Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 14/69/01. The protocol was agreed in November 2014. The assessment report began editorial review in March 2015 and was accepted for publication in July 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Rob Riemsma is a member of the National Institute for Health Research Health Technology Assessment editorial board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Riemsma et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and definition of the decision problem(s)
Population
Diabetes affects an estimated 3.75 million people in the UK;1,2 approximately 250,000 of these affected people have type 1 diabetes mellitus (T1DM). 3
Type 1 diabetes arises when the body does not produce insulin and is most commonly first diagnosed in the teenage years. T1DM accounts for around 5–15% of all diabetes cases. Type 2 diabetes mellitus (T2DM), which arises when the body develops a resistance to insulin, usually affects people over the age of 40 years. However, T2DM is becoming increasingly more prevalent in younger people, and may be more common in people of South Asian, African Caribbean or Middle Eastern descent. People who are overweight, have inactive lifestyles or a family history of diabetes are at greater risk of developing diabetes. 2,4,5
The characteristic feature of diabetes is high blood glucose (BG) levels, also known as hyperglycaemia; low BG levels is called hypoglycaemia. Optimal BG levels for most people are 4–7 mmol/l before meals, 6–10 mmol/l at bedtime and 5–15 mmol/l before exercise. 6
Type 1 diabetes is caused by the destruction of the pancreatic beta cells which produce insulin, and the mainstay of treatment are insulin injections, which are necessary to sustain life. The Diabetes Control and Complications Trial (DCCT)7 and other studies8 have shown that intensive insulin treatment, aimed at tightly controlling BG, reduces the risk of the long-term complications of diabetes, such as retinopathy and renal disease. Diabetes is one of the most common causes of blindness and end-stage renal failure. 9–11
Intensive insulin treatment is a package of care consisting of either multiple daily insulin injections (MDIs) or continuous subcutaneous insulin infusion (CSII) with an insulin pump, frequent testing of BG, self-adjustment of insulin dosages in response to BG levels, as well as lifestyle interventions such as a restricted diet and undertaking required levels of physical activity.
However, insulin injections cannot provide the sort of fine tuning that can be achieved by a healthy pancreas controlled by the body’s normal feedback mechanisms, and many people with T1DM do not succeed in achieving good control of their diabetes. This is particularly true in children. The best measure of BG control is glycated haemoglobin (HbA1c). An audit of diabetic control in Scottish children showed that only about 10% achieved the National Institute for Health and Care Excellence (NICE) target of a HbA1c level of ≤ 7.5%. 12 In England and Wales, approximately 17% of children and young people with diabetes achieved this NICE target. 13 In 2008, NICE recommended CSII (‘insulin pump’) therapy as a treatment option for adults and children, aged ≥ 12 years, with T1DM. 14 NICE concluded that CSII therapy had a valuable effect on BG control by reducing HbA1c levels and also reducing associated complications.
The provision of an insulin pump alone is not enough; for a pump to be used effectively, it should be accompanied by intensive management. Hyperglycaemia can be controlled by increasing the amount of insulin injected. However, this can lower BG too far. Low BG is called hypoglycaemia, and this is often the limiting factor in attempts to control hyperglycaemia. NICE was also persuaded that CSII therapy could reduce the rate of hypoglycaemic episodes, and it heard from patient experts that when hypoglycaemia occurs in people using CSII therapy, it does so gradually and there is sufficient time for the pump user to take remedial action. 14
The symptoms of hypoglycaemia range from feelings of hunger, faintness, sweating, anxiety and sleepiness at the mild end of the spectrum, to confusion, difficulty in speaking and disturbances of behaviour; and at the severe end of the spectrum, loss of consciousness, convulsions and, rarely, death can occur. Hypoglycaemia is assumed to be the main cause of the ‘found dead in bed’ cases,15 which, fortunately, are rare.
Hypoglycaemic events can be very frightening, especially in children and for their parents, and fear of hypoglycaemia is very common, not just among those with diabetes but also among relatives and friends. There is particular anxiety among parents of young children, some of whom may allow BG levels to run high in order to avoid hypoglycaemia (‘hypo avoidance behaviour’). 16
Parents of young children express considerable anxiety, and may feel a need to get up during the night to check BG levels in their children. BG control may be easier if children are on an insulin pump, but even then parents are likely to set alarms to get up during the night to check that their child is not experiencing hypoglycaemia. Many severe hypoglycaemic events in children occur at night.
As soon as people with diabetes recognise the symptoms, they can consume fast-acting carbohydrates in the form of a sugar-containing food, or just sugar itself, and thereby raise BG levels again. However, there is a particular problem, known as hypoglycaemia unawareness, whereby some people do not develop any warning symptoms. Being unaware of impending hypoglycaemia, such people may not consume sugar-rich foods or sugar in time to prevent a serious hypoglycaemic event. Hypoglycaemia unawareness usually occurs after frequent hypoglycaemic events, and a vicious circle can develop where frequent hypoglycaemic events cause hypoglycaemia unawareness, which leads to more, and more severe, hypoglycaemia, associated with the failure of the body to release the counter-regulatory hormones, such as adrenaline, that cause warning symptoms.
Until recently, self-monitoring of blood glucose (SMBG) meant pricking a part of the body, such as the fingertip, with a needle to make it bleed (sometimes up to 15 times a day), putting a drop of blood on a test strip and measuring BG levels with the aid of a meter. Depending on the result, the patient could then adjust their insulin dose or diet in order to try and keep BG levels within the optimum range.
