Notes
Article history
This issue of Health Technology Assessment contains a project originally commissioned by the MRC but managed by the Efficacy and Mechanism Evaluation Programme. The EME programme was created as part of the National Institute for Health Research (NIHR) and the Medical Research Council (MRC) coordinated strategy for clinical trials. The EME programme is funded by the MRC and NIHR, with contributions from the CSO in Scotland and NISCHR in Wales and the HSC R&D, Public Health Agency in Northern Ireland. It is managed by the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC) based at the University of Southampton.
The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from the material published in this report.
Declared competing interests of authors
David A Richards receives funding support from the National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care. Simon Gilbody was a NIHR Health Technology Assessment Clinical Evaluation and Trials Board member during the conduct of this study (tenure 23 June 2008 to 30 September 2014). Glyn Lewis is currently a NIHR Efficacy and Mechanism Evaluation Board member. Peter Bower reports personal fees from the British Association for Counselling & Psychotherapy, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Richards et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
This chapter utilises material from three1–3 of the four Open Access articles previously published by the research team in accordance with the terms of the Creative Commons Attribution licences (CC BY 2.0, CC BY 3.0 and CC BY 4.0).
Depression
Depression results in substantial disability and is recognised as a major health problem; it is currently the second largest cause of global disability. 4 Around 350 million people are impacted by depression across the world and each year up to 5.8% of men and 9.5% of women will suffer from an episode of depression. 5 Depression has a very significant impact on physical health, occupational functioning and the social lives of sufferers. 6 Often anxiety is also present, causing further disability. 7
Depression is the acknowledged reason for two-thirds of all suicides. 8 The nature of depression is that it can frequently be chronic, with regular bouts of relapse and subsequent new episodes. After one depressive episode, around 50% of people will experience additional episodes. The risk of subsequent relapse is 70% after a second bout of depression and as much as 90% following three or more episodes. 9 Among other diagnostic criteria, depressive symptomatology incorporates depressed mood, loss of interest or pleasure in activities, insomnia or sleeping too much and fatigue or loss of energy. 10
Although effective pharmacological and psychological treatments for depression are available, people are often treated with a less than optimal programme. Internationally, there is often poor patient adherence to pharmacological treatment11 and further problems caused by organisational barriers between generalists and specialist mental health professionals. 12,13 There is often very limited support for primary care doctors when treating participants with both psychosocial interventions and pharmacological methods. Such support may be critical given that, in systems such as that in the UK and elsewhere, the general practitioner (GP) is the sole responsible medical clinician for 90–95% of patients. 14
Collaborative care
In a previous systematic review of 36 studies testing organisational interventions,15 it was concluded that guidelines, practitioner education and other simple interventions were not effective in studies attempting to improve the management of depression. However, there is better evidence for the role of organisational interventions in improving the management of a range of chronic conditions generally. Organisational strategies have been used in the management of depression, including ‘collaborative care’. This is a complex intervention developed in the USA that has been supported by previous reviews. Collaborative care has been identified as the most effective of the range of organisational approaches studied. 15–20
Collaborative care incorporates a multiprofessional approach to patient care; a structured management plan; scheduled patient follow-ups; and enhanced interprofessional communication. 21 In practice, this is achieved by the introduction of a care manager into primary care, responsible for delivering care to depressed patients under supervision from a professionally qualified mental health specialist and for liaising between primary care clinicians and specialists. Care management has been described as a health worker taking responsibility for proactively following up a patient, assessing patient adherence to psychological and pharmacological treatments, monitoring patient progress, taking action when treatment is unsuccessful and delivering psychological support. 18 Care managers work closely with the primary care provider (who retains overall clinical responsibility) and can receive regular supervision from a mental health specialist. 15,22 The specific disciplines vary by country context but can include counsellors, paraprofessionals or nurses as care managers, and psychiatrists, psychologists and mental health nurses acting in the specialist role.
Systematic reviews23,24 demonstrate that collaborative care improves depression outcomes, with some studies showing benefit for up to 5 years. Before developing the CollAborative DEpression Trial (CADET), our 2006 systematic review24 of 28 collaborative care studies showed collaborative care to be effective [standardised mean difference (SMD) –0.24, 95% confidence interval (CI) –0.17 to –0.32]. The I2 estimates of inconsistency were 80% for antidepressant use and 54% for depressive outcomes. In metaregression analyses three intervention content variables predicted improvement in depressive symptoms, recruitment by systematic identification (p = 0.061), care managers having a specific mental health background (p = 0.004) and provision of regular supervision for care managers (p = 0.033), which reduced the overall heterogeneity (I2) from 54% to 48% for systematic identification, 43% for case manager background and 49% for supervision.
More recently (after the initiation of the CADET trial) we have undertaken a Cochrane review. 23 Our new analyses show greater improvement in depression outcomes for adults with depression treated with the collaborative care model compared with usual care in the short term (0–6 months) [SMD –0.34, 95% CI –0.41 to –0.27; relative risk (RR) 1.32, 95% CI 1.22 to 1.43], medium term (7–12 months) (SMD –0.28, 95% CI –0.41 to –0.15; RR 1.31, 95% CI 1.17 to 1.48) and long term (13–24 months) (SMD –0.35, 95% CI –0.46 to –0.24; RR 1.29, 95% CI 1.18 to 1.41). However, these significant benefits were not demonstrated into the very long term (≥ 25 months) (RR 1.12, 95% CI 0.98 to 1.27). In metaregression of this significantly larger study data set (n = 79) collaborative care that included psychological interventions predicted improvement in depression (beta-coefficient 20.11, 95% CI 20.01 to 20.20; p = 0.03). These new data include the results of our CADET trial along with another nine UK studies and a greatly expanded study data set. We include them here for completeness and refer to them further in the discussion.
In 2008, at the commencement of the CADET trial, collaborative care had generally been developed and tested in the USA within managed health-care settings. It is possible that the overall effectiveness of collaborative care programmes might vary when it is implemented and evaluated in non-US settings. In other areas of mental health care results from US-developed organisational interventions have not generalised outside the original health-care context. 25 For collaborative care, there was some supportive evidence from other contexts, including the developing world,26,27 but prior to the CADET trial there has been uncertainty around the standardised effect size in UK trials (SMD 0.24, 95% CI –0.060 to 0.547) and elsewhere. 24 These limited non-US data and the relatively small effect size in trials of patients with depression alone led the UK National Institute for Health and Care Excellence (NICE)28 to issue a research recommendation that ‘The efficacy of organisational interventions, such as chronic disease management programmes or other programmes of enhanced care for depression, should be tested in large-scale multicentre trials in the NHS’ (research recommendation 5.6.8.1, p. 103). This provided us with the rationale to undertake a fully powered UK evaluation of collaborative care.
Development of the CollAborative DEpression Trial
In published studies there is considerable between-study heterogeneity in terms of the duration and intensity of collaborative care and the training and background of care managers. Therefore, to investigate the clinical effectiveness and cost-effectiveness of collaborative care in the UK, we conducted a series of Medical Research Council (MRC)-funded preparatory studies. We wished to develop an intervention in anticipation of a fully powered randomised controlled trial. We carefully developed our collaborative care intervention to be applicable outside the USA, in health-care systems with a well-developed primary care sector. 29–31
In our Phase II testing of this intervention,30 we found preliminary evidence that collaborative care adapted to the UK was acceptable to patients and clinicians and may be effective outside the USA, but that a cluster randomised controlled trial was required to guard against potential contamination between trial arms. We amended the clinical protocol studied in our pilot trial to take account of acceptability data in our qualitative interviews and designed a cluster randomised controlled trial of sufficient power to detect clinically meaningful and achievable differences between collaborative care and usual care. We now report the results of this pragmatic cluster randomised controlled trial1 to determine whether or not collaborative care is more clinically effective and cost-effective than usual care in the management of patients with moderate to severe depression. This report is divided into chapters detailing the methods and results of our primary clinical effectiveness and cost-effectiveness questions followed by similar chapters for our process evaluation. We have undertaken an additional long-term follow-up of clinical outcomes and report this in a separate chapter. Finally, we conclude with a discussion chapter summarising our results and considering their implications for the management of depression.
Chapter 2 Trial methods
This chapter utilises material from three1–3 of the four Open Access articles previously published by the research team in accordance with the terms of the Creative Commons Attribution licences (CC BY 2.0, CC BY 3.0 and CC BY 4.0).
Research question
Is collaborative care more clinically effective and cost-effective than usual care in the management of participants with moderate to severe depression in UK primary care?
Study design
The CADET trial was a multicentre, two-group, cluster randomised controlled trial with allocation of general practice clusters to two trial arms: collaborative care (experimental group) or usual care (GP management). We chose a cluster design given that our Phase II trial30 described in the previous chapter demonstrated that a participant-randomised trial of collaborative care could be vulnerable to contamination and open to type II error, underestimating the true effect size of the intervention through potential intervention ‘leakage’.
Patient and public involvement
We involved patient and public representatives at all stages of the project. A patient and public involvement (PPI) advisor (CM) was a trial applicant, investigator and full member of the Trial Management Group (TMG). He attended all meetings of the TMG and advised on patient-facing materials including ethics materials and participant therapeutic manuals and on the conduct of the trial including project management, questionnaire development, data collection and project dissemination. There were two PPI representatives on the Trial Steering Committee (TSC), one from a depression consumer advocacy group and another with lived experience of depression. Both provided important checks and balances as part of the independent TSC oversight of the trial.
In addition, the trial was initially co-ordinated from the Mood Disorders Centre at the University of Exeter and latterly from the University of Exeter’s Medical School. Both the Mood Disorders Centre and the Medical School operate within a culture of PPI, guided by published theories of participation, empowerment and engagement, through the Mood Disorders Centre’s 20-strong Lived Experience Group and the National Institute for Health Research (NIHR) CLAHRC (Collaboration for Leadership in Applied Health Research and Care) for the South West’s patient involvement group PenPIG (Peninsula Public Involvement Group).
Setting and participants
We recruited participants between June 2009 and January 2011 from the electronic case records of primary care general practices in three UK sites: Bristol, London and Greater Manchester.
Inclusion criteria
Our eligibility criteria were as follows:
-
Adults aged ≥ 18 years meeting International Classification of Diseases, Tenth Edition (ICD-10) criteria for a depressive episode. 32 Diagnosis was determined by research personnel interviewing potential participants using the Clinical Interview Schedule – Revised (CIS-R),33 a computerised interview schedule that establishes the nature and severity of neurotic symptoms and identifies a categorical diagnosis of mild, moderate or severe depression.
-
Newly identified as depressed, including those with or without previous episodes; in treatment for an existing diagnosis of depression but not responding; suffering from peri- or postnatal depression; or suffering with comorbid physical illness or comorbid psychological disorders such as anxiety.
-
People were eligible to participate whether or not they were in receipt of antidepressant medication in line with the pragmatic nature of this trial and to reflect usual primary care management of depression in the UK.
Exclusion criteria
We excluded people for whom there was a sufficiently severe risk of suicide that they required immediate specialist mental health crisis management; those with type I and type II bipolar disorder; those with psychosis; those with depression that was associated with a recent bereavement; those with an alcohol or drug abuse primary presenting problem; and those who, at the time of interview, were receiving specialist mental health treatment for their depression, including psychotherapy.
Randomisation
We randomly allocated primary care practices to either collaborative care or treatment as usual as they were recruited into the trial. We minimised randomisation within our three sites using the Index of Multiple Deprivation (IMD) rank,34 the number of GPs and practice size.
Allocation concealment
We concealed the allocation sequence from the researchers as they recruited practices by ensuring that researchers were unaware of prior allocations or the allocation sequence. Randomisation was undertaken by the trial statistician using Minim (www.sghms.ac.uk/depts/phs/guide/randser.htm). 35 We managed participant identification and the trial databases through our partnership with the Peninsula Clinical Trials Unit (PenCTU), who undertook these functions remotely from the trial team and trial statistician.
Blinding
In this type of trial, in which interventions are complex and clearly different from each other, it is not possible to blind participants, care managers or GPs and so our procedures focused on helping to keep research workers blind to participant allocation and protecting the study against assessment interpretation bias through the use of self-report measures. Our research workers were blinded to practice allocation. To help control for the effect of any potential unblinding after research workers assessed and confirmed that people were eligible for the trial, they then collected participant outcomes as self-report measures.
After assessments had been completed, research workers recorded participants’ data on a remote, web-based system. This database, administered by PenCTU, allocated each participant an identification number and, if in a collaborative care cluster practice, automatically advised the relevant care manager to contact the participant. The system also automatically communicated with each participant’s GP by letter.
Recruitment
Potential participants were identified by clinical studies officers (CSOs) or practice staff from July 2009 to January 2011. These workers searched the computerised records of participating practices over a 19-month period, looking for records of people with at least one identification code for depression recorded against their name by their GP in the previous 4 weeks. We searched for those codes most widely used by GPs to classify participants as depressed.
The lists of people generated by the searches were screened by GPs to remove the names of anyone whom GPs knew would not meet our inclusion criteria or who would be excluded at interview. Staff then sought permission from potentially eligible people for researchers to contact them. Potentially eligible people were sent an information sheet and reply slip in the post. Practice staff or CSOs followed up this letter by telephone after 1 week.
Those who gave research interview consent were contacted by a researcher trained in the specific interview procedures by study investigators and an interview was organised at the convenience of the participant. Interviews took place either in their home or at their GP practice no earlier than 48 hours after they had received the trial information letter. The first part of the research interview consisted of the researcher outlining the trial in detail and answering questions. Once the potential participants were fully briefed and willing to enter the trial, researchers asked them to fill in and sign the trial consent form, following which the diagnostic component of the baseline interview was undertaken.
If the diagnostic component of the baseline interview – the CIS-R33 – confirmed that a potentially eligible person met our depression diagnostic inclusion criteria, he or she was included as a participant in the trial, the research interview proceeded and a full baseline data set was taken.
Intervention and comparator groups
Intervention: collaborative care
Our experimental intervention was collaborative care. As detailed earlier, the specific components of the intervention had been developed, tested and amended in our earlier trial and process evaluation. 30 Care managers in three UK sites provided the intervention under supervision from specialists in mental health care. A copy of the complete clinical protocol is provided in Appendix 1. A summary of the collaborative care protocol is given in the following sections.
Care management
All participants received usual care from their GP. Collaborative care consisted of 6–12 contacts between the care manager and the trial participant, with contacts spanning a period of no more than 14 weeks. The initial appointment was of 30–40 minutes’ duration and was conducted in a face-to-face manner. Subsequent appointments were undertaken on the telephone and were of 15–20 minutes’ duration. Although most follow-on appointments were by telephone, care managers could arrange to meet the participant face-to-face if either party thought that this was desirable. Routinely, however, the telephone was the preferred contact medium for the majority of follow-on appointments.
Although the frequency of contacts was determined by a participant’s needs, in our protocol we suggested that contacts should be undertaken weekly during the first month or so of care management. We recommended that fortnightly appointments could be arranged after this. Once again, we designed our protocol to be sufficiently flexible to permit more frequent sessions if either party regarded this as important, given the progress of the participant or his or her clinical presentation. We recommended short frequent sessions to care managers as opposed to lengthy appointments on a less frequent basis. We asked care managers to be flexible with appointment schedules to permit sessions to be delivered outside usual 0900–1800 working hours, but our expectation was that the majority of sessions would occur during these hours. We advised care managers to try many times to contact participants if they did not manage to get through on the telephone at first. This is an important component of collaborative care protocols worldwide, because many people with depression avoid contact with other people because of their mood state, with social avoidance being a common symptom.
During appointments, care managers would:
-
assess participants’ views about psychological and medication treatments
-
negotiate a treatment programme that was acceptable to participants
-
help participants with their management of any prescribed antidepressants
-
support participants to use behavioural activation, a brief low-intensity psychosocial treatment for depression
-
provide advice on relapse prevention.
Symptom assessment
Care managers conducted a symptom assessment every time they had a session with a participant, whether face to face or by telephone. They used the Hospital Anxiety and Depression Scale (HADS)36 to evaluate and record common symptoms of depression and then engaged participants in a discussion regarding these symptoms. We chose the HADS so as not to use the same measure [the Patient Health Questionnaire-9 (PHQ-9)37] as our primary research outcome measure. Care managers also conducted a risk assessment to assess the level of risk to self and others for each participant. These assessments were undertaken at the beginning of each appointment.
Medication management
We instructed care managers to help participants engage appropriately with any medicines that they had been prescribed for their depression. Each participant’s GP remained the responsible medical practitioner in terms of medication prescription but the care manager helped participants understand the reason for their prescription, reinforced information from their GP and problem solved any difficulties that participants had in tolerating their medicines.
Behavioural activation
Behavioural activation is a psychological treatment with good evidence that it is as effective as cognitive–behavioural therapy in depression. 38 Behavioural activation is a brief psychological treatment that helps people interrupt patterns of avoidance that maintain depression. Behavioural activation assists people to increase their levels of activity to help them experience more examples of situations likely to lead to a positive mood. Behavioural activation was suitable for care managers to use given its simplicity and brevity and had been tested previously in our pilot work. We provided participants with support information prepared by the trial team. In summary, participants were supported through a self-guided behavioural activation treatment programme that helped them to increase the frequency and range of activities in their day-to-day lives.
Communicating with general practitioners
We outlined three levels of care manager contact with GPs:
-
Level 1. Care managers communicated a brief statement of the participant’s main problem and treatment plan to the GP after the first treatment session, using a structure outlined in our protocol. If participants were progressing satisfactorily and/or willing to engage in the treatment plan, routine records of each contact were recorded.
-
Level 2. If participants were not progressing satisfactorily, or they wanted to change their pharmacological treatment regime, the case manager could alert their GP as required. In these instances care managers could inform the GP about changes that may need to be made to the treatment plan. Care managers could also let the GP know if a participant had been advised to make an appointment with his or her GP.
-
Level 3. We instructed care managers to contact GPs in person directly or by telephone should there be an urgent need to do so. Circumstances requiring level 3 contact included a participant experiencing intolerable medication side effects, a substantial worsening in a participant’s mental health or a participant being at acute risk to self or others.
Care managers
Care managers were existing NHS mental health workers working in a primary care environment. They had been previously trained as paraprofessional mental health workers. They continued to treat patients in their existing NHS role, undertaking care management of CADET participants alongside their NHS caseload. Care managers were supervised each week by specialist mental health workers including clinical psychologists, psychiatrists, academic GPs with a special interest in mental health or senior nurse psychotherapists. Every CADET participant was discussed at least once a month. Discussions were organised using a bespoke computerised patient case management information system [PC-MIS (see www.pc-mis.co.uk; accessed 17 November 2015)]. PC-MIS includes automated alerts so that supervisors and care managers are informed of their supervision discussion schedules automatically, including algorithms driven by routine outcome measures that identify participants not responding to treatment.
Specialist mental health worker members of the investigator team trained care managers using a 5-day collaborative care instruction programme. The training consisted of protocol instruction, modelling and treatment session role play. All components of the collaborative care protocol were included in the programme. This included the initial contact, subsequent appointments, telephone working, GP liaison and supervision. The training instructed care managers on both specific case management skills such as participant education, medication support and behavioural activation and non-specific factors necessary to develop therapeutic engagement.
Each care manager received a handbook to accompany the training programme (see Appendix 1). The handbook included a collaborative care management session-by-session guide and participant information materials on depression, medication and behavioural activation. The handbook contained all of the worksheets and diaries that care managers were to use in supporting participants with their collaborative care activity programme. It also included information on, and examples of, how care managers should communicate with GPs and provided worksheets to help them prepare case materials for supervision discussions.
Supervision
Specialist mental health professionals – psychiatrists and psychological therapists (RA, JC, LG, DK, KL and SP) – supervised the care managers. Supervisors helped and supported care managers through discussion with them about participant progress. Care managers discussed participant symptom levels, treatment plans and their own care management activities. In this, they were prompted by alerts on PC-MIS. In addition to routinely triggered discussions they were also able to bring any problems experienced in managing specific cases to supervision. Supervisors assisted care managers with any communications that they needed to have with GPs, for example communicating medication advice for individual participants. Supervisors undertook sessions with care managers over the telephone, either on an individual basis or in groups on a weekly basis. At each supervision session the following cases were reviewed:
-
all new participants
-
participants who had reached a scheduled supervision review point after being in the trial for 4, 8 or 12 weeks
-
participants who were not improving as expected, for example when an adequate trial of antidepressant medication was not having a therapeutic effect or when participants were not benefiting from or engaging in the behavioural activation programme
-
overdue participants; that is, when the care manager had not been able to make contact with a participant as previously arranged
-
any other participants requiring discussion and/or an overview of current caseloads.
Supervision was principally informed by ratings collected from participants using the HADS, assessments of participant risk to self and others, details of participant concordance with treatment plans and discussions of care management strategies. Supervision focused care managers’ attention on their overall decision-making for individual participants while also helping them practice care management principles for their whole caseload.
Control condition: usual care
General practitioners provided control participants with care that reflected their standard practice. For control participants this included antidepressant therapy and/or referral to specialist mental health care. Given that the CADET trial was a pragmatic trial, we did not specify any clinical protocols for usual care. However, we did measure the components of usual care received by participants.
Outcomes
Primary clinical outcome
The primary clinical outcome was depression severity at 4 months’ follow-up using the PHQ-9. 37
Secondary clinical outcomes
Secondary outcomes were the PHQ-9 at 12 months, quality of life [Short Form questionnaire-36 items (SF-36)39], worry and anxiety [General Anxiety Disorder-7 (GAD-7)40] at 4 and 12 months, health state values (health-related quality of life) [European Quality of Life-5 Dimensions three-level version (EQ-5D-3L)41,42] at 4 and 12 months and participant satisfaction [Client Satisfaction Questionnaire-8 (CSQ-8)43] at 4 months.
Demographic data on sex, age, ethnic origin, education level, employment, marital status, presence or absence of antidepressant treatment, previous history of depression, severity of depression, any secondary diagnoses of an anxiety disorder and any participant self-reported long-standing physical illness were also collected at baseline.
Economic outcomes
The health economic end point was the cost per quality-adjusted life-year (QALY) at 12 months. We used the recommended area under the curve approach for assessing repeated measures. 44,45 Resource use pertaining to the collaborative care intervention as delivered by care managers and supervisors in the trial was collected directly from our trial and case records. These data included care manager and specialist supervision contact time. Participant-level health and social care resource use data, information on informal care from friends/relatives and other participant costs (e.g. over-the-counter costs, one-off participant costs) were collected at the 4-month and 12-month follow-up points using a self-report format with assistance from interviewers. These same data were collected at baseline using the same self-report data collection approach, asking participants to report their resource use during the 6-month period prior to the baseline assessment. Given the difficulties in collecting data on medication use using the self-report format we did not include medication use in the estimates of health and social care resource use. However, data are reported on the proportion of participants on antidepressant medications at baseline and 12 months’ follow-up.
