, i.v. immunoglobulin, botulinum toxin and acupuncture – are sufficiently effective and safe to be considered as treatments for tics in children and young people with TS.
-
There is no evidence that i.v. immunoglobulin or penicillin are an effective treatments for tics in children and young people identified with PANDAS.
-
There is a high degree of uncertainty with respect to the benefits and harms of plasma exchange treatment of tics in children and young people identified with PANDAS.
Access to care and delay in diagnosis
-
Young people with TS and their parents frequently reported concerns about lack of knowledge of TS and its treatment among health professionals both in primary care (GPs) and secondary care (CAMHS and paediatrics).
-
Delays in recognition and referral for diagnosis were common with the average delay of 3 years from onset of tics to diagnosis of TS.
-
There appeared to be a lack of clear care pathways for children and young people with tics and TS. Inadequate care for children and young people with TS may result from a lack of integration in the commissioning and provision of physical and mental health services for children and young people with TS care falling between this gap.
Provision of information and psychoeducation
Qualitative study of Experiences of Services and Treatment national survey and qualitative study
-
Young people with TS and their parents placed great emphasis on the provision of information about TS and its management. Often this information was not provided by health services or was viewed as inadequate. An important finding was the lack of information provided by health services to schools on TS and its management. In only one-quarter of children and young people surveyed with TS had health professionals contacted and provided information to the school.
Recognition of associated emotional symptoms including anxiety
Qualitative study of Experiences of Services and Treatment national survey and qualitative study
-
Young people with TS and their parents highlighted the importance of recognising and managing anxiety symptoms associated with TS. This is a complex issue as anxiety can be both a cause and consequence of tics and is also related to premonitory urges.
What treatment outcomes are important to young people with Tourette syndrome and their parents?
Qualitative study of Experiences of Services and Treatment national survey and qualitative study
-
Young people with TS and their parents regarded reducing the frequency and intensity of tics and increasing control over tics as the most important outcomes of treatment.
-
Reducing anxiety and stress associated with tics was also viewed by young people in particular as being important.
Implications of findings for practice and research
Drawing together the results from the part 1 and part 2 study, there are a number of implications for practice as well as identified gaps in the evidence requiring further research. Detailed research recommendations are presented in Recommendations for further research.
-
It is clear that both medication (antipsychotics and noradrenergic agents) and behavioural intervention (HRT/CBIT) can be effective in treating tics. However, the order in which these treatments should be delivered, and if they are more effective in combination, is unclear. Therefore, RCTs are required comparing the effectiveness of pharmacological and behavioural interventions delivered alone with a combined pharmacological/behavioural intervention.
-
Aripiprazole was perceived by parents of young people with TS as the most helpful medication with a relatively favourable adverse effect profile. However, lack of information on dosing and comparison with a control intervention means that this cannot be interpreted as evidence of effectiveness or lack of harm. Currently, there are no placebo controlled studies available for aripiprazole in the treatment of tics, although trials may be ongoing. Therefore, evidence from controlled trials is needed for aripiprazole before firm conclusions regarding its clinical effectiveness and cost-effectiveness and safety can be drawn.
-
There remains uncertainty regarding the clinical effectiveness and cost-effectiveness of pharmacological and behavioural interventions for tics in the presence of comorbidities (e.g. ADHD, OCD, ASD). So far, clinical trials have been inadequately designed to address moderation of outcome by comorbidity and tic severity. Clinically, this is an important question as comorbidity is the rule with TS rather than the exception.
-
Behavioural intervention (HRT/CBIT) is effective and well received by young people with TS and their parents. However, access is limited with up to three-quarters of young people with TS surveyed not receiving this intervention. Factors affecting access are likely to be limited availability of trained HRT/CBIT therapists, as well as travel to specialist centres and the need for frequent appointments (e.g. up to 10–12 face-to-face sessions of HRT/CBIT). Access to treatment may be increased if the behavioural intervention could be delivered remotely (via video consultation/telemedicine) and/or in digital formats using apps available through mobile devices. An important question for research is whether or not these forms of delivery of behavioural interventions are practicable and acceptable, genuinely increase access and are cost-effective.
-
The findings highlight difficulties in accessing specialist services and delays in diagnosis. Health services research is required to understand the key processes and barriers in the referral and care pathway in order to target health service system change. Furthermore, the factors contributing to delayed diagnosis need to be better understood as well as the impact of duration of untreated tics on outcome.
-
Current lack of provision of information to schools should be addressed by health services. However, further research is also needed to identify what aspect of information on TS and its management are most relevant to schools, who it should be delivered to, in what format and what outcomes are important. Cluster RCTs will be needed to evaluate the clinical effectiveness and cost-effectiveness of information packages for schools.
-
The importance placed on tic reduction by young people with TS and their parents suggests that current outcome measures, such as the YGTSS, remain relevant and appropriate tools for clinical practice and research trials. However, the findings also highlight the importance of anxiety symptoms and further research is required to determine how these symptoms are best measured in children and young people with TS. Finally, the clinical importance of changes on rating scales can be difficult to interpret – both in clinical practice and from the results of trials. Therefore, research is needed to confirm what constitutes clinically meaningful change using rating scales such as the YGTSS.
Overall conclusions
The findings of this systematic review and evidence synthesis show that there are effective pharmacological (e.g. antipsychotics and noradrenergic agents) and behavioural interventions (e.g. HRT/CBIT) available for the treatment of tics in children and young people with TS. However, the number and quality of clinical trials is low and this downgrades the strength of the evidence and conclusions. Larger and better conducted trials addressing important clinical uncertainties are required. Access to behavioural interventions is currently limited and delays in diagnosis of TS are common. Knowledge of TS and its management among health professionals is often inadequate and information provision to schools is generally poor. Information packages for schools should be developed and evaluated. The relevance of associated anxiety and emotional symptoms is often overlooked and requires greater attention from both clinical practice and research. Further research is needed to inform the development of clinical guidelines for children and young people with TS, in particular to answer questions about the order in which interventions should be given, how interventions should be combined and how their clinical effectiveness is affected by comorbidity and tic severity. Aripiprazole appears a promising drug treatment for tics; however, further clinical trial evidence is required to establish its efficacy, dosing strategy and safety. Poor access to behavioural interventions is an important issue and research is needed to test whether or not technological innovations (e.g. mobile digital and video technology) can be used to widen access while maintaining the effectiveness of the intervention. Tic reduction is a relevant primary outcome for both clinical practice and research trials and secondary outcomes should include associated anxiety, stress and self-esteem.
Recommendations for further research
The TEG has made the following recommendations for research, based on its review of evidence:
-
Is the combination of a behavioural intervention together with medication management more clinically effective and cost-effective in the short term than either behavioural intervention alone or medication management alone for the treatment of moderate and severe tics in children and young people with Tourette syndrome?
The behavioural intervention would be an eight-session manualised therapy package that includes HRT, psychoeducation, relaxation training and contingency management. Medication management would be protocol-based and allow use of either clonidine, risperidone or aripiprazole. The key outcomes should include symptoms, impairment, QoL, treatment acceptability, experience of care, level of psychosocial functioning and the cost-effectiveness of the interventions.
The programme of research would compare the clinical effectiveness and cost-effectiveness of behavioural intervention alone, compared with medication management alone, and compared with a behavioural intervention and medication management combined, for the treatment of moderate and severe tics in children and young people with TS. The study would use a randomised controlled superiority trial design. The trial would be adequately powered to evaluate the moderating effects of (1) common comorbid conditions (e.g. ADHD, OCD) and (2) symptom severity on outcome.
Why is this important?
Currently, about half of children and young people receive medication as treatment for TS (the most commonly prescribed medications are clonidine, risperidone or aripiprazole), and about one-quarter receive some form of behavioural intervention (e.g. HRT). Medication carries the risk of adverse effects and is often required for the long term as treatment is symptomatic. Therefore, psychological interventions tailored for treatment of tics (e.g. HRT) may either be an effective alternative treatment or in combination with medication allow lower effective doses to be used, and potentially the combination treatment may result in better outcomes. RCTs have shown modest benefits of behavioural interventions based on HRT and for medication (noradrenergic and antipsychotic drugs). However, it is unclear (1) if a combination of a behavioural intervention and medication is superior to either intervention alone, (2) if a behavioural intervention alone is as effective as medication alone, and (3) whether or not the clinical effectiveness of these interventions (a behavioural intervention, medication and combination therapy) is moderated by comorbid conditions (e.g. ADHD, OCD) and tic severity.
The suggested programme of research would need to test out, using an adequately powered, randomised placebo controlled design, the clinical effectiveness and cost-effectiveness of using aripiprazole, compared with (1) a noradrenergic agent (clonidine) and with (2) placebo plus standard care, for the treatment of moderate and severe tics in children and young people with TS. The outcomes considered should include QoL, symptomatic and functional improvements, treatment acceptability and adverse effects.
-
What is the feasibility, acceptability, clinical effectiveness and cost-effectiveness of a behavioural intervention for tics that is delivered remotely [e.g. via telemedicine/videoconference or Skype™ (Microsoft Corporation, Redmond, WA, USA)] compared with traditional face-to-face delivery of therapy?
The suggested programme of research would need to test out, using an adequately powered, randomised controlled design, the clinical effectiveness and cost-effectiveness of using remotely delivered behavioural interventions (e.g. via telemedicine/videoconference or Skype) compared with standard face-to-face delivery of behaviour therapy. The feasibility and acceptability of a remotely delivered behavioural intervention would need to be established first before evaluating clinical effectiveness and cost-effectiveness of the intervention using a non-inferiority trial design. The outcomes considered should include QoL, symptomatic and functional improvements, number of sessions received, treatment acceptability and cost-effectiveness.
Why is this important?
Behavioural interventions (CBIT) have been shown to be effective for reducing tics in children with TS. Unfortunately, there remain significant barriers to behavioural intervention with no more than one-quarter of young people with TS accessing behavioural intervention in the UK. Behavioural intervention is delivered over 8–10 weekly sessions, which is challenging for routine NHS practice to achieve and in a national survey, almost half of young people received fewer than five sessions. There is low-quality evidence that videoconference CBIT is acceptable and equally effective in reducing tics as face-to-face CBIT. Therefore, remote delivery of a behavioural intervention may increase both access and adherence to it and allow the intervention to be delivered to more young people at less cost.
-
What is the feasibility, acceptability, clinical effectiveness and cost-effectiveness of a behavioural intervention (HRT/CBIT) and self-monitoring mobile app for tics compared with traditional face-to-face delivery of therapy?
The suggested programme of research would need to test out, using an adequately powered, randomised controlled design, the clinical effectiveness and cost-effectiveness of using a HRT/CBIT mobile app compared with standard face to face delivery of behavioural therapy. The first phase of programme of research would develop an app (computerised version of CBIT – eCBIT) that could be accessed on a personal computer (PC), tablet and smartphone platforms. The feasibility and acceptability of the HRT/CBIT app for tics would need to be established first before evaluating clinical effectiveness and cost-effectiveness of the intervention in an adequately powered clinical trial using a non-inferiority design. The outcomes considered should include QoL, symptomatic and functional improvements, number of sessions received, treatment acceptability and cost-effectiveness.
Why is this important?
Computerised delivery of behavioural therapy is increasingly available for conditions such as depression. Young people (16- to 24-year olds) have the greatest smartphone usage in the population. 219 Therefore, the advantages of a behavioural intervention and monitoring app include increased accessibility, more frequent opportunities for rehearsal and practice, and self-pacing. Furthermore, monitoring of tics by traditional rating scales is subjective and retrospectively averaged over the previous 7 days. The development of automated tic monitoring via a webcam on a mobile telephone, tablet or PC has the potential to allow the impact of computerised behaviour therapy to be assessed in real time and fed back to the young person and their therapist.
-
What is the clinical effectiveness and cost-effectiveness of aripiprazole for the treatment of tics in children and young people with TS?
The suggested programme of research would need to test out, using an adequately powered, randomised placebo controlled design, the clinical effectiveness and cost-effectiveness of using aripiprazole, compared with (1) a noradrenergic agent (clonidine) and with (2) placebo plus standard care, for the treatment of moderate and severe tics in children and young people with TS. The outcomes considered should include QoL, symptomatic and functional improvements, treatment acceptability and adverse effects.
Why is this important?
Currently, aripiprazole is prescribed in about one-quarter of children and young people receiving medication for tics. Survey data from parents and young people suggest that aripiprazole may be an effective treatment and is better tolerated than either clonidine or risperidone. Despite its increasing use, there is only one low-quality randomised head-to-head trial of aripiprazole in children and young people with TS and no RCT with a placebo control arm, which is necessary to establish clinical effectiveness.
-
What is the clinical effectiveness and cost-effectiveness of a psychoeducation/information package on TS and its management designed for schools?
The suggested programme of work involves developing a TS information package for schools in collaboration with clinical experts, teachers and service users. Different modes of delivery will be piloted. The outcomes considered should include both the uptake of the package as well as its impact on tic management in schools, knowledge and attitudes of teachers, school performance/attendance and acceptance/reduced victimisation by peers. Following development and piloting of the package, its clinical effectiveness and cost-effectiveness should be evaluated using both a cluster RCT and qualitative research.
Why is this important?
Less than half of parents/carers (38%) in a national survey reported that a health-care professional contacted their child’s school to give information about managing tics at school. Among those parents/carers whose child’s school was contacted, most of them (70%), reported that the information given to the school was somewhat helpful or very helpful.
-
What are the barriers to care, including diagnosis and treatment, for children and young people with TS?
This programme of health services research would involve analysis of routes of referral, types of care and barriers to care for tics and the common comorbidities of TS, as well as access to liaison for educational and social liaison. The methodology would be similar to that used by Sayal et al. 220 for ADHD and would explore services accessed by children identified in an epidemiological sample and establish their care and outcomes.
Why is this important?
Findings from the qualitative literature review and national survey (QuEST) show that delays in referral to specialist services are common, with an average duration of 3 years between onset of tics and diagnosis of TS.
-
What is the clinical effectiveness and cost-effectiveness of an exercise programme for the treatment of tics in children and young people with TS?
The suggested programme of research would need to test out, using an adequately powered, randomised controlled design, the clinical effectiveness and cost-effectiveness of using an exercise programme plus TAU compared with usual care for treatment of tics in children and young people with TS. The first phase of programme of research would develop an exercise programme that is acceptable and enjoyable for young people of different ages as well as effective in reducing tics. The outcomes considered should include QoL, symptomatic and functional improvements (both short and long term), number of sessions received, treatment acceptability and cost-effectiveness.
Why is this important?
Many young people with tics report that exercise is helpful in reducing tics and anxiety levels and increases well-being and self-confidence. Furthermore, young people with tics may be excluded (or exclude themselves) from traditional forms of exercise and sport at school, while medications for tics can also cause overweight and reduce exercise tolerance. Currently, it is unclear which forms of exercise are most helpful in reducing tics and whether or not the benefits are sustained outside the periods of exercise.
-
What constitutes a clinical meaningful change on the YGTSS from the perspective of children and young people with TS and their carers?
The suggested programme of research would establish the minimum change in tic score on the YGTSS that is associated with a clinically meaningful change in function from the perspective of children and young people with TS and their carers. An adequately powered study would collect longitudinal observational data from the YGTSS and independent measures of clinical change (e.g. CGI-I scale) rated by children, young people and carers.
Why is this important?
The YGTSS is the most widely used outcome scale used in clinical practice and research trials. Currently, there is no clear consensus of what constitutes a clinically meaningful change on the YGTSS when results are reported from clinical trials. Furthermore, what work that does exist on this topic has focused on assessing meaningful clinical change from the perspective of clinicians only.
Acknowledgements
Contribution of authors
Professor Chris Hollis (Professor of Child and Adolescent Psychiatry) is the principal investigator of the study and chairperson of the TEG. He initiated the project and substantial contributions to conception and design of project, wrote parts of the report and critically revised the report during development and final editing.
Dr Mary Pennant (systematic reviewer, NCCMH) conducted the systematic review including the acquisition of data, data analysis and drafting the report (Chapters 2 and 3 qualitative systematic review).
Dr José Cuenca (research assistant, Institute of Mental Health, University of Nottingham) conducted the QuEST survey and interviews including acquisition of data, data analysis and drafting the report (Chapter 3 QuEST survey and interviews).
Professor Cris Glazebrook (Professor of Health Psychology and Head of Division of Psychiatry, University of Nottingham) made substantial contributions to the conception and design of the project, led the analysis of the survey and interview data and supervised the drafting the QuEST study findings (Chapter 3 QUEST survey and interviews).
Professor Tim Kendall (Director, NCCMH and Medical Director/Consultant Psychiatrist, Sheffield Health and Social Care NHS Foundation Trust) is the facilitator of the project and TEG. He made substantial contributions to conception and design of the project and critically revised the report during development.
Dr Craig Whittington (Associate Director – Clinical Effectiveness, NCCMH) oversaw the systematic review work in Chapter 2 of the report, designed the systematic review protocol, contributed to the analysis and interpretation of data and critically revised the report during development.
Ms Sarah Stockton (senior information scientist, NCCMH) conducted the searches for Chapters 2 and 3 of the report.
Dr Linnéa Larsson (project manager and research assistant, NCCMH), drafted sections of the report (Chapter 2), contributed to the acquisition of data (Chapter 2) and managed the development of the report and the overall project.
Dr Penny Bunton (Consultant Clinical Psychologist, University of Manchester) critically revised the behavioural interventions section of the report.
Mrs Suzanne Dobson (CEO Tourettes Action) drafted sections of the main introduction of the report.
Dr Madeleine Groom (Lecturer in Developmental Cognitive Neuroscience, University of Nottingham) critically revised the physical interventions section of the report.
Dr Tammy Hedderly (Consultant in Paediatric Neurology, Kings College Hospital NHS Foundation Trust, London) critically revised the physical interventions section of the report and drafted the sections of the main introduction of the report.
Dr Isobel Heyman (Consultant in Child & Adolescent Psychiatry, Great Ormond Street Hospital for Children, London) critically revised the pharmacological interventions section of the report and drafted sections of the main introduction of the report.
Professor Georgina M Jackson (Professor of Cognitive Neuropsychology, University of Nottingham) critically revised the behavioural interventions section of the report.
Professor Stephen Jackson (Professor of Cognitive Neuroscience, University of Nottingham) critically revised the physical interventions section of the report.
Dr Tara Murphy (Consultant Clinical Psychologist, Great Ormond Street Hospital for Children, London) critically revised the behavioural interventions section of the report.
Dr Hugh Rickards (Consultant Neuropsychiatrist, National Centre for Mental Health, Birmingham) critically revised the pharmacological interventions section of the report.
Professor Mary Robertson (Professor of Neuropsychiatry, St Georges Hospital NHS Foundation Trust, London) drafted the main introduction of the report.
Dr Jeremy Stern (Consultant Neurologist, St Georges Hospital NHS Foundation Trust, London) critically revised the pharmacological interventions section of the report.
Tourette Expert Group members
Professor Chris Hollis (chairperson): see Contribution of authors.
Professor Tim Kendall (facilitator): see Contribution of authors.
Ms Seonaid Anderson: managed Tourettes Action website and portal for national parents survey (part 2).
Dr Penny Bunton: see Contribution of authors.
Mrs Suzanne Dobson: see Contribution of authors.
Dr Madeleine Groom: see Contribution of authors.
Dr Tammy Hedderly: see Contribution of authors.
Dr Isobel Heyman: see Contribution of authors.
Professor Georgina M Jackson: see Contribution of authors.
Professor Stephen Jackson: see Contribution of authors.
Dr Tara Murphy: see Contribution of authors.
Dr Hugh Rickards: see Contribution of authors.
Professor Mary Robertson: see Contribution of authors.
Dr Jeremy Stern: see Contribution of authors.
Mr Nick Bingham: Patient and Public Involvement (PPI) representative. Expert in lived experience of Tourette syndrome.
Mr David Jones: Patient and Public Involvement (PPI) representative. Expert in lived experience of Tourette syndrome.
Ms Katherine Leggett, Senior Project Manager, NCCMH managed the project from 12 June – 14 August 2013.
Dr Clare Taylor, senior editor: edited manuscript.
Ms Nuala Ernest, assistant editor: edited manuscript.
Data sharing statement
Requests for data sharing for secondary research purposes should be sent to the corresponding author.
Publications
Cuenca J, Glazebrook C, Kendall T, Hedderly T, Heyman I, Jackson G, et al. Perceptions of treatment for tics among young people with Tourette syndrome and their parents: a mixed methods study. BMC Psychiatr 2015;15:46.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Diagnostic and Statistical Manual of Mental Disorders. DSM-5. Washington, DC: American Psychiatric Association; 2013.
- International Classification of Diseases and Health-Related Problems. Geneva: WHO; 1992.
- Robertson MM. The Gilles de la Tourette syndrome: the current status. Br J Psychiatry 1989;154:147-69. http://dx.doi.org/10.1192/bjp.154.2.147.
- Robertson MM. Annotation: Gilles de la Tourette syndrome – an update. J Child Psychol Psychiatry 1994;35:597-611. http://dx.doi.org/10.1111/j.1469-7610.1994.tb01209.x.
- Robertson MM, Trimble MR, Lees AJ. Self-injurious behaviour and the Gilles de la Tourette syndrome: a clinical study and review of the literature. Psychol Med 1989;19:611-25. http://dx.doi.org/10.1017/S0033291700024211.
- Kurlan R, Daragjati C, Como PG, McDermott MP, Trinidad KS, Roddy S, et al. Non–obscene complex socially inappropriate behavior in Tourette’s syndrome. J Neuropsychiatry Clin Neurosci 1996;8:311-17. http://dx.doi.org/10.1176/jnp.8.3.311.
- Robertson MM. Gilles de la Tourette Syndrome: The Current Status. London: Royal College of Psychiatrists; 2013.
- Robertson MM, Banerjee S, Kurlan R, Cohen DJ, Leckman JF, McMahon W, et al. The Tourette syndrome diagnostic confidence index: development and clinical associations. Neurology 1999;53:2108-12. http://dx.doi.org/10.1212/WNL.53.9.2108.
- Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed 2012;97:166-75. http://dx.doi.org/10.1136/archdischild-2011-300585.
- Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: The epidemiological and prevalence studies. J Psychosom Res 2008;65:461-72. http://dx.doi.org/10.1016/j.jpsychores.2008.03.006.
- Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 2: Tentative explanations for differing prevalence figures in GTS, including the possible effects of psychopathology, aetiology, cultural differences, and differing phenotypes. J Psychosom Res 2008;65:473-86. http://dx.doi.org/10.1016/j.jpsychores.2008.03.007.
- Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol 2012;47:77-90. http://dx.doi.org/10.1016/j.pediatrneurol.2012.05.002.
- Ferrao YA, de Alvarenga PG, Hounie AG, de Rosario MC, Miguel EC, Martino D, et al. Tourette Syndrome. New York, NY: Oxford University Press; 2013.
- Cath DC, Ludolph AG, Martino D, Leckman JF. Tourette Syndrome. New York, NY: Oxford University Press; 2013.
- Khalifa N, von Knorring AL. Prevalence of tic disorders and Tourette syndrome in a Swedish school population. Dev Med Child Neurol 2003;45:315-19. http://dx.doi.org/10.1111/j.1469-8749.2003.tb00402.x.
- Khalifa N, von Knorring AL. Tourette syndrome and other tic disorders in a total population of children: clinical assessment and background. Acta Paediatr 2005;94:1608-14. http://dx.doi.org/10.1111/j.1651-2227.2005.tb01837.x.
- Mol Debes NMM, Hjalgrim H, Skov L. Validation of the presence of comorbidities in a Danish clinical cohort of children with Tourette syndrome. J Child Neurol 2008;23:1017-27. http://dx.doi.org/10.1177/0883073808316370.
- Scahill L, Dalsgaard S, Bradbury K, Martino D, Leckman JF. Tourette Syndrome. New York, NY: Oxford University Press; 2013.
- Robertson MM, Eapen V, Martino D, Leckman JF. Tourette Syndrome. New York, NY: Oxford University Press; 2013.
- Fernandez TV, State MW, Martino D, Leckman JF. Tourette Syndrome. New York, NY: Oxford University Press; 2013.
- Pauls DL, Leckman JF, Cohen DJ. Evidence against a genetic relationship between Tourette’s syndrome and anxiety, depression, panic and phobic disorders. Br J Psychiatry 1994;164:215-21. http://dx.doi.org/10.1192/bjp.164.2.215.
- Robertson MM, Trimble MR, Lees AJ. The psychopathology of the Gilles de la Tourette syndrome. A phenomenological analysis. Br J Psychiatry 1988;152:383-90. http://dx.doi.org/10.1192/bjp.152.3.383.
- Robertson MM, Trimble MR. Some personality variables in functional neurological disorders. Behav Neurol 1988;1:23-8. http://dx.doi.org/10.1155/1988/839812.
- Robertson MM, Channon S, Baker J, Flynn D. The psychopathology of Gilles de la Tourette’s syndrome. A controlled study. Br J Psychiatry 1993;162:114-17. http://dx.doi.org/10.1192/bjp.162.1.114.
- Robertson MM, Banerjee S, Hiley PJ, Tannock C. Personality disorder and psychopathology in Tourette’s syndrome: a controlled study. Br J Psychiatry 1997;171:283-6. http://dx.doi.org/10.1192/bjp.171.3.283.
- Rickards H, Robertson M. A controlled study of psychopathology and associated symptoms in Tourette syndrome. World J Biol Psychiatry 2003;4:64-8. http://dx.doi.org/10.3109/15622970309167953.
- Carter AS, O’Donnell DA, Schultz RT, Scahill L, Leckman JF, Pauls DL. Social and emotional adjustment in children affected with Gilles de la Tourette’s syndrome: associations with ADHD and family functioning. Attention Deficit Hyperactivity Disorder. J Child Psychol Psychiatry 2000;41:215-23. http://dx.doi.org/10.1017/S0021963099005156.
- Robertson MM, Banerjee S, Eapen V, Fox-Hiley P. Obsessive compulsive behaviour and depressive symptoms in young people with Tourette syndrome. A controlled study. Eur Child Adolesc Psychiatry 2002;11:261-5. http://dx.doi.org/10.1007/s00787-002-0301-3.
- Robertson MM, Orth M. Behavioral and affective disorders in Tourette syndrome. Adv Neurol 2006;99:39-60.
- Sukhodolsky DG, Scahill L, Zhang H, Peterson BS, King RA, Lombroso PJ, et al. Disruptive behavior in children with Tourette’s syndrome: association with ADHD comorbidity, tic severity, and functional impairment. J Am Acad Child Adolesc Psychiatry 2003;42:98-105. http://dx.doi.org/10.1097/00004583-200301000-00016.
- Rizzo R, Curatolo P, Gulisano M, Virzi M, Arpino C, Robertson MM. Disentangling the effects of Tourette syndrome and attention deficit hyperactivity disorder on cognitive and behavioral phenotypes. Brain Dev 2007;29:413-20. http://dx.doi.org/10.1016/j.braindev.2006.12.003.
- Haddad AD, Umoh G, Bhatia V, Robertson MM. Adults with Tourette’s syndrome with and without attention deficit hyperactivity disorder. Acta Psychiatr Scand 2009;120:299-307. http://dx.doi.org/10.1111/j.1600-0447.2009.01398.x.
- Elstner K, Selai CE, Trimble MR, Robertson MM. Quality of Life (QOL) of patients with Gilles de la Tourette’s syndrome. Acta Psychiatr Scand 2001;103:52-9. http://dx.doi.org/10.1034/j.1600-0447.2001.00147.x.
- Cavanna AE, Luoni C, Selvini C, Blangiardo R, Eddy CM, Silvestri PR, et al. Disease-specific quality of life in young patients with tourette syndrome. Pediatr Neurol 2013;48:111-14. http://dx.doi.org/10.1016/j.pediatrneurol.2012.10.006.
- Eddy CM, Cavanna AE, Gulisano M, Cali P, Robertson MM, Rizzo R. The effects of comorbid obsessive–compulsive disorder and attention-deficit hyperactivity disorder on quality of life in tourette syndrome. J Neuropsychiatry Clin Neurosci 2012;24:458-62. http://dx.doi.org/10.1176/appi.neuropsych.11080181.
- Robertson MM, Walkup JT, Mink JW, St. P. McNaught K. A Family Guide to Tourette Syndrome. Bloomington, IN: iUniverse Inc., Tourette Syndrome Association Inc.; 2012.
- Sukhodolsky DG, Eicher VW, Leckman JF, Martino D, Leckman JF. Tourette Syndrome. New York, NY: Oxford University Press; 2013.
- Bawden HN, Stokes A, Camfield CS, Camfield PR, Salisbury S. Peer relationship problems in children with Tourette’s disorder or diabetes mellitus. J Child Psychol Psychiatry 1998;39:663-8. http://dx.doi.org/10.1017/S0021963098002480.
- Lee MY, Chen YC, Wang HS, Chen DR. Parenting stress and related factors in parents of children with Tourette syndrome. J Nurs Res 2007;15:165-74. http://dx.doi.org/10.1097/01.JNR.0000387612.85897.9a.
- Cooper C, Robertson MM, Livingston G. Psychological morbidity and caregiver burden in parents of children with Tourette’s disorder and psychiatric comorbidity. J Am Acad Child Adolesc Psychiatry 2003;42:1370-5. http://dx.doi.org/10.1097/01.CHI.0000085751.71002.48.
- Robertson MM, Bedard MA, Agid Y, Chouinard S, Fahn S, Korczyn AD, et al. Mental and Behavioral Dysfunction in Movement Disorders. Totowa, NJ: Humana Press; 2003.
- Grados MA, Mathews CA. Tourette Syndrome Association International Consortium for Genetics; Latent class analysis of Gilles de la Tourette syndrome using comorbidities: clinical and genetic implications. Biol Psychiatry 2008;64:219-15. http://dx.doi.org/10.1016/j.biopsych.2008.01.019.
- Cavanna AE, Critchley HD, Orth M, Stern JS, Young M-B, Robertson MM. Dissecting the Gilles de la Tourette spectrum: a factor analytic study on 639 patients. J Neurol Neurosurg Psychiatry 2011;82:1320-3. http://dx.doi.org/10.1136/jnnp.2010.225029.
- Rodgers S, Müller M, Kawohl W, Knöpfli D, Rössler W, Castelao E, et al. Sex-related and non sex-related comorbidity subtypes of tic disorders: a latent class approach. Eur J Neurol 2014;21:700-7. http://dx.doi.org/10.1111/ene.12274.
- Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain 2000;123:425-62. http://dx.doi.org/10.1093/brain/123.3.425.
- Robertson MM. Gilles de la Tourette syndrome: the complexities of phenotype and treatment. Br J Hosp Med 2011;72:100-7. http://dx.doi.org/10.12968/hmed.2011.72.2.100.
- Erenberg G, Cruse RP, Rothner AD. The natural history of Tourette syndrome: a follow-up study. Ann Neurol 1987;22:383-5. http://dx.doi.org/10.1002/ana.410220317.
- Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics 1998;102:14-9. http://dx.doi.org/10.1542/peds.102.1.14.
- Coffey BJ, Biederman J, Geller D, Frazier J, Spencer T, Doyle R, et al. Reexamining tic persistence and tic-associated impairment in Tourette’s Disorder findings from a naturalistic follow-up study. J Nerv Ment Dis 2004;192:776-80. http://dx.doi.org/10.1097/01.nmd.0000144696.14555.c4.
- Bloch MH, Sukhodolsky DG, Leckman JF, Schultz RT. Fine-motor skill deficits in childhood predict adulthood tic severity and global psychosocial functioning in Tourette’s syndrome. J Child Psychol Psychiatry 2006;47:551-9. http://dx.doi.org/10.1111/j.1469-7610.2005.01561.x.
- Pappert EJ, Goetz CG, Louis ED, Blasucci L, Leurgans S. Objective assessments of longitudinal outcome in Gilles de la Tourette’s syndrome. Neurology 2003;61:936-40. http://dx.doi.org/10.1212/01.WNL.0000086370.10186.7C.
- Lin H, Katsovich L, Ghebremichael M, Findley DB, Grantz H, Lombroso PJ, et al. Psychosocial stress predicts future symptom severities in children and adolescents with Tourette syndrome and/or obsessive–compulsive disorder. J Child Psychol Psychiatry 2007;48:157-66. http://dx.doi.org/10.1111/j.1469-7610.2006.01687.x.
- Rizzo R, Gulisano M, Cali PV, Curatolo P. Long term clinical course of Tourette syndrome. Brain Dev 2012;34:667-73. http://dx.doi.org/10.1016/j.braindev.2011.11.006.
- Ferenczi S. Psychoanalytic observations on tic. Int J Psychoanal 1921;2:1-30.
- Mahler MS, Rangell L. A psychosomatic study of maladie des tics (Gilles de la Tourette’s Disease). Psychiatr Q 1943;17:579-603. http://dx.doi.org/10.1007/BF01561841.
- The Tourette Syndrome Association International Consortium for Genetics . A complete genome scan in sib-pairs affected with Gilles de la Tourette Syndrome. Am J Hum Gen 1999;65:1428-36. http://dx.doi.org/10.1086/302613.
- Scharf JM, Miller LL, Mathews CA, Ben-Shlomo Y. Prevalence of Tourette syndrome and chronic tics in the population-based Avon longitudinal study of parents and children cohort. J Am Acad Child Adolesc Psychiatry 2012;51:192-201. http://dx.doi.org/10.1016/j.jaac.2011.11.004.
- Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, et al. Genome-wide association study of Tourette’s syndrome. Mol Psychiatry 2014;18:721-8. http://dx.doi.org/10.1038/mp.2012.69.
- Abelson JF, Kwan KY, O’Roak BJ, Baek DY, Stillman AA, . Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science 2005;310:317-20. http://dx.doi.org/10.1126/science.1116502.
- Scharf JM, Moorjani P, Fagerness J, Platko JV, Illmann C, Galloway B, et al. Lack of association between SLITRK1var321 and Tourette syndrome in a large family-based sample. Neurology 2008;70:1495-6. http://dx.doi.org/10.1212/01.wnl.0000296833.25484.bb.
- Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O’Roak BJ, Mason CE, et al. L-Histidine decarboxylase and Tourette’s syndrome. N Engl J Med 2010;362:1901-8. http://dx.doi.org/10.1056/NEJMoa0907006.
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 1998;155:264-71.
- Murphy TK, Sajid M, Soto O, Shapira N, Edge P, Yang M, et al. Detecting pediatric autoimmune neuropsychiatric disorders associated with streptococcus in children with obsessive–compulsive disorder and tics. Biol Psychiatry 2004;55:61-8. http://dx.doi.org/10.1016/S0006-3223(03)00704-2.
- Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies in movement disorders. Pediatrics 1993;92:39-43.
- Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies: tics and obsessive–compulsive symptoms. J Dev Behav Pediatr 1994;15:421-5. http://dx.doi.org/10.1097/00004703-199412000-00005.
- Muller N, Riedel M, Straube A, Gunther W, Wilske B. Increased anti-streptococcal antibodies in patients with Tourette’s syndrome. Psychiatry Res 2000;94:43-9. http://dx.doi.org/10.1016/S0165-1781(00)00125-6.
- Muller N, Kroll B, Schwarz MJ, Riedel M, Straube A, Lutticken R, et al. Increased titers of antibodies against streptococcal M12 and M19 proteins in patients with Tourette’s syndrome. Psychiatry Res 2001;101:187-93. http://dx.doi.org/10.1016/S0165-1781(01)00215-3.
- Church AJ, Dale RC, Lees AJ, Giovannoni G, Robertson MM. Tourette’s syndrome: a cross sectional study to examine the PANDAS hypothesis. J Neurol Neurosurg Psychiatry 2003;74:602-7. http://dx.doi.org/10.1136/jnnp.74.5.602.
- Rizzo R, Gulisano M, Pavone P, Fogliano F, Robertson MM. Increased antistreptococcal antibody titers and anti-basal ganglia antibodies in patients with Tourette syndrome: Controlled cross-sectional study. J Child Neurol 2006;21:747-53. http://dx.doi.org/10.1177/08830738060210091001.
- Peterson BS, Leckman JF, Tucker D, Scahill L, Staib L, Zhang H, et al. Preliminary findings of antistreptococcal antibody titers and basal ganglia volumes in tic, obsessive–compulsive, and attention deficit/hyperactivity disorders. Arch Gen Psychiatry 2000;57:364-72. http://dx.doi.org/10.1001/archpsyc.57.4.364.
- Singer HS, Gause C, Morris C, Lopez P. Serial immune markers do not correlate with clinical exacerbations in pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Pediatrics 2008;121:1198-205. http://dx.doi.org/10.1542/peds.2007-2658.
- Murphy TK, Martino D, Leckman JF. Tourette Syndrome. New York, NY: Oxford University Press; 2013.
- Rhee H, Cameron DJ. Lyme disease and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): an overview. Int J Gen Med 2012;5:163-74. http://dx.doi.org/10.2147/IJGM.S24212.
- Riedel M, Straube A, Schwarz MJ, Wilske B, Muller N. Lyme disease presenting as Tourette’s syndrome. Lancet 1998;351:418-19. http://dx.doi.org/10.1016/S0140-6736(05)78357-4.
- Court A. One Bite Changed My Life Forever 2013. www.yours.co.uk.
- Editorial . Yours Magazine 2013:20-1. www.yours.co.uk/ (accessed November 2015).
- Leckman JF, Bedard MA, Agid Y, Chouinard S, Fahn S, Korczyn AD, et al. Mental and Behavioral Dysfunction in Movement Disorders. Totowa, NJ: Humana Press; 2003.
- Pasamanick B, Kawi A. A study of the association of prenatal and paranatal factors with the development of tics in children; a preliminary investigation. J Pediatr 1956;48:596-601. http://dx.doi.org/10.1016/S0022-3476(56)80095-4.
- Burd L, Severud R, Klug MG, Kerbeshian J. Prenatal and perinatal risk factors for Tourette disorder. J Perinat Med 1999;27:295-302. http://dx.doi.org/10.1515/jpm.1999.042.