In recent years, meters for continuous monitoring of interstitial fluid glucose have been introduced to help people with T1DM to achieve better control of their disease. Increasingly sophisticated integrated methods of glucose monitoring and insulin delivery are designed to provide a closer approximation to the body’s natural system and achieve acceptable glycaemic control, while minimising the risk of hypoglycaemic episodes. Interventions designed to help people with T1DM to achieve better control include structured education (the dose adjustment for normal eating course17 or similar courses) and CSII with an insulin pump.
The aim of CSII is to provide a flexible method for administering insulin, which tries to mimic the body’s natural pattern of a small amount of insulin being present all the time (basal infusion) and peaks of insulin release after meals (boluses), aided by SMBG by capillary blood testing.
However, there are limits to what can be done with capillary blood testing (and it is painful – even more so than insulin injections). In recent years, devices which continually measure BG (strictly speaking, they actually measure the level of glucose in the subcutaneous tissue) have been introduced. These use a cannula inserted under the skin, which is connected to a glucose meter. The first of these continuous glucose monitoring (CGM) systems merely recorded BG levels for later downloading. However, there are now CGM devices that display interstitial glucose levels – so-called ‘real-time CGM’ – so that users can see their most recent glucose level (CGM is not actually continuous, as the name suggests, but measures glucose levels every 5–10 minutes). The psychosocial impact of CGM is mixed however, with both positive results with regard to the greater control over diabetes, but also negative impacts resulting from intrusive false alarms and the additional burden and visibility of the disease. 18,19 In addition, CGM does not make capillary blood testing redundant; a minimum of two tests per day is still required to calibrate CGM devices.
The next step in the development of CGM systems was to have integrated alarm facilities, whereby the CGM meter could alert the user to BG levels that are too high or too low. In theory, the user can then adjust insulin dosage, by, for example, reducing the insulin infusion rate if BG levels are too low or showing a decreasing trend. These integrated systems are called ‘sensor-augmented pump therapy’ (SAPT).
Current CGM systems rely on the user taking action, and this may not occur, particularly at night. Hypoglycaemic events at night are known as nocturnal hypoglycaemia. Alarms may wake people up, but those having nocturnal hypoglycaemic events often sleep through these alarms and recurrent hypoglycaemic events can lead to hypoglycaemia unawareness.
CGM may initially raise anxiety, because it provides much more data on BG levels, and this can lead to more anxiety among patients and parents. False alarms are a particular problem, leading to distrust of the device and a lack of willingness to take appropriate action.
A recent development in CGM/pump technology, which has been available in the UK since 2009, is the Medtronic Veo suspend combination (Medtronic Inc., Northridge, CA, USA); this CGM device can stop (suspend) the insulin infusion from the pump for up to 2 hours. After that, insulin infusion is restored at a basal rate. In practice, few suspensions are for as long as 2 hours because, in most cases, the pump user takes corrective action. 20 A small study (31 patients used this device for 3 weeks), performed in UK centres, reported that 66% of suspend durations were for ≤ 10 minutes, that most long episodes of suspension occurred at night and that there was a reduction in nocturnal hypoglycaemia.
After insulin infusion stops, it takes 30 minutes for BG levels to increase,21 so hypoglycaemic events may be shortened or made less severe, rather than always avoided.
Suspension can be controlled manually by the user, in response to an alarm or after checking real-time results, or automatically by the device. Patients can over-ride the pump and cancel suspension, using food to increase BG levels instead. One problem reported is that sleeping position may cause inaccurately low readings because of tissue compression. 22
This assessment will focus on the use of integrated SAPT systems in T1DM. 14
The populations for the current assessment were adults and children with T1DM. The interventions assessed (integrated CGM and insulin pump systems with or without a suspend function) aim to provide better monitoring and dose adjustment and hence achieve acceptable glycaemic control while minimising hypoglycaemic episodes.
Description of the technologies under assessment
The MiniMed® Paradigm™ Veo system (Medtronic Inc., Northridge, CA, USA) and the Vibe™ (Animas® Corporation, West Chester, PA, USA) and G4® PLATINUM CGM system (Dexcom Inc., San Diego, CA, USA) are integrated CGM and insulin pump systems intended to aid the effective management of diabetes. The MiniMed Paradigm Veo System has an added insulin suspend function intended to prevent hypoglycaemia, including nocturnal hypoglycaemia.
The MiniMed Paradigm Veo system
The MiniMed Paradigm Veo system has three components:
-
a small glucose sensor, placed under the skin, which measures glucose levels every 5 minutes, 24 hours per day (this sensor must be replaced every 6 days)
-
the MiniLink™ transmitter (Medtronic Inc., Northridge, CA, USA), which sends the information to the Paradigm Veo insulin pump
-
the Paradigm Veo insulin pump.
The system is complete and stand alone and not directly interchangeable with other manufacturers’ pumps or sensors. Many insulin formulations can be used in the insulin pump. In this report, we will focus on only fast-acting insulin formulations, because this type of formulation in the preferred clinical option for use with insulin pumps in the UK. 23
Continuous glucose monitors measure the level of tissue glucose electronically on a continuous basis (every few minutes). They use a subcutaneous, disposable glucose sensor placed just under the skin to measure interstitial glucose levels. The glucose sensor of the Veo system is replaced every 6 days. The sensor is connected to a non-implanted transmitter (MiniLink) which communicates glucose levels wirelessly to the Paradigm Veo pump. The pump displays BG levels with nearly continuous updates, as well as monitoring rising and falling trends. The pump can prompt a person with diabetes, