Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 13/46/01. The protocol was agreed in June 2014. The assessment report began editorial review in January 2015 and was accepted for publication in April 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Lesley Kay has received sponsorship to attend meetings by AbbVie and Merck Sharp & Dohme Limited in 2014. Helena Marzo-Ortega has received grants, sponsorship and/or honoraria from AbbVie, Janssen, Merck Sharp & Dohme Limited, Pfizer and UCB.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Corbett et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Spondyloarthritis (SpA) encompasses a heterogeneous group of inflammatory rheumatologic diseases including ankylosing spondylitis (AS), reactive arthritis, psoriatic arthritis, inflammatory bowel disease-related arthritis and undifferentiated SpA. 1 SpA can be categorised as having predominantly axial (sacroiliac joints or spine) or peripheral involvement. In people with axial spondyloarthritis (axSpA), the predominant symptom is back pain (due to inflammation of the sacroiliac joints, the spine, or both) but there may also be extra-articular and peripheral joint manifestations.
In practice, and in clinical trials, AS is commonly diagnosed using the modified New York criteria (Box 1); sometimes in practice radiography may not be performed routinely (because of the radiation doses involved) or magnetic resonance imaging (MRI) may be preferred as a diagnostic tool. The recently developed Assessment of SpondyloArthritis International (ASAS) Society classification criteria encompass a broad range of patients with axSpA, including patients with AS and patients with non-radiographic axial spondyloarthritis (nr-AxSpA). 3 All axSpA patients will have developed chronic back pain (≥ 3 months) before age 45 years. Classifications can be made using the imaging or clinical arms of the criteria. The imaging arm requires evidence of joint damage (erosions or fusion) due to sacroiliitis, using either radiography (when the disease is classified as AS) or MRI (when the disease is classified as nr-AxSpA);4 additionally, at least one of the following SpA features is also required: inflammatory back pain, arthritis, enthesitis (heel), uveitis, dactylitis, psoriasis, Crohn’s/colitis, good response to non-steroidal anti-inflammatory drugs (NSAIDs), family history of SpA, human leucocyte antigen (HLA)-B27 genetic marker, and elevated C-reactive protein (CRP). People with axSpA often have the genetic marker HLA-B27. To be classified as having axSpA via the clinical arm of the criteria, patients must be HLA-B27 positive and also have at least three of the aforementioned SpA features.
-
Low back pain and stiffness for more than 3 months that improves with exercise but is not relieved by rest.
-
Limitation of motion of the lumbar spine in the sagittal and frontal planes.
-
Limitation of chest expansion relative to normal values correlated for age and sex.
-
Sacroiliitis grade > 2 bilaterally or grade 3–4 unilaterally.
-
Definite AS if the radiological criterion is associated with at least one clinical criterion.
The use of MRI allows for earlier detection of axSpA, as joint damage may not become evident on radiography for many years. Patients with nr-AxSpA may, or may not, have signs of sacroiliac joint inflammation on a magnetic resonance image. There may be other objective signs of inflammation such as an abnormally raised erythrocyte sedimentation rate (ESR) or CRP level, although these are less sensitive and specific for AS. A MRI diagnosis may therefore provide the opportunity for treatment to reduce the possibility of long-term structural damage (and associated burden of symptoms). 5 However, there is some concern that the diagnostic criteria for nr-AxSpA may be too liberal and may include patients who do not have axSpA and will never progress to AS, particularly with respect to patients who are diagnosed without evidence of imaging (MRI) changes. 6–8 The differences between AS and nr-AxSpA are explored further in Chapter 3.
Prognosis
Axial spondyloarthritis is a painful, progressive form of inflammatory arthritis. It mainly affects the spine but can also affect other joints, tendons and ligaments. Other areas such as the eyes and bowel can also sometimes be involved in non-radiographic and radiographic (AS) forms of axSpA. 9 The pain and stiffness of axSpA adversely affects optimal daily functioning. These symptoms are a result of a combination of reversible components of the disease, such as inflammation and flares, and irreversible components, such as syndesmophytes and vertebral bridging (bony deposition). 10 Most patients with AS develop the first symptoms at 25–45 years of age. 11 Progression of the disease is variable and difficult to predict. 12 There is often a delay of many years between patients first noticing symptoms and the diagnosis of axSpA being received. Many people with axSpA have AS, with evidence of bony deposition as well as inflammation. In later-stage AS, joints and bones may fuse together, a process that can occur over a long period of time and cause restricted movement. The functional impairment because of inflammation and/or bony deposition can have a profound effect on health and quality of life, and lead to withdrawal from active employment, with resultant adverse financial consequences; the burden of disease is greater in more socially deprived patients. 13 The prognosis is poor, although there is some evidence that deterioration plateaus in well-established AS. 14 Paradoxically, early disease (nr-AxSpA) may be less readily diagnosed and patients offered fewer treatment options even though it can be as, or even more, debilitating that established AS. 15
Ankylosing spondylitis is associated with an increased risk of death; it is estimated that patients have a standardised mortality ratio (SMR) of ≥ 1.5. The increased risk appears to be greater in men, with one study reporting a statistically significant increase in SMR of 1.63 in men but no significant increase in women (SMR 1.38) with AS. 16 This study found that, after correcting for age, sex, disease duration and pre-existing cardiovascular disease, independent predictors of increased mortality were elevated CRP level, diagnostic delay, not using NSAIDs and work disability. According to British Society for Rheumatology (BSR) guidelines, the excess mortality is mainly accounted for by cardiac valvular disease, amyloidosis and fractures. 17 nr-AxSpA affects approximately equal numbers of men and women, but it is more likely that men will develop AS. 18
Epidemiology
Currently, only limited epidemiological data are available for axSpA defined according to ASAS criteria. For AS, the prevalence is thought to be around 0.25% in European populations. 19 It is around three times more common in men than in women. 20 A recent study published in the USA reported an estimated AS prevalence of 0.52–0.55%, and the prevalence of axSpA as approximately 1.0–1.4%. 21 The proportion of nr-AxSpA among patients with axSpA is estimated to be between 20% and 80%. 22 Each year in the UK an estimated 2% of patients in a general practice will present with back pain and up to 5% of these will show features of AS. 23
Measurement of disease
There are a number of components and measures of disease activity in axSpA;24 a patient’s health-related quality of life (HRQoL) is determined by both by physical functioning and by disease activity. In turn, physical function is determined by spinal mobility and disease activity, and spinal mobility is determined by structural damage and inflammation of the spine. 24 In nr-AxSpA, a patient may have significant inflammation but no detectable structural damage; in AS, a patient may have both significant inflammation and structural damage; and in late AS, there may be less inflammation but extensive structural damage.
The main tools used for the assessment of various components of the disease are listed in Table 1.
| Assessment measures | ||
|---|---|---|
| Tool | Disease component | Description |
| BASDAI | Disease activity | Consists of a 1–10 scale (1 being no problem and 10 being the worst problem) which is used to answer six questions pertaining to the five major symptoms of AS:
|
| BASFI | Functional ability | Patient assesses difficulty on a 10-point scale (1 is easy and 10 is impossible) for each of 10 items:
|
| BASMI | Disease activity, spinal mobility | Clinician assessment of cervical rotation, tragus-to-wall distance, lumbar side flexion, modified Schober’s, intermalleolar distance |
| ASDAS | Disease activity | Calculated from BASDAI questions on spinal pain, peripheral arthritis and duration of morning stiffness, patients global assessment of disease activity and CRP level (or ESR if CRP level not available) |
| mSASSS | Structural damage | In the mSASSS the anterior vertebral corners of the cervical (lower border of C2 to upper border of T1) and lumbar (lower border of T12 to upper border of S1) segments (a total of 24 VCs) are scored at a lateral view, for the presence of erosion and/or sclerosis and/or squaring (1 point), syndesmophyte (2 points) and bridging syndesmophyte (3 points). The total score ranges from 0 to 72. The mSASSS has shown better reliability and sensitivity to change than other radiographic scoring methods25 |
| MRI assessments | ||
| Measures of response | ||
| BASDAI 50 | Response criterion | ≥ 50% improvement in BASDAI |
| ASAS 20 | Response criterion | ≥ 20% improvement and ≥ 1 unit absolute improvement (range 1–10) in three of four domains with no worsening of ≥ 20% improvement and ≥ 1 unit absolute in the fourth domain: BASFI, spinal pain, patient GDA and inflammation (BASDAI Q5 and 6) |
| ASAS 40 | Response criterion | ≥ 40% improvement and ≥ 2 units absolute improvement (range 1–10) in three of four domains with no worsening at all in the fourth domain: BASFI, spinal pain, patient GDA and inflammation (BASDAI Q5 and 6) |
| ASAS partial remission | Response criterion | A value of ≥ 2 units absolute improvement (range 1–10) in each of four domains: ASFI, spinal pain, patient GDA and inflammation (BASDAI Q5 and 6) |
| ASAS 5/6 | Response criterion | Improvement in five out of six domains (using pre-defined % improvements) without deterioration in the sixth domain: pain, patient global assessment, function, inflammation, spinal mobility, CRP level |
| ASDAS major improvement | Response criterion | ≥ 2 units improvement in ASDAS |
Placebo response
The term ‘placebo effect’ can be used to describe different types of ‘effect’ but it generally encompasses one or more of three different meanings. First, there is the temporal (before–after) change after placebo medication, in which the effects of a placebo intervention cannot be distinguished from the natural course of the disease or regression to the mean. Second, there is the causal effect of placebo intervention associated with the treatment ritual, and, finally, there is the effect of all the psychological processes involved in the interaction between doctor and patient. 26 For the placebo-controlled trials in AS and nr-AxSpA these non-pharmacological components can be assumed to act equally in the anti-tumour necrosis factor (TNF) and placebo arms. Results from the placebo arms measure the non-pharmacological effects and the difference between the anti-TNF and placebo arms measures the pharmacological effect. All three components of the placebo effect could be important to consider when evaluating trials in this assessment, although once the trial treatment periods have ended, it is likely that the effect of the natural course of the disease becomes the most important factor of any ‘placebo’ effect. Estimated cost-effectiveness ratios and associated policy decisions may be sensitive to assumptions regarding the mechanism underlying placebo responses. 27
The natural course of disease activity in AS is known to vary over time with exacerbations, or flares, being common. In a study of flares in patients with AS, clinically relevant changes in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI; but not in function) were noted during minor/localised flares (which occurred in 59% of patients in any given week). Although major/generalised flares were less common (reported in 12% of patients in any given week) they were associated with clinically relevant changes in both disease activity and function. 28 Pain is a key component of BASDAI and the ASAS responder outcomes; a Cochrane systematic review of placebos for all clinical conditions found that placebo interventions can influence patient-reported outcomes, especially pain (and nausea). 29 The authors also concluded that it was difficult to distinguish patient-reported effects of placebo from biased reporting, and that the effect on pain varied from negligible to clinically important, even among trials with low risk of bias.
Current service provision
Management of disease
Short- and long-term treatment goals for axSpA include minimising pain and stiffness, maintaining function and posture, arresting disease progression and maintaining quality of life and ability to work. Current conventional therapy for axSpA includes acute anti-inflammatory treatment with NSAIDs and physiotherapy and exercise.
Conventional therapy for AS is limited to NSAIDs (despite very limited supporting clinical trial evidence)30 and recommendations regarding appropriate physical activity. Other statements in the ASAS/EULAR (European League Against Rheumatism) recommendations for the management of AS include analgesics such as paracetamol and opioid-like drugs that may be considered for residual pain. Glucocorticoid injections into the direct site of inflammation (but not systemic) may be of benefit. The use of disease-modifying antirheumatic drugs (DMARDs, such as methotrexate and sulfasalazine) has been all but abandoned after evidence of lack of benefit. The cornerstone of non-pharmacological treatment of patients with AS is patient education and regular exercise; home exercises are effective. Physical therapy with supervised exercises, land- or water-based, individually or in a group, should be preferred, as these are more effective than home exercises. Patient associations and self-help groups may be useful. A Cochrane review of 11 trials concluded that the current best available evidence suggests that physiotherapy is beneficial for people with AS, but that it is still not clear which treatment protocol, duration and intensity should be recommended in the management of AS. 31 Physiotherapy is universally recommended32 but variable in practice.
Biologic drugs are the only treatment shown to be efficacious in the treatment of symptoms and signs of disease activity in axSpA and AS. Current National Institute for Health and Care Excellence (NICE) and BSR guidance recommends treatment with the anti-TNFs adalimumab, etanercept and golimumab in adults with active (severe) AS only if certain criteria are fulfilled, but it does not recommend infliximab for AS. 17,33
Description of technology under assessment
Tumour necrosis factor-α inhibitors (adalimumab, certolizumab pegol, etanercept, golimumab and infliximab), also referred to as anti-TNFs, are typically used when the disease has not responded adequately to conventional therapy. They target the activation of TNF-α and its subsequent activation of downstream inflammatory processes, and as such have the potential to offer symptom control as well as alter disease progression. Adalimumab, certolizumab pegol, golimumab and infliximab are monoclonal antibodies, whereas etanercept is a recombinant human TNF receptor fusion protein.
Adalimumab, etanercept, golimumab and infliximab are licensed in the UK for the treatment of adults with severe active AS that has responded inadequately to conventional therapy. Certolizumab pegol is licensed for the treatment of adults with severe active AS whose disease has responded inadequately to, or who are intolerant of, NSAIDs.
Adalimumab, etanercept and certolizumab pegol are also licensed for the treatment of adults with severe nr-AxSpA with objective signs of inflammation (including elevated CRP level and/or positive MRI), whose disease has responded inadequately to, or who are intolerant of, NSAIDs. Golimumab and infliximab do not currently have a UK marketing authorisation for nr-AxSpA. Current NICE guidance recommends treatment with adalimumab, etanercept or golimumab in adults with active (severe) AS only if certain criteria are fulfilled (including a stipulation that patients must have tried at least two different NSAIDs, which have failed to control symptoms), but it does not recommend infliximab for AS. 17,33 Anti-TNFs for patients with nr-AxSpA have not previously been appraised by NICE.
Chapter 2 Definition of decision problem
Decision problem in terms of Population, Intervention, Comparator, Outcome, Study design and other key issues
The decision problem relates to the optimal use of adalimumab, certolizumab pegol, etanercept, golimumab and infliximab, within their licensed indications, for the treatment of severe/active AS or severe axSpA without radiographic evidence of AS (but with objective signs of inflammation).
Previous National Institute for Health and Care Excellence appraisals
In the previous NICE technology appraisal (TA) 143, adalimumab, etanercept and infliximab were evaluated for AS, while in TA23333 golimumab was evaluated for AS. A number of key areas of uncertainty and potential limitations of the evidence base were identified from these appraisals. These include:
-
a lack of direct head-to-head trial evidence evaluating the relative efficacy and safety of the TNF-α inhibitors
-
a lack of evidence on the efficacy and safety of the sequential use of TNF-α inhibitors
-
the long-term effectiveness of TNF-α inhibitors in controlling disease activity
-
the rate of disease progression in responders and non-responders to treatment, and in those on placebo
-
the proportion of patients who may experience a significant improvement in their condition without TNF-α inhibitor treatment
-
the rate of treatment withdrawal on TNF-α inhibitors and the degree to which a patient’s condition might be expected to rebound if therapy is withdrawn
-
the adverse effects associated with the long-term use of TNF-α inhibitors
-
the impact of TNF-α inhibitors on the progression of structural damage in the spine and functional disability associated with ankylosis
-
the time horizon appropriate for considering the cost-effectiveness of TNF-α inhibitors
-
a lack of registry data of patients receiving TNF-α inhibitors for severe active AS.
This assessment would consider each of these areas of uncertainty and identify the relevant evidence available to inform the limitations of the previous appraisals.
Overall aims and objectives of assessment
The aim of the study is to determine the clinical effectiveness, safety and cost-effectiveness within the NHS of adalimumab, certolizumab pegol, etanercept, golimumab and infliximab, within their licensed indications, for the treatment of severe active AS or severe axSpA without radiographic evidence of AS (but with objective signs of inflammation). If evidence allows, the clinical effectiveness and cost-effectiveness of sequential use of these treatments will also be evaluated.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
Inclusion criteria
Two reviewers independently screened all titles and abstracts. Full manuscripts of any titles/abstracts that were relevant were obtained where possible and the relevance of each study assessed by two reviewers according to the criteria below. Any discrepancies were resolved by consensus and, when necessary, a third reviewer was consulted. Studies available only as abstracts were included.
Study design
For the review of clinical efficacy randomised controlled trials (RCTs) were eligible, including any open-label extensions of RCTs. Adverse event (AE) data were sought from existing reviews and other appropriately large studies. For studies of natural history, long-term effectiveness, adherence and sequential use, published analyses based on large and long-term data sets (including studies of registry data) were eligible.
Interventions
Adalimumab, certolizumab pegol, etanercept, golimumab, infliximab or any of their biosimilars were eligible.
Comparators
Relevant comparators were conventional management strategies (either with or without placebo) and also the different TNF-α inhibitors listed above (i.e. head-to-head trials).
Participants
Studies of adults with either severe active AS or severe axSpA without radiographic evidence of AS but with objective signs of inflammation (such as elevated CRP levels or a positive MRI) were eligible. Patients with predominantly peripheral spondyloarthritis were excluded. Data relating to serious adverse effects associated with anti-TNF agents used in other indications were also considered.
Outcomes
Studies reporting the following outcomes were eligible:
-
multiple domain response criteria: (e.g. ASAS 20, ASAS 40, ASAS 5/6 and ASAS partial remission)
-
disease activity (e.g. BASDAI)
-
functional capacity [e.g. Bath Ankylosing Spondylitis Functional Index (BASFI)]
-
disease progression [e.g. modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS)]
-
pain [e.g. visual analogue scale (VAS) scores]
-
peripheral symptoms (including enthesitis, peripheral arthritis and dactylitis)
-
symptoms of extra-articular manifestations (including anterior uveitis, inflammatory bowel disease and psoriasis)
-
HRQoL [e.g. European Quality of Life-5 Dimensions (EQ-5D)]
-
rates of treatment discontinuation and withdrawal
-
AEs.
For AEs the evaluation specifically focused on known possible AEs of anti-TNFs, such as reactivation of latent tuberculosis, malignancies, non-melanoma skin cancer, severe infections, congestive heart failure, and injection site reactions. Withdrawals because of AEs and events categorised as serious adverse events (SAEs) were also evaluated.
Searches
The following databases were searched for relevant clinical effectiveness and cost-effectiveness research:
-
MEDLINE
-
EMBASE
-
Cumulative Index to Nursing and Allied Health Literature Plus
-
Science Citation Index
-
ClinicalTrials.gov
-
Cochrane Central Register of Controlled Trials
-
Cochrane Database of Systematic Reviews
-
Database of Abstracts of Reviews of Effects
-
International Prospective Register of Systematic Reviews (PROSPERO)
-
Health Technology Assessment Database
-
Conference Proceedings Citation Index – Science
-
National Guidelines Clearinghouse
-
NHS Evidence
-
NHS Clinical Knowledge Summaries
-
NHS Economic Evaluation Database (NHS EED).
The terms for search strategies were identified through discussion within the research team, by scanning the background literature and browsing the MEDLINE medical subject headings. No date or language limits were applied. As several databases were searched, some degree of duplication resulted. To manage this issue, the titles and abstracts of bibliographic records were imported into EndNote bibliographic management software (version X7, Thomson Reuters, CA, USA) to remove duplicate records. Databases were searched from inception, with most of the searches being performed in June or July 2014. The full search strategies used in each database, together with the search dates, are listed in Appendix 1.
Data extraction
Data relating to study design, outcome results and quality were extracted by one reviewer using a standardised data extraction form and independently checked for accuracy by a second reviewer. Disagreements were resolved through consensus, and, when necessary, a third reviewer was consulted. Data from studies with multiple publications were extracted and reported as a single study. Data were also extracted from the manufacturer’s submissions when they were not available from other sources. 34–37 Clinicaltrials.gov records and relevant US Food and Drug Administration (FDA) or European Medicines Agency reports were also used to extract any missing data. When data could only be estimated from graphs, the estimates used in the previous assessment report38 were used when available. In the light of the multidomain outcomes which incorporated pain scores (the ASAS and BASDAI outcomes), it was decided that pain scores on their own would not be extracted.
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Critical appraisal
The quality of RCTs was assessed using the Cochrane risk of bias tool,39 with additional assessments made for baseline imbalance of important prognostic indicators. 40 The relevant prognostic and treatment response indicators were identified from both published research and clinical advice. The risk of bias assessments were performed by one reviewer, and independently checked by a second. Disagreements were resolved through consensus, and, when necessary, a third reviewer was consulted. Open-label extension studies were evaluated based on the imputation methods and patient withdrawal criteria used.
Methods of data synthesis
This section describes the data set construction and meta-analyses conducted for the different outcomes individually. Chapter 5 provides detailed evidence synthesis methods that incorporate different outcomes within one analysis and presents clinical outcome estimates appropriate for the economic model.
Results of the data extraction in terms of study characteristics and quality assessment are presented in tables and summarised narratively. Results of open-label studies, drug survival and switching studies and natural history studies were also summarised narratively. As several of the RCTs were placebo-controlled up to 24 weeks, only time points beyond 24 weeks were evaluated in the open-label studies. AE data from the RCTs were pooled when enough data were identified; otherwise, the AE data and the other studies relating specifically to AEs were summarised narratively.
Clinical effectiveness data were synthesised using Bayesian meta-analysis methods. The main analysis was of outcomes reported from 10 to 16 weeks. A sensitivity analysis was done of outcomes reported from 24 to 30 weeks.
Dosage and pooling of trial arms
The doses included in the analyses were:
-
adalimumab – 40 mg every other week
-
certolizumab pegol – 200 mg every 2 weeks, 400 mg every 4 weeks
-
etanercept – 25 mg twice weekly, 50 mg weekly
-
golimumab – 50 mg every month
-
infliximab – 5 mg/kg at 0, 2 or ≥ 6 weeks.
Golimumab of 100 mg every 4 weeks was excluded when it was not used according to its licence.
Data from active treatment arms were pooled in trials which studied different doses. This occurred for certolizumab pegol 200 mg every 2 weeks and 400 mg every 4 weeks and for etanercept 25 mg twice weekly and 50 mg weekly.
Data imputation and assumptions
Medians were treated as means. Although the median may not be exactly the same as the mean, the median was considered to give sufficiently accurate information. Standard deviations (SDs) were estimated from interquartile ranges, the method of which is described in Appendix 2. Where no SD was reported, the highest SD from the other trials was used as a conservative estimate.
In the meta-analyses, ‘change from baseline’ outcomes were used in the analysis for continuous outcomes. Where these were not reported, but adequate baseline and final value outcomes were reported, the change from baseline and its SD were derived from the baseline and final values and their SDs. The detailed methods are described in Appendix 2.
The imputation of change from baseline or final values required a within-trial correlation estimate, and trials that reported the SDs of baseline, change from baseline and final values were used to estimate the within-trial correlation. For BASDAI the within-study correlation varied from 0.33 to 0.67 across four trials. Given the small samples of some trials, the within-study correlation can vary significantly from trial to trial. For the base-case analysis, a correlation estimate of 0.3 was used and an estimate of 0.7 was tested in sensitivity analysis. For the calculation of final values, the lowest possible correlation was used when 0.3 or 0.7 were not feasible solutions (see Appendix 2).
Change from baseline was imputed for three trials for BASDAI, five trials for BASFI, one trial for Bath Ankylosing Spondylitis Metrology Index (BASMI), two trials for Short Form questionnaire-36 items (SF-36) physical component summary (PCS), and one trial for SF-36 mental component summary (MCS). For each of these outcomes, one of the imputations was for a trial with a non-radiographic population.
Binary event outcomes
Odds ratios (ORs) were derived for binary event outcomes. Relative risks were also derived from the ORs using the placebo absolute risks estimated from all the trials measuring the relevant outcome within weeks 10 to 16. The relative risk estimates are therefore based on the population distribution of the trials across the interventions. As the placebo absolute risk was based on more trials than those informing the ORs for some outcomes, the 95% credible interval (CrI) estimates of the relative risk were narrower than the CrI estimates of the OR. The placebo absolute risk was estimated using both fixed- and random-effect models within WinBUGS (Medical Research Council Biostatistics Unit, Cambridge, UK). As the random-effect model for the placebo absolute risk was a better fit than the fixed effect model according to the deviance information criterion (DIC) statistic, the placebo absolute risks from the random-effect models were used. For the ASAS outcomes, fewer trials reported the greater response outcomes, so a prior distribution was used for the between-study SD based on the closest ASAS outcome (see Appendix 2).
Analyses
Analyses were conducted in WinBUGS version 1.4.3. See Chapter 5 for more details on the models. For each outcome, multiple-treatment meta-analyses were conducted assuming that the treatments had independent effects [related to models A1 (fixed effect) and A2 (random effects) in Chapter 5]. They were also run assuming that they had a common class effect [related to models A3 (fixed effect) and A4 (random effects) in Chapter 5] and that the DIC statistic was used to determine the model that best fitted the data. The random-effect models with independent treatment effects were assumed to have a common between-study variance across the comparisons in the network.
The sensitivity of random-effect models to the between-study SD priors was tested. I2 statistics for heterogeneity were calculated for random-effect models that were insensitive to change in the prior distribution for the between-study SD. Results were only presented for random-effect models.
Clinical effectiveness results
Quantity and quality of research available
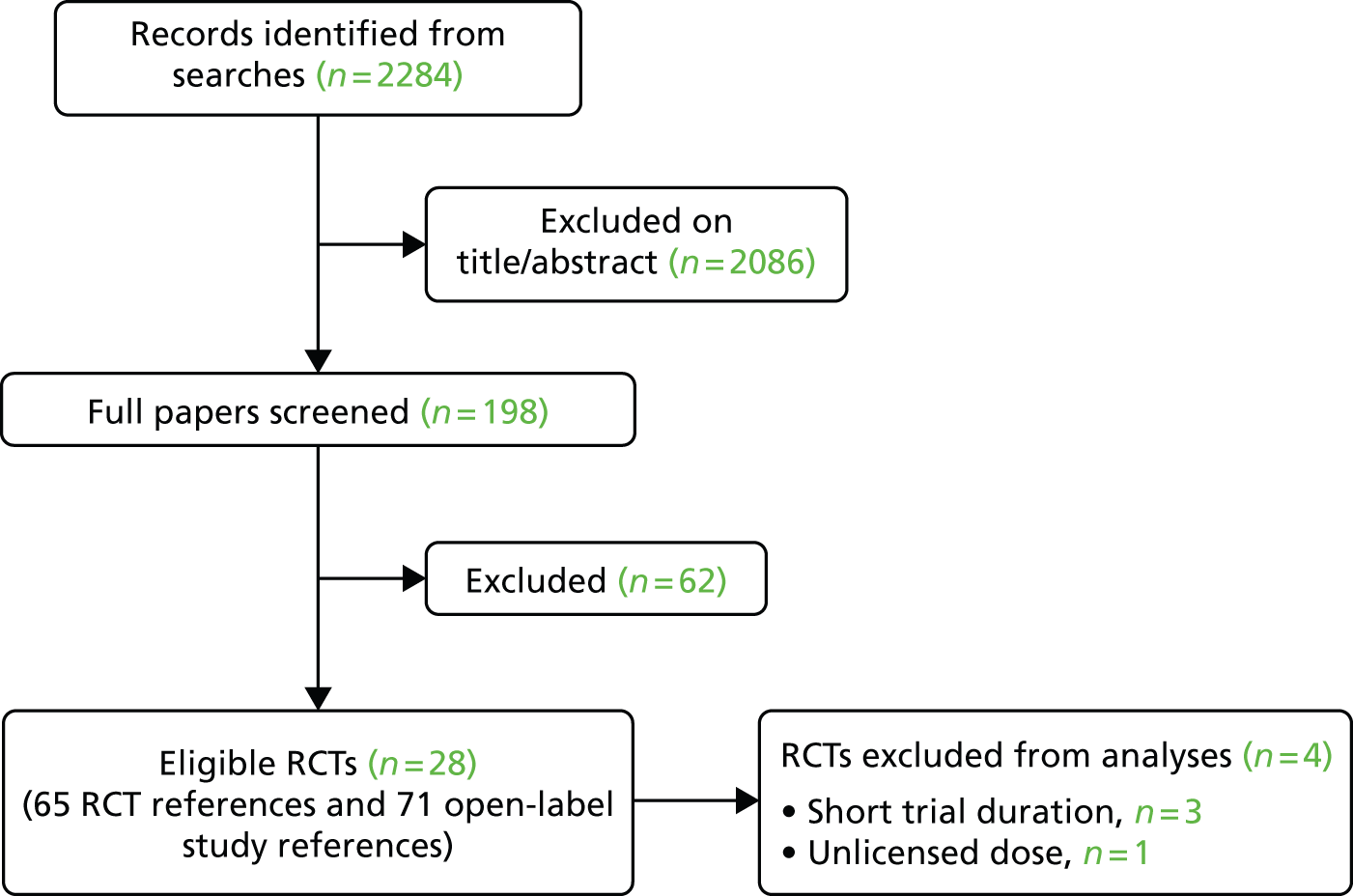
The electronic database searches identified 2284 references. After screening titles and abstracts, full copies of 198 papers were assessed for inclusion in the review. Three trials of axSpA populations were excluded because results were not available separately for the AS and nr-AxSpA populations. 41–43 One study of adalimumab appeared likely to be eligible but was excluded as it was only available as a ClinicalTrials.gov record, without any results or further study details. 44 One excluded study was an ongoing trial of golimumab (called GO-AHEAD). 45
Twenty-eight eligible RCTs were identified, with 24 being suitable for data synthesis. Three etanercept trials were not suitable for data synthesis because the study durations were only 6 weeks,46–48 and one infliximab trial was unsuitable because a (currently) unlicensed dose (3 mg/kg) had been studied. 49 The Barkham 2009 trial50,51 of infliximab in nr-AxSpA patients (see Table 2) was included in the clinical efficacy section because, even though infliximab is not currently licensed for patients with nr-AxSpA, the dose used in this trial was the same as that licensed for AS. Furthermore, there was no reason to think it could not be considered in the same class as the other anti-TNFs when treating a nr-AxSpA population. The results of the trial therefore had the potential to be useful to help inform the relative efficacy of anti-TNFs for nr-AxSpA.
Of the 17 RCTs in which participants were studied beyond the randomised phase (i.e. in open-label studies), 71 additional full publications or conference abstracts were identified. Figure 1 illustrates the flow of studies through the review process.
FIGURE 1.
Flow chart showing the number of studies identified and included.
Study characteristics
Table 2 lists the 24 eligible RCTs (and all the RCT-related references) which were eligible for inclusion in the network meta-analysis. Six trials compared adalimumab versus placebo, one compared certolizumab pegol versus placebo, seven compared etanercept versus placebo, three compared golimumab versus placebo, five compared infliximab versus placebo, one compared etanercept with infliximab and one compared infliximab with an infliximab biosimilar (CT-P13). Most placebo-controlled phases lasted for 12 weeks. All but seven of the trials were extended into open-label (unblinded) phases, with 11 studies having a total duration of at least 1 year.
| Study | Interventions | Anti-TNF dose | Country/continent | Population | Duration of placebo-controlled phase (weeks) | Total duration of study, including any open-label extension phase |
|---|---|---|---|---|---|---|
| Haibel 200852–54 | Adalimumab; placebo | 40 mg every other week | Germany | nr-AxSpA with inflammation, inadequate response/intolerance to NSAIDs | 12 | 1 year |
| Hu 201255 | Adalimumab; placebo | 40 mg every other week | China | AS, inadequate response/intolerance to NSAIDs | 12 | 24 weeks |
| Huang 201456 | Adalimumab; placebo | 40 mg every other week | China | AS, inadequate response/intolerance to NSAIDs | 12 | 24 weeks |
| Lambert 200757 | Adalimumab; placebo | 40 mg every other week | Canada | AS, inadequate response to a NSAID or DMARD | 12 | 1 year |
| ABILITY-1 201358–60 | Adalimumab; placebo | 40 mg every other week | Australia, Europe, North America | nr-AxSpA with inflammation, inadequate response/contraindication to NSAIDs | 12 | 3 years |
| ATLAS 200661–63 | Adalimumab; placebo | 40 mg every other week | USA, Europe | AS, inadequate response to a NSAID or DMARD | 12 | 5 years |
| RAPID-axSpA 201464–70 | Certolizumab pegol; placebo | 200 mg every 2 weeks or 400 mg every 4 weeks | Europe, North America, Latin America | AS, nr-AxSpA with inflammation, inadequate response/intolerance to NSAIDs | 12 | 96 weeks |
| Barkham 201071 | Etanercept; placebo | 25 mg twice weekly | UK | AS | 12 | 12 weeks |
| Davis 200372,73 | Etanercept; placebo | 25 mg twice weekly | North America, Europe | AS | 24 | 168 weeks |
| Dougados 201174,75 | Etanercept; placebo | 50 mg weekly | Europe | AS, inadequate response to NSAIDs | 12 | 24 weeks |
| Dougados 201476–78 | Etanercept; placebo | 50 mg weekly | Europe, Asia, South America | nr-AxSpA, inadequate response to NSAIDs | 12 | 48 weeks |
| Gorman 200279–82 | Etanercept; placebo | 25 mg twice weekly | USA | AS | 16 | 40 weeks |
| Calin 200483–85 | Etanercept; placebo | 25 mg twice weekly | Europe | AS | 12 | 5 years |
| van der Heijde 200686,87 | Etanercept; placebo | 25 mg twice weekly or 50 mg weekly | Europe | AS | 12 | 12 weeks |
| Giardina 201088,89 | Etanercept; infliximab | 50 mg weekly; 5 mg/kg (at week 0, 2, 6 and every 6 weeks) | Italy | AS, inadequate response to NSAIDs | N/A | 12 weeks |
| GO-RAISE 200890–94 | Golimumab; placebo | 50 mg or 100 mg every 4 weeks | North America, Europe, Asia | AS | 16 | 4 years |
| Bao 201495,96 | Golimumab; placebo | 50 mg every 4 weeks | China | AS | 14 | 1 year |
| Tam 201497 | Golimumab; placebo | 50 mg every 4 weeks | China (Hong Kong) | AS, inadequate response to NSAIDs | 24 | 1 year |
| Barkham 200950,51 | Infliximab; placebo | 5 mg/kg (at 0, 2, 6 and 12 weeks) | UK | nr-AxSpA with inflammation | 16 | 16 weeks |
| Braun 200298,99 | Infliximab; placebo | 5 mg/kg (at weeks 0, 2 and 6) | Germany | AS | 12 | 8 years |
| Marzo-Ortega 2005100 | Infliximab + methotrexate; placebo + methotrexate | 5 mg/kg (at weeks 0, 2, 6, 14 and 22) | UK | AS | 30 | 30 weeks |
| Van den Bosch 2002101 | Infliximab; placebo | 5 mg/kg (at weeks 0, 2 and 6) | Belgium | AS | 12 | 12 weeks |
| ASSERT102–109 | Infliximab; placebo | 5 mg/kg (at weeks 0, 2, 6, 12 and 18) | North America, Europe | AS, inadequate response/intolerance to NSAIDs | 24 | 2 years |
| PLANETAS 2013110,111 | CT-P13 biosimilar; infliximab | Both 5 mg/kg | Europe, Asia, Latin America | AS | N/A | 2 years (using randomised interventions up to 54 weeks) |
Of the trials suitable for analysis, most were conducted in Europe and/or North America; four were conducted in China. Four studies recruited a nr-AxSpA population, 19 an AS population and one recruited both populations. 64 Table 3 details the baseline characteristics of the populations studied. In the nr-AxSpA studies around half of the participants were male, whereas in the AS studies around three-quarters were male. All trials recruited participants with active disease; half the trials specified that participants had to have failed one or more NSAID, and a BASDAI score of ≥ 4 was used as an entry requirement in most, with the exception of six early trials in which a BASDAI criterion was not stated. 72,79,83,86,100 Notwithstanding these entry criteria, the recruited participants mostly still took a NSAID (around between 80% and 90% of participants, although reported in only 12 trials) and had quite high mean (or median) BASDAI scores: most were between 5.5 and 6.5 (the range across all trial arms was 5.3–7.0). BASFI scores varied more widely, ranging between 3.2 and 6.7. Variation in CRP levels was also apparent, with lower values in the nr-AxSpA trials being evident. Trials which reported both mean and median CRP showed skewed distributions, with means being higher than medians. 58,64,95 The upper limits of normal used for defining elevated CRP level in the nr-AxSpA trials were either unclear58 or varied, being 3 mg/l,76 6 mg/l51 or 7.9 mg/l. 64 One nr-AxSpA study recruited only MRI-positive patients. 50 In the remaining nr-AxSpA trials the proportion of MRI-positive patients ranged from 51%58 to 81%. 76
| Trial | Patient group | Trial arm | n | % male | Age (years) | % on a NSAID | Symptom duration (years) | BASDAI score | BASFI score | BASMI score | CRP level mg/l, (SD) | % HLA-B27 positive | SF-36 MCS score | SF-36 PCS score | ASQoL score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haibel 200852 | nr-AxSpA | Adalimumab | 22 | 41 | Mean 38 | NR | Mean 7 | Mean 6.5 (SD 1.2) | Mean 5.4 (SD 2) | Mean 1.3 (SD 1.2) | Mean 6.2 (SD 5.8) | 59 | Mean 41.3 (SD 12.5) | Mean 28.8 (SD 7.6) | Mean 10.8 (SD 3.7) |
| nr-AxSpA | Placebo | 24 | 50 | Mean 37 | NR | Mean 8 | Mean 6.2 (SD 1.3) | Mean 4.9 (SD 1.6) | Mean 1.3 (SD 1.6) | Mean 7.8 (SD 7.0) | 75 | Mean 43.6 (SD 11.1) | Mean 30.7 (SD 6) | Mean 9.5 (SD 3) | |
| Hu 201255 | AS | Adalimumab | 26 | 92 | Mean 28.2 (SD 6.9) | NR | Mean 7.4 | Mean 5.9 (SD 1.4) | Mean 3.7 (SD 2.1) | – | Mean 24.6 | 96 | – | – | – |
| AS | Placebo | 20 | 100 | Mean 27.4 (SD 7.2) | NR | Mean 7.6 | Mean 6.2 (SD 1.1) | Mean 3.9 (SD 2) | – | Mean 32.1 | 95 | – | – | – | |
| Huang 201456 | AS | Adalimumab | 229 | 81 | Mean 30.1 (SD 8.7) | 80 | Mean 8.1 | Mean 6.0 (SD 1.4) | Mean 4.3 (SD 2.3) | Mean 3.4 (SD 1.4) | Mean 22.4 (SD 24) | 96 | Mean 36.2 (SD 10.7) | Mean 33.8 (SD 7) | – |
| AS | Placebo | 115 | 83 | Mean 29.6 (SD 7.5) | 78 | Mean 7.7 | Mean 6.2 (SD 1.4) | Mean 4.4 (SD 2.3) | Mean 3.4 (SD 1.5) | Mean 23 (SD 30) | 95 | Mean 35 (SD 10.6) | Mean 32.2 (SD 6.7) | – | |
| Lambert 200757 | AS | Adalimumab | 38 | 76 | Mean 41.9 (SD 11.1) | NR | Mean 14.5 | Mean 6.2 (SD 1.7) | Mean 5.3 (SD 2) | – | Mean 18 | 87 | – | – | – |
| AS | Placebo | 44 | 82 | Mean 40 (SD 10.9) | NR | Mean 12.1 | Mean 6.5 (SD 1.6) | Mean 5.6 (SD 2.2) | – | Mean 23 | 82 | – | – | – | |
| aABILITY-1 201358 | nr-AxSpA | Adalimumab | 69 | 46 | Mean 38.3 (SD 11.7) | NR | Mean 10.7 | Mean 6.4 (SD 1.6) | Mean 4.5 (SD 2.1) | Mean 2.7 | Mean 8.6 (SD 13.1) | NR | – | Mean 33.3 (SD 7.8) | – |
| nr-AxSpA | Placebo | 73 | 45 | Mean 38.3 (SD 10.5) | NR | Mean 10.5 | Mean 6.4 (SD 1.5) | Mean 4.8 (SD 2.3) | Mean 2.7 | Mean 9.3 (SD 10.9) | NR | – | Mean 33.2 (SD 8.2) | – | |
| ATLAS 200661 | AS | Adalimumab | 208 | 76 | Mean 41.7 (SD 11.7) | 80 | Mean 11.3 | Mean 6.3 (SD 1.7) | Mean 5.2 (SD 2.2) | Mean 3.8 (SD 2.2) | Mean 18 | 78 | Mean 43.4 (SD 12) | Mean 32.9 (SD 8) | Mean 10.2 (SD 4) |
| AS | Placebo | 107 | 74 | Mean 43.4 (SD 11.3) | 79 | Mean 10 | Mean 6.3 (SD 1.7) | Mean 5.6 (SD 2.2) | Mean 4.2 (SD 2.1) | Mean 22 | 79 | Mean 44.4 (SD 12) | Mean 31.8 (SD 8) | Mean 10.6 (SD 4) | |
| RAPID-axSpA 201464 | AS | Certolizumab pegol 200 mg | 65 | 72 | Mean 41 (SD 10.8) | 91 | Median 8.8 | Mean 6.5 (SD 1.7) | Mean 5.6 (SD 2.3) | Mean 4.2 (SD 1.6) | Median 14 | 82 | – | – | – |
| AS | Certolizumab pegol 400 mg | 56 | 73 | Mean 41.9 (SD 11.5) | 91 | Median 8.8 | Mean 6.2 (SD 1.3) | Mean 5.7 (SD 2.3) | Mean 4.3 (SD 1.8) | Median 12.9 | 79 | – | – | – | |
| AS | Placebo | 57 | 72 | Mean 41.6 (SD 12.8) | 90 | Median 10.2 | Mean 6.4 (SD 1.9) | Mean 6.0 (SD 2) | Mean 4.7 (SD 1.6) | Median 16.6 | 84 | – | – | – | |
| RAPID-axSpA 201464 | nr-AxSpA | Certolizumab pegol 200 mg | 46 | 44 | Mean 36.6 (SD 13) | 83 | Median 4.8 | Mean 6.5 (SD 1.4) | Mean 4.8 (SD 2.2) | Mean 3.1 (SD 1.4) | Median 10 | 74 | – | – | – |
| nr-AxSpA | Certolizumab pegol 400 mg | 51 | 53 | Mean 37.5 (SD 10.8) | 86 | Median 7.3 | Mean 6.6 (SD 1.6) | Mean 5.1 (SD 2.4) | Mean 3.3 (SD 1.5) | Median 12.1 | 73 | – | – | – | |
| nr-AxSpA | Placebo | 50 | 48 | Mean 38 (SD 11.8) | 82 | Median 4.5 | Mean 6.4 (SD 1.5) | Mean 4.9 (SD 2.2) | Mean 3.1 (SD 1.6) | Median 13.5 | 78 | – | – | – | |
| Barkham 201071 | AS | Etanercept | 20 | 75 | Mean 40.8 (SD 9.7) | NR | Median 11 | Mean 6.1 (SD 1.7) | Mean 5.6 (SD 2.0) | – | – | NR | – | – | – |
| AS | Placebo | 20 | 85 | Mean 39.4 (SD 10.1) | NR | Median 20 | Mean 5.5 (SD 1.7) | Mean 5.3 (SD 1.8) | – | – | NR | – | – | – | |
| Davis 200372 | AS | Etanercept | 138 | 76 | Mean 42.1 | 91 | Mean 10.1 | Mean 5.8 (SE 0.15) | Mean 5.2 | – | Mean 19 | 84 | – | – | – |
| AS | Placebo | 139 | 76 | Mean 41.9 | 92 | Mean 10.5 | Mean 6.0 (SE 0.14) | Mean 5.6 | – | Mean 20 | 84 | – | – | – | |
| Dougados 201174 | AS | Etanercept | 39 | 95 | Mean 46 (SD 11) | NR | Mean 19 | Mean 6.4 (SD 1.2) | Mean 6.3 (SD 2.0) | Mean 5.7 (SD 1.4) | Mean 25 (31) | 79 | – | – | – |
| AS | Placebo | 43 | 91 | Mean 48 (SD 10) | NR | Mean 23 | Mean 5.8 (SD 1.5) | Mean 5.7 (SD 1.9) | Mean 5.8 (SD 1.3) | Mean 17 (19) | 86 | – | – | – | |
| bDougados 201476 | nr-AxSpA | Etanercept | 106 | 64 | Mean 31.9 (SD 7.8) | CiC information has been removed | Mean 2.4 | CiC information has been removed | CiC information has been removed | Mean 1.4 (SD 1.3) | Mean 6.8 | 67 | – | CiC information has been removed | CiC information has been removed |
| nr-AxSpA | Placebo | 109 | 57 | Mean 32 (SD 7.8) | CiC information has been removed | Mean 2.5 | CiC information has been removed | CiC information has been removed | Mean 1.2 (SD 1.3) | Mean 6.4 | 76 | – | CiC information has been removed | CiC information has been removed | |
| Gorman 200279 | AS | Etanercept | 20 | 65 | CiC information has been removed | 80 | CiC information has been removed | – | Mean 4.5 (SD 2.1) | – | Mean 20 | 95 | – | – | – |
| AS | Placebo | 20 | 90 | CiC information has been removed | 95 | CiC information has been removed | – | Mean 3.2 (SD 2.5) | – | Mean 15 | 90 | – | – | – | |
| Calin 200483 | AS | Etanercept | 45 | 80 | Mean 45.3 (SD 9.5) | 89 | Mean 15.0 | Mean 6.1 | Mean 6.0 | – | Median 154 | NR | – | – | – |
| AS | Placebo | 39 | 77 | Mean 40.7 (SD 11.4) | 85 | Mean 9.7 | Mean 5.9 | Mean 5.7 | – | Median 97 | NR | – | – | – | |
| van der Heijde 200686 | AS | Etanercept 25 mg | 150 | 76 | Mean 39.8 (SD 10.7) | 85 | Mean 10.0 | Mean 5.9 (SD 1.7) | Mean 5.8 (SD 2.0) | – | Mean 19.8 (SD 20.8) | NR | – | – | – |
| AS | Etanercept 50 mg | 155 | 70 | Mean 41.5 (SD 11) | 80 | Mean 9.0 | Mean 6.2 (SD 1.7) | Mean 6.1 (SD 2.0) | – | Mean 21.7 (SD 24.6) | NR | – | – | – | |
| AS | Placebo | 51 | 78 | Mean 40.1 (SD 10.9) | 78 | Mean 8.5 | Mean 6.1 (SD 1.4) | Mean 6.0 (SD 1.9) | – | Mean 22 (SD 22.9) | NR | – | – | – | |
| Giardina 201088 | AS | Etanercept | 25 | 80 | Mean 32.6 (SD 6.8) | NR | Mean 15.7 | Mean 6.6 (SD 1.1) | Mean 6.5 (SD 1.1) | Mean 3.9 (SD 1.7) | Mean 22.9 | 96 | – | – | – |
| AS | Infliximab | 25 | 76 | Mean 31.9 (SD 9.2) | NR | Mean 15.4 | Mean 6.5 (SD 1.2) | Mean 6.1 (SD 0.9) | Mean 3.7 (SD 1.6) | Mean 25 | 92 | – | – | – | |
| GO-RAISE 200890 | AS | Golimumab 50 mg | 138 | 74 | Median 38 | 90 | Median 11 | Median 6.6 (IQR 5.6–7.6) | Median 5 (IQR 3.2–6.7) | Median 3 (IQR 2–4) | Mean 11 | 82 | Median 46.5 (IQR 36.8–54.1) | Median 29.7 (IQR 22.5–35.3) | – |
| AS | Golimumab 100 mg | 140 | 70 | Median 38 | 88 | Median 9.5 | Median 7 (IQR 6.0–7.9) | Median 5.4 (IQR 3.4 to 7.3) | Median 3 (IQR 2–5) | Mean 9 | 84 | Median 43.1 (IQR 33.5–53.5) | Median 29.8 (IQR 25.2–35.5) | – | |
| AS | Placebo | 78 | 71 | Median 41 | 92 | Median 16.0 | Median 6.6 (IQR 5.7–7.7) | Median 4.9 (IQR 3.5–6.8) | Median 4 (IQR 2–5) | Mean 11.5 | 85 | Median 46.2 (IQR 37.1–54.8) | Median 28.3 (IQR 23.8–34.1) | – | |
| Bao 201495 | AS | Golimumab | 108 | 83 | Mean 30.5 (SD 10.3) | 67 | Mean 6.8 | Mean 6.6 (1.3) | Mean 5 (SD 2.4) | Mean 4 (SD 1.9) | Mean 20.6 | – | Mean 36.5 (SD 10.5) | Mean 33.2 (SD 7.8) | – |
| AS | Placebo | 105 | 83 | Mean 30.6 (SD 8.6) | 72 | Mean 7.5 | Mean 6.5 (1.5) | Mean 5 (SD 2.4) | Mean 3.8 (SD 1.6) | Mean 18.6 | – | Mean 36.2 (SD 11.5) | Mean 33.9 (SD 7.7) | – | |
| Tam 201497 | AS | Golimumab | 20 | 90 | Mean 35.6 (SD 9.9) | 85 | Mean 8.0 | Mean 6.2 (1.0) | Mean 4.6 (SD 1.9) | Median 5.0 (IQR 4.0–7.0) | Mean 23.9 (SD 18.6) | – | – | – | – |
| AS | Placebo | 21 | 90 | Mean 34.2 (SD 10) | 100 | Mean 11.0 | Mean 6.2 (1.5) | Mean 4.1 (SD 2.3) | Median 3 (IQR 2.0–5.5) | Mean 19.9 (SD 14.0) | – | – | – | – | |
| Barkham 200950 | nr-AxSpA | Infliximab | 20 | 75 | Mean 29.5 | 90 | Mean 13.4 | Mean 5.9 | Mean 4.4 | – | Median 5 | 100 | – | – | Mean 10 |
| nr-AxSpA | Placebo | 20 | 75 | Mean 28.2 | 90 | Mean 17.2 | Mean 5.8 | Mean 4.1 | – | Median 11.5 | 100 | – | – | Mean 11 | |
| Braun 200298 | AS | Infliximab | 34 | 68 | Mean 40.6 (SD 8) | NR | Mean 16.4 | Mean 6.5 (1.2) | Mean 5.4 (SD 1.8) | Mean 3.7 (SD 2.0) | Mean 24 | 91 | Mean 51.5 (SD 22.6) | Mean 46.5 (SD 22.6) | - |
| AS | Placebo | 35 | 63 | Mean 39 (SD 9.1) | NR | Mean 14.9 | Mean 6.3 (1.4) | Mean 5.1 (SD 2.2) | Mean 3.7 (SD 2.2) | Mean 18 | 88 | Mean 65.4 (SD 18.4) | Mean 47.6 (SD 23.4) | – | |
| Marzo-Ortega 2005100 | AS | Infliximab | 28 | 82 | Mean 41 | 89 | Median 8 | Mean 6.5 (1.9) | Median 6.7 | – | Median 30.5 | 96 | – | – | Median 14 |
| AS | Placebo | 14 | 79 | Mean 39 | 86 | Median 10 | Mean 6.6 (2.1) | Median 6 | – | Median 30 | 86 | – | – | Median 13.5 | |
| Van den Bosch 2002101 | AS | Infliximab | 9 | 78 | Mean 44.3 | NR | Mean 10 | Median 5.9 | Median 4.7 | Median 5 | Mean 41.0 | 89 | – | – | – |
| AS | Placebo | 12 | 83 | Mean 46.4 | NR | Mean 17 | Median 5.3 | Median 5.9 | Median 4 | Mean 25.7 | 75 | – | – | – | |
| ASSERT 2005102 | AS | Infliximab | 201 | 78 | Median 40 | NR | Mean 7.7 | Median 6.6 (IQR 5.2– 7.1) | Median 5.7 (IQR 4.5–7.1) | – | Mean 15 | 87 | Median 47.6 (IQR 37.6–54.9) | Median 28.8 (IQR 23.8–33.7) | – |
| Park 2013110 | AS | Placebo | 78 | 87 | Median 41 | NR | Mean 13.2 | Median 6.5 (IQR 5.3–7.6) | Median 6 (IQR 4.1–7.2) | – | Mean 17 | 89 | Median 45 (IQR 33.7–55.5) | Median 30.1 (IQR 24.9–36.2) | – |
| PLANETAS 2013110 | AS | CT-P13 | 125 | 79 | Median 38 | NR | – | Mean 6.7 (SD 1.4) | Mean 6.2 (SD 1.9) | Mean 4 (SD 2.1) | Median 11 | – | – | – | – |
| AS | Infliximab | 125 | 82 | Median 38 | NR | – | Mean 6.6 (SD 1.6) | Mean 6.2 (SD 2.2) | Mean 4.1 (SD 2.1) | Median 14 | – | – | – | – |
Risk of bias
Results of the risk of bias judgements are presented in Table 4. Further details, including judgement reasons and the prognostic indicators of important baseline imbalance, are available in Appendix 3. Most trials were judged to have a low risk of bias overall; when possible bias was detected, there was little indication to suggest that this varied across the different anti-TNF trials.
| Trial | Bias domain | |||||||
|---|---|---|---|---|---|---|---|---|
| 1. Sequence generation | 2. Allocation concealment | 3. Important baseline imbalance | Selection bias based on 1, 2, and 3 | 4. Blinding of participants and personnel | 5. Blinding of outcome assessment | 6. Incomplete outcome data | 7. Selective reporting | |
| Risk of bias judgement | ||||||||
| Adalimumab vs. placebo | ||||||||
| Haibel 200852 | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| Hu 201255 | Unclear | Unclear | Low | Low | Unclear | Low | Unclear | Unclear |
| Huang 201456 | Low | Low | Low | Low | Low | Low | Low | Low |
| Lambert 200757 | Unclear | Unclear | Low | Low | Unclear | Low | Low | Low |
| ABILITY-1 201358 | Low | Low | Low | Low | Low | Low | Low | Low |
| ATLAS 200661 | Unclear | Unclear | Low | Low | Low | Low | Low | Low |
| Certolizumab pegol vs. placebo | ||||||||
| RAPID-axSpA 201464 | Low | Low | Low | Low | Unclear | Low | Low | Low |
| Etanercept vs. placebo | ||||||||
| Barkham 201071 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Low |
| Davis 200372 | Unclear | Unclear | Low | Low | Low | Low | Low | Low |
| Dougados 201174 | Unclear | Unclear | Low | Low | Low | Low | Low | Low |
| Dougados 201476 | Low | Low | Low | Low | Low | Low | Low | Low |
| Gorman 200279 | Low | Low | Higha | Higha | Low | Low | Low | Low |
| Calin 200483 | Unclear | Unclear | High | High | Low | Low | Low | Low |
| van der Heijde 200686 | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| Etanercept vs. infliximab | ||||||||
| Giardina 201088 | High | High | Low | Low | High | High | Low | Low |
| Golimumab vs. placebo | ||||||||
| GO-RAISE 200890 | Low | Low | Low | Low | Low | Low | Low | Low |
| Bao 201495 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Low |
| Tam 201497 | Low | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| Infliximab vs. placebo | ||||||||
| Barkham 200950 | Unclear | Unclear | High | High | Low | Low | Low | Low |
| Braun 200298 | Low | Low | Low | Low | Low | Low | Low | Low |
| Marzo-Ortega 2005100 | Low | Low | Unclear | Low | Low | Low | Unclear | Low |
| Van den Bosch 2002101 | Unclear | Unclear | High | High | Low | Low | Low | Low |
| ASSERT102 | Unclear | Unclear | Low | Low | Low | Low | Low | Low |
| Infliximab vs. biosimilar infliximab (InflectraCT-P13) | ||||||||
| PLANETAS 2013110 | Low | Low | Unclear | Low | Low | Low | Low | Low |
Over half the trials did not report adequate details about methods of randomisation and allocation concealment, although in the majority of those trials (8 out of 14) an assessment could be made of whether or not groups were balanced in all five of the important prognostic indicators of treatment response. Using both randomisation method details and a baseline assessment to judge the risk of selection bias, 15 trials were judged as having a low risk of selection bias, five trials were judged as having an unclear risk51,71,86,96,112 and four as having a high risk;50,79,83,101 in one of these four trials the risk was deemed likely to be a result of a chance effect. 79
The risk of performance bias arising from lack of blinding of participants and personnel was low in 20 trials, unclear in three trials55,57,64 and high in the one head-to-head trial, in which blinding would have been difficult to achieve because of the different modes and timings of delivery (weekly injection for etanercept vs. 6-weekly infusion for infliximab). 88 All except one of the trials were at low risk of detection bias, as they were all adequately placebo controlled (except the head-to-head trial), with nearly all the key outcomes being self-reported by patients (a notable exception being BASMI). The blinded patients were the outcome assessors, and the effect of any unblinded study personnel on patient questionnaire responses was likely to be minimal at most. The proportion of patients withdrawing or dropping out of trials was generally low; most trials received low risk judgements for attrition bias. In two of the trials with unclear risk judgements, there were nevertheless reasons to suspect the possibility of important bias (see Appendix 3). 55,71 Of the studies with missing data which also reported details on the populations and imputations used in analyses, ‘last observation carried forward’ (LOCF) was used; this was done using a modified intention-to-treat (mITT) approach in just over half the trials (in which patients had to have received at least one dose of treatment) and an intention-to-treat (ITT) approach in the remaining trials (see Appendix 3). There was no evidence of reporting bias in any of the trials with all being judged as low risk, except for one trial with an unclear risk of bias. 55
Clinical effectiveness results: efficacy results from randomised controlled trials
Individual results for all 24 trials are presented in Appendix 4.
Exclusions from the meta-analyses
Of the trials with results at between 10 and 16 weeks, one small head-to-head trial (n = 50) comparing etanercept with infliximab was excluded, as it was redundant in a class-effect model (in addition, blinding was not feasible in this trial). 88 One trial110 was excluded because it compared infliximab and CT-P13, and therefore did not include any of the relevant comparators needed for meta-analysis. The maximum number of studies included for any one outcome was 16.
Exclusions from the sensitivity analyses
Five studies were excluded in the sensitivity analyses because of risk of bias judgements. 55,71,79,83,101 Further details can be found in Appendix 3. A sensitivity analysis of the nr-AxSpA trials was not performed, as the one trial judged to have a high risk of bias had only 40 patients;50 any effect arising from the removal of such a small study would have been likely to have been minimal.
The best model
Models were run when it was assumed that:
-
there were different independent treatment effects
-
there was just one treatment class effect.
In addition, fixed-effect and random-effects models were run when there were sufficient data. These models relate to models A1, A2, A3 and A4 in Chapter 5. For the non-radiographic population, there were very few studies and therefore only fixed-effect analyses were conducted.
The DIC and I2 results for each outcome (other than injection site reactions) are shown for the AS population in Table 5 and for the nr-AxSpA population in Table 6. The lower the DIC for a given outcome, the better the model fit. I2 varies between 0% and 100%, with 0% representing no heterogeneity in the results and 100% indicating that all of the variation in the results can be explained by heterogeneity. The greater the value of I2, the more likely it is that a random-effects model would be a better fit. But this is not always the case, as if there are few studies then there will be significant uncertainty around the between-study variance and therefore the I2 also. Random-effect model results and I2 results are not presented for some outcomes because of sensitivity to prior distributions in the model.
| Outcome | Independent effects | Class effect | I2 (%) | ||
|---|---|---|---|---|---|
| Fixed effect | Random effects | Fixed effect | Random effects | ||
| Related model in Chapter 5 | A1 | A2 | A3 | A4 | – |
| BASDAI 50 | 16.82 | – | 10.86 | 12.71 | 21 |
| BASDAI | 16.76 | 18.22 | 13.53 | 15.12 | 21 |
| BASFI | 18.96 | 20.87 | 14.79 | 16.80 | 10 |
| ASAS 20 | 10.68 | 17.05 | 9.98 | 8.73 | 16 |
| ASAS 40 | 10.36 | 14.07 | 8.50 | 10.29 | 27 |
| ASAS 50 | 8.38 | – | 6.68 | 8.11 | 52 |
| ASAS 70 | 2.92 | – | – | – | – |
| BASMI | –0.87 | – | 0.12 | –3.01 | 77 |
| SF-36 PCS | 19.64 | – | 20.20 | 17.71 | 76 |
| MASES | 5.99 | – | 4.17 | – | – |
| SF-36 MCS | 19.20 | – | 16.67 | 18.26 | 47 |
| Outcome | Independent effects | Class effect | I2 (%) | ||
|---|---|---|---|---|---|
| Fixed effect | Random effects | Fixed effect | Random effects | ||
| Related model in Chapter 5 | A1 | A2 | A3 | A4 | – |
| BASDAI 50 | 6.74 | – | 4.85 | – | – |
| BASDAI | 10.80 | – | 11.07 | 11.51 | 69 |
| BASFI | 11.45 | – | 13.74 | 10.70 | 83 |
| ASAS 20 | 6.72 | – | 5.23 | – | – |
| ASAS 40 | 11.17 | – | 7.96 | 9.30 | 49 |
| ASAS 50 | – | – | – | – | – |
| ASAS 70 | – | – | – | – | – |
| BASMI | 1.80 | – | 4.74 | 2.42 | 89 |
| SF-36 PCS | 16.67 | – | 20.18 | – | – |
| MASES | – | – | – | – | – |
| SF-36 MCS | 14.61 | – | 14.08 | – | – |
Overall, assuming a class effect for the treatments produced a better-fitting model than assuming independent treatment effects. In addition, a fixed-effect analysis was more often than not appropriate. The mean and median effects of the two analyses were also similar. Hence the fixed-effect results are reported in this chapter; these represent a common class effect.
For AS, the common class-effect model was found to be a much better fit than the independent treatment effect model. As described in Chapter 5 the exchangeable class-effect model, not explored here, also fitted the data well, although not so well as the common class-effect model. It should be noted here that the common class-effect model may possibly underestimate the uncertainty around the treatment effect estimate. As explained in Chapter 5, if the differences between treatments are a result of systematic differences in study design between treatments, then an exchangeable class-effect model may be appropriate. However, if in fact there is a true difference between treatments, such as between infliximab and the other TNF-inhibitors, then an exchangeable class-effect model may overestimate the uncertainty around the effect estimates. As the common class-effect model had a lower DIC than the exchangeable class-effect model, this is the model evaluated in this chapter. The economic model explores the assumption that treatment effect differences are in fact because of systematic differences in study design between treatments.
As there was very little difference between the results in which change from baseline was imputed assuming a within-study correlation of 0.3 or 0.7, only the results assuming a within-study correlation of 0.3 are reported here. A comparison of the results assuming different within-study correlations is presented in Appendix 5.
Individual anti-tumour necrosis factors compared with placebo
Binary responder outcomes at between 10 and 16 weeks
The results of the analyses of the responder outcomes between 10 and 16 weeks for patients with AS are presented in Table 7.
| Intervention | Type of analysis | ASAS 20 | ASAS 40 | ASAS 50 | BASDAI 50 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of trials (number of patients) | Relative risk (95% CrI) | OR (95% CrI) | Number of trials (number of patients) | Relative risk (95% CrI) | OR (95% CrI) | Number of trials (number of patients) | Relative risk (95% CrI) | OR (95% CrI) | Number of trials (number of patients) | Relative risk (95% CrI) | OR (95% CrI) | ||
| Adalimumab | Main | 3 (741) | 2.28 (1.98 to 2.62) | 4.52 (3.23 to 6.33) | 2 (659) | 3.42 (2.57 to 4.55) | 5.67 (3.56 to 8.97) | 1 (82) | 2.75 (1.11 to 5.45) | 3.58 (1.12 to 11.17) | 2 (659) | 3.16 (2.40 to 4.16) | 4.68 (3.14 to 7.03) |
| Sensitivity | 3 (741) | 2.27 (1.97 to 2.62) | 4.52 (3.23 to 6.33) | 2 (659) | 3.34 (2.53 to 4.40) | 5.67 (3.56 to 8.97) | As in the main analysis | 2 (659) | 3.11 (2.37 to 4.09) | 4.68 (3.14 to 7.03) | |||
| Certolizumab pegol | Main | 1 (178) | 1.80 (1.24 to 2.39) | 2.61 (1.37 to 5.01) | 1 (178) | 2.53 (1.47 to 3.98) | 3.38 (1.59 to 7.15) | – | – | – | 1 (178) | 3.60 (2.02 to 5.74) | 5.97 (2.39 to 15.03) |
| Sensitivity | 1 (178) | 1.80 (1.24 to 2.39) | 2.61 (1.37 to 5.01) | 1 (178) | 2.49 (1.46 to 3.87) | 3.38 (1.59 to 7.15) | – | – | – | 1 (178) | 3.53 (2.00 to 5.58) | 5.97 (2.39 to 15.03) | |
| Etanercept | Main | 5 (839) | 2.23 (1.93 to 2.55) | 4.23 (3.05 to 5.88) | 3 (478) | 2.75 (1.88 to 3.88) | 3.86 (2.21 to 6.72) | 2 (359) | 3.43 (2.40 to 4.90) | 5.04 (2.98 to 8.51) | 3 (478) | 3.17 (2.20 to 4.49) | 4.74 (2.71 to 8.28) |
| Sensitivity | 3 (715) | 2.17 (1.84 to 2.53) | 3.98 (2.78 to 5.73) | 2 (436) | 2.65 (1.80 to 3.72) | 3.72 (2.11 to 6.53) | As in the main analysis | 2 (436) | 3.03 (2.08 to 4.31) | 4.50 (2.52 to 8.01) | |||
| Golimumab | Main | 2 (429) | 2.14 (1.75 to 2.53) | 3.82 (2.47 to 5.86) | 2 (429) | 3.11 (2.24 to 4.26) | 4.77 (2.85 to 7.98) | – | – | – | 2 (429) | 3.57 (2.51 to 5.00) | 5.85 (3.31 to 10.28) |
| Sensitivity | 2 (429) | 2.13 (1.74 to 2.53) | 3.82 (2.47 to 5.86) | 2 (429) | 3.05 (2.21 to 4.13) | 4.77 (2.85 to 7.98) | – | – | 2 (429) | 3.50 (2.48 to 4.88) | 5.85 (3.31 to 10.28) | ||
| Infliximab | Main | 2 (111) | 2.45 (1.73 to 3.06) | 5.54 (2.41 to 12.71) | – | – | – | 1 (69) | 5.59 (2.44 to 9.81) | 14.71 (3.07 to 72.69) | 1 (69) | 4.86 (2.41 to 7.82) | 12.07 (3.09 to 46.37) |
| Sensitivity | 2 (111) | 2.44 (1.72 to 3.06) | 5.54 (2.41 to 12.71) | – | – | – | As in the main analysis | 1 (69) | 4.72 (2.38 to 7.54) | 12.07 (3.09 to 46.37) | |||
| Anti-TNFs as a class | Main | 13 (2298) | 2.21 (2.01 to 2.43) | 4.12 (3.40 to 4.99) | 8 (1744) | 3.06 (2.52 to 3.76) | 4.61 (3.51 to 6.05) | 4 (510) | 3.51 (2.55 to 4.86) | 5.23 (3.31 to 8.27) | 9 (1813) | 3.37 (2.75 to 4.16) | 5.22 (4.00 to 6.79) |
| Sensitivity | 11 (2174) | 2.18 (1.97 to 2.42) | 4.04 (3.32 to 4.92) | 7 (1702) | 2.99 (2.47 to 3.66) | 4.57 (3.48 to 6.02) | As in the main analysis | 8 (1771) | 3.29 (2.68 to 4.07) | 5.16 (3.94 to 6.72) | |||
Assessment in Ankylosing Spondylitis improvement criteria: Assessment in Ankylosing Spondylitis 20, Assessment in Ankylosing Spondylitis 40, Assessment in Ankylosing Spondylitis 50 and Assessment in Ankylosing Spondylitis 70
For the AS population ASAS 20 data were available for all five anti-TNFs, although the number of participants studied varied considerably, ranging from 839 patients in five etanercept trials to 111 patients in two infliximab trials. A consistent effect was evident across the treatments with the pooled relative risks ranging from 1.80 (certolizumab pegol) to 2.45 (infliximab). ASAS 40 data were available for four anti-TNFs (no data were available for infliximab); the number of data available ranged from 178 patients in one certolizumab trial to 659 patients in two adalimumab trials. Again, a consistent effect was found, with relative risks ranging from 2.53 (certolizumab pegol) to 3.42 (adalimumab); all the relative risks were greater than the corresponding ASAS 20 estimates. For ASAS 50 there were two trials of etanercept (totalling 359 participants) and small single trials in adalimumab (n = 82) and infliximab (n = 69). A wider range of relative risks and CrIs resulted, ranging from 2.75 (adalimumab) to 5.59 (infliximab), which may be a consequence of the smaller numbers of patients studied. Only two trials, both of etanercept (n = 359), reported actual numbers of ASAS 70 responders. The pooling of these data showed that patients taking etanercept were more than three times more likely to be ASAS 70 responders than patients taking placebo (relative risk 3.59, 95% CrI 2.18 to 5.87).
For the nr-AxSpA population, each of the relative risks for certolizumab pegol and etanercept were based on single, quite large trials; the estimate for adalimumab was based on a similar number of patients (to etanercept and certolizumab) across two trials, whereas infliximab was represented by a single small trial (n = 40). ASAS 20 results were similar across treatments but for ASAS 40 heterogeneity of effect appeared evident; the smallest estimate was for etanercept and the largest estimate was seen in the small infliximab trial (Table 8). However, this infliximab trial was the only nr-AxSpA trial judged to be at high risk of bias. Only one trial (ABILITY-158) reported ASAS 50 or ASAS 70 results. For ASAS 50 the relative risk was 4.23 (95% CrI 1.84 to 9.72; OR 5.96, 95% CrI 2.40 to 14.80). For ASAS 70 the relative risk was 4.58 (95% CrI 1.37 to 15.40; OR 5.42, 95% CrI 1.54 to 19.11).
| Intervention | ASAS 20 | ASAS 40 | BASDAI 50 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of trials (number of patients) | Relative risk (95% CrI) | OR (95% CrI) | Number of trials (number of patients) | Relative risk (95% CrI) | OR (95% CrI) | Number of trials (number of patients) | Relative risk (95% CrI) | OR (95% CrI) | |
| Adalimumab | 2 (188) | 1.92 (1.47 to 2.56) | 3.71 (2.02 to 6.75) | 2 (188) | 3.14 (1.99 to 4.68) | 5.04 (2.44 to 10.32) | 2 (188) | 2.52 (1.65 to 3.83) | 3.97 (1.97 to 7.86) |
| Certolizumab pegol | 1 (147) | 1.59 (1.10 to 2.21) | 2.32 (1.15 to 4.67) | 1 (147) | 3.04 (1.74 to 4.81) | 4.75 (2.01 to 11.17) | 1 (147) | 2.80 (1.71 to 4.47) | 4.92 (2.09 to 11.58) |
| Etanercept | 1 (215) | 1.46 (1.08 to 1.94) | 1.94 (1.13 to 3.37) | 1 (215) | 2.07 (1.26 to 3.20) | 2.55 (1.32 to 4.92) | 1 (215) | 1.92 (1.27 to 2.82) | 2.45 (1.37 to 4.43) |
| Infliximab | – | – | – | 1 (40) | 3.63 (1.41 to 6.44) | 6.85 (1.52 to 31.03) | – | – | – |
| Anti-TNFs as a class | 4 (550) | 1.65 (1.37 to 2.04) | 2.52 (1.78 to 3.59) | 5 (590) | 2.74 (2.08 to 3.62) | 3.92 (2.61 to 5.91) | 4 (550) | 2.31 (1.76 to 3.10) | 3.33 (2.24 to 4.96) |
Bath Ankylosing Spondylitis Disease Activity Index 50
For the AS population BASDAI 50 data were available for all five anti-TNFs; the number of participants studied varied widely, ranging from 69 patients in one infliximab trial to 659 patients in two adalimumab trials. Although a consistent beneficial effect was evident across treatments, some heterogeneity of effect could be seen with the relative risks ranging from 3.16 (adalimumab) to 4.86 (infliximab).
For the nr-AxSpA population the relative risks were lower than for the AS population being 2.52 (95% CrI 1.65 to 3.83, two trials) for adalimumab, 2.80 (95% CrI 1.71 to 4.47, one trial) for certolizumab and 1.92 (95% CrI 1.27 to 2.82, one trial) for etanercept (see Table 8).
Results of the AS sensitivity analyses were very similar to those of the main analyses (see Table 7).
Continuous outcomes at between 10 and 16 weeks
The results of the analyses of the continuous efficacy outcomes for patients with AS are presented in Table 9.
| Intervention | Type of analysis | BASDAI score | BASFI score | BASMI score | SF-36 PCS score | SF-36 MCS score | MASES | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of trials (number of patients) | Mean difference in change from baseline (95% CrI) | Number of trials (number of patients) | Mean difference in change from baseline (95% CrI) | Number of trials (number of patients) | Mean difference in change from baseline (95% CrI) | Number of trials (number of patients) | Mean difference in change from baseline (95% CrI) | Number of trials (number of patients) | Mean difference in change from baseline (95% CrI) | Number of trials (number of patients) | Mean difference in change from baseline (95% CrI) | ||
| Adalimumab | Main | 3 (705) | –1.55 (–1.88 to –1.22) | 2 (390) | –1.25 (–1.63 to –0.87) | 2 (659) | –0.37 (–0.50 to –0.23) | 2 (659) | 3.53 (2.37 to 4.68) | 2 (659) | 1.41 (–0.19 to 3.02) | 2 (659) | –0.50 (–0.89 to –0.11) |
| Sensitivity | 2 (659) | –1.55 (–1.89 to –1.21) | 1 (344) | –1.28 (–1.68 to –0.88) | Same as the main analysis | Same as the main analysis | Same as the main analysis | Same as the main analysis | |||||
| Certolizumab pegol | Main | 1 (178) | –1.46 (–2.17 to –0.74) | 1 (178) | –1.10 (–1.83 to –0.37) | 1 (178) | –0.26 (–0.55 to 0.03) | 1 (178) | 5.64 (3.64 to 7.66) | 1 (178) | 1.25 (–2.08 to 4.61) | – | – |
| Sensitivity | Same as the main analysis | Same as the main analysis | Same as the main analysis | Same as the main analysis | Same as the main analysis | – | – | ||||||
| Etanercept | Main | 4 (483) | –1.75 (–2.14 to –1.37) | 5 (523) | –1.43 (–1.82 to –1.04) | 1 (82) | –0.37 (–0.65 to –0.09) | – | – | – | – | – | |
| Sensitivity | 2 (359) | –1.72 (–2.16 to –1.29) | 2 (359) | –1.29 (–1.76 to –0.84) | Same as the main analysis | – | – | – | – | – | |||
| Golimumab | Main | – | – | 2 (429) | –1.45 (–1.84 to –1.05) | 2 (429) | –0.11 (–0.26 to 0.04) | 2 (429) | 5.06 (3.71 to 6.40) | 2 (429) | 2.75 (1.08 to 4.40) | 1 (216) | –0.70 (–1.53 to 0.11) |
| Sensitivity | – | – | Same as the main analysis | Same as the main analysis | Same as the main analysis | Same as the main analysis | Same as the main analysis | ||||||
| Infliximab | Main | 3 (132) | –2.28 (–3.18 to –1.38) | 3 (132) | –2.16 (–3.18 to –1.12) | – | – | – | – | – | – | – | – |
| Sensitivity | 2 (111) | –2.18 (–3.14 to –1.21) | 2 (111) | –1.94 (–3.07 to –0.80) | – | – | – | – | – | – | – | – | |
| Anti-TNFs as a class | Main | 11 (1498) | –1.66 (–1.88 to –1.43) | 13 (1652) | –1.38 (–1.59 to –1.18) | 6 (1348) | –0.27 (–0.36 to –0.18) | 5 (1266) | 4.40 (3.60 to 5.21) | 5 (1266) | 1.93 (0.12 to 3.72) | 3 (875) | –0.54 (–0.89 to –0.19) |
| Sensitivity | 7 (1305) | –1.63 (–1.88 to –1.39) | 8 (1419) | –1.34 (–1.57 to –1.12) | Same as the main analysis | Same as the main analysis | Same as the main analysis | Same as the main analysis | |||||
For the AS population, when compared with placebo, adalimumab (n = 705), certolizumab pegol (n = 178), etanercept (n = 483) and infliximab (n = 132) produced statistically significant reductions in disease activity, when assessed using BASDAI. The magnitude of the reductions in change from baseline BASDAI score ranged from 1.46 units (certolizumab pegol) to 2.28 units (infliximab). None of the three golimumab trials reported BASDAI as a continuous outcome. The number of data available for BASFI in patients with AS ranged from 132 patients in three infliximab trials, to 523 patients in five etanercept trials. When compared with placebo, all five anti-TNFs produced statistically significant improvements in function. The magnitude of the reductions in change from baseline BASFI score ranged from 1.1 units (certolizumab pegol) to 2.16 units (infliximab). When compared with placebo, statistically significant improvements in BASMI scores were found for AS patients taking adalimumab (mean difference in change from baseline –0.37 units, 95% CrI –0.50 to –0.23 units) and etanercept (mean difference in change from baseline –0.37 units, 95% CrI –0.65 to –0.09 units) but not for certolizumab pegol (mean difference in change from baseline –0.26 units, 95% CrI –0.55 to 0.03 units) and golimumab (mean difference in change from baseline –0.11 units, 95% CrI –0.26 to 0.04 units). Results for SF-36 MCS, SF-36 PCS and ethesitis [Maastricht Ankylosing Spondylitis Enthesitis Score (MASES)] are presented in Table 9.
For the nr-AxSpA population, a heterogeneity of effect on BASDAI and BASFI appears evident from the relative risks of the individual anti-TNFs. The smallest estimates were for etanercept and the largest estimates were seen in the small infliximab trial, although this trial was the only nr-AxSpA trial judged to be at high risk of bias (Table 10).
| Intervention | BASDAI score | BASFI score | BASMI score | SF-36 PCS score | SF-36 MCS score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of trials (number of patients) | Mean difference in change from baseline (95% CrI) | Number of trials (number of patients) | Mean difference in change from baseline (95% CrI) | Number of trials (number of patients) | Mean difference in change from baseline (95% CrI) | Number of trials (number of patients) | Mean difference in change from baseline (95% CrI) | Number of trials (number of patients) | Mean difference in change from baseline (95% CrI) | |
| Adalimumab | 2 (188) | –1.23 (–1.83 to –0.62) | 2 (188) | –0.90 (–1.44 to –0.36) | 2 (188) | –0.02 (–0.24 to 0.20) | 2 (188) | 4.98 (2.74 to 7.20) | 2 (188) | 1.13 (–1.86 to 4.13) |
| Certolizumab pegol | 1 (147) | –1.85 (–2.83 to –0.88) | 1 (147) | –1.90 (–2.87 to –0.94) | 1 (147) | –0.55 (–0.89 to –0.20) | 1 (147) | 6.99 (4.23 to 9.76) | 1 (147) | 4.01 (0.44 to 7.53) |
| Etanercept | 1 (215) | –0.70 (–1.54 to 0.12) | 1 (215) | –0.60 (–1.16 to –0.06) | – | – | – | – | – | – |
| Infliximab | 1 (40) | –2.67 (–4.21 to –1.13) | 1 (40) | –2.24 (–3.67 to –0.80) | 1 (40) | 0.00 (–0.44 to 0.44) | 1 (40) | 2.10 (–0.21 to 4.37) | – | – |
| Anti-TNFs as a class | 5 (590) | –1.32 (–1.74 to –0.90) | 5 (590) | –0.99 (–1.34 to –0.64) | 4 (375) | –0.15 (–0.32 to 0.02) | 4 (375) | 4.41 (3.04 to 5.81) | 3 (335) | 2.33 (0.07 to 4.62) |
Results of the AS sensitivity analyses were very similar to those of the main analyses (see Table 9).
When the mean baseline BASDAI and BASFI are presented by treatment response at week 12 (or 14 for golimumab) for three of the five anti-TNFs (see Appendix 6), it can be seen that in patients with AS and patients with nr-AxSpA, on average baseline BASDAI does not differ greatly between responders and non-responders to either placebo or active anti-TNF therapy. In patients with AS or nr-AxSpA from the trials of adalimumab [ATLAS (Adalimumab Trial Evaluating Long-term Efficacy and Safety for Ankylosing Spondylitis)61 and ABILITY-158] and golimumab (GO-RAISE90) on average baseline BASFI was higher in non-responders compared with responders. However, this was not seen in the etanercept trials.
Individual anti-tumour necrosis factors compared with each other
For efficacy outcomes, all of the comparisons that could be made between different anti-TNFs at 10–16 weeks resulted in no statistically significant differences between treatments. For the full results see Appendix 7.
One small trial, which could not be included in the meta-analysis (see Study characteristics), compared infliximab with etanercept in a 2-year unblinded randomised study of 50 AS patients. 88 At 12 weeks there were statistically significant differences between groups in terms of BASDAI score (3.5 vs. 5.6; p < 0.005) and BASFI score (3.5 vs. 5; p < 0.005), favouring treatment with infliximab. By week 48, the BASDAI and BASFI scores were almost identical across the treatment groups (data were only presented graphically). In addition, at 12 weeks 19 of 25 infliximab patients were ASAS 20 responders compared with 15 of 25 etanercept patients (not a statistically significant difference). This study concluded that infliximab produces a more rapid clinical improvement, but, at the end of the study, treatment with both etanercept and infliximab was effective and safe. The results of this trial may explain why at 10–16 weeks the meta-analysis results for infliximab were a little better than those of the other anti-TNFs.
Another trial which could not be included in the meta-analysis compared infliximab with an infliximab biosimilar called CT-P13 in 250 AS patients. 110 The ASAS 40 response rates at week 14 were 42% for CT-P13 and 46% for infliximab [OR 0.85; 95% confidence interval (CI) 0.51 to 1.42] and at week 30 they were 52% for CT-P13 and 47% for infliximab (OR 1.19, 95% CI 0.70 to 2.00). At week 14 BASDAI median change from baseline scores were identical (–2.7) and at week 30 they differed slightly (–3.1 CT-P13 vs. –2.5 infliximab). For BASFI the median change from baseline scores were –2.2 CT-P13 versus –2.4 infliximab at week 14 and –2.6 CT-P13 versus –2.2 infliximab at week 30. The study concluded that CT-P13 had a comparable efficacy and safety profile with that of infliximab.
Anti-tumour necrosis factors as a class compared with placebo
Within this section the class effect, calculated as a common effect across all the TNF-inhibitors under consideration, assumes a single treatment effect for all the TNF-inhibitors. It is calculated as the pooled treatment effect using a fixed effect model. The common class-effect model may possibly underestimate the uncertainty around the treatment effect estimate. As explained in Chapter 5, if the differences between treatments is a result of systematic differences in study design between treatments then an exchangeable class-effect model may be appropriate. However, if in fact there is a true difference between treatments, such as between infliximab and the other TNF-inhibitors, then an exchangeable class-effect model may overestimate the uncertainty around the mean class-effect estimates. As the common class-effect model had a lower DIC than the exchangeable class-effect model, this is the model evaluated in this chapter. The economic model in Chapter 6 explores the assumption that treatment effect differences are because of differences in study design between treatments.
Binary responder outcomes at between 10 and 16 weeks
When compared with placebo, anti-TNFs as a common class were more than twice as likely to result in patients with AS achieving an ASAS 20 response (relative risk 2.21, 95% CrI 2.01 to 2.43; 13 trials, see Table 7). Anti-TNFs were also around three times as likely to result in patients achieving an ASAS 40 response (relative risk 3.06, 95% CrI 2.52 to 3.76; eight trials) and three and a half times as likely to result in patients achieving an ASAS 50 response (relative risk 3.51, 95% CrI 2.55 to 4.86; four trials). Only two trials, both of etanercept, reported data suitable for the ASAS 70 analysis; the results are presented in Individual anti-TNFs compared with placebo. There was little evidence of heterogeneity for ASAS 20 (I2 = 16%) and ASAS 40 (I2 = 27%) but heterogeneity was evident for ASAS 50 (I2 = 52%). For ASAS 50, three of the four trials were small (i.e. fewer than 100 participants), which may partly explain the heterogeneity estimate.
For the nr-AxSpA population anti-TNFs as a common class were statistically significantly more effective than placebo, although the relative risks being lower than for the AS population. For ASAS 20 the relative risk was 1.65 (95% CrI 1.37 to 2.04; four trials) and for ASAS 40 the relative risk was 2.74 (95% CrI 2.08 to 3.62; five trials). Only one trial presented ASAS 50 and ASAS 70 results (see Clinical effectiveness results: efficacy results from randomised controlled trials). A heterogeneity estimate could be calculated for only ASAS 40 (I2 = 49%).
Anti-TNFs as a common class resulted in patients with AS being more than three times more likely to achieve a BASDAI 50 response than patients taking placebo (relative risk 3.37, 95% CrI 2.75 to 4.16; nine trials). There was little evidence of heterogeneity (I2 = 21%).
For the nr-AxSpA population, anti-TNFs as a common class were also statistically significantly more effective than placebo in terms of achieving a BASDAI 50, although the relative risk was lower than for the AS population (relative risk 2.31, 95% CrI 1.76 to 3.10; four trials). Results of the AS sensitivity analyses were very similar to the main analyses (see Table 7).
Binary responder outcomes at between 24 and 30 weeks
Four AS trials reported outcomes at between 24 and 30 weeks (see Table 2). Anti-TNFs as a common class were statistically significantly more effective than placebo at 24–30 weeks; for ASAS 20 the relative risk was 1.69 (95% CrI 1.30 to 2.14; four trials). No studies reported BASDAI 50 or ASAS 70 results, and only single studies reported on ASAS 40 (relative risk 4.01, 95% CrI 2.13 to 7.55)102 and ASAS 50 (relative risk 4.17, 95% CrI 2.45 to 7.12). 72
Continuous outcomes at between 10 and 16 weeks
When considered together as a group compared with placebo (see Table 9), treatment with an anti-TNF in patients with AS produced statistically significant improvements (calculated using mean difference in change from baseline) in the following areas: disease activity (BASDAI mean difference –1.66 units, 95% CrI –1.88 to –1.43 units; 11 trials); function (BASFI mean difference –1.38 units, 95% CrI –1.59 to –1.18 units; 13 trials); spinal mobility (BASMI mean difference –0.27 units, 95% CrI –0.36 to –0.18 units); physical health (SF-36 PCS mean difference 4.40 units, 95% CrI 3.60 to 5.21 units; five trials); mental health (SF-36 MCS mean difference 1.96 units, 95% CrI 0.87 to 3.05 units; five trials); and enthesitis (MASES mean difference –0.54 units, 95% CrI –0.89 to –0.19 units; three trials). There was little evidence of heterogeneity for BASDAI (I2 = 21%) and BASFI (I2 = 10%), but evidence of substantial heterogeneity for BASMI (I2 = 77%), SF-36 PCS (I2 = 76%), SF-36 MCS (I2 = 47%) and MASES (I2 = 91%).
In the nr-AxSpA population the mean differences achieved with anti-TNFs (see Table 10) were also statistically significant, although slightly lower than for the AS population. For BASDAI the mean difference was –1.32 units (95% CrI –1.74 to –0.90; I2 = 69%) and for BASFI the mean difference was –0.99 units (95% CrI –1.34 to –0.64 units; I2 = 83%) but there was evidence of substantial heterogeneity. The results for SF-36 MCS and SF-36 PCS were similar to those for AS (see Table 10).
Results of the AS sensitivity analyses were very similar to the main analyses (see Table 9). As the results of the independent treatment effects showed a trend that infliximab had a greater, although not statistically significant, effect on the change in BASDAI and BASFI from baseline, an additional sensitivity analysis was conducted for which infliximab was assumed to be different from the rest of the anti-TNFs. The results are presented in Table 11. The low weight of evidence available for infliximab ensures that the class effect for the other anti-TNFs does not change greatly. Although it is possible that infliximab has a greater effect than the other anti-TNFs at least at 12 weeks, there is no strong evidence from these analyses to suggest that it does.
| Category of intervention | BASDAI score | BASFI score | ||
|---|---|---|---|---|
| Mean | 95% CrI | Mean | 95% CrI | |
| All TNFs | –1.66 | –1.88 to –1.43 | –1.38 | –1.59 to –1.18 |
| TNFs other than infliximab | –1.62 | –1.85 to –1.38 | –1.35 | –1.56 to –1.14 |
| Infliximab | –2.28 | –3.18 to –1.38 | –2.15 | –3.18 to –1.11 |
Continuous outcomes at between 24 and 30 weeks
Four AS trials reported outcomes at between 24 and 30 weeks (see Table 2). The mean differences in change from baseline were –1.98 units (95% CrI –2.27 to –1.68 units, four trials) for BASDAI, –0.87 units (95% CrI –1.11 to –0.62 units; three trials)72,100,102 for BASFI, and –1.00 unit (95% CrI –1.19 to –0.81 units; two studies)97,102 for BASMI. One study reported SF-36 outcomes, with differences of 9.40 units (95% CrI 7.88 to 10.92 units) for SF-36 PCS and 0.70 units (95% CrI –1.36 to 2.76 units) for SF-36 MCS. 102
Outcomes not included in the meta-analyses
Very few data were available on peripheral symptoms (other than enthesitis, see the MASES results in Table 9) or symptoms of extra-articular manifestations. One trial reported five cases of inflammatory bowel disease flare up to the 24-week time point: three occurred in patients on etanercept and two in patients on placebo. 72 Another study reported that there were no cases of inflammatory bowel disease at 12 weeks. 86 Incidence of uveitis was also reported in one trial; up to the 24-week time point there were three cases in the etanercept arm and eight cases in the placebo arm. 72
One trial (ABILITY-158) reported statistically significantly improved quality of life, using EQ-5D index scores, in patients taking adalimumab [change from baseline 0.15 units (SD 0.30 units)] when compared with those taking placebo [change from baseline 0.06 units (SD 0.28 units)]. A study of adalimumab reported no statistically significant difference in EQ-5D between groups at 12 weeks (0.78 units for adalimumab vs. 0.72 units for placebo; p = 0.32). 51
For Ankylosing Spondylitis Quality of Life (ASQoL), a quality of life instrument specific to AS, ATLAS61 was the only trial which reported results together with SDs or standard errors (SEs); significant improvements were found favouring treatment with adalimumab at week 12 [mean change from baseline –3.2 units (SD 0.3 units) for adalimumab vs. –1 unit (SD 0.4 units) for placebo]. 62 Similar statistically significant results were reported in an etanercept trial at 12 weeks (mean change from baseline –3.3 units for etanercept vs. –0.1 units for placebo; p = 0.02)71 and in an infliximab trial at 16 weeks (mean change from baseline –6.2 units for infliximab vs. –1 unit for placebo; p = 0.007). 50 Another small study of infliximab did not find a significant difference between groups at 30 weeks (p = 0.14). 100
‘Placebo’ response in ankylosing spondylitis and non-radiographic axial spondyloarthritis
To inform insight into the extent of any ‘placebo’ effects (outlined in Chapter 1, Description of health problem), Table 12 compares the placebo response rates in trials which reported ASAS 20 results and at least one of ASAS 40 or BASDAI 50 results. These data highlight the relatively high rates of ASAS 20 response (median 31%, range 21–40%) when compared with ASAS 40 response (median 15%, range 10–23%) and BASDAI 50 response (median 16%, range 5–24%).
| Population and study | Placebo compared with | Time point (weeks) | Number of patients on placebo | Number of responders | % of responders | Difference in response (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASAS 20 | ASAS 40 | BASDAI 50 | ASAS 20 | ASAS 40 | BASDAI 50 | ASAS 20 vs. ASAS 40 | ASAS 20 vs. BASDAI 50 | ASAS 40 vs. BASDAI 50 | ||||
| Nr-axSpA51 | Adalimumab | 12 | 24 | 6 | 3 | 5 | 25 | 13 | 21 | 13 | 4 | –8 |
| AS56 | Adalimumab | 12 | 115 | 35 | 11 | 19 | 30 | 10 | 17 | 21 | 14 | –7 |
| Nr-axSpA58 | Adalimumab | 12 | 73 | 23 | 10 | 10 | 32 | 14 | 14 | 18 | 18 | 0 |
| AS61 | Adalimumab | 12 | 107 | 22 | 14 | 17 | 21 | 13 | 16 | 7 | 5 | –3 |
| AS64 | Certolizumab | 12 | 57 | 21 | 11 | 6 | 37 | 19 | 11 | 18 | 26 | 9 |
| Nr-axSpA64 | Certolizumab | 12 | 50 | 20 | 8 | 8 | 40 | 16 | 16 | 24 | 24 | 0 |
| AS74 | Etanercept | 12 | 43 | 14 | 10 | 10 | 33 | 23 | 23 | 9 | 9 | 0 |
| Nr-axSpA76 | Etanercept | 12 | 109 | 39 | 17 | 26 | 36 | 16 | 24 | 20 | 12 | –8 |
| AS86 | Etanercept | 12 | 51 | 19 | 11 | 10 | 37 | 22 | 20 | 16 | 18 | 2 |
| AS90 | Golimumab | 14 | 78 | 17 | 12 | 12 | 22 | 15 | 15 | 6 | 6 | 0 |
| AS95 | Golimumab | 14 | 105 | 26 | 10 | 5 | 25 | 10 | 5 | 15 | 20 | 5 |
| AS98 | Infliximab | 12 | 35 | 10 | – | 3 | 29 | – | 9 | – | 20 | – |
However, the extent of the ‘placebo’ response on the ASAS 20 results might result in an underestimation of anti-TNF efficacy, notably when ASAS 20 is the only ASAS improvement outcome reported in a trial. An increase in the likelihood of being a responder (i.e. the relative risks when compared with placebo) when moving up the ASAS thresholds seems apparent from the results in Clinical effectiveness results: efficacy results from randomised controlled trials. This might be explained by considering the subset of patients who achieve an ASAS 20 response largely because of regression to the mean (i.e. because of natural variation in repeated data measurements, such as patients transitioning from flare at randomisation to no flare at 12 weeks). For those patients who experience regression to the mean after taking an anti-TNF, the true benefit of treatment may be hidden in the ASAS 20 outcome for some patients, and the proportion of ASAS 20 responders might therefore differ only moderately between the anti-TNF and placebo groups. As the bar for response is raised, from ASAS 20 through to ASAS 70, this difference in the proportion of responders between active treatment and placebo groups is likely to increase as an effect because regression to the mean becomes less probable. The diluting effect of a placebo response on the relative risks therefore diminishes as the ASAS thresholds increase (and more informative estimates of treatment benefit can be seen). Regardless of the reason, these results highlight the limited applicability of ASAS 20 as a clinically informative outcome measure. ASAS 20 was nevertheless the most commonly reported responder outcome across the trials.
