Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 12/42/01. The protocol was agreed in July 2013. The assessment report began editorial review in March 2014 and was accepted for publication in October 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Crathorne et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Aim of the review
The aim of this assessment was to review and update research evidence as necessary to inform National Institute for Health and Care Excellence (NICE) guidance to the NHS in England and Wales on the clinical effectiveness and cost-effectiveness of erythropoiesis-stimulating agents (ESAs) for the treatment of cancer treatment-induced anaemia (CIA) (see Current service provision).
The previous guidance [technology appraisal (TA)1421] was primarily based on evidence presented to NICE in the assessment report by Wilson and colleagues. 2 We have incorporated relevant evidence presented in the previous report and report new evidence gathered since 2004.
Description of the health problem
Anaemia is defined as ‘a reduction of the haemoglobin (Hb) concentration, red blood cell (RBC) count, or packed cell volume below normal levels’ (p. v244). 3 A commonly used classification of anaemia according to Hb level is shown in Table 1. 3
| Severity | WHO, Hb level (g/dl) | NCI, Hb level (g/dl) |
|---|---|---|
| Grade 0 (WNL) | ≥ 11 | WNL |
| Grade 1 (mild) | 9.5–10.9 | > 10 WNL |
| Grade 2 (moderate) | 8.0–9.4 | 8–10 |
| Grade 3 (serious/adverse) | 6.5–7.9 | 6.5–7.9 |
| Grade 4 (life-threatening) | < 6.5 | < 6.5 |
It is the most frequent haematological manifestation in patients with cancer; > 50% of all cancer patients will be anaemic, regardless of the treatment received, and approximately 20% of all patients undergoing chemotherapy will require a red blood cell transfusion (RBCT). 4
The cause of anaemia is usually multifactorial and may be patient, disease or treatment related. 4 The haematological features in anaemic patients depend on the different types of malignant disease, stage and duration of the disease, the regimen and intensity of tumour therapy and possible intercurrent infections or surgical interventions. Tumour-associated factors, such as tumour bleeding, haemolysis and deficiency in folic acid and vitamin B12, can be acute or chronic. In the advanced stages of haematological malignancy, bone marrow involvement often leads to progressive anaemia. In addition, interaction between tumour cell populations and the immune system can lead to the release of cytokines, especially interferon-gamma, interleukin-1 and tumour necrosis factor. This disrupts endogenous erythropoietin synthesis in the kidney and suppresses differentiation of erythroid precursor cells in the bone marrow. As a result, patients with tumour anaemia may have relatively low levels of erythropoietin for the grade of anaemia observed. Moreover, activation of macrophages can lead to a shorter erythrocyte half-life and a decrease in iron utilisation.
Chemotherapy may cause both transient and sustained anaemia. 4 Mechanisms of drug-induced anaemia in patients with cancer include stem cell death, blockage or delay of haematopoietic factors, oxidant damage to mature haematopoietic cells, long-term myelodysplasia and immune-mediated haematopoietic cell destruction. 4 Patients treated with platinum-based regimens develop anaemia most often and frequently need transfusions. 4 As a consequence, dose-intensified regimens or shortened treatment intervals, as well as multimodal therapies, are associated with a higher degree of anaemia. 4 Anaemia can also compromise the effect of treatment because low tissue oxygenation is associated with a reduced sensitivity of tumours to radiation and some forms of chemotherapy, contributing to the progression of cancer and reduction in survival. 4
Among those patients with solid tumours, the incidence of anaemia is highest in patients with lung cancer (71%) or gynaecological cancer (65%); these patients have the highest frequency of anaemia and the highest rate of transfusion requirements. 4,5 The frequency of RBCT requirements in these patients varies from 47% to 100% depending on the cumulative dose of platinum chemotherapy received and other risk factors, for example age, disease stage and pretreatment Hb level. In haematological cancers, anaemia is an almost invariable feature of the disease. 4 In addition, some of the newer chemotherapeutic agents, such as taxanes or vinorelbine, are strongly myelosuppressive and frequently cause anaemia. 6
The clinical manifestation and severity of anaemia can vary considerably among individual patients. 4 Mild-to-moderate anaemia can typically cause such symptoms as headache, palpitations, tachycardia and shortness of breath. 4 Chronic anaemia can result in severe organ damage affecting the cardiovascular system, immune system, lungs, kidneys and the central nervous system. 4 In addition to physical symptoms, the subjective impact of cancer-related anaemia on quality of life, mental health and social activities may be substantial. 4 A common anaemia-related problem is fatigue, which impairs the patient’s ability to perform normal daily activities. 4
Relationship between cancer treatment-induced anaemia and survival
Although the evidence is uncertain, some researchers hypothesise that anaemia in cancer patients is associated with a worse prognosis. According to Bohlius and colleagues,7 one explanation may be that, as a result of a low Hb level, the tumour cells become hypoxic and are subsequently less sensitive to cytotoxic drugs, in particular oxygen-dependent chemotherapies. 8–10 Evidence for this, as reported in the study by Tonia and colleagues,11 exists in studies in which tumour control and overall survival (OS) are improved in solid tumour patients with better tumour oxygenation. 10,12 There is also the practical implication that severe anaemia may require a dose reduction or delay of chemotherapy, subsequently leading to a poorer outcome. It is therefore plausible that efforts taken to reduce anaemia may improve tumour response and OS. 7 That said, it should be noted that Hb levels elevated to > 14 g/dl in women and > 15 g/dl in men are undesirable and may lead to increased viscosity, impaired tumour oxygenation and thromboembolic events. 13
As an intervention used to increase Hb, and by association improve prognosis, some studies actually report a detrimental effect of ESAs on survival and tumour progression. 14–20 This effect is postulated to be caused by the presence of erythropoietin receptors on various cancers,21–25 whereby the endogenously produced or exogenously administered erythropoietin promotes the proliferation and survival of erythropoietin receptor-expressing cancer cells. 7 However, controversy about the functionality of these receptors remains26–30 and several studies show no effect on tumour progression for patients receiving ESAs. 17,31–33
It should be noted that the majority of the studies examined in the systematic reviews by Bohlius and colleagues7 and Tonia and colleagues11 used a wide range of administration frequencies and dosages of ESAs (generally exceeding the licence), which may result in an increase in adverse events (AEs) and mortality. This knowledge, along with the generally poor reporting and data omission on factors such as tumour stage and method of assessment, led to the conclusion by Tonia and colleagues11 that no clear evidence was found to either exclude or prove a tumour-promoting effect of ESAs.
Current management
Red blood cell transfusions
Anaemia in cancer patients can be treated with RBCTs, with 15% of people with solid tumours treated with RBCTs. 34
Different cut-off values are used for transfusions, depending on clinical symptoms and patient characteristics, with a Hb level of < 9 g/dl commonly used. 34 After administration of 1 unit of RBCs, the Hb level rises by 1 g/dl, with the lifespan of transfused RBCs being 100–110 days. Complications related to RBCT are procedural problems, iron overload, viral and bacterial infections and immune injury. 34
Erythropoietin-stimulating agents
Erythropoietin is an acidic glycoprotein hormone. Approximately 90% of the hormone is synthesised in the kidney and 10% is synthesised in the liver. Erythropoietin is responsible for regulating RBC production. Erythropoietin for clinical use is produced by recombinant DNA technology. 1
Exogenously administered erythropoietin is used to shorten the period of symptomatic anaemia in patients receiving cytotoxic chemotherapy. It is used in addition to, rather than as a complete replacement for, the existing treatments. Blood transfusion, in particular, may still be needed. 1
Marketing authorisations: haemoglobin levels
Initially, all ESAs were recommended for use at Hb levels of ≤ 11 g/dl, with target Hb levels not exceeding 13 g/dl. However, because of data showing a consistent, unexplained, excess mortality in cancer patients with anaemia treated with ESAs, a safety review of all available data on ESA treatment of patients with CIA was conducted in 2008 by the Pharmacovigilance Working Party at the request of the Committee for Medicinal Products for Human Use. As a result of this safety review, the European Medicines Agency (EMA) requested that the Summary of Product Characteristics (SPCs) for all ESAs be changed to highlight that ESAs should be used only if anaemia is associated with symptoms; to establish a uniform target Hb range for all ESAs; to mention the observed negative benefit risk balance in patients treated with high target Hb concentrations; and to include the relevant results of the trials triggering the safety review. SPCs for all ESAs were therefore revised in 2008 to decrease the Hb value for treatment initiation to ≤ 10 g/dl and to amend Hb treatment target values to 10–12 g/dl and Hb levels for stopping treatment to > 13 g/dl.
The EMA labels the use of ESAs as follows:
-
in patients treated with chemotherapy and with a Hb level of ≤ 10 g/dl, treatment with ESAs might be considered to increase Hb (to within the target range of 10–12 g/dl) or to prevent further decline in Hb
-
in patients not treated with chemotherapy, there is no indication for the use of ESAs and there might be an increased risk of death when ESAs are administered to a target Hb level of 12–14 g/dl
-
in patients with curative intent, ESAs should be used with caution.
These changes to the licence (Table 2) were introduced subsequent to the previous NICE appraisal.
| Pre 2008 | 2008 onwards |
|---|---|
|
|
Details of current licence recommendations are summarised in Table 3.
| Product characteristics | Epoetin alfa, epoetin zeta | Epoetin beta | Epoetin theta | Darbepoetin alfa |
|---|---|---|---|---|
| Manufacturer (product) | Janssen-Cilag Ltd (Eprex®),35 Sandoz Ltd (Binocrit®),36 Hospira UK Ltd (Retacrit®)37 | Roche Products Ltd (NeoRecormon®)38 | Teva Pharmaceuticals Ltd (Eporatio®)39 | Amgen Inc. (Aranesp®)40 |
| Marketing authorisation | Treatment of anaemia and reduction of RBCT requirements in adults receiving chemotherapy for solid tumours, malignant lymphoma or multiple myeloma, who are at risk of transfusion as assessed by their general status (e.g. cardiovascular status, pre-existing anaemia at the start of chemotherapy) | Treatment of symptomatic anaemia in adults with non-myeloid malignancies receiving chemotherapy | ||
| Starting Hb level | ≤ 10 g/dl | ≤ 10 g/dl | ≤ 10 g/dl | ≤ 10 g/dl |
| Target Hb level | 10–12 g/dl | 10–12 g/dl | 10–12 g/dl | 10–12 g/dl |
| Initial treatment | 150 IU/kg SC TIW (or 450 IU/kg SC QW) | 150 IU/kg SC TIW (or 450 IU/kg SC QW) | 20,000 IU/QW | 2.25 µg/kg SC QW [or 500 µg (6.75 µg/kg) SC Q3W] |
| Dose increase | 4 weeks Hb increase < 1 g/dl and reticulocyte increase ≥ 40,000 cells/µl dose is doubled to 300 IU/kg TIW or 900 IU/kg QW | 300 IU/kg SC TIW | 4 weeks Hb increase < 1 g/dl dose is doubled to 40,000 IU/QW; if Hb increase insufficient at 8 weeks increase to 60,000 IU/QW | Not specified |
| Dose reduction | If Hb increases by ≥ 2 g/dl: 25–50%; if Hb > 12 g/dl: 25–50% | If Hb > 12 g/dl or increase is > 2 g/dl in 4 weeks: 25–50% | If Hb increases by ≥ 2 g/dl: 25–50%; if Hb ≥ 12 g/dl: 25–50% | |
| Dose withholding | If Hb > 13 g/dl, until 12 g/dl reinitiate at 25% lower dose | If Hb > 13 g/dl, until 12 g/dl reinitiate at 25% lower dose | If Hb > 13 g/dl, until 12 g/dl reinitiate at 25% lower dose | |
Current service provision
National Institute for Health and Care Excellence guidance (TA142)1 currently recommends ESAs in combination with intravenous iron as an option for:
-
the management of CIA in women receiving platinum-based chemotherapy for ovarian cancer who have symptomatic anaemia with a Hb level of ≤ 8 g/dl. The use of ESAs does not preclude the use of existing approaches to the management of anaemia, including blood transfusion when necessary
-
people who cannot be given blood transfusions and who have profound cancer treatment-related anaemia that is likely to have an impact on survival.
When indicated, the ESA used should be the one with the lowest acquisition cost.
Description of the technologies under assessment
Several short- and long-acting ESAs are available, including epoetin alfa, epoetin beta and darbepoetin beta. Since the last appraisal (2004) [the Health Technology Assessment (HTA) monograph relating to this was published in 20072], an additional two ESAs have become available: epoetin theta and epoetin zeta. All are administered by subcutaneous injection. This technology assessment report will consider six pharmaceutical interventions: epoetin alfa (Eprex®, Janssen-Cilag Ltd; Binocrit®, Sandoz Ltd), epoetin beta (NeoRecormon®, Roche Products Ltd), epoetin theta (Eporatio®, Teva Pharmaceuticals Ltd), epoetin zeta (Retacrit®, Hospira UK Ltd) and darbepoetin alfa (Aranesp®, Amgen Inc.). 1 Two of the six ESAs, Binocrit and Retacrit, are biosimilars of epoetin alfa. A ‘biosimilar’ medicine is similar to a biological medicine (the ‘reference medicine’) that is already authorised in the European Union and contains a similar active substance to the reference medicine. The reference medicine for both Binocrit and Retacrit is Eprex/Erypo®, which contains epoetin alfa. Unlike generic medicines, biosimilars are similar but not identical to the original biological medicine. 41,42 Treatment recommendations according to licence are summarised for each pharmaceutical intervention in Table 3.
This NICE appraisal focuses on the treatment of CIA. As such, the appraisal does not cover all aspects of the licensed indications, such as the prevention of anaemia or the treatment of symptomatic anaemia as a result of chronic renal failure.
Clinical guidelines
European Organisation for Research and Treatment of Cancer
In Europe, treatment guidelines for CIA have been formulated by the European Organisation for Research and Treatment of Cancer (EORTC), who most recently updated its recommendations on the use of ESAs in September 2007. 41 In 2010, joint treatment guidelines were issued by American Society of Clinical Oncology/American Society of Hematology (ASCO/ASH). 42
The EORTC guidelines recommend that patients whose Hb level is < 9 g/dl should be assessed for the need for RBCT in addition to ESAs. 41 The joint ASCO/ASH guidelines suggest that RBCT is also an option for patients with CIA and a Hb level of < 10 g/dl, depending on the severity of the anaemia or clinical circumstances, and may also be warranted by clinical conditions in patients with a Hb level of ≥ 10 g/dl but < 12 g/dl. 42
Recommendations for ESA therapy for CIA are broadly similar between the EORTC guidelines and the joint ASCO/ASH guidelines, with small differences in the threshold for initiation of ESA therapy and variation in the wording related to Hb levels. 41,42
The EORTC guidelines41 emphasise that reducing the need for RBCT is a major goal of therapy in anaemic cancer patients and highlight that ESAs can achieve a sustained increase in Hb levels, unlike intermittent transfusions. The guidelines also state that there is no evidence that oral iron supplements increase the response to erythropoietic proteins, although there is evidence of a better response to erythropoietic proteins with intravenous iron.
British Columbia Cancer Agency
The British Columbia Cancer Agency (BCCA) guidelines recommend treatment with ESAs for the treatment of CIA when the Hb level is 10 g/dl and there is a minimum of 2 months of planned chemotherapy. 43
The guidelines also state that the benefits of treatment must be weighed against the possible risks for individual patients: ESAs may increase the risk of death, serious cardiovascular events, thromboembolic events and stroke and they may shorten survival and/or increase the risk of tumour progression or recurrence, as shown in clinical trials in patients with breast, head and neck, lymphoid, cervical non-small-cell lung cancers and patients with active malignancies who are not treated with either chemotherapy or radiotherapy. 43
Existing evidence
Existing systematic reviews of effectiveness
There have been a number of well-conducted systematic reviews evaluating the effects of ESAs for treating CIA in cancer patients. We identified 11 systematic reviews (reported in 14 publications) that fulfilled the definition of a systematic review prespecified in the protocol; a summary of the eligible systematic reviews and a quality assessment [compared with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement44] is provided in Appendix 1.
Cochrane review
The Cochrane review by Tonia and colleagues11 was the most recent and authoritative review. The Cochrane review’s conclusions were that ESAs reduce the need for RBCTs but increase the risk for thromboembolic events and deaths. ESAs may improve quality of life but the effect of ESAs on tumour control is uncertain. The review concluded that ‘Further research is needed to clarify cellular and molecular mechanisms and pathways of the effects of ESAs on thrombogenesis and their potential effects on tumour growth (p. 2). 11
This was an update of a Cochrane review first published in 2004. 7 Searches were conducted in the Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, MEDLINE and other databases. Searches were carried out for the periods January 1985 to December 2001 for the first review, January 2002 to April 2005 for the first update and up to November 2011 for the most recent update. The authors of the review also contacted experts in the field and pharmaceutical companies [for access to individual patient data (IPD)]. Inclusion, quality assessment and data abstraction were undertaken in duplicate by several reviewers. Eligibility criteria are detailed and compared with those in the Peninsula Technology Assessment Group (PenTAG) review in Table 4. The Cochrane review differed from the PenTAG review in respect of the population (cancer-related anaemia vs. chemotherapy-induced anaemia) and the intervention [all ESAs irrespective of licence vs. ESAs within licence (defined based on start dose)].
| Criteria | Tonia and colleagues11 | Current systematic review |
|---|---|---|
| Population | Patients diagnosed with malignant disease (using clinical and histological/cytological criteria) and at risk of transfusion as assessed by their general status (e.g. cardiovascular status, pre-existing anaemia at the start of chemotherapy). Excluded trials in which > 80% of participants were diagnosed with an acute leukaemia | Patients had to be receiving chemotherapy for solid tumours, malignant lymphoma, multiple myeloma or non-myeloid malignancies and at risk of transfusion as assessed by their general status (e.g. cardiovascular status, pre-existing anaemia at the start of chemotherapy) |
| Intervention | ESAs to prevent or reduce anaemia, given singly or concomitantly with chemotherapy, radiotherapy or combination therapy | ESAsa to prevent or reduce anaemia, given concomitantly with chemotherapy |
| Dose: included studies or study arms with low doses | Dose: licensed dose defined by start dose even if it did not align with other criteria specified by the licence | |
| Comparator | Placebo or ‘no treatment’ was not required for inclusion but was considered in evaluating study quality | Placebo, standard care, no treatment/usual care |
| Outcomes | HaemR,b Hb change, RBCT, RBC units, OS, mortality, tumour response (CR), AEs, HRQoL | HaemR,b Hb change, RBCT, RBC units, OS, tumour response (CR), AEs, HRQoL |
| Study design | RCTs | RCTs, SRs of RCTsc |
A total of 91 studies with 20,102 participants were included in the Cochrane review by Tonia and colleagues. 11 The results from the Cochrane review are summarised in Table 5 and compared with the results of the PenTAG HTA review throughout Chapter 3.
| Outcomes measured | Results |
|---|---|
| Anaemia-related outcomes | |
| Hb changea | WMD 1.57, 95% CI 1.51 to 1.62; χ2(het) = 564.37, df = 74; p < 0.001 75 trials, n = 11,609 |
| HaemRb | RR 3.39, 95% CI 3.10 to 3.71; χ2(het) = 95.56, df = 45; p < 0.001 46 trials, n = 6413 |
| RBCT | RR 0.65, 95% CI 0.62 to 0.68; χ2(het) = 217.08, df = 87; p < 0.001 88 trials, n = 16,093 |
| Units transfused | WMD –0.98, 95% CI –1.17 to –0.78; χ2(het) = 34.52, df = 24; p = 0.080 25 trials, n = 4715 |
| Malignancy-related outcomes | |
| Tumour response | RR 1.02, 95% CI 0.98 to 1.06; χ2(het) = 16.10, df = 18; p = 0.59 19 trials, n = 5012 |
| OS | HR 1.05, 95% CI 1.00 to 1.11; χ2(het) = 95.40, df = 75; p = 0.060 80 trials, n = 19,003 |
| Mortality | HR 1.17, 95% CI 1.06 to 1.29; χ2(het) = 59.49, df = 63; p = 0.600 64 trials, n = 14,179 |
| Safety-related outcomes | |
| Thromboembolic events | RR 1.52, 95% CI 1.34 to 1.74; χ2(het) = 34.99, df = 55; p = 0.980 60 trials, n = 15,498 |
| Hypertension | RR 1.30, 95% CI 1.08 to 1.56; χ2(het) = 26.87, df = 34; p = 0.800 35 trials, n = 7006 |
| Thrombocytopenia/haemorrhage | RR 1.21, 95% CI 1.04 to 1.42; χ2(het) = 14.50, df = 20; p = 0.800 21 trials, n = 4220 |
| Seizures | RR 0.77, 95% CI 0.42 to 1.41; χ2(het) = 6.19, df = 6; p = 0.400 7 trials, n = 2790 |
| Pruritus | RR 1.49, 95% CI 0.99 to 2.24; χ2(het) = 13.18, df = 15; p = 0.590 16 trials, n = 4346 |
| HRQoL-related outcomes | |
| FACT-F 13 items (score 0–52) | MD 2.08, 95% CI 1.43 to 2.72; χ2(het) = 36.48, df = 17; p = 0.004 18 trials, n = 4965 |
| Any subgroup effect | Yes: imputed vs. non-imputed data, baseline Hb level, type of anticancer therapy, duration of ESA treatment and ITT analysis |
Cochrane review: meta-analysis based on individual patient data
Another Cochrane review7 examined the effect of ESAs and identified factors that modify the effects of ESAs on OS, progression-free survival (PFS) and thromboembolic and cardiovascular events, as well as the need for transfusions and other important safety and efficacy outcomes in cancer patients. It concluded that ‘ESA treatment in cancer patients increased on study mortality and worsened OS. For patients undergoing chemotherapy the increase was less pronounced, but an adverse effect could not be excluded’ (p. 2).
The review was conducted in 2009. Searches were conducted in The Cochrane Library, MEDLINE, EMBASE and conference proceedings for eligible trials and manufacturers of ESAs were contacted to identify additional trials. The review included randomised controlled trials (RCTs) comparing ESAs plus RBCT (as necessary) with RBCT (as necessary) alone to prevent or treat anaemia in adult or paediatric cancer patients with or without concurrent antineoplastic therapy. Inclusion, quality assessment and data abstraction were undertaken in duplicate by several reviewers. A meta-analysis of RCTs was conducted and patient-level data were obtained and analysed by independent statisticians.
A total of 13,933 cancer patients from 53 trials were analysed; 1530 patients died on study and 4993 died overall. ESAs increased on-study mortality [combined hazard ratio (cHR) 1.17; 95% confidence interval (CI) 1.06 to 1.30] and worsened OS (cHR 1.06; 95% CI 1.00 to 1.12), with little heterogeneity between trials (I2 = 0%, p = 0.87, and I2 = 7.1%, p = 0.33 respectively). Thirty-eight trials enrolled 10,441 patients receiving chemotherapy (Table 6). The cHR for on-study mortality was 1.10 (95% CI 0.98 to 1.24) and that for OS was 1.04 (95% CI 0.97 to 1.11). There was little evidence of a difference between trials of patients receiving different cancer treatments (p-value for interaction = 0.42).
| Outcomes measured | Results |
|---|---|
| Malignancy-related outcomes | |
| OS | cHR 1.04, 95% CI 0.97 to 1.11 38 trials, n = 10,441 |
| On-study mortality | cHR 1.10, 95% CI 0.98 to 1.24 38 trials, n = 10,441 |
Previous Health Technology Assessment review
The previous HTA review (Wilson and colleagues2) informed NICE guidance (TA1421). It assessed the effectiveness and cost-effectiveness of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment. The review concluded that ESAs are effective in improving the haematological response and reducing RBCT requirements, but that the effect on health-related quality of life (HRQoL) is uncertain and the incidence of side effects and the effect on survival are highly uncertain. If there is no effect on survival it seems highly unlikely that ESAs would be considered a cost-effective use of health-care resources.
Using the Cochrane review45 published in 2004 as the start point, Wilson and colleagues2 conducted a systematic review of RCTs comparing ESAs with standard care. MEDLINE, EMBASE, The Cochrane Library and other databases were searched from 2000 (1996 in the case of darbepoetin alfa) to September 2004. Inclusion, quality assessment and data abstraction were undertaken in duplicate. Eligibility criteria are detailed and compared with those of the PenTAG review in Table 7. When possible, meta-analysis was employed. The economic assessment consisted of a systematic review of past economic evaluations, an assessment of economic models submitted by the manufacturers of the three ESAs and development of a new individual sampling model (see Chapter 4, Wilson and colleagues: summary).
| Eligibility criteria | Wilson and colleagues2 | Current systematic review |
|---|---|---|
| Population | Patients diagnosed with malignant disease (using clinical and histological/cytological criteria) and at risk of transfusion as assessed by the patient’s general status (e.g. cardiovascular status, pre-existing anaemia at the start of chemotherapy) | Patients had to be receiving chemotherapy for solid tumours, malignant lymphoma, multiple myeloma or non-myeloid malignancies and be at risk of transfusion as assessed by the patient’s general status (e.g. cardiovascular status, pre-existing anaemia at the start of chemotherapy) |
| Intervention | ESAs to prevent or reduce anaemia, given singly or concomitantly with chemotherapy, radiotherapy or combination therapy | ESAsa to prevent or reduce anaemia, given concomitantly with chemotherapy |
| Dose: included studies or study arms with low doses | Dose: licensed dose, defined by start dose, even if studies did not align with other criteria specified by the licence | |
| Comparator | Placebo or ‘no treatment’ was not required for inclusion but was considered in evaluating study quality | Placebo, standard care, no treatment/usual care |
| Outcomes | HaemR,b Hb change, RBCT, RBC units, OS, mortality, tumour response (CR), AEs, HRQoL | HaemR,b Hb change, RBCT, RBC units, OS, tumour response (CR), AEs, HRQoL |
| Study design | RCTs | RCTs, SRs of RCTsc |
A total of 46 RCTs were included in the review, 27 of which had been included in the Cochrane review. 7 All 46 studies compared ESA plus supportive care for anaemia (including transfusions) with supportive care for anaemia (including transfusions alone). Outcomes assessed were anaemia-related outcomes (haematological response, Hb change, RBCT requirements), malignancy-related outcomes (tumour response and OS), HRQoL and AEs.
Results from the previous HTA review2 (Table 8) are compared with the results of the PenTAG review throughout Chapter 3.
| Outcomes measured | Results |
|---|---|
| Anaemia-related outcomes | |
| Hb changea | WMD 1.63, 95% CI 1.46 to 1.80; χ2(het) = 23.74, df = 19; p = 0.21 10 trials, n = 1620 |
| HaemRb | RR 3.40, 95% CI 3.01 to 3.83; χ2(het) = 23.60, df = 32; p = 0.86 21 trials, n = 3740 |
| RBCT | RR 0.63, 95% CI 0.58 to 0.67; χ2(het) = 94.75, df = 48; p = 0.001 35 trials, n = 5564 |
| Units transfused | WMD –1.05, 95% CI –1.32 to –0.78; χ2(het) = 8.96, df = 16; p = 0.91 14 trials, n = 2353 |
| Malignancy-related outcomes | |
| Tumour response | RR 1.31, 95% CI 1.08 to 1.60; χ2(het) = NR; df = NR; p = NR 9 trials, n = 1260 |
| OS | HR 1.03, 95% CI 0.92 to 1.16; χ2(het) = 37.74, df = 27; p = 0.08 28 trials, n = 5308 |
| Mortality | NR |
| Safety-related outcomes | No safety-related meta-analysis |
| HRQoL-related outcomes | No HRQoL meta-analyses |
Key points
-
Anaemia is defined as a deficiency in RBCs. It is the most frequent haematological manifestation in patients with cancer; > 50% of all cancer patients will be anaemic, regardless of the treatment received, and approximately 20% of all patients undergoing chemotherapy will require a RBCT. The cause is multifactorial: patient, disease or treatment related.
-
Anaemia is associated with many symptoms, all of which affect quality of life. These symptoms include dizziness, shortness of breath on exertion, palpitations, headache and depression. Severe fatigue is probably the most commonly reported symptom and can lead to an inability to perform everyday tasks. However, fatigue in people with cancer can also have other causes, for example the disease itself, chemotherapy, radiotherapy, anxiety or depression.
-
Many people are anaemic when cancer is diagnosed, before any cancer treatment starts. The degree of anaemia caused by treatments such as chemotherapy often fluctuates depending on the nature of the treatment and the number of courses administered, but is typically at its worst 2–4 weeks after chemotherapy is given. Once cancer treatments are stopped, a period of ‘normalisation’ is likely, during which the Hb may return to pretreatment levels.
-
Options available for the management of CIA include adjustments to the cancer treatment regimen, iron supplementation and blood transfusion. The majority of people who become anaemic do not receive any treatment for their anaemia, but those who become moderately or severely anaemic are usually given blood transfusions. Complications related to RBCT include procedural problems, iron overload, viral and bacterial infections and immune injury.
-
Current evidence suggests that ESAs reduce the need for RBCT but increase the risk of thromboembolic events and death. There is suggestive evidence that ESAs may improve quality of life. Whether and how ESAs affect tumour control remains uncertain.
-
Based on the previous assessment,2 NICE guidance (TA142)1 recommended the use of ESAs in combination with intravenous iron for the treatment of CIA in women with ovarian cancer receiving platinum-based chemotherapy with symptomatic anaemia (Hb ≤ 8 g/dl). The recommendation made in TA142 did not prohibit the use of other management strategies for the treatment of CIA, for example blood transfusion. 1 In addition, guidance set out in TA142 recommended ESAs in combination with intravenous iron for people with profound CIA who cannot be given blood transfusions. 1 The ESA with the lowest acquisition cost should be used. 1
Chapter 2 Definition of the decision problem
Decision problem
The purpose of this assessment was to review and update as necessary guidance to the NHS in England and Wales on the clinical effectiveness and cost-effectiveness of ESAs [epoetin alfa (Eprex and Binocrit), epoetin beta (NeoRecormon), epoetin theta (Eporatio), epoetin zeta (Retacrit) and darbepoetin alfa (Aranesp)] within their licensed indications for the treatment of CIA.
The project was undertaken based on a published scope46 and in accordance with a predefined protocol. There were no major departures from this protocol. The protocol stated that interventions would be evaluated in line with their UK marketing authorisations. However, as none of the included studies was completely aligned with the current licence we applied a definition of ‘within licence’, which was not predefined. Given the recent publication of the 2012 Cochrane review,11 which considered all ESAs, irrespective of their licence, ‘within licence’ was defined as a licensed starting dose, irrespective of how other licence criteria were dealt with.
Population
The population was people receiving chemotherapy for solid tumours, malignant lymphoma or multiple myeloma and people with non-myeloid malignancies at risk of transfusion as assessed by their general status (e.g. cardiovascular status, pre-existing anaemia at the start of chemotherapy).
Haematological malignancy specifically refers to non-myeloid malignancy (chronic lymphocytic leukaemia, non-Hodgkin’s lymphoma, Hodgkin’s disease and multiple myeloma).
Interventions
The interventions considered were ESAs: epoetin alfa (Eprex and Binocrit), epoetin beta (NeoRecormon), epoetin theta (Eporatio), epoetin zeta (Retacrit) and darbepoietin alfa (Aranesp).
All interventions were considered according to their UK marketing authorisation with respect to the starting dose administered (see Table 3).
Comparators
The following comparators were considered:
-
best supportive care (including adjustment to the cancer treatment regimen, RBCT and iron supplementation)
-
one of the other interventions under consideration, provided it was used in line with its marketing authorisation.
Outcomes
Evidence in relation to the following kinds of outcomes were considered:
-
haematological response to treatment: defined as a transfusion-free increase in Hb of ≥ 2 g/dl or a haematocrit increase of 6 percentage points
-
need for blood transfusion after treatment: number of patients transfused and number of units transfused per patient
-
tumour response: time to cancer progression
-
OS
-
AEs of treatment: hypertension, rash/irritation, pruritus, mortality, thromboembolic events, seizure, haemorrhage/thrombocytopenia, fatigue, pure red cell aplasia (a note was made of other AEs described within the trial reports)
-
HRQoL: validated quality-of-life measures, for example the Functional Assessment of Cancer Therapy – General (FACT-G), Functional Assessment of Cancer Therapy – Fatigue (FACT-F) and Functional Assessment of Cancer Therapy – Anaemia (FACT-An), the European Quality of Life-5 Dimensions (EQ-5D) and the Short Form questionnaire-36 items (SF-36).
Research question
This assessment addressed the following research question: ‘What is the effectiveness and cost-effectiveness of ESAs (epoetin alfa, beta, theta and zeta and darbepoetin alfa) for treating CIA (including review of TA142)?’
Chapter 3 Assessment of clinical effectiveness
The review commissioned by NICE was to update the previous guidance (TA1421) based on the HTA review conducted by Wilson and colleagues. 2 The differences between the remit of the previous review and that of the current review are discussed in Chapter 1 (see Previous Health Technology Assessment review). The project was undertaken in accordance with a predefined protocol. There were no major departures from this protocol. The protocol stated that interventions would be evaluated in line with their UK marketing authorisations. However, as none of the included studies was completely aligned with the current licence we applied a definition of ‘within licence’, which was not predefined. Given the recent publication of the 2012 Cochrane review,11 which considered all ESAs irrespective of their licence, ‘within licence’ was defined as a licensed starting dose irrespective of how other licence criteria were dealt with.
A scoping search was undertaken to identify existing reviews and other background material. Among this literature two recent Cochrane reviews were identified that assessed the effectiveness of ESAs. 7,47
The aim was to systematically review the effectiveness of ESAs with regard to treating cancer treatment-related anaemia, their effects on patients regarding their underlying malignancy and survival and their effectiveness in improving quality of life and reducing the impact of AEs. Given the recent publication of the Cochrane review,11 the focus for this review was to identify and consider trials in which ESAs have been used in a manner consistent with or closest to their respective marketing authorisations (see Eligibility criteria, Dose).
Methods
Identification of studies
The search strategy was based on the strategy used in the previous multiple technology appraisal (MTA) on this topic by Wilson and colleagues. 2 It combined free-text and medical subject heading (MeSH) terms for epoetin (generic and brand names), cancer and anaemia (see Appendix 1). Search filters were applied to retrieve RCTs, cost-effectiveness studies and quality-of-life studies. The search terms and structure of the search were mainly the same as in the study by Wilson and colleagues,2 with additional search terms for epoetin theta, epoetin zeta and corresponding drug brand names. The search filters for RCTs, cost-effectiveness studies and quality-of-life studies were different from those used in Wilson and colleagues. 2 The filters were developed by an information specialist to ensure an appropriate balance of sensitivity and specificity. Changes to the previous MTA search strategy, including the filters, were made in MEDLINE and translated as appropriate for other databases. The MEDLINE randomised controlled trial (RCT) search strategy was checked by a clinical expert for inaccuracies and omissions relating to drug and cancer terms.
The databases were searched from the search end date of the previous MTA on this topic2 (search end date 2004). Although epoetin alfa (Binocrit), epoetin theta and epoetin zeta were not covered in the previous report, we believe that relevant interventional research is highly unlikely to have been published on these drugs before this date given that the drugs were launched in 2007 (Binocrit and epoetin zeta) and 2009 (epoetin theta). All searches were also limited to English-language papers, although some foreign-language papers would have been identified by virtue of being included in other systematic reviews.
The following databases were searched: MEDLINE (Ovid), MEDLINE In-Process & Other Non-Indexed Citations (Ovid), EMBASE (Ovid), The Cochrane Library including CENTRAL, the Cochrane Database of Systematic Reviews (CDSR), the Database of Abstracts of Reviews of Effects (DARE), the HTA database, the NHS Economic Evaluation Database (NHS EED) and the Office for Health Economics Health Economic Evaluations Database (HEED), Web of Science (Thomson Reuters), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost), the British Nursing Index (ProQuest) and Health Management Information Consortium (HMIC) (Ovid). The US Food and Drug Administration (FDA) and EMA websites were also searched.
In addition, the following websites were searched for background information (all accessed 26 June 2015):
-
medical societies:
-
British Society for Haematology: www.b-s-h.org.uk/
-
Association of Cancer Physicians: www.cancerphysicians.org.uk/
-
ASH: www.hematology.org/
-
ASCO: www.asco.org/
-
Canadian Oncology Societies: www.cos.ca/
-
Haematology Society of Australia and New Zealand: www.hsanz.org.au/
-
Clinical Oncology Society of Australia: www.cosa.org.au/
-
New Zealand Society for Oncology: www.nzsoncology.org.nz/
-
-
UK charities:
-
Cancer Research UK: www.cancerresearchuk.org/home/
-
Macmillan: www.macmillan.org.uk/
-
Marie Curie: www.mariecurie.org.uk/
-
-
non-UK charities:
-
American Cancer Society: www.cancer.org/
-
Canadian Cancer Society: www.cancer.ca/
-
Cancer Council Australia: www.cancer.org.au/
-
Cancer Society of New Zealand: www.cancernz.org.nz/
-
World Cancer Research Fund: www.wcrf-uk.org/.
-
The database search results were exported to, and deduplicated using, EndNote X5 (Thomson Reuters, CA, USA). Deduplication was also performed using manual checking. The search strategies and the numbers of references retrieved for each database are detailed in Appendix 1. After the reviewers completed the screening process, the bibliographies of included papers were scrutinised for further potentially includable studies.
A supplementary search was carried out in MEDLINE (Ovid) to search for utilities as a function of Hb levels and for information on Hb levels after chemotherapy ends. A systematic search was not required for this part of the review and so the search strategy was limited to MEDLINE. These searches are detailed in Appendix 1.
Wilson and colleagues2
Studies included in the previous HTA review2 were screened against the inclusion criteria for the PenTAG review for includable studies.
Reference lists
Reference lists of included guidelines, systematic reviews and clinical trials were scrutinised for additional information.
Ongoing trials
A search for ongoing trials was also undertaken. Terms for the intervention (‘epoetin’ OR ‘darbepoetin’) and condition of interest (cancer* OR carcinoma* OR leukemia OR malignan* OR neoplasm* OR tumo?r OR myelo* OR lymphoma* OR oncolog* OR chemotherapy*) were used to search the trial registers ClinicalTrials.gov and Current Controlled Trials (International Standard Randomised Controlled Trial Number) for ongoing trials. Trials that did not relate to cancer-induced or chemotherapy-related anaemia were removed by hand sorting. Finally, duplicates, identified through their study identification numbers when possible, were removed. Searches were carried out on 28 August 2013.
Eligibility criteria
Study design
Only RCTs were included. Non-RCTs and quasi-randomised trials (such as when allocation is based on date of birth or day of month) were excluded.
Population
People receiving chemotherapy for solid tumours, malignant lymphoma or multiple myeloma and at risk of transfusion as assessed by their general status (e.g. cardiovascular status, pre-existing anaemia at the start of chemotherapy) and people with non-myeloid malignancies who are receiving chemotherapy were relevant to the scope of this review. There were no age restrictions; however, it is recognised that the licences for all of the interventions of interest do not cover the use of ESAs in children for this indication. Studies in which ESAs were given in the context of myeloablative chemotherapy ahead of bone marrow or peripheral blood stem cell transplantation or for short-term preoperative treatment to correct anaemia or to support collection of autologous blood before cancer surgery were excluded.
Interventions
Studies evaluating the use of ESAs were included if ESAs were given to treat CIA. The ESAs of interest for this appraisal were epoetin alfa (Eprex and Binocrit), epoetin beta (NeoRecormen), epoetin theta (Eporatio), epoetin zeta (Retacrit) or darbepoetin alfa (Aranesp).
Concomitant anaemia therapy, such as iron or granulocyte colony-stimulating factor (G-CSF) supplementation, was permitted, as was RBCT. However, G-CSF had to be administered to patients in both the treatment and the control arms.
Dose
For the main analysis for this systematic review, studies were considered eligible for inclusion if they evaluated a licensed (weight-based) starting dose, irrespective of how they dealt with other criteria stipulated by the licence (see Table 3).
With respect to European labelling, inclusion Hb levels ≤ 11 g/dl and > 11 g/dl and target Hb levels ≤ 13 g/dl and > 13 g/dl were considered in subgroup analyses; start dose plus an inclusion Hb level ≤ 11 g/dl and start dose plus an inclusion Hb level ≤ 11 g/dl plus a target Hb level ≤ 13 g/dl were also considered in post-hoc analyses.
Comparator
The main comparators of interest were placebo and best supportive care (including adjustment to the cancer treatment regimen, blood transfusion and iron supplementation). In addition, the comparator could be one of the other ESAs under consideration, provided that it was administered in line with the relevant marketing authorisation.
Outcomes
Outcomes sought from the studies fell into four categories: anaemia-related outcomes, malignancy-related outcomes, AE data and patient-specific outcomes such as quality-of-life outcomes and patient preferences:
-
Anaemia-related outcomes: haematological response to treatment (defined as a transfusion-free increase in Hb of ≥ 2 g/dl or a haematocrit increase of 6%), mean Hb change and RBCT requirements [including number of patients transfused, number of units transfused per patient and number of units transfused per average patient (i.e. including participants not requiring transfusion)].
-
Tumour response.
-
OS.
-
On-study mortality.
-
AEs: hypertension, rash/irritation, pruritus, mortality, thromboembolic events, seizure, haemorrhage/thrombocytopenia, fatigue and pure red cell aplasia. A note was made of other AEs described within the trial.
-
HRQoL: data on validated HRQoL measures was sought – anticipated HRQoL measures included Functional Assessment of Cancer Therapy (FACT) (including FACT-G, FACT-F and FACT-An) [see www.facit.org/FACITOrg/Questionnaires (accessed July 2015)]. A note was made of any other HRQoL measure reported.
Selection of studies
Studies retrieved from the update searches were selected for inclusion according to the inclusion/exclusion criteria specified in Eligibility criteria. First, titles and abstracts returned by the search strategy were screened for inclusion independently by four researchers. Disagreements were resolved by discussion, with the involvement of a fifth reviewer. Full texts of identified studies were obtained and screened in the same way. Abstract-only studies were included on the provision that sufficient methodological details were reported to allow critical appraisal of study quality.
In addition, studies included in the review conducted by Wilson and colleagues2 were screened for inclusion against the eligibility criteria for this review (see Chapter 1, Previous Health Technology Assessment review).
On completion of the first round of screening, eligible studies were then rescreened. For this stage, studies were eligible for inclusion in the review only if the ESA treatments evaluated were administered in accordance with their European marketing authorisations with respect to the starting dose, irrespective of how the study dealt with other criteria stipulated by the licence (see Table 3).
Data extraction and management
Included full papers were split between four reviewers for the purposes of data extraction using a standardised data extraction form. Data extraction was checked independently by another reviewer and discrepancies were resolved by discussion, with the involvement of an additional review team member if necessary. Information extracted and tabulated included details of the study’s design and methodology, baseline characteristics of participants and results for the outcomes of interest (see Appendix 2).
If several publications were identified for one study, the data from the most recent publication were evaluated and these data were amended with information from other publications.
For studies comparing more than one experimental arm with one control arm, we assigned a separate reference for each study arm, using the author and publication year of the main publication and adding the suffixes a and b, etc. For example, the study by Tjulandin and colleagues48 compared two different experimental study arms with one control group. Because of this referencing system a study may appear more than twice in the list of included studies.
When there was incomplete information on key data, we referred to the 2012 Cochrane review. 11 For the Cochrane review the authors evaluated documents presented at the Oncologic Drugs Advisory Committee (ODAC) hearing at the US FDA held in May 2004, May 2007 and May 2008. These documents were reported to include briefing documents plus additional PowerPoint presentations prepared by medical review authors of the FDA, as well as documents and additional PowerPoint presentations prepared by the companies Roche, Johnson & Johnson and Amgen Inc.
Critical appraisal
The protocol stated that the Cochrane risk of bias tool would be used for quality appraisal; however, for consistency, assessments of study quality were performed using the same criteria as in the previous review. 2 The criteria used to critically appraise the included studies are summarised in Table 9. The results were tabulated and the relevant aspects described on the data extraction forms. Methodological notes were made for each included study on the data extraction forms, including the reviewer’s observations on sample size, power calculations, participant attrition, methods of data analysis and conflicts of interest. In addition, GRADE (Grading of Recommendations Assessment, Development and Evaluation) analysis was carried out; the results are presented in Appendix 3.
| Domain | Description |
|---|---|
| Treatment allocation | 1. Was allocation truly random? (Yes: random numbers, coin toss, shuffle, etc.; no: patient ID number, date of birth, alternate; unclear: if the method not stated) |
| 2. Was treatment allocation concealed? (Yes: central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware; inadequate: allocation was alternate or based on information known to the triallist; unclear: insufficient information given) | |
| Similarity of groups | 3. Were the patients’ characteristics at baseline similar in all groups? |
| Implementation of masking | 4. Was the treatment allocation masked from the participants? (either stated explicitly or an identical placebo used) |
| 5. Was the treatment allocation masked from clinicians? | |
| Completeness of trial | 6. Were the numbers of withdrawals, dropouts and those lost to follow-up in each group stated? |
| 7. Did the analysis include an ITT analysis or were < 10% of the study arm excluded? |
Methods of data analysis/synthesis
When data permitted, the results of individual studies were pooled using the methods described below.
Because of heterogeneity, a random-effects model was assumed for all meta-analyses. For binary data, risk ratio (RR) was used as a measure of treatment effect and the DerSimonian–Laird method was used for pooling. For continuous data, standardised mean differences were calculated if the outcome was measured on the same scale in all trials. For HRQoL, only identical scales and subscales were combined in a given meta-analysis. For time-to-event data, that is, OS, data were extracted from the Cochrane review. 11 In the Cochrane review,11 hazard ratios (HRs) were based on IPD; when IPD were not available, HRs were calculated from published reports including secondary analyses, using methods reported in Parmar and colleagues,49 or binary mortality data. 11 Similarly, data from the Cochrane review11 were used for mean Hb change, transfusion requirement, mean units of blood transfused, complete tumour response, HRQoL and AEs if this information was not available in the published trial reports.
One study48 had two intervention arms that were separately compared with the control arm. To take account of the fact that some study-specific estimates would use the same control arm, the information was divided across the number of comparisons from the study. When pooling RRs, the number of events and the total sample size in the control arm were divided equally across the comparisons and, when pooling mean differences, the total sample size in the control arm was adjusted and divided equally across the comparisons. However, if only one experimental arm was eligible for the analysis,50–53 all participants assigned to the control arm were included.
The following prespecified subgroup analyses were conducted, if appropriate:
-
Hb level at study entry (< 10 g/dl vs. < 11 g/dl vs. < 12 g/dl vs. < 14.5 g/dl vs. not reported)
-
Hb inclusion criteria (≤ 11 g/dl vs. < 11 g/dl)
-
target Hb (≤ 12 g/dl and > 12 g/dl)
-
solid tumours compared with haematological malignancies (solid vs. haematological vs. mixed vs. not reported)
-
ovarian cancer compared with other cancers
-
type of chemotherapy treatment (platinum chemotherapy vs. non-platinum chemotherapy vs. chemotherapy plus radiotherapy vs. mixed chemotherapy vs. not reported)
-
short-lasting ESAs compared with long-lasting ESAs (erythopoietins vs. darbepoetin)
-
iron supplementation (iron supplementation given vs. no iron supplementation vs. iron handled differently in study arm vs. not reported)
-
duration of ESA medication (6–9 weeks vs. 12–16 weeks vs. 17–20 weeks vs. > 20 weeks)
-
study design (placebo vs. standard care).
In addition, based on subgroup analyses, meta-regression models were conducted including random effects and a subgroup as a covariate to assess the effects of subgroups on the outcomes. These analyses were conducted if there was a sufficient number of studies in each subgroup. The DerSimonian–Laird method was used to estimate between-study variance in meta-regression. All covariates showing a significant effect (p < 0.05) in a univariate analysis were further considered in a model selection. However, these analyses should be interpreted with caution as they can be exploratory only and should be considered as hypothesis-generating rather than hypothesis-testing analyses. 54,55
We stated in the protocol that we would consider the use of iron supplementation plus ESAs; people with any type of cancer receiving platinum-based chemotherapy; people with head and neck malignancies; women with ovarian cancer; women with ovarian cancer receiving platinum-based chemotherapy; and people unable to receive blood transfusions.
All analyses were performed using Stata version 12 (StataCorp LP, College Station, TX, USA).
Sensitivity analysis
To allow comparison with the Cochrane review11 and with the previous HTA review,2 fixed-effects meta-analyses for the main analysis were also conducted.
Assessment of bias
Identified research evidence was interpreted according to the assessment of methodological strengths and weaknesses and the possibility of potential biases. Publication bias for the main outcomes was assessed using funnel plots. The Egger test56 was used for continuous outcomes [mean difference, standard error (SE)] and the Harbord test57 was used for binary outcomes [odds ratio (OR), log SE]. However, it should be noted that these tests typically have low power to detect funnel plot asymmetry and so the possibility of publication bias existing in the meta-analysis cannot be excluded even if there is no statistically significant evidence of publication bias. In addition, meta-regression models including random effects and using publication year as a covariate to assess the effect of publication year on the considered outcome were conducted.
Graphical representation of summary trial information
We present a summary of information relating to each trial at the end of each comparison section using Graphical Overview for Evidence Reviews (Gofer) software (developed by Dr Will Stahl-Timmins at the University of Exeter Medical School in association with PenTAG and the European Centre for Environment and Human Health). These figures graphically represent the study design, study quality and results in a format that allows quick comparison between trials.
Note
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Results
Studies identified
We screened the titles and abstracts of 1404 unique references identified by the PenTAG searches and additional sources and retrieved 292 papers for detailed consideration. Of these, 244 were excluded, five because they were unobtainable and 239 for other reasons (a list of these papers with reasons for their exclusion can be found in Appendix 4). Forty-eight studies met the prespecified criteria set out in the protocol and were considered eligible for inclusion. In assessing titles and abstracts, agreement between the two reviewers was good (κ = 0.693, 95% CI 0.648 to 0.738). At the full-text stage, agreement was substantial (κ = 0.792, 95% CI 0.705 to 0.879). At both stages, initial disagreements were easily resolved by consensus.
Twenty-nine studies from the previous HTA review2 were also considered eligible for inclusion in the update review. We also searched the citations of all of the includable studies and systematic reviews (including the 2012 Cochrane review;11 see Appendix 5). This process revealed an additional five primary studies. 58–62
In restricting eligibility to ESA treatments evaluated in accordance with their European marketing authorisation with respect to starting dose, 47 studies were excluded (a list of these studies together with the study characteristics can be found in Appendix 6). In total, 23 primary studies17,48,50–53,62–78 reported in 34 publications17,48,50–53,58–60,62–86 were judged to meet the inclusion criterion for the review (Table 10); study characteristics are summarised in Appendix 7. Primary studies are linked to multiple secondary publications, as shown in Appendix 8.
| Author, year | n | Agent | Control | Malignancy | Treatment | Outcomes | Multiple publications identified |
|---|---|---|---|---|---|---|---|
| Included studies from Wilson and colleagues2 meeting the inclusion criteria for the PenTAG review | |||||||
| Abels 199363 | 413a | Epoetin alfa | Placebo | Haematologicalb | Chemotherapy: mixed | HaemR, Hb, HCT, RBCT, HRQoL,c AEc | Abels 1996,59 Case 1993,86 Henry 1994,85 Henry 199558 |
| Aravantinos 200364 | 47 | Epoetin alfa | Standard | Solid | Chemotherapy: platinum based | Hb, HCT, RBCT | NA |
| dBoogaerts 200365 | 262 | Epoetin beta | Standard | Solid and haematologicalb | Chemotherapy: NR | HaemR, Hb, RBCT, HRQoL | NA |
| Dammacco 200166 | 145 | Epoetin alfa | Placebo | Haematologicalb | Chemotherapy: mixede | HaemR, Hb, RBCT, HRQoL, AEs | NA |
| Del Mastro 199767 | 62 | rHuEPOf | Standard | Solid (breast) | Chemotherapy: non-platinum based | Hb, RBCT, HRQoL, AEs | NA |
| Dunphy 199968 | 30 | rHuEPOf | Standard | Solid (head and neck, lung) | Chemotherapy: mixed | Hb, RBCT | NA |
| Hedenus 200253 | 33g | Darbepoetin alfa | Placebo | Haematologicalb | Chemotherapy: NR | HaemR, Hb, RBCT, AEs | NA |
| Hedenus 200317 | 349 | Darbepoetin alfa | Placebo | Haematologicalb | Chemotherapy: NR | HaemR, Hb, RBCT, AEs, HRQoL | Littlewood 200683 |
| Kotasek 200350 | 249 | Darbepoetin alfa | Placebo | Solid | Chemotherapy: NR | HaemR, Hb, RBCT, HRQoL | NA |
| Kurz 199769 | 35 | Epoetin alfa | Placebo | Solid (cervix, ovary, uterus) | Chemotherapy: mixed | HaemR, Hb, RBCT, HRQoL, AEs | NA |
| Littlewood 200170 | 375 | Epoetin alfa | Placebo | Mixed | Chemotherapy: non-platinum based | HaemR, Hb, RBCT, HRQoL, AEs | Aapro 2004,82 Bajetta 2004,81 Patrick 200360 |
| Österborg 200271 | 349 | Epoetin beta | Placebo | Haematologicalb | Chemotherapy: non-platinum based | HaemR, Hb, RBCT, HRQoL, AEs | Österborg 200579 |
| Silvestris 199572 | 54 | Epoetin alfa | Standard | Haematologicalb | Chemotherapy: NR | HaemR, Hb, AEs | NA |
| ten Bokkel Huinink 199851 | 122 | Epoetin beta | Standard | Solid (ovary) | Chemotherapy: platinum based | Hb, RBCT, AEs | NA |
| Thatcher 199952 | 130 | Epoetin alfa | Standard | Solid (SCLC) | Chemotherapy: mixed | Hb, RBCT, HRQoL, AEs | NA |
| Vansteenkiste 200273 | 314 | Darbepoetin alfa | Placebo | Solid (lung) | Chemotherapy: platinum based | HaemR, Hb, RBCT, HRQoL, AE, disease progression, survival | Vansteenkiste 200484 |
| PenTAG review update 2004 to July 2007 | |||||||
| Grote 200574 | 224 | Epoetin alfa | Placebo | Solid (SCLC) | Chemotherapy: mixed | Hb, RBCT, TR, survival, AEs | NA |
| Moebus 201362 | 643 | Epoetin alfa | Standard | Solid (breast) | Chemotherapy: non-platinum based | Hb, RBCT, HRQoL,f survival, AEs | NA |
| Ray-Coquard 200975 | 218 | Epoetin alfa | Standard | Mixed | Chemotherapy: NR | RBCT, OS, HRQoL, AEs | NA |
| Österborg 200579 | 349 | Epoetin beta | Placebo | Haematologicalb | Chemotherapy: non-platinum based | HaemR, Hb, RBCT, HRQoL, AEs | Österborg 200271 |
| Strauss 200876 | 74 | Epoetin beta | Standard | Solid (cervix) | Chemotherapy + radiotherapy | Hb, RBCT, TR, survival, AEs | NA |
| Tjulandin 201048 | 223 | Epoetin theta, epoetin beta | Placebo | Solid | Chemotherapy: platinum based | HaemR, RBCT, HRQoL,h AEs | NA |
| Tjulandin 201177 | 186 | Epoetin theta | Placebo | Mixed | Chemotherapy: non-platinum based | HaemR, RBCT, HRQoL, AEs | NA |
| iUntch 201178 | 733 | Darbepoetin alfa | Standard | Solid (breast) | Chemotherapy: non-platinum based | Hb, pathological response, disease progression, survival, AEs | Untch 201180i |
Update searches were conducted on 2 December 2013 using the same methodology as described earlier. In total, 68 records were screened by two reviewers (LC and MH) and eight records were selected for full-text retrieval. No studies were judged eligible on full-text appraisal. A list of these papers with reasons for their exclusion can be found in Appendix 4.
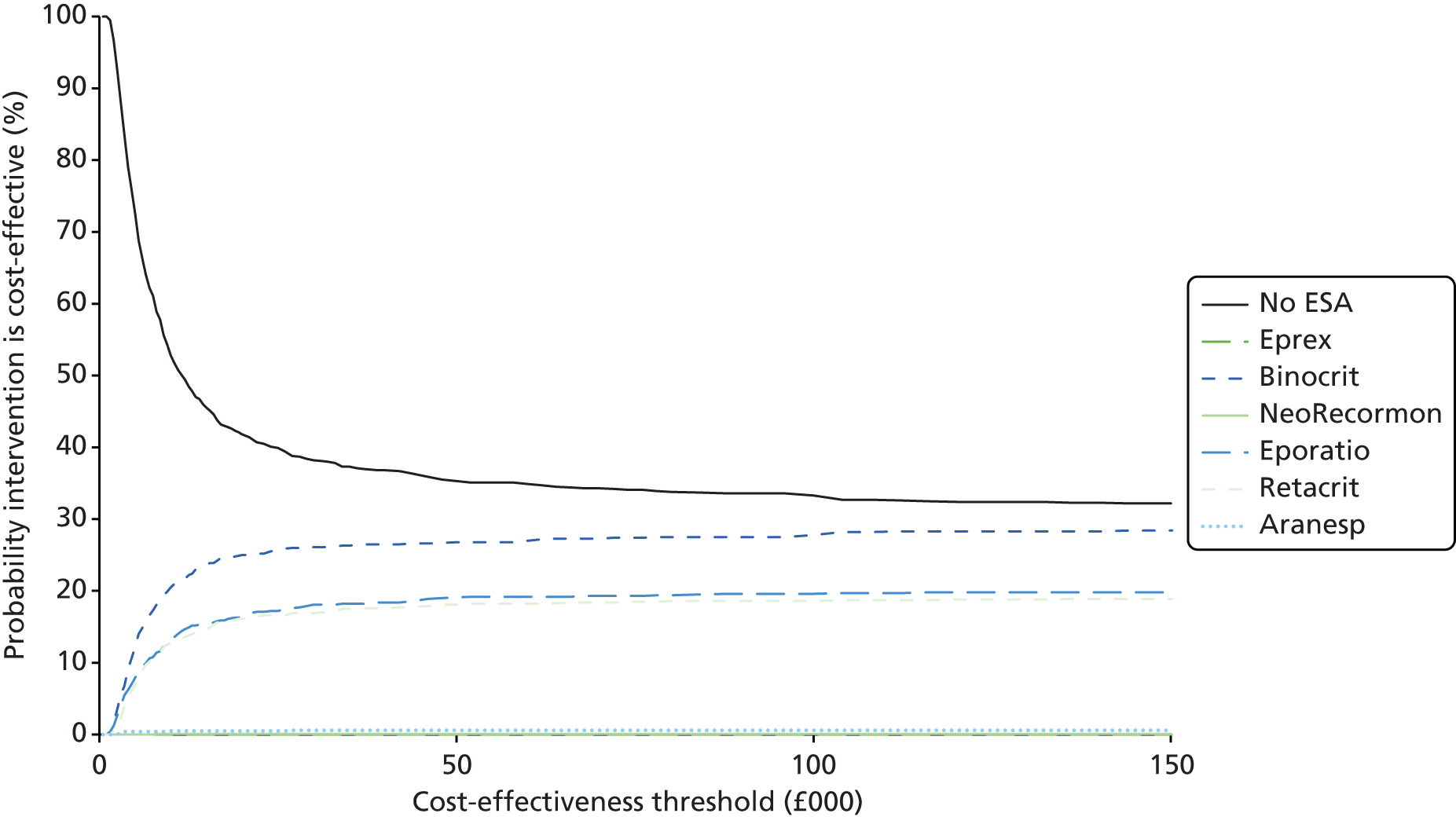
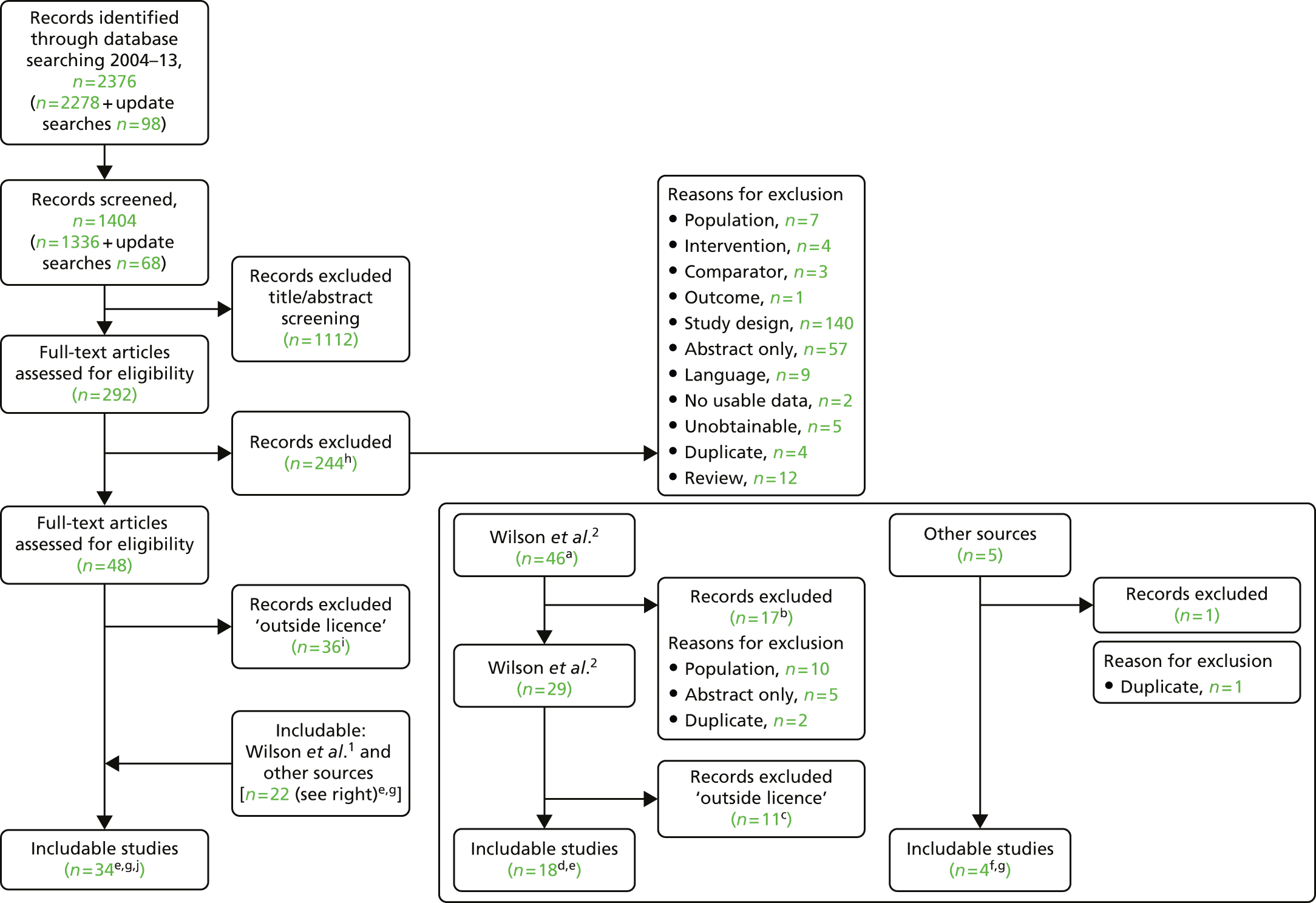
The process of identifying studies is illustrated in detail in Figure 1.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart: clinical effectiveness review. a, Number of included studies in the previous HTA review. 2 b, Excluded studies listed in Appendix 4 (see Wilson and colleagues2 excluded studies). c, Baseline characteristics for studies excluded on dose (‘outside of licence’, n = 11) are reported in Appendix 6. Note that, for four included trials50–53 that evaluated different ESA doses, only the within-license doses were included in the PenTAG review. As a result, the number of studies in the subsection of the table ‘Wilson and colleagues2’ is 15. d, Coiffier and colleagues87 was included in the review by Wilson and colleagues. 2 The full paper65 for this abstract was identified by citation chasing and included in the PenTAG review. e, Wilson and colleagues:2 16 primary trials17,50–53,63–73 reported in 18 publications. 17,50–53,58,63–73,86 f, Other sources: 11 systematic reviews2,7,11,41,88–94 reported in 12 publications. 2,7,11,41,88–95 g, Other sources: four studies59,60,62,85 were identified as eligible for inclusion. h, Excluded studies listed in Appendix 4 (see Clinical effectiveness review: excluded studies). i, Baseline characteristics for studies excluded on dose (‘outside of licence’, n = 36) are reported in Appendix 6. j, PenTAG searches 2004–13: seven primary trials48,74–78,80 reported in 12 publications. 48,74–84
Study characteristics
No head-to-head trials were identified in either the 2007 review2 or the update searches. One three-arm trial compared epoetin beta and epoetin theta with placebo;48 however, comparison was made only between each intervention and placebo. The majority of trials (> 50%) compared an ESA plus standard care with placebo plus standard care. Of these, four trials were identified in the update searches. 48,74,77,79 Of note, the Österborg and colleagues trial79 evaluated long-term survival for epoetin beta plus standard care compared with placebo plus standard care from the earlier 2002 RCT. 71 The remaining trials compared an ESA plus standard care with standard care alone. Of these, four trials (reported in five publications) were identified in the update searches. 62,75,76,78,80
Interventions and comparators
The following interventions were evaluated in the included studies: epoetin alfa, beta and theta and darbepoetin alfa (Table 11). In two of the included studies it was uncertain which ESA was evaluated [reported as recombinant human erythropoietin (rHuEPO)],67,68 although it was assumed to be either epoetin alfa or epoetin beta based on the study dates and the doses evaluated. Of note, no studies of epoetin zeta met the eligibility criteria for this review (study design).
| Intervention | Number of studiesa | vs. placebo + SC | vs. SC alone | Total population,b n | Treated with ESA, n (%) |
|---|---|---|---|---|---|
| Epoetin alfac | 10c | 5 | 5c | 2284 | 1135 (56) |
| Epoetin betac | 4c,d | 1d | 3c | 768 | 382 (50) |
| Epoetin theta | 2d | 2d | 0 | 409 | 171 (42) |
| Epoetin zeta | 0 | – | – | – | – |
| Darbepoetin alfa | 5 | 4 | 1 | 1678 | 727e (43) |
| rHuEPOf | 2 | – | 2 | 92 | 46 (50) |
| Total | 23d | 12d | 11 |
The ESA administration and dosing strategies varied considerably in the literature in terms of starting dose (fixed or weight based), trigger Hb level (the point below which ESAs should be administered, ≤ 10.0 g/dl), target Hb level (the point above which ESAs should be stopped or titrated, 10–12 g/dl), dose escalation (used if people do not achieve a haematological response within a specified time period), stopping rules for non-responders and duration of use following each chemotherapy session. These aspects will have an impact on clinical effectiveness. The majority (82%) of studies were initiated before the 2008 update of the SPCs and no studies were completely aligned with the UK marketing authorisation for these drugs in respect of these criteria (see Appendix 9).
This review focused only on those studies evaluating ESA treatment in accordance with UK marketing authorisations with respect to the starting dose (see Dose), irrespective of other aspects of the licence (e.g. starting or target Hb levels or stopping rules). For darbepoetin alfa, two studies50,53 were dose ranging studies and therefore evaluated doses under and over the current licence recommendations, and two studies51,52 included a second intervention group evaluating epoetin alfa at a start dose of 300 IU/kg. Only the licensed start doses from these studies were included in the PenTAG review. In addition, one study78,80 evaluated darbepoetin alfa at a dose of 4.5 µg/kg once every 2 weeks. This was considered to be within licence, as the equivalent dose per week (2.25 µg/kg) is a licensed dose.
Of note, none of the included studies evaluated ESAs entirely within the remit of their marketing authorisations, in particular with respect to trigger and target Hb levels and stopping rules, all of which were generally higher than specified in the licence. Appendix 9 provides a summary of the administration of ESAs within the included studies in relation to their respective licences. Two additional definitions of ‘within licence’ were considered in post-hoc analyses: (1) licensed start dose plus inclusion Hb level ≤ 11 g/dl and (2) licensed start dose plus inclusion Hb level ≤ 11 g/dl plus target Hb level ≤ 13 g/dl.
The majority of the trials gave ESA therapy over the course of the chemotherapy, with many continuing with ESA therapy for 4 weeks after chemotherapy, which is permissible within the licensed indications. The average time on erythropoietin treatment was 12 weeks, with trial duration clustering around 12–28 weeks. One study reported follow-up data. 79
Concomitant treatments
There were several possible concomitant treatments – G-CSF, iron supplementation and RBCT, with some protocols giving recommendations for when transfusions should be given (referred to in this review as transfusion triggers) (see Appendix 7). Two studies were identified in which G-CSF was given. In one study67 G-CSF was given at a dose of 5 µg/kg from day 4 until day 11 during the first five chemotherapy cycles, to allow accelerated chemotherapy. The second study75 stated that G-CSF could be used in primary or secondary prophylaxis as recommended by ASCO and French Federation of Cancer Centre guidelines. However, it was unclear whether G-CSF was administered to any of the study participants during the study period.
In the majority of studies (n = 1417,48,64,65,67–72,75–80) iron supplementation was given. Reporting of details in this respect varied. A fixed daily dose of oral iron (either 200 mg or 325 mg) for all patients was most common, although in a few studies administration of oral iron supplementation was dependent on transferrin saturation levels (i.e. ≤ 20% or < 10%); in one study70 daily oral iron supplementation was recommended, but if (during the study) transferrin saturation fell to ≤ 20% intravenous iron was recommended. In two studies that enrolled patients with a baseline transferrin saturation level of < 25%71,79 and < 20%,76 participants were given intravenous iron supplementation at a dose of 100 mg per week before the start of study treatment. In cases in which patients were contraindicated or the drug was not available, oral iron supplementation was administered. In one study69 intravenous iron supplementation was administered following each dose of chemotherapy, beginning with the next cycle. One trial was identified in which concomitant iron supplementation was given only to patients receiving an erythropoietin. 78,80 Several studies reported that iron supplementation was allowed during the study without specifying details or that iron supplementation was given at the investigators’ discretion. Nine studies did not report concomitant treatment and in two studies52,73 iron supplementation during the study period was not permitted.
Population characteristics
Population characteristics of the included trials are summarised in Tables 12 and 13; characteristics are described in more detail in Appendix 7.
| Malignancy | Mixed types | Specific malignancies |
|---|---|---|
| Solid tumours | Tjulandin 2010;48 Aravantinos 2003;64 Kotasek 2003;50 Dunphy 1999;68 Kurz 199769 | Moebus 201362 (breast); Untch 201178,80 (breast); Strauss 200876 (cervix); Grote 200574 (SCLC); Vansteenkiste 200273 (lung); Thatcher 199952 (SCLC); ten Bokkel Huinink 199851 (ovary); Del Mastro 199767 (breast) |
| Haematologicala | Hedenus 2003;17 Österborg 2002,71 bÖsterborg 2005;79 Hedenus 2002;53 Dammacco 200166 | Silvestris 199572 (MM) |
| Mixed solid and haematologicala | Tjulandin 2011;77 Ray-Coquard 2009;75 Boogaerts 2003;65 Littlewood 2001;70 cAbels 199363 |
| Malignancy | Trials |
|---|---|
| Chemotherapy: platinum based | Tjulandin 2010;48 Vansteenkiste 2002;73 Aravantinos 2003;64 aten Bokkel Huinink 1998;51 Abels 199363 |
| Chemotherapy: non-platinum based | Moebus 2013;62 Tjulandin 2011;77 Untch 2011;78,80 Österborg 2002,71 b2005;79 Littlewood 2001;70 aDel Mastro 1997;67 Abels 199363 |
| Chemotherapy: type unknown | Ray-Coquard 2009;75 Boogaerts 2003;65 Hedenus 2003;17 Kotasek 2003;50 Hedenus 2002;53 Silvestris 199572 |
| Mixed chemotherapy | Grote 2005;74 Dammacco 2001;66 Dunphy 1999;68 Thatcher 1999;52 Kurz 199769 |
| Chemotherapy + radiotherapy | Strauss 200876 |
The age range of trial participants was 18–92 years. In the majority of included studies there was an equal distribution of men and women, with the obvious exception of trials whose populations had gynaecological and breast malignancies (within the breast malignancies group one patient was male70). However, in one study68 (head, neck and lung tumours) gender was not distributed equally between the two treatment groups; in the treatment arm 92% of participants were men, compared with an equal distribution of men and women in the control arm (50% each).
The studies included a variety of malignancies (see Table 12). Five trials included patients with a mix of solid tumours. 48,50,64,68,69 One of the retrospective analyses identified81 was a subgroup analysis of a breast cancer cohort enrolled in the study conducted by Littlewood and colleagues;70 however, the overall study was not powered to discriminate treatment differences within subgroups. Eight of the included studies concentrated on specific solid tumour types (breast n = 3;62,67,78,80 ovary n = 1;51 cervix n = 1;76 lung n = 352,73,74). Four studies included a mix of haematological malignancies (specifically haematological non-myeloid malignancies: chronic lymphocytic leukaemia, non-Hodgkin’s lymphoma, Hodgkin’s disease and multiple myeloma);17,53,66,71,79 of these, one study was reported in two papers,71,79 with the later paper79 reporting long-term survival data from the earlier study. 71 One study focused on multiple myeloma. 72 Five studies included participants with a mix of solid and haematological malignancies. 63,65,70,75,77
Malignancy treatments consisted of chemotherapy (platinum based and non-platinum based) and chemotherapy plus radiotherapy. In four studies participants received platinum-based chemotherapy,48,51,64,73 in six studies participants were on non-platinum-based chemotherapy,62,67,70,71,77–80 in one study participants received platinum-based and non-platinum-based chemotherapy,63 in six studies participants were receiving chemotherapy but the type was unknown17,50,53,65,72,75 and in five studies participants were on mixed chemotherapy treatment. 52,66,68,69,74 Of the group of trials in which participants received mixed chemotherapy, two52,69 reported that the majority of participants received platinum-based chemotherapy (proportion not reported) and in one66 of the studies the majority of participants received non-platinum-based chemotherapy (proportion not reported). One trial involved participants on chemotherapy plus radiotherapy. 75
The majority of included studies specified the required baseline degree of anaemia in the eligibility criteria, with three studies not specifying this. The highest cut-off was a Hb level of ≤ 14.5 g/dl74 and the lowest was a Hb level of ≤ 8 g/dl. 72 Despite this, the mean/median Hb level at baseline ranged from 9.2 g/dl to 14.1 g/dl in the intervention group and from 9.1 g/dl to 14.1 g/dl in the control group.
Quality of the included studies
It was originally intended to use the Cochrane risk of bias tool to assess study quality; however, all trials were assessed using the same quality assessment tool as in the previous HTA review. 2 Quality assessment criteria are presented in Table 9 and the study quality appraisal is presented in Table 14. However, there is some variation in the method of quality assessment between the previous review and the current review. In the current appraisal, only information published in the primary studies was considered when conducting the quality appraisal, whereas the previous HTA review also used quality assessment information published in the 2004 Cochrane review. 45 Cochrane review authors contacted the trial investigators to request missing data, including information on study conduct. In addition, we have access to new information from papers published after the inclusion date for the previous review. Only primary studies were appraised, with secondary analyses of previously published data not assessed. Similarly, if a trial was reported in multiple publications, only one quality assessment of the trial was conducted. In total, 23 trials were assessed,17,48,50–53,62,63–78 including eight trials not included in the previous HTA review. 2 In addition, GRADE analysis was carried out, with the results presented in Appendix 3.
| Author, year | Random allocation | Concealment of allocation | Baseline similarity | Patients blinded | Physicians blinded | Losses | ITT or < 10% dropout |
|---|---|---|---|---|---|---|---|
| Abels 199363 | Uncleara | NR | Unclearb | Yes | Yes | Partially | Yes |
| Aravantinos 200364 | Uncleara | NR | Unclearb | No | No | NR | Yes |
| Boogaerts 200365 | Uncleara | NR | No: previous chemotherapy, FACT-F | No | No | Partially | Yes |
| Dammacco 200166 | Uncleara | Unclearc | Unclearb | Yes | Yes | Yes | Yes, primary end point and HRQoL only |
| Del Mastro 199767 | Yes | NR | Unclearb | No | NR | Partially | Yes, apart from HRQoL (87% and 84% of participants were analysed in the treatment and control groups, respectively) |
| Dunphy 199968 | Uncleara | NR | No: gender | No | No | Yes | No |
| Grote 200574 | Yes | NR | Unclearb | Yes | Yes | Partiallyd | Yes |
| Hedenus 200253 | Yes | Unclearc | No: gender, platelet and neutrophil counts | Yes | Yes | Partially | Yese |
| Hedenus 200317 | Yes | NR | Unclear | Yes | Yes | Partiallyd | Yese |
| Kotasek 200350 | Uncleara | NR | Yesf | Yes | Yes | Partiallyd | Yese |
| Kurz 199769 | Yes | Unclearc | Yes | Yes | Yes | NR | Yes, results report response for all participants; assumed ITT |
| Littlewood 200170 | Uncleara | NR | Unclearb | Yes | Yes | Yes | Yes, apart from HRQoL (80% and 73% of participants were analysed in the treatment and control groups, respectively) |
| Moebus 201362 | Yes | Unclearc | Unclearb | NR | NR | Yes | Yes |
| Österborg 200271 | Uncleara | NR | Unclearb | Yes | Yes | Partiallyd | Yes |
| Ray-Coquard 200975 | Uncleara | Unclearc | No: HRQoLb | No | No | Partially | Yes, apart from HRQoL (54% and 57% of participants were analysed in the treatment and control groups, respectively) |
| Silvestris 199572 | Yes | NR | NR | No | No | Yes | No |
| Strauss 200876 | Uncleara | Unclearc | Yes | No | No | Yes | Yes |
| ten Bokkel Huinink 199851 | Uncleara | NR | Unclearb | No | No | Partially | Yes, but two participants were excluded from ITT analyses |
| Thatcher 199952 | Uncleara | NR | Unclearb | No | NR | Yes | Yes, apart from HRQoL (75% and 61% of participants were analysed in the treatment and control groups, respectively) |
| Tjulandin 201177 | Yes | NR | Unclearb | Yes | Yes | Yes | Yes, apart from HRQoL (89.5–97.9% and 85.7–96.7% of participants were analysed in the treatment and control groups, respectively) |
| Tjulandin 201048 | Yes | Unclearg | Unclearb | Yes | Yes | Yes | Yes |
| Untch 201178 | Uncleara | NR | NRh | No | No | Partiallyd | Yes |
| Vansteenkiste 200273 | Uncleara | Unclearc | Unclearb | Yes | Yes | Partially | Yes,e apart from HRQoL (81% of participants were analysed in both the treatment group and the control group) |
Overall assessment
The 23 included RCTs were of variable quality but all are flawed, some because of reporting issues but others more substantially. For most of the trials it was difficult to make a general assessment about study quality because of reporting omissions. In fact, 1051,52,62,64,66,67,70,71,73,78–80 of the 23 trials either did not report, or lacked clarity on, at least three of the seven items constituting the quality appraisal tool used (see Table 9). Most notably, all trials lacked clarity in the reporting of allocation methods (the procedure for randomisation and/or allocation concealment). Three of the studies were of generally high quality,48,69,77 with each of these satisfactorily addressing five of the seven items of the quality appraisal tool used. However, even the reports of these three studies omitted important information relating to study quality. The study by Dunphy and colleagues68 has the poorest quality profile, followed by that by Boogaerts and colleagues,65 Ray-Coquard and colleagues75 and Silvestris and colleagues. 72 Further details of the quality of the included studies according to individual items on the quality appraisal tool used are provided in the following sections.
Treatment allocation
Random allocation
The method of random allocation was clearly stated and sufficient in nine trials,17,48,53,62,67,69,72,74,77 whereas 14 trials50–52,63–66,68,70,71,73,75,76,78–80 did not specify the method used.
Concealment of allocation
The method of concealment of allocation was not clearly reported in any of the included trials. Fourteen trials17,50–52,63–65,67,68,70–72,74,78–80 did not report any information on allocation concealment, whereas eight trials48,53,62,66,69,73,75,76 provided some information. A centralised system for randomisation was reported in seven trials53,62,66,69,73,75,76 and authors of one trial48 stated that only the person administering study medication was unblinded. It is therefore possible that the allocation sequence was concealed in these eight trials. However, as no specific details on allocation concealment were reported, this remains unclear.
Similarity of groups
Only three trials50,69,76 fully reported baseline characteristics, including p-values for baseline group comparisons. Authors of 14 trials17,48,51,52,62–64,66,67,70,71,73,74,77,79 stated that there was ‘similarity between groups’; however, no statistical information was reported to support this. Another four studies53,65,68,75 reported some baseline differences for one or more outcomes, whereas no baseline characteristics were reported for two trials;72,78,80 one78,80 of these two trials used a Latin square design and baseline characteristics are reported for groups randomised by chemotherapy but not for the erythropoietin randomisation.
Implementation of masking
Treatment allocation masked from participants
Participants were blinded to treatment allocation in 12 trials. 17,48,50,53,63,66,69–71,73,74,77,79 Ten trials51,52,64,65,67,68,72,75,76,78,80 did not blind participants from treatment allocation and one trial62 did not report any information about blinding participants to treatment allocation.
Treatment allocation masked from clinicians
The 12 trials17,48,50,53,63,66,69,70,71,73,74,77,79 that blinded participants to treatment allocation also masked treatment allocation from clinicians. Eight trials51,64,65,68,72,75,76,78,80 did not blind clinicians to treatment allocation and three trials52,62,67 did not report any information about blinding of clinicians to treatment allocation; these three trials compared erythropoietin groups with standard care.
Completeness of the trial
Reporting of losses to follow-up, withdrawals and dropouts
Losses to follow-up, withdrawals and dropouts were fully reported in nine trials48,52,62,66,68,70,72,76,77 and partially reported in 12 trials. 17,50,51,53,63,65,67,71,79,73–75,78,80 Among the 12 trials in which this information was partially reported, five trials17,50,71,74,78–80 reported withdrawals and dropouts until the end of the trials but did not provide any data on the follow-up period. Two trials64,69 did not report any information on losses to follow-up, withdrawals and dropouts.
Intention-to-treat analysis or < 10% of participants lost
Intention-to-treat (ITT) analysis or < 10% of participants lost was reported in 14 studies17,48,50,51,53,62,63–65,69,71,74,76,78–80 for all measured outcomes. ITT analysis or < 10% of participants lost was reported in seven studies52,66,67,70,73,75,77 for the primary outcome and most of the secondary outcomes. Only two trials68,72 did not use ITT analysis or reported ≥ 10% of participants lost.
Manufacturers’ reviews of clinical effectiveness
Two submissions were presented summarising evidence on the effectiveness of darbepoetin alfa (Aranesp)96 and epoetin alfa (Binocrit). 97
One was a systematic review submitted by Amgen Inc. summarising evidence of the effectiveness of darbepoetin alfa (Aranesp) and the other was an evidence summary submitted by Sandoz Ltd, summarising trials from its clinical development programme and post-approval trials (biosimilar epoetin alfa; Binocrit). Although neither are part of the PenTAG systematic review, they are presented here for convenience and because the results are compared.
Epoetin alfa (Binocrit)
Sandoz Ltd submitted an evidence summary that contained a number of publications that were excluded from the PenTAG review because they did not meet the inclusion criteria. A list of these publications with reasons for their exclusion can be found in Appendix 10.
The evidence summary consisted of:
-
Details of the clinical development programme for Binocrit:
-
Three Phase I studies: multiple intravenous doses of Binocrit compared with epoetin alfa 100 IU/kg three times a week;98 multiple subcutaneous doses of Binocrit compared with epoetin alfa 100 IU/kg three times a week;99 and multiple subcutaneous doses of Binocrit compared with epoetin beta 100 IU/kg three times a week. 100 All studies were of 4 weeks’ duration.
-
Pivotal data: two Phase III studies. 101,102 Both of the Phase III studies were identified in the PenTAG review; one was excluded on population (chronic renal failure)102 and the other was excluded on comparator (epoetin alfa assessed by class). 101
-
-
Post-approval data: four retrospective studies were identified, of which three were abstracts (one observational study,103 one single-centre audit104 and one retrospective, matched-cohort analysis105) and one was a fully published retrospective study. 106 These were not included in the PenTAG review as they were non-randomised studies.
Results from the identified studies were reported narratively. One Phase III trial101 evaluated the efficacy and safety of Binocrit in the treatment of CIA in cancer patients (n = 114; n = 94 ITT population). The comparator was epoetin alfa (Erypo/Eprex) and the primary end point was haematological response (absolute increase in Hb of ≥ 2 g/dl between the screening/baseline period and the evaluation period in the absence of RBCT during the preceding 4 weeks). Haematological response (as defined) was reported in 62% (n = 37/60) (95% CI 48.2% to 78.9%) of participants treated with Binocrit and RBCT requirement was 32% (n = 19/60) compared with 38% (n = 13/34) in the epoetin alfa (Erypo/Eprex) group. The study reported comparable efficacy and a similar safety profile to that expected for the therapeutic area.
Results from non-RCT and observational studies were presented to support the application with regard to the effectiveness of ESAs in terms of the haematological response (Hb change, RBCT requirement). The reported results are consistent with existing evidence in respect of these outcomes.
Evidence was also presented to support the following additional aspects:
-
pharmacoeconomic rationale for the use of biosimilars
-
adjusting the current recommendation regarding the trigger Hb level (≤ 8 g/dl) to align with UK marketing authorisation, product SPCs and clinical guidelines (≤ 10 g/dl)
-
advantages of using Binocrit over alternative ESAs, for example syringes have an innovative safety needle protector, extended shelf-life of 24 months.
Darbepoetin alfa (Aranesp)
Amgen Inc. presented a meta-analysis of pivotal trials as part of its submission. Searches for the systematic review were based on the previous HTA appraisal2 and included RCT evidence published since 2004 evaluating the efficacy and safety of ESAs for the treatment of CIA in cancer patients, specifically darbepoetin alfa. Studies that used a licensed starting dose (500 µg, 6.75 µg/kg once every 3 weeks or 2.25 µg/kg once a week) were considered eligible for inclusion.
A total of nine studies were identified that evaluated darbepoetin alfa compared with best supportive care (placebo, no treatment, usual care) for the treatment of CIA in cancer patients. Four were included in the PenTAG review. 17,50,53,73 Five studies were abstracts107–111 and as such were not included in the PenTAG systematic review as there was not enough information to quality appraise the abstracts; they are described in Appendix 10.
The pooled summary estimates presented for the effect of darbepoetin alfa on CIA in cancer patients are provided in Table 15.
| Outcome | Results from meta-analyses |
|---|---|
| Anaemia-related outcomes | |
| Hb changea,b | WMD 1.06, 95% CI 0.86 to 1.26, p < 0.00001; χ2(het) = 10.79, df = 2; p = 0.005; I2 = 81% 3 trials, n = 1645 |
| HaemRb,c | RR 3.67, 95% CI 2.73 to 4.94, p < 0.00001; χ2(het) = 1.77, df = 3; p = 0.62; I2 = 0% 4 trials, n = 528 |
| RBCTb | RR 0.56, 95% CI 0.49 to 0.64, p < 0.00001; χ2(het) = 4.43, df = 6; p = 0.62; I2 = 0% 7 trials, n = 1744 |
| Units transfusedb | WMD –1.25, 95% CI –1.84 to –0.66; p < 0.00001; heterogeneity NA 1 trial, n = 298 |
| Malignancy-related outcomes | |
| Tumour responseb | RR 0.99, 95% CI 0.89 to 1.09; p = 0.84; heterogeneity NA 1 trial, n = 599 |
| OSb | HR 0.88, 95% CI 0.72 to 1.06; p = 0.18; χ2(het) = 4.74, df = 3; p = 0.19; I2 = 37% 4 trials, n = NR |
| HRQoL | |
| FACT-F | 3 trials: results indicated darbepoetin alfa and PBO have a similar effect on HRQoL; 1 study reported a non-significant difference in favour of darbepoetin alfa vs. PBO |
| FACT-An | 1 trial: results indicated darbepoetin alfa and PBO have a similar effect on HRQoL (no significant difference between studies); 1 study reported a non-significant difference in favour of darbepoetin alfa vs. PBO |
| FACT-G | 1 trial: results indicated darbepoetin alfa and PBO have a similar effect on HRQoL (no significant difference between studies) |
| Safety-related outcomes | |
| No. of AEsb,d | RR 1.03, 95% CI 0.94 to 1.12; p = 0.51; χ2(het) = 0.02, df = 1; p = 0.90; I2 = 0% 1 trial, n = 665 |
| No. of SAEsb,e | RR 1.13, 95% CI 0.99 to 1.29; p = 0.08; χ2(het) = 0.03, df = 1; p = 0.86; I2 = 0% 2 trials, n = 1798 |
| Thromboembolic eventsb,f | RR 2.15, 95% CI 1.41 to 3.28; p = 0.0004; χ2(het) = 0.88, df = 2; p = 0.64; I2 = 0% 3 trials, n = 2112 |
The pooled summary estimates presented for the effect of ESAs (specifically darbepoetin alfa for this analysis) were largely consistent with the summary estimates in the PenTAG systematic review, particularly with respect to improvements in haematological response and reduction in RBCT requirements. No significant difference was observed for the outcome of Hb change. Estimates for the malignancy-related outcomes – tumour response and survival – suggested a benefit of treatment compared with the control; however, the results were not statistically significant and there was evidence of heterogeneity in the case of OS. In addition, data were insufficient in this respect to rule out detrimental effects; however, this uncertainty is consistent with previously reported estimates. Estimates for thromboembolic events (RR 2.15, 95% CI 1.41 to 3.28) were worse than estimates in the PenTAG review.
Ongoing studies
Searches of ClinicalTrials.gov and Current Controlled Trials yielded a total of 218 trials. Of these, 95 trials were considered to be relevant to this review; however, in all cases it was not possible to ascertain whether ESAs were evaluated in accordance with their licensed indications. Seven studies were identified as ongoing (n = 2) or recruiting (n = 5). In six trials the current status was recorded as ‘unknown’. Ten trials had terminated and, of these, three had results available. Finally, 72 studies had been completed. An overview of these trials is provided in Appendix 11.
Effectiveness
Anaemia-related outcomes
Anaemia-related outcomes included mean Hb change [measured as a change in Hb level (g/dl) from baseline until the end of the treatment period], haematological response (defined as the proportion of participants with an increase in Hb level of ≥ 2 g/dl or as an increase in haematocrit of ≥ 6 percentage points, unrelated to transfusion) and RBCT requirements [number of participants transfused and number of units transfused per average patient (i.e. including participants not requiring transfusion)].
Haemoglobin change
The mean Hb change was measured as a change in Hb level (g/dl) from baseline until the end of the treatment period. In total, 20 trials17,48,50,51,53,62–72,74,76–79 measured Hb change, of which 1617,48,50,51,53,63–67,69,70,74,77–79 were included in the meta-analysis. Two studies62,76 [the study by Moebus and colleagues62 was included as an abstract33 in the Cochrane review by Tonia and colleagues11) reported only the median change in Hb (g/dl) without any measure of variance and in two studies,68,72 no point estimates were reported and the results were presented graphically. These four studies were excluded from the analyses.
Overall, the analysis included 16 trials with 3170 participants. 17,48,50,51,53,63–67,69,70,74,77–79 Four trials were newly identified in the update searches. 48,74,77,78 As some trials with multiple experimental arms were split into subsets,48,63 the number of trials displayed is 18.
The random-effects meta-analysis demonstrated a statistically significant difference in Hb change in favour of treatment [weighted mean difference (WMD) 1.59 g/dl, 95% CI 1.33 to 1.84 g/dl; Figure 2]. Although all individual studies indicated a beneficial effect of ESAs with regard to Hb change and varied only in magnitude, there was statistically significant heterogeneity between the trials [I2 = 75.9%, p < 0.001; χ2 = 70.52, degrees of freedom (df) = 17; p < 0.01]. To assess whether publication bias was likely, a funnel plot was constructed (see Appendix 12). The funnel plot analysis did not show statistically significant asymmetry (p = 0.133). In addition, a meta-regression using publication year as a covariate (to assess the effect of publication year on Hb change) showed that the effects of ESA on Hb change were independent of any effect of publication year (p = 0.180); the meta-regression plot is presented in Appendix 12. The fixed-effects meta-analysis undertaken as a sensitivity analysis also showed a statistically significant difference in Hb change in favour of treatment (WMD 1.49 g/dl, 95% CI 1.37 to 1.60 g/dl; I2 = 75.9%; p < 0.001); the forest plot of this analysis is provided in Appendix 12.
FIGURE 2.
Forest plot: Hb change overall (g/dl). Random-effects meta-analysis (DerSimonian–Laird). SD, standard deviation.
To identify sources of heterogeneity, subgroup analyses were conducted (Table 16). In addition, meta-regression models that included random effect and subgroup as covariates (to assess the effects of subgroups on Hb change) were performed; the F-statistics from these analyses are reported in Table 16. All covariates showing a significant effect (p < 0.05) in a univariate analysis were considered further in model selection.
| Subgroup | Number of trials | WMD | 95% CI | I 2 | Tau2 |
|---|---|---|---|---|---|
| Overall | 18 | 1.59 | 1.33 to 1.84 | 75.9%; p < 0.01 | 0.22 |
| Inclusion Hb level (g/dl) | |||||
| ≤ 11.0 | 13 | 1.52 | 1.30 to 1.75 | 48.1%; p = 0.03 | 0.08 |
| > 11.0 | 5 | 1.75 | 1.03 to 2.47 | 91.4%; p < 0.01 | 0.60 |
| F (between : within) | F1,16 = 0.47; p = 0.50 | ||||
| Baseline Hb level (g/dl) | |||||
| ≤ 10.0 | 13 | 1.51 | 1.29 to 1.72 | 43.6%; p = 0.05 | 0.06 |
| ≤ 11.0 | 1 | 1.98 | 1.42 to 2.54 | NA | 0 |
| ≤ 12.0 | 1 | 1.23 | 0.48 to 1.98 | NA | 0 |
| ≤ 14.5 | 3 | 1.94 | 0.68 to 3.19 | 95.5%; p < 0.01 | 1.17 |
| F (between : within) | F3,14 = 0.60; p = 0.63 | ||||
| Target Hb level (g/dl) | |||||
| ≤ 13.0 | 4 | 1.29 | 0.90 to 1.67 | 61.9%; p = 0.05 | 0.10 |
| > 13.0 | 11 | 1.59 | 1.27 to 1.91 | 74.0%; p < 0.01 | 0.21 |
| NR | 3 | 2.03 | 1.42 to 2.65 | 46.0%; p = 0.16 | 0.14 |
| F (between : within) | F2,15 = 1.33; p = 0.29 | ||||
| Malignancy type | |||||
| Solid tumours | 9 | 1.65 | 1.11 to 2.18 | 85.2%; p < 0.01 | 0.53 |
| Haematological tumours | 6 | 1.63 | 1.33 to 1.93 | 49.2%; p = 0.08 | 0.07 |
| Mixed | 3 | 1.44 | 1.15 to 1.74 | 28.1%; p = 0.25 | 0.02 |
| F (between : within) | F2,15 = 0.12; p = 0.89 | ||||
| Ovarian cancer | |||||
| Ovarian cancer | 1 | 1.23 | 0.48 to 1.98 | NA | 0 |
| Other cancers | 17 | 1.60 | 1.34 to 1.87 | 77.2%; p < 0.01 | 0.23 |
| F (between : within) | F1,16 = 0.34; p = 0.57 | ||||
| Chemotherapy treatmenta | |||||
| Platinum containing | 5 | 1.42 | 1.10 to 1.75 | 0%; p = 0.77 | 0 |
| Non-platinum containing | 6 | 1.62 | 1.20 to 2.03 | 82.4%; p < 0.01 | 0.21 |
| F (between : within) | F1,9 = 0.40; p = 0.54 | ||||
| Iron supplementation | |||||
| Iron in both arms | 10 | 1.60 | 1.38 to 1.82 | 40.7%; p = 0.09 | 0.05 |
| Iron in an intervention arm | 1 | 0.91 | 0.65 to 1.17 | NA | 0 |
| NR | 7 | 1.62 | 1.07 to 2.16 | 79.2%; p < 0.01 | 0.42 |
| F (between : within) | F2,15 = 1.07; p = 0.37 | ||||
| Study design | |||||
| RCT | 13 | 1.70 | 1.43 to 1.97 | 64.9%; p < 0.01 | 0.15 |
| ROL | 5 | 1.30 | 0.86 to 1.73 | 72.0%; p < 0.01 | 0.16 |
| F (between : within) | F1,16 = 1.97; p = 0.18 | ||||
| Study duration (weeks) | |||||
| 12–16 | 12 | 1.65 | 1.40 to 1.89 | 50.4%; p = 0.02 | 0.09 |
| 17–20 | 2 | 1.92 | 0.34 to 3.51 | 90.8%; p < 0.01 | 1.19 |
| > 20 | 4 | 1.24 | 0.86 to 1.62 | 69.6%; p = 0.02 | 0.10 |
| F (between : within) | F2,15 = 1.67; p = 0.22 | ||||
| ESA | |||||
| Erythropoietin | 14 | 1.74 | 1.49 to 2.00 | 62.7%; p < 0.01 | 0.14 |
| Darbepoetin | 4 | 1.07 | 0.61 to 1.52 | 71.4%; p = 0.02 | 0.14 |
| F (between : within) | F1,16 = 6.32; p = 0.02 | ||||
Univariate analyses identified significant differences for one of the subgroups, ESA therapy [short-acting (erythropoietin) vs. long-acting (darbepoetin)] (p = 0.023; Figure 3). For subgroup analysis by erythropoietin treatment type, the short-acting ESA treatment (WMD 1.74 g/dl, 95% CI 1.49 to 2.00 g/dl; I2 = 62.7%; p = 0.001) appeared to have a greater benefit than the long-acting ESA treatment (WMD 1.06 g/dl, 95% CI 0.61 to 1.52 g/dl; I2 = 71.4%; p = 0.015). The results were also investigated visually. One small study69 (n = 35) appeared to differ from most of the other included trials; this study reported the highest mean difference between the ESA group and the control group. Excluding this study from the meta-analysis did not change the overall conclusions (data not reported). We therefore included all 18 trials in the analysis of Hb change.
FIGURE 3.
Forest plot: Hb change by treatment drug (g/dl). Note: random-effects meta-analysis (DerSimonian–Laird). SD, standard deviation.
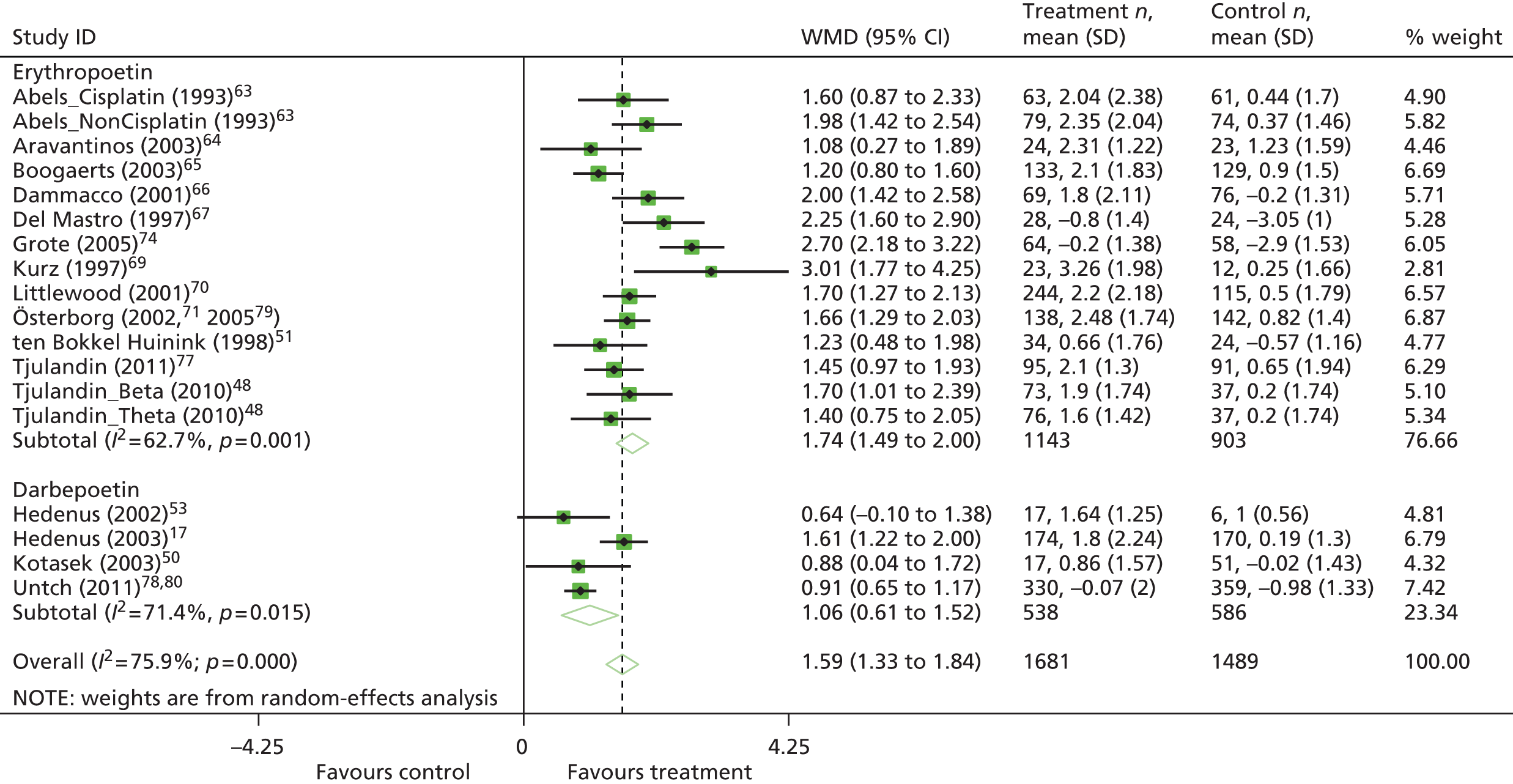
Overall, there is a statistically significant effect of ESAs on Hb change. Compared with the control group, patients receiving ESAs achieve a weighted mean Hb increase of 1.59 g/dl from baseline to the end of treatment (95% CI 1.33 to 1.84 g/dl). We identified statistically significant heterogeneity between the trials (I2 = 75.9%; p < 0.001); however, all individual studies indicated a beneficial effect of ESAs with regard to Hb change. Subgroup analyses suggested that short-acting (erythropoietin) ESA treatment may offer greater benefits than long-acting (darbepoetin) ESA treatment. However, as the number of studies in the subgroup analysis was very small, this analysis may not have statistical power to detect the effects of short- or long-acting ESAs on Hb change, if such effects exist. Overall, the data confirm the results from previous analyses: compared with control groups, patients receiving ESAs improved their Hb levels.
Haematological response
This binary outcome was defined as the proportion of participants with an increase in Hb level of ≥ 2 g/dl or as an increase in haematocrit of ≥ 6 percentage points, unrelated to transfusion. Eight trials defined haematological response as the proportion of participants with an increase in Hb level of ≥ 2 g/dl,17,48,50,65,66,70,77,79 one study defined haematological response as an increase in haematocrit of ≥ 6 percentage points63 and one trial reported haematological response using both definitions;53 for consistency, haematological response as defined by an increase in Hb level was used in the analyses. Two studies69,73 described haematological response as an increase in Hb level of ≥ 2 g/dl or as a Hb level > 12 g/dl and were therefore excluded from the analyses.
Although both the previous HTA review2 and the study by Tonia and colleagues11 used the same definition of haematological response, only the study by Tonia and colleagues11 excluded both the trial by Kurz and colleagues69 and the trial by Vansteenkiste and colleagues73 from the analyses. The previous HTA review2 argued that most of the data in the trial by Vansteenkiste and colleagues73 would have been derived from an increase in Hb of 2 g/dl (considering baseline Hb values) and included it in the analyses. Vansteenkiste and colleagues73 reported mean baseline Hb levels of 10.28 g/dl [standard deviation (SD) 1.08 g/dl] and 9.93 g/dl (SD 1.01 g/dl) in the treatment and control groups respectively. Kurz and colleagues69 reported mean baseline Hb levels of 9.88 g/dl (SD 0.89 g/dl) and 9.85 g/dl (SD 0.60 g/dl) in the treatment and control groups respectively. For consistency with the previous HTA review,2 sensitivity analyses including the trials by Vansteenkiste and colleagues73 and Kurz and colleagues69 were performed.
Overall, the analysis of haematological response included 10 trials with 2228 participants. 17,48,50,53,63,65,66,70,77,79 Two trials were newly identified in the update searches. 48,77 As some trials with multiple experimental arms were split into subsets,48,63 the number of trials displayed is 12.
Haematological response was observed in 759 out of 1213 participants in the ESA-treated groups, compared with 182 out of 1015 participants in the control groups. The random-effects meta-analysis showed a statistically significant difference in haematological response in favour of treatment (RR 3.29, 95% CI 2.84 to 3.81; Figure 4). Heterogeneity between the trials was not significant (I2 = 6.4%, p = 0.383; χ2 = 11.75, df = 11, p = 0.383), with all individual studies indicating a beneficial effect of ESAs with regard to haematological response. To test whether publication bias was present in the meta-analysis, funnel plot asymmetry was investigated (see Appendix 12). The funnel plot analysis did not suggest statistically significant asymmetry (p = 0.275). A meta-regression using publication year as a covariate to assess the effect of publication year on haematological response suggested that earlier published studies tended to report higher effects than later published studies (p = 0.044). The earlier studies also tended to be smaller trials (see the meta-regression plot in Appendix 12).
FIGURE 4.
Forest plot: haematological response overall. Note: random-effects meta-analysis (DerSimonian–Laird). Events, treatment = number of events/number of participants in the treatment group; events, control = number of events/number of participants in the control group.

The fixed-effects meta-analysis undertaken as a sensitivity analysis also showed a statistically significant difference in haematological response in favour of treatment (RR 3.41, 95% CI 2.96 to 3.92; I2 = 6.4%; p = 0.383); the forest plot of the analysis is provided in Appendix 12. Including the trials by Kurz and colleagues69 and Vansteenkiste and colleagues73 in the meta-analysis did not affect the overall conclusions (RR 3.21, 95% CI 2.81 to 3.68; I2 = 8.2%, p = 0.363; the forest plot of the analysis is provided in Appendix 12). Similarly to the Hb change outcome, the trial by Kurz and colleagues69 (n = 35) appeared to differ from most of the other included trials. This study reported the highest RR for haematological response, with wide CIs (RR 14.63, 95% CI 0.94 to 226.68).
Prespecified subgroup analysis was performed (Table 17). None of the studies with available haematological response data included ovarian cancer patients. Therefore, the planned ovarian cancer subgroup analysis was not completed. In addition, meta-regression models including random effect and subgroups as covariates to assess the effects of a subgroup on haematological response were performed; the F-statistics from these analyses are reported in Table 17. All covariates showing a significant effect (p < 0.05) in a univariate analysis were further considered in a model selection.
| Subgroup | No. of trials | RR | CI | I 2 | Tau2 |
|---|---|---|---|---|---|
| Analyses using all main trials | |||||
| Overall | 12 | 3.29 | 2.84 to 3.81 | 6.4%; p = 0.383 | < 0.01a |
| Chemotherapy treatmentb | |||||
| Platinum containing | 3 | 3.93 | 2.50 to 6.17 | 11.9%; p = 0.32 | 0.02 |
| Non-platinum containing | 4 | 3.05 | 2.43 to 3.82 | 29.9%; p = 0.23 | 0.02 |
| F (between : within) | F1,5 = 1.24; p = 0.32 | ||||
| Iron supplementation | |||||
| Iron in both arms | 7 | 3.05 | 2.63 to 3.54 | 0%; p = 0.67 | 0 |
| NR | 5 | 4.94 | 3.38 to 7.20 | 0%; p = 0.72 | 0 |
| F (between : within) | F1,10 = 11.94; p < 0.01 | ||||
| Study design | |||||
| RCT | 11 | 3.31 | 2.81 to 3.90 | 13.8%; p = 0.32 | 0.01 |
| ROL | 1 | 3.59 | 2.23 to 5.80 | NA | 0 |
| F (between : within) | F1,10 = 0.12; p = 0.73 | ||||
| Study duration (weeks) | |||||
| 12–16 | 10 | 3.29 | 2.73 to 3.97 | 18.6%; p = 0.27 | 0.02 |
| > 20 | 2 | 3.65 | 2.71 to 4.92 | 0%; p = 0.94 | 0 |
| F (between : within) | F1,10 = 0.23; p = 0.64 | ||||
| ESA | |||||
| Erythropoietin | 9 | 3.41 | 2.80 to 4.16 | 29.7%; p = 0.18 | 0.03 |
| Darbepoetin | 3 | 3.35 | 2.45 to 4.58 | 0%; p = 0.83 | 0 |
| F (between : within) | F1,10 = 0; p = 0.96 | ||||
| Analyses using results for Hb inclusion subgroups70 | |||||
| Overall | 13 | 3.29 | 2.81 to 3.85 | 13.4%; p = 0.31 | 0.01 |
| Inclusion Hb level (g/dl) | |||||
| ≤ 11.0 | 12 | 3.20 | 2.78 to 3.68 | 2.0%; p = 0.43 | < 0.01 |
| > 11.0 | 1 | 25.52 | 1.66 to 392.30 | NA | 0 |
| F (between : within) | F1,11 = 104.53; p < 0.01 | ||||
| Baseline Hb level (g/dl) | |||||
| ≤ 10.0 | 11 | 3.15 | 2.72 to 3.63 | 1.9%; p = 0.42 | < 0.01 |
| ≤ 11.0 | 1 | 4.31 | 2.35 to 7.90 | NA | 0 |
| ≤ 12.0 | 1 | 25.52 | 1.66 to 392.30 | NA | 0 |
| F (between : within) | F2,10 = 49.43; p < 0.01 | ||||
| Target Hb level (g/dl) | |||||
| ≤ 13.0 | 3 | 3.06 | 2.28 to 4.09 | 0%; p = 0.79 | 0 |
| > 13.0 | 8 | 3.25 | 2.63 to 4.01 | 24.5%; p = 0.23 | 0.02 |
| NR | 2 | 5.00 | 2.99 to 8.37 | 0%; p = 0.35 | 0 |
| F (between : within) | F2,10 = 0.31; p = 0.74 | ||||
| Analyses using results for malignancy subgroups70 | |||||
| Overall | 13 | 3.28 | 2.84 to 3.79 | 4.3%; p = 0.40 | < 0.01c |
| Malignancy type | |||||
| Solid tumours | 4 | 3.70 | 2.63 to 5.18 | 0%; p = 0.844 | 0 |
| Haematological tumours | 7 | 3.55 | 2.70 to 4.67 | 43%; p = 0.10 | 0.05 |
| Mixed | 2 | 3.13 | 2.33 to 4.20 | 0%; p = 0.47 | 0 |
| F (between : within) | F2,10 = 0.89; p = 0.44 | ||||
One study70 provided separate results for the subgroups malignancy type (solid and haematological malignancy) and baseline Hb level [≤ 10.5 g/dl and > 10.5 g/dl (but ≤ 12 g/dl)]. In addition to results from the ITT population, results for these subgroups were also used in the PenTAG meta-analyses: using baseline Hb levels the effect estimate was RR 3.29 (95% CI 2.81 to 3.85, I2 = 13.4%; p = 0.310; see Appendix 12) and using malignancy type the effect estimate was RR 3.28 (95% CI 2.84 to 3.78, I2 = 13.4%; p = 0.403; see Appendix 12). In addition, the trial by Vansteenkiste and colleagues73 also reported subgroup results for participants with baseline Hb levels < 10.0 g/dl and for participants with baseline Hb levels ≥ 10.0 g/dl (but ≤ 11 g/dl) (reported in Vansteenkiste and colleagues84). Including the trials by Kurz and colleagues69 and Vansteenkiste and colleagues73 in the meta-analyses with subgroup results had no impact on the overall conclusions.
Univariate analyses identified significant differences between trials reporting use of iron supplementation and trials not reporting use of iron supplementation (p = 0.006; Figure 5). Trials that did not report whether they used iron supplementation appeared to offer greater benefits (RR 4.94, 95% CI 3.38 to 7.20, I2 = 0%; p = 0.752) than trials using iron supplementation (RR 3.05, 95% CI 2.63 to 3.54, I2 = 0%; p = 0.669). The meta-regression model with iron subgroups is presented in Appendix 12. However, including the trials by Kurz and colleagues69 and Vansteenkiste and colleagues73 in the meta-regression model with iron supplementation as a covariate provided different results; the difference between trials using iron supplementation and trials not reporting iron supplementation was no longer significant (p = 0.735). As noted earlier, the trial by Kurz and colleagues69 appeared to differ from the other included studies. A sensitivity analysis including the trial by Vansteenkiste and colleagues73 but excluding that by Kurz and colleagues69 again suggested that trials not reporting iron supplementation offer greater benefits (p = 0.037). The studies not reporting whether they used iron supplementation tended to be smaller (see Figure 5). Univariate analyses using the Hb subgroup results identified significant differences based on baseline and inclusion Hb levels (see Table 17). However, these results seemed to be driven mainly by the study by Littlewood and colleagues70 [Hb subgroup < 12 g/dl; RR 25.52, 95% CI 1.66 to 392.3; I2 = not applicable (NA); Figure 6] for both the baseline and the inclusion Hb levels. Because of collinearity we did not combine the baseline and inclusion Hb level subgroups in the same model. A model using the Hb baseline subgroup as a covariate suggests that participants with a higher baseline Hb level (< 12 g/dl; only one study was included in this subgroup) favoured treatment significantly more (RR 25.52, 95% CI 1.66 to 392.3, I2 = NA) than participants with Hb baseline values of < 11 g/dl (RR 3.76, 95% CI 2.62 to 5.39, I2 = 0%; p = 0.583) and participants with Hb baseline values of < 10 g/dl (RR 3.10, 95% CI 2.64 to 3.64, I2 = 19.7%; p = 0.244; see Figure 6). The meta-regression with baseline Hb subgroup as a covariate is presented in Appendix 12. Including the trials by Kurz and colleagues69 and Vansteenkiste and colleagues73 in the meta-analyses with Hb subgroup results had no impact on the conclusions. However, it should be highlighted that only one trial (n = 56) contributed to the subgroup with Hb baseline levels of < 12 g/dl.
FIGURE 5.
Forest plot: haematological response by iron supplementation. Note: random-effects meta-analysis (DerSimonian–Laird). Events, treatment = number of events/number of participants in the treatment group; events, control = number of events/number of participants in the control group.
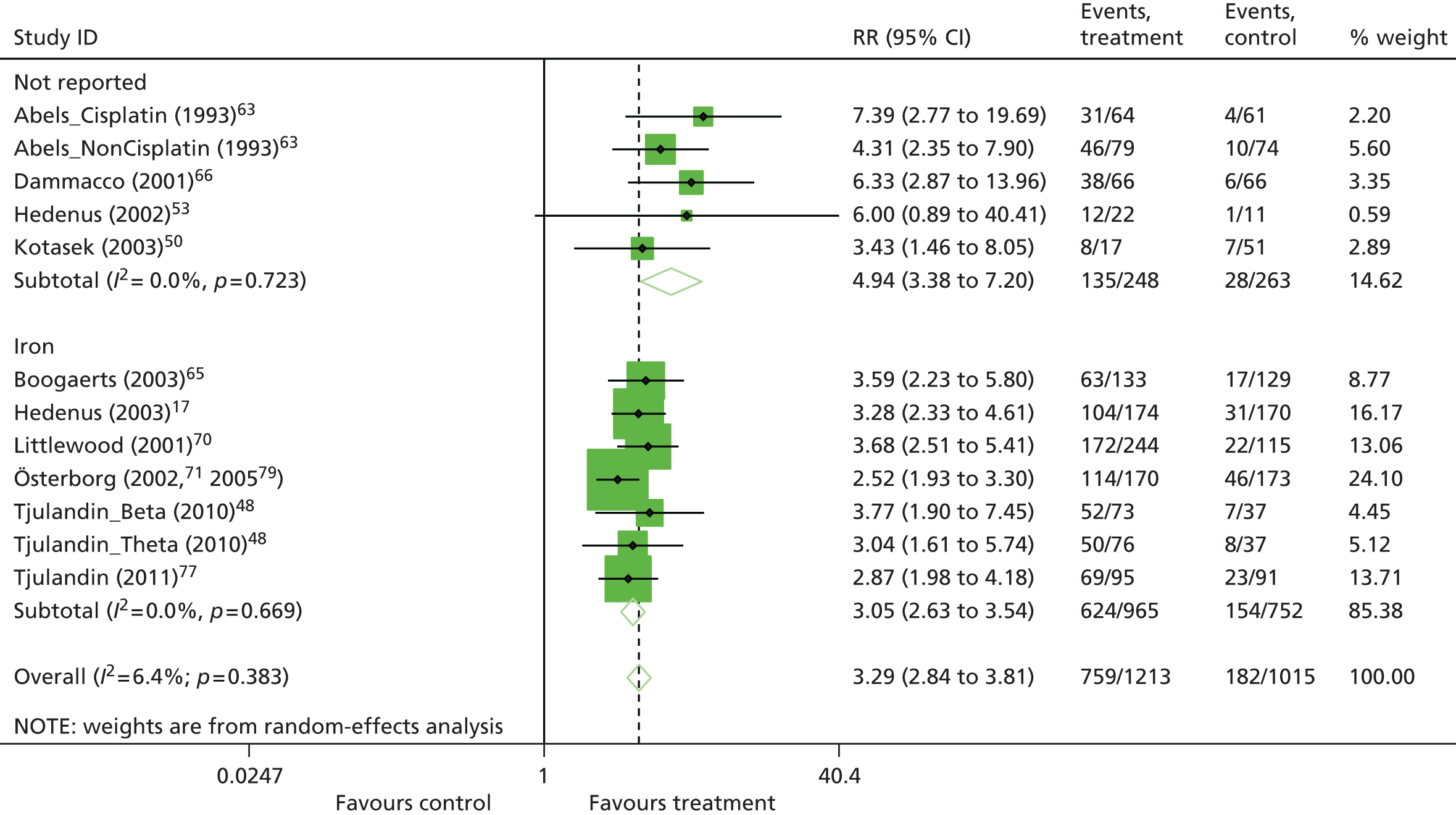
FIGURE 6.
Forest plot: haematological response using Hb subgroups by Hb baseline level. Note: random-effects meta-analysis (DerSimonian–Laird). Events, treatment = number of events/number of participants in the treatment group; events, control = number of events/number of participants in the control group.

Because of the small number of studies in the meta-analysis, these meta-regressions and subgroup analyses have to be interpreted with caution (see Methods of data analysis/synthesis). The Cochrane Handbook for Systematic Reviews of Interventions54 recommends at least 10 studies per subgroup. In addition, sensitivity analyses (e.g. including data from the trials by Kurz and colleagues69 and Vansteenkiste and colleagues73) suggest that there are differences in the impact of covariates.
Analyses suggest that ESA treatment in CIA is effective in producing a haematological response as defined by an increase in Hb level of ≥ 2 g/dl or an increase in haematocrit of ≥ 6 percentage points. In total, 63% (n = 759/1213) of participants who received ESA treatment had a haematological response compared with 18% (n = 182/1015) of control patients. The heterogeneity between the trials was non-significant (I2 = 6.4%; p = 0.383), with all individual studies indicating a beneficial effect of ESAs with regard to Hb response. The results of the subgroup analyses were non-conclusive, suggesting that the analyses may not have the statistical power to detect effects of subgroups on haematological response if such effects exist. Overall, the results support previous analyses.
Red blood cell transfusion requirement
This binary outcome was defined as the proportion of participants requiring a RBCT. Overall, the analysis of RBCT requirement included 22 trials17,48,50–53,62,63–70,73–79 with 4779 participants. Seven trials were newly identified in the update searches. 48,62,74–80 As some trials with multiple experimental arms were split into subsets,48,63 the number of studies displayed is 24.
A RBCT was required by 554 of 2480 participants treated with ESAs compared with 835 of 2299 participants receiving placebo/no treatment. The random-effects meta-analysis showed a statistically significant difference in RBCT requirement in favour of the treatment group (RR 0.63, 95% CI 0.57 to 0.69; Figure 7). The heterogeneity between the trials was not significant (I2 = 10.5%, p = 0.315; χ2 = 25.71, df = 23, p = 0.315). All but one individual study78,80 indicated a beneficial effect of ESAs with regard to RBCT requirement. To test whether publication bias was present in the sample included in the meta-analysis, funnel plot asymmetry was investigated (see Appendix 12). The funnel plot analysis did not show statistically significant asymmetry (p = 0.234). A meta-regression using publication year as a covariate to assess the effect of publication year on RBCT requirement was not statistically significant (p = 0.208; see meta-regression plots in Appendix 12).
FIGURE 7.
Forest plot: RBCT. Notes: random-effects meta-analysis (DerSimonian–Laird); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 report data for epoetin theta and epoetin beta and Abels and colleagues63 report data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy. Events, treatment = number of events/number of participants in the treatment group; events, control = number of events/number of participants in the control group.

The fixed-effects meta-analysis undertaken as a sensitivity analysis showed a statistically significant difference in RBCT requirement in favour of treatment (RR 0.62, 95% CI 0.51 to 0.67); the forest plot of this analysis is provided in Appendix 12).
Prespecified subgroup analyses were performed (Table 18). In addition, meta-regression models including random effect and subgroups as a covariate to assess the effects of a subgroup on RBCT requirement were performed. The F statistics from these analyses are reported in Table 18. All covariates showing a significant effect (p < 0.05) in a univariate analysis were considered further in model selection.
| Subgroup | Number of trials | RR | CI | I 2 | Tau2 |
|---|---|---|---|---|---|
| Analyses using all main trials | |||||
| Overall | 24 | 0.63 | 0.57 to 0.69 | 10.5%; p = 0.32 | 0.01 |
| Chemotherapy treatmenta | |||||
| Platinum containing | 6 | 0.52 | 0.37 to 0.72 | 60.0%; p = 0.03 | 0.08 |
| Non-platinum containing | 7 | 0.65 | 0.53 to 0.79 | 31.1%; p = 0.19 | 0.02 |
| F (between : within) | F1,11 = 0.21; p = 0.66 | ||||
| Iron supplementation | |||||
| Iron in both arms | 14 | 0.61 | 0.54 to 0.68 | 0%; p = 0.460 | 0 |
| Iron in an intervention arm | 1 | 3.18 | 0.13 to 77.7 | NA | 0 |
| Iron not used | 1 | 0.77 | 0.50 to 1.16 | NA | 0 |
| NR | 8 | 0.66 | 0.55 to 0.80 | 29.4%; p = 0.193 | 0.02 |
| F (between : within) | F3,20 = 1.08; p = 0.38 | ||||
| Study design | |||||
| RCT | 14 | 0.66 | 0.60 to 0.73 | 0%; p = 0.78 | 0 |
| ROL | 10 | 0.56 | 0.45 to 0.71 | 37.7%; p = 0.11 | 0.04 |
| F (between : within) | F1,22 = 0.61; p = 0.44 | ||||
| Study duration (weeks) | |||||
| 6–9 | 2 | 0.76 | 0.40 to 1.47 | 0%; p = 0.39 | 0 |
| 12–16 | 14 | 0.66 | 0.60 to 0.74 | 0%; p = 0.73 | 0 |
| 17–20 | 3 | 0.50 | 0.38 to 0.66 | 26.5%; p = 0.26 | 0.02 |
| > 20 | 5 | 0.62 | 0.45 to 0.85 | 48.0%; p = 0.10 | 0.05 |
| F (between : within) | F3,20 = 0.57; p = 0.64 | ||||
| ESA | |||||
| Erythropoietin | 19 | 0.62 | 0.55 to 0.70 | 27.1%; p = 0.13 | 0.02 |
| Darbepoetin | 5 | 0.63 | 0.52 to 0.75 | 0%; p = 0.89 | 0 |
| F (between : within) | F1,22 = 0.03; p = 0.86 | ||||
| Analysed using results for baseline Hb subgroups70,73 | |||||
| Overall | 26 | 0.61 | 0.55 to 0.68 | 22.4%; p = 0.15 | 0.02 |
| Inclusion Hb level (g/dl) | |||||
| ≤ 11.0 | 16 | 0.64 | 0.57 to 0.71 | 7.3%; p = 0.37 | < 0.01 |
| > 11.0 | 10 | 0.56 | 0.44 to 0.72 | 39.1%; p = 0.10 | 0.05 |
| F (between : within) | F1,24 = 0.72; p = 0.40 | ||||
| Baseline Hb level (g/dl) | |||||
| ≤ 10.0 | 15 | 0.64 | 0.58 to 0.71 | 0%; p = 0.69 | 0 |
| ≤ 11.0 | 2 | 0.60 | 0.31 to 1.18 | 81.4%; p = 0.02 | 0.19 |
| ≤ 12.0 | 3 | 0.38 | 0.14 to 1.00 | 74.1%; p = 0.02 | 0.52 |
| ≤ 14.5 | 5 | 0.69 | 0.52 to 0.92 | 0%; p = 0.69 | 0 |
| NR | 1 | 0.47 | 0.34 to 0.66 | NA | NA |
| F (between : within) | F1,24 = 0.28; p = 0.60 | ||||
| Target Hb level (g/dl) | |||||
| ≤ 13.0 | 4 | 0.52 | 0.34 to 0.80 | 48.4; p = 0.14 | 0.04 |
| > 13.0 | 19 | 0.60 | 0.53 to 0.67 | 0%; p = 0.70 | 0 |
| NR | 3 | 0.71 | 0.51 to 1.00 | 22.4%; p = 0.15 | 0.02 |
| F (between : within) | F2,23 = 0.82; p = 0.45 | ||||
| Analysed using results for malignancy subgroups70 | |||||
| Overall | 25 | 0.62 | 0.56 to 0.68 | 15.8%; p = 0.24 | 0.01 |
| Malignancy type | |||||
| Solid tumours | 15 | 0.56 | 0.48 to 0.66 | 17.2%; p = 0.26 | 0.01 |
| Haematological tumours | 7 | 0.68 | 0.59 to 0.79 | 15.3%; p = 0.31 | 0.02 |
| Mixed | 3 | 0.61 | 0.50 to 0.75 | 0%; p = 0.92 | 0 |
| F (between : within) | F2,22 = 0.70; p = 0.51 | ||||
One study70 reported results for the subgroups malignancy type (solid and haematological malignancy) and baseline Hb level [≤ 10.5 g/dl and > 10.5 g/dl (but ≤ 12 g/dl)]. Vansteenkiste and colleagues73 also reported subgroup results for participants with baseline Hb levels < 10.0 g/dl and ≥ 10.0 g/dl (but ≤ 11 g/dl) (reported in Vansteenkiste and colleagues84). In addition to results from the ITT population, results for these subgroups were included in the PenTAG meta-analyses: using the Hb subgroups the effect estimate was RR 0.61 (95% CI 0.55 to 0.68, I2 = 22.4%; p = 0.015) and using the malignancy subgroups the effect estimate was RR 0.62 (95% CI 0.56, 0.68, I2 = 15.8%; p = 0.239; see Appendix 12).
Univariate analyses did not identify any significant differences based on the predefined subgroups (see Table 18).
The RR for receiving a RBCT was statistically significantly reduced by 37% in the study groups receiving ESAs (RR 0.63, 95% CI 0.57 to 0.69). Heterogeneity between the studies was non-significant (I2 = 10.5%; p = 0.315). Overall, the data confirm the results from previous analyses that ESAs reduce the RR for receiving a RBCT in patients with CIA.
Number of red blood cell units transfused
Overall, 10 trials51,52,63,65,66,69,73,74,77,79 evaluating a total of 1920 participants were included in the analysis of RBC units transfused. As one study63 was split into subsets, the number of trials displayed is 11. Two trials were newly identified;74,77 neither was included in the Cochrane review11 for the analysis of this outcome. All except one study77 reported the mean number of units transfused per average participant (i.e. regardless of whether participants had received a RBCT). For Tjulandin and colleagues77 this was calculated from the data presented in the published paper.
The overall mean difference between groups showed a statistically significant benefit for participants receiving ESAs (WMD –0.87, 95% CI –1.28 to –0.46; Figure 8); the ESA group received fewer units of blood per participant than the control group. The heterogeneity between the studies was significant (I2 = 59.3%; p = 0.006). All but one study indicated a reduced need for RBCs in participants receiving ESAs compared with control subjects. A funnel plot analysis did not suggest statistically significant asymmetry (p = 0.137; see Appendix 12).
FIGURE 8.
Forest plot: mean number of RBC units transfused. Notes: random-effects meta-analysis (DerSimonian–Laird); trial with multiple experimental arms split into subsets in the analysis: Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy; mean units transfused per average participant (i.e. regardless of whether participants had received a RBCT).
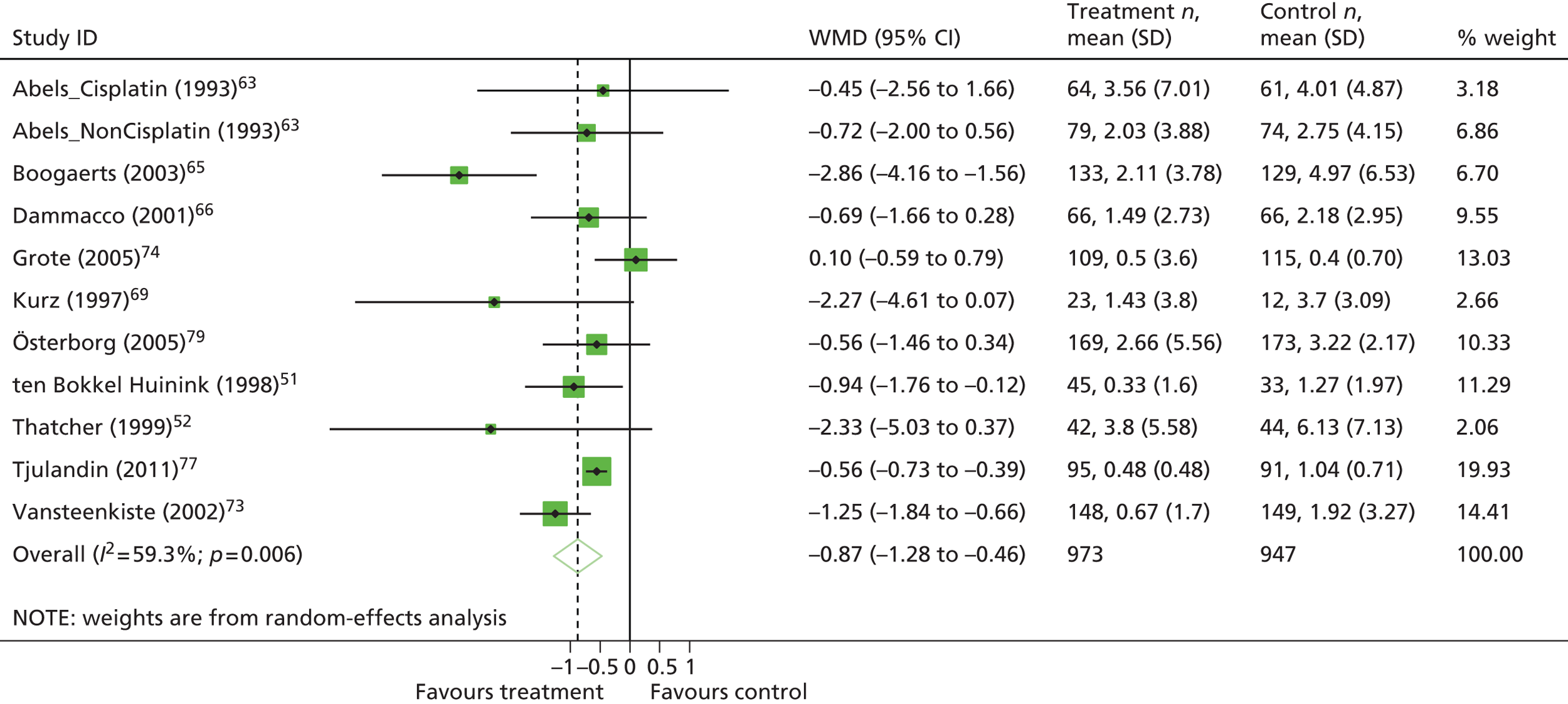
One study73 provided separate results for participants with baseline Hb levels of < 10.0 g/dl, and ≥ 10.0 g/dl (but ≤ 11.0 g/dl). Meta-analysis including these subgroup results was conducted (WMD –0.87, 95% CI –1.24 to –0.50; see Appendix 12). The fixed-effects meta-analysis undertaken as a sensitivity analysis showed a statistically significant difference for number of RBC units transfused in favour of treatment (WMD –0.64, 95% CI –0.79 to –0.48); the forest plot of this analysis is provided in Appendix 12.
To identify sources of heterogeneity, subgroup analyses were conducted (Table 19). In addition, meta-regression models including random effect and a subgroup as a covariate to assess the effects of subgroups on Hb change were performed; the F-statistics from these analyses are reported in Table 19. All covariates showing a significant effect (p < 0.05) in a univariate analysis were considered further in a model selection.
| Subgroup | No. of trials | WMD | 95% CI | I 2 | Tau2 |
|---|---|---|---|---|---|
| Overall | 11 | –0.87 | –1.28 to –0.46 | 59.3%; p = 0.02 | 0.21 |
| Chemotherapy treatmenta | |||||
| Platinum containing | 3 | –1.11 | –1.58 to –0.64 | 0%; p = 0.69 | 0 |
| Non-platinum containing | 3 | –0.56 | –0.73 to –0.39 | 0%; p = 0.97 | 0 |
| F (between : within) | F1,4 = 4.63; p = 0.10 | ||||
| Iron supplementation | |||||
| Iron in both arms | 4 | –1.30 | –2.31 to –0.29 | 78.3%; p < 0.01 | 0.73 |
| Iron not used | 1 | –2.30 | –5.03 to –0.37 | NA | 0 |
| NR | 6 | –0.70 | –1.19 to –0.20 | 43.7%; p = 0.11 | 0.16 |
| F (between : within) | F2,8 = 0.09; p = 0.44 | ||||
| Study design | |||||
| RCT | 8 | –0.63 | –0.97 to –0.30 | 35.4%; p = 0.15 | 0.07 |
| ROL | 3 | –1.91 | –3.37 to –0.44 | 68.6%; p = 0.04 | 1.10 |
| F (between : within) | F1,9 = 4.25; p = 0.07 | ||||
| Study duration (weeks) | |||||
| 12–16 | 7 | –0.70 | –0.96 to –0.44 | 11.7%; p = 0.34 | 0.02 |
| 17–20 | 1 | 0.10 | –0.59 to 0.79 | NA | 0 |
| > 20 | 3 | –1.91 | –3.37 to –0.44 | 68.6%; p = 0.04 | 1.08 |
| F (between : within) | F2,8 = 3.72; p = 0.07 | ||||
| ESA | |||||
| Erythropoietin | 10 | –0.89 | –1.43 to –0.35 | 53.8%; p = 0.02 | 0.36 |
| Darbepoetin | 1 | –1.25 | –1.84 to –0.66 | NA | 0 |
| F (between : within) | F1,9 = 0.27; p = 0.61 | ||||
| Malignancy type | |||||
| Solid tumours | 5 | –0.95 | –1.73 to –0.17 | 65.7%; p = 0.02 | 0.44 |
| Haematological tumours | 4 | –0.63 | –1.19 to –0.06 | 0%; p = 0.99 | 0 |
| Mixed | 2 | –1.62 | –3.86 to –0.63 | 91.6%; p < 0.01 | 2.42 |
| F (between : within) | F2,88 = 0.50; p = 0.62 | ||||
| Ovarian cancer | |||||
| Ovarian cancer | 1 | –0.94 | –1.76 to –0.12 | NA | 0 |
| Other cancers | 10 | –0.88 | –1.34 to –0.42 | 62.5%; p < 0.01 | 0.25 |
| F (between : within) | F1,9 = 0.00; p = 0.98 | ||||
| Analysed using results for baseline Hb subgroups73 | |||||
| Overall | 12 | –0.87 | –1.24 to –0.50 | 55.6%; p = 0.01 | 0.17 |
| Inclusion Hb level (g/dl) | |||||
| ≤ 11.0 | 9 | –0.99 | –1.41 to –0.56 | 56.2%; p = 0.02 | 0.18 |
| > 11.0 | 3 | –0.63 | –1.67 to 0.41 | 64.7%; p = 0.06 | 0.49 |
| F (between : within) | F1,10 = 0.76; p = 0.41 | ||||
| Baseline Hb level (g/dl) | |||||
| ≤ 10.0 | 7 | –1.13 | –1.76 to –0.49 | 65.3%; p = 0.01 | 0.39 |
| ≤ 11.0 | 2 | –0.88 | –1.35 to –0.40 | 0%; p = 0.80 | 0 |
| ≤ 12.0 | 1 | –0.94 | –1.76 to –0.12 | NA | 0 |
| ≤ 14.5 | 2 | –0.75 | –3.02 to –1.52 | 65.8%; p = 0.09 | 1.94 |
| F (between : within) | F3,8 = 0.36; p = 0.79 | ||||
| Target Hb level (g/dl) | |||||
| ≤ 13.0 | 1 | –0.56 | –0.74 to –0.39 | NA | 0 |
| > 13.0 | 8 | –1.01 | –1.57 to –0.45 | 65.7%; p < 0.01 | 0.39 |
| NR | 3 | –0.94 | –1.93 to –0.05 | 0%; p = 0.46 | 0 |
| F (between : within) | F2,9 = 0.20; p = 0.82 | ||||
Univariate analyses identify any significant differences based on the predefined subgroups.
Overall, there is a statistically significant effect of ESAs on the number of RBC units transfused. The WMD in RBC units was –0.87 (95% CI –1.28 to –0.46), suggesting that fewer units per participant were used in the treatment arm than in the control arm. We identified statistically significant heterogeneity between the trials (I2 = 59.3%; p = 0.006); however, all but one of the individual studies indicated a beneficial effect of ESAs with regard to RBC units transfused. Overall, the data confirm the results from previous analyses that there is only a slight difference in the number of RBC units transfused between the intervention group and the control group.
Anaemia-related outcomes: overall summary
All studies included in the analyses of anaemia-related outcomes were of moderate or poor quality. The general problem of reporting of trials on this topic was greatly assisted by the recent Cochrane review,11 as the authors had gathered further details from investigators and manufacturers, which were used in the meta-analysis for this review.
In total, 20 studies measured Hb change, of which 16 were included in the meta-analysis. All of the studies indicated a beneficial effect of ESAs with regard to Hb change, which varied only in magnitude. The overall WMD for Hb level increase was 1.59 g/dl. Hb change was not restricted to patients who were transfusion free; therefore, the results may have been confounded by transfusion in some of the patients.
Haematological response was defined as the proportion of participants with an increase in Hb level of ≥ 2 g/dl or an increase in haematocrit of ≥ 6 percentage points, unrelated to transfusion. In total, 10 trials reported this outcome and all were included in the meta-analysis. The analysis showed that participants treated with ESAs were three times more likely to experience a ≥ 2 g/dl increase in Hb than participants in the control group, with 63% (n = 759/1213) of participants who received ESAs having a haematological response compared with 18% (n = 182/1015) of control patients. Estimates of haematological response were considered robust, with no marked heterogeneity or subgroup effects.
The number of patients receiving RBCTs was the third outcome assessed to investigate the effects of ESAs on CIA. In total, 22 trials reported this outcome and all were included in the meta-analysis. Data were reported for the trial period; the RR of receiving a RBCT was 0.63 in favour of ESAs, equating to 22% of participants in the ESA treatment groups receiving RBCT compared with 33% in the control groups. The number of transfusions per patient was also investigated. Only 10 trials reported this outcome and many of these data were obtained by the Cochrane review authors through further questions to the trial authors. There was little difference between the ESA group and the control group with regard to the amount of blood transfused. Estimates of numbers transfused were considered robust, with no marked heterogeneity or subgroup effects.
Effectiveness estimates were consistent with previously reported estimates for anaemia-related outcomes (Table 20). A graphical summary of the study characteristics, quality appraisal and results for these outcomes is presented in Figure 9.
| Outcome | aWilson and colleagues2 | aTonia and colleagues11 | PenTAGa | PenTAGb |
|---|---|---|---|---|
| Hb change (g/dl)c,d | WMD 1.63, 95% CI 1.46 to 1.80; χ2(het) = 23.74, df = 19; p = 0.21 10 trials, n = 1620 |
WMD 1.57, 95% CI 1.51 to 1.62; χ2(het) = 564.37, df = 74; p < 0.001 75 trials, n = 11,609 |
WMD 1.49, 95% CI 1.37 to 1.60; χ2(het) = 70.52, df = 17; p < 0.001 18 trials, n = 3170 |
WMD 1.59, 95% CI 1.33 to 1.84; χ2(het) = 70.52, df = 17; p < 0.001 18 trials, n = 3170 |
| HaemRd,e | RR 3.40, 95% CI 3.01 to 3.83; χ2(het) = 23.60, df = 32; p = 0.86 21 trials, n = 3740 |
RR 3.39, 95% CI 3.10 to 3.71; χ2(het) = 95.56, df = 45; p < 0.001 46 trials, n = 6413 |
RR 3.41, 95% CI 2.96 to 3.92; χ2(het) = 11.75, df = 11; p = 0.383 12 trials, n = 2228 |
RR 3.29, 95% CI 2.84 to 3.81; χ2(het) = 11.75, df = 11; p = 0.383 12 trials, n = 2228 |
| RBCTd | RR 0.63, 95% CI 0.58 to 0.67; χ2(het) = 94.75, df = 48; p = 0.001 35 trials, n = 5564 |
RR 0.65, 95% CI 0.62 to 0.68; χ2(het) = 217.08, df = 87; p < 0.001 88 trials, n = 16,093 |
RR 0.62, 95% CI 0.58 to 0.67; χ2(het) = 25.71, df = 23; p = 0.315 24 trials, n = 4799 |
RR 0.63, 95% CI 0.57 to 0.69; χ2(het) = 25.71, df = 23; p = 0.315 24 trials, n = 4799 |
| Units transfusedd | WMD –1.05, 95% CI –1.32 to –0.78; χ2(het) = 8.96, df = 16; p = 0.91 14 trials, n = 2353 |
WMD –0.98, 95% CI –1.17 to –0.78; χ2(het) = 34.52, df = 24; p = 0.080 25 trials, n = 4715 |
WMD –0.64, 95% CI –0.79 to –0.48; χ2(het) = 24.55, df = 10; p = 0.006 11 trials, n = 1920 |
WMD –0.87, 95% CI –1.28 to –0.46; χ2(het) = 24.55, df = 10; p = 0.006 11 trials, n = 1920 |
FIGURE 9.
Anaemia-related outcomes: graphical summary. Notes: for some of the included trials, data for some anaemia-related outcomes were not available for all participants; where different, the numbers included are reported in notes 1–8 and 10–12. 1: Hb change: epo alfa n = 63; 2: HaemR and units: epo alfa n = 66 and placebo n = 66; 3: Hb change: rHuEPO n = 28 and control n = 24; 4: Hb change: epo alfa n = 64 and control n = 58; 5: Hb change: darbe alfa n = 17 and placebo n = 6; 6: RBCT: darbe alfa n = 167 and placebo n = 165; 7: HaemR = increase in Hb of ≥ 2 g/dl and/or Hb level > 12 g/dl; 8: RBCT: epo alfa n = 251 and placebo n = 124; 9: Latin square design; 10: Hb change: epo beta n = 138 and placebo n = 142; 11: Hb change: licensed epo alfa n = 34 and control n = 24; 12: Hb change: darbe alfa n = 330 and placebo n = 359. ?, unknown; chemo, chemotherapy; darbe, darbepoetin; epo, epoetin; haem, haematological; haemR, haematological response; non-plat, non-platinum; plat, platinum; QA, quality appraisal; units, units transfused.


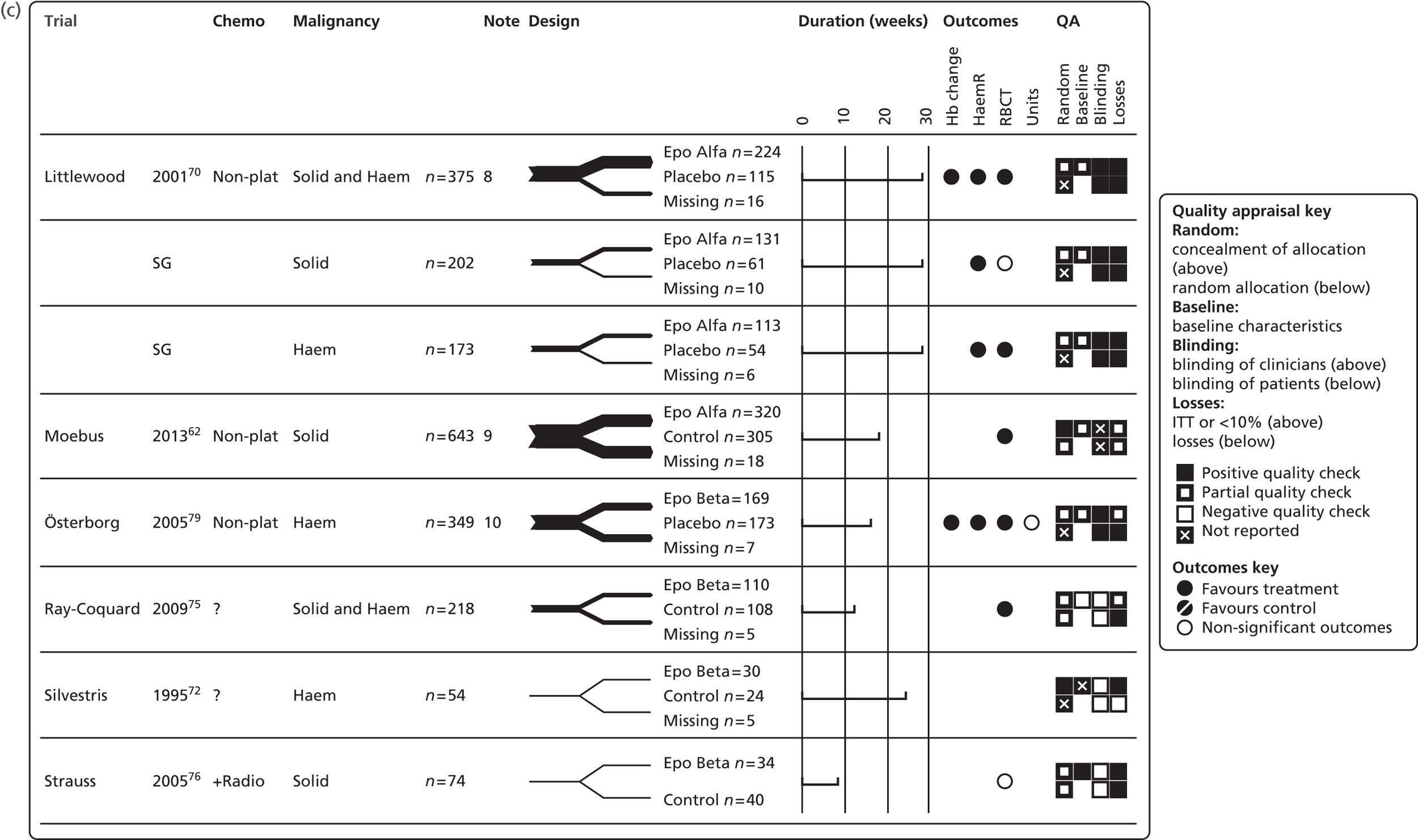

Tumour response
We identified seven trials51,66,70,74,76,78,79 that measured a complete tumour response. Overall, the analysis included seven trials with 1909 participants. Three trials were newly identified in the update searches. 74,76,78
A complete tumour response was reported in 177 out of 1003 participants in the ESA-treated groups and 142 out of 906 participants in the control groups. The random-effects meta-analysis showed a RR of 1.10 (95% CI 0.86 to 1.41; Figure 10), which was not statistically significant. There was non-significant heterogeneity between the trials (I2 = 37.5%, p = 0.143; χ2 = 9.59, df = 6, p = 0.143); however, the direction of effects of the individual studies varied (see Figure 10). Because there were only seven primary studies included in the meta-analysis, the funnel plot analysis to assess whether publication bias was likely was not conducted. 54 The fixed-effects meta-analysis undertaken as a sensitivity analysis showed similar non-significant results (RR 1.20, 95% CI 0.85 to 1, 71, I2 = 37.5%; p = 0.143); the forest plot of the analysis is included in Appendix 12.
FIGURE 10.
Forest plot: tumour response (complete response). Note: random-effects meta-analysis (DerSimonian–Laird). Events, treatment = number of events/number of participants in the treatment group; events, control = number of events/number of participants in the control group.

The previous HTA review,2 using a fixed-effects model, suggested that ESAs have detrimental effects with regard to tumour response (RR 1.31, 95% CI 1.08 to 1.60). However, the Cochrane review11 did not find any differences between the control group and the treatment group with regard to tumour response (RR 1.02, 95% CI 0.98 to 1.06). It must be emphasised that the current analysis included only studies complying with the licenced ESA dose, whereas the HTA review and the Cochrane review did not apply any restrictions regarding the ESA posology. The HTA meta-analyses included nine trials with 1260 participants and the Cochrane review included 19 trials with 5012 participants.
Prespecified subgroup analyses and meta-regression models with subgroups as covariates were not conducted because only seven trials were included in the meta-analysis.
In addition, Tonia and colleagues11 used additional quality criteria to assess the quality of trials reporting data on tumour control. The study population had to be homogeneous (i.e. all participants had to have the same tumour type/stage), all participants had to receive a predefined, identical anticancer therapy and the study had to be designed to assess tumour outcomes prospectively and/or tumour outcomes were defined as the primary or secondary study outcome. Trials were also considered if they were stratified by treatment and/or by tumour type (tumour stage). Only two studies76,78,80 included in the current review met the additional criteria of Tonia and colleagues. 11
Summary
Seven trials reported tumour response, all of which were included in the meta-analysis. All were of moderate or poor quality. The general problem of reporting of trials on this topic was greatly assisted by the recent Cochrane review,11 as the authors had gathered further details from investigators and manufacturers, which were used in the meta-analysis for this review. Analyses suggest that treatment with ESAs in patients with cancer-induced anaemia did not have a significant effect on complete tumour response (RR 1.10, 95% CI 0.86 to 1.41). In total, 18% (n = 177/1003) of participants who received ESAs had a complete tumour response compared with 16% (n = 142/906) of patients in the control groups. There was non-significant heterogeneity between the trials (I2 = 37.5%; p = 0.143); however, the direction of the effects of ESAs with regard to tumour response varied across the individual trials. The data from the seven trials suggest that there is no difference between patients treated with ESAs and patients in the control groups with regard to tumour response; however, the data are insufficient to exclude detrimental effects. It should also be noted that this is a difficult area of assessment, especially in a heterogeneous mix of tumour types, and the results should be treated with caution. Data are presented alongside the results from previous analyses in Table 21. (See Figure 13 for a graphical summary of the study characteristics, quality appraisal and results for this outcome.)
| Outcome | aWilson and colleagues2 | aTonia and colleagues11 | PenTAGa | PenTAGb |
|---|---|---|---|---|
| Tumour responsec | RR 1.31, 95% CI 1.08 to 1.60; χ2(het) = NR, df = NR; p = NR 10 trials, n = 1260 |
RR 1.02, 95% CI 0.98 to 1.06; χ2(het) = 16.10, df = 18; p = 0.59 19 trials, n = 5012 |
RR 1.20, 95% CI 0.85 to 1.71; χ2(het) = 9.59, df = 6; p = 0.14 7 trials, n = 1909 |
RR 1.10, 95% CI 0.86 to 1.41; χ2(het) = 9.59, df = 6; p = 0.14 7 trials, n = 1909 |
Overall survival
For OS, data were extracted from the Cochrane review. 11 In the Cochrane review the reported HRs were based on IPD. When IPD were not available, the authors extracted HRs from published reports, including secondary analyses, using methods reported in Parmar and colleagues49 or from binary mortality data. OS was calculated from the longest follow-up available and varied between studies.
Overall survival data were available from 21 trials17,48,50–53,62,63,65–70,73–79 including 5054 participants. Seven studies48,62,74–78 were newly identified. Two studies48,63 were split into subsets, two studies53,69 reported zero events and three studies50–52 reported events/effect size for a combined treatment arm (studies evaluated different ESA doses) and as such included unlicensed doses; as a result, the number of studies included in the meta-analysis is 18.
The OS estimate is provided in Figure 11 (HR 0.97, 95% CI 0.83 to 1.13). The heterogeneity between trials was significant, with an I2 of 42.4% (p = 0.030; χ2 = 29.5, df = 17, p = 0.030); the forest plot suggested that there was a tendency for smaller studies to favour treatment. Funnel plot analysis identified one outlier68 and also suggested that smaller studies had a tendency to favour treatment; a funnel plot without the outlier is presented in Appendix 12. The Harbord test was not performed because raw data were not available.
FIGURE 11.
Forest plot: OS. Notes: random-effects meta-analysis (DerSimonian–Laird); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta and Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy; effect sizes reported are HRs; IPD data as reported in Tonia and colleagues11 (Cochrane review) for Abels and colleagues,63 Boogaerts and colleagues,65 Dammacco and colleagues,66 Grote and colleagues,74 Hedenus and colleagues,17 Littlewood and colleagues,70 Österborg and colleagues,71 Ray-Coquard and colleagues,75 Strauss and colleagues76 and Vansteenkiste and colleagues. 73 HRs reported for other trials calculated using other accepted methods. ES, effect size.

A meta-regression using publication year as a covariate (to assess the effect of publication year on OS) showed that the effects of ESAs on OS were independent of any effect of publication year (p = 0.579; the meta-regression plot is presented in Appendix 12).
To identify sources of heterogeneity we performed subgroup analyses (Table 22). In addition, meta-regression models that included random effect and subgroups as covariates (to assess the effects of a subgroup on OS) were performed. The F statistics from these analyses are reported in Table 22. All covariates showing a significant effect (p < 0.05) in a univariate analysis were considered further in model selection.
| Subgroup | No. of trials | HR | 96% CI | I 2 | Tau2 |
|---|---|---|---|---|---|
| Overall | 18 | 0.97 | 0.83 to 1.13 | 42.4%; p = 0.03 | 0.04 |
| Inclusion Hb level (g/dl) | |||||
| ≤ 11.0 | 10 | 0.91 | 0.70 to 1.20 | 51.7%; p = 0.03 | 0.07 |
| > 11.0 | 8 | 0.99 | 0.81 to 1.20 | 35.5%; p = 0.15 | 0.02 |
| F (between : within) | F1,16 = 0.09; p = 0.77 | ||||
| Baseline Hb level (g/dl) | |||||
| ≤ 10.0 | 11 | 0.88 | 0.71 to 1.08 | 53.0%; p = 0.02 | 0.05 |
| ≤ 11.0 | 1 | 1.11 | 0.45 to 2.73 | NA | NA |
| ≤ 12.0 | 1 | 2.00 | 0.65 to 1.13 | NA | NA |
| ≤ 14.5 | 4 | 1.20 | 0.96 to 1.50 | 0%; p = 0.56 | 0 |
| NR | 1 | 0.97 | 0.67 to 1.41 | NA | NA |
| F (between : within) | F4,13 = 0.78; p = 0.56 | ||||
| Target Hb level (g/dl) | |||||
| ≤ 13.0 | 4 | 0.73 | 0.32 to 1.64 | 61.8%; p = 0.05 | 0.41 |
| > 13.0 | 12 | 0.97 | 0.82 to 1.14 | 46.6%; p = 0.04 | 0.03 |
| NR | 2 | 0.88 | 0.46 to 1.70 | 0%; p = 0.47 | 0 |
| F (between : within) | F2,15 = 0.03; p = 0.97 | ||||
| Malignancy type | |||||
| Solid tumours | 9 | 0.96 | 0.74 to 1.25 | 46.3%; p = 0.06 | 0.06 |
| Haematological tumours | 5 | 1.01 | 0.73 to 1.40 | 48.5%; p = 0.10 | 0.05 |
| Mixed | 4 | 0.84 | 0.69 to 1.02 | 0%; p = 0.40 | 0 |
| F (between : within) | F2,15 = 0.40; p = 0.68 | ||||
| Chemotherapy treatmenta | |||||
| Platinum containing | 4 | 0.67 | 0.46 to 0.98 | 14.5%; p = 0.32 | 0.03 |
| Non-platinum containing | 7 | 0.99 | 0.86 to 1.14 | 0%; p = 0.42 | 0 |
| F (between : within) | F1,9 = 3.48; p = 0.10 | ||||
| Iron supplementation | |||||
| No iron | 12 | 0.96 | 0.79 to 1.17 | 38.9%; p = 0.08 | 0.03 |
| Iron in an intervention arm | 1 | 1.33 | 0.91 to 1.95 | NA | 0 |
| NR | 5 | 0.87 | 0.61 to 1.23 | 54.0%; p = 0.07 | 0.07 |
| F (between : within) | F2,15 = 0.72; p = 0.50 | ||||
| Study design | |||||
| RCT | 11 | 0.92 | 0.75 to 1.13 | 52.4%; p = 0.02 | 0.05 |
| ROL | 7 | 1.05 | 0.81 to 1.36 | 28.1%; p = 0.21 | 0.03 |
| F (between : within) | F1,16 = 0.50; p = 0.49 | ||||
| Study duration (weeks) | |||||
| 6–9 | 2 | 1.90 | 0.63 to 5.76 | 0%; p = 0.51 | 0 |
| 12–16 | 11 | 0.86 | 0.68 to 1.08 | 48.8%; p = 0.03 | 0.05 |
| 17–20 | 2 | 1.10 | 0.88 to 1.37 | 0%; p = 0.43 | 0 |
| > 20 | 3 | 1.10 | 0.72 to 1.67 | 66.4%; p = 0.05 | 0.09 |
| F (between : within) | F3,14 = 0.87; p = 0.48 | ||||
| ESA | |||||
| Erythropoietin | 15 | 0.92 | 0.77 to 1.10 | 31.2%; p = 0.12 | 0.03 |
| Darbepoetin | 3 | 1.10 | 0.77 to 1.58 | 74.6%; p = 0.03 | 0.08 |
| F (between : within) | F1,16 = 0.92; p = 0.35 | ||||
Univariate analyses did not identify any significant differences based on the predefined subgroups (see Table 22). The fixed-effects meta-analysis undertaken as a sensitivity analysis showed similar results (HR 0.98, 95% CI 0.89 to 1.08); the forest plot of this analysis is included in Appendix 12. Both fixed- and random-effects estimates suggested that there was no difference in OS between the control arm and the treatment arm. Interestingly, the fixed-effects estimate reported in the recent Cochrane review11 favoured the control arm, suggesting that higher mortality occurred in patients treated with ESAs (HR 1.05, 95% CI 1.00 to 1.11). The previous HTA review2 did not find a significant difference between the control arm and the treatment arm with regard to survival (HR 1.03, 95% CI 0.92 to 1.16). It must be emphasised that the current analysis included only studies complying with the licenced ESA dose, whereas the Cochrane review did not apply any restrictions regarding the ESA posology. The Cochrane review included 76 studies in the OS meta-analysis; however, subgroup analyses comparing studies using licensed and unlicensed ESA doses were not conducted.
Summary
In total, 21 trials reported OS. All were of moderate or poor quality. The general problem of reporting of trials on this topic was greatly assisted by the recent Cochrane review,11 as the authors had gathered further details from investigators and manufacturers, which were used in the meta-analysis for this review. Eighteen trials were included in the meta-analysis. Analyses suggest that treatment with ESAs in patients with CIA did not have a significant effect on OS. In total, 35% (n = 818/2317) of participants who received ESAs died and 35% (n = 744/2137) of patients in the control groups died. The risk of death was 0.97 (HR 0.97, 95% CI 0.83 to 1.13). However, there was significant heterogeneity between the trials (I2 = 42.4%; p = 0.030), for which no explanation could be provided. In addition, OS was calculated from the longest follow-up available (no minimum was required) and, as such, this variation between the studies (short-term and long-term studies) should be considered when interpreting the results. Overall, data suggest that, if the licensed ESA dose is followed, there are no detrimental effects of ESAs on OS; however, these results are subject to the limitations acknowledged and should be interpreted with caution. Effectiveness estimates are presented alongside previously reported estimates for OS in Table 23. (See Figure 13 for a graphical summary of the study characteristics, quality appraisal and results for this outcome.)
| Outcome | aWilson and colleagues2 | aTonia and colleagues11 | PenTAGa | PenTAGb |
|---|---|---|---|---|
| OSc | HR 1.03, 95% CI 0.92 to 1.16; χ2(het) = 37.74, df = 27; p = 0.08 28 trials, n = 5308 |
HR 1.05, 95% CI 1.00 to 1.11; χ2(het) = 95.40, df = 75; p = 0.060 80 trials, n = 19,003 |
HR 0.98, 95% CI 0.89 to 1.08; χ2(het) = 29.50, df = 17; p = 0.03 18 trials, n = 4454 |
HR 0.97, 95% CI 0.83 to 1.13; χ2(het) = 29.50, df = 17; p = 0.03 18 trials, n = 4454 |
On-study mortality
For on-study mortality, data were extracted from the Cochrane review. 11 In the Cochrane review, reported HRs were based on IPD. When IPD were not available, the authors extracted HRs from published reports, including secondary analyses, using the methods reported in Parmar and colleagues. 49 On-study mortality was defined as deaths occurring up to 30 days after the active study period.
Mortality data were available from 21 trials17,48,50–53,62,63,65–70,73–79 including 5085 participants. Seven studies48,62,74–78 were newly identified. Two studies48,63 were split into subsets, six studies62,67,69,76,78,79 reported zero events and four studies50–53 reported events/effect size for combined treatment arms (studies evaluated different ESA doses) and as such included unlicensed doses. As a result, the number of trials included in the meta-analysis is 14 (including 2967 participants). One study reported mortality events in the control arm, whereas there were no deaths recorded in the treatment arm (HR 0.14, 95% CI 0.00 to 6.82). 68
The results from the on-study mortality meta-analysis are provided in Figure 12 (HR 0.86, 95% CI 0.67 to 1.11). Heterogeneity between trials was not significant (I2 = 16.4%, p = 0.274; χ2 = 15.55, df = 13, p = 0.274); however, the forest plot may suggest a tendency for smaller studies to favour treatment (see Figure 12). Similarly to the OS data, funnel plot analysis (see Appendix 12) identified one outlier68 and was also suggestive of a tendency for smaller studies to favour treatment; a funnel plot without the outlier is presented in Appendix 12. The Harbord test was not performed because raw data were not available.
FIGURE 12.
Forest plot: on-study mortality. Notes: random-effects meta-analysis (DerSimonian–Laird); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta and Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy; IPD data as reported in Tonia and colleagues11 (Cochrane review) for Abels and colleagues,63 Boogaerts and colleagues,65 Dammacco and colleagues,66 Grote and colleagues,74 Hedenus and colleagues,17 Littlewood and colleagues,70 Österborg and colleagues,71 Ray-Coquard and colleagues,75 Strauss and colleagues76 and Vansteenkiste and colleagues. 73 HRs reported for other trials calculated using other accepted methods.