- Mathews CA, Bimson B, Lowe TL, Herrera LD, Budman CL, Erenberg G, et al. Association between maternal smoking and increased symptom severity in Tourette’s syndrome. Am J Psychiatry 2006;163:1066-73. http://dx.doi.org/10.1176/ajp.2006.163.6.1066.
- Hoekstra P, Martino D, Leckman JF. Tourette Syndrome. New York, NY: Oxford University Press; 2013.
- Murphy T, Eddy CM, Martino D, Leckman JF. Tourette Syndrome. New York, NY: Oxford University Press; 2013.
- Mueller SC, Jackson GM, Dhalla R, Datsopoulos S, Hollis CP. Enhanced cognitive control in young people with Tourette’s syndrome. Curr Biol 2006;16:570-3. http://dx.doi.org/10.1016/j.cub.2006.01.064.
- Jackson SR, Parkinson A, Jung J, Ryan SE, Morgan PS, Hollis C, et al. Compensatory neural reorganization in Tourette syndrome. Curr Biol 2011;21:580-5. http://dx.doi.org/10.1016/j.cub.2011.02.047.
- Greene DJ, Black KJ, Schlagger BJ, Martino D, Leckman JF. Tourette Syndrome. New York, NY: Oxford University Press; 2013.
- Sandor P, Robertson MM, Myers ES, Chung S, Shapiro C. Tourette’s with Dignity. Don’t Tic Me Off. The Youthdale Series. Toronto, ON: Joli Joco Publications; 2013.
- Eddy CM, Rizzo R, Gulisano M, Agodi A, Barchitta M, Calı P, et al. Quality of life in young people with Tourette syndrome: a controlled study. J Neurol 2011;258:291-30. http://dx.doi.org/10.1007/s00415-010-5754-6.
- Storch EA, Merlo LJ, Lack C, Milsom VA, Geffken GR, Goodman WK, et al. Quality of life in youth with Tourette’s syndrome and chronic tic disorder. J Clin Child Adolesc Psychol 2007;36:217-27. http://dx.doi.org/10.1080/15374410701279545.
- Burd L, Kauffman DW, Kerbeshian J. Tourette Syndrome and learning-disabilities. J Learn Disabil 1992;25:598-604. http://dx.doi.org/10.1177/002221949202500909.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2011. www.cochrane-handbook.org (accessed 17 July 2015).
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380-2. http://dx.doi.org/10.1016/j.jclinepi.2010.09.011.
- Shapiro AK, Shapiro E. Treatment of Gilles de la Tourette’s Syndrome with haloperidol. Br J Psychiatry 1968;114:345-50. http://dx.doi.org/10.1192/bjp.114.508.345.
- Harris K, Singer HS. Tic disorders: neural circuits, neurochemistry, and neuroimmunology. J Child Neurol 2006;21:678-89. http://dx.doi.org/10.1177/08830738060210080901.
- Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psych 2011;20:173-96. http://dx.doi.org/10.1007/s00787-011-0163-7.
- Pringsheim T, Steeves T. Pharmacological treatment for Attention Deficit Hyperactivity Disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev 2011;4. http://dx.doi.org/10.1002/14651858.cd007990.pub2.
- Waldon K, Hill J, Termine C, Balottin U, Cavanna AE. Trials of pharmacological interventions for Tourette syndrome: a systematic review. Behav Neurol 2013;26:265-73. http://dx.doi.org/10.1155/2013/626410.
- Roessner V, Schoenefeld K, Buse J, Bender S, Ehrlich S, Munchau A. Pharmacological treatment of tic disorders and Tourette Syndrome. Neuropharmacology 2013;68:143-9. http://dx.doi.org/10.1016/j.neuropharm.2012.05.043.
- Sallee FR, Nesbitt L, Jackson C, Sine L, Sethuraman G. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry 1997;154:1057-62. http://dx.doi.org/10.1176/ajp.154.8.1057.
- Shapiro E, Shapiro AK, Fulop G, Hubbard M, Mandeli J, Nordlie J, et al. Controlled study of haloperidol, pimozide, and placebo for the treatment of Gilles de la Tourette’s syndrome. Arch Gen Psychiatry 1989;46:722-30. http://dx.doi.org/10.1001/archpsyc.1989.01810080052006.
- Shapiro AK, Shapiro E. Controlled study of pimozide vs. placebo in Tourette’s syndrome. J Am Acad Child Psychiatry 1984;23:161-73. http://dx.doi.org/10.1097/00004583-198403000-00007.
- Scahill L, Leckman JF, Schultz RT, Katsovich L, Peterson BS. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology 2003;60:1130-5. http://dx.doi.org/10.1212/01.WNL.0000055434.39968.67.
- Dion Y, Annable L, Sandor P, Chouinard G. Risperidone in the treatment of Tourette syndrome: A double-blind, placebo-controlled trial. J Clin Psychopharmacol 2002;22:31-9. http://dx.doi.org/10.1097/00004714-200202000-00006.
- Sallee FR, Kurlan R, Goetz CG, Singer H, Scahill L, Law G, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: A pilot study. J Am Acad Child Adolesc Psychiatry 2000;39:292-9. http://dx.doi.org/10.1097/00004583-200003000-00010.
- Gilbert DL, Batterson JR, Sethuraman G, Sallee FR. Tic reduction with risperidone versus pimozide in a randomized, double-blind, crossover trial. J Am Acad Child Adolesc Psychiatry 2004;43:206-14. http://dx.doi.org/10.1097/00004583-200402000-00017.
- Bruggeman R, Van der Linden C, Buitelaar JK, Gericke GS, Hawkridge SM, Temlett JA. Risperidone versus pimozide in Tourette’s disorder: A comparative double-blind parallel-group study. J Clin Psychiatry 2001;62:50-6. http://dx.doi.org/10.4088/JCP.v62n0111.
- Ross MS, Moldofsky H. A comparison of pimozide and haloperidol in the treatment of Gilles de la Tourette’s syndrome. Am J Psychiatry 1978;135:585-7. http://dx.doi.org/10.1176/ajp.135.5.585.
- Liu ZS, Chen YH, Zhong YQ, Zou LP, Wang H, Sun D, et al. A multicenter controlled study on aripiprazole treatment for children with Tourette syndrome in China. Zhonghua Er Ke Za Zhi 2011;49:572-6.
- Yoo HK, Lee JS, Paik KW, Choi SH, Yoon SJ, Kim JE, et al. Open-label study comparing the efficacy and tolerability of aripiprazole and haloperidol in the treatment of pediatric tic disorders. Eur Child Adolesc Psychiatry 2011;20:127-35. http://dx.doi.org/10.1007/s00787-010-0154-0.
- Gulisano M, Cali PV, Cavanna AE, Eddy C, Rickards H, Rizzo R. Cardiovascular safety of aripiprazole and pimozide in young patients with Tourette syndrome. Neurol Sci 2011;32:1213-17. http://dx.doi.org/10.1007/s10072-011-0678-1.
- Ji W-d, Li Y, Li N, Guo B-y. Olanzapine for treatment of Tourette syndrome: a double-blind randomised controlled trial. Chin J Clin Rehabil 2005;9:66-8.
- Fulop G, Phillips RA, Shapiro AK, Gomes JA, Shapiro E, Nordlie JW. ECG changes during haloperidol and pimozide treatment of Tourette’s disorder. Am J Psychiatry 1987;144:673-5. http://dx.doi.org/10.1176/ajp.144.5.673.
- Flockhart DA, Drici MD, Kerbusch T, Soukhova N, Richard E, Pearle PL, et al. Studies on the mechanism of a fatal clarithromycin–pimozide interaction in a patient with Tourette syndrome. J Clin Psychopharmacol 2000;20:317-24. http://dx.doi.org/10.1097/00004714-200006000-00005.
- Kurlan R, Goetz CG, McDermott MP, Plumb S, Singer H, Dure L, et al. Treatment of ADHD in children with tics: A randomized controlled trial. Neurology 2002;58:527-36. http://dx.doi.org/10.1212/WNL.58.4.527.
- Goetz CG, Tanner CM, Wilson RS. Clonidine and Gilles de la Tourette’s syndrome: double-blind study using objective rating methods. Ann Neurol 1987;21:307-10. http://dx.doi.org/10.1002/ana.410210313.
- Singer HS, Brown J, Quaskey S, Rosenberg LA, Mellits ED, Denckla MB. The treatment of attention-deficit hyperactivity disorder in Tourette’s syndrome: a double-blind placebo-controlled study with clonidine and desipramine. Pediatrics 1995;95:74-81.
- Leckman JF, Hardin MT, Riddle MA, Stevenson J, Ort SI, Cohen DJ. Clonidine treatment of Gilles de la Tourette’s syndrome. Arch Gen Psychiatry 1991;48:324-8. http://dx.doi.org/10.1001/archpsyc.1991.01810280040006.
- Du YS, Li HF, Vance A, Zhong YQ, Jiao FY, Wang HM, et al. Randomized double-blind multicentre placebo-controlled clinical trial of the clonidine adhesive patch for the treatment of tic disorders. Aust N Z J Psychiatry 2008;42:807-13. http://dx.doi.org/10.1080/00048670802277222.
- Zhong YQ, Zhou WZ, Hu WG. Randomized double-blind controlled study on treatment of tic disorders in children with transcutaneous patch of clonidine. Zhonghua Er Ke Za Zhi 2007;45:785-7.
- Gancher S, Conant ND, Angell R. Treatment of Tourette’s syndrome with transdermal clonidine: a pilot study. J Neuropsychiatry Clin Neurosci 1990;2:66-9. http://dx.doi.org/10.1176/jnp.2.1.66.
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry 2001;158:1067-74. http://dx.doi.org/10.1176/appi.ajp.158.7.1067.
- Cummings DD, Singer HS, Krieger M, Miller TL, Mahone EM. Neuropsychiatric effects of guanfacine in children with mild Tourette syndrome: a pilot study. Clin Neuropharmacol 2002;25:325-32. http://dx.doi.org/10.1097/00002826-200211000-00009.
- Allen AJ, Kurlan RM, Gilbert DL, Coffey BJ, Linder SL, Lewis DW, et al. Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology 2005;65:1941-9. http://dx.doi.org/10.1212/01.wnl.0000188869.58300.a7.
- Spencer TJ, Sallee FR, Gilbert DL, Dunn DW, McCracken JT, Coffey BJ, et al. Atomoxetine treatment of ADHD in children with comorbid tourette syndrome. J Atten Disord 2008;11:470-81. http://dx.doi.org/10.1177/1087054707306109.
- Gadow KD, Nolan EE, Sverd J. Methylphenidate in hyperactive boys with comorbid tic disorder: II. Short-term behavioral effects in school settings. J Am Acad Child Adolesc Psychiatry 1992;31:462-71. http://dx.doi.org/10.1097/00004583-199205000-00012.
- Gadow KD, Sverd J, Nolan EE, Sprafkin J, Schneider J. Immediate-release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. J Am Acad Child Adolesc Psychiatry 2007;46:840-8. http://dx.doi.org/10.1097/chi.0b013e31805c0860.
- Castellanos FX, Giedd JN, Elia J, Marsh WL, Ritchie GF, Hamburger SD, et al. Controlled stimulant treatment of ADHD and comorbid Tourette’s syndrome: Effects of stimulant and dose. J Am Acad Child Adolesc Psychiatry 1997;36:589-96. http://dx.doi.org/10.1097/00004583-199705000-00008.
- Lyon GJ, Samar SM, Conelea C, Trujillo MR, Lipinski CM, Bauer CC, et al. Testing tic suppression: Comparing the effects of dexmethylphenidate to no medication in children and adolescents with attention-deficit/hyperactivity disorder and Tourette’s disorder. J Child Adolesc Psychopharmacol 2010;20:283-9. http://dx.doi.org/10.1089/cap.2010.0032.
- Ondo WG, Jankovic J, Connor GS, Pahwa R, Elble R, Stacy MA, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology 2006;66:672-7. http://dx.doi.org/10.1212/01.wnl.0000200779.03748.0f.
- Smith-Hicks CL, Bridges DD, Paynter NP, Singer HS. A double blind randomized placebo control trial of levetiracetam in Tourette syndrome. Mov Disord 2007;22:1764-70. http://dx.doi.org/10.1002/mds.21615.
- Awaad Y, Michon AM, Minarik S, Rizk T. Levetiracetam in Tourette syndrome: a randomized double blind, placebo controlled study. J Pediatr Neurol 2009;7:257-63.
- Jankovic J, Jimenez-Shahed J, Brown LW. A randomised, double-blind, placebo-controlled study of topiramate in the treatment of Tourette syndrome. J Neurol Neurosurg Psychiatry 2010;81:70-3. http://dx.doi.org/10.1136/jnnp.2009.185348.
- Feigin A, Kurlan R, McDermott MP, Beach J, Dimitsopulos T, Brower CA, et al. A controlled trial of deprenyl in children with Tourette’s syndrome and attention deficit hyperactivity disorder. Neurology 1996;46:965-8. http://dx.doi.org/10.1212/WNL.46.4.965.
- Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med 2007;356:39-46. http://dx.doi.org/10.1056/NEJMoa054830.
- Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med 2007;356:29-38. http://dx.doi.org/10.1056/NEJMoa062222.
- Gilbert DL, Sethuraman G, Sine L, Peters S, Sallee FR. Tourette’s syndrome improvement with pergolide in a randomized, double-blind, crossover trial. Neurology 2000;54:1310-15. http://dx.doi.org/10.1212/WNL.54.6.1310.
- Gilbert DL, Dure L, Sethuraman G, Raab D, Lane J, Sallee FR. Tic reduction with pergolide in a randomized controlled trial in children. Neurology 2003;60:606-11. http://dx.doi.org/10.1212/01.WNL.0000044058.64647.7E.
- Kurlan R, Crespi G, Koval S, Wunderlich G, Mueller-Vahl K, Ludolph A, et al. A multicenter randomized placebo-controlled clinical trial of pramipexole for Tourette’s syndrome. Mov Disord 2012;27:775-8. http://dx.doi.org/10.1002/mds.24919.
- Nicolson R, Craven-Truss B, Smith J, McKinlay BD, Castellanos FX. A randomized, double-blind, placebo-controlled trial of metoclopramide for the treatment of Tourette’s disorder. J Am Acad Child Adolesc Psychiatry 2005;44:640-6. http://dx.doi.org/10.1097/01.chi.0000163279.39598.44.
- Toren P, Weizman A, Ratner S, Cohen D, Laor N. Ondansetron treatment in Tourette’s disorder: a 3-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2005;66:499-503. http://dx.doi.org/10.4088/JCP.v66n0413.
- Singer HS, Wendlandt J, Krieger M, Giuliano J. Baclofen treatment in Tourette syndrome: a double-blind, placebo-controlled, crossover trial. Neurology 2001;56:599-604. http://dx.doi.org/10.1212/WNL.56.5.599.
- Spencer T, Biederman J, Coffey B, Geller D, Crawford M, Bearman SK, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2002;59:649-56. http://dx.doi.org/10.1001/archpsyc.59.7.649.
- Rickards H, Cavanna AE, Worrall R. Treatment practices in Tourette syndrome: the European perspective. Eur J Paediatr Neurol 2012;16:361-4. http://dx.doi.org/10.1016/j.ejpn.2011.12.001.
- Kurlan R, Como PG, Deeley C, McDermott M, McDermott MP. A pilot controlled study of fluoxetine for obsessive–compulsive symptoms in children with Tourette’s syndrome. Clin Neuropharmacol 1993;16:167-72. http://dx.doi.org/10.1097/00002826-199304000-00009.
- Scahill L, Riddle MA, King RA, Hardin MT, Rasmusson A, Makuch RW, et al. Fluoxetine has no marked effect on tic symptoms in patients with Tourette’s syndrome: a double-blind placebo-controlled study. J Child Adolesc Psychopharmacol 1997;7:75-8. http://dx.doi.org/10.1089/cap.1997.7.75.
- Sanberg PR, Shytle RD, Silver AA. Treatment of Tourette’s syndrome with mecamylamine. Lancet 1998;352:705-6. http://dx.doi.org/10.1016/S0140-6736(05)60822-7.
- Silver AA, Shytle RD, Sheehan KH, Sheehan DV, Ramos A, Sanberg PR. Multicenter, double-blind, placebo-controlled study of mecamylamine monotherapy for tourette’s disorder. J Am Acad Child Adolesc Psychiatry 2001;40:1103-10. http://dx.doi.org/10.1097/00004583-200109000-00020.
- Silver AA, Shytle RD, Philipp MK, Wilkinson BJ, McConville B, Sanberg PR. Transdermal nicotine and haloperidol in Tourette’s disorder: a double-blind placebo-controlled study. J Clin Psychiatry 2001;62:707-14. http://dx.doi.org/10.4088/JCP.v62n0908.
- Howson AL, Batth S, Ilivitsky V, Boisjoli A, Jaworski M, Mahoney C, et al. Clinical and attentional effects of acute nicotine treatment in Tourette’s syndrome. Eur Psychiatry 2004;19:102-12. http://dx.doi.org/10.1016/j.eurpsy.2003.11.002.
- Gabbay V, Babb JS, Klein RG, Panzer AM, Katz Y, Alonso CM, et al. A double-blind, placebo-controlled trial of omega-3 fatty acids in Tourette’s disorder. Pediatrics 2012;129:e1493-500. http://dx.doi.org/10.1542/peds.2011-3384.
- Gaffney GR, Perry PJ, Lund BC, Bever-Stille KA, Arndt S, Kuperman S. Risperidone versus clonidine in the treatment of children and adolescents with Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 2002;41:330-6. http://dx.doi.org/10.1097/00004583-200203000-00013.
- George MS, Trimble MR, Robertson MM. Fluvoxamine and sulpiride in comorbid obsessive–compulsive disorder and Gilles de la Tourette Syndrome. Hum Psychopharmacol 1993;8:327-34. http://dx.doi.org/10.1002/hup.470080505.
- Hedderick EF, Morris CM, Singer HS. Double-Blind, Crossover Study of Clonidine and Levetiracetam in Tourette Syndrome. Pediatric Neurology 2009;40:420-5. http://dx.doi.org/10.1016/j.pediatrneurol.2008.12.014.
- Kang H, Zhang YF, Jiao FY, Guo XY, Gao XM. Efficacy of clonidine transdermal patch for treatment of Tourette’s syndrome in children. Zhongguo Dang Dai Er Ke Za Zhi 2009;11:537-9.
- Azrin N, Nunn R. Habit-reversal: a method of eliminating nervous habits and tics. Behav Res Ther 1973;11:619-28. http://dx.doi.org/10.1016/0005-7967(73)90119-8.
- Carr JE. Competing responses for the treatment of Tourette syndrome and tic disorders. Behav Res Ther 1995;33:455-6. http://dx.doi.org/10.1016/0005-7967(94)00066-S.
- Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, et al. Behavior therapy for children with tourette disorder: A randomized controlled trial. JAMA 2010;303:1929-37. http://dx.doi.org/10.1001/jama.2010.607.
- Ganos C, Kahl U, Schunke O, Kuhn S, Haggard P, Gerloff C, et al. Are premonitory urges a prerequisite of tic inhibition in Gilles de la Tourette syndrome?. J Neurol Neurosurg Psychiatry 2012;83:975-8. http://dx.doi.org/10.1136/jnnp-2012-303033.
- Verdellen C, van de Griendt J, Hartmann A, Murphy T. ESSTS Guidelines Group . European clinical guidelines for Tourette Syndrome and other tic disorders. Part III: behavioural and psychosocial interventions. Eur Child Adolesc Psychiatry 2011;20:197-20. http://dx.doi.org/10.1007/s00787-011-0167-3.
- Steeves T, McKinlay B, Gorman D, Billinghurst L, Day L, Carroll A, et al. Canadian guidelines for the evidence-based treatment of tic disorders: behavioural therapy, deep brain stimulation, and transcranial magnetic stimulation. Can J Psychiatry 2012;57:144-51.
- Hoogduim K, Verdellen C, Cath D. Exposure and response prevention in the treatment of Gilles de la Tourette’s Syndrome: four case studies. Clin Psychol Psychother 1998;4:125-35. http://dx.doi.org/10.1002/(SICI)1099-0879(199706)4:2<125::AID-CPP125>3.0.CO;2-Z.
- Yates AJ. The application of learning theory to the treatment of tics. J Abnorm Soc Psychol 1958;56:175-82. http://dx.doi.org/10.1037/h0040699.
- Peterson AL, Azrin NH. An evaluation of behavioral treatments for Tourette syndrome. Behav Res Ther 1992;30:167-74. http://dx.doi.org/10.1016/0005-7967(92)90140-C.
- Sukhodolsky DG, Kassinove H, Gorman BS. Cognitive-behavioral therapy for anger in children and adolescents: a meta-analysis. Aggress Violent Beh 2004;9:247-69. http://dx.doi.org/10.1016/j.avb.2003.08.005.
- Scahill L, Sukhodolsky DG, Bearss K, Findley D, Hamrin V, Carroll DH, et al. Randomized trial of parent management training in children with tic disorders and disruptive behavior. J Child Neurol 2006;21:650-6. http://dx.doi.org/10.1177/08830738060210080201.
- Barkley RA. Psychosocial treatments for attention-deficit/hyperactivity disorder in children. J Clin Psychiatry 2002;63:36-43.
- Wilhelm S, Peterson AL, Piacentini J, Woods DW, Deckersbach T, Sukhodolsky DG, et al. Randomized trial of behavior therapy for adults with Tourette syndrome. Arch Gen Psychiatry 2012;69:795-803. http://dx.doi.org/10.1001/archgenpsychiatry.2011.1528.
- Wilhelm S, Deckersbach T, Coffey BJ, Bohne A, Peterson AL, Baer L. Habit reversal versus supportive psychotherapy for Tourette’s disorder: a randomized controlled trial. Am J Psychiatry 2003;160:1175-7. http://dx.doi.org/10.1176/appi.ajp.160.6.1175.
- Deckersbach T, Rauch S, Buhlmann U, Wilhelm S. Habit reversal versus supportive psychotherapy in Tourette’s disorder: A randomized controlled trial and predictors of treatment response. Behav Res Ther 2006;44:1079-90. http://dx.doi.org/10.1016/j.brat.2005.08.007.
- Azrin NH, Peterson A.L. Treatment of Tourette Syndrome by habit reversal: a waiting-list control group comparison. Behav Ther 1990;21:305-18. http://dx.doi.org/10.1016/S0005-7894(05)80333-8.
- Azrin N, Nunn R, Frantz S. Habit reversal vs negative practice treatment of nervous tics. Behav Ther 1980;11:169-78. http://dx.doi.org/10.1016/S0005-7894(80)80017-7.
- Verdellen CWJ, Keijsers GPJ, Cath DC, Hoogduin CAL. Exposure with response prevention versus habit reversal in Tourette’s syndrome: A controlled study. Behav Res Ther 2004;42:501-11. http://dx.doi.org/10.1016/S0005-7967(03)00154-2.
- Himle MB, Freitag M, Walther M, Franklin SA, Ely L, Woods DW. A randomized pilot trial comparing videoconference versus face-to-face delivery of behavior therapy for childhood tic disorders. Behav Res Ther 2012;50:565-70. http://dx.doi.org/10.1016/j.brat.2012.05.009.
- Bergin A, Waranch HR, Brown J, Carson K, Singer HS. Relaxation therapy in Tourette syndrome: a pilot study. Pediatr Neurol 1998;18:136-42. http://dx.doi.org/10.1016/S0887-8994(97)00200-2.
- Sukhodolsky DG, Vitulano LA, Carroll DH, McGuire J, Leckman JF, Scahill L. Randomized trial of anger control training for adolescents with Tourette’s syndrome and disruptive behavior. J Am Acad Child Adolesc Psychiatry 2009;48:413-21. http://dx.doi.org/10.1097/CHI.0b013e3181985050.
- Woods DW, Piacentini JC, Scahill L, Peterson AL, Wilhelm S, Chang S, et al. Behavior therapy for tics in children: acute and long-term effects on psychiatric and psychosocial functioning. J Child Neurol 2011;26:858-65. http://dx.doi.org/10.1177/0883073810397046.
- Anderson WS, Lenz FA. Surgery insight: Deep brain stimulation for movement disorders. Nature clinical practice. Neurology 2006;2:310-20. http://dx.doi.org/10.1038/ncpneuro0193.
- Cavanna AE, Eddy CM, Mitchell R, Pall H, Mitchell I, Zrinzo L, et al. An approach to deep brain stimulation for severe treatment-refractory Tourette syndrome: the UK perspective. Br J Neurosurg 2011;25:38-44. http://dx.doi.org/10.3109/02688697.2010.534200.
- Lyons MK. Deep brain stimulation: current and future clinical applications. Mayo Clinic Proc 2011;86:662-72. http://dx.doi.org/10.4065/mcp.2011.0045.
- Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet 1999;353. http://dx.doi.org/10.1016/S0140-6736(98)05964-9.
- Piedad JC, Rickards HE, Cavanna AE. What patients with Gilles de la tourette syndrome should be treated with deep brain stimulation and what is the best target?. Neurosurgery 2012;71:173-92. http://dx.doi.org/10.1227/NEU.0b013e3182535a00.
- Hariz MI, Robertson MM. Gilles de la Tourette syndrome and deep brain stimulation. Eur J Neurosci 2010;32:1128-34. http://dx.doi.org/10.1111/j.1460-9568.2010.07415.x.
- Mink JW, Walkup J, Frey KA, Como P, Cath D, Delong MR, et al. Patient selection and assessment recommendations for deep brain stimulation in Tourette syndrome. Mov Disord 2006;21:1831-8. http://dx.doi.org/10.1002/mds.21039.
- Muller-Vahl KR, Cath DC, Cavanna AE, Dehning S, Porta M, Robertson MM, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part IV: deep brain stimulation. Eur Child Adolesc Psychiatry 2011;20:209-17. http://dx.doi.org/10.1007/s00787-011-0166-4.
- Edwards MJ, Talelli P, Rothwell JC. Clinical applications of transcranial magnetic stimulation in patients with movement disorders. Lancet Neurol 2008;7:827-40. http://dx.doi.org/10.1016/S1474-4422(08)70190-X.
- Gilbert DL, Garvey MA, Bansal AS, Lipps T, Zhang J, Wassermann EM. Should transcranial magnetic stimulation research in children be considered minimal risk?. Clin Neurophysiol 2004;115:1730-9. http://dx.doi.org/10.1016/j.clinph.2003.10.037.
- Hoekstra PJ, Kallenberg CG, Korf J, Minderaa RB. Is Tourette’s syndrome an autoimmune disease?. Mol Psychiatry 2002;7:437-45. http://dx.doi.org/10.1038/sj.mp.4000972.
- Hoekstra PJ, Minderaa RB, Kallenberg CG. Lack of effect of intravenous immunoglobulins on tics: a double-blind placebo-controlled study. J Clin Psychiatry 2004;65:537-42. http://dx.doi.org/10.4088/JCP.v65n0413.
- Kivity S, Katz U, Daniel N, Nussinovitch U, Papageorgiou N, Shoenfeld Y. Evidence for the use of intravenous immunoglobulins – a review of the literature. Clin Rev Allergy Immunol 2010;38:201-69. http://dx.doi.org/10.1007/s12016-009-8155-9.
- Anton C. Botulinum toxins: Adverse effects. Adverse Drug React Bull n.d.:1027-30. http://dx.doi.org/10.1097/FAD.0b013e328346f575.
- Bloch MH. Emerging treatment for Tourette’s disorder. Curr Psychiatry Rep 2008;10:323-30. http://dx.doi.org/10.1007/s11920-008-0052-z.
- Berardelli A, Abbruzzese G, Bertolasi L, Cantarella G, Carella F, Curra A, et al. Guidelines for the therapeutic use of botulinum toxin in movement disorders. Italian Study Group for Movement Disorders. Ital Soc Neurol 1997;18:261-9. http://dx.doi.org/10.1007/BF02083302.
- Simpson DM, Blitzer A, Brashear A, Comella C, Dubinsky R, Hallett M, et al. Assessment: botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008;70:1699-706. http://dx.doi.org/10.1212/01.wnl.0000311389.26145.95.
- Cui Y, Liu Z, Zhang W. Acupuncture for Gilles de la Tourette’s syndrome. Cochrane Database Syst Rev 2009. http://dx.doi.org/10.1002/14651858.cd007832.
- Ackermans L, Duits A, Van Der Linden C, Tijssen M, Schruers K, Temel Y, et al. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain 2011;134:832-44. http://dx.doi.org/10.1093/brain/awq380.
- Welter ML, Mallet L, Houeto JL, Karachi C, Czernecki V, Cornu P, et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol 2008;65:952-7. http://dx.doi.org/10.1001/archneur.65.7.952.
- Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg 2007;107:1004-14. http://dx.doi.org/10.3171/JNS-07/11/1004.
- Orth M, Kirby R, Richardson MP, Snijders AH, Rothwell JC, Trimble MR, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol 2005;116:764-8. http://dx.doi.org/10.1016/j.clinph.2004.10.003.
- Chae JH, Nahas Z, Wassermann E, Li X, Sethuraman G, Gilbert D, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndrome. Cogn Behav Neurol 2004;17:109-17. http://dx.doi.org/10.1097/01.wnn.0000116253.78804.3a.
- Munchau A, Bloem BR, Thilo KV, Trimble MR, Rothwell JC, Robertson MM. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology 2002;59:1789-91. http://dx.doi.org/10.1212/01.WNL.0000036615.25044.50.
- Marras C, Andrews D, Sime E, Lang AE. Botulinum toxin for simple motor tics: A randomized, double-blind, controlled clinical trial. Neurology 2001;56:605-10. http://dx.doi.org/10.1212/WNL.56.5.605.
- Chi X, Jin Z, Cui SZ. Clinical study on treatment of tourette’s syndrome with acupuncture-Chinese herbs combination. Journal of Acupuncture and Tuina Science 2003;1:15-6. http://dx.doi.org/10.1007/BF02845419.
- Kurlan R. Tourette’s syndrome and ‘PANDAS’: will the relation bear out? Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Neurology 1998;50:1530-4. http://dx.doi.org/10.1212/WNL.50.6.1530.
- Kurlan R, Kaplan EL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) etiology for tics and obsessive–compulsive symptoms: hypothesis or entity? Practical considerations for the clinician. Pediatrics 2004;113:883-6. http://dx.doi.org/10.1542/peds.113.4.883.
- Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive–compulsive disorder and tic disorders in childhood. Lancet 1999;354:1153-8. http://dx.doi.org/10.1016/S0140-6736(98)12297-3.
- Garvey MA, Perlmutter SJ, Allen AJ, Hamburger S, Lougee L, Leonard HL, et al. A pilot study of penicillin prophylaxis for neuropsychiatric exacerbations triggered by streptococcal infections. Biol Psychiatry 1999;45:1564-71. http://dx.doi.org/10.1016/S0006-3223(99)00020-7.
- Snider LA, Lougee L, Slattery M, Grant P, Swedo SE. Antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders. Biol Psychiatry 2005;57:788-92. http://dx.doi.org/10.1016/j.biopsych.2004.12.035.
- Elliott RA, Warmough DE, Gray NJ, Glazebrook C, Conroy S, Lakhanpaul M, et al. Talking About Medicines (Tabs): Involving Children and Young People With Chronic Illness in Managing Their Medicines. Final Report. Southampton: NIHR Health Services and Delivery Research programme; 2013.
- Rivera-Navarro J, Cubo E, Almazan J. The diagnosis of Tourette’s Syndrome: communication and impact. Clin Child Psychol Psychiatry 2009;14:13-2. http://dx.doi.org/10.1177/1359104508100127.
- Mol Debes NM, Hjalgrim H, Skov L. Limited knowledge of Tourette syndrome causes delay in diagnosis. Neuropediatrics 2008;39:101-5. http://dx.doi.org/10.1055/s-2008-1081457.
- Kompoliti K, Goetz CG, Morrissey M, Leurgans S. Gilles de la Tourette syndrome: Patient’s knowledge and concern of adverse effects. Mov Disord 2006;21:248-52. http://dx.doi.org/10.1002/mds.20680.
- Woods DW, Conelea CA, Himle MB. Behavior Therapy for Tourette’s Disorder: Utilization in a Community Sample and an Emerging Area of Practice for Psychologists. Prof Psychol Res Pr 2010;41:518-25. http://dx.doi.org/10.1037/a0021709.
- Conelea CA, Woods DW, Zinner SH, Budman C, Murphy T, Scahill LD, et al. Exploring the impact of chronic tic disorders on youth: results from the Tourette Syndrome Impact Survey. Child Psychiatry Hum Dev 2011;42:219-42. http://dx.doi.org/10.1007/s10578-010-0211-4.
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale – Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989;28:566-73. http://dx.doi.org/10.1097/00004583-198907000-00015.
- Woods DW, Piacentini JC, Chang S, Deckersbach T, Ginsberg G, Peterson AL, et al. Managing Tourette’s syndrome: A behavioral Intervention for Children and Adults (Therapist Guide). New York, NY: Oxford University Press; 2008.
- Cuenca J, Glazebrook C, Kendall T, Hedderly T, Heyman I, Jackson G, et al. Perceptions of treatment for tics among young people with Tourette syndrome and their parents: a mixed methods study. BMC Psychiatr 2015;15. http://dx.doi.org/10.1186/s12888-015-0430-0.
- Boyatzis RE. Transforming Qualitative Information: Thematic Analysis and Code Development. London: Sage; 1998.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2013;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Irvine I, Drew P, Sainsbury R. Mode Effects in Qualitative Interviews: A Comparison of Semistructured Face-to-Face and Telephone Interviews Using Conversation Analysis, Research Works, 2010–03. York: Social Policy Research Unit, University of York; 2010.
- Ofcom . The Communications Market Report n.d. http://stakeholders.ofcom.org.uk/market-data-research/market-data/communications-market-reports/cmr15/.
- Sayal K, Goodman R, Ford T. Barriers to the identification of children with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry 2006;47:744-50. http://dx.doi.org/10.1111/j.1469-7610.2005.01553.x.
Appendix 1 Search strategies
Search summary
A systematic search strategy was developed to locate all of the relevant evidence. The balance between sensitivity (the power to identify all studies on a particular topic) and specificity (the ability to exclude irrelevant studies from the results) was carefully considered and a decision made to utilise a broad approach to searching to maximise retrieval of evidence to all parts of the review. The sensitivity of this approach was aimed at minimising the risk of overlooking relevant publications owing to potential weaknesses that result from more focused search strategies. The search strategies were initially developed for MEDLINE before being translated for use in other databases/interfaces.
Study design filters
To aid retrieval of relevant and sound studies, study design filters were used to limit the results of searches to evidence of systematic reviews, RCTs, observational studies, qualitative studies and survey literature. For standard mainstream bibliographic databases, search terms for the population were combined with filters for each of the study designs under review. For searches generated in The Cochrane Library (CENTRAL, CDSR, DARE, HTA) and HMIC, search terms for the population were used without the appendage of a filter.
The study design filters for systematic reviews and RCTs are adaptations of filters of designed by the CRD and the Health Information Research Unit of McMaster University, Ontario. The study design filters for observational studies, qualitative studies and survey literature were developed in-house. Each filter comprises index terms relating to the study type(s) and associated text words for the methodological description of the design(s).
Date and language restrictions
Searches for RCTs and observational studies were generated from the inception of the databases to January 2013. Searches for systematic reviews, primary qualitative studies and survey literature were limited to research published from 1995 as older research was thought to be less useful.
No language restrictions were applied at the searching stage.
Other search methods
Other search methods involved: (1) scanning the reference lists of all eligible publications (systematic reviews and included studies) for more published reports and citations of unpublished research; (2) sending lists of studies meeting the inclusion criteria to subject experts (identified through searches and expert group members) and asking them to check the lists for completeness, and to provide information of any published or unpublished research for consideration; (3) checking the tables of contents of key journals for studies that might have been missed by the database and reference list searches; and (4) tracking key papers in the Science Citation Index (prospectively) over time for further useful references.
Databases searched
Australian Education Index.
Allied and Complementary Medicine Database.
Applied Social Sciences Index and Abstracts.
BIOSIS Citation Index.
British Education Index.
Cochrane Database of Systematic Reviews (The Cochrane Library).
NHS Database of Abstracts of Reviews of Effectiveness (The Cochrane Library).
Education Resources in Curriculum.
Cochrane Central Register of Controlled Trials (The Cochrane Library).
Cumulative Index to Nursing and Allied Health Literature.
Health Management Information Consortium.
Health Technology Assessment database (The Cochrane Library).
EMBASE.
International Bibliography of Social Science.
MEDLINE.
PREMEDLINE In-Process & Other Non-Indexed Citations.
PsycBOOKS.
PsycEXTRA.
PsycINFO.
Social Services Abstracts.
Sociological Abstracts.
Social Sciences Citation Index.
Web of Science.
Full details of the search strategies and filters used for the systematic review of clinical evidence are provided below.
Search strategies used in the major electronic databases
Population search terms
EMBASE, MEDLINE, PREMEDLINE In-Process & Other Non-Indexed Citations, PsycINFO (via Ovid)
-
exp tic/ or gilles de la tourette syndrome/
-
1 use emez
-
exp tic disorders/
-
3 use mesz, prem
-
tics/ or tourette syndrome/
-
5 use psyh
-
(tic or tics or tourette$).ti,ab,id.
-
or/2,4,6-7
Allied and Complementary Medicime Database (via Ovid)
-
exp tic/
-
(tic or tics or tourette$).ti,ab.