Summary of the randomised controlled trial clinical efficacy results
For both the AS and nr-AxSpA populations the results of the meta-analyses demonstrated that anti-TNFs produce statistically significant and clinically relevant benefits to patients in terms of improving function and reducing disease activity. The common class-effect model used may have underestimated the uncertainty in the effect estimates. Although there is a possibility that infliximab is more effective than other TNF inhibitors, at least at 12 weeks, there is no strong evidence to support this. For the disease activity, function and responder outcomes, the class-efficacy estimates were consistently slightly smaller for nr-AxSpA than for AS, most noticeably for BASFI and BASDAI 50.
The included RCTs were generally subject to low risks of bias and no important variation in baseline characteristics was evident, with the exception of CRP levels: in the nr-AxSpA trial populations CRP levels were much lower than in the AS populations. Although heterogeneity of CRP levels was evident across both the AS trials and the nr-AxSpA trials, in almost all the AS trials the CRP levels were higher than the 14 mg/l threshold identified as being a key predictor of treatment response (in AS, higher CRP levels are associated with an increased likelihood of BASDAI 50 response). 112 In the nr-AxSpA trials only the RAPID-axSpA64 population came close to this cut-off point. These lower CRP levels may therefore have had an impact on the efficacy estimates for the nr-AxSpA population.
Statistical heterogeneity was more apparent in the nr-AxSpA analyses than in the AS analyses. This may be a result of both clinical heterogeneity in the nr-AxSpA trials (such as variation in CRP thresholds, or the proportion of MRI positive patients) and the fact that fewer studies were available for analysis. In the light of the statistical heterogeneity across the nr-AxSpA trials, both the reliability of the nr-AxSpA-pooled estimates and their true relevance to patients seen in clinical practice are questionable.
The clinical relevance of the efficacy of anti-TNFs can be evaluated in part by considering the literature on minimum clinically important differences (MCIDs) or minimum clinically important improvements. In a study of 125 AS patients, Pavy et al. 113 reported a MCID of 1 unit (or a 20% relative change) for BASDAI and 0.7 units (17.5% relative change) for BASFI. All the effect estimates from this review for both BASDAI and BASFI were considerably higher than these MCIDs. The small effect on spinal mobility (a group effect reduction of around 0.3 BASMI units) appears unlikely to be clinically important.
Summary of some key issues arising from the Food and Drug Administration assessments of the ABILITY-158 and RAPID-axSpA64 trials
The FDA Arthritis Advisory Committee met in July 2013 to discuss licence applications for adalimumab for patients with active nr-AxSpA (with objective signs of inflammation) and certolizumab pegol for patients with active axSpA, including patients with AS. 114 An important issue which arose in both trials was the differences in diagnoses arising from radiograph images evaluated centrally compared with images being evaluated locally. The implications for efficacy were explored via further analyses.
RAPID-axSpA64 trial (certolizumab pegol)
This trial aimed to recruit both AS and nr-AxSpA patients. 64 The nr-AxSpA patients had to have a positive MRI or an elevated CRP level; the definition used for CRP level elevation was 7.9 mg/l.
In AS males predominated (72%), whereas in nr-AxSpA the male-to-female ratio was roughly equal. The AS population had a mean age of 41.5 years, which was around four years older than the nr-AxSpA population. Baseline BASFI, BASMI and CRP levels suggested more functional and mobility impairment and more inflammation in the AS group when compared with the nr-AxSpA group. However, baseline back pain severity and BASDAI scores were similar between the AS and nr-AxSpA subgroups (Table 13).
| Trial and population | Characteristic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years), mean | % male | Duration (years) of symptoms | Weight (kg), mean | % HLA-B27 positive | % on NSAIDs | CRP level | % MRI positive | BASDAI score, mean | BASFI score, mean | |
| ABILITY-1,58 nr-AxSpA (n = 142) | 38 | 46 | Median 8, mean 11 | 80 | 80 | 81 | Median ≈4, mean 9 | 51 | 6 | 4.7 |
| RAPID-axSpA,64 nr-AxSpA (n = 147) | 37 | 48 | Median 5.5, mean 8.6 | 82 | 75 | 84 | Median 11.9, mean 16 | 54 | 6.5 | 4.9 |
| RAPID-axSpA,64 AS (n = 178) | 42 | 73 | Median 9.1, mean 11.9 | 82 | 82 | 91 | Median 14.3, mean 21.3 | N/A | 6.4 | 5.7 |
In the trial, many patients had their disease reclassified when radiographs were evaluated centrally, rather than being evaluated locally. Two readers were involved in the central evaluation of the radiographs, they were blinded to both the assigned subgroup and the treatment group; a third reader was used in cases of disagreement. Twenty-one per cent of locally classified AS patients were reclassified as nr-AxSpA by central readers and 51% of locally classified nr-AxSpA patients were reclassified as AS by the central readers. Based on the central assessments 184 patients had AS and 98 patients had nr-AxSpA. Central reads could not be made for 43 patients as radiographs were not available (37 AS patients and six nr-AxSpA patients).
ABILITY-158 trial (adalimumab)
This trial intended to recruit only nr-AxSpA patients, although this included patients (n = 43) who had nr-AxSpA but neither a positive MRI nor an elevated CRP level. 58 The population with these 43 patients excluded is referred to as the ‘adalimumab target population’ (ATP). As in the RAPID-axSpA64 trial, central rereading of radiographs was performed (in addition to local evaluation), although this was only done for per-protocol patients who also reached week 104 [n = 102 (out of 185) patients]. Thirty-eight of the 102 patients were identified as having AS rather than nr-AxSpA. The FDA statistician analysed the results in these 38 patients and compared them to those for patients with centrally confirmed nr-AxSpA. The FDA document reported results for the subpopulations based on local or central diagnosis, including ATP analyses.
Comparison of ankylosing spondylitis and non-radiographic axial spondyloarthritis results and impact of reclassification in the trials
For certolizumab pegol the FDA statistical review stated that ‘efficacy findings were consistent in both AS and nr-AxSpA subpopulations regardless of the discrepancy in pelvic X-ray readings at local or central lab for modified New York criteria’115 (Table 14).
| Outcomes at week 12 | ABILITY-158 ATP population | RAPID-axSpA64 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Local laboratory nr-AxSpAa | Central laboratory nr-AxSpAb | Local laboratory nr-AxSpAc | Central laboratory nr-AxSpAd | Local laboratory ASe | Central laboratory ASf | |||||
| Adalimumab, 40 mg, % (95% CI) | Adalimumab, 40 mg, % (95% CI) | Certolizumab, 200 mg, % (95% CI) | Certolizumab, 400 mg, % (95% CI) | Certolizumab, 200 mg, % (95% CI) | Certolizumab, 400 mg, % (95% CI) | Certolizumab, 200 mg, % (95% CI) | Certolizumab, 400 mg, % (95% CI) | Certolizumab, 200 mg, % (95% CI) | Certolizumab, 400 mg, % (95% CI) | |
| ASAS 20 | 28 (12 to 44) | 15 (–14 to 44) | 19 (1 to 38) | 23 (4 to 42) | 23 (2 to 44) | 23 (1 to 44) | 20 (3 to 37) | 27 (10 to 45) | 17 (1 to 33) | 23 (7 to 39) |
| ASAS 40 | 27 (13 to 41) | 11 (–16 to 38) | 32 (14 to 49) | 31 (14 to 48) | 18 (0 to 36) | 27 (8 to 47) | 21 (5 to 36) | 31 (14 to 47) | 28 (13 to 43) | 33 (17 to 48) |
| BASDAI 50 | 25 (11 to 39) | 19 ( –8 to 46) | – | – | – | – | – | – | – | – |
For ABILITY-158 a notably higher proportion of patients in the AS subgroup responded to adalimumab (ASAS 40) than placebo compared with patients with confirmed nr-AxSpA. This suggests that the treatment benefit in the whole trial population may be driven by benefit in AS patients rather than in nr-AxSpA patients, skewing the results for the ATP (see Table 14). It should be noted, however, that this may be an atypical AS population; the trial had intended to recruit only nr-AxSpA patients.
Owing to the fact that only a select group of patients could be subject to central confirmation of their nr-AxSpA status, the FDA statistician explored assumptions around the proportion of true nr-AxSpA patients in the whole trial population. Given that the treatment difference in the non-centrally read patients was 23%:
-
Assuming that all non-centrally read patients were true negatives and therefore including them in the analysis with the centrally read negatives, the treatment difference for the centrally read and non-centrally read negatives was 15%.
-
Assuming that a fraction (i.e. 63%) of non-centrally read patients were true negatives and including only this fraction of non-centrally read patients with the centrally read negatives, the treatment difference was 14%.
The FDA document stated that:
Because there was a differential treatment effect between the centrally-read positive and centrally-read negative, it is safe to assume that the difference of 23% is an overestimate of the treatment effect because this includes both positive and negative x-ray groups. If there is a fraction of patients who are negative in the non-centrally-read group, treatment difference among this negative group would be smaller. Therefore, the treatment difference for negative x-rays (i.e. centrally-read and non-centrally-read) should be at most 15%. Based on the data provided, the estimate of the treatment effect in ASAS40 response for nr-AxSpA should be no bigger than 15%
FDA Briefing Package116
Overall, the results suggest reduced efficacy of anti-TNFs in the centrally diagnosed nr-AxSpA population compared with the locally diagnosed population. Nevertheless, there was noticeable variation across the two trials. In RAPID-axSpA64 (certolizumab) the difference between the central and local populations appears small (and is not evident for 400-mg vs. placebo results). Conversely, in ABILITY-158 (adalimumab) the locally diagnosed population had notably more responders than the centrally diagnosed population, although the treatment group sample sizes were small.
Long-term efficacy results from open-label extensions of randomised controlled trials
Of the 24 included RCTs, 17 reported data from an open-label extension phase. Results for all studies are presented in Appendix 8. Considerable effort has been put into patient follow-up in anti-TNF trials with the result that data up to 5 years are available (there are data up to 8 years for infliximab but these included an involuntary treatment break which is not discussed further). The longest follow-up durations in patients with AS by anti-TNF are adalimumab 260 weeks, etanercept 264 weeks, infliximab 156 weeks, golimumab 268 weeks and certolizumab pegol 96 weeks. However, the data were reported across numerous publications and in various formats. Results were reported as observed, as completer analyses, using imputation (and rarely LOCF) for non-responders and LOCF for missing continuous data, but these related to differing populations (at varying time points): all patients randomised, all patients who took active drug at any point in the study or all patients who took active drug just during the open-label phase. The follow-up protocols were not clearly reported, with stopping rules unclear, but it appears that not all non-responders discontinued therapy. Therefore, the results may not reflect clinical practice should response be required for treatment continuation.
Table 15 presents the results based on non-responder imputation (NRI) analyses for the main studies when these results could be extracted. For AS the results show that across all the anti-TNFs after approximately 2 years of treatment, around half of patients are still achieving a good level of response to therapy. The results for golimumab look particularly strong with around 60% of all randomised patients achieving ASAS 40 and BASDAI 50 after 5 years. However, this is probably not reflective of clinical practice, as many of the normal weight patients took the 100-mg dose of golimumab rather than the 50-mg dose: the licence permits the use of 100-mg dose only in patients with a body weight of more than 100 kg who do not achieve an adequate clinical response after three or four doses. The equivalent results for adalimumab and etanercept are approximately 30% and 50%, although it is unknown if the difference may be because of variations in follow-up protocols rather than true treatment difference.
| Outcome | Trial | 52 weeks, n/N (%) | 104 weeks, n/N (%) | 156 weeks, n/N (%) | 5 years (approximately 264 weeks), n/N (%) |
|---|---|---|---|---|---|
| Adalimumab | |||||
| ASAS 20 | ATLAS61 | 193/311 (62)a | 135/311 (43)a | – | 111/311 (36)a |
| ASAS 40 | ATLAS61 | 138/311 (44)a | 109/311 (35)a | – | 88/311 (28)a |
| BASDAI 50 | ATLAS61 | 167/311 (54)a | 122/311 (39)a | – | 96/311 (31)a |
| Certolizumab | |||||
| ASAS 20 | RAPID-axSpA64 (AS) | (48 weeks) 89/121 (74)b | (96 weeks) 78/121 (64)b | – | – |
| ASAS 40 | RAPID-axSpA64 (AS) | (48 weeks) 70/121 (58)b | (96 weeks) 61/121 (50)b | – | – |
| BASDAI 50 | – | – | – | – | – |
| Etanercept | |||||
| ASAS 20 | Calin 200483 | – | (108 weeks) 52/81 (64)c | – | – |
| ASAS 40 | Calin 200483 | – | (108 weeks) 44/81 (54)c | – | 40/81 (49)c |
| BASDAI 50 | Calin 200483 | – | (108 weeks) 42/81 (52)c | – | 39/81 (48)c |
| Golimumab | |||||
| ASAS 20 | GO-RAISE90 | – | 235/356 (66)b | (160 weeks) 246/356 (69)b | 235/356 (66)b |
| ASAS 40 | GO-RAISE90 | – | 203/356 (57)b | (160 weeks) 208/356 (58)b | 203/356 (57)b |
| BASDAI 50 | GO-RAISE90 | – | 199/356 (58)b | – | 199/356 (58)b |
| Infliximab | |||||
| ASAS 20 | PLANETAS 2013110 | (78 weeks) 125/174 (72)c,d | (102 weeks) 127/174 (73)c,d | – | – |
| ASAS 40 | – | (78 weeks) 93/174 (53)c,d | (102 weeks) 101/174 (58)c,d | – | – |
| ASAS 40 | ASSERT 2005102 | (102 weeks) | 33/78 (42)b,e | – | – |
| BASDAI 50 | Braun 200298 | (54 weeks) 33/69 (48)b | (102 weeks) 30/69 (43)b | – | – |
The long-term follow-up for nr-AxSpA patients (Table 16) shows continued high proportions of responders. At 1 year around half of patients are achieving an ASAS 40 or BASDAI 50 level response and with certolizumab this is maintained at 2 years and with adalimumab at 3 years.
| Outcome | Trial | 52 weeks, n/N (%) | 104 weeks, n/N (%) | 156 weeks, n/N (%) | 5 years (approximately 264 weeks), n/N (%) |
|---|---|---|---|---|---|
| Adalimumab | |||||
| ASAS 20 | ABILITY-158 | – | – | 83/142 (58)a | – |
| ASAS 40 | Haibel 200852 | 23/46 (50)b | – | – | – |
| ASAS 40 | ABILITY-158 | (68 weeks) 77/142 (54)a | – | 67/142 (47)a | – |
| BASDAI 50 | Haibel 200852 | 24/46 (52) | – | – | – |
| BASDAI 50 | ABILITY-158 | (68 weeks) 74/142 (52)a | – | 70/142 (49)a | – |
| Certolizumab | |||||
| ASAS 20 | RAPID-axSpA64 (AS) | (48 weeks) 68/97 (70)b | (96 weeks) 59/97 (61)b | – | – |
| ASAS 40 | RAPID-axSpA64 (AS) | (48 weeks) 56/97 (58)b | (96 weeks) 49/97 (51)b | – | – |
| BASDAI 50 | – | – | – | – | – |
| Etanercept | |||||
| ASAS 20 | Dougados 201476 | (48 weeks) (CiC information has been removed) | – | – | – |
| ASAS 40 | – | (48 weeks) 108/205 (53)b | – | – | – |
| BASDAI 50 | – | (48 weeks) (CiC information has been removed) | – | – | – |
When the long-term data are presented as observed or completer analyses, the long-term results are similarly good; withdrawal rates are not high and a high proportion of those who remain on treatment continue to achieve a good response, see the example data available from one trial of adalimumab and one of certolizumab pegol (Table 17).
| Trial, anti-TNF (population) | Time point | Type of analysis | ASAS 20, n/N (%) | ASAS 40, n/N (%) | BASDAI 50, n/N (%) |
|---|---|---|---|---|---|
| ATLAS 2006,61 adalimumab (AS) | 52 weeks | Observed | 193/276 (70) | 138/276 (50) | 167/276 (61) |
| 104 weeks | Observed | 135/173 (78) | 109/173 (63) | 122/173 (71) | |
| 5 years | Completer | 111/125 (89) | 88/125 (70) | 96/124 (77) | |
| RAPID-axSpA,64 certolizumab pegol; all (AS) | 96 weeks | Observed | 78/93 (84) | 61/93 (66) | – |
| RAPID-axSpA,64 certolizumab pegol; all (nr-AxSpA) | 96 weeks | Observed | 59/74 (80) | 49/74 (66) | – |
At long-term follow-up mean final values or mean change from baseline for BASDAI, BASFI and BASMI, when reported, were generally maintained at clinically meaningful levels.
For adalimumab, data from the large ATLAS trial61 showed that mean changes from baseline at 1, 2 and 3 years remain stable and clinically meaningful at around –3.7 units for BASDAI and at around –2.9 units for BASFI. Similarly, the mean final value for BASMI remains at a level indicative of clinically significant treatment benefit (3.1 to 3.7 units). At 5 years the mean final values are BASDAI 1.8 units, BASFI 2.1 units, and BASMI 3.7 units. Clearly these results relate only to those patients who have remained on adalimumab in the long-term (40% of those who started adalimumab). They do, however, demonstrate continued benefit in a significant proportion of patients.
For certolizumab, results for these outcomes are available up to 96 weeks. At this time point the mean BASDAI and BASFI are indicative of clinically significant treatment benefit (both around 3 units).
The long-term data from Calin et al. 83 for etanercept, with 81 patients at 2 years and 59 (73%) remaining at 5 years also report mean BASDAI and BASFI scores of around 3.
From GO-RAISE90 at 2 years for those who took golimumab throughout the trial and follow-up (n = 138), median BASDAI score was around 3 and median BASFI score was around 2. These values are from a LOCF analysis of all patients randomised to golimumab 50 mg.
For infliximab, the Braun et al. 98 and follow-up studies found, from 1 to 3 years, a stable mean BASDAI score of around 2.6, a stable mean BASFI score of around 3 and a stable mean BASMI score of around 2.7.
Overall, the reported data (although not particularly robust) do indicate that significant proportions of patients continue to derive real benefit from continued use of anti-TNFs. There is nothing to indicate any difference between them.
Almost no data were available regarding radiographic progression of bony disease in patients with AS. Furthermore, it should be noted that radiographic changes and progression of these take many years to appear and radiography is an insensitive tool by which to evaluate the progression of AS. Therefore, evidence, particularly that from relatively short-term studies, has to be interpreted with caution. The limited evidence includes mSASSS change from baseline, reported for golimumab from the GO-RAISE90 study at 4 years (208 weeks): 1.3 units (SD 4.1 units) based on the 111 of 138 patients randomised to 50 mg. As results from untreated cohorts suggest a progression rate of 2 units/2 years, a rate of 1.3 units (or even 2 units) over 4 years seems beneficial. For further discussion of this issue see Effect of anti-tumour necrosis factors on radiographic progression. MASES was reported only for adalimumab from ATLAS;61 in patients remaining on therapy at 2 years the mean change from baseline was 2.2 units (n = 217).
For nr-AxSpA patients long-term data for the continuous outcomes was limited to 1 year’s follow-up. For adalimumab, data were available from only one small study (Haibel 2008,52 n = 46): BASDAI change from baseline 2.8 units (95% CI 2.1 to 3.6 units); BASFI change from baseline 2 units (95% CI 1.4 to 2.6 units); BASMI change from baseline –0.4 units (95% CI –0.7 to –0.04 units); and MASES change from baseline of 0.9 units (95% CI –0.02 to 1.9 units). In addition, of 26 patients with magnetic resonance images at baseline and 52 weeks’ follow-up, none showed a change in sclerosis or in erosions. For etanercept, data were available on 205 patients randomised to etanercept or placebo and then on long-term etanercept (Dougados 201476): [commercial-in-confidence (CiC) information has been removed]. For certolizumab, LOCF analysis at 96 weeks (n = 97) gave a BASDAI final value score of 3.0, and a BASFI score of 2.4. Overall, the 1-year results in nr-AxSpA patients are similar to each other and also reflect those seen in AS patients. Again, the short-term nature of this follow-up relative to the 8–10 years over which radiographic changes develop must be borne in mind.
Findings from anti-tumour necrosis factor patient registry studies
Effect of anti-tumour necrosis factors on radiographic progression
A total of seven studies were identified that provided some comparative results on the effect of anti-TNFs on radiographic progression (Table 18).
| Study | Methods | Results |
|---|---|---|
| van der Heijde et al. 2009117 | Study used 2-year data from active treatment arms of two adalimumab trials (total n = 397) and compared them with OASIS cohort118 (186 with radiographs at 2 years). Note: primary analysis set = 307 adalimumab (minimum of 1.5 years exposure to drug) and 169 anti-TNF naive (OASIS) | There were significant differences between adalimumab and OASIS118 patients at baseline for BASDAI, BASFI and other measures. Increase in mSASSS was very similar in the two groups: adalimumab mean 0.8 (SD 2.6) and OASIS mean 0.9 (SD 3.3). When only patients who would have qualified for the adalimumab trials were included in the OASIS cohort (n = 77) the results were not changed. Note: in the light of these van der Heijde results, it would have been good to test effect of baseline BASDAI (mean 6.2 in adalimumab cohort and 3.4 in OASIS), as without treatment progression in adalimumab cohort would have been expected to be higher than in the OASIS one, so there might have been some effect of adalimumab |
| van der Heijde, et al. 2008103 | Study compared 2-year data from infliximab trial (ASSERT102) (n = 201) with that from OASIS118 (n = 192). OASIS patients not treated with any anti-TNF | There were significant differences between infliximab and OASIS118 patients at baseline for BASDAI, BASFI and other measures (higher disease activity and worse function in trial patients). Mean increase in mSASSS was very similar in the two groups: infliximab 0.9 (SD 2.6) and OASIS 1.0 (SD 3.2). When only patients who would have qualified for the infliximab trials were included in the OASIS cohort (n = 70), the results changed very little [mean mSASSS increase 1.2 (SD 3.9)] |
| van der Heijde, et al. 2008119 | Study compared 2-year data from etanercept trial (Davis et al.72) (n = 257) with that from OASIS118 (n = 175). OASIS patients not treated with any anti-TNF | There were significant differences between infliximab and OASIS118 patients at baseline for BASDAI, BASFI and other measures (higher disease activity and worse function in trial patients). Mean increase in mSASSS was very similar in the two groups: etanercept 0.91 (SD 2.5) and OASIS 0.95 (SD 3.2). When only patients who would have qualified for the etanercept trials were included in the OASIS cohort (n = 76), the results changed very little [mean mSASSS increase 1.3 (SD 3.6)] |
| Braun et al. 2014120 | Long-term data on golimumab (2- and 4-year radiographic data) (n = 233). No comparison with OASIS118 made | Mean increase in mSASSS to 2 years was 0.9 (SD 2.7) (50 mg) and 0.9 (SD 3.9) (100 mg). Mean increase in mSASSS to 4 years was 1.3 (SD 4.1) (50 mg) and 2.0 (SD 5.6) (100 mg). Note: 2-year results are very similar to those with other anti-TNFs and OASIS,118 that is there is no benefit of golimumab evident |
| Haroon et al. 2013121 | Cohort study (n = 334) with at least two spinal radiographs at 2-year intervals (patients with total spinal fusion at baseline excluded). Logistic regression analysis tested for baseline mSASSS, ESR, BASDAI, smoking, male vs. female, age at onset, disease duration, HLA-B27, anti-TNF use and NSAID index. Further analysis tested factors that could influence exposure to anti-TNFs using propensity matching | In total, 201 out of 334 patients had received anti-TNFs for a mean of 2.5 years (SD 2.6 years). No radiographic abnormality of the spine was seen at baseline in 144 patients (43%) and 102 patients (30.5%) showed no progression (> 1 mSASSS unit/year). Mulitvariate regression found baseline mSASSS (OR 1.06, 95% CI 1.04 to 1.08), ESR and smoking significantly increased and anti-TNF use significantly increased odds of radiographic progression (OR 0.47, 95% CI 0.24 to 0.94). Further analysis using the 142 patients who could be included post propensity matching confirmed these findings except for ESR: baseline mSASSS (OR 1.05, 95% CI 1.02 to 1.08) and anti-TNF (OR 0.30, 95% CI 0.11 to 0.78). Note: the association with anti-TNF use is explained by the more severe patients with radiographic changes at baseline being treated with anti-TNFs |
| Barialiakos et al. 2014122 | Comparison of long-term (8 years) treatment with infliximab with historical cohort (infliximab n = 22 and Herne cohort n = 34) | Progression as assessed by mSASSS increased equally in infliximab treated patients and in the Herne cohort from baseline to 2, 4 and 6 years but while progression increased only slightly in the infliximab group between 6 and 8 years it increased greatly in the Herne cohort so that at 8 years there was a difference in infliximab’s favour of 4.5 mSASSS (p = 0.047). Result was adjusted for baseline mSASSS. Other factors (age, symptom duration, BASDAI, BASFI) not significant confounders |
| Barialiakos 2007123 | 4-year radiographic progression in AS patients treated with infliximab (n = 33). Crude comparison made with OASIS cohort118 results at 4 years | At baseline, mean mSASSS was 11.6 (15.3 SD), mean BASDAI was 6.6 (1.4 SD) and mean BASFI was 3.5 (1.9 SD). Progression assessed by mSASSS. Mean change over 4 years was 1.6 (SD 2.6) mSASSS units. Published results for OASIS are 4.4 units in 4 years |
Four studies reported on disease progression over 2 years of follow-up in terms of mSASSS in patients taking adalimumab,117 infliximab,103 etanercept119 and golimumab. 120 All four open-label, uncontrolled follow-up studies found that mSASSS increased by a mean of around 0.9 units over 2 years. Three of these studies compared their rates with those from the Outcomes in Ankylosing Spondylitis International Study (OASIS) cohort118 (of patients not taking an anti-TNF) and found no difference (mean rate over 2 years for OASIS was 0.9 units, Table 19). As stated in the previous section, radiographic changes and progression of these take many years to appear and, therefore, the evidence from these relatively short-term studies should be interpreted with caution.
| Trial, anti-TNF | Increase in mSASSS over 2 years, patients on an anti-TNF | Increase in mSASSS over 2 years, patients from OASIS cohort118 | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| van der Heijde 2009,117 adalimumab | 397 | 0.8 (SD 2.6) | 186 | 0.9 (SD 3.3) |
| van der Heijde 2008, etanercept119 | 257 | 0.91 (SD 2.45) | 175 | 0.95 (SD 3.2) |
| Infliximab103 | 201 | 0.9 (SD 2.6) | 192 | 1.0 (SD 3.2) |
| Golimumab120 | 111 | 50 mg, 0.9 (SD 2.7) | – | – |
| 122 | 100 mg, 0.9 (SD 3.9) | – | – | |
Comparison of the rates calculated from the OASIS cohort118 in these studies with those from the studies by Ramiro124,125 highlight a discrepancy; the latter reported rates of 2 mSASSS units every 2 years, rather than the 0.9 units/2 years used here to compare with individual anti-TNFs.
Two very small studies of infliximab reported some inhibiting effect on radiographic progression. 122,123 The first123 compared findings in 22 infliximab patients with 34 from the HERNE cohort, over 2, 4, 6 and 8 years. Progression as assessed by mSASSS increased equally in infliximab treated patients and in the untreated HERNE cohort from baseline to 2, 4 and 6 years but then while progression increased only slightly in the infliximab group between 6 and 8 years it increased greatly in the HERNE cohort so that at 8 years there was a difference in infliximab’s favour of 4.5 mSASSS units. The result was adjusted for baseline mSASSS (other factors, age, symptom duration, BASDAI, BASFI, etc., were not statistically significant confounders). The other study of 33 patients123 found the mean progression over 4 years was 1.6 mSASSS units (SD 2.6 units), lower than the 4.4 units seen in the untreated OASIS cohort118 at 4 years.
Another study examined a cohort of 334 patients with at least two spinal radiographs at 2-year intervals (patients with total spinal fusion at baseline were excluded). 121 In this study 201 out of 334 patients had received anti-TNFs for a mean of 2.5 years (SD 2.6 years) and no radiographic abnormality of the spine was seen at baseline in 144 patients (43%). At follow-up 102 patients (30.5%) showed no progression (≥ 1 mSASSS unit/year). Mulitvariate regression found baseline mSASSS (OR 1.06, 95% CI 1.04 to 1.08), ESR and smoking significantly increased the odds of radiographic progression, but anti-TNF use was significantly associated with a > 50% reduction in the (adjusted) odds of progression (0.47, 95% CI 0.24 to 0.94). Further analysis that tested factors that could influence exposure to anti-TNFs using propensity matching confirmed the association with mSASSS and found a stronger association with anti-TNF use (OR 0.30, 95% CI 0.11 to 0.78).
In conclusion, there is evidence of disease progression over time, although the disease course is highly variable. Best estimates of yearly disease progression rates without anti-TNF therapy are around 1.0 mSASSS units and 0.035–0.07 BASFI units. Whether or not there is any impact of anti-TNF treatment is unclear; a beneficial effect can neither be assumed, and nor, given the short-term nature of the follow-up and the insensitivity of radiography as a tool for the evaluation of disease progression in AS, can one be discounted.
Drug survival and anti-tumour necrosis factor switching
The EndNote Library generated by the searches for RCTs of all the anti-TNFs were separately screened to identify patient registry studies of any or all of the anti-TNFs. This was possible because the search strategy for RCTs was very sensitive and will have identified any clinical study including any of the named anti-TNFs.
A total of 25 potentially relevant studies were screened fully and 12 publications that reported some data on drug survival or the efficacy of anti-TNFs after switching were identified (see Table 20 for summary details of each). Across the 12 studies, the sources of data were either retrospective cohort studies or prospective registers (although analysis plans may have been retrospective), from a range of regions: USA (two studies), Canada (one study) and Europe (nine studies). No data from a UK-based cohort were available. Most of the cohorts and registries included experience with the three oldest anti-TNFs: infliximab, etanercept and adalimumab. One study (of the RHAPSODY cohort) included results from 326 patients treated with adalimumab as second anti-TNF after infliximab or etanercept. Small numbers of patients provided data on golimumab (three studies) and even smaller numbers on certolizumab (two studies). The population in 10 of the 12 studies was AS, although the diagnostic criteria used to specify AS were rarely given. One study provided results specifically for nr-AxSpA and one study provided results for axial SpA (nr-AxSpA or AS).
| Citation | Study/registry and method | n (duration, where stated) | Population | Anti-TNFs included | Drug survival | Efficacy on switching |
|---|---|---|---|---|---|---|
| Bonafede 2012126 | Market Scan (administrative claims data) 2005–9, USA; retrospective | 308 (360 days) | AS | Etanercept, adalimumab and infliximab | n (%) who stopped treatment and did not switch/who switched:
|
NR |
| Choquette 2013127 (abstract only) | Rhumadata register, Canada; unknown | 119 (5 years) | AS, previous NSAIDs and BASDAI score of ≥ 4 | Etanercept, adalimumab and infliximab | n who remained on same anti-TNF was 80% at 1 year; 70% at 2 years; and 55% at 5 years (no difference between anti-TNFs) | NR |
| Gulfe 2014128 | SSATG registry, Sweden; prospective | 112 (2 years) | Nr-axSpA not AS, demographic summary available | Etanercept, adalimumab, infliximab, golimumab and certolizumab | Kaplan–Meier estimates drug survival was 76% at 1 year and 65% at 2 years | NR |
| Nell-Duxneuner 2012129 | Drug reimbursement data, Austria; retrospective | 694 (2 years) | AS | Etanercept, adalimumab and infliximab | Starting in 2007 drug survival was:
|
NR |
| Yeaw 2014130 | LifeLink Health Plan Claims database 2004–10, USA; retrospective | 632 | AS patients who had discontinued an anti-TNF | Etanercept, adalimumab and infliximab | % who restart within 360 days after stopping:
|
NR |
% who switch to another anti-TNF or biologic:
|
||||||
% who switch to non-biologic:
|
||||||
% who switch to no new treatment:
|
||||||
| Scire et al. 2013131 | MonitorNet database (Italian Society of Rhuematology) to 2012, Italy; multiple imputation used for missing data | 498 | AS | Etanercept, adalimumab and infliximab | Unadjusted Kaplan–Meier estimates of drug survival at:
|
NR |
| Adjusted HR discontinuation rate (median follow-up 17 months) 0.59 (95% CI 0.46 to 0.75) (adjusted for age, sex, number of comorbidities, disease duration, number of previous DMARDs, concurrent DMARDS, baseline BASDAI score and BASFI score) | ||||||
| Zufferey 2014132 | Single centre in Switzerland (Centre Hospitalier Universitaire Vaudois) 2011–12; retrospective | 112, of whom 77 were AS (follow-up at 12 and 24 months) | SpA (AxSpA and AS) | Etanercept, adalimumab, infliximab and golimumab | Median drug survival across all anti-TNFs 12 months (IQR 7–19 months) for AxSpA and 8 months (IQR 6–13 months) for AS | NR |
Drug survival for AS:
|
||||||
| No difference between anti-TNFs | ||||||
| Pavelka 2009133 | ATTRA national registry, Czech Republic; prospective | 310 (1 year) | AS (note mean BASDAI score 6.4 at baseline) | Etanercept, adalimumab and infliximab | Drug survival at 1 year was 84%; at 2 years was 76%; and at 3 years was 72% | NR |
| Lie 2011134 | NOR-DMARD register 2000–9, Norway; prospective | 514 (2 years) | AS | Etanercept, adalimumab and infliximab | In total 77 patients switched from first anti-TNF; 437 did not. In the 77 switchers, median drug survival on first anti-TNF was 266 days on the first anti-TNF (range 1–1392) and the second anti-TNF was a median of 77 days (range 0–1608 after the first was stopped). Finding may just be a consequence of the stopping rules in Denmark (patients given around 6 months to achieve a response) | Non-switchers: response to first anti-TNF at 3 months (n = 362):
|
| % on treatment after 1 and 2 years: First anti-TNF: 76% and 65% Second anti-TNF: 67% and 60% |
Switchers: response to first anti-TNF at 3 months:
Median (IQR) BASDAI, score: 4.8 (3.3–7.01) (n = 63) |
|||||
Response to second anti-TNF at 3 months:
Median (IQR) BASDAI score: 4.1 (1.9–6.1) (n = 62) |
||||||
| Data also available by reason for withdrawal | ||||||
| Glintborg 2010112 | DANIBO registry, Denmark; prospective | 842 (8 years) | AS | Etanercept, adalimumab and infliximab | Median drug survival was 4.3 years (unadjusted 1- and 2-year retention rates 74% and 63%) which was similar across three anti-TNFs; only male sex, low baseline VAS fatigue and high CRP level (> 14 mg/l) were associated with better drug survival | NR |
| Glintborg 2012135 | DANBIO registry, Denmark; prospective | 1436 (432 switchers; median 2.4 years) | AS (switchers only; had received at least two anti-TNFs during follow-up) | Etanercept, adalimumab, infliximab and golimumab (certolizumab and other biologics had less than 1% between them and only to first treatment course) | Median (95% CI) years of drug survival (n) (% on treatment after 2 years) for sequential anti-TNFs:
|
Median (IQR) BASDAI at 3 months for sequential anti-TNFs:
|
Median (IQR) BASFI at 3 months for sequential anti-TNFs:
|
||||||
% BASDAI 50/20 mm responders at 6 months (NR at 3 months):
|
||||||
| Rudwaleit 2009136 | RHAPSODY, European cohort; prospective uncontrolled cohort of patients treated with adalimumab | 1250 (12-week response data only) | AS | Adalimumab | NR | 12-week response rates: anti-TNF naive (n = 924); BASDAI 50–63%; ASAS 40–59%; anti-TNF exposed (etanercept and/or infliximab, n = 326); BASDAI 50–41%; and ASAS 40–38% |
| Logistic regression with backward elimination found that younger age, higher CRP level, HLA-B27 positive and anti-TNF naivety all predictive of better response (table 1134) |
Drug survival on first anti-TNF for all anti-TNFs was around 70–80% at 1 year, around 65–75% at 2 years, around 70% at 3 years and 55% at 5 years. Little difference between the three older anti-TNFs was identified, although one analysis using Cox proportional hazard estimates found statistically lower rates of discontinuation with etanercept and adalimumab compared with infliximab. 131
The median drug survival in AS patients across all anti-TNFs reported varied (Table 21). Based on the largest registry (DANBIO)135 the median drug survival for a first anti-TNF was 3.1 years (95% CI 2.6 to 3.7 years) (n = 1436), with 58% of patients remaining on treatment at 2 years. Median drug survival for a second anti-TNF was 1.6 years (95% CI 1.0 to 2.2 years) (n = 432), with 47% of patients remaining on treatment at 2 years, and for a third, 1.8 years (95% CI 0.9 to 2.7 years) (n = 137) (49% on treatment at 2 years).
| Anti-TNF | Drug survival for sequential anti-TNFs | |
|---|---|---|
| Median (95% CI) | % on treatment after 2 years | |
| First (n = 1436) | 3.1 (2.6 to 3.7) | 58 |
| Second (n = 432) | 1.6 (1.0 to 2.2) | 47 |
| Third (n = 137) | 1.8 (0.9 to 2.7) | 49 |
The efficacy of second or third anti-TNFs after switching in AS patients was reported in only a small number of studies. One analysis based on the NOR-DMARD registry134 showed how the response rate and BASDAI and BASFI achieved at 3 months in patients who remain on their first therapy is (not surprisingly) better than in patients who switch. Median BASDAI and BASFI achieved with a second anti-TNF were not as low (not as good) as was achieved with a first anti-TNF in non-switchers. An analysis of the DANBIO registry indicated that response (BASDAI 50) at 6 months reduced with subsequent anti-TNFs, as did the median improvement in BASDAI and BASFI achieved (Table 22). 135 These results are supported by the RHAPSODY study that found higher response rates with adalimumab in anti-TNF naive patients (BASDAI 50–63%; ASAS 40–59%)(n = 924) than in anti-TNF exposed (BASDAI 50–41%; ASAS 40–38%) (n = 326). 136
| Anti-TNF | % BASDAI score 50/20 mm responders at 6 months (at 3 NR) | BASDAI score at 0 months for sequential anti-TNFs, median (IQR) | BASDAI score at 3 months for sequential anti-TNFs, median (IQR) | BASFI score at 0 months for sequential anti-TNFs, median (IQR) | BASFI score at 3 months for sequential anti-TNFs, median (IQR) |
|---|---|---|---|---|---|
| First (n = 1436) | 54 | 5.9 (4.5–7.1) | 2.8 (1.1–4.8) | 5.0 (3.4–6.7) | 2.8 (1.1–4.8) |
| Second (n = 432) | 37 | 5.6 (3.8–7.3) | 3.6 (1.9–6.4) | 5.2 (3.5–7.0) | 3.6 (1.7–6.0) |
| Third (n = 137) | 30 | 6.4 (4.8–7.9) | 5.1 (3.6–6.7) | 6.4 (4.2–7.9) | 5.1 (3.0–7.3) |
The registries and cohort studies provided no data on the efficacy of anti-TNFs as second or third, after switching in nr-AxSpA patients.
In summary, sequential treatment with anti-TNFs can be worthwhile in patients with AS but the response rates and benefits are reduced with second and third anti-TNFs, with the proportion of BASDAI 50 responders falling approximately 10% with each subsequent anti-TNF and the median BASDAI and BASFIs achieved increasing (worsening). The lower efficacy of a second anti-TNF relative to a first is reflected in lower median drug survival and proportion of patients remaining on therapy at 2 years. Interestingly, despite a further reduction in response and efficacy with a third anti-TNF, drug survival does not fall, suggesting that at this stage in their treatment history patients may continue with a less than optimally effective anti-TNF given any better alternative.
Clinical effectiveness results: adverse events
Randomised trials
We focused on the following outcomes, known to have possible associations with anti-TNF treatment: serious infections, tuberculosis (including tuberculosis reactivation), injection/infusion site reactions, congestive heart failure, cancer, non-melanoma skin cancer, SAEs and withdrawals due to SAEs. For the randomised phases of the trials included in the review, the reporting of AE data was generally limited. For three of the 24 trials no information on AEs was available. 55,56,74 Several trials provided AE data only at time points after which placebo patients may have switched to receive an anti-TNF (so true placebo comparisons were not available).
Analysable data on injection/infusion site reactions were available for 10 trials, although these studies were only of etanercept or infliximab. The data for certolizumab, golimumab and adalimumab trials either were not reported or were only provided at time points after which placebo patients could ‘escape’ to receive an anti-TNF; these data would not allow for an accurate comparison with placebo. Results for injection/infusion site reactions analyses from this review for etanercept and infliximab showed a statistically significant increase in reactions associated with etanercept (relative risk 2.69, 95% CrI 1.82 to 3.89) compared with placebo but no significant difference between infliximab and placebo. Compared with each other, the risk of an injection/infusion site reaction was statistically significantly higher with etanercept than with infliximab (relative risk 2.27, 95% CrI 1.01 to 5.37). Incidence of serious infections was reported in only eight trials, although such events were rare (nine cases in total). Of the eight trials which reported incidence of tuberculosis, only four cases were identified; three cases were reported in the longest study, the 54-week trial which compared infliximab with an infliximab biosimilar (CT-P13). 110 Four trials reported on congestive heart failure (no cases reported), six trials reported on cancer (one case) and three trials reported on non-melanoma skin cancer (two cases, one in each group of the ABILITY-158 trial). In most trials few SAEs were reported; group rates ranged from 0% to around 9%. Similarly, most trials had few withdrawals because of AEs; rates ranged from 0% to around 12%. Full results are reported in Appendix 9.
Large systematic reviews
Overall, the number and size of trials, and the short duration of their placebo-controlled phases, were too limited to provide enough data for meaningful analyses of AE. This common problem, of having too few data to evaluate AEs, underpinned the rationale for a Cochrane review (and network meta-analysis) of AEs of nine biologics in adults with any disease, except HIV/AIDS. 137 In order to provide a better understanding of toxicity, data were pooled across diseases by assuming a similar rate of AEs (across diseases). For the present assessment, estimates of AE rates have therefore been derived from the Cochrane review, which included 160 RCTs (n = 48,676) and 46 open-label extension studies (n = 11,954). The median durations were 6 months for RCTs and 13 months for open-label extension studies. The biologics included were abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, rituzimab and tocilizumab. The anti-TNFs included in the present assessment were studied in 115 (72%) of the RCTs and 40 (87%) of the open-label studies included in the Cochrane review. Most studies assessed etanercept or infliximab in cancer or rheumatoid arthritis patients; 10 RCTs were of AS (fewer than in this assessment, as in the Cochrane review databases were searched up until January 2010). The biologics were evaluated both as a group and as individual interventions. The results from the RCTs, what the review classified as ‘major’ outcomes, are in Tables 23 and 24. Biologics as a group were associated with statistically significantly higher rates of total AEs, withdrawals because of AEs, serious infections and tuberculosis reactivation compared with control treatments. When the individual anti-TNFs were analysed separately, compared with control treatments only infliximab and certolizumab were statistically significantly associated with AEs: infliximab with higher rates of total AEs [number needed to harm (NNH) 13, 95% CrI 8 to 505] and withdrawals because of AEs (NNH 10, 95% CrI 5 to 30), and certolizumab pegol with higher rates of serious infections (NNH 12, 95% CrI 4 to 79) and SAEs (NNH 18, 95% CrI 9 to 162) (see Table 24).
| AE | Risk with comparator, per 1000 patients unless otherwise stated | Risk with intervention, per 1000 patients, unless otherwise stated (95% CrI) | OR (95% CrI) | Number of participants (studies) |
|---|---|---|---|---|
| SAEs | 118 | 127 (115 to 142) | 1.09 (0.97 to 1.24) | 21,152 (76) |
| Total AEs | 724 | 770 (741 to 797) | 1.28 (1.09 to 1.50) | 14,959 (48) |
| Withdrawal due to AEs | 98 | 137 (115 to 168) | 1.47 (1.20 to 1.86) | 22,636 (83) |
| Serious infections | 26 | 35 (27 to 46) | 1.37 (1.04 to 1.82) | 21,853 (70) |
| Tuberculosis reactivation | 4 per 10,000 | 20 per 10,000 | 4.68 (1.18 to 18.6) | 30,671 (71) |
| Lymphoma | 9 per 10,000 | 1 | 0.53 (0.17 to 1.66) | 21,260 (52) |
| Congestive heart failure | 8 | 6 (1 to 21) | 0.69 (0.18 to 2.69) | 8847 (24) |
| Anti-TNF | Risk with comparator, per 1000 patients unless otherwise stated | Risk with intervention, per 1000 patients, unless otherwise stated (95% CrI) | OR (95% CrI) | Number of participants (studies) |
|---|---|---|---|---|
| SAEs | ||||
| Adalimumab | 118 | 114 (90 to 145) | 0.96 (0.74 to 1.27) | 4662 (15) |
| Certolizumab | 118 | 174 (124 to 237) | 1.57 (1.06 to 2.32) | 2421 (6) |
| Etanercept | 118 | 142 (111 to 184) | 1.24 (0.93 to 1.69) | 3931 (21) |
| Golimumab | 118 | 123 (82 to 184) | 1.05 (0.67 to 1.69) | 1564 (8) |
| Infliximab | 118 | 133 (102 to 174) | 1.15 (0.85 to 1.57) | 3403 (14) |
| Total AEs | ||||
| Adalimumab | 724 | 730 (637 to 802) | 1.03 (0.67 to 1.54) | 3266 (10) |
| Certolizumab | 724 | 754 (651 to 837) | 1.17 (0.71 to 1.95) | 1829 (5) |
| Etanercept | 724 | 784 (677 to 866) | 1.38 (0.80 to 2.46) | 1600 (7) |
| Golimumab | 724 | 765 (672 to 839) | 1.24 (0.78 to 1.98) | 1187 (6) |
| Infliximab | 724 | 803 (726 to 860) | 1.55 (1.01 to 2.35) | 2330 (9) |
| Withdrawal due to AEs | ||||
| Adalimumab | 98 | 128 (81 to 194) | 1.35 (0.82 to 2.22) | 5268 (18) |
| Certolizumab | 98 | 125 (70 to 226) | 1.32 (0.69 to 2.69) | 2421 (6) |
| Etanercept | 98 | 124 (82 to 191) | 1.30 (0.82 to 2.17) | 5189 (25) |
| Golimumab | 98 | 127 (64 to 241) | 1.34 (0.63 to 2.92) | 1549 (7) |
| Infliximab | 98 | 203 (132 to 310) | 2.34 (1.40 to 4.14) | 2973 (15) |
| Serious infections | ||||
| Adalimumab | 26 | 32 (17 to 60) | 1.23 (0.65 to 2.40) | 4847 (15) |
| Certolizumab | 26 | 113 (39 to 330) | 4.75 (1.52 to 18.45) | 1683 (4) |
| Etanercept | 26 | 33 (19 to 61) | 1.29 (0.72 to 2.45) | 4630 (19) |
| Golimumab | 26 | 29 (12 to 65) | 1.11 (0.45 to 2.59) | 1334 (6) |
| Infliximab | 26 | 36 (20 to 65) | 1.41 (0.75 to 2.62) | 2652 (13) |
| Tuberculosis reactivation | ||||
| All nine biologics | 4 per 10,000 | 20 per 10,000 | 4.68 (1.18 to 18.60) | 30,671 (71) |
| Lymphoma | ||||
| All nine biologics | 9 per 10,000 | 1 | 0.53 (0.17 to 1.66) | 21,260 (52) |
| Congestive heart failure | ||||
| All nine biologics | 8 | 6 (1 to 21) | 0.69 (0.18 to 2.69) | 8847 (24) |
For total AEs, the Cochrane review team judged the strength of evidence to be high; for SAEs, withdrawals because of AEs and serious infections, the strength of evidence was judged to be moderate; and for tuberculosis reactivation, lymphoma and congestive heart failure, the strength of evidence was judged to be low. For tuberculosis reactivation, lymphoma and congestive heart failure, the network meta-analysis statistical models did not converge (because of low numbers of events) therefore estimates for individual anti-TNFs were not available. Outcomes which were classed in the review as ‘minor’ were not analysed by the review authors because of the low numbers of events and the complexity of the analyses for the major outcomes. The minor outcomes included cardiac AEs, infusion and injection site reactions, allergic reactions, neurological outcomes, deaths, all cancers, serious lung infections or pneumonia, fungal infections and opportunistic infections. For the purposes of the present assessment, further large studies on cancer risk were therefore sought. An individual patient data meta-analysis of 22,904 adults (from 74 RCTs) which assessed the cancer risk of taking adalimumab, etanercept or infliximab in the short term (median duration < 6 months) was identified. 138 Although funded by manufacturers, this study was requested by the European Medicines Agency and was planned and conducted by independent researchers working with an independent academic steering committee. For all three anti-TNFs as a group, there was no increase in risk of cancers excluding non-melanoma skin cancer (relative risk 0.99, 95% CI 0.61 to 1.68) but there was a doubling in the risk of non-melanoma skin cancer associated with taking an anti-TNF (relative risk 2.02, 95% CI 1.11 to 3.95). Evaluation of drug-specific effects was hampered by statistical precision and by differences in baseline cancer risk and reporting detail across trials. 138
Another review of AE effects of etanercept, adalimumab and infliximab was based on systematic searches for systematic reviews of the safety of biologic agents. 139 Six reviews that were sufficiently rigorous to meet the Database of Abstracts of Reviews of Effects inclusion criteria were included in the overview. This review also included large RCTs and non-randomised studies (≥ 500 patients), and was focused on serious potential AEs, such as serious infections, reactivation of latent tuberculosis and cancer. 139 Table 25, which summarises the rates of SAEs among the included non-randomised studies and large RCTs, indicates that the rates of SAEs cover a broadly similar range across the three different biologic agents. However, all estimates were derived from a highly heterogeneous group of studies in terms of participants (e.g. inflammatory condition or disease severity), study design (e.g. length of follow-up) and treatment regimens (e.g. dose and frequency). Consequently, reliable estimates of the relative rate of SAEs for each drug could not be made.
| Drug | Serious infections (%) | Cancer (%) | Tuberculosis (%) | Mortality (%) | Withdrawals due to AE (%) |
|---|---|---|---|---|---|
| Etanercept | 0.6–13.2 | 1–5.7 | 0–1.4 | 0–3.1 | 0–13.6 |
| Infliximab | 0.8–13.8 | 0.16–5.1 | 0.06–4.6 | 0.06–2.0 | 6.4–12.8 |
| Adalimumab | 0.4–5.1 | 0.1–1.1 | 0–0.4 | 0.5–0.9 | 5.8–10.7 |
Withdrawal rates due to AEs were typically < 10% for all drugs, with the highest reported single estimate being 13.6% for one etanercept study. This suggested that the majority of patients can tolerate biologic treatment in the medium term, although again the estimates were derived from a highly heterogeneous group of studies; therefore, the possibility of poorer tolerability in specific patient groups was not ruled out.
Open-label extensions of randomised trials
Of the longer-term follow-up studies included in our present review we evaluated those reporting AEs after 6 months (as the Cochrane review covered events occurring up to 6 months); 13 trial cohorts had studies which reported data after 6 months. Both the type of AEs assessed, and the periods over which they were assessed, varied across studies. Table 26 compares results for studies with at least around 2 years of follow-up. The ATLAS61 and GO-RAISE90 trials both had extension study publications at the 2-year and 5-year time points. 140–143 Both cohorts were analysed using mITT data, in which patients had to have received at least one dose of treatment. This amounted to 99% of the randomised patients in both studies (311 out of 315 in ATLAS61 and 353 out of 356 in GO-RAISE90). Davis72,73 reported results for the 257 patients who enrolled in a 168-week open-label study following week 24 of the randomised phase; 277 patients had taken part in the earlier randomised study. All 257 patients in the open-label study had received at least one dose of etanercept. 144,145 The Calin trial83–85 randomised 84 patients, with 81 patients enrolling in the open-label extension study. Results were presented separately for the 12-week to 2-year time points and the 2- to 5-year time points. 146,147 RAPID-axSpA64 data at 96 weeks were reported in the manufacturer’s submission. These data related to the mITT population: 315 (97%) of the 325 originally randomised patients.
| Event outcome | Adalimumab | Golimumab | Etanercept | Certolizumab | Infliximab | ||||
|---|---|---|---|---|---|---|---|---|---|
| ATLAS61 | GO-RAISE90 | Davis72,73 | Calin83–85 | RAPID-axSpA65 | aASSERT102 | ||||
| 2 years (n = 311) | 5 years (n = 311) | 2 years (n = 353) | 5 years (n = 353) | 24–192 weeks (n = 257)b | 12–108 weeks (n = 81)b | 2–5 years (n = 59) | 96 weeks (n = 315) | 24–102 weeks (n = 74) | |
| SAEs | 48 events (15%) 10.5/100 PY | 140 events (45%) 11.7/100 PY | 40 events (11%) | 72 events (20%) | 33 events (13%) 8/100 PY | 19 events (23%) | 21/100 PY | AiC information has been removed | 15 events (20%) |
| Withdrawals because of AEs | 24 (8%) events 4.5/100 PY | – | 19 events (5%) | 32 events (9%) 2.13/100 PY | 14 events (5%) | 15 events (19%) | 7 events (12%) | AiC information has been removed | – |
| Serious infections | 6 (2%) events 1.1/100 PY | 17 events (5%) 1.4/100 PY | 11 events (3%) | 21 events (6%) 2.1/100 PY | 6 events (2%) 2/100 PY | 5 events (6%) | 3 events (5%) 3/100 PY | AiC information has been removed | 3 events (4%) |
| Cancer | 4 events (1%) 0.7/100 PY | 3 events (1%) 0.2/100 PY | 2 events (0.6%) | 3 events (0.8%) 0.21/100 PY | – | 4 events (5%) | 3 events (5%) | – | 1 events (1%) |
| NMSC | 0.4/100 PY | – | – | – | – | – | – | – | – |
| Congestive heart failure | 0 events | 2 events (0.6%) 0.2/100 PY | – | – | – | – | – | – | – |
| Injection site reactions | 42 events (14%) 17.6/100 PY | – | 38 events (11%) | 43 events (12%) | 57 events (22%) | 30 events (37%) | 7 events (12%) | AiC information has been removed | 9 events (12%) |
| Tuberculosis | 0 events | 0 events | – | – | – | 0 events | 0 events | AiC information has been removed | – |
The 2-year study of the ASSERT (Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy)102 (infliximab) cohort allowed dose escalation whereby, from week 36, patients with BASDAI scores of ≥ 3 could increase their dose to 7.5 mg/kg, which is a currently unlicensed dose. Results for the 5 mg/kg group (74 patients) between weeks 24 and 102 have therefore been presented in Table 26. The Braun cohort148 was followed up for 8 years, but it was a small study which reported only SAEs and withdrawals due to SAEs.
Table 26 illustrates that rates of SAEs, cancer and serious infections were similar across all four anti-TNFs when using incidence per 100 patient-years as estimates. At 5 years, SAEs appeared more prevalent with adalimumab (45%) than golimumab (20%), although it is possible this difference is because of the way the data were reported; it was unclear whether the ATLAS61 data related to the total number of SAEs or to the number of patients experiencing a SAE. At 2 years, the incidence of injection site reactions was higher in patients taking etanercept than in patients taking adalimumab, golimumab or certolizumab pegol. Withdrawal rates due to AEs were broadly similar across treatments. The reporting of tuberculosis and congestive heart failure was limited.
Summary of adverse event data
Data from large systematic reviews, which included patients with a wide range of diseases, suggest that, in the short term, anti-TNFs as a group are associated with significantly higher rates of serious infections, tuberculosis reactivation, non-melanoma skin cancer, total AEs and withdrawals because of AEs than control treatments. Specifically, infliximab is associated with significantly higher rates of total AEs and withdrawals because of AEs, and certolizumab pegol is associated with significantly higher rates of serious infections and SAEs. Analyses from the present review showed etanercept to be statistically significantly more likely to result in an injection/infusion site reaction compared with infliximab, although analysable data on such reactions were not reported for the three other anti-TNFs. Evaluations of longer-term data are more scarce, although suggest similar safety profiles across anti-TNFs. Data from the open-label studies included in this review also do not suggest that there are important differences between treatments, other than a higher incidence of injection site reactions following treatment with etanercept. These open-label data are, however, limited by the small sample sizes and non-randomised study designs.
Review of natural history of ankylosing spondylitis and non-radiographic axial spondyloarthritis
In order to get some understanding of what happens to patients who, although eligible for anti-TNF therapy for their AS or nr-AxSpA, do not receive it, we conducted a rapid review of relevant literature. This was not a systematic review but one that started with the library of papers found by the main searches for RCTs of the anti-TNFs and then followed relevant citations to papers on AS and axSpA in patients not receiving an anti-TNF. Potentially relevant papers were those that reported on the pattern of disease, AS or nr-AxSpA or axSpA, without treatment with anti-TNFs over time. This process identified a number of relevant registries: OASIS,118 Scotland and Ireland Registry for Ankylosing Spondylitis (SIRAS), Devenir des Spondylarthropathies Indifferenciées Récentes, Esperanza, Spanish Registry of spondyloarthritis, German Spondyloarthritis Inception Cohort (GESPIC) and St Mary RheumaToid Arthritis (SMART). Additional searches of MEDLINE were conducted using these specific registry names. All relevant studies identified through this process are presented in Table 27.
| Study | Description | Population characteristics | Summary of findings |
|---|---|---|---|
| Landewe 200910 | Examined the relationship between disease activity, radiographic damage and physical function in AS. Based on (European) OASIS cohort118 baseline and 2-year data. n = 217 consecutive (from 1996) patients with AS (no specific criteria). BASDAI score mean 3.4 (SD 2.1), with 38% ≥ 4 | BASFI score mean 3.4 (SD 2.6), 41% ≥ 4. mSASSS median 5, 69% > 0. Note: does mSASSS < 0 mean nr-AxSpA? None of the patients in the cohort had used anti-TNFs. Subgroup (n = 188) baseline BASDAI score of ≤ 6 | Univariate correlation between baseline mSASSS and BASFI score = 0.45 (Spearman’s rank correlation coefficient), but this was modified by baseline BASDAI:
|
| Ramiro 2014124 | Analysed long-term relationship between disease activity (ASDAS, BASDAI) and radiographic damage (mSASSS) in AS. Used OASIS cohort118 over 12 years | Subgroup used patients (n = 184) who had at least two sets of radiographs. Baseline characteristics of this subgroup: BASDAI score mean 3.4 (SD 2.0); mSASSS mean 10.8 (SD 15.2), 81% > 0. None of the patients had used anti-TNFs | On average patients had a progression of 1.9 mSASSS units/2 years. This varied with baseline ASDAS:
|
| Ramiro 2013125 | Earlier analysis of OASIS cohort118 12-year data to describe the evolution of radiographic abnormalities in AS patients | Subgroup used (n = 186) who had at least two sets of radiographs. Baseline characteristics of this subgroup: BASDAI score mean 3.4 (SD 2.0); mSASSS mean 11.6 (SD 16.2). None of the patients had used anti-TNFs | Long-term radiographic progression in AS highly variable at the patient level, but is more severe in men who are HLA-B27 positive. Over whole follow-up, 24% of patients (and 18% of the 68 patients who were followed for 12 years) had no progression on mSASSS. Duration of disease is not relevant. At the group level, progress is linear at 2 mSASSS units/2 years |
| Baraliakos 200912 | Natural course of radiographic progression in AS. Retrospective cohort, single clinic (Herne, Germany), 1993–2005. Mean follow-up 3.8 years (SD 1.7 years) | n = 146 anti-TNF naive patients. Baseline:
|
Mean mSASSS change was 1.3 units/year (SD 2.5 units/year). Note: range was mSASSS 0–22.8. Thirty-four (23%) patients showed no progression |
| Dean 2014,149 poster at BSR meeting | SIRAS cohort. Study of BASDAI over time | BASDAI score at diagnosis data available for only 240 patients (out of the 1210 patient cohort). Baseline BASDAI score (at diagnosis) of 4.9 (SD 2.3). High disease activity group BASDAI score of 6.3 (SD 1.4) and low disease activity group BASDAI score of 2.5 (SD 1.3) | Baseline BASDAI remained fairly stable over time: across the whole cohort and in the high and low disease activity groups. The subgroup treated with anti-TNFs had higher mean BASDAI score [5.7 (SD 2.0)] than non-biologic patients [4.2 (SD 2.5)] and this remained so until around a year after treatment with anti-TNFs began, when mean BASDAI fell to the level of the non-biologic patients |
| Healey 201314 | Cohort study, single centre, England. Followed patients over 10 years [n = 69 who provided assessments at baseline (1998) and at 10 years (2008)]. Assessments using RLDQ, BASDAI, ASQoL and EQ-5D (and others) | At study entry patients were 84% male, mean age 49 years, disease duration 15.5 years, symptom duration 21.4 years. 1.5% on an anti-TNF at 10 years | Only RLDQ changed significantly over time for assessment 1 (1998) and 2 (2008):
|
| Stone 2007150 | Analysis of longitudinal data from SMART (Bath, UK) data set (n = 224). Regression analysis of BASDAI score on symptom duration and BASFI score adjusted for BASDAI score > 4 at baseline. Duration of follow-up was unclear | Overall, 68% had a baseline BASDAI score of ≥ 4. Mean symptom duration was 28.8 years | Only 20% experienced a significant change in BASDAI score over time (13% a decrease; 7% an increase). BASFI score increases over time by 0.035 units/symptom-year. In patients with baseline BASDAI score of ≥ 4, those who would be treated with anti-TNFs, the increase over time is 0.039 units/symptom-year |
| Machado 2010151 | Baseline data from ASSERT.102 Analysis of relation between mSASSS and MRI inflammation and BASMI | n = 214 AS patients (mNY criteria). Baseline median (IQR):
|
Concluded that spinal mobility (BASMI) independently determined both by irreversible (mSASSS) and reversible spinal damage (MRI), the former in late disease and the latter in early disease |
| Machado 201124 | Baseline data from ASSERT.102 Analysis of relation between SF-36 and BASFI and BASDAI, ASDAS, CRP level, mSASSS, MRI inflammation and BASMI | n = 214 AS patients (mNY criteria) | Regression coefficients for associations reported in the publication. Briefly, SF-36 is determined by BASFI and BASDAI; and BASFI is determined by BASDAI, mSASSS and BASMI |
| Kobelt 2004152 | A modelling study of infliximab but refers to large UK observational data set and generates an estimate for BASFI over time. Survey in 2002 (n = 1413). Value generated from patients who were captured in two surveys at two time points, 1992/1994 and November 2002, approximately 8 years apart (n = 1100). Data from a cohort of 493 patients who had been followed up for more than 3 years were used as a check for the result based on the survey | – | From the whole survey (n = 1413) mean BASDAI score 4.2 (SD 2.3) and mean BASFI score 4.4 (SD 2.8). The population was broader than that eligible for anti-TNFs, with 47% having a BASDAI score of < 4. It appears (but is unclear) that this is the BASDAI at the later time (2002) point not the earlier (1992/4). Estimate of annual BASFI progression was 0.07 points. Note: progression was faster (0.1 points) in patients with BASFI score of < 4 at baseline, but was stable (0?) in patients with BASFI score above 7. (Ceiling effect of BASFI?) When only patients with BASDAI score of ≥ 4 included BASFI progression was estimated as 0.054. Data from the cohort study generated similar findings; however, the number was not actually reported for whole survey. BASFI progression was 0.059 for patients with a BASDAI score of ≥ 4 |
| Nr-axSpA | |||
| Kiltz 2012153 | Comparison of characteristics of patients with AS and nr-AxSpA. Cohort of 100 patients seen in 2010 in Herne clinic, Germany. Analysis tested if the proportion of patients reaching pre-specified cut-off criteria (markers of disease severity) differed between AS and nr-AxSpA | Consecutive, diagnosed with axSpA. None of the patients had used anti-TNFs. n = 100 AxSpA: n = 44 nr-AxSpA and n = 56 AS
|
Differences were statistically significant for ASDAS, CRP level, mSASSS and number of inflamed lesions. Proportion of males also significantly different. Results:
|
| Rudwaleit 200918 | Cross-sectional study of GESPIC cohort (n = 462) patients with axSpA. Divided into AS (n = 236) and nr-AxSpA (with ≤ 5 years of symptoms, n = 226) | Baseline mean:
|
When AS patients were divided into those with more than 5 years of symptoms and those no more than 5 years, there were no differences in characteristics at baseline. When AS (≤ 5 years) and nr-AxSpA were compared there were statistically significant differences (worse for patients with AS) in Physicians Global assessment, BASFI, BASMI, spinal mobility, lateral spinal flexion, CRP, ESR and all radiographic measures (mSASSS 4.9 in AS vs. 1.4 in nr-AxSpA). mSASSS was significantly worse in males vs. females and in CRP level > 6 vs. ≤ 6. Note: the AS patients had a very short symptom duration and must have progressed to AS rapidly. In addition, nr-AxSpA patients had only a short time from the start of symptoms and may therefore not reflect patients who remain nr-AxSpA for a longer time. |
| Poddubnyy 2011154 | Study of radiographic progression of sacroiliitis in AS and nr-AxSpA. Radiographic evidence of sacroiliitis is a criterion in the mNY for AS; therefore, it is useful to see this analysis of progression rather than only the mSASSS | German cohort (GESPIC) n = 210 (n = 115 AS and n = 95 nr-AxSpA), 2 years’ follow-up. (Baseline BASDAI score was 4 and BASFI score was 3 across AS and nr-AxSpA.) Overall the cohort had a short symptom duration of 4.2 years (5.2 years AS and 3.2 years nr-AxSpA). Only 3.5% had had treatment with anti-TNFs (3.5% AS and 1.1% nr-AxSpA) | After 2 years’ follow-up, n = 11 of the 95 nr-AxSpA patients (11.6%, 95% CI 6.6% to 19.6%) fulfilled the mNY for AS. In addition, after 2 years approximately 10.5% of patients in the nr-AxSpA cohort had progressed by at least one mNY grade, compared with 8.7% of patients in the AS group (difference not statistically significant). Predictors of sacroiliitis progression were raised CRP level for both AS and nr-AxSpA. Male sex and HLA-B27 positive predicted lower progression in nr-AxSpA but higher progression in AS |
| Poddubnyy 2012155 | GESPIC cohort. Radiographs of spine and SIJ at baseline and at 2 years | Baseline, all patients (n = 210): of the 2.4% patients treated with anti-TNFs, the BASDAI score was 4 and the BASFI score was 3. AS (n = 115): of the 3.5% patients treated with anti-TNFs the BASDAI score was 4 and the BASFI was score 3. nr-AxSpA (n = 95): of the 1.1% of patients treated with anti-TNFs, the BASDAI score was 4 and the BASFI score was 3 | Regression analysis found syndemophytes at baseline, elevated ESR and CRP level and smoking were significantly associated with spinal progression (≥ mSASSS/2 years) in AS but only syndemophytes at baseline in axSpA. In AS patients mSASSS increased significantly from 5.86 (SD 10.30) to 6.81 (SD 11.71), mean difference 0.95 (SD 2.78). In nr-AxSpA patients mSASSS increased significantly from 2.30 (SD 4.24) to 2.76 (SD 5.26), mean difference 0.46 (SD 1.63). The difference between mean progression in AS and nr-AxSpA patients was not statistically significant, and neither was the difference between those with symptom duration of ≤ 5 years and > 5 years. Percentage that progressed by > 2 mSASSS units/2 years: all axSpA 14.3%; AS 20.0% (95% CI 13.7% to 28.2%); nr-AxSpA 7.4% (95% CI 3.6% to 14.4%). There was no difference in mSASSS change between patients not progressing to AS (0.49 units) and those who progressed to AS (0.27 units); p = 0.53 |
| Flares | |||
| Cooksey 201028 | Cohort derived from full population of a trial comparing probiotic and placebo treatment in AS. Followed up for 1216 person-weeks and recorded localised/minor flares and generalised/major flares, plus BASDAI, BASFI and pain VAS | AS patients n = 134. Baseline mean BASDAI score of 3.7 (SD 2.1); mean BASFI score of 3.6 (SD 2.8). Mean duration of symptoms 21 years (SD 13 years, range 0–58 years) | The overall flare rate was 71.4 per 100 person-weeks: major flare rate of 12 per 100 person-weeks and minor flare rate of 59.4 per 100 person-weeks. Mean BASDAI scores were 5.5 (major flare), 3.1 (minor flare) and 2–2.5 (flare free). Mean BASFI scores were 5.5 (major flare), 3.1 (minor flare) and 2.5–3.5 (flare free). Note: these means are not from whole population but only from patients who experienced major flares plus flare-free periods (n = 27) and minor flares plus flare-free periods (n = 77) |
| Stone 2008156 | A pilot study to investigate pattern of disease and impact of disease flares. It used the SMART cohort (Bath, UK). Patients were asked about four patterns of disease (see under Summary of findings in this table) | AS patients, although the diagnostic criteria was not stated, n = 114 (although not n = 114 for all of the percentage). Mean BASDAI score of 4.2 and BASFI score of 4.0 | Overall, 96% of patients reported experiencing flares. The duration varied by patient: days (40%), weeks (30%) and months (30%). Of these, 83% reported experiencing symptoms between flares. The percentage of patients for the four patterns of the disease were:
|
The studies collectively explore the associations between the various components of axSpA: disease activity, structural damage and spinal mobility. The exploration of the ASSERT trial baseline data24,124 reveals that HRQoL as determined by SF-36 physical and mental components, is determined by BASFI and BASDAI; BASFI is determined by BASDAI, mSASSS and BASMI (spinal mobility); and BASMI is independently determined both by irreversible spinal damage (mSASSS) in late disease and reversible spinal damage (MRI) in early disease.
The studies identified that from a clinical practice and patients’ point of view disease progression in terms of BASFI, a measure of the patient’s functional ability, is very important. A number of studies on the disease progression of AS have been based on the European OASIS cohort118 (a consecutive cohort, started in 1996, although there were no further specific eligibility criteria); the total cohort numbers 217 patients. One of these, a study by Landewe et al. ,10 demonstrated that physical function impairment (BASFI) is independently affected by both disease activity (BASDAI) and bony progression, usually assessed using mSASSS despite this being a measure of bony growth in the spine only (and not in the sacroiliac joints). Other studies by Ramiro124,125 have demonstrated that radiographic progression, increases on average by around 2 mSASSS units every 2 years. 124,125 However, this progression is highly variable; the average patient with inactive disease [Ankylosing Spondylitis Disease Activity Score (ASDAS) 0] would progress by 5 mSASSS units over 12 years compared with a patient with ‘very active disease’ (ASDAS 4) who would have 19 units of progression. 124 In addition, of 68 patients who were followed for 12 years, 18% had no progression on mSASSS. 125 The variability is also demonstrated by the results based on a different cohort: a single German clinic (n = 146). 12 Baseline characteristics were similar to those in the OASIS cohort118 (see Table 27). Mean follow-up was 3.8 years (SD 1.7 years) and mean mSASSS change was 1.3 units/year (SD 2.5 units/year) with a range of 0–22.8 mSASSS units. Thirty-four (23%) patients showed no progression.
There is evidence that BASDAI is relatively constant over time. An analysis of data from a UK registry, SIRAS, demonstrated that patients stratified into high or low disease activity (BASDAI) remain in their separate groups over many (12) years. 149 Data on the long-term pattern of patient function (BASFI) in patients not being treated with anti-TNFs are more scarce. A cohort study, from a single centre in England, provided data on 69 patients followed over 10 years [two data points: at baseline (1998) and at 10 years (2008)]. 14 The assessment of BASDAI confirmed that it remains relatively constant [mean at baseline 4.1 units (SD 2.5 units) and after 10 years 4.4 units (SD 2.7 units) (p = 0.36)]. Patient function was assessed using the Revised Leeds Disability Questionnaire (RLDQ) rather than BASFI, but provided evidence of deteriorating function over time: mean RLDQ at baseline was 10.4 (SD 8.3), and after 10 years was 13.6 (SD 10.9) (p = 0.002). Analysis of longitudinal data from the SMART (Bath, UK) data set (n = 223) found that BASFI increased over time by 0.035 units/symptom year. 150 In patients with baseline BASDAI of ≥ 4 (those that would be treated with anti-TNFs and 68% of the total cohort) the rate of BASFI increase was 0.039 units/symptom year. Estimates of the rate of change in BASFI over time were also reported in a cost-effectiveness modelling study. 152 The data were from patients who were captured in two surveys at two time points 1992/1994 and November 2002 approximately 8 years apart (n = 1100). The estimate of annual BASFI progression was 0.07 points, but when only patients with BASDAI score of ≥ 4 were included in the analysis, BASFI progression was estimated as 0.054. It was reported that data from a cohort of 493 patients who had been followed up for more than 3 years generated similar findings; the number was not actually reported for the whole survey but was 0.059 for the BASDAI score of ≥ 4 subgroup.
Natural history data from patients with nr-AxSpA are even more scarce than those for AS patients, with no long-term data identified. A comparison of AS and nr-AxSpA patients from a cohort of 100 consecutive patients (Herne clinic, Germany) (AxSpA n = 100, nr-AxSpA n = 44, AS n = 56) found that slightly higher proportions of AS patients met pre-specified cut-off points of disease severity than did nr-AxSpA patients, but the differences were statistically significant only for ASDAS, CRP level, mSASSS and the number of inflamed lesions; the proportion of males was also statistically significantly different. 153 The results are given in Table 27. The difference for BASFI was very close to statistical significance.
A larger cross-sectional study of the GESPIC cohort [n = 462 patients with axSpA (AS or nr-AxSpA)] also found differences between AS and nr-AxSpA patients. 18 When AS (≤ 5 years) and nr-AxSpA were compared, there were statistically significant differences in Physician Global Assessment, BASFI (3.1 in AS vs. 2.5 in nr-AxSpA), BASMI (1.9 in AS vs. 1.1 in nr-AxSpA), spinal mobility and lateral spinal flexion, CRP level and ESR, and all radiographic measures (mSASSS 4.9 in AS vs. 1.4 in nr-AxSpA). mSASSS was statistically significantly worse in males versus females and between CRP level > 6 and < 6, although it is unclear whether or not this is a meaningful cut-off point for CRP level.
In two longitudinal studies of progression in nr-AxSpA,154,155 also using the GESPIC cohort, progression in terms of sacroiliitis and in terms of radiographic progression in the spine (mSASSS) was slightly more rapid in AS than in nr-AxSpA but not statistically significantly so. Raised CRP level at baseline was a predictor of both measures of progression in AS but only for sacroiliitis in nr-AxSpA. The presence of syndesmophytes was predictive of higher progression rates as assessed by mSASSS in both AS and nr-AxSpA. Of the 95 patients with nr-AxSpA, 11 (11.6%) fulfilled the modified New York criteria for AS after 2 years of follow-up. A review of the burden of illness in nr-AxSpA157 cited this (11.6%) progression rate along with a 10% rate over 2 years and a 24% rate over 10 years. However, the 10-year rate was derived from a broader, more heterogeneous population than the GESPIC cohort: patients had undifferentiated spondyloarthropathies with over half not having inflammatory low back pain. 156,158 The GESPIC study recruited only patients with axSpA (AS or nr-AxSpA).
Studies of disease progression in nr-AxSpA focus on aspects of the disease that can be assessed through imaging techniques: radiography or MRI. This may appear reasonable given the subjective, patient-questionnaire basis of the BASFI score.
Finally, there is evidence that as well as being progressive, the course of AS includes flares. A study based on the population of a trial comparing probiotic and placebo treatment in AS found that the overall flare rate was 71.4 per 100 person-weeks; the major flare rate was 12/100 person-weeks and the minor flare rate was 59.4/100 person-weeks. 28 BASDAI and BASFI varied with type of flare: mean BASDAI scores were 5.5 (major flare), 3.1 (minor flare) and 2–2.5 (flare free), and mean BASFI scores were 5.5 (major flare), 3.1 (minor flare) and 2.5–3.5 (flare free). A pilot study used the SMART cohort (Bath, UK) to investigate the pattern of disease and impact of disease flares. 159 Of the 114 patients, 96% reported experiencing flares. Flare duration varied by patient: days (40%), weeks (30%) and months (30%). Fifty per cent of patients reported flares on a background of symptoms, while 26% reported gradually developing and resolving flares after which symptoms were worse than before the start of the flare. These patterns were associated with higher BASFI scores. Around 20% reported flares with no symptoms between. A small proportion (7%) reported gradually developing and resolving flare with periods of no symptoms.
In summary, the available studies indicate that in AS and nr-AxSpA disease activity (BASDAI) is fairly stable over time and does not generally progress, although it can be at a high (severe) level early in the disease. Patients function (as assessed by BASFI) does deteriorate over time, although the course is not constant or predictable. BASFI is determined by both disease activity and bone neo-formation; progression of BASFI score over time is driven by progression of bony disease as assessed by imaging scores such as mSASSS, or the presence of syndesmophytes. Best estimates of yearly disease progression rates without anti-TNF therapy are around 1.0 mSASSS units and 0.035–0.07 BASFI units. Information on the natural history of nr-AxSpA is relatively sparse. While disease progression appears to be faster in AS, patients with nr-AxSpA can have severe disease activity and hence poor function.
Clinical effectiveness summary and conclusions
Summary of randomised controlled trial results
The quality of the trial evidence was generally high; most studies were unlikely to have produced results which were biased. For both the AS and nr-AxSpA populations, the results of the meta-analyses demonstrated that anti-TNFs produce statistically significant and clinically relevant benefits to patients in terms of improving function and reducing disease activity. The common class-effect model used may have underestimated the uncertainty in the effect estimates. Although there is a possibility that infliximab is more effective than other TNF inhibitors at least at 12 weeks, there is no strong evidence to support this. For the disease activity, function and responder outcomes, the class-efficacy estimates were consistently slightly smaller for nr-AxSpA than for AS, most noticeably for BASFI and BASDAI 50. Statistical heterogeneity was more apparent in the nr-AxSpA analyses than in the AS analyses. This may be a result of both clinical heterogeneity in the nr-AxSpA trials (such as variation in CRP levels or the proportion of MRI positive patients) and the fact that fewer studies were available for analysis. In the light of the statistical heterogeneity across the nr-AxSpA trials, both the reliability of the nr-AxSpA-pooled estimates and their true relevance to patients seen in clinical practice is questionable.
The US FDA reanalyses of two key nr-AxSpA trials further emphasised the heterogeneity in the nr-AxSpA population. Results for an adalimumab trial in nr-AxSpA patients suggested reduced efficacy in a centrally diagnosed nr-AxSpA population than in a locally diagnosed population and that the treatment benefit in the whole trial population may have been driven by benefit in patients who actually had AS, not nr-AxSpA. Conversely, in a certolizumab pegol trial which recruited both populations, the efficacy findings were consistent across the AS and nr-AxSpA subpopulations, regardless of the discrepancy in local or central pelvic radiograph readings.
Long-term efficacy
The longest follow-up durations in patients with AS by anti-TNF were 5 years for adalimumab, 5 years for etanercept, 3 years for infliximab, around 5 years for golimumab and nearly 2 years for certolizumab pegol. The results showed that across all the anti-TNFs after approximately 2 years of treatment, around half of patients still achieved a good level of response to therapy. At 5 years around 60% of golimumab patients, 50% of etanercept patients and 30% of adalimumab patients still achieved a good treatment response. However, the long-term studies were not as well-reported as the RCTs, and their results were derived from less reliable data; it is therefore unknown if these are true treatment differences or a result of differences in follow-up protocols, and/or imputation and analysis methods.
The long-term follow-up for nr-AxSpA patients showed a continued high proportion of responders. At 1 year around half of patients on adalimumab, etanercept or certolizumab still achieved an ASAS 40 or BASDAI 50 level response. With certolizumab this is maintained at 2 years and with adalimumab at 3 years.
When the long-term data are presented as observed or as completer analyses, the long-term results are similarly good: withdrawal rates are not high and those patients who remain on treatment continue to achieve a good response.
For all anti-TNFs, at long-term follow-up mean final values or mean change from baseline for BASDAI, BASFI and BASMI, when reported, were generally maintained at levels indicative of clinically significant treatment benefit for those patients with AS and those with nr-AxSpA.
Four studies reported on radiographic disease progression over 2 years of follow-up in terms of mSASSS in patients taking adalimumab, infliximab, etanercept and golimumab. All four open-label, uncontrolled follow-up studies found that mSASSS increased by a mean of around 0.9 over 2 years. Three of these studies compared their rates with those from the OASIS cohort118 (of patients not taking an anti-TNF) and found no difference. In conclusion, there is no real evidence for the impact of anti-TNF treatment on radiographic disease progression; a beneficial effect cannot be assumed, nor, given the short-term nature of the follow-up and the insensitivity of radiography as a tool for the evaluation of disease progression in AS, can one be discounted. There are some data to suggest an identifiable benefit from around 4 years but results from ongoing long-term studies should help to clarify this issue.
Registry data demonstrate that around 60% of patients with AS treated with a first anti-TNF will still be taking their therapy at 2 years, with median drug survival of 3.1 years (based on Danish registry n = 1436). Sequential treatment with anti-TNFs can be worthwhile but the drug survival response rates and benefits are reduced with second and third anti-TNFs, with the proportion of BASDAI 50 responders falling approximately 10% with each subsequent anti-TNF and the median BASDAI and BASFIs achieved increasing (worsening). The lower efficacy of a second anti-TNF relative to a first is reflected in lower median drug survival and proportion of patients remaining on therapy at 2 years. Interestingly, despite a further reduction in response and efficacy with a third anti-TNF, drug survival does not fall further, suggesting that patients may be allowed to, and be prepared to, continue with a less than optimally effective anti-TNF at this stage in their treatment history.
Adverse effects
Data from large systematic reviews, which included patients with a wide range of diseases, suggest that, in the short term, anti-TNFs as a group are associated with significantly higher rates of serious infections, tuberculosis reactivation, non-melanoma skin cancer, total AEs and withdrawals because of AEs, when compared with control treatments. Specifically, infliximab is associated with significantly higher rates of total AEs and withdrawals because of AEs, and that certolizumab pegol is associated with significantly higher rates of serious infections and SAEs. Analyses from the present review showed etanercept to be statistically significantly more likely to result in an injection/infusion site reaction compared with infliximab, although analysable data on such reactions were not reported for the other three anti-TNFs. Evaluations of longer-term data are more scarce, although they suggest similar safety profiles across anti-TNFs. Data from the open-label studies included in this review also do not suggest that there are important differences between treatments, other than a higher incidence of injection site reactions following treatment with etanercept. These open-label data are, however, limited by the small sample sizes and non-randomised study designs.
Natural history
The available studies indicate that in AS and nr-AxSpa disease activity (BASDAI) is fairly stable over time and does not generally progress, although it can be at a high (severe) level early in the disease. Patient function (as assessed by BASFI) does deteriorate over time, although the course is not constant or predictable. BASFI is determined by both disease activity and bony disease; progression of BASFI over time is driven by progression of bony disease as assessed by imaging scores such as mSASSS, or the presence of syndesmophytes. Best estimates of yearly disease progression rates without anti-TNF therapy are around 1.0 mSASSS units and 0.035 to 0.07 BASFI units. Information on the natural history of nr-AxSpA is relatively sparse. While disease progression appears to be faster in AS, patients with nr-AxSpA can have severe disease activity and hence poor function.
Overall conclusions
-
For both the AS and nr-AxSpA populations the results of the meta-analyses demonstrated that anti-TNFs produce statistically significant and clinically important benefits to patients in terms of improving function and reducing disease activity. The efficacy estimates were consistently slightly smaller for nr-AxSpA than for AS.
-
In AS, although there is a little variation in treatment effects and it is possible that infliximab may be more effective than other anti-TNFs at 12 weeks, the evidence for this is not strong and it is plausible that anti-TNFs may have a common class effect, with the treatments being equally effective.
-
Statistical heterogeneity was more apparent in the nr-AxSpA analyses than in the AS analyses. This may be because of both clinical heterogeneity in the nr-AxSpA trials and the fact that fewer studies were available for analysis. In the light of this heterogeneity, both the reliability of the nr-AxSpA-pooled estimates and their true relevance to patients seen in clinical practice is questionable.
-
Effectiveness was maintained over time. About 50% of patients maintained a benefit at 2 and 5 years.
-
Evidence for an effect of anti-TNFs on radiographic disease progression was limited. The relatively short-term follow-up available to date and the insensitivity of radiography as an imaging tool precluded the drawing of firm conclusions regarding the role of anti-TNFs in preventing or delaying the progression of AS; there are some data to suggest an identifiable benefit from around 4 years, but results from ongoing long-term studies should help to clarify this issue.
-
Sequential treatment with anti-TNFs can be worthwhile in patients with AS but the drug survival response rates and benefits are reduced with second and third anti-TNFs.
Chapter 4 Assessment of existing cost-effectiveness evidence
Systematic review of existing cost-effectiveness evidence
The following sections provide an overview of existing cost-effectiveness evidence and an assessment of the relevance of the data from the perspective of the UK NHS. The differences in the approaches and assumptions used across the studies are examined in order to explain any discrepancies in the findings and to identify key areas of remaining uncertainty. The findings from the review provide the basis for the development of a new decision-analytic model reported in Chapter 6, Independent economic assessment: York model.
Methods
An initial systematic search was undertaken in the NHS EED using a combination of technology names and disease terms. Further searches were undertaken in MEDLINE and EMBASE for modelling and utility studies using disease terms only (as known references were not identified from the initial search in NHS EED). Only full economic evaluations that compared two or more options and consider both costs and consequences (including cost-effectiveness, cost–utility and cost–benefit analyses) were included in the review of existing economic literature. No language and date limits were initially applied, although eligibility of studies was subsequently restricted to those reporting results which were specific to the UK. Full details of the search strategies used are reported in Appendix 1.
In addition, as part of the current multiple TAs process, each manufacturer submitted de novo evidence on the cost-effectiveness of the anti-TNFs in line with their indications for the treatment of AS and nr-AxSpA. 34–37 These submissions are reviewed and the findings compared with those found in the review of previously published studies.
Results of review of existing cost-effectiveness evidence
The combined searches retrieved 210 citations. A total of six UK studies reporting on the cost-effectiveness of anti-TNFs for the treatment of AS were identified. No previously published studies were identified for patients with nr-AxSpA.
Four of these studies were industry-funded assessments of the following anti-TNFs: infliximab (Kobelt et al. 2004152 and Kobelt et al. 2007160 both funded via an unrestricted grant by Schering-Plough), etanercept (Ara et al. 161 funded by Wyeth pharmaceutical P.C.) and adalimumab (Botteman et al. 162 funded by Abbott Laboratories). The three studies published in 2007 are largely based on the economic analyses originally submitted by the manufacturers to NICE as part of the previous multiple TA (TA143). 17 As the earlier publication by Kobelt has been superseded by the 2007 publication, only the latter publication is further considered in this review. The remaining two UK studies were publications of the assessments and/or critiques undertaken by the independent Assessment Group/Evidence Review Group (ERG) for infliximab, etanercept and adalimumab for TA14338 and golimumab for TA233. 163 Therefore, a total of five studies met the inclusion criteria and are included in this review.
The following sections provide a narrative discussion of each publication. The quality assessment of these studies is shown in Appendix 10. A single critique section is used to highlight the key issues and potential limitations of existing published cost-effectiveness evidence. These issues are then revisited with respect to the de novo analyses submitted by the manufacturers considering how these key issues and potential limitations have been addressed in the two separate indications. The final section highlights the remaining issues and uncertainties and provides the basis for informing the development of a separate independent analysis of the cost-effectiveness of anti-TNFs for AS and nr-AxSpA relevant to informing decisions for the NHS.
Assessment of published cost-effectiveness studies
Kobelt et al.:160 Comparison of the Cost-Effectiveness of Infliximab in the Treatment of Ankylosing Spondylitis in the UK Based on Two Different Clinical Trials
Kobelt et al. 160 estimated the cost-effectiveness of infliximab for the treatment of AS compared with standard care over a lifetime horizon (60 years). Results were presented from both societal and NHS/personal social services (PSS) perspectives, although only the latter are reported here in line with the current NICE reference case. Short-term effectiveness data were derived from two separate clinical trials (Braun et al. 98 and ASSERT102,164) to inform the proportion and magnitude of initial response to treatment expressed in terms of BASDAI 50 (or a BASDAI ≤ 4) response (12–24 weeks) and changes in BASDAI and BASFI scores. These were combined with longer-term observational evidence on disease progression (BASFI only) and other external sources on costs and utilities to estimate cost-effectiveness. Results were reported separately based on each trial. Costs and benefits were discounted at 3.5% and presented at 2005 prices.
Methods
The cost-effectiveness model was based on a short-term decision tree representing the double-blind periods of the trials (12–24 weeks) and a longer-term Markov model to estimate subsequent progression. The Markov model comprised three states: ‘Off treatment’, ‘On treatment’ and ‘Dead’. Only patients responding to treatment as defined by the following criteria [BASDAI ≤ 4 (scale 0–10) or a ≥ 50% improvement in BASDAI] remain on treatment at the end of the double-blind periods. Differential BASDAI and BASFI scores (scale 0–10) were derived from Braun [‘Off treatment’ BASDAI score = 6.3 and BASFI score = 5.4; ‘On treatment’ (responders) BASDAI score = 1.8 and BASFI score = 2.0] and ASSERT102 [‘Off treatment’ BASDAI score = 6.4 and BASFI score = 5.8; ‘On treatment’ (responders) BASDAI score = 1.4 and BASFI score = 1.9]. Disease progression was expressed in terms of changes in BASFI and was estimated from two surveys conducted 10 years apart (n = 1110). 152 The mean absolute annual change in BASFI applied was + 0.07 (scale 0–10) and this was used to characterise the natural history of progression for patients with AS without infliximab. Three main scenarios were presented reflecting different assumptions concerning the impact of infliximab on disease progression: (1) no progression while on treatment; (2) 50% of natural history (0.035/year); and (3) same as natural history (0.07/year).
Fifteen per cent of patients were assumed to discontinue from infliximab annually based on data specific to responders from the open-label extension period in the Braun trial. Interestingly, the authors noted that the persistence rate was lower in responders compared with the entire sample in the Braun trial98 and its extension (approximately 10% withdrawal rate per annum). The BASDAI and BASFI scores for patients who withdrew from infliximab were assumed to return to the mean score of the non-treated group. Mortality was modelled from general population life-tables applying a SMR of 1. Hence no additional mortality was assumed to be related to AS and no direct or indirect benefits for mortality were assumed for infliximab.
Disease costs and HRQoL were derived from a cross-sectional retrospective survey conducted at the University of Bath, with the sample covering the full range of BASDAI and BASFI (1–10). The annual cost of infliximab was based on 5 mg/kg body weight (weeks 0, 2 and 6 and then every 6 weeks). An initial cost was assigned to all patients starting treatment (£79.25) and an outpatient cost was applied to each infusion.
Results
From a NHS perspective, the cost per quality-adjusted life-years (QALYs) gained ranged from £28,332 and £26,751 (no progression while on treatment) to £49,417 and £46,167 (no effect of treatment on progression) as shown in Table 28. The model was also sensitive to the time horizon and the withdrawal rate. Using a 10-year horizon resulted in incremental cost-effectiveness ratios (ICERs) between 63% and 66% higher than the base-case lifetime horizon (60 years) and a withdrawal rate of 5% resulted in ICERs between 22% and 33% higher than the base case (15%).
| Scenario | Incremental cost | QALY gain | Incremental cost-effectiveness ratio (£/QALY) |
|---|---|---|---|
| Braun98 | |||
| No progression on treatment | 36,378 | 1.28 | 28,332 |
| 50% progression on treatment | 35,756 | 1.01 | 35,332 |
| Same progression on treatment | 39,336 | 0.80 | 49,417 |
| ASSERT | |||
| No progression on treatment102 | 33,920 | 1.27 | 26,751 |
| 50% progression on treatment102 | 34,408 | 1.01 | 34,067 |
| Same progression on treatment102 | 39,242 | 0.86 | 46,167 |
Ara et al.:161 The Cost-Effectiveness of Etanercept in Patients with Severe Ankylosing Spondylitis in the UK
Ara et al. 161 estimated the cost-effectiveness of etanercept for the treatment of severe AS in the UK in accordance with BSR guidelines from a NHS/PSS perspective over a 25-year time horizon. Effectiveness data were derived from individual patient data from a large multicentre European RCT to inform the proportion and magnitude of initial response to treatment and associated changes in BASDAI and BASFI scores. These were combined with longer-term observational evidence on disease progression (BASFI) and other external sources on costs and utilities to estimate cost-effectiveness. Costs and benefits were discounted at 3.5%. The price year was not formally stated.
Methods
An individual patient model was used to estimate short-term and longer-term costs and outcomes. Patients in the model were assumed to have tried and failed at least two consecutive NSAIDs and have a BASDAI measurement ≥ 40 (scale 0–100). Response was defined as a ≥ 50% reduction in BASDAI (or all fall of ≥ 20 units) and a reduction of the spinal VAS by ≥ 2 units. Response rates at 12 and 24 weeks were derived from two RCTs (67% and 55% for etanercept and 24% and 16% for comparator arm at each respective time point). Individual patient data at 12 and 24 weeks were used to estimate the magnitude of change in BASDAI and BASFI for responders and non-responders. The mean BASDI and BASI scores at week 12 and 24 for responders and non-responders are reported in Table 29 together with observed utility at week 12 and the predicted utility values mapped from BASDAI and BASFI at week 24.
| Patient response | Week 12 | Week 24 | ||||
|---|---|---|---|---|---|---|
| BASDAI | BASFI | EQ-5Da | BASDAI | BASFI | EQ-5Db | |
| Treatment non-responder | 53.02 | 54.86 | 0.48 | 56.87 | 56.87 | 0.46 |
| Treatment responder | 19.52 | 25.39 | 0.79 | 18.32 | 21.41 | 0.80 |
| Comparator non-responder | 55.60 | 57.55 | 0.46 | 47.67 | 47.78 | 0.42 |
| Comparator responder | 22.97 | 29.88 | 0.74 | 25.11 | 20.92 | 0.79 |
For patients who continued responding to treatment it was assumed that BASDAI and BASFI measures remained constant at the levels observed at week 24. For patients who withdrew after week 24, it was assumed patients would immediately revert back to their baseline values of BASDAI and BASFI. After 24 weeks in the model it was also assumed that patients with AS, not receiving anti-TNFs [conventional care (CC) and etanercept non-responders], would experience a worsening BASFI. A mean absolute change in BASFI of 0.7 (scale 0–100) was assumed based on a cross-sectional study of over 1000 UK patients. 152
Quality-adjusted life-years were estimated using a relationship derived from BASDAI, BASFI and EQ-5D from a single European RCT (utility = 0.9235 – 0.004 × BASFI – 0.004 × BASDAI). Disease costs were derived from a separate costing study of 147 patients attending the Staffordshire Rheumatology Centre in Stoke-on-trent. 165 A relationship between BASDAI and BASFI measurements and costs was used to estimate the disease costs and impact of etanercept (annual costs = 5.862 + 0.006 × BASDAI + 0.016 × BASFI). An annual cost of £9372 was included to reflect the acquisition and monitoring costs associated with etanercept. An initial cost of £71 was also applied to the first 3-month period for etanercept, although no further details were provided by the authors concerning what this cost represented. The costs and/or HRQoL associated with AEs were not included.
The authors assumed that 10% of patients withdraw from etanercept every year. These data were derived from external sources and no explanation was provided concerning whether or not these data specifically applied to the post-24-week period or not and/or whether they were derived from responders to treatment or not. Mortality was modelled from general population life-tables applying a SMR of 1.50. No direct or indirect benefits for mortality were assumed for etanercept.
Separate scenarios were presented to explore alternative assumptions related to disease progression, long-term annual withdrawal and the model time horizon.
Results
The main results are summarised in Table 30. From a NHS perspective, the base-case cost per QALY gained was £22,704 for etanercept over a 25-year horizon. In contrast to the study by Kobelt et al. ,160 the impact of alternative progression assumptions appeared to have limited impact on the ICER, with alternative scenario results ranging from between £23,625 (50% progression on treatment) and £25,679 per QALY (same progression on treatment). The ICERs for alternative annual withdrawal rates ranged from £15,103 (5% withdrawal rate) to £29,428 per QALY (15% withdrawal rate). The ICERs for alternative time horizons ranged between £27,594 (2 years) and £22,704 (25 years).
| Scenario | Incremental cost | QALY gain | ICER (£/QALY) |
|---|---|---|---|
| Base case | 35,978 | 1.59 | 22,704 |
| No progression for any patient | 36,825 | 1.43 | 25,679 |
| 50% progression on treatment (0.035 BASFI score) | 36,032 | 1.56 | 23,155 |
| Same progression on treatment (0.07 BASFI score) | 36,088 | 1.53 | 23,625 |
| Annual withdrawal rate, 5% | 33,976 | 2.25 | 15,103 |
| Annual withdrawal rate, 15% | 36,968 | 1.26 | 29,428 |
Botteman et al.:162 Cost-Effectiveness of Adalimumab for the Treatment of Ankylosing Spondylitis in the UK
Botteman et al. 162 evaluated the cost-effectiveness of adalimumab versus conventional therapy in patients with active AS from a NHS perspective over a 30-year time horizon. Effectiveness data were derived from pooled data from two Phase III studies in patients with an inadequate response to ≥ 1 NSAID. Micro-simulation methods were subsequently applied to these studies to simulate treatment decisions in accordance with BSR guidelines and associated outcomes. These were combined with author assumptions on disease progression (BASFI only), utility and cost data from the clinical trials and other external sources to estimate cost-effectiveness. Costs and benefits were discounted at 3.5% using a 2004 price year.
Methods
Micro-simulation methods were applied to patients (n = 397) recruited into two adalimumab RCTs: ATLAS61 and M03-606. In the adalimumab clinical trials, patients were kept on active treatment even when response had not been achieved. Consequently, simulation methods were applied to the patients in the clinical trial to mimic treatment decisions which more closely reflected treatment guidelines and the requirements of the economic model. In accordance with BSR guidelines, a response in the model was defined as a reduction of BASDAI of 50% or a decrease of ≥ 2 cm (scale 0–10) accompanied by a reduction of spinal pain VAS of ≥ 2 cm. Assessment of initial response was assumed to take place 8 weeks after treatment initiation. If the response criteria were not met at 8 weeks, a second response assessment was assumed at 12 weeks. Failure to achieve response on both occasions was assumed to lead to withdrawal of adalimumab therapy. Therapeutic responses were then assumed to be reviewed every 3 months until the end of the simulation (year 30). Failure to maintain the original response led to repeat assessments after 6–12 weeks in the first 48 weeks. Failure to maintain response on both occasions led to withdrawal of adalimumab. After week 48, the simulation model defined inadequate response on the basis of BASDAI scores only. In the RCTs, patients were allowed to switch to open-label adalimumab at week 24; for these patients, LOCF at time of switch for BASDAI, BASFI and VAS values were used in the model.
The BASDAI, BASFI and spinal pain scores were based on directly observed trial scores (until week 48) and additional assumptions about disease progression (after week 48). The BASDAI, BASFI and spinal pain scores were adjusted at each time point by a fixed value equal to the average difference between adalimumab and CC patients observed at baseline. BASDAI scores after week 48 were assumed to remain constant at these levels for patients continuing to respond to adalimumab and for CC patients. BASFI was assumed, for CC patients, to worsen after week 48 by 0.05 units (scale 0–10) annually. The estimate applied to the increase in BASFI appears to be based on the authors’ own assumption but is argued to be consistent with previous cost-effectiveness/epidemiological studies. In contrast, BASFI scores were assumed to remain stable for adalimumab while patients remained on therapy, which was argued to be consistent with the assumptions applied in previous published cost-effectiveness studies. It was assumed that patients who discontinued would revert back to the BASFI scores of CC patients within 12 weeks (i.e. any benefits in BASFI were not maintained over a longer period). This was argued by the authors to be a conservative assumption.
Utilities were derived from the Health Utilities Index 3 (HUI-3) from data at baseline and at 24 weeks from both adalimumab trials. A subsequent regression was estimated to predict utilities based on BASDAI, BASFI, sex and race [utility = 0.948857 – 0.041528 × BASDAI – 0.034481 × BASFI + 0.047080 × Gender(1 = male, 0 = female) – 0.063801 × Race(1 = white, 0 = other)].
Estimates of disease costs were based on 2-year data from 208 patients in the OASIS study, conducted in the Netherlands, Belgium and France. 118 An ordinary least squares regression was estimated using only BASDAI (and only BASFI in a sensitivity analysis). The regression utilised in the base-case was £708.45 + £750 × BASDAI. Hence each increase in BASDAI of 1 unit (scale 0–10) was assumed to be associated with an increase in costs of £750.
Additional acquisition costs were applied to adalimumab (£357.50 per injection). No additional administration costs were incorporated as patients were assumed to self-administer their injections. All patients, regardless of treatment, were assumed to require at least two rheumatologist visits per year. Routine safety monitoring costs were based on national guidance and included the cost of nursing and physician time. The cost of a routine tuberculosis screening test via chest radiography was assumed before and 6 months after initiation of therapy and tuberculosis skin testing before initiation of therapy. The cost of AEs was based on data collected from the two clinical trials. A cost of £5100 was applied to an active tuberculosis case.
An annual rate of withdrawal of 10% was applied based on a assumption by the authors. The estimate was argued to be consistent with estimates reported in previously published cost-effectiveness analyses.
Results
The main results are summarised in Table 31. From a NHS perspective, the base-case cost per QALY gained was £23,097 for adalimumab over a 30-year horizon. Similar to the study by Ara et al. ,161 the impact of alternative progression assumptions appeared to have limited impact on the ICER, with alternative scenario results ranging from between £23,802 (no BASFI progression on any treatment) and £23,812 per QALY (same BASFI progression on treatment). However, in contrast to Ara et al. ,161 the ICERs appeared more sensitive to the alternative time horizons with estimates ranging between £47,083 (48 weeks), £26,332 (5 years) and £23,097 (30 years).
| Scenario | Incremental cost | QALY gain | ICER (£/QALY) |
|---|---|---|---|
| Base case | 23,857 | 1.03 | 23,097 |
| No progression for any patient | NR | NR | 23,802 |
| Same progression on treatment (0.05 BASFI) | NR | NR | 23,812 |
McLeod et al.:38 Adalimumab, Etanercept and Infliximab for the Treatment of Ankylosing Spondylitis: A Systematic Review and Economic Evaluation
McLeod et al. 38 evaluated the clinical effectiveness and cost-effectiveness of adalimumab, etanercept and infliximab compared with conventional treatment for AS. The publication is based on the independent assessment undertaken by Liverpool Reviews and Implementation Group (LRiG) for NICE TA143. 17 The cost-effectiveness of these interventions over the short term (1 year) and over alternative time horizons of up to 20 years was reported.
Methods
The authors assumed that all three interventions were of equal clinical effectiveness and analysed the anti-TNFs as a class versus placebo. Short-term effectiveness over 1 year was modelled using individual patient data from two RCTs (including an open-label extension from week 24) for adalimumab from 397 patients (246 adalimumab, 151 placebo). Of these, 315 of 397 patients were deemed to meet the BSR guidelines and were included within the Abbott economic model. There is a lack of transparency regarding the values used because of the commercial nature of the data. However, the estimates for response rates were reported to be similar to those reported by LRiG in a separate pooled analysis at weeks 12 and 24, which were 59% and 49.2%, respectively, for the TNF-α inhibitors (vs. 22.5% and 14%, respectively, for placebo). No information is reported on the magnitude of changes assumed. From week 30 onwards it was assumed that spontaneous recovery without treatment (for placebo patients) would occur at a rate of 17.1%, as estimated by LRiG from the patient-level analysis of the two adalimumab RCTs. This assumption was explored in a separate sensitivity analyses.
The assessment group model assumed that patients withdraw from TNF-α inhibitor treatment at a rate of 15% per year, which was considered to represent the ‘central value’ of the studies that were identified reporting longer-term discontinuation rates. This estimate is also the same as the annual rate reported in the open-label extension study for infliximab (Braun trial) also reported in the review undertaken by LRiG. Rates of 7% and 24% were also explored in separate sensitivity analyses, representing the range of values reported across the studies considered. The annual withdrawal rate (after the first 12 months, as observed rates are used in the first 12 months) was applied to the difference in response rate between the two arms of the evaluation, rather than the absolute number of responders. This was to account for a potential anomaly that could arise through the assumption of a constant level (17.1%) of spontaneous recovery without treatment.
The assessment group model took into account the cost of drug acquisition, administration, monitoring and AEs. No administration costs were assumed for etanercept and adalimumab, as it was assumed that both would be self-administered at home without supervision. The authors assumed an additional cost of £267 to administer infliximab infusions based on NHS Reference Costs estimates for the regular attender cost for chemotherapy with musculoskeletal primary diagnosis. Quarterly monitoring and testing was assumed for all patients receiving long-term treatment with TNF-α inhibitors. However, two of these assessments were assumed to take place at the patient’s routine follow-up outpatient visit, so only the additional costs of tests for monitoring (£25) were applied to these assessments. The remaining two assessments were assumed to be undertaken at a general practitioner’s surgery and an additional cost of £25 was assumed for nurse/general practitioner time in addition to the costs of tests for monitoring (£25). AEs costs were based on estimates reported by one manufacturer (Abbott) of £95.29 in the first year of treatment and £47.65 per patient-year thereafter.
Disease-related costs to the NHS were estimated by fitting an exponential cost model to the weighted aggregate data from the OASIS study; a 2-year prospective study of 208 AS patients from four centres in France, Belgium and the Netherlands (n = 208). 118 The exponential model estimated NHS cost = £1585.30 × exp(0.1832 × BASFI). The OASIS data were considered by the authors to provide a more reliable source than other published studies from Stoke and Bath, being prospective in design and over a longer period. BASFI was used by the authors as the major predictor of costs because it was considered to better reflect long-term disease progression compared with BASDAI.
Health-related quality of life was estimated using the utility model provided by Schering-Plough developed from the Bath Survey data set on the grounds that it used a comparatively larger sample of UK AS patients (n = 1144), and also because it incorporated age and sex variables: utility = 0.8772129 – 0.0384087 × BASDAI – 0.0322519 × BASFI – 0.0278913 × Male + 0.0016809 × Age.
The assessment group adopted a long-term increase in BASFI scores of 0.07 units per year for the conventional treatment comparator arm of the model. This progression rate is applied for all periods after week 20 in the model. In the base-case analysis, the same value was used in the intervention arm adjusted pro rata to the proportion remaining of the maximal excess response seen at 12 weeks. In effect, this assumes that patients withdrawn from anti-TNFs are assumed to return to the same trajectory as non-responders, such that there is no ensuring benefit associated with being an initial responder.
Results
Over a 1-year time horizon, base-case ICERs for adalimumab and etanercept versus CC were essentially the same (approximately £57,000 per QALY). In contrast, the ICER for infliximab was over £120,000 per QALY. With respect to modelling beyond 12 months, the results for adalimumab were considered as representative of etanercept, and only the former were provided. In contrast with other published models, the ICERs increased steadily from year 2 onwards. At a 20-year horizon the ICERs for adalimumab/etanercept increased to £98,910 per QALY and to £175,000 per QALY for infliximab.
Additional National Institute for Health and Care Excellence Decision Support Unit analyses
Given the discrepancy between the results reported by McLeod et al. 38 and the manufacturer’s submissions34–37 (largely reflected in the three industry-funded publications previously discussed in this section)160–162 also submitted as part of TA143,17 additional work was undertaken by NICE’s Decision Support Unit (DSU) to reconcile the different models and to explore whether or not differences were a result of different parameter inputs or alternative structural assumptions.
A common set of parameter values were applied by the DSU to the three manufacturer models and the LRiG model. The purpose of this was to attempt to identify whether or not differences between the results of the models persisted once this common set of values were used. The specific parameter values which were implemented were:
-
no improvement in BASFI or BASDAI for patients not on anti-TNFs
-
BASFI progression prevented while on anti-TNFs
-
BASFI progresses at 0.07 per annum when patients are not on anti-TNFs
-
annual withdrawal rate of 7% from anti-TNFs
-
baseline BASDAI/BASFI score averages 6.5/5.6
-
utility model as in the Schering-Plough submission
-
assessment group parameters for cost parameters (drug costs only)
-
a 20-year time horizon.
These parameter values were reported to have been the values agreed at a separate NICE committee meeting and consequently the rationale for these values and assumptions is not formally stated by the NICE DSU.
The results of the DSU analysis found that the manufacturer models all gave relatively consistent results for each of the drugs. For Schering-Plough, the ICERs over 20 years for etanercept/adalimumab were £27,000 or £24,000 and for infliximab were £58,000 and £50,000. Two figures were presented because Schering-Plough presented two different versions of the model which reflected two different trials. The Wyeth model gave results of £20,000 for etanercept and £39,000 for infliximab. Abbott gave results of £17,000 for adalimumab and £43,000 for infliximab (over a 30-year time horizon). These ICERs were markedly different from those reported by the independent assessment group. Using a similar set of parameters the results for etanercept/adalimumab using the LRiG model were £42,000 and for infliximab £82,000.
Further work by the DSU revealed that the differences appeared largely driven by two key assumptions which differed between the LRiG and industry models relating to:
-
the modelling of a ‘placebo’ effect
-
the longer-term functions fitted to BASDAI and BASFI for responders to anti-TNFs.
The LRiG model applied a 17.1% rate of spontaneous recovery without treatment from week 30 onwards (i.e. akin to assuming a long-term ‘placebo’ response for CC) in contrast to the manufacturers who either assumed there would be no response with CC or that any response would be transient and dissipate quickly after the 12-week period.
The LRiG model also applied a quadratic function to the BASDAI and BASFI scores of responders over a longer-time horizon, compared with the linear functions used by the manufacturers. The use of a quadratic function assumes that that the difference compared with CC was decreasing (initially) with time; that is, over time, the differences in BASDAI/BASFI would slowly reduce in responders and eventually be the same as for CC. However, the logical problem of applying a quadratic function is clear. While the scores are reducing for a period, at longer-time periods the function starts to increase again. The issues were addressed by LRiG by using various assumptions and logical constraints (i.e. BASDAI/BASFI score not allowed to be higher than CC).
To further reconcile the models, the DSU incorporated a series of alternative structural assumptions within the LRiG model. These assumptions included removing the 17.1% rate of spontaneous improvement applied to CC and assuming constant BASDAI/BASFI scores after 1 year for responders. Applying these assumptions resulted in an ICER for etanercept/adalimumab of £30,100 per QALY (estimates for infliximab not reported) which were considered to be more consistent with the manufacturer results.
Importantly, the DSU highlighted that, although these analyses helped to reconcile the different model results, any progression in terms of BASDAI or BASFI over time while on treatment would cause the ICER to increase beyond £30,100. Similarly, the DSU concluded that the exclusion of the 17.1% spontaneous recovery, without a comparable adjustment made to the intervention group was favourable towards the cost-effectiveness of TNF-α inhibitors and any adjustment for this issue would similarly lead to a higher ICER.
Armstrong et al.:163 Golimumab for the Treatment of Ankylosing Spondylitis: a National Institute for Health and Care Excellence Single Technology Appraisal
Armstrong et al. 163 summarises the report undertaken by the ERG on the clinical effectiveness and cost-effectiveness of golimumab for AS for a NICE single technology appraisal (TA233). 33 The ERG provided a critique of the manufacturer’s submission (Merck Sharp & Dohme)37 and undertook additional exploratory analyses. The manufacturer’s model applied a 20-year time horizon in the base-case and a separate lifetime analysis (60.1 years) was presented in a separate sensitivity analysis. The discount rate applied was 3.5% for utilities and costs, and costs are considered from a NHS and PSS perspective.
Methods
The manufacturer’s submission for golimumab37 was based on a single trial versus placebo (GO-RAISE90). A total of seven additional placebo controlled trials were included of other anti-TNFs; five RCTs for etanercept and two for adalimumab. In the absence of head-to-head studies directly comparing the relative effectiveness of the alternative anti-TNFs, the manufacturer undertook a Bayesian random-effects mixed-treatment comparison (MTC) including BASDAI 50 response, discontinuations and SAEs. All treatments were reported by the manufacturer to be statistically significantly more effective than placebo in terms of BASDAI 50 response. No statistically significant differences were reported between each of the alternative anti-TNFs in terms of discontinuations and SAEs. When the alternative anti-TNFs were compared with each other, no significant differences between golimumab, adalimumab and etanercept were identified for BASDAI 50. A higher risk of discontinuation was reported for golimumab versus etanercept (relative risk 4.30, 95% CrI 1.01 to 18.50), although golimumab was associated with significant improvements in BASDAI versus etanercept (mean difference –0.88, 95% CrI –1.58 to –0.14) and BASMI versus adalimumab (mean difference 0.52, 95% CrI 0.23 to 0.80).
The manufacturer cost-effectiveness model37 was based on a short-term decision tree (12 weeks) and a longer-term Markov model. The short-term tree was used to characterise response to each TNF-α inhibitor treatment based on the MTC results for BASDAI 50. After the short-term tree, patients entered a separate Markov model with a cycle length of 12 weeks and time horizon of 20 years. If patients were already receiving a TNF-α inhibitor, they either stayed on therapy (‘on TNF inhibitor’ state) or discontinued therapy because of lack of efficacy or AEs (‘not on TNF-inhibitor’ state). It was assumed that discontinuations occurred at a rate of 15% per year in line with NICE TA143. 17 To model the lower disease activity just after discontinuation of TNF-α inhibitor therapy, two 12-week tunnel states (‘just discontinued’ and ‘discontinued’) were also incorporated into the model. Patients who are in the health state ‘on TNF-α inhibitor’ are assumed to have at least a 50% improvement in BASDAI (BASDAI 50) during the first 12 weeks of treatment and do not discontinue. Treatment is discontinued in patients whose condition does not respond to treatment and they are switched to conventional therapy. Patients in the CC arm enter the Markov model in the ‘not on TNF-α inhibitor’ state. Patients could die at any point in the model.
Disease progression was incorporated in the model using BASDAI and BASFI scores. Data from the GO-RAISE90 trial and the open-label extension period were used to develop predictive equations of mean change from baseline in BASDAI and BASFI scores over time. Two separate equations were developed based on the 24-week data for all patients and post-24-week data from GO-RAISE90 for responders only. These equations were used for all anti-TNFs and the manufacturer assumed that the scores followed the GO-RAISE90 data for 2 years before they either levelled off (BASDAI) or started to deteriorate [BASFI at 50% of the rate of CC, equivalent to an increase of 0.035 (scale 0–10) units per year].
Although the equations are critical to the model structure and parameter estimates, these are not reported in the paper by Armstrong et al. 163 A separate examination of the full ERG report33 revealed that these were reported as CiC by the manufacturer and hence it is not possible to report the assumptions made in relation to the magnitude of change in BASDAI and BASFI over the initial 24-week period and subsequent post-24-week period for the anti-TNFs (responders, non-responders) and CC. BASFI scores for CC were reported to deteriorate according to the GO-RAISE trial (short-term equations were available only) after which they were assumed to deteriorate at a rate of 0.07 units per year. The assumptions related to the impact of discontinuation of anti-TNFs are not formally stated in the paper by Armstrong et al. 163 However, the structure of the model implies that patients will revert back to the subsequent trajectories of CC for both BASDAI and BASFI after 2 cycles (24 weeks).
Utilities were derived from the previous NICE TA (TA14317) and incorporated age, sex, BASFI and BASDAI. Costs included in the model comprised drug acquisition, short-term (12-week) costs, longer-term disease costs and AEs. Longer-term disease costs were based on BASFI scores from the GO-RAISE90 trial using the same regression equation used for NICE TA guidance 143. Mortality was included in the model and was considered to be a constant across the comparator treatments at a relative risk of 1.47.
Results
The main base-case results from the manufacturer are summarised in Table 32. From a NHS perspective, the base-case cost per QALY gained was £26,597 for golimumab compared with CC over a 20-year horizon. Both etanercept and adalimumab were reported to be extendedly dominated by golimumab.
| Technology | Costs (£) | QALYs | Incremental costs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 88,667 | 6.6581 | – | – | – |
| Adalimumab | 93,601 | 6.8426 | 4934 | 0.1845 | N/A (extendedly dominated) |
| Etanercept | 93,782 | 6.8504 | 5115 | 0.1923 | N/A (extendedly dominated) |
| Golimumab | 93,786 | 6.8506 | 5119 | 0.1925 | 26,597 |
The ERG undertook a limited validation of the model and reported various errors which were corrected. However, they concluded that questions remained concerning the integrity of the manufacturer model. The ERG subsequently presented results based on an exploratory reanalysis of the manufacturer’s submission, using results from a separate MTC analysis and employing a lifetime horizon. The results of the ERG reanalysis are reported in in Table 33. The results of this re-analysis resulted in golimumab being extendedly dominated by the other two anti-TNFs.
| Technology | Costs (£) | QALYs | Incremental costs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 95,227 | 7.8762 | – | – | – |
| Golimumab | 99,361 | 8.0296 | 4134 | 0.1534 | N/A (extendedly dominated) |
| Adalimumab | 108,295 | 8.3683 | 8934 | 0.3387 | N/A (extendedly dominated) |
| Etanercept | 108,347 | 8.3712 | 52 | 0.0029 | 26,505 |
There is no discussion by Armstrong et al. 163 of the appropriateness of the assumptions applied to BASFI progression, despite this being a critical structural assumption. However, a separate sensitivity analysis was presented in the full ERG report which uses the same rate of disease progression for BASFI (0.07 units per year) for all patients after 2 years. As part of this analysis, the ERG corrected errors identified in the way the BASFI regression equations were incorporated by the manufacturer.
Table 34 reports the ERG results based only on correcting the error identified and Table 35 reports the results of also applying a common rate of disease progression for all patients after 2 years as well as correcting for the error. Golimumab was reported to be extendedly dominated by the other two anti-TNFs in both scenarios. It is also worth noting that the ICER for etanercept versus CC exceeded £30,000 per QALY in both scenarios.
| Technology | Costs (£) | QALYs | Incremental costs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 77,505 | 6.7336 | – | – | – |
| Golimumab | 81,849 | 6.8746 | 4334 | 0.1410 | N/A (extendedly dominated) |
| Adalimumab | 91,340 | 7.1703 | 9491 | 0.2937 | N/A (extendedly dominated) |
| Etanercept | 91,408 | 7.1734 | 68 | 0.0031 | 31,612 |
| Technology | Costs (£) | QALYs | Incremental costs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 74,980 | 6.8267 | – | – | – |
| Golimumab | 79,330 | 6.9675 | 4350 | 0.1408 | N/A (extendedly dominated) |
| Adalimumab | 88,994 | 7.2567 | 9664 | 0.2892 | N/A (extendedly dominated) |
| Etanercept | 89,055 | 7.2600 | 61 | 0.0033 | 32,483 |
Summary and critique of published cost-effectiveness studies
No previously published studies were identified which assessed the cost-effectiveness of anti-TNFs for nr-AxSpA. Consequently, the de novo submissions provided by the manufacturers provide the only existing evidence which can be considered to inform decisions for the NHS. Of the previously published UK cost-effectiveness study identified, there appear marked differences between the results of the industry-funded assessments and the results from the independent assessment by LRiG. Importantly, the results of the independent critique and exploratory reanalysis by the ERG for TA23333 also appear potentially less favourable than the industry-funded published assessments. Although the DSU review of models submitted as part of TA14317 has reconciled many of the key differences and highlighted the key assumptions, a number of key uncertainties remain. The remainder of this section provides an overview of the issues and uncertainties identified based on existing published studies and the DSU reports. This summary provides an important basis for considering the extent to which the de novo submissions provided by the manufacturers for this appraisal have adequately addressed these.
All existing models are based on similar two part structures:
-
initial-response period (short-term model used to determine initial response rate)
-
post-response period (longer-term model used to characterise natural history of disease (i.e. without anti-TNFs) and impact of anti-TNFs (while on therapy and when therapy is stopped).
All models use changes in BASDAI and/or BASFI to quantitatively model the short- and longer-term costs and quality-of-life implications (using QALYs) of the use of anti-TNFs versus CC alone.
Although there are differences between the modelling of the initial response period, existing models are broadly comparable being based on an assessment around 12 weeks (and potentially at 24 weeks as well) using a particular variant of existing BSR guidelines. Patients receiving anti-TNFs who meet the response criteria at the 12-/24-week assessment are continued on anti-TNFs. Anti-TNFs are withdrawn in non-responders at the 12-/24-week assessment point and patients subsequently receive CC alone.
However, there are marked differences between existing studies in relation to the modelling of the post-response period and the assumptions used. This period is often separated into different time intervals allowing different assumptions to be made regarding the effect of anti-TNFs (i.e. initially improving with time in responders but then later ‘levelling off’ or even deteriorating over a longer-term time horizon relative to CC). An important difference between existing models is the timing of this ‘levelling off’ period and assumptions employed over a longer time horizon. The differences in approaches and the timing of this ‘flattening off’ period are also closely linked to the data used, that is whether or not the changes in BASDAI/BASFI used in the model are restricted to the 12- to 24-week data from RCT evidence reported during the double-blind phase (Kobelt et al. 2007160 and Ara et al. 2007161) or also incorporate longer-term data from the open-label extensions. Studies which use change in BASDAI/BASFI data directly in the model, from the double-blind phase, appear to use shorter ‘levelling off’ periods than studies using data from the open-label extension phase (Botteman et al. 2007,162 McLeod et al. 200738 and Armstrong et al. 2007163).
Those studies incorporating an open-label extension typically assume continuing changes in BASDAI/BASFI for responders to anti-TNFs versus non-responders/CC beyond the initial 12/24-week period. Importantly, none of the studies using open-label extension data appear to provide any discussion of the potential for selection bias (e.g. related to the initial consent for patients to participate and/or agree to switch treatments as well as ongoing selection issues concerning retention over a longer period) and how these should be considered and/or adjusted for in the economic model. However, the implication of this is important, as the assumption being made by several models appears to incorporate an assumption of an increasing effect of anti-TNFs in responders over time (i.e. in terms of continuing improvements in BASDAI/BASFI), which does not appear to be adequately justified or related to any underlying clinical/pharmacological mechanism. In the absence of the counter-factual (i.e. comparable data in patients who did not participate or were subsequently withdrawn from the open-label study) it is unclear whether the apparent increasing effect is simply a function of the selection issue or is a real effect of the anti-TNFs. Importantly, those studies which only use data from the double-blind periods of RCTs often cite the open-label data as providing supportive evidence regarding the maintenance of the effects observed at 12/24 weeks but do not use it to support an assumption of an increasing effect over time.
The longer-term impact on costs and utilities beyond the initial response period are subsequently quantified by estimating separate BASDAI/BASFI ‘trajectories’ for different patient categories. The three main categories are:
-
CC
-
non-responder to anti-TNFs at 12-/24-week assessment
-
initial responder to anti-TNFs at 12-/24-week assessment.
The ‘trajectory’ for patients who are responders to anti-TNFs at the initial 12-/24-week assessment are further separated into (1) the period up to the point that anti-TNFs are subsequently withdrawn (i.e. because of loss of efficacy or AEs) and (2) the period post TNF-α inhibitor withdrawal.
After the ‘levelling off’ period for BASDAI, the majority of existing studies assume BASDAI is constant over the longer term, that is the BASDAI of responders to anti-TNFs is assumed to be lower than the equivalent BASDAI value (lower disease activity) applied to CC/non-responders and a constant difference is assumed to be retained until patients discontinue. At the point of discontinuation of anti-TNFs, patients subsequently revert back to the same value assumed for CC/placebo and non-responders to anti-TNFs at 12/24 weeks. Hence, any improvement in BASDAI is assumed to dissipate immediately or within a short period (3–6 months) after discontinuation of anti-TNFs.
All existing studies model BASFI as a linearly increasing function over the longer term for non-responders/CC, that is a constant rate of change is subsequently applied which is used to characterise the impact of disease progression on functional ability, typically a worsening of 0.07 (0–10 scale) units per annum. Again, the same assumptions applied to BASDAI for non-responders to anti-TNFs are applied to BASFI, that is beyond 12/24 weeks non-responders are assumed to follow an identical BASFI ‘trajectory’ as that of CC/placebo patients. By contrast, patients who respond to anti-TNFs are typically assumed not to ‘progress’ further in terms of functional disability, or progress at a lower rate than CC patients, while continuing to receive anti-TNFs. Hence the difference in individual mean BASFI scores increases over time in existing economic models between patients who continue to receive anti-TNFs and non-responders/CC.
The only study which employs a markedly different approach to the modelling of BASDAI and BASFI for responders is the study undertaken by the previous independent assessment group (LRiG) for TA143. 17 Instead, LRiG applied a quadratic function to the BASDAI and BASFI scores of responders. This approach assumed that the difference compared with CC was decreasing (initially) with time, that is, over time, the differences in BASDAI/BASFI would slowly reduce in responders and eventually be the same as for CC. While the logical problems of applying a quadratic function over a longer period were recognised by the authors (i.e. function begins to increase after a particular period) and was addressed using a series of logical restrictions (i.e. BASDAI/BASFI score constrained to be the same or better than CC), the clinical ‘face’ validity of this approach also appears questionable in the context of longer-term projections which are required for appropriate assessments of cost-effectiveness.
Another key difference between existing studies relates to the assumptions made concerning the subsequent trajectory of BASFI for patients who withdraw from active treatment. Given that BASFI is linearly increasing with time for CC, the assumption of the subsequent BASFI trajectory is potentially an important driver of cost-effectiveness. This is often referred to as ‘rebound’. Typically, two scenarios are used:
-
Rebound equal to gain: when patients fail therapy (after initially responding), their BASFI deteriorates by the same amount by which it improves when they responded to therapy.
-
Rebound back to natural history/CC: when patients fail therapy (after initially responding), their BASFI deteriorates to the level and subsequent trajectory it would have been had they not initially responded to therapy.
In the absence of evidence on the magnitude of any rebound, these alternative scenarios represent the ‘best-case’ and ‘worst-case’ scenarios possible. In other words, the reality regarding rebound is likely to be somewhere between these two scenarios which should, therefore, be seen as the limits.
The implications of the different rebound scenarios are clearly illustrated in Figures 2 and 3. Studies which are based on assumptions of rebound equal to gain incorporate an ongoing benefit of anti-TNFs in patients in whom therapy is subsequently withdrawn after an initial response. Hence, such an assumption is more optimistic than assuming no continuing benefit at the point treatment is withdrawn.
FIGURE 2.
Illustration of the scenario of rebound equal to gain.
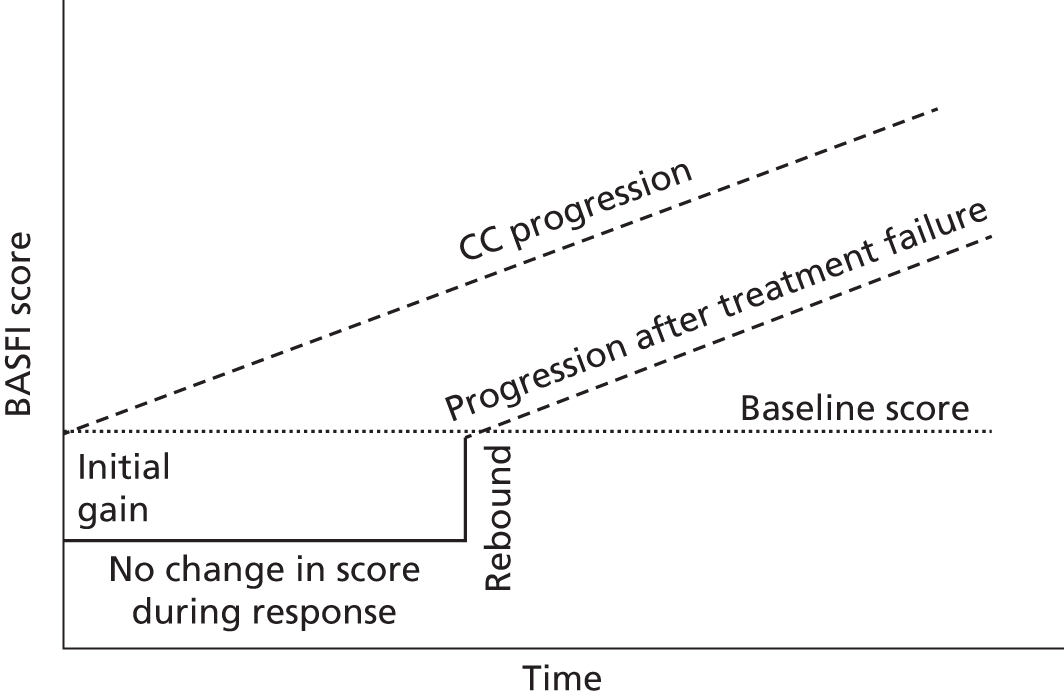
FIGURE 3.
Illustration of the scenario of rebound to CC.
Although the impact of discontinuation in patients who initially respond is clearly an important issue, the assumptions underpinning the subsequent trajectories of patients who are non-responders at 12/24 weeks to anti-TNFs are rarely explicitly justified. The most common assumption applied is that non-responders during the initial period follow the same subsequent trajectory for BASDAI/BASFI as CC/placebo patients beyond the 12-/24-week assessment point. However, the appropriateness of this assumption does not appear to have been discussed in existing studies. Essentially, for this assumption to hold, the initial response to anti-TNFs has to be independent of baseline patient characteristics, such that response to treatment is effectively a random process. However, if response is not independent of patient characteristics, the implication is that responders/non-responders to TNF-α inhibitors may be systematically different from each other. This has implications for the appropriateness of current assumptions being applied to non-responders at 12/24 weeks and subsequent responders who later withdraw. For example, all other things being equal, if patients with more severe disease (high BASDAI/high BASFI) were more likely not to respond, then assuming that the non-responders at 12/24 weeks follow the same trajectory as the ‘average’ CC/placebo patient is likely to be optimistic towards the anti-TNFs (and vice versa for if less severe patients are more likely to respond). Hence, rather than following the trajectory of an ‘average’ placebo/CC patient, a non-responder may actually follow a different trajectory, that is that of an equivalent more/less severe CC patient. Inevitably, the impact of different patient characteristics is likely to be more complex than the simplistic scenarios presented above.
As previously noted, all models use changes in BASDAI and/or BASFI to quantitatively model the short- and longer-term costs and quality-of-life implications (using QALYs) of the use of anti-TNFs versus CC. The justification for using these measures appears largely driven by the existence of external sources of costs and health utility estimates which can be directly linked to these measures and not to others (e.g. BASMI, ASDAS, mSASSS, etc.). Hence current models appear more of a function of the data which are available to link to costs and utilities rather than being based on a clear underlying biological or clinical process. This raises more general conceptual concerns regarding existing models and also regarding the generalisability of findings in an AS population to the separate nr-AxSpA population.
The use of BASDAI/BASFI per se is perhaps not the most significant issue, as in the absence of alternative mapping functions to costs and/or utilities it is unclear how to estimate longer-term costs and QALYs without ultimately linking to these measures. However, it is concerning that the majority of existing studies do not appear to link the data and assumptions applied to these measures to any coherent clinical underpinning regarding differences between population characteristics and the effect of anti-TNFs. Consequently, ‘progression’ over time is currently modelled entirely via changes in BASFI, as BASDAI is assumed to remain constant. However, no attempt is made to justify why BASFI increases, the rate at which it increases and how this rate might differ across different groups as well as the impact that anti-TNFs might have (i.e. any effect on BASFI which may be independent of the effect on BASDAI).
Modelling ‘progression’ implicitly (i.e. employing natural history estimates of the rate of change of BASFI from external studies) rather than explicitly (i.e. attempting to explain how BASFI evolves over time in relation to inflammatory and other processes and how these may differ within populations and across the AS and nr-AxSpA groups) has led to a series of implicit/evidence-free assumptions. These include:
-
No change in BASFI while receiving anti-TNFs (i.e. assuming implicitly that these act as disease modifiers and that while patients respond and continue to receive them, further deterioration in functional progression is completely prevented).
-
Lower BASFI changes while receiving anti-TNFs (i.e. assuming that anti-TNFs do not completely halt further deterioration in functional progression but that the rate of progression is reduced relative to progression on CC).
-
Similar natural history rates of change in BASFI across different subgroups and populations (i.e. assuming that rate of change in BASFI is independent of time and/or patient characteristics).
Similar conceptual concerns were also highlighted by the NICE DSU in their work to support TA143, noting that in inflammatory arthritis a clearer conceptual relationship is assumed between disease activity, radiographic progression and physical functioning, such that changes in physical functioning can be more clearly related to different processes and evidence for the anti-TNFs on each separate process. In highlighting these issues, the DSU cited emerging longer-term data reported for anti-TNFs based on measures of radiographic progression (mSASSS) in AS. Although this evidence was not formally included in their analyses, the evidence was cited to indicate that an assumption of no further progression while on anti-TNFs for AS was potentially optimistic based on emerging longer-term radiographic progression data.
Importantly, the only UK study published since the NICE DSU review did subsequently use a less favourable assumption concerning the impact of anti-TNFs on functional progression (BASFI). The assumption used by the manufacturer for golimumab37 assumed that the longer-term rate of change in BASFI for responders who continued on treatment would be 50% of that assumed for CC/non-responders. Although this assumption is a significant departure from the base-case assumptions applied within previous industry-funded studies, no justification appeared to be identified to support this by Armstrong et al. 163 in the review of the manufacturer’s submission.
In summary, there appear to be significant differences between the cost-effectiveness results reported in existing UK published studies. Many of these differences appear largely because of differences in data sources (i.e. double-blind period vs. open-label extensions), subsequent assumptions and estimates related to the magnitude and duration of the differences in BASDAI and BASFI measurements between responders and non-responders in the short to medium term (i.e. the ‘levelling off’ period) and then longer term in relation to assumptions concerning BASFI progression, and issues around ‘placebo’ effect and the withdrawal of anti-TNFs. Some of the main differences between existing studies have been highlighted in a separate review by the NICE DSU. However, while this review is helpful in identifying the impact of parameter and structural assumptions, it does not provide a basis for informing which assumptions appear most justified based on existing data and clinical understanding of the progression of AS and the impact of anti-TNFs. It is also concerning that many of the existing studies are based on CiC data and hence lack transparency regarding specific inputs and assumptions.
To date, only two UK studies have attempted to assess the cost-effectiveness of the alternative anti-TNFs. One of these studies, McLeod et al. ,38 assumed that the alternative treatments were identical in terms of clinical effectiveness and hence only considered differences in the acquisition, administration and monitoring costs. The justification provided by the authors was based on the lack of statistically significant differences across key outcome measures based on indirect comparisons. The other study, Armstrong et al. ,163 assumed differences in the clinical effectiveness of the alternative anti-TNFs based on a separate MTC. However, differences between the anti-TNFs appeared sensitive to the studies included and the specific outcomes considered. Hence different conclusions could be drawn concerning the most ‘efficient’ intervention depending on the analysis considered. However, the magnitude of differences in clinical effect and QALYs remained small and the clinical and economic value of this might appear questionable.
There are conceptual concerns surrounding all existing models relating to the subsequent projection of BASDAI and BASFI over a longer time horizon which are required in order to generate more appropriate lifetime estimates of costs and QALYs required for cost-effectiveness assessments. The speculative nature of these projections was highlighted as a significant concern by the previous independent assessment group (LRiG) and hence their longer-term results were presented as exploratory scenarios. However, it appears that all existing models are largely based on implicit approaches and assumptions, and lack a clearer conceptual basis which might help to more appropriately inform parameter estimates and structural assumptions, and facilitate a more evidence-based assessment of the potential longer-term impact of anti-TNFs.
The following sections present a summary of the de novo submissions provided by the manufacturers34–37 for the separate AS and nr-AxSpA indications. Brief overviews of the manufacturers’ submissions for AS and nr-AxSpA are provided alongside a summary of the base-case cost-effectiveness results. This is followed by a more in-depth comparison of key parameter and structural assumptions across the manufacturers and the separate indications. The issues and concerns regarding existing published studies are used as the basis for a more critical assessment of these submissions and the extent to which these concerns have been adequately addressed and key uncertainties which still remain have been highlighted is investigated.
It should be noted that although fully incremental results were routinely presented by each manufacturer, there were differences between manufacturers in terms of how the results were presented and also whether or not the correct calculations based on dominance and extended dominance were included. Consequently, the fully incremental ICER tables reported are based on our own calculations to ensure accuracy and consistency between the various manufacturer results tables.
Summary of manufacturers’ de novo submissions
Manufacturers submitted de novo analyses for both AS (AbbVie,34 UCB,35 Pfizer36 and Merck Sharp & Dohme37) and nr-AxSpA (AbbVie,34 UCB35 and Pfizer36) populations.
Overview of AbbVie (adalimumab) model
The economic model presented by AbbVie34 compared the cost-effectiveness of adalimumab versus conventional therapy and other licensed anti-TNFs for nr-AxSpA and AS. Separate state-transition models were developed for the two indications separately based on the ASAS guidelines for the use of anti-TNFs. All patients were assumed to take conventional therapy/background therapy (e.g. NSAIDs) during the modelled horizon and also receive one of the licensed anti-TNFs or placebo (conventional therapy only). Specifically, patients were assumed to stay on therapy as long as they had an adequate therapeutic response (i.e. ASAS 40 for nr-AxSpA and ASAS 20 for AS) and patients were assumed to discontinue therapy when insufficient response occurred. Discontinuations due to AEs or reasons other than therapeutic failures were also included.
The model consists of a short-term component (first 12 weeks) and a longer-term component to estimate lifetime cost-effectiveness (40 years). In common with previously published models, the model was based on the estimation of BASDAI and BASFI scores over time. The model used the available long-term open-label extension data of trials of adalimumab (up to 156 weeks in ABILITY-158 for nr-AxSpA and 260 weeks in ATLAS61 for AS, Figures 4 and 5) as well as including assumptions beyond these study durations to inform the life-time cost-effectiveness results. To avoid extrapolating life-time improvement by applying a functional form to the BASDAI/BASFI data, the manufacturer applied the mean observed BASDAI and BASFI scores until the last available data point and carried forward the last observed values to the end of horizon.
FIGURE 4.
Observed mean BASDAI and BASFI scores for adalimumab ASAS 20 responders in the licensed population from ATLAS61 (AS).
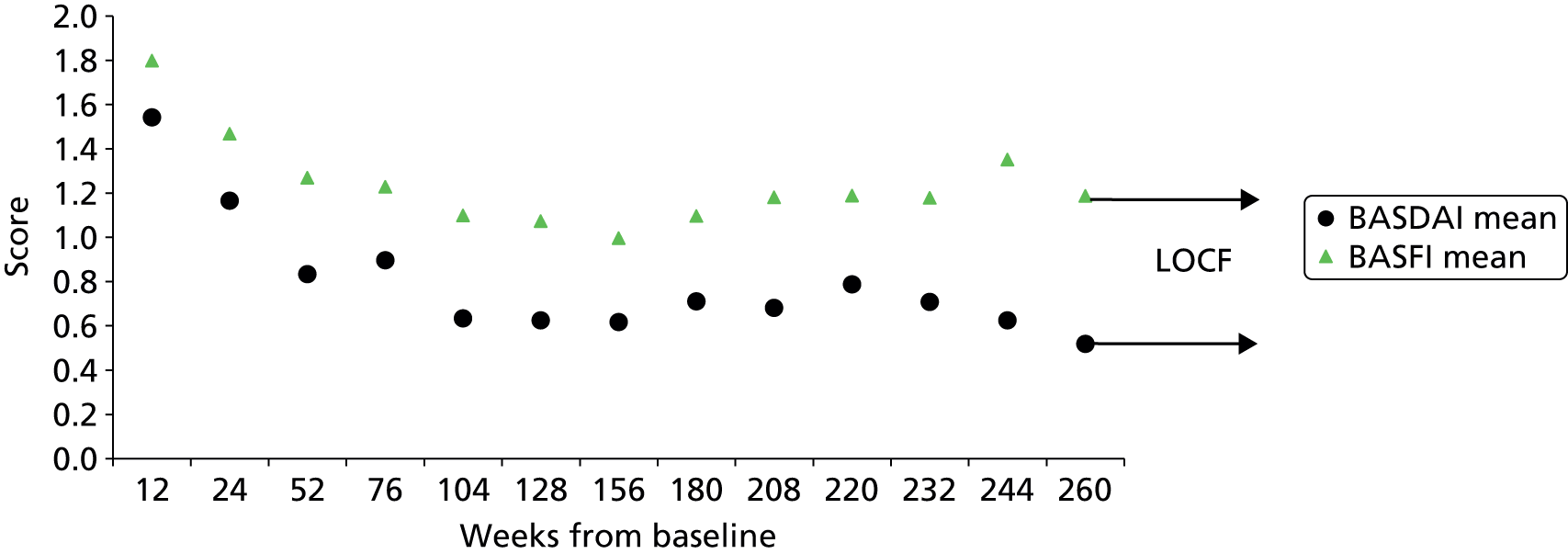
FIGURE 5.
Observed mean BASDAI and BASFI scores for adalimumab ASAS 40 responders in the licensed population from ABILITY-158 (nr-AxSpA).
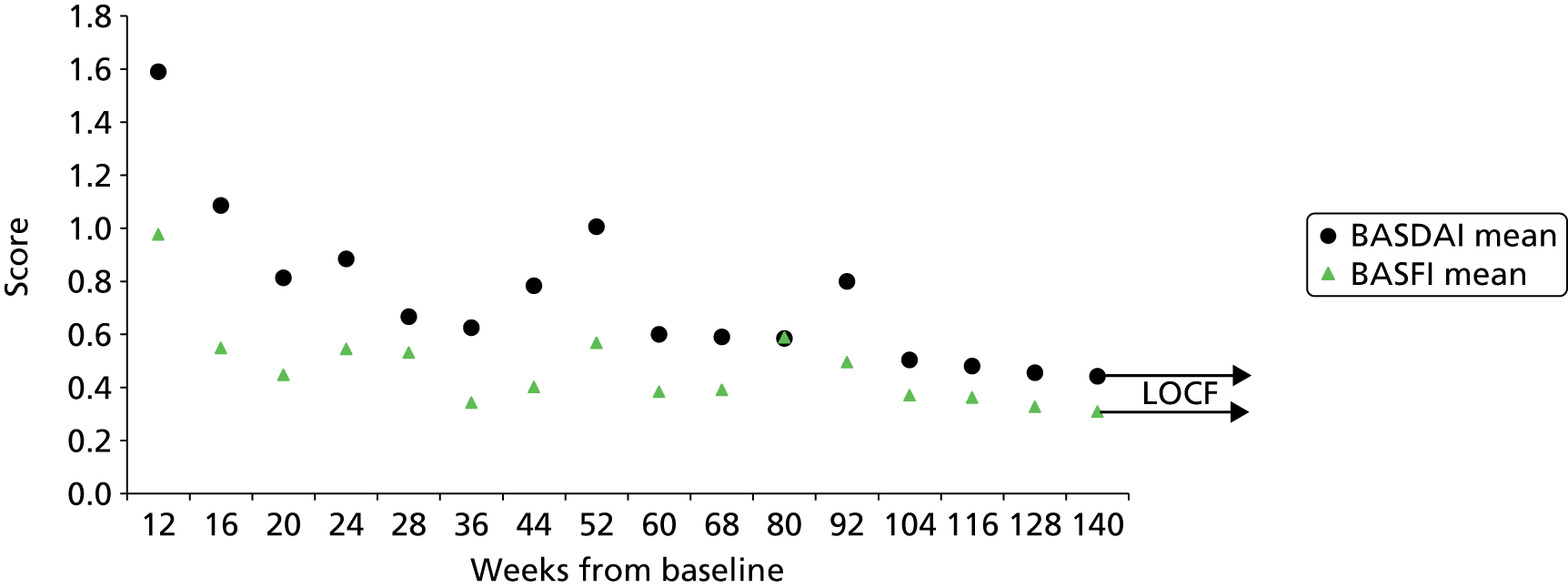
Response rates and other select treatment efficacy end points were based on a separate systematic review and network meta-analysis. In the base case, ASAS 40 for nr-AxSpA and ASAS 20 for AS were used to define clinical response at week 12, based on the primary outcome measures from the clinical trials of adalimumab. In the base-case analysis, placebo responders at week 12 were assumed to lose response and return to baseline disease severity. Patients who subsequently withdrew from TNF-α treatment at any time point were also assumed to return to baseline disease severity (rebound equal to gain). Longer-term discontinuation was assumed to be time-dependent and was based on a log-normal parametric distribution from the separate open-label RCTs adjusting for subsequent loss of response.
In the base-case model, the BASFI score for all patients not on TNF-α inhibitor treatment increases in a linear fashion by 0.084 (scale 0–10) per year in patients with nr-AxSpA, in line with the evidence from the ABILITY-1 trial,58 in which each additional year of baseline symptom duration was reported to be associated with a significant (+0.084; p = 0.0005) increase in baseline BASFI score, adjusting for the age of onset (age at first reported axial SpA symptom) to control for the age effect on functional damage. An estimate of +0.056 was applied to patients with AS based on applying a similar approach to the ATLAS trial,61 adjusting for age at disease diagnosis. Hence, a higher BASFI progression was applied to patients not on anti-TNFs in the nr-AxSpA population compared with the AS population.
The BASDAI and BASFI scores were used jointly to estimate quality of life associated with AS, using the relationship observed between the utility scores (measured in HUI-3) and the BASDAI and BASFI scores in the ATLAS trial. 61 Observed EQ-5D scores were mapped to BASDAI and BASFI for the relationship in the base case for nr-AxSpA from ABILITY-1. 58
The relationship between BASDAI and costs, derived from a reanalysis of the OASIS data, was applied in the base case. Costs of drug, administration, initiation and monitoring, and AEs were also included. Discounting was applied at 3.5% for both costs and outcomes. SMRs of 1 and 1.5 were assumed for nr-AxSpA and AS, respectively. Uncertainty surrounding results was addressed using probabilistic sensitivity analyses (PSAs).
Base-case results from AbbVie (adalimumab) model
The main base-case ICER results from the manufacturer are summarised in Table 36 for the AS population. From a NHS perspective, the base-case cost per QALY gained versus CC ranged from £16,391 per QALY (adalimumab) and £44,448 per QALY (infliximab).
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 112,762 | 8.62 | – | – | – |
| Adalimumab | 139,860 | 10.28 | 27,098 | 1.65 | 16,391 |
| Certolizumab | 133,273 | 9.82 | 20,511 | 1.20 | 17,067 |
| Etanercept | 139,574 | 10.21 | 26,812 | 1.59 | 16,897 |
| Golimumab | 138,385 | 10.17 | 25,624 | 1.55 | 16,535 |
| Infliximab | 197,100 | 10.52 | 84,339 | 1.90 | 44,448 |
Table 37 reports the results based on the fully incremental analysis. In the manufacturer base-case analysis, certolizumab and etanercept were ruled out by extended dominance. The ICER of adalimumab was £16,391 per QALY compared with CC. The ICER of the next more costly (and non-dominated) TNF-α inhibitor was £238,500 per QALY for the comparison between infliximab and adalimumab.
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 112,762 | 8.62 | – | – | – |
| Certolizumab | 133,273 | 9.82 | – | – | Extendedly dominated |
| Golimumab | 138,385 | 10.17 | – | – | Extendedly dominated |
| Etanercept | 139,574 | 10.21 | – | – | Extendedly dominated |
| Adalimumab | 139,860 | 10.28 | 27,098 | 1.66 | 16,391 |
| Infliximab | 197,100 | 10.52 | 57,240 | 0.24 | 238,500 |
The main base-case ICER results from the manufacturer and fully incremental analysis are summarised in Tables 38 and 39 for the nr-AxSpA population. The ICERs versus CC ranged from £12,866 (certolizumab) to £13,288 per QALY (adalimumab). In the fully incremental comparison, adalimumab was extendedly dominated and hence the ICER for certolizumab versus CC is the only ICER reported (£12,866).
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 126,075 | 8.88 | – | – | – |
| Adalimumab | 142,218 | 10.10 | 16,143 | 1.22 | 13,228 |
| Certolizumab | 142,608 | 10.16 | 16,532 | 1.28 | 12,866 |
| Etanercept | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 126,075 | 8.88 | – | – | – |
| Adalimumab | 142,218 | 10.10 | – | – | Extendedly dominated |
| Certolizumab | 142,608 | 10.16 | 390 | 0.06 | 12,866 |
| Etanercept | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
The manufacturer reported more favourable ICERs versus CC in the nr-AxSpA population compared with the AS population. This appears largely driven by two inputs: (1) the lower BASDAI/BASFI scores assumed for responders based on ABILITY-158 (compared with ATLAS61) and (2) the higher annual BASFI progression rate assumed for non-responders/CC in the nr-AxSpA population (0.084 vs. 0.056).
Overview of UCB (certolizumab) model
The economic model presented by UCB34 compared the cost-effectiveness of certolizumab with conventional therapy and other licensed anti-TNFs for nr-AxSpA and AS. Separate Markov cohort models were developed for the two indications separately based on the subpopulations of the RAPID-axSpA trial. 64 Separate analyses were argued to be necessary given that the comparators differed for each subpopulation. Analyses performed for the AS subpopulation consisted of all patients with AS from the RAPID-axSpA study,64 including those who were anti-TNF therapy experienced or therapy naive. The nr-AxSpA subpopulation consisted of anti-TNF therapy-naive patients only, as there were no anti-TNF therapy-experienced patients in this subpopulation.
The analyses used a lifetime time horizon in the base case. An alternative time horizon of 20 years was tested in a scenario analysis. A NHS and PSS perspective was used and an annual discount rate of 3.5% was applied to costs and outcomes. All costs are reported at 2013 values.
The model consists of a short-term component and a longer-term component to estimate lifetime cost-effectiveness. The duration of the short-term component varied between the models used for the AS and the nr-AxSpA subpopulations based on the response end point assumed. Response was assessed at 24 weeks in the AS subpopulation which was argued by the manufacturer to be in accordance with clinical practice as indicated key British opinion leaders. For the nr-AxSpA subpopulation, response assessment was assumed at 12 weeks, as comparator data were available only at that time point. In their base case, the manufacturer used ASAS 20 to determine response in line with the primary outcome measure in the RAPID-axSpA. 64 However, it should be noted that ASAS 20 response at week 12 was the primary outcome in the RAPID-axSpA trial. Hence, although the measure of response used is in accordance with the primary outcome of the RAPID-axSpA trial, the differential timing of this applied across the separate populations clearly deviates from this. This has potential issues because at week 16 patients were allowed an ‘early escape’ from placebo and hence results at week 24 used for the AS subpopulation are no longer based on the original randomised population.
Assessment in Ankylosing Spondylitis 20 response rates for certolizumab and relative treatment effects for the other anti-TNFs were derived based on a separate systematic review and MTC. The base-case model inputs applied in the manufacturer’s submission34 are replicated (and associated footnotes) in Tables 40 and 41.
| Treatment | ASAS 20 response (%)a | SE | Relative riskb | CI | Source |
|---|---|---|---|---|---|
| Certolizumab pegol | CiC information has been removed | CiC information has been removed | – | – | MTC |
| Adalimumabb | – | – | AiC information has been removed | AiC information has been removed | MTC |
| Etanerceptb | – | – | AiC information has been removed | AiC information has been removed | MTC |
| Golimumabb | – | – | AiC information has been removed | AiC information has been removed | MTC |
| Infliximabb | – | – | AiC information has been removed | AiC information has been removed | MTC |
| Treatmenta | ASAS 20 response (%)b | SE | Relative riska | CI | Source |
|---|---|---|---|---|---|
| Certolizumab pegol | AiC information has been removed | AiC information has been removed | – | – | MTC |
| Adalimumaba | – | – | AiC information has been removed | AiC information has been removed | MTC |
| Etanercepta | – | – | AiC information has been removed | AiC information has been removed | MTC |
The MTC was also used to determine change in baseline BASFI and BASDAI scores. The base-case inputs for change from baseline in BASFI and BASDAI at week 24 for the AS subpopulation reported by the manufacturer are replicated in Tables 42 and 43. The manufacturer noted that the mean change from baseline reported in the tables is that observed per trial arm, which includes both the ASAS 20 responders and non-responders in each arm. In order to determine the change in BASFI and BASDAI for responders alone, the manufacturer used the equation:
| Treatment | Change from baseline in BASFI score at Week 24: initial response assessment period | Source | |
|---|---|---|---|
| Mean | SD | ||
| Certolizumab pegol | AiC information has been removed | AiC information has been removed | MTC |
| Adalimumab | AiC information has been removed | AiC information has been removed | MTC |
| Etanercept | AiC information has been removed | AiC information has been removed | MTC |
| Golimumaba | AiC information has been removed | AiC information has been removed | MTCa |
| Infliximab | AiC information has been removed | AiC information has been removed | MTC |
| CCb | AiC information has been removed | AiC information has been removed | Assumed zero in base caseb |
| Treatment | Change from baseline in BASDAI Score at week 24: initial response assessment period | Source | |
|---|---|---|---|
| Mean | SD | ||
| Certolizumab pegol | AiC information has been removed | AiC information has been removed | MTC |
| Adalimumab | AiC information has been removed | AiC information has been removed | MTC |
| Etanercept | AiC information has been removed | AiC information has been removed | MTC |
| Golimumab | AiC information has been removed | AiC information has been removed | MTC |
| Infliximab | AiC information has been removed | AiC information has been removed | MTC |
| CCa | AiC information has been removed | AiC information has been removed | Assumed zero in base casea |
This approach assumed that the change in BASFI (and BASDAI) score among ASAS 20 non-responders is equal to that of the CC arm. Thus, the equation is used to algebraically solve for change in BASFI (and BASDAI) score among ASAS 20 responders. The manufacturer stated that:
As an example for the AS subpopulation base case, the change in BASFI among ASAS 20 responders for CZP [certolizumab pegol] is: [academic-in-confidence (AiC) information removed]. Thus, in this example, the actual change from baseline in AS responders to CZP is [AiC information removed]. The same approach was used for change from baseline for BASDAI. This approach, where the change from baseline for BASDAI and BASFI is calculated among responders only, is consistent with previous evaluations pharmacoeconomic evaluations conducted for AS
Manufacturer’s submission, pp. 69–7034
The manufacturer base-case inputs for change from baseline in BASFI and BASDAI at week 12 for the nr-AxSpA subpopulation are replicated in Tables 44 and 45.
| Treatment | Change from baseline in BASFI score at week 12: initial response assessment period | Source | |
|---|---|---|---|
| Mean | SD | ||
| Certolizumab pegol | AiC information has been removed | AiC information has been removed | MTC |
| Adalimumab | AiC information has been removed | AiC information has been removed | MTC |
| Etanercept | AiC information has been removed | AiC information has been removed | MTC |
| CCa | AiC information has been removed | AiC information has been removed | Assumed zero in base casea |
| Treatment | Change from baseline in BASDAI score at week 12: initial response assessment period | Source | |
|---|---|---|---|
| Mean | SD | ||
| Certolizumab | AiC information has been removed | AiC information has been removed | MTC |
| Adalimumab | AiC information has been removed | AiC information has been removed | MTC |
| Etanercept | AiC information has been removed | AiC information has been removed | MTC |
| CCa | AiC information has been removed | AiC information has been removed | Assumed zero in base caseb |
The manufacturer’s submission34 assumed no change in BASDAI and BASFI for CC during the response period. The manufacturer justified this assumption with reference to evidence from RAPID-axSpA,64 ATLAS61 and ABILITY-158 studies, although no specific data were reported to support this.
These change scores are assumed to be maintained for BASDAI as long as a patient continues to receive an anti-TNF. For AS patients on CC, an additional annual increase of 0.07 points (scale 0–10) in BASFI is assumed and justified by the manufacturer according to the assumptions deemed reasonable by a previous NICE committee. Hence, while the change scores are assumed constant, the absolute difference between patients receiving anti-TNFs and CC is increasing over time given the underlying progression assumed for BASFI for patients receiving CC. The assumption of no progression in BASFI for patients receiving anti-TNFs is not explicitly discussed within the manufacturer’s submission, neither are separate results provided for alternative assumptions.
The same annual rate (0.07) in BASFI progression for CC is also applied to the nr-AxSpA subpopulation. In addition, it is assumed that some nr-AxSpA patients may progress to AS during their course of treatment. The manufacturer’s model adopts an estimate for disease progression for the nr-AxSpA subpopulation based on a German cohort of axSpA patients, GESPIC. In this cohort, the rates and predictors of radiographic spinal progression over 2 years were estimated based on mSASSS. In total, 7.4% of the 95 nr-AxSpA patients were reported to show spinal radiographic progression, which was defined as a worsening of mSASSS by ≥ 2 units over 2 years. As this 7.4% progression represents a proportion, it was converted to a rate for use in the economic model, assuming an exponential distribution through the following formula:
The manufacturer’s submission34 is not explicit about how this additional aspect of progression subsequently alters the BASDAI/BASFI trajectories within the nr-AxSpA model. However, examination of the electronic model submitted by the manufacturer reveals that once patients are assumed to show spinal radiographic progression, they effectively become AS patients by picking up the same absolute values of BASDAI and BASFI (on and off treatment) applied in their AS subpopulation model. The justification for this approach and the values subsequently assigned are not formally discussed by the manufacturer and the validity of the approach appears questionable (i.e. given other differences, e.g. disease duration, severity of radiographic disease etc., that may differ between the two populations even after radiographic progression has occurred in the nr-AxSpA subpopulation).
Patients who subsequently withdrew from TNF-α treatment at any time point were assumed to revert back to the same trajectory as CC over a 6-month period (i.e. rebound back to CC/natural history). A constant annual rate of discontinuation of 7% was assumed for all anti-TNFs over the longer-term period in both the AS and nr-AxSpA populations. The estimate of 7% applied to the AS subpopulation was justified by citing the rate apparently assumed by the NICE committee for TA143 and the lack of long-term evidence more generally. This estimate was referred to earlier in the review section of our report when the additional analyses undertaken by the NICE DSU were considered (see Data extraction). Identical assumptions for discontinuation rates were assumed for the nr-AxSpA subpopulations, although no justification was provided by the manufacturer.
The BASDAI and BASFI scores were used jointly to estimate quality of life in both subpopulations based on EQ-5D data collected in the RAPID-axSpA64 study. Data from patients having EQ-5D, BASDAI and BASFI scores available at baseline and at weeks 12 and 24 were used to estimate a relationship between utility and the BASDAI and BASFI scores. Utilities were subsequently converted using a logistic transformation with the justification based on possible floor and ceiling effects, as they are bounded by 0 and 1. Without access to the original data, it is not possible to determine the impact of this transformation, although it should be noted that EQ-5D is not bounded by 0 (i.e. negative values are possible). The manufacturer used a repeated-measures logistic regression to model the relationship between utility and the BASDAI and BASFI scores.
The relationship between BASFI and costs, derived from the OASIS study and used by the previous independent assessment group in TA143,17 was applied in the base case. Costs of drug, administration, initiation and monitoring were included. The costs and HRQoL of AEs were not included. Discounting was applied at 3.5% for both costs and outcomes. Uncertainty surrounding outcomes was addressed using PSA.
Base-case results from UCB (certolizumab) model
The main base-case ICER results from the manufacturer35 are summarised in Table 46 for the AS population, together with a fully incremental comparison of ICERs in Table 47. The ICERs versus CC ranged from £16,647 per QALY (certolizumab) and £42,671 per QALY (infliximab). In the fully incremental analysis, certolizumab dominated (i.e. less costly and more expensive) all other TNF-α treatments apart from infliximab. However, it should be noted that the costs of certolizumab are based on a patient access scheme (PAS) which has been proposed but is not yet formally agreed with the Department of Health and NICE. Results without the PAS were not reported by the manufacturer. UCB will make certolizumab pegol (Cimzia,® USB Pharma) available free of charge to all NHS patients for the first 3 months of therapy, at which point clinical response should be clear. Only after this 3-month stage will the NHS be charged for continuing to use this therapy.
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | – |
| Adalimumab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 19,932 |
| Certolizumab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 16,647 |
| Etanercept | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 19,272 |
| Golimumab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 19,049 |
| Infliximab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 42,671 |
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | – |
| Certolizumab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 16,647 |
| Golimumab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | Dominated |
| Adalimumab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | Dominated |
| Etanercept | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | Dominated |
| Infliximab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 113,871 |
The ICER of certolizumab was £16,647 per QALY versus CC and the ICER for infliximab was £113,871 (vs. certolizumab).
The main base-case ICER results from the manufacturer35 are summarised in Table 48 for the nr-AxSpA population, together with a fully-incremental comparison of ICERs in Table 49. In contrast to the results for AS, there was a more marked difference between the ICERs of the alternative anti-TNFs and CC. The ICERs versus CC ranged from £15,615 (certolizumab) to £50,692 per QALY (etanercept). The higher differential ICERs appears to be largely a result of the more heterogeneous trials included in the MTC for the nr-AxSpA populations and a higher differential effect assumed for certolizumab vis-à-vis the other alternative anti-TNFs compared with the AS population. Importantly, other manufacturers (Pfizer) argue that the results for certolizumab in this population may be confounded by population characteristics which could invalidate the indirect comparison of certolizumab versus the other comparator treatments in the current nr-AxSpA MTC. In the fully incremental analysis, certolizumab dominated adalimumab and etanercept.
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | – |
| Adalimumab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 30,370 |
| Certolizumab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 15,615 |
| Etanercept | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 50,692 |
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | – |
| Certolizumab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 15,615 |
| Etanercept | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | Dominated |
| Adalimumab | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | Dominated |
Overview of Pfizer (etanercept) model
The economic model submitted by Pfizer36 compared the cost-effectiveness of etanercept versus conventional therapy and other licensed anti-TNFs for AS, nr-AxSpA and a combined population (axSpA). The results for the combined population are not summarised in this review but are reported separately in the manufacturer’s submission. The model is based on a lifetime time horizon and costs and benefits are discounted at an annual rate of 3.5%. The reference year for costs was reported to be 2014.
The model was based on a patient-level simulation model based on a discrete event simulation (DES). The analysis was conducted from a NHS/PSS perspective. Data to populate the model were derived from key clinical trials for etanercept, the results of a clinical systematic review, MTC and, in a separate analysis presented for the nr-AxSpA population, a match-adjusted indirect comparison (MAIC). The model structure was reported to be developed in accordance with current OMERACT (Outcome Measures in Rheumatology) guidance and was constructed around the BASDAI and the BASFI in line with other published studies.
The AS population was defined based on current NICE guidance in TA14317 and TA233. 33 The nr-AxSpA population was defined based on the scope issued by NICE and was defined by the manufacturer as people with severe axSpA without radiographic evidence of AS but with objective signs of inflammation, whose disease has responded inadequately to, or who are intolerant to, NSAIDs.
An important aspect of the submission for the nr-AxSpA population was an attempt to adjust analyses for differences in the baseline patient characteristics between the trials included. The manufacturer reported that:
The clinical systematic review identified that the baseline characteristics of nr-AxSpA patients within the randomised controlled trials of certolizumab pegol and adalimumab were heterogeneous, and potentially differed in characteristics that could act as treatment effect modifiers. Furthermore, the populations of these trials also included sizable proportions of AS patients who were originally classified as nr-AxSpA on the basis of a difference between centralised and localised readings of X-rays.
To address the differences in the proportions of AS patents in the trials due to reclassification upon central assessment, analyses were conducted using match adjusted indirect comparison (MAIC) and simulated treatment comparison (STC) techniques that incorporated AS patients from the etanercept 314-EU trial. These analyses are referred to collectively as ‘analyses adjusting for differences in study baseline characteristics’. A comparison of the results from the MAIC and STC approaches show that while the results of the two analyses are similar, when considering comparisons between etanercept and both adalimumab and certolizumab, the MAIC analysis provides a lower overall comparative estimate of the benefit of etanercept, and is therefore considered overall to be the more conservative of the two approaches. To maintain consistency in the analysis utilised in the economic section, the MAIC was used throughout as the adjusted comparative efficacy measure between etanercept versus adalimumab and etanercept versus certolizumab. For the analysis comparing etanercept against certolizumab pegol, it was possible to address the issue of patient reclassification and differences in baseline characteristics by utilising the RAPID-axSpA trial results that were also available at the level of AxSpA patients, an approach not possible in the comparison of etanercept versus adalimumab. We note that although not explicitly detailed within the scope, the AxSpA population encompasses both nr-AxSpA and AS patients, thus making it a relevant comparison to the decision problem outlined in the scope.
Manufacturer’s submission, pp. 226–736
The manufacturer argued that the use of DES conferred potential advantages in relation to modelling non-linearity because of heterogeneous patient characteristics and in relation to modelling time dependency. The latter was also argued as an advantage to considering the impact of sequential therapy which was argued to be complex within a more conventional Markov type structure. Pfizer’s model was the only model which explicitly explored issues of treatment sequences. However, in the base case the use of second-line TNF-α inhibitor treatment was restricted to those patients who withdrew because of AEs and was assumed equal efficacy to first line usage. The schematic of the model provided by the manufacturer is replicated in Figure 6.
Etanercept RCT data were used to predict an initial 12-week response (in terms of reduction in BASDAI and BASFI) for etanercept for both nr-AxSpA and AS populations. Separate multivariate regressions were used to account for correlation between BASDAI and BASFI. A range of variables were initially included in the regression models based on potential predictors of response identified from their review of economic studies. The statistical significance and direction of effect were evaluated before final models were specified. The 12-week models of BASDAI and BASFI for the nr-AxSpA had R2 values of (AiC information has been removed) respectively. For the AS population, the equivalent R2 values were (AiC information has been removed). The regressions were used to estimate mean change in BASDAI and BASFI which through the patient level simulation were used to assign patients into BASDAI 50 responder/non-responder categories and to assess the associated magnitude of change at 12 weeks for these categories.
Relative effects from the MTC (or MAIC in the analyses adjusting for differences in study baseline characteristics), in terms of mean differences in BASDAI and BASFI, were applied in order to predict equivalent response and change scores for the other anti-TNF agents and CC at 12 weeks.
From week 12, the BASFI scores for CC were assumed to increase at a rate of 0.7 units per annum (0–100 scale). The modelling of change in BASDAI and BASFI at week 48 for responders to etanercept was conducted using the same approach used for the week 12 treatment response. However, change in BASDAI and BASFI from baseline at week 12 were included as additional covariates within the resulting models in order to ensure that an individual’s response at week 48 was dependent on their response at week 12. The 48-week models of BASDAI and BASFI for the nr-AxSpA had R2 values of (AiC information has been removed), respectively. For the AS population, the equivalent R2 values were (AiC information has been removed). In the absence of relative effect estimates at week 48 for other therapies, it was assumed that patients who remained on TNF-α inhibitor treatment beyond week 12 (i.e. responders) would converge at the BASDAI and BASFI levels predicted for etanercept by week 48. Constant BASDAI and BASFI scores for TNF-α inhibitor responders were assumed at the level observed at week 48 for subsequent periods.
Treatment discontinuation was modelled by fitting separate parametric survival curves to long-term open-label study data from etanercept for the AS and nr-AxSpA populations. In order to predict treatment cessation in the population that was likely to continue treatment after 12 weeks, parametric curves were fitted only to subjects who achieved a BASDAI 50 response at week 12. Only patients who were randomised to etanercept at baseline were retained within these survival analyses and patients who began etanercept during open-label phases of studies were excluded. The distributions that provided the best fit were exponential (AiC information has been removed) and log-normal (AiC information has been removed), based on the minimisation of the Akaike information criterion and the Bayesian information criterion. The exponential model was chosen based, in part, on the goodness of fit but also because the use of hazard ratios, which were applied to estimate the effect of other anti-TNFs on the rate of discontinuation, required the use of a proportional hazard survival model [to avoid making further assumptions when applying the hazard ratio to the log-normal (accelerated failure time) model].
The same risk of discontinuation was applied to all individuals in the model. The models of discontinuation translate into annual probabilities of discontinuation for etanercept, for patients who achieve a BASDAI 50 response, of 5% and 11% for nr-AxSpA and AS populations, respectively. Based on data from the DANBIO registry, it was assumed that other anti-TNFs have an increased risk of discontinuation compared with etanercept: a hazard ratio of 1.3 is applied for infliximab and 1.12 for adalimumab. In the absence of evidence for golimumab and certolizumab, it was assumed that the relative effect is the same as for adalimumab on the basis that these have common molecular structure and belong to monoclonal antibodies.
After discontinuation of the first treatment, an alternative TNF-α inhibitor was modelled as second-line treatment for patients who discontinued due to AEs [(CiC information has been removed)% for AS and (CiC information has been removed)% for nr-AxSpA]. The same efficacy as applied for first-line treatments was assumed for second-line treatments for patients switching because of AEs. For patients who discontinued because of loss of efficacy, no further TNF-α treatment was modelled. These assumptions were considered by the manufacturer to be consistent with current NICE guidance. For the base-case model, it was assumed that following discontinuation from anti-TNFs, patients would rebound back to their baseline BASDAI and BASFI scores and that the rebound takes 6 months based on the approach used within the TA23333 submission to NICE.
In the absence of previously published studies reporting the relationship between BASDAI/BASFI and EQ-5D utility scores in the nr-AxSpA population, a de novo relationship was estimated from the 1031 study;166 variables included age, sex, baseline BASDAI and BASFI. Ordinary least squares regression models were used, with SEs clustered around each subject to account for repeated observations. For consistency, a similar relationship was estimated for the AS population using the 314-EU study. 167 Alternative linear and non-linear relationships were evaluated and final model selection based on Akaike information criterion statistics. In the nr-AxSpA population, the final model included squared terms for BASDAI and BASFI and an interaction between BASDAI and BASFI, while in the AS population, the covariates for the interaction term, age and male were not included. Scenario analyses considered using alternative model specifications for mapping. The manufacturer reported that according to visual inspection, the estimated models were very similar between populations and reported a high degree of similarity between the results of the de novo estimated models and those published previously.
Figures 7 and 8 replicate the relationships reported by the manufacturer between EQ-5D, BASDAI and BASFI in the nr-AxSpA and AS populations, respectively. Additional figures were also presented by the manufacturer for predicted versus observed EQ-5D in each of the populations. The manufacturer concluded that the models overpredicted EQ-5D at low observed EQ-5D and underpredicted at higher observed EQ-5D values. The manufacturer argued that this was a common feature of mapping algorithms and argued that the approach would be conservative towards the use of anti-TNFs.
FIGURE 7.
AiC information has been removed. 36
FIGURE 8.
AiC information has been removed. 36
The manufacturer included the acquisition, administration and pre-treatment monitoring costs of TNF-α inhibitors. Subsequent monitoring costs were not included in order to avoid potential double counting of the costs which were estimated as a function of BASDAI and BASFI. In the base-case analysis the manufacturer used data from Rafia et al. 168 based on BASDAI scores only. A categorical approach was applied to BASDAI scores based on the following annual costs: BASDAI score of < 40 = £151.96; 40 ≤ BASDAI score < 60 = £311.08; and BASDAI score of ≥ 60 = £1039.16. The manufacturer justified the use of this source as it provides the most recent UK specific data reported and permitted separation of particular cost items. The costs and HRQoL of AEs (serious infections only) were included in the base-case analysis (none observed in the nr-AxSpA trial 1031 study;166 however, serious infections were observed in the AS trial 314-EU study167). A separate sensitivity analysis included the costs of serious infections.
A SMR of 1 for the nr-AxSpA population and 1.5 for the AS population were applied to general population life-tables.
Results of Pfizer (etanercept) model
The main base-case ICER results from the manufacturer36 are summarised in Table 50 for the AS population, together with a fully incremental comparison of ICERs in Table 51. The ICERs versus CC ranged from £19,586 per QALY (certolizumab) and £37,741 per QALY (infliximab). In common with the UCB model, it should be noted that the costs of certolizumab assumed within Pfizer’s model were also based on the PAS for certolizumab which has been proposed but is not yet formally agreed with the Department of Health and NICE. Hence the ICER for certolizumab versus CC without the PAS will be higher than the estimates reported here.
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 18,122 | 7.318 | – | – | – |
| Adalimumab | 57,535 | 9.203 | 39,413 | 1.885 | 20,909 |
| Certolizumab | 51,843 | 9.040 | 33,721 | 1.722 | 19,586 |
| Etanercept | 60,338 | 9.334 | 42,216 | 2.016 | 20,938 |
| Golimumab | 62,698 | 9.412 | 44,576 | 2.094 | 21,288 |
| Infliximab | 98,340 | 9.443 | 80,218 | 2.125 | 37,741 |
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 18,122 | 7.318 | – | – | – |
| Certolizumab | 51,843 | 9.040 | 33,721 | 1.722 | 19,586 |
| Adalimumab | 57,535 | 9.203 | – | – | Extendedly dominated |
| Etanercept | 60,338 | 9.334 | 8495 | 0.294 | 28,834 |
| Golimumab | 62,698 | 9.412 | 2360 | 0.078 | 30,376 |
| Infliximab | 98,340 | 9.443 | 35,642 | 0.031 | 1,131,181 |
In the fully incremental analysis, adalimumab was extendedly dominated. Of the remaining non-dominated treatments, the ICERs of the next most costly interventions compared with the previous non-dominated alternative were £19,586 (certolizumab vs. CC), £28,834 (etanercept vs. certolizumab), £30,376 (golimumab vs. etanercept) and £1,131,181 (infliximab vs. golimumab).
The main base-case ICER results from the manufacturer36 are summarised in Table 52 for the nr-AxSpA population together with a fully incremental comparison of ICERs (Table 53). The ICERs versus CC ranged from £23,195 (etanercept) and £23,575 (certolizumab). In contrast to the UCB analysis, the ICERs for the nr-AxSpA population were marginally less favourable than the results for the AS population. There was also less of a marked difference between the ICERs for each of the anti-TNFs and CC compared with the UCB results, although a large difference was evident relating to the magnitude of the incremental QALY estimates for certolizumab vis-à-vis the other anti-TNFs. Table 54 reports the results of the fully incremental analysis. None of the anti-TNFs was ruled out via dominance or extended dominance, and the ICER of each comparison remained below £30,000 per QALY for each successively more expensive and effective treatment.
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 20,609 | 10.221 | – | – | – |
| Adalimumab | 62,667 | 12.030 | 42,058 | 1.809 | 23,242 |
| Certolizumab | 74,282 | 12.497 | 53,673 | 2.276 | 23,575 |
| Etanercept | 59,635 | 11.903 | 39,026 | 1.682 | 23,195 |
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 20,609 | 10.221 | – | – | – |
| Etanercept | 59,635 | 11.903 | 39,026 | 1.683 | 23,195 |
| Adalimumab | 62,667 | 12.030 | 3033 | 0.127 | 23,871 |
| Certolizumab | 74,282 | 12.497 | 11,615 | 0.467 | 24,864 |
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| Adalimumab | 48,494 | 11.473 | – | – | – |
| Etanercept | 60,404 | 11.928 | 11,910 | 0.455 | 26,176 |
To address the concerns noted by Pfizer relating to the heterogeneity across the different trials in the nr-AxSpA population, a separate matched indirect comparison was presented for etanercept versus adalimumab. A separate comparison was also presented versus certolizumab for the combined axSpA population in the manufacturer’s submission. Using the MAIC approach, the ICER for etanercept versus adalimumab was £23,195 per QALY. Total cost and QALYs estimates were reversed in the MAIC approach compared with the base-case analysis (adalimumab generated greater QALYs at increased cost), demonstrating the potential impact of trying to minimise observable sources of possible confounding.
Overview of Merck Sharp & Dohme (golimumab, infliximab) model
The economic models submitted by Merck Sharp & Dohme37 compared the cost-effectiveness of golimumab and infliximab with conventional therapy and other licensed anti-TNFs for AS. Although the manufacturer made separate submissions for golimumab and infliximab, the model structures and data sources used to inform the economic models are identical across the submissions. Hence this review focuses on the specific submission for golimumab but also considers key data sources and assumptions specific to infliximab. The model base case is based on a lifetime time horizon (approximately 60 years), and costs and benefits are discounted at an annual rate of 3.5%. A NHS and PSS perspective is used for costs. The reference year for costs was reported to be 2012/13.
The economic model submitted by the manufacturer for golimumab37 is based on the same model structure submitted as part of NICE TA23333 and summarised previously in Data extraction (Armstrong et al. 2013)163 Hence, a description of the structure of the model is not repeated in this section. In summary, the manufacturer’s cost-effectiveness model was based on a short-term decision tree (based on an assessment of BASDAI 50 response at 12 weeks in the base case) and a longer-term Markov model.
The proportion of patients achieving BASDAI 50 at week 12 (± 2 weeks) for each TNF-α inhibitor was obtained from a systematic review and MTC undertaken by the manufacturer. The results are summarised in Table 55.
| Treatment | BASDAI 50 | |
|---|---|---|
| OR (95% CrI) | Probability | |
| Golimumab | 5.54 (2.12 to 12.13) | 0.49 |
| Infliximab | 22.44 (2.78 to 89.05) | 0.79 |
| Adalimumab | 5.20 (2.14 to 10.62) | 0.47 |
| Etanercept | 5.46 (2.03 to 11.74) | 0.60 |
| Certolizumab pegol | 6.62 (1.66 to 17.59) | 0.53 |
| Conventional therapy | – | 0.15 |
Data from the GO-RAISE90 trial and the open-label extension period (up to week 108) were used to develop predictive equations of mean change from baseline in BASDAI and BASFI scores over time. Two separate equations were developed based on the 24-week data (0–24 weeks) for all patients and post-24-week (week 24–108) data from GO-RAISE90 for patients who remained on treatment. The variables applied in each equation are summarised in Tables 56 and 57.
| Variable | Parameter | SE |
|---|---|---|
| BASFI | ||
| Intercept | 0.1008 | 0.557 |
| Age | –0.0284 | 0.009874 |
| Baseline BASFI | 0.1780 | 0.05429 |
| Treatment | 1.8096 | 0.2551 |
| Male | 0.04156 | 0.2767 |
| Week–2 | 5.226 | 0.2767 |
| Treatment × week–2 | –14.6396 | 2.2699 |
| BASDAI | ||
| Intercept | 0.4685 | 0.8126 |
| Age | –0.03399 | 0.0105 |
| Baseline BASDAI | 0.2212 | 0.08436 |
| Treatment | 2.0620 | 0.2742 |
| Male | 0.2652 | 0.2953 |
| Week–2 | –3.4664 | 2.1365 |
| Treatment × week–2 | –7.1029 | 2.6887 |
| Variable | Parameter | SE |
|---|---|---|
| BASFI | ||
| Intercept | 0.4933 | 0.7364 |
| Age | –0.03915 | 0.01321 |
| Baseline BASFI | 0.5706 | 0.07292 |
| Male | 0.6523 | 0.4001 |
| Log (week) | 0.09524 | 0.04938 |
| BASDAI | ||
| Intercept | 0.6277 | 1.0303 |
| Age | –0.03531 | 0.01367 |
| Baseline BASDAI | 0.5762 | 0.1055 |
| Male | 0.2196 | 0.4094 |
| Log (week) | 0.2196 | 0.06908 |
The treatment coefficient (and interaction term) in the short-term regression equation is used to estimate separate BASDAI/BASFI scores for anti-TNFs and CC. Hence, up to week 24, the same estimate of BASDAI/BASFI appears to be applied to all TNFs (i.e. regardless of the differential response rates assumed). Beyond week 24, the same BASDAI/BASFI score is applied to a responder to any of the TNFs, although a different response rate for each TNF-α inhibitor is assumed based on the MTC. The BASDAI/BASFI regressions are applied to responders who continue on TNF-α inhibitor therapy up to week 108 for BASDAI and up to week 108 for BASFI.
The BASDAI and BASFI scores beyond week 108 for responders who continue to receive anti-TNFs beyond this period in the model are assumed to remain constant (at the week 108 value). The BASFI scores beyond week 256 for responders who continue to receive anti-TNFs beyond this period in the model are assumed to remain constant (at the week 108 value) but are also subject to an annual progression rate of BASFI at this point which is set to half the rate of CC in the baseline (0.035 units per annum, 0–10 scale). The justification for this is not explicitly made by the manufacturer. For the base-case model, BASFI scores for CC patients on conventional therapy are assumed to progress at a rate of 0.07 units per year after week 24.
An annual discontinuation rate of 6.1% is applied for the entire time horizon after week 12 in the base-case analysis. This estimate is derived from data reported between weeks 24 and 256 in the 50 mg arm of golimumab from the GO-RAISE90 extension period. The manufacturer does not formally state whether or not this is specific to those patients who were identified to be responders at 12 weeks. However, it appears to be based on all patients who continued to receive golimumab beyond 24 weeks regardless of their response status. The same discontinuation rate is applied to all TNF-α inhibitors. Following discontinuation from anti-TNFs, the BASFI and BASDAI scores are assumed to deteriorate/rebound over a 24-week period back to their baseline BASFI and BASDAI scores (i.e. rebound equal to gain). Therefore, in common with other models which apply this rebound assumption, patients are assumed to achieve a lifetime benefit from treatment with anti-TNFs for BASFI.
Utilities were derived from a NICE TA (TA14317) and incorporated age, sex, BASFI score and BASDAI score. Costs included in the model comprised drug acquisition, short-term (12-week) costs, longer-term disease costs and AEs. Longer-term disease costs were based on BASFI scores from the GO-RAISE90 trial using the same regression equation used for NICE TA143. 17
The proportion of males and females recruited in the GO-RAISE90 trial is used to estimate a weighted average mortality risk by sex. The sex-specific SMR for AS from a study by Bakland et al. 16 is applied to the mortality rates from the general population to calculate adjusted mortality rates for AS patients in the model. The study by Bakland reported a SMR of 1.63 (95% CI 1.29 to 1.97) for males and 1.38 (95% CI 0.48 to 2.28) for females.
Results of the Merck Sharp & Dohme (golimumab, infliximab) model
The main base-case ICER results from the manufacturer37 are summarised in Table 58 for the AS population, together with a fully incremental comparison of ICERs in Table 59. The ICERs versus CC ranged from £19,070 (golimumab) to £42,532 (infliximab). In the fully incremental analysis, golimumab and certolizumab were the non-dominated anti-TNFs. The ICER for golimumab versus CC was £19,070 and for certolizumab versus golimumab was £21,441 per QALY.
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 160,837 | 10.5529 | – | – | – |
| Adalimumab | 181,589 | 11.6296 | 20,752 | 1.0766 | 19,275 |
| Certolizumab | 183,017 | 11.6962 | 22,180 | 1.1432 | 19,401 |
| Etanercept | 183,540 | 11.5862 | 22,703 | 1.0332 | 21,972 |
| Golimumab | 181,427 | 11.6326 | 20,590 | 1.0797 | 19,070 |
| Infliximab | 208,856 | 11.6819 | 48,019 | 1.1290 | 42,532 |
| Technology | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CC | 160,837 | 10.5529 | – | – | – |
| Golimumab | 181,427 | 11.6326 | 20,590 | 1.0797 | 19,070 |
| Adalimumab | 181,589 | 11.6296 | – | – | Dominated |
| Certolizumab | 183,017 | 11.6962 | 1590 | 0.0636 | 21,441 |
| Etanercept | 183,540 | 11.5862 | – | – | Dominated |
| Infliximab | 208,856 | 11.6819 | – | – | Dominated |
Summary and critique of de novo cost-effectiveness submissions
In general, the manufacturer models34–37 appeared to be constructed to a high standard and it is evident that significant work had been undertaken by each to identify and use previously published studies and to exploit existing individual patient data from their own RCTs and open-label extension periods to generate estimates that were appropriate for the requirements of the model.
Despite the different model structures and assumptions applied across the various manufacturer’s submissions,34–37 the ICERs reported for the anti-TNFs versus CC were remarkably consistent in the AS population. Table 60 presents a summary of the ICER reported by each manufacturer for each of the anti-TNFs versus CC. It is perhaps expected that the majority of manufacturer’s reported the lowest ICER versus CC for their own products. The only exception to this is the Pfizer model, which estimated the lowest ICER versus CC for certolizumab in this population (etanercept was the next lowest), although it should be noted that Pfizer included the proposed PAS costs for certolizumab which was not universally applied across the different manufacturer’s submissions. Hence, although differences between the ICER versus CC were quite similar, the variation in approaches used by each manufacturer appears partially driven by maximising any potential comparative advantage considered vis-à-vis other manufacturer products (i.e. in terms of assumptions made about similarities and differences for response rates, magnitude of changes in BASDAI and BASFI and withdrawal rates). However, it should be noted that no manufacturer makes a strong claim regarding differential efficacy between the alternative anti-TNFs which is borne out in the relatively small differentials reported between the different products in each of the submissions.
| Technology | AbbVie34 (adalimumab), ICER (£) | UCB35 (certolizumab), ICER (£) | Pfizer36 (etanercept), ICER (£) | Merck Sharp & Dohme37 (golimumab, infliximab), ICER (£) |
|---|---|---|---|---|
| CC | – | – | – | – |
| Adalimumab | 16,391 | 19,932 | 20,909 | 19,275 |
| Certolizumab | 17,067 | 16,647 | 19,586 | 19,401 |
| Etanercept | 16,897 | 19,272 | 20,938 | 21,972 |
| Golimumab | 16,535 | 19,049 | 21,288 | 19,070 |
| Infliximab | 44,448 | 42,671 | 37,741 | 42,532 |
Table 61 presents a summary of the ICERs reported by each manufacturer for each of the anti-TNFs versus CC for the nr-AxSpA population. There appears to be much more heterogeneity across the manufacturer’s submissions34–36 compared with the AS population. There appears an almost twofold difference in the ICERs reported across the submissions for each of the anti-TNFs. Importantly, there also appears variation across the populations with more favourable ICERs reported than CC for the nr-AxSpA population vis-à-vis the estimates by AbbVie (both adalimumab and certolizumab) and UCB (certolizumab only). Hence, the differences in structural and parameter assumptions appear more evident in the results for the nr-AxSpA population compared with results for the AS population.
| Technology | AbbVie34 (adalimumab), ICER (£) | UCB35 (certolizumab), ICER (£) | Pfizer36 (etanercept), ICER (£) |
|---|---|---|---|
| CC | – | – | – |
| Adalimumab | 13,228 | 30,370 | 23,242 |
| Certolizumab | 12,866 | 15,615 | 23,575 |
| Etanercept | Not assessed | 50,692 | 23,195 |
To assist in identifying possible reasons for the differences between populations, summaries of the key structural assumptions used by each manufacturer are provided in Tables 62 and 63. A more micro-level analysis of comparison of specific parameter estimates is reported separately in Appendix 11.
| Parameter | Merck Sharp & Dohme economic model37 (infliximab, golimumab) | AbbVie34 economic model (adalimumab) | UCB35 economic model (certolizumab) | Pfizer36 economic model (etanercept) |
|---|---|---|---|---|
| Model type | Decision tree followed by Markov model | Markov model | Markov model | Patient-level simulation model (DES) |
| Time horizon | Lifetime | 40 years | Lifetime | Lifetime |
| Response criterion | BASDAI 50 response at week 12 | ASAS 20 response at week 12 | ASAS 20 response at week 24 | BASDAI 50 response at week 12 |
| Response criterion justification | Efficacy outcome in GO-RAISE90 study; recommended by the ASAS Working Group (Keat 2005)169 | Primary end point of ATLAS61 study | ASAS 20 is the primary end point of RAPID-axSpA64 study | Based on the current NICE definition of treatment response (TA14317) |
| Progression assumption BASDAI | ||||
| Anti-TNFs responders | Constant after week 108 | Constant after week 260 | Constant after week 24 | Constant after week 48 |
| Anti-TNFs non-responders | Constant | Constant | Constant | Constant |
| CC | Constant after week 24 | Constant | Constant | Constant after week 12 |
| Progression assumption BASFI | ||||
| Anti-TNFs responders | Constant after week 108; 0.035 after week 256 | Constant after week 260 | Constant after week 24 | Constant after week 48 |
| Anti-TNFs non-responders | 0.07 | 0.056 | 0.07 | 0.07 |
| CC | 0.07 after week 24 | 0.056 | 0.07 | 0.07 after week 12 |
| Rebound assumption | Rebound to baseline | Rebound to baseline | Rebound to conventional therapy | Rebound to baseline |
| Rebound assumption duration | Over a 6-month period | Immediately | Over a 6-month period | Over a 6-month period |
| Placebo response | 14.5% at week 12; loss or maintenance of placebo response not reported | BASDAI and BASFI scores return to baseline at week 12 | No placebo response | BASDAI and BASFI scores return to baseline at 12 weeks |
| Parameter | AbbVie34 economic model (adalimumab) | UCB35 economic model (certolizumab) | Pfizer36 economic model (etanercept) |
|---|---|---|---|
| Model type | Markov model | Markov model | Patient-level simulation model (DES) |
| Time horizon | 40 years | Lifetime | Lifetime |
| Response criterion | ASAS 40 response at week 12 | ASAS 20 response at week 12 | BASDAI 50 response at week 12 |
| Response criterion justification | Primary end point of ABILITY-158 study | Primary end point of RAPID-axSpA64 study | Based on the current NICE definition of treatment response (TA14317) |
| Progression assumption BASDAI | |||
| Anti-TNFs responders | Constant after week 140 | Constant after week 12 | Constant after week 48 |
| Anti-TNFs non-responders | Constant | Constant | Constant |
| CC | Constant | Constant | Constant after week 12 |
| Progression assumption BASFI | |||
| Anti-TNFs responders | Constant after week 140 | Constant after week 12 | Constant after week 48 |
| Anti-TNFs non-responders | 0.084 | 0.07 | Constant/0.07 |
| CC | 0.084 | 0.07 | 0.07 after week 12 |
| Rebound assumption | Rebound to baseline | Rebound to conventional therapy | Rebound to baseline |
| Rebound assumption duration | Immediately | Over a 6-month period | Over a 6-month period |
| Placebo response | BASDAI and BASFI scores return to baseline at week 12 | No placebo response | BASDAI and BASFI scores return to baseline at 12 weeks |
In general, it is difficult to identify the specific factors which can easily explain differences within and between the two populations across the manufacturer’s submissions. In general, similar model structures were applied by each manufacturer across the separate populations. However, it is evident that there are important differences based on a number of key structural issues: (1) the response criteria and timings applied; (2) the magnitude of change scores and particularly the assumption concerning the time at which these were assumed to ‘level off’ (generally longer in the AS populations because of the longer open-label extension periods); (3) the underlying rate of progression of BASFI with CC and the impact of anti-TNFs on this rate; and (4) the rebound assumption and timing of this.
Given the complex inter-relationship between these structural assumptions and subsequent parameter estimates, it is difficult to identify single specific reasons for differences. However, the structural differences clearly lead to marked differences in the BASDAI and BASFI scores estimated over time by each manufacturer for each population. Figures 9–11 provide a graphical summary of the cohort BASDAI and BASFI scores, for the AS population, from three of the manufacturers. These highlight the significant differences in subsequent parameter estimates applied at a cohort level. Equivalent estimates are not presented for the Pfizer model because of the complexities of generating this data from the DES model. The BASDAI and BASFI scores are presented here only for the case made by each manufacturer for their own product.
FIGURE 9.
Comparison of cohort BASDAI/BASFI scores for AS population from AbbVie model (adalimumab). 34
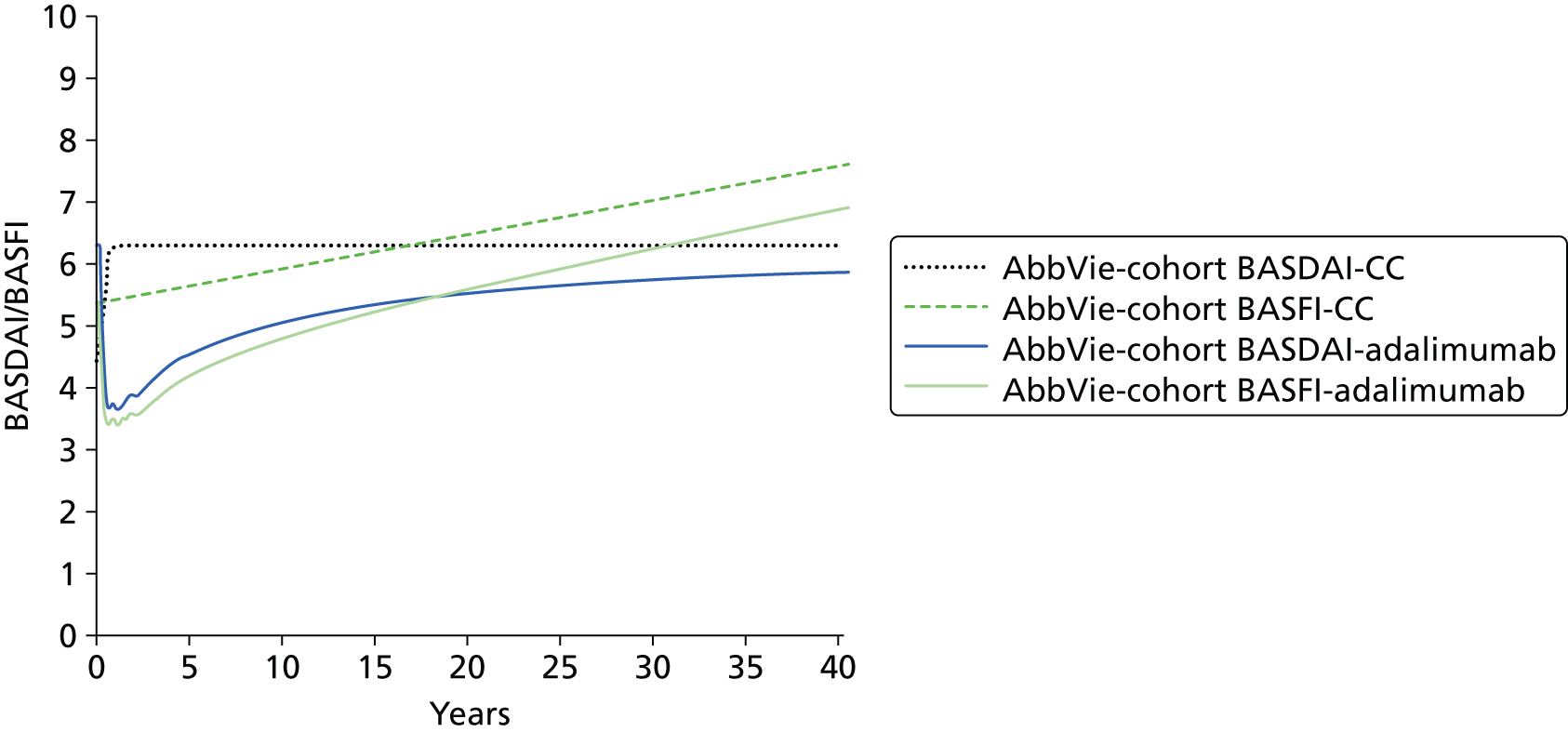
FIGURE 10.
Comparison of cohort BASDAI/BASFI scores for AS population from UCB model (certolizumab). 35
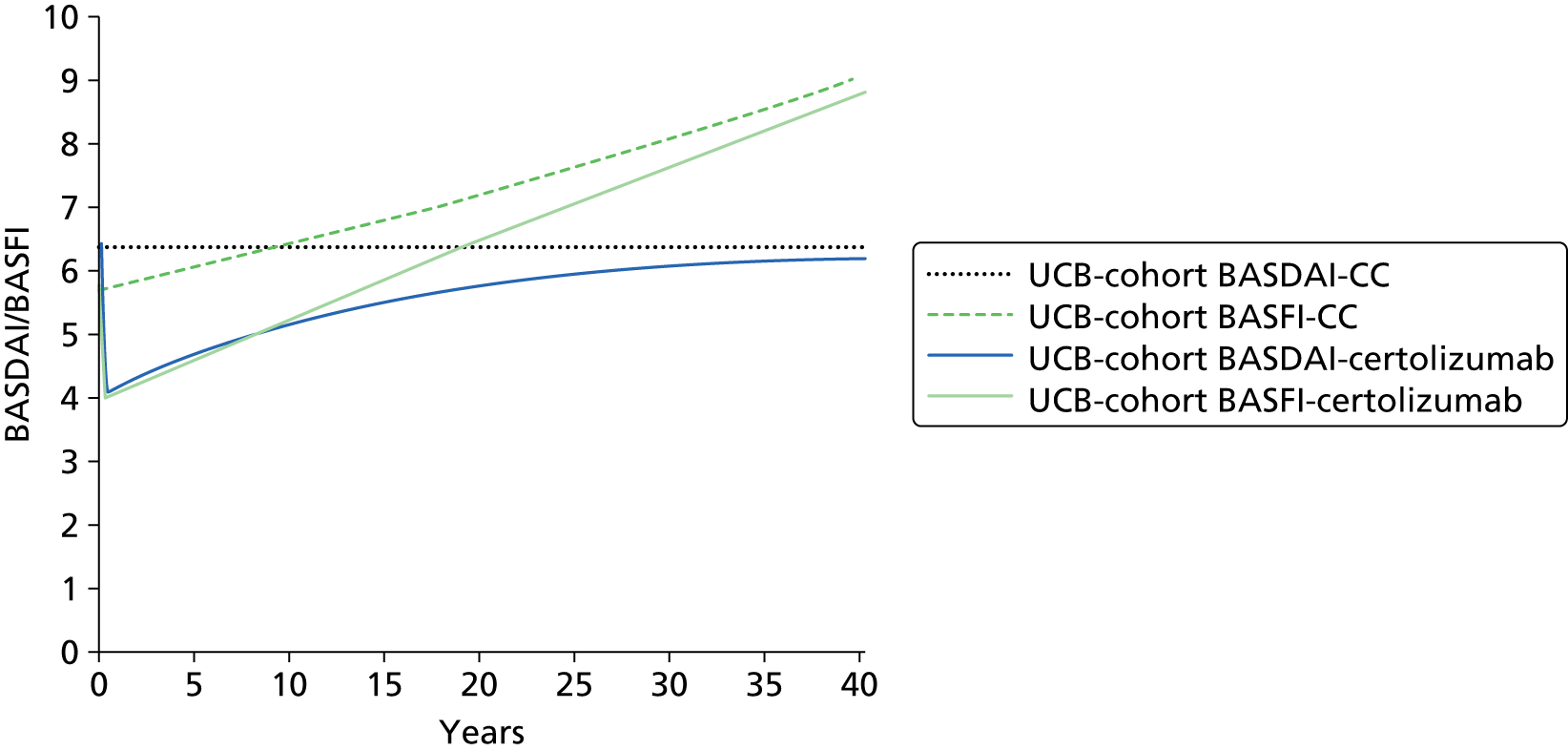
FIGURE 11.
Comparison of cohort BASDAI/BASFI scores for AS population from Merck Sharp & Dohme models (golimumab/infliximab). 37 MSD, Merck Sharp & Dohme.
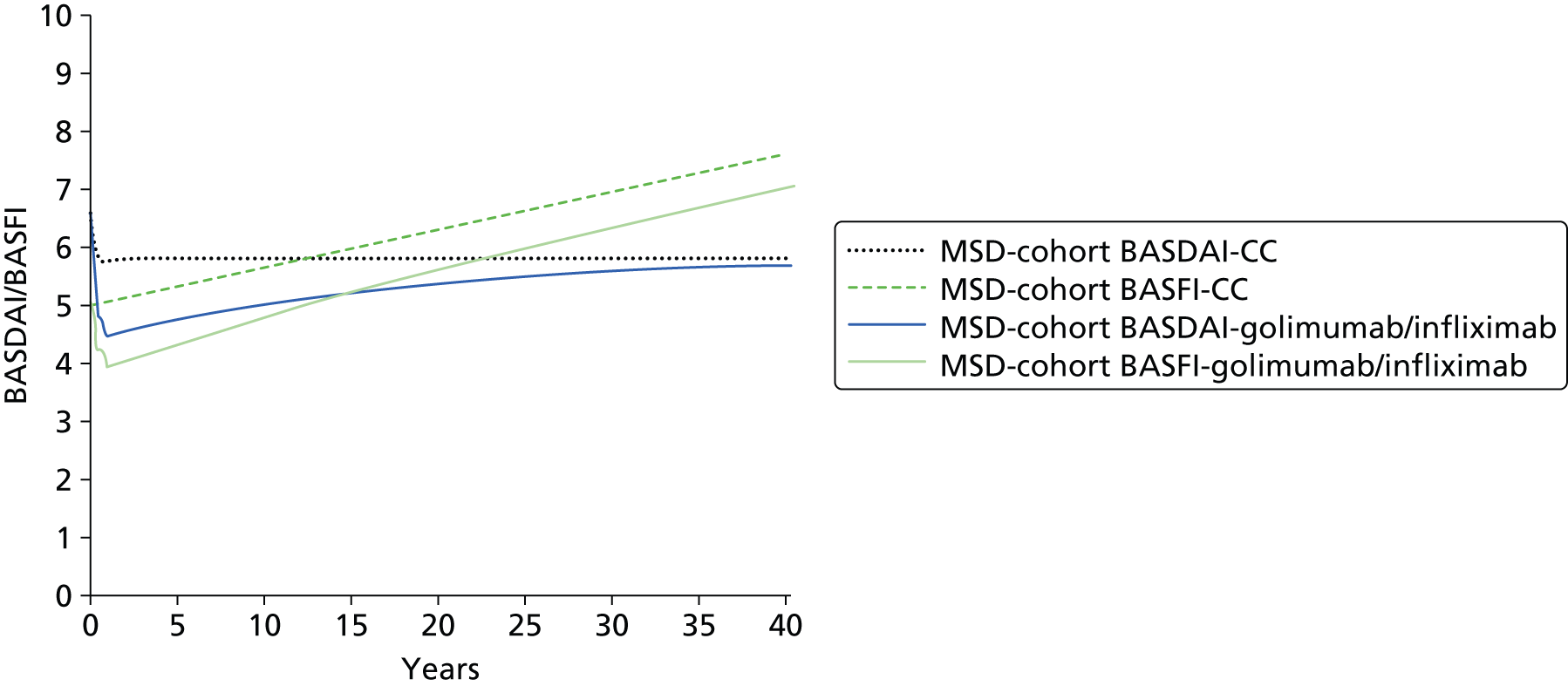
Tables 64 and 65 summarise the mean difference in BASDAI and BASFI scores applied to responders to anti-TNFs and those applied to CC at various time points in each model. The tables clearly highlight the range of different values applied across the separate manufacturers. This further emphasises the variation in approaches, sources and assumptions.
| Time | Adalimumab vs. CC | Certolizumab vs. CC | Infliximab/golimumab vs. CC |
|---|---|---|---|
| 12 weeks | –2.98 | AiC information has been removed | –2.01 |
| 24 weeks | –4.42 | AiC information has been removed | –2.05 |
| 1 year | –4.9 | AiC information has been removed | –2.77 |
| 3 years | –5.23 | AiC information has been removed | –2.83 |
| 5 years | –5.31 | AiC information has been removed | –2.83 |
| 10 years | –5.31 | AiC information has been removed | –2.83 |
| 20 years | –5.31 | AiC information has been removed | –2.83 |
| 40 years | –5.31 | CiC information has been removed | –2.83 |
| Time | Adalimumab vs. CC | Certolizumab vs. CC | Infliximab/golimumab vs. CC |
|---|---|---|---|
| 12 weeks | –2.03 | AiC information has been removed | –1.68 |
| 24 weeks | –3.28 | AiC information has been removed | –1.74 |
| 1 year | –3.71 | AiC information has been removed | –2.49 |
| 3 years | –4.25 | AiC information has been removed | –2.59 |
| 5 years | –4.25 | AiC information has been removed | –2.66 |
| 10 years | –4.53 | AiC information has been removed | –2.85 |
| 20 years | –5.09 | AiC information has been removed | –3.18 |
| 40 years | –6.21 | AiC information has been removed | –3.75 |
The equivalent figures and tables are reported for the nr-AxSpA population (Figures 12 and 13 and Tables 66 and 67).
FIGURE 12.
Comparison of cohort BASDAI/BASFI scores for nr-AxSpA population from AbbVie model (adalimumab). 34

FIGURE 13.
Comparison of cohort BASDAI/BASFI scores for nr-AxSpA population from UCB model (certolizumab). 35
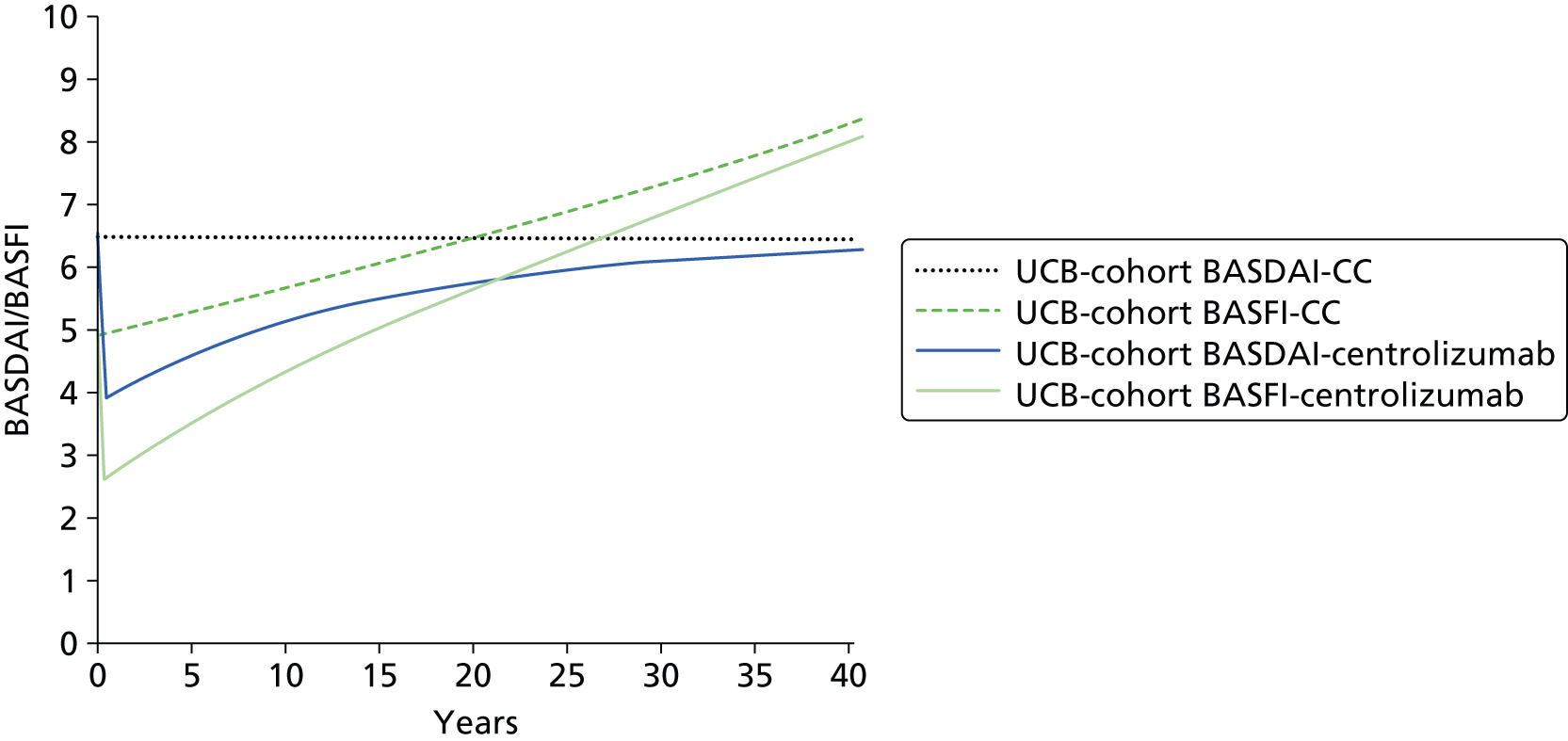
| Time | Adalimumab vs. CC | Certolizumaba vs. CC |
|---|---|---|
| 12 weeks | –3.89 | AiC information has been removed |
| 24 weeks | –5.54 | AiC information has been removed |
| 1 year | –5.42 | AiC information has been removed |
| 3 years | –5.99 | AiC information has been removed |
| 5 years | –5.99 | AiC information has been removed |
| 10 years | –5.99 | AiC information has been removed |
| 20 years | –5.99 | AiC information has been removed |
| 40 years | –5.99 | AiC information has been removed |
| Time | Adalimumab vs. CC | Certolizumaba vs. CC |
|---|---|---|
| 12 weeks | –2.95 | AiC information has been removed |
| 24 weeks | –4.11 | AiC information has been removed |
| 1 year | –4.12 | AiC information has been removed |
| 3 years | –4.55 | AiC information has been removed |
| 5 years | –4.72 | AiC information has been removed |
| 10 years | –5.14 | AiC information has been removed |
| 20 years | –5.98 | AiC information has been removed |
| 40 years | –7.66 | AiC information has been removed |
The differences across manufacturers and between the populations are further illustrated by the summary of key parameter inputs reported in Appendix 11. As well as reporting the main parameter inputs, Appendix 11 also explores differences in approaches at a parameter level for key inputs (e.g. withdrawal, costs, etc.).
It is evident from these comparisons that there are significant differences across the manufacturers in terms of key structural and parameter estimates. While it might appear reassuring that these differences do not appear to lead to significant differences across the ICER estimates reported for the AS population, the greater heterogeneity reported in the ICER estimates for the nr-AxSpA is clearly an issue. However, even within the AS population, any reassurance that one might have regarding the robustness and appropriateness that these estimates have for informing NHS practice needs to be carefully considered in relation to the key conceptual issues and concerns highlighted in Summary and critique of published cost-effectiveness studies for previously published cost-effectiveness studies. Despite significant work undertaken by each manufacturer in support of existing and new indications for their products, it is particularly concerning that many of the key conceptual issues and concerns appear to have not been fully addressed. Indeed, many of these issues seem to have not been addressed at all, such that many models still seem reliant on the use of open-label extension data (and even more so with the extended follow-up reported in the AS population) without any formal consideration of the potential issues with selection that the use of these studies inevitably are subject to. Consequently, the benefits of anti-TNFs are being projected over significant periods of time without any evidence on the counterfactual (i.e. what happens to patients who do not enter into the open-label extension periods? What happens to patients who subsequently withdraw from anti-TNFs? And what would have happened to patients over a longer-time horizon who did not receive anti-TNFs?).
It appears that much of the case being made concerning the cost-effectiveness of the anti-TNFs rests on comparison of single-arm studies (the subject of open-label data) and retrospective comparisons against historical cohorts (as the counterfactual, for patients not on treatment, is unknown). While such a comparison may be necessitated by the short-term nature of the double-blind periods, the lack of a more detailed consideration of the appropriateness of the comparisons being made in relation to sources of natural history data (and subsequent assumptions made concerning the BASDAI/BASFI trajectories of the different patient categories) is concerning and, hence, current ICER estimates reported by the manufacturers must be considered to be both speculative and highly uncertain.
Many of these problems can be associated with whether or not BASDAI and BASFI scores provide an appropriate conceptual basis for modelling the chronic and progressive nature of AS and nr-AxSpA. Hence current models appear largely driven by data availability (i.e. the extensive evidence which has been generated and continues to be generated investigating the relationship between BASDAI/BASFI and costs/utilities) rather than trying to develop a clearer underlying biological or clinical process which may better characterise the disease and subsequent progression across the separate populations.
Until such time that sufficient data-linking costs and utilities to other measures are reported, it seems inevitable that models will continue to be driven largely by BASDAI and BASFI scores over time together with assumptions concerning the longer-term effect of anti-TNFs. However, given the nature of existing models and the reliance on uncontrolled longer-term follow-up of anti-TNFs, and comparison with historical ‘controls’ (particularly in relation to BASFI progression over time and the assumptions being made concerning the potential disease modification properties of anti-TNFs in both AS and nr-AxSpA populations), it is surprising that greater efforts have not been made by the manufacturers to try to more formally link to the increasing evidence base being generated in relation to radiographic progression in the AS population.
It is also surprising that more thought has also not been given to characterising the potential difference in BASFI progression across the separate populations and how generalisable assumptions may be between these. The result is that many of the key assumptions concerning whether the anti-TNFs are primarily symptom control treatments or whether they are also potential disease modifiers remains implicitly dealt with within existing submissions. The result is that several manufacturers use identical assumptions across populations with respect to BASFI progression and the effect of the anti-TNFs. Interestingly, only one manufacturer appears to use differential rates of BASFI progression across the populations (AbbVie), although the same structural assumption concerning the effect of anti-TNFs is still made. Interestingly, this manufacturer applies a higher rate of change in BASFI for patients receiving CC in the nr-AxSpA population vis-à-vis the AS population. However, while such a difference is interesting, the basis of and implication for this differential is not fully explained or justified by the manufacturer.
The issue of intermittent and sequential use of anti-TNFs remain important clinical questions but the existing models do not provide a robust basis for informing these decisions. The cost-effectiveness of intermittent therapy versus continuous therapy was not formally considered in any model identified. However, it could be argued that such a comparison might be deemed outside the scope of a NICE appraisal. Although one manufacturer (Pfizer36) explored the potential cost-effectiveness of sequential therapy, much of this has been done via assumptions (e.g. assuming equal efficacy second line in patients who discontinue first line as a result of an AE) or via adjustments applied to first-line efficacy estimates based on ‘real-world’ evidence reported from large-scale registries (which typically show anti-TNFs to be clinically effective but with lower response rates than reported in naive patients). Consequently, existing attempts to model sequential therapy are largely based on applying adjustments to first-line efficacy data using observational evidence which are clearly subject to potential confounding. In large part, the limitations of existing cost-effectiveness models for informing these clinical questions appears less a function of the models themselves but rather that robust clinical data to date has not been generated to inform unbiased estimates of relative efficacy of alternative strategies for using the anti-TNFs.
The following sections report the development of a de novo model to address some of the key issues and uncertainties which have been identified in this review. Chapter 5 reports the results of an extended synthesis which has been developed to provide a more generalisable framework for synthesising clinical efficacy data ensuring that appropriate estimates are generated for the model which make use of all relevant and available evidence. This is followed in Chapter 6 by a description of the de novo model (York model) which attempts to link this framework to a more coherent conceptual model of the chronic and progressive nature of AS and nr-AxSpA.
Chapter 5 Independent economic assessment: extended synthesis
Existing evidence on the short-term clinical effectiveness of anti-TNF drugs has been presented and discussed in Chapter 3. The methods of evidence synthesis are extended in this section to more directly address the decision problem and the parameter inputs required for the economic model. There were two specific aims to these analyses. Firstly, we aimed to more formally explore the differences between individual anti-TNF treatments to inform the most appropriate assumption for the economic model (i.e. equivalence or drug-specific differences). Within Chapter 3 of this report, the assumption of independent treatment effects was evaluated alongside the assumption of a common (equal) treatment effect across anti-TNFs, for every outcome of interest. While there is no evidence that supports differences in the effectiveness of these drugs, assuming equal effectiveness means that the trials are pooled as if the same drug had been trialled; this leads to an arguably overly precise estimate of effect for the class of drugs. For this reason, we explore an additional scenario in which treatments are assumed to have a similar, but not equal, effectiveness, that is there are differences between the effectiveness of treatments that we may not be able to explain but that we should consider.
The second aim was to generate appropriate effect-size estimates and their associated uncertainty to inform the main input parameters of the economic model by synthesising together evidence on BASDAI and BASFI outcomes jointly. Initially, we considered the two related BASDAI outcomes relevant to the decision model reported in the effectiveness evidence available: changes in BASDAI scores over a certain period of time and a probability of response to BASDAI 50 (that is, a 50% change in the BASDAI score in relation to baseline). The BASDAI 50 is important, as patients are expected to discontinue anti-TNFs if, at 12 weeks, they have not been able to achieve response to this criterion (according to NICE guidance). 17,33 Changes in BASDAI scores observed at this same time point determine the magnitude of initial response to treatment and have often been used in economic modelling as the basis for extrapolating treatment effects. Given that these outcomes are both central to informing effect parameters in the decision model, a synthesis model that considers the relation between these two outcomes provides a more consistent and coherent basis for informing these parameters.
We developed a synthesis model that pools evidence on the change in BASDAI score by considering both those studies that report this measure directly and also those that report the proportion of patients achieving a BASDAI 50 response. We expressed BASDAI 50 as a function of the absolute change in BASDAI and we use this relationship in the extended synthesis. We also aim to simultaneously synthesise information on BASFI score, a measure that is used together with BASDAI score to determine the long-term QALY and cost burden of the disease in the economic model. Treatments improving AS symptoms are expected to affect both disease activity and function, and thus we expect a reduction in both BASDAI and BASFI scores, this means that we expect the changes to these two measures to be correlated. Extending the synthesis modelling to consider BASFI scores not only allows all relevant evidence to contribute to the synthesis but also ensures that all measures are synthesised together to reflect the expected correlations between the two outcomes. Uncertainty is also more appropriately quantified than synthesising each outcome separately.
In the decision model, prognosis, costs and QALYs are determined by absolute BASDAI and BASFI scores. Given that treatment continuation is determined by response to BASDAI 50 at 12 weeks, it is important for the economic model to estimate the absolute change in BASDAI and BASFI separately for responders and non-responders, that is the conditional scores. However, the published clinical effectiveness evidence does not report the conditional scores. Consequently, we requested the conditional data from the pivotal trials in both the AS and nr-AxSpA indications from each manufacturer. These data were subsequently provided by the manufacturers for their four pivotal trials. However, this information was not available for the other trials in the evidence-base (there were 15 trials in the evidence base and conditional scores were only available for four). In view of the limited data available on the conditional scores, another important extension of the synthesis approach was the evaluation of these. We used the results from the extended synthesis model to evaluate the conditional scores by simulating BASDAI and BASFI scores for two equivalent cohorts of patients the only difference being that one cohort was treated and the other was not.
This section provides only a summary of these analyses; full details are in Appendices 5, 12 and 13. We will describe first the approach for the synthesis of evidence on the AS population, followed by the approaches and results for the nr-AxSpA population.
Ankylosing spondylitis population
Brief description of the data
Based on study population and follow-up (i.e. around 12 weeks in duration), 16 of the RCTs are considered directly relevant to the decision problem for the AS population (studies 1–16 in Table 68). One of these studies did not report BASDAI or BASFI outcomes (study 357) and thus could not be included in the analyses. The 15 remaining studies reported at least one outcome measure of BASDAI 50 score and/or change from baseline on BASDAI and BASFI scores.
| Study number | Trial name | Treatments | Number in treatment group | Number in placebo group | BASDAI 50 score | Change BASDAI score | Change BASFI score |
|---|---|---|---|---|---|---|---|
| 1 | Hu (2012)55 | 1 | 26 | 20 | ✗ | ✗ | |
| 2 | Huang (2014)56 | 1 | 229 | 115 | ✗ | ✗ | ✗ |
| 3 | Lambert (2007)57 | 1 | 38 | 44 | |||
| 4 | ATLAS (2006)61 | 1 | 208 | 107 | ✗ | ✗ | |
| 5 | RAPID-axSpA (2014)64 | 2 | 121 | 57 | ✗ | ✗ | ✗ |
| 6 | Barkham (2010)71 | 3 | 20 | 20 | ✗ | ✗a | ✗a |
| 7 | Davis (2003)72 | 3 | 138 | 139 | ✗ | ✗ | |
| 8 | Dougados (2011)74 | 3 | 39 | 43 | ✗ | ✗ | ✗ |
| 9 | Gorman (2002)79 | 3 | 20 | 20 | ✗ | ||
| 10 | Calin (2004)83 | 3 | 45 | 39 | ✗ | ✗ | |
| 11 | van der Heijde (2006)86 | 3 | 305 | 51 | ✗ | ||
| 12 | GO-RAISE (2008)90 | 4 | 138 | 78 | ✗ | ✗ | |
| 13 | Bao (2012)96 | 4 | 108 | 105 | ✗ | ✗ | |
| 14 | Braun (2002)98 | 5 | 34 | 35 | ✗ | ✗a | ✗a |
| 15 | Marzo-Ortega (2005)100 | 5 | 28 | 14 | ✗ | ✗a | |
| 16 | Van den Bosch (2002)101 | 5 | 9 | 12 | ✗a | ✗a |
General aspects of implementation and software
The synthesis was conducted from a Bayesian perspective, using WinBUGS [a Markov chain Monte Carlo (MCMC) simulation-based software for Bayesian inference]. For burn-in, we ran 100,000 simulations and another 100,000 were used in inferences. Convergence was assessed by running two chains and convergence was assumed if the Gelman–Rubin statistic was equal to 1. Goodness of fit was assessed using the DIC, a criterion developed by Spiegelhalter et al. 170 based on the trade-off between the fit of the data to the model and the complexity of the model. Fit is measured using the deviance, and complexity is included using a measure of the ‘effective number of parameters’ (i.e. posterior mean deviance minus deviance evaluated at the posterior mean of the parameters). Models with smaller DIC are better supported by the data: that is, the lower the DIC, the better the data fit the model. In the presence of autocorrelation, the MCMC simulation for inference was increased to 200,000 and a thin of 20 was applied (yielding a sample for inference of 10,000 for each chain).
The main synthesis models (approaches B and C described next) pooled differences between treatment and control in change in scores from baseline (BASDAI and BASFI). The treatment associated with the lowest (most negative) mean change score is expected to be best. However, it is important to quantify the uncertainty around the estimates and for this reason SDs were reported alongside expected values. When averaged ORs were presented median values instead of means were used, as ORs tend to follow a skewed distribution.
Relative effectiveness estimates for models assuming exchangeability across treatments are based on the predictive distribution, representing the distribution of the data averaged over all possible parameter values. This summary statistic best reflects the impact of uncertainty in the parameters of the model and is here judged as a more appropriate basis to be used in the decision model. 171
When possible, meta-regression analyses were conducted to evaluate potential treatment effect modifiers. Meta-regression is a tool aimed at examining the impact of variables on effect size using regression-based techniques. In these explorations, the following baseline characteristics were considered: BASDAI score, BASFI score, age, sex, duration of symptoms (years) and CRP level.
Exploring assumptions for the relative effectiveness of individual anti-tumour necrosis factor treatments (modelling approach A)
In AS, pivotal trials for the licensed anti-TNFs do not perform head-to-head comparisons with other agents but instead compare the effect of treatments against standard care. These trials show anti-TNFs to be effective in relation to standard care. In view of the available evidence, previous NICE guidance (TA14317 and TA23333) concluded that there was no compelling evidence on which it could reliably distinguish between the anti-TNFs on the basis of clinical effectiveness when making recommendations.
Our analysis, based on the most up-to-date evidence base, aimed to evaluate anti-TNF drugs using indirect comparisons across trials. Within this subsection, alternative assumptions of equivalence in the effectiveness of anti-TNF treatments will be more formally assessed. Note that at this stage each outcome was synthesised independently.
Brief description of synthesis methods
In brief, the synthesis model directly aggregates relative treatment effects, that is log OR for BASDAI 50 response and the difference between treatment and placebo in change in BASDAI from baseline (the data set analysed is shown in Appendix 12). In common with the approach implemented in Chapter 3, all outcomes are here assumed normally distributed. We implemented alternative models that differ in the way treatment effects are considered; a summary of each is presented below.
Model A1 (treatments: independent; studies: fixed effect): This model considers treatments to be independent: that is, it assumes the effects to differ between treatments. This is a fixed-effect model in that multiple studies evaluating the same treatment are considered to measure the same treatment effect.
Model A2 (treatments: independent; studies: random effects): This model differs from model A1 in that a random effect is assumed to describe the findings of multiple studies evaluating the same treatment.
Model A3 (treatments: equal; studies: fixed effect): This model differs from model A1 in that treatments are not assumed to differ. The model thus evaluates a common relative effectiveness for all anti-TNFs.
Model A4 (treatments: equal; studies: random effects): This model differs from model A3 in that a random effect is assumed to describe the findings of multiple studies evaluating the same treatment.
Model A5 (treatments: exchangeable; studies: fixed effect): This model differs from model A1 in that a random effect is used to describe any differences between treatments (exchangeability is assumed). This model thus assumes the treatments to have a similar, but not equal, effectiveness: there are differences between the effectiveness of treatments that we may not be able to explain but that we should consider.
There is some evidence that health outcomes may depend on patients’ characteristics such as age, BASFI score, enthesitis, therapy, CRP level and HLA-B27 genotype. 172 There is, however, no evidence on which factors may modify the effects of treatment with anti-TNFs (note that Lord et al. 2010173 studied predictors of BASDAI 50 response in patients receiving anti-TNFs, but by not including a placebo arm this study was not able to evaluate treatment effect modifiers). To our knowledge, previous meta-analyses of studies in AS have not explored how the effect of treatment may depend on characteristics of the patients or of their disease. Within this modelling approach we explored potential heterogeneity in treatment effects using meta-regression (i.e. potential treatment effect modifiers). We did so by extending the modelling approach in A1 to include treatment effect interactions with baseline characteristics (centred on their means where relevant). We have explored the inclusion of alternative covariates by evaluating the DIC associated with alternative models.
Results of modelling approach A
All models implemented synthesise results on each of the outcomes separately. The results of each modelling approach are shown in Table 69.
| A1. Treatment: independent; studies: fixed effect | A2. Treatment: independent; studies: random effects | A3. Treatment: common; studies: fixed effect | A4. Treatment: common; studies: random effects | A5. Treatment: exchangeable; studies: fixed effect | |
|---|---|---|---|---|---|
| Outcome 1: OR on BASDAI 50 | Median (SD) | Median (SD) | Median (SD) | Median (SD) | Median (SD) |
| Adalimumab | 4.71 (1.00) | 4.69 (6.11) | 5.21 (0.72) | 5.30 (0.98) | 5.34 (9.79) |
| Certolizumab | 6.02 (3.33) | 6.04 (22.87) | |||
| Etanercept | 4.73 (1.43) | 4.72 (3.32) | |||
| Golimumab | 5.86 (1.81) | 6.10 (7.45) | |||
| Infliximab | 11.9 (11.94) | 12.10 (44.00) | |||
| Outcome 2: change in BASDAI | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Adalimumab | –1.56 (0.16) | –1.57 (0.27) | –1.66 (0.11) | –1.67 (0.15) | –1.70 (0.87) |
| Certolizumab | –1.45 ( 0.37) | –1.46 (0.51) | |||
| Etanercept | –1.76 (0.20) | –1.73 (0.28) | |||
| Golimumab | N/A | N/A | |||
| Infliximab | –2.28 (0.46) | –2.27 (–2.28) | |||
| Outcome 3: change in BASFI | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Adalimumab | –1.22 (0.18) | –1.18 (0.29) | –1.38 (0.11) | –1.39 (0.13) | –1.41 (0.49) |
| Certolizumab | –1.10 (0.37) | –1.11 (0.47) | |||
| Etanercept | –1.48 (0.19) | –1.50 (0.24) | |||
| Golimumab | –1.45 (0.20) | –1.44 (0.29) | |||
| Infliximab | –2.16 (0.53) | –2.17 (0.56) | |||
| DIC | 52.4 | 57.0 | 39.1 | 44.3 | 43.6 |
Models A1 and A2 consider that anti-TNF have distinct relative effects. Applying the assumptions of model A1, adalimumab is expected to be the least effective of the set of treatments analysed in terms of BASDAI 50 (the expected OR is 4.71), but in terms of the differences in change scores it is certolizumab that is expected to be the least effective, with differences of –1.45 and –1.10 in BASDAI and BASFI scores, respectively. It should be noted that studies on golimumab (studies 1290 and 1396 in Table 68) do not report absolute changes in BASDAI scores, and thus using this modelling approach we were unable to estimate a treatment effect for this outcome measure. Model A2 reports similar results to model A1, but the SE of the estimates is slightly higher, reflecting increased uncertainty because of the use of the random effects to characterise between study results. The DIC is lower in model A1 (52 vs. 57), indicating that model A1 is preferable to model A2.
Outcome 1 is OR for BASDAI 50; outcome 2 is the difference between treatment and placebo on change in BASDAI from baseline; and outcome 3 is the difference between treatment and placebo on change in BASFI from baseline.
Models A3 and A4 consider the treatments as equal in terms of their effectiveness in each of the three outcomes. This means drugs are assumed equally effective and results from trials are pooled together as if these trials evaluated the same drug, which will return more precise estimates (i.e. less uncertainty) and interpretations of this evidence may thus be overly confident. The DIC of these models is substantially lower than that of models A1 and A2, indicating that the data supports the assumption of equivalence, rather than one of independence. As with models A1 and A2, the random-effect assumption was also not deemed worthwhile.
Model A5 assumes the treatments to have a similar, but not equal, effectiveness; this model introduces more flexibility than assuming treatment effects to be equal (model A3), but does not fully assume treatments to differ as in model A1. It does imply that there are differences between the effectiveness of treatments that we may not be able to explain but that we should consider. These may be a result of differences between the treatments themselves or because of differences in the design of the trials used to evaluate each treatment. Ignoring such difference could lead to misrepresenting uncertainty, with overprecise results. Model A5 shows a slightly higher DIC than model A3 but this difference is not significant (i.e. lower than 5 units), which means that both models represent equally well the existing data. Given the underlying assumptions, results differ to those of model A3 particularly in relation to the measures of uncertainty. As expected, results from model A3 are more precise than the results of model A5. The results from model A5 in Table 69 relate to the predictive distribution which reflects uncertainty in all model parameters; in this case, such uncertainty explicitly accounts for the observed differences in the effects treatments.
Despite our preferred summary from model A5 in this evaluation being the common effect for the ‘class of drugs’ (see Table 69), the assumption of treatment effects being drug specific may still retain some plausibility. From model A5, drug-specific estimates can be retrieved (Table 70). Within this model drug-specific inferences will borrow strength from the common-class effect and estimates are thus shrunken towards the mean of this class effect (that is estimates are closer to the value reported for the class in Table 2).
| Treatment | Model A5 | ||
|---|---|---|---|
| Outcome 1: OR on BASDAI 50 (median, SD) | Outcome 2: change in BASDAI (mean, SD) | Outcome 3: change in BASFI (mean, SD) | |
| Adalimumab | 5.05 (0.87) | –1.60 (0.15) | –1.31 (0.16) |
| Certolizumab | 5.42 (1.71) | –1.59 (0.26) | –1.31 (0.23) |
| Etanercept | 5.13 (1.08) | –1.72 (0.17) | –1.43 (0.15) |
| Golimumab | 5.47 (1.25) | –1.69 (0.84) | –1.42 (0.16) |
| Infliximab | 5.70 (3.30) | –1.88 (0.34) | –1.55 (0.33) |
Explorations of heterogeneity suggested only sex to potentially modify the effect of anti-TNF treatment, specifically for change in BASDAI as outcome; however, when sex is used together with all covariates, such evidence on effect modification disappears.
Interpretation/discussion
The models implemented above show that there is no significant heterogeneity across trials evaluating each treatment, that is the DIC of model A2 is higher than that of model A1, indicating the use of a random effect across studies to be unnecessary.
The statistical analysis has also shown the effectiveness of the different treatments to be similar. This is in line with the published evidence, in AS, that does not demonstrate one anti-TNF treatment to be significantly more effective than another. Specifically, we implemented a model considering a common effect for all anti-TNFs when compared with placebo (model A3). This model shows a better fit than the one estimating a different effect for each anti-TNF (model A1). However, unless we believe this assumption to hold AND the trials to be homogeneous in design and in the populations included, we believe adopting model A3 would misrepresent uncertainty in the estimates.
For this reason, we evaluated an alternative model (model A5) that assumes treatments to have a similar (but not equal) effect. In this model, the treatment effects for the anti-TNFs are assumed to come from a ‘common’ distribution, assumed Normal with a common mean, that is a ‘class effect’. This is an assumption of exchangeability across treatments within the class, which we also refer to as a random-effect distribution. The DIC for this model is not significantly different from that of model A3, and it allows a more appropriate description of the uncertainty over the effects of anti-TNFs. However, it should be noted that this model is not explicit about the source of the differences in the effects of treatments.
The evidence available does not appear to suggest obvious treatment effect modifiers. However, because only aggregate data were available, the results may be prone to ecological fallacy in which statistical associations between variables present or absent at the group level may not be reflective of associations at the individual level. 174
Extending the modelling approach to jointly relate outcomes (modelling approach B)
In the previous chapter the two outcomes based on BASDAI scores were synthesised separately; however, BASDAI 50 is the probability of having a reduction in BASDAI score of 50%, and thus it should be possible to relate the proportion of BASDAI 50 responders to the change in absolute BASDAI scores from baseline observed in each study. Such structural constraints should be incorporated into the synthesis, when possible, by expressing it algebraically. 174 Within this chapter, we use this structural relation within the synthesis, allowing change scores from baseline to be informed not only from direct data on this quantity but also from data on BASDAI 50 (Joint synthesis of Bath Ankylosing Spondylitis Disease Activity Index outcomes). We then extend the modelling framework further to consider BASFI outcomes [(Extending the modelling framework to synthesise change in Bath Ankylosing Spondylitis Functional Index scores (modelling approach C)].
Joint synthesis of Bath Ankylosing Spondylitis Disease Activity Index outcomes
The model implemented here pools the change in BASDAI score from baseline to evaluate the difference between treatment and placebo, using evidence reported in trials directly on the change scores for each arm and also data on BASDAI 50.
The following description briefly explains the approach used to model these data.
Brief description of synthesis methods
Data on the mean change in BASDAI score from baseline, alongside the SE for this measure, were assumed normally distributed (likelihood). The mean of this distribution was the treatment effect, defined as the sum of the change score for the placebo arm plus the difference in change score for the treatments. Some studies also reported the number of responders to BASDAI 50 (a 50% reduction in BASDAI score), out of the total individuals in the study. The likelihood for the BASDAI 50 data was expressed as a binomial distribution. The probability parameter of this distribution was then related to the change score as follows. The BASDAI score at baseline and the change score were assumed correlated using a bivariate normal distribution. To define the bivariate distribution a number of quantities were needed. First, the mean score at baseline was reported in the data and was thus assumed known. Second, the variability on BASDAI score at baseline was assumed equal to that of the change score. This was also reported in the data and was thus assumed known. Finally, the unknown correlation between baseline and change score was estimated within the model by assuming this quantity was independent of study. The correlation parameter was estimated separately for placebo and anti-TNF treatment. Under these assumptions, the probability parameter from BASDAI 50 data was expressed algebraically as a function of the change score. For treatment effects, our preferred approach was to assume a common class effect (i.e. exchangeable effects across treatments, analogous assumption to model A5). See Appendix 12 for a fuller description of the methods used in analyses.
Results of modelling approach B
The summary results regarding relative treatment effects from this modelling approach are reported in Table 71 for model B. The treatment effect reported here represents difference between treatment and placebo on BASDAI score changes from baseline.
| Treatment | Estimated | Assumeda | Predicted | |
|---|---|---|---|---|
| Difference in change score from baseline, mean (SD) | Probability of having a BASDAI 50 response, placebo, mean (SD) | Probability of having a BASDAI 50 response, anti-TNF, mean (SD) | OR for BASDAI 50 response, anti-TNF vs. placebo, median (SD) | |
| Anti-TNFs | –1.91 (0.48) | 0.10 (–) | 0.40 (0.08) | 5.94 (4.06) |
With model B, we were now able to consider the evidence from trials only reporting information on BASDAI 50 to estimate the change in BASDAI score, an example being evidence on golimumab. The class effect of anti-TNFs is evaluated to be slightly higher –1.91 (SD 0.48) in comparison to model A5 [reporting a class effect on the change score of –1.70 (SD 0.87)], reflecting the inclusion of BASDAI 50 evidence. By using the indirect evidence on BASDAI 50, model B returns more precise estimates of the pooled change score than model A5 (SE of 0.48 in model B compared with 0.87 in model A5). This modelling approach, despite pooling absolute change scores, can be used to evaluate BASDAI 50 response for a specific baseline BASDAI score and change score in the placebo arm. We assumed a baseline BASDAI score of 6.11 (SD 1.56) and a change score for placebo of 0.61 (SD 1.44), which represent the average across trials (weighted by number of patients). According to these, the assumed probability of having a BASDAI 50 response to placebo is evaluated at 0.10. Based on the change score evaluated in the synthesis model, the probability of having a BASDAI 50 response when on anti-TNFs is evaluated at 0.40 (SD 0.08), which results in an OR for BASDAI 50 response of 5.94 (SD 4.06).
Drug-specific (shrunken) estimates from model B are shown in Table 72.
| Treatment | Change in BASDAI, mean (SD) |
|---|---|
| Adalimumab | –1.77 (0.25) |
| Certolizumab | –2.01 (0.37) |
| Etanercept | –1.88 (0.18) |
| Golimumab | –1.92 (0.30) |
| Infliximab | –2.02 (0.32) |
Interpretation/discussion
The current modelling approach, by synthesising together evidence on both BASDAI outcomes, is a theoretically coherent approach to the synthesis. Moreover, it allows using the whole of the evidence on this outcome. In addition, given these outcomes are to be both used in the decision model, the combined synthesis model will generate consistent estimates by considering their structural relation explicitly.
The results of modelling approach B show that using information on BASDAI 50 alongside direct evidence on change scores from baseline results in slightly higher estimates of effectiveness compared with approach A. There are two possible explanations for this. One is that higher treatment effects are observed in the trials only reporting BASDAI 50 compared with the remaining studies. The few studies that only report BASDAI 50 are studies 11,86 1290 and 13;96 these report ORs for BASDAI of, respectively, 5.9, 4.4 and 10.42. The second possible explanation relates to the assumptions used when defining the relation between the outcomes in the model. While we expected the model to use the BASDAI 50 evidence in such a way that would exactly predict the value of change score observed in the sample, we cannot guarantee this is the case as our analysis is based on assumptions over the distribution of BASDAI scores across patients. Given we did not have access to individual patient data when developing this relationship, and thus the validity of the assumptions of analysis cannot be established. The differences observed are, however, not significant and any misspecification of the model can be thus deemed irrelevant.
Extending the modelling framework to synthesise change in Bath Ankylosing Spondylitis Functional Index scores (modelling approach C)
The models implemented here extend those in Joint synthesis of Bath Ankylosing Spondylitis Disease Activity Index outcomes by adding the syntheses of changes in BASFI score. This is of particular relevance to the economic modelling because BASFI scores are used together with BASDAI scores. Given we expect that, within each trial, changes to BASDAI scores to be related to changes in BASFI scores, this section will model the trial evidence to reflect this correlation. Figure 14 plots the BASDAI change scores against the BASFI change scores observed in the trials, showing support for the existence of correlation.
FIGURE 14.
Scatterplot of BASDAI and BASFI change scores observed in the trials (AS).

Brief description of synthesis methods
The data on mean change in BASFI score reported in some of the studies was assumed normally distributed. The mean of this distribution was the treatment effect, defined as the sum of the change score for the placebo arm plus the difference in change score for the treatments (analogous to BASDAI). Treatment effects on BASFI were considered correlated to those on BASDAI across trials. The variation in treatment effects for both BASDAI and BASFI, and the correlation parameter between these were estimated from the data. As in Joint synthesis of Bath Ankylosing Spondylitis Disease Activity Index outcomes, we assumed again exchangeability across the effects of the different treatments (analogous to models ‘5’ in modelling approach A).
Results of modelling approach C
The results on differences between treatment and placebo on change score form baseline are reported in Table 73, for both BASDAI and BASFI scores.
| Outcome | Estimated | Assumeda | Predicted | |
|---|---|---|---|---|
| Difference in change score from baseline, mean (SD) | Probability of having a BASDAI 50 response, placebo, mean (SD) | Probability of having a BASDAI 50 response, anti-TNF, mean (SD) | OR for BASDAI 50 response, anti-TNF vs. placebo, median (SD) | |
| Effect of anti-TNFs on BASDAI | –1.95 (0.30) | 0.10 (–) | 0.41 (0.05) | 6.30 (1.56) |
| Effect of anti-TNFs on BASFI | –1.40 (0.28) | – | – | – |
Based on the change score evaluated in the synthesis model, the probability of having a BASDAI 50 response when on anti-TNFs is evaluated at 0.41 (SD 0.05), which returns an OR for BASDAI 50 response of 6.3 (SD 1.56). Note that estimates on BASDAI treatment effects are more precise than in modelling approach B, reflecting the support to inferences from the data on BASFI; the correlation between outcomes observed in the data and allowed in the synthesis model allows inferences in BASDAI to borrow strength from those on BASFI. Drug-specific (shrunken) estimates from model C are shown in Table 74.
| Treatment | Change in BASDAI, mean (SD) | Change in BASFI, mean (SD) |
|---|---|---|
| Adalimumab | –1.89 (0.22) | –1.34 (0.17) |
| Certolizumab | –2.02 (0.28) | –1.36 (0.21) |
| Etanercept | –1.94 (0.18) | –1.43 (0.16) |
| Golimumab | –1.98 (0.25) | –1.42 (0.17) |
| Infliximab | –2.03 (0.27) | –1.49 (0.25) |
Interpretation/discussion
We hypothesised that treatments improving AS symptoms are expected to affect both disease activity and function, and therefore we expected changes to these two measures to be correlated. We have thus extended the synthesis model to consider BASFI scores. This not only allows all relevant evidence to contribute to the synthesis but also ensures that all measures are synthesised together to reflect the expected correlations between the two outcomes.
The results obtained with this modelling approach for BASDAI outcomes are similar to those of modelling approach B, the difference being that estimates are now more precise because of the borrowing of strength between outcomes.
Non-radiographic axial spondyloarthritis population
This section examines the evidence on the effectiveness of anti-TNFs on the nr-AxSpA population.
Brief description of the data
On the nr-AxSpA population, five RCTs were considered directly relevant to the decision problem (studies 17–2150,51,58,64,76 in Table 75). All studies reported BASDAI and BASFI outcomes and one study did not report BASDAI 50 (study 2150).
| Study number | Trial name | Treatments | Number in treatment group | Number in placebo group | BASDAI 50 score | Change BASDAI score | Change BASFI score |
|---|---|---|---|---|---|---|---|
| 17 | Haibel 200852 | Adalimumab | 22 | 24 | ✗ | ✗ | ✗ |
| 18 | ABILITY-1 201358 | Adalimumab | 69 | 73 | ✗ | ✗ | ✗ |
| 19 | RAPID-axSpA 201464 | Certolizumab pegol | 46 + 51 | 50 | ✗ | ✗ | ✗ |
| 20 | Dougados 201476 | Etanercept 50 | 106 | 109 | ✗ | ✗ | ✗ |
| 21 | Barkham 200950 | Infliximab | 20 | 20 | ✗ | ✗ |
Description of approaches to the synthesis
To synthesise these data we used the same implementation and software specifications as described in Chapter 5, Ankylosing spondylitis population. Analyses explored two different scenarios to consider these data:
-
scenario 1 – data from nr-AxSpA trials were considered in isolation
-
scenario 2 – data from AS population were also used, no difference between the populations was assumed.
All models implemented here jointly synthesise BASDAI and BASFI outcomes [for our preferred modelling approach, C, see description in Extending the modelling framework to synthesise change in Bath Ankylosing Spondylitis Functional Index scores (modelling approach C)].
Results of the synthesis
In what concerns scenario 1, in which only data from the nr-AxSpA trials has been considered, we implemented two models: one assuming an equal effect across treatments and another assuming exchangeable treatment effects. Both models represented the data equally well (DIC of 87.6 vs. 88.7), and thus we only present results in Table 76 for the latter model [the preferred model; see Exploring assumptions for the relative effectiveness of individual anti-tumour necrosis factor treatments (modelling approach A)]. Results are qualitatively similar to those in AS but slightly lower estimates for both change scores: BASDAI –1.95 in AS and –1.86 in the nr-AxSpA population; and BASFI –1.40 in AS and –1.30 in the nr-AxSpA population. The uncertainty over these estimates is higher in the nr-AxSpA population, which was expected as the number of trials (and overall number of patients in the set of trials) is substantially lower.
| Estimated | Assumeda | Predicted | ||
|---|---|---|---|---|
| Difference in change score from baseline, mean (SD) | Probability of having a BASDAI 50 response, placebo, mean (SD) | Probability of having a BASDAI 50 response, anti-TNF, mean (SD) | OR for BASDAI 50 response, anti-TNF versus placebo, median (SD) | |
| Scenario 1: data from nr-AxSpA trials | ||||
| Effect of anti-TNFs on BASDAI | –1.86 (0.79) | 0.20 (–) | 0.53 (0.13) | 4.39 (6.59) |
| Effect of anti-TNFs on BASFI | –1.30 (0.84) | – | – | – |
| Scenario 2: data from AS and nr-AxSpA trials, no difference between the populations | ||||
| Effect of anti-TNFs on BASDAI | –1.97 (0.32) | 0.20 (–) | 0.55 (0.06) | 4.94 (1.48) |
| Effect of anti-TNFs on BASFI | –1.37 (0.3) | – | – | – |
When the data from the nr-AxSpA trials were considered together with data on AS (scenario 2), inferences were more precise. As treatment effects in AS trials are not significantly different from those observed in the nr-AxSpA population, pooled treatment effect estimates do not differ significantly from those reported in AS.
Interpretation/discussion
The evidence base of the effect of anti-TNFs in the nr-AxSpA population consists of five trials that observed four treatments and CC in a total of 590 patients. The effect measures pooled across the five trials were not significantly different from the outcomes expected in the AS population. Thus, it may be reasonable to consider the evidence in nr-AxSpA and AS together for inferences over treatment effects.
Bath Ankylosing Spondylitis Disease Activity Index and Bath Ankylosing Spondylitis Functional Index scores conditional on Bath Ankylosing Spondylitis Disease Activity Index response
We previously highlighted that NICE guidance determines that BASDAI 50 at 12 weeks defines treatment continuation with anti-TNFs in clinical practice. Given much of the evidence on prognosis, costs and utility scores links to the absolute values of BASDAI and BASFI scores, it is important to consider absolute changes in BASDAI and BASFI separately for responders and non-responders, that is the conditional scores. However, the published clinical effectiveness evidence does not report the conditional scores. In this section we use the results from the extended synthesis model to evaluate the conditional scores by simulating BASDAI and BASFI scores for two equivalent cohorts of patients, one treated with an anti-TNF and the other with conventional therapy.
Brief description of methods
From the inferences obtained using the synthesis model above, it is possible to derive the conditional change score in responders and non-responders using simulation. While the synthesis focuses on the pooling of mean estimates of change scores and proportion of responders to BASDAI 50, to derive conditional mean scores there is the need to consider the distributions at the individual patient level. Hence, conditional scores could not directly be derived from the synthesis, but through a simulation procedure based on the assumptions and results of the synthesis model. The simulation procedure is described in detail in Appendix 5. Briefly, we used a simulation sample size of 10,000 patients. Given results depend on the baseline distributions of BASDAI and BASFI scores and on the change scores from baseline for placebo, we used the averages across trials (weighted by the number of patients in each trial) in AS. Baseline BASDAI scores were thus assumed normally distributed with mean 6.11 and SD 1.56; the change from baseline for placebo was simulated from a normal distribution with mean –0.61 and SD 1.44. For BASFI, the baseline was assumed to have a mean of 5.27 and a SD 1.79 and the change from baseline for placebo a mean of –0.19 and a SD 0.22. The correlation between baseline BASFI and BASDAI scores was valued at (AiC information has been removed). This value was based on the sample correlation on BASDAI and BASFI at baseline from etanercept studies [the individual patient data were available in the Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) file for the etanercept submission]; the Spearman’s rank correlation coefficient was (AiC information has been removed) in 314-EU study167 in AS and (AiC information has been removed) in 1031 study166 in nr-AxSpA.
Results for ankylosing spondylitis
The conditional change scores derived from the synthesis model (and underlying assumptions) are reported in Table 77. While it is natural to consider that conditional change in BASDAI scores differ between respondents and non-respondents, differences in the baseline of respondents and non-respondents may be less intuitive. These are, however, natural. If we consider two patients that obtained the same change score in BASDAI from anti-TNF treatment, for example –2 units, but one started with a baseline of 4 and another with a baseline of 5, the first would be considered a responder and the second would not. For this reason, respondents are expected to have a lower BASDAI than non-responders. Results of the prediction of conditional scores using the synthesis model are presented in Table 77.
| BASDAI | BASFI | |||
|---|---|---|---|---|
| Control | Treatment | Control | Treatment | |
| Base case | ||||
| % responders to BASDAI 50 | 0.09 | 0.42 | ||
| Change in score | ||||
| Responders | –2.89 | –3.86 | –1.72 | –3.08 |
| Non-responders | –0.36 | –1.64 | –0.04 | –0.44 |
| All | –0.59 | –2.57 | –0.19 | –1.55 |
| Baseline | ||||
| Responders | 4.01 | 4.80 | 3.52 | 4.20 |
| Non-responders | 6.33 | 7.08 | 5.46 | 6.07 |
| All | 6.12 | 6.12 | 5.28 | 5.28 |
Results show, as expected, that the change in BASDAI score for respondents is more negative than the mean change score (–3.86 for the 42% predicted anti-TNF responders vs. –2.63 for all anti-TNF users; in the control arm, responders were predicted to have a change score of –2.70 vs. –0.66 for all participants). Non-respondents were still expected to have a negative change score in both arms revealing some level of symptom control but this was lower than the mean (–1.73 vs. –2.63 for anti-TNF users and –0.45 vs. –0.66 in control arm). The baseline BASDAI and BASFI were predicted to be lower for respondents than non-respondents (e.g. the BASDAI baseline for responders to treatment was 4.76 in respondents when the group baseline was 6.08).
We requested the conditional data from the pivotal trials in AS from each manufacturer. These data were subsequently provided by all manufacturers for their pivotal trials. Conditional scores observed in the trials are summarised in Table 78. The results show that there are some differences between the conditional results predicted using the synthesis and the ones observed in trials. Differences are especially relevant for the conditional baseline scores; although the synthesis model predicts, for example, that treated patients that respond have a baseline BASDAI score of 4.76 and those that do not respond have a baseline score of 7.03, the trials show much smaller differences. Despite incorporating all evidence available at the aggregate level, the predictive ability of the conditional baseline score from the synthesis could only be improved if we had access to the individual patient data, as this methodology is strongly dependent on assumptions over the distribution of scores across patients.
| BASDAI | BASFI | |||
|---|---|---|---|---|
| Control | Treatment | Control | Treatment | |
| ATLAS61 trial (adalimumab, studies = 4) | ||||
| % responders to BASDAI 50 | 0.16 | 0.46 | ||
| Change in score | ||||
| Conditional on response | –4.5 | –4.64 | –2.74 | –2.92 |
| Conditional on non-response | –0.2 | –0.82 | –0.17 | –0.72 |
| Total | –0.90 | –2.58 | –0.59 | –1.73 |
| Baseline | ||||
| Conditional on response | 6.31 | 6.14 | 4.50 | 4.53 |
| Conditional on non-response | 6.37 | 6.35 | 5.91 | 5.78 |
| Total | 6.36 | 6.25 | 5.68 | 5.21 |
| GO-RAISE90 (golimumab, studies = 12) | ||||
| % responders to BASDAI 50 | 0.15 | 0.46 | ||
| Change in score | ||||
| Conditional on response | –4.25 | –4.74 | –1.80 | –3.03 |
| Conditional on non-response | –0.18 | –1.22 | 0.38 | –0.53 |
| Total | –0.81 | –2.84 | 0.05 | –1.68 |
| Baseline | ||||
| Conditional on response | 6.52 | 6.25 | 3.56 | 4.45 |
| Conditional on non-response | 6.63 | 6.69 | 5.39 | 5.48 |
| Total | 6.61 | 6.49 | 5.11 | 5.01 |
| RAPID-axSpA64 (certolizumab, studies = 5) | ||||
| % responders to BASDAI 50 | CiC information has been removed | CiC information has been removed | ||
| Change in score | ||||
| Conditional on response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Conditional on non-response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Total | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Baseline | ||||
| Conditional on response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Conditional on non-response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Total | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| 314-EU study167 (etanercept, studies = 11)a | ||||
| % responders to BASDAI 50 | CiC information has been removed | CiC information has been removed | ||
| Change in score | ||||
| Conditional on response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Conditional on non-response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Total | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Baseline | ||||
| Conditional on response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Conditional on non-response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Total | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
Results for non-radiographic axial spondyloarthritis
The conditional results were also predicted for the nr-AxSpA population using both scenarios implemented of the synthesis model.
For this population, conditional data were provided by only two manufacturers (Pfizer and AbbVie). Conditional scores observed are summarised in Tables 79 and 80.
| BASDAI | BASFI | |||
|---|---|---|---|---|
| Control | Treatment | Control | Treatment | |
| Scenario 1 | ||||
| % responders to BASDAI 50 | AiC information has been removed | AiC information has been removed | ||
| Change in score | AiC information has been removed | AiC information has been removed | ||
| Responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Non-responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| All | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Baseline | AiC information has been removed | AiC information has been removed | ||
| Responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Non-responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| All | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Scenario 2 | ||||
| % responders to BASDAI 50 | AiC information has been removed | AiC information has been removed | ||
| Change in score | AiC information has been removed | AiC information has been removed | ||
| Responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Non-responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| All | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Baseline | AiC information has been removed | AiC information has been removed | ||
| Responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Non-responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| All | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| BASDAI | BASFI | |||
|---|---|---|---|---|
| Control | Treatment | Control | Treatment | |
| ABILITY-158 trial (adalimumab, studies = 18) | ||||
| % responders to BASDAI 50 | 0.14 | 0.40 | ||
| Change in score | ||||
| Conditional on response | –3.9 | –4.79 | –2.78 | –2.75 |
| Conditional on non-response | –0.69 | –0.55 | –0.40 | –0.32 |
| Total | –1.16 | –2.23 | –0.75 | –1.29 |
| Baseline | ||||
| Conditional on response | 5.64 | 6.21 | 4.37 | 3.60 |
| Conditional on non-response | 6.46 | 6.53 | 4.91 | 4.97 |
| Total | 6.34 | 6.40 | 4.83 | 4.43 |
| RAPID-axSpA64 (certolizumab, study = 19) | ||||
| % responders to BASDAI 50 | CiC information has been removed | CiC information has been removed | ||
| Change in score | ||||
| Conditional on response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Conditional on non-response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Total | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Baseline | ||||
| Conditional on response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Conditional on non-response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Total | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| EU-1031166 (etanercept, studies = 20) | ||||
| % responders to BASDAI 50 | CiC information has been removed | CiC information has been removed | ||
| Change in score | ||||
| Conditional on response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Conditional on non-response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Total | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Baseline | ||||
| Conditional on response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Conditional on non-response | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Total | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
Prediction results are consistent with those in AS, and the differences between the conditional results predicted using the synthesis and the ones observed in trials are also present in this analysis.
Interpretation/discussion
Conditional scores predicted using synthesis model C differ from those seen in the trials. Differences are probably because of distributional assumptions over the baseline and change scores. Only with access to the individual patient data could such predictions be improved. Note that the synthesis model itself does not rely as heavily on such assumptions, and thus any concerns should not be transposed to the results obtained in Ankylosing spondylitis population and Non-radiographic axial spondyloarthritis population.
Discussion/conclusion
The analyses developed in this section focused on extending the synthesis evidence on the short-term clinical effectiveness of anti-TNF drugs in Chapter 3 that considered individually multiple outcomes of interest reported in the trials, namely the mean change in BASDAI scores at 12 weeks, the proportion of BASDAI 50 responders (i.e. those who had, at 12 weeks, a change in the baseline BASDAI score of 50% or more) and the mean change in BASFI scores at 12 weeks.
Initially, within such a univariate framework, we further explored assumptions over the relative effectiveness of anti-TNFs. We evaluated the possibility of the evidence suggesting treatment effects to be independent, equal or similar effects (treatment effects were assumed to come from a ‘common’ distribution, i.e. a ‘class effect’). Independence was ruled out through statistical checks of goodness of fit; this is in line with the published evidence, in AS, that does not demonstrate one anti-TNF treatment to be significantly more effective than another. The data were as well represented by the other two models. However, unless we believe the equality assumption to hold AND the trials to be homogeneous in design and in the populations included, assuming equality in treatment effects will provide overprecise estimates. For this reason, our preferred assumption was that of similarity; however, it should be noted that this model is not explicit about the source of the differences in the effects of treatments. Whereas heterogeneity may be a plausible explanation, further research needs to examine data at the individual patient level to avoid the potential for ecological bias.
We also extended the synthesis in a way that allowed multiple outcomes to be jointly modelled. We did so by (1) structurally relating the BASDAI-based outcomes, allowing for trials reporting BASDAI 50 to inform BASDAI change scores, and (2) by concomitantly synthesising BASFI outcomes, allowing correlation between outcomes and the borrowing of strength between results to BASDAI and BASFI. For these reasons, the synthesis model developed here more directly addresses the decision problem. It also generates appropriate effect-size estimates and their associated uncertainty to inform the main input parameters of the economic model.
In the decision model, treatment continuation is determined by response to BASDAI 50 at 12 weeks. Given that prognosis, costs and QALYs are determined by absolute BASDAI and BASFI scores, it is important to evaluate the absolute change in BASDAI and BASFI separately for responders and non-responders: that is, the conditional scores. We used the results from the extended synthesis model to develop a simulation model that allowed prediction of the conditional scores. The results obtained differ from those seen in three pivotal trials (data provided by the manufacturers on request), probably because of distributional assumptions over the baseline and change scores. Only with access to the individual-patient data such predictions could be improved.
Chapter 6 Independent economic assessment: York model
Overview
Chapter 4 indicates that there are significant conceptual concerns and uncertainties arising from previously published studies and the submissions made by manufacturers. For this reason, it has been necessary to develop a de novo model (hereafter referred to as the ‘York model’). Although it shares some of the assumptions and parameter estimates from the manufacturer models, it has a different conceptual structure and applies a more generalised framework for the synthesis of data from the double-blind periods of existing RCTs, combined with a more explicit approach to modelling the progressive nature of AS and nr-AxSpA and the potential impact of the anti-TNFs.
The aim of the York model is to assess the cost-effectiveness of adalimumab, certolizumab, etanercept, golimumab and infliximab, in accordance with their respective licences, for the treatment of AS and nr-AxSpA. The model uses short-term trial data, based on the extended evidence synthesis, to model the response of patients to TNF-α inhibitor therapy at 12 weeks based on BASDAI 50 measured in the trials. In contrast to the models submitted by the manufacturers, the York model is based on an assumption of similar (but not identical) effects for the alternative biologics based on the results of the extended synthesis reported in Chapter 5.
In common with all existing cost-effectiveness studies, measures of disease activity (BASDAI) and functioning (BASFI) are used to characterise the chronic, progressive nature of AS and nr-AxSpA and the effect of anti-TNFs. However, the York model uses an alternative conceptual model applied to estimate longer-term BASFI scores. The effect of response to TNF-α therapy is modelled in terms of the short- and longer-term impact on BASDAI and BASFI scores.
The NHS and PSS costs are based on the cost of the TNF-α therapies (acquisition, administration and monitoring) and disease costs linked to BASFI scores. HRQoL, in terms of utility, is based on both BASDAI and BASFI scores. Health effects are subsequently expressed in terms of QALYs. Both costs and QALYs are discounted at 3.5% per annum. Costs are presented based on current prices.
The model is developed in accordance with the NICE reference case. The model has a lifetime horizon (60 years) and considers costs from the perspective of the NHS and PSS.
Contribution of the York model
Although the York model shares some of the assumptions and parameters from existing studies and manufacturer’s submissions, it also provides a number of significant developments to existing cost-effectiveness analyses. First, the short-term clinical effectiveness inputs are based on an evidence-synthesis approach which is based on all available trial data for each biological therapy and which jointly synthesises ‘related’ parameters ensuring uncertainty is more appropriately characterised. Second, the evidence-synthesis approach is more explicitly linked to the decision problem and the requirements of the economic model, that is the model requires estimates of response and the impact on BASDAI/BASFI conditional upon this. As the conditional response scores are not conventionally reported in existing publications, existing models have largely been based on selective approaches (i.e. using conditional scores from single studies or assumptions) or appear to have ignored the conditional scores entirely and instead use estimates from longer-term follow-up and/or open-label sources (i.e. implicitly assuming that patients who continue to participate in longer-term follow-up and open-label sources are more likely to be responders than patients who do not). Neither approach appears satisfactory in terms of meeting the requirements of the economic model and ensuring that all relevant evidence is considered. The evidence synthesis approach which underpins the York model is based on a joint synthesis of related parameters which makes fuller use of existing evidence and which can more appropriately estimate the input parameters which are required to populate existing models and better characterise the uncertainty surrounding these.
Another important development of the York model is the approach to modelling longer-term BASFI changes over time to characterise the progressive nature of AS and nr-AxSpA. In previous sections we highlighted our concerns over how this has been previously modelled and the implicit assumptions underlying the effect of anti-TNFs (i.e. potential disease modification properties resulting in halting further ‘progression’, or reducing the rate of progression, while patients respond and continue to receive anti-TNFs). Within the York model, we attempt to model the impact of different processes on BASFI over time, relating the changes more explicitly to the existing clinical effectiveness data for anti-TNFs on these different processes. Specifically, we consider the independent effects on BASFI because of disease activity (BASDAI) and the extent and progression of radiographic disease (as measured by the mSASSS) for AS. For the nr-AxSpA population, we assume a similar underlying clinical process relating to BASFI.
This approach confers several advantages over current approaches by linking changes in BASFI to a more explicit clinical/biological process and facilitating a more formal consideration of the potential impact of anti-TNFs on BASFI, via the specific effects these drugs have on the different processes which independently relate to this parameter. This approach allows consideration of the impact on BASFI that might be achieved via symptomatic improvements (i.e. in terms of reductions in disease activity) and those which might be conferred by disease modification properties (i.e. the effect on the likelihood and/or rate of further radiographic progression). The latter aspect is particularly important given the increasing amount of published evidence reported on the potential impact of anti-TNFs on radiographic progression which has not been formally considered or incorporated within existing cost-effectiveness studies.
Comparators
Table 81 summarises the comparators included in each of the populations, in line with the relevant existing (or likely to be granted by the time of the NICE appraisal) marketing authorisations for each manufacturer.
| Comparator | Manufacturer | AS | nr-AxSpA |
|---|---|---|---|
| CC | – | Yes | Yes |
| Adalimumab | AbbVie | Yes | Yes |
| Certolizumab | UCB | Yes | Yes |
| Etanercept | Pfizer | Yes | Yes |
| Golimumab | Merck Sharp & Dohme | Yes | No |
| Infliximab | Merck Sharp & Dohme | Yes | No |
Model structure
The York model is a cohort model and takes the form of a modified decision tree for AS and nr-AxSpA. A simplified version of the structure is shown in Figure 15. A similar structure has been previously been used to evaluate the cost-effectiveness of anti-TNFs in psoriatic arthritis. 175
FIGURE 15.
A simplified schematic of the York model structure.
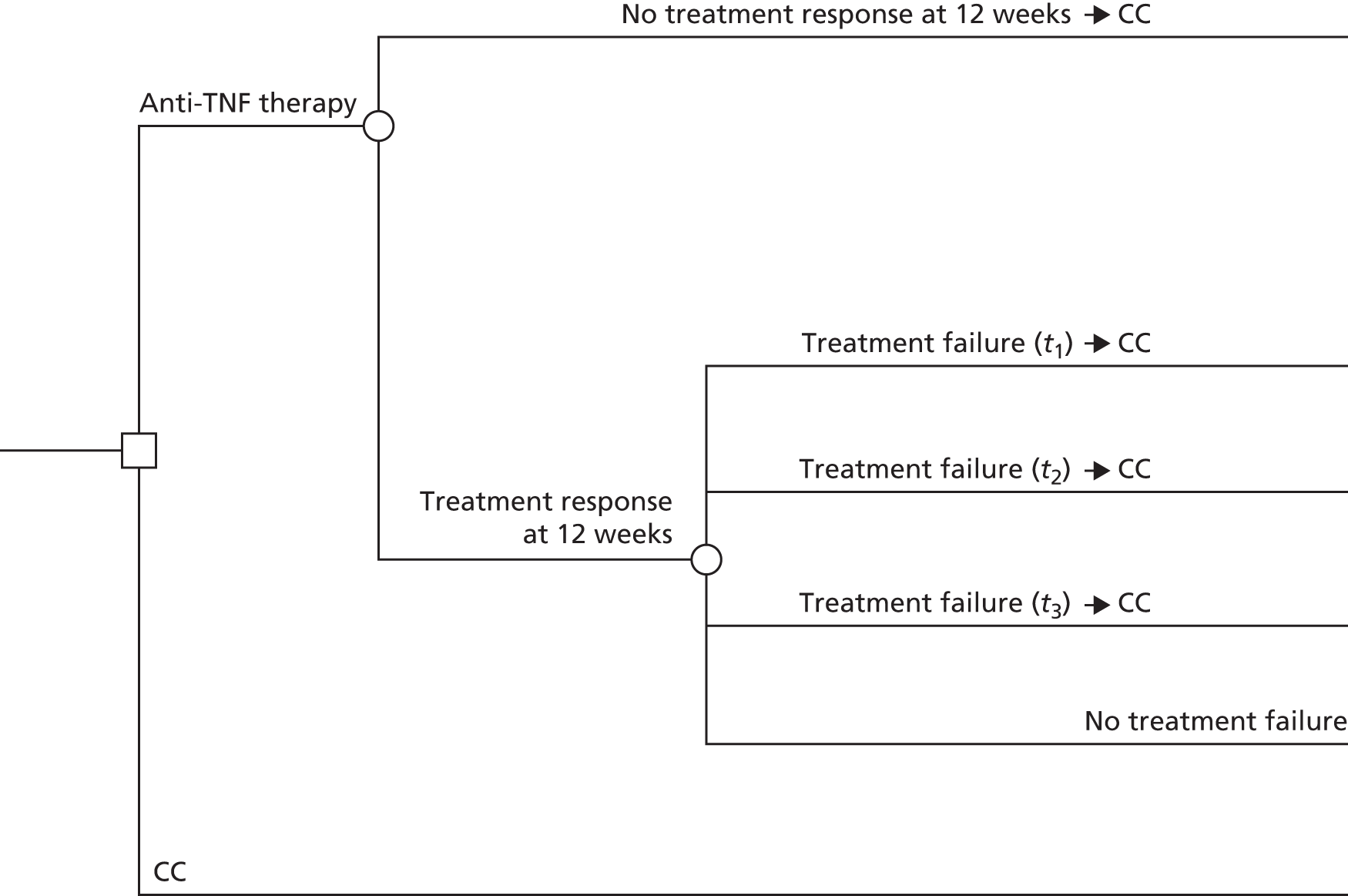
For the alternative TNF-α inihibitors, initial response is determined on the basis of a short-term BASDAI 50 response (12 weeks). For those who respond, there is then an ongoing risk of withdrawal of treatment at any time point in the model. Initial or later treatment failures are assumed to move on to CC. The use of BASDAI 50 is consistent with existing BSR guidelines and previous NICE appraisals for AS. 17,33,169 Ensuring consistency in the response measure between the various appraisals provides a more comparable basis for exploring any subsequent differences in results. In addition, using BASDAI 50 as a response measure for the economic model maximises the evidence base used to inform the various clinical-effectiveness parameters required and, as outlined in Chapter 5, uses the same clinical constructs to inform response and subsequent BASDAI score changes.
Those patients who receive anti-TNFs will experience an initial improvement which is based on results of the evidence synthesis (average of mean change in BASDAI and BASFI scores estimated for responders and non-responders). From week 12, patients who continue to receive anti-TNFs are assigned the conditional mean change in BASDAI and BASFI scores estimated from the evidence synthesis which is assumed to remain constant for the treatment duration period. In addition to this initial improvement in BASDAI and BASFI, patients continuing on anti-TNFs treatment are also assumed to experience a slower progression rate in BASFI as long as they are responding (see Longer-term discontinuation).
Patients who fail on TNF-α inhibitor therapy after the initial (12-week) period will experience some form of rebound in terms of BASDAI/BASFI score, but the trial data are too short term to be able to characterise this accurately. The model, therefore, considers two rebound scenarios:
-
Rebound equal to gain (BASDAI and BASFI). When patients fail therapy (after initially responding), their BASDAI and BASFI deteriorates by the same amount by which it improves when they responded to therapy.
-
Rebound back to natural history/CC (BASFI only). When patients fail therapy (after initially responding), their BASFI deteriorates to the level and subsequent trajectory it would have been had they not initially responded to therapy. As BASDAI is not assumed to progress over time on CC, the same assumptions are applied to BASDAI in both scenarios.
Given the absence of evidence on rebound, both scenarios (rebound equal to gain and rebound back to natural history) are presented as the ‘best-case’ and ‘worst-case’ scenarios possible. In other words, the reality regarding rebound is likely to be somewhere between these two scenarios which should, therefore, be seen as the limits.
Importantly, the York model explores the impact of assuming different baseline BASDAI and BASFI scores for responders and non-responders. Hence, in contrast to existing models, the York model assumes that response is unlikely to be independent of baseline patient characteristics and hence the baseline characteristics of responders/non-responders to anti-TNFs may be systematically different from each other. Importantly, the results from the extended synthesis model estimated higher baseline BASDAI and BASFI scores for non-responders vis-à-vis responders and a similar relationship was also reported by those manufacturers who provided conditional response data requested by the assessment group. Consequently, assuming that non-responders revert back to the ‘average’ of the baseline BASDAI/BASFI score of all patients randomised to receive TNF-α inhibitor treatment, or the ‘average’ of patients receiving CC, is likely to be overly optimistic towards the subsequent cost-effectiveness of anti-TNFs. The model thus use different baselines for responders and non-responders (at 12 weeks) and at the point of discontinutation patients are assumed to revert to their respective baseline BASDAI and BASFI scores (i.e. at 12 weeks non-responders revert back to the non-responder baseline and after 12 weeks patients who subsequently discontinue from their TNF-α therapy revert back to their responder baseline). The impact of using these data is explored as part of the sensitivity analysis.
Patients are at risk of all-cause mortality at every time point in the model but no differential mortality risk between the therapies being evaluated. Aside from the cost of the TNF-α therapies themselves (i.e. acquisition, administration, monitoring and AEs), all other costs of AS and nr-AxSpA are assumed to vary according to BASFI score. Costs are presented based on current prices. HRQoL (in terms of utility) is implemented as a function of BASDAI and BASFI scores.
Model input parameters
The parameter estimates used in the York model, together with their sources, are detailed in Tables 82 and 83.
| Parameter | Mean value | SE | Distribution | Source |
|---|---|---|---|---|
| Annual discount rate costs/QALYs | 3.5% | – | Fixed | – |
| Time horizon (years) | 60 | – | Fixed | – |
| Cycle length (years) | 0.25 | – | Fixed | – |
| Baseline patient characteristics | ||||
| Average age (years) | 40 | – | Fixed | Assumption |
| Proportion male | 0.7 | – | Fixed | Assumption |
| Average weight (kg) | 73 | – | Fixed | Assumption |
| Average baseline BASDAI | 6.12 | N/A | Derived from responder and non-responder baseline | Evidence synthesis (see Chapter 5) |
| Average baseline BASFI | 5.28 | N/A | ||
| Baseline BASDAI CC responders | 4.01 | N/A | From evidence synthesis | Evidence synthesis (see Chapter 5) |
| Baseline BASDAI CC non-responders | 6.33 | N/A | From evidence synthesis | |
| Baseline BASFI CC responders | 3.52 | N/A | From evidence synthesis | |
| Baseline BASFI CC non-responders | 5.46 | N/A | From evidence synthesis | |
| Baseline BASDAI anti-TNF responders | 4.80 | N/A | From evidence synthesis | |
| Baseline BASDAI anti-TNF non-responders | 7.08 | N/A | From evidence synthesis | |
| Baseline BASFI anti-TNF responders | 4.20 | N/A | From evidence synthesis | |
| Baseline BASFI anti-TNF non-responders | 6.07 | N/A | From evidence synthesis | |
| Response (12-week BASDAI 50) | ||||
| Anti-TNF | 42.0% | N/A | From evidence synthesis | Evidence synthesis (see Chapter 5) |
| Conventional therapy | 9.1% | N/A | From evidence synthesis | |
| Treatment effect | ||||
| Initial BASDAI Change| Tx response: anti-TNF | –3.86 | N/A | From evidence synthesis | Evidence synthesis (see Chapter 5) |
| Initial BASDAI Change| Tx response: CC | –2.89 | N/A | From evidence synthesis | |
| Initial BASDAI Change| Tx no response: anti-TNF | –1.64 | N/A | From evidence synthesis | |
| Initial BASDAI Change| Tx no response: CC | –0.36 | N/A | From evidence synthesis | |
| Initial BASFI Change| Tx response: anti-TNF | –3.08 | N/A | From evidence synthesis | |
| Initial BASFI Change| Tx response: CC | –1.72 | N/A | From evidence synthesis | |
| Initial BASFI Change| Tx no response: anti-TNF | –0.44 | N/A | From evidence synthesis | |
| Initial BASFI Change| Tx no response: CC | –0.04 | N/A | From evidence synthesis | |
| Long-term annual BASFI progression | ||||
| BASFI annual progression anti-TNF | 0.034 | – | Derived from probabilistic inputs (the following four rows) | – |
| BASFI annual progression CC | 0.082 | – | – | |
| Annual rate of mSASSS change for mSASSS ≥ 10 | 1.44 | 0.133 | Normal | Ramiro et al. (2013)148 |
| BASFI change with 1 unit change in mSASSS | 0.057 | 0.0049 | Normal | Landewe et al. (2009)10 |
| Treatment effect on progression (relative risk) | 0.42 | 0.122 | Normal | Haroon et al. (2013)121 |
| Time to treatment effect (years) | 4 | – | – | Haroon et al. (2013),121 Baraliakos et al. (2014)122 |
| Long-term annual BASDAI progression | ||||
| BASDAI annual progression anti-TNF | 0 | N/A | ||
| BASDAI annual progression CC | 0 | N/A | ||
| Annual withdrawal probability | ||||
| Constant rate of annual withdrawal | 0.11 | AiC information has been removed | Log-normal; from exponential model [coefficient (AiC information has been removed); SE (AiC information has been removed)] | Pfizer submission34 |
| Mortality | ||||
| SMR women | 1.38 | 0.163 | Normal | Bakland (2011)16 |
| SMR men | 1.63 | 0.163 | Normal | Bakland (2011)16 |
| Quality of life | ||||
| Intercept | (AiC information has been removed) | Uncertainty from reported variance–covariance matrix | Multivariate normal | Pfizer submission34 |
| BASDAI coefficient | (AiC information has been removed) | Multivariate normal | ||
| BASFI coefficient | (AiC information has been removed) | Multivariate normal | ||
| BASDAI2 coefficient | (AiC information has been removed) | Multivariate normal | ||
| BASFI2 coefficient | (AiC information has been removed) | Multivariate normal | ||
| Initial 12-week period costs (drug + initiation + administration) | ||||
| Adalimumab | 2422 | – | Fixed | As discussed in Resource use and costs |
| Certolizumab pegol | 3884 | – | Fixed | |
| Etanercept | 2454 | – | Fixed | |
| Golimumab | 2415 | – | Fixed | |
| Infliximab | 6878 | – | Fixed | |
| Certolizumab pegol PAS | 309 | – | Fixed | |
| Subsequent 12-week costs (drug + monitoring + administration) | ||||
| Adalimumab | 2171 | – | Fixed | As discussed in Resource use and costs |
| Certolizumab pegol | 2203 | – | Fixed | |
| Etanercept | 2203 | – | Fixed | |
| Golimumab | 2164 | – | Fixed | |
| Infliximab | 3435 | – | Fixed | |
| Certolizumab pegol PAS | 2203 | – | Fixed | |
| Disease-related costs: annual | ||||
| Intercept | 1284 | 0.165 | Log-normal | OASIS data,118 AbbVie submission34 |
| BASFI coefficient | 0.213 | 0.038 | Normal | |
| AE costs (£ per patient) | ||||
| Year 1 | 18.2 | – | Fixed | Excess rates for anti-TNFs from Cochrane review,137 costs from NHS Reference Costs 2012/13176 |
| Subsequent years | 0 | – | Fixed | |
| Parameter | Mean value | SE | Distribution | Source |
|---|---|---|---|---|
| Annual discount rate costs/QALYs | 3.5% | – | Fixed | – |
| Time horizon (years) | 60 | – | Fixed | – |
| Cycle length (years) | 0.25 | – | Fixed | – |
| Baseline patient characteristics | ||||
| Average age (years) | 40 | – | Fixed | Assumption |
| Proportion male | 0.5 | – | Fixed | Assumption |
| Average weight (kg) | 73 | – | Fixed | Assumption |
| Average baseline BASDAI | 6.42 | N/A | Derived from responder and non-responder baseline | Evidence synthesis (see Chapter 5) |
| Average baseline BASFI | 4.92 | N/A | ||
| Baseline BASDAI CC responders | 4.54 | N/A | From evidence synthesis | Evidence synthesis (see Chapter 5) |
| Baseline BASDAI CC non-responders | 6.86 | N/A | From evidence synthesis | |
| Baseline BASFI CC responders | 2.95 | N/A | From evidence synthesis | |
| Baseline BASFI CC non-responders | 5.38 | N/A | From evidence synthesis | |
| Baseline BASDAI anti-TNF responders | 5.45 | N/A | From evidence synthesis | |
| Baseline BASDAI anti-TNF non-responders | 7.51 | N/A | From evidence synthesis | |
| Baseline BASFI anti-TNF responders | 3.92 | N/A | From evidence synthesis | |
| Baseline BASFI anti-TNF non-responders | 6.04 | N/A | From evidence synthesis | |
| Response (12-week BASDAI 50) | ||||
| Anti-TNF | 52.9% | N/A | From evidence synthesis | Evidence synthesis (see Chapter 5) |
| Conventional therapy | 18.9% | N/A | From evidence synthesis | |
| Treatment effect | ||||
| Initial BASDAI Change| Tx response: anti-TNF | –4.31 | N/A | From evidence synthesis | Evidence synthesis (see Chapter 5) |
| Initial BASDAI Change| Tx response: CC | –3.34 | N/A | From evidence synthesis | |
| Initial BASDAI Change| Tx no response: anti-TNF | –2.28 | N/A | From evidence synthesis | |
| Initial BASDAI Change| Tx no response: CC | –1.06 | N/A | From evidence synthesis | |
| Initial BASFI Change| Tx response: anti-TNF | –3.24 | N/A | From evidence synthesis | |
| Initial BASFI Change| Tx response: CC | –1.88 | N/A | From evidence synthesis | |
| Initial BASFI Change| Tx no response: anti-TNF | 0.08 | N/A | From evidence synthesis | |
| Initial BASFI Change| Tx no response: CC | –0.05 | N/A | From evidence synthesis | |
| Long-term annual BASFI progression | ||||
| BASFI annual progression anti-TNF | 0.017 | Derived from probabilistic inputs (the following four rows) | – | |
| BASFI annual progression CC | 0.039 | – | ||
| Annual rate of mSASSS change for mSASSS < 10 | 0.69 | 0.031 | Normal | Ramiro et al. (2013)148 |
| BASFI change with 1 unit change in mSASSS | 0.057 | 0.0049 | Normal | Landewe et al. (2009)10 |
| Treatment effect on progression (relative risk) | 0.42 | 0.122 | Normal | Haroon et al. (2013)121 |
| Time to treatment effect (years) | 4 | – | – | Haroon et al. (2013),121 Baraliakos et al. (2014)122 |
| Long-term annual BASDAI progression | ||||
| BASDAI annual progression anti-TNF | 0 | N/A | – | – |
| BASDAI annual progression CC | 0 | N/A | – | – |
| Annual withdrawal probability | ||||
| Constant rate of annual withdrawal | 0.06 | (AiC information has been removed) | Log-normal; from exponential model [coefficient (AiC information has been removed); SE (AiC information has been removed)] | Pfizer submission36 |
| Mortality | ||||
| SMR women | 1.38 | 0.163 | Normal | Bakland (2011)16 |
| SMR men | 1.63 | 0.163 | Normal | Bakland (2011)16 |
| Quality of life | ||||
| Intercept | AiC information has been removed | Uncertainty from reported variance–covariance matrix | Multivariate normal | Pfizer submission36 |
| BASDAI coefficient | AiC information has been removed | Multivariate normal | ||
| BASFI coefficient | AiC information has been removed | Multivariate normal | ||
| Male coefficient | AiC information has been removed | Multivariate normal | ||
| Age coefficient | AiC information has been removed | Multivariate normal | ||
| BASDAI2 coefficient | AiC information has been removed | Multivariate normal | ||
| BASFI2 coefficient | AiC information has been removed | Multivariate normal | ||
| BASFI × BASDAI coefficient | AiC information has been removed | Multivariate normal | ||
| Initial 12-week period costs (drug + initiation + administration) | ||||
| Adalimumab | 2573 | – | Fixed | As discussed in Resource use and costs |
| Certolizumab pegol | 4035 | – | Fixed | |
| Etanercept | 2606 | – | Fixed | |
| Golimumab | 2566 | – | Fixed | |
| Infliximab | 7213 | – | Fixed | |
| Certolizumab pegol PAS | 460 | – | Fixed | |
| Subsequent 12-week costs (drug + monitoring + administration) | ||||
| Adalimumab | 2177 | – | Fixed | As discussed in Resource use and costs |
| Certolizumab pegol | 2210 | – | Fixed | |
| Etanercept | 2210 | – | Fixed | |
| Golimumab | 2170 | – | Fixed | |
| Infliximab | 3441 | – | Fixed | |
| Certolizumab pegol PAS | 2210 | – | Fixed | |
| Disease-related costs: annual | ||||
| Intercept | 1284 | 0.165 | Log-normal | OASIS data,118 AbbVie submission34 |
| BASFI coefficient | 0.213 | 0.038 | Normal | |
| AE costs (£ per patient) | ||||
| Year 1 | 18.2 | – | Fixed | Excess rates for anti-TNFs from Cochrane review,137 costs from NHS Reference Costs 2012/13176 |
| Subsequent years | 0 | – | Fixed | |
Baseline patient characteristics
Baseline characteristics applied to the AS and nr-AxSpA populations are summarised in Tables 82 and 83, respectively.
Response, change in Bath Ankylosing Spondylitis Disease Activity Index/Bath Ankylosing Spondylitis Functional Index and conditional baselines
The BASDAI 50 response, the conditional change scores for BASDAI and BASFI at 12 weeks and the separate conditional baselines estimated for BASDAI and BASFI (responders vs. non-responders) were derived directly from the results of the extended synthesis model reported in Chapter 5. In the base case, it was assumed that the percentage of BASDAI 50 responders, change in BASDAI/BASFI and conditional baselines were the same for all anti-TNFs. The outputs [CODA (Convergence Diagnostic and Output Analysis) file format] from the simulations were incorporated directly into the model to maintain correlation and to avoid any additional distributional assumptions.
Longer-term Bath Ankylosing Spondylitis Functional Index progression
As previously highlighted in the overview section, the York model attempts to address some of the conceptual concerns outlined in Chapter 4 surrounding the assumptions applied within existing models in relation to modelling BASFI progression over time. Specifically, we assume that BASFI is a function of separate processes which are independently related to disease severity/activity (BASDAI) and to the extent and subsequent progression of radiographic disease (mSASSS). The rationale for this is that the association between BASDAI and BASFI is already accounted for in the separate mean change scores applied to both BASDAI and BASFI for responders versus non-responders/CC patients. Differences in BASDAI are assumed to remain constant over the longer-term horizon (an assumption which is common across all models). Hence any additional changes which might affect BASFI need to be more explicitly related to a separate clinical process (or processes). Based on the studies included in the reviews reported in Chapter 3 for natural history (see Review of natural history of ankylosing spondylitis and non-radiographic axial spondyloarthritis) and the effect on anti-TNFs on radiographic progression (see Effect of anti-tumour necrosis factors on radiographic progression), we modelled longer-term changes in BASFI (for CC and anti-TNFs) as a function of mSASSS scores.
The approach applied in the AS population is based on the following studies and assumptions:
-
The multivariate relationship reported in Landewe et al. ,10 based on longitudinal assessments of BASFI, BASDAI and mSASSS, was used to estimate the independent effect of a 1-unit change in mSASSS on BASFI scores (mean 0.057 units, SE 0.0049 units).
-
Data from a 12-year prospective follow-up of the OASIS study was used to estimate the annual rate of change in mSASSS. Although at the individual level progression of mSASSS is highly variable, the study by Ramiro et al. 124 demonstrated that at a group level (i.e. akin to the cohort approach applied in the York model) changes in mSASSS were stable, progressing at an annual rate of 0.98 mSASSS units per year. 125 Combining the estimates reported across the studies implies a change in BASFI of 0.056 units per annum (0–10 scale). However, as the population included in the study by Ramiro et al. 124 included patients who would not be eligible to receive anti-TNFs, we used data in the subgroup of patients with baseline mSASSS ≥ 10 units. The annual rate of mSASSS progression in this subgroup was 1.44 units (95% CI 1.18 to 1.70 units) per year with an implied annual BASFI score change of 0.082 units per year. This compares with an annual change of BASFI score of between 0.056 and 0.07 units assumed across the manufacturer’s submissions. The specific subgroup (mSASSS ≥ 10 units) was chosen to reflect that AS patients eligible to receive anti-TNFs are likely to be more similar to this subgroup than the entire cohort reported by Ramiro et al. 124 This also provided a basis for differentiating between the AS and nr-AxSpA populations which is discussed in the following section.
Given the uncertainties noted in Chapter 3 (see Effect of anti-tumour necrosis factors on radiographic progression), surrounding the effect of anti-TNFs on radiographic progression, we explored alternative scenarios in the decision model. In the base case, we assumed that the effect was related to the duration of therapy which has been reported in recent studies by Haroon et al. 121 and Baraliakos et al. 122 Both studies consistently reported evidence that the difference in mSASSS between patients who received anti-TNFs and historical controls became different only in patients who had received treatment for approximately 4 years or more. In the absence of any relative-effect measure reported by Baraliakos et al. ,122 we used results reported by Haroon et al. 121 applying a zero-inflated binomial model with a relative rate of mSASSS change of 0.42 units (95% CI 0.18 to 0.98 units). Hence, in the model, no effect on mSASSS was assumed until year 4 of the model and then only applied to patients who continued to receive therapy beyond this period.
-
Given the inherent uncertainties regarding the effect of anti-TNFs on radiographic progression we explored alternative scenarios based on (1) an assumption of no impact on radiographic progression; and (2) an immediate effect, applying the estimate of 0.42 from the outset.
For the nr-AxSpA population, we assume a similar underlying clinical process relating to BASFI but model separate BASFI processes for patients depending upon the probability of developing radiographic disease over time and thereafter modelling the extent and progression of radiographic disease via mSASSS changes. Hence, our intention in the nr-AxSpA model was to employ a constant BASFI score (on and off treatment) until a patient develops radiographic progression. At the time point of ‘progression’ an increasing BASFI would be assumed using a similar approach applied to the AS population. However, programming the additional transition to allow separate BASFI progression estimates based on the time of progression (and time since progression for patients who had previously progressed) proved more complex than anticipated. Consequently, a more simplified assumption was made such that all patients were assumed to incur progression in BASFI albeit at a lower rate relative to the AS population.
The approach we intended to apply in the nr-AxSpA population was based on the following studies and assumptions:
-
Poddubnyy et al. 155 is used to estimate the probability of nr-AxSpA patients progressing to radiographic disease based on the outcome ‘% progressed by ≥ 2 mSASSS over 2 years’ (7.4%) reported. These estimates are converted into a rate to estimate the cycle-specific probability.
Following progression, the mSASSS scores of patients are subsequently assumed to increase at a rate of 0.69 units (95% CI 0.63 to 0.75 units) per year, based on the subgroup of patients with baseline mSASSS < 10 reported by Ramiro et al. 148 BASFI is assumed to remain constant for patients who do not progress in each cycle of the model.
The same results reported by Haroon et al. ,121 applying a zero-inflated binomial model with a relative rate of mSASSS change of 0.42 units (95% CI 0.18 to 0.98 units), were applied to the mSASSS scores for patients who progressed to estimate the treatment effect of anti-TNFs. Hence, in common with the AS model, no effect on mSASSS was assumed until year 4 and then it was only applied to patients who continued to receive therapy beyond this period.
-
Given the inherent uncertainties regarding the effect of anti-TNFs on radiographic progression we explored alternative scenarios based on (1) an assumption of no impact on radiographic progression and (2) an immediate effect – applying the estimate of 0.42 from the outset. We also considered an exploratory scenario where we assumed no radiographic progression for nr-AxSpA for patients receiving anti-TNFs, to investigate the untested hypothesis that early intervention in patients, prior to established radiographic disease, might halt subsequent progression.
Given the additional programming challenges that could not be overcome within the remaining time and funding constraints, the mSASSS scores of all nr-AxSpA patients were assumed to increase at the rate of 0.69 units per year. Hence the subsequent results reported for the nr-AxSpA population are potentially optimistic, as not all patients will develop radiographic progression. However, the use of mSASSS in this context inevitably represents an uncertain proxy process for BASFI changes. Further, it should also be noted that the BASFI trajectory of nr-AxSpA patients has been reported in publications to be similar to early AS patients. 177 Consequently, applying the change in mSASSS reported in the subgroup of patients with baseline mSASSS < 10 reported by Ramiro et al. 148 may not be an unreasonable proxy for the purposes of predicting future changes in BASFI over longer periods.
Longer-term discontinuation
Patients who achieve a response at 12 weeks are subsequently assumed to remain on that treatment until the treatment is discontinued (i.e. because of loss of efficacy or AEs) and hence the evidence required to inform the decision model is the post-12 week withdrawal data for responders. The rationales for this are (1) that discontinuation for lack of efficacy is higher during the first 3 months, and this has already been accounted for in the model using the probability of no BASDAI 50 response during the initial treatment period; and (2) that discontinuation rates in responders may differ from withdrawal rates in studies which potentially include both responders and non-responders. Although Chapter 3, Drug survival and anti-tumour necrosis factor switching identified 12 studies reporting on longer-term drug survival from registries, none of these appears to directly inform the model requirements (i.e. either including the initial 3-month period and/or not being specific to responders).
The most relevant estimates appeared to be those presented in previous and current submissions by the manufacturers. Three alternative approaches and sources were identified which appeared to meet the requirements of the economic model. These included:
-
A constant annual probability of 15% applied in the study by Kobelt et al. ,160 based on data from infliximab responders (BASDAI 50) reported as part of the 2nd year of the open-label extension period of the Braun trial (n = 18).
-
Separate time-dependent estimates of the probability for AS and nr-AxSpA reported in the AbbVie submission. 34 These estimates were based on a parametric function (log-normal distribution) estimated from responders (ASAS 20 for AS and ASAS 40 for nr-AxSpA at week 12) from the open-label extensions of ATLAS61 (up to 260 weeks; n = not stated) and ABILITY-158 (up to 156 weeks; n = 28).
-
A constant annual estimate (approximately 5% for nr-AxSpA and 11% for AS) reported in the Pfizer submission. 36 These estimates were based on a parametric function (exponential distribution) estimated from responders (BASDAI 50 at week 12) from the open-label extensions of studies 311-EU,83–85 312-EU146 and 907-EU147 (up to approximately 250 weeks for 311-EU; n = not stated) for the AS population and 1031 study166 (up to approximately 110 weeks; n = 46) for the nr-AxSpA population.
Figure 16 provides a comparison of the different estimates in terms of the subsequent drug survival over a longer-time horizon for AS.
FIGURE 16.
Comparison of withdrawal rates: AS population.
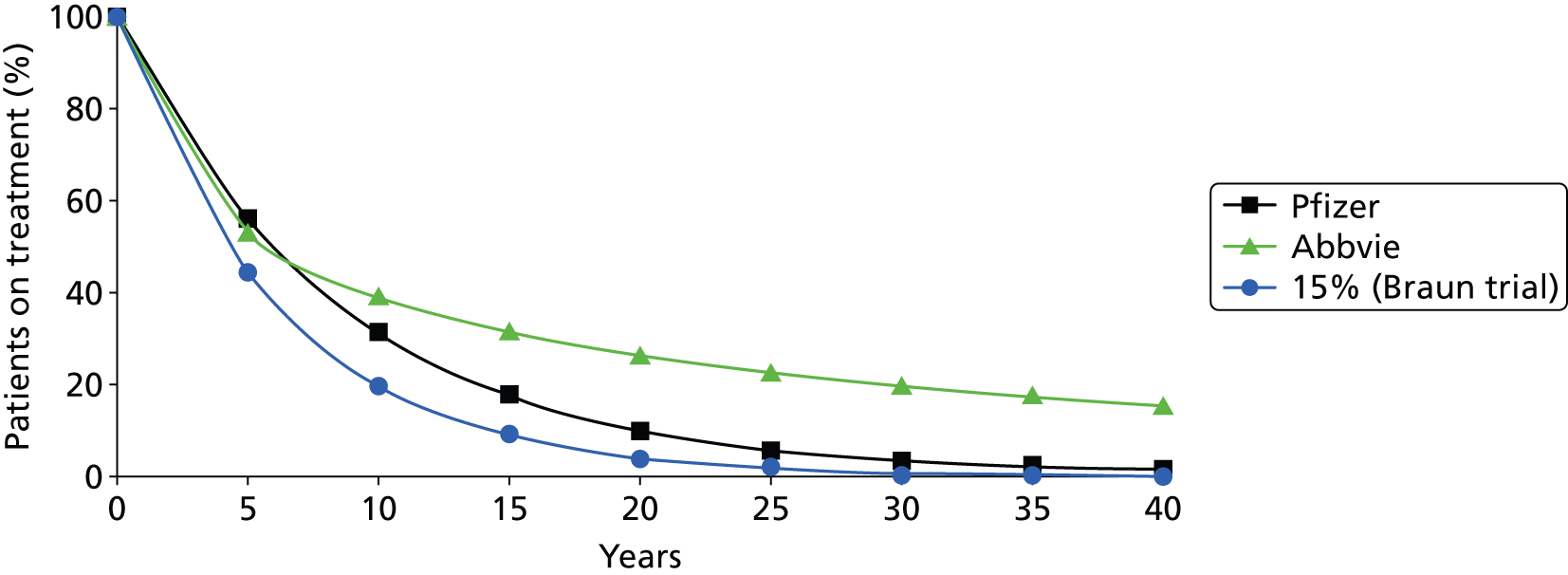
Figure 17 provides a comparison of the different estimates in terms of the subsequent drug survival over a longer-time horizon for nr-AxSpA.
FIGURE 17.
Comparison of withdrawal rates: nr-AxSpA population.

The base case of the York model is based on the estimates reported in the submission by Pfizer for both populations. The justification for this is that (1) the estimates relate to the response end point used in the York model (BASDAI 50); (2) full details were reported by Pfizer concerning the alternative parametric models and associated goodness-of-fit statistics and the exponential model appeared the most appropriate function; and (3) the continued use of a time-dependent function with long tails such as the log-normal distribution results in a significant proportion of patients who would still be assumed to be on TNF-α therapy even after 40 years. Although it is not possible to completely rule out this possibility, the approach by Pfizer was deemed to be a more appropriate basis for informing the York model based on a series of considerations.
Health-related quality of life
The current manufacturer’s submissions are based on alternative mapping algorithms to link BASDAI and BASFI scores to a generic utility measure. The approach used by AbbVie in their base case was based on separate mapping algorithms for the AS and nr-AxSpA populations using data from the ATLAS61 and ABILITY-158 trials, respectively. For the nr-AxSpA population, BASDAI and BASFI were mapped to EQ-5D, whereas the algorithm for the AS population mapped to HUI-3; reflecting the use of different generic utility measures used in the two trials. The approach employed by Pfizer in their base case was similarly based on separate algorithms for each population estimated using data from the 1031 study166 (nr-AxSpA) and the 314-EU study167 (AS) both mapped to EQ-5D. Both regressions were based on the relationships between BASDAI, BASFI and EQ-5D. The approach employed by UCB in their base case was based on the same single-mapping algorithm from the RAPID-axSpA64 trial that included both patient populations. Merck Sharp & Dohme adopted the algorithm reported in McLeod et al. 38
We undertook a separate search for other published utility algorithms and identified only the algorithm reported in Ara et al. ,161 which was based on the cost-effectiveness analysis submitted by Pfizer to NICE for TA143. 17 Full details of the search and an associated review of utility studies are reported in Appendices 1 and 14, respectively. A summary of the alternative algorithms based on EQ-5D is provided in Tables 84 and 85.
| AS | Ara 2007161 | Merck Sharp & Dohme | UCB | Pfizer |
|---|---|---|---|---|
| BASDAI/BASFI scale | 0–10 | 0–10 | 0–10 | 0–100 |
| Regression model | Linear | Linear | Logistic | Non-linear |
| Intercept | 0.92300000 | 0.877213 | AiC information has been removed | AiC information has been removed |
| BASFI | –0.04318800 | –0.032252 | AiC information has been removed | AiC information has been removed |
| BASDAI | –0.04019000 | –0.038409 | AiC information has been removed | AiC information has been removed |
| Male | 0.00000000 | –0.027891 | AiC information has been removed | AiC information has been removed |
| Age | 0.00000000 | 0.001681 | AiC information has been removed | AiC information has been removed |
| BASFI2 | 0.00000000 | 0.000000 | AiC information has been removed | AiC information has been removed |
| BASDAI2 | 0.00000000 | 0.000000 | AiC information has been removed | AiC information has been removed |
| BASFI × BASDAI | 0.00000000 | 0.000000 | AiC information has been removed | AiC information has been removed |
| Nr-axSpA | UCB | AbbVie | Pfizer |
|---|---|---|---|
| BASDAI/BASFI scale | 0–10 | 0–10 | 0–100 |
| Regression model | Logistic | Linear | Non-linear |
| Intercept | AiC information has been removed | 0.9220000 | AiC information has been removed |
| BASFI | AiC information has been removed | –0.0411700 | AiC information has been removed |
| BASDAI | AiC information has been removed | –0.0392400 | AiC information has been removed |
| Male | AiC information has been removed | 0.0000000 | AiC information has been removed |
| Age | AiC information has been removed | 0.0000000 | AiC information has been removed |
| BASFI2 | AiC information has been removed | 0.0000000 | AiC information has been removed |
| BASDAI2 | AiC information has been removed | 0.0000000 | AiC information has been removed |
| BASFI × BASDAI | AiC information has been removed | 0.0000000 | AiC information has been removed |
Figures 18–21 provide a comparison of the utility predictions for each algorithm in each population. For each population, two separate figures are presented. Each figure is based on the impact of holding either BASDAI or BASFI constant (at the mean value) and allowing the other measure to vary across the entire range. The baseline characteristics (BASDAI, BASFI and age) were derived from a weighted average of the baseline characteristics of the clinical trials for the AS population used in the manufacturer’s economic models. For nr-AxSpA, baseline characteristics (BASDAI, BASFI and age) of the nr-AxSpA subpopulation from the RAPID-axSpA64 study were used. Sex was assumed to be 65% male in AS and 35% male in nr-AxSpA.
FIGURE 18.
Illustration of predicted EQ-5D values using different mapping algorithms: constant BASDAI and varying BASFI (AS). MSD, Merck Sharp & Dohme.
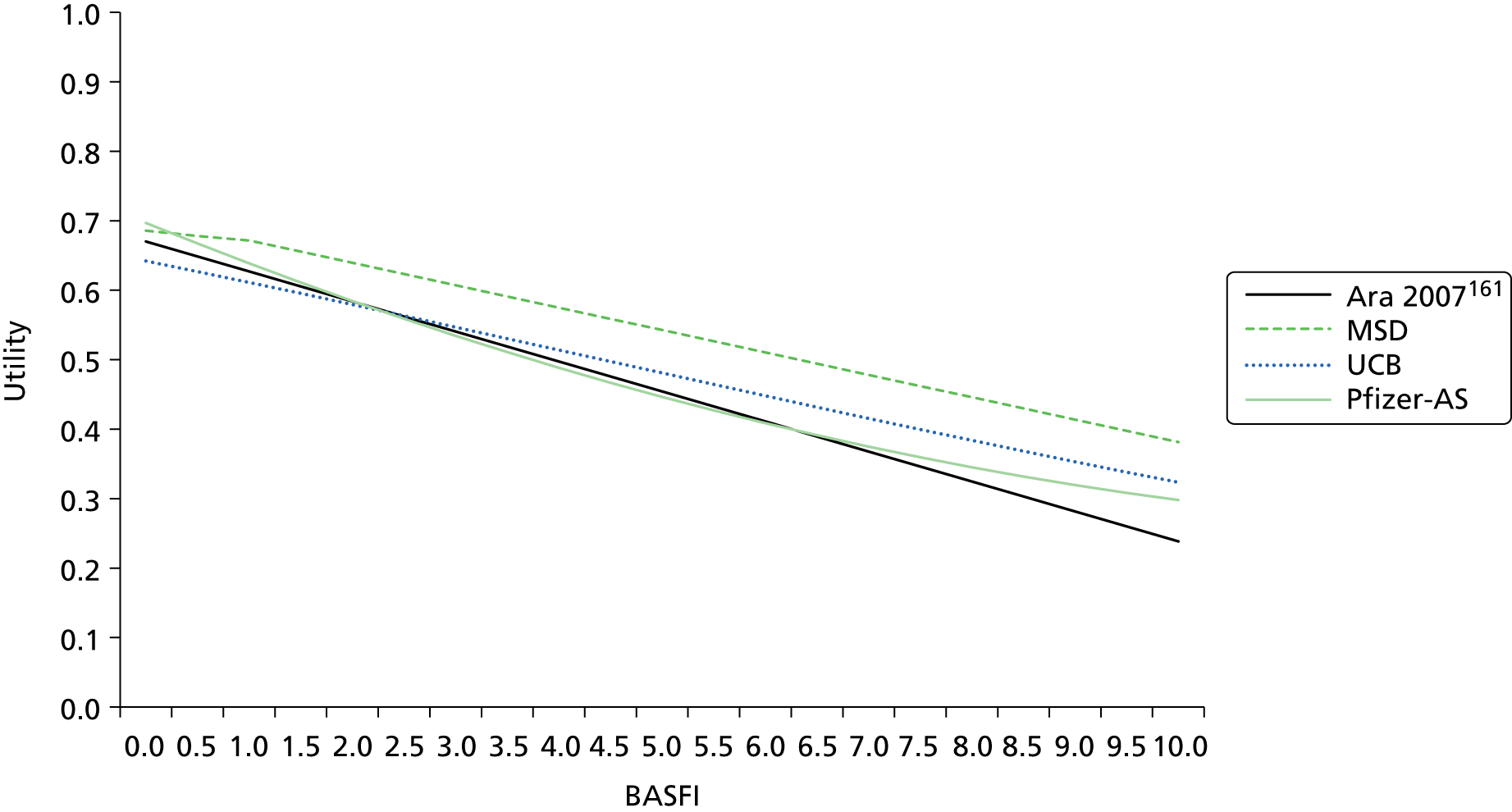
FIGURE 19.
Illustration of predicted EQ-5D values using different mapping algorithms: constant BASFI and varying BASDAI (AS). MSD, Merck Sharp & Dohme.
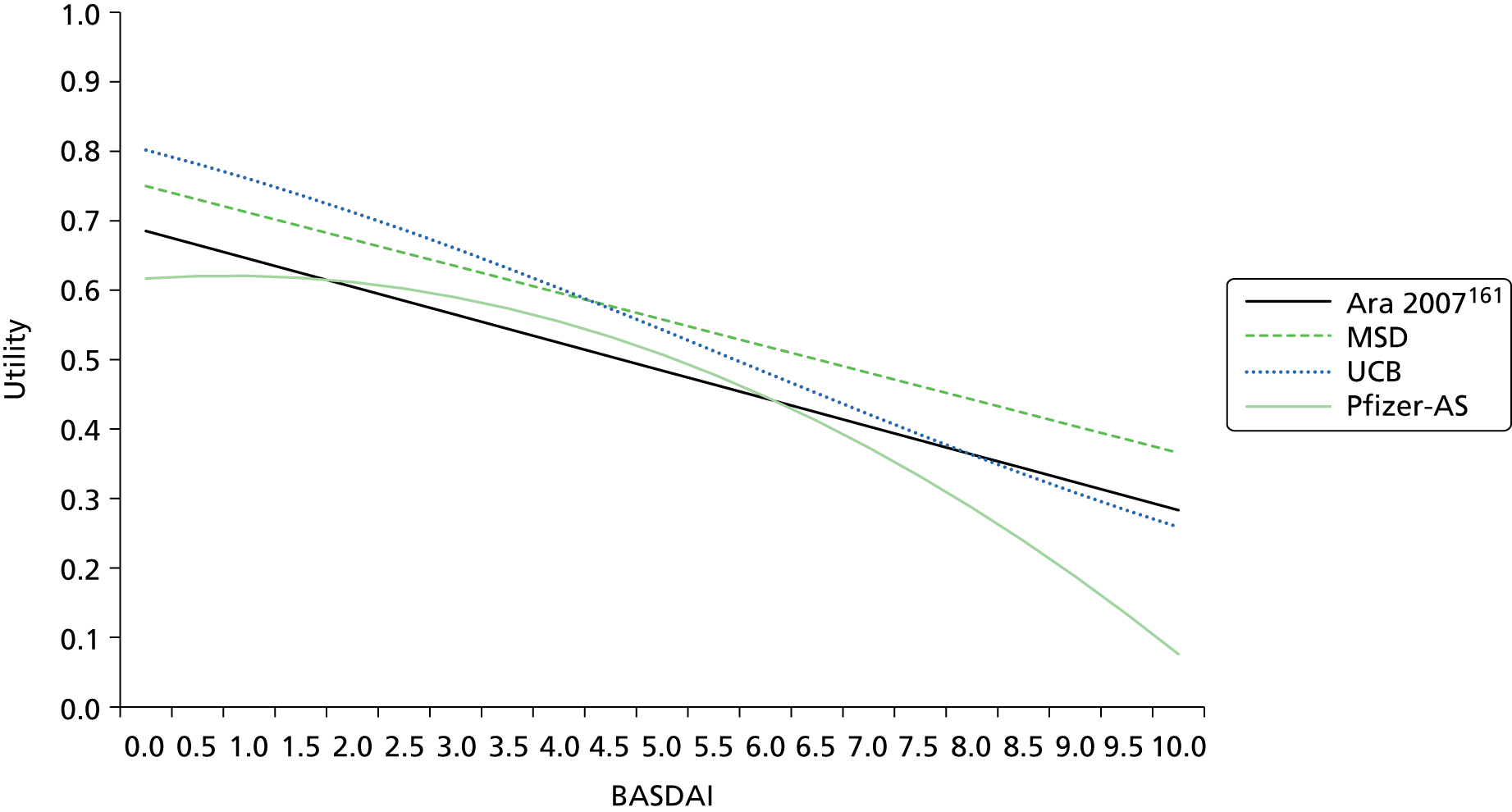
FIGURE 20.
AiC information has been removed.
FIGURE 21.
AiC information has been removed.
It is evident that there is significant variation in the utility predictions arising from each separate algorithm. In particular, the non-linear function estimated by Pfizer results in important differences across several of the figures at the extremes of the BASDAI/BASFI ranges. However, limited details were provided in relation to goodness of fit and/or predictive performance for the majority of algorithms and hence a formal assessment of the validity of the different approaches is problematic. Only the submission by Pfizer36 reported additional detail on these aspects and hence was subsequently used in the York model base case (separate algorithms for the different populations). The non-linear function for utilities was also considered to be more consistent with the non-linear approach applied to costs.
A potential limitation of all the manufacturer analyses is that their algorithms are based on trial data. These data may represent a more limited range of BASDAI and BASFI values and hence there may be issues associated with their subsequent predictive performance in the context of the longer-term economic model, although, from the data reported by Pfizer36 at least, it appeared as if the full range of BASDAI and BASFI scores were represented in the sample used. However, a separate sensitivity analysis was also undertaken based on the algorithm used by Merck Sharp & Dohme. This algorithm is based on a reanalysis of the Kobelt et al. 152 data from patients (n = 1144) who had BASDAI and BASFI scores across the whole 0–10 scale and was previously used by McLeod et al. 38 for the previous multiple TA. Hence this scenario also provides a more consistent basis for comparing the results from our new analysis.
Resource use and costs
Drug acquisition costs
The unit costs of anti-TNFs were sourced from the British National Formulary. Doses were calculated in accordance with their respective licences. Tables 86 and 87 summarise the drug acquisition costs and the licensed dosage for AS and nr-AxSpA patients.
| Drug | Dose | Cost (£) | Source |
|---|---|---|---|
| Infliximab (Remicade®, Merck Sharp & Dohme) | i.v. infusion: 100-mg vial | 419.62 | BNF,178 November 2014 |
| Golimumab (Simponi®, Merck Sharp & Dohme) | Injection: 50-mg prefilled pen or prefilled syringe | 762.97 | BNF,178 November 2014 |
| Injection: 100-mg prefilled pen | 1525.94 | ||
| Adalimumab (Humira®, AbbVie) | Injection: 40-mg prefilled pen/prefilled syringe or 40-mg/0.8-ml vial | 352.14 | BNF,178 November 2014 |
| Certolizumab (Cimzia®, UCB) | Injection: 200-mg prefilled syringe | 357.5 | BNF,178 November 2014 |
| Etanercept (Enbrel®, Pfizer) | Injection: powder for reconstitution, 25-mg vial or 25-mg prefilled syringe | 89.38 | BNF,178 November 2014 |
| Injection: 50-mg prefilled pen or prefilled syringe | 178.75 | BNF,178 November 2014 |
| Drug | Licensed dosage in AS and nr-AxSpA |
|---|---|
| Infliximab (Remicade®, Merck Sharp & Dohme) | Dose of 5 mg/kg given as an intravenous infusion followed by additional 5-mg/kg infusion doses at 2 and 6 weeks after the first infusion, then every 6 to 8 weeks. If a patient does not respond by 6 weeks (i.e. after two doses), no additional treatment with infliximab should be given |
| Golimumab (Simponi®, Merck Sharp & Dohme) | Dose of 50 mg given once a month, on the same date each month. For patients with a body weight of more than 100 kg who do not achieve an adequate clinical response after three or four doses, increasing the dose of golimumab to 100 mg once a month may be considered |
| Adalimumab (Humira®, AbbVie) | Recommended dose for patients with AS and axSpA without radiographic evidence of AS is 40 mg of adalimumab administered every other week as a single dose via subcutaneous injection |
| Certolizumab (Cimzia®, UCB) | The recommended starting dose of Cimzia for adult patients is 400 mg (given as 2 subcutaneous injections of 200 mg each) at weeks 0, 2 and 4. After the starting dose, the recommended maintenance dose of Cimzia for adult patients with AS is 200 mg every 2 weeks or 400 mg every 4 weeks |
| Etanercept (Enbrel®, Pfizer) | The recommended dose is 25 mg of Enbrel administered twice weekly, or 50 mg administered once weekly |
Patient access scheme details
Certolizumab with PAS: UCB will make Cimzia available free of charge to all NHS patients for the first 3 months of therapy, at which point clinical response should be clear. Only after this 3-month stage will the NHS be charged for continuing to use this therapy. However, it should be noted that the proposed PAS is not yet formally agreed with the Department of Health and NICE.
Golimumab PAS: the manufacturer provides the 100-mg dose of golimumab at the same cost as the 50-mg dose, agreed as part of the PAS.
Drug administration costs
Administration costs for intravenous therapies were based on a regular chemotherapy cost [Healthcare Resource Group (HRG) code SB15Z, Deliver subsequent elements of a chemotherapy cycle], similar to NICE TA143 (Table 88). 17 Therapies administered subcutaneously were assumed to be self-administered following instruction. The cost of instruction in the model was based on 1 hour of nurse time [Personal Social Services Research Unit (PSSRU) 2013]. 179 Drug administration did not differ between the AS and nr-AxSpA indications.
Initiation and monitoring costs
The initiation and monitoring costs for anti-TNF therapies were restricted to the additional costs incurred compared with patients receiving CC alone, as these drugs are used in addition to current practice. The resource use assumptions for laboratory testing for anti-TNF initiation and monitoring have been sourced from the York model for psoriatic arthritis (TA199175) and conform to guidelines from the BSR169 for the use of biologics.
Specifically, during the initial 12-week period AS patients on anti-TNF therapy are assumed to undertake a series of tests at treatment initiation and at week 12 when assessing treatment response (i.e. a full blood count, ESR, liver function test, and urea and electrolytes). Additional testing is conducted once during the initial period (i.e. chest radiography, tuberculosis Heaf test, antinuclear antibody and a double-stranded deoxyribonucleic acid test). AS patients on anti-TNF therapy are also assumed to visit a specialist twice during the initial 12-week period (at treatment initiation and when assessing 12-week response) and two times per year thereafter for monitoring. For quarterly monitoring, AS patients are assumed to receive a series of laboratory tests once every 3 months (i.e. a full blood count, ESR, liver function test, and urea and electrolytes).
Non-radiographic axial spondyloarthritis patients, in addition to the initiation and monitoring resource use assumed for AS patients on anti-TNF therapy, are also assumed to get a MRI test and a CRP test at treatment initiation, as well as a radiograph once per year after the initial period for monitoring, in order to assess radiographic progression.
Cost estimates for laboratory testing have been sourced from the York model for psoriatic arthritis (TA199175) and have been inflated to 2012/13 prices, using the Hospital and Community Health Services Pay and Prices Index. 175,179 The CRP test cost is derived from Henriksson 2010. 180 Specialist visits are costed at £100 (outpatient rheumatology follow-up attendance), using the NHS Reference Costs 2012/13. 176
A summary of the initiation and monitoring resource use assumptions for anti-TNF therapies and the subsequent costs for the AS and nr-AxSpA populations is reported in Tables 89 and 90.
| Item | Resource use | Cost | ||
|---|---|---|---|---|
| Initiation period (12 weeks) | Quarterly monitoring | Initiation period (12 weeks) (£) | Quarterly monitoring (£) | |
| Full blood count | 2 | 1 | 5.97 | 2.98 |
| ESR | 2 | 1 | 5.90 | 2.95 |
| Liver function test | 2 | 1 | 1.50 | 0.75 |
| Urea and electrolytes | 2 | 1 | 2.77 | 1.38 |
| Chest radiography | 1 | 0 | 26.19 | 0.00 |
| Tuberculosis Heaf test | 1 | 0 | 8.72 | 0.00 |
| Antinuclear antibody | 1 | 0 | 4.65 | 0.00 |
| Double-stranded DNA test | 1 | 0 | 4.65 | 0.00 |
| Specialist visit | 2 | 0.5 | 200.00 | 50.00 |
| CRP level | 0 | 0 | 0.00 | 0.00 |
| Total | – | – | 260 | 58 |
| Item | Resource use | Cost | ||
|---|---|---|---|---|
| Initiation period (12 weeks) | Quarterly monitoring | Initiation period (12 weeks) (£) | Quarterly monitoring (£) | |
| Full blood count | 2 | 1 | 5.97 | 2.98 |
| ESR | 2 | 1 | 5.90 | 2.95 |
| Liver function test | 2 | 1 | 1.50 | 0.75 |
| Urea and electrolytes | 2 | 1 | 2.77 | 1.38 |
| Chest radiography | 1 | 0.25 | 26.19 | 6.55 |
| Tuberculosis Heaf test | 1 | 0 | 8.72 | 0.00 |
| Antinuclear antibody | 1 | 0 | 4.65 | 0.00 |
| Double-stranded DNA test | 1 | 0 | 4.65 | 0.00 |
| Specialist visit | 2 | 0.5 | 200.00 | 50.00 |
| MRI | 1 | 0 | 144.45 | 0.00 |
| CRP level | 1 | 0 | 6.62 | 0.00 |
| Total | – | – | 411 | 65 |
Summary of drug acquisition, administration and monitoring costs
Tables 91 and 92 summarise the drug acquisition, administration and monitoring costs applied in the economic model, for the initial 12-week period and on an annual basis thereafter.
| Treatment (dosage) | Initial period (3 months) | Annual cost (after initial 3 months) | Total costs | |||||
|---|---|---|---|---|---|---|---|---|
| Acquisition cost (£) | Administration cost (£) | Monitoring costs (£) | Acquisition cost (£) | Administration cost (£) | Monitoring costs (£) | Initial period (3 months) (£) | Subsequent annual costs (£) | |
| Adalimumab, 40 mg eow | 2112.8 | 49.0 | 260.4 | 8451.4 | 0.0 | 232.3 | 2422.2 | 8683.6 |
| Certolizumab, 200 mg/2 weeks | 3575.0 | 49.0 | 260.4 | 8580.0 | 0.0 | 232.3 | 3884.4 | 8812.3 |
| Certolizumab, 200 mg/2 weeks, with PAS | 0.0 | 49.0 | 260.4 | 8580.0 | 0.0 | 232.3 | 309.4 | 8812.3 |
| Etanercept, 25 mg twice/week | 2145.1 | 49.0 | 260.4 | 8580.5 | 0.0 | 232.3 | 2454.5 | 8812.8 |
| Etanercept, 50 mg once/week | 2145.0 | 49.0 | 260.4 | 8580.0 | 0.0 | 232.3 | 2454.4 | 8812.3 |
| Golimumab, 50 mg once monthly, with PAS | 2105.6 | 49.0 | 260.4 | 8422.4 | 0.0 | 232.3 | 2415.0 | 8654.7 |
| Infliximab, 5 mg/kg every 7 weeks, four vials | 5639.7 | 978.8 | 260.4 | 11,509.6 | 1997.5 | 232.3 | 6878.8 | 13,739.3 |
| Treatment (dosage) | Initial period (3 months) | Annual cost (after initial 3 months) | Total costs | |||||
|---|---|---|---|---|---|---|---|---|
| Acquisition drug cost (£) | Administration cost (£) | Monitoring costs (£) | Acquisition drug cost (£) | Administration cost (£) | Monitoring costs (£) | Initial period (3 months) (£) | Subsequent annual costs (£) | |
| Adalimumab, 40 mg eow | 2112.8 | 49.0 | 411.4 | 8451.4 | 0.0 | 258.5 | 2573.3 | 8709.8 |
| Certolizumab, 200 mg/2 weeks | 3575.0 | 49.0 | 411.4 | 8580.0 | 0.0 | 258.5 | 4035.4 | 8838.5 |
| Certolizumab, 200 mg/2 weeks, with PAS | 0.0 | 49.0 | 411.4 | 8580.0 | 0.0 | 258.5 | 460.4 | 8838.5 |
| Etanercept, 25 mg twice/week | 2145.1 | 49.0 | 411.4 | 8580.5 | 0.0 | 258.5 | 2605.5 | 8838.9 |
| Etanercept, 50 mg once/week | 2145.0 | 49.0 | 411.4 | 8580.0 | 0.0 | 258.5 | 2605.4 | 8838.5 |
| Golimumab, 50 mg once monthly, with PAS | 2105.6 | 49.0 | 411.4 | 8422.4 | 0.0 | 258.5 | 2566.0 | 8680.9 |
| Infliximab, 5 mg/kg every 7 weeks, four vials | 5796.08 | 1005.9 | 411.4 | 11,509.6 | 1997.5 | 258.5 | 7213.4 | 13,765.5 |
Long-term disease management costs
Patients who remain on anti-TNF treatment incur disease management costs. Previously published economic evaluations employed observational cohort studies to estimate disease management costs and modelled these according to BASDAI and/or BASFI (e.g. NICE TA14317). In addition, as discussed in Chapter 4, the majority of the manufacturer’s submissions within this appraisal (and the LRiG model in TA14317) have analysed health-care resource use data from the OASIS118 to estimate disease management costs. The submission by Pfizer36 estimated disease-related costs using data from Rafia et al. 168 arguing that it is a more recent study and provides a UK-specific cost estimate. However, the comparative analysis of the different long-term cost models in Appendix 11 showed that the Rafia model provided considerably lower cost estimates; the reasons for this discrepancy are not clear.
In NICE TA14317 the committee judged that the OASIS data were the most reliable source, being a 2-year prospective study of 208 AS patients from four centres in France, Belgium and the Netherlands, and collecting clinical assessments and economic data including BASDAI and BASFI every 2 or 6 months. The NICE committee also decided that only BASFI should be employed as the major predictor of costs as it reflects long-term disease progression, while BASDAI appears to fluctuate but not increase over time.
The base case of the York model uses the exponential BASFI regression model from the AbbVie submission, which is a reanalysis of the OASIS resource utilisation data using up-to-date published tariffs (NHS Reference Costs 2012/13176 and PSSRU 2013179) (Table 93).
Adverse events
Only serious infections and tuberculosis reactivation were included in the economic model. Anti-TNF excess rates versus CC for serious infections and tuberculosis reactivation for were sourced from the Cochrane review of AEs137 which has been discussed in Chapter 3, Cost-effectiveness results: adverse events. The cost of a serious infection was sourced from the Pfizer submission36 and was assumed to be £1457 based on a weighted average of relevant HRG costs from NHS Reference Costs 2012/13176 (Table 94). The cost of tuberculosis was estimated to be £3204.50 per episode and was based on a weighted average of the relevant HRG codes with different levels of severity (codes DZ14C, DZ14D and DZ14E) from NHS Reference Costs 2012/13.
| HRG code | HRG description | Activity | National average unit cost (£) |
|---|---|---|---|
| WA03C | Septicaemia, with CC score 0–1 | 44,956 | 1792 |
| DZ23G | Bronchopneumonia with CC score 0–4 | 5231 | 1252 |
| LA04M | Kidney or urinary tract infections, with interventions, with CC score 0–2 | 2587 | 2289 |
| PA16B | Major infections with CC score 0 | 7859 | 1573 |
| DZ22J | Unspecified acute lower respiratory infection with CC score 0–1 | 21,109 | 657 |
| DZ21U | Chronic obstructive pulmonary disease or bronchitis, without NIV, without intubation, with CC Score 0–3 | 52,421 | 1453 |
| Weighted average cost (£) | 1457 | ||
Mortality
Sex-specific SMRs are applied to the mortality rates from the general population to calculate separate adjusted mortality rates for AS and nr-AxSpA populations in the model. 16
Analytic methods
The expected costs and QALYs of the alternative anti-TNFs are estimated and cost-effectiveness assessed based on the incremental cost per additional QALY gained. As an assumption is made concerning the similarity in terms of clinical effect between the alternative anti-TNFs, the differences between each of the treatments are driven entirely by their respective acquisition, administration and monitoring costs. Under this assumption, inevitably the lowest cost TNF-α inhibitor would clearly dominate (i.e. lower cost and equal effect) in a fully incremental comparison of cost-effectiveness. Consequently, each TNF-α inhibitor is compared separately versus CC alone. This provides a more consistent basis for assessing the impact that the different drug costs have across each separate scenario.
Probabilistic sensitivity analysis is used to assess the implications of parameter uncertainty (the imprecision with which input parameters are estimated). The mean costs and QALY reported in the tables are derived from the PSA and the probabilities that each TNF-α inhibitor is more cost-effective than CC alone are reported at thresholds of £20,000 and £30,000 per QALY.
Sensitivity analyses
A number of separate scenarios are presented to assess the implications of key parameter assumptions and sources of structural uncertainty in the model. These include the following.
Scenario 1: no response to conventional care assumed at 12 weeks
The base-case model incorporates the probability of response to CC at 12 weeks and assigns separate baselines to responders and non-responders. Although the changes in BASDAI/BASFI estimated at 12 weeks for CC are assumed to disappear in the following 12-week cycle, the separate baselines estimated for responders and non-responders are retained for the remainder of the model horizon. Given uncertainties surrounding the nature of the ‘placebo’ response assumed to apply to CC and whether or not this would be evident in actual clinical practice, a separate scenario was modelled which assumed that no patients receiving CC would achieve a BASDAI 50 response. This scenario was based on a separate simulation using the extended synthesis model where the magnitude of ‘placebo’ effect was assumed to be 0. Hence, employing this scenario, the impact of the ‘placebo’ effect is effectively netted out of the model for both CC and the anti-TNFs. Hence, although the difference in response rates and BASDAI/BASFI scores for responders to anti-TNFs remains similar to the base-case model, the absolute response rate for anti-TNFs and the absolute BASDAI/BASFI scores are lower when the adjustment is applied. In addition, as no response is assumed for CC, a single baseline BASDAI and BASFI score is applied to CC patients.
Scenario 2: different baselines assumed for responders and non-responders
In the base-case analysis, the extended synthesis model is used to estimate both changes in BASDAI and BASFI conditional upon BASDAI 50 response as well as different baseline BASDAI/BASFI scores for responder and non-responders. It was noted in Chapter 5 that there appeared a disparity in the magnitude of the difference in the conditional baseline scores estimated from the extended synthesis model compared with the differences reported by those manufacturers who provided additional data on request. Specifically, the difference between responders and non-responders appeared higher in our extended synthesis compared with the direct data reported by manufacturers. To explore the potential impact of this difference on the cost-effectiveness results, a separate scenario was undertaken wherein the difference in the conditional baselines was based on a pooled estimate of the differences across the trials provided by manufacturers rather than those estimated by the extended synthesis model.
In addition to exploring the impact of assuming different baselines, this scenario also included a pooled estimate of the change in BASDAI/BASFI scores for responders and non-responders reported by manufacturers. Hence, in this scenario, the extended synthesis model is used only to predict the response to BASDAI 50, the differences in the conditional baselines and change scores being derived from pooled estimates from the data reported by manufacturers.
Scenario 3: no effect of anti-tumour necrosis factors on Bath Ankylosing Spondylitis Functional Index progression
In the base-case model, a treatment effect is applied from year 4 of the model on the rate of further BASFI progression for patients who continue to receive TNF-α inhibitors beyond this time point. Given the uncertainty reported in Chapter 3 surrounding existing evidence for anti-TNFs in relation to disease modification, a separate scenario was explored which assumed that the rate of BASFI progression would be the same for patients receiving anti-TNFs and CC alone.
Scenario 4: treatment effect of anti-tumour necrosis factors applied from start of model (Bath Ankylosing Spondylitis Functional Index progression)
A separate scenario was also undertaken assuming that the treatment effect on further BASFI progression would be incurred from the start of the model, as opposed to year 4. This scenario assumes that any disease modification would be achieved immediately compared with the delayed effect assumed in the base case.
Scenario 5: utilities – linear Bath Ankylosing Spondylitis Disease Activity Index/Bath Ankylosing Spondylitis Functional Index model
The base-case analysis in both the AS and nr-AxSpA populations are based on the non-linear mapping algorithms reported in the submission by Pfizer. A separate scenario was run in both populations using an alternative linear model which has been applied in previous NICE appraisals (referred to as the ‘Merck Sharp & Dohme’ algorithm in Health-related quality of life). This scenario was incorporated to explore the impact of using a linear model and to provide results which are more consistent with the utility approach applied in previous NICE appraisals (TA14317 and TA23333).
Scenario 6 (non-radiographic axial spondyloarthritis only): trials in non-radiographic axial spondyloarthritis and ankylosing spondylitis populations combined
The base-case analysis for the nr-AxSpA population is based on the extended synthesis model using only the trials reporting in this population. A separate scenario was undertaken based on the results from the extended synthesis model which combined the AS and nr-AxSpA trials.
Following the consultation process to the NICE appraisal, additional analyses were undertaken to address comments received on Chapters 5 and 6. These focused on the conditional baseline BASDAI scores used in Chapter 5 and on the existence of a biosimilar product for infliximab with a lower list price. Further details on these analyses and their results are shown in Appendix 15.
Model validation
The conceptualisation of the model and related structural assumptions were informed by the review of existing models and discussions with two clinical advisors. The face validity of the model structure, data sources and key assumptions were addressed using inputs based on systematic reviews, targeted searching and clinical input. Verification of the model and the associated inputs was undertaken using a staged process. One researcher developed the initial model structure and the preliminary coding. This was then checked and extended for the final model by a second researcher. Both researchers were subsequently involved in the subsequent quality-assurance process entailing detailed cross-checks of input data against their sources and undertook extensive logical checks and scenarios to assess the performance of the model. Two other researchers were involved in further checks of key aspects including the integration of the results from the extended synthesis within the Excel model. A fifth researcher was involved in all stages with preparing and checking parameter inputs for the model. Cross-validation was assessed by comparing the results with existing models and identifying differences and their causes.
Results of the independent economic assessment
Base-case results: ankylosing spondylitis population
The base-case results for the AS population, for the alternative rebound assumptions, are reported in Tables 95 and 96.
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.245 | – | 110,821 | – | – | – | – |
| Certolizumab with PAS | 8.163 | 0.918 | 128,485 | 17,665 | 19,240 | 0.550 | 0.895 |
| Golimumab | 8.163 | 0.918 | 130,173 | 19,352 | 21,079 | 0.427 | 0.841 |
| Adalimumab | 8.163 | 0.918 | 130,257 | 19,436 | 21,170 | 0.423 | 0.839 |
| Etanercept | 8.163 | 0.918 | 130,630 | 19,810 | 21,577 | 0.402 | 0.826 |
| Certolizumab | 8.163 | 0.918 | 132,059 | 21,238 | 23,133 | 0.299 | 0.761 |
| Infliximab | 8.163 | 0.918 | 148,073 | 37,252 | 40,576 | 0.001 | 0.089 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.265 | – | 109,933 | – | – | – | – |
| Certolizumab with PAS | 7.867 | 0.603 | 130,277 | 20,344 | 33,762 | 0.035 | 0.399 |
| Golimumab | 7.867 | 0.603 | 131,960 | 22,027 | 36,554 | 0.019 | 0.299 |
| Adalimumab | 7.867 | 0.603 | 132,045 | 22,111 | 36,695 | 0.017 | 0.293 |
| Etanercept | 7.867 | 0.603 | 132,423 | 22,489 | 37,322 | 0.017 | 0.275 |
| Certolizumab | 7.867 | 0.603 | 133,851 | 23,918 | 39,693 | 0.011 | 0.203 |
| Infliximab | 7.867 | 0.603 | 150,022 | 40,088 | 66,529 | 0.000 | 0.001 |
In the rebound equal to gain scenario, the ICER of the alternative anti-TNFs varied between £19,240 (certolizumab with the proposed PAS) to £40,576 per additional QALY (infliximab). Infliximab had the highest ICER (£40,576 per QALY) and the lowest probability of being cost-effective at a £20,000 and £30,000 per QALY threshold (0.001 and 0.089, respectively). Excluding infliximab, the ICERs of the other anti-TNFs were similar, ranging from £19,240 (certolizumab with the proposed PAS) to £23,133 (certolizumab without the proposed PAS).
As previously highlighted, the difference in the ICERs between the individual anti-TNFs is driven entirely by the different acquisition and administration costs associated with each. Excluding infliximab, the probability that each TNF-α inhibitor was more cost-effective than CC alone ranged between 0.299 and 0.550 at a £20,000 per QALY threshold and between 0.761 and 0.895 at a £30,000 threshold. There was less variation in these probabilities when the proposed PAS for certolizumab was included, ranging from 0.402 to 0.550 at a £20,000 per QALY threshold and from 0.826 to 0.895 at a £30,000 threshold.
In the rebound to CC scenario, the ICER of the alternative anti-TNFs varied between £33,762 (certolizumab with the proposed PAS) to £66,529 per additional QALY (infliximab). Infliximab had the highest ICER (£66,529 per QALY) and the lowest probability of being cost-effective at a £20,000 and £30,000 per QALY threshold (0.000 and 0.001, respectively). Excluding infliximab, the ICERs of the other anti-TNFs varied between £33,762 (certolizumab with the proposed PAS) to £39,693 (certolizumab without the proposed PAS) and the probability that each TNF-α inhibitor was more cost-effective than CC alone ranged between 0.011 and 0.035 at a £20,000 per QALY threshold and between 0.203 and 0.399 at a £30,000 threshold. There was less variation in these probabilities when the proposed PAS for certolizumab was included, ranging from 0.017 to 0.035 at a £20,000 per QALY threshold and from 0.275 to 0.399 at a £30,000 threshold.
Base-case results: non-radiographic axial spondyloarthritis population
The base-case results for the nr-AxSpA population, for the alternative rebound assumptions, are reported in Tables 97 and 98.
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.956 | – | 89,493 | – | – | – | – |
| Certolizumab with PAS | 11.351 | 1.395 | 128,911 | 39,418 | 28,247 | 0.139 | 0.591 |
| Adalimumab | 11.351 | 1.395 | 130,316 | 40,823 | 29,253 | 0.106 | 0.545 |
| Etanercept | 11.351 | 1.395 | 131,057 | 41,563 | 29,784 | 0.093 | 0.529 |
| Certolizumab | 11.351 | 1.395 | 132,484 | 42,991 | 30,807 | 0.066 | 0.482 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.963 | – | 90,219 | – | – | – | – |
| Certolizumab with PAS | 11.200 | 1.237 | 131,714 | 41,495 | 33,555 | 0.057 | 0.396 |
| Adalimumab | 11.200 | 1.237 | 133,109 | 42,890 | 34,684 | 0.038 | 0.343 |
| Etanercept | 11.200 | 1.237 | 133,859 | 43,640 | 35,290 | 0.035 | 0.318 |
| Certolizumab | 11.200 | 1.237 | 135,286 | 45,067 | 36,444 | 0.029 | 0.284 |
In the rebound equal to gain scenario, the ICER of the alternative anti-TNFs varied between £28,247 (certolizumab with the proposed PAS) and £30,807 per additional QALY (certolizumab without the proposed PAS). The probability that each TNF-α inhibitor was more cost-effective than CC alone ranged between 0.066 and 0.139 at a £20,000 per QALY threshold and between 0.482 and 0.591 at a £30,000 threshold. Again, there was less variation in these probabilities when only the proposed PAS for certolizumab was considered, ranging from 0.093 to 0.139 at a £20,000 per QALY threshold and from 0.529 to 0.591 at a £30,000 threshold.
In the rebound to CC scenario, the ICER of the alternative anti-TNFs varied between £32,528 (certolizumab with the proposed PAS) and £35,365 per additional QALY (certolizumab without the proposed PAS). The probability that each TNF-α inhibitor was more cost-effective than CC alone varied between 0.030 and 0.062 at a £20,000 per QALY threshold and between 0.312 and 0.429 at a £30,000 threshold. Again, there was less variation in these probabilities when only the proposed PAS for certolizumab was included, ranging from 0.039 to 0.062 at a £20,000 per QALY threshold and from 0.369 to 0.429 at a £30,000 threshold.
Sensitivity analyses results: ankylosing spondylitis population
Table 99 summarises the scenarios undertaken for the AS population.
| Number | Parameter/structural | Approach in scenario | Approach in base case |
|---|---|---|---|
| 1 | CC (‘placebo’) response | No response to CC assumed at 12 weeks | Response to CC included at 12 weeks |
| 2 | Different baselines assumed for responders and non-responders and change in BASDAI/BASFI scores | Separate baselines based on pooled estimates provided by manufacturers. Changes in BASDAI/BASFI conditioned on response also based on pooled estimates provided by manufacturers | Separate baselines and changes in BASDAI/BASFI conditioned on responses estimated via extended synthesis model |
| 3 | BASFI progression | No effect of anti-TNFs on BASFI progression | Treatment effect applied from year 4 onwards |
| 4 | BASFI progression | Treatment effect of anti-TNFs applied from start of model | Treatment effect applied from year 4 onwards |
| 5 | Utilities | Linear BASDAI/BASFI model (based on Kobelt et al.152) | Non-linear BASDAI/BASFI model (Pfizer submission36) |
Each of these scenarios was undertaken for the two alternative rebound assumptions. Tables 100 and 101 summarise the ICER estimates for each scenario. Full ICER tables for each scenario are reported in Appendix 16.
| Strategy | Base case (£) | Scenario (£) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Conventional therapy | – | – | – | – | – | – |
| Certolizumab (with PAS) | 19,240 | 20,319 | 11,527 | 20,655 | 18,466 | 23,290 |
| Golimumab | 21,079 | 22,920 | 12,785 | 22,581 | 20,213 | 25,469 |
| Adalimumab | 21,170 | 23,013 | 12,851 | 22,677 | 20,301 | 25,579 |
| Etanercept | 21,577 | 23,425 | 13,143 | 23,106 | 20,695 | 26,073 |
| Certolizumab | 23,133 | 25,495 | 14,220 | 24,739 | 22,180 | 27,926 |
| Infliximab | 40,576 | 43,510 | 26,699 | 43,125 | 39,037 | 49,021 |
| Strategy | Base case (£) | Scenario (£) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Conventional therapy | – | – | – | – | – | – |
| Certolizumab (with PAS) | 33,762 | 34,229 | 25,530 | 36,518 | 32,222 | 29,414 |
| Golimumab | 36,554 | 38,068 | 27,986 | 39,483 | 34,910 | 31,827 |
| Adalimumab | 36,695 | 38,207 | 28,107 | 39,634 | 35,045 | 31,950 |
| Etanercept | 37,322 | 38,824 | 28,652 | 40,306 | 35,647 | 32,499 |
| Certolizumab | 39,693 | 41,885 | 30,731 | 42,828 | 37,928 | 34,554 |
| Infliximab | 66,529 | 68,815 | 54,045 | 71,565 | 63,684 | 58,022 |
The ICER estimates appeared to remain relatively stable across the majority of scenarios compared with the base-case ICER estimates. The exception to this appeared to be scenario 2 which used data submitted on request by several manufacturers which was used to inform the differences in the conditional baselines and the change scores assumed for responders versus non-responders. In summary, when the manufacturer’s data were used, the ICER estimates became more favourable towards the anti-TNFs. The more favourable results are driven by smaller differences between responders and non-responders in terms of their conditional baselines and marginally higher differences in the conditional change scores. Both differences result in improvements in the ICER estimates compared with the base-case results derived from the extended synthesis model.
Sensitivity analyses results: non-radiographic axial spondyloarthritis population
Table 102 summarises the scenarios undertaken for the nr-AxSpA population.
| Number | Parameter/structural | Approach in scenario | Approach in base case |
|---|---|---|---|
| 1 | CC (‘placebo’) response | No response to CC assumed at 12 weeks | Response to CC included at 12 weeks |
| 2 | Different baselines assumed for responders and non-responders and change in BASDAI/BASFI scores | Separate baselines based on pooled estimates provided by manufacturers. Changes in BASDAI/BASFI conditioned on response also based on pooled estimates provided by manufacturers | Separate baselines and changes in BASDAI/BASFI conditioned on responses estimated via extended synthesis model |
| 3 | BASFI progression | No effect of anti-TNFs on BASFI progression | Treatment effect applied from year 4 onwards |
| 4 | BASFI progression | Treatment effect of anti-TNFs applied from start of model | Treatment effect applied from year 4 onwards |
| 5 | Utilities | Linear BASDAI/BASFI model (based on Kobelt et al.152) | Non-linear BASDAI/BASFI model (Pfizer submission36) |
| 6 | Treatment effect of anti-TNFs | Trials in nr-AxSpA and AS populations combined | Only trials in nr-AxSpA included |
Each of these scenarios was undertaken for the two alternative rebound assumptions. Tables 103 and 104 summarise the ICER estimates for each scenario. Full ICER tables for each scenario are reported in Appendix 16.
| Strategy | Base case (£) | Scenario (£) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Conventional therapy | – | – | – | – | – | – | – |
| Certolizumab (with PAS) | 28,247 | 34,841 | 25,482 | 28,643 | 27,471 | 25,324 | 28,282 |
| Adalimumab | 29,988 | 37,884 | 27,302 | 29,670 | 28,466 | 29,228 | 29,512 |
| Etanercept | 29,253 | 38,507 | 27,821 | 30,208 | 28,988 | 29,753 | 30,041 |
| Certolizumab | 30,807 | 40,949 | 29,378 | 31,250 | 29,996 | 30,732 | 31,034 |
| Strategy | Base case (£) | Scenario (£) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Conventional therapy | – | – | – | – | – | – | – |
| Certolizumab (with PAS) | 32,528 | 40,928 | 29,884 | 34,416 | 31,841 | 26,900 | 33,184 |
| Adalimumab | 33,639 | 44,365 | 31,942 | 35,615 | 32,940 | 27,850 | 34,270 |
| Etanercept | 34,232 | 45,078 | 32,528 | 36,241 | 33,523 | 28,343 | 34,866 |
| Certolizumab | 35,365 | 47,842 | 34,288 | 37,456 | 34,642 | 29,303 | 35,985 |
In common with the AS scenarios, the ICER estimates appeared to remain relatively stable across the majority of scenarios compared with the base-case ICER estimates. However, the impact of applying adjustments to the conditional baseline estimates and BASDAI/BASFI scores provided by the manufacturers (scenario 2) had less of an impact in the nr-AxSpA population. The scenario which showed the largest variation compared with the base-case analysis was scenario 1. This scenario was based on results from the extended synthesis which excluded any placebo effect and resulted in a single baseline applied to all CC patients. The differences in the ICERs appear largely as a result of the impact of ignoring the non-linear relationship between baseline BASDAI/BASFI scores because of variation in the baseline of responders versus non-responders in scenario 1. Interestingly, the impact of this approach appears more marked in the nr-AxSpA population, compared with the AS population, which is likely to be driven by several inter-related factors including the magnitude of difference assumed between the conditional baseline scores and the absolute BASDAI and BASFI scores which differ across the populations.
Discussion and comparison with manufacturer models
Based on an underlying assumption of similarity in the clinical effectiveness of each of the anti-TNFs, the York model demonstrates that the cost-effectiveness results are dependent on several factors, including (1) the different acquisition and administration costs; (2) the rebound assumption applied to patients who discontinue therapy; (3) the magnitude of the change in BASDAI/BASFI scores assumed for responders versus non-responders; (4) the different baseline BASDAI/BASFI scores assumed for responders versus non-responders; and (5) the impact of anti-TNFs on the rate of longer-term BASFI progression.
Interestingly, the importance of specific factors also appears to vary across the separate indications. For example, the impact of the alternative rebound assumptions appears more marked in the AS population compared with the nr-AxSpA population. This appears largely driven by the smaller rate of BASFI progression applied in the York model to the nr-AxSpA population, such that the impact of alternative assumptions regarding possible rebound effects has a less significant impact within this population. This difference also has an important bearing on the subsequent interpretation of the base-case ICERs estimated by the York model in the separate populations. Our findings suggest that the ICER estimates for anti-TNFs appear more favourable for the AS population, relative to those estimated for the nr-AxSpA population, based on the rebound equal to gain scenario. The more favourable results in the AS population based on the rebound equal to gain scenario appear to be driven by two main factors: (1) the smaller conditional change in BASDAI/BASFI scores estimated for the nr-AxSpA population and (2) the lower rate of BASFI progression assumed for the nr-AxSpA population. However, this finding appears reversed in the rebound to CC scenario. Interestingly, within this scenario, the lower conditional change in BASDAI/BASFI scores appears offset by the less significant influence of BASFI progression in the nr-AxSpA model, that is the impact on the ICERs of the two rebound assumptions is closely related to the underlying rate of BASFI progression assumed and the contribution that this makes to the ICER estimates under the separate scenarios. However, it should also be noted that, although the ICERs for the nr-AxSpA population appear more favourable in this scenario compared with those estimated for the AS population, all of the ICER estimates exceeded £30,000 per QALY in the York base case across both populations.
Tables 105 and 106 compare the results of the York model with the base-case results reported by each manufacturer for the alternative populations. In contrast to the manufacturer models which reported a single base case based on an assumption of either rebound equal to gain (AbbVie, Pfizer, Merck Sharp & Dohme) or rebound to CC (UCB), the York model presents both rebound scenarios in order to represent the potential limits to the ICER; recognising that the reality lies somewhere between these scenarios.
| Strategy | AbbVie, ICER (£) | UCB, ICER (£) | Pfizer, ICER (£) | Merck Sharp & Dohme, ICER (£) | York (rebound equal to gain), ICER (£) | York (rebound to CC), ICER (£) |
|---|---|---|---|---|---|---|
| CC | – | – | – | – | – | – |
| Adalimumab | 16,391 | 19,932 | 20,909 | 19,275 | 21,170 | 36,695 |
| Certolizumab | 17,067 | 16,647a | 19,586a | 19,401a | 19,240a | 33,762a |
| Etanercept | 16,897 | 19,272 | 20,938 | 21,972 | 21,577 | 37,322 |
| Golimumab | 16,535 | 19,049 | 21,288 | 19,070 | 21,079 | 36,554 |
| Infliximab | 44,448 | 42,671 | 37,741 | 42,532 | 40,576 | 66,529 |
| Strategy | AbbVie (adalimumab), ICER (£) | UCB (certolizumab), ICER (£) | Pfizer (etanercept), ICER (£) | York (rebound equal to gain), ICER (£) | York (rebound to CC), ICER (£) |
|---|---|---|---|---|---|
| CC | – | – | – | – | – |
| Adalimumab | 13,228 | 30,370 | 23,242 | 29,988 | 33,639 |
| Certolizumab | 12,866 | 15,615a | 23,575a | 28,247a | 32,528a |
| Etanercept | Not assessed | 50,692 | 23,195 | 29,253 | 34,232 |
Although there are a number of important differences in approaches both among the different manufacturer models and compared with the York model, the comparison of ICERs based on the York rebound equal to gain scenario appear broadly consistent in the AS population. This might appear surprising given that the York model is based on two key assumptions that appear less favourable than those used by manufacturers, specifically: (1) incorporating separate baseline BASDAI/BASFI scores for responders and non-responders which assume that responders are likely to be less severe in terms of their baseline BASDAI and BASFI scores than non-responders; and (2) only incorporating an effect of anti-TNFs on disease progression for patients remaining on therapy for at least 4-years. However, these appear counterbalanced by the higher rate of BASFI progression applied to AS patients [0.082 (0–10 scale) units per annum compared with estimates between 0.056 and 0.07 assumed by the manufacturers]. As we highlighted at the start of this section, it is our view that the York model has a more coherent basis for modelling longer-term BASFI progression.
Another important counterbalancing effect is the use of the conditional scores for responders and non-responders obtained via the extended synthesis within the York model. This contrasts with the selective approaches (i.e. using conditional scores from single studies or assumptions) or use of longer-term follow-up and/or open-label sources (i.e. implicitly assuming that patients who continue to participate in longer-term follow-up and open label sources are more likely to be responders than patients who do not). Consequently, the change scores assumed in the York model for BASDAI 50 responders appear higher than those assumed by several of the manufacturers. The approach applied within the York model is based on a more generalised framework for synthesis and hence utilises more evidence than considered by the manufacturers. This approach directly informs the conditional change scores which are fundamental to an appropriate assessment of the cost-effectiveness when a response-based assessment is incorporated to determine eligibility for continued treatment.
In Chapter 4 it was noted that there appeared more variation in the ICER estimates reported across the manufacturer’s submissions in the nr-AxSpA population compared with those reported in the AS population. Again, the ICER estimates reported by the York model in the nr-AxSpA population do not appear inconsistent with the range of ICERs reported across the separate manufacturers. However, any attempt to formally cross-validate the results from the York model with those reported by the manufacturers is difficult given the contrasting approaches and assumptions employed. As the York model uses several of the key parameter inputs reported in the submission by Pfizer,36 a comparison may be more usefully made by comparing the results of the York model and those reported by Pfizer. In general, the ICER estimates appear less favourable in the York model compared with those reported by Pfizer. One possible explanation for these differences is that the York model uses a lower rate of BASFI progression and only assumes that anti-TNFs affect this rate after at least 4 years of treatment. However, our results have also shown that the impact of progression appears less of a driver of cost-effectiveness in the nr-AxSpA model. Another possible explanation is the use of different baselines assumed for responders and non-responders assumed in the York model, that is the York model assumes that responders and typically less severe in terms of baseline BASDAI/BASFI scores compared with non-responders. Consequently, an additional scenario was undertaken using the York model to further assist in cross-validation. For this scenario, an assumption was made that the responders and non-responders did not differ in terms of baseline BASDAI/BASFI scores.
The results of the additional validation scenario are reported in Table 107. The ICERs in this scenario appeared closer to those reported by Pfizer. 36 Hence this additional validation scenario is important in helping to identify potential drivers of difference between the results of the York model and those reported by the manufacturers. The scenario also demonstrates that the assumption made concerning potential differences (and the magnitude of any difference) between the baseline BASDAI/BASFI scores of responders and non-responders has an important impact on the cost-effectiveness results. Hence, studies which are based on similar baselines are likely to be potentially overly optimistic in the subsequent ICER estimates reported for anti-TNFs. Equally, it might be argued that the results from the York base-case model may be conservative towards the anti-TNFs because the magnitude of differences in the baseline scores estimated from the extended synthesis model appeared higher than those obtained on request from manufacturers (although the direction of the difference was consistent). Hence, in a similar manner to which the different rebound assumptions represent the potential limits on the ICER given uncertainties surrounding rebound, the differences in the ICERs based on assuming no difference in baselines and the magnitude of differences employed in the York base case may also represent the limits of the ICER based on uncertainty surrounding the magnitude of this difference. Given the potential importance of this assumption, Appendix 16 reports the full ICER results for each population (and under each rebound assumption) assuming identical baselines for responders and non-responders.
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.977 | – | 88,692 | – | – | – | – |
| Certolizumab (with PAS) | 11.551 | 1.574 | 125,205 | 36,513 | 23,199 | 0.390 | 0.759 |
| Adalimumab | 11.551 | 1.574 | 126,606 | 37,914 | 24,089 | 0.341 | 0.733 |
| Etanercept | 11.551 | 1.574 | 127,350 | 38,658 | 24,562 | 0.319 | 0.720 |
| Certolizumab | 11.551 | 1.574 | 128,777 | 40,085 | 25,469 | 0.272 | 0.702 |
Although the York model provides a number of significant developments to existing cost-effectiveness analyses, there are still several potential limitations. First, in common with all existing models, subsequent linkages to costs and QALYs are related to BASDAI and BASFI, largely because of the existence of data. Second, the cost-effectiveness estimates are based on uncertain projections of BASDAI and BASFI over a longer time horizon in order to generate more appropriate lifetime estimates of costs and QALYs required for cost-effectiveness assessments. Although extensive efforts have been made to identify a more appropriate basis for informing these longer-term estimates (particularly for BASFI), inevitably, significant uncertainty remains. Third, it should be noted that there are potential benefits which have not been formally captured and quantified within the current model, specifically any potential impact on productivity costs and any additional benefits that anti-TNFs may confer for other comorbidities (e.g. inflammatory bowel disease, psoriasis, etc.). A final limitation is that it was not possible to include the biosimilar version of infliximab (CT-P13) within the analysis as a formal list price was not available at the time of the assessment.
In addition, the York model has not specifically addressed important clinical questions concerning the issue of intermittent and sequential use of anti-TNFs. However, in the absence of robust clinical evidence from RCTs, existing evidence is clearly subject to potential confounding. Consequently, existing attempts to model sequential therapy within the current manufacturer’s submissions (Pfizer36 only) are largely based on applying simple adjustments to first-line efficacy but which are unlikely to provide a robust basis for informing these decisions. Clearly, until such time that more robust data are available, a rough rule of thumb could similarly be applied to the results presented from the York model, such that the ICERs of a second-line TNF-α inhibitor in a patient who had previously responded but subsequently lost response, might be in the order of one-third higher than the results presented here.
Finally, it is important to appreciate that the assessments of cost-effectiveness reported in the York model are based on a normative approach, that is they are based on the assumption that 12-week continuation rules (and ongoing monitoring of response) would be fully adhered to in clinical practice. Hence, they do not necessarily reflect the cost-effectiveness of how anti-TNFs are currently used in the management of AS within the NHS or how they might be used, in the event of positive guidance from the NICE in nr-AxSpA. The findings of West Midland Rheumatology Audit from 2010 give some grounds for potential concern. 181 This regional audit was undertaken to assess compliance with the NICE guidelines (TA14317) in 17 rheumatology centres across the East and West Midlands. The findings from this audit revealed that (1) the proportion of patients being assessed at 12 weeks after treatment initiation was suboptimal; (2) fewer than 20% of patients with an inadequate response at 12 weeks had their treatment discontinued; and (3) fewer than half of the patients received regular 12-weekly assessments. During the course of our assessment we contacted the British Society for Rheumatology Biologics Register (BSRBR) Ankylosing Spondylitis Register to assess the feasibility of obtaining access to data which has been collected since the register was set up in 2012. Although our request was positively received, it was clear during ongoing discussions that the data and analyses requested could not be undertaken within the time frame of our assessment.
Chapter 7 Assessment of factors relevant to the NHS and other parties
The results of this technology assessment have some implications for clinical practice. Existing NICE guidance recommends adalimumab, certolizumab, etanercept and golimumab for the treatment of AS and therefore the use of these drugs is already widespread in the NHS. However, in the light of the additional evidence presented here, the use of these agents in AS may increase further.
Furthermore, the available clinical evidence indicates that adalimumab, certolizumab and etanercept are effective in patients with nr-AxSpA, although there is some uncertainty regarding the definition of the nr-AxSpA patient population who would benefit most from these anti-TNFs. The effectiveness demonstrated in the nr-AxSpA population suggests that early treatment of AS/nr-AxSpA patients is warranted. A key study on flares in AS suggested that the 12-week period required to confirm sustained active spinal disease in AS patients commencing an anti-TNF may be too long. The findings suggest that shorter time periods may therefore be considered in future guidance, which would minimise the delay in starting treatment and the discomfort experienced by patients.
The potential extra cost to the NHS of providing anti-TNFs for patients with nr-AxSpA in addition to AS patients is unclear because the prevalence of nr-AxSpA in the UK is somewhat uncertain. The potentially large volume of new patients to be assessed for eligibility for anti-TNF treatment could add a large burden to existing services. NICE guidance recommending the use of adalimumab, certolizumab and etanercept in nr-AxSpA would further increase the impact of these agents on the NHS budget.
Chapter 8 Discussion
Statement of principal findings
The systematic review of clinical efficacy identified a substantial and, generally, high-quality evidence base on the efficacy and safety of anti-TNFs in patients with AS, either as individual treatments or as a common class; there was limited evidence to suggest meaningful differences between the therapies in terms of efficacy, other than infliximab providing more rapid improvements during the first few months of treatment. The results of our meta-analyses demonstrated that anti-TNFs (when compared with placebo) produce statistically significant and clinically important benefits in patients with AS in terms of improving function and reducing disease activity over a 3- to 6-month period (none of the trials maintained randomised treatment allocations across groups beyond 6 months). Of the limited number of trials which reported quality-of-life outcomes, significant improvements were found following anti-TNF therapy but very few data were available on efficacy relating to any peripheral symptoms (other than enthesitis) or other possible symptoms such as uveitis, inflammatory bowel disease and psoriasis.
Although far fewer trials have been performed in the nr-AxSpA population, similar, although slightly smaller, benefits were achieved. The smaller benefit was most noticeable for the function (BASFI) and disease-activity (BASDAI 50) outcomes. However, in the nr-AxSpA trials, both clinical and statistical heterogeneity were evident, bringing into question both the reliability of the nr-AxSpA meta-analysis results and their true relevance to patients seen in clinical practice. This heterogeneity may have been compounded by the inclusion criteria applied in previous nr-AxSpA trials. For example, ABILITY-158 recruited patients who fulfilled the ASAS classification criteria and relied on the expertise of the local clinicians and/or radiologists to read sacroiliac joint radiographs and MRI images, as happens in real clinical practice. RAPID-axSpA64 selected its population carefully by requiring objective evidence of disease activity at study entry by either a positive MRI showing signs of sacroiliac joint inflammation according to the ASAS/OMERACT definition or an elevated than normal CRP level. The difficulty of identifying which nr-AxSpA patients should receive anti-TNFs remains.
Results from open-label trial extension studies suggested that across all the anti-TNFs around half of patients still achieve a good level of response after around 2 years of treatment. The data also suggest that at 5 years around 60% of golimumab patients, 50% of etanercept patients and 30% of adalimumab patients still achieve a good treatment response. However, these longer-term studies were not as well-reported as the RCTs, and their results were derived from less reliable data; it is therefore unknown if these are true treatment differences or if they are a result of differences in the follow-up protocols (e.g. stopping rules) and/or methods used to impute missing data.
Evidence for an effect of anti-TNFs on radiographic disease progression was limited; the relatively short-term follow-up available to date and the insensitivity of radiography as an imaging tool precluded the drawing of firm conclusions regarding the role of anti-TNFs in preventing or delaying the progression of AS. There are some data to suggest an identifiable benefit from around 4 years but results from ongoing long-term studies should help to clarify this issue.
The results from studies based on registry data demonstrated that sequential treatment with anti-TNFs can be worthwhile in patients with AS. However, the drug survival, response rates and benefits were reduced with second and third anti-TNFs, with the proportion of BASDAI 50 responders falling approximately 10% with each subsequent anti-TNF and the median BASDAIs and BASFIs achieved increasing (worsening).
Data from large systematic reviews, which included patients with a wide range of diseases, suggest that, in the short-term, anti-TNFs as a group are associated with significantly higher rates of serious infections, tuberculosis reactivation, non-melanoma skin cancer, total AEs and withdrawals because of AEs than control treatments. Specifically, infliximab is associated with significantly higher rates of total AEs and withdrawals because of AEs, and certolizumab pegol is associated with significantly higher rates of serious infections and SAEs. Evaluations of longer-term data are more scarce and are limited by small sample sizes and uncontrolled designs. They suggest similar safety profiles across anti-TNFs, other than a higher incidence of injection site reactions following treatment with etanercept.
The systematic review of cost-effectiveness studies revealed significant conceptual issues and uncertainties arising from previously published studies and the submissions made by manufacturers. For this reason, a de novo model (‘York model’) was developed. Although it shared some of the assumptions and parameter estimates from the manufacturer models, it was based on a different conceptual structure and applies a more generalised framework for the synthesis of data from the double-blind periods of existing RCTs, combined with a more explicit approach to modelling the progressive nature of AS and nr-AxSpA and the potential impact of the TNF-α inhibitors.
Based on an underlying assumption of similarity in the clinical effectiveness of each of the TNF-α inhibitors, the York model demonstrates that the cost-effectiveness results are dependent on several factors, including (1) the different acquisition and administration costs; (2) the rebound assumption applied to patients who discontinue therapy; (3) the magnitude of the change in BASDAI/BASFI scores assumed for responders versus non-responders; (4) the different baseline BASDAI/BASFI scores assumed for responders versus non-responders; and (5) the impact of TNF-α inhibitors on the rate of longer-term BASFI progression.
Although there are a number of important differences in approaches both among the different manufacturer models and compared with the York model, the comparison of ICERs based on the York rebound equal to gain scenario appear broadly consistent with those reported by the manufacturers in both populations.
Strengths and limitations of the assessment
Strengths
Through our comprehensive searches we sought to identify all relevant published and unpublished trials, which minimised the possibility of publication or language biases affecting the review results. A full evaluation of the risk of bias in each RCT was performed, which incorporated an additional assessment of key baseline characteristics to allow firmer judgements to be made on the risk of selection bias. The use of multiple-treatment meta-analyses allowed for greater precision in random-effect models, and the calculation of relative risks was based on the population risk across all the trials. A key further strength of our review lies in the extensive breadth of other types of study we included, such as non-randomised trial extension studies, registry studies of patients taking anti-TNFs, systematic reviews and other large studies of adverse effects of anti-TNFs and a review of the natural history of AS and nr-AxSpA. Our review of AEs incorporated a wealth of data from RCTs in patients on anti-TNFs with diseases other than AS and nr-AxSpA, although the results only relate to short-term use. Our review was performed according to Centre for Reviews and Dissemination guidance, so the potential for reviewer errors and biases was minimised. Our review was reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.
The York model confers several advantages over current cost-effectiveness studies by linking changes in BASFI to a more explicit clinical/biological process and facilitating a more formal consideration of the potential impact of TNF-α inhibitors on BASFI, via the specific effects these drugs have on the different processes which independently relate to this parameter. This approach allows consideration of the impact on BASFI that might be achieved via symptomatic improvements (i.e. in terms of reductions in disease activity) and those which might be conferred by disease modification properties (i.e. the effect on the likelihood and/or rate of further radiographic progression). The latter aspect is particularly important given the increasing amount of published evidence reported on the potential impact of TNF-α inhibitors on radiographic progression that has not been formally considered or incorporated within existing cost-effectiveness studies. In addition, the evidence synthesis approach which underpins the York model is based on a joint synthesis of related parameters which makes fuller use of existing evidence and which can more appropriately estimate the input parameters and better characterise the uncertainty surrounding these.
Limitations
A key limitation of the systematic review was the variation in the reporting of outcomes across trials. ASAS 20 was the most commonly reported responder outcome, but its value in determining efficacy was somewhat limited by the relatively high rates of ‘placebo’ response associated with the 20% threshold. Results for 40%, 50% and 70% improvements (i.e. ASAS 40, ASAS 50 and ASAS 70) were reported less frequently, despite the fact that trial investigators would have had the data available to do so. Many trials did not report HRQoL outcomes and most trials were also limited in their assessment (or reporting) of improvement in any peripheral symptoms or symptoms of extra-articular manifestations. Although largely free of important biases, most RCTs had quite short durations (generally around 3 months) and several were limited by their small sample sizes (increasing the possibility of chance results for some outcomes).
Although we sought data beyond those available from RCTs, much of the data reported in studies using other designs may have been affected by biases or confounding; furthermore, key method details (e.g. imputation methods, or anti-TNF stopping rules) were often absent from publications. Much less reliability and certainty could therefore be ascribed to the results obtained from these other studies.
The York model did not directly address important clinical questions concerning the issue of intermittent and sequential use of anti-TNFs because of the lack of robust clinical evidence from RCTs.
Uncertainties
-
The magnitude of treatment effect of anti-TNFs in patients with nr-AxSpA remains uncertain because of the heterogeneous nature of the trials performed to date.
-
The limited design and reporting of the studies looking at the long-term use of anti-TNFs means there is uncertainty whether or not there are differences in efficacy between the different anti-TNFs in the long term.
-
The evidence on the long-term risk of AEs is uncertain because of small study sample sizes and the study designs used.
-
The long-term impact of anti-TNFs on other important outcomes in AS and nr-AxSpA remain uncertain, such as AS-related causes of death (cardiac valvular disease, amyloidosis and fractures), and extra-articular symptoms such as uveitis, inflammatory bowel disease and psoriasis. Studies based on ongoing anti-TNF registries (e.g. BSRBR) that record such data should inform this.
-
With the patents of some anti-TNFs studied in this assessment due to expire shortly, biosimilars are likely to become available in the next few years (CT-P13 became available early in 2015). As they are difficult to produce, the number of biosimilars which will become available, and their price, is uncertain.
Chapter 9 Conclusions
Meta-analysis results derived from a substantial and, generally, high-quality evidence base on the efficacy of anti-TNFs in patients with AS (considered either as individual treatments or as a common class) show statistically significant and clinically important benefits in terms of improved function and reduced disease activity following around 3 months of treatment with an anti-TNF. Smaller benefits were seen across outcomes in patients with nr-AxSpA, being most noticeably smaller for the function and disease-activity outcomes. However, in the light of the clinical and statistical heterogeneity seen across the nr-AxSpA trials, both the reliability of the nr-AxSpA-pooled estimates and their true relevance to patients seen in clinical practice are questionable. Data from (less robust) observational studies suggest that good levels of treatment response are maintain in around 50% of patients after around 2 years of treatment. Evidence for an effect of anti-TNFs on radiographic disease progression is limited, although results from ongoing studies should clarify whether or not progression rates are reduced in the longer term. The results from studies based on registry data demonstrated that sequential treatment with anti-TNFs can be worthwhile in patients with AS, although drug survival, response rates and benefits were reduced with second and third anti-TNFs. Data from large systematic reviews, which included patients with a wide range of diseases, suggested that, in the short term, anti-TNFs as a group were associated with significantly higher rates of serious infections, tuberculosis reactivation, non-melanoma skin cancer, total AEs and withdrawals because of AEs than control treatments. Longer-term data on AEs were limited.
Implications for service provision
-
From our review of natural history a key study on flares suggested that the 12-week period required to confirm sustained active spinal disease in AS patients commencing an anti-TNF may be too long. The findings suggest that shorter time periods might therefore be considered in future guidance, which would minimise the delay in starting treatment and the discomfort experienced by patients.
Suggested research priorities
-
Randomised trials are needed to identify the nr-AxSpA population that will benefit the most from TNF-inhibitors; trials using stratified randomisation and pre-planned analyses by stratified group should inform this issue. Groups could be stratified according to their imaging status (i.e. MRI positive or not) and their CRP level; both the cut-off points to be used for CRP level elevation, and the eligibility criteria used for CRP level elevation, should be given careful consideration, given the variation evident in previous trials. These studies should help to inform clearer guidance as to what ASAS and the anti-TNF licences mean when referring to ‘elevated CRP level’ in patients with nr-AxSpA. There is also a clear need for more accurate biomarkers, or other measures of disease activity, to be developed. In the previous nr-AxSpA trials the placebo-controlled phases typically lasted around 3 months; a placebo-controlled follow-up period of at least 6 months in future trials would therefore be useful for studying persistence of response.
-
Long-term longitudinal studies are needed on the natural history of nr-AxSpA to help clarify the characteristics of patients who do (or do not) eventually develop AS. Similar to the RCT recommendations, these studies should include analyses stratified by how patients were diagnosed; a comparison of patients with imaging (MRI) evidence of nr-AxSpA versus patients who are diagnosed with only clinical criteria evidence, would be particularly useful, albeit difficult to perform.
-
Large, long-term longitudinal, cohort studies are needed to clarify the effect of anti-TNFs on the progression of structural damage in AS. In the absence of a gold standard imaging tool across the spectrum from nr-AxSpA to AS, sequential MRI and radiography assessment should be used at pre-defined end points to ascertain the true sensitivity and specificity of these tools in the diagnosis and assessment of neo-formation, and ankyloses characteristic of structural progression in the spine and sacroiliac joints of these patients.
Studies are also needed to better inform the efficacy estimates relating to sequential use of anti-TNFs. An ongoing study is looking at comparing the effect of intermittent versus standard use of anti-TNFs in patients with stable (low-active) disease. 182
Acknowledgements
We acknowledge Nicky Welton, Reader in Evidence Synthesis, School of Social and Community Medicine, University of Bristol, for the guidance provided in the development of the synthesis model.
Rider on responsibility for report
The views expressed in this report are those of the authors and not necessarily those of the NIHR HTA programme. Any errors are the responsibility of the authors.
Contributions of authors
Mark Corbett contributed to the protocol, study selection, data extraction, validity assessment, synthesis of the included studies, interpretation of the results and the writing of the report.
Marta Soares developed the extended synthesis model and undertook the evidence synthesis for the purposes of the economic model. She also contributed to the development of the economic model and the writing of the report.
Gurleen Jhuti contributed to the protocol, the development of the economic model, the review of economic analyses, the interpretation of the results and the writing of the report.
Stephen Rice contributed to data extraction, validity assessment, undertook synthesis of the included studies for the clinical effectiveness section, contributed to the interpretation of the results and the writing of the report.
Eldon Spackman contributed to the development of the economic model, performed the economic analyses and contributed to the interpretation of the results.
Eleftherios Sideris contributed to the review of economic evaluations, the development of the economic model and the writing of the report.
Thirimon Moe-Byrne contributed to the protocol, study selection, data extraction and validity assessment of the included studies.
Dave Fox contributed to the protocol development, developed the search strategies, conducted a range of searches to locate studies and wrote the sections of the report relating to the searches.
Helena Marzo-Ortega provided expert clinical advice, contributed to the protocol, interpretation of the results and commented on drafts of the report.
Lesley Kay provided expert clinical advice, contributed to the protocol, interpretation of the results and commented on drafts of the report.
Nerys Woolacott contributed to the protocol, study selection, data extraction, validity assessment, synthesis of the included studies, interpretation of the results and the writing of the report, and took overall responsibility for the clinical effectiveness section.
Stephen Palmer had overall responsibility for the cost-effectiveness sections. He contributed to the protocol development and to all aspects of the cost-effectiveness work including the writing of the report.
Data sharing statement
All available data are either included within the main report or available in the appendices.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Lipton S, Deodhar A. The new ASAS classification criteria for axial and peripheral spondyloarthritis. Int J Clin Rheumatol 2012;7:675-82. http://dx.doi.org/10.2217/ijr.12.61.
- Vanderlinden S, Valkenburg HA, Cats A. Evaluation of diagnostic-criteria for ankylosing-spondylitis – a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361-8. http://dx.doi.org/10.1002/art.1780270401.
- Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68:1-44. http://dx.doi.org/10.1136/ard.2008.104018.
- Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777-83. http://dx.doi.org/10.1136/ard.2009.108233.
- Goh L, Samanta A. Update on biologic therapies in ankylosing spondylitis: a literature review. Int J Rheum Dis 2012;15:445-54. http://dx.doi.org/10.1111/j.1756-185X.2012.01765.x.
- Robinson PC, Wordsworth BP, Reveille JD, Brown MA. Viewpoint axial spondyloarthritis: a new disease entity, not necessarily early ankylosing spondylitis. Ann Rheum Dis 2013;72:162-4. http://dx.doi.org/10.1136/annrheumdis-2012-202073.
- van Tubergen A, Weber U. Diagnosis and classification in spondyloarthritis: identifying a chameleon. Nat Rev Rheumatol 2012;8:253-61. http://dx.doi.org/10.1038/nrrheum.2012.33.
- Deodhar A. Rheumatologists Make Progress Defining Spectrum of Axial Spondyloarthritis 2014. www.the-rheumatologist.org/issue/may-2014 (accessed 1 December 2015).
- National Ankylosing Spondylitis Society . National Ankylosing Spondylitis Society (NASS) Website n.d. http://nass.co.uk/ (accessed 11 December 2014).
- Landewe R, Dougados M, Mielants H, van der Tempel H, van der Heijde D. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis 2009;68:863-7. http://dx.doi.org/10.1136/ard.2008.091793.
- Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007;369:1379-90. http://dx.doi.org/10.1016/S0140-6736(07)60635-7.
- Baraliakos X, Listing J, von der Recke A, Braun J. The natural course of radiographic progression in ankylosing spondylitis – evidence for major individual variations in a large proportion of patients. J Rheumatol 2009;36:997-1002. http://dx.doi.org/10.3899/jrheum.080871.
- Healey EL, Haywood KL, Jordan KP, Garratt AM, Packham JC. Disease severity in ankylosing spondylitis: variation by region and local area deprivation. J Rheumatol 2010;37:633-8. http://dx.doi.org/10.3899/jrheum.090899.
- Healey EL, Haywood KL, Jordan KP, Garratt AM, Packham JC. Patients with well-established ankylosing spondylitis show limited deterioration in a ten-year prospective cohort study. Clin Rheumatol 2013;32:67-72. http://dx.doi.org/10.1007/s10067-012-2092-3.
- Callhoff J, Sieper J, Weiss A, Zink A, Listing J. Efficacy of TNFα blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a meta-analysis. Ann Rheum Dis 2015;74:1241-8. http://dx.doi.org/10.1136/annrheumdis-2014-205322.
- Bakland G, Gran JT, Nossent JC. Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis 2011;70:1921-5. http://dx.doi.org/10.1136/ard.2011.151191.
- Technology Appraisal TA143: Ankylosing Spondylitis – Adalimumab, Etanercept and Infliximab. London: NICE; 2008.
- Rudwaleit M, Haibel H, Baraliakos X, Listing J, Marker-Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717-27. http://dx.doi.org/10.1002/art.24483.
- Reveille JD, Sims A-M, Danoy P, Evans DM, Leo P, Pointon JJ, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet 2010;42:123-7. http://dx.doi.org/10.1038/ng.513.
- NHS Choices . Ankylosing Spondylitis n.d. www.nhs.uk/conditions/Ankylosing-spondylitis/Pages/Introduction.aspx (accessed 29 April 2014).
- Reveille JD, Weisman MH. The epidemiology of back pain, axial spondyloarthritis and HLA-B27 in the United States. Am J Med Sci 2013;345:431-6. http://dx.doi.org/10.1097/MAJ.0b013e318294457f.
- Sieper J, van der Heijde D. Review: nonradiographic axial spondyloarthritis: new definition of an old disease?. Arthritis Rheum 2013;65:543-51. http://dx.doi.org/10.1002/art.37803.
- National Institute for Health and Care Excellence . Ankylosing Spondylitis: Prevalence n.d. http://cks.nice.org.uk/ankylosing-spondylitis#!backgroundsub:2 (accessed 29 April 2014).
- Machado P, Landewe R, Braun J, Hermann KG, Baraliakos X, Baker D, et al. A stratified model for health outcomes in ankylosing spondylitis. Ann Rheum Dis 2011;70:1758-64. http://dx.doi.org/10.1136/ard.2011.150037.
- Wanders AJB, Landewe RBM, Spoorenberg A, Dougados M, van der Linden S, Mielants H, et al. What is the most appropriate radiologic scoring method for ankylosing spondylitis?. Arthritis Rheum 2004;50:2622-32. http://dx.doi.org/10.1002/art.20446.
- Hróbjartsson A. What are the main methodological problems in the estimation of placebo effects?. J Clin Epidemiol 2002;55:430-5. http://dx.doi.org/10.1016/S0895-4356(01)00496-6.
- Hawkins N, Scott DA. Cost-effectiveness analysis: discount the placebo at your peril. Med Decis Making 2010;30:536-43. http://dx.doi.org/10.1177/0272989X10362106.
- Cooksey R, Brophy S, Gravenor MB, Brooks CJ, Burrows CL, Siebert S. Frequency and characteristics of disease flares in ankylosing spondylitis. Rheumatology 2010;49:929-32. http://dx.doi.org/10.1093/rheumatology/kep435.
- Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Sys Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd003974.pub3.
- Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011;70:896-904. http://dx.doi.org/10.1136/ard.2011.151027.
- Dagfinrud H, Hagen KB, Kvien TK. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Sys Rev 2008;1. http://dx.doi.org/10.1002/14651858.cd002822.pub3.
- Looking Ahead: Best Practice for the Care of People with Ankylosing Spondylitis (AS). Richmond: NASS; 2013.
- Technology Appraisal TA233: Ankylosing Spondylitis – Golimumab. London: NICE; 2011.
- AbbVie . National Institute for Health and Care Excellence (NICE) Multiple Technology Appraisal: TNF-Alpha Inhibitors for Severe Active Ankylosing Spondylitis and Severe Axial Spondyloarthritis Without Radiographic Evidence of Ankylosing Spondylitis n.d.
- UCB . National Institute for Health and Care Excellence (NICE) Multiple Technology Appraisal: Certolizumab Pegol for the Treatment of Ankylosing Spondylitis and Axial Spondyloarthritis Without Radiographic Evidence of Ankylosing Spondylitis. Submission by UCB, Manufacturer of Certolizumab Pegol, September 2014 n.d.
- Pfizer . National Institute for Health and Care Excellence (NICE) Multiple Technology Appraisal: Ankylosing Spondylitis and Axial Spondyloarthritis (Non-Radiographic) – Adalimumab, Etanercept Infliximab and Golimumab n.d.
- Merck Sharp & Dohme . National Institute for Health and Care Excellence (NICE) Multiple Technology Appraisal: TNF-Alpha Inhibitors for Ankylosing Spondylitis and Axial Spondyloarthritis Without Radiographic Evidence of Ankylosing Spondylitis (Including a Review of TA143 and TA233) n.d.
- McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al. Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11280.
- Higgins JPT, Altman DG, Gotzsche PC, Jueni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Corbett MS, Higgins JPT, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. JRSM 2014;5:79-85. http://dx.doi.org/10.1002/jrsm.1090.
- Pedersen SJ, Sorensen IJ, Loft AG, Hindrup JS, Thamsborg G, Asmussen K, et al. Efficacy of Adalimumab in Patients With Axial Spondyloarthritis: Results of an Investigator-Initiated 12-Weeks Randomized Double-Blind Placebo Controlled Trial With a 12 Weeks Open-Label Extension Phase n.d.
- Sieper J, Lenaerts J, Wollenhaupt J, Rudwaleit M, Mazurov VI, Myasoutova L, et al. Efficacy and safety of infliximab plus naproxen versus naproxen alone in patients with early, active axial spondyloarthritis: results from the double-blind, placebo-controlled INFAST study, Part 1. Ann Rheum Dis 2014;73:101-7. http://dx.doi.org/10.1136/annrheumdis-2012-203201.
- Dougados M, Wood E, Combe B, Schaeverbeke T, Miceli-Richard C, Berenbaum F, et al. Evaluation of the nonsteroidal anti-inflammatory drug-sparing effect of etanercept in axial spondyloarthritis: results of the multicenter, randomized, double-blind, placebo-controlled SPARSE study. Arthritis Res Ther 2014;16. http://dx.doi.org/10.1186/s13075-014-0481-5.
- ClinicalTrials.gov . Danish Multicenter Study of Adalimumab in Spondyloarthritis n.d. http://ClinicalTrials.gov/show/NCT00477893 (accessed 1 December 2014).
- ClinicalTrials.gov . Effect of Golimumab in Participants With Active Axial Spondyloarthritis (P07642 AM2) n.d. http://ClinicalTrials.gov/show/NCT01453725 (accessed 1 December 2014).
- Brandt J, Khariouzov A, Listing J, Haibel H, Sorensen H, Grassnickel L, et al. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum 2003;48:1667-75. http://dx.doi.org/10.1002/art.11017.
- Zhang J, Huang F, Chen SL, Gu JR, Li ZG, Wu DH, et al. A multicenter, double-blind, placebo-controlled, randomized III clinical study of etanercept in the treatment of Chinese subjects with active ankylosing spondylitis. Int J Rheum Dis 2010;13.
- Zhang J, Zhang YM, Zhang JL, Deng XH, Huang F. Efficacy of etanercept in patients with ankylosing spondylitis: a double-blind, randomized, placebo controlled trial. Chin J New Drugs 2009;18:1846-9.
- Inman RD, Maksymowych WP. Candle Study Group . A double-blind, placebo-controlled trial of low dose infliximab in ankylosing spondylitis. J Rheumatol 2010;37:1203-10. http://dx.doi.org/10.3899/jrheum.091042.
- Barkham N, Keen HI, Coates LC, O’Connor P, Hensor E, Fraser AD, et al. Clinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging-determined early sacroiliitis. Arthritis Rheum 2009;60:946-54. http://dx.doi.org/10.1002/art.24408.
- Barkham N, Keen HI, Coates LC, O’Connor P, Hensor EMA, Fraser A, et al. A randomized controlled trial of infliximab shows clinical and MRI efficacy in HLA B27 positive very early ankylosing spondylitis. Clin Exp Rheumatol 2008;26.
- Haibel H, Rudwaleit M, Listing J, Heldmann F, Wong RL, Kupper H, et al. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum 2008;58:1981-91. http://dx.doi.org/10.1002/art.23606.
- Haibel H, Rudwaleit M, Heldmann F, Listing J, Wong R, Kupper H, et al. Adalimumab is effective in patients with preradiographic axial spondyloarthritis: results of a 12-week, randomized controlled trial. Rheumatology 2007;46.
- Haibel H, Rudwaleit M, Heldmann F, Listing J, Wong R, Kupper H, et al. Efficacy of adalimumab in the treatment of preradiographic axial spondyloarthritis: results of a 12-week, randomized controlled trial. Arthritis Rheum 2006;54:4044-5. http://dx.doi.org/10.1002/art.23606.
- Hu Z, Xu M, Li Q, Lin Z, Liao Z, Cao S, et al. Adalimumab significantly reduces inflammation and serum DKK-1 level but increases fatty deposition in lumbar spine in active ankylosing spondylitis. Int J Rheum Dis 2012;15:358-65. http://dx.doi.org/10.1111/j.1756-185X.2012.01734.x.
- Huang F, Gu J, Zhu P, Bao C, Xu J, Xu H, et al. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis 2014;73:587-94. http://dx.doi.org/10.1136/annrheumdis-2012-202533.
- Lambert RG, Salonen D, Rahman P, Inman RD, Wong RL, Einstein SG, et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2007;56:4005-14. http://dx.doi.org/10.1002/art.23044.
- Sieper J, van der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis 2013;72:815-22. http://dx.doi.org/10.1136/annrheumdis-2012-201766.
- Sieper J, Van Der Heijde D, Dougados M, Mease PJ, Brown LS, Pangan A. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis results from a phase 3 study. Arthritis Rheum 2011;63. http://dx.doi.org/10.1136/annrheumdis-2012-201766.
- Maksymowych WP, Mease PJ, Rao S, Pangan A, Brown LS, Arora V, et al. Effect of adalimumab on function, health-related quality of life, work productivity, and daily activities in patients with non-radiographic axial spondyloarthritis. Arthritis Rheum 2011;63.
- van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BA, Braun J, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;54:2136-46. http://dx.doi.org/10.1002/art.21913.
- Davis JC, Revicki D, van der Heijde DM, Rentz AM, Wong RL, Kupper H, et al. Health-related quality of life outcomes in patients with active ankylosing spondylitis treated with adalimumab: results from a randomized controlled study. Arthritis Rheum 2007;57:1050-7. http://dx.doi.org/10.1002/art.22887.
- Davis J, Kivitz A, Schiff M, Sieper J, Dijkmans B, Braun J, et al. Major clinical response and partial remission in ankylosing spondylitis subjects treated with adalimumab: the ATLAS trial. Arthritis Rheum 2005;52:S208-9.
- Landewe R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis 2014;73:39-47. http://dx.doi.org/10.1136/annrheumdis-2013-204231.
- van der Heijde D, Maksymowych WP, Landewe R, Stach C, Hoepken B, Fichtner A, et al. Effect of certolizumab pegol on inflammation of spine and sacroiliac joints in patients with axial spondyloarthritis: 12-week magnetic resonance imaging results of rapid-axSpA study. Ann Rheum Dis 2013;72:A515-16. http://dx.doi.org/10.1136/annrheumdis-2013-eular.1546.
- Sieper J, Kivitz A, Van Tubergen A, Deodhar A, Coteur G, Woltering F, et al. Rapid improvements in patient-reported outcomes with certolizumab pegol in patients with axial spondyloarthritis, including ankylosing spondylitis: 24-week results of rapid-axSpA study. Ann Rheum Dis 2013;72. http://dx.doi.org/10.1136/annrheumdis-2013-eular.888.
- Landewe R, Rudwaleit M, van der Heijde D, Dougados M, Maksymowych WP, Braun J, et al. Effect of certolizumab pegol on signs and symptoms of axial spondyloarthritis, including ankylosing spondylitis and non-radiographic axial spondyloarthritis: 24-week results of rapid-axSpA study. Ann Rheum Dis 2013;72:A86-7. http://dx.doi.org/10.1136/annrheumdis-2013-eular.311.
- Sieper J, Kivitz A, Van Tubergen A, Deodhar A, Coteur G, Woltering F, et al. Rapid improvements in patient-reported outcomes with certolizumab pegol in patients with axial spondyloarthritis, including ankylosing spondylitis and non-radiographic axial spondyloarthritis: 24-week results of a phase 3 double blind randomized placebo-controlled study. Value Health 2013;16. http://dx.doi.org/10.1016/j.jval.2013.03.1150.
- Landewe RBM, Rudwaleit M, van der Heijde D, Dougados M, Maksymowych WP, Braun J, et al. Effect of certolizumab pegol on signs and symptoms of ankylosing spondylitis and non-radiographic axial spondyloarthritis: 24 week results of a double blind randomized placebo-controlled phase 3 axial spondyloarthritis study. Arthritis Rheum 2012;64:S336-7.
- Sieper J, Kivitz AJ, Van Tubergen AM, Deodhar AA, Coteur G, Woltering F, et al. Rapid improvements in patient reported outcomes with certolizumab pegol in patients with axial spondyloarthritis, including ankylosing spondylitis and non-radiographic axial spondyloarthritis: 24 week results of a phase 3 double blind randomized placebo-controlled study. Arthritis Rheum 2012;64.
- Barkham N, Coates LC, Keen H, Hensor E, Fraser A, Redmond A, et al. Double-blind placebo-controlled trial of etanercept in the prevention of work disability in ankylosing spondylitis. Ann Rheum Dis 2010;69:1926-8. http://dx.doi.org/10.1136/ard.2009.121327.
- Davis JC, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg DO, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003;48:3230-6. http://dx.doi.org/10.1002/art.11325.
- Davis JC, van der Heijde D, Braun J, Dougados M, Cush JJ, Clegg DO, et al. Etanercept (Enbrel®) improves signs and symptoms of ankylosing spondylitis: Results of a phase 3 multicenter clinical trial. Ann Rheum Dis 2003;62.
- Dougados M, Braun J, Szanto S, Combe B, Elbaz M, Geher P, et al. Efficacy of etanercept on rheumatic signs and pulmonary function tests in advanced ankylosing spondylitis: results of a randomised double-blind placebo-controlled study (SPINE). Ann Rheum Dis 2011;70:799-804. http://dx.doi.org/10.1136/ard.2010.139261.
- Dougados M, Braun J, Szanto S, Combe B, Elbaz M, Geher P, et al. Efficacy of etanercept on rheumatic signs and pulmonary function tests in advanced ankylosing spondylitis. Results of a randomized double blind placebo-controlled study (SPINE). Arthritis Rheum 2009;60. http://dx.doi.org/10.1136/ard.2010.139261.
- Dougados M. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2014;66:2091-102. http://dx.doi.org/10.1002/art.38721.
- Dougados M, van der Heijde D, Sieper J, Braun J, Maksymowych WP, Citera G, et al. Clinical and imaging efficacy of etanercept in early non-radiographic axial spondyloarthritis: a 12-week, randomized, double-blind, placebo-controlled trial. Ann Rheum Dis 2013;72:A87-8. http://dx.doi.org/10.1136/annrheumdis-2013-eular.313.
- Dougados M, Tsai WC, Saaibi DL, Bonin R, Bukowski J, Pedersen R, et al. Patient-reported outcomes of etanercept in early non-radiographic axial spondyloarthritis: a 12-week, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2013;65:S656-7.
- Gorman JD, Sack KE, Davis JC. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med 2002;346:1349-56. http://dx.doi.org/10.1056/NEJMoa012664.
- Gorman JD, Sack KE, Davis JC. Efficacy of etanercept (Enbrel®) in the treatment of ankylosing spondylitis: a randomized, placebo-controlled, double-blind study. Arthritis Rheum 2001;44.
- Gorman JD, Sack KE, Davis JC. A randomized, double-blind, placebo-controlled trial of (Enbrel®) in the treatment of ankylosing spondylitis. Arthritis Rheum 2001;44.
- Gorman JD, Sack KE, Davis JC. Etanercept in the treatment of ankylosing spondylitis: a randomized, double-blind, placebo-controlled study. Arthritis Rheum 2000;43.
- Calin A, Dijkmans BA, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, et al. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004;63:1594-600. http://dx.doi.org/10.1136/ard.2004.020875.
- Calin A, Dijkmans B, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, et al. Assessments of disease activity and functionality by Enbrel-treated ankylosing spondylitis patients in a multicenter, placebo-controlled trial. Arthritis Rheum 2003;48.
- Calin A, Dijkmans B, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, et al. A multicentre, placebo-controlled trial of enbrel in ankylosing spondylitis. Ann Rheum Dis 2003;62.
- van der Heijde D, Da Silva JC, Dougados M, Geher P, van der Horst-Bruinsma I, Juanola X, et al. Etanercept 50 mg once weekly is as effective as 25 mg twice weekly in patients with ankylosing spondylitis. Ann Rheum Dis 2006;65:1572-7. http://dx.doi.org/10.1136/ard.2006.056747.
- Braun J, McHugh N, Singh A, Wajdula JS, Sato R. Improvement in patient-reported outcomes for patients with ankylosing spondylitis treated with etanercept 50 mg once-weekly and 25 mg twice-weekly. Rheumatology 2007;46:999-1004. http://dx.doi.org/10.1093/rheumatology/kem069.
- Giardina AR, Ferrante A, Ciccia F, Impastato R, Miceli MC, Principato A, et al. A 2-year comparative open label randomized study of efficacy and safety of etanercept and infliximab in patients with ankylosing spondylitis. Rheumatol Int 2010;30:1437-40. http://dx.doi.org/10.1007/s00296-009-1157-3.
- Impastato R, Ciccia F, Ferrante A, Principato A, Giardina A, Cadelo M, et al. Infliximab and etanercept are both effective and safe in patients with ankylosing spondylitis. A two-year randomised study. Ann Rheum Dis 2007;66. http://dx.doi.org/10.1007/s00296-009-1157-3.
- Inman RD, Davis JC, van der Heijde D, Diekman L, Sieper J, Kim SI, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 2008;58:3402-12. http://dx.doi.org/10.1002/art.23969.
- Braun J, Davis J, Van Der Heijde D, Deodhar A, Diekman L, Sieper J, et al. Golimumab, a new, human, TNFA antibody, in ankylosing spondylitis: 24-week efficacy and safety results of the golimumab, a new, human, a antibody, in ankylosing spondylitis: 24-week efficacy and safety results of the GO-RAISE study. Intern Med J 2009;39.
- Braun J, Davis J, Van Der Heijde D, Deodhar A, Diekman L, Sieper J, et al. Golimumab, a new, human, TNFalpha antibody, in ankylosing spondylitis (AS): 24-week efficacy and safety results of the go-raise study. Rheumatology 2009;48.
- Braun J, Davis JC, van der Heijde D, Diekman L, Sieper J, Kim SI, et al. Golimumab, a new, human, TNF-alpha antibody administered subcutaneously every 4 weeks, in ankylosing spondylitis: 24-week efficacy and safety results of the randomized, placebo-controlled GO-RAISE study. Clin Exp Rheumatol 2008;26.
- Braun J, Davis JC, Van der Heijde D, Diekman L, Sieper J, Kim SI, et al. Golimumab, a new, human, TNF-alpha antibody administered as a monthly subcutaneous injection in ankylosing spondylitis (AS): 24-week efficacy and safety results of the randomized, placebo-controlled GO-RAISE study. Arthritis Rheum 2007;56:4236-7.
- Bao C, Huang F, Khan MA, Fei K, Wu Z, Han C, et al. Safety and efficacy of golimumab in Chinese patients with active ankylosing spondylitis: 1-year results of a multicentre, randomized, double-blind, placebo-controlled phase III trial. Rheumatology 2014;53:1654-63. http://dx.doi.org/10.1093/rheumatology/keu132.
- Bao C, Huang F, Khan MA, Fei K, Wu Z, Hsia EC. Golimumab administered subcutaneously every 4 weeks in Chinese patients with active ankylosing spondylitis: week 24 safety and efficacy results from a randomized, placebo-controlled study. Arthritis Rheum 2012;64.
- Tam L-S, Shang Q, Kun EW, Lee K-L, Yip M-L, Li M, et al. The effects of golimumab on subclinical atherosclerosis and arterial stiffness in ankylosing spondylitis – a randomized, placebo-controlled pilot trial. Rheumatology 2014;53:1065-74. http://dx.doi.org/10.1093/rheumatology/ket469.
- Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359:1187-93. http://dx.doi.org/10.1016/S0140-6736(02)08215-6.
- Brandt J, Listing I, Alten R, Krause A, Gromnica-Ihle E, Kellner H, et al. Quality of life improvement in patients with severe ankylosing spondylitis upon treatment with the anti-TNF alpha antibody infliximab in a placebo controlled multicenter trial. Arthritis Rheum 2001;44.
- Marzo-Ortega H, McGonagle D, Jarrett S, Haugeberg G, Hensor E, O’Connor P, et al. Infliximab in combination with methotrexate in active ankylosing spondylitis: a clinical and imaging study. Ann Rheum Dis 2005;64:1568-75. http://dx.doi.org/10.1136/ard.2004.022582.
- Van den Bosch F, Kruithof E, Baeten D, Herssens A, de Keyser F, Mielants H, et al. Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis Rheum 2002;46:755-65. http://dx.doi.org/10.1002/art.511.
- van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582-91. http://dx.doi.org/10.1002/art.20852.
- van der Heijde D, Landewe R, Baraliakos X, Houben H, van Tubergen A, Williamson P, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063-70. http://dx.doi.org/10.1002/art.23901.
- van der Heijde D, Han C, DeVlam K, Burmester G, van den Bosch F, Williamson P, et al. Infliximab improves productivity and reduces workday loss in patients with ankylosing spondylitis: results from a randomized, placebo-controlled trial. Arthritis Rheum 2006;55:569-74. http://dx.doi.org/10.1002/art.22097.
- van der Heijde D, Landewe R, Hermann K, Han J, Williamson P, Braun J. The effect of infliximab therapy on spinal inflammation assessed by magnetic resonance imaging in a randomized, placebo-controlled trial of 279 patients with ankylosing spondylitis. Ann Rheum Dis 2005;64.
- van der Heijde D, Han C, Bala M, Williamson P, Han J, Braun J. Infliximab improves fatigue and pain in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Ann Rheum Dis 2005;64:318-19.
- Braun J, Maksymowych WP, Dougados M, Steinfeld S, Dewoody K, Williamson P, et al. Efficacy of infliximab in subgroups of patients with ankylosing spondylitis: results from the ankylosing spondylitis study for the evaluation of recombinant infliximab therapy (ASSERT). Ann Rheum Dis 2004;63.
- Braun J, Kellner H, Deodhar A, Inman R, Han C, DeWoody K, et al. Infliximab improves quality of life in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Ann Rheum Dis 2004;63.
- van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson R, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a 24-week randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582-91.
- Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis 2013;72:1605-12. http://dx.doi.org/10.1136/annrheumdis-2012-203091.
- Park W, Hrycaj P, Kovalenko V, Miranda P, Gutierrez-Urena S, Lee Y, et al. A randomized, double-blind, phase 1 study demonstrates equivalence in pharmacokinetics, safety, and efficacy of CT-P13 and infliximab in patients with ankylosing spondylitis. Ann Rheum Dis 2013;71.
- Glintborg B, Ostergaard M, Krogh NS, Dreyer L, Kristensen HL, Hetland ML. Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti-tumor necrosis factor: results from 8 years’ surveillance in the Danish nationwide DANBIO registry. Ann Rheum Dis 2010;69:2002-8. http://dx.doi.org/10.1136/ard.2009.124446.
- Pavy S, Brophy S, Calin A. Establishment of the minimum clinically important difference for the bath ankylosing spondylitis indices: a prospective study. J Rheumatol 2005;32:80-5.
- Summary Minutes of the Arthritis Advisory Committee Meeting July 23, 2013. Silver Spring, MD: FDA; 2013.
- FDA Briefing Package: sBLA 125160/215: Cimzia® (Certolizumab) for the Treatment of Active Axial Spondyloarthritis, Including Patients with Ankylosing Spondylitis. Silver Spring, MD: FDA; 2013.
- FDA Briefing Package: sBLA 125057/323: Adalimumab for the Treatment of Active Non-Radiographic Axial Spondyloarthritis in Adults with Objective Signs of Inflammation by Elevated C-reactive Protein (CRP) or Magnetic Resonance Imaging (MRI), Who Have Had an Inadequate Response to, or are Intolerant to, a Nonsteroidal Anti-Inflammatory Drug. Silver Spring, MD: FDA; 2013.
- van der Heijde D, Salonen D, Weissman BN, Landewe R, Maksymowych WP, Kupper H, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 2009;11. http://dx.doi.org/10.1186/ar2794.
- Boonen A, van der Heijde D, Landewe R, Guillemin F, Rutten-van Molken M, Dougados M, et al. Direct costs of ankylosing spondylitis and its determinants: an analysis among three European countries. Ann Rheum Dis 2003;62:732-40. http://dx.doi.org/10.1136/ard.62.8.732.
- van der Heijde D, Landewe R, Einstein S, Ory P, Vosse D, Ni L, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 2008;58:1324-31. http://dx.doi.org/10.1002/art.23471.
- Braun J, Baraliakos X, Hermann KG, Deodhar A, van der Heijde D, Inman R, et al. The effect of two golimumab doses on radiographic progression in ankylosing spondylitis: results through 4 years of the GO-RAISE trial. Ann Rheum Dis 2014;73:1107-13. http://dx.doi.org/10.1136/annrheumdis-2012-203075.
- Haroon N, Inman RD, Learch TJ, Weisman MH, Lee M, Rahbar MH, et al. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013;65:2645-54. http://dx.doi.org/10.1002/art.38070.
- Baraliakos X, Haibel H, Listing J, Sieper J, Braun J. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis 2014;73:710-15. http://dx.doi.org/10.1136/annrheumdis-2012-202698.
- Baraliakos X, Listing J, Brandt J, Haibel H, Rudwaleit M, Sieper J, et al. Radiographic progression in patients with ankylosing spondylitis after 4 yrs of treatment with the anti-TNF-alpha antibody infliximab. Rheumatology 2007;46:1450-3. http://dx.doi.org/10.1093/rheumatology/kem166.
- Ramiro S, van der Heijde D, van Tubergen A, Stolwijk C, Dougados M, van den Bosch F, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014;73:1455-61. http://dx.doi.org/10.1136/annrheumdis-2014-205178.
- Ramiro S, Stolwijk C, van Tubergen A, van der Heijde D, Dougados M, van den Bosch F, et al. Evolution of radiographic damage in ankylosing spondylitis over 12 years of follow-up. Ann Rheum Dis 2013;72:665-6. http://dx.doi.org/10.1136/annrheumdis-2013-eular.1973.
- Bonafede M, Fox KM, Watson C, Princic N, Gandra SR. Treatment patterns in the first year after initiating tumor necrosis factor blockers in real-world settings. Adv Ther 2012;29:664-74. http://dx.doi.org/10.1007/s12325-012-0037-5.
- Choquette D, Sauvageau D, Raynauld JP. Retention rate of adalimumab, etanercept and infliximab at 5 years in patients with ankylosing spondylitis: report from the rhumadata computerized database. Ann Rheum Dis 2013;71.
- Gulfe A, Kapetanovic ML, Kristensen E. Efficacy and drug survival of anti-tumor necrosis factor-alpha therapies in patients with non-radiographic axial spondyloarthritis: an observational cohort study from Southern Sweden. Scand J Rheumatol 2014;43:493-7. http://dx.doi.org/10.3109/03009742.2014.918173.
- Nell-Duxneuner V, Schroeder Y, Reichardt B, Bucsics A. The use of TNF-inhibitors in ankylosing spondylitis in Austria from 2007 to 2009 – a retrospective analysis. Int J Clin Pharmacol Ther 2012;50:867-72. http://dx.doi.org/10.5414/CP201707.
- Yeaw J, Watson C, Fox KM, Schabert VF, Goodman S, Gandra SR. Treatment patterns following discontinuation of adalimumab, etanercept, and infliximab in a US managed care sample. Adv Ther 2014;31:410-25. http://dx.doi.org/10.1007/s12325-014-0110-3.
- Scire CA, Caporali R, Sarzi-Puttini P, Frediani B, Di Franco M, Tincani A, et al. Drug survival of the first course of anti-TNF agents in patients with rheumatoid arthritis and seronegative spondyloarthritis: analysis from the MonitorNet database. Clin Exp Rheumatol 2013;31:857-63.
- Zufferey P, Ghosn J, Becce F, Ciurea A, Aubry-Rozier B, Finckh A, et al. Anti-tumor necrosis factor drug survival in axial spondyloarthritis is independent of the classification criteria. Rheumatol Int 2014;35:295-302. http://dx.doi.org/10.1007/s00296-014-3094-z.
- Pavelka K, Forejtova S, Stolfa J, Chroust K, Buresova L, Mann H, et al. Anti-TNF therapy of ankylosing spondylitis in clinical practice. Results from the Czech national registry ATTRA. Clin Exp Rheumatol 2009;27:958-63.
- Lie E, van der Heijde D, Uhlig T, Mikkelsen K, Rødevand E, Koldingsnes W, et al. Effectiveness of switching between TNF inhibitors in ankylosing spondylitis: data from the NOR-DMARD register. Ann Rheum Dis 2011;70:157-63. http://dx.doi.org/10.1136/ard.2010.131797.
- Glintborg B, Ostergaard M, Krogh NS, Tarp U, Manilo N, Loft AGR, et al. Clinical response, drug survival and predictors thereof in 432 ankylosing spondylitis patients after switching tumour necrosis factor alpha inhibitor therapy: results from the Danish nationwide DANBIO registry. Ann Rheum Dis 2013;72:1149-55. http://dx.doi.org/10.1136/annrheumdis-2012-201933.
- Rudwaleit M, Claudepierre P, Wordsworth P, Cortina EL, Sieper J, Kron M, et al. Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol 2009;36:801-8. http://dx.doi.org/10.3899/jrheum.081048.
- Singh JA, Wells GA, Christensen R, Ghogomu TE, Maxwell L, MacDonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011;2. http://dx.doi.org/10.1002/14651858.cd008794.pub2.
- Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf 2011;20:119-30. http://dx.doi.org/10.1002/pds.2046.
- Rodgers M, Epstein D, Bojke L, Yang H, Craig D, Fonseca T, et al. Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15100.
- van der Heijde D, Schiff MH, Sieper J, Kivitz AJ, Wong RL, Kupper H, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis 2009;68:922-9. http://dx.doi.org/10.1136/ard.2007.087270.
- Sieper J, van der Heijde D, Dougados M, Brown LS, Lavie F, Pangan AL. Early response to adalimumab predicts long-term remission through 5 years of treatment in patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:700-6. http://dx.doi.org/10.1136/annrheumdis-2011-200358.
- Braun J, Deodhar A, Inman RD, van der Heijde D, Mack M, Xu S, et al. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 104-week results of the GO-RAISE study. Ann Rheum Dis 2012;71:661-7. http://dx.doi.org/10.1136/ard.2011.154799.
- Deodhar A, Braun J, Inman RD, van der Heijde D, Zhou Y, Xu S, et al. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 5-year results of the GO-RAISE study. Ann Rheum Dis 2015;74:757-61. http://dx.doi.org/10.1136/annrheumdis-2014-205862.
- Davis JC, van der Heijde DM, Braun J, Dougados M, Clegg DO, Kivitz AJ, et al. Efficacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis. Ann Rheum Dis 2008;67:346-52. http://dx.doi.org/10.1136/ard.2007.078139.
- Davis JC, van der Heijde DM, Braun J, Dougados M, Cush J, Clegg D, et al. Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann Rheum Dis 2005;64:1557-62. http://dx.doi.org/10.1136/ard.2004.035105.
- Dijkmans B, Emery P, Hakala M, Leirisalo-Repo M, Mola EM, Paolozzi L, et al. Etanercept in the longterm treatment of patients with ankylosing spondylitis. J Rheumatol 2009;36:1256-64. http://dx.doi.org/10.3899/jrheum.081033.
- Martin-Mola E, Sieper J, Leirisalo-Repo M, Dijkmans BA, Vlahos B, Pedersen R, et al. Sustained efficacy and safety, including patient-reported outcomes, with etanercept treatment over 5 years in patients with ankylosing spondylitis. Clin Exp Rheumatol 2010;28:238-45.
- Baraliakos X, Listing J, Fritz C, Haibel H, Alten R, Burmester GR, et al. Persistent clinical efficacy and safety of infliximab in ankylosing spondylitis after 8 years – early clinical response predicts long-term outcome. Rheumatology 2011;50:1690-9. http://dx.doi.org/10.1093/rheumatology/ker194.
- Dean LE, Macfarlane GJ, MacDonald AG. The Natural History of Ankylosing Spondylitis – Results from the Scotland and Ireland Registry for Ankylosing Spondylitis (SIRAS) n.d.
- Stone MA, Sengupta R, Gordon D, Pomeroy E, Mogg R, Keat A. Longitudinal analyses of disease outcomes in ankylosing spondylitis yield insight into the natural history of AS. Ann Rheum Dis 2007;66.
- Machado P, Landewe R, Braun J, Hermann KG, Baker D, van der Heijde D. Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheum Dis 2010;69:1465-70. http://dx.doi.org/10.1136/ard.2009.124206.
- Kobelt G, Andlin-Sobocki P, Brophy S, Jonsson L, Calin A, Braun J. The burden of ankylosing spondylitis and the cost-effectiveness of treatment with infliximab (Remicade®). Rheumatology 2004;43:1158-66. http://dx.doi.org/10.1093/rheumatology/keh271.
- Kiltz U, Baraliakos X, Karakostas P, Igelmann M, Kalthoff L, Klink C, et al. Do patients with non-radiographic axial spondylarthritis differ from patients with ankylosing spondylitis?. Arthritis Care Res 2012;64:1415-22. http://dx.doi.org/10.1002/acr.21688.
- Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Maerker-Hermann E, Zeidler H, et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis 2011;70:1369-74. http://dx.doi.org/10.1136/ard.2010.145995.
- Poddubnyy D, Haibel H, Listing J, Marker-Hermann E, Zeidler H, Braun J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum 2012;64:1388-98. http://dx.doi.org/10.1002/art.33465.
- Stone MA, Pomeroy E, Keat A, Sengupta R, Hickey S, Dieppe P, et al. Assessment of the impact of flares in ankylosing spondylitis disease activity using the Flare Illustration. Rheumatology 2008;47:1213-18. http://dx.doi.org/10.1093/rheumatology/ken176.
- Sampaio-Barros PD, Bortoluzzo AB, Conde RA, Costallat LTL, Samara AM, Bertolo MB. Undifferentiated spondyloarthritis: a longterm followup. J Rheumatol 2010;37:1195-9. http://dx.doi.org/10.3899/jrheum.090625.
- Sampaio-Barros PD, Bertolo MB, Kraemer MHS, Marques-Neto JF, Samara AM. Undifferentiated spondyloarthropathies: a 2-year follow-up study. Clin Rheumatol 2001;20:201-6. http://dx.doi.org/10.1007/s100670170066.
- Boonen A, Sieper J, van der Heijde D, Dougados M, Bukowski JF, Valluri S, et al. The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum 2015;44:556-62. http://dx.doi.org/10.1016/j.semarthrit.2014.10.009.
- Kobelt G, Sobocki P, Sieper J, Braun J. Comparison of the cost-effectiveness of infliximab in the treatment of ankylosing spondylitis in the United Kingdom based on two different clinical trials. Int J Technol Assess Health Care 2007;23:368-75. http://dx.doi.org/10.1017/S0266462307070432.
- Ara RM, Reynolds AV, Conway P. The cost-effectiveness of etanercept in patients with severe ankylosing spondylitis in the UK. Rheumatology 2007;46:1338-44. http://dx.doi.org/10.1093/rheumatology/kem133.
- Botteman MF, Hay JW, Luo MP, Curry AS, Wong RL, Hout BA. Cost effectiveness of adalimumab for the treatment of ankylosing spondylitis in the United Kingdom. Rheumatology 2007;46:1320-8. http://dx.doi.org/10.1093/rheumatology/kem031.
- Armstrong N, Joore M, van Asselt T, Misso K, Manning N, Tomini F, et al. Golimumab for the treatment of ankylosing spondylitis: a NICE single technology appraisal. Pharmacoeconomics 2013;31:415-25. http://dx.doi.org/10.1007/s40273-013-0049-2.
- ClinicalTrials.gov . Study Comparing Etanercept (ETN) Against a Placebo for Etanercept on a Background Nonsteroidal Anti Inflammatory Drug (NSAIDs) in the Treatment of Early Spondyloarthritis (SpA) Patients Who Do Not Have X-Ray Structural Changes (AS EARLY) n.d.
- ClinicalTrials.gov . Study Comparing Etanercept 50 Mg Once Weekly to 25 Mg Twice Weekly in Patients With Ankylosing Spondylitis n.d.
- Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, et al. Two year maintenance of efficacy and safety of infliximab in the treatment of ankylosing spondylitis. Ann Rheum Dis 2005;64:229-34. http://dx.doi.org/10.1136/ard.2004.025130.
- Ara RM, Packham J, Haywood K. The direct healthcare resource costs associated with ankylosing spondylitis patients attending a UK secondary care rheumatology unit. Ann Rheum Dis 2006;65.
- Rafia R, Ara R, Packham J, Haywood K, Healey E. Healthcare costs and productivity losses directly attributable to ankylosing spondylitis. Clin Exp Rheumatol 2012;30:246-53.
- Keat A, Barkham N, Bhalla A, Gaffney K, Marzo-Ortega H, Paul S, et al. BSR guidelines for prescribing TNF-alpha blockers in adults with ankylosing spondylitis. Report of a working party of the British Society for Rheumatology. Rheumatology 2005;44:939-47. http://dx.doi.org/10.1093/rheumatology/keh669.
- Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Ser B 2002;64:583-616. http://dx.doi.org/10.1111/1467-9868.00353.
- Welton N, Ades AE. Research decisions in the face of heterogeneity: what can a new study tell us?. Health Econ 2012;21:1196-200. http://dx.doi.org/10.1002/hec.1797.
- Vastesaeger N, van der Heijde D, Inman RD, Wang YX, Deodhar A, Hsu B, et al. Predicting the outcome of ankylosing spondylitis therapy. Ann Rheum Dis 2011;70:973-81. http://dx.doi.org/10.1136/ard.2010.147744.
- Lord PA, Farragher TM, Lunt M, Watson KD, Symmons DP, Hyrich KL, et al. Predictors of response to anti-TNF therapy in ankylosing spondylitis: results from the British Society for Rheumatology Biologics Register. Rheumatology 2010;49:563-70. http://dx.doi.org/10.1093/rheumatology/kep422.
- Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity – subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making 2013;33:618-40. http://dx.doi.org/10.1177/0272989X13485157.
- Technology Appraisal TA199: Etanercept, Infliximab and Adalimumab for the Treatment of Psoriatic Arthritis. London: NICE; 2010.
- NHS Reference Costs 2012/13. London: Department of Health; 2013.
- Poddubnyy D, Haibel H, Braun J, Rudwaleit M, Sieper J. Patients with non-radiographic axial spondyloarthritis and ankylosing spondylitis demonstrate the same clinical disease course over two years: results from the GESPIC cohort. Ann Rheum Dis 2014;73. http://dx.doi.org/10.1136/annrheumdis-2014-eular.3599.
- British National Formulary (Online). London: BMJ Group and Pharmaceutical Press; n.d.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Henriksson M, Palmer S, Chen R, Damant J, Fitzpatrick NK, Abrams K, et al. Assessing the cost effectiveness of using prognostic biomarkers with decision models: case study in prioritising patients waiting for coronary artery surgery. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.b5606.
- Rees F, Peffers G, Bell C, Obrenovic K, Sandhu R, Packham J, et al. Compliance with NICE guidance on the use of anti-TNF alpha agents in ankylosing spondylitis: an east and west Midlands regional audit. Clin Med 2012;12:324-7. http://dx.doi.org/10.7861/clinmedicine.12-4-324.
- University Hospital Montpellier . Effect of Spacing of Anti-TNF Drugs in Ankylosing Spondylitis With Low Disease Activity (SPACING) 2012. http://ClinicalTrials.gov/show/NCT01610947 (accessed 11 December 2014).
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2011.
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. http://dx.doi.org/10.1002/sim.1186.
- Haibel H, Rios V, Hermann KG, Althoff C, Rudwaleit M, Kupper H, et al. No progression of chronic sacroiliac changes in patients with active, non-radiographic axial spondyloarthritis treated with adalimumab over 52 weeks. Arthritis Rheum 2009;60.
- Haibel H, Rudwaleit M, Amtenbrink A, Heldmann F, Listing J, Wong R, et al. Adalimumab is effective in patients with preradiographic axial spondyloarthritis (PRSPA): results of a 12-week, randomised controlled trial with a 40-week open-label extension (OLE). Rheumatology 2008;47.
- Haibel H, Rudwaleit M, Amtenbrink A, Heldmann F, Listing J, Kupper H, et al. Efficacy of adalimumab in the treatment of preradiographic axial spondyloarthritis: results of a 20-week, randomized controlled trial with an open-label extension phase. Z Rheumatol 2007;66.
- Haibel H, Rudwaleit M, Amtenbrink A, Heldmann F, Listing J, Wong R, et al. Efficacy of adalimumab in the treatment of preiradiographic axial spondyloarthritis: results of a 20-week, randomized controlled trial with an open-label extension phase. Ann Rheum Dis 2007;66. http://dx.doi.org/10.1002/art.23606.
- van der Heijde D, Maksymowych W, Sieper J, Lambert R, Brown MA, Rathmann S, et al. Relationship between MRI and clinical remission in patients with non-radiographic axial spondyloarthritis after two years of adalimumab therapy. Ann Rheum Dis 2013;72. http://dx.doi.org/10.1136/annrheumdis-2013-eular.889.
- Sieper J, Baeten DL, Van Den Bosch F, Rathmann SS, Anderson J, Pangan AL. Sustained clinical remission in patients with non-radiographic axial spondyloarthritis after two years of adalimumab treatment. Ann Rheum Dis 2013;72. http://dx.doi.org/10.1136/annrheumdis-2013-eular.314.
- Sieper J, Van Der Heijde D, Dougados M, Van Den Bosch F, Goupille P, Sarkar S, et al. Sustained efficacy of adalimumab in patients with non-radiographic axial spondyloarthritis: week 68 results from ability 1. Ann Rheum Dis 2013;71.
- van der Heijde DM, Mease PJ, Pangan AL, Rao SA, Chen N, Cifaldi M. Improvement in physical function, health-related quality of life, and work productivity with adalimumab treatment in non-radiographic axial spondyloarthritis. Arthritis Rheum 2013;65.
- Sieper J, Van Der Heijde D, Dougados M, Van Den Bosch F, Goupille P, Rathmann SS, et al. Sustained efficacy of adalimumab in patients with non-radiographic axial spondyloarthritis with positive MRI of the sacroiliac joints or spine or elevated C-reactive protein at baseline. Rheumatology 2013;52:i52-3.
- van der Heijde D, Mease P, Pangan AL, Rao S, Chen N, Cifaldi MA. Improvement in physical function, health-related quality of life, and work productivity with adalimumab treatment in nonradiographic axial SPA: WK-52 results from ability-1. Arthritis Rheum 2012;64.
- Kimel M, Revicki D, Rao S, Fryback D, Feeny D, Harnam N, et al. Norms-based assessment of patient-reported outcomes associated with adalimumab monotherapy in patients with ankylosing spondylitis. Clin Exp Rheumatol 2011;29:624-32.
- van der Heijde DM, Revicki DA, Gooch KL, Wong RL, Kupper H, Harnam N, et al. Physical function, disease activity, and health-related quality-of-life outcomes after 3 years of adalimumab treatment in patients with ankylosing spondylitis. Arthritis Res Ther 2009;11. http://dx.doi.org/10.1186/ar2790.
- Revicki DA, Luo MP, Wordsworth P, Wong RL, Chen N, Davis JC, et al. Adalimumab reduces pain, fatigue, and stiffness in patients with ankylosing spondylitis: results from the adalimumab trial evaluating long-term safety and efficacy for ankylosing spondylitis (ATLAS). J Rheumatol 2008;35:1346-53.
- van der Heijde D, Breban MA, Halter DG, DiVittorio G, Bratt J, Cantini F, et al. Sustained improvement of spinal mobility, physical function, and quality of life in patients with ankylosing spondylitis: 5-year results. Arthritis Rheum 2011;63. http://dx.doi.org/10.1093/rheumatology/keu438.
- Maksymowych W, Van Der Heijde D, Landewe R, Pangan A, Brown S, Lavie F. Predictors of radiographic progression in adalimumab-treated patients with Ankylosing Spondylitis. Arthritis Rheum 2010;62. http://dx.doi.org/10.1186/ar2794.
- van der Heijde D, Emery P, Patra K, Lavie F. Relationship between bone formation in patients with ankylosing spondylitis and clinical signs and symptoms. Rheumatology 2010;49.
- Scott DG, Van Der Heijde D, Schiff MH, Sieper J, Kivitz A, Dijkmans BA, et al. Improvement in long-term spinal mobility in patients with ankylosing spondylitis (AS) is sustained during up to 3 years of adalimumab treatment: 3-year atlas results. Rheumatology 2009;48. http://dx.doi.org/10.1136/ard.2007.087270.
- Landewe RBM, Rudwaleit M, Van Der Heijde DM, Dougados M, Mease PJ, Reveille JD, et al. Effect of certolizumab pegol over 48 weeks in patients with axial spondyloarthritis, including ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Rheum 2013;65. http://dx.doi.org/10.1136/annrheumdis-2013-204231.
- Sieper J, Kivitz A, Van Tubergen A, Deodhar A, Coteur G, Singh P, et al. Long-term maintenance of improvements in patient-reported outcomes with certolizumab pegol in patients with axial spondyloarthritis, including ankylosing spondylitis and non-radiographic axial spondyloarthritis: 48-week results of the rapid-axspa study. Value Health 2013;16. http://dx.doi.org/10.1016/j.jval.2013.08.1523.
- Maksymowych W, Landewe R, Rudwaleit M, van der Heijde D, Dougados M, Mease P, et al. Effect of certolizumab pegol over 48 weeks in patients with axial spondyloarthritis, including ankylosing spondylitis and non-radiographic axial spondyloarthritis. J Rheumatol 2014;41:1496-7.
- Boonen A, Patel V, Traina S, Chiou CF, Maetzel A, Tsuji W. Rapid and sustained improvement in health-related quality of life and utility for 72 weeks in patients with ankylosing spondylitis receiving etanercept. J Rheumatol 2008;35:662-7.
- Davis JC, van der Heijde D, Dougados M, Woolley JM. Reductions in health-related quality of life in patients with ankylosing spondylitis and improvements with etanercept therapy. Arthritis Rheum 2005;53:494-501. http://dx.doi.org/10.1002/art.21330.
- Dougados M, Van Der Heijde D, Sieper J, Braun J, Maksymowych WP, Citera G, et al. Safety and efficacy of etanercept in early non-radiographic axial spondyloarthritis: a randomized, double-blind, placebo-controlled trial at 24 weeks. Arthritis Rheum 2013;65:S642-3.
- Davis J, Webb A, Lund S, Sack K. Results from an open-label extension study of etanercept in ankylosing spondylitis. Arthritis Rheum 2004;51:302-4. http://dx.doi.org/10.1002/art.20241.
- Bao C, Huang F, Khan MA, Fei K, Wu Z, Zhuang Y, et al. Safety and efficacy of golimumab, a human anti-TNF monoclonal antibody injected subcutaneously every 4 weeks, in Chinese patients with active ankylosing spondylitis: one-year results of a phase 3, randomized, placebo-controlled study. Ann Rheum Dis 2013;72. http://dx.doi.org/10.1136/annrheumdis-2013-eular.2835.
- Bao C, Huang F, Khan A, Fei K, Wu Z, Zhuang Y, et al. Safety and efficacy of golimumab, a human anti-tumor necrosis factor monoclonal antibody injected subcutaneously every 4 weeks, in Chinese patients with active ankylosing spondylitis: 1-year results of a phase 3, randomized, placebo-controlled study. Arthritis Rheum 2013;65.
- van der Heijde D, Braun J, Deodhar A, Inman RD, Xu S, Mack ME, et al. Comparison of three enthesitis indices in a multicentre, randomized, placebo-controlled trial of golimumab in ankylosing spondylitis (GO-RAISE). Rheumatology 2013;52:321-5. http://dx.doi.org/10.1093/rheumatology/kes251.
- Deodhar A, Braun J, Inman R, Van Der Heijde D, Zhou Y, Hsu B. Long-term safety and efficacy of golimumab in the treatment of ankylosing spondylitis: results through 5 years of the go-raise trial. Ann Rheum Dis 2013;72. http://dx.doi.org/10.1136/annrheumdis-2013-eular.880.
- Braun J, Van Der Heijde D, Hermann KG, Baraliakos X, Deodhar A, Beutler A, et al. The effect of anti-tumor necrosis factor therapy with golimumab on radiographic progression in definite ankylosing spondylitis: 4-year results. Ann Rheum Dis 2013;71. http://dx.doi.org/10.1136/annrheumdis-2012-eular.2243.
- van der Heijde D, Deodhar A, Braun J, Mack M, Hsu B, Gathany T, et al. Achieving ASDAS-CRP major improvement and inactive disease in patients with ankylosing spondylitis after treatment with golimumab is associated with normalized health related quality of life: two-year results from the go-raise trial. Ann Rheum Dis 2013;71. http://dx.doi.org/10.1136/annrheumdis-2012-eular.1853.
- van der Heijde D, Deodhar A, Braun J, Mack M, Hsu B, Gathany T, et al. Achieving ankylosing spondylitis disease activity score c-reactive protein major improvement and inactive disease in patients with ankylosing spondylitis after treatment with golimumab is associated with normalized health-related quality of life: 2-year results from go-raise. Rheumatology 2013;52:i161-2.
- Braun J, Beutler A, Deodhar A, Hsu B, Van Der Heijde D, Hermann KG, et al. The effect of anti-tumor necrosis factor therapy with golimumab on radiographic progression in definite ankylosing spondylitis: 4-year results. Int J Rheum Dis 2012;15. http://dx.doi.org/10.1136/annrheumdis-2012-eular.2243.
- Braun J, Van Der Heijde D, Hermann KG, Baraliakos X, Deodhar A, Beutler A, et al. The effect of anti-tumor necrosis factor therapy with two different doses of golimumab on radiographic progression in definite ankylosing spondylitis: 4-year results. Arthritis Rheum 2011;1. http://dx.doi.org/10.1136/annrheumdis-2012-203075.
- Braun J, Van Der Heijde D, Deodhar A, Beutler A, Mack M, Hsu B, et al. Sustained clinical response with golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 104-week results of a randomized, placebo-controlled study. Arthritis Rheum 2011;1.
- Braun J, Van Der Heijde D, Deodhar A, Diekman L, Sieper J, Kim SI, et al. Golimumab, a new, human, TNF-alpha antibody administered subcutaneously every 4 weeks, in ankylosing spondylitis: 104-week efficacy and safety results of the randomized, placebo-controlled go-raise study. Scand J Rheumatol 2010;39.
- Kim SI, Braun J, Van Der Heijde D, Deodhar A, Dieckman L, Sieper J, et al. Golimumab, a new, human, TNF-alpha antibody administered subcutaneously every 4 weeks, in ankylosing spondylitis: 104 week efficacy and safety results of the randomized, placebo-controlled GO-RAISE study. Int J Rheum Dis 2010;13:152-3.
- Braun J, Van Der Heijde DMFM, Deodhar AA, Diekman L, Sieper J, Kim SI, et al. Golimumab, a new, human, TNF-antibody administered subcutaneously every 4 weeks, in ankylosing spondylitis (AS): 104-week efficacy and safety results of the randomized, placebo-controlled GO-RAISE study. Arthritis Rheum 2009;60.
- Braun J, van der Heijde D, Deodhar A, Diekman L, Sieper J, Kim SI, et al. Golimumab, a new, human, TNF-alpha antibody administered subcutaneously every 4 weeks, in ankylosing spondylitis: 104-week efficacy and safety results of the randomized, placebo controlled GO-RAISE study. Clin Exp Rheumatol 2010;28.
- Park W, Miranda P, Brzosko M, Wiland P, Gutierrez-Urena S, Mikazane H, et al. Efficacy and safety of CT-P13 (infliximab biosimilar) over two years in patients with ankylosing spondylitis: comparison between continuing with CT-P13 and switching from infliximab to CT-P13. Arthritis Rheum 2013;65.
- Braun J, Baraliakos X, Listing J, Fritz C, Alten R, Burmester G, et al. Persistent clinical efficacy and safety of anti-tumour necrosis factor alpha therapy with infliximab in patients with ankylosing spondylitis over 5 years: evidence for different types of response. Ann Rheum Dis 2008;67:340-5. http://dx.doi.org/10.1136/ard.2007.075879.
- Braun J, Baraliakos X, Brandt J, Listing J, Zink A, Alten R, et al. Persistent clinical response to the anti-TNF-alpha antibody infliximab in patients with ankylosing spondylitis over 3 years. Rheumatology 2005;44:670-6. http://dx.doi.org/10.1093/rheumatology/keh584.
- Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, et al. Long-term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum 2003;48:2224-33. http://dx.doi.org/10.1002/art.11104.
- Baraliakos X, Brandt J, Listing J, Rudwaleit M, Sieper J, Braun J. Clinical response to long-term therapy with infliximab in patients with ankylosing spondylitis – results after 3 years. Ann Rheum Dis 2004;63.
- Baraliakos X, Brandt J, Listing J, Rudwaleit M, Sieper J, Braun J. Clinical response to withdrawal of anti-TNF therapy in patients with ankylosing spondylitis (AS) after 3 years of continuous treatment with infliximab. Ann Rheum Dis 2004;63.
- Brandt J, Listing J, Alten R, Bumester G, Gromnica-Ihle E, Kellner H, et al. Two year follow up results of a controlled trial of the anti-TNF alpha antibody infliximab in active ankylosing spondylitis. Arthritis Rheum 2003;48:S172-3.
- Brandt J, Listing J, Alten R, Burmester G, Gromnica-Ihle E, Schneider M, et al. One year results of a double-blind placebo controlled, phase-III clinical trial of infliximab in active ankylosing spondylitis. Arthritis Rheum 2002;46.
- Heldmann F, Brandt J, van der Horst-Bruinsma IE, Landewe R, Sieper J, Burmester GR, et al. The European ankylosing spondylitis infliximab cohort (EASIC): a European multicentre study of long term outcomes in patients with ankylosing spondylitis treated with infliximab. Clin Exp Rheumatol 2011;29:672-80.
- Braun J, Deodhar A, Dijkmans B, Geusens P, Sieper J, Williamson P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis over a two-year period. Arthritis Rheum 2008;59:1270-8. http://dx.doi.org/10.1002/art.24001.
- Machado P, Landewe R, Braun J, Baraliakos X, Hermann KGA, Hsu B, et al. MRI inflammation and its relation with measures of clinical disease activity and different treatment responses in patients with ankylosing spondylitis treated with a TNF inhibitor. Ann Rheum Dis 2013;71.
- Heldmann F, Brandt J, Braun J. European ankylosing spondylitis infliximab cohort (EASIC): long-term (5 years) efficacy of infliximab on disease activity and function: a real life experience after the end of ASSERT. Arthritis Rheum 2009;60.
- Braun J, Han C, Kellner H, Deodhar A, Inman R, Xu W, et al. Infliximab improves spinal mobility in patients with ankylosing spondylitis: two-year findings from the assert clinical trial. Rheumatology 2008;47.
- Braun J, Han C, Kellner H, Deodhar A, Inman R, Xu W, et al. Infliximab improves spinal mobility in patients with ankylosing spondylitis: two-year findings from the assert clinical trial. Ann Rheum Dis 2007;66:390-1.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Pumford N, Wade A, Crawford G, McConnachie A. Sensitivity of PRO’s to detect changes in quality of life in patients treated with a biologic agent. Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.08.450.
- Wade AG, Crawford GM, Pumford N, Koscielny V, Maycock S, McConnachie A. Baseline characteristics and patient reported outcome data of patients prescribed etanercept: web-based and telephone evaluation. BMC Med Res Methodol 2011;11. http://dx.doi.org/10.1186/1471-2288-11-91.
- Lee TJ, Park BH, Son HK, Kim JW, Shin K, Lee EB, et al. Differences in cost-of-illness and quality of life between rheumatoid arthritis and ankylosing spondylitis in South Korea. Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.08.407.
- Lee T-J, Park B-H, Kim JW, Shin K, Lee EB, Song Y-W. Cost-of-illness and quality of life in patients with ankylosing spondylitis at a tertiary hospital in Korea. J Korean Med Sci 2014;29:190-7. http://dx.doi.org/10.3346/jkms.2014.29.2.190.
- Joore M, Brunenberg D, Nelemans P, Wouters E, Kuijpers P, Honig A, et al. The impact of differences in EQ-5D and SF-6D utility scores on the acceptability of cost–utility ratios: results across five trial-based cost-utility studies. Value Health 2010;13:222-9. http://dx.doi.org/10.1111/j.1524-4733.2009.00669.x.
- Van Tubergen A, Boonen A, Landewe R, Rutten-Van Molken M, Van der Heijde D, Hidding A, et al. Cost effectiveness of combined spa-exercise therapy in ankylosing spondylitis: a randomized controlled trial. Arthritis Rheum-Arthritis Care Res 2002;47:459-67. http://dx.doi.org/10.1002/art.10658.
- Kobelt G, Andlin-Sobocki P, Collantes-Estevez E, Gratacos-Masmitja J, Pocovi A, Mulero-Mendoza J. The cost effectiveness of infliximab in the treatment of ankylosing spondylitis in Spain. Comparison of clinical trial and clinical practice data. Ann Rheum Dis 2006;65:282-3. http://dx.doi.org/10.1080/03009740701607224.
- Kobelt G, Sobocki P, Mulero J, Gratacos J, Collantes-Estevez E, Braun J. The cost-effectiveness of infliximab in the treatment of ankylosing spondylitis in Spain: comparison of clinical trial and clinical practice data. Scand J Rheumatol 2008;37:62-71. http://dx.doi.org/10.1080/03009740701607224.
- Boonen A, van der Heijde D, Landewe R, Spoorenberg A, Schouten H, Rutten-van Molken M, et al. Work status and productivity costs due to ankylosing spondylitis: comparison of three European countries. Ann Rheum Dis 2002;61:429-37. http://dx.doi.org/10.1136/ard.61.5.429.
- Boonen A, van der Heijde D, Landewe R, Guillemin F, Spoorenberg A, Schouten H, et al. Costs of ankylosing spondylitis in three European countries: the patient’s perspective. Ann Rheum Dis 2003;62:741-7. http://dx.doi.org/10.1136/ard.62.8.741.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Boonen A, van der Heijde D, Landewe R, van Tubergen A, Mielants H, Dougados M, et al. How do the EQ-5D, SF-6D and the well-being rating scale compare in patients with ankylosing spondylitis?. Ann Rheum Dis 2007;66:771-7. http://dx.doi.org/10.1136/ard.2006.060384.
- Gordeev VS, Maksymowych WP, Evers SM, Ament A, Schachna L, Boonen A. Role of contextual factors in health-related quality of life in ankylosing spondylitis. Ann Rheum Dis 2010;69:108-12. http://dx.doi.org/10.1136/ard.2008.100164.
- Haywood KL, Garratt AM, Dziedzic K, Dawes PT. Generic measures of health-related quality of life in ankylosing spondylitis: reliability, validity and responsiveness. Rheumatology 2002;41:1380-7. http://dx.doi.org/10.1093/rheumatology/41.12.1380.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. http://dx.doi.org/10.1136/bmj.316.7133.736.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: University of York Centre for Health Economics; 1995.
- Dolan P, Gudex C, Kind P, Williams A. The time trade-off method: results from a general population study. Health Econ 1996;5:141-54. http://dx.doi.org/10.1002/(SICI)1099-1050(199603)5:2<141::AID-HEC189>3.0.CO;2-N.
- Haywood KL, Garratt AM, Jordan KP, Healey EL, Packham JC. Evaluation of ankylosing spondylitis quality of life (EASi-QoL): reliability and validity of a new patient-reported outcome measure. J Rheumatol 2010;37:2100-9. http://dx.doi.org/10.3899/jrheum.091359.
- Kvamme MK, Kristiansen IS, Lie E, Kvien TK. Identification of cutpoints for acceptable health status and important improvement in patient-reported outcomes, in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2010;37:26-31. http://dx.doi.org/10.3899/jrheum.090449.
- Osnes-Ringen H, Kvamme MK, Kristiansen IS, Thingstad M, Henriksen JE, Kvien TK, et al. Cost-effectiveness analyses of elective orthopaedic surgical procedures in patients with inflammatory arthropathies. Scand J Rheumatol 2011;40:108-15. http://dx.doi.org/10.3109/03009742.2010.503661.
- Lindstrom U, Bremander A, Bergman S, Haglund E, Petersson IF, Jacobsson LT. Patients with non-as axial SPA have similar prevalence compared to as, but worse perceived health. Results from a population based study. Ann Rheum Dis 2013;72:A667-8. http://dx.doi.org/10.1136/annrheumdis-2013-eular.1978.
Appendix 1 Search strategies for clinical and economic reviews
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (Ovid)
Date range searched: 1946 to present.
Searched: 5 June 2014 via OVID interface.
Search strategy
-
spondylarthritis/ or spondylitis, ankylosing/ (12,386)
-
((ankyl$ or axial) adj2 spondyl$).ti,ab. (10,322)
-
(ankyl$ adj2 (spine$ or spinal or vertebra$)).ti,ab. (402)
-
((Bechtere$ or Bekhtere$ or “Marie Strumpell$” or “Marie Struempell$”) adj2 (disease or syndrome)).ti,ab. (451)
-
1 or 2 or 3 or 4 (14,886)
-
(adalimumab or humira or 331731-18-1).af. (3751)
-
(certolizumab or CDP870 or cimzia or 428863-50-7).af. (497)
-
(etanercept or enbrel or altebrel or 185243-69-0).af. (5540)
-
(golimumab or CNTO 148 or simponi or 476181-74-5).af. (328)
-
(infliximab or remicade or 170277-31-3 or inflectra or remsima or CT-P13).af. (9166)
-
6 or 7 or 8 or 9 or 10 (13,950)
-
randomized controlled trial.pt. (375,396)
-
controlled clinical trial.pt. (88,473)
-
randomized.ab. (295,232)
-
placebo.ab. (154,473)
-
drug therapy.fs. (1,704,080)
-
randomly.ab. (213,686)
-
trial.ab. (306,623)
-
groups.ab. (1,359,351)
-
12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 (3,348,700)
-
animals/ not (animals/ and humans/) (3,855,883)
-
20 not 21 (2,872,482)
-
5 and 11 and 22 (1008)
EMBASE
Date range searched: 1974 to week 22, 2014.
Searched: 5 June 2014 via OVID interface.
Search strategy
-
exp spondylarthritis/ or exp ankylosing spondylitis/ (20,531)
-
((ankyl$ or axial) adj2 spondyl$).ti,ab. (14,760)
-
(ankyl$ adj2 (spine$ or spinal or vertebra$)).ti,ab. (542)
-
((Bechtere$ or Bekhtere$ or “Marie Strumpell$” or “Marie Struempell$”) adj2 (disease or syndrome)).ti,ab. (551)
-
1 or 2 or 3 or 4 (22,426)
-
(adalimumab or humira or 331731-18-1).af. (15,439)
-
(certolizumab or CDP870 or cimzia or 428863-50-7).af. (3097)
-
(etanercept or enbrel or altebrel or 185243-69-0).af. (19,368)
-
(golimumab or CNTO 148 or simponi or 476181-74-5).af. (2124)
-
(infliximab or remicade or 170277-31-3 or inflectra or remsima or CT-P13).af. (29,667)
-
6 or 7 or 8 or 9 or 10 (41,065)
-
(random$ or factorial$ or crossover$ or cross over$ or cross-over$ or placebo$ or (doubl$ adj blind) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).ti,ab,kw. (1,351,644)
-
crossover-procedure/ or double-blind procedure/ or randomized controlled trial/ or single-blind procedure/ (390984)
-
12 or 13 (1,428,385)
-
5 and 11 and 14 (603)
-
limit 15 to embase (581)
-
animals/ not (animals/ and humans/) (1,188,711)
-
16 not 17 (581)
Cumulative Index to Nursing and Allied Health Literature Plus
Date range searched: inception to 5 June 2014.
Searched: 5 June 2014 via EBSCOhost interface.
Search strategy
S19 S6 AND S12 AND S18 (87)
S18 S13 OR S14 OR S15 OR S16 OR S17 (148,267)
S17 singl* N blind* or doubl* N blind* or singl* N mask* or doubl* N mask (285)
S16 (ZT “randomized controlled trial”) (38,240)
S15 (allocate* or assign* or divid*) N5 (condition* or experiment* or treatment* or control* or group*) (26,737)
S14 crossover or “cross over” or “latin square” or placebo* (41,898)
S13 randomi* or random N allocate* or random N assign* or random N divid* or random N trial* or random N study or random N studies (108,710)
S12 S7 OR S8 OR S9 OR S10 OR S11 (3091)
S11 TX (infliximab or remicade or 170277-31-3 or inflectra or remsima or CT-P13) (1792)
S10 TX (golimumab or CNTO 148 or simponi or 476181-74-5) (119)
S9 TX (etanercept or enbrel or altebrel or 185243-69-0) (1298)
S8 TX (certolizumab or CDP870 or cimzia or 428863-50-7 (91)
S7 TX (adalimumab or humira or 331731-18-1) (647)
S6 S1 OR S2 OR S3 OR S4 OR S5 (2566)
S5 TX ((Bechtere* or Bekhtere* or “Marie Strumpell*” or “Marie Struempell*”) N2 (disease or syndrome)) (3)
S4 TX (ankyl* N2 (spine* or spinal or vertebra*)) (91)
S3 TX ((ankyl* or axial) N2 spondyl*) (2277)
S2 MH spondylitis, ankylosing (1803)
S1 MH spondylarthritis (500)
Science Citation Index
Date range searched: 1900 to 2014.
Searched: 16 June 2014 via Web of Science.
Indexes=SCI-EXPANDED Timespan=1900-2014.
Search strategy
#13 #12 AND #11 AND #5 (1001)
#12 TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow-up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*) (2,435,907)
#11 #10 OR #9 OR #8 OR #7 OR #6 (20,446)
#10 TOPIC: ((infliximab or remicade or 170277-31-3 or inflectra or remsima or CT-P13)) (13,285)
#9 TOPIC: ((golimumab or CNTO 148 or simponi or 476181-74-5)) (494)
#8 TOPIC: ((etanercept or enbrel or altebrel or 185243-69-0)) (7138)
#7 TOPIC: ((certolizumab or CDP870 or cimzia or 428863-50-7)) (916)
#6 TOPIC: ((adalimumab or humira or 331731-18-1)) (4754)
#5 #4 OR #3 OR #2 OR #1 (14,918)
#4 TOPIC: (((Bechtere* or Bekhtere* or “Marie Strumpell*” or “Marie Struempell*”) NEAR/2 (disease or syndrome))) (191)
#3 TOPIC: ((ankyl* NEAR/2 (spine* or spinal or vertebra*))) (644)
#2 TOPIC: (((ankyl* or axial) NEAR/2 spondyl*)) (13,854)
#1 TOPIC: (spondylarthritis OR spondyloarthritis) (2394)
National Institutes of Health ClinicalTrials.gov register
Searched: 23 July 2014 online at http://clinicaltrials.gov/ct2/search.
Search strategy
((spondylarthritis OR spondyloarthritis OR spondylitis) AND (infliximab OR remicade OR inflectra OR remsima OR golimumab OR simponi OR etanercept OR enbrel OR altebrel OR certolizumab OR cimzia OR adalimumab OR humira))
160 results.
Cochrane Library (includes Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Health Technology Assessment Database, Cochrane Central Register of Controlled Trials and NHS Economic Evaluation Database)
Searched: 5 June 2014 online at http://onlinelibrary.wiley.com/o/cochrane/cochrane_search_fs.html.
Search strategy
#1 MeSH descriptor: [Spondylitis, Ankylosing] explode all trees
#2 MeSH descriptor: [Spondylarthritis] explode all trees
#3 ((ankyl* or axial) near/2 spondyl*):ti,ab,kw (Word variations have been searched)
#4 (ankyl* near/2 (spine* or spinal or vertebra*)):ti,ab,kw (Word variations have been searched)
#5 ((Bechtere* or Bekhtere* or “Marie Strumpell*” or “Marie Struempell*”) near/2 (disease or syndrome)):ti,ab,kw
#6 #1 or #2 or #3 or #4 or #5
#7 (adalimumab or humira or 331731-18-1):ti,ab,kw
#8 (certolizumab or CDP870 or cimzia or 428863-50-7):ti,ab,kw
#9 (etanercept or enbrel or altebrel or 185243-69-0):ti,ab,kw
#10 (golimumab or CNTO 148 or simponi or 476181-74-5):ti,ab,kw
#11 (infliximab or remicade or 170277-31-3 or inflectra or remsima or CT-P13):ti,ab,kw
#12 #7 or #8 or #9 or #10 or #11
#13 #6 and #12
284 total results comprised two Cochrane Database of Systematic Reviews, five Database of Abstracts of Reviews of Effects, 21 HTA, 233 Cochrane Central Register of Controlled Trials and 14 NHS Economic Evaluation Database.
Conference Proceedings Citation Index – Science
Searched: 2 September 2014 via Wiley Web of Science interface.
Indexes=CPCI-S Timespan=1900-2014.
Search strategy
#12 #11 AND #5 (341)
#11 #10 OR #9 OR #8 OR #7 OR #6 (4745)
#10 TOPIC: ((infliximab or remicade or 170277-31-3 or inflectra or remsima or CT-P13)) (2537)
#9 TOPIC: ((golimumab or CNTO 148 or simponi or 476181-74-5)) (141)
#8 TOPIC: ((etanercept or enbrel or altebrel or 185243-69-0)) (1221)
#7 TOPIC: ((certolizumab or CDP870 or cimzia or 428863-50-7)) (291)
#6 TOPIC: ((adalimumab or humira or 331731-18-1)) (1140)
#5 #4 OR #3 OR #2 OR #1 (2117)
#4 TOPIC: (((Bechtere* or Bekhtere* or “Marie Strumpell*” or “Marie Struempell*”) NEAR/2 (disease or syndrome))) (4)
#3 TOPIC: ((ankyl* NEAR/2 (spine* or spinal or vertebra*))) (55)
#2 TOPIC: (((ankyl* or axial) NEAR/2 spondyl*)) (1906)
#1 TS=(spondylarthritis OR spondyloarthritis) (393)
International Prospective Register of Systematic Reviews (PROSPERO)
Searched: 7 October 2014 online at www.crd.york.ac.uk/prospero/search.asp.
Search strategy
spondylitis [In All Fields]
OR
spondylarthritis [In All Fields]
OR
spondyloarthritis [In All Fields]
6 results.
National Guideline Clearinghouse
Searched: 7 October 2014 online at www.guideline.gov.
Search strategy
spondylitis OR spondylarthritis OR spondyloarthritis
15 results.
NHS Evidence
Searched 27 October 2014 online at www.evidence.nhs.uk.
Search strategy
((((ankyl* or axial) near/2 spondyl*) OR (ankyl* near/2 (spine* or spinal or vertebra*)) AND (adalimumab or humira or certolizumab or CDP870 or cimzia or etanercept or enbrel or altebrel or golimumab or CNTO 148 or simponi or infliximab or remicade or inflectra or remsima or CT-P13))
350 results.
NHS Clinical Knowledge Summaries
Searched: 27 October 2014 online at http://cks.nice.org.uk/#?char=A.
1 result for ankylosing spondylitis.
Searches for economic review
NHS Economic Evaluation Database
Searched: 5 June 2014 online at http://onlinelibrary.wiley.com/o/cochrane/cochrane_search_fs.html.
Search strategy
#1 MeSH descriptor: [Spondylitis, Ankylosing] explode all trees
#2 MeSH descriptor: [Spondylarthritis] explode all trees
#3 ((ankyl* or axial) near/2 spondyl*):ti,ab,kw (Word variations have been searched)
#4 (ankyl* near/2 (spine* or spinal or vertebra*)):ti,ab,kw (Word variations have been searched)
#5 ((Bechtere* or Bekhtere* or “Marie Strumpell*” or “Marie Struempell*”) near/2 (disease or syndrome)):ti,ab,kw
#6 #1 or #2 or #3 or #4 or #5
#7 (adalimumab or humira or 331731-18-1):ti,ab,kw
#8 (certolizumab or CDP870 or cimzia or 428863-50-7):ti,ab,kw
#9 (etanercept or enbrel or altebrel or 185243-69-0):ti,ab,kw
#10 (golimumab or CNTO 148 or simponi or 476181-74-5):ti,ab,kw
#11 (infliximab or remicade or 170277-31-3 or inflectra or remsima or CT-P13):ti,ab,kw
#12 #7 or #8 or #9 or #10 or #11
#13 #6 and #12
14 results.
Searches for European Quality of Life-5 Dimensions
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (Ovid)
Date range searched: 1946 to present.
Searched: 16 June 2014 via OVID interface.
Search strategy
-
spondylarthritis/ or spondylitis, ankylosing/ (12,394)
-
((ankyl$ or axial) adj2 spondyl$).ti,ab. (10,334)
-
(ankyl$ adj2 (spine$ or spinal or vertebra$)).ti,ab. (402)
-
((Bechtere$ or Bekhtere$ or “Marie Strumpell$” or “Marie Struempell$”) adj2 (disease or syndrome)).ti,ab. (451)
-
1 or 2 or 3 or 4 (14,899)
-
(5d or 5-d or 5 dimension or eq-5d or eq5d or eq 5d).ti,ab. (13,976)
-
5 and 6 (27)
EMBASE (June 2014)
Searched: 16 June 2014 via OVID interface.
Search strategy
-
exp spondylarthritis/ or exp ankylosing spondylitis/ (20,653)
-
((ankyl$ or axial) adj2 spondyl$).ti,ab. (14,855)
-
(ankyl$ adj2 (spine$ or spinal or vertebra$)).ti,ab. (545)
-
((Bechtere$ or Bekhtere$ or “Marie Strumpell$” or “Marie Struempell$”) adj2 (disease or syndrome)).ti,ab. (552)
-
1 or 2 or 3 or 4 (22,550)
-
(5d or 5-d or 5 dimension or eq-5d or eq5d or eq 5d).ti,ab. (17,019)
-
5 and 6 (60)
-
limit 7 to embase (55)
Searches for economic models
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (Ovid)
Date range searched: 1946 to present.
Searched: 25 July 2014 via OVID interface.
Search strategy
-
spondylarthritis/ or spondylitis, ankylosing/ (12,505)
-
((ankyl$ or axial) adj2 spondyl$).ti,ab. (10,436)
-
(ankyl$ adj2 (spine$ or spinal or vertebra$)).ti,ab. (407)
-
((Bechtere$ or Bekhtere$ or “Marie Strumpell$” or “Marie Struempell$”) adj2 (disease or syndrome)).ti,ab. (455)
-
1 or 2 or 3 or 4 (15,038)
-
exp models, economic/ (10,268)
-
((economic$ or cost$ or pric$ or value or statistic$) and model$).ti,ab. (245,686)
-
6 or 7 (250,668)
-
5 and 8 (107)
EMBASE
Date range searched: 1974 to 24 July 2014.
Searched: 25 July 2014 via OVID interface.
Search strategy
-
exp spondylarthritis/ or exp ankylosing spondylitis/ (20,858)
-
((ankyl$ or axial) adj2 spondyl$).ti,ab. (14,996)
-
(ankyl$ adj2 (spine$ or spinal or vertebra$)).ti,ab. (553)
-
((Bechtere$ or Bekhtere$ or “Marie Strumpell$” or “Marie Struempell$”) adj2 (disease or syndrome)).ti,ab. (553)
-
1 or 2 or 3 or 4 (22,760)
-
statistical model/ (102,203)
-
((economic$ or cost$ or pric$ or value or statistic$) adj2 model$).ti,ab. (24,642)
-
6 or 7 (119,366)
-
5 and 8 (63)
-
limit 9 to embase (55)
Appendix 2 Synthesis methods for clinical efficacy network meta-analyses
Estimating standard deviations from interquartile ranges
Where Q1 is the lower quartile, Q3 is the upper quartile and σ is the SD, then the SD was estimated as:
Calculating change from baseline outcomes and standard deviations
Given baseline and final values and their SDs, the change from baseline values and SDs can be calculated if the within-study correlation between baseline and final values is known. Similarly, the final values can be computed.
The within-study correlation ρ between baseline and final values can be calculated as follows, as stated in the Cochrane Handbook,183 where
The SD of the change from baseline can be found by rearranging the above equation. The SD of the final value can be found by rearranging the above equation which produces a quadratic. As a range of correlation estimates were obtained from the studies available, we tested 0.3 and 0.7 correlation estimates in our analyses. In calculating the SD of final values, this sometimes resulted in complex roots. In these cases, the lowest correlation estimate that allowed a real root was used in the calculation.
Prior distribution for the between-study standard deviation for the placebo absolute risk
In running fixed-effect and random-effect models to estimate the placebo absolute risk, the random-effect models had better fit. For ASAS 70 response, there were insufficient trials to run a random-effects model, so a prior distribution for the between-study SD was specified. This was derived from the between-study SD from the ASAS 40 analysis. The prior distribution was specified as a log-normal distribution, and the log-normal distribution parameters µ and σ2 were derived from the following equations:
I2
As noted in Higgins et al. ,184 I2 was calculated as
where τ2 is the between-study variance estimated in the multiple-treatment meta-analysis,
which was calculated in Excel, and wi is the precision of study i.
Correlation
Table 108 presents the results for BASDAI change from baseline assuming a class effect and independent treatment effects, and assuming 0.3 and 0.7 within-study correlation. It is clear that the different correlation assumptions make no difference in this case. This is perhaps because the studies affected by the correlation assumption were small studies.
| Intervention | 0.3 correlation | 0.7 correlation | ||
|---|---|---|---|---|
| Mean | 95% CrI | Mean | 95% CrI | |
| Class | –1.66 | –1.89 to –1.43 | –1.66 | –1.88 to –1.43 |
| Adalimumab | –1.55 | –1.88 to –1.23 | –1.56 | –1.88 to –1.24 |
| Centrolizumab | –1.46 | –2.16 to –0.74 | –1.46 | –2.16 to –0.74 |
| Etanercept | –1.76 | –2.15 to –1.37 | –1.76 | –2.15 to –1.37 |
| Infliximab | –2.28 | –3.18 to –1.38 | –2.28 | –3.18 to –1.38 |
Appendix 3 Risk-of-bias data
| Trial | Sequence generation | Allocation concealment | Important baseline imbalance | Blinding of participants and researchers | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Exclude in sensitivity analysis? |
|---|---|---|---|---|---|---|---|---|
| Haibel 200852 | Unclear | Unclear | Unclear | Low | Low | Low | Low | No |
| – | – | Imbalance for HLA-B27 positive and MRI positive | – | – | Number of withdrawals and dropouts: 0 | All main relevant outcomes reported | ||
| Imputation used for continuous outcomes: N/A | ||||||||
| Hu 201255 | Unclear | Unclear | Low | Unclear | Low | Unclear | Unclear | Yes |
| There is no description of the randomisation procedure and no explanation for the imbalance in number of patients in treatment arms, 26 vs. 20 | No details reported | Main prognostic indicators were similar across trial arms at baseline | No details reported | – | Number of withdrawals and dropouts: NR | No reporting of AEs | ||
| Imputation used for continuous outcomes: NR | ||||||||
| Huang 201456 | Low | Low | Low | Low | Low | Low | Low | No |
| Centralised computer-based system | Centralised computer-based system | Groups comparable for all important factors | Matching placebo and all study personnel and patients stated to be blinded | – | Number of withdrawals and dropouts: 12 | All main outcomes reported | ||
| Imputation used for continuous outcomes: LOCF | ||||||||
| Lambert 200757 | Unclear | Unclear | Low | Unclear | Low | Low | Low | No |
| – | – | No imbalances in possible prognostic factors | Stated to be double blind | – | Number of withdrawals and dropouts: 0 at week 12; two from placebo arm at week 52 | BASDAI score not reported at follow-up | ||
| Imputation used for continuous outcomes: no imputation for missing SPARCC enthesitis index score | ||||||||
| ABILITY-1 201358 | Low | Low | Low | Low | Low | Low | Low | No |
| Centralised randomisation with interactive voice response system | – | – | Matching placebo | – | Number of withdrawals and dropouts: n = 6 | – | ||
| Imputation used for continuous outcomes: LOCF imputed values | ||||||||
| ATLAS 200661 | Unclear | Unclear | Low | Low | Low | Low | Low | No |
| – | – | Balanced across treatment arms | Matching placebo | – | Number of withdrawals and dropouts: by week 12, n = 4 placebo and n = 4 active; by week 24, n = 6 placebo and n = 13 active (Note: week 24 was still RCT although no responders permitted early escape after week 12). Imputation used for continuous outcomes: LOCF | Primary and all main outcomes reported | ||
| RAPID-axSpA (Landewe 2014)64 | Low | Low | Low | Unclear | Low | Low | Low | No |
| Central randomisation | Central randomisation | Small difference in baseline CRP level and HLA-B27 positive, making the placebo group have slightly increased risk (but unclear possible impact) | Administration of treatment was by unblinded trained personnel; their role in assessment is unclear and so the impact of their unblinded status is unclear | – | Number of withdrawals and dropouts: unclear at 12 weeks but at 24 weeks, n = 10 placebo, n = 6 200 mg and n = 9 400 mg | Hierarchical analysis plan adhered to | ||
| Imputation used for continuous outcomes: LOCF | ||||||||
| Barkham 201071 | Unclear | Unclear | Unclear | Low | Low | Unclear | Low | Yes |
| – | – | – | – | – | Number of withdrawals and dropouts: unclear, although it appears to be n = 9 for etanercept and n = 8 for placebo (the number for which ASAS 40 data were available) | – | ||
| Imputation used for continuous outcomes: ITT LOCF | ||||||||
| Davis 200372 | Unclear | Unclear | Low | Low | Low | Low | Low | No |
| – | – | – | – | – | Number of withdrawals and dropouts: at 12 weeks, n = 6 etanercept and n = 5 placebo; at 24 weeks, n = 12 etanercept, n = 19 placebo | – | ||
| Imputation used for continuous outcomes: LOCF using etanercept n = 138, and using placebo n = 139 | ||||||||
| Dougados 201174 | Unclear | Unclear | Low | Low | Low | Low | Low | No |
| – | – | – | – | – | Number of withdrawals and dropouts: n = 1 etanercept and n = 4 placebo. Imputation used for continuous outcomes: mITT (at least one dose) with LOCF | – | ||
| Dougados 201476 | Low | Low | Low | Low | Low | Low | Low | No |
| – | – | – | – | – | Number of withdrawals and dropouts: n = 6 etanercept and n = 3 placebo. In addition to this, five patients in each group were excluded from analyses because of misdiagnosis | – | ||
| Imputation used for continuous outcomes: LOCF in mITT population, n = 106 etanercept and n = 109 placebo | ||||||||
| Gorman 200279 | Low | Low | High (chance imbalance) | Low | Low | Low | Low | Yes |
| – | – | BASFI | – | – | Number of withdrawals and dropouts: n = 3 | – | ||
| Imputation used for continuous outcomes: not totally clear, but appears to be proper ITT with LOCF | ||||||||
| Calin 200483 | Unclear | Unclear | High | Low | Low | Low | Low | Yes |
| – | – | Important difference in CRP level, borderline important difference in age | – | – | Number of withdrawals and dropouts: n = 2 etanercept | – | ||
| Imputation used for continuous outcomes: LOCF for mITT population (placebo n = 39 and etanercept n = 45) | ||||||||
| van der Heijde 200686 | Unclear | Unclear | Unclear | Low | Low | Low | Low | No |
| – | – | No data for HLA-B27 | – | – | Number of withdrawals and dropouts: n = 14 in 50-mg group, n = 14 in 25-mg group and n = 7 in placebo group. In addition to this, five patients did not receive one dose of treatment (no further details) | – | ||
| Imputation used for continuous outcomes: mITT population analysed (had at least one dose) using the n = 155, n = 150, n = 51 group sizes and LOCF was used to impute missing data | ||||||||
| Giardina 201088 | High | High | Low | High | High | Low | Low | Yes |
| – | – | – | – | – | Number of withdrawals and dropouts: n = 0 | – | ||
| Imputation used for continuous outcomes: none needed | ||||||||
| GO-RAISE 200890 | Low | Low | Low | Low | Low | Low | Low | No |
| Random assignment using voice response system | Random assignment using voice response system | No important imbalance in key prognostic variables | Matching placebo used | – | Number of withdrawals and dropouts: n = 17 to week 24 (n = 2 placebo, n = 9 50 mg and n = 6 100 mg). Not clear how many at week 14 (primary time point) | Primary end point and all other main outcomes (BASDAI, BASFI, BASMI and SF-36) reported | ||
| Imputation used for continuous outcomes: LOCF (ITT population) | ||||||||
| Bao 201296 | Unclear | Unclear | Unclear | Low | Low | Unclear | Low | No |
| – | – | No HLA-B27 data | – | – | Number of withdrawals and dropouts: not reported for week 14 | Primary outcome and other main outcomes reported | ||
| Imputation used for continuous outcomes: NR | ||||||||
| Tam 201497 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear | No |
| Abstract only, very small study (Chinese) | Abstract only, very small study (Chinese) | Abstract only, very small study (Chinese) | Abstract only, very small study (Chinese) | – | Number of withdrawals and dropouts: NR | Abstract only, very small study (Chinese) | ||
| Imputation used for continuous outcomes: NR | ||||||||
| Barkham 200950 | Unclear | Unclear | High | Low | Low | Low | Low | Yes |
| – | – | Median CRP level 11.5 placebo vs. 5 infliximab; likely a result of chance, as there was higher CRP level in placebo group (and higher CRP level was associated with better responses) | – | – | Number of withdrawals and dropouts: n = 1 in the placebo group (at 12 weeks) | – | ||
| Imputation used for continuous outcomes: NR but ITT population analysed | ||||||||
| Braun 200298 | Low | Low | Low | Low | Low | Low | Low | No |
| – | – | – | – | – | Number of withdrawals and dropouts: n = 0 | – | ||
| Imputation used for continuous outcomes: none required | ||||||||
| Marzo-Ortega 2005100 | Low | Low | Unclear | Low | Low | Unclear | Low | No |
| – | – | The only issue is with age, and the difference of 2 years could be caused by rounding | – | – | Number of withdrawals and dropouts: n = 5 out of 14 for placebo and n = 2 out of 28 for infliximab | – | ||
| Imputation used for continuous outcomes: ITT with LOCF | ||||||||
| Van den Borsch 2002101 | Unclear | Unclear | High; | Low | Low | Low | Low | Yes |
| – | – | BASFI > 1 point | – | – | Number of withdrawals and dropouts: n = 0 | – | ||
| Imputation used for continuous outcomes: N/A | ||||||||
| ASSERT 2005102 | Unclear | Unclear | Low | Low | Low | Low | Low | No |
| – | – | – | – | – | Number of withdrawals and dropouts: n = 8 (n = 4 in each group) | – | ||
| Imputation used for continuous outcomes: not reported, but ITT population analysed. LOCF was used for ASAS 20 | ||||||||
| PLANETAS 2013110 | Low | Low | Unclear | Low | Low | Low | Low | No |
| – | – | HLA-B27 was not reported | – | – | Number of withdrawals and dropouts: n = 21 (n = 12 CT-P13 vs. n = 9 infliximab) | – | ||
| Imputation used for continuous outcomes: NR, although ITT population was analysed |
| Possible prognostic indicator | Results of association | Implications for baseline imbalance across groups within a trial (and variation in efficacy across trials) | ||
|---|---|---|---|---|
| Glintborg 2010 (DANBIO registry)112 (n = 842); 6-month time point; adalimumab, etanercept, infliximab | Vastesaeger 2011172 (ASSERT102 and GO-RAISE90 trial data)a (n = 635); 3-month time point; infliximab, golimumab | Lord 2009 (BSRBR)173 (n = 261); 6-month time point; adalimumab, etanercept, infliximab | ||
| HLA-B27 status | No data | Moderate association | No data | HLA-B27 positive patients have a better outcome. Use 20% group difference as an important imbalance? |
| CRP level | ≤ 14 mg/l vs. > 14 mg/l (OR 0.45, p < 0.001) | ≤ 6 mg/l vs. > 6 mg/l to 20 mg/l: moderate; ≤ 6 mg/l vs. > 20 mg/l: moderate to strong | ‘Raised inflammatory markers’ an important predictor, but result only available for CRP level or ESR (not CRP level alone) | Higher CRP levels are associated with a better outcome. Use Glintborg and Vastesaeger cut-offs (providing there’s at least a 2 mg/l difference between groups) |
| Age | OR 0.98 per year; p = 0.03 | < 40 years vs. > 40 years: weak to moderate | No significant association | Younger age associated with a better outcome. Use Vastesaeger cut-off point providing at least a 2-year difference between groups |
| BASFI score | OR 0.87 per cm increase; p = 0.008 | < 6.5 vs. > 6.5 moderate to strong | Per unit increase, OR 0.78 (95% CI 0.64 to 0.99) | Lower BASFI scores associated with a better outcome. Use a 1-point difference as an indication of important imbalance? Use < 6.5 vs. > 6.5 providing there’s at least a 0.5-point difference between groups |
| BASDAI | Not analysed | No significant association | Per unit increase, OR 1.30 (95% CI 1.04 to 1.62) | Higher BASDAI scores associated with a better outcome. Use a 1-point difference as an indication of important imbalance? |
| Disease duration | No significant association | No significant association | No significant association | Do not assess |
| Sex | No significant association | No significant association | No significant association | Do not assess |
Appendix 4 Trial results
| Trial | Population | Treatment arm | Dose | Time point (weeks) | Patients | Mean final values | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BASDAI | BASFI | BASMI | MASES | SF-36 PCS | SF-36 MCS | ||||||
| Haibel 200852 | Nr-axSpA | Adalimumab | 40 mg every 2 weeks | 12 | 22 | 3.8 (SD 2.5) | 3 (SD 2.4) | 1.3 (SD 1.4) | 2.5 (SD 3.5) | 38.8 (SD 11.8) | 44.6 (SD 12.7) |
| Nr-axSpA | Placebo | – | 12 | 24 | 5 (SD 2.4) | 4.1 (SD 2.6) | 1.7 (SD 1.5) | 2.8 (SD 3.4) | 34.9 (SD 9.6) | 43.9 (SD 11.8) | |
| Hu 201255 | AS | Adalimumab | 40 mg every 2 weeks | 12 | NR | 2.3 (SD 1.8) | 1.8 (SD 1.6) | – | – | – | – |
| AS | Placebo | – | 12 | NR | 4.2 (SD 2.6) | 2.9 (SD 1.9) | – | – | – | – | |
| Huang 201456 | AS | Adalimumab | 40 mg every 2 weeks | 12 | 229 | – | – | – | – | – | – |
| AS | Placebo | – | 12 | 115 | – | – | – | – | – | – | |
| Lambert 200757 | AS | Adalimumab | 40 mg every 2 weeks | 12 | 38 | – | – | – | – | – | – |
| AS | Placebo | – | 12 | 44 | – | – | – | – | – | – | |
| aABILITY-1 201358 | Nr-axSpA | Adalimumab | 40 mg every 2 weeks | 12 | 69 | – | – | – | – | – | – |
| Nr-axSpA | Placebo | – | 12 | 73 | – | – | – | – | – | – | |
| ATLAS 200661 | AS | Adalimumab | 40 mg every 2 weeks | 12 | 208 | – | 1.414 | – | – | – | – |
| AS | Placebo | – | 12 | 107 | – | – | – | – | – | – | |
| RAPID-axSpA 201464 | AS | Certolizumab pegol | 200 mg every 2 weeks | 12 | 65 | – | – | – | – | – | – |
| AS | Certolizumab pegol | 400 mg every 4 weeks | 12 | 56 | – | – | – | – | – | – | |
| AS | Placebo | – | 12 | 57 | – | – | – | – | – | – | |
| RAPID-axSpA 201464 | Nr-axSpA | Certolizumab pegol | 200 mg every 2 weeks | 12 | 46 | – | – | – | – | – | – |
| Nr-axSpA | Certolizumab pegol | 400 mg every 4 weeks | 12 | 51 | – | – | – | – | – | – | |
| Nr-axSpA | Placebo | – | 12 | 50 | – | – | – | – | – | – | |
| Barkham 201071 | AS | Etanercept | 25 mg twice weekly | 12 | 20 | – | – | – | – | – | – |
| AS | Placebo | – | 12 | 20 | – | – | – | – | – | – | |
| Davis 200372 | AS | Etanercept | 25 mg twice weekly | 12 | 138 | – | – | – | – | – | – |
| AS | Placebo | – | 12 | 139 | – | – | – | – | – | – | |
| AS | Etanercept | 25 mg twice weekly | 24 | 138 | 3.45 (SE 0.21) | 3.6 (SE 0.22) | – | – | – | – | |
| AS | Placebo | – | 24 | 139 | 5.51 (SE 0.2) | 5.47 (SE 0.22) | – | – | – | – | |
| Dougados 201174 | AS | Etanercept | 50 mg weekly | 12 | 39 | 3.7 (SD 2.6) | 4.1 (SD 2.9) | 5.1 (SD 1.7) | – | – | – |
| AS | Placebo | – | 12 | 43 | 4.5 (SD 1.9) | 4.8 (SD 2.1) | 5.6 (SD 1.3) | – | – | – | |
| Dougados 201476 | AS | Etanercept | 50 mg weekly | 12 | 106 | – | – | – | – | 43.7 (SD 8.9) | – |
| AS | Placebo | – | 12 | 109 | – | – | – | – | 41 (SD 7.8) | – | |
| Nr-axSpA | Etanercept | 50 mg weekly | 12 | 94 | – | – | – | – | – | – | |
| Nr-axSpA | Placebo | – | 12 | 95 | – | – | – | – | – | – | |
| Gorman 200279 | AS | Etanercept | 25 mg twice a week | 16 | 20 | – | 2.2 (SD 2.1) | – | – | – | – |
| AS | Placebo | – | 16 | 20 | – | 3.1 (SD 3) | – | – | – | – | |
| Calin 200483 | AS | Etanercept | 25 mg twice weekly | 12 | 45 | 3.38 | 3.96 | – | – | – | – |
| AS | Placebo | – | 12 | 39 | 5.01 | 5.39 | – | – | – | – | |
| van der Heijde 200686 | AS | Etanercept | 25 mg twice weekly | 12 | 150 | – | – | – | – | – | – |
| AS | Etanercept | 50 mg weekly | 12 | 155 | – | – | – | – | – | – | |
| AS | Placebo | – | 12 | 51 | – | – | – | – | – | – | |
| Giardina 201088 | AS | Etanercept | 50 mg weekly | 12 | 25 | – | 5 | – | – | – | – |
| AS | Infliximab | 5 mg/kg at 0, 2 and ≥ 6 weeks | 12 | 25 | – | 3.5 | – | – | – | – | |
| GO-RAISE 200890 | AS | Golimumab | 50 mg (two every 4 weeks) | 14 | 138 | – | – | – | – | – | – |
| AS | Golimumab | 100 mg (two every 4 weeks) | 14 | 140 | – | – | – | – | – | – | |
| AS | Placebo | – | 14 | 78 | – | – | – | – | – | – | |
| Bao 201296 | AS | Golimumab | 50 mg every 4 weeks | 14 | – | – | – | – | – | – | – |
| AS | Placebo | – | 14 | – | – | – | – | – | – | – | |
| Tam 201497 | AS | Golimumab | 50 mg monthly | 26 | NR | – | – | – | – | – | – |
| AS | Placebo | – | 26 | NR | – | – | – | – | – | – | |
| Barkham 200950 | Nr-axSpA | Infliximab | 5 mg/kg (0, 2 and ≥ 6 weeks) | 16 | 20 | – | – | – | – | – | – |
| Nr-axSpA | Placebo | – | 16 | 20 | – | – | – | – | – | – | |
| Braun 200298 | AS | Infliximab | 5 mg/kg (0, 2 and 6 weeks) | 12 | 34 | 3.3 | – | – | – | – | – |
| AS | Placebo | – | 12 | 35 | 5.7 | – | – | – | – | – | |
| Marzo-Ortega 2005100 | AS | Infliximab + methotrexate | 5 mg/kgb | 10 | 28 | 3.34 (SD 2.56) | 4.96 | – | – | – | – |
| AS | Placebo + methotrexate | –c | 10 | 14 | 5.19 (SD 2.52) | 6.1 | – | – | – | – | |
| AS | Infliximab + methotrexate | 5 mg/kgb | 30 | 28 | 4.6 (SD 2.85) | 5.04 | – | – | – | – | |
| AS | Placebo + methotrexate | –c | 30 | 14 | 5.74 (SD 2.34) | 5.68 | – | – | – | – | |
| Van den Bosch 2002101 | AS | Infliximab | 5 mg/kg (0, 2 and 6 weeks) | 12 | 9 | 2.66 | 2.74 | 4 | – | – | – |
| AS | Placebo | – | 12 | 12 | 5.01 | 7.19 | 4 | – | – | – | |
| ASSERT 2005102 | AS | Infliximab | 5 mg/kg (0, 2 and ≥ 6 weeks) | 24 | 201 | – | – | – | – | – | – |
| AS | Placebo | – | 24 | 78 | – | – | – | – | – | – | |
| PLANETAS 2013110 | AS | CT-P13 | 5 mg/kg | 14 | 125 | – | – | – | – | – | – |
| AS | Infliximab | 5 mg/kg | 14 | 125 | – | – | – | – | – | – | |
| AS | CT-P13 | 5 mg/kg | 30 | 125 | – | – | – | – | – | – | |
| AS | Infliximab | 5 mg/kg | 30 | 125 | – | – | – | – | – | – | |
| AS | CT-P13 | 5 mg/kg | 54 | 125 | – | – | – | – | – | – | |
| AS | Infliximab | 5 mg/kg | 54 | 125 | – | – | – | – | – | – | |
| Trial | Population | Treatment arm | Dose | Time point (weeks) | Patients | Mean change from baseline | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BASDAI | BASFI | BASMI | MASES | SF-36 PCS | SF-36 MCS | ||||||
| Haibel 200852 | Nr-axSpA | Adalimumab | 40 mg every 2 weeks | 12 | 22 | – | – | – | – | – | – |
| Nr-axSpA | Placebo | – | 12 | 24 | – | – | – | – | – | – | |
| Hu 201255 | AS | Adalimumab | 40 mg every 2 weeks | 12 | NR | – | – | – | – | – | – |
| AS | Placebo | – | 12 | NR | – | – | – | – | – | – | |
| Huang 201456 | AS | Adalimumab | 40 mg every 2 weeks | 12 | 229 | –2.8 (SD 1.9) | –1.75 (SD 2.02) | –0.5 (SD 0.6) | –1.2 (SD 2.1) | 6.6 (SD 6.4) | 5.1 (SD 9.9) |
| AS | Placebo | – | 12 | 115 | –1.4 (SD 1.9) | –0.47 (SD 1.64) | –0.2 (SD 0.7) | –0.8 (SD 1.7) | 4 (SD 6.3) | 2.8 (SD 9.4) | |
| Lambert 200757 | AS | Adalimumab | 40 mg every 2 weeks | 12 | 38 | – | – | – | – | – | – |
| AS | Placebo | – | 12 | 44 | – | – | – | – | – | – | |
| aABILITY-1 201358 | Nr-axSpA | Adalimumab | 40 mg every 2 weeks | 12 | 69 | –2.2 (SD 2.5) | –1.28 (SD 2.02) | –0.2 (SD 0.73) | –0.7 (SD 2.78) | 6.9 (SD 9.32) | 1.4 (SD 8.63) |
| Nr-axSpA | Placebo | – | 12 | 73 | –1.1 (SD 1.96) | –0.63 (SD 1.79) | –0.2 (SD 0.64) | –1 (SD 2.71) | 2.3 (SD 6.81) | 0.7 (SD 11.38) | |
| ATLAS 200661 | AS | Adalimumab | 40 mg every 2 weeks | 12 | 208 | –2.6 (SE 0.2) | – | –0.5 (SE 0.1) | –2.7 (SE 0.4) | 6.9 (SE 0.6) | 2.7 (SE 0.7) |
| AS | Placebo | – | 12 | 107 | –0.8 (SE 0.2) | – | 0.1 (SE 0.1) | –1.3 (SE 0.5) | 1.6 (SE 0.8) | 2.4 (SE 1) | |
| RAPID-axSpA 201464 | AS | Certolizumab | 200 mg every 2 weeks | 12 | 65 | –2.5 (SE 0.3) | –1.7 (SE 0.3) | –0.6 (SE 0.1) | – | 8.73 (SD 7.63) | 2.42 (SD 9.08) |
| AS | Certolizumab | 400 mg every 4 weeks | 12 | 56 | –2.4 (SE 0.3) | –1.7(SE 0.3) | –0.3 (SE 0.2) | – | 7.6 (SD 7.65) | 2.22 (SD 10.44) | |
| AS | Placebo | – | 12 | 57 | –1 (SE 0.3) | –0.6 (SE 0.3) | –0.2 (SE 0.1) | – | 2.56 (SD 5.67) | 1.07 (SD 10.92) | |
| RAPID-axSpA 201464 | Nr-axSpA | Certolizumab | 200 mg | 12 | 46 | –3.3 (SE 0.4) | –2.3 (SE 0.4) | –0.6 (SE 0.2) | – | 9.56 (SD 9.46) | 4.59 (SD 9.7) |
| Nr-axSpA | Certolizumab | 400 mg | 12 | 51 | –3.4 (SE 0.4) | –2.3 (SE 0.4) | –0.5 (SE 0.2) | – | 8.72 (SD 8.84) | 6.12 (SD 10.94) | |
| Nr-axSpA | Placebo | – | 12 | 50 | –1.5 (SE 0.4) | –0.4 (SE 0.4) | 0 (SE 0.1) | – | 2.13 (SD 7.47) | 1.39 (SD 10.24) | |
| Barkham 201071 | AS | Etanercept | 25 mg twice weekly | 12 | 20 | –1.97 | –1.35 | – | – | – | – |
| AS | Placebo | – | 12 | 20 | –0.1 | 0.21 | – | – | – | – | |
| Davis 200372 | AS | Etanercept | 25 mg twice weekly | 12 | 138 | –2.36 (SE 0.19) | –1.67 (SE 0.2) | – | – | – | – |
| AS | Placebo | – | 12 | 139 | –0.45 (SE 0.18) | –0.33 (SE 0.21) | – | – | – | – | |
| AS | Etanercept | 25 mg twice weekly | 24 | 138 | – | – | – | – | – | – | |
| AS | Placebo | – | 24 | 139 | – | – | – | – | – | – | |
| Dougados 201174 | AS | Etanercept | 50 mg weekly | 12 | 39 | –2.6 (SD 2) | –2.2 (SD 1.8) | –0.57 (SD 0.65) | – | – | – |
| AS | Placebo | – | 12 | 43 | –1.4 (SD 2) | –1 (SD 1.8) | –0.2 (SD 0.65) | – | – | – | |
| Dougados 201476 | AS | Etanercept | 50 mg weekly | 12 | 106 | –2 (SE 0.3) | –1.4 (SE 0.2) | –0.3 (SE 0.2) | – | – | – |
| AS | Placebo | – | 12 | 109 | –1.3 (SE 0.3) | –0.8 (SE 0.2) | –0.3 (SE 0.1) | – | – | – | |
| Nr-axSpA | Etanercept | 50 mg weekly | 12 | 94 | – | – | – | – | – | – | |
| Nr-axSpA | Placebo | – | 12 | 95 | – | – | – | – | – | – | |
| Gorman 200279 | AS | Etanercept | 25 mg twice a week | 16 | 20 | – | – | – | – | – | – |
| AS | Placebo | – | 16 | 20 | – | – | – | – | – | – | |
| Calin 200483 | AS | Etanercept | 25 mg twice weekly | 12 | 45 | –2.72 (SE 0.34) | –2.06 (SE 0.33) | – | – | – | – |
| AS | Placebo | – | 12 | 39 | –0.85 (SE 0.35) | –0.33 (SE 0.31) | – | – | – | – | |
| van der Heijde 200686 | AS | Etanercept | 25 mg twice weekly | 12 | 150 | – | – | – | – | – | – |
| AS | Etanercept | 50 mg weekly | 12 | 155 | – | – | – | – | – | – | |
| AS | Placebo | – | 12 | 51 | – | – | – | – | – | – | |
| Giardina 201088 | AS | Etanercept | 50 mg weekly | 12 | 25 | – | – | – | – | – | – |
| AS | Infliximab | 5 mg/kg (0, 2 and ≥ 6 weeks) | 12 | 25 | – | – | – | – | – | – | |
| GO-RAISE 200890 | AS | Golimumab | 50 mg (two every 4 weeks) | 14 | 138 | – | –1.4 (IQR –3.1 to –0.1) | 0 (IQR –1 to 0) | –0.5 (SD 2.6) | 7.3 (IQR 1.5 to 15.3) | 1.5 (IQR –2.2 to 7.8) |
| AS | Golimumab | 100 mg (two every 4 weeks) | 14 | 140 | – | –1.5 (IQR –3.0 to –0.1) | 0 (IQR –1 to 0) | –1.3 (SD 3.11) | 8.4 (IQR 2.3 to 14.1) | 3.7 (IQR –3.2 to 12.1) | |
| AS | Placebo | – | 14 | 78 | – | 0.1 (IQR –1.1 to 1.1) | 0 (SD –1 to 0) | –0.2 (SD 2.99) | 2.4 (IQR –1.4 to 7.8) | 0.1 (IQR –4.3 to 5.3) | |
| Bao 201296 | AS | Golimumab | 50 mg every 4 weeks | 14 | – | –1.26 (SD 2.57) | –0.42 (SD 0.91) | – | 6.25 (SD 7.95) | 3.86 (SD 8.92) | |
| AS | Placebo | – | 14 | – | 0.11 (SD 2.1) | –0.19 (SD 0.72) | – | 1.59 (SD 6.12) | 0.82 (SD 9.44) | ||
| Tam 201497 | AS | Golimumab | 50 mg monthly | 26 | NR | –1.82 (SD 1.64) | –0.13 (SD 0.25)b | –1 (IQR –2 to 0) | – | – | – |
| AS | Placebo | – | 26 | NR | –0.66 (SD 1.24) | 0.17 (SD 0.72)b | 0 (IQR –1 to 0) | – | – | – | |
| Barkham 200950 | Nr-axSpA | Infliximab | 5 mg/kg (0, 2 and ≥ 6 weeks) | 16 | 20 | –3.41 (SD 2.53) | –2.7 (SD 2.36) | – | – | – | – |
| Nr-axSpA | Placebo | – | 16 | 20 | –0.75 (SD 2.42) | –0.47 (SD 2.25) | – | – | – | – | |
| Braun 200298 | AS | Infliximab | 5 mg/kg (0, 2 and ≥ 6 weeks) | 12 | 34 | –3.2 | –2.1 | – | – | – | – |
| AS | Placebo | – | 12 | 35 | –0.6 | –0.1 | – | – | – | – | |
| Marzo-Ortega 2005100 | AS | Infliximab + methotrexate | 5 mg/kgc | 10 | 28 | –3.11 (SD 2.23) | – | – | – | – | – |
| AS | Placebo + methotrexate | –d | 10 | 14 | –1.38 (SD 2.11) | – | – | – | – | – | |
| AS | Infliximab + methotrexate | 5 mg/kgc | 30 | 28 | –1.85 (SD 2.84) | – | – | – | – | – | |
| AS | Placebo + methotrexate | –d | 30 | 14 | –0.84 (SD 1.8) | – | – | – | – | – | |
| Van den Bosch 2002101 | AS | Infliximab | 5 mg/kg (0, 2 and 6 weeks) | 12 | 9 | – | – | – | – | – | – |
| AS | Placebo | – | 12 | 12 | – | – | – | – | – | – | |
| ASSERT 2005102 | AS | Infliximab | 5 mg/kg (0, 2 and ≥ 6 weeks) | 24 | 201 | –2.9 (IQR –4.9 to –0.9) | –1.7 (IQR –3.6 to –0.6) | –1 (IQR –1 to 0) | – | 10.2 (IQR 3.9 to 17.1) | 2.7 (IQR –2.9 to 8.8) |
| AS | Placebo | – | 24 | 78 | –0.4 (IQR –1.4 to 0.7) | 0 (IQR –1 to 1) | 0 (IQR –1 to 0) | – | 0.8 (IQR –1.9 to 6) | 2 (IQR –2.6 to 7.5) | |
| PLANETAS 2013110 | AS | CT-P13 | 5 mg/kg | 14 | 125 | –2.91 (SD 2.17) | –2.51 (SD 2.14) | –0.7 (SD 1.2) | – | – | – |
| AS | Infliximab | 5 mg/kg | 14 | 125 | –2.77 (SD 2.08) | –2.47 (SD 2.18) | –0.7 (SD 1.4) | – | – | – | |
| AS | CT-P13 | 5 mg/kg | 30 | 125 | –3.04 (SD 2.23) | –2.6 (SD 2.19) | –1 (SD 1.4) | – | 7.6 | 6.5 | |
| AS | Infliximab | 5 mg/kg | 30 | 125 | –2.71 (SD 2.24) | –2.54 (SD 2.17) | –0.9 (SD 1.4) | – | 8.5 | 5.2 | |
| AS | CT-P13 | 5 mg/kg | 54 | 125 | – | – | – | – | – | – | |
| AS | Infliximab | 5 mg/kg | 54 | 125 | – | – | – | – | – | – | |
| Trial | Population | Treatment arm | Dose | Time point (weeks) | Patients | Number of responders (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ASAS 20 | ASAS 40 | ASAS 50 | ASAS 70 | BASDAI 50 | ||||||
| Haibel 200852 | Nr-axSpA | Adalimumab | 40 mg every 2 weeks | 12 | 22 | 15 (68) | 12 (55) | – | – | 11 (50) |
| Nr-axSpA | placebo | – | 12 | 24 | 6 (25) | 3 (13) | – | – | 5 (21) | |
| Hu 201255 | AS | Adalimumab | 40 mg every 2 weeks | 12 | 26 | – | – | – | – | – |
| AS | Placebo | – | 12 | 20 | – | – | – | – | – | |
| Huang 201456 | AS | Adalimumab | 40 mg every 2 weeks | 12 | 229 | 154 (67) | 102 (45) | – | – | 114 (50) |
| AS | Placebo | – | 12 | 115 | 35 (30) | 11 (10) | – | – | 19 (17) | |
| Lambert 200757 | AS | Adalimumab | 40 mg every 2 weeks | 12 | 38 | 18 (47) | – | 12 (32) | – | – |
| AS | Placebo | – | 12 | 44 | 12 (27) | – | 5 (11) | – | – | |
| aABILITY-1 201358 | Nr-axSpA | Adalimumab | 40 mg every 2 weeks | 12 | 69 | 41 (59) | 28 (41) | 24 (35) | 13 (19) | 27 (39) |
| Nr-axSpA | Placebo | – | 12 | 73 | 23 (32) | 10 (14) | 6 (8) | 3 (4) | 10 (14) | |
| ATLAS 200661 | AS | Adalimumab | 40 mg every 2 weeks | 12 | 208 | 121 (58) | 83 (40) | – | – | 94 (45) |
| AS | Placebo | – | 12 | 107 | 22 (21) | 14 (13) | – | – | 17 (16) | |
| RAPID-axSpA 201464 | AS | Certolizumab pegol | 200 mg every 2 weeks | 12 | 65 | 37 (57) | 26 (40) | – | – | 27 (42) |
| AS | Certolizumab pegol | 400 mg every 4 weeks | 12 | 56 | 36 (64) | 28 (50) | – | – | 23 (41) | |
| AS | Placebo | – | 12 | 57 | 21 (37) | 11 (19) | – | – | 6 (11) | |
| Nr-axSpA | Certolizumab pegol | 200 mg | 12 | 46 | 27 (59) | 22 (48) | – | – | 23 (50) | |
| Nr-axSpA | Certolizumab pegol | 400 mg | 12 | 51 | 32 (63) | 24 (47) | – | – | 24 (47) | |
| Nr-axSpA | Placebo | – | 12 | 50 | 20 (40) | 8 (16) | – | – | 8 (16) | |
| Barkham 201071 | AS | Etanercept | 25 mg twice weekly | 12 | 20 | – | 4 (20) | – | – | 7 (35) |
| AS | Placebo | – | 12 | 20 | – | 0 (0) | – | – | 1 (5) | |
| Davis 200372 | AS | Etanercept | 25 mg twice weekly | 12 | 138 | 82 (59) | – | 62 (45) | 40 (29) | – |
| AS | Placebo | – | 12 | 139 | 39 (28) | – | 18 (13) | 10 (7) | – | |
| AS | Etanercept | 25 mg twice weekly | 24 | 138 | 78 (57) | – | 58 (42) | – | – | |
| AS | Placebo | – | 24 | 139 | 31 (22) | – | 14 (10) | – | – | |
| Dougados 201174 | AS | Etanercept | 50 mg weekly | 12 | 39 | 25 (64) | 17 (44) | 15 (38) | 10 (26) | 18 (46) |
| AS | Placebo | – | 12 | 43 | 14 (33) | 10 (23) | 6 (14) | 4 (9) | 10 (23) | |
| Dougados 201476 | AS | Etanercept | 50 mg weekly | 12 | 106 | 55 (52) | 34 (32) | – | – | 46 (43) |
| AS | Placebo | – | 12 | 109 | 39 (36) | 17 (16) | – | – | 26 (24) | |
| Nr-axSpA | Etanercept | 50 mg weekly | 12 | 94 | – | 33 (35) | – | – | – | |
| Nr-axSpA | Placebo | – | 12 | 95 | – | 16 (17) | – | – | – | |
| Gorman 200279 | AS | Etanercept | 25 mg twice a week | 16 | 20 | 16 (80) | – | – | – | – |
| AS | Placebo | – | 16 | 20 | 6 (30) | – | – | – | – | |
| Calin 200483 | AS | Etanercept | 25 mg twice weekly | 12 | 45 | 26 (58) | – | – | – | – |
| AS | Placebo | – | 12 | 39 | 9 (23) | – | – | – | – | |
| van der Heijde 200686 | AS | Etanercept | 25 mg twice weekly | 12 | 150 | 107 (71) | 80 (53) | – | – | 87 (58) |
| AS | Etanercept | 50 mg weekly | 12 | 155 | 115 (74) | 90 (58) | – | – | 93 (60) | |
| AS | Placebo | – | 12 | 51 | 19 (37) | 11 (22) | – | – | 10 (20) | |
| Giardina 201088 | AS | Etanercept | 50 mg weekly | 12 | 25 | 15 (60) | 11 (44) | – | – | – |
| AS | Infliximab | 5 mg/kg (0, 2 and ≥ 6 weeks) | 12 | 25 | 19 (76) | 14 (56) | – | – | – | |
| GO-RAISE 200890 | AS | Golimumab | 50 mg (two every 4 weeks) | 14 | 138 | 82 (59) | 62 (45) | – | – | 61 (44) |
| AS | Golimumab | 100 mg (two every 4 weeks) | 14 | 140 | 84 (60) | 69 (49) | – | – | 56 (40) | |
| AS | Placebo | – | 14 | 78 | 17 (22) | 12 (15) | – | – | 12 (15) | |
| Bao 201296 | AS | Golimumab | 50 mg every 4 weeks | 14 | 108 | 53 (49) | 38 (35) | – | – | 37 (34) |
| AS | Placebo | – | 14 | 105 | 26 (25) | 10 (10) | – | – | 5 (5) | |
| Tam 201497 | AS | Golimumab | 50 mg monthly | 26 | 20 | 11 (55) | – | – | – | – |
| AS | Placebo | – | 26 | 21 | 3 (14) | – | – | – | – | |
| Barkham 200950 | Nr-axSpA | Infliximab | 5 mg/kg (0, 2 and ≥ 6 weeks) | 16 | 20 | – | 11 (55) | – | – | – |
| Nr-axSpA | Placebo | – | 16 | 20 | – | 3 (15) | – | – | – | |
| Braun 200298 | AS | Infliximab | 5 mg/kg (0, 2 and 6 weeks) | 12 | 34 | 23 (68) | – | 16 (47) | – | 18 (53) |
| AS | Placebo | – | 12 | 35 | 10 (29) | – | 2 (6) | – | 3 (9) | |
| Marzo-Ortega 2005100 | AS | Infliximab + methotrexate | 5 mg/kgb | 10 | 28 | 20 (71) | – | – | – | – |
| AS | Placebo + methotrexate | –c | 10 | 14 | 4 (29) | – | – | – | – | |
| AS | Infliximab + methotrexate | 5 mg/kgb | 30 | 28 | 14 (50) | – | – | – | – | |
| AS | Placebo + methotrexate | –c | 30 | 14 | 3 (21) | – | – | – | – | |
| Van den Bosch 2002101 | AS | Infliximab | 5 mg/kg (0, 2 and 6 weeks) | 12 | 9 | – | – | – | – | – |
| AS | Placebo | – | 12 | 12 | – | – | – | – | – | |
| ASSERT 2005102 | AS | Infliximab | 5 mg/kg (0, 2 and ≥ 6 weeks) | 24 | 201 | 123 (61) | 93 (46) | – | – | – |
| AS | Placebo | – | 24 | 78 | 15 (19) | 9 (12) | – | – | – | |
| PLANETAS 2013110 | AS | CT-P13 | 5 mg/kg | 14 | 125 | 72 (58) | 48 (38) | – | – | – |
| AS | Infliximab | 5 mg/kg | 14 | 125 | 79 (63) | 56 (45) | – | – | – | |
| AS | CT-P13 | 5 mg/kg | 30 | 125 | 79 (63) | 58 (46) | – | – | – | |
| AS | Infliximab | 5 mg/kg | 30 | 125 | 84 (67) | 55 (44) | – | – | – | |
| AS | CT-P13 | 5 mg/kg | 54 | 125 | 71 (57) | 51 (41) | – | – | – | |
| AS | Infliximab | 5 mg/kg | 54 | 125 | 75 (60) | 46 (37) | – | – | – | |
Appendix 5 Bath Ankylosing Spondylitis Disease Activity Index and Bath Ankylosing Spondylitis Functional Index scores conditional on Bath Ankylosing Spondylitis Disease Activity Index response
In this appendix we use the results from the extended synthesis model (Appendices 12 and 13) to evaluate the conditional scores by simulating BASDAI and BASFI scores for two equivalent cohorts of patients one treated with an anti-TNF and the other with conventional therapy.
Description of methods
From the inferences obtained using the synthesis model in Appendix 13 it is possible to derive the conditional change score in responders and non-responders using simulation. Whereas the synthesis focuses on the pooling of mean estimates of change scores and proportion of responders to BASDAI 50, to derive conditional mean scores there is the need to consider the distributions at the individual patient level. Hence, conditional scores could not directly be derived from the synthesis but instead were derived through a simulation procedure based on the assumptions and results of the synthesis model.
The steps undertaken within the simulation procedure were:
-
Simulate baseline BASDAI scores, xBASDAI*, from beliefs over its distribution, X∼N(ν, σ)
-
Simulate yk=1∗ from beliefs over the mean (µ) of this quantity considering correlation with xBASDAI*:
-
Simulate yk=2∗ (where k = 2 represents treatment with anti-TNF) by considering:
-
Calculate final score for placebo and treatment separately, by summing xfinalBASDAI∗=ykBASDAI∗+xBASDAI∗
-
Compute response variables for both groups as ykBASDAI*+x∗/2 <0
Repeat steps 1 to 4 until the desired sample size is achieved, and calculate conditional scores based on response variable and change in scores.
To evaluate BASFI conditional on BASDAI scores one needs to firstly consider we have available information on the BASFI scores at baseline: XBASFI∼N(νBASFI,(seBASFI)2), and also on correlation with BASDAI scores, φ (at individual level). By considering xBASDAI*, one can:
-
Simulate from the distribution of the baseline BASFI score conditional on the baseline BASDAI score being x*:
Note the correlation parameter, φ which represents the individual-level correlation between baseline BASFI and BASDAI scores.
-
Simulate the change from baseline on BASFI for placebo yk=1BASFI* from belief over this quantity, consider this to be correlated with the yk=1∗ simulated for BASDAI (use correlation parameter estimated within the synthesis):
-
Simulate the change from baseline for anti-TNF treatment:
Note that d represents the mean of the predictive distribution from the synthesis model.
We used a simulation sample size of 10,000 patients. Given results depend on the baseline distributions of BASDAI and BASFI and on the change scores from baseline for placebo, we used the averages across trials (weighted by the number of patients in each trial) in AS. Baseline BASDAI scores were thus assumed normally distributed with mean 6.11 and SD of 1.56; change from baseline for placebo was simulated from a normal distribution with mean –0.61 and SD of 1.44. For BASFI, the baseline was assumed to have a mean of 5.27 and a SD of 1.79 and change from baseline for placebo a mean of –0.19 and a SD of 0.22. The correlation between baseline BASFI and BASDAI scores was valued at 0.7 (φ). Average scores from the RAPID-axSpA64 trial for certolizumab were used for the nr-AxSpA analysis.
Results
Results of the prediction of conditional scores using the synthesis model in the AS population are presented in Table 114 and for the nr-AxSpA population in Table 115.
| BASDAI | BASFI | |||
|---|---|---|---|---|
| Control | Treat | Control | Treat | |
| Scenario 1 | ||||
| % responders to BASDAI 50 | 0.10 | 0.42 | – | – |
| Change in score | ||||
| Responders | –2.70 | –3.86 | –1.41 | –3.02 |
| Non-responders | –0.45 | –1.73 | –0.17 | –0.63 |
| All | –0.66 | –2.63 | –0.29 | –1.64 |
| Baseline | ||||
| Responders | 3.83 | 4.76 | 3.42 | 4.17 |
| Non-responders | 6.31 | 7.03 | 5.43 | 6.02 |
| All | 6.08 | 6.08 | 5.24 | 5.24 |
| BASDAI | BASFI | |||
|---|---|---|---|---|
| Control | Treat | Control | Treat | |
| Scenario 1 | ||||
| % responders to BASDAI 50 | AiC information has been removed | AiC information has been removed | – | – |
| Change in score | AiC information has been removeda | AiC information has been removeda | – | – |
| Responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Non-responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| All | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Baseline | AiC information has been removeda | AiC information has been removeda | – | – |
| Responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Non-responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| All | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Scenario 2 | ||||
| % responders to BASDAI 50 | AiC information has been removed | AiC information has been removed | – | – |
| Change in score | AiC information has been removeda | AiC information has been removeda | – | – |
| Responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Non-responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| All | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Baseline | AiC information has been removeda | AiC information has been removeda | – | – |
| Responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Non-responders | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| All | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
Appendix 6 Bath Ankylosing Spondylitis Disease Activity Index and Bath Ankylosing Spondylitis Functional Index scores conditional on response data
| Anti-TNF Population (trial) | Response criterion | Treatment | Response | n | Mean | SD |
|---|---|---|---|---|---|---|
| Adalimumab AS (ATLAS61) | ASAS 20 | Adalimumab 40 mg | Non-responder | 83 | 6.23 | 1.929 |
| Responder | 121 | 6.27 | 1.542 | |||
| Placebo | Non-responder | 82 | 6.29 | 1.712 | ||
| Responder | 22 | 6.64 | 1.468 | |||
| ASAS 40 | Adalimumab 40 mg | Non-responder | 119 | 6.21 | 1.802 | |
| Responder | 85 | 6.32 | 1.568 | |||
| Placebo | Non-responder | 89 | 6.37 | 1.714 | ||
| Responder | 15 | 6.34 | 1.362 | |||
| BASDAI 50 | Adalimumab 40 mg | Non-responder | 119 | 6.21 | 1.802 | |
| Responder | 85 | 6.32 | 1.568 | |||
| Placebo | Non-responder | 89 | 6.37 | 1.714 | ||
| Responder | 15 | 6.34 | 1.362 | |||
| Golimumab AS (GO-RAISE90) | ASAS 20 | Golimumab 50 mg | Non-responder | 56 | 6.51 | 1.687 |
| Responder | 82 | 6.49 | 1.494 | |||
| Placebo | Non-responder | 61 | 6.65 | 1.622 | ||
| Responder | 17 | 6.46 | 1.120 | |||
| ASAS 40 | Golimumab 50 mg | Non-responder | 76 | 6.54 | 1.680 | |
| Responder | 62 | 6.45 | 1.433 | |||
| Placebo | Non-responder | 66 | 6.65 | 1.579 | ||
| Responder | 12 | 6.41 | 1.194 | |||
| BASDAI 50 | Golimumab 50 mg | Non-responder | 72 | 6.69 | 1.523 | |
| Responder | 61 | 6.25 | 1.638 | |||
| Placebo | Non-responder | 66 | 6.63 | 1.581 | ||
| Responder | 12 | 6.51 | 1.194 | |||
| Etanercept AS (314-EU167 study) | ASAS 20 | Etanercept 25 mg twice weekly | Non-responder | 43 | CiC information has been removed | CiC information has been removed |
| Responder | 107 | CiC information has been removed | CiC information has been removed | |||
| Etanercept 50 mg once weekly | Non-responder | 40 | CiC information has been removed | CiC information has been removed | ||
| Responder | 115 | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | 32 | CiC information has been removed | CiC information has been removed | ||
| Responder | 19 | CiC information has been removed | CiC information has been removed | |||
| ASAS 40 | Etanercept 25 mg twice weekly | Non-responder | 70 | CiC information has been removed | CiC information has been removed | |
| Responder | 80 | CiC information has been removed | CiC information has been removed | |||
| Etanercept 50 mg once weekly | Non-responder | 65 | CiC information has been removed | CiC information has been removed | ||
| Responder | 90 | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | 40 | CiC information has been removed | CiC information has been removed | ||
| Responder | 11 | CiC information has been removed | CiC information has been removed | |||
| BASDAI 50 | Etanercept 25 mg twice weekly | Non-responder | 63 | CiC information has been removed | CiC information has been removed | |
| Responder | 87 | CiC information has been removed | CiC information has been removed | |||
| Etanercept 50 mg once weekly | Non-responder | 62 | CiC information has been removed | CiC information has been removed | ||
| Responder | 93 | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | 41 | CiC information has been removed | CiC information has been removed | ||
| Responder | 10 | CiC information has been removed | CiC information has been removed | |||
| Adalimumab ABILITY-158 (nr-AxSpA sub-population with a positive MRI and/or elevated CRP level) | ASAS 20 | Adalimumab 40 mg | Non-responder | 27 | 6.31 | 1.66 |
| Responder | 41 | 6.46 | 1.49 | |||
| Placebo | Non-responder | 46 | 6.49 | 1.37 | ||
| Responder | 23 | 6.05 | 1.77 | |||
| ASAS 40 | Adalimumab 40 mg | Non-responder | 40 | 6.60 | 1.63 | |
| Responder | 28 | 6.13 | 1.41 | |||
| Placebo | Non-responder | 59 | 6.41 | 1.55 | ||
| Responder | 10 | 5.93 | 1.27 | |||
| BASDAI 50 | Adalimumab 40 mg | Non-responder | 41 | 6.53 | 1.69 | |
| Responder | 27 | 6.21 | 1.31 | |||
| Placebo | Non-responder | 59 | 6.46 | 1.52 | ||
| Responder | 10 | 5.64 | 1.34 | |||
| Etanercept (1031 study,166 nr-AxSpA) | ASAS 20 | Etanercept 50 mg | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | ||
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| ASAS 40 | Etanercept 50 mg | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | ||
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| BASDAI 50 | Etanercept 50 mg | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | ||
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Anti-TNF (trial) | Response criterion | Treatment | Response | n | Mean | SD |
|---|---|---|---|---|---|---|
| Adalimumab AS (ATLAS61) | ASAS 20 | Adalimumab 40 mg | Non-responder | 83 | 53.03 | 23.881 |
| Responder | 121 | 51.38 | 20.843 | |||
| Placebo | Non-responder | 82 | 57.96 | 23.089 | ||
| Responder | 22 | 52.27 | 16.661 | |||
| ASAS 40 | Adalimumab 40 mg | Non-responder | 119 | 53.05 | 22.864 | |
| Responder | 85 | 50.65 | 21.005 | |||
| Placebo | Non-responder | 89 | 57.05 | 22.954 | ||
| Responder | 15 | 54.98 | 14.996 | |||
| BASDAI 50 | Adalimumab 40 mg | Non-responder | 110 | 57.79 | 21.015 | |
| Responder | 94 | 45.34 | 21.514 | |||
| Placebo | Non-responder | 87 | 59.06 | 21.989 | ||
| Responder | 17 | 44.98 | 17.979 | |||
| Golimumab AS (GO-RAISE90) | ASAS 20 | Golimumab 50 mg | Non-responder | 56 | 5.35 | 2.530 |
| Responder | 82 | 4.76 | 2.249 | |||
| Placebo | Non-responder | 59 | 5.38 | 2.260 | ||
| Responder | 17 | 4.13 | 1.985 | |||
| ASAS 40 | Golimumab 50 mg | Non-responder | 76 | 5.33 | 2.488 | |
| Responder | 62 | 4.60 | 2.184 | |||
| Placebo | Non-responder | 64 | 5.33 | 2.247 | ||
| Responder | 12 | 3.88 | 1.932 | |||
| BASDAI 50 | Golimumab 50 mg | Non-responder | 72 | 5.48 | 2.412 | |
| Responder | 61 | 4.45 | 2.288 | |||
| Placebo | Non-responder | 64 | 5.39 | 2.179 | ||
| Responder | 12 | 3.56 | 2.070 | |||
| Etanercept AS (314-EU167) | ASAS 20 | Etanercept 25 mg twice weekly | Non-responder | 43 | CiC information has been removed | CiC information has been removed |
| Responder | 107 | CiC information has been removed | CiC information has been removed | |||
| Etanercept 50 mg once weekly | Non-responder | 40 | CiC information has been removed | CiC information has been removed | ||
| Responder | 115 | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | 32 | CiC information has been removed | CiC information has been removed | ||
| Responder | 19 | CiC information has been removed | CiC information has been removed | |||
| ASAS 40 | Etanercept 25 mg twice weekly | Non-responder | 70 | CiC information has been removed | CiC information has been removed | |
| Responder | 80 | CiC information has been removed | CiC information has been removed | |||
| Etanercept 50 mg once weekly | Non-responder | 65 | CiC information has been removed | CiC information has been removed | ||
| Responder | 90 | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | 40 | CiC information has been removed | CiC information has been removed | ||
| Responder | 11 | CiC information has been removed | CiC information has been removed | |||
| BASDAI 50 | Etanercept 25 mg twice weekly | Non-responder | 63 | CiC information has been removed | CiC information has been removed | |
| Responder | 87 | CiC information has been removed | CiC information has been removed | |||
| Etanercept 50 mg once weekly | Non-responder | 62 | CiC information has been removed | CiC information has been removed | ||
| Responder | 93 | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | 41 | CiC information has been removed | CiC information has been removed | ||
| Responder | 10 | CiC information has been removed | CiC information has been removed | |||
| Adalimumab nr-AxSpA, ABILITY-158 (subpopulation with a positive MRI and/or elevated CRP level) | ASAS 20 | Adalimumab 40 mg | Non-responder | 27 | 45.17 | 22.07 |
| Responder | 40 | 43.05 | 19.31 | |||
| Placebo | Non-responder | 47 | 48.07 | 22.99 | ||
| Responder | 23 | 47.91 | 23.75 | |||
| ASAS 40 | Adalimumab 40 mg | Non-responder | 40 | 47.61 | 22.60 | |
| Responder | 27 | 39.09 | 15.41 | |||
| Placebo | Non-responder | 60 | 48.26 | 23.46 | ||
| Responder | 10 | 46.54 | 21.67 | |||
| BASDAI 50 | Adalimumab 40 mg | Non-responder | 40 | 49.71 | 20.05 | |
| Responder | 27 | 35.97 | 18.12 | |||
| Placebo | Non-responder | 59 | 49.06 | 23.25 | ||
| Responder | 10 | 43.66 | 23.07 | |||
| Etanercept (1031 study166 nr-AxSpA) | ASAS 20 | Etanercept 50 mg | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | ||
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| ASAS 40 | Etanercept 50 mg | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | ||
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| BASDAI 50 | Etanercept 50 mg | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| Placebo | Non-responder | CiC information has been removed | CiC information has been removed | CiC information has been removed | ||
| Responder | CiC information has been removed | CiC information has been removed | CiC information has been removed |
Summary
The mean baseline BASDAI and BASFI are presented by treatment response at week 12 (or 14 for golimumab) for three of the five anti-TNFs. This reveals that in patients with AS and patients with nr-AxSpA, on average baseline BASDAI score does not differ greatly between responders and non-responders either to placebo or to active anti-TNF therapy. In patients with AS or nr-AxSpA from the trials of adalimumab (ATLAS61 and ABILITY-158) and golimumab (GO-RAISE90) on average baseline BASFI score was higher in non-responders compared with responders. However, this was not seen in the etanercept trials.
Appendix 7 Relative effects of anti-tumour necrosis factors
Ankylosing spondylitis population
In the following tables, the intervention is stated in the top row and the comparator is in the left-hand column, which is reverse to normal.
| Anti-TNF | ADA | CER | ETA | GOL | INF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 1.15 | 0.61 to 1.86 | 1.01 | 0.65 to 1.50 | 1.13 | 0.75 to 1.66 | 1.55 | 0.74 to 2.50 |
| CER | 0.87 | 0.54 to 1.63 | – | – | 0.88 | 0.50 to 1.70 | 0.99 | 0.58 to 1.89 | 1.34 | 0.61 to 2.74 |
| ETA | 0.99 | 0.67 to 1.53 | 1.14 | 0.59 to 1.98 | – | – | 1.12 | 0.71 to 1.78 | 1.53 | 0.72 to 2.66 |
| GOL | 0.88 | 0.60 to 1.33 | 1.01 | 0.53 to 1.74 | 0.89 | 0.56 to 1.40 | – | – | 1.37 | 0.65 to 2.30 |
| INF | 0.65 | 0.40 to 1.35 | 0.74 | 0.37 to 1.65 | 0.65 | 0.38 to 1.38 | 0.73 | 0.43 to 1.54 | – | – |
| Anti-TNF | ADA | CER | ETA | GOL | INF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 1.28 | 0.47 to 3.48 | 1.01 | 0.51 to 2.02 | 1.25 | 0.62 to 2.48 | 2.58 | 0.62 to 10.60 |
| CER | 0.78 | 0.29 to 2.14 | – | – | 0.79 | 0.27 to 2.32 | 0.98 | 0.33 to 2.89 | 2.02 | 0.39 to 10.33 |
| ETA | 0.99 | 0.50 to 1.97 | 1.26 | 0.43 to 3.71 | – | – | 1.23 | 0.56 to 2.73 | 2.55 | 0.58 to 11.01 |
| GOL | 0.80 | 0.40 to 1.61 | 1.02 | 0.35 to 3.03 | 0.81 | 0.37 to 1.80 | – | – | 2.06 | 0.47 to 8.91 |
| INF | 0.39 | 0.09 to 1.62 | 0.50 | 0.10 to 2.56 | 0.39 | 0.09 to 1.72 | 0.49 | 0.11 to 2.12 | – | – |
| Anti-TNF | ADA | CER | ETA | GOL | INF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 0.79 | 0.53 to 1.07 | 0.98 | 0.82 to 1.17 | 0.94 | 0.75 to 1.15 | 1.07 | 0.75 to 1.38 |
| CER | 1.27 | 0.93 to 1.88 | – | – | 1.24 | 0.91 to 1.83 | 1.19 | 0.85 to 1.77 | 1.35 | 0.88 to 2.09 |
| ETA | 1.03 | 0.86 to 1.22 | 0.81 | 0.55 to 1.10 | – | – | 0.96 | 0.76 to 1.18 | 1.10 | 0.77 to 1.41 |
| GOL | 1.07 | 0.87 to 1.34 | 0.84 | 0.56 to 1.18 | 1.04 | 0.85 to 1.31 | – | – | 1.14 | 0.79 to 1.53 |
| INF | 0.93 | 0.73 to 1.34 | 0.74 | 0.48 to 1.14 | 0.91 | 0.71 to 1.31 | 0.87 | 0.66 to 1.27 | – | – |
| Anti-TNF | ADA | CER | ETA | GOL | INF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 0.57 | 0.28 to 1.20 | 0.94 | 0.58 to 1.50 | 0.85 | 0.49 to 1.46 | 1.23 | 0.50 to 3.01 |
| CER | 1.74 | 0.84 to 3.57 | – | – | 1.62 | 0.78 to 3.35 | 1.47 | 0.67 to 3.16 | 2.13 | 0.74 to 6.13 |
| ETA | 1.07 | 0.67 to 1.71 | 0.62 | 0.30 to 1.28 | – | – | 0.90 | 0.52 to 1.55 | 1.31 | 0.54 to 3.20 |
| GOL | 1.18 | 0.69 to 2.05 | 0.68 | 0.32 to 1.49 | 1.11 | 0.65 to 1.91 | – | – | 1.46 | 0.57 to 3.70 |
| INF | 0.82 | 0.33 to 1.99 | 0.47 | 0.16 to 1.36 | 0.76 | 0.31 to 1.86 | 0.69 | 0.27 to 1.75 | – | – |
| Anti-TNF | ADA | CER | ETA | GOL | ||||
|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 0.74 | 0.41 to 1.22 | 0.80 | 0.51 to 1.20 | 0.91 | 0.61 to 1.32 |
| CER | 1.35 | 0.82 to 2.45 | – | – | 1.09 | 0.61 to 2.04 | 1.23 | 0.72 to 2.26 |
| ETA | 1.24 | 0.83 to 1.95 | 0.92 | 0.49 to 1.63 | – | – | 1.13 | 0.72 to 1.81 |
| GOL | 1.10 | 0.76 to 1.64 | 0.81 | 0.44 to 1.38 | 0.88 | 0.55 to 1.38 | – | – |
| Anti-TNF | ADA | CER | ETA | GOL | ||||
|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 0.59 | 0.25 to 1.45 | 0.68 | 0.33 to 1.40 | 0.84 | 0.42 to 1.67 |
| CER | 1.68 | 0.69 to 4.04 | – | – | 1.14 | 0.45 to 2.90 | 1.42 | 0.57 to 3.50 |
| ETA | 1.47 | 0.71 to 3.02 | 0.87 | 0.35 to 2.24 | – | – | 1.23 | 0.58 to 2.63 |
| GOL | 1.19 | 0.60 to 2.38 | 0.71 | 0.29 to 1.75 | 0.81 | 0.38 to 1.72 | – | – |
| Anti-TNF | ADA | ETA | INF | |||
|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 1.24 | 0.60 to 3.31 | 2.00 | 0.73 to 5.87 |
| ETA | 0.81 | 0.30 to 1.66 | – | – | 1.63 | 0.68 to 2.95 |
| INF | 0.50 | 0.17 to 1.36 | 0.61 | 0.34 to 1.46 | – | – |
| Anti-TNF | ADA | ETA | INF | |||
|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 1.40 | 0.40 to 5.05 | 4.11 | 0.59 to 29.29 |
| ETA | 0.71 | 0.20 to 2.49 | – | – | 2.92 | 0.55 to 15.51 |
| INF | 0.24 | 0.03 to 1.71 | 0.34 | 0.06 to 1.81 | – | – |
| Anti-TNF | ADA | CER | ETA | INF | ||||
|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 0.10 | –0.68 to 0.88 | –0.20 | –0.71 to 0.30 | –0.73 | –1.69 to 0.24 |
| CER | –0.10 | –0.88 to 0.68 | – | – | –0.30 | –1.12 to 0.52 | –0.82 | –1.98 to 0.33 |
| ETA | 0.20 | –0.30 to 0.71 | 0.30 | –0.52 to 1.12 | – | – | –0.53 | –1.50 to 0.47 |
| INF | 0.73 | –0.24 to 1.69 | 0.82 | –0.33 to 1.98 | 0.53 | –0.47 to 1.50 | – | – |
| Anti-TNF | ADA | CER | ETA | GOL | INF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 0.15 | –0.67 to 0.97 | –0.18 | –0.73 to 0.36 | –0.20 | –0.75 to 0.35 | –0.91 | –2.00 to 0.20 |
| CER | –0.15 | –0.97 to 0.67 | – | – | –0.33 | –1.16 to 0.49 | –0.35 | –1.17 to 0.47 | –1.05 | –2.31 to 0.22 |
| ETA | 0.18 | –0.36 to 0.73 | 0.33 | –0.49 to 1.16 | – | – | –0.02 | –0.57 to 0.55 | –0.72 | –1.83 to 0.39 |
| GOL | 0.20 | –0.35 to 0.75 | 0.35 | –0.47 to 1.17 | 0.02 | –0.55 to 0.57 | – | – | –0.71 | –1.82 to 0.42 |
| INF | 0.91 | –0.20 to 2.00 | 1.05 | –0.22 to 2.31 | 0.72 | –0.39 to 1.83 | 0.71 | –0.42 to 1.82 | – | – |
| Anti-TNF | ADA | CER | ETA | GOL | ||||
|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 0.11 | –0.21 to 0.42 | 0.00 | –0.32 to 0.31 | 0.26 | 0.06 to 0.46 |
| CER | –0.11 | –0.42 to 0.21 | – | – | –0.11 | –0.51 to 0.30 | 0.15 | –0.17 to 0.48 |
| ETA | 0.00 | –0.31 to 0.32 | 0.11 | –0.30 to 0.51 | – | – | 0.26 | –0.06 to 0.58 |
| GOL | –0.26 | –0.46 to –0.06 | –0.15 | –0.48 to 0.17 | –0.26 | –0.58 to 0.06 | – | – |
| Anti-TNF | ADA | CER | GOL | |||
|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 2.11 | –0.20 to 4.44 | 1.52 | –0.24 to 3.30 |
| CER | –2.11 | –4.44 to 0.20 | – | – | –0.59 | –2.99 to 1.85 |
| GOL | –1.52 | –3.30 to 0.24 | 0.59 | –1.85 to 3.00 | – | – |
| Anti-TNF | GOL | |
|---|---|---|
| Mean difference | 95% CrI | |
| ADA | –0.20 | –1.12 to 0.70 |
| Anti-TNF | ADA | CER | GOL | |||
|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | –0.15 | –3.83 to 3.54 | 1.33 | –0.97 to 3.63 |
| CER | 0.15 | –3.53 to 3.83 | – | – | 1.51 | –2.24 to 5.21 |
| GOL | –1.33 | –3.63 to 0.98 | –1.51 | –5.20 to 2.24 | – | – |
Relative effects of anti-tumour necrosis factors: non-radiographic axial spondyloarthritis population
In the following tables, the intervention is stated in the top row and the comparator is in the left-hand column, which is reverse to normal.
| Anti-TNF | ADA | CER | ETA | |||
|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 1.11 | 0.62 to 1.96 | 0.76 | 0.44 to 1.30 |
| CER | 0.90 | 0.51 to 1.61 | – | – | 0.69 | 0.38 to 1.22 |
| ETA | 1.31 | 0.77 to 2.28 | 1.46 | 0.82 to 2.62 | – | – |
| Anti-TNF | ADA | CER | ETA | |||
|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 1.24 | 0.42 to 3.75 | 0.62 | 0.25 to 1.55 |
| CER | 0.81 | 0.27 to 2.40 | – | – | 0.50 | 0.18 to 1.40 |
| ETA | 1.62 | 0.65 to 3.99 | 2.01 | 0.72 to 5.68 | – | – |
| Anti-TNF | ADA | CER | ETA | |||
|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 0.83 | 0.54 to 1.20 | 0.77 | 0.52 to 1.08 |
| CER | 1.20 | 0.84 to 1.87 | – | – | 0.92 | 0.60 to 1.44 |
| ETA | 1.31 | 0.93 to 1.94 | 1.09 | 0.70 to 1.67 | – | – |
| Anti-TNF | ADA | CER | ETA | |||
|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 0.62 | 0.25 to 1.59 | 0.52 | 0.23 to 1.19 |
| CER | 1.60 | 0.63 to 3.98 | – | – | 0.83 | 0.34 to 2.01 |
| ETA | 1.92 | 0.84 to 4.33 | 1.20 | 0.50 to 2.92 | – | – |
| Anti-TNF | ADA | CER | ETA | INF | ||||
|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 0.97 | 0.51 to 1.78 | 0.66 | 0.35 to 1.21 | 1.16 | 0.42 to 2.29 |
| CER | 1.04 | 0.56 to 1.98 | – | – | 0.68 | 0.35 to 1.35 | 1.20 | 0.43 to 2.55 |
| ETA | 1.51 | 0.83 to 2.82 | 1.46 | 0.74 to 2.85 | – | – | 1.74 | 0.63 to 3.70 |
| INF | 0.86 | 0.44 to 2.37 | 0.84 | 0.39 to 2.33 | 0.57 | 0.27 to 1.58 | – | – |
| Anti-TNF | ADA | CER | ETA | INF | ||||
|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 0.94 | 0.31 to 2.90 | 0.51 | 0.19 to 1.35 | 1.36 | 0.26 to 7.22 |
| CER | 1.07 | 0.34 to 3.25 | – | – | 0.54 | 0.18 to 1.58 | 1.45 | 0.25 to 8.10 |
| ETA | 1.98 | 0.74 to 5.23 | 1.86 | 0.63 to 5.51 | – | – | 2.68 | 0.52 to 13.91 |
| INF | 0.73 | 0.14 to 3.91 | 0.69 | 0.12 to 3.93 | 0.37 | 0.07 to 1.91 | – | – |
| Anti-TNF | ADA | CER | ETA | INF | ||||
|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | –0.63 | –1.77 to 0.52 | 0.53 | –0.51 to 1.56 | –1.43 | –3.08 to 0.22 |
| CER | 0.63 | –0.52 to 1.77 | – | – | 1.15 | –0.12 to 2.42 | –0.81 | –2.62 to 1.00 |
| ETA | –0.53 | –1.56 to 0.51 | –1.15 | –2.42 to 0.12 | – | – | –1.97 | –3.70 to –0.21 |
| INF | 1.43 | –0.21 to 3.08 | 0.81 | –1.00 to 2.62 | 1.97 | 0.21 to 3.70 | – | – |
| Anti-TNF | ADA | CER | ETA | INF | ||||
|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | –1.00 | –2.10 to 0.10 | 0.30 | –0.48 to 1.08 | –1.33 | –2.86 to 0.19 |
| CER | 1.00 | –0.10 to 2.10 | – | – | 1.30 | 0.19 to 2.41 | –0.33 | –2.05 to 1.38 |
| ETA | –0.30 | –1.08 to 0.48 | –1.30 | –2.41 to –0.19 | – | – | –1.63 | –3.15 to –0.09 |
| INF | 1.33 | –0.19 to 2.86 | 0.33 | –1.38 to 2.05 | 1.63 | 0.09 to 3.15 | – | – |
| Anti-TNF | ADA | CER | ETA | |||
|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | –0.53 | –0.93 to –0.12 | 0.02 | –0.47 to 0.51 |
| CER | 0.53 | 0.12 to 0.93 | – | – | 0.55 | –0.02 to 1.10 |
| INF | –0.02 | –0.51 to 0.47 | –0.55 | –1.10 to 0.02 | – | – |
| Anti-TNF | ADA | CER | ETA | |||
|---|---|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 2 | –1.53 to 5.57 | –2.88 | –6.11 to 0.31 |
| CER | –2.00 | –5.57 to 1.53 | – | – | –4.88 | –8.52 to –1.29 |
| INF | 2.88 | –0.31 to 6.11 | 4.88 | 1.29 to 8.52 | – | – |
| Anti-TNF | ADA | CER | ||
|---|---|---|---|---|
| Mean difference | 95% CrI | Mean difference | 95% CrI | |
| ADA | – | – | 2.87 | –1.78 to 7.49 |
| CER | –2.87 | –7.49 to 1.78 | – | – |
Appendix 8 Long-term efficacy data
| Study characteristics | Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of responders (%) | Other outcomes | ||||||||
| Trial cohort and references of open-label studies | Population | Treatment and dose | Time point | Number of patients | Imputation methods and withdrawal criteria | ASAS 20 | ASAS 40 | BASDAI 50 | Other results ASAS 50, ASAS 70, BASDAI, BASFI, BASMI, mSASSS, MASES, SF-36 MCS, SF-36 PCS, EQ-5D |
| Haibel 200852–54,185–188 | Nr-axSpA with inflammation | Adalimumab, 40 mg every other week. Non-responders at the end of the double-blind trial (week 12) and after open-label therapy for at least 12 weeks were eligible for dose escalation to 40 mg/week | 52 weeks | 46 | ITT. Patients who withdrew from the study were counted as non-responders for categorical data. LOCF was used for continuous variables | – | 23/46 (50) | 24/46 (52) | BASDAI change from baseline 2.8 (95% CI 2.1 to 3.6) |
| BASFI change from baseline 2 (95% CI 1.4 to 2.6) | |||||||||
| BASMI change from baseline –0.4 (95% CI –0.7 to –0.04) | |||||||||
| EQ-5D change from baseline 0.22 (95% CI 0.13 to 0.31) | |||||||||
| SF-36 MCS change from baseline 4.9 (95% CI 1.6 to 8.1) | |||||||||
| SF-36 PCS change from baseline 10.3 (95% CI 6.9 to 13.8) | |||||||||
| ASQoL change from baseline 5.3 (95% CI 3.8 to 6.7) | |||||||||
| MASES change from baseline 0.9 (95% CI –0.02 to 1.9) | |||||||||
| In total, 26 patients with MRIs at baseline and 52 weeks showed no change in sclerosis or in erosions | |||||||||
| ABILITY-1 201358,189–194 | Nr-axSpA with inflammation | Adalimumab, placebo/adalimumab | 52 weeks | 61 | – | – | – | – | SF-36 PCS change from baseline 10.0 (SD 9.91) |
| Adalimumab, adalimumab/adalimumab | 52 weeks | 55 | – | – | – | – | SF-36 PCS change from baseline 11.0 (SD 9.93) | ||
| Adalimumab, 40 mg every other week | 68 weeks | 111 (patients MRI positive or CRP positive) | Observed (n = 142 at week 12) | 77/111 (69) | 74/111 (67) | – | |||
| Adalimumab, 40 mg every other week | 104 weeks | 102 | – | – | – | – | – | ||
| Adalimumab, 40 mg every other week | 156 weeks | 97 (patients MRI positive or CRP positive) | Observed (n = 142 at week 12) | 83/97 (86) | 67/97 (69) | 70/97 (72) | ASAS 50 responders: n = 58 | ||
| ASAS 70 responders: n = 47 | |||||||||
| ATLAS 200661,140,141,195–201 | AS | Adalimumab, 40 mg every other week | 52 weeks | 311 had at least one dose | Observed | 193/276 (70) | 138/276 (50) | 167/276 (61) | BASDAI change from baseline –3.5 (SD 2.55), n = 274 |
| BASFI change from baseline –2.6 (SD 2.04), n = 274 | |||||||||
| BASMI final value 3.2 (SD 2.2), n = 273 | |||||||||
| SF-36 MCS change from baseline 5.6 (SD 10.35), n = 265 | |||||||||
| SF-36 PCS change from baseline 10.19 (SD 9.5), n = 265 | |||||||||
| ASQoL change from baseline –4.8 (SD 4.41), n = 274 | |||||||||
| MASES final value 2.4 (SD 4.6), n = 279 | |||||||||
| Adalimumab, 40 mg every other week | 76 weeks | – | Observed | – | – | – | BASDAI change from baseline –3.8 (SD 2.33), n = 270 | ||
| BASFI change from baseline –2.8 (SD 2.1), n = 270 | |||||||||
| SF-36 MCS change from baseline 5.1 (SD 11.06), n = 263 | |||||||||
| SF-36 PCS change from baseline 10.8 (SD 9.88), n = 263 | |||||||||
| ASQoL change from baseline –5 (SD 4.32), n = 270 | |||||||||
| Adalimumab, 40 mg every other week | 104 weeks | 173 | Observed | 135/173 (78) | 109/173 (63) | 122/173 (71) | BASDAI change from baseline –3.9 (SD 2.44), n = 262 | ||
| BASFI change from baseline –2.9 (SD 2.14), n = 261 | |||||||||
| BASMI final value 3.1 (SD 2.2), n = 173 | |||||||||
| SF-36 MCS change from baseline 5.7 (SD 10.96), n = 255 | |||||||||
| SF-36 PCS change from baseline 11 (SD 9.88), n = 255 | |||||||||
| ASQoL change from baseline –5.4 (SD 4.28), n = 263 | |||||||||
| MASES change from baseline 2.2 (SD 4.4), n = 217 | |||||||||
| Adalimumab, 40 mg every other week | 128 weeks | – | Observed | – | – | – | BASDAI change from baseline –3.9 (SD 2.39), n = 242 | ||
| BASFI change from baseline –2.9 (SD 2.17), n = 242 | |||||||||
| SF-36 MCS change from baseline 4.1 (SD 10.84), n = 229 | |||||||||
| SF-36 PCS change from baseline 11.3 (SD 9.68), n = 229 | |||||||||
| ASQoL change from baseline –5.3 (SD 4.35), n = 242 | |||||||||
| Adalimumab, 40 mg every other week | 156 weeks | – | Observed | – | – | – | BASDAI change from baseline –3.9 (SD 3.39), n = 236 | ||
| BASFI change from baseline –3 (SD 2.1), n = 236 | |||||||||
| BASMI final value 3.7 (SD 1.8), n = 233 | |||||||||
| SF-36 MCS change from baseline 5.6 (SD 11.59), n = 227 | |||||||||
| SF-36 PCS change from baseline 11.6 (SD 9.65), n = 227 | |||||||||
| ASQoL change from baseline –5.4 (SD 4.36), n = 236 | |||||||||
| Adalimumab, 40 mg every other week | 5 years | 125 (patients randomised to adalimumab and completed 5 years) | Observed | 111/125 (89) | 88/125 (70) | 96/124 (77) | BASDAI score final value 1.8 (SD 1.9), n = 124 | ||
| BASFI score final value 2.1 (SD 2.1), n = 125 | |||||||||
| BASMI score final value 3.7 (SD 1.8), n = 124 | |||||||||
| SF-36 PCS score final value 44.4 (SD 10), n = 165 | |||||||||
| ASQoL score final value 4.8 (SD 4.8), n = 169 | |||||||||
| RAPID-axSpA 201464,202–204 | AS | Certolizumab pegol 200 mg every 2 weeks | 48 weeks | 65 | NRI + LOCF | 47/65 (72) | 34/65 (52) | – | BASDAI score final value 3.3 |
| BASFI score final value 3 | |||||||||
| Certolizumab pegol 400 mg every 4 weeks | 48 weeks | 56 | 42/56 (75) | 36/56 (64) | – | BASDAI score final value 3 | |||
| BASFI score final value 3.2 | |||||||||
| Certolizumab pegol, all | 48 weeks | 121 | AiC information has been removed | AiC information has been removed | – | – | |||
| Certolizumab pegol, 200 mg every 2 weeks | 96 weeks | 65 | NRI | 39 | – | – | – | ||
| Certolizumab pegol, 400 mg every 4 weeks | 96 weeks | 56 | NRI | 39 | – | – | – | ||
| Certolizumab pegol, all | 96 weeks | 121 | NRI | 78/121 (64) | 61/121 (50) | – | – | ||
| Certolizumab pegol, all | 96 weeks | 93 | Observed case | 78/93 (84) | 61/93 (66) | – | – | ||
| Nr-axSpA with inflammation | Certolizumab pegol, 200 mg every 2 weeks | 48 weeks | 46 | NRI was used for categorical measures and LOCF for quantitative measures (48-week data) | 32/46 (70) | 25/46 (54) | – | BASDAI score final value 2.9 | |
| BASFI score final value 2.1 | |||||||||
| Certolizumab pegol, 400 mg every 4 weeks | 48 weeks | 51 | 35/51 (69) | 30/51 (59) | – | BASDAI score final value 3.3 | |||
| BASFI score final value 2.8 | |||||||||
| Certolizumab pegol, all | 48 weeks | 97 | AiC information has been removed | AiC information has been removed | – | – | |||
| Certolizumab pegol 200 mg every 2 weeks | 96 weeks | 46 | NRI | 30 | – | – | |||
| Certolizumab pegol 400 mg every 4 weeks | 96 weeks | 51 | NRI | 29 | – | – | |||
| Certolizumab pegol, all | 96 weeks | 97 | NRI | 59/97 (61) | 49/97 (51) | – | – | ||
| Certolizumab pegol, all | 96 weeks | 74 | Observed case | 59/74 (80) | 49/74 (66) | – | – | ||
| Davis 200372,119,144,145,205,206 | AS | Placebo then etanercept | 72 weeks | 105 | Observed case | – | – | – | BASFI score final value 32.3 (SD 2.5) |
| Etanercept then etanercept | 96 weeks | 95 | Observed case | 70/95 (74) | – | – | ASAS 70 responders 44 | ||
| BASFI score final value 25.4 (SD 2.4) | |||||||||
| Combined groups | 96 weeks | 257 | – | – | – | – | mSASSS change from baseline 0.91 (SD 2.45) | ||
| Placebo then etanercept | 168 weeks | 127 | LOCF | 77/127 (61) | 64/127 (50) | – | – | ||
| Etanercept then etanercept | 192 weeks | 124 | LOCF | 83/124 (67) | 61/124 (49) | – | – | ||
| Dougados 201476,207 | Nr-axSpA mixed | Etanercept then etanercept, 50 mg weekly | 32 weeks | 100 | NRI | – | AiC information has been removed | – | – |
| Placebo then etanercept, 50 mg weekly | 32 weeks | 105 | NRI | – | AiC information has been removed | – | – | ||
| Etanercept then etanercept, 50 mg weekly | 40 weeks | 100 | NRI | – | AiC information has been removed | – | – | ||
| Placebo then etanercept, 50 mg weekly | 40 weeks | 105 | NRI | – | AiC information has been removed | – | – | ||
| Etanercept plus placebo groups together, 50 mg weekly | 48 weeks | AiC information has been removed (189 observed) | NRI + LOCF | 147 | 108 | 128 | AiC information has been removed | ||
| Etanercept then etanercept, 50 mg weekly | 48 weeks | 100 | – | – | AiC information has been removed | – | – | ||
| Placebo then etanercept, 50 mg weekly | 48 weeks | 105 | – | – | AiC information has been removed | – | – | ||
| Gorman 200279,206,208 | Etanercept then etanercept | 28 weeks | 19 | NRI | AiC information has been removed | – | – | AiC information has been removed | |
| Placebo then etanercept | 28 weeks | 19 | NRI | AiC information has been removed | – | – | AiC information has been removed | ||
| Etanercept then etanercept | 40 weeks | 19 | NRI | AiC information has been removed | – | – | AiC information has been removed | ||
| Placebo then etanercept | 40 weeks | 19 | NRI | AiC information has been removed | – | – | AiC information has been removed | ||
| Calin 200483,146,147 | AS | Etanercept then etanercept | 60 weeks | 42 | LOCF | – | – | – | BASDAI score final value 2.1 |
| BASFI score final value 2.9 | |||||||||
| mSASSS change from baseline 0.36 (95% CI –0.1 to 0.8), n = 33 | |||||||||
| Placebo then etanercept | 60 weeks | 39 | LOCF | – | – | – | BASDAI final value 2.7 | ||
| BASFI final value 3.4 | |||||||||
| mSASSS change from baseline –0.15 (95% CI –0.7 to 0.4), n = 34 | |||||||||
| Combined group | 108 weeks | 81 | LOCF | AiC information has been removed | 44/81 (54) | AiC information has been removed | AiC information has been removed | ||
| BASFI final value: 2.9 | |||||||||
| Etanercept then etanercept | 108 weeks | 42 | LOCF | – | – | – | BASDAI score final value 2.3 | ||
| BASFI score final value 3 | |||||||||
| Placebo then etanercept | 108 weeks | 39 | LOCF | – | – | – | BASDAI score final value 2.9 | ||
| BASFI score final value 3.5 | |||||||||
| Combined group | 264 weeks | 59 | LOCF | 40/59 (68) | 39/59 (66) | AiC information has been removed | |||
| BASDAI score final value 2.7 | |||||||||
| BASFI score final value 3.2 | |||||||||
| Bao 201295,96,209,210 | AS | Golimumab, 50 mg | 52 weeks | 108 | ITT | 76/108 (70) | 53/108 (49) | 62/108 (57) | – |
| GO-RAISE 200890,120,142,143,211–222 | AS | Golimumab placebo, 50 mg | 104 weeks | 78 | ITT | 30/78 (38) | 30/78 (38) | – | BASDAI score final value median 6 (IQR 1.36 to 7.79) |
| BASFI score final value median 4.9 (IQR 0.98 to 7.07) | |||||||||
| mSASSS score change from baseline 1.6 (SD 4.6), n = 66 | |||||||||
| Golimumab, 50 mg | 104 weeks | 138 | ITT | 83/138 (60) | 77/138 (56) | – | BASDAI score final value median 2.7 (IQR 0.84 to 6.08) | ||
| BASFI score final value median 2.2 (IQR 0.52 to 5.80) | |||||||||
| mSASSS score change from baseline 0.9 (SD 2.7), n = 111 | |||||||||
| Golimumab, 100 mg | 104 weeks | 140 | ITT | 100/140 (71) | 76/140 (54) | – | BASDAI score final value median 2.7 (IQR 1.08 to 5.34) | ||
| BASFI score final value median 1.8 (IQR 0.49 to 4.79) | |||||||||
| mSASSS score change from baseline 0.9 (SD 3.9), n = 122 | |||||||||
| All patients randomised (all golimumab from week 24) | 104 weeks | 356 | NRI + LOCF | 249/356 (70) | 213/356 (60) | – | – | ||
| All patients randomised (all golimumab from week 24) | 160 weeks | 356 | NRI + LOCF | 246/356 (69) | 208/356 (58) | – | – | ||
| Golimumab placebo, 50 mg | 208 weeks | 78 | – | – | – | – | mSASSS change from baseline 2.1 (SD 5.2), n = 66 | ||
| Golimumab, 50 mg | 208 weeks | 138 | – | – | – | – | mSASSS change from baseline 1.3 (SD 4.1), n = 111 | ||
| Golimumab, 100 mg | 208 weeks | 140 | – | – | – | – | mSASSS change from baseline 2 (SD 5.6), n = 122 | ||
| All patients randomised (all golimumab from week 24) | 256 weeks | 356 | NRI + LOCF | 235/356 (66) | 203/356 (57) | 199/356 (58) | – | ||
| Tam 201497 | AS | Golimumab, 50 mg monthly | 54 weeks | 19 | UC | 18 | – | – | – |
| Placebo/golimumab | 54 weeks | 17 | UC | 14 | – | – | – | ||
| Placebo/placebo | 54 weeks | 3 | UC | 1 | – | – | – | ||
| PLANETAS 2013110,223 | AS | CT-P13 (biosimilar to infliximab) 5 mg/kg | 78 weeks | 88 | ITT | 61/88 (69) | 50/88 (57) | – | – |
| CT-P13 then infliximab (switched at week 54) 5 mg/kg | 78 weeks | 86 | ITT | 64/86 (74) | 43/86 (50) | – | – | ||
| CT-P13 (biosimilar to infliximab) 5 mg/kg | 102 weeks | 88 | ITT | 67/88 (76) | 53/88 (60) | – | – | ||
| CT-P13 then infliximab (switched at week 54) 5 mg/kg | 102 weeks | 86 | ITT | 60/86 (70) | 48/86 (56) | – | – | ||
| Braun 200298,148,164,224–230 | AS | Infliximab 5 mg/kg (infusion at 0, 2 and 6 weeks) | 54 weeks | 34 | NRI for binary data. A completer analysis was conducted | – | – | 47% | mSASSS reported for two groups: patients with worsening of BASFI score of > 1 and those with score of < 1 |
| Placebo/infliximab | 54 weeks | 35 | – | – | 51% | ||||
| Aggregate | 54 weeks | 69 | – | – | 33/69 (48) | BASDAI score final value: 2.5 (SD 1.7), n = 52 | |||
| BASFI score final value: 3.0 (SD 2.2), n = 52 | |||||||||
| BASMI score final value: 2.4 (SD 2.0), n = 52 | |||||||||
| SF-36 MCS score final value: 50.9 (SD 8.9), n = 52 | |||||||||
| SF-36 PCS score final value: 40.6 (SD 10.6), n = 52 | |||||||||
| BASDAI score final value: 2.4 (SD 1.6), n = 46 | |||||||||
| BASFI score final value: 3.1 (SD 2.2), n = 46 | |||||||||
| BASMI score final value: 2.4 (SD 1.9), n = 46 | |||||||||
| SF-36 MCS score final value: 51.5 (SD 8.6), n = 46 | |||||||||
| SF-36 PCS score final value: 40.2 (SD 10.8), n = 46 | |||||||||
| Aggregate | 102 weeks | 69 | NRI for binary data. A completer analysis was conducted | – | – | 30/69 (43) | BASDAI score final value: 2.6 (SD 2), n = 52 | ||
| BASFI score final value: 3.0 (SD 2.2), n = 52 | |||||||||
| BASMI score final value: 2.7 (SD 2.1), n = 52 | |||||||||
| SF-36 MCS score final value: 50.2 (SD 9.5), n = 52 | |||||||||
| SF-36 PCS score final value: 40.9 (SD 11.1), n = 52 | |||||||||
| BASDAI score final value: 2.6 (SD 2), n = 46 | |||||||||
| BASFI score final value: 3.1 (SD 2.3), n = 46 | |||||||||
| BASMI score final value: 2.7 (SD 2.1), n = 46 | |||||||||
| SF-36 MCS score final value: 51.4 (SD 8.9), n = 46 | |||||||||
| SF-36 PCS score final value: 40.5 (SD 11.4), n = 46 | |||||||||
| Aggregate | 156 weeks | 46 | Completer analysis. To calculate means, LOCF was used | – | – | 28/46 (61) | BASDAI score final value: 2.7 (SD 2), n = 46? | ||
| BASFI score final value: 3.1 (SD 2.5) | |||||||||
| BASMI score final value: 2.8 (SD 2.2) | |||||||||
| SF-36 MCS score final value: 48.8 (SD 10.4) | |||||||||
| SF-36 PCS score final value: 41.6 (SD 11.7) | |||||||||
| ASSERT 2005102,231–236 | AS | Infliximab (on placebo 0–24 weeks) 5 mg | Week 102 | 78 (remaining study patients may have taken high unlicensed dose of infliximab) | Completer analysis and ITT LOCF | – | Completer analysis 28/61 (46) | – | BASMI score change from baseline: –1 (IQR –2.0 to 0.0) |
| ITT LOCF 33/78 (42) | SF-36 MCS change from baseline: 2.3 (IQR –3.6 to 11.9) | ||||||||
| SF-36 PCS change from baseline: 8.3 (IQR 2.5 to 17.7) | |||||||||
Appendix 9 Adverse events
| Trial name | Population | Treatment arm | Dose | Time point (weeks) | Number of patients randomised | SAEs | Serious infections | Tuberculosis (including tuberculosis reactivation) | Injection site reactions | Congestive heart failure | Malignancies | Non-melanoma skin cancer | Withdrawals because of AEs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haibel 200852 | Nr-axSpA | Adalimumab | 40 mg | 12 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Nr-axSpA | Placebo | 0 | 12 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Hu 201255 | AS | Adalimumab | 40 mg | 12 | 26 | ||||||||
| AS | Placebo | 0 | 12 | 20 | |||||||||
| Huang 201456 | AS | Adalimumab | 40 mg | 12 | 229 | 1 | 1 | 0 | 0 | 0 | 0 | 4 | |
| AS | Placebo | 0 | 12 | 115 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Lambert 200757 | AS | Adalimumab | 40 mg | 12 | 38 | ||||||||
| AS | Placebo | 0 | 12 | 44 | |||||||||
| ABILITY-1 2013 (licensed population)58 | Nr-axSpA | Adalimumab | 40 mg | 12 | 95 | 3 | 0 | 0 | 0 | 1 | |||
| Nr-axSpA | Placebo | 0 | 12 | 97 | 1 | 0 | 0 | 0 | 1 | ||||
| ATLAS 200661 | AS | Adalimumab | 40 mg | 12 | 208 | ||||||||
| AS | Placebo | 0 | 12 | 107 | |||||||||
| RAPID-axSpA 201464 | AS | Certolizumab pegol | 200 mg | 12 | 65 | a | a | a | |||||
| AS | Certolizumab pegol | 400 mg | 12 | 56 | |||||||||
| AS | Placebo | 0 | 12 | 57 | |||||||||
| Nr-axSpA | Certolizumab pegol | 200 mg | 12 | 46 | |||||||||
| Nr-axSpA | Certolizumab pegol | 400 mg | 12 | 51 | |||||||||
| Nr-axSpA | Placebo | 0 | 12 | 50 | |||||||||
| Barkham 201071 | AS | Etanercept | 25 mg twice weekly | 12 | 20 | 0 | 0 | ||||||
| AS | Placebo | 0 | 12 | 20 | 0 | 0 | |||||||
| Davis 200372 | AS | Etanercept | 25 mg | 12 | 138 | ||||||||
| AS | Placebo | 0 | 12 | 139 | |||||||||
| AS | Etanercept | 25 mg | 24 | 138 | 0 | 0 | 41 | 7 | |||||
| AS | Placebo | 0 | 24 | 139 | 1 | 0 | 13 | 1 | |||||
| Dougados 201174 | AS | Etanercept | 50 mg | 12 | 39 | ||||||||
| AS | Placebo | 0 | 12 | 43 | |||||||||
| Dougados 201476 | Nr-axSpA mixed | Etanercept | 50 mg | 12 | 106 | 2 | 3 | 1 | 1 | ||||
| Nr-axSpA mixed | Placebo | 0 | 12 | 109 | 1 | 0 | 0 | 0 | |||||
| Nr-axSpA | Etanercept | 50 mg | 12 | 94 | |||||||||
| Nr-axSpA | Placebo | 0 | 12 | 95 | |||||||||
| Gorman 200279 | AS | Etanercept | 25 mg | 16 | 20 | 0 | 5 | 0 | |||||
| AS | Placebo | 0 | 16 | 20 | 0 | 1 | 0 | ||||||
| Calin 200483 | AS | Etanercept | 25 mg | 12 | 45 | 1 | 0 | 15 | 0 | ||||
| AS | Placebo | 0 | 12 | 39 | 0 | 0 | 6 | 0 | |||||
| van der Heijde 200686 | AS | Etanercept | 25 mg | 12 | 150 | 1 | 0 | 32 | 0 | 6 | |||
| AS | Etanercept | 50 mg | 12 | 155 | 1 | 0 | 34 | 0 | 8 | ||||
| AS | Placebo | 0 | 12 | 51 | 0 | 0 | 6 | 0 | 0 | ||||
| Giardina 201088 | AS | Etanercept | 50 mg | 104 | 25 | 1 | 0 | 5 | 0 | 0 | 0 | ||
| AS | Infliximab | 5 mg/kg | 104 | 25 | 2 | 0 | 1 | 0 | 0 | 0 | |||
| GO-RAISE 200890 | AS | Golimumab | 50 mg | 16 | 138 | 5 | |||||||
| AS | Golimumab | 100 mg | 16 | 140 | 7 | ||||||||
| AS | Placebo | 0 | 16 | 78 | 4 | ||||||||
| Bao 201495 | AS | Golimumab | 50 mg | 14 | 108 | ||||||||
| AS | Placebo | 0 | 14 | 105 | |||||||||
| Tam 201497 | AS | Golimumab | 50 mg | 24 | 20 | 0 | |||||||
| AS | Placebo | 0 | 24 | 21 | 1 | ||||||||
| Barkham 200950 | Nr-axSpA | Infliximab | 5 mg/kg | 16 | 20 | 0 | 1 | ||||||
| Nr-axSpA | Placebo | 0 | 16 | 20 | |||||||||
| Braun 200298 | AS | Infliximab | 5 mg/kg | 12 | 34 | 3 | 1 | 0 | 4 | ||||
| AS | Placebo | 0 | 12 | 35 | 0 | 0 | 0 | ||||||
| Marzo-Ortega 2005100 | AS | Infliximab + methotrexate | 5 mg/kg | 10 | 28 | ||||||||
| AS | Placebo + metotrexate | 0 | 10 | 14 | |||||||||
| AS | Infliximab + methotrexate | 5 mg/kg | 30 | 28 | 0 | 1 | 0 | ||||||
| AS | Placebo + metotrexate | 0 | 30 | 14 | 0 | 0 | 0 | ||||||
| Van den Bosch 2002101 | AS | Infliximab | 5 mg/kg | 12 | 9 | Unclear | Unclear | ||||||
| AS | Placebo | 0 | 12 | 12 | Unclear | Unclear | |||||||
| ASSERT 2005102 | AS | Infliximab | 5 mg/kg | 24 | 202 | 7 | 2 | 0 | 22 | 0 | 2 | ||
| AS | Placebo | 0 | 24 | 75 | 2 | 0 | 0 | 7 | 0 | 1 | |||
| PLANETAS 2013110 | AS | CT-P13 | 5 mg/kg | 14 | 125 | ||||||||
| AS | Infliximab | 5 mg/kg | 14 | 125 | |||||||||
| AS | CT-P13 | 5 mg/kg | 30 | 128 | 6 | 2 | 5 | 8 | |||||
| AS | Infliximab | 5 mg/kg | 30 | 122 | 8 | 1 | 6 | 5 | |||||
| AS | CT-P13 | 5 mg/kg | 54 | 125 | |||||||||
| AS | Infliximab | 5 mg/kg | 54 | 125 |
Appendix 10 Quality assessment of studies included in the cost-effectiveness review
| Question | Ara et al. 2007161 | Botteman et al. 2007162 | Kobelt et al. 2007160 | McLeod et al. 200738 | Armstrong et al. 2013163 |
|---|---|---|---|---|---|
| 1. Was a well-defined question posed in answerable form? | Yes | Yes | Yes | Yes | Yes |
| 2. Was a comprehensive description of the competing alternatives given (i.e. can you tell who did what to whom, where, and how often)? | Yes | Yes | No | Yes | Yes |
| 3. Was the effectiveness of the programme or services established? | Yes (short–medium term) | Yes (short–medium term) | Yes (short term) | Yes (short–medium term) | Yes (short–medium term) |
| 4. Were all the important and relevant costs and consequences for each alternative identified? | No | Yes | Yes | Yes | Yes (consequences); cannot tell (costs) |
| 5. Were costs and consequences measured accurately in appropriate physical units (e.g. hours of nursing time, number of physician visits, lost work-days, gained life-years)? | Cannot tell | Cannot tell | Cannot tell | Yes | Cannot tell |
| 6. Were the cost and consequences valued credibly? | Cannot tell | Cannot tell | Cannot tell | Yes | Cannot tell |
| 7. Were costs and consequences adjusted for differential timing? | Yes | Yes | Yes | Yes | Cannot tell |
| 8. Was an incremental analysis of costs and consequences of alternatives performed? | Yes | Yes | Yes | Yes | Yes |
| 9. Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | Yes | Yes | Yes | Yes |
| 10. Did the presentation and discussion of study results include all issues of concern to users? | No | No | No | Yes | No |
Appendix 11 Comparison of parameter inputs across manufacturer models
Tables 146 and 147 provide an overview of the main parameter inputs applied in each of the manufacturer models for the AS and nr-AxSpA populations.
| Parameter | Merck Sharp & Dohme economic model37 (infliximab, golimumab) | AbbVie economic model34 (adalimumab) | UCB economic model35 (certolizumab) | Pfizer economic model36 (etanercept) |
|---|---|---|---|---|
| Time horizon | Lifetime | 40 years | Lifetime | Lifetime |
| Discount rate (%) | 3.5 | 3.5 | 3.5 | 3.5 |
| Average age (years) | 39 | 42 | 41 | 41 |
| Percentage male | 72 | 75 | 72 | 74 |
| Average weight (kg) | 70 | 81.1 | 81.7 | 76.4 |
| Baseline BASDAI score | 6.5 | 6.3 | 6.4 | 6.1 |
| Baseline BASFI score | 5.0 | 5.3 | 5.7 | 5.9 |
| Source of baseline characteristics | GO-RAISE90 | ATLAS61 | RAPID-axSpA64 trial | 314-EU study167 |
| Mortality (SMR) | Male 1.63; female 1.38 | 1.5 | 1.5 | 1.5 |
| Response criterion | BASDAI 50 response at week 12 | ASAS 20 response at week 12 | ASAS 20 response at week 24 | BASDAI 50 response at week 12 |
| Percentage of responders |
|
|
|
|
| Placebo response | Loss or maintenance of placebo response not clearly reported | BASDAI and BASFI score return to baseline at week 12 | No placebo response | BASDAI and BASFI score return to baseline at 12 weeks |
| Annual long-term rate of anti-TNFs withdrawal | 6.1% (GO-RAISE90), a common rate for all anti-TNFs | Time-dependent discontinuation; log-normal model fitted to adalimumab week 12 responder data (ATLAS61). Less than 15% projected to stay on treatment at year 40, a common rate for all anti-TNFs | 7% (NICE TA14317), a common rate for all anti-TNFs | Exponential model fitted to etanercept data; model translates to 11% annual discontinuation for etanercept. Hazard ratios applied for other anti-TNFs (Glintborg 2010)112 |
| Natural history: annual rate of BASFI progression | 0.07 points (Kobelt et al. 2004152) | 0.056 points (ATLAS61) | 0.07 points (Kobelt et al. 2004152) | 0.07 points (Kobelt et al. 2004152) |
| AEs included; annual probability/rate | Serious AEs and ISRs included. Convent. care rates from GO-RAISE study. ORs from the NMA applied for each anti-TNF | Only infectious AEs included; excess proportion for adalimumab 29.7% annually (ATLAS61 trial). Same rate applied to all anti-TNFs | No AEs included | Serious infections for etanercept: 3.8% annually. Relative effects from a published NMA (Singh 2011137) applied for other anti-TNFs |
| HRQoL algorithm (EQ-5D) | 0.877121 – 0.03841 × BASDAI – 0.03225 × BASFI – 0.02789 × male + 0.00168 × age (NICE TA14317) | 0.899 – 0.031 × BASDAI-0.041 × BASFI (HUI-3, data from ATLAS61) | 2.126 – 0.132 × BASFI – 0.245 × BASDAI (RAPID-axSpA64 study) | 0.887 – 0.006030 × BASFI + 0.001030 × BASDAI + 0.000020 × BASFI2 – 0.0000064 × BASDAI2 (314-EU study167) |
| Annual health-care resource use costs | 1902.49 × exp(0.1832 × BASFI) (NICE TA14317) | £1124.619 × exp(0.264 × BASDAI) (OASIS118) | 1909.33 × exp(0.1832 × BASFI) (NICE TA14317) |
|
| Parameter | AbbVie economic model34 (adalimumab) | UCB economic model35 (certolizumab) | Pfizer economic model36 (etanercept) |
|---|---|---|---|
| Time horizon | 40 years | Lifetime | Lifetime |
| Discount rate (%) | 3.5 | 3.5 | 3.5 |
| Average age (years) | 38 | 37 | 32 |
| Percentage male | 45 | 48 | 60 |
| Average weight (kg) | NR | 82 | 74 |
| Baseline BASDAI score | 6.4 | 6.5 | 6.0 |
| Baseline BASFI score | 4.6 | 4.9 | 4.0 |
| Source of baseline characteristics | ABILITY-158 | RAPID-axSpA64 trial | 1031 study166 |
| Mortality (SMR) | 1.0 | 1.5 | 1.0 |
| Response criterion | ASAS 40 response at week 12 | ASAS 20 response at week 12 | BASDAI 50 response at week 12 |
| Percentage of responders |
|
|
|
| Placebo response | BASDAI and BASFI score return to baseline at week 12 | No placebo response | BASDAI and BASFI score return to baseline at 12 weeks |
| Annual long-term rate of anti-TNFs withdrawal | Time-dependent discontinuation; Log-normal model fitted to adalimumab week 12 responder data (ABILITY-158). Less than 10% projected to stay on treatment at year 40, a common rate for all anti-TNFs | 7% (NICE TA143), common rate for all anti-TNFs | Exponential model fitted to etanercept week 12 responder data; model translates to 5% annual discontinuation for etanercept. Hazard ratios applied for other anti-TNFs (Glintborg et al. 2010)112 |
| Progression rate from nr-AxSpA to AS | – | 3.84% per year | – |
| Natural history: annual rate of BASFI progression | 0.084 points (ABILITY-158) | 0.07 points (Kobelt et al. 2004152) | 0.07 points (Kobelt et al. 2004152) |
| AEs included; annual probability/rate | Only tuberculosis AEs and non-tuberculosis SAEs included; excess rate for adalimumab 7.3% for non tuberculosis SAEs and 0.16% for tuberculosis AEs annually (ABILITY-158 trial). Same rate applied to all anti-TNFs | No AEs included | No AEs included |
| HRQoL algorithm | 0.922 – 0.039 × BASDAI – 0.041 × BASFI (ABILITY-158) | 2.1262 – 0.1323 × BASFI – 0.2450 × BASDAI (RAPID-axSpA64 study) | 0.919 – 0.00431 × BASFI + 0.000788 × BASDAI + 0.0000511 × BASFI2 – 0.0000194 × BASDAI2 – 0.00102 × Age + 0.0478 × male – 0.0000754 × BASDAI × BASFI (1031 study166) |
| Annual health-care resource use costs | £1124.62 × exp(0.264 × BASDAI) (OASIS118) | 1909.33 × exp(0.1832 × BASFI) (NICE TA14317) |
|
Comparison of disease costs assumed for the ankylosing spondylitis and non-radiographic axial spondyloarthritis populations
A variety of alternative regressions were applied across the submissions to estimate the annual disease costs associated with BASDAI and BASFI scores. Merck Sharp & Dohme37 and UCB35 used the same exponential regression function estimated by LRiG, uprated to current prices, as part of NICE TA14317 based on the OASIS study. 118
Regression in NICE TA14317 based on OASIS data and cost element uprated to current prices: £1902.492 × exp(0.1832 × BASFI).
AbbVie undertook their own reanalysis of the OASIS data set based on current prices. In their base-case regression an exponential model based on BASDAI was assumed. However, results from separate linear and exponential models were also presented.
Base-case regression used by AbbVie:34
Alternative regressions presented by AbbVie:
The submission by Pfizer36 was based on a recent UK study by Rafia et al. 168 Rather than employing a regression approach, the manufacturer used results based on a categorical analysis of the annual costs for BASDAI: BASDAI score of < 4 = £151.96, 4 ≤ BASDAI score < 6 = £311.08; and BASDAI score of ≥ 6 = £1039.16.
However, the paper by Rafia et al. 170 also specified a separate two-part regression function which was not included within the Pfizer submission36 but is used in the subsequent comparisons of regressions to provide a more comparable approach to assessing the alternative costs sources used across the manufacturer’s submissions and the predictions across a range of different BASDAI and BASFI scores.
Two-part model in Rafia et al. 168:
Logistic regression model to derive probability of incurring costs:
Generalised linear model to obtain 3-month costs:
Figures 22 and 23 provide a comparison of the predictions from the alternative cost regressions using the separate sources identified across the manufacturer models. The baseline characteristics (BASDAI, BASFI, age and disease duration) are derived from a weighted average of the baseline characteristics of the clinical trials for the AS population from the manufacturer’s submissions.
FIGURE 22.
Comparison of main manufacturer cost regressions: assuming constant BASDAI score.
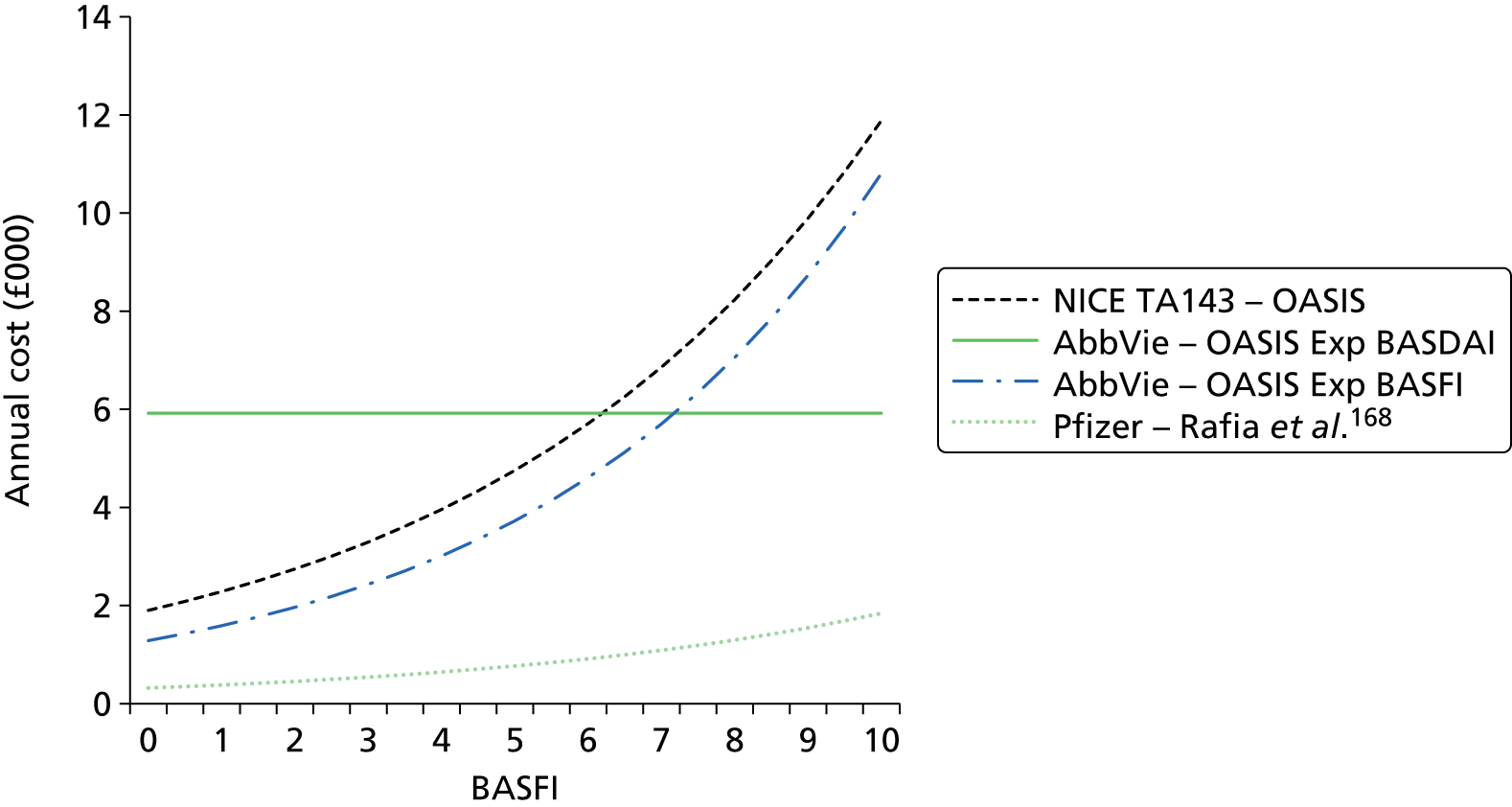
FIGURE 23.