A meta-regression using publication year as a covariate (to assess the effect of publication year on on-study mortality) suggested that the effects of ESAs on mortality were independent of when the trial results were published (p = 0.465; the meta-regression plot is presented in Appendix 12).
The fixed-effects meta-analysis undertaken as a sensitivity analysis showed similar results (HR 0.87, 95% CI 0.70 to 1.09); the forest plot of this analysis is provided in Appendix 12. Both fixed- and random-effects estimates suggested no difference in on-study mortality between the control arm and the treatment arm. Interestingly, the fixed-effects estimate reported in the recent Cochrane review11 favoured the control arm, suggesting that higher mortality occurred in patients treated with ESAs (HR 1.17, 95% CI 1.03 to 1.29). Again, it must be emphasised that the current analysis included only studies complying with the licensed ESA dose, whereas the Cochrane review did not apply any restrictions regarding the ESA posology. The Cochrane review included 64 studies in the on-study mortality meta-analysis, but subgroup analyses comparing studies using licensed and unlicensed ESA doses were not conducted.
Predefined subgroup analyses were performed (Table 24). None of the studies with available Hb response data included ovarian cancer patients. Therefore, the planned ovarian cancer subgroup analysis was not completed. In addition, to assess the effects of subgroups on mortality, meta-regression models were performed that included random effect and subgroups as covariates; the F statistics from these analyses are reported in Table 24. All covariates showing a significant effect (p < 0.05) in a univariate analysis were considered further in model selection.
| Subgroup | No. of trials | HR | 95% CI | I 2 | Tau2 |
|---|---|---|---|---|---|
| Overall | 14 | 0.86 | 0.67 to 1.11 | 16.4%; p = 0.27 | 0.04 |
| Inclusion Hb level (g/dl) | |||||
| ≤ 11.0 | 10 | 0.89 | 0.61 to 1.30 | 37.7%; p = 0.11 | 0.13 |
| > 11.0 | 4 | 0.77 | 0.55 to 1.08 | 0%; p = 0.98 | 0 |
| F (between : within) | F1,12 = 0.74; p = 0.41 | ||||
| Baseline Hb level (g/dl) | |||||
| ≤ 10.0 | 11 | 0.84 | 0.62 to 1.15 | 33.2%; p = 0.13 | 0.09 |
| ≤ 11.0 | 1 | 1.11 | 0.45 to 2.73 | NA | 0 |
| ≤ 14.5 | 2 | 0.78 | 0.41 to 1.50 | 0%; p = 0.67 | 0 |
| F (between : within) | F2,11 = 0.14; p = 0.87 | ||||
| Target Hb level (g/dl) | |||||
| ≤ 13.0 | 3 | 0.50 | 0.20 to 1.22 | 29.7%; p = 0.24 | 0.19 |
| > 13.0 | 9 | 0.92 | 0.70 to 1.22 | 20.0%; p = 0.27 | 0.04 |
| NR | 2 | 0.88 | 0.46 to 1.70 | 0%; p = 0.47 | 0 |
| F (between : within) | F2,11 = 0.89; p = 0.44 | ||||
| Malignancy type | |||||
| Solid tumours | 5 | 0.71 | 0.44 to 1.15 | 17.6%; p = 0.30 | 0.06 |
| Haematological tumours | 5 | 0.98 | 0.54 to 1.79 | 52.7%; p = 0.08 | 0.24 |
| Mixed | 4 | 0.83 | 0.58 to 1.17 | 0%; p = 0.88 | 0 |
| F (between : within) | F2,11 = 0.61; p = 0.56 | ||||
| Chemotherapy treatmenta | |||||
| Platinum containing | 4 | 0.64 | 0.34 to 1.18 | 36.0%; p = 0.20 | 0.14 |
| Non-platinum containing | 4 | 1.01 | 0.71 to 1.43 | 0%; p = 0.65 | 0 |
| F (between : within) | F1,6 = 1.36; p = 0.29 | ||||
| Iron supplementation | |||||
| Iron in both arms | 9 | 0.89 | 0.63 to 1.26 | 25.6%; p = 0.22 | 0.07 |
| NR | 5 | 0.82 | 0.55 to 1.21 | 14.5%; p = 0.32 | 0.03 |
| F (between : within) | F1,12 = 0.09; p = 0.77 | ||||
| Study design | |||||
| Blinded (RCT) | 11 | 0.86 | 0.63 to 1.17 | 33.0%; p = 0.14 | 0.09 |
| Unblinded (ROL) | 3 | 0.82 | 0.49 to 1.35 | 0.0%; p = 0.77 | 0 |
| F (between : within) | F1,12 = 0.07; p = 0.80 | ||||
| Study duration (weeks) | |||||
| 6–9 | 1 | 0.14 | 0 to 365.61 | NA | 0 |
| 12–16 | 10 | 0.85 | 0.59 to 1.23 | 39.6%; p = 0.09 | 0.13 |
| 17–20 | 1 | 0.79 | 0.41 to 1.52 | NA | 0 |
| > 20 | 2 | 0.84 | 0.53 to 1.32 | 0.0%; p = 0.61 | 0 |
| F (between : within) | F3.10 = 0.070; p = 0.97 | ||||
| ESA | |||||
| Erythropoietin | 12 | 0.80 | 0.63 to 1.02 | 1.0%; p = 0.43 | < 0.01 |
| Darbepoetin | 2 | 1.42 | 0.66 to 3.05 | 43.0%; p = 0.19 | 0.14 |
| F (between : within) | F1.12 = 2.51; p = 0.14 | ||||
Univariate analyses did not identify any significant differences based on the predefined subgroups.
Summary
On-study mortality was assessed in 12 trials of moderate or poor quality. Analyses suggested that treatment with ESAs in patients with CIA did not have a significant effect on on-study mortality. In total, 11% (n = 174/1586) of participants who received ESAs had died within 30 days of the active study period compared with 12% (n = 164/1381) of patients in the control groups. The risk of death was 0.86 (HR 0.86, 95% CI 0.67 to 1.1). There was no significant heterogeneity between the trials (I2 = 16.4%, p = 0.274). Overall, data suggested that, if the licensed ESA dosage is followed, there are no detrimental effects of ESAs on on-study mortality. However, these results should be interpreted with caution. Effectiveness estimates are compared with previously reported estimates in Table 25 and a graphical summary of the study characteristics, quality appraisal and results for this outcome is presented in Figure 13.
| Outcome | aWilson and colleagues2 | aTonia and colleagues11 | PenTAGa | PenTAGb |
|---|---|---|---|---|
| Mortalityc | NR | HR 1.17, 95% CI 1.03 to 1.29; χ2(het) = 59.49, df = 63; p = 0.600 72 trials,c n = 15,935 |
HR 0.87, 95% CI 0.70 to 1.09; χ2(het) = 15.55, df = 13; p = 0.274 14 trials, n = 2967 |
HR 0.86, 95% CI 0.67 to 1.11; χ2(het) = 15.55, df = 13; p = 0.274 14 trials, n = 2967 |
FIGURE 13.
Tumour response, OS and mortality outcomes: graphical summary. Notes: for some of the included trials, data for some anaemia-related outcomes were not available for all participants; where different, the numbers included are reported in notes 1–4. 1: tumour response reported for epo alfa n = 64 and placebo n = 63; 2: tumour response reported for epo beta n = 154 and placebo n = 162; 3: OS reported for darbe alfa n = 345 and control n = 369 and mortality reported for darbe alfa n = 353 and control n = 376; 4: OS and mortality reported for all randomised participants (n = 320), whereas other outcomes were reported using all randomised participants receiving at least one dose of study treatment (n = 314). ?, unknown; chemo, chemotherapy; darbe, darbepoetin; epo, epoetin; haem, haematological; non-plat, non-platinum; plat, platinum; QA, quality appraisal.
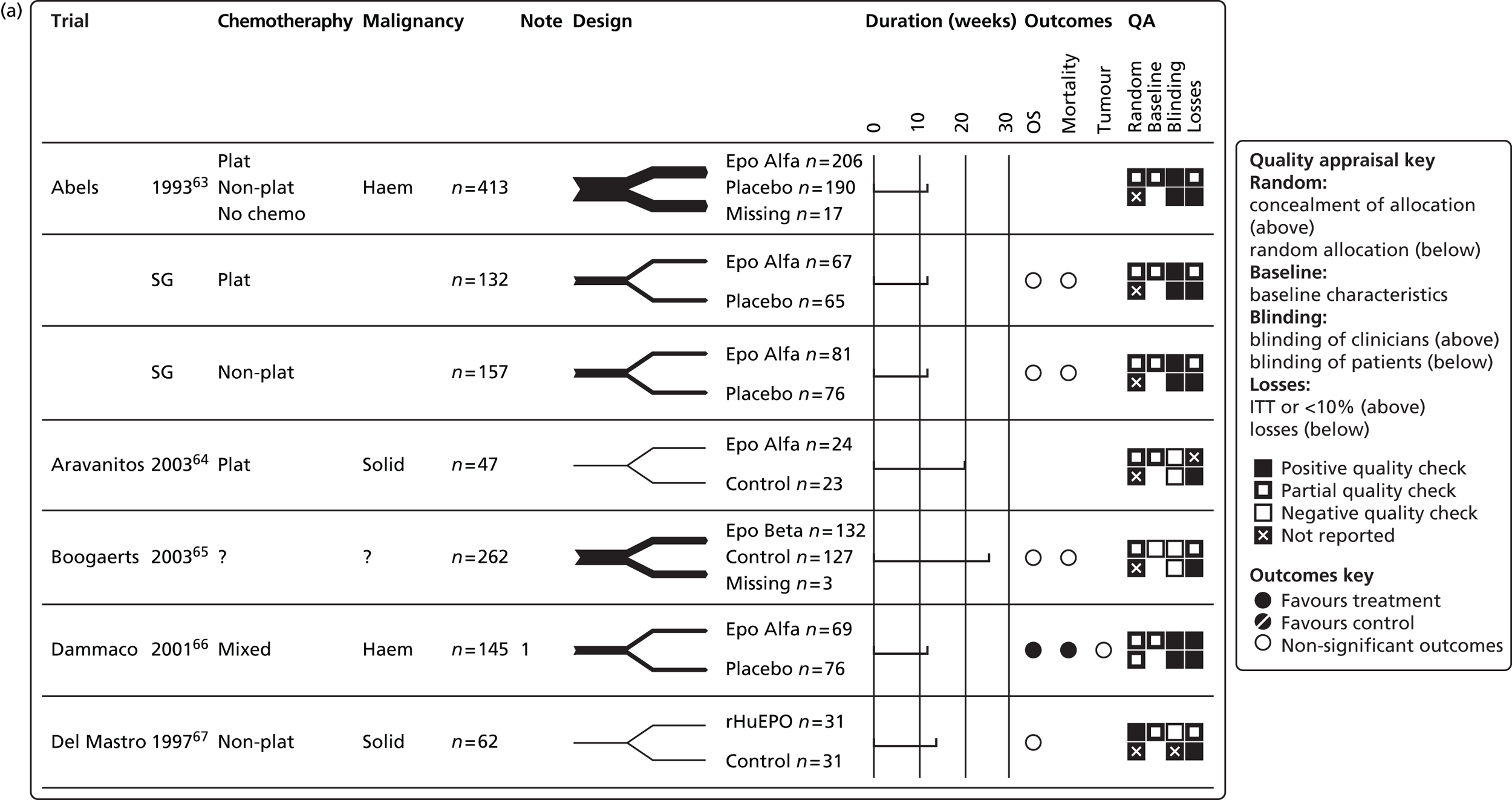
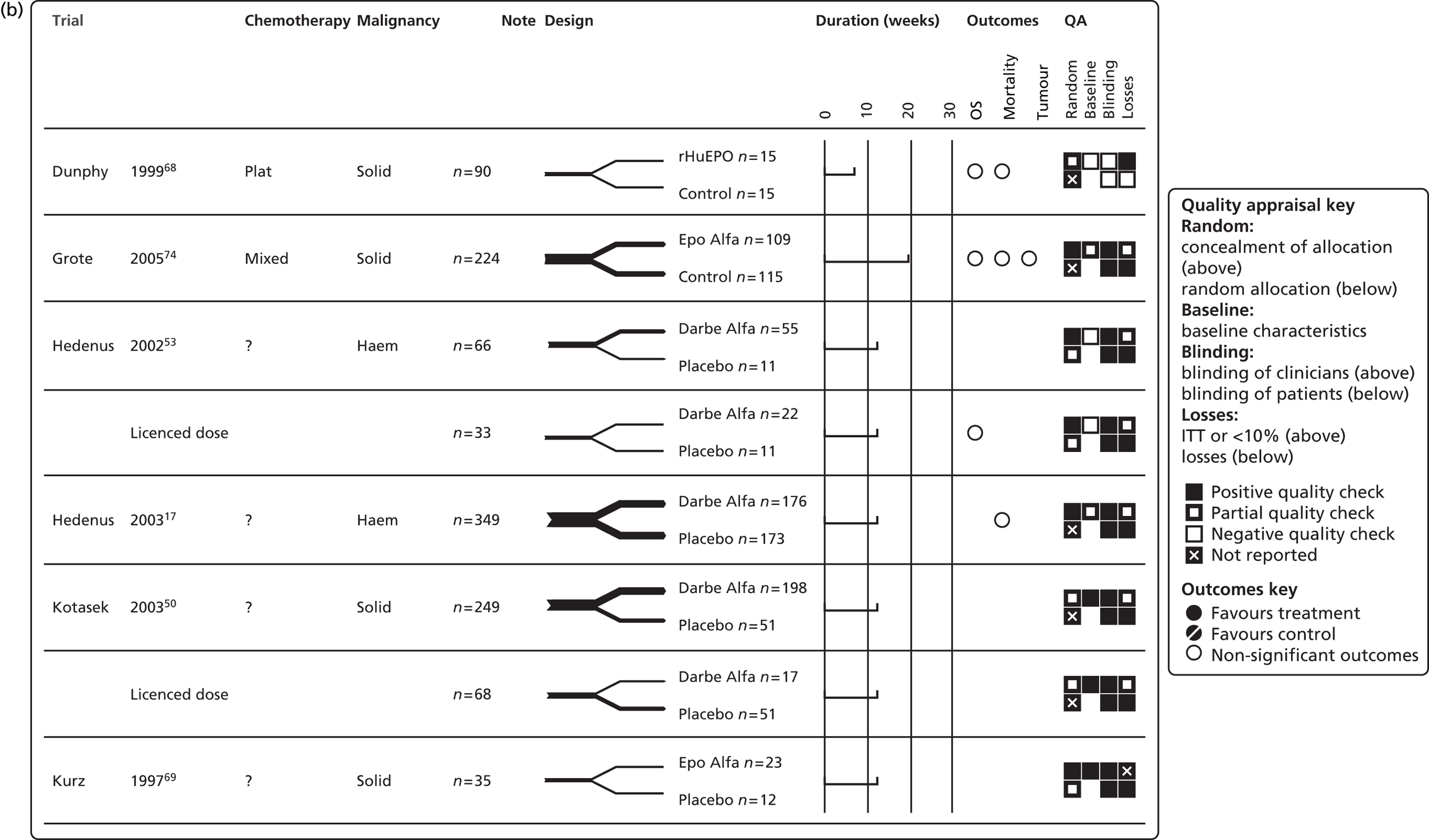


Safety
Adverse events of relevance to this review included thromboembolic events, hypertension, thrombocytopenia/haemorrhage, seizures, pruritus and red cell aplasia.
All studies reporting AEs were of moderate or poor quality. There was considerable variability in the reporting of AEs among the included studies, for example some reported AEs that occurred in > 5% of patients, some reported AEs that occurred in > 10% of patients and some reported the overall number of events. In addition, there was some variability in the definitions of AEs used in the studies. Given the greater access to data in the Cochrane review,11 these data were used to conduct meta-analyses for the following AEs: thromboembolic events, thrombocytopenia and haemorrhage, hypertension, seizures and pruritus (defined as pruritus, rash and irritation).
No studies were identified that reported red cell aplasia. In addition, this safety outcome was not analysed in the Cochrane review.
Thromboembolic events
We identified 14 trials17,51,52,62,63,66,70,73–79 that measured thromboembolic events, including 4013 participants. Of these, 2029 participants were treated with ESAs. As one multiarm study63 was split into subsets, the number of studies displayed is 15. Five included studies were newly identified in the update searches. 62,75–78 If thromboembolic events were not reported, data from the Cochrane review by Tonia and colleagues11 were used in the PenTAG analyses. One study52 did not report any thromboembolic events in the treatment or placebo arms and was excluded from the meta-analysis.
Data from Moebus and colleagues62 were used in the PenTAG meta-analyses (whereas the analysis in Tonia and colleagues11 used data from Moebus and colleagues33). The Moebus and colleagues62 trial showed an increased risk for patients treated with ESAs compared with control participants (RR 2.26, 95% CI 1.09 to 4.70), whereas there was no difference between the treatment arm and the control arm in the study by Moebus and colleagues. 33
Thromboembolic events were reported in 103 out of 2029 participants treated with ESAs, compared with 66 out of 1984 participants in the control group. The random-effects meta-analysis showed a RR of 1.46 (95% CI 1.07 to 1.99), favouring the control group (Figure 14). There was no heterogeneity between the trials (I2 = 0%, p = 0.733; χ2 = 9.52, df = 13, p = 0.733), with 11 studies indicating detrimental effects of ESA treatment and three studies indicating beneficial effects of ESA treatment with regard to thromboembolic events. To test whether publication bias was present in the sample included in the meta-analysis, a funnel plot was constructed (see Appendix 12). The funnel plot analysis did not show statistically significant asymmetry (p = 0.627). In addition, a meta-regression using publication year as a covariate to assess the effect of publication year on thromboembolic events suggested that the effects of ESAs on thromboembolic events were independent of when the trial results were published (p = 0.871); the meta-regression plot is presented in Appendix 12.
FIGURE 14.
Forest plot: thromboembolic events (overall). Notes: random-effects meta-analysis (DerSimonian–Laird); trial with multiple experimental arms split into subsets in the analysis: Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy. Events, treatment = number of events/number of participants in the treatment group; events, control = number of events/number of participants in the control group.

The fixed-effects meta-analysis undertaken as a sensitivity analysis showed similar results, favouring the control participants over those receiving ESAs (RR 1.52, 95% CI 1.13 to 2.05, I2 = 0%; p = 0.733); the forest plot of the analysis is included in Appendix 12.
Prespecified subgroup analyses were conducted (Table 26). In addition, meta-regression models including random effect and a subgroup as a covariate to assess the effects of subgroups on thomboembolic events were performed; the F statistics from these analyses are reported in Table 26. All covariates showing a significant effect (p < 0.05) in a univariate analysis were considered further in a model selection.
| Subgroup | Number of trials | RR | 95% CI | I 2 | Tau2 |
|---|---|---|---|---|---|
| Overall | 14 | 1.46 | 1.08 to 1.99 | 0%; p = 0.73 | 0 |
| Inclusion Hb level (g/dl) | |||||
| ≤ 11.0 | 7 | 1.29 | 0.66 to 2.54 | 12.2%; p = 0.34 | 0.10 |
| > 11.0 | 7 | 1.55 | 1.08 to 2.21 | 0%; p = 0.88 | 0 |
| F (between : within) | F1,12 = 0.35; p = 0.57 | ||||
| Baseline Hb level (g/dl) | |||||
| ≤ 10.0 | 8 | 1.34 | 0.82 to 2.21 | 0%; p = 0.52 | 0 |
| ≤ 11.0 | 1 | 0.63 | 0.11 to 3.64 | NA | 0 |
| ≤ 12.0 | 2 | 3.58 | 0.40 to 31.59 | 0%; p = 0.97 | 0 |
| ≤ 14.5 | 2 | 1.33 | 0.82 to 2.17 | 0%; p = 0.64 | 0 |
| NR | 1 | 2.26 | 1.09 to 4.70 | NA | 0 |
| F (between : within) | F4,9 = 0.53; p = 0.72 | ||||
| Target Hb level (g/dl) | |||||
| ≤ 13.0 | 2 | 1.38 | 0.75 to 2.57 | 0%; p = 0.36 | 0 |
| > 13.0 | 10 | 1.73 | 1.72 to 2.54 | 0%; p = 0.82 | 0 |
| NR | 2 | 0.70 | 0.29 to 1.68 | 0%; p = 0.88 | 0 |
| F (between : within) | F2,11 = 1.75; p = 0.22 | ||||
| Malignancy type | |||||
| Solid tumours | 6 | 1.59 | 1.09 to 2.32 | 0%; p = 0.82 | 0 |
| Haematological tumours | 5 | 1.57 | 0.57 to 4.34 | 35.1%; p = 0.19 | 0.46 |
| Mixed | 3 | 1.21 | 0.57 to 2.61 | 0%; p = 0.69 | 0 |
| F (between : within) | F2,11 = 1.09; p = 0.37 | ||||
| Ovarian cancer | |||||
| Ovarian cancer | 1 | 3.97 | 0.18 to 74.51 | NA | 0 |
| Other cancers | 13 | 1.45 | 1.06 to 1.97 | 0%; p = 0.69 | 0 |
| F (between : within) | F1,12 = 0.61; p = 0.45 | ||||
| Chemotherapy treatmenta | |||||
| Platinum containing | 3 | 1.06 | 0.51 to 2.20 | 0%; p = 0.47 | 0 |
| Non-platinum containing | 6 | 1.57 | 1.04 to 2.37 | 0%; p = 0.66 | 0 |
| F (between : within) | F1,7 = 0.54; p = 0.49 | ||||
| Iron supplementation | |||||
| Iron in both arms | 7 | 1.86 | 1.13 to 3.07 | 0%; p = 0.73 | 0 |
| Iron in an intervention arm | 1 | 1.47 | 0.78 to 2.75 | NA | 0 |
| NR | 6 | 1.15 | 0.70 to 1.89 | 0%; p = 0.53 | 0 |
| F (between : within) | F2,11 = 0.21; p = 0.82 | ||||
| Study design | |||||
| RCT | 9 | 1.24 | 0.81 to 1.90 | 0%; p = 0.55 | 0 |
| ROL | 5 | 1.74 | 1.12 to 2.69 | 0%; p = 0.83 | 0 |
| F (between : within) | F1,12 = 0.01; p = 0.94 | ||||
| Study duration (weeks) | |||||
| 6–9 | 1 | 3.44 | 0.15 to 81.71 | NA | 0 |
| 12–16 | 8 | 1.24 | 0.72 to 2.13 | 0%; p = 0.45 | 0 |
| 17–20 | 2 | 1.64 | 0.84 to 3.18 | 35.7%; p = 0.21 | 0.08 |
| > 20 | 3 | 1.48 | 0.88 to 2.51 | 0%; p = 0.83 | 0 |
| F (between : within) | F3,10 = 0.17; p = 0.91 | ||||
| ESA | |||||
| Erythropoietin | 11 | 1.40 | 0.96 to 2.04 | 0%; p = 0.65 | 0 |
| Darbepoetin | 3 | 1.60 | 0.94 to 2.71 | 0%; p = 0.46 | 0 |
| F (between : within) | F1,12 = 037; p = 0.56 | ||||
Univariate analyses did not identify any significant differences based on the predefined subgroups (see Table 26).
Analyses suggest that treatment with ESAs in patients with CIA increases the risk for thromboembolic events (RR 1.46, 95% CI 1.08 to 1.99). In total, 5% (n = 103/2029) of participants who received ESAs reported thromboembolic events compared with 3% (n = 66/1984) of patients in the control groups. There was no heterogeneity between the trials (I2 = 0%; p = 0.733). Overall, the data confirm results from previous trials that there is an increased risk of thromboembolic events in patients treated with ESAs compared with control participants.
Hypertension
We identified 10 trials48,51,52,63,66,70,72,73,77,79 that measured hypertension, including 2086 participants. Of these, 1152 participants were treated with ESAs. As two multiarm studies48,63 were split into subsets, the number of studies displayed is 12. Two included studies48,77 were newly identified in the update searches. If hypertension was not reported, we used data from the Cochrane review by Tonia and colleagues11 in the PenTAG analyses.
Hypertension was reported in 62 out of 1152 participants (5%) treated with ESAs compared with 27 out of 934 participants (3%) in the control groups. The random-effects meta-analysis showed a risk ratio of 1.80 (95% CI 1.14 to 2.85; Figure 15), favouring the control. There was no statistical heterogeneity between the trials (I2 = 0%; χ2 = 7.10, df = 11; p = 0.791); however, the direction of the effects of ESAs with regard to hypertension varied across the individual trials (see Figure 15). To test whether publication bias was present in the sample included in the meta-analysis, a funnel plot was constructed (see Appendix 12). The funnel plot analysis did not show statistically significant asymmetry (p = 0.689). In addition, a meta-regression using publication year as a covariate to assess the effect of publication year on hypertension suggests that the effects of ESAs on hypertension were independent of when the trial results were published (p = 0.735); the meta-regression plot is presented in Appendix 12.
FIGURE 15.
Forest plot: hypertension (overall). Notes: random-effects meta-analysis (DerSimonian–Laird); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta and Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy. Events, treatment = number of events/number of participants in the treatment group; events, control = number of events/number of participants in the control group.
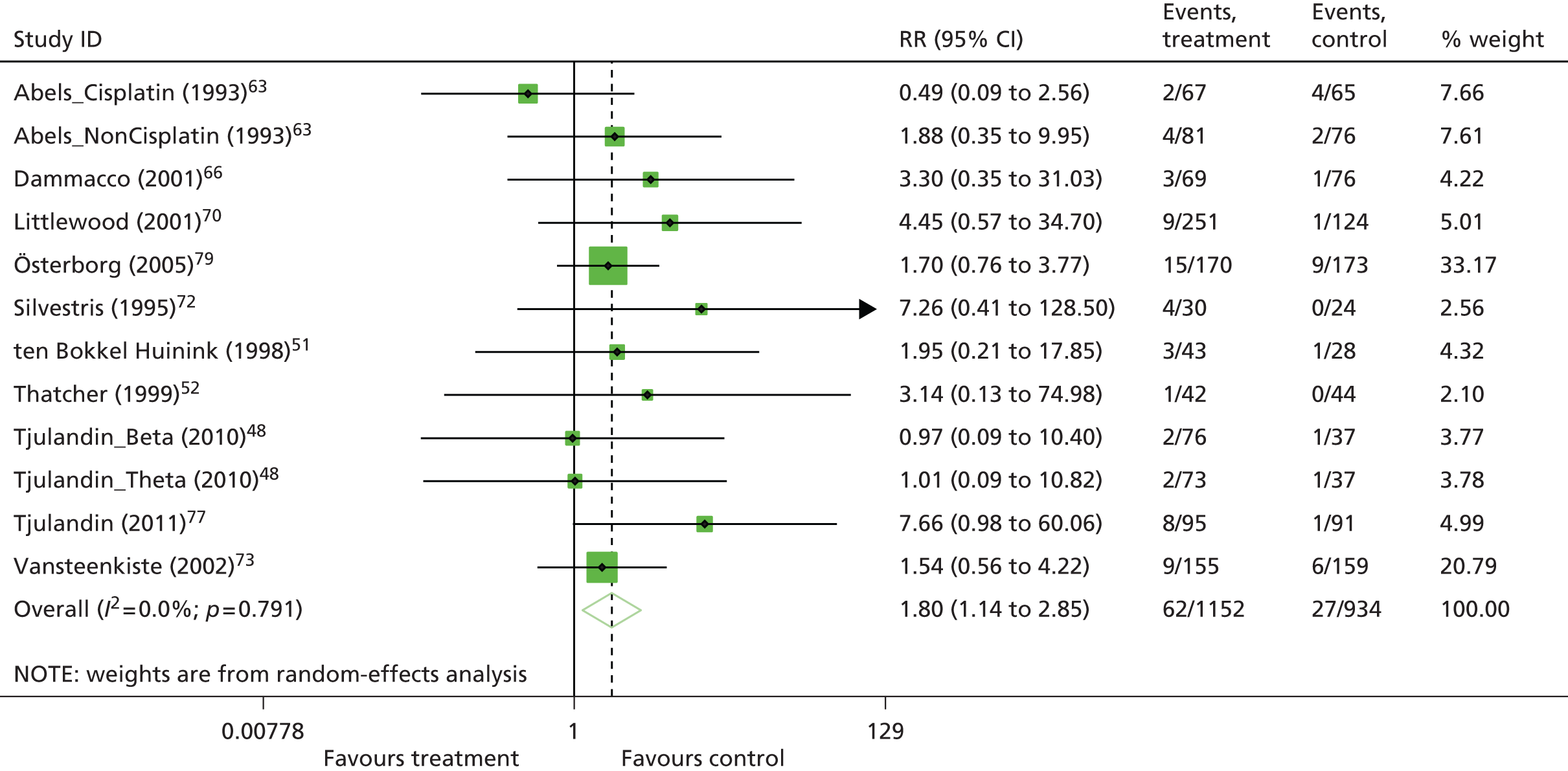
The fixed-effects meta-analysis undertaken as a sensitivity analysis showed similar results (RR 1.97, 95% CI 1.27 to 3.07, I2 = 0%; p = 0.791); the forest plot of the analysis is included in Appendix 12.
Prespecified subgroup analyses were conducted (Table 27). In addition, meta-regression models including random effect and a subgroup as a covariate to assess the effects of subgroups on hypertension were performed; the F statistics from these analyses are reported in Table 27. All covariates showing a significant effect (p < 0.05) in a univariate analysis were considered further in a model selection.
| Subgroup | No. of trials | RR | 95% CI | I 2 | Tau2 |
|---|---|---|---|---|---|
| Overall | 12 | 1.80 | 1.14 to 2.85 | 0%; p = 0.79 | 0 |
| Inclusion Hb level (g/dl) | |||||
| ≤ 11.0 | 9 | 1.68 | 1.03 to 2.74 | 0%; p = 0.64 | 0 |
| > 11.0 | 3 | 3.06 | 0.78 to 11.91 | 0%; p = 0.86 | 0 |
| F (between : within) | F1,10 = 0.07; p = 0.79 | ||||
| Baseline Hb level (g/dl) | |||||
| ≤ 10.0 | 9 | 1.76 | 1.07 to 2.89 | 0%; p = 0.54 | 0 |
| ≤ 11.0 | 1 | 1.88 | 0.35 to 9.95 | NA | 0 |
| ≤ 12.0 | 1 | 1.95 | 0.21 to 17.85 | NA | 0 |
| ≤ 14.5 | 1 | 3.14 | 0.13 to 74.98 | NA | 0 |
| F (between : within) | F3,8 = 0.10; p = 0.96 | ||||
| Target Hb level (g/dl) | |||||
| ≤ 13.0 | 3 | 2.19 | 0.53 to 9.12 | 16.8%; p = 0.30 | 0.27 |
| > 13.0 | 6 | 1.89 | 1.09 to 3.28 | 0%; p = 0.94 | 0 |
| NR | 3 | 1.39 | 0.35 to 5.53 | 32.9%; p = 0.23 | 0.49 |
| F (between : within) | F2,9 = 0.07; p = 0.93 | ||||
| Malignancy type | |||||
| Solid tumours | 5 | 1.51 | 0.69 to 3.28 | 0%; p = 0.97 | 0 |
| Haematological tumours | 5 | 1.63 | 0.88 to 3.02 | 0%; p = 0.48 | 0 |
| Mixed | 2 | 5.83 | 1.36 to 24.98 | 0%; p = 0.71 | 0 |
| F (between : within) | F2,9 = 4.07; p = 0.06 | ||||
| Ovarian cancer | |||||
| Ovarian cancer | 1 | 1.95 | 0.21 to 17.85 | NA | 0 |
| Other cancers | 11 | 1.79 | 1.12 to 2.87 | 0%; p = 0.72 | 0 |
| F (between : within) | F1,10 = 0.14; p = 0.71 | ||||
| Chemotherapy treatment | |||||
| Platinum containing | 5 | 1.17 | 0.57 to 2.41 | 0%; p = 0.81 | 0 |
| Non-platinum containing | 4 | 2.20 | 1.15 to 4.19 | 0%; p = 0.49 | 0 |
| F (between : within) | F1,7 = 3.89; p = 0.09 | ||||
| Iron supplementation | |||||
| Iron in both arms | 6 | 2.13 | 1.13 to 3.99 | 0%; p = 0.55 | 0 |
| No iron supplementation | 1 | 3.14 | 0.13 to 74.98 | NA | 0 |
| NR | 5 | 1.44 | 0.72 to 2.86 | 0%; p = 0.552 | 0 |
| F (between : within) | F2,9 = 0.96; p = 0.42 | ||||
| Study design | |||||
| RCT | 9 | 1.70 | 1.05 to 2.76 | 0%; p = 0.65 | 0 |
| ROL | 3 | 3.17 | 0.68 to 14.72 | 0%; p = 0.77 | 0 |
| F (between : within) | F1,10 = 0.84; p = 0.38 | ||||
| Study duration (weeks) | |||||
| 12–16 | 8 | 1.61 | 0.98 to 2.64 | 0%; p = 0.66 | 0 |
| > 20 | 4 | 3.58 | 1.05 to 12.24 | 0%; p = 0.90 | 0 |
| F (between : within) | F1,10 = 1.69; p = 0.22 | ||||
| ESA | |||||
| Erythropoietin | 11 | 1.88 | 1.12 to 3.15 | 0%; p = 0.73 | 0 |
| Darbepoetin | 1 | 1.54 | 0.56 to 4.22 | NA | 0 |
| F (between : within) | F1,10 = 0.38; p = 0.55 | ||||
Univariate analyses did not identify any significant differences based on the predefined subgroups (see Table 27).
Analyses suggest that treatment with ESAs in people with CIA increases the number of hypertension events (RR 1.80, 95% CI 1.14 to 2.85). In total, 5% (n = 62/1152) of participants who received ESAs reported hypertension compared with 3% (n = 27/934) of participants in the control groups. There was no heterogeneity between the trials (I2 = 0%; p = 0.791). Overall, the data confirm the results from previous analyses that there is an increased risk of hypertension in patients receiving ESAs compared with control participants.
Thrombocytopenia/haemorrhage
Data for thrombocytopenia (decrease of platelets in the blood)/haemorrhage were available from seven trials. 52,65–67,70,76,78 Overall, the analysis included all seven studies with 1715 participants. If thrombocytopenia/haemorrhage were not reported, data were obtained from the Cochrane review by Tonia and colleagues. 11
Thrombocytopenia/haemorrhage was reported in 55 out of 877 participants treated with ESAs, compared with 54 out of 838 participants in the control groups. The random-effects meta-analysis showed a RR of 0.93 (95% CI 0.65 to 1.34; Figure 16), which was not statistically significant. There was no statistical heterogeneity between the trials (I2 = 0%; χ2 = 3.02, df = 6, p = 0.807); however, the direction of the effects of ESAs with regard to hypertension varied across the individual trials. Because there were only seven primary studies included in the meta-analysis, the funnel plot analysis to test whether publication bias was present was not conducted. 54
FIGURE 16.
Forest plot: thrombocytopenia/haemorrhage (overall). Note: random-effects meta-analysis (DerSimonian–Laird). Events, treatment = number of events/number of participants in the treatment group; events, control = number of events/number of participants in the control group.
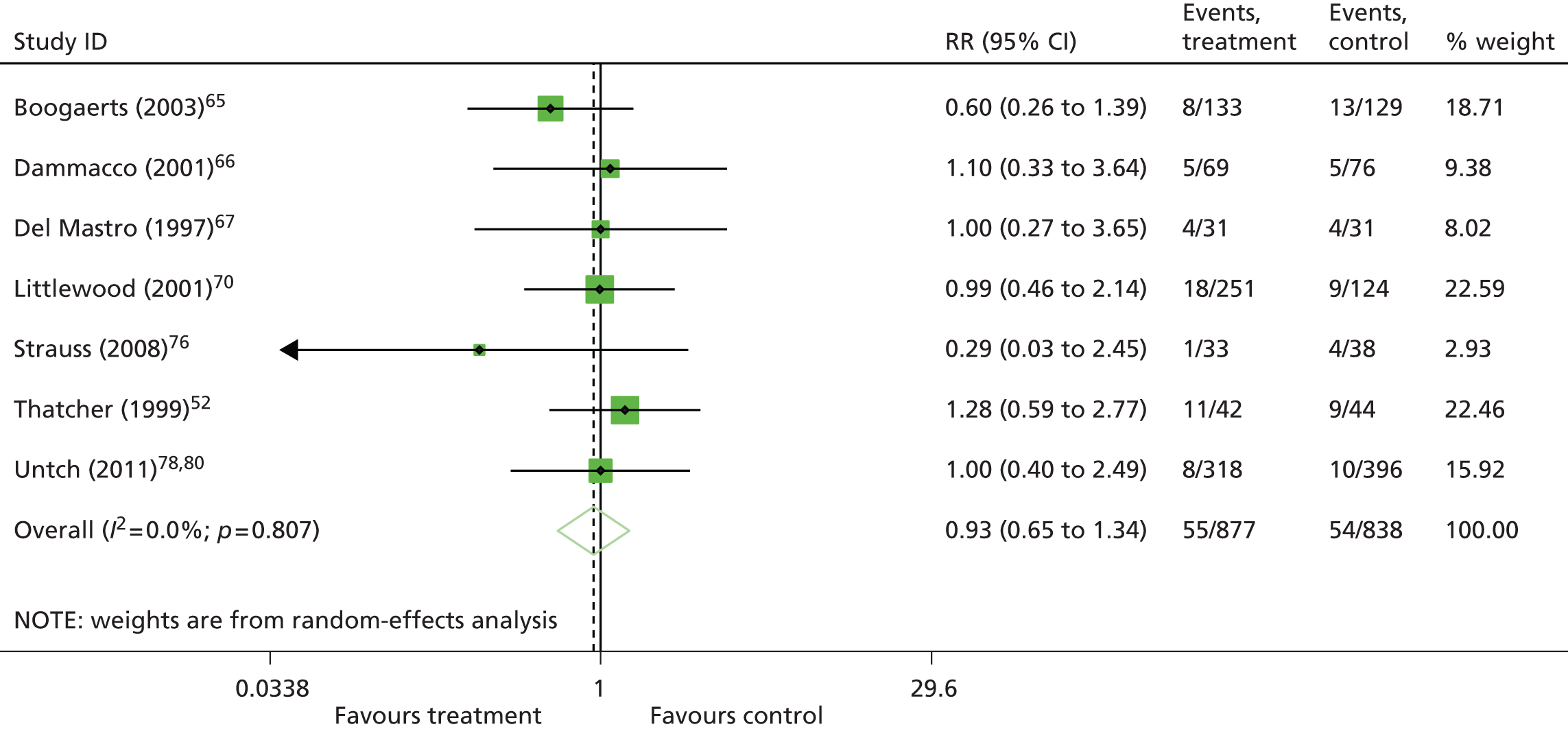
The fixed-effects meta-analysis undertaken as a sensitivity analysis showed similar non-significant results (RR 0.91, 95% CI 0.63 to 1.30; see Appendix 12).
Prespecified subgroup analyses and meta-regression models with subgroups as covariates were not conducted because only seven trials were included in the meta-analysis.
Analyses suggest that treatment with ESAs in people with CIA did not have an effect on thrombocytopenia/haemorrhage (RR 0.93, 95% CI 0.65 to 1.34). In total, 6% (n = 55/877) of participants who received ESAs and 6% (n = 54/838) of participants in the control groups reported thrombocytopenia/haemorrhage. There was no heterogeneity between the trials (I2 = 0%; p = 0.807). Overall, the data seem to be different from previous analyses in suggesting that ESAs do not have a detrimental effect on thrombocytopenia/haemorrhage.
Seizures
Data on seizures were available from one trial63 including 289 participants. As this trial was split into subsets, the number of studies displayed in the forest plot is two. If seizures were not reported, we used data from the Cochrane review by Tonia and colleagues11 in the PenTAG analyses.
Overall, five seizure events were reported in the ESA-treated group (n = 148) and four in the control group (n = 141), resulting in a RR of 1.19 (RR 1.19; 95% CI 0.33 to 4.38; Figure 17). There was no heterogeneity between the trials (I2 = 0%, p = 0.742; χ2 = 0.11, df = 5, p = 0.742), although the two included trials indicated effects in opposite directions. Because only two primary studies were included in the meta-analysis, the funnel plot analysis to test whether publication bias was present was not conducted. 54 The fixed-effects meta-analysis undertaken as a sensitivity analysis showed similar non-significant results (RR 1.19, 95% CI 0.33 to 4.35, I2 = 0%; p = 0.742; see Appendix 12).
FIGURE 17.
Forest plot: seizures (overall). Notes: random-effects meta-analysis (DerSimonian–Laird); trial with multiple experimental arms split into subsets in the analysis: Abels and colleagues63 reported data for participants on platinum-based and non-platinum-based chemotherapy. Events, treatment = number of events/number of participants in the treatment group; events, control = number of events/number of participants in the control group.
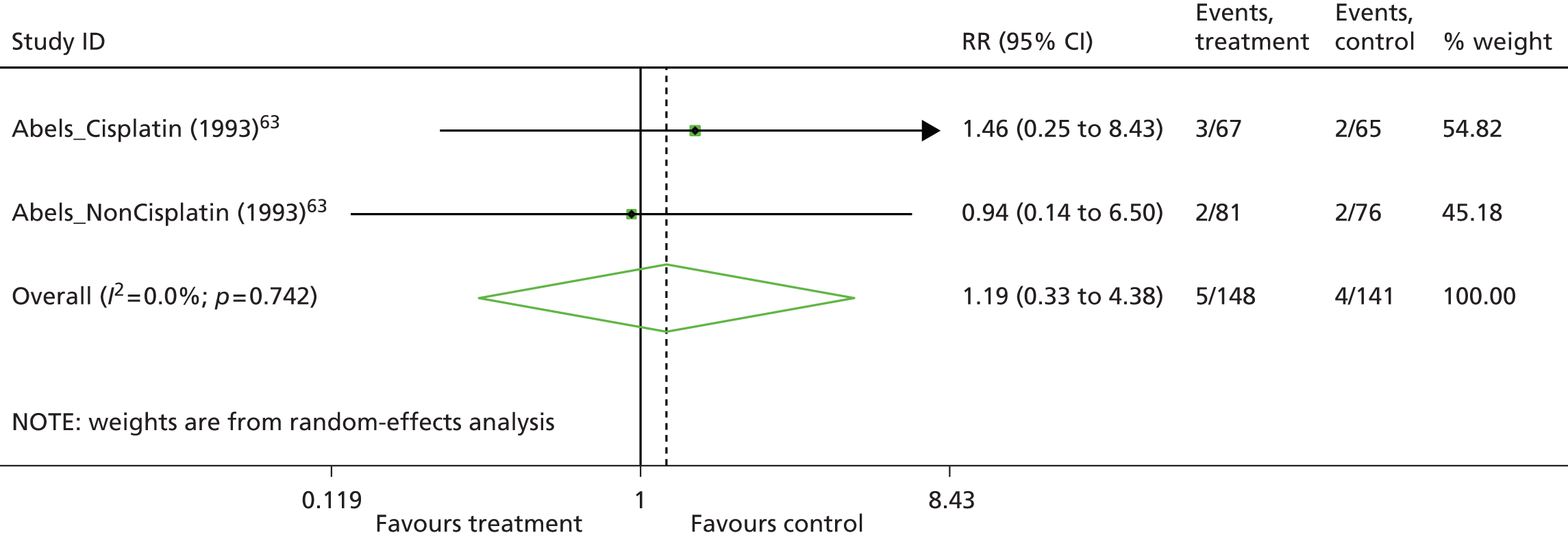
Prespecified subgroup analyses and meta-regression models with subgroups as covariates were not conducted because only two trials were included in the meta-analysis.
Analyses suggest that treatment with ESAs in patients with CIA did not have a significant effect on seizures (RR 1.19, 95% CI 0.33 to 4.38). In total, 3% (5/148) of participants who received ESAs had a seizure; similarly, 3% (4/141) of participants in the control groups had a seizure. There was no heterogeneity between the trials (I2 = 0%; p = 0.742). Although data from one study suggests that ESAs do not have a detrimental effect on seizures, there was no significant difference between groups. The possibility of detrimental effects of ESAs on the number of seizures, however, cannot be excluded. Overall, the data confirm the results from previous analyses.
Pruritus (pruritus, rash and irritation)
We identified seven trials52,63,67,69,76,77,79 that measured pruritus (pruritus, rash and irritation were considered11) including 904 participants. Of these, 450 participants were treated with ESAs. Two included studies were newly identified in the update searches. 76,77 If pruritus events were not reported, we used data from the Cochrane review by Tonia and colleagues11 in the PenTAG analyses. One study69 did not report any pruritus events in the treatment and placebo arms and was excluded from the meta-analysis.
The random-effects meta-analysis showed a risk ratio of 2.04 (95% CI 1.11 to 3.75; Figure 18), favouring the control. There was no heterogeneity between the trials (I2 = 0%, p = 0.872; χ2 = 1.83, df = 5, p = 0.872), with all of the individual studies indicating a detrimental effect of treatment with ESAs with regard to the number of pruritus events. Because only six primary studies were included in the meta-analysis, the funnel plot analysis to test whether publication bias was present was not conducted. 54 The fixed-effects meta-analysis undertaken as a sensitivity analysis showed similar results (RR 2.16, 95% CI 1.18 to 3.92, I2 = 0%; p = 0.872); the forest plot of the analysis is included in Appendix 12.
FIGURE 18.
Forest plot: pruritus (overall). Note: random-effects meta-analysis (DerSimonian–Laird). Events, treatment = number of events/number of participants in the treatment group; events, control = number of events/number of participants in the control group.

The prespecified subgroup analyses and meta-regression models with subgroups as covariates were not conducted because only six trials were included in the meta-analysis.
Analyses suggest that treatment with ESAs in people with CIA increases the number of cases of pruritus (RR 2.04, 95% CI 1.11 to 3.75). In total, 7% (30/450) of participants who received ESAs reported pruritus compared with 3% (13/454) of participants in the control groups. There was no heterogeneity between the trials (I2 = 0%; p = 0.872), with all of the individual studies indicating a detrimental effect of treatment with ESAs with regard to pruritus. Overall, the data seem to be different compared with those from previous analyses. The data suggest that ESAs increase the number of cases of pruritus in patients with chemotherapy-induced anaemia. The definition of pruritus encompassed pruritus, rash and irritation (as defined in the Cochrane review11) and the marked variation in event rates may be a result of the definition of pruritus used. These results should be interpreted with caution.
Safety-related outcomes: summary
All studies were of moderate or poor quality. In addition, there was considerable variability in the reporting of AEs among the included studies. Given the greater access to data in the Cochrane review11 than in the primary papers, relevant data from the Cochrane review11 were used to conduct meta-analyses for the AEs of interest. Overall, the data suggested that there is an increased risk of thromboembolic events and hypertension after treatment with ESAs, consistent with previous estimates (Table 28). Data for seizures are also consistent with previous meta-analyses, showing no effects of ESAs on seizures (see Table 28). Of note is that all AEs are relatively rare compared with other outcomes considered in this report (e.g. RBCT, Hb change and mortality).
| Outcome | aWilson and colleagues2 | aTonia and colleagues11 | PenTAGa | PenTAGb |
|---|---|---|---|---|
| Thromboembolic eventsc,d | NR | RR 1.52, 95% CI 1.34 to 1.74; χ2(het) = 34.99, df = 55; p = 0.980 60 trials, n = 15,498 |
RR 1.52, 95% CI 1.13 to 2.05; χ2(het) = 9.52, df = 14; p = 0.872 15 trials, n = 4013 |
RR 1.46, 95% CI 1.07 to 1.99; χ2(het) = 9.52, df = 14; p = 0.872 15 trials, n = 4013 |
| Hypertensiond | NR | RR 1.30, 95% CI 1.08 to 1.56; χ2(het) = 26.87, df = 34; p = 0.800 35 trials, n = 7006 |
RR 1.97, 95% CI 1.27 to 3.07; χ2(het) = 7.10, df = 11; p = 0.791 12 trials, n = 2086 |
RR 1.80, 95% CI 1.14 to 2.85; χ2(het) = 7.10, df = 11; p = 0.791 12 trials, n = 2086 |
| Thromobocytopenia/haemorrhage | NR | RR 1.21, 95% CI 1.04 to 1.42; χ2(het) = 14.50, df = 20; p = 0.800 21 trials, n = 4220 |
RR 0.91, 95% CI 0.63 to 1.30; χ2(het) = 3.02, df = 11; p = 0.807 7 trials, n = 1715 |
RR 0.93, 95% CI 0.65 to1.34; χ2(het) = 3.02, df = 11; p = 0.807 7 trials, n = 1715 |
| Seizured | NR | RR 0.77, 95% CI 0.42 to 1.41; χ2(het) = 6.19, df = 6; p = 0.400 7 trials, n = 2790 |
RR 1.19, 95% CI 0.33 to 4.35; χ2(het) = 0.11, df = 1; p = 0.742 2 trials, n = 289 |
RR 1.19, 95% CI 0.33 to 4.38; χ2(het) = 0.11, df = 1; p = 0.742 2 trials, n = 289 |
| Pruritusc | NR | RR 1.49, 95% CI 0.99 to 2.24; χ2(het) = 13.18, df = 15; p = 0.590 16 trials, n = 4346 |
RR 2.16, 95% CI 1.18 to 3.92; χ2(het) = 1.83, df = 5; p = 0.872 7 trials, n = 904 |
RR 2.04, 95% CI 1.11 to 3.75; χ2(het) = 1.83, df = 5; p = 0.872 7 trials, n = 904 |
The PenTAG analyses suggest that there is an increased risk of pruritus, with a significant difference found between patients treated with ESAs and participants in the control arms (RR 2.04, 95% CI 1.11 to 3.75). In comparison, the Cochrane review11 did not find a significant difference between patients treated with ESAs and participants in the control arms (RR 1.49, 95% CI 0.99 to 2.24). It must be highlighted that both the current review and the Cochrane review11 combined events of skin rash, irritation and pruritus in the meta-analyses. However, the rates of skin rash, irritation and pruritus may differ and the way that this outcome has been defined may be the cause of the marked variation in event rates.
Also, the summary estimate for risk of thrombocytopenia/haemorrhage associated with ESA treatment found in the PenTAG review was a RR of 0.93 (95% CI 0.65 to 1.34), suggesting that treatment with ESAs in patients with CIA did not have an effect on thrombocytopenia/haemorrhage. However, the Cochrane review11 found a RR of 1.21 (95% CI 1.04 to 1.42), suggesting detrimental effects of ESAs with regard to thrombocytopenia/haemorrhage.
It must be emphasised that the current analyses included only studies complying with the licenced ESA dose, whereas the Cochrane review did not apply any restrictions regarding the ESA posology. However, these results should be interpreted with caution (see Chapter 6, Strengths and limitations of the systematic review of studies of effectiveness for more details).
A graphical summary of the study characteristics, quality appraisal and results for the safety outcomes is presented in Figure 19.
FIGURE 19.
Safety-related outcomes: graphical summary. Notes: for some of the included trials, data for some anaemia-related outcomes were not available for all participants; where different, the numbers included are reported in notes 1–4. 1: hypertension reported for epo beta n = 43 and control n = 28; 2: participants who received at least one dose of study treatment even if they did not meet the eligibility criteria were included in the safety population; 3: one participant randomised to the darbe alfa arm did not receive the study treatment and was included in the placebo arm for the safety evaluation. ?, unknown; chemo, chemotherapy; darbe, darbepoetin; epo, epoetin; haem, haematological; non-plat, non-platinum; plat, platinum; QA, quality appraisal; thrombo/haemor, thrombosis/haemorrhage.



Subgroup analyses
The results of the subgroup analyses by iron supplementation and platinum-based chemotherapy are reported throughout this chapter (see Effectiveness).
Use of iron supplementation varied among the studies, for example oral iron supplementation given as needed (dosage and trigger level differed between studies) or as standard and/or intravenous iron supplementation (see Concomitant treatments). In addition, limited details in the publications hindered the interpretation of this outcome. Subgroup analyses did not identify any significant differences between groups.
Five studies48,51,63,64,73 evaluated the use of ESAs in people with any type of cancer receiving platinum-based chemotherapy. The point estimates for this subgroup are reported in Table 29.
| Outcome measure | Results |
|---|---|
| Anaemia-related outcomes | |
| Hb change (g/dl) | WMD 1.42, 95% CI 1.10 to 1.75; I2 = 0%, p = 0.774 Trials: 5a |
| HaemR | RR 3.93, 95% CI 2.50 to 6.17; I2 = 11.9%, p = 0.321 Trials: 3a |
| RBCT | RR 0.52, 95% CI 0.37 to 0.72; I2 = 60.0%, p = 0.029 Trials: 6a |
| RBC units | WMD –1.11, 95% CI –1.58 to –0.64; I2 = 0%, p = 0.685 Trials: 3 |
| Malignancy-related outcomes | |
| Tumour response | RR 0.91, 95% CI 0.62 to 1.33; I2 = NA Trials: 1 |
| OS | HR 0.67, 95% CI 0.46 to 0.98; I2 = 14.5%, p = 0.319 Trials: 4a |
| On-study mortality | HR 0.63, 95% CI 0.34 to 1.18; I2 = 36.0%, p = 0.196 Trials: 4a |
| Safety-related outcomes | |
| Thromboembolic events | RR 1.06, 95% CI 0.51 to 2.20; I2 = 0%, p = 0.473 Trials: 3 |
| Hypertension | RR 1.17, 95% CI 0.57 to 2.41; I2 = 0%, p = 0.808 Trials: 5a |
| Seizures | RR 1.46, 95% CI 0.25 to 8.43; I2 = NA Trials: 1 |
| Pruritus | RR 3.40, 95% CI 0.73 to 15.74; I2 = NA Trials: 1 |
Results from this subgroup analysis are consistent with the findings from the overall analysis for the anaemia-related outcomes, that is, an improved haematological response and a reduction in RBCT requirements, and are different from the results reported in the Cochrane review. 11 Similarly to the overall analysis, the results for the malignancy-related outcomes (OS and on-study mortality) suggest fewer detrimental effects for people with chemotherapy-induced anaemia treated with ESAs. These effects are also reflected in the decrease in the number of people experiencing thromboembolic events. However, these results should be interpreted with caution. The number of studies per subgroup is small, some of the changes are not statistically significant and the CIs remain wide. It is also important to remember that multiple testing issues arise when subgroups are tested and that CIs presented here have not been adjusted for multiple testing.
We also investigated women with ovarian cancer and women with ovarian cancer receiving platinum-based chemotherapy. Only one study evaluated participants with ovarian cancer;51 all participants (n = 122) received platinum-based chemotherapy. The outcomes measured were Hb change, RBCT, RBC units transfused, tumour response and safety. The point estimates for these outcomes are reported in Table 30. Other studies may have included some ovarian cancer patients; however, the results are reported for whole study populations and not by malignancy type.
| Outcome measure | Results |
|---|---|
| Anaemia-related outcomes | |
| HaemR | NR |
| Hb change (g/dl) | WMD 1.23, 95% CI 0.48 to 1.98; I2 = NA Trials: 1 |
| RBCT | RR 0.11, 95% CI 0.03 to 0.47; I2 = NA Trials: 1 |
| RBC units | WMD –0.94, 95% CI –1.76 to –0.12; I2 = NA Trials: 1 |
| Malignancy-related outcomes | |
| Tumour response | RR 0.91, 95% CI 0.62 to 1.33; I2 = NA Trials: 1 |
| OS | NR |
| On-study mortality | NR |
| Safety-related outcomes | |
| Thromboembolic events | RR 3.70, 95% CI 0.18 to 74.51; I2 = NA Trials: 1 |
| Hypertension | RR 0.11, 95% CI 0.03 to 0.47; I2 = NA Trials: 1 |
No studies were identified that evaluated people with head and neck malignancies receiving platinum-based chemotherapy. Similarly, no trials were identified that evaluated people unable to receive RBCTs (e.g. Jehovah’s Witnesses and people who have multiple antibodies to RBCs because they have required regular transfusions in the past). Clinical advice suggests that it is reasonable to assume that ESAs are likely to improve Hb levels in this subpopulation. It is also considered reasonable to believe that, if people can be supported through the period of life-threatening anaemia, they will recover; if ESAs are not allowed they run the risk of death.
Definition of ‘within licence’
For this HTA review, studies were considered eligible for inclusion if they evaluated starting doses of ESAs according to European labelling, irrespective of how they dealt with Hb levels (see Chapter 1, Marketing authorisations: haemoglobin levels).
With respect to European labelling, additional measures of dose efficiency [inclusion Hb level (≤ 11 g/dl and > 11 g/dl) and target Hb level (≤ 13 g/dl and > 13 g/dl)] were also considered in post-hoc analyses. Studies contributing to these subgroups are listed in Table 31. In addition, we also considered measures relating to the administration of ESAs in conjunction with study quality, specifically blinding (double-blind RCTs).
| Subgroup by ‘closer to’ licence recommendations | Trials | References |
|---|---|---|
| Starting dose criteria met | 23 | aAbels 1993;63 Aravantinos 2003;64 Boogaerts 2003;65 Dammacco 2001;66 Del Mastro 1997;67 Dunphy 1999;68 Grote 2005;74 Hedenus 2002;53 Hedenus 2003;17 Kotasek 2003;50 Kurz 1997;69 Littlewood 2001;70Moebus 2013;62 bÖsterborg 2002;71 bÖsterborg 2005;79 Ray-Coquard 2009;75 Silvestris 1995;72 Straus 2008;76 ten Bokkel Huinink 1998;51 aThatcher 1999;52 Tjulandin 2010;48 cTjulandin 2011;77 bUntch 2011;78,80 Vansteenkiste 200273 |
| Starting dose criteria met and inclusion Hb ≤ 11 g/dl | 14 | aAbels 1993;63 Aravantinos 2003;64 Boogaerts 2003;65 Dammacco 2001;66 Hedenus 2002;53 Hedenus 2003;17 Kotasek 2003;50 Kurz 1997;69 Littlewood 200170 (≤ 10 g/dlc); bÖsterborg 2002;71 bÖsterborg 2005;79 Silvestris 1995;72 aTjulandin 2010;48 Tjulandin 2011;77 Vansteenkiste 200273 (Hb < 10 g/dl and ≥ 10 and ≤ 11 g/dld) |
| Starting dose criteria met, inclusion Hb ≤ 11 g/dl and target Hb ≤ 13 g/dl | 2 | aTjulandin 2010;48 Tjulandin 201177 |
Results from these subgroup analyses are summarised in Table 32.
| Outcome | Start dose, double-blind RCT | Licence start dose, double-blind RCT | Licence start dose, inclusion Hb ≤ 11 g/dl | Licence start dose, inclusion Hb ≤ 11 g/dl | Licence start dose, inclusion Hb ≤ 11 g/dl and target Hb ≤ 13 g/dl | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | ES (95% CI) | I2, p-value | Number of studies | ES (95% CI) | I2, p-value | Number of studies | ES (95% CI) | I2, p-value | Number of studies | ES (95% CI) | I2, p-value | Number of studies | ES (95% CI) | I2, p-value | |
| Hb change (g/dl)a,b | 18 | WMD 1.59 (1.33 to 1.84) | 75.9%, p < 0.01 | 13 | WMD 1.70 (1.43 to 1.97) | 64.9%, p < 0.01 | 13 | WMD 1.52 (1.30 to 1.75) | 48.1%, p = 0.03 | 11 | WMD 1.59 (1.35 to 1.84) | 46.4%, p = 0.05 | 3 | WMD 1.50 (1.16 to 1.83) | 0%, p = 0.80 |
| HaemRa,b,c | 13 | RR 3.29 (2.81 to 3.85) | 13.4%, p = 0.31 | 12 | RR 3.30 (2.77 to 3.93) | 19.5%, p = 0.25 | 12 | RR 3.20 (2.78 to 3.68) | 2.0%, p = 0.43 | 11 | RR 3.20 (2.74 to 3.75) | 8.9%, p = 0.36 | 3 | RR 3.06 (2.28 to 4.09) | 0%, p = 0.79 |
| RBCTa,b,d | 26 | RR 0.61 (0.55 to 0.68) | 22.4%, p = 0.15 | 16 | RR 0.64 (0.58 to 0.72) | 6.4%, p = 0.38 | 16 | RR 0.64 (0.57 to 0.71) | 7.3%, p = 0.37 | 14 | RR 0.66 (0.59 to 0.74) | 0%, p = 0.52 | 3 | RR 0.50 (0.33 to 0.77) | 0%, p = 0.92 |
| RBC unitsa,e | 12 | WMD –0.87 (–1.24 to –0.50) | 55.6%, p = 0.01 | 9 | WMD –0.9 (–0.93 to –0.36) | 28.0%, p = 0.20 | 9 | WMD –0.99 (–1.41 to –0.56) | 56.2%, p = 0.01 | 8 | WMD –0.63 (–0.79 to –0.47) | 0.6%, p = 0.43 | 1 | WMD –0.56 (–0.74 to –0.39) | NA |
| Tumour response | 7 | RR 1.10 (0.86 to 1.41) | 37.5%, p = 0.14 | 4 | RR 1.50 (1.01 to 2.23) | 21.5%, p = 0.28 | 2 | RR 1.60 (0.88 to 2.90) | 0%, p = 0.70 | 2 | RR 1.60 (0.88 to 2.90) | 0%, p = 0.70 | 0 | NA | NA |
| OSa,b | 18 | HR 0.97 (0.83 to 1.13) | 42.4%, p = 0.03 | 11 | HR 0.92 (0.75 to 1.13) | 52.4%, p = 0.02 | 10 | HR 0.91 (0.70 to 1.20) | 51.7%, p = 0.03 | 9 | HR 0.87 (0.65 to 1.15) | 53.7%, p = 0.03 | 3 | HR 0.50 (0.20 to 1.23) | 29.7%, p = 0.24 |
| On study mortalitya,b | 14 | HR 0.86 (0.67 to 1.11) | 16.4%, p = 0.27 | 11 | HR 0.86 (0.63 to 1.17) | 33.0%, p = 0.14 | 10 | HR 0.89 (0.61 to 1.30) | 37.7%, p = 0.11 | 9 | HR 0.86 (0.56 to 1.32) | 44.5%, p = 0.07 | 3 | HR 0.50 (0.20 to 1.23) | 29.7%, p = 0.24 |
| Thromboembolic eventsa | 14 | RR 1.46 (1.07 to 1.99) | 0%, p = 0.73 | 9 | RR 1.24 (0.81 to 1. 90) | 0%, p = 0.55 | 7 | RR 1.29 (0.66 to 2.54) | 12.2%, p = 0.34 | 7 | RR 1.29 (0.66 to 2.54) | 12.2%, p = 0.34 | 1 | RR 0.32 (0.01 to 7.74) | NA |
| Hypertensiona,b | 12 | RR 1.80 (1.14 to 2.85) | 0%, p = 0.79 | 9 | RR 1.70 (1.05 to 2.76) | 0%, p = 0.65 | 9 | RR 1.68 (1.03 to 2.74) | 0%, p = 0.64 | 8 | RR 1.61 (0.98 to 2.64) | 0%, p = 0.66 | 3 | RR 2.19 (0.53 to 9.12) | 16.8%, p = 0.30 |
| Thrombocytopenia/haemorrhage | 7 | RR 0.93 (0.65 to 1.34) | 0%, p = 0.81 | 5 | RR 0.89 (0.57 to 1.39) | 0%, p = 0.58 | 2 | RR 0.73 (0.37 to 1.46) | 0%, p = 0.41 | 1 | RR 1.10 (0.33 to 3.64) | NA | 0 | NA | NA |
| Seizuresa | 2 | RR 1.19 (0.33 to 4.38) | 0%, p = 0.74 | 2 | RR 1.19 (0.33 to 4.38) | 0%, p = 0.74 | 2 | RR 1.19 (0.33 to 4.38) | 0%, p = 0.74 | 2 | RR 1.19 (0.33 to 4.38) | 0%, p = 0.74 | 0 | NA | NA |
| Pruritus | 6 | RR 2.04 (1.11 to 3.75) | 0%, p = 0.87 | 3 | RR 2.20 (1.05 to 4.58) | 0%, p = 0.66 | 3 | RR 2.20 (1.05 to 4.58) | 0%, p = 0.66 | 3 | RR 2.20 (1.05 to 4.58) | 0%, p = 0.66 | 1 | RR 1.78 (0.74 to 4.26) | NA |
Post-hoc analyses offer some limited evidence to suggest that compliance with European labelling results in better outcomes: there are no detrimental effects of ESAs on either on-study or overall mortality in patients with chemotherapy-induced anaemia. These effects are consistent with an improved tumour response and a decrease in the number of thromboembolic events. However, these analyses must be interpreted with caution. The number of studies per subgroup is small, some of the effect sizes are not statistically significant and the CIs remain wide. In addition, the analyses may not have the statistical power to detect the effects of adherence to European labelling on outcomes, if such effects exist. It should also be noted that this is a difficult area of assessment, especially in a heterogeneous mix of tumour types. Furthermore, we have not sought to address multiple testing issues that arise when considering subgroups, and so inference is not straightforward.
Health-related quality of life
Health-related quality of life has become a key clinical outcome. Anaemia is often associated with cancer, either because of the disease itself or because of the subsequent treatment. Therefore, the patient may experience exhaustion, fatigue, weakness, impaired concentration, respiratory distress and chest pain, which will, in turn, significantly impact on HRQoL. 70 As ESAs may relieve CIA by increasing Hb levels, HRQoL is a particular outcome of interest for the interventions under review.
Methods
A search specifically targeted at HRQoL was conducted (see Identification of studies). Titles and abstracts identified in the quality of life searches were screened according to the eligibility criteria presented earlier (see Eligibility criteria); however, these titles and abstracts were screened specifically for HRQoL outcomes.
Results from included studies were tabulated and narratively reported. In addition, meta-analyses were used to provide an overview with an estimate of overall effect.
Results
In total, 13 trials17,50,52,63,65–67,69–71,73,75,77 (reported in 23 publications17,50,52,58–60,63,65–67,69–71,73,75,77,79,81–86) were identified (full details relating to the selection of studies and a PRISMA flow diagram are provided in Appendix 13).
Study characteristics
Study characteristics are reported in Table 10. A summary of the HRQoL measures included in the studies is provided in Table 33.
| HRQoL measure | Studies |
|---|---|
| FACT-G | Österborg 2002;71 Littlewood 2001;70 Aapro 2004;82 Bajetta 2004;81 Patrick 2003;60 Tjulandin 201177 |
| FACT-An | Österborg 2002;71 Tjulandin 201177 |
| FACT-F | Österborg 2002;71 Littlewood 2001;70 Boogaerts 2003;65 Hedenus 2003;17 Vansteenkiste 2002;73 Aapro 2004;82 Bajetta 2004;81 Kotasek 2003;50 Littlewood 2006;83 Patrick 2003;60 Tjulandin 201177 |
| FACT-An-An | Aapro 2004;82 Boogaerts 2003;65 Littlewood 2001;70 Österborg 200271 |
| SF-36 | Boogaerts 2003;65 Patrick 200360 |
| CLAS/LASA | Dammacco 2001;66 Littlewood 2001;70 Aapro 2004;82 Bajetta 2004;81 Patrick 200360 |
| PDI | Del Mastro 199767 |
| EORTC QLQ-C30 | Ray-Coquard 200975 |
| BSI | Littlewood 200683 |
| NHP | Dammacco 200166 |
| VAS | Abels 1993;63 Boogaerts 2003;65 Kurz 1997;69 Thatcher 199952 |
A range of questionnaires was used to measure HRQoL and subsequent changes in response to treatment. The scales are summarised in Appendix 13; however, this review focuses on the FACT tool, as it is has been widely used in ESA trials and is considered to have good responsiveness to change and good convergent and discriminant validity. 11 Furthermore, the FACT tool is the only tool in this review for which there are sufficient studies to enable meta-analysis.
The FACT tool, which asks patients to focus on HRQoL issues over the previous 7 days, is part of a collection of HRQoL questionnaires (Figure 20), beginning with a generic questionnaire called the FACT-G. There are now over 50 different scales and symptom indexes, some of which have been modified over time. The FACT scales used in this review are listed in Table 33. Copies of these questionnaires and details of scoring and interpretation are available at: http://www.facit.org/FACITOrg/Questionnaires (accessed September 2015). It should be noted that since 1997 the scale has been known as FACIT.
FIGURE 20.
Overview of the FACT scales used in this review. Fact-An-An, FACT-Anaemia Anaemia subscale.

Using both anchor-based and distribution-based methods to analyse FACT-F, FACT-G and FACT-An, data from three samples of patients (n = 50, n = 131 and n = 2402) determined the minimal clinically important difference to be FACT-F = 3.0, FACT-G = 4.0 and FACT-An = 7.0. 112
Trials identified in the previous Health Technology Assessment review
Of the 11 trials identified in the previous HTA review,2 nine indicated that there was a statistically significant difference in HRQoL between patients treated with ESAs and control participants (Figure 21). Of the two studies that did not show ESAs to be effective compared with placebo, one used an unvalidated assessment tool (Health State Utility Scale)69 and the other used the Psychological Distress Inventory (PDI). 67
FIGURE 21.
Health-related quality of life: graphical summary. Notes: 1: Study population included patients not receiving chemotherapy; 2: 87% and 84% of participants were analysed in the treatment and control groups, respectively; 3: 80% and 73% of participants were analysed in the treatment and control groups, respectively; 4: 54% and 57% of participants were analysed in the treatment and control groups, respectively; 5: 75% and 61% of participants were analysed in the treatment and control groups, respectively; 6: 90–98% and 86–97% of participants were analysed in the treatment and control groups, respectively; 7: 81% of participants were analysed in the treatment and control groups. ?, unknown; BSC, best supportive care; BSI, Brief Symptom Inventory; CLAS, Cancer Linear Analog Scale; D&A, depression and anxiety subscale of the BSI; darbe, darbepoetin; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30; epo, epoetin; FACT-An-An, FACT–Anaemia Anaemia subscale; haem, haematological; LASA, Linear Analogue Scale Assessment; NHP, Nottingham Health Profile; non-plat, non-platinum; PDI, Psychological Distress Inventory; plat, platinum; VAS, visual analogue scale.



Thatcher and colleagues52 reported that only the overall HRQoL level revealed a statistically significant improvement favouring epoetin alfa (p < 0.05). Evaluation of World Health Organization (WHO) performance scores revealed similar findings, with no significant between- or within-group differences. 52
Trials identified, 2004 to current
Three trials were identified following the previous HTA review. 2 Of these, one was a follow-up study79 of a study identified previously. 71 Österborg and colleagues79 report a statistically significant increase in favour of epoetin beta; however, the variability between patients was considerable. Ray-Coquard and colleagues75 stated that there were no statistically detectable differences during the study period, although none of these data were reported. Tjulandin and colleagues77 also found no significant differences between the epoetin theta group and the placebo group.
Post-hoc studies identified, 2004 to current
Five studies60,81–84 reported trends that favour ESA. However, Bajetta and colleagues81 and Littlewood and colleagues83 did not analyse this statistically.
Additional results to the primary study73 provided by Vansteenkiste and colleagues84 indicated a significant difference (p = 0.0147) in HRQoL between the darbepoetin alfa group and the placebo group for those with a baseline Hb level of < 10 g/dl. In contrast, no difference was apparent between groups for those with a baseline Hb of ≥ 10 g/dl.
Meta-analysis: Functional Assessment of Cancer Therapy – Fatigue (13 items) score (random effects)
Given the variability of reporting in the published papers, FACT-F data were extracted from the Cochrane review by Tonia and Colleagues11 for use in the PenTAG analyses. Functional Assessment of Cancer Therapy – Fatigue scores were available from seven studies17,50,65,70,71,73,77,79 including 1794 participants. One new primary study was identified. 77
The WMD was 2.54 (95% CI 1.42 to 3.65; Figure 22). There was low heterogeneity between the trials (I2 = 14.9%; p = 0.32) (Table 34). Because only seven primary studies were included in the meta-analysis, the funnel plot analysis to test whether publication bias was present was not conducted in accordance with published giudelines. 54 The fixed-effects meta-analysis undertaken as a sensitivity analysis showed similar significant results (see Appendix 13, Figure 108). All of the studies were similar in terms of quality; however, the trial reported by Boogaerts and colleagues65 did not employ blinding for participants. Removing this study from the meta-analysis had a minimal impact on the results (WMD 2.21, 95% CI 1.131 to 3.280; see Appendix 13, Figure 113), but did improve heterogeneity (I2 = 0%, p = 0.51).
FIGURE 22.
Health-related quality of life: FACT-F (13 items) – overall. Note: random-effects meta-analysis (DerSimonian–Laird). BSI, Brief Symptom Inventory; CLAS, Cancer Linear Analogue Scale; D&A, depression and anxiety subscale of the BSI; darbe, darbepoetin; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30; epo, epoetin; FACT-An-An, FACT-An Anaemia subscale; haem, haematological; LASA, Linear Analogue Scale Assessment; NHP, Nottingham Health Profile; non-plat, non-platinum; plat, platinum; VAS, visual analogue scale.

| FACT scale | aWilson and colleagues2 | aTonia and colleagues11 | PenTAGa | PenTAGb |
|---|---|---|---|---|
| FACT-F (13 items; score 0–52)c | NR | WMD 2.08, 95% CI 1.43 to 2.72; χ2(het) = 36.48, df = 17; p = 0.004 18 trials, n = 4965 |
WMD 2.49, 95% CI 1.48 to 3.51; χ2(het) = 7.05, df = 6; p = 0.000 7 trials, n = 1794 |
WMD 2.54, 95% CI 1.42 to 3.65; χ2(het) = 7.05, df = 6; p = 0.000 7 trials, n = 1794 |
| Any subgroup effect | NR | Yes: imputed vs. non-imputed data, baseline Hb level, type of anticancer therapy, duration of ESA treatment and ITT analysis | – | Possible: malignancy, intervention and duration |
| cFACT-F (13 items; score 0–52) without Boogaerts 200365 | – | – | – | WMD 2.21, 95% CI 1.13 to 3.28; χ2(het) = 4.31, df = 5; p = 0.000 6 trials, n = 1581 |
| FACT-G (27 items; score 0–108)c | NR | NR | WMD 3.16,e 95% CI 1.11 to 5.21; χ2(het) = 6.82, df = 2; p = 0.003 3 trials, n = 686 |
WMD 2.98,e 95% CI –0.83 to 6.78; χ2(het) = 6.82, df = 2; p = 0.13 3 trials, n = 686 |
| FACT-An-An (seven items; score 0–28)c,d | NR | NRd | WMD 1.05,f 95% CI 0.93 to 1.12; χ2(het) = 80.66, df = 2; p = 0.00 3 trials, n = 686 |
WMD 2.60,f 95% CI –0.52 to 5.72; χ2(het) = 80.66, df = 2; p = 0.00 3 trials, n = 686 |
Meta-analysis was performed on FACT-G and FACT-An Anaemia subscale (FACT-An-An) data; however, only three studies70,71,77,79 were suitable for inclusion for each scale with high levels of heterogeneity [see Table 34 and Appendix 13, Figures 114 and 115 (FACT-G), and Figures 116 and 117 (FACT-An)]. The results of no statistical difference between the intervention and the control must therefore be treated with caution.
Univariate subgroup analyses were conducted for FACT-F outcomes according to chemotherapy type, malignancy type, intervention (epoetin or darbepoetin) and study duration and showed significant results; however, the number of studies included was small (see Table 34 and Appendix 13, Figures 109–112).
Health-related quality-of-life outcomes: overall summary
Effectiveness estimates are presented alongside previously reported estimates for HRQoL (see Table 34). A graphical summary of the study characteristics, quality appraisal and results for these outcomes is presented in Figures 21 (HRQoL) and 23 (FACT-F).
FIGURE 23.
Forest plot: HRQoL overall. Note: random effects meta-analysis (DerSimonian–Laird).
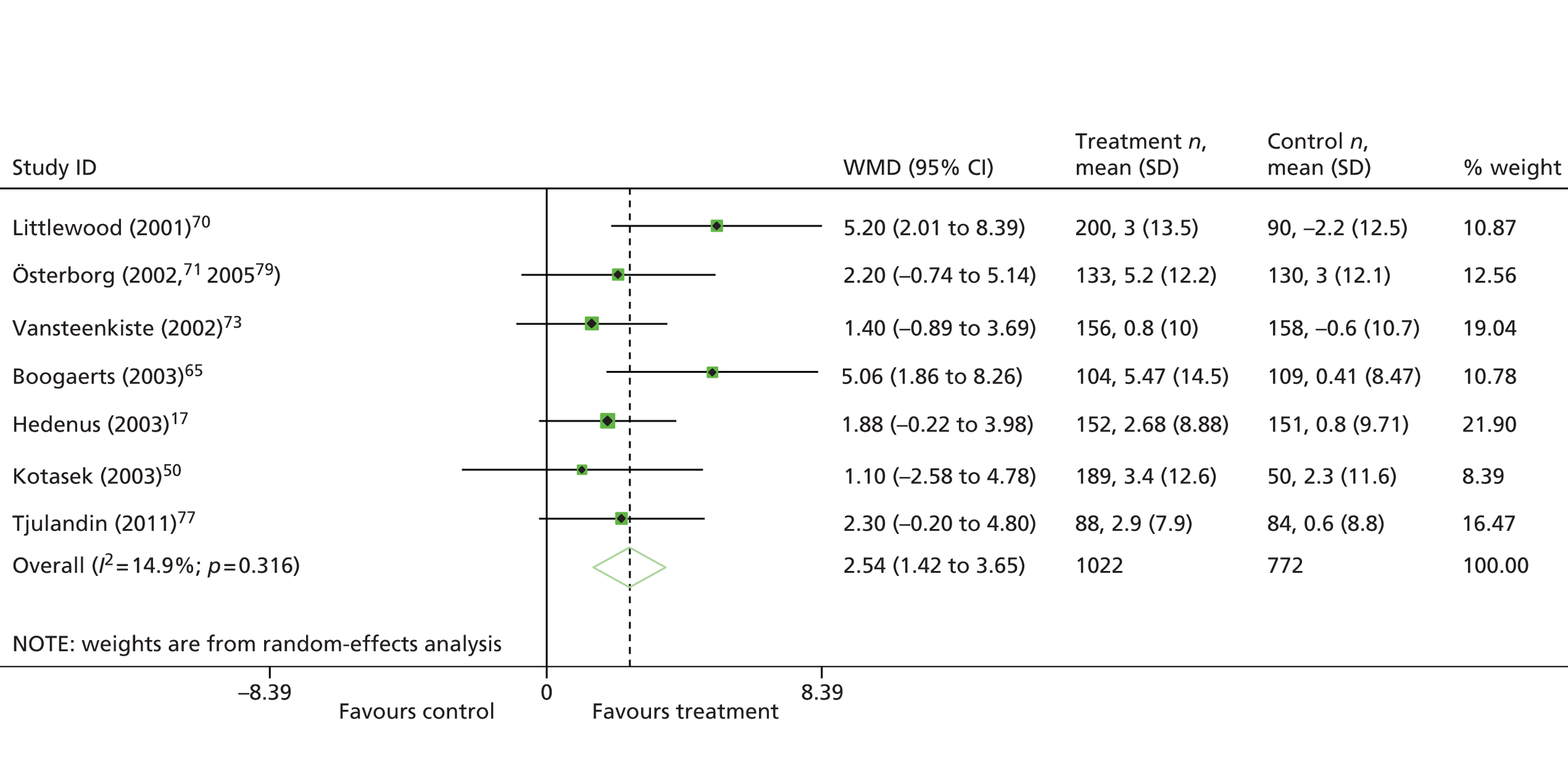
Overall, the conclusions of the PenTAG review are in agreement with those of the Cochrane review11 in that there is a statistically significant difference between patients treated with ESAs and control participants when combining HRQoL parameters; however, this is probably not clinically important (minimal clinically important difference = 3.0112). As with previous reviews, however, it should be noted that there are several methodological concerns that may result in bias, for example the substantial quantity of missing data, expecting patients to complete repeated questionnaires leading to a shift in patient response and the various modes of administration of the questionnaires.
Key points
-
From a total of 1517 titles and abstracts screened, 11 systematic reviews (reported in 12 publications) and 23 RCTs (reported in 34 publications) were found that matched the inclusion criteria and were considered ‘within licence’ based on the start dose administered.
-
Of note, none of the included studies evaluated ESAs entirely within the remit of their marketing authorisations. In particular, starting and target Hb levels and stopping rules were all generally higher than specified in the licences. This could be because the majority of studies (82%) were initiated before the changes to the licences in 2008.
-
Overall, the included trials were of moderate or poor quality. All were flawed because of reporting issues but some were more flawed than others. Most notably, all trials lacked clarity in the reporting of allocation methods (the procedure for randomisation and/or allocation concealment). For most of the studies it was difficult to make a general assessment about quality because of reporting omissions.
-
Pooled estimates for anaemia-related outcomes were consistent with previous estimates in terms of both haematological response and requirement for RBCT and were in favour of ESA treatment. The estimates for haematological response and numbers transfused seem to be robust, with no marked heterogeneity or subgroup effects. However, the analyses for Hb change did include important heterogeneity, which may possibly indicate subgroup effects; analyses in this respect were inconclusive.
-
The HR for OS was 0.97 (95% CI 0.83 to 1.13) although the forest plot suggested that there was a tendency for smaller studies to favour treatment. However, this estimate is subject to uncertainty and no definitive conclusions can be drawn.
-
The HR for on-study mortality (deaths occurring up to 30 days after the active study period) was 0.86 (95% CI 0.67 to 1.11).
Adverse events
-
Overall, pooled data suggest that treatment with ESAs is associated with an increased risk for thromboembolic events, hypertension, seizure and rash, consistent with previous estimates. The risk for thrombocytopenia/haemorrhage associated with ESA treatment remains unclear and there were too few data to rule out detrimental effects.
-
Adverse events are mainly affected by the quality of information available, the variability in the definition of AEs used and the width of the CIs.
Health-related quality of life
-
There is a statistically significant difference in HRQoL between patients treated with ESAs and control participants when combining HRQoL parameters, which is, however, probably not clinically important (minimal clinically important difference = 3.0112).
-
Meta-analysis was performed for the FACT-G and FACT-An-An subscales; however, only three studies were suitable for inclusion for each scale with high levels of heterogeneity. The result of no statistical difference between the intervention and the control must therefore be treated with caution.
-
Publication bias was noted in the Cochrane review,11 suggesting over-reporting of studies that showed beneficial effects of ESAs. It was not possible to examine publication bias using funnel plots because there were fewer than 10 included studies; therefore, it was not possible to confirm or refute the claims made in the Cochrane review.
-
Health-related quality of life is affected by the variability of instruments used and the moderate or poor study quality, for example patients and physicians were not blinded in the majority of trials, which is considered to have a significant impact on HRQoL assessed by self-reporting. Significant numbers were lost to follow-up for HRQoL outcomes in at least six trials.
Subgroup and exploratory analyses
-
Only one study evaluated the use of ESAs in women with ovarian cancer. All participants in this study received platinum-based chemotherapy.
-
Subgroup analyses of platinum-based chemotherapy in people with any type of cancer showed a trend towards a slight benefit associated with ESA treatment in terms of on-study mortality or OS in patients with chemotherapy-induced anaemia. However, these results should be treated with caution because of the small number of studies included in the analysis.
-
No studies were identified that considered the use of ESAs among people unable to receive RBCTs. However, it is reasonable to assume that ESAs are likely to work in improving Hb in this subpopulation. It is also reasonable to believe that, if patients can be supported through the period of life-threatening anaemia, their Hb level will recover; if ESAs are not allowed, they run the risk of death.
-
Post-hoc analyses (starting dose plus inclusion Hb level ≤ 11.0 g/dl and starting dose plus inclusion Hb level ≤ 11.0 g/dl plus target Hb level ≤ 13.0 g/dl) offer some limited evidence to suggest that compliance to European labelling results in better outcomes, although results should be interpreted with caution (when considering the subgroup of trials. Data suggest that, if the licensed recommendations for ESA administration are followed, there are no detrimental effects of ESAs on on-study mortality or overall mortality in patients with chemotherapy treatment-induced anaemia. Although these effects are consistent with improved tumour response and a decrease in the number of thromboembolic events, these results should be interpreted with caution, as the point estimates are not statistically significant and the CIs around the estimates remain wide. Furthermore, we have not sought to address multiple testing issues that arise when considering subgroups and so inference is not straightforward.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
The cost-effectiveness of ESAs within their licensed indications for the treatment of chemotherapy-induced anaemia compared with each other and with best supportive care was assessed in a systematic review of the literature.
This systematic review of cost-effectiveness evidence was an update of a systematic review reported by Wilson and colleagues,2 which informed previous NICE guidance TA142. 1 The methods and results of the previous systematic review are summarised in the following section and the methods and results for this update review are described in Update review.
Economic evaluations submitted by manufacturers would have been included in the systematic review but no such evaluations were submitted.
Wilson and colleagues:2 summary
A systematic review of cost-effectiveness evidence was reported by Wilson and colleagues,2 which informed previous NICE guidance TA142. 1
Objective
The objective of this systematic review was ‘to identify and appraise past economic evaluations of erythropoietin in the treatment of anaemia associated with cancer treatment’ (p. 83). 2
Methods
Searches were conducted in a range of databases, as detailed in Table 35. Industry submissions were also evaluated and searched for additional references.
| Database | Interface | Date range |
|---|---|---|
| MEDLINE | Ovid | 1966 to July Week 4 2004 |
| EMBASE | Ovid | 1980 to Week 30 2004 |
| DARE | – | 2004 Issue 3 |
| NHS EED | – | 2004 Issue 3 |
| OHE HEED | – | July 2004 |
Separate search strategies were developed for costs, economic models and quality-of-life studies, which are detailed in Appendix 3 of Wilson and colleagues. 2
The inclusion criteria were such that included studies were ‘all economic evaluations (cost–benefit, cost–utility, cost-effectiveness and cost–consequence analyses) of erythropoietin for anaemia associated with cancer treatment from 1995 to July 2004’ (p. 83). 2 Screening was performed by one reviewer.
Included studies were critically appraised using the checklist suggested by Drummond and colleagues. 113 Single points were assigned to all but one criterion on the Drummond checklist when met; these were summed to give an overall quality score for a study.
Data were abstracted from the studies using a framework used by the West Midlands group in previous technology appraisals. Data abstraction was performed by one reviewer and checked by another.
Qualitative analysis was performed by one reviewer based on manually identified patterns in tabulated data. Conclusions were scrutinised by two other reviewers.
Results
The electronic database searches retrieved 491 citations. No additional citations were identified from industry submissions. Full texts were retrieved for 44 citations (the remainder being excluded as irrelevant on the basis of title and/or abstract). Five studies114–118 were included following full-text screening (the remainder generally being excluded for not considering both costs and benefits). Figure 24 provides the study flow diagram for the systematic review.
FIGURE 24.
Study flow diagram for the systematic review of cost-effectiveness evidence reported by Wilson and colleagues. 2 Adapted from the PRISMA flow diagram.

Three cost–utility studies114–116 included in the systematic review reported by Wilson and colleagues2 are also included in the update review and are hence not reported here.
Of the other two included studies, Ortega and colleagues117 used a willingness-to-pay experiment to determine the societal benefit of epoetin alfa in monetary terms and compare this to the predicted incremental costs of epoetin alfa. The benefit described was avoidance of transfusion and was separately valued by cancer patients and the general population. The benefit of reversing anaemia was not valued. The incremental costs outweighed the benefits in monetary terms and the conclusion was therefore that epoetin alfa was less cost-effective than standard care with RBCT. Sheffield and colleagues118 used a decision tree to model the costs and consequences of epoetin alfa use and concluded that epoetin alfa would be dominated by standard care with RBCT; that is, it would be more expensive and produce worse outcomes. Wilson and colleagues2 highlighted several assumptions made that seemed implausible.
Update review
Objective
The objective of the update review was specified in the appraisal protocol (see www.crd.york.ac.uk/PROSPEROFILES/5812_PROTOCOL_20130824.pdf): this systematic review aims to update the systematic review of cost-effectiveness studies that was conducted in 2004 as part of the review of evidence to inform NICE’s earlier guidance on these drugs (TA142). 1
The review aimed to summarise the main results of past studies and identify any key economic costs and trade-offs relevant to the decision problem. It also aimed to indicate the strengths and weaknesses of different modelling approaches in this treatment area.
Therefore, data were extracted and studies quality assessed only for those economic evaluations or costing studies published since 2004 that are of relevance to the current decision problem.
Methods
Searches
Search strategies were designed by an information specialist (SB) and were based on the searches for clinical effectiveness evidence with additional terms to limit the results to economic evaluations (see Appendix 1). Table 36 provides a summary of the databases searched. When possible, searches were limited to publications since 2004.
| Database | Interface | Date range |
|---|---|---|
| MEDLINE | Ovid | 1946 to May Week 3 2013 |
| MEDLINE In-Process & Other Non-Indexed Citations | Ovid | To 28 May 2013 |
| EMBASE | Ovid | 1980 to Week 21 2013 |
| NHS EED | The Cochrane Library | April 2013, Issue 2 of 4 |
| Web of Science | Thomson Reuters | Searched 29 May 2013 |
| CINAHL | EBSCOhost | Searched 29 May 2013 |
| OHE HEED | The Cochrane Library | Searched 29 May 2013 |
In addition, supplementary searches not limited to cost-effectiveness were conducted in the following databases on 24–30 May 2013 (see Appendix 1):
-
CDSR (via The Cochrane Library): April 2013, Issue 4 of 12
-
DARE and HTA database (via The Cochrane Library): April 2013, Issue 4 of 12
-
HMIC (via Ovid): 1979 to March 2013.
Screening
Inclusion and exclusion criteria were the same as for the clinical effectiveness systematic review (see Chapter 3, Eligibility criteria), with the following exceptions (as specified in the appraisal protocol):
-
non-randomised studies were included (e.g. decision model-based analyses or analyses of patient-level cost and effectiveness data alongside observational studies)
-
full cost-effectiveness analyses, cost–utility analyses, cost–benefit analyses and cost–consequence analyses were included (economic evaluations that reported only average cost-effectiveness ratios were included only if the incremental ratios could be easily calculated from the published data)
-
stand-alone cost analyses based in the UK NHS were also sought and appraised.
For the purpose of this review, ‘administered in accordance with licensed indications’ was taken to mean the frequency of administration but not the dose quantity. Licences allowed for all ESAs to be administered weekly, for darbepoetin alfa to be administered every 3 weeks, for epoetin alfa and epoetin zeta to be administered three times a week and for epoetin beta to be administered three to seven times a week. Fixed dosages and weight-based dosages were allowed; this is a different application of the licence from that in the systematic review of clinical effectiveness evidence (see Changes from the protocol).
Titles and abstracts were screened for relevance by two reviewers (NH and TS), with disagreements resolved by discussion. Full texts were retrieved for references judged to be relevant and these were screened for eligibility by the same reviewers, with disagreements resolved by discussion.
The bibliographies of review articles not judged to be eligible for inclusion were examined by one reviewer (TS) to identify other potentially relevant references. These references were retrieved and checked for eligibility in the same way as full texts from the database searches.
Data extraction
Study characteristics and results were abstracted by one reviewer (TS) using a template adapted from the systematic review by Wilson and colleagues. 2 In addition, parameters that could be used in the construction of an independent economic model were identified and noted.
Selection of studies for detailed appraisal and reporting
Data extraction was conducted for all included studies but, for reasons of expediency, not all studies that were eligible according to the inclusion and exclusion criteria were selected for detailed appraisal and reporting. Instead, only systematic reviews (n = 2) and cost–utility studies (n = 3) were selected for detailed appraisal and reporting. Data extraction for these studies was checked by a second reviewer (HC).
Quality appraisal
Selected studies (all new systematic reviews and cost–utility studies) were quality assessed by one reviewer (TS) using the checklist developed by Evers and colleagues. 119 In line with the instructions accompanying the final checklist, when there was insufficient information available in the article to assess the quality of an item, the item was marked ‘no’. In contrast to the previous review there was no attempt to assign scores to studies on the basis of the quality appraisal checklist.
When studies were based on decision models they were further quality assessed using the checklist developed by Philips and colleagues. 120
Analysis
The results of the included studies were qualitatively analysed on the basis of visual inspection of the tabulated extracted data. Draft conclusions were drawn by one reviewer (TS) and scrutinised by all authors from PenTAG.
Changes from the protocol
For the purpose of the cost-effectiveness review, ‘administered in accordance with licensed indications’ was taken to mean the frequency of administration but not the dose quantity or calculation (i.e. fixed and weight-based doses were accepted). Had the same criteria been used as for the systematic review of clinical effectiveness evidence then several cost–utility analyses would have been excluded:
-
Cremieux and colleagues,115 Fagnoni and colleagues121 and Tonelli and colleagues88 would have been excluded for using fixed doses
-
the Roche and Ortho Biotec submissions would have been excluded as the doses were not reported in Wilson and colleagues2
-
the de novo analysis in Wilson and colleagues2 would have been excluded as doses were not reported.
Given the importance of the above studies to the conclusions of this review, it appears reasonable to have not included dose quantity or calculation method in the assessment of study eligibility for the cost-effectiveness review.
At the full-text screening stage only one study was excluded for using an unlicensed dosing schedule; the study by Glaspy and colleagues,122 which was published only as an abstract, used darbepoetin alfa once every two weeks.
Data extraction was conducted for all included studies, but only a subset of studies (systematic reviews and cost–utility studies) was selected for detailed appraisal and reporting. This change was to ensure that efforts were focused on the studies that were most relevant to the appraisal given the significant number of non-quality-adjusted life-year (QALY) outcomes of limited utility to decision-makers attempting to maximise the total health benefit across health-care spending. This resulted in the exclusion of 12 studies in abstract form only (characteristics and results of these studies are provided in Appendix 14) and six studies in full paper form (for characteristics and results of these studies see the following section). 9,123–127 Also, the monograph by Wilson and colleagues2 and TA1421 were not considered as part of the update review.
Results
Figure 25 shows the study flow diagram for this update review. The electronic database searches for cost-effectiveness evidence identified 1131 records and the supplementary searches identified 32 records. After deduplication 843 records remained, all of which were screened by title and abstract. Of these, 47 were identified for full-text screening and 43 full texts were retrieved and assessed for eligibility. The bibliographies of six reviews128–133 (which were excluded as they were not deemed to be systematic) were examined by one reviewer (TS) and a further seven records were identified for full-text screening, of which six were retrieved. A total of five records could not be retrieved.
FIGURE 25.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of update review.

One study by Roungrong and colleagues,134 a cost–utility analysis of epoetin alfa for cancer patients with anaemia in Thailand, could not be obtained. The Centre for Reviews and Dissemination produced a critical appraisal of the study for NHS EED,135 which revealed that the study was generally well conducted except for the limited reporting of clinical data sources and that it concluded that epoetin alfa would not be a cost-effective alternative to standard care with RBCT.
Three studies published in 1997/1998 also could not be obtained, one of which was by Sheffield and colleagues118 and was included in the previous systematic review by Wilson and colleagues. 2 Of the other two studies, one appears to be a conference abstract of a cost–utility study136 and the other appears to be a full paper but is likely to be a review rather than a primary study. 137
Finally, one conference abstract by Malonne and colleagues138 could not be obtained, although the title suggests that this study may have evaluated only costs.
Of the full texts assessed for eligibility, 291,2,123,127,139–158 were deemed to meet the eligibility criteria. The reasons for exclusion after full-text screening are detailed in Appendix 15. Five texts123,139–142 were deemed to be multiple publications, including four abstracts and a peer-reviewed journal paper by Klarenbach and colleagues142 deemed to be a multiple publication of the Canadian Agency for Drugs and Technologies in Health technology assessment report by Tonelli and colleagues88 (see Appendix 16), leaving 24 primary studies2,1,124–127,143–158 from which data were abstracted. Twelve primary publications were conference abstracts,143–154 three were or included systematic reviews2,88,155 and two were related to the previous NICE appraisal. 1,2 The results of Wilson and colleagues,2 which informed TA142,1 are discussed in Chapter 1 (see Existing systematic reviews of effectiveness) and, although not appraised as a part of this update review, the results are considered as conclusions are drawn.
Summary tables of study characteristics, key parameters and findings for the abstracts are provided in Appendix 14. (See Tables 44–46 for summary tables of the study characteristics, key parameters and findings for the full papers.)
Of the eligible studies, four88,121,155,156 were selected for detailed appraisal. These consisted of one stand-alone systematic review by Duh and colleagues155 and three new cost–utility studies,88,121,156 of which one also contained a systematic review. 88
Summaries of the identified systematic reviews
Duh and colleagues155 conducted a systematic review of the medical literature to identify cost and cost-effectiveness studies of epoetin alfa, epoetin beta and darbepoetin alfa. MEDLINE and ‘all other PubMed databases’ were searched from January 2000 to April 2007 for English-language references with human subjects and combinations of the following sets of terms:
-
intervention terms: epoetin, darbepoetin, Procrit®, Aranesp, Epogen®, erythropoietin, erythropoietic agent
-
outcome terms: cost, effectiveness, pharmacoeconomic.
It is notable that the authors did not include studies comparing ESAs with standard care not including ESA therapy.
The authors identified 67 studies in the field of oncology, in addition to 39 in the field of chronic kidney disease (CKD) and 46 in other areas. We report only the aspects of the report relating to oncology. Ten of the 67 studies were selected for review and a further nine studies were identified through conferences (meetings of ASCO, ASH, European Society for Medical Oncology and European Hematology Association in the period 2003–6) or bibliographies to give a total of 19 studies reviewed.
The authors appear to have conducted some limited critical appraisal, although no specific critical appraisal tool appears to have been used. A narrative synthesis was conducted using textual descriptions and tabulation.
All 19 studies identified compared epoetin alfa with darbepoetin alfa, with three studies additionally including epoetin beta as a comparator. No evaluations included standard care without ESA therapy as a comparator.
Various outcome measures were used and in five studies no effectiveness measures were reported. No cost–utility studies (i.e. studies with QALYs as the outcome measure) were identified.
Cost ratios are presented for all but one study and suggest that epoetin alfa is cheaper than darbepoetin alfa in most cases, although the authors acknowledge that many studies do not include costs other than drug acquisition costs.
Cost-effectiveness results were not always presented when effectiveness outcomes were listed as being included; only measures of drug costs were given for nine of the fourteen studies with listed effectiveness outcomes, and in all five studies in which cost-effectiveness results were presented epoetin alfa has a lower average cost-effectiveness ratio than darbepoetin alfa.
The authors made a number of arguments that seemed to be designed to undermine the results from existing cost–utility studies that produced incremental cost-effectiveness ratios (ICERs) above cost–utility thresholds, notably:
-
The studies are outdated and corresponding changes in pricing and practice patterns, as well as emerging clinical effectiveness evidence, should be considered.
-
ESAs approach acceptable cost–utility thresholds only when a survival benefit is assumed. As a survival benefit is not a ‘main outcome’ of ESA therapy and such benefits are uncertain, cost–utility results ‘may be best used to augment evidence from studies that measure costs and effectiveness separately’ (pp. 115–16). 155
The authors also suggested that cumulative changes in Hb levels are more relevant for payers than overall responses at a particular point in time, suggesting that failing to use cumulative measures will underestimate the value of epoetin alfa, which it is claimed achieves a response more rapidly than darbepoetin alfa (the authors cited an earlier publication sharing two authors with the systematic review, including the primary author).
The authors acknowledged that financial support was provided by Ortho Biotec (manufacturers of epoetin alfa), who provided editorial review and approval of the manuscript. There was inconsistent reporting of study results, which may have biased the apparent results in favour of epoetin alfa.
Tonelli and colleagues88 conducted a systematic review of the medical literature and health economic literature to identify economic evaluations of ESAs in adult patients with malignancy and anaemia. MEDLINE, EMBASE, EconLit and NHS EED were searched on 11–21 October 2007 using search strategies listed in an appendix.
Studies were included if they met the following criteria (reproduced verbatim as permitted):88
-
Evaluated the incremental impact of an ESA against a comparator group on relevant costs and health outcomes
-
Included one of the following in the comparator group: placebo, no therapy with ESAs, different ESA or same ESA but varying hemoglobin target, dose or schedule
-
Included (in a cost-minimization analysis) comparisons of different ESAs or comparisons of alternative route or schedule of administration of ESAs to achieve a similar hemoglobin target, only if based on RCT data for effectiveness
-
Examined a cohort of adult patients with malignancy and anemia
Included studies were quality appraised using a checklist adapted from the literature and relevant data (including industry funding) were extracted.
A qualitative synthesis of included studies was planned as a small number of studies was expected.
The combined searches produced 1134 citations, of which 58 were identified for full-text scrutiny. Forty-seven studies were excluded, leaving 11 primary studies included in the systematic review.
Five of the 11 studies were cost–utility analyses:
-
the HTA review by Wilson and colleagues2 was carried out for the previous NICE appraisal
-
the study by Fagnoni and colleagues121 was also identified in this update review
-
the studies by Martin and colleagues,116 Cremieux and colleagues115 and Barosi and colleagues114 were all included in the systematic review reported by Wilson and colleagues. 2
Quality appraisal of these studies demonstrated that none met all of the quality criteria but all met most of the quality criteria.
A narrative review identified that only one study116 reported an attractive incremental cost–utility ratio. Tonelli and colleagues88 noted that this was an industry-sponsored study and that a subgroup of RCT patients with stage IV breast cancer who demonstrated a survival advantage with epoetin use (although this survival advantage did not reach statistical significance) was identified to inform the model; the favourable cost-effectiveness results did not remain when the whole population of the RCT was used instead.
The six non-cost–utility studies were:
-
a discrete choice experiment by Ossa and colleagues159 to ascertain the utility of anaemia-related health states and the willingness to pay for epoetin alfa
-
a model-based cost-effectiveness analysis by Borget and colleagues157 of darbepoetin alfa compared with standard care without ESA use in patients with lung cancer, with an effectiveness measure related to the final Hb level achieved
-
a cost–consequences analysis by Reed and colleagues,160 based on an open-label RCT of epoetin alfa once weekly and darbepoetin alfa every 2 weeks in patients with solid malignancies
-
a study by Casadevall and colleagues161 of epoetin and recombinant human granulocyte colony-stimulating factor and supportive care in patients with myelodysplastic syndrome (this was excluded from this review because of concomitant treatment with G-CSF)
-
the studies by Ortega and colleagues117 and Sheffield and colleagues,118 both identified in the systematic review reported by Wilson and colleagues. 2
Tonelli and colleagues88 noted in their discussion that ESA use leads to large incremental costs that do not tend to be significantly altered across a range of costs for RBCT. They noted that, when health outcomes were converted to a common metric (QALYs for cost–utility analyses, costs for cost–benefit analyses), most of the base-case analyses indicated that ESAs were not a cost-effective use of health resources.
Tonelli and colleagues88 identified that the lack of preference-based utility scores from RCTs was a weakness and that, even with many opportunities for confounding and bias, which could favour ESA use, nevertheless, most studies produced unfavourable estimates of cost-effectiveness.
Characteristics of the new cost–utility studies
In this study the authors retrospectively identified 192 consecutive breast cancer patients receiving either of two standard adjuvant chemotherapy regimens between 1999 and 2004, of whom 91 were treated before the use of epoetin was allowed (1999–2001) and 101 could have received epoetin (2002–4). Patients were excluded if their disease progressed during the 22-week study period or if they failed to complete the chemotherapy course within the study period. A cost–utility analysis was conducted from a health-care perspective by modelling costs and quality of life for patients in the study according to individual patient records.
Per-patient costs were calculated by extracting resource use from individual patient computerised records and applying unit costs (see Table 42). All costs were in euros at 2004 prices. Exact doses administered were recorded and priced. An official tariff was used for the cost of blood transfusions per RBCT unit. Blood transfusions were recorded separately but were also accounted for in hospitalisation costs. Thus, to avoid double counting, RBCT costs that had been collected for each patient were removed from this per diem cost.
Quality of life was modelled as a function of Hb level, according to the Linear Analogue Scale Assessment (LASA) methodology described by Crawford and colleagues. 162 Hb levels were measured at least every 3 weeks (i.e. at least once per chemotherapy cycle). The lowest Hb level measured was taken as the Hb level for each chemotherapy cycle.
Four sensitivity analyses were conducted. In the first, different methodologies were explored for modelling quality of life as a function of Hb level. In the second, unit costs were all scaled up or down by 30%. In the third, subgroups were identified by age or chemotherapy regimen. In the fourth, indirect costs relating to sick leave were included, reducing the population to those initially active and for whom French public health insurance data were available.
In this study the authors constructed an economic model based on the model presented by Wilson and colleagues2 to evaluate the cost–utility (measured in euros or Swedish kronor per QALY) of epoetin alfa compared with RBCT.
Two epoetin alfa strategies were included, in both of which RBCTs were given and epoetin alfa treatment was initiated if the Hb level fell below 10 g/dl. In the first epoetin alfa strategy, called EPOLOW, patients received epoetin alfa until they reached a target Hb level of 12 g/dl (reflecting Swedish treatment guidelines at the time of writing). In the second epoetin alfa strategy, called EPOHIGH, the target Hb level was 13 g/dl (reflecting earlier Swedish treatment guidelines). Patients responding to epoetin alfa were classed as responders and did not discontinue epoetin alfa until the target Hb was reached. Patients not responding were treated with epoetin alfa for two chemotherapy cycles (each 4 weeks) before being discontinued. No dose doubling was included in the base-case analysis.
Three RBCT strategies were included, with trigger Hb levels of 9, 10 and 11 g/dl for transfusion of 2 units of RBCs.
After chemotherapy cessation (six treatment cycles of 4 weeks each), Hb levels normalise to 13 g/dl at a rate of 1 g/dl per 4 weeks.
The effectiveness of epoetin alfa in achieving a Hb response was estimated by calibrating to a study in which doses were doubled if a response was not achieved within 4 weeks, with some adjustment (perhaps arbitrary) to remove the impact of dose doubling.
A health-care perspective was adopted and the following costs were included: drug acquisition, nurse-led hospital oncology clinic (one-off drug administration for epoetin alfa), acquisition of filtered RBCs and RBCT administration. Unit costs for drug acquisition were derived from Pharmaceutical Specialities in Sweden [Farmaceutiska Specialiteter i Sverige (FASS); it is not clear whether these are list prices or acquisition prices]; other unit costs were derived from the price list of the Swedish Southern Health Care Region for 2007.
Utilities were mapped from Hb levels using data from Wilson and colleagues. 2
In this study the authors constructed an economic model to examine the cost–utility of ESA use in adults matching those enrolled in trials of ESAs for the treatment of anaemia related to cancer.
The economic model consisted of two submodels, one representing the 15 weeks during which ESAs are administered in RCTs and another representing the following year, during which the impact of ESAs on long-term survival is assessed.
Inputs for the model were drawn from a systematic review of clinical effectiveness evidence conducted by the authors and included:
-
quality-of-life improvement (calculated using a relationship between Hb levels and HRQoL)
-
Hb level improvement from baseline to the end of the trial period
-
reduction in RBC units transfused
-
short-term mortality (within 15 weeks)
-
long-term mortality (within 1 year).
Although an increase in all AEs was found in the systematic review of clinical effectiveness, this was not included in the base-case analysis because of the heterogeneous nature of these AEs and the lack of data regarding resource utilisation and the costs of these AEs.
A health-care perspective was adopted and costs were included for:
-
ESA acquisition (epoetin alfa in the base case, darbepoetin alfa in a scenario analysis)
-
RBCT (acquisition and administration).
In the base-case analysis, gains in Hb level for patients receiving ESA therapy over patients not receiving ESA therapy were assumed to be instantaneous (acting in favour of ESA cost-effectiveness) but the gains were not assumed to persist beyond the 15 weeks of the RCTs (i.e. instantaneous normalisation, acting against the cost-effectiveness of ESA therapy). In a scenario analysis the gains were assumed to persist for an additional 11 weeks.
Quality of the new cost–utility studies
The quality appraisal checklist developed by Evers and colleagues119 was applied to the three new cost–utility studies (Table 37). None of the studies reported the use of discounting, although, given the short time horizons used, discounting would have been unlikely to materially affect the results. All three studies performed an incremental analysis and included some sensitivity or scenario analyses, but only that by Tonelli and colleagues88 was judged to have included sensitivity analyses of all important variables. No study produced a probabilistic sensitivity analysis (PSA).
| Item | Fagnoni and colleagues113 | Borg and colleagues145 | Tonelli and colleagues114 |
|---|---|---|---|
| 1. Is the study population clearly described? | Yes | Yes | Yes |
| 2. Are competing alternatives clearly described? | Yes | Yes | Yes |
| 3. Is a well-defined research question posed in an answerable form? | Yes | Yes | Yes |
| 4. Is the economic study design appropriate to the stated objective? | Yes | Yes | Yes |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | No | Yes | No |
| 6. Is the actual perspective chosen appropriate?a | Yes | Yes | Yes |
| 7. Are all important and relevant costs for each alternative identified? | No | Yes | No |
| 8. Are all costs measured appropriately in physical units? | Yes | Yes | Yes |
| 9. Are costs valued appropriately? | No | Yes | Yes |
| 10. Are all important and relevant outcomes for each alternative identified? | No | Yes | Yes |
| 11. Are all outcomes measured appropriately? | Yes | Yes | Yes |
| 12. Are outcomes valued appropriately? | Yes | Yes | Yes |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | Yes | Yes | Yes |
| 14. Are all future costs and outcomes discounted appropriately? | No | No | No |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | No | No | Yes |
| 16. Do the conclusions follow from the data reported? | Yes | Yes | Yes |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | No | No | Yes |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | Yes | No | No |
| 19. Are ethical and distributional issues discussed appropriately? | No | No | Yes |
In addition, the quality appraisal checklist developed by Philips and colleagues120 was applied to the two new model-based cost–utility studies (Table 38). The reviewer (TS) believes that the only item for which quality was not indicated that would materially affect the conclusions is that Borg and colleagues156 did not subject many of the key parameters, the values of which were uncertain, to sensitivity analyses.
| Item | Borg and colleagues145 | Tonelli and colleagues114 |
|---|---|---|
| Structure (S) | ||
| S1: Statement of decision problem/objective | No | Yes |
| S2: Statement of scope/perspective | Yes | Yes |
| S3: Rationale for structure | No | No |
| S4: Structural assumptions | No | Yes |
| S5: Strategies/comparators | No | No |
| S6: Model type | Yes | Yes |
| S7: Time horizon | Yesa | No |
| S8: Disease states/pathways | Yes | Yes |
| S9: Cycle length | No | NA |
| Data (D) | ||
| D1: Data identification | No | Yes |
| D2: Pre-model data analysis | (No)b | (Yes)b |
| D2a: Baseline data | Yes | Yes |
| D2b: Treatment effects | No | Yes |
| D2c: Quality of life weights (utilities) | Yes | Yes |
| D3: Data incorporation | Yes | No |
| D4: Assessment of uncertainty | (No)b | (No)b |
| D4a: Methodological | No | Yes |
| D4b: Structural | No | Yes |
| D4c: Heterogeneity | No | Yes |
| D4d: Parameter | No | No |
| Consistency (C) | ||
| C1: Internal consistency | No | Yes |
| C2: External consistency | Yes | Yes |
Key parameters of all cost–utility studies
ESA dosing strategies vary significantly in the literature (Table 39) in terms of:
-
start dose (fixed or weight based)
-
trigger Hb level (i.e. the point below which ESAs should be administered)
-
target Hb level (i.e. the point above which ESAs should be stopped or titrated)
-
dose escalation (sometimes used if patients do not achieve a haematological response within a specified time period)
-
ESA abandonment for persistent non-responders
-
duration of continued ESA use following chemotherapy cessation.
| Study | Start dose | Trigger Hb level (g/dl) | Target Hb level (g/dl) | Dose escalation | ESA abandonment | Duration of continued use |
|---|---|---|---|---|---|---|
| Barosi 1998114 | Epoetin alfa Q3W: 150 IU/kg | 10.7 | None | None | None | NR |
| Cremieux 1999115 | Epoetin alfa Q3W: 10,000 IU | NR | None | None | None | None |
| Martin 2003116 | Epoetin alfa Q3W: 150 IU/kg | 10.5 | None | Dose doubled after 4 weeks (no further details) | NR | 4 weeks (expected) |
| Amgen Inc. model2 | Darbepoetin alfa QW: 2.25 µg/kg | NRa | NRa | NRa | NRa | NRa |
| Ortho Biotec model2 | NRa | NRa | NRa | NRa | NRa | NRa |
| Roche model2 | NRa | NRa | NRa | NRa | NRa | NRa |
| Wilson 20072 | Not clear | 13 | 13b | None | 12 weeks | NR |
| Fagnoni 2006121 | Epoetin alfa QW: 40,000 IU | 11.5 | NR | Dose doubled if no response after 6 weeks | 12 weeks | NR |
| Borg 2008156 | Epoetin alfa Q3W: 150 IU/kg | 10 | 12 | None | 8 weeks | NRc |
| Tonelli 200988 | Epoetin alfa QW: 42,148 IU | Noned | None | None | None | NR |
These aspects of dosing will potentially affect clinical effectiveness (see Chapter 3, Dose, and Appendix 9) and will almost certainly affect cost-effectiveness.
Start doses were generally well reported and were broadly consistent with licensed doses. Trigger Hb levels were not always reported and varied from 10 g/dl in Borg and colleagues156 to 13 g/dl in Wilson and colleagues. 2 The target Hb level was reported in only two studies and was 13 g/dl in Wilson and colleagues2 and 12 g/dl in Borg and colleagues. 156
Dose escalation was included in the analyses by Martin and colleagues116 and Fagnoni and colleagues;121 in both cases the dose was doubled, after 4 and 6 weeks of inadequate response, respectively. Dose escalation may improve clinical effectiveness, but it adds costs, which may lead to an overall worsening of cost-effectiveness (indeed, Borg and colleagues156 found that dose doubling was not cost-effective relative to non-escalated dosing).
Abandonment of ESA therapy was included in the analyses by Wilson and colleagues2 at 12 weeks, Fagnoni and colleagues121 at 12 weeks and Borg and colleagues156 at 8 weeks. Abandoning ESA therapy for non-responders is likely to improve cost-effectiveness; as such, patients are unlikely to benefit from further therapy that would incur significant costs. Earlier abandonment may improve the cost-effectiveness of ESA therapy.
Continuation of ESA therapy following chemotherapy cessation was explicitly reported only in the study by Martin and colleagues,116 in which patients were expected to receive ESA therapy for 4 weeks following chemotherapy cessation, although delays in chemotherapy treatment would reduce the duration of continued use. Continuation of ESA therapy is allowed for in ESA licenses up to 4 weeks, which could hasten the return to normal Hb levels for patients receiving ESAs and increase the QALY benefit estimated to arise in the normalisation period.
The impact of ESA use on utility or HRQoL in all of the cost–utility studies is shown in Table 40.
| Study | Utility/HRQoL estimation method | Utility profile over time |
|---|---|---|
| Barosi 1998114 | Baseline HRQoL from Glaspy and colleagues164 adjusted according to Abels165 (visual analogue scale) | Instantaneous improvement (not explicitly stated) |
| Cremieux 1999115 | HRQoL reported by randomised placebo-controlled trial patients (LASA method)63 | Not clear |
| Martin 2003116 | NAa | NA |
| Amgen Inc. model2 | Hb level (six levels) mapped to utility using unpublished data from Amgen Inc. study (EQ-5D data from Phase III active controlled darbepoetin alfa trial; data collected weekly from around 100 patients over 16 weeks)2 | Gradual improvement2 |
| Ortho Biotec model2 | Hb level (four levels) mapped to utility using unpublished data from Ortho Biotec study (TTO from community values of different levels of fatigue)2 | NRb |
| Roche model2 | Hb level (four levels) mapped to utility using unpublished data from Roche study (TTO study of general population)2 | Hb levels from RCTsc |
| Wilson 20072 | Hb level (seven levels) mapped to utility using unpublished data provided by Ortho Biotec | Gradual improvement for responders |
| Fagnoni 2006121 | Hb level (11 levels ever experienced by patients) mapped to HRQoL (LASA) following Crawford and colleagues155 | Clinical study |
| Borg 2008156 | Hb level (seven levels) mapped to utility following Wilson and colleagues2 | Gradual improvement for responders |
| Tonelli 200988 | Hb increment linearly mapped to utility following Ossa and colleagues159 | Instantaneous improvement |
All cost–utility studies except that by Martin and colleagues116 included an improvement in utility or HRQoL as a result of ESA use. Several studies (those published most recently) estimated utility or HRQoL as a function of Hb level and therefore indirectly estimated the impact of ESA use on utility or HRQoL by estimating the impact of ESA use on Hb levels. Fagnoni and colleagues121 estimated the impact of Hb level on quality of life as measured by LASA. Barosi and colleagues114 and Cremieux and colleagues115 both estimated the impact of ESA use on HRQoL directly.
It was not always clear whether the impact of ESA use on utility/HRQoL was instantaneous. Wilson and colleagues2 and Borg and colleagues156 explicitly modelled the proportion of patients in different Hb levels over time. This approach results in a gradual improvement in utility for patients responding to ESA treatment. A gradual improvement in utility is also seen in the Amgen Inc. model in the previous NICE appraisal. 2 Tonelli and colleagues explicitly stated that the improvement in Hb levels, and hence utility, was assumed to be instantaneous (which acts in favour of ESA use in their analysis). Fagnoni and colleagues121 mapped the Hb levels of patients in a retrospective observational study to HRQoL, hence the improvement in Hb levels was translated exactly into HRQoL improvement.
Normalisation is the process of Hb recovering to normal levels following chemotherapy cessation. This was explicitly modelled in all three submissions in the previous NICE appraisal, as reported by Wilson and colleagues. 2 Wilson and colleagues2 also included normalisation in their base-case analysis, although they assumed a slightly faster reversion to normal Hb levels. Borg and colleagues156 followed the model design of Wilson and colleagues2 and, as a result, used the same rate of recovery (Table 41).
| Study | Time frame for normalisation | Rate of normalisation (g/dl/week) (slower rate favours ESA use) | Normal Hb level (g/dl) (higher level favours ESA use) | Duration of continued ESA use |
|---|---|---|---|---|
| Amgen Inc. model2 | 12 weeks | 0.1 | NRa | NRa |
| Ortho Biotec model2 | Overall time frame 36 months | 0.2 | 13 | NRa |
| Roche model2 | NR in Wilson and colleagues2 | 0.2 | Solid tumours: 13; haematological tumours: 11.9 | NRa |
| Wilson 20072 | NR | 0.25 | 13 | NR |
| Borg 2008145 | 32 weeks | 0.25 | 13 | NR |
Earlier studies did not include normalisation. Fagnoni and colleagues121 produced a cost–utility analysis based on a retrospective observational study in which patients were followed up for up to 7 weeks following chemotherapy cessation. If normalisation did occur it would have been measured and included in the analysis, but there is no mention of normalisation in the text. Tonelli and colleagues88 did not assume normalisation in their base-case analysis, but in a sensitivity analysis they extended the utility benefit of ESA use for 11 weeks after chemotherapy cessation. No explicit rate of normalisation or normal Hb level was defined.
In general, assuming a slower rate of normalisation or a higher ‘normal’ Hb level favours ESA use.
None of the studies explicitly stated whether or for how long ESA treatment was continued beyond chemotherapy cessation, which could impact on the rate of normalisation as well as increase costs.
The drug acquisition costs for ESAs would be expected to have a significant impact on the cost-effectiveness of ESAs given that these costs tend to account for the majority of the total incremental costs. The quality of reporting with regard to drug acquisition costs was variable, notably with Wilson and colleagues2 reporting cost per dose rather than unit costs for epoetin alfa and epoetin beta (Table 42). None of the studies appears to be an outlier with regard to drug acquisition costs, but it is notable that the current NHS list prices appear to be lower than the prices used in the UK studies and that pharmacies may reasonably be expected to obtain some discount on list prices.
| Study | Price year, currency | Epoetin alfa (per 1000 IU) | Epoetin beta (per 1000 IU) | Darbepoetin alfa (per µg) |
|---|---|---|---|---|
| Barosi 1998114 | NR, US dollars | ≈10.00 | – | – |
| Cremieux 1999115 | 1997, US dollars | 9.50 | – | – |
| Martin 2003116 | 2000, UK pounds | 8.38 | – | – |
| Amgen Inc. model2 | NRa | – | 1.68 | |
| Ortho Biotec model2 | NRa | 83.30 per dose (Q3W) | – | – |
| Roche model2 | NRa | 83.80 per dose (Q3W) | – | |
| Wilson 20072 | NR, UK pounds | 83.30 per dose (Q3W) | 83.80 per dose (Q3W) | 1.68 |
| Fagnoni 2006121 | 2004, euros | 8.90 | – | – |
| Borg 2008156 | 2007, eurosb | 10.55 | – | – |
| Tonelli 200988 | 2008, Canadian dollars | 14.40 | – | 2.88 |
| NHS list price166 | 2013, UK pounds | Eprex: 5.53; Binocrit: 5.09 | 7.01 | 1.47 |
Results of all cost–utility studies
Table 43 compares the base-case results across the cost–utility studies identified in this review. More detailed reporting of the results is provided in the following sections.
| Study | Costs | QALYs | Incremental costs | Incremental QALYs | ICER (cost per QALY) |
|---|---|---|---|---|---|
| Barosi 1998114 | Epoetin alfa: US$4568; no ESA: US$206 | +US$4362 | +0.023 | US$190,000 | |
| Cremieux 1999115 | Epoetin alfa: US$7551; no ESA: US$1416 | No base case | +US$6135 | No base case | US$111,000–US$214,000 |
| Martin 2003116 | Epoetin alfa: £10,768; no ESA: £6515 | Epoetin alfa: 1.0375; no ESA: 0.5570 | +£4253 | +0.4805 | £8851 |
| Amgen Inc. model2 (short-term analysis) | Darbepoetin alfa: £3570; no ESA: £1156 | Darbepoetin alfa: 0.0309; no ESA: 0.0146 | +£2594 | +0.0163 | £159,000 |
| Amgen Inc. model2 (long-term analysis) | – | – | – | – | £23,600 |
| Ortho Biotec model2 | – | – | +£4021 | – | £13,000 |
| Roche model2 (solid tumours) | – | – | +£3727 | +0.132 | £28,200 |
| Roche model2 (haematological tumours) | – | – | +£3510 | +0.042 | £83,700 |
| Wilson 20072 | – | – | +£4450 | +0.030 | £150,000 |
| Fagnoni 2006121 | Epoetin alfa: €1649; no ESA: €34 | – | +€1615 | +0.0052 | €311,000 |
| Borg 2008156 | Epoetin alfa: €3750; no ESA: €2881 | Epoetin alfa: 0.5687; no ESA: 0.5334 | +€870 | +0.035 | €24,700 |
| Tonelli 200988 (short-term analysis) | – | – | +CA$8643 | +0.03 | CA$267,000 |
| Tonelli 200988 (long-term analysis) | – | – | +CA$8643 | −0.086 | ESA use dominated by no ESA use |
The combination of improved quality of life and reduced risk from blood-borne diseases transmitted through RBCTs resulted in a gain of 0.023 QALYs (8.4 quality-adjusted life-days) at an additional cost of US$4362, resulting in an ICER of US$190,000 per QALY.
Various sensitivity analyses were considered, including varying the risk of blood-borne infections, extending survival for cancer patients to match the general population life expectancy, adjusting patient age and varying the quality of life improvement from ESA use, of which most did not result in ICERs of < US$100,000 per QALY. If the ESA acquisition cost was reduced by 50% the ICER fell to < US$100,000 per QALY. A scenario analysis was considered in which all patients receiving ESAs had no RBCTs and anaemia was improved in all patients; for this the ICER remained high at US$146,000 per QALY. Using the base-case drug acquisition cost, ESA use was cost-effective (ICER < US$100,000 per QALY) only if used in patients who would be heavily transfused and could avoid at least 4.5 RBC units.
Patients in the epoetin alfa arm accrued total costs of US$7551, whereas those in the standard care arm accrued total costs of US$1416. These costs included indirect costs for patients who needed to attend hospital three times weekly for epoetin alfa administration and for patients requiring transfusion. Opportunity costs accounted for US$723 in the epoetin alfa arm and US$176 in the standard care arm. Reduced transfusion usage in the epoetin alfa arm resulted in cost savings of US$428, but these were more than offset by epoetin alfa costs of US$6563. Drug acquisition was the most expensive resource, accounting for US$4560 in the epoetin alfa arm.
Analysis of the data using cumulative Hb gains yielded a cumulative effectiveness measure of 21.0 for the epoetin arm and 3.2 for the standard care arm, giving an incremental effectiveness of 17.8 (units g/dl/week).
Quality of life was measured at baseline and at the end of the study using the LASA. Epoetin alfa patients gained 8.3 mm (the scale is 100 mm in length), whereas standard care patients lost 1.0 mm; therefore, the incremental effectiveness was 9.3 mm. Two methods were suggested for converting LASA measurements to ‘utilities’: the first assumed that a 9.3-mm gain would correspond to a 0.093 gain in utility; the second assumed that a 9.3-mm gain would correspond to a 0.184 gain in utility (based on the mean measurement of 50.6 mm). Neither of these methods actually produces a preference-based utility estimate. Using transfusion rates and cumulative doses from the RCT that provided the LASA measurements resulted in ICERs of $214,000 per QALY when the utility gain was assumed to be 0.093 and $111,000 per QALY when the utility gain was assumed to be 0.184.
Various sensitivity analyses were performed but in all the ICER was > $100,000 per QALY for epoetin alfa use.
In the base-case analysis epoetin alfa use resulted in greater discounted mean costs (£10,768 vs. £6515; difference +£4253) and greater discounted mean QALYs (1.0375 vs. 0.5570; difference + 0.4805). The base-case ICER was £8851 per QALY.
The increased costs for epoetin alfa patients were a result of the epoetin alfa costs (£3995) and increased costs in the follow-up phase (because of greater time spent in the follow-up phase). Increased costs were partially compensated for by decreased costs in the active, supportive and terminal phases and a very small reduction in blood unit costs.
The difference in QALYs came about solely through improved survival; that is, there is no QALY gain from relieving the symptoms of anaemia. Patients receiving epoetin alfa accrued 0.5079 more QALYs in the follow-up phase, with very small reductions in QALYs in the active, supportive and terminal phases.
A joint sensitivity analysis was conducted by bootstrapping effectiveness and cost estimates from the RCT. This analysis demonstrated a 94% probability of cost-effectiveness at the £30,000-per-QALY threshold.
A number of scenario analyses were conducted in which the resulting ICER was < £30,000 per QALY. When all patients from the RCT (rather than only stage IV breast cancer patients) were used to estimate the effectiveness of epoetin alfa the ICER was £39,300 per QALY.
The authors state that ‘The population studied in both groups had no difference in terms of clinical and therapeutic characteristics when one takes into account the evolution of the diagnostic diagrams and recommended treatment strategies between the two studied periods (1999–2001 and 2002–2004)’ (p. 1032). 121 The initial Hb and haematocrit levels were very similar for both patient groups. The median number of Hb measurements per patient was the same (n = 6) for both groups.
In the possible use of treatment with epoetin alfa group, 46/101 (45.5%) participants actually received epoetin alfa. The mean Hb level at initiation of epoetin alfa treatment was 11.3 g/dl (range 9.4–12.5 g/dl). No RBCTs occurred in either group and a similar proportion of patients was hospitalised because of anaemia in both groups (2.0% in the possible use of treatment with epoetin alfa group vs. 2.2% in the no epoetin alfa group). On average, patients in the possible use of treatment with epoetin alfa group spent almost 6 weeks with a Hb level > 13.49 g/dl, whereas those in the no epoetin alfa group spent just over 3 weeks with a Hb level > 13.49 g/dl. Mapping Hb levels to quality of life resulted in an increase of 0.0052 QALYs after the introduction of epoetin alfa over the 22-week study period.
The average cost of epoetin alfa treatment was €1593 per patient. The average cost of hospitalisation was €56 per patient for the possible use of treatment with epoetin alfa group and €34 for the no epoetin alfa group, although this was not statistically significant. The base-case ICER was €311,000 per QALY.
None of the sensitivity analyses reduced the ICER for possible epoetin alfa treatment compared with no epoetin alfa treatment to < €160,000 per QALY. The different methodologies for estimating quality of life according to Hb level produced some differences in the QALY difference between the groups: using the relationship between Hb level and FACT-G resulted in the greatest QALY difference (0.0099 QALYs), whereas an alternative LASA methodology resulted in the smallest QALY difference (0.0046 QALYs). It should be noted that none of these HRQoL measures is preference based.
The base-case comparison was between the epoetin alfa arm with a target Hb level of 12 g/dl and the RBCT arm with a trigger level of 10 g/dl (the same trigger level as in the epoetin alfa arm). Patients in the epoetin alfa arm were estimated to incur total costs of €3750, whereas those in the RBCT arm were expected to incur total costs of €2881 (difference +€870). The additional cost of epoetin alfa (€2054) was partially compensated for by savings in RBCT costs (€1185). Patients were expected to accrue 0.5687 QALYs in the epoetin alfa arm and 0.5334 QALYs in the RBCT arm (difference +0.0353 QALYs). The base-case ICER was €24,700 per QALY.
A scenario analysis was conducted in which the rate of normalisation was doubled from 1 g/dl per 4-week model cycle to 2 g/dl per cycle; the resulting ICER (epoetin alfa vs. RBCT) was €29,500 per QALY.
Another scenario analysis was conducted, in which patients not responding to epoetin alfa after 4 weeks had their dose doubled; the resulting ICER (epoetin alfa double dose vs. standard epoetin alfa dose) was €136,900 per QALY.
An epoetin alfa strategy with a higher target Hb level of 13 g/dl was more expensive than the base-case epoetin alfa strategy (+€609) but generated very little benefit (+0.0018 QALYs), resulting in an ICER of €336,500 per QALY.
Red blood cell transfusion strategies with trigger levels of 9 g/dl and 11 g/dl were also considered. An increased trigger level led to increased costs and QALYs. With a trigger level of 9 g/dl, RBCT cost €2360 and resulted in 0.4948 QALYs. With a trigger level of 11 g/dl, RBCT cost €3340 and resulted in 0.5605 QALYs. All strategies were on the cost-effectiveness frontier (i.e. no strategies were dominated or extendedly dominated); therefore, if RBCT with a trigger level of 11 g/dl was to be considered a valid comparator, the ICER for epoetin alfa would be €50,000 per QALY.
In the base-case analysis epoetin alfa use resulted in increased costs (CA$8643) and increased benefits (0.03 QALYs) over 15 weeks, resulting in an ICER of CA$267,000 per QALY. Over a 1-year time frame costs were unchanged, but increased long-term mortality resulted in decreased benefits (−0.086 QALYs); epoetin alfa use was dominated by standard care as a result. Similar results were obtained with darbepoetin alfa.
Several univariate sensitivity analyses and scenario analyses were conducted. When the mortality parameters were varied within their 95% CIs, ESA use remained not cost-effective, even at a threshold of CA$100,000 per QALY. When alternative methods of estimating the relationship between Hb levels and utility were used, ESA use became less cost-effective. A number of other scenario analyses were conducted, the most favourable of which involved limiting the studies informing the model to those with a target Hb level of ≤ 12 g/dl and/or an initial Hb level of ≤ 10 g/dl, but even in these the ICERs remained above CA$70,000 per QALY.
Summary tables for the other full non-selected studies
The study characteristics, key parameters and results for the other full non-selected studies are summarised in Tables 44–46 respectively.
| Characteristic | Ben-Hamadi and colleagues148 | Persson and colleagues125 | Borget and colleagues157 | Spaepen and colleagues126 | Aapro and colleagues158 | Pashos and colleagues127 |
|---|---|---|---|---|---|---|
| Evaluation type | Cost-effectiveness analysis | Cost–consequences analysis | Cost-effectiveness analysis | Cost–consequences analysis | Cost-minimisation study | Cost–consequences analysis |
| Modelling used | Limited | Yes | Yes | Limited | Minimal | No |
| Nature of modelling | Integration of drug acquisition costs based on dose escalation rate | Calculation of drug costs | Markov model | Statistical matching of patients receiving different ESAs to estimate costs | Multiplication of dosing level by unit price | |
| Perspective | Payer | Health carea | Health care | Health care | Health care | Drug costs only |
| Country (setting) | USA (not explicitly stated) | Sweden | France (not explicitly stated) | Belgium (hospital) | Germany, France, UK, Italy, Spain | USA |
| Intervention/comparator | Epoetin alfa QW: 40,000 IU, escalated to 60,000 IU after 4 weeks if Hb increase < 1 g/dl; darbepoetin alfa QW: 2.25 µg/kg, escalated to 4.5 µg/kg if Hb increase < 1 g/dl and/or given RBCT | Epoetin alfa TIW: 150 IU/kg;b darbepoetin alfa QW: 2.25 µg/kgb | Darbepoetin alfa QW; standard care: RBCT if Hb < 8 g/dl or 8–10 g/dl and signs of poor tolerance of anaemia | Darbepoetin alfa; epoetin alfa; epoetin betac | Originator epoetin alfa QW: 40,000 IU, 450 IU/kg; biosimilar epoetin alfa QW: 30,000 or 40,000 IU, 450 IU/kg; epoetin beta QW: 30,000 IU, 450 IU/kg; darbepoetin alfa QW: 150 µg, 2.25 µg/kg; darbepoetin alfa Q3W: 500 µg, 6.75 µg/kg | Epoetin alfa QW: 40,000 IU; darbepoetin alfa Q3W: 500 µg |
| Population | Patients with chemotherapy-induced anaemia | Patients with cancer therapy-related anaemia receiving epoetin alfa or darbepoetin alfa | Lung cancer patients | Adult cancer patients receiving ESA support at some point | Patients with chemotherapy-induced anaemia | Adult cancer patients receiving ESA therapy |
| Outcomes considered | Treatment success (proportion of patients not requiring RBCT) | Haematological response, AUCHB, proportion of patients receiving RBCT, number of RBC units transfused | Proportion of patients receiving RBCT, number of RBC units transfused, mean Hb level | TA-free survival (composite of transfusion-free survival and anaemia-related readmission-free survival) | None (costs only) | Proportion of patients requiring packed RBCT, units of packed RBC transfused per patient, increase in Hb level from baseline |
| Time frame | 16 weeks | 16 weeks | 36 weeks | For duration of records until loss of follow-up at end of calendar year | 18 weeks | Duration of ESA treatment up to maximum of 16 weeks |
| Discounting | Not stated | Not stated | Not stated | Not stated | Not discounted | Not stated |
| Funding | Ortho Biotec (manufacturer of epoetin alfa) | Johnson & Johnson (manufacturer of epoetin alfa) | Not stated | Amgen Inc. (manufacturer of darbepoetin alfa) | Sandoz Biopharmaceuticals [manufacturer of biosimilar epoetin alfa (Binocrit)] | Ortho Biotec (manufacturer of epoetin alfa) |
| Parameter | Ben-Hamadi and colleagues148 | Persson and colleagues125 | Borget and colleagues157 | Spaepen and colleagues126 | Aapro and colleagues158 | Pashos and colleagues127 |
|---|---|---|---|---|---|---|
| Effectiveness (source): transfusion, response rate, survival, QALYs | Epoetin alfa: Witzig and colleagues;167 darbepoetin alfa: Kotasek and colleagues168 | Retrospective chart review performed at three Swedish hospitals | 2-year retrospective study | Retrospective analysis of Belgian national patient database | Assumed equivalent | Dosing and Outcomes Study of Erythropoiesis-Stimulating Therapies (DOSE) |
| Effectiveness (data): transfusion, response rate | Treatment success (proportion of patients not requiring RBCT) Weeks 0–16: epoetin alfa 75%; darbepoetin alfa 63% Weeks 5–16: epoetin alfa 85%; darbepoetin alfa 73% |
Results by day 112:a Haematological response (Hb increase ≥ 1 g/dl): epoetin alfa 100%; darbepoetin alfa 80% Haematological response (Hb increase ≥ 2 g/dl): epoetin alfa 86%; darbepoetin alfa 63% AUCHB (Hb g/dl/day, mean ± SD): epoetin alfa 203.0 ± 122.9; darbepoetin alfa 157.0 ± 162.3 Patients receiving one or more RBCT: epoetin alfa 14/29; darbepoetin alfa 14/30 Mean units of RBC transfused: epoetin alfa 1.71; darbepoetin alfa 1.95 |
Proportion of patients receiving RBCT: darbepoetin alfa 19.1%; standard care 33.6% Mean number of RBC units transfused: darbepoetin alfa 2.11 ± 0.47; standard care 2.97 ± 1.47 |
TA-free survival (composite of transfusion-free survival and anaemia-related readmission-free survival) (95% CI): darbepoetin alfa 84.37% (79.22% to 88.35%); epoetin alfa 84.60% (80.72% to 87.75%); epoetin beta 84.94% (80.03% to 88.72%) Transfusion-free survival (95% CI): darbepoetin alfa 84.46% (79.29% to 88.43%); epoetin alfa 84.86% (81.00% to 87.99%); epoetin beta 85.51% (80.70% to 89.19%) Anaemia-related readmission-free survival (95% CI): darbepoetin alfa 89.16% (84.71% to 92.38%); epoetin alfa 88.66% (85.18% to 91.36%); epoetin beta 87.91% (83.31% to 91.29%) |
NA | Proportion of patients requiring RBCT: epoetin alfa 13.9%; darbepoetin alfa 22.5% (p = 0.026) RBC units: epoetin alfa 0.4; darbepoetin alfa 0.7 (p = 0.020) Increase in Hb from baseline (g/dl) at week 12: epoetin alfa 0.6; darbepoetin alfa 0.1 (p = 0.032) |
| Effectiveness (data): survival, QALYs | NA | NA | NA | NA | NA | NA |
| HRQoL/utility (source) | NA | NA | NA | NA | NA | NA |
| HRQoL/utility (data) | NA | NA | NA | NA | NA | NA |
| Costs (source) | Medi-Span® Master Drug Data Base (MDDB), May 2005 | Drug acquisition: list price in 2003 Swedish Pharmacopoeia; hospitalisation and RBCT: official list of regional administrative prices | Transfusion costs from national unit costs; darbepoetin alfa drug costs from drug purchase prices paid by the hospital | Belgian national databases | This study; list price (Germany, France, Italy, Spain); negotiated price (UK) | Wholesale acquisition costs |
| Cost year | 2005 | 2003 | Not stated | 2003–5 (patient specific); 2006 across all patients in sensitivity analysis | 2010 | May 2009 |
| Parameter | Ben-Hamadi and colleagues148 | Persson and colleagues125 | Borget and colleagues157 | Spaepen and colleagues126 | Aapro and colleagues158 | Pashos and colleagues127 |
|---|---|---|---|---|---|---|
| Measure | Cost per 1% successful treatment | Cost | Cost per g/dl Hb | Cost per patient | Relative savings with use of biosimilar epoetin alfaa | Cumulative drug cost per patient |
| Cost year, currency | 2005, US$ | 2003, SEK | Not stated, US$ | 2003–5, € | 2010, € | 2009, US$ |
| Base case | Epoetin alfa dominates Average ICERs: Weeks 0–16: epoetin alfa US$121; darbepoetin alfa US$215 Weeks 5–16: epoetin alfa US$106; darbepoetin alfa US$186 |
Total treatment cost at day 112: epoetin alfa SEK 74,701; darbepoetin alfa SEK 85,285 | Darbepoetin alfa: mean Hb 13.0 ± 0.5 g/dl; mean cost US$1732 ± 897 Standard care: mean Hb 11.9 ± 1.0 g/dl; mean cost US$996 ± US$643 ICER: US$669 per g/dl Hb |
Overall costs: darbepoetin alfa €16,949 ± €1025; epoetin alfa €19,472 ± €901; epoetin beta €19,295 ± €1048 | Fixed dosing using biosimilar epoetin alfa 40,000/30,000 IU per week: Originator epoetin alfa 13.8%/35.4%; epoetin beta 16.4%/37.3%; darbepoetin alfa QW 25.5%/44.2%; darbepoetin alfa Q3W 33.0%/49.7% Weight-based dosing using biosimilar epoetin alfa: Originator epoetin alfa 13.8%; epoetin beta 16.4%; darbepoetin alfa QW 44.2%; darbepoetin alfa Q3W 44.2% |
Epoetin alfa US$4261; darbepoetin alfa US$8643 |
| Probabilistic results | NR | NR | NA | NA | NA | NA |
| Sensitivity analyses | Using average sale price + 6% (as used for reimbursement for Medicare Part B-covered drugs) Epoetin alfa dominates Average ICERs: Weeks 0–16: epoetin alfa US$97; darbepoetin alfa US$159 Weeks 5–16: epoetin alfa US$86; darbepoetin alfa US$137 |
Costs modelled for observed/fixed patient body weights and response rates | Reducing cost of transfusion or baseline prevalence of anaemia led to standard care having a lower average ICER | Applying 2006 prices does not alter the conclusion that darbepoetin alfa is significantly cheaper than epoetin alfa and epoetin beta | Results presented individually for five treatment scenarios; relative savings are unchanged | NA |
Discussion
All cost–utility studies presenting favourable results were funded or produced by industry.
Martin and colleagues116 produced an analysis demonstrating good cost-effectiveness in a subgroup of cancer patients on the basis of a substantial survival advantage in a RCT, but there are numerous problems with this analysis:
-
the stage IV breast cancer subgroup was not identified a priori (nor indeed were any subgroups identified a priori) and was likely selected as the subgroup in which the observed survival benefit was greatest
-
survival was not a primary outcome of the RCT and indeed the RCT was not powered to detect survival differences and survival was added as a supplementary outcome after the trial started;81 this leaves open the possibility of reporting bias of survival results
-
the RCT was neither powered nor stratified for subgroup analyses and there were baseline differences between the epoetin alfa and the placebo arms
The three industry submissions in the previous NICE appraisal2 achieved ICERs of < £30,000 per QALY only by the inclusion of survival benefits that have not generally been reproduced in more recent meta-analyses. Analyses not including survival benefits seem to predict small incremental benefits of ESA therapy in the range of 0.0052–0.035 QALYs.
The only analysis not including a survival benefit and producing a favourable estimate of cost-effectiveness was that by Borg and colleagues,156 which demonstrated a significantly lower incremental cost of ESAs than other analyses, including those funded or produced by industry. The average cumulative dose predicted by the model may be calculated by dividing the total cost of epoetin alfa (€2054) by the cost of epoetin alfa per 4-week cycle (€1329) to estimate an average 1.546 cycles, giving an average cumulative dose of approximately 195,000 IU (based on a 70-kg patient, as chosen by the authors), whereas data from the clinical study informing the model by Persson and colleagues125 suggest a cumulative dose of 460,000 IU. Dose doubling was included in the clinical study, but this would not account for the discrepancy; indeed, the maximum mean dosage for those receiving epoetin alfa was 37,143 IU, compared with a start mean dosage of 31,786 IU. This suggests that the analysis by Borg and colleagues156 assumes that patients discontinue epoetin alfa sooner than expected from the study from which clinical effectiveness estimates were drawn, leading to questions about the internal validity of the study.
None of the studies incorporated any impact of ESA therapy on chemotherapy management.
Conclusions
For ESA therapy to be cost-effective some or all of the following seem to be necessary:
-
a significant survival advantage for patients receiving ESA therapy
-
utility improvements as a result of improvements in Hb level
-
a low cumulative dose of ESA
-
a normalisation period in which the benefits of ESAs persist beyond chemotherapy cessation (and beyond ESA cessation).
A significant survival advantage has not been shown in general either by the recent Cochrane review11 or by the systematic review in Chapter 3.
The primary claimed benefit of ESA therapy is improved HRQoL following correction of anaemia, but this has not been demonstrated on general HRQoL measures (such as the EQ-5D) in published and peer-reviewed RCTs. Significant predicted improvements in utility have resulted from the application of the results of Ossa and colleagues,159 but this study has several methodological weaknesses (see Chapter 5, Clinical effectiveness parameters). Tonelli and colleagues88 have noted that, as a result of using utility estimates derived from Ossa and colleagues,159 a 0.15 difference in utility between the ESA and the non-ESA arms was predicted, on a par with the utility associated with a kidney transplant for a patient with end-stage kidney disease on dialysis, which they regarded as a potential overestimation.
Achieving a low cumulative dose of ESAs (without sacrificing significant clinical effectiveness) will likely result from identifying non-responders as early as possible and discontinuing ESA therapy in them; focusing ESA therapy on patients with moderate to severe anaemia, in whom it is likely to impact on quality of life and survival, rather than continuing ESA therapy to achieve Hb levels of > 12 g/dl; and employing dose escalation only if it is shown to be clinically effective. These strategies have largely been included in current licences and guidance notes, but there is not yet RCT evidence of clinical effectiveness when ESAs are used fully within licence.
Some amount of normalisation would logically be expected, but no clinical evidence for this has been presented in the economic analyses, even from observational studies. If normalisation is a significant contributor to the benefit of ESAs in analyses it should be subjected to extensive sensitivity analysis to reflect the significant amount of uncertainty.
Wilson and colleagues2 concluded that AEs relating to ESA therapy or RBCT would be unlikely to impact on cost-effectiveness. The two new model-based cost–utility analyses88,156 did not include AEs and provide no further insight into this. Fagnoni and colleagues121 included anaemia-related hospitalisation costs, but it appears that these costs are valued according to average costs of hospitalisation rather than AE-specific hospitalisation costs. They do not demonstrate a significant difference in costs in this area.
The new cost–utility studies did not demonstrate a significant impact on cost-effectiveness of the cost of RBCT.
All studies appear to include greater drug acquisition costs than would be expected now in the NHS as the list price has come down. As drug acquisition costs are the largest component of the incremental costs in all analyses, any discounts would be expected to impact total incremental costs. However, disaggregated total costs as well as incremental costs would be needed to make an appropriate adjustment, and these have not been reported by Wilson and colleagues. 2 Furthermore, NHS hospitals could be expected to achieve discounts from the list price, further improving cost-effectiveness.
Following this update review there remains some uncertainty about the cost-effectiveness of ESAs given the recent reduction in drug acquisition costs and changes to licences designed to address safety concerns. If no survival benefit is assumed then a maximum QALY gain of 0.030–0.035 seems reasonable based on the results from Wilson and colleagues,2 Borg and colleagues156 and Tonelli and colleagues. 88 This could be an overestimate, as there is a lack of high-quality evidence that ESA therapy improves HRQoL on generic measures such as the EQ-5D.
There is a need for an up-to-date analysis of the cost-effectiveness of ESAs in the NHS to reflect reduced drug acquisition costs, changes to licences and market entry of additional comparators. This analysis will need to explore the significant amount of uncertainty that still remains.
Strengths and limitations
This review included a comprehensive search of the literature and inclusion and exclusion criteria were not unnecessarily restrictive, unlike those of the systematic review by Duh and colleagues,155 which excluded standard care without ESAs as a comparator. The two systematic reviews by Duh and colleagues155 and Tonelli and colleagues88 did not identify cost–utility studies that were not identified in this review. The full text of one cost–utility study by Roungrong and colleagues134 could not be obtained, but the Centre for Reviews and Dissemination135 critical appraisal of this study suggests that it would not change the conclusions of the review.
The methods and results of the included cost–utility studies were described and critically appraised and conclusions were drawn by comparing the methods and results of all cost–utility studies.
Records from database searches published pre 2004 were excluded, although it was not possible to assess whether these had been screened for eligibility in the systematic review presented by Wilson and colleagues. 2
The reviewers (TS and NH) excluded darbepoetin alfa given once every 2 weeks as an allowed intervention as biweekly administration is not allowed within the licence for darbepoetin alfa. This could be viewed as a limitation of the review, but at the full paper screening stage this resulted in the exclusion of only a single abstract not describing a cost–utility analysis.
No critical appraisal or narrative synthesis of non-cost–utility studies was performed, which could also be viewed as a limitation of this review. Cost–utility analyses are preferred for NICE appraisals, therefore this is not a significant limitation within the NICE appraisal context, but the value of this review to other audiences may have been limited, although cost–utility analyses are also preferred by many other decision-makers.
The analyses identified in this review are outdated in some ways because of changes in ESA costs and licences and the market entry of new ESAs, but this is a drawback of the published literature rather than the review methods.
Areas of uncertainty
It is not clear what incremental costs could be expected by the introduction of ESAs at current list prices or wholesale acquisition prices. The cost of drug administration is also uncertain and dependent on whether patients are assumed to self-administer drugs. The cost of RBCT in the NHS has not been recently evaluated by the studies identified, but there is evidence that cost-effectiveness may not be particularly sensitive to the cost of RBCT (although this is from studies in which drug acquisition costs dominate to a greater extent than would now be expected). Studies did not include the costs of blood tests or outpatient clinics and so it is not clear how these might impact on cost-effectiveness. Cumulative doses of ESAs, when given within licence, are also uncertain.
The benefits from ESAs are highly uncertain. If ESAs impact on survival then this will have a significant effect on cost-effectiveness, even though ESAs are not given to enhance survival. A systematic review and meta-analysis was conducted as a part of this appraisal and several others exist that do not rule out an impact on survival. If ESA therapy does not result in a meaningful improvement in quality of life then this will also have a significant impact on cost-effectiveness. There is an absence of high-quality evidence in this area. Benefits from normalisation are also highly uncertain and have a significant impact on cost-effectiveness.
Overall, the clinical effectiveness of ESAs measured in QALYs is highly uncertain, as are the costs of ESAs.
Update searches
Update searches were conducted on 2 December 2013 using the same methodology as described earlier. Fifty-one records were screened by two reviewers (TS and LC) and one record was selected for full-text retrieval. The study was judged to be eligible on full-text appraisal by TS and NH. The study was neither a cost–utility study nor a systematic review and its results do not alter the conclusions of this review (see Appendix 17 for further details).
Economic evaluations submitted by the manufacturers
No economic evaluations were submitted by any of the manufacturers.
Key points
-
Ten cost–utility analyses and two systematic reviews were identified by updating an existing review by Wilson and colleagues. 2
-
Five cost–utility analyses suggested that ESA therapy is cost-effective; these were all funded by industry116,156 or conducted by industry (submissions by Amgen Inc., Roche and Ortho Biotec as reported by Wilson and colleagues2).
-
The inclusion of survival benefits was common to four favourable analyses (Martin and colleagues116 and the industry submissions as reported by Wilson and colleagues2), although no statistically significant survival benefit has been shown.
-
The fifth favourable analysis156 may suffer from problems of internal validity as it appears that the cumulative dose of epoetin alfa in the analysis was less than half that in the clinical study informing the effectiveness estimates; this would account for the lower than usual incremental drug acquisition costs.
-
A key assumption in almost all of the analyses was that raising Hb levels would improve HRQoL, although in no case was this assumption based on published RCT evidence using a preference-based quality-of-life measure.
-
A number of studies assumed a period following treatment during which Hb levels would gradually return to normal (termed ‘normalisation’), during which patients in the ESA arm would continue to accrue incremental benefits in terms of quality of life over patients in the no ESA arm; no evidence for or against normalisation has been presented.
-
In the absence of survival benefits the expected health gain from ESA therapy is small (up to 0.035 QALYs) and is subject to uncertainty.
-
Studies did not incorporate current list prices or wholesale acquisition costs, which could significantly reduce the drug acquisition cost component of ESA therapy and improve cost-effectiveness.
Chapter 5 Independent economic assessment
Methods
Model structure
In the PenTAG assessment, the economic evaluation takes the form of a simple, empirical model, informed directly by the systematic review of clinical effectiveness. This differs from standard mechanistic modelling approaches (such as Markov or discrete event simulation models), which require specific states and processes to be modelled.
The model compares patients receiving ESA therapy with patients not receiving ESA therapy (referred to as the ESA arm and the control arm) and is split into two temporal sections, one to evaluate the short-term costs and QALYs (while patients are anaemic) and one to evaluate long-term QALYs.
Short-term costs are accrued in the form of ESA drug acquisition and administration costs, RBCT costs and costs of AEs. Although patients may incur significant costs through cancer treatment (e.g. chemotherapeutic agents), these costs are not modelled as they are assumed to be equal for the ESA arm and the control arm (the potential ramifications of this assumption are discussed in Chapter 6, Chemotherapy costs). Short-term QALYs are accrued as HRQoL is improved by ESA therapy correcting anaemia and associated symptoms (e.g. fatigue); no difference in time spent in the short-term phase is modelled between the arms.
Long-term QALYs are accrued because of potential differences in OS between the two arms; it is assumed that HRQoL is equal for both arms in this phase as patients no longer have CIA and HRQoL is driven by symptoms of cancer. Although patients may incur significant ongoing costs related to cancer treatment (e.g. costs of maintenance chemotherapy, subsequent chemotherapy cycles or relapse), because these are highly uncertain (because of the wide range of cancers that patients may have and the treatments for them) and because the inclusion of such costs could perversely worsen cost-effectiveness for the arm with greater OS, these costs are not modelled in the base case. The potential ramifications of this assumption are explored through a univariate sensitivity analysis in Univariate sensitivity analysis, Long-term costs, and are discussed in Chapter 6 (see Chemotherapy costs).
Short-term costs and quality-adjusted life-years
Short-term costs in the model include ESA drug acquisition and administration costs, RBCT-related costs and costs relating to AEs. In all cases resource use and unit costs are estimated separately. Resource use for ESA drug acquisition and administration is estimated in ESA withdrawal rate and mean weekly dose and Duration of ESA treatment. Resource use for RBCT is estimated in Number of red blood cell transfusions. Resource use for AEs is estimated in Adverse event costs. Unit costs are estimated in Costs.
We have considered three possible model structures for the estimation of short-term QALYs (Table 47):
-
Using reported HRQoL outcomes directly from RCTs of ESAs – Hb levels are not modelled. Ideally, this would be the preferred model structure. However, this option is not available because:
-
Although many RCTs report outcomes measured by disease-specific health questionnaires, such as FACT-An, FACT-F and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30), no RCTs report generic preference-based HRQoL measures such as the EQ-5D or Short Form questionnaire-6 Dimensions (SF-6D), which are required to estimate health utilities. Indeed, this limitation has been noted by Grant and colleagues. 89
-
Very little information can be gained from mapping from the disease-specific health questionnaires to the EQ-5D (see Estimation of the impact of erythropoiesis-stimulating agents on health utilities from mapping disease-specific questionnaires to the European Quality of Life-5 Dimensions).
Despite this, some previous cost-effectiveness analyses (e.g. Cremieux and colleagues115) have taken this approach, using quality of life based on visual analogue scales (VASs) or LASA methodology, which is not recommended as health state values elicited using these scales are not based on stated trade-offs between quantity and quality of life by surveyed individuals. 169
-
A variant of this method is seen in Fagnoni and colleagues,121 in which Hb levels over time were taken directly from a clinical trial and then mapped to utility, although this was not according to generic HRQoL measures such as the EQ-5D.
-
-
Mechanistic modelling of the exact Hb level over time during ESA treatment. It is necessary to model many processes, including:
-
doses of ESAs at all times, which are driven by Hb levels, and Hb responses to ESAs
-
times when RBCTs are given and Hb responses to these
-
starting Hb levels.
One of the motivations for modelling Hb levels over time is that these are widely reported in the ESA RCTs and it is possible to estimate health utilities as a function of Hb level.
This option has the attraction of flexibility to depart from the characteristics of the RCTs. However, we have not chosen this option because (1) data for many of the required parameters are simply not available and (2) it is not possible to incorporate many of the outcomes from the systematic review of clinical effectiveness (see Table 49).
-
-
Empirical observation of Hb over time.
Here, Hb levels over time are taken directly from clinical trials. This approach attempts to bolt on an economic evaluation to the RCTs of ESAs. This option has been chosen because (1) good estimates of all of the necessary parameters are available and (2) the method can use many of the outcomes from the systematic review of clinical effectiveness (see Chapter 3).
| Criteria assessed | Model structures | ||
|---|---|---|---|
| Quality of life from trial | Mechanistic modelling of Hb over time | Empirical observation of Hb over time | |
| Complexity | Simplest | Complex, more parameters required | Intermediate |
| Flexibility to depart from characteristics of the RCTs, e.g. patient age, initial Hb level, subsequent Hb level, ESA doses | Less flexibility | More flexibility, e.g. to mirror difference in clinical practice compared with RCTs, changes to licences | Less flexibility |
| Data availability | Preference-based HRQoL data not available from RCTs | Quality data for many parameters not available, e.g. impact on Hb of increase in ESA dose | Yes, taken from the PenTAG systematic review of RCTs |
| Ability to use outcomes from multiple RCTs (PenTAG systematic review of RCTs) | Yes | Not for some parameters, e.g. incremental change in Hb level. Also, some parameters are a function of the characteristics of RCTs, e.g. OS HR of ESAs | Yes, with exception of HRQoL outcomes |
| Accuracy of utilities during ESA treatment and normalisation | Accurate, but excluding Hb outcomes | Assumes HRQoL impact of ESAs captured through the Hb level. Quality of life as a result of AEs is captured independently | |
| Examples of previous economic evaluations | Barosi and colleagues;114 Cremieux and colleagues115 | Wilson and colleagues;2 Borg and colleagues156 | Tonelli and colleagues;88 Fagnoni and colleagues121 |
A summary model diagram is presented in Figure 26. This diagram demonstrates how Hb levels are modelled according to the baseline Hb level [see Initial (baseline) haemoglobin level], the expected change in Hb level for patients not receiving ESA therapy (see Change in haemoglobin level for patients not receiving erythropoiesis-stimulating agent therapy), the expected final difference in Hb level between arms (see Table 49) and the average difference in Hb levels between arms as a proportion of the final difference (see Mean difference in haemoglobin levels between treatment arms as a proportion of the difference at the end of the trial). The concept of normalisation, which takes place after cancer treatment has ended, is described fully in Normalisation of haemoglobin levels following chemotherapy cessation.
FIGURE 26.
Diagram indicating model assumptions about Hb levels by model stage. Note: Hb levels mapped to utility to calculate short-term QALYs.