Although all baseline interview data were collected during a face-to-face interview, we were more flexible with our follow-up assessments. We used face-to-face, telephone or postal methods of data collection in accordance with participants’ wishes and to maximise data collection. For resource use data we used face-to-face or telephone methods of data collection, all using an interviewer-assisted self-report format.
Sample size
The CADET trial was powered at 90% (alpha = 0.05) to detect an effect size of 0.4. This effect size is regarded as a clinically meaningful difference between interventions of this type. 46 The proposed effect size was also within the 95% CI of the effect that we had predicted following our analysis of our feasibility and pilot study. 30 This study had shown a potential effect size of 0.63 with a 95% CI from 0.18 to 1.07. However, an effect size of 0.4 is greater than that in the meta-analysis results of trials published at the time that we were commissioned to undertake the CADET trial (effect size 0.25, 95% CI 0.18 to 0.32). 24
In a two-arm randomised controlled trial in which participants are the unit of randomisation, we would have required 132 participants per group to detect a difference of 0.4 between groups. However, cluster randomisation produces a design effect and inflates the required sample size. In our trial, we planned for 12 participants in each primary care cluster and we used data from our pilot study30 to estimate the intracluster correlation (ICC) as 0.06. The design effect was calculated as 1.65 and we estimated our required sample size without any attrition to be 440. We estimated that the CADET trial might suffer from around 20% participant attrition at our primary end point. In this scenario, to be able to follow up 440 participants, we planned to randomly allocate 550 participants across the trial arms. To deliver this target we decided to recruit 48 practices with up to 14 participants per practice, given that our recruitment rate would not be exactly even between practices.
Statistical methods and analyses
Clinical outcomes
All of our analyses for all of our outcomes used the intention-to-treat principle. We reported all outcomes according to Consolidated Standards of Reporting Trials (CONSORT) guidelines. 47 We analysed all data in Stata 10.1 (StataCorp LP, College Station, TX, USA). We wrote, and agreed with the TSC, an a priori analysis plan. We used ordinary least squares or logistic regression, allowing for clustering by use of robust standard errors, to analyse outcomes at 4 and 12 months. We adjusted at the cluster level for minimisation variables and site and at the individual level for age and, when appropriate, the baseline measurement of the variable. We undertook sensitivity analyses to assess the effect of missing data. We estimated this by chained regression equations multiple imputation48 using all available scale clinical scores, age, sex, practice variables, site and treatment group.
Standardised effect sizes for our outcome variables were calculated by taking the mean difference between the intervention group and the control group and dividing the difference by the pooled standard deviation (SD). We also calculated the degree of clustering within our participant clusters by GP practice. We have reported these as ICC coefficients.
We wanted to ensure that our results could be easily interpreted from a clinical perspective and compared with existing published studies. Therefore, using the baseline SD for all participants, we calculated rates of ‘recovery’ and ‘response’. These commonly used metrics can help service commissioners, managers, clinicians and patients translate continuous outcome variables into a meaningful clinical figure. The rate of recovery can be regarded as the proportion of participants with a PHQ-9 score of ≤ 9 at the end of the trial whereas the response rate can be regarded as a ≥ 50% reduction in scores from baseline. Finally, to further aid interpretation the numbers needed to treat were deduced from the inverse of the absolute risk reduction adjusted for clustering by practice.
Economic outcomes
In our economic evaluation we undertook our economic analyses from the UK NHS and Personal Social Services perspective (third-party payer perspective). We also undertook a sensitivity analysis using the broader participant and carer perspective. We estimated the costs associated with health and social care service use and the additional cost of the delivery of the collaborative care intervention and estimated QALYs.
Data on resource use were combined with published unit costs to estimate the mean cost per participant. We used nationally available data sources, in UK pounds sterling at 2011 costs (Table 1), adjusted for inflation when necessary, to compute health-care resource values from unit costs. To estimate the intervention cost for collaborative care, we based our calculations on costs for UK NHS Agenda for Change (AfC) Band 5 staff. We chose a unit cost of £65 per hour for patient contact time,49 a rate equivalent to that for a qualified mental health nurse. All staff cost components are included in this unit cost, for example telephone and travel time, including an allowance of contact time to non-contact time of 1 : 0.89. 49 Supervision costs were calculated by selecting the full costs for specialist mental health professionals at NHS AfC Band 8a49 from a unit cost of £135 per hour for clinical supervisors. QALYs were estimated over the 12-month follow-up period using the EQ-5D trial data, applying UK tariffs obtained from a UK general population survey to value the EQ-5D health states. 53
| Resource item | Unit cost (£)a | Source | Basis of estimate |
|---|---|---|---|
| GP (surgery/practice) | 36.00 | Curtis49 | GP appointment/surgery; based on costing at 11.7 minutes |
| GP (home) | 121.00 | Curtis49 | |
| Practice nurse (surgery) | 15.00 | Curtis49 | Assuming average contact time of 15.5 minutes and using hourly rate for nurse contact time |
| Practice nurse (home) | 30.00 | Curtis49 | Assuming average contact time of 25 minutes and using hourly rate for nurse contact time |
| Walk-in centre (appointment) | 41.00 | Curtis49 | Walk-in service (not admitted) |
| Counsellor | 60.00 | Curtis49 | Per consultation |
| Mental health worker | 76.00 | Curtis49 | Mental health nurse, £76 per 1-hour contact (assumed 1 hour) |
| Social worker/care manager | 212.00 | Curtis49 | Per 1-hour contact (assumed 1 hour) |
| Home help/care worker | 18.00 | Curtis49 | Per weekday hour |
| Occupational therapist | 82.00 | Curtis49 | Community-based occupational therapist per 1 hour of client contact (assumed 1 hour) |
| Voluntary group (e.g. Mind) | 21.73 | Curtis50 | Cost per user session, voluntary/non-profit organisation (£21 per session in 2010) |
| Acute psychiatric ward (bed-day) | 312.00 | Curtis49 | Cost per bed-day |
| Long-stay ward (bed-day) | 222.52 | Curtis50 | Cost per bed-day (£215 in 2010) |
| General medical ward (bed-day) | 321.00 | Curtis49 | Weighted average of all adult mental health inpatient days |
| Accident and emergency (contact) | 106.00 | Curtis49 | Contact, not admitted |
| Day hospital (day) | 126.00 | Curtis49 | Cost per day, weighted average of all adult attendances |
| Psychiatrist (outpatient contact) | 161.38 | bDepartment of Health51 | 2008–9 cost per consultation (£155; code MHOPFUA2) |
| Psychologist (outpatient contact) | 135.00 | Curtis49 | Cost per contact hour (assumed 1 hour) |
| Community psychiatric nurse/care co-ordinator (outpatient contact) | 76.00 | Curtis49 | Mental health nurse, £76 per 1-hour contact (assumed 1 hour) |
| Other outpatient contact | 143.00 | Curtis49 | Outpatient consultant services, weighted average |
| Day care centre (community services/social care) | 34.00 | Curtis49 | Cost per user session |
| Drop-in club (community services/social care) | 34.00 | Curtis49 | Assume the same cost as day care centre, cost per user session |
| Help from friends/relatives | 18.00 | Curtis49 | Use cost per hour, based on unit cost for home help/care worker |
| Lost work (day) (friends/relatives) | 99.6 | ONS52 | Based on median gross weekly earnings in 2011 for full-time employees of £498 |
| Travel cost per mile (participant’s own car) | 0.44 | Estimate of reclaim/expense rate (running cost per mile) |
We estimated mean costs and QALYs for our primary economic evaluation by treatment allocation. We used covariates that were prespecified for age (at the individual level) and deprivation (IMD), site and practice size (at the cluster level). A multilevel regression model (Stata, xtmixed) was used for the primary analyses. This took into account the hierarchical (clustered) nature of the data, presenting the ICC for the main analyses. We undertook data analyses using generalised linear modelling, with appropriate family and link components to account for the non-normally distributed nature of cost data. Analyses were undertaken in Stata 12.
We conducted a number of sensitivity analyses for areas of uncertainty in the cost-effectiveness analyses:
-
We considered the effect of missing data, estimated by multiple imputation (Stata MI command, with 25 replicated data sets), using all available data on the target variable together with covariates for individual and cluster variables used in the base-case regression analyses. 54
-
We undertook analyses using a broader analytical perspective, including estimated costs for informal care and participant out-of pocket expenses.
-
We analysed data for a scenario using trial data from the SF-36 to estimate QALYs using the Short Form questionnaire-6 dimensions (SF-6D),55 which presents tariffs obtained from a UK general population survey to value health states as an alternative QALY outcome measure.
-
We considered uncertainty in the intervention costs.
-
We analysed a scenario in which one participant, with an extremely high level of self-reported resource use, was excluded, as this potentially offers a more likely and policy-relevant estimate of cost-effectiveness.
We combined estimates of incremental costs and incremental benefits to present incremental cost-effectiveness ratios (ICERs), allowing decision-makers to assess value for money using the cost per QALY estimates [ICER = (CostCC – CostTAU)/(QALYCC – QALYTAU), where CC represents collaborative care and TAU represents treatment as usual]. We used the NICE threshold of £20,000–30,000 per QALY,56,57 that is the expected payer willingness to pay per unit of additional outcome, to assess the cost-effectiveness of collaborative care, with ICERs below these values regarded as cost-effective. We used the non-parametric bootstrap approach,58 with 10,000 replications, to estimate 95% CIs around estimated cost differences and QALY differences to address uncertainty. To present the level of uncertainty in the cost-effectiveness estimates we used the cost-effectiveness plane to present combinations of incremental cost and incremental QALY data from bootstrap replicates and used the cost-effectiveness acceptability curve (CEAC) with the ‘net benefit statistic’ [net monetary benefit = (incremental QALYs × willingness to pay per QALY) – incremental cost)]59,60 to present the probability that the intervention is cost-effective (i.e. incremental net benefit statistic is 0) against a range of potential cost-effectiveness thresholds.
Participant consent and ethical approval
We were granted ethical approval by the NHS Health Research Authority, National Research Ethics Service (NRES) Committee South West (NRES/07/H1208/60). We ensured that informed consent was gathered from participants before they undertook any engagement with the study, including data collection and treatment allocation and receipt. In detail, the process was as follows.
First, potential participants had to indicate their potential interest in the trial. They then consented to a researcher-led discussion. Everyone who reached this stage was sent the full participant information sheet by a member of the CADET research team and an appointment was also made. At the initial appointment the trial was explained in detail by the research interviewer, who also answered potential participant questions.
We informed all potential trial participants that being consented into the trial would not replace or adversely affect usual care delivered by their GP. All interviewees were told that they could avail themselves of other services or treatments and that they could withdraw from the trial without incurring any penalty to their health or treatment choices. Having considered these facts and agreed to trial participation, we asked potential trial participants to sign a formal consent form. All of our researchers undertaking this consent process were trained and supervised by the CADET investigator team.
Chapter 3 Results of the clinical and economic analyses
This chapter utilises material from two2,3 of the four Open Access articles previously published by the research team in accordance with the terms of the Creative Commons Attribution licences (CC BY 3.0 and CC BY 4.0).
Participant flow and retention
Allocation of practices
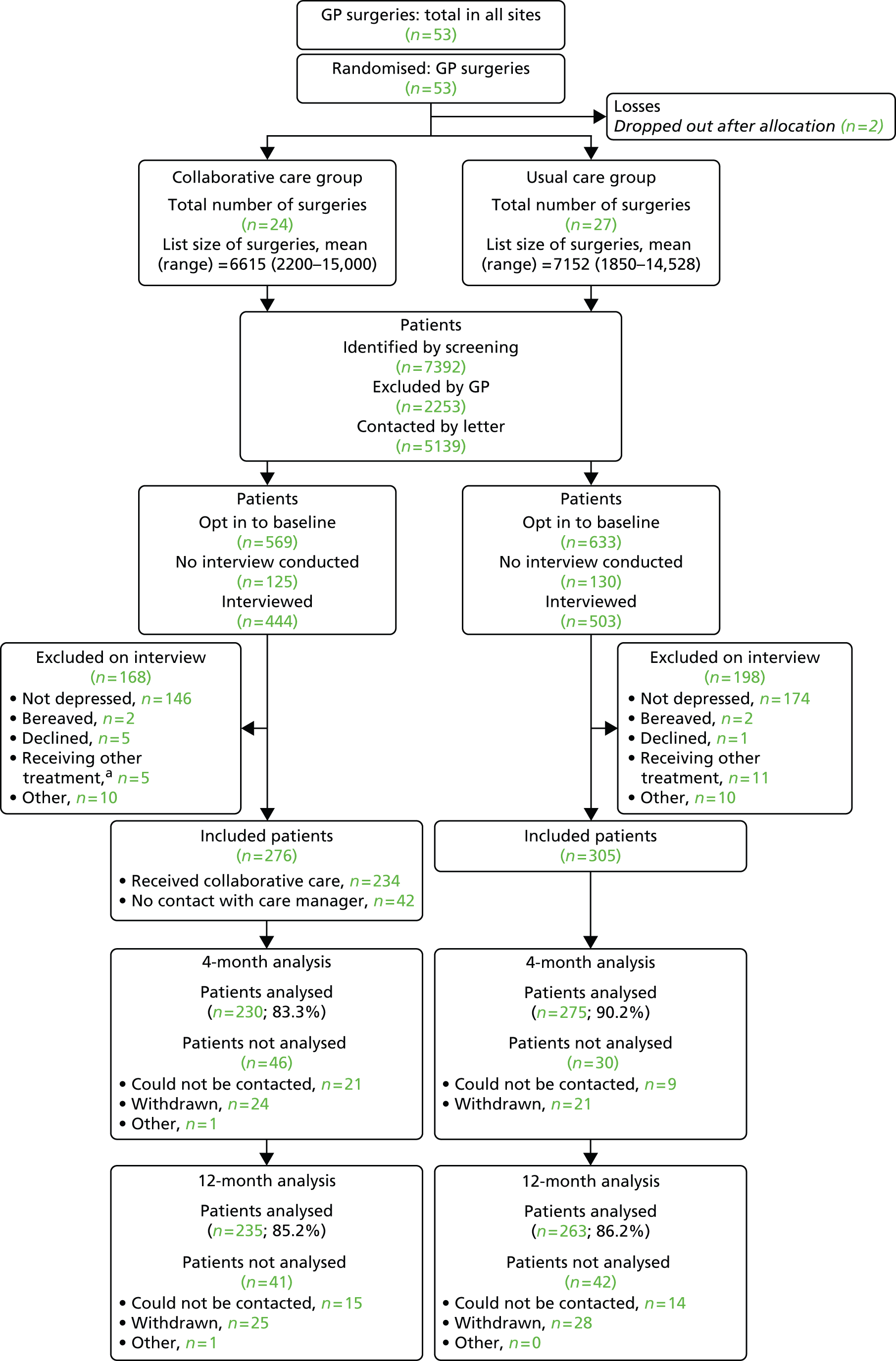
In total, 53 practices were randomised, two of which dropped out after allocation (Figure 1). These practices were removed from the minimisation schedule and their data did not influence later allocations. During recruitment, we found that the cut-off adopted for the IMD had been set far too high, with all practices so far recruited being below the cut-off. We changed this cut-off to one close to the median of practices so far recruited, retaining allocations so far. One practice was found to have been mistakenly recorded in the wrong geographical area; it was moved to the correct group, retaining its allocation. Of the remaining 51 practices, two did not recruit any participants.
FIGURE 1.
Trial CONSORT diagram. a, The number of patients in the collaborative care group who were excluded on interview because they were receiving treatment from secondary care or ‘another mental health provider’ (n = 5) includes one participant who was initially allocated in error and who was subsequently excluded.
Tables 2 and 3 show the geographical distribution of practices and minimisation variables (IMD, number of GPs and number of registered patients per practice), respectively, by intervention group. There was a wide range for all three practice characteristics.
| Site | Collaborative care | Usual care | Total |
|---|---|---|---|
| Greater Manchester | 7 | 9 | 16 |
| Bristol | 8 | 9 | 17 |
| London | 9 | 9 | 18 |
| Total | 24 | 27 | 51 |
| Variable | Group | Number of practices | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| IMD | Collaborative care | 24 | 9210 | 7416 | 317 | 27,365 |
| Usual care | 27 | 8449 | 6012 | 265 | 19,536 | |
| Total | 51 | 8807 | 6651 | 265 | 27,365 | |
| Number of GPs (whole-time equivalents) | Collaborative care | 24 | 3.8 | 2.0 | 1.0 | 10.0 |
| Usual care | 27 | 4.0 | 1.9 | 1.0 | 7.8 | |
| Total | 51 | 3.9 | 1.9 | 1.0 | 10.0 | |
| Number of patients | Collaborative care | 24 | 6615 | 3282 | 2200 | 15,000 |
| Usual care | 27 | 7152 | 3781 | 1850 | 14,528 | |
| Total | 51 | 6899 | 3530 | 1850 | 15,000 |
Participant recruitment
The mean number of participants recruited for the remaining 49 practice clusters was 11.9 (SD 3.9, range 4 to 20). We recruited 581 participants in total and followed up 505 (87%) and 498 (86%) at 4 and 12 months, respectively. The CONSORT diagram in Figure 1 illustrates the flow of participants through the trial.
Baseline characteristics of participants
The mean age of participants was 44.8 years (SD 13.3) and 72% were women. Fewer than half (44%) of participants were in full- or part-time paid employment. More than half (56%) of the participants fulfilled ICD-10 criteria for a moderately severe depressive episode, with a further 30% meeting criteria for severe depression and 14% meeting criteria for mild depression and 73% of all participants having had depression in the past (Table 4). Almost all (98%) participants had a secondary diagnosis of an anxiety disorder, the most common being generalised anxiety disorder. Almost two-thirds of participants (64%) reported a long-standing physical illness (e.g. diabetes, asthma, heart disease). At baseline, 83% of participants had been prescribed antidepressant drugs by their primary care doctor.
| Characteristic | Collaborative care (n = 276) | Usual care (n = 305) | Total (n = 581) |
|---|---|---|---|
| GP practices by centre, n | |||
| Bristol | 8 | 9 | 17 |
| London | 9 | 9 | 18 |
| Greater Manchester | 7 | 9 | 16 |
| Minimisation variables, mean (SD) | |||
| IMD | 9210 (7416) | 8449 (6012) | 8807 (6651) |
| Number of GPs | 3.8 (2.0) | 4.0 (1.9) | 3.9 (1.9) |
| Number of patients | 6615 (3282) | 7152 (3781) | 6899 (3530) |
| Sex, n (%) | |||
| Female | 202 (73.2) | 216 (70.8) | 418 (71.9) |
| Male | 74 (26.8) | 89 (29.2) | 163 (28.1) |
| Age (years) | |||
| Mean (SD) | 45.0 (13.2) | 44.5 (13.4) | 44.8 (13.3) |
| Range | 18 to 82 | 17 to 79 | 17 to 82 |
| Ethnic origin, n (%) | |||
| White British | 233 (84.4) | 261 (85.6) | 494 (85.0) |
| Other | 43 (15.6) | 44 (14.4) | 87 (15.0) |
| Education, n (%) | |||
| None | 54 (19.6) | 74 (24.3) | 128 (22.0) |
| GCSE/O-level | 65 (23.6) | 81 (26.6) | 146 (25.1) |
| Post GCSE/O-level | 84 (30.4) | 79 (25.9) | 163 (28.1) |
| Degree or higher | 49 (17.8) | 53 (17.4) | 102 (17.6) |
| Other or don’t know | 24 (8.7) | 18 (5.9) | 42 (7.2) |
| Employment, n (%)a | |||
| Employed/self-employed | 130 (47.4) | 122 (40.0) | 252 (43.5) |
| Not working | 144 (52.6) | 183 (60.0) | 327 (56.5) |
| Married/cohabiting, n (%) | 127 (46.0) | 114 (37.4) | 241 (41.5) |
| Prescribed antidepressants, n (%) | 231 (83.7) | 249 (81.6) | 480 (82.6) |
| CIS-R score, mean (SD) | 28.8 (9.3) | 30.3 (8.9) | 29.6 (9.1) |
| ICD-10 diagnosis, n (%)b | |||
| Mild | 42 (15.2) | 41 (13.4) | 83 (14.3) |
| Moderate | 156 (56.5) | 167 (54.8) | 323 (55.7) |
| Severe | 78 (28.3) | 96 (31.5) | 174 (30.0) |
| Previous history of depression, n (%) | 202 (73.2) | 220 (72.1) | 422 (72.6) |
| Secondary diagnosis, n (%) | |||
| Any anxiety disorder | 269 (97.5) | 301 (98.7) | 570 (98.1) |
| Long-standing physical illness | 171 (62.0) | 199 (65.2) | 370 (63.7) |
| Baseline outcomes, mean (SD) | |||
| PHQ-9 score | 17.4 (5.2) | 18.1 (5.0) | 17.8 (5.1) |
| GAD-7 score | 12.9 (5.3) | 13.6 (4.7) | 13.3 (5.0) |
| SF-36 MCS score | 23.2 (10.4) | 22.3 (10.3) | 22.7 (10.3) |
| SF-36 PCS score | 44.8 (12.4) | 44.5 (12.3) | 44.6 (12.3) |
| EQ-5D score | 0.50 (0.29) | 0.46 (0.31) | 0.48 (0.30) |
| SF-6D score | 0.54 (0.08) | 0.54 (0.09) | 0.54 (0.08) |
The distribution of PHQ-9 scores at baseline was negatively skewed, with the majority of scores being in the higher part of the range. The mean PHQ-9 score overall was 17.8 (SD 5.1), with the usual care group having a slightly higher average score than the collaborative care group (18.1 vs. 17.4 respectively) (Figure 2 and see Table 2). All subjects were selected for the trial using a different measure of depression (CIS-R) from that used in the analysis to avoid problems of regression towards the mean. Low scores represent random variation because of measurement error.
FIGURE 2.
Distribution of PHQ-9 scores at baseline.

Delivery and receipt of the intervention
A total of 10 care managers provided collaborative care for 276 participants. The mean number of participants managed per care manager was 27.6 (SD 16.42, range 4 to 46).
Patients received a mean of 5.6 (SD 4.01, range 0 to 15) sessions with their care manager. Forty-two (15.2%) participants did not attend any sessions with their care manager, 213 (77.2%) had two or more contacts and 171 (62.0%) had four or more contacts. The mean total time in collaborative care was 3.03 hours (SD 2.18 hours) over a period of 12 weeks (SD 7.75 weeks). For those participants who attended at least one session, the mean duration of the sessions was 34.5 minutes (SD 8.2 minutes). Most participants in both collaborative care and usual care remained on antidepressant medication (74.8% vs. 73.8% at 4 months; 69.7% vs. 69.2% at 12 months).