Comparison of main manufacturer cost regressions: assuming constant BASFI score.

In Figure 22, BASDAI scores are held constant at the mean value and the impact of varying BASFI across the range (0–10 scale) are reported. In Figure 23, BASFI scores are held constant at the mean value and the impact of varying BASDAI across the range (0–10 scale) are reported.
Figures 24 and 25 compare the alternative regression functions reported in the submission by AbbVie based on their reanalysis of the OASIS study.
FIGURE 24.
Comparison of AbbVie cost regressions: assuming constant BASDAI score.

FIGURE 25.
Comparison of AbbVie cost regressions: assuming constant BASFI score.
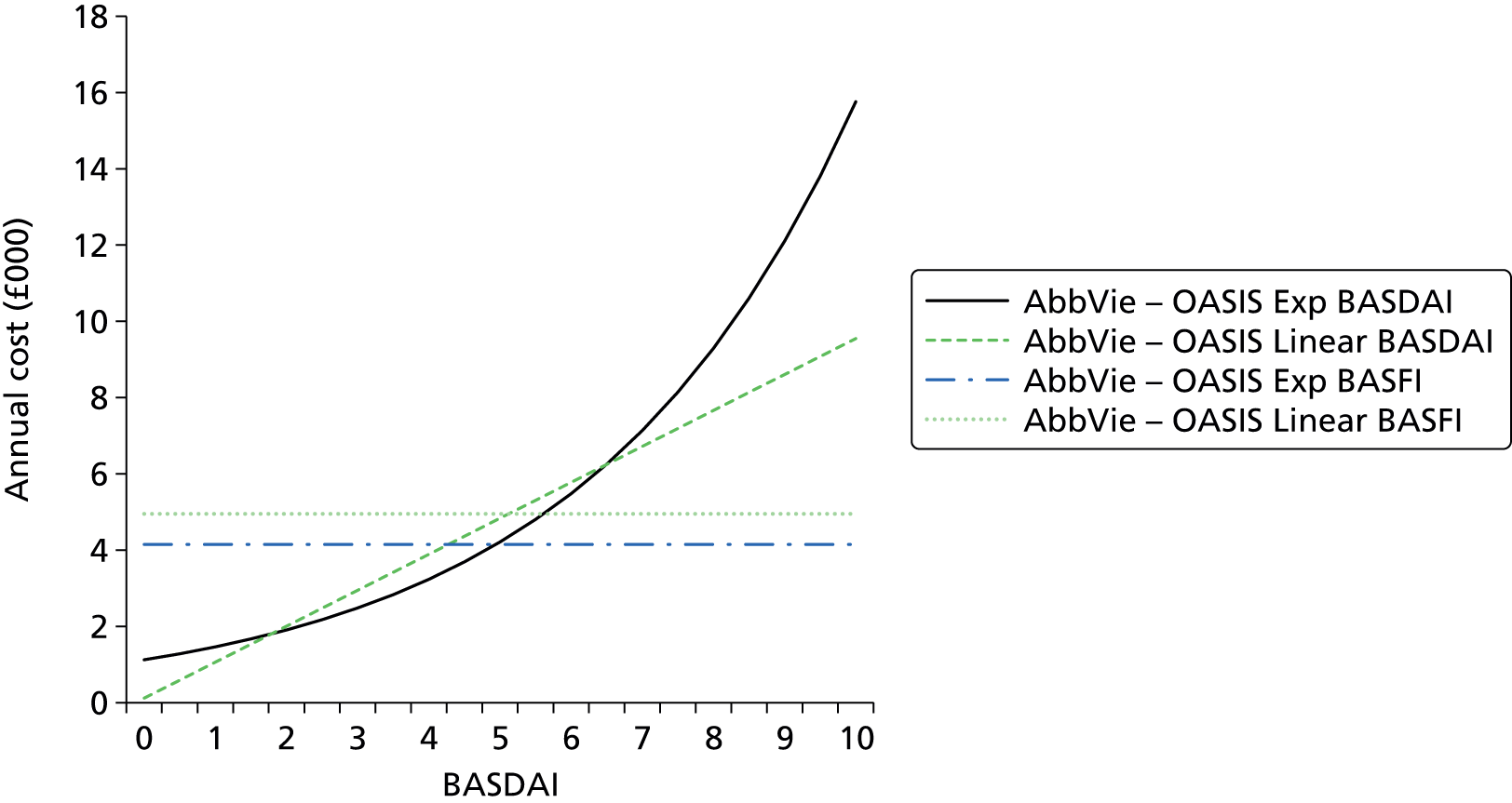
| Parameter | Merck Sharp & Dohme economic model37 (infliximab and golimumab) | AbbVie economic model34 (adalimumab) | UCB economic model35 (certolizumab) | Pfizer economic model36 (etanercept) |
|---|---|---|---|---|
| Administration costs | Subcutaneous therapies: no administration cost | Subcutaneous therapies: no administration cost | Subcutaneous therapies: £49 cost of nurse training for self-administration (PSSRU179) | Subcutaneous therapies: £49 cost of nurse training for self-administration (PSSRU179) |
| Intravenous therapies: cost of £109 per administration (no reference provided) | Intravenous therapies: cost of £99 per administration (no reference provided) | Intravenous therapies: cost of £398 per administration (PSSRU179) | Intravenous therapies: cost of £302 per administration (NICE TA14317) | |
| Doses and unit costs | Costs estimated in line with licensed doses | Costs estimated in line with licensed doses | Costs estimated in line with licensed doses | Costs estimated in line with licensed doses |
| PAS included for certolizumab and golimumab | PAS included for golimumab, not included for certolizumab | PAS included for certolizumab and golimumab | PAS included for certolizumab and golimumab | |
| Infliximab dosage: average weight of 70 kg assumed (four vials), subsequent administration every 7 weeks | Infliximab dosage: average weight of 81.1 kg assumed (five vials), subsequent administration every 6 weeks | Infliximab dosage: average weight of 81.7 kg assumed (4.88 vials), subsequent administration every 7 weeks | Infliximab dosage: average weight of 76.4 kg assumed (four vials), subsequent administration every 6 weeks | |
| Monitoring costs | Short-term treatment costs applied in first cycle only for CC and anti-TNFs. Costs were informed by key opinion leader interviews. Anti-TNFs: £873.2. CC: £1459.5 | Initiation and quarterly monitoring costs included. Common for all anti-TNFs comparators (York Model TA199175). Initiation: £470.09. Monitoring: £110.98 per cycle | No monitoring costs included | No monitoring costs included in the base case |
| Annual health-care resource use costs | 1902.49 × exp(0.1832 × BASFI) (NICE TA14317) | £1124.619 × EXP(0.264 × BASDAI) (OASIS118) | 1909.33 × exp(0.1832 × BASFI) (NICE TA14317) |
|
| Parameter | Merck Sharp & Dohme economic model37 (infliximab, golimumab) | AbbVie economic model34 (adalimumab) | UCB economic model35 (certolizumab) | Pfizer economic model36 (etanercept) |
|---|---|---|---|---|
| Annual long-term rate of anti-TNF withdrawal: AS population | 6.1% (GO-RAISE90 study, data of patients on treatment with golimumab from week 24 to week 256), common rate for all anti-TNFs | Time-dependent discontinuation rate; log-normal model fitted to adalimumab week-12 responder data up to week 260 (ATLAS61). Fewer than 15% of week-12 responders were projected to stay on treatment at year 40 for AS, a common rate for all anti-TNFs | 7% (NICE TA143), common rate for all anti-TNFs | Exponential model fitted to etanercept data; model translates to 11% annual discontinuation for etanercept. Hazard ratios applied for other anti-TNFs (Glintborg 2010).112 Annual discontinuation:
|
| Annual long-term rate of anti-TNF withdrawal: nr-AxSpA population | Not applicable | Time-dependent discontinuation; log-normal model fitted to adalimumab week-12 responder data up to week 156 (ABILITY-158). Fewer than 10% of week-12 responders were projected to stay on treatment at year 40, a common rate for all anti-TNFs | 7% (NICE TA143), common rate for all anti-TNFs | Exponential model fitted to etanercept week-12 responder data; model translates to 5% annual discontinuation for etanercept. Hazard ratios applied for other anti-TNFs (Glintborg 2010).112 Annual discontinuation:
|
| Parameter | Merck Sharp & Dohme economic model37 (infliximab and golimumab) | AbbVie economic model34 (adalimumab) | UCB economic model35 (certolizumab) | Pfizer economic model36 (etanercept) |
|---|---|---|---|---|
| AEs included; annual probability | SAEs and ISRs included. CC rates from GO-RAISE90 study at 24 weeks. OR of SAEs and ISRs from the NMA applied for each anti-TNF | Only infectious AEs included; excess proportion for adalimumab was 29.7% annually (ATLAS61 trial) | No AEs included | Only serious infections included. Annual probability: 3.8% (312-EU146) |
Annual probability (%) of SAEs:
|
Same rate applied to all anti-TNFs | Relative effects for other anti-TNF agents were applied in the model, obtained from a published NMA (Singh 2011)137 | ||
Annual probability (%) of ISRs:
|
Annual probability (%):
|
|||
| Unit cost of AE | Cost per serious AE episode (weighted average): £214.26 anti-TNFs, £397.32 for CC (GO-RAISE90). Cost of injection site reaction £94.18 per episode | Cost per infectious AE episode: £45 (one GP visit assumed per infectious AE) | – | Cost per serious infection episode: £1457 (weighted average) (NHS Reference Costs 2012/13)176 |
| Disutility of AE | Only disutility associated with SAEs applied; utility decrement of 0.01 applied for one cycle (NICE TA23333) | No disutility applied | – | 0.156 for 28 days |
| Parameter | AbbVie economic model34 (adalimumab) | UCB economic model35 (certolizumab) | Pfizer economic model36 (etanercept) |
|---|---|---|---|
| AEs included; annual probability | Only tuberculosis AEs and non-tuberculosis SAEs included; the excess percentage for adalimumab was 7.3% for non-tuberculosis SAEs and 0.16% for tuberculosis AEs annually (ABILITY-158 trial), same rate applied to all anti-TNFs | No AEs included | No AEs included |
| Unit cost of AE | Non-tuberculosis SAEs: £4216 per episode (NHS Reference Costs 2012/13)176 | – | – |
| Tuberculosis AEs: £6559.76 per episode (Botteman 2007)162 | |||
| Disutility of AE | No disutility applied | – | – |
Appendix 12 Extended synthesis models
In this appendix we describe in more detail the data and modelling approaches implemented in Chapter 5. Note that while this appendix aims to provide a methodological description of methods, a full description of findings and its interpretations are in Chapter 5.
General aspects of implementation and software
The synthesis was conducted from a Bayesian perspective, using WinBUGS (a MCMC simulation based software for Bayesian inference). For burn-in, we ran 100,000 simulations and another 100,000 were used in inferences. Convergence was assessed by running two chains and convergence was assumed if the Gelman–Rubin statistic was equal to 1. Goodness of fit was assessed using the DIC. 170 Models with smaller DIC are better supported by the data. In the presence of autocorrelation, the MCMC simulation for inference was increased to 200,000 and a thin of 20 was applied (yielding a sample for inference of 10,000 for each chain).
The main synthesis models will pool differences between treatment and control in change scores from baseline (BASDAI and BASFI). The treatment associated with the lowest (most negative) mean change score is expected to be best. However, it is important to quantify the uncertainty around the estimates and for this reason SDs will be reported alongside expected values. When ORs are presented, median values instead of means were used to summarise inferences.
When possible, meta-regression analyses were conducted to evaluate potential treatment effect modifiers. Meta-regression is a tool aimed at examining the impact of variables on effect size using regression-based techniques. In these explorations, the following baseline characteristics were considered: BASDAI score, BASFI score, age, sex, duration of symptoms (years) and CRP level.
Relative effectiveness estimates for models assuming exchangeability across treatments (model A5) are based on the predictive distribution, representing the distribution of the data averaged over all possible parameter values. This summary statistic best reflects the impact of uncertainty in the parameters of the model and is here judged as a more appropriate basis to be used in the decision model. 171
Modelling approach A
Brief description of the data
Based on study populations and follow-up (i.e. around 12 weeks in duration), 16 of the RCTs are considered directly relevant to the decision problem for the AS population (studies 1 to 16 in Table 152). One of these studies did not report BASDAI or BASFI outcomes (study 3) and thus could not be included in analyses. The 15 remaining studies reported at least one outcome measure: BASDAI 50 score and/or change from baseline on BASDAI and BASFI scores.
| Study number | Trial name | Treatment | Number in treatment group | Number in placebo group | BASDAI 50 score | Change BASDAI score | Change BASFI score |
|---|---|---|---|---|---|---|---|
| 1 | Hu 201255 | 1 | 26 | 20 | ✗ | ✗ | |
| 2 | Huang 201456 | 1 | 229 | 115 | ✗ | ✗ | ✗ |
| 3 | Lambert 200757 | 1 | 38 | 44 | |||
| 4 | ATLAS 200661 | 1 | 208 | 107 | ✗ | ✗ | |
| 5 | RAPID-axSpA 201464 | 2 | 121 | 57 | ✗ | ✗ | ✗ |
| 6 | Barkham 201071 | 3 | 20 | 20 | ✗ | ✗a | ✗a |
| 7 | Davis 200372 | 3 | 138 | 139 | ✗ | ✗ | |
| 8 | Dougados 201174 | 3 | 39 | 43 | ✗ | ✗ | ✗ |
| 9 | Gorman 200279 | 3 | 20 | 20 | ✗ | ||
| 10 | Calin 200483 | 3 | 45 | 39 | ✗ | ✗ | |
| 11 | van der Heijde 200686 | 3 | 305 | 51 | ✗ | ||
| 12 | GO-RAISE 200890 | 4 | 138 | 78 | ✗ | ✗ | |
| 13 | Bao 201296 | 4 | 108 | 105 | ✗ | ✗ | |
| 14 | Braun 200298 | 5 | 34 | 35 | ✗ | ✗a | ✗a |
| 15 | Marzo-Ortega 2005100 | 5 | 28 | 14 | ✗ | ✗a | |
| 16 | Van den Bosch 2002101 | 5 | 9 | 12 | ✗a | ✗a |
This modelling approach directly evaluates relative treatment effects, that is log OR for BASDAI 50 response and the difference between treatment and placebo in change in BASDAI and BASFI scores from baseline. The data set analysed is shown in Table 153.
| Study, j | Treatment, t | Outcome, o | y | SE |
|---|---|---|---|---|
| 1 | 1 | 1 | – | – |
| 2 | 1 | 1 | 1.61 | 0.28 |
| 3 | 1 | 1 | – | – |
| 4 | 1 | 1 | 1.47 | 0.30 |
| 5 | 2 | 1 | 1.79 | 0.42 |
| 6 | 3 | 1 | 2.30 | 1.13 |
| 7 | 3 | 1 | – | – |
| 8 | 3 | 1 | 1.04 | 0.48 |
| 9 | 3 | 1 | – | – |
| 10 | 3 | 1 | – | – |
| 11 | 3 | 1 | 1.78 | 0.37 |
| 12 | 4 | 1 | 1.47 | 0.36 |
| 13 | 4 | 1 | 2.34 | 0.50 |
| 14 | 5 | 1 | 2.45 | 0.69 |
| 15 | 5 | 1 | – | – |
| 16 | 5 | 1 | – | – |
| 1 | 1 | 2 | –1.60 | 0.67 |
| 2 | 1 | 2 | –1.40 | 0.22 |
| 3 | 1 | 2 | – | – |
| 4 | 1 | 2 | –1.80 | 0.28 |
| 5 | 2 | 2 | –1.45 | 0.36 |
| 6 | 3 | 2 | –1.87 | 0.90a |
| 7 | 3 | 2 | –1.91 | 0.26 |
| 8 | 3 | 2 | –1.20 | 0.44 |
| 9 | 3 | 2 | – | – |
| 10 | 3 | 2 | –1.87 | 0.49 |
| 11 | 3 | 2 | – | – |
| 12 | 4 | 2 | – | – |
| 13 | 4 | 2 | – | – |
| 14 | 5 | 2 | –2.60 | 0.69a |
| 15 | 5 | 2 | –1.73 | 0.70 |
| 16 | 5 | 2 | –2.97 | 1.26a |
| 1 | 1 | 3 | –0.90 | 0.68 |
| 2 | 1 | 3 | –1.28 | 0.20 |
| 3 | 1 | 3 | – | – |
| 4 | 1 | 3 | – | – |
| 5 | 2 | 3 | –1.10 | 0.37 |
| 6 | 3 | 3 | –1.56 | 0.93a |
| 7 | 3 | 3 | –1.34 | 0.29 |
| 8 | 3 | 3 | –1.20 | 0.40 |
| 9 | 3 | 3 | –2.20 | 0.92 |
| 10 | 3 | 3 | –1.73 | 0.45 |
| 11 | 3 | 3 | – | – |
| 12 | 4 | 3 | –1.50 | 0.27 |
| 13 | 4 | 3 | –1.37 | 0.32 |
| 14 | 5 | 3 | –2.00 | 0.71a |
| 15 | 5 | 3 | –1.82 | 1.00a |
| 16 | 5 | 3 | –3.21 | 1.28a |
Description of synthesis methods for modelling approach A
Consider we have available information on J trials comparing an individual treatment, k (out of the total number of treatments T) to placebo. Trials report one or more outcomes, o. Information on outcome o for treatment k in a study j is represented by yjko and is used alongside the SE for this measure, sejko2. In common with the approach implemented in Chapter 3, all outcomes are here assumed normally distributed, with mean θjko. We implemented alternative models that differ in the way treatment effects are considered; a summary of each is presented below. Note that at this stage each outcome was synthesised independently.
Model A1 (treatments; independent, studies; fixed effect): this model considers the j treatments to be independent, that is it assumes the effects to differ between treatments, d[k,o]. This is a fixed effect model in that multiple studies evaluating the same treatment are considered to measure the same treatment effect.
The model used was:
Likelihood:
Model:
Priors:
Model A2 (treatments; independent, studies; random effects): this model differs from A1 in that a random effect is assumed to describe the findings of multiple studies evaluating the same treatment.
The model used was:
Likelihood:
Model:
Priors:
The random effect is defined using a variance parameter for each outcome but common across treatments, σo2.
Model A3 (treatments; equal, studies; fixed effect): this model differs from model A1 in that treatments are not assumed to differ. The model thus evaluates a common relative effectiveness for all anti-TNFs, d[o], for each outcome.
The model used was:
Likelihood:
Model:
Priors:
Model A4 (treatments; equal, studies; random effects): this model differs from model A3 in that a random effect is assumed to describe the findings of multiple studies evaluating the same treatment.
The model used was:
Likelihood:
Model:
Priors:
Model A5 (treatments; exchangeable, studies; fixed effect): this model differs from model A1 in that a random effect is used to describe any differences between treatments (exchangeability is assumed). This model thus assumes the treatments to have a similar, but not equal, effectiveness; there are differences between the effectiveness of treatments that we may not be able to explain but that we should consider.
The model used was:
Likelihood:
Model:
Priors:
The parameter γo2 is the variance parameter defining the random effect across treatment. The priors differ for outcome 1 because this is a log odds, while outcomes 2 and 3 are assumed continuous measures.
Within this modelling approach we explored potential heterogeneity in treatment effects using metaregression (i.e. potential treatment effect modifiers). We did so by extended the modelling approach in model A1 to include treatment effect interactions with baseline characteristics (centred on their means when relevant). We have explored the inclusion of alternative covariates by evaluating the DIC associated with alternative models.
Results of modelling approach A
The results of each modelling approach are shown in Table 154.
| A1. Treatment: independent; studies: fixed effect | A2. Treatment: independent; studies: random effects | A3. Treatment: common; studies: fixed effect | A4. Treatment: common; studies: random effects | A5. Treatment: exchangeable; studies: fixed effect | |
|---|---|---|---|---|---|
| Outcome 1: OR on BASDAI 50 score | Median (SD) | Median (SD) | Median (SD) | Median (SD) | Median (SD) |
| Adalimumab | 4.71 (1.00) | 4.69 (6.11) | 5.21 (0.72) | 5.30 (0.98) | 5.34 (9.79)a |
| Certolizumab | 6.02 (3.33) | 6.04 (22.87) | |||
| Etanercept | 4.73 (1.43) | 4.72 (3.32) | |||
| Golimumab | 5.86 (1.81) | 6.10 (7.45) | |||
| Infliximab | 11.9 (11.94) | 12.10 (44.00) | |||
| σ1 | – | 0.31 (0.30) | – | 0.15 (0.14) | – |
| D1 | – | – | – | – | 1.69 (0.23) |
| γ1 | – | – | – | – | 0.27 (0.28) |
| Outcome 2: change in BASDAI score | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Adalimumab | –1.56 (0.16) | –1.57 (0.27) | –1.66 (0.11) | –1.67 (0.15) | |
| Certolizumab | –1.45 (0.37) | –1.46 (0.51) | |||
| Etanercept | –1.76 (0.20) | –1.73 (0.28) | –1.70 (0.87)a | ||
| Golimumab | N/A | N/A | |||
| Infliximab | –2.28 (0.46) | –2.27 (–2.28) | |||
| σ2 | – | 0.25 (0.24) | – | 0.25 (0.19) | – |
| D2 | – | – | – | – | –1.63 (0.57) |
| γ2 | – | – | – | – | 0.43 (0.63) |
| Outcome 3: change in BASFI score | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Adalimumab | –1.22 (0.18) | –1.18 (0.29) | –1.38 (0.11) | –1.39 (0.13) | |
| Certolizumab | –1.10 (0.37) | –1.11 (0.47) | |||
| Etanercept | –1.48 (0.19) | –1.50 (0.24) | –1.41 (0.49)a | ||
| Golimumab | –1.45 (0.20) | –1.44 (0.29) | |||
| Infliximab | –2.16 (0.53) | –2.17 (0.56) | |||
| σ3 | – | 0.22 (0.19) | – | 0.14 (0.12) | – |
| D3 | – | – | – | – | –1.40 (0.22) |
| γ3 | – | – | – | – | 0.28 (0.33) |
| DIC | 52.4 | 57.0 | 39.1 | 44.3 | 43.6 |
From model A5, drug-specific estimates can be retrieved (Table 155). Within this mode drug-specific inferences will borrow strength from the common class effect and estimates are thus shrunken towards the mean of this class effect (i.e. estimates are closer to the value reported for the class in Table 153).
| Model A5 | |||
|---|---|---|---|
| Outcome 1: OR on BASDAI 50 score, median (SD) | Outcome 2: change in BASDAI score, median (SD) | Outcome 3: change in BASFI score, median (SD) | |
| Adalimumab | 5.05 (0.87) | –1.60 (0.15) | –1.31 (0.16) |
| Certolizumab | 5.42 (1.71) | –1.59 (0.26) | –1.31 (0.23) |
| Etanercept | 5.13 (1.08) | –1.72 (0.17) | –1.43 (0.15) |
| Golimumab | 5.47 (1.25) | –1.69 (0.84) | –1.42 (0.16) |
| Infliximab | 5.70 (3.30) | –1.88 (0.34) | –1.55 (0.33) |
Explorations of heterogeneity suggested only sex potentially modified the effect of anti-TNF treatment, specifically for change in BASDAI as outcome; however, when sex is used together with all covariates, such evidence on effect modification disappears (results not shown but available on request).
Modelling approach B
In the previous section the two outcomes based on BASDAI scores were synthesised separately; however, BASDAI 50 is the probability of having a reduction in BASDAI score of 50%, and thus it should be possible to relate the proportion of BASDAI 50 responders to the change in absolute BASDAI scores from baseline observed in each study. Within this section, we use this structural relation within the synthesis, allowing change scores from baseline to be informed not only from direct data on this quantity but also from data on BASDAI 50.
Brief description of the data
The model implemented here pools the change in BASDAI score from baseline to evaluate the difference between treatment and placebo, using evidence reported in trials directly on the change scores for each arm and also data on BASDAI 50. The data modelled within this approach are shown in Table 156.
| s[] | t[] | n[] | r[] | b[] | sd[] | y[] | y.se[] | y.f[] | y.f.se[] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 20 | N/A | 6.2 | 1.1 | –2 | 0.560 | –1 | 0.34 |
| 1 | 2 | 26 | N/A | 5.9 | 1.4 | –3.6 | 0.377 | –1.9 | 0.29 |
| 2 | 1 | 115 | 19 | 6.2 | 1.4 | –1.4 | 0.177 | –0.47 | 0.15 |
| 2 | 2 | 229 | 114 | 6 | 1.4 | –2.8 | 0.126 | –1.75 | 0.13 |
| 3 | 1 | 44 | N/A | 6.5 | 1.6 | N/A | N/A | N/A | N/A |
| 3 | 2 | 38 | N/A | 6.2 | 1.7 | N/A | N/A | N/A | N/A |
| 4 | 1 | 107 | 17 | 6.3 | 1.7 | –0.8 | 0.2 | N/A | N/A |
| 4 | 2 | 208 | 94 | 6.3 | 1.7 | –2.6 | 0.2 | N/A | N/A |
| 5 | 1 | 57 | 8 | 6.4 | 1.9 | –1.0 | 0.3 | –0.6 | 0.30 |
| 5 | 3 | 121 | 50 | 6.36 | 1.54 | –2.45 | 0.206 | –1.7 | 0.21 |
| 6 | 1 | 20 | 1 | 5.46 | 1.74 | –0.1 | 0.632 | 0.21 | 0.71 |
| 6 | 4 | 20 | 7 | 6.05 | 1.71 | –1.97 | 0.645 | –1.35 | 0.56 |
| 7 | 1 | 139 | N/A | 5.96 | 1.65 | –0.45 | 0.18 | –0.33 | 0.21 |
| 7 | 4 | 138 | N/A | 5.81 | 1.76 | –2.36 | 0.19 | –1.67 | 0.20 |
| 8 | 1 | 43 | 10 | 5.8 | 1.5 | –1.4 | 0.305 | –1 | 0.27 |
| 8 | 4 | 39 | 18 | 6.4 | 1.2 | –2.6 | 0.320 | –2.2 | 0.29 |
| 9 | 1 | 20 | N/A | N/A | N/A | N/A | N/A | –0.1 | 0.49 |
| 9 | 4 | 20 | N/A | N/A | N/A | N/A | N/A | –2.3 | 0.36 |
| 10 | 1 | 39 | N/A | 5.86 | 2.05 | –0.85 | 0.35 | –0.33 | 0.31 |
| 10 | 4 | 45 | N/A | 6.1 | 1.87 | –2.72 | 0.34 | –2.06 | 0.33 |
| 11 | 1 | 51 | 10 | 6.11 | 1.37 | N/A | N/A | N/A | N/A |
| 11 | 4 | 305 | 180 | 6.09 | 1.69 | N/A | N/A | N/A | N/A |
| 12 | 1 | 78 | 12 | 6.6 | 1.49 | N/A | N/A | 0.1 | 0.19 |
| 12 | 5 | 138 | 61 | 6.6 | 1.49 | N/A | N/A | –1.4 | 0.19 |
| 13 | 1 | 105 | 5 | 6.5 | 1.54 | N/A | N/A | 0.11 | 0.20 |
| 13 | 5 | 108 | 37 | 6.6 | 1.31 | N/A | N/A | –1.26 | 0.25 |
| 14 | 1 | 35 | 3 | 6.3 | 1.4 | –0.6 | 0.478 | –0.1 | 0.55 |
| 14 | 6 | 34 | 18 | 6.5 | 1.2 | –3.2 | 0.495 | –2.1 | 0.44 |
| 15 | 1 | 14 | N/A | 6.57 | 2.05 | –1.38 | 0.564 | 0.1 | 0.88 |
| 15 | 6 | 28 | N/A | 6.45 | 1.87 | –3.11 | 0.42 | –1.72 | 0.49 |
| 16 | 1 | 12 | N/A | 5.27 | 2.05 | –0.26 | 0.816 | 1.3 | 0.95 |
| 16 | 6 | 9 | N/A | 5.89 | 1.87 | –3.23 | 0.961 | –1.91 | 0.86 |
Description of synthesis methods
Consider we have available information on J trials comparing an individual treatment, k (out of the total number of treatments T) to placebo. Study j may report yjk, the mean change in BASDAI from baseline, alongside the SE for this measure, sejk. The likelihood for the data on change score was assumed normally distributed and was expressed as:
The mean of this distribution was the treatment effects, θjk, defined as the sum of the change score for the placebo arm plus the difference in change score for the treatments:
Some studies also reported the number of responders to BASDAI 50 (a 50% reduction in BASDAI score), rjk, out of the total number of individuals in the study, njk. The likelihood for the BASDAI 50 data was binomially distributed and thus expressed as:
Consider the BASDAI score at baseline for study j and treatment k, Xjk, as normally distributed, with a mean score at baseline of νjk and variability on BASDAI score at baseline represented by σjk2:
The probability parameter of the binomial distribution can be expressed as a function of the baseline and final BASDAI scores:
This can help us establish an algebraic relation between pjk and the change score Yjk, for a given baseline value, Xjk. This requires some assumptions over the distribution of scores, which are described next.
Across individuals, the BASDAI scores at baseline and the change score are assumed correlated and are described using a bivariate normal distribution:
For simplicity, the variability on BASDAI score at baseline, σjk2, was assumed equal to that of the change score. The correlation parameter is represented by ρ.
We would like to further explore the following relationship:
To do so, first consider expressing Y by conditioning on the baseline value, Xjk = x (for simplicity we will drop the jk subscript in the next few formulas):
So, we can standardise and relate this probability to a standard Normal distribution
To obtain the joint distribution, one needs to average over Xjk∼N(νjk,σjk2), which means integrating over this distribution with respect to x:
Note that one can express the expectation over the cdf of a normal distribution as:
Here, a=−(1/2+ρ)σ(1−ρ2) and b=−θ+ρνσ(1−ρ2). Therefore:
The relations established above thus allow the probability parameter from BASDAI 50 data to be expressed algebraically as a function of the change score:
In computations, we used the mean score at baseline, vjk, and the associated SD, σjk, as reported in the data (these were thus assumed known). The correlation between baseline and change score was estimated within the model by assuming this quantity to be independent of study but assumed to differ between placebo and anti-TNF treatments.
In what concerns the treatment effects, all trials in our evidence base compare against CC: δjk=dk. Our preferred approach to model these was to assume a common class effect (i.e. exchangeable effects across treatments, analogous assumption to model A5 above). This means:
where k = 1 is standard care.
The priors used to implement this model were:
FIGURE 26.
WinBUGS code for modelling approach B.

Results of modelling approach B
The summary results regarding relative treatment effects from this modelling approach are reported in Table 157 for model B.
| Estimated | Assumeda | Predicted | ||
|---|---|---|---|---|
| Difference in change score from baseline, mean (SD) | Probability of having a BASDAI 50 response, placebo, mean (SD) | Probability of having a BASDAI 50 response, anti-TNF, mean (SD) | OR for BASDAI 50 response, anti-TNF vs. placebo, median (SD) | |
| Anti-TNFs | –1.91 (0.48)b | 0.10 (–) | 0.40 (0.08) | 5.94 (4.06) |
| Other model summaries | ||||
| D | –1.91 (0.28) | – | – | – |
| γ | 0.30 (0.28) | – | – | – |
| ρplacebo | 0.26 (0.33) | – | – | – |
| ρanti-TNF | 0.69 (0.26) | – | – | – |
| DIC | 146.3 | – | – | – |
Drug-specific (shrunken) estimates from model B are shown in Table 158.
| Treatment | Change in BASDAI score, mean (SD) |
|---|---|
| Adalimumab | –1.77 (0.25) |
| Certolizumab | –2.01 (0.37) |
| Etanercept | –1.88 (0.18) |
| Golimumab | –1.92 (0.30) |
| Infliximab | –2.02 (0.32) |
Modelling approach C
The models implemented here extend those in the previous section by adding the syntheses of changes in BASFI score. The data used are presented in Table 156.
Description of synthesis methods
Data on mean change in BASFI score reported in some of the studies available have been described as normally distributed (the likelihood):
The treatment effects over BASFI θjkBASFI were then defined as:
Treatment effects on BASFI were assumed correlated to those on BASDAI across trials:
with o = {BASDAI, BASFI} and k = 1 is placebo.
The additional priors used to implement this model were:
The variation in treatment effects for both BASDAI and BASFI and the correlation parameter between these were estimated from the data. As in model B, we assumed exchangeability across the effects of the different treatments.
FIGURE 27.
WinBUGS code for modelling approach C.

Results of modelling approach C
The results on differences between treatment and placebo on change score form baseline are reported in Table 159, both for BASDAI and BASFI scores.
| Estimated | Assumeda | Predicted | ||
|---|---|---|---|---|
| Difference in change score from baseline, mean (SD) | Probability of having a BASDAI 50 response, placebo, mean (SD) | Probability of having a BASDAI 50 response, anti-TNF, mean (SD) | OR for BASDAI 50 response, anti-TNF vs. placebo, mean (SD) | |
| Effect of anti-TNFs on BASDAI | –1.95 (0.30) | 0.10 (–) | 0.41 (0.05) | 6.30 (1.56) |
| Effect of anti-TNFs on BASFI | –1.40 (0.28) | – | – | – |
| Other model summaries | ||||
| DBASDAI | –1.99 (0.20) | – | – | – |
| DBASFI | –1.40 (0.16) | – | – | – |
| γBASDAI | 0.13 (0.10) | – | – | – |
| γBASFI | 0.11 (0.09) | – | – | – |
| ρplacebo | 0.42 (0.26) | – | – | – |
| ρanti-TNF | 0.71 (0.23) | – | – | – |
| ρm | 0.51 (0.29) | – | – | – |
| σ2re | 0.16 (0.14) | – | – | – |
| DIC | 181.9 | – | – | – |
Drug-specific (shrunken) estimates from model C are shown in Table 160.
| Treatment | Change in BASDAI score, mean (SD) | Change in BASFI score, mean (SD) |
|---|---|---|
| Adalimumab | –1.89 (0.22) | –1.34 (0.17) |
| Certolizumab | –2.02 (0.28) | –1.36 (0.21) |
| Etanercept | –1.94 (0.18) | –1.43 (0.16) |
| Golimumab | –1.98 (0.25) | –1.42 (0.17) |
| Infliximab | –2.03 (0.27) | –1.49 (0.25) |
Appendix 13 Synthesis of evidence on the non-radiographic axial spondyloarthritis population
This section analyses the evidence on the effectiveness of anti-TNFs on the nr-AxSpA population.
Brief description of the data
On the nr-AxSpA population, five RCTs were considered directly relevant to the decision problem (studies 17–21 in Table 161). All studies reported BASFI outcomes and one study did not report BASDAI 50 (study 21).
| Study number | Trial name | Treatment | Number in treatment group | Number in placebo group | BASDAI 50 score | Change BASDAI score | Change BASFI score |
|---|---|---|---|---|---|---|---|
| 17 | Haibel 200852 | Adalimumab | 22 | 24 | ✗ | ✗ | ✗ |
| 18 | ABILITY-1 201358 | Adalimumab | 69 | 73 | ✗ | ✗ | ✗ |
| 19 | RAPID-axSpA 201464 | Certolizumab pegol | 46 + 51 | 50 | ✗ | ✗ | ✗ |
| 20 | Dougados 201476 | ETA50 | 106 | 109 | ✗ | ✗ | ✗ |
| 21 | Barkham 200950 | Infliximab | 20 | 20 | ✗ | ✗ |
The data on these five studies are shown in Table 162.
| s[] | t[] | n[] | r[] | b[] | sd[] | y[] | y.se[] | y.f[] | y.f.se[] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 24 | 5 | 6.20 | 0.59 | –1.20 | 7.79 | –0.80 | 6.87 |
| 1 | 2 | 22 | 11 | 6.50 | 0.69 | –2.70 | 6.30 | –2.40 | 7.24 |
| 2 | 1 | 73 | 10 | 6.38 | 0.44 | –1.10 | 19.00 | –0.63 | 22.78 |
| 2 | 2 | 69 | 27 | 6.43 | 0.42 | –2.20 | 11.04 | –1.28 | 16.91 |
| 3 | 1 | 50 | 8 | 6.40 | 0.44 | –1.50 | 6.25 | –0.40 | 6.25 |
| 3 | 3 | 97 | 47 | 6.55 | 0.43 | –3.35 | 11.64 | –2.30 | 12.21 |
| 4 | 1 | 109 | 26 | 6.00 | 0.28 | –1.30 | 11.11 | –0.80 | 25.00 |
| 4 | 4 | 106 | 46 | 6.00 | 0.31 | –2.00 | 11.11 | –1.40 | 25.00 |
| 5 | 1 | 20 | N/A | 5.76 | 0.28 | –0.75 | 3.42 | –0.47 | 3.95 |
| 5 | 5 | 20 | N/A | 5.85 | 0.31 | –3.41 | 3.12 | –2.70 | 3.59 |
Description of approaches to the synthesis
To synthesise these data we used the same implementation and software specifications as described in Appendix 12. Analyses explored two different scenarios to consider these data:
-
scenario 1: data from nr-AxSpA trials were considered in isolation
-
scenario 2: data from AS population were also used, no difference between the populations was assumed.
All models implemented here jointly synthesise BASDAI and BASFI outcomes (our preferred modelling approach, C).
Results of the synthesis
Results of the analysis are in Table 163.
| Estimated | Assumeda | Predicted | ||
|---|---|---|---|---|
| Difference in change score from baseline, mean (SD) | Probability of having a BASDAI 50 response, placebo, mean (SD) | Probability of having a BASDAI 50 response, anti-TNF, mean (SD) | OR for BASDAI 50 response, anti-TNF vs. placebo, median (SD) | |
| Scenario 1: data from nr-AxSpA trials | ||||
| Effect of anti-TNFs on BASDAI | –1.86 (0.79) | 0.20 (–) | 0.53 (0.13) | 4.39 (6.59) |
| Effect of anti-TNFs on BASFI | –1.30 (0.84) | – | – | – |
| Other model summaries | ||||
| DBASDAI | –1.86 (0.53) | – | – | – |
| DBASFI | –1.30 (0.65) | – | – | – |
| γBASDAI | 0.41 (0.43) | – | – | – |
| γBASFI | 0.68 (0.53) | – | – | – |
| ρplacebo | 0.60 (0.27) | – | – | – |
| ρanti-TNF | 0.57 (0.28) | – | – | – |
| ρm | 0.51 (0.29) | – | – | – |
| σ2re | 0.55 (0.29) | – | – | – |
| DIC | 88.6 | – | – | – |
| Scenario 2: data from AS and nr-AxSpA trials, no difference between the populations | ||||
| Effect of anti-TNFs on BASDAI | –1.97 (0.32) | 0.20 (–) | 0.55 (0.06) | 4.94 (1.48) |
| Effect of anti-TNFs on BASFI | –1.37 (0.3) | – | – | – |
| Other model summaries | ||||
| DBASDAI | –1.97 (0.20) | – | – | – |
| DBASFI | –1.37 (0.18) | – | – | – |
| γBASDAI | 0.12 (0.09) | – | – | – |
| γBASFI | 0.18 (0.11) | – | – | – |
| ρplacebo | 0.50 (0.26) | – | – | – |
| ρanti-TNF | 0.74 (0.22) | – | – | – |
| ρm | 0.54 (0.29) | – | – | – |
| σ2re | 0.19 (0.16) | – | – | – |
| DIC | 269.0 | – | – | – |
Appendix 14 Utility review
In accordance with the NICE reference case,238 utility values should be based on the EQ-5D instrument. Therefore, a systematic review of utility studies was carried out to identify relevant studies which (1) directly estimate EQ-5D utility values; and (2) establish the relationship between generic measures of utility (in particular the EQ-5D) and measures of disease progression (including mapping studies). The review of utility studies focuses on anti-TNFs for AS and AxSpA without radiographic evidence of AS (nr-AxSpA).
Methods
Searches were undertaken in EMBASE and MEDLINE/MEDLINE In-Process & Other Non-Indexed Citations (Ovid). A combination of disease terms and terms associated with the EQ-5D were used. Upon initial review, it was evident that the results of the search did not identify the studies found in the cost-effectiveness review that also reported on the quality of life of AS patients, for example Ara et al. 161 Therefore, a separate search in NHS EED, MEDLINE and EMBASE for published modelling studies was also subsequently undertaken. No language and date limits were applied. Full details of the search strategy used are presented in Appendix 1.
Studies that reported utility values consistent with the NICE reference case were included in the review, that is studies reporting utilities for AS or nr-AxSpa patients generated using:
-
the EQ-5D
-
HRQoL or changes in HRQoL measured directly by patients
-
changes in HRQoL should be valued using public preferences from a representative sample of the UK population using a choice-based method (or this could be reasonably assumed from the publication).
When a mapping algorithm was reported, eligibility of studies was restricted to those that mapped from BASDAI score and/or BASFI score to EQ-5D.
Results
Identified studies
The combined search retrieved 210 citations. After screening titles and abstracts, 28 citations were retrieved for full review. The abstract by Pumford et al. 239 was excluded, as the full publication by Wade et al. 240 reported on the same study. The abstract by Lee et al. 241 was excluded as a more recent full publication of the study (Lee et al. 242) reported that a non-UK valuation set was used. Joore et al. 243 was also excluded, as primary data were reported in Van Tubergen et al. 244 A further three studies were excluded because the manuscripts were in a language other than English.
Kobelt et al. have reported costs/quality of life/cost-effectiveness of AS patients in multiple references (e.g. Kobelt et al. 2004,152 Kobelt et al. 2006,245 Kobelt et al. 2007160 and Kobelt et al. 2008246). Kobelt et al. 152,160 are relevant to a UK population and are preferred to the other Kobelt publications that are relevant to non-UK populations. Of these, Kobelt et al. 152 reports utility data collected and used in the analysis and is, therefore, included in this review.
In total, 12 studies were deemed to meet the NICE reference case238 and are summarised in Table 164.
| Study | Population characteristics | Interventions | Utility assessment methods | Utilities reported, mean (SD) [95% CI] | Reviewer comments |
|---|---|---|---|---|---|
| Ara et al. (2007)161 The cost-effectiveness of etanercept in patients with severe ankylosing spondylitis in the UK (mapping algorithm to EQ-5D values also reported) |
|
|
EQ-5D
|
European RCT week 12 (observed) for patient with a BASDAI score of ≥ 4:
|
|
| Boonen et al. (2002)247 and (2003)248 2002: Work status and productivity costs due to ankylosing spondylitis: comparison of three European countries 2003: Costs of ankylosing spondylitis in three European countries: the patient’s perspective |
AS patients diagnosed using mNY criteria | N/A | EQ-5D
|
Baseline:
|
|
There were 130 patients from the Netherlands. Patients were sampled from the Dutch standard diagnosis register of rheumatic diseases:
|
Time averaged across 2-year follow-up period:
|
||||
There were 53 patients from France. Consecutive in- and outpatients at a hospital rheumatology department
|
|||||
There were 26 patients from Belgium. Consecutive outpatients at a hospital rheumatology department
|
|||||
| Boonen et al. (2007)250 How do the EQ-5D, SF-6D and the well-being rating scale compare in patients with ankylosing spondylitis? |
AS patients diagnosed using mNY criteria | OASIS: N/A (prevalence cohort) | EQ-5D
|
|
|
| There were 134 patients from the prevalence-based OASIS cohort (Boonen et al. 2002/3)247,248 | |||||
| There were 120 patients from a RCT comparing spa treatment (n = 80) with usual care (n = 40) (Van Tuburgen et al. 2002244) | RCT: spa treatment (3 weeks) and usual care | ||||
Both data sets were merged as authors found that QoL instruments provided similar results in the two populations:
|
|||||
| Boonen et al. (2008)205 Rapid and sustained improvement in health-related quality of life and utility for 72 weeks in patients with ankylosing spondylitis receiving etanercept |
In total 257 AS patients were diagnosed using mNY criteria who had completed 24 weeks of treatment in a previous RCT (277 patients enrolled) comparing etanercept with placebo. Patients were treated with etanercept in the open-label extension study
|
|
EQ-5D
|
Baseline (n = 232):
|
|
| Braun et al. (2007)85 Improvement in patient-reported outcomes for patients with ankylosing spondylitis treated with etanercept 50 mg once-weekly and 25 mg twice-weekly |
In total 356 active AS patients were diagnosed using mNY criteria defined by a VAS score for mean morning stiffness ≥ 30, and by at least two of the following: VAS score for patient global assessment of disease activity ≥ 30, average VAS score for nocturnal and total pain ≥ 30 or BASFI score of ≥ 30
|
|
EQ-5D
|
Mean increase between 0 to 12 weeks reported in figure 2 in Braun et al.:205
|
|
| Gordeev et al. (2010)251 Role of contextual factors in health-related quality of life in ankylosing spondylitis |
In total 764 patients with AS were diagnosed using mNY criteria, in Canada and Australia were sent a questionnaire in the post. Overall, 522 (68%) responded and were included in the analysis
|
|
EQ-5D
|
|
|
| Haywood et al. (2002)252 Generic measures of health-related quality of life in ankylosing spondylitis: reliability, validity and responsiveness |
A random sample of 451 patients with AS, diagnosed using mNY criteria, were sent a postal questionnaire
|
N/A | EQ-5D
|
Reliability analysis using data from patients whose health remained the same at 2 weeks (n = 321): 0.53 (0.35) Longitudinal construct validity analysis at 6 months |
|
AS:
|
|||||
General health:
|
|||||
| Healey et al. (2013)14 Patients with well-established ankylosing spondylitis show limited deterioration in a ten-year prospective cohort study |
In total 269 patients with AS, diagnosed using mNY criteria, were invited to participate at a rheumatology centre 159 patients participated at baseline 69 patients participated at the 10 year assessment
|
N/A | EQ-5D
|
Baseline assessment in 1998 (n = 159): 0.64 (0.28) 10-year follow-up assessment: 0.61 (0.30) |
|
| Kobelt et al. (2004)152 The burden of ankylosing spondylitis and the cost-effectiveness of treatment with infliximab (Remicade®) |
Clinical trial, hospital cohort and survey data for AS patients were utilised in this study Utilities were estimated from a survey of 3000 patients. 1413 (57%) patients responded and were included in the analysis Survey responders had the following characteristics:
|
N/A | EQ-5D
|
Survey mean: 0.67 (0.21) |
|
BASDAI subgroups:
|
|||||
BASFI subgroups:
|
|||||
| McLeod et al. (2007)38 Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation (mapping algorithm to EQ-5D values reported) |
Utilities were estimated from a reanalysis of the Kobelt et al.152 survey data by the manufacturer of infliximab (n = 1144)
|
N/A | EQ-5D
|
Algorithm used in the assessment group (LRiG) model:
|
|
| Van Tubergen et al. (2002)244 Cost effectiveness of combined spa-exercise therapy in ankylosing spondylitis: a randomized controlled trial |
120 AS patients, diagnosed using mNY criteria 111 included in the analysis
|
|
EQ-5D
|
Spa treatment in Austria (n = 36):
|
|
Spa treatment in the Netherlands (n = 38):
|
|||||
Usual care (n = 37):
|
|||||
| Wade et al. (2011)240 Baseline characteristics and patient reported outcome data of patients prescribed etanercept: web-based and telephone evaluation |
There were 43 patients prescribed etanercept for AS (diagnostic criteria not reported) RA, PsA and psoriasis patients were also included in the study
|
|
EQ-5D
|
Baseline: 0.37 (0.37)a |
|
The main reasons for excluding studies at the title/abstract and at full review stage were (1) utilities were not reported (e.g. Haywood et al. 256), (2) valuation set not reported or a non-UK valuation was used (e.g. Kvamme et al. 257), (3) utilities were reported for a mixed population with different inflammatory arthropies or in a population not relevant to the decision problem (e.g. Osnes-Ringen et al. 258).
Studies meeting the National Institute for Health and Care Excellence reference case
The 12 studies meeting the reference case238 have been summarised in Table 164. The table includes a primary study to Boonen et al. ,250 reported in Boonen et al. 247,248 The study by Boonen et al. 250 has been retained as it reports utility values for patients with a BASDAI score of ≥ 4.
Ankylosing spondylitis population
All studies included in Table 164 are of AS patients. Five studies reported utility values (or mapping algorithms) generated from data specifically collected from the UK population (Haywood et al. ,252 Healey et al. ,14 Kobelt et al. ,152 McLeod et al. 38 and Wade et al. 240). Four studies included interventions specific to this appraisal, all of these studies were of etanercept (Ara et al. ,161 Boonen et al. ,205 Braun et al. 87 and Wade et al. 240). Utility values reported ranged from values at baseline to at 10 years’ follow-up.
Non-radiographic axial spondyloarthritis population
Two citations were identified in the review that reported utilities for nr-AxSpA patients (Dougados et al. 78 and Lindstrom et al. 259). However, these studies did not explicitly report which population valuation sets were used and, therefore, were excluded from the review.
Mapping algorithms
Of the 12 studies in Table 164, two report mapping algorithms between disease-specific measures and the EQ-5D (Ara et al. 161 and McLeod et al. 38). Both have been reported as part of a cost-effectiveness analysis and provide limited information on methodology employed (e.g. covariates tested, correlation considerations and goodness of fit). McLeod et al. 38 reports on an algorithm generated using data from UK AS patients.
Appendix 15 Additional cost-effectiveness results
Following the consultation process to the NICE appraisal, additional analyses were undertaken to address comments received on Chapters 5 and 6. These focused on the conditional baseline BASDAI scores used in Chapter 5 and on the existence of a biosimilar product for infliximab with a lower list price.
A. Truncated baseline Bath Ankylosing Spondylitis Disease Activity Index scores
One of the consultees identified that the simulation procedure undertaken to evaluate conditional scores in Chapter 5 was using a non-truncated distribution for the baseline BASDAI score. This meant that it was possible for simulated individuals to have a baseline BASDAI score of < 4, which is inconsistent with clinical practice for which treatment with anti-TNFs is only provided to patients with a baseline BASDAI score of > 4. This was a result of assuming a normal distribution for baseline BASDAI scores. Together with the SD applied, this resulted in the simulation model sampling population characteristics which we acknowledge would subsequently fall outside of the decision problem. We have rerun our base-case results assuming a truncated distribution (i.e. excluding the possibility of sampling patients with a baseline BASDAI score of < 4). The results are presented in Table 165 and confirm only a minimal impact on the conditional scores.
| Patient population | BASDAI | BASFI | ||
|---|---|---|---|---|
| Control | Treatment | Control | Treatment | |
| BASDAI baseline score truncated to a minimum value of 4 | ||||
| % responders to BASDAI 50 | 0.08 | 0.45 | – | – |
| Change in score | ||||
| Responders | –2.93 | –3.77 | –1.49 | –3.01 |
| Non-responders | –0.39 | –1.56 | –0.06 | –0.39 |
| All | –0.60 | –2.56 | –0.18 | –1.58 |
| Baseline | ||||
| Responders | 4.52 | 4.86 | 3.92 | 4.24 |
| Non-responders | 6.30 | 7.23 | 5.40 | 6.15 |
| All | 6.15 | 6.15 | 5.28 | 5.28 |
Tables 166 and 167 report the revised base-case cost-effectiveness results for the AS population using a truncated distribution for baseline BASDAI (the original base-case cost-effectiveness results are reported in Tables 95 and 96 for the AS population).
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.240 | – | 111,532 | – | – | – | – |
| Certolizumab pegol PAS | 8.249 | 1.009 | 131,909 | 20,377 | 20,195 | 0.482 | 0.877 |
| Golimumab | 8.249 | 1.009 | 133,543 | 22,011 | 21,814 | 0.381 | 0.830 |
| Adalimumab | 8.249 | 1.009 | 133,637 | 22,105 | 21,907 | 0.376 | 0.827 |
| Etanercept | 8.249 | 1.009 | 134,054 | 22,522 | 22,321 | 0.345 | 0.811 |
| Certolizumab pegol | 8.249 | 1.009 | 135,483 | 23,951 | 23,737 | 0.258 | 0.758 |
| Infliximab | 8.249 | 1.009 | 153,255 | 41,723 | 41,350 | 0.000 | 0.081 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.251 | – | 111,666 | – | – | – | – |
| Certolizumab pegol PAS | 7.898 | 0.647 | 134,955 | 23,289 | 35,982 | 0.013 | 0.333 |
| Golimumab | 7.898 | 0.647 | 136,585 | 24,919 | 38,500 | 0.009 | 0.245 |
| Adalimumab | 7.898 | 0.647 | 136,679 | 25,013 | 38,646 | 0.008 | 0.239 |
| Etanercept | 7.898 | 0.647 | 137,100 | 25,434 | 39,296 | 0.006 | 0.222 |
| Certolizumab pegol | 7.898 | 0.647 | 138,528 | 26,862 | 41,503 | 0.004 | 0.172 |
| Infliximab | 7.898 | 0.647 | 156,420 | 44,754 | 69,146 | 0.000 | 0.000 |
These results demonstrate that the ICERs show only small variation using a truncated baseline BASDAI distribution. The ICERs appear marginally less favourable than the original base-case results. Hence, any potential bias in the original analysis appears to work in favour of the TNF-inhibitors. However, the magnitude is small and could equally be a result of simulation error as opposed to any bias.
Non-radiographic axial spondyloarthritis population
We have rerun our base-case results assuming a truncated distribution (i.e. excluding the possibility of sampling patients with a baseline BASDAI score of < 4) for the nr-AxSpA population. The results are presented in Table 168 and confirm only a minimal impact on the conditional scores.
| Patient population | BASDAI | BASFI | ||
|---|---|---|---|---|
| Control | Treatment | Control | Treatment | |
| BASDAI baseline score truncated to a minimum value of 4 | ||||
| % responders to BASDAI 50 | 0.21 | 0.55 | – | – |
| Change in score | ||||
| Responders | –3.23 | –4.29 | –1.77 | –3.23 |
| Non-responders | –1.05 | –2.25 | 0.02 | 0.17 |
| All | –1.50 | –3.37 | –0.35 | –1.70 |
| Baseline | ||||
| Responders | 4.80 | 5.40 | 3.24 | 3.86 |
| Non-responders | 6.83 | 7.64 | 5.34 | 6.20 |
| All | 6.41 | 6.41 | 4.91 | 4.91 |
Tables 169 and 170 report the revised base-case cost-effectiveness results for the nr-AxSpA population using a truncated distribution for baseline BASDAI (the original base-case cost-effectiveness results are reported in Tables 97 and 98).
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.906 | – | 90,850 | – | – | – | – |
| Certolizumab pegol PAS | 11.291 | 1.386 | 130,974 | 40,124 | 28,958 | 0.102 | 0.576 |
| Adalimumab | 11.291 | 1.386 | 132,373 | 41,523 | 29,968 | 0.069 | 0.506 |
| Etanercept | 11.291 | 1.386 | 133,119 | 42,269 | 30,506 | 0.059 | 0.484 |
| Certolizumab pegol | 11.291 | 1.386 | 134,547 | 43,696 | 31,536 | 0.045 | 0.454 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.963 | – | 90,219 | – | – | – | – |
| Certolizumab pegol PAS | 11.200 | 1.237 | 131,714 | 41,495 | 33,555 | 0.057 | 0.396 |
| Adalimumab | 11.200 | 1.237 | 133,109 | 42,890 | 34,684 | 0.038 | 0.343 |
| Etanercept | 11.200 | 1.237 | 133,859 | 43,640 | 35,290 | 0.035 | 0.318 |
| Certolizumab pegol | 11.200 | 1.237 | 135,286 | 45,067 | 36,444 | 0.029 | 0.284 |
As Tables 169 and 170 demonstrate, the ICERs show only small variation employing a truncated distribution for baseline BASDAI. The ICERs appear marginally less favourable than the original base-case results. Hence, any potential bias in the original analysis appears to work in favour of the TNF-inhibitors. However, the magnitude is small and could equally be a result of simulation error as opposed to any bias.
B. Biosimilar
One of the consultees provided the list price for Remsima™, a biosimilar for infliximab marketed by Celltrion Healthcare. In view of this, we updated the base-case analysis for the AS population using the list price for Remsima. The results, of rerunning the analysis excluding infliximab and replacing it with Remsima using the list price provided, are reported in Tables 171 and 172. Minor differences in the ICERs for the other TNF inhibitors compared with the original base case are a result of sapling variation (i.e. all ICERs are derived from the probabilistic analysis and rerunning the simulation results in minor differences each time).
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.266 | – | 111,696 | – | – | – | – |
| Certolizumab pegol PAS | 8.179 | 0.913 | 129,281 | 17,586 | 19,257 | 0.551 | 0.899 |
| Golimumab | 8.179 | 0.913 | 130,969 | 19,274 | 21,106 | 0.428 | 0.848 |
| Adalimumab | 8.179 | 0.913 | 131,053 | 19,357 | 21,197 | 0.421 | 0.846 |
| Etanercept | 8.179 | 0.913 | 131,426 | 19,731 | 21,606 | 0.390 | 0.835 |
| Certolizumab pegol | 8.179 | 0.913 | 132,855 | 21,159 | 23,171 | 0.295 | 0.762 |
| Remsima™ | 8.179 | 0.913 | 145,256 | 33,561 | 36,751 | 0.004 | 0.204 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.250 | – | 111,647 | – | – | – | – |
| Certolizumab pegol PAS | 7.854 | 0.604 | 131,922 | 20,275 | 33,578 | 0.040 | 0.397 |
| Golimumab | 7.854 | 0.604 | 133,605 | 21,958 | 36,366 | 0.016 | 0.307 |
| Adalimumab | 7.854 | 0.604 | 133,690 | 22,043 | 36,506 | 0.016 | 0.305 |
| Etanercept | 7.854 | 0.604 | 134,067 | 22,420 | 37,131 | 0.012 | 0.287 |
| Certolizumab pegol | 7.854 | 0.604 | 135,496 | 23,849 | 39,497 | 0.007 | 0.215 |
| Remsima™ | 7.854 | 0.604 | 148,010 | 36,363 | 60,222 | 0.000 | 0.003 |
Appendix 16 Full incremental cost-effectiveness ratio tables for scenarios
Ankylosing spondylitis population
| Strategy number | Parameter/structural | Approach in scenario | Approach in base case |
|---|---|---|---|
| 1 | CC (‘placebo’) response | No response to CC assumed at 12 weeks | Response to CC included at 12 weeks |
| 2 | Different baselines assumed for responders and non-responders and change in BASDAI/BASFI scores | Separate baselines based on pooled estimates provided by manufacturers. Changes in BASDAI/BASFI score conditioned on response also based on pooled estimates provided by manufacturers | Separate baselines and changes in BASDAI/BASFI conditioned on responses estimated via extended synthesis model |
| 3 | BASFI progression | No effect of anti-TNFs on BASFI progression | Treatment effect applied from year 4 onwards |
| 4 | BASFI progression | Treatment effect of anti-TNFs applied from start of model | Treatment effect applied from year 4 onwards |
| 5 | Utilities | Linear BASDAI/BASFI model (based on Kobelt et al.152) | Non-linear BASDAI/BASFI model (Pfizer submission36) |
| 6 | Baseline BASDAI score truncated at 4 | Baseline BASDAI scores used in the NMA are limited to be between 4 and 10 | Baseline BASDAI scores used in the NMA are limited to be between 0 and 10 |
| 7 | Price of biosimilar infliximab | The price of the biosimilar for infliximab provided by the manufacturer was used in the model | BNF price of infliximab |
Ankylosing spondylitis scenario results: rebound equal to gain
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.262 | – | 111,702 | – | – | – | – |
| Certolizumab pegol PAS | 7.952 | 0.691 | 125,734 | 14,033 | 20,319 | 0.462 | 0.861 |
| Golimumab | 7.952 | 0.691 | 127,531 | 15,829 | 22,920 | 0.313 | 0.764 |
| Adalimumab | 7.952 | 0.691 | 127,594 | 15,893 | 23,013 | 0.308 | 0.761 |
| Etanercept | 7.952 | 0.691 | 127,879 | 16,178 | 23,425 | 0.292 | 0.741 |
| Certolizumab pegol | 7.952 | 0.691 | 129,308 | 17,607 | 25,495 | 0.188 | 0.651 |
| Infliximab | 7.952 | 0.691 | 141,750 | 30,048 | 43,510 | 0.000 | 0.063 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.100 | – | 112,768 | – | – | – | – |
| Certolizumab pegol PAS | 8.120 | 1.019 | 127,856 | 15,088 | 14,803 | 0.840 | 0.988 |
| Golimumab | 8.120 | 1.019 | 129,536 | 16,768 | 16,451 | 0.727 | 0.979 |
| Adalimumab | 8.120 | 1.019 | 129,621 | 16,853 | 16,535 | 0.720 | 0.977 |
| Etanercept | 8.120 | 1.019 | 130,001 | 17,233 | 16,907 | 0.696 | 0.975 |
| Certolizumab pegol | 8.120 | 1.019 | 131,430 | 18,662 | 18,309 | 0.561 | 0.955 |
| Infliximab | 8.120 | 1.019 | 147,674 | 34,906 | 34,246 | 0.015 | 0.232 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.253 | – | 109,379 | – | – | – | – |
| Certolizumab pegol PAS | 8.128 | 0.875 | 127,455 | 18,075 | 20,655 | 0.462 | 0.843 |
| Golimumab | 8.128 | 0.875 | 129,140 | 19,760 | 22,581 | 0.348 | 0.775 |
| Adalimumab | 8.128 | 0.875 | 129,224 | 19,845 | 22,677 | 0.341 | 0.771 |
| Etanercept | 8.128 | 0.875 | 129,600 | 20,220 | 23,106 | 0.319 | 0.760 |
| Certolizumab pegol | 8.128 | 0.875 | 131,028 | 21,649 | 24,739 | 0.234 | 0.698 |
| Infliximab | 8.128 | 0.875 | 147,118 | 37,739 | 43,125 | 0.001 | 0.063 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.239 | – | 111,036 | – | – | – | – |
| Certolizumab pegol PAS | 8.201 | 0.962 | 128,804 | 17,767 | 18,466 | 0.589 | 0.929 |
| Golimumab | 8.201 | 0.962 | 130,485 | 19,448 | 20,213 | 0.462 | 0.878 |
| Adalimumab | 8.201 | 0.962 | 130,570 | 19,533 | 20,301 | 0.453 | 0.875 |
| Etanercept | 8.201 | 0.962 | 130,949 | 19,912 | 20,695 | 0.429 | 0.862 |
| Certolizumab pegol | 8.201 | 0.962 | 132,377 | 21,341 | 22,180 | 0.345 | 0.808 |
| Infliximab | 8.201 | 0.962 | 148,597 | 37,560 | 39,037 | 0.005 | 0.124 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 10.272 | – | 111,187 | – | – | – | – |
| Certolizumab pegol PAS | 11.043 | 0.771 | 129,139 | 17,953 | 23,290 | 0.217 | 0.891 |
| Golimumab | 11.043 | 0.771 | 130,819 | 19,632 | 25,469 | 0.099 | 0.755 |
| Adalimumab | 11.043 | 0.771 | 130,904 | 19,717 | 25,579 | 0.094 | 0.750 |
| Etanercept | 11.043 | 0.771 | 131,285 | 20,098 | 26,073 | 0.074 | 0.724 |
| Certolizumab pegol | 11.043 | 0.771 | 132,713 | 21,526 | 27,926 | 0.048 | 0.593 |
| Infliximab | 11.043 | 0.771 | 148,974 | 37,787 | 49,021 | 0.000 | 0.003 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.240 | – | 111,532 | – | – | – | – |
| Certolizumab pegol PAS | 8.249 | 1.009 | 131,909 | 20,377 | 20,195 | 0.482 | 0.877 |
| Golimumab | 8.249 | 1.009 | 133,543 | 22,011 | 21,814 | 0.381 | 0.830 |
| Adalimumab | 8.249 | 1.009 | 133,637 | 22,105 | 21,907 | 0.376 | 0.827 |
| Etanercept | 8.249 | 1.009 | 134,054 | 22,522 | 22,321 | 0.345 | 0.811 |
| Certolizumab pegol | 8.249 | 1.009 | 135,483 | 23,951 | 23,737 | 0.258 | 0.758 |
| Infliximab | 8.249 | 1.009 | 153,255 | 41,723 | 41,350 | 0.000 | 0.081 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.266 | – | 111,696 | – | – | – | – |
| Certolizumab pegol PAS | 8.179 | 0.913 | 129,281 | 17,586 | 19,257 | 0.551 | 0.899 |
| Golimumab | 8.179 | 0.913 | 130,969 | 19,274 | 21,106 | 0.428 | 0.848 |
| Adalimumab | 8.179 | 0.913 | 131,053 | 19,357 | 21,197 | 0.421 | 0.846 |
| Etanercept | 8.179 | 0.913 | 131,426 | 19,731 | 21,606 | 0.390 | 0.835 |
| Certolizumab pegol | 8.179 | 0.913 | 132,855 | 21,159 | 23,171 | 0.295 | 0.762 |
| Remsima | 8.179 | 0.913 | 145,256 | 33,561 | 36,751 | 0.004 | 0.204 |
Ankylosing spondylitis scenario results: rebound to conventional care
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.295 | – | 112,675 | – | – | – | – |
| Certolizumab pegol PAS | 7.762 | 0.467 | 128,654 | 15,979 | 34,229 | 0.038 | 0.385 |
| Golimumab | 7.762 | 0.467 | 130,446 | 17,771 | 38,068 | 0.014 | 0.257 |
| Adalimumab | 7.762 | 0.467 | 130,511 | 17,836 | 38,207 | 0.013 | 0.256 |
| Etanercept | 7.762 | 0.467 | 130,799 | 18,124 | 38,824 | 0.010 | 0.245 |
| Certolizumab pegol | 7.762 | 0.467 | 132,228 | 19,553 | 41,885 | 0.004 | 0.161 |
| Infliximab | 7.762 | 0.467 | 144,800 | 32,125 | 68,815 | 0.000 | 0.000 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.092 | – | 114,772 | – | – | – | – |
| Certolizumab pegol PAS | 7.756 | 0.664 | 132,257 | 17,485 | 26,348 | 0.136 | 0.666 |
| Golimumab | 7.756 | 0.664 | 133,945 | 19,174 | 28,892 | 0.076 | 0.531 |
| Adalimumab | 7.756 | 0.664 | 134,029 | 19,257 | 29,018 | 0.075 | 0.521 |
| Etanercept | 7.756 | 0.664 | 134,402 | 19,631 | 29,580 | 0.068 | 0.493 |
| Certolizumab pegol | 7.756 | 0.664 | 135,831 | 21,059 | 31,733 | 0.042 | 0.380 |
| Infliximab | 7.756 | 0.664 | 151,831 | 37,059 | 55,842 | 0.000 | 0.008 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.252 | – | 110,930 | – | – | – | – |
| Certolizumab pegol PAS | 7.818 | 0.566 | 131,610 | 20,679 | 36,518 | 0.021 | 0.339 |
| Golimumab | 7.818 | 0.566 | 133,289 | 22,359 | 39,483 | 0.009 | 0.249 |
| Adalimumab | 7.818 | 0.566 | 133,374 | 22,444 | 39,634 | 0.008 | 0.245 |
| Etanercept | 7.818 | 0.566 | 133,755 | 22,824 | 40,306 | 0.006 | 0.230 |
| Certolizumab pegol | 7.818 | 0.566 | 135,183 | 24,253 | 42,828 | 0.003 | 0.166 |
| Infliximab | 7.818 | 0.566 | 151,457 | 40,526 | 71,565 | 0.000 | 0.000 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.268 | – | 108,817 | – | – | – | – |
| Certolizumab pegol PAS | 7.894 | 0.626 | 128,999 | 20,182 | 32,222 | 0.047 | 0.429 |
| Golimumab | 7.894 | 0.626 | 130,683 | 21,866 | 34,910 | 0.022 | 0.341 |
| Adalimumab | 7.894 | 0.626 | 130,767 | 21,951 | 35,045 | 0.020 | 0.339 |
| Etanercept | 7.894 | 0.626 | 131,144 | 22,327 | 35,647 | 0.016 | 0.310 |
| Certolizumab pegol | 7.894 | 0.626 | 132,573 | 23,756 | 37,928 | 0.008 | 0.234 |
| Infliximab | 7.894 | 0.626 | 148,706 | 39,889 | 63,684 | 0.000 | 0.000 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 10.272 | – | 112,648 | – | – | – | – |
| Certolizumab pegol PAS | 10.967 | 0.695 | 133,103 | 20,455 | 29,414 | 0.012 | 0.511 |
| Golimumab | 10.967 | 0.695 | 134,781 | 22,133 | 31,827 | 0.005 | 0.340 |
| Adalimumab | 10.967 | 0.695 | 134,866 | 22,218 | 31,950 | 0.005 | 0.333 |
| Etanercept | 10.967 | 0.695 | 135,248 | 22,600 | 32,499 | 0.004 | 0.300 |
| Certolizumab pegol | 10.967 | 0.695 | 136,677 | 24,028 | 34,554 | 0.002 | 0.165 |
| Infliximab | 10.967 | 0.695 | 152,997 | 40,349 | 58,022 | 0.000 | 0.000 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.251 | – | 111,666 | – | – | – | – |
| Certolizumab pegol PAS | 7.898 | 0.647 | 134,955 | 23,289 | 35,982 | 0.013 | 0.333 |
| Golimumab | 7.898 | 0.647 | 136,585 | 24,919 | 38,500 | 0.009 | 0.245 |
| Adalimumab | 7.898 | 0.647 | 136,679 | 25,013 | 38,646 | 0.008 | 0.239 |
| Etanercept | 7.898 | 0.647 | 137,100 | 25,434 | 39,296 | 0.006 | 0.222 |
| Certolizumab pegol | 7.898 | 0.647 | 138,528 | 26,862 | 41,503 | 0.004 | 0.172 |
| Infliximab | 7.898 | 0.647 | 156,420 | 44,754 | 69,146 | 0.000 | 0.000 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.250 | – | 111,647 | – | – | – | – |
| Certolizumab pegol PAS | 7.854 | 0.604 | 131,922 | 20,275 | 33,578 | 0.040 | 0.397 |
| Golimumab | 7.854 | 0.604 | 133,605 | 21,958 | 36,366 | 0.016 | 0.307 |
| Adalimumab | 7.854 | 0.604 | 133,690 | 22,043 | 36,506 | 0.016 | 0.305 |
| Etanercept | 7.854 | 0.604 | 134,067 | 22,420 | 37,131 | 0.012 | 0.287 |
| Certolizumab pegol | 7.854 | 0.604 | 135,496 | 23,849 | 39,497 | 0.007 | 0.215 |
| Remsima | 7.854 | 0.604 | 148,010 | 36,363 | 60,222 | 0.000 | 0.003 |
Non-radiographic axial spondyloarthritis population
| Strategy number | Parameter/structural | Approach in scenario | Approach in base case |
|---|---|---|---|
| 1 | CC (‘placebo’) response | No response to CC assumed at 12 weeks | Response to CC included at 12 weeks |
| 2 | Different baselines assumed for responders and non-responders and change in BASDAI/BASFI scores | Separate baselines based on pooled estimates provided by manufacturers. Changes in BASDAI/BASFI score conditioned on response also based on pooled estimates provided by manufacturers | Separate baselines and changes in BASDAI/BASFI conditioned on responses estimated via extended synthesis model |
| 3 | BASFI progression | No effect of anti-TNFs on BASFI progression | Treatment effect applied from year 4 onwards |
| 4 | BASFI progression | Treatment effect of anti-TNFs applied from start of model | Treatment effect applied from year 4 onwards |
| 5 | Utilities | Linear BASDAI/BASFI model (based on Kobelt et al.152) | Non-linear BASDAI/BASFI model (Pfizer submission36) |
| 6 | Treatment effect of anti-TNFs | Trials in nr-AxSpA and AS populations combined | Only trials in nr-AxSpA included |
| 7 | Baseline BASDAI score truncated at 4 | Baseline BASDAI scores used in the NMA are limited to be between 4 and 10 | Baseline BASDAI scores used in the NMA are limited to be between 0 and 10 |
Non-radiographic axial spondyloarthritis scenario results: rebound equal to gain
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 10.012 | – | 87,215 | – | – | – | – |
| Certolizumab pegol PAS | 11.864 | 1.852 | 122,480 | 35,265 | 19,040 | 0.550 | 0.861 |
| Adalimumab | 11.864 | 1.852 | 123,883 | 36,668 | 19,797 | 0.500 | 0.844 |
| Etanercept | 11.864 | 1.852 | 124,625 | 37,410 | 20,198 | 0.481 | 0.838 |
| Certolizumab pegol | 11.864 | 1.852 | 126,052 | 38,837 | 20,968 | 0.421 | 0.815 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.988 | – | 87,947 | – | – | – | – |
| Certolizumab pegol PAS | 11.666 | 1.678 | 124,455 | 36,508 | 21,757 | 0.377 | 0.764 |
| Adalimumab | 11.666 | 1.678 | 125,858 | 37,910 | 22,593 | 0.337 | 0.739 |
| Etanercept | 11.666 | 1.678 | 126,600 | 38,653 | 23,036 | 0.317 | 0.722 |
| Certolizumab pegol | 11.666 | 1.678 | 128,027 | 40,080 | 23,886 | 0.276 | 0.683 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.891 | – | 91,479 | – | – | – | – |
| Certolizumab pegol PAS | 11.262 | 1.370 | 130,734 | 39,254 | 28,643 | 0.138 | 0.576 |
| Adalimumab | 11.262 | 1.370 | 132,141 | 40,662 | 29,670 | 0.102 | 0.528 |
| Etanercept | 11.262 | 1.370 | 132,879 | 41,399 | 30,208 | 0.093 | 0.505 |
| Certolizumab pegol | 11.262 | 1.370 | 134,306 | 42,827 | 31,250 | 0.076 | 0.460 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.923 | – | 90,625 | – | – | – | – |
| Certolizumab pegol PAS | 11.338 | 1.415 | 129,492 | 38,867 | 27,471 | 0.154 | 0.627 |
| Adalimumab | 11.338 | 1.415 | 130,899 | 40,274 | 28,466 | 0.127 | 0.574 |
| Etanercept | 11.338 | 1.415 | 131,637 | 41,012 | 28,988 | 0.116 | 0.549 |
| Certolizumab pegol | 11.338 | 1.415 | 133,064 | 42,440 | 29,996 | 0.087 | 0.501 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 10.973 | – | 89,400 | – | – | – | – |
| Certolizumab pegol PAS | 12.527 | 1.554 | 128,760 | 39,361 | 25,324 | 0.120 | 0.781 |
| Adalimumab | 12.527 | 1.554 | 130,165 | 40,765 | 26,227 | 0.086 | 0.725 |
| Etanercept | 12.527 | 1.554 | 130,905 | 41,506 | 26,704 | 0.071 | 0.692 |
| Certolizumab pegol | 12.527 | 1.554 | 132,333 | 42,933 | 27,622 | 0.053 | 0.629 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.944 | – | 88,563 | – | – | – | – |
| Certolizumab pegol PAS | 11.382 | 1.437 | 129,592 | 41,030 | 28,282 | 0.068 | 0.612 |
| Adalimumab | 11.382 | 1.437 | 130,978 | 42,415 | 29,228 | 0.040 | 0.570 |
| Etanercept | 11.382 | 1.437 | 131,737 | 43,175 | 29,753 | 0.032 | 0.546 |
| Certolizumab pegol | 11.382 | 1.437 | 133,165 | 44,602 | 30,732 | 0.020 | 0.483 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.906 | – | 90,850 | – | – | – | – |
| Certolizumab pegol PAS | 11.291 | 1.386 | 130,974 | 40,124 | 28,958 | 0.102 | 0.576 |
| Adalimumab | 11.291 | 1.386 | 132,373 | 41,523 | 29,968 | 0.069 | 0.506 |
| Etanercept | 11.291 | 1.386 | 133,119 | 42,269 | 30,506 | 0.059 | 0.484 |
| Certolizumab pegol | 11.291 | 1.386 | 134,547 | 43,696 | 31,536 | 0.045 | 0.454 |
Non-radiographic axial spondyloarthritis scenario results: rebound to conventional care
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.998 | – | 88,600 | – | – | – | – |
| Certolizumab pegol PAS | 11.646 | 1.648 | 124,101 | 35,501 | 21,537 | 0.386 | 0.787 |
| Adalimumab | 11.646 | 1.648 | 125,519 | 36,919 | 22,397 | 0.342 | 0.746 |
| Etanercept | 11.646 | 1.648 | 126,246 | 37,646 | 22,839 | 0.318 | 0.733 |
| Certolizumab pegol | 11.646 | 1.648 | 127,674 | 39,074 | 23,705 | 0.267 | 0.712 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.989 | – | 87,391 | – | – | – | – |
| Certolizumab pegol PAS | 11.460 | 1.472 | 124,660 | 37,268 | 25,326 | 0.230 | 0.618 |
| Adalimumab | 11.460 | 1.472 | 126,073 | 38,682 | 26,287 | 0.193 | 0.590 |
| Etanercept | 11.460 | 1.472 | 126,805 | 39,414 | 26,784 | 0.182 | 0.573 |
| Certolizumab pegol | 11.460 | 1.472 | 128,232 | 40,841 | 27,754 | 0.163 | 0.542 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.891 | – | 91,602 | – | – | – | – |
| Certolizumab pegol PAS | 11.066 | 1.175 | 132,047 | 40,445 | 34,416 | 0.052 | 0.396 |
| Adalimumab | 11.066 | 1.175 | 133,456 | 41,854 | 35,615 | 0.036 | 0.348 |
| Etanercept | 11.066 | 1.175 | 134,192 | 42,590 | 36,241 | 0.031 | 0.330 |
| Certolizumab pegol | 11.066 | 1.175 | 135,620 | 44,017 | 37,456 | 0.026 | 0.290 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.948 | – | 90,402 | – | – | – | – |
| Certolizumab pegol PAS | 11.223 | 1.275 | 131,015 | 40,613 | 31,841 | 0.063 | 0.456 |
| Adalimumab | 11.223 | 1.275 | 132,416 | 42,014 | 32,940 | 0.047 | 0.415 |
| Etanercept | 11.223 | 1.275 | 133,160 | 42,758 | 33,523 | 0.040 | 0.395 |
| Certolizumab pegol | 11.223 | 1.275 | 134,587 | 44,185 | 34,642 | 0.027 | 0.337 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 10.975 | – | 90,413 | – | – | – | – |
| Certolizumab pegol PAS | 12.462 | 1.487 | 130,404 | 39,991 | 26,900 | 0.069 | 0.678 |
| Adalimumab | 12.462 | 1.487 | 131,817 | 41,404 | 27,850 | 0.050 | 0.599 |
| Etanercept | 12.462 | 1.487 | 132,549 | 42,136 | 28,343 | 0.042 | 0.572 |
| Certolizumab pegol | 12.462 | 1.487 | 133,976 | 43,563 | 29,303 | 0.028 | 0.498 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.953 | – | 89,196 | – | – | – | – |
| Certolizumab pegol PAS | 11.228 | 1.275 | 131,515 | 42,319 | 33,184 | 0.013 | 0.398 |
| Adalimumab | 11.228 | 1.275 | 132,901 | 43,704 | 34,270 | 0.007 | 0.353 |
| Etanercept | 11.228 | 1.275 | 133,661 | 44,464 | 34,866 | 0.002 | 0.332 |
| Certolizumab pegol | 11.228 | 1.275 | 135,088 | 45,891 | 35,985 | 0.001 | 0.284 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.963 | – | 90,219 | – | – | – | – |
| Certolizumab pegol PAS | 11.200 | 1.237 | 131,714 | 41,495 | 33,555 | 0.057 | 0.396 |
| Adalimumab | 11.200 | 1.237 | 133,109 | 42,890 | 34,684 | 0.038 | 0.343 |
| Etanercept | 11.200 | 1.237 | 133,859 | 43,640 | 35,290 | 0.035 | 0.318 |
| Certolizumab pegol | 11.200 | 1.237 | 135,286 | 45,067 | 36,444 | 0.029 | 0.284 |
Additional validation scenarios assuming same baselines for responders and non-responders
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.262 | – | 111,636 | – | – | – | – |
| Certolizumab pegol PAS | 8.317 | 1.054 | 126,238 | 14,601 | 13,851 | 0.803 | 0.975 |
| Golimumab | 8.317 | 1.054 | 127,917 | 16,281 | 15,444 | 0.732 | 0.958 |
| Adalimumab | 8.317 | 1.054 | 128,002 | 16,366 | 15,525 | 0.730 | 0.958 |
| Etanercept | 8.317 | 1.054 | 128,383 | 16,746 | 15,886 | 0.708 | 0.952 |
| Certolizumab pegol | 8.317 | 1.054 | 129,811 | 18,175 | 17,241 | 0.645 | 0.931 |
| Infliximab | 8.317 | 1.054 | 146,079 | 34,443 | 32,673 | 0.044 | 0.376 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 7.274 | – | 109,511 | – | – | – | – |
| Certolizumab pegol PAS | 7.958 | 0.684 | 127,164 | 17,654 | 25,809 | 0.277 | 0.632 |
| Golimumab | 7.958 | 0.684 | 128,850 | 19,339 | 28,273 | 0.183 | 0.554 |
| Adalimumab | 7.958 | 0.684 | 128,934 | 19,423 | 28,396 | 0.178 | 0.550 |
| Etanercept | 7.958 | 0.684 | 129,309 | 19,799 | 28,945 | 0.165 | 0.534 |
| Certolizumab pegol | 7.958 | 0.684 | 130,738 | 21,227 | 31,034 | 0.107 | 0.473 |
| Infliximab | 7.958 | 0.684 | 146,808 | 37,298 | 54,528 | 0.000 | 0.010 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 9.977 | – | 88,692 | – | – | – | – |
| Certolizumab pegol PAS | 11.551 | 1.574 | 125,205 | 36,513 | 23,199 | 0.390 | 0.759 |
| Adalimumab | 11.551 | 1.574 | 126,606 | 37,914 | 24,089 | 0.341 | 0.733 |
| Etanercept | 11.551 | 1.574 | 127,350 | 38,658 | 24,562 | 0.319 | 0.720 |
| Certolizumab pegol | 11.551 | 1.574 | 128,777 | 40,085 | 25,469 | 0.272 | 0.702 |
| Strategy | Total QALYs | Incremental QALYs | Total costs (£) | Incremental costs (£) | ICER (£) | Probability of CE £20,000 | Probability of CE £30,000 |
|---|---|---|---|---|---|---|---|
| Conventional therapy | 10.030 | – | 88,389 | – | – | – | – |
| Certolizumab pegol PAS | 11.391 | 1.361 | 126,116 | 37,727 | 27,721 | 0.218 | 0.617 |
| Adalimumab | 11.391 | 1.361 | 127,525 | 39,136 | 28,756 | 0.176 | 0.586 |
| Etanercept | 11.391 | 1.361 | 128,261 | 39,872 | 29,297 | 0.160 | 0.574 |
| Certolizumab pegol | 11.391 | 1.361 | 129,689 | 41,299 | 30,345 | 0.133 | 0.537 |
Glossary
- Adverse effect
- An abnormal or harmful effect caused by, and attributable to, exposure to a chemical (e.g. a drug), which is indicated by some result such as death, a physical symptom or visible illness. An effect may be classed as adverse if it causes functional or anatomical damage, causes irreversible change in the homeostasis of the organism or increases the susceptibility of the organism to other chemical or biological stress.
- Ankylosing spondylitis
- A rheumatic disease that affects the spine and may lead to some degree of stiffness in the back. As the inflammation reduces and healing takes place, bone grows out from both sides of the vertebrae and may join the two together; this stiffening is called ankylosis. If definite, changes to spinal and/or pelvic joints are present on plain radiographs.
- Articular
- Of or relating to the joints.
- Axial spondyloarthritis
- Refers to a form of arthritis in which the predominant symptom is back pain due to inflammation of spinal and/or pelvic joints. If definite changes on plain radiographs are present, the disease is classified as ankylosing spondylitis, but if they are absent the disease is classified as non-radiographic axial spondyloarthritis. Further tests may indicate that in some patients it is very likely that non-radiographic axial spondyloarthritis is ankylosing spondylitis, only at an earlier stage of disease.
- Between-study variance
- Between-study variance is a measure of statistical heterogeneity that depends on the scale of the outcome measured. It represents the variation in reported study effects over and above the variation expected given the within-study variation.
- Biologic therapies (synonym: biological)
- Medical preparations derived from living organisms. Includes anti-tumour necrosis factor drugs and other new drugs which target pathologically active T cells.
- Biosimilar
- An imitation biological medical product (such as an anti-tumour necrosis factor) usually marketed by a different manufacturer to the original biological product, once a patent has expired. The biosimilar should be similar to the original licensed product in terms of safety and efficacy.
- C-reactive protein
- Concentrations of this protein in the blood can be measured as a test of inflammation or disease activity, for example in ankylosing spondylitis and non-radiographic axial spondyloarthritis.
- Corticosteroid
- A synthetic hormone, similar to that produced naturally by the adrenal glands, which is available in pill, topical and injectable forms.
- Cost–benefit analysis
- An economic analysis that converts the effects or consequences of interventions into the same monetary terms as the costs and compares them using a measure of net benefit or a cost–benefit ratio.
- Cost-effectiveness analysis
- An economic analysis that expresses the effects or consequences of interventions on a single dimension. This would normally be expressed in ‘natural’ units (e.g. cases cured, life-years gained or additional strokes prevented). The difference between interventions in terms of costs and effects is typically expressed as an incremental cost-effectiveness ratio (e.g. the incremental cost per life-year gained).
- Cost–utility analysis
- The same as a cost-effectiveness analysis but the effects or consequences of interventions are expressed in generic units of health gain, usually quality-adjusted life-years.
- Credible interval
- In Bayesian statistics, a credible interval is a posterior probability interval estimation which incorporates problem-specific contextual information from the prior distribution. Credible intervals are used for the purposes similar to those of confidence intervals in frequentist statistics.
- Disease-modifying antirheumatic drugs
- Disease-modifying antirheumatic drugs are drugs capable of modifying the progression of rheumatic disease. The term is, however, applied to what are now considered to be traditional disease-modifying drugs, in particular sulphasalazine, methotrexate and ciclosporin, as well as azathioprine, cyclophosphamide, antimalarials, penicillamine and gold. The newer agent leflunomide may be included as a disease-modifying antirheumatic drug. The biologics such as etanercept and infliximab are not generally referred to as disease-modifying antirheumatic drugs.
- Erythrocyte sedimentation rate
- One of the tests designed to measure the degree of inflammation.
- Fixed-effect model
- A statistical model that stipulates that the units under analysis (e.g. people in a trial or study in a meta-analysis) are the ones of interest, and thus constitute the entire population of units. Only within-study variation is taken to influence the uncertainty of results (as reflected in the confidence interval) of a meta-analysis using a fixed-effect model.
- Heterogeneity
- In systematic reviews heterogeneity refers to variability or differences between studies in the estimates of effects. A distinction is sometimes made between ‘statistical heterogeneity’ (differences in the reported effects), ‘methodological heterogeneity’ (differences in study design) and ‘clinical heterogeneity’ (differences between studies in key characteristics of the participants, interventions or outcome measures).
- I-squared (I2)
- A measure of ‘statistical heterogeneity’ (differences in the reported effects). It varies between 0 and 1, for which 0 indicates that the differences in reported effects are entirely consistent with the within-study uncertainty and 1 indicates that the differences in reported effects are entirely explained by study characteristics that vary across studies.
- Intention to treat
- An intention-to-treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether or not they received it.
- Monoclonal antibody
- An antibody produced in a laboratory from a single clone that recognises only one antigen.
- Non-radiographic axial spondyloarthritis
- Axial spondyloarthritis for which definite changes to spinal and/or pelvic joints on plain radiographs are not present. Further tests may indicate that in some patients it is very likely that non-radiographic axial spondyloarthritis ankylosing spondylitis, only at an earlier stage of disease.
- Non-steroidal anti-inflammatory drugs
- Consists of a large range of drugs of the aspirin family, prescribed for different kinds of arthritis which reduce inflammation and control pain, swelling and stiffness.
- Open-label study
- A type of study in which both participants and researchers know which treatment is being administered.
- Placebo
- An inactive substance or procedure administered to a patient, usually to compare its effects with those of a real drug or other intervention but sometimes for the psychological benefit to the patient through a belief that s/he is receiving treatment.
- Quality-adjusted life-year
- An index of health gain in which survival duration is weighted or adjusted by the patient’s quality of life during the survival period. Quality-adjusted life-years have the advantage of incorporating changes in both quantity (mortality) and quality (morbidity) of life.
- Quality of life
- A concept incorporating all the factors that might impact on an individual’s life, including factors such as the absence of disease or infirmity as well as other factors which might affect their physical, mental and social well-being.
- Random-effects model
- A statistical model sometimes used in meta-analysis in which both within-study sampling error (variance) and between-studies variation are included in the assessment of the uncertainty (confidence interval) of the results of a meta-analysis.
- Randomised controlled trial (synonym: randomised clinical trial)
- An experiment in which investigators randomly allocate eligible people into intervention groups to receive, or not to receive, one or more interventions that are being compared.
- Relative risk (synonym: risk ratio)
- The ratio of risk in the intervention group to the risk in the control group. The risk (proportion, probability or rate) is the ratio of people with an event in a group to the total in the group. A relative risk of one indicates no difference between comparison groups. For undesirable outcomes, a relative risk that is less than one indicates that the intervention was effective in reducing the risk of that outcome.
- Sensitivity analysis
- An analysis used to determine how sensitive the results of a study or systematic review are to changes in how it was done. Sensitivity analyses are used to assess how robust the results are to uncertain decisions or assumptions about the data and the methods that were used.
- Tumour necrosis factor
- One of the cytokines, or messengers, known to be involved in the process of systemic inflammation.
- Weighted mean difference (in meta-analysis)
- A method of meta-analysis used to combine measures on continuous scales, where the mean, standard deviation and sample size in each group are known. The weight given to each study is determined by the precision of its estimate of effect and is equal to the inverse of the variance. This method assumes that all of the trials have measured the outcome on the same scale.
List of abbreviations
- AE
- adverse event
- AiC
- academic in confidence
- AS
- ankylosing spondylitis
- ASAS
- Assessment in Ankylosing Spondylitis
- ASDAS
- Ankylosing Spondylitis Disease Activity Score
- ASQoL
- Ankylosing Spondylitis Quality of Life
- ASSERT
- Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy
- ATLAS
- Adalimumab Trial Evaluating Long-term Efficacy and Safety for Ankylosing Spondylitis
- ATP
- adalimumab target population
- axSpA
- axial spondyloarthritis
- BASDAI
- Bath Ankylosing Spondylitis Disease Activity Index
- BASFI
- Bath Ankylosing Spondylitis Functional Index
- BASMI
- Bath Ankylosing Spondylitis Metrology Index
- BSR
- British Society for Rheumatology
- BSRBR
- British Society for Rheumatology Biologics Register
- CC
- conventional care
- CI
- confidence interval
- CiC
- commercial in confidence
- CrI
- credible interval
- CRP
- C-reactive protein
- DES
- discrete event simulation
- DIC
- deviance information criterion
- DMARD
- disease-modifying antirheumatic drug
- DSU
- Decision Support Unit
- EQ-5D
- European Quality of Life-5 Dimensions
- ERG
- Evidence Review Group
- ESR
- erythrocyte sedimentation rate
- FDA
- Food and Drug Administration
- GESPIC
- German Spondyloarthritis Inception Cohort
- HLA
- human leucocyte antigen
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HUI
- Health Utilities Index
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LOCF
- last observation carried forward
- LRiG
- Liverpool Reviews and Implementation Group
- MAIC
- match-adjusted indirect comparison
- MASES
- Maastricht Ankylosing Spondylitis Enthesitis Score
- MCID
- minimum clinically important difference
- mCMC
- Markov chain Monte Carlo
- MCS
- mental component summary
- mITT
- modified intention to treat
- MRI
- magnetic resonance imaging
- mSASSS
- modified Stoke Ankylosing Spondylitis Spinal Score
- MTC
- mixed treatment comparison
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NNH
- number needed to harm
- nr-AxSpA
- non-radiographic axial spondyloarthritis
- NRI
- non-responder imputation
- NSAID
- non-steroidal anti-inflammatory drug
- OASIS
- Outcomes in Ankylosing Spondylitis International Study
- OMERACT
- Outcome Measures in Rheumatology
- OR
- odds ratio
- PAS
- patient access scheme
- PCS
- physical component summary
- PSA
- probabilistic sensitivity analysis
- PSS
- personal social services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RLDQ
- Revised Leeds Disability Questionnaire
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- SIRAS
- Scotland and Ireland Registry for Ankylosing Spondylitis
- SMART
- St Mary RheumaToid Arthritis
- SMR
- standardised mortality ratio
- SpA
- spondyloarthritis
- TA
- technology appraisal
- TNF
- tumour necrosis factor
- VAS
- visual analogue scale
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed academic-in-confidence and commercial-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of academic-in-confidence data and commercial-in-confidence removed and replaced by the statement ‘academic-in-confidence and/or commercial-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.