It is important to note that we model the average Hb profiles across the patient population rather than modelling individual patients’ Hb profiles. As such, the Hb profile is considerably smoother than that expected for an individual patient.
Long-term quality-adjusted life-years
Long-term QALYs are calculated by estimating OS in each arm and applying a long-term utility that is common to both arms; that is, it is assumed that long-term QALY differences come about only through a difference in survival as a result of ESA therapy, not through any enduring impact on HRQoL. Long-term utility is estimated in Peninsula Technology Assessment Group base-case utilities after erythropoiesis-stimulating agent discontinuation.
The systematic review of clinical effectiveness provided estimates for the HR for survival between the ESA arm and the control arm, but to implement this in the model required an estimate of baseline survival for patients without ESA treatment. As ESAs can be administered to individuals with a range of cancers, a wide range of OS estimates appear in clinical studies.
Review of best practice
Here, we briefly outline key points from Latimer170,171 on best practice, as they apply to this setting (note that this paper principally advises on best practice in the case of patient-level data from a single study rather than summary data from multiple studies):
1. Mean time-to-event should be estimated rather than medians.
2. Parametric models should be used, rather than restricted means approaches, unless data is almost entirely complete.
3. The analyst should demonstrate that a range of parametric models have been considered and compared, in order to make evident that the model choice has not been arbitrary. . . .
4. The fit of alternative models should be assessed systematically. . . .
5. [Proportional hazards] modelling should only be used if the proportional hazards assumption can be clearly justified. . . .
6. Where parametric models are fitted separately to individual treatment arms it is sensible to use the same ‘type’ of model. . . .
7. The duration of treatment effect assumption is important when a PH approach is taken, and in the extrapolated portion of survival curves when individual parametric models are fitted to treatment arms. . . .
8. The process of excluding data points should only be undertaken when it can be clearly demonstrated that certain points are erroneous outliers. . . .
Modelling approach
We examined OS curves from all studies included in the systematic review of clinical effectiveness in which such survival curves were shown for patients receiving and not receiving ESA therapy.
For each survival curve we constructed the corresponding cumulative hazard curve to assess how the hazard function behaved over time. Plots of cumulative hazard over time can be useful in identifying candidate parametric survival functions; for example, if the cumulative hazard curve is a straight line then an exponential distribution may be appropriate and if the cumulative hazard function has a sigmoid shape this suggests the need for a survival function with a non-monotonic hazard.
When OS figures were provided as vector graphics (as was the case for Ray-Coquard and colleagues75 and Moebus and colleagues62) the exact survival curve was extracted using Inkscape [freely available from www.inkscape.org/ (accessed 24 June 2015)] and transformed appropriately using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA). When OS figures were provided as raster graphics the underlying image was extracted using Inkscape and then transformed using the GNU Image Manipulation Program [freely available from www.gimp.org/ (accessed 24 June 2015)] and MathMap [freely available from www.complang.tuwien.ac.at/schani/mathmap/ (accessed 24 June 2015)], as outlined in Appendix 18. This approach meant that no data points were excluded.
We additionally constructed the corresponding Weibull plot (a plot of log cumulative hazard vs. log time) using the same methodology. A straight line on a Weibull plot suggests that a Weibull distribution may be appropriate and parallel straight lines for different arms suggests that a proportional hazards Weibull model can be used.
Visual inspection of the cumulative hazard plots suggested that an exponential survival function would fit both arms in the studies by Vansteenkiste and colleagues73 and Österborg and colleagues79 (Table 48).
| Study | Survival curve | Cumulative hazard curve | Weibull plot |
|---|---|---|---|
| Vansteenkiste 200273 |  |

|

|
| Österborg 200579 | 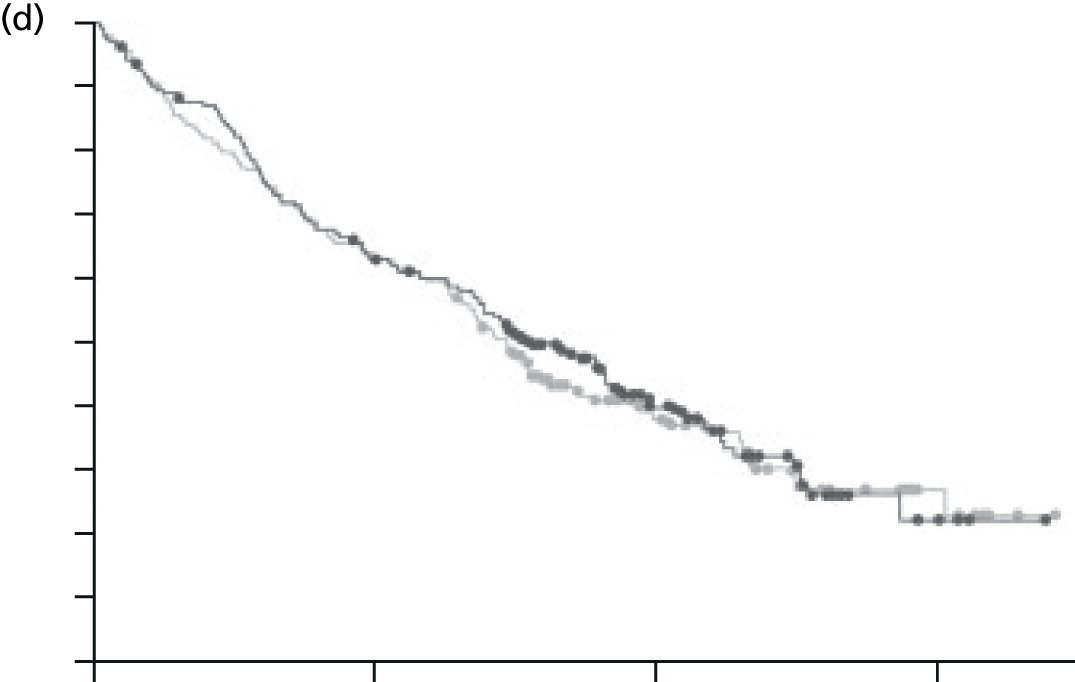 |
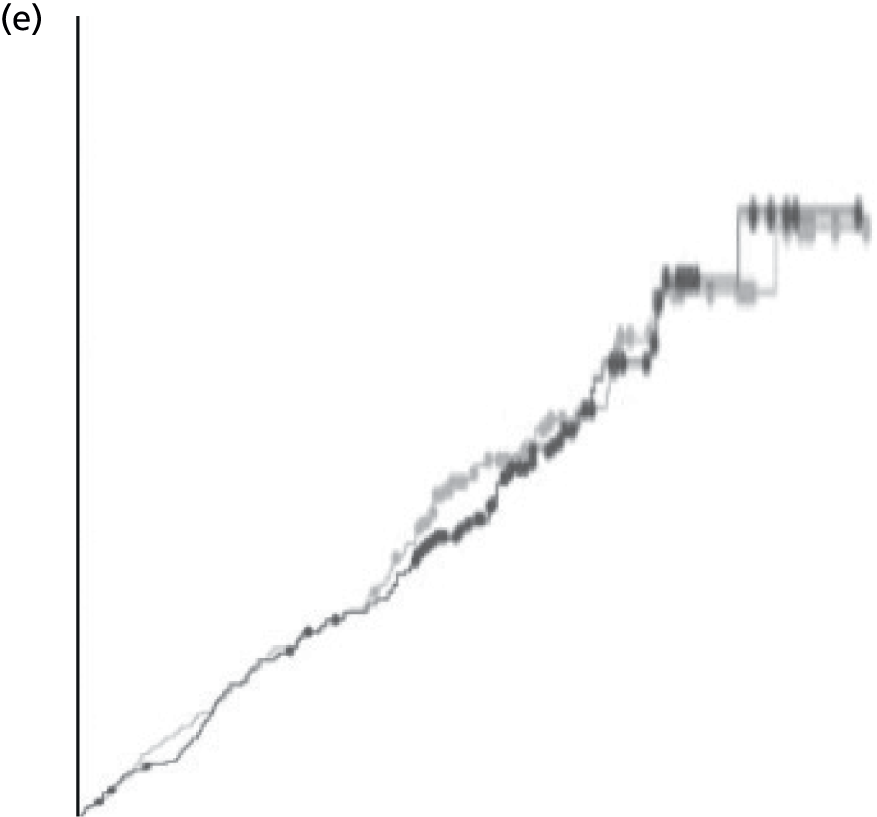
|

|
| Ray-Coquard 200975 |

|

|

|
| Untch 201178,80 |  |
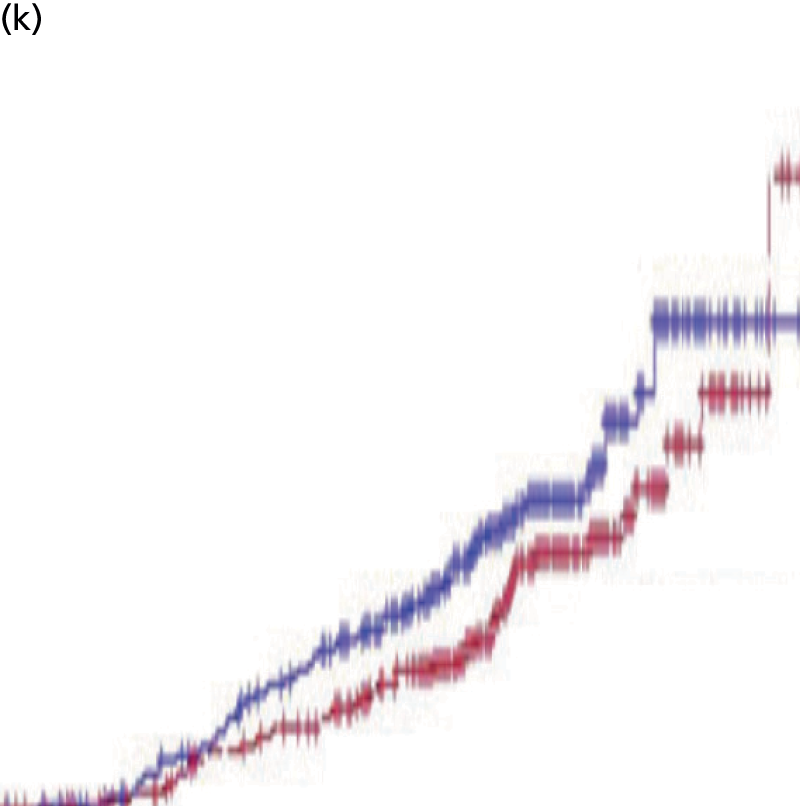
|
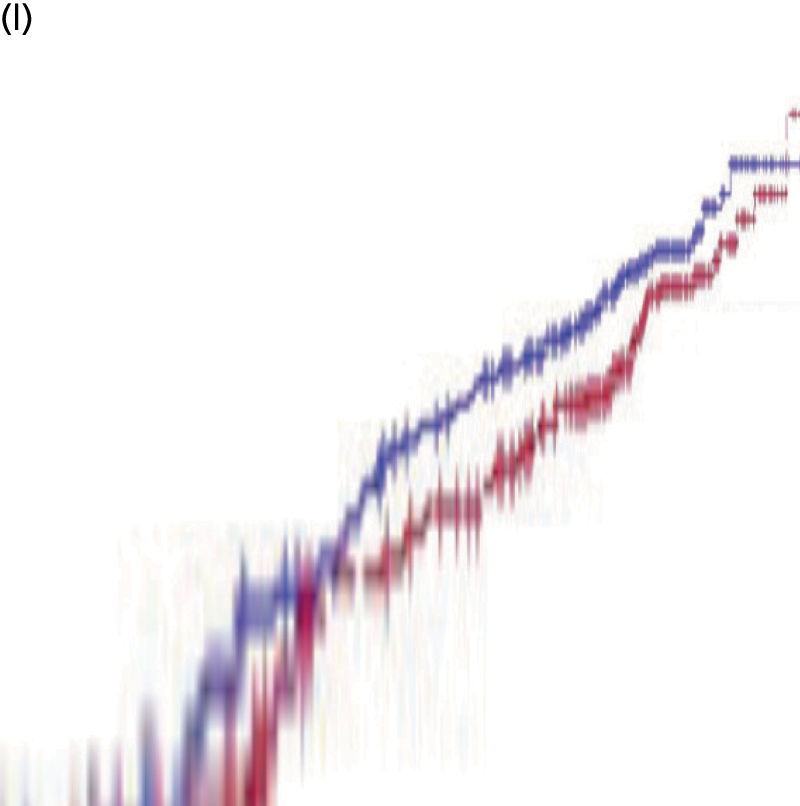
|
| Moebus 201362 |

|
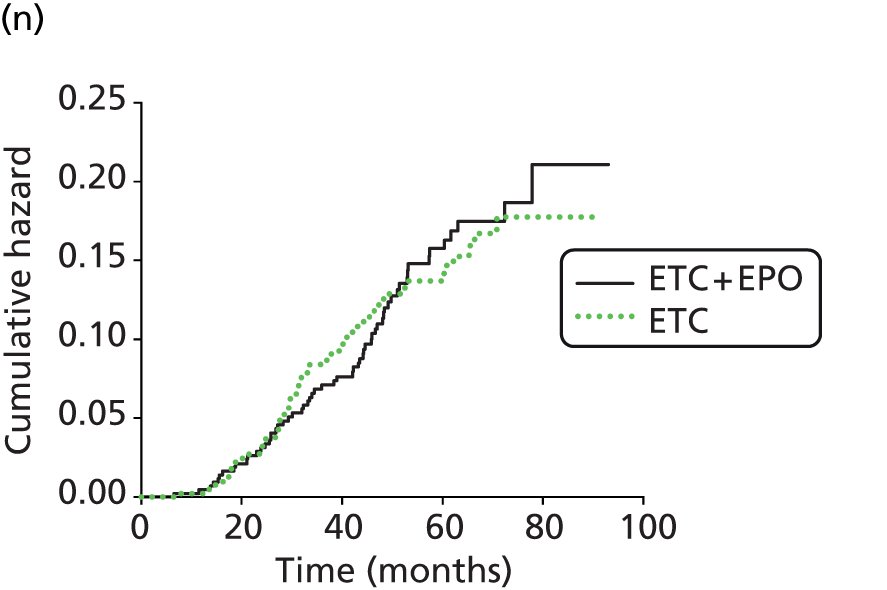
|

|
The plots for Littlewood and colleagues70 suggest that neither a Weibull nor an exponential survival function would fit the arms well. It is also not clear whether a proportional hazards assumption would be valid, as the survival curves converge after significant censoring.
The plots for Grote and colleagues74 suggest that an exponential survival function could be valid, as the cumulative hazard plot diverges from being linear only after significant censoring, although if the Kaplan–Meier curve beyond divergence is considered informative it could suggest a delayed treatment effect on OS.
The survival plot for Ray-Coquard and colleagues75 suggests that OS data are mature in this study (as 0% Kaplan–Meier survival is reached), but in fact the vast majority of patients are censored after around 12 months’ follow-up. Up to this time exponential survival does not seem unreasonable.
The plots for Untch and colleagues80 suggest that exponential survival may not be appropriate (see Table 48). Examination of the Weibull plot suggests that a Weibull survival function may be appropriate. It might also be appropriate to use piecewise exponential survival with a very low hazard rate for the first year and then a higher hazard rate thereafter given that the rightmost upturn in the cumulative hazard plot occurs only after significant censoring. A proportional hazards assumption would not be unreasonable given the Weibull plot.
The plots for Moebus and colleagues62 are noteworthy as they seem to suggest a non-monotonic hazard function, ruling out exponential, Weibull and Gompertz distributions for fitting (see Table 48). This study evaluated performance in breast cancer (stages II–IIIa) patients, who might be expected to have a reasonable prognosis, and hence a long tail (as would be associated with a log-logistic or log-normal distribution) might not be inappropriate as it could be for other cancers.
Given that some included studies supported the use of an exponential survival function and that the exponential survival function is frequently used in the modelling of cancer, we used an exponential survival function in the base case with proportional hazards.
To explore the significant structural uncertainty we also performed three scenario analyses:
-
The survival in the control arm was unchanged from the base case and survival for patients receiving ESA therapy was estimated using proportional hazards for the first 3 years followed by an equal hazard rate to that of the control arm (as though the effect of ESA therapy on mortality lasts for only 3 years). The length of follow-up is not reported for a number of studies contributing to the HR for OS, although it is likely for a number of studies that follow-up was extremely limited. Of the studies providing Kaplan–Meier curves, follow-up was > 3 years only for Untch and colleagues80 (median follow-up 43.5 months) and Moebus and colleagues62 (median follow-up 62 months).
-
A Weibull survival function was fitted to the control arm survival curve from Untch and colleagues80 and a proportional hazards assumption was applied using the same HR as applied in the base case.
-
Two log-normal survival functions were fitted to the two arms in the study by Littlewood and colleagues70 and were extrapolated to a mean life expectancy for 59-year-old members of the general population (weighted average of male and female life expectancy according to the gender balance in the study). Limiting the extrapolation to life expectancy was carried out to approximate the inclusion of background mortality, which was otherwise not modelled and would not have been adequately represented in the Kaplan–Meier curve (which covers only approximately 3 years of follow-up).
We were able to perform a PSA for the first two scenarios (although OS in the control arm was not varied probabilistically in the second scenario), but a PSA was not performed for the third scenario as we had no adequate information to incorporate uncertainty about OS in this instance.
Closed-form expressions for the expected discounted life-years in each arm were available for the exponential distribution and for the first scenario (assuming a rate of continuous discounting of rc):
Closed-form expressions for the expected discounted life-years were not available for the Weibull or log-normal distributions, so these were calculated numerically using trapezoidal integration with a step size of 0.1 years.
Overall survival describes how the OS models were parameterised.
Model parameters
On guidance from NICE, and so that a larger set of clinical study results could be used, clinical effectiveness parameters are not given for individual ESAs but for ESAs as a whole. In other words, in the PenTAG cost-effectiveness modelling there are assumed to be no differences in clinical effectiveness between the alternative ESAs. The only exceptions are for parameters unique to each of the ESAs, such as drug doses and costs.
Appendix 19 provides a summary table which includes all of the model parameters.
Clinical effectiveness parameters
As explained in the previous section, the PenTAG economic evaluation is intended to link directly to the clinical evidence from the RCTs of ESAs. In this section we outline the relevant parameters and their estimates taken from the RCTs.
To ensure consistency between costs and benefits, all parameters were estimated on the basis of ITT. For example, we used the mean weekly dosage of ESAs averaged over all patients at baseline for the full intended treatment duration. This average includes some patients who withdrew from ESA treatment during the trial. This ensures consistency with clinical outcomes, such as the mean difference between treatment arms in the change in Hb level from baseline and the mean difference in the number of units of RBCs transfused between the ESA arm and the control arm, as these quantities are also estimated from all randomised patients.
The ESA withdrawal rate and mean weekly dose are incorporated in the economic model but are often reported only indirectly in trials. The derivations of these parameters (see Table 50) are provided in Erythropoietin-stimulating agent withdrawal rate and mean weekly dose. Similarly, the mean difference in Hb levels between treatment arms over the entire ESA treatment period (as a proportion of the difference at the end of the trial) is another key parameter in the economic model, but one that is often reported only indirectly. The derivation of this parameter is provided in Mean difference in haemoglobin levels between treatment arms as a proportion of the difference at the end of the trial.
The mean weekly dose and frequency of administration can differ between ESAs because of differences in licensing. These differences are discussed in Erythropoietin-stimulating agent withdrawal rate and mean weekly dose and Cost of administering erythropoiesis-stimulating agents, respectively.
Some parameters were taken directly from random-effects meta-analyses in the PenTAG systematic review of clinical evidence (Table 49):
-
OS HR
-
difference in Hb change from baseline
-
difference in number of RBC units transfused
-
relative risk of AEs (thromboembolic events, hypertension and thrombocytopenia).
| Parameter | Pooled mean used in the PenTAG model base case (SE) | Pooled mean used in scenario analysis (SE) | Section |
|---|---|---|---|
| OS (HR) | 0.967 (0.079) | 0.914 (0.137) | See Chapter 3, Overall survival |
| Change in Hb from baseline to end of ESA treatment: difference between ESA and control arms | 1.59 (0.130) | 1.52 (0.115) | See Chapter 3, Haemoglobin change |
| Mean number of RBC units transfused in control arm | 2.09 | 2.30 | Calculated from reported outcomes of the RBC units meta-analysis (see Chapter 3, Number of red blood cell units transfused) |
| Mean difference in number of RBC units between the ESA and control arms | −0.87 (0.21) | −0.99 (0.22) | See Chapter 3, Number of red blood cell units transfused |
| Relative risk of AEs in ESA vs. control arm (reported on natural log scale) | |||
| Thromboembolic events | ln(1.46) = 0.378 (0.158) | ln(1.29) = 0.255 (0.344) | See Chapter 3, Thromboembolic events |
| Hypertension | ln(1.8) = 0.588 (0.234) | ln(1.68) = 0.519 (0.250) | See Chapter 3, Hypertension |
| Thrombocytopenia | ln(0.93) = −0.073 (0.185) | ln(0.73) = −0.315 (0.350) | See Chapter 3, Thrombocytopenia/haemorrhage |
| Probability of AEs in control arm (%) | |||
| Thromboembolic events | 3.3 (0.4) | 3.7 (0.8) | Calculated from reported numbers of AEs (see Chapter 3, Safety) |
| Hypertension | 2.9 (0.5) | 1.8 (1.0) | |
| Thrombocytopenia | 6.4 (0.8) | 2.5 (0.8) | |
Other parameters were calculated from the inputs in those meta-analyses (see Table 49):
-
Hb change from baseline in the control arm
-
number of RBC units transfused in the control arm
-
absolute risk of AEs in the control arm.
Further parameters were not extracted as part of the systematic review of clinical effectiveness evidence and needed to be additionally extracted for the economic analysis:
-
OS in the control arm
-
baseline Hb level
-
mean weekly ESA dose (adjusted for dose escalation, interruption and withdrawal)
-
mean difference between Hb change curves as a proportion of the final difference in Hb change from baseline
-
duration of ESA treatment
-
age
-
weight.
Table 50 provides the estimates for some of these outcomes from clinical studies, which are then pooled as described in later sections. The methods used to incorporate the other parameters are discussed in later sections.
| Study | Mean weekly ESA dosea | Hb | Mean OS | ||
|---|---|---|---|---|---|
| Mean baseline Hb level (g/dl) | Mean increase in Hb level (g/dl) in control arm | Mean difference in Hb levels between treatment arms as a proportion of the difference at the end of the trial (%)b | |||
| Wilson and colleagues:2 included studies meeting inclusion criteria for the PenTAG review | |||||
| Abels 199363 | 307 IU/kgc | NR | NR | NR | NR |
| Aravantinos 200364 | NR | EA 9.80, No tx 9.32 | +1.23 | 23 | NR |
| Boogaerts 200365 | 463 IU/kg | EB 9.0, No tx 9.2 | +0.9d | 68d | NR |
| Dammacco 200166 | 496 IU/kg | EA 9.3, PBO 9.6 | 0.0 | 56 | NR |
| Del Mastro 199767 | 429 IU/kg | EA 13.0, No tx 13.1 | −3.05 | 73 | NR |
| Dunphy 199968 | 467 IU/kg | 14.1 | −2.8 | 77 | NR |
| Hedenus 200253 | 2.20 µg/kg | DA (2.25 µg/kg QWe) 9.4 (SD 1.3), PBO 9.5 (1.0) | +1.00 | 59 | NR |
| Hedenus 200317 | NR | 9.54 | +0.19 | NR | NR |
| Kotasek 200350 | 2.025 µg/kg | DA 9.93,f PBO 9.87 | −0.02 | NR | NR |
| Kurz 199769 | NR | EA 9.88, No tx 9.85 | +0.25 | 50 | NR |
| Littlewood 200170 | NR | 9.8 | +0.5 | 110 | 12-month survival: EA 60%, PBO 49%; median survival (months): EA 17, PBO 11 |
| Österborg 2002,71 200579 | NR | EB 9.2, PBO 9.3 | NR | NR | EB 17.4 months,d PBO 18.0 monthsd |
| Silvestris 199572 | 733 IU/kg | (From figure) Non-transfusion dependent: EA 7.6,d No tx 7.8;d transfusion dependent: EA 7.4,d No tx 7.8d | (From figure) Combining transfusion dependent and non-transfusion dependent: +0.22d | (Combining transfusion dependent and non-transfusion dependent) 84d | NR |
| ten Bokkel Huinink 199851 | 302 IU/kg | EA (150 IU/kg TIW) 12.0,d No tx 11.8d | NR | NR | NR |
| Thatcher 199952 | 335 IU/kg | EA 13.7,d PBO 13.4d | NR | 92 | NR |
| Vansteenkiste 200273 | 161 µgg | 10.11 | NR | NR | DA 46 weeks,d PBO 36 weeksd |
| PenTAG review update 2004 onwards | |||||
| Grote 200574 | Cannot be calculated as intended treatment duration not fixed | EA 12.8, PBO 13.0 | −2.7 | 232h | EA 10.5 months,d PBO 10.4 monthsd |
| Moebus 201362 | 414 IU/kg | EA 12.40,d No tx 12.80d | −2.20 | 77 | 5-year OS: EA 81%, No tx 83% |
| Ray-Coquard 200975 | NR | EA 10.0, No tx 10.0 | NR | NR | EA 7.6 months,d No tx 6.0 monthsd |
| Strauss 200876 | 26,338 IU | EB 11.4, No tx 11.6 | −0.7 | 76 | NR |
| Tjulandin 201048 | ET 23,594 IU, EB 31,251 IU | ESA 9.5, PBO 9.4 | +0.2 | ET 62, EB 60 | NR |
| Tjulandin 201177 | ET 22,235 IU | ET 9.2, PBO 9.1 | +0.65 | 50 | NR |
| Untch 201178,80 | NR | DA 13.64, No tx 13.61 | −0.98 | NR | At median follow-up (43.5 months): DA 88.0%, No tx 91.8% |
We found no evidence from RCTs of normalisation of Hb levels following chemotherapy cessation, therefore this part of the model had to be parameterised on the basis of clinical expert opinion (see Normalisation of haemoglobin levels following chemotherapy cessation).
In the base case we used all 24 studies included in the systematic review of clinical effectiveness evidence (see Chapter 3). There is some heterogeneity in this collection of studies, which may be the result of treatment intention differences; for example, in some studies the intention may be to correct anaemia, whereas in others the intention may be to prevent anaemia.
In an attempt to produce an analysis more consistent with the licensed use of ESAs (for anaemia correction) we performed a scenario analysis in which the subgroup of studies with an inclusion Hb level of ≤ 11.0 g/dl (or lower) was used. Although this subgroup still includes 13 studies, the precision of some effectiveness estimates was reduced (particularly as not all studies include all outcomes) and the subgroup may still include studies in which a higher target Hb level than recommended in the licence was chosen.
If target Hb level is used to identify subgroups, the number of included studies falls significantly; only two studies have an inclusion Hb level ≤ 11.0 g/dl and a target Hb level ≤ 13.0 g/dl. 48,77 One additional study80 had a target Hb level ≤ 13.0 g/dl (but not an inclusion Hb level ≤ 11.0 g/dl). We did not believe these subgroups to be adequate to inform the model because of a lack of precision and possible bias, as Untch and colleagues78,80 did not meet a number of study quality standards.
Two notable clinical outcomes from the RCTs were not used in the economic model: the haematological response rate and the tumour response rate. The haematological response rate is defined as the proportion of patients achieving either an increase in Hb of at least 2 g/dl or a haematocrit increase of at least 6%. We did not use this outcome in the model for two reasons. First, we used more detailed information on the change in Hb level from the RCTs. Second, as far as we are aware, the impact of haematocrit levels on quality of life is unknown. Tumour response rate RCT data were not used in the PenTAG model because the tumour response rate is modelled indirectly by its impact on survival and we did not model the cancer disease pathway.
There is significant uncertainty surrounding a number of clinical effectiveness parameters and it is important that the impact of this uncertainty on the decision problem is demonstrated. We performed a PSA in which model parameters were varied according to probability distributions with expected values equal to the deterministic parameter values. Although it would be best practice for certain parameters to be correlated in the PSA, there were not enough data for such an approach and, as such, all parameters were drawn independently.
It would also be best practice to have the distributions of parameters in the PSA reflect the between-study variance after accounting for the within-study variance; however, the within-study variance was not reported or not extracted for outcomes not included in the systematic review of clinical effectiveness. As a result, for some parameters we used the sample SD of the extracted outcomes from studies as the SE in the model. This is preferable to using the sample SE as this would underestimate uncertainty (as it would not incorporate the within-study variance). The sample SD was also weighted using the same weights as the central estimate.
Number of red blood cell transfusions
The systematic review of clinical effectiveness evidence provides a summary estimate for the difference in number of RBC units transfused per patient between patients receiving and patients not receiving ESAs of −0.87 (95% CI −1.28 to −0.46). The CI corresponds to a SE of 0.21 units. This summary estimate is from a random-effects meta-analysis and we used the same weights to estimate the absolute mean number of RBC units transfused for patients not receiving ESA therapy (2.09 units). As the absolute mean number of RBC units transfused does not affect cost-effectiveness, this was not varied in the PSA.
In the scenario analysis with the subgroup of studies in which the inclusion Hb level was ≤ 11.0 g/dl the difference in number of RBC units transfused was −0.99 (95% CI −1.41 to −0.56) and the absolute mean number of RBC units transfused in the no ESA arm was 2.30 units.
Assuming that the average number of RBC units per transfusion is equal regardless of ESA use, we can calculate the average number of transfusions that occur for each transfused patient. In the base case we used an average number of units per transfusion of 2.7 units. 172 A normal distribution was used for this parameter in the PSA, with the SE equal to 20% of the mean.
Erythropoietin-stimulating agent withdrawal rate and mean weekly dose
Erythropoietin-stimulating agent dosages are adaptive, in many cases being increased when an inadequate initial response is obtained and decreased or interrupted if Hb levels rise too fast or too high. Furthermore, patients may withdraw from ESA therapy for a number of reasons. As most of the clinical effectiveness data informing the model was calculated on an ITT basis (the general exception being AE data), it is important that the amount of ESA drug use is commensurate.
The modelling approach adopted was to combine the withdrawal rate, dose escalation, dose reduction, etc., into a single parameter, the ITT mean weekly dose. This was estimated, when possible, from data published in the studies included in the systematic review of clinical effectiveness. No single method of estimation would work for all studies, so we briefly outline the most common methods employed:
-
if the mean dose actually administered (denoted D) is reported, as well as the mean treatment duration (T) and intended treatment duration (T*), the ITT mean weekly dose is calculated as D × T ÷ T*
-
the mean treatment duration can also be estimated if it is not reported: if the number or proportion of patients remaining on ESA therapy is reported at various time points, these can be interpolated and then the area under the proportion–time curve is approximately equal to the mean treatment duration
-
if the mean cumulative dose per patient is given, this can be divided by the intended treatment duration to calculate the ITT mean weekly dose.
Table 51 lists the clinical effectiveness studies with estimates of ITT mean weekly dose and the corresponding weights of those studies in the random-effects meta-analysis of Hb change. In the base case the weights were taken from the full set of RCTs. In a scenario analysis the weights were used from the subgroup in which the initial Hb level was ≤ 11 g/dl. An average weight of 66.6 kg was assumed to convert from weight-based to fixed doses and produce the estimates in Table 52. As no studies were found with epoetin zeta ITT mean weekly doses, we assumed the same mean weekly dose as for epoetin alfa because of the similarity of their licences.
| Study | ESA | ITT mean weekly dose | Weighta | |
|---|---|---|---|---|
| Base caseb | Scenario analysisc | |||
| Abels 199363 | Epoetin alfa | 307 IU/kgd | 10.72e | 14.61e |
| Boogaerts 200365 | Epoetin beta | 463 IU/kg | 6.69 | 11.14 |
| Dammacco 200166 | Epoetin alfa | 496 IU/kg | 5.71 | 8.11 |
| Del Mastro 199767 | Epoetin alfa | 429 IU/kg | 5.28 | NA |
| Dunphy 199968 | Epoetin alfa | 467 IU/kg | NA | NA |
| Hedenus 200253 | Darbepoetin alfa | 2.20 µg/kg | 4.81 | 6.01 |
| Kotasek 200350 | Darbepoetin alfa | 2.025 µg/kg | 4.32 | 5.07 |
| Silvestris 199572 | Epoetin alfa | 733 IU/kg | NA | NA |
| ten Bokkel Huinink 199851 | Epoetin alfa | 302 IU/kg | 4.77 | NA |
| Thatcher 199952 | Epoetin alfa | 335 IU/kg | NA | NA |
| Vansteenkiste 200273 | Darbepoetin alfa | 161 µgf | NA | NA |
| Moebus 201362 | Epoetin alfa | 414 IU/kg | NA | NA |
| Strauss 200876 | Epoetin beta | 26,338 IU | NA | NA |
| Tjulandin 201048 | Epoetin theta | 23,594 IU | 5.34 | 7.18 |
| Epoetin beta | 31,251 IU | 5.10 | 6.64 | |
| Tjulandin 201177 | Epoetin theta | 22,235 IU | 6.29 | 9.78 |
| ESA | Base case | Scenario analysis |
|---|---|---|
| Epoetin alfa (IU/week) | 24,729 | 24,745 |
| Epoetin beta (IU/week) | 31,021 | 30,840 |
| Epoetin theta (IU/week) | 22,859 | 22,810 |
| Epoetin zeta (IU/week) | 24,729 | 24,745 |
| Darbepoetin alfa (µg/week) | 141.1 | 140.1 |
As there is significant uncertainty in the ITT mean weekly dose, we assumed a gamma distribution with means as shown in Table 52 and SEs equal to 20% of the means.
Duration of erythropoiesis-stimulating agent treatment
As stated in Clinical effectiveness parameters, clinical effectiveness parameters were estimated on an ITT basis. As such, the duration of ESA treatment was taken to be 12 weeks in this analysis, as this is the estimate acquired from the majority (13/23) of the RCTs included in the PenTAG meta-analysis. Some RCTs included longer treatment durations, but 17 of 23 reported a treatment duration of ≤ 18 weeks and all but one study with unambiguous reporting reported a duration of ≤ 24 weeks. In a univariate sensitivity analysis we explored the impact of varying treatment duration up to 24 weeks, which is also the maximum duration included in the study by Wilson and colleagues. 2 It is noted that the duration of ESA treatment affects the short-term QALY gain, as a longer duration of treatment allows time for more QALYs to accrue.
Erythropoietin-stimulating agent drug administration was modelled per protocol rather than on an ITT basis (i.e. withdrawals were not incorporated). In the base case this does give a higher cost of administration for ESAs than we would otherwise expect; however, this increase in the cost of drug administration is small enough that it does not greatly influence the overall costs. This cost is further discussed in Cost of administering erythropoiesis-stimulating agents.
Initial (baseline) haemoglobin level
The initial Hb level of patients has an impact on the Hb level after chemotherapy has finished and therefore has an impact on how long it takes for Hb levels to return to normal. Initial Hb levels are well reported in the included RCTs. Figure 27 shows the range of baseline Hb levels recorded. There is heterogeneity in the initial Hb levels, which is likely to be a result of the different inclusion criteria used.
FIGURE 27.
Initial Hb level by study.
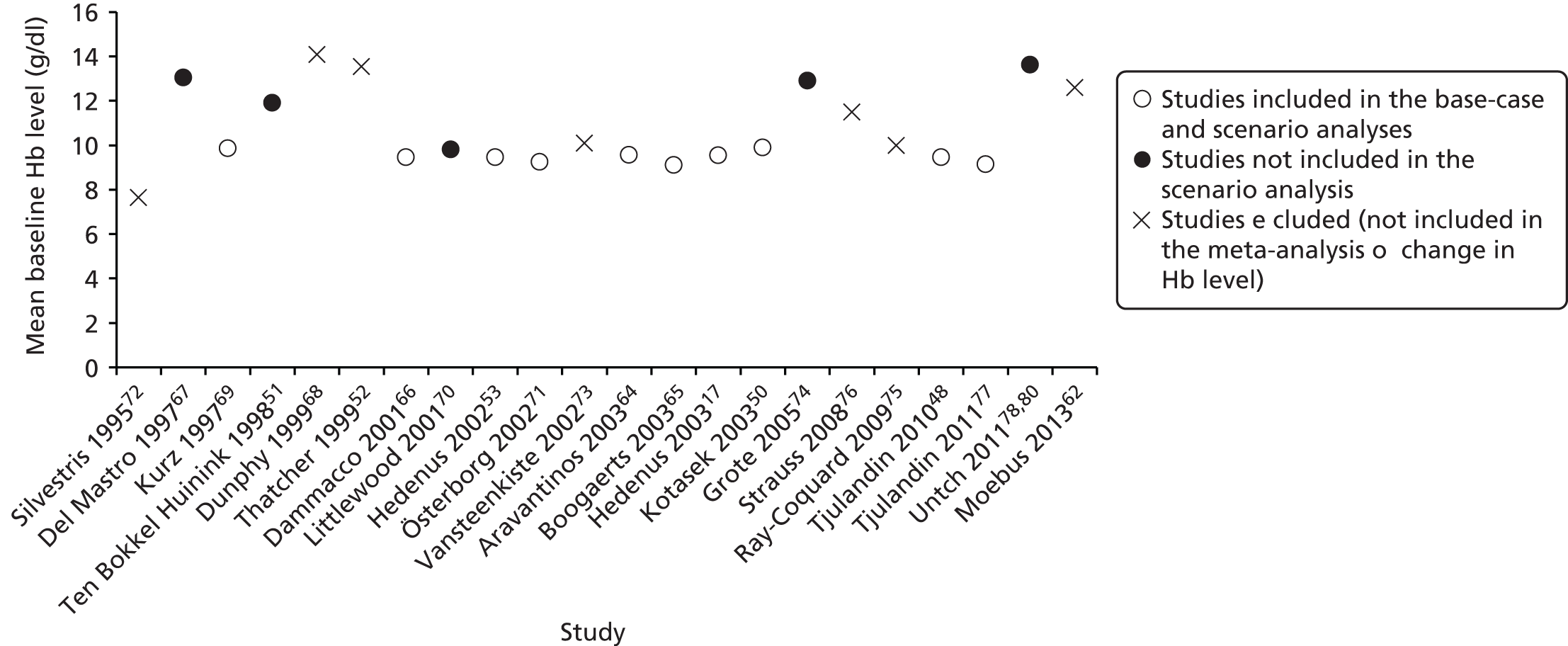
In the base case we calculated a weighted average baseline Hb level with weights taken from the random-effects meta-analysis of mean Hb change. In a scenario analysis the weights from the subgroup with an inclusion Hb level of ≤ 11.0 g/dl were used.
The resulting baseline Hb levels are 10.38 g/dl (base case) and 9.40 g/dl (scenario analysis), as shown in Table 53. The SE in the base case was estimated from the weighted SD of the baseline Hb levels as 1.59 g/dl, with the effect that 95% of simulated values fall in the range 7.28–13.49 g/dl. The SE in the scenario analysis was calculated as 0.22 g/dl, with the effect that 95% of simulated values fall in the range 8.97–9.84 g/dl.
| Study | Baseline Hb level (g/dl) | Weighta | |
|---|---|---|---|
| Base caseb | Scenario analysisc | ||
| Aravantinos 200364 | 9.56 | 4.46 | 5.34 |
| Boogaerts 200365 | 9.1 | 6.69 | 11.14 |
| Dammacco 200166 | 9.45 | 5.71 | 8.11 |
| Del Mastro 199767 | 13.05 | 5.28 | NA |
| Dunphy 199968 | 14.1 | NA | NA |
| Hedenus 200253 | 9.45 | 4.81 | 6.01 |
| Hedenus 200317 | 9.54 | 6.79 | 11.51 |
| Kotasek 200350 | 9.90 | 4.32 | 5.07 |
| Kurz 199769 | 9.865 | 2.81 | 2.78 |
| Littlewood 200170 | 9.8 | 6.57 | NA |
| Österborg 2002,71 200579 | 9.25 | 6.87 | 11.82 |
| Silvestris 199572 | 7.65 | NA | NA |
| ten Bokkel Huinink 199851 | 11.9 | 4.77 | NA |
| Thatcher 199952 | 13.55 | NA | NA |
| Vansteenkiste 200273 | 10.11 | NA | NA |
| Grote 200574 | 12.9 | 6.05 | NA |
| Moebus 201362 | 12.60 | NA | NA |
| Ray-Coquard 200975 | 10.0 | NA | NA |
| Strauss 200876 | 11.5 | NA | NA |
| Tjulandin 201048 | 9.45 | 10.44d | 13.82d |
| Tjulandin 201177 | 9.15 | 6.29 | 9.78 |
| Untch 201178,80 | 13.625 | 7.42 | NA |
| Summary estimate (base case) | 10.38 | 89.28 (100%) | – |
| Summary estimate (scenario) | 9.40 | – | 85.38 (100%) |
Change in haemoglobin level for patients not receiving erythropoiesis-stimulating agent therapy
Haemoglobin levels are expected to vary over time for patients even if they do not receive ESA therapy. This has an important impact on how long Hb levels take to return to normal. It is expected that the Hb trajectories for patients in different studies will vary because of the differing effects of chemotherapy regimens and cancers on Hb levels.
Figure 28 shows the change in Hb level for patients not receiving ESAs in the different RCTs and Table 54 shows how these data are combined to form the parameter values in the base-case analysis and the scenario analysis in the subgroup of studies with an inclusion Hb level of ≤ 11.0 g/dl.
FIGURE 28.
Change in Hb level for patients not receiving ESAs at the end of the study period.
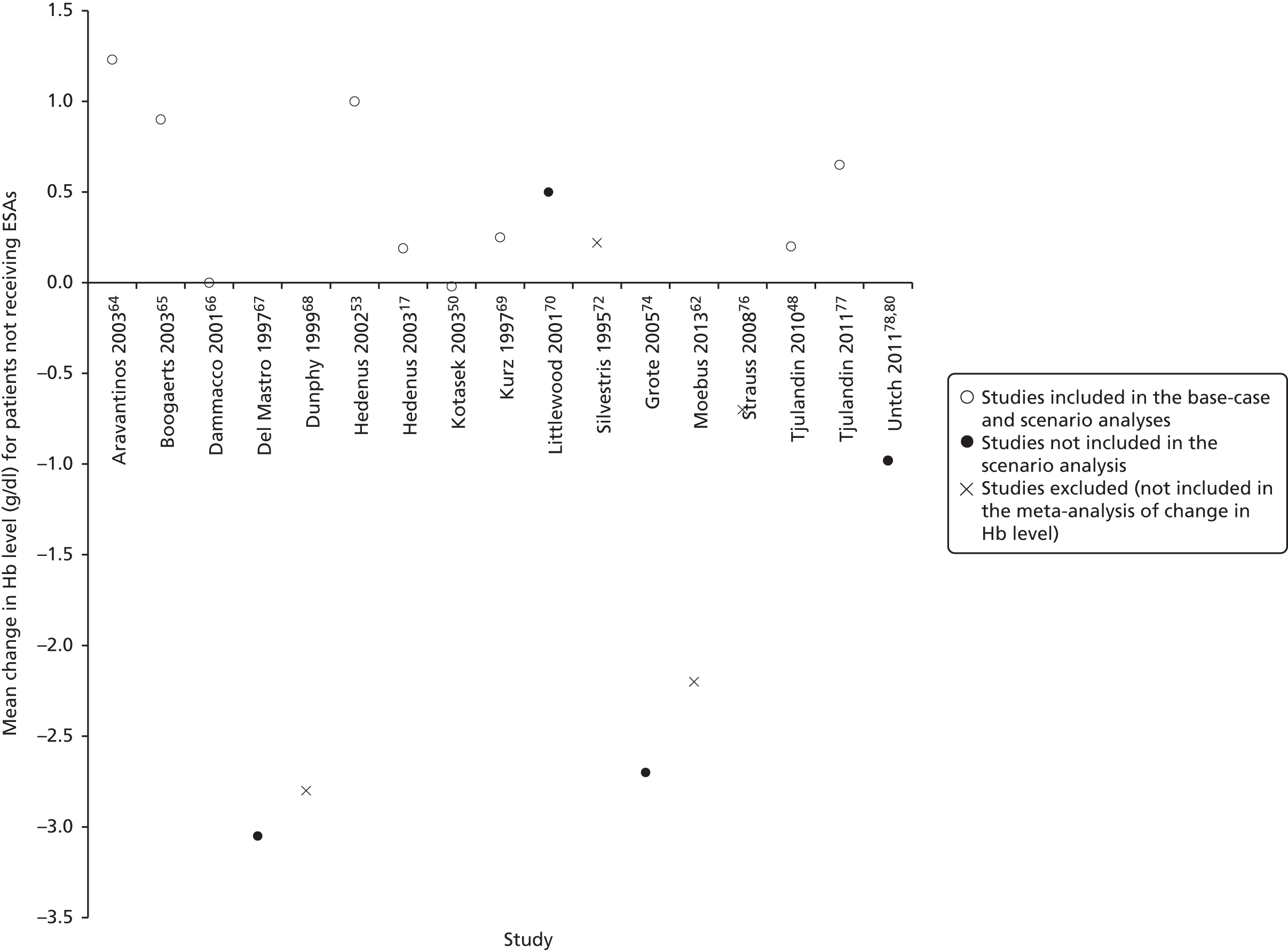
| Study | Change in Hb level (g/dl) | Weighta | |
|---|---|---|---|
| Base caseb | Scenario analysisc | ||
| Aravantinos 200364 | 1.23 | 4.46 | 5.34 |
| Boogaerts 200365 | 0.9 | 6.69 | 11.14 |
| Dammacco 200166 | 0.0 | 5.71 | 8.11 |
| Del Mastro 199767 | –3.05 | 5.28 | NA |
| Dunphy 199968 | –2.8 | NA | NA |
| Hedenus 200253 | 1.00 | 4.81 | 6.01 |
| Hedenus 200317 | 0.19 | 6.79 | 11.51 |
| Kotasek 200350 | –0.02 | 4.32 | 5.07 |
| Kurz 199769 | 0.25 | 2.81 | 2.78 |
| Littlewood 200170 | 0.5 | 6.57 | NA |
| Österborg 2002,71 200579 | NR | 6.87 | 11.82 |
| Silvestris 199572 | 0.22 | NA | NA |
| ten Bokkel Huinink 199851 | NR | 4.77 | NA |
| Thatcher 199952 | NR | NA | NA |
| Vansteenkiste 200273 | NR | NA | NA |
| Grote 200574 | –2.7 | 6.05 | NA |
| Moebus 201362 | –2.20 | NA | NA |
| Ray-Coquard 200975 | NR | NA | NA |
| Strauss 200876 | –0.7 | NA | NA |
| Tjulandin 201048 | 0.2 | 10.44d | 13.82d |
| Tjulandin 201177 | 0.65 | 6.29 | 9.78 |
| Untch 201178,80 | –0.98 | 7.42 | NA |
| Summary estimate (base case) | −0.155 | 77.64 (100%) | |
| Summary estimate (scenario analysis) | 0.469 | 73.56 (100%) | |
The resulting change in Hb level for patients not receiving ESA therapy is −0.155 g/dl in the base case and 0.469 g/dl in the scenario analysis. The weighted sample SD was used to estimate the SE in the base case as 1.25 g/dl, meaning that 95% of the simulated values range from −2.60 to 2.29 g/dl. In the scenario analysis the SE was estimated as 0.41 g/dl, meaning that 95% of the simulated values range from −0.33 to 1.27 g/dl.
Mean difference in haemoglobin levels between treatment arms as a proportion of the difference at the end of the trial
The mean difference in Hb levels between treatment arms over the entire ESA treatment period, as a proportion of the difference at the end of the trial, is another key parameter for the economic model, but one that is often reported only indirectly.
We therefore calculated, for each week, the improvement in Hb level from baseline in each treatment arm and this quantity as a proportion of the improvement from baseline to the end of treatment. We then took an average to give the mean difference over the treatment period (see Appendix 20 for details).
Figure 29 shows the values from included studies. While for most studies the parameter value is under 100%, for two studies the parameter value is over 100% because the final difference in Hb level is less than at earlier times in the trial (i.e. the Hb trajectories of the two arms converge over time). Table 55 shows the derivation of the parameter values used in the model (on the basis of a weighted-average using weights from the random-effects meta-analysis of Hb level change).
FIGURE 29.
Mean difference in Hb levels between treatment arms as a proportion of the difference at the end of the trial.

| Study | Mean difference in Hb levels as proportion of the difference at the end of the trial (%) | Weighta | |
|---|---|---|---|
| Base caseb | Scenario analysisc | ||
| Aravantinos 200364 | 23 | 4.46 | 5.34 |
| Boogaerts 200365 | 68 | 6.69 | 11.14 |
| Dammacco 200166 | 56 | 5.71 | 8.11 |
| Del Mastro 199767 | 73 | 5.28 | NA |
| Dunphy 199968 | 77 | NA | NA |
| Hedenus 200253 | 59 | 4.81 | 6.01 |
| Hedenus 200317 | NR | 6.79 | 11.51 |
| Kotasek 200350 | NR | 4.32 | 5.07 |
| Kurz 199769 | 50 | 2.81 | 2.78 |
| Littlewood 200170 | 110 | 6.57 | NA |
| Österborg 2002,71 200579 | NR | 6.87 | 11.82 |
| Silvestris 199572 | 84 | NA | NA |
| ten Bokkel Huinink 199851 | NR | 4.77 | NA |
| Thatcher 199952 | 92 | NA | NA |
| Vansteenkiste 200273 | NR | NA | NA |
| Grote 200574 | 232 | 6.05 | NA |
| Moebus 201362 | 77 | NA | NA |
| Ray-Coquard 200975 | NR | NA | NA |
| Strauss 200876 | 76 | NA | NA |
| Tjulandin 201048 | ET 62, EB 60; midpoint 61 | 10.44d | 13.82d |
| Tjulandin 201177 | 50 | 6.29 | 9.78 |
| Untch 201178,80 | NR | 7.42 | NA |
| Summary estimate (base case) | 80.6 | 59.11 (100%) | |
| Summary estimate (scenario analysis) | 55.5 | 56.98 (100%) | |
The parameter value in the base case is 80.6% and the value in the scenario analysis is 55.5%. The weighted sample SD was used to estimate the SE, calculated as 55.0% in the base case and 12.0% in the scenario analysis. A gamma distribution was assumed such that in the base case 95% of simulated values fall in the range 10.9–218.6% and in the scenario analysis 95% of simulated values fall in the range 34.4–81.4%.
Normalisation of haemoglobin levels following chemotherapy cessation
It has been assumed in some previous economic evaluations of ESAs2,156 that after chemotherapy cessation Hb levels will return to ‘normal’ (see Chapter 4). Although this is an intuitive assumption that is generally supported by clinical expert opinion, we have not found direct evidence of this process (termed normalisation) in the published literature. Given that approximately half of the QALY gain from ESA therapy could be accrued during normalisation,2 the modelling of normalisation is likely to be very important in determining overall cost-effectiveness.
The PenTAG modelling approach matches that adopted in previous economic evaluations, namely that in the normalisation period Hb levels rise at a constant rate (the same rate for all patients regardless of treatment) until they reach a ‘normal level’. Assuming a slower rate of normalisation results in improved incremental effectiveness of ESA therapy over standard care, as does assuming a higher normal Hb level.
Table 56 provides normalisation parameters from previous economic evaluations and those suggested by clinical experts. A normal Hb level of 12 g/dl appears to be a good compromise with regard to the values suggested (this figure may be lower for haematological cancers, but this is not modelled). This was varied in the PSA with a distribution N(µ,σ2), with µ = 12.0 and σ = 0.51, with the result that 95% of simulated values lie in the range 11.0–13.0 g/dl. It is possible for patients receiving ESA therapy in the model to finish ESA therapy with a higher Hb level than the ‘normal level’, in which case their actual Hb level is assumed to be the normal level on the basis that clinicians would not seek to raise Hb levels above normal levels for a patient. We also assumed that the same utility gradient with respect to Hb level is observed (contrary to some studies that show a levelling off), on the basis that clinicians would raise Hb levels in such patients only to improve HRQoL and therefore utility. If it is actually the case that utility levels off, then this method will overestimate the short-term QALY gain when Hb levels of ≥ 12 g/dl are modelled.
| Source | Rate of normalisation (g/dl/week) | Normal Hb level (g/dl) |
|---|---|---|
| Previous economic evaluations | ||
| Amgen Inc. model2 | 0.1 | ≥ 12 |
| Roche model2 | 0.2 | 13 (solid tumours), 11.9 (haematological tumours) |
| Ortho Biotec model2 | 0.2 | 13 |
| Birmingham model2 | 0.25 | 13 |
| Borg 2008145 | 0.25 | 13 |
| Clinical expert opinion | ||
| Expert 1 (KS) | Normalised within 3 months | |
| Expert 2 (CR) | 0.125 | 11 |
| Expert 3 (MN) | 0.25 | 11 |
| Expert 4 (NR) | Normalised within 6–8 weeks | 12 |
Given that the base-case initial Hb level is 10.38 g/dl and the base-case change in Hb for patients not receiving ESAs is −0.15 g/dl, normalisation is expected to take the Hb level from 10.23 g/dl to 12.00 g/dl, a rise of 1.77 g/dl. One clinical expert suggested that normalisation could be complete within 6–8 weeks; this would suggest a rate of normalisation of 0.22–0.30 g/dl/week, which is consistent with other estimates.
A normalisation rate of 0.2 g/dl/week is broadly consistent with previous evaluations and clinical expert opinion and this was used as the PenTAG base-case value. In PSA this was varied according to N(µ,σ2), with µ = 0.2 and σ = 0.051, with the result that 95% of simulated values lie in the range 0.1–0.3 g/dl/week.
It was assumed on the basis of clinical opinion that normalisation will be complete within 3 month; this was incorporated in the model as a cap on the maximum time to normalisation, with the rate of normalisation effectively being increased when necessary to meet this cap.
Overall survival
To parameterise the base case (exponential survival function with proportional hazards) we calculated what rate parameter (λ) would be necessary to achieve either the reported median survival or the reported Kaplan–Meier survival at a specified point in time in the control arm for each included study. We then calculated a weighted geometric mean of the rates (using the weights from the random-effects meta-analysis of the OS HR) using the formula:
where λi is the estimate of λ from a study and wi is the weight given to that study. The weighted geometric mean was chosen, as the same mean OS is obtained whether the average of λ values or the average of OS is used.
Table 57 provides the calculation of the summary estimates in the base case (all studies included) and in the scenario analysis (including only studies with an inclusion Hb level of ≤ 11.0 g/dl).
| Study | Reported OS | Calculated λ | Weighta | |
|---|---|---|---|---|
| Base caseb | Scenario analysisc | |||
| Littlewood 200170 | KM at 1 year: 49% | 0.713 | 11.32 | NA |
| Vansteenkiste 200273 | Median: 34 weeks | 1.060 | 11.22 | 21.13 |
| Grote 200574 | Median: 10.4 months | 0.800 | 6.05 | NA |
| Österborg 200579 | Median: 18.0 months | 0.462 | 12.40 | 22.46 |
| Ray-Coquard 200975 | Median: 6.0 months | 1.386 | 10.22 | NA |
| Untch 201178,80 | KM at 43.5 weeks: 91.8% | 0.024 | 8.48 | NA |
| Moebus 201362 | KM at 5 years: 83% | 0.037 | 8.69 | NA |
| Summary estimate (base case) | 0.374 | 100% | ||
| Summary estimate (scenario analysis) | 0.691 | 100% | ||
The resulting values for λ correspond to a mean OS in the control arm of 2.670 years in the base case and 1.447 years in the scenario analysis. In the PSA the baseline OS was set to follow a gamma distribution, with a SE of 50% of the mean to capture the high level of uncertainty and the range of cancers from which patients receiving ESA therapy may suffer.
Overall survival for patients in the ESA arm was calculated by applying the HR provided in the clinical effectiveness review to the OS for patients not receiving ESA therapy. In the base case the hazard rate is 0.967, giving a mean undiscounted survival for patients on ESA therapy of 2.762 years. In the scenario analysis the hazard rate is 0.914, resulting in a mean undiscounted survival for patients on ESAs of 1.583 years. In the PSA the HR was distributed as log-normal to match the result of the random-effects meta-analysis (as the HR was meta-analysed following log-transformation). Using a HR possibly derived from Cox proportional hazards and other non-parametric analyses to adjust a parametric survival function could result in a different result from that obtained after derivation of the HR by parametric fitting, but given the limited data we believe that this is the most appropriate approach. We allowed for the alternative survival distributions to examine whether our results were robust to the adopted base-case assumptions.
In the first scenario analysis exploring structural uncertainty in the modelling of OS, the HR for the first 3 years was set to be equal to the HR used in the base case and thereafter a HR of exactly 1 was used.
In the second scenario analysis exploring structural uncertainty in the modelling of OS (in which a Weibull curve was fitted to the control arm of Untch and colleagues80 and a proportional hazards assumption was applied), the HR derived from the systematic review of clinical effectiveness evidence was used, as in the base case. The Weibull curve was fitted to the control arm of the survival plot by extracting several data points and then finding the fit that minimised the sum of squared errors using Solver in Microsoft Excel. The resulting parameters [using the proportional hazards parameterisation: S(t) = exp(–λ × tγ); t in years] were λ = 0.010987 and γ = 1.950282. Figure 30 shows the Weibull function overlaid on the original Kaplan–Meier curve, demonstrating a very good fit.
FIGURE 30.
Weibull distribution fitted to the Kaplan–Meier survival curve from Untch and colleagues. 78,80 Black dashed line = Weibull curve fitted to the control arm. Reproduced from Untch M, Minckwitz G, Konecny GE et al. PREPARE trial: a randomised phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel, and CMF versus a standard-dosed epirubicin-cyclophosphamide followed by paclitaxel with or without darbepoetin alfa in primary breast cancer – outcome on prognosis. Ann Oncol: official journal of the European Society for Medical Oncology/ESMO. 2011; 22, 1999–200680 by permission of Oxford University Press (UK) © European Society for Medical Oncology (ESMO) All rights reserved (URL: http://annonc.oxfordjournals.org/content/22/9/1999.full.pdf+html).
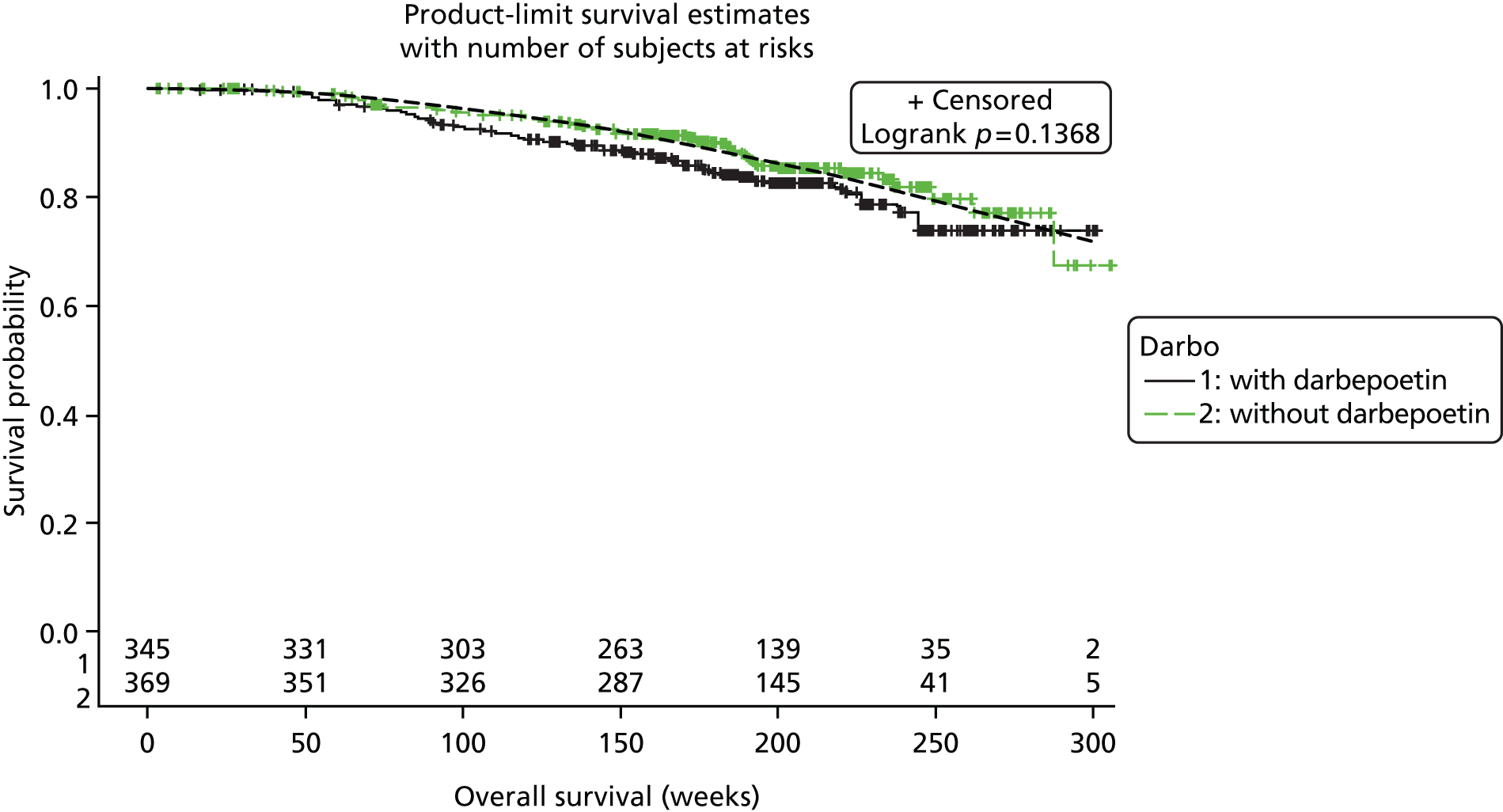
In the third scenario analysis exploring structural uncertainty all parameters were estimated by fitting to the survival curves in Littlewood and colleagues. 70 The HR from the systematic review of clinical effectiveness evidence cannot be applied in this case as a log-normal curve is used, which cannot be used in conjunction with a proportional hazards assumption. The resulting parameters (time measured in months) were µ = 2.501676 in the control arm and 2.826619 in the ESA arm and σ = 1.483129 in the control arm and 1.348525 in the ESA arm. According to interim life tables for England and Wales (2010–12),173 the additional life expectancy for an individual aged 59 years (the approximate mean age of patients in the study by Littlewood and colleagues70) is 23.2 years for men and 26.0 years for women. As 251 of 375 participants were female, we estimated an additional life expectancy of 25.1 years. Log-normal functions overlaid on the original Kaplan–Meier plots appear to demonstrate a reasonable fit. Under 2% of the population in both arms was modelled as still alive at 25.1 years, after which it was assumed that survival is zero.
Figures 31 and 32 show the various OS distributions employed for the control and ESA arms respectively.
FIGURE 31.
Overall survival distributions used for the control arm.

FIGURE 32.
Overall survival distributions used for the ESA arm.

Figures 33–37 show the OS distributions for both arms under each OS modelling assumption.
FIGURE 33.
Overall survival distributions used in the deterministic base case.
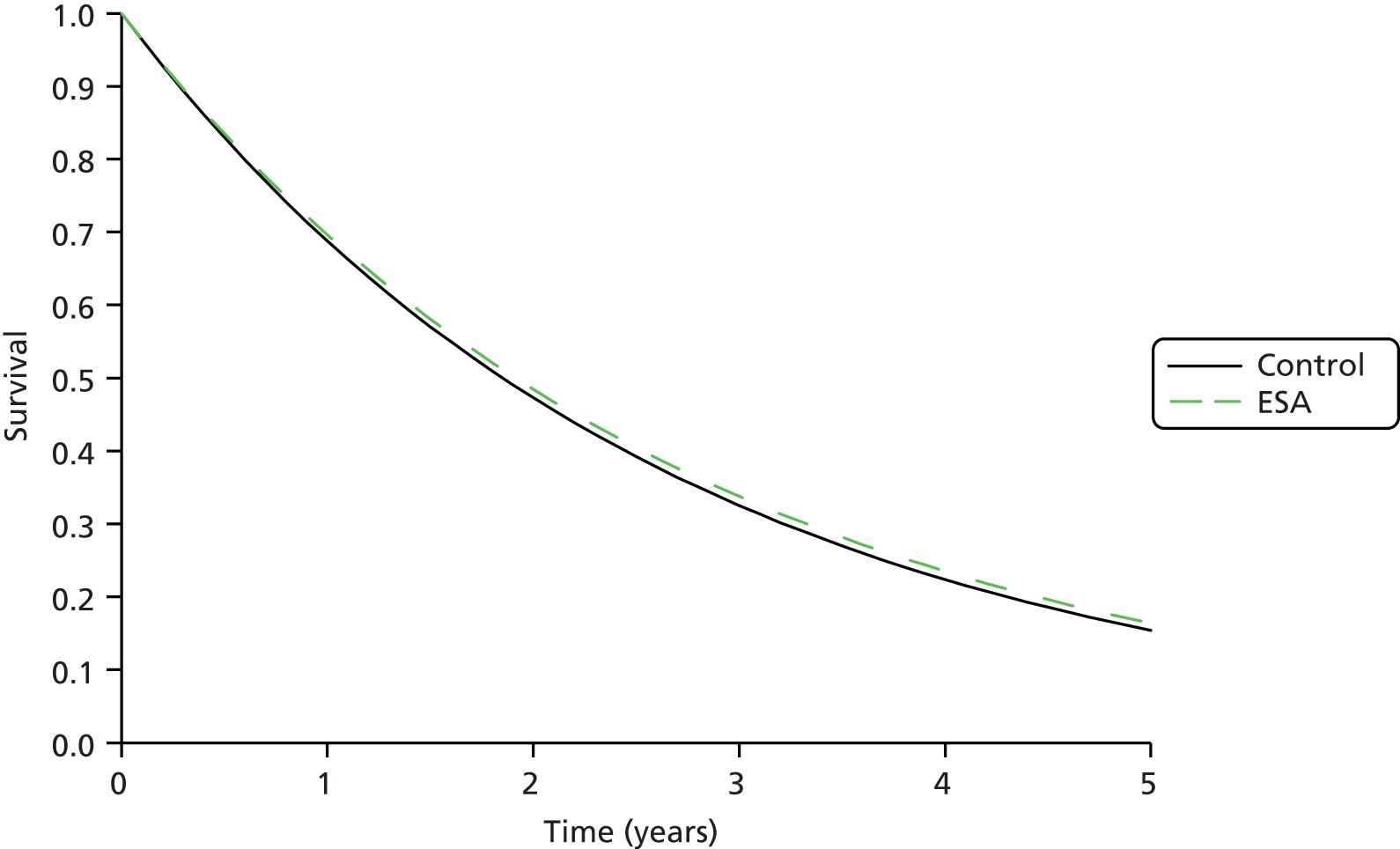
FIGURE 34.
Overall survival distributions used in the subgroup analysis in which the inclusion Hb level is ≤ 11.0 g/dl.

FIGURE 35.
Overall survival distributions used in the first scenario analysis (as in the base case except that the HR applies only for the first 3 years).
FIGURE 37.
Overall survival distributions used in the third scenario analysis (log-normal distributions fitted to the survival curves in Littlewood and colleagues70 and truncated at 25.1 years).

Utilities
As explained in Model structure, the PenTAG model requires two sources of utility values: (1) utility as a function of Hb levels during ESA treatment and during normalisation to reflect the impact of ESAs on HRQoL and (2) a constant utility value after normalisation, equal in all treatment arms.
The cost-effectiveness of ESAs is likely to be very sensitive to both of these, depending on how survival is accounted for in the model. In particular, cost-effectiveness is sensitive to the rate at which utilities change with respect to changes in Hb (i.e. the gradient of the utility/Hb graph) and this appears to be an area that has not been researched in depth for previous cost-effectiveness reviews. It is therefore necessary to research this carefully and in detail.
As explained in Model structure, utility is modelled as a function of Hb level during ESA treatment and during normalization to reflect the impact of ESAs on HRQoL. As such, we implicitly assume that ESAs do not impact on HRQoL in any other way. However, it is possible that ESAs affect some other aspect of health that is not captured by changes in Hb levels.
We used only RCTs to populate these parameters, as only RCTs can support valid causal inferences about the effects of a particular treatment on quality of life. 89 With RCTs, potentially confounding factors such as disease severity, which may affect both direct treatment outcomes and quality of life, should be distributed equally among the trial arms and in order not to bias estimates of the effect of treatment on quality of life. 89
Utilities in cost-effectiveness models of erythropoiesis-stimulating agents
In Model structure we outlined approaches to estimating utilities in published economic evaluations of ESAs for cancer-related anaemia. Here, we elaborate on this (Table 58) to assess the usefulness of approaches to the incorporation of utilities in published economic evaluations.
| Study | During ESA treatment | After ESA treatment | |||
|---|---|---|---|---|---|
| Method of utility estimation | Source data and method of utility estimation | Critique | Source data and method of utility estimation | Critique | |
| Barosi 1998114 | ESAs affect HRQoL directly | VAS from Abels157 | VAS method not recommended by NICEa | Not modelled as short time horizon | |
| Cremieux 1999115 | ESAs affect HRQoL directly | LASA from Abels63 | LASA scale not recommended | Not modelled as short time horizon | |
| Martin 2003116 | ESAs do not affect quality of life | NA | Justification not given | Utilities: 0.13–0.73 depending on stage of breast cancer. Estimated from 30 nurses using standard gamble | Poor methodology and restricted to breast cancer |
| Amgen Inc. model2 | Utility distribution per Hb level | Unpublished study of EQ-5D according to Hb level during Amgen Inc. RCT of darbepoetin. Data collected weekly from approximately 100 patients over 16 weeks | Details unpublished, therefore unable to critique | 0.66 (assumed same as baseline) | Justification not given |
| Ortho Biotec model2 | Function of Hb level | Data from Ossa and colleagues166 on community values of different levels of fatigue (using TTO method), sponsored by Ortho Biotec | Abstract only. Translation of anaemia states to Hb levels unreported | Not reported | |
| Roche model2 | Function of Hb level | Utilities from Ossa and colleagues,166 TTO and regression analysis | Abstract only. Authors include employee of Roche | 0.81 (assumed same as baseline) | Justification not given; mix of utility measurements used to choose baseline (standard gamble, TTO and EQ-5D) |
| Wilson 20072 | Function of Hb level | Unpublished data from Ortho Biotec | Unpublished, therefore unable to critique | Not reported | |
| Fagnoni 2006121 | Function of Hb level | LASA from Crawford and colleagues155 | LASA not recommended as no value set | Not modelled as short time horizon | |
| Borg 2008156 | Function of Hb level | Following model in Wilson and colleagues2 | Based on unpublished utilities study, therefore unable to critique | Not modelled | |
| Tonelli 200988 | Function of Hb level | Ossa and colleagues152 | See critique in Studies reporting utilities as a function of Hb level | Not reported | |
All studies except that by Martin and colleagues116 assume that ESAs affect HRQoL during ESA treatment. Most studies, including the previous HTA review,2 estimate the impact of ESAs on HRQoL through the impact of ESAs on Hb levels.
Only two analyses modelled the impact of ESAs on HRQoL directly rather than through the impact on Hb levels. One of these114 used the VAS and the other115 used the LASA to estimate HRQoL. We believe that both instruments are seriously flawed in terms of assessing utilities as they do not allow trading off life expectancy with quality of life, as required by NICE. 169
Of the seven studies that modelled the impact of ESAs on HRQoL through the impact of ESAs on Hb levels, we consider the approach of Fagnoni and colleagues121 to be inappropriate because it also used the LASA.
Both the Ortho Biotec and Roche models2 use utility data from Ossa and colleagues. 174 This is reported only in abstract form but is reported fully in Ossa and colleagues,159 which we have identified and critiqued in Studies reporting utilities as a function of haemoglobin levels. The industry submissions differ in their partitioning of Hb levels into anaemia states.
The Amgen Inc. submission2 relied on unpublished data and used utility values elicited from patients on both experimental and licensed doses of darbepoetin (patients who discontinued darbepoetin were not followed up).
The data underlying the estimates of utilities as a function of Hb levels from Borg and colleagues156 also relied on unpublished data.
Utilities after ESA treatment are reported in three cost-effectiveness studies, that by Martin and colleagues,116 the Amgen Inc. model2 and the Roche model. 2 We do not consider the corresponding utilities further because the values from Martin and colleagues116 relate to breast cancer only, minimal detail is given for the value used in the Amgen Inc. model2 and both the Amgen Inc. and Roche models2 use the baseline utility to inform the utility after treatment. This means that the utility is not specific to post treatment and instead relies on the assumed baseline utility for this population. Some studies (e.g. Cremieux and colleagues115 and Fagnoni and colleagues121) do not report utilities after ESA treatment because they consider only a short time horizon.
Principles for the identification of studies to inform the choice of utilities
In this section we follow the principles for the identification, review and synthesis of health state utility values from the literature, as recommended by the NICE Decision Support Unit in the UK. 175 There are no agreed reporting standards for studies of utilities, but the following information is key to understanding the nature, quantity and quality of evidence:175
-
the population describing the health state (e.g. age, sex, disease severity)
-
the approach used to describe the health state
-
the utility value elicitation technique, for example time trade-off, standard gamble, visual analogue score
-
sample size
-
respondent selection and recruitment and inclusion and exclusion criteria
-
survey response rates, numbers lost to follow-up (and reasons), methods of handling missing data.
Clearly, the relevance of the data to the decision model and to the agency to which the model will be submitted is important. In the current project, the NICE reference case169 is used. Modification of utility values from the literature for use in economic models, and sensitivity analyses using less relevant utility values, should be considered. 175
A systematic search for studies reporting utilities should be undertaken. 175 For the current project, the search method is given in Appendix 1. In addition, sources of utility values were obtained from published models on the cost-effectiveness of ESAs (see Utilities in cost-effectiveness models of erythropoiesis-stimulating agents).
Studies reporting utilities as a function of haemoglobin level
Our search for studies to inform utility values as a function of Hb levels yielded 235 publications. On inspection of titles and abstracts, four papers were deemed sufficiently relevant to read in full. 176–179
Three papers reported studies that measured HRQoL as a function of Hb level. 176–178 Wisloff and colleagues179 did not provide estimates of utilities as a function of Hb level. Instead, in a study of multiple myeloma patients, the authors concluded that Hb level has limited impact on HRQoL as measured by the cancer-specific questionnaire EORTC QLQ-C30. They stressed that Hb level may be correlated with tumour type, disease severity and response to treatment, which themselves may affect quality of life. The authors therefore concluded that it is essential to adjust for these variables to assess the impact of Hb level on HRQoL.
In addition, we critiqued two further studies, the first of which was that by Ossa and colleagues,159 whose preliminary results174 were used in the cost-effectiveness analysis of two of the TA142 industry submissions and therefore formed the basis of the utility values reported in the Wilson and colleagues2 model. It was also used in the cost-effectiveness analysis of Tonelli and colleagues. 88 In addition, we critiqued the study by Crawford and colleagues,162 used in the cost-effectiveness analysis of Fagnoni and colleagues. 121 The key characteristics and results of all five fully critiqued studies are provided in Table 59. We did not critique the industry submissions from Wilson and colleagues,2 as the data underpinning the Roche and Ortho Biotec submissions are presented in Ossa and colleagues159 and in the methods of the Amgen Inc. submission utility was not explicitly reported as a function of Hb.
| Characteristic | aHarrow and colleagues176 | Tajima and colleagues178 | Ossa and colleagues159 | Lloyd and colleagues177 | Crawford and colleagues162 | Amgen Inc. submission |
|---|---|---|---|---|---|---|
| Health elicitation by patients? | Yes | Yes | No | First study: yes; second study: no | Yes | Yes |
| Preference elicitation instrument | SF-6D | EQ-5D | Health state vignettes reflecting chemotherapy-induced anaemia based on FACT-An and EQ-5D. Validated by three oncology specialists and six cancer-related anaemia patients | Health state vignettes reflecting cancer-related anaemia. Reviewed by clinicians and quality of life experts | LASA | EQ-5D |
| Preference valuation | General public used standard gamble | Japanese general publication used TTO | General public used TTO | First study: general public used standard gamble; second study: cancer patients used TTO | None | NR, presumably TTO |
| Study population size | 13,433 | 537 | 110 | First study: 85 members of the general public; second study: 26 cancer patients | Approx. 4000 | NR |
| Study population | Women with cancer aged 50–79 years, mean age 63 years | CKD patients; 52% male, mean age 55 years, mean Hb 12.7 g/dl | 100 members of the general population | First study: general population; second study: cancer patients receiving chemotherapy, some anaemia, mean age 60 years | Cancer patients undergoing chemotherapy, mean age 63 years | Patients on darbepoetin, some on experimental dosing |
| Country | USA | Japan | UK | UK | USA | NR |
| Year | 1993–8 | 2008 | 2004 | Not stated but assume 2000s | 1990s | NR, pre 2004 |
| Loss to follow-up? | NA as measurement at baseline | NA as measurement at baseline | NA | NA | Appears not to be large | Numbers NR but there was loss to follow-up |
| Study funding | Study funded by US government. Analysis funded by industry (Pfizer) | Funded by Japanese government | Industry (Roche) | Industry (Ortho Biotec) | Industry (Ortho Biotec) | Industry (Amgen Inc.) |
| Results: ΔUtility for ΔHb of 1 g/dl | 0.009 over Hb 9–12 g/dl | 0.016 | 0.109 over Hb 8.7–11.0 g/dl | First study: 0.032; second study: 0.062, over Hb 8.5–11.5 g/dl | 0.029 over Hb 9–11 g/dl | 0.030 over Hb 8.5–11.5 g/dl |
| Major strengths | Sample size very large; health elicited by patients, as required by NICE;89 generic preference elicitation instrument SF-6D | EQ-5D is preferred by NICE;169 public valued using TTO, as preferred by NICE;169 sample size large; health elicited by patients, as required by NICE169 | None | In second study, health elicited by patients with experience of CIA | Sample size very large; health elicited by patients, as required by NICE;169 patients with CIA | Health elicited by patients, as required by NICE;169 patients with CIA; EQ-5D is preferred by NICE169 |
| Minor strengths | Preference valuation standard gamble appropriate (although TTO preferred by NICE89); government funded | Government funded | UK based; TTO preferred by NICE169 | UK based | ||
| Major weaknesses | Observational study, hence possibly unmeasured confounding variables;b however, many covariates were controlled for in the analysis | Patients with CKD, not cancer. Observational study, hence possibly unmeasured confounding variables; however, many covariates were controlled for in the analysis, e.g. albumin, creatinine, GFR, age, gender | Health status not elicited from patients, a requirement for the NICE reference case;169 health state vignettes assessed by experts, whereas NICE prefers patient self-reports using classification systems of generic questionnaires;169 small sample size of 110 | Health state vignettes, whereas NICE prefers patient self-reports using generic questionnaires;169 very small sample size of 26 cancer patients | LASA instrument utilities are not obtained by a choice-based method, which is required by NICE169 | Observational study, hence possibly unmeasured confounding variables;b all utilities taken from patients on the same dose of ESA (utilities not taken when ESA use discontinued); poorly reported |
| Minor weaknesses | Women only; US, not UK based; NICE prefers use of the EQ-5D to the SF-6D;169 patients not necessarily having chemotherapy | Utility values of health states derived elicited from Japanese, not UK, general public | Although health vignettes reported to reflect CIA, descriptions could equally apply to cancer-related anaemia; population under-represents ethnic minorities and over-represents wealthy people;b industry funded | Industry funded | US not UK based; industry funded | Industry funded |
In the study by Harrow and colleagues,176 13,433 women with cancer completed the SF-6D questionnaire at baseline. This represents a useful data set as the sample size was very large, health was appropriately elicited by patients and an appropriate preference elicitation instrument, the SF-6D, was used (see Table 59). However, the main weakness is that this was an observational study, which means that there could have been unmeasured covariates that contributed to the observed relationship between utility values and Hb levels. For example, patients with low Hb levels may have been more likely to have had more advanced cancer. This would tend to bias the apparent impact of Hb level on utilities, probably in the direction of a steeper gradient. However, the authors tried to minimise the risk of confounding by controlling for many covariates in their analysis. Utilities were found to increase only slightly from 9 to 14 g/dl of Hb and decrease thereafter (Figures 38 and 39 and see Table 59).
FIGURE 38.
Utilities as a function of Hb level by study. a, Tajima and colleagues178 report the relationship between change in Hb level and utility (slope of the line) but not absolute utility at each Hb level. We set the utility at Hb 7 g/dl to equal 0.6 so that the utility estimates fall within plausible Hb ranges, as reported by other studies. b, Utilities cannot be directly compared, as they are reported on different scales and elicited through different tools. c, Data presented as part of the HTA review for TA142 published as Wilson and colleagues. 2
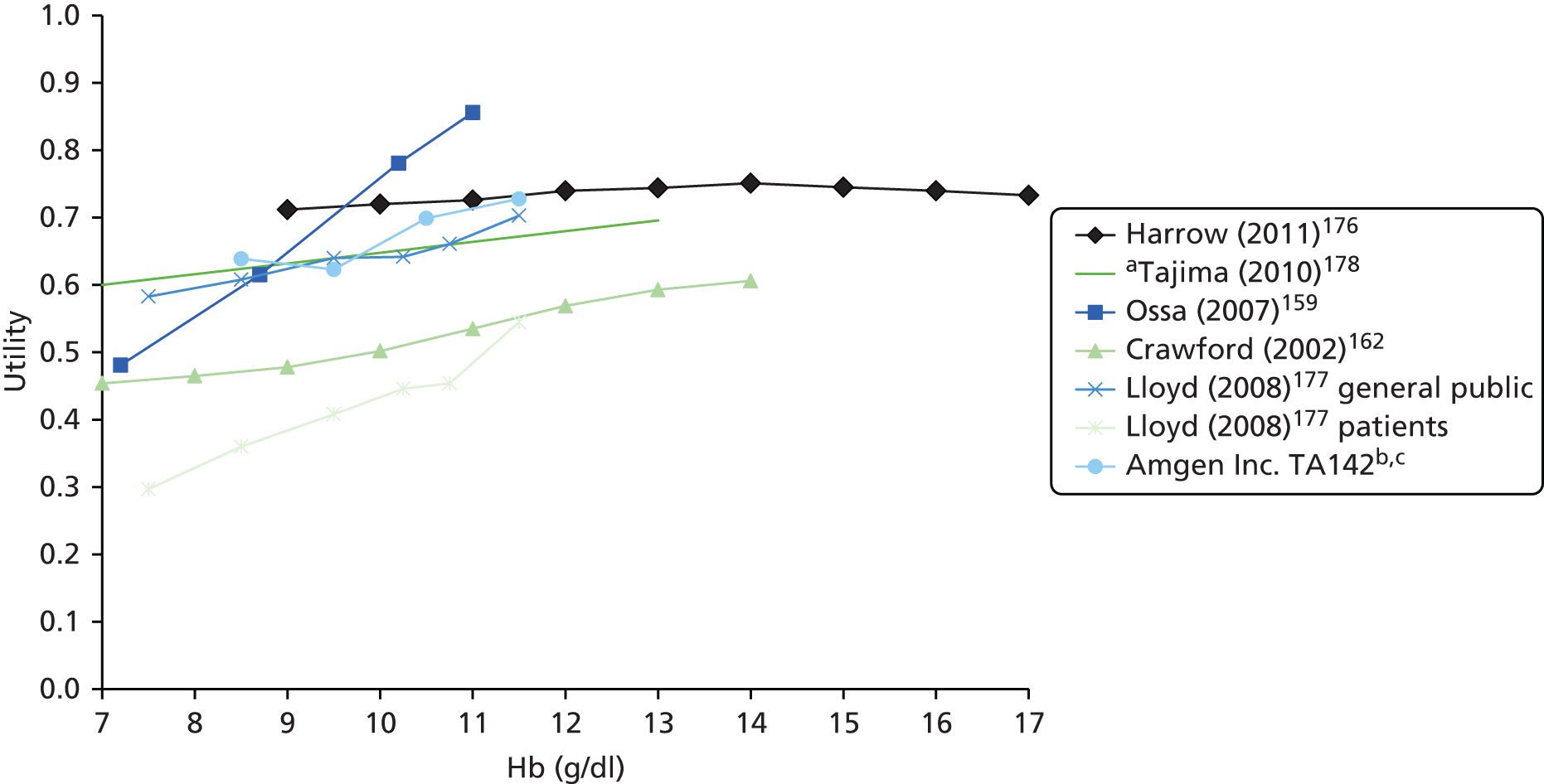
FIGURE 39.
Utilities as a function of Hb level from Harrow and colleagues. Source: Harrow and colleagues. 176
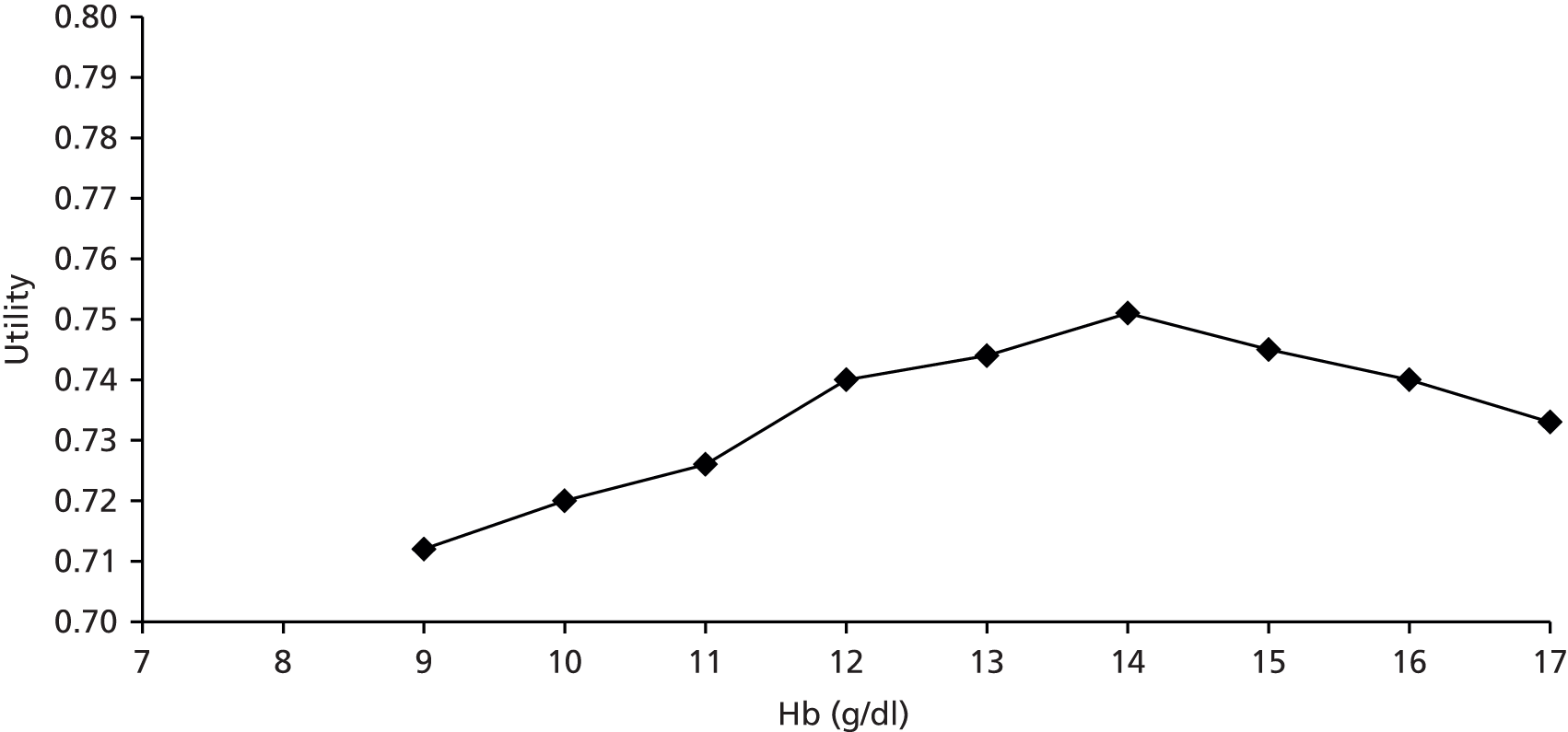
The study by Tajima and colleagues178 was also an observational study, which, among other factors, investigated the impact of Hb level on utilities for patients with CKD in Japan. This is also a useful data set because, as preferred by NICE,169 health was self-reported by patients using the EQ-5D classification system and the resulting health states were valued using utilities elicited from the general public using the time trade-off technique. However, the two main weaknesses are that (1) this was an observational study, which means that there could have been unmeasured covariates that contributed to the observed relationship between utility values and Hb levels and (2) patients had CKD, not cancer. Any bias resulting from (1) was minimised as several potentially confounding variables were included in the regression analysis. As for (2), it would be only a minor weakness if one could plausibly assume that the comorbidity of anaemia impacts HRQoL additively and in the same way in different patient groups. In this study utilities were found to increase only slightly, at a rate of 0.016 per unit change in Hb (see Table 59). It should also be noted that, as this study was conducted in Japan, the results may not entirely translate to a British population.
We believe that there are substantial weaknesses in the remaining three studies. There are many weaknesses in the study by Ossa and colleagues,159 including the use of health state vignettes (see Table 59). Hence, we attach little importance to the finding that utility increases steeply from 7 to 11 g/dl of Hb (see Figure 38).
The study by Lloyd and colleagues177 also has many important weaknesses, including the use of health state vignettes and the very small sample size. Hence, we attach little importance to the finding that utility increases steeply from 7.5 to 11.5 g/dl of Hb (see Figure 38).
In the study by Crawford and colleagues,162 health was appropriately elicited from patients. However, the one important weakness of the study was that the health preference elicitation instrument used was the LASA, whose self-assessment consists of five questions on physical, emotional, spiritual, intellectual and overall well-being, rated on a scale from 0 to 10. As such, utilities are not obtained by a choice-based method, such as the time trade-off or standard gamble, which is required by NICE. 169 Hence, we attach little importance to the finding that utility increases moderately from 7 to 14 g/dl of Hb (see Figure 38).
As stated above, the cost-effectiveness of ESAs may be very sensitive to the rate at which utilities change with respect to changes in Hb (i.e. the gradient of the utility/Hb graph). Cost-effectiveness is likely to be insensitive to the absolute utilities during the period of treatment with ESAs because mortality is assumed to be zero during this period for both the ESA treatment arm and the best supportive care arm.
Estimation of the impact of erythropoiesis-stimulating agents on health utilities from mapping disease-specific questionnaires to the European Quality of Life-5 Dimensions
As mentioned in Clinical effectiveness parameters, very little information can be gained from mapping from the disease-specific health questionnaires to the EQ-5D. Of the RCTs included in the PenTAG systematic review of clinical effectiveness, one study75 used the EORTC QLQ-C30 questionnaire and one study77 used the FACT-G questionnaire. These have been mapped to the EQ-5D by Dakin. 180
However, in the first case, it is not possible to perform such a mapping because the required EORTC QLQ-C30 information is not provided. In the second case, it is possible to make an approximate estimation of the impact of epoetin alfa on utilities. At the end of treatment we can estimate the difference in utilities between arms; in the case of Tjulandin and colleagues77 this is 0.007 × 6.1 = 0.04, where 6.1 is the difference in FACT-G total score in Littlewood and colleagues70 (2.5 + 3.6) and 0.007 is the coefficient from the utility mapping paper. 180 The authors of this paper found a better mapping function using the dimensions of the FACT-G questionnaire rather than the total score.
All of the other RCTs in the PenTAG systematic review that reported HRQoL use questionnaires for which we understand there is no mapping to the EQ-5D nor to the SF-6D. 180
Peninsula Technology Assessment Group base-case utilities by haemoglobin level
As mentioned in the previous section, we consider the studies by Harrow and colleagues176 and Tajima and colleagues178 to be the most methodologically robust. The key differences between the two studies are:
-
the study by Harrow and colleagues176 has the advantage of relating to people with cancer, whereas the study by Tajima and colleagues178 concerns people with CKD
-
the study by Tajima and colleagues178 has the advantage of using the EQ-5D valued using time trade-off, both preferred by NICE,174 whereas Harrow and colleagues176 used the SF-6D valued using the standard gamble.
Both studies find that the impact of Hb level on utilities is rather slight. In Harrow and colleagues,176 over the range Hb 9–12 g/dl, utilities increase by 0.009 per unit increase in Hb. This scales to 0.028 per unit increase in Hb on the EQ-5D, using the results of Brazier and colleagues’181 regression analysis. In Tajima and colleagues,178 over a similar Hb range, utilities increase by 0.016 per unit increase in Hb.
These results are consistent with the findings of Wisloff and colleagues179 and with our review of HRQoL that there is only weak evidence that ESAs improve HRQoL (see Chapter 3, Health-related quality-of-life outcomes: overall summary).
The results are also consistent with the estimated impact of epoetin alfa on utilities (see the previous section). At the end of treatment, the estimated difference in utilities between arms is 0.04. Given that we estimate a coefficient for Hb of 0.016 and that the difference in Hb between arms in the study by Littlewood and colleagues70 was 1.7 g/dl, we would estimate a difference in utility of 0.022 for Littlewood and colleagues,70 which is plausibly close.
For our base-case utilities we used the scaled utility value from Harrow and colleagues. 176 This was chosen over the EQ-5D results from Tajima and colleagues178 mainly on the basis that Harrow and colleagues’ population of people with cancer more closely matches our own. We therefore assumed that utilities increase by 0.028 per unit increase in Hb. This utility was then applied until the end of normalisation and adjusted for the mean difference in Hb levels between the ESA arm and the no ESA arm at the relevant time points to calculate the short-term QALY gain.
For the PSA we assumed a gamma distribution with a mean of 0.028 and a SE of 20% of the mean, reflecting Harrow and colleagues. 176 We also performed univariate sensitivity analyses using the estimate from Tajima and colleagues178 (0.016), as well as the unscaled value from Harrow and collegues176 (0.009) and the estimate used in the previous HTA review2 (0.060).
As stated above, the main weakness of both studies is that they are observational. This means that the estimated relation between utility and Hb level may be biased because of unmeasured confounding variables. However, as suggested by Tonelli and colleagues,88 any such bias is likely to lead to an overestimate of the rate of change of utility as a function of Hb. This is because (1) people with low Hb levels may be more likely to have more advanced cancer and hence lower reported utilities and (2) people who are told that their Hb level is low may underestimate their reported quality of life. This bias has the effect of biasing cost-effectiveness in favour of ESAs compared with no ESAs.
Peninsula Technology Assessment Group base-case utilities after erythropoiesis-stimulating agent discontinuation
The value of utilities after ESA discontinuation is difficult to generalise as the patient populations in source studies cover a wide range of cancers. The average age (59.1 years) taken from the RCTs is equivalent to a utility of 0.830, using the formula published by Ara and Brazier182 (Equation 4) and assuming the probability of being male to be 46% based on ONS cancer registration statistics for 2011183 for people aged 50–60 years.
We can therefore surmise that the utility must be lower than this after ESA discontinuation. In the previous HTA review,2 once people had returned to a Hb level of ≥ 13 g/dl, their utility was 0.810. In this assessment people normalise to a lower Hb value than in the previous HTA review2 and, given the similarity of this value to that in people in the general population, we use a lower utility value for people in the long term. Tengs and Wallace184 reported a utility for cancer of 0.83–0.92 (irrespective of age) using a time trade-off method. Applying this to the age-related utility gives a range of values from 0.68 to 0.76. Comparing this range to the values reported in Utilities in cost-effectiveness models of erythropoiesis-stimulating agents, as well as to those reported in previous PenTAG cancer HTA assessments,185,186 we conclude that using the higher estimate of 0.76 is the most appropriate utility.
Again, this is a parameter that is highly uncertain (because of the lack of data), which could have a potentially large impact on the overall QALYs accrued in the analysis. As such, in the PSA we vary the utility multiplier 0.92 as a beta distribution with a SE of 20% of the mean (0.184). The resulting SE of the long-term utility is 0.830 × 0.184 = 0.153.
Utilities not included in the Peninsula Technology Assessment Group model
In the previous sections we have described two sources of utility values within the model. An additional source of disutility can come from the AEs associated with ESA use. These utilities are not modelled explicitly and instead the disbenefit associated with AEs is accounted for only by cost.
This decision was made for several reasons, the main reason being that AE data in the RCTs are extremely poorly defined. First, the AEs themselves are poorly defined and, for example, a thromboembolic event can refer to several events, including pulmonary embolism and deep-vein thrombosis. These specific AEs are often not specified within the RCTs or different RCTs will include different AEs within their definition. Second, the severity and length of impact of the AEs are not consistent across the RCTs and are undefined for the pooled results. These poor definitions make it difficult to assign either costs or QALYs to AEs, and make it especially difficult to define the disutility of an AE and translate this into a QALY; indeed, there were no data to define these results.
One area in which the long-term disbenefit of AEs is implicitly included is survival. As with short-term mortality, any mortality associated with AEs should be implicitly identified by the survival estimates encountered in the RCTs, as these are extracted from the same pool of studies.
We acknowledge the lack of utilities associated with AEs as a limitation of the model and discuss this in Chapter 6 (see Adverse events).
Costs
In this analysis we model the following costs: blood test costs, cost of ESAs, RBCT costs (unit cost of blood and cost of the transfusion appointment) and costs of AEs. We do not model long-term costs in the base case given the uncertainty attached to these values as a result of the wide patient population. Additionally, any arbitrary cost added to long-term survival would disadvantage any arm with a survival benefit, which will be demonstrated in a sensitivity analysis.
Adjustments to 2014/15 prices
All costs and prices in the model were inflated to 2011/12 prices using the Hospital and Community Health Services (HCHS) Pay and Prices Index187 and then further inflated by 3.65% per annum for 2 years to 2014/15 prices, where 3.65% is the average (geometric mean) inflation of the index between 2006/7 and 2011/12.
Erythropoietin-stimulating agent prices
Table 60 presents the 2013 drug prices for ESAs, which have been taken from the British National Formulary. 166 Separately we report the expected wholesale acquisition costs (see Wholesale acquisition costs), which we used to conduct a sensitivity analysis on plausible actual costs to the NHS.
| Units | Epoetin alfa (£) | Epoetin beta (£) | Epoetin theta (£) | Epoetin zeta (£) | µg | Darbepoetin alfa (£) | |
|---|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormon | Eporatio | Retacrit | Aranesp | ||
| 500 | 3.51 | 10 | 14.68 | ||||
| 1000 | 5.53 | 5.09 | 5.99 | 5.66 | 15 | 22.02 | |
| 2000 | 11.06 | 10.18 | 14.03 | 11.98 | 11.31 | 20 | 29.36 |
| 3000 | 16.59 | 15.27 | 21.04 | 17.98 | 16.97 | 30 | 44.04 |
| 4000 | 22.12 | 20.36 | 28.06 | 23.97 | 22.63 | 40 | 58.73 |
| 5000 | 27.65 | 25.46 | 35.07 | 29.96 | 28.28 | 50 | 73.41 |
| 6000 | 33.19 | 30.55 | 42.08 | 33.94 | 60 | 88.09 | |
| 8000 | 44.25 | 40.73 | 45.25 | 80 | 117.45 | ||
| 10,000 | 55.31 | 50.91 | 70.14 | 59.92 | 56.57 | 100 | 146.81 |
| 20,000 | 110.62 | 140.29 | 119.84 | 113.13 | 130 | 190.86 | |
| 30,000 | 199.11 | 210.43 | 179.75 | 169.70 | 150 | 220.22 | |
| 40,000 | 265.48 | 226.26 | 300 | 440.43 | |||
| 50,000 | 374.48 | 500 | 734.05 | ||||
The majority of ESA dosages are calculated based on weight, with the exception of epoetin theta. As such, there is no standard dose for each patient and Table 60 demonstrates the various vial sizes for the ESAs that can make up a dose. Given the wide variety of vial sizes, we believe that drug wastage will be minimal and therefore did not account for this in our analysis.
Using the various vial sizes we calculated the costs per 1000 IU for epoetin alfa, beta, theta and zeta and per µg for darbepoetin. These depend on the vial size of the ESA for some of the ESAs, for example for a vial size no greater than 20,000 IU for Eprex the cost is £5.53 per 1000 IU, but if a larger vial size is used the cost is £6.64 per 1000 IU. In the base case we used the lowest cost per 1000 IU, for each of the ESAs, as this covered the largest range of vial sizes. These base-case costs are provided in Table 61.
| ESA | Per 1000 IU (£) | Per µg (£) | |
|---|---|---|---|
| Epoetin alfa | Eprex | 5.53 | |
| Binocrit | 5.09 | ||
| Epoetin beta | NeoRecormon | 7.01 | |
| Epoetin theta | Eporatio | 5.99 | |
| Epoetin zeta | Retacrit | 5.66 | |
| Darbepoetin alfa | Aranesp | 1.47 | |
The overall cost per dose for each ESA was then calculated using the number of units/µg per week.
The ESA unit costs were not varied in the PSA.
Wholesale acquisition costs
Drug manufacturers are free to sell to hospitals below the list price and acquisition costs under these sales would usually be commercially confidential. Manufacturers will typically employ a price–volume methodology in which more substantial savings are available to purchasers if commitments are made regarding the minimum quantity to be purchased. Because of different purchasing decisions by hospitals (in part because of different patient population sizes), the same drug will be acquired at a range of prices. Ideally, in an economic evaluation one would wish to use the average acquisition cost for each drug in the base case, but such information is generally kept confidential.
In this appraisal the manufacturers consented at the NICE Consultee Information Meeting (7 August 2013) to pharmacists revealing the confidential prices to PenTAG. We received the latest tenderings to London hospitals (South East England Specialist Pharmacy Services, Commercial Medicines Unit, 27 September 2013, personal communication). These were understood to be from the most recent tendering process and therefore the most representative prices going forwards.
As shown in Table 62, all manufacturers were prepared to offer some level of discount from the list prices and some (not all) were prepared to offer a discount with minimal commitment to volume. It can also be seen that the London hospitals did not secure the cheapest prices for all ESAs.
| ESA | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
|---|---|---|---|---|
| Epoetin alfa (Eprex) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Epoetin alfa (Binocrit) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Epoetin beta (NeoRecormon) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Epoetin zeta (Retacrit) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Darbepoetin alfa (Aranesp) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
If PenTAG were to adopt the strike prices agreed by London hospitals this would represent a significant bias in favour of the ESAs for which significant discounts were obtained. London hospitals entered contracts committing to a volume of at least 8000 people, which would have been sufficient to command the best offer from any manufacturer had all volume been promised to a single manufacturer.
If all ESAs are deemed to be equally effective then all purchasers should exclusively purchase the ESA that minimises total costs (i.e. that with the lowest combined drug acquisition and administration costs). By concentrating full purchasing power it should be possible for all purchasers to get the best offer price from each manufacturer.
We therefore believe that the best offer to London hospitals is the best unbiased estimate of the wholesale acquisition cost of ESAs. PenTAG noted that epoetin theta is not included in the list of ESAs offered to the London hospitals and therefore no wholesale acquisition cost can be estimated for this ESA.
The best offer prices cannot be guaranteed to last beyond the contract agreed between the manufacturer and the purchaser – in the case of the London hospitals the contract was for 12 months with the option to extend by a further 24 months.
Cost of administering erythropoiesis-stimulating agents
There are multiple dosing options for most of the ESAs and we chose the base-case dosing schedule for each on the basis of both the evidence available in the RCTs and the advice of our clinical experts. This allowed us to be consistent with our other evidence as well as clinical practice, including incorporating information on missed doses. In the base case we assumed that dosing occurs once a week for all ESAs. In sensitivity analysis we investigated the different dosing schedules for each ESA, as shown in Table 63.
| ESA | Base-case dose | Sensitivity analyses |
|---|---|---|
| Epoetin alfa | Once weekly | 3 times a week |
| Epoetin beta | Once weekly | 3–7 times a week |
| Epoetin theta | Once weekly | 3 times a week |
| Epoetin zeta | Once weekly | 3 times a week |
| Darbepoetin alfa | Once weekly | Once every 3 weeks |
In the context of CKD, ESAs are typically self-administered by the patient when possible (advice from MN) and, in the case of the industry submissions presented in this review,2 the majority of patients are expected to self-administer. However, consultations with our clinical experts (KS, MN, CR, NR) suggested a more varied view on ESA administration, with some indicating that, for the therapy under review (CIA), with a comparatively short period of treatment, it may be more likely for patients not to self-administer. As our experts covered a range of cancers and backgrounds, we decided that the most appropriate decision in the base case was to take an average of the opinions on how ESAs should be administered in practice. Therefore, of the ESAs administered each week, in the base case 16.25% are administered during patients’ chemotherapy appointments, 43.13% are administered during a general practitioner appointment or by a district nurse and 40.63% are self-administered (Table 64). We did not allocate these values to specific patients, as patients are likely to encounter a combination of these practices during their time on ESAs (advice from CR). This also means that we did not explicitly account for instances such as the weeks when patients do not have a chemotherapy appointment, as this is factored into the average values. Given the uncertainty around these values, as part of our sensitivity analysis we examined the situation in which ESAs are administered to cancer patients in a similar manner to that for CKD patients. The costs of each type of administration and the overall average cost for ESA administration are presented in Table 64. In the PSA the probabilities were drawn from a Dirichlet distribution.
| ESA administration | Cost (£) | Source | % of ESAsa | Source |
|---|---|---|---|---|
| Appointment with district nurse | 18.80 | PSSRU188 | 21.56 | Clinical experts NR, KS, MN, CR |
| Appointment with general practice nurse | 10.74 | PSSRU188 | 21.56 | |
| Appointment with hospital staff nurse | 11.01 | PSSRU188 | 16.25 | |
| Self-administered | 0 | Assumed | 40.63 | |
| Average cost per ESA administration | 8.16 |
As stated in Duration of erythropoiesis-stimulating agent treatment, the duration of ESA treatment is calculated on an ITT basis and, as such, the cost of administration may be slightly exaggerated. However, as the average cost per ESA administration is £8.16, the cost does not have a significant impact on the results compared with the cost of ESA drug prices in the base case.
Additional blood tests for erythropoiesis-stimulating agents
Another additional cost for ESAs is incurred by an increase in the number of blood tests (advice from clinical experts KS and NR). Opinion appears to be divided on how much of an increase this would be. In the base case we assumed that blood tests would occur regularly for both patients who are on ESAs and those who are not while patients are undergoing chemotherapy treatment, but that additional blood tests would continue post chemotherapy for those patients on ESAs. In the base case we costed for four additional blood tests. We assumed that these were carried out by a general practice nurse at a cost of £42.98 per hour (£40 in 2012/13188 inflated to 2014 prices) and that the appointment takes 15.5 minutes of nurse time, based on the average surgery consultation time,189 resulting in a cost of £11.10. We also added the NHS reference cost for phlebotomy [Healthcare Resource Group (HRG) code DAPS08) of £3.91 (inflated from £3.64 in 2012/13). 190 The total cost of a blood test is then £15.01. As the cost of blood tests is relatively small compared with the other costs associated with CIA, we do not expect any increase or reduction in the number of blood tests to have a significant impact on the results. To represent uncertainty in these parameters all parameters were drawn from gamma distributions in the PSA, with SE equal to 20% of the mean.
Adverse event costs
The AEs that we accounted for in this cost-effectiveness analysis were identified through the clinical effectiveness review. In particular, we accounted for the costs of:
-
thromboembolic events
-
hypertension
-
thrombocytopenia.
Resource use for patients not receiving ESA therapy was estimated from the systematic review of clinical effectiveness evidence by simple pooling of all studies to calculate how many patients did and did not experience at least one AE. A beta distribution was constructed for the PSA on the basis of these figures. Patients were assumed to experience, at most, one AE of each type. Resource use for patients receiving ESA therapy was calculated similarly but also applying the relative risk obtained from the systematic review of clinical effectiveness evidence (see Chapter 3).
The unit costs of managing thromboembolic events (particularly pulmonary embolism and deep-vein thrombosis), hypertension and thrombocytopenia were identified through NHS reference costs 2012–13190 and updated to 2014/15 prices. These figures are presented in Table 65 and are the weighted averages dependent on HRG codes. No decision was made to specify HRG codes beyond the particular AE, to reflect the fact that the relative risks identified in the PenTAG clinical effectiveness systematic review refer to any AE, regardless of severity. These costs are significantly larger than those reported in TA142,2 in which the cost of an AE was only £101, but attempts to identify how this figure arose were unsuccessful, beyond identifying it in the Ortho Biotec submission. The previous Roche submission in TA142 had previously attached a cost of monitoring for hypertension of £4 a week and the Amgen Inc. submission a cost of £185 for a deep-vein thrombosis, although sources of these costs were unclear. The NHS reference costs themselves report a wide range of costs for managing each of the AEs and, as such, these costs were altered in the PSA following a gamma distribution with SEs equal to 20% of the means.
| AE | PenTAG base case (£) | HRG codes |
|---|---|---|
| Thromboembolic events | 1243 | DZ09D, DZ09E, DZ09F, DZ09G, DZ09H (pulmonary embolus), QZ20A, QZ20B, QZ20C, QZ20D, QZ20E (DVT) |
| Hypertension | 826 | EB04Z (hypertension) |
| Thrombocytopenia | 744 | SA12G, SA12H, SA12J, SA12K (thrombocytopenia) |
Red blood cell acquisition costs
Unit costs for the supply of RBCs were taken directly from NHS Blood and Transplant (NHSBT) 2012/13 costs (£122 per unit)191 and uprated to 2014/15 prices. This cost is significantly different from the cost of blood products in outpatient care reported in the NHS reference costs 2012–13190 (average cost around £1300). However this cost is for all blood products, not just RBCs, and as such it has a skewed distribution: for HRG code XD05Z (Blood Products, Band 1) the average unit cost is £1269, but the upper quartile cost is £482. We did not use the NHS reference costs because of the imprecision around the term ‘blood products’. Furthermore, the cost of RBCs from the NHSBT is similar to the unit cost reported in a publicly accessible letter detailing the outcomes of the National Commissioning Group for Blood meeting on 9 October 2007,192 which detailed the cost of RBCs for 2008/9 as £139.72. A gamma distribution was used for the cost of a RBC unit with the SE equal to 20% of the mean.
Cost of a transfusion appointment
The closest cost reported in the NHS reference costs for an outpatient blood transfusion appointment is the outpatient cost for a blood and bone marrow transplant. As with the cost of blood products, this covers more than the specific figure needed for our analysis. Returning to the TA142 analysis2 the cost value reported came from the study by Varney and Guest. 172 Attempts were made to find updated versions of the figure reported in this paper, with marginal success. Audits from the NHSBT indicate that the numbers of transfusions, as well as the percentages of associated complications, have decreased since the Varney and Guest172 study was conducted, but the associated costs were not available for this analysis. As such, we used the same figure as reported in Varney and Guest172 and uprated the cost to 2014/15 costs (Table 66). A gamma distribution was used for the unit cost of a RBC transfusion appointment, with the SE equal to 20% of the mean.
Intravenous iron supplementation
National Institute for Health and Care Excellence guidance states that, in circumstances in which ESA therapy is recommended, it should be used in combination with intravenous iron as this is associated with a greater probability of a haematological response. 1
Intravenous iron supplementation was not included in any of the cost–utility studies identified in the update systematic review of cost-effectiveness (see Chapter 4, Update review).
Iron supplementation is likely to be given to anaemic patients independently of whether they receive ESA therapy, therefore differences in resource use between patients receiving and patients not receiving ESA therapy are likely to be very small (e.g. if anaemia is corrected sooner then iron supplementation would be used for less time) and we have not sought studies from which to estimate such resource use differences.
The cost of intravenous iron has been assumed to be negligible in previous economic studies. To check that this is a reasonable assumption we briefly estimated the cost of the acquisition and administration of intravenous iron. Assuming that intravenous iron would be given in the form of iron dextran 100 mg once weekly (alongside ESA administration), the acquisition cost of CosmoFer® (Pharmacosmos) would be £7.97 per week (2-ml ampule of 50 mg/ml iron dextran). 166
Resource use for drug administration is difficult to estimate, as patients may already be attending an outpatient clinic for chemotherapy and ESA therapy. We assumed that the incremental resource use for intravenous iron supplementation is minimal and of the same order of magnitude as the drug acquisition cost.
Given that resource use is likely to be very similar between patients receiving and patients not receiving ESA therapy (and that no clinical data would directly inform an estimate of the difference), and given that unit costs are also small in comparison to the cost of ESA acquisition and RBCT, we assumed that the cost of intravenous iron supplementation can be ignored as it will be very similar for all arms.
Other model characteristics
Time horizon, perspective and discounting
A lifetime time horizon was used in the model. The perspective adopted was that of the NHS and Personal Social Services. Costs and benefits were discounted at 3.5% per annum.
Patient characteristics
The age and weight of patients in the model were estimated from the age and weight reported in clinical studies included in the systematic review of clinical effectiveness evidence. A simple average was taken across the studies to estimate the mean, and the SD across studies was used to estimate the SE used in the PSA.
The mean age in the base case was estimated as 59.1 years (SE 5.3 years) and in the scenario analysis with an inclusion Hb level of ≤ 11.0 g/dl it was estimated as 60.8 years (SE 4.2 years).
The mean weight in the base case was estimated as 66.6 kg (SE 3.3 kg) and in the scenario analysis it was estimated as 66.1 kg (SE 3.6 kg).
The proportion of male patients was estimated as 46% based on cancer registration statistics in England in 2011 (individuals aged 50–59 years). 183
Key points
-
Our economic model consists of two components: short term and long term.
-
In the short-term component:
-
The mean Hb levels across the population were estimated as a function of time for those receiving and not receiving ESA therapy. Hb levels were mapped to utilities to derive QALYs.
-
The difference in Hb levels between the ESA arm and the non-ESA arm at the end of treatment was taken from the systematic review of clinical effectiveness (see Chapter 3).
-
Anaemia correction was not assumed to be instantaneous in the ESA arm; instead, the average difference in Hb levels between the ESA arm and the non-ESA arm across the duration of treatment was set to a proportion of the final difference in Hb levels based on results from the randomised trials.
-
The short-term component includes a period during which Hb levels return to normal, a process called ‘normalisation’. We found no published data on normalisation, so clinical expert advice and previous economic models were used to inform our modelling.
-
Dose adjustment, dose interruption and treatment withdrawal from ESA therapy were incorporated into an ITT mean weekly dose estimated from randomised trials to attempt to achieve consistency between drug acquisition costs and effectiveness outcomes.
-
The relationship between Hb levels and utilities was estimated from the published literature and assumed to be linear in the range of interest.
-
The drug acquisition costs for ESAs were taken from NHS list prices.
-
Some patients (41%) were assumed to self-administer ESAs, whereas the rest required an appointment with a nurse.
-
Thromboembolic events, hypertension and thrombocytopenia were included as AEs that incurred costs but which did not incur disutility.
-
The units of blood required in RBCTs for the ESA and no ESA arms were estimated from the clinical effectiveness review. Units per transfusion were estimated from the published literature and were assumed to be the same for all arms. Costs associated with transfusions were estimated from NHSBT and the published literature. The cost of a transfusion appointment was inflated from 2001 estimates, which were the most recent available.
-
-
In the long-term component:
-
A constant rate of mortality was assumed with an expected survival duration of 2.67 years for those not receiving ESA therapy, calculated from studies identified in the systematic review of clinical effectiveness. The rate of mortality was adjusted for those receiving ESA therapy using the HR derived in the systematic review of clinical effectiveness (see Chapter 3).
-
A constant utility of 0.76 was assumed for the whole population to derive QALYs.
-
Results
We first present the base-case cost-effectiveness results, comparing six different ESA anaemia treatments with usual treatment not involving ESAs, for adult patients with CIA. The options for anaemia treatment are either RBCTs only or ESAs with RBCTs. Given the differing cost of ESAs, the results for patients on ESAs are examined across the different manufacturers.
Next, we present the cost-effectiveness results under a number of scenarios and the PSA results. These scenarios include:
-
analysis in which survival is assumed to be equal in both the ESA arm and the no ESA arm
-
the impact of wholesale acquisition costs for ESAs, when applied to both the base-case results and the scenario analysis, in which survival is assumed to be equal in both arms
-
subgroup analysis based on studies in which the initial Hb level of patients was ≤ 11 g/dl
-
analyses investigating the OS assumptions.
We also present a comparison of our base-case results with the results presented in TA142. 1
We do not present results for either of the subgroups originally recommended for ESA therapy from TA142: ovarian cancer patients on platinum-based chemotherapy and patients unable to undergo a blood transfusion (on medical or religious grounds). This is because of the lack of suitable data on these two subgroups (see Chapter 3).
Base case
For our base case we present the summary results but emphasise the uncertainty in the model through scenario analyses and the PSA, as the deterministic results do not account for such uncertainty.
Cost-effectiveness results
The summary cost-effectiveness results are presented in Table 67 and Figure 40. Costs, which all occur within the first year, and short-term QALY gains remain undiscounted, but QALYs gained in the long term are discounted.
| Treatment arm | No ESA | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | ||
| Total costs per strategy (£) | 912 | 2414 | 2283 | 3384 | 2416 | 2451 | 3258 |
| Total incremental costs vs. no ESA (£) | – | 1502 | 1371 | 2472 | 1504 | 1539 | 2346 |
| Total discounted QALYs gained vs. no ESA | – | 0.0706 | 0.0706 | 0.0706 | 0.0706 | 0.0706 | 0.0706 |
| ICER vs. no ESA (£/QALY) | – | 21,279 | 19,429 | 35,018 | 21,309 | 21,804 | 33,233 |
| ICER (£/QALY) | – | Dominated by Binocrit | 19,429 | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit |
| INHB vs. no ESA at WTP of £20,000/QALY | – | –0.005 | 0.002 | –0.053 | –0.005 | –0.006 | –0.047 |
| INHB vs. no ESA at WTP of £30,000/QALY | – | 0.021 | 0.025 | –0.012 | 0.020 | 0.019 | –0.008 |
FIGURE 40.
Incremental costs and QALYs per patient by anaemia treatment strategy.

As Table 67 shows, the ICERs for the ESA strategies compared with no ESA use in the deterministic base-case analysis range from £19,429 to £35,018 per QALY gained. Five of these ICERs are all above the NICE-designated willingness-to-pay threshold of £20,000 per QALY and two (NeoRecormen and Aranesp) lie above the upper £30,000 per QALY willingness-to-pay threshold. One ESA (Binocrit) lies below the £20,000-per-QALY threshold but is very close to this threshold, with an ICER of £19,429 per QALY gained. These results are represented pictorially in Figure 40. As our ICERs cover a range from < £20,000 to > £30,000 per QALY and are highly sensitive to the parameter estimates, it is important that we demonstrate the impact of the uncertainty in these ICERs and quantify the probability that these ICERs represent the true results.
When the ICERs are translated into incremental net health benefit (INHB) compared with no ESA use, the INHB ranges from –0.053 to 0.002 QALYs at a willingness-to-pay of £20,000 per QALY and from –0.012 to 0.025 QALYs at a willingness-to-pay of £30,000 per QALY, depending on the ESA. This represents a slight net health benefit from the use of ESAs for most ESAs at the £30,000 per QALY willingness-to-pay threshold, but only a net health benefit for one ESA (Binocrit) at the £20,000-per-QALY threshold. Again, it is important to assess the likelihood of this very modest potential net benefit. Inevitably, given the assumed identical effectiveness, we also find that when the ESA strategies are compared with each other, they are dominated by the ESA with the lowest total ESA cost (in this case Binocrit). This is because the only model parameter that differs between each type of ESA is the cost of the drug itself. Therefore, ESAs with a higher cost are dominated by the ESA with the lowest cost when they are directly compared.
We now briefly describe the breakdown of costs and QALY results that give our overall results.
Costs
In the base case, costs are accrued only in the short term (within the first year) so that long-term costs unrelated to anaemia do not disadvantage a treatment with a survival benefit. The costs reported in the base case are therefore not discounted.
Table 68 shows that the total cost per patient in all arms is not particularly high, implying that small changes to these costs may have large impacts on the overall results. The largest cost for all ESA arms is the cost of the ESA itself (£1510–£2485). The largest cost for a patient not on ESA therapy is the cost of a RBCT (£799).
| Treatment arm | No ESA | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | ||
| Total cost per strategy (£) | 912 | 2414 | 2283 | 3384 | 2416 | 2451 | 3258 |
| ESA cost (£) | 0 | 1641 | 1510 | 2611 | 1643 | 1678 | 2485 |
| ESA administration cost (£) | 0 | 98 | 98 | 98 | 98 | 98 | 98 |
| AE cost (£) | 113 | 148 | 148 | 148 | 148 | 148 | 148 |
| RBCT cost (£) | 799 | 467 | 467 | 467 | 467 | 467 | 467 |
| Cost of additional blood tests (£) | 0 | 60 | 60 | 60 | 60 | 60 | 60 |
| Incremental results | |||||||
| Incremental cost vs. no ESA (£) | – | 1502 | 1371 | 2472 | 1504 | 1539 | 2346 |
| ESA cost (£) | – | 1641 | 1510 | 2611 | 1643 | 1678 | 2485 |
| ESA administration cost (£) | – | 98 | 98 | 98 | 98 | 98 | 98 |
| AE cost (£) | – | 35 | 35 | 35 | 35 | 35 | 35 |
| RBCT cost (£) | – | –332 | –332 | –332 | –332 | –332 | –332 |
| Cost of additional blood tests (£) | – | 60 | 60 | 60 | 60 | 60 | 60 |
Adverse events have the one of the lowest total costs, in both the ESA arm and the no ESA arm. However, it is important to note that the data from the RCTs used to populate the values of the AE model parameters were available only as the probabilities of having at least one AE (hypertension, thrombocytopenia, thromboembolic events) and the model costs this as only one AE. Given the uncertainty around the AE data, we explored their impact on the results in sensitivity analyses (see Univariate sensitivity analysis, Adverse event costs).
As we have assumed the same dosing schedule for all ESAs in the base case (once weekly) and that all ESAs are likely to be administered in the same manner, the administration cost for each ESA is equal. Similarly, because of assumptions of equal effectiveness, the costs of AEs, RBCT and additional blood tests are the same for all ESAs.
Incremental results (see Table 68 and Figure 41) demonstrate that, although there is an estimated cost saving of £332 for RBCTs avoided, this is outweighed by the additional costs accrued in each ESA arm.
FIGURE 41.
Incremental costs vs. no ESA use in the base case.

Quality-adjusted life-years and survival gain
As Table 69 demonstrates, there is a life-year and QALY gain for patients on ESAs compared with no ESAs, both in the short term (QALY gain as a consequence of an increase in Hb level) and in the long term (a survival gain resulting in a QALY increase). We do not report QALYs for the no ESA arm, instead reporting all QALYs as incremental compared with no ESA treatment. This is because in the short term we do not allocate a specific utility value to each Hb level, instead assigning an increase in utility per Hb increase of 1 g/dl. We therefore do not calculate the short-term utility for the patients in the no ESA arm, instead calculating the difference in utility between arms using the Hb levels. QALYs gained (or lost) by the ESA arm compared with the no ESA arm are then calculated by applying this difference in utility across the appropriate time frame. For consistency, the long-term utility is applied to the difference in survival between the arms, giving the QALYs gained (or lost) by the ESA arm rather than specific QALYs for each arm.
| Treatment arm | Incremental life-year and QALY gains (ESA vs. no ESA) |
|---|---|
| Undiscounted life-years gained vs. no ESAs | 0.0911 |
| Discounted life-years gained vs. no ESAs | 0.0762 |
| Total discounted QALYs gained vs. no ESAs | 0.0706 |
| Total short term | 0.0124 |
| Short term during cancer treatment | 0.0083 |
| Short term during normalisation | 0.0042 |
| Long term | 0.0582 |
As the results are based on new meta-analyses in PenTAG’s clinical effectiveness review, these results are not conducted separately for each ESA product.
Figure 42 demonstrates where these QALYs are accrued. Over three-quarters of the QALY gain results from the modelled increased survival.
FIGURE 42.
Incremental QALY gain vs. no ESAs.

Short-term QALYs are accrued during chemotherapy and in the post-chemotherapy period designated as normalisation. Again, all ESA types are treated as equal in this regard and, as with the costs, these values are not discounted because of the short time frame within which they occur. In our analysis we do not explicitly model any additional ESA use during the normalisation period (it is possible for patients to still be on ESA therapy for up to 4 weeks after chemotherapy); therefore this QALY gain could be greater. Our estimated short-term QALY gain, 0.0124, is lower than that in other comparable studies (e.g. Wilson and colleagues,2 in which the short-term gain in the base case is 0.030) because of PenTAG’s smaller utility gain associated with an increase in Hb level.
The long-term QALY gain for patients on ESAs compared with those not on ESAs is a direct result of the life-years gained, as the utility is assumed to be the same in both arms once patients’ Hb levels have normalised. Given the time frame of this section of the model, the life-years gained and associated QALYs are discounted in the final results. The number of discounted life-years gained for patients on ESAs is 0.0762. This translates to a discounted QALY gain of 0.0582, which is significantly larger than the QALY gain from short-term Hb level improvement. This demonstrates the importance of the estimated survival effect of ESA usage. Although our base case includes a survival benefit associated with ESA use, this survival benefit is not demonstrated with statistical significance, as discussed in the PenTAG clinical effectiveness review, and is one parameter that is investigated thoroughly in sensitivity analysis in an attempt to quantify its effects on the results. It is this parameter in particular that drives the cost-effectiveness results and emphasises the importance of our PSA.
Probabilistic sensitivity analysis of the Peninsula Technology Assessment Group base case
Here, we present the results of the PSA for our base case. Table 70 presents the average PSA results compared with the ICERs in the deterministic base case. On average, the ICERs are slightly reduced in the PSA compared with the deterministic base case. However, as we can see from the 95% CIs for the costs and QALYs, the true ICERs are likely to cover a wide range. Indeed, the credible intervals (CrIs) cover a range from £2500 per QALY to the point where they are dominated (with ESAs having higher costs and lower QALYs than the no ESA arm).
| Treatment arm | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | |
| Deterministic ICER vs. no ESA (£/QALY) | 21,279 | 19,429 | 35,018 | 21,309 | 21,804 | 33,233 |
| Mean probabilistic ICER vs. no ESA (95% CrI) (£/QALY) | 16,135 (2529 to Dtd) | 14,724 (2322 to Dtd) | 27,226 (4067 to Dtd) | 16,312 (2581 to Dtd) | 16,484 (2439 to Dtd) | 25,684 (3841 to Dtd) |
| Incremental QALYs vs. no ESA (95% CI) | 0.092 (–0.264 to 0.447) | 0.092 (–0.264 to 0.447) | 0.092 (–0.264 to 0.447) | 0.092 (–0.264 to 0.447) | 0.092 (–0.264 to 0.447) | 0.092 (–0.264 to 0.447) |
| Incremental short-term QALYs vs. no ESA (95% CI) | 0.014 (0.001 to 0.028) | 0.014 (0.001 to 0.028) | 0.014 (0.001 to 0.028) | 0.014 (0.001 to 0.028) | 0.014 (0.001 to 0.028) | 0.014 (0.001 to 0.028) |
| Incremental long-term QALYs vs. no ESA (95% CI) | 0.077 (–0.278 to 0.433) | 0.077 (–0.278 to 0.433) | 0.077 (–0.278 to 0.433) | 0.077 (–0.278 to 0.433) | 0.077 (–0.278 to 0.433) | 0.077 (–0.278 to 0.433) |
| Incremental costs vs. no ESA (95% CI) (£) | 1478 (792 to 2164) | 1349 (710 to 1987) | 2494 (1401 to 3586) | 1494 (826 to 2163) | 1510 (720 to 2249) | 2353 (1327 to 3379) |
| Incremental ESA costs vs. no ESA (95% CI) (£) | 1624 (986 to 2262) | 1495 (908 to 2082) | 2640 (1588 to 3693) | 1641 (1005 to 2277) | 1656 (953 to 2360) | 2499 (1492 to 3507) |
| Incremental ESA administration costs vs. no ESA (95% CI) (£) | 97 (4 to 191) | 97 (4 to 191) | 97 (4 to 191) | 97 (4 to 191) | 97 (4 to 191) | 97 (4 to 191) |
| Incremental AE costs vs. no ESA (95% CI) (£) | 37 (1 to 74) | 37 (1 to 74) | 37 (1 to 74) | 37 (1 to 74) | 37 (1 to 74) | 37 (1 to 74) |
| Incremental RBCT costs vs. no ESA (95% CI) (£) | –341 (–556 to –125) | –341 (–556 to –125) | –341 (–556 to –125) | –341 (–556 to –125) | –341 (–556 to –125) | –341 (–556 to –125) |
| Cost of additional blood tests vs. no ESA (95% CI) (£) | 60 (41 to 79) | 60 (41 to 79) | 60 (41 to 79) | 60 (41 to 79) | 60 (41 to 79) | 60 (41 to 79) |
| INHB vs. no ESA at WTP of £20,000 per QALY (95% CI) | 0.018 (–0.339 to 0.375) | 0.024 (–0.332 to 0.381) | –0.033 (–0.392 to –0.326) | 0.017 (–0.338 to 0.372) | 0.016 (–0.342 to 0.374) | –0.026 (–0.386 to –0.334) |
The average INHB for each ESA in the PSA is slightly higher than in the deterministic base case, especially when the ESA was close to the boundary of the £20,000 per QALY willingness-to-pay threshold in the deterministic base case. However, when the 95% CI for each INHB is examined, it is clear that there is quite a range that each INHB can lie on. The breakdown of costs and QALYs indicates where the majority of the uncertainty in the overall costs and QALYs comes from. Unsurprisingly, as they appeared to be the main drivers in the deterministic scenario, the ESA costs and the long-term QALY gain appear to have the largest impact on the uncertainty around the overall costs and QALYs.
To represent the uncertainty further, we plotted the simulation results for Binocrit (currently the cheapest of the different ESAs) in Figure 43.
FIGURE 43.
Probabilistic sensitivity analysis base-case incremental costs and QALYs: scatterplot.

The scatterplot demonstrates that all data points fall within the north-west and north-east quadrants so that none of the simulations resulted in a cost saving from ESA use. The four quadrants and their proportions of data points are summarised in Table 71. From examining the cost results of the model, in 100% of simulations the ESA arm had higher costs for ESA use and reduced costs for RBCTs and in 0.8% of simulations there was a reduction in the costs of AEs compared with the no ESA arm. This 0.8% occurs when the RR of thrombocytopenia is favourable for ESA use and the additional costs of thrombocytopenia in the control arm outweigh the costs in the ESA arm. However, as the simulations demonstrate, this reduction in cost for AEs does not produce an overall cost saving (the cost saving for the ESA arm in these occurrences is < £10).
| Cost | Health loss | Health gain |
|---|---|---|
| Increase | 31.4 | 68.6 |
| Saving | 0 | 0 |
A significant proportion (31.4%) of the data points also reflect an estimated loss of QALYs. This suggests the possibility that ESAs may actually result in a reduction in QALYs while still having an increased cost. There is always a QALY gain from ESA use in the short term, as the CI for the difference in Hb level at the end of the trial between the ESA arm and the no ESA arm never favours no ESA use, therefore this loss of QALYs is a direct result of the wide CI for the OS HR. The model shows that 36% of simulations have a QALY loss in the long term (as a result of the OS HR favouring no ESA therapy over ESA use) and in the majority of these simulations (≈87.2%) this is larger than the QALY gain in the short term, resulting in an overall QALY loss. This suggests that the OS HR is the primary driver of the QALY results for the simulations.
Table 72 shows that, at a willingness-to-pay threshold of £20,000 per QALY, 50.9% of simulations fall above this threshold (of which 31.4% are dominated by the no ESA arm). The percentage of simulations that therefore put ESAs within the region of being cost-effective at a willingness-to-pay threshold of £20,000 per QALY is 48.1%. Comparing this value to the 31.4% of simulations in which ESA use is dominated, we can conclude that the likelihood of ESAs being cost-effective is highly uncertain.
| ESA dominated vs. no ESA | ICERs > £20,000 per QALY vs. no ESA | Total in which ESA is not cost-effective (at £20,000 threshold) | |
|---|---|---|---|
| Probability | 31.4 | 19.5 | 50.9 |
When we compare the cost-effectiveness acceptability curves (CEACs) of all of the ESA strategies (Figure 44), we see that below a willingness-to-pay threshold of £150,000 per QALY, no single ESA strategy is as probable to be cost-effective as the current practice arm, with the majority converging to a probability far below that of the no ESA arm. The probability of the no ESA arm being cost-effective reduces swiftly as the willingness-to-pay threshold lowers, to the extent that, by a willingness-to-pay threshold of £20,000 per QALY, it falls to < 50%. However, at this £20,000-per-QALY threshold we also see that the ESA arm most likely to be cost-effective still has a < 25% probability of being cost-effective. All other ESA arms have a probability of being cost-effective of < 20% for any willingness-to-pay threshold of < £150,000 per QALY.
FIGURE 44.
Cost-effectiveness acceptability curves from the base-case PSA.
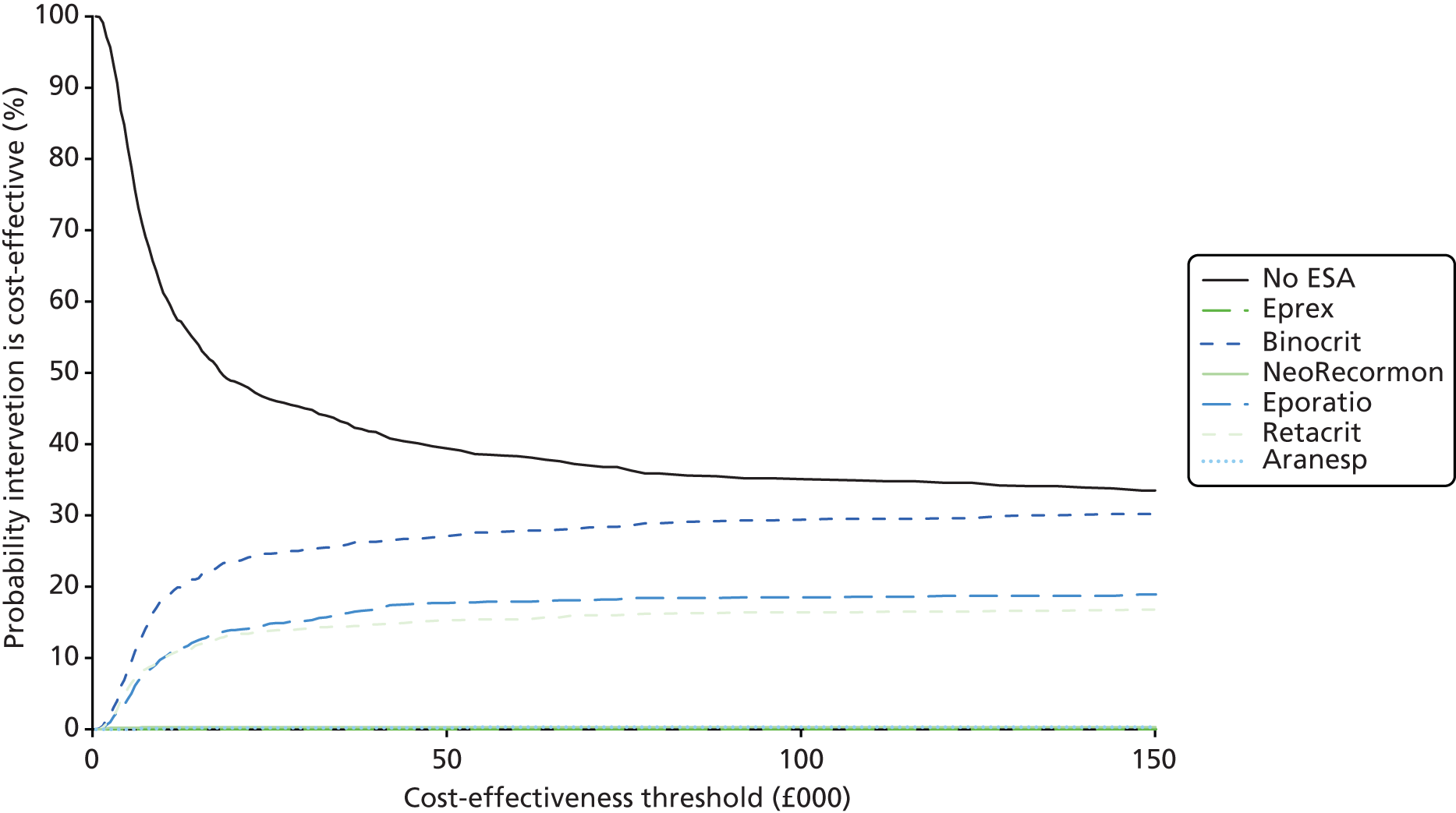
The cost-effectiveness acceptability frontier (CEAF) (Figure 45) compares the expected net health benefits of strategies at various willingness-to-pay thresholds. Given the higher average costs and equal QALY gains of the other ESAs, Binocrit consistently has the highest net health benefit of the ESAs and therefore is the only ESA to appear on the CEAF. We see that, at a willingness to pay of £15,000 per QALY, Binocrit appears to be the most favourable option (i.e. it has the highest probability of producing the highest net health benefit).
FIGURE 45.
Cost-effectiveness acceptability frontier for the base-case PSA.

Overall, the PSA results demonstrate that the uncertainty inherent in the parameter estimates, particularly those relating to long-term QALY gains, is highly influential on the results. There appears to be the potential for ESAs to be cost-effective at a £20,000-per-QALY threshold, depending on their cost, but this is to be viewed with caution given that there is also the possibility of ESAs producing a survival loss and uncertainty which ESAs would be cost-effective.
Scenario analysis 1: setting overall survival as equal across arms
As the long-term QALYs from any potential survival benefit are highly influential on the cost-effectiveness results and both the clinical and the statistical significance of any survival benefit may be disputed, we present a scenario in which the OS HR is set to exactly 1 (and not varied in the PSA). For the purposes of this scenario we present first the deterministic results, then a threshold analysis of the mean weekly cost to establish the cost at which ESAs become cost-effective and, finally, a PSA to investigate how removing the long-term survival benefit in the model affects the model results.
Deterministic analysis: scenario analysis 1
As this scenario is identical to the base case, but with the long-term aspect effectively removed, the costs and short-term QALYs in the deterministic analysis are the same as those in the base case except that the long-term incremental QALYs become equal to 0. This can be demonstrated by comparing Table 73 with Table 67.
| Treatment arm | No ESA | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | ||
| Total cost per strategy (£) | 912 | 2414 | 2283 | 3384 | 2416 | 2451 | 3258 |
| Total incremental cost vs. no ESA (£) | – | 1502 | 1371 | 2472 | 1504 | 1539 | 2346 |
| Total discounted QALYs gained vs. no ESA | – | 0.0124 | 0.0124 | 0.0124 | 0.0124 | 0.0124 | 0.0124 |
| ICER vs. no ESA (£/QALY) | – | 120,995 | 110,477 | 199,118 | 121,166 | 123,983 | 188,968 |
| ICER (£/QALY) | – | Dominated by Binocrit | £110,477 | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit |
| INHB vs. no ESA at WTP of £20,000/QALY | – | –0.063 | –0.056 | –0.111 | –0.063 | –0.065 | –0.105 |
| INHB vs. no ESA at WTP of £30,000/QALY | – | –0.038 | –0.033 | –0.070 | –0.038 | –0.039 | –0.066 |
The overall QALY gain is now greatly reduced from 0.0706 in the base case to 0.0124, a reduction of 82%. As the costs have remained the same we see that the ICERs are greatly increased, such that all ESAs have an ICER > £110,000 per QALY compared with the no ESA arm. These ICERs lie well above the £30,000-per-QALY threshold depicted in Figure 46. This therefore suggests that, if no survival benefit is assumed, ESAs do not appear to be cost-effective compared with current practice.
FIGURE 46.
Incremental costs and QALYs for scenario analysis 1.
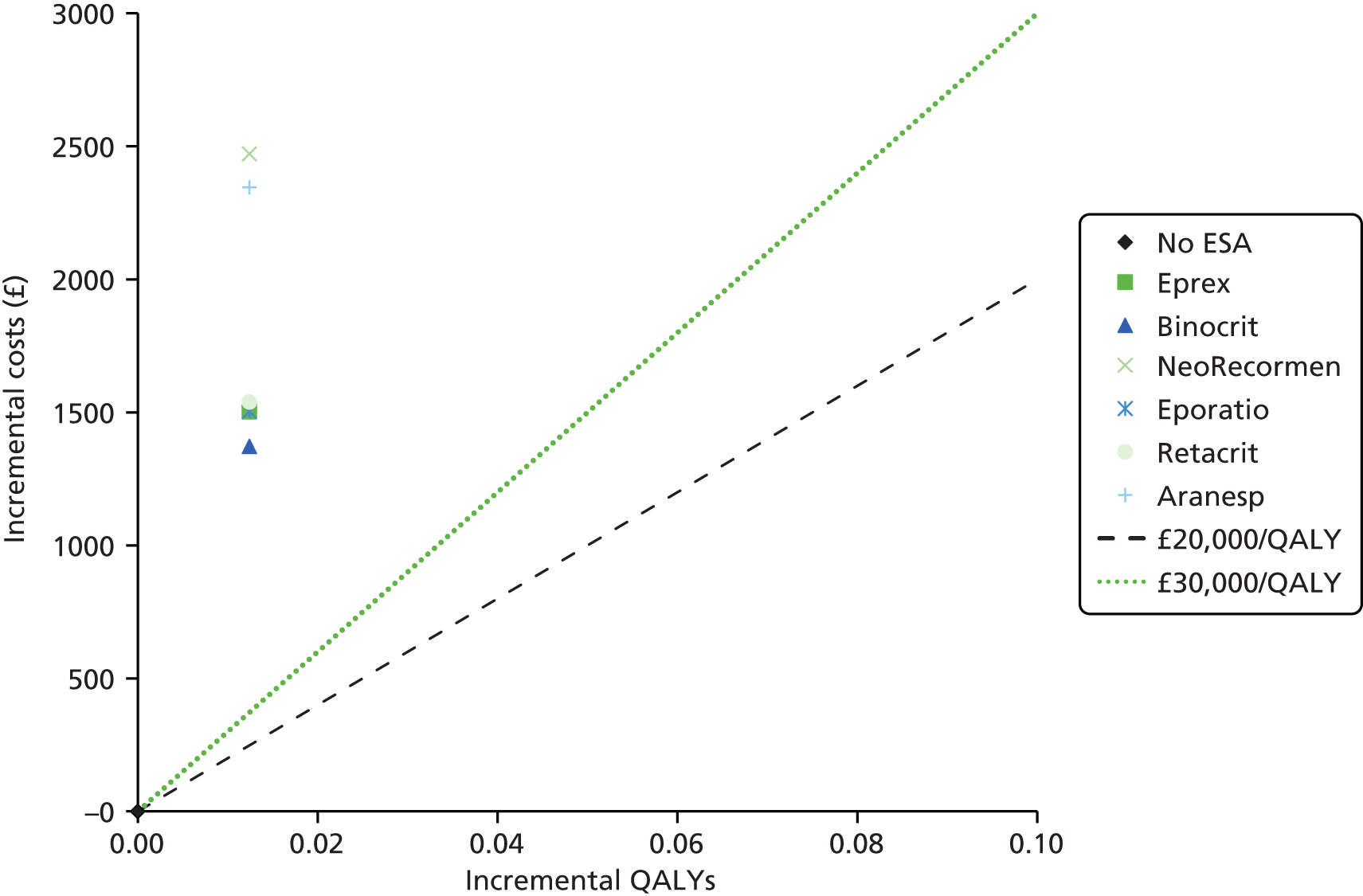
Threshold analysis of erythropoiesis-stimulating agent costs: scenario analysis 1
As part of this scenario analysis, which assumes no impact on OS, we considered what cost of ESA therapy would reduce the ICERs to below the £20,000-per-QALY threshold. As the ESA dose cost depends on both the unit cost and the size of the dose, we performed a threshold analysis on the weekly ESA cost. In the base case we see that the dose cost per week ranges from £137 to £218. By testing a range of dose costs per week and fixing all other values, for the ICER to fall below £20,000 per QALY gained in this scenario, the weekly cost of ESA therapy must fall below £32 (Table 74). As any alteration in dose would likely affect the effectiveness of the ESAs, the only variation in the base-case analysis implied by this scenario is a reduction in the unit cost of between 75% and 85%.
| Dose cost per week (£) | Total ESA cost (£) | ICER (£/QALY) |
|---|---|---|
| 30 | 360 | 17,799 |
| 31 | 372 | 18,765 |
| 32 | 384 | 19,732 |
| 33 | 396 | 20,699 |
| 34 | 408 | 21,666 |
| 35 | 420 | 22,632 |
| Minimum base-case value: 137 | 2283 | 110,477 |
(Commercial-in-confidence information has been removed). An analysis of the wholesale acquisition costs is provided separately in Scenario analysis 2: using wholesale acquisition costs, but this analysis does indicate that, for a certain cost, ESAs may be cost-effective even without a modelled survival gain.
Probabilistic sensitivity analysis: scenario analysis 1
We also performed a PSA on this scenario to see how uncertain the results are once the uncertainty around survival is removed. As the results in Table 75 show, the 95% CIs around the incremental QALYs and INHBs are much reduced compared with the base case, suggesting that a large component of the uncertainty has been removed by eliminating the uncertainty surrounding OS. This is also consistent with no ESA therapy being cost-effective at the highest cost-effectiveness threshold. The lower limit of the 95% CrI for the ICERs does not fall below £30,000 per QALY gained for any of the ICERs, suggesting that in this scenario ESAs are unlikely to be cost-effective.
| Treatment arm | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbe alfa | |
|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | |
| Deterministic ICER vs. no ESA (£/QALY) | 120,995 | 110,477 | 199,118 | 121,166 | 123,983 | 188,968 |
| Mean probabilistic ICER vs. no ESA (95% CrI) (£/QALY) | 106,007 (40,506 to > 300,000) | 96,754 (36,897 to > 300,000) | 174,193 (71,732 to > 500,000) | 104,706 (41,987 to > 300,000) | 106,745 (40,827 to > 300,000) | 166,848 (69,324 to > 500,000) |
| Incremental QALYs (95% CI) | 0.014 (0.001 to 0.027) | 0.014 (0.001 to 0.027) | 0.014 (0.001 to 0.027) | 0.014 (0.001 to 0.027) | 0.014 (0.001 to 0.027) | 0.014 (0.001 to 0.027) |
| Incremental cost (95% CI) (£) | 1504 (777 to 2232) | 1373 (695 to 2051) | 2472 (1387 to 3556) | 1486 (816 to 2156) | 1515 (787 to 2242) | 2368 (1311 to 3425) |
| INHB vs. no ESA at WTP of £20,000 per QALY (95% CI) | –0.061 (–0.100 to –0.022) | –0.054 (–0.091 to –0.018) | –0.109 (–0.165 to –0.054) | –0.060 (–0.096 to –0.024) | –0.062 (–0.100 to –0.023) | –0.104 (–0.159 to –0.050) |
Indeed, when we examine the scatterplot of the simulations (Figure 47), we see that the distribution of points along the horizontal axis is greatly reduced, both because there is no longer a QALY loss and because the QALY benefit is not spread across such a wide area. In fact, if we consider the scatterplot on the same axes as for the base-case result (Figure 48), we see a much narrower distribution of QALY estimates. Given the much smaller QALY difference estimates in this case and the same differences in costs compared with the base case, we find that 99.7% of the data points lie above the £20,000-per-QALY threshold.
FIGURE 47.
Incremental costs and QALYs by PSA simulation for scenario analysis 1.
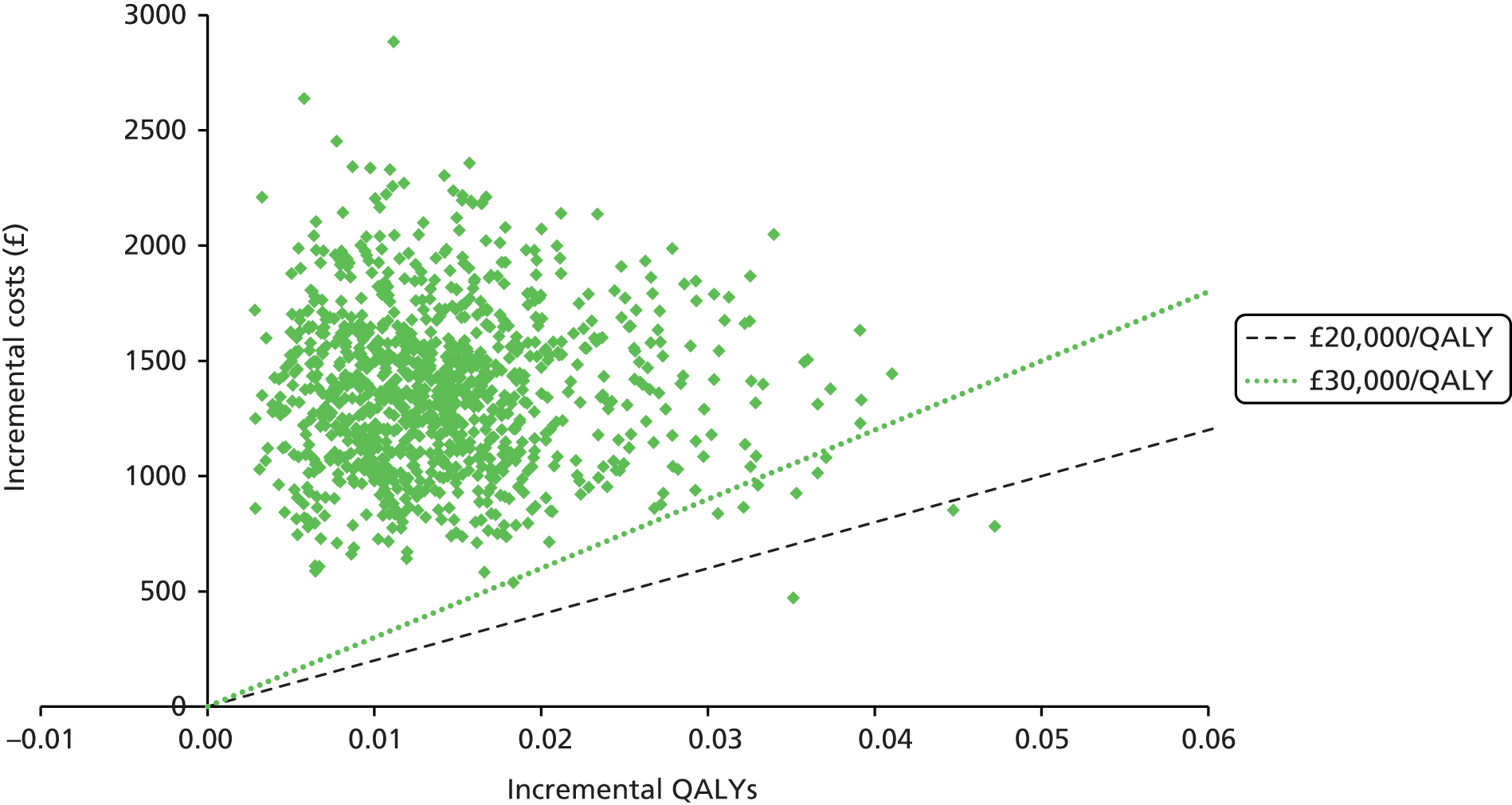
FIGURE 48.
Incremental costs and QALYs by PSA simulation for scenario analysis 1 and scaled to the axes used in the base case.
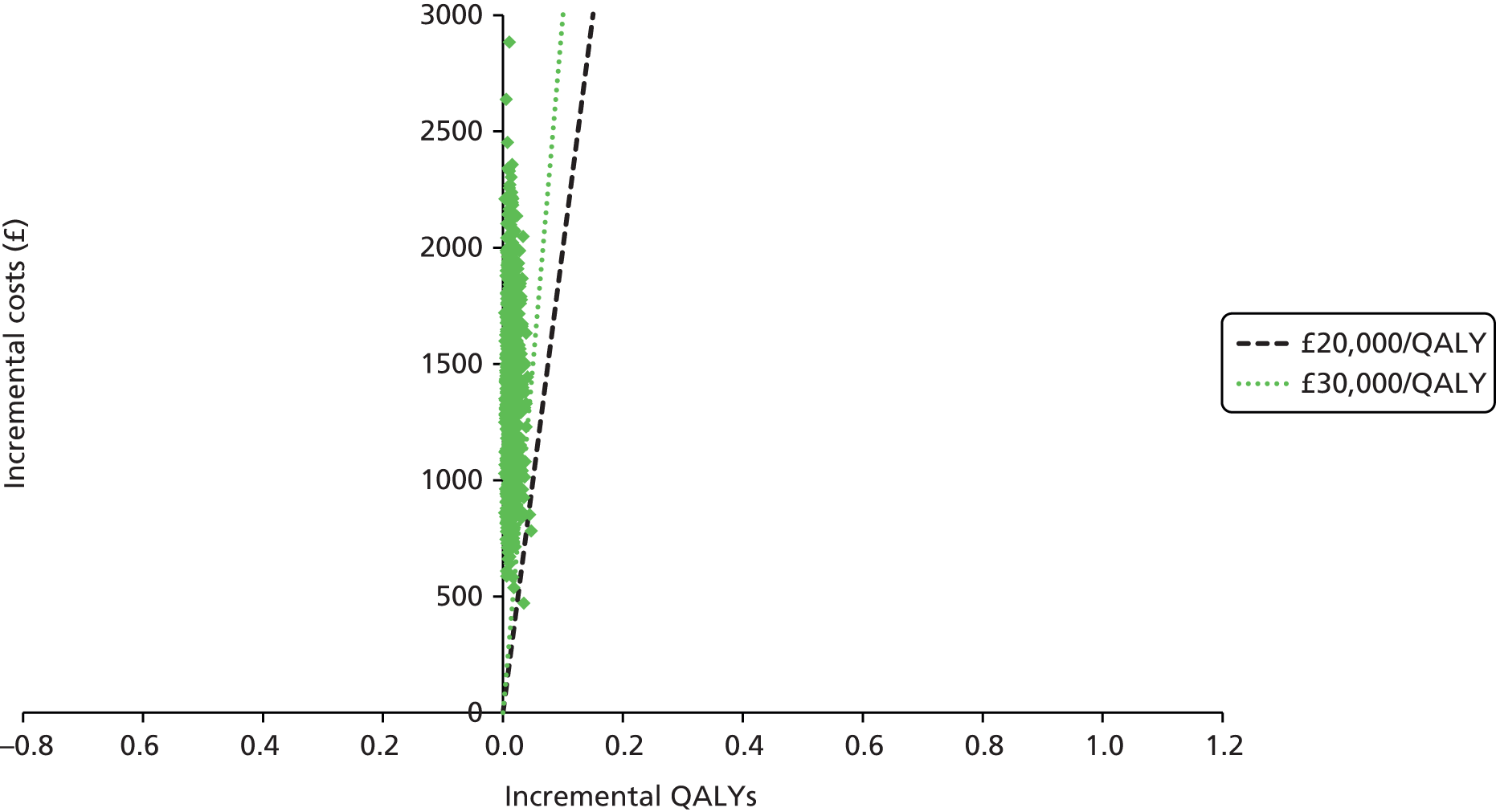
The CEACs for this scenario (Figure 49) demonstrate a much more gradual decline in the probability of cost-effectiveness for the no ESA arm, with increase in the cost-effectiveness threshold, as well as an increase in the ESA arms, compared with the base case. The ESA arms also begin to converge at a higher probability than in the base case, although this is still well below 50%. The CEAF (Figure 50) also demonstrates a much higher willingness-to-pay threshold (£100,000 per QALY) at which one of the ESAs (Binocrit) may produce a higher net health benefit compared with the no ESA arm.
FIGURE 49.
Cost-effectiveness acceptability curves for scenario analysis 1.

FIGURE 50.
Cost-effectiveness acceptability frontier for scenario analysis 1.

The results from this PSA suggest that, if ESA use is assumed to have exactly no impact on survival, the current practice of not using ESAs appears to be the most cost-effective option at a willingness-to-pay threshold of £30,000 per QALY.
Scenario analysis 2: using erythropoiesis-stimulating agent wholesale acquisition costs
Although we have partly investigated the impact of reducing the costs of the ESAs in scenario 1, we also consider it important to apply the actual costs that we have available into the model. To give a complete picture, we apply these costs both in the base case and in scenario 1, in which there is no survival benefit accounted for. This allows us to investigate the impact of these costs, regardless of the beliefs about survival.
As we did not receive any cost information for epoetin theta, epoetin theta is omitted from these results.
Scenario analysis 2a: application to the base-case results
As Table 76 shows, all costs in this scenario are greatly reduced compared with the base case and the ICERs range from (Commercial-in-confidence information has been removed) per QALY gained, depending on the ESA, in the deterministic case. As with the base case, when the averages are taken from the PSA results, we see that the ICERs are further reduced, but in either case they are all far below the willingness-to-pay threshold of £20,000 per QALY gained. Although the ICERs indicate that the most cost-effective ESA is Retacrit (having the lowest cost), the INHB PSA results indicate that the 95% CIs for INHB overlap for all ESAs, suggesting that the cost-effectiveness of the ESAs is similar. (Commercial-in-confidence information has been removed).
| Treatment arm | No ESA | Epoetin alfa | Epoetin beta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Retacrit | Aranesp | ||
| Deterministic results | ||||||
| Total cost per strategy (£) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Total incremental cost vs. no ESA (£) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Total discounted QALYs gained vs. no ESA | – | 0.0706 | 0.0706 | 0.0706 | 0.0706 | 0.0706 |
| ICER vs. no ESA (£/QALY) | – | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| ICER (£/QALY) | – | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Probabilistic results | ||||||
| Total cost per strategy (£) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Total incremental cost vs. no ESA (95% CI) (£) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
| Total discounted QALYs gained vs. no ESA (95% CI) | 0.083 (–0.251 to 0.418) | 0.083 (–0.251 to 0.418) | 0.083 (–0.251 to 0.418) | 0.083 (–0.251 to 0.418) | 0.083 (–0.251 to 0.418) | |
| ICER vs. no ESA (£/QALY) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
| INHB vs. no ESA at WTP of £20,000/QALY (95% CI) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
If we examine the PSA results for the most cost-effective ESA in this scenario (Figure 51), we see that the majority of data points lie around the origin. A summary of where the data points lie is provided in Table 77 and shows that in 26.4% of simulations ESA therapy was dominated by no ESA therapy (cost increase and QALY loss), but in 5% of cases ESA therapy dominated the no ESA arm (cost saving and QALY gain). ESAs dominate when the cost saving from a reduction in RBCT use outweighs the additional costs from ESA use. For a significant proportion of the simulations (37.1%) the cost of ESA therapy (dose and administration) is smaller than the cost saving from RBCT use, but the additional AE costs and blood test costs prevent the majority of these simulations from having an overall cost saving. Therefore, when the unit costs of the ESA are reduced, the other potential costs associated with ESA use become more important.
FIGURE 51.
Incremental costs and QALYs: PSA results for scenario analysis 2a. Commercial-in-confidence information has been removed.
| Cost | Health loss | Health gain |
|---|---|---|
| Increase | 26.4 | 65.9 |
| Saving | 2.7 | 5.0 |
The CEACs for this scenario (Figure 52) show that, at a willingness-to-pay threshold of at least £3500 per QALY, Retacrit has the highest probability of being cost-effective. Furthermore, the probability of no ESA use being cost-effective is greatly reduced for all thresholds. The CEAF (Figure 53) demonstrates that Retacrit becomes the optimal strategy at a willingness-to-pay threshold of £2000 per QALY.
FIGURE 52.
Cost-effectiveness acceptability curves: scenario analysis 2a.
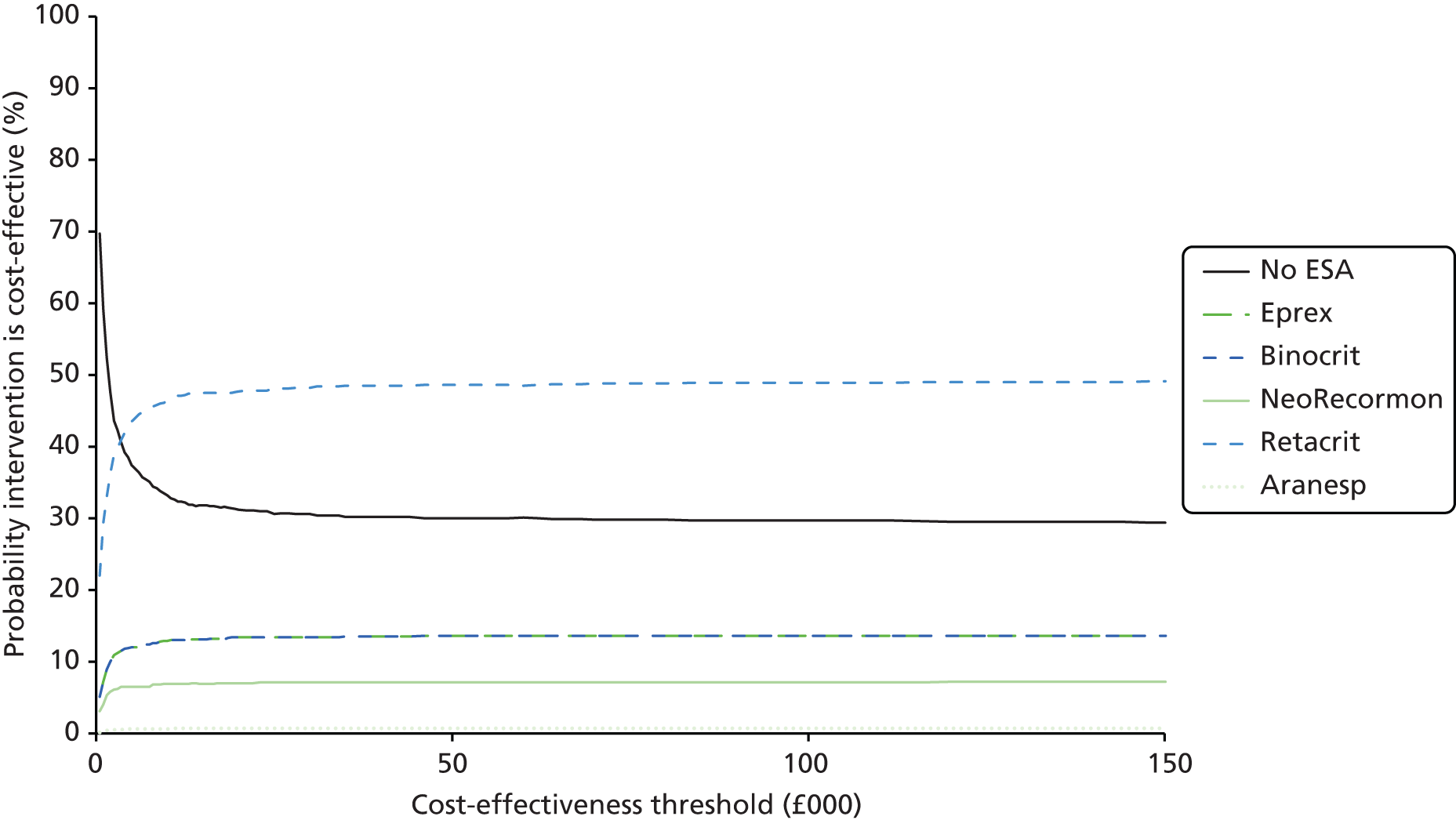
FIGURE 53.
Cost-effectiveness acceptability frontier: scenario analysis 2a.
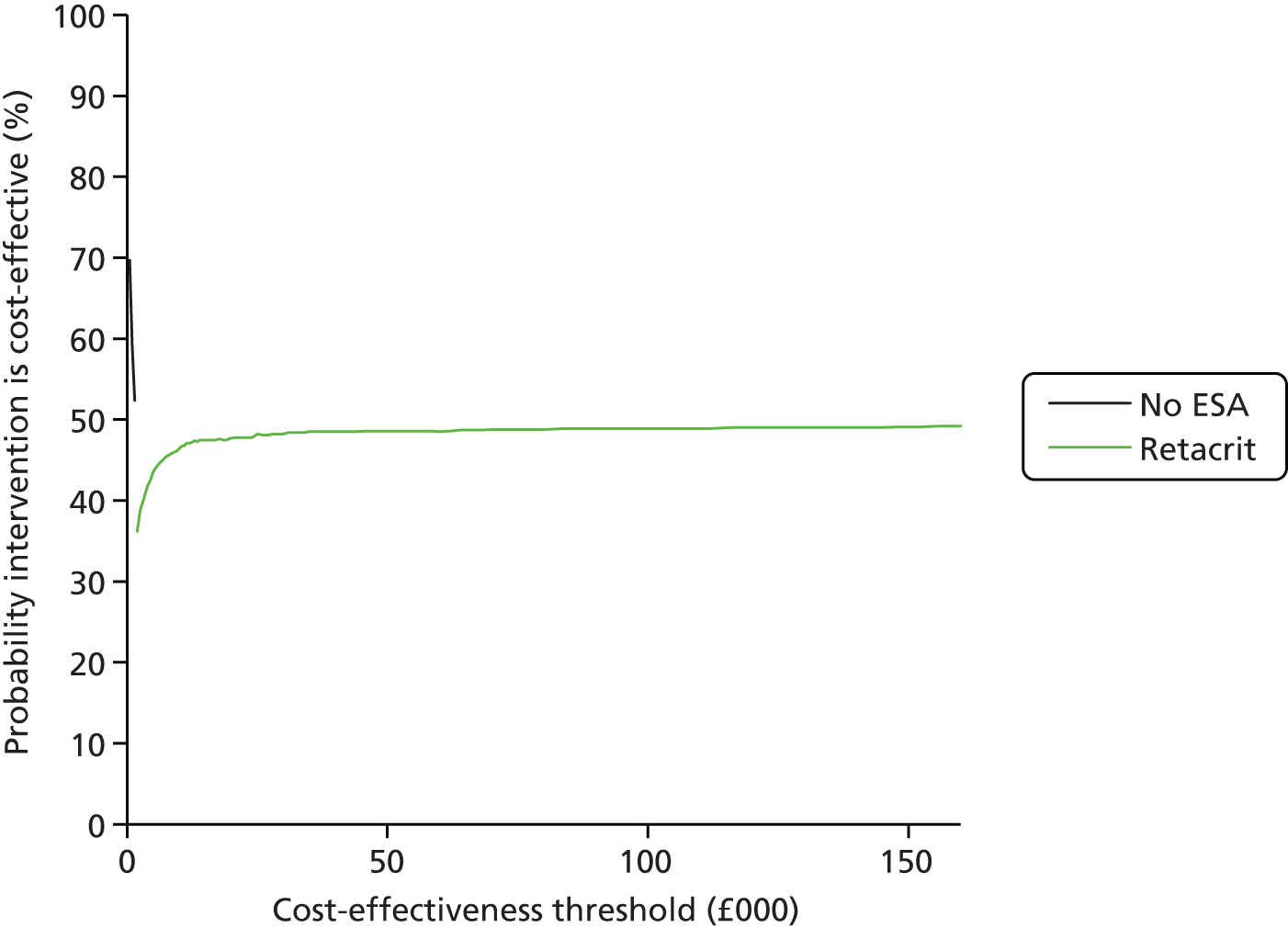
The results of this scenario suggest that the ESAs are more cost-effective than in the base case. However, the long-term QALYs are still highly uncertain and the reduction in costs makes the impact of their uncertainty more influential than in the base case. As such, the probability of ESAs being cost-effective is still uncertain.
Scenario analysis 2b: application to scenario analysis 1 results, no survival benefit
The summary results for the wholesale acquisition costs applied to scenario analysis 1, in which survival is assumed to be equal for both the ESA arm and the no ESA arm (Commercial-in-confidence information has been removed) (Table 78). As expected, the ICERs for both the deterministic and the average probabilistic results are larger than those found when the wholesale acquisition costs are applied to the base case. However, the majority of ESAs have ICERs that are <£20,000 per QALY and there is therefore an indication that, at the prices paid for the ESAs, they could be cost-effective, regardless of the survival benefit. However, the upper limit of the 95% CrIs is still > £30,000 per QALY for all ESAs. It is noted that there is still much crossover in the INHB 95% CIs, suggesting that it is difficult to choose between the ESAs.
As with scenario analysis 1, when the survival component is removed from the model, the distribution of data points is greatly reduced (Figure 54). In this scenario, 8.2% of simulations are both cost saving and QALY increasing, but 34.4% still lie above the £20,000-per-QALY threshold. As before, for a significant proportion of the simulations (36.7%), the cost of ESA therapy (dose and administration) is smaller than the cost saving from a reduction in RBCT use, but the additional AE costs and blood test costs prevent the majority of these simulations from having an overall cost saving. This value is slightly different from that when wholesale acquisition costs are applied in the base case because of a different run of the simulations.
FIGURE 54.
Incremental costs and QALYs: PSA results for scenario analysis 2b. Commercial-in-confidence information has been removed.
The CEACs for this scenario (Figure 55) appear to be quite different from those in the previous scenarios. At a willingness-to-pay threshold of £75,000 per QALY, all ESAs have a higher probability of being cost-effective than no ESA. Furthermore, the probability that Retacrit is cost-effective at a willingness-to-pay threshold of £20,000 per QALY is > 50%, higher than in the other scenarios.
FIGURE 55.
Cost-effectiveness acceptability curves: scenario analysis 2b.

The CEAF for this scenario indicates that Retacrit is the most optimal choice at a willingness-to-pay threshold of £9500 per QALY (Figure 56).
FIGURE 56.
Cost-effectiveness acceptability frontier for scenario analysis 2b.
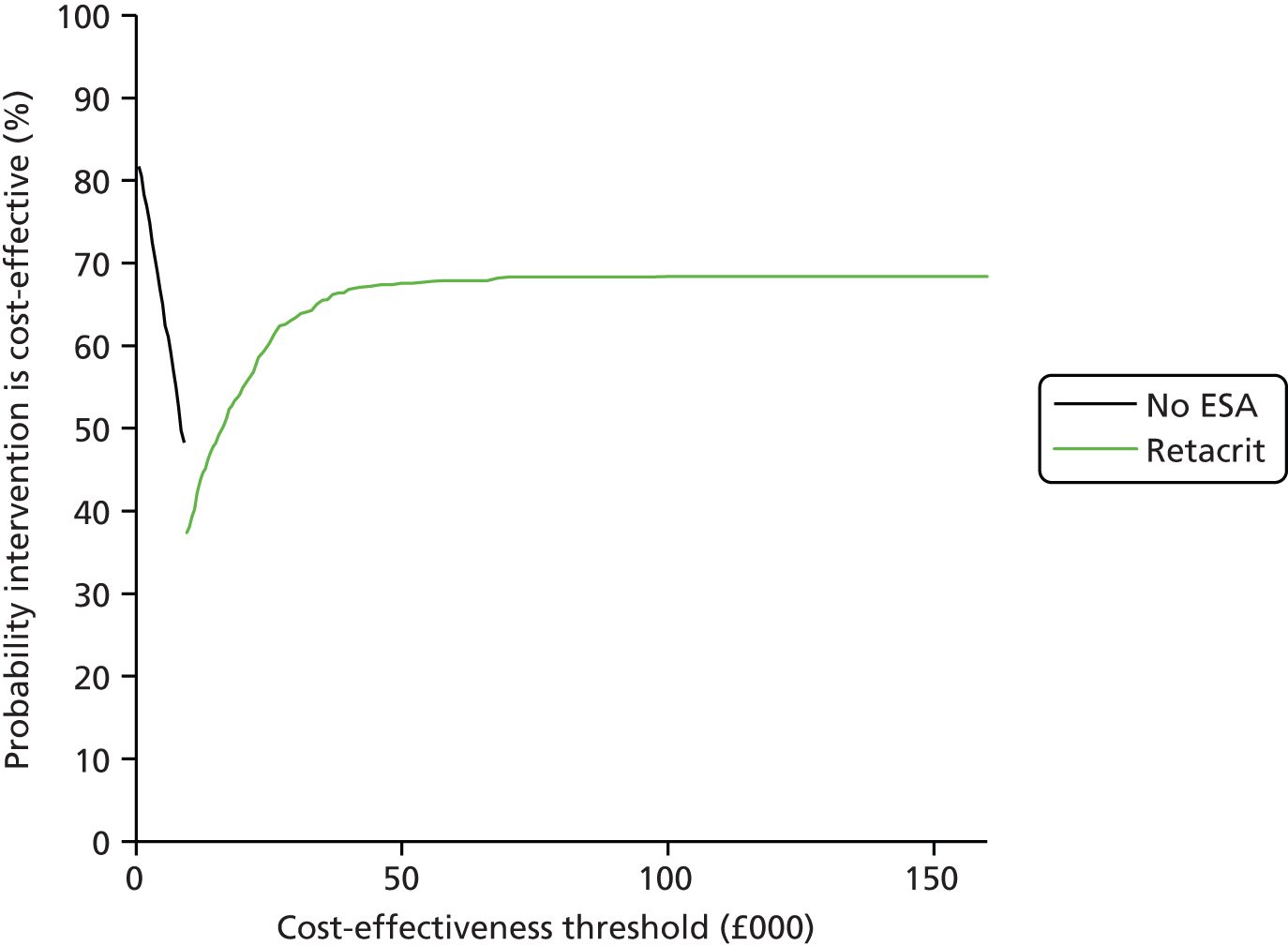
The overall results of this scenario demonstrate that, when the ESA prices are lowered to those that are available currently to the NHS, the cost-effectiveness of ESAs appears to be much improved, regardless of whether survival is accounted for in the model. If survival is assumed to be equal in both the ESA arm and the no ESA arm then ESA therapy being cost-effective seems plausible; however, it is equally plausible that it is not cost-effective. Even if survival is not assumed to be equal, there is still a significant proportion of the simulations in which a survival disbenefit occurs and, as such, there is the possibility of ESAs being dominated by current practice.
Scenario analysis 3: subgroup of randomised controlled trials based on the initial haemoglobin level
Deterministic analysis: scenario analysis 3
We next present a scenario analysis that uses only data from RCTs in which only patients with an inclusion Hb level of ≤ 11 g/dl are included. This subgroup was used in an attempt to get closer to the licensed indication while maintaining a large enough subgroup of studies to gain estimates for all parameters. Summary estimates are presented in Table 79. The input parameters for this scenario are provided in Appendix 19 and described in Methods. One of the main changes in input parameters in this scenario analysis is a higher estimated gain in OS from the use of ESA therapy [HR reduced from 0.97 (95% CI 0.83 to 1.13) to 0.91 (95% CI 0.70 to 1.20)]
| Treatment arm | No ESA | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | ||
| Total cost per strategy (£) | 956 | 2396 | 2266 | 3350 | 2394 | 2434 | 3222 |
| Total incremental cost vs. no ESA (£) | – | 1441 | 1310 | 2394 | 1438 | 1478 | 2267 |
| Total discounted QALYs gained vs. no ESA | – | 0.1040 | 0.1040 | 0.1040 | 0.1040 | 0.1040 | 0.1040 |
| ICER vs. no ESA (£/QALY) | – | 13,849 | 12,593 | 23,013 | 13,826 | 14,206 | 21,785 |
| ICER (£/QALY) | – | Dominated by Binocrit | £12,593 | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit |
| INHB vs. no ESA at WTP of £20,000/QALY | – | 0.032 | 0.039 | –0.016 | 0.032 | 0.030 | –0.009 |
| INHB vs. no ESA at WTP of £30,000/QALY | – | 0.056 | 0.060 | 0.024 | 0.056 | 0.055 | 0.028 |
As Table 79 shows, the ICERs for the ESA strategies compared with no ESA use in the deterministic base case range from £12,593 to £23,013 per QALY gained. Four of these ICERs are below the NICE-designated willingness-to-pay threshold of £20,000 per QALY and two (NeoRecormen and Aranesp) lie above this threshold but below the upper £30,000 per QALY willingness-to-pay threshold. These results are represented pictorially in Figure 57. As with our base case, the ICERs cover a range around the £20,000-per-QALY threshold, therefore we felt that it was important to demonstrate the impact of the uncertainty in these ICERs and quantify the probability that these ICERs represent the true results.
FIGURE 57.
Incremental costs and QALYs for scenario analysis 3.

When these ICERs are translated into INHBs compared with no ESA use, the INHB ranges from –0.016 to 0.039 QALYs at a willingness-to-pay threshold of £20,000 per QALY and from 0.024 to 0.060 QALYs at a willingness-to-pay threshold of £30,000 per QALY, depending on the ESA. This represents a net health benefit from the use of ESAs for all ESAs at the £30,000 per QALY willingness-to-pay threshold and a net health benefit for most ESAs at the £20,000-per-QALY threshold.
When the ESA strategies are compared with each other, we find that, as in the base case, they are dominated by the ESA with the lowest total ESA cost (in this case Binocrit). This is mostly expected as the parameters in the base case that are altered for this scenario analysis are those relevant to effectiveness; however, there are also differences in the mean weekly dose of each ESA compared with the base case, which alters costs.
Figure 58 shows that the incremental costs in this scenario are similar to those in the PenTAG base case (see Figure 40). One difference from the base case is that the incremental QALYs gained during normalisation are similar to the incremental QALYs gained during treatment (Figure 59). This happens because of a lower starting Hb level and a longer normalisation period in this scenario compared with the base case. The total incremental QALYs gained in the short term, however, are broadly similar to those in the base case (0.011 as opposed to 0.012). The total QALYs gained as a result of the mortality difference are also higher than in the base case (0.093 as opposed to 0.058). This gives a much higher overall QALY gain of 0.104 (compared with 0.071 in the base case) and explains why the ICERs appear much reduced.
FIGURE 58.
Incremental costs vs. no ESA for scenario analysis 3.

FIGURE 59.
Incremental QALYs gained vs. no ESA in scenario analysis 3.

Probabilistic analysis: scenario analysis 3
We also conducted a PSA for this scenario to see what effect limiting the subgroup had on the uncertainty in the results. A summary of the average results is presented in Table 80; as in the base case, the average PSA ICERs are slightly less than the estimates from the deterministic results, but the upper limit of the CrIs is still dominated by no ESA use. Figure 60 shows that, compared with the equivalent plot for the base case, there appears to be just as much, if not more, uncertainty in this subgroup of studies, particularly in terms of QALY gains.
| Treatment arm | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | |
| Deterministic ICER vs. no ESA (£/QALY) | 13,849 | 12,593 | 23,013 | 13,826 | 14,206 | 21,785 |
| Mean probabilistic ICER vs. no ESA (95% CrI) (£/QALY) | 11,403 (1916 to Dtda) | 10,363 (1706 to Dtda) | 19,157 (3473 to Dtda) | 11,339 (1888 to Dtda) | 11,573 (1929 to Dtda) | 17,745 (3351 to Dtda) |
| Incremental QALYs (95% CI) | 0.126 (–0.276 to 0.528) | 0.126 (–0.276 to 0.528) | 0.126 (–0.276 to 0.528) | 0.126 (–0.276 to 0.528) | 0.126 (–0.276 to 0.528) | 0.126 (–0.276 to 0.528) |
| Incremental cost (95% CI) (£) | 1436 (701 to 2171) | 1305 (620 to 1991) | 2413 (1305 to 3521) | 1428 (729 to 2128) | 1458 (722 to 2193) | 2235 (1193 to 3277) |
| INHB vs. no ESA at WTP of £20,000 per QALY (95% CI) | 0.054 (–0.350 to 0.458) | 0.061 (–0.343 to 0.465) | 0.005 (–0.399 to 0.409) | 0.055 (–0.350 to 0.459) | 0.053 (–0.352 to 0.458) | 0.014 (–0.390 to 0.418) |
FIGURE 60.
Incremental costs and QALYs of PSA simulations for scenario analysis 3.
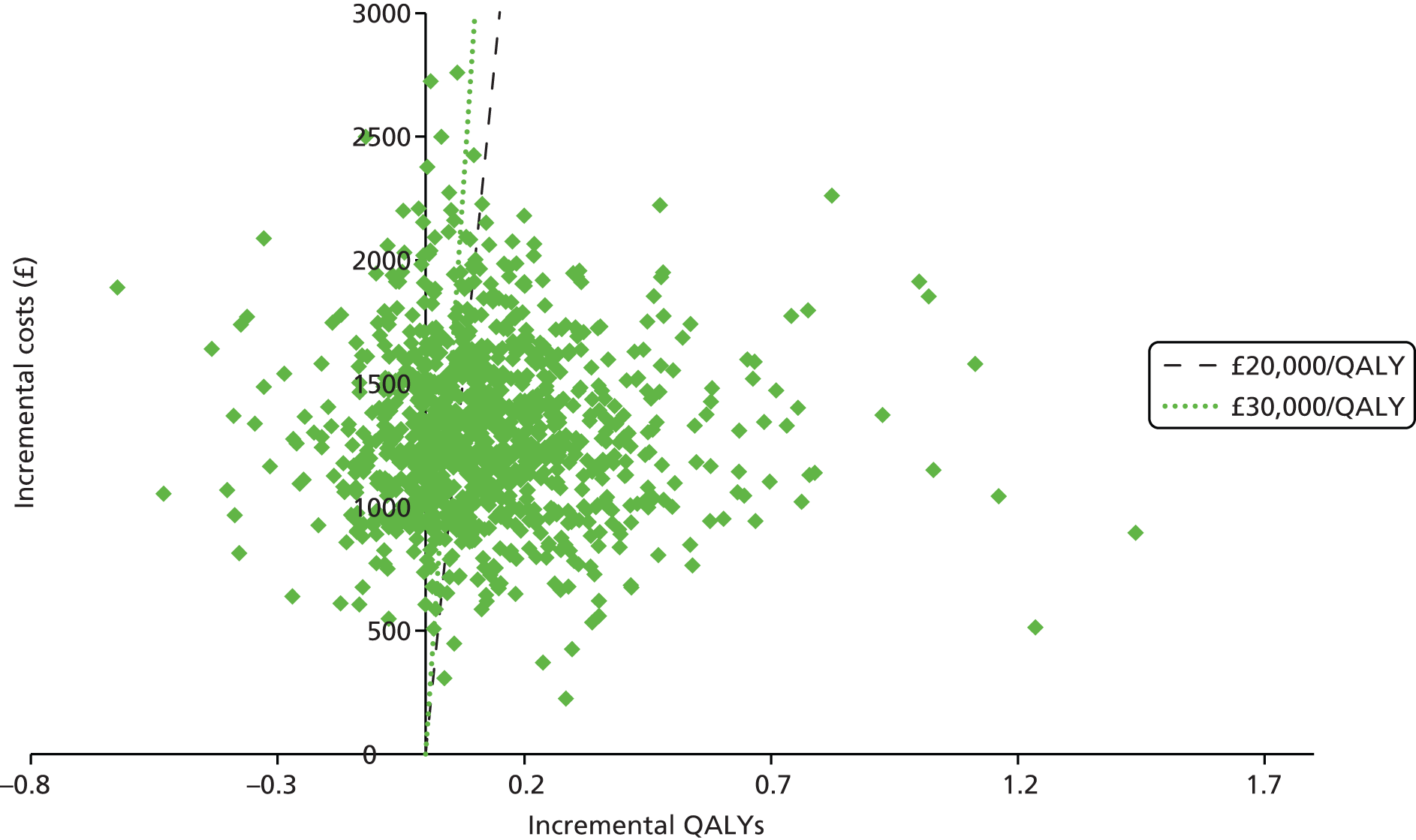
As with the base case, we see that a significant proportion (23.1%) of data points incur an increase in cost with a loss in QALYs and another 19.4% have a health gain but are above the £20,000-per-QALY threshold. In total, 3.2% of simulations had an overall QALY gain but a survival disbenefit, with QALYs gained only in the short term. The percentage of simulations in which ESAs are within the region of being cost-effective at £20,000 per QALY is 57.5%, which is slightly larger than in the base case.
The CEACs for this PSA (Figure 61) suggest that Binocrit may have a higher probability of being cost-effective than no ESA use at a willingness-to-pay threshold of £42,000 per QALY, but that this probability is still fairly low (< 35%). All other ESA arms have a probability of being cost-effective of < 25% for any willingness-to-pay threshold < £150,000 per QALY.
FIGURE 61.
Cost-effectiveness acceptability curves for scenario analysis 3.