-
1 or 2
The Cochrane Library (Wiley)
-
mesh descriptor tics, this term only
-
mesh descriptor tourette syndrome, this term only
-
(tic or tics or tourette*):ti or (tic or tics or tourette*):ab
-
(#1 or #2 or #3)
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost)
-
( s1 or s2 )
-
ti ( tic or tics or tourette* ) or ab ( tic or tics or tourette* )
-
(mh “tic+”)
Australian Education Index, Applied Social Sciences Index and Abstracts, British Education Index, Education Resources in Curriculum, International Bibliography of Social Science, Social Services Abstracts, Sociological Abstracts (ProQuest)
su=((tics) or (tourette’s syndrome) or (vocal tics)) or ti=(tic or tics or tourette*) or ab=(tic or tics or tourette*)
-
( s1 or s2 )
-
ti ( tic or tics or tourette* ) or ab ( tic or tics or tourette* )
-
(mh “tic+”)
-
( s1 or s2 )
-
ti ( tic or tics or tourette* ) or ab ( tic or tics or tourette* )
-
(mh “tic+”)
BIOSIS Citation Index, Web of Science (Thomson Reuters)
-
topic=(tic or tics or tourette*) or title=(tic or tics or tourette*)
Health Management Information Consortium (Healthcare Databases Advanced Search)
-
(tic or tics or tourette*).af.
PsycBOOKS, PsycEXTRA (via OvidSP)
-
(tic or tics or tourette$).ti,ab,hw.
Study design filters
Systematic review filters
EMBASE, MEDLINE, PREMEDLINE In-Process & Other Non-Indexed Citations, PsycINFO (via Ovid)
Quantitative systematic review
-
meta analysis/ or systematic review/
-
1 use emez
-
meta analysis.sh,pt. or “meta-analysis as topic”/ or “review literature as topic”/
-
3 use mesz, prem
-
(literature review or meta analysis).sh,id,md. or systematic review.id,md.
-
5 use psyh
-
(exp bibliographic database/ or (((electronic or computer$ or online) adj database$) or bids or cochrane or embase or index medicus or isi citation or medline or psyclit or psychlit or scisearch or science citation or (web adj2 science)).ti,ab.) and (review$.ti,ab,sh,pt. or systematic$.ti,ab.)
-
7 use emez
-
(exp databases, bibliographic/ or (((electronic or computer$ or online) adj database$) or bids or cochrane or embase or index medicus or isi citation or medline or psyclit or psychlit or scisearch or science citation or (web adj2 science)).ti,ab.) and (review$.ti,ab,sh,pt. or systematic$.ti,ab.)
-
9 use mesz, prem
-
(computer searching.sh,id. or (((electronic or computer$ or online) adj database$) or bids or cochrane or embase or index medicus or isi citation or medline or psyclit or psychlit or scisearch or science citation or (web adj2 science)).ti,ab.) and (review$.ti,ab,pt. or systematic$.ti,ab.)
-
11 use psyh
-
((analy$ or assessment$ or evidence$ or methodol$ or quantitativ$ or systematic$) adj2 (overview$ or review$)).tw. or ((analy$ or assessment$ or evidence$ or methodol$ or quantitativ$ or systematic$).ti. and review$.ti,pt.) or (systematic$ adj2 search$).ti,ab.
-
(metaanal$ or meta anal$).ti,ab.
-
(research adj (review$ or integration)).ti,ab.
-
reference list$.ab.
-
bibliograph$.ab.
-
published studies.ab.
-
relevant journals.ab.
-
selection criteria.ab.
-
(data adj (extraction or synthesis)).ab.
-
(handsearch$ or ((hand or manual) adj search$)).ti,ab.
-
(mantel haenszel or peto or dersimonian or der simonian).ti,ab.
-
(fixed effect$ or random effect$).ti,ab.
-
((pool$ or combined or combining) adj2 (data or trials or studies or results)).ti,ab.
-
or/2,4,6,8,10,12-25
Qualitative systematic review
-
(cross case analys$ or eppi approach or metaethno$ or meta ethno$ or metanarrative$ or meta narrative$ or meta overview or metaoverview or metastud$ or meta stud$ or metasummar$ or meta summar$ or qualitative overview$).ti,ab.
-
(((critical interpretative or evidence or meta or mixed methods or multilevel or multi level or narrative or parallel or realist) adj synthes$) or metasynthes$).ti,ab.
-
(qualitative$ and (metaanal$ or meta anal$ or synthes$ or systematic review$)).ti,ab,hw,pt.
-
or/1-3
Allied and Complementary Medicine Database (via Ovid)
Quantitative systematic review
-
meta analysis/
-
(databases bibliographic/ or (((electronic or computer$ or online) adjdatabase$) or bids or cochrane or embase or index medicus or isi citation or medline or psyclit or psychlit or scisearch or science citation or (web adj2 science)).ti,ab.) and (review$.ti,ab,pt. or systematic$.ti,ab.)
-
((analy$ or assessment$ or evidence$ or methodol$ or qualitativ$ or quantativ$ or systematic$) adj2 (overview$ or review$)).tw. or ((analy$ or assessment$ or evidence$ or methodol$ or quantativ$ or qualitativ$ or systematic$).ti. and review$.ti,pt.) or (systematic$ adj2 search$).ti,ab.
-
(evidence synthesis or meta anal$ or metaanal$ or meta ethnograph$ or metaethnograph$ or meta study or metastud$ or meta synthesis or metasynthesis or (qualitative and synthesis) or realist synthesis).ti,ab.
-
(research adj (review$ or integration)).ti,ab.
-
reference list$.ab.
-
published studies.ab.
-
relevant journals.ab.
-
selection criteria.ab.
-
(data adj (extraction or synthesis)).ab.
-
(handsearch$ or ((hand or manual) adj search$)).ti,ab.
-
(mantel haenszel or peto or dersimonian or der simonian).ti,ab.
-
(fixed effect$ or random effect$).ti,ab.
-
or/1-13
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost)
Quantitative systematic review
| s33 | s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 or s9 or s10 or s11 or s12 or s13 or s14 or s15 or s16 or s22 or s23 or s26 or s27 or s28 or s29 or s30 or s31 or s32 |
| s32 | ti ( analy* n5 review* or assessment* n5 review* or evidence* n5 review* or methodol* n5 review* or quantativ* n5 review* or systematic* n5 review* ) or ab ( analy* n5 review* or assessment* n5 review* or evidence* n5 review* or methodol* n5 review* or quantativ* n5 review* or systematic* n5 review* ) |
| s31 | ti ( analy* n5 overview* or assessment* n5 overview* or evidence* n5 overview* or methodol* n5 overview* or quantativ* n5 overview* or systematic* n5 overview* ) or ab ( analy* n5 overview* or assessment* n5 overview* or evidence* n5 overview* or methodol* n5 overview* or quantativ* n5 overview* or systematic* n5 overview* ) |
| s30 | ti ( pool* n2 results or combined n2 results or combining n2 results ) or ab ( pool* n2 results or combined n2 results or combining n2 results ) |
| s29 | ti ( pool* n2 studies or combined n2 studies or combining n2 studies ) or ab ( pool* n2 studies or combined n2 studies or combining n2 studies ) |
| s28 | ti ( pool* n2 trials or combined n2 trials or combining n2 trials ) or ab ( pool* n2 trials or combined n2 trials or combining n2 trials ) |
| s27 | ti ( pool* n2 data or combined n2 data or combining n2 data ) or ab ( pool* n2 data or combined n2 data or combining n2 data ) |
| s26 | s24 and s25 |
| s25 | ti review* or pt review* |
| s24 | ti analy* or assessment* or evidence* or methodol* or quantativ* or systematic* |
| s23 | ti “systematic* n5 search*” or ab “systematic* n5 search*” |
| s22 | (s17 or s18 or s19) and (s20 or s21) |
| s21 | ti systematic* or ab systematic* |
| s20 | tx review* or mw review* or pt review* |
| s19 | (mh "cochrane library") |
| s18 | ti ( bids or cochrane or index medicus or “isi citation” or psyclit or psychlit or scisearch or “science citation” or web n2 science ) or ab ( bids or cochrane or index medicus or “isi citation” or psyclit or psychlit or scisearch or “science citation” or web n2 science ) |
| s17 | ti ( “electronic database*” or “bibliographic database*” or “computeri?ed database*” or “online database*” ) or ab ( “electronic database*” or “bibliographic database*” or “computeri?ed database*” or “online database*” ) |
| s16 | (mh "literature review") |
| s15 | pt systematic* or pt meta* |
| s14 | ti ( “fixed effect*” or “random effect*” ) or ab ( “fixed effect*” or “random effect*” ) |
| s13 | ti ( “mantel haenszel” or peto or dersimonian or “der simonian” ) or ab ( “mantel haenszel” or peto or dersimonian or “der simonian” ) |
| s12 | ti ( handsearch* or "hand search*" or "manual search*" ) or ab ( handsearch* or "hand search*" or "manual search*" ) |
| s11 | ab "data extraction" or "data synthesis" |
| s10 | ab "selection criteria" |
| s9 | ab "relevant journals" |
| s8 | ab "published studies" |
| s7 | ab bibliograph* |
| s6 | ab "reference list*" |
| s5 | ti ( “research review*” or “research integration” ) or ab ( “research review*” or “research integration” ) |
| s4 | ti ( metaanal* or “meta anal*”) or ab ( metaanal* or “meta anal*”) |
| s3 | (mh "meta analysis") |
| s2 | (mh "systematic review") |
| s1 | (mh "literature searching+") |
| s33 | s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 or s9 or s10 or s11 or s12 or s13 or s14 or s15 or s16 or s22 or s23 or s26 or s27 or s28 or s29 or s30 or s31 or s32 |
| s32 | ti ( analy* n5 review* or assessment* n5 review* or evidence* n5 review* or methodol* n5 review* or quantativ* n5 review* or systematic* n5 review* ) or ab ( analy* n5 review* or assessment* n5 review* or evidence* n5 review* or methodol* n5 review* or quantativ* n5 review* or systematic* n5 review* ) |
| s31 | ti ( analy* n5 overview* or assessment* n5 overview* or evidence* n5 overview* or methodol* n5 overview* or quantativ* n5 overview* or systematic* n5 overview* ) or ab ( analy* n5 overview* or assessment* n5 overview* or evidence* n5 overview* or methodol* n5 overview* or quantativ* n5 overview* or systematic* n5 overview* ) |
| s30 | ti ( pool* n2 results or combined n2 results or combining n2 results ) or ab ( pool* n2 results or combined n2 results or combining n2 results ) |
| s29 | ti ( pool* n2 studies or combined n2 studies or combining n2 studies ) or ab ( pool* n2 studies or combined n2 studies or combining n2 studies ) |
| s28 | ti ( pool* n2 trials or combined n2 trials or combining n2 trials ) or ab ( pool* n2 trials or combined n2 trials or combining n2 trials ) |
| s27 | ti ( pool* n2 data or combined n2 data or combining n2 data ) or ab ( pool* n2 data or combined n2 data or combining n2 data ) |
| s26 | s24 and s25 |
| s25 | ti review* or pt review* |
| s24 | ti analy* or assessment* or evidence* or methodol* or quantativ* or systematic* |
| s23 | ti “systematic* n5 search*” or ab “systematic* n5 search*” |
| s22 | (s17 or s18 or s19) and (s20 or s21) |
| s21 | ti systematic* or ab systematic* |
| s20 | tx review* or mw review* or pt review* |
| s19 | (mh "cochrane library") |
| s18 | ti ( bids or cochrane or index medicus or “isi citation” or psyclit or psychlit or scisearch or “science citation” or web n2 science ) or ab ( bids or cochrane or index medicus or “isi citation” or psyclit or psychlit or scisearch or “science citation” or web n2 science ) |
| s17 | ti ( “electronic database*” or “bibliographic database*” or “computeri?ed database*” or “online database*” ) or ab ( “electronic database*” or “bibliographic database*” or “computeri?ed database*” or “online database*” ) |
| s16 | (mh "literature review") |
| s15 | pt systematic* or pt meta* |
| s14 | ti ( “fixed effect*” or “random effect*” ) or ab ( “fixed effect*” or “random effect*” ) |
| s13 | ti ( “mantel haenszel” or peto or dersimonian or “der simonian” ) or ab ( “mantel haenszel” or peto or dersimonian or “der simonian” ) |
| s12 | ti ( handsearch* or "hand search*" or "manual search*" ) or ab ( handsearch* or "hand search*" or "manual search*" ) |
| s11 | ab "data extraction" or "data synthesis" |
| s10 | ab "selection criteria" |
| s9 | ab "relevant journals" |
| s8 | ab "published studies" |
| s7 | ab bibliograph* |
| s6 | ab "reference list*" |
| s5 | ti ( “research review*” or “research integration” ) or ab ( “research review*” or “research integration” ) |
| s4 | ti ( metaanal* or “meta anal*”) or ab ( metaanal* or “meta anal*”) |
| s3 | (mh "meta analysis") |
| s2 | (mh "systematic review") |
| s1 | (mh "literature searching+") |
Qualitative systematic review
| s1 | ti (“cross case analys*” or “eppi approach” or metaethno* or “meta ethno*” or metanarrative* or “meta narrative*” or “meta overview” or metaoverview or metastud* or “meta stud*” or metasummar* or “meta summar*” or “qualitative overview*”) or ab (“cross case analys*” or “eppi approach” or metaethno* or “meta ethno*” or metanarrative* or “meta narrative*” or “meta overview” or metaoverview or metastud* or “meta stud*” or metasummar* or “meta summar*” or “qualitative overview*”) |
| s2 | ti (((“critical interpretative” or evidence or meta or “mixed methods” or multilevel or “multi level” or narrative or parallel or realist) n1 synthes*) or metasynthes*) or ab (((“critical interpretative” or evidence or meta or “mixed methods” or multilevel or “multi level” or narrative or parallel or realist) n1 synthes*) or metasynthes*) |
| s3 | ti qualitative* or ab qualitative* or mw qualitative* or pt qualitative* |
| s4 | ti (metaanal* or “meta anal*” or synthes* or “systematic review*”) or ab (metaanal* or “meta anal*” or synthes* or “systematic review*”) or mw (metaanal* or “meta anal*” or synthes* or “systematic review*”) or pt (metaanal* or “meta anal*” or synthes* or “systematic review*”) |
| s5 | s3 and s4 |
| s6 | s1 or s2 or s5 |
| s1 | ti (“cross case analys*” or “eppi approach” or metaethno* or “meta ethno*” or metanarrative* or “meta narrative*” or “meta overview” or metaoverview or metastud* or “meta stud*” or metasummar* or “meta summar*” or “qualitative overview*”) or ab (“cross case analys*” or “eppi approach” or metaethno* or “meta ethno*” or metanarrative* or “meta narrative*” or “meta overview” or metaoverview or metastud* or “meta stud*” or metasummar* or “meta summar*” or “qualitative overview*”) |
| s2 | ti (((“critical interpretative” or evidence or meta or “mixed methods” or multilevel or “multi level” or narrative or parallel or realist) n1 synthes*) or metasynthes*) or ab (((“critical interpretative” or evidence or meta or “mixed methods” or multilevel or “multi level” or narrative or parallel or realist) n1 synthes*) or metasynthes*) |
| s3 | ti qualitative* or ab qualitative* or mw qualitative* or pt qualitative* |
| s4 | ti (metaanal* or “meta anal*” or synthes* or “systematic review*”) or ab (metaanal* or “meta anal*” or synthes* or “systematic review*”) or mw (metaanal* or “meta anal*” or synthes* or “systematic review*”) or pt (metaanal* or “meta anal*” or synthes* or “systematic review*”) |
| s5 | s3 and s4 |
| s6 | s1 or s2 or s5 |
Australian Education Index, Applied Social Sciences Index and Abstracts, British Education Index, Education Resources in Curriculum, International Bibliography of Social Science, Social Services Abstracts, Sociological Abstracts (ProQuest)
Quantitative systematic review
-
su.exact(“literature reviews”)
-
su.exact(“meta-analysis”)
-
su.exact(“systematic reviews”)
-
ti ((analy* or assessment* or evidence* or methodol* or quantitativ* or systematic*) near/2 (overview* or review*)) or (systematic* near/2 search*) or ab ((analy* or assessment* or evidence* or methodol* or quantitativ* or systematic*) near/2 (overview* or review*)) or (systematic* near/2 search*)
-
ti ((analy* or assessment* or evidence* or methodol* or quantitativ* or systematic*) and review*
-
ti (metaanal* or “meta anal*”) or ab (metaanal* or “meta anal*”)
-
ti (research near/1 (review* or integration)) or ab (research near/1 (review* or integration))
-
ti “reference list*” or ab “reference list*”
-
ti bibliograph* or ab bibliograph*
-
ti “published studies” or ab “published studies”
-
ti “relevant journals” or ab “relevant journals”
-
ti “selection criteria” or ab “selection criteria”
-
ti (data near/1 (extraction or synthesis)) or ab (data near/1 (extraction or synthesis))
-
ti (handsearch* or ((hand or manual) near/1 search*)) or ab (handsearch* or ((hand or manual) near/1 search*))
-
ti (mantel haenszel or peto or dersimonian or “der simonian”) or ab (mantel haenszel or peto or dersimonian or “der simonian”)
-
ti (fixed effect* or “random effect*”) or ab (fixed effect* or “random effect*”)
-
ti ((pool* or combined or combining) near/2 (data or trials or studies or results)) or ab ((pool* or combined or combining) near/2 (data or trials or studies or results))
-
s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 or s9 or s10 or s11 or s12 or s13 or s14 or s15 or s16 or s17
Qualitative systematic review
-
ti (“cross case analys*” or “eppi approach” or metaethno* or “meta ethno*” or metanarrative* or “meta narrative*” or “meta overview” or metaoverview or metastud* or “meta stud*” or metasummar* or “meta summar*” or qualitative overview*) or ab (“cross case analys*” or “eppi approach” or metaethno* or “meta ethno*” or metanarrative* or “meta narrative*” or “meta overview” or metaoverview or metastud* or “meta stud*” or metasummar* or “meta summar*” or qualitative overview*)
-
ti (((“critical interpretative” or evidence or meta or “mixed methods” or multilevel or “multi level” or narrative or parallel or realist) near/1 synthes*) or metasynthes*) or ab (((“critical interpretative” or evidence or meta or “mixed methods” or multilevel or “multi level” or narrative or parallel or realist) near/1 synthes*) or metasynthes*)
-
ti (qualitative* and (metaanal* or “meta anal*” or synthes* or “systematic review*”)) or ab (qualitative* and (metaanal* or “meta anal*” or synthes* or “systematic review*”))
-
s1 or s2 or s3
BIOSIS Citation Index, Web of Science (Thomson Reuters)
Quantitative systematic review filter
| #1 | title=(“electronic database*” or “computer* database*” or “online database*” or bids or cochrane or embase or “index medicus” or “isi citation” or medline or psyclit or psychlit or scisearch or “science citation” or “web of science”) |
| #2 | title=(review* or systematic*) or topic=(review* or systematic*) |
| #3 | #1 and #2 |
| #4 | topic=((systematic* near search* or metaanal* or “meta anal*” or “research review*” or “research integration” or “reference list*” or bibliograph* or “published studies” or “relevant journals” or “selection criteria” or “data extraction” or “data synthesis” or handsearch* or “hand search*” or “manual search*” or “mantel haenszel” or peto or dersimonian or “der simonian” or “fixed effect*” or “random effect*” or ((pool* or combined or combining) near (data or trials or studies or results)))) or title=((systematic* near search* or metaanal* or “meta anal*” or “research review*” or “research integration” or “reference list*” or bibliograph* or “published studies” or “relevant journals” or “selection criteria” or “data extraction” or “data synthesis” or handsearch* or “hand search*” or “manual search*” or “mantel haenszel” or peto or dersimonian or “der simonian” or “fixed effect*” or “random effect*”) or ((pool* or combined or combining) near (data or trials or studies or results)))) |
| #5 | topic=(((analy* or assessment* or evidence* or methodol* or quantitativ* or systematic*) near (overview* or review*))) or title=(((analy* or assessment* or evidence* or methodol* or qualitativ* or quantitativ* or systematic*) near (overview* or review*))) |
| #6 | #3 or #4 or #5 |
| #1 | title=(“electronic database*” or “computer* database*” or “online database*” or bids or cochrane or embase or “index medicus” or “isi citation” or medline or psyclit or psychlit or scisearch or “science citation” or “web of science”) |
| #2 | title=(review* or systematic*) or topic=(review* or systematic*) |
| #3 | #1 and #2 |
| #4 | topic=((systematic* near search* or metaanal* or “meta anal*” or “research review*” or “research integration” or “reference list*” or bibliograph* or “published studies” or “relevant journals” or “selection criteria” or “data extraction” or “data synthesis” or handsearch* or “hand search*” or “manual search*” or “mantel haenszel” or peto or dersimonian or “der simonian” or “fixed effect*” or “random effect*” or ((pool* or combined or combining) near (data or trials or studies or results)))) or title=((systematic* near search* or metaanal* or “meta anal*” or “research review*” or “research integration” or “reference list*” or bibliograph* or “published studies” or “relevant journals” or “selection criteria” or “data extraction” or “data synthesis” or handsearch* or “hand search*” or “manual search*” or “mantel haenszel” or peto or dersimonian or “der simonian” or “fixed effect*” or “random effect*”) or ((pool* or combined or combining) near (data or trials or studies or results)))) |
| #5 | topic=(((analy* or assessment* or evidence* or methodol* or quantitativ* or systematic*) near (overview* or review*))) or title=(((analy* or assessment* or evidence* or methodol* or qualitativ* or quantitativ* or systematic*) near (overview* or review*))) |
| #6 | #3 or #4 or #5 |
Qualitative systematic review filter
#1 topic=((“cross case analys*” or “eppi approach” or metaethno* or “meta ethno*” or metanarrative* or “meta narrative*” or “meta overview” or metaoverview or metastud* or “meta stud*” or metasummar* or “meta summar*” or “qualitative overview*”)) or title=((“cross case analys*” or “eppi approach” or metaethno* or “meta ethno*” or metanarrative* or “meta narrative*” or “meta overview” or metaoverview or metastud* or “meta stud*” or metasummar* or “meta summar*” or “qualitative overview*”))
#2 topic=((((“critical interpretative” or evidence or meta or “mixed methods” or multilevel or “multi level” or narrative or parallel or realist) near synthes*) or metasynthes*)) or title=((((“critical interpretative” or evidence or meta or “mixed methods” or multilevel or “multi level” or narrative or parallel or realist) near synthes*) or metasynthes*))
#3 topic=((qualitative* and (metaanal* or “meta anal*” or synthes* or “systematic review*”))) or title=((qualitative* and (metaanal* or “meta anal*” or synthes* or “systematic review*”)))
#4 #1 or #2 or #3
Randomised controlled trial filters
EMBASE, MEDLINE, PREMEDLINE In-Process & Other Non-Indexed Citations, PsycINFO (via Ovid)
-
exp “clinical trial (topic)”/ or exp clinical trial/ or crossover procedure/ or double blind procedure/ or placebo/ or randomization/ or random sample/ or single blind procedure/
-
1 use emez
-
exp clinical trial/ or exp “clinical trials as topic”/ or cross-over studies/ or double-blind method/ or placebos/ or random allocation/ or single-blind method/
-
3 use mesz, prem
-
(clinical trials or placebo or random sampling).sh,id.
-
5 use psyh
-
(clinical adj2 trial$).ti,ab.
-
(crossover or cross over).ti,ab.
-
(((single$ or doubl$ or trebl$ or tripl$) adj2 blind$) or mask$ or dummy or doubleblind$ or singleblind$ or trebleblind$ or tripleblind$).ti,ab.
-
(placebo$ or random$).ti,ab.
-
treatment outcome$.md. use psyh
-
animals/ not human$.mp. use emez
-
animal$/ not human$/ use mesz, prem
-
(animal not human).po. use psyh
-
(or/2,4,6-11) not (or/12-14)
Allied and Complementary Medicine Database (via Ovid)
-
(clinical trials or double blind method or placebos or random allocation).sh.
-
(clinical adj2 trial$).ti,ab.
-
(crossover or cross over).ti,ab.
-
(((single$ or doubl$ or trebl$ or tripl$) adj5 blind$) or mask$ or dummy or singleblind$ or doubleblind$ or trebleblind$ or tripleblind$).ti,ab.
-
(placebo$ or random$).ti,ab.
-
or/1-5
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost)
| s10 | s9 not s8 |
| s9 | s1 or s2 or s3 or s4 or s5 or s6 or s7 |
| s8 | (mh "animals") not (mh "human") |
| s7 | (pt "clinical trial") or (pt "randomized controlled trial") |
| s6 | ti ( placebo* or random* ) or ab ( placebo* or random* ) |
| s5 | ti ( single blind* or double blind* or treble blind* or mask* or dummy* or singleblind* or doubleblind* or trebleblind* ) or ab ( single blind* or double blind* or treble blind* or mask* or dummy* or singleblind* or doubleblind* or trebleblind* ) |
| s4 | ti ( crossover or cross over ) or ab ( crossover or cross over ) |
| s3 | ti clinical n2 trial* or ab clinical n2 trial* |
| s2 | (mh "crossover design") or (mh "placebos") or (mh "random assignment") or (mh "random sample") |
| s1 | (mh "clinical trials+") |
| s10 | s9 not s8 |
| s9 | s1 or s2 or s3 or s4 or s5 or s6 or s7 |
| s8 | (mh "animals") not (mh "human") |
| s7 | (pt "clinical trial") or (pt "randomized controlled trial") |
| s6 | ti ( placebo* or random* ) or ab ( placebo* or random* ) |
| s5 | ti ( single blind* or double blind* or treble blind* or mask* or dummy* or singleblind* or doubleblind* or trebleblind* ) or ab ( single blind* or double blind* or treble blind* or mask* or dummy* or singleblind* or doubleblind* or trebleblind* ) |
| s4 | ti ( crossover or cross over ) or ab ( crossover or cross over ) |
| s3 | ti clinical n2 trial* or ab clinical n2 trial* |
| s2 | (mh "crossover design") or (mh "placebos") or (mh "random assignment") or (mh "random sample") |
| s1 | (mh "clinical trials+") |
Australian Education Index, Applied Social Sciences Index and Abstracts, British Education Index, Education Resources in Curriculum, International Bibliography of Social Science, Social Service Abstracts, Sociological Abstracts (ProQuest)
-
su.exact.explode(“clinical randomized controlled trials” or “cluster randomized controlled trials” or “double blind randomized controlled trials” or “randomized consent design” or “randomized controlled trials” or “single blind randomized controlled trials” or “urn randomization”)
-
su.exact(“clinical trials”)
-
su.exact(“crossover trials”)
-
su.exact(“placebos”)
-
su.exact(“random sampling”)
-
su.exact(“randomization”)
-
su.exact(“random samples”)
-
su.exact(“placebo effect”)
-
ti (clinical near/2 trial*) or ab (clinical near/2 trial*)
-
ti (crossover or “cross over”) or ab (crossover or “cross over”)
-
ti (((single* or doubl* or trebl* or tripl*) near/2 blind*) or mask* or dummy or doubleblind* or singleblind* or trebleblind* or tripleblind*) or ab (((single* or doubl* or trebl* or tripl*) near/2 blind*) or mask* or dummy or doubleblind* or singleblind* or trebleblind* or tripleblind*)
-
ti (placebo* or random*) or ab (placebo* or random*)
-
s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 or s9 or s10 or s11 or s12
BIOSIS Citation Index, Web of Science (Thomson Reuters)
| #1 | topic=(((clinical near trial* or crossover or “cross over”) or ((single* or doubl* or trebl* or tripl*) near (blind* or mask* or dummy)) or (singleblind* or doubleblind* or trebleblind* or tripleblind* or placebo* or random*))) or title=(((clinical near trial* or crossover or “cross over”) or ((single* or doubl* or trebl* or tripl*) near (blind* or mask* or dummy)) or (singleblind* or doubleblind* or trebleblind* or tripleblind* or placebo* or random*))) |
| #1 | topic=(((clinical near trial* or crossover or “cross over”) or ((single* or doubl* or trebl* or tripl*) near (blind* or mask* or dummy)) or (singleblind* or doubleblind* or trebleblind* or tripleblind* or placebo* or random*))) or title=(((clinical near trial* or crossover or “cross over”) or ((single* or doubl* or trebl* or tripl*) near (blind* or mask* or dummy)) or (singleblind* or doubleblind* or trebleblind* or tripleblind* or placebo* or random*))) |
Observational study filters
EMBASE, MEDLINE, PREMEDLINE In-Process & Other Non-Indexed Citations, PsycINFO (via Ovid)
-
exp case control study/ or cohort analysis/ or cross-sectional study/ or follow up/ or longitudinal study/ or observational study/ or prospective study/ or
-
retrospective study/
-
1 use emez
-
exp case control studies/ or exp cohort studies/ or cross-sectional studies/ or epidemiologic studies/
-
3 use mesz, prem
-
(cohort analysis or followup studies or longitudinal studies or prospective studies or retrospective studies).sh,id. or (followup study or longitudinal study or
-
prospective study or retrospective study).md.
-
5 use psyh
-
((epidemiologic$ or observational) adj (study or studies)).ti,ab.
-
(cohort$1 or cross section$ or crosssection$ or followup$ or follow up$ or followed or longitudinal$ or prospective$ or retrospective$).ti,ab.
-
(case adj2 (control or series)).ti,ab.
-
or/2,4,6-9
Allied and Complementary Medicine Database (via Ovid)
-
case control studies/ or cohort studies/ or follow up studies/ or longitudinal studies/ or prospective studies/ or retrospective studies/
-
((epidemiologic$ or observational) adj (study or studies)).ti,ab.
-
(cohort$1 or cross section$ or crosssection$ or followup$ or follow up$ or followed or longitudinal$ or prospective$ or retrospective$).ti,ab.
-
(case and (control$ or series)).ti,ab.
-
or/1-4
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost)
| s4 | s1 or s2 or s3 |
| s3 | ti ( “case control” or cohort* or “cross sectional” or followup* or “follow up*” or followed or longitudinal or prospective* or retrospective* ) or ab ( “case control” or cohort* or “cross sectional” or followup* or “follow up*” or followed or longitudinal or prospective* or retrospective* ) |
| s2 | ti ( “cross sectional” n2 study or “cross sectional” n2 studies or epidemiologic* n2 study or epidemiologic* n2 studies or observational* n2 study or observational* n2 studies ) or ab ( “cross sectional” n2 study or “cross sectional” n2 studies or epidemiologic* n2 study or epidemiologic* n2 studies or observational* n2 study or observational* n2 studies ) |
| s1 | (mh “correlational studies”) or (mh “case control studies+”) or (mh “cross sectional studies”) or (mh “prospective studies+”) |
Australian Education Index, Applied Social Sciences Index and Abstracts, British Education Index, Education Resources in Curriculum, International Bibliography of Social Science, Social Services Abstracts, Sociological Abstracts
-
su.exact(“case controlled studies”)
-
su.exact(“case studies”)
-
su.exact(“cohort analysis”)
-
su.exact(“cohorts”)
-
su.exact(“cross sectional analysis”)
-
su.exact(“cross sectional studies”)
-
su.exact(“followup”)
-
su.exact(“followup studies”)
-
su.exact(“longitudinal analysis”)
-
su.exact(“longitudinal studies”)
-
su.exact(“prospective controlled trials”)
-
su.exact(“prospective studies”)
-
su.exact(“retrospective studies”)
-
ti ((epidemiologic* or observational) near/1 (study or studies)) or ((epidemiologic* or observational) near/1 (study or studies))
-
ti (cohort* or “cross section*” or crosssection* or followup* or “follow up*” or followed or longitudinal* or prospective* or retrospective*) or ab (cohort* or “cross section*” or crosssection* or followup* or “follow up*” or followed or longitudinal* or prospective* or retrospective*)
-
ti (case near/2 (control or series)) or ab (case near/2 (control or series))
-
s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 or s9 or s10 or s11 or s12 or s13 or s14 or s15 or s16
BIOSIS Citation Index, Web of Science (Thomson Reuters)
-
topic=(((epidemiologic* or observational) near (study or studies)) or cohort* or cross section* or crosssection* or followup* or “follow up*” or followed or longitudinal* or prospective* or retrospective* or (case near (control or series))) or title=(((epidemiologic* or observational) near (study or studies)) or cohort* or cross section* or crosssection* or followup* or “follow up*” or followed or longitudinal* or prospective* or retrospective* or (case near (control or series)))
Primary qualitative studies/survey literature filter
EMBASE, MEDLINE, PREMEDLINE In-Process & Other Non-Indexed Citations, PsycINFO (via Ovid)
-
cluster analysis/ or constant comparative method/ or content analysis/ or cultural anthropology/ or discourse analysis/ or ethnographic research/ or ethnography/ or ethnology/ or ethnonursing research/ or field study/ or grounded theory/ or information processing/ or nursing methodology research/ or personal experience/ or phenomenology/ or purposive sample/ or qualitative research/ or exp recording/ or semi structured interview/ or storytelling/ or structured interview/ or thematic analysis/ or theoretical sample/
-
1 use emez
-
anthropology, cultural/ or focus groups/ or exp tape recording/ or interview/ or personal narratives/ or exp interviews as topic/ or narration/ or nursing methodology research/ or observation/ or qualitative research/ or sampling studies/ or cluster analysis/ or videodisc recording/
-
3 use mesz, prem
-
“culture (anthropological)”/ or cluster analysis/ or content analysis/ or discourse analysis/ or ethnography/ or “experiences (events)”/ or grounded theory/ or interviews/ or life experiences/ or narratives/ or observation methods/ or phenomenology/ or qualitative research/ or structured clinical interview/ or exp tape recorders/ or storytelling/ or (field study or interview or focus group or qualitative study).md.
-
5 use psyh
-
(action research or audiorecord$ or ((audio or tape or video$) adj5 record$) or colaizzi$ or (constant adj (comparative or comparison)) or content analy$ or critical social$ or (data adj1 saturat$) or discourse analys?s or emic or ethical enquiry or ethno$ or etic or fieldnote$ or (field adj (note$ or record$ or stud$ or research)) or (focus adj4 (group$ or sampl$)) or ((focus$ or structured) adj2 interview$) or giorgi$ or glaser or (grounded adj (theor$ or study or studies or research)) or heidegger$ or hermeneutic$ or heuristic or human science or husserl$ or ((life or lived) adj experience$) or maximum variation or merleau or narrat$ or ((participant$ or nonparticipant$) adj3 observ$) or ((philosophical or social) adj research$) or (pilot testing and survey) or purpos$ sampl$ or qualitative$ or ricoeur or semiotics or shadowing or snowball or spiegelberg$ or stories or story or storytell$ or strauss or structured categor$ or tape record$ or taperecord$ or testimon$ or (thematic$ adj3 analys$) or themes or theoretical sampl$ or unstructured categor$ or van kaam$ or van manen or videorecord$ or video record$ or videotap$ or video tap$).ti,ab.
-
or/2,4,6-7
-
(((adult$ or attender$ or carer$ or caregiv$ or care giv$ or client$ or community based or consumer$ or couples or customer$ or daughter$ or famil$ or father$ or friend$ or (home adj2 (care or caring or nurs$)) or home based or husband$ or individual$ or ((informal or non professional) adj5 (care or nursing)) or marital$ or men or mentor$ or mother$ or multifam$ or neighbo?r$ or next of kin or niece or nephew$ or parent$1 or partner$1 or patient$ or people$ or person$ or relative or relatives or sibling$ or significant other$ or spous$ or step relationship$ or teacher$ or wife$1 or wives or women or user$) or (adolescen$ or boy$ or child$ or delinquen$ or girl$ or graders or infant$ or junior$ or juvenile$ or kindergarten or minors or p?ediatric$ or postpubert$ or postpubescen$ or preadolescen$ or prepubert$ or prepubescen$ or preschool$ or preteen$ or pubert$ or pubescen$ or school$ or teen$ or toddler$ or (young$ adj1 (people or person$ or patient$ or population$)) or youngster$ or youth$)) adj3 (account$ or anxieties or attitude$ or barriers or belief$ or buyin or buy in$1 or cooperat$ or co operat$ or expectation$ or experienc$ or feedback or involv$ or opinion$ or participat$ or perceived need$ or (perception$ not speech perception) or perspective$ or preferen$ or satisf$ or view$ or voices or worry)).ti,ab.
-
((information adj (need$ or requirement$ or support$)) or health information).ti,ab.
-
(service$ adj2 (acceptab$ or unacceptab$)).ti,ab.
-
or/8-11
-
health care survey/ or health survey/
-
13 use emez
-
health care surveys/ or exp health surveys/
-
15 use mesz, prem
-
exp surveys/
-
17 use psyh
-
(survey$ or question$).ti,ab.
-
exp attitude/ or exp patient acceptance of health care/ or exp patient attitude/ or patient-centered care/ or patient education/ or patient satisfaction/
-
20 use emez
-
exp attitude to health/ or exp patient acceptance of health care/ or patient-centered care/ or patient education/ or patient education handout/ or patient satisfaction/
-
22 use mesz, prem
-
exp client attitudes/ or client education/ or exp consumer attitudes/ or exp health
-
attitudes/ or exp patient attitude/ or patient-centered care/ or patient education/ or
-
patient satisfaction/
-
24 use psyh
-
(account$ or anxieties or attitude$ or barriers or belief$ or buyin or buy in$1 or
-
cooperat$ or co operat$ or expectation$ or experienc$ or feedback or involv$ or
-
opinion$ or participat$ or perceived need$ or (perception$ not speech perception)
-
or perspective$ or preferen$ or satisf$ or view$ or voices or worry).ti,ab.