The mean number of collaborative care sessions per participant in which medication was discussed was 3.2 (SD 3.43, range 0–13). The mean number of sessions incorporating behavioural activation was 5.4 (SD 3.4, range 0–13). The mean number of face-to-face contacts per participant was 1.17 (SD 0.92, range 0–6). The mean number of telephone contacts was 5.3 (SD 3.5, range 0–14).
We collected 220 supervision records reporting a mean supervision time of 35 minutes per week, with six participants discussed on average per session. Participants were discussed in an average of three supervisory sessions over the course of their intervention, at 6 minutes per participant per session. Care managers, therefore, received the intended level of supervision and number of sessions, with the number of participants discussed being dependent on the caseload of individual care managers.
Primary outcome: Patient Health Questionnaire-9 at 4 months
The primary and secondary outcomes at 4 and 12 months are presented in Table 5 and data on recovery, response and numbers needed to treat are presented in Table 6. With regard to the primary outcome (PHQ-9 at 4 months) we found a significant effect of collaborative care. The estimated mean depression score was 1.33 PHQ-9 points lower (95% CI –2.31 to –0.35; p = 0.009) for participants receiving collaborative care than for participants receiving usual care after adjustment for baseline depression. More participants receiving collaborative care than those receiving usual care met criteria for recovery [odds ratio (OR) 1.67, 95% CI 1.22 to 2.29; number needed to treat 8.4] and response (OR 1.77, 95% CI 1.22 to 2.58; number needed to treat 7.8).
| Outcome | Collaborative care | Usual care | Adjusted difference | 95% CI | p-value | Effect size | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||||
| Primary outcome | ||||||||||
| PHQ-9 score at baseline | 276 | 17.4 | 5.2 | 305 | 18.1 | 5.0 | ||||
| PHQ-9 score at 4 monthsa | 230 | 11.1 | 7.3 | 275 | 12.7 | 6.8 | –1.33 | –2.31 to –0.35 | 0.009 | 0.26 |
| Secondary outcomes | ||||||||||
| PHQ-9 score at 12 months | 235 | 10.0 | 7.1 | 263 | 11.7 | 6.8 | –1.36 | –2.64 to –0.07 | 0.04 | 0.28 |
| GAD-7 score at baseline | 276 | 12.9 | 5.3 | 305 | 13.6 | 4.7 | ||||
| GAD-7 score at 4 months | 228 | 9.1 | 6.8 | 273 | 9.8 | 5.8 | –0.39 | –1.30 to 0.53 | 0.4 | 0.08 |
| GAD-7 score at 12 months | 227 | 7.7 | 6.2 | 253 | 9.1 | 6.2 | –1.09 | –2.21 to 0.03 | 0.06 | 0.22 |
| SF-36 MCS score at baseline | 276 | 23.2 | 10.4 | 305 | 22.3 | 10.3 | ||||
| SF-36 MCS score at 4 months | 227 | 34.6 | 15.4 | 268 | 30.7 | 13.7 | 3.4 | 1.1 to 5.7 | 0.005 | 0.33 |
| SF-36 MCS score at 12 months | 223 | 36.4 | 15.0 | 249 | 33.4 | 14.5 | 2.5 | –0.6 to 5.5 | 0.1 | 0.24 |
| SF-36 PCS score at baseline | 276 | 44.8 | 12.4 | 305 | 44.5 | 12.3 | ||||
| SF-36 PCS score at 4 months | 227 | 45.8 | 13.2 | 268 | 45.6 | 13.8 | –0.05 | –1.67 to 1.56 | 0.9 | –0.004 |
| SF-36 PCS score at 12 months | 223 | 46.1 | 13.2 | 249 | 44.9 | 13.3 | –0.93 | –0.93 to 3.01 | 0.3 | 0.08 |
| CSQ-8 score at 4 months | 232 | 25.3 | 5.8 | 269 | 22.1 | 6.2 | 3.13 | 1.87 to 4.39 | < 0.001 | 0.52 |
| Collaborative care | Usual care | ORa | 95% CIb | p-valueb | Number needed to treatc | |||
|---|---|---|---|---|---|---|---|---|
| n | Recovered/responded, n (%) | n | Recovered/responded, n (%) | |||||
| Recoveryd | ||||||||
| 4 months | 230e | 108 (47.0) | 275 | 96 (34.9) | 1.67 | 1.22 to 2.29 | 0.001 | 8.4 |
| 12 months | 235 | 131 (55.7) | 263 | 106 (40.3) | 1.88 | 1.28 to 2.75 | 0.001 | 6.5 |
| Responsef | ||||||||
| 4 months | 230e | 99 (43.0) | 275 | 83 (30.2) | 1.77 | 1.22 to 2.58 | 0.003 | 7.8 |
| 12 months | 235 | 115 (48.9) | 263 | 93 (35.4) | 1.73 | 1.22 to 2.44 | 0.002 | 7.3 |
Secondary outcomes
Depression at 12 months
At 12 months’ follow-up, PHQ-9 data were available for 498 participants, 86% of those recruited (see Table 5). There was a significant effect of collaborative care on depression at 12 months. The mean PHQ-9 score was 1.36 points lower (95% CI –2.64 to –0.07; p = 0.04) in participants receiving collaborative care than in those receiving usual care (standardised effect size 0.28, 95% CI 0.01 to 0.52). More participants in collaborative care than in usual care met criteria for recovery (OR 1.88, 95% CI 1.28 to 2.75; number needed to treat 6.5) and response (OR 1.73, 95% CI 1.22 to 2.44; number needed to treat 7.3) (see Table 6).
Anxiety
We found no significant effect of collaborative care on anxiety at 4 months, as measured by the GAD-7 (see Table 5). The adjusted difference between the groups in the anxiety score at 4 months was 0.39 (95% CI –1.30 to 0.53; p = 0.4). At 12 months there was also no significant effect of collaborative care on anxiety. The adjusted difference between the groups in the anxiety score at 12 months was 1.09 (95% CI –2.21 to 0.03; p = 0.06).
Quality of life
Mental health
We found a highly significant effect of collaborative care on the mental component summary (MCS) score of the SF-36 at 4 months, with the mean score higher by 3.4 T-score points (95% CI 1.1 to 5.7 T-score points; p = 0.005) in the collaborative care group. This corresponds to an effect size of 0.33 SD (95% CI 0.11 to 0.56 SD). At 12 months this effect was no longer significant (mean difference 2.5 T-score points, 95% CI −0.6 to 5.5 T-score points; p = 0.1).
Physical health
We found no significant effect of collaborative care on the quality of physical health at 4 months, as measured by the SF-36. The difference between the groups in the physical component summary (PCS) score of the SF-36 was 0.05 T-score points (95% CI –1.67 to 1.56; p = 0.9). The same was true at 12 months, with a difference in PCS T-score points between the groups of 1.04 (95% CI −0.93 to 3.01; p = 0.3).
Table 7 shows the adjusted effect of collaborative care on each SF-36 subscale at 4 months. Four of the subscales indicated significant benefits of collaborative care: mental health, role limitations (emotional) and vitality of the mental components and general health of the physical components. Although not significant, the other element of the mental dimension, social functioning, showed a small estimated benefit, too.
| Subscale | Coefficient | Robust standard error | t | p-value | 95% CI |
|---|---|---|---|---|---|
| Physical functioning | 0.038 | 0.078 | 0.49 | 0.6 | −0.119 to 0.194 |
| Role limitations, physical | 0.065 | 0.096 | 0.68 | 0.5 | −0.128 to 0.258 |
| Bodily pain | −0.021 | 0.095 | −0.22 | 0.8 | −0.211 to 0.170 |
| General health perceptions | 0.271 | 0.071 | 3.81 | < 0.001 | 0.128 to 0.415 |
| Social functioning | 0.157 | 0.114 | 1.37 | 0.2 | −0.074 to 0.387 |
| Mental health | 0.289 | 0.103 | 2.79 | 0.007 | 0.081 to 0.497 |
| Role limitations, emotional | 0.264 | 0.100 | 2.63 | 0.01 | 0.062 to 0.465 |
| Vitality | 0.337 | 0.097 | 3.48 | 0.001 | 0.142 to 0.531 |
Client satisfaction at 4 months
We found a highly significant effect of collaborative care on client satisfaction (see Table 5). The adjusted difference between the groups in satisfaction score at 4 months was 3.13 (95% CI 1.87 to 4.39; p < 0.001). The estimated effect size for the CSQ-8 was 0.52 (95% CI 0.31 to 0.73). This has been calculated slightly differently from the effect sizes for the PHQ-9, GAD-7 and SF-36 because there is no baseline SD. The crude SD within intervention groups for all participants at 4 months was used.
Missing data
In Table 8 we present the results after multiple imputation for the effect of collaborative care at 4 and 12 months on the main scales used, which also shows the results of the analyses using the available data, which were reported in the preceding sections. The imputed estimates are very similar to the available data estimates and so we can conclude that for all of these analyses the effects of collaborative care are little affected by missing data. The lower GAD-7 anxiety score at 12 months in the collaborative care group, which is not significant in the available data analysis, is just statistically significant in this simulation. However, the difference in the p-value (0.06 or 0.05) is very small.
| Scale | Data | Coefficient | Robust standard error | t | p-value | 95% CI |
|---|---|---|---|---|---|---|
| PHQ-9 score at 4 months | Imputed | −1.31 | 0.53 | −2.49 | 0.02 | −2.37 to −0.26 |
| Available data | −1.33 | 0.49 | −2.72 | 0.009 | −2.31 to −0.35 | |
| PHQ-9 score at 12 months | Imputed | −1.29 | 0.62 | −2.08 | 0.04 | −2.54 to −0.04 |
| Available data | −1.36 | 0.64 | −2.13 | 0.04 | −2.64 to −0.07 | |
| GAD-7 score at 4 months | Imputed | −0.37 | 0.45 | −0.82 | 0.4 | −1.27 to 0.53 |
| Available data | −0.39 | 0.45 | −0.85 | 0.4 | −1.30 to 0.53 | |
| GAD-7 score at 12 months | Imputed | −1.07 | 0.52 | −2.04 | 0.05 | −2.12 to −0.01 |
| Available data | −1.09 | 0.56 | −1.95 | 0.06 | −2.21 to 0.03 | |
| SF-36 PCS score at 4 months | Imputed | 0.03 | 0.78 | 0.04 | 1.0 | −1.53 to 1.59 |
| Available data | −0.05 | 0.80 | −0.07 | 0.9 | −1.67 to 1.56 | |
| SF-36 PCS score at 12 months | Imputed | 0.98 | 0.91 | 1.08 | 0.3 | −0.84 to 2.80 |
| Available data | 1.04 | 0.98 | 1.06 | 0.3 | −0.93 to 3.01 | |
| SF-36 MCS score at 4 months | Imputed | 3.6 | 1.2 | 3.13 | 0.003 | 1.3 to 6.0 |
| Available data | 3.4 | 1.2 | 2.97 | 0.005 | 1.1 to 5.7 | |
| SF-36 MCS score at 12 months | Imputed | 2.6 | 1.4 | 1.86 | 0.07 | −0.2 to 5.4 |
| Available data | 2.5 | 1.5 | 1.60 | 0.1 | −0.1 to 5.5 | |
| CSQ-8 score at 4 months | Imputed | 3.20 | 0.61 | 5.23 | < 0.001 | 1.97 to 4.44 |
| Available data | 3.13 | 0.63 | 4.98 | < 0.001 | 1.87 to 4.39 |
Missing data were related to intervention group. Table 9 shows missing PHQ-9 data at 4 months and 12 months by intervention group. At 4 months, missing data were significantly more likely in the collaborative care group. At 12 months the difference between the groups was much smaller and not significant. As far as we can tell, this difference in missingness at 4 months does not produce a difference in the outcome variables between the two groups and does not explain the observed lower PHQ-9 score in the collaborative care group. This difference persists at 12 months, when the difference in missingness is much smaller.
| Time point of missing PHQ-9 data | Intervention group, n (%) | OR for missing data in collaborative care group | p-value (robust standard error) | |
|---|---|---|---|---|
| Collaborative care | Usual care | |||
| 4 months | 46 (16.7) | 30 (9.8) | 1.83 | 0.03 |
| 12 months | 41 (14.9) | 42 (13.8) | 1.09 | 0.7 |
Results of the economic analyses
Our estimated mean cost per participant for the delivery of the collaborative care intervention was £272.50. This cost estimate includes care manager costs at £232 and clinical supervision costs of £40.50. Our probabilistic analyses used to explore uncertainty around the main cost component, drawing from the distribution of contact time for care managers, showed that in 95% of simulations (cost estimates) the estimated cost of collaborative care was between £101 and £592 per participant (median £249 per participant).
NHS and social care resource use and costs
We found no statistically significant differences between treatment groups in use of resources prior to the baseline assessment. Table 10 presents resource use over the 12-month follow-up period and Table 11 presents the costs associated with the resource use over the 12-month follow-up period. Table 12 presents cost data by category with comparison by treatment group. We found a broadly similar pattern of resource use across groups, with estimated mean costs of NHS and social care (third-party payer perspective), excluding the collaborative care intervention, of £1571 and £1614 for usual care and collaborative care participants respectively. After adjustment for baseline costs and individual and cluster covariates the cost difference was not statistically significant, with wide CIs. When including the cost of the collaborative care intervention, the mean total NHS and social care costs were £1571 and £1887 for usual care and collaborative care participants, respectively, but, similarly, after adjustment the cost difference of £271 was not statistically significant. Excluding the intervention cost, the one area of substantial cost difference between groups was for hospital stay, with a mean cost difference of £161 (regression-adjusted estimate). This estimated difference in hospital costs was driven by one participant in the collaborative care group who reported an acute psychiatric hospital stay of 100 days. When we excluded this participant from the analyses, the cost difference for hospital stay was adjusted to £34.27 (95% CI –£119 to £189) and the related differences in NHS and social care costs, without collaborative care costs and with collaborative care costs, were adjusted to –£209 (lower cost for collaborative care) and £63 (additional cost for collaborative care), respectively.
| Resource item | Usual care (n = 305) | Collaborative care (n = 276) | ||
|---|---|---|---|---|
| n | Mean (SD) [range] | n | Mean (SD) [range] | |
| Primary/community care (contacts) | ||||
| GP (surgery/practice) | 244 | 8.21 (6.69) [0–56] | 217 | 7.77 (6.78) [0–45] |
| GP (home) | 247 | 0.12 (0.80) [0–11] | 218 | 0.05 (0.27) [0–3] |
| Nurse (surgery/practice) | 247 | 1.77 (3.08) [0–24] | 215 | 1.68 (3.10) [0–32] |
| Nurse (home) | 247 | 0.06 (0.46) [0–4] | 218 | 0.05 (0.45) [0–6] |
| Walk-in centre | 247 | 0.32 (0.87) [0–8] | 217 | 0.31 (0.86) [0–5] |
| Counsellor | 246 | 3.58 (11.26) [0–116] | 212 | 2.67 (7.21) [0–48] |
| Mental health worker | 247 | 0.58 (3.51) [0–50] | 215 | 0.79 (3.72) [0–36] |
| Social worker | 247 | 0.34 (1.79) [0–14] | 218 | 0.58 (3.94) [0–33] |
| Home help/care worker | 247 | 4.35 (47.27) [0–722] | 218 | 1.24 (15.07) [0–220] |
| Occupational therapist | 247 | 0.22 (0.98) [0–9] | 218 | 0.13 (0.61) [0–5] |
| Voluntary group | 247 | 0.94 (5.80) [0–64] | 218 | 0.22 (1.39) [0–16] |
| Secondary care | ||||
| Hospital admissions, n | 247 | 34 | 218 | 28 |
| Acute psychiatric ward (days) | 247 | – | 218 | 0.78 (11.51) [0–170]a |
| Psychiatric rehabilitation ward (days) | 247 | – | 218 | – |
| Long-stay ward (days) | 247 | 0.06 (0.94) [0–15] | 218 | – |
| Psychiatric ICU ward (days) | 247 | – | 218 | – |
| General medical ward (days) | 247 | 0.48 (2.02) [0–21] | 217 | 0.42 (1.67) [0–12] |
| Other hospital ward (days) | 247 | 0.28 (1.58) [0–17] | 218 | 0.39 (2.12) [0–24] |
| Accident and emergency (attendance) | 247 | 0.40 (0.93) [0–7] | 218 | 0.34 (0.76) [0–5] |
| Day hospital (attendance) | 247 | 0.60 (2.22) [0–24] | 218 | 0.36 (1.19) [0–12] |
| Outpatient appointment | 247 | 2.62 (5.60) [0–58] | 217 | 2.63 (5.63) [0–65] |
| Social care (contact/session) | ||||
| Used day care services (%)b | 247 | 3/2 | 218 | 4/3 |
| Day care centre | 247 | 0.28 (4.54) [0–70] | 218 | 0.07 (1.08) [0–16] |
| Drop-in club | 247 | 0.56 (5.26) [0–70] | 218 | 0.12 (1.40) [0–20] |
| Day care other | 247 | 0.39 (2.85) [0–28] | 217 | 0.65 (5.67) [0–74] |
| Informal care from friends/relatives | ||||
| Had help/care from friends/relatives (%)b | 45/48 | 38/35 | ||
| Hours per week help from friends/relativesc | 230 | 6.11 (15.44) [0–112] | 209 | 3.95 (10.11) [0–104] |
| Report time off work for friends/relatives (%)b | 7/9 | 7/10 | ||
| Days off work lost by friends/relatives | 246 | 4.05 (29.14) [0–360] | 217 | 1.65 (11.28) [0–144] |
| Participant other costs | ||||
| OTC cost (£) | 246 | 28.40 (57.90) [0–429]d | 215 | 40.31 (68.61) [0–507]d |
| Travel costs (£) | 246 | 10.98 (30.30) [0–320] | 216 | 14.33 (37.57) [0–202] |
| Own car travel (miles) | 246 | 26.12 (77.68) [0–600] | 214 | 31.53 (138.95) [0–1862] |
| Other ‘one-off’ costs (£) | 246 | 35.77 (144.35) [0–1569] | 218 | 51.58 (213.53) [0–1998] |
| Resource item | Usual care (n = 305) | Collaborative care (n = 276) | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| Primary/community care | ||||
| GP (surgery/practice) | 244 | 295.52 (241) | 217 | 279.76 (243) |
| GP (home) | 247 | 14.21 (97) | 218 | 5.55 (32) |
| Nurse (surgery/practice) | 247 | 26.54 (46) | 215 | 25.19 (46) |
| Nurse (home) | 247 | 1.94 (14) | 218 | 1.38 (13) |
| Walk-in centre (attendance) | 247 | 13.28 (35) | 217 | 12.66 (35) |
| Counsellor | 246 | 214.63 (676) | 212 | 160.47 (433) |
| Mental health worker | 247 | 44 (267) | 215 | 59.74 (283) |
| Social worker | 247 | 72.10 (379) | 218 | 122.53 (835) |
| Home help/care worker | 247 | 78.27 (851) | 218 | 22.38 (271) |
| Occupational therapist | 247 | 18.26 (80) | 218 | 10.53 (50) |
| Voluntary group | 247 | 20.50 (126) | 218 | 4.88 (30) |
| Secondary care | ||||
| Acute psychiatric ward | 247 | 0 | 218 | 243.30 (3,592) |
| Psychiatric rehabilitation ward | 247 | 0 | 218 | 0 |
| Long-stay ward | 247 | 13.51 (212) | 218 | 0 |
| Psychiatric ICU ward | 247 | 0 | 218 | 0 |
| General medical ward | 247 | 154.65 (649) | 217 | 134.61 (535) |
| Other hospital ward/stay | 247 | 90.97 (507) | 218 | 123.69 (682) |
| Accident and emergency | 247 | 43.06 (99) | 218 | 36.47 (81) |
| Day hospital | 247 | 74.99 (280) | 218 | 45.08 (150) |
| Outpatient appointment, psychiatrist | 247 | 26.79 (148) | 217 | 43.88 (170) |
| Outpatient appointment, psychologist | 247 | 25.14 (313) | 217 | 25.51 (296) |
| Outpatient appointment, community psychiatric nurse | 247 | 8.92 (67) | 217 | 12.61 (166) |
| Outpatient appointment, other | 246 | 306.93 (588) | 215 | 285.34 (498) |
| Social care | ||||
| Day care centre | 247 | 9.64 (151) | 218 | 2.50 (37) |
| Drop-in club | 247 | 19.13 (179) | 218 | 4.06 (48) |
| Day care other | 247 | 13.35 (97) | 217 | 22.09 (193) |
| Informal care from friends/relatives | ||||
| Help from friends/relatives | 230 | 5714.73 (14,455) | 209 | 3698.50 (9462) |
| Days off work lost by friends/relatives | 246 | 403.26 (2902) | 217 | 164.78 (1123) |
| Participant other costs | ||||
| OTC costs (£) | 246 | 28.40 (58) | 215 | 40.31 (69) |
| Travel costs (£) | 246 | 10.98 (30) | 216 | 14.33 (38) |
| Own car travel | 246 | 11.75 (35) | 214 | 14.19 (63) |
| ‘One-off’ costs (£) | 246 | 35.77 (144) | 218 | 51.58 (213) |
| Resource item | Usual care (n = 305) | Collaborative care (n = 276) | Difference, no adjustment (£) | Difference, adjusted for baseline and participant/cluster covariates,a mean (95% CI)b (£) | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |||
| Primary and community services/care | 243 | 801.49 (1476.98) | 208 | 715.86 (1220.06) | –85.63 | –116.48 (–341.06 to 110.91) |
| Secondary care: hospital stay | 247 | 259.14 (835.40) | 217 | 402.65 (2282.88) | 143.51 | 160.92 (–70.81 to 481.70) |
| Secondary care: outpatient care | 246 | 368.03 (781.43) | 215 | 368.09 (692.60) | 0.06 | –30.68 (–148.85 to 111.70) |
| Secondary care: day hospital | 247 | 74.99 (280.31) | 218 | 45.08 (149.65) | –29.91 | –14.52 (–50–13 to 17.94) |
| Accident and emergency | 247 | 42.06 (98.66) | 218 | 36.47 (80.51) | –5.59 | –5.87 (–22.39 to 9.99) |
| Day services and care | 247 | 42.12 (334.85) | 217 | 28.67 (203.43) | –13.45 | 1.83 (–38.51 to 41.01) |
| Total NHS and Personal Social Services (excluding collaborative care) | 242 | 1570.70 (2441.55) | 205 | 1614.32 (3714.49) | 43.62 | 1.78 (–454.82 to 640.81) |
| Collaborative care | – | 272.50 | 272.50 | – | ||
| Total NHS and Personal Social Services | 242 | 1570.70 (2441.55) | 205 | 1886.82 (3714.49) | 316.12 | 270.72 (–202.98 to 886.04)c |
| Patient personal costs (OTC costs/medications and travel costs plus patient ‘one-off’ costs) | 244 | 86.64 (175.50) | 211 | 120.79 (260.37) | 34.15 | 24.95 (–12.41 to 65.61) |
| Informal care costs | 230 | 5714.73 (14,455.18) | 209 | 3698.50 (9642.61) | –2016.23 | –1114.13 (–3366.09 to 1117.32) |
| Total costs (NHS and patient/related costs) | 223 | 7010.59 (13,492.42) | 195 | 5764.48 (10,796.40) | –1246.11 | –312.83 (–2339.92 to 2035.27)c |
Broader participant-level and social costs
In Tables 10 and 11 we report resource use and cost estimates associated with informal care from friends and/or relatives and other participant out-of-pocket expenses. Our findings show that informal care costs, when estimated using a shadow price for informal care (an estimate of £18 per hour; see Table 1), represented the largest resource and cost burden associated with participants’ depression. Participants in the usual care group reported a high use of informal care, which resulted in a higher mean (SD) cost estimate over 12 months of £5715 (£14,455); this compared with £3699 (£9462) in the collaborative care group. However, there is wide variation in the self-report data as shown by the large SDs. When adjusting for baseline costs and other covariates the difference in estimated cost for informal care was –£1114 (95% CI –£3366 to £1117), with lower costs for the collaborative care group and, therefore lower total costs for the collaborative care group (see Table 12).
Quality-adjusted life-years
In Table 13 we report data on health state values for the EQ-5D and SF-6D and the estimated QALY values over the 12-month follow-up period. When adjusted for baseline and for individual and cluster covariates we found a difference of 0.02 QALYs (95% CI –0.02 to 0.06 QALYs) over 12 months for the EQ-5D and 0.017 QALYs (95% CI 0.000 to 0.032 QALYs) for the SF-6D. Both measures show a QALY gain for collaborative care, although the EQ-5D difference is not statistically significant.
| Resource item | Usual care (n = 305) | Collaborative care (n = 276) | Difference, no adjustment | Difference, adjusted for baseline and participant/cluster covariates,a mean (95% CI) | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) [range] | n | Mean (SD) [range] | |||
| EQ-5D: baseline | 305 | 0.464 (0.313) [–0.29 to 1.00] | 276 | 0.504 (0.288) [–0.349 to 1.00] | 0.040 | |
| EQ-5D: 4 months | 273 | 0.557 (0.331) [–0.239 to 1.00] | 228 | 0.599 (0.341) [–0.484 to 1.00] | 0.042 | |
| EQ-5D: 12 months | 254 | 0.593 (0.338) [–0.349 to 1.00] | 227 | 0.650 (0.317) [–0.484 to 1.00] | 0.057 | |
| EQ-5D: QALYs (12 months) | 248 | 0.554 (0.286) [–0.27 to 0.97] | 218 | 0.605 (0.261) [–0.29 to 0.97] | 0.051b | 0.019 (–0.019 to 0.06)c |
| SF-6D: baseline | 303 | 0.538 (0.86) [0.30 to 0.77] | 274 | 0.540 (0.83) [0.30 to 0.82] | 0.002 | |
| SF-6D: 4 months | 269 | 0.597 (0.126) [0.30 to 1.00] | 227 | 0.614 (0.140) [0.32 to 1.00] | 0.017 | |
| SF-6D 12 months | 250 | 0.605 (0.131) [0.30 to 1.00] | 223 | 0.634 (0.144) [0.30 to 1.00] | 0.029b | |
| SF-6D: QALYs (12 months) | 241 | 0.591 (0.109) [0.30 to 0.90] | 211 | 0.609 (0.114) [0.35 to 0.91] | 0.018 | 0.0168 (0.000 to 0.032) |
Cost-effectiveness analyses
In Table 14 we present estimates of cost per QALY, based on participants with data on costs and outcomes at follow-up. The base-case cost per QALY for collaborative care was £14,248, adopting a NHS and social care perspective, with uncertainty around this estimate illustrated in Figure 3 (cost-effectiveness plane) and Figure 4 (CEACs). The probability that collaborative care is cost-effective compared with treatment as usual is 0.58 at a willingness to pay of £20,000 per QALY and 0.65 at a willingness to pay of £30,000 per QALY.
| Scenario/analysis | Difference, adjusted for baseline and participant/cluster covariates,a mean (95% CI) | ICER, cost (£) per QALY | Probability collaborative care cost-effective at WTPb per QALY gained of | |
|---|---|---|---|---|
| £20,000 per QALY | £30,000 per QALY | |||
| Base case | ||||
| Total NHS and Personal Social Services costs (£) | 270.72 (–202.98 to 886.04) | |||
| EQ-5D: QALYs (12 months) | 0.019 (–0.019 to 0.06) | 14,248 | 0.58 | 0.65 |
| Sensitivity analyses | ||||
| 1. Base-case cost-effectiveness analysis with multiple imputation of missing data | ||||
| Total NHS and Personal Social Services costs (£) | 292.08 (–216.88 to 801.04) | |||
| EQ-5D: QALYs (12 months) | 0.017 (–0.020 to 0.054) | 17.490 | NA | NA |
| 2. Cost-effectiveness analysis using SF-6D QALY data | ||||
| SF-6D: QALYs (12 months) | 0.0168 (0.000 to 0.032) | 16,114 | 0.57 | 0.72 |
| 3. Cost-effectiveness analysis when excluding one high-cost participant | ||||
| Total NHS and Personal Social Services costs (£) | 63.34 (–295.98 to 422.67) | |||
| EQ-5D: QALYs (12 months) | 0.019 (–0.018 to 0.06) | 3334 | 0.76 | 0.79 |
| 4. Cost-effectiveness analysis using higher cost estimate for collaborative care (mean cost of £338.80) | ||||
| Total NHS and Personal Social Services costs (£) | 337.02 (–136.67 to 952.34) | 17,738 | 0.54 | 0.62 |
| 5. Cost-effectiveness analysis using a broader perspective, including patient costs and informal care costs | ||||
| Total costs (NHS and patient/related costs) | –£312.83 (–2339.93 to 2035.27) | Collaborative care is dominantc | NA | NA |
FIGURE 3.
Cost-effectiveness plane (payer perspective).
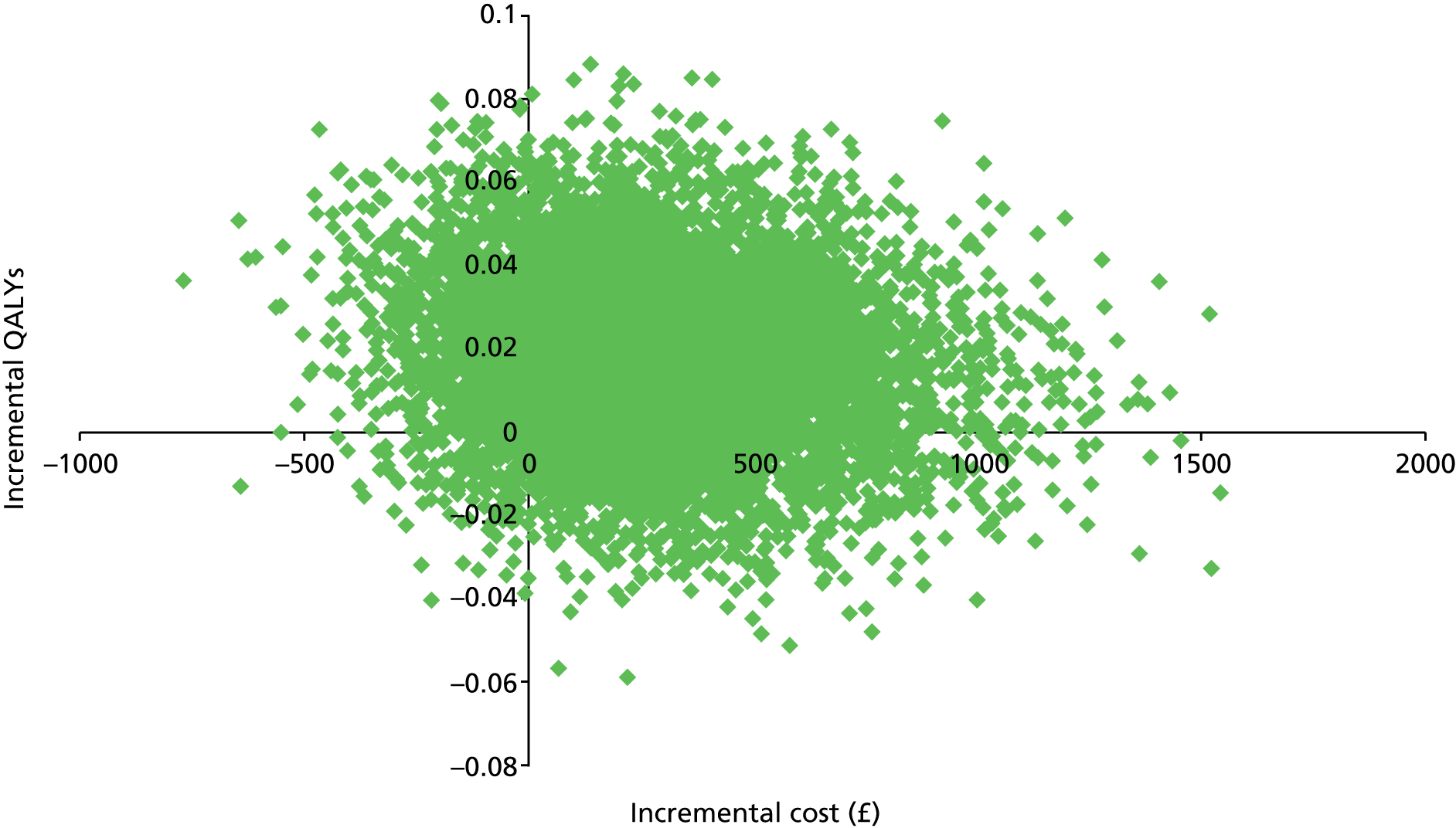
FIGURE 4.
Cost-effectiveness acceptability curves. CEA, cost-effectiveness analysis; INB, incremental net benefit; SA, sensitivity analysis.

Sensitivity analyses
The results of the sensitivity analyses are also presented in Table 14, in which we estimated incremental costs and QALYs and cost per QALY using alternative assumptions. In the base-case analysis 23% of the cost data are missing at the 12-month follow-up (21% control, 25% collaborative care) and 20% of the QALY (EQ-5D) data are missing at the 12-month follow-up (19% control, 21% collaborative care).
Imputation of missing data resulted in an estimated incremental cost of £292 and an incremental EQ-5D QALY gain of 0.017, with a cost per QALY of £17,490. When we adopted a broader analytical perspective, including all participants with data on costs and outcomes at follow-up, we estimated a mean cost saving of £313 with collaborative care, alongside an estimated incremental gain in QALYs of 0.02. This therefore represents a position of dominance for the collaborative care intervention compared with usual care. Using the SF-6D QALY estimate the cost per QALY increased to £16,114, with 0.57 and 0.72 probability of collaborative care being cost-effective at a willingness to pay of £20,000 and £30,000 per QALY, respectively. When we used an alternative cost for the collaborative care intervention, assuming a cost of £338.80 per participant (compared with the base case of £272.50) to allow for additional clinical supervision time for the care manager, and therefore per participant, and for supervision from a psychiatrist (unit cost per hour £267),49 the cost per QALY estimate increased to £17,738. When we excluded from the analysis the participant with extremely high resource use the cost per QALY was reduced to £3334, with a 0.76 and 0.79 probability of collaborative care being cost-effective at a willingness to pay of £20,000 and £30,000 per QALY, respectively.
Chapter 4 Results of the process evaluation
Alongside the main clinical and economic evaluation we undertook a process evaluation to investigate the implementation of the intervention, moderators of outcome and possible mechanisms of effect. This chapter utilises material from one61 of the four Open Access articles previously published by the research team in accordance with the terms of the Creative Commons Attribution (CC BY 2.0)61 licence. Qualitative data from this work are reproduced verbatim to preserve the integrity of the data analysis.
Objectives
The objectives of the process evaluation were to investigate:
Methods
To investigate moderators and mechanisms of effect we recorded a number of baseline covariates together with intermediate process variables that were targets of the intervention, regressing these against depression severity (PHQ-9) at 4 and 12 months using multilevel multiple linear regression.
Measures
Moderators
We recorded six possible moderators at baseline: patients’ attitudes towards antidepressant medication, patients’ attitudes towards behavioural activation, depression severity (PHQ-9), history of depression (number of previous episodes), physical health (comorbidity) and socioeconomic status. To measure attitudes towards treatment we asked ‘How acceptable is it to you to use antidepressant medication?’ and ‘How acceptable is it to you to review and change your routines and increase your daily activities as a way of helping with depression?’ Response options were from 1 (definitely acceptable) to 4 (definitely not acceptable). To investigate the moderating effect of patients’ socioeconomic status we used their postcode to obtain an IMD34 score at the lower super output area level.
Mechanisms of change
We measured participants’ adherence to antidepressant medication and level of behavioural activation at 4 and 12 months through self-report of medication adherence and the Behavioural Activation for Depression Scale – Short Form. 64
Process of implementation
We conducted face-to-face interviews with six care managers and five supervisors involved in delivering and supervising collaborative care and undertook telephone interviews with a sample of GPs from intervention practices. Telephone interviews were offered to GPs in such a way as to cause minimum disruption to their working day. We sampled GPs purposively based on location, GP surgery, years of experience and practice demographics. We ceased recruitment when category saturation of data was achieved (n = 15). We used a flexible topic guide for all interviews with open-ended questions to encourage discussion. All interviews were audio-recorded with consent, anonymised and transcribed verbatim.
Further, we used routinely collected data from session audio tapes collected by care managers for supervision to analyse the process of implementation. We purposively sampled 30 files for transcription and analysis from 656 collected, to cover as wide a range as possible of care managers, patient sex and different treatment sessions, from assessment to the final session.
Analysis
Consistent with the mechanisms of change framework described by Kraemer and colleagues for randomised controlled trials, all of our analyses were exploratory, hypotheses generating activities. 62 Analyses were undertaken in Stata 12.1 following a predefined analysis plan.
To explore the role of moderators we analysed the direct effects of our six baseline covariates on depression severity (PHQ-9) at 4 and 12 months using multilevel multiple linear regression, with a preliminary step to assess ‘overall moderation’ (regression including all moderator variables), thereby controlling for type I error inflation as a result of performing a large number of statistical tests. 65 We used the xtmixed command in Stata, modelling surgery as a random effect, to account for nested structure of the data (patients nested within surgeries). Trial site location was modelled as a fixed effect rather than the top random-effects level to reduce model complexity and avoid non-convergence issues. Each of the six baseline covariates and their interactions with the intervention (through which we investigated moderation) were included in the overall model; all variables were appropriately centred. 66 We planned to proceed with individual moderator analyses only in the presence of ‘overall moderation’.
Potential mediating effects of collaborative care were investigated using structural equation modelling (SEM) in Stata. We analysed available data on the effect of medication adherence and behavioural activation at 4 months on PHQ-9 scores at 4 and 12 months and we explored the effect of medication adherence and behavioural activation at 12 months on the 12-month PHQ-9 scores. To explore and control for possible confounding,63,67 for mediators that were found to have a statistically significant effect on outcome, we analysed the effect of pre-randomisation variables on mediation by including all of the baseline covariates (hypothesised moderators) in the respective structural equation model. To investigate the effect of possible post-randomisation confounding variables on mediation, we analysed the direct effects of the collaborative care participants’ care manager and number of treatment sessions on the intervention group’s 4- and 12-month PHQ-9 scores using multilevel multiple linear regressions, with GP surgery modelled as a random effect and trial site as a fixed effect. We planned to proceed with mediation analyses (SEM) allowing for post-randomisation confounding variables only if there was evidence of a direct effect of care manager or number of treatment sessions on depression severity.
In all mediation and confounding analyses we analysed the effect of missing process data in sensitivity analysis, in which we used 1000 sample bootstraps. For the post-randomisation confounding investigation we performed an additional sensitivity analysis in which we controlled for all of the baseline covariates in the regression model and bootstrapped 1000 times.
Qualitative interview analysis
The transcripts from each interview formed the data. We used an iterative approach using constant comparison techniques68 and topic guides that we reviewed and adapted after each interview following discussions between authors as the study progressed, allowing for emerging themes to be incorporated into the topic guides. CCG, NC, EA and PS conducted an initial thematic analysis and coding,69 independently at first, and themes were agreed through discussion between researchers of different professional backgrounds (general practice, nursing, psychology). Following the thematic analysis we conducted a further theory-driven analysis of the data guided by the four main constructs of normalisation process theory24 (coherence, cognitive participation, collective action and reflexive monitoring), detailed in Box 1, building on a previous process evaluation in which we had used the normalisation process model71 to identify the work required to implement collaborative care for depression. 72 Our analyses aimed to identify barriers to and facilitators of the successful implementation of collaborative care into UK primary care.
Coherence: a set of ideas about the meaning, uses and utility of a practice (defined as an ensemble of beliefs, behaviours and acts that manipulate or organise objects and others), which hold the practice together and make it possible to share and enact it.
-
This is the sense-making work that people do individually and collectively when they are faced with the problem of operationalising some set of practices.
Cognitive participation: the symbolic and real enrolments and engagements of human actors that position them for the interactional and material work of collective action.
-
This is the relational work that people do to build and sustain a community of practice around a new technology or complex intervention.
Collective action: the chains of interactions that are the site of mental and material work to organise and enact practice, which might include reshaping behaviours or actions, employing objects or artefacts or reorganising relationships and contexts.
-
This is the operational work that people do to enact a set of practices, whether these represent a new technology or complex health-care intervention.
Reflexive monitoring: the continuous evaluation, both formally and informally, of implementation processes by participants, which may involve judgements about the utility and effectiveness of a new practice with reference to socially patterned and institutionally shared beliefs.
-
This is the appraisal work that people do to assess and understand the ways that a new set of practices affect them and others around them.
Source: from May and Finch70 and www.normalizationprocess.org (accessed 18 November 2015).
This analysis was conducted individually by CCG, NC, EA and LG and the final analysis was agreed through discussion, with data being tabulated to illustrate the four constructs of normalisation process theory. Disconfirmatory evidence was sought in the data throughout the analysis.
Audio-tape analysis
We analysed the implementation of the intervention by initial transcription of 30 audio files and then analysis of the files by reading the transcriptions, referring in detail to the trial manual. We undertook a thematic analysis69 similar to that for the interview data, with initial open coding of themes carried out first by SB and then by LG, utilising MAXQDA version 10 qualitative software [VERBI Software GmBH, Berlin, Germany; www.maxqda.com/ (accessed 10 December 2015)] to manage the data and develop codes and categories within the data set. Memos were used in the development of emerging themes, specifically comparing the interviews with the CADET manual and model of intervention. LG and SB met regularly to discuss, clarify and characterise the themes.
Results
Moderation
The level of participants’ depressive symptoms, number of previous depressive episodes, attitudes to antidepressant medication, attitudes to behavioural activation, number of limiting physical health problems and socioeconomic status at baseline are summarised in Table 15. There was little evidence of overall moderation of depression severity at 4 months (χ2 = 10.01; p = 0.35) or 12 months (χ2 = 5.63; p = 0.78). Multiple imputation data produced similar results (overall moderator effect at 4 months F9,37799.1 = 0.76; p = 0.66; at 12 months F9,30924.1 = 0.42; p = 0.93).
| Moderator (n)a | Mean (SD) score or %, CC vs. UC | 4-month coefficient (95% CI)b | 12-month coefficient (95% CI)c |
|---|---|---|---|
| PHQ-9 (505) | 17.54 (5.18) vs. 17.96 (5.02) | 0.49 (0.37 to 0.60) | 0.37 (0.25 to 0.49) |
| Number of previous depressive episodes (475) | |||
| 0 | 28.4 vs. 29.3 | Reference category | Reference category |
| 1 | 10.7 vs. 9.7 | –2.15 (–4.25 to –0.05) | –1.76 (–3.95 to 0.42) |
| 2–4 | 30.1 vs. 33.6 | –1.31 (–2.77 to 0.16) | –0.005 (–1.52 to 1.53) |
| 5+ | 16.2 vs. 15.4 | –0.004 (–1.85 to 1.84) | 1.46 (–0.47 to 3.40) |
| Chronically | 14.8 vs. 12.0 | 1.66 (–0.22 to 3.53) | 2.23 (0.27 to 4.19) |
| Positive attitude towards ADM (504) | 76.42 vs. 79.27 | –1.66 (–3.08 to –0.24) | –1.48 (–2.97 to 0.001) |
| Positive attitude towards BA (504) | 94.32 vs. 93.09 | –0.48 (–2.92 to 1.95) | 1.82 (–0.72 to 4.36) |
| Limiting physical problems (454) | 1.40 (1.46) vs. 1.59 (1.64) | 1.16 (0.78 to 1.54) | 0.77 (0.37 to 1.17) |
| Socioeconomic status (483) | 29.22 (16.13) vs. 33.47 (15.53) | 0.04 (–0.002 to 0.07) | 0.04 (–0.003 to 0.07) |
Mediation
Participants’ levels of behavioural activation, medication adherence and PHQ-9 scores at 4 and 12 months are provided in Table 16.
| Variable | 4-month score | 12-month score | ||
|---|---|---|---|---|
| Collaborative care | Usual care | Collaborative care | Usual care | |
| Behavioural activation, mean (SD) | 19.60 (11.11) | 16.04 (10.63) | 20.80 (11.73) | 17.27 (10.35) |
| Medication adherence (% incomplete) | 39.51 | 35.83 | 35.97 | 37.74 |
| Depression severity (PHQ-9), mean (SD) | 11.10 (7.28) | 12.68 (6.85) | 10.03 (7.10) | 11.74 (6.79) |
Effect of 4-month mediators on depression at 4 months
We found strong evidence of an effect of behavioural activation (coefficient 4.00, 95% CI 1.46 to 6.55) but not medication adherence (coefficient –0.03, 95% CI –0.14 to 0.08) on the intervention. The effect of behavioural activation led to strong evidence of an indirect effect of collaborative care on depression severity (coefficient –1.53, 95% CI –2.49 to –0.57). This is larger than the effect of collaborative care if the model did not include behavioural activation and medication adherence (coefficient –1.22, 95% CI –2.86 to 0.41). The effect of collaborative care at 4 months was therefore mediated in full by behavioural activation at 4 months.