The CEAF (Figure 62) suggests that, at a willingness-to-pay threshold of at least £10,500 per QALY, Binocrit appears to be the most favourable option.
FIGURE 62.
Cost-effectiveness acceptability frontier for scenario analysis 3.

This scenario suggests that ESAs may be more cost-effective when patients are limited to this subgroup. This could be interpreted as an indication that, when ESAs are used within their correct licensing, they appear to be more cost-effective. However, the PSA clearly shows that there is still a high level of uncertainty and this should be kept in mind when considering these results. In particular, in 23.1% of the simulations for Binocrit, ESA use is dominated, having fewer QALYs and higher costs than no ESA use.
Overall survival scenario analyses
As described in Modelling approach, we performed three scenario analyses exploring the structural assumptions with regard to OS.
Hazard ratio applying for only 3 years
Overall survival in the base case is estimated for both arms using an exponential distribution, with the OS in the control arm estimated by synthesising outcomes from included RCTs and the OS in the ESA arm estimated by applying a constant HR to the survival in the control arm, with the HR taken from the systematic review of clinical effectiveness evidence. As follow-up is limited for trials, we explored the impact of assuming that the HR applies for only the first 3 years, after which patients in both arms experience the same rate of mortality. Deterministic and probabilistic results are both available in this scenario.
Deterministic analysis
The short-term costs and QALYs remain unchanged for both arms. The long-term life-years and QALYs are unchanged in the control arm, but in the ESA arm they are slightly reduced:
-
the mean incremental undiscounted life-years are estimated at 0.028, reduced from 0.091 in the base case
-
the mean incremental discounted long-term QALYs are estimated at 0.0198, reduced from 0.0582 in the base case
-
the mean incremental discounted total QALYs are estimated at 0.0322, reduced from 0.0706 in the base case.
These results suggest that 66% of the long-term QALY gain and 54% of the total QALY gain in the base case are accrued over 3 years after ESA treatment.
The reduction in QALY gain means that cost-effectiveness is worsened and now none of the ESAs are cost-effective at a willingness-to-pay threshold of £20,000 or £30,000 per QALY (Table 81). Binocrit remains the most cost-effective of the ESAs, but its ICER is estimated at £42,584 per QALY.
| Treatment arm | No ESA | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | ||
| Total cost per strategy (£) | 912 | 2414 | 2283 | 3384 | 2416 | 2451 | 3258 |
| Total incremental cost vs. no ESA (£) | – | 1502 | 1371 | 2472 | 1504 | 1539 | 2346 |
| Total discounted QALYs gained vs. no ESA | – | 0.0322 | 0.0322 | 0.0322 | 0.0322 | 0.0322 | 0.0322 |
| ICER vs. no ESA (£/QALY) | – | 46,638 | 42,584 | 76,751 | 46,704 | 47,790 | 72,839 |
| ICER (£/QALY) | – | Dominated by Binocrit | 42,584 | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit |
| INHB vs. no ESA at WTP of £20,000/QALY | – | –0.043 | –0.036 | –0.091 | –0.043 | –0.045 | –0.085 |
| INHB vs. no ESA at WTP of £30,000/QALY | – | –0.018 | –0.014 | –0.050 | –0.018 | –0.019 | –0.046 |
Probabilistic analysis
The PSA scatterplot for the incremental cost-effectiveness of Binocrit compared with no ESA is given in Figure 63 (with the same axis scales as presented in the base case). The scatterplot shows that a considerable amount of uncertainty about the incremental QALYs has been eliminated by assuming a HR of 1 from 3 years onwards. Even so, approximately one in four simulations predicts an overall QALY loss for patients receiving ESA therapy because of an adverse impact on OS in the first 3 years (Table 82 and Figure 63).
FIGURE 63.
Incremental costs and QALYs: PSA results for the scenario analysis in which the OS HR applies for only 3 years.

| Cost | Health loss | Health gain |
|---|---|---|
| Increase | 24.9 | 75.1 |
| Saving | 0 | 0 |
The summary probabilistic cost-effectiveness results are shown in Table 83; the ICERs are not changed significantly from the deterministic results, with the ICER for Binocrit remaining the lowest compared with no ESA therapy at £39,836 per QALY.
| Treatment arm | No ESA | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | ||
| Total cost per strategy (£) | 941 | 2440 | 2308 | 3398 | 2436 | 2475 | 3293 |
| Total incremental cost vs. no ESA (£) | – | 1499 | 1367 | 2457 | 1495 | 1534 | 2352 |
| Total discounted QALYs gained vs. no ESA | – | 0.0343 | 0.0343 | 0.0343 | 0.0343 | 0.0343 | 0.0343 |
| ICER vs. no ESA (95% CrI) (£/QALY) | – | 43,667 (9371 to Dtda) | 39,836 (8523 to Dtda) | £71,589 (15,002 to Dtda) | 43,568 (9422 to Dtda) | 44,689 (8795 to Dtda) | 68,532 (14,287 to Dtda) |
| INHB vs. no ESA at WTP of £20,000/QALY | – | –0.041 | –0.034 | –0.089 | –0.040 | –0.042 | –0.083 |
| INHB vs. no ESA at WTP of £30,000/QALY | – | –0.016 | –0.011 | –0.048 | –0.016 | –0.017 | –0.044 |
Cost-effectiveness acceptability curves and the CEAF are provided in Figures 64 and 65, respectively. The CEAF switches from no ESA to Binocrit at a willingness-to-pay threshold of ≥ £40,000 per QALY.
FIGURE 64.
Cost-effectiveness acceptability curves for the scenario analysis in which the OS HR applies for only 3 years.

FIGURE 65.
Cost-effectiveness acceptability frontier for the scenario analysis in which the OS HR applies for only 3 years.

Weibull curve fitted to the overall survival in Untch and colleagues
In this scenario the HR from the systematic review of clinical effectiveness is maintained as in the base case, but the OS in the control arm is set to a Weibull distribution fitted to the OS in Untch and colleagues. 80 Deterministic and probabilistic results are given for this scenario analysis, although the OS in the control arm is not varied in the PSA.
Deterministic results
The short-term costs and QALYs remain unchanged for both arms. The long-term incremental life-years and QALYs are increased:
-
the mean incremental undiscounted life-years are estimated at 0.156, increased from 0.091 in the base case
-
the mean incremental discounted long-term QALYs are estimated at 0.0807, increased from 0.0582 in the base case
-
the mean incremental discounted total QALYs are estimated at 0.0931, increased from 0.0706 in the base case.
The increase in QALY gain means that cost-effectiveness is improved and now four of the ESAs are cost-effective at a willingness-to-pay threshold of £20,000 per QALY (Table 84). Binocrit remains the most cost-effective of the ESAs, with an ICER estimated at £14,726 per QALY.
| Treatment arm | No ESA | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | ||
| Total cost per strategy (£) | 912 | 2414 | 2283 | 3384 | 2416 | 2451 | 3258 |
| Total incremental cost vs. no ESA (£) | – | 1502 | 1371 | 2472 | 1504 | 1539 | 2346 |
| Total discounted QALYs gained vs. no ESA | – | 0.0931 | 0.0931 | 0.0931 | 0.0931 | 0.0931 | 0.0931 |
| ICER vs. no ESA (£/QALY) | – | 16,128 | 14,726 | 26,541 | 16,150 | 16,526 | 25,188 |
| ICER (£/QALY) | – | Dominated by Binocrit | 14,726 | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit |
| INHB vs. no ESA at WTP of £20,000/QALY | – | 0.018 | 0.025 | –0.030 | 0.018 | 0.016 | –0.024 |
| INHB vs. no ESA at WTP of £30,000/QALY | – | 0.043 | 0.047 | 0.011 | 0.043 | 0.042 | 0.015 |
Probabilistic analysis
The PSA scatterplot for the incremental cost-effectiveness of Binocrit compared with no ESA is given in Figure 66 (with the same axis scales as presented in the base case). The scatterplot shows that changing the baseline OS function does not have a significant impact on the considerable uncertainty regarding the incremental QALYs (Table 85).
| Cost | Health loss | Health gain |
|---|---|---|
| Increase | 30.7 | 69.3 |
| Saving | 0 | 0 |
The summary probabilistic cost-effectiveness results are shown in Table 86; the ICERs are not changed significantly from the deterministic results, with the ICER for Binocrit remaining the lowest compared with no ESA therapy, at £12,649 per QALY.
| Treatment arm | No ESA | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | ||
| Total cost per strategy (£) | 932 | 2438 | 2307 | 3379 | 2428 | 2471 | 3279 |
| Total incremental cost vs. no ESA (£) | – | 1506 | 1375 | 2447 | 1496 | 1539 | 2347 |
| Total discounted QALYs gained vs. no ESA | – | 0.1087 | 0.1087 | 0.1087 | 0.1087 | 0.1087 | 0.1087 |
| ICER vs. no ESA (95% CrI) (£/QALY) | – | 13,857 (2297 to Dtd) | 12,649 (2091 to Dtd) | 22,516 (4004 to Dtd) | 13,767 (2243 to Dtd) | 14,160 (2319 to Dtd) | 21,590 (3573 to Dtd) |
| INHB vs. no ESA at WTP of £20,000/QALY (95% CI) | – | 0.033 (−0.400 to 0.467) | 0.040 (−0.393 to 0.473) | −0.014 (−0.449 to 0.422) | 0.034 (−0.402 to 0.470) | 0.032 (−0.405 to 0.468) | −0.009 (−0.447 to 0.429) |
| INHB vs. no ESA at WTP of £30,000/QALY (95% CI) | – | 0.058 (−0.374 to 0.491) | 0.063 (−0.370 to 0.496) | 0.027 (−0.407 to 0.461) | 0.059 (−0.376 to 0.494) | 0.057 (−0.378 to 0.492) | 0.030 (−0.405 to 0.466) |
Cost-effectiveness acceptability curves and the CEAF are provided in Figures 67 and 68, respectively The CEAF switches from no ESA to Binocrit at a willingness-to-pay threshold of ≥ £13,000 per QALY. It is notable that the no ESA arm is cost-effective in more simulations than any of the ESAs at cost-effectiveness thresholds up to £150,000 per QALY.
Log-normal curves fitted to Littlewood and colleagues70
Kaplan–Meier curves from Littlewood and colleagues70 suggest that neither exponential nor Weibull curves would fit OS in the population accurately. A log-normal distribution was shown graphically to give a reasonable fit and so for this scenario analysis separate log-normal survival functions were fitted to the Kaplan–Meier curves for the two arms and extrapolated. The HR from the systematic review of clinical effectiveness cannot be applied, as the log-normal distribution allows only accelerated failure time modelling and not proportional hazards.
No probabilistic results are presented for this scenario as we did not attempt to quantify the uncertainty in the fitting of the log-normal distributions, but given that the improved survival in the ESA arm was not statistically significant (p = 0.13 by the log-rank test) it is likely that uncertainty would remain in the cost-effectiveness results, given how critical OS is to cost-effectiveness.
The short-term costs and QALYs remain unchanged for both arms. The long-term incremental life-years and QALYs are significantly increased:
-
the mean incremental undiscounted life-years are estimated at 0.471, increased from 0.091 years in the base case
-
the mean incremental discounted long-term QALYs are estimated at 0.3087, increased from 0.0582 in the base case
-
the mean incremental discounted total QALYs are estimated at 0.3211, increased from 0.0706 in the base case.
The increase in QALY gain means that cost-effectiveness is improved and now all of the ESAs are cost-effective at a threshold of £20,000 per QALY (Table 87). Binocrit remains the most cost-effective of the ESAs, with an ICER estimated at £4271 per QALY.
| Treatment arm | No ESA | Epoetin alfa | Epoetin beta | Epoetin theta | Epoetin zeta | Darbepoetin alfa | |
|---|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | ||
| Total cost per strategy (£) | 912 | 2414 | 2283 | 3384 | 2416 | 2451 | 3258 |
| Total incremental cost vs. no ESA (£) | – | 1502 | 1371 | 2472 | 1504 | 1539 | 2346 |
| Total discounted QALYs gained vs. no ESA | – | 0.3211 | 0.3211 | 0.3211 | 0.3211 | 0.3211 | 0.3211 |
| ICER vs. no ESA (£/QALY) | – | 4678 | 4271 | 7698 | 4684 | 4793 | 7306 |
| ICER (£/QALY) | – | Dominated by Binocrit | 4271 | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit | Dominated by Binocrit |
| INHB vs. no ESA at WTP of £20,000/QALY | – | 0.246 | 0.253 | 0.197 | 0.246 | 0.244 | 0.204 |
| INHB vs. no ESA at WTP of £30,000/QALY | – | 0.271 | 0.275 | 0.239 | 0.271 | 0.270 | 0.243 |
It is worth noting that the study by Littlewood and colleagues70 is just one study out of a number of studies to which we could have fitted OS curves, including two74,80 that suggested a survival disbenefit from ESA use (although not statistically significant) and two62,79 that showed no clear effect on survival of ESA therapy. We are not presenting this scenario as an alternative base case but simply demonstrating the very significant impact that assumptions about OS have on cost-effectiveness.
Univariate sensitivity analysis
As the scenario analyses examine in depth the impact of ESA costs and OS, as well as the overall uncertainty in the model parameters, the univariate sensitivity analysis is used to investigate particular aspects identified or not covered by the PSA. A summary of these univariate sensitivity analyses is provided in Table 88.
| Parameter | Value in base case | Sensitivity analysis alternative values | ICERs vs. no ESA (£/QALY) | |||||
|---|---|---|---|---|---|---|---|---|
| Eprex | Binocrit | NeoRecormen | Eporatio | Retacrit | Aranesp | |||
| Base case | 21,279 | 19,429 | 35,018 | 21,309 | 21,804 | 33,233 | ||
| Long-term costs | £0 | £20,000/year | 42,877 | 41,027 | 56,616 | 42,907 | 43,402 | 54,831 |
| Utility associated with increase in Hb level of 1 g/dl | 0.028 | 0.009 | 24,162 | 22,062 | 39,763 | 24,196 | 24,759 | 37,736 |
| 0.016 | 23,013 | 21,013 | 37,872 | 23,046 | 23,582 | 35,942 | ||
| 0.060 | 17,718 | 16,177 | 29,157 | 17,743 | 18,155 | 27,671 | ||
| ESA dosing schedule | Once per week, all ESAs | DA Q3W | 21,279 | 19,429 | 35,018 | 21,309 | 21,804 | 32,308 |
| EA TIW | 24,053 | 22,204 | 35,018 | 21,309 | 21,804 | 33,233 | ||
| EB TIW | 21,279 | 19,429 | 37,792 | 21,309 | 21,804 | 33,233 | ||
| EB 7 times/week | 21,279 | 19,429 | 43,342 | 21,309 | 21,804 | 33,233 | ||
| EZ TIW | 21,279 | 19,429 | 35,018 | 21,309 | 24,579 | 33,233 | ||
| ESA administration | 43.1%a and 16.3%b nurse, 40.6% self administered | 25% nurse, 75% self administered | 20,519 | 18,669 | 34,258 | 20,549 | 21,045 | 32,473 |
| RBCT appointment cost | £688 | £344 | 22,849 | 20,999 | 36,588 | 22,879 | 23,375 | 34,803 |
| £1376 | 18,138 | 16,288 | 31,877 | 18,168 | 18,663 | 30,092 | ||
| AE costs | ||||||||
| Thromboembolic event | £1243 | £621 | 21,144 | 19,294 | 34,883 | 21,174 | 21,670 | 33,098 |
| £2486 | 21,548 | 19,698 | 35,287 | 21,578 | 22,074 | 33,502 | ||
| Hypertension | £826 | £413 | 21,143 | 19,294 | 34,882 | 21,173 | 21,669 | 33,097 |
| £1652 | 21,549 | 19,700 | 35,288 | 21,579 | 22,075 | 33,503 | ||
| Thrombocytopenia | £744 | £372 | 21,302 | 19,453 | 35,041 | 21,332 | 21,828 | 33,257 |
| £1488 | 21,231 | 19,381 | 34,970 | 21,261 | 21,757 | 33,185 | ||
| Duration of ESA treatment | 12 weeks | 24 weeks | 41,108 | 37,796 | 65,710 | 41,162 | 42,049 | 62,514 |
| Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | ||
Long-term costs
As discussed in Methods, long-term costs are not accounted for in the base case of the model, partly as the difference in costs between arms was problematic given the range of cancers included and partly because to set an equal annual cost in both arms would disadvantage any arm with a survival benefit. Therefore, this sensitivity analysis is not supposed to be an account of true costs, as it is not unexpected that patients with a survival benefit would have different cancer treatment costs and that these costs may even be reduced. Instead, the long-term annual costs are set to an arbitrary £20,000 (regardless of ESA use) to demonstrate how this value disadvantages the ESAs in the base case. Indeed in this analysis, the additional long-term costs increase the ICERs of all ESAs to > £30,000 per QALY gained.
Utility associated with an increase in haemoglobin level of 1 g/dl
A range of values for the utility associated with Hb level was investigated in the methods section. PenTAG’s chosen base-case value was based on a cancer population and was transformed to the EQ-5D, as preferred by NICE. In the sensitivity analysis, the original SF-6D value (0.009), the EQ-5D value identified from CKD patients (0.016) and the original value from Wilson and colleagues2 (0.060) were used as alternatives.
As the model is quite sensitive to changes in QALYs, by reducing the utility to 0.009 or 0.016 the ICERs of the ESAs increase, with the effect that they all lie above the £20,000-per-QALY threshold, with the most cost-effective ICERs calculated as £22,062 per QALY gained for a short-term utility of 0.009 (equal to a short-term gain of 0.004 QALYs) and £21,013 per QALY gained for a short-term utility of 0.016 (equal to a short-term gain of 0.007 QALYs). Increasing the utility to 0.06, as in the case of the TA142 model,2 increased the short-term QALY gain to 0.027 QALYs and reduced the ICERs with the result that all ESAs have an ICER of > £30,000 per QALY compared with no ESA.
Erythropoietin-stimulating agent dosing schedule
The licensed doses for ESAs can be given on different schedules. In the base case this was set to once per week, as this was in line both with what licensing allows and with what occurred most frequently in the RCTs. However, previous assessments, including TA142, assumed that doses would be given three times a week. 2,106–108,145 As such, we explored the alternative dosing schedules for each of the ESAs, as applicable.
Darbepoetin alfa has the option of being given once every 3 weeks, reducing its total administration cost from £98 to £33. This had a fairly minor impact on the ICER for Aranesp, reducing it from £33,200 per QALY gained to £32,300 per QALY gained (rounded to the nearest hundred). As none of the other ESAs was affected, their ICERs remained the same as in the base case.
Epoetin alfa and epoetin zeta can be given three times a week, increasing their total administration cost to £294. In the scenario in which epoetin alfa is increased to a three times a week schedule and the other ESAs are held as in the base case, Binocrit no longer has an ICER below £20,000 per QALY gained and Eporatio becomes the least costly ESA. When epoetin zeta is assumed to have a three times a week schedule, the ICER for Retacrit increases from £21,800 to £24,600 per QALY gained (rounded to the nearest hundred).
Epoetin beta can be given either once weekly or have the dose divided and administered three to seven times per week. This dosing schedule gives ICERs for NeoRecormen of between £37,792 and £43,342 per QALY gained, an increase of 8–24% from the base case ICER of £35,018 per QALY gained.
These results demonstrate that, even though ESA administration is a small component of the overall costs in the base case, it can have a larger impact on the results if the ESAs are to be administered more than once a week. This is particularly true if the ICERs lie close to the threshold: in the base case Binocrit is cost-effective at a threshold of £20,000 per QALY but, if ESAs are administered three times per week as opposed to once, Binocrit no longer appears to be cost-effective at this threshold.
Although this sensitivity analysis demonstrates the impact of changes to the administration costs, it is possible that, when the dosing schedule is altered in practice, how the dose is administered may also change. For example, it is possible that if a dose was required daily, patients could more frequently be expected to self-administer.
Erythropoietin-stimulating agent administration
As was discussed in Methods, who administers ESAs is not entirely agreed by clinicians. There may be many reasons for this, including factors such as patient ability/preferences and chemotherapy schedule. As such, our base case reflects an average view across the clinicians’ opinions available.
In this analysis we examined the possibility that ESAs would be given on a schedule closer to that of CKD patients, being administered 25% of the time by a nurse and 75% of the time by self-administering. If this approach was adopted for cancer patients, the overall cost of ESA administration would be reduced to £44 and the ICERs for all ESAs would be reduced so that, in particular, Eprex and Eporatio would have ICERs of £20,500 per QALY gained (rounded to the nearest hundred), very close to the £20,000-per-QALY threshold.
Red blood cell transfusion appointment costs
The cost of the transfusion appointment was taken from a very old source172 and uprated to 2014/15 prices. As such, the true cost may vary considerably. Therefore, this sensitivity analysis attempted to investigate the impact that altering the cost of a RBCT appointment has, by halving and then doubling the cost.
When the cost of a RBCT appointment is halved to £344, the ICERs for the ESAs compared with no ESA increase by around £1500 each, resulting in all lying above the willingness-to-pay threshold of £20,000 per QALY. This is a result of the reduced RBCT cost making the cost saving from ESA use smaller. Similarly, when the cost of the RBCT appointment is doubled to £1376, the cost saving between the ESA arm and the no ESA arm increases and the ICERs are reduced, so that four of the ESAs have ICERs below the £20,000-per-QALY threshold.
It therefore appears that the cost of a RBCT appointment can have an effect on the ICERs, particularly if they are close to the willingness-to-pay threshold in the base case.
Adverse event costs
Another cost parameter for which limited data were found in the base case was the cost of AEs and, as such, this sensitivity analysis investigates the impact of changing these costs. As with the RBCT costs, they were halved and doubled to demonstrate the impact rather than to demonstrate alternative values.
The results in Table 88 show that altering individual AE costs has very little impact on the overall cost-effectiveness results, with ICERs altering by only a few hundred pounds in each case. As in the base case, the no ESA arm is more likely to suffer from thrombocytopenia; the ICERs alter differently for thrombocytopenia compared with the other AEs, with a reduction in the cost of thrombocytopenia causing an increase in the ICERs for the ESAs compared with no ESA use.
The reason that changing the costs of AE costs appears to have such a small impact is because the costs of AEs are very similar for patients in both arms. This is primarily driven by the lack of information on the number of each type of AE that occurs in each arm and their severity, as both are likely to affect the overall cost of the AEs. In the model the arms are assumed to have the same level of severity of AEs and the model costs for one instance of any individual AE rather than multiple instances. As such, further information is required to properly evaluate the effect of a change in AE costs on the overall results.
Duration of erythropoiesis-stimulating agent treatment
Another parameter that varied quite substantially in the RCTs was the duration of ESA therapy. As such, we assessed the impact of increasing the duration of therapy to 24 weeks, as described in Methods.
Doubling the duration of treatment increases both the short-term QALYs (from 0.0124 in the base case to 0.0207 when the duration is doubled) and the costs. This is because doubling the duration doubles both the QALYs gained on ESA treatment and the costs directly associated with ESA use. However, as treatment duration is a small component of the overall QALYs gained but a large component of the overall costs, the ICERs for the ESAs compared with no ESA use are greatly increased when the treatment duration increases. All of the ICERs are above the £30,000-per-QALY threshold, with the lowest ICER being £37,796 per QALY gained compared with no ESA use.
Of note, when the ESA costs are reduced to those of their wholesale acquisition costs, the ICERs all fall below the £20,000-per-QALY threshold.
Comparison with Wilson and colleagues2
As we are conducting an update of the HTA review by Wilson and colleagues,2 we attempted to compare our results with those previously reported. Table 89 demonstrates that there is a large difference between the most cost-effective ESA in the PenTAG base case and the most cost-effective ESA in the base case reported in TA142,2 with ICERs of £19,429 and £150,342 per QALY, respectively.
| PenTAG base case (Binocrit) | TA142 base case2 | PenTAG model, adapted to use TA142 base-case parameters | |
|---|---|---|---|
| Short-term QALY gain vs. no ESA | 0.012 | 0.030 | 0.059 |
| Long-term QALY gain vs. no ESA | 0.058 | 0.000 | 0.000 |
| Incremental QALYs, ESA vs. no ESA | 0.071 | 0.030 | 0.059 |
| Incremental cost (£), ESA vs. no ESA | 1371 | 4450 | 6448 |
| ICER (£/QALY), ESA vs. no ESA | 19,429 | 150,342 | 109,055 |
To attempt to account for these differences, we adjusted the PenTAG model to incorporate parameters used in the TA142 report. 2 Parameters that we were able to identify and enter into the model included baseline and normalised Hb levels, utility associated with Hb level and long-term utility, mean survival, the OS HR, ESA weekly cost (dose and administration), transfusion costs and probabilities, AE costs and probabilities and ESA treatment duration. The values for these parameters are reported in Appendix 19. Preferably, we would have updated the TA142 model to match our parameters, but no model copy was available. We attempted to discover whether the differences in the results were caused primarily by model structure or by the updated parameters. To make the results of our adjusted model comparable, costs were kept as reported in the TA142 monograph. 2
Unfortunately, as only limited outputs were reported in Wilson and colleagues,2 the comparison of the models is also restricted. Certain parameters in the PenTAG model, particularly those crucial to short-term utility, could not be accounted for using the parameters given in the TA142 monograph. 2 One specific example of this is the mean difference in Hb levels between treatment arms as a proportion of the difference at the end of the trial, which, as a parameter in our model, identifies when the benefit to the Hb level from ESA use occurs. This was obviously not parameterised in the Wilson model, as the Hb level changes were modelled mechanistically. Also, the normalisation rate was only approximated to 0.2 g/dl in the TA142 model but had to be entered as exactly 0.2 g/dl in the PenTAG model.
Table 89 shows that, when the PenTAG model is adapted to use the parameters reported in TA142, the ICER increases to £110,680 per QALY gained, a value much closer to that in the original TA142 model results.
By comparing the adjusted PenTAG model and the TA142 base case, we see that the altered PenTAG model has both a larger QALY gain and a larger cost than those reported in TA142. We believe that this is mostly the result of not being able to substitute all of the parameters from TA142 into the PenTAG model or having to use parameters from the TA142 model in a different manner from which they were intended, based on underlying model assumptions.
One particular example of this is the use of the maximum duration of ESA treatment from TA142 of 24 weeks because an average value could not be calculated, which would result in both higher costs and higher QALYs in the PenTAG model than those reported in TA142. Furthermore, the weekly cost is taken as a maximum and does not reflect the dose reduction that could occur in the TA142 model. This occurs because the PenTAG model accounts for dose changes by setting the input parameter for mean dose to reflect the ITT basis, whereas the Wilson and colleagues2 model approaches this mechanistically, adjusting the dose depending on the health state. Unfortunately, no information on the size of the initial dose was reported in the TA142 model, therefore we do not know what size dose the cost is equivalent to. Comparing our weekly ESA dose cost (£126–218) with the weekly ESA cost calculated using the TA142 values (£251) we can see that there is a slight increase in cost per week for ESAs in TA142, which is partly because of a change in the unit cost but primarily because of the difference in size of the dose.
As previously discussed, one parameter that greatly affects the QALY gains in the short term in the PenTAG model, but which was not available from the TA142 monograph, is the mean difference in Hb levels between treatment arms as a proportion of the difference at the end of the trial. The larger this value is, the larger the benefits of ESAs and the greater the QALY gain in the short term. In the PenTAG base case this value is set to 81%. By varying this parameter we can see how easily this alters the results (Table 90). We do not use this analysis to find the appropriate value for this parameter (as there are other factors affecting the QALY gain, some of which are also linked to cost results) but merely to show that this is one parameter in the PenTAG model that could not be altered based on the information given in the TA142 monograph but which is likely to be different and, as such, has an impact on the ICERs.
| Value (%) | Total QALY gain | ICER (£/QALY) |
|---|---|---|
| Base case: 81 | 0.059 | 109,055 |
| 10 | 0.027 | 235,633 |
| 20 | 0.032 | 202,366 |
| 30 | 0.036 | 177,331 |
| 40 | 0.041 | 157,808 |
| 50 | 0.045 | 142,157 |
| 60 | 0.050 | 129,330 |
| 70 | 0.054 | 118,627 |
| 80 | 0.059 | 109,560 |
| 90 | 0.063 | 101,780 |
| 100 | 0.068 | 95,032 |
We also note that the measure for the utility gain in the short term for the TA142 model was elicited using the time trade-off method, whereas the values in the PenTAG model have been converted to the EQ-5D. The utility value from TA142 could not be converted to the EQ-5D, which also accounts for some of the differences in QALYs between the PenTAG adapted model and the TA142 model. This is discussed in more detail in Utilities.
We believe that these model differences account for the difference between the adjusted PenTAG model and the TA142 base case.
Notwithstanding the comparison difficulties just described, by comparing the adjusted PenTAG results with the PenTAG base case we can identify which updated parameters have had the most impact:
-
The short-term QALY gain is reduced in the PenTAG base case as a result of our much reduced utility gain associated with increases in Hb level.
-
The PenTAG base case also includes a long-term QALY gain from a modelled favourable impact on survival, which was not assumed in the TA142 modelling. The OS HR was 1 in the TA142 model but is 0.97 in the PenTAG base case, based on a pooled estimate from 18 studies identified as more closely reflecting current licensed usage (i.e. patients receiving chemotherapy and receiving the licensed start dose).
-
The costs of receiving ESAs are also greatly reduced in the PenTAG base case as a result of a reduction in the costs of ESAs (in terms of both unit costs and dose reduction), a reduction in the number of administrations of ESAs and a reduced time frame over which ESAs are administered.
Key points
-
The cost of ESA therapy is the largest cost component in any ESA arm and the cost of RBCT is the largest cost component for no ESA use.
-
The costs of AEs, RBCTs and additional blood tests are equal across ESA arms.
-
When ESAs are used there are cost savings in terms of a reduction in the number of RBCTs.
-
ICERs for ESA treatment compared with no ESA treatment range from £19,429 to £35,018 per QALY gained in the deterministic case. The PSA gave ICERs that were lower than in the deterministic base case (£14,724–27,226 per QALY gained). On average, 0.092 (95% CI –0.264 to 0.447) QALYs were gained for ESA treatment compared with no ESA treatment. The incremental cost for the most cost-effective ESA (Binocrit; epoetin alfa) was £1349 (95% CI £710 to £1,987). The ICER for Binocrit had a 95% CrI that was dominated by no ESA use (fewer QALYs and higher costs) at its upper interval, with a lower value of £2332 per QALY gained. In total, 36% of simulations from the PSA had an OS loss, with 31.4% of simulations having an overall QALY loss.
-
Three important scenario analyses considered were (1) setting the OS HR to exactly 1, so that survival is the same for both patients on ESA therapy and those not on ESA therapy; (2) setting ESA costs to wholesale acquisition costs in an attempt to establish the real costs to the NHS; and (3) setting the OS HR to exactly 1 and the ESA costs to wholesale acquisition costs.
-
In the first of these scenarios, when survival is assumed to be equal for both treatment arms, the QALY gain is greatly reduced [as well as the 95% CI 0.014 (0.001 to 0.027)] compared with the base case. The most cost-effective ESA achieved an ICER of £96,754 per QALY gained (95% CrI £36,897 to > £300,000 per QALY gained) in the PSA.
-
In the second scenario, when wholesale acquisition costs were used there was a reduction in the expected mean ICER from the PSA to (Commercial-in-confidence information has been removed) (for the least costly ESA – Retacrit) per QALY gained. However, in this scenario the 95% CrI went from ESA dominating, with more QALYs and lower costs than no ESA use, to being dominated by the no ESA arm.
-
In the third scenario, when survival was assumed to be equal for both treatment arms and wholesale acquisition costs were used, the expected ICER from the PSA for Retacrit is (Commercial-in-confidence information has been removed).
-
We also conducted scenario analyses on a subgroup of studies in which the initial inclusion Hb level for participants was ≤ 11 g/dl. This resulted in changes to many of the parameters; in particular the OS HR reduced to 0.91 in the deterministic results. The expected ICERs were reduced compared with the base case, but the level of uncertainty was maintained.
-
Scenario analyses were conducted on the OS modelling assumptions. Although all affected the ICERs, the most significant result showed that, when the impact of any survival benefit is included for only 3 years, ESAs appear to become much less cost-effective, with all ICERs > £30,000 per QALY.
-
Univariate sensitivity analyses were also conducted, with the most significant of these appearing to be related to the duration of ESA treatment.
Chapter 6 Discussion
Aim
The remit for this report has been to update the evidence used to inform the previous NICE guidance on ESAs for the treatment of anaemia in cancer patients, particularly as laid out in the report by the West Midlands Health Technology Assessments Centre (WMHTAC). 2 In general, they considered evidence up to 2004, which is the start date we have used for this report.
Based on the previous assessment,2 NICE guidance (TA142)1 recommended the use of ESAs in combination with intravenous iron for the treatment of CIA in women with ovarian cancer receiving platinum-based chemotherapy with symptomatic anaemia (Hb ≤ 8 g/dl). The recommendation made in TA142 did not prohibit the use of other management strategies for the treatment of CIA, for example blood transfusion. 1 In addition, guidance set out in TA142 recommended ESAs in combination with intravenous iron for people with profound CIA who cannot be given blood transfusions. 1 The ESA with the lowest acquisition cost should be used. 1
Initially, all ESAs were recommended for use at a Hb level of ≤ 11 g/dl, with target Hb levels not exceeding 13 g/dl. Following a safety review by the Pharmacovigilance Working Party at the request of the Committee for Medicinal Products for Human Use in 2008, changes were made to the SPCs for all ESAs at the EMA’s request. These changes came into effect in 2008 – after the previous guidance was issued – and included a decrease in the Hb value for treatment initiation to ≤ 10 g/dl (to either increase Hb or to prevent further decline); an amendment to the Hb target values to 10–12 g/dl; and an amendment to the Hb level for stopping treatment to > 13 g/dl.
The scope of this update review differed from that of the previous HTA review2 in respect of the population under consideration. Whereas the review conducted by Wilson and colleagues2 considered cancer-related anaemia, the population covered in the PenTAG review is narrower, being restricted to cancer patients with treatment-induced anaemia (specifically chemotherapy treatment). Similarly, the recent Cochrane review11 considered the broader population. In light of the publication of the Cochrane review, the PenTAG review aimed to include only studies evaluating ESAs as close to the licensed recommendations as possible. This was defined in the first instance, based on the start dose administered, irrespective of the other criteria specified in the licence, for example start and target Hb levels. In sensitivity analyses the definition of ‘within licence’ was tightened to (1) a licensed start dose plus an inclusion Hb level of ≤ 11 g/dl and (2) a licensed start dose plus an inclusion Hb level of ≤ 11 g/dl plus a target Hb level of ≤ 13 g/dl.
Clinical effectiveness
A total of 1515 titles/abstracts were screened. In total, 23 primary studies17,48,50–53,62–70,72–79 reported in 34 publications17,48,50–53,58–60,62–86 were judged to meet the inclusion criteria for the review. These included studies eligible for inclusion from the previous HTA review2 and more recent studies identified by the PenTAG review team. Included studies were cross-checked with the recent Cochrane review11 to ensure completeness. Only one study62 was identified that was not included in the Cochrane review;11 however, it had been included in abstract format. 33
Taken as a whole, the quality of the studies ranged from moderate to poor. For most of the trials it was difficult to make a general assessment about study quality because of omissions in study reporting. Most notably, all trials lacked clarity in the reporting of allocation methods (the procedure for randomisation and/or allocation concealment).
All of the included trials evaluated ESAs as administered in accordance with the start dose recommended in the current licence specifications. However, none of the included studies evaluated ESAs entirely within the remit of their marketing authorisation; in particular, start and target Hb levels and stopping levels were all generally higher than specified in the licence. Twelve studies17,48,50,53,63,66,69–71,73,74,77 compared ESAs plus supportive care (including transfusions) with placebo plus supportive care (including transfusions). The remaining studies51,52,62,64,65,67,68,72,75,76,78 compared ESAs plus supportive care (including transfusions) with supportive care (including transfusions) alone.
Analysis of haematological response (defined as an improvement in Hb of ≥ 2 g/dl or an increase in haematocrit of ≥ 6 percentage points) showed that there was a statistically significant difference in Hb response in favour of treatment (RR 3.29, 95% CI 2.84 to 3.81; 12 trials, n = 2228). In total, 63% (759/1213) of participants who received ESAs achieved a haematological response, compared with 18% (182/1015) of participants who did not receive ESAs. Subgroup analyses were inconclusive. This and previous analyses provide consistent evidence that ESAs reduced the requirement for RBCT by an estimated 37%. The point estimate generated in the current update is in line with previous and other systematic reviews and meta-analyses. The analysis also provides consistent evidence that ESAs reduce the average number of RBC units transfused.
We identified no evidence for a beneficial effect of ESAs on tumour response (RR 1.10, 95% CI 0.86 to 1.41; seven trials, n = 1909). The results of previous reviews with respect to survival have varied and there is much debate surrounding the impact of ESAs on survival. The HR was 0.97 (95% CI 0.83 to 1.13; 18 trials, n = 4455). Although this estimate differed from those reported by Wilson and colleagues2 and Tonia and colleagues11 (1.05, 95% CI 1.00 to 1.11; 80 trials, n = 19,003, and 1.03, 95% CI 0.83 to 1.13; 28 trials, n = 5308 respectively), there was considerable uncertainty around the estimate (statistically significant heterogeneity identified: I2 = 42.4%; p = 0.03). In addition, subgroup analyses could not identify groups at lower or higher risk. In summary, the data with respect to OS remain inconclusive.
On-study mortality was defined as death occurring up to 30 days after the active study period. Data extracted from the Cochrane review11 were available for 21 studies including 5085 participants. Analyses suggest that treatment with ESAs in patients with CIA did not have a significant effect on mortality (HR 0.86, 95% CI 0.67 to 1.11; 14 trials, n = 2967). In total, 11% (174/1586) of participants who received ESAs had died within 30 days of the active study period, compared with 12% (164/1381) of patients in the control groups.
In agreement with the Cochrane review11 there was a statistically significant difference between patients treated with ESAs and control patients when combining HRQoL parameters, although this is probably not clinically important. Univariate subgroup analyses conducted for FACT-F outcomes according to chemotherapy type, malignancy type, intervention (epoetin or darbepoetin) and study duration also showed similarly statistically significant differences between the intervention group and the control group; however, the number of included studies was small, therefore the results must be treated with caution.
All AEs were relatively rare compared with the other outcomes considered in this report. The AE with the highest rate was thrombocytopenia/haemorrhage: 6% (55/877) of participants who received ESA treatment reported thrombocytopenia/haemorrhage and 6% (54/838) of participants in the control groups reported thrombocytopenia/haemorrhage. The summary estimate from the random-effects meta-analysis for thrombocytopenia/haemorrhage in the PenTAG review was a RR of 0.93 (95% CI 0.65 to 1.34) compared with a RR of 1.21 (95% CI 1.04 to 1.42) in the Cochrane review. 11 The data are insufficient to rule out detrimental effects of ESAs. Overall, the data suggest that there is an increased risk for thromboembolic events (RR 1.46, 95% CI 1.08 to 1.99), hypertension (RR 1.80, 95% CI 1.14 to 2.85), seizures (RR 1.19, 95% CI 0.33 to 4.38) and pruritus (skin rash, irritation and pruritus were combined in the analyses) (RR 2.04, 95% CI 1.11 to 3.75), consistent with previous estimates.
Important gaps in the evidence remain with respect to survival, mortality, AEs and impact on quality of life.
Subgroup analyses
Two of the subgroups evaluated corresponded with the current NICE recommendations:1 women with ovarian cancer receiving platinum-based chemotherapy and people unable to receive a RBCT.
Only one included trial51 evaluated the use of ESAs in women with ovarian cancer; all participants received platinum-based chemotherapy. The data confirm the results from previous analyses that ESAs reduce the risk of a RBCT (RR 0.11, 95% CI 0.03 to 0.47) and improve physiological parameters, such as Hb level (Hb change WMD 1.23, 95% CI 0.48 to 1.98), but increase the risk of thromboembolic events (RR 3.70, 95% CI 0.18 to 74.51). OS was not measured in this study.
No trials were identified that evaluated people unable to receive blood transfusions. However, it is reasonable to generalise from the wider RCT pool that ESAs are likely to work in improving Hb levels in this subpopulation. It is also reasonable to believe that, if people can be supported through the period of life-threatening anaemia, their Hb level will recover; if ESAs are not allowed they run the risk of death in the absence of a RBCT.
In addition, subgroup analyses considering any type of cancer and platinum-based chemotherapy, platinum-based chemotherapy in head and neck malignancies and iron supplementation were conducted. Five trials48,51,63,64,73 evaluated the use of ESAs in people with any type of cancer receiving platinum-based chemotherapy. Results from this subgroup analysis are consistent with findings from the overall analysis for the anaemia-related outcomes, that is, an improved haematological response and a reduction in RBCT requirements, and are different from the results reported in the Cochrane review. 11 Similar to the overall analysis, the results for the malignancy-related outcomes (OS and on-study mortality) suggest that there are less detrimental effects for people with chemotherapy-induced anaemia treated with ESAs. These effects are also reflected in the decrease in the number of people experiencing thromboembolic events. However, these results should be interpreted with caution. The number of studies per subgroup is small, some of the changes are not statistically significant and the CIs remain wide. It is also important to remember that multiple testing issues arise when subgroups are tested and that the CIs presented here have not been adjusted for this.
Subgroup analyses for the use of ESAs plus iron supplementation did not identify any significant differences between groups. Use of iron supplementation varied between the studies, hindering comparison of the results. No trials were identified that considered the use of ESAs in people with head and neck malignancies receiving platinum-based chemotherapy.
The impact of administering erythropoiesis-stimulating agents ‘within licence’
In addition, post-hoc sensitivity analyses considered the impact of administering ESAs ‘closer to licence’. For the purposes of these analyses this was defined as a licensed start dose plus an inclusion Hb level of ≤ 11 g/dl and a licensed start dose plus an inclusion Hb level of ≤ 11 g/dl plus a target Hb level of ≤ 13 g/dl. It appeared that the effectiveness of some outcomes was improved when ESAs were evaluated closer to their licensed indications. Anaemia-related outcomes showed improvements consistent with previous analyses. Malignancy-related outcomes appeared to be affected by the licence application and point estimates were notably lower than those reported in previous analyses when ESAs were administered in accordance with licence recommendations (licensed start dose plus inclusion Hb level of ≤ 11 g/dl). Importantly, although the results for thromboembolic events from the PenTAG review agree with those from the Cochrane review,11 suggesting an increase in thromboembolic events in patients undergoing ESA therapy compared with control patients, the closer the studies were to the licence recommendations, the smaller the point estimates (suggesting fewer detrimental effects of ESA).
Although the evidence is uncertain, some researchers have hypothesised that anaemia in cancer patients is associated with a worse prognosis. According to Bohlius and colleagues,7 one explanation may be that, as a result of a low Hb level, tumour cells become hypoxic and are subsequently less sensitive to cytotoxic drugs, in particular oxygen-dependent chemotherapies. 9,10,193 Evidence for this, as reported in Tonia and colleagues,11 exists in studies in which tumour control and OS are improved in solid tumour patients with better tumour oxygenation. 10,12 There is also the practical implication that severe anaemia may require a dose reduction or delay of chemotherapy, subsequently leading to a poorer outcome. It is therefore plausible that efforts taken to reduce anaemia may improve tumour response and OS. 7 That said, it should be noted that Hb levels elevated to > 14 g/dl in women and > 15 g/dl in men are undesirable and may lead to increased viscosity and impaired tumour oxygenation. 186
As an intervention used to increase Hb, and by association improve prognosis, some studies actually report a detrimental effect of ESAs on survival and tumour progression. 14–20 This effect is postulated to result from the presence of erythropoietin receptors on various cancers,21,22,24,25,194 whereby the endogenously produced or exogenously administered erythropoietin promotes the proliferation and survival of erythropoietin receptor-expressing cancer cells. 7 However, controversy about the functionality of these receptors remains26–30 and there are several studies that show no effect on tumour progression for patients receiving ESAs. 17,31–33
It should be noted that the majority of studies examined in the systematic reviews by Bohlius and colleagues7 and Tonia and colleagues11 used a wide range of administration frequencies and dosages of ESAs (generally exceeding the licences), which may cause a rise in the number of AEs and a rise in mortality. This knowledge, along with the generally poor reporting and omission of data on factors such as tumour stage and method of assessment, has led to the conclusion by Tonia and colleagues11 that no clear evidence was found to either exclude or prove a tumour-promoting effect of ESAs.
Importantly, all subgroup analyses must be interpreted with caution. The number of studies per subgroup is small and the CIs remain wide. The analyses may not have the statistical power to detect the effects of licence application on the effectiveness of outcomes, if such effects exist. Furthermore, we have not sought to address multiple testing issues that arise when considering subgroups, therefore inference is not straightforward.
Cost-effectiveness
Published economic evaluations
Ten cost–utility analyses2,88,114–116,121,156 and two systematic reviews88,156 were identified by updating an existing review by Wilson and colleagues. 2 Five cost–utility analyses suggested that ESA therapy is cost-effective; these were all funded by industry116,156 or conducted by industry (submissions by Amgen Inc., Roche and Ortho Biotec, as reported by Wilson and colleagues2).
The inclusion of survival benefits was common to four favourable analyses (that by Martin and colleagues116 and the industry submissions as reported by Wilson and colleagues2), although no statistically significant survival benefit has been shown. The fifth favourable analysis156 may suffer from problems of internal validity, as it appears that the cumulative dose of epoetin alfa in the analysis was less than half that in the clinical study informing the effectiveness estimates; this would account for the lower than usual incremental drug acquisition costs.
A key assumption in almost all of the analyses was that raising Hb levels would improve HRQoL, although in no case was this assumption based on published RCT evidence using a preference-based quality-of-life measure.
A number of studies assumed a period following treatment over which Hb levels would gradually return to normal (termed normalisation), during which patients in the ESA arm would continue to accrue incremental benefits in quality of life over patients in the no ESA arm; however, no evidence for or against normalisation has been presented.
In the absence of a survival benefit the expected health gain from ESA therapy is small (up to 0.035 QALYs) and is subject to uncertainty.
Studies did not incorporate current list prices or wholesale acquisition costs, which could reduce the drug acquisition cost component of ESA therapy and improve cost-effectiveness.
Strengths and limitations of the systematic review of studies of clinical effectiveness
The systematic review of studies of clinical effectiveness was conducted by an independent experienced research team using the latest evidence and working to a prespecified protocol (registered as PROSPERO CRD42013005812). This technology assessment builds on existing secondary research and economic evaluations. However, there are some important sources of uncertainty that impact on the conclusions:
-
Relative effectiveness. We did not address the relative effectiveness of different ESAs. The lack of head-to-head RCT evidence would have been an important limitation if we had tried to do this.
-
Dose. The protocol stated that ESAs should be evaluated in accordance with their UK marketing authorisations. However, given the fact that no studies were completely aligned with their current UK marketing authorisation, we identified studies that were closest to the current UK marketing authorisations, focusing initially on the starting dose. It is important to note that beyond the start dose there were still significant differences from the current licence recommendations in the included studies. Also, we did not prespecify the criteria used to define ‘closest to the current UK authorisation’, but we did explore alternative, stricter ways of making this definition.
-
Generalisability. There may be other challenges to the applicability of the included trials, which were carried out up to 20 years ago. Chemotherapy has changed during this period, as has the quality of supportive treatment.
-
Study quality. The included trials were of variable quality but all were flawed to some degree. Most notably, all trials lacked clarity about randomisation and allocation concealment. The general problem of poor reporting of trials on this topic was greatly assisted by the recent Cochrane review. 11 The authors had gathered further information from investigators and manufacturers, which was used in the meta-analysis for the current review.
-
Heterogeneity. There is considerable unexplained statistical heterogeneity for a number of outcomes, particularly survival.
-
Publication bias. There was some evidence in both the previous review2 and the Cochrane review11 that the results from small negative trials may not be available for inclusion in the systematic reviews, suggesting the possibility of publication bias. For some outcomes in this review, for example HRQoL, this could not be further investigated because of the small number of included studies; for others, such as survival, there was continuing support for the possibility of publication bias. Industry-sponsored trials predominate.
-
Precision. Although there is an apparent wealth of RCTs, only a minority of these were included because of the desire to address effectiveness as closely as possible to current UK marketing authorisations. In consequence, 95% CIs were often wide and included values indicating no difference in effect. In addition, it is not clear whether the total number of patients in the trials included was sufficient to establish the true presence or absence of an effect, either because events are uncommon, such as AEs, or because the effect size that would be deemed to be clinically important is small, as would be the case with survival.
-
Multiple testing. Although we were aware of the possibility of spuriously positive tests for statistical significance arising because of the multiple subgroup analyses carried out, we did not formally make adjustments for this.
The limitations identified here impact on the key outcomes as follows:
-
Haematological response and numbers transfused seem to be robust estimates, with no marked heterogeneity or subgroup effects.
-
Hb change does have important heterogeneity, which may possibly indicate subgroup effects; however, analyses in this respect were inconclusive.
-
HRQoL is affected by the variability of instruments used and study quality.
-
AEs are mainly affected by the quality of information available, the variability in the definitions of individual AEs used and the width of the CIs.
-
Survival is subject to all of the limitations outlined above. Marked heterogeneity was identified for which no explanation could be provided. In addition, OS was calculated from the longest follow-up available and, as a result, there was a mix of short- and long-term studies.
Strengths and limitations of the systematic review of studies of cost-effectiveness
The systematic review of cost-effectiveness evidence was conducted by an independent research team using the latest evidence and to a prespecified protocol. Two new systematic reviews88,156 were identified, neither of which identified studies that would have been eligible for this review, but which were not included.
Limitations were identified as follows:
-
The searches were limited to English-language studies because of resource limitations.
-
Only systematic reviews and cost–utility studies were fully critically appraised and considered in the narrative synthesis.
-
Records from database searches published pre 2004 were excluded, although it was not possible to assess whether these had been screened for eligibility in the systematic review presented by Wilson and colleagues. 2 Studies using darbepoetin alfa once every 2 weeks were excluded as being out of licence, although these could have usefully contributed to the review.
Strengths and limitations of the economic modelling by Peninsula Technology Assessment Group
The PenTAG model is an independent model that is not sponsored by any of the manufacturers producing ESAs. We have used up-to-date clinical effectiveness data that have been acquired through a systemic review of current evidence. As such, although we have built on past economic analyses of ESAs, we have also been able to identify key areas where information is scarce or uncertain and, when possible, have attempted to address some of these limitations. These limitations are discussed in the following sections.
Data quality for erythropoiesis-stimulating agent dose
According to licence, the dose of ESAs can be varied in a number of situations. Doses may be escalated if patients do not achieve an adequate response or may be reduced or withdrawn if a patient’s Hb level rises at an unacceptable pace or to an unacceptable level.
We estimated the mean weekly dose for patients on an ITT basis to ensure consistency between modelled costs and benefits. The mean weekly dose was estimated by pooling estimates from a number of studies, which could improve external validity, but the individual estimates from studies typically required assumptions such as a uniform withdrawal rate. As a result, estimates from individual studies may not be accurate.
We estimated a mean weekly dose for epoetin alfa of 24,729 IU over a course of 12 weeks, resulting in a modelled cumulative dose of approximately 297,000 IU. Tonelli and colleagues88 estimate a weekly dose of 30,150 IU over a course of 15 weeks, resulting in a modelled cumulative dose of approximately 452,000 IU (52% larger than our cumulative dose). Tonelli and colleagues88 did not attempt to model dose adjustment and this, combined with the assumption of 3 weeks of extra treatment, may explain the difference.
Uncertainty in overall survival
Differing assumptions regarding OS for patients receiving and not receiving ESA therapy have a significant impact on the estimated cost-effectiveness of ESAs.
Systematic reviews of the clinical effectiveness of ESAs (including our own) have included meta-analyses of HRs for OS but, to our knowledge, the assumption of proportional hazards (which must be made when calculating HRs) has never been formally tested. Furthermore, it is likely that follow-up for a number of trials for which HRs have been estimated has been very short, therefore there is considerable uncertainty as to the effect of ESA therapy on long-term mortality. IPD have been shared with the Cochrane review group7 on this subject and these could potentially be scrutinised to address these concerns.
Even when the assumption of proportional hazards is made and the random-effects meta-analysis HR is used, there remains significant parameter and structural uncertainty.
Parameter uncertainty exists in that the CI for the OS HR is very wide (95% CI 0.83 to 1.13). There have not been many studies that are sufficiently powered to detect differences in OS for this parameter to be estimated precisely. Parameter uncertainty also exists in that the OS HR appears to be somewhat sensitive to the choice of inclusion criteria for studies. Further parameter uncertainty exists regarding the OS estimated for patients not receiving ESA therapy. This also has a significant impact on cost-effectiveness, but it is uncertain and likely to differ according to the patient population.
Structural uncertainty exists in that even when assuming proportional hazards there are a number of distributions that permit the proportional hazards assumption: exponential, Weibull and Gompertz distributions. These distributions allow for quite different mortality rates over time, but none appears to be compatible with all reported survival data.
We have demonstrated that uncertainty surrounding OS is the principal contributor to the uncertainty regarding cost-effectiveness by exploring cost-effectiveness when exactly no difference in OS is assumed. This should not be seen as advocacy of the view that there is exactly no difference in OS, as there are biologically plausible explanations for beneficial and detrimental effects of ESA therapy on OS and there is no reason to suppose that these would cancel out.
Normalisation of haemoglobin levels
Clinical expert opinion seems to be in agreement that following chemotherapy cessation Hb levels will gradually increase, potentially up to pre-chemotherapy levels. Unfortunately, we have not found any published clinical studies documenting normalisation, therefore the modelled behaviour of Hb levels during normalisation is based entirely on clinical expert opinion.
Our economic evaluation suggests that the QALY gain from normalisation accounts for approximately one-third of the short-term QALY gain and 6% of the total QALY gain estimated in the base case. This is a significant proportion of the predicted benefits to be largely based on clinical expert opinion, even though the expert opinion was at least not conflicting.
Exclusion of transfusion-dependent haemoglobin measurements
Some clinical studies (e.g. Tjulandin and colleagues48) excluded Hb measurements from certain statistical analyses if the patient had received a transfusion in the previous 28 days. The rationale for this exclusion is that transfusions are assumed to increase Hb levels temporarily and that including measurements that could be affected by transfusion could lead to biased estimates of effectiveness.
Our economic evaluation assumes that Hb outcomes reported in trials are unbiased estimates of Hb outcomes for patients in clinical practice where transfusions may be used. Transfusion costs are modelled to ‘pay’ for the transient benefits in terms of Hb level; if the impact of transfusions on Hb level is stripped from the effectiveness results then we are modelling the costs but not the benefits of transfusion. As there is greater utilisation of transfusions in patients not receiving ESA therapy, it is possible that the cost-effectiveness of ESA therapy has been overestimated.
Ideally, we would ensure that all outcomes relating to Hb levels used in the model are based on Hb levels from all patients (i.e. not excluding patients who had recently received a transfusion), but there are insufficient data reported in clinical studies to achieve this.
Ultimately, the QALY gains from short-term correction of anaemia are dwarfed by the highly uncertain impact of ESAs on OS, so small biases such as these in the estimation of short-term QALYs are unlikely to materially affect cost-effectiveness.
Haemoglobin to utility mapping
The short-term QALYs associated with anaemia require mapping of Hb levels to utility. This is a surrogate outcome and requires several assumptions. First, the relationship between Hb level and utilities is assumed to be linear. Although our review of previous studies suggests that this is appropriate for Hb levels of < 12 g/dl, the model does allow for normalisation at Hb levels > 12 g/dl in the PSA, in which this assumption of linearity no longer seems to hold. Furthermore, the review of previous studies showed that the evidence base for mapping Hb levels to utility appears to include many different measurement tools for utility (SF-6D, EQ-5D, health state vignettes, LASA), suggesting that if all studies could be mapped to the same scale then this linear relationship may not hold. However, as linear scaling was used to scale the SF-6D results to the EQ-5D, this was not a problem in our base case. To assess the impact of scaling this utility, we also conducted a sensitivity analysis using the unscaled SF-6D value and a sensitivity analysis using an unscaled EQ-5D value from a population of CKD patients. In both instances the QALY gains for ESA use were lower and the ICERs compared with no ESA use increased.
There were several additional problems with the base-case source of our utility estimate associated with a change in Hb level. Aside from having to map the reported outcomes to the EQ-5D, the patient population was restricted to female cancer patients and did not include patients on ESA therapy. This meant that the study examined the association of anaemia and utility rather than the association of anaemia correction and utility improvement, which our analysis was attempting to model. Furthermore, the study design was observational, although this appeared to be the case for most of the studies identified in our review. This does mean that the estimated relationship between utility and Hb level may be biased because of unmeasured confounding variables. As discussed in Methods, the results from Tonelli and colleagues114 suggest that this would likely bias the results in favour of the ESA arm compared with the no ESA arm. Bias may also have occurred in the mapping study of the SF-6D to the EQ-5D because of measurement error in the SF-6D values, which would result in an underestimation of the relationship between the two measures (i.e. attenuation bias).
Chemotherapy costs
The PenTAG model assumes that chemotherapy costs are equal between the groups both in the short term and in the long term, regardless of ESA use. Short-term chemotherapy costs may differ in accordance with on-study mortality or with compliance to chemotherapy treatments, whose effects are not captured in the short term. Although the review of clinical effectiveness studies did not identify any statistically significant difference between the ESA arm and the control arm for on-study mortality, the overall estimate (HR 0.87, 95% CI 0.70 to 1.09) suggests a trend in favour of improved survival with ESA therapy. There is also the possibility that ESA use may affect adherence to a chemotherapy regimen: ESA use appears to reduce the time in hospital for RBCT and this may impact patient attitudes to their treatment. However, it is difficult to speculate about the possible impact that this may have on costs as there appears to be no evidence currently with which to make any claim. Furthermore, in the long term, if an impact on OS is assumed then the chemotherapy costs are likely to differ between groups. Again, is difficult to speculate how these costs might differ, as a longer survival might mean a longer follow-up and larger chemotherapy costs, a better prognosis and therefore fewer chemotherapy costs or a different approach to treatment. There may also be a follow-on cost difference according to the effects of chemotherapy adherence in the short term. Without a clear clinical understanding of the impact of ESAs on survival and patient preferences, it is difficult to address how chemotherapy costs may alter, which is why they are assumed to be equal in both arms for the base case.
Adverse events
Adverse event rates associated with ESAs are also highly uncertain. The level of severity and specificity of each AE is not well reported. The model specifically does not include rash or seizure as an AE, even though they are reported in the clinical effectiveness systematic review, as these cover a wide, non-specific symptom base. The model included thromboembolic events, hypertension and thrombocytopenia, which cover a more specific symptom base, but these are still not well defined within or across the studies in the review of clinical effectiveness. As such, the review of clinical effectiveness included all definitions of AEs at all levels of severity. This makes it problematic to assign either costs or disutilities to these AEs in the model. Base-case costs were extracted from the NHS reference costs 2012–13 for events likely to fall into the categories of AEs, but these costs are averages from a wide variety of scenarios and as such are highly uncertain. Our sensitivity analyses (doubling and halving the costs) did not appear to make a significant difference to the results, but this assumes that the underlying event costs for AEs are the same in both arms. The model identifies only the proportion of patients who had at least one AE, regardless of severity or number. As such, the costs in the model reflect only one AE and do not account for the possibility that AEs may be more severe in one arm than the other. It is therefore probable that the unit cost for an AE is less likely to have an impact on the overall costs than the number of events or the cost according to severity.
Assigning utilities to the AEs is even more problematic, in terms of both estimating the utility and estimating the time that the disutility should apply for. As such, the model does not account for utility loss associated with AEs and the disbenefit of AEs is reflected only in their costs. Given the sensitivity of the model to changes in QALYs, this could have a significant impact on the overall results. The findings from the clinical effectiveness review were mostly in favour of the control arm: thromboembolic events and hypertension occurred more frequently in patients on ESA therapy, but thrombocytopenia appeared to be more common in the control arm. As such, the addition of AE utilities into the model would likely worsen the cost-effectiveness of ESAs. However, this situation would also lead to the slightly unusual result that the group with the highest risk of AEs has a better survival outcome. This could be explained by the lack of detail on AEs (the higher risk may not actually correspond to the more severe AEs) or by the possible spurious nature of the survival benefit. Again, without a clear clinical understanding of the possible difference in OS, it is difficult to speculate.
Other considerations
The base-case cost for each of the ESAs may not be representative of the actual cost currently paid by individual organisations within the NHS. We therefore used the data collected on wholesale acquisition prices in a sensitivity analysis, with the caveat that these prices cannot be guaranteed.
The cost of administering ESAs was not adjusted in the model for missed doses, which therefore gives an increased cost for patients in the ESA arm. The sensitivity analysis in which alternative dosing schedules were considered does not address this issue directly, but does demonstrate that altering these costs does not have a big impact on the model. Similarly, although it appears that there is debate among clinicians over how ESAs are administered or who should administer them, our results demonstrate that administering ESAs in a similar way to how they are administered for CKD patients has little impact on the overall results.
There is also uncertainty over the implications of other tests that may occur during ESA use. Some clinicians advise that additional blood tests should be carried out and one of the manufacturer submissions from the previous HTA review2 costed for additional blood pressure checks, but other clinicians believe that no additional tests are required as patients will be under fairly high surveillance during their cancer treatment. However, the impact of this cost seems minimal and the base-case results of the model seem fairly insensitive to changes in this cost.
The model also does not include AEs associated with RBCTs. However, we believe this risk to be minimal and the consequences are not easily defined or accounted for.
The cost of RBCTs has been updated from a particularly old source, making it unlikely to be representative of current costs. Without current information to better inform this cost, we altered it in sensitivity analyses to show the potential impact that changing this cost may have. Again, the results demonstrate that the model is not overly sensitive to this cost, particularly if the cost is reduced.
The model also assumed that the number of RBC units per transfusion is equal in both arms, as we found no evidence to inform a difference between arms. This may not be an accurate representation of the actual number of RBCs per transfusion. If the number of units transfused per transfusion is less for the ESA arm than for the no ESA arm, then the number of transfusions for the ESA arm will increase and the cost-effectiveness of the ESAs will be reduced.
Chapter 7 Assessment of factors relevant to the NHS and other parties
Existing safety concerns
When seeking clinical experts to advise us in this assessment we found that most relevant clinicians (i.e. oncologists, haematologists and gynaecologists) did not use ESA therapy in their clinical practice. This was generally because of concerns about their safety and effectiveness (OS), in addition to restrictions set by the previous NICE guidance (TA142). 1
As this assessment is unlikely to have reduced any safety concerns, it is relevant to the NHS that many clinicians appear to have judged that the potential risks of ESAs outweigh their potential benefits.
Current usage
It is difficult to assess how frequently ESA therapy is used within the indication of CIA because prescription records do not routinely link medication with indication and ESA therapy is widely used in individuals with CKD. Some indirect evidence of the use of ESA therapy for CIA is available from recorded costs for ESAs, which are categorised by cost centre (in particular, oncology and haematology).
We were provided with data (South East England Specialist Pharmacy Services, 3 October 2013, personal communication) detailing how much had been spent on erythropoietin and darbepoetin alfa by hospital trusts (anonymised) in the East of England. These data were provided for the haematology and oncology cost centres. The oncology cost centre would be unlikely to include CKD patients but would not necessarily include all patients with CIA. The haematology cost centre could include CKD patients. By including only the haematology and oncology cost centres, 87.4% of ESA expenditure was excluded, although the proportion varied according to hospital trust (Figure 69), which suggests that trusts may record ESA prescriptions differently. Total ESA expenditure is highly variable but appears to be somewhat correlated with the size of population served (Figure 70). This correlation disappears when only haematology and oncology are considered (Figure 71). This is suggestive of significant variability in current usage, consistent with the experience that many clinicians do not use ESAs because of safety concerns, although data quality is low and interpretation challenging.
FIGURE 69.
Total ESA expenditure for hospital trusts in the East of England.
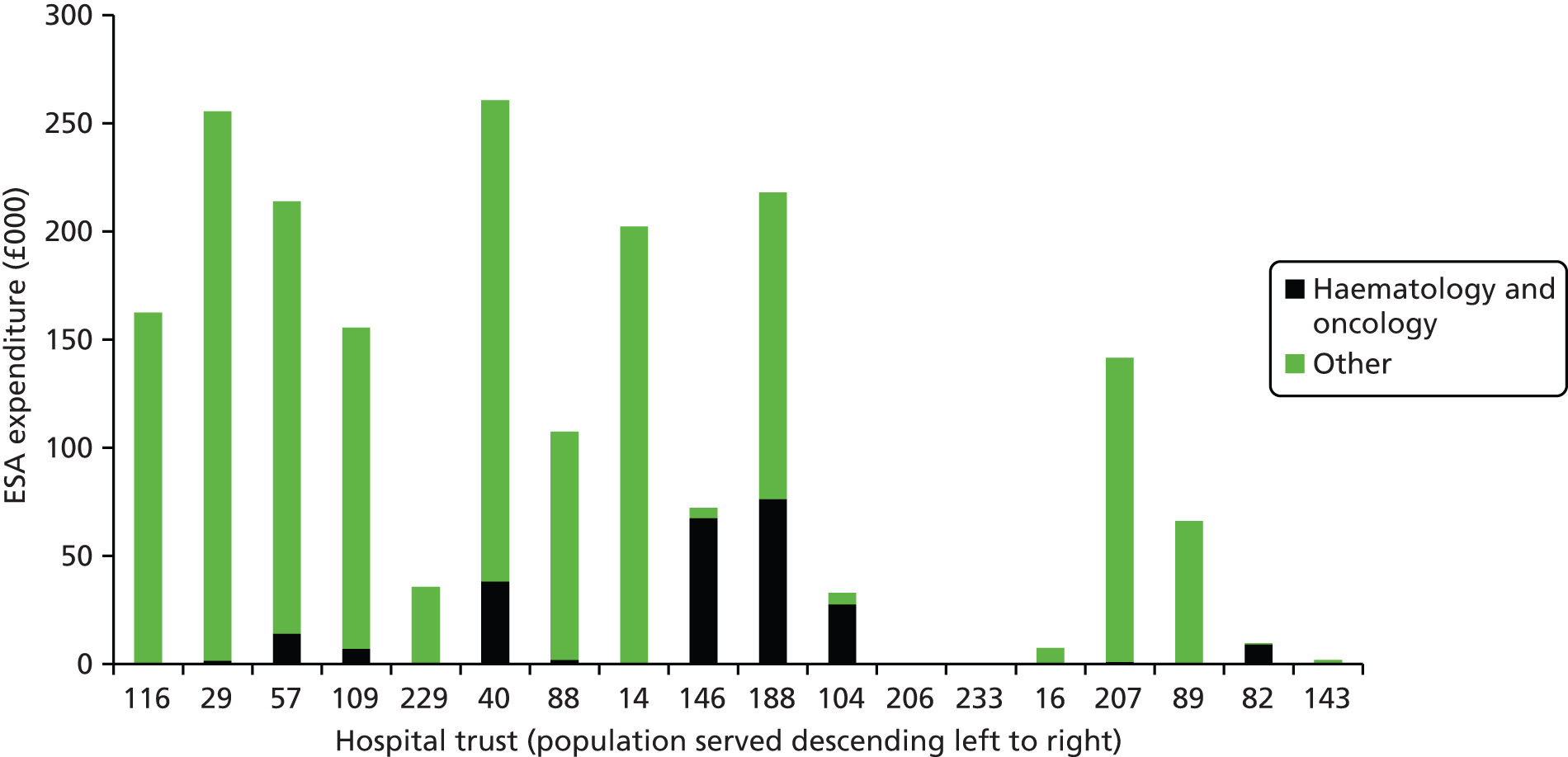
FIGURE 70.
Comparison of total ESA expenditure with population served.
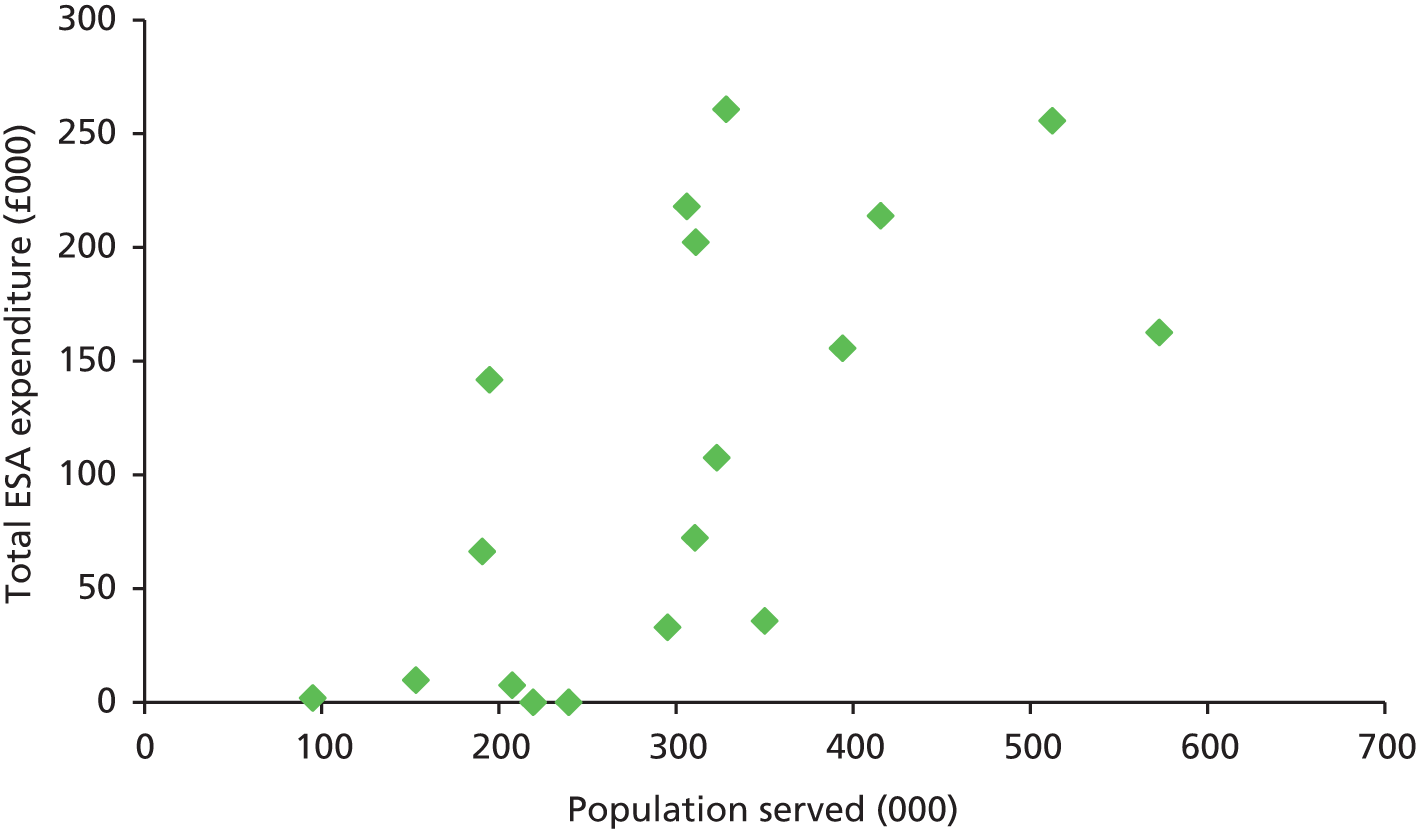
FIGURE 71.
Comparison of ESA expenditure in haematology and oncology with population served.