-
(or/14,16,18,19) and (or/21,23,25-26)
-
or/12,27
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost)
| s43 | S28 or s39 or s40 or s41 or s42 | ||
| s42 | ti ( ( (service* n2 (acceptab* or unacceptab*)) ) ) or ab ( ( (service* n2 (acceptab* or unacceptab*)) ) ) | ||
| s41 | ti ( ( ((information n1 (need* or requirement* or support*)) or patient information) ) ) or ab ( ( ((information n1 (need* or requirement* or support*)) or patient information) ) ) | ||
| s40 | ti (((adult* or attender* or carer* or caregiv* or “care giv*” or client* or “community based” or consumer* or couples or customer* or daughter* or famil* or father* or friend* or (home n2 (care or caring or nurs*)) or “home based” or husband* or individual* or ((informal or “non professional”) n5 (care or nursing)) or marital* or men or mentor* or mother* or multifam* or neighbo?r* or “next of kin” or niece or nephew* or parent* or partner* or patient* or people* or person* or relative or relatives or sibling* or “significant other*” or spous* or “step relationship*” or teacher* or wife* or wives or women or user*) or (adolescen* or boy* or child* or delinquen* or girl* or graders or infant* or junior* or juvenile* or kindergarten or minors or p?ediatric* or postpubert* or postpubescen* or preadolescen* or prepubert* or prepubescen* or preschool* or preteen* or pubert* or pubescen* or school* or teen* or toddler* or (young* n1 (people or person* or patient* or population*)) or youngster* or youth*)) n3 (account* or anxieties or attitude* or barriers or belief* or buyin or "buy in*" or cooperat* or "co operat*" or expectation* or experienc* or feedback or involv* or opinion* or participat* or perceived need* or (perception* not "speech perception") or perspective* or preferen* or satisf* or view* or voices or worry)) or ab (((adult* or attender* or carer* or caregiv* or “care giv*” or client* or “community based” or consumer* or couples or customer* or daughter* or famil* or father* or friend* or (home n2 (care or caring or nurs*)) or “home based” or husband* or individual* or ((informal or “non professional”) n5 (care or nursing)) or marital* or men or mentor* or mother* or multifam* or neighbo?r* or “next of kin” or niece or nephew* or parent* or partner* or patient* or people* or person* or relative or relatives or sibling* or “significant other*” or spous* or “step relationship*” or teacher* or wife* or wives or women or user*) or (adolescen* or boy* or child* or delinquen* or girl* or graders or infant* or junior* or juvenile* or kindergarten or minors or p?ediatric* or postpubert* or postpubescen* or preadolescen* or prepubert* or prepubescen* or preschool* or preteen* or pubert* or pubescen* or school* or teen* or toddler* or (young* n1 (people or person* or patient* or population*)) or youngster* or youth*)) n3 (account* or anxieties or attitude* or barriers or belief* or buyin or "buy in*" or cooperat* or "co operat*" or expectation* or experienc* or feedback or involv* or opinion* or participat* or perceived need* or (perception* not "speech perception") or perspective* or preferen* or satisf* or view* or voices or worry)) | ||
| S39 | ti (#29 or #30) and (#31 or #32 or #33 or #34 or #35 or #36 or #37 or #38) | ||
| S38 | ti ( (account* or anxieties or attitude* or barriers or belief* or buyin or "buy in*" or cooperat* or "co operat*" or expectation* or experienc* or feedback or involv* or opinion* or participat* or perceived need* or (perception* not "speech perception") or perspective* or preferen* or satisf* or view* or voices or worry) ) or ab ( (account* or anxieties or attitude* or barriers or belief* or buyin or "buy in*" or cooperat* or "co operat*" or expectation* or experienc* or feedback or involv* or opinion* or participat* or perceived need* or (perception* not "speech perception") or perspective* or preferen* or satisf* or view* or voices or worry) ) | ||
| S37 | (mh "consumer attitudes") | ||
| S36 | (mh "consumer satisfaction") | ||
| s35 | (mh "patient satisfaction") | ||
| s34 | (mh "patient education (iowa nic) (non-cinahl)") | ||
| s33 | (mh "patient education") or (mh "hiv education") or (mh "patient discharge education") | ||
| s32 | (mh "patient centered care") | ||
| s31 | (mh "attitude to health+") | ||
| s30 | ( (survey* or question*) ) or ab ( (survey* or question*) ) | ||
| S29 | (mh "surveys") | ||
| s28 | s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 or s9 or s10 or s11 or s12 or s13 or s14 or s15 or s16 or s17 or s18 or s19 or s20 or s21 or s22 or s23 or s24 or s25 or s26 or s27 | ||
| s27 | ti (qualitative* or ethno* or emic or etic or heuristic or semiotics or ((focus* or structured) n2 interview*) or ((audio or tape or video*) n5 record*) or audiorecord* or taperecord* or videorecord* or videotap* or story or stories or storytell* or “story tell*” or testimon* or (focus n4 (group* or sampl*)) or narrat* or ((life or lived) n1 experience*) or ((participant* or nonparticipant*) n3 observ*) or (constant n1 (comparative or comparison)) or “content analy*” or (field n1 (note* or record* or stud* or research)) or fieldnote* or (data n1 saturat*) or “discourse analys?s” or (grounded n1 (theor* or study or studies or research)) or hermeneutic* or heidegger* or husserl* or colaizzi* or giorgi* or glaser or spiegelberg* or strauss or “maximum variation” or snowball or “purpos* sampl*” or “structured categor*” or “unstructured categor*” or ((thematic* n3 analys*) or themes) or “theoretical sampl*” or ricoeur or spiegelberg* or merleau or “van kaam*” or “van manen” or “constant compar*” or “action research” or “human science” or “critical social*” or “ethical enquiry” or (“pilot testing” and survey) or shadowing or ((philosophical or social) n1 research*)) or ab (qualitative* or ethno* or emic or etic or heuristic or semiotics or ((focus* or structured) n2 interview*) or ((audio or tape or video*) n5 record*) or audiorecord* or taperecord* or videorecord* or videotap* or story or stories or storytell* or “story tell*” or testimon* or (focus n4 (group* or sampl*)) or narrat* or ((life or lived) n1 experience*) or ((participant* or nonparticipant*) n3 observ*) or (constant n1 (comparative or comparison)) or “content analy*” or (field n1 (note* or record* or stud* or research)) or fieldnote* or (data n1 saturat*) or “discourse analys?s” or (grounded n1 (theor* or study or studies or research)) or hermeneutic* or heidegger* or husserl* or colaizzi* or giorgi* or glaser or spiegelberg* or strauss or “maximum variation” or snowball or “purpos* sampl*” or “structured categor*” or “unstructured categor*” or ((thematic* n3 analys*) or themes) or “theoretical sampl*” or ricoeur or spiegelberg* or merleau or “van kaam*” or “van manen” or “constant compar*” or “action research” or “human science” or “critical social*” or “ethical enquiry” or (“pilot testing” and survey) or shadowing or ((philosophical or social) n1 research*)) | ||
| s26 | (mh "theoretical sample") | ||
| s25 | (mh "purposive sample") | ||
| s24 | (mh "observational methods+") | ||
| s23 | (mh "methodological research") | ||
| s22 | (mh "grounded theory") | ||
| s21 | (mh "information processing (iowa noc)") | ||
| s20 | (mh "focus groups") | ||
| s19 | (mh "field studies") | ||
| s18 | (mh "ethnonursing research") | ||
| s17 | (mh "ethnological research") | ||
| s16 | (mh "ethnography") | ||
| s15 | (mh "thematic analysis") | ||
| s14 | (mh "content analysis") | ||
| s13 | (mh "cluster analysis") | ||
| s12 | (mh "discourse analysis") | ||
| s11 | (mh "constant comparative method") | ||
| s10 | (mh "attitude+") | ||
| s9 | (mh "audiorecording") | ||
| s8 | (mh "videorecording") | ||
| s7 | (mh "storytelling+") | ||
| s6 | (mh "narratives") | ||
| s5 | (mh "life change events") | ||
| s4 | (mh "life experiences") | ||
| s3 | (mh "qualitative studies+") | ||
| s2 | (mh "qualitative validity") | ||
| s1 | (mh "phenomenology") | ||
Australian Education Index, Applied Social Sciences Index and Abstracts, British Education Index, Education Resources in Curriculum, International Bibliography of Social Science, Social Services Abstracts, Sociological Abstracts (ProQuest)
-
su.exact(“cluster analysis”)
-
su.exact(“content analysis”)
-
su.exact(“cultural anthropology”)
-
su.exact(“discourse analysis”)
-
su.exact(“ethnography”)
-
su.exact(“focus groups”)
-
su.exact(“focused interviews”)
-
su.exact(“grounded theory”)
-
su.exact(“narration”)
-
su.exact(“narratives”)
-
su.exact(“personal narratives”)
-
su.exact.explode(“phenomenology” or “phenomenoological analysis”)
-
su.exact(“qualitative analysis”)
-
su.exact(“qualitative data”)
-
su.exact(“qualitative methods”)
-
su.exact(“qualitative research”)
-
su.exact(“qualitative studies”)
-
su.exact(“semi structured interviews”)
-
su.exact(“semistructured interviews”)
-
su.exact(“semistructured questionnaires”)
-
su.exact(“story telling”)
-
su.exact(“storytelling”)
-
su.exact(“tape recordings”)
-
su.exact.explode(“tape recordings” or “selfhelp tape recordings”)
-
su.exact(“videodisc recordings”)
-
su.exact(“videotape recording”)
-
su.exact(“videotape recordings”)
-
ti (“action research” or audiorecord* or ((audio or tape or video*) near/5 record*)
-
or colaizzi* or (constant near/1 (comparative or comparison)) or “content analy*” or “critical social*” or (data near/1 saturat*) or “discourse analys?s” or emic or “ethical enquiry” or ethno* or etic or fieldnote* or (field near/1 (note* or record* or stud* or research)) or (focus near/4 (group* or sampl*)) or ((focus* or
-
structured) near/2 interview*) or giorgi* or glaser or (grounded near/1 (theor* or study or studies or research)) or heidegger* or hermeneutic* or heuristic or “human science” or husserl* or ((life or lived) near/1 experience*) or “maximum variation” or merleau or narrat* or ((participant* or nonparticipant*) near/3 observ*) or ((philosophical or social) near/1 research*) or (“pilot testing” and survey) or “purpos* sampl*” or qualitative* or ricoeur or semiotics or shadowing or snowball or spiegelberg* or stories or story or storytell* or strauss or “structured categor*” or “tape record*” or taperecord* or testimon* or (thematic* near/3 analys*) or themes or “theoretical sampl*” or “unstructured categor*” or “van kaam*” or “van manen” or videorecord* or “video record*” or videotap* or “video tap*”) or ab (“action research” or audiorecord* or ((audio or tape or video*) near/5 record*) or colaizzi* or (constant near/1 (comparative or comparison)) or “content analy*” or “critical social*” or (data near/1 saturat*) or “discourse analys?s” or emic or “ethical enquiry” or ethno* or etic or fieldnote* or (field near/1 (note* or record* or stud* or research)) or (focus near/4 (group* or sampl*)) or ((focus* or structured) near/2 interview*) or giorgi* or glaser or (grounded near/1 (theor* or study or studies or research)) or heidegger* or hermeneutic* or heuristic or “human science” or husserl* or ((life or lived) near/1 experience*) or “maximum variation” or merleau or narrat* or ((participant* or nonparticipant*) near/3 observ*) or ((philosophical or social) near/1 research*) or (“pilot testing” and survey) or “purpos* sampl*” or qualitative* or ricoeur or semiotics or shadowing or snowball or spiegelberg* or stories or story or storytell* or strauss or “structured categor*” or “tape record*” or taperecord* or testimon* or (thematic* near/3 analys*) or themes or “theoretical sampl*” or “unstructured categor*” or “van kaam*” or “van manen” or videorecord* or “video record*” or videotap* or “video tap*”)
-
ti (((adult* or attender* or carer* or caregiv* or “care giv*” or client* or
-
“community based” or consumer* or couples or customer* or daughter* or famil*
-
or father* or friend* or (home near/2 (care or caring or nurs*)) or “home based”
-
or husband* or individual* or ((informal or “non professional”) near/5 (care or
-
nursing)) or marital* or men or mentor* or mother* or multifam* or neighbo?r*
-
or “next of kin” or niece or nephew* or parent* or partner* or patient* or people*
-
or person* or relative or relatives or sibling* or “significant other*” or spous* or
-
“step relationship*” or teacher* or wife* or wives or women or user*) or
-
(adolescen* or boy* or child* or delinquen* or girl* or graders or infant* or
-
junior* or juvenile* or kindergarten or minors or p?ediatric* or postpubert* or
-
postpubescen* or preadolescen* or prepubert* or prepubescen* or preschool* or
-
preteen* or pubert* or pubescen* or school* or teen* or toddler* or (young*
-
near/1 (people or person* or patient* or population*)) or youngster* or youth*))
-
near/3 (account* or anxieties or attitude* or barriers or belief* or buyin or “buy
-
in*” or cooperat* or “co operat*” or expectation* or experienc* or feedback or
-
involv* or opinion* or participat* or perceived need* or (perception* not “speech
-
perception”) or perspective* or preferen* or satisf* or view* or voices or worry))
-
or ab (((adult* or attender* or carer* or caregiv* or “care giv*” or client* or
-
“community based” or consumer* or couples or customer* or daughter* or famil*
-
or father* or friend* or (home near/2 (care or caring or nurs*)) or “home based”
-
or husband* or individual* or ((informal or “non professional”) near/5 (care or
-
nursing)) or marital* or men or mentor* or mother* or multifam* or neighbo?r*
-
or “next of kin” or niece or nephew* or parent* or partner* or patient* or people*
-
or person* or relative or relatives or sibling* or “significant other*” or spous* or
-
“step relationship*” or teacher* or wife* or wives or women or user*) or
-
(adolescen* or boy* or child* or delinquen* or girl* or graders or infant* or
-
junior* or juvenile* or kindergarten or minors or p?ediatric* or postpubert* or
-
postpubescen* or preadolescen* or prepubert* or prepubescen* or preschool* or
-
preteen* or pubert* or pubescen* or school* or teen* or toddler* or (young*
-
near/1 (people or person* or patient* or population*)) or youngster* or youth*))
-
near/3 (account* or anxieties or attitude* or barriers or belief* or buyin or “buy
-
in*” or cooperat* or “co operat*” or expectation* or experienc* or feedback or
-
involv* or opinion* or participat* or perceived need* or (perception* not “speech
-
perception”) or perspective* or preferen* or satisf* or view* or voices or worry))
-
ti ((information near/1 (need* or requirement* or support*)) or “health
-
information”) or ab ((information near/1 (need* or requirement* or support*)) or
-
“health information”)
-
ti (service* near/2 (acceptab* or unacceptab*)) or ab (service* near/2 (acceptab*
-
or unacceptab*))
-
s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 or s9 or s10 or s11 or s12 or s13 or s14
-
or s15 or s16 or s17 or s18 or s19 or s20 or s21 or s22 or s23 or s24 or s25 or s26
-
or s27 or s28 or s29 or s30 or s31
-
ti ((survey* or question*) and (account* or anxieties or attitude* or barriers or
-
belief* or buyin or “buy in*” or cooperat* or co operat* or expectation* or
-
experienc* or feedback or involv* or opinion* or participat* or perceived need*
-
or perspective* or preferen* or satisf* or view* or voices or worry)) or ab
-
((survey* or question*) and (account* or anxieties or attitude* or barriers or
-
belief* or buyin or “buy in*” or cooperat* or co operat* or expectation* or
-
experienc* or feedback or involv* or opinion* or participat* or perceived need*
-
or perspective* or preferen* or satisf* or view* or voices or worry))
-
32 or 33
BIOSIS Citation Index, Web of Science (Thomson Reuters)
-
topic=((“action research” or audiorecord* or ((audio or tape or video*) near record*) or colaizzi* or (constant near (comparative or comparison)) or “content analy*” or “critical social*” or (data near saturat*) or “discourse analys?s” or emic or “ethical enquiry” or ethno* or etic or fieldnote* or (field near (note* or record* or stud* or research)) or (focus near (group* or sampl*)) or ((focus* or structured) near interview*) or giorgi* or glaser or (grounded near (theor* or study or studies or research)) or heidegger* or hermeneutic* or heuristic or “human science” or husserl* or ((life or lived) near experience*) or “maximum variation” or merleau or narrat* or ((participant* or nonparticipant*) near observ*) or ((philosophical or social) near research*) or (“pilot testing” and survey) or “purpos* sampl*” or qualitative* or ricoeur or semiotics or shadowing or snowball or spiegelberg* or stories or story or storytell* or strauss or “structured categor*” or “tape record*” or taperecord* or testimon* or (thematic* near analys*) or themes or “theoretical sampl*” or “unstructured categor*” or “van kaam*” or “van manen” or videorecord* or “video record*” or videotap* or “video tap*”)) or title= ((“action research” or audiorecord* or ((audio or tape or video*) near record*) or colaizzi* or (constant near (comparative or comparison)) or “content analy*” or “critical social*” or (data near saturat*) or “discourse analys?s” or emic or “ethical enquiry” or ethno* or etic or fieldnote* or (field near (note* or record* or stud* or research)) or (focus near (group* or sampl*)) or ((focus* or structured) near interview*) or giorgi* or glaser or (grounded near (theor* or study or studies or research)) or heidegger* or hermeneutic* or heuristic or “human science” or husserl* or ((life or lived) near experience*) or “maximum variation” or merleau or narrat* or ((participant* or nonparticipant*) near observ*) or ((philosophical or social) near research*) or (“pilot testing” and survey) or “purpos* sampl*” or qualitative* or ricoeur or semiotics or shadowing or snowball or spiegelberg* or stories or story or storytell* or strauss or “structured categor*” or “tape record*” or taperecord* or testimon* or (thematic* near analys*) or themes or “theoretical sampl*” or “unstructured categor*” or “van kaam*” or “van manen” or videorecord* or “video record*” or videotap* or “video tap*”))
-
topic= (((adult* or attender* or carer* or caregiv* or “care giv*” or client* or “community based” or consumer* or couples or customer* or daughter* or famil* or father* or friend* or (home near (care or caring or nurs*)) or “home based” or husband* or individual* or ((informal or “non professional”) near (care or nursing)) or marital* or men or mentor* or mother* or multifam* or neighbo?r* or “next of kin” or niece or nephew* or parent* or partner* or patient* or people* or person* or relative or relatives or sibling* or “significant other*” or spous* or “step relationship*” or teacher* or wife* or wives or women or user*) or (adolescen* or boy* or child* or delinquen* or girl* or graders or infant* or junior* or juvenile* or kindergarten or minors or p?ediatric* or postpubert* or postpubescen* or preadolescen* or prepubert* or prepubescen* or preschool* or preteen* or pubert* or pubescen* or school* or teen* or toddler* or (young* near (people or person* or patient* or population*)) or youngster* or youth*)) near (account* or anxieties or attitude* or barriers or belief* or buyin or “buy in*” or cooperat* or “co operat*” or expectation* or experienc* or feedback or involv* or opinion* or participat* or perceived need* or (perception* not “speech perception”) or perspective* or preferen* or satisf* or view* or voices or worry)) or title= (((adult* or attender* or carer* or caregiv* or “care giv*” or client* or “community based” or consumer* or couples or customer* or daughter* or famil* or father* or friend* or (home near (care or caring or nurs*)) or “home based” or husband* or individual* or ((informal or “non professional”) near (care or nursing)) or marital* or men or mentor* or mother* or multifam* or neighbo?r* or “next of kin” or niece or nephew* or parent* or partner* or patient* or people* or person* or relative or relatives or sibling* or “significant other*” or spous* or “step relationship*” or teacher* or wife* or wives or women or user*) or (adolescen* or boy* or child* or delinquen* or girl* or graders or infant* or junior* or juvenile* or kindergarten or minors or p?ediatric* or postpubert* or postpubescen* or preadolescen* or prepubert* or prepubescen* or preschool* or preteen* or pubert* or pubescen* or school* or teen* or toddler* or (young* near (people or person* or patient* or population*)) or youngster* or youth*)) near (account* or anxieties or attitude* or barriers or belief* or buyin or “buy in*” or cooperat* or “co operat*” or expectation* or experienc* or feedback or involv* or opinion* or participat* or perceived need* or (perception* not “speech perception”) or perspective* or preferen* or satisf* or view* or voices or worry))
-
topic= ((information near (need* or requirement* or support*)) or “health information”) or title= ((information near (need* or requirement* or support*)) or “health information”)
-
topic= (service* near (acceptab* or unacceptab*)) or title= (service* near (acceptab* or unacceptab*))
-
1 or 2 or 3 or 4
-
topic= ((survey* or question*) and (account* or anxieties or attitude* or barriers or belief* or buyin or “buy in*” or cooperat* or co operat* or expectation* or experienc* or feedback or involv* or opinion* or participat* or perceived need* or perspective* or preferen* or satisf* or view* or voices or worry)) or title= ((survey* or question*) and (account* or anxieties or attitude* or barriers or belief* or buyin or “buy in*” or cooperat* or co operat* or expectation* or experienc* or feedback or involv* or opinion* or participat* or perceived need* or perspective* or preferen* or satisf* or view* or voices or worry))
-
5 or 6
Appendix 2 Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram
FIGURE 237.
A PRISMA flow diagram of included studies. MAO-B, monoamine oxidase type B.

Appendix 3 Study characteristics
Included studies
| Study | Study design | Population | Treatment | Attrition | |
|---|---|---|---|---|---|
| Antipsychotics | |||||
| Sallee 199798 | Haloperidol and pimozide vs. placebo Crossover RCT (2 weeks’ washout) Inclusion criteria:
|
22 children Mean age 10.2 years, SD 2.5 years Age range 7–16 years 77% male, 100% TS 59% ADHD, 23% OCD 0% on medication TSGS 28.5, SD 14.5 |
Haloperidol Mean dose of 3.5 mg/day, SD 2.2 mg/day Range 1–8 mg/day Start dose of 1 mg/day Pimozide Mean dose of 3.4 mg/day, SD 1.6 mg/day Range 1–6 mg/day Start dose of 1 mg/day Administered 1 × day 6 weeks’ duration |
95% completed 100% analysed |
|
| Shapiro 198999 | Haloperidol and pimozide vs. placebo Parallel RCT (crossover but first-phase data used) Inclusion criteria:
|
57 adults and children Mean age 21.1 years, SD 11 years Age range NR 72% male, 100% TS 19% ADHD, % OCD NR 0% on medication Shapiro TSSS 4.1, SD 2.0 |
Haloperidol Maximum dose of 10 mg/day Start dose of 0.5 mg/day Pimozide Maximum dose of 0.3 mg/kg/day or 20 mg/day Start dose of 1 mg/day Administered 1 × day 6 weeks’ duration |
84% completed 84% analysed |
|
| Shapiro 1984100 | Pimozide vs. placebo Crossover RCT (2 weeks’ washout) Inclusion criteria:
|
20 adults and children Mean age 24.7 years, SE 2.7 years Age range 11–53 years 65% male 100% TS 30% ADHD, % OCD NR 0% on medication |
Maximum dose of 20 mg/day for adults, 10 mg/day or ≈ 0.2 mg/kg/day for children aged ≤ 12 years Start dose of 1 mg/day Administered 1 × day 6 weeks’ duration |
83% completed 83% analysed |
|
| Ross 1978106 | Pimozide vs. haloperidol Crossover RCT (6 day washout) Inclusion criteria:
|
Nine adults and children Mean age 18.7 years, SD NR Age range 8–28 years 78% male 100% TS % ADHD, % OCD NR 0% current medication Mean tic frequency 44 per 5 minutes, SD 26 tics |
Pimozide Maximum dose of 12 mg/day Start dose of 2 mg/day Haloperidol Maximum dose of 12 mg/day Start dose of 2 mg/day Administered 1 × day 12 days’ duration |
Only available cases reported | |
| Gulisano 2011109 | Pimozide vs. aripiprazole Parallel controlled before-and-after study Inclusion criteria:
|
50 children Mean age 11.1 years, SD 2.1 years Age range 6–18 years 86% male, 100% TS 56% ADHD, 48% OCD 0% on medication YGTSS global score 49.3, SD 2.7 |
Pimozide mean dose of 4.4 mg/2 × day, SD 1.5 mg/2 × day Aripiprazole mean dose of 5.3 mg/2 × day, SD 2.4 mg/2 × day 24 months’ duration |
% completed NR % analysed NR |
|
| Scahill 2003101 | Risperidone vs. placebo Parallel RCT Inclusion criteria:
|
26 children Mean age 11.1 years, SD 2.2 years Age range 6–NR years 96% male, 100% TS 42% ADHD, 15% OCD 0% on medication YGTSS total tic score 27.9, SD 6.7 |
Maximum dose of 3 mg/day, 4 mg/day for older adolescents Start dose of 0.5 mg/day Administered 2 × day 8 weeks’ duration |
92% completed 100% analysed |
|
| Dion 2002102 | Risperidone vs. placebo Parallel RCT Inclusion criteria:
|
48 adults and children Median age 32 years Age range 14–49 years 18% male, 100% TS % ADHD NR, % OCD NR 0% on medication Shapiro TSSS 5.3, SD 1.3 |
Maximum dose of 6 mg/day Start dose of 0.25 mg/day Administered 2 × day 8 weeks’ duration |
81 completed 96% analysed |
|
| Sallee 2000103 | Ziprasidone vs. placebo Parallel RCT Inclusion criteria:
|
28 children Mean age 11.6 years, SD NR Age range 7–16 years 79% male 96% TS, 4% CTD 54% ADHD, 36% OCD 0% on medication YGTSS global score 46.9, SD 16 |
Maximum dose of 20 mg/day Start dose of 5 mg/day Administered 2 × day 8 weeks’ duration |
86% completed 96% analysed |
|
| Gilbert 2004104 | Risperidone vs. pimozide Crossover RCT (2 weeks’ washout) Inclusion criteria:
|
19 children Mean age 11 years, SD 2.5 years Age range 7–17 years 79% male 84% TS, 16% CTD 37% ADHD, 2% OCD 0% current medication Mean YGTSS global score 43.3, SD 17.5 |
Risperidone Maximum dose of 4 mg/day Start dose of 1 mg/day Split into two equal doses per day administered morning and night time Pimozide Maximum dose of 4 mg/day Start dose of 1 mg/day Administered 1 × day (+ placebo 1 × day) 4 weeks’ duration |
68% completed 68% analysed |
|
| Bruggerman 2001105 | Risperidone vs. pimozide Parallel RCT Inclusion criteria:
|
50 adults and children Median age 22 years Range 11–50 years 88% male 4% ADHD, 46% OCD 0% current medicate Mean Shapiro TSSS total score 4.3 |
Risperidone Maximum dose of 6 mg/day Start dose of 1 mg/day Pimozide Maximum dose of 6 mg/day 1 mg/week increments Administered 1 × day 8 weeks’ duration |
82% completed 100% analysed |
|
| Liu 2011107 | Aripiprazole vs. tiapride Parallel quasi-randomised trial Inclusion criteria:
|
200 children Mean age 10.1 years, SD 2.7 years Range 5–17 years 80% male 100% TS % on current medication NR Mean YGTSS global score 52.5, SD 14.7 |
Aripiprazole Maximum dose of 15–25 mg/day Start dose of 2.5–5 mg/day Tiapride Maximum dose of 300–500 mg/day Start dose of 25–50 mg/day 12 weeks’ duration |
98% completed 95% analysed |
|
| Yoo 2011108 | Aripiprazole vs. haloperidol Parallel comparative (non-randomised) study Inclusion criteria:
|
48 children Mean age 10.3 years, SD 3.5 years Range 6–15 years 69% male 54% TS, 22% CTD 31% ADHD, 6% OCD % on current medication NR YGTSS total tic score 26.9, SD 5.8 |
Aripiprazole Maximum dose of 20 mg/day Start dose of 5 mg/day Haloperidol Maximum dose of 4.5 mg/day Start dose of 0.75 mg/day 8 weeks’ duration |
77% competed 100% analysed |
|
| Ji 2005110 | Olanzapine vs. haloperidol Parallel RCT Inclusion criteria:
|
60 children Mean age 10.5 years, SD 3.7 years Range 8–16 years 95% male 100% TS % on current medication NR YGTSS total tic score 31, SD 9 |
Olanzapine Maximum dose of 10 mg/day Start dose of 2.5–5 mg/day Haloperidol Maximum dose of 12 mg/day Start dose of 2–4 mg/day 4 weeks’ duration |
90% completed 90% analysed |
|
| Noradrenergic agents | |||||
| Kurlan 2002113 | Clonidine and methylphenidate and clonidine + methylphenidate vs. placebo Parallel RCT Inclusion criteria:
|
136 children Mean age 10.2 years, SD 2 years Age range 7–14 years 85% male 94% TS 6% CTD 100% ADHD, 16% OCD 0% on other medication YGTSS global score 40.6, SD 18 |
Clonidine Maximum dose of 0.6 mg/day Start dose of 0.2 mg/day Methylphenidate Maximum dose of 60 mg/day Both clonidine and methylphenidate Administered 1 × day 16 weeks’ duration Outcome assessed at 4, 8, 12 and 16 weeks |
89% completed 100% analysed |
|
| Leckman 1991116 | Clonidine vs. placebo Parallel RCT Inclusion criteria:
|
47 adults and children Mean age 15.6 years, SD 10.4 years Age range 7–48 years 80% male 100% TS 55% ADHD, 20% OCD 0% on other medication TSGS 35.7, SD 8.3 |
0.25 mg/day Start dose of 4–5 mg/kg/day Increased over 2 weeks and maintained for 10 weeks Administered 2, 3 or 4 times/day 12 weeks’ duration Outcomes assessed at 12 weeks |
89% completed 85% analysed |
|
| Goetz 1987114 | Clonidine vs. placebo Crossover RCT (1 week of washout) Inclusion criteria:
|
24 child [IPD from mixed study (n = 30)] Mean age 12.9 years, SD 2.6 years Age range 8–17 years 79% male 100% TS 8% ADHD, 8% OCD 46% on other medication Motor: 8 severe, 17 moderate, 5 mild Vocal: 1 severe, 4 moderate, 16 mild (for n = 30 population) |
Maximum dose of 0.0075 mg/kg/day or 0.015 mg/kg/day Start dose of 0.0025 mg/kg/day, escalated over 6 weeks and maintained for 6 weeks Administered 1 × day 12 weeks’ duration |
% completed NR % analysed NR |
|
| Singer 1995115 | Clonidine and desipramine vs. placebo Crossover RCT (1 week of washout) Inclusion criteria:
|
37 children Mean age 10.6 years, SD NR Age range 7–14 years 91% male 100% TS 100% ADHD, 0% OCD Baseline severity NR |
Clonidine Maximum dose of 0.2 mg/day Start dose of 0.05 mg/day Desipramine Maximum dose of 100 mg/day Start dose of 25 mg/day Administered up to 4 × day 6 weeks’ duration |
92% completed 92% analysed |
|
| Du 2008117 | Clonidine patch vs. placebo patch Parallel RCT Inclusion criteria:
|
437 children Mean age 10.1 years, SD 2.8 years Age range 6–18 years 85% male % ADHD NR, % OCD NR YGTSS motor tic score 15.6, SD 5 |
1 mg, 1.5 mg or 2 mg for body weight < 20 kg, 20–40 kg or 40–60 kg, respectively Patch applied 1 × day 4 weeks’ duration |
87% completed 100% analysed |
|
| Zhong 2007118 | Clonidine patch vs. placebo patch Parallel RCT Inclusion criteria:
|
76 children Median age 8–9 years Age range 6–18 years 82% male % ADHD or OCD NR YGTSS global tic score 56, SD 14 |
1 mg, 1.5 mg or 2 mg for body weight < 20 kg, 20–40 kg or 40–60 kg, respectively Patch applied 1 × day 4 weeks’ duration |
% completed NR % analysed NR |
|
| Gancher 1990119 | Clonidine patch vs. placebo patch Crossover RCT (2 weeks’ washout) Inclusion criteria:
|
10 adults and children Mean age adults 28 years, children 12 years, SD NR Age range NR 100% TS % ADHD NR, % OCD NR 0% on other medication TSGS 8.5, range 6–19 |
Maximum dose of 0.4 mg/day Start dose of 0.1 mg/day Patch applied once a week 2 months’ duration |
90% completed 90% analysed |
|
| Cummings 2002121 | Guanfacine vs. placebo Parallel RCT Inclusion criteria:
|
24 children Mean age 10.4 years, SD 2.3 years Age range NR 83% male 96% TS, 4% CTD 0% on other medication 17% ADHD, 0% OCD YGTSS global score 32.2, SD 13 |
Maximum dose of 2 mg/day Start dose of 0.5 mg/day Administered 2 × day 4 weeks’ duration |
% completed NR % analysed NR |
|
| Scahill 2001120 | Guanfacine vs. placebo Parallel RCT Inclusion criteria:
|
34 children Mean age 10.4 years, SD 2.0 years Age range 7–14 years 91% male 59% TS, 35% CTD 100% ADHD, % OCD NR 0% on other medication YGTSS global score 15.3, SD 6.7 |
Maximum dose of 4 mg/day Start dose of 0.5 mg/day Administered 1, 2 or 3 times/day 8 weeks’ duration |
% completed NR 100% analysed |
|
| SSRI antidepressants | |||||
| Kurlan 1993143 | Fluoxetine vs. placebo Parallel RCT Inclusion criteria:
|
11 children Mean age 13.1 years, SD 2.6 years Age range 10–18 years 100% male 100% TS % ADHD NR, 100% OCD symptoms (LOI-CV ≥ 14, OCD cut-off point ≥ 20) Six patients on haloperidol and/or clonidine Goetz motor tic frequency 5.4, SD 2.6 |
Maximum dose of 40 mg/day Initial dose of 20 mg/day 4 months’ duration |
82% competed 82% analysed |
|
| Scahill 1997144 | Fluoxetine vs. placebo Crossover RCT (4 weeks’ washout) but first-phase data used Inclusion criteria:
|
Seven adults and seven children Mean age 19 years, SD 8.2 years Age range 9–34 years 64% male 100% TS 43% ADHD, 36% OCD 0% on other medication YGTSS total tic score 25.4, SD 5.8 |
Fixed dose of 20 mg/day 8 weeks’ duration |
71% completed 71% analysed |
|
| TCA | |||||
| Singer 1995115 | Desipramine (and clonidine) vs. placebo Crossover RCT (1 week of washout) Inclusion criteria:
|
37 children Mean age 10.6 years SD NR Age range 7–14 years 91% male, 100% TS 100% ADHD, 0% OCD Baseline severity NR |
Desipramine Maximum dose of 100 mg/day Start dose of 25 mg/day Administered up to 4 × day 6 weeks’ duration |
92% completed 92% analysed |
|
| Spencer 2002141 | Desipramine vs. placebo Parallel RCT Inclusion criteria:
|
39 children (with tics, two without tics not included in tic outcomes) Mean age 10.9 years, SD 3 years Age range NR 83% male 87% TS, 13% CTD 100% ADHD, 29% OCD 0% on other medication YGTSS global score 64.0, SD 17 |
Maximum dose of 3.5 mg/kg/day Administered 2 × day 6 weeks’ duration |
95% completed 100% analysed |
|
| Antiemetic and gastroprokinetic agents | |||||
| Nicolson 2005138 | Metoclopramide vs. placebo Parallel RCT Inclusion criteria:
|
27 children Mean age 11.5 years, SD ≈2.6 years Age range 7–18 years 70% male 96% TS, 4% CTD 67% ADHD, % OCD NR On other medications for tics or comorbidities YGTSS total tic score 22.4, SD ≈6 |
Maximum dose of 40 mg/day Start dose of 5 mg/day Administered 2 or 3 times/day 8 weeks’ duration |
86% completed 96% analysed |
|
| Toren 2005139 | Ondansetron vs. placebo Parallel RCT Inclusion criteria:
|
15 children (IPD from mixed n = 30 sample) Mean age 14 years, SD 2.9 years Age range 12–18 years 60% male, 100% TS 20% ADHD, 13% OCD YGTSS total tic score 26.3, SD 8.2 |
Maximum dose of 24 mg/day Start dose of 8 mg/day Administered 3 × day (increased from 1 to 2 to 3 times/day) 3 weeks’ duration |
87% completed 87% analysed |
|
| Anticonvulsants | |||||
| Smith-Hicks 2007129 | Levetiracetam vs. placebo Crossover RCT (2 weeks’ washout) Inclusion criteria:
|
22 children Mean age 12.2 years, SD 2.3 years Age range 8–16 years 95% male 100% TS 50% ADHD, 9% OCD, 45% OCD behaviours 50% on other medications |
Maximum dose of 30 mg/kg/day Start dose of 10 mg/kg/day Administered 2 × day 4 week duration |
91% completed 91% analysed |
|
| Jankovic 2010131 | Topiramate vs. placebo Parallel RCT Inclusion criteria:
|
29 adults and children Mean age 16.5 years, SD 9.9 years Age range 7–65 years 90% male 100% TS 34% ADHD,% OCD NR 0% on other medications |
Maximum dose of 200 mg/day Start dose of 25 mg/day Administered 1 × day 6 week duration |
67% completed 93% analysed |
|
| Stimulants | |||||
| Kurlan 2002113 | Methylphenidate (and clonidine and clonidine + methylphenidate) vs. placebo Parallel RCT Inclusion criteria:
|
136 children (in three arms) Mean age 10.2 years, SD ≈1.9 years Age range 7–14 years 85% male 94% TS, 6% CTD 100% ADHD, 16% OCD 0% on medications for tics, ADHD or other behavioural symptoms YGTSS global score 40.6, SD 18 |
Methylphenidate Maximum dose of 60 mg/day Clonidine Maximum dose of 0.6 mg/day Methylphenidate and clonidine Administered 1 × day 16 weeks’ duration Outcome assessed at 4, 8, 12 and 16 weeks |
86% completed (117/136) 100% analysed |
|
| Castellanos 1997126 | Methylphenidate (and dextroamphetamine) vs. placebo Crossover RCT (no washout) Inclusion criteria:
|
12 children mean age 9.4 years, SD 2 years Range 6–13 years 100% male 95% TS, 5% CTD 100% ADHD, 10% OCD 25% (n = 3) subjects taking haloperidol Mean YGTSS global score 37.3, SD 14.9 |
Methylphenidate Maximum dose of 1.2 mg/kg Start dose of 0.43 mg/kg/day Dextroamphetamine Maximum dose of 0.64 mg/kg Start dose of 0.2 mg/kg Administered 2 × day 3 weeks’ duration |
83% completed 83% analysed |
|
| Gadow 2007125 | Immediate-release methylphenidate vs. placebo Crossover RCT (no washout) Inclusion criteria:
|
71 children Mean age 9.0 years, SD 1.4 years Age range 6–12 years 80% male 96% TS, 4% CTD 100% ADHD, 4% OCD 0% on other medications YGTSS global score 36.9, SD 14.6 |
Maximum dose of 0.1 mg/kg/day, 0.3 mg/kg/day and 0.5 mg/kg/day Administered 2 × day 2 weeks’ duration |
% completed NR % analysed NR |
|
| Gadow 1992124 | Methylphenidate vs. placebo Crossover RCT (no washout) Inclusion criteria:
|
11 children Mean age 8.3 years, SD 1.96 years Range 6–11 years 100% male 91% TS, 9% CTD 100% ADHD, % OCD NR 0% on other medications YGTSS global score 40.6, SD 16.6 |
Maximum dose of 0.1 mg/kg/day, 0.3 mg/kg/day and 0.5 mg/kg/day Administered 2 × day 2 weeks’ duration |
% completed NR % analysed NR |
|
| Lyon 2010127 | Immediate-release dexmethylphenidate vs. no treatment Crossover RCT (1 day washout) Inclusion criteria:
|
13 children Mean age 12.7 years, SD 2.6 years Age range 8–16 years 90% male 100% TS 100% ADHD, 0% OCD 70% on other medications YGTSS global score 41.8, SD 13.4 |
0.15 mg/kg One-off dose Studied on 1 day |
77% completed 77% analysed |
|
| Dopamine agonists | |||||
| Gilbert 2000135 | Pergolide vs. placebo Crossover RCT (2 weeks’ washout) Inclusion criteria:
|
24 children Mean age NR Age range 7–17 years 79% male % TS NR 68% ADHD, 32% OCD 0% on other medication YGTSS global score 48.0, SD 13.3 |
Maximum dose of 300 µg/day Start dose of 25 µg Administered 1 × day 6 weeks’ duration Outcome assessed at 6 weeks |
79% completed 79% analysed |
|
| Gilbert 2003136 | Pergolide vs. placebo Parallel RCT Inclusion criteria:
|
57 children Mean age 10.7 years, SD 2.4 years Age range 7–17 years 73% male % TS NR 24% ADHD, 19% OCD 0% on other medication YGTSS global score 49.0, SD 13 |
Maximum dose of 150 µg/day Start dose of 25 µg/day Administered 3 × day 8 weeks’ duration |
84% completed 89% analysed |
|
| Kurlan 2012137 | Pramipexole vs. placebo Parallel RCT Inclusion criteria:
|
63 children Mean age NR Age range 6–17 years % male NR % ADHD, % OCD NR 0% on other medication Baseline tic severity NR |
Maximum dose of 0.5 mg/day Start dose of 0.0625 mg 2 × day Administered 2 × day 6 weeks’ duration |
% completed NR 98% analysed |
|
| Atomoxetine | |||||
| Allen 2005122 | Atomoxetine vs. placebo Parallel RCT Inclusion criteria:
|
148 children Mean age 11.2 years, SD 2.5 years Age range 7–17 years 88.5% male 79% TS, 21% CTD 100% ADHD, 3% OCD 0% on other medication YGTSS total tic score 22, SD 8 |
Maximum dose of 1.5 mg/kg Start dose of 0.5 mg/kg Administered 1 × day 18 weeks’ duration |
70% completed 98% analysed |
|
| Spencer 2008123 (subgroup of Allen 2005122) | Atomoxetine vs. placebo Parallel RCT Inclusion criteria:
|
117 children Mean age 11.2 years, SD 2.4 years Age range 7–17 years 87% male 100% TS 100% ADHD, % OCD NR 0% on other medication YGTSS total tic score 23.3, SD 8 |
Maximum dose of 1.5 mg/kg Start dose of 0.5 mg/kg Administered 1 × day 18 weeks’ duration |
73% completed 99% analysed |
|
| Selegiline | |||||
| Feigin 1996132 | Selegiline (deprenyl) vs. placebo Crossover RCT (6 weeks’ washout) Inclusion criteria:
|
24 children Mean age 12 years, SD 2.5 years Age range 7–16 years 88% male 100% TS 100% ADHD, % OCD NR Current medication: haloperidol (n = 4), clonidine (n = 6), imipramine (n = 5), fluphenazine (n = 4), pimozide (n = 1), molidone (n = 1) YGTSS global score 44.2, SD ≈17.3 |
Maximum dose of 10 mg/day Starting dose of 5 mg/day 8 weeks’ duration |
63% completed % analysed NR |
|
| Mecamylamine | |||||
| Silver 2001146 | Mecamylamine vs. placebo Parallel Inclusion criteria:
|
61 children Mean age 11.3 years Age range 8–17 years 88% male 100% TS 60% ADHD, 30% OCD 0% on other medications YGTSS global score 48.8, SD NR |
Maximum dose of 7.5 mg/day Start dose of 2.5 mg/day Administered 2 × day 8 weeks’ duration |
62% completed 82% analysed |
|
| Baclofen | |||||
| Singer 2001140 | Baclofen vs. placebo Crossover (tapered over 12 days’ and 5 days’ drug-free washout) Inclusion criteria:
|
10 children Mean age 11.7 years, SD 2 years Age range 8–14 years 70% male 100% TS 50% ADHD, 30% OCD 30% on other medications YGTSS global score 63.5, SD 5.5 |
Maximum dose of 60 mg/day Start dose of 15 mg/day Administered 3 × day 4 weeks’ duration |
90% completed 90% analysed |
|
| Transdermal nicotine | |||||
| Silver 2001147 | Nicotine patch + haloperidol vs. placebo + haloperidol Parallel RCT Inclusion criteria:
|
70 children Mean age 11.1 years, SD 2 years Age range 8–17 years 90% male, 100% TS % ADHD, % OCD NR 100% on haloperidol YGTSS global score 46.7, SD 15 |
Maximum dose of 7 mg/day Nicotine patch for first 19 days. Haloperidol at optimised dose for first 5 days and then reduced by 50% for rest of study Outcome assessed at 19 and 33 days |
80% completed 80% analysed |
|
| Howson 2004148 | Nicotine patch + antipsychotic drug vs. placebo + antipsychotic drug Crossover RCT (8 weeks’ washout) Inclusion criteria:
|
23 children Mean age 11.9 years, SD 2.9 years Age range 8–17 years 82% male, 100% TS 86% ADHD, 32% OCD 96% on one or more antipsychotic drug YGTSS total tic score 21, SD 3 |
7 mg one-off dose Outcome assessed at 1 week |
61% completed 61% analysed |
|
| Omega-3 fatty acids | |||||
| Gabbay 2012149 | Omega-3 fatty acids vs. olive oil Parallel RCT Inclusion criteria:
|
33 children Mean age 11.3 years, SD 3 years Age range 6–18 years 82% male 100% TS 61% ADHD, 55% OCD 76% on other medications YGTSS global score 47.1, SD 8 |
Omega-3 fatty acids Maximum dose of 6000 mg Start dose of 500 mg Olive oil placebo Administered 1 × day 20 weeks’ duration |
76% completed 100% analysed |
|
| Head-to-head comparisons between different classes of drugs | |||||
| Gaffney 2002150 | Risperidone vs. clonidine Parallel RCT Inclusion criteria:
|
21 children Mean age 11.4 years, SD 2.9 years Range 7–17 years 90% male 100% TS 90% ADHD, 95% OCD Mean YGTSS global score 52.1, SD ≈15.5 |
Risperidone Maximum dose of 0.06 mg/kg/day Start dose of 0.03 mg/kg/day Administered 1 × day 8 weeks’ duration Clonidine Maximum dose of 0.005 mg/kg/day Start dose of 0.0025 mg/kg/day Administered 1 × day 8 weeks’ duration |
95% completed 100% analysed |
|
| George 1993151 | Fluvoxamine vs. sulpiride Crossover RCT (2 weeks’ washout) Inclusion criteria:
|
11 adults and children Mean age 28.4 years, SD 3.0 years Age range NR 73% male 100% TS % ADHD NR, 100% OCD 0% current medication Mean YGTSS global score 74.6, SD 14.8 |
Fluvoxamine Maximum dose of 300 mg/day Start dose of 50 mg/day Administered 1 × day 6 weeks’ duration Sulpiride Maximum dose of 1 g/day Start dose of 200 mg/day Administered 1 × day 6 weeks’ duration |
73% completed 91% analysed |
|
| Hedderick 2009152 | Levetiracetam vs. clonidine Crossover RCT (2 weeks’ washout) Inclusion criteria:
|
10 adults and children Mean age 14.9 years, SD 5.5 years Age range 8–27 years 70% male 100% TS 20% ADHD, % OCD NR 0% current medication YGTSS total tic score 24.0, SD 4.9 |
Levetiracetam Maximum dose of 50 mg/kg/day or 2500 mg/day Start dose of 10 mg/kg/day Administered 2 × day 6 weeks’ duration Clonidine Maximum dose of 0.4 mg/day Start dose of 0.05 mg/day 2 × day Administered 2 × day 6 weeks’ duration |
83% completed 83% analysed |
|
| Singer 1995115 | Clonidine and desipramine vs. placebo Crossover RCT (1 week of washout) Inclusion criteria:
|
37 children Mean age 10.6 years, SD NR years Age range 7–14 years 91% male 100% TS 100% ADHD, 0% OCD Baseline severity NR |
Clonidine Maximum dose of 0.2 mg/day Start dose of 0.05 mg/day Administered up to 4 × day 6 weeks’ duration Desipramine Maximum dose of 100 mg/day Start dose of 25 mg/day Administered up to 4 × day 6 weeks’ duration |
92% completed 92% analysed |
|
| Kang 2009153 | Clonidine patch vs. haloperidol Controlled study Inclusion criteria:
|
119 children Mean age 8.5 years, SD 1.3 years Range 2–15 years 72% male 100% TS % ADHD NR, % OCD NR 0% on other medication YGTSS total tic score 39.3, SD 4.2 |
Clonidine patch 1–2 mg/week Administered 1 × week 4 weeks’ duration Haloperidol Maximum dose of 2 mg/day Start dose of 0.5–0.7 mg/day Administered 2 × day 4 weeks’ duration |
% completed NR % analysed NR |
|
| Study | Study design | Population | Treatment 1 | Treatment 2 | Attrition |
|---|---|---|---|---|---|
| HRT vs. waiting list control | |||||
| Azrin 1990169 | HRT vs. waiting list Parallel RCT Inclusion criteria:
|
7 childrena Mean age 11.9 years, SD 3.5 years Age range 6–16 years 71% male 100% TS, 0% CTD % ADHD NR, % OCD NR 43% on medication Tic countb 290 per hour, SD 218 per hour |
HRT Mean 20 sessions (range 13–30 sessions) over 8–11 months Primary component: competing response training Awareness training, self-monitoring, relaxation training, competing response training, contingency management Assessed each month for 8–11 months |
Waiting list 3 months, no treatment Assessed at 3 months |
64% completedc 64% analysed |
| HRT/CBIT vs. SP | |||||
| Deckersbach 2006168 | HRT vs. SP Parallel RCT Inclusion criteria:
|
32 adults Mean age 35.1 years, SD 12.2 years Age range NR 53% male 100% TS, 0% CTD % ADHD NR, 30% OCD 53% on medication YGTSS total tic scoreb 28.5, SD ≈6.1 |
HRT Fourteen 50-minute sessions over 20 weeks Self-monitoring, competing response training, relaxation training, contingency management Assessed at 10 and 20 weeks |
SP Fourteen 50-minute sessions over 5 months Encouragement, reassurance, reframing, clarification, ventilation, modelling appropriate behaviour, absolution and advice giving Assessed at 10 and 20 weeks |
88% completed 94% analysed |
| Piacentini 2010156 | CBIT vs. SP Parallel RCT Inclusion criteria:
|
126 children Mean age 11.7 years, SD 2.3 years Age range 9–17 years 79% male 94% TS, CTD 6% 26% ADHD, 19% OCD 37% on medication for tics YGTSS total tic scoreb 24.7, SD 6.1 |
CBIT Eight sessions (two 90 minutes, six 60 minutes) over 10 weeks Primary component: HRT Awareness training, competing response training, relaxation training, functional behavioural strategies to reduce influence of tic enhancing situations Assessed at 5 and 10 weeks |
SP Eight sessions (two 90 minutes, six 60 minutes) over 10 weeks Information about tic disorders and discussion, designed to mimic adjunctive components of psychopharmacological treatment Assessed at 5 and 10 weeks |
90% completed 100% analysed |
| Wilhelm 2003167 | HRT vs. SP Parallel RCT Inclusion criteria:
|
32 adults Mean age 34.9 years, SD ≈12.5 years Age range NR 55% male 100% TS, 0% CTD % ADHD NR, % OCD NR 48% on medication YGTSS total tic scoreb 28.8, SD ≈7.5 |
HRT 14 sessions over 20 weeks Awareness training, self-monitoring, relaxation training, competing response training, contingency management and inconvenience review. Asked to practice at home Assessed at 20 weeks |
SP 14 sessions over 20 weeks Patients selected topic sessions and therapists were non-directive. Focus on experiencing, reflecting and expressing feelings about current life issues and problem solving Assessed at 20 weeks |
81% completed 91% analysed |
| Wilhelm 2012166 | CBIT vs. SP Parallel RCT Inclusion criteria:
|
122 adults Mean age 31.6 years, SD ≈13.8 years Age range 16–NR years 64% males 84% TS, 16% CTD 28% ADHD, 18% OCD 25% on medication YGTSS total tic scoreb 22.9, SD ≈6.6 |
CBIT Eight sessions (two 90 minutes, six 60 minutes) over 10 weeks Extension of HRT Psychoeducation, awareness training, competing response training, relaxation training, functional behavioural strategies to reduce influence of tic enhancing situations Assessed at 10 weeks |
SP Eight sessions (two 90 minutes, six 60 minutes) over 10 weeks Information about the course, genetics, underlying neurobiology and rationale for current treatments. Discussion of tics and related issues Assessed at 10 weeks |
86% completed 93% analysed |
| Videoconference CBIT vs. face-to-face CBIT | |||||
| Himle 2012172 | Videoconference CBIT vs. face-to-face CBIT parallel RCT Inclusion criteria:
|
20 children Mean age 11.6 years, SD 2.7 years Age range 8–17 years 94% male %TS NR, % CTD NR 28% ADHD, 22% OCD 28% on medication YGTSS total tic scoreb 23.7, SD 6.0 |
Videoconference CBIT 10 weekly sessions delivered via videoconference with therapist. Sessions conducted according to the CBIT manual Parents given CBIT parent workbook Assessed at 10 weeks |
Face-to-face CBIT 10 weekly sessions delivered face-to-face with therapist. Sessions conducted according to the CBIT manual Parents given CBIT parent workbook Assessed at 10 weeks |
82% completed 82% analysed |
| Negative practice vs. HRT | |||||
| Azrin 1980170 | Negative practice vs. HRT Parallel RCT Inclusion criteria:
|
22 adults and children Mean age 30.0 years, SD NR years Age range 11–62 years 77% male 9% TS, 91% CTD % ADHD NR, % OCD NR 18% on medication Tic score NR |
HRT One or two sessions of ≈2.5 hours and telephone contact Primary component: competing response training Review of inconveniences caused by tics, identification of people or situations associated with tics, awareness training, relaxation training. Methods to be practised and utilised at home Follow-up over next 18 months |
Negative practice One session of ≈2.5 hours and telephone contact Written instructions and discussion of rationale. Purposeful performance of tic in front of mirror saying ‘this is what I’m supposed not to do’. Methods to be practised and utilised at home Follow-up over next 18 months |
100% completed 100% analysed |
| ERP vs. HRT | |||||
| Verdellen 2004171 | ERP vs. HRT Parallel RCT Inclusion criteria:
|
25 childrend Mean age 11.9 years, SD 2.7 years Age range 7–17 years 80% male 100% TS, 0% CTD 48% on medication YGTSS total tic scoreb 19.6, SD 5.8 |
ERP 12 weekly 2-hour sessions Learned to suppress tics, practised tic suppression with optimised exposure (patients asked to concentrate on tics and bring with them tic-eliciting objects). Encouraged to practice at home Assessed at 12 weeks |
HRT 10 weekly 1-hour treatment sessions Awareness training and competing response training. Encouraged to practice at home Assessed at 10 weeks |
80% completed 100% analysed |
| Relaxation therapy vs. minimal therapy | |||||
| Bergin 1998173 | Relaxation training vs. minimal therapy Parallel RCT Inclusion criteria:
|
23 children Mean age 11.3 years, SD 3.0 years Age range 7–18 years 83% male 100% TS, 0% CTD 57% ADHD, 0% OCD 13% on medication YGTSS global scoreb 49.9, SD ≈12.9 (total tic score NR) |
Relaxation training Six weekly 1-hour sessions Awareness training, diaphragmatic breathing, behavioural relaxation training, applied relaxation techniques and electromyographic biofeedback. Practice and homework assignments of ≈25 minutes/day Assessed at 6 weeks |
Minimal therapy Six weekly 1-hour sessions Awareness training and quiet time. Practice and homework assignments of ≈25 minutes/day Assessed at 6 weeks |
70% completed 70% analysed |
| AC training vs. TAU | |||||
| Sukhodolsky 2009174 | AC training vs. TAU Parallel RCT Inclusion criteria:
|
26 children Mean age 12.7 years, SD ≈0.88 years Age range 11–16 years 92% male % TS NR, % CTD NR 69% ADHD, 35% OCD 69% on medication YGTSS total tic scoreb 17.5, SD ≈7.2 |
AC training 10 1-hour sessions over 10 weeks Arousal management (education about triggers, experience, expression and anger management), cognitive restructuring of hostile attributions and practising problem-solving skills, and behavioural practice for preventing or solving anger-provoking situations Continued to see treating clinician Assessed at 10 weeks |
TAU Continued to see treating clinician Assessed at 10 weeks |
100% completed 100% analysed |
| Parent training vs. TAU | |||||
| Scahill 2006164 | Parent training vs. TAU Parallel RCT Inclusion criteria:
|
24 children Mean age 8.9 years, SD 2.0 years Age range 6–12 years 75% male 75% TS, 25% CTD 42% ADHD, 17% OCD 83% on medication YGTSS total tic scoreb 18.2, SD 8.7 |
Parent training 10 sessions Core skills: providing positive reinforcement for appropriate behaviour, communicating directions effectively and being consistent with consequences for disruptive behaviour Techniques learnt: positive attending, selective ignoring, token economies and time-out Assignments to implement skills at home given after each session TAU continued |
TAU Commonly included patient education, clinical monitoring, medication management and school consultations as needed Could receive other community clinical services, e.g. child individual psychotherapy and school-based mental health services but were not to initiate new treatments, or stop existing treatments, during the course of the study |
96% completed 96% analysed |
| Study | Population | Study design | Treatment | Control | Attrition |
|---|---|---|---|---|---|
| DBS | |||||
| Ackermans 2011194 | Crossover RCT (no washout period) (Randomised treatment order but agreement with ethical committee that patients could switch order. 5/6 patients had DBS first) Inclusion criteria:
|
Six adults Mean age 40.33 years, SD NR years Age range 35–48 years 100% male 100% TS, 0% CTD 0% ADHD and OCD 83% on medication YGTSS total tic score 42.3, SD 3.1 |
DBS Frequency, pulse width and voltage adapted to obtain the best clinical effect on tic reduction. Monopolar or bipolar electrode active contacts chosen Constant over 3 months Outcome assessment after 3-month treatment |
Sham Constant over 3 months Outcome assessment after 3-month treatment |
86% completed 86% analysed |
| Welter 2008195 | Crossover RCT (no washout period) Inclusion criteria:
|
Three adults Mean age 32 years, SD 3.5 years Age range 30–36 years 33% male 100% TS, 0% CTD 0% ADHD and OCD 100% on medication YGTSS total tic score 33.7, SD 3.5 |
DBS Bilateral thalamic Bilateral pallidal Bilateral thalamic and pallidal Applied at level bellow that inducing adverse events during 24–48 hours, pulse width 60 microseconds, frequency 130 Hz Constant for 2 months Outcome assessment after 2-month treatment |
Sham Constant for 2 months Outcome assessment after 2-month treatment |
% completed NR % analysed NR |
| Maciunas 2007196 | Crossover RCT (no washout period) Inclusion criteria:
|
Five adults Mean age 28.2 years, SD NR Age range 18–34 years 100% male 100% TS, 0% CTD 60% ADHD, 80% OCD % on medication NR YGTSS total tic score 37.2, range 26–49 |
DBS with bilateral implants on right and left sides off–on on–off on–on Optimum set of parameters chosen for each side depending on tics and adverse events Constant for 1 week Outcome assessment after 1-week treatment |
Sham with bilateral implants off–off Constant for 1 week Outcome assessment after 1-week treatment |
100% completed 100% analysed |
| rTMS | |||||
| Munchau 2002199 | Crossover RCT (2 weeks’ washout) Inclusion criteria:
|
16 adults Mean age 38 years, SD 13.2 years Age range 18–NR years 75% male 100% TS, 0% CTD 0% ADHD, 44% OCD 68.75% on medication YGTSS global score 57.2, SD 20.5 |
rTMS left pre-motor motor Two 20-minute sessions on consecutive days. 1200 pulses, 1 Hz at 80% of active motor threshold Immediate outcome assessment following treatment |
Left motor sham Two 20-minute sessions on consecutive days Immediate outcome assessment following treatment |
% completed NR % analysed NR |
| Orth 2005197 | Crossover RCT (pseudorandom order) (4 weeks’ washout) Inclusion criteria:
|
Five adults Median age 29 years Age range 19–52 years 80% male 100% TS, 0% CTD 40% ADHD, 0% OCD 80% on medication YGTSS total tic score 23.1, SD ≈4 |
rTMS left pre-motor cortex followed by right pre-motor cortex left pre-motor cortex followed by sham stimulation of the right pre-motor cortex Two sessions on consecutive days. 1800 stimuli, 1 Hz, 80% active motor threshold Immediate outcome assessment following treatment |
Sham stimulation of left pre-motor cortex followed by sham stimulation of right pre-motor cortex Two sessions on consecutive days Immediate outcome assessment following treatment |
100% completed 100% analysed |
| Chae 2004198 | Crossover RCT (no washout) Inclusion criteria:
|
Eight adults Mean age 34.9 years, SD 16.4 years Age range 22–60 years 63% male 100% TS, 0% CTD 38% ADHD, 50% OCD 63% on medication YGTSS global score 70.2, SD 22.4 |
rTMS motor cortex low frequency [10 minutes 1 Hz, 600 stimuli per session (2400 per day)] motor cortex high frequency [15 Hz for 3 seconds and then lower frequency (overall 2400 per day)] pre-frontal cortex low frequency pre-frontal cortex high frequency For each intervention, four sessions in 1 day with 50-minute rest between sessions Interventions on five consecutive days with outcome assessment at end of each treatment day |
Sham four sessions in 1 day with 50-minute rest between sessions interventions on five consecutive days with outcome assessment at end of each treatment day |
100% completed 100% analysed |
| i.v. immunoglobulin vs. placebo | |||||
| Hoekstra 2004187 | Parallel RCT Inclusion criteria:
|
30 adults and children Mean age 29.8 years, SD NR years Age range 14–63 years 62% male 90% TS, 10% CTD % ADHD NR, 0% OCD 55% on medication YGTSS total tic score 25.0, SD 9.6 |
i.v. immunoglobulin 1 g/kg of immunoglobulin/day over 5–6 hours on two consecutive days Outcome assessment at 2, 6, 8, 12 and 14 weeks |
Albumin solution of same volume over 5–6 hours on two consecutive days Outcome assessment at 2, 6, 8, 12 and 14 weeks |
97% completed 97% analysed |
| Botulinum toxin | |||||
| Marras 2001200 | Crossover RCT (at least 8 weeks’ washout) (when botulinum given first, outcomes measured over 12 or more weeks and, when tic levels back down to baseline, placebo phase started) Inclusion criteria:
|
18 adults and children Median age 31.5 years Age range 15–55 years 72% male 78% TS, 22% CTD % ADHD and OCD NR 44% on medication YGTSS motor tic score median 9, range ≈5–14 |
Botulinum toxin Variable doses, similar to those used for dystonia Single injection Outcome assessment at 2 weeks |
i.v. saline Single injection Outcome assessment at 2 weeks |
90% completed 90% analysed |
| Acupuncture and Chinese herbs vs. haloperidol and artane | |||||
| Chi 2003201 | Parallel RCT Inclusion criteria:
|
60 children Mean age NR Age range 4–20 years 75% male 100% TS, 0% CTD % ADHD and OCD NR % on medication NR Tic score NR |
Acupuncture and Chinese herbs 20 sessions, once daily, 30-minute needle retaining Outcome assessment point NR |
Haloperidol and artane 1.5–8 mg/day Starting dose: 4-year-olds – 0.5 mg/day, 5-year-olds – 1 mg/2 × day Outcome assessment point NR |
% completed NR % analysed NR |
| Study | Study design | Population | Treatment | Control | Attrition |
|---|---|---|---|---|---|
| i.v. immunoglobulin and plasma exchange vs. placebo | |||||
| Perlmutter 1999204 | Parallel RCT Inclusion criteria:
|
29 children defined as having PANDAS Mean age 9.6 years, SD ≈2.5 years Age range 5–14 years % male NR % TS NR % ADHD NR, 86% OCD 62% on medication TS unified rating scale 13.2, SD ≈9.3 |
i.v. immunoglobulin: 1 g/kg of i.v. immunoglobulin on two consecutive days or Plasma exchange: one plasma volume (45 ml/kg) exchanged per procedure. 5–6 procedures over 10–12 days. Citrate anticoagulant. 80% of replacement fluid was 5% albumin, remaining was saline Outcome assessment at 1 month Follow-up of i.v. immunoglobulin and plasma exchange groups at 1 year |
i.v. saline (1 g/kg of i.v. immunoglobulin) on two consecutive days Outcome assessment at 1 month |
97% completed 97% analysed |
| Penicillin vs. placebo | |||||
| Garvey 1999205 | Crossover RCT (no washout between treatments) Inclusion criteria:
|
40 children defined as having PANDAS Mean age 9.6 years, SD 2.6 years Age range 52–15.9 years 73% male 97% tics, 73% OCD 51% ADHD % on medication NR YGTSS total tic score 15.4, SD 9.0 |
Penicillin prophylaxis 250 mg twice a day for 4 months. Dose adjustments if needed Outcome assessed at 4 months |
Placebo Twice a day for 4 months. Dose adjustments if needed Outcome assessed at 4 months |
88% completed 88% analysed |
| Penicillin vs. azithromycin | |||||
| Snider 2005206 | Parallel RCT Inclusion criteria:
|
24 children defined as having PANDAS Mean age 7.9 years, SD 1.3 years Age range 5–10 years 65% male % TS and CTD NR 26% ADHD, 69% OCD % on medication NR Tic score NR |
Penicillin prophylaxis vs. potassium 250 mg twice a day, once a week. Placebo capsules taken 2 × day on other days 12 months’ duration Outcome assessed at 12 months |
Azithromycin prophylaxis 250 mg 2 × day, once a week. Placebo capsules taken 2 × day on other days 12 months’ duration Outcome assessed at 12 months |
96% completed 96% analysed |
| Study | Study design | Setting | Subjects | Study aim | Outcomes/themes |
|---|---|---|---|---|---|
| Kompoliti 2006210 | Structured interview survey of consecutive patients | Tertiary referral centre Chicago, USA |
100 TS patients or their parents For adult and child patients Mean age 19.4 years, SD 14 years Mean age symptoms onset 7.3 years, SD 5.4 years Median YGTSS rating 4, range 0–10 42% ADHD 37% OCD 11% affective disorders 7% learning difficulties 4% pervasive developmental disorders 2% psychosis 55% previous antipsychotic drug use |
To assess awareness and concern about the side effects of antipsychotic drugs | Side effect awareness Side effects experienced Side effects concerna |
| Mol Debes 2008209 | Structured interview survey | University Hospital TS clinic Glostrup, Denmark |
314 children with TS Mean age 12.41 years, range 5.3–20 years Median age of symptom onset 5.5 years 30.9% motor tics 9.2% vocal tics 19.1% ADHD 8.3% OCB 5.1% rage attacks 4.8% sleep disturbance 2.9% stuttering 19.7% other behavioural problems |
To examine the TS diagnostic process | Person who first noticed symptoms Professionals consulted before diagnosisa Professional who made diagnosisa |
| Rivera-Navarro 2009208 | Qualitative focus groups | Neutral settings Madrid, Spain |
29 doctors, adult and adolescent TS patients and their relatives Aged > 11 years No other concomitant disease Multiple motor tics as well as one or more vocal tic |
To explore experiences of the communication and impact of TS diagnosis | Difficulties in diagnosisa Impact of diagnosis Communication of diagnosisa |
| Woods 2010211 | Structured internet survey | National internet survey USA |
465 adults with TS and 487 parents of children with TS Child sample Mean age 10.6 years, SD 2.9 years Mean parent tic questionnaire score 39.2, SD 23.2 97% formal TS diagnosis 50.9% comorbid psychiatric disorder |
To describe treatment utilisation patterns | Proportions of patients undergoing behavioural, medical and complementary interventions Barriers to carea Factors inhibiting use of behavioural therapya |
Appendix 4 Grading of Recommendations Assessment, Development and Evaluation profile tables
Grading of Recommendations Assessment, Development and Evaluation assessment of efficacy was conducted on child studies where available and mixed/adult studies where child studies were not available. GRADE assessments of safety outcomes were conducted on all studies.
Pharmacological interventions
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Haloperidol | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 6 weeks; measured with: TS global scale and Shapiro TSSS; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.50 lower (1.06 lower to 0.06 higher) | ⊕⊕OO LOW | Critical |
| Motor tic score (follow-up mean 6 weeks; measured with: TS global scale motor tic score; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.63 lower (1.04 to 0.22 lower) | ⊕⊕OO LOW | Critical |
| Vocal tic score (follow-up mean 6 weeks; measured with: TS global scale vocal tic score; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.24 lower (0.62 lower to 0.14 higher) | ⊕⊕OO LOW | Critical |
| Global tic score (follow-up mean 6 weeks; measured with: TS global scale global score; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.37 lower (0.75 lower to 0.02 higher) | ⊕⊕OO LOW | Critical |
| Impairment (follow-up mean 6 weeks; measured with: C-GAS; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.49 lower (0.88 lower to 0.09 higher) | ⊕⊕OO LOW | Important |
| Overall clinical impression (follow-up mean 6 weeks; measured with: CGI scale; range of scores: 0–7; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 1.23 lower (1.73 to 0.74 lower) | ⊕⊕OO LOW | Important |
| EPS (follow-up mean 6 weeks; measured with: ESRS; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 6d | 5 | – | SMD 0.51 higher (0.06 lower to 1.07 higher) | ⊕⊕OO LOW | Critical |
| AIMS (follow-up mean 6 weeks; measured with: abnormal involuntary movements; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.11 higher (0.27 lower to 0.48 higher) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Pimozide | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 6 weeks; measured with: TS global scale and Shapiro TSSS; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.81 lower (1.24 to 0.38 lower) | ⊕⊕OO LOW | Critical |
| Motor tic score (follow-up mean 6 weeks; measured with: TS global scale motor tic score; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.75 lower (1.17 to 0.32 lower) | ⊕⊕OO LOW | Critical |
| Vocal tic score (follow-up mean 6 weeks; measured with: TS global scale vocal tic score; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.66 lower (1.07 to 0.25 lower) | ⊕⊕OO LOW | Critical |
| Global tic score (follow-up mean 6 weeks; measured with: TS global scale global score; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.65 lower (1.06 to 0.23 lower) | ⊕⊕OO LOW | Critical |
| Impairment (follow-up mean 6 weeks; measured with: C-GAS; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.64 lower (1.05 to 0.23 lower) | ⊕⊕OO LOW | Important |
| Overall clinical impression (follow-up mean 6 weeks; measured with: CGI scale; range of scores: 0–7; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 1.23 lower (1.73 to 0.74 lower) | ⊕⊕OO LOW | Important |
| EPS (follow-up mean 6 weeks; measured with: ESRS; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 6d | 5 | – | SMD 0.2 higher (0.33 lower to 0.73 higher) | ⊕⊕OO LOW | Critical |
| AIMS (follow-up mean 6 weeks; measured with: Abnormal Involuntary Movements; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.22 higher (0.16 lower to 0.6 higher) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Risperidone | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 8 weeks; measured with: YGTSS total tic score; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 12 | 14 | – | SMD 1.10 lower (1.94 to 0.26 lower) | ⊕⊕OO LOW | Critical |
| Overall clinical impression (follow-up mean 8 weeks; assessed with: CGI scale) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 9/12 (75%) | 1/14 (7.1%) | RR 10.50 (1.54 to 71.38) | 679 more per 1000 (from 39 more to 1000 more) | ⊕⊕OO LOW | Important |
| Fatigue (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 2b | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa,c | None | 19/39 (48.7%) | 5/41 (12.2%) | RR 3.72 (1.57 to 8.85) | 332 more per 1000 (from 70 more to 957 more) | ⊕⊕OO LOW | Important |
| Somnolence/sedation (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 2b | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousc | None | 11/39 (28.2%) | 2/41 (4.9%) | RR 5.38 (1.24 to 23.3) | 214 more per 1000 (from 12 more to 1000 more) | ⊕⊕OO LOW | Important |
| Headache (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 2b | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousc | None | 4/39 (10.3%) | 12/41 (29.3%) | RR 0.40 (0.15 to 1.04) | 176 fewer per 1000 (from 249 fewer to 12 more) | ⊕⊕OO LOW | Important |
| Increased appetite/weight gain (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 2b | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousc | None | 12/39 (30.8%) | 1/41 (2.4%) | RR 7.68 (1.46 to 40.43) | 163 more per 1000 (from 11 more to 962 more) | ⊕⊕OO LOW | Important |
| Dizziness (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1b | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousd | None | 9/23 (39.1%) | 6/23 (26.1%) | RR 1.50 (0.64 to 3.53) | 130 more per 1000 (from 94 fewer to 660 more) | ⊕⊕OO LOW | Important |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Ziprasidone | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 8 weeks; measured with: YGTSS total tic score; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 16 | 11 | – | SMD 1.10 lower (1.93 to 0.27 lower) | ⊕⊕OO LOW | Critical |
| Global tic score (follow-up mean 8 weeks; measured with: YGTSS global score; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 16 | 11 | – | SMD 1.02 lower (1.84 to 20 lower) | ⊕⊕OO LOW | Critical |
| CGI scale (follow-up mean 8 weeks; measured with: CGI-Severity; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 16 | 11 | – | SMD 0.71 lower (1.5 lower to 0.09 higher) | ⊕⊕OO LOW | Important |
| Adverse events (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 16/16 (100%) | 7/11 (63.6%) | RR 1.55 (0.99 to 2.43) | 350 more per 1000 (from 6 fewer to 910 more) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Risperidone | Pimozide | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 4 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 13c | – | – | SMD 0.54 lower (1.32 lower to 0.24 higher) | ⊕OOO VERY LOW | Critical |
| Impairment (follow-up mean 4 weeks; measured with: YGTSS impairment scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 13c | – | – | SMD 0.77 lower (1.57 lower to 0.02 higher) | ⊕OOO VERY LOW | Important |
| CGI scale (follow-up mean 4 weeks; measured with: CGI-I; range of scores: 1–7; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 13c | – | – | SMD 0.63 lower (1.42 lower to 0.16 higher) | ⊕OOO VERY LOW | Important |
| Weight gain (follow-up mean 4 weeks; measured with: investigator measurement; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 13c | – | – | Mean 0.7 higher (0.18 lower to 1.73 higher) | ⊕OOO VERY LOW | Important |
| EPS (follow-up mean 4 weeks; measured with: ESRS; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 13c | – | – | SMD 0.05 lower (0.77 lower to 0.66 higher) | ⊕OOO VERY LOW | Critical |
| Adverse events (follow-up mean 4 weeks; measured with: Side Effects Checklist; range of scores: ---; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 13c | – | – | SMD 0.12 lower (0.84 lower to 0.59 higher) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Haloperidol | Pimozide | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 6 weeks; measured with: TS global scale tic score; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.25 higher (0.13 lower to 0.63 higher) | ⊕⊕OO LOW | Critical |
| Motor tic score (follow-up mean 6 weeks; measured with: TS global scale motor tic score; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.04 higher (0.33 lower to 0.42 higher) | ⊕⊕OO LOW | Critical |
| Vocal tic score (follow-up mean 6 weeks; measured with: TS global scale vocal tic score; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.38 higher (0.01 lower to 0.76 higher) | ⊕⊕OO LOW | Critical |
| Impairment (follow-up mean 6 weeks; measured with: C-GAS; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.14 higher (0.24 lower to 0.52 higher) | ⊕⊕OO LOW | Important |
| Overall clinical impression (follow-up mean 6 weeks; measured with: C-GAS; range of scores: 0–100; better indicated by higher values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.14 lower (0.52 lower to 0.24 higher) | ⊕⊕OO LOW | Critical |
| CGI scale (follow-up mean 6 weeks; measured with: CGI-Severity; range of scores: 1–7; better indicated by higher values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0 higher (0.37 lower to 0.37 higher) | ⊕⊕OO LOW | Important |
| EPSs (follow-up mean 6 weeks; measured with: ESRS; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 6d | 5 | – | SMD 0.40 higher (0.15 lower to 0.94 higher) | ⊕⊕OO LOW | Critical |
| Abnormal involuntary movements (follow-up mean 6 weeks; measured with: Abnormal Involuntary Movements scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 22c | – | – | SMD 0.09 lower (0.47 lower to 0.28 higher) | ⊕⊕OO LOW | Critical |
| Treatment-limiting side effects (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 9/22 (40.9%) | 3/22 (13.6%) | RR 3.0 (0.94 to 9.62) | 273 more per 1000 (from 8 fewer to 1000 more) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Aripiprazole | Antipsychotic | Relative (95% CI) | Absolute | ||
| Global/total tic score (follow-up 8–12 weeks; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousa | Seriousb | No serious indirectness | Seriousc | None | 129 | 114 | – | SMD 0.17 higher (0.41 lower to 0.75 higher) | ⊕OOO VERY LOW | Critical |
| Impairment score (follow-up mean 12 weeks; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousd | No serious inconsistency | No serious indirectness | Seriouse | None | 98 | 97 | – | SMD 0.04 lower (0.32 lower to 0.24 higher) | ⊕⊕OO LOW | Critical |
| Clinically much or very much improved (follow-up 8–12 weeks) | ||||||||||||
| 2 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousc | None | 80/129 (62%) | 67/114 (58.8%) | RR 1.05 (0.85 to 1.29) | 29 more per 1000 (from 88 fewer to 170 more) | ⊕⊕OO LOW | Critical |
| Adverse events – hypersomnia (follow-up 8–12 weeks) | ||||||||||||
| 2 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousc | None | 23/129 (17.8%) | 19/114 (16.7%) | RR 0.73 (0.51 to 1.04) | 45 fewer per 1000 (from 82 fewer to 7 more) | ⊕⊕OO LOW | Critical |
| Adverse events – EPS (follow-up mean 8 weeks) | ||||||||||||
| 1 | Randomised trials | Very seriousf | No serious inconsistency | No serious indirectness | Seriousg | None | 6/31 (19.4%) | 7/17 (41.2%) | RR 0.47 (0.19 to 1.17) | 218 fewer per 1000 (from 334 fewer to 70 more) | ⊕OOO VERY LOW | Critical |
| Adverse events – headache (follow-up 8–12 weeks) | ||||||||||||
| 2 | Randomised trials | Seriousa | Serioush | No serious indirectness | Seriousc | None | 7/129 (5.4%) | 12/114 (10.5%) | RR 0.39 (0.13 to 1.21) | 64 fewer per 1000 (from 92 fewer to 22 more) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Olanzapine | Haloperidol | Relative (95% CI) | Absolute | ||
| Tics (follow-up mean 4 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 25 | 29 | – | SMD 0.37 lower (0.91 lower to 0.17 higher) | ⊕⊕OO LOW | Critical |
| Overall clinical outcome (follow-up mean 4 weeks; measured with: CGI-Severity; range of scores: 0–7; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 25 | 29 | – | SMD 1.39 lower (1.99 to 0.79 lower) | ⊕⊕OO LOW | Important |
| Side effects (follow-up mean 4 weeks; measured with: STESS side effects scale; range of scores: 0–7; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 25 | 29 | – | SMD 0.57 lower (1.12 to 0.03 lower) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Antipsychotics | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 6–8 weeks; measured with: YGTSS total tic scale and TS global scale tic score; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 3 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 39c | 36 | – | SMD 0.74 lower (1.08 to 0.41 lower) | ⊕⊕OO LOW | Critical |
| Global tic score (follow-up mean 6–8 weeks; measured with: YGTSS global and TS global scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousd | None | 26c | 23 | – | SMD 0.51 lower (0.86 to 0.15 lower) | ⊕⊕OO LOW | Critical |
| CGI scale (follow-up mean 6–8 weeks; measured with: CGI-Severity; range of scores: 1–7; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousd | None | 26c | 23 | – | SMD 1.01 lower (1.49 to 0.53 lower) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Clonidine | Placebo | Relative (95% CI) | Absolute | ||
| Motor tic score (follow-up mean 12–16 weeks; measured with: TSGS motor tic score, YGTSS motor tic score and motor tic severity score; better indicated by lower values) | ||||||||||||
| 3 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 67 | 63 | – | SMD 0.55 lower (0.9 to 0.2 lower) | ⊕⊕⊕O MODERATE | Critical |
| Vocal tic score (follow-up mean 12–16 weeks; measured with: TSGS vocal tic score, YGTSS vocal tic score and vocal tic severity score; better indicated by lower values) | ||||||||||||
| 3 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 67 | 63 | – | SMD 0.30 lower (0.62 lower to 0.01 higher) | ⊕⊕⊕O MODERATE | Critical |
| Impairment score (follow-up mean 12–16 weeks; measured with: TSGS school and occupational function and YGTSS impairment score; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousb | None | 55 | 51 | – | SMD 0.54 lower (0.93 to 0.16 lower) | ⊕⊕⊕O MODERATE | Important |
| Global tic score (follow-up mean 12–16 weeks; measured with: TSGS total score YGTSS global score; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousb | None | 55 | 51 | – | SMD 0.71 lower (1.1 to 0.31 lower) | ⊕⊕⊕O MODERATE | Important |
| CGI proportion improved (follow-up mean 16 weeks; assessed with: CGI-I) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousc | None | 23/34 (67.6%) | 9/32 (28.1%) | RR 2.41 (1.32 to 4.39) | 397 more per 1000 (from 90 more to 953 more) | ⊕⊕⊕O MODERATE | Important |
| CGI-I scale (follow-up mean 12 weeks; measured with: CGI-Severity; range of scores: 1–7; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousd | None | 21 | 19 | – | SMD 1.04 lower (1.71 to 0.38 lower) | ⊕⊕⊕O MODERATE | Important |
| Proportion with adverse events (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriouse | None | 28/34 (82.4%) | 15/34 (44.1%) | RR 1.87 (1.24 to 2.81) | 384 more per 1000 (from 106 more to 799 more) | ⊕⊕⊕O MODERATE | Critical |
| Sedation/fatigue (follow-up mean 12–16 weeks; assessed with: self-reported) | ||||||||||||
| 2 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousb | None | 19/55 (34.5%) | 7/51 (13.7%) | RR 2.46 (1.34 to 4.5) | 200 more per 1000 (from 47 more to 480 more) | ⊕⊕⊕O MODERATE | Critical |
| Dry mouth (follow-up mean 12 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousd | None | 12/21 (57.1%) | 5/19 (26.3%) | RR 2.17 (0.94 to 5.02) | 308 more per 1000 (from 16 fewer to 1000 more) | ⊕⊕⊕O MODERATE | Critical |
| Faintness/dizziness (follow-up mean 12 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousd | None | 9/21 (42.9%) | 4/19 (21.1%) | RR 2.04 (0.75 to 5.54) | 219 more per 1000 (from 53 fewer to 956 more) | ⊕⊕⊕O MODERATE | Critical |
| Irritability (follow-up mean 12 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousd | None | 7/21 (33.3%) | 1/19 (5.3%) | RR 6.33 (0.86 to 46.86) | 281 more per 1000 (from 7 fewer to 1000 more) | ⊕⊕⊕O MODERATE | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Guanfacine | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 4–8 weeks; measured with: YGTSS total tic score; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 29 | 29 | – | SMD 0.73 lower (1.26 to 0.2 lower) | ⊕⊕⊕O MODERATE | Critical |
| Motor tic score (follow-up mean 4 weeks; measured with: YGTSS motor tic score; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousb | None | 12 | 12 | – | SMD 0.64 lower (1.46 lower to 0.18 higher) | ⊕⊕⊕O MODERATE | Critical |
| Vocal tic score (follow-up mean 4 weeks; measured with: YGTSS vocal tic score; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousb | None | 12 | 12 | – | SMD 0.70 lower (1.53 lower to 0.12 higher) | ⊕⊕⊕O MODERATE | Critical |
| Impairment (follow-up mean 4 weeks; measured with: YGTSS impairment score; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousb | None | 12 | 12 | – | SMD 0.27 higher (0.53 lower to 1.08 higher) | ⊕⊕⊕O MODERATE | Important |
| Global tic score (follow-up mean 4 weeks; measured with: YGTSS global score; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousb | None | 12 | 12 | – | SMD 0.29 lower (1.1 lower to 0.51 higher) | ⊕⊕⊕O MODERATE | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Clonidine patch | Placebo | Relative (95% CI) | Absolute | ||
| Total tics (follow-up mean 4 weeks; measured with: YGTSS total tic score; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecisionb | None | 326 | 111 | – | SMD 0.10 lower (0.32 lower to 0.12 higher) | ⊕⊕⊕O MODERATE | Critical |
| Dry mouth (follow-up mean 4 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Very seriousc | No serious inconsistency | No serious indirectness | Seriousd | None | 5/58 (8.6%) | 1/18 (5.6%) | RR 1.55 (0.19 to 12.43) | 31 more per 1000 (from 45 fewer to 635 more) | ⊕OOO VERY LOW | Critical |
| Itchy skin (follow-up mean 4 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Very seriousc | No serious inconsistency | No serious indirectness | Seriousd | None | 5/58 (8.6%) | 2/18 (11.1%) | RR 0.78 (0.16 to 3.66) | 24 fewer per 1000 (from 93 fewer to 296 more) | ⊕OOO VERY LOW | Critical |
| Dizziness (follow-up mean 4 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Very seriousc | No serious inconsistency | No serious indirectness | Seriousd | None | 4/58 (6.9%) | 1/18 (5.6%) | RR 1.24 (0.15 to 10.41) | 13 more per 1000 (from 47 fewer to 523 more) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Noradrenergic agents | Placebo | Relative (95% CI) | Absolute | ||
| Total tics/global tic score (follow-up mean 4–16 weeks; measured with: YGTSS global score, TS global score or YGTSS total tic score; better indicated by lower values) | ||||||||||||
| 4 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 84 | 80 | – | SMD 0.72 lower (1.03 to 0.4 lower) | ⊕⊕⊕O MODERATE | Critical |
| Impairment (follow-up mean 4–16 weeks; measured with: YGTSS impairment score; better indicated by lower values) | ||||||||||||
| 3 | Randomised trials | No serious risk of bias | Seriousb | No serious indirectness | Seriousc | none | 67 | 63 | – | SMD 0.32 lower (0.84 lower to 0.2 higher) | ⊕⊕OO LOW | Important |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Fluoxetine | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 2–4 months; measured with: YGTSS total tic scale and Goetz simple motor tic scale; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 12c | 13 | – | SMD 0.41 lower (1.23 lower to 0.42 higher) | ⊕⊕OO LOW | Critical |
| Motor restlessnessd (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriouse | No serious inconsistency | No serious indirectness | Seriousf | None | 7/12 (58.3%) | 2/12 (16.7%) | RR 3.50 (0.91 to 13.53) | 417 more per 1000 (from 15 fewer to 1000 more) | ⊕⊕OO LOW | Critical |
| Insomniad (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriouse | No serious inconsistency | No serious indirectness | Seriousf | None | 5/12 (41.7%) | 2/12 (16.7%) | RR 2.50 (0.6 to 10.46) | 250 more per 1000 (from 67 fewer to 1000 more) | ⊕⊕OO LOW | Critical |
| Decreased appetited (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriouse | No serious inconsistency | No serious indirectness | Seriousf | None | 3/12 (25%) | 0/12 (0%) | RR 7.00 (0.4 to 122.44) | – | ⊕⊕OO LOW | Critical |
| Diarrhoead (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriouse | No serious inconsistency | No serious indirectness | Seriousf | None | 3/12 (25%) | 1/12 (8.3%) | RR 3.00 (0.36 to 24.92) | 167 more per 1000 (from 53 fewer to 1000 more) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Desipramine | Placebo | Relative (95% CI) | Absolute | ||
| ≥ 30% reduction in total tic score (follow-up mean 6 weeks; assessed with: YGTSS total tic scale) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 11/19 (57.9%) | 2/20 (10%) | RR 5.79 (1.47 to 22.77) | 479 more per 1000 (from 47 more to 1000 more) | ⊕⊕OO LOW | Critical |
| ≥ 30% reduction in motor tic score (follow-up mean 6 weeks; assessed with: YGTSS motor tic scale) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 10/19 (52.6%) | 4/20 (20%) | RR 2.63 (0.99 to 6.98) | 326 more per 1000 (from 2 fewer to 1000 more) | ⊕⊕OO LOW | Critical |
| ≥ 30% reduction in vocal tic score (follow-up mean 6 weeks; assessed with: YGTSS vocal tic scale) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 11/19 (57.9%) | 2/20 (10%) | RR 5.79 (1.47 to 22.77) | 479 more per 1000 (from 47 more to 1000 more) | ⊕⊕OO LOW | Critical |
| Global tic score (follow-up mean 6 weeks; measured with: YGTSS global scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 19 | 20 | – | MD 0.96 lower (1.63 to 0.29 lower) | ⊕⊕OO LOW | Critical |
| ≥ 30% reduction in global tic score (follow-up mean 6 weeks; assessed with: YGTSS global scale) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 12/19 (63.2%) | 1/20 (5%), 0% | RR 12.63 (1.81 to 87.98) | 581 more per 1000 (from 40 more to 1000 more) | ⊕⊕OO LOW | Critical |
| Much or very much improved (follow-up mean 6 weeks; assessed with: CGI-I) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 11/19 (57.9%) | 1/20 (5%) | RR 11.58 (1.65 to 81.26) | 529 more per 1000 (from 32 more to 1000 more) | ⊕⊕OO LOW | Important |
| Proportion of children with adverse events (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Very seriousb | No serious inconsistency | No serious indirectness | Seriousc | None | 26/34 (76.5%) | 15/34 (44.1%) | RR 1.73 (1.14 to 2.64) | 322 more per 1000 (from 62 more to 724 more) | ⊕OOO VERY LOW | |
| Decreased appetite (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 5/19 (26.3%) | 0/20 (0%) | RR 11.55 (0.68 to 195.63) | – | ⊕⊕OO LOW | Critical |
| Difficulty sleeping (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 4/19 (21.1%) | 1/20 (5%) | RR 4.21 (0.52 to 34.36) | 161 more per 1000 (from 24 fewer to 1000 more) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Metoclopramide | Placebo | Relative (95% CI) | Absolute | ||
| Total tics (follow-up mean 8 weeks; measured with: YGTSS total tic score; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 14 | 13 | – | SMD 1.43 lower (2.28 to 0.59 lower) | ⊕⊕OO LOW | Critical |
| Motor tics (follow-up mean 8 weeks; measured with: YGTSS motor tic score; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 14 | 13 | – | SMD 1.44 lower (2.28 to 0.59 lower) | ⊕⊕OO LOW | Critical |
| Vocal tics (follow-up mean 8 weeks; measured with: YGTSS vocal tic score; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 14 | 13 | – | SMD 0.79 lower (1.58 to 0.01 lower) | ⊕⊕OO LOW | Critical |
| CGI scale (follow-up mean 8 weeks; measured with: CGI-Severity score; range of scores: 1–7; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 14 | 13 | – | SMD 1.07 lower (1.88 to 0.27 lower) | ⊕⊕OO LOW | Important |
| Sedation (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 3/14 (21.4%) | 1/13 (7.7%) | RR 2.79 (0.33 to 23.52) | 138 more per 1000 (from 52 fewer to 1000 more) | ⊕⊕OO LOW | Critical |
| Dysphoria (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 1/14 (7.1%) | 1/13 (7.7%) | RR 0.93 (0.06 to 13.37) | 5 fewer per 1000 (from 72 fewer to 952 more) | ⊕⊕OO LOW | Critical |
| Increased appetite (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 3/14 (21.4%) | 1/13 (7.7%) | RR 2.79 (0.33 to 23.52) | 138 more per 1000 (from 52 fewer to 1000 more) | ⊕⊕OO LOW | Critical |
| Weight gain (follow-up mean 8 weeks; measured with: investigator assessed; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 14 | 13 | – | MD 0.5 higher (0.75 lower to 1.75 higher) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Ondansetron | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 3 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9 | 6 | – | SMD 0.38 lower (1.42 lower to 0.66 higher) | ⊕OOO VERY LOW | Critical |
| Motor tic score (follow-up mean 3 weeks; measured with: YGTSS motor tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9 | 6 | – | SMD 0.14 lower (1.18 lower to 0.89 higher) | ⊕OOO VERY LOW | Critical |
| Vocal tic score (follow-up mean 3 weeks; measured with: YGTSS vocal tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9 | 6 | – | SMD 0.69 lower (1.76 lower to 0.37 higher) | ⊕OOO VERY LOW | Critical |
| CGI scale (follow-up mean 3 weeks; measured with: CGI-Severity; range of scores: 1–7; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9 | 6 | – | SMD 0.50 lower (1.55 lower to 0.55 higher) | ⊕OOO VERY LOW | Important |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Levetiracetam | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 4 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 11 | 9 | – | SMD 0.23 lower (0.85 lower to 0.39 higher) | ⊕⊕OO LOW | Critical |
| Global tic score (follow-up mean 4 weeks; measured with: YGTSS global scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 11 | 9 | – | SMD 0.09 higher (0.53 lower to 0.7 higher) | ⊕⊕OO LOW | Critical |
| Anxiety (follow-up mean 4 weeks; measured with: MASC; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 11 | 9 | – | SMD 0.04 lower (0.66 lower to 0.58 higher) | ⊕⊕OO LOW | Important |
| Depression (follow-up mean 4 weeks; measured with: CDI-S; range of scores: 1–10; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 11 | 9 | – | SMD 0.20 higher (0.43 lower to 0.82 higher) | ⊕⊕OO LOW | Important |
| CGI scale (follow-up mean 4 weeks; measured with: CGI-Severity; range of scores: 1–7; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 11 | 9 | – | SMD 0.14 higher (0.49 lower to 0.76 higher) | ⊕⊕OO LOW | Important |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Topiramate | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 6 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 14 | 13 | – | SMD 0.88 lower (1.68 to 0.08 lower) | ⊕⊕OO LOW | Critical |
| Motor tic score (follow-up mean 6 weeks; measured with: YGTSS motor tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 14 | 13 | – | SMD 0.86 lower (1.65 to 0.06 lower) | ⊕⊕OO LOW | Critical |
| Vocal tic score (follow-up mean 6 weeks; measured with: YGTSS vocal tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 14 | 13 | – | SMD 0.64 lower (1.41 lower to 0.14 higher) | ⊕⊕OO LOW | Critical |
| Global tic score (follow-up mean 6 weeks; measured with: YGTSS global scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 14 | 13 | – | SMD 1.19 lower (2.02 to 0.36 lower) | ⊕⊕OO LOW | Critical |
| Adverse events (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousb | None | 11/15 (73.3%) | 13/14 (92.9%) | RR 0.79 (0.56 to 1.11) | 195 fewer per 1000 (from 409 fewer to 102 more) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Methylphenidate | Placebo | Relative (95% CI) | Absolute | ||
| Motor tic score (follow-up mean 2–16 weeks; measured with: YGTSS and global tic rating scale; better indicated by lower values) | ||||||||||||
| 3 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 79c | 72 | – | SMD 0.03 lower (0.2 lower to 0.15 higher) | ⊕⊕OO LOW | Critical |
| Vocal tic score (follow-up mean 2–16 weeks; measured with: global tic rating and YGTSS scale; better indicated by lower values) | ||||||||||||
| 3 | Randomised trials | Seriousa | Seriousd | No serious indirectness | Seriousb | None | 79c | 72 | – | SMD 0.28 lower (0.73 lower to 0.16 higher) | ⊕OOO VERY LOW | Critical |
| Impairment (follow-up mean 2–16 weeks; measured with: YGTSS scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousa | Seriouse | No serious indirectness | Seriousf | None | 73c | 67 | – | SMD 0.29 lower (0.89 lower to 0.3 higher) | ⊕OOO VERY LOW | Important |
| Global tic score (follow-up mean 2–16 weeks; measured with: YGTSS scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousa | Seriousg | No serious indirectness | Seriousf | None | 73c | 67 | – | SMD 0.32 lower (0.85 lower to 0.22 higher) | ⊕OOO VERY LOW | Critical |
| Mood (follow-up mean 2 weeks; measured with: SSEC mood index;h better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousi | No serious inconsistency | No serious indirectness | Seriousj | None | 71k | – | – | SMD 0.27 lower (0.48 to 0.05 lower) | ⊕OOO VERY LOW | Important |
| Much or very much improved: parent rated (follow-up mean 16 weeks; assessed with: CGI-I) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousl | None | 20/37 (54.1%) | 9/32 (28.1%) | RR 1.92 (1.03 to 3.6) | 259 more per 1000 (from 8 more to 731 more) | ⊕⊕⊕O MODERATE | Important |
| Much or very much improved: teacher rated (follow-up mean 16 weeks; assessed with: CGI-I) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousl | None | 24/37 (64.9%) | 11/32 (34.4%) | RR 0 (0 to 3.22) | 344 fewer per 1000 (from 344 fewer to 763 more) | ⊕⊕⊕O MODERATE | Important |
| Much or very much improved: investigator rated (follow-up mean 16 weeks; assessed with: CGI-I) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousl | None | 16/37 (43.2%) | 9/32 (28.1%) | RR 1.54 (0.79 to 2.99) | 152 more per 1000 (from 59 fewer to 560 more) | ⊕⊕⊕O MODERATE | Important |
| Moderate or severe sedation (follow-up mean 16 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousl | None | 16/37 (43.2%) | 9/32 (28.1%) | RR 1.30 (0.23 to 7.28) | 84 more per 1000 (from 217 fewer to 1000 more) | ⊕⊕⊕O MODERATE | Critical |
| Side effects: teacher rated (follow-up mean 2 weeks; measured with: SSEC Somatic index; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousi | No serious inconsistency | No serious indirectness | Seriousj | None | 71k | – | – | SMD 0.09 higher (0.12 lower to 0.3 higher) | ⊕OOO VERY LOW | Critical |
| Side effects: parent rated (follow-up mean 2 weeks; measured with: SSEC Somatic index; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousi | No serious inconsistency | No serious indirectness | Seriousj | None | 71k | – | – | SMD 0.90 higher (0.3 to 1.5 higher) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Dexmethylphenidate | Placebo | Relative (95% CI) | Absolute | ||
| Motor tic score (follow-up mean 1 days; measured with: YGTSS motor tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 10c | – | – | SMD 0.60 lower (1.13 to 0.07 lower) | ⊕OOO VERY LOW | Critical |
| Vocal tic score (follow-up mean 1 days; measured with: YGTSS vocal tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 10c | – | – | SMD 0.62 lower (1.15 to 0.09 lower) | ⊕OOO VERY LOW | Critical |
| Impairment (follow-up mean 1 days; measured with: YGTSS impairment scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 10c | – | – | SMD 0.41 lower (1.3 lower to 0.47 higher) | ⊕OOO VERY LOW | Important |
| Global tic score (follow-up mean 1 days; measured with: YGTSS global scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 10c | – | – | SMD 0.66 lower (1.27 lower to 0.04 higher) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Stimulants | Placebo | Relative (95% CI) | Absolute | ||
| Motor tic score (follow-up mean 0.1–16 weeks; measured with: YGTSS and global tic rating scale; better indicated by lower values) | ||||||||||||
| 4 | Randomised trials | Seriousa | Seriousb | No serious indirectness | Seriousc | None | 84d | 77 | – | SMD 0.17 lower (0.46 lower to 0.11 higher) | ⊕OOO VERY LOW | Critical |
| Vocal tic score (follow-up mean 0.1–16 weeks; measured with: YGTSS and global tic rating scale; better indicated by lower values) | ||||||||||||
| 4 | Randomised trials | Seriousa | Seriouse | No serious indirectness | Seriousc | None | 84e | 77 | – | SMD 0.36 lower (0.76 lower to 0.03 higher) | ⊕OOO VERY LOW | Critical |
| Impairment (follow-up mean 0.1–16 weeks; measured with: YGTSS and global tic rating scale; better indicated by lower values) | ||||||||||||
| 3 | Randomised trials | Seriousa | Seriousf | No serious indirectness | Seriousg | None | 78d | 72 | – | SMD 0.30 lower (0.76 lower to 0.15 higher) | ⊕OOO VERY LOW | Important |
| Global tic score (follow-up mean 0.1–16 weeks; measured with: YGTSS and global tic rating scale; better indicated by lower values) | ||||||||||||
| 3 | Randomised trials | Seriousa | Serioush | No serious indirectness | Seriousg | None | 78d | 72 | – | SMD 0.40 lower (0.84 lower to 0.04 higher) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Pergolide | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 8 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 36 | 15 | – | SMD 0.59 lower (1.21 lower to 0.02 higher) | ⊕⊕OO LOW | Critical |
| Global tic score (follow-up mean 6–8 weeks; measured with: YGTSS global tic scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousb | No serious inconsistency | No serious indirectness | Seriousc | None | 46d | 24 | – | SMD 0.87 lower (1.39 to 0.34 lower) | ⊕⊕OO LOW | Critical |
| Impairment (follow-up mean 8 weeks; measured with: YGTSS impairment scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 36 | 15 | – | SMD 0.53 lower (1.14 lower to 0.08 higher) | ⊕⊕OO LOW | Important |
| CGI scale (follow-up mean 6–8 weeks; measured with: CGI-Severity; range of scores: 1–7; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousb | No serious inconsistency | No serious indirectness | Seriousc | None | 46d | 24 | – | SMD 0.28 lower (0.78 lower to 0.23 higher) | ⊕⊕OO LOW | Important |
| Adverse events (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriouse | No serious inconsistency | No serious indirectness | Seriousf | None | 7/19 (36.8%) | 8/19 (42.1%) | RR 0.88 (0.4 to 1.93) | 51 fewer per 1000 (from 253 fewer to 392 more) | ⊕⊕OO LOW | Critical |
| Side effects (follow-up mean 6–8 weeks; measured with: STESS; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousb | No serious inconsistency | No serious indirectness | Seriousc | None | 46d | 24 | – | SMD 0.05 lower (0.49 lower to 0.38 higher) | ⊕⊕OO LOW | Critical |
| Gastrointestinal adverse events (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 26/36 (72.2%) | 11/15 (73.3%) | RR 0.98 (0.68 to 1.42) | 15 fewer per 1000 (from 235 fewer to 308 more) | ⊕⊕OO LOW | Critical |
| Headaches (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 16/36 (44.4%) | 9/15 (60%) | RR 0.74 (0.43 to 1.29) | 156 fewer per 1000 (from 342 fewer to 174 more) | ⊕⊕OO LOW | Critical |
| Mild insomnia (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 6/36 (16.7%) | 0/15 (0%) | RR 5.62 (0.34 to 93.94) | – | ⊕⊕OO LOW | Critical |
| QRS width (follow-up mean 8 weeks; measured with: self-reported; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 36 | 15 | – | MD 0.70 higher (3.35 lower to 4.75 higher) | ⊕⊕OO LOW | Critical |
| Rash (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 4/36 (11.1%) | 1/15 (6.7%) | RR 1.67 (0.2 to 13.7) | 45 more per 1000 (from 53 fewer to 847 more) | ⊕⊕OO LOW | Critical |
| PR interval (follow-up mean 8 weeks; measured with: self-reported; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 36 | 15 | – | MD 9.90 higher (5.27 lower to 25.07 higher) | ⊕⊕OO LOW | Critical |
| QT interval (follow-up mean 8 weeks; measured with: self-reported; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 36 | 15 | – | MD 13.50 higher (4.29 lower to 31.29 higher) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Pramipexole | Placebo | Relative (95% CI) | Absolute | ||
| Total tics (follow-up mean 6 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 42 | 20 | – | SMD 0.00 lower (0.53 lower to 0.53 higher) | ⊕⊕OO LOW | Critical |
| Global tic score (follow-up mean 6 weeks; measured with: YGTSS global scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 42 | 20 | – | SMD 0.01 lower (0.54 lower to 0.52 higher) | ⊕⊕OO LOW | Critical |
| Depression (follow-up mean 6 weeks; measured with: CDI-S; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousc | None | 42 | 19 | – | SMD 0.44 lower (0.99 lower to 0.11 higher) | ⊕⊕OO LOW | Important |
| Anxiety (follow-up mean 6 weeks; measured with: MASC; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousc | None | 42 | 19 | – | SMD 0.30 lower (0.85 lower to 0.24 higher) | ⊕⊕OO LOW | Important |
| CGI improved (follow-up mean 6 weeks; assessed with: CGI scale) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 10/42 (23.8%) | 4/20 (20%) | RR 1.19 (0.42 to 3.33) | 38 more per 1000 (from 116 fewer to 466 more) | ⊕⊕OO LOW | Important |
| CGI unchanged (follow-up mean 6 weeks; assessed with: CGI scale) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 32/42 (76.2%) | 16/20 (80%) | RR 0.95 (0.72 to 1.26) | 40 fewer per 1000 (from 224 fewer to 208 more) | ⊕⊕OO LOW | Important |
| 0% | – | |||||||||||
| Adverse events (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 17/42 (40.5%) | 5/20 (25%) | RR 1.62 (0.7 to 3.76) | 155 more per 1000 (from 75 fewer to 690 more) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Atomoxetine | Placebo | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 18 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 74 | 71 | – | SMD 0.32 lower (0.05 lower to 0.01 higher) | ⊕⊕⊕O MODERATE | Critical |
| Motor tic score (follow-up mean 18 weeks; measured with: YGTSS motor tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 74 | 71 | – | SMD 0.30 lower (0.62 lower to 0.03 higher) | ⊕⊕⊕O MODERATE | Critical |
| Vocal tic score (follow-up mean 18 weeks; measured with: YGTSS vocal tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 74 | 71 | – | SMD 0.22 lower (0.54 lower to 0.11 higher) | ⊕⊕⊕O MODERATE | Critical |
| CGI scale (follow-up mean 18 weeks; measured with: CGI-Tic/Neuro-S; range of scores: 1–7; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 74 | 71 | – | SMD 0.54 lower (0.87 to 0.21 lower) | ⊕⊕⊕O MODERATE | Important |
| Decreased appetite (follow-up mean 18 weeks; assessed with: self-reported) | ||||||||||||
| 1b | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousc | None | 12/74 (16.2%) | 2/71 (2.8%) | RR 5.68 (1.32 to 24.52) | 132 more per 1000 (from 9 more to 663 more) | ⊕⊕⊕O MODERATE | Important |
| Nausea (follow-up mean 18 weeks; assessed with: self-reported) | ||||||||||||
| 1b | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousc | None | 12/76 (15.8%) | 1/72 (1.4%) | RR 11.37 (1.52 to 85.22) | 144 more per 1000 (from 7 more to 1000 more) | ⊕⊕⊕O MODERATE | Important |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Selegiline | Placebo | Relative (95% CI) | Absolute | ||
| Motor tic score (follow-up mean 8 weeks; measured with: YGTSS motor tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 15c | – | – | SMD 0.64 lower (1.37 lower to 0.09 higher) | ⊕⊕OO LOW | Critical |
| Vocal tic score (follow-up mean 8 weeks; measured with: YGTSS vocal tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 15c | – | – | SMD 0.30 lower (1.02 lower to 0.41 higher) | ⊕⊕OO LOW | Critical |
| Impairment score (follow-up mean 8 weeks; measured with: YGTSS impairment scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 15c | – | – | SMD 0.75 lower (1.49 to 0.01 lower) | ⊕⊕OO LOW | Critical |
| Global tic score (follow-up mean 8 weeks; measured with: YGTSS global scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 15c | – | – | SMD 0.72 lower (1.45 lower to 0.02 higher) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Mecamylamine | Placebo | Relative (95% CI) | Absolute | ||
| BP > 90/60 (follow-up mean 8 weeks; assessed with: diastolic and systolic BP) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 4/25 (16%) | 0/25 (0%) | RR 9.00 (0.51 to 158.85) | – | ⊕⊕OO LOW | Critical |
| Weakness (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1c | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousd | None | 8/29 (27.6%) | 3/32 (9.4%) | RR 2.94 (0.86 to 10.05) | 182 more per 1000 (from 13 fewer to 848 more) | ⊕⊕OO LOW | Critical |
| Aggression (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1c | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousd | None | 7/29 (24.1%) | 3/32 (9.4%) | RR 2.57 (0.73 to 9.04) | 147 more per 1000 (from 25 fewer to 754 more) | ⊕⊕OO LOW | Critical |
| Heart rate after 1 week of treatment (follow-up mean 8 weeks; measured with: monitor; better indicated by higher values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousd | None | 29 | 32 | – | MD 8.50 higher (2.22 to 14.78 higher) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Baclofen | Placebo | Relative (95% CI) | Absolute | ||
| Tic score (follow-up mean 4 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9c | – | – | SMD 0.54 lower (1.50 lower to 0.42 higher) | ⊕OOO VERY LOW | Critical |
| Impairment (follow-up mean 4 weeks; measured with: YGTSS impairment scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9c | – | – | SMD 1.38 lower (2.46 to 0.30 lower) | ⊕OOO VERY LOW | Important |
| Global score (follow-up mean 4 weeks; measured with: YGTSS global scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9c | – | – | SMD 0.95 lower (1.93 lower to 0.02 higher) | ⊕OOO VERY LOW | Critical |
| Overall clinical outcome (follow-up mean 4 weeks; measured with: CGI-Severity; range of scores: 1–7; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9c | – | – | SMD 1.05 lower (2.03 to 0.07 lower) | ⊕OOO VERY LOW | Important |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Nicotine patch | Placebo | Relative (95% CI) | Absolute | ||
| Motor tics (follow-up mean 1–3 weeks; measured with: YGTSS motor tic scale and TSSL simple motor tic scale; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousa | No serious inconsistencyb | No serious indirectness | Seriousc | None | 34 | 36 | – | SMD 0.03 lower (0.49 lower to 0.43 higher) | ⊕⊕OO LOW | Critical |
| Vocal tics (follow-up mean 1–3 weeks; measured with: YGTSS vocal tic scale and TSSL simple vocal tic scale; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousa | Seriousd | No serious indirectness | Seriousc | None | 34 | 36 | – | SMD 0.34 higher (0.37 lower to 1.05 higher) | ⊕OOO VERY LOW | Critical |
| Impairment (follow-up mean 1–3 weeks; measured with: YGTSS impairment and Conners’ Global Index social problems scale; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousa | No serious inconsistencye | No serious indirectness | Seriousc | None | 34 | 36 | – | SMD 0.30 lower (0.75 lower to 0.15 higher) | ⊕⊕OO LOW | Important |
| Global tic score (follow-up mean 3 weeks; measured with: YGTSS global scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousf | None | 27 | 29 | – | SMD 0.43 lower (0.96 lower to 0.18 higher) | ⊕⊕OO LOW | Critical |
| Anxiousness/shyness (follow-up mean 1 weeks; measured with: Conners’ Parent rating scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousg | None | 14h | – | – | SMD 0.08 lower (0.55 lower to 0.39 higher) | ⊕⊕OO LOW | Important |
| Emotional liability (follow-up mean 1 weeks; measured with: Conners’ Parent rating scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousg | None | 14h | – | – | SMD 0.29 lower (0.77 lower to 0.19 higher) | ⊕⊕OO LOW | Important |
| Global improvement (follow-up mean 3 weeks; measured with: parent rated; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousf | None | 27 | 29 | – | SMD 0.79 lower (1.34 lower to 0.25 higher) | ⊕⊕OO LOW | Important |
| Global improvement (follow-up mean 3 weeks; measured with: clinician rated; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousf | None | 27 | 29 | – | SMD 0.53 lower (1.06 lower to 0.01 higher) | ⊕⊕OO LOW | Important |
| Nausea (follow-up mean 3 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousc | None | 25/35 (71.4%) | 6/35 (17.1%) | RR 4.17 (1.95 to 8.89) | 543 more per 1000 (from 163 more to 1000 more) | ⊕⊕OO LOW | Critical |
| Itching at patch site (follow-up mean 3 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousc | None | 20/35 (57.1%) | 15/35 (42.9%) | RR 1.33 (0.83 to 2.15) | 141 more per 1000 (from 73 fewer to 493 more) | ⊕⊕OO LOW | Critical |
| Headache (follow-up mean 3 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousc | None | 17/35 (48.6%) | 14/35 (40%) | RR 1.21 (0.71 to 2.06) | 84 more per 1000 (from 116 fewer to 424 more) | ⊕⊕OO LOW | Critical |
| Vomiting (follow-up mean 3 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousc | None | 14/35 (40%) | 3/35 (8.6%) | RR 4.67 (1.47 to 14.82) | 315 more per 1000 (from 40 more to 1000 more) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Omega-3 fatty acids | Olive oil | Relative (95% CI) | Absolute | ||
| Total tics (follow-up mean 20 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 17 | 16 | – | SMD 0.24 lower (0.92 lower to 0.45 higher) | ⊕⊕OO LOW | Critical |
| Impairment (follow-up mean 20 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 17 | 16 | – | SMD 0.76 lower (1.47 to 0.05 lower) | ⊕⊕OO LOW | Important |
| Global tic score (follow-up mean 20 weeks; measured with: YGTSS global tic scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 17 | 16 | – | SMD 0.67 lower (1.38 lower to 0.03 higher) | ⊕⊕OO LOW | Critical |
| Proportion of children with ≥ 30% reductions in tic score (follow-up mean 20 weeks; assessed with: YGTSS total tic score) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 9/17 (52.9%) | 6/16 (37.5%) | RR 1.41 (0.65 to 3.07) | 154 more per 1000 (from 131 fewer to 776 more) | ⊕⊕OO LOW | Critical |
| Proportion of children with ≥ 30% reductions in impairment (follow-up mean 20 weeks; assessed with: YGTSS total tic score) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 10/17 (58.8%) | 4/16 (25%) | RR 2.35 (0.92 to 6.01) | 337 more per 1000 (from 20 fewer to 1000 more) | ⊕⊕OO LOW | Important |
| Proportion of children with ≥ 30% reductions in global tic score (follow-up mean 20 weeks; assessed with: YGTSS global tic score) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 9/17 (52.9%) | 5/16 (31.3%) | RR 1.69 (0.72 to 3.98) | 216 more per 1000 (from 87 fewer to 931 more) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Risperidone | Clonidine | Relative (95% CI) | Absolute | ||
| Global tic score (follow-up mean 8 weeks; measured with: YGTSS global scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 9 | 12 | – | SMD 0.19 higher (0.68 lower to 1.05 higher) | ⊕⊕OO LOW | Critical |
| ≥ 30% reduction in global tic score (follow-up mean 8 weeks; assessed with: YGTSS global scale) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 4/9 (44.4%) | 6/12 (50%) | RR 0.89 (0.35 to 2.24) | 55 fewer per 1000 (from 325 fewer to 620 more) | ⊕⊕OO LOW | Critical |
| Depression (follow-up mean 8 weeks; measured with: HAM-D; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 9 | 12 | – | SMD 0.03 higher (0.83 lower to 0.89 higher) | ⊕⊕OO LOW | Important |
| CGI scale (follow-up mean 8 weeks; measured with: CGI-Severity; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 9 | 12 | – | SMD 0.65 higher (0.24 lower to 1.54 higher) | ⊕⊕OO LOW | Important |
| Clinically significant adverse events (follow-up mean 8 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 3/9 (33.3%) | 7/12 (58.3%) | RR 0.57 (0.2 to 1.62) | 251 fewer per 1000 (from 467 fewer to 362 more) | ⊕⊕OO LOW | Critical |
| Weight gain (kg) (follow-up mean 8 weeks; measured with: Investigator assessed; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 9 | 12 | – | MD 2.00 higher (1.66 lower to 5.66 higher) | ⊕⊕OO LOW | Important |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Fluvoxamine | Sulpiride | Relative (95% CI) | Absolute | ||
| Global tic score (follow-up mean 6 weeks; measured with: YGTSS global tic scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 11d | – | – | SMD 0.23 higher (0.3 lower to 0.77 higher) | ⊕OOO VERY LOW | Critical |
| Mild-to-moderate depression (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1e | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 0/11 (0%)d | 3/11 (27.3%) | RR 0.14 (0.01 to 2.48) | 235 fewer per 1000 (from 270 fewer to 404 more) | ⊕OOO VERY LOW | Critical |
| Akathisia (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1e | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 2/11 (18.2%)d | 2/11 (18.2%) | RR 1.00 (0.17 to 5.89) | 0 fewer per 1000 (from 151 fewer to 889 more) | ⊕OOO VERY LOW | Critical |
| Mild and transient nausea (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1e | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 4/11 (36.4%)d | 0/11 (0%) | RR 9.00 (0.54 to 149.5) | – | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Levetiracetam | Clonidine | Relative (95% CI) | Absolute | ||
| Total tic score (follow-up mean 6 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 10d | – | – | SMD 0.22 lower (0.78 lower to 0.34 higher) | ⊕⊕OO LOW | Critical |
| Global tic score (follow-up mean 6 weeks; measured with: YGTSS global tic scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 10d | – | – | SMD 0.33 lower (0.9 lower to 0.24 higher) | ⊕⊕OO LOW | Critical |
| Anxiety (follow-up mean 6 weeks; measured with: MASC; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 10d | – | – | SMD 0.16 lower (0.72 lower to 0.4 higher) | ⊕⊕OO LOW | Important |
| Depression (follow-up mean 6 weeks; measured with: CDI-S; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 10d | – | – | SMD 0.08 higher (0.48 lower to 0.63 higher) | ⊕⊕OO LOW | Important |
| CGI scale (follow-up mean 6 weeks; measured with: CGI-Severity; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 10d | – | – | SMD 0.16 lower (0.72 lower to 0.4 higher) | ⊕⊕OO LOW | Important |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Clonidine | Desipramine | Relative (95% CI) | Absolute | ||
| Adverse events (follow-up mean 6 weeks; assessed with: self-reported) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 28/34 (82.4%) | 26/34 (76.5%) | RR 1.08 (0.84 to 1.37) | 61 more per 1000 (from 122 fewer to 283 more) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Clonidine patch | Haloperidol | Relative (95% CI) | Absolute | ||
| Global score (follow-up mean 4 weeks; measured with: YGTSS global scale; range of scores: 0–100; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 65 | 54 | – | SMD 0.38 lower (0.75 to 0.02 lower) | ⊕OOO VERY LOW | Critical |
| 50% reduction in global score (follow-up mean 4 weeks; assessed with: YGTSS global scale) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 53/65 (81.5%) | 36/54 (66.7%) | RR 0.55 (0.29 to 1.05) | 300 fewer per 1000 (from 473 fewer to 33 more) | ⊕OOO VERY LOW | Critical |
Behavioural interventions
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Habit reversal/CBIT | Control | Relative (95% CI) | Absolute | ||
| Change in YGTSS total tic score (follow-up mean 10 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectnessb | No serious imprecisionc | None | 61 | 65 | – | SMD 0.64 lower (1 to 0.29 lower) | ⊕⊕⊕O MODERATE | Critical |
| Change in YGTSS total tic score at 10 m follow up (follow-up mean 10 months; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 2 | Randomised trials | Seriousd | No serious inconsistency | Seriouse | Seriousf | None | 18 | 21 | – | SMD 1.11 lower (1.8 to 0.42 lower) | ⊕OOO VERY LOW | Critical |
| Change in YGTSS motor tic score (follow-up mean 10 weeks; measured with: YGTSS motor tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 61 | 65 | – | SMD 0.45 lower (0.81 to 0.1 lower) | ⊕⊕⊕O MODERATE | Critical |
| Change in YGTSS vocal tic score (follow-up mean 10 weeks; measured with: YGTSS vocal tic scale; range of scores: 0–25; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 61 | 65 | – | SMD 0.54 lower (0.9 to 0.19 lower) | ⊕⊕⊕O MODERATE | Critical |
| Change in YGTSS impairment score (follow-up mean 10 weeks; measured with: YGTSS impairment scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 61 | 65 | – | SMD 0.51 lower (0.87 to 0.16 lower) | ⊕⊕⊕O MODERATE | Critical |
| Change in YGTSS impairment at 10 m follow-up (follow-up mean 10 months; measured with: YGTSS impairment scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousd | No serious inconsistency | Seriouse | Seriousf | None | 10 | 11 | – | SMD 0.42 lower (1.29 lower to 0.45 higher) | ⊕OOO VERY LOW | Critical |
| CGI much or very much improved (follow-up mean 10 weeks; assessed with: CGI-I) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 32/61 (52.5%) | 12/65 (18.5%) | RR 2.84 (1.62 to 4.99) | 34 more per 100 (from 11 more to 74 more) | ⊕⊕⊕O MODERATE | Important |
| Change in anxiety: child rated (follow-up mean 10 weeks; measured with: screen for child anxiety related emotional disorders; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 61 | 65 | – | SMD 0.10 lower (0.45 lower to 0.25 higher) | ⊕⊕⊕O MODERATE | Important |
| Change in anxiety: parent rated (follow-up mean 10 weeks; measured with: screen for child anxiety related emotional disorders; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 61 | 65 | – | SMD 0.15 lower (0.5 lower to 0.2 higher) | ⊕⊕⊕O MODERATE | Important |
| Change in depression (follow-up mean 10 weeks; measured with: children’s depression inventory: total score; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectnessb | Seriousc | None | 61 | 65 | – | SMD 0.22 lower (0.57 lower to 0.13 higher) | ⊕⊕⊕O MODERATE | Important |
| Change in family functioning: parent total (follow-up mean 10 weeks; measured with: family assessment measure:-III short form; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectness | Seriousc | None | 61 | 65 | – | SMD 0.16 higher (0.19 lower to 0.51 higher) | ⊕⊕⊕O MODERATE | Important |
| Change in family functioning: child total (follow-up mean 10 weeks; measured with: family assessment measure-III short form; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectness | Seriousc | None | 61 | 65 | – | SMD 0.02 lower (0.37 lower to 0.33 higher) | ⊕⊕⊕O MODERATE | Important |
| Change in caregiver strain: total score (follow-up mean 10 weeks; measured with: Caregiver Strain Questionnaire; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectness | Seriousc | None | 61 | 65 | – | SMD 0.02 lower (0.37 lower to 0.33 higher) | ⊕⊕⊕O MODERATE | Important |
| Change in social adjustment: Family (follow-up mean 10 weeks; measured with: social adjustment scale self-report; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectness | Seriousc | None | 61 | 65 | – | SMD 0.15 lower (0.5 lower to 0.2 higher) | ⊕⊕⊕O MODERATE | Important |
| Change in social adjustment: friends (follow-up mean 10 weeks; measured with: social adjustment scale self-report; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectness | Seriousc | None | 61 | 65 | – | SMD 0.08 lower (0.43 lower to 0.27 higher) | ⊕⊕⊕O MODERATE | Important |
| Change in social adjustment: school (follow-up mean 10 weeks; measured with: social adjustment scale self-report; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasa | No serious inconsistency | No serious indirectness | Seriousc | None | 61 | 65 | – | SMD 0.10 higher (0.25 lower to 0.45 higher) | ⊕⊕⊕O MODERATE | Important |
| Adverse eventsg (follow-up mean 10 weeks; assessed with: self-report) | ||||||||||||
| 1 | Randomised trials | No serious risk of biash | No serious inconsistency | No serious indirectness | Seriousc | None | – | – | – | – | ⊕⊕⊕O MODERATE | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Videoconference CBIT | face-to-face CBIT | Relative (95% CI) | Absolute | ||
| Change in YGTSS total tic score (follow-up mean 10 weeks; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 10 | 8 | – | SMD 0.18 lower (1.11 lower to 0.75 higher) | ⊕⊕OO LOW | Critical |
| Change in YGTSS total tic score at 4 month follow-up (follow-up mean 4 months; measured with: YGTSS total tic scale; range of scores: 0–50; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasb | No serious inconsistency | No serious indirectness | Very seriousa | None | 9 | 7 | – | SMD 0.32 lower (1.32 lower to 0.67 higher) | ⊕⊕OO LOW | Critical |
| CGI much or very much improved (follow-up mean 10 weeks; assessed with: CGI-I) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousa | None | 8/10 (80%) | 6/8 (75%) | RR 1.07 (0.64 to 1.77) | 5 more per 100 (from 27 fewer to 58 more) | ⊕⊕OO LOW | Important |
| CGI much or very much improved at 4 month follow-up (follow-up mean 4 months; assessed with: CGI-I) | ||||||||||||
| 1 | Randomised trials | No serious risk of biasb | No serious inconsistency | No serious indirectness | Very seriousa | None | 5/9 (55.6%) | 3/7 (42.9%) | RR 1.30 (0.46 to 3.65) | 13 more per 100 (from 23 fewer to 100 more) | ⊕⊕OO LOW | Important |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Negative practice | HRT | Relative (95% CI) | Absolute | ||
| Number of tics per day (follow-up mean 4 weeks; measured with: tic count; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | Seriousb | Seriousc | None | 12 | 10 | – | SMD 1.74 higher (0.76 to 2.72 higher) | ⊕OOO VERY LOW | Critical |
| Proportion of patients with < 1 tic per day (follow-up mean 4 weeks; assessed with: tic count) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | Seriousb | Seriousc | None | 2/12 (16.7%) | 8/10 (80%) | RR 4.80 (1.3 to 17.66) | 1000 more per 1000 (from 240 more to 1000 more) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | ERP | HRT | Relative (95% CI) | Absolute | ||
| YGTSS total tic score (follow-up mean 10 weeks; measured with: YGTSS total tic scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 11 | 14 | – | SMD 0.43 lower (1.23 lower to 0.37 higher) | ⊕⊕OO LOW | Critical |
| YGTSS motor tic score (follow-up mean 10 weeks; measured with: YGTSS motor tic scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousb | None | 11 | 14 | – | SMD 0.08 higher (0.71 lower to 0.87 higher) | ⊕⊕OO LOW | Critical |
| YGTSS vocal tic score (follow-up mean 10 weeks; measured with: YGTSS vocal tic scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousb | None | 11 | 14 | – | SMD 0.73 lower (1.55 lower to 0.09 higher) | ⊕⊕OO LOW | Critical |
| YGTSS impairment score (follow-up mean 10 weeks; measured with: YGTSS impairment scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousb | None | 11 | 14 | – | SMD 0.04 higher (0.75 lower to 0.83 higher) | ⊕⊕OO LOW | Important |
| > = 30% reduction YGTSS total tic score (follow-up mean 10 weeks; assessed with: YGTSS total tic scale) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | No serious indirectness | Very seriousb | None | 7/11 (63.6%) | 6/14 (42.9%) | RR 1.48 (0.7 to 3.15) | 206 more per 1000 (from 129 fewer to 921 more) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Relaxation training | Minimal therapy | Relative (95% CI) | Absolute | ||
| Proportion with improved YGTSS global score (follow-up mean 6 weeks; assessed with: YGTSS global scale) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 6/7 (85.7%) | 6/9 (66.7%) | RR 1.29 (0.74 to 2.23) | 193 more per 1000 (from 173 fewer to 820 more) | ⊕OOO VERY LOW | Critical |
| Proportion with improved YGTSS global score at follow-up (follow-up mean 3 months; assessed with: YGTSS global scale) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 5/7 (71.4%) | 5/9 (55.6%) | RR 0.64 (0.16 to 2.56) | 200 fewer per 1000 (from 467 fewer to 867 more) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | AC training | TAU | Relative (95% CI) | Absolute | ||
| YGTSS total tic score (follow-up mean 10 weeks; measured with: YGTSS total tic scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 13 | 13 | – | SMD 0.58 lower (1.37 lower to 0.2 higher) | ⊕OOO VERY LOW | Critical |
| CBCL: Total competence score (follow-up mean 10 weeks; measured with: CBCL; better indicated by higher values) | ||||||||||||
| 1 | Randomised trials | Very seriousa,c | No serious inconsistency | Seriousd | Very seriousb | None | 13 | 13 | – | SMD 1.18 lower (2.01 to 0.35 lower) | ⊕OOO VERY LOW | Important |
| Family functioning (follow-up mean 10 weeks; measured with: family assessment device general functioning scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa,c | No serious inconsistency | Seriousd | Very seriousb | None | 13 | 13 | – | SMD 0.42 lower (1.2 lower to 0.35 higher) | ⊕OOO VERY LOW | Important |
| CGI much or very much improved (follow-up mean 10 weeks; assessed with: CGI-I) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousd | Very seriousb | None | 9/13 (69.2%) | 2/13 (15.4%) | RR 0.36 (0.16 to 0.85) | 98 fewer per 1000 (from 23 fewer to 129 fewer) | ⊕OOO VERY LOW | Important |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Parent training | TAU | Relative (95% CI) | Absolute | ||
| YGTSS total tic score (follow-up mean 10 weeks; measured with: YGTSS total tic scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa,b | No serious inconsistency | No serious indirectness | Very seriousc | None | 11 | 12 | – | SMD 0.29 higher (0.53 lower to 1.12 higher) | ⊕OOO VERY LOW | Critical |
| Parenting stress (follow-up mean 10 weeks; measured with: Parenting Stress Index-Short Form; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa,b | No serious inconsistency | Seriousd | Very seriousc | None | 11 | 12 | – | SMD 0.12 lower (0.94 lower to 0.7 higher) | ⊕OOO VERY LOW | Important |
| CGI much or very much improved (follow-up mean 10 weeks; assessed with: CGI-I) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousd | Very seriousc | None | 7/11 (63.6%) | 2/12 (16.7%) | RR 0.44 (0.19 to 0.99) | 93 fewer per 1000 (from 2 fewer to 135 fewer) | ⊕OOO VERY LOW | Important |
Physical interventions
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | i.v. immunoglobulin | i.v. saline | Relative (95% CI) | Absolute | ||
| YGTSS total tic score (follow-up mean 14 weeks; measured with: YGTSS total tic scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | Seriousa | Seriousb | None | 14 | 15 | – | SMD 0.51 lower (1.25 lower to 0.23 higher) | ⊕⊕OO LOW | Critical |
| CGI much or very much improved (follow-up mean 14 weeks; assessed with: CGI-I) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | Seriousa | Seriousb | None | 4/14 (28.6%) | 1/15 (6.7%) | RR 4.29 (0.54 to 33.85) | 219 more per 1000 (from 31 fewer to 1000 more) | ⊕⊕OO LOW | Important |
| Adverse events (follow-up mean 14 weeks) | ||||||||||||
| 1 | Randomised trials | No serious risk of bias | No serious inconsistency | Seriousa | Seriousb | None | 13/14 (92.9%) | 4/15 (26.7%) | RR 3.48 (1.49 to 8.16) | 661 more per 1000 (from 131 more to 1000 more) | ⊕⊕OO LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | i.v. Botulinum toxin | i.v. saline | Relative (95% CI) | Absolute | ||
| Shapiro TSSS (follow-up mean 2 weeks; measured with: Shapiro TSSS; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 18 | – | – | SMD 0.02 higher (0.63 lower to 0.67 higher) | ⊕OOO VERY LOW | Critical |
| Proportion of patients who felt better (follow-up mean 2 weeks) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Very seriousb,d | Seriousc | None | 4/18 (22.2%) | 1/18 (5.6%)e | RR 4.0 (0.49 to 32.39) | 167 more per 1000 (from 28 fewer to 1000 more) | ⊕OOO VERY LOW | Important |
| Pain score (follow-up mean 2 weeks; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 18 | – | – | SMD 0.52 higher (0.15 lower to 1.18 higher) | ⊕OOO VERY LOW | Critical |
| Weakness (follow-up mean 2 weeks) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 12/18 (66.7%) | 2/18 (11.1%)e | RR 6.00 (1.56 to 23.07) | 556 more per 1000 (from 62 more to 1000 more) | ⊕OOO VERY LOW | Critical |
| Neck discomfort (follow-up mean 2 weeks) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 3/18 (16.7%) | 1/18 (5.6%)e | RR 3.00 (0.34 to 26.19) | 111 more per 1000 (from 37 fewer to 1000 more) | ⊕OOO VERY LOW | Critical |
| Blurry vision (follow-up mean 2 weeks) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 1/18 (5.6%) | 0/18 (0%)e | RR 3.00 (0.13 to 69.09) | – | ⊕OOO VERY LOW | Critical |
| Swallowing difficulty (follow-up mean 2 weeks) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 2/18 (11.1%) | 0/18 (0%)e | RR 5.00 (0.26 to 97.37) | – | ⊕OOO VERY LOW | Critical |
| Motor restlessness (follow-up mean 2 weeks) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 2/18 (11.1%) | 0/18 (0%)e | RR 5.00 (0.26 to 97.3) | – | ⊕OOO VERY LOW | Critical |
| Increased urge to tic (follow-up mean 2 weeks) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 1/18 (5.6%) | 0/18 (0%)e | RR 3.00 (0.13 to 69.09) | – | ⊕OOO VERY LOW | Critical |
| New tics (follow-up mean 2 weeks) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 2/18 (11.1%) | 0/18 (0%)e | RR 5.00 (0.26 to 97.37) | – | ⊕OOO VERY LOW | Critical |
| YGTSS Motor tic frequency (treated tic) (follow-up mean 2 weeks; measured with: YGTSS; better indicated by higher values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 18 | – | – | SMD 0.14 lower (0.8 lower to 0.51 higher) | ⊕OOO VERY LOW | Critical |
| YGTSS Motor tic intensity (treated tic) (follow-up mean 2 weeks; measured with: YGTSS; better indicated by higher values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 18 | – | – | SMD 0.91 lower (1.59 to 0.22 lower) | ⊕OOO VERY LOW | Critical |
| YGTSS Motor tic interference (treated tic) (Copy) (follow-up mean 2 weeks; measured with: YGTSS; better indicated by higher values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | Seriousb | Seriousc | None | 18 | – | – | SMD 0.05 lower (0.7 lower to 0.61 higher) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Acupuncture and Chinese herbs | Haloperidol and artane | Relative (95% CI) | Absolute | ||
| YGTSS global score proportion ≥ 30% reduction (assessed with: YGTSS global scale) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | Seriousb | Seriousc | None | 28/30 (93.3%) | 23/30 (76.7%) | RR 1.22 (0.98 to 1.52) | 169 more per 1000 (from 15 fewer to 399 more) | ⊕OOO VERY LOW | Critical |
| YGTSS global score proportion ≥ 60% reduction (assessed with: YGTSS global scale) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | Seriousb | Seriousc | None | 17/30 (56.7%) | 9/30 (30%) | RR 1.89 (1.01 to 3.55) | 267 more per 1000 (from 3 more to 765 more) | ⊕OOO VERY LOW | Critical |
Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection studies
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | i.v. immunoglobulin | i.v. saline | Relative (95% CI) | Absolute | ||
| TS rating score (follow-up mean 1 month; measured with: TS rating scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9 | 10 | – | SMD 0.00 higher (0.9 lower to 0.9 higher) | ⊕OOO VERY LOW | Critical |
| NIMH impairment (follow-up mean 1 month; measured with: NIMH global impairment scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9 | 10 | – | SMD 1.87 lower (3 to 0.75 lower) | ⊕OOO VERY LOW | Important |
| NIMH anxiety (follow-up mean 1 month; measured with: NIMH anxiety scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9 | 10 | – | SMD 1.03 lower (2 to 0.05 lower) | ⊕OOO VERY LOW | Important |
| NIMH depression (follow-up mean 1 month; measured with: NIMH global depression scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9 | 10 | – | SMD 0.64 lower (1.57 lower to 0.29 higher) | ⊕OOO VERY LOW | Important |
| Overall clinical outcome (follow-up mean 1 month; measured with: CGI-I; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 9 | 10 | – | SMD 1.86 lower (2.98 to 0.74 lower) | ⊕OOO VERY LOW | Important |
| Adverse events (follow-up mean 1 month) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Very seriousb | None | 6/9 (66.7%) | 2/10 (20%) | RR 3.33 (0.89 to 12.51) | 466 more per 1000 (from 22 fewer to 1000 more) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Plasma exchange | i.v. saline | Relative (95% CI) | Absolute | ||
| TS rating score (follow-up mean 1 month; measured with: TS rating scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 10 | 10 | – | SMD 0.88 lower (1.81 to 0.05 lower) | ⊕OOO VERY LOW | Critical |
| NIMH impairment (follow-up mean 1 month; measured with: NIMH global impairment scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 10 | 10 | – | SMD 1.42 lower (2.42 to 0.41 lower) | ⊕OOO VERY LOW | Important |
| NIMH anxiety (follow-up mean 1 month; measured with: NIMH anxiety scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 10 | 10 | – | SMD 1.24 lower (2.21 to 0.26 lower) | ⊕OOO VERY LOW | Important |
| NIMH depression (follow-up mean 1 month; measured with: NIMH global depression scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 10 | 10 | – | SMD 1.06 lower (2 to 0.11 lower) | ⊕OOO VERY LOW | Important |
| Overall clinical outcome (follow-up mean 1 month; measured with: CGI-I; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 10 | 10 | – | SMD 2.38 lower (3.58 to 1.18 lower) | ⊕OOO VERY LOW | Important |
| Adverse events (follow-up mean 1 month) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 7/10 (70%) | 2/10 (20%) | RR 3.50 (0.95 to 12.9) | 500 more per 1000 (from 10 fewer to 1000 more) | ⊕OOO VERY LOW | Critical |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Penicillin | Placebo | Relative (95% CI) | Absolute | ||
| YGTSS total tic score (follow-up mean 4 weeks; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 37 | – | – | SMD 0.26 lower (0.72 lower to 0.21 higher) | ⊕⊕OO LOW | Critical |
| YGTSS motor tic score (follow-up mean 4 months; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 37 | – | – | SMD 0.14 lower (0.61 lower to 0.32 higher) | ⊕⊕OO LOW | Critical |
| YGTSS vocal tic score (follow-up mean 4 months; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 37 | – | – | SMD 0.23 lower (0.69 lower to 0.24 higher) | ⊕⊕OO LOW | Critical |
| Rate of streptococcal infection (follow-up mean 4 months) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 14/35 (40%) | 21/37 (56.8%) | RR 0.70 (0.43 to 1.15) | 170 fewer per 1000 (from 324 fewer to 85 more) | ⊕⊕OO LOW | Important |
| Rate of neuropsychiatric exacerbations (follow-up mean 4 months) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 18/35 (51.4%) | 19/37 (51.4%) | RR 1.00 (0.64 to 1.57) | 0 fewer per 1000 (from 185 fewer to 293 more) | ⊕⊕OO LOW | Critical |
| Impairment (follow-up mean 4 months; measured with: C-GAS; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 37 | – | – | SMD 0.20 lower (0.66 lower to 0.27 higher) | ⊕⊕OO LOW | Important |
| Anxiety (follow-up mean 4 months; measured with: NIMH anxiety scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 37 | – | – | SMD 0.52 lower (0.99 to 0.05 lower) | ⊕⊕OO LOW | Important |
| Depression (follow-up mean 4 months; measured with: NIMH depression scale; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 37 | – | – | SMD 0.62 lower (1.1 to 0.15 lower) | ⊕⊕OO LOW | Important |
| Overall global clinical outcome (follow-up mean 4 months; measured with: CGI scale: global improvement; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 37 | – | – | SMD 0.07 lower (0.53 lower to 0.39 higher) | ⊕⊕OO LOW | Important |
| Overall TS clinical outcome (follow-up mean 4 months; measured with: CGI scale: TS improvement; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 37 | – | – | SMD 0.01 lower (0.47 lower to 0.45 higher) | ⊕⊕OO LOW | Important |
| Quality assessment | Number of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Penicillin | Azithromycin | Relative (95% CI) | Absolute | ||
| Neuropsychiatric symptom exacerbations (follow-up mean 1 year; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 11 | 12 | – | SMD 0.77 lower (1.63 lower to 0.08 higher) | ⊕OOO VERY LOW | Critical |
| Streptococcal infections (follow-up mean 1 year; better indicated by lower values) | ||||||||||||
| 1 | Randomised trials | Very seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 11 | 12 | – | SMD 0.00 higher (0.82 lower to 0.82 higher) | ⊕OOO VERY LOW | Important |
Appendix 5 Sensitivity analyses
Sensitivity analysis was conducted on the largest meta-analyses in the review to test the effect of assumptions made (paucity of data for other interventions made sensitivity analysis less useful). The following shows the most conservative scenario with the following changes made:
-
removal of studies with higher risk of bias (in meta-analyses with low and unclear risk of bias studies, unclear risk of bias studies removed. In meta-analysis with unclear and high risk of bias studies, high risk of bias studies removed)
-
when SDs have been calculated for within-group changes, an assumed correlation coefficient of 0.3, rather than 0.6, has been used
-
removal of all crossover studies.
Antipsychotic studies sensitivity analysis
Crossover RCTs were removed98,100 and a correlation coefficient of 0.3 was assumed for Scahill et al. 101 (in Shapiro et al. ,99 there was first-phase parallel data but only post-treatment results were reported so no SDs were assumed; in Dion et al. ,102 change scores were reported so no SDs were assumed; and in Sallee et al. ,103 change scores were reported so no SDs were assumed). The overall SMD was reduced from the original level (SMD –0.79, 95% CI –1.13 to –0.45) to SMD –0.68 (95% CI –1.10 to –0.34) but the interpretation of findings remains the same.
FIGURE 238.
Sensitivity analysis for studies of antipsychotics compared with placebo. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

Noradrenergic agents sensitivity analysis
A correlation coefficient of 0.3 was assumed for Leckman et al. ,116 Scahill et al. 120 and Cummings et al. 121 (Kurlan et al. 113 presented net change scores so no SDs were assumed). The overall effect size was reduced from the original level (SMD –0.65, 95% –0.96 to –0.33; n = 164) to SMD –0.55 (95% CI –0.86 to –0.24; n = 164) but the interpretation of findings remains the same.
FIGURE 239.
Sensitivity analysis for studies of noradrenergic agent compared with placebo. df, degrees of freedom; IV, independent variable; ROB, risk of bias; SE, standard error.

Habit reversal training/comprehensive behavioural intervention for tics sensitivity analysis
Studies with a high or unclear risk of bias were removed. There was no difference in the interpretation of findings.
FIGURE 240.
Sensitivity analysis for studies of HRT/CBIT compared with SP. df, degrees of freedom; IV, independent variable; ROB, risk of bias.
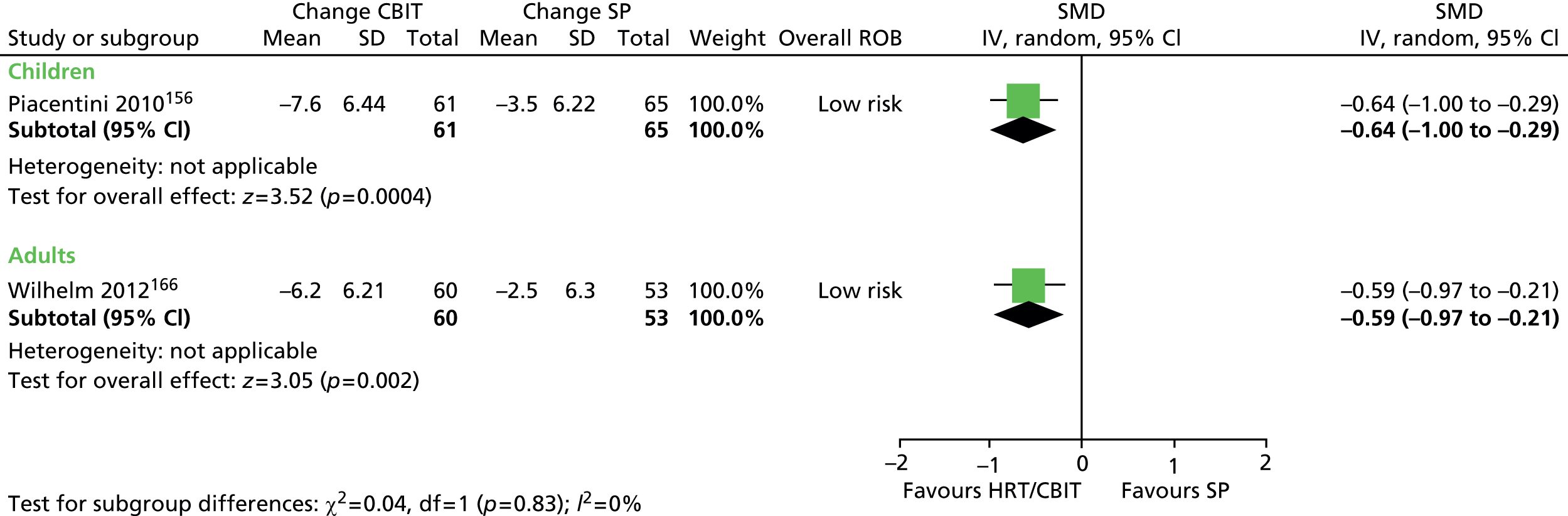
Appendix 6 Excluded studies
| Reference | Reason for exclusion |
|---|---|
| Quantitative systematic review | |
| Ackermans L, Duits A, Temel Y, Winogrodzka A, Peeters F, Beuls EaM, et al. Long-term outcome of thalamic deep brain stimulation in two patients with Tourette syndrome. J Neurol Neurosurg Psychiatry 2010;81:1068–72 | Inappropriate study design |
| Ackermans L, Temel Y, Cath D, van der Linden C, Bruggeman R, Kleijer M, et al. Deep brain stimulation in Tourette’s syndrome: two targets? Mov Disord 2006;21:709–13 | Inappropriate study design |
| Ackermans L, Temel Y, Bauer NJC, Visser-Vandewalle V. Vertical gaze palsy after thalamic stimulation for Tourette syndrome: case report. Neurosurgery 2007;61:E1100 | Inappropriate study design |
| Ahmed MA, Martinez A, Yee A, Cahill D, Besag FM, Ahmed MAS, et al. Psychogenic and organic movement disorders in children. Dev Med Child Neurol 2008;50:300–4 | Inappropriate study design |
| Alacqua M, Trifirò G, Arcoraci V, Germanò E, Magazù A, Calarese T, et al. Use and tolerability of newer antipsychotics and antidepressants: a chart review in a paediatric setting. Pharm World Sci 2008;30:44–50 | Inappropriate study design |
| Anca MH, Giladi N, Korczyn AD. Ropinirole in Gilles de la Tourette syndrome. Neurology 2004;62:1626–7 | Inappropriate study design |
| Asher SWA, Aminoff MJ. Tetrabenazine and movement disorders. Neurology 1981;31:1051–4 | Inappropriate population |
| Awaad YM, Michon AM, Minarik S. Use of levetiracetam to treat tics in children and adolescents with Tourette syndrome. Mov Disord 2005;20:714–18 | Inappropriate study design |
| Awaad YM, Michon AM, Minarik S. Long-term use of levetiracetam to treat tics in children and adolescents with Tourette syndrome. J Pediatr Neurol 2007;5:209–14 | Inappropriate study design |
| Awaad Y. Double-blind controlled randomized study of the use of levetiracetam to treat tics in children and adolescents with Tourette syndrome. Eur J Neurol 2009;16:170 | Inappropriate outcomes |
| Bajo S, Battaglia M, Pegna C, Bellodi L. Citalopram and fluvoxamine in Tourette’s disorder. J Am Acad Child Adolesc Psychiatry 1999;38:230–1 | Inappropriate study design |
| Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: A systemic, placebo-controlled evaluation. Pediatrics 1990;86:184–92 | Inappropriate population |
| Blair J, Scahill L, State M, Martin A. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry 2005;44:73–9 | Inappropriate study design |
| Boon-yasidhi V, Kim YS, Scahill L. An open-label, prospective study of guanfacine in children with ADHD and tic disorders. J Med Assoc Thailand 2005;88(Suppl. 8):S156–62 | Inappropriate study design |
| Bruun RD, Budman CL. Paroxetine treatment of episodic rages associated with Tourette’s disorder. J Clin Psychiatry 1998;59:581–4 | Inappropriate study design |
| Budman C, Coffey BJ, Shechter R, Schrock M, Wieland N, Spirgel A, et al. Aripiprazole in children and adolescents with Tourette disorder with and without explosive outbursts. J Child Adolesc Psychopharmacol 2008;18:509–15 | Inappropriate study design |
| Budman CL, Gayer A, Lesser M, Shi Q, Bruun RD. An open-label study of the treatment efficacy of olanzapine for Tourette’s disorder. J Clin Psychiatry 2001;62:290–4 | Inappropriate study design |
| Buitelaar JKC. Remoxipride in adolescents with Tourette’s syndrome: an open pilot study. J Child Adolesc Psychopharmacol 1995;5:121–8 | Inappropriate study design |
| Caine ED, Polinsky RJ, Ebert MH, Rapoport JL, Mikkelsen EJ. Trial of chlorimipramine and desipramine for Gilles de la Tourette syndrome. Ann Neurol 1979;5:305–6 | Inappropriate study design |
| Chappell PB, Leckman JF, Scahill LD, Hardin MT, Anderson G, Cohen DJ. Neuroendocrine and behavioral effects of the selective kappa agonist spiradoline in Tourette’s syndrome: A pilot study. Psychiatry Res 1993;47:267–80 | Inappropriate study design |
| Chappell PB, Riddle MA, Scahill L, Lynch KA, Schultz R, Arnsten A, et al. Guanfacine treatment of comorbid attention-deficit hyperactivity disorder and Tourette’s syndrome: Preliminary clinical experience. J Am Acad Child Adolesc Psychiatry 1995;34:1140–6 | Inappropriate study design |
| Cheng Y, Jiang DH. [Therapeutic effect of inosine in Tourette syndrome and its possible mechanism of action.] Zhonghua Shen Jing Jing Shen Ke Za Zhi 1990;23:90–7 | Inappropriate study design |
| Clarke MA, Bray MA, Kehle TJ, Truscott SD. A school-based intervention designed to reduce the frequency of tics in children with Tourette’s syndrome. Sch Psychol Rev 2001;30:11 | Inappropriate study design |
| Coffey BJ, Jummani R, Hirsch S, Lyon G, Spirgel A, Goldman R, et al. Aripiprazole in tourette’s disorder: an open label safety and tolerability study in youth. J Child Adolesc Psychopharmacol 2009;19:783–4 | Inappropriate study design |
| Cohen DJ, Detlor J. Clonidine ameliorates Gilles de la Tourette syndrome. Arch Gen Psychiatry 1980;37:1350–7 | Inappropriate study design |
| Connell PH. Drug treatment of adolescent tiqueurs: a double-blind trial of diazepam and haloperidol. Br J Psychiatry 1967;113:375–81 | Inappropriate study design |
| Cubo E, Fernández Jaén A, Moreno C, Anaya B, González M, Kompoliti K. Donepezil use in children and adolescents with tics and attention-deficit/hyperactivity disorder: an 18-week, single-center, dose-escalating, prospective, open-label study. Clin Ther 2008;30:182–9 | Inappropriate study design |
| Cui Y-H, Zheng Y, Yang Y-P, Liu J, Li J. Effectiveness and tolerability of aripiprazole in children and adolescents with Tourette’s disorder: a pilot study in China. J Child Adolesc Psychopharmacol 2010;20:291–8 | Inappropriate study design |
| Dalery J, Chauvin C, Maillet J, De VR. Clinical trial of tiapride in children with TICS disorder. J Neurol 1985;232:160 | Inappropriate outcomes |
| Davies LS, Stern J, Agrawal NM, Robertson M. A case series of patients with Tourette’s Syndrome in the United Kingdom treated with aripiprazole. Hum Psychopharmacol 2006;21:447–53 | Inappropriate study design |
| De Bruijn SV. Risperidone versus behaviour therapy in the treatment of tic disorders – a randomized single-blinded trial. Parkinsonism Relat Disord 2012;18(Suppl.2):S61–2 | Protocol |
| de Jonge JL, Cath DC, van Balkom AJ. Quetiapine in patients with Tourette’s disorder: an open-label, flexible-dose study. J Clin Psychiatry 2007;68:1148 | Inappropriate study design |
| Drtilkova IB. Therapeutical effects of clonidine and clonazepam in children with tick syndrome. Homeost Health Dis 1994;35:296 | Inappropriate study design |
| Dueck A, Wolters A, Wunsch K, Bohne-Suraj S, Mueller JU, Haessler F, et al. Deep brain stimulation of globus pallidus internus in a 16-year-old boy with severe tourette syndrome and mental retardation. Neuropediatrics 2009;40:239–42 | Inappropriate study design |
| Eggers C, Rothenberger A, Berghaus U. Clinical and neurobiological findings in children suffering from tic disease following treatment with tiapride. Eur Arch Psychiatry Neurol Sci 1988;237:223–9 | Inappropriate outcomes |
| Erdogan A, Karaman MG, Ozdemir E, Yurteri N, Tufan AE, Kurcer MA. Six months of treatment with risperidone may be associated with nonsignificant abnormalities of liver function tests in children and adolescents: a longitudinal, observational study from Turkey. J Child Adolesc Psychopharmacol 2010;20:407–13 | Inappropriate study design |
| Fernández-Jaén A, Fernández-Mayoralas DM, Muñoz-Jareño N, Calleja-Pérez B. An open-label, prospective study of levetiracetam in children and adolescents with Tourette syndrome. Eur J Paediatr Neurol 2009;13:541–5 | Inappropriate study design |
| Findling RL, Bukstein OG, Melmed RD, Lopez FA, Sallee FR, Arnold LE, et al. A randomized, double-blind, placebo-controlled, parallel-group study of methylphenidate transdermal system in pediatric patients with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2008;69:149–59 | Inappropriate population |
| Franklin ME, Best S, Wilson MA, Loew B, Compton SN. Habit reversal training and acceptance and commitment therapy for Tourette syndrome: a pilot project. J Dev Phys Disabil 2011;23:49–60 | Inappropriate study design |
| Fulop G, Phillips RA, Shapiro AK, Gomes JA, Shapiro E, Nordlie JW. ECG changes during haloperidol and pimozide treatment of Tourette’s disorder. Am J Psychiatry 1987;144:673–5 | Inappropriate outcomes |
| Gadow KD, Sverd J, Sprafkin J, Nolan EE, Grossman S. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Arch Gen Psychiatry 1999;56:330–6 | Inappropriate study design |
| Gilbert DL, Sallee FR, Sine L, Sethuraman G. Behavioral and hormonal effects of low-dose pergolide in children and adolescents with Gilles de la Tourette’s syndrome. Curr Ther Res Clin Exp 2000;61:378–87 | Inappropriate study design |
| Gilbert DL, Zhang J, Lipps TD, Natarajan N, Brandyberry J, Wang Z, et al. Atomoxetine treatment of ADHD in Tourette Syndrome: Reduction in motor cortex inhibition correlates with clinical improvement. Clin Neurophysiol 2007;118:1835–41 | Inappropriate study design |
| Himle JA, Fischer DJ, Van Etten ML, Janeck AS, Hanna GL. Group behavioral therapy for adolescents with tic-related and non-tic-related obsessive–compulsive disorder. Depress Anxiety 2003;17:73–7 | Inappropriate study design |
| Himle MB, Olufs E, Himle J, Tucker BTP, Woods DW. Behavior therapy for tics via videoconference delivery: an initial pilot test in children. Cogn Behav Pract 2010;17:329–37 | Inappropriate study design |
| Himle MB, Woods DW, Conelea CA, Bauer CC, Rice KA. Investigating the effects of tic suppression on premonitory urge ratings in children and adolescents with Tourette’s syndrome. Behav Res Ther 2007;45:2964–76 | Inappropriate study design |
| Ho C-S, Chen H-J, Chiu N-C, Shen E-Y, Lue H-C. Short-term sulpiride treatment of children and adolescents with Tourette syndrome or chronic tic disorder. J Formos Med Assoc 2009;108:788–93 | Inappropriate study design |
| Jankovic J. Botulinum toxin in the treatment of dystonic tics. Mov Disord 1994;9:347–9 | Inappropriate study design |
| Jankovic J, Glaze DG, Frost JD. Effect of tetrabenazine on tics and sleep of Gilles de la Tourette’s syndrome. Neurology 1984;34:688–92 | Inappropriate study design |
| Jimenez-Jimenez FJ. Nicardipine improves motor tics. Eur J Neurol 1997;4:498–501 | Inappropriate study design |
| Kaido T, Otsuki T, Kaneko Y, Takahashi A, Omori M, Okamoto T. Deep brain stimulation for Tourette syndrome: a prospective pilot study in Japan. Neuromodulation 2011;14:123–8 | Inappropriate study design |
| Katuwawela IC. Good response to clonidine in tourette syndrome associated with chromosomal translocation involving the IMMP2L gene. J Neuropsychiatry Clin Neurosci 2012;24:E17 | Inappropriate outcomes |
| Kenney CJ, Hunter CB, Mejia NI, Jankovic J. Tetrabenazine in the treatment of Tourette syndrome. J Pediatr Neurol 2007;5:9–13 | Inappropriate population |
| Kim B-N, Lee C-B, Hwang J-W, Shin M-S, Cho S-C. Effectiveness and safety of risperidone for children and adolescents with chronic tic or tourette disorders in Korea. J Child Adolesc Psychopharmacol 2005;15;318–24 | Inappropriate study design |
| Kohen DP. Coping with the stress of Tourette syndrome in children and adolescents: use of self-hypnosis techniques. Aust J Clin Exp Hypnosis 1995;23:145–57 | Inappropriate study design |
| Kuo SH, Jimenez-Shahed J. Topiramate in treatment of Tourette syndrome. Clin Neuropharmacol 2010;33:32–4 | Inappropriate study design |
| Lacruz F, Obeso JA, Martinez VE, Artieda J, Luquin R, Martinez JM. Tiapride for the treatment of Gilles de la Tourette Syndrome. Arch Neurobiol 1985:247 | Inappropriate study design |
| Law SFS, Schachar RJ. Do typical clinical doses of methylphenidate cause tics in children treated for attention-deficit hyperactivity disorder? J Am Acad Child Adolesc Psychiatry 1999;38:944–51 | Inappropriate population |
| Leckman JF, Detlor J, Harcherik DF, Young JG, Anderson GM, Shaywitz BA, et al. Acute and chronic clonidine treatment in Tourette’s syndrome: a preliminary report on clinical response and effect on plasma and urinary catecholamine metabolites, growth hormone, and blood pressure. J Am Acad Child Psychiatry 1983;22:433–40 | Inappropriate outcomes |
| Leckman JFD. Short- and long-term treatment of Tourette’s syndrome with clonidine: a clinical perspective. Neurology 1985;35:343–51 | Inappropriate study design |
| Lichter DG, Jackson LA. Predictors of clonidine response in Tourette syndrome: implications and inferences. J Child Neurol 1996;11:93–7 | Inappropriate study design |
| Lombroso PJ, Scahill L, King RA, Lynch KA, Chappell PB, Peterson BS, et al. Risperidone treatment of children and adolescents with chronic tic disorders: a preliminary report. J Am Acad Child Adolesc Psychiatry 1995;34:1147–52 | Inappropriate study design |
| Luo J. [Observation on therapeutic effects of acupuncture on gilles de la tourette syndrome.] Zhongguo Zhen Jiu 1997;17:429–30 | Inappropriate study design |
| Lyon GJ, Samar S, Jummani R, Hirsch S, Spirgel A, Goldman R, et al. Aripiprazole in children and adolescents with tourette’s disorder: an open-label safety and tolerability study. J Child Adolesc Psychopharmacol 2009;19:623–33 | Inappropriate study design |
| March JSF. Tics moderate treatment outcome with sertraline but not cognitive-behavior therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry 2007;61:344–7 | Inappropriate outcomes |
| McConville BJ, Sanberg PR, Fogelson MH, King J, Cirino P, Parker KW, et al. The effects of nicotine plus haloperidol compared to nicotine only and placebo nicotine only in reducing tic severity and frequency in Tourette’s disorder. Biol Psychiatry 1992;31:832–40 | Inappropriate study design |
| McCracken JT, Suddath R, Chang S, Thakur S, Piacentini J. Effectiveness and tolerability of open label olanzapine in children and adolescents with Tourette syndrome. J Child Adolesc Psychopharmacol 2008;18:501–8 | Inappropriate study design |
| McDougle CJG. Haloperidol addition in fluvoxamine-refractory obsessive–compulsive disorder: a double-blind, placebo-controlled study in patients with and without tics. Arch Gen Psychiatry 1994;51:302–8 | Inappropriate population |
| McKeith IG, Williams A, Nicol AR. Clonidine in Tourette syndrome. Lancet 1981;1:270–1 | Inappropriate study design |
| Merikangas JR, Merikangas KR, Kopp U, Hanin I. Blood choline and response to clonazepam and haloperidol in Tourette’s syndrome. Acta Psychiatr Scand 1985;72:395–9 | Inappropriate outcomes |
| Micheli F, Gatto M, Lekhuniec E, Mangone C, Fernandez Pardal M, Pikielny R, et al. Treatment of Tourette’s syndrome with calcium antagonists. Clin Neuropharmacol 1990;13:77–83 | Inappropriate study design |
| Miltenberger RG, Fuqua RW. A comparison of contingent vs non-contingent competing response practice in the treatment of nervous habits. J Behav Ther Exp Psychiatry 1985;16:195–200 | Inappropriate study design |
| Moriarty J, Schmitz B, Trimble MR, De Koning P. A trial of eltoprazine in the treatment of aggressive behaviours in two populations: patients with epilepsy or gilles de la tourette’s syndrome. Hum Psychopharmacol 1994;9:253–8 | Inappropriate outcomes |
| Mukaddes NM, Abali O. Quetiapine treatment of children and adolescents with tourette’s disorder. J Child Adolesc Psychopharmacol 2003;13:295–9 | Inappropriate study design |
| Müller-Vahl KR, Schneider U, Prevedel H, Theloe K, Kolbe H, Daldrup T, et al. Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: A 6-week randomized trial. J Clin Psychiatry 2003;64:459–65 | Inappropriate intervention |
| Muller-Vahl KR, Schneider U, Koblenz A, Jobges M, Kolbe H, Daldrup T, et al. Treatment of Tourette’s syndrome with Delta 9-tetrahydrocannabinol (THC): a randomized crossover trial. Pharmacopsychiatry 2002;35:57–61 | Inappropriate intervention |
| Müller-Vahl KR, Koblenz A, Jobges M, Kolbe H, Emrich HM, Schneider U. Influence of treatment of Tourette syndrome with delta9-tetrahydrocannabinol (delta9-THC) on neuropsychological performance. Pharmacopsychiatry 2001;34:19–24 | Inappropriate intervention |
| Murphy TK, Bengtson MA, Soto O, Edge PJ, Sajid MW, Shapira N, Yang M. Case series on the use of aripiprazole for Tourette syndrome. Int J Neuropsychopharmacol 2005;8:489–90 | Inappropriate study design |
| Murphy TK, Mutch J, Reid JM, Edge PJ, Storch EA, Bengtson M, et al. Open label aripiprazole in the treatment of youth with tic disorders. J Child Adolesc Psychopharmacol 2009;19:441–7 | Inappropriate study design |
| Niederhofer H, Staffen W, Mair A. A placebo-controlled study of lofexidine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. J Psychopharmacol 2003;17:113–19 | Paper retracted |
| Nolan EE, Gadow KD, Sprafkin S. Stimulant medication withdrawal during long-term therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Pediatrics 1999;103:730–7 | Inappropriate study design |
| O’Connor K, Gareau D, Borgeat F. A comparison of a behavioural and a cognitive–behavioural approach to the management of chronic tic disorders. Clin Psychol Psychother 1997;4:15–17 | Inappropriate study design |
| O’Connor KP, Laverdure A, Taillon A, Stip E, Borgeat F, Lavoie M. Cognitive behavioral management of Tourette’s syndrome and chronic tic disorder in medicated and unmedicated samples. Behav Res Ther 2009;47:1090–5 | Inappropriate population |
| O’Connor KP, Brault M, Robillard S, Loiselle J, Borgeat F, Stip E. Evaluation of a cognitive–behavioural program for the management of chronic tic and habit disorders. Behav Res Ther 2001;39:667–81 | Inappropriate study design |
| Ondo WG, Jong D, Davis A. Comparison of weight gain in treatments for Tourette syndrome: tetrabenazine versus neuroleptic drugs. J Child Neurol 2008;23:435–7 | Inappropriate study design |
| Peterson AL, Azrin NH. An evaluation of behavioral treatments for Tourette syndrome. Behav Res Ther 1992;30:167–74 | Inappropriate study design |
| Porta MB. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology 2009;73:1375–80 | Inappropriate study design |
| Pringsheim T, Pearce M. Complications of antipsychotic therapy in children with tourette syndrome. Pediatr Neurol 2010;43:17–20 | Inappropriate study design |
| Ramos-Rios R, Gago-Ageitos AM, Vidal-Millares M, Mazaira-Castro JA, Dominguez-Santos MD. Clinical effects and tolerability of aripiprazole in children and adolescents with psychiatric disorders. Eur Neuropsychopharmacol 2009;19:S691 | Inappropriate study design |
| Rasmusson AM, Anderson GM, Lynch KA, McSwiggan HM, Scahill LD, Mazure CM, et al. A preliminary study of tryptophan depletion on tics, obsessive–compulsive symptoms, and mood in Tourette’s syndrome. Biol Psychiatry 1997;41:117–21 | Inappropriate study design |
| Riddle MA, Hardin MT, King R, Scahill L, Woolston JL. Fluoxetine treatment of children and adolescents with Tourette’s and obsessive compulsive disorders: Preliminary clinical experience. J Am Acad Child Adolesc Psychiatry 1990;29:45–8 | Inappropriate study design |
| Saccomani LR. Combined treatment with haloperidol and trazodone in patients with tic disorders. J Child Adolesc Psychopharmacol 2000;10:307–10 | Inappropriate study design |
| Sallee FR, Miceli JJ, Tensfeldt T, Robarge L, Wilner K, Patel NC, et al. Single-dose pharmacokinetics and safety of ziprasidone in children and adolescents. J Am Acad Child Adolesc Psychiatry 2006;45:720–8 | Inappropriate outcomes |
| Sallee FR, Sethuraman G, Rock CM. Effects of pimozide on cognition in children with Tourette syndrome: Interaction with comorbid attention deficit hyperactivity disorder. Acta Psychiatr Scand 1994;90:4–9 | Inappropriate study design |
| Sandor P, Stephens RT. Risperidone treatment of aggressive behavior in children with Tourette syndrome. J Clin Psychopharmacol 2000;20:710–12 | Inappropriate study design |
| Sehgal N. Short-term versus longer term pimozide therapy in Tourette’s syndrome: a preliminary study. Neurology 1999;52:874–7 | Inappropriate intervention |
| Semerci BS. Case series on the use of aripiprazole for tic disorder. Eur Neuropsychopharmacol 2008;18:S432 | Inappropriate study design |
| Seo WS, Sung H-M, Sea HS, Bai DS. Aripiprazole treatment of children and adolescents with Tourette disorder or chronic tic disorder. J Child Adolesc Psychopharmacol 2008;18:197–205 | Inappropriate study design |
| Shapiro AK, Shapiro E, Eisenkraft GJ. Treatment of Gilles de la Tourette syndrome with pimozide. Am J Psychiatry 1983;140:1183–6 | Inappropriate study design |
| Song DH. Aripiprazole valuable in pediatric tic disorder or Tourette syndrome. Pharm Ther 2006;31:727–8 | Inappropriate study design |
| Spencer T, Biederman J, Steingard R, Wilens T. Bupropion exacerbates tics in children with attention-deficit hyperactivity disorder and Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 1993;32:211–14 | Inappropriate study design |
| Stamenkovic M, Schindler SD, Aschauer HN, Kasper S. Olanzapine in Gilles de la Tourette Syndrome patients. Paris: 11th European College of Neuropsychopharmacology Congress; 1998 | Inappropriate study design |
| Stamenkovic M, Schindler SD, Aschauer HN, de Zwaan M, Willinger U, Resinger E, et al. Effective open-label treatment of Tourette’s disorder with olanzapine. Int Clin Psychopharmacol 2000;15:23–8 | Inappropriate study design |
| Steingard R, Biederman J, Spencer T, Wilens T, Gonzalez A. Comparison of clonidine response in the treatment of attention-deficit hyperactivity disorder with and without comorbid tic disorders. J Am Acad Child Adolesc Psychiatry 1993;32:350–3 | Inappropriate study design |
| Stephens RJ, Bassel C, Sandor P. Olanzapine in the treatment of aggression and tics in children with Tourette’s syndrome – a pilot study. J Child Adolesc Psychopharmacol 2004;14:255–66 | Inappropriate study design |
| Storch EA, Morgan JE, Caporino NE, Brauer L, Lewin AB, Piacentini J, et al. Psychosocial treatment improved resilience and reduce impairment in youth with tics: an intervention case series of eight youth. [References]. J Cogn Psychother 2012;26:56–70 | Inappropriate study design |
| Sverd J, Cohen S, Camp JA. Brief report: Effects of propranolol in Tourette syndrome. J Autism Dev Disord 1983;13:207–13 | Inappropriate study design |
| Sverd J, Gadow KD, Paolicelli LM. Methylphenidate treatment of attention-deficit hyperactivity disorder in boys with Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 1989;28:574–9 | Inappropriate study design |
| Toren PL. Ondansetron treatment in patients with Tourette’s syndrome. Int Clin Psychopharmacol 1999;14:373–6 | Inappropriate study design |
| van der Linden C, Bruggeman R, van Woerkom TC. Serotonin-dopamine antagonist and Gilles de la Tourette’s syndrome: an open pilot dose-titration study with risperidone. Mov Disord 1994;9:687–8 | Inappropriate study design |
| Verdellen CW, Hoogduin CA, Keijsers GP, Verdellen CWJ, Hoogduin CAL, Keijsers GPJ. Tic suppression in the treatment of Tourette’s syndrome with exposure therapy: the rebound phenomenon reconsidered. Mov Disord 2007;22:1601–6 | Inappropriate study design |
| Werry JS, Beiderman J, Thisted R, Greenhill L, Ryan N. Resolved: cardiac arrhythmias make desipramine an unacceptable choice in children. J Am Acad Child Adolesc Psychiatry 1995;34:1239–48 | Inappropriate population |
| Wetterneck CT, Woods DW. An evaluation of the effectiveness of exposure and response prevention on repetitive behaviors associated with Tourette’s syndrome. J Appl Behav Anal 2006;39:441–4 | Inappropriate study design |
| Woods DW, Twohig MP. Using habit reversal to treat chronic vocal tic disorder in children. Behav Interv 2002;17:159–68 | Inappropriate study design |
| Woods DW, Himle MB, Miltenberger RG, Carr JE, Osmon DC, Karsten AM, et al. Durability, negative impact, and neuropsychological predictors of tic suppression in children with chronic tic disorder. J Abnorm Child Psychol 2008;36:237–45 | Inappropriate outcomes |
| Woods DW, Walther MR, Bauer CC, Kemp JJ, Conelea CA. Controlled evaluation of an educational intervention used to modify peer attitudes and behavior toward persons with Tourette’s syndrome. Behav Mod 2005;29:900–12 | Inappropriate study design |
| Wu M, Xiao G-H, Yao M, Zhang J-M, Zhang X, Zhou Y-B, et al. The development of stimulus control over tics: A potential explanation for contextually-based variability in the symptoms of Tourette syndrome. Behav Res Ther 2009;47:41–7 | Inappropriate study design |
| Wu MX. Multicenter clinical study on the treatment of children’s tic disorder with Qufeng Zhidong Recipe. Chin J Integr Med 2009;15:254–60 | Inappropriate intervention |
| Wu MX. Clinical research into Qufeng Zhidong recipe used to treat 31 children with tic disorder. J Trad Chin Med 2010;30:163–70 | Inappropriate intervention |
| Xiang S-J, Cai Y-H, Zhang Z-D. [Observation on therapeutic effect of tic disorders treated with local acupuncture.] Zhongguo Zhen Jiu 2010;30:469–72 | Inappropriate study design |
| Yoo HK, Choi S-H, Park S, Wang H-R, Hong J-P, Kim C-Y. An open-label study of the efficacy and tolerability of aripiprazole for children and adolescents with tic disorders. J Clin Psychiatry 2007;68:1088–93 | Inappropriate study design |
| Young JG, Cohen DJ, Hattox SE, Kavanagh ME, Erson GM, Shaywitz BA, et al. Plasma free MHPG and neuroendocrine responses to challenge doses of clonidine in Tourette’s syndrome: preliminary report. Life Sci 1981;29:1467–75 | Inappropriate outcomes |
| Zhao LL, Li AY, Lv H, Liu FY, Qi FH. Traditional Chinese medicine ningdong granule: the beneficial effects in tourette’s disorder. J IntMed Res 2010;38:169–75 | Inappropriate intervention |
| Qualitative systematic review | |
| Bastiaens L. Pediatric psychopharmacology in a capitated managed care system: how do patients fare? J Child Adolesc Psychopharmacol 1998;8:115–24 | Inappropriate population |
| Christie D. ‘Oh no he doesn’t!’, ‘Oh yes he does!’: comparing parent and teacher perceptions in Tourette’s syndrome. Clinical Child Psychology and Psychiatry 2002;7:553–8 | Did not examine experience of intervention, access to care or service quality |
| Cutler D, Murphy T, Gilmour J, Heyman I. The quality of life of young people with Tourette syndrome. Child Care Health Dev 2009;35:496–504 | Did not examine experience of intervention, access to care or service quality |
| De Lange N, Meyer LW, Olivier MAJ. Tourette’s Syndrome: isn’t that the foul mouth disease? Early Child Dev Care 2003;173:613–23 | Did not examine experience of intervention, access to care or service quality |
| Hansen BHS. Comparison of sleep problems in children with anxiety and attention deficit/hyperactivity disorders. Eur Child Adolesc Psychiatry 2011;20:321–30 | Inappropriate population |
| Kompoliti K, Fan W, Leurgans S, Kompoliti K, Fan W, Leurgans S. Complementary and alternative medicine use in Gilles de la Tourette syndrome. Mov Disord 2009;24:2015–19 | Did not examine experience of intervention, access to care or service quality |
| Lewin AB, Storch EA, Storch HD. Risks from antipsychotic medications in children and adolescents. JAMA 2010;303:729–30; author reply 30–1 | Did not examine experience of intervention, access to care or service quality |
| Silva RR, Munoz DM, Barickman J, Friedhoff AJ. Environmental factors and related fluctuation of symptoms in children and adolescents with Tourette’s disorder. J Child Psychol Psychiatry 1995;36:305–12 | Did not examine experience of intervention, access to care or service quality |
| Wigley K, Mason A, Lambert S, Collins J, Lask B, Christie D. A specialist service for children and adolescents with Tourette’s syndrome: problems and attempted solutions. Clin Child Psychol Psychiatry 2000;5:247–57 | Did not examine experience of intervention, access to care or service quality |
| Wilkinson BJ, Marshall RM, Curtwright B. Impact of Tourette’s disorder on parent reported stress. J Child Fam Stud 2008;17:582–98 | Did not examine experience of intervention, access to care or service quality |
Appendix 7 Qualitative study of Experiences of Services and Treatment interviewee identifying codes and characteristics
| Co-occurring conditions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participant identification code | Sex | Age (years) | Has taken medication for tics | Has received some form of behavioural intervention for tics | Other treatment/intervention for tics | ADHD | OCD | ASD | Anxiety | Other |
| 1 | Male | 14 | Yes | No | Cognitive–behavioural therapy; relaxation training | No | Yes | No | No | No |
| 2 | Male | 11 | Yes | Yes | No | No | No | No | No | |
| 3 | Male | 12 | No | No | No | Yes | No | Yes | No | |
| 4 | Male | 17 | No | No | Yes | No | No | No | No | |
| 5 | Male | 15 | Yes | Yes | No | No | No | No | No | |
| 6 | Female | 14 | Yes | Yes | No | No | No | No | No | |
| 7 | Female | 11 | Yes | No | No | Yes | No | No | No | |
| 8 | Male | 17 | Yes | No | No | Yes | Yes | No | Yes | |
| 9 | Female | 17 | Yes | No | No | No | No | No | No | |
| 10 | Female | 17 | Yes | No | Massage | No | No | Yes | No | No |
| 11 | Male | 15 | Yes | No | Relaxation training | Yes | No | No | Yes | No |
| 12 | Male | 17 | Yes | No | Yes | No | No | Yes | No | |
| 13 | Male | 13 | No | No | Psychotherapy | No | No | No | No | No |
| 14 | Male | 11 | Yes | No | No | No | No | No | No | |
| 15 | Female | 13 | Yes | No | No | No | No | No | Yes | |
| 16 | Male | 13 | No | No | No | No | No | No | No | |
| 17 | Male | 10 | No | No | Auditory listening programme | No | No | Yes | No | Yes |
| 18 | Male | 16 | Yes | Yes | No | No | No | No | No | |
| 19 | Male | 12 | Yes | No | Relaxation training | No | No | No | No | No |
| 20 | Male | 12 | No | No | No | No | No | No | No | |
| 21 | Male | 11 | No | No | Yes | No | No | No | No | |
| 22 | Male | 16 | Yes | No | No | Yes | No | Yes | No | |
| 23 | Male | 13 | No | No | No | Yes | No | No | No | |
| 24 | Female | 13 | Yes | No | Yes | No | No | No | Yes | |
| 25 | Male | 13 | No | No | No | No | No | No | No | |
| 26 | Male | 11 | No | No | No | No | No | No | No | |
| 27 | Male | 15 | Yes | No | Anger management | No | No | No | No | No |
| 28 | Male | 13 | No | No | No | No | No | No | No | |
| 29 | Male | 16 | Yes | No | Relaxation training | No | No | No | No | No |
| 30 | Male | 12 | Yes | No | No | No | No | No | No | |
| 31 | Male | 12 | Yes | Yes | No | No | No | No | No | |
| 32 | Male | 15 | No | No | No | No | No | No | No | |
| 33 | Male | 10 | No | No | No | Yes | No | No | No | |
| 34 | Female | 11 | No | No | No | No | No | No | No | |
| 35 | Female | 12 | No | No | Play therapy; cognitive–behavioural therapy; counselling | No | No | No | No | No |
| 36 | Female | 13 | No | No | No | No | No | No | No | |
| 37 | Male | 13 | No | No | Training to suppress tics (not habit reversal) | Yes | No | No | No | No |
| 38 | Male | 17 | Yes | Yes | Yes | No | No | No | No | |
| 39 | Male | 14 | Yes | Yes | No | No | No | No | No | |
| 40 | Male | 11 | No | No | Yes | No | Yes | No | No | |
Appendix 8 Qualitative study of Experiences of Services and Treatment interview schedule
Introduction
This interview is about you and what you think about different treatments for Tourette syndrome. Take your time to think about and answer the questions. Some of the questions are about personal things like your friendships and your feelings. You don’t have to answer a question if you don’t want to. I am audio (sound) recording this interview, but I will not put your name on the recording or the written summary of this project. Do you have any questions before we start? Would you like to start the interview?
Background information
-
How old are you?
-
Do you go to school/college at the moment? (If yes, which year are you in? Have you got a favourite subject? How are you finding school/college?)
About having TS and tics
I am just going to ask you a little bit about having TS and tics.
-
Can you tell me a bit about your tics? (prompts for vocal tics: for example, sniffing, coughing, grunting, words, phrases. If no vocal tics at the moment, in the past? Prompts for motor tics: for example, eye blinking, head jerk, shoulder shrugs, leg/feet or arm/hand movements)
-
Have the tics affected you in some way? (If yes, how have tics affected you? Which areas of your life are most affected? [prompts: family life/home, school, friendships, leisure activities/hobbies])
-
What is it about tics that you find most troublesome? (prompts: urges, unwanted movements or sounds, other people’s reactions, effort to suppress tics, physical pain or fatigue).
-
How long have you been aware that you have tics?
-
When did you first realise that you had TS? (as diagnosis)
-
What sorts of things make your tics better? (prompts: specific activities, how you are feeling, time of day/year)?
-
What sorts of things make your tics worse? (prompts: specific classes, specific topic, teacher, other pupils, time, why?)
About treatment for TS and tics
-
Who do you see about your treatment for TS? (prompts: doctor, psychologist, nurse, etc.)
-
Has anyone explained to you or given you information about how best to manage/control your tics? (If yes, who has explained or given you this information? How did you find this information? Was it helpful? Did you do anything differently? Can you give me an example?)
-
Can you tell me what treatment you have had for your TS/tics? (prompts for medicationa; any other treatment/intervention?; have you taken or done something else to help with your TS/tics?)
If participant has taken medication:
-
Can you remember the names of any medication you have taken for TS? (prompts for medicationa)
-
How many times a day did you take the medication?
-
Do you remember the dose?
-
For how long did you take the medication? (If stopped taking medication, why was it stopped? Whose decision was it?)
For any treatment for tics reported (including medication/behavioural interventions/other treatments):
-
How do you feel about that treatment? (prompts: how well do you feel your treatment works/worked for you?; Did the treatment/medicine helped? If yes, in what sense?; Did it become more helpful/less helpful with time?; Was there something that you didn’t like of your treatment/medication)
-
How easy is it to follow your treatment advice (or what the doctor/psychologist/nurse ask you to do for your tics)? (prompts: are there times you’re not able to follow advice, for example not take tablets, not practice behavioural interventions?; if yes, how often would you say you don’t follow your treatment [for example, miss doses]?; what makes it hard for you to follow your treatment?)
-
Have you received information about your treatment? Has someone explained to you what it was about, how does it work? (If yes, was the information sufficient/informative)
-
Who helps you with your treatment for TS/tics? (prompts: role of parents, school, friends and health professionals)
-
-
Have you heard of any treatments/other treatments for TS/tics? (If yes, what have you heard?)
If participant has not heard of medication for tics—describe this treatment using the guide that is shown below; if participant has not heard of behavioural interventions for tics—describe this treatment using the guide that is shown below.
Before providing treatment description(s):
Now I would like to give you a short description of a treatment to see what you think about it OK?; it doesn’t mean that the treatment is better than others or that I think that you or others should try it—I only would like to know what do you think about it.
After providing treatment description(s):
-
How do you feel about that treatment?
-
Is there anything that would make that treatment difficult for you?
-
| Guide to describe medication for tics | Guide to describe behavioural interventions for tics |
|---|---|
Sometimes medication is given to help with tics. The medication can help:
Children who take medication for tics often need to:
|
Sometimes behavioural interventions are given to help with tics. Behavioural interventions can help to:
|
| Medicines may have other effects, which may make children/young people feel drowsy, sleepy or dizzy | Family members are often involved to support the child and help them to know that they are making progress with the therapy by practising their strategies/exercises. Children/young people who are in behaviour therapy often need to: meet with a doctor (or psychologist/nurse) a number of times to practice/learn the treatment, sometimes weekly and for a couple of months; practice the strategies/exercises at home, often with the help of the parent/carer |
About expectations of treatment for TS and tics
Now I would like to ask you about what you would hope/like a treatment for TS/tics to do.
-
What would you most like a treatment for tics to achieve or make different? (prompts: reduce or eliminate tics; give you better control over tics; feel less bothered/embarrassed by tics; make other people understand tics better; have someone to talk to who understands your tics and what it’s like) Why would you like that?
-
Is there something else you would like or hope a treatment for tics to do?
-
How well has the treatment you’ve received met these goals or things you would like from treatment?
Would you like to add something else?
Background information (final questions)
-
I am just going to finish with a couple of questions about you.
-
Household members (who do you live with?).
-
If siblings – where do you come in the family?
-
Does anyone else in the family have TS or tics?
-
Depending on family composition – Does your mum/dad/carer work? (If yes, do you know what his/her job is?)
Prompts for medication
Clonidine.
Risperidone.
Aripiprazole.
Sulpiride.
Haloperidol.
Olanzapine.
Quetiapine.
Pimozide.
Clonazepam.
Lorazepam.
Appendix 9 Demographic characteristics
| Characteristic | n | % | Mean | SD |
|---|---|---|---|---|
| Age (years) | 256 | 44.0 | 6.3 | |
| Relationship to the child | ||||
| Mother | 237 | 92.2 | ||
| Father | 18 | 7.0 | ||
| Grandparent | 1 | 0.4 | ||
| Other | 1 | 0.4 | ||
| Marital status | ||||
| Married/cohabitating | 201 | 78.2 | ||
| Divorced/separated | 37 | 14.4 | ||
| Single (never married) | 17 | 6.6 | ||
| Widowed | 2 | 0.8 | ||
| Highest level of education | ||||
| Did not complete secondary school/compulsory education | 7 | 2.7 | ||
| Secondary school | 58 | 22.7 | ||
| Further education (e.g. A-level) | 85 | 33.2 | ||
| Undergraduate | 58 | 22.7 | ||
| Postgraduate | 48 | 18.8 | ||
| Characteristic | n | % | Mean | SD |
|---|---|---|---|---|
| Age (years) | 295 | 12.4 | 3.0 | |
| Age of tic onset (years) | 293 | 5.9 | 2.8 | |
| Age at diagnosis of TS (years) | 273 | 9.1 | 2.7 | |
| YGTSS tic-related impairment score | 291 | 2.3 | 1.5 | |
| Sex | ||||
| Male | 234 | 79.3 | ||
| Female | 61 | 20.7 | ||
| Co-occurring conditions | ||||
| OCD | 106 | 35.9 | ||
| ADHD | 89 | 30.2 | ||
| Anxiety | 73 | 24.7 | ||
| ASD | 60 | 20.3 | ||
| Depression | 32 | 10.8 | ||
| Learning disability | 29 | 9.8 | ||
| Dyspraxia | 17 | 5.8 | ||
| Dyslexia | 16 | 5.4 | ||
| Epilepsy | 7 | 2.4 | ||
| Other | 14 | 4.7 | ||
Glossary
- PR
- The interval between the beginning of the P wave and the beginning of the QRS complex of the electrocardiogram.
- QRS
- The QRS complex is a series of three deflections (Q, R and S waves) in an electrocardiogram that represent electrical activity generated by ventricular depolarisation prior to contraction of the ventricles.
- QT
- The interval between the start of the Q wave and the end of the T wave in the electrocardiogram (ECG).
- QTC
- QT corrected for heart rate.
List of abbreviations
- AC
- anger control
- ADHD
- attention deficit hyperactivity disorder
- AEI
- Australian Education Index
- AIMS
- Abnormal Involuntary Movement Scale
- ASD
- autism spectrum disorder
- ASSIA
- Applied Social Sciences Index and Abstracts
- BCI
- BIOSIS Citation Index
- BP
- blood pressure
- BREI
- British Education Index
- C-GAS
- Children’s Global Assessment Scale
- CAMHS
- child and adolescent mental health services
- CBCL
- Child Behaviour Checklist
- CBIT
- comprehensive behavioural intervention for tics
- CDI
- Child Depression Inventory
- CDI-S
- Child Depression Inventory – short version
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CGB
- caregiver burden
- CGI
- Clinical Global Impressions
- CGI-I
- Clinical Global Impressions – Improvement
- CGI-Tic/Neuro-S
- Clinical Global Impressions – severity of tics and other neurological symptoms
- CGI-TS
- Clinical Global Impressions – Tic Severity
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CTD
- chronic tic disorder
- DARE
- Database of Abstracts of Reviews of Effectiveness
- DBS
- deep brain stimulation
- ECG
- electrocardiogram
- EPS
- extrapyramidal symptoms
- ERIC
- Education Resources in Curriculum
- ERP
- exposure and response prevention
- ESRS
- Extrapyramidal Symptom Rating Scale
- FDA
- Food and Drug Administration
- GABA
- gamma-aminobutyric acid
- GABHS
- group A beta-haemolytic streptococcus
- GP
- general practitioner
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- GWAS
- Genome Wide Association Study
- HAM-D
- Hamilton Rating Scale for Depression
- HMIC
- Health Management Information Consortium
- HRT
- habit reversal training
- HTA
- Health Technology Assessment
- i.v.
- intravenous
- IBSS
- International Bibliography of Social Science
- IPD
- individual patient data
- IQ
- intelligence quotient
- MASC
- Multidimensional Anxiety Scale for Children
- MD
- mean difference
- MDD
- major depressive disorder
- NCCMH
- National Collaborating Centre for Mental Health
- NICE
- National Institute for Health and Care Excellence
- NIMH
- National Institute for Mental Health
- OCB
- obsessive–compulsive behaviour
- OCD
- obsessive–compulsive disorder
- OCS
- obsessive–compulsive symptom
- PANDAS
- Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection
- PC
- personal computer
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QoL
- quality of life
- QuEST
- Qualitative study of Experiences of Services and Treatment
- RCT
- randomised controlled trial
- RR
- risk ratio
- rTMS
- repetitive transcranial magnetic stimulation
- SD
- standard deviation
- SMD
- standardised mean difference
- SP
- supportive psychotherapy
- SSA
- Social Services Abstracts
- SSCI
- Social Sciences Citation Index
- SSEC
- Stimulant Side Effects Checklist
- SSRI
- selective serotonin reuptake inhibitor
- STESS
- Subjective Treatment Emergent Symptom Scale
- TA
- Tourettes Action
- TAU
- treatment as usual
- TCA
- tricyclic antidepressant
- TEG
- Tourette Expert Group
- TODS-CR
- Tourette’s Disorder Scale – Clinician Rated
- TS
- Tourette syndrome
- TSGS
- Tourette’s Syndrome Global Scale
- TSSL
- Tourette Syndrome Symptom List
- TSSL-C
- Tourette Syndrome Symptom List – Child’s report
- TSSS
- Tourette Syndrome Severity Scale
- UTRS
- Unified Tic Rating Scale
- YGTSS
- Yale Global Tic Severity Scale