When we undertook a structural equation model including behavioural activation at 4 months but not medication adherence we also found strong evidence for the effect of behavioural activation on the intervention (coefficient 3.56, 95% CI 1.78 to 5.34) and an indirect effect of collaborative care on depression severity (coefficient –1.33, 95% CI 1.99 to –0.67). There was little evidence of a direct effect of collaborative care (coefficient –0.08, 95% CI –1.10 to 0.94), suggesting that its effect on depression severity at 4 months was mediated in full by behavioural activation at 4 months. However, including the pre-randomisation variables in the model produced little evidence of an indirect effect of collaborative care, although the size of the effect was similar (coefficient –1.33, 95% CI –3.53 to 0.86). The direct effect of the intervention remained small and non-significant (coefficient –0.15, 95% –1.03 to 0.73). These results of the structural equation models were verified after a 1000-replication bootstrap.
Effect of 4-month mediators on depression at 12 months
When we analysed the effect of medication adherence and behavioural activation at 4 months on PHQ-9 scores at 12 months we found strong evidence of an effect of behavioural activation (coefficient 3.86, 95% CI 1.30 to 6.42) but not medication adherence (coefficient –0.01, 95% CI –0.12 to 0.17) on the intervention. There was strong evidence of an indirect effect of collaborative care on depression (coefficient –1.20, 95% CI –2.00 to –0.39), larger than the equivalent effect if the model did not include behavioural activation and medication adherence (coefficient –0.97, 95% CI –2.89 to 0.96). The effect of collaborative care at 12 months was therefore mediated in full by behavioural activation at 4 months.
Moreover, when we undertook SEM including behavioural activation at 4 months but not medication adherence we found strong evidence for the effect of behavioural activation on the intervention (coefficient 3.57, 95% CI 1.78 to 5.37) and an indirect effect of collaborative care on depression severity at 12 months (coefficient –1.03, 95% CI –1.54 to –0.52). Although there was little evidence of a direct effect of collaborative care and the magnitude was relatively small (coefficient –0.42, 95% CI –1.63 to 0.80), we observed partial (not full) mediation; the effect of the intervention on depression severity at 12 months did not completely pass through levels of behavioural activation at 4 months. Including the pre-randomisation variables in the model produced little evidence of an indirect effect of collaborative care (coefficient –0.86, 95% CI –2.53 to 0.80). The direct effect of the intervention was larger but non-significant (coefficient –0.77, 95% CI –1.91 to 0.36). The results of the structural equation models were verified after a 1000-replication bootstrap.
Effect of 12-month mediators on depression at 12 months
When we undertook a structural equation model including medication adherence and behavioural activation at 12 months we found weak evidence of a moderately sized effect of behavioural activation (coefficient 2.52, 95% CI –0.80 to 5.85) but not medication adherence (coefficient 0.02, 95% CI –0.12 to 0.15) on the intervention. There was weak evidence of an indirect effect of collaborative care on depression severity (coefficient –0.96, 95% CI –2.19 to 0.28), which was similar to the size and strength of the direct effect (coefficient –0.95, 95% CI –2.29 to 0.40). We therefore conclude that the effect of the intervention on depression severity at 12 months partly passed through levels of behavioural activation at 12 months.
Using similar procedures to those at 4 months, including behavioural activation at 12 months but not medication adherence we found that the effect of the intervention on depression severity at 1 year was partly mediated by level of behavioural activation at 12 months. There was strong evidence for the effect of behavioural activation on the intervention (coefficient 3.53, 95% CI 1.09 to 5.97) and an indirect effect of collaborative care on depression severity (coefficient –1.40, 95% CI –2.34 to –0.45). However, although the direct effect of the intervention is relatively small and non-significant (coefficient –0.31, 95% CI –1.38 to 0.76), the effect of the intervention on depression severity is not fully mediated by behavioural activation at 12 months. Including the pre-randomisation variables produced strong evidence of a larger indirect effect of collaborative care (coefficient –2.87, 95% CI –4.94 to –0.80) and little evidence of a direct effect (coefficient –0.62, 95% CI –1.74 to 0.50). Results were verified after a 1000-replication bootstrap.
Effect of post-randomisation confounding variables on mediation
When we undertook multilevel multiple linear regression to explore the effect of participants’ care manager and number of treatment sessions on the intervention group’s 4-month PHQ-9 scores we found little evidence of an effect of care manager (χ2 = 2.21; p = 0.99) or number of sessions (coefficient –0.11, 95% CI –0.38 to 0.15). We also found little evidence of an effect of care manager (χ2 = 7.57; p = 0.58) or number of treatment sessions (coefficient –0.07, 95% CI –0.32 to 0.17) on depression severity at 12 months. We observed no difference between the result of the multilevel multiple linear regression including observed data and the sensitivity analyses. The mediating effects of behavioural activation on treatment outcome were not confounded by care manager or number of treatment sessions.
Results of the qualitative interview analyses
We present our results using the a priori normalisation process theory concepts of coherence, cognitive participation, collective action and reflexive monitoring with respect to the implementation of collaborative care as described in the methods section. We present data to support the analysis, which is labelled by identifier (CM = care manager, S = supervisor, GP = general practitioner) and number.
The demographics of care managers and supervisors have not been included to ensure the anonymity of participants. GP demographics can be seen in Table 17. The initial thematic analysis is summarised in Table 18, with some illustrative data provided.
| GP | Sex | Years of experience as a GP | Practice population | Practice size | IMD rank | CADET recruitment figures | Actively involved in commissioning |
|---|---|---|---|---|---|---|---|
| GP001 | Female | 25 | African Caribbean, Asian, Eastern European and Turkish population, long stay, suburban | 14,000 | 4339 | 16 | No |
| GP002 | Male | 17 | 50% Caucasian, 50% Asian population, urban, deprived, socioeconomic mix, many family residents | 2800 | 2938 | 12 | Yes |
| GP003 | Male | 39 | Urban, mixed social class – less deprived (groups 1 and 2) | 8000 | 26,048 | 13 | No |
| GP004 | Male | 31 | Urban, mixed social class – less deprived (groups 1 and 2) | 8000 | 26,048 | 13 | No |
| GP005 | Female | 25–26 | Almost totally white population, not deprived, urban edges/semi-rural. Core of family-based patients | 2350 | 14,588 | 11 | No, but is mental health lead for primary care trust |
| GP006 | Male | 28 | 5–10% Asian population, one-third transient, two-thirds settled (lots of families), high-deprivation area, over-represented in mental health compared with other practices | 3500 | 1128 | 9 | No |
| GP007 | Female | 21 | White British population, high-deprivation area, high unemployment, many patients with smoking-related illnesses | 6000 | 317 | 13 | Yes in future |
| GP008 | Male | 15 | African Caribbean, Asian, Eastern European and Turkish population, long stay, suburban | 14,000 | 4339 | 16 | No |
| GP009 | Male | 14 | Younger population, high turnover. Eastern European, African Caribbean, South Asian, minority Far East Asian population, higher than ‘normal’ number of patients with mental health issues | 7500 | 1809 | 8 | Yes but resigning because of political nature |
| GP010 | Male | 30 | Diverse, multiethnic. Top 10% most deprived areas in the country. A lot of mental health issues | 8000 | 3428 | 12 | Not for last 18 months |
| GP011 | Male | 18 | Two branches, slightly different demographics in each. One has new Eastern European immigrants; other has significant Asian and African Caribbean population. Suburban teaching/training practice | 8300 | 9601/128,182 (two branches) | 12 | Not any more |
| GP012 | Male | 7 | Same surgery as above. This GP says this is an inner-city practice. Lots of people with English as a second language. Mobile patient population (high turnover) | 8300 | 9601/128,182 (two branches) | 12 | Not asked |
| GP013 | Male | 17 | Mainly white males aged 25–35 years, a few Asian, Chinese and black people | 7600 | 8179 | 16 | No |
| GP014 | Female | 10 | Mainly white males aged 25–35 years, a few Asian, Chinese and black people | 7600 | 8179 | 16 | No |
| GP015 | Male | 22 | Majority white British, very few black and minority ethnic groups | 7750 | 317 | 13 | No |
| Main theme | Subthemes | Illustrative data |
|---|---|---|
| Recognising the need for change | GPs’ understanding of current services | Theoretically we have access to counselling services. There is a group commissioned by the PCT [primary care trust] called [names team] which I think has changed over the years from being a purely sort of counselling service to one with a range of psychological servicesGP011 |
| Limited access to services | [P]sychological services as opposed to psychiatric acute services are dire locally, absolutely dire . . . we have such limited access, there’s just such a burden of . . . mild to moderate psychiatric illness and that isn’t well catered for at allGP001 | |
| Reflections on the past | The structure, I think, the way we used to work in the old days we used to work collaboratively anyway, which was really good, erm, but we haven’t got that structure now, so it’s about number crunching really, you know, in terms of referrals coming through to you, and being based at . . . a main health centre where they have to come to youCM102 | |
| Operationalising collaborative care | Understanding collaborative care | I was rereading the protocol for this session [interview] and thinking, should I have been doing more with GPs? Talking with them more about medication? So I thought, maybe I’ve done something kind of wrong and not quite completely as collaborative as I could have been, I think I probably could’ve done moreCM106 |
| Delivering the intervention | I didn’t really understand collaborative care; I’ll be quite honest . . . I didn’t know what collaborative care was, although I could have had a guess. Collaborative care would have meant care that involved both myself and someone else, if you see what I meanGP004 | |
| It’s a better experience for the therapist, I’ve kind of had a really positive experience of CADET, which I think if I’d purely had experience of IAPT [Improving Access to Psychological Therapies] I wouldn’t be feeling quite so positive about BA [behavioural activation] or telephone support or telephone supervision or whatever, so 100% I think it’s greatCM105 | ||
| Facilitating communication | Something that is quite helpful . . . if a client’s got an issue, especially something that is about medication, I will say you know, ‘why don’t you speak to your GP about that?’ and I will say ‘I will be writing to your GP just to let him know that this is what we’ve discussed’, so the client would go, I would write a letter on the other side as well, and it’s quite nice because the client would then come back and go ‘Oh yeah, the GP got your letter’ and when I speak to the GP they say ‘Oh yeah the client did come back to me after what you said’ so I think, it really does workCM104 | |
| [T]here’s that sort of linking where the GP was linked in, and I think that he was really pleased that erm, he was actually able to have a conversation with me about the medication, because he was actually feeling stuck and I think [names CM] was feeling a bit stuckS102 | ||
| [A] lot of the time I’ve also noticed that through the GP if you do mention that through supervision what I have been told is X, Y and Z, then they could be, you know, they could be more likely to listen as well, to accept your opinion, so yeah, I think that works quite well as well, if you do tell them ‘after discussing this in supervision, this is what we thought’CM104 | ||
| I’ve had very little, if any, involvement with the study except notification from you that a particular patient has been included on the studyGP004 | ||
| Enhanced supervision | I think sometimes I’ll write to them asking them something or asking their opinion of something, then the GP will kind of contact me, get back to me, and I think on one or two occasions I have had a GP ringing just to ask if I’d seen a client or when am I next seeing a client, so yeah, I think that’s the only thing, it’d not something that happens that often, one or two occasionsCM104 | |
| Communication vs. collaboration | It’s such a big problem; I’m not blaming anybody because GPs don’t have the time . . . You could try to make it happen, it would be nice just to see that, increasing that contact . . . it sounds like a very desirable thing that would be helpful for everybody . . . I think collaborative is too strong a word for collaborative care, it’s not truly collaborative in my opinion, but that’s my opinionS105 | |
| Catering for complexity | Recognition of complexity | I don’t think there is such a thing as pure depression, it comes in a package with lots of other things so when I say comorbid things, very often comorbid psychiatric problems, but also physical problems and never to forget, lots of social problems around, so you’ve got those three things there that are all competing, so there is a person with depression but at the same time there is obsessive–compulsive disorder, or query, you knowS104 |
| The need to avoid mind–body dualism | I think that would be really helpful actually, for us to have more understanding of physical health problems and how they affect people . . . we need to recognise physical health problems and long-term conditions and how they affect people . . . I think knowledge about those is really important, we just need to know moreCM106 | |
| I would have thought logically yes, it’s likely to be those sorts of people, the more complex your problem the more likely you are to benefit from it, erm, yeah, I would say comorbidity, absolutelyGP015 | ||
| Usefulness of a collaborative care approach for people with complex problems | I think that the whole thing about collaborative care isn’t about the interventions, it’s actually about the system, and so that case management role is great . . . you know, I guess if you’re saying, well there’s the system which is about active follow-up, is absolutely right and that covers all of these peopleS101 |
Understanding the collaborative care framework (coherence)
Behavioural activation, which formed the psychological intervention component of collaborative care in this study, was described by care managers as a user-friendly intervention and easy to understand, not just for themselves as practitioners but also for the patients, as they didn’t find it ‘too overcomplicated’ (CM105). The care managers did find that the intervention encouraged them to develop joint plans with patients to a greater extent than in their usual practice:
By collaborative care what do I mean? Erm, I mean more that sense of working with the patient . . . and I think it’s more about reaching a shared understanding and working towards shared goals with enough input from other professionals that are involved in that person’s care.
CM101
Supervisors and care mangers understandably demonstrated a good understanding of the collaborative care framework in addition to the intervention itself. For supervisors, this level of understanding was because of their role as co-investigators in the trial. Care mangers reported that the CADET training had provided them with sufficient information and opportunities to clarify and improve their understanding of collaborative care, the intervention they were to deliver to patients and the expectation of working with GPs. Care mangers described how their understanding of collaborative care and their role had been changed by the training prior to working on the trial:
I’d assumed [collaborative care] would be self-help-based stuff because we were primary care, and collaborating with other professionals. Since doing the training it’s mainly GPs that I’ve learned, but I kind of had the idea that it would be collaborating with other mental health workers, but not specifically GPs.
CM103
Only a minority of GPs demonstrated a good understanding of collaborative care, either because of their self-declared interest in mental health or because of previous experience of working within a collaborative care framework:
So we’ve got more likelihood of being aware of what’s happening in terms of the management and then that can affect any input that we might have, say in terms of medication if we’re treating patients with antidepressants, we can get a feel for whether things were moving in the right direction and get the therapists’ input as well as our own assessment. So it can potentially improve our understanding of how the patient is progressing and responding and aid our management.
GP010
However, the majority of GP respondents did not fully understand the collaborative care framework and could not differentiate between the management of patients with depression in collaborative care and routine care. As a result, some GPs used the qualitative interview as an opportunity for further clarification, perhaps suggesting a lack of such opportunities during their initial discussions about involvement in the trial:
Are you able to define collaborative care for me so I know what you’re talking about, or not?
Erm, I mean what we’re trying to do it get an understanding of your understanding of it, so if you’re not aware.
I mean they’re all buzz words, so collaborative care, what it actually means?
Some GPs described the main benefit of participating in the trial as the potential for increased support in their management of patients with depression in the context of limited access to psychological therapy services to which to refer patients:
The CADET trial offered to me a resource which I thought would be beneficial. Another opportunity for somebody else to look at these patients, talk to them and share the workload in a way, with me.
GP011
This GP is not reflecting specifically on the collaborative care framework; rather, she seems to be reflecting on the benefits of participation in any trial in which patients can access an additional ‘service’.
Most GPs identified the potential benefits of adopting a more collaborative approach to patient care, particularly for patients with more complex problems:
[I]t’s likely to be those sorts of people, the more complex your problem the more likely you are to benefit from it, erm, yeah, I would say comorbidity, absolutely . . . the more complicated the things are, the more likely it is that the collaborative approach is going to help.
GP015
It was not clear, even with probing in the interviews, what GPs actually meant by a ‘collaborative approach’ and GPs were not clear whether or not a collaborative care intervention would fit with their existing ways of working.
Establishing relationships (cognitive participation)
A number of new relationships needed to be established within the collaborative care intervention. Supervisors and care managers reported well-structured, weekly, scheduled supervisory sessions that were arranged as part of the trial. Supervisors and care managers reported the value of an initial face-to face meeting to establish the relationship, followed by weekly telephone supervision. Supervision was also supported by the PC-MIS, a web-based patient management system, demonstrating evidence of the work carried out for both establishing and sustaining collaboration between these two parties:
The supervision has been excellent I must say. It’s really nice to have it weekly, and it’s great to have PC-MIS because it means we’re both looking at the same screen, so it’s been really good.
CM103
Supervision as part of the CADET trial was also considered by care managers as ongoing learning, affirming to their practice and confidence boosting:
[T]hey might point stuff out to me or they might anticipate problems before they arrived which in my lesser experience maybe wouldn’t have foreseen so therefore they gave me some advice about how I might manage certain situations or what I might say to prepare a patient for something, erm, so yeah, it was fantastic, really, really good.
CM105
Supervisors also considered that supervision in the trial was superior to that received in usual care and highlighted the importance of such supervision to the success of collaborative care, with one describing it as an ‘integral part of . . . the whole collaborative care process’ (S102).
However, supervisors identified potential problems around identifying the right people to provide supervision outside of the research study, including finding people who are both willing and able to provide the same level of supervision as was delivered in the CADET study.
I think the biggest issue is the amount of supervisor time, and I think that, I think that we’re fairly generous in CADET in that the same supervisor is involved in following people up, and that means that you do get, that means that people do get really good supervision, but it’s quite, there’s quite a lot of time involved in that . . . It’s not that there’d be less time, there’d be less people that, erm, that are used to doing that kind of supervision.
S102
In contrast, there was limited evidence of new relationships being established between the care managers and GPs in participating practices. Any liaison between care managers and GPs consisted of written information from the care manager to the practice, with direct contact unusual and reported to have occurred only when risk was deemed high, with few reports of care managers having direct access to the practice information technology system:
[E]very 4 weeks we send a review letter, obviously you send the initial assessment letter to say ‘we’ve assessed this person, their main problem is, their scores are’ and then follow-up letters every 4 weeks.
CM105
Have you been able to access to the patient records, has there been a sharing of information?
Erm, there’s a couple where I’ve needed to, and I can’t remember what practice it was but I went there and she said I had to send them a letter, so I had to come back here to fax them and then they faxed me a letter back, it was a bit, kind of ridiculous.
One care manager did report having access to the patient records at some GP practices, but encountered different information technology systems in different practices, which was initially problematic, and she reported that developing good relationships with the practice administrative staff was essential to enable utilisation of these:
[T]he other barrier I had was using the different computer systems in different surgeries, so that was dead complicated, but I got past that, and I found the staff were great because they’d just come and sign you on and things like that, because I couldn’t remember the password.
CM102
As care managers were already working within existing services and were seconded to the trial, a minority of care managers described pre-existing relationships with GPs that they found beneficial to engaging GPs in the collaborative care framework. Care managers also described a number of strategies that they had attempted to use to enhance opportunities for collaboration with GPs, including identifying the GPs’ preferred method of communication at the beginning of the trial in anticipation of the need to communicate with them when working within a collaborative care framework:
Initially with the study, what I did was, I went out and visited the GPs . . . and just said ‘what’s the best way for communicating?’ . . . so it’s looking at what’s best for that GP, you know if you do get a relationship with them.
CM102
Data suggest that the work carried out around setting up supervision and establishing care manager–supervisor relationships was important and appreciated by both parties. However, direct contact between care managers and GPs seemed to be the exception rather than the rule, and occurred at a time of crisis for an individual patient. Additional work was needed by care managers, as well as building on prior knowledge of practices, to establish working relationships with GPs that would enable engagement as a routine.
Working within a collaborative care framework (collective action)
Care managers identified few difficulties in delivering the psychosocial intervention to patients; rather, they focused on the difficulties encountered in liaising or collaborating with GPs. Despite care managers reporting sending regular summary letters to GPs, the majority of GPs reported limited or no communication with care managers. It is unclear, therefore, whether GPs did not receive these letters or whether they did not have time to read them:
I’ve had very little, if any, involvement with the study except notification from you that a particular patient has been included on the study.
GP004
I don’t think I had any contact personally with the case manager. I think I saw a letter or two, but no sort of telephone or e-mail or anything of that sort.
GP007
Either way, the limited communication reported by some GPs may account for their lack of awareness of the involvement of the care managers in the trial and the work that was being carried out with their patients.
You said that there would be someone with more specialist interest might be involved, erm, did you know who else was going to be involved?
Recruiting patients?
Erm, so the person you would be collaborating with?
No.
No. OK. Erm, and so, are you aware now about the case managers that were involved in the study? That was involved in seeing the patient therapeutically?
No.
The lack of GP involvement is supported by some care managers’ reports that, although GPs were helpful once they had managed to contact them, GPs rarely initiated contact, which left care managers feeling that communication was one-sided:
[S]ince I’ve been working here, and that’s been 2 years now, I think I’ve only ever had GPs initiate contact with me twice. Yep. It’s really, really rare, which is a shame really.
CM105
Despite the difficulties identified in contacting GPs, care managers reported improved relationships with participating GPs, along with identifying the benefits of this:
Yeah, I think, I mean there are some GPs who are really difficult to get hold of or, you do write to them and you don’t get a response and you have to try to chase them up, but a lot of the time what I have found is that they are quite helpful, you know, certain GPs are very easy to talk to on the phone, or make appointments with, so that’s been quite helpful, and erm, yeah, kind of discussing the patient as well, it’s, you know, I can suggest something, they can give me their side of what they’re doing, again, come to some sort of conclusion.
CM104
Some care managers suggested that co-location within GP practices could bring more opportunities for collaboration with GPs because of the increased possibility of informal communication and they compared this with their previous ways of working:
[I]n the old days if we worked at a surgery, based there, it’s that relationship building that you have a chance to do, erm, and so at the moment we don’t do that as part of normal care, it’s harder to do, I think it’s impossible to do really, so what we get is, we’re based at one health centre and we get people from all different surgeries being referred through to that one health centre so we don’t get a chance to build those relationships.
CM102
Supervisors recognised the difficulty experienced in achieving true collaboration between care managers and GPs:
I mean you’ve got to have people together to collaborate, you know, I just wonder to what extent this really is collaboration, because it’s only collaboration in name, in a way and the interested parties don’t really get down and talk to each other very much . . . It’s such a big problem . . . I’m not blaming anybody because GPs don’t have the time. . . . You could try to make it happen, it would be nice just to see that, increasing that contact . . . it sounds like a very desirable thing that would be helpful for everybody . . . I think collaborative is too strong a word for collaborative care, it’s not truly collaborative in my opinion, but that’s my opinion.
S105
The supervisors recognised that the collaborative care framework did not seem to fit within existing working practices of GPs.