Acquisition cost of erythropoiesis-stimulating agents
As noted in Chapter 5, the cost at which hospitals acquire ESAs may be significantly lower than the list price for these drugs. These prices are the subject of confidential negotiations and are commercially sensitive.
The NICE methods guide169 states that in the reference case analysis, prices should be chosen to best reflect those relevant to the NHS, if these are transparent and consistently applied across the NHS, and can be guaranteed for a period. In the absence of such conditions, prices from the Commercial Medicines Unit Electronic Marketing Information Tool, or public list prices, should be used in the reference case.
At the time of writing no manufacturer has agreed a Patient Access Scheme with the Department of Health and no contracts have been negotiated by the NHS Commercial Medicines Unit. Current acquisition prices are confidential and therefore not transparent and there are no guarantees that current prices will continue into the future.
At present, acquisition prices will largely be driven by demand for ESAs for individuals with CKD. Current prices could be disturbed if there are developments in the management of CKD or if demand for ESAs increases for patients with CIA.
Chapter 8 Conclusions
The previous HTA review2 concluded that ‘Epo is effective in improving haematological response and reducing RBCT requirements. It also appears to improve HRQoL. Its impact on side-effects and survival remains highly uncertain. If there is no impact on survival, it seems highly unlikely to be considered that epo is a cost-effective use of healthcare resources’ (p. iv).
Additional clinical effectiveness evidence identified in this updated systematic review continues to suggest that there is clinical benefit from ESAs with respect to anaemia-related outcomes; that is, improvements in haematological response and a reduction in RBCT requirements. Data also suggest that there is an improvement in HRQoL and this is better quantified than in the previous HTA review. 2 The impact on side effects and survival, however, remains highly uncertain. Although the point estimates for both survival and thromboembolic events are lower than previously reported estimates, the 95% CIs are wide and not statistically significant.
Conclusions concerning cost-effectiveness are also no clearer. Base-case ICERs for ESA treatment compared with no ESA treatment ranged from £19,429 to £35,018 per QALY gained, but sensitivity and scenario analyses demonstrate that there is considerable uncertainty in these ICERs. In line with the previous HTA review,2 survival was an influential parameter. If the survival benefit reported in the clinical effectiveness review (HR 0.97, 95% CI 0.83 to 1.13) is used, ESAs appear to be cost-effective on average, but this is highly uncertain and QALY losses cannot be ruled out (31.4% of simulations in the base case had a QALY loss from ESA therapy). However, if exactly equal survival is assumed in both groups, regardless of ESA therapy, ESAs are predicted not to be cost-effective unless wholesale acquisition costs are used, in which case ESAs are predicted to be cost-effective on average, although approximately one in three simulations give an ICER of > £20,000 per QALY.
In summary, ESAs could be cost-effective but there is considerable uncertainty, mainly because of unknown impacts on OS.
Suggested research priorities
-
If ESAs are thought to have a major potential for improving cancer care, large RCTs meeting current methodological and reporting standards with adequate follow-up are needed to evaluate ESAs as administered in line with current marketing authorisations (including licence criteria for Hb levels).
-
There is a need for improved estimates of the impact of ESAs on tumour response and mortality; if these estimates are neutral or slightly beneficial it is plausible that ESAs could be cost-effective.
-
There should be assessment of the frequency of the key potential AEs related to ESA administration.
-
More data are needed to assess the impact of ESAs on HRQoL. Such studies should include the effect of ESAs on the EQ-5D.
-
More evidence is needed to assess the impact of Hb normalisation on utility. If clinical studies of normalisation are conducted it would also be valuable for HRQoL outcomes to be measured, preferably using the EQ-5D or another universal HRQoL questionnaire, so that incremental QALYs resulting from normalising from a higher Hb level can be modelled directly rather than by using the surrogate of Hb level.
-
In addition to new trials it may be valuable to revisit the Cochrane IPD meta-analysis7 and select studies that better fit ‘licensed recommendations’ with respect to Hb criteria and dose administered.
-
It may also be helpful to explore reasons why improved anaemia may lead to better outcomes; that is, whether ESAs allow better compliance with chemotherapy.
Acknowledgements
We would like to acknowledge the help of Professor Nicholas Reed (Consultant Clinical Oncologist, Beatson Oncology Centre, Gartnavel General Hospital, Glasgow) for clinical input into the design of the model; Dr Claudius Rudin and Dr Kate Scatchard (Royal Devon & Exeter NHS Foundation Trust, Exeter) for clinical input into the systematic review and the design of the model; and Paul Foster (Clinical Director, Pharmacy and Prescribing, South Devon Healthcare NHS Foundation Trust), Chris Roome (Head of Clinical Effectiveness, Clinical Effectiveness and Medicines Optimisation Team, Northern, Eastern and Western Devon Clinical Commissioning Group), Clint Botha (Technical Pharmacist, Commercial Medicines Unit) and Kevan Wind (Medicines Procurement Specialist, South East England Specialist Pharmacy Services) for information relating to the current use of ESAs in the indication under review and acquisition costs.
We would like to thank Dr Obi Ukoumunne and Dr Jaime Peters (University of Exeter Medical School, Exeter) for providing statistical advice on the clinical effectiveness meta-analysis; Dr Will Stahl-Timmins [BMJ (previously University of Exeter Medical School, Exeter)] for summary graphics; Mrs Mary Bond (University of Exeter Medical School, Exeter) and Dr Emily Youngman (Public Health Devon and University of Exeter Medical School, Exeter) for reviewing and commenting on the draft report; Dr Rob Anderson (University of Exeter Medical School, Exeter) for reviewing and commenting on the draft report; Mrs Sue Whiffin and Ms Jenny Lowe (University of Exeter Medical School, Exeter) for administrative support (organisation and recording of meetings, collation of the report for review and liaison with external experts) throughout the project; and Mrs Sue Whiffin for proofreading the draft report.
Contribution of authors
Louise Crathorne provided overall project management and led the systematic review of clinical effectiveness, including the assessment of all abstracts and titles for possible inclusion and the meta-analysis for the clinical effectiveness outcomes. She also drafted and/or edited all sections of the report.
Nicola Huxley led the development and execution of the economic model and wrote the sections on the design and results of the economic model. She also assessed titles and abstracts for inclusion in the cost-effectiveness review.
Marcela Haasova assessed titles and abstracts for inclusion in the systematic review of clinical effectiveness, led the meta-analysis for the clinical effectiveness review and contributed to the writing and editing of the report.
Tristan Snowsill led the systematic review of economic evaluations, contributed to the design and parameterisation of the model and performed model checking. He also contributed to the writing and editing of all sections of the report.
Tracey Jones-Hughes led the systematic review of quality-of-life outcomes, including the assessment of all abstracts and titles for possible inclusion, and contributed to the writing and editing of the report.
Martin Hoyle led the design of the economic model and contributed to the write-up of the economic model.
Simon Briscoe designed and carried out the literature searches for the systematic reviews and identification of model parameters and contributed to the writing and editing of the report.
Helen Coelho contributed to the clinical effectiveness, quality-of-life and cost-effectiveness systematic reviews and to the editing of the report.
Linda Long assessed abstracts and titles for inclusion in the systematic review of quality-of-life outcomes, assessed the quality of the included systematic reviews, compiled the summary table of review characteristics and conducted the GRADE assessment. She also contributed to the editing of the report.
Antonieta Medina-Lara provided advice on the cost-effectiveness (utilities) and quality-of-life systematic reviews and contributed to the writing and editing of the report.
Ruben Mujica-Mota provided advice on the cost-effectiveness systematic review and the design and results of the cost-effectiveness modelling and contributed to the writing and editing of the report.
Mark Napier provided clinical input into the design of the model and advised on clinical matters.
Chris Hyde contributed to the clinical effectiveness, quality-of-life and cost-effectiveness reviews, the design of the model and the writing and editing of the report and was overall director of the project and guarantor of the report.
Data-sharing statement
This is a systematic review; therefore, there is no primary data to share. Further information can be obtained from the lead author if required.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Epoetin Alfa, Epoetin Beta and Darbepoetin Alfa for Cancer Treatment-Induced Anaemia. London: NICE; 2008.
- Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al. A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11130.
- Schrijvers D, De Samblanx H, Roila F. Erythropoiesis-stimulating agents in the treatment of anaemia in cancer patients: ESMO Clinical Practice Guidelines for use. Ann Oncol 2010;21:v244-7. http://dx.doi.org/10.1093/annonc/mdq202.
- Mercadante S, Gebbia V, Marrazzo A, Filosto S. Anaemia in cancer: pathophysiology and treatment. Cancer Treat Rev 2000;26:303-11. http://dx.doi.org/10.1053/ctrv.2000.0181.
- Schrijvers D, Roila F. Esmo Guidelines Working Group . Erythropoiesis-stimulating agents in cancer patients: ESMO recommendations for use. Ann Oncol 2009;20:159-61.
- Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst 1999;91:1616-34. http://dx.doi.org/10.1093/jnci/91.19.1616.
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Erythropoietin or darbepoetin for patients with cancer – meta-analysis based on individual patient data. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.cd007303.pub2.
- Vaupel P, Mayer A. Hypoxia and anemia: effects on tumour biology and treatment resistance. Transfus Clin Biol 2005;12:5-10. http://dx.doi.org/10.1016/j.tracli.2004.11.005.
- Schrijvers D, Highley M, De Bruyn E, Van Oosterom A, Vermorken J. Role of red blood cells in pharmacokinetics of chemotherapeutic agents. Anticancer Drugs 1999;10:147-53. http://dx.doi.org/10.1097/00001813-199902000-00002.
- Hockel M, Knoop C, Schlenger K, Vorndran B, Baussmann E, Mitze M, et al. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol 1993;26:45-50. http://dx.doi.org/10.1016/0167-8140(93)90025-4.
- Tonia T, Mettler A, Robert N, Schwarzer G, Seidenfeld J, Weingart O, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12. http://dx.doi.org/10.1002/14651858.cd003407.pub5.
- Knocke TH, Weitmann HD, Feldmann HJ, Selzer E, Potter R. Intratumoral pO2-measurements as predictive assay in the treatment of carcinoma of the uterine cervix. Radiother Oncol 1999;18:156-69. http://dx.doi.org/10.1016/s0167-8140(99)00139-5.
- Vaupel P, Thews O, Mayer A, Hockel S, Hockel M. Oxygenation status of gynecologic tumors: what is the optimal hemoglobin level?. Strahlenther Onkol 2002;178:727-31. http://dx.doi.org/10.1007/s00066-002-1081-x.
- Leyland-Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet 2003;4:459-60. http://dx.doi.org/10.1016/S1470-2045(03)01163-X.
- Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet 2003;362:1255-60. http://dx.doi.org/10.1016/S0140-6736(03)14567-9.
- Smith RE, Aapro MS, Ludwig H, Pinter T, Smakal M, Ciuleanu TE, et al. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a Phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol 2008;26:1040-50. http://dx.doi.org/10.1200/JCO.2007.14.2885.
- Hedenus M, Adriansson M, San Miguel J, Kramer MH, Schipperus MR, Juvonen E, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol 2003;122:394-403. http://dx.doi.org/10.1046/j.1365-2141.2003.04448.x.
- Overgaard J, Hoff C, Sand Hansen H, Specht L, Overgaard M, Grau C. Randomized study of the importance of novel erythropoiesis stimulating protein (Aranesp®) for the effect of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC) – the Danish Head and Neck Cancer Group DAHANCA 10 randomized trial. Eur J Cancer Suppl 2007;5.
- Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol 2007;25:1027-32. http://dx.doi.org/10.1200/JCO.2006.07.1514.
- Goss G, Feld R, Bezjak A, Perry G, Melosky Y, Smith C, et al. Impact of maintaining Hb with epoetin alfa on time to progression (TTP), overall survival (OS), quality of life (QOL) and transfusion reduction in limited disease SCLC patients. Lung Cancer 2005;49. http://dx.doi.org/10.1016/S0169-5002(05)80288-0.
- McBroom JW, Acs G, Rose GS, Krivak TC, Mohyeldin A, Verma A. Erythropoeitin receptor function and expression in epithelial ovarian carcinoma. Gynecol Oncol 2005;99:571-7. http://dx.doi.org/10.1016/j.ygyno.2005.06.038.
- Arcasoy MO, Jiang X, Haroon ZA. Expression of erythropoietin receptor splice variants in human cancer. Biochem Biophys Res Comm 2003;307:999-1007. http://dx.doi.org/10.1016/S0006-291X(03)01303-2.
- Arcasoy MO, Amin K, Vollmer RT, Jiang X, Demark-Wahnefried W, Haroon ZA. Erythropoietin and erythropoietin receptor expression in human prostate cancer. Mod Pathol 2005;18:421-30. http://dx.doi.org/10.1038/modpathol.3800288.
- Dagnon K, Pacary E, Commo F, Antoine M, Bernaudin M, Bernaudin JF, et al. Expression of erythropoietin and erythropoietin receptor in non-small cell lung carcinomas. Clin Cancer Res 2005;11:993-9.
- Leo C, Horn LC, Rauscher C, Hentschel B, Liebmann A, Hildebrandt G, et al. Expression of erythropoietin and erythropoietin receptor in cervical cancer and relationship to survival, hypoxia, and apoptosis. Clin Cancer Res 2006;12:6894-900. http://dx.doi.org/10.1158/1078-0432.CCR-06-1285.
- Feldman L, Wang Y, Rhim JS, Bhattacharya N, Loda M, Sytkowski AJ. Erythropoietin stimulates growth and STAT5 phosphorylation in human prostate epithelial and prostate cancer cells. Prostate 2006;66:135-45. http://dx.doi.org/10.1002/pros.20310.
- Yasuda Y, Fujita Y, Matsuo T, Koinuma S, Hara S, Tazaki A, et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis 2003;24:1021-9. http://dx.doi.org/10.1093/carcin/bgg060.
- Mohyeldin A, Lu H, Dalgard C, Lai SY, Cohen N, Acs G, et al. Erythropoietin signaling promotes invasiveness of human head and neck squamous cell carcinoma. Neoplasia 2005;7:537-43. http://dx.doi.org/10.1593/neo.04685.
- Henke M, Mattern D, Pepe M, Bezay C, Weissenberger C, Werner M, et al. Do erythropoietin receptors on cancer cells explain unexpected clinical findings?. J Clin Oncol 2006;24:4708-13. http://dx.doi.org/10.1200/JCO.2006.06.2737.
- Jelkmann W, Bohlius J, Hallek M, Sytkowski AJ. The erythropoietin receptor in normal and cancer tissues. Crit Rev Oncol Hematol 2008;67:39-61. http://dx.doi.org/10.1016/j.critrevonc.2008.03.006.
- Machtay M, Pajak TF, Suntharalingam M, Shenouda G, Hershock D, Stripp DC, et al. Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: a randomized trial of the Radiation Therapy Oncology Group (RTOG 99-03). Int J Radiat Oncol Biol Phys 2007;69:1008-17. http://dx.doi.org/10.1016/j.ijrobp.2007.04.063.
- Chang J, Couture F, Young S, McWatters KL, Lau CY. Weekly epoetin alfa maintains hemoglobin, improves quality of life, and reduces transfusion in breast cancer patients receiving chemotherapy. J Clin Oncol 2005;23:2597-605. http://dx.doi.org/10.1200/JCO.2004.12.027.
- Moebus V, Lueck H, Thomssen C, Harbeck N, Nitz U, Kreienberg R. The impact of epoetin-alpha on anemia, red blood cell (RBC) transfusions, and survival in breast cancer patients (pts) treated with dose-dense sequential chemotherapy: mature results of an AGO Phase III study (ETC trial). J Clin Oncol 2007;25.
- Schrijvers D. Management of anemia in cancer patients: transfusions. Oncologist 2011;16:12-8. http://dx.doi.org/10.1634/theoncologist.2011-S3-12.
- Summary Product Characteristics: Eprex 40,000 IU/ml Solution for Injection in Pre-Filled Syringe. High Wycombe: Janssen-Cilag; 2012.
- Summary of Product Characteristics: Binocrit Solution for Injection in a Pre-Filled Syringe. Camberley: Sandoz Ltd; 2012.
- Summary of Product Characteristics: Retacrit Solution for Injection in Pre Filled Syringe. Leamington Spa: Hospira UK Ltd; 2012.
- Summary of Product Characteristics: Neorecormon Solution for Injection in Pre-Filled Syringe. Welwyn Garden City: Roche Products Ltd; 2012.
- Summary of Product Characteristics: Eporatio 1,000 IU/0.5 ml Solution for Injection in Pre-Filled Syringe. Harlow: Teva Pharmaceuticals Ltd; 2012.
- Summary of Product Characteristics: Aranesp PFS. Cambridge: Amgen Inc; 2012.
- Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Österborg A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 2007;43:258-70. http://dx.doi.org/10.1016/j.ejca.2006.10.014.
- Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spicak JL, et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol 2010;28:4996-5010. http://dx.doi.org/10.1200/JCO.2010.29.2201.
- BCCA Protocol Summary Guidelines for the Use of Erythropoiesis-Stimulating Agents (ESAs) in Patients with Cancer. Vancouver, BC: British Columbia Cancer Agency; 2012.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2535.
- Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennett C, et al. Erythropoietin for patients with malignant disease. Cochrane Database Syst Rev 2004;3. http://dx.doi.org/10.1002/14651858.cd003407.pub2.
- Final Scope: Anaemia (Cancer-Treatment Induced) – Erythropoiesis-Stimulating Agents (Epoetin and Darbepoetin). London: NICE; 2014.
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Mortality in Cancer Patients Receiving Recombinant Human Erythropoiesis-Stimulating Agents: Individual Patient Data Meta-Analysis Based on Randomized Trials n.d.
- Tjulandin SA, Bias P, Elsasser R, Gertz B, Kohler E, Buchner A. Epoetin theta in anaemic cancer patients receiving platinum-based chemotherapy: a randomised controlled trial. Arch Drug Inf 2010;3:45-53. http://dx.doi.org/10.1111/j.1753-5174.2010.00030.x.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. http://dx.doi.org/10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8.
- Kotasek D, Steger G, Faught W, Underhill C, Poulsen E, Colowick AB, et al. Darbepoetin alfa administered every 3 weeks alleviates anaemia in patients with solid tumours receiving chemotherapy; results of a double-blind, placebo-controlled, randomised study. Eur J Cancer 2003;39:2026-34. http://dx.doi.org/10.1016/S0959-8049(03)00456-8.
- ten Bokkel Huinink WW, de Swart CA, van Toorn DW, Morack G, Breed WP, Hillen HF. Controlled multicentre study of the influence of subcutaneous recombinant human erythropoietin on anaemia and transfusion dependency in patients with ovarian carcinoma treated with platinum-based chemotherapy. Med Oncol 1998;15:174-82. http://dx.doi.org/10.1007/BF02821936.
- Thatcher N, De Campos ES, Bell DR, Steward WP, Varghese G, Morant R, et al. Epoetin alpha prevents anaemia and reduces transfusion requirements in patients undergoing primarily platinum-based chemotherapy for small cell lung cancer. Br J Cancer 1999;80:396-402. http://dx.doi.org/10.1038/sj.bjc.6690369.
- Hedenus M, Hansen S, Taylor K, Arthur C, Emmerich B, Dewey C, et al. Randomized, dose-finding study of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies. Br J Haematol 2002;119:79-86. http://dx.doi.org/10.1046/j.1365-2141.2002.03774.x.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration; 2011.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: Introduction to Evidence Synthesis for Decision Making. n.d. www.nicedsu.org.uk (accessed 31 August 2015).
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. http://dx.doi.org/10.1136/bmj.315.7109.629.
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443-57. http://dx.doi.org/10.1002/sim.2380.
- Henry DH, Brooks BJ, Case DC, Fishkin E, Jacobson R, Keller AM, et al. Recombinant human erythropoietin therapy for anemic cancer patients receiving cisplatin chemotherapy. Canc J Sci Am 1995;1:252-60.
- Abels RI, Larholt KM, Krantz KD, Bryant EC. Recombinant human erythropoietin (rHuEPO) for the treatment of the anemia of cancer. Oncologist 1996;1:140-50.
- Patrick DL, Gagnon DD, Zagari MJ, Mathijs R, Sweetenham J. Epoetin Alfa Study Group . Assessing the clinical significance of health-related quality of life (HrQOL) improvements in anaemic cancer patients receiving epoetin alfa. Eur J Cancer 2003;39:335-45. http://dx.doi.org/10.1016/S0959-8049(02)00628-7.
- Wagner LM, Billups CA, Furman WL, Rao BN, Santana VM. Combined use of erythropoietin and granulocyte colony-stimulating factor does not decrease blood transfusion requirements during induction therapy for high-risk neuroblastoma: a randomized controlled trial. J Clin Oncol 2004;22:1886-93. http://dx.doi.org/10.1200/JCO.2004.01.002.
- Moebus V, Jackisch C, Schneeweiss A, Huober J, Lueck HJ, du Bois A, et al. Adding epoetin alfa to intense dose-dense adjuvant chemotherapy for breast cancer: randomized clinical trial. J Natl Cancer Inst 2013;105:1018-26. http://dx.doi.org/10.1093/jnci/djt145.
- Abels R. Erythropoietin for anaemia in cancer patients. Eur J Cancer 1993;29A:S2-8. http://dx.doi.org/10.1016/S0959-8049(05)80281-3.
- Aravantinos G, Linardou H, Makridaki D, Laiou E, Zafiropoulos A, Janninis J, et al. Recombinant human erythropoietin for platinum-based chemotherapy-induced anaemia: a single-centre randomised study. J BUON 2003;8:127-32.
- Boogaerts M, Coiffier B, Kainz C. Impact of epoetin beta on quality of life in patients with malignant disease. Br J Cancer 2003;88:988-95. http://dx.doi.org/10.1038/sj.bjc.6600801.
- Dammacco F, Castoldi G, Rodjer S. Efficacy of epoetin alfa in the treatment of anaemia of multiple myeloma. Br J Haematol 2001;113:172-9. http://dx.doi.org/10.1046/j.1365-2141.2001.02715.x.
- Del Mastro L, Venturini M, Lionetto R, Garrone O, Melioli G, Pasquetti W, et al. Randomized Phase III trial evaluating the role of erythropoietin in the prevention of chemotherapy-induced anemia. J Clin Oncol 1997;15:2715-21.
- Dunphy FR, Harrison BR, Dunleavy TL, Rodriguez JJ, Hilton JG, Boyd JH. Erythropoietin reduces anemia and transfusions: a randomized trial with or without erythropoietin during chemotherapy. Cancer 1999;86:1362-7. http://dx.doi.org/10.1002/(SICI)1097-0142(19991001)86:7<1362::AID-CNCR36>3.0.CO;2-T.
- Kurz C, Marth C, Windbichler G, Lahousen M, Medl M, Vavra N, et al. Erythropoietin treatment under polychemotherapy in patients with gynecologic malignancies: a prospective, randomized, double-blind placebo-controlled multicenter study. Gynecol Oncol 1997;65:461-6. http://dx.doi.org/10.1006/gyno.1997.4675.
- Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B. Epoetin Alfa Study Group . Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2001;19:2865-74.
- Österborg A, Brandberg Y, Molostova V, Iosava G, Abdulkadyrov K, Hedenus M, et al. Randomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. J Clin Oncol 2002;20:2486-94. http://dx.doi.org/10.1200/JCO.2002.08.131.
- Silvestris F, Romito A, Fanelli P, Vacca A, Dammacco F. Long-term therapy with recombinant human erythropoietin (rHu-EPO) in progressing multiple myeloma. Ann Hematol 1995;70:313-18. http://dx.doi.org/10.1007/BF01696618.
- Vansteenkiste J, Pirker R, Massuti B, Barata F, Font A, Fiegl M, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 2002;94:1211-20. http://dx.doi.org/10.1093/jnci/94.16.1211.
- Grote T, Yielding AL, Castillo R, Butler D, Fishkin E, Henry DH, et al. Efficacy and safety analysis of epoetin alfa in patients with small-cell lung cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2005;23:9377-86. http://dx.doi.org/10.1200/JCO.2005.01.8507.
- Ray-Coquard I, Dussart S, Goillot C, Mayeur D, Debourdeau P, Ghesquieres H, et al. A risk model for severe anemia to select cancer patients for primary prophylaxis with epoetin alpha: a prospective randomized controlled trial of the ELYPSE study group. Ann Oncol 2009;20:1105-12. http://dx.doi.org/10.1093/annonc/mdn750.
- Strauss HG, Haensgen G, Dunst J, Hayward CR, Burger HU, Scherhag A, et al. Effects of anemia correction with epoetin beta in patients receiving radiochemotherapy for advanced cervical cancer. Int J Gynecol Cancer 2008;18:515-24. http://dx.doi.org/10.1111/j.1525-1438.2007.01032.x.
- Tjulandin SA, Bias P, Elsasser R, Gertz B, Kohler E, Buchner A. Epoetin theta with a new dosing schedule in anaemic cancer patients receiving nonplatinum-based chemotherapy: a randomised controlled trial. Arch Drug Inf 2011;4:33-41. http://dx.doi.org/10.1111/j.1753-5174.2011.00035.x.
- Untch M, Fasching PA, Konecny GE, von Koch F, Conrad U, Fett W, et al. PREPARE trial: a randomized Phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel and CMF versus a standard-dosed epirubicin/cyclophosphamide followed by paclitaxel +/– darbepoetin alfa in primary breast cancer – results at the time of surgery. Ann Oncol 2011;22:1988-98. http://dx.doi.org/10.1093/annonc/mdq709.
- Österborg AB, Brandberg Y, Hedenus M. Impact of epoetin-beta on survival of patients with lymphoproliferative malignancies: long-term follow up of a large randomized study. Br J Haematol 2005;129:206-9. http://dx.doi.org/10.1111/j.1365-2141.2005.05440.x.
- Untch M, Minckwitz G, Konecny GE, Conrad U, Fett W, Kurzeder C, et al. PREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel, and CMF versus a standard-dosed epirubicin-cyclophosphamide followed by paclitaxel with or without darbepoetin alfa in primary breast cancer – outcome on prognosis. Ann Oncol 2011;22:1999-2006. http://dx.doi.org/10.1093/annonc/mdq713.
- Bajetta E, Vercammen E, Reinhardt U, Janmohamed R, da Costa RM, Matulonis U, et al. Efficacy of epoetin alfa in a retrospective non-stratified subgroup analysis of a breast cancer cohort receiving non-platinum chemotherapy. Tumori 2004;90:449-57.
- Aapro M, Bajetta E, Freund M, Littlewood TJ, Nortier JWR, Rapoport B. Is there a possible survival benefit to increasing hemoglobin levels with epoetin alfa during chemotherapy?. Eur J Cancer Suppl 2004;2:20-8. http://dx.doi.org/10.1016/S1359-6349(03)00104-6.
- Littlewood TJ, Kallich JD, San Miguel J, Hendricks L, Hedenus M. Efficacy of darbepoetin alfa in alleviating fatigue and the effect of fatigue on quality of life in anemic patients with lymphoproliferative malignancies. J Pain Symptom Manage 2006;31:317-25. http://dx.doi.org/10.1016/j.jpainsymman.2005.08.013.
- Vansteenkiste J, Tomita D, Rossi G, Pirker R. Darbepoetin alfa in lung cancer patients on chemotherapy: a retrospective comparison of outcomes in patients with mild versus moderate-to-severe anaemia at baseline. Support Care Cancer 2004;12:253-62. http://dx.doi.org/10.1007/s00520-003-0583-0.
- Henry DH, Abels RI. Recombinant human erythropoietin in the treatment of cancer and chemotherapy-induced anemia: results of double-blind and open-label follow-up studies. Semin Oncol 1994;21:21-8.
- Case DC, Bukowski RM, Carey RW, Fishkin EH, Henry DH, Jacobson RJ, et al. Recombinant human erythropoietin therapy for anemic cancer patients on combination chemotherapy. J Natl Cancer Inst 1993;85:801-6. http://dx.doi.org/10.1093/jnci/85.10.801.
- Coiffier B, Boogaerts M, Kaine C. Impact of Epoetin Beta Versus Standard Care on Quality of Life in Patients With Malignant Disease n.d.
- Tonelli M, Lloyd A, Weibe N, Hemmelgarn B, Reiman T, Manns B, et al. Erythropoiesis-Stimulating Agents for Anemia of Cancer or of Chemotherapy: Systematic Review and Economic Evaluation. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009.
- Grant MD, Piper M, Bohlius J, Tonia T, Nadege R, Vikrant V, et al. Epoetin and Darbepoetin for Managing Anemia in Patients Undergoing Cancer Treatment: Comparative Effectiveness Update. Chicago, IL: Blue Cross and Blue Shield Association Technology Evaluation Center; 2013.
- Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr 2004:40-5. http://dx.doi.org/10.1093/jncimonographs/lgh027.
- Ross SD, Allen IE, Probst CA, Sercus B, Crean SM, Ranganathan G. Efficacy and safety of erythropoiesis-stimulating proteins in myelodysplastic syndrome: a systematic review and meta-analysis. Oncologist 2007;12:1264-73. http://dx.doi.org/10.1634/theoncologist.12-10-1264.
- Shehata N, Walker I, Meyer R, Haynes AE, Imrie K. Cancer Care Ontario Hematology Disease Site Group . The use of erythropoiesis-stimulating agents in patients with non-myeloid hematological malignancies: a systematic review. Ann Hematol 2008;87:961-73. http://dx.doi.org/10.1007/s00277-008-0525-5.
- Kvam AK, Fayers P, Hjermstad M, Gulbrandsen N, Wisloff F. Health-related quality of life assessment in randomised controlled trials in multiple myeloma: a critical review of methodology and impact on treatment recommendations. Eur J Haematol 2009;83:279-89. http://dx.doi.org/10.1111/j.1600-0609.2009.01303.x.
- Minton O, Stone P, Richardson A, Sharpe M, Hotopf M. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd006704.pub3.
- Bohlius JS, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet 2009;373:1532-42. http://dx.doi.org/10.1016/S0140-6736(09)60502-X.
- Amgen Inc . Erythropoiesis-Stimulating Agents (Epoetin and Darbepoetin) for Treating Cancer-Treatment Induced Anaemia (Including Review of TA 142): Aranesp® (Darbepoetin Alfa) 2013.
- Sandoz Ltd . Erythropoiesis Agents for Treatment of Cancer-Treatment Induced Anaemia (Incl Review of TA 142): Binocrit® (Epoetin Alfa) 2013.
- Sorgel F, Thyroff-Friesinger U, Vetter A, Vens-Cappell B, Kinzig M. Bioequivalence of HX575 (recombinant human epoetin alfa) and a comparator epoetin alfa after multiple intravenous administrations: an open-label randomised controlled trial. BMC Clin Pharmacol 2009;9. http://dx.doi.org/10.1186/1472-6904-9-10.
- Sorgel F, Thyroff-Friesinger U, Vetter A, Vens-Cappell B, Kinzig M. Bioequivalence of HX575 (recombinant human epoetin alfa) and a comparator epoetin alfa after multiple subcutaneous administrations. Pharmacology 2009;83:122-30. http://dx.doi.org/10.1159/000189027.
- Sorgel F, Thyroff-Friesinger U, Vetter A, Vens-Cappell B, Kinzig M. Biosimilarity of HX575 (human recombinant epoetin alfa) and epoetin beta after multiple subcutaneous administration. Int J Clin Pharmacol Ther 2009;47:391-40. http://dx.doi.org/10.5414/CPP47391.
- Weigang-Köhler K, Vetter A, Thyroff-Friesinger U. HX575, recombinant human epoetin alfa, for the treatment of chemotherapy-associated symptomatic anaemia in patients with solid tumours. Onkologie 2009;32:168-74. http://dx.doi.org/10.1159/000200783.
- Haag-Weber M, Vetter A, Thyroff-Friesinger U. INJ-Study Group . Therapeutic equivalence, long-term efficacy and safety of HX575 in the treatment of anemia in chronic renal failure patients receiving hemodialysis. Clin Nephrol 2009;72:380-90.
- Desrame J, Stamerra O, Labourey JL, Toledano A, Dauriac C, Ianotto JC, et al. Haemoglobin Outcomes With Biosimilar Epoetin Alfa in the Management of Chemotherapy-Induced Anaemia in Cancer Patients: First Results From OnCoBOS, A French Observational Study n.d.
- Rodriguez Garzotto A, Cortijo Cascajares S, Pernaut Sanchez C, Otero Blas I, Ruiz Ares G, Rebollo Laserna FJ, et al. Use of Erythropoiesis-Stimulating Agents and Comparison of Different Products for the Treatment of Chemotherapy-Induced Anaemia (CIA) n.d.
- Lorenz A, Heine O. First Comparison of Biosimilar Epoetin Alfa and Darbepoetin Alfa for the Treatment of Chemotherapy-Induced Anaemia n.d.
- Kerkhofs L, Boschetti G, Lugini A, Stanculeanu DL, Palomo AG. Use of biosimilar epoetin to increase haemoglobin levels in patients with chemotherapy-induced anemia: real-life clinical experience. Future Oncol 2012;8:751-6. http://dx.doi.org/10.2217/fon.12.39.
- Delarue R, Haioun C, Coiffier B, Fornecker L, Fournier M, Mounier N, et al. Survival effect of darbepoetin alfa in patients with diffuse large B-cell lymphoma (DLBCL) treated with immunochemotherapy: the LNH03-6B study. J Clin Oncol 2011;29.
- Hartmann JT, Metzner B, Binder C, Mergenthaler HG, Rick O, Sayer HG, et al. Addition of darbepoetin alfa to sequential high-dose VIP chemotherapy for patients with advanced metastatic germ cell cancer. J Clin Oncol 2012;30.
- Katsumata N, Fujiwara Y, Katakami N, Nishiwaki Y, Tsuboi M, Takeda K, et al. Randomized, Double Blind, Placebo-Controlled Phase III Study of Weekly Administration of Darbepoetin Alfa in Anemic Patients With Lung or Gynecologic Cancer Receiving Platinum-Containing Chemotherapy n.d.
- Nitz U, Oberhoff C, Reimer T, Schumacher C, Hackmann J, Warm M, et al. Adjuvant chemotherapy with or without darbepoetin in node-positive breast cancer: a safety analysis from the Phase III ARA plus trial. Cancer Res 2009;69. http://dx.doi.org/10.1158/0008-5472.SABCS-4100.
- Suzuki Y, Tokuda Y, Okamoto R, Nakagawa K, Ando K, Iwata H, et al. Randomized, placebo-controlled Phase II study of darbepoetin alfa (DA) administered every three weeks (Q3W) in patients with chemotherapyinduced anemia (CIA). 34th Congress of the European Society for Medical Oncology (ESMO), Stockholm, Sweden, September 2008. Ann Oncol 2008;19.
- Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage 2002;24:547-61. http://dx.doi.org/10.1016/S0885-3924(02)00529-8.
- Drummond MF, Sculpher MJ, Forrance GN, O‘Brien BS, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd edn. Oxford: Oxford University Press; 2005.
- Barosi G, Marchetti M, Liberato NL. Cost-effectiveness of recombinant human erythropoietin in the prevention of chemotherapy-induced anaemia. Br J Cancer 1998;78:781-7. http://dx.doi.org/10.1038/bjc.1998.579.
- Cremieux PY, Finkelstein SN, Berndt ER, Crawford J, Slavin MB. Cost effectiveness, quality-adjusted life-years and supportive care: recombinant human erythropoietin as a treatment of cancer-associated anaemia. Pharmacoeconomics 1999;16:459-72. http://dx.doi.org/10.2165/00019053-199916050-00004.
- Martin SC, Gagnon DD, Zhang L, Bokemeyer C, Van Marwijk Kooy M, van Hout B. Cost-utility analysis of survival with epoetin-alfa versus placebo in stage IV breast cancer. Pharmacoeconomics 2003;21:1153-69. http://dx.doi.org/10.2165/00019053-200321160-00002.
- Ortega A, Dranitsaris G, Puodziunas AL. What are cancer patients willing to pay for prophylactic epoetin alfa? A cost–benefit analysis 1998;83:2588-96.
- Sheffield RE, Sullivan SD, Saltiel E, Nishimura L. Cost comparison of recombinant human erythropoietin and blood transfusion in cancer chemotherapy-induced anemia. Ann Pharmacother 1997;31:15-22.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Philips Z, Ginnely L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Fagnoni P, Limat S, Chaigneau L, Briaud S, Schmitt B, Merrouche Y, et al. Clinical and economic impact of epoetin in adjuvant-chemotherapy for breast cancer. Support Care Cancer 2006;14:1030-7. http://dx.doi.org/10.1007/s00520-006-0062-5.
- Glaspy J, Tchekmedyian N, Gupta S. PCN17: comparing the cost-effectiveness of 3 mcg/kg Q2W darbepoetin alfa with standard dose epoetin alfa for anemia management in chemotherapy-treated cancer patients in United States. Value Health 2002;5. http://dx.doi.org/10.1016/S1098-3015(10)61433-7.
- Borget I, Tilleul P, Joly AC, Chouaid C. Incremental cost-effectiveness ratio of darbepoetin alfa (Aranesp (R)) in the treatment of chemotherapy-induced anemia in lung cancer patients. Value Health 2006;9:A278-9. http://dx.doi.org/10.1016/S1098-3015(10)63440-7.
- Ben-Hamadi R, Duh MS, Aggarwal J, Henckler A, McKenzie RS, Tak Piech C. The cost-effectiveness of weekly epoetin alfa relative to weekly darbepoetin alfa in patients with chemotherapy-induced anemia. Curr Med Res Opin 2005;21:1677-82. http://dx.doi.org/10.1185/030079905X65501.
- Persson U, Borg S, Jansson S, Ekman T, Franksson L, Friesland S, et al. Epoetin alfa and darbepoetin alfa for the treatment of chemotherapy-related anemia in cancer patients in Sweden: comparative analysis of drug utilization, costs, and hematologic response. Adv Ther 2005;22:208-24. http://dx.doi.org/10.1007/BF02849930.
- Spaepen E, Demarteau N, Van Belle S, Annemans L. Health economic evaluation of treating anemia in cancer patients receiving chemotherapy: a study in Belgian hospitals. Oncologist 2008;13:596-607. http://dx.doi.org/10.1634/theoncologist.2007-0219.
- Pashos CL, Larholt K, Fraser KA, McKenzie RS, Senbetta M, Piech CT. Outcomes of erythropoiesis-stimulating agents in cancer patients with chemotherapy-induced anemia. Support Care Cancer 2012;20:159-65. http://dx.doi.org/10.1007/s00520-010-1083-7.
- Cornes P, Coiffier B, Zambrowski JJ. Erythropoietic therapy for the treatment of anemia in patients with cancer: a valuable clinical and economic option. Curr Med Res Opin 2007;23:357-68. http://dx.doi.org/10.1185/030079906X167282.
- Herrmann R. Erythropoietin therapy in cancer-related anaemia, yes or no?. Intern Med J 2008;38:749-50. http://dx.doi.org/10.1111/j.1445-5994.2008.01767.x.
- Marchetti M, Barosi G. Clinical and economic impact of epoetins in cancer care. Pharmacoeconomics 2004;22:1029-45. http://dx.doi.org/10.2165/00019053-200422160-00001.
- Reeder CE. Anemia in cancer and critical care patients: pharmacoeconomic considerations. Am J Health Syst Pharm 2007;64:S22-7. http://dx.doi.org/10.2146/ajhp060603.
- Stasi R, Amadori S, Littlewood TJ, Terzoli E, Newland AC, Provan D. Management of cancer-related anemia with erythropoietic agents: doubts, certainties, and concerns. Oncologist 2005;10:539-54. http://dx.doi.org/10.1634/theoncologist.10-7-539.
- Repetto L, Moeremans K, Annemans L. European guidelines for the management of chemotherapy-induced anaemia and health economic aspects of treatment. Cancer Treat Rev 2006;32:S5-9. http://dx.doi.org/10.1016/j.ctrv.2006.04.007.
- Roungrong J. Cost utility analysis of recombinant human erythropoietin in anemic cancer patients induced by chemotherapy in Thailand. J Med Assoc Thai 2008;91:S119-25.
- Roungrong J, Teerawattananon Y, Chaikledkaew U. Cost–utility analysis of recombinant human erythropoietin in anaemic cancer patients induced by chemotherapy in Thailand. J Med Assoc Thailand 2008;91:S119-25.
- Griggs JJ. Cost–utility of erythropoietin in the treatment of cancer-related anemia. Med Decis Making 1997;17.
- Griggs JJ. Recombinant erythropoietin and blood transfusions in cancer chemotherapy-induced anemia. Anticancer Drugs 1998;9:925-32. http://dx.doi.org/10.1097/00001813-199811000-00012.
- Malonne H. Cost Evaluation of Erythropoiesis Stimulating Agents in the Treatment of Platinum Chemotherapy Induced Anaemia n.d.
- Borget I, Tilleul P, Baud M, Joly AC, Chouaid C. Routine once-weekly darbepoetin alfa administration is cost-effective in lung cancer patients with chemotherapy-induced anemia: a Markov analysis. Pharm World Sci 2007;29.
- Chouaid C, Borget I, Baud M, Joly AC, Daguenel A, Tilleul P. Routine once-weekly darbepoetin alfa administration is cost-effective in lung cancer patients with chemotherapy-induced anemia: a Markov analysis. Lung Cancer 2005;49. http://dx.doi.org/10.1016/S0169-5002(05)80192-8.
- Finek J, Holubec L, Wiesnerova A, Pav Z, Dusek V. Darbepoetin alfa versus epoetin alfa for treatment of chemotherapy-induced anemia: a health economic evaluation. Ann Oncol 2010;21.
- Klarenbach S, Manns B, Reiman T, Reaume MN, Lee H, Lloyd A, et al. Economic evaluation of erythropoiesis-stimulating agents for anemia related to cancer. Cancer 2010;116:3224-32. http://dx.doi.org/10.1002/cncr.25052.
- Szucs TD, Boogaerts M, Coiffier B, Kainz C. Cost-Effectiveness of Epoetin Beta in the Management of Cancer-Related Anaemia n.d.
- Cremieux P, Greenberg P, Piech CT. Epoetin alfa is more effective and less costly relative to darbepoetin alfa in lung cancer patients receiving treatment for chemotherapy-induced anemia. Proc Am Soc Clin Oncol 2003;22.
- Fragoulakis V, Maniadakis N. Economic evaluation of darbepoetin alpha in the management of patients with chemotherapy induced anemia (CIA) in Greece. Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.08.1139.
- Mark TL, McKenzie SR, Piech CT. Better early and overall hematologic outcomes and lower drug cost with epoetin alfa (EPO) compared with darbepoetin alfa (DARB). Blood 2003;102.
- van Hout BA, Gagnon DD. The cost-effectiveness of epoetin alfa (Eprex) and darbepoetin alfa (Aranesp) in the treatment of chemotherapy-related anemia. Hematol J 2004;5:S176-7.
- Ben-Hamadi R, Duh MS, Henckler A, McKenzie S, Fastenau J, Piech CT. Cost-effectiveness of once weekly epoetin alfa and darbepoetin alfa in treating chemotherapy-induced anemia. J Clin Oncol 2005;23. http://dx.doi.org/10.1016/s1098-3015(10)62574-0.
- Van Bellinghen LA, Demarteau N, Annemans L, Bracco A. A cost–consequence analysis of darbepoetin alfa administered every 3 weeks (Q3W_DA) compared to weekly epoetin alfa (QW_EA) or epoetin beta (QW_EB) in patients with chemotherapy-induced anemia (CIA): a retrospective study. Value Health 2006;9. http://dx.doi.org/10.1016/S1098-3015(10)63463-8.
- Esposito G, Lamotte M, Annemans L, Bracco A. Cost–consequence analysis of darbepoetin alfa administered every 3 weeks (Q3W_DA) compared to weekly epoetin alfa (QW_EA) or epoetin beta (QW_EB) in patients with chemotherapy-induced anemia (CIA): the Italian case. Value Health 2007;10. http://dx.doi.org/10.1016/S1098-3015(10)65166-2.
- Van Bellinghen LA, Esposito G, Lamotte M, Bracco A, Goertz A, Annemans L. A cost–consequence analysis of darbepoetin alfa administered every 3 weeks (Q3W_DA) compared to weekly epoetin alfa (QW_EA) or epoetin beta (QW_EB) in patients with chemotherapy-induced anemia (CIA): the German case. Value Health 2007;10. http://dx.doi.org/10.1016/S1098-3015(10)65177-7.
- Finek J, Holubec L, Wiesnerova A, Pav Z, Dusek L. Darbepoetin alfa versus epoetin alfa for treatment of chemotherapy-induced anemia: a health economic evaluation. Value Health 2010;13. http://dx.doi.org/10.1016/S1098-3015(11)72978-3.
- Liwing J, Nasman P, Kernell H, Gagnon DD, Wu Y. Cost-effectiveness modelling of epoetin alfa and darbepoetin alfa in the treatment of chemotherapy-related anaemia in Sweden. Value Health 2010;13. http://dx.doi.org/10.1016/S1098-3015(10)72167-7.
- Walter E, Ribnicsek E, Kutikova L. Economic evaluation of darbepoetin alfa (Aranesp) compared to epoetin alfa (Erypo) and epoetin beta (NeoRecormon) in the treatment of chemotherapy-induced anemia (CIA) in Austria. Value Health 2010;13. http://dx.doi.org/10.1016/S1098-3015(11)72530-X.
- Duh MS, Weiner JR, White LA, Lefebvre P, Greenberg PE. Management of anaemia: a critical and systematic review of the cost effectiveness of erythropoiesis-stimulating agents. Pharmacoeconomics 2008;26:99-120. http://dx.doi.org/10.2165/00019053-200826020-00002.
- Borg S, Glenngard AH, Österborg A, Persson U. The cost-effectiveness of treatment with erythropoietin compared to red blood cell transfusions for patients with chemotherapy induced anaemia: a Markov model. Acta Oncol 2008;47:1009-17. http://dx.doi.org/10.1080/02841860701744498.
- Borget I, Tilleul P, Baud M, Joly AC, Daguenel A, Chouaid C. Routine once-weekly darbepoetin alfa administration is cost-effective in lung cancer patients with chemotherapy-induced anemia: a Markov analysis. Lung Cancer 2006;51:369-76. http://dx.doi.org/10.1016/j.lungcan.2005.10.024.
- Aapro M, Cornes P, Sun D, Abraham I. Comparative cost efficiency across the European G5 countries of originators and a biosimilar erythropoiesis-stimulating agent to manage chemotherapy-induced anemia in patients with cancer. Ther Adv Med Oncol 2012;4:95-105. http://dx.doi.org/10.1177/1758834012444499.
- Ossa DF, Briggs A, McIntosh E, Cowell W, Littlewood T, Sculpher M. Recombinant erythropoietin for chemotherapy-related anaemia: economic value and health-related quality-of-life assessment using direct utility elicitation and discrete choice experiment methods. Pharmacoeconomics 2007;25:223-37. http://dx.doi.org/10.2165/00019053-200725030-00005.
- Reed SD, Radeva JI, Daniel DB, Mody SH, Forlenza JB, McKenzie RS, et al. Economic evaluation of weekly epoetin alfa versus biweekly darbepoetin alfa for chemotherapy-induced anaemia: evidence from a 16-week randomised trial. Pharmacoeconomics 2006;24:479-94. http://dx.doi.org/10.2165/00019053-200624050-00006.
- Casadevall N, Durieux P, Dubois S, Hemery F, Lepage E, Quarre MC, et al. Health, economic, and quality-of-life effects of erythropoietin and granulocyte colony-stimulating factor for the treatment of myelodysplastic syndromes: a randomized, controlled trial. Blood 2004;104:321-7. http://dx.doi.org/10.1182/blood-2003-07-2252.
- Crawford J, Cella D, Cleeland CS, Cremieux PY, Demetri GD, Sarokhan BJ, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 2002;95:888-95. http://dx.doi.org/10.1002/cncr.10763.
- Evers S. CHEC Project 2015. www.maastrichtuniversity.nl/web/Institutes/Theme/DepartmentsCAPHRI/HealthServicesResearch/ResearchHSR/CHECProject.htm (accessed 12 November 2015).
- Glaspy J, Bukowski R, Steinberg D, Taylor C, Tchekmedyian S, Vadhan-Raj S. Impact of therapy with epoetin alfa on clinical outcomes in patients with nonmyeloid malignancies during cancer chemotherapy in community Oncology practice. Procrit Study Group. J Clin Oncol 1997;15:1218-34.
- Abels RI. Recombinant human erythropoietin in the treatment of the anaemia of cancer. Acta Haematol 1992;87:4-11. http://dx.doi.org/10.1159/000204780.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- Witzig TE, Silberstein PT, Loprinzi CL, Sloan JA, Novotny PJ, Mailliard JA, et al. Phase III, randomized, double-blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. J Clin Oncol 2005;23:2606-17. http://dx.doi.org/10.1200/JCO.2004.10.020.
- Kotasek D, Canon J-L, San Miguel J, Hedenus M, Hendricks L, Rossi G, et al. Correction/maintenance dosing (front loading) of darbepoetin alfa: final results from a randomized Phase 3 active controlled trial. Blood 2004;104.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Latimer N. NICE DSU Technical Support Document 14: Undertaking Survival Analysis for Economic Evaluations Alongside Clinical Trials – Extrapolation With Patient-Level Data n.d. www.nicedsu.org.uk/ (accessed 16 August 2015).
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making 2013;33:743-54.
- Varney SJ, Guest JF. The annual cost of blood transfusions in the UK. Transfusion medicine (Oxford, England) 2003;13:205-18. http://dx.doi.org/10.1046/j.1365-3148.2003.00443.x.
- Interim Life Tables, 2010–2012. London: ONS; 2013.
- Ossa DF, Briggs A, Cowell W, Littlewood T, Sculpher M. Utility associated with severity of cancer-related anaemia (CRA): a societal valuation. Value Health 2004;7. http://dx.doi.org/10.1016/s1098-3015(10)65767-1.
- Papaioannou D, Brazier J, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values from the Literature 2010. www.nicedsu.org.uk/ (accessed 16 August 2015).
- Harrow BS, Eaton CB, Roberts MB, Assaf AR, Luo X, Chen Z. Health utilities associated with hemoglobin levels and blood loss in postmenopausal women: the Women’s Health Initiative. Value Health 2011;14:555-63. http://dx.doi.org/10.1016/j.jval.2010.11.008.
- Lloyd A, van Hanswijck de Jonge P, Doyle S, Cornes P. Health state utility scores for cancer-related anemia through societal and patient valuations. Value Health 2008;11:1178-85. http://dx.doi.org/10.1111/j.1524-4733.2008.00394.x.
- Tajima R, Kondo M, Kai H, Saito C, Okada M, Takahashi H, et al. Measurement of health-related quality of life in patients with chronic kidney disease in Japan with EuroQol (EQ-5D). Clin Exp Nephrol 2010;14:340-8. http://dx.doi.org/10.1007/s10157-010-0304-1.
- Wisloff F, Gulbrandsen N, Hjorth M, Lenhoff S, Fayers P. Quality of life may be affected more by disease parameters and response to therapy than by haemoglobin changes. Eur J Haematol 2005;75:293-8. http://dx.doi.org/10.1111/j.1600-0609.2005.00509.x.
- Dakin H. Review of studies mapping from quality of life or clinical measures to EQ-5D: an online database. Health Qual Life Outcomes 2013;11. http://dx.doi.org/10.1186/1477-7525-11-151.
- Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ 2004;13:873-84. http://dx.doi.org/10.1002/hec.866.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Statistical Bulletin: Cancer Registration Statistics, England, 2011. London: ONS; 2011.
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583-637. http://dx.doi.org/10.1097/00005650-200006000-00004.
- Hoyle M, Crathorne L, Peters J, Jones-Hughes T, Cooper C, Napier M, et al. The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal no.150 and part review of technology appraisal no. 118): a systematic review and economic model. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17140.
- Pavey T, Hoyle M, Ciani O, Crathorne L, Jones-Hughes T, Cooper C, et al. Dasatinib, nilotinib and standard-dose imatinib for the first-line treatment of chronic myeloid leukaemia: systematic reviews and economic analyses. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16420.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit; 2012.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit; 2013.
- 2006/07 UK General Practice Workload Survey. Leeds: The Information Centre; 2007.
- NHS Reference Costs 2012–2013. London: Department of Health; 2013.
- NHSBT Price List 2013/2014. London: Department of Health; 2013.
- Update on the Cost of Blood Components and Prices for the Provision of Diagnostic Services for 2008/09 from the National Blood Service (NBS). London: Department of Health; 2007.
- Vaupel P. Hypoxia and aggressive tumor phenotype: implications for therapy and prognosis. Oncologist 2008;13:21-6. http://dx.doi.org/10.1634/theoncologist.13-S3-21.
- Arcasoy MO, Amin K, Chou SC, Haroon ZA, Varia M, Raleigh JA. Erythropoietin and erythropoietin receptor expression in head and neck cancer: relationship to tumor hypoxia. Clin Cancer Res 2005;11:20-7.
- Nunnaly JC. Psychometric Theory. New York, NY: McGraw Hill; 1978.
- Cazzola M, Beguin Y. New tools for clinical evaluation of erythron function in man. Br J Haematol 1992;80:278-84. http://dx.doi.org/10.1111/j.1365-2141.1992.tb08133.x.
- Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 1975;36:842-54. http://dx.doi.org/10.1002/1097-0142(197509)36:3<842::AID-CNCR2820360303>3.0.CO;2-U.
- Moebus V, Jackisch C, Lueck HJ, du Bois A, Thomssen C, Kurbacher C, et al. Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: mature results of an AGO phase III study. J Clin Oncol 2010;28:2874-80. http://dx.doi.org/10.1200/JCO.2009.24.7643.
- Moebus V, Untch M, du Bois A, Lueck HJ, Thomssen C, Kuhn W, et al. Dose-dense sequential chemotherapy with epirubicin (E), paclitaxel (T) and cyclophosphamide (C) (ETC) is superior to conventional dosed chemotherapy in high-risk breast cancer patients (≥ 4 +LN). First results of an AGO-trial. J Clin Oncol 2004;22.
- Moebus VJ, Lueck HJ, Thomssen C, Kuhn W, Kurbacher C, Nitz U, et al. Dose-dense sequential chemotherapy with epirubicin (E), paclitaxel (T) and cyclophosphamide (C) (ETC) in comparison to conventional dosed chemotherapy in high-risk breast cancer patients (≥ 4 + LN). Mature results of an AGO-trial. Breast Cancer Res Treat 2007;103.
- Ray-Coquard I, Le Cesne A, Rubio MT, Mermet J, Maugard C, Ravaud A, et al. Risk model for severe anemia requiring red blood cell transfusion after cytotoxic conventional chemotherapy regimens. The Elypse 1 Study Group. J Clin Oncol 1999;17:2840-6.
- Tchekmedyian NS, Kallich J, McDermott A, Fayers P, Erder MH. The relationship between psychologic distress and cancer-related fatigue. Cancer 2003;98:198-203. http://dx.doi.org/10.1002/cncr.11463.
- Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Österborg A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer. Eur J Cancer 2004;40:2201-16. http://dx.doi.org/10.1016/j.ejca.2004.07.015.
- Minton O, Stone P, Richardson A, Sharpe M, Hotopf M. Drug therapy for the management of cancer related fatigue. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.cd006704.pub2.
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst 2008;100:1155-66. http://dx.doi.org/10.1093/jnci/djn250.
- Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2006;3. http://dx.doi.org/10.1002/14651858.cd003407.pub4.
- Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennett C, et al. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst 2005;97:489-98. http://dx.doi.org/10.1093/jnci/dji087.
- Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006;98:708-14. http://dx.doi.org/10.1093/jnci/djj189.
- Bohlius JF, Langensiepen S, Engert A, Schwarzer G, Bennett CL. Effectiveness of erythropoietin in the treatment of patients with malignancies: methods and preliminary results of a Cochrane review. Best Pract Res Clin Haematol 2005;18:449-54. http://dx.doi.org/10.1016/j.beha.2005.01.022.
- Bamias A, Aravantinos G, Kalofonos C, Timotheadou N, Siafaka V, Vlahou I, et al. Prevention of anemia in patients with solid tumors receiving platinum-based chemotherapy by recombinant human erythropoietin (rHuEpo): a prospective, open label, randomized trial by the Hellenic Cooperative Oncology Group. Oncology 2003;64:102-10. http://dx.doi.org/10.1159/000067766.
- Cascinu S, Fedeli A, Del Ferro E, Luzi Fedeli S, Catalano G. Recombinant human erythropoietin treatment in cisplatin-associated anemia: a randomized, double-blind trial with placebo. J Clin Oncol 1994;12:1058-62.
- Cazzola M, Messinger D, Battistel V, Bron D, Cimino R, Enller-Ziegler L, et al. Recombinant human erythropoietin in the anemia associated with multiple myeloma or non-Hodgkin’s lymphoma: dose finding and identification of predictors of response. Blood 1995;86:4446-53.
- Iconomou G, Koutras A, Rigopoulos A, Vagenakis AG, Kalofonos HP. Effect of recombinant human erythropoietin on quality of life in cancer patients receiving chemotherapy: results of a randomized, controlled trial. J Pain Symptom Manage 2003;25:512-18. http://dx.doi.org/10.1016/S0885-3924(03)00070-8.
- Kunikane H, Watanabe K, Fukuoka M, Saijo N, Furuse K, Ikegami H, et al. Double-blind randomized control trial of the effect of recombinant human erythropoietin on chemotherapy-induced anemia in patients with non-small cell lung cancer. Int J Clin Oncol 2001;6:296-301. http://dx.doi.org/10.1007/s10147-001-8031-y.
- Oberhoff C, Neri B, Amadori D, Petry KU, Gamucci T, Rebmann U, et al. Recombinant human erythropoietin in the treatment of chemotherapy-induced anemia and prevention of transfusion requirement associated with solid tumors: a randomized, controlled study. Ann Oncol 1998;9:255-60. http://dx.doi.org/10.1023/A:1008296622469.
- Österborg A, Boogaerts MA, Cimino R, Essers U, Holowiecki J, Juliusson G, et al. Recombinant human erythropoietin in transfusion-dependent anemic patients with multiple myeloma and non-Hodgkin’s lymphoma – a randomized multicenter study. The European Study Group of Erythropoietin (Epoetin Beta) Treatment in Multiple Myeloma and Non-Hodgkin’s Lymphoma. Blood 1996;87:2675-82.
- Rosen FR, Haraf DJ, Kies MS, Stenson K, Portugal L, List MA, et al. Multicenter randomized Phase II study of paclitaxel (1-hour infusion), fluorouracil, hydroxyurea, and concomitant twice daily radiation with or without erythropoietin for advanced head and neck cancer. Clin Cancer Res 2003;9:1689-97.
- Throuvalas N, Antonadu D, Boufi M, Lavey RS, Malamos N. Erythropoietin Decreases Transfusion Requirements During Radiochemotherapy n.d.
- Welch RS, James RD, Wilkinson PM, Belli F, Cowan RA. Recombinant human erythropoietin and platinum-based chemotherapy in advanced ovarian cancer. Canc J Sci Am 1995;1:261-6.
- Aapro M, Scherhag A, Burger HU. Effect of treatment with epoetin-beta on survival, tumour progression and thromboembolic events in patients with cancer: an updated meta-analysis of 12 randomised controlled studies including 2301 patients. Br J Cancer 2008;99:14-22. http://dx.doi.org/10.1038/sj.bjc.6604408.
- Aapro M, Scherhag A, Osterwalder B, Ukarma L, Burger HU. Epoetin beta treatment in patients with cancer chemotherapy induced anaemia: the impact of initial haemoglobin and target haemoglobin levels on survival, tumour progression and thromboembolic events. International MASCC/IS00 Symposium, Rome, June 2009. Support Care Cancer 2009;17:1036-7.
- Berndt E, Kallich J, McDermott A, Xu X, Lee H, Glaspy J. Reductions in anaemia and fatigue are associated with improvements in productivity in cancer patients receiving chemotherapy. Pharmacoeconomics 2005;23:505-14. http://dx.doi.org/10.2165/00019053-200523050-00009.
- Blohmer JU, Paepke S, Sehouli J, Boehmer D, Kolben M, Wurschmidt F, et al. Randomized Phase III trial of sequential adjuvant chemoradiotherapy with or without erythropoietin alfa in patients with high-risk cervical cancer: results of the NOGGO-AGO intergroup study. J Clin Oncol 2011;29:3791-7. http://dx.doi.org/10.1200/JCO.2010.30.4899.
- Cabanillas ME, Kantarjian H, Thomas DA, Mattiuzzi GN, Rytting ME, Bruera E, et al. Epoetin alpha decreases the number of erythrocyte transfusions in patients with acute lymphoblastic leukemia, lymphoblastic lymphoma, and Burkitt leukemia/lymphoma: results of a randomized clinical trial. Cancer 2012;118:848-55. http://dx.doi.org/10.1002/cncr.26341.
- Chang J, Couture FA, Young SD, Lau CY, McWatters KL. Weekly administration of epoetin alfa improves cognition and quality of life in patients with breast cancer receiving chemotherapy. Support Cancer Ther 2004;2:52-8. http://dx.doi.org/10.3816/SCT.2004.n.023.
- Charu V, Belani CP, Gill AN, Bhatt M, Tomita D, Rossi G, et al. Efficacy and safety of every-2-week darbepoetin alfa in patients with anemia of cancer: a controlled, randomized, open-label Phase II trial. Oncologist 2007;12:727-37. http://dx.doi.org/10.1634/theoncologist.12-6-727.
- Christodoulou C, Dafni U, Aravantinos G, Koutras A, Samantas E, Karina M, et al. Effects of epoetin-alpha on quality of life of cancer patients with solid tumors receiving chemotherapy. Anticancer Res 2009;29:693-702.
- Engert A, Josting A, Haverkamp H, Villalobos M, Lohri A, Sokler M, et al. Epoetin alfa in patients with advanced-stage Hodgkin’s lymphoma: results of the randomized placebo-controlled GHSG HD15EPO trial. J Clin Oncol 2010;28:2239-45. http://dx.doi.org/10.1200/JCO.2009.25.1835.
- Fujisaka Y, Sugiyama T, Saito H, Nagase S, Kudoh S, Endo M, et al. Randomised, Phase III trial of epoetin-beta to treat chemotherapy-induced anaemia according to the EU regulation. Br J Cancer 2011;105:1267-72. http://dx.doi.org/10.1038/bjc.2011.395.
- Glaspy J, Vadhan-Raj S, Patel R, Bosserman L, Hu E, Lloyd RE, et al. Randomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia: the 20030125 study group trial. J Clin Oncol 2006;24:2290-7. http://dx.doi.org/10.1200/JCO.2005.03.8570.
- Gupta S, Singh PK, Bisth SS, Bhatt ML, Pant M, Gupta R, et al. Role of recombinant human erythropoietin in patients of advanced cervical cancer treated ‘by chemoradiotherapy.’. Cancer Biol Ther 2009;8:13-7. http://dx.doi.org/10.4161/cbt.8.1.7089.
- Hernandez E, Ganly P, Charu V, Dibenedetto J, Tomita D, Lillie T, et al. Randomized, double-blind, placebo-controlled trial of every-3-week darbepoetin alfa 300 micrograms for treatment of chemotherapy-induced anemia. Curr Med Res Opin 2009;25:2109-20. http://dx.doi.org/10.1185/03007990903084164.
- Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol 2005;23:5960-72. http://dx.doi.org/10.1200/JCO.2005.06.150.
- Milroy R, Bajetta E, van den Berg PM, O’Brien MER, Perez-Manga G, Georgoulias V, et al. Effects of epoetin alfa on anemia and patient-reported outcomes in patients with non-small cell lung cancer receiving chemotherapy: results of a European, multicenter, randomized, controlled study. Eur J Clin Med Oncol 2011;3:49-56.
- Nagel S, Kellner O, Engel-Riedel W, Guetz S, Schumann C, Gieseler F, et al. Addition of darbepoetin alfa to dose-dense chemotherapy: results from a randomized Phase II trial in small-cell lung cancer patients receiving carboplatin plus etoposide. Clin Lung Cancer 2011;12:62-9. http://dx.doi.org/10.3816/CLC.2011.n.009.
- O’Shaughnessy JA, Vukelja SJ, Holmes FA, Savin M, Jones M, Royall D, et al. Feasibility of quantifying the effects of epoetin alfa therapy on cognitive function in women with breast cancer undergoing adjuvant or neoadjuvant chemotherapy. Clin Breast Cancer 2005;5:439-46. http://dx.doi.org/10.3816/CBC.2005.n.002.
- Pirker R, Ramlau RA, Schuette W, Zatloukal P, Ferreira I, Lillie T, et al. Safety and efficacy of darbepoetin alpha in previously untreated extensive-stage small-cell lung cancer treated with platinum plus etoposide. J Clin Oncol 2008;26:2342-9. http://dx.doi.org/10.1200/JCO.2007.15.0748.
- Pronzato P, Cortesi E, van der Rijt CC, Bols A, Moreno-Nogueira JA, de Oliveira CF, et al. Epoetin alfa improves anemia and anemia-related, patient-reported outcomes in patients with breast cancer receiving myelotoxic chemotherapy: results of a European, multicenter, randomized, controlled trial. Oncologist 2010;15:935-43. http://dx.doi.org/10.1634/theoncologist.2009-0279.
- Razzouk BI, Hord JD, Hockenberry M, Hinds PS, Feusner J, Williams D, et al. Double-blind, placebo-controlled study of quality of life, hematologic end points, and safety of weekly epoetin alfa in children with cancer receiving myelosuppressive chemotherapy. J Clin Oncol 2006;24:3583-9. http://dx.doi.org/10.1200/JCO.2005.03.4371.
- Reed SD, Radeva JI, Daniel DB, Fastenau JM, Williams D, Schulman KA. Early hemoglobin response and alternative metrics of efficacy with erythropoietic agents for chemotherapy-related anemia. Curr Med Res Opin 2005;21:1527-33. http://dx.doi.org/10.1185/030079905X65394.
- Waltzman R, Croot C, Justice GR, Fesen MR, Charu V, Williams D. Randomized comparison of epoetin alfa (40,000 U weekly) and darbepoetin alfa (200 microg every 2 weeks) in anemic patients with cancer receiving chemotherapy. Oncologist 2005;10:642-50. http://dx.doi.org/10.1634/theoncologist.10-8-642.
- Rosenzweig MQ, Bender CM, Lucke JP, Yasko JM, Brufsky AM. The decision to prematurely terminate a trial of R-HuEPO due to thrombotic events. J Pain Symptom Manage 2004;27:185-90. http://dx.doi.org/10.1016/j.jpainsymman.2003.06.010.
- Savonije JH, van Groeningen CJ, Wormhoudt LW, Giaccone G. Early intervention with epoetin alfa during platinum-based chemotherapy: an analysis of quality-of-life results of a multicenter, randomized, controlled trial compared with population normative data. Oncologist 2006;11:197-205. http://dx.doi.org/10.1634/theoncologist.11-2-197.
- Savonije JH, van Groeningen CJ, Wormhoudt LW, Giaccone G. Early intervention with epoetin alfa during platinum-based chemotherapy: an analysis of the results of a multicenter, randomized, controlled trial based on initial hemoglobin level. Oncologist 2006;11:206-16. http://dx.doi.org/10.1634/theoncologist.11-2-206.
- Savonije JH, van Groeningen CJ, van Bochove A, Honkoop AH, van Felius CL, Wormhoudt LW, et al. Effects of early intervention with epoetin alfa on transfusion requirement, hemoglobin level and survival during platinum-based chemotherapy: results of a multicenter randomised controlled trial. Eur J Cancer 2005;41:1560-9. http://dx.doi.org/10.1016/j.ejca.2005.03.024.
- Schwartzberg LS, Yee LK, Senecal FM, Charu V, Tomita D, Wallace J, et al. A randomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia in patients with breast, lung, or gynecologic cancer. Oncologist 2004;9:696-707. http://dx.doi.org/10.1634/theoncologist.9-6-696.
- Senecal FM, Yee L, Gabrail N, Charu V, Tomita D, Rossi G, et al. Treatment of chemotherapy-induced anemia in breast cancer: results of a randomized controlled trial of darbepoetin alfa 200 microg every 2 weeks versus epoetin alfa 40,000 U weekly. Clin Breast Cancer 2005;6:446-54. http://dx.doi.org/10.3816/CBC.2005.n.050.
- Thomas G, Ali S, Hoebers FJ, Darcy KM, Rodgers WH, Patel M, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dl with erythropoietin vs. above 10.0 g/dl without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol 2008;108:317-25. http://dx.doi.org/10.1016/j.ygyno.2007.10.011.
- Tsuboi M, Ezaki K, Tobinai K, Ohashi Y, Saijo N. Weekly administration of epoetin beta for chemotherapy-induced anemia in cancer patients: results of a multicenter, Phase III, randomized, double-blind, placebo-controlled study. Jpn J Clin Oncol 2009;39:163-8. http://dx.doi.org/10.1093/jjco/hyn151.
- Wilkinson PM, Antonopoulos M, Lahousen M, Lind M, Kosmidis P. Epoetin alfa in platinum-treated ovarian cancer patients: results of a multinational, multicentre, randomised trial. Br J Cancer 2006;94:947-54. http://dx.doi.org/10.1038/sj.bjc.6603004.
- Winquist E, Julian JA, Moore MJ, Nabid A, Sathya J, Wood L, et al. Randomized, double-blind, placebo-controlled trial of epoetin alfa in men with castration-resistant prostate cancer and anemia. J Clin Oncol 2009;27:644-6. http://dx.doi.org/10.1200/JCO.2008.20.4966.
- Strauss HG, Haensgen G, Dunst J, Hayward C, Koelbl H. Effects of anaemia correction with epoetin beta in patients with advanced cervical cancer and radiochemotherapy. J Clin Oncol 2005;23.
- Delarue R, Tilly H, Mounier N, Petrella T, Salles G, Thieblemont C, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 2013;14:525-33. http://dx.doi.org/10.1016/S1470-2045(13)70122-0.
- Boccia R, Malik IA, Raja V, Kahanic S, Liu R, Lillie T, et al. Darbepoetin alfa administered every three weeks is effective for the treatment of chemotherapy-induced anemia. Oncologist 2006;11:409-17. http://dx.doi.org/10.1634/theoncologist.11-4-409.
- Boccia R, Lillie T, Tomita D, Balducci L. The effectiveness of darbepoetin alfa administered every 3 weeks on hematologic outcomes and quality of life in older patients with chemotherapy-induced anemia. Oncologist 2007;12:584-93. http://dx.doi.org/10.1634/theoncologist.12-5-584.
- Glaspy J. Assessment of early and standard intervention with procrit® (epoetin alfa) 120,000 units once every three weeks (q3w) in patients with cancer receiving chemotherapy. Blood 2006;108.
- Gregory SA. Efficacy of darbepoetin alfa in the treatment of chemotherapy-induced anemia in non-Hodgkin’s lymphoma. Support Cancer Ther 2006;3:232-9. http://dx.doi.org/10.3816/SCT.2006.n.021.
- Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the MD Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer 2007;110:1629-40. http://dx.doi.org/10.1002/cncr.22943.
- Larholt K, Pashos CL, Wang Q, Bookhart B, McKenzie RS, Piech CT. Dosing and Outcomes Study of Erythropoiesis-Stimulating Therapies (DOSE): a registry for characterizing anaemia management and outcomes in oncology patients. Clin Drug Investig 2008;28:159-67. http://dx.doi.org/10.2165/00044011-200828030-00003.
- Dammacco F. A Placebo-Controlled Study on the Effect of Epoetin Alfa in Subjects With Multiple Myeloma Followed by an Open-Label Extension 1997.
- Dammacco F. A placebo-controlled study on the effect of epoetin alfa in subjects with multiple myeloma followed by an open-label extension. Eur J Cancer 1997;33. http://dx.doi.org/10.1016/S0959-8049(97)84828-9.
- Dammacco F, Castoldi G, Roedjer S. A placebo-controlled study on the effect of epoetin alfa in subjects with multiple myeloma followed by an open-label extension. Blood 1997;90.
- Reddy PK, Williams D, Wilhelm FE. An open-label pilot to evaluate a flexible dosing regimen of epoetin alfa for the treatment of chemotherapy-induced anemia: 60,000 units weekly followed by 60,000 units every 2 weeks. Support Cancer Ther 2006;4:56-62. http://dx.doi.org/10.3816/SCT.2006.n.032.
- Justice G, Kessler JF, Jadeja J, Campos L, Weick J, Chen CF, et al. A randomized, multicenter study of subcutaneous and intravenous darbepoetin alfa for the treatment of chemotherapy-induced anemia. Ann Oncol 2005;16:1192-8. http://dx.doi.org/10.1093/annonc/mdi218.
- Kotasek D, Canon JL, Mateos MV, Hedenus M, Rossi G, Taylor K. A randomized, controlled trial comparing darbepoetin alfa correction/maintenance dosing with weekly dosing for treating chemotherapy-induced anemia. Curr Med Res Opin 2007;23:1387-401. http://dx.doi.org/10.1185/030079907X188053.
- Glaspy J, Vadhan-Raj S, Patel R, Bosserman L, Hu E, . 20030125 Study Group Trial . Randomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia: the 20030125 Study Group Trial. J Clin Oncol 2006;24:2290-7. http://dx.doi.org/10.1200/JCO.2005.03.8570.
- Montoya VP, Xie J, Williams D, Woodman RC, Wilhelm FE. An extended maintenance dosing regimen of epoetin alfa 80,000 U every 3 weeks in anemic patients with cancer receiving chemotherapy. Support Care Cancer 2007;15:1385-92. http://dx.doi.org/10.1007/s00520-007-0263-6.
- Charu V, Saidman B, Ben-Jacob A, Justice GR, Maniam AS, Tomita D, et al. A randomized, open-label, multicenter trial of immediate versus delayed intervention with darbepoetin alfa for chemotherapy-induced anemia. Oncologist 2007;12:1253-63. http://dx.doi.org/10.1634/theoncologist.12-10-1253.
- Bastit L, Vandebroek A, Altintas S, Gaede B, Pintér T, Suto TS, et al. Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol 2008;26:1611-18. http://dx.doi.org/10.1200/JCO.2006.10.4620.
- Gregory SA, Williams D. Epoetin alfa 60,000 U QW followed by Q2W maintenance dosing corrects anemia and maintains hemoglobin (Hb) in anemic patients with cancer receiving chemotherapy. Blood 2003;102.
- Baltz B, Gregory SA, Ehmann WC, Williams D. Initial dosing of epoetin alfa 60,000 U qw followed by q2w maintenance for anemic patients with cancer receiving chemotherapy. J Clin Oncol 2004;22.
- Gregory SA, Baltz B, Williams D. Every-two-week (Q2W) maintenance dosing with epoetin alfa in anemic patients with cancer receiving chemotherapy: 60,000 U qw to target Hb 12 g/dl, followed by 60,000 U q2w. Blood 2004;104.
- Gregory SA, Xie J, Woodman RC. Maintenance dosing with epoetin alfa every two weeks (q2w) in anemic patients with cancer receiving chemotherapy every one or four weeks: final results. Blood 2005;106.
- Glaspy JA, Charu V, Luo D, Moyo V, Kamin M, Wilhelm FE. Initiation of epoetin-alpha therapy at a starting dose of 120,000 units once every 3 weeks in patients with cancer receiving chemotherapy: an open-label, multicenter study with randomized and nonrandomized treatment arms. Cancer 2009;115:1121-31. http://dx.doi.org/10.1002/cncr.24127.
- Steensma DP, Molina R, Sloan JA, Nikcevich DA, Schaefer PL, Rowland KM, et al. Phase III study of two different dosing schedules of erythropoietin in anemic patients with cancer. J Clin Oncol 2006;24:1079-89. http://dx.doi.org/10.1200/JCO.2005.02.7276.
- Steensma DP, Molina R, Sloan JA, Nikcevich DA, Schaefer PL, Roland KM, et al. A Phase III randomized trial of two different dosing schedules of erythropoietin (EPO) in patients with cancer-associated anemia: North Central Cancer Treatment Group (NCCTG) study N02C2. J Clin Oncol 2005;23.
- Straus DJ, Testa MA, Sarokhan BJ, Czuczman MS, Tulpule A, Turner RR, et al. Quality-of-life and health benefits of early treatment of mild anemia: a randomized trial of epoetin alfa in patients receiving chemotherapy for hematologic malignancies. Cancer 2006;107:1909-17. http://dx.doi.org/10.1002/cncr.22221.
- Glaspy J, Henry D, Patel R, Tchekmedyian S, Applebaum S, Berdeaux D, et al. Effects of chemotherapy on endogenous erythropoietin levels and the pharmacokinetics and erythropoietic response of darbepoetin alfa: a randomised clinical trial of synchronous versus asynchronous dosing of darbepoetin alfa. Eur J Cancer 2005;41:1140-9. http://dx.doi.org/10.1016/j.ejca.2005.01.021.
- Gordon D, Nichols G, Ben-Jacob A, Tomita D, Lillie T, Miller C. Treating anemia of cancer with every-4-week darbepoetin alfa: final efficacy and safety results from a phase II, randomized, double-blind, placebo-controlled study. Oncologist 2008;13:715-24. http://dx.doi.org/10.1634/theoncologist.2007-0241.
- Canon JL, Vansteenkiste J, Bodoky G, Mateos MV, Bastit L, Ferreira I, et al. Randomized, double-blind, active-controlled trial of every-3-week darbepoetin alfa for the treatment of chemotherapy-induced anemia. J Natl Cancer Inst 2006;98:273-84. http://dx.doi.org/10.1093/jnci/djj053.
- Canon JL, Vansteenkiste J, Hedenus M, Gascon P, Bokemeyer C, Ludwig H, et al. Transfusion risk in cancer patients with chemotherapy-induced anemia when initiating darbepoetin alfa therapy at a baseline hemoglobin level of < 9 g/dl versus 9 to < 10 g/dl versus ≥ 10 g/dl: an exploratory analysis of a phase 3 trial. Med Oncol 2012;29:2291-9. http://dx.doi.org/10.1007/s12032-011-0103-x.
- Vansteenkiste J, Hedenus M, Gascon P, Bokemeyer C, Ludwig H, Vermorken J, et al. Darbepoetin alfa for treating chemotherapy-induced anemia in patients with a baseline hemoglobin level < 10 g/dl versus > or = 10 g/dl: an exploratory analysis from a randomized, double-blind, active-controlled trial. BMC Cancer 2009;9. http://dx.doi.org/10.1186/1471-2407-9-311.
- Schwartzberg L, Burkes R, Mirtsching B, Rearden T, Silberstein P, Yee L, et al. Comparison of darbepoetin alfa dosed weekly (QW) vs. extended dosing schedule (EDS) in the treatment of anemia in patients receiving multicycle chemotherapy in a randomized, phase 2, open-label trial. BMC Cancer 2010;10. http://dx.doi.org/10.1186/1471-2407-10-581.
- Schouwink JH, Codrington H, Sleeboom HP, Kerkhofs LG, Wormhoudt LW. Prevention of anaemia by early intervention with once weekly epoetin alfa during chemotherapy. Eur J Cancer 2008;44:819-29. http://dx.doi.org/10.1016/j.ejca.2008.02.017.
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570-9.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. http://dx.doi.org/10.1097/00005650-199206000-00002.
- Hunt SM, McEwen J, McKenna SP. Measuring health status: a new tool for clinicians and epidemiologists. J R Coll Gen Pract 1985;35:185-8.
- Bowling A. Measuring Health, A Guide to Rating Scales and Questionnaires. Buckingham: Oxford University Press; 1997.
- Bretscher M, Rummans T, Sloan J, Kaur J, Bartlett A, Borkenhagen L, et al. Quality of life in hospice patients. A pilot study. Psychosomatics 1999;40:309-13. http://dx.doi.org/10.1016/S0033-3182(99)71224-7.
- Sloan JA, Loprinzi CL, Kuross SA, Miser AW, O’Fallon JR, Mahoney MR, et al. Randomized comparison of four tools measuring overall quality of life in patients with advanced cancer. J Clin Oncol 1998;16:3662-73.
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med 1983;13:595-60. http://dx.doi.org/10.1017/S0033291700048017.
- Morasso G, Costantini M, Baracco G, Borreani C, Capelli M. Assessing psychological distress in cancer patients: validation of a self-administered questionnaire. Oncology 1996;53:295-302. http://dx.doi.org/10.1159/000227576.
- de Haes J, Curran D, Young T, Bottomley A, Flechtner H, Aaronson N, et al. Quality of life evaluation in oncological clinical trials – the EORTC model. The EORTC Quality of Life Study Group. Eur J Cancer 2000;36:821-5. http://dx.doi.org/10.1016/S0959-8049(00)00007-1.
- Michallet M, Goldet K, Sobh M, Morisset S, Chelghoum Y, Thomas X, et al. Prospective study of erythropoietin use on quality of life and cost effectiveness in acute myeloid leukemia and allogeneic hematopoietic stem cell transplantation patients. Cancer 2013;119:107-14. http://dx.doi.org/10.1002/cncr.27686.
Appendix 1 Literature search strategies
Clinical effectiveness
MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to May Week 3 2013.
Date searched: 24 May 2013.
Searcher: SB.
Hits: 342.
Strategy
-
(erythropoietin* or EPO).tw.
-
Erythropoietin/
-
Receptors, erythropoietin/
-
erythropoiesis.tw.
-
Erythropoiesis/
-
(epoetin adj1 (alfa or beta or theta or zeta)).tw.
-
darbepoetin.tw.
-
CERA.tw.
-
(eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp).tw.
-
or/1-9
-
an?emi?.tw.
-
exp anemia/
-
11 or 12
-
(cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumo?r* or myelo* or lymphoma* or oncolog* or chemotherap*).tw.
-
exp neoplasms/
-
14 or 15
-
(random* or rct* or “controlled trial*” or “clinical trial*”).tw.
-
randomized controlled trial.pt.
-
17 or 18
-
10 and 13 and 16 and 19
-
limit 20 to (english language and yr=”2004 -Current”)
MEDLINE(R) In-Process & Other Non-Indexed Citations
Host: Ovid.
Data parameters: 23 May 2013.
Date searched: 24 May 2013.
Searcher: SB.
Hits: 28.
Strategy
-
(erythropoietin* or EPO).tw.
-
erythropoiesis.tw.
-
(epoetin adj1 (alfa or beta or theta or zeta)).tw.
-
darbepoetin.tw.
-
CERA.tw.
-
(eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp).tw.
-
or/1-6
-
an?emi?.tw.
-
(cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumo?r* or myelo* or lymphoma* or oncolog* or chemotherap*).tw.
-
(random* or rct* or “controlled trial*” or “clinical trial*”).tw.
-
7 and 8 and 9 and 10
-
limit 11 to yr=”2004 -Current”
EMBASE
Host: Ovid.
Data parameters: 1980 to Week 21 2013.
Date searched: 29 May 2013.
Searcher: SB.
Hits: 865.
Strategy
-
(erythropoietin* or EPO).tw.
-
Erythropoietin/
-
Receptors, erythropoietin/
-
recombinant erythropoietin/
-
erythropoiesis.tw.
-
Erythropoiesis/
-
(epoetin adj1 (alfa or beta or theta or zeta)).tw.
-
darbepoetin.tw.
-
novel erythropoiesis stimulating protein/
-
CERA.tw.
-
continuous erythropoiesis receptor activator/
-
(eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp).tw.
-
or/1-12
-
an?emi?.tw.
-
exp anemia/
-
14 or 15
-
(cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumo?r* or myelo* or lymphoma* or oncolog* or chemotherap*).tw.
-
exp neoplasms/
-
17 or 18
-
(random* or rct* or “controlled trial*” or “clinical trial*”).tw.
-
13 and 16 and 19 and 20
-
limit 21 to (english language and yr=”2004 -Current”)
Cochrane Central Register of Controlled Trials
Host: The Cochrane Library.
Data parameters: Issue 4 of 12, April 2013.
Date Searched: 24 May 2013.
Searcher: SB.
Hits: 219.
Strategy
-
(erythropoietin* or EPO):ti or (erythropoietin* or EPO):ab from 2004, in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials, Technology Assessments and Economic Evaluations
-
MeSH descriptor: [Erythropoietin] explode all trees
-
MeSH descriptor: [Receptors, Erythropoietin] explode all trees
-
erythropoiesis:ti or erythropoiesis:ab from 2004, in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials, Technology Assessments and Economic Evaluations
-
MeSH descriptor: [Erythropoiesis] explode all trees
-
(epoetin near/1 (alfa or beta or theta or zeta)):ti or (epoetin near/1 (alfa or beta or theta or zeta)):ab from 2004, in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials, Technology Assessments and Economic Evaluations
-
darbepoetin:ti or darbepoetin:ab from 2004, in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials, Technology Assessments and Economic Evaluations
-
CERA:ti or CERA:ab from 2004, in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials, Technology Assessments and Economic Evaluations
-
(eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp):ti or (eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp):ab from 2004, in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials, Technology Assessments and Economic Evaluations
-
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
-
anemi? or anaemi?:ti or anemi? or anaemi?:ab from 2004, in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials, Technology Assessments and Economic Evaluations
-
MeSH descriptor: [Anemia] explode all trees
-
#11 or #12
-
(cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumour* or tumor* or myelo* or lymphoma* or oncolog* or chemotherap*):ti or (cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumour* or tumor* or myelo* or lymphoma* or oncolog* or chemotherap*):ab from 2004, in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials, Technology Assessments and Economic Evaluations
-
MeSH descriptor: [Neoplasms] explode all trees
-
#14 or #15
-
#10 and #13 and #16 from 2004, in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials, Technology Assessments and Economic Evaluations
Web of Science
Host: Thomson Reuters.
Data parameters: not applicable.
Date searched: 28 May 2013.
Searcher: SB.
Hits: 745.
Strategy
-
Title=((erythropoietin* or EPO)) OR Topic=((erythropoietin* or EPO))
-
Title=(erythropoiesis) OR Topic=(erythropoiesis)
-
Title=((epoetin near/0 (alfa or beta or theta or zeta))) OR Topic=((epoetin near/0 (alfa or beta or theta or zeta)))
-
Title=(darbepoetin) OR Topic=(darbepoetin)
-
Title=(CERA) OR Topic=(CERA)
-
Title=((eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp)) OR Topic=((eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp))
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6
-
Title=(anemi* OR anaemi*) OR Topic=(anemi* OR anaemi*)
-
TI=((cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumour* or tumor* or myelo* or lymphoma* or oncolog* or chemotherap*)) OR TS=((cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumour* or tumor* or myelo* or lymphoma* or oncolog* or chemotherap*))
-
Title=((random* or rct* or “controlled trial*” or “clinical trial*”)) OR Topic=((random* or rct* or “controlled trial*” or “clinical trial*”))
-
#7 AND #8 AND #9 AND #10 Timespan=2004-2013
Cumulative Index to Nursing and Allied Health Literature
Host: EBSCOhost.
Data parameters: not applicable.
Date searched: 29 May 2013.
Searcher: SB.
Hits: 79.
Strategy
-
TI(erythropoietin* or EPO) OR AB(erythropoietin* or EPO)
-
(MH “Erythropoietin”)
-
TI(erythropoiesis) OR AB(erythropoiesis)
-
(MH “Erythropoiesis”)
-
TI(epoetin n0 (alfa or beta or theta or zeta)) OR AB(epoetin n0 (alfa or beta or theta or zeta))
-
TI(darbepoetin) OR AB(darbepoetin)
-
TI(CERA) OR AB(CERA)
-
TI(eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp) OR AB(eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp)
-
S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8
-
TI(anemi* or anaemi*) OR AB(anemi* or anaemi*)
-
(MH “Anemia+”)
-
S10 OR S11
-
TI(cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumor* or tumour* or myelo* or lymphoma* or oncolog* or chemotherap*) OR AB(cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumor* or tumour* or myelo* or lymphoma* or oncolog* or chemotherap*)
-
(MH “Neoplasms+”)
-
S13 OR S14
-
TI(random* or rct* or “controlled trial*” or “clinical trial*”) OR AB(random* or rct* or “controlled trial*” or “clinical trial*”)
-
PT(randomized controlled trial)
-
S16 OR S17
-
S9 AND S12 AND S15 AND S18
Date limited 2004–current.
Numbers of references retrieved and deduplicated: clinical effectiveness review
| Database | Hits |
|---|---|
| MEDLINE | 342 |
| MEDLINE In-Process & Other Non-Indexed Citations | 28 |
| EMBASE | 865 |
| CENTRAL | 219 |
| Web of Science | 745 |
| CINAHL | 79 |
| Total | 2278 |
| Automatically deduplicated | 845 |
| Manually deduplicated | 97 |
| Total records to screen | 1336 |
Cost-effectiveness
MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to May Week 3 2013.
Date searched: 29 May 2013.
Searcher: SB.
Hits: 144.
Strategy
Lines 1–16: see MEDLINE clinical effectiveness strategy.
-
(pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or “health utilit*” or “value for money”).tw.
-
(fiscal or funding or financial or finance* or expenditure* or budget*).tw.
-
(“resource* alloca*” or “resource* use”).tw.
-
exp Economics/
-
exp models, economic/
-
exp “Costs and Cost Analysis”/
-
Cost of illness/
-
ec.fs.
-
(decision adj2 (model* or tree* or analy*)).tw.
-
markov.tw.
-
decision trees/
-
or/17-27
-
10 and 13 and 16 and 28
-
limit 29 to (english language and yr=”2004 -Current”)
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations
Host: Ovid.
Data parameters: 28 May 2013.
Date searched: 29 May 2013.
Searcher: SB.
Hits: 13.
Strategy
Lines 1–9: see MEDLINE In-Process & Other Non-Indexed Citations clinical effectiveness strategy.
-
(pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or “health utilit*” or “value for money”).tw.
-
(fiscal or funding or financial or finance* or expenditure* or budget*).tw.
-
(“resource* alloca*” or “resource* use”).tw.
-
(decision adj2 (model* or tree* or analy*)).tw.
-
markov.tw.
-
or/10-14
-
7 and 8 and 9 and 15
-
limit 16 to yr=”2004 -Current”
EMBASE
Host: Ovid.
Data parameters: 1980 to Week 21 2013.
Date searched: 29 May 2013.
Searcher: SB.
Hits: 677.
Strategy
Lines 1–19: see EMBASE clinical effectiveness strategy.
-
(pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or “health utilit*” or “value for money”).tw.
-
(fiscal or funding or financial or finance* or expenditure* or budget*).tw.
-
(“resource* alloca*” or “resource* use”).tw.
-
exp Economics/
-
models, economic/
-
exp health economics/
-
exp “Costs and Cost Analysis”/
-
Cost of illness/
-
resource allocation/
-
pe.fs.
-
(decision adj2 (model* or tree* or analy*)).tw.
-
markov.tw.
-
decision trees/
-
or/20-32
-
13 and 16 and 19 and 33
-
limit 34 to (english language and yr=”2004 -Current”)
NHS Economic Evaluation Database
Host: The Cochrane Library.
Data parameters: Issue 2 of 4, April 2013.
Date searched: 24 May 2013.
Searcher: SB.
Hits: 10.
Strategy
See CENTRAL clinical effectiveness strategy.
Web of Science
Host: Thomson Reuters.
Data parameters: not applicable.
Date searched: 29 May 2013.
Searcher: SB.
Hits: 173.
Strategy
Lines 1–9: see Web of Science clinical effectiveness strategy.
-
TI=((pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or “health utilit*” or “value for money”)) OR TS=((pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or “health utilit*” or “value for money”))
-
Title=((fiscal or funding or financial or finance* or expenditure* or budget*)) OR Topic=((fiscal or funding or financial or finance* or expenditure* or budget*))
-
Title=((“resource* alloca*” or “resource* use”)) OR Topic=((“resource* alloca*” or “resource* use”))
-
Title=((decision near/1 (model* or tree* or analy*))) OR Topic=((decision near/1 (model* or tree* or analy*)))
-
Title=(markov) OR Topic=(markov)
-
#14 OR #13 OR #12 OR #11 OR #10
-
#15 AND #9 AND #8 AND #7 Timespan=2004-2013
Cumulative Index to Nursing and Allied Health Literature
Host: EBSCOhost.
Data parameters: not applicable.
Date searched: 29 May 2013.
Searcher: SB.
Hits: 81.
Strategy
Lines 1–15: see CINAHL clinical effectiveness strategy.
-
TI(pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or “health utilit*” or “value for money”) OR AB(pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or “health utilit*” or “value for money”)
-
TI(fiscal or funding or financial or finance* or expenditure* or budget*) OR AB(fiscal or funding or financial or finance* or expenditure* or budget*)
-
TI(“resource* alloca*” or “resource* use”) OR AB(“resource* alloca*” or “resource* use”)
-
(MH “Economics+”)
-
TI(decision n1 (model* or tree* or analy*)) OR AB(decision n1 (model* or tree* or analy*))
-
TI(markov) OR AB(markov)
-
(MH “Decision Trees”)
-
S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22
-
S9 AND S12 AND S15 AND S23
Date limited 2004–current.
Health Economic Evaluations Database
Host: The Cochrane Library.
Data parameters: not applicable.
Date searched: 29 May 2013.
Searcher: SB.
Hits: 33.
Strategy
-
TI=(erythropoietin* or EPO)
-
TI=erythropoiesis
-
TI=(epoetin alfa or epoetin beta or epoetin theta or epoetin zeta)
-
TI=darbepoetin
-
TI=CERA
-
TI=(eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp)
-
AB=(erythropoietin* or EPO)
-
AB=erythropoiesis
-
AB=(epoetin alfa or epoetin beta or epoetin theta or epoetin zeta)
-
AB=darbepoetin
-
AB=CERA
-
AB=(eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp)
-
CS=(1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12)
-
TI=(anaemi* or anemi*)
-
AB=(anaemi* or anemi*)
-
CS=(14 or 15)
-
TI=(cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumor* or tumour* or myelo* or lymphoma* or oncolog* or chemotherap*)
-
AB=(cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumor* or tumour* or myelo* or lymphoma* or oncolog* or chemotherap*)
-
CS=(17 or 18)
-
CS=(13 and 16 and 19)
Numbers of references retrieved and deduplicated: cost-effectiveness review
| Database | Hits |
|---|---|
| MEDLINE | 144 |
| MEDLINE In-Process & Other Non-Indexed Citations | 13 |
| EMBASE | 677 |
| NHS EED | 10 |
| Web of Science | 173 |
| CINAHL | 81 |
| HEED | 33 |
| Total | 1131 |
| Automatically de-duplicated | 279 |
| Manually de-duplicated | 38 |
| Total records to screen | 814 |
Quality of life
MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to May Week 4 2013.
Date searched: 30 May 2013.
Searcher: SB.
Hits: 369.
Strategy
Lines 1–16: see MEDLINE clinical effectiveness strategy.
-
(“quality of life” or QoL or HRQL or HRQoL).tw.
-
quality of life/
-
(“quality adjusted life year*” or QALY*).tw.
-
quality-adjusted life years/
-
“activities of daily living”.tw.
-
activities of daily living/
-
(“quality of wellbeing” or QWB or “QWB SA”).tw.
-
(“health* year* equivalent*” or HYE*).tw.
-
“health status”.tw.
-
health status/
-
health status indicators/
-
Psychometrics/
-
psychometric*.tw.
-
(“short form 36” or “SF-36” or SF36).tw.
-
(“short form 20” or “SF-20” or SF20).tw.
-
(“short form 12” or “SF-12” or SF12).tw.
-
(“short form 8” or “SF-8” or SF8).tw.
-
(Euroqol or “EQ-5D”).tw.
-
exp Questionnaires/
-
or/17-35
-
10 and 13 and 16 and 36
-
limit 37 to (english language and yr=”2004 -Current”)
MEDLINE(R) In-Process & Other Non-Indexed Citations
Host: Ovid.
Data parameters: 29 May 2013.
Date searched: 30 May 2013.
Searcher: SB.
Hits: 19.
Strategy
Lines 1–9: see MEDLINE In-Process & Other Non-Indexed Citations clinical effectiveness strategy.
-
(“quality of life” or QoL or HRQL or HRQoL).tw.
-
(“quality adjusted life year*” or QALY*).tw.
-
“activities of daily living”.tw.
-
(“quality of wellbeing” or QWB or "QWB SA").tw.
-
(“health* year* equivalent*" or HYE*).tw.
-
“health status”.tw.
-
psychometric*.tw.
-
(“short form 36” or “SF-36” or SF36).tw.
-
(“short form 20” or “SF-20” or SF20).tw.
-
(“short form 12” or “SF-12” or SF12).tw.
-
(“short form 8” or “SF-8” or SF8).tw.
-
(Euroqol or “EQ-5D”).tw.
-
or/10-21
-
7 and 8 and 9 and 22
-
limit 23 to yr=”2004 -Current”
EMBASE
Host: Ovid.
Data parameters: 1980 to Week 21 2013.
Date searched: 30 May 2013.
Searcher: SB.
Hits: 952.
Strategy
Lines 1–19: see EMBASE clinical effectiveness strategy.
-
(“quality of life” or QoL or HRQL or HRQoL).tw.
-
exp quality of life/
-
(“quality adjusted life year*” or QALY*).tw.
-
“activities of daily living”.tw.
-
daily life activity/
-
(“quality of wellbeing” or QWB or “QWB SA”).tw.
-
(“health* year* equivalent*” or HYE*).tw.
-
“health status”.tw.
-
health status/
-
health status indicators/
-
psychometric*.tw.
-
psychometry/
-
(“short form 36” or “SF-36” or SF36).tw.
-
(“short form 20” or “SF-20” or SF20).tw.
-
(“short form 12” or “SF-12” or SF12).tw.
-
(“short form 8” or “SF-8” or SF8).tw.
-
exp questionnaire/
-
or/20-36
-
13 and 16 and 19 and 37
-
limit 38 to (english language and yr=”2004 -Current”)
Web of Science
Host: Thomson Reuters.
Data parameters: not applicable.
Date searched: 30 May 2013.
Searcher: SB.
Hits: 646.
Strategy
Lines 1–9: see Web of Science clinical effectiveness strategy.
-
Title=((“quality of life” or QoL or HRQL or HRQoL)) OR Topic=((“quality of life” or QoL or HRQL or HRQoL))
-
Title=((“quality adjusted life year*” or QALY*)) OR Topic=((“quality adjusted life year*” or QALY*))
-
Title=(“activities of daily living”) OR Topic=(“activities of daily living”)
-
Title=((“quality of wellbeing" or QWB or "QWB SA")) OR Topic=((“quality of wellbeing” or QWB or “QWB SA”))
-
Title=((“health* year* equivalent*” or HYE*)) OR Topic=((“health* year* equivalent*” or HYE*))
-
Title=(“health status”) OR Topic=(“health status”)
-
Title=(psychometric*) OR Topic=(psychometric*)
-
Title=((“short form 20” or “SF-20” or SF20)) OR Topic=((“short form 20” or “SF-20” or SF20))
-
Title=((“short form 12” or “SF-12” or SF12)) OR Topic=((“short form 12” or “SF-12” or SF12))
-
Title=((“short form 8” or “SF-8” or SF8)) OR Topic=((“short form 8” or “SF-8” or SF8))
-
Title=((Euroqol or “EQ-5D”)) OR Topic=((Euroqol or “EQ-5D”))
-
#20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10
-
#21 AND #9 AND #8 AND #7 Timespan=2004-2013
Cumulative Index to Nursing and Allied Health Literature
Host: EBSCOhost.
Data parameters: not applicable.
Date searched: 30 May 2013.
Searcher: SB.
Hits: 111.
Strategy
Lines 1–15: see CINAHL clinical effectiveness strategy.
-
TI(“quality of life” or QoL or HRQL or HRQoL) OR AB(“quality of life” or QoL or HRQL or HRQoL)
-
(MH “Quality of Life+”)
-
TI(“quality adjusted life year*” or QALY*) OR AB(“quality adjusted life year*” or QALY*)
-
(MH “Quality-Adjusted Life Years”)
-
TI(“activities of daily living”) OR AB(“activities of daily living”)
-
(MH “Activities of Daily Living+”)
-
TI(“quality of wellbeing” or QWB or “QWB SA”) OR AB(“quality of wellbeing” or QWB or “QWB SA”)
-
TI(“health* year* equivalent*” or HYE*) OR AB(“health* year* equivalent*” or HYE*)
-
TI(“health status”) OR AB(“health status”)
-
(MH “Health Status+”)
-
(MH “Health Status Indicators”)
-
TI(psychometric*) OR AB(psychometric*)
-
(MH “Psychometrics”)
-
TI(“short form 36” or “SF-36” or SF36) OR AB(“short form 36” or “SF-36” or SF36)
-
TI(“short form 20” or “SF-20” or SF20) OR AB(“short form 20” or “SF-20” or SF20)
-
TI(“short form 12” or “SF-12” or SF12) OR AB(“short form 12” or “SF-12” or SF12)
-
TI(“short form 8” or “SF-8” or SF8) OR AB(“short form 8” or “SF-8” or SF8)
-
TI(Euroqol or “EQ-5D”) OR AB(Euroqol or “EQ-5D”)
-
(MH “Questionnaires+”)
-
S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34
-
S9 AND S12 AND S15 AND S35
Date limited 2004-current.
British Nursing Index
Host: ProQuest.
Data parameters: not applicable.
Date searched: 31 May 2013.
Searcher: SB.
Hits: 43.
Strategy
(TI,AB((erythropoietin* or EPO or erythropoiesis) OR (epoetin near/1 (alfa or beta or theta or zeta)) OR (eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp))) AND (TI,AB(anaemi* or anemi*)) AND (TI,AB(cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumo?r* or myelo* or lymphoma* or oncolog* or chemotherap*))
Date limited 2004–current.
Numbers of references retrieved and deduplicated: quality of life
| Database | Hits |
|---|---|
| MEDLINE | 369 |
| MEDLINE In-Process & Other Non-Indexed Citations | 19 |
| EMBASE | 952 |
| Web of Science | 646 |
| CINAHL | 111 |
| British Nursing Index | 43 |
| Total | 2140 |
| Automatically deduplicated | 805 |
| Manually deduplicated | 67 |
| Total records to screen | 1268 |
Update searches
Numbers of references retrieved and deduplicated
All update searches were run on 2 December 2013 and date limited from 1 January 2013 to 2 December 2013.
| Database | Hits |
|---|---|
| Clinical effectiveness | |
| MEDLINE | 11 |
| MEDLINE In-Process & Other Non-Indexed Citations | 8 |
| EMBASE | 44 |
| CENTRAL | 2 |
| Web of Science | 32 |
| CINAHL | 1 |
| Total | 98 |
| Automatically deduplicated | 30 |
| Manually deduplicated | 0 |
| Total records to screen | 68 |
| Cost effectiveness | |
| MEDLINE | 8 |
| MEDLINE In-Process & Other Non-Indexed Citations | 5 |
| EMBASE | 47 |
| NHS EED | 2 |
| Web of Science | 11 |
| CINAHL | 0 |
| HEED | 0 |
| Total | 73 |
| Automatically deduplicated | 17 |
| Manually deduplicated | 5 |
| Total records to screen | 51 |
| Quality of life | |
| MEDLINE | 9 |
| MEDLINE In-Process & Other Non-Indexed Citations | 8 |
| EMBASE | 46 |
| Web of Science | 25 |
| CINAHL | 0 |
| British Nursing Index | 0 |
| Total | 88 |
| Automatically deduplicated | 24 |
| Manually deduplicated | 3 |
| Total records to screen | 61 |
Supplementary searches (1): reviews and reports
Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects and Health Technology Assessment database
Host: The Cochrane Library.
Data parameters: CDSR: Issue 4 of 12, April 2013; DARE and HTA database: Issue 2 of 4, April 2013.
Date searched: 24 May 2013.
Searcher: SB.
Hits: CDSR = 8; DARE = 16; HTA database = 6.
Strategy
See CENTRAL clinical effectiveness strategy.
Health Management Information Consortium
Host: Ovid.
Data parameters: 1979 to March 2013.
Date searched: 30 May 2013.
Searcher: SB.
Hits: 2.
Strategy
-
(erythropoietin* or EPO).tw.
-
erythropoiesis.tw.
-
(epoetin adj1 (alfa or beta or theta or zeta)).tw.
-
darbepoetin.tw.
-
CERA.tw.
-
(eprex or erypo or HEXAL or procrit or abseamed or epogen or binocrit or neorecormon or eporatio or retacrit or silapo or aranesp).tw.
-
or/1-6
-
an?emi?.tw.
-
(cancer* or carcinom* or leukemia or neoplasm* or malignan* or tumo?r* or myelo* or lymphoma* or oncolog* or chemotherap*).tw.
-
7 and 8 and 9
-
limit 10 to yr=“2004 -Current”
Numbers of references retrieved and deduplicated: reviews and reports
| Database | Hits |
|---|---|
| CDSR | 8 |
| DARE | 16 |
| HTA | 6 |
| HMIC | 2 |
| Total | 32 |
| Manually deduplicated | 3 |
| Total records to screen | 29 |
Supplementary searches (2): haemoglobin level
The references retrieved for these two searches were not deduplicated because the searches were carried out only in MEDLINE and each search was sent to the review team as a separate EndNote file.
Haemoglobin level over time after stopping chemotherapy
MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to September Week 1 2013.
Date searched: 17 September 2013.
Searcher: SB.
Hits: 159.
Strategy
-
(haemoglobin* or hemoglobin*).tw.
-
exp Hemoglobins/
-
(hgb or hb).tw.
-
or/1-3
-
((post or after* or subsequent* or following) adj5 chemo*).tw.
-
postchemo*.tw.
-
or/5-6
-
an?emi?.tw.
-
exp anemia/
-
or/8-9
-
4 and 7 and 10
Utilities as a function of haemoglobin level
MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to September Week 1 2013.
Date searched: 18 September 2013.
Searcher: SB.
Hits: 258.
Strategy
-
(haemoglobin* or hemoglobin*).tw.
-
exp hemoglobins/
-
(hgb or hb).tw.
-
or/1-3
-
an?emi?.tw.
-
exp anemia/
-
or/5-6
-
(utility or utilities or “EQ-5D” or “SF-6D” or “EORTC-QLQ-C30” or HUI2 or “time trade-off” or TTO or “standard gamble” or SG or “quality-adjusted life year*” or QALY? or “discrete choice” or “stated preference”).tw.
-
Quality-Adjusted Life Years/
-
8 or 9
-
4 and 7 and 10
Appendix 2 Data extraction forms
Clinical effectiveness and health-related quality-of-life review: data extraction forms (primary studies)
| EndNote ref. ID: 2706 | Malignancy type: anaemic cancer (primary myeloid malignancies and acute leukaemias excluded) | |
| Treatment: rHuEPO (Amgen Inc.; assumed epoetin alfa) | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Abels 199363 | n = 413. Three populations: cyclic non-cisplatin-containing chemotherapy (n = 157), cyclic cisplatin-containing chemotherapy (n = 132) and no chemotherapy (n = 124) vs. placebo (n = 200)a |
| Objective | To examine the safety of rHuEPO treatment and its impact on haematocrit, transfusion requirements and quality of life | Inclusion criteria: > 18 years of age; biopsy-proven diagnosis of cancer (with primary myeloid malignancies and acute leukaemias excluded); anaemia: haematocrit of ≤ 32% or Hb level ≤ 10.5 g/dl; ECOG score of ≤ 3; life expectancy ≥ 3 months; cyclic cisplatin- and non-cisplatin-containing chemotherapy administered < 5 days every 3–4 weeks Exclusion criteria: known cerebral metastases; uncontrolled hypertension; acute illness within 7 days of study entry; radiation or surgery within 30 days of study entry; experimental therapy within 30 days of study entry; androgen therapy within 2 months of study entry; evidence of renal insufficiency (i.e. serum creatinine ≥ 2 mg/dl); evidence of folate, B12 and/or iron deficiency, autoimmune haemolysis or presence of gastrointestinal bleeding |
| No. of centres | NR | |
| Other references/aliases | Abels 1996,59 Henry 1994,85 Henry 1995,58 Case 1993,86 see note for more details | |
| Geographical setting | NR | |
| Duration of treatment | 12 weeks | |
| Length of follow-up (if different) | After completion of double-blind therapy, patients were eligible to receive rHuEPO on an open-label basis. Henry and colleagues85 provide results for the first 6 months of epoetin therapy (combined double-blind and open-label data: the mean duration of epoetin therapy was 17.1, 18.2 and 15.8 weeks for no chemotherapy, non-cisplatin-containing chemotherapy and cisplatin-containing chemotherapy respectively | |
| Country of corresponding author | USA | |
| Language of publication | English | |
| Sources of funding | NR | |
| Randomisation and allocation | Series of double-blind, placebo-controlled trials: three populations of anaemic cancer patients were randomised to rHuEPO or placebo. The three populations were (1) patients not receiving concomitant chemotherapy, (2) patients receiving chemotherapeutic regimens that did not contain cisplatin and (3) patients receiving chemotherapeutic regimens that contained cisplatin | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Epoetin | Placebo |
| n | 206 (efficacy population) | 190 (efficacy population) |
| Dose and frequency (once daily, twice daily, etc.) | 150 U/kg three times a week | 150 U/kg three times a week |
| Dose adjustment (yes/no) | Yes | |
| Route of administration | Subcutaneously | Subcutaneously |
| Duration of epoetin treatment | ||
| Adjuvant anaemia treatment | NR | NR |
| Transfusion trigger | NR | NR |
| Outcomes | |
|---|---|
| Primary outcome | – |
| Other outcomes | RBCT (number of units of blood transfused per patient and the proportion of patients transfused requirements); haematological response (haematocrit: change from baseline, mean weekly haematocrit, number of correctors and responders,a neutrophil analyses; platelet analyses; HRQoL (100-mm VAS: energy level, ability to perform daily activities and overall quality of life) |
| Analysis | |
|---|---|
| Statistical technique used | Fischer’s exact test was used for statistical inference for dichotomous variables (e.g. sex by treatment group) formulated as 2 × 2 tables. The extended Mantel–Haenszel test with integer scores was used for other types of discrete data. Between-group comparisons of means were analysed with two-sample t-tests and changes from baseline to final value were analysed with paired t-tests. A linear model approach was used for inference on major efficacy variables such as transfusion requirements. These models were constructed with treatment group and covariant factors such as endogenous serum erythropoietin level, haematocrit, performance score, etc. All statistical tests were two-sided, with α = 0.05 |
| ITT analysis? | Patients were considered evaluable for efficacy if they completed > 15 days on the study. All patients were evaluable for safety |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation)? | NR |
| Attrition rate (loss to follow-up)? | Unclear: ITT n = 413 (epoetin n = 213 and placebo n = 200); efficacy population n = 396 (epoetin n = 206, placebo n = 190) |
| Was attrition rate adequately dealt with? | NR |
| No. (%) followed up from each condition? | Unclear; Henry and colleagues85 provide results for the first 6 months of epoetin therapy (combined double-blind and open-label data): n = 363, efficacy population n = 347 |
| Baseline characteristics | ||
|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Anaemic cancer (primary myeloid malignancies and acute leukaemias excluded) | |
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Cyclic non-cisplatin chemotherapy (n = 157) and cyclic cisplatin chemotherapy (n = 132) | |
| Adjuvant anaemia treatment | Iron | NR |
| G-CSF | NR | |
| Transfusion trigger | NR | |
| Hb inclusion criterion level | Anaemia: haematocrit of ≤ 32% or a Hb level ≤ 10.5 g/dl | |
| Arm 1 = epoetin (n = 213) | Arm 2 = placebo (n = 200) | p-value | |
|---|---|---|---|
| Baseline demographics and clinical characteristics are reported for the entire enrolled patient population and are not separated out by chemotherapy treatment | |||
| Sex | |||
| Male, n | 102 | 95 | |
| Female, n | 111 | 105 | |
| Age (years), mean (SD) | 61.2 (13) | 62.5 (14.1) | |
| Patients evaluable for efficacy | n = 206 | n = 190 | |
| Patients transfused, % | 44.7 | 48.4 | |
| No. of RBC units transfused per patient per month prior to the study, mean (SD) | 0.67 (1.08) | 0.73 (1.04) | |
| Mean haematocrit, mean (SD) | 29.1 (4) | 28.5 (3.8) | |
| Haematocrit, mean (SD) | |||
| Non-cisplatin chemotherapy | (n = 79) 28.6 (3.9) | (n = 74) 29.4 (3) | |
| Cisplatin chemotherapy | (n = 64) 29.4 (4) | (n = 61) 28.4 (14.5) | |
| Endogenous serum erythropoietin level (mU/ml), mean (SD) [median] | 146 (260) [76] | 149 (217) [85] | |
| Median serum erythropoietin level (mU/ml) | NR | NR | |
| Overall quality of life (mm), mean (SD) | 50 (24) | 50.4 (26) | |
| Tumour type | |||
| Haematological, % | 32 | 32.1 | |
| Non-haematological, % | 68 | 67.9 | |
| Prostate | 11.2 | 9 | |
| Breast | 10.7 | 12.6 | |
| Gastrointestinal | 10.2 | 5.3 | |
| Lung, non-small cell | 10.2 | 9 | |
| Gynaecological | 9.2 | 12.1 | |
| Lung, small cell | 3.9 | 8 | |
| Head and neck | 2.4 | 1.6 | |
| Oesophagus | 1.0 | 1.6 | |
| Unknown primary | 3.4 | 1.1 | |
| Other | 5.8 | 7.9 | |
| Were intervention and control groups comparable? | No p-values reported; authors stated that ‘Pooling patients across all trials shows equivalent demographic characteristics between the patients randomised to r-HuEPO and the patients randomised to placebo’ (p. S3) | ||
| Results (reported for platinum-based chemotherapy and non-platinum-based chemotherapy; data available for no chemotherapy but outside the scope of this appraisal) | ||||
|---|---|---|---|---|
| Patients evaluable for efficacy | n = 206 | n = 190 | Notes | p-value |
| Response of haematocrit to therapy | ||||
| Non-cisplatin chemotherapy | n = 79 | n = 74 | ||
| Change in haematocrit (%), mean (SE) | 6.9 (6) | 1.1 (4.3) | Figure 2 represents mean (SE) weekly haematocrit comparing epoetin and placebo in all three populations | < 0.004 |
| Final haematocrit (%), mean (SE) | 35.5 (6) | 30.5 (4) | ||
| Correctors (%) | 40.5 | 4.1 | < 0.008 | |
| Responders (%) | 58.2 | 13.5 | < 0.008 | |
| Area under the curve for neutrophil count vs. time (cells × week/µl) | 30,203 | 34,189 | As reported in Case and colleagues86 | |
| Platelet counts/µl (% change from baseline to final value) | –39 | –48 | ||
| Rise in haematocrit to ≥ 38% unrelated to transfusion, n (%) | 32 (40.5) | 3 (4.1) | ||
| ≥ 6% point rise in haematocrit from baseline unrelated to transfusion, n (%) | 46 (58.2) | 10 (13.5) | ||
| Cisplatin chemotherapy | n = 64 | n = 61 | ||
| Change in haematocrit (%), mean (SE) | 6 (7) | 1.3 (5) | Figure 2 represents mean (SE) weekly haematocrit comparing epoetin and placebo in all three populations | < 0.004 |
| Final haematocrit (%), mean (SE) | 35.4 (7) | 29.7 (4.5) | ||
| Correctors (%) | 35.9 | 1.6 | < 0.008 | |
| Responders (%) | 48.4 | 6.6 | < 0.008 | |
| Haematological response (≥ 6 percentage points without a transfusion in the 4 weeks prior to that haematocrit value), change from baseline (mean ± SD) | 6.0 ± 7.0 | 1.3 ± 5.0 | As reported in Henry and colleagues;58 also reports baseline haematocrit 29.4 ± 4.0% (rHuEPO) and 28.4 ± 4.5% (placebo) | |
| Difference 4.7; p < 0.0001 (favours epoetin) | ||||
| Transfusions | ||||
| Non-cisplatin chemotherapy | n = 79 | n = 74 | ||
| Proportion of patients transfused (%), overall | 40.5 | 48.6 | When the non-cisplatin and cisplatin chemotherapy populations were combined there was a significant difference for the proportion of patients transfused at months 2–3 (p ≤ 0.005) and the mean units per patients at months 2–3 (p = 0.009) | |
| Mean units transfused per patient, overall (unclear whether SD or SE) | 2.03 (3.88) | 2.75 (4.15) | ||
| Proportion of patients transfused (%), month 1 | 25.3 (n = 70) | 27 (n = 68) | ||
| Mean units per patient, month 1 | 0.69 | 0.71 | ||
| Proportion of patients transfused (%), months 2–3 | 28.6 (n = 70) | 36.8 (n = 68) | ||
| Mean units per patient, months 2–3 | 0.91 | 1.65 | 0.056 | |
| Patients transfused, n (%) | As reported in Case and colleagues86 | |||
| Month 1 (n = 79) | 20 (25.3) | 20 (27.0) | ||
| Months 2–3 (n = 70) | 20 (28.6) | 25 (36.8) | ||
| Transfusion rate (least-squares mean from linear analysis), mean ± SE | ||||
| Month 1 (n = 79) | 0.69 ± 0.16 | 0.71 ± 0.16 | ||
| Months 2–3 (n = 70) | 0.91 ± 0.27 | 1.65 ± 0.27 | 0.056 | |
| Cisplatin chemotherapy | n = 64 | n = 61 | ||
| Proportion of patients transfused (%), overall | 53.1 | 68.9 | When the non-cisplatin and cisplatin chemotherapy populations were combined there was a significant difference for the proportion of patients transfused at months 2–3 (p ≤ 0.005) and the mean units per patients at months 2–3 (p = 0.009) | |
| Mean units transfused per patients, overall, mean (unclear whether SD or SE) | 3.56 (7.01) | 4.01 (4.87) | ||
| Proportion of patients transfused (%), month 1 | 43.8 | 44.3 | ||
| Mean units per patients, month 1 | 1.71 | 1.2 | ||
| Proportion of patients transfused (%), months 2–3 | 26.8 (n = 56) | 56.4 (n = 55) | ≤ 0.005 | |
| Mean units per patients, months 2–3 | 1.2 | 2.02 | 0.089 | |
| RBCT least-squares mean | ||||||
|---|---|---|---|---|---|---|
| All participants, n | Patients transfused, n (%) | All participants, n | Patients transfused, n (%) | |||
| Patients transfused | ||||||
| n | 64 | 34 (53.1) | 61 | 42 (68.9) | As reported in Henry and colleagues58 | |
| Month 1 | 64 | 28 (43.8) | 61 | 27 (44.3) | > 0.05 | |
| Month 2 | 56 | 12 (21.4) | 55 | 27 (49.1) | < 0.005 | |
| Month 3 | 47 | 8 (17.0) | 46 | 13 (28.3) | ||
| Months 2–3 | 56 | 15 (26.8) | 55 | 31 (56.4) | ||
| Units transfused, mean ± SE | ||||||
| n | 56 | 53 | ||||
| Units transfused | 4.01 ± 0.85 | 3.95 ± 0.84 | > 0.05 | |||
| Month 1 | 1.71 ± 0.28 | 1.20 ± 0.29 | > 0.05 | |||
| Month 2 | 0.71 ± 0.22 | 1.30 ± 0.22 | 0.0572 | |||
| Month 3 | 0.42 ± 0.16 | 0.62 ± 0.16 | – | |||
| Months 2–3 | 1.20 ± 0.33 | 2.02 ± 0.33 | 0.0893 | |||
| Haematological vs. non-haematological tumour | ||||||
| Change in haematocrit from baseline to final value by tumour type (%) | ||||||
| Chronic lymphocytic leukaemia | 6.0 (n = 7) | 0.9 (n = 9) | As the data for any turnout type may include patients from the no chemotherapy, non-cisplatin-containing chemotherapy and cisplatin-containing chemotherapy treatment groups, duration of therapy can range from 8 weeks (no chemotherapy) to 12 weeks (non-cisplatin-containing chemotherapy, cisplatin-containing chemotherapy) | 0.077 | ||
| Myeloma | 3.7 (n = 19) | 0.3 (n = 23) | 0.058 | |||
| Lymphoma | 6.0 (n = 40) | 0.5 (n = 29) | ≤ 0.05 | |||
| Breast cancer | 6.5 (n = 22) | 1.6 (n = 24) | ≤ 0.05 | |||
| Lung cancer | 6.4 (n = 29) | 1.1 (n = 32) | ≤ 0.05 | |||
| Prostate cancer | 2.3 (n = 23) | 0.1 (n = 17) | ||||
| Gastrointestinal cancer | 5.8 (n = 21) | 1.6 (n = 10) | ≤ 0.05 | |||
| Gynaecological cancer | 7.7 (n = 18) | –0.3 (n = 23) | ≤ 0.05 | |||
| HRQoL | ||||
|---|---|---|---|---|
| Reported for the entire enrolled patient population and not separated by chemotherapy treatment. Data presented graphically (Figures 3 and 4) | ||||
| As reported in Case and colleagues:86 Pre-study and post-study quality-of-life assessments were available for 124 patients (rHuEPO n = 63; placebo n = 61); the rHuEPO-treated population as a whole had a statistically significant (p ≤ 0.05) increase in the baseline to final evaluation for energy level and ability to perform daily activities, as well as a near statistically significant (p = 0.86) improvement for overall quality of life. No similar improvements in quality-of-life assessments were seen in placebo-treated patients. The changes in quality-of-life scores were of somewhat greater magnitude in the rHuEPO-treated populations, with an increase in haematocrit to ≥ 38% or an increase of ≥ 6 percentage points (both unrelated to transfusion), than in the rHuEPO-treated population as a whole | ||||
| As reported in Henry and colleagues:58 patients in the rHuEPO-treated group experienced a significant (p ≤ 0.05) pre-study to post-study improvement in energy level, ability to perform daily activities and overall quality of life. Patients in the placebo group also experienced a significant (p ≤ 0.05) pre-study to post-study improvement in energy level, but not in ability to perform daily activities or overall quality of life. Comparing the two treatment arms there was a significantly greater pre-study to post-study change in overall quality of life for the rHuEPO-treated group than for the placebo-treated group (p = 0.013). When only responders in the rHuEPO-treated group were compared with the placebo-treated group, the quality-of-life changes were even greater in favour of the rHuEPO group but did not achieve significance because of the smaller numbers involved | ||||
| Adverse effects of treatment (%) | ||||
| Reported for the entire enrolled patient population and not separated out by chemotherapy treatment | ||||
| Reported by at least 10% of patients | n = 213 | n = 200 | ||
| Nausea | 23 | 29 | ||
| Pyrexia | 22 | 21 | ||
| Asthenia | 17 | 16 | ||
| Fatigue | 15 | 20 | ||
| Vomiting | 15 | 18 | ||
| Diarrhoea | 15 | 9 | ||
| Oedema | 14 | 8 | ||
| Dizziness | 10 | 9 | ||
| Skin reaction at medication site | 10 | 10 | ||
| Constipation | 10 | 9 | ||
| Shortness of breath | 8 | 15 | < 0.03 | |
| Decreased appetite | 8 | 12 | ||
| Chills | 7 | 10 | ||
| Trunk pain | 8 | 12 | ||
| Hypertension | 5 | 3.5 | > 0.05 | |
| Non-cisplatin chemotherapy | ||||
|---|---|---|---|---|
| ITT population | n = 81 | n = 76 | As reported in Case and colleagues86 | > 0.05 |
| No. (%) of patients completing the study | 63 (78) | 63 (83) | > 0.05 | |
| No. (%) of patients who discontinued the study prematurely because of an adverse experience, death or disease progression | 13 (16) | 8 (11) | ||
| Diarrhoea, n (%) | 18 (22) | 8 (10) | 0.05 | |
| Diaphoresis, n (%) | 9 (11) | 1 (1) | < 0.05 | |
| Hypertension, na | 4 | 2 | ||
| Seizure, nb | 2 | 2 | ||
| Thromboembolic events, n | 4 | 4 | ||
| Cisplatin chemotherapy | ||||
|---|---|---|---|---|
| n | 67 | 65 | As reported in Henry 199558 | |
| Overall, n (%) | 58 (87) | 58 (89) | ||
| ≥ 10% of patients, n (%) | ||||
| Fever | 16 (24) | 17 (26) | ||
| Nausea | 15 (22) | 25 (28) | ||
| Vomiting | 13 (19) | 17 (26) | ||
| Fatigue | 11 (16) | 12 (18) | ||
| Diarrhoea | 10 (15) | 4 (6) | ||
| Abdominal/trunk pain | 10 (15) | 12 (18) | ||
| Asthenia | 9 (13) | 9 914) | ||
| Oedema | 9 (13) | 6 (9) | ||
| Anorexia | 7 (10) | 10 (15) | ||
| Bacterial infection | 7 (10) | 7 (11) | ||
| Paraesthesia | 7 (10) | 5 (8) | ||
| Skin reaction at medication site | 7 (10) | 4 (6) | ||
| Constipation | 7 (10) | 3 (5) | ||
| Rash | 7 (10) | 2 (3) | ||
| Shortness of breath | 5 (7) | 13 (20) | ||
| Arthralgia | 5 (7) | 7 (11) | ||
| < 10% of patients, selected AEs, n (%) | ||||
| Thrombosis | 6 (9) | 2 (3) | ||
| Headache | 5 (7) | 3 (5) | ||
| Seizure | 3 (4) | 2 (3) | ||
| Hypertension | 2 (3) | 4 (6) | ||
| Quality appraisal | ||||
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear (states randomised but no details given) | |||
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR | |||
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Unclear; no p-values reported, authors stated that ‘Pooling patients across all trials shows equivalent demographic characteristics between the patients randomised to r-HuEPO and the patients randomised to placebo’ (p. S3) | |||
| 4. Were the eligibility criteria specified? | Yes | |||
| 5. Were the participants blind to treatment allocation? | Yes (states double blind) | |||
| 6. Were the outcome assessors blind to treatment allocation? | Yes (states double blind) | |||
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Partially | |||
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No | |||
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Unclear | |||
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | After completion of double-blind therapy, patients were eligible to receive rHuEPO on an open-label basis | |||
| Other | |
|---|---|
| Generalisability | |
| Author conclusions | rHuEPO increases the haematocrit and corrects anaemia in cancer patients whether or not they are receiving chemotherapy and apparently without regard to type of cancer. With a dose of 150 U/kg three times weekly, rHuEPO appears to decrease transfusion requirements after the first month of therapy but not earlier. This therapy also appears to improve functional capacity in those anaemic cancer patients who show a significant increase in haematocrit in response to therapy. rHuEPO also appears to be well tolerated in this patient population |
| Reviewer comments | |
| EndNote ref. ID: 2685 | Malignancy type: solid (ovarian, lung and stomach) | |
| Treatment: rHuEPO (epoetin alfa) | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Aravantinos 200364 | n = 47 |
| Objective | To evaluate the safety and efficacy of rHuEPO for the management of anaemia in cancer patients receiving platinum-based chemotherapy | Inclusion criteria: Adults with confirmed (histologically proven) malignancies about to start or already receiving platinum-based chemotherapy. Diagnosis of recent-onset anaemia as a result of malignant disease, performance status of 0–2 according to ECOG and life expectancy ≥ 3 months. Patients with Hb values < 10.5 g/dl before initiation or during chemotherapy, receiving platinum-based combinations on a 3- to 4-weekly schedule lasting for not more than 5 days per cycle. Laboratory requirements: white blood cell count > 3500/µl, platelet count > 100,000/µl, serum creatinine < 2 mg/dl, negative direct Cooms reaction (to exclude haemolytic anaemia) and normal iron levels (to exclude iron-deficiency anaemia) Exclusion criteria: Uncontrolled hypertension (diastolic blood pressure > 100 mmHg) and suspicion of iron, folic acid or vitamin B12 deficiency; patients who had received radiotherapy, who had undergone surgery 2 weeks prior to study entry or who had received a RBCT the week before |
| No. of centres | 1 | |
| Other references/aliases | NA | |
| Geographical setting | Greece | |
| Duration of treatment | Unclear; median five cycles | |
| Length of follow-up (if different) | NR | |
| Country of corresponding author | Greece | |
| Language of publication | English | |
| Sources of funding | NR | |
| Randomisation and allocation | Randomised, unblinded, single-centre study. Stratified by type of malignancy, type of platinum compound (cisplatin or carboplatin) and chemotherapy cycle number at study entry (first vs. second vs. third) | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | rHuEPO | Control: no rHuEPO |
| n | 24 | 23 |
| Dose and frequency (once daily, twice daily, etc.) | 150 IU/kg Q3W | NA |
| Dose adjustment (yes/no) | Yes: Hb value > 14 g/dl rHuEPO administration was interrupted and reinitiated in a dose reduced by 25% when Hb was < 12.5 g/dl. No escalation of the rHuEPO dose was attempted in case of failure to increase Hb by > 1 g/dl in a month. Dose adjustments were made according to body weight on the first day of the following chemotherapy cycle | NA |
| Route of administration | Subcutaneous | NA |
| Duration of epoetin treatment | NR | NR |
| Adjuvant anaemia treatment | 200 mg elementary iron daily | 200 mg elementary iron daily |
| Transfusion trigger | Discretion of treating physician but avoided if Hb level > 9 g/dl | Discretion of treating physician but avoided if Hb level > 9 g/dl |
| Outcomes | |
|---|---|
| Primary outcome | Reduction in transfusion requirement, number of transfusions (per group and per patient) |
| Other outcomes | Hb level (change per cycle), haematocrit level (change per cycle), number of RBCTs required (change per cycle), number of patients requiring transfusion |
| Analysis | |
|---|---|
| Statistical technique used | ANOVA with two parameters was used for the administration of epoetin, follow-up, RBCTs and cycle number. Statistical significance was tested in relation to epoetin administration (with or without epoetin) and in relation to cycle number. ANOVA with one parameter was used to identify statistically significant differences in relation to the use of epoetin and cycle number. Post-hoc comparisons and Scheffe tests followed in order to assess the statistical significance of differences between the two groups. Independent Mann–Whitney tests were performed to study the differences concerning the number of transfusions and all data were also studied with descriptive statistics. A p-value < 0.05 was considered significant |
| ITT analysis? | Unclear; likely ITT analysis as no crossover and results were reported for the full data set but not mentioned in the study write-up |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation) | NR |
| Attrition rate (loss to follow-up)? | NR |
| Was attrition rate adequately dealt with? | Unclear; attrition rate not reported |
| No. (%) followed up from each condition? | NA |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Solid – ovarian, lung, stomach, other | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Platinum based (cisplatin or carboplatin) | ||||
| Adjuvant anaemia treatment | Iron | 200 mg elementary iron daily | |||
| G-CSF | NR | ||||
| Transfusion trigger | Discretion of treating physician but avoided if Hb level > 9 g/dl | ||||
| Hb inclusion criterion level | < 10.5 g/dl | ||||
| Arm 1 = rHuEPO (n = 24) | Arm 2 = no rHuEPO (n = 23) | Notes | p-value | ||
| Sex, n (%) | |||||
| Male | 2 (8) | 7 (30) | |||
| Female | 22 (92) | 16 (70) | |||
| Age (years), median (range) | 59 (18–76) | 64 (23–75) | |||
| Performance status: ECOG score, n (%) | |||||
| 0 | 11 (45.8) | 14 (60.9) | |||
| 1 | 8 (33.3) | 4 (17.4) | |||
| 2 | 5 (20.9) | 5 (21.7) | |||
| Type of solid tumour, n (%) | |||||
| Ovarian | 16 (67) | 10 (43) | |||
| Lung | 3 (12.5) | 5 (22) | |||
| Stomach | 2 (8) | 2 (9) | |||
| Other | 3 (12.5) | 6 (26) | |||
| No. of chemotherapy cycle at study entry, n (%) | |||||
| 1 | 9 (37.5) | 5 (21.7) | |||
| 2 | 9 (37.5) | 13 (56.5) | |||
| 3 | 3 (12.5) | 2 (8.6) | |||
| 4 | 3 (12.5) | 3 (13.0) | |||
| Hb at baseline (g/dl) | NR | NR | |||
| Hb at cycle 1 (g/dl) | 9.8 (0.5) | 9.32 (0.8) | Reported values are assumed to be means and SDs | ||
| Iron at baseline (U/l) | NR | NR | |||
| Epoetin at baseline (mU/ml) | NR | NR | |||
| Target Hb | NR | NR | |||
| Were intervention and control groups comparable? | No p-values reported; authors stated that ‘all characteristics were well-balanced between the two groups’ (p. 129) | ||||
| Results | ||||
|---|---|---|---|---|
| Median no. of chemotherapy cycles | 5 | 5 | ||
| Mean Hb level by cycle | ||||
| Cycle 1 | 9.8 ± 0.5 | 9.32 ± 0.8 | Reported values are assumed to be means and SDs | p-value for all cycles: < 0.0002 |
| Cycle 2 | 10.36 ± 1.08 | 10.2 ± 1.01 | ||
| Cycle 3 | 10.66 ± 1.3 | 10.07 ± 1.32 | ||
| Cycle 4 | 11.47 ± 1.67; Hb increase compared with control group: p < 0.03 | 10.31 ± 1.56 | ||
| Cycle 5 | 12.11 ± 1.39; Hb increase compared with control group: p < 0.03 | 10.55 ± 1.83 | ||
| Mean haematocrit by cycle | ||||
| Cycle 1 | 28.56 ± 4.92 | 28.74 ± 2.68 | Reported values are assumed to be means and SDs | |
| Cycle 2 | 31.5 ± 0.47 | 31.09 ± 3.14 | ||
| Cycle 3 | 32 ± 4.06 | 30.57 ± 4.21 | ||
| Cycle 4 | 34.9 ± 4.48; haematocrit increase compared with control group: p < 0.002 | 31.58 ± 4.54 | ||
| Cycle 5 | 36.43 ± 4.33 | 32.2 ± 5.63 | ||
| RBC count (×104/mm3) by cycle | ||||
| Cycle 1 | 3.46 ± 0.42 | 3.46 ± 0.59 | Reported values are assumed to be means and SDs | |
| Cycle 2 | 3.62 ± 0.50 | 3.71 ± 0.59 | ||
| Cycle 3 | 3.64 ± 0.57 | 3.61 ± 0.62 | ||
| Cycle 4 | 3.77 ± 0.55 | 3.54 ± 0.66 | ||
| Cycle 5 | 4.01 ± 0.4 | 3.61 ± 0.61 | ||
| RBCT | ||||
| No. (%) of patients requiring a RBCT | 9 (37.5) | 23 (100%) | < 0.0001 | |
| No. of transfusions | ||||
| Total | 20 | 73 | < 0.04 | |
| Per patient | 2.22 | 3.17 | ||
| Quality appraisal | ||||
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear; stratification | |||
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR | |||
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Unclear; p-values NR; authors stated that ‘all characteristics were well-balanced between the two groups’ (p. 129) | |||
| 4. Were the eligibility criteria specified? | Yes | |||
| 5. Were the participants blind to treatment allocation? | No | |||
| 6. Were the outcome assessors blind to treatment allocation? | No | |||
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Partially (variability can be calculated from data presented in the paper) | |||
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No | |||
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes; results reported for full population and no crossover, so appears to be ITT analysis but not mentioned in study description | |||
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | NR | |||
| Other | |
|---|---|
| Generalisability | Mixed population – majority women (81%) (majority of women had ovarian cancer); other solid tumours included lung and stomach cancer |
| Author conclusions | Administration of rHuEPO is an effective intervention for the management of chemotherapy-induced anaemia, significantly reducing RBCT requirements in patients receiving platinum-based chemotherapy. Hb and haematocrit levels proved reliable indicators for the response to rHuEPO treatment |
| Reviewer comments | Trial unblinded |
| EndNote ref. ID: 2710 | Malignancy type: haematological and solid | |
| Treatment: epoetin beta | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Boogaerts 200365 | n = 262 |
| Objective | To assess the impact of epoetin beta on quality of life compared with standard care in anaemic patients with lymphoid or solid tumour malignancies | Inclusion criteria: Adult outpatients; Hb level ≤ 11 g/dl associated with non-Hodgkin’s lymphoma or chronic lymphocytic leukaemia and any solid tumour treated with myelosuppressive chemotherapy with at least three cycles remaining; WHO performance status of ≤ 2 and a life expectancy of 6 months Exclusion criteria: Anaemia arising for other reasons (iron or vitamin B12 deficiency, acute bleeding, haemolytic anaemia); refractory hypertension; severe renal insufficiency [serum creatinine of > 2.5 mg/dl (> 220 µmol/l)]; epilepsy or acute infection; pregnant or lactating women and women of childbearing age practising unreliable contraception; any patient scheduled to undergo bone marrow or peripheral stem cell transplantation during the study period or 4 weeks prior to the study |
| No. of centres | Multicentre; conducted between October 1996 and September 1998 | |
| Other references/aliases | Coiffier 200187 (abstract) (see note) | |
| Geographical setting | Eight countries: Austria, Belgium, France, Germany, Italy, South Africa, Sweden, UK | |
| Duration of treatment | 12 weeks (plus run-in period of up to 2 weeks) | |
| Length of follow-up (if different) | 26 weeks? | |
| Country of corresponding author | Belgium | |
| Language of publication | English | |
| Sources of funding | NR | |
| Randomisation and allocation | Patients were randomised 1 : 1, stratified according to centre to receive either epoetin beta or standard care with transfusion support | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Epoetin beta | Standard care |
| n | 133 | 129 |
| Dose and frequency (once daily, twice daily, etc.) | 150 U/kg Q3W. Average dose of epoetin over the study period was 174 IU/kg per administration | NA |
| Dose adjustment (yes/no) | Dose increased to 300 U/kg Q3W for those patients in whom Hb level increased by < 0.5 g/dl after 3–4 weeks or by < 1 g/dl after 6–8 weeks. The dose was reduced by 50% if the Hb level increased by > 2 g/dl per month, whereas treatment was interrupted if the Hb level increased to > 14 g/dl. Treatment was recommenced at half the previous dose once the Hb level had declined to < 12 g/dl | NA |
| Route of administration | Subcutaneous | NA |
| Duration of epoetin treatment | 12 weeks | NR |
| Adjuvant anaemia treatment | Oral iron supplementation (200–300 mg elemental iron per day) as indicated (transferrin saturation < 15%) | Oral iron supplementation (200–300 mg elemental iron per day) as indicated (transferrin saturation < 15%) |
| Transfusion trigger | Hb 8.5 g/dl was a guide to initiate transfusion throughout the centres | Hb 8.5 g/dl was a guide to initiate transfusion throughout the centres |
| Outcomes | |
|---|---|
| Primary outcome | HRQoL (change from baseline to week 12 in SF-36, FACT-An and FACT-F) |
| Other outcomes | Haematological response [defined as an increase in Hb level of ≥ 2 g/dl without transfusion requirement after the first 4 weeks (also measured haematological response as an increase in Hb level of ≥ 2 g/dl or an increase in Hb level to ≥ 12 g/dl)]; change in Hb from baseline to week 12 (plus changes in Hb and corresponding changes in quality of life); RBCTs; HRQoLa (change from baseline to week 12 in VAS and FACT-An Global); AEsb (including no. of hospitalisations) |
| Analysis | |
|---|---|
| Statistical technique used | Psychometric evaluation was performed to evaluate how well the quality-of-life scale items satisfied the assumptions underlying the Likert method for summated rating. The internal consistency reliability of each scale score was estimated using Cronbach’s alpha. Cronbach’s alpha, which ranges from 0 to 1, where ‘1’ equals perfect reliability, is based on the average inter-item correlation and the number of items. Minimum values ≥ 0.70 have been recommended for group-level comparisons.195 For quality-of-life assessments only patients for whom values were available at baseline and at least one follow-up visit were included in the analysis. The data are presented in their raw form and using the LOCF approach for patients with missing values at the final visit. For the percentage of clinical responders, Kaplan–Meier estimates and CIs for time to treatment response were determined and curves were compared using the log-rank test. The observed/predicted log serum erythropoietin ratio was derived from reference regression at the particular haematocrit or Hb level and was calculated for responders and non-responders to epoetin beta. The relation between endogenous erythropoietin level and response to treatment was explored using the OR and RRs.196 Appropriate parametric and non-parametric tests were used to analyse between-group differences for continuous and categorical variables respectively. All tests were two-sided and p < 0.05 was considered significant. Assessment of statistical significance was not adjusted for multiple comparisons |
| ITT analysis? | Yes; ITT = 262 |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation)? | Based on expected change in SF-36 PCS score. To detect a between-group difference in SF-36 PCS score of at least 4 points, assuming a SD of 10 using a two-sided test with a statistical power of 80% and α = 2.5%, at least 121 patients/group were required to complete the study and be evaluable for efficacy. To allow for dropouts approximately 310 patients were to be enrolled; however, this was not achieved |
| Attrition rate (loss to follow-up)? | 51 patients were withdrawn from the study (epoetin beta n = 30; control n = 21); 20 were withdrawn because of AEs (epoetin beta n = 15; control n = 5). Other reasons for withdrawal included death, loss to follow-up, withdrawal of consent and protocol violation |
| Was attrition rate adequately dealt with? | LOCF for patients with missing values at final visit |
| No. (%) followed up from each condition? | NR |
| Baseline characteristics | ||
|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Haematological and solid | |
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Chemotherapy, NR | |
| Adjuvant anaemia treatment | Iron | Oral iron supplementation (200–300 mg elemental iron per day) was indicated (transferrin saturation < 15%) |
| G-CSF | NR | |
| Transfusion trigger | Hb 8.5 g/dl | |
| Hb inclusion criterion level | ≤ 11 g/dl | |
| Arm 1 = epoetin | Arm 2 = control | Notes | p-value | |
|---|---|---|---|---|
| Evaluable population | n = 133 | n = 129 | ||
| Sex | ||||
| Male, n (%) | 46 (35) | 52 (40) | ||
| Female, n (%) | 87 (65) | 77 (60) | ||
| Age (years), median (range) | 62 (24–85) | 62 (24–85) | ||
| Hb (g/dl), median (range) | 9.0 (5–13) | 9.2 (5–12) | ||
| Erythropoietin (mU/ml), median (range) (n = 25) | 54 (7–1650) | 58 (5–4300) | ||
| Iron, serum (µg/dl), median (range) (n = 26) | 63.7 (6–472) | 78.8 (4–510) | ||
| Iron saturation (%), median (range) (n = 26) | 20.6 (1–97) | 29.0 (2–100) | ||
| Folic acid (mg/ml), mean (SD) (n = 25) | NR | NR | ||
| Vitamin B12 (pg/ml), mean (SD) (n = 24) | NR | NR | ||
| Baseline quality-of-life score | ||||
| SF-36 PCS, mean (SD); median (range) | 35 (8.4); 35 (17–60) | 38 (9.5); 38 (15–60) | ||
| FACT-F, mean (SD); median (range) | 27 (12); 28 (1–49) | 31 (11); 33 (2–51) | 0.02a | |
| FACT-AN, mean (SD); median (range) | 20 (3.8); 21 (6–27) | 21 (4.4); 22 (2–28) | ||
| VAS, mean (SD); median (range) | 56 (17); 53 (11–96) | 62 (17); 60 (18–96) | ||
| Were intervention and control groups comparable? | Authors report that ‘Overall, there were no significant differences between groups in baseline demographics and clinical characteristics, except for a significantly higher proportion of patients in the control group having received prior chemotherapy 80 vs. 68%; p = 0.025)’ (p. 990). With respect to quality-of-life measures, baseline scores on the FACT-An subscale were comparable between treatment groups, although those randomised to epoetin beta therapy had a lower FACT-F subscale score relative to the control group (p = 0.02) | |||
| Results | ||||
|---|---|---|---|---|
| Haematological response, n (%) | ||||
| Responders: increase ≥ 2 g/dl | 63 (47) | 17 (13) | Figure 2 provides a graph showing response | < 0.001 |
| Responders: increase ≥ 2 g/dl or increase to ≥ 12 g/dl | 65 (49) | 19 (15) | ||
| Hb baseline to week 12, median increase (range) | ||||
| All patients | 2.1 (–3 to 8) (n = 112) | 0.9 (–3 to 6) (n = 112) | Figure 3 provides a graph showing Hb change | < 0.001 |
| Patients with solid tumours | 2.1 (–1 to 8) (n = 45) | 0.9 (–3 to 4) (n = 51) | No p-values provided for subgroup analyses | |
| Patients with lymphoid tumours | 1.9 (–3 to 8) (n = 67) | 0.9 (–3 to 6) (n = 61) | ||
| With chemotherapy | 2.1 (NR) (n = 74) | (NR) (n = 88) | ||
| Without chemotherapy | 2.0 (NR) (n = 38) | 0.2 (NR) (n = 24) | ||
| Transfusions | ||||
| Hb level before transfusion (g/dl), median | 7.64 | 7.8 | Units transfused per patient reduced by 45% during the treatment period with epoetin beta | |
| Patients transfused in last 8 weeks of study (%) | 22 | 43 | < 0.001 | |
| Patients transfused overall (%) | 32 | 52 | 0.001 | |
| HRQoL | ||||
| Cronbach’s alpha: reliabilities for SF-36 subscales varied from 0.83 to 0.90 for the pooled population, apart from the general health subscale (0.75). The FACT-F subscale and the FACT-An global scale showed high consistency (> 0.9), whereas the FACT-An seven-item subscale reached 0.68 using the pooled population | ||||
| Median change baseline to week 12, LOCF data | ||||
| SF-36 PCS | +3.1 (n = 104) | NR (n = 109) | p-value vs. control | < 0.05 |
| FACT-F | +3.0 (n = 104) | NR (n = 109) | < 0.05 | |
| FACT-AN | +1.0 (n = 104) | NR (n = 109) | 0.076 | |
| VAS | +10.0 (n = 111) | +1.0 (n = 112) | 0.004 | |
| Median change baseline to week 12, data without LOCF | ||||
| SF-36 PCS | +3.3 (n = 77) | NR | p-value vs. control | 0.01 |
| FACT-F | +4.0 (n = 90) | NR | 0.001 | |
| FACT-AN | +1.0 (n = 89) | NR | 0.068 | |
| VAS | +10.0 (n = 89) | +3.0 (n = 98) | 0.001 | |
| AEs in ≥ 5% of patients in at least one treatment group, n (%) | ||||
|---|---|---|---|---|
| Malignancy progression | 33 (25) | 42 (33) | ||
| Anaemia | 18 (14) | 33 (26) | ||
| Leucopenia | 20 (15) | 19 (15) | ||
| Thrombocytopenia | 8 (6) | 13 (10) | ||
| Bronchitis | 7 (5) | 8 (6) | ||
| Fever | 5 (4) | 10 (8) | ||
| Nausea | 6 (5) | 8 (6) | ||
| Pain | 9 (7) | 5 (4) | ||
| Pneumonia | 9 (7) | 5 (4) | ||
| Asthenia | 6 (5) | 7 (5) | ||
| Diarrhoea | 11 (8) | 2 (2) | ||
| Infection | 8 (6) | 4 (3) | ||
| Sepsis | 3 (2) | 7 (5) | ||
| Vomiting | 9 (7) | 1 (< 1) | ||
| Depression | 8 (6) | 1 (< 1) | ||
| Headache | 7 (5) | 2 (2) | ||
| No. of hospitalisations per patient, mean (SD) | 3.8 (4.5) | 4.1 (4.9) | 0.52 | |
| No. of hospital days, mean (SD) | 11.7 (13.7) | 9.4 (10.3) | 0.46 | |
| Admissions related to anaemia, mean (SD) | 0.8 (2.2) | 1.5 (3.6) | 0.043 | |
| Iron | ||||
| Iron supplementation (mostly oral), n | 30 | 28 | ||
| Parenteral iron, n | 9 | 2 | ||
| Serum iron deficit, baseline to study end | 4.5 µg/dla | 16.8 mg/dla | < 0.01 | |
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear; randomised but method not specified |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | No; higher proportion of participants in the control group had received chemotherapy previously (80% vs. 68%; p = 0.025); participants randomised to epoetin beta had lower FACT-F scores relative to the control group (p = 0.02) |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | No (open label) |
| 6. Were the outcome assessors blind to treatment allocation? | No (open label) |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Yes |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Partially; total number and number withdrawing because of AEs reported per group. Other reasons stated but numbers not reported |
| Other | |
|---|---|
| Generalisability | |
| Author conclusions | Compared with transfusion therapy, epoetin beta produced a clinically significant improvement in quality of life in patients with anaemia associated with malignancy. Epoetin beta improved physical function and well-being as a result of diminished anaemia-related symptoms as measured by the FACT-An and FACT-F questionnaires. These improvements in quality of life accompany and are mediated through improvements in Hb concentration and can be achieved after a few weeks of epoetin beta therapy. In addition, baseline erythropoietin serum levels and the observed/predicted ratio might identify those patients with lymphoproliferative malignancies who are more likely respond to epoetin beta; however, this requires further study |
| Reviewer comments | Abstract by Coiffier and colleagues included in the Wilson and colleagues2 review. We have included the full paper in this review |
| EndNote ref. ID: 2689 | Malignancy type: haematological (multiple myeloma) | |
| Treatment: epoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Dammacco 200166 | n (ITT) = 145 |
| Objective | To evaluate the efficacy of epoetin alfa in correcting anaemia in patients with multiple myeloma thereby decreasing transfusion requirements | Inclusion criteria: Men and women aged 40–80 years with multiple myeloma; life expectancy of at least 3 months and an ECOG score of 0–3; receiving chemotherapy for at least 6 months; baseline Hb level < 11.0 g/dl Exclusion criteria: Patients with uncontrolled hypertension or evidence of untreated iron, folate or vitamin B12 deficiency; received a blood transfusion within 7 days of study entry; major infection within 1 month or an acute illness within 7 days of study entry |
| No. of centres | 31 | |
| Other references/aliases | None | |
| Geographical setting | 12 countries (Italy, Poland, UK, Norway, Sweden, Czech Republic, Hungary, Belgium, Israel, Denmark, Spain, Switzerland) | |
| Duration of treatment | 12 weeks double blind | |
| Length of follow-up (if different) | 12 weeks open label optional | |
| Country of corresponding author | Italy | |
| Language of publication | English | |
| Sources of funding | Supported by a grant from the RW Johnson Pharmaceutical Research Institute, Bassersdorf, Switzerland | |
| Randomisation and allocation | Stratified according to receipt of blood transfusion within the preceding 3 months. Patients within each transfusion stratum were then randomised to treatment or control. Patients in both arms who completed the 12 weeks had an option to receive epoetin for up to 12 weeks in the open-label extension of the study | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Epoetin alfa | Placebo |
| n | 69 | 76 |
| Dose and frequency (once daily, twice daily, etc.) | 150 IU/kg Q3W (each dose separated by at least 1 day) | Matched to epoetin alfa dose |
| Dose adjustment (yes/no) | Yes: if Hb level had not increased by ≥ 1 g/dl after 4 weeks of treatment the dose was doubled to 300 IU/kg Q3W for the remaining 8 weeks of the study; if Hb level increased to > 14 g/dl treatment was withheld until Hb level was < 12 g/dl and was then reinitiated at a dose approximately 25% lower than the start dose; if Hb level increased by ≥ 2 g/dl within a 4-week period the dose was reduced by approximately 25% to maintain an increase of < 2 g/dl | Matched to epoetin alfa dose |
| Route of administration | Subcutaneous | Subcutaneous |
| Duration of epoetin treatment | 12 weeks (double blind) | 12 weeks (double blind) |
| Adjuvant anaemia treatment | NR | NR |
| Transfusion trigger | < 8 g/dl; to be avoided if possible if Hb > 8 g/dl | To be avoided if possible if Hb > 8 g/dl |
| Outcomes | |
|---|---|
| Primary outcome | Transfusion requirement stratified by baseline transfusion status |
| Other outcomes | HRQoL (change in quality-of-life scores: NHP and CLAS/LASA). AEs reported (by questioning patients at study visits); all AEs together with investigators’ assessments of their seriousness, severity and presumed relationship to study medication were recorded |
| Analysis | |
|---|---|
| Statistical technique used | Proportion of patients transfused stratified by pre-study transfusion history was analysed with the Cochran–Mantel–Haenszel test. Only data for months 2 and 3 were analysed (effects not expected before this time).156 Between-group changes in haematological parameters from baseline to last determination were compared using t-tests; between-group differences in the proportions of responders and correctors were compared using Fisher’s exact test. For quality of life, the Kruskal–Wallis test was performed to ensure that no bias had been introduced by deleting patients. Assessments were evaluated in univariate analyses using t-tests; multivariate analyses were also performed. Changes in performance scores between treatment groups, categories of response to chemotherapy and the treatment groups stratified by response to chemotherapy were analysed using Kruskal–Wallis and Cochran–Mantel–Haenszel tests. Between-group differences in the physician’s global assessment were analysed using the Kruskal–Wallis test. All statistical tests were two-sided |
| ITT analysis? | Results for the primary efficacy evaluation of transfusion requirements and safety are reported for the ITT population. Results for the secondary efficacy parameters are reported for the efficacy population (patients randomised to a treatment group who remained in the study for at least 2 months – it was believed that this duration would allow patients, including those who required a dose increase at week 4, sufficient time to respond). Quality-of-life analyses were performed for the ITT population minus patients who died during the double-blind phase of the study, for whom quality-of-life data were incomplete |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation) | NR |
| Attrition rate (loss to follow-up)? | Yes. Five epoetin patients discontinued [n = 2 AEs (death from septic shock n = 1 and disease progression n = 1); n = 3 for personal reasons]. 15 placebo patients discontinued [n = 3 AEs (pneumonia n = 1, death from septic shock n = 1, death from acute renal failure n = 1); n = 6 disease progression; n = 3 personal reasons; n = 3 other unspecified reasons] |
| Was attrition rate adequately dealt with? | Partially: ITT population was not used for secondary efficacy parameters and HRQoL data |
| No. (%) followed up from each condition? | NA |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Haematological: multiple myeloma | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Unclear, although most commonly used non-platinum-based chemotherapy | ||||
| Adjuvant anaemia treatment | Iron | NR | |||
| G-CSF | NR | ||||
| Transfusion trigger | To be avoided if possible if Hb > 8 g/dl | ||||
| Hb inclusion criterion level | < 11.0 g/dl | ||||
| Arm 1 = epoetin alfa (n = 69) | Arm 2 = placebo (n = 76) | Notes | p-value | ||
| Sex | |||||
| Male, n (%) | 34 (49%) | 31 (41%) | |||
| Female, n (%) | 35 (51%) | 45 (59%) | |||
| Age (years), median (range) | 67.3 (43.0–80.4) | 65.0 (38.2–88.9) | |||
| ECOG performance score (0–4; higher score indicates worse the performance status), (%) | |||||
| Missing | 1 | 0 | |||
| 0 | 9 | 8 | |||
| 1 | 51 | 50 | |||
| 2 | 33 | 34 | |||
| 3 | 6 | 8 | |||
| Creatinine (µmol/l), mean ± SD | 106.3 ± 42.29 | 102.4 ± 35.60 | |||
| No. of chemotherapy cycles within 6 months prestudy, mean ± SD (range) | 3 ± 2.5 (0–15) (n = 68) | 4 ± 2.0 (0–8) (n = 75) | |||
| Malignancy staging (%)197 | |||||
| IA | 4 | 5 | |||
| IB | 0 | 1 | |||
| IIA | 33 | 34 | |||
| IIB | 4 | 0 | |||
| IIIA | 46 | 54 | |||
| IIIB | 12 | 5 | |||
| Hb baseline (g/dl), mean ± SD (median; range) | 9.3 ± 1.27 (9.6; 5.7–11.5) | 9.6 ± 0.95 (9.7; 7.4–11.8) | |||
| Hb level (g/dl) at transfusion (for patients receiving transfusions at baseline), mean ± SD | 8.1 ± 1.08 (n = 25) | 8.1 ± 0.93 (n = 28) | |||
| Serum erythropoietin level (mU/ml), median (range) | 116 (18–5220) (n = 36) | 93 (10–408) (n = 36) | |||
| Were intervention and control groups comparable? | No p-values reported but authors state that ‘baseline demographic and clinical characteristics were comparable between treatment groups’ (p. 174). Based on the reported values the groups appear comparable | ||||
| Results | |||
|---|---|---|---|
| ITT population | n = 69 | n = 76 | |
| RBCT | |||
| Patients transfused during months 2 and 3 (double-blind study), n (%) | 19 (27.5) | 36 (47.4) | 0.017 |
| Transfused (by transfusion history) (either having or not having received one or more transfusions during the previous 3 months) | |||
| Transfused pre study, n (%) | 14 (56.0) | 22 (78.6) | 0.006 |
| Not transfused pre study, n (%) | 5 (11.4) | 14 (29.2) | |
| Hb level (g/dl) triggering RBCT, mean (range) | 7.66 (6.1–9.7) | 7.89 (6.47–9.45) | |
| Adverse effects of treatment (reported in ≥ 10% of patients in any treatment group), n (%) | |||
| Any AE | 50 (72.5) | 57 (75.0) | |
| Fever | 5 (7.2) | 10 (13.2) | |
| Pain | 9 (13.0) | 3 (3.9) | |
| Skeletal pain | 5 (7.2) | 2 (2.6) | |
| Leukopenia | 9 (13.0) | 6 (7.9) | |
| Granulocytopenia | 3 (4.3) | 4 (5.3) | |
| Dyspnoea | 2 (2.9) | 3 (3.9) | |
| Hypertension | 3 (4.3) | 1 (1.3) | |
| Infection | 1 (1.4) | 4 (5.3) | |
| Deaths, na | 1 | 7 | |
| ECOG score | n = 66 | n = 66 | |
|---|---|---|---|
| Change from baseline | NR | NR | 0.038 |
| 1-point improvement, n (%) | 13 (19.7) | 4 (6.1) | |
| 2-point deterioration, n (%) | 1 (1.5) | 5 (7.6) |
| Response to anaemia treatment (rated by physician) (%)a | ||||
|---|---|---|---|---|
| Excellent | 19.7 | 0.0 | ||
| Very good | 19.7 | 3.0 | ||
| Good | 13.6 | 9.1 | ||
| Fair | 18.2 | 24.2 | ||
| Poor | 28.8 | 63.6 | ||
| Efficacy population | n = 66 | n = 66 | |
|---|---|---|---|
| Hb | |||
| Change in Hb level (g/dl) baseline to last value, mean ± SD | 1.8 ± 2.05 | 0.0 ± 1.18 | < 0.001 |
| Mean Hb level (g/dl) week 12 | 11.2 | 9.7 | |
| Responders, n (%) | 38 (57.6) | 6 (9.1) | < 0.001 |
| Mean time (days) for responders to achieve Hb level ≥ 2 g/dl above baseline | 46 | 35a | |
| Correctors, n (%) | 30 (45.5) | 2 (3) | < 0.001 |
| Mean time (days) for correctors to achieve Hb level ≥ 12 g/dl | 50 | 23a | |
| HRQoL | ||
| Quality of life population | n = 66 | n = 72 |
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Unclear; no p-values reported but groups appear comparable based on the values reported |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | Yes (although not blinded to dose – placebo dose matched epoetin alfa dose) |
| 6. Were the outcome assessors blind to treatment allocation? | Yes (although not blinded to dose – placebo dose matched epoetin alfa dose) |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Partially (variability can be calculated from data presented in the paper) |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Partially: primary end point and HRQoL only |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes |
| Other | |
|---|---|
| Generalisability | Haematological cancer |
| Author conclusions | Epoetin alfa is an effective and well-tolerated agent for the management of myeloma-associated anaemia. Benefits include prevention or amelioration of anaemia, reduction in transfusion requirements and improvements in quality of life |
| Reviewer comments | |
| EndNote ref. ID: 2700 | Malignancy type: solid (breast) | |
| Treatment: epoetin (assumed epoetin alfa) | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Del Mastro 199767 | n = 62 |
| Objective | To evaluate the ability of epoetin to prevent the development of clinically significant anaemia in patients treated with chemotherapy | Inclusion criteria: Stage II breast cancer patients receiving accelerated (every 14 days) adjuvant chemotherapy after radical mastectomy or breast-conserving surgery; Hb ≤ 12 g/dl; normal mean corpuscular volume of RBCs (within 80–100 fl) Exclusion criteria: Uncontrolled hypertension; inadequate iron reserves as evidenced by serum iron level less than normal (37 µg/dl) associated with a ferritin level < 10 ng/ml and/or transferrin saturation < 20% |
| No. of centres | 1 (February 1993–June 1995) | |
| Other references/aliases | None | |
| Geographical setting | Italy | |
| Duration of treatment | 12 weeks: 6 cycles plus 2 weeks (starting on day 1 of chemotherapy until 2 weeks after the last chemotherapy cycle); 36 administrations planned per participant | |
| Length of follow-up (if different) | A blood count was performed 6 months after the last chemotherapy cycle | |
| Country of corresponding author | Italy | |
| Language of publication | English | |
| Sources of funding | Supported in part by a grant from the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy | |
| Randomisation and allocation | Randomisation was performed by a telephone call to a central office. Randomisation was balanced with blocks of variable size. No stratification was planned. Two-arm Phase III study | |
| Treatment arms | ||
| Arm drug name(s) | Epoetin | Best supportive care |
| n | 31 | 31 |
| Dose and frequency (once daily, twice daily, etc.) | 150 U/kg Q3W | NA |
| Dose adjustment (yes/no) | Yes. If Hb increased to 15 g/dl in two consecutive weekly assays, epoetin treatment was stopped until Hb < 13 g/dl (n = 4) | NA |
| Route of administration | Subcutaneous | NA |
| Duration of epoetin treatment | 12 weeks: 6 cycles plus 2 weeks (starting on day 1 of chemotherapy until 2 weeks after last chemotherapy cycle); 36 administrations planned per patient | 12 weeks |
| Adjuvant anaemia treatment | G-CSF 5 µg/kg SC day 4 to day 11 during the first 5 cycles; it was withdrawn after the sixth cycle. Oral iron supplement (ferrous sulphate 325 mg/d) was started at the occurrence of serum iron < 37 µg/dl; serum ferritin < 10 ng/ml; or transferrin saturation < 20% (n = 4) | G-CSF 5 µg/kg SC day 4 to day 11 during the first 5 cycles; it was withdrawn after the sixth cycle. Oral iron supplement (ferrous sulphate 325 g/d) was started at the occurrence of serum iron < 37 µg/dl; serum ferritin < 10 ng/ml; or transferrin saturation < 20% (n = 3) |
| Transfusion trigger | Hb < 8 g/dl or in presence of anaemia-related symptoms (dyspnea, tachycardia, severe asthenia) | Hb < 8 g/dl or in presence of anaemia-related symptoms (dyspnea, tachycardia, severe asthenia) |
| Outcomes | |
|---|---|
| Primary outcome | Unclear |
| Other outcomes | RBC count (mean corpuscular volume, mean corpuscular Hb level, mean corpuscular Hb concentration); haematocrit; reticulocyte count; HRQoL (PDI) |
| Analysis | |
|---|---|
| Statistical technique used | Student’s t-test for dependent and independent samples was used. ANCOVA for repeated measures was used to evaluate differences in terms of Hb, iron-related parameters and psychological distress after adjustment for baseline values. The probability of maintaining Hb levels at > 10 g/dl was calculated using the Kaplan–Meier method. The log-rank test was used to assess the difference between the curves. Patients who did not develop anaemia were censored at the cycle at which they were taken off treatment. Patients who received a RBCT were considered as events |
| ITT analysis? | Yes [HRQoL: PDI available only for 53 (85.5%) patients] |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation)? | Yes. Previous study indicated that 50% of patients treated with accelerated CEF chemotherapy developed clinically significant anaemia defined as Hb level ≤ 10 g/dl. Study interested in reducing anaemia occurrence to 10% of patients; 30 patients per arm had to be randomised to ensure a significance of 0.05 (two-sided) and a power of 0.90 |
| Attrition rate (loss to follow-up)? | Partially: two patients in the control group and three in the epoetin group did not complete all six cycles of chemotherapy. Two patients refused accelerated chemotherapy and epoetin treatment. They were treated with CEF chemotherapy at the same doses but every 3 weeks. No attrition rate provided for the last measurement (2 weeks post sixth cycle) or for HRQoL data |
| Was attrition rate adequately dealt with? | Unclear as attrition rate not fully reported |
| No. (%) followed up from each condition? | Yes |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Solid (breast) | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Chemotherapy: six cycles of CEF, cycles repeated every 2 weeks (unless delayed until hematological recovery) | ||||
| Adjuvant anaemia treatment | Iron | Oral iron supplement (ferrous sulphate 325 mg/day) started on occurrence of serum iron < 37 µg/dl, serum ferritin < 10 ng/ml or transferrin saturation < 20% | |||
| G-CSF | G-CSF 5 µg/kg subcutaneously from day 4 to day 11 during the first five cycles; withdrawn after the sixth cycle | ||||
| Transfusion trigger | Hb < 8 g/dl or in presence of anaemia-related symptoms (dyspnoea, tachycardia, severe asthenia) | ||||
| Hb inclusion criterion level | ≤ 12 g/dl | ||||
| Arm 1 = epoetin (n = 31) | Arm 2 = control (n = 31) | Notes | p-value | ||
| Age (years), median (range) | 54 (31–68) | 56 (29–68) | |||
| Hb (g/dl), mean ± SD | 13.0 ± 0.7 | 13.1 ± 0.6 | |||
| White blood cell count (×109/l), mean ± SD | 7.2 ± 2.0 | 7.1 ± 2.0 | |||
| Platelet count (×109/l), mean ± SD | 247 ± 60.7 | 241 ± 51.3 | |||
| RBC count (×1012/l), mean ± SD | 4.4 ± 0.2 | 4.4 ± 0.3 | |||
| Haematocrit (%), mean ± SD | 39.8 ± 2.2 | 40.0 ± 2.0 | |||
| Reticulocyte count (%), mean ± SD | 8.8 ± 6.8 | 7.0 ± 5.6 | |||
| Corpuscular volume (fl), mean ± SD | 91.2 ± 3.7 | 90.7 ± 4.7 | |||
| Corpuscular Hb (pg), mean ± SD | 29.9 ± 1.1 | 29.8 ± 1.6 | |||
| Corpuscular Hb concentration (g/dl), mean ± SD | 32.7 ± 1.1 | 32.8 ± 1.0 | |||
| Serum iron (mmol/l), mean ± SD | 77.3 ± 46.2 | 93.1 ± 37.0 | |||
| Transferrin saturation (%),mean ± SD | 20.7 ± 14.5 | 27.1 ± 11.4 | |||
| Ferritin (ng/ml) | 61.2 ± 48.1 | 45.1 ± 35.1 | |||
| Total iron-binding capacity (µg/dl), mean ± SD | 348 ± 55.6 | 352 ± 51.3 | |||
| Serum erythropoietin (mU/ml), median (range) | 21.0 (0–512) | 25.5 (0–800) | Evaluated in 16 patients per arm; note that methods section states 15 patients | ||
| Observed/predictive ratio, median (range) | 1.13 (0.82–1.31) | 1.19 (0.87–2.34) | |||
| No. (%) receiving conservative surgery | 22 (71) | 26 (84) | |||
| HRQoL: PDI, mean ± SD | 27.5 ± 8.6 (n = 27) | 27.1 ± 7.3 (n = 26) | |||
| Were intervention and control groups comparable? | Unclear. p-values NR but authors state that there was ‘no statistically significant difference in baseline haematological and iron-related parameters’ (p. 2717) | ||||
| Results | |||||
| RBC-related parameters at end of treatment period, mean ± SD | |||||
| RBC count (×1012/l) | 4.1 ± 0.5 | 3.3 ± 0.4 | 0.000 | ||
| Hb (g/dl) | 12.2 ± 1.2 | 10.0 ± 1.1 | 0.000 | ||
| Hb decrease at the end of chemotherapya | 0.8 ± 1.4 (95% CI 0.3 to 1.4) | 3.05 ± 1 (95% CI 2.6 to 3.5) | < 0.001 | ||
| Hb (g/dl) at 6 months’ follow-up | 13.2 ± 0.87 | 13.2 ± 0.61 | > 0.05 | ||
| Anaemia (Hb level ≤ 10 g/dl), n (%)b | 0 (0) (95% CI 0 to 14) | 16 (52) (95% CI 33 to 69) | 0.00001 | ||
| RBCT (no. of patients requiring) | 2 | ||||
| Haematocrit (%) | 37.8 ± 3.9 | 31.0 ± 3.8 | 0.000 | ||
| Reticulocyte count (%) | 10.1 ± 9.8 | 11.8 ± 8.7 | 0.6 | ||
| Mean corpuscular volume (fl) | 91.9 ± 6.7 | 94.7 ± 4.3 | 0.08 | ||
| Mean corpuscular Hb level (pg) | 29.6 ± 2.3 | 30.7 ± 1.8 | 0.07 | ||
| Mean corpuscular Hb concentration (g/dl) | 32.3 ± 1.3 | 32.4 ± 1.2 | 0.8 | ||
| Observed/predictive ratio, median (range) | 1.32 (0.85–2.19) | 1.05 (0.63–2.14) | Evaluated in 16 patients per arm; note that methods section states 15 patients | ||
| Serum erythropoietin (mU/ml), median (range)a | 83 (18–774) | 66 (14.5–469) | |||
| O/P ratio < 1, % | 1 | 37 | |||
| Iron metabolism | Throughout six cycles of treatment, serum iron (p < 0.001) and transferrin saturation (p = 0.0002) significantly decreased, regardless of the treatment arm. Differences between the two arms for serum iron (p = 0.33) and transferrin saturation (p = 0.79) were not statistically significant. After the first cycle of chemotherapy a sharp increase in mean serum ferritin was observed in both arms. After this time ANCOVA showed that the ferritin values did not change significantly (p = 0.14) during treatment, but levels were significantly lower in the epoetin group than in the control group (p = 0.0015). Results were presented graphically | |||
| Total iron-binding capacity at the end of chemotherapy, mean ± SD | 356.4 ± 62.0 | 338.5 ± 58.6 | ||
| HRQoL | ||||
|---|---|---|---|---|
| Health state utility scale: PDI score | n = 27 | n = 26 | ||
| During treatment, mean ± SD | 30.6 ± 10.4 | 28.3 ± 8.0 | ||
| Follow-up, mean ± SD | 27.4 ± 11.2 | 26.3 ± 9.8 | ||
| Adverse effects of treatment, n (%) | |||||
|---|---|---|---|---|---|
| WHO grade | |||||
| Leukopenia | I–II | – | 4 (13) | No grade IV toxicity reported. No statistically significant differences in the main toxicities were observed between the two arms | |
| III | 2 (7) | – | |||
| Thrombocytopenia | I | 4 (13) | 4 (13) | ||
| Nausea/vomiting | I–II | 22 (71) | 23 (74) | ||
| III | 6 (19) | 3 (10) | |||
| Alopecia | III | 31 (100) | 31 (100) | ||
| Mucositis | I–II | 16 (52) | 15 (48) | ||
| III | 3 (10) | 4 (13) | |||
| Diarrhoea | I–II | 1 (3) | 3 (10) | ||
| III | 1 (3) | 1 (3) | |||
| Bone pain | I–II | 12 (39) | 10 (32) | ||
| III | 4 (13) | 4 (13) | |||
| Fatigue | I–II | 18 (58) | 19 (61) | ||
| III | 1 (3) | 1 (3) | |||
| Fever | I–II | 5 (16) | 5 (16) | ||
| III | – | 1 (3) | |||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Yes |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | Unclear; randomisation was performed by a telephone call to a central office |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Unclear: no p-values reported but authors state that there were no significant differences between groups |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | No |
| 6. Were the outcome assessors blind to treatment allocation? | NR |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | No; no primary outcome stated |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | Partial; some evidence, e.g. white blood cell count mentioned but data NR |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes; however, for HRQoL only 87% and 84% of participants were analysed in the epoetin and control groups respectively |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Partially |
| Other | |
| Generalisability | Women only (breast cancer patients) |
| Author conclusions | Epoetin prevents anaemia in patients undergoing chemotherapy. Further trials are required to identify subsets of patients in which the preventative use of this drug could be cost-effective |
| Reviewer comments | |
| EndNote ref. ID: 2701 | Malignancy type: advanced head and neck or lung carcinoma | |
| Treatment: epoetin (assumed epoetin alfa) | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Dunphy 199968 | n = 30 |
| Objective | The effects of paclitaxel and carboplatin with or without concurrent epoetin in the treatment of patients with head and neck carcinoma and lung carcinoma on anaemia and the number of transfusions required | Inclusion criteria: Patients with head and neck carcinoma and lung carcinoma treated at Saint Louis University Health Sciences Center in a Phase II trial using paclitaxel and carboplatin; histologically confirmed advanced head and neck carcinoma (clinical stage III and IV) or advanced non-small cell lung carcinoma (stage IV); no previous therapy was permitted and all patients had measurable or evaluable disease; serum iron saturation ≥ 15%; Zubrod performance status of ≤ 2; serum creatinine < 3 mg/dl; serum bilirubin < 1.5 mg/dl; granulocyte count > 1500/µl; platelet count > 100,000/µL; life expectancy > 4 months; Hb level NR (see dose adjustment) Exclusion criteria: NR (see inclusion criteria) |
| No. of centres | 1 | |
| Other references/aliases | ||
| Geographical setting | USA | |
| Duration of treatment | Unclear – while on chemotherapy. The mean number of chemotherapy courses administered was three for each group (6 weeks?) | |
| Length of follow-up (if different) | NR | |
| Country of corresponding author | USA | |
| Language of publication | English | |
| Sources of funding | NR | |
| Randomisation and allocation | Patients were randomised in a non-blinded fashion either to receive epoetin or to not receive epoetin | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Epoetin | Control |
| n | 15 | 15 |
| Dose and frequency (once daily, twice daily, etc.) | 150 U/kg three times per week | NA |
| Dose adjustment (yes/no) | Yes. If the Hb fell by ≥ 1 g/dl (course 1) the dose was escalated to 300 U/kg (course 2); if the Hb fell by ≥ 1 g/dl the dose was escalated to 450 U/kg (course 3). Epoetin was not initiated if the Hb level was ≥ 16 g/dl. Once epoetin was initiated the Hb level was checked weekly. If the Hb level rose to 18 g/dl, epoetin was discontinued until it fell to 16 g/dl, at which point treatment was reinitiated | NA |
| Route of administration | NR | NR |
| Duration of epoetin treatment | NR | NR |
| Adjuvant anaemia treatment | Oral iron and folic acid for the duration of chemotherapy (ferrous sulfate, 325 mg orally, three times per day and folic acid, 1 mg orally, twice per day) | Oral iron and folic acid for the duration of chemotherapy (ferrous sulfate, 325 mg orally, three times per day and folic acid, 1 mg orally, twice per day) |
| Transfusion trigger | Hb < 8.0 g/dl or development of cardiovascular symptoms of anaemia | Hb < 8.0 g/dl or development of cardiovascular symptoms of anaemia |
| Outcomes | |
|---|---|
| Primary outcome | |
| Other outcomes | Haematological response, RBCT |
| Analysis | |
|---|---|
| Statistical technique used | Accrual was limited by the number of patients who were to be enrolled in local phase II protocols for the treatment of carcinoma of the head and neck and lung carcinoma with paclitaxel and carboplatin. Therefore, the sample size was insufficient to ensure adequate power for subset analyses. Repeated analysis of variance was used to compare the difference in post-chemotherapy Hb levels between the two groups during the first two courses of chemotherapy. Fisher’s exact test was used to compare the difference in the rate of transfusion between the two groups. The Mann–Whitney U-test and Fisher’s exact test were used to compare characteristics between the two groups. An a priori level of significance of 0.05 was used for all comparisons |
| ITT analysis? | Of 30 people randomised, three were not evaluable [n = 2 in the epoetin group (n = 1 non-compliance and n = 1 epoetin initiated on day 8) and n = 1 in the control group (early death)] |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation) | Yes. A minimum of 20 evaluable patients was required to detect a difference of 2.5 g/dl in post-chemotherapy Hb levels between epoetin and control participants with a power of > 90% at a significance level of 0.05 |
| Attrition rate (loss to follow-up)? | Partially: two participants (non-compliance and epoetin was initiated on day 8) in the epoetin group and one participant (early death) in the control group were not evaluable |
| Was attrition rate adequately dealt with? | NR |
| No. (%) followed up from each condition? | NR |
| Baseline characteristics | ||
|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Advanced head and neck or lung carcinoma | |
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Paclitaxel and carboplatin, repeated every 21 days. Patients with advanced lung carcinoma were treated until best response or for six courses of chemotherapy. After two to three preoperative chemotherapy courses, patients with head and neck carcinoma were treated with radiation if they were observed to have a > 50% response or surgery if a < 50% response was observed. They were then followed up with no further treatment until they developed a recurrence | |
| Adjuvant anaemia treatment | Iron | Oral iron and folic acid for the duration of chemotherapy (ferrous sulfate, 325 mg orally, three times per day and folic acid, 1 mg orally, twice per day) |
| G-CSF | NR | |
| Transfusion trigger | Hb < 8.0 g/dl or development of cardiovascular symptoms of anaemia | |
| Hb inclusion criterion level | NR | |
| Evaluable population | Arm 1 = epoetin (n = 13) | Arm 2 = control (n = 14) | Notes | p-value |
|---|---|---|---|---|
| Sex | ||||
| Male, n (%) | 12 (92) | 7 (50) | Gender was not distributed equally between the two treatment groups | 0.003 |
| Female, n (%) | 1 (8) | 7 (50) | ||
| Age (years), median (range) | 59 (42–76) | 67 (32–82) | ||
| Hb g/dl mean (SD) | 14.1 (2.1) | 14.1 (1.6) | 0.68 | |
| Epoetin (mU/ml), mean (SD) (n = 25) | 8.8 (5.1) | 7.3 (4.4) | ||
| Iron, serum (mg/dl), mean (SD) (n = 26) | 67.2 (22.9) | 75.7 (51.1) | ||
| Iron saturation (%), mean (SD) (n = 26) | 26.8 (8.7) | 31.9 (24.3) | ||
| Folic acid (mg/ml), mean (SD) (n = 25) | 8.3 (4.2) | 6.1 (3.1) | ||
| Vitamin B12 (pg/ml), mean (SD) (n = 24) | 552 (243) | 445 (139) | ||
| Type of solid tumour, randomised participants | ||||
| Head and neck, n (%) | 10 (66.7) | 11 (77.3) | ||
| Lung, n (%) | 5 (33.3) | 4 (26.7) | ||
| Were intervention and control groups comparable? | No | |||
| Results | ||||
| Hb | ||||
| Change in Hb (g/dl) after two courses of chemotherapy | 1.2 | 2.8 | 0.037 | |
| Transfusions | ||||
|---|---|---|---|---|
| No. (%) transfused during two courses of chemotherapy | 1 (8) | 2 (14) | > 0.05 | |
| No. (%) of transfused participant at four courses of chemotherapy | 2 (15) | 5 (36) | These differences were not compared statistically because after the second chemotherapy session fewer patients were treated in subsequent courses (see Figure 2) | |
| Units received per participant at four courses of chemotherapy | 3 | 2.8 | ||
| Serum epoetin, n | 10 | 10 | Three epoetin and four control participants had no follow-up serum epoetin data. Epoetin levels increased significantly over time for both groups (p = 0.007); however, the increase in the group treated with epoetin was significantly greater than the increase in the control group (p = 0.002) (see Figure 3) | |
| HRQoL | ||||
| NR | ||||
| Adverse effects of treatment | ||||
| NR | ||||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear; not specified |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Gender was not distributed equally between the two treatment groups (p = 0.003) |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | No |
| 6. Were the outcome assessors blind to treatment allocation? | NR |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | NA; no primary outcome specified |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | No |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes |
| Other | |
|---|---|
| Generalisability | Gender was not distributed equally between the two treatment groups (p = 0.003) |
| Author conclusions | There was significantly less anaemia and transfusions were reduced by 50% in patients randomised to receive epoetin during chemotherapy with paclitaxel and carboplatin |
| Reviewer comments | No Hb inclusion criterion; epoetin was not initiated if the Hb level was ≥ 16 g/dl. In addition, after two to three preoperative chemotherapy courses, patients with head and neck carcinoma were treated with radiation if they were observed to have a > 50% response or surgery if a < 50% response was observed. They were then followed up with no further treatment until they developed a recurrence |
| EndNote ref. ID: 362 | Malignancy type: SCLC | |
| Treatment: epoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Grote 200574 | n = 224 |
| Objective | To evaluate the effects of epoetin alfa on tumour response to chemotherapy and survival in patients with SCLC | Inclusion criteria: Age ≥ 18 years; newly diagnosed both extensive-stage and limited-stage SCLC scheduled for at least three chemotherapy cycles; ECOG score 0–2; life expectancy ≥ 3 months; Hb ≤ 14.5 g/dl Exclusion criteria: Previous cytotoxic chemotherapy or radiotherapy or scheduled curative-intent radiotherapy during the first three chemotherapy cycles; uncontrolled underlying disease not attributable to malignancy; uncontrolled hypertension; evidence of untreated iron, folate or vitamin B12 deficiency or ongoing haemolysis |
| No. of centres | 35 sites | |
| Other references/aliases | N93–004 | |
| Geographical setting | USA | |
| Duration of treatment | 12 weeks = from start of treatment until 3 weeks after chemotherapy completed (the mean number of chemotherapy cycles was 4 and 4.1 in the epoetin and placebo groups respectively; duration of a cycle = 3 weeks) | |
| Length of follow-up (if different) | 3 years after treatment | |
| Country of corresponding author | USA | |
| Language of publication | English | |
| Sources of funding | Johnson & Johnson LLC | |
| Randomisation and allocation | Randomised double-blind, parallel-group, placebo-controlled trial; 1 : 1 computer-generated randomisation, no details provided on allocation concealment | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Epoetin alfa | Placebo |
| n | 109 | 115 |
| Dose and frequency (once daily, twice daily, etc.) | 150 U/kg three times a week | 150 U/kg three times a week |
| Dose adjustment (yes/no) | Yes. Epoetin stopped if Hb level was > 16 g/dl until Hb level was < 14 g/dl and resumed at 75 U/kg; no dose escalation | |
| Route of administration | Subcutaneous | Subcutaneous |
| Duration of epoetin treatment | Until approximately 3 weeks after final chemotherapy cycle | Until approximately 3 weeks after final chemotherapy cycle |
| Adjuvant anaemia treatment | NR | NR |
| Transfusion trigger | NR | NR |
| Outcomes | |
|---|---|
| Primary outcome | |
| Other outcomes | Haematological response (weekly Hb, after three cycles and after final cycle); RBCT (proportion of patients transfused, no. of units transfused, time to first transfusion); tumour response (after three cycles and final cycle); survival (up to 3 years); AEs |
| Analysis | |
|---|---|
| Statistical technique used | Overall tumour response rate CRa plus PRb and 95% CIs reported. Kaplan–Meier estimates for survival data |
| ITT analysis? | Yes for all efficacy data. Safety population consisted of all patients receiving at least one dose of study drug with available safety data |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation) | Yes (based on 15% one-sided decrease in overall tumour response rate in the epoetin arm). However, the trial was terminated early and thus there were power issues |
| Attrition rate (loss to follow-up)? | No. of patients and reasons for treatment discontinuation reported for both arms |
| Was attrition rate adequately dealt with? | NR except to say that ITT analysis was conducted |
| No. (%) followed up from each condition? | NR |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | SCLC | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Etoposide and cisplatin | ||||
| Adjuvant anaemia treatment | Iron | NR | |||
| G-CSF | NR | ||||
| Transfusion trigger | NR | ||||
| Hb inclusion criterion level | ≤ 14.5 | ||||
| Arm 1 = epoetin (n = 109) | Arm 2 = placebo (n = 115) | Notes | p-value | ||
| Sex, n (%) | |||||
| Male | 59 (54.1) | 64 (55.7) | |||
| Female, | 50 (45.9) | 51 (44.3) | |||
| Age (years), mean (SD) [range] | 64.4 (8.7) [37–78] | 63.2 (8.9) [37–78] | |||
| Performance status: ECOG score, n (%) | |||||
| 0–1 | 73 (67) | 83 (72.2) | |||
| 2 | 34 (31.2) | 32 (27.8) | |||
| 3 | 1 (0.9) | 0 (0) | |||
| 4 | 0 (0) | 0 (0) | |||
| Missing | 1 (0.9) | 0 (0) | |||
| Baseline Hb (g/dl), mean (SD) | 12.8 (1.5) | 13 (1.5) | |||
| Baseline iron (U/l), mean (SD) | 75.3 (65.41) | 81.6 (66.35) | |||
| Ferritin (ng/dl), mean (SD) | 471.7 (856.3) | 460.3 (632.9) | |||
| No. of chemotherapy cycles received, mean (SD); median (range) | 4 (2.1); 4 (1–10) | 4.1 (2.2); 4 (1–12) | |||
| Received radiotherapy, all cycles, n (%) | 16 (14.7) | 14 (12.2) | |||
| Extensive-stage SCLC, n (%) | 72 (66.1) | 68 (59.1) | |||
| Were intervention and control groups comparable? | No p-values reported; authors stated that the ‘demographics and clinical characteristics were generally similar between groups’ (p. 9379) | ||||
| Results | ||||
|---|---|---|---|---|
| Difference epoetin – placebo | 95% CI | |||
| Tumour response | ||||
| Tumour response: CR or PR at final cycle, n (%) | 65 (59.6) | 64 (55.7) | 4% | –9 to 17% |
| Tumour response: CR at final cycle, n (%) | 20 (18.3) | 21 (18.3) | ||
| Hb | ||||
| Mean Hb at cycle 3 (g/dl) | 12.5 | 10.6 | 1.9 | 1.4 to 2.4 |
| Mean Hb at final cycle (g/dl) | 12.2 | 10.3 | 1.9 | 1.4 to 2.4 |
| Mean change in Hb from baseline to end of treatment (g/dl) | –0.6 | –2.7 | ||
| Mean change in Hb at time of median exposure to study drug (13 weeks) (g/dl) | –0.2 | –2.9 | ||
| Hb at 13 weeks estimated from Figure 2, mean (SD) | 12.5 (0.6) (n = 64) | 10.24 (0.4) (n = 58) | ||
| HRQoL | ||||
| NR | ||||
| OS | ||||
| Median survival (Kaplan–Meier) (months)a | 10.5 | 10.4 | ||
| Transfusions | ||||
| Participants, n (%) | 26 (24) | 42 (37) | HR 0.597, 95% CI 0.365 to 0.977 | |
| No. of units, mean (SD) | 0.5 (3.6)b | 0.4 (0.7) | ||
| Safety dataa | ||
|---|---|---|
| Discontinued chemotherapy because of AEs, n (%) | 23 (21) | 32 (28) |
| Deaths (at 3 years’ follow-up), n (%) | 100 (91.7) | 101 (87.8) |
| Cause of death = disease progression, % | 91 | 84 |
| Nausea, n (%) | 80 (73.4) | 79 (68.7) |
| Vomiting, n (%) | 56 (51.4) | 58 (50.4) |
| Fatigue, n (%) | 32 (29.4) | 40 (34.8) |
| Constipation, n (%) | 34 (31.2) | 40 (34.8) |
| Clinically relevant thrombovascular events, n (%) | 12 (11) | 11 (9.6) |
| Thromboembolic events, n (%) | 1 (0.9) | 0 (0.9) |
| Hypertension | NR; patients with uncontrolled hypertension were excluded | |
| ECOG score | At baseline 98% and 100% patients had an ECOG score of ≤ 2 in the epoetin and placebo groups, respectively; at the end of treatment 71% of patients had an ECOG score of ≤ 2 in both groups | |
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Yes |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Unclear; no p-values reported |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | Yes |
| 6. Were the outcome assessors blind to treatment allocation? | Yes |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Yes |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes, during treatment period only |
| Other | |
|---|---|
| Generalisability | |
| Author conclusions | Results suggest that in newly diagnosed patients with SCLC epoetin alfa does not affect tumour response to chemotherapy or survival. However, the early trial closure makes these conclusions preliminary |
| Reviewer comments | Divergence in survival curves after 12 months (see Figure 1). The authors note that there is no information on patients’ medication after the end of treatment, nor on the possible differences in the proportion of patients with extensive-stage SCLC. However, the paper does not report whether any significant differences were found for outcomes measured at baseline. In addition, although unknown, the medication could be expected to be similar for all patients |
| EndNote ref. ID: 2703 | Malignancy type: lymphoproliferative | |
| Treatment: darbepoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Hedenus 200253 | n = 66 |
| Objective | To assess the safety and dose–response relationship of darbepoetin alfa in patients with different types of lymphoproliferative malignancies receiving multicycle chemotherapy | Inclusion criteria: Patients with a diagnosis of lymphoproliferative malignancy (multiple myeloma, low- and intermediate-grade non-Hodgkin’s lymphoma, Hodgkin’s disease or chronic lymphocytic leukaemia); life expectancy ≥ 6 months; ECOG performance status 0–2; at least 12 more weeks of chemotherapy; adequate iron stores (transferrin saturation ≥ 15% or ferritin ≥ 10 µg/l); normal serum vitamin B12 and folate concentrations; adequate liver function (serum bilirubin ≤ 1.5 times the upper limit of the normal range); adequate renal function (serum creatinine ≤ 177 µmol/l); not received two RBCTs within 4 weeks of randomisation or any RBCT within 2 weeks of randomisation; Hb level: ≤ 11.0 g/dl Exclusion criteria: High-grade non-Hodgkin’s lymphoma; myeloablative chemotherapy or radiotherapy for transplantation or chemotherapy regimens using investigational agents; primary or metastatic malignancy involving the central nervous system; clinical evidence of active infection or inflammatory disease; other disorders that could potentially interfere with the response of darbepoetin |
| No. of centres | 15 | |
| Other references/aliases | NR | |
| Geographical setting | Europe/Australia | |
| Duration of treatment | 12 weeks | |
| Length of follow-up (if different) | 4 weeks after treatment period | |
| Country of corresponding author | Sweden | |
| Language of publication | English | |
| Sources of funding | Amgen, Inc. | |
| Randomisation and allocation | Multicentre, randomised (1 : 2 : 2 : 1 ratioa), double-blind, placebo-controlled, dose-finding study. Randomisation was performed using a central computerised system and was stratified to balance the treatment groups with respect to malignancy type (myeloma vs. lymphoma) | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Darbepoetin alfa | Placebo |
| n | 22 | 11 |
| Dose and frequency (once daily, twice daily, etc.) | 2.25 µg/kg once weekly | NR |
| Dose adjustment (yes/no) | Yes; doses reduced by 50% for patients who had a ≥ 2 g/dl increase in Hb during any 28-day period in the absence of RBCT; withheld for patients with Hb concentrations > 15.0 g/dl (men) or > 14.0 g/dl (women) and reinstated at 50% of weekly dose once Hb concentrations decreased to ≤ 13.0 g/dl | NR |
| Route of administration | Subcutaneous | Subcutaneous |
| Duration of epoetin treatment | 12 weeks | 12 weeks |
| Adjuvant anaemia treatment | NR | NR |
| Transfusion trigger | RBCTs were recommended for patients with Hb concentrations ≤ 8.0 g/dl | NR but assumed to match darbepoetin group |
| Outcomes | |
|---|---|
| Primary outcome | Haematological response (defined as an increase in Hb of ≥ 2.0 g/dl from baseline in the absence of RBCT; haematopoietic response defined as Hb response or increase in Hb concentration to ≥ 12.0 g/dl in the absence of RBCT; sustained Hb response defined as Hb response maintained for 28 days or until the end of treatment; Hb concentrations measured weekly |
| Other outcomes | RBCT (from week 5 until the end of the treatment period); AEs (AEs, excess increases in Hb, changes in laboratory variables and vital signs, antibody formation resulting from darbepoetin administration) |
| Analysis | |
|---|---|
| Statistical technique used | Rates of Hb response and haematopoietic response estimated using the Kaplan–Meier method. Logistic regression was used to assess treatment effect, dose–response relationships and the effect of covariates |
| ITT analysis? | Described as ITT analysis but defined as all randomised who received at least one dose of study drug, so not strict ITT analysis |
| Does statistical technique adjust for confounding? | Covariates included in models were malignancy type, sex, baseline Hb (categorical variable), RBCTs in the 4 weeks before randomisation, baseline serum endogenous erythropoietin concentration (categorical variable) |
| Power calculation (a priori sample calculation) | NR |
| Attrition rate (loss to follow-up)? | Three of the 66 patients recruited to the four study groups (two patients in the darbepoetin groups withdrawn because of a delay in chemotherapy;a one in the placebo group withdrew consent) |
| Was attrition rate adequately dealt with? | Not clear, although attrition rate low |
| No. (%) followed up from each condition? | NR |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Lymphoproliferative | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Chemotherapy (type NR) | ||||
| Adjuvant anaemia treatment | Iron | NR | |||
| G-CSF | NR | ||||
| Transfusion trigger | RBCTs were recommended for patients with Hb concentrations ≤ 8.0g/dl | ||||
| Hb inclusion criterion level | ≤ 11.0 g/dl | ||||
| Arm 1 = darbepoetin (n = 22) | Arm 2 = placebo (n = 11) | Notes | p-value | ||
| Sex, n (%) | |||||
| Male | 14 (64) | 2 (18) | |||
| Female | 8 (36) | 9 (82) | |||
| Age (years), median (range) | 69 (20–84) | 63 (25 –80) | |||
| Neutrophil count (×109/l), mean (SD) | 2.9 (2.2) | 7.0 (7.5) | |||
| RBCT during 4 weeks pre randomisation, n (%) | 4 (18) | 2 (18) | |||
| Hb (g/dl), mean (SD) | 9.4 (1.3) | 9.5 (1.0) | |||
| Platelet count (×109/l), mean (SD) | 232.4 (157.6) | 283.1 (188.6) | |||
| Endogenous serum erythropoietin (U/l), median (range) | 69 (12–1362) | 45 (12–132) | |||
| Lymphoma, n (%) | |||||
| Hodgkin’s disease | 4 (18) | 3 (27) | |||
| Non-Hodgkin’s lymphoma | 11 (50) | 3 (27) | |||
| Chronic lymphocytic leukaemia | 1 (5) | 2 (18) | |||
| Multiple myeloma | 6 (27) | 3 (27) | |||
| Serum ferritin (µg/l), median (range) | 430 (15–1288) | 524 (14–2178) | |||
| Transferrin saturation (%), median (range) | 25 (6–71) | 18 (9–37) | |||
| Were intervention and control groups comparable? | No; stated that there was a higher proportion of women in the placebo group and that neutrophil and platelet counts were higher in the placebo group. No analyses presented to support these statements | ||||
| Results | |||
|---|---|---|---|
| Haematology | |||
| Proportion of participants with a haematological response | 55 | 10 | Analysis comparing these two groups NR |
| Time to response (weeks), median (range) | 13 weeks (1–13) | Not estimated | |
| Proportion of participants with a haematopoietic response (95% CI) | 60 (39 to 81) | 19 (0 to 43) | |
| Mean change (95% CI) in Hb from baseline to week 13 | 1.64 (1.05 to 2.24) | 1.00 (0.55 to 1.45) | |
| Transfusions | |||
| Proportion of patients transfused (95% CI) | 27 (9 to 46) | 45 (16 to 75) | |
| HRQoL | |||
| NR | |||
| Adverse effects of treatment | |||
| Darbepoetin (n = 55) | Placebo (n = 11) | ||
| At least one AE during the study period, n (%) | 52 (95) | 10 (91) | |
| Rapid rise in Hb of ≥ 2g/dl within 28-day period, n (%) | 22 (40) | 1 (9) | |
| Quality appraisal | ||
|---|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Yes, computerised stratified system | |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | Unclear; stated as central computer but further details not provided | |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Higher proportion of women in the placebo group (but sex included as confounder in models); neutrophil and platelet counts higher in the placebo group | |
| 4. Were the eligibility criteria specified? | Yes | |
| 5. Were the participants blind to treatment allocation? | Yes | |
| 6. Were the outcome assessors blind to treatment allocation? | Yes | |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Yes | |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No | |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes | |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Partially. No CONSORT flow chart, but withdrawals and reasons are noted in the text: ‘Two patients receiving darbepoetin alfa were withdrawn. However, it is unclear from which of the three darbepoetin alfa groups the participants withdrew. In addition, one patient randomised to receive placebo withdrew consent’ (p. 81) | |
| Other | ||
| Generalisability | Small sample sizes. Analyses conducted using combined data from the three darbepoetin groups vs. placebo, but only one of the darbepoetin groups is relevant to this review | |
| Author conclusions | The results of the study indicated that darbepoetin alfa, administered once weekly at doses of 1.0 µg/kg, 2.25 µg/kg and 4.5 µg/kg, was associated with greater effects on Hb levels than placebo in patients with lymphoproliferative malignancies | |
| Reviewer comments | Difficult to interpret results specifically for the dosage relevant to this review | |
| EndNote ref. ID: 2704 | Malignancy type: lymphoproliferative | |
| Treatment: darbepoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Hedenus 200317 | n = 344 |
| Objective | To evaluate the efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies. The study included patients with myeloma and lymphoma and was stratified to enable a comparison of darbepoetin alfa and placebo within each malignancy type | Inclusion criteria: Men and women aged ≥ 18 years; lymphoproliferative malignancies (Hodgkin’s disease, non-Hodgkin’s lymphoma, chronic lymphocytic leukaemia or multiple myeloma); anaemia (Hb ≤ 11 g/dl), primarily because of cancer or chemotherapy (i.e. serum folate ‡ 4.5 nmol/l and vitamin B12 ‡ 148 pmol/l, no haemolysis, and no gastrointestinal bleeding); ECOG performance status 0–3; scheduled to receive cytotoxic chemotherapy for at least 12 additional weeks; adequate renal and liver function (serum creatinine concentration ≤ 177 µmol/l, serum bilirubin ≤ 1.5 times the central laboratory upper limit of normal); life expectancy of ≥ 4 months Exclusion criteria: Burkitt’s or lymphoblastic lymphoma; scheduled to receive a stem cell transplant within 16 weeks of randomisation; received myeloablative chemotherapy, radiotherapy for transplantation or chemotherapy regimens containing investigational agents; transferrin saturation < 15% and ferritin < 10 µg/l; significant central nervous system, cardiac or inflammatory diseases; any known primary haematological disorders that could cause anaemia; patients not to have received epoetin within 8 weeks, more than two RBCTs within 4 weeks or any RBCT within 2 weeks of randomisation |
| No. of centres | 49 | |
| Other references/aliases | Secondary analysis in Littlewood 200683 (see note) | |
| Geographical setting | Europe, Australia and Canada | |
| Duration of treatment | 12 weeks | |
| Length of follow-up (if different) | Unclear; a median follow-up period of approximately 11 months | |
| Country of corresponding author | Sweden | |
| Language of publication | English | |
| Sources of funding | This study was supported by Amgen, Inc., Thousand Oaks, CA, USA | |
| Randomisation and allocation | Central randomisation 1 : 1 to receive darbepoetin alfa or placebo. Randomisation was stratified to balance the treatment groups with respect to malignancy type (lymphoma vs. myeloma), region (Australia vs. Canada vs. Western Europe) and chemotherapy before randomisation (heavily pretreated vs. not heavily pretreated; note: patients were considered to have been heavily pretreated if they had received two or more lines of chemotherapy or one line of chemotherapy and a stem cell transplant) | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Darbepoetin alfa | Placebo |
| n | 174 | 170 |
| Dose and frequency (once daily, twice daily, etc.) | 2.25 µg/kg, QW | NA, QW |
| Dose adjustment (yes/no) | Yes. Dose doubled for patients who had a ≤ 1 g/dl increase in Hb from baseline after 4 weeks of treatment. It was withheld if Hb value increased to > 15 g/dl for men or > 14 g/dl for women and was reinstated at 50% once Hb level was ≤ 13 g/dl | |
| Route of administration | Subcutaneous | |
| Duration of epoetin treatment | 12 weeks | |
| Adjuvant anaemia treatment | Iron therapy was at the discretion of the investigators | |
| Transfusion trigger | Transfusion policies were left to the discretion of the investigators; recommended if Hb ≤ 8 g/dl | |
| Outcomes | ||
| Primary outcome | Haematological response (proportion of participants with a Hb responsea) | |
| Other outcomes | Haematological response (proportion of participants with a haematopoietic responseb); RBCT [incidence of transfusions from week 5 to the end of the treatment (and from week 1 to the end of the treatment)]; tumour response (continued to be collected during a long-term follow-up period); survival (continued to be collected during a long-term follow-up period); AEs (AEs and antibody formation); HRQoL (FACT-F every 4 weeks on day 1 of each cycle of chemotherapy, before any other study procedures) | |
| Analysis | |
|---|---|
| Statistical technique used | The Kaplan–Meier method was used to estimate the percentages of patients with a Hb response, haematopoietic response or RBCT because of the anticipated withdrawal rate and approximate 95% CIs were calculated using Greenwood’s formula. Statistical comparisons of these percentages between treatment groups were based on the chi-squared test. Cox proportional hazards modelling was performed as an exploratory analysis to evaluate the effect of baseline serum erythropoietin (≤ 100 vs. > 100 IU/l) on the time to Hb response. The mean (± SEM) change in Hb concentration was assessed in two ways: first, by subtracting the baseline Hb value from the last value during the treatment phase and, second, by evaluating the completers analysis.a Efficacy end points were analysed with and without adjusting for the stratification factors of malignancy type, region and chemotherapy before randomisation. Results of these analyses were similar; thus, only the results of the unadjusted analyses are presented. Exploratory analyses of changes in the FACT-F subscale were conducted using analysis of variance. The relationship between the change in the FACT-F subscale and the change in Hb was investigated using simple linear regression |
| ITT analysis? | Yes. All patients who received at least one dose of the study drug were included in the analyses of efficacy and safety (ITT analysis set n = 344), with the exception of transfusion end points, valuated during week 5 to the end of the treatment phase. For these end points, patients who did not complete the first 4 weeks of treatment were excluded from the analysis (n = 332). For Hb and haematopoietic response, patients who withdrew from the study early for any reason were censored at the time of withdrawal. For the RBCT end points, patients who withdrew from the study before the completion of the treatment period were considered to have been transfused and patients who withdrew because of either disease progression or death were censored at the time of withdrawal |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation) | Yes. Sample size to detect an increase in Hb response rate from 25% in the placebo group to 50% in the darbepoetin alfa group within each malignancy type, with 90% power at a two-sided significance level of 0.05 (estimated withdrawal rate of 10% during the 12-week study) |
| Attrition rate (loss to follow-up)? | Yes, until the end of treatment |
| Was attrition rate adequately dealt with? | Yes |
| No. (%) followed up from each condition? | NR |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Lymphoproliferative | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Chemotherapy – no further details given | ||||
| Adjuvant anaemia treatment | Iron | Iron therapy was at the discretion of the investigators | |||
| G-CSF | None | ||||
| Transfusion trigger | At the discretion of the investigators; recommended if Hb ≤ 8 g/dl | ||||
| Hb inclusion criterion level | ≤ 11 g/dl | ||||
| Darbepoetin alfa (n = 174) | Placebo (n = 170) | Difference | p-value | ||
| Sex | |||||
| Male (%) | 87 (50) | 78 (46) | |||
| Female (%) | 87 (50) | 92 (54) | |||
| Age (years), mean (SD) | 64.8 (13.8) | 64.6 (12.2) | |||
| ECOG score, n (%) | |||||
| 0 | 54 (31) | 43 (25) | |||
| 1 | 80 (46) | 92 (54) | |||
| 2 | 32 (18) | 28 (16) | |||
| > 2 | 8 (5) | 6 (4) | |||
| Missing | 0 (0) | 1 (1) | |||
| Hb baseline (g/dl), mean (SD) | 9.59 (1.22) | 9.5 (1.21) | |||
| Ferritin (µg/l), median (range) | 324.5 (5–5352) | 253.5 (15–5027) | |||
| Transferrin saturation (%), median (range) | 26.5 (5–95) | 25 (4–95) | |||
| Serum erythropoietin baseline (mU/ml), median (range) | 68.99 (2.3–1522.7) | 54.49 (10.9–3169.1) | |||
| Previous chemotherapy, n (%) | |||||
| Heavily pretreateda | 46 (26) | 47 (28) | |||
| Not heavily pretreated | 128 (74) | 123 (72) | |||
| Malignancy type, n (%) | |||||
| Lymphoma (Hodgkin’s disease, non-Hodgkin’s lymphoma, chronic lymphocytic leukaemia) | 85 (49) | 86 (51) | |||
| Multiple myeloma | 89 (51) | 84 (49) | |||
| Were intervention and control groups comparable? | No p-values reported; authors stated that ‘baseline demographic and clinical characteristics were generally well balanced between the treatment groups’ (p. 397) | ||||
| Results | ||||
|---|---|---|---|---|
| Hb response (%) (95% CI) | 60 (52 to 68) | 18 (12 to 24) | 42 (32 to 52) | < 0.001 |
| Haematopoietic response (%)a (95% CI) | 65 (57 to 73) | 24 (18 to 31) | < 0.001 | |
| Mean change in Hb (SEM); ITTb | 1.8 (0.17) | 0.19 (0.1) | < 0.001 | |
| Mean change in Hb (SEM); completer’s analysisb | 2.66 (0.2) | 0.69 (0.14) | < 0.001 | |
| Lymphoma subgroup | ||||
| Hb response (%) | 64 (n = 85) | 13 (n = 86) | 51 | < 0.001 |
| Myeloma subgroup | ||||
| Hb response (%) | 56 (n = 89) | 23 (n = 84) | 33 | < 0.001 |
| Baseline serum erythropoietin levels ≤ 100 IU/l | ||||
| Hb response (%) (95% CI) | 69 (60 to 79) (n = 89) | 16 (9 to 22) (n = 84) | ||
| Baseline serum erythropoietin levels > 100 IU/l | ||||
| Hb response (%) (95% CI) | 44 (31 to 58) (n = 89) | 25 (11 to 39) (n = 84) | 19 (0 to 38) | |
| Transfusions; from week 5 to end of treatment (%) (95% CI)c | 31 (24 to 38) (n = 167) | 48 (41 to 56) (n = 165) | < 0.001 | |
| Transfusions; from week 1 to end of treatment (%) (95% CI)d | (n = 167) | (n = 165) | 17 (6 to 27) | < 0.001 |
| Change in FACT-F subscale score from baseline to end of treatment period (84% of patients completed the FACT-F subscale at week 13): improvement in FACT-F subscale score compared with placebo regardless of level of fatigue at baseline. Patients with the lowest baseline FACT-F subscale scores reported the largest improvement in FACT-F subscale score at EOTP. After adjusting for the effect of baseline score, increases in FACT-F subscale scores with darbepoetin alfa treatment were significantly greater than those observed with placebo (p = 0.032). For every 1 g/dl increase in Hb, the estimated mean increase in FACT-F subscale score was 1.39 (95% CI 0.83 to 1.94; p < 0.001). For FACT-F change scores in the lymphoma and myeloma subgroups, see Littlewood and colleagues83 | ||
| Adverse effects | ||
|---|---|---|
| Deaths (during the study or within 30 days of the last dose of study drug), n (%) | 10 (6) | 4 (2) |
| Withdrawal because of adverse effects (not including death) (%) | 3 | 4 |
| No evidence of neutralising antibodies to darbepoetin alfa was detected for any patient | ||
| Iron supplementation received (%) | ||
| Oral | 6 | 7 |
| Subcutaneous | 0 | 1 |
| Survival (median follow-up period of approximately 11 months) | ||
| PFS, n (%) | 82 (47) | 76 (45) |
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Yes |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | No p-values reported; authors stated that ‘baseline demographic and clinical characteristics were generally well balanced between the treatment groups’ (p. 397) |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | Yes |
| 6. Were the outcome assessors blind to treatment allocation? | Yes |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Yes |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes, ITT defined as all randomised who received one or more dose of the study drug |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes, until the end of treatment |
| Other | |
|---|---|
| Generalisability | Yes |
| Author conclusions | The efficacy of darbepoetin alfa was consistent for patients with lymphoma or myeloma. Improvements in quality of life were also observed with darbepoetin alfa. The overall safety profile of darbepoetin alfa was consistent with that expected for this patient population. Darbepoetin alfa significantly increased Hb levels and reduced RBCTs in patients with lymphoproliferative malignancies receiving chemotherapy. Darbepoetin alfa demonstrated clinically important improvements in response rate relative to placebo, regardless of baseline endogenous erythropoietin level |
| Reviewer comments | |
| EndNote ref. ID: 2705 | Malignancy type: solid – breast, gynaecological, gastrointestinal, lung, other | |
| Treatment: chemotherapy, darbepoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Kotasek 200350 | n = 249 |
| Objective | To assess the safety of darbepoetin alfa in patients with cancer receiving chemotherapy, to assess the feasibility of administering darbepoetin alfa Q3W and to characterise the dose–response relationships for darbepoetin alfa when given Q3W | Inclusion criteria: Age ≥ 18 years with solid tumours receiving cyclic chemotherapy; life expectancy ≥ 6 months; ECOG performance status 0–2; adequate liver and renal function; anaemia (Hb level ≤ 11.0 g/dl) because of cancer and/or chemotherapy Exclusion criteria: Iron deficient (transferrin saturation < 15% and ferritin < 10 µg/l); received recombinant human erythropoietin within 8 weeks of randomisation; more than two RBCTs within 4 weeks of randomisation; any RBCT within 2 weeks of randomisation; known primary haematological disorders that could cause anaemia and central nervous system, cardiac or inflammatory diseases |
| No. of centres | 26 | |
| Other references/aliases | None | |
| Geographical setting | Australia, Canada, Costa Rica and Europe | |
| Duration of treatment | 12 weeks (double-blind treatment). NB: study in two parts (part B open-label treatment period weeks 12–24) | |
| Length of follow-up (if different) | Unclear: 8-week observation period after last dose of study drug at week 12 (Figure 1 shows part A of study has observation period running to week 18, thus 6 weeks); however, results for the observation period are not reported | |
| Country of corresponding author | Australia | |
| Language of publication | English | |
| Sources of funding | Supported by Amgen, Inc., USA | |
| Randomisation and allocation | Randomised, double-blind, placebo-controlled, dose-finding study of darbepoetin alfa. Randomised 4 : 1 to receive darbepoetin alfa (4.5 µg/kg, 6.75 µg/kg, 9.0 µg/kg or 13.5 µg/kg) or placebo. Later, after review of data by the safety monitoring committee, dose cohorts of 12.0 µg/kg and 15.0 µg/kg were added | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Darbepoetin alfa | Placebo |
| n | 17 | 51 |
| Dose and frequency (once daily, twice daily, etc.) | 6.75 µg/kg Q3Wa (2.2 µg/kg QW) The mean administered number of darbepoetin alfa doses over the 12-week treatment phase was 3.6 |
NR |
| Dose adjustment (yes/no) | Yes. No dose increase for inadequate response was allowed in the double-blind part of the study. If Hb level increased to > 15.0 g/dl for men or ≥ 14.0 g/dl for women, treatment was interrupted and reinstated at a lower dose when Hb level was ≤ 13.0 g/dl | NR |
| Route of administration | Subcutaneous | NR |
| Duration of epoetin treatment | 12 weeks | 12 weeks |
| Adjuvant anaemia treatment | NR | NR |
| Transfusion trigger | NR | NR |
| Outcomes | |
|---|---|
| Primary outcome | AEsa (incidence of AE by dose and treatment group and formation of antibodies) |
| Other outcomes | Haematological response [responders;b haematopoietic response;c Hb level (change from baseline); RBCT (week 5 to end of treatment period); HRQoL (FACT-G, FACT-F)] |
| Analysis | |
|---|---|
| Statistical technique used | Proportion of patients per dose group (Hb response, haematopoietic response) estimated by taking 1 minus the Kaplan–Meier estimate of the survivor function at the time of the last observed end point. Approximate 95% CIs for the Kaplan–Meier estimate of the proportion were calculated using Greenwood’s estimate of the variance and assuming a normal distribution for the Kaplan–Meier estimate. For the incidence of RBCT a subset was used (transfusions from week 5 to end of treatment period) including all patients who received at least one dose of study drug and who ended their treatment phase during week 5 or later. Patients who had more than one transfusion were counted only once in calculating the incidence of transfusions Change in Hb from baseline:
|
| ITT analysis? | Analyses conducted on patients randomised to study drug who received at least one dose |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation) | Sample size was statistically based on the secondary objectives to determine a clinically effective dose, by means of estimating Hb response rates. The 4 : 1 randomisation allowed for 36 darbepoetin alfa patients per dose cohort. Anticipated premature withdrawal rate of approximately 20%, and therefore a sample size of 29, allows estimation of the Hb response rate within a SE of 0.09. The exact number of patients in each cohort was determined by the rate of enrolment and how long it took the data monitoring committee to determine safety before allowing dose escalation |
| Attrition rate (loss to follow-up)? | Yes (detailed in patient flow chart in Figure 2) |
| Was attrition rate adequately dealt with? | Yes |
| No. (%) followed up from each condition? | NR |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Solid – breast, gynaecological, gastrointestinal, lung, other | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Chemotherapy: NR | ||||
| Adjuvant anaemia treatment | Iron | NR | |||
| G-CSF | NR | ||||
| Transfusion trigger | NR | ||||
| Hb inclusion criterion level | ≤ 11.0 g/dl | ||||
| Arm 1 = darbepoetin alfa (n = 198) | Arm 2 = placebo (n = 51) | Notes | p-value | ||
| Baseline demographics and clinical characteristics reported for all darbepoetin alfa patients not separated out by dose | |||||
| Sex, n (%) | |||||
| Male | 56 (28) | 16 (31) | |||
| Female | 142 (72) | 35 (69) | |||
| Age (years), mean (SD) | 58.3 (11.9) | 56.2 (12.4) | |||
| ECOG performance status, n (%) | |||||
| < 2 | 180 (91) | 45 (88) | |||
| Type of solid tumour, n (%) | |||||
| Breast | 61 (31) | 13 (25) | |||
| Gynaecological | 46 (23) | 9 (18) | |||
| Gastrointestinal | 34 (17) | 13 (25) | |||
| Lung | 33 (17) | 10 (20) | |||
| Other | 24 (12) | 6 (12) | |||
| Hb (g/l), mean (SD) | 99.3 (10.0) | 98.7 (11.2) | |||
| Hb (g/dl), mean (SD) (PenTAG calculated) | 9.93 (1.00) | 9.87 (1.12) | |||
| Ferritin (µg/l) < 50, mean (SD) | 21 (11) | 3 (6) | |||
| Endogenous erythropoietin baseline (patients with ≥ 100 mU/ml), n (%) | 32 (17) (n = 183) | 7 (15) (n = 47) | |||
| FACT-F score, mean (SD) (darbepoetin and placebo groups combined; n = 239) | 27.2 (12.4) | ||||
| Were intervention and control groups comparable? | No p-values reported; authors stated that ‘In general, baseline demographic and clinical characteristics of patients were well balanced between the darbepoetin alfa and placebo groups (p. 2029). Some imbalances were noted in the 12.0 µg/kg group in respect of disease type and mean Hb concentration at baseline. In addition, the authors state that ‘No clinically meaningful differences in pretreatment chemotherapy were seen between the darbepoetin alfa and placebo patients (data not shown)’ (p. 2029) | ||||
| Results (data extraction for 6.75 µg/kg Q3W and placebo arms only) | |||
|---|---|---|---|
| Arm 1 = darbepoetin alfa 6.75 µg/kg Q3W [n = 17 (of total 198] | Arm 2 = placebo (n = 51) | ||
| Hb | |||
| Responders, Kaplan–Meier proportion (95% CI) | 52 (27 to 78) | 31 (16 to 45) | Hb values within 28 days of a RBCT have been omitted |
| Change in Hb from baseline to EOTP (g/l), mean (SE) | 8.6 (3.8) | –0.2 (2.0) | |
| Change in Hb from baseline to EOTP (g/dl), mean (SE) (PenTAG calculated) | 0.86 (0.38) | –0.02 (0.2) | |
| Change in Hb from baseline after 12 weeks (g/l),a mean (SE) | 10.2 (5.4) (n = 11) | 3.1 (2.4) (n = 37) | |
| Change in Hb from baseline after 12 weeks (g/dl), mean (SE) (PenTAG calculated) | 1.02 (0.54) (n = 11) | 0.31 (0.24) (n = 37) | |
| Safety: withdrawal because of, n (%) | ||
| Deaths | 7 (4)a | 3 (6) |
| Tumour progression | 6 (3)a | 0 |
| AEsb | 1 (1)a | 0 |
| RBCT (week 5 to end of treatment period)c | n = 188 | n = 50 |
| HRQoL: change in FACT-F score from baseline to EOTP by change in Hbd | ||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear; process not described |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | No.a ‘In general, baseline demographic and clinical characteristics of patients were well balanced between the darbepoetin alfa and placebo groups’ (p. 2029). Some imbalances were noted in the 12.0 µg/kg group in respect of disease type and mean Hb concentration at baseline. In addition, the authors state that ‘No clinically meaningful differences in pretreatment chemotherapy were seen between the darbepoetin alfa and placebo patients (data not shown)’ (p. 2029) |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | Yes |
| 6. Were the outcome assessors blind to treatment allocation? | Yes |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Partially: results for AEs occurring with ≥ 15% incidence are presented graphically in Figure 3, not separated out by dose |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes, all randomised who received one or more dose of study drug were analysed (100% and 95% of participants in darbepoetin alfa and placebo groups respectively) |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Partially; only until the end of the double-blind study |
| Other | |
|---|---|
| Generalisability | Dose-finding study |
| Author conclusions | Darbepoetin alfa Q3W is well tolerated and effective in the treatment of anaemic patients receiving chemotherapy. Need for further research to investigate the proportion of patients responding to treatment and the time to achieve a response in this setting. Ability to administer Q3W as well as the possibility of administering darbepoetin alfa to coincide with chemotherapy that is administered Q3W represents an opportunity to simplify the treatment of anaemia and fatigue in cancer patients undergoing chemotherapy |
| Reviewer comments | |
| EndNote ref. ID: 2691 | Malignancy type: solid | |
| Treatment: rHuEPO, assume epoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Kurz 199769 | n = 35 |
| Objective | To evaluate the effectiveness of rHuEPO with respect to increasing Hb levels and decreasing RBCT requirements and to assess its influence on quality-of-life parameters | Inclusion criteria: Age 18–75 years; Hb level < 11 g/dl; ferritin serum level > 29 ng/ml; stool negative for occult blood; life expectancy > 3 months Exclusion criteria: Clinically significant disease or dysfunction of the pulmonary, cardiovascular, endocrine, neurological, gastrointestinal or genitourinary system not attributable to the underlying malignancy; uncontrolled hypertension (diastolic blood pressure > 100 mmHg); anaemia attributable to factors other than chronic neoplastic disease, such as vitamin B12 deficiency, iron deficiency and ferritin serum levels < 29 ng/ml; gastrointestinal bleeding or autoimmune haemolysis; acute illness within the last 7 days; creatinine > 2.5 mg/dl |
| No. of centres | 4 | |
| Other references/aliases | None | |
| Geographical setting | Austria | |
| Duration of treatment | 12 weeks | |
| Length of follow-up (if different) | NR | |
| Country of corresponding author | Austria | |
| Language of publication | English | |
| Sources of funding | Supported in part by Janssen-Cilag Austria | |
| Randomisation and allocation | Random permuted blocks and a corresponding randomisation list was used in the randomisation office at Janssen-Cilag. A 2 : 1 ratio between rHuEPO and placebo was implemented. The randomisation code was broken after documentation of all data | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | rHuEPO (Erypo; epoetin alfa) | Placebo |
| n | 23 | 12 |
| Dose and frequency (once daily, twice daily, etc.) | 150 U/kg Q3W | 150 U/kg Q3W |
| Dose adjustment (yes/no) | Yes. If Hb levels at week 4 were < 1 g/dl above the baseline value each dose was increased to 300 U/kg Q3W. If at week 4 Hb levels were > 1 g/dl above the baseline value but still within the anaemic range, the patient received 150 U/kg subcutaneously Q3W for the next 8 weeks | Yes. If Hb levels at week 4 were < 1 g/dl above the baseline value each dose was increased to 300 U/kg Q3W. If at week 4 Hb levels were > 1 g/dl above the baseline value, but still within the anaemic range, the patient received 150 U/kg subcutaneously Q3W for the next 8 weeks |
| Route of administration | Subcutaneous | Subcutaneous |
| Duration of epoetin treatment | 12 weeks | 12 weeks |
| Adjuvant anaemia treatment | Iron saccharate substitution following each dose of chemotherapy beginning with the next cycle | Iron saccharate substitution following each dose of chemotherapy beginning with the next cycle |
| Transfusion trigger | Hb < 8 g/dl | Hb < 8 g/dl |
| Outcomes | |
|---|---|
| Primary outcome | |
| Other outcomes | Haematological response (Hb levels measured every 4 weeks); RBCT (number of transfusions documented); HRQoL (beginning of treatment and then every 4 weeks before receiving chemotherapy patients completed a standardised questionnaire (10 items) – VAS (1–5); self-administration – collected by a nurse but results not read immediately and physician did not comment on the results) |
| Analysis | |
|---|---|
| Statistical technique used | Significance of the number of responders and the number of transfusions evaluated using chi-squared test. Differences between the treatment group and the control group shown using Kruskall–Wallis test for variables with a non-parametric distribution. Quality of life described per patient by 10 different scores, each of which was calculated as the average value of the scores for weeks 4, 8 and 12 minus the pretreatment value. Described the average of these 10 scores separately for each treatment and evaluated the significance in an exploratory mode using the Student’s t-test for unpaired samples. A multivariate evaluation of all 10 different scores was carried out using Hotelling’s T2 test. The effect of achieving a response from a state of non-response was described for each responding patient (n = 13) by the difference in average quality-of-life score values for the items for feeling of well-being, level of activity, physical ability and social activities under response and non-response |
| ITT analysis? | Assumed ITT but unclear. Results reported for the total patient population and no reported crossover; however, not reported explicitly in the paper |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation) | NR |
| Attrition rate (loss to follow-up)? | NR |
| Was attrition rate adequately dealt with? | Unclear as attrition rate NR |
| No. (%) followed up from each condition? | NA |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Solid – ovarian, cervical, uterine | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Platinum-based chemotherapy [n = 28 (n = 17 epoetin; n = 11 placebo)] and non-platinum-based chemotherapy [n = 7 (n = 6 epoetin; n = 1 placebo)] | ||||
| Adjuvant anaemia treatment | Iron | Iron saccharate substitution following each dose of chemotherapy beginning with the next cycle | |||
| G-CSF | NR | ||||
| Transfusion trigger | Hb < 8 g/dl | ||||
| Hb inclusion criterion level | < 11 g/dl | ||||
| Arm 1 = rHuEPO (epoetin alfa) (n = 23) | Arm 2 = placebo (n = 12) | Notes | p-value | ||
| Age (years), mean ± SD (range) | 54.4 ± 9.7 (32–68) | 52.7 ± 7.5 (43–63) | 0.36a | ||
| WHO performance status, n | |||||
| 0–1 | 17 | 9 | 0.88b | ||
| 1–2 | 6 | 3 | |||
| Type of solid tumour, n | |||||
| Ovarian | 17 | 8 | 0.64b | ||
| Uterine sarcoma | 3 | 1 | |||
| Cervical carcinoma | 3 | 3 | |||
| Hb baseline (g/dl), mean ± SD | 9.88 ± 0.889 | 9.85 ± 0.60 | 0.63a | ||
| Haematocrit baseline (ng/ml), mean ± SD | 29.9 ± 3.1 | 29.9 ± 1.7 | |||
| Ferritin baseline (ng/ml), mean ± SD | 300 ± 255 | 245 ± 196 | |||
| Were intervention and control groups comparable? | Yes; no statistically significant differences between the groups were reported | ||||
| Results | ||||
|---|---|---|---|---|
| Hb level (g/dl), mean | ||||
| Week 4 | 11.3 | No change | ||
| Week 8 | 11.9 | No change | ||
| Week 12 | 13.1 | No change | ||
| Haematological response, n (%)a | ||||
| Yes | 13 (56.5)b | 0 (0) | χ2 = 10.79 | 0.001 |
| No | 10 (43.5) | 12 (100) | ||
| Hb | Epoetin alfa (n = 23) | Placebo (n = 12) | ||
|---|---|---|---|---|
| 4 weeks, mean (SD) | 11.34 (1.75) | 9.82 (1.75) | ||
| 8 weeks, mean (SD) | 11.87 (2.25) | 10.32 (2.25) | ||
| 12 weeks, mean (SD) | 13.14 (2.25) | 10.1 (2.25) | ||
| RBCT | ||||
| RBCT requirement, n (%)a | 5 (21.7) | 8 (66.6) | χ2 = 6.81 | 0.009 |
| HRQoL (not validated questionnaire) | ||||
|---|---|---|---|---|
| rHuEPO (n = 23) | Placebo (n = 12) | t-test, p-valuea | ||
| Health state utility scale (see notes in Outcomes regarding the questionnaire used) | ||||
| Feeling of well-being | 0.004 | –0.16 | 0.77 | |
| Mood | –0.21 | –0.18 | 0.94 | |
| Level of activity | 0.26 | 0.58 | 0.71 | |
| Pain | 0.37 | –0.26 | 0.32 | |
| Nausea | –0.11 | –0.43 | 0.17 | |
| Appetite | –0.32 | –0.07 | 0.61 | |
| Physical ability | –0.33 | –0.32 | 0.53 | |
| Social activities | –0.04 | –0.51 | 0.89 | |
| Anxiety | 1.92 | 2.45 | 0.38 | |
| Treatment is helping | 1.76 | 2.34 | 0.11 | |
| Adverse effects | ||||
| Adverse effects of treatment | Well tolerated without any significant side effects (data not reported). No local reactions at the injection area nor any dermatitis or eruption could be observed | |||
| Quality appraisal | ||
|---|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Yes | |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | Unclear; randomisation was performed in the randomisation office but details of allocation concealment were not reported | |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Yes | |
| 4. Were the eligibility criteria specified? | Yes | |
| 5. Were the participants blind to treatment allocation? | Yes | |
| 6. Were the outcome assessors blind to treatment allocation? | Yes | |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | No; variability measure NR, unclear what the primary end point was | |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No | |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes; results include response for all patients and no crossovers so assume ITT | |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | NR | |
| Other | |
|---|---|
| Generalisability | Women only |
| Author conclusions | rHuEPO significantly increases Hb levels and decreases RBCT requirements while maintaining quality of life in patients with gynaecological malignancies who are undergoing polychemotherapy |
| Reviewer comments | rHuEPO is epoetin alfa (Erypo, Janssen-Cilag) |
| EndNote ref. ID: 2692 | Malignancy type: solid or non-myeloid haematological malignancies | |
| Treatment: epoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Littlewood 200170 | n = 375 |
| Objective | To assess the effects of epoetin alfa on RBCT requirements, haematopoietic parameters, quality of life and safety in patients receiving non-platinum-based chemotherapy | Inclusion criteria: Age ≥ 18 years; confirmed diagnosis of solid or non-myeloid hematological malignancy and receiving or scheduled to receive non-platinum-based chemotherapy (with a minimum cycle duration of 3 weeks); life expectancy ≥ 6 months; Hb level ≤ 10.5 g/dl or > 10.5 g/dl but ≤ 12.0 g/dl with at least a 1.5-g decrease in Hb per cycle/month since beginning chemotherapy Exclusion criteria: Patients with acute leukaemia and myeloid malignancies; uncontrolled hypertension or untreated iron, folate or vitamin B12 deficiency; previous myeloablative chemotherapy; acute major infection or bleeding within 1 month; radiotherapy or allogeneic blood transfusion within 14 days; severe illness or surgery within 7 days of study entry |
| No. of centres | 73 sites | |
| Other references/aliases | Patrick 2003,60 Aapro 200482 and Bajetta 200481 and all retrospective analyses of this trial | |
| Geographical setting | 15 countries (Germany, the Netherlands, UK, Ireland, Belgium, Luxembourg, Italy, South Africa, France, Greece, Switzerland, Poland, Portugal, Hungary, Czech Republic) | |
| Duration of treatment | Up to 28 weeks: 12–24 weeks (three to six cycles) of chemotherapy and a 4-week period after the last dose of chemotherapy | |
| Length of follow-up (if different) | Survival rates were determined based on data collected during the 12-month period after the study was completed by the last patient | |
| Country of corresponding author | UK | |
| Language of publication | English | |
| Sources of funding | Supported by a research grant from RW Johnson Pharmaceutical Research Institute and Ortho Biotech Europe/Janssen-Cilag | |
| Randomisation and allocation | Stratified by tumour stratum (solid or haematological) and Hb level (≤ 10.5 g/dl or ≤ 12.0 g/dl but > 10.5 g/dl). Double-blind trail but concealed allocation not reported | |
| Treatment arms | ||
| Arm drug name(s) | Epoetin alfa | Placebo |
| n | 251 | 124 |
| Dose and frequency (once daily, twice daily, etc.) | 150 IU/kg three times a week | Matching volume to epoetin alfa |
| Dose adjustment (yes/no) | Yes, at 4 weeks. Dose was doubled if Hb increase was < 1 g/dl and the reticulocyte count increase was < 40,000 above baseline. Dose reduction by 25% if Hb increased by ≥ 2 g/dl per month or cycle. If at any time the Hb level was > 15 g/dl, medication was interrupted until the Hb level was < 12g/dl and restarted with a 25% dose reduction | |
| Route of administration | Subcutaneously | Subcutaneously |
| Duration of epoetin treatment | Up to 28 weeks | Up to 28 weeks |
| Adjuvant anaemia treatment | Oral daily dose of 200 mg of elemental iron daily | Oral daily dose of 200 mg of elemental iron daily |
| Transfusion trigger | ||
| Outcomes | ||
| Primary outcome | RBCT (proportion of patients transfused after first 4 weeks of treatment) | |
| Other outcomes | Haematological responsea [change in Hb level (baseline to last value); proportion of responders]; survival; HRQoL [change in quality-of-life score (baseline to last value) on five cancer-specific scales: FACT-An, FACT-G Total, FACT-An Fatigue, CLAS, LASA]) | |
| Analysis | |
|---|---|
| Statistical technique used | Primary end point analysed for ITT and efficacy populations (see below). Patients on study for ≤ 28 days were counted as transfused for the ITT analysis. The analyses were performed using a logistic regression model that included terms correcting for the main effects of treatment group, primary tumour stratum (solid or haematological) and haematological stratum (Hb ≤ 10.5 g/dl or > 10.5 g/dl). As the interaction terms for treatment by tumour stratum and treatment by Hb stratum were not significant at the 10% level, they were not included in the model. Secondary efficacy variables (other than quality of life) were analysed for the efficacy population. Changes in Hb level from baseline to last value were compared using t-tests and the proportions of responders were compared using the Fisher’s exact test Univariate analysis was performed to test within-group mean quality-of-life change scores for differences from 0 with a paired t-test and differences in mean change scores between the treatment groups were examined using independent-sample t-tests (two-sided). The p-values for the primary quality-of-life measures were adjusted for multiple comparisons using a sequentially rejective version of the Bonferroni procedure. All hypothesis tests were performed on the adjusted p-values. In a separate analysis Pearson correlation coefficients were calculated to assess the relationship between change in Hb level and quality-of-life scores for each primary quality-of-life measure Protocol not designed or powered for survival, was amended before unblinding and study end to permit prospective analysis of survival. Information for this analysis was collected 12 months after the study end and survival distributions were estimated using Kaplan–Meier curves, which were compared by means of log-rank tests. In addition, to compensate for the variable survival times associated with different malignancies, Kaplan–Meier estimates of survival by tumour strata (haematological vs. solid) were also performed. Further analysis with the Cox regression model was performed using a stepwise selection procedure to correct for effects of potential prognostic factors on patient survival. Eight factors were tested for; four – tumour stratum, baseline Hb level, age and area under the curve for neutrophils – were found to be significant and were included in the model For all analyses p < 0.05 was considered significant. The study was not powered for subgroups |
| ITT analysis? | Partially. Three populations were used for the efficacy analyses: the ITT population, which included all randomised patients; the efficacy group, which included all randomised patients on study for > 28 days (efficacy); and the quality of life population, which was defined as all patients who had been randomised, received the study drug and had a baseline and at least one follow-up quality-of-life assessment. Safety population was the same as for the ITT population Baseline demographics and primary efficacy variable were analysed for both the ITT population and the efficacy population. Secondary variables other than quality of life domains were analysed for the efficacy population |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation) | NR |
| Attrition rate (loss to follow-up)? | Yes; 16 patients (seven receiving epoetin alfa and nine receiving placebo) were excluded from the efficacy evaluation, 14 (six receiving epoetin alfa and eight receiving placebo) because they discontinued before completing treatment (no reasons reported) and two (one per patient group) because the blind on their treatment codes was broken permanently. The remaining 359 patients were assessable for efficacy. Numbers were also reported for the quality-of-life data set |
| Was attrition rate adequately dealt with? | Yes; patients on study for ≤ 28 days were counted as transfused for the ITT analysis (although no sensitivity analysis was performed) |
| No. (%) followed up from each condition? | Yes; two epoetin alfa patients and one placebo patient were lost to follow-up for the 12-month survival analysis |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Solid: breast | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Non-platinum-based chemotherapy | ||||
| Adjuvant anaemia treatment | Iron | An oral daily dose of 200 mg of iron was recommended if transferrin saturation was ≤ 20%; intravenous iron was recommended, use of iron dextran was not allowed | |||
| G-CSF | No | ||||
| Transfusion trigger | At the discretion of the physician, with a recommended Hb level of < 8 g/dl unless clinically indicated | ||||
| Hb inclusion criterion level | ≤ 10.5 g/dl or > 10.5 g/dl but ≤ 12.0 g/dl after a ≥ 1.5 g/dl decrease in Hb level per cycle or month since beginning chemotherapy | ||||
| Arm 1 = epoetin alfa (n = 251) | Arm 2 = placebo (n = 124) | Notes | p-value | ||
| Sex, n (%) | |||||
| Male | 85 (34) | 39 (31) | |||
| Female | 166 (66) | 85 (69) | |||
| Age (years), mean ± SD | 58.3 ± 14.2 | 59.5 ± 13.9 | |||
| WHO/ECOG performance status | NR | NR | |||
| Hb baseline (g/dl), mean ± SD | 9.9 ± 1.1 | 9.7 ± 1.1 | |||
| Hb stratum, n (%) | |||||
| ≤ 10.5 g/dl | 209 (83) | 109 (88) | |||
| > 10.5 g/dl | 42 (17) | 15 (12) | |||
| Chemotherapy within 3 months of study start, n (%) | 231 (92) | 114 (92) | |||
| Pre-study transfusions within 3 months of study start, n (%) | 71 (28) | 44 (35) | |||
| Iron baseline (U/l), median (range) | NR | NR | |||
| Erythropoietin baseline (mU/ml) | NR | NR | |||
| Target Hb | NR | NR | |||
| Tumour stratum, n (%)70 | |||||
| Solid | 136 (54) | 66 (53) | |||
| Haematological | 115 (46) | 58 (47) | |||
| Were intervention and control groups comparable? | No p-values reported; authors stated that ‘Demographic and baseline characteristics of the patients in the ITT population were generally comparable between the epoetin alfa and placebo treatment groups (p. 2867). However, there were proportionally fewer previously transfused patients at baseline in the epoetin alfa group than in the placebo group (28% vs. 36% respectively) (p. 2867)’ | ||||
| Results: ITT and efficacy populations | |||
|---|---|---|---|
| Patients transfused, n/N (%) (ITT population) | |||
| Overall | 62/251 (24.7) | 49/124 (39.5) | 0.0057 (adjusted) |
| Solid tumour | 33/136 (24.3) | 24/66 (36.4) | |
| Haematological tumour | 29/155 (25.2) | 25/58 (43.1) | |
| Change in Hb after 28 weeks (g/dl), mean (SD) (efficacy population)a | 2.2 (2.18) (n = 244) | 0.5 (1.79) (n = 115) | < 0.001 |
| Responders (increase in Hb level of 2 g/dl during the study without transfusion in the previous 30 days), n/N (%) (efficacy population) | |||
| Overall | 172 (70.5) (n = 244) | 22 (19.1) (n = 115) | < 0.001 (Fisher’s exact test) |
| Solid tumour | 87/131 (66.4) | 13/61 (21.3) | |
| Haematological tumour | 85/113 (75.2) | 9/54 (16.7) | |
| Hb ≤ 10.5 g/dl | 139/203 (68.5) | 22/100 (22.0) | |
| Hb > 10.5 g/dl | 33/41 (80.5) | 0/15 (0.0) | |
| Survival 12 months after the last patient enrolled completed the study (median follow-up 26 months) | |||
| OS at 12-month assessment (safety population), n (%) | n = 251 | n = 124 | |
| Alive | 94 (37) | 41 (33) | |
| Dead | 155 (62) | 82 (66) | |
| Lost to follow-up | 2 (1) | 1 (1) | |
| Median survival (months) (ITT population) | 17 | 11 | |
| OS (note study insufficiently powered for this outcome) Kaplan–Meier 12-month survival estimates (ITT population) (%) | 60 | 49 | 0.13 |
| Estimated HR (placebo vs. epoetin alfa) | 1.309b | 0.052 | |
| OS at 12-month assessment (safety population), n (%) | ||||
| Haematological malignancies (n = 173) | n = 115 | n = 58 | ||
| Alive | 60 (52) | 28 (48) | ||
| Dead | 54 (47) | 30 (52) | ||
| Lost to follow-up | 1 (1) | 0 (0) | ||
| Solid tumours (n = 202) | n = 136 | n = 66 | ||
| Alive | 34 (25) | 13 (20) | ||
| Dead | 101 (74) | 52 (79) | ||
| Lost to follow-up | 1 (1) | 1 (2) | ||
| Health state utility scale | Of the 375 ITT patients, 349 were evaluated for changes in quality-of-life parameters. Presented as change from baseline to last assessment (unclear when) | |||
| Results for FACT and CLAS | ||||
| Mean change score: FACT-An Fatigue | 3.0 (n = 200) | –2.2 (n = 90) | 0.004 | |
| Mean change score: FACT-An Anaemia | 4 (n = 200) | –2.6 (n = 90) | 0.0007 (not adjusted for multiple comparisons) | |
| Mean change score FACT-G | 2.5 (n = 194) | –3.6 (n = 88) | 0.004 | |
| CLAS-Energy | 8.1 (n = 228) | –5.8 (n = 108) | 0.0007 | |
| CLAS-Daily activities | 7.5 (n = 228) | –6.0 (n = 108) | 0.0018 | |
| CLAS-Overall quality of life | 4.8 (n = 228) | –6.0 (n = 107) | 0.0048 | |
| Adverse effects of treatment (safety population), n (%)c | n= 251 | n = 124 | ||
| Any AE | 216 (86) | 101 (81) | ||
| Fever | 55 (22) | 21 (17) | ||
| Granulocytopenia | 49 (20) | 16 (13) | ||
| Disease progression | 44 (18) | 27 (22) | ||
| Nausea | 46 (18) | 17 (14) | ||
| Abdominal pain | 30 (12) | 13 (10) | ||
| Constipation | 30 (12) | 16 (13) | ||
| Leukopenia | 31 (12) | 13 (10) | ||
| Diarrhoea | 27 (11) | 10 (8) | ||
| Vomiting | 24 (10) | 13 (10) | ||
| Fatigue | 17 (7) | 15 (12) | ||
| Dyspnoea | 15 (6%) | 14 (11%) | ||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear: stratified by tumour stratum (solid or haematological) and Hb level (≤ 10.5 g/dl or ≤ 12.0 g/dl but > 10.5 g/dl) |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Uncleara |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | Yes |
| 6. Were the outcome assessors blind to treatment allocation? | Yes |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Partially, no variability |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes, apart from HRQoL (only 80% and 73% of participants analysed in the epoetin and placebo groups, respectively)b |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes, numbers given but detailed reasons not provided |
| Other | |
|---|---|
| Generalisability | Yes – broad population |
| Author conclusions | Epoetin alfa safely and effectively ameliorates anaemia and significantly improves quality of life in cancer patients receiving non-platinum-based chemotherapy. Encouraging results regarding increased survival warrant another trial designed to confirm these findings |
| Reviewer comments | Caution required with regard to survival results because of being underpowered. Also, concern over lack of explanation for dropouts/withdrawals; some explanation reported in Littlewood and colleagues70 but reasons for withdrawals not specified in detail |
| EndNote ref. ID: 2680 | Malignancy type: breast cancer | |
| Treatment: epoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Moebus 201362 | n = 1284, of whom 643 in the intense dose-dense arm sequential chemotherapy (IDD-ETC) arm were randomised to epoetin alfa (n = 324) or epoetin alfa (n = 319) |
| Objective | The AGO-ETC trial compared intense dose-dense sequential chemotherapy every 2 weeks with conventional scheduled therapy in high-risk breast cancer patients. The objective of this study was to evaluate the safety and efficacy of epoetin alfa in a second randomisation of the IDD-ETC arm | Inclusion criteria: Age 18–65 years; histologically confirmed primary breast cancer of stages II–IIIa with four or more tumour-infiltrated axillary lymph nodes, M0 status and R0 resection of the primary tumour and axilla with a minimum of 10 axillary lymph nodes removed; ECOG performance status of 0–1; normal left ventricular ejection fraction; neutrophils ≥ 1,500/µl; platelets ≥ 100,000/µl; serum creatinine, transaminases and total bilirubin < 1.25; alkaline phosphatase < 3.0 times the institutional upper normal limit Exclusion criteria: History of severe cardiac disease; previous systemic tumour therapy; simultaneous contralateral breast cancer or any other cancer except for basal cell skin carcinoma |
| No. of centres | 165 (recruitment between November 1998 and April 2003) | |
| Other references/aliases | EPO-GER-10, AGO-ETC trial, Moebus 2010198 and presented in part at the 40th Annual Meeting of the American Society of Clinical Oncology, New Orleans, LA, 5–8 June 2004,199 and at the 29th Annual San Antonio Breast Cancer Symposium, San Antonio, TX, 14–17 December 2006200 | |
| Geographical setting | Germany | |
| Duration of treatment | Median 18 weeks (mean 16.9 weeks) | |
| Length of follow-up (if different) | Median follow-up was 62 months but the study is ongoing for continued 10-year follow-up | |
| Country of corresponding author | Germany | |
| Language of publication | English | |
| Sources of funding | Bristol-Myers Squibb, Amgen Inc., Pharmacia and Johnson & Johnson | |
| Randomisation and allocation | Patients stratified by centre, menopausal status (pre- vs. post menopausal) and the number of affected lymph nodes (4–9 vs. ≥ 10) at central fax randomisation. Computer-generated lists with permuted blocks of randomly variable size were used | |
| Treatment arms | ||
| Arm drug name/s | Epoetin alfa | Control, standard care |
| n | 324 | 319 |
| Dose and frequency (once daily, twice daily, etc.) | 150 IU/kg three times weekly | NA |
| Dose adjustment (yes/no) | To maintain Hb level of 12.5–13.0 g/dl. Dose doubled if Hb dropped > 2 g/dl within a 4-week period. Epoetin was withdrawn when Hb > 14.0 g/dl and was restarted when Hb < 13.0 g/dl | NA |
| Route of administration | Subcutaneously | NA |
| Duration of epoetin treatment | Started on day 1 and continued up to 14 days after the last dose of cyclophosphamide | NA |
| Adjuvant anaemia treatment | 200 mg/day oral iron | 200 mg/day oral iron |
| Transfusion trigger | Patients with a Hb level < 9.0 g/dl were evaluated for transfusion by the physician. The indication for RBCT depended on the symptoms of the patients and was at the discretion of the physician | Patients with a Hb level < 9.0 g/dl were evaluated for transfusion by the physician |
| Outcomes | |
|---|---|
| Primary outcome | Hb levels baseline to cycle 9 |
| Other outcomes | RBCT (no. of blood transfusions); survival (OS, recurrence-free survival); HRQoL (EORTC QLQ, version 3 [assessed before the start of treatment, at every second cycle, at the end of treatment and at each follow-up visit)]; AEs |
| Analysis | |
|---|---|
| Statistical technique used | All statistical tests were two-sided except for the primary end point of transfusion, for which a one-sided hypothesis was prospectively defined. Comparison of Hb levels was evaluated with ANOVA and Wilcoxon tests. Numbers with at least one on-study RBCT were compared between the two groups using Fisher’s exact test. On-study was defined as the period from randomisation to the date of the last cycle of chemotherapy plus 14 days or the date of withdrawal, whichever occurred first. Kaplan–Meier estimates of the relapse-free survival rate were compared using a two-sided log-rank test with and without the stratification factors for menopausal status and number of positive lymph nodes. Cox regression models, with and without adjustment for the stratification factors, were performed to calculate HRs and 95% CIs |
| ITT analysis? | Yes, ITT and per-protocol analyses for primary outcomes and relapse-free survival. The safety population included 627 subjects (309 epoetin arm, 318 control arm): all epoetin patients receiving epoetin and all control subjects with no epoetin treatment. The per-protocol population included 511 subjects (258 epoetin arm, 253 control arm). Patients were excluded if unknown ECOG/WHO performance status; less than four positive lymph nodes at baseline; not receiving the assigned treatment (the majority of patients were excluded if they failed to receive nine cycles of chemotherapy) |
| Does statistical technique adjust for confounding? | NA |
| Power calculation (a priori sample calculation)? | Yes, based on the size needed to detect any difference in Hb levels and proportions needing transfusion. In addition, the sample size had approx. 85% power to detect a 10% difference in the 5-year relapse-free survival rate after a median follow-up of 5 years using a log-rank test |
| Attrition rate (loss to follow-up)? | Yes |
| Was attrition rate adequately dealt with? | Yes |
| No. (%) followed up from each condition? | Yes |
| Baseline characteristics | ||
|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Solid tumour: breast cancer | |
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Nine cycles of three sequential cycles of epirubicin (150 mg/m2), paclitaxel (225 mg/m2) and cyclophosphamide (2500 mg/m2) every 2 weeks (IDD arm A) | |
| Adjuvant anaemia treatment | Iron | 200 mg/day oral iron |
| G-CSF | All cycles were administered in 3-week intervals without growth factor support | |
| Transfusion trigger | Patients with a Hb level < 9.0 g/dl were evaluated for transfusion by the physician | |
| Hb inclusion criterion level | NR | |
| Arm 1 = epoetin alfa (n = 324) | Arm 2 = control (n = 319) | Notes | p-value | |
|---|---|---|---|---|
| Age (years), median (range) | 50 (29–65) | 52 (28–67) | ||
| ECOG performance status, n (%) | (n = 315) | (n = 312) | ||
| 0 | 254 (81) | 260 (83) | ||
| 1 | 61 (19) | 52 (17) | ||
| Body mass index (kg/m2), median (range) | 24.5 (17–42) | 24.4 (17–48) | ||
| Hb baseline (g/dl), median (IQR) | 12.4 (11.7–13.3) (n = 313) | 12.8 (12.2–13.6) (n = 303) | ||
| Tumour stage, n (%) | ||||
| pT1 | 81 (25) | 100 (31) | ||
| pT2 | 190 (59) | 172 (54) | ||
| pT3 | 50 (15) | 46 (14) | ||
| Were intervention and control groups comparable? | No p-values reported; authors stated that ‘the two treatment groups were generally similar with respect to the demographic and baseline characteristics’ (p. 1020) | |||
| Results | ||||
|---|---|---|---|---|
| Change in Hb (cycle 9)a | 0 | –2.2 | < 0.001 | |
| No. of transfusions, assessed during the period from randomisation to the date of the last cycle of chemotherapy plus 14 days or the date of withdrawal, whichever occurred first (ITT)b | 41 (12.8) | 86 (28.1) | HR 0.37 (95% CI 0.25 to 0.57) | < 0.0001 |
| HRQoLc | ||||
| Survival (ITT), n | 324 | 317 | ||
| 5-year relapse-free survival (%) (95% CI) | 71 (66 to 76) | 72 (67 to 77) | HR 1.03 (95% CI 0.77 to 1.37) | p = 0.86 |
| 5-year OS (%) (95% CI) | 81 (76 to 86) | 83 (78 to 87) | HR 0.97 (95% CI 0.67 to 1.41) | p = 0.89 |
| Safetyd | (n = 309) | (n = 318) | ||
| Total no. of subjects with AEs | 10 (3) | 22 (7) | ||
| Embolism, n (%) | 1 (< 1) | 0 (0) | ||
| No. (%) of patients with thromboembolic vascular event while on chemotherapy | 39 (13) | 22 (7) | ||
| No. (%) of patients with clinically relevant thromboembolic vascular events | 22 (7) | 10 (3) | p = 0.03 | |
| Vascular disorders, n (%) | ||||
| Thrombosis | 21 (7) | 9 (3) | ||
| Venous thrombosis | 2 (1) | 0 (0) | ||
| Arterial thrombosis | 1 (< 1) | 0 (0) | ||
| Deep-vein thrombosis | 1 (< 1) | 0 (0) | ||
| Embolism | 0 (0) | 1 (< 1) | ||
| Subclavian vein thrombosis | 0 (0) | 1 (< 1) | ||
| Respiratory, thoracic, and mediastinal disorders, n (%) | ||||
| Pulmonary embolism | 0 (0) | 1 (< 1) | ||
| Serious AE (%) | 10% | 13% | ||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Yes |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | Unclear |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Unclear; no p-values reported |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | No |
| 6. Were the outcome assessors blind to treatment allocation? | NR |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Yes |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | Yes; this study had a Latin square design and this paper reports second randomisation (first randomisation results published in an alternative reference,198 excluded as epo vs. epo not measured) |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes |
| Other | |
|---|---|
| Generalisability | Females only (breast cancer) |
| Author conclusions | Epoetin alfa resulted in improved Hb levels and decreased transfusions without an impact on relapse-free or OS. However, epoetin alfa had an adverse effect, resulting in increased thrombosis |
| Reviewer comments | Although epoetin alfa dosing information had to be reported in the case report form as the number of units administered per kilogram of body weight, a fixed dose of 10,000 IU was specified for some subjects. In these instances, a per-kg dose was calculated using the subject’s body weight |
| EndNote ref. ID: 2693 | Malignancy type: lymphoproliferative malignancies – NHL, CLL, MM | |
| Treatment: ESA – epoetin beta | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Österborg 200579 | n = 349 (ITT n = 343) |
| Objective | To investigate the efficacy of epoetin beta in eliminating severe anaemia and transfusion dependency and concomitant effects on quality of life using the FACT scale in participants with advanced MM, low-grade NHL and CLL | Inclusion criteria: Age ≥ 18 years; confirmed diagnosis of NHL, CLL or MM; Hb < 10 g/dl with a transfusion requirement of ≥ 2 units of RBCs in the 3 months before the study; inadequately low endogenous serum erythropoietin concentration ≤ 100 IU/l if Hb > 9 g/dl to < 10 g/dl) ≤ 180 IU/l if Hb level > 8 g/dl to ≤ 9 g/dl or ≤ 300 IU/l if Hb level ≤ 8 g/dl; scheduled to receive antitumour therapy for the next 4 months; life expectancy > 4 months; WHO performance score 0–3. Exclusion criteria: Therapy-resistant hypertension; relevant acute or chronic bleeding in 3 months before study commencement; thrombocytopenia or thrombocytosis (platelets < 20 and > 450 × 109/l respectively); vitamin B12 or folic acid deficiency; creatinine level > 2.5 mg/dl; haemolysis (haptoglobin level < 50 mg/dl); epilepsy; known hypersensitivity to preservatives used in study medication injection formulation; evidence of functional iron deficiency (transferrin < 25%) |
| No. of centres | 63, conducted between June 1997 and July 1999 | |
| Other references/aliases | Österborg 200271 | |
| Geographical setting | 12 countries | |
| Duration of treatment | 16 weeks | |
| Length of follow-up (if different) | Participants followed up for at least 1 year after the end of the treatment period. The minimum length of follow-up was approx. 17.5 months in both treatment groups, with only 4 participants in each group having a shorter follow-up (reported in Österborg and colleagues79) | |
| Country of corresponding author | Sweden | |
| Language of publication | English | |
| Sources of funding | F. Hoffmann-La Roche | |
| Randomisation and Allocation | Randomised, double-blind, placebo-controlled trial. Stratified according to malignancy type and study centre. After a run-in period of approximately 2 weeks, patients suitable for inclusion were randomised | |
| Treatment arms | ||
| Arm drug name(s) | Epoetin beta | Placebo |
| n | 170 | 173 |
| Dose and frequency (once daily, twice daily, etc.) | 150 IU/kg three times a week | |
| Dose adjustment (yes/no) | The dose was increased to 300 IU/kg if Hb level was < 8.5 g/dl or if increase in Hb from baseline was < 0.5 g/dl after 4 weeks. Dose was decreased by 50% if Hb increased by > 2 g/dl within this period. If Hb was > 14 g/dl, the study medication was suspended until Hb level declined to ≤ 13 g/dl, when epoetin beta was reinstated at 50% of the previous dose | |
| Route of administration | Subcutaneous | Subcutaneous |
| Duration of epoetin treatment | 16 weeks | 16 weeks |
| Adjuvant anaemia treatment | Enrolled participants with a baseline transferrin saturation of < 25% received intravenous iron substitution (100 mg iron) before the start of study treatment. When the transferrin saturation level decreased to < 25% during the course of the study, intravenous iron substitution therapy was administered at a dose of 100 mg of iron per week until transferrin saturation reached ≥ 25%. Oral iron substitution therapy (200–300 mg) was administered to those in whom intravenous iron was precluded | Enrolled participants with a baseline transferrin saturation of < 25% received intravenous iron substitution (100 mg iron) before the start of study treatment. When the transferrin saturation level decreased to < 25% during the course of the study, intravenous iron substitution therapy was administered at a dose of 100 mg of iron per week until transferrin saturation reached ≥ 25%. Oral iron substitution therapy (200–300 mg) was administered to those in whom intravenous iron was precluded |
| Transfusion trigger | Hb < 8.5 g/dl or at higher levels if medically indicated, i.e. the presence of marked anaemic symptoms such as angina pectoris | Hb < 8.5 g/dl or at higher levels if medically indicated, i.e. the presence of marked anaemic symptoms such as angina pectoris |
| Outcomes | ||
| Primary outcome | Transfusion-free survival (during weeks 5–16 of the study). Also analysed severe anaemia-free survival (Hb ≥ 8.5 g/dl) during weeks 5–16. Death without a previous event was considered a failure | |
| Other outcomes | Haematological response (increase in Hb level of ≥ 2 g/dl above baseline without the need for a blood transfusion in the previous 6 weeks); Hb nadir (measured at 4-week long intervals); HRQoL (subjective quality of life was assessed at baseline and every 4 weeks during the study using FACT-An;a questionnaires were completed before any examination or treatment so that participant assessments could not be influenced by references to current Hb level); AEs (AEs, hematological parameters, concomitant medications, blood transfusions and antitumour therapy were documented throughout the course of the study) | |
| Analysis | |||||
|---|---|---|---|---|---|
| Statistical technique used | Subgroup analyses with a one-sided Wald chi-squared test (α = 0.05) performed if the difference in the total study population was significant and no significant interaction between study treatment and strata was present (p > 0.1). HRs were calculated to estimate the relative risk of failure and event-free curves were displayed that were based on Kaplan–Meier estimates. Multivariate Cox proportional hazard methods were used to assess the contribution of other baseline characteristics to event-free rates. Cumulative response rates were analysed by the stratified log-rank test and displayed as Kaplan–Meier curves. Analysis of covariance techniques were used to analyse the changes from baseline in quality of life and Hb data, with baseline values considered as covariates Long-term survival data (Österborg 200579): Survival data were analysed by standard Kaplan–Meier methods and differences in survival between groups were assessed using a log-rank test. The median time to patients being censored was 27.8 months in the epoetin beta group and 27.5 months in the placebo group |
||||
| ITT analysis? | Yes; the primary efficacy variable was analysed on an ITT basis via a Cox proportional hazard model adjusted for the type of underlying malignant disease at a significance level of 5%, although the ITT population is defined as all participants receiving study treatment (n = 343), whereas 349 participants were randomised. ITT population = safety population in this study (n = 343) | ||||
| Does statistical technique adjust for confounding? | Yes | ||||
| Power calculation (a priori sample calculation)? | Yes; 150 patients with low-grade NHL, CLL or MM needed to detect an improvement in the primary variable from 25% to 50% with a power of 85% via a Cox proportional hazard model. In an amendment to the study protocol, the sample size was increased: enrol at least 100 participants per stratum (MM, NHL, CLL; 50 participants per treatment group) to achieve a power of 80% for the three corresponding subgroup analyses). A lost-to follow-up rate of ≤ 10% in weeks 5–16 weeks was assumed | ||||
| Attrition rate (loss to follow-up)? | Three participants in each treatment group were withdrawn before receiving study medication because of withdrawal of consent (n = 5) and protocol violation (n = 1). In total, of 349 participants, 281 completed the study. The main reasons for withdrawal were death (n = 35), withdrawal of consent (n = 12) and AEs (n = 11) | ||||
| Was attrition rate adequately dealt with? | In case of premature withdrawal from study treatment, participants were observed and Hb level, number of blood transfusions and vital status were recorded whenever possible until study week 16 | ||||
| No. (%) followed up from each condition? | Yes, until the end of treatment only | ||||
| Baseline characteristics | |||||
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | NHL, CLL, MM | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Non-platinum-based chemotherapy | ||||
| Adjuvant anaemia treatment | Iron | Yes, see treatment description above for more details | |||
| G-CSF | NR | ||||
| Transfusion trigger | Hb < 8.5 g/d or at higher levels if medically indicated, i.e. the presence of marked anaemic symptoms such as angina pectoris | ||||
| Hb inclusion criterion level | < 10 g/dl | ||||
| Arm 1 = epoetin beta (n = 170) | Arm 2 = placebo (n = 173) | Notes | p-value | ||
| Sex, n (%) | |||||
| Male | 91 (54.0) | 82 (47.0) | |||
| Female | 79 (46.0) | 91 (53.0) | |||
| Age (years), median (range) | 63 (32–86) | 64 (28–83) | |||
| WHO performance status, n (%) | |||||
| 0 | 10 (6.0) | 13 (7.5) | |||
| 1 | 57 (33.5) | 62 (36.0) | |||
| 2 | 73 (43.0) | 68 (39.0) | |||
| 3 | 30 (17.5) | 30 (17.5) | |||
| Body weight (kg), mean ± SD | 69 ± 12 | 69 ± 13 | |||
| Underlying malignancy, n (%) | |||||
| CLL | 59 (35.0) | 66 (38.0) | |||
| MM | 58 (34.0) | 58 (33.5) | |||
| NHL | 53 (31.0) | 49 (28.5) | |||
| Transfusion requirement, n (%) | |||||
| None | 11 (6.5) | 6 (3.5) | |||
| 1 unit | 4 (2.5) | 6 (3.5) | |||
| 2–5 units | 126 (74.0) | 131 (76.0) | |||
| ≥ 6 units | 29 (17.0) | 30 (17.0) | |||
| Haematological parameters | |||||
| Hb (g/dl), mean ± SD | 9.2 ± 1.1 | 9.3 ± 1.0 | |||
| Haematocrit (%), mean ± SD | 28.2 ± 4.7 | 28.6 ± 4.2 | |||
| Neutrophil count (×109/l), mean ± SD | 2.8 ± 2.5 | 3.0 ± 3.1 | |||
| Platelet count, (×109/l), mean (IQR) | 149 (100–195) | 141 (94–190) | |||
| Serum erythropoietin (IU/l), median (IQR) | 38 (20–72) | 41 (21–77) | |||
| Serum ferritin (ng/ml), median (IQR) | 586 (235–1121) | 514 (195–1183) | |||
| Transferrin saturation (%), mean ± SD | 38 ± 22 | 39 ± 23 | |||
| Quality-of-life scores, mean ± SD | |||||
| FACT-An | 115.2 ± 28.0 | 114.0 ± 28.3 | |||
| FACT-G | 69.1 ± 14.4 | 68.5 ± 15.0 | |||
| FACT-F | 28.8 ± 10.7 | 29.2 ± 11.0 | |||
| FACT-An subscale | 17.3 ± 4.6 | 17.0 ± 5.0 | |||
| Were intervention and control groups comparable? | Unclear: no p-values reported; author states that ‘There were no major differences in the demographics and clinical characteristics of the two treatment groups’ (p. 207) | ||||
| Results | ||||
|---|---|---|---|---|
| Haematological | ||||
| Hb response (≥ 2 g/dl increase in Hb without transfusion) at 16 weeks (%)a | 67 | 27 | < 0.0001 | |
| Hb response MM patients (n = 116) (%) | 76 | 29 | < 0.0001 | |
| Hb response NHL patients (n = 102) (%) | 62 | 24 | < 0.0001 | |
| Hb response CLL patients (n = 125) (%) | 63 | 26 | < 0.0001 | |
| Hb nadir (g/dl), mean ± SDb | ||||
| 1–4 weeks | 9.1 ± 1.4 (n = 169) | 8.7 ± 1.2 (n = 173) | 0.0003 | |
| 5–8 weeks | 10.0 ± 1.9 (n = 161) | 8.8 ± 1.5 (n = 165) | 0.0001 | |
| 9–12 weeks | 10.5 ± 2.0 (n = 152) | 8.9 ± 1.5 (n = 153) | 0.0001 | |
| 13–16 weeks | 10.8 ± 2.0 (n = 146) | 9.2 ± 1.6 (n = 147) | 0.0001 | |
| Prediction of response (Cox’s multivariate regression analysis of factors in transfusion-free survival during weeks 5–16)c | ||||
| Treatment, epoetin beta vs. placebo | HR 0.555, 95% CI 0.369 to 0.776; p = 0.0006 | |||
| Platelet count, ≥ 100 vs. < 100 × 109 g/l | HR 0.416, 95% CI 0.292 to 0.592; p = 0.0001 | |||
| Hb level, ≥ 9 g/dl vs. < 9 g/dl | HR 0.589, 95% CI 0.423 to 0.821; p = 0.0018 | |||
| Pretreatment transfusion requirement, ≤ 2 vs. 3 units | HR 0.645, 95% CI 0.458 to 0.909; p = 0.0123 | |||
| Underlying malignancy | HR 0.803, 95% CI 0.565 to 1.140; p = 0.2198 | |||
| Severe anaemia and transfusion-free survival | ||||
| Participants with blood transfusions in first 4 weeks of study treatment (%) | 29.0 | 27.2 | 0.707 | |
| Transfusion-free survival during 16 weeks of treatment (%) | 66.7 | 47.6 | Risk reduction of 43% favouring epoetin beta | 0.0012 |
| Severe anaemia- and transfusion-free survivald | Risk reduction of 51% favouring epoetin beta | 0.0001 | ||
| Interaction between underlying malignant disease and treatment | > 0.1 | |||
| Survival (long-term follow-up) (eÖsterborg and colleagues79) | ||||
| No. (%) of deaths | 110 (65%), censored n = 60 | 109 (63%), censored n = 64 | ||
| Kaplan–Meier: survival (months), median (95% CI) | 17.4 (15.0 to 20.5) | 18 (16 to 22.3) | HR 1.04 (0.8 to 1.36) | 0.76 |
| HRQoL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline and change from baseline in quality-of-life questionnairesVariableBaselineWeek 4Week 8Week 12Week 16Score, mean ± SDNo. of participantsScore, mean ± SDNo. of participantsScore, mean ± SDNo. of participantsScore, mean ± SDNo. of participantsScore, mean ± SDNo. of participantsFACT-An, 49 items, range 0–196Epoetin beta115.2 ± 28.01284.9 ± 21.41277.9 ± 25.711813.1 ± 27.6a11414.8 ± 28.0a105Placebo114.0 ± 28.31215.3 ± 19.51197.4 ± 22.71107.1 ± 26.31028.7 ± 28.9101FACT-G, 29 items, range 0–116Epoetin beta69.1 ± 14.41291.7 ± 11.81283.7 ± 13.01185.9 ± 14.5a1146.5 ± 13.8a106Placebo68.5 ± 15.01222.2 ± 10.11202.9 ± 11.51122.6 ± 12.91043.1 ± 14.4103FACT-F subscale, 13 items, range 0–52Epoetin beta28.8 ± 10.71602.2 ± 8.71572.8 ± 10.81484.2 ± 11.71455.2 ± 12.2133Placebo29.2 ± 11.01571.8 ± 8.41571.9 ± 9.81452.5 ± 10.91353.0 ± 12.1130FACT-An subscale, seven items, range 0–28Epoetin beta17.3 ± 4.61600.9 ± 3.31571.2 ± 4.21481.7 ± 4.41452.0 ± 4.3133Placebo17.0 ± 5.01570.8 ± 3.51571.2 ± 4.11451.2 ± 4.51351.7 ± 5.2130ap < 0.5 (after 12 and 16 weeks, the improvement in FACT-An and FACT-G scores was greater in the epoetin beta arm).NoteAnalysis of the dimensions of the FACT-G scale revealed statistically significant differences after 12 weeks: p < 0.01 and p < 0.05 favouring epoetin beta for social and family well-being and emotional well-being respectively. | Variable | Baseline | Week 4 | Week 8 | Week 12 | Week 16 | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | FACT-An, 49 items, range 0–196 | Epoetin beta | 115.2 ± 28.0 | 128 | 4.9 ± 21.4 | 127 | 7.9 ± 25.7 | 118 | 13.1 ± 27.6a | 114 | 14.8 ± 28.0a | 105 | Placebo | 114.0 ± 28.3 | 121 | 5.3 ± 19.5 | 119 | 7.4 ± 22.7 | 110 | 7.1 ± 26.3 | 102 | 8.7 ± 28.9 | 101 | FACT-G, 29 items, range 0–116 | Epoetin beta | 69.1 ± 14.4 | 129 | 1.7 ± 11.8 | 128 | 3.7 ± 13.0 | 118 | 5.9 ± 14.5a | 114 | 6.5 ± 13.8a | 106 | Placebo | 68.5 ± 15.0 | 122 | 2.2 ± 10.1 | 120 | 2.9 ± 11.5 | 112 | 2.6 ± 12.9 | 104 | 3.1 ± 14.4 | 103 | FACT-F subscale, 13 items, range 0–52 | Epoetin beta | 28.8 ± 10.7 | 160 | 2.2 ± 8.7 | 157 | 2.8 ± 10.8 | 148 | 4.2 ± 11.7 | 145 | 5.2 ± 12.2 | 133 | Placebo | 29.2 ± 11.0 | 157 | 1.8 ± 8.4 | 157 | 1.9 ± 9.8 | 145 | 2.5 ± 10.9 | 135 | 3.0 ± 12.1 | 130 | FACT-An subscale, seven items, range 0–28 | Epoetin beta | 17.3 ± 4.6 | 160 | 0.9 ± 3.3 | 157 | 1.2 ± 4.2 | 148 | 1.7 ± 4.4 | 145 | 2.0 ± 4.3 | 133 | Placebo | 17.0 ± 5.0 | 157 | 0.8 ± 3.5 | 157 | 1.2 ± 4.1 | 145 | 1.2 ± 4.5 | 135 | 1.7 ± 5.2 | 130 | ap < 0.5 (after 12 and 16 weeks, the improvement in FACT-An and FACT-G scores was greater in the epoetin beta arm).NoteAnalysis of the dimensions of the FACT-G scale revealed statistically significant differences after 12 weeks: p < 0.01 and p < 0.05 favouring epoetin beta for social and family well-being and emotional well-being respectively. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Variable | Baseline | Week 4 | Week 8 | Week 12 | Week 16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FACT-An, 49 items, range 0–196 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Epoetin beta | 115.2 ± 28.0 | 128 | 4.9 ± 21.4 | 127 | 7.9 ± 25.7 | 118 | 13.1 ± 27.6a | 114 | 14.8 ± 28.0a | 105 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Placebo | 114.0 ± 28.3 | 121 | 5.3 ± 19.5 | 119 | 7.4 ± 22.7 | 110 | 7.1 ± 26.3 | 102 | 8.7 ± 28.9 | 101 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FACT-G, 29 items, range 0–116 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Epoetin beta | 69.1 ± 14.4 | 129 | 1.7 ± 11.8 | 128 | 3.7 ± 13.0 | 118 | 5.9 ± 14.5a | 114 | 6.5 ± 13.8a | 106 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Placebo | 68.5 ± 15.0 | 122 | 2.2 ± 10.1 | 120 | 2.9 ± 11.5 | 112 | 2.6 ± 12.9 | 104 | 3.1 ± 14.4 | 103 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FACT-F subscale, 13 items, range 0–52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Epoetin beta | 28.8 ± 10.7 | 160 | 2.2 ± 8.7 | 157 | 2.8 ± 10.8 | 148 | 4.2 ± 11.7 | 145 | 5.2 ± 12.2 | 133 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Placebo | 29.2 ± 11.0 | 157 | 1.8 ± 8.4 | 157 | 1.9 ± 9.8 | 145 | 2.5 ± 10.9 | 135 | 3.0 ± 12.1 | 130 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FACT-An subscale, seven items, range 0–28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Epoetin beta | 17.3 ± 4.6 | 160 | 0.9 ± 3.3 | 157 | 1.2 ± 4.2 | 148 | 1.7 ± 4.4 | 145 | 2.0 ± 4.3 | 133 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Placebo | 17.0 ± 5.0 | 157 | 0.8 ± 3.5 | 157 | 1.2 ± 4.1 | 145 | 1.2 ± 4.5 | 135 | 1.7 ± 5.2 | 130 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ap < 0.5 (after 12 and 16 weeks, the improvement in FACT-An and FACT-G scores was greater in the epoetin beta arm).NoteAnalysis of the dimensions of the FACT-G scale revealed statistically significant differences after 12 weeks: p < 0.01 and p < 0.05 favouring epoetin beta for social and family well-being and emotional well-being respectively. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quality of life results at baseline and change from baseline in epoetin beta responders and non-respondersVariableBaselineWeek 4Week 8Week 12Week 16Score, mean ± SDNo. of participantsScore, mean ± SDNo. of participantsScore, mean ± SDNo. of participantsScore, mean ± SDNo. of participantsScore, mean ± SDNo. of participantsFACT-An, 49 items, range 0–196Responder118.9 ± 25.1925.1 ± 21.6919.7 ± 25.2a8715.2 ± 26.3a8817.4 ± 25.9a82Non-responder105.7 ± 32.9364.3 ± 21.0363.0 ± 27.0315.8 ± 31.0265.8 ± 33.723FACT-G, 29 items, range 0–116Responder70.6 ± 12.9921.5 ± 12.2914.9 ± 12.4a876.9 ± 14.3a887.8 ± 13.4a83Non-responder65.6 ± 17.2372.0 ± 10.8370.5 ± 14.2312.6 ± 14.7261.9 ± 14.523FACT-F subscale, 13 items, range 0–52Responder30.4 ± 10.11142.5 ± 8.3*1123.8 ± 10.5a1085.3 ± 10.5a1106.3 ± 10.5a102Non-responder24.8 ± 11.2461.3 ± 9.5450.2 ± 11.4400.5 ± 14.3351.7 ± 15.031FACT-An subscale, seven items, range 0–28Responder17.8 ± 4.41141.0 ± 3.21121.3 ± 4.31082.1 ± 3.9a1102.2 ± 4.0a102Non-responder16.0 ± 4.7460.7 ± 3.5451.2 ± 4.2400.4 ± 5.4351.3 ± 5.231ap < 0.05.NoteAnalysis of the relationship between the final Hb level in week 16 and change in the total FACT-An score from baseline in the epoetin beta group was undertaken by regression analysis. A statistically significant correlation was found on the basis of a log-linear relationship regression (r = 0.3167; p = 0.001), but the variability between participants was considerable and a uniform target Hb value associated with an optimal quality of life could not be identified. | Variable | Baseline | Week 4 | Week 8 | Week 12 | Week 16 | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | FACT-An, 49 items, range 0–196 | Responder | 118.9 ± 25.1 | 92 | 5.1 ± 21.6 | 91 | 9.7 ± 25.2a | 87 | 15.2 ± 26.3a | 88 | 17.4 ± 25.9a | 82 | Non-responder | 105.7 ± 32.9 | 36 | 4.3 ± 21.0 | 36 | 3.0 ± 27.0 | 31 | 5.8 ± 31.0 | 26 | 5.8 ± 33.7 | 23 | FACT-G, 29 items, range 0–116 | Responder | 70.6 ± 12.9 | 92 | 1.5 ± 12.2 | 91 | 4.9 ± 12.4a | 87 | 6.9 ± 14.3a | 88 | 7.8 ± 13.4a | 83 | Non-responder | 65.6 ± 17.2 | 37 | 2.0 ± 10.8 | 37 | 0.5 ± 14.2 | 31 | 2.6 ± 14.7 | 26 | 1.9 ± 14.5 | 23 | FACT-F subscale, 13 items, range 0–52 | Responder | 30.4 ± 10.1 | 114 | 2.5 ± 8.3* | 112 | 3.8 ± 10.5a | 108 | 5.3 ± 10.5a | 110 | 6.3 ± 10.5a | 102 | Non-responder | 24.8 ± 11.2 | 46 | 1.3 ± 9.5 | 45 | 0.2 ± 11.4 | 40 | 0.5 ± 14.3 | 35 | 1.7 ± 15.0 | 31 | FACT-An subscale, seven items, range 0–28 | Responder | 17.8 ± 4.4 | 114 | 1.0 ± 3.2 | 112 | 1.3 ± 4.3 | 108 | 2.1 ± 3.9a | 110 | 2.2 ± 4.0a | 102 | Non-responder | 16.0 ± 4.7 | 46 | 0.7 ± 3.5 | 45 | 1.2 ± 4.2 | 40 | 0.4 ± 5.4 | 35 | 1.3 ± 5.2 | 31 | ap < 0.05.NoteAnalysis of the relationship between the final Hb level in week 16 and change in the total FACT-An score from baseline in the epoetin beta group was undertaken by regression analysis. A statistically significant correlation was found on the basis of a log-linear relationship regression (r = 0.3167; p = 0.001), but the variability between participants was considerable and a uniform target Hb value associated with an optimal quality of life could not be identified. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | Week 4 | Week 8 | Week 12 | Week 16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | Score, mean ± SD | No. of participants | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FACT-An, 49 items, range 0–196 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Responder | 118.9 ± 25.1 | 92 | 5.1 ± 21.6 | 91 | 9.7 ± 25.2a | 87 | 15.2 ± 26.3a | 88 | 17.4 ± 25.9a | 82 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-responder | 105.7 ± 32.9 | 36 | 4.3 ± 21.0 | 36 | 3.0 ± 27.0 | 31 | 5.8 ± 31.0 | 26 | 5.8 ± 33.7 | 23 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FACT-G, 29 items, range 0–116 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Responder | 70.6 ± 12.9 | 92 | 1.5 ± 12.2 | 91 | 4.9 ± 12.4a | 87 | 6.9 ± 14.3a | 88 | 7.8 ± 13.4a | 83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-responder | 65.6 ± 17.2 | 37 | 2.0 ± 10.8 | 37 | 0.5 ± 14.2 | 31 | 2.6 ± 14.7 | 26 | 1.9 ± 14.5 | 23 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FACT-F subscale, 13 items, range 0–52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Responder | 30.4 ± 10.1 | 114 | 2.5 ± 8.3* | 112 | 3.8 ± 10.5a | 108 | 5.3 ± 10.5a | 110 | 6.3 ± 10.5a | 102 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-responder | 24.8 ± 11.2 | 46 | 1.3 ± 9.5 | 45 | 0.2 ± 11.4 | 40 | 0.5 ± 14.3 | 35 | 1.7 ± 15.0 | 31 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FACT-An subscale, seven items, range 0–28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Responder | 17.8 ± 4.4 | 114 | 1.0 ± 3.2 | 112 | 1.3 ± 4.3 | 108 | 2.1 ± 3.9a | 110 | 2.2 ± 4.0a | 102 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-responder | 16.0 ± 4.7 | 46 | 0.7 ± 3.5 | 45 | 1.2 ± 4.2 | 40 | 0.4 ± 5.4 | 35 | 1.3 ± 5.2 | 31 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ap < 0.05.NoteAnalysis of the relationship between the final Hb level in week 16 and change in the total FACT-An score from baseline in the epoetin beta group was undertaken by regression analysis. A statistically significant correlation was found on the basis of a log-linear relationship regression (r = 0.3167; p = 0.001), but the variability between participants was considerable and a uniform target Hb value associated with an optimal quality of life could not be identified. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AEs | |||
|---|---|---|---|
| Participants reporting at least one AE, n (%) | 122 (72) | 132 (76) | |
| Hypertension (%) | 9 | 5 | |
| Local transient reaction after injection (%) | 1 | 0 | |
| Serious AE, n (%) | 57 (33) | 55 (32) | |
| Deaths, n (%) | 28 (16) | 22 (13) | |
| Death from pulmonary embolism, n | 1 | 0 | |
| Stable disease or partial remission (%) | 68 | 68 | Reported in Österborg and colleagues79 |
| Remission, n (%) | 9 (5) | 5 (3) | |
| Progressive disease, n (%) | 31 (18) | 40 (23) | |
| Iron | |||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Unclear |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | Yes |
| 6. Were the outcome assessors blind to treatment allocation? | Yes |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Partially (variability can be calculated from data provided in the paper |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes, until the end of treatment only |
| Other | |
|---|---|
| Generalisability | Reasonably sized, broad sample |
| Author conclusions | This randomised, placebo-controlled study has demonstrated that epoetin beta treatment is effective in relieving anaemia and improving quality of life in severely anaemic, transfusion-dependent patients with advanced-phase NHL, CLL and MM. Overall, the improvement in quality of life was particularly apparent in participants with Hb increases of ≥ 2 g/dl. This suggests that the minimum increase in Hb may be a more important determinant of improved quality of life than a uniform and close to normal target Hb level Österborg 2005: Treatment with epoetin beta was found to have ‘no significant effect on the risk of progressive disease or long term survival in patients with lymphoproliferative malignancies. . . . a limitation of these data is that the 16-week treatment period was relatively short compared with the median survival time of patients’ (p. 208) |
| Reviewer comments | |
| EndNote ref. ID: 1119 | Malignancy type: solid tumours and hematological cancer | |
| Treatment: epoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Ray-Coquard 200975 | n = 218 |
| Objective | This randomised Phase III study aimed to identify the effects of epoetin alfa in patients at high risk for anaemia requiring RBCT: patients receiving chemotherapy with a Hb level < 12 g/dl, performance statusa > 1 and/or lymphocytes ≤ 700/µl (score of ≥ 4 according to the ELYPSE risk modelb) | Inclusion criteria: Histologically documented solid tumours or hematological cancer necessitating chemotherapy; age ≥ 18 years; Hb < 12 g/dl (on day 1 of chemotherapy) and lymphocytes ≤ 700/µl or performance status > 1; negative human immunodeficiency virus test in patients with non–Hodgkin’s lymphoma; chemotherapy not requiring haematopoietic stem cell support, chemotherapy planned for at least 3 months and inclusion during first or second course of chemotherapy (regardless of line of treatment) Exclusion criteria: Systematic administration of epoetin during chemotherapy; uncontrolled hypertension (i.e. diastolic blood pressure > 95 mmHg); patient refusal; anaemia in cancer patients not receiving chemotherapy; history of nervous or psychiatric disorder that would preclude informed consent or compliance; anaemia resulting from factors other than cancer or its treatment; untreated folate or vitamin B12 deficiency; pregnancy; history of thrombovascular events in the preceding 6 months; current dose-intensification chemotherapy for bone marrow or stem cell transplant in the preceding 8 weeks |
| No. of centres | Nine sites; September 2000–January 2005 | |
| Other references/aliases | Ray-Coquard 1999201 (not eligible for inclusion in the review – reports more detail about the development of the ELYPSE model) | |
| Geographical setting | France | |
| Duration of treatment | 12 weeks | |
| Length of follow-up (if different) | Median follow-up 12 months (95% CI 12 to 12.4 months) | |
| Country of corresponding author | France | |
| Language of publication | English | |
| Sources of funding | Ministry of Health; Ligue Contre le Cancer (Ain, Rhône and Savoie) | |
| Randomisation and allocation | Randomisation was centralised and stratified according to the participating centres and the number of prognostic factors for severe anaemia, with two vs. three of the following criteria: Hb level at day 0 < 12 g/dl, lymphocytes ≤ 700/µl and performance status > 1 | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Epoetin alfa | No treatment |
| n | 110 | 108 |
| Dose and frequency (once daily, twice daily, etc.) | 150 UI/kg three times a week | |
| Dose adjustment (yes/no) | Decreased to 75% if Hb increase > 2 g/dl. If after 4 weeks Hb level was < 10.5 g/dl with < 1 g/dl decrease and reticulocyte count was < 40,000 cells/µl, dose increased to 60,000 UI weekly. If Hb increased to > 12 g/dl, dose interrupted until Hb is 12 g/dl | |
| Route of administration | Subcutaneous | NA |
| Duration of epoetin treatment | 12 weeks | |
| Adjuvant anaemia treatment | Oral iron supplementation was administered to support erythropoiesis in patients with iron deficiency as no information on the improved efficacy of intravenous iron treatment was available at the initiation of the trial | Oral iron supplementation was administered to support erythropoiesis in patients with iron deficiency as no information on the improved efficacy of intravenous iron treatment was available at the initiation of the trial |
| Transfusion trigger | Incidence of severe anaemia | Incidence of severe anaemia |
| Outcomes | |
|---|---|
| Primary outcome | |
| Other outcomes | RBCTc (rate of transfusion, number of transfusions); survival (OS, time to disease progression); HRQoL (EORTC QLQ-C30); AEs |
| Analysis | |
|---|---|
| Statistical technique used | OS was the time interval from randomisation to date of death or last follow-up. Kaplan–Meier survival estimates and differences were assessed by the log-rank test. Safety variables were analysed using the safety population (all randomly assigned patients with at least one safety assessment). Quality-of-life scores were compared between the two arms for each chemotherapy cycle and variations from baseline were calculated for each patient and compared between arms after stratification into three levels on the assumption that a 10-point disparity represented a clinically pertinent differential |
| ITT analysis? | Yes |
| Does statistical technique adjust for confounding? | NA |
| Power calculation (a priori sample calculation)? | Yes, to detect a 15% difference in RBCTs with a power of 80% and a one-sided significance level of 5% |
| Attrition rate (loss to follow-up)? | NR; numbers of patients and reasons for treatment discontinuation reported (but not by study arm) |
| Was attrition rate adequately dealt with? | NR |
| No. (%) followed up from each condition? | NR |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Solid tumours and hematological cancer | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Unspecified chemotherapy | ||||
| Adjuvant anaemia treatment | Iron | Yes; oral supplementation in patients with iron deficiency | |||
| G-CSF | Yes; could be used in primary or secondary prophylaxis | ||||
| Transfusion trigger | Incidence of severe anaemia | ||||
| Hb inclusion criterion level | < 12 g/dl | ||||
| Arm 1 = epoetin alfa (n = 110) | Arm 2 = control (n = 108) | Notes | p-value | ||
| Sex, n (%) | |||||
| Male | 52 (47.3) | 41 (38) | |||
| Female | 58 (52.7) | 67 (62) | |||
| Age (years), mean (SD) | 62.7 (11.6) | 61.7 (11.6) | |||
| ECOG score | |||||
| 0–1 | 8 (7.3) | 8 (7.4) | |||
| 2 | 87 (79.1) | 8 (76.9) | |||
| 3–4 | 15 (13.6) | 17 (15.7) | |||
| Hb baseline (g/dl), mean (SD) | 10 (1.2) | 10 (1.2) | |||
| Haematocrit (%), mean (SD) | 30.3 (3.4) | 30.4 (3.8) | |||
| Ferritin (µg/dl), mean (SD) | 585 (697) | 701 (1005) | |||
| Stage, n (%) | |||||
| Local | 16 (14.5) | 12 (11.1) | |||
| Metastatic | 92 (83.6) | 94 (87.0) | |||
| NA | 2 (1.8) | 2 (1.9) | |||
| Two strata (prognostic factor), n (%) | 84 (76.4) | 79 (73.1) | |||
| Three strata (prognostic factor), n (%) | 26 (23.6) | 29 (26.9) | |||
| Health state utility scale (EORTC QLQ-C30) score | 0.048 | ||||
| Were intervention and control groups comparable? | No p-values reported apart from HRQoL data; authors stated that ‘Patient distribution was well balanced between the two groups of treatments’ (p. 1107) | ||||
| Results | ||||
|---|---|---|---|---|
| HRQoL:a,b health state utility scale (EORTC QLQ-C30) | ||||
| Baseline | p = 0.048 | |||
| Follow-up | ||||
| Survival | ||||
| OSc,d | ||||
| Median survival (months) (95% CI) | 7.6 (5.3 to 10.4) | 6 (5.0 to 8.0) | p = 0.148 | |
| Median PFS (months) (95% CI) | 5 (4.3 to 6.6) | 4.4 (3.8 to 5.2) | p = 0.17 | |
| Transfusionsc | ||||
| Participants, n (%) | 39 (36.1) | 61 (58) | Relative risk 0.62 (95% CI 0.46 to 0.84) | p = 0.001 |
| Safety data | ||||
| At least one AE, n (%) | 59 (53.6) | 50 (46.7) | p = 0.31 | |
| At least one serious AE, n (%) | 54 (49.1) | 49 (45.4) | p = 0.58 | |
| Fatal AE, n (%) | 20 (18.2) | 20 (18.5) | p = 0.95 | |
| Deaths | The majority (73%) of patients had died at the time of the final analysis | |||
| Cause of death = thrombovascular events (%) | 1.3 | 0.6 | ||
| Cause of death = disease progression (%) | 27 | 22 | ||
| Thrombovascular events (%) | 4.5 | 3.7 | ||
| Hematological toxic effects (%) | 18.2 | 13 | ||
| Serious AEs were considered related to the study drug (%) | 4.6 | 2.9 | p = 0.72 | |
| Incidence of serious AEs, including deaths (%) | 50 | 46.7 | p = 0.63 | |
| Quality appraisal | ||||
|---|---|---|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear; method was centralised but not reported | |||
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | Unclear | |||
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Unclear; no p-values reported but the authors report that patient distribution was well balanced between the two treatment groups (p. 1107). However, significant differences in favour of the epoetin alfa arm were noted for quality-of-life scores at inclusion and so the groups were unbalanced in this respect | |||
| 4. Were the eligibility criteria specified? | Yes | |||
| 5. Were the participants blind to treatment allocation? | No (open label) | |||
| 6. Were the outcome assessors blind to treatment allocation? | No (open label) | |||
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Yes | |||
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No | |||
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes, apart from HRQoL (54% and 57% participants analysed in epoetin alfa and control groups respectively) | |||
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | NR; see Analysis section | |||
| Other | |
|---|---|
| Generalisability | A very specific population group |
| Author conclusions | Patients at high risk for RBCT according to Hb < 12 g/dl and lymphocytes ≤ 700/µl and/or performance status > 1 could be given prophylactic epoetin alfa, with a significantly reduced requirement for RBCT and no significant impact on side effects, PFS or OS |
| Reviewer comments | Not a very well-reported trial, e.g. data in results and methods section only partially reported |
| EndNote ref. ID: 2695 | Malignancy type MM | |
| Treatment: second induction chemotherapy, rHuEPO – assume epoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Silvestris 199572 | n = 54 |
| Objective | Not stated – paper reports the results of a long-term trial using rHuEPO in MM patients undergoing second-induction chemotherapy | Inclusion criteria: MM stages I–IIIA, resistant to conventional melphalan-prednisone; chronic anaemia (Hb level ≤ 8.0 g/dl) with or without transfusional supplementation; commencement of second induction chemotherapy; preserved kidney function; Karnofsky performance status < 50 Exclusion criteria: NR |
| No. of centres | NR | |
| Other references/aliases | None | |
| Geographical setting | NR | |
| Duration of treatment | NR – according to graph 24 weeks | |
| Length of follow-up (if different) | NR | |
| Country of corresponding author | Italy | |
| Language of publication | English | |
| Sources of funding | This work was supported in part by the Finalised Project ‘Clinical Application of Oncology Research’ of the Italian National Research Council. No further details provided | |
| Randomisation and allocation | Randomisation was carried out directly by the biostatistical department of the pharmaceutical company providing the recombinant hormone (Cilag AG, Schaffhausen, Switzerland) | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | rHuEPO | Control (assumed as this arm is not mentioned) |
| n | 30 | 24 |
| Dose and frequency (once daily, twice daily, etc.) | 150 IU/kg, three times a week, started within the first month of the conventional cytotoxic protocol | |
| Dose adjustment (yes/no) | Dose increased to 300 IU/kg by the sixth week of treatment | |
| Route of administration | Subcutaneous | |
| Duration of epoetin treatment | ||
| Adjuvant anaemia treatment | Regular iron supplementation was provided throughout the study | |
| Transfusion trigger | Hb 9.5 g/dl | |
| Outcomesa | ||
| Primary outcome | ||
| Other outcomes | Haematological response (an increase of ≥ 2 g/dl above the original Hb level or no further RBC supplementation in transfusion-dependent participants was taken as response to treatment) | |
| Analysis | |
|---|---|
| Statistical technique used | As the majority of laboratory parameter studies are not normally distributed, ANOVA was performed by evaluating the median of each parameter and its range between minimum and maximum. The Wilcoxin test was adopted as a non-parametric method to compare different groups |
| ITT analysis? | NR |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation)? | NR |
| Attrition rate (loss to follow-up)? | Four participants withdrawn although the results table suggests five |
| Was attrition rate adequately dealt with? | Yes |
| No. (%) followed up from each condition? | NR |
| Baseline characteristics | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | MM | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Second induction chemotherapy | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adjuvant anaemia treatment | Iron | Regular iron was provided | |||||||||||||||||||||||||||||||||||||||||||||||||||
| G-CSF | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transfusion trigger | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hb inclusion criterion level | ≤ 8.0 g/dl | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arm 1 = rHuEPO (n = 30) | Arm 2 = control (n = 24) | Notes | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Male | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Female | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), median (range) | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Neutrophil count, (cell/µL), median | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients transfused, n (%) | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| No. of RBC units transfused per patient over 3 months prior to study start, mean (range) | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean haematocrit, n (%) | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Endogenous erythropoietin level (mU/ml), mean (median) [range] | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of solid tumour, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Haematological | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Breast | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Gynaecological | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Gastrointestinal | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Lung (SCLC and NSCLC) | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Prostate | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Head and neck | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Other | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Unknown primary | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Distribution of patients enrolled into the trialChemotherapy groupsArm 1 = rHuEPO (n = 30) (n = 27 evaluable)Arm 2 = control (n = 24) (n = 22 evaluable)NTD, nRespondersa/evaluable patientsTD, nRespondersa/evaluable patientsNTD, nTD, n VMCP119/1095/812b5c VMCP + α-IFN55/5––3– VED132/312 CTX–1–/1–1CTX, high dose cyclophosphamide; IFN, interferon; NTD, non-transfusion dependant; TD, transfusion dependant; VED, vincristine + epirubicin + dexamethasone; VMCP, vincristine + melphalan + cyclophosphamide + prednisone.aResponse defined as ≥ 2 g/dl increase in Hb concentration.bEleven patients were evaluable at the end of the study.cFour patients were evaluable at the end of the study. | Chemotherapy groups | Arm 1 = rHuEPO (n = 30) (n = 27 evaluable) | Arm 2 = control (n = 24) (n = 22 evaluable) | NTD, n | Respondersa/evaluable patients | TD, n | Respondersa/evaluable patients | NTD, n | TD, n | VMCP | 11 | 9/10 | 9 | 5/8 | 12b | 5c | VMCP + α-IFN | 5 | 5/5 | – | – | 3 | – | VED | 1 | 3 | 2/3 | 1 | 2 | CTX | – | 1 | –/1 | – | 1 | CTX, high dose cyclophosphamide; IFN, interferon; NTD, non-transfusion dependant; TD, transfusion dependant; VED, vincristine + epirubicin + dexamethasone; VMCP, vincristine + melphalan + cyclophosphamide + prednisone.aResponse defined as ≥ 2 g/dl increase in Hb concentration.bEleven patients were evaluable at the end of the study.cFour patients were evaluable at the end of the study. | |||||||||||||||||
| Chemotherapy groups | Arm 1 = rHuEPO (n = 30) (n = 27 evaluable) | Arm 2 = control (n = 24) (n = 22 evaluable) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| NTD, n | Respondersa/evaluable patients | TD, n | Respondersa/evaluable patients | NTD, n | TD, n | ||||||||||||||||||||||||||||||||||||||||||||||||
| VMCP | 11 | 9/10 | 9 | 5/8 | 12b | 5c | |||||||||||||||||||||||||||||||||||||||||||||||
| VMCP + α-IFN | 5 | 5/5 | – | – | 3 | – | |||||||||||||||||||||||||||||||||||||||||||||||
| VED | 1 | 3 | 2/3 | 1 | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| CTX | – | 1 | –/1 | – | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| CTX, high dose cyclophosphamide; IFN, interferon; NTD, non-transfusion dependant; TD, transfusion dependant; VED, vincristine + epirubicin + dexamethasone; VMCP, vincristine + melphalan + cyclophosphamide + prednisone.aResponse defined as ≥ 2 g/dl increase in Hb concentration.bEleven patients were evaluable at the end of the study.cFour patients were evaluable at the end of the study. | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Were intervention and control groups comparable? | No baseline characteristics reported. However, the authors do report the distribution of participants by their chemotherapy protocols, transfusion dependency and response to the recombinant erythropoietin treatment | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Results | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 = rHuEPO (n = 30) (n = 27 evaluable) | Arm 2 = control (n = 24) (n = 22 evaluable) | Notes | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||
| Hb response (≥ 2g/dl), n (%) | 21 (77.7) after a median period of 8 weeks | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Median Hb (g%) (approximate interpretation from graphs by PenTAG)Week 0Week 4Week 8Week 12Week 16Week 20Week 24NTD – VMCP + EPO (n = 9)7.68.29.510.510.410.410.2NTD – VCMP – EPO (n = 11)7.87.67.57.67.67.67.6TD – VMCP + EPO (n = 5)7.49.09.59.49.49.29.4TD – VCMP – EPO (n = 4)7.88.48.18.69.08.99.0EPO, epoetin; NTD, non-transfusion dependant; TD, transfusion dependant; VMCP, vincristine + melphalan + cyclophosphamide + prednisone. | Week 0 | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 | NTD – VMCP + EPO (n = 9) | 7.6 | 8.2 | 9.5 | 10.5 | 10.4 | 10.4 | 10.2 | NTD – VCMP – EPO (n = 11) | 7.8 | 7.6 | 7.5 | 7.6 | 7.6 | 7.6 | 7.6 | TD – VMCP + EPO (n = 5) | 7.4 | 9.0 | 9.5 | 9.4 | 9.4 | 9.2 | 9.4 | TD – VCMP – EPO (n = 4) | 7.8 | 8.4 | 8.1 | 8.6 | 9.0 | 8.9 | 9.0 | EPO, epoetin; NTD, non-transfusion dependant; TD, transfusion dependant; VMCP, vincristine + melphalan + cyclophosphamide + prednisone. | ||||||||||||
| Week 0 | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 | ||||||||||||||||||||||||||||||||||||||||||||||
| NTD – VMCP + EPO (n = 9) | 7.6 | 8.2 | 9.5 | 10.5 | 10.4 | 10.4 | 10.2 | |||||||||||||||||||||||||||||||||||||||||||||
| NTD – VCMP – EPO (n = 11) | 7.8 | 7.6 | 7.5 | 7.6 | 7.6 | 7.6 | 7.6 | |||||||||||||||||||||||||||||||||||||||||||||
| TD – VMCP + EPO (n = 5) | 7.4 | 9.0 | 9.5 | 9.4 | 9.4 | 9.2 | 9.4 | |||||||||||||||||||||||||||||||||||||||||||||
| TD – VCMP – EPO (n = 4) | 7.8 | 8.4 | 8.1 | 8.6 | 9.0 | 8.9 | 9.0 | |||||||||||||||||||||||||||||||||||||||||||||
| EPO, epoetin; NTD, non-transfusion dependant; TD, transfusion dependant; VMCP, vincristine + melphalan + cyclophosphamide + prednisone. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| HRQoL | NR | NR |
| Adverse effectsa | ||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | NR |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | Unclear |
| 6. Were the outcome assessors blind to treatment allocation? | Unclear |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | No |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | No, ≥ 10% of dropouts in the epoetin group |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes |
| Other | |
|---|---|
| Generalisability | Unable to assess |
| Author conclusions | Our data suggest that α-interferon plus rHuEPO treatment in MM patients is effective in restoring normal B-cell function. These results may reflect in vivo the modulation of normal human B-cells and lymphoblasts by rHuEPO observed in vitro |
| Reviewer comments | Small sample size, no baseline characteristics, poorly reported outcomes |
| EndNote ref. ID: 341 | Malignancy type: cervical cancer | |
| Treatment: epoetin beta | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Strauss 200876 | n = 74 |
| Objective | To investigate whether in patients with cervical cancer the effectiveness and outcome of radiotherapy plus cisplatin could be positively influenced by treatment with epoetin beta. The design of the second stage was to be adapted or rejected depending on the outcome of the first stage of the trial. The primary objective of the first stage was to investigate whether there was a correlation between anaemia correction with epoetin beta and treatment failure in women with cervical cancer receiving radiochemotherapy. After the first stage had been analysed the study protocol outlined a continuation of the study in which a further 450 patients were to be enrolled to investigate the potential impact of anaemia correction with epoetin on survival | Inclusion criteria: Age ≥ 18 years; histologically confirmed diagnosis of cervical cancer; FIGO (International Federation of Gynecology and Obstetrics) stage IIB–IVA (except chorion carcinoma and neuroendocrine small cell carcinoma); Hb levels between 9 and 13 g/dl at screening; WHO performance status of 0–2; life expectancy of at least 3 months; adequate bone marrow function (platelets > 100 × 109/l and leucocytes > 3.0 × 109/l); adequate liver function (transaminases and/or alkaline phosphatises no greater than 2.5 × upper normal limit; bilirubin no greater than 1.5 × normal limit); adequate renal function (calculated creatinine clearance > 60 ml/minute); no previous systemic antineoplastic therapy or radiotherapy for cervical cancer except previous single brachytherapy fraction of the protocol-prescribed radiotherapy course as clinically indicated Exclusion criteria: Patients with distant metastasis (M1 disease); positive para-aortic lymph nodes; chronic heart failure [New York Heart Association (NYHA ≥ 2]; uncontrolled arterial hypertension (systolic blood pressure ≥ 170 mmHg, diastolic blood pressure ≥ 100 mmHg); known history of deep-vein thrombosis; thrombocytosis; known haemoglobinopathies; vitamin B12 and/or folic acid deficiencies; haemolytic anaemia; bleeding requiring transfusion within 3 months before planned start of treatment; acute infection; transferrin saturation < 20%; known presence of other neoplasias within the last five years; pregnancy or lactation; exposure to epoetins within 3 months; contraindications against cisplatin therapy |
| No. of centres | 20 | |
| Other references/aliases | Full paper | |
| Geographical setting | Europe, Turkey and Thailand | |
| Duration of treatment | Unclear – participants scheduled to receive radiotherapy over 6 weeks (to a maximum of 50 days) plus concomitant cisplatin | |
| Length of follow-up (if different) | 447–513 days (unclear if this starts after the end of the treatment period) | |
| Country of corresponding author | Germany | |
| Language of publication | English | |
| Sources of funding | F. Hoffman-La Roche | |
| Randomisation and allocation | Open-label, randomised, two-arm, parallel-group, two-stage adaptive study. Patients were centrally randomised to the epoetin arm or the control arm. No details given on randomisation procedure | |
| Treatment armsa | ||
| Arm drug name(s) | Epoetin beta | Control (standard care) |
| n | 34 | 40 |
| Dose and frequency (once daily, twice daily, etc.) | 450 IU/kg in three divided doses | |
| Dose adjustment (yes/no) | Yes. If insufficient Hb response (increase in Hb of < 0.5 g/dl after 4 weeks of treatment or requirement for RBCT in the fourth week of treatment) the dose could be doubled to 900 IU/kg. If Hb > 15 g/dl epoetin was stopped and resumed at 50% of the previous dose until Hb ≤ 14 g/dl. If Hb increased by > 2 g/dl in 4 weeks dose reductions of 50% were applied | |
| Route of administration | Subcutaneous | |
| Duration of epoetin treatment | Median duration of epoetin beta treatment was 63 days (range 3–98 days) | |
| Adjuvant anaemia treatment | If transferrin saturation was < 20%, intravenous iron supplementation with a dose of 100 mg of Fe3+ was recommended. If contraindicated or not available, daily oral iron supplementation at a dose of 200–300 mg of Fe3+ could be used. Iron was received by 27 participants (79%) in the epoetin group. Of these, 15 received iron intravenously and 12 orally. In the control group, iron was received by 22 participants (55%), with 12 receiving iron intravenously and 10 orally | |
| Transfusion trigger | At physicians’ discretion if Hb level < 8.5 g/dl and to be avoided in participants with a Hb level > 8.5 g/dl | |
| Outcomes | |
|---|---|
| Primary outcome | Treatment failures in correlation with Hb change from baseline to study end (defined as participants with no complete response or relapsing within 6 months after initiation of radiochemotherapy) |
| Other outcomes | Tumour response; progression-/relapse-free survival; OS; overall response rate; AEs |
| Analysis | |
|---|---|
| Statistical technique used | The effect of Hb change from baseline on treatment failure (defined as no complete response or relapse within 6 months after initiation of radiochemotherapy) was analysed using a logistical regression analysis (two-sided test at α = 5% with change in Hb from baseline as main factor in the model). A proof of concept for the first stage of the study was to be accepted if a positive correlation between change in Hb level from baseline to the end of the treatment period and treatment failure could be established and no important safety concerns were raised in an initial group of approximately 80 participants. Progression-free and OS were analysed by log-rank testing and Cox regression analysis. Multivariate analysis was performed using a stepwise Cox regression procedure. The overall response was analysed using the Chi–squared test with Schouten correction and 95% Clopper–Pearson CIs. Change in Hb from baseline at the end of the treatment period was tested in an ANCOVA model, with Hb at baseline as a covariate. Hb change from baseline was assessed at week 4 and at the end of the treatment period |
| ITT analysis? | Yes; all randomised participants were included in the ITT population and all efficacy results are provided for this population. The safety population consisted of all patients who received at least one dose of the trial medication (radiochemotherapy and/or epoetin in the epoetin beta group and at least one dose of radiochemotherapy in the control group |
| Does statistical technique adjust for confounding? | NR (only baseline Hb values mentioned as a covariate in ANCOVA) |
| Power calculation (a priori sample calculation)? | NR |
| Attrition rate (loss to follow-up)? | See below |
| Was attrition rate adequately dealt with? | Three participants were excluded from the safety analysis (one from the treatment arm and two from the control arm) as they did not receive the study treatment. A total of 12 participants (16%) were withdrawn prematurely from the study, eight in the epoetin arm and four in the control arm. There were no withdrawals because of AEs in either group. Reasons for withdrawal were death (not related to study medication), refusal of further treatment, failure to return for treatment, inclusion criteria not being met or exclusion criteria being fulfilled |
| No. (%) followed up from each condition? | Median follow-up for survival was 482 (IQR 447–617) days in the epoetin beta group and 466 (IQR 446–513) days in the control group |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Cervical | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Chemotherapy + radiotherapy | ||||
| Adjuvant anaemia treatment | Iron | Enrolled participants with a transferrin saturation of < 20% were recommended to receive intravenous iron supplementation with a dose of 100 mg Fe3+. If contraindicated or not available, daily oral iron supplementation at a dose of 200–300 mg Fe3+ could be used | |||
| G-CSF | No | ||||
| Transfusion trigger | Blood transfusions were given according to decision of physician if Hb level was < 8.5 g/dl and were to be avoided in participants with a Hb level > 8.5 g/dl | ||||
| Hb inclusion criterion level | Between 9 and 13 g/dl at screening | ||||
| Arm 1 = epoetin beta (n = 34) | Arm 2 = control (n = 40) | Notes | p-value | ||
| Sex, n (%) | |||||
| Male | – | – | |||
| Female (%) | 34 (100) | 40 (100) | 0.957 | ||
| Age (years), mean (SD) | 48.8 (10.2) | 49.2 (12.8) | |||
| WHO performance status, n (%) | |||||
| 0 | 21 (61.8) | 27 (67.5) | 0.689 | ||
| 1 | 13 (38.2) | 12 (30.0) | 0.529 | ||
| 2 | 0 | 1 (2.5) | – | ||
| 3 | |||||
| 4 | |||||
| Hb baseline (g/dl), median (IQR) | 11.4 (10.8–12.0) | 11.6 (10.9–12.4) | 0.371 | ||
| Hb before radiochemotherapy (g/dl), median (IQR) | 11.8 (10.6–13.1) | 11.7 (10.9–12.4) | 0.633 | ||
| Epoetin baseline (mU/ml) | |||||
| Were intervention and control groups comparable? | Yes | ||||
| Results | |||
|---|---|---|---|
| Haematological outcomes and transfusions | |||
| Median change in Hb (g/dl) (baseline to last value)a | 1.3 | –0.7 | |
| Transfusion-free participants | 25 (73.5) | 28 (70) | |
| RBC units received, median (range) | 3.3 (0.9–6.4) | 12 (0.9–6.0) | Not significant |
| Survival, n (%) | |||
| OS, deathsb | 8 (23.5) | 5(12.5) | 0.22 |
| Treatment failures | 11 (32.4) | 12 (30.0) | 0.32 |
| Complete response | 18 (52.9) | 23 (57.5) | 0.86 |
| Partial response | 4 (11.8) | 6 (15.0) | 0.83 |
| Stable disease | 0 | 3 (7.5) | |
| Progressive disease | 7 (20.6) | 3 (7.5) | 0.12 |
| PFS (%) | 10 (29.4) | 13 (32.5) | 0.96 |
| Tumour response | (n = 29) | (n = 35) | |
| Complete response, n | 18 | 23 | |
| HRQoL: health state utility scale | Not collected | ||
| Adverse effects, n (%)c | |||
| Total | 19 (58) | 26 (68) | 0.409 |
| Deaths | 8 (23.5) | 5 (12.5) | 0.22 |
| Thromboembolic events | |||
| Hypertension | |||
| Haemorrhage/thrombocytopenia | 1 (3) | 4 (11) | 0.313 |
| Rash/irritation/pruritus | 1 (3) | 0 (0) | |
| Seizures | |||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | Unclear; although described as ‘centrally randomised’, further details were not provided |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | No |
| 6. Were the outcome assessors blind to treatment allocation? | No |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Partially (variability can be calculated from data presented in the paper) |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes |
| Other | |
| Generalisability | |
| Author conclusions | This study shows that epoetin beta rapidly, effectively and safely increases Hb levels in patients with cervical cancer receiving radiochemotherapy. Because no positive correlation between Hb increase and improvement in clinical outcomes, such as a reduction in treatment failure, could be demonstrated in stage 1 of this study, this study was not expanded to its second stage, which was designed to investigate the potential benefits of anaemia correction on survival. Therefore, this study does not allow any definite conclusions to be drawn with respect to the positive or negative effects of epoetin therapy on survival or disease progression in patients with cervical cancer receiving radiochemotherapy |
| Reviewer comments | |
| EndNote ref ID: 2696 | Malignancy type: Ovarian carcinoma | |
| Treatment: epoetin beta (assumed) | ||
| Study design | Participants | |
|---|---|---|
| Author, year | ten Bokkel Huinink 199851 | n = 122 |
| Objective | To investigate the influence of rHuEPO on anaemia and transfusion requirement in patients with ovarian carcinoma treated with platinum-based therapy | Inclusion criteria: Age ≥ 18 years; ovarian cancer stage IIb–IV [according to the International Federation of Gynecology and Obstetrics (FIGO) classification]; WHO performance status 0–2; Hb < 13 g/dl prior to treatment; overall life expectancy > 2 months; previously treated patients who had achieved a complete remission (CR) and who had not received treatment for at least 1 year could be enrolled into the study; receiving cisplatin ≥ 75 mg/m2 or carboplatin ≥ 350 mg/m2 because lower doses induce anaemia in only a small proportion of patients Exclusion criteria: Previous chemotherapy or radiotherapy for ovarian cancer if requirements for previously treated patients as detailed in the inclusion criteria not met; white blood cell count ≤ 3.5 × 109/l; platelet count ≤ 100 × 109/l; hypertension (systolic blood pressure > 160 mmHg or diastolic blood pressure > 95 mmHg); impaired liver function (bilirubin > 25 mmol/l); impaired renal function (creatinine > 120 µmol/l); thrombocytosis (≥ 500 × 109/l); other reasons for anaemia; severely impaired coagulation; iron deficiency; epilepsy; blood transfusion < 1 week prior to protocol treatment; haemoglobinopathies; acute infections; second primary tumours; administration of an investigational drug within 30 days preceding the first dose of the study drug |
| No. of centres | NR | |
| Other references/aliases | None | |
| Geographical setting | NR | |
| Duration of treatment | Treatment with epoetin and chemotherapy began 2 days after randomisation in most patients. Epoetin was continued throughout the course of chemotherapy and for a further 3–24 weeks after the last cycle of treatment, depending on the duration of chemotherapy. Median duration of observation (between randomisation and last examination) was 170 days in the control group and 167 days in group 1 | |
| Length of follow-up (if different) | ||
| Country of corresponding author | The Netherlands | |
| Language of publication | English | |
| Sources of funding | NR | |
| Randomisation and allocation | Participants were randomly allocated to three study groups. Randomisation was performed centrally using permuted blocks stratified by institute and previous treatment (previously untreated, first line or recurrent disease) | |
| Treatment arms | |
|---|---|
| Arm drug name(s) | Not given – assume epoetin alfa |
| n | 46 |
| Dose and frequency (once daily, twice daily, etc.) | 150 µg/kg three times a week |
| Dose adjustment (yes/no) | The dose of epoetin was reduced by 50% if Hb increased by > 2 g/dl during chemotherapy. If Hb exceeded 15 g/dl at any time, epoetin administration was stopped until Hb returned to < 14 g/dl and was then resumed at half the previous dose. Epoetin was withheld while platelet count was < 20 × 109 g/l. If chemotherapy was delayed because of thrombocytopenia but platelet count was > 20 × 109 g/l, epoetin was continued |
| Route of administration | Subcutaneous |
| Duration of epoetin treatment | Treatment with epoetin and chemotherapy began 2 days after randomisation in most patients. Epoetin continued throughout the course of chemotherapy and for a further 3–24 weeks after the last cycle of treatment, depending on the duration of chemotherapy |
| Adjuvant anaemia treatment | NR |
| Transfusion trigger | Hb < 9.7 g/dl |
| Outcomes | |
|---|---|
| Primary outcome | Time from randomisation to first erythrocyte transfusion; RBCT (no. of patients in study period) |
| Other outcomes | No. and volume of RBCTs per patient and per chemotherapy cycle; course of Hb per chemotherapy cycle; response to chemotherapy; number of deaths; AEsa |
| Analysis | |
|---|---|
| Statistical technique used | The time to first erythrocyte transfusion was analysed using failure-time methods (Kaplan–Meier estimates) using log-rank tests based on months from randomisation and number of cycles of chemotherapy. Univariate and multiple failure-time analyses (Cox proportional hazard method, maximum likelihood methods) were also performed. Laboratory parameters were analysed by means of parametric and non-parametric statistics |
| ITT analysis? | Described as ITT but two patients were excluded from the analysis because of insufficient data |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation)? | NR |
| Attrition rate (loss to follow-up)? | Numbers unclear – author states 84 patients completed the protocol. Of 120 patients, one patient in group 1 and one in group 2 dropped out of the study before the start of treatment. Twenty-nine plus seven withdrew (because of death, non-compliance, etc.) to give 82 patients |
| Was attrition rate adequately dealt with? | Safety was assessed according to ITT analysis, but unclear how missing data were handled |
| No. (%) followed up from each condition? | NR |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Ovarian cancer | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Platinum-based chemotherapy | ||||
| Adjuvant anaemia treatment | Iron | NR | |||
| G-CSF | NA | ||||
| Transfusion trigger | Hb < 9.7 g/dl | ||||
| Hb inclusion criterion level | < 13 g/dl | ||||
| Arm 2 = rHuEPO (n = 45) | Arm 1 = control (n = 33) | Notes | p-value | ||
| Sex (%) | |||||
| Male | |||||
| Female | 100 | 100 | |||
| Age (years), mean | 58.81 | 58.83 | |||
| WHO performance status, n (%) | |||||
| 0 | 24 (53.3) | 20 (60.6) | |||
| 1 | 19 (42.2) | 13 (39.4) | |||
| 2 | 2 (4.4) | 0 | |||
| Previous chemotherapy, n (%) | |||||
| Carboplatin ≤ 350 mg/m2 | 17 (37) | 15 (45) | |||
| Carboplatin > 350 mg/m2 | 9 (20) | 8 (24) | |||
| Cisplatin < 75 mg/m2 | 0 | 1 (3) | |||
| Cisplatin ≥ 75 to < 100 mg/m2 | 16 (35) | 7 (21) | |||
| Cisplatin ≥ 100 mg/m2 | 3 (6) | 2 (6) | |||
| Haematological parameters, median (range) | |||||
| Hb (g/dl) | 12.0 (10.3–12.6) | 11.8 (10.6–12.5) | There appears to be a ’typo’ in Table 1 for the epoetin baseline Hb value: 12.0 (1.3–12.6) assumed to be 12.0 (10.3–12.6) | ||
| Haematocrit (%) | 37.0 (34.2–38.5) | 37.0 (33.0–38.0) | |||
| Erythrocytes (×109/l) | 4.0 (3.7–4.3) | 4.0 (3.8–4.3) | |||
| Reticulocytes (%) | 10.5 (7.6–14.0) | 12.8 (15.7–16.9) | |||
| Platelet count (×109/l) | 383 (304–433) | 395 (302–505) | |||
| Neutrophil count (×109/l) | 4.3 (3.3–5.7) | 5.1 (3.4–6.2) | |||
| Iron (U/l), median (range) | NR | NR | |||
| Epoetin (mU/ml) | NR | NR | |||
| Target Hb (g/dl) | NR | NR | |||
| Were intervention and control groups comparable? | No p-values reported; authors stated that ‘The three groups were comparable with respect to age, stage of disease, WHO performance status, primary and recurrent disease, previous chemotherapy and baseline haematological parameters’ (p. 176) | ||||
| Results | |||
|---|---|---|---|
| Arm 2 = rHuEPO (n = 44a) | Arm 1 = control (n = 33) | ||
| Response to chemotherapy, n | (n = 40) | (n = 30) | |
| Progression | 6 | 2 | |
| Complete remission | 23 | 19 | |
| Deaths | 1 | 2 | |
| Transfusions | |||
| Time to first transfusion (months) | Longer in epoetin group than in control group | 0.0002 | |
| No. (%) of patients receiving at least one transfusion | 2 (4.4) | (39.4) | |
| No. of units | 15 units in six transfusion events | 41 units in 19 transfusion events | |
| Haematological outcomes | |||
| Patients with Hb < 10 g/dl, n (%) | |||
| Cycle 1 | 2 (4.5) | 8 (24.2) | |
| Cycle 2 | 1 (2.4) | 10 (32.3) | |
| Cycle 3 | 1 (2.5) | 15 (50) | |
| Cycle 4 | 3 (8.1) | 15 (53.7) | |
| Cycle 5 | 6 (16.7) | 13 (50) | |
| Cycle 6 | 6 (17.6) | 12 (50) | |
| Serum EPO (mU/ml) | |||
| No. of patients evaluable | 31 | 19 | |
| Median (range) | 9 (9–584) | 8 (2–29) | |
| O/P ratio | |||
| No. of patients evaluable | 28 | 18 | |
| O/P ratiob ≥ 0.8 | 16 | 7 | |
| O/P ratiob < 0.8 | 12 | 11 | |
| HRQoL | NR | NR | |
| AEsc | |||
| Thromboembolic events, n | 1 | ||
| Hypertension, n/N (%) | 1/43 (2.3) | 1/28 (3.6) | |
| Participants suffering at least one AE, n/N (%) | 39/45 (86.7) | 28/33 (84.8) | |
| Participants suffering more than one AE, n/N (%) | (20.0) | (15.2) | |
| Superficial thrombophlebitis, n | 1 | ||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | No |
| 6. Were the outcome assessors blind to treatment allocation? | No |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | No |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Partially; number unclear |
| Other | |
|---|---|
| Generalisability | Small sample size – all-female population |
| Author conclusions | The use of rHuEPO should be considered in patients with ovarian cancer receiving platinum-based chemotherapy, particularly if they have an endogenous erythropoietin deficiency, to delay the onset of anaemia and reduce the need for RBCT |
| Reviewer comments | Some of the results for the two dosing arms (one of which is not applicable to this review) have been combined and therefore not extracted |
| EndNote ref. ID: 2697 | Malignancy type: small-cell lung cancer (SCLC) | |
| Treatment: epoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Thatcher 199952 | n = 130 |
| Objective | To determine the efficacy and safety of epoetin alfa in preventing the decline in Hb level in patients undergoing cyclic chemotherapy for SCLC and to evaluate whether a reduction in RBCT requirements could also be achieved. The impact of epoetin alfa therapy on patients’ quality of life was also assessed | Inclusion criteria: Male or female aged 18–75 years; planned treatment with four to six cycles of combination chemotherapy, primarily platinum based; SCLC; required to be ambulatory and capable of self-care (WHO performance status ≤ 2); Hb ≤ 10.5 g/dl; neutrophil count > 3000/µl; platelet count > 100,000/µl; no clinically relevant abnormalities of renal or hepatic function; serum calcium < 10.6 mg/dl; stool samples negative for occult blood Exclusion criteria: Pregnant or of childbearing potential and not taking adequate contraceptive measures; any clinically significant disease; history of primary haematological disease; history of seizures or acute illness within 7 days of study entry; received androgen therapy within 2 months of study entry or received any experimental treatment, immunosuppressive drugs or other agents known to affect haematocrit within 1 month of study entry; receiving haematopoietic growth factors (including epoetin alfa); participating in another trial |
| No. of centres | NR | |
| Other references/aliases | None | |
| Geographical setting | Unclear | |
| Duration of treatment | Maximum study duration was 26 weeks | |
| Length of follow-up (if different) | NR | |
| Country of corresponding author | UK | |
| Language of publication | English | |
| Sources of funding | NR | |
| Randomisation and allocation | Participants randomised to one of three groups: epoetin alfa 150 IU/kg, epoetin alfa 300 IU/kg (outside licence therefore and therefore not applicable) and control | |
| Treatment arms | |||
|---|---|---|---|
| Arm drug name(s) | Epoetin alfa | Control (standard care) | |
| n | 42 | 44 | |
| Dose and frequency (once daily, twice daily, etc.) | 150 IU/kg three times a week. Treatment started 1 day after administration of each cycle of chemotherapy and continued until 3 days prior to the following cycle; treatment continued for 1 month after the final cycle | ||
| Dose adjustment (yes/no) | If Hb level exceeded 15 g/dl, epoetin alfa was discontinued until the value had fallen to < 13 g/dl, at which point treatment was reinstated at half the initial dose | ||
| Route of administration | Subcutaneous | ||
| Duration of epoetin treatment | Maximum study duration was 26 weeks | ||
| Adjuvant anaemia treatment | Transfusions were allowed as necessary. No participants received iron supplementation | ||
| Transfusion trigger | NR | ||
| Outcomes | |||
| Primary outcome | Haematological response (prevention of anaemia defined as maintenance of Hb level at ≥ 10 g/dl) | ||
| Other outcomes | HRQoL [participant well-being in the week prior to each cycle of chemotherapy was assessed using a quality-of-life questionnaire, in which participant responses to three levels (energy level, daily activity and overall quality of life) were scored on a 100-mm VAS, and WHO performance status score]; AEs [safety assessments included participant discontinuation information, vital signs (recorded in the treated groups only) and the incidence and severity of AEs, laboratory parameters at the start of each cycle and epoetin alfa antibody titre at study end compared with baseline] | ||
| Analysis | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistical technique used | Comparability of the three groups with regard to demographic and clinical characteristics at baseline was tested by means of ANOVA, Kruskal–Wallis tests or chi-squared tests, as appropriate. Differences between treatment groups for mid-cycle Hb and haematocrit through cycles 1–6 were tested using ANOVA. Within-group differences from baseline for efficacy parameters were tested using a paired Student’s t-test. The proportion of participants transfused was compared between treatment groups using a Cochran–Mantel–Haenszel analysis. For pairwise comparisons of treatment groups, the sequentially rejective Bonferroni–Holm procedure was applied to adjust for three multiple comparisons. The time to become anaemic or require transfusion was analysed by survival analysis using Kaplan–Meier estimates and the log-rank test. All tests were conducted at the two-sided, 0.05 significance level | |||||||||||||||||||||
| ITT analysis? | Yes | |||||||||||||||||||||
| Does statistical technique adjust for confounding? | NR | |||||||||||||||||||||
| Power calculation (a priori sample calculation)? | NR | |||||||||||||||||||||
| Attrition rate (loss to follow-up)? | Reasons for premature study discontinuation ParameterControl (n = 42)Epoetin alfa (n = 44)AEs24Death31Intercurrent illness11Othera810Total1416aIncluding personal reasons, loss to follow-up, non-responder to chemotherapy, disease progression or remission, discontinuation of chemotherapy, toxicity of chemotherapy, elevated Hb, deterioration of general condition and physician decision. |
Parameter | Control (n = 42) | Epoetin alfa (n = 44) | AEs | 2 | 4 | Death | 3 | 1 | Intercurrent illness | 1 | 1 | Othera | 8 | 10 | Total | 14 | 16 | aIncluding personal reasons, loss to follow-up, non-responder to chemotherapy, disease progression or remission, discontinuation of chemotherapy, toxicity of chemotherapy, elevated Hb, deterioration of general condition and physician decision. | ||
| Parameter | Control (n = 42) | Epoetin alfa (n = 44) | ||||||||||||||||||||
| AEs | 2 | 4 | ||||||||||||||||||||
| Death | 3 | 1 | ||||||||||||||||||||
| Intercurrent illness | 1 | 1 | ||||||||||||||||||||
| Othera | 8 | 10 | ||||||||||||||||||||
| Total | 14 | 16 | ||||||||||||||||||||
| aIncluding personal reasons, loss to follow-up, non-responder to chemotherapy, disease progression or remission, discontinuation of chemotherapy, toxicity of chemotherapy, elevated Hb, deterioration of general condition and physician decision. | ||||||||||||||||||||||
| Was attrition rate adequately dealt with? | NR | |||||||||||||||||||||
| No. (%) followed up from each condition? | NR | |||||||||||||||||||||
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | SCLC | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Platinum-based chemotherapy | ||||
| Adjuvant anaemia treatment | Iron | None | |||
| G-CSF | None | ||||
| Transfusion trigger | NR | ||||
| Hb inclusion criterion level | ≥ 10.5 g/dl | ||||
| Arm 2 = epoetin alfa (n = 42) | Arm 1 = control (n = 44) | Notes | p-value | ||
| Sex, n | |||||
| Male | 26 | 27 | |||
| Female | 16 | 17 | |||
| Age (years), median (range) | 59.0 (43–72) | 60 (39–74) | |||
| Hb (g/dl), median (range) | 13.7 (10.7–16.1) | 13.4 (10.9–16.4) | |||
| Haematocrit (%), median (range) | 41.0 (32.6–50.3) | 39.4 (32.3–46.8) | |||
| Reticulocyte count (×109/l), median (range) | 40.1 (1.0–76.2) | 39.3 (0.1–109.1) | |||
| Neutrophil count (×109/l), median (range) | 6.0 (1.7–11.3) | 5.9 (2.9–16.4) | |||
| WHO performance status (0–4), median (range) | 1.0 (0–3) | 1.0 (0–2) | |||
| Quality-of-life scores (0–100 mm), median (range) | |||||
| Energy level | 47.0 (11–100) | 51.0 (0–94) | |||
| Daily activity | 46.0 (5–100) | 32.0 (0–97) | |||
| Overall quality of life | 44.0 (1–100) | 49.0 (0–98) | |||
| Chemotherapy regimen, n | |||||
| Carboplatin based | 34 | 38 | |||
| Cisplatin based | 2 | 2 | |||
| Other | 6 | 4 | |||
| Were intervention and control groups comparable? | No p-values reported; authors stated that there were ‘no statistically significant between-group differences’ (p. 398) | ||||
| Results | |||
|---|---|---|---|
| Haematological and transfusion outcomes | |||
| Participants experiencing Hb < 10g/dl (%) | 48 | 66 | < 0.05 |
| Participants requiring a transfusion, n/N (%) | 19/42 (45) | 26/44 (59) | < 0.05 |
| Total no. of transfusions | 41 | 73 | |
| Cumulative transfusion rate for 6 cycles of chemotherapy, mean ± SD | 3.84 ± 5.58 | 6.13 ± 7.13 | < 0.01 |
| Median time (days) to become anaemic/require first transfusion | 116/98 | 59/48 | |
| HRQoL | |||
| Parameters assessed by the quality-of-life questionnaire did not show any marked changes from baseline at the end of the study in any group, with the exception of significant improvement in overall quality of life in the epoetin alfa 150 IU/kg group (p < 0.05). There were no significant between-group differences, which may be related to the fact that all groups had similar Hb values at study end. Evaluation of WHO performance status scores revealed similar findings, with no significant between- or within-group differences | |||
| Change in quality-of-life parameter from baseline (0–100 mm), mean ± SD | (n = 33) | (n = 27) | |
| Energy level | –2.3 ± 31.9 | 1.6 ± 23.9 | |
| Daily activity | 3.0 ± 31.7 | 10.8 ± 35.6 | |
| Overall quality of life | 11.7 ± 30.6a | 7.5 ± 29.1 | |
| Adverse effects reported by ≥ 5% of participants in any treatment group, n (%)b,c,d | |||
| Anaemia | 19 (43) | 14 (33) | |
| Thrombocytopenia | 9 (20) | 11 (26) | |
| Bacterial infection | 10 (23) | 8 (19) | |
| Nausea | 6 (14) | 3 (7) | |
| Neutropeniac | 8 (18) | 5 (12) | |
| Pyrexia | 7 (16) | 7 (17) | |
| Dyspnoea | 1 (2) | 1 (2) | |
| Vomiting | 5 (11) | 5 (12) | |
| Dizziness | 1 (2) | 3 (7) | |
| Cough | 0 | 0 | |
| Headache | 1 (2) | 2 (5) | |
| Constipation | 1 (2) | 2 (5) | |
| Malaise | 0 | 2 (5) | |
| Urinary tract infection | 0 | 0 | |
| Alopecia | 3 (7) | 1 (2) | |
| Oedema | 0 | 4 (10) | |
| Diarrhoea | 2 (5) | 5 (12) | |
| Rash | 4 (9) | 5 (12) | |
| Decreased white blood cell count | 3 (7) | 1 (2) | |
| Lethargy | 3 (7) | 1 (2) | |
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | No |
| 6. Were the outcome assessors blind to treatment allocation? | NR |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Partially (variability can be calculated from data presented in the paper) |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes (except for quality of life) |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes |
| Other | |
|---|---|
| Generalisability | Relatively small sample size |
| Author conclusions | This study has demonstrated that epoetin alfa is effective and well tolerated in maintaining a Hb level ≥ 10 g/dl and reducing transfusion requirements in patients with SCLC undergoing platinum-based cyclic combination chemotherapy |
| Reviewer comments | Quality of life of limited used because of unvalidated scale. WHO performance status scores were measured |
| EndNote ref. ID: 435 | Malignancy type: solid tumours | |
| Treatment: epoetin theta and beta and placebo | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Tjulandin 201048 | n = 223 |
| Objective | To assess the effects of epoetin theta compared with placebo for efficacy and to compare the efficacy and safety profiles of epoetin theta and epoetin beta | Inclusion criteria: Secondary anaemia (Hb ≤ 11 g/dl) related to platinum-containing chemotherapy; age ≥ 18 years; histologically or cytologically proven diagnosis of a solid tumour; at least one platinum-based chemotherapy cycle as treatment for the current malignancy during the last 4 weeks (Hb concentration of ≤ 11 g/dl after the last chemotherapy); ECOG score of ≥ 3 Exclusion criteria: Head and neck tumours; uncontrolled severe hypertension; receiving concomitant radiotherapy |
| No. of centres | 54 sites; between October 2005 and July 2007 | |
| Other references/aliases | Trial registration: ISRCTN09530309 | |
| Geographical setting | International: 10 countries (Argentina, Belarus, Brazil, Bulgaria, Croatia, India, Moldova, Romania, Russia, Ukraine) | |
| Duration of treatment | 12 weeks. The mean ± SD treatment duration was comparable in all three groups (75.0 ± 16.9 days epoetin theta vs. 71.0 ± 19.7 days epoetin beta vs. 70.5 ± 23.7 days placebo) | |
| Length of follow-up (if different) | NR | |
| Country of corresponding author | Germany | |
| Language of publication | English | |
| Sources of funding | Sponsored by BioGeneriX AG, a company of the ratiopharm Group SA | |
| Randomisation and allocation | Randomised using a computer-generated allocation schedule in a 1 : 1: 1 ratio stratified by country to double-blind treatment for 12 weeks with epoetin theta, epoetin beta or placebo. Randomisation list generated by the Department of Biostatistics, Merckle GmbH. Only the person administering the study medication was unblinded (because of the difference in dosing schemes). An unblinded data monitoring committee closely monitored for safety | |
| Treatment arms | |||
|---|---|---|---|
| Arm drug name/s | Epoetin theta | Epoetin beta | Placebo |
| n | 76 | 73 | 74 |
| Dose and frequency (once daily, twice daily, etc.) | 20,000 IU once per weeka | 450 IU/kg three times per week | Same schedule as epoetin theta for blinding purposes |
| Dose adjustment (yes/no) | Yes. After 4 weeks increase to 40 000 IU if Hb increase is < 1 g/dl, with a further increase to 60,000 IU if after the next 4 weeks there is still an insufficient response. Reduce by 50% if Hb increase is > 2 g/dl at 4 weeks. If Hb is > 13 g/dl, dose interruption or 50% dose reduction | Yes. After 4 weeks dose doubled if Hb increase is < 1 g/dl. Reductions the same as for epoetin theta | NA |
| Route of administration | Subcutaneous | Subcutaneous | Subcutaneous |
| Duration of epoetin treatment | 12 weeks | 12 weeks | NA |
| Adjuvant anaemia treatment | Iron substitution was allowed during the study | Iron substitution was allowed during the study | Iron substitution was allowed during the study |
| Transfusion trigger | At the discretion of the investigator but should be avoided if Hb level is ≥ 8.5 g/dl | At the discretion of the investigator but should be avoided if Hb level is ≥ 8.5 g/dl | At the discretion of the investigator but should be avoided if Hb level is ≥ 8.5 g/dl |
| Outcomes | |
|---|---|
| Primary outcome | Haematological response (increase in Hb of ≥ 2 g/dl from baseline without the benefit of a transfusion within the previous 4 weeks) |
| Other outcomes | Haematological response (partial Hb response of ≥ 1 g/dl from baseline; number of patients having a complete Hb response with the initial dose; time course of Hb, haematocrit and reticulocytes; dose of epoetin theta or epoetin beta at the time of complete/partial Hb response); RBCT (no. of patients requiring a RBCT; no. of blood units transfused); HRQoL [FACT-An (including FACT-G and FACT-F)]; AEs (safety laboratory variables, vital signs, incidence of AEs, adverse drug reactions, overall tolerability and screening for antidrug antibodies to epoetin theta and epoetin beta at the beginning and end of the study and 60 days after the end of the individual treatment period) |
| Analysis | |
|---|---|
| Statistical technique used | Logistic regression analysis with treatment and baseline Hb level as explanatory variables was performed to estimate the difference in the proportion of complete Hb responders for epoetin theta vs. placebo, epoetin beta vs. placebo and epoetin theta vs. epoetin beta in the confirmatory analysis of the primary end point. For other binary secondary efficacy end points the same logistic regression model as for the primary end point was used. Changes in quality of life (FACT score) from baseline to the end of the treatment period were compared pairwise among treatment groups with the Wilcoxon–Mann–Whitney test; treatment groups for other secondary end points were only compared descriptively |
| ITT analysis? | Yes. Full analysis set used for efficacy end points; no crossovers and end points reported for full patient numbers |
| Does statistical technique adjust for confounding? | NR; however, logistic regression analysis was adjusted for baseline Hb level to estimate the effects of treatment on Hb response |
| Power calculation (a priori sample calculation)? | Partial; sample size calculation given for the statistical superiority test comparing epoetin theta and placebo but not overall (two-sided α = 5%, assuming the actual Hb response rates for epoetin theta and placebo were 50% and 20%, respectively) |
| Attrition rate (loss to follow-up)? | Yes; placebo n = 21 withdrawals (n = 4 because of AEs, n = 12 patient request, n = 2 loss to follow-up, n = 3 other); epoetin beta n = 9 withdrawals (n = 1 because of AE, n = 7 patient request, n = 1 other); epoetin theta n = 12 withdrawals (n = 2 because of AEs, n = 2 patient request, n = 1 because of inclusion/exclusion criteria, n = 3 loss to follow-up, n = 4 other) |
| Was attrition rate adequately dealt with? | Unclear |
| No. (%) followed up from each condition? | NA; no follow-up reported |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Solid tumours | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Platinum-containing chemotherapy | ||||
| Adjuvant anaemia treatment | Iron | Iron substitution was allowed during the study | |||
| G-CSF | |||||
| Transfusion trigger | Hb ≤ 8.5 g/dl | ||||
| Hb inclusion criterion level | ≤ 11.0 g/dl | ||||
| Arm 1 = epoetin theta (n = 76) | Arm 2 = epoetin beta (n = 73) | Arm 3 = placebo (n = 74) | p-value | ||
| Sex, n (%) | |||||
| Male | 30 (39.5) | 22 (30.1) | 19 (25.7) | ||
| Female | 46 (60.5) | 51 (69.9) | 55 (74.3) | ||
| Age (years), mean ± SD, median (range) | 53.7 ± 10.3, 53.5 (19.0–76.0) | 57.3 ± 10.5, 57.0 (28.0–83.0) | 57.3 ± 11.5, 59.5 (26.0–76.0) | ||
| ECOG performance status, n (%) | |||||
| 0 | 6 (7.9) | 9 (12.3) | 5 (6.8) | ||
| 1 | 55 (72.4) | 40 (54.8) | 48 (64.9) | ||
| 2 | 15 (19.7) | 24 (32.9) | 20 (27.0) | ||
| 3 | 0 | 0 | 1 (1.4) | ||
| Hb (g/dl), mean ± SD | 9.6 ± 1.1 | 9.5 ± 0.8 | 9.4 ± 1.2 | ||
| Iron (U/l), median (range) | NR | NR | NR | ||
| Epoetin (mU/ml) | NR | NR | NR | ||
| Target Hb | NR | NR | NR | ||
| Most common tumour types, n (%) | |||||
| Ovarian epithelial cancer | 14 (18.4) | 21 (28.8) | 20 (27.0) | ||
| Gastric cancer | 6 (7.9) | 5 (6.8) | 7 (9.5) | ||
| Lung squamous cell carcinoma | 4 (5.3) | 5 (6.8) | 7 (9.5) | ||
| Breast cancer | 6 (7.9) | 3 (4.1) | 6 (8.1) | ||
| Ovarian epithelial cancer metastatic | 6 (7.9) | 6 (8.2) | 3 (4.1) | ||
| Most common on-study treatment | |||||
| Cisplatin | 55 (72.4) | 48 (65.8) | 42 (56.8) | ||
| Carboplatin | 22 (28.9) | 29 (39.7) | 24 (32.4) | ||
| Cyclophosphamide | 18 (23.7) | 17 (23.3) | 15 (20.3) | ||
| Etoposide | 20 (26.3) | 11 (15.1) | 14 (18.9) | ||
| Were intervention and control groups comparable? | No p-values reported; authors stated that ‘no relevant differences between treatment groups with regard to medical history, prior or concomitant medications, ECOG performance status, blood transfusions prior to study entry, concomitant diseases, tumour types and on-study chemotherapies’ were found (p. 48) | ||||
| Results | ||||
|---|---|---|---|---|
| Hb | ||||
| Hb at end of study (g/dl), mean (SD) | 11.2 (2) | 11.4 (2) | 9.6 (1.2) | |
| Change in Hb level (g/dl), mean (SD)a | 1.6 | 1.9 | 0.2 | |
| Complete Hb response without blood transfusion (increase of ≥ 2 g/dl from baseline), n (%) | 50 (65.8) | 52 (71.2) | 15 (20.3) | |
| Epoetin beta vs. placebo | OR 10.25 (95% CI 4.86 to 22.83) | < 0.0001 | ||
| Epoetin theta vs. placebo | OR 8.06 (95% CI 3.89 to 17.63) | < 0.0001 | ||
| Epoetin theta vs. epoetin beta | OR 0.79 (95% CI 0.39 to 1.58) | 0.5004 | ||
| Complete Hb response without blood transfusion and dose adjustment, n (%) | 26 (34.2) | 29 (39.7) | 8 (10.8) | |
| Epoetin beta vs. placebo | OR 5.40 (95% CI 2.35 to 13.68) | 0.0001 | ||
| Epoetin theta vs. placebo | OR 4.24 (95% CI 1.84 to 10.76) | 0.0012 | ||
| Epoetin theta vs. epoetin beta | OR 0.79 (95% CI 0.40 to 1.53) | 0.4765 | ||
| Partial Hb response without blood transfusion (increase of ≥ 1 g/dl from baseline), n (%) | 69 (90.8) | 66 (90.4) | 37 (50) | |
| Epoetin beta vs. placebo | OR 9.39 (95% CI 4.01 to 24.93) | < 0.0001 | ||
| Epoetin theta vs. placebo | OR 9.8 (95% CI 4.19 to 26.00) | < 0.0001 | ||
| Transfusions | ||||
| Received blood transfusion, n (%) | 8 (10.5) | 9 (12.3) | 18 (24.3) | |
| Epoetin beta vs. placebo | NR | 0.1042 | ||
| Epoetin theta vs. placebo | OR 0.38 (95% CI 0.14 to 0.95) | 0.0433 | ||
| Epoetin theta vs. epoetin beta | OR 1.04 (95% CI 0.34 to 3.20) | 0.9394 | ||
| No. of blood units transfused, mean (SD) | 3.3 (2.2) | 1.8 (0.7) | 2.8 (2.9) | |
| HRQoL | ||||
| FACT-An including FACT-F and FACT-G | NR | NR | NR | |
| Adverse effects of treatment, n (%) | ||||
| Any TEAE | 58 (76.3) | 63 (86.3) | 63 (85.1) | |
| TEADR | 14 (18.4) | 16 (21.9) | 13 (17.6) | |
| Serious TEAE | 9 (11.8) | 9 (12.3) | 15 (20.3) | |
| Serious TEADR | 1 (1.3) | 1 (1.4) | 0 | |
| Deathb | 5 (6.6) | 4 (5.5) | 12 (16.2) | |
| Discontinuation | 4 | 3 | 6 | |
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Yes |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | Uncleara |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Unclear, no p-values reported; similar ECOG scores between groups; other characteristics similar |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | Yesb |
| 6. Were the outcome assessors blind to treatment allocation? | Yes |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Partially (variability can be calculated from data presented in the paper) |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | Yes; quality-of-life data not reported |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes; however four and three participants withdrew for unspecified ‘other’ reasons in the epoetin and placebo groups respectively |
| Other | |
|---|---|
| Generalisability | Yes |
| Author conclusions | No conclusions regarding epoetin beta. Epoetin theta with a weekly starting dose of 20,000 IU is superior to placebo in terms of complete Hb response without blood transfusion. Epoetin theta is a safe and effective treatment for the treatment of anaemia resulting from platinum-based chemotherapy in patients with solid tumours |
| Reviewer comments | The differences between epoetin beta and placebo and between epoetin beta and epoetin theta were estimated with the same statistical model |
| EndNote ref. ID: 436 | Malignancy type: solid tumour or non-myeloid haematological tumour | |
| Treatment: epoetin theta | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Tjulandin 201177 | n = 186 |
| Objective | The objective of this study was to demonstrate the superiority of epoetin theta compared with placebo for efficacy during the treatment period of 12 weeks in patients with solid tumours or non-myeloid haematological malignancies receiving non-platinum-based chemotherapy | Inclusion criteria: Age ≥ 18 years; histologically or cytologically proven diagnosis of a solid tumour or non-myeloid haematological tumour; anaemia caused by non-platinum-based chemotherapy defined by a documented Hb concentration of ≤ 11 g/dl after the last chemotherapy cycle prior to inclusion; at least one previous non-platinum-based chemotherapy cycle as treatment for the current malignancy during the last 4 weeks; ECOG performance status = 0, 1, 2 or 3 Exclusion criteria: Any other primary haematological disorder that would cause anaemia; head and neck tumours; uncontrolled severe hypertension; concomitant radiotherapy |
| No. of centres | 72 sites; between November 2005 and May 2007 | |
| Other references/aliases | Trial registration: ISRCTN08063129 | |
| Geographical setting | International; 10 countries (Argentina, Belarus, Brazil, Bulgaria, Croatia, India, Moldova, Romania, Russia, Ukraine) | |
| Duration of treatment | 12 weeks. The mean ± SD treatment duration was comparable in both groups (71.9 ± 6.9 days placebo vs.72.1 ± 15.7 days epoetin theta | |
| Length of follow-up (if different) | NA | |
| Country of corresponding author | Germany | |
| Language of publication | English | |
| Sources of funding | Sponsored by BioGeneriX AG, a company of the ratiopharm Group SA | |
| Randomisation and allocation | A total of 186 patients were randomised using a computer-generated allocation schedule in a 1 : 1 ratio stratified by country to double-blind treatment for 12 weeks with either epoetin theta (n = 95) or placebo (n = 91). All persons involved in the conduct of the study were blinded with respect to the study medication. The investigator and all other study personnel were kept blinded and performed all assessments of the patient without knowledge of treatment. An unblinded independent data safety monitoring committee closely monitored safety to ensure that patients were not exposed to an unjustifiable risk | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Epoetin theta | Placebo |
| n | 95 | 91 |
| Dose and frequency (once daily, twice daily, etc.) | 20,000 IU once per week. The mean ± SD average weekly dose was 25,905 ± 10,956 IU in the epoetin theta group | |
| Dose adjustment (yes/no) | Yes. After 4 weeks increase to 40,000 IU if Hb increase is < 1 g/dl, with a further increase to 60,000 IU if after the next 4 weeks there is still an insufficient response. Reduce by 50% if Hb increase is > 2 g/dl at 4 weeks. If Hb level is > 13 g/dl, dose interruption or 50% dose reduction | Yes, according to the same schedule as for epoetin theta for blinding purposes |
| Route of administration | Subcutaneous | Subcutaneous |
| Duration of epoetin treatment | 12 weeks | 12 weeks |
| Adjuvant anaemia treatment | Iron substitution was allowed during the study | Iron substitution was allowed during the study |
| Transfusion trigger | At the discretion of the investigator but should be avoided if Hb ≥ 8.5 g/dl | At the discretion of the investigator but should be avoided if Hb ≥ 8.5 g/dl |
| Outcomes | ||
| Primary outcome | Haematological response (increase in Hb of ≥ 2 g/dl from baseline without the benefit of a transfusion within the previous 4 weeks) | |
| Other outcomes | Haematological response (partial Hb response of ≥ 1 g/dl from baseline; no. of patients having a complete and partial Hb response with the initial dose; time course of Hb, haematocrit and reticulocytes; dose of epoetin theta at the time of Hb response); RBCT (no. of patients requiring a RBCT; no. of blood units transfused); HRQoL [FACT-An (including FACT-G and FACT-F)]; AEs (immunogenicity was assessed by a predefined cascade of antibody assays; this cascade was structured into a sequential scheme comprising screening, confirmation and characterisation of clinical specimens; confirmed positive samples were investigated for neutralising antibodies in a cellular assay using an erythropoietin-dependent UT-7 cell line) | |
| Analysis | |
|---|---|
| Statistical technique used | A logistic regression analysis with treatment and type of cancer as explanatory variables and baseline Hb value as a continuous variable was performed to estimate the difference in the proportion of complete Hb responders between the epoetin theta group and the placebo group in the confirmatory analysis of the primary efficacy end point. For the primary efficacy end point a subgroup analysis with type of malignancy (solid, non-myeloid haematological) was performed. For the other binary secondary efficacy end points the same logistic regression model as for the primary end point was estimated. Changes in quality of life from baseline to end of study were compared pairwise with the Wilcoxon–Mann–Whitney test. Other secondary efficacy end points were compared only descriptively. Descriptive p-values were calculated with appropriate statistical tests but were regarded as supportive only |
| ITT analysis? | Yes. Full analysis set used for efficacy end points; no crossovers and end points reported for full patient numbers |
| Does statistical technique adjust for confounding? | NR; however, logistic regression analysis was adjusted for baseline Hb level to estimate the effects of treatment on Hb response |
| Power calculation (a priori sample calculation)? | Partial; sample size calculation given for statistical superiority test comparing epoetin theta and placebo but not overall: n = 80 patients per treatment group to achieve a power of 90% for the statistical superiority test comparing epoetin theta and placebo assuming a response rate of 45% for epoetin theta and 20% for placebo |
| Attrition rate (loss to follow-up)? | Yes; n = 25 prematurely discontinued: n = 15 in placebo group (n = 6 AEs, n = 4 patient request, n = 2 lack of efficacy, n = 1 lost to follow-up and n = 2 other) and n = 10 in epoetin theta group (n = 4 AEs, n = 5 patient request, n = 0 lack of efficacy, n = 1 lost to follow-up and n = 0 other) |
| Was attrition rate adequately dealt with? | Unclear; full analysis set used for efficacy end points |
| No. (%) followed up from each condition? | NA; no follow-up reported |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Solid tumour or non-myeloid haematological tumour | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Non-platinum-based chemotherapy | ||||
| Adjuvant anaemia treatment | Iron | Iron substitution was allowed during the study | |||
| G-CSF | NR | ||||
| Transfusion trigger | Hb ≤ 8.5 g/dl at the discretion of the investigator | ||||
| Hb inclusion criterion level | ≤ 11.0 g/dl | ||||
| Arm 1 = epoetin theta (n = 95) | Arm 3 = placebo (n = 91) | Notes | p-value | ||
| Sex, n (%) | |||||
| Male | 30 (31.6) | 34 (37.4) | |||
| Female | 65 (68.4) | 57 (62.6) | |||
| Age (years), mean ± SD, median (range) | 56.9 ± 14.7, 60 (18.0–83.0) | 55.8 ± 14.3, 57.0 (18.0–82.0) | |||
| ECOG performance status, n (%) | |||||
| 0 | 14 (14.7) | 9 (9.9) | |||
| 1 | 53 (55.8) | 60 (65.9) | |||
| 2 | 28 (29.5) | 21 (23.1) | |||
| 3 | 0 | 1 (1.1) | |||
| Hb (g/dl), mean ± SD | 9.2 ± 1.3 | 9.1 ± 1.3 | |||
| Iron (U/l), median (range) | NR | NR | |||
| Epoetin (mU/ml) | NR | NR | |||
| Target Hb (g/dl) | NR | NR | |||
| Most common malignancies, n (%) | |||||
| Multiple myeloma | 19 (20) | 17 (18.7) | |||
| Breast cancer | 16 (16.8) | 17 (18.7) | |||
| Chronic lymphocytic leukaemia | 5 (5.3) | 7 (7.7) | |||
| Gastric cancer | 6 (6.3) | 3 (3.3) | |||
| Most common on-study chemotherapy, n (%) | |||||
| Cyclophosphamide | 50 (52.6) | 47 (51.6) | |||
| Doxorubicin | 32 (33.7) | 29 (31.9) | |||
| Vincristine | 26 (27.4) | 28 (30.8) | |||
| Dexamethasone | 22 (23.2) | 21 (23.1) | |||
| Prednisolone | 14 (14.7) | 26 (28.6) | |||
| Were intervention and control groups comparable? | No p-values reported; authors stated that ‘There were no relevant differences between treatment groups with regard to medical history, prior or concomitant medications, ECOG performance status, previous chemotherapy, concomitant diseases, and primary malignant disease (Table 1). There were no clinically noteworthy differences between the treatment groups with regard to on-study chemotherapies’ (p. 35) | ||||
| Results | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hb | |||||||||||||||||||||||||||||||
| Hb at end of study (g/dl), mean (SD) | 11.3 (2) | < 10 | |||||||||||||||||||||||||||||
| Change in Hb levels (g/dl), mean (SD) | 2.1 (NR) | < 0.0001 | |||||||||||||||||||||||||||||
| Results for Hb change from baseline presented graphically (Figure 3) Hb (estimated from Figure 3)Arm 1 = epoetin theta (n = 95)Arm 3 = placebo (n = 91)MeanSEMSDMeanSEMSDAt the end of study (g/dl)11.310.222.149.890.222.10SEM, standard error of the mean. |
Hb (estimated from Figure 3) | Arm 1 = epoetin theta (n = 95) | Arm 3 = placebo (n = 91) | Mean | SEM | SD | Mean | SEM | SD | At the end of study (g/dl) | 11.31 | 0.22 | 2.14 | 9.89 | 0.22 | 2.10 | SEM, standard error of the mean. | ||||||||||||||
| Hb (estimated from Figure 3) | Arm 1 = epoetin theta (n = 95) | Arm 3 = placebo (n = 91) | |||||||||||||||||||||||||||||
| Mean | SEM | SD | Mean | SEM | SD | ||||||||||||||||||||||||||
| At the end of study (g/dl) | 11.31 | 0.22 | 2.14 | 9.89 | 0.22 | 2.10 | |||||||||||||||||||||||||
| SEM, standard error of the mean. | |||||||||||||||||||||||||||||||
| Complete Hb response without blood transfusion (increase of ≥ 2 g/dl from baseline), n (%) | 69 (72.6) | 23 (25.3) | |||||||||||||||||||||||||||||
| Epoetin beta vs. placebo | Hb-adjusted OR 7.944 (95% CI 4.182 to 15.632) | <0.0001 | |||||||||||||||||||||||||||||
| Complete Hb response without blood transfusion and dose adjustment (increase of ≥ 2 g/dl from baseline), n (%) | 43 (45.3) | 9 (9.9) | |||||||||||||||||||||||||||||
| Epoetin beta vs. placebo | OR 7.728 (95% CI 3.59 to 18.285) | < 0.0001 | |||||||||||||||||||||||||||||
| Partial Hb response without blood transfusion (increase of ≥ 1 g/dl from baseline), n (%) | 78 (82.1) | 56 (61.5) | |||||||||||||||||||||||||||||
| Epoetin beta vs. placebo | OR 2.841 (95% CI 1.462 to 5.694) | 0.0025 | |||||||||||||||||||||||||||||
| Partial Hb response without blood transfusion and dose adjustment, n (%) | 56 (58.9) | 24 (26.4) | |||||||||||||||||||||||||||||
| Epoetin beta vs. placebo | OR 4.028 (95% CI 2.179 to 7.632) | < 0.0001 | |||||||||||||||||||||||||||||
| Transfusions | |||||||||||||||||||||||||||||||
| Patients received blood transfusions, n (%) | 13 (13.7) | 23 (25.3) | |||||||||||||||||||||||||||||
| Epoetin beta vs. placebo | OR 0.352 (95% CI 0.133 to 0.868) | 0.0277 | |||||||||||||||||||||||||||||
| No. of blood units transfused, mean (SD) | 3.5 (3.5) | 4.1 (2.8) | |||||||||||||||||||||||||||||
| HRQoL | |||||||||||||||||||||||||||||||
| FACT-An total, mean (SD) | 6.3 (21.7) | 0.6 (22) | 0.243 | ||||||||||||||||||||||||||||
| FACT-An trial outcome index, mean (SD) | 5.6 (17.1) | 1.2 (18.8) | 0.222 | ||||||||||||||||||||||||||||
| FACT-F, mean (SD) | 2.9 (7.9) | 0.6 (8.8) | 0.142 | ||||||||||||||||||||||||||||
| FACT-G, mean (SD) | 3.0 (12.7) | –0.2 (12.4) | 0.224 | ||||||||||||||||||||||||||||
| AEs | |||||||||||||||||||||||||||||||
| Any AE | 76 (80.0) | 71 (78.0) | |||||||||||||||||||||||||||||
| Related AE = ADR | 27 (28.4) | 18 (19.8) | |||||||||||||||||||||||||||||
| Serious AE | 11 (11.6) | 14 (15.4) | |||||||||||||||||||||||||||||
| Serious ADR | 0 | 1 (1.1) | |||||||||||||||||||||||||||||
| Deatha | 6 (6.3) | 5 (5.5) | |||||||||||||||||||||||||||||
| Discontinuationb | 4 (4.2) | 6 (6.6) | |||||||||||||||||||||||||||||
| Hypertension | 8 (8.4) | 1 (1.1) | < 0.05 | ||||||||||||||||||||||||||||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Yes |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Unclear – no p-values reported; authors stated that ‘There were no relevant differences between treatment groups with regard to medical history, prior or concomitant medications, ECOG performance status, previous chemotherapy, concomitant diseases, and primary malignant disease (Table 1). There were no clinically noteworthy differences between the treatment groups with regard to on-study chemotherapies’ (p. 35) |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | Yes |
| 6. Were the outcome assessors blind to treatment allocation? | Yes. An unblinded independent data safety monitoring committee closely monitored safety to ensure that patients were not exposed to an unjustifiable risk |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Partially (variability can be calculated from data presented in the paper) |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes, apart from HRQoL (89.5–97.9% and 85.7–96.7% of participants analysed in the epoetin and placebo groups, respectively) |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Yes |
| Other | |
|---|---|
| Generalisability | Yes |
| Author conclusions | Epoetin theta showed a superior efficacy to placebo in terms of complete Hb response without blood transfusion within the previous 4 weeks. Treatment with epoetin theta resulted in a statistically significant increase in mean Hb level compared with placebo. The overall frequencies of AEs were similar in both treatment groups |
| Reviewer comments | |
| EndNote ref. ID: 961 | Malignancy type: breast cancer | |
| Treatment: darbepoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Untch 201178 | n = 736 enrolled, with 733 randomly allocated |
| Objective | Latin square design – in a second randomisation, the short- and long-term effects of primary use of darbepoetin alfa independent from Hb levels on tumour response and safety were investigated. The toxicity and response data are described here and the effect on DFS and OS is reported in Untch and colleagues80 | Inclusion criteria: Age 18–65 years with histologically confirmed primary breast cancer by core biopsy; the primary tumour had to be 2 cm based on either clinical or ultrasound measurement; inflammatory breast cancer was also included; no systemic metastasis according to chest radiography, sonography or computed tomography scan of upper abdomen and bone scan; ECOG score of< 2; adequate organ function: aspartate aminotransferase and bilirubin = 1.5 × upper limit, white blood cells = 3000/µl, neutrophils = 1000/µl, platelets = 100,000/µl and serum creatinine < 2.0 mg/dl; normal left ventricular ejection fraction Exclusion criteria: NR (but see inclusion criteria above) |
| No. of centres | 78 | |
| Other references/aliases | PREPARE trial, Untch 2011,80 NCT00544232 | |
| Geographical setting | Germany | |
| Duration of treatment | 26 weeks; there were 24 weeks of chemotherapy – darbepoetin alfa was administered with the first dose of epirubicin (day 1) until 14 days after the last dose of paclitaxel | |
| Length of follow-up (if different) | Median follow-up 43.5 months | |
| Country of corresponding author | Germany | |
| Language of publication | English | |
| Sources of funding | Amgen Inc. Bristol-Myers Squibb | |
| Randomisation and allocation | Latin square design – patients were randomised in a 1 : 1 allocation to receive standard dose or dose-intensified preoperative chemotherapy. Patients within each treatment arm were further randomised in a 1 : 1 allocation to receive darbepoetin alfa or no darbepoetin alfa therapy | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Darbepoetin alfa | Control (standard care) |
| n | 356 | 377 |
| Dose and frequency (once daily, twice daily, etc.) | 4.5 µg/kg every two weeks | NA |
| Dose adjustment (yes/no) | To achieve the target Hb level of 12.5–13 g/dl, the dose was doubled if the Hb increase was < 1 g/dl during the first 4 weeks or discontinued if Hb was > 14 g/dl. Treatment was re-induced at 50% of the dose if Hb was ≤ 13.0 g/dl | NA |
| Route of administration | NR | |
| Duration of epoetin treatment | Starting with the first dose of epirubicin (day 1) until 14 days after the last dose of paclitaxel | NA |
| Adjuvant anaemia treatment | 200 mg oral iron daily | NA |
| Transfusion trigger | NR | None |
| Outcomes | |
|---|---|
| Primary outcome | |
| Other outcomes | RBCT; tumour response [pCR at surgery (defined as regression Grades 4–5 according to the modified regression grading system)]; survival (DFS, OS); AEs (haematological and non-haematological, cardiovascular and thromboembolic) |
| Analysis | |
|---|---|
| Statistical technique used | Comparisons between intensified or standard chemotherapy and between treatments with and without darbepoetin alfa used the chi- squared test. All secondary end point tests were two sided and 95% CIs were provided for relevant estimates. The change in Hb level difference between the treatments with and without darbepoetin alfa used ANCOVA with baseline Hb level as a covariate. Binary logistic regression analysis was employed to adjust for major predictive factors. Kaplan–Meier curves were used to estimate DFS and OS probabilities. DFS was defined as the time from informed consent to first documentation of relapse or death from any cause. OS was the time from the date of informed consent to the date of death from any cause. Local DFS was defined as time in weeks between the date of signing the informed consent and the date of local recurrence. Patients with no local recurrence reported were censored at the date of the last contact |
| ITT analysis? | Yes. The change in Hb level was analysed on the full analysis set (all patients who met all eligibility criteria and were randomly allocated to the chemotherapy treatment) using the last observation carried forward approach. Patients who did not meet eligibility criteria but who received at least one dose of study treatment were included only in the safety (toxicity) evaluation |
| Does statistical technique adjust for confounding? | Yes; OS and DFS were analysed adjusted for baseline factors. Binary logistic regression analysis was employed to adjust for major predictive factors. For multivariable analysis, Cox proportional hazards models for adjusting survival end points were used; adjustments were made for age, hormone receptor status, clinical tumour size and nodal status, grade, chemotherapy arm, darbepoetin alfa application and pCR |
| Power calculation (a priori sample calculation)? | Yes; 720 patients needed to detect an improvement of 10% in PFS with the dose-dense regimen with an expected proportion of relapses of 30% after 5 years in the standard treatment arm. This is equal to a HR of 1.4 with a type 1 error of α = 5% using a one-sided test |
| Attrition rate (loss to follow-up)? | Partially – until the point of surgery (as reported in supplemental online materials). In total, 733 participants were randomly allocated and 19 did not receive any study treatment; 318/356 patients randomly allocated to darbepoetin alfa actually received the treatment. Most of the patients had surgery after chemotherapy: n = 326 in the darbepoetin group and n = 343 in the control group remained at that point |
| Was attrition rate adequately dealt with? | Partially; the change in Hb level was analysed on the ‘full analysis set’ using the last observation carried forward approach, but patient flow and numbers used in the analysis were difficult to follow and remain unclear |
| No. (%) followed up from each condition? | NR |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Breast cancer | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Preoperative chemotherapy of epirubicin, cyclophosphamide and paclitaxel each 3-weekly (n = 370) for four cycles or epirubicin and paclitaxel with pegfilgrastim followed by CMF (combination chemotherapy with cyclophosphamide, methotrexate and fluorouracil) each 2-weekly and for three cycles (n = 363). There were eight and nine planned cycles in the standard and intensified regimen respectively | ||||
| Adjuvant anaemia treatment | Iron | 200 mg oral iron in the darbepoetin alfa arm | |||
| G-CSF | Yes, in the intensified regimen chemotherapy only (5 µg/kg/day) | ||||
| Transfusion trigger | NR | ||||
| Hb inclusion criterion level | NR | ||||
| Arm 1 = darbepoetin alfa (n = 356) | Arm 2 = control (n = 377) | Notes | p-value | ||
| Sex | NR | NR | |||
| Age (years), median (range) | NR; median age reported separately for the intensified and standard chemotherapy arms only; the median age at randomisation was 48 years (range 23–65 years) | ||||
| < 50 years, n (%) | 183 (51.4) | 213 (56.4) | |||
| ≥ 50 years, n (%) | 173 (43.6) | 164 (43.6) | |||
| ECOG performance status, n (%) | |||||
| 0 | 306 (86.0) | 323 (85.7) | |||
| 1 | 20 (5.6) | 29 (7.7) | |||
| 2/3 | 2 (0.6) | 4 (1.1) | |||
| Missing | 28 (7.9) | 21 (5.6) | |||
| Hb (g/dl), mean (SD)a | (n = 333) 13.64 (1.17) | (n = 360) 13.61 (1.16) | As reported in supplemental online materials | ||
| Clinical tumour stage | |||||
| T1–T3 | 315 (88.5) | 334 (88.6) | |||
| T4 | 27 (7.6) | 31 (8.2) | |||
| Missing | 14 (3.9) | 12 (3.2) | |||
| Tumour grade | |||||
| 1–2 | 118 (33.1) | 120 (31.8) | |||
| 3 | 97 (27.3) | 117 (31) | |||
| Missing | 141 (39.6) | 140 (37.2) | |||
| Were intervention and control groups comparable? | No p-values are reported; authors stated that ‘baseline characteristics were similar in the treatment arms’ (p. 1991). It is assumed that this refers to the chemotherapy arms and it is not clear whether it also refers to the epoetin vs. no epoetin arms | ||||
| Results | |||
|---|---|---|---|
| Hb | |||
| Hb at the end of chemotherapy (g/dl), mean (SD)a | (n = 342) 13.59 (1.7) | (n = 368) 12.61 (1.38) | |
| Change in Hb (g/dl), mean (SD) (95% CI)b | (n = 330) –0.07 (0.11) (–0.28 to 0.14) | (n = 359) –0.98 (0.07) (–1.12 to –0.84) | |
| Tumour response, n (%)c | |||
| pCR | 57 (16) | 60 (15.9) | 0.972 (pCR vs. no pCR) |
| CR (by most appropriate method) | 46 (12.9) | 54 (14.3) | 0.580 |
| Toxicity (safety analysis set), n (%) | (n = 318) | (n = 396) | |
| Cardiovascular and thromboembolic events | 20 (6.3) | 17 (4.3) | 0.232 |
| Thromboembolic events: embolism/thrombosis | 18 (5.7) | 12 (3) | 0.055 |
| Nausea grades 1–4 | 251 (78.9) | 315 (79.5) | |
| Nausea grades 3–4 | 19 (6.0) | 19 (4.8) | |
| Anaemia grades 1–4 | 31 (9.7) | 35 (8.8) | |
| Anaemia grades 3–4 | 1 (0.3) | 1 (0.3) | |
| Transfusions, n | 1 | 0 | |
| Survivald | |||
| DFS | (n = 345) | (n = 369) | |
| Estimated at 3 years (%) | 74.3 | 78 | HR 1.31 (95% CI 0.99 to 1.74); p = 0.061 |
| Events, n | 106 | 90 | |
| Events adjusted for baseline, n (%)e | 104 (30) | 88 (24) | HR 1.23 (95% CI 0.83 to 1.83); p = 0.296 in multivariate analyses adjusted for chemotherapy, age, initial tumour size, grading, ER/PgR status |
| DFS subgroup analyses: no pCR vs. pCR (better outcome observed for patients who achieved a pCR)e | With darbepoetin alfa: HR 2.38 (95% CI 1.2 to 4.71); p = 0.013; without darbepoetin alfa: HR 2.13 (95% CI 1.03 to 4.41); p = 0.041 | ||
| OS | |||
| Estimated at 3 years (%) | 88 | 91.8 | HR 1.33 (95% CI 0.91 to 1.95); p = 0.139 |
| Events, n | 59 | 48 | HR 1.33 (95% CI 0.91 to 1.95); p = 0.139 in univariate analysis |
| Events adjusted for baseline, n (%)e | 59 (17) | 48 (13) | HR 1.24 (95% CI 0.71 to 2.19); p = 0.4502 in multivariate analyses adjusted for chemotherapy, age, initial tumour size, grading, ER/PgR status |
| Subgroup analyses: no pCR vs. pCR (better outcome observed for patients who achieved a pCR)b | With darbepoetin alfa: HR 4.02 (95% CI 1.26 to 12.85); p = 0.019; without darbepoetin alfa: HR 3.08 (95% CI 0.95 to 9.92); p = 0.060 | ||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | NR |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | NR – p-values for baseline comparisons are not reported; authors stated that ‘baseline characteristics were similar in the treatment arms’ (p. 1991). It is assumed that this refers to the chemotherapy arms and it is not clear whether it also refers to the epoetin vs. no epoetin arms |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | No (open label) |
| 6. Were the outcome assessors blind to treatment allocation? | No (open label) |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Yes |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yes |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Partially – only until the point of surgery; n = 326 darbepoetin alfa group and n = 343 control group |
| Other | |
|---|---|
| Generalisability | |
| Author conclusions | Primary use of darbepoetin alfa did not affect pCR whereas darbepoetin alfa might have detrimental effects on DFS. Patients should not be treated with ESAs in the neoadjuvant setting under the assumption of better tumour oxygenation because a negative influence of darbepoetin alfa on DFS cannot completely be ruled out. The dose-intensified regimen was found to be superior to conventional chemotherapy in terms of pCR, but no difference in DFS or OS was found |
| Reviewer comments | Patient flow and numbers used in analysis were difficult to follow and remain unclear |
| EndNote ref. ID: 2698 (HTA) | Malignancy type: lung cancer | |
| Treatment: darbepoetin alfa | ||
| Study design | Participants | |
|---|---|---|
| Author, year | Vansteenkiste 200273 | n = 314 |
| Objective | The safety and efficacy of darbepoetin alfa compared with placebo in patients with lung cancer receiving chemotherapy | Inclusion criteria: Lung cancer; expected to receive at least 12 additional weeks of platinum-containing chemotherapy; age ≥ 18 years; life expectancy of at least 6 months; ECOG performance status 0 –2; anaemia (i.e. Hb ≤ 11.0 g/dl) primarily because of cancer or chemotherapy; adequate serum folate, vitamin B12, ferritin, and saturated transferrin levels; adequate renal and hepatic function Exclusion criteria: Iron deficiency; primary or metastatic malignancy of the central nervous system; more than two RBCTs within 4 weeks of randomisation or received any RBCT within 2 weeks of randomisation; rHuEPO therapy within 8 weeks of randomisation or any previous treatment with darbepoetin alfa; pregnant, breastfeeding or not using adequate birth control measures; history of seizure disorders, active cardiac disease, uncontrolled hypertension, active infection or inflammation or a primary hematological disorder as the cause of the present anaemia |
| No. of centres | 70 | |
| Other references/aliases | NESP 980297, Tchekmedyian 2003202 [examined the correlation between psychological distress (anxiety and depression) and fatigue over time], secondary analysis in Vantenkeenste 200484 (determined whether the degree of benefit obtained from treatment with darbepoetin alfa is affected by a patient’s Hb level at the start of treatment) | |
| Geographical setting | Australia, Canada, Western Europe and Central and Eastern Europe | |
| Duration of treatment | 12 weeks | |
| Length of follow-up (if different) | 4-week follow-up period after the last dose of study drug and long-term follow-up to determine tumour status and survival (in this paper 6 months after the last patient completed the study; planned for at least 1 year) | |
| Country of corresponding author | Belgium | |
| Language of publication | English | |
| Sources of funding | R Pirker received research and travel grants and consulting fees from Amgen, Inc. and D Tomita holds stock in Amgen, Inc., the maker of darbepoetin alfa and epoetin alfa | |
| Randomisation and allocation | A double-blind, placebo-controlled, randomised Phase III study; patients were randomly assigned by a central randomisation service for all sites in a 1 : 1 ratio. Randomisation was stratified by tumour type (small-cell lung cancer or non-small-cell lung cancer) and geographical region (Australia, Canada, Western Europe or Central and Eastern Europe) | |
| Treatment arms | ||
|---|---|---|
| Arm drug name(s) | Darbepoetin alfa | Placebo |
| n | 156 | 158 |
| Dose and frequency (once daily, twice daily, etc.) | 2.25 µg/kg/week | Volume equivalent to darbepoetin alfa treatment |
| Dose adjustment (yes/no) | Yes. At week 6 if Hb was ≤ 1.0 g/dl over baseline Hb the dose of the study drug was doubled to 4.5 µg/kg/week, or the volume equivalent, beginning at week 7 (and continuing for the remainder of the study). Treatment was withheld if Hb was > 15.0 g/dl for men or > 14.0 g/dl for women. Once Hb decreased to ≤ 13.0 g/dl, the dose was reinstated at 50% | Yes, same as darbepoetin alfa (see above) |
| Route of administration | Subcutaneous | Subcutaneous |
| Duration of epoetin treatment | 12 weeks | |
| Adjuvant anaemia treatment | ||
| Transfusion trigger | Recommended when Hb was ≤ 8.0 g/dl and based on clinical judgement (transfusion policies can vary widely from country to country) | As for darbepoetin alfa |
| Outcomes | ||
| Primary outcome | RBCT (proportion of participants who received a RBCT during a specific time period – from week 5 until the end of treatmenta) | |
| Other outcomes | Haematological response (haematopoietic response,b Hb collected weekly); RBCT (the incidence of RBCT from week 1 until the end of treatment, the incidence of transfusion or Hb concentration ≤ 8.0 g/dl, number of units of blood transfused); tumour response (tumour status and survival information are being collected during an open-label, long-term follow-up period); survival (disease progression and survival were also assessed quarterly for a minimum of 1 year if applicable); HRQoL (FACT-F, collected every 3–4 weeks on the first day of each cycle of chemotherapy, before any other study procedures); AEs (AE profile; incidence and duration of hospitalisation) | |
| Analysis | |
|---|---|
| Statistical technique used | Kaplan–Meier estimates were used for the proportion of patients who received at least one transfusion during week 5 until the end of treatment and for secondary transfusion-related end points and OS and PFS. The SE of the Kaplan–Meier proportion was calculated using Greenwood’s formula; 95% CIs were also reported. Efficacy end points were analysed with and without adjusting for the two factors used to stratify the randomisation: tumour type and geographical region. Results of both types of analyses were consistent and so only the results of the unstratified analyses are presented. Cox proportional hazards and logistic regression were used to compare treatment groups after adjusting for tumour type, geographical region and other potentially prognostic factors once it had been determined that data complied with assumptions for this method. No adjustments were made for multiple significance tests. The percentage of change from baseline for the FACT–F score was analysed as two dichotomous variables (any improvement and at least a 25% improvement) in patients who had the baseline and at least one post-treatment score using the uncorrected chi-squared test. Safety was evaluated in all patients who received at least one dose of study drug |
| ITT analysis? | NR. All patients randomly assigned into the study who received at least one dose of study drug were included in the analyses. In total, 314 participants received the study drug and were included in the analysis for all end points (including OS and PFS). However this does not seem to apply to analyses of FACT-F. However, in the analysis of transfusions during week 5 until the end-of-treatment phase, patients who withdrew (n = 17) before study day 29 were excluded. In total, 297 participants (93%) completed the first 28 days of the study and were included in the analysis of the primary end point |
| Does statistical technique adjust for confounding? | NR |
| Power calculation (a priori sample calculation)? | Yes; 90% power to detect a 50% reduction (from 40% to 20%) in the proportion of participants with at least one transfusion during week 5 until the end of treatment (anticipated that 30% of patients would withdraw) |
| Attrition rate (loss to follow-up)? | Yes, CONSORT flow diagram provided. A total of 101 participants withdrew from the study (49 in the darbepoetin alfa group and 52 in the placebo group). Reasons for withdrawal included death, tumour progression, chemotherapy delayed or discontinued, AEs, withdrew consent, administrative decision and loss-to-follow-up. The numbers of participants withdrawn before study day 29 were also reported |
| Was attrition rate adequately dealt with? | NR |
| No. (%) followed up from each condition? | Partially |
| Baseline characteristics | |||||
|---|---|---|---|---|---|
| Malignancy type (e.g. solid/solid head, neck, lung, ovarian, cervical/haematological/myelodysplastic syndrome/mixed) | Lung cancer | ||||
| Treatment (e.g. chemotherapy platinum/non-platinum based; chemotherapy + radiotherapy; no specific malignancy treatment; NR) | Platinum-based chemotherapy | ||||
| Adjuvant anaemia treatment | Iron | NR | |||
| G-CSF | NR | ||||
| Transfusion trigger | Recommended when Hb ≤ 8.0 g/dl and based on clinical judgement | ||||
| Hb inclusion criterion level | < 11 g/dl | ||||
| Arm 1 = darbepoetin alfa (n = 156) | Arm 2 = placebo (n = 158) | Notes | p-value | ||
| Sex, n (%) | |||||
| Male | 111 (71) | 116 (73) | |||
| Female | 45 (29) | 42 (27) | |||
| Age (years), mean (SD), median (range) | 61.6 (9.2), 62.5 (39–80) | 61.3 (8.8), 61 (36–79) | |||
| WHO/ECOG performance status, n (%) | |||||
| 0 | 22 (14) | 23 (15) | |||
| 1 | 109 (70) | 98 (62) | |||
| 2 | 24 (15) | 37 (23) | |||
| > 2 | 1 (1) | 0 | |||
| Hb (g/dl), mean (SD), median (range) | 10.28 (1.08), 10.4 (7.4–13.6) | 9.93 (1.01), 10.15 (6.6–12.3) | |||
| Iron (U/l), median (range) | |||||
| Epoetin (mU/ml) | |||||
| Target Hb | |||||
| Ferritin (µg/l), mean (SD), median (range) | 552.22 (453.45), 431 (36–3046) | 534.5 (528.1), 402 (14–4895) | |||
| Transferrin saturation (%), mean (SD), median (range) | 20.98 (13.25), 18 (5–90) | 18.95 (12.26), 16 (6–73) | |||
| Data from secondary analyses (Vansteenkiste 200484) | |||||
| Baseline Hb (g/dl), mean (SD) | |||||
| Hb < 10 g/dl | 9.1 (0.7) (n = 51) | 9 (0.7) (n = 69) | |||
| Hb ≥ 10 g/dl | 10.9 (0.7) (n = 105) | 10.7 (0.5) (n = 89) | |||
| Were intervention and control groups comparable? | No p-values are reported; authors stated that ‘Baseline demographics and clinical characteristics were similar between the two treatment groups’ (p. 1214) | ||||
| Results | ||||
|---|---|---|---|---|
| Haematological and transfusions | ||||
| Transfusions | n = 148 and n = 149 for darbepoetin alfa and placebo groups respectively | |||
| Participants with RBCTs from week 5 to end of treatment period (%) (95% CI) | 27 (20 to 35) | 52 (44 to 66) | Difference 25% (95% CI 14% to 36%) | < 0.001 |
| First RBCT or Hb ≤ 8 g/dl (%) (95% CI) | 32 (24 to 39) | 62 (54 to 71) | < 0.001 | |
| RBC units transfused, mean (SD) | 0.67 (1.7) | 1.92 (3.27) | Difference 1.25 (95% CI 0.65 to 0.84) | < 0.001 |
| Haematopoietic response (%) (95% CI) | 66 (58 to 74) (103 participants calculated) | 24 (16 to 31) (38 participants calculated) | Difference 42 (31 to 53) | < 0.001 |
| Participants with RBCTs from week 1 to EOTP (%) (95% CI)a | 28 (21 to 35) | 57 (49 to 65) | ||
| Time to disease progression or death (weeks), median (95% CI)a | 23 (19 to 31) | 20 (17 to 23) | ||
| Data from secondary analyses (Vansteenkiste 200484) | ||||
| Hb < 10 g/dl (%) (95% CI) | (n = 51) 65 (50 to 80) (33 participants calculated) | (n = 69) 31 (17 to 45) 21 participants calculated | < 0.002 | |
| Hb ≥ 10 g/dl (%) (95% CI) | (n = 105) 67 (57 to 77) (70 participants calculated) | (n = 89) 20 (11 to 29) (17 participants calculated) | < 0.001 | |
| HRQoL | n = 127 and n = 128 in the darbepoetin and placebo groups, respectively, completed the scale through study week 4; also completed baseline and at least one time from week 5 until the end of the treatment phase | |||
| Improvement in FACT-F scale (%) (95% CI) | 56 (47 to 65) | 44 (35 to 52) | 0.052 | |
| Patients with at least a 25% improvement from baseline in FACT-F scale (%) (95% CI) | 32 (23 to 40) | 19 (12 to 26) | Difference 13 (CI 2 to 23) | 0.019 |
| Adverse effects of treatment | ||||
| Deaths, n (%) | 22 (14) | 19 (12) | ||
| Death because of disease progression (%) | 61 | 58 | ||
| Thrombotic events, n (%) | 7 (5) | 5 (3) | ||
| Hypertension, n (%) | 9 (6) | 6 (4) | ||
| Hospitalisations for overnight stays (days), mean (SD) | 10.3 (13.7) | 13 (17.7) | ||
| Average of 1 year of follow-up after participants’ first dose of study drug (n = 156 and n = 158 for darbepoetin and placebo groups, respectively) | ||||
| OS (weeks), median (95% CI) | 46 (39 to 53) | 34 (29 to 39) | ||
| Deaths, n (%) | 92 (59) | 109 (69) | ||
| PFS (weeks), median (95% CI) | 22 (18 to 31) | 20 (17 to 23) | ||
| Disease progression or died, n (%) | 129 (83) | 141 (89) | ||
| Quality appraisal | |
|---|---|
| 1. Was the method used to generate random allocations adequate? (Yes = random numbers, coin toss, shuffle, etc.; no = patient’s number, date of birth, alternate; unclear = method not stated) | Unclear; no randomisation details given |
| 2. Was the treatment allocation adequately concealed? (Yes = central allocation at trial office/pharmacy, sequentially numbered coded vials, other methods in which the triallist allocating treatment could not be aware of treatment allocation; inadequate = allocation alternate or based on information known to the triallist) | Unclear; randomisation was performed using a centralised system, but details on allocation concealment were not reported |
| 3. Were the groups similar at baseline in terms of prognostic factors, e.g. severity of disease? | Unclear – no p-values are reported; authors stated that ‘Baseline demographics and clinical characteristics were similar between the two treatment groups’ (p. 35) |
| 4. Were the eligibility criteria specified? | Yes |
| 5. Were the participants blind to treatment allocation? | Yes |
| 6. Were the outcome assessors blind to treatment allocation? | Yes |
| 7. Were the point estimates and measure of variability presented for the primary outcome measure? | Yes |
| 8. Is there evidence to suggest that the authors collected more outcome data than they reported? | No |
| 9. Did the analyses include an ITT analysis or was < 10% of each study arm excluded? | Yesa – not for HRQoL; only 81% of patients analysed in both treatment groups |
| 10. Were withdrawals, dropouts and loss to follow-up in each group stated? | Partially |
| Other | |
|---|---|
| Generalisability | The majority of participants were male |
| Author conclusions | Patients with chemotherapy-associated anaemia can safely and effectively be treated with weekly darbepoetin alfa therapy. Darbepoetin alfa decreased RBCT requirements, increased Hb concentration and decreased fatigue. Although no conclusions can be drawn about survival from this study, the potential salutary effect on disease outcome warrants further investigation in a prospectively designed study |
| Reviewer comments | |
Appendix 3 Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessment
GRADE table for the use of erythropoiesis-stimulating agents for the treatment of treatment-induced anaemia in cancer patients: anaemia-related outcomes
| No. of participants (no. of studies) | Quality assessment | Summary of findings | |||||
|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence | ||
| Hb change (overall) (measured by change in Hb levels (g/dl) from baseline until the end of treatment period; better indicated by higher values) | |||||||
| 3170 (16 trials reported in 26 papers17,48,50,51,53,58–60,63–67,69–71,74,77–83,85,86) Meta-analysis: 18 trialsa |
Seriousb | Serious; significant heterogeneity (I2 = 75.9%; p < 0.01) | No serious indirectness | No serious imprecision | Undetected; funnel plot analysis did not show statistically significant asymmetry (p = 0.13) | ⊕⊕⊖⊖ LOW because of risk of bias, inconsistency | Sample sizes, n: control 1489, ESAs 1681; WMD 1.59 (95% CI 1.33 to 1.84) The random-effects meta-analysis demonstrated a statistically significant difference in Hb change (increase from baseline) in favour of treatment |
| Haematological response (overall) (assessed by proportion of participants with an increase in Hb level of ≥ 2 g/dl or an increase in haematocrit of ≥ 6 percentage points, unrelated to transfusion) | |||||||
| 2228 (10 trials reported in 19 papers17,48,50,53,58–60,63,65,66,70,71,77,79,81–83,85,86) Meta-analysis: 12 trialsa |
Seriousb | No serious inconsistency | No serious indirectness | No serious imprecision | Undetected; funnel plot analysis did not show statistically significant asymmetry (p = 0.28) | ⊕⊕⊕⊖ MODERATE because of risk of bias | Study event rates, n/N (%): control 182/1015 (17.9), ESAs 759/1213 (62.6); RR 3.29 (95 CI 2.84 to 3.81) The random-effects meta-analysis demonstrated a statistically significant difference in haematological response in favour of treatment |
| RBCT requirements (overall) (assessed by proportion of participants requiring RBCT | |||||||
| 4779 (22 trials reported in 33 papers17,48,50–53,58–60,62,63–71,73–86) Meta-analysis: 24 trialsa |
Seriousb | No serious inconsistency | No serious indirectness | No serious imprecision | Undetected; funnel plot analysis did not show statistically significant asymmetry (p = 0.23) | ⊕⊕⊕⊖ MODERATE because of risk of bias | Study event rates, n/N (%): control 835/2299 (36.3), ESAs 554/2480 (22.3); RR 0.63 (95% CI 0.57 to 0.69) The random-effects meta-analysis demonstrated a statistically significant difference in RBCT requirement in favour of treatment |
| RBC units (overall) [assessed by no. of units transfused per average patient (i.e. including participants not requiring transfusion)] | |||||||
| 1920 (10 trials reported in 16 papers51,52,58,59,63,65,66,69,71,73,74,77,79,84–86) Meta-analysis: 11 trialsa |
Seriousb | Serious; significant heterogeneity (I2 = 59.3%; p = 0.01) | No serious indirectness | No serious imprecision | Undetected; funnel plot analysis did not show statistically significant asymmetry (p = 0.14) | ⊕⊕⊖⊖ LOW because of risk of bias, inconsistency | Sample sizes, n: control 947, ESAs 973; WMD –0.87 (95% CI –1.28 to –0.46) The random-effects meta-analysis demonstrated a statistically significant difference in RBC units used in favour of the treatment |
GRADE table for the use of erythropoiesis-stimulating agents for the treatment of treatment-induced anaemia in cancer patients: malignancy-related outcomes
| No. of participants (no. of studies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence | Summary of findings |
|---|---|---|---|---|---|---|---|
| Complete tumour response (overall) (assessed by total disappearance of all known malignant disease) | |||||||
| 1909 (seven trials published in 12 papers51,60,66,70,71,74,76,78–82) Meta-analysis: 7 trials |
Seriousa | No serious inconsistency | No serious indirectness | Seriousb | Undetected; funnel plot analysis not conducted because of low number of primary studies (n = 7) | ⊕⊕⊖⊖ LOW because of risk of bias, imprecision | Study event rates, n/N (%): control 142/906 (15.7), ESAs 177/1003 (17.6); RR 1.10 (95% CI 0.86 to 1.41) The random-effects meta-analysis demonstrated a statistically non-significant difference in complete tumour response in favour of the treatment |
| OS (calculated from the longest follow-up available using HRs)c | |||||||
| 4454 (21 trials published in 32 papers17,48,50–53,58–60,62,63,65–71,73–86) Meta-analysis: 18 trialse,f |
Seriousd | Serious; significant heterogeneity (I2 = 42.4%; p = 0.03) | No serious indirectness | No serious imprecision | Undetected; funnel plot analysis did not suggest asymmetry; the Harbord test could not be performed because raw data were not available | ⊕⊕⊖⊖ LOW because of risk of bias, inconsistency | Study event rates, n/N (%): control 744/2137 (35), ESAs 818/2317 (35); HR 0.97 (95% CI 0.83 to 1.13) The random-effects meta-analysis demonstrated no statistically significant difference in survival in favour of treatment |
| Mortality (assessed by deaths occurring up to 30 days after the active study period) | |||||||
| 2967 (21 trials published in 32 papers17,48,50–53,58–60,62,63,65–70,71,73–86) Meta-analysis: 14 trialse,g |
Seriousd | No serious inconsistency | No serious indirectness | Seriousb | Undetected; funnel plot analysis did not suggest asymmetry; the Harbord test could not be performed because raw data were not available | ⊕⊕⊖⊖ LOW because of risk of bias, imprecision | Study event rates, n/N (%): control 164/1381 (12), ESAs 174/1586 (11); HR 0.86 (95% CI 0.67 to 1.11) The random-effects meta-analysis demonstrated no statistically significant difference in mortality in favour of treatment |
GRADE table for the use of erythropoiesis-stimulating agents for the treatment of treatment-induced anaemia in cancer patients: safety outcomes
| No. of participants (no. of studies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence | Summary of findings |
|---|---|---|---|---|---|---|---|
| Thromboembolic events (overall) | |||||||
| 4013 (14 trials published in 25 papers17,51,52,58–60,62,63,66,70,71,73–86) Meta-analysis: 15 trialsb |
Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | Undetected; funnel plot analysis did not show statistically significant asymmetry (p = 0.63) | ⊕⊕⊕⊖ MODERATE because of risk of bias | Study event rates, n/N (%): control 66/1984 (3.3), ESAs 103/2029 (5.1); RR 1.46 (95% CI 1.07 to 1.99) The random-effects meta-analysis demonstrated a statistically significant difference favouring the control |
| Hypertension (overall) | |||||||
| 2086 (10 trials published in 19 papers48,51,52,58–60,63,66,70–73,77,79,81,82,84–86) Meta-analysis: 12 trialsb |
Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | Undetected; funnel plot analysis did not show statistically significant asymmetry (p = 0.69) | ⊕⊕⊕⊖ MODERATE because of risk of bias | Study event rates, n/N (%): control 27/934 (2.9), ESAs 62/1152 (5.4); RR 1.8 (95% CI 1.14 to 2.85) The random-effects meta-analysis demonstrated a statistically significant difference favouring the control |
| Thrombocytopenia/haemorrhage events (assessed by decrease of platelets in the blood/haemorrhage) | |||||||
| 1715 (seven trials published in 11 papers52,60,65–67,70,76,78,80–82) | Seriousa | No serious inconsistency | No serious indirectness | Very seriousc | Undetected; funnel plot analysis not conducted because of low number of primary studies (n = 7) | ⊕⊖⊖⊖ VERY LOW because of risk of bias, imprecision | Study event rates, n/N (%): control 54/838 (6.4), ESAs 55/877 (6.3); RR 0.93 (95% CI 0.65 to 1.34) The random-effects meta-analysis demonstrated no statistically significant difference in thrombocytopenia/haemorrhage favouring treatment |
| Seizures (overall) | |||||||
| 289 (one trial published in five papers58,59,63,85,86) Meta-analysis: two trialsb |
Seriousa | No serious inconsistency | No serious indirectness | Very seriousc | Undetected; funnel plot analysis not conducted because of low number of primary studies (n = 2) | ⊕⊕⊖⊖ VERY LOW because of risk of bias, imprecision | Study event rates, n/N (%): control 4/141 (2.8), ESAs 5/148 (3.4); RR 1.19 (95% CI 0.33 to 4.38) The random-effects meta-analysis demonstrated no statistically significant difference in seizures favouring treatment |
| Pruritus (overall) | |||||||
| 904 (seven trials published in 12 papers52,58,59,63,67,69,71,76,77,79,85,86) Meta-analysis: six trialsd |
Seriouse | No serious inconsistency | No serious indirectness | No serious imprecision | Undetected; funnel plot analysis not conducted because of low number of primary studies (n = 6) | ⊕⊕⊕⊖ MODERATE because of risk of bias | Study event rates, n/N (%): control 13/454 (2.9), ESAs 30/450 (6.7); RR 2.04 (95% CI 1.11 to 3.75) The random-effects meta-analysis demonstrated a statistically significant difference in pruritus favouring the control |
Appendix 4 Excluded studies
Clinical effectiveness review: excluded studies
Reason for exclusion: population (n = 7)
Abdelrazik N, Fouda M. Once weekly recombinant human erythropoietin treatment for cancer-induced anemia in children with acute lymphoblastic leukemia receiving maintenance chemotherapy: a randomized case-controlled study. Hematology 2007;12:533–41.
Agnihotri P, Telfer M, Butt Z, Jella A, Cella D, Kozma CM, et al. Chronic anemia and fatigue in elderly patients: results of a randomized, double-blind, placebo-controlled, crossover exploratory study with epoetin alfa. J Am Geriatr Soc 2007;55:1557–65.
Cella D, Kallich J, McDermott A, Xu X. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from five randomized clinical trials. Ann Oncol 2004;15:979–86.
Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med 2007;357:965–76.
Gordon D, Nichols G, Ben-Jacob A, Tomita D, Lillie T, Miller C. Symptom management and supportive care. Treating anemia of cancer with every-4-week darbepoetin alfa: final efficacy and safety results from a Phase II, randomized, double-blind, placebo-controlled study. Oncologist 2008;13:715–24.
Gurion R, Gafter-Gvili A, Paul M, Vidal L, Ben-Bassat I, Yeshurun M, et al. Hematopoietic growth factors in aplastic anemia patients treated with immunosuppressive therapy-systematic review and meta-analysis. Haematologica 2009;94:712–19.
Henke M, Mattern D, Pepe M, Bezay C, Weissenberger C, Werner M, et al. Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol 2006;24:4708–13.
Reason for exclusion: intervention (n = 4)
Radiation Therapy Oncology Group. Radiation therapy with or without epoetin alfa in treating anemic patients with head and neck cancer. ClinicalTrials.gov identifier: NCT00004917. URL: https://clinicaltrials.gov/ct2/show/NCT00004917 (accessed 17 July 2010).
Krzakowski M. Epoetin delta: efficacy in the treatment of anaemia in cancer patients receiving chemotherapy. Clin Oncol 2008;20:705–13.
Lindholm E, Daneryd P, Korner U, Hyltander A, Fouladiun M, Lundholm K. Effects of recombinant erythropoietin in palliative treatment of unselected cancer patients. Clin Cancer Res 2004;10:6855–64.
Macdougall IC. CERA (continuous erythropoietin receptor activator): a new erythropoiesis-stimulating agent for the treatment of anemia. Curr Hematol Rep 2005;4:436–40.
Reason for exclusion: comparator (n = 3)
Casadevall N, Durieux P, Dubois S, Hemery F, Lepage E, Quarre MC, et al. Health, economic, and quality-of-life effects of erythropoietin and granulocyte colony-stimulating factor for the treatment of myelodysplastic syndromes: a randomized, controlled trial. Blood 2004;104:321–7.
Laurie SA, Ding K, Whitehead M, Feld R, Murray N, Shepherd FA, et al. The impact of anemia on outcome of chemoradiation for limited small-cell lung cancer: a combined analysis of studies of the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol 2007;18:1051–5.
Weigang-Kohler K, Vetter A, Thyroff-Friesinger U. HX575, recombinant human epoetin alfa, for the treatment of chemotherapy-associated symptomatic anaemia in patients with solid tumours. Onkologie 2009;32:168–74.
Reason for exclusion: outcomes (n = 1)
Littlewood TJ, Schenkel B, Liss M. Effect of patient exclusion criteria on the efficacy of erythropoiesis-stimulating agents in patients with cancer-related anemia. Oncologist 2005;10:357–60.
Reason for exclusion: study design (n = 140)
Aapro M. Emerging topics in anaemia and cancer. Ann Oncol 2012;23(Suppl. 10):x289–93.
Aapro M, Coiffier B, Dunst J, Österborg A, Burger HU. Effect of treatment with epoetin beta on short-term tumour progression and survival in anaemic patients with cancer: a meta-analysis. Br J Cancer 2006;95:1467–73.
Aapro M, Jelkmann W, Constantinescu SN, Leyland-Jones B. Effects of erythropoietin receptors and erythropoiesis-stimulating agents on disease progression in cancer. Br J Cancer 2012;106:1249–58.
Aapro M, Spivak JL. Update on erythropoiesis-stimulating agents and clinical trials in oncology. Oncologist 2009;14(Suppl. 1):6–15.
Bennett CL. The blue cross blue shield assessment technology review: summary of findings. Best Pract Res Clin Haematol 2005;18:423–31.
Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008;299:914–24.
Beutel G, Ganser A. Risks and benefits of erythropoiesis-stimulating agents in cancer management. Semin Hematol 2007;44:157–65.
Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennett C, et al. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst 2005;97:489–98.
Bohlius J, Tonia T, Schwarzer G. Twist and shout: one decade of meta-analyses of erythropoiesis-stimulating agents in cancer patients. Acta Haematol 2011;125:55–67.
Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2006;3:CD003407.
Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006;98:708–14.
Bohlius JF, Langensiepen S, Engert A, Schwarzer G, Bennett CL. Effectiveness of erythropoietin in the treatment of patients with malignancies: methods and preliminary results of a Cochrane review. Best Pract Res Clin Haematol 2005;18:449–54.
Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, A-Sterborg A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer. Eur J Cancer 2004;40:2201–16.
Boogaerts M, Oberhoff C, ten Bokkel Huinink W, Nowrousian MR, Hayward CRW, Burger HU. Epoetin beta (NeoRecormon) therapy in patients with solid tumours receiving platinum and non-platinum chemotherapy: a meta-analysis. Anticancer Res 2006;26:479–84.
Booton R, Thatcher N. The value of erythropoietin therapy in cancer patients. Am J Cancer 2005;4:87–103.
Boulaamane L, Goncalves A, Boutayeb S, Viens P, M’Rabti H, Bertucci F, et al. Prognostic impact of the combination of erythropoiesis-stimulating agents to cancer treatment: literature review. Support Care Cancer 2013;21:2359–69.
Cersosimo RJ, Jacobson DR. Epoetin alfa versus darbepoetin alfa in chemotherapy-related anemia. Ann Pharmacother 2006;40:58–65
Cheer SM, Wagstaff AJ. Epoetin beta – a review of its clinical use in the treatment of anaemia in patients with cancer. Drugs 2004;64:323–46.
Coiffier B, Boogaerts M, Aapro M, Hayward C, Burger H-U. Effect of treatment with epoetin beta on thromboembolic events in anemic patients with cancer: a metaanalysis. Support Cancer Ther 2006;4:49–55.
Cornes P, Coiffier B, Zambrowski JJ. Erythropoietic therapy for the treatment of anemia in patients with cancer: a valuable clinical and economic option. Curr Med Res Opin 2007;23:357–68.
Cortesi E, Gascon P, Henry D, Littlewood T, Milroy R, Pronzato P, et al. Standard of care for cancer-related anemia: improving hemoglobin levels and quality of life. Oncology 2005;68(Suppl. 1):22–32.
Couture F, Turner AR, Melosky B, Xiu L, Plante RK, Lau CY, et al. Prior red blood cell transfusions in cancer patients increase the risk of subsequent transfusions with or without recombinant human erythropoietin management. Oncologist 2005;10:63–71.
Crawford J. Erythropoiesis-stimulating protein support and survival. Oncology (Williston Park) 2006;20:39–43.
Crawford J, Robert F, Perry MC, Belani C, Williams D. A randomized trial comparing immediate versus delayed treatment of anemia with once-weekly epoetin alfa in patients with non-small cell lung cancer scheduled to receive first-line chemotherapy. J Thoracic Oncol 2007;2:210–20.
De Los Santos JF, Thomas GM. Anemia correction in malignancy management: threat or opportunity? Gynecol Oncol 2007;105:517–29.
Desai J, Demetri GD. Recombinant human erythropoietin in cancer-related anemia: an evidence-based review. Best Pract Res Clin Haematol 2005;18:389–406.
Donato H. Erythropoietin: an update on the therapeutic use in newborn infants and children. Exp Opin Pharmacother 2005;6:723–34.
Eton DT, Cella D. Do erythropoietic-stimulating agents relieve fatigue? A review of reviews. Cancer Treat Res 2011;157:181–94.
Ferrario E, Ferrari L, Bidoli P, De Candis D, Del Vecchio M, De Dosso S, et al. Treatment of cancer-related anemia with epoetin alfa: a review. Cancer Treat Rev 2004;30:563–75.
Feusner J. Guidelines for epo use in children with cancer. Pediatr Blood Cancer 2009;53:308–9.
Folloder J. Effects of darbepoetin alfa administered every two weeks on hemoglobin and quality of life of patients receiving chemotherapy. Oncology Nurs Forum 2005;32:81–9.
Gao S, Ma JJ, Lu C. Venous thromboembolism risk and erythropoiesis-stimulating agents for the treatment of cancer-associated anemia: a meta-analysis. Tumour Biol 2014;35:603–13.
Gascon P. Evaluating erythropoietic agents for the treatment of anaemia in the oncology setting. Eur J Cancer 2005;41:2601–12.
Glaspy J, Beguin Y. Anaemia management strategies: optimising treatment using epoetin beta (NeoRecormon (R)). Oncology 2005;69:8–16.
Glaspy J, Crawford J, Vansteenkiste J, Henry D, Rao S, Bowers P, et al. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer 2010;102:301–15.
Glaspy J, Rossi G. A randomized, active-control, pilot trial of front-loaded dosing regimens of darbepoetin-alfa for the treatment of patients with anemia during chemotherapy for malignant disease – reply. Cancer 2004;10:1546.
Glaspy JA. Randomized controlled trials of the erythroid-stimulating agents in cancer patients. Cancer Treat Res 2011;157:195–215.
Goodnough LT, Shander A. Update on erythropoiesis-stimulating agents. Best Pract Res Clin Anaesthesiol 2013;27:121–9.
Gosselin A, McKenzie RS, Lefebvre P, Mody SH, Piech CT, Duh MS. Dose-conversion ratio for epoetin alfa and darbepoetin alfa in chemotherapy patients with anemia and cancer. PT 2006;31(10).
Hedenus M, Österborg A, Tomita D, Bohac C, Coiffier B. Effects of erythropoiesis-stimulating agents on survival and other outcomes in patients with lymphoproliferative malignancies: a study-level meta-analysis. Leuk Lymphoma 2012;53:2151–8.
Hedenus M, Vansteenkiste J, Kotasek D, Austin M, Amado RG. Darbepoetin alfa for the treatment of chemotherapy-induced anemia: disease progression and survival analysis from four randomized, double-blind, placebo-controlled trials. J Clin Oncol 2005;23:6941–8.
Henry DH. Epoetin alfa for the treatment of cancer- and chemotherapy-related anaemia: product review and update. Exp Opin Pharmacother 2005;6:295–310.
Henry DH. Epoetin alfa treatment for patients with chemotherapy-induced anemia. Support Cancer Ther 2007;4:78–91.
Heuser M, Ganser A. Recombinant human erythropoietin in the treatment of nonrenal anemia. Ann Hematol 2006;85:69–78.
Jadersten M, Hellstrom-Lindberg E. Myelodysplastic syndromes: biology and treatment. J Intern Med 2009;265:307–28.
Jadersten M, Malcovati L, Dybedal I, Della Porta MG, Invernizzi R, Montgomery SM, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol 2008;26:3607–13.
Janecka IP. Erythropoletin to treat anaemia in patients with head and neck cancer. Lancet 2004;363:993–4.
Jones M, Schenkel B, Just J, Fallowfield L. Epoetin alfa improves quality of life in patients with cancer: results of metaanalysis. Cancer 2004;101:1720–32. [Erratum published in Cancer 2005;103:1984].
Juneja V, Keegan P, Gootenberg JE, Rothmann MD, Shen YL, Lee KY, et al. Continuing reassessment of the risks of erythropoiesis-stimulating agents in patients with cancer. Clin Cancer Res 2008;14:3242–7.
Kaanders JH, van der Kogel AJ. Erythropoietin to treat anaemia in patients with head and neck cancer. Lancet 2004;363:78–9; author reply 81–2.
Katodritou E, Dimopoulos MA, Zervas K, Terpos E. Update on the use of erythropoiesis-stimulating agents (ESAs) for the management of anemia of multiple myeloma and lymphoma. Cancer Treat Rev 2009;35:738–43.
Kelaidi C, Fenaux P. Darbepoetin alfa in anemia of myelodysplastic syndromes: present and beyond. Exp Opin Biol Ther 2010;10:605–14.
Kimel M, Leidy NK, Mannix S, Dixon J. Does epoetin alfa improve health-related quality of life in chronically ill patients with anemia? Summary of trials of cancer, HIV/AIDS, and chronic kidney disease. Value Health 2008;11:57–75.
Klarenbach S, Manns B, Reiman T, Reaume MN, Lee H, Lloyd A, et al. Economic evaluation of erythropoiesis-stimulating agents for anemia related to cancer. Cancer 2010;116:3224–32.
Kowalczyk M, Banach M, Mikhailidis DP, Rysz J. Erythropoietin update 2011. Med Sci Monit 2011;17:RA240–7.
Lambin P, Ramaekers BL, van Mastrigt GA, Van den Ende P, de Jong J, De Ruysscher DK, et al. Erythropoietin as an adjuvant treatment with (chemo) radiation therapy for head and neck cancer. Cochrane Database Syst Rev 2009;3:CD006158.
Larsson AM, Landberg G, Pahlman S, Albertsson M. Erythropoietin enhances response to treatment in patients with advanced breast cancer. Acta Oncol 2004;43:594–7.
Leonard RC, Untch M, Von Koch F. Management of anaemia in patients with breast cancer: role of epoetin. Ann Oncol 2005;16:817–24.
Littlewood T. Epoetin alfa (Eprex) and quality of life. Curr Med Res Opin 2005;21(Suppl. 2):S1–2.
Littlewood T, Collins G. Epoetin alfa: basic biology and clinical utility in cancer patients. Exp Rev Anticancer Ther 2005;5:947–56.
Lonnroth C, Svensson M, Wang W, Korner U, Daneryd P, Nilsson O, et al. Survival and erythropoietin receptor protein in tumours from patients randomly treated with rhEPO for palliative care. Med Oncol 2008;25:22–9.
Ludwig H, Crawford J, Österborg A, Vansteenkiste J, Henry DH, Fleishman A, et al. Pooled analysis of individual patient-level data from all randomized, double-blind, placebo-controlled trials of darbepoetin alfa in the treatment of patients with chemotherapy-induced anemia. J Clin Oncol 2009;27:2838–47.
Ludwig H, Wedding U, Van Belle S. Anaemia in elderly patients with cancer: focus on chemotherapy-induced anaemia. J Geriatr Oncol 2012;3:256–64.
Lyman GH, Glaspy J. Are there clinical benefits with early erythropoietic intervention for chemotherapy-induced anemia? A systematic review. Cancer 2006;106:223–33.
Marec-Berard P, Chastagner P, Kassab-Chahmi D, Casadevall N, Marchal C, Misset JL, et al. 2007 standards, options, and recommendations: use of erythropoiesis- stimulating agents (ESA: epoetin alfa, epoetin beta, and darbepoetin) for the management of anemia in children with cancer. Pediatr Blood Cancer 2009;53:7–12.
McKinney M, Arcasoy MO. Erythropoietin for oncology supportive care. Exp Cell Res 2011;317:1246–54.
Melosky BL. Erythropoiesis-stimulating agents: benefits and risks in supportive care of cancer. Curr Oncol 2008;15:S10–15.
Metzger NL, Francis KE, Voils SA. A comparative review of erythropoiesis stimulating agents. J Pharm Pract 2008;21:424–30.
Milano M, Schneider M. EPO in cancer anemia: benefits and potential risks. Crit Rev Oncol Hematol 2007;62:119–25.
Miller CP, Lowe KA, Valliant-Saunders K, Kaiser JF, Mattern D, Urban N, et al. Evaluating erythropoietin-associated tumor progression using archival tissues from a Phase III clinical trial. Stem Cells 2009;27:2353–61.
Minisini A, Atalay G, Bottomley A, Puglisi F, Piccart M, Biganzoli L. What is the effect of systemic anticancer treatment on cognitive function? Lancet Oncol 2004;5:273–82.
Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst 2008;100:1155–66.
Morere J-F. Role of epoetin in the management of anaemia in patients with lung cancer. Lung Cancer 2004;46:149–56.
Morreale A, Plowman B, DeLattre M, Boggie D, Schaefer M. Clinical and economic comparison of epoetin alfa and darbepoetin alfa. Curr Med Res Opin 2004;20:381–95.
Moyo V, Lefebvre P, Duh MS, Yektashenas B, Mundle S. Erythropoiesis-stimulating agents in the treatment of anemia in myelodysplastic syndromes: a meta-analysis. Ann Hematol 2008;87:527–36.
Mystakidou K, Potamianou A, Tsilika E. Erythropoietic growth factors for children with cancer: a systematic review of the literature. Curr Med Res Opin 2007;23:2841–7.
Newland AM, Black CD. Tumor progression associated with erythropoiesis-stimulating agents. Ann Pharmacother 2008;42:1865–70.
Nowrousian MR. Regarding ‘andomized comparison of epoetin alfa and darbepoetin alfa in anemic patients with cancer receiving chemotherapy’. Oncologist 2006;11:535–6; author reply 536–7.
Nowrousian MR, Dunst J, Vaupel P. Erythropoiesis-stimulating agents: favorable safety profile when used as indicated. Strahlenther Und Onkol 2008;184:121–36.
Oberhoff C. Speed of haemoglobin response in patients with cancer: a review of the erythropoietic proteins. Support Care Cancer 2007;15:603–11.
Ohashi Y, Uemura Y, Fujisaka Y, Sugiyama T, Ohmatsu H, Katsumata N, et al. Meta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: individual patient data from Japanese randomized, placebo-controlled trials. Cancer Sci 2013;104:481–5.
Oster HS, Hoffman M, Prutchi-Sagiv S, Katz O, Neumann D, Mittelman M. Erythropoietin in clinical practice: current use, effect on survival, and future directions. Isr Med Assoc J 2006;8:703–6.
Oster HS, Neumann D, Hoffman M, Mittelman M. Erythropoietin: the swinging pendulum. Leuk Res 2012;36:939–44.
Österborg A. New erythropoietic proteins: rationale and clinical data. Semin Oncol 2004;31:12–18.
Ots PMS, Carrizosa CL, Perez AR, de Dios Saez Garrido J, Perez JMD. Darbepoetin versus epoetin alfa for the correction of anemia in cancer patients receiving radiotherapy or chemoradiotherapy treatment. Clin Med Oncol 2008;2:393–9.
Parrish C, Owen RG. Waldenstrom macroglobulinemia with a durable clinical response to epoetin beta. Leuk Lymphoma 2012;53:1811–13.
Pedrazzoli P, Cinieri S, Lorusso V, Gamucci T, Secondino S, Silvestris N. Darbepoetin alpha coming of age. Anticancer Res 2007;27:4419–24.
Pelegri A. Impact of erythropoietin treatment on the quality of life of oncologic patients. Clin Transl Oncol 2007;9:645–51.
Petru E, Stummvoll W, Angleitner-Boubenizek L, Scholl T, Sevelda P, Benedicic C, et al. A literature review-based clinical guide on the use of erythropoiesis-stimulating agents (ESA) in the treatment of patients with gynaecological malignancies and related anaemia. Geburtshilfe Frauenheilkd 2010;70:646–57.
Pirker R. Darbepoetin alfa for the treatment of cancer-related anemia: an update. Exp Rev Anticancer Ther 2004;4:735–44.
Pirker R. Safety considerations for erythropoietin treatment in patients with cancer. Exp Opin Drug Saf 2007;6:63–9.
Pirker R. Clinical use of erythropoiesis-stimulating agents: defining the benefits. J Thoracic Oncol 2009;4:S153–5.
Pirker R. Erythropoiesis-stimulating agents in patients with cancer: update on safety issues. Exp Opin Drug Saf 2009;8:515–22.
Pronzato P, Jassem J, Mayordomo J. Epoetin beta therapy in patients with solid tumours. Crit Rev Oncol Hematol 2006;58:46–52.
Pujade-Lauraine E, Topham C. Once-weekly treatment of anemia in patients with cancer: a comparative review of epoetins. Oncology 2005;68:122–9.
Quercia RA, Keating KP, Goldman MC. Management of epoetin alpha use in the intensive care unit: a drug use evaluation. Formulary 2006;41:442–9.
Quirt I, Kovacs M, Couture F, Turner AR, Noble M, Burkes R, et al. Patients previously transfused or treated with epoetin alfa at low baseline hemoglobin are at higher risk for subsequent transfusion: an integrated analysis of the Canadian experience. Oncologist 2006;11:73–82.
Rades D, Golke H, Schild SE, Kilic E. The impact of tumor expression of erythropoietin receptors and erythropoietin on clinical outcome of esophageal cancer patients treated with chemoradiation. Int J Radiat Oncol Biol Phys 2008;71:152–9.
Reed SD, Radeva JI, Daniel DB, Mody SH, Forlenza JB, McKenzie RS, et al. Economic evaluation of weekly epoetin alfa versus biweekly darbepoetin alfa for chemotherapy-induced anaemia: evidence from a 16-week randomised trial. Pharmacoeconomics 2006;24:479–94.
Revicki DA, Stull D, Vernon M, Rader M, Tomita D, Viswanathan HN. Assessing the effect of darbepoetin alfa on patient-reported fatigue in chemotherapy-induced anemia in four randomized, placebo-controlled clinical trials. Qual Life Res 2012;21:311–21.
Rigolin GM, Castoldi G. The role of rHuEpo in low-risk myelodysplastic syndrome patients. Leuk Lymphoma 2005;46:823–31.
Rizzo JD. Evidence-based medicine: can it be applied to stimulation of erythropoiesis for patients with malignancy? Best Pract Res Clin Haematol 2005;18:439–48.
Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol 2010;28:4996–5010.
Rizzo JD, Somerfield MR, Hagerty KL, Seidenfeld J, Bohlius J, Bennett CL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol 2008;26:132–49.
Rosberg JH, Ben-Hamadi R, Cremieux PY, Fastenau JM, Piech CT. Dose conversion and cost effectiveness of erythropoietic therapies in chemotherapy-related anaemia: a meta-analysis. Clin Drug Invest 2005;25:33–48.
Ross SD, Allen IE, Henry DH, Seaman C, Sercus B, Goodnough LT. Clinical benefits and risks associated with epoetin and darbepoetin in patients with chemotherapy-induced anemia: a systematic review of the literature. Clin Ther 2006;28:801–31.
Saintigny P, Besse B, Soria LC, Bernaudin JF, Callard P. Does erythropoietin promote tumor growth? Reply. Clin Cancer Res 2008;14:1920–1.
Santini V. Treatment of low-risk myelodysplastic syndrome: hematopoietic growth factors erythropoietins and thrombopoietins. Semin Hematol 2012;49:295–303.
Savona MR, Silver SM. Erythropoietin-stimulating agents in oncology. Cancer J 2008;14:75–84.
Schrijvers D, De Samblanx H, Roila F. Erythropoiesis-stimulating agents in the treatment of anaemia in cancer patients: ESMO clinical practice guidelines for use. Ann Oncol 2010;21:v244–7.
Schwartz RN. Anemia in patients with cancer: incidence, causes, impact, management, and use of treatment guidelines and protocols. Am J Health System Pharm 2007;64:S5–S13.
Siddiqui MAA, Keating GM. Darbepoetin alfa – a review of its use in the treatment of anaemia in patients with cancer receiving chemotherapy. Drugs 2006;66:997–1012.
Siddiqui MAA, Keating GM. Spotlight on darbepoetin alfa in the treatment of anemia in patients with cancer receiving chemotherapy. BioDrugs 2006;20:321–3. [Reprint of Drugs 2006;66:997–1012].
Spaeth D. Epoetin beta once weekly: review of its efficacy and safety in patients with chemotherapy-induced anemia. Exp Rev Anticancer Ther 2008;8:875–85.
Spano JP, Khayat D. Treatment options for anemia, taking risks into consideration: erythropoiesis-stimulating agents versus transfusions. Oncologist 2008;13:27–32.
Spivak JL. The anaemia of cancer: death by a thousand cuts. Nature Rev Cancer 2005;5:543–55.
Stasi R, Amadori S, Littlewood TJ, Terzoli E, Newland AC, Provan D. Management of cancer-related anemia with erythropoietic agents: doubts, certainties, and concerns. Oncologist 2005;10:539–54.
Steensma DP, Loprinzi CL. Erythropoietin use in cancer patients: a matter of life and death? J Clin Oncol 2005;23:5865–8.
Steensma DP, Witzig TE. Does treatment with recombinant human erythropoietin affect the survival of anemic patients with cancer? Nature Clin Pract Oncol 2005;2:444–5.
Straus DJ. Treatment of anemia with erythropoietic agents in patients with hematologic malignancies. Support Cancer Ther 2005;2:215–24.
Straus DJ. Management of anemia in patients with hematologic malignancies. Oncology (Williston Park) 2006;20:8–11.
Szenajch J, Wcislo G, Jeong JY, Szczylik C, Feldman L. The role of erythropoietin and its receptor in growth, survival and therapeutic response of human tumor cells. From clinic to bench – a critical review. Biochim Biophys Acta Rev Cancer 2010;1806:82–95.
Testa U. Erythropoietic stimulating agents. Exp Opin Emerg Drugs 2010;15:119–38.
Tonia T, Bohlius J. Ten years of meta-analyses on erythropoiesis-stimulating agents in cancer patients. Cancer Treat Res 2011;157:217–38.
Tonia T, Schwarzer G, Bohlius J. Cancer, meta-analysis and reporting biases: the case of erythropoiesis-stimulating agents. Swiss Med Wkly 2013;143:w13776.
Tovari J, Pirker R, Timar J, Ostoros G, Kovacs G, Dome B. Erythropoietin in cancer: an update. Curr Mol Med 2008;8:481–91.
Vansteenkiste J, Glaspy J, Henry D, Ludwig H, Pirker R, Tomita D, et al. Benefits and risks of using erythropoiesis-stimulating agents (ESAs) in lung cancer patients: study-level and patient-level meta-analyses. Lung Cancer 2012;76:478–85.
Vansteenkiste J, Hedenus M, Gascon P, Bokemeyer C, Ludwig H, Vermorken J, et al. Darbepoetin alfa for treating chemotherapy-induced anemia in patients with a baseline hemoglobin level < 10 g/dL versus ≥ 10 g/dL: an exploratory analysis from a randomized, double-blind, active-controlled trial. BMC Cancer. 2009;9:311.
Vansteenkiste J, Wauters I. The use of darbepoetin alfa for the treatment of chemotherapy-induced anaemia. Exp Opin Pharmacother 2005;6:429–40.
Vansteenkiste J, Wauters I, Elliott S, Glaspy J, Hedenus M. Chemotherapy-induced anemia: the story of darbepoetin alfa. Curr Med Res Opin 2013;29:325–37.
Vaupel P, Dunst J, Engert A, Fandrey J, Feyer P, Freund M, et al. Effects of recombinant human erythropoietin (rHuEPO) on tumor control in patients with cancer-induced anemia. Onkologie 2005;28:216–21.
Vaupel P, Mayer A, Gemici C, Henke M, Pajonk F, Janecka IP. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet 2004;363:992–4.
Volgger B, Petru E, Angleitner-Boubenizek L, Weigert M, Reinthaller A, Lass H, et al. Erythropoetin beta twice weekly versus standard therapy in patients with gynaecological malignancies – a randomised Austrian AGO trial. Anticancer Res 2008;28:3977–84.
Waltzman RJ. A randomized, active-control, pilot trial of front-loaded dosing regimens of darbepoetin-alfa for the treatment of patients with anemia during chemotherapy for malignant disease. Cancer 2004;100:1545–6; author reply 1546.
Wauters I, Pat K, Vansteenkiste J. Flexible dosing with darbepoetin alfa for the treatment of chemotherapy-induced anemia. Ther Clin Risk Manag 2006;2:175–86.
Wauters I, Vansteenkiste J. Darbepoetin alfa in the treatment of chemotherapy-induced anaemia. Exp Opin Biol Ther 2009;9:221–30.
Wauters I, Vansteenkiste J. Erythropoiesis-stimulating agents in cancer patients: reflections on safety. Exp Rev Clin Pharmacol 2011;4:467–76.
Wauters I, Vansteenkiste J. Darbepoetin alfa in the treatment of anemia in cancer patients undergoing chemotherapy. Exp Rev Anticancer Ther 2012;12:1383–90.
Wiffen PJ. Evidence-based pain management and palliative care in issue one for 2009 of The Cochrane Library. J Pain Palliat Care Pharmacother 2009;23:166–8.
Ziegler JA, Herrington JD. Current and future options for the treatment of chemotherapy-induced anaemia. Exp Opin Invest Drugs 2006;15:1051–65.
Reason for exclusion: study design (systematic reviews of randomised controlled trials that were scrutinised for references) (n = 12)
Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet 2009;373:1532–42.
Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Erythropoietin or darbepoetin for patients with cancer – meta-analysis based on individual patient data. Cochrane Database Syst Rev 2009;3:CD007303.
Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Österborg A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 2007;43:258–70.
Grant MD, Piper M, Bohlius J, Tonia T, Nadege R, Vikrant V, et al. Epoetin and Darbepoetin for Managing Anaemia in Patients Undergoing Cancer Treatment: Comparative Effectiveness Update. Chicago, IL: Blue Cross and Blue Shield Association Technology Evaluation Center; 2013.
Kvam AK, Fayers P, Hjermstad M, Gulbrandsen N, Wisloff F. Health-related quality of life assessment in randomised controlled trials in multiple myeloma: a critical review of methodology and impact on treatment recommendations. Eur J Haematol 2009;83:279–89.
Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr 2004;(32):40–50.
Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2010;7:CD006704.
Ross SD, Allen IE, Probst CA, Sercus B, Crean SM, Ranganathan G. Efficacy and safety of erythropoiesis-stimulating proteins in myelodysplastic syndrome: a systematic review and meta-analysis. Oncologist 2007;12:1264–73.
Shehata N, Walker I, Meyer R, Haynes AE, Imrie K, Cancer Care Ontario Hematology Disease Site Group. The use of erythropoiesis-stimulating agents in patients with non-myeloid hematological malignancies: a systematic review. Ann Hematol 2008;87:961–73.
Tonelli M, Hemmelgarn B, Reiman T, Manns B, Reaume MN, Lloyd A, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ 2009;180:E62–71.
Tonia T, Mettler A, Robert N, Schwarzer G, Seidenfeld J, Weingart O, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12:CD003407.
Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al. A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technol Assess 2007;11(13).
Reason for exclusion: duplicate (n = 4)
Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennet C, et al. Erythropoietin for patients with malignant disease. Cochrane Database Syst Rev 2004;3:CD003407.
Ohashi Y, Uemura Y, Fujisaka Y, Sugiyama T, Ohmatsu H, Katsumata N, et al. Meta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: individual patient data from Japanese randomized, placebo-controlled trials. Cancer Sci 2013;104:481–5.
Razzouk BI, Hord JD, Hockenberry M, Hinds PS, Feusner J, Williams D, et al. Double-blind, placebo-controlled study of quality of life, hematologic end points, and safety of weekly epoetin alfa in children with cancer receiving myelosuppressive chemotherapy. J Clin Oncol 2006;24:3583–9.
Schwartzberg LS, Yee LK, Senecal FM, Charu V, Tomita D, Wallace J, et al. Symptom management and supportive care. A randomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia in patients with breast, lung, or gynecologic cancer. Oncologist 2004;9:696–707.
Reason for exclusion: language (n = 9)
Hernandez MAL, Lopez EU. [Weekly beta erythropoietin high doses in patients with lymphoblastic acute leukemia in remission and under chemotherapy, its effects in transfusion requirements]. Med Intern Mex 2008;24:375–80.
Liang J, Bi Q, Shen LD, Cheng HY. [The clinical study on recombinant human erythropoietin for chemotherapy-related anemia]. Tumor 2009;29:58–60.
Meric JB, Morere JF. Anemia in lung cancer patients. Bull Cancer 2005;92:439–44.
Ray-Coquard I, Kassab-Chahmi D, Casadevall N, Chastagner P, Marchal C, Marec-Berard P, et al. Clinical practice guidelines for the use of erythropoiesis-stimulating agents (ESA: epoetin alfa, epoetin beta, darbepoetin) in anaemic patients with cancer: 2007 update (summary report). Bull Cancer 2008;95:433–41.
Ray-Coquard I, Kassab-Chahmi D, Casadevall N, Chastagner P, Marchal C, Marec-Berard P, et al. Standards, options and recommendations for the use of erythropoiesis-stimulating agents (ESA: epoetin alfa, epoetin beta, darbepoetin) in anaemic patients with cancer: 2007 update. Oncologie 2008;10:160–6.
Sanchez CO, Gonzalez GP, Sanchez LFO. Erythropoietin and cancer-related anaemia. Light and shade. Med Clin 2005;124:186–95.
Schipper J, Henke M. [Erythropoietin in patients with head and neck carcinomas?]. Laryngorhinootologie 2004;83:292–7.
Spaeth D, Casadevall N, Daouphars M, Marchal C, Marec-Berard P, Fabre N, et al. Summary version of the standards, options and recommendations for the use of recombinant erythropoietin (epoietin-alpha and beta, darbepoietin-alpha, EPO) in the management of anaemia in oncology – update 2003. Bull Cancer 2004;91:179–88.
Zemelka T, Rolski J, Ziobro M, Michalczyk A. [Opinion on influence of erythropoietin on quality of life and survival in patients with advanced non-small cell lung cancer]. Wspolczesna Onkol 2007;11:37–40.
Reason for exclusion: unobtainable (n = 5)
Coiffier B, Milpied N, Facon T, Beris P. Epoetin beta (NeoRecormon) once weekly or three-times weekly produces a rapid haemoglobin response in anaemic patients with lymphoproliferative malignancies. 2004. URL: wwwehaweborg.
Costa EC, Martin EG, Vilaplana PG. Current approach in the use of erythropoietin. Cancer Chemother Rev 2007;2:121–32.
Littlewood T, Zagari M, Schenkel B. Effects of population definitions and study endpoints on efficacy of epoetin alfa vs placebo in anemic patients with hematologic malignancies receiving chemotherapy. 2004. URL: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/246/CN-00525246/frame.html (accessed 9 August 2015).
Vandebroek A, Altintas S, Gaede B, Smith K, Yao B, Schupp M. Darbepoetin alfa administered every 3 weeks with or without parenteral iron in anaemic patients with nonmyeloid malignancies receiving chemotherapy: interim results from a randomised open-label study. Haematologica 2006;91(Suppl. 1):0029.
Wang R. Safety of erythropoiesis-stimulating agents: lessons and limitations of meta-analyses. 2009 Annual Meeting Student Poster Abstract.
Reason for exclusion: no usable data (n = 2)
Benefits of epoetin alfa for cancer patients’ quality of life are confirmed after modelling to account for missing data. Curr Med Res Opin 2005;21(Suppl. 2):S6–8.
Golshayan AR, Jin T, Maciejewski J, Fu AZ, Bershadsky B, Kattan MW, et al. Efficacy of growth factors compared to other therapies for low-risk myelodysplastic syndromes. Br J Haematol 2007;137:125–32.
Reason for exclusion: abstract only (n = 57)
Alexopoulos CG, Kotsori AA. A randomized comparison of rHuEPO with darbepoetin for cancer related anaemia. Ann Oncol 2004;15:219.
Canon JL, Vansteenkiste J, Bodoky G, Mateos MV, Bastit L, Ferreira I, et al. Final results of a randomized, double-blind, active-controlled trial of darbepoetin alfa administered once every 3 weeks (Q3W) for the treatment of anemia in patients receiving multicycle chemotherapy. J Clin Oncol 2005;23:799S.
Canon JL, Vansteenkiste J, Bodoky G, Mateos MV, Bastit L, Ferreira I, et al. Darbepoetin alfa administered once every 3 weeks (Q3W) is effective for treating anaemia in patients receiving multicycle chemotherapy: results of a randomised, double-blind, active-controlled trial. EJC Suppl 2005;3:370.
Charu V, Belani CP, Gill AN, Bhatt M, Ben-Jacob A, Tomita D, et al. A controlled, randomized, open-label study to evaluate the effect of every-2-week darbepoetin alfa for anemia of cancer. J Clin Oncol 2004;22:749S.
Charu V, Belani CP, Gill AN, Bhatt M, Ben-Jacob A, Tomita D, et al. A controlled, randomized, open-label study to evaluate the effects of every-2-week darbepoetin alfa for anemia of cancer. J Support Oncol 2005;3:12–13.
Charu V, Saidman B, Ben-Jacob A, Justice GR, Maniam AS, Rearden T, et al. Improvements in fatigue are associated with early treatment with every 3-week (Q3W) darbepoetin alfa (DA) treatment in anemic patients (pts) receiving chemotherapy. Blood 2004;104:233.
Crawford J, Glaspy J, Vansteenkiste J, Henry DH, Tomita D, Bridges K, et al. Use of erythropoiesis-stimulating agents (ESAs) in lung cancer patients: study-level and patient-level meta-analyses of safety outcomes. J Thoracic Oncol 2010;5:S552–3.
Delarue R. Survival impact of prophylactic administration of darbepoetin alfa in patients with diffuse large B-cell lymphoma treated with immunochemotherapy: the LNH03-6B study. Educational Cancer Convention Lugano of the European School of Oncology, Lugano, Switzerland, April 2012.
Delarue R, Haioun C, Broussais-Guillaumot F, Sibon D, Fournier M, Mounier N, et al. Efficacy and safety of prophylactic use of darbepoetin alfa in patients with diffuse large B-cell lymphoma (DLBCL) treated with immunochemotherapy: results of the interim analysis of the LNH03-6B gela study. Blood 2009;114:1701.
Freemantle N, Yao B, Calvert M, Lillie T. Impact of darbepoetin alfa on transfusion, hemoglobin response, and survival in cancer patients with chemotherapy-induced anemia: results of a meta-analysis of randomized, placebo-controlled trials. Blood 2005;106:871A.
Gupta S, Singh PK, Bhatt ML, Pant MC, Sundar S, Verma J, et al. Clinical benefits of epoetin beta in patients with advanced stage hormone refractory prostate cancer. Eur Urol Suppl 2011;10:337.
Hartmann JT, Metzner B, Binder C, Mergenthaler HG, Rick O, Sayer HG, et al. Addition of darbepoetin alfa to sequential high-dose VIP chemotherapy for patients with advanced metastatic germ cell cancer. J Clin Oncol 2012;30(Suppl. 1):e15026.
Heras P, Hatzopoulos A, Karagiannis S. Efficacy and safety of epoetin beta 30,000 IU once weekly in patients with solid tumors and chemotherapy-induced anemia. J Clin Oncol 2006;24(18S):697.
Heras P, Hatzopoulos A, Karagiannis S, Kritikos K. Epoetin beta (30000) vs. epoetin alfa (40000) for chemotherapy induced anemia in patients with colorectal cancer: a randomized comparative study. Ann Oncol 2007;18:VII77.
Hernandez E, Di Benedetto J, Kotasek D, Ganly P, Silberstein P, Tomita D, et al. Effectiveness of darbepoetin alfa 300 mcg every 3 weeks in patients with chemotherapy-induced anemia. J Clin Oncol 2006;24:S691.
Hinds PS, Hockenberry M, Feusner J, Hord JD, Rackoff W, Rozzouk BI. Hemoglobin response and improvements in quality of life in anemic children with cancer receiving myelosuppressive chemotherapy. J Support Oncol 2005;3(Suppl. 4):10–11.
Houben R, Pijls-Johannesma M, Ramaekers B, Van Den Ende P, De Jong J, De Huysscher D, et al. Erythropoietin as an adjuvant treatment with (chemo) radiation therapy for head and neck cancer: updated systematic review with additional data and new methodology. European Society for Therapeutic Radiology and Oncology (ESTRO) 29 Congress, Barcelona, Spain, September 2010.
Katsumata N, Fujiwara Y, Sugiyama T, Goto I, Ohmatsu H, Okamoto R, et al. Erythropoiesis-stimulating agents for the treatment of chemotherapy-induced anemia and mortality: a meta-analysis of individual patient data from Japanese randomized trials. Eur J Cancer 2011;47:S242.
Kelada OJ, Marignol L. Does the use of erythropoietin-stimulating agents in breast cancer patients with chemotherapy-induced anaemia impact on clinical outcomes? A critical review of the literature. European Society for Therapeutic Radiology and Oncology (ESTRO) 29 Congress, Barcelona, Spain, September 2010.
Kotsori AA, Alexopoulos CG. A randomized comparison of darbepoetin alfa with epoetin for chemotherapy induced anemia in nonhematological tumors. J Clin Oncol 2006;24:S692.
Langer CJ. Managing anemia in lung cancer. J Thoracic Oncol 2009;4:S144–5.
Ludwig H, Crawford J, Österborg A, Fleishman A, Lillie T, Sueto T, et al. Patient-level integrated analysis of data from 6 randomized, double-blind, placebo-controlled trials of darbepoetin alfa (DA) in patients (pts) with chemotherapy-induced anemia (CIA). EJC Suppl 2007;5:142–3.
Marangolo M, Lang I, Beato C, Colomer R, Ukarma L. Breast Cancer – Anaemia and the Value of Erythropoietin (BRAVE): preliminary results from a study of the efficacy of epoetin beta 30,000 IU once weekly in patients with metastatic breast cancer receiving chemotherapy. Eur J Cancer 2005;3:388.
Marangolo M, Malamos N, Pedrini JL, Rotarski, M. Epoetin beta in patients with metastatic breast cancer receiving chemotherapy: results from the Breast Cancer – Anaemia and the Value of Erythropoietin (BRAVE) study. J Clin Oncol 2005;23(Suppl.):764.
Marinaccio M, Mele E, Poma S, Cantinieri C, Cocca M, Latiano T. Pretreatment normalisation of mild anemia with epoetin alfa predicts long-term outcome for women with epithelail ovarian cancer. J Clin Oncol 2004:22:S5132.
Markus R, Henry D, Gascon P, Fleishman A, Borenstein J. Design and rationale of a double-blind, randomized, placebo-controlled study to evaluate the long-term safety and efficacy of darbepoetin alfa administered 500 mcg once every three weeks (Q3W) in anemic patients with advanced non-small cell lung cancer (NSCLC) receiving multi-cycle chemotherapy. J Thoracic Oncol 2009;4:S431.
Mihaylov G, Tsekova V, Koytchev R. Epoetin zeta: safety data from an open-label, Phase III trial in patients with chemotherapyinduced anaemia. Ann Oncol 2008;19(Suppl. 8):278.
Moehler M, Geissler M, Raedle J, Ebert MD, Flieger D, Seufferlein T, et al. Epoetin beta once weekly in anaemic patients with advanced cancer of the stomach or gastroesophageal junction: interim results of a randomized German AIO Phase II trial. Onkologie 2007;30:140–1.
Nitz U, Oberhoff C, Reimer T, Schumacher C, Hackmann J, Warm M, et al. Adjuvant chemotherapy with or without darbepoetin in node-positive breast cancer: a safety analysis from the Phase III ARA plus trial. Cancer Res 2009;69(Suppl.):4100.
Norris L, Mattison D, Qureshi ZP, Bennett C. Location, location, location: reassessing erythropoiesis stimulating agents (ESAs) in the United States (US), Canada, and Europe (2007–2011). Blood 2011;118:4753.
Norris L, Qureshi Z, Barnato S, Lai S, Bennett C. Serious adverse drug reactions (sADRS) associated with hematopoietic growth factors: a systematic review from the Southern Network on Adverse Reactions (SONAR) program. 7th Annual Hematology/Oncology Pharmacy Association (HOPA) Conference, Salt Lake City, UT, USA, March 2011.
Ohmatsu H, Nishiwaki Y, Ichinose Y, Ohe Y, Yamada Y, Takeda K, et al. Randomized Phase II study of weekly administration of darbepoetin alfa (DA) in anemic patients with lung cancer and ovarian cancer receiving platinum-based chemotherapy. Ann Oncol 2006;17:291–2.
Paladini L, Clark O, Clark LG, Engel T, Pegoretti B, Faleiros E. Erythropoiesis stimulating agents (ESA) for the treatment of chemotherapy induced anemia in patients with hemoglobin levels (HB) < 11 g/dl – a systematic review and meta-analysis. ISPOR 12th Annual European Congress, Paris, France, October 2009. Value Health 2009;12:A493.
Pirker R, Collins H, Legg JC, Vansteenkiste J. F. Rate of hemoglobin (Hb) decline from less than 10 g/dl to less than 9 g/dl in placebo-treated patients (pts) receiving chemotherapy: a pooled analysis of data from six randomized darbepoetin alfa trials. J Clin Oncol 2011;29(Suppl. 1):e19637.
Pollera CF, Nelli F, Gamucci T, Sperduti I, Giampaolo AM, Moscetti L, et al. Prospective evaluation of epoetin-alfa (EA) vs epoetin-beta (EB) vs darbepoetin (DE) in anemic cancer patients (pts) receiving chemotherapy (CT): early results of an independent observational survey by the Italian ReVERTO network. J Clin Oncol 2006;24:S692.
Rearden TP, Charu V, Saidman B, Ben-Jacob A, Justice GR, Manaim AS, et al. Results of a randomized study of every three-week dosing (Q3W) of darbepoetin alfa for chemotherapy-induced anemia (CIA). J Clin Oncol 2004;22:745S.
Reed N, Chan S, Hayward C, Burger H, Bokkel Huinink W. Impact of epoetin beta on the survival of anemic patients with ovarian cancer receiving platinum-based chemotherapy. J Clin Oncol 2005;23:S5102.
Rosti G, Secondino S, Giordano L, Garassino I, Gandini C, Auerbach M, et al. Intravenous iron supplementation and erythropoiesis stimulating agents (ESAs): meta-analysis of randomized trials in patients with chemotherapy-induced anemia. Joint ECCO 15 – 34th ESMO Multidisciplinary Congress, Berlin, Germany, September 2009. Eur J Cancer 2009;7:256.
Schwartzberg L, Yee L, Charu V, Tomita D, Rossi G, Senecal F. Comparable efficacy and safety of darbepoetin alfa 200 mug every 2 weeks and epoetin alfa 40,000 U weekly in patients with breast cancer: results of a randomized comparison. J Support Oncol 2005;3:30–1.
Sevinir B, Durmaz O. Once a week erythropoietin in children with cancer. Pediatr Blood Cancer 2004;43:491–2.
Shah B. Darbepoetin in cancer related anemia. 54th Annual Conference of the Indian Society of Haematology and Blood Transfusion (Haematacon), Mumbai, India, 2013.
Steensma DP, Dakhil SR, Novotny PJ, Sloan JA, Johnson DB, Anderson DM, et al. A randomized comparison of standard weekly epoetin alfa to every-3-week epoetin alfa and every-3-week darbepoetin alfa: a study of the Mayo Clinic Cancer Research Consortium (MCCRC). 51st Annual Meeting of the American Society of Hematology (ASH), New Orleans, LA, USA, December 2009. Blood 2009;114:abstract 3008.
Strauss H, Haensgen G, Dunst J, Hayward C, Koelbl H. Effects of anaemia correction with epoetin beta in patients with advanced cervical cancer and radiochemotherapy. J Clin Oncol 2005;23:S5121.
Suzuki Y, Tokuda Y, Okamoto R, Nakagawa K, Ando K, Iwata H, et al. Randomized, placebo-controlled Phase II study of darbepoetin alfa (DA) administered every three weeks (Q3W) in patients with chemotherapy-induced anemia (CIA). 34th Congress of the European Society for Medical Oncology (ESMO), Stockholm, Sweden, 2008. Ann Oncol 2008;19(Suppl. 8):viii277.
Taylor K, Ganly P, Charu V, Di Benedetto J, Kracht K, Rossi G, et al. Randomized, double-blind, placebo-controlled study of darbepoetin alfa every 3 weeks for the treatment of chemotherapy-induced anemia. Blood 2005;106:3556.
Tesch H, Liberati AM, Ifrah N. Assessment of cognitive effects of once-weekly epoetin alfa in anemic patients with hematologic malignancies receiving chemotherapy: results of the EPOLYM trial. Haematologica 2006;91(Suppl. 1):abstract 022.
Toma A, Chevret S, Kosmider O, Delaunay J, Stamatoullas A, Rose C, et al. , editors. A randomized study of lenalidomide (LEN) with or without EPO in RBC transfusion dependent (TD) IPSS low and int-1 (lower risk) myelodysplastic syndromes (MDS) without del 5q resistant to EPO. 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, USA, June 2013. J Clin Oncol 2013;31(Suppl.):7002.
Tzekova V, Koytchev R. Efficacy of epoetin zeta in patients with chemotherapy-induced anemia and hematological malignancies: interim results. Haematologica 2008;93:S444.
Tzekova V, Mihaylov G, Koytchev R. Epoetin zeta: efficacy data from an open-label, Phase III trial in patients with chemotherapyinduced anaemia. Ann Oncol 2008;19(Suppl. 8):278.
Van Groeningen CJ, Kok TC, Biesma B, Melissant CF, De Klerk G, Brok R, et al. Epoetin alfa and darbepoetin alfa in clinical practice in patients with chemotherapy induced anemia in the Netherlands (EVALUATE). Eur J Cancer 2011;47(Suppl. 1):237.
Vandebroek A, Gaede B, Altintas S, Smith K, Yao B, Schupp M, et al. A randomized open-label study of darbepoetin alfa administered every 3 weeks with or without parenteral iron in anemic subjects with nonmyeloid malignancies receiving chemotherapy. J Clin Oncol 2006;24:S8612.
Vandebroek A, Gaede B, Altintas S, Smith K, Yao B, Schupp M, et al. A randomized open-label study of darbepoetin alfa administered every 3 weeks with or without parenteral iron in anemic subjects with nonmyeloid malignancies receiving chemotherapy. J Support Oncol 2007;5:24–6.
Walter E, Ribnicsek E, Kutikova L. Economic evaluation of darbepoetin alfa (Aranesp) compared to epoetin alfa (Erypo) and epoetin beta (NeoRecormon) in the treatment of chemotherapy-induced anemia (CIA) in Austria. Value Health 2010;13:A377.
Waltzman RJ, Fesen M, Justice GR, Croot C, Williams D. Epoetin alfa 40,000 U QW vs darbepoetin alfa 200 mcg Q2W in anemic cancer patients receiving chemotherapy: preliminary results of a comparative trial. J Clin Oncol 2004;22:S8153.
Waltzman R, Williams D. Head-to-head comparison of epoetin alfa (EPO) 40,000 U QW vs. darbepoetin alfa (DARB) 200 µg Q2W in anemic cancer patients receiving chemotherapy (CT): final results of a planned interim analysis (IA). Blood 2004;104:4233.
Watanbabe M, Ezaki K, Tobinai K, Tsuboi M, Ohashi Y, Hirashima K, et al. A multicenter Phase III randomized double-blind placebo-controlled study of epoetin beta administered once-weekly for chemotherapy-induced anemia (CIA) in cancer patients: Japan erythropoietin study group. Ann Oncol 2006;17:294.
Youssef LA, Hussien DH, Sulaiman S. The effectiveness of a fixed low dose of erythropoietin (EPO) in anemic solid tumor patients receiving concomitant chemotherapy: a prospective, randomized, controlled study. 53rd Annual Meeting of the American Society of Hematology (ASH), San Diego, CA, USA, December 2011. Abstract 2092.
Quality-of-life review: excluded studies
Reason for exclusion: population (n = 9)
Abdelrazik N, Fouda M. Once weekly recombinant human erythropoietin treatment for cancer-induced anemia in children with acute lymphoblastic leukemia receiving maintenance chemotherapy: a randomized case-controlled study. Hematology 2007;12:533–41.
Carroll JK, Kohli S, Mustian KM, Roscoe JA, Morrow GR. Pharmacologic treatment of cancer-related fatigue. Oncologist 2007;12:43–51.
Casadevall N, Durieux P, Dubois S, Hemery F, Lepage E, Quarre MC, et al. Health, economic, and quality-of-life effects of erythropoietin and granulocyte colony-stimulating factor for the treatment of myelodysplastic syndromes: a randomized, controlled trial. Blood 2004;104:321–7.
Hoskin PJ, Robinson M, Slevin N, Morgan D, Harrington K, Gaffney C. Effect of epoetin alfa on survival and cancer treatment-related anemia and fatigue in patients receiving radical radiotherapy with curative intent for head and neck cancer. J Clin Oncol 2009;27:5751–6.
Lindholm E, Daneryd P, Korner U, Hyltander A, Fouladiun M, Lundholm K. Effects of recombinant erythropoietin in palliative treatment of unselected cancer patients. Clin Cancer Res 2004;10:6855–64.
Mystakidou K, Kalaidopoulou C, Katsouda E, Parpa E, Kouskouni E, Chondros C, et al. Evaluation of epoetin supplemented with oral iron in patients with solid malignancies and chronic anemia not receiving anticancer treatment. Anticancer Res 2005;25:3495–500.
Norager CB, Jensen MB, Madsen MR, Qvist N, Laurberg S. Effect of darbepoetin alfa on physical function in patients undergoing surgery for colorectal cancer – a randomized, double-blind, placebo-controlled study. Oncology 2006;71:212–20.
Oliansky DM, Antin JH, Bennett JM, Deeg HJ, Engelhardt C, Heptinstall KV, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of myelodysplastic syndromes: an evidence-based review. Biol Blood Marrow Transplant 2009;15:137–72.
Smith RE, Aapro MS, Ludwig H, Pinter T, Smakal M, Ciuleanu TE, et al. Darbepoetin alfa for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a Phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol 2008;26:1040–50.
Reason for exclusion: intervention (n = 5)
Berndt E, Kallich J, McDermott A, Xu X, Lee H, Glaspy J. Reductions in anaemia and fatigue are associated with improvements in productivity in cancer patients receiving chemotherapy. Pharmacoeconomics 2005;23:505–14.
Blair S, Bardwell WA, Podbelewicz-Schuller Y, Mortimer JE. Correlation between hemoglobin and fatigue in women undergoing adjuvant chemotherapy without erythropoietin-stimulating-agent support. Clin Breast Cancer 2008;8:522–6.
Cheng Z, Wu JL, Chen JF. Clinical observation on the treatment of male neoplastic anemia with Yixuesheng capsule combined with recombination human erythropoietin. Chin J Integr Med 2009;15:63–5.
Gascon P, Pirker R, Del Mastro L, Durrwell L. Effects of CERA (continuous erythropoietin receptor activator) in patients with advanced non-small-cell lung cancer (NSCLC) receiving chemotherapy: results of a Phase II study. Ann Oncol 2010;21:2029–39.
Krzakowski M. Epoetin delta: efficacy in the treatment of anaemia in cancer patients receiving chemotherapy. Clin Oncol 2008;20:705–13.
Reason for exclusion: comparator (n = 11)
Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol 2004;22:1301–7.
Balleari E, Rossi E, Clavio M, Congiu A, Gobbi M, Grosso M, et al. Erythropoietin plus granulocyte colony-stimulating factor is better than erythropoietin alone to treat anemia in low-risk myelodysplastic syndromes: results from a randomized single-centre study. Ann Hematol 2006;85:174–80.
Bastit L, Vandebroek A, Altintas S, Gaede B, Pinter T, Suto TS, et al. Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alfa administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol 2008;26:1611–18.
Canon JL, Vansteenkiste J, Bodoky G, Mateos MV, Bastit L, Ferreira J, et al. Randomized, double-blind, active-controlled trial of every-3-week darbepoetin alfa for the treatment of chemotherapy-induced anemia. J Natl Cancer Inst 2006;98:273–84.
Courneya KS, Jones LW, Peddle CJ, Sellar CM, Reiman T, Joy AA, et al. Effects of aerobic exercise training in anemic cancer patients receiving darbepoetin alfa: a randomized controlled trial. Oncologist 2008;13:1012–20.
Crawford J, Robert F, Perry MC, Belani C, Williams D, Anemia Prevention in NSCLC Group. A randomized trial comparing immediate versus delayed treatment of anemia with once-weekly epoetin alfa in patients with non-small cell lung cancer scheduled to receive first-line chemotherapy. J Thorac Oncol 2007;2:210–20.
Glaspy J, Henry D, Patel R, Tchekmedyian D, Applebaum S, Berdeaux D, et al. Effects of chemotherapy on endogenous erythropoietin levels and the pharmacokinetics and erythropoietic response of darbepoetin alfa: a randomised clinical trial of synchronous versus asynchronous dosing of darbepoetin alfa. Eur J Cancer 2005;41:1140–9.
Gupta S, Singh PK, Bhatt ML, Pant MC, Sundar S, Verma J, et al. Clinical benefits of two different dosing schedules of recombinant human erythropoietin in anemic patients with advanced head and neck cancer. Biosci Trends 2010;4:267–72.
Justice G, Kessler JF, Jadeja J, Campos L, Weick J, Chen CF, et al. A randomized, multicenter study of subcutaneous and intravenous darbepoetin alfa for the treatment of chemotherapy-induced anemia. Ann Oncol 2005;16:1192–8.
Larsson AM, Landberg G, Pahlman S, Albertsson M. Erythropoietin enhances response to treatment in patients with advanced breast cancer. Acta Oncol 2004;43:594–7.
Steensma DP, Sloan JA, Dakhil SR, Dalton R, Kahanic SP, Prager DJ, et al. Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy-associated anemia. J Clin Oncol 2011;29:97–105.
Reason for exclusion: outcomes (n = 20)
Bell D, Grimes D, Gurney H, Dalley D, Blackwell T, Fox R, et al. Outcomes and predicting response in anaemic chemotherapy patients treated with epoetin alfa. A multicentre, 4-month, open-label study in Australia and New Zealand. Intern Med J 2008;38:751–7.
Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008;299:914–24.
Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennett C, et al. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst 2005;97:489–98.
Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet 2009;373:1532–42. [Erratum published in Lancet 2009;374:28].
Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Erythropoietin or darbepoetin for patients with cancer – meta-analysis based on individual patient data. Cochrane Database Syst Rev 2009;3:CD007303.
Bohlius J, Trelle S, Weingart O, Schwarzer G, Brillant C, Clarke MJ, et al. Erythropoietin or darbepoetin for patients with cancer – meta-analysis based on individual patient data. Cochrane Database Syst Rev 2008;3:CD007303.
Devon KM, McLeod RS. Pre and peri-operative erythropoietin for reducing allogeneic blood transfusions in colorectal cancer surgery. Cochrane Database Syst Rev 2009;1:CD007148.
Duh MS, Weiner JR, White LA, Lefebvre P, Dreenberg PE. Management of anaemia – a critical and systematic review of the cost effectiveness of erythropoiesis-stimulating agents. Pharmacoeconomics 2008;26:99–120.
Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 2007;12:231–42.
Henry DH, Gordan LN, Charu V, Wilhelm FE, Williams D, Xie J, et al. Randomized, open-label comparison of epoetin alfa extended dosing (80000 U Q2W) vs. weekly dosing (40000 U QW) in patients with chemotherapy-induced anemia. Curr Med Res Opin 2006;22:1403–13.
Machtay M, Pajak TF, Suntharalingam M, Shenouda G, Hershock D, Stripp DC, et al. Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: a randomized trial of the Radiation Therapy Oncology Group (RTOG 99-03). Int J Radiat Oncol Biol Phys 2007;69:1008–17.
Moyo V, Lefebvre P, Duh MS, Yektashenas B, Mundle S. Erythropoiesis-stimulating agents in the treatment of anemia in myelodysplastic syndromes: a meta-analysis. Ann Hematol 2008;87:527–36.
Reed SD, Radeva JI, Daniel DB, Fastenau JM, Williams D, Schulman KA. Early hemoglobin response and alternative metrics of efficacy with erythropoietic agents for chemotherapy-related anemia. Curr Med Res Opin 2005;21:1527–33.
Reed SD, Radeva JI, Daniel DB, Mody SH, Forlenza JB, McKenzie RS, et al. Economic evaluation of weekly epoetin alfa versus biweekly darbepoetin alfa for chemotherapy-induced anaemia – evidence from a 16-week randomised trial. Pharmacoeconomics 2006;24:479–94.
Rosenzweig MQ, Bender CM, Lucke JP, Yasko JM, Brufsky AM. The decision to prematurely terminate a trial of R-HuEPO due to thrombotic events. J Pain Symptom Manage 2004;27:185–90.
Schwartzberg L, Yee L, Charu V, Tomita D, Rossi G, Senecal F. Comparable efficacy and safety of darbepoetin alfa 200 mug every 2 weeks and epoetin alfa 40,000 U weekly in patients with breast cancer: results of a randomized comparison. J Support Oncol 2005;3(Suppl. 1):30–1.
Schwartzberg LS, Yee LK, Senecal FM, Charu V, Tomita D, Wallace J, et al. Symptom management and supportive care. A randomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia in patients with breast, lung, or gynecologic cancer. Oncologist 2004;9:696–707.
Senecal FM, Yee L, Gabrail N, Charu V, Tomita D, Rossi G, et al. Treatment of chemotherapy-induced anemia in breast cancer: results of a randomized controlled trial of darbepoetin alfa 200 microg every 2 weeks versus epoetin alfa 40,000 U weekly. Clin Breast Cancer 2005;6:446–54.
Strauss HG, Haensgen G, Dunst J, Hayward CRW, Burger HU, Scherhag A, et al. Effects of anemia correction with epoetin beta in patients receiving radiochemotherapy for advanced cervical cancer. Int J Gynecol Cancer 2008;18:515–24.
Tonia T, Bohlius J. Ten years of meta-analyses on erythropoiesis-stimulating agents in cancer patients. Cancer Treat Res 2011;157:217–38.
Reason for exclusion: study design (n = 64)
Aapro M, Coiffier B, Dunst J, Österborg A, Burger HU. Effect of treatment with epoetin beta on short-term tumour progression and survival in anaemic patients with cancer: a meta-analysis. Br J Cancer 2006;95:1467–73.
Aapro M, Osterwalder B, Scherhag A, Burger HU. Epoetin-beta treatment in patients with cancer chemotherapy-induced anaemia: the impact of initial haemoglobin and target haemoglobin levels on survival, tumour progression and thromboembolic events. Br J Cancer 2009;101:1961–71.
Aapro M, Scherhag A, Burger HU. Effect of treatment with epoetin-beta on survival, tumour progression and thromboembolic events in patients with cancer: an updated meta-analysis of 12 randomised controlled studies including 2301 patients. Br J Cancer 2008;99:14–22.
Aapro MS, Dale DC, Blasi M, Sarokhan B, Ahmed F, Woodman RC. Epoetin alfa increases hemoglobin levels and improves quality of life in anemic geriatric cancer patients receiving chemotherapy. Support Care Cancer 2006;14:1184–94.
Ariganello O, Mancuso A, Di Molfetta M, Diana F, Beccaglia P, Cortesi E, et al. A new induction schedule of epoetin alfa 40.000 IU in anemic patients with advanced lung cancer. Lung Cancer 2004;46:119–24.
Azzara A, Carulli G, Galimberti S, Barate C, Fazzi R, Cervetti G, et al. High-dose (40,000 IU twice/week) alpha recombinant human erythropoietin as single agent in low/intermediate risk myelodysplastic syndromes: a retrospective investigation on 133 patients treated in a single institution. Am J Hematol 2011;86:762–7.
Badzek S, Curic Z, Krajina Z, Plestina S, Golubic-Cepulic B, Radman I. Treatment of cancer-related anemia. Coll Antropol 2008;32:615–22.
Beer TM, Bergenstock M, Birt K, Higano CS. Darbepoetin alfa administered every 4 weeks for anemia in patients with advanced prostate cancer. Clin Genitourin Cancer 2007;5:329–33.
Bennett CL. The blue cross blue shield assessment technology review: summary of findings. Best Pract Res Clin Haematol 2005;18:423–31.
Boccia R, Lillie T, Tomita D, Balducci L. The effectiveness of darbepoetin alfa administered every 3 weeks on hematologic outcomes and quality of life in older patients with chemotherapy-induced anemia. Oncologist 2007;12:584–93.
Bogdanos J, Karamanolakis D, Milathianakis K, Repousis P, Chloraki-Bobota A, Majed H, et al. Epoetin beta (NeoRecormon) corrects anaemia in patients with hormone-refractory prostate cancer and bone metastases. Anticancer Res 2004;24:1957–61.
Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006;98:708–14.
Boogaerts M, Oberhoff C, Huinink WTB, Nowrousian MR, Hayward CRW, Burger HU. Epoetin beta (NeoRecormon (R)) therapy in patients with solid tumours receiving platinum and non-platinum chemotherapy: a meta-analysis. Anticancer Res 2006;26:479–84.
Boulaamane L, Goncalves A, Boutayeb S, Viens P, M’Rabti H, Bertucci F, et al. Prognostic impact of the combination of erythropoiesis-stimulating agents to cancer treatment: literature review. Support Care Cancer 2013;21:2359–69.
Burstein HJ, Parker LM, Keshaviah A, Doherty J, Partridge AH, Schapira L, et al. Efficacy of pegfilgrastim and darbepoetin alfa as hematopoietic support for dose-dense every-2-week adjuvant breast cancer chemotherapy. J Clin Oncol 2005;23:8340–7.
Cacic DL, Hervig T, Seghatchian J. Anemia treatment of lymphoproliferative malignancies with erypoiesis: an overview of state of the art. Transfus Apheresis Sci 2013;48:277–81.
Caocci G, La Nasa G, Efficace F. Health-related quality of life and symptom assessment in patients with myelodysplastic syndromes. Exp Rev Hematol 2009;2:69–80.
Cella D, Kallich J, McDermott A, Xu X. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from rive randomized clinical trials. Ann Oncol 2004;15:979–86.
Cella D, Viswanathan H, Hays RD, Mendoza T, Stein K, Pasta D, et al. Development of the Brief Functional Capacity Tool (BFCT): a brief instrument to identify functional capacity deficits in anemic cancer patients. J Support Oncol 2007;5(Suppl. 2):10–11.
Cersosimo RJ, Jacobson DR. Epoetin alfa versus darbepoetin alfa in chemotherapy-related anemia. Ann Pharmacother 2006;40:58-65; quiz 169–70.
Chu E, Einhorn LH, Lefebvre P. Clinical benefits of once-weekly epoetin alfa in anemic patients with colorectal cancer receiving chemotherapy. J Support Oncol 2006;4:243–50.
Corapcioglu F, Aksu G, Basar EZ, Demirel A, Oncel S, Mutlu A. Recombinant human erythropoietin beta therapy: an effective strategy to reduce transfusion requirement in children receiving anticancer treatment. Pediatr Hematol Oncol 2008;25:509–21.
Cornes P, Coiffier B, Zambrowski J-J. Erythropoietic therapy for the treatment of anemia in patients with cancer: a valuable clinical and economic option. Curr Med Res Opin 2007;23:357–68.
Couture F, Turner AR, Melosky B, Xiu L, Plante RK, Lau CY, et al. Prior red blood cell transfusions in cancer patients increase the risk of subsequent transfusions with or without recombinant human erythropoietin management. Oncologist 2005;10:63–71.
da Silva Costa MT, Schimassek A, Fontes P, Castro V, Fernandes T, Oliveira A, et al. Erythropoiesis-stimulating agents in the treatment of anemia in patients with head and neck tumors ? Analysis of quality of life. Radiother Oncol 2006;81:S339.
Durmaz O, Demirkaya M, Sevinir B. Recombinant human erythropoietin: the effect of weekly dosing on anemia, quality of life, and long-term outcomes in pediatric cancer patients. Pediatr Hematol Oncol 2011;28:461–8.
Esquerdo G, Llorca C, Cervera JM, Orts D, Juarez A, Carrato A. Effectiveness of darbepoetin alfa in a cohort of oncology patients with chemotherapy-induced anaemia. Relationship between variation in three fatigue-specific quality of life questionnaire scores and change in haemoglobin level. Clin Transl Oncol 2011;13:341–7.
Eton DT, Cella D. Do erythropoietic-stimulating agents relieve fatigue? A review of reviews. Cancer Treat Res 2011;157:181–94.
Fagnoni P, Limat S, Chaigneau L, Briaud S, Schmitt B, Merrouche Y, et al. Clinical and economic impact of epoetin in adjuvant-chemotherapy for breast cancer. Support Care Cancer 2006;14:1030–7.
Folloder J. Effects of darbepoetin alfa administered every two weeks on hemoglobin and quality of life of patients receiving chemotherapy. Oncol Nurs Forum 2005;32:81–91.
Gascon P. Evaluating erythropoietic agents for the treatment of anaemia in the oncology setting. Eur J Cancer 2005;41:2601–12.
Gaston KE, Kouba E, Moore DT, Pruthi RS. The use of erythropoietin in patients undergoing radical prostatectomy: effects on hematocrit, transfusion rates and quality of life. Urol Int 2006;77:211–15.
Glaspy J, Crawford J, Vansteenkiste J, Henry D, Rao S, Bowers P, et al. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer 2010;102:301–15.
Glaspy JA. Randomized controlled trials of the erythroid-stimulating agents in cancer patients. Cancer Treat Res 2011;157:195–215.
Gregory SA. Efficacy of darbepoetin alfa in the treatment of chemotherapy-induced anemia in non-Hodgkin’s lymphoma. Support Cancer Ther 2006;3:232–9.
Jones M, Schenkel B, Just J, Fallowfield L. Epoetin alfa improves quality of life in patients with cancer: results of metaanalysis. Cancer 2004;101:1720–32. [Erratum published in Cancer 2005;103:1984].
Kallich J, McDermott A, Xu X, Fayers P, Cella D. The relationship between patient knowledge of hemoglobin levels and health-related quality of life. Qual Life Res 2006;15:57–68.
Kimel M, Leidy NK, Mannix S, Dixon J. Does epoetin alfa improve health-related quality of life in chronically ill patients with anemia? Summary of trials of cancer, HIV/AIDS, and chronic kidney disease. Value Health 2008;11:57–75.
Larsson G, Janson ET. Anemia in patients with midgut carcinoid, treated with alpha interferon: effects by erythropoietin treatment on the perceived quality of life. Eur J Cancer Care 2008;17:200–4.
Latagliata R, Oliva EN, Volpicelli P, Carmosino I, Breccia M, Vincelli I, et al. Twice-weekly high-dose rHuEpo for the treatment of anemia in patients with low-risk myelodysplastic syndromes. Acta Haematol 2008;120:104–7.
Ludwig H, Crawford J, Österborg A, Vansteenkiste J, Henry DH, Fleishman A, et al. Pooled analysis of individual patient-level data from all randomized, double-blind, placebo-controlled trials of darbepoetin alfa in the treatment of patients with chemotherapy-induced anemia. J Clin Oncol 2009;27:2838–47.
Lyman GH, Glaspy J. Are there clinical benefits with early erythropoietic intervention for chemotherapy-induced anemia? A systematic review. Cancer 2006;106:223–33.
Maisnar V, Chroust K. Treatment of associated anemia in different hematological disorders with epoetin alpha. Neoplasma 2004;51:379–84.
Michallet M, Goldet K, Sobh M, Morisset S, Chelghoum Y, Thomas X, et al. Prospective study of erythropoietin use on quality of life and cost effectiveness in acute myeloid leukemia and allogeneic hematopoietic stem cell transplantation patients. Cancer 2013;119:107–14.
Moul JW, Dawson N. Quality of life associated with treatment of castration-resistant prostate cancer: a review of the literature. Cancer Invest 2012;30:1–12.
Newland AM, Black CD. Tumor progression associated with erythropoiesis-stimulating agents. Ann Pharmacother 2008;42:1865–70.
Ohashi Y, Uemura Y, Fujisaka Y, Sugiyama T, Ohmatsu H, Katsumata N, et al. Meta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: individual patient data from Japanese randomized, placebo-controlled trials. Cancer Sci 2013;104:481–5.
Oliva EN, Nobile F, Alimena G, Specchia G, Danova M, Rovati B, et al. Darbepoetin alfa for the treatment of anemia associated with myelodysplastic syndromes: efficacy and quality of life. Leuk Lymphoma 2010;51:1007–14.
Ots PMS, Carrizosa CL, Perez AR, de Dios Saez Garrido J, Perez JMD. Darbepoetin versus epoetin alfa for the correction of anemia in cancer patients receiving radiotherapy or chemoradiotherapy treatment. Clin Med Oncol 2008;2:393–9.
Palumbo A, Petrucci MT, Lauta VM, Musto P, Caravita T, Barbui AM, et al. Correlation between fatigue and hemoglobin level in multiple myeloma patients: results of a cross-sectional study. Haematologica 2005;90:858–60.
Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Addition of iron to erythropoiesis-stimulating agents in cancer patients: a meta-analysis of randomized trials. J Cancer Res Clin Oncol 2012;138:179–87.
Quirt I, Kovacs M, Couture F, Turner AR, Noble M, Burkes R, et al. Patients previously transfused or treated with epoetin alfa at low baseline hemoglobin are at higher risk for subsequent transfusion: an integrated analysis of the Canadian experience. Oncologist 2006;11:73–82.
Reinhardt U, Tulusan A, Angermund R, Lutz H. Increased hemoglobin levels and improved quality-of-life assessments during epoetin alfa treatment in anemic cancer patients: results of a prospective, multicenter German trial. Oncologist 2005;10:225–37.
Reinhardt U, Tulusan A, Angermund R, Lutz H. Symptom management and supportive care. Increased hemoglobin levels and improved quality-of-life assessments during epoetin alfa treatment in anemic cancer patients: results of a prospective, multicenter German trial. Oncologist 2005;10:225–37.
Revicki DA, Stull D, Vernon M, Rader M, Tomita D, Viswanathan HN. Assessing the effect of darbepoetin alfa on patient-reported fatigue in chemotherapy-induced anemia in four randomized, placebo-controlled clinical trials. Qual Life Res 2012;21:311–21.
Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 2010;116:4045–59.
Ross SD, Allen E, Henry DH, Seaman C, Sercus B, Goodnough LT. Clinical benefits and risks associated with epoetin and darbepoetin in patients with chemotherapy-induced anemia: a systematic review of the literature. Clin Ther 2006;28:801–31.
Steensma DP, Loprinzi CL. Epoetin alfa and darbepoetin alfa go head to head. J Clin Oncol 2006;24:2232–6.
Stephens JM, Gramegna P, Laskin B, Botteman MF, Pashos CL. Chronic lymphocytic leukemia: economic burden and quality of life: literature review. Am J Ther 2005;12:460–6.
Stull DE, Vernon MK, Legg JC, Viswanathan HN, Fairclough D, Revicki DA. Use of latent growth curve models for assessing the effects of darbepoetin alfa on hemoglobin and fatigue. Contemp Clin Trials 2010;31:172–9.
Tonelli M, Hemmelgarn B, Reiman T, Manns B, Reaume MN, Lloyd A, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ 2009;180:E62–71.
Vansteenkiste J, Glaspy J, Henry D, Ludwig H, Pirker R, Tomita D, et al. Benefits and risks of using erythropoiesis-stimulating agents (ESAs) in lung cancer patients: study-level and patient-level meta-analyses. Lung Cancer 2012;76:478–85.
Vansteenkiste J, Wauters I, Elliott S, Glaspy J, Hedenus M. Chemotherapy-induced anemia: the story of darbepoetin alfa. Curr Med Res Opin 2013;29:325–37.
Yang S, Jun M, Hong-Li Z, Jian-Min W, Chun W, Lu-Gui Q, et al. A multi-center open-labeled study of recombinant erythropoietin-beta in the treatment of anemic patients with multiple myeloma, low-grade non-Hodgkin’s lymphoma, or chronic lymphocytic leukemia in Chinese population. Int J Hematol 2008;88:139–44.
Reason for exclusion: study design (systematic reviews of randomised controlled trials that were scrutinised for references) (n = 8)
Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Österborg A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 2007;43:258–70.
Kvam AK, Fayers P, Hjermstad M, Gulbrandsen N, Wisloff F. Health-related quality of life assessment in randomised controlled trials in multiple myeloma: a critical review of methodology and impact on treatment recommendations. Eur J Haematol 2009;83:279–89.
Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr 2004;(32):40–50.
Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2010;7:CD006704.
Ross SD, Allen IE, Probst CA, Sercus B, Crean SM, Ranganathan G. Efficacy and safety of erythropoiesis-stimulating proteins in myelodysplastic syndrome: a systematic review and meta-analysis. Oncologist 2007;12:1264–73.
Shehata N, Walker I, Meyer R, Haynes AE, Imrie K, Cancer Care Ontario Hematology Disease Site Group. The use of erythropoiesis-stimulating agents in patients with non-myeloid hematological malignancies: a systematic review. Ann Hematol 2008;87:961–73.
Tonia T, Mettler A, Robert N, Schwarzer G, Seidenfeld J, Weingart O, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12:CD003407.
Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al. A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technol Assess 2007;11(13).
Reason for exclusion: abstract only (n = 54)
Benefits of epoetin alfa for cancer patients’ quality of life are confirmed after modelling to account for missing data. Curr Med Res Opin 2005;21(Suppl. 2):S6–8.
Darbepoetin during RT boosts Hb level, with QOL benefit. Oncol News Int 2006;15:37.
Epoetins and darbepoetin alfa in malignant disease. Drug Ther Bull 2004;42:21–3.
Meta-analysis of the effects of epoetin alfa treatment on quality of life in anaemic cancer patients. Curr Med Res Opin 2005;21(Suppl. 2):16–18.
Retacrit (epoetin zeta) is an effective treatment for chemotherapy-induced anaemia. EJHP Pract 2009;15:38.
Treating anaemia with epoetin alfa is associated with improvements in quality of life in cancer patients receiving chemotherapy. Curr Med Res Opin 2005;21(Suppl. 2):S9–11.
Aapro M, Scherhag A, Osterwalder B, Ukarma L, Burger HU. Epoetin beta treatment in patients with cancer chemotherapy induced anaemia: the impact of initial haemoglobin and target haemoglobin levels on survival, tumour progression and thromboembolic events. 2009 International MASCC/IS00 Symposium, Rome, Italy, June 2009.
Addeo R, Caraglia M, Frega N, Del Prete S. Two faces for Janus: recombinant human erythropoiesis-stimulating agents and cancer mortality. Exp Rev Hematol 2009;2:513–15.
Altintas S, Bastit L, Vandebroek A, Mossman T, Suto T, Gaede B. Analysis of quality of life responses by efficacy response status in cancer patients with chemotherapy-induced anaemia who received darbepoetin alfa 500 mcg every 3 weeks and IV iron. Haematologica 2007;92:286–7.
Anonymous. Highlights. CMAJ 2009;180:1085.
Antonadou D, Kyprianou C, Apostolou D, Coliarakis N, Athanasiou H, Papadopoulos V, et al. A multicenter, open study evaluating the impact of darbepoetin alfa on anaemia and quality of life, in cancer patients undergoing radiotherapy. EJC Suppl 2005;3:374.
Auerbach M, Silberstein PT, Webb RT, Averyanova S, Ciuleanu TE, Cam L, et al. Darbepoetin alfa (DA) 500 mcg or 300 mcg once every three weeks (Q3W) with or without iron in patients (PTS) with chemotherapy-induced anemia (CIA). Ann Oncol 2008;19(S8):3.
Blumberg N, Heal JM. Erythropoietin to treat anaemia in patients with head and neck cancer. Lancet 2004;363:80–1; author reply 81–2.
Brett AS. An erythropoietin-stimulating agent for cancer-related anemia – no benefit. J Watch Gen Med 8 April 2008. URL: www.jwatch.org/jw200804080000003/2008/04/08/erythropoietin-stimulating-agent-cancer-related (accessed 28 September 2015).
Bruun KH, Norgaard A, Johansson P, Daugaard G, Sorensen M. HaemOPtimal: randomized feasibility study of the optimal haemoglobin trigger for red blood cell transfusion (RBC) of anaemic cancer patients (PTS) treated with chemotherapy (CT). 12th Annual NATA Symposium, Dublin, Ireland, April 2011. Tranfus Altern Transfus Med 2011;12:19–20.
Burton MJ, Deschler DG, Rosenfeld RM. Extracts from The Cochrane Library: erythropoietin as an adjuvant treatment with (chemo) radiation therapy for head and neck cancer. Otolaryngol Head Neck Surg 2009;141:438–41.
Caravita T, Siniscalchi A, Montanaro M, Niscola P, Stasi R, Amadori S, et al. High-dose epoetin alfa as induction treatment for severe anemia in multiple myeloma patients. Int J Hematol 2009;90:270–2.
Charu V, Belani C, Gill A, Bhatt M, Ben-Jacob A, Tomita D, et al. A controlled, randomized, open-label study to evaluate the effects of every-2-week darbepoetin alfa for anemia of cancer. J Support Oncol 2005;3(Suppl. 1):12–13.
Charu V, Saidman B, Ben-Jacob A, Justice GR, Maniam AS, Rearden T, et al. Improvements in fatigue are associated with early treatment with darbepoetin alfa every 3 weeks in anemic patients receiving chemotherapy. J Support Oncol 2005;3(Suppl. 1):14–15.
Dintinjana RD, Nacinovic AD, Dintinjana M, Petranovic D. Influence of anaemia on clinical symptoms, quality of life and cognitive functions in chemotherapy naive cancer patients. IPOS 12th World Congress of Psycho-Oncology, Quebec City, QC, Canada, May 2010. Psychooncology 2010;19(Suppl. 2):S234.
Engert M, Haverkamp H, Borchmann P, Josting A, Fuchs M, Diehl V. A prospectively randomized placebo-controlled trial of epoetin-a in patients with advanced-stage Hodgkin lymphoma: final analysis of the GHSG HD15-EPO trial. 14th Congress of the European Hematology Association, Berlin, Germany, June 2009. Haematologica 2009;94:438.
Fagim M, Dina S, Svetlana D. Anaemic syndrome correction in patients receiving chemoradiation therapy with eprex for uterine cervical cancer. Radiother Oncol 2011;99:S308.
Gabrilove J, Paquette R, Lyons RM, Mushtaq C, Sekeres MA, Lam H, et al. The efficacy and safety of darbepoetin alfa for treating anemia in low-risk myelodysplastic syndrome patients: results after 53/55 weeks. J Support Oncol 2007;5(Suppl. 2):14–15.
Gascon P. Intravenous iron: supplement or substitute to ESAs? Tumor Biol 2012;33:S62–3.
Green D. Erythropoietin for myelodysplastic syndromes. J Watch Oncol Hematol 20 October 2009. URL: www.jwatch.org/oh200910200000003/2009/10/20/erythropoietin-myelodysplastic-syndromes (accessed 28 September 2015).
Gregory SA, Blayney DW, Vadhan-Raj S, Tomita DK, Rossi G, Mirtsching B. Efficacy of darbepoetin alfa in the treatment of chemotherapy-induced anemia in non-Hodgkin’s lymphoma. J Support Oncol 2005;3(Suppl. 1):24–5.
Gupta S, Singh PK, Bhatt ML, Pant MC, Sundar S, Verma J, et al. Clinical benefits of epoetin beta in patients with advanced stage hormone refractory prostate cancer. Eur Urol Suppl 2011;10:337.
Hassan MA, Sleem MM. Phase II trial comparing darbepoetin alfa every 3-week versus weekly epoetin alfa for the treatment of chemotherapy-induced anemia. J Clin Oncol 2009;27(Suppl. 1):e20724.
Hellstrm-Lindberg E. Erythropoiesis-stimulating agents in myelodysplastic syndromes. Leuk Lymphoma 2010;51:1155–6.
Henry DH, Dahl NV, Auerbach MA. Thrombocytosis and venous thromboembolism in cancer patients with chemotherapy induced anemia may be related to ESA induced iron restricted erythropoiesis and reversed by administration of IV iron. Am J Hematol 2012;87:308–10.
Heras P, Hatzopoulos A, Mitsibounas D. Effectivness of recombinant human erythropoietin (epoetin beta, EPO) in improving hematological parameters and QOL in patients with chemotherapy-induced anemia. A double-blind, parallel-group, dose-finding study. EJC Suppl 2005;3:373.
Hinds PS, Hockenberry M, Feusner J, Hord JD, Rackoff W, Rozzouk BI. Hemoglobin response and improvements in quality of life in anemic children with cancer receiving myelosuppressive chemotherapy. J Support Oncol 2005;3(Suppl. 4):10–11.
Kaanders JH, der Kogel V. Erythropoietin to treat anaemia in patients with head and neck cancer. Lancet 2004;363:78–9; author reply 81–2.
Katsumata N, Fujiwara Y, Saijo N, Ohashi Y. Once-weekly epoetin beta improves hemoglobin and quality of life in anemic cancer patients receiving chemotherapy. EJC Suppl 2005;3:375.
Katsumata N, Fujiwara Y, Sugiyama T, Goto I, Ohmatsu H, Okamoto R, et al. Erythropoiesis-stimulating agents for the treatment of chemotherapy-induced anemia and mortality: a meta-analysis of individual patient data from Japanese randomized trials. Eur J Cancer 2011;47:S242.
Leyland-Jones B, Mahmud S. Erythropoietin to treat anaemia in patients with head and neck cancer. Lancet 2004;363:80–2.
Mel JR, Salar A, Rodriguez CA, Alegre A, Gonzalez A, Cassinello J, et al. Weekly fixed dose of darbepoetin alfa (DA) is efficacious and improves health-related quality of life in patients with chemotherapy induced anemia (CIA). On behalf of the AMG-DAR-2002-01 study group. Ann Oncol 2006;17:292–3.
Mhaskar R, Wao H, Kumar A, Miladinovic B, Djulbegovic B. Role of iron supplementation to erythropoiesis stimulating agents in the management of chemotherapy-induced anemia in cancer patients: a systematic review and meta-analysis. Blood 2010;116:2055.
Michallet M, Goldet K, Morisset S, Sobh M, Chelghoum Y, Thomas X, et al. Erythropoietin use in patients with AML or undergoing allogeneic HSCT significantly improves quality of life and reduces red blood cells and platelets transfusions without any survival effect. Blood 2010;116:3810.
Michallet M, Goldet K, Morisset S, Sobh M, Ducastelle S, Chelghoum Y, et al. Erythropoietin use in patients undergoing allogeneic HSCT does not impact on quality of life, reduces red blood cells transfusions without any survival effect. 37th Annual Congress of the European Group for Blood and Marrow Transplantation (EBMT), Paris, France, April 2011.
Nitz U, Oberhoff C, Reimer T, Schumacher C, Hackmann J, Warm M, et al. Adjuvant chemotherapy with or without darbepoetin in node-positive breast cancer: a safety analysis from the Phase III ARA plus trial. Cancer Res 2009;69(Suppl.):4100.
Oliva EN, Latagliata R, Danova M, Specchia G, Impera S, Rovati B, et al. Darbepoetin for the treatment of anemia of myelodysplastic syndromes: efficacy and quality of life. Leuk Res 2007;31:S117–18.
Oliva EN, Latagliata R, Danova M, Vincelli I, Rovati B, Ronco F, et al. Darbepoetin for the treatment of anemia of myelodysplastic syndromes: efficacy and improvements in quality of life. Blood 2006;108:754A.
Ordonez A, Gonzalez-Baron M, De Castro J, Isla D, Sanchez A, Arrivi A, et al. Epoetin beta (NeoRecormon (R)) prevents anaemia and improves quality of life in lung cancer patients receiving platinum-based chemotherapy. Lung Cancer 2005;49:S339.
Patrick D, Gagnon DD, Zagari MJ. Improvements in quality of life associated with epoetin alfa treatment are clinically, as well as statistically, significant. Curr Med Res Opin Suppl 2005;21:S3–5.
Ross SD, Allen IE, Henry D, Seaman C, Sercus B, Goodnough LT. Clinical benefits and risks associated with epoetin (alfa/beta) and darbepoetin alfa in patients with chemotherapy-induced anemia: a systematic review of the literature. J Support Oncol 2007;5(Suppl. 2):20–1.
Sakai H, Saijo N, Ohashi Y. Once-weekly epoetin beta improves hemoglobin levels and quality of life in patients with chemotherapy-induced anemia: a randomized, double-blind, parallel-group, dose-finding study. Ann Oncol 2004;15:219.
Steensma DP, Dakhil SR, Novotny PJ, Sloan JA, Johnson DB, Anderson DM, et al. A randomized comparison of standard weekly epoetin alfa to every-3-week epoetin alfa and every-3-week darbepoetin alfa: a study of the Mayo Clinic Cancer Research Consortium (MCCRC). 51st Annual Meeting of the American Society of Hematology (ASH), New Orleans, LA, USA, December 2009. Blood 2009;114:abstract 3008.
Suzuki Y, Tokuda Y, Okamoto R, Nakagawa K, Ando K, Iwata H, et al. Randomized, placebo-controlled Phase II study of darbepoetin alfa (DA) administered every three weeks (Q3W) in patients with chemotherapy-induced anemia (CIA). Ann Oncol 2008;19:viii277.
Wilkinson PM, Antonopoulos M, Lahousen M, Lind M, Kosmidis P. Epoetin alfa in platinum-treated ovarian cancer patients: results of a multinational, multicentre, randomised trial. Br J Cancer 2006;94:947–54.
Winquist E, Julian JA, Moore MJ, Nabid A, Sathya J, Wood L, et al. Randomized, double-blind, placebo-controlled trial of epoetin alfa in men with castration-resistant prostate cancer and anemia. J Clin Oncol 2009;27:644–6.
Witzig TE, Silbertstein PT, Loprinzi CL, Spigel DR. Weekly erythropoietin improves anemia associated with cancer treatment. J Clin Outcomes Manage 2004;11:751–2.
Yoshizaki A, Kumagai S, Sugiyama T, Goto I, Saito H, Ariyoshi Y, et al. A Phase III, randomized double-blind placebocontrolled study of epoetin beta in lung and gynecological cancer receiving platinum-based chemotherapy: Japan erythropoietin study group. 35th ESMO Congress, Milan, Italy, October 2010. Ann Oncol 2010;21:viii385.
Youssef LA, Hussien DH, Sulaiman S. The effectiveness of a fixed low dose of erythropoietin (EPO) in anemic solid tumor patients receiving concomitant chemotherapy: a prospective, randomized, controlled study. 53rd Annual Meeting of the American Society of Hematology (ASH), San Diego, CA, USA, 2011. Blood 2011;118:abstract 2092.
Reason for exclusion: duplicate (n = 6)
Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006;98:708–14.
Palumbo A, Petrucci MT, Lauta VM, Musto P, Caravita T, Barbui AM, et al. Correlation between fatigue and hemoglobin level in multiple myeloma patients: results of a cross-sectional study. Haematologica 2005;90:858–60.
Schwartzberg LS, Yee LK, Senecal FM, Charu V, Tomita D, Wallace J, et al. Symptom management and supportive care. A randomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia in patients with breast, lung, or gynecologic cancer. Oncologist 2004;9:696–707.
Steensma DP, Dakhil SR, Dalton R, Kahanic SP, Kugler JW, Stella PJ, et al. A Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy-associated anemia: a study of the Mayo Clinic Cancer Research Consortium (MCCRC). 51st Annual Meeting of the American Society of Hematology (ASH), New Orleans, LA, USA, December 2009. Blood 2009;114:abstract 630.
Vansteenkiste J, Wauters I, Elliott S, Glaspy J, Hedenus M. Chemotherapy-induced anemia: the story of darbepoetin alfa. Curr Med Res Opin 2013;29:325–37.
Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al. A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technol Assess 2007;11(13).
Reason for exclusion: language (n = 2)
Ludwig H, Auberger T, Burghuber OC, Gnant M, Hopfinger G, Jager U, et al. Replacement of erythropoese stimulated protein in anemic patients with malignant disease. Wien Klin Wochenschr 2008;120:507–13.
Zemelka T, Rolski J, Ziobro M, Michalczyk A. Opinion on influence of erythropoietin on quality of life and survival in patients with advanced non-small cell lung cancer. Contemp Oncol 2007;11:37–40.
Reason for exclusion: no usable data (n = 11)
Earlier initiation of treatment recommended in using erythropoietic agents for chemotherapy-induced anemia. J Support Oncol 2004;2:319.
Anthony LB, Gabrail NY, Ghazal H, Woytowitz DV, Flam MS, Drelichman A, et al. IV iron sucrose for cancer and/or chemotherapy-induced anemia in patients treated with erythropoiesisstimulating agents. Community Oncol 2011;8:270–8.
Arcasoy MO, Amin K, Chou SC, Haroon ZA, Varia M, Raleigh JA. Erythropoietin and erythropoietin receptor expression in head and neck cancer: relationship to tumor hypoxia. Clin Cancer Res 2005;11:20–7.
Borg S, Glenngard AH, Österborg A, Persson U. The cost-effectiveness of treatment with erythropoietin compared to red blood cell transfusions for patients with chemotherapy induced anaemia: a Markov model. Acta Oncol 2008;47:1009–17.
Clark J, Schergen A. Advantages of every-3-week dosing of erythropoietic agents to manage chemotherapy-induced anemia. Oncology 2006;20:795–800.
Dale DC. The benefits of haematopoietic growth factors in the management of gynaecological oncology. Eur J Gynaecol Oncol 2004;25:133–44.
Greaves P, Agrawal S. Safe and efficacious use of recombinant human erythropoietin in malignancy. Clin Med 2007;7:617–20.
Jadersten M, Malcovati L, Dybedal I, Della Porta MG, Invernizzi R, Montgomery SM, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol 2008;26:3607–13.
Jadersten M, Montgomery SM, Dybedal I, Porwit-MacDonald A, Hellstrom-Lindberg E. Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood 2005;106:803–11.
Moebus V, Jackisch C, Schneeweiss A, Huober J, Lueck HJ, du Bois A, et al. Adding epoetin alfa to intense dose-dense adjuvant chemotherapy for breast cancer: randomized clinical trial. J Natl Cancer Inst 2013;105:1018–26.
Roungrong J, Teerawattananon Y, Chaikledkaew U. Cost–utility analysis of recombinant human erythropoietin in anemic cancer patients induced by chemotherapy in Thailand. J Med Assoc Thai 2008;91(Suppl. 2):119–25.
Reason for exclusion: unobtainable (n = 1)
Adamson JW. Erythropoietic-stimulating agents: the cancer progression controversy and collateral damage to the blood supply. Transfusion 2009;49:824–6.
Reason for exclusion: unlicensed dose (n = 36)
Aapro M, Scherhag A, Burger HU. Effect of treatment with epoetin-beta on survival, tumour progression and thromboembolic events in patients with cancer: an updated meta-analysis of 12 randomised controlled studies including 2301 patients. Br J Cancer 2008;99:14–22.
Aapro M, Scherhag A, Osterwalder B, Ukarma L, Burger HU. Epoetin beta treatment in patients with cancer chemotherapy induced anaemia: the impact of initial haemoglobin and target haemoglobin levels on survival, tumour progression and thromboembolic events. International MASCC/IS00 Symposium, Rome, Italy, June 2009. Support Care Cancer 2009;17:1036–7.
Berndt E, Kallich J, McDermott A, Xu X, Lee H, Glaspy J. Reductions in anaemia and fatigue are associated with improvements in productivity in cancer patients receiving chemotherapy. Pharmacoeconomics 2005;23:505–14.
Blohmer JU, Paepke S, Sehouli J, Boehmer D, Kolben M, Wurschmidt F, et al. Randomized Phase III trial of sequential adjuvant chemoradiotherapy with or without erythropoietin alfa in patients with high-risk cervical cancer: results of the NOGGO-AGO intergroup study. J Clin Oncol 2011;29:3791–7.
Cabanillas ME, Kantarjian H, Thomas DA, Mattiuzzi GN, Rytting ME, Bruera E, et al. Epoetin alpha decreases the number of erythrocyte transfusions in patients with acute lymphoblastic leukemia, lymphoblastic lymphoma, and Burkitt leukemia/lymphoma: results of a randomized clinical trial. Cancer 2012;118:848–55.
Chang J, Couture F, Young S, McWatters KL, Lau CY. Weekly epoetin alfa maintains hemoglobin, improves quality of life, and reduces transfusion in breast cancer patients receiving chemotherapy. J Clin Oncol 2005;23:2597–605.
Chang J, Couture FA, Young SD, Lau CY, Lee McWatters K. Weekly administration of epoetin alfa improves cognition and quality of life in patients with breast cancer receiving chemotherapy. Support Cancer Ther 2004;2:52–8.
Charu V, Belani CP, Gill AN, Bhatt M, Tomita D, Rossi G, et al. Efficacy and safety of every-2-week darbepoetin alfa in patients with anemia of cancer: a controlled, randomized, open-label Phase II trial. Oncologist 2007;12:727–37.
Christodoulou C, Dafni U, Aravantinos G, Koutras A, Samantas E, Karina M, et al. Effects of epoetin-alpha on quality of life of cancer patients with solid tumors receiving chemotherapy. Anticancer Res 2009;29:693–702.
Engert A, Josting A, Haverkamp H, Villalobos M, Lohri A, Sokler M, et al. Epoetin alfa in patients with advanced-stage Hodgkin’s lymphoma: results of the randomized placebo-controlled GHSG HD15EPO trial. J Clin Oncol 2010;28:2239–45.
Fujisaka Y, Sugiyama T, Saito H, Nagase S, Kudoh S, Endo M, et al. Randomised, Phase III trial of epoetin-beta to treat chemotherapy-induced anaemia according to the EU regulation. Br J Cancer 2011;105:1267–72.
Glaspy J, Vadhan-Raj S, Patel R, Bosserman L, Hu E, Lloyd RE, et al. Randomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia: the 20030125 study group trial. J Clin Oncol 2006;24:2290–7.
Gupta S, Singh PK, Bisth SS, Bhatt ML, Pant M, et al. Role of recombinant human erythropoietin in patients of advanced cervical cancer treated ‘by chemoradiotherapy.’ Cancer Biol Ther 2009;8:13–17.
Hernandez E, Ganly P, Charu V, Dibenedetto J, Tomita D, Lillie T, et al. Randomized, double-blind, placebo-controlled trial of every-3-week darbepoetin alfa 300 micrograms for treatment of chemotherapy-induced anemia. Curr Med Res Opin 2009;25:2109–20.
Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol 2005;23:5960–72.
Milroy R, Bajetta E, van den Berg PM, O’Brien MER, Perez-Manga G, Georgoulias V, et al. Effects of epoetin alfa on anemia and patient-reported outcomes in patients with non-small cell lung cancer receiving chemotherapy: results of a European, multicenter, randomized, controlled study. Eur J Clin Med Oncol 2011;3:49–56.
Nagel S, Kellner O, Engel-Riedel W, Guetz S, Schumann C, Gieseler F, et al. Addition of darbepoetin alfa to dose-dense chemotherapy: results from a randomized Phase II trial in small-cell lung cancer patients receiving carboplatin plus etoposide. Clin Lung Cancer 2011;12:62–9.
O’Shaughnessy JA, Vukelja SJ, Holmes FA, Savin M, Jones M, Royall D, et al. Feasibility of quantifying the effects of epoetin alfa therapy on cognitive function in women with breast cancer undergoing adjuvant or neoadjuvant chemotherapy. Clin Breast Cancer 2005;5:439–46.
Pirker R, Ramlau RA, Schuette W, Zatloukal P, Ferreira I, Lillie T, et al. Safety and efficacy of darbepoetin alpha in previously untreated extensive-stage small-cell lung cancer treated with platinum plus etoposide. J Clin Oncol 2008;26:2342–9.
Pronzato P, Cortesi E, van der Rijt CC, Bols A, Moreno-Nogueira JA, de Oliveira CF, et al. Epoetin alfa improves anemia and anemia-related, patient-reported outcomes in patients with breast cancer receiving myelotoxic chemotherapy: results of a European, multicenter, randomized, controlled trial. Oncologist 2010;15:935–43.
Razzouk BI, Hord JD, Hockenberry M, Hinds PS, Feusner J, Williams D, et al. Double-blind, placebo-controlled study of quality of life, hematologic end points, and safety of weekly epoetin alfa in children with cancer receiving myelosuppressive chemotherapy. J Clin Oncol 2006;24:3583–9.
Reed SD, Radeva JI, Daniel DB, Fastenau JM, Williams D, Schulman KA. Early hemoglobin response and alternative metrics of efficacy with erythropoietic agents for chemotherapy-related anemia. Curr Med Res Opin 2005;21:1527–33.
Rosenzweig MQ, Bender CM, Lucke JP, Yasko JM, Brufsky AM. The decision to prematurely terminate a trial of R-HuEPO due to thrombotic events. J Pain Symptom Manage 2004;27:185–90.
Savonije JH, van Groeningen CJ, Wormhoudt LW, Giaccone G. Early intervention with epoetin alfa during platinum-based chemotherapy: an analysis of quality-of-life results of a multicenter, randomized, controlled trial compared with population normative data. Oncologist 2006;11:197–205.
Savonije JH, van Groeningen CJ, van Bochove A, Honkoop AH, van Felius CL, Wormhoudt LW, et al. Effects of early intervention with epoetin alfa on transfusion requirement, hemoglobin level and survival during platinum-based chemotherapy: results of a multicenter randomised controlled trial. Eur J Cancer 2005;41:1560–9.
Savonije JH, van Groeningen CJ, Wormhoudt LW, Giaccone G. Early intervention with epoetin alfa during platinum-based chemotherapy: an analysis of the results of a multicenter, randomized, controlled trial based on initial hemoglobin level. Oncologist 2006;11:206–16.
Schwartzberg LS, Yee LK, Senecal FM, Charu V, Tomita D, Wallace J, et al. A randomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia in patients with breast, lung, or gynecologic cancer. Oncologist 2004;9:696–707.
Senecal FM, Yee L, Gabrail N, Charu V, Tomita D, Rossi G, et al. Treatment of chemotherapy-induced anemia in breast cancer: results of a randomized controlled trial of darbepoetin alfa 200 microg every 2 weeks versus epoetin alfa 40,000 U weekly. Clin Breast Cancer 2005;6:446–54.
Thomas G, Ali S, Hoebers FJ, Darcy KM, Rodgers WH, Patel M, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dl with erythropoietin vs. above 10.0 g/dl without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol 2008;108:317–25.
Tsuboi M, Ezaki K, Tobinai K, Ohashi Y, Saijo N. Weekly administration of epoetin beta for chemotherapy-induced anemia in cancer patients: results of a multicenter, Phase III, randomized, double-blind, placebo-controlled study. Jpn J Clin Oncol 2009;39:163–8.
Wagner LM, Billups CA, Furman WL, Rao BN, Santana VM. Combined use of erythropoietin and granulocyte colony-stimulating factor does not decrease blood transfusion requirements during induction therapy for high-risk neuroblastoma: a randomized controlled trial. J Clin Oncol 2004;22:1886–93.
Waltzman R, Croot C, Justice GR, Fesen MR, Charu V, Williams D. Randomized comparison of epoetin alfa (40,000 U weekly) and darbepoetin alfa (200 microg every 2 weeks) in anemic patients with cancer receiving chemotherapy. Oncologist 2005;10:642–50.
Wilkinson PM, Antonopoulos M, Lahousen M, Lind M, Kosmidis P. Epoetin alfa in platinum-treated ovarian cancer patients: results of a multinational, multicentre, randomised trial. Br J Cancer 2006;94:947–54.
Winquist E, Julian JA, Moore MJ, Nabid A, Sathya J, Wood L, et al. Randomized, double-blind, placebo-controlled trial of epoetin alfa in men with castration-resistant prostate cancer and anemia. J Clin Oncol 2009;27:644–6.
Witzig TE, Silberstein PT, Loprinzi CL, Sloan JA, Novotny PJ, Mailliard JA, et al. Phase III, randomized, double-blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. J Clin Oncol 2005;23:2606–17.
Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol 2007;25:1027–32.
Wilson and colleagues2 excluded studies
Reason for exclusion: population (n = 10)
Blohmer JU, Wurschmidt F, Petry KU, Weise G, Sehouli J, Kimmig R. 6th interim analysis of a prospective, randomised, open and controlled AGO- and NOGGO-intergroup study: sequential adjuvant chemo-radiotherapy with vs without epoetin alfa with patients with high-risk cervival cancer. Proc Am Soc Clin Oncol 2003;22:447.
Henke M, Guttenberger R, Barke A, Pajonk F, Potter R, Frommhold H. Erythropoietin for patients undergoing radiotherapy: a pilot study. Radiother Oncol 1999;50:185–90.
Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet 2003;362:1255–60.
Henze G, Michon J, Morland B, Perek D, Rizzari C, Zoubek A. Phase III randomised study: efficacy of epoetin alfa in reducing blood transfusions in newly diagnosed pediatric cancer patients receiving chemotherapy. Proc Am Soc Clin Oncol 2002;21:387a.
Italian Cooperative Study Group for rHuEpo in Myelodysplastic Syndromes, Ferrini PR, Grossi A, Vannucchi AM, Barosi G, Guarnone R, et al. A randomized double-blind placebo-controlled study with subcutaneous recombinant human erythropoietin in patients with low-risk myelodysplastic syndromes. Br J Haematol 1998;103:1070–4.
Rose E, Rai KR, Revicki DA, Brown R, Rebalndo J. Clinical and health status assessments in anemia chronic lymphocytic leukemia (CLL) patients treated with epoetin alfa (EPO). Blood 1994;84(Suppl. A):526a.
Smith RE Jr, Tchekmedyian NS, Chan D, Meza LA, Northfelt DW, Patel R, et al. A dose- and schedule-finding study of darbepoetin alpha for the treatment of chronic anaemia of cancer. Br J Cancer 2003;88:1851–8.
Sweeney PJ, Nicolae D, Ignacio L, Chen L, Roach M, Wara W, et al. Effect of subcutaneous recombinant human erythropoietin in cancer patients receiving radiotherapy: final report of a randomised, open-labelled, Phase II trial. Br J Cancer 1998;77:1996–2002.
Thompson JA, Gilliland DG, Prchal JT, Bennett JM, Larholt K, Nelson RA, et al. Effect of recombinant human erythropoietin combined with granulocyte/ macrophage colony-stimulating factor in the treatment of patients with myelodysplastic syndrome. GM/EPO MDS Study Group. Blood 2000;95:1175–9.
Wurnig C, Windhager R, Schwameis E, Kotz R, Zoubek A, Stockenhuber F, et al. Prevention of chemotherapy-induced anemia by the use of erythropoietin in patients with primary malignant bone tumors (a double-blind, randomized, Phase III study). Transfusion 1996;36:155–9.
Reason for exclusion: duplicate (n = 2)
Casadevall N, Durieux P, Dubois S, Hemery F, Lepage E, Quarre MC, et al. Health, economic, and quality-of-life effects of erythropoietin and granulocyte colony-stimulating factor for the treatment of myelodysplastic syndromes: a randomized, controlled trial. Blood 2004;104:321–7.
Rosenzweig MQ, Bender CM, Lucke JP, Yasko JM, Brufsky AM. The decision to prematurely terminate a trial of R-HuEPO due to thrombotic events. J Pain Symptom Manage 2004;27:185–90.
Reason for exclusion: abstract only (n = 5)
Carabantes FJ, Benavides M, Trujillo R, Cobo M, Herbrero ML, Garcia S. Epoetin alfa in the prevention of anaemia in cancer patients undergoing platinum-based chemotherapy (CT). A prospective randomised study. 35th Annual meeting of the American Society of Clinical Oncology, Atlanta, GA, USA, May 1999. Abstract 2303.
Huddart RA, Welch RS, Chan S, Perren T, Atkinson R. A prospective randomised comparative group evaluation of epoetin alfa for the treatment of anaemia in UK cancer patients receivin platinum-based chemotherapy. Ann Oncol 2002;13:177.
Janinis D, Dafni U, Aravantinos G, Kalofonos HP, Papakostas D, Tsavdaridis D, et al. Quality of life (QoL) outcome of epoetin alfa (EPO-A) in anemic cancer patients undergoing platinum or non-platinum-based chemotherapy: a randomised study conducted by the Hellenic Cooperative Oncology Group. Proc Am Soc Clin Oncol 2003;22:789.
Quirt I, Micucci S, Moran LA, Pater J, Browman J. The role of recombinant human erythropoietin (EPO) in reducing red blood cell transfusions and maintaining quality of life (QoL) in patients with lymphoma and solid tumours receiving cytotoxic chemotherapy. Results of a randomized, double-blind, placebo-controlled clinical trial. Blood 1996;88:347a.
Thomas H, McAdam KF, Thomas RJ, Joffe JK, Sugden EM, Awwad ST, et al. Early intervention with epoetin alfa for treatment of anaemia and improvement of quality of life in cancer patients undergoing myelotoxic chemotherapy. Ann Oncol 2002;13:177.
Reason for exclusion: unlicensed dose (n = 11)
Bamias A, Aravantinos G, Kalofonos C, Timotheadou N, Siafaka V, Vlahou I, et al. Prevention of anemia in patients with solid tumors receiving platinum-based chemotherapy by recombinant human erythropoietin (rHuEpo): a prospective, open label, randomized trial by the Hellenic Cooperative Oncology Group. Oncology 2003;64:102–10.
Cascinu S, Fedeli A, Del Ferro E, Luzi Fedeli S, Catalano G. Recombinant human erythropoietin treatment in cisplatin-associated anemia: a randomized, double-blind trial with placebo. J Clin Oncol 1994;12:1058–62.
Cazzola M, Messinger D, Battistel V, Bron D, Cimino R, Enller-Ziegler L, et al. Recombinant human erythropoietin in the anemia associated with multiple myeloma or non-Hodgkin’s lymphoma: dose finding and identification of predictors of response. Blood 1995;86:4446–53.
Iconomou G, Koutras A, Rigopoulos A, Vagenakis AG, Kalofonos HP. Effect of recombinant human erythropoietin on quality of life in cancer patients receiving chemotherapy: results of a randomized, controlled trial. J Pain Symptom Manage 2003;25:512–18.
Kunikane H, Watanabe K, Fukuoka M, Saijo N, Furuse K, Ikegami H, et al. Double-blind randomized control trial of the effect of recombinant human erythropoietin on chemotherapy-induced anemia in patients with non-small cell lung cancer. Int J Clin Oncol 2001;6:296–301.
Leyland-Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet 2003;4:459–60.
Oberhoff C, Neri B, Amadori D, Petry KU, Gamucci T, Rebmann U, et al. Recombinant human erythropoietin in the treatment of chemotherapy-induced anemia and prevention of transfusion requirement associated with solid tumors: a randomized, controlled study. Ann Oncol 1998;9:255–60.
Österborg A, Boogaerts MA, Cimino R, Essers U, Holowiecki J, Juliusson G, et al. Recombinant human erythropoietin in transfusion-dependent anemic patients with multiple myeloma and non-Hodgkin’s lymphoma – a randomized multicenter study. The European Study Group of Erythropoietin (Epoetin Beta) Treatment in Multiple Myeloma and Non-Hodgkin’s Lymphoma. Blood 1996;87:2675–82.
Rosen FR, Haraf DJ, Kies MS, Stenson K, Portugal L, List MA, et al. Multicenter randomized Phase II study of paclitaxel (1-hour infusion), fluorouracil, hydroxyurea, and concomitant twice daily radiation with or without erythropoietin for advanced head and neck cancer. Clin Cancer Res 2003;9:1689–97.
Throuvalas N, Antonadu D, Boufi M, Lavey RS, Malamos N. Erythropoietin decreases transfusion requirements during radiochemotherapy. 36th Annual Meeting of the American Society of Clinical Oncology, New Orleans, LA, USA, May 2000. Abstract 1558.
Welch RS, James RD, Wilkinson PM, Belli F, Cowan RA. Recombinant human erythropoietin and platinum-based chemotherapy in advanced ovarian cancer. Cancer J Sci Am 1995;1:261–6.
Reason for exclusion: unlicensed arms from four of the included studies (n = 4)
Hedenus M, Hansen S, Taylor K, Arthur C, Emmerich B, Dewey C, et al. Randomized, dose-finding study of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies. Br J Haematol 2002;119:79–86.
Kotasek D, Steger G, Faught W, Underhill C, Poulsen E, Colowick AB, et al. Darbepoetin alfa administered every 3 weeks alleviates anaemia in patients with solid tumours receiving chemotherapy: results of a double-blind, placebo-controlled, randomised study. Eur J Cancer 2003;39:2026–34.
ten Bokkel Huinink WW, de Swart CA, van Toorn DW, Morack G, Breed WP, Hillen HF. Controlled multicentre study of the influence of subcutaneous recombinant human erythropoietin on anaemia and transfusion dependency in patients with ovarian carcinoma treated with platinum-based chemotherapy. Med Oncol 1998;15:174–82.
Thatcher N, De Campos ES, Bell DR, Steward WP, Varghese G, Morant R, et al. Epoetin alpha prevents anaemia and reduces transfusion requirements in patients undergoing primarily platinum-based chemotherapy for small cell lung cancer. Br J Cancer 1999;80:396–402.
Appendix 5 Systematic reviews
| Author, year | Title (no. of included studies) | Participants | Intervention | Comparator | Outcomes | Design | Results | Comment |
|---|---|---|---|---|---|---|---|---|
| Lawrence 200490 | Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients (27 studies included) | All cancer patients (or cancer survivors) with, or assessed for, fatigue | Various; one study included on epoetin alfa | Various; PBO used in single epoetin alfa study | Fatigue as determined by haemR and QoL measures | Variety; only RCTs included for treatment of CRF | For the epoetin alfa vs. PBO study there was a strong statistically significant correlation between Hb levels and QoL. The mean increase in Hb level from baseline to last value was significantly greater in the epoetin alfa group than in the PBO group (2.2 g/dl vs. 0.5 g/dl; p < 0.001). Significant differences were observed for epoetin alfa for all five cancer- and anaemia-specific primary QoL measures (p ≤ 0.0048) | Only one relevant study involving epoetin alfa was included in this SR |
| aBokemeyer 200741 | EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update (43 studies included in updated search plus additional 78 relevant abstracts) | All anaemic adults with cancer or lymphoproliferative malignancies | ESAs | Various (few individual study details given) | HaemR, RBCT requirement, QoL, OS | Variety; 19 studies were level 1 standard (meta-analysis of good-quality controlled studies or RCTs) | Level 1 evidence exists for a positive impact of erythropoietin proteins on Hb levels when administered to patients with chemotherapy-induced anaemia or anaemia of chronic disease, when used to prevent cancer anaemia, and in patients undergoing cancer surgery | |
| Ross 200791 | Efficacy and safety of erythropoiesis-stimulating proteins in myelodysplastic syndrome: a systematic review and meta-analysis (59 studies included) | Anaemic adults with MDS | ESAs | SC, PBO | HaemR, QoL | Uncontrolled case studies and controlled trials including RCTs (four RCTs included for epoetin vs. control) | Significant increase in haemR (OR 5.2; 95% CI 2.5 to 10.8) found for patients receiving epoetin compared with control patients. Patients receiving erythropoiesis-stimulating proteins attained a pre–post change (measured using FACT-F) that exceeded minimum clinically important differences | Only four relevant studies (RCTs of epoetin vs. control) were included in this SR |
| bWilson 20072 | A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment (46 studies included) | Anaemic adults with cancer | ESAs plus supportive care for anaemia (including RBCT) | SC for anaemia (including RBCT) alone | HaemR, RBCT, Hb change, HRQoL, TR, OS, AEs | RCTs | Epoetin improves haemR (defined as an improvement in Hb of 2 g/dl) (RR 3.4, 95% CI 3 to 3.8; response rate for epoetin of 53%). Hb change showed a WMD of 1.63 g/dl (95% CI 1.46 to 1.8) in favour of epoetin. The number of CIA patients receiving RBCTs reduced by an estimated 18%. A positive effect was observed in favour of an improved HRQoL for patients receiving epoetin | The incidence of side effects and effects on survival remain highly uncertain. Authors suggest that, if there is no impact on survival, it seems highly unlikely that epoetin would be considered a cost-effective use of health-care resources |
| Shehata 200892 | The use of erythropoiesis-stimulating agents in patients with non-myeloid hematological malignancies: a systematic review [22 studies included (17 published reports and five abstracts)] | Adults with non-myeloid hematological malignancies | ESAs | PBO | RBCT, HRQoL, OS | RCTs | Statistically significant decrease in transfusion requirements. No evidence that the use of ESAs improved survival. Impact on QoL was difficult to assess because of limitations in the available studies | Authors state that more data are required to confirm improvements in QoL and inferior survival associated with ESA use |
| Kvam 200993 | Health-related quality-of-life assessment in randomised controlled trials in multiple myeloma: a critical review of methodology and impact on treatment recommendations (15 studies included) | Adults with MM receiving chemotherapy (total n = 2200; epoetin n = 1207) | Epoetin alfa, epoetin beta, darbepoetin alfa | PBO (in relevant studies) | RBCT, Hb change, transfusion-free survival, HRQoL outcomes and HRQoL influence on clinical decision-making (authors’ statement) | RCTs | Statistically significant decrease in RBCT and rise in Hb levels in patients receiving ESAs. Improvement in HRQoL for epoetin beta (one study); improvement in HRQoL for darbepoetin alfa (one study); improvement in cancer- and anaemic-specific HRQoL domains for epoetin alfa (one study) | Only four relevant studies were included in this SR. Epoetin alfa was recommended based on better clinical outcomes and improvement in HRQoL (two studies). Epoetin beta was recommended based on improved HRQoL and better clinical outcomes (one study). Darbepoetin alfa was recommended based on better clinical outcomes and less fatigue (one study). However, the average HRQoL benefit of ESAs in these trials appears to be of limited subjective importance, despite HRQoL data being used widely for marketing of ESAs |
| Tonelli 200988 | Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis (52 trials included) | Anaemic adults with cancer | Epoetin alfa, epoetin beta, darbepoetin alfa | No treatment, PBO | Mortality, CV events, HTN, HRQoL, RBCT, TR | RCTs | Pooled all-cause mortality during treatment was significantly higher in the group receiving erythropoiesis-stimulating therapy than in the control group (RR 1.15, 95% CI 1.03 to 1.29). Compared with no treatment, use of ESAs led to clinically detectable improvements in disease-specific measures of QoL. It also reduced the use of RBCTs (RR 0.64, 95% CI 0.56 to 0.73) | Use of ESAs resulted in increased risk of thromboembolic events (RR 1.69, 95% CI 1.27 to 2.24) and serious AEs (RR 1.16, 95% CI 1.08 to 1.25) |
| cBohlius 20097 | Erythropoietin or Darbepoetin for patients with cancer – meta-analysis based on individual patient data (53 studies included) | Paediatric and adult cancer patients | Epoetin alfa, epoetin beta or darbepoetin alfa plus RBCT (as necessary) | RBCT alone (as necessary) | Mortality during the active study period, OS | RCTs | 1530 patients died during the active study period and 4993 overall (out of a total of 13,933 cancer patients). ESAs increased mortality during the active study period (cHR 1.17, 95% CI 1.06 to 1.30) and worsened OS (cHR 1.06, 95% CI 1 to 1.12), with little heterogeneity between trials. The cHR for mortality during the active period for patients on chemotherapy was 1.10 (95% CI 0.98 to 1.24) and for OS was 1.04 (95% CI 0.97 to 1.11). There was little evidence of a difference between trials of patients given different anticancer treatments | Authors conclude that treatment with ESAs in patients with cancer increased mortality during active study periods and worsened OS. They recommend that the increased risk of death associated with treatment with these drugs should be balanced against their benefits |
| dMinton 201094 | Drug therapy for the management of cancer-related fatigue (50 studies included) | Adult cancer patients with CRF | Drug therapy for CRF (haemopoietic growth factors e.g. ESAs) | PBO, usual care or a non-pharmacological intervention for CRF | Hb concentration and subsequent change in fatigue scores | RCTs (11 relevant studies for epoetin; four relevant studies for darbepoetin alfa) | A meta-analysis of studies of ESAs showed an effect of ESAs over SC or PBO for the treatment of CRF. A meta-analysis of darbepoetin studies showed a small but statistically significant difference between darbepoetin and PBO for the treatment of CRF | Authors note increased safety concerns raised regarding ESAs and recommend that they are not used in practice. There was a very high degree of statistical and clinical heterogeneity in the trials |
| Grant 201389 | Epoetin and darbepoetin for managing anemia in patients undergoing cancer treatment: comparative effectiveness update (54 studies included) | Anaemic adults undergoing chemotherapy and/or radiotherapy for malignancy | ESAs | Control (various) | OS (on-study and longest available follow-up), PFS, QoL, haemR, RBCT, TR, thromboembolic complications, AEs | RCTs, observational studies | In 38 trials, ESAs decreased the risk of transfusion (pooled RR 0.58, 95% CI 0.53 to 0.64). In 37 trials, thromboembolic event rates were higher in ESA-treated patients (pooled RR 1.51, 95% CI 1.3 to 1.74). In 14 trials reporting QoL (FACT-F subscale), scores decreased by –0.6 in the control arms (95% CI –6.4 to 5.2) and increased by 2.1 in the ESA arms (95% CI –3.9 to 8.1). In 37 trials, mortality was increased during the on-study period (pooled HR 1.17, 95% CI 1.04 to 1.31) | Authors conclude that ESAs reduce the need for RBCT and increase the risk of thromboembolism. FACT-F scores were better with ESA use but the magnitude was less than the minimal clinically important difference. An increase in mortality accompanied the use of ESAs |
| eTonia, 201211 | Erythropoietin or darbepoetin for patients with cancer (91 studies included) | Paediatric and adult cancer patients with anaemia with/without chemotherapy, radiotherapy or combination therapy | ESAs ± RBCT | PBO, no treatment, RBCT ± PBO | HaemR, RBCT, changes in QoL, TR, on-study mortality, OS, AEs | RCTs | Use of ESAs significantly reduces the relative risk of RBCT (RR 0.65, 95% CI 0.62 to 0.68). HaemR was observed more often in participants receiving ESAs (RR 3.93, 95% CI 3.10 to 3.71). There was suggestive evidence that ESAs may improve QoL. There was strong evidence that ESAs increase mortality during the active study period (HR 1.17, 95% CI 1.06 to 1.29) and some evidence that ESA decrease OS (HR 1.05, 95% CI 1 to 1.11). Risk of thromboembolic complications was increased in patients receiving ESAs compared with control patients, whereas HTN and thrombocytopenia/haemorrhage may be increased in patients receiving ESAs compared with control patients | Authors conclude that ESAs reduce the need for RBCTs but increase the risk for thromboembolic events and death. Authors recommend that the increased risk of death and thromboembolic events should be balanced against the potential benefits of ESA treatment |
| Section/topic | Item | Checklist item | Studies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Ba | C | Db | E | F | G | Hc | Id | Je | K | |||
| Title | |||||||||||||
| Title | 1 | Identify the report as a systematic review, meta-analysis or both | N | N | Y | Y | Y | N | Y | Y | N | N | N |
| Abstract | |||||||||||||
| Structured summary | 2 | Provide a structured summary including, as applicable, background, objectives, data sources, study eligibility criteria, participants, interventions, study appraisal and synthesis methods, results, limitations, conclusions and implications of key findings, systematic review registration number | N | N | Pf | Pg | N | Ph | Pi | Pj | Pk | Pl | Pm |
| Introduction | |||||||||||||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Objectives | 4 | Provide an explicit statement of questions being addressed, with reference to participants, interventions, comparisons, outcomes and study design | Pn | N | Po | Yp | Pq | Pr | Ys | Pt | Pu | Pt | Y |
| Methods | |||||||||||||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed and, if available, provide registration information including registration number | N | N | N | Y | N | N | N | N | N | N | Y |
| Eligibility criteria | 6 | Specify study characteristics and report characteristics used as criteria for eligibility, giving rationale | Yv | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Information sources | 7 | Describe all information sources in the search and date last searched | Pw | Y | Y | Y | Y | Y | Y | Y | Pw | Y | Y |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | N | N | Y | Y | Y | Y | Yx | Yy | Yz | Yaa | Yab |
| Study selection | 9 | State the process for selecting studies | N | N | N | Yac | Y | Y | Y | Y | Y | Y | Y |
| Data collection process | 10 | Describe method of data extraction from reports and any processes for obtaining and confirming data from investigators | N | N | Pad | Y | Pad | N | Pad | Yae | Pad | Yad | Paf |
| Data items | 11 | List and define all variables for which data are sought and any assumptions and simplifications made | Y | N | N | Y | Y | N | N | Y | Pag | Y | Y |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies and how this information is to be used in any data synthesis | N | N | Y | Y | Y | Pah | Yai | Y | Y | Y | Y |
| Summary measures | 13 | State the principal summary measures | N/A | N/A | Y | N | N/A | Y | Y | Y | Y | Y | Y |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if carried out, including measures of consistency for each meta-analysis | N/A | N/A | Y | Y | N/A | N/A | Y | Y | Y | Y | Y |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence | N/A | N/A | N | Y | N/A | Y | N | Y | N | Y | Y |
| Additional analyses | 16 | Describe methods of additional analyses, if carried out, indicating which were prespecified | N | N | Y | Y | Paj | N | Y | Y | N | Y | Y |
| Results | |||||||||||||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility and included in the review, with reasons for exclusions at each stage, ideally in a flow diagram | N | N | N | Y | N | Pak | Y | Y | Y | Y | Y |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted and provide the citations | Y | N | N | Y | Y | Y | Yal | Y | Y | Y | Y |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome-level assessments | Y | Pam | N | Y | Y | Pan | Yao | Y | Y | Y | Y |
| Results of individual studies | 20 | For all outcomes considered, present for each study (a) simple summary data for each intervention group and (b) effect estimates and confidence intervals, ideally with a forest plot | N | N | Pap | Y | N | N | Y | Y | Y | Y | Y |
| Synthesis of results | 21 | Present results of each meta-analysis carried out, including confidence intervals and measure of consistency | N | N | Paq | Y | N | N | Y | Y | Y | Y | Y |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies | N | N | N | Y | N | Y | N | Y | N | Y | N |
| Additional analysis | 23 | Give results of additional analyses, if carried out | N/A | N/A | Y | Y | Yar | N/A | Y | Y | N/A | Y | Y |
| Discussion | |||||||||||||
| Summary of evidence | 24 | Summarise the main findings, including the strength of evidence for each main outcome; consider their relevance for key groups | N | Y | Pas | Y | Y | Y | Pat | Y | Y | Y | Pau |
| Limitations | 25 | Discuss limitations at study and outcome level and at review level | N | N | N | Pav | Y | Y | Y | N | Y | Paw | N |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence and implications for future research | Y | Y | Y | Y | Yax | Y | Y | Y | Y | Y | Y |
| Funding | |||||||||||||
| Funding | 27 | Describe sources of funding for the systematic review and other support and role of funders for the systematic review | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Appendix 6 Study and baseline characteristics of excluded unlicensed studies
| Study, year | Intervention group characteristicsa | Control group characteristicsa | Study interventiona | Control | Adjuvant anaemia treatment | Malignancy type and treatment | Outcomes sought | Included in Cochrane review 2012,11 Y/N |
|---|---|---|---|---|---|---|---|---|
| Wilson and colleagues2 | ||||||||
| Bamias 2003210 ROL |
n = 2; age (years): 60 (18–77); male, n (%): 35 (49); Hb BL (g/dl): 11.5 (11.1–11.9); epoetin BL (mU/ml): 24.8 (16.6–37) | n = 72; age (years): 62 (19–80); male, n (%): 39 (54); Hb BL (g/dl): 11.5 (11.2–11.8); epoetin BL (mU/ml): 12.5 (8.7–18) | Brand: epoetin alfa; dose: 30,000 IU QW; dose adjustment: Y, ↓; duration of epoetin tx: 21–24 weeks (duration of chemotherapy); duration of trial: duration of chemotherapy + 3 weeks; follow-up | SC | Iron: NR; G-CSF: NR; RBCT trigger: prn (Hb inclusion criterion level: < 13 g/dl) | Disease: solid; treatment: chemotherapy platinum containing | Hb, RBCT, HRQoL measured in a subset | Y |
| Cascinu 1994211 RCT |
n = 50; age (years): 58 (44–72); male, n (%): 24 (48); Hb BL (g/dl): 8.63 ± 0.62; epoetin BL (mU/ml): 67.9 ± 66.6 | n = 50; age (years): 57 (45–68); male, n (%): 29 (58); Hb BL (g/dl): 8.73 ± 0.52; epoetin BL (mU/ml): 49.3 ± 39.9 | Brand: epoetin alfa; dose: 300 IU/kg QW; dose adjustment: Y; duration of epoetin tx: 9 weeks; duration of trial: 9 weeks; follow-up: NR | PBO | Iron: Y, oral (as indicated by serum iron, serum ferritin, transferrin saturation); G-CSF: NR; RBCT trigger: < 8 g/dl (Hb inclusion criterion level: < 9 g/dl) | Disease: solid; treatment: chemotherapy, platinum containing | Hb, RBCT, AEs | Y |
| Cazzola 1995212 ROL |
Epoetin beta 1000 IU/kg: n = 31 (analysed n = 31); age (years): 67 (48–82); male, n (%): 16 (51.6); Hb BL (g/dl): 9.3 (0.9); epoetin BL (mU/ml): 43 (22–69) Epoetin beta 2000 IU/kg: n = 29 (analysed n = 29); age (years): 65 (40–82); male, n (%): 19 (65.5); Hb BL (g/dl): 9.4 (0.9); epoetin BL (mU/ml): 30 (21–64) Epoetin beta 5000 IU/kg: n = 31 (analysed n = 31); age (years): 68 (42–85); male, n (%): 14 (45.2); Hb BL (g/dl): 9.4 (1.2); epoetin BL (mU/ml): 24 (11–47) Epoetin beta 10,000 IU/kg: n = 26 (analysed n = 26); age (years): 63 (28–80); male, n (%): 16 (61.5); Hb BL (g/dl): 9.4 (1.0); epoetin BL (mU/ml): 38 (18–70) |
n = 29 (analysed n = 29); age (years): 68 (28–82); male, n (%): 16 (55.2); Hb BL (g/dl): 9.5 (1.1); epoetin BL (mU/ml): 32 (19–66) | Brand: epoetin beta; dose: 5000 IU/day or 10,000 IU/day; dose adjustment: NR; duration of epoetin tx: NR; duration of trial: NR; follow-up: NR | SC | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: NR) | Disease: haematological; treatment: chemotherapy, non-platinum containing | HaemR, Hb, RBCT, AEs | Y |
| bHedenus 200253 RCT, dose–response study; only licensed dose included in current review |
Darbepoetin alfa 1.0 µg/kg QW:b n = 11; age (years): 64 (26–80); male, n (%): 7 (64); Hb BL (g/dl): 9.7 (0.8); epoetin BL (mU/ml): 46 (12–208) Darbepoetin alfa 4.5 µg/kg QW:b n = 22; age (years): 70 (52–84); male, n (%): 14 (64); Hb BL (g/dl): 9.7 (0.9); epoetin BL (mU/ml): 57 (12–227) |
n = 11; age (years): 63 (25–80); male, n (%): 2 (18); Hb BL (g/dl): 9.5 (1.0); epoetin BL (mU/ml): 45 (12–132 | Brand: darbepoetin alfa; dose: 1.0 and 4.5 µg/kg QW;b dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 16 weeks; follow-up: unclear | PBO | Iron: NR; G-CSF: NR; RBCT trigger: Hb ≤ 8 g/dl (Hb inclusion criterion level: ≤ 11.0 g/dl) | Disease: haematological; treatment: chemotherapy, NR | HaemR, Hb, RBCT, AEs | Y |
| Iconomou 2003213 ROL |
n = 57; age (years): 60.6 (33–85); male, n (%): 22 (39); Hb BL (g/dl): 10.1 ± 0.6; epoetin BL (mU/ml): NR | n = 55; age (years): 62.6 (34–80); male, n (%): 24 (44); Hb BL (g/dl): 10.1 ± 0.6; epoetin BL (mU/ml): NR | Brand: rHuEPO; dose: 30,000 IU QW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: NR | SC | Iron: Y; G-CSF: NR; RBCT trigger: Hb 7.5 g/dl or prn (Hb inclusion criterion level: ≤ 11 g/dl) | Disease: solid; treatment: chemotherapy, mixed | HaemR, HRQoL | Y |
| bKotasek 200350 RCT, dose–response study; only licensed dose included in current review |
Darbepoetin alfa 1.5 µg/kg QW: n = 32; male, n (%): 9 (28) Darbepoetin alfa 3.0 µg/kg QW: n = 46; male, n (%): 13 (28) Darbepoetin alfa 4.0 µg/kg QW: n = 28; male, n (%): 8 (28) Darbepoetin alfa 4.5 µg/kg QW: n = 35; male, n (%): 10 (28) Darbepoetin alfa 5.0 µg/kg QW: n = 40; male, n (%): 11 (28) Age (years): 58.3 (11.9);c Hb BL (g/dl): 9.93 (1.0);c epoetin BL (mU/ml): 17% patients ≥ 100 mU/mlc |
n = 51; age (years): 56.2 (12.4); male, n (%): 16 (31); Hb BL (g/dl): 9.87 (1.12); epoetin BL (mU/ml): NR | Brand: darbepoetin alfa; dose: 1.0, 3.0, 4.0, 4.5 and 5.0 µg/kg QW; dose adjustment: Y, ↓; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: unclear | PBO | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 11.0 g/dl) | Disease: solid (breast, gynaecological, gastrointestinal, lung); treatment: chemotherapy, NR | HaemR, Hb, RBCT, HRQoL,c AEc | Y |
| Kunikane 2001214 RCT |
Epoetin beta 300 IU/kg QW n = 16; age (years): NR; male, n (%): NR; Hb BL (g/dl): > 12; epoetin BL (mU/ml) Epoetin beta 600 IU/kg QW n = 18; age (years): male, n (%): Hb BL (g/dl): > 12; epoetin BL (mU/ml) |
n = 38; age (years): NR; male, n (%): NR; Hb BL (g/dl): > 12; epoetin BL (mU/ml): NR | Brand: epoetin beta; dose: 300 and 600 IU/kg QW; dose adjustment: NR; duration of epoetin tx: 6 weeks; duration of trial: NR; follow-up: NR | PBO | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: NR) | Disease: solid (non-small-cell lung cancer); treatment: chemotherapy, platinum containing | Hb, RBCT | Y |
| Leyland-Jones 200314 RCT |
n = NR (total for trial 939); age (years): NR; male, n (%): NR; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | n = NR (total for trial 939); age (years): NR; male, n (%): NR; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: NR; dose adjustment: NR; duration of epoetin tx: NR; duration of trial: 12–19 months; follow-up: NR | PBO | Iron: NR; G-CSF: NR; RBCT trigger: NR [Hb inclusion criterion level: 13 g/dl (aim of study to keep Hb > 12 g/dl to < 14 g/dl)] | Disease: solid (metastatic breast); treatment: NR | Survival | Y |
| Oberhoff 1998215 ROL |
n = 114; age (years): NR; male, n (%): NR; Hb BL (g/dl): ≤ 10; epoetin BL (mU/ml): NR | n = 104; age (years): NR; male, n (%): NR; Hb BL (g/dl): 10; epoetin BL (mU/ml): NR | Brand: epoetin beta; dose: 5000 IU/day; dose adjustment: 12 weeks; duration of epoetin tx: NR; duration of trial: NR; follow-up: NR | SC | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: NR) | Disease: solid; treatment: chemotherapy, platinum containing | HaemR, Hb, RBCT, AEs | Y |
| Österborg 1996216 ROL |
Epoetin beta 10,000 IU/day: n = 47; age (years): NR; male, n (%): NR; Hb BL (g/dl): ≤ 10; epoetin BL (mU/ml): NR Epoetin beta 2000 IU/day: n = 48; age (years): NR; male, n (%): NR; Hb BL (g/dl): ≤ 10; epoetin BL (mU/ml): NR |
n = 49; age (years): NR; male, n (%): NR; Hb BL (g/dl): ≤ 10; epoetin BL (mU/ml): NR | Brand: epoetin beta; dose: 2000 and 10,000 IU/day; dose adjustment: NR; duration of epo tx: 24 weeks; duration of trial: NR; follow-up: NR | SC | Iron: NR; G-CSF: Y; RBCT trigger: NR (Hb inclusion criterion level: NR) | Disease: haematological; treatment: chemotherapy, non-platinum containing | HaemR, Hb, RBCT, AEs | Y |
| Rosen 2003217 ROL |
n = 47; age (years): 56 (35–80); male, n (%): 33 (71); Hb BL (g/dl): < 10; epoetin BL (mU/ml): NR | n = 43; age (years): 56 (35–80); male, n (%): 31 (71); Hb BL (g/dl): < 10; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: NR; duration of epoetin tx: 14 weeks + 4 weeks continuation; duration of trial: 48 months; follow-up: NR | SC | Iron: Y (tx arm only); G-CSF: NR; RBCT trigger: Hb < 10 g/dl (Hb inclusion criterion level: ≤ 16 g/dl) | Disease: solid (head/neck); treatment: chemotherapy + radiotherapy | Hb, RBCT | Y |
| bten Bokkel Huinink 199851 ROL, three-arm study; only comparison with licensed dose included in current review |
n = 42 (analysed 42);b age (years): 60.97; male, n (%): all female; Hb BL (g/dl): 12.0 (1.3–12.6); epoetin BL (mU/ml): NR | n = 34 (analysed 33); age (years): 58.83; male, n (%): all female; Hb BL (g/dl): 11.8 (10.6–12.5); epoetin BL (mU/ml): NR | Brand: epoetin beta; dose: 300 IU/kg TIW;b dose adjustment: Y; duration of epoetin tx: 24 weeks; duration of trial: 24 weeks; follow-up: NR | SC | Iron: NR; G-CSF: N; RBCT trigger: Hb < 9.7 g/dl (Hb inclusion criterion level: < 13 g/dl) | Disease: solid (ovary); treatment: chemotherapy, platinum containing | RBCT, AEs | Y |
| bThatcher 199952 ROL, three-arm study; only comparison with licensed dose included in current review |
n = 44;b age (years): 58.5 (30–72); male, n (%): 29 (66); Hb BL (g/dl): 13.6 (10.9–17.0); epoetin BL (mU/ml): NR | n = 44; age (years): 60 (39–74); male, n (%): 27 (61.3); Hb BL (g/dl): 13.4 (10.9–16.4); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 300 IU/kg TIW;b dose adjustment: Y; duration of epoetin tx: 26 weeks; duration of trial: 26 weeks; follow-up: NR | SC | Iron: N; G-CSF: N; RBCT trigger: prn (Hb inclusion criterion level: ≥ 10.5 g/dl) | Disease: solid (small-cell lung cancer); treatment: chemotherapy, mixedd | Hb, RBCT, HRQoL, AEs | Y |
| Throuvalas 2000218 ROL |
n = 28 (analysed 28); age (years): NR; male, n (%): NR; Hb BL (g/dl): > 10 to ≤ 12; epoetin BL (mU/ml): NR | n = 27 (analysed 26); age (years): NR; male, n (%): NR; Hb BL (g/dl): > 10 to ≤ 12; epoetin BL (mU/ml): NR | Brand: rHuEPO; dose: 50,000 IU QW; dose adjustment: unclear; duration of epoetin tx: NR; duration of trial: 6 weeks; follow-up: NR | SC | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: NR) | Disease: solid (cervix, bladder); treatment: chemotherapy (platinum containing) + radiotherapy | RBCT | Y |
| Welch 1995219 ROL |
n = 15; age (years): NR; male, n (%): NR; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | n = 15; age (years): NR; male, n (%): NR; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 300 IU/kg TIW; dose adjustment: NR; duration of epoetin tx: NR; duration of trial: 24 weeks; follow-up: NR | SC | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: NR) | Disease: solid (ovary); treatment: chemotherapy, platinum containing | Hb, RBCT, HRQoL, AEs | Y |
| PenTAG review: 2004 to current | ||||||||
| Aapro 2008220 ROL (Breast Cancer – Anemia and the Value of Erythropoietin; BRAVE), supplementary reference Aapro 2009221 |
n = 231; age (years): 56 (27–78); male, n (%): all female; Hb BL (g/dl): 11.2 ± 1.2; epoetin BL (mU/ml): NR | n = 232; age (years): 57.5 (29–83); male, n (%): all female; Hb BL (g/dl): 11.5 ± 1.1; epoetin BL (mU/ml): NR | Brand: epoetin beta; dose: 450 IU/kg QW; dose adjustment: Y; duration of epoetin tx: 24 weeks; duration of trial: 24 weeks; follow-up: 18 months | SC | Iron: Y, oral or intravenous; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: < 12.9 g/dl) | Disease: solid (breast); treatment: chemotherapy, non-platinum containing | HaemR, Hb, RBCT, HRQoL, AEs, TR, survival | Y |
| Aapro 2009221 Subgroup analysis of Aapro 2008220 |
n = 231; age (years): 56 (27–78); male, n (%): all female; Hb BL (g/dl): 11.2 ± 1.2; epoetin BL (mU/ml): NR | n = 232; age (years): 57.5 (29–83); male, n (%): all female; Hb BL (g/dl): 11.5 ± 1.1; epoetin BL (mU/ml): NR | Brand: epoetin beta; dose: 450 IU/kg QW; dose adjustment: Y; duration of epoetin tx: 24 weeks; duration of trial: 24 weeks; follow-up: 18 months | SC | Iron: Y, oral or intravenously; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: < 12.9 g/dl) | Disease: solid (breast); treatment: chemotherapy, non-platinum containing | AEs (subgroup analysis, TVEs among patients receiving and not receiving antithromboembolic therapy; hypothesis generating) | N |
| Berndt 2005222 ROL, subgroup analysis of Glaspy 2002122 (not included in previous HTA review2) |
n = 300;e age (years): 60.8 (12.3) (20–91); male, n (%): 91 (30.3); Hb BL (g/dl): 9.9 (0.9) (6.2–12.2); epoetin BL (mU/ml): NR | Brand: darbepoetin alfa; dose: 0.5, 1.0, 1.5, 2.25, 4.5, 6.0 and 8.0 µg QW and 3.0, 5.0, 7.0 and 9.0 µg Q2W; dose adjustment: Y, ↓; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: NR Brand: epoetin alfa; dose: 150 IU/kg TIW and 40,000 IU/kg QW; dose adjustment: Y;f duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: NR |
Head-to-head | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 11 g/dl) | Disease: solid; treatment: chemotherapy, mixed | Analysis explores the impact of fatigue on productivity and caregiver burden (although includes licensed doses of intervention under review, results reported are based on pooled data) | N | |
| Blohmer 2011223 ROL [Nordostdeutsche Gesellschaft fur Gynaekologische Onkologie Arbeitsgemeinschaft Gynaekologische Onkologie (NOGGO-AGO)] |
n = 127; age (years): 41 (24–73); male, n (%): all female; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | n = 129; age (years): 42 (25–66); male, n (%): all female; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: rHuEPO; dose: 10,000 IU TIW; dose adjustment: Y; duration of epoetin tx: unclear; duration of trial: unclear; follow-up: 3 years | No epoetin | Iron: Y, oral; G-CSF: NR; RBCT trigger: Hb < 9 g/dl (Hb inclusion criterion level: NR) | Disease: solid (cervix); treatment: chemotherapy + radiotherapy | Hb, RBCT, HRQoL (ECOG performance status), AEs, survival | Y |
| Cabanillas 2012224 ROL |
n = 55; age (years): 41 ± 16.7; male, n (%): 33 (58); Hb BL (g/dl): 9 ± 1.5; epoetin BL (mU/ml): 473 ± 570 | n = 54; age (years): 42 ± 17.3; male, n (%): 24 (42); Hb BL (g/dl): 8.9 ± 1.5; epoetin BL (mU/ml): 326 ± 514 | Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: unclear; duration of trial: unclear; follow-up: 3 years | No epoetin | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: > 10 g/dl) | Disease: haematological; treatment: chemotherapy, non-platinum containing | RBCT, HRQoL, AEs, TR, survival | N, excluded trials if > 80% of participants were diagnosed with an acute leukaemia |
| Chang 200532 ROL |
n = 175; age (years): 50.4 (11.1) (27–78); male, n (%): all female; Hb BL (g/dl): 11.2 (0.9) (8.15–12.6);g epoetin BL (mU/ml): NR | n = 175; age (years): 50.1 (10.0) (31–85); male, n (%): all female; Hb BL (g/dl): 11.3 (0.8) (7.8–13.4);g epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 16 weeks; follow-up: NR | SC | Iron: Y, oral, daily (as indicated by transferrin saturation); G-CSF: NR; RBCT trigger: Hb < 8 g/dl, prn (Hb inclusion criterion level: ≤ 15 g/dl at screening; ≤ 12 g/dl randomised) | Disease: solid (breast); treatment: chemotherapy, NR | HaemR,h Hb, RBCT, HRQoL, AEs | Y |
| Chang 2004225 ROL, subgroup analysis of Chang 200532 (published online ahead of print 2004) |
n = 176; age (years): 50.4 (11.1) (27–78); male, n (%): all female; Hb BL (g/dl): 11.2 (0.9) (8.15–12.6); epoetin BL (mU/ml): NR | n = 178; age (years): 50.1 (10.0) (31–85); male, n (%): all female; Hb BL (g/dl): 11.3 (0.8) (7.8–13.4); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 16 weeks; follow-up: NR | SC | Iron: Y, oral, daily (as indicated by transferrin saturation); G-CSF: NR; RBCT trigger: Hb < 8 g/dl, prn (Hb inclusion criterion level: ≤ 15 g/dl at screening; ≤ 12 g/dl randomised) | Disease: solid (breast); treatment: chemotherapy, NR | Analysis of the effect of epoetin alfa on changes in quality of life and utility scales at 12 weeks | Y |
| Charu 2007226 ROL |
n = 226; age (years): 71.7 (10.4); male, n (%): 95 (42); Hb BL (g/dl): 10.1 (0.9) (for n = 220); epoetin BL (mU/ml): NR | n = 59; age (years): 67.2 (12.5); male, n (%): 23 (39); Hb BL (g/dl): 10.3 (0.9) (for n = 55); epoetin BL (mU/ml): NR | Brand: darbepoetin alfa; dose: 3 µg/kg Q2W; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks;i follow-up: NR | No epoetin | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 11 g/dl) | Disease: mixed; treatment: chemotherapy, NR | HaemR, Hb, RBCT, HRQoL, AEs, numbert of hospitalisations | Y |
| Christodoulou 2009227 ROL |
n = 167; age (years): 61 (22–82); male, n (%): 88 (53); Hb BL (g/dl): 10.15 ± 0.69; epoetin BL (mU/ml): NR | n = 170; age (years): 63 (30–89); male, n (%): 81 (48); Hb BL (g/dl): 10.30 ± 0.58); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 10,000 IU TIW; dose adjustment: Y; duration of epoetin tx: NR; duration of trial: NR; follow-up: NR | No epoetin | Iron: Y, oral; G-CSF: NR; RBCT trigger: Hb < 8.5 g/dl prn (Hb inclusion criterion level: ≤ 12 g/dl) | Disease: solid; treatment: chemotherapy, mixed | Hb, RBCT, HRQoL, TR, survival | Y |
| Engert 2010228 RCT [German Hodgkin Study Group (GHSG)-HD15-EPO] |
n = 648; age (years): 34 (18–60); male, n (%): 402 (62); Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | n = 685; age (years): 34 (18–60); male, n (%): 406 (62); Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: unclear; duration of trial: unclear; follow-up: unclear | PBO | Iron: Y, oral (as indicated by BL transferrin saturation or serum ferritin level); G-CSF: NR; RBCT trigger: Hb < 8 g/dl (Hb inclusion criterion level: NR) | Disease: haematological; treatment: chemotherapy, non-platinum containing | Hb, RBCT, HRQoL, AEs, survival | Y |
| Fujisaka 2011229 RCT | n = 89; age (years): 67 (40–79); male, n (%): 47 (53); Hb BL (g/dl): 9.4 (8.1–11.4); epoetin BL (mU/ml): 43 (7.78–577) | n = 92; age (years): 63.5 (44–79); male, n (%): 40 (43); Hb BL (g/dl): 9.3 (7.2–11.4); epoetin BL (mU/ml): 43.6 (10.5–320) | Brand: epoetin beta; dose: 36,000 IU QW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: 12 months | PBO | Iron: Y, oral daily (as indicated by transferrin saturation); G-CSF: NR; RBCT trigger: prn (Hb inclusion criterion level: ≤ 8 g/dl/≤ 10 g/dl) | Disease: solid (lung or gynaecological); treatment: chemotherapy, platinum containing | Hb, RBCT, HRQoL, AEs, survival | Y |
| Glaspy 2006230 ROL, active control |
Darbepoetin alfa: n = 606; age (years): 63.2 (12.4); male, n (%): 191 (32); Hb BL (g/dl): 10.2 (0.9); epoetin BL (mU/ml): NR |
Epoetin alfa: n = 603; age (years): 63.7 (11.6); male, n (%): 222 (37); Hb BL (g/dl): 10.2 (0.9); epoetin BL (mU/ml): NR |
Brand: darbepoetin alfa; dose: 200 µg Q2W; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 16 weeks; follow-up: NR Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 16 weeks; follow-up: NR |
NA (active control; see left) | Iron: NR; G-CSF: NR; RBCT trigger: Hb > 8 g/dl prn (Hb inclusion criterion level: ≤ 11 g/dl) | Disease: solid; treatment: chemotherapy, NR | Hb, RBCT, HRQoL, AEs; haemR measured but defined as achievement of target Hb range 11–13 g/dl) | N; excluded as ESAs were given in context with surgery, stem cell transplantation |
| Gupta 2009231 RCT |
n = 60 (analysed n = 58); age (years): 48.27 (18–70); male, n (%): NR; Hb BL (g/dl): 10.45 (9.5–11.0); epoetin BL (mU/ml): NR | n = 60 (analysed n = 57); age (years): 48.18 (20–65); male, n (%): NR; Hb BL (g/dl): 10.70 (10.0–12.5); epoetin BL (mU/ml): NR | Brand: epoetin beta; dose: 10,000 IU TIW; dose adjustment: NR; duration of epoetin tx: unclear; duration of trial: unclear; follow-up: 24 months | PBO | Iron: Y, oral 10–15 days before chemotherapy + radiotherapy (unclear whether given in control arm); G-CSF: NR; RBCT trigger: Hb ≤ 10 g/dl (Hb inclusion criterion level: 9.5–12.5 g/dl) | Disease: solid (cervix); treatment: chemotherapy (platinum containing) + radiotherapy | Hb, RBCT, HRQoL, AE, TR, survival | Y |
| Hernandez 2009232 RCT |
n = 193; age (years): 64.5 (12.1); male, n (%): 76 (39); Hb BL (g/dl): 10.1 (0.9); epoetin BL (mU/ml): 90.3 (96.1) (n = 186) | n = 193; age (years): 63.6 (12.3); male, n (%): 76 (39); Hb BL (g/dl): 10.0 (0.9); epoetin BL (mU/ml): 109.9 (186.4) (n = 184) | Brand: darbepoetin alfa; dose: 300 µg Q3W; dose adjustment: Y; duration of epoetin tx: 13 weeks; duration of trial: 16 weeks; follow-up: 29 weeks | PBO | Iron: Y, not specified, although based on serum iron, serum ferritin or transferrin saturation; G-CSF: NR; RBCT trigger: Hb ≤ 8 g/dl or if > 8 g/dl and signs of anaemia present (Hb inclusion criterion level: < 11 g/dl) | Disease: solid and haematological?; treatment: chemotherapy, mixed | Hb, RBCT, HRQoL, AEs, TR, survival | Y |
| Leyland-Jones 2005233 RCT (Breast Cancer Erythropoietin Survival Trial; BEST) |
n = 469; age (years): 55.8 (11.1; 24–83); male, n (%): all female; Hb BL (g/dl): 12.5 (1.8); epoetin BL (mU/ml): NR | n = 470; age (years): 55.1 (10.5; 30–84); male, n (%): all female; Hb BL (g/dl): 12.5 (1.7); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: 12 months; duration of trial: 12 months;j follow-up: NR | PBO | Iron: Y, oral (as indicated by transferrin saturation); G-CSF: NR; RBCT trigger: prn (Hb inclusion criterion level: NR) | Disease: solid (breast); treatment: chemotherapy, NR | Hb, RBCT, HRQoL, AEs, TR, survival | Y |
| Milroy 2011234 ROL |
n = 214 (analysed n = 189); age (years): 61.6 ± 8.7 (41–82); male, n (%): 142 (75.1); Hb BL (g/dl): 12.8 ± 1.4; epoetin BL (mU/ml): NR | n = 191 (analysed n = 191); age (years): 60.1 ± 9.3 (34–83); male, n (%): 148 (77.5); Hb BL (g/dl): 12.6 ± 1.6; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 10,000 IU TIW; dose adjustment: Y; duration of epoetin tx: 22–28 weeks; duration of trial: 22–28 weeks; follow-up: 6 and 12 months | SC | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 15 g/dl men; ≤ 14 g/dl women) | Disease: solid (non-small-cell lung cancer); treatment: chemotherapy, platinum containing | HaemR, Hb, RBCT, HRQoL, AEs, TR, survival | Y |
| Nagel 2011235 ROL |
n = 37; age (years): 61 (41–88); male, n (%): 29 (77.8); Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | n = 37; age (years): 59 (37–80); male, n (%): 24 (63.9); Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: darbepoetin alfa; dose: 300 µg Q2W; dose adjustment: Y; duration of epoetin tx: up to 28 weeks; duration of trial: up to 28 weeks; follow-up: 24 months | SC | Iron: Y, oral; G-CSF: Y, pegfilgastrim D4 each cycle; RBCT trigger: prn (Hb inclusion criterion level: < 12 g/dl; treatment initiated at this point in the intervention group) | Disease: solid (small-cell lung cancer); treatment: chemotherapy, platinum containing | HRQoL, AEs, TR, survival | N; excluded as too many patients in experimental arm did not receive ESAs |
| O’Shaugnessy 2005236 RCT |
n = 47; age (years): 53.3 ± 9.7; male, n (%): all female; Hb BL (g/dl): 12.8 ± 1.0; epoetin BL (mU/ml): NR | n = 47; age (years): 54.3 ± 12.0; male, n (%): all female; Hb BL (g/dl): 13.0 ± 1.0; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 16 weeks; follow-up: 6 months | PBO | Iron: Y, not specified but prn; G-CSF: NR; RBCT trigger: Hb < 8 g/dl (Hb inclusion criterion level: 9–14 g/dl) | Disease: solid (breast); treatment: chemotherapy, platinum containing | Hb, cognitive function and mood [Executive Interview (EXIT25), Clock Drawing task (CLOX) 1 (prompted) and 2 (unprompted), Profile of Mood States (POMS)], HRQoL, AEs | Y |
| Pirker 2008237 RCT |
n = 298; age (years): NR; male, n (%): NR; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | n = 298; age (years): NR; male, n (%): NR; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: darbepoetin alfa; dose: 300 µg QW for 4 weeks then every 3 weeks up to six cycles of chemotherapy; dose adjustment: Y; duration of epoetin tx: 13 weeks?; duration of trial: 24 weeks; follow-up: 12 months | PBO | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≥ 9 g/dl and ≤ 13 g/dl) | Disease: solid (small-cell lung cancer); treatment: chemotherapy, platinum containing | Hb, RBCT, HRQoL, AEs, survival, disease progression | Y |
| Pronzato2010238 ROL (EPO-INT-47) |
n = 107; age (years): 58.3 ± 10.3 (29–76); male, n (%): all female; Hb BL (g/dl): 10.6; epoetin BL (mU/ml): NR | n = 109; age (years): 54.3 ± 11.6 (27–77); male, n (%): all female; Hb BL (g/dl): 10.8; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 10,000 IU TIW (5000 IU QW if < 45 kg); dose adjustment: Y; duration of epoetin tx: up to 28 weeks; duration of trial: up to 28 weeks; follow-up: 6 and 12 months | SC | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 12 g/dl) | Disease: solid; treatment: chemotherapy, platinum containing | HaemR, Hb, HRQoL, AEs, TR, survival | Y |
| kRazzouk 2006239 RCT |
n = 111; age (years): 12.4 (3.6); male, n (%): 63 (56.8); Hb BL (g/dl): 9.8 (1.3); epoetin BL (mU/ml): NR | n = 111; age (years): 10.8 (4.0); male, n (%): 58 (52.3); Hb BL (g/dl): 9.5 (1.0); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 600 IU/kg QW; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 16 weeks; follow-up: NR | PBO | Iron: Y, oral (as indicated by transferrin saturation or ferritin level); G-CSF: NR; RBCT trigger: Hb ≤ 7 g/dl (Hb inclusion criterion level: < 10.5, < 11.0 or < 12.0 g/dl dependent on age) | Disease: haematological; treatment: chemotherapy, NR | Hb, RBCT, HRQoL, AEs | Y |
| Reed 2005240 ROL, active control; subgroup analysis of Waltzman 2005241 |
n = 274; age (years): 62.4 (11.7); male, n (%): 93 (34); Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 16 weeks; follow-up: NR Brand: darbepoetin alfa; dose: 200 µg Q2W; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 16 weeks; follow-up: NR |
Head-to-head | Iron: Y, oral, daily or intravenously if contraindicated; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 11 g/dl) | Disease: solid; treatment: chemotherapy, mixed | Analysis of patients with a baseline Hb value and at least one post-randomisation Hb value or documentation of RBCT. Patients were dichotomised based on whether they experienced an early Hb response, regardless of treatment assignment | N; compared different ESA products (epoetin vs. darbepoetin) | |
| Rosenzweig 2004242 Retrospective analysis; original trial unknown |
n = 14; age (years): 55.9 ± 11.7; male, n (%): all female; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | n = 13; age (years): 53.9 ± 14.20; male, n (%): all female; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: rHuEPO; dose: 40,000 IU QW; dose adjustment: NR; duration of epoetin tx: NR; duration of trial: NR;l follow-up: NA | SC | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: < 12 g/dl) | Disease: solid (breast); treatment: chemotherapy, NR | Analysis to determine the efficacy of rHuEPO in reducing cancer-related fatigue and improving quality of life and fatigue in patients with metastatic breast cancer experiencing mild anaemia (retrospective review over an 18-month period) | Y |
| Savonije 2006243 RCT, subgroup analysis of Savonije 2006244 |
n = 211; age (years): 57 ± 11 (20–80); male, n (%): 117 (55); Hb BL (g/dl): 10.7 ± 1.0 (7.6–13.8); epoetin BL (mU/ml): NR | n = 104; age (years): 58 ± 10 (27–78); male, n (%): 61 (59); Hb BL (g/dl): 10.8 ± 10 (8.5–12.7); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 10,000 IU TIW; dose adjustment: Y; duration of epoetin tx: NR; duration of trial: 13.9 weeks vs. 14.5 weeks (mean intervention vs. control); follow-up: 12 months | SC | Iron: Y, oral (as indicated by BL transferrin saturation ± serum ferritin level); G-CSF: NR; RBCT trigger: Hb ≥ 9.7 g/dl prn (Hb inclusion criterion level: ≤ 12.1 g/dl) | Disease: solid; treatment: chemotherapy, platinum containing | HRQoL in patients with solid tumours and mild-to-moderate anaemia receiving platinum-containing chemotherapy relative to population norms | Y |
| Savonije 2006244 RCT, subgroup analysis of Savonije 2006244 |
n = 211; age (years): 57 ± 11 (20–80); male, n (%): 117 (55); Hb BL (g/dl): 10.7 ± 1.0 (7.6–13.8); epoetin BL (mU/ml): NR | n = 104; age (years): 58 ± 10 (27–78); male, n (%): 61 (59); Hb BL (g/dl): 10.8 ± 10 (8.5–12.7); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 10,000 IU TIW; dose adjustment: Y; duration of epoetin tx: NR; duration of trial: 13.9 weeks vs. 14.5 weeks (mean intervention vs. control); follow-up: 12 months | SC | Iron: Y, oral (as indicated by BL transferrin saturation ± serum ferritin level); G-CSF: NR; RBCT trigger: Hb ≥ 9.7 g/dl prn (Hb inclusion criterion level: ≤ 12.1 g/dl) | Disease: solid; treatment: chemotherapy, platinum containing | Analysed the effect of BL Hb level on HaemR, Hb, RBCT, and HRQoL | N |
| Savonije 2005245 RCT (NCT00283465) |
n = 211; age (years): 57 ± 11 (20–80); male, n (%): 117 (55); Hb BL (g/dl): 10.7 ± 1.0 (7.6–13.8); epoetin BL (mU/ml): NR | n = 104; age (years): 58 ± 10 (27–78); male, n (%): 61 (59); Hb BL (g/dl): 10.8 ± 10 (8.5–12.7); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 10,000 IU TIW; dose adjustment: Y; duration of epoetin tx: NR; duration of trial: 13.9 weeks vs. 14.5 weeks (mean intervention vs. control); follow-up: 12 months | SC | Iron: Y, oral (as indicated by BL transferrin saturation ± serum ferritin level); G-CSF: NR; RBCT trigger: Hb ≥ 9.7 g/dl prn (Hb inclusion criterion level: ≤ 12.1 g/dl) | Disease: solid; treatment: chemotherapy, platinum containing | HaemR, Hb, RBCT, HRQoL, AEs, survival (6 and 12 months) | Y |
| Schwartzberg 2004246 ROL, active control; integrated analysis of three RCTs including Senecal 2005247 |
Darbepoetin alfa: n = 157; age (years): 58.7 (11.5); male, n (%): 23 (15); Hb BL (g/dl): 10.4 (0.8); epoetin BL (mU/ml): NR |
Epoetin alfa: n = 155; age (years): 61.7 (12.1); male, n (%): 26 (17); Hb BL (g/dl): 10.4 (0.8); epoetin BL (mU/ml): NR |
Brand: darbepoetin alfa; dose: 200 µg Q2W; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 19/20 weeks; follow-up: NR Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 19/20 weeks; follow-up: NR |
Head-to-head | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 11 g/dl) | Disease: solid (breast, lung, gynaecological); treatment: chemotherapy, mixed | Hb, RBCT, HRQoL, PSQ, AEs; subgroup analysis by BL Hb category < 10 g/dl and ≥ 10 g/dl) | N; excluded as compared darbepoetin with epoetin |
| Senecal 2005247 RCT, active control; also reported in Schwartzberg 2004246 |
Darbepoetin alfa: n = 72; age (years): 53.6 ± 11.4 (35–81); male, n (%): all female; Hb BL (g/dl): 10.5 ± 0.8; epoetin BL (mU/ml): NR |
Epoetin alfa: n = ; age (years): 58.4 ± 12.5 (34–81); male, n (%): all female; Hb BL (g/dl): 10.6 ± 0.7; epoetin BL (mU/ml): NR |
Brand: darbepoetin alfa; dose: 200 µg Q2W; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 19/20 weeks; follow-up: NR Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 19/20 weeks; follow-up: NR |
Head-to-head | Iron: Y, not specified, per institution standards; G-CSF: NR; RBCT trigger: Hb < 8 g/dl (Hb inclusion criterion level: ≤ 11 g/dl) | Disease: solid (breast); treatment: chemotherapy, mixed | HaemR, Hb, RBCT, PSQ, AEs; subgroup analysis by BL Hb category < 10 g/dl and ≥ 10 g/dl) | N; excluded as ESAs were given in context with surgery, stem cell transplantation |
| Thomas 2008248 ROL (GOG-0191; NCT00017004; CAN-NCIC-CX4) |
n = 57; age (years): 46 (25–77); male, n (%): all female; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | n = 52; age (years): 50 (32–78); male, n (%): all female; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: rHuEPO; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: unclear; duration of trial: unclear; follow-up: 37 months (9.8–50.4 months) | SC | Iron: NR; G-CSF: NR; RBCT trigger: Hb < 12 g/dl (as a result of vaginal bleeding, intervention arm) (Hb inclusion criterion level: < 12 g/dl for randomisation; < 14 g/dl at study entry) | Disease: solid (cervix); treatment: chemotherapy (platinum containing) + radiotherapy | Hb, RBCT, AEs (TVEs), survival; trial terminated early with < 25% of planned accrual because of concerns over TVEs with rHuEPO | Y |
| Tsuboi 2009249 RCT |
n = 61; age (years): 61.8 ± 11.9; male, n (%): 34 (56); Hb BL (g/dl): 10.0 ± 1.0; epoetin BL (mU/ml): 67.3 ± 72.0 | n = 56; age (years): 62.1 ± 9.6; male, n (%): 33 (59); Hb BL (g/dl): 10.4 ± 1.0; epoetin BL (mU/ml): 49.1 ± 33.4 | Brand: epoetin beta; dose: 36,000 IU QW; dose adjustment: Y; duration of epoetin tx: 8 weeks; duration of trial: 8 weeks; follow-up: median follow-up 670 days | PBO | Iron: Y, oral, if indicated by serum iron saturation or MCV); G-CSF: NR; RBCT trigger: prn (Hb inclusion criterion level: ≤ 8.0 g/dl and ≤ 11 g/dl) | Disease: solid (lung) and haematological; treatment: chemotherapy, mixed | HaemR, Hb, RBCT, HRQoL, AEs, survivalm | Y |
| Wagner 200461 ROLk |
n = 18; age (years): 3.2 (1.2–19.4); male, n (%): NR; Hb BL (g/dl): 8.85 (6.10–11.20); epoetin BL (mU/ml): NR | n = 20; age (years): 3.2 (1.1–7.3); male, n (%): NR; Hb BL (g/dl): 9.35 (7.00–15.3); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 200 IU/kg/day; dose adjustment: Y; duration of epoetin tx: day 6 of cycle 1 continuing to 48 hours before start of cycle 2 – in subsequent cycles given 24 hours after completion of chemotherapy; duration of trial: unclear; follow-up: NR | SC | Iron: Y, oral, daily (as indicated by transferrin saturation); G-CSF: 10 µg/kg/day both tx arms; RBCT trigger: Hb ≤ 8 g/dl (Hb inclusion criterion level: NR) | Disease: haematological; treatment: chemotherapy, mixed | Hb, RBCT, survival | N; excluded as no usable data for any outcome |
| Waltzman 2005241 ROL, active control |
Epoetin alfa: n = 178; age (years): 62.1 ± 11.8; male, n (%): 69 (38.8); Hb BL (g/dl): 10.16 ± 0.749; epoetin BL (mU/ml): NR |
Darbepoetin alfa: n = 180; age (years): 63.4 ± 11.8; male, n (%): 61 (33.9); Hb BL (g/dl): 10.07 ± 0.787; epoetin BL (mU/ml): NR |
Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 16 weeks; follow-up: NR Brand: darbepoetin alfa; dose: 200 µg Q2W; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 16 weeks; follow-up: NR |
Head-to-head | Iron: Y, oral daily or intravenously if contraindicated; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 11 g/dl) | Disease: solid; treatment: chemotherapy, mixed | HaemR, Hb, RBCT, HRQoL, AEs | N; excluded as ESAs were given in context with surgery, stem cell transplantation |
| Wilkinson 2006250 ROL |
n = 114; age (years): 59.1 ± 10.6 (35–87); male, n (%): all female; Hb BL (g/dl): 10.75 ± 0.94; epoetin BL (mU/ml): NR | n = 59; age (years): 60.3 ± 11.2 (30–79); male, n (%): all female; Hb BL (g/dl): 10.66 ± 0.83; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 10,000 IU TIW; dose adjustment: Y; duration of epoetin tx: up to 28 weeks; duration of trial: up to 28 weeks; follow-up: NR | SC | Iron: Y, oral (as indicated by transferrin saturation); G-CSF: Y, not specified; RBCT trigger: Hb < 9 g/dl prn (Hb inclusion criterion level: ≤ 12 g/dl) | Disease: solid (ovary); treatment: chemotherapy, platinum containing | HaemR, Hb, RBCT, HRQoL, AEs, TR | Y |
| Winquist 2009251 RCT |
n = 26; age (years): 71 (53–87); male, n (%): all male; Hb BL (g/dl): 104 (73–120); epoetin BL (mU/ml): NR | n = 30; age (years): 71 (50–83); male, n (%): all male; Hb BL (g/dl): 104 (81–120); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 40,000 IU TIW; dose adjustment: Y; duration of epoetin tx: 16 weeks;n duration of trial: 16 weeks;n follow-up: NAn | PBO | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 12 g/dl) | Disease: solid (prostate); treatment: unclear, NR | Hb, RBCT, HRQoL, AEs, survival | Y |
| Witzig 2005167 RCT (NCT00003600; CDR0000066673; NCCTG-979253; NCI-P98-0133) |
n = 174; age (years): 63.6 (11.89); male, n (%): 75 (45); Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | n = 170; age (years): 63.7 (13.0); male, n (%): 71 (43); Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: 16 weeks; follow-up: 12 months | PBO | Iron: Y, oral, daily; G-CSF: NR; RBCT trigger: Hb prn (Hb inclusion criterion level: < 11.5 g/dl men; < 10.5 g/dl women) | Disease: solid and haematological; treatment: chemotherapy, mixed | HaemR, Hb, RBCT, HRQoL, AEs, TR, survival | Y |
| Wright 200719 RCT |
n = 33; age (years): 68 (47–86); male, n (%): 17 (52); Hb BL (g/dl): 103 (72–118); epoetin BL (mU/ml): NR | n = 37; age (years): 70 (39–87); male, n (%): 20 (54); Hb BL (g/dl): 103 (76–120); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 40,000 IU QW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: 26 weeks+ | PBO | Iron: Y, not specified, prn; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: NR) | Disease: solid (non-small-cell lung cancer); treatment: chemotherapy, non-platinum containingo | Hb, RBCT, HRQoL, AEs, survival | Y |
Appendix 7 Study and baseline characteristics of included licensed studies
| Study, year | Intervention group characteristicsa | Control group characteristicsa | Study interventiona | Control | Adjuvant anaemia treatment | Malignancy type and treatment | Outcomes sought | Included in Cochrane review 2012,11 Y/N |
|---|---|---|---|---|---|---|---|---|
| Wilson and colleagues 2007:2 primary studies | ||||||||
| Abels 199363 RCT; multiple publications: Abels 1996,59 Henry 1995,58 Henry 1994,85 Case 199386 |
n = 153/213 (analysed n = 143/206);b age (years): 61.2 ± 13.0; male, n (%): 102 (47.8); Hb BL (g/dl): NR; epoetin BL (mU/ml): 146 ± 260 | n = 200 (analysed n = 135/190); age (years): 62.5 ± 12.3; male, n (%): 95 (47.5); Hb BL (g/dl): NR; epoetin BL (mU/ml): 149 ± 217 | Brand: rHuEPO;c dose: 150 IU/kg TIW; dose adjustment: NR; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks;d follow-up: NR | PBO | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 10.5 g/dl) | Disease: haematological and solid; treatment: chemotherapy, mixed | HaemR, haematocrit, RBCT, HRQoL,b AEsb | Y |
| Aravantinos 200364 ROL |
n = 24; age (years): 59 (18–76); male, n (%): 2 (8); Hb BL (g/dl): 9.8 ± 0.5; epoetin BL (mU/ml): NR | n = 23; age (years): 64 (23–75); male, n (%): 7 (30%); Hb BL (g/dl): 9.3 ± 0.8; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y, ↓prn; duration of epoetin tx: unclear, median five cycles; duration of trial: NR; follow-up: NR | SC | Iron: Y, oral, fixed; G-CSF: NR; RBCT trigger: Hb < 9 g/dl (Hb inclusion criterion level: < 10.5 g/dl) | Disease: solid (including ovarian); treatment: chemotherapy, platinum-containing | Hb, haematocrit, RBCT | Y |
| Boogaerts 200365 ROL; identified: citation checking (abstract of this paper – Coiffier 200187 was included in Wilson and colleagues’2 review) |
n = 133; age (years): 62 (24–85); male, n (%): 46 (35); Hb BL (g/dl): 9.0 (5–13); epoetin BL (mU/ml): 54 (7–1650) | n = 129; age (years): 62 (24–85); male, n (%): 52 (40); Hb BL (g/dl): 9.2 (5–12); epoetin BL (mU/ml): 58 (5–4300) | Brand: epoetin beta; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: NR | SC | Iron: Y, oral (as indicated by transferrin saturation level); G-CSF: NR; RBCT trigger: Hb < 8.5 g/dl (Hb inclusion criterion level: ≤ 11.0 g/dl) | Disease: haematological and solid; treatment: chemotherapy, NR | HaemR, Hb, RBCT, HRQoL, AEs | Y |
| Dammacco 200166 RCT NCT00270101; CR005911 |
n = 69; age (years): 67 (43–80); male, n (%): 34 (49); Hb BL (g/dl): 9.3 ± 1.27; epoetin BL (mU/ml): 116 (18–5220) | n = 76; age (years): 65 (38–89); male, n (%): 31 (41); Hb BL (g/dl): 9.6 ± 0.95; epoetin BL (mU/ml): 93 (10–408) | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks;d follow-up: NR | PBO | Iron: NR; G-CSF: NR; RBCT trigger: Hb < 8 g/dl (Hb inclusion criterion level: < 11.0 g/dl) | Disease: haematological; treatment: chemotherapy, mixede | HaemR, Hb, RBCT, HRQoL, AEs | Y |
| Del Mastro 199767 ROL |
n = 31; age (years): 54 (31–68); male, n (%): NR; Hb BL (g/dl): 13.0 ± 0.7; epoetin BL (mU/ml): 21.0 (0–512) | n = 31; age (years): 56 (29–68); male, n (%): NR; Hb BL (g/dl): 13.1 ± 0.6; epoetin BL (mU/ml): 25.5 (0–800) | Brand: rHuEPO;c dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: 14 weeks; duration of trial: 14 weeks; follow-up: 6 months | SC | Iron: Y, oral iron (as indicated by serum iron, ferritin and transferrin saturation levels); G-CSF: Y, 5 µg/kg subcutaneously days 4–11, cycles 1–5; RBCT trigger: Hb < 8 g/dl (Hb inclusion criterion level: ≥ 12.0 g/dl) | Disease: solid (breast); treatment: chemotherapy, non-platinum containing | Hb, RBCT, HRQoL, AEs | Y |
| Dunphy 199968 ROL |
n = 15 (analysed n = 13); age (years): 59 (42–76); male, n (%): 12 (92); Hb BL (g/dl): 14.1 ± 2.1; epoetin BL (mU/ml): 8.8 (5.1) | n = 15 (analysed n = 14); age (years): 67 (32–82); male, n (%): 7 (50); Hb BL (g/dl): 14.1 ± 1.6; epoetin BL (mU/ml): 7.3 (4.4) | Brand: rHuEPO;c dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: unclear, ≈6 weeks; duration of trial: unclear; follow-up: NR | SC | Iron: Y, oral, dailly; G-CSF: NR; RBCT trigger: Hb < 8 g/dl (Hb inclusion criterion level: NR) | Disease: solid (including head and neck, lung); treatment: chemotherapy, mixed | Hb, RBCT | Y |
| Hedenus 200253 RCT, dose–response study; two unlicensed doses excluded |
n = 22;f age (years): 69 (20–84); male, n (%): 14 (64); Hb BL (g/dl): 9.4 (1.3); epoetin BL (mU/ml): 69 (12–1362) | n = 11; age (years): 63 (25–80); male, n (%): 2 (18); Hb BL (g/dl): 9.5 (1.0); epoetin BL (mU/ml): 45 (12–132) | Brand: darbepoetin alfa; dose: 2.25 μg/kg QW;f dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 16 weeks; follow-up: unclear | PBO | Iron: prn; G-CSF: NR; RBCT trigger: Hb ≤ 8 g/dl prn (Hb inclusion criterion level: ≤ 11.0 g/dl) | Disease: haematological; treatment: chemotherapy, NR | HaemR, Hb, RBCT, AEs | Y |
| Hedenus 200317 RCT; multiple publications: Littlewood 200683 (see PenTAG results below) |
n = 176 (analysed n = 174);f age (years): 64.8 (13.8); male, n (%): 87 (50); Hb BL (g/dl): 9.59 (1.22); epoetin BL (mU/ml): 68.99 (2.3–1522.7) | n = 173 (analysed n = 170); age (years): 64.6 (12.2); male, n (%): 78 (46); Hb BL (g/dl): 9.5 (1.21); epoetin BL (mU/ml): 54.49 (10.9–3169.1) | Brand: darbepoetin alfa; dose: 2.25 µg/kg QW;f dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 16 weeks; follow-up: median ≈11 months | PBO | Iron: prn; G-CSF: NR; RBCT trigger: Hb ≤ 8 g/dl prn (Hb inclusion criterion level: ≤ 11.0 g/dl) | Disease: haematological; treatment: chemotherapy, NR | HaemR, RBCT, HRQoL, AEs | Y |
| Kotasek 200350 RCT, dose–response study; five unlicensed doses excluded |
n = 17/198;b,f age (years): 58.3 (11.9);b male, n (%): 56 (28);b Hb BL (g/dl): 9.93 (1.00);b epoetin BL (mU/ml): NR | n = 51; age (years): 56.2 (12.4); male, n (%): 16 (31); Hb BL (g/dl): 9.87 (1.12); epoetin BL (mU/ml): NR | Brand: darbepoetin alfa; dose: 2.25 µg/kg QW;f dose adjustment: Y, ↓; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks;d follow-up: unclear | PBO | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 11.0 g/dl) | Disease: solid (breast, gynaecological, gastrointestinal, lung); treatment: chemotherapy, NR | HaemR, Hb, RBCT, HRQoL,b AEsb | Y |
| Kurz 199769 RCT |
n = 23; age (years): 54.4 ± 9.7; male, n (%): NR; Hb BL (g/dl): 9.88 ± 0.8; epoetin BL (mU/ml): NR | n = 12; age (years): 52.7 ± 7.5; male, n (%): NR; Hb BL (g/dl): 9.85 ± 0.60; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: NR | PBO | Iron: Y, intravenous iron; G-CSF: NR; RBCT trigger: Hb < 8 g/dl (Hb inclusion criterion level: < 11.0 g/dl) | Disease: solid (ovary, cervix, uterus); treatment: chemotherapy, mixedg | HaemR, RBCT, HRQoL, AEs | Y |
| Littlewood 200170 RCT (EPO-INT-1); multiple publications: Aapro, 2004,82 Bajetta 2004,81 Patrick 200360 (see PenTAG results below) |
n = 251 (analysed n = 244); age (years): 58.3 ± 14.2; male, n (%): 85 (34); Hb BL (g/dl): 9.9 ± 1.1; epoetin BL (mU/ml): NR | n = 124 (analysed n = 115); age (years): 59.5 ± 13.9; male, n (%): 39 (31); Hb BL (g/dl): 9.7 ± 1.1; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: up to 28 weeks; duration of trial: up to 28 weeks; follow-up: 12 monthsh | PBO | Iron: Y, oral (or intravenous as indicated by transferrin saturation level); G-CSF: N; RBCT trigger: Hb < 8 g/dl prn (Hb inclusion criterion level: ≤ 10.5 g/dl or > 10.5 g/dl but ≤ 12.0 g/dl after a ≥ 1.5 g/dl decrease in Hb) | Disease: solid and haematological; treatment: chemotherapy, non-platinum containing | HaemR, Hb, RBCT, HRQoL, AEs, survival (at 12 months’ follow-up) | Y |
| Österborg 200271 RCT; multiple publications: Österborg 200579 (see PenTAG results below) |
n = 173 (analysed n = 170); age (years): 63 (32–86); male, n (%): 91 (54); Hb BL (g/dl): 9.2 ± 1.1; epoetin BL (mU/ml): 38 (20–72) | n = 176 (analysed n = 173); age (years): 64 (28–83); male, n (%): 82 (47); Hb BL (g/dl): 9.3 ± 1.0; epoetin BL (mU/ml): 41 (21–77) | Brand: epoetin beta; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: up to 16 weeks; follow-up: min. 17.5 months both tx groups | PBO | Iron: Y, intravenously (or orally if intravenously precluded); G-CSF: NR; RBCT trigger: Hb < 8.5 g/dl or increase in Hb of < 0.5 g/dl vs. BL (Hb inclusion criterion level: < 10 g/dli) | Disease: haematological; treatment: chemotherapy, non-platinum containing | HaemR, Hb, RBCT, HRQoL, AEs, survival, long-term survival | Y |
| Silvestris 199572 ROL |
n = 30 (analysed n = 27); age (years): NR; male, n (%): NR; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | n = 24 (analysed n = 22); age (years): NR; male, n (%): NR; Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: up to 24 weeks; duration of trial: up to 24 weeks; follow-up: NR | SC | Iron: Y, not specified; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 8 g/dl) | Disease: haematological; treatment: chemotherapy, NR | HaemR, Hb, AEs | Y |
| ten Bokkel Huinink 199851 ROL; multiple treatment arms, one unlicensed dose excluded |
n = 46 (analysed n = 45);f age (years): 58.81; male, n (%): all female; Hb BL (g/dl): 12.0 (1.3–12.6); epoetin BL (mU/ml): NR | n = 34 (analysed n = 33); age (years): 58.83; male, n (%): all female; Hb BL (g/dl): 11.8 (10.6–12.5); epoetin BL (mU/ml): NR | Brand: epoetin beta; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: 24 weeks; duration of trial: 24 weeks; follow-up: NR | SC | Iron: NR; G-CSF: NR; RBCT trigger: Hb < 9.7 g/dl (Hb inclusion criterion level: < 13 g/dl) | Disease: solid (ovary); treatment: chemotherapy, platinum containing | RBCT, AEs | Y |
| Thatcher 199952 ROL; multiple treatment arms, one unlicensed dose excluded |
n = 42;f age (years): 59 (43–72); male, n (%): 26 (61.9); Hb BL (g/dl): 13.7 (10.7–16.1); epoetin BL (mU/ml): NR | n = 44; age (years): 60 (39–74); male, n (%): 27 (61.3); Hb BL (g/dl): 13.4 (10.9–16.4); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: 26 weeks; duration of trial: 26 weeks; follow-up: NR | SC | Iron: NR; G-CSF: NR; RBCT trigger: prn (Hb inclusion criterion level: ≥ 10.5 g/dl) | Disease: solid (small-cell lung cancer); treatment: chemotherapy: mixedg | Hb, RBCT, HRQoL, AEs | Y |
| Vansteenkiste 200273 RCT; multiple publications: Vansteenkiste 200484 (see PenTAG results below) |
n = 156; age (years): 61.6 (9.2); male, n (%): 111 (71); Hb BL (g/dl): 10.28 (1.08); epoetin BL (mU/ml): 53.17 (58.87)j | n = 158; age (years): 61.3 (8.8); male, n (%): 116 (73); Hb BL (g/dl): 9.93 (1.01); epoetin BL (mU/ml): 51.10 (71.72)j | Brand: darbepoetin alfa; dose: 2.25 µg/kg QW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: 12 months | PBO | Iron: NR; G-CSF: NR; RBCT trigger: Hb ≤ 8 g/dl (Hb inclusion criterion level: ≤ 11.0 g/dl) | Disease: solid (lung); treatment: chemotherapy, platinum containing | HaemR, Hb, RBCT (from week 5 and week 1), HRQoL, AEs, disease progression, survival | Y |
| Wilson and colleagues 2007:2 multiple publications | ||||||||
| Abels 199659 Double-blind data + unified analysis ; identified: citation checking; primary study: Abels 199363 |
n = 153/213 (analysed n = 143/206);b age (years): 61.2 ± 13.0; male, n (%): 102 (47.8); Hb BL (g/dl): NR; epoetin BL (mU/ml): 146 ± 260 | n = 200 (analysed n = 135/190); age (years): 62.5 ± 12.3; male, n (%): 95 (47.5); Hb BL (g/dl): NR; epoetin BL (mU/ml): 149 ± 217 | Brand: rHuEPOb; dose: 150 IU/kg TIW; dose adjustment: NR; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks;d follow-up: NR | PBO | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: ≤ 10.5 g/dl) | Disease: haematological and solid; treatment: chemotherapy, mixed | HaemR, haematocrit, RBCT, HRQoL,b AEsb | Y |
| Case 199386 RCT; primary study: Abels 199363 |
n = 81 (analysed n = 79); age (years): 64 (27–92); male, n (%): 33 (40.7); Hb BL (g/dl): NR; epoetin BL (mU/ml): 95.0 (16–1262) | n = 76 (analysed n = 79); age (years): 64 (30–88); male, n (%): 29 (38.1); Hb BL (g/dl): NR; epoetin BL (mU/ml): 93.5 (16–1734) | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y, ↓prn; duration of epoetin tx: NR; duration of trial: NR; follow-up: NR | SC | Iron: Y, oral iron, fixed; G-CSF: N; RBCT trigger: Hb < 9 g/dl (Hb inclusion criterion level: < 10.5 g/dl) | Disease: solid (including ovarian); treatment: chemotherapy, platinum containing | Hb, haematocrit, RBCT | Y |
| Henry 199558 ROL; primary study: Abels 199363 |
n = 69 (analysed n = 64); age (years): 58; male, n (%): 30 (45); Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | n = 61 (analysed n = 65); age (years): 59; male, n (%): 32 (49); Hb BL (g/dl): NR; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y, ↓prn; duration of epoetin tx: 12 weeks or until haematocrit = 38−40%; duration of trial: 12 weeks; follow-up: NR | PBO | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: NR) | Disease: haematological and solid; treatment: chemotherapy, platinum containing | Haematocrit, RBCT, AEs | Y |
| Henry 199485 ROL Double-blind and open-label extension data; identified: citation checking; primary study: Abels 199363 |
n = 67 (analysed n = 64); age (years): NR;k male, n (%): NR;k Hb BL (g/dl): NR;k epoetin BL (mU/ml): NRk | n = 65 (analysed n = 61); age (years): NR;k male, n (%): NR;k Hb BL (g/dl): NR;k epoetin BL (mU/ml): NRk | Brand: epoetin alfa; dose: 450 IU/kg QW; dose adjustment: NR; duration of epoetin tx: 12 weeks; duration of trial: NR; follow-up: NR | SC | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: NR) | Disease: solid and haematological; treatment: chemotherapy, platinum containing | HaemR, RBCT, HRQoL, AEs | Y |
| PenTAG review: study characteristics – primary studies 2004 to current | ||||||||
| Grote 200574 ROL (N93-004) |
n = 109; age (years): 64.4 (8.7); male, n (%): 59 (54.1); Hb BL (g/dl): 12.8 (1.5); epoetin BL (mU/ml): NR | n = 115; age (years): 63.2 (8.9); male, n (%): 64 (55.7); Hb BL (g/dl): 13 (1.5); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: unclear; duration of trial: unclear; follow-up: 3 years | PBO | Iron: NR; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: < 14.5 g/dl) | Disease: solid (small-cell lung cancer); treatment: chemotherapy, platinum containing | Hb, RBCT, AEs, TR, survival | Y |
| Moebus 201362 ROL [Arbeitsgemeinschaft für Gynäkologische Onkologie epirubicin, paclitaxel, and cyclophosphamide (AGO ETC) trial] |
n = 324; age (years): 50 (29–65); male, n (%): all female; Hb BL (g/dl): 12.4 (9–16); epoetin BL (mU/ml): NR | n = 319; age (years): 52 (28–67); male, n (%): all female; Hb BL (g/dl): 12.8 (9–16); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: 18 weeks; duration of trial: 18 weeks; follow-up: 10 years (ongoing) | SC | Iron: Y, oral; G-CSF: NR; RBCT trigger: Hb < 9 g/dl and investigator discretion (Hb inclusion criterion level: NR) | Disease: solid (breast); treatment: chemotherapy, non-platinum containing | Hb, RBCT, HRQoL, AEs, survival | N; however, Moebus 2004192 and 200733 included |
| Ray-Coquard 200975 ROL (ELYPSE study) |
n = 110; age (years): 62.7 (11.6); male, n (%): 52 (47.3); Hb BL (g/dl): 10 (1.2); epoetin BL (mU/ml): NR | n = 108; age (years): 61.7 (11.6); male, n (%): 41 (38); Hb BL (g/dl): 10 (1.2); epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: 12 months (95% CI 12–12.4 months) | SC | Iron: Y, oral; G-CSF: Y; RBCT trigger: NR (Hb inclusion criterion level: ≤ 12.0 g/dl) | Disease: solid and haematological; treatment: chemotherapy, NR | RBCT, OS, HRQoL, AEs | Y |
| Strauss 200876 RCT (NCT00046969; Roche MO16375; Strauss 2005252) |
n = 34; age (years): 48.8 ± 10.2; male, n (%): all female; Hb BL (g/dl): 11.4 (10.8–12.0); epoetin BL (mU/ml): NR | n = 40; age (years): 49.2 ± 12.8); male, n (%): all female; Hb BL (g/dl): 11.6 (10.9–12.4); epoetin BL (mU/ml): NR | Brand: epoetin beta; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: 6 months | PBO | Iron: Y, orally or intravenously; G-CSF: NR; RBCT trigger: ≤ 8.5 g/dl prn (Hb inclusion criterion level: 9–13 g/dl) | Disease: solid (cervix); treatment: chemotherapy + radiotherapy | Hb, RBCT TR, survival, AEs | Y |
| Tjulandin 201048 RCT (ISRCTN09530309) |
Epoetin theta: n = 76; age (years): 53.7 ± 10.3; male, n (%): 30 (39.5); Hb BL (g/dl): 9.6 ± 1.1; epoetin BL (mU/ml): NR Epoetin beta: n = 73; age (years): 57.3 ± 10.5; male, n (%): 22 (30.1); Hb BL (g/dl): 9.5 ± 0.8; epoetin BL (mU/ml): NR |
n = 74; age (years): 57.3 ± 11.5; male, n (%): 19 (25.7); Hb BL (g/dl): 9.4 ± 1.2; epoetin BL (mU/ml): NR | Brand: epoetin beta; dose: 150 IU/kg TIW; dose adjustment: Y Brand: epoetin theta; dose: 20,000 IU QW Dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: NR |
PBO | Iron: Y, not specified; G-CSF: NR; RBCT trigger: ≤ 8.5 g/dl prn (Hb inclusion criterion level: ≤ 11.0 g/dl) | Disease: solid; treatment: chemotherapy, platinum containing | HaemR, Hb, RBCT, RBC units, HRQoL, AEs | Y |
| Tjulandin 201177 RCT |
n = 95; age (years): 56.9 ± 14.7; male, n (%): 30 (31.6); Hb BL (g/dl): 9.2 ± 1.3; epoetin BL (mU/ml): NR | n = 91; age (years): 55.8 ± 14.3; male, n (%): 34 (37.4); Hb BL (g/dl): 9.1 ± 1.3; epoetin BL (mU/ml): NR | Brand: epoetin theta; dose: 20,000 IU QW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: NR | PBO | Iron: Y, not specified; G-CSF: NR; RBCT trigger: ≤ 8.5 g/dl (Hb inclusion criterion level: ≤ 11.0 g/dl) | Disease: solid and haematological; treatment: chemotherapy, non-platinum containing | HaemR, Hb, RBCT, RBC units, HRQoL, AEs | Y |
| Untch 201178,80 RCT |
n = 356; age (years): 48 (23–65);b male, n (%): NR; Hb BL (g/dl): 13.64 ± 1.17; epoetin BL (mU/ml): NR | n = 377; age (years): 48 (23–65);b male, n (%): NR; Hb BL (g/dl): 13.61 ± 1.16; epoetin BL (mU/ml): NR | Brand: darbepoetin alfa; dose: 4.5 mg/kg Q2W;l dose adjustment: Y; duration of epoetin tx: NR; duration of trial: NR; follow-up: median 43.5 months | SC | Iron: Y, orally; G-CSF: NR; RBCT trigger: NR (Hb inclusion criterion level: NR | Disease: solid (breast); treatment: chemotherapy, non-platinum containing | Hb, pathological response, disease progression, survival, AEs | Y |
| Multiple publications: PenTAG review | ||||||||
| Aapro 200482 Primary study: Littlewood 200170 |
n = 251 (analysed n = 244); age (years): 58.3 ± 14.2; male, n (%): 85 (34); Hb BL (g/dl): 9.9 ± 1.1; epoetin BL (mU/ml): NR | n = 124 (analysed n = 115); age (years): 59.5 ± 13.9; male, n (%): 39 (31); Hb BL (g/dl): 9.7 ± 1.1; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: up to 28 weeks; duration of trial: up to 28 weeks; follow-up: 12 monthsh | PBO | Iron: Y, orally or intravenously; G-CSF: N; RBCT trigger: Hb < 8 g/dl prn (Hb inclusion criterion level: ≤ 10.5 g/dl or > 10.5 g/dl but ≤ 12.0 g/dl after a ≥ 1.5 g/dl decrease in Hb) | Disease: solid + haematological; treatment: chemotherapy, non-platinum containing | HaemR, Hb, RBCT, HRQoL, AEs, survival (at 12 months’ follow-up) | N |
| Bajetta 200481 Primary study: Littlewood 200170 |
Subgroup: breast population: n = 78 (analysed n = 75); age (years): 54.6; male, n (%): 1 (1); Hb BL (g/dl): 10.0 ± 1.6; epoetin BL (mU/ml): NR |
Subgroup: breast population: n = 36 (analysed n = 35); age (years): 52.9; male, n (%): all female; Hb BL (g/dl): 9.9 ± 1.01; epoetin BL (mU/ml): NR |
Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: up to 28 weeks; duration of trial: up to 28 weeks; follow-up: 12 monthsh | PBO | Iron: Y, orally (or intravenously as indicated by transferrin saturation level); G-CSF: N; RBCT trigger: Hb < 8 g/dl prn (Hb inclusion criterion level: ≤ 10.5 g/dl or > 10.5 g/dl but ≤ 12.0 g/dl after a ≥ 1.5 g/dl decrease in Hb) | Disease: solid and haematological; treatment: chemotherapy, non-platinum containing | HaemR, Hb, RBCT, HRQoL, AEs (retrospective analysis of breast cancer cohort70) | N |
| Littlewood 200683 Primary study: Hedenus, 200317 |
Subgroup: HRQoL sample: n = 303;m age (years): 64.8 (12.8); male, n (%): 146 (48.2); Hb BL (g/dl): 9.6 (1.2); epoetin BL (mU/ml): NR |
Brand: darbepoetin alfa; dose: 2.25 µg/kg QW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: median ≈11 months | PBO | Iron: prn; G-CSF: NR; RBCT trigger: Hb ≤ 8 g/dl (Hb inclusion criterion level: ≤ 11.0 g/dl) | Disease: haematological; treatment: chemotherapy, NR | HRQoL (alleviating fatigue and effect of fatigue on quality of life) | N | |
| Österborg 2005,79 survival data from Österborg 200271 RCT; primary study: Österborg 200271 |
n = 173 (analysed n = 170); age (years): 63 (32–86); male, n (%): 91 (54); Hb BL (g/dl): 9.2 ± 1.1; epoetin BL (mU/ml): 38 (20–72) | n = 176 (analysed n = 173); age (years): 64 (28–83); male, n (%): 82 (47); Hb BL (g/dl): 9.3 ± 1.0; epoetin BL (mU/ml): 41 (21–77) | Brand: epoetin beta; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: 16 weeks; duration of trial: up to 16 weeks; follow-up: minimum 17.5 months both tx groups | PBO | Iron: Y, oral iron or intravenous iron if transferrin saturation ≤ 20%; G-CSF: N; RBCT trigger: Hb < 8.5 g/dl or increase in Hb of < 0.5 g/dl vs. BL (Hb inclusion criterion level: < 10 g/dli) | Disease: haematological; treatment: chemotherapy, non-platinum containing | Long-term survival | Y |
| Patrick 200360 Primary study: Littlewood 200170 |
n = 251 (analysed n = 244); age (years): 58.3 ± 14.2; male, n (%): 85 (34); Hb BL (g/dl): 9.9 ± 1.1; epoetin BL (mU/ml): NR | n = 124 (analysed n = 115); age (years): 59.5 ± 13.9; male, n (%): 39 (31); Hb BL (g/dl): 9.7 ± 1.1; epoetin BL (mU/ml): NR | Brand: epoetin alfa; dose: 150 IU/kg TIW; dose adjustment: Y; duration of epoetin tx: up to 28 weeks; duration of trial: up to 28 weeks; follow-up: 12 monthsh | PBO | Iron: Y, orally (or intravenously as indicated by transferrin saturation level); G-CSF: N; RBCT trigger: Hb < 8 g/dl prn (Hb inclusion criterion level: ≤ 10.5 g/dl or > 10.5 g/dl but ≤ 12.0 g/dl after a ≥ 1.5 g/dl decrease in Hb) | Disease: solid and haematological; treatment: chemotherapy, non-platinum containing | HRQoL (minimally important difference in HRQoL) | N |
| Vansteenkiste 200484 Primary study: Vansteenkiste, 200273 |
Subgroup Hb < 10 g/dl: n = 51; age (years): 63 (47–76); male, n (%): 42 (82); Hb BL (g/dl): 9.2 (7.4–9.9); epoetin BL (mU/ml): 50.3 (13.3–739.8) Subgroup Hb ≥ 10 g/dl n = 105; age (years): 62 (39–80); male, n (%): 69 (66); Hb BL (g/dl): 10.8 (10.0–13.6); epoetin BL (mU/ml): 28.8 (12.0–106.1) |
Subgroup Hb < 10 g/dl: n = 69; age (years): 60 (42–78); male, n (%): 52 (75); Hb BL (g/dl): 9.2 (6.6–9.9); epoetin BL (mU/ml): 52.2 (14.3–1998.6) Subgroup Hb ≥ 10 g/dl n = 89; age (years): 62 (36–76); male, n (%): 64 (72); Hb BL (g/dl): 10.6 (10.0–12.3); epoetin BL (mU/ml): 30.2 (12.0–109.8) |
Brand: darbepoetin alfa; dose: 2.25 µg/kg QW; dose adjustment: Y; duration of epoetin tx: 12 weeks; duration of trial: 12 weeks; follow-up: 12 months | PBO | Iron: N; G-CSF: N; RBCT trigger: Hb ≤ 8 g/dl and prn (Hb inclusion criterion level: ≤ 11.0 g/dl) | Disease: solid (lung); treatment: chemotherapy, platinum containing | HaemR, Hb, RBCT (from week 5 and week 1), HRQoL, AEs, disease progression, survival | Y |
Appendix 8 Multiple publications in clinical-effectiveness review
Primary study
Hedenus M, Adriansson M, San Miguel J, Kramer MH, Schipperus MR, Juvonen E, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol 2003;122:394–403.
Secondary publication
Littlewood TJ, Kallich JD, San Miguel J, Hendricks L, Hedenus M. Efficacy of darbepoetin alfa in alleviating fatigue and the effect of fatigue on quality of life in anemic patients with lymphoproliferative malignancies. J Pain Symptom Manage 2006;31:317–25.
Primary study
Österborg A, Brandberg Y, Molostova V, Iosava G, Abdulkadyrov K, Hedenus M, et al. Randomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. J Clin Oncol 2002;20:2486–94.
Secondary publication
Österborg A, Brandberg Y, Hedenus M. Impact of epoetin-beta on survival of patients with lymphoproliferative malignancies: long-term follow up of a large randomized study. Br J Haematol 2005;129:206–9.
Primary study
Vansteenkiste J, Pirker R, Massuti B, Barata F, Font A, Fiegl M, et al. Double-blind, placebo-controlled, randomized Phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 2002;94:1211–20.
Secondary publication
Vansteenkiste J, Tomita D, Rossi G, Pirker R. Darbepoetin alfa in lung cancer patients on chemotherapy: a retrospective comparison of outcomes in patients with mild versus moderate-to-severe anaemia at baseline. Support Care Cancer 2004;12:253–62.
Primary study
Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B, Epoetin Alfa Study Group. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2001;19:2865–74.
Secondary publications
Bajetta E, Vercammen E, Reinhardt U, Janmohamed R, da Costa RM, Matulonis U, et al. Efficacy of epoetin alfa in a retrospective non-stratified subgroup analysis of a breast cancer cohort receiving non-platinum chemotherapy. Tumori 2004;90:449–57.
Aapro M, Bajetta E, Freund M, Littlewood TJ, Nortier JWR, Rapoport B. Is there a possible survival benefit to increasing hemoglobin levels with epoetin alfa during chemotherapy? Eur J Cancer Suppl 2004;2:20–8.
Patrick DL, Gagnon DD, Zagari MJ, Mathijs R, Sweetenham J, Epoetin Alfa Study Group. Assessing the clinical significance of health-related quality of life (HrQOL) improvements in anaemic cancer patients receiving epoetin alfa. Eur J Cancer 2003;39:335–45.
Primary study
Abels R. Erythropoietin for anaemia in cancer patients. Eur J Cancer 1993;29A(Suppl. 2):S2–8.
Secondary publications
Case DC Jr, Bukowski RM, Carey RW, Fishkin EH, Henry DH, Jacobson RJ, et al. Recombinant human erythropoietin therapy for anemic cancer patients on combination chemotherapy. J Natl Cancer Inst 1993;85:801–6.
Henry DH, Abels RI. Recombinant human erythropoietin in the treatment of cancer and chemotherapy-induced anemia: results of double-blind and open-label follow-up studies. Semin Oncol 1994;21:21–8.
Henry DH, Brooks BJ Jr, Case DC Jr, Fishkin E, Jacobson R, Keller AM, et al. Recombinant human erythropoietin therapy for anemic cancer patients receiving cisplatin chemotherapy. Cancer J Sci Am 1995;1:252–60.
Abels RI, Larholt KM, Krantz KD, Bryant EC. Recombinant human erythropoietin (rHuEPO) for the treatment of the anemia of cancer. Oncologist 1996;1:140–50.
Primary study
Untch M, Fasching PA, Konecny GE, von Koch F, Conrad U, Fett W, et al. PREPARE trial: a randomized Phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel and CMF versus a standard-dosed epirubicin/cyclophosphamide followed by paclitaxel ± darbepoetin alfa in primary breast cancer – results at the time of surgery. Ann Oncol 2011;22:1988–98.
Secondary publication
Untch M, Minckwitz G, Konecny GE, Conrad U, Fett W, Kurzeder C, et al. PREPARE trial: a randomized Phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel, and CMF versus a standard-dosed epirubicin/cyclophosphamide followed by paclitaxel with or without darbepoetin alfa in primary breast cancer – outcome on prognosis. Ann Oncol 2011;22:1999–2006.
Appendix 9 Application of licence in the included studies
| Study | n | Arms | Malignancy | Treatment | Initial treatmenta | Start Hb levela | Target Hb levela | Dose adjustmenta |
|---|---|---|---|---|---|---|---|---|
| Licence details for epoetin alfa and epoetin betab | Initial treatment: 150 IU/kg SC TIW or 450 IU/kg SC QW Start Hb level: ≤ 10 g/dl Target Hb level: 10−12 g/dl Dose adjustment: 4 weeks: Hb increase < 1 g/dl and reticulocyte increase ≥ 40,000 cells/µl: 300 IU/kg Q3W or 900 IU/kg QW; Hb increase ≥ 2 g/dl: reduce dose by 25–50%; Hb ≥ 12 g/dl: reduce dose by 25–50%; Hb ≥ 13 g/dl: stop and reinitiate at Hb 12 g/dl at a 25% lower dose |
|||||||
| Abels 199363 | 413c | Epoetin alfa vs. placebo | Solid and haematological | Chemotherapy: mixed | 150 IU/kg TIW | ≤ 10.5 g/l or haematocrit ≤ 32% | NR | No dose escalation used. Dosing continued for 12 weeks; if haematocrit ≥ 38% withheld until haematocrit fell below 38% |
| Aravantinos 200364 | 47 | Epoetin alfa vs. standard | Solid | Chemotherapy: mixed | 150 IU/kg TIW | < 10.5 g/dl | NR | No dose escalation used Hb > 14 g/dl: stop and reinitiate at Hb < 12.5 g/dl at 25% lower than the start dose |
| Boogaerts 200365 | 262 | Epoetin beta vs. standard | Solid and haematological | Chemotherapy: NR | 150 IU/kg TIW | ≤ 11 g/dl | 12–14 g/dl | 3–4 weeks Hb increase < 0.5 g/dl or 6 weeks Hb increase < 1 g/dl: dose doubled Hb increase > 2 g/dl: dose reduced by 50% Hb > 14 g/dl: stop and reinitiate at Hb < 12 g/dl at 50% lower than the start dose |
| Dammacco 200166 | 145 | Epoetin alfa vs. placebo | Haematological | Chemotherapy: mixed | 150 IU/kg TIW | < 11 g/dl | 12–14 g/dl | 4 weeks Hb increase < 1 g/dl: dose doubled 4 weeks Hb increase ≥ 2 g/dl: dose reduced by 25% Hb > 14 g/dl: stop and reinitiate at Hb ≤ 12 g/dl at 25% lower than the start dose |
| Del Mastro 199767 | 62 | rHuEPOd vs. standard | Solid (breast) | Chemotherapy: non-plat | 150 IU/kg TIW | ≤ 12 g/dl | NR | If Hb > 15.0 g/dl in two consecutive weekly assays, treatment stopped until Hb < 13.0 g/dl |
| Dunphy 199968 | 30 | rHuEPOd vs. standard | Solid (head and neck, lung) | Chemotherapy: mixed | 150 IU/kg TIW | NR; note rHuEPO was initiated if Hb ≤ 16 g/dl | NR | First course of chemotherapy: Hb increase < 1 g/dl: dose doubled Second course of chemotherapy: Hb increase < 1 g/dl: dose increased to 450 IU/kg Hb ≥ 18 g/dl: stop and reinitiate at Hb ≤ 16 g/dl |
| Grote 200574 | 224 | Epoetin alfa vs. placebo | Solid (SCLC) | Chemotherapy: mixed | 150 IU/kg TIW | ≤ 14.5 g/dl | 14–16 g/dl | Dose escalation not permitted Hb > 16 g/dl: stop and reinitiate at Hb < 14 g/dl at a 50% lower dose |
| Kurz 199769 | 35 | Epoetin alfa vs. placebo | Solid (cervix, ovary, uterus) | Chemotherapy: mixed | 150 IU/kg TIW | < 11 g/dl | NR | 4 weeks Hb increase < 1 g/dl: dose doubled |
| Littlewood 200170 | 375 | Epoetin alfa vs. placebo | Mixed | Chemotherapy: non-plat | 150 IU/kg TIW | ≤ 10.5 g/dl or > 10 and ≤ 12 g/dl with ≥ 1.5 g/dl decrease in Hb per cycle per month | 12–15 g/dl | 4 weeks Hb increase < 1 g/dl and reticulocyte count increase < 40,000 cells/µl: dose doubled to 300 IU/kg 4 weeks Hb increase ≥ 2 g/dl: reduce dose by 25% If Hb > 15 g/dl: stop and reinitiate at Hb < 12 g/dl at a 25% lower dose |
| Moebus 201362 | 643 | Epoetin alfa vs. standard | Solid (breast) | Chemotherapy: non-plat | 150 IU/kg TIW | NR | 12.5−13 g/dl | 4 weeks Hb increase < 2 g/dl: dose doubled If Hb > 14 g/dl: stop and reinitiate at Hb < 13 g/dl |
| Österborg 2002,71 200579 | 349 | Epoetin beta vs. placebo | Haematological | Chemotherapy: non-plat | 150 IU/kg TIW | < 10 g/dl e | 13–14 g/dl | 4 weeks Hb increase < 0.5 g/dl: dose doubled 4 weeks Hb < 8.5 g/dl or transfusion: dose doubled 4 weeks Hb increase > 2 g/dl: reduce dose by 50% If Hb > 14 g/dl: stop and reinitiate at Hb ≤ 13 g/dl at 50% |
| Ray-Coquard 200975 | 218 | Epoetin alfa vs. standard | Mixed | Chemotherapy: NR | 150 IU/kg TIW | < 12 g/dl | 12–14 g/dl | 4 weeks Hb increase < 1 g/dl and Hb < 10.5 g/dl and reticulocyte count < 40,000 cells/µl: dose increased to 60,000 IU weekly 4 weeks Hb increase ≥ 2 g/dl: reduce dose by 25% If Hb > 12 g/dl: stop and reinitiate at Hb ≤ 12 g/dl |
| Silvestris 199572 | 54 | Epoetin alfa vs. standard | Haematological | Chemotherapy: NR | 150 IU/kg TIW | ≤ 8 g/dl | NR | By sixth week: dose doubled |
| Strauss 200876 | 74 | Epoetin beta vs. standard | Solid (cervix) | Chemotherapy + radiotherapy | 150 IU/kg TIW | 9−13 g/dl | 14–15 g/dl | 4 weeks Hb increase < 0.5 g/dl: dose doubled 4 weeks Hb increase > 2 g/dl: reduce dose by 50% If Hb > 15 g/dl: stop and reinitiate at Hb ≤ 14 g/dl at 50% lower dose If Hb < 8.5 g/dl: dose doubled |
| ten Bokkel Huinink 199851 | 122 | Epoetin beta vs. standard | Solid (ovary) | Chemotherapy: plat | 150 IU/kg TIW | < 13 g/dl | 14–15 g/dl | 4 weeks Hb increase ≥ 2 g/dl: reduce dose by 50% Hb > 15 g/dl: stop and reinitiate at Hb ≤ 14 g/dl at 50% lower dose Epoetin withheld while platelet counts < 20,000 µg/l |
| Thatcher 199952 | 130 | Epoetin alfa vs. standard | Solid (SCLC) | Chemotherapy: plat | 150 IU/kg TIW | ≤ 10.5 g/dl | ≥ 10 g/dl | Hb > 15 g/dl: stop and reinitiate at Hb ≤ 13 g/dl at 50% lower dose |
| Licence details for epoetin theta | Dose: 20,000 IU QW Start Hb level: ≤ 10 g/dl Target HB level: 10−12 g/dl Dose adjustment: 4 weeks Hb increase < 1 g/dl: dose doubled; increase to 60,000 IU if Hb increase insufficient at 8 weeks; Hb > 12 g/dl should be avoided; 12 weeks Hb increase < 1 g/dl: discontinue |
|||||||
| Tjulandin 201048 | 223 | Epoetin theta and epoetin beta vs. placebo | Solid | Chemotherapy: plat | Epoetin theta: 20,000 IU QW | ≤ 11 g/dl | NR | 4 weeks Hb increase < 1 g/dl: dose doubled; further increase to 60,000 IU if no response at 8 weeks 4 weeks Hb increase > 2 g/dl: reduce dose by 50% Hb > 13 g/dl: stop or reduce dose by 50% |
| Epoetin beta: 150 IU/kg Q3W≤ | ≤ 11 g/dl | NR | 4 weeks Hb increase < 1 g/dl: dose doubled; no further increase allowed 4 weeks Hb increase > 2 g/dl: dose reduced by 50% Hb > 13 g/dl: stop or reduce dose by 50% |
|||||
| Tjulandin 201177 | 186 | Epoetin theta vs. placebo | Mixed | Chemotherapy: non-plat | 20,000 IU QW | ≤ 11 g/dl | NR | 4 weeks Hb increase < 1 g/dl: dose doubled; further increase to 60,000 IU if no response at 8 weeks 4 weeks Hb increase > 2 g/dl: reduce dose by 50% Hb > 13 g/dl: stop or reduce dose by 50% |
| Licence details for darbepoetin alfa | Dose: 2.25 µg/kg SC QW; 500 µg (6.75 µg/kg) SC Q3W Start Hb level: ≤ 10 g/dl Target HB level: 10−12 g/dl Dose adjustment: 4 weeks Hb increase < 1 g/dl: dose doubled; 4 weeks Hb increase ≥ 2 g/dl: reduce dose by 25–50%; Hb ≥ 12 g/dl: reduce dose by 25–50%; Hb ≥ 13 g/dl: stop and reinitiate at Hb 12 g/dl at a 25% lower dose |
|||||||
| Hedenus 200253 | 33f | Darbepoetin alfa vs. placebo | Haematological | Chemotherapy: NR | 2.25 µg/kg QW | ≤ 11 g/dl | NR | 4 weeks Hb increase ≥ 2 g/dl: 50% dose reduction If Hb > 15.0 g/dl (men) or > 14.0 g/dl (women): stop and reinitiate at Hb ≤ 13.0 g/dl at 50% lower dose |
| Hedenus 200317 | 349 | Darbepoetin alfa vs. placebo | Haematological | Chemotherapy: NR | 2.25 µg/kg QW | ≤ 11 g/dl | 13–14 g/dl | 4 weeks Hb increase ≤ 1 g/dl: dose doubled If Hb > 15.0 g/dl (men) or > 14.0 g/dl (women): stop and reinitiate at Hb ≤ 13.0 g/dl at a 50% lower dose |
| Kotasek 200350 | 249 | Darbepoetin alfa vs. placebo | Solid | Chemotherapy: NR | 6.75 µg/kg Q3W | ≤ 11 g/dl | 13–14 g/dl (women), 13–15 g/dl (men) | Dose increase not allowed If Hb > 15.0 g/dl (men) or > 14.0 g/dl (women): stop and reinitiate at Hb ≤ 13.0 g/dl at a 50% lower dose |
| Untch 201178,80 | 733 | Darbepoetin alfa vs. standard | Solid (breast) | Chemotherapy: non-plat | 4.5 µg/kg (every 2 weeks)g | NR | 12.5−13 g/dl | 4 weeks Hb increase < 1 g/dl: dose doubled If Hb > 14.0 g/dl: stop and reinitiate at Hb ≤ 13.0 g/dl at a 50% lower dose |
| Vansteenkiste 200273 | 314 | Darbepoetin alfa vs. placebo | Solid (lung) | Chemotherapy: plat | 2.25 µg/kg QW | ≤ 11 g/dl | 13–14 g/dl (women), 13–15 g/dl (men) | 6 weeks Hb increase < 1 g/dl: dose doubled If Hb > 15.0 g/dl (men) or > 14.0 g/dl (women): stop and reinitiate at Hb ≤ 13.0 g/dl at a 50% lower dose |
Appendix 10 Comparison of search results with the manufacturer submissions
| Citation | Reason for exclusion from the PenTAG review |
|---|---|
| Haag-Weber M, Eckardt KU, Hörl WH, Roger SD, Vetter A, Roth K. Safety, immunogenicity and efficacy of subcutaneous biosimilar epoetin-alpha (HX575) in non-dialysis patients with renal anemia: a multi-center, randomized, double-blind study. Clinical Nephrol 2012;77:8–17 | Comparator (epoetin alfa vs. epoetin alfa); no control |
| Weigang-Köhler K, Vetter A, Thyroff-Friesinger U. HX575, recombinant human epoetin alfa, for the treatment of chemotherapy-associated symptomatic anaemia in patients with solid tumours. Onkologie 2009;32:168–74 | Comparator (epoetin alfa vs. epoetin alfa); no control |
| Desrame J, Stamerra O, Labourey JL, Toeldano A, Dauriac C, Ianotto JC, et al. Haemoglobin outcomes with biosimilar epoetin alfa in the management of chemotherapy-induced anaemia in cancer patients: first results from the French OncoBOS observational study. Poster presented at the European Cancer Congress, Amsterdam, the Netherlands, 27 September–1 October 2013 | Abstract only; observational study |
| Kerkhofs L, Boschetti G, Lughini A, Stanculeanu DL, Palomo AG. Use of biosimilar epoetin to increase haemoglobin levels in patients with chemotherapy-induced anaemia: real-life clinical experience. Future Oncol 2012;8:751–6 | Abstract only; retrospective analysis |
| Lorenz A, Heine O. First comparison of biosimilar epoetin alfa and darbepoetin alfa for the treatment of chemotherapy-induced anaemia. Poster presented at the European Cancer Congress, Amsterdam, the Netherlands, 27 September–1 October 2013 | Abstract only; retrospective, matched-cohort analysis |
| Rodriguez Garzotto A, Cortijo Cascajares S, Pernaut Sanchez C, Otero Blas I, Ruiz Ares G, Rebollo Laserna FJ, et al. Use of erythropoiesis-stimulating agents and comparison of different products for the treatment of chemotherapy-induced anaemia. Poster presented at the European Cancer Congress, Amsterdam, the Netherlands, 27 September–1 October 2013 | Abstract only; study design single centre audit |
| Citation | Reason for exclusion from the PenTAG review |
|---|---|
| Delarue R. Survival impact of prophylactic administration of darbepoetin alfa in patients with diffuse large B-cell lymphoma treated with immunochemotherapy: the LNH03-6B study. Educational Cancer Convention Lugano of the European School of Oncology, Lugano, Switzerland, April 2012. Crit Rev Oncol Haematol 2012;82(Suppl. 1):12–13 | Abstract only; included in Appendix 11 (current trial status unknown) |
| Hartmann JT, Metzner B, Binder C, Mergenthaler HG, Rick O, Sayer HG, et al. Addition of darbepoetin alfa to sequential high-dose VIP chemotherapy for patients with advanced metastatic germ cell cancer. J Clin Oncol 2012;30(Suppl. 1):e15026 | Abstract only |
| Katsumata N, Fujiwara Y, Katakami N, Nishiwaki Y, Tsuboi M, Takeda K, et al. Randomized, double blind, placebo-controlled Phase III study of weekly administration of darbepoetin alfa in anemic patients with lung or gynecologic cancer receiving platinum-containing chemotherapy. 20th Regional Congress of the International Society of Blood Transfusion, Nagoya, Japan, November 2009 | Abstract only |
| Nitz U, Oberhoff C, Reimer T, Schumacher C, Hackmann J, Warm M, et al. Adjuvant chemotherapy with or without darbepoetin in node-positive breast cancer: a safety analysis from the Phase III ARA plus trial. Cancer Res 2009;69(Suppl.):4100 | Abstract only; included in Appendix 11 (current trial status unknown) |
| Suzuki Y, Tokuda Y, Okamoto R, Nakagawa K, Ando K, Iwata H, et al. Randomized, placebo-controlled Phase II study of darbepoetin alfa (DA) administered every three weeks (Q3W) in patients with chemotherapy-induced anemia (CIA). Ann Oncol 2008;19:viii277 | Abstract only |
Appendix 11 Ongoing studies
| Register/identifier number (if not available study ID cited) | Sponsor/collaborators | Trial name | Investigator | Country | Established/anticipated sample size | Phase | Status | Included in PenTAG Review |
|---|---|---|---|---|---|---|---|---|
| Active not recruiting | ||||||||
| NCT00482716/CDR0000549549/BARTS-06/Q0605/93/ISRCTN11830961/EU-20731 | St Bartholomew’s Hospital, London | Epoetin alfa or epoetin beta with or without iron infusion in treating anemia in patients with cancer | Samir G Agrawal (St Bartholomew’s Hospital) | UK | 80 | Phase III | Active, not recruiting | NA |
| NCT01444456/20101123 | Amgen Inc. | Assessment of quality of life in patients with symptomatic chemotherapy-induced anaemia | MD, Amgen Inc. | Austria, Belgium, France, Germany, Greece, Italy, Netherlands, Poland, Romania | 1264 | ? | Ongoing, not recruiting | NA |
| Recruiting | ||||||||
| NCT00875004/CDR0000633325/CLCC-PLATON/CLCC-VA-2007/21/CLCC-AFSSAPS-A70755–52/INCA-RECF0639/EUDRACT-2007–003615–31/ROCHE-CLCC-PLATON | Centre Val d’Aurelle – Paul Lamarque, Montpelier | Epoetin beta in patients undergoing chemotherapy for solid tumors | Damien Pouessel and Paul Lamarque (Centre Val d’Aurelle) | France | 300 | ? | Recruiting | NA |
| NCT00338286/CR005143/EPOANE3010/CR005143/2005–001817–17 | Janssen Research & Development, LLC | A Study of epoetin alfa plus standard supportive care versus standard supportive care only in anemic patients with metastatic breast cancer receiving standard chemotherapy | Janssen Research & Development, LLC | USA, Argentina, Australia, Brazil, Bulgaria, Chile, Colombia, Ecuador, Georgia, Hong Kong, India, Indonesia, Macedonia, Malaysia, Mexico, Philippines, Poland, Romania, Russian Federation, South Africa, Taiwan, Ukraine | 2100 | Phase III | Recruiting | NA |
| NCT00858364/20070782 | Amgen Inc. | Anemia treatment for advanced non-small cell lung cancer (NSCLC) patients receiving chemotherapy | MD, Amgen Inc. | USA, Austria, Belgium, Brazil, Bulgaria, Canada, Chile, China, Croatia, Czech Republic, Germany, Greece, Hong Kong, India, Ireland, Israel, Italy, Korea, Luxembourg, Malaysia, Mexico, Netherlands, Philippines, Poland, Puerto Rico, Romania, Russian Federation, Serbia, Slovenia, South Africa, Spain, Switzerland, Taiwan, Ukraine, UK | 3000 | Phase III | Recruiting | NA |
| NCT01374373/BIOS-012010 | Bio Sidus SA | Epoetin alfa (Hemax®) Phase IV study in chemotherapy induced anemia | Roberto Diez, MD (Bio Sidus SA) | Argentina | 30 | Phase IV | Recruiting | NA |
| NCT01795690/iOMTAn | iOMEDICO AG | Clinical registry on anemia therapy (TAn-Registry) | ? | Germany | 1000 | ? | Recruiting | NA |
| Status unknown | ||||||||
| NCT00381836/2005–005658–37/LM: 2612–3148/Ethical: 20060074/Data Protection: 2005–41–6015 | University of Aarhus, Denmark, and Amgen Inc. | Effect of darbepoetin alfa (Aranesp) on anemia in patients with advanced hormone independent prostate cancer | Michael Borre (Department of Urology, Aarhus University Hospital,Aarhus) | Denmark | 140 | Phase III (anaemia) | Unknown | NA |
| NCT00400686/CASE-CCF-5497/P30CA043703/CASE-CCF-5497/ORTHO-CASE-CCF-5497 | Cleveland Clinic | Epoetin alfa in treating anemia in patients undergoing chemotherapy for multiple myeloma | Ronald M Sobecks (Case Comprehensive Cancer Center, Cleveland, OH) | USA | 50 | ? | Unknown | NA |
| NCT00144755 | Lymphoma Study Association | R-CHOP-14 versus R-CHOP-21 and darbepoetin alpha in patients aged 60–80 years with diffuse large B-cell lymphoma | Richard Delarue (Lymphoma Study Association) | Belgium, France, Switzerland | 600 | Phase III | Unknown (some results published Delarue and colleagues253) | NA |
| NCT00039884/01/155A | Ramin Mirhashemi, MD | Will radiation/chemotherapy treatment of cervical cancer work better with medication that may improve anemia? | Ramin Mirhashemi | USA | 64 | Phase II | Unknown | NA |
| NCT00309920/CDR0000458037/WGSG-ARA-PLUS/AVENTIS-WGSG-ARA-PLUS/SANOFI-WGSF-ARA-PLUS/EU-205108 | Heinrich-Heine University, Dusseldorf | Combination chemotherapy with or without darbepoetin alfa in treating women with stage III breast cancer | Ulrike Nitz (Heinrich-Heine University, Dusseldorf) | Germany | 1234 | ? | Unknown (some results published Nitz and colleagues102) | NA |
| NCT00281892/ | German CLL Study Group | Fludarabine and darbepoetin alfa in treating older patients with chronic lymphocytic leukemia | Michael Hallek, MD (Medizinische Universitaetsklinik I, University of Cologne) | Germany | 348 | Phase III | Unknown | – |
| Terminated | ||||||||
| NCT00386152/CR012985/EPOANE2007 | Johnson & Johnson Pharmaceutical Research & Development, LLC | A study comparing two different PROCRIT doses to a dose of ARANESP in anemic cancer patients receiving chemotherapy | Johnson & Johnson Pharmaceutical Research & Development, LLC | USA | 235 | Phase II | Terminated, has results | – |
| NCT01736215/CR016558/EPOCAN4028 | Janssen-Cilag Ltd, Thailand | An observational study to predict the response of erythropoietin treatment in participants with cancer related anemia receiving chemotherapy | Janssen-Cilag Ltd, Thailand | Thailand | 33 | Phase IV | Terminated, has results | – |
| NCT00258440/CDR0000445450/OHSU-ONC-03017-LP/OHSU-1616/OHSU-7754/ORTHO-ONC-03017-L | Oregon Health & Science University Knight Cancer Institute, Portland, OR | Epoetin alfa in treating patients with anemia who are undergoing chemotherapy for cancer | Joseph Bubalo (Oregon Health & Science University Knight Cancer Institute) | USA | 7 | ? | Terminated, has results | – |
| NCT00989092/20000219 | Amgen Inc. | Darbepoetin alfa and anemia of cancer | Amgen Inc. | ? | 287 | Phase II | Terminated (slow enrolment and change in product development strategy) | – |
| NCT00254436/ID00–264 | MD Anderson Cancer Center, Houston, TX | A double-blind, randomized, placebo-controlled study of the efficacy and safety of weekly Procrit given to gastric or rectal patients | Saroj Vadhan-Raj, MD (MD Anderson Cancer Center) | USA | 50 | Phase III | Terminated | – |
| NCT00246597/CR002305 | Ortho Biotech Products, LP | A Phase III clinical trial of PROCRIT (epoetin alfa) versus placebo in women undergoing adjuvant chemotherapy for stage I, II or III breast cancer | Ortho Biotech Products, LP | ? | 37 | Phase III | Terminated | – |
| NCT00189371/AGO-OVAR 2.7 | Christian Jackisch MD (AGO Study Group) | Reinduction chemotherapy containing carboplatin and paclitaxel with or without epoetin alpha in recurrent platinum sensitive ovarian cancer, cancer of the fallopian tube or peritoneum | AGO Study Group | Germany | 300 | Phase III | Terminated | – |
| NCT00306267/CR10540 | Johnson & Johnson Pharmaceutical Research & Development, LLC | A study of PROCRIT (epoetin alfa) 80,000 units (U) once every four weeks (Q4W) vs. 40,000 U once every two weeks (Q2W) in cancer patients not receiving chemotherapy | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 61 | Phase II | Terminated | –; unlicensed/fixed dose or botha |
| NCT00310232/CTA-Control-076080/HC File 9427-J0921–22C | Ontario Clinical Oncology Group (OCOG) | Epoetin alfa in advanced non-small cell lung cancer (EPO-CAN-20) | Ontario Clinical Oncology Group (OCOG) | Canada | 70 | Phase III | Terminated | – |
| NCT00495378/CR005128 | Ortho Biotech Products, LP | RAPID-2. A study to evaluate the effectiveness of alternate dosing of PROCRIT (epoetin alfa) in maintaining hemoglobin levels in patients with chemotherapy related anemia | Ortho Biotech Products, LP | ? | 25 | Phase IV | Terminated (25/200 patients enrolled) | –; non-randomised studya |
| Completed | ||||||||
| NCT00117039/20030206 | Amgen Inc. | A study to evaluate the effectiveness of Aranesp for cancer patients with anaemia | MD, Amgen Inc. | ? | 1500 | Phase IV | Completed (results published Boccia and colleagues254,255) | Identified but excluded as studies non-randomised |
| NCT00272662/AFX01–05, 2005–003354–10 | Affymax, Inc. | Study of subcutaneously administered peginesatide in anemic cancer patients receiving chemotherapy | Study director, Affymax, Inc. | Czech Republic, Poland, UK | 60 | Phase II | Completed | –; non-randomised, dose finding, new ESAa |
| NCT00210600/CR003196 | Johnson & Johnson Pharmaceutical Research & Development, LLC | Early and standard intervention with 120,000 units of PROCRIT (epoetin alfa) every three weeks in patients receiving chemotherapy | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 186 | Phase II | Completed (results published Glaspy256) | –; unlicensed/fixed dose or botha |
| NCT00117117/20020132 | Amgen Inc. | A study to assess symptom burden in subjects with nonmyeloid malignancies receiving chemotherapy and Aranesp | MD, Amgen Inc. | ? | 2423 | Phase IV | Completed (results published Gregory257 and Gabrilove and colleagues258) | Identified Gabrilove and colleagues258 but not Gregory;257 however, both non-randomised, single-arm studies |
| NCT00072059/ROCHE-NA17101/UCLA-0303085/CDR0000335429 | Jonsson Comprehensive Cancer Center, Los Angeles, CA | Ro 50–3821[Mircera® epoetin beta] in treating anemia in patients receiving antineoplastic therapy for stage IIIB or stage IV non-small cell lung cancer | John Glaspy, MD (Jonsson Comprehensive Cancer Center) | USA | 210 | Phase II | Completed | – |
| NCT00212862/CR004561/ABT-OP-03–02 | Ortho Biotech Products, LP | Dosing and outcomes study of erythropoietic stimulating therapies in patients with chemotherapy induced anemia (DOSE) | Ortho Biotech Products, LP | ? | 2130 | Phase IV | Completed (results published Larholt and colleagues259) | Not identified as beyond scope of review – observational cohort study |
| NCT00270101/CR005911 | Johnson & Johnson Pharmaceutical Research & Development, LLC | The effect of epoetin alfa on the anemia of patients with multiple myeloma | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 156 | Phase III | Completed (results published Dammacco and colleagues260–262) | Not identified as pre-2004 (PenTAG searches 2004–13) |
| NCT00158379/3002000 | North-Eastern German Society of Gynaecologic Oncology | Taxol carboplatin and erythropoietin | Jalid Sehouli, Charité Campus Virchow-Klinikum, Berlin | ? | 105 | Phase II | Completed | –; non-randomised studya |
| NCT00315484/CR004609 | Ortho Biotech Products, LP | Hematologic response of epoetin alfa (PROCRIT) versus darbepoetin alfa (ARANESP) in chemotherapy induced anemia | Ortho Biotech Products, LP | ? | 358 | Phase IV | Completed (results published Waltzman and colleagues241) | Identified; excluded as unlicensed/fixed dose or both |
| NCT00540384/980291 | Amgen Inc. | Dose-finding study of novel erythropoiesis stimulating protein (NESP) for the treatment of anaemia in subjects with solid tumours receiving multicycle chemotherapy | MD, Amgen Inc. | ? | 405 | Phase II | Completed (results published Kotasek and colleagues50) | Identified; included treatment arm evaluating a licensed dosage |
| NCT00344409/KRN321-SC/05-A54 | Kyowa Hakko Kirin Company, Ltd | A double-blind study of KRN321 for the treatment of anemia in cancer patients | Nagahiro Saijo, MD (National Cancer Center Hospital East) | Japan | 200 | Phase III | Completed | – |
| NCT00144482/EPO307JP | Chugai Pharmaceutical Company Ltd | A study of recombinant human erythropoietin in anemic cancer patients undergoing chemotherapy | Yoshiharu Ishikura (Chugai Pharmaceutical Company, Ltd) | ? | 122 | Phase III | Completed | –; unlicensed/fixed dose or botha |
| NCT00628043/EPO316JP | Chugai Pharmaceutical Company Ltd | Clinical study of epoetin beta to chemotherapy-induced anaemia (CIA) patients | Yoshito Suzuki (Chugai Pharmaceutical Company, Ltd) | Japan | 160 | Phase III | Completed | –; unlicensed/fixed dose or botha |
| NCT00338299/CR005098 | Johnson & Johnson Pharmaceutical Research & Development, LLC | Alternate dosing of PROCRIT (epoetin alfa) in patients with cancer and chemotherapy induced anemia | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 51 | Phase III | Completed (results published Reddy and colleagues263) | Not identified; study was a non-randomised, single-arm study |
| NCT00144495/EPO308JP | Chugai Pharmaceutical Company Ltd | A study of recombinant human erythropoietin in anemic cancer patients undergoing chemotherapy | Yoshiharu Ishikura (Chugai Pharmaceutical Company, Ltd) | ? | 104 | Phase III | Completed | –; non-randomised, unlicensed/fixed dose or botha |
| NCT00776425/ML20197 | Hoffmann-La Roche | A study of the quality of life and treatment response to once weekly NeoRecormon (epoetin beta) treatment in anemic patients with solid and lymphoid malignancies | Clinical Trials, Hoffman-La Roche | Russian Federation | 125 | Phase IV | Completed | –; non-randomised, single arm, unlicensed/fixed dosea |
| NCT00035607/20010199 | Amgen Inc. | Chemotherapy related anemia | MD, Amgen Inc. | ? | 120 | Phase III | Completed (results published Justice and colleagues264) | Identified; excluded as comparison of darbepoetin alfa vs. darbepoetin alfa – beyond scope |
| NCT00711958/2003–31-INJ-11 | Novartis | Study to assess the efficacy and safety of HX575 in the treatment of chemotherapy associated anemia in cancer patients | Andrea Vetter, MD (Hexal AG) | Germany, Romania | 105 | Phase III | Completed | –; unlicensed/fixed dose or both, bioequivalence studya |
| NCT00117624/20020118 | Amgen Inc. | A study of darbepoetin alfa for the treatment of anemia in subjects with a non-myeloid malignancy | MD, Amgen Inc. | ? | ? | Phase III | Completed (results published Kotasek and colleagues265) | Identified; excluded as comparison of darbepoetin alfa vs. darbepoetin alfa – beyond scope |
| NCT00046969/AGOSG-OVAR-MO16375-MARCH/CDR0000257189, EU-20217/ROCHE-MO16375 ROCHE-RO2053859 | AGO Study Group | Epoetin beta in treating anemia in patients with cervical cancer | Heinz Koelbl, MD (Martin-Luther-Universität of Halle-Wittenberg) | Germany | 450 | Phase IV | Completed | – |
| NCT00261313/20040137 | Amgen Inc. | ACCELERATE: doxorubicin and cyclophosphamide followed by paclitaxel with pegfilgrastim and darbepoetin alfa support for the treatment of women with breast cancer | MD, Amgen Inc. | ? | 80 | Phase II | Completed | –; appears G-CSF not given in both treatment armsa |
| NCT00148421/20030125 | Amgen Inc. | Study for the treatment of anemia in patients with non-myeloid malignancies receiving multicycle chemotherapy | MD, Amgen Inc. | ? | ? | Phase III | Completed (results published Glaspy and colleagues266) | Identified; excluded as unlicensed/fixed dose or both |
| NCT00401544/20060103 | Amgen Inc. | Darbepoetin alfa with or without IV iron | MD, Amgen Inc. | ? | ? | Phase III | Completed | – |
| NCT00338416/CR004612 | Johnson & Johnson Pharmaceutical Research & Development, LLC | An efficacy and safety study of PROCRIT (epoetin alfa) in cancer patients receiving chemotherapy every three weeks | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 115 | Phase II | Completed (results published Montoya and colleagues267) | Not identified; study non-randomised, single arm |
| NCT00269984/CR005833 | Johnson & Johnson Pharmaceutical Research & Development, LLC | A study to determine the safety and effectiveness of epoetin alfa versus placebo in patients with persistent anemia caused by advanced cancer | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 56 | Phase II | Completed | –; non-randomised, unlicensed/fixed dose or botha |
| NCT00559195/CDR0000574173/CHUL-NEOPALIA/RECF0359 | Centre Hospital Regional Universitaire de Limoges | Epoetin beta in treating fatigue and anemia in patients receiving palliative care for malignant solid tumors | Jean-Luc Labourey (Centre Hospital Regional Universitaire de Limoges) | France | 40 | Phase II | Completed | –; non-randomised studya |
| NCT00120705/20020167 | Amgen Inc. | Treatment for anemic subjects with non-myeloid malignancies receiving chemotherapy | MD, Amgen Inc. | ? | 204 | Phase II | Completed (results published Charu and colleagues268) | Identified; excluded – unlicensed/fixed dose or both |
| NCT00364455 | Janssen-Ortho Inc., Canada, and Ontario Clinical Oncology Group | Impact of erythropoietin treatment versus placebo on quality-of-life in patients with advanced prostate cancer | ? | ? | 56 | Phase III | Completed | – |
| NCT00135317/ | Amgen Inc. | AIM 3: anemia and iron management with every 3 week dosing in anemic subjects with nonmyeloid malignancies | MD, Amgen Inc. | ? | ? | Phase III | Completed (results published Bastit and colleagues269) | Identified; excluded – unlicensed/fixed dose or both |
| NCT00269997/CR005839 | Johnson & Johnson Pharmaceutical Research & Development, LLC | A study to evaluate the safety and effectiveness of epoetin alfa versus placebo in patients with persistent anemia as a result of cancer treatment with cisplatin, a platinum-containing chemotherapy drug | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 72 | Phase II | Completed | – |
| NCT00266617/CR005845 | Johnson & Johnson Pharmaceutical Research & Development, LLC | A study to evaluate the safety and effectiveness of epoetin alfa in patients with anemia as a result of advanced cancer and treatment with aggressive chemotherapy | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 86 | Phase II | Completed | – |
| NCT00110955/20030232 | Amgen Inc. | Treatment of anemia in subjects with non-myeloid malignancy receiving multicycle chemotherapy | MD, Amgen Inc. | ? | 391 | Phase III | Completed (results published Hernandez and colleagues232) | Identified; excluded – unlicensed/fixed dose or both |
| NCT00337948/CR004615 | Johnson & Johnson Pharmaceutical Research & Development, LLC | An efficacy and safety study of PROCRIT (epoetin alfa) in cancer patients receiving chemotherapy every week or every four weeks | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 129 | Phase II | Completed (results published Gregory and Williams,270 Baltz and colleagues271 and Gregory and colleagues272,273) | Not identified; non-randomised, single-arm study |
| NCT00255749/CDR0000449950/UCLA-0504038/ORTHO-PR04–27–018 | Jonsson Comprehensive Cancer Center, Los Angeles, CA | Epoetin alfa in treating patients with anemia who are undergoing chemotherapy for cancer | John A Glaspy, MD (Jonsson Comprehensive Cancer Center) | USA | 89 | Phase II | Completed (results published Glaspy and colleagues274) | Identified; excluded – includes randomised and non-randomised studies, unlicensed/fixed dose used |
| NCT00058331/CDR0000288821/NCCTG-N02C2 | North Central Cancer Treatment Group | Epoetin alfa in treating anemia in patients with solid tumors | David P Steensma, MD (Mayo Clinic) | USA | ? | Phase II | Completed (results published Steensma and colleagues275,276) | Identified; excluded – unlicensed/fixed dose or both |
| NCT00003600/CDR0000066673, NCCTG-979253, NCI-P98–0133 | North Central Cancer Treatment Group | Epoetin alfa in treating anemia in patients who are receiving chemotherapy | Thomas E Witzig, MD (Mayo Clinic) | USA | ? | Phase III | Completed | – |
| NCT00524407/CR005125 | Ortho Biotech Products, LP | Effect of epoetin alfa on hemoglobin, symptom distress, and quality of life in patients receiving chemotherapy | Ortho Biotech Products, LP | ? | 273 | Phase IV | Completed (result published Straus and colleagues277) | Identified; excluded – unlicensed/fixed dose or both |
| NCT00261677/CR002296 | Ortho Biotech Products, LP | A study to evaluate the effect of weekly PROCRIT (epoetin alfa) or placebo on anemia and quality of life in children with cancer undergoing chemotherapy | Ortho Biotech Products, LP | ? | 224 | Phase III | Completed (results published Razzouk and colleagues239) | Identified; excluded – unlicensed/fixed dose or both (paediatric population) |
| NCT00036023/NCT00039247(obsolete)/20010162 | Amgen Inc. | Chemotherapy related anemia in patients with non-myeloid malignancies | MD, Amgen Inc. | ? | ? | Phase II | Completed (results published Glaspy and colleagues278) | |
| NCT00145652 | Sundsvall Hospital, Sweden | Adjuvant IV iron therapy during erythropoietin treatment of anemic patients with lymphoproliferative disorders | ? | ? | 66 | ? | Completed | – |
| NCT00270166/CR005923 | Johnson & Johnson Pharmaceutical Research & Development, LLC | The effect of epoetin alfa on the anemia of patients with selected cancers receiving chemotherapy | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 201 | Phase III | Completed | – |
| NCT01626547/ORHEO | Hospira, Inc. | Biosimilar Retacritä (epoetin zeta) in the treatment of chemotherapy-induced symptomatic anaemia in haematology and oncology | ? | Germany | 240 | ? | Completed | –; non-randomised studya |
| NCT00121030/20020166 | Amgen Inc. | Treatment for patients with gynecological malignancies who suffer from anemia due to chemotherapy | MD, Amgen Inc. | ? | ? | Phase II | Completed (results published Schwartzberg and colleagues246) | Identified; excluded – unlicensed/fixed dose or both |
| NCT00038064/NCT00046982 [obsolete], 20010101 | Amgen Inc. | Anemia in patients with a non-myeloid malignancy | MD, Amgen Inc. | ? | 707 | Phase III | Completed | –; unlicensed/fixed dose or botha |
| NCT00120679/20020165 | Amgen Inc. | Treatment for patients with non-small cell lung cancer who developed anemia due to chemotherapy | MD, Amgen Inc. | ? | ? | Phase II | Completed (results published Schwartzberg and colleagues246) | |
| NCT00264108/CR002455/EPOCAN4015 | Janssen-Cilag BV | Cost-effectiveness study of epoetin alfa and darbepoetin alfa in adult patients with cancer who have anemia | Clinical Trials Janssen-Cilag BV | ? | 492 | Phase IV | Completed | –; non-randomised, unlicensed/fixed dose or botha |
| NCT00239239/20040232 | Amgen Inc. | Fractionated dosing study: study to evaluate darbepoetin alfa for the treatment of anemia in subjects with non-myeloid malignancies | MD, Amgen Inc. | ? | 44 | Phase III | Completed | –; non-randomised, pharmacokinetic studya |
| NCT00146562/03–154 | Harold J Burstein, MD, PhD, and Dana-Farber Cancer Institute; Massachusetts General Hospital; Beth Israel Deaconess Medical Center; Lowell General Hospital; Brigham and Women’s Hospital; North Shore Medical Center | Pegfilgrastim and darbepoetin alfa in support of adjuvant chemotherapy for breast cancer | Harold Burstein, MD, PhD (Dana-Farber Cancer Institute, Boston, MA) | USA | 109 | Phase II | Completed | –; non-randomiseda |
| NCT00120692/20020152 | Amgen Inc. | Treatment for patients suffering from anemia due to chemotherapy | MD, Amgen Inc. | ? | ? | Phase II | Completed (results published Senecal and colleagues247) | Identified; excluded – unlicensed/fixed dose or both |
| NCT00119613/20010145 | Amgen Inc. | A study of subjects with previously untreated extensive-stage small-cell lung cancer (SCLC) treated with platinum plus etoposide chemotherapy with or without darbepoetin alfa | MD, Amgen Inc. | ? | 600 | Phase II | Completed (results published Pirker and colleagues266) | Identified; excluded – unlicensed/fixed dose or both |
| NCT00028938/CDR0000069148/CCWFU-62299/CCCWFU-BG01–193/NCI-P01–0200 | Wake Forest Baptist Health/National Cancer Institute | Chemotherapy and radiation therapy with or without epoetin alfa in treating patients with stage IIIA or stage IIIB non-small cell lung cancer | Arthur William Blackstock, MD (Comprehensive Cancer Center of Wake Forest University, Winston-Salem, NC) | USA | 202–232 | Phase III | Completed | – |
| NCT00111137/20020139 | Amgen Inc. | Treatment for patients with non-myeloid malignancies receiving chemotherapy | MD, Amgen Inc. | ? | 718 | Phase III | Completed | –; unlicensed/fixed dose or botha |
| NCT00022386/ORTHO-PR-00–27–012/UCLA-0011004/CDR0000068811/ORTHO-PR-01–27–003, NCI-G01–2002 | Jonsson Comprehensive Cancer Center,, Los Angeles, CA, and National Cancer Institute | Epoetin alfa in treating chemotherapy-related anemia in women with stage I, stage II, or stage III breast cancer | John A Glaspy, MD (Jonsson Comprehensive Cancer Center) | ? | 2500 | Phase IV | Completed | –; non-randomised studya |
| NCT00017004/CDR0000068641/GOG-0191/CAN-NCIC-CX4 | Gynecologic Oncology Group/National Cancer Institute and NCIC Clinical Trials Group | Radiation therapy and cisplatin with or without epoetin alfa in treating patients with cervical cancer and anemia | Gillian M Thomas (Odette Cancer Center at Sunnybrook, Toronto) and Peter S Craighead (Tom Baker Cancer Center, Calgary) | USA, Canada, Norway, UK | 460 | Phase III | Completed | – |
| NCT00270127/CR005917/EPO-C111–457/EPO-INT-10 | Johnson & Johnson Pharmaceutical Research & Development, LLC | Epoetin alfa for anemia in patients with cancer receiving non-platinum chemotherapy | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 375 | Phase III | Completed | – |
| NCT00211133/CR004414 | Johnson & Johnson Pharmaceutical Research & Development, LLC | A study to evaluate the impact of maintaining hemoglobin levels using epoetin alfa in patients with metastatic breast cancer receiving chemotherapy | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 939 | Phase III | Completed (results published Leyland-Jones and colleagues233) | Identified; excluded – unlicensed/fixed dose or both |
| NCT00283465/CR002047 | Janssen-Cilag BV | A study of the effectiveness and safety of treatment with epoetin alfa on hemoglobin levels, red blood cell transfusions, and quality of life in patients with cancer receiving platinum-containing chemotherapy | Clinical Trials Janssen-Cilag BV | ? | 316 | Phase IV | Completed (results published Savonije and colleagues245) | Identified; excluded – unlicensed/fixed dose or both |
| NCT00095277/20030204 | Amgen Inc. | Darbepoetin alfa administered once every 4 weeks in the treatment of subjects with anemia of cancer | MD, Amgen Inc. | ? | 220 | Phase II | Completed (results published Gordon and colleagues279) | Identified; excluded as patients did not receive chemotherapy |
| NCT00118638/20030231 | Amgen Inc. | A study of darbepoetin alfa for the treatment of anemia in subjects with non-myeloid malignancy receiving multicycle chemotherapy | MD, Amgen Inc. | ? | 705 | Phase III | Completed (results published Canon and colleagues280,281 and Vansteenkiste and colleagues282) | All identified; excluded Canon and colleagues280 as comparison of darbepoetin alfa vs. of darbepoetin alfa and beyond scope and excluded Canon and colleagues281 and Vansteenkiste and colleagues282 as retrospective analyses |
| NCT00144131/20040262 | Amgen Inc. | Flexibility: a study to assess the impact of darbepoetin alfa in subjects with non-myeloid malignancies with anemia due to chemotherapy | MD, Amgen Inc. | ? | 750 | Phase II | Completed (results published Schwartzberg and colleagues283) | Identified; excluded as comparison of darbepoetin alfa vs. of darbepoetin alfa and beyond scope |
| NCT00091858/NCT00098696 (obsolete)/20010103 | Amgen Inc. | Study of darbepoetin alfa for the treatment of anemia of cancer | MD, Amgen Inc. | ? | 1000 | Phase III | Completed (results published Smith and colleagues16) | Identified; excluded as patients not receiving chemotherapy |
| NCT00216541/CR003541 | Janssen-Cilag BV | A study of the safety and effectiveness of epoetin alfa on hemoglobin levels and blood transfusions in cancer patients receiving chemotherapy | Clinical Trials Janssen-Cilag BV | ? | 110 | Phase IV | Completed (results published Schouwink and colleagues284) | Identified; excluded as comparison of epoetin alfa vs. epoetin alfa and beyond scope and unlicensed/fixed dose or both |
| NCT00245895/03–6503-A | University of Washington, Seattle/Amgen | Study of Aranesp to treat anemia in prostate cancer patients | Celestia S Higano, MD (University of Washington) and Tomasz M Beer, MD (Oregon Health & Science University) | USA | 20 | Phase II | Completed | –; non-randomised studya |
| NCT00039247/20010162 | Amgen Inc. | Chemotherapy related anemia in patients with non-myeloid malignancies | MD, Amgen Inc. | ? | Phase II | Completed (results published Glaspy and colleagues278) | Identified; excluded as comparison of darbepoetin alfa vs. darbepoetin alfa | |
| NCT01099202 | MD Anderson Cancer Center, Houston, TX | Procrit versus no Procrit in acute lymphocytic leukemia, lymphoblastic lymphoma, or Burkitt’s undergoing induction/consolidation chemotherapy | Jorge Cortes, (University of Texas, MD, Anderson Cancer Center) | USA | 109 | Not provided | Completed | – |
| NCT00661999 | National Cancer Institute | Darbepoetin alfa with or without iron in treating anemia caused by chemotherapy in patients with cancer | Charles L Loprinzi (Mayo Clinic) | USA | 502 | Phase III | Completed | – |
| NCT01394991/CR010543/EPOANE4008 | Johnson & Johnson Pharmaceutical Research & Development, LLC | A safety study of epoetin alfa in patients with cancer who have chemotherapy-related anemia | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 504 | Phase IV | Completed | – |
| NCT00236951 | Luitpold Pharmaceuticals, Inc. | Intravenous (IV) iron vs. no iron as the treatment of anemia in cancer patients undergoing chemotherapy and erythropoietin therapy | Marc Tokars (Senior Director of Clinical Operations, Luitpold Pharmaceuticals, Inc.) | ? | 224 | Phase III | Completed | – |
| NCT00270049/CR005905 | Johnson & Johnson Pharmaceutical Research & Development, LLC | Epoetin alfa for the treatment of anemia resulting from chronic lymphocytic leukemia | Johnson & Johnson Pharmaceutical Research & Development, LLC | ? | 195 | Phase II | Completed | – |
| NCT00003341/97–125/MSKCC-97125/ORTHO-PR-96–27–031/RPCI-DS-97–38/NCI-G98–1436 | Memorial Sloan Kettering Cancer Center, New York | Epoetin alfa in treating anemia in patients with lymphoma, chronic lymphocytic leukemia, or multiple myeloma and anemia caused by chemotherapy | David J Straus (Memorial Sloan Kettering Cancer Center) | USA | 275 | Phase III | Completed | – |
| NCT00070382/CDR0000333213/P30CA016042/UCLA-0306021/AMGEN-20030125 | Jonsson Comprehensive Cancer Center, Los Angeles, CA, and National Cancer Institute | Darbepoetin alfa compared with epoetin alfa in treating anemia in patients receiving chemotherapy for cancer | John A Glaspy, MPH (Jonsson Comprehensive Cancer Center) | ? | 14 | Phase III | Completed (published Glaspy and colleagues266) | Identified; excluded – unlicensed/fixed dose or both. Possibly duplicate of NCT00148421 |
| NCT00416624/CDR0000522677/P30CA015083/RC05CB/06–002991/EPOANE3015 | Mayo Clinic | Epoetin alfa or darbepoetin alfa in treating patients with anemia caused by chemotherapy | Charles L Loprinzi (Mayo Clinic) | USA | 320 | ? | Completed | – |
Appendix 12 Supplementary analyses
Anaemia-related outcomes
Haemoglobin change
Publication bias
FIGURE 72.
Haemoglobin change: publication bias – funnel plot with pseudo 95% confidence limits.
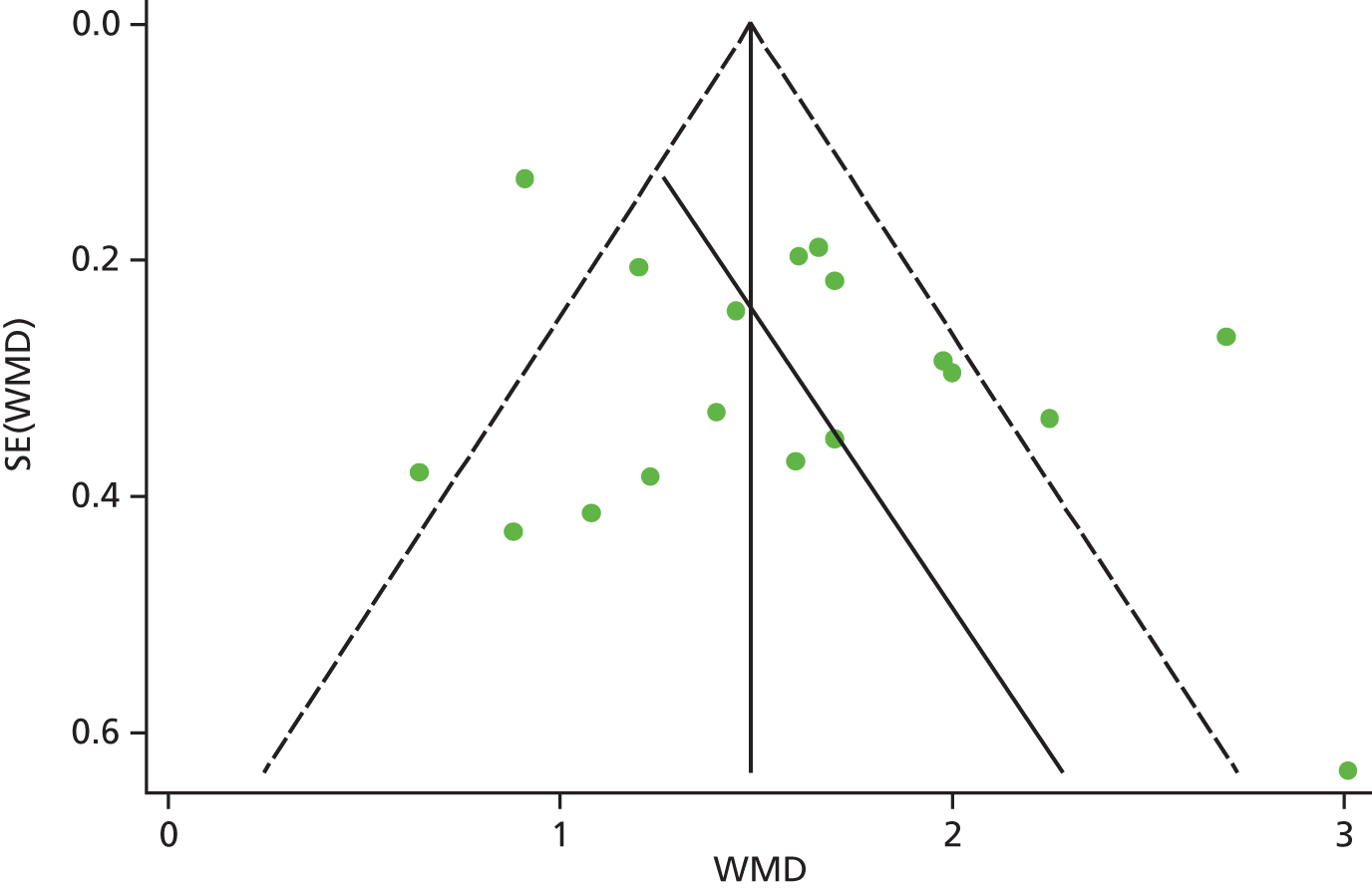
| Study effect | Coefficient | SE | t | p > |t| | 95% CI |
|---|---|---|---|---|---|
| Slope | 1.002 | 0.33 | 3.06 | < 0.01 | 0.31 to 1.70 |
| Bias | 2.020 | 1.28 | –1.16 | 0.13 | –0.69 to 4.73 |
| Test of H0 no small study effects p = 0.133 | |||||
FIGURE 73.
Haemoglobin change: publication bias – meta-regression plot with year of publication as a covariate.

Fixed effects
FIGURE 74.
Forest plot: haemoglobin change. Fixed-effects meta-analysis (Mantel–Haenszel); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta and Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy.

Meta-regression
| Variable | Mean difference | SE | p-value |
|---|---|---|---|
| Intercept (other chemotherapy and erythropoietin) | 1.576 | 0.115 | < 0.001 |
| Darbepoetin | –0.491 | 0.212 | 0.035 |
| Mixed chemotherapy | 0.879 | 0.006 | 0.018 |
Haematological response
Publication bias
FIGURE 75.
Haematological response: publication bias – funnel plot with pseudo 95% confidence limits.

FIGURE 76.
Haematological response: publication bias – meta-regression plot with year of publication as a covariate.
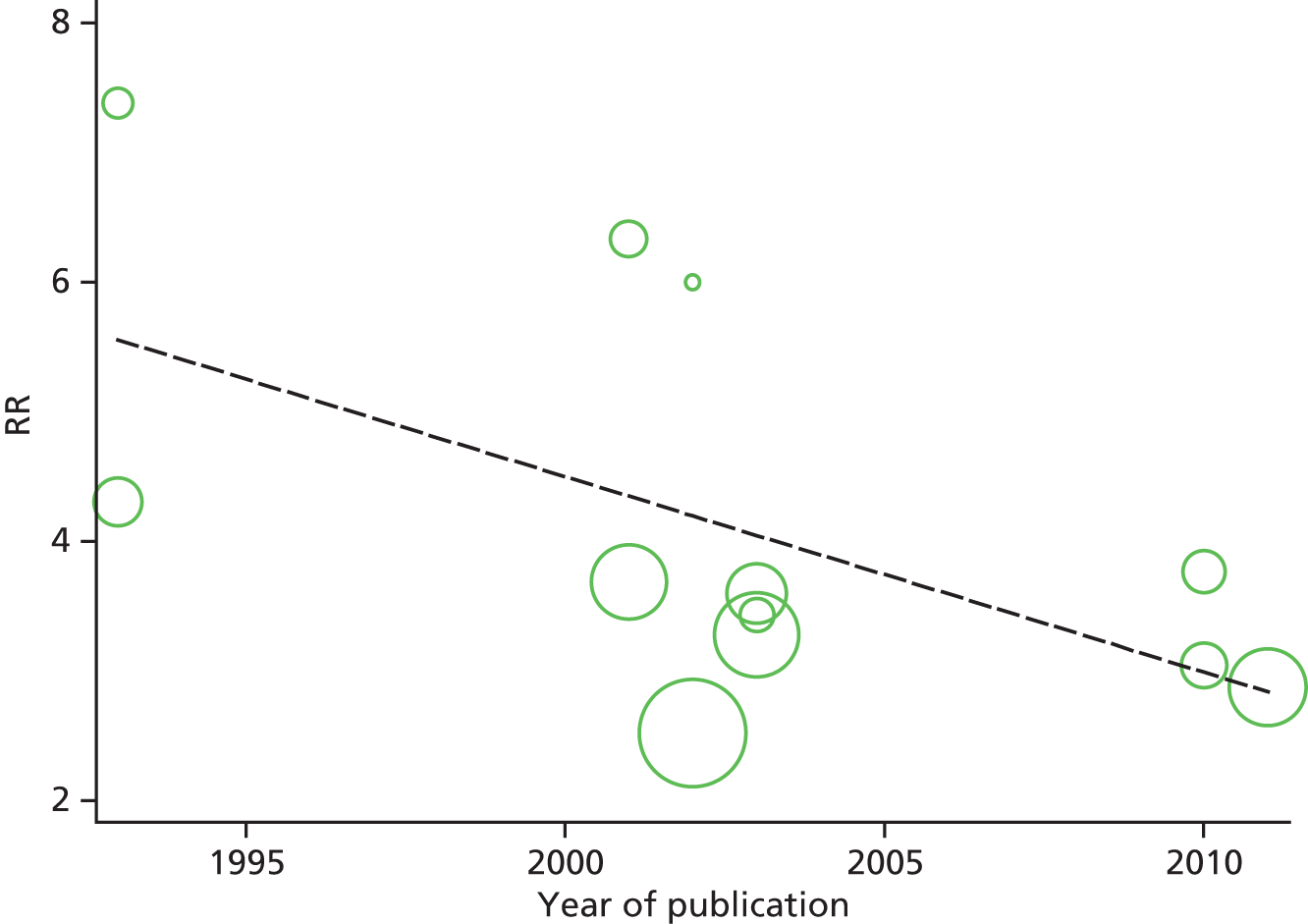
Fixed effects
FIGURE 77.
Forest plot: haematological response. Fixed-effects meta-analysis (Mantel–Haenszel); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta and Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy. Events, treatment = number of events/number of participants in treatment group; events, control = number of events/number of participants in control group.

Additional analyses
FIGURE 78.
Forest plot: haematological response (including Kurz and colleagues69 and Vansteenkiste and colleagues73). Random-effects meta-analysis (DerSimonian–Laird); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta and Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy. Events, treatment = number of events/number of participants in treatment group; events, control = number of events/number of participants in control group.

FIGURE 79.
Forest plot: haematological response using Hb subgroups. 65 Random-effects meta-analysis (DerSimonian–Laird); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta, Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy; and Littlewood and colleagues70 reported data by baseline Hb ≤ 10.5 g/dl and > 10.5 g/dl. Events, treatment = number of events/number of participants in treatment group; events, control = number of events/number of participants in control group.

FIGURE 80.
Forest plot: haematological response using malignancy subgroups. 65 Random-effects meta-analysis (DerSimonian–Laird); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta, Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy; and Littlewood and colleagues70 reported data by malignancy type (solid and haematological tumours). Events, treatment = number of events/number of participants in treatment group; events, control = number of events/number of participants in control group.

Meta-regression
| Variable | RR | SE | p-value |
|---|---|---|---|
| Intercept (NR) | 5.163 | 0.497 | < 0.001 |
| Iron | –2.163 | 0.626 | 0.006 |
| Variable | RR | SE | p-value |
|---|---|---|---|
| Intercept (Hb < 12 g/dl) | 25.524 | 2.108 | < 0.001 |
| Hb < 11 g/dl | –21.480 | 2.642 | < 0.001 |
| Hb < 10 g/dl | –21.215 | 2.163 | < 0.001 |
Red blood cell transfusion
Publication bias
FIGURE 81.
Red blood cell transfusion: publication bias – funnel plot with pseudo 95% confidence limits.
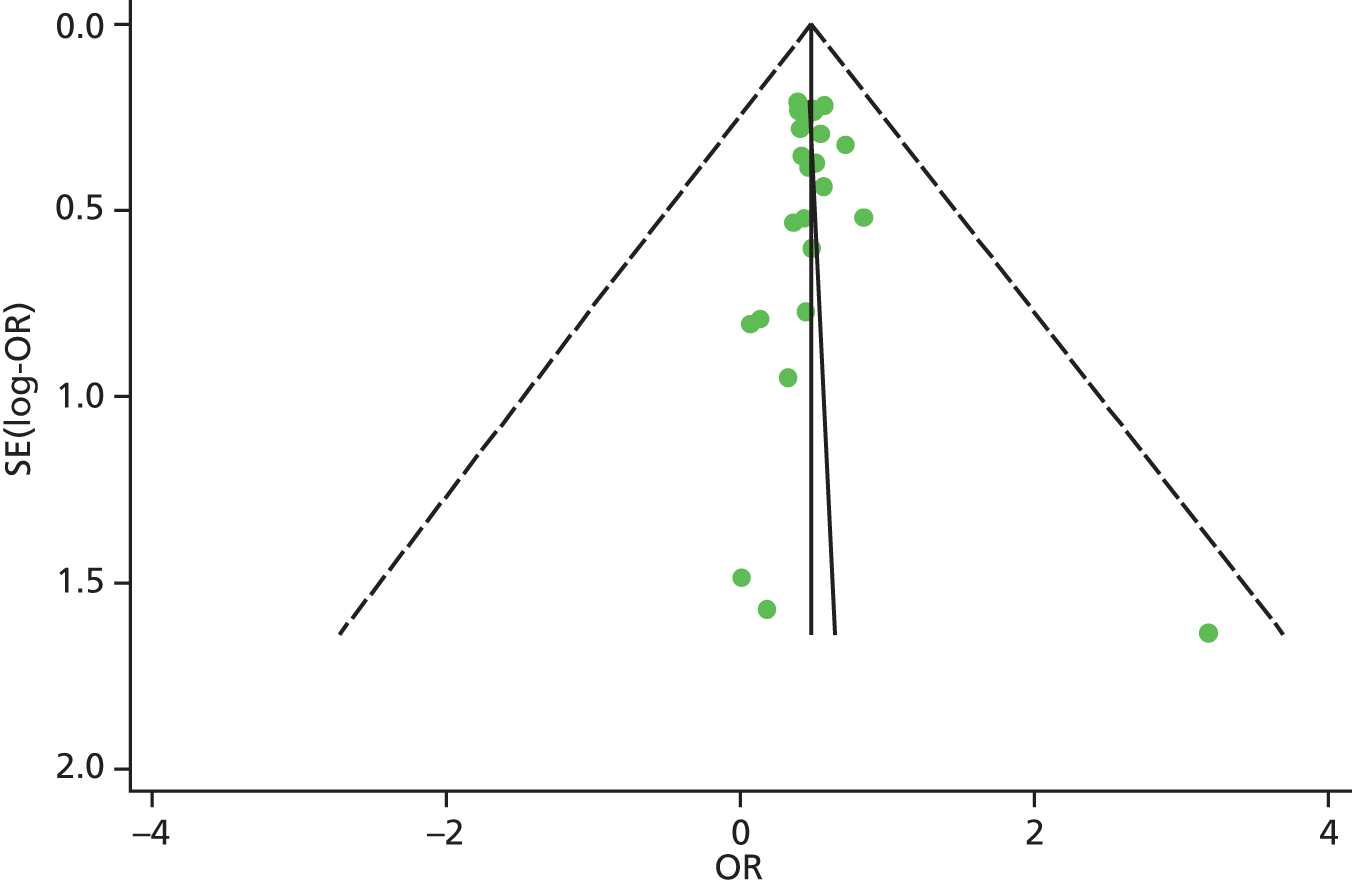
| Z/sqrt(V) | Coefficient | SE | t | p > |t| | 95% CI |
|---|---|---|---|---|---|
| sqrt(V) | –0.60 | 0.17 | –3.44 | 0.002 | –0.96 to –0.24 |
| Bias | –0.62 | 0.51 | –1.22 | 0.234 | –1.68 to 0.43 |
| Test of H0 no small-study effects p = 0.234 | |||||
FIGURE 82.
Red blood cell transfusion: publication bias – analysis of year of publication. pubyear, publication year.

Fixed effects
FIGURE 83.
Forest plot: red blood cell transfusion. Fixed-effects meta-analysis (Mantel–Haenszel); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta and Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy. Events, treatment = number of events/number of participants in treatment group; events, control = number of events/number of participants in control group.
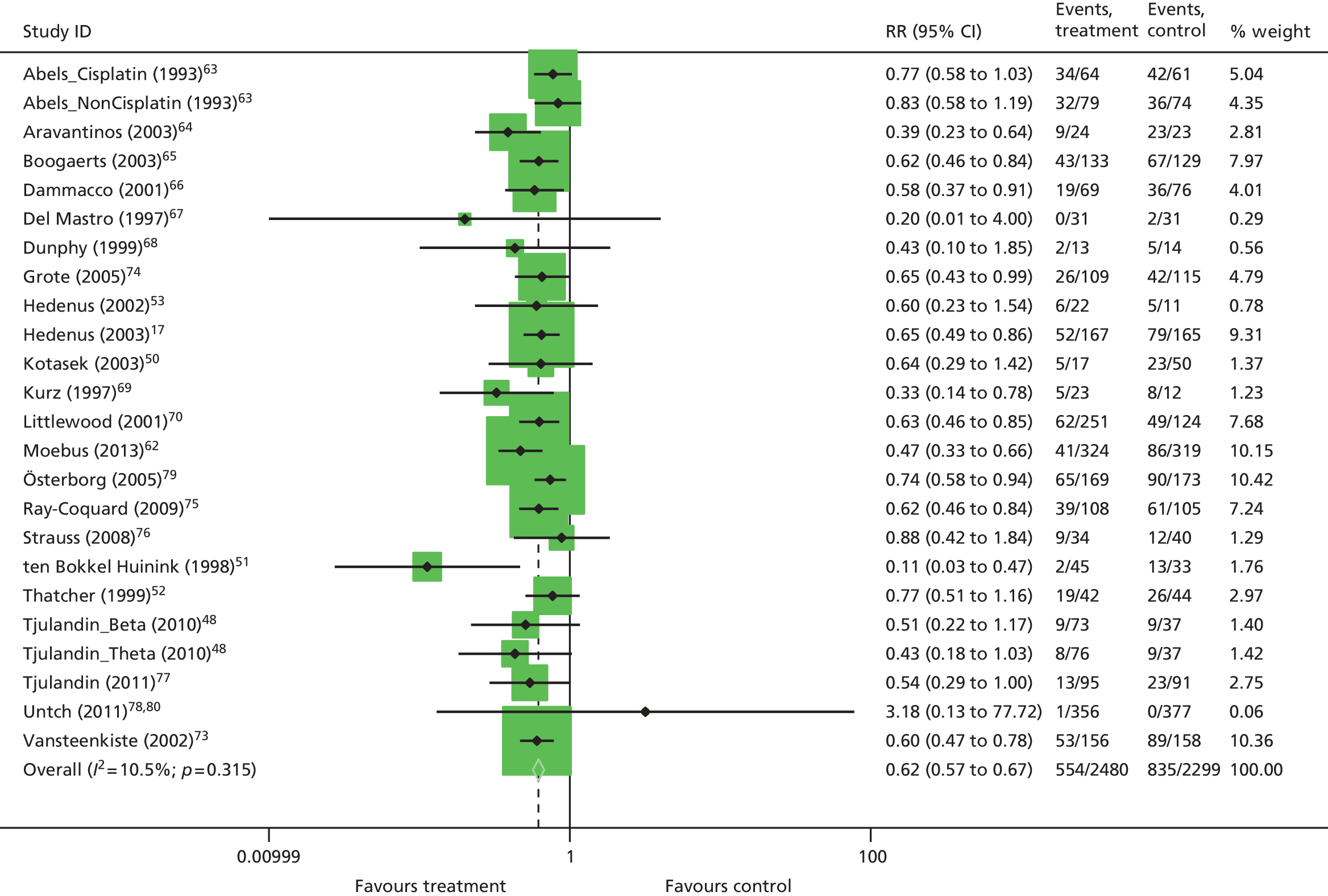
Additional analyses
FIGURE 84.
Forest plot: red blood cell transfusion using Hb subgroups. 65,73 Random-effects meta-analysis (DerSimonian–Laird); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta and Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy. Events, treatment = number of events/number of participants in treatment group; events, control = number of events/number of participants in control group.
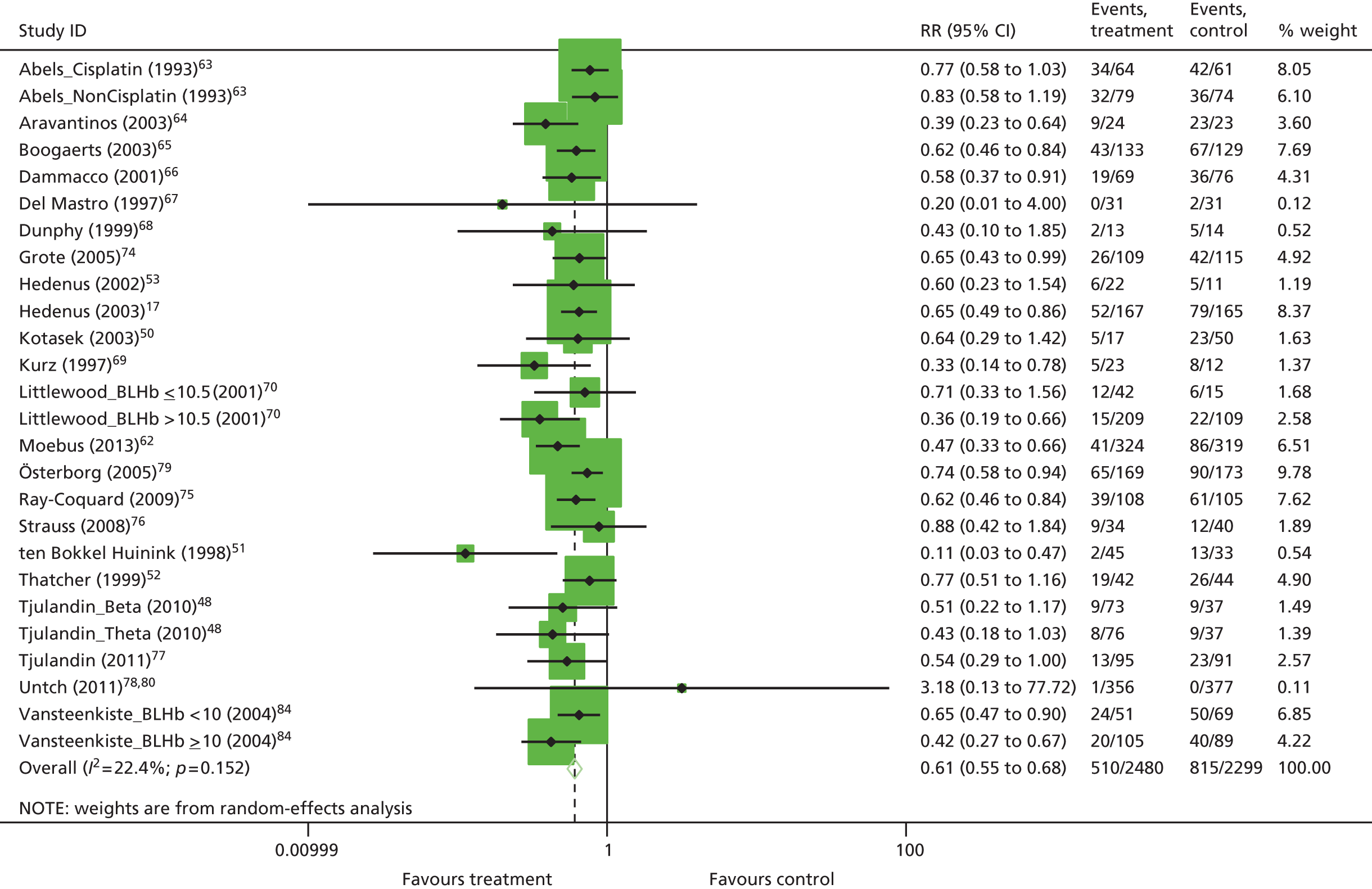
FIGURE 85.
Forest plot: red blood cell transfusion using malignancy subgroups. 65 Random-effects meta-analysis (DerSimonian–Laird); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta and Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy. Events, treatment = number of events/number of participants in treatment group; events, control = number of events/number of participants in control group.

Red blood cell units transfused
Publication bias
FIGURE 86.
Red blood cell units transfused: publication bias – Egger’s test; funnel plot with pseudo 95% confidence limits.

| Study effect | Coefficient | SE | t | p > |t| | 95% CI |
|---|---|---|---|---|---|
| Slope | –0.4604 | 0.16 | –2.96 | 0.02 | –0.81 to –0.11 |
| Bias | –1.986 | 0.60 | –1.63 | 0.14 | –2.35 to 0.38 |
| Test of H0 no small study effects p = 0.137 | |||||
Additional analyses
FIGURE 87.
Forest plot: red blood cell units transfused using Hb subgroups. 73 Random-effects meta-analysis (DerSimonian–Laird); trial with multiple experimental arm split into subsets in the analysis: Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy.
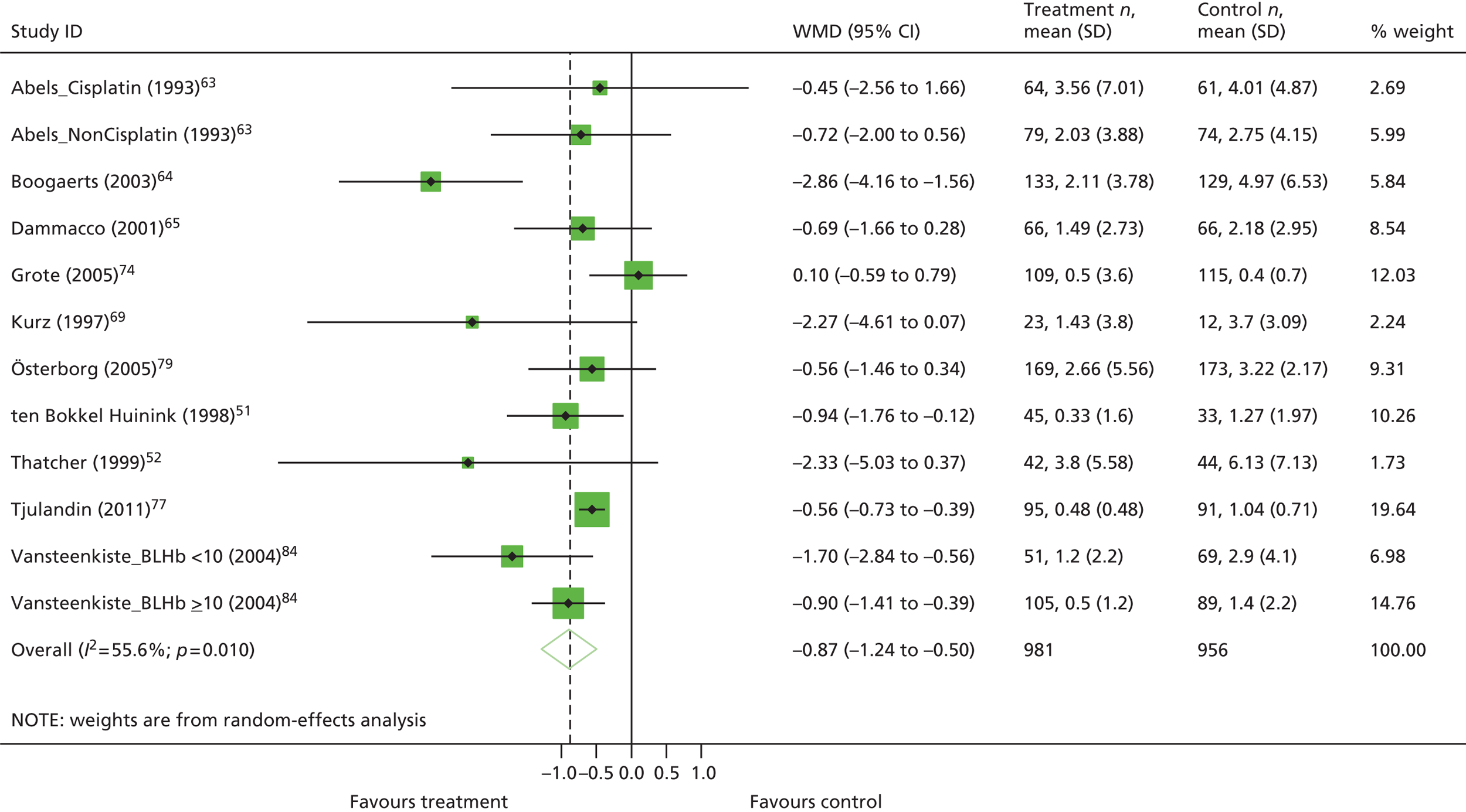
Fixed effects
FIGURE 88.
Forest plot: red blood cell units transfused. Fixed-effects meta-analysis (Mantel–Haenszel); trial with multiple experimental arm split into subsets in the analysis: Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy.
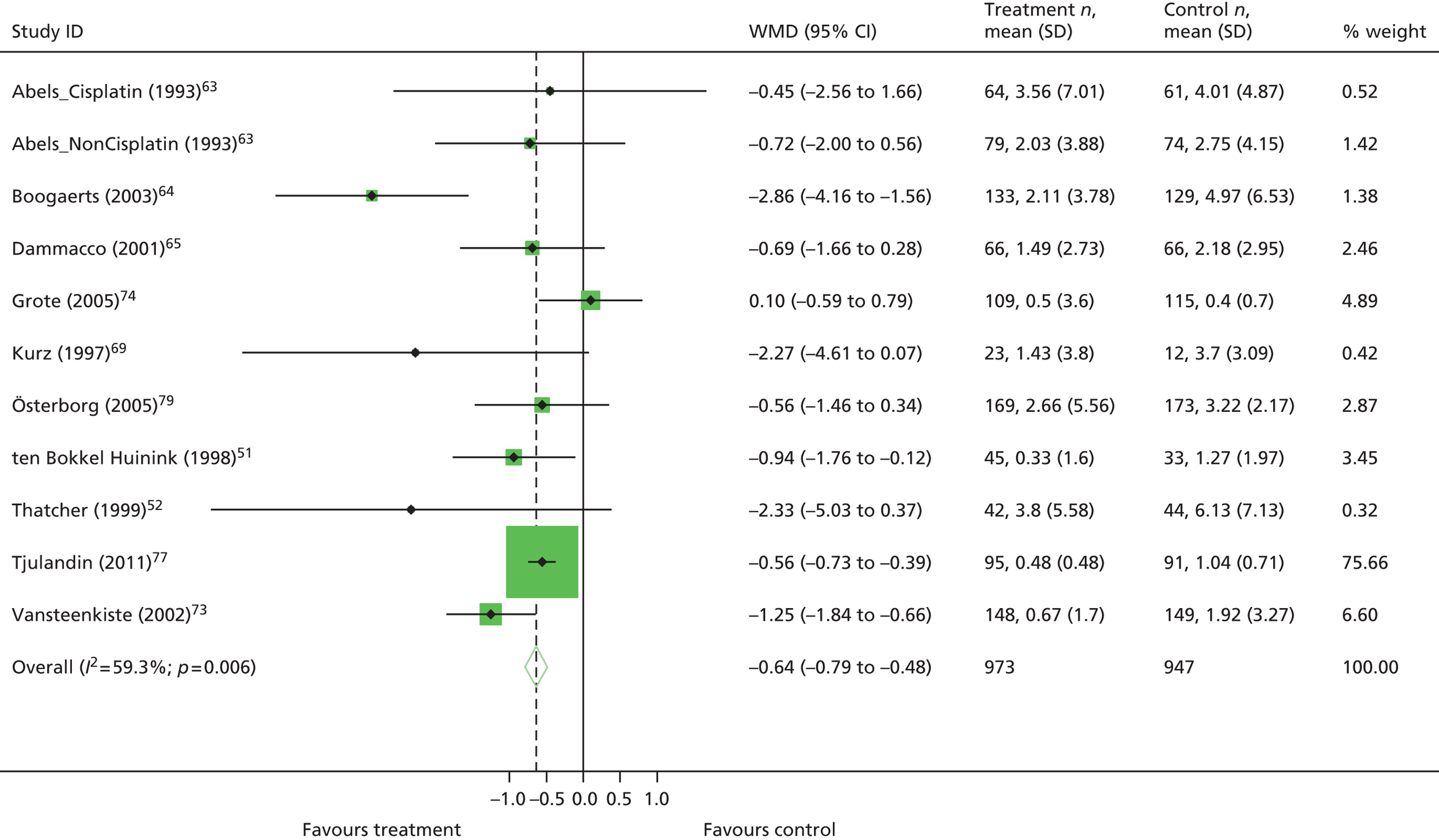
Malignancy-related outcomes
Tumour response
Fixed effects
FIGURE 89.
Forest plot: tumour response. Fixed-effects meta-analysis (Mantel–Haenszel). Events, treatment = number of events/number of participants in treatment group; events, control = number of events/number of participants in control group.

Overall survival
Publication bias
FIGURE 90.
Funnel plot with pseudo 95% confidence limits: OS.

FIGURE 92.
Overall survival: publication bias – meta-analysis using year of publication as covariate. pubyear, publication year.

Fixed effects
FIGURE 93.
Forest plot: overall survival. Fixed-effects meta-analysis; trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta and Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy; effect sizes reported are HRs; IPD data as reported in Tonia and colleagues11 (Cochrane review) for Abels and colleagues,63 Boogaerts and colleagues,65 Dammacco and colleagues,66 Grote and colleagues,74 Hedenus and colleagues,17 Littlewood and colleagues,70 Österborg and colleagues,71 Ray-Coquard and colleagues,75 Strauss and colleagues76 and Vansteenkiste and colleagues. 73 HRs reported for other trials calculated using other accepted methods. ES, effect size.
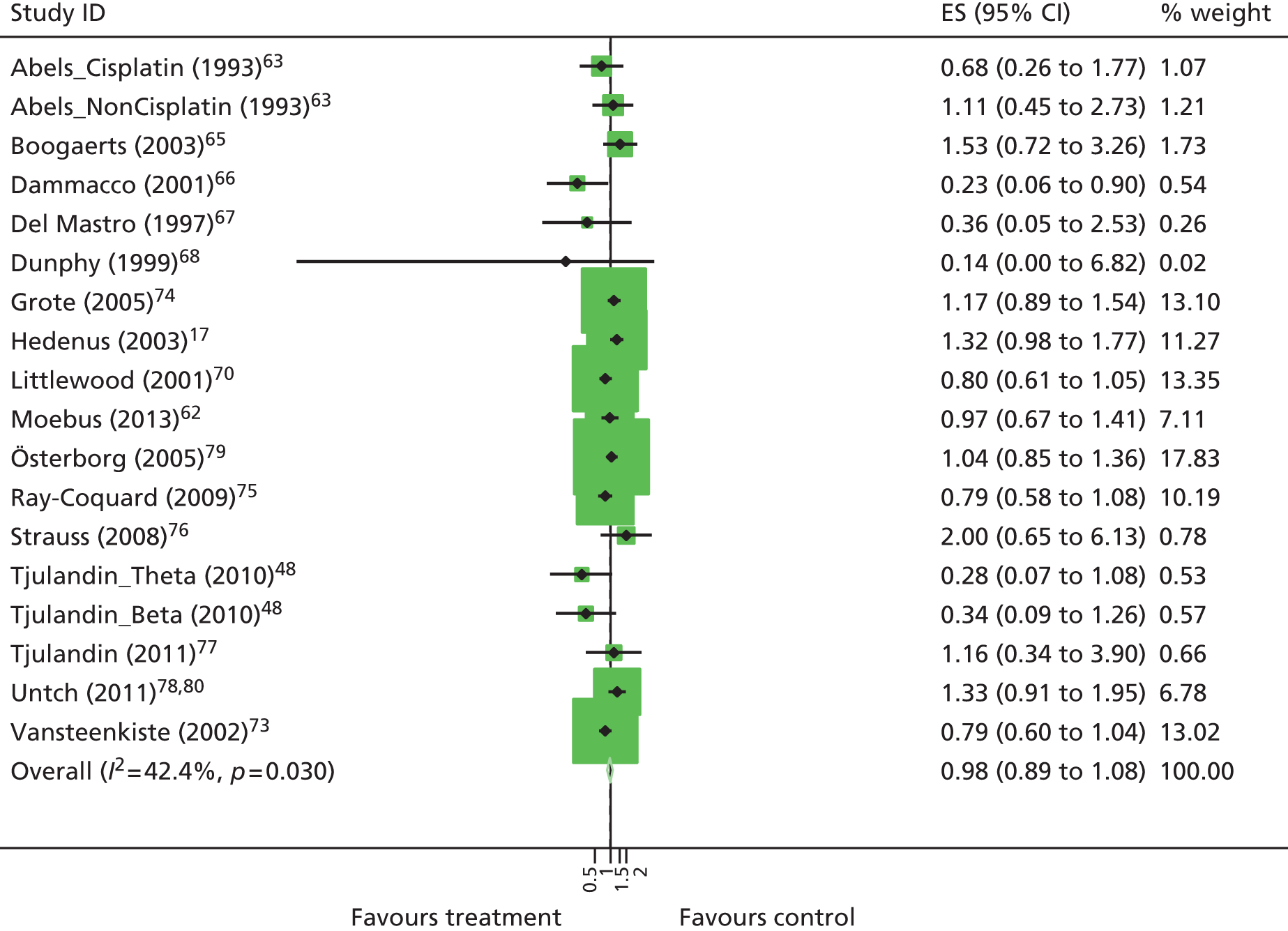
On-study mortality
Publication bias
FIGURE 94.
Funnel plot with pseudo 95% confidence limits: mortality.

FIGURE 95.
Funnel plot with pseudo 95% confidence limits: mortality excluding Dunphy and colleagues. 68
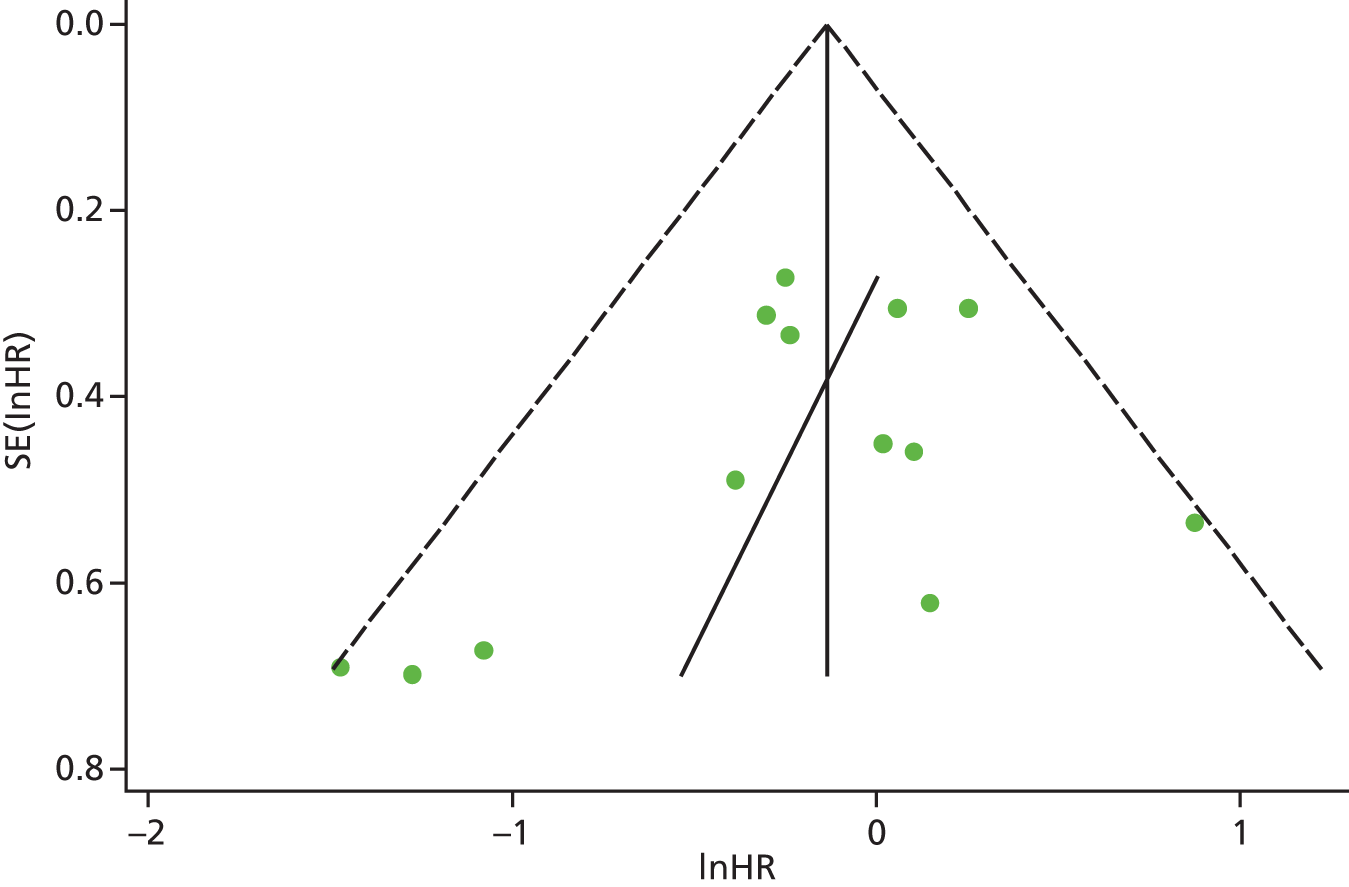
FIGURE 96.
Meta-regression plot: mortality.

Fixed effects
FIGURE 97.
Forest plot: mortality. Fixed-effects meta-analysis (DerSimonian–Laird); trials with multiple experimental arms split into subsets in the analysis: Tjulandin and colleagues48 reported data for epoetin theta and epoetin beta and Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy; IPD data as reported in Tonia and colleagues11 (Cochrane review) for Abels and colleagues,63 Boogaerts and colleagues,65 Dammacco and colleagues,66 Grote and colleagues,74 Hedenus and colleagues,17 Littlewood and colleagues,70 Österborg and colleagues,71 Ray-Coquard and colleagues75 and Vansteenkiste and colleagues. 73 HRs reported for other trials calculated using other accepted methods.

Safety-related outcomes
Thromboembolic events
Publication bias
FIGURE 98.
Thromboembolic events: publication bias – funnel plot with pseudo 95% confidence limits.
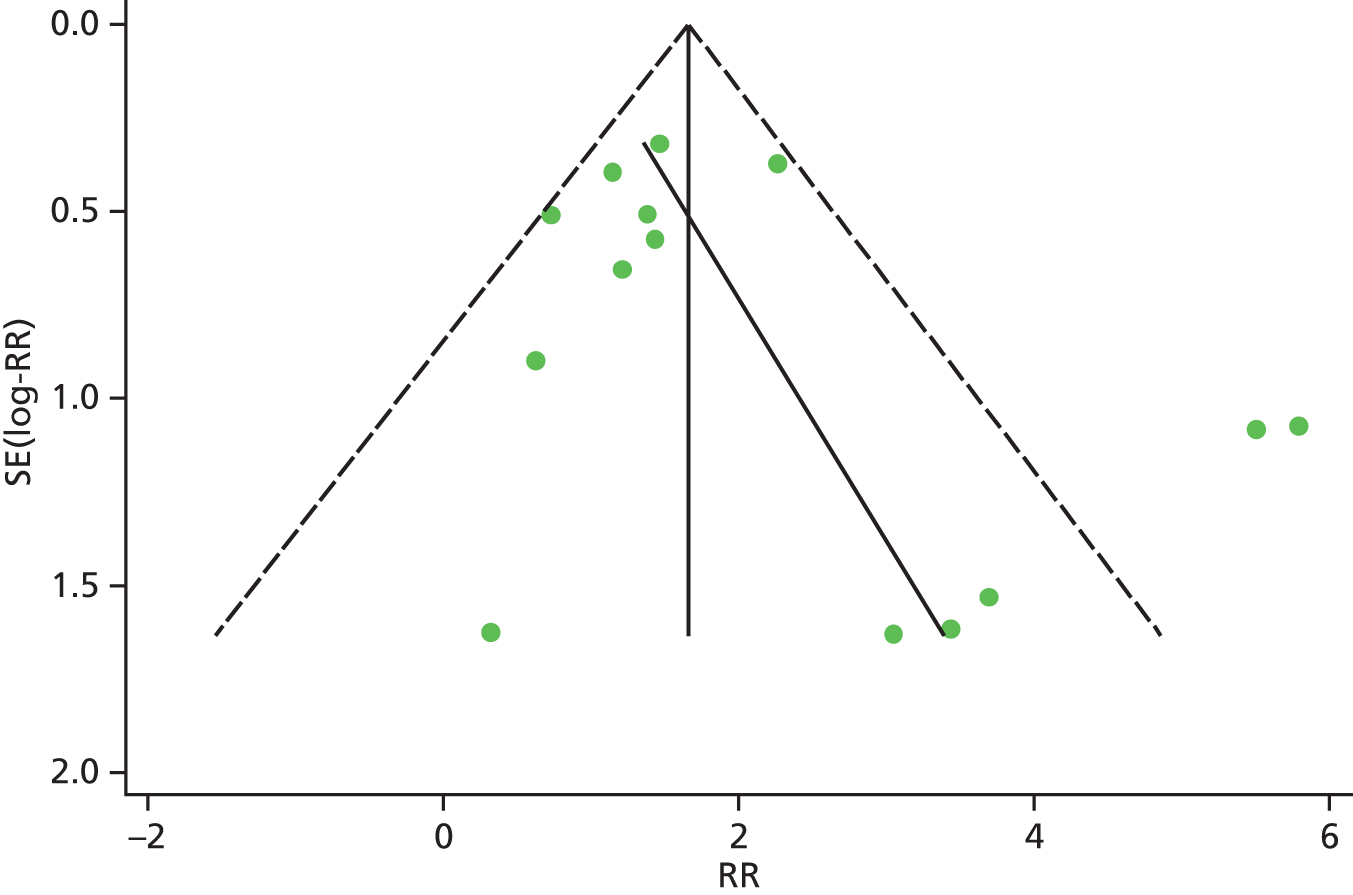
| Z/sqrt(V) | Coefficient | SE | t | p > |t| | 95% CI |
|---|---|---|---|---|---|
| sqrt(V) | 0.30 | 0.33 | 0.91 | 0.38 | –0.42 to 1.03 |
| Bias | 0.28 | 0.56 | 0.63 | 0.63 | –0.94 to 1.50 |
| Test of H0 no small study effects p = 0.627 | |||||
FIGURE 99.
Thromboembolic events: publication bias – meta-regression plot with year of publication as a covariate.
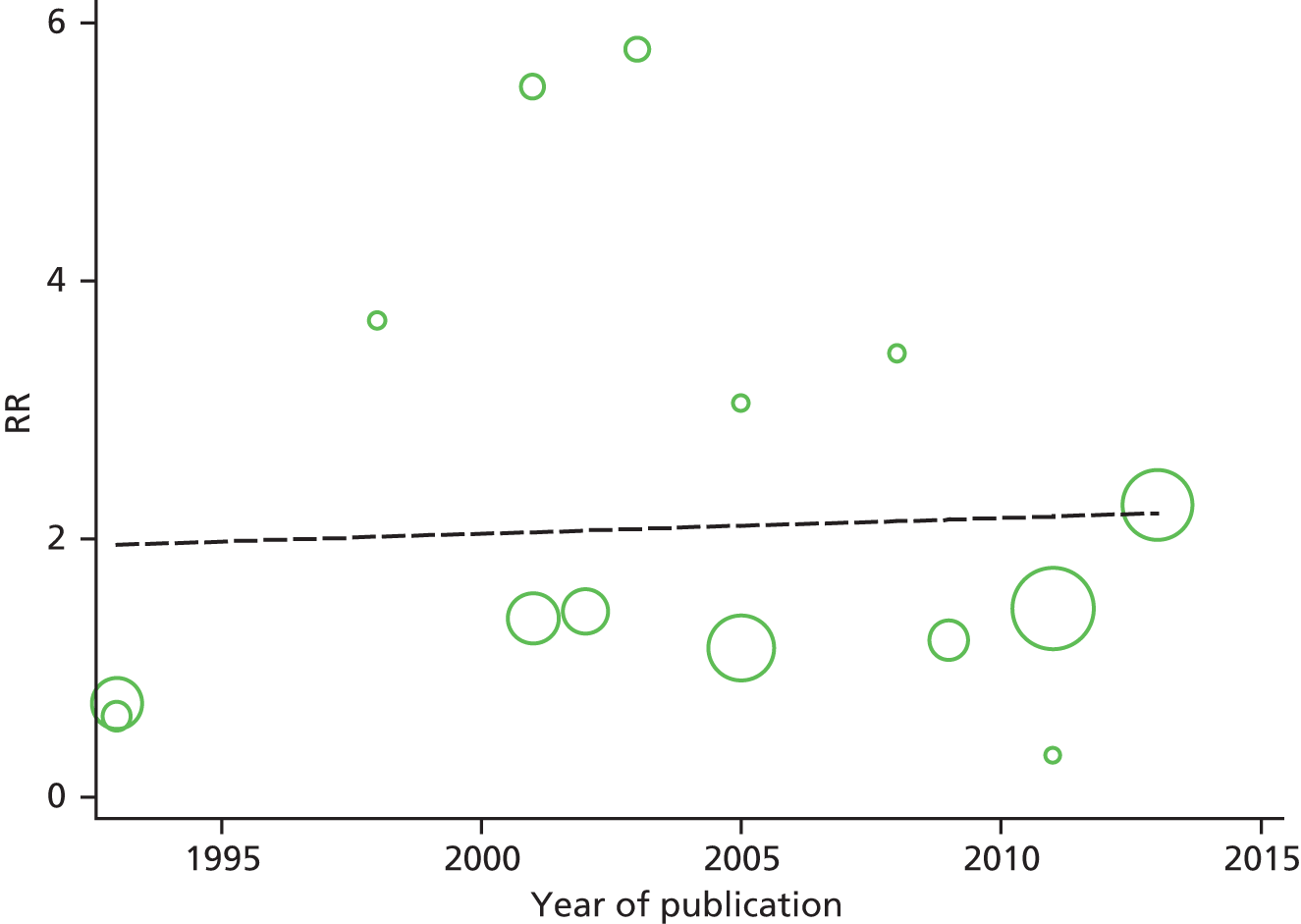
Fixed effects
FIGURE 100.
Forest plot: thromboembolic events. Fixed-effects meta-analysis (Mantel–Haenszel); trial with multiple experimental arms split into subsets in the analysis: Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy. Events, treatment = number of events/participants in the treatment group; events, control = number of events/participants in the control group.
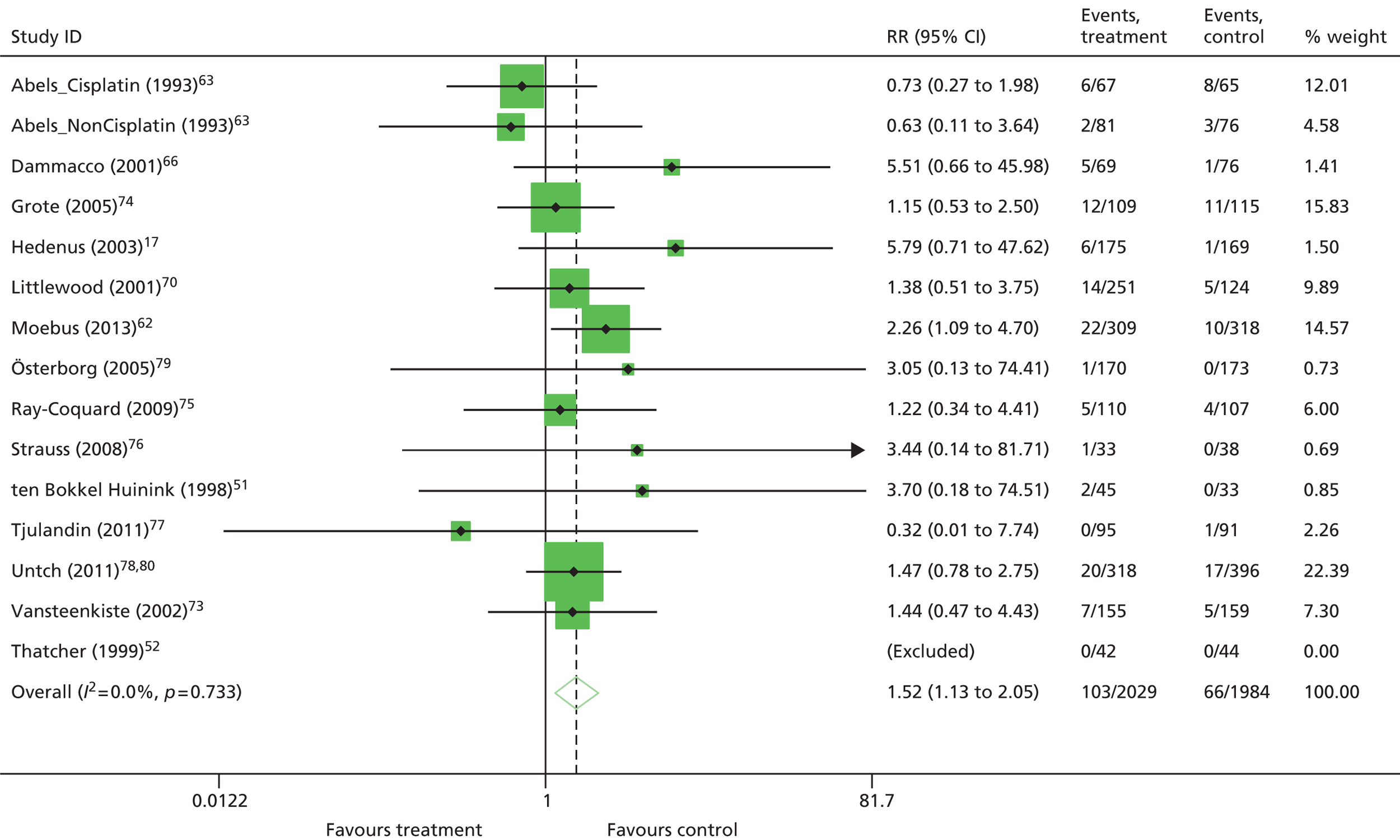
Hypertension
Publication bias
FIGURE 101.
Hypertension: publication bias – funnel plot with pseudo 95% confidence limits (fixed effects).

| Z/sqrt(V) | Coefficient | SE | t | p > |t| | 95% CI |
|---|---|---|---|---|---|
| sqrt(V) | 0.49 | 0.51 | 0.95 | 0.364 | –0.66 to 1.63 |
| Bias | 0.28 | 0.68 | 0.41 | 0.689 | –1.22 to 1.79 |
| Test of H0 no small study effects p = 0.689 | |||||
FIGURE 102.
Hypertension: publication bias – meta-regression plot with year of publication as a covariate.

Fixed effects
FIGURE 103.
Forest plot: hypertension. Fixed-effects meta-analysis (Mantel–Haenszel); trial with multiple experimental arms split into subsets in the analysis: Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy. Events, treatment = number of events/participants in the treatment group; events, control = number of events/participants in the control group.

Thrombocytopenia/haemorrhage
Fixed effects
FIGURE 104.
Forest plot: thrombocytopenia/haemorrhage. Fixed-effects meta-analysis (Mantel–Haenszel). Events, treatment = number of events/participants in the treatment group; events, control = number of events/participants in the control group.
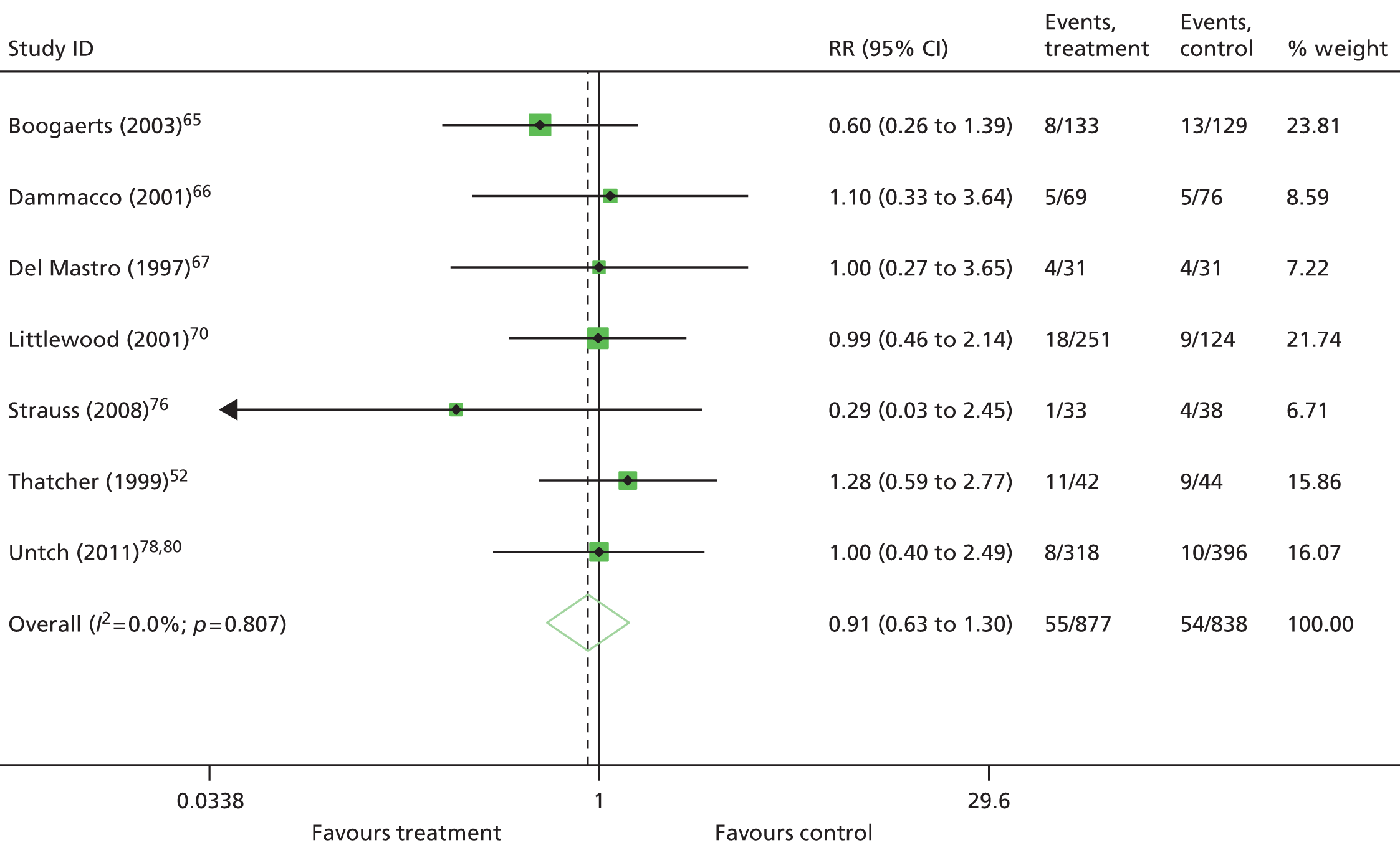
Seizure
Fixed effects
FIGURE 105.
Forest plot: seizure. Fixed-effects meta-analysis (Mantel–Haenszel); trial with multiple experimental arms split into subsets in the analysis: Abels and colleagues63 reported data for participants on platinum-based chemotherapy and non-platinum-based chemotherapy. Events, treatment = number of events/participants in the treatment group; events, control = number of events/participants in the control group.

Pruritus
Fixed effects
FIGURE 106.
Forest plot: pruritus. Fixed-effects meta-analysis (Mantel–Haenszel). Events, treatment = number of events/participants in the treatment group; events, control = number of events/participants in the control group.
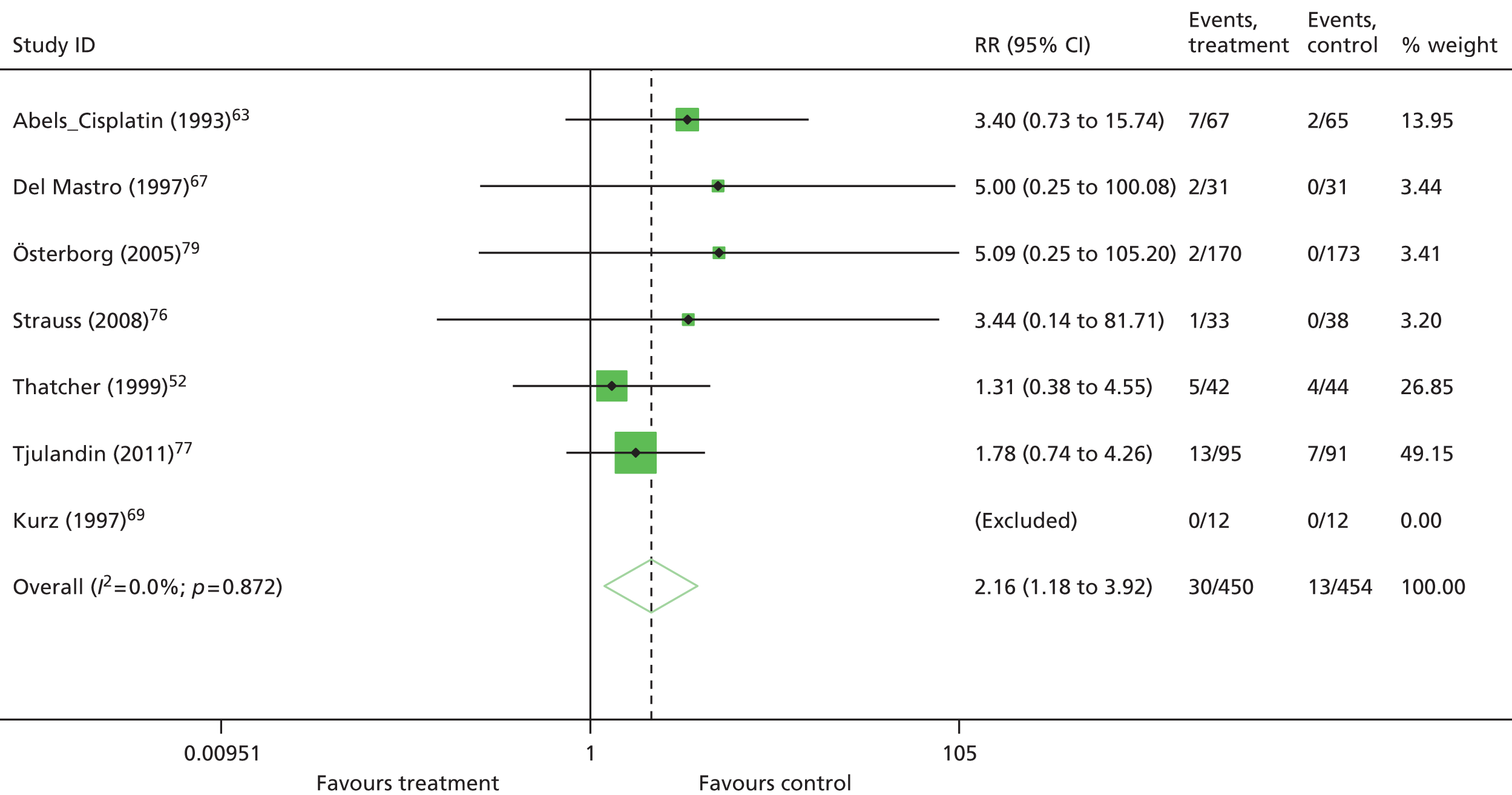
Sensitivity ‘close to licence’ analyses
| Licence | Outcome | Trials | ES (95% CI) | I 2 |
|---|---|---|---|---|
| Starting dose criteria met | Hb changea,b | 18 | WMD 1.59 (1.33 to 1.84) | 75.9%; p < 0.01 |
| HaemRa,b,c | 13 | RR 3.29 (2.81 to 3.85) | 13.4%; p = 0.31 | |
| RBCTa,b,d | 26 | RR 0.61 (0.55 to 0.68) | 22.4%; p = 0.15 | |
| Unitsa,e | 12 | WMD –0.87 (–1.24 to –0.50) | 55.6%; p = 0.01 | |
| Tumour response | 7 | RR 1.10 (0.86 to 1.41) | 37.5%; p = 0.14 | |
| OSa,b | 18 | HR 0.97 (0.83 to 1.13) | 42.4%; p = 0.03 | |
| On-study mortalitya,b | 14 | HR 0.86 (0.67 to 1.11) | 16.4%; p = 0.27 | |
| Thromboembolic eventsa | 14 | RR 1.46 (1.07 to 1.99) | 0%; p = 0.73 | |
| Hypertensiona,b | 12 | RR 1.80 (1.14 to 2.85) | 0%; p = 0.79 | |
| Thrombocytopenia/haemorrhage | 7 | RR 0.93 (0.65 to 1.34) | 0%; p = 0.81 | |
| Seizuresa | 2 | RR 1.19 (0.33 to 4.38) | 0%; p = 0.74 | |
| Pruritus | 6 | RR 2.04 (1.11 to 3.75) | 0%; p = 0.87 | |
| Starting dose criteria met and inclusion Hb ≤ 11 g/dl | Hb changea,b | 13 | WMD 1.52 (1.30 to 1.75) | 48.1%; p = 0.03 |
| HaemRa,b,c | 12 | RR 3.20 (2.78 to 3.68) | 2.0%; p = 0.43 | |
| RBCTa,b,d | 16 | RR 0.64 (0.57 to 0.71) | 7.3%; p = 0.37 | |
| Unitsa,e | 9 | WMD –0.99 (–1.41 to –0.56) | 56.2%; p = 0.02 | |
| Tumour response | 2 | RR 1.60 (0.88 to 2.90) | 0%; p = 0.70 | |
| OSa,b | 10 | HR 0.91 (0.70 to 1.20) | 51.7%; p = 0.03 | |
| On-study mortalitya,b | 10 | HR 0.89 (0.61 to 1.30) | 37.7%; p = 0.11 | |
| Thromboembolic eventsa | 7 | RR 1.29 (0.66 to 2.54) | 12.2%; p = 0.34 | |
| Hypertensiona,b | 9 | RR 1.68 (1.03 to 2.74) | 0%; p = 0.64 | |
| Thrombocytopenia/haemorrhage | 2 | RR 0.73 (0.37 to 1.46) | 0%; p = 0.41 | |
| Seizuresa | 2 | RR 1.19 (0.33 to 4.38) | 0%; p = 0.74 | |
| Pruritus | 3 | RR 2.20 (1.05 to 4.58) | 0%; p = 0.66 | |
| Starting dose criteria met and target Hb ≤ 13 g/dl | Hb changeb | 4 | WMD 1.29 (0.90 to 1.67) | 61.9%; p = 0.05 |
| HaemRb | 3 | RR 3.06 (2.28 to 4.09) | 0%; p = 0.79 | |
| RBCTb | 4 | RR 0.52 (0.34 to 0.80) | 48.4%; p = 0.14 | |
| Unitse | 1 | WMD –0.56 (–0.74 to 0.39) | NA | |
| Tumour response | 1 | RR 0.90 (0.63 to 1.3) | NA | |
| OSb | 4 | HR 0.73 (0.32 to 1.64) | 61.8%; p = 0.05 | |
| On-study mortalityb | 3 | HR 0.50 (0.20 to 1.23) | 29.7%; p = 0.24 | |
| Thromboembolic events | 2 | RR 1.38 (0.75 to 2.57) | 0%; p = 0.36 | |
| Hypertensionb | 3 | RR 2.19 (0.53 to 9.12) | 16.8%; p = 0.30 | |
| Thrombocytopenia/haemorrhage | 1 | RR 1.00 (0.40 to 2.50) | NA | |
| Seizures | 0 | NA | NA | |
| Pruritus | 1 | RR 1.78 (0.74 to 4.26) | NA | |
| Starting dose criteria met, inclusion Hb ≤ 11 g/dl and target Hb ≤ 13 g/dl | Hb changeb | 3 | WMD 1.50 (1.16 to 1.83) | 0%; p = 0.80 |
| HaemRb | 3 | RR 3.06 (2.28 to 4.09) | 0%; p = 0.79 | |
| RBCTb | 3 | RR 0.50 (0.33 to 0.77) | 0%; p = 0.92 | |
| Unitse | 1 | WMD –0.56 (–0.74 to 0.39) | NA | |
| Tumour response | 0 | NA | NA | |
| OSb | 3 | HR 0.50 (0.20 to 1.23) | 29.7%; p = 0.24 | |
| On-study mortalityb | 3 | HR 0.50 (0.20 to 1.23) | 29.7%; p = 0.24 | |
| Thromboembolic events | 1 | RR 0.32 (0.01 to 7.74) | NA | |
| Hypertensionb | 3 | RR 2.19 (0.53 to 9.12) | 16.8%; p = 0.30 | |
| Thrombocytopenia/haemorrhage | 0 | NA | NA | |
| Seizures | 0 | NA | NA | |
| Pruritus | 1 | RR 1.78 (0.74 to 4.26) | NA |
Appendix 13 Supplementary material: health-related quality-of-life review
Health-related quality-of-life review: methods
The search strategy was based on the strategy used in the previous MTA on this topic by Wilson and colleagues,2 with additional search terms for epoetin theta, epoetin zeta and corresponding drug brand names. It combined free-text and MeSH terms for epoetin (generic and brand names), cancer and anaemia. A search filter was developed by an information scientist to retrieve HRQoL studies, ensuring an appropriate balance of sensitivity and specificity (see Chapter 3, Studies identified, and Appendix 1 for further details).
The database search results were exported to EndNote (X5) and deduplicated using the software and manual checking. The search strategies and the numbers retrieved for each database are detailed in Appendix 1. After the reviewers completed the screening process, the bibliographies of included papers were scrutinised for further potentially includable studies.
Inclusion criteria were the same as for the main review (see Chapter 3, Eligibility criteria). Data were tabulated and analysed by meta-analysis to provide an overview with an estimate of overall effect.
Health-related quality-of-life review: results
Studies identified
We screened the titles and abstracts of 1268 unique references identified by the PenTAG searches and additional sources and retrieved 224 papers for detailed consideration. Of these, 191 were excluded (a list of these items with reasons for their exclusion can be found in Appendix 4). Update searches conducted on 2 December 2013 yielded 61 titles and abstracts, none of which was considered eligible for inclusion. Thirty-three studies met the prespecified criteria set out in the protocol and were considered eligible for inclusion in the HRQoL review. Fifteen studies were considered eligible from the previous HTA review. 2 At both stages, initial disagreements were easily resolved by consensus.
A total of 48 publications were considered eligible for inclusion. As for the clinical effectiveness review, we further specified that eligible interventions should be assessed as administered in accordance with their licensed indications. This criterion was applied after the first round of full-paper screening to make sure that we captured all relevant evidence (see Chapter 3, Methods, Selection of studies for details). In applying this criterion, a further 25 studies were excluded, as they evaluated an unlicensed dose. In total, 13 studies reported in 23 publications17,50,52,58–60,63,65–67,69–71,73,76,77,79,81–86 were considered eligible for inclusion in the HRQoL review. This process is illustrated in detail in Figure 107.
FIGURE 107.
Quality-of-life review: PRISMA flow diagram. SR, systematic review. a, ‘within licence’, based on the administration of ESAs at the licensed weight-based start dose.
Health-related quality-of-life measures
| Scale | Type of HRQoL instrument | Domains | Items | Implication of value |
|---|---|---|---|---|
| FACT-G284 | Specific for use with patients of any tumour type |
|
27 items – response between 0 and 4 for each question with a maximum score of 108 | Higher score indicates improved HRQoL |
| FACT-F284 | Symptom specific (fatigue) | Fatigue-related questions often used in isolation or as a component of other FACT questionnaires | 13 items – response between 0 and 4 for each question with a maximum score of 52 | Higher score indicates improved HRQoL |
| FACT-An284 | Symptom specific (fatigue or anaemia) | Composed of FACT-G, FACT-F and FACT-An-An | 47 items – response between 0 and 4 for each question with a maximum score 188 | Higher score indicates improved HRQoL |
| FACT-An-An285 | Symptom specific (additional concerns for anaemia) | Anaemia-related questions that do not include fatigue | Seven items – response between 0 and 4 for each question with a maximum score of 28 | Higher score indicates improved HRQoL |
| SF-36286 | Generic |
|
36 items – each scale is directly transformed into a 0–100 scale | The lower the score, the greater the disability |
| NHP287 | Generic |
|
38 items – scores on the first component are weighted to give a score between 0 and 100 | The higher the score, the lower the HRQoL; however, it should be noted the NHP was not originally intended to measure HRQoL and is not considered highly sensitive2,288 |
| Cancer Linear Analog Scale or LASA289,290 | Specific for cancer patients to indicate feelings |
|
25 items – 100 mm lines | Higher score indicates improved HRQoL |
| Brief Symptom Inventory291 | Generic psychiatry/psychology |
|
53 items – scores between 0 and 4 with a maximum score of 212 | The higher the score the greater the distress |
| Psychological Distress Inventory292 | Specific for cancer patients |
|
13 items – scores between 0 and 5 | A higher score indicates a higher level of distress |
| EORTC-QLQ-C30293 | Specific for cancer patients | A range of questions including on daily activities, sleep, pain, mobility, emotions and health | 30 items – 28 items with a score between 0 and 4 and two items with a score between 0 and 7 with a maximum score of 126 | The higher the score, the higher the level of functioning |
Meta-analysis: health-related quality of life
FACT-F: fixed effects
FIGURE 108.
Forest plot: HRQoL [change in FACT-F score – overall (fixed effects – Mantel–Haenszel pooled WMD)].
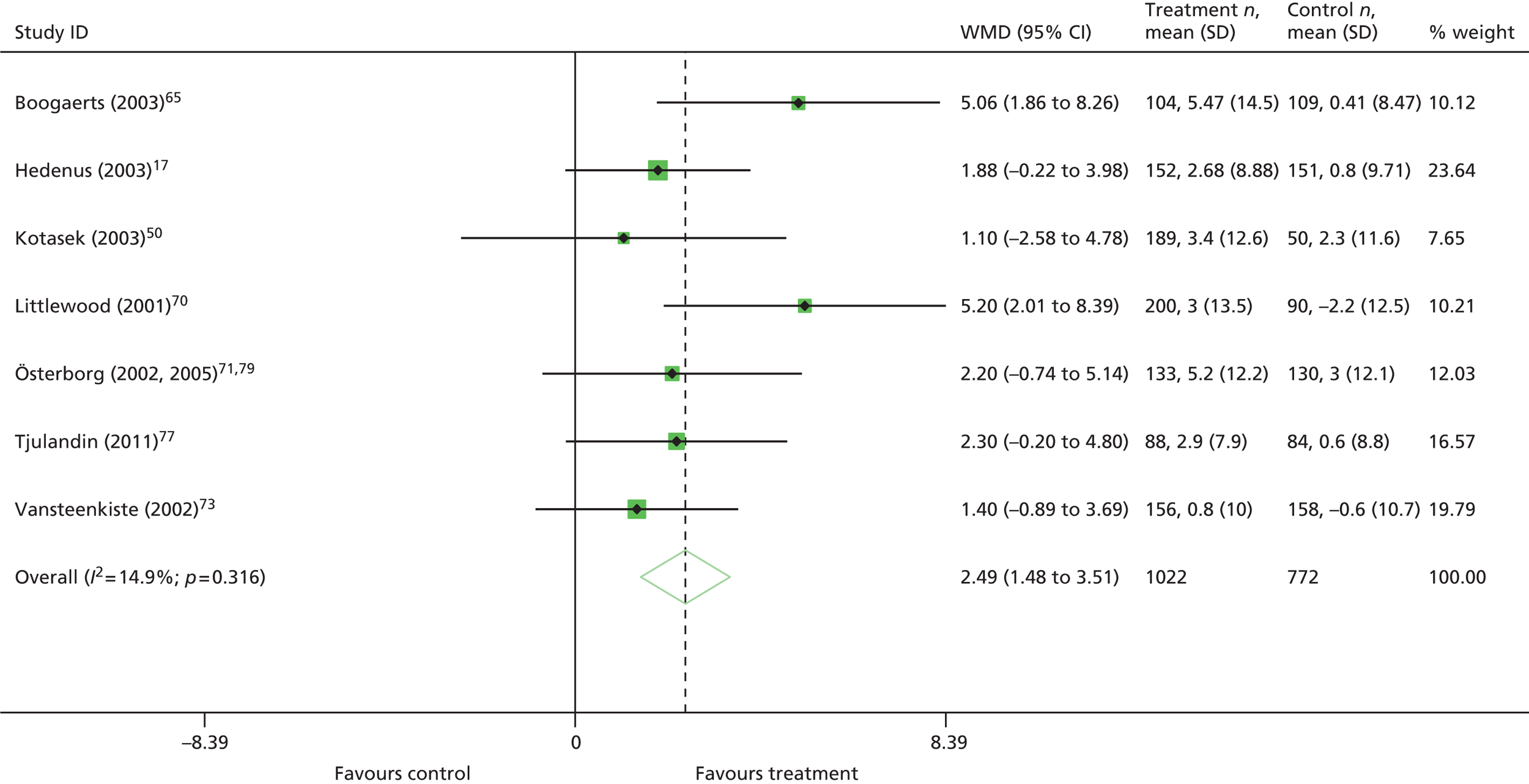
FACT-F: subgroup analyses (random effects)
FIGURE 109.
Forest plot: HRQoL [change in FACT-F score by chemotherapy type (random effects – DerSimonian–Laird pooled WMD)].
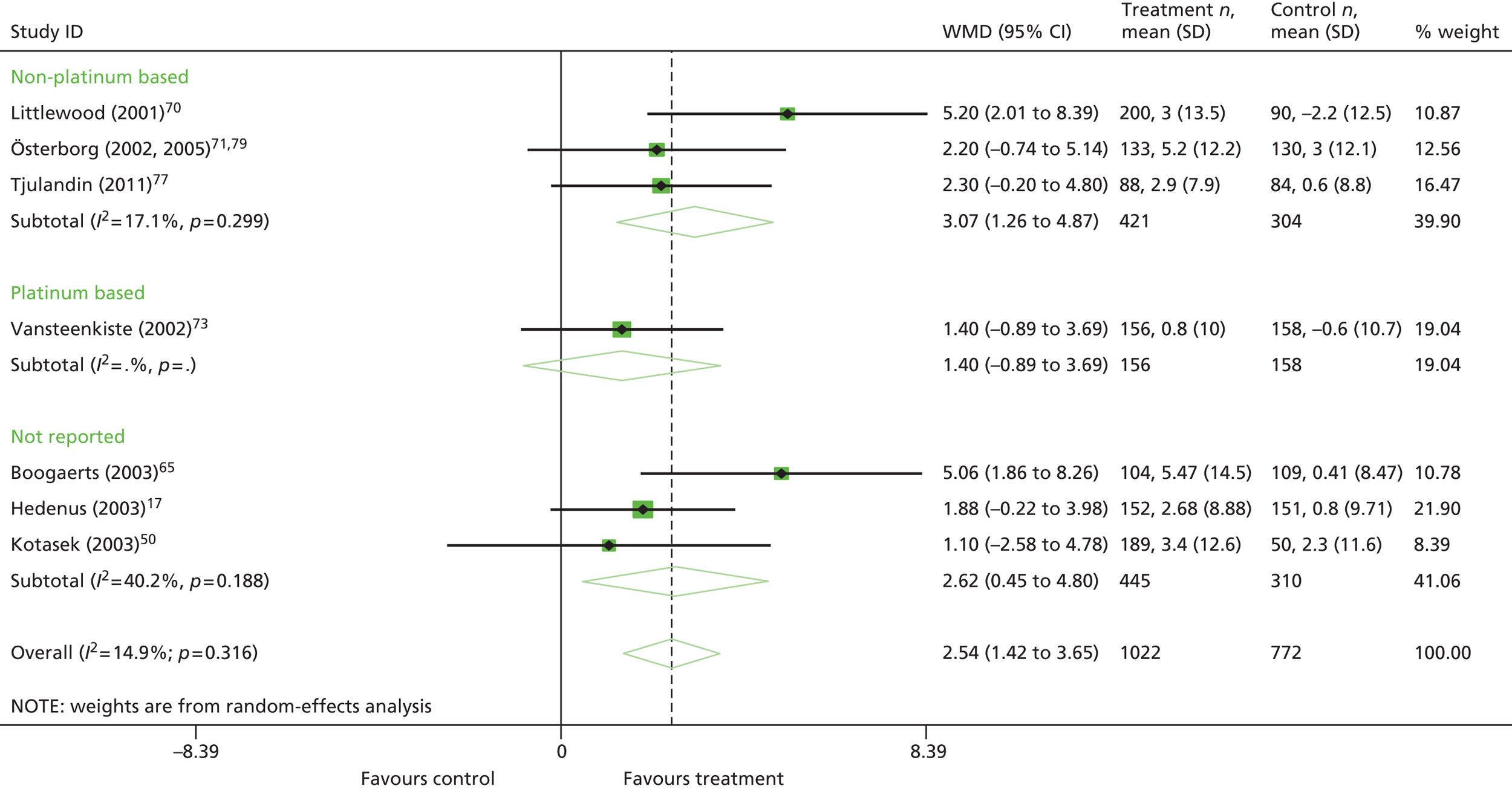
FIGURE 110.
Forest plot: HRQoL [change in FACT-F score by malignancy (random effects – DerSimonian–Laird pooled WMD)].

FIGURE 111.
Forest plot: HRQoL [change in FACT-F score by intervention (random effects – DerSimonian–Laird pooled WMD)].
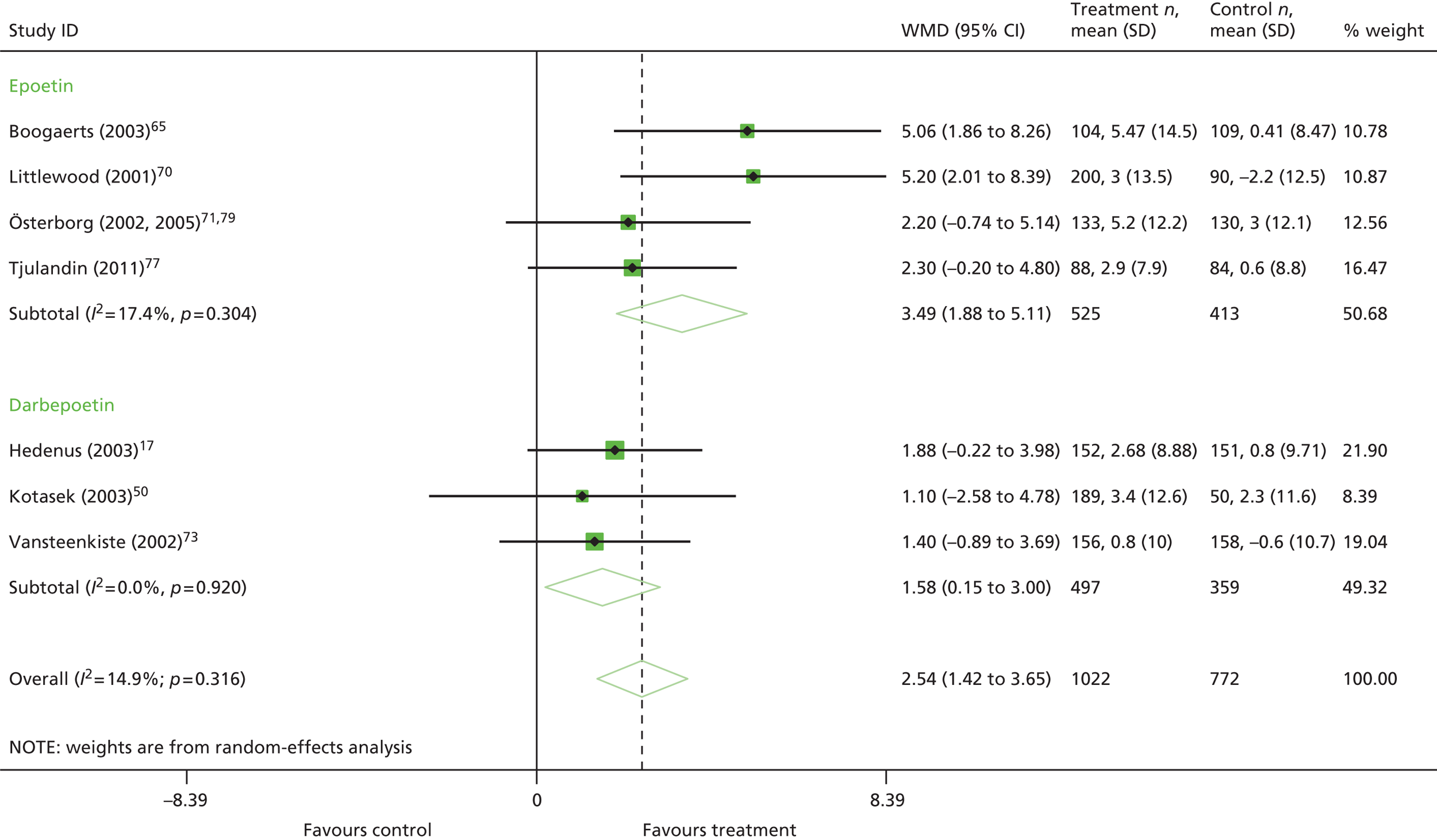
FIGURE 112.
Forest plot: HRQoL [change in FACT-F score by study duration (random effects – DerSimonian–Laird pooled WMD)].
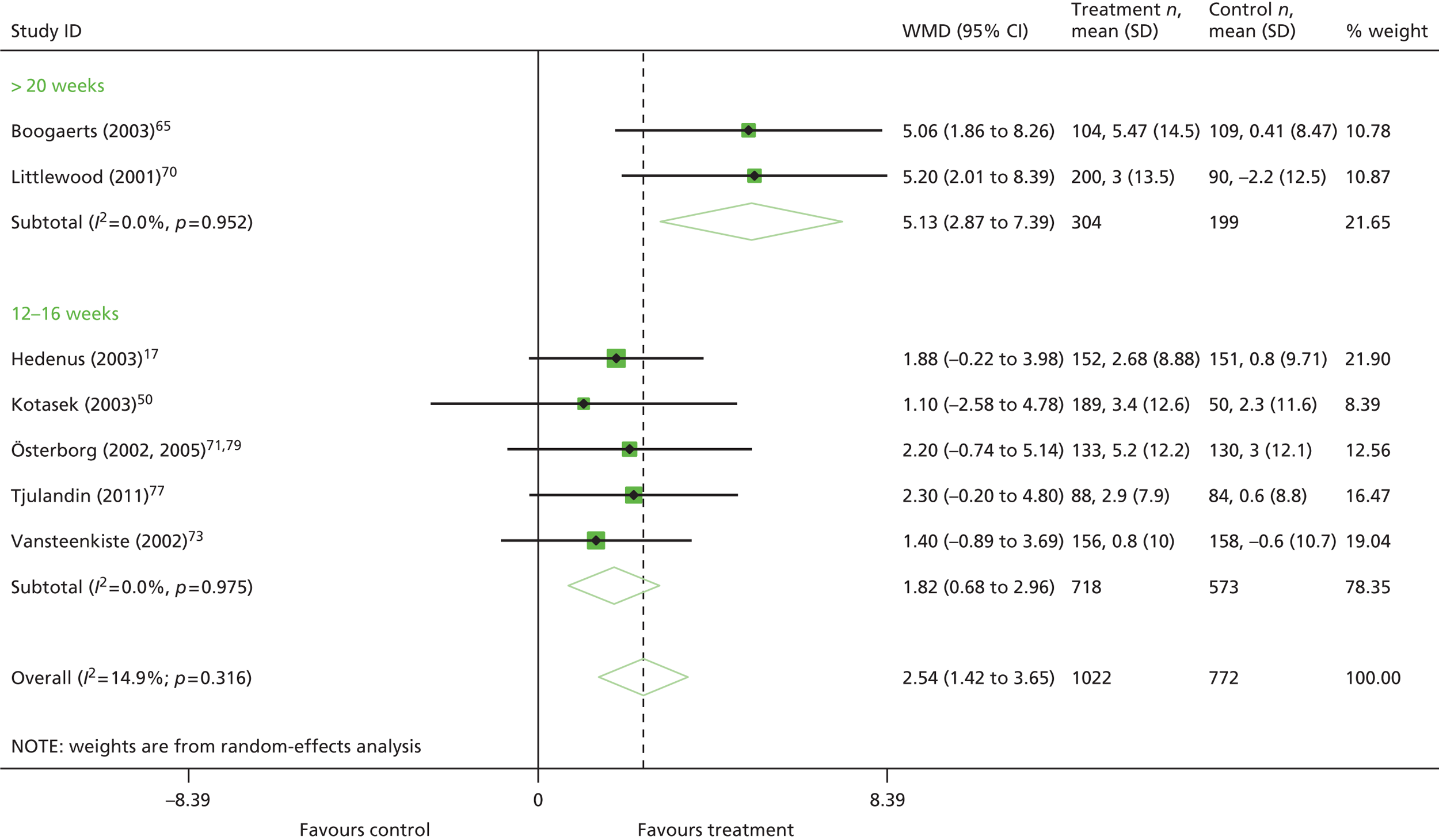
FIGURE 113.
Forest plot: HRQoL [change in FACT-F score with data from Boogaerts and colleagues65 removed (random effects – DerSimonian–Laird pooled WMD)].
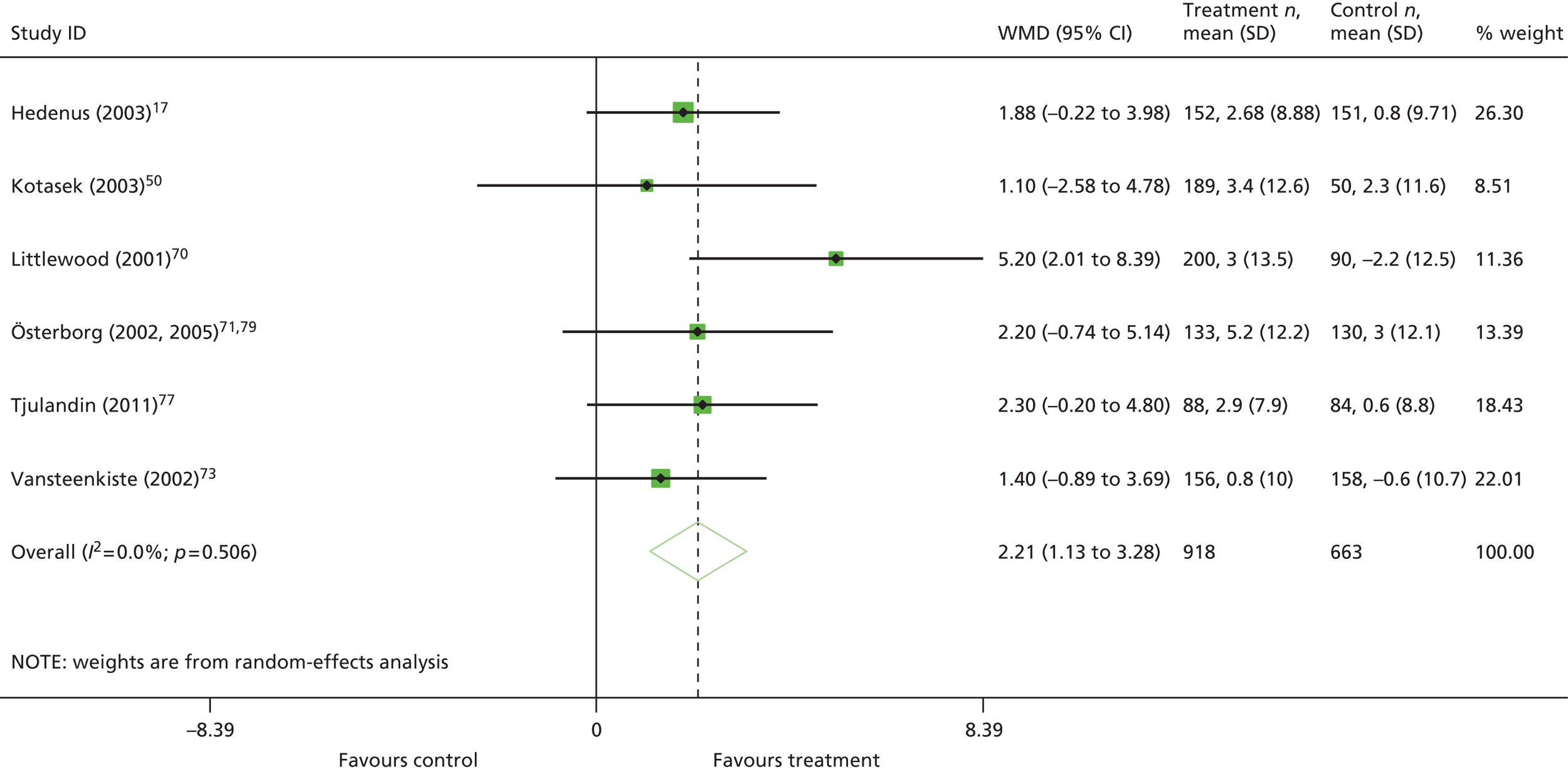
FACT-G
FIGURE 114.
Forest plot: HRQoL [change in FACT-G score – overall (random effects – DerSimonian–Laird pooled WMD)].

FIGURE 115.
Forest plot: HRQoL [change in FACT-G score – overall (fixed effects – Mantel–Haenszel pooled WMD)].

FACT-An
FIGURE 116.
Forest plot: HRQoL [change in FACT-An score – overall (fixed effects – Mantel–Haenszel pooled WMD)].

FIGURE 117.
Forest plot: HRQoL [change in FACT-An score – overall (fixed effects – Mantel–Haenszel pooled WMD)].

Appendix 14 Study characteristics, key parameters and results of conference abstracts identified in the cost-effectiveness review
| Parameter | Szucs and colleagues132 | Cremieux and colleagues133 | Mark and colleagues135 | van Hout and Gagnon136 |
|---|---|---|---|---|
| Evaluation type | Cost-effectiveness analysis | Cost-effectiveness analysis | Cost–consequences analysis | Cost-effectiveness analysis |
| Modelling used | No | No | No | Yes |
| Nature of modelling | NA | NA | NA | ‘Bayesian simulation model’ |
| Perspective | Societal | Societal | Drug cost only | Health carea |
| Country (setting) | Multiple (France, Germany, Italy, Sweden and UK) | Not stated (probably USA) | Not stated (probably USA) | UKa |
| Intervention/comparator | Epoetin beta TIW: 150 IU/kg; standard care | Epoetin alfa QW: 40,000 IU; darbepoetin alfa QW: 2.25 µg/kg | Epoetin alfa; darbepoetin alfa | Epoetin alfa QW: 150 IU/kg;b darbepoetin alfa QW: 2.25 µg/kgc |
| Population | Patients with solid or lymphoid tumours | Patients with lung cancer receiving chemotherapy | Non-myeloid cancer patients with chemotherapy-related anaemia | Anaemic cancer patients receiving chemotherapy |
| Outcomes considered | SF-36 PCS, FACIT-F, FACT-An | Cumulative change in Hb (AUC), change in FACIT-F | Proportion of patients requiring transfusion, change in Hb from baseline, Hb AUC | Hb response (≥ 2 g/dl change or Hb ≥ 12 g/dl unrelated to transfusion), dose escalation, avoidance of transfusion |
| Time frame | 12 weeks | 12 weeks | 12 weeks | 12 weeks |
| Discounting | Not stated | Not stated | Not stated | Not stated |
| Funding | Not stated | Ortho Biotec (manufacturer of epoetin alfa) | Ortho Biotec (manufacturer of epoetin alfa) | Johnson & Johnson (manufacturer of epoetin alfa) |
| Parameter | Ben-Hamadi and colleagues137 | Van Bellinghen and colleagues138 | Esposito and colleagues139 | Van Bellinghen and colleagues140 |
|---|---|---|---|---|
| Evaluation type | Cost-effectiveness | Cost–consequences analysis | Cost–consequences analysis | Cost–consequences analysis |
| Modelling used | Minimal | Yes | Yes | Yes |
| Nature of modelling | Integration of costs with Hb levels from separate placebo-controlled RCTs | Decision tree | Decision tree | Decision tree |
| Perspective | Societal | Societal | Health care | Societal |
| Country (setting) | Not stated (probably USA) | France | Italy | Germany |
| Intervention/comparator | Epoetin alfa QW: 40,000 IU; darbepoetin alfa QW: 2.25 µg/kg | Darbepoetin alfa Q3W: 500 µg; epoetin alfa QW: European label dose; epoetin beta QW: European label dose | Darbepoetin alfa Q3W: 500 µg; epoetin alfa QW: European label dose; epoetin beta QW: European label dose | Darbepoetin alfa Q3W: 500 µg; epoetin alfa QW: European label dose; epoetin beta QW: European label dose |
| Population | Patients with chemotherapy-induced anaemia | Patients with chemotherapy-induced anaemia | Patients with chemotherapy-induced anaemia | Patients with chemotherapy-induced anaemia |
| Outcomes considered | Area under the Hb change curve over 12 weeks | Hb levels | Hb levels | Hb levels |
| Time frame | 12 weeks | 16 weeks (assumed based on trial length) | 16 weeks (assumed based on trial length) | 16 weeks (assumed based on trial length) |
| Discounting | Not stated | Not stated | Not stated | Not stated |
| Funding | Ortho Biotec (manufacturer of epoetin alfa) | Amgen Inc. (manufacturer of darbepoetin alfa) | Amgen Inc. (manufacturer of darbepoetin alfa) | Amgen Inc. (manufacturer of darbepoetin alfa) |
| Parameter | Finek and colleagues141 | Liwing and colleagues142 | Walter and colleagues143 | Fragoulakis and Maniadakis134 |
|---|---|---|---|---|
| Evaluation type | Cost-effectiveness analysis | Cost-effectiveness analysis | Cost-effectiveness analysis | Cost-effectiveness analysisa |
| Modelling used | Minimal | Yes | Yes | Yes |
| Nature of modelling | Integration of drug acquisition costs with retrospective, single-centre analysis | Simulation model | Decision tree | Decision tree |
| Perspective | Not stated | Not stated (probably health care) | Health care | Health care (plus patient transportation) |
| Country (setting) | Czech Republic (not explicitly stated) | Sweden | Austria | Greece |
| Intervention/comparator | Epoetin alfa QW: 40,000 IU; darbepoetin alfa Q3 W: 500 µg | Epoetin alfa; darbepoetin alfa | Darbepoetin alfa Q3W: 500 µg; darbepoetin alfa QW: 150 µg; epoetin alfa QW: 40,000 IU; epoetin beta QW: 30,000 IU; epoetin beta TIW: 30,000 IU (per week) | Darbepoetin alfa Q3W: 500 µg; darbepoetin alfa QW: 150 µg; epoetin alfa QW: 40,000 IU; epoetin beta QW: 30,000 IU; epoetin beta TIW: 30,000 IU (per week) |
| Population | Patients with chemotherapy-induced anaemia | Patients with chemotherapy-related anaemia | Patients with chemotherapy-induced anaemia | Patients with chemotherapy-induced anaemia |
| Outcomes considered | Clinical response (Hb ≥ 11 g/dl) | Haematopoietic response rates, dose escalation rates, mean number of RBCTs required | Hb response rate | Hb response (≥ 2 g/dl) |
| Time frame | Not stated | 12 weeks | 12 weeks | Not stated |
| Discounting | Not stated | Not stated | Not stated | Not stated |
| Funding | None | Johnson & Johnson Pharmaceutical Service (parent company of Janssen-Cilag, manufacturers of epoetin alfa) | Amgen Inc. (manufacturers of darbepoetin alfa) | Genesis Pharma (distributor of darbepoetin alfa) |
Appendix 15 Excluded studies: cost-effectiveness review
| Study | Notes |
|---|---|
| Could not be obtained | |
| Sheffield R, Sullivan S, Saltiel E, Nishimura L. Cost comparison of recombinant human erythropoietin and blood transfusion in cancer chemotherapy-induced anemia. Ann Pharmacother 1997;31:15–22 | Published pre 2004 |
| Roungrong J, Teerawattananon Y, Chaikledkaew IU. Cost utility analysis of recombinant human erythropoietin in anemic cancer patients induced by chemotherapy in Thailand. J Med Assoc Thai 2008;91(Suppl. 2):119–25 | |
| Griggs JJ, Sorbero MES. Cost–utility of erythropoietin in the treatment of cancer-related anemia. Med Decis Making 1997;17:529 | Published pre 2004 |
| Griggs JJ, Blumberg N. Recombinant erythropoietin and blood transfusions in cancer chemotherapy-induced anemia. Anticancer Drugs 1998;9:925–32 | Published pre 2004 |
| Malonne H, editor. Cost evaluation of erythropoiesis stimulating agents in the treatment of platinum chemotherapy induced anaemia. 20th Annual Meeting of the Belgian Haematology Society, Genval, Belgium, January 2005 | |
| Study design | |
| Reeder CE. Anemia in cancer and critical care patients: pharmacoeconomic considerations. Am J Health System Pharm 2007;64:S22–7 | Not a systematic review |
| Dale DC. The benefits of haematopoietic growth factors in the management of gynaecological oncology. Eur J Gynaecol Oncol 2004;25:133–44 | Expert commentary |
| Marchetti M, Barosi G. Clinical and economic impact of epoetins in cancer care. Pharmacoeconomics 2004;22:1029–45 | Not a systematic review |
| Scarpace SL, Miller K, Elefante A, Czuczman MS, McCarthy P, Chanan-Khan A. Cost–utility of darbepoetin alfa (DARBE) on an every-2 week (QOW) schedule in anemic non-myeloid hematologic malignancies: a positive overall impact on the healthcare system (HCS). J Clin Oncol 2004;22:797S | Cost study, not UK |
| Steensma DP, Loprinzi CL. Epoetin alfa and darbepoetin alfa go head to head. J Clin Oncol 2006;24:2232–6 | Review/commentary |
| Cornes P, Coiffier B, Zambrowski J-J. Erythropoietic therapy for the treatment of anemia in patients with cancer: a valuable clinical and economic option. Curr Med Res Opin 2007;23:357–68 | Not a systematic review |
| Herrmann R. Erythropoietin therapy in cancer-related anaemia, yes or no? Intern Med J 2008;38:749–50 | Not a systematic review |
| Repetto L, Moeremans K, Annemans L. European guidelines for the management of chemotherapy-induced anaemia and health economic aspects of treatment. Cancer Treat Rev 2006;32:S5–9 | Not a systematic review |
| Stasi R, Amadori S, Littlewood TJ, Terzoli E, Newland AC, Provan D. Management of cancer-related anemia with erythropoietic agents: doubts, certainties, and concerns. Oncologist 2005;10:539–54 | Not a systematic review |
| Reichardt B. Evidence-based, novel comparison between epoetin alfa, epoetin beta, and darbepoetin alfa based on drug use, efficacy and treatment costs in daily oncological clinical practice. Hematol J 2004;5(Suppl. 2):177 | Cost study, not UK |
| Population | |
| Wadelin FR, Myers B. Darbepoetin is more cost-effective than regular transfusion: a review of the use of erythropoietin in haematology patients. 49th Annual Scientific Meeting of the British Society for Haematology, Brighton, April 2009. Br J Haematol 2009;145(Suppl. S1):58 | Results not presented separately for malignancy subgroup |
| Intervention | |
| Glaspy J, Tchekmedyian N, Gupta S. PCN17 comparing the cost-effectiveness of 3 mcg/kg Q2W darbepoetin alfa with standard dose epoetin alfa for anemia management in chemotherapy-treated cancer patients in united states. Value Health 2002;5:543 | Abstract; uses unlicensed dosing (once every 2 weeks) for darbepoetin alfa; published pre 2004 |
| Outcome | |
| Ben-Hamadi R, Duh MS, Aggarwal J, Henckler A, McKenzie S, Fastenau J, et al. Cost-effectiveness of once weekly epoetin alfa and darbepoetin alfa in treating chemotherapy-induced anemia. Value Health 2005;8:238 | Abstract; cannot calculate ICERs from reported data |
| Gozzo M, Lucioni C, Mazzi S. Economics evaluation of erythropoiesis-stimulating agents for the treatment of chemotherapy-induced anaemia in Italy. Eur J Hosp Pharm 2012;19:202 | Abstract; cannot calculate ICERs from reported data |
| No usable data | |
| Coiffier B, Schlag R, Velasco A, Yao B, Schupp M, Demarteau N, et al. Cost and effectiveness of darbepoetin alfa administered every 3 weeks (Q3W DA) compared with weekly epoetin alfa (QW EA) or epoetin beta (QW EB) in patients (PTS) with chemotherapy-induced anaemia (CIA): a retrospective study. Ann Oncol 2006;17:293 | Abstract |
| Grocott R, Metcalfe S, Moodie P. PHARMAC and erythropoietin for cancer patients. N Z Med J 2006;119:U2039 | Study not complete at time of publication |
| Published pre 2004 | |
| Cremieux P-Y, Finkelstein SN, Berndt ER, Crawford J, Slavin MB. Cost-effectiveness, quality-adjusted life-years and supportive care: recombinant human erythropoietin as a treatment of cancer-associated anaemia. Pharmacoeconomics 1999;16:459–72 | Included in Wilson and colleagues2 |
| Barosi G, Marchetti M, Liberato NL. Cost-effectiveness of recombinant human erythropoietin in the prevention of chemotherapy-induced anaemia. Br J Cancer 1998;78:781–7 | Included in Wilson and colleagues2 |
| Language (not English) | |
| Borget I, Chouaid C, Demarteau N, Annemans L, Pujol JL. Cost-effectiveness of darbepoetin alpha in an every-3-weeks schedule. Bull Cancer 2008;95:465–73 | French language |
| Danish Centre for Evaluation and Health Technology Assessment (DACEHTA). Epoetin (EPO) for Anaemic Cancer Patients. Copenhagen: DACEHTA; 2004. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32005000197/frame.html (accessed 19 August) | Danish language |
Appendix 16 Multiple publications in cost-effectiveness review
Primary study
Borget I, Tilleul P, Baud M, Joly AC, Daguenel A, Chouaid C. Routine once-weekly darbepoetin alfa administration is cost-effective in lung cancer patients with chemotherapy-induced anemia: a Markov analysis. Lung Cancer 2006;51:369–76.
Secondary publications
Chouaid C, Borget I, Baud M, Joly AC, Daguenel A, Tilleul P. Routine once-weekly darbepoetin alfa administration is cost-effective in lung cancer patients with chemotherapy-induced anemia: a Markov analysis. Lung Cancer 2003;49:S23.
Borget I, Tilleul P, Joly AC, Chouaid C. Incremental cost-effectiveness ratio of darbepoetin alfa (Aranesp (R)) in the treatment of chemotherapy-induced anemia in lung cancer patients. Value Health 2006;9:A278–9.
Borget I, Tilleul P, Baud M, Joly AC, Chouaid C. Routine once-weekly darbepoetin alfa administration is cost-effective in lung cancer patients with chemotherapy-induced anemia: a Markov analysis. Pharm World Sci 2007;29:454.
Primary study
Finek J, Holubec L, Wiesnerova A, Pav Z, Dusek L. Darbepoetin alfa versus epoetin alfa for treatment of chemotherapy-induced anemia: a health economic evaluation. Value Health 2010;13:A465.
Secondary publication
Finek J, Holubec L, Wiesnerova A, Pav Z, Dusek L. Darbepoetin alfa versus epoetin alfa for treatment of chemotherapy-induced anemia: a health economic evaluation. Ann Oncol 2010;21(Suppl. 8):344.
Primary study
Tonelli M, Lloyd A, Weibe N, Hemmelgarn B, Reiman T, Manns B, et al. Erythropoiesis-stimulating agents for anemia of cancer or of chemotherapy: systematic review and economic evaluation. HTA issue 119. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2009.
Secondary publication
Klarenbach S, Manns B, Reiman T, Reaume MN, Lee H, Lloyd A, et al. Economic evaluation of erythropoiesis-stimulating agents for anemia related to cancer. Cancer 2010;116:3224–32.
Appendix 17 Update of cost-effectiveness review
All searches were updated on 2 December 2013 and date limited from 1 January 2013 to 2 December 2013. Seventy-three records were obtained from the main database searches, resulting in 51 records following deduplication. Two additional records were obtained from DARE, resulting in a total of 53 records identified for title/abstract screening.
Independent, blinded screening was performed by two reviewers (TS and LC) and both reviewers included exactly one (and the same) study. The full text of this study was retrieved and assessed for eligibility by two reviewers (TS and NH), who both judged it to be eligible.
Data extraction was conducted by TS.
The included study by Michallet and colleagues294 describes itself as including a cost-effectiveness analysis, although on inspection it is a combined assessment of various effectiveness outcomes, as well as a cost analysis. As such, it would normally be considered a cost–consequences analysis.
The study by Michallet and colleagues294 is a historically controlled study matching patients receiving ESA therapy with those in the past known not to have received ESA therapy. Not all outcomes were recorded for the control group and so only transfusion requirement and survival (overall and event-free) are evaluated comparatively.
The study found that patients receiving ESA therapy experienced an improvement in HRQoL compared with baseline, but patients receiving ESA therapy were not compared with patients not receiving ESA therapy. The study found that patients receiving ESA therapy had a lower transfusion need; in addition, no statistically significant difference was found in OS or event-free survival between patients receiving ESA therapy and control patients. RBCT costs were lower for patients receiving ESA therapy, but these did not sufficiently offset the increased cost of ESA acquisition/administration.
The tables below show the characteristics, key parameters and results of the study.
| Parameter | Michallet and colleagues294 |
|---|---|
| Evaluation type | Cost–consequences analysis |
| Modelling used | No |
| Nature of modelling | NA |
| Perspective | Health care |
| Country (setting) | France |
| Intervention/comparator | Darbepoetin alfa QW: 150 µg; no treatment |
| Population | Patients with anaemia following consolidation chemotherapy for AML |
| Outcomes considered | HRQoL (FACT-G, FACT-F, FACT-An), Hb response (CR = Hb ≥ 12 g/dl; PR = Hb increase ≥ 2 g/dl), AEs, costs, Hb levels, transfusion need, survival (overall and event-free) |
| Time-frame | NA |
| Discounting | Not stated |
| Funding | Not disclosed |
| Parameter | Michallet and colleagues294 |
|---|---|
| Effectiveness (source): transfusion, response rate, survival, QALYs | Historically controlled study (this study) |
| Effectiveness (data): transfusion, response rate | Transfusion requirement – median reduction RBC units: 3.9 (p = 0.0002); median reduction platelet units: 1.7 (p = 0.029) |
| Effectiveness (data): survival | Not statistically significant (OS, p = 0.77; event-free survival, p = 0.57) |
| Effectiveness (data): QALYs | NA |
| QoL/utility (source) | This study |
| QoL/utility (data) | NA (not evaluated for control group) |
| Costs (source) | This study |
| Cost year | Not stated |
| Parameter | Michallet and colleagues294 |
|---|---|
| Measure | Costs, transfusion requirement, survival |
| Cost year; currency | NR; euros |
| Base case | ESA cost: darbepoetin alfa €3904, no treatment €0; RBCT cost: darbepoetin alfa €2568, no treatment €4280; total cost: darbepoetin alfa €6472, no treatment €4280 |
| Probabilistic results | NA |
| Sensitivity analyses | NA |
Appendix 18 Use of MathMap to construct cumulative hazard and Weibull plots
MathMap [freely available from www.complang.tuwien.ac.at/schani/mathmap/ (accessed 20 August 2015)] is a flexible tool and programming language for constructing and manipulating raster graphics with support for general mathematical transformations.
To construct cumulative hazard and Weibull plots we made use of functionality in which the result image, B, can be based on the input image, A, using an arbitrary mathematical backward mapping; that is, expressions of the form B(x, y) = A(f(x, y), g(x, y)).
The cumulative hazard graph plots ln(−S(t)) versus t and therefore the backward mapping functions are f(x, y) = x and g(x, y) = exp(−y).
The Weibull graph plots ln(−n(−S(t))) versus ln(t) and therefore the backward mapping functions are f(x, y) = exp(x) and g(x, y) = exp(−exp(y)).
The code for performing these mappings additionally must account for the dimensions of B and the location of the survival graph in A.
We show example code for transforming the survival plot from Littlewood and colleagues. 70 Note that ‘#’ is used to create a comment (non-functioning line) and has been used to ‘comment out’ a number of statements that would otherwise create different plots. The code as presented constructs the Weibull plot (time plotted from 1 to 40 and cumulative hazard plotted from 0.1 to 1.2).
Appendix 19 Summary of parameters used in the Peninsula Technology Assessment Group cost-effectiveness model
| Parameter | Base case (SE) | Subgroup inclusion Hb level ≤ 11.0 g/dl (SE) | Location in report | Wilson and colleagues’2 value | |
|---|---|---|---|---|---|
| OS ESA vs. control (HR, SE in log scale) | 0.967 (0.079) | 0.914 (0.137) | Chapter 3, Assessment of clinical effectiveness, Results, Effectiveness, Overall survival (Table 23) | 1 | |
| OS (control arm) | 2.670 (1.335) | 1.447 (0.723) | Chapter 5, Clinical effectiveness parameters, Overall survival | 1.54 | |
| Change in Hb from baseline to end of ESA treatment: difference between ESA arm and control arm | 1.59 (0.130) | 1.52 (0.115) | Chapter 3, Assessment of clinical effectiveness, Results, Effectiveness, Anaemia-related outcomes, Anaemia-related outcomes: overall summary (Table 20) | 1.63 (clinical effectiveness review) | |
| Mean no. of units transfused in control arm | 2.09 | 2.30 | Chapter 5, Clinical effectiveness parameters, Number of red blood cell transfusions | 2 | |
| Mean difference in no. of units of RBCs transfused between the ESA arm and the control arm | −0.87 (0.21) | −0.99 (0.22) | Chapter 3, Assessment of clinical effectiveness, Results, Effectiveness, Anaemia-related outcomes, Anaemia-related outcomes: overall summary (Table 20) | −1.05 | |
| Relative risk of AEs in the ESA arm vs. the control arm (reported on natural log scale) | |||||
| Thromboembolic events | ln(1.46) = 0.378 (0.158) | ln(1.29) = 0.255 (0.344) | Chapter 3, Assessment of clinical effectiveness, Results, Effectiveness, Safety, Thromboembolic events | ||
| Hypertension | ln(1.80) = 0.588 (0.234) | ln(1.68) = 0.519 (0.250) | Chapter 3, Assessment of clinical effectiveness, Results, Effectiveness, Safety, Hypertension | ||
| Thrombocytopenia | ln(0.93) = −0.073 (0.185) | ln(0.73) = −0.315 (0.350) | Chapter 3, Assessment of clinical effectiveness, Results, Effectiveness, Safety, Thrombocytopenia/haemorrhage | ||
| Probability of AEs in the control arm | 0%, but 5% for SAEs on epoetin | ||||
| Thrombotic events | 3.3 (0.4) | 3.7 (0.8) | Chapter 5, Clinical effectiveness parameters (Table 49) | ||
| Hypertension | 2.9 (0.5) | 1.8 (1.0) | |||
| Thrombocytopenia | 6.4 (0.8) | 2.5 (0.8) | |||
| Baseline Hb level (g/dl) | 10.38 (1.59) | 9.40 (0.22) | Chapter 5, Clinical effectiveness parameters, Initial (baseline) haemoglobin level | 9.9 (calculated using reported figures at baseline) | |
| Change in Hb level (no ESA) (g/dl) | −0.155 (1.25) | 0.469 (0.41) | Chapter 5, Clinical effectiveness parameters, Change in haemoglobin level for patients not receiving erythropoiesis-stimulating agent therapy | ||
| Mean difference in Hb levels between treatment arms over the trial duration as a proportion of the difference at the end of the trial (%) | 80.6 (55.0) | 55.5 (12.0) | Chapter 5, Clinical effectiveness parameters, Mean difference in haemoglobin levels between treatment arms as a proportion of the difference at the end of the trial | ||
| Mean age (years) | 59.1 (5.3) | 60.8 (4.2) | Chapter 5, Patient characteristics | Not used | |
| Mean weight (kg) | 66.6 (3.3) | 66.1 (3.6) | Not used | ||
| Probability patient is male | 0.46 | ||||
| Mean OS (no ESA) (years) | 2.670 (1.335) | 1.447 (0.724) | Chapter 5, Clinical effectiveness parameters, Overall survival | 1.54 | |
| Mean weekly ESA dose | |||||
| Epoetin alfa (IU) | 24,721 (4944) | 24,947 (4989) | Chapter 5, Clinical effectiveness parameters, erythropoiesis-stimulating agent withdrawal rate and mean weekly dose | ||
| Epoetin beta (IU) | 31,138 (6228) | 30,997 (6199) | |||
| Epoetin theta (IU) | 22,859 (4572) | 22,810 (4562) | |||
| Epoetin zeta (IU) | 24,721 (4944) | 24,947 (4989) | |||
| Darbepoetin alfa (µg) | 141.1 (28.2) | 141.2 (28.2) | |||
| No. of RBC units per transfusion | 2.7 (0.54) | Chapter 5, Clinical effectiveness parameters, Number of red blood cell transfusions | |||
| Duration of ESA treatment (weeks) | 12 | ||||
| Normalised Hb level (g/dl) | 12a (0.51) | Chapter 5, Clinical effectiveness parameters, Normalisation of haemoglobin levels following chemotherapy cessation | 24 | ||
| Normalisation rate (g/dl/week) | 0.2 (0.051) | 13 | |||
| Utility increase per Hb level increase (1g/dl) | 0.028 (0.006) | Chapter 5, Peninsula Technology Assessment Groupbase-case utilities by haemoglobin level | 0.2 (approx.) | ||
| Long-term utility | 0.763 (0.183) | 0.756 (0.151) | Chapter 5, Peninsula Technology Assessment Groupbase-case utilities after erythropoiesis-stimulating agent discontinuation | 0.06 | |
| ESA acquisition cost (£) | 276.70/week (including SAEs) | ||||
| Epoetin alfa (per 1000 IU) | Eprex | 5.53 | Chapter 5, Erythropoietin-stimulating agent prices | ||
| Binocrit | 5.09 | ||||
| Epoetin beta (per 1000 IU) | Neo Recormon | 7.01 | |||
| Epoetin theta (per 1000 IU) | Eporatio | 5.99 | |||
| Epoetin zeta (per 1000 IU) | Retacrit | 5.66 | |||
| Darbepoetin alfa (per µg) | Aranesp | 1.47 | |||
| Dosing schedule of ESA | Once weekly | Chapter 5, Cost of administering erythropoiesis-stimulating agents | Three times per week | ||
| Average cost per ESA administration (£) | 9.13 | 8.01 | |||
| Additional blood tests for ESA | 4 | Chapter 5, Additional blood tests for erythropoiesis-stimulating agents | |||
| Cost of blood test (£) | 15.14 | ||||
| Cost of AEs (£) | 101 | ||||
| Thrombotic events | 1243 (249) | Chapter 5, Adverse event costs | |||
| Hypertension | 826 (165) | ||||
| Thrombocytopenia | 744 (149) | ||||
| Cost per unit cost of RBCs transfused (£) | 127 (25) | Chapter 5, Red blood cell acquisition costs | |||
| Cost of transfusion appointment (£) | 688 | Chapter 5, Cost of transfusion appointment | |||
| Time frame | Lifetime | ||||
| Cycle length | NA | ||||
| HaemR RR (ESA vs. control) | NA | Chapter 5, Clinical effectiveness parameters | |||
Appendix 20 Mean difference in haemoglobin level as a proportion of the final difference in haemoglobin level
The PenTAG economic model uses a parameter corresponding to the mean difference in Hb level as a proportion of the final difference in Hb level. The final difference in Hb level is a commonly reported outcome, but cumulative differences (which incorporate information about Hb levels over time between measurement of baseline and final Hb levels) are not generally reported succinctly. In some studies figures are presented showing the trajectory of Hb levels.
When this parameter is set to 100%, the average difference in Hb level between the intervention arm and the control arm over time is the same as the final difference in Hb level (adjusting for any differences at baseline).
Two calculation methods were applied to estimate this parameter, with the easiest method to apply being used for each figure.
Method 1
Measuring tools of Adobe Acrobat X Pro (Adobe Systems, Inc., San Jose, CA, USA) were used to estimate:
-
the area bounded above by the intervention arm Hb level curve and below by the control arm Hb level curve (denoted by A)
-
the (vertical) distance between the intervention arm Hb level curve and the control arm Hb level curve at baseline (denoted by L0; positive if baseline Hb is higher in the intervention arm)
-
the (vertical) distance between the intervention arm Hb level curve and the control arm Hb level curve at the final Hb level measurement (denoted by L1; positive if final Hb is higher in the intervention arm)
-
the (horizontal) distance between the times of the baseline and final Hb level measurements (denoted by W).
The required parameter is then calculated as (A – L0 × W)/[W × (L1 – L0)].
Method 2
An appropriate tool was used to estimate the mean Hb level at each measurement for both the intervention arm and the control arm.
The area under each Hb level curve was calculated by summing the areas of trapezoids (denoted by AUCIntervention and AUCControl). These were adjusted to become area under Hb change curves by subtracting the hypothetical area under the curve if the Hb level did not change (denoted ΔAUCIntervention and ΔAUCControl).
The hypothetical area under the curve if the Hb level instantaneously jumped to the final Hb level difference was calculated (denoted ΔAUCInstantaneous).
The required parameter is then calculated as (ΔAUCIntervention – ΔAUCControl)/ΔAUCInstantaneous.
Results
| Study | Calculation steps | Result (%) | |||
|---|---|---|---|---|---|
| A | L 0 | L 1 | W | ||
| Method 1 | |||||
| Littlewood 201170 | 1.00 | 0.05 | 0.38 | 2.42 | 110 |
| Grote 200574 | 2.39 | −0.09 | 0.24 | 3.53 | 232 |
| Tjulandin 201048 | ET 1.66 EB 1.79 |
ET 0.00 EB 0.00 |
ET 0.69 EB 0.78 |
ET 3.85 EB 3.85 |
ET 62 EB 60 |
| Tjulandin 201177 | 2.11 | 0.11 | 1.10 | 3.47 | 50 |
| Moebus 201362 | 0.69 | 0.00 | 0.39 | 2.29 | 77 |
| ΔAUCIntervention | ΔAUCControl | ΔAUCInstantaneous | |||
| Method 2 | |||||
| Silvestris 199572 | 46.15 | 3.04 | 51.08 | 84 | |
| Del Mastro 199767 | 0.70 | −9.35 | 13.80 | 73 | |
| Kurz 199769 | 20.32 | 2.26 | 36.12 | 50 | |
| Dunphy 199968 | −5.35 | −13.00 | 9.92 | 77 | |
| Thatcher 199952 | −6.37 | −11.03 | 5.04 | 92 | |
| Dammacco 200166 | 12.37 | −0.04 | 22.15 | 56 | |
| Hedenus 200253 | 9.43 | 4.11 | 9.04 | 59 | |
| Aravantinos 200364 | 4.245 | 3.235 | 4.32 | 23 | |
| Boogaerts 200365 | 15.98 | 7.02 | 13.12 | 68 | |
| Strauss 200876 | 11.80 | −6.40 | 24.00 | 76 | |
List of abbreviations
- AE
- adverse event
- ASCO
- American Society of Clinical Oncology
- ASH
- American Society of Hematology
- CDSR
- Cochrane Database of Systematic Reviews
- CEAC
- cost-effectiveness acceptability curve
- CEAF
- cost-effectiveness acceptability frontier
- CENTRAL
- Cochrane Central Register of Controlled Trials
- cHR
- combined hazard ratio
- CI
- confidence interval
- CIA
- cancer treatment-induced anaemia
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CKD
- chronic kidney disease
- CrI
- credible interval
- DARE
- Database of Abstracts of Reviews of Effects
- df
- degrees of freedom
- EMA
- European Medicines Agency
- EORTC
- European Organisation for Research and Treatment of Cancer
- EORTC QLQ-C30
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30
- EQ-5D
- European Quality of Life-5 Dimensions
- ESA
- erythropoiesis-stimulating agent
- FACIT
- Functional Assessment of Chronic Illness Therapy
- FACT
- Functional Assessment of Cancer Therapy
- FACT-An
- Functional Assessment of Cancer Therapy – Anaemia
- FACT-An-An
- Functional Assessment of Cancer Therapy – Anaemia Anaemia subscale
- FACT-F
- Functional Assessment of Cancer Therapy – Fatigue
- FACT-G
- Functional Assessment of Cancer Therapy – General
- FDA
- Food and Drug Administration
- G-CSF
- granulocyte colony-stimulating factor
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- Hb
- haemoglobin
- HMIC
- Health Management Information Consortium
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- INHB
- incremental net health benefit
- IPD
- individual patient data
- ITT
- intention to treat
- LASA
- Linear Analogue Scale Assessment
- MeSH
- medical subject heading
- MTA
- multiple technology appraisal
- NA
- not applicable
- NHP
- Nottingham Health Profile
- NHSBT
- NHS Blood and Transplant
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- OS
- overall survival
- PDI
- Psychological Distress Inventory
- PenTAG
- Peninsula Technology Assessment Group
- PFS
- progression-free survival
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RBC
- red blood cell
- RBCT
- red blood cell transfusion
- RCT
- randomised controlled trial
- rHuEPO
- recombinant human erythropoietin
- RR
- risk ratio
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SPC
- Summary of Product Characteristics
- TA
- technology appraisal
- VAS
- visual analogue scale
- WHO
- World Health Organization
- WMD
- weighted mean difference
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed commercial-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence data removed and replaced by the statement ‘commercial-in-confidence information removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.