Probably because of the set-up and frequency of supervision, supervisors and care managers reported good professional relationships with each other. Supervisors and care managers reported being impressed with each other’s skills, suggesting confidence in each other’s abilities. More specifically, supervisors reported satisfaction with the care managers’ skills for delivering behavioural activation within a collaborative care framework, even to those patients identified as complex:
I’ve been pretty impressed by the ability of the case managers to assess and manage some people who have not always been that straightforward, by any means, and these are people who are supposed to have, you know, these are people who have I suppose moderate degrees of depression, but they’ve got complicated life problems as well, some of them have been in crisis, and they’ve managed them. I think it’s gone pretty well.
S102
Likewise, care managers were enthusiastic about what they considered to be enhanced supervision, because of its increased frequency and the supervisors’ wealth of experience and knowledge:
[T]hey might point stuff out to me or they might anticipate problems before they arrived which in my lesser experience maybe wouldn’t have foreseen so therefore they gave me some advice about how I might manage certain situations or what I might say to prepare a patient for something, erm, so yeah, it was fantastic, really, really good.
CM105
There was little evidence in the GP data that the work conducted by the care managers and supervisors had any impact on GPs’ routine consultations or their work with patients:
[A]s far as the CADET study is concerned, we’ve not . . . it’s happened alongside us really, it hasn’t had . . . it certainly hasn’t been detrimental to anything that we’ve been doing, but that’s not really what I mean. What I mean is that we identified patients but then didn’t need to change what we were doing very much.
GP007
Care managers reported that they had taken or planned to take many elements from collaborative care (such as increased collaboration with GPs and medication management, as well as the behavioural activation psychosocial intervention) back into their routine work, which demonstrates that this approach is acceptable to care managers and has the potential to become normalised within their routine practice:
What I will probably take back is a lot more information on medication . . . when I was working prior to that [CADET], the focus wasn’t so much on the medication, yeah, and I don’t think that I had much idea of medication, and I think now, there was a time when I wasn’t too keen on medication myself, I wasn’t too sure if medication really worked, whereas now I’ve seen that it is quite helpful so I would probably emphasise the medication with my patients, yeah, and I probably will take the whole BA [behavioural activation] in terms of being active and how that helps with the depression, so yeah, as a whole, the whole thing, but if there’s one thing I’m going to focus on more it’ll be the medication, yeah.
CM104
Our data suggest that organisational changes within practices would be required to establish relationships between care managers and GPs and facilitate successful collaboration, such as integrated information technology systems and enhanced opportunities for GP/care manager communication and possibly co-location of professionals. Collaborative care would need to be seen as fitting in with the routine work of the practice for GPs to make changes to accommodate the work involved.
Evaluating collaborative care (reflexive monitoring)
The weekly supervision presented regular opportunities for care managers and supervisors to reflect on patients and monitor their progress jointly. Collaborative care and the psychosocial intervention were described as effective and acceptable by care managers and supervisors, although it seems that care managers reflected on the perceived effectiveness of the psychosocial intervention (which formed the majority of their work with individual patients) rather than the collaborative care framework as a whole. The care managers described how they monitored patients through the collection of routine data (HADS), their own perceptions of patients’ progress and discussions within supervision:
[A] couple of people who, especially one, he’s had long-standing social anxiety so a bit more of a complicated problem, but also depression, and we just worked away on the depression and we saw an improvement, so just by doing that behavioural activation, so sometimes even though someone’s got more complex problems, for some people behavioural activation just saw quite an improvement, you know.
CM102
I think it’s effective . . . I think that has been the most satisfying part, that I know it can work, I’ve seen BA [behavioural activation] work.
CM105
Although care managers and supervisors identified some problems around delivering the trial psychological intervention (behavioural activation) in line with the protocol for those with comorbid mental health and complex social problems, the principles of intervention were still perceived to be acceptable in reducing symptoms of depression:
I think I would’ve liked to work on anxiety a bit more, but at the same time . . . we’ve watched those depression scores come down.
CM101
Some GPs did report receiving positive feedback from patients about their experiences with the care managers and of the intervention, which led the GPs to believe that there was some value in the intervention. This ‘second-hand’ knowledge was the only evidence on which GPs could reflect on the intervention, or on the collaborative care framework:
A significant amount of them have reported personally that they have felt better after participating in the trial, in the study and then whatever the numbers there is some benefit in it.
GP002
I think certainly with a number of patients they did seem to gain considerable benefit and their depression was improved and their general social functioning seemed to be improved . . . I didn’t get any negative feedback about the process.
GP006
In contrast to the care managers’ reports, GPs reported that they did not actively seek feedback from patients regarding their experience of collaborative care, and feedback was received only when volunteered by the patients:
Generally from the patients we have had very positive feedback, and often our patients are generally kind of if there is something they don’t like they will come and tell us.
GP009
Similarly, some GPs suggested that the results of the trial rather than their views would determine their opinion on the future possibility of working in a new way:
[O]ne of my managers doesn’t see how, if CADET really works, so, and at the same time I’m not sure because I’m waiting, I look for the actual, you know sometimes I think it hasn’t worked, sometimes I think it has worked . . . I suppose that’s where the results will show, whether that’s worked.
CM101
[W]e’re talking small numbers and I think we need to see some outcome data rather than just my anecdotal subjective views of possibilities.
GP010
The supervisors raised concerns about who would take on the responsibility of supervision of the care managers if collaborative care was implemented into routine practice, because of both the expertise and the time required to deliver supervision to the same standard and frequency as was delivered in the trial. Care managers also identified time as the biggest resource necessary to implement collaborative care, which included the time needed to maintain the prompt commencement of the intervention following referral, the time required for the administration involved in communicating with GPs and the time invested in supervision:
I think the collaborative care part of it, because, writing a letter after assessment and then keeping a GP updated with letters, often what happens at [names team], the GPs are sent a letter on discharge with a summary of what happened, so that’s kind of like no collaboration at all, for a lot of people there’s absolutely no collaboration, and that’s just down to time really and just the number of patients that everybody has.
CM106
However, GPs felt that the main obstacle to implementing collaborative care would be the financial cost of commissioning collaborative care services, which they perceived would be more expensive than current care:
What are your views on whether collaborative care should be commissioned as a service for management of people with depression in primary care?
I would say it is an excellent way forward. However, it couldn’t really have come at a worse time could it?
Could you explain that?
Well in terms of all the financial restrictions and all the changes that are going to be happening at the moment.
Thus, care managers and supervisors valued the care manager role encompassing expert supervision as well as the specific psychological intervention, including the behavioural activation and medication management components. Care managers placed less emphasis on the liaison between care manager and GP. GPs did not report actively reflecting on and monitoring the collaborative aspect of collaborative care, between care managers and GPs, but care managers described examples of liaison and how it might be facilitated. Care managers were positive about implementing collaborative care into routine practice, although possibly the emphasis was on the psychosocial intervention rather than the broader collaborative care framework; however, lack of time, concerns over supervisory arrangements in routine practice and the perceived cost of implementation were identified by all participants as barriers to this.
Results of the analysis of therapy recordings
Our analysis of the 30 session tapes sampled led to three emergent themes describing the process of treatment delivery: (1) engaging the patient, (2) adopting a counselling model and (3) variations in the delivery of behavioural activation. We describe these in the following sections.
Engaging the patient
The theme ‘engaging the patient’ describes the efforts and strategies employed by care managers to develop a therapeutic relationship with participants. The theme covers not only these strategies and skills but also communication examples in which care managers failed to connect with participants before trying to engage in the more functional aspects of the CADET clinical protocol.
In terms of achieving engagement, care managers would use scripts, not necessarily those provided in the CADET treatment manual, but rather from a routine framed by the organisation in which they worked. Care managers were not directly employed by the trial team but were working with CADET participants alongside their other responsibilities in the provider organisation for whom they worked. As such, their ‘script’ could represent standard introductory information that they were required to give as part of their usual clinical practice. In most cases, this made specific reference to the limits of confidentiality:
OK so I’ve got a bit of a script to read out first but the rest of it is just completely free-flowing conversation. It’s just to make sure I give you the information I need to begin with so I don’t miss anything out.
Care manager, assessment T4
It was clear, however, that one of the difficult tasks for the care managers was to merge the script described above with the need to address the immediate needs of the participant and to engage them in the CADET clinical protocol. Non-specific therapeutic skills such as simply providing encouragement and positive feedback seemed to help with achieving and maintaining engagement. A key skill that we observed here was the verbal expression of empathy:
Oh, I don’t really have much confidence in myself let’s put it that way, I just plod on, I just keep going through it.
You’ve obviously had a struggle for quite some time but somehow you have managed to keep going.
Assessment T3
Care managers did not always demonstrate engagement with participants and a number of examples of styles of communication that seemed to result in a failure of engagement could be identified in the sessions. There were examples of the care manager almost having a parallel dialogue in which the cues provided by the participant were not picked up on, because of the need to progress with the CADET interview task. One task, collecting factual assessment information, sometimes resulted in prolonged interrogative sequences in which the opportunity to pick up on key emotional cues was lost. Likewise, premature reassurance without understanding, acknowledging and empathising with the nature of the problems was not a successful intervention in achieving engagement:
I feel useless, like I’m a bad mother.
And what makes you say that?
I don’t know.
Because it seems like, although it is really hectic, it seems like there is a routine there and there is control and just having a routine is a base for children, because they come home and –
They’re always playing up and that so I just think I don’t have that much control over them that way, sometimes I just sit there and cry for nothing, I just feel like this is all there is to life, what happened to me basically, because I used to be such a bubbly, outgoing person.
Assessment T2
Adopting a counselling model
The theme ‘adopting a counselling model’ describes two particular types of interaction that moved beyond simply being empathic to engage the participant and towards something more recognisable as counselling. The first (delivered in response to life events/emotional cues from the participant) involved offering not simple empathic comments but explanatory hypotheses as a response to the participant talking about life difficulties, an approach more associated with psychodynamic therapies. The second counselling focus was when care managers talked about relationships rather than exploring the impact of depression on behaviours, triggers and consequences, and working towards goals. In several of the recordings, open-ended discussions of relationship issues rather than a focus on strategies to manage and address low mood were observed:
I all too easily see the negative side in me, what I, what’s wrong with me and what I haven’t done or what I did or shouldn’t have done or –
Sounds like you’re very hard on yourself.
I am yeah.
Well that’s going to have an impact on your confidence isn’t it? You know if, again if you’re seeing the redeeming features in everybody then perhaps any situation that comes up you might be more likely to take that on as if something bad happens then that’s something that you’ve done rather than –
Yes, yes, like it couldn’t be their fault because they’re not like that it must be me.
Yeah, ok, so you recognise that yet you still find that this has impacted on you in certain ways. Well you’re not alone with that, certainly and it sounds again like I mean how long were you with your wife for?
Assessment T4
This discussion topic could then become a primary feature of the shared problem statement, making it difficult to move on to functional aspects of the CADET protocol such as symptom monitoring, medication management, behavioural activation and GP liaison.
Variations in the delivery of behavioural activation
The theme ‘variations in the delivery of behavioural activation’ describes how care managers explained and delivered behavioural activation, a core element of the CADET clinical protocol. Behavioural activation was clearly described in the manual provided to the care managers and they received 5 days of training in how to deliver it. Nonetheless, we observed differing approaches from care managers in terms of initial explanations, identification of behavioural exercises and goal setting with participants. We also identified care managers focusing on the content of participants’ cognitions, a cognitive treatment strategy not part of the CADET clinical protocol.
In terms of explaining behavioural activation, some of the care managers managed a reasonably accurate explanation of it. In some sessions the explanation was brief and reduced simply to the idea of ‘getting going and doing things again’, with quite cursory reference to it during later sessions. In a similar way to the behaviours that we observed in the theme ‘engaging the patient’, this sometimes sounded rather like a one-way preprescribed ‘script’, with little opportunity for the participant to ask questions:
The idea is that depression leads to changes in how we behave, our routines change, we withdraw from things that we enjoy and we tend to avoid doing necessary and important things, so the idea is that by setting goals we act our way out of depression rather than waiting until we think that we’re ready to think our way out of depression. So behavioural activation is a structured and active method of helping yourself and it focuses on re-establishing daily routines, increasing the pleasurable activities that we do and addressing necessary issues so we’ll help you to regain the functions that may have been lost or reduced since you felt low. Does that make sense or –
Yeah, of course, get my brain back and functioning again, I’d appreciate that!
Assessment T5
There were examples of conversations in which care managers focused on the therapeutic effect of increasing physical activity or exercise, whereas the behavioural activation protocol instructed care managers to help participants increase a much broader range of personally relevant activities with the objective of re-establishing routines that had been reduced or disturbed during their depression. Likewise, we did observe variation in the way that care managers helped participants to set personal goals, another key element of the behavioural activation protocol. Some care managers left goals rather vague whereas others helped participants narrow down to specifics:
OK, what are your expectations and your ideas of what you’d like to achieve in the time that we’ve got together?
Just to be what people call normal, I suppose.
What does normal mean? What are you not doing now that you’d like to do by the time we’re finished working together?
Assessment T9
We saw how care managers could help participants to translate overall goals into specific activities. Planning of activities could be detailed to help participants know what activity they were going to do and how and when they were going to try and carry out the activity. The next two extracts show this variation clearly; in the first extract the first care manager tries to establish clear procedural detail whereas, in the second extract, the details remain somewhat vague and after cursory attention being paid to the detail of how a goal was to be achieved the interview moves on to the care manager’s own agenda:
Yeah, so what do you think you want to do over the next week to look at that, do you think there’s anything you could change to put that in your diary or?
Yeah, I think the first thing I should do is clean myself up really. I mean I’m not dirty or anything like that, don’t get me wrong, but my hygiene has gone out of the window looking at this, it really has.
So if you just look at the personal hygiene in a morning and work on that bit first that sounds reasonable doesn’t it, so what are your aims there, is it a wash or a shower or a wash one day and a shower the next?
Follow-up T7 (3)
Yeah, I think maybe make that as a goal for this week or the next time we speak, maybe to contact the Citizens Advice Bureau and see what you could do there, that’s quite an important thing to do at the moment. Well that’s good, excellent. I am going to go on to the HADS scale, the HADS questionnaire, do you have the paper in front of you?
Care manager, follow-up T2 (1)
In terms of care managers’ fidelity to the CADET clinical protocol, we did observe some use of clinical strategies that we had not included in the manualised protocol. As noted previously in this section, our care managers had established NHS clinical roles and were used to using a range of approaches as part of their work. One common element of their established working practices – addressing cognitions directly and using cognitive therapy concepts – could be seen to creep into their work with CADET participants even though this was not in the CADET clinical protocol:
[W]hen we’re feeling low we have those automatic negative thoughts and if you’re a person who tends to actually personalise things which is a thought bias then sometimes we can beat ourselves up about it. I’m certainly not thinking this is your fault, I’m thinking oh a sinus infection, that’s terribly painful.
Care manager, follow-up T4 (2)
Chapter 5 Results of long-term follow-up at 36 months
Introduction
Our original funding and protocol1 included only a maximum 12-month follow-up period. However, we were able to use some grant underspend to facilitate a no-cost extension from the MRC/NIHR to examine the long-term effects of collaborative care.
Our procedures were adapted from those described in the preceding chapters. Given our limited resources, 36-month follow-up interviews could be conducted only over the telephone or by post from a central site in Exeter.
Sample
Participants were those who were recruited and allocated at baseline to the intervention or control groups of the CADET study.
Ethical considerations
We applied for and received ethics approval for this trial extension from the NHS Health Research Authority, NRES Committee South West (NRES/07/H1208/60). Because of the unplanned nature of the 36-month follow-up, participants had not been warned that we would be contacting them at this point. Therefore, we sent them a letter explaining the trial extension with an opt-out slip attached that they could return to the research team if they did not wish to be contacted. If a participant could not be contacted by the research team, the GP and/or clinical commissioning group were contacted as appropriate and all reasonable attempts to re-establish contact were made.
Measures
Our outcomes at the 36-month follow-up point were depression (PHQ-9), quality of life (SF-36) and worry and anxiety (GAD-7).
Analysis
We assessed the baseline characteristics of participants followed up and compared them with the baseline characteristics of those lost to follow-up, using logistic regression predicting follow-up, adjusting for clustering, age, site, practice size and IMD. When differences between those followed up and those lost to follow-up were shown to be significant, we undertook further logistic regressions of treatment group on significant baseline variables, adjusted for age and minimisation variables.
We analysed outcome data by ordinary least squares or logistic regression, allowing for clustering by use of robust standard errors, adjusting at the cluster level for minimisation variables and site and at the individual level for age and, when appropriate, the baseline measurement of the variable.
We analysed the effect of missing data as a sensitivity analysis, estimated by chained regression equations multiple imputation48 using all available scale clinical scores, age, sex, practice variables, site and treatment group.
Results
At 36 months we obtained follow-up data, defined as primary outcome (PHQ-9) data, from 354 of the 581 participants who were observed at baseline (61% of those recruited).
Comparison between those followed up and those lost to follow-up
We compared the following variables between those followed up at 36 months and those lost to follow-up: intervention group, age, sex, baseline PHQ-9 score, baseline GAD-7 score and baseline SF-36 PCS score and MCS score. We defined a participant as being present at 36 months if he or she returned a PHQ-9 questionnaire. We successfully followed up 63.8% of the collaborative care group and 58.4% of the usual care group. Table 19 shows the comparison between those followed up at 36 months and those not followed up at 36 months. Table 20 shows the same analysis broken down by treatment group. There is little evidence of a difference at baseline between those followed up and those not followed up apart from a significant tendency for participants with a higher level of education to be followed up at 36 months. Table 21 shows education level by treatment allocation for those participants with data at 36 months. Our logistic regression of group on education, adjusted for age and minimisation variables, gives χ2 (4 degrees of freedom) = 7.8 (p = 0.1) indicating that there is no evidence that education differs between treatment groups in those followed up for 36 months.
| Variable at baseline | PHQ-9 data at 36 months | No PHQ-9 data at 36 months | p-valuea |
|---|---|---|---|
| n | 354 | 227 | |
| Collaborative care group, n (%) | 176 (49.7) | 100 (44.1) | 0.2 |
| Male sex, n (%) | 102 (28.8) | 61 (26.9) | 0.7 |
| Age (years), mean (SD) | 44.5 (12.4) | 45.1 (14.6) | 0.6 |
| Ethnic origin white British, n (%) | 305 (86.2) | 189 (83.3) | 0.2 |
| Education, n (%) | |||
| None | 67 (18.9) | 61 (26.9) | 0.004 |
| GCSE/O-level | 87 (24.6) | 59 (26.0) | |
| Post GCSE/O-level | 94 (26.6) | 69 (30.4) | |
| Degree or higher | 78 (22.0) | 24 (10.6) | |
| Other or don’t know | 28 (7.9) | 14 (6.2) | |
| Employed or self-employed, n (%) | 105 (29.7) | 61 (26.9) | 0.2 |
| Married or cohabiting, n (%) | 149 (42.1) | 92 (40.5) | 0.7 |
| Prescribed antidepressants, n (%) | 292 (82.5) | 188 (82.8) | 0.9 |
| CIS-R score, mean (SD) | 28.8 (9.3) | 30.3 (8.9) | 0.06 |
| ICD-10 diagnosis, n (%)b | |||
| Mild | 50 (14.2) | 33 (14.5) | 0.5 |
| Moderate | 203 (57.5) | 120 (52.9) | |
| Severe | 100 (28.3) | 74 (32.6) | |
| History of depression, n (%) | 258 (72.9) | 164 (72.2) | 0.9 |
| Anxiety disorder, n (%) | 344 (97.2) | 226 (99.6) | 0.06 |
| Long-standing physical illness, n (%) | 230 (65.0) | 140 (61.7) | 0.3 |
| PHQ-9 score, mean (SD) | 17.5 (5.1) | 18.2 (5.1) | 0.1 |
| GAD-7 score, mean (SD) | 13.0 (5.1) | 13.7 (5.0) | 0.2 |
| SF-36 PCS score, mean (SD) | 44.5 (12.1) | 44.8 (12.7) | 0.6 |
| SF-36 MCS score, mean (SD) | 23.4 (10.0) | 21.7 (10.7) | 0.08 |
| Variable at baseline | Collaborative care | Usual care | ||
|---|---|---|---|---|
| PHQ-9 data at 36 months | No PHQ-9 data at 36 months | PHQ-9 data at 36 months | No PHQ-9 data at 36 months | |
| n | 176 | 100 | 178 | 127 |
| Male sex, n (%) | 48 (27.3) | 26 (26.0) | 54 (30.3) | 35 (27.6) |
| Age (years), mean (SD) | 44.7 (12.1) | 45.6 (15.0) | 44.3 (12.8) | 44.8 (14.2) |
| Ethnic origin white British, n (%) | 149 (84.7) | 84 (84.0) | 156 (87.6) | 105 (82.7) |
| Education, n (%) | ||||
| None | 28 (15.9) | 26 (26.0) | 39 (21.9) | 35 (27.6) |
| GCSE/O-level | 37 (21.0) | 28 (28.0) | 50 (28.1) | 31 (24.4) |
| Post GCSE/O-level | 57 (32.4) | 27 (27.0) | 37 (20.8) | 42 (33.1) |
| Degree or higher | 38 (21.6) | 11 (11.0) | 40 (22.5) | 13 (10.2) |
| Other or don’t know | 16 (9.1) | 8 (8.0) | 12 (6.7) | 6 (4.7) |
| Employed or self-employed, n (%) | 55 (31.3) | 27 (27) | 50 (28.1) | 34 (26.8) |
| Married or cohabiting, n (%) | 87 (49.4) | 40 (40.0) | 62 (34.8) | 52 (40.9) |
| Prescribed antidepressants, n (%) | 144 (81.8) | 87 (87.0) | 148 (83.1) | 101 (79.5) |
| CIS-R score, mean (SD) | 28.4 (9.7) | 29.4 (8.6) | 29.6 (9.0) | 31.3 (8.6) |
| ICD-10 diagnosis, n (%)a | ||||
| Mild | 27 (15.3) | 15 (15.0) | 23 (13.0) | 18 (14.2) |
| Moderate | 97 (55.1) | 59 (59.0) | 106 (59.9) | 61 (48.0) |
| Severe | 52 (29.6) | 26 (26.0) | 48 (27.1) | 48 (37.8) |
| History of depression, n (%) | 131 (74.4) | 71 (71.0) | 127 (71.3) | 93 (73.2) |
| Anxiety disorder, n (%) | 169 (96.0) | 100 (100.0) | 175 (98.3) | 126 (99.2) |
| Long-standing physical illness, n (%) | 112 (63.6) | 59 (59.0) | 118 (66.3) | 81 (63.8) |
| PHQ-9 score, mean (SD) | 17.2 (5.3) | 17.7 (5.1) | 17.8 (4.9) | 18.5 (5.1) |
| GAD-7 score, mean (SD) | 12.5 (5.4) | 13.6 (5.2) | 13.5 (4.7) | 13.8 (4.9) |
| SF-36 PCS score, mean (SD) | 44.7 (11.6) | 44.8 (13.6) | 44.2 (12.6) | 44.8 (12.0) |
| SF-36 MCS score, mean (SD) | 23.5 (9.2) | 22.7 (12.1) | 23.3 (10.7) | 20.9 (9.5) |
| Education | Collaborative care, n (%) | Usual care, n (%) |
|---|---|---|
| None | 28 (15.9) | 39 (21.9) |
| GCSE/O level | 37 (21.0) | 50 (28.1) |
| Post GCSE/O level | 57 (32.4) | 37 (20.8) |
| Degree or higher | 38 (21.6) | 40 (22.5) |
| Other or don’t know | 16 (9.1) | 12 (6.7) |
Outcomes
Summary statistics for available data are presented in Table 22. The results for the effect of collaborative care at 36 months using the available data are reported in Tables 23 and 24.
| Measure | Collaborative care | Usual care | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Minimum | Maximum | n | Mean | SD | Minimum | Maximum | |
| PHQ-9 score baseline | 176 | 17.2 | 5.3 | 5 | 27 | 178 | 17.8 | 4.9 | 4 | 27 |
| PHQ-9 score 36 months | 176 | 10.2 | 7.2 | 0 | 27 | 178 | 10.4 | 7.1 | 0 | 27 |
| GAD-7 score baseline | 139 | 12.3 | 5.5 | 1 | 21 | 141 | 13.2 | 4.6 | 3 | 21 |
| GAD-7 score 36 months | 140 | 8.2 | 6.5 | 0 | 20 | 141 | 8.1 | 6.3 | 0 | 20 |
| SF-36 MCS score baseline | 137 | 23.7 | 9.4 | 1.3 | 44.8 | 137 | 23.9 | 10.6 | –3.9 | 50.4 |
| SF-36 MCS score 36 months | 139 | 37.1 | 15.2 | –1.8 | 63.6 | 138 | 37.0 | 14.6 | 5.4 | 63.4 |
| SF-36 PCS score baseline | 137 | 44.7 | 12.2 | 17.7 | 70.7 | 137 | 43.9 | 12.3 | 18.2 | 68.2 |
| SF-36 PCS score 36 months | 139 | 45.4 | 12.1 | 15.9 | 66.2 | 138 | 44.3 | 12.9 | 13.5 | 66.0 |
| Measure | Collaborative care | Usual care | Adjusted difference | 95% CI | p-value | Effect size | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||||
| PHQ-9 score 36 months | 176 | 10.2 | 7.2 | 178 | 10.4 | 7.1 | –0.04 | –1.59 to 1.66 | 1.0 | 0.01 |
| GAD-7 score 36 months | 140 | 8.2 | 6.5 | 141 | 8.1 | 6.3 | 0.53 | –0.78 to 1.85 | 0.4 | 0.10 |
| SF-36 MCS score 36 months | 139 | 37.1 | 15.2 | 138 | 37.0 | 14.6 | 0.42 | –3.40 to 4.24 | 0.8 | 0.04 |
| SF-36 PCS score 36 months | 139 | 45.4 | 12.1 | 138 | 44.3 | 12.9 | 0.69 | –1.79 to 3.17 | 0.6 | 0.06 |
| Collaborative care | Usual care | ORa | 95% CIb | p-valueb | Number needed to treatc | |||
|---|---|---|---|---|---|---|---|---|
| n | Recovered/responded, n (%) | n | Recovered/responded, n (%) | |||||
| Recoveryd | ||||||||
| 36 months | 176 | 87 (49.4) | 178 | 78 (43.8) | 1.29 | 0.77 to 2.15 | 0.3 | 18.8 |
| Responsee | ||||||||
| 36 months | 177 | 81 (46.0) | 178 | 70 (39.3) | 1.33 | 0.80 to 2.21 | 0.3 | 15.6 |
Depression
There was no significant effect of collaborative care on depression (n = 354). The mean PHQ-9 score was 0.04 scale points lower (95% CI −1.59 to 1.66; p = 1.0) in participants receiving collaborative care than in those receiving usual care (standardised effect size 0.01, 95% CI −0.31 to 0.33) (see Table 23). More participants in collaborative care than those in usual care met criteria for recovery (OR 1.29, 95% CI 0.77 to 2.15; number needed to treat 18.8) and response (OR 1.33, 95% CI 0.80 to 2.21; number needed to treat 15.6) but neither difference was significant (p = 0.3 for both) (see Table 24).
Anxiety
With data available for 281 (48.4%) participants we found no significant effect of collaborative care on anxiety as measured by the GAD-7 (mean difference between collaborative care and usual care 0.53, 95% CI −0.78 to 1.85; p = 0.4) (see Table 23).
Quality of life
Mental health
With data available for 277 (48%) participants we found a non-significant difference (mean difference 0.42, 95% CI −3.40 to 4.24; p = 0.8) in the SF-36 MCS score between collaborative care and usual care.
Physical health
We found no significant effect of collaborative care on quality of physical health at 36 months (n = 277) as measured by the SF-36 PCS score (mean difference between collaborative care and usual care 0.69, 95% CI −1.79 to 3.17; p = 0.6).
Missing data
We undertook additional analyses using imputation. The imputed estimates in Table 25 are very similar to the available data estimates (see Table 23) so we can conclude that for all of these analyses the effects of collaborative care are little affected by missing data. Missing data were also not related to intervention group (Table 26). At 36 months, we conclude, therefore, that, despite the inevitable large losses to follow-up over 3 years, there is no evidence of bias in the treatment effect estimates from available data.
| Scale | Data | Coefficient | Robust standard error | t | p-value | 95% CI |
|---|---|---|---|---|---|---|
| PHQ-9 score, 36 months | Imputed | −0.18 | 0.73 | −0.25 | 0.8 | −1.65 to 1.30 |
| Available data | 0.04 | 0.81 | 0.05 | 1.0 | −1.59 to 1.66 | |
| GAD-7 score, 36 months | Imputed | 0.53 | 0.67 | 0.79 | 0.4 | −0.84 to 1.90 |
| Available data | 0.53 | 0.65 | 0.82 | 0.4 | −0.78 to 1.85 | |
| SF-36 MCS score, 36 months | Imputed | −0.12 | 1.71 | −0.07 | 0.9 | −3.62 to 3.38 |
| Available data | 0.42 | 1.90 | 0.22 | 0.8 | −3.40 to 4.24 | |
| SF-36 PCS score, 36 months | Imputed | 0.94 | 1.14 | 0.83 | 0.4 | −1.41 to 3.29 |
| Available data | 0.69 | 1.23 | 0.56 | 0.6 | −1.79 to 3.17 |
| Intervention group, n (%) | OR for missing data in collaborative care group | p-value (robust standard error) | ||
|---|---|---|---|---|
| Collaborative care | Usual care | |||
| Missing PHQ-9 data at 36 months | 99 (35.9) | 127 (41.6) | 1.26 | 0.20 |
Summary
Our results at 36 months’ follow-up showed that, after 3 years, participants in this trial were greatly improved with regard to depression, anxiety and quality of mental health. There were no longer any treatment differences, suggesting that the usual care participants had caught up with the earlier greater improvements in the collaborative care group. There was little change in physical quality of life; however, we would not have expected this as both collaborative care and usual care in this population were directed towards participants’ mental health. This provides evidence for the validity of the trial.
Chapter 6 Discussion
This chapter utilises material from three2,3,61 of the four open-access articles previously published by the research team in accordance with the terms of the Creative Commons Attribution (CC BY 3.0) licence explicitly permitting the unrestricted distribution, remixing, adaption and reuse of these works.
We found that collaborative care improved depression at our primary end point of 4 months compared with usual care, effects that persisted up to 12 months. Collaborative care is cost-effective when service commissioners are willing to pay up to £20,000 per QALY gained and was preferred by patients over usual care. The differences in clinical outcomes between participants treated by collaborative care and participants treated by usual care were no longer apparent at 36 months’ follow-up. In our process analyses we demonstrated that only one variable, the amount of behavioural activation undertaken by participants, predicted better outcomes, despite the fact that there was considerable variation in how behavioural activation was both explained and operationalised by care managers in sessions. We also found that case managers and supervisors regarded collaborative care as coherent but that the collective action required to implement elements of collaborative care was made difficult by GPs’ lack of engagement with the collaborative care framework.
Clinical outcomes
Collaborative care improved depression compared with usual care at both 4 and 12 months’ follow-up. Our observed effect size was less than that used to power the study, although the 95% CI around it (0.26, 95% CI 0.07 to 0.46) encompassed our original target (0.4). Our result also lies within the 95% CI of the SMD found in the most recent meta-analysis of collaborative care,73 which includes our results (overall SMD 0.28, 95% CI 0.23 to 0.33), and is no different from US (SMD 0.28, 95% CI 0.21 to 0.35), non-US excluding the UK (SMD 0.36, 95% CI 0.13 to 0.59), and other UK (SMD 0.32, 95% CI 0.07 to 0.57) trials. 23 Collaborative care is as effective in the UK health-care system, an example of an integrated health system with a well-developed primary care sector, as in the USA. Our study adds to the emerging international literature from countries such as Chile26 and India27 indicating that collaborative care is a model that reliably generalises outside the USA.
We also found that participants rated their satisfaction with treatment more highly in the collaborative care group than in the usual care group, the largest difference between groups of any of our measures. These results are in line with data showing that most participants were adherent to collaborative care, with the majority receiving more than two contacts with care managers and the average contact rate being between five and six sessions. Collaborative care is therefore not only effective but patients receiving it are more satisfied with their care than those receiving usual care and are adherent to the intervention.
In the long-term, at 36 months, there was no significant difference between the groups. This confirms the picture described in the recent Cochrane review23 in which the clinical benefits of collaborative care were not found beyond 2 years after the intervention. Despite the lack of significant difference it is worth noting that in the collaborative care group > 5% more participants had responded or recovered at 36 months.
Economic outcomes
Although previous reviews74,75 have identified evidence from cost–utility (cost per QALY) studies to support the economic value of collaborative care for depression in the US health-care system, the CADET trial is the first study to estimate the cost-effectiveness of collaborative care in a UK primary care setting. We have shown that collaborative care is cost-effective compared with usual care in treating people with depression in a UK primary care setting when providers are willing to pay up to £20,000 per QALY gained. Furthermore, when taking a broader analytical perspective and including costs associated with informal care, the results show that collaborative care is expected to be cost saving, with expected health gains, and therefore dominates the usual care comparator.
Our cost-effectiveness analyses report an expected modest mean QALY gain for collaborative care at a relatively low cost. Although the mean QALY gain is modest, it is comparable, and favourable, to that recently reported in the evaluation of a UK Improving Access to Psychological Therapies (IAPT) service,76 which estimated a mean EQ-5D QALY gain of 0.014 (SF-6D gain of 0.008). Our estimated costs for health and social care in the CADET trial are similar to those reported in the IAPT service evaluation for IAPT service or comparator mental health-care services. 76 Furthermore, despite differences in the populations, the QALY gain from the collaborative care intervention is in a similar range to that reported in an evaluation of therapist-delivered cognitive–behavioural therapy for depression,77 in which the mean incremental QALY benefit was reported as 0.027 (95% CI –0.012 to 0.066).
Our base-case difference in health and social care (NHS and Personal Social Services) costs over 12 months (£272.50) and the subsequent cost per QALY estimate of £14,248 are heavily influenced by one participant who reported extremely high levels of service use for specialist care, including a 100-day stay in an acute psychiatric hospital. This participant, in the collaborative care group, had an estimated service use cost of £48,522 compared with a mean cost of £1637 for all other trial participants with cost data over 12 months (n = 446) averaged across both groups; 94% of participants had cost estimates of < £5000, all but four participants had cost estimates of < £10,000 and three participants had costs estimated between £10,000 and £24,000. When we excluded this one participant from the analyses, the difference in NHS and personal social services costs between collaborative care and usual care when including the cost of collaborative care was £63, with an estimated cost per QALY of £3334.
Our probabilistic analyses indicate that collaborative care has a 58% and 65% probability of being cost-effective at commonly assumed UK NICE willingness-to-pay thresholds of £20,000 and 30,000 per QALY respectively. When we considered the uncertainty around the cost-effectiveness estimate that excludes one participant with high service use and costs (£3334 per QALY), the probability of collaborative care being cost-effective at these cost per QALY thresholds was > 75%. The most conservative expectation (based on the intention-to-treat principle) would be that the introduction of collaborative care will involve an additional cost of £272.50 per participant for the UK NHS and this potential cost, alongside estimated EQ-5D QALY gains, will result in an expected cost per QALY of £14,342, which is similar to the base-case analysis presented here and represents a cost-effective use of NHS resources. We would suggest that the likely cost-effectiveness of collaborative care in practice might be closer to the estimate in the sensitivity analysis with the very high-cost participant excluded.
Process analyses
The principal finding from our moderation and mediation analyses was that the effects of collaborative care on depression at 4 months’ follow-up can be entirely attributed to the amount of behavioural activation undertaken by participants and the effects at 12 months are strongly mediated by behavioural activation. Despite the fact that collaborative care is a complex intervention that also includes medication management, we found evidence only for the mediating effect of behavioural activation. We found no evidence that depression severity, number of previous depressive episodes, attitudes towards treatment components, physical health problems or socioeconomic status influenced treatment outcome, and nor did we find that the care manager, number of contacts between care manager and participant and adherence to medication influenced outcome, albeit our study was not powered to detect these effects per se.
The second component of our process analysis, interviews with care managers, supervisors and GPs, showed that collaboration around the management of patients with depression in primary care was valued by professionals. However, GPs’ understanding of collaborative care, compounded by long-standing organisational barriers, hindered their engagement in the intervention. It is unclear whether or not more GP engagement would have led to better outcomes. Enhanced supervision as reported in this study may be collaboration enough to result in improved patient care.
Our final process evaluation element, in which we analysed care manager/participant contact audio-tape recordings, revealed variation in the way that care managers engaged participants, how they behaved clinically and how they undertook behavioural activation. Verbal expression of empathy, encouragement and positive feedback seemed to be key in achieving both initial engagement and its maintenance. Some care managers strayed from merely demonstrating empathy into a style of treatment more closely identifiable as counselling. There was variation in how the elements of behavioural activation were demonstrated by care managers, with considerable difference in how behavioural activation was both explained and operationalised. Despite the protocol focus on behaviour alone, some care managers also chose to address cognitions.
Strengths and limitations of the study
The CADET study is one of the largest studies of collaborative care with an integrated economic evaluation. Less than 50% of published collaborative care trials have followed up participants for ≥ 12 months and our levels of attrition at 4 and 12 months are comparable with those in 70% of collaborative care trials and better than those in other trials of brief interventions in this area. 78 There was no evidence that missing follow-up data biased the findings, even at 36 months when, not surprisingly, attrition was higher than at other follow-up time points.
Although our cluster design protected against contamination of the usual care arm by changes in behaviour being tested in the collaborative care arm, cluster trials are prone to selection bias. We minimised this bias by recruiting participants through electronic case note searches rather than clinician referral. Given the nature of the intervention and comparator we could not blind GPs, patients or care managers to treatment allocation but we used self-report outcome measures to minimise the impact of detection bias. The supervisors who we interviewed were also CADET co-investigators and therefore their views are likely to be framed by their academic investment in the study. As CADET trial researchers conducted the qualitative interviews some researcher bias may be evident, as this is likely to have affected the participants’ responses, particularly those of the supervisors and care managers. 79 We attempted to use interviewers from another study site to reduce this bias. Although purposive sampling of GPs was attempted, GPs were difficult to recruit to this qualitative study, with a majority of those who refused citing lack of time or limited involvement in the trial as reasons for this. However, category saturation was achieved within the data, although the difficulties experienced in recruiting GPs may mean that the data may not represent the views and experiences of GPs in all participating practices.
Our within-trial analysis demonstrates cost-effectiveness at the willingness-to-pay threshold of £20,000 per QALY, without the need to extrapolate potential benefits over the longer term. Our analyses used data collected within the trial to estimate resource use and costs associated with delivery of the intervention, but relied on self-report data from interviewer-administered questionnaires to estimate health and social care service use and broader resource impacts. Routinely collected service use data may have provided a more rigorous estimate of service use, particularly for primary care contacts. 80 However, there would be difficulties and costs related to the collection of service use data from 42 general practices and to necessary routine data collection for aspects of care not recorded in GP records and therefore we chose to use participant self-report data. We also relied on self-reported records of care manager contacts and so have no means to assess record accuracy.
Difficulties in collecting detailed data on medication use by self-report methods can lead to errors in self-reporting. These errors include the potential for variation in medication names, variation in reported dose and complexity in relation to the use of medications for a wide range of comorbid conditions. These issues led us to exclude medication costs from the economic analysis plan1 and this may be a limitation in the results presented here, as medication adherence has been shown to be one of the potential benefits of collaborative care. 23 However, most participants in both the collaborative care group and the usual care group remained on antidepressant medication (74.8% vs. 73.8% at 4 months; 69.7% vs. 69.2% at 12 months). Finally, the collection of resource use data using self-report methods over 4-month and 8-month durations may have introduced recall bias and this has not been explored as part of the current analyses.
Although care managers were already employed by organisations providing primary care mental health services in the UK NHS, supervisors were senior members of the investigator group and so it is unclear how much their, albeit minimal, supervision can be generalised beyond the trial. Our intervention was brief and it is possible that a more intensive intervention might have improved outcomes further, particularly for the more complex cases. We could have chosen a different psychological intervention such as cognitive–behavioural therapy81 but a review38 and a randomised controlled trial82 showed that behavioural activation is as effective as cognitive–behavioural therapy, potentially more so for severe cases,83 and can be delivered effectively by junior, less intensively trained health-care personnel. 82
The perspective on the analyses does not extend to the broad welfare and economic impacts of depression, including impact on productivity costs, as such costs are not included in the reference case analyses suggested by NICE56 for UK analyses. However, data collection did cover aspects of care and support and patient costs, which has extended the primary perspective (of NHS and Personal Social Services costs) to a broader patient- and societal-orientated perspective. We accept that the use of a relatively small number of categories for these broader considerations may be a limitation in the analyses. However, as in other studies (e.g. Romeo et al. 84), we found that resources and estimated costs associated with informal care were a dominant aspect when taking a wider perspective and this gives us clear guidance on the magnitude of these wider perspective costs.
Implications of the clinical and economic findings for the NHS
During the time that we undertook the CADET study the number of international trials of collaborative care more than doubled, albeit with many of them still conducted in the USA. Although the generalisability of a US collaborative care model to the UK had been suggested by previous small-scale studies, the CADET trial has provided definitive confirmation of generalisability and that collaborative care is preferred over usual care by depressed patients and is cost-effective in the UK. We have therefore answered a specific research need highlighted in the NICE guidelines for depression,28 providing critical evidence for service delivery improvement. The NICE guidelines can now be reviewed to reassess the place of collaborative care in the stepped care pathway in light of our findings that collaborative care is clinically effective and cost-effective for a range of depressed patients, not necessarily those who fail at other treatments.
Although our results sit within the expected effect range of collaborative care reported in the latest meta-analysis of international collaborative care trials,23 the clinical implications of our results are more difficult to interpret given that the average difference in treatment response was less than that we had anticipated (actual effect size 0.26 vs. 0.4 anticipated). Between-group differences can obscure response rates in individual patients. We have therefore presented the data on meaningful clinical difference using numbers needed to treat and two criteria commonly applied in the depression literature and regarded as clinically meaningful: that of recovery (falling below a recognised point on the PHQ-9 symptom scale) and response (a ≥ 50% reduction in symptoms of depression). Using these metrics it is particularly noteworthy that at 12 months 56% of patients receiving collaborative care were ‘recovered’, 15% more than in usual care. Health services would therefore need to treat 6.5 patients using collaborative care to produce one additional patient with a sustained recovery compared with usual care.
Studies that have achieved higher effects have been undertaken in countries with less developed primary care services26,27 or have used more highly qualified workers such as nurses or social workers. 85 These workers are in acute short supply in the UK and hence this would not have been a translatable model for our health system.
Our results represent a slightly higher recovery rate than that reported by the UK IAPT programme. Recovery rates of around 45% have been achieved in the IAPT programme following an investment of £700M over 6 years. 86 We suggest that integration of our CADET protocol into IAPT services might enhance outcomes for depressed patients receiving treatment and provide guidance to international mental health services that this model can be applicable outside the USA.
The finding that collaborative care is the dominant intervention compared with usual care when we included informal care costs in our analyses is an important issue. Family members and others involved in informal care contribute to the care of depression in a substantial manner. As Richard Layard87 and others have often asserted, the implications of these care costs on productivity are a significant burden on the economic activity of a nation. The introduction of collaborative care has the potential to relieve some of the significant burden that falls on informal carers and reduce this economic load.
Given that collaborative care is more effective over a sustained period of time than usual care and represents value for money to the NHS at commonly used thresholds for cost per QALY, we suggest that commissioners of health care in the UK might review the organisation of their routine depression management services and consider using a collaborative care model. Evidence here indicates that services could benefit from being commissioned to support the management of patients with depression in UK primary care using a collaborative care model, as it would be both clinically effective and cost-effective to do so.
Implications of the results for treatment development and future research
Although collaborative care is an organisational intervention that improves outcomes, much remains to be done to improve the effectiveness of treatments for depression. Even intensive psychological treatments for depression have been shown to achieve only modest gains (effect size of 0.42 in 51 studies88). Our careful selection of intervention ingredients, directed by our identification of components present in the better-performing trials from our previous metaregression,89 did not succeed in achieving the larger effects we had hoped for. In the trial, 44% of participants receiving collaborative care had PHQ-9 scores that remained above the PHQ-9 depression threshold at 12 months’ follow-up.
The strong finding that behavioural activation was the only mediator of effect is important. Behavioural activation is hypothesised to operate by providing people with more opportunities for positively reinforcing experiences and reducing the amount of negatively reinforced avoidance behaviours, thereby improving affect. This hypothesis would appear to be supported by our results. We would encourage future researchers to measure the mediational contribution of behavioural activation to patient outcomes in other trials, to determine the universality or otherwise of our findings. If replicated, this would have considerable implications for the inclusion of activating strategies in other effective depression management strategies. Further, in the context of the findings from our audio-tape analysis that there was variation in the delivery of behavioural activation in care managers’ contacts with participants, we may have achieved better outcomes, more in keeping with our predictions, had care managers been able to deliver a more rigorous and consistent behavioural activation programme. We may have achieved better outcomes, more in keeping with our predictions, had care managers been able to deliver a more rigorous and consistent behavioural activation programme. Our supervision model did not require supervisors to listen to audio tapes and give care managers specific clinical feedback on these tapes. It might be that we could optimise the delivery of behavioural activation to ensure that it is more consistent by amending our supervision model to require supervisors to sample audio tapes and feed back their analyses. It seems clear that clinical and supervisory practice should focus much more on enhancing the quality and consistency of behavioural activation within the collaborative care model. However, our supervisory system was delivered by professionals with considerable expertise in case management and behavioural activation. The ability of routine services to replicate this standard of supervision would need to be tested and then monitored in any implementation programme.
The limited liaison reported between GPs and care managers could suggest that more work is needed to facilitate collaboration around individual patients. Some structural aspects were identified that may facilitate liaison, including shared place of working, shared information technology systems, facilitating opportunities for informal meetings and building in formal collaboration into the collaborative care framework. However, although considered by many to be desirable, it is unclear if such an increase in collaboration between care managers and GPs would improve clinical outcomes. Despite being a core component of the collaborative care model, the extent to which additional collaboration between care managers, supervisors and GPs beyond established communication lines is actually necessary for effective patient management of depression is as yet undetermined. For example, a recent trial of collaborative care for people with long-term physical health conditions and depression90 introduced joint sessions with primary care staff (practice nurses) and mental health-care managers to reflect the comorbid problems that patients had, but the effects were no different from those in the CADET study.
Although the vast majority (98%) of our participants had a secondary diagnosis of anxiety we found no differential effect of collaborative care compared with usual care on anxiety at any follow-up point. Patients in both groups had less anxiety at 12 months in particular but the difference between the two groups at this point fell just short of being significant. It seems that a fruitful area of potential treatment development would be the addition of specific anxiety-directed treatment components to the basic collaborative care package. In addition, the high prevalence of long-term physical health conditions in our study population is worthy of note and suggests that specific attention given to this aspect of collaborative care might reap rewards. Indeed, several studies in the USA91,92 and more recently the UK90 have done this. Unfortunately, the addition of this focus in the UK did not deliver significantly enhanced outcomes compared with the CADET study. 90
Future trials should therefore test enhancements of the basic collaborative care model by developing, testing and delivering better treatments within the effective collaborative care organisational framework or improve the delivery of existing treatments through more rigorous supervision, rather than test collaborative care per se, given that the effects of collaborative care are now firmly established.
Acknowledgements
We would like to thank all of the participants, care managers and GPs involved in the study. Special thanks go to Emma Anderson, Fatima Bibi, Samantha Carter, Nia Coupe, Nathan Filer, Lone Gale, Jocelyne Kenny, Liz Salter, Paul Sykes, Debbie Tallon and Helen Thorp who were the project researchers and to the Mental Health Research Network and Primary Care Research Network staff who helped with practice and participant recruitment.
This report uses material from four1–3,61 Open Access articles previously published by the research team and distributed in accordance with the terms of the Creative Commons Attribution licences (CC BY 2.0, CC BY 3.0 and CC BY 4.0) which, provided the authors and original source are properly cited permit the unrestricted reuse of these works [for full details see http://creativecommons.org/licenses/by/2.0/, https://creativecommons.org/licenses/by/3.0/ and http://creativecommons.org/licenses/by/4.0/].
Contributions of authors
David A Richards, Peter Bower, Carolyn Chew-Graham, Linda Gask, Karina Lovell, John Cape, Stephen Pilling, Ricardo Araya, David Kessler, Michael Barkham, J Martin Bland, Simon Gilbody, Colin Green, Glyn Lewis and Chris Manning designed the study and were responsible for its conduct.
Jacqueline J Hill, Adwoa Hughes-Morley and Abigail Russell were responsible for study management and data collection.
David A Richards, J Martin Bland, Colin Green, Evangelos Kontopantelis and Jacqueline J Hill undertook data analysis.
All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the writing and editing of the report.
Publications
Richards DA, Hughes-Morley A, Hayes RA, Araya R, Barkham M, Bland JM, et al. Collaborative Depression Trial (CADET): multi-centre randomised controlled trial of collaborative care for depression – study protocol. BMC Health Serv Res 2009;9:188.
Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, et al. CADET: clinical effectiveness of collaborative care for depression in UK primary care. A cluster randomised controlled trial. BMJ 2013;347;f4913.
Coupe N, Anderson E, Gask L, Sykes P, Richards DA, Chew-Graham C. Facilitating professional liaison in collaborative care for depression in UK primary care; a qualitative study utilising normalisation process theory. BMC Fam Pract 2014;15:78.
Green C, Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, et al. Cost-effectiveness of collaborative care for depression in UK primary care: economic evaluation of a randomised controlled trial (CADET). PLOS ONE 2014;9:e104225.
Data sharing statement
The authors confirm that all data underlying the findings are fully available without restriction. The authors have made the clinical and economic data set available through the University of Exeter’s Institutional Repository – Open Research Exeter (see https://ore.exeter.ac.uk). Access to these data is permitted but controlled through requests made via the repository to the chief investigator (Professor Richards: d.a.richards@exeter.ac.uk). Although use is permitted, this will be on the basis that the source of the data is acknowledged (including the funder) and it includes reference to the data set ‘handle’.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the HTA programme, the EME programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme, the EME programme or the Department of Health.
References
- Richards DA, Hughes-Morley A, Hayes RA, Araya R, Barkham M, Bland JM, et al. Collaborative Depression Trial (CADET): multi-centre randomised controlled trial of collaborative care for depression – study protocol. BMC Health Serv Res 2009;9. http://dx.doi.org/10.1186/1472-6963-9-188.
- Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, et al. CADET: clinical effectiveness of collaborative care for depression in UK primary care. A cluster randomised controlled trial. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4913.
- Green C, Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, et al. Cost-effectiveness of collaborative care for depression in UK primary care: economic evaluation of a randomised controlled trial (CADET). PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0104225.
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLOS Med 2013;10. http://dx.doi.org/10.1371/journal.pmed.1001547.
- Mental and Neurological Disorder – Factsheet 265. Geneva: World Health Organization; 2001.
- Ormel J, Vonkorff M, Oldehinkel AJ, Simon G, Tiemens BG, Ustun TB. Onset of disability in depressed and non-depressed primary care patients. Psychol Med 1999;29:847-53. http://dx.doi.org/10.1017/S0033291799008600.
- Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994.
- Sartorius N. The economic and social burden of depression. J Clin Psychiatry 2001;62:8-11.
- Kupfer DJ. Long-term treatment of depression. J Clin Psychiatry 1991;52:28-34.
- Diagnostic Criteria From DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
- Simon GE, Ludman EJ, Tutty S, Operskalski B, Von Korff M. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: a randomized controlled trial. JAMA 2004;292:935-42. http://dx.doi.org/10.1001/jama.292.8.935.
- Gask L. Overt and covert barriers to the integration of primary and specialist mental health care. Soc Sci Med 2005;61:1785-94. http://dx.doi.org/10.1016/j.socscimed.2005.03.038.
- Richards DA, Lovell K, McEvoy P. Access and effectiveness in psychological therapies: self-help as a routine health technology. Health Soc Care Community 2003;11:175-82. http://dx.doi.org/10.1046/j.1365-2524.2003.00417.x.
- Meltzer H, Gill B, Petticrew M, Hinds K. Physical Complaints, Service Use and Treatment of Adults with Psychiatric Disorders. London: HMSO; 1995.
- Gilbody S, Whitty P, Grimshaw J, Thomas R. Educational and organizational interventions to improve the management of depression in primary care: a systematic review. JAMA 2003;289:3145-51. http://dx.doi.org/10.1001/jama.289.23.3145.
- Bower P, Gilbody S. Managing common mental health disorders in primary care: conceptual models and evidence base. BMJ 2005;330:839-42. http://dx.doi.org/10.1136/bmj.330.7495.839.
- Neumeyer-Gromen A, Lampert T, Stark K, Kallischnigg G. Disease management programs for depression: a systematic review and meta-analysis of randomized controlled trials. Med Care 2004;42:1211-21. http://dx.doi.org/10.1097/00005650-200412000-00008.
- Von Korff M, Goldberg D. Improving outcomes in depression. BMJ 2001;323:948-9. http://dx.doi.org/10.1136/bmj.323.7319.948.
- Badamgarav E, Weingarten SR, Henning JM, Knight K, Hasselblad V, Gano A, et al. Effectiveness of disease management programs in depression: a systematic review. Am J Psychiatry 2003;160:2080-90. http://dx.doi.org/10.1176/appi.ajp.160.12.2080.
- Gensichen J, von Korff M, Peitz M, Muth C, Beyer M, Guthlin C, et al. Case management for depression by health care assistants in small primary care practices: a cluster randomized trial. Ann Intern Med 2009;151:369-78. http://dx.doi.org/10.7326/0003-4819-151-6-200909150-00001.
- Gunn J, Diggens J, Hegarty K, Blashki G. A systematic review of complex system interventions designed to increase recovery from depression in primary care. BMC Health Serv Res 2006;6. http://dx.doi.org/10.1186/1472-6963-6-88.
- Katon W, Von Korff M, Lin E, Simon G. Rethinking practitioner roles in chronic illness: the specialist, primary care physician, and the practice nurse. Gen Hosp Psychiatry 2001;23:138-44. http://dx.doi.org/10.1016/S0163-8343(01)00136-0.
- Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10. http://dx.doi.org/10.1002/14651858.cd006525.pub2.
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314-21. http://dx.doi.org/10.1001/archinte.166.21.2314.
- Killaspy H, Bebbington P, Blizard R, Johnson S, Nolan F, Pilling S, et al. The REACT study: randomised evaluation of assertive community treatment in north London. BMJ 2006;332:815-20. http://dx.doi.org/10.1136/bmj.38773.518322.7C.
- Araya R, Rojas G, Fritsch R, Gaete J, Rojas M, Simon G, et al. Treating depression in primary care in low-income women in Santiago, Chile: a randomised controlled trial. Lancet 2003;361:995-1000. http://dx.doi.org/10.1016/S0140-6736(03)12825-5.
- Patel V, Weiss HA, Chowdhary N, Naik S, Pednekar S, Chatterjee S, et al. Effectiveness of an intervention led by lay health counsellors for depressive and anxiety disorders in primary care in Goa, India (MANAS): a cluster randomised controlled trial. Lancet 2010;376:2086-95. http://dx.doi.org/10.1016/S0140-6736(10)61508-5.
- Depression: Management of Depression in Primary and Secondary Care. London: NICE; 2004.
- Richards DA, Lankshear AJ, Fletcher J, Rogers A, Barkham M, Bower P, et al. Developing a UK protocol for collaborative care: a qualitative study. Gen Hosp Psychiatry 2006;28:296-305. http://dx.doi.org/10.1016/j.genhosppsych.2006.03.005.
- Richards DA, Lovell K, Gilbody S, Gask L, Torgerson D, Barkham M, et al. Collaborative care for depression in UK primary care: a randomized controlled trial. Psychol Med 2008;38:279-87. http://dx.doi.org/10.1017/S0033291707001365.
- Simpson A, Richards D, Gask L, Hennessy S, Escott D. Patients’ experiences of receiving collaborative care for the treatment of depression in the UK: a qualitative investigation. Ment Health Fam Med 2008;5:95-104.
- International Classification of Diseases. Geneva: World Health Organization; 2015.
- Lewis G, Pelosi AJ, Araya R, Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol Med 1992;22:465-86. http://dx.doi.org/10.1017/S0033291700030415.
- The English Indices of Multiple Deprivation 2007. London: HMSO; 2008.
- Evans SJW, Day SJ, Royston P. Minim: Minimisation Program for Allocating Patients to Treatments in Clinical Trials. London: London Hospital Medical College; 1990.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. http://dx.doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychol Med 2008;38:611-23. http://dx.doi.org/10.1017/S0033291707001614.
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: Health Institute, New England Medical Centre; 1993.
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. http://dx.doi.org/10.1001/archinte.166.10.1092.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- EQ-5D User Guide. Rotterdam: EuroQol Group; 1996.
- Attkisson C, Zwick R. The client satisfaction questionnaire: psychometric properties and correlations with service utilisation and psychotherapy outcome. Eval Program Plann 2003;5:233-7. http://dx.doi.org/10.1016/0149-7189(82)90074-X.
- Brazier J, Ratcliffe J, Salomon J, Tsuchiya A. Measuring and Valuing Health Benefits for Economic Evaluation. Oxford: Oxford University Press; 2007.
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230-5. http://dx.doi.org/10.1136/bmj.300.6719.230.
- Elliott R, Stiles WB, Shapiro DA, Giles TR. Handbook of Effective Psychotherapy. New York: Plenum Press; 1993.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. CONSORT Group . CONSORT 2010 statement: extension to cluster randomised trials. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5661.
- Goldberg D, Huxley P. Common Mental Disorders: A Bio-Social Model. London: Routledge; 1992.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU, University of Kent; 2011.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; n.d.
- Department of Health . NHS Reference Costs 2008–09 n.d. https://data.gov.uk/dataset/nhs-reference-costs2008-09 (accessed 18 May 2012).
- Office for National Statistics . 2011 Annual Survey of Hours and Earnings (Based on SOC 2010) n.d. www.ons.gov.uk/ons/dcp171778_256900.pdf (accessed 18 May 2012).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Royston P. Multiple imputation of missing values. Stata J 2004;4:227-41.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. http://dx.doi.org/10.1016/S0167-6296(01)00130-8.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8. http://dx.doi.org/10.1192/bjp.187.2.106.
- Drummond F, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Coupe N, Anderson E, Gask L, Sykes P, Richards DA, Chew-Graham C. Facilitating professional liaison in collaborative care for depression in UK primary care; a qualitative study utilising normalisation process theory. BMC Fam Pract 2014;15. http://dx.doi.org/10.1186/1471-2296-15-78.
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 2002;59:877-83. http://dx.doi.org/10.1001/archpsyc.59.10.877.
- Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res 2010;19:237-70. http://dx.doi.org/10.1177/0962280209105014.
- Manos RC, Kanter JW, Luo W. The Behavioral Activation for Depression Scale – Short Form: development and validation. Behav Ther 2011;42:726-39. http://dx.doi.org/10.1016/j.beth.2011.04.004.
- Frazier PA, Tix AP, Barnett CL. The relational context of social support: relationship satisfaction moderates the relations between enacted support and distress. Pers Soc Psychol Bull 2003;29:1133-46. http://dx.doi.org/10.1177/0146167203254545.
- Kraemer H, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol 2008;27. http://dx.doi.org/10.1037/0278-6133.27.2(Suppl.).S101.
- Bullock JG, Green DP, Ha SE. Yes, but what’s the mechanism? (don’t expect an easy answer). J Pers Soc Psychol 2010;98:550-8. http://dx.doi.org/10.1037/a0018933.
- Glaser B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. New York, NY: Aldine de Gruyter; 1967.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. http://dx.doi.org/10.1177/0038038509103208.
- May C. A rational model for assessing and evaluating complex interventions in health care. BMC Health Serv Res 2006;6. http://dx.doi.org/10.1186/1472-6963-6-86.
- Gask L, Bower P, Lovell K, Escott D, Archer J, Gilbody S, et al. What work has to be done to implement collaborative care for depression? Process evaluation of a trial utilizing the normalization process model. Implement Sci 2010;5. http://dx.doi.org/10.1186/1748-5908-5-15.
- Coventry PA, Hudson JL, Kontopantelis E, Archer J, Richards DA, Gilbody S, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0108114.
- Gilbody S, Bower P, Whitty P. Costs and consequences of enhanced primary care for depression: systematic review of randomised economic evaluations. Br J Psychiatry 2006;189:297-308. http://dx.doi.org/10.1192/bjp.bp.105.016006.
- Jacob V, Chattopadhyay SK, Sipe TA, Thota AB, Byard GJ, Chapman DP. Economics of collaborative care for management of depressive disorders: a community guide systematic review. Am J Prev Med 2012;42:539-49. http://dx.doi.org/10.1016/j.amepre.2012.01.011.
- Mukuria C, Brazier J, Barkham M, Connell J, Hardy G, Hutten R, et al. Cost-effectiveness of an improving access to psychological therapies service. Br J Psychiatry 2013;202:220-7. http://dx.doi.org/10.1192/bjp.bp.111.107888.
- Hollinghurst S, Peters TJ, Kaur S, Wiles N, Lewis G, Kessler D. Cost-effectiveness of therapist-delivered online cognitive–behavioural therapy for depression: randomised controlled trial. Br J Psychiatry 2010;197:297-304. http://dx.doi.org/10.1192/bjp.bp.109.073080.
- Cape J, Whittington C, Buszewicz M, Wallace P, Underwood L. Brief psychological therapies for anxiety and depression in primary care: meta-analysis and meta-regression. BMC Med 2010;8. http://dx.doi.org/10.1186/1741-7015-8-38.
- Kvale S. Doing Interviews. London: Sage; 2007.
- Petrou S, Murray L, Cooper P, Davidson LL. The accuracy of self-reported healthcare resource utilization in health economic studies. Int J Technol Assess Health Care 2002;18:705-10. http://dx.doi.org/10.1017/S026646230200051X.
- Beck AT, Rush A, Shaw B, Emery G. Cognitive Therapy of Depression: A Treatment Manual. New York, NY: Guilford Press; 1979.
- Ekers D, Richards D, McMillan D, Bland JM, Gilbody S. Behavioural activation delivered by the non-specialist: Phase II randomised controlled trial. Br J Psychiatry 2011;198:66-72. http://dx.doi.org/10.1192/bjp.bp.110.079111.
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol 2006;74:658-70. http://dx.doi.org/10.1037/0022-006X.74.4.658.
- Romeo R, Knapp M, Hellier J, Dewey M, Ballard C, Baldwin R, et al. Cost-effectiveness analyses for mirtazapine and sertraline in dementia: randomised controlled trial. Br J Psychiatry 2013;202:121-8. http://dx.doi.org/10.1192/bjp.bp.112.115212.
- Unutzer J, Katon W, Callahan CM, Williams JW, Hunkeler E, Harpole L, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288:2836-45. http://dx.doi.org/10.1001/jama.288.22.2836.
- IAPT Three-Year Report. The First Million Patients. London: Department of Health; 2012.
- Layard R. The Depression Report: A New Deal for Depression and Anxiety Disorders. London: London School of Economics; 2006.
- Cuijpers P, Smit F, Bohlmeijer E, Hollon SD, Andersson G. Efficacy of cognitive–behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry 2010;196:173-8. http://dx.doi.org/10.1192/bjp.bp.109.066001.
- Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care. Making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry 2006;189:484-93. http://dx.doi.org/10.1192/bjp.bp.106.023655.
- Coventry P, Lovell K, Dickens C, Bower P, Chew-Graham C, McElvenny D, et al. Integrated primary care for patients with mental and physical multimorbidity: cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h638.
- Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611-20. http://dx.doi.org/10.1056/NEJMoa1003955.
- Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, et al. The Pathways study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004;61:1042-9. http://dx.doi.org/10.1001/archpsyc.61.10.1042.
Appendix 1 The CollAborative DEpression Trial care manager’s guide
Appendix 2 Consolidated Standards of Reporting Trials checklist
| Section/topic | Item number | Checklist item | Reported on page number |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | vii | |
| 1b | Structured summary of trial design, methods, results and conclusions (for specific guidance see CONSORT for abstracts) | vi–vii | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | 1–3 |
| 2b | Specific objectives or hypotheses | 5 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 5 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | NA | |
| Participants | 4a | Eligibility criteria for participants | 5–6 |
| 4b | Settings and locations where the data were collected | 7 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 7–10 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 10 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | NA | |
| Sample size | 7a | How the sample size was determined | 11 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | NA | |
| Randomisation | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | 6 |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 6 | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 6 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants and who assigned participants to interventions | 6 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | 6 |
| 11b | If relevant, description of the similarity of interventions | NA | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 11–13 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 11–13 | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment and were analysed for the primary outcome | 15–16 |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | 16 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 7 |
| 14b | Why the trial ended or was stopped | NA | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 17–18 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 16 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group and the estimated effect size and its precision (such as 95% confidence interval) | 20–23 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | NA | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory analyses | 20–23 |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | NA |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision and, if relevant, multiplicity of analyses | 65–66 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | 66–68 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms and considering other relevant evidence | 66–68 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | viii |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | 71 |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | viii |
Appendix 3 Consolidated Standards of Reporting Trials abstract checklist
| Item | Description | Reported on line number |
|---|---|---|
| Title | Identification of the study as randomised | vii |
| Authorsa | Contact details for the corresponding author | vii |
| Trial design | Description of the trial design (e.g. parallel, cluster, non-inferiority) | vii |
| Methods | ||
| Participants | Eligibility criteria for participants and the settings where the data were collected | vii |
| Interventions | Interventions intended for each group | viii |
| Objective | Specific objective or hypothesis | vii |
| Outcome | Clearly defined primary outcome for this report | viii |
| Randomisation | How participants were allocated to interventions | vii |
| Blinding (masking) | Whether or not participants, care givers and those assessing the outcomes were blinded to group assignment | viii |
| Results | ||
| Numbers randomised | Number of participants randomised to each group | viii |
| Recruitment | Trial status | NA |
| Numbers analysed | Number of participants analysed in each group | viii |
| Outcome | For the primary outcome, a result for each group and the estimated effect size and its precision | viii |
| Harms | Important adverse events or side effects | NA |
| Conclusions | General interpretation of the results | viii |
| Trial registration | Registration number and name of trial register | viii |
| Funding | Source of funding | viii |
Appendix 4 The CollAborative DEpression Trial ethics documents
List of abbreviations
- AfC
- Agenda for Change
- CADET
- CollAborative DEpression Trial
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CIS-R
- Clinical Interview Schedule – Revised
- CONSORT
- Consolidated Standards of Reporting Trials
- CSO
- clinical studies officer
- CSQ-8
- Client Satisfaction Questionnaire-8
- EQ-5D-3L
- European Quality of Life-5 Dimensions three-level version
- GAD-7
- General Anxiety Disorder-7
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- IAPT
- Improving Access to Psychological Therapies
- ICC
- intracluster correlation
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- MCS
- mental component summary (of the SF-36)
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRES
- National Research Ethics Service
- OR
- odds ratio
- PC-MIS
- patient case management information system
- PCS
- physical component summary (of the SF-36)
- PenCTU
- Peninsula Clinical Trails Unit
- PHQ-9
- Patient Health Questionnaire-9
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RR
- relative risk
- SD
- standard deviation
- SEM
- structural equation modelling
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 dimensions
- SMD
- standardised mean difference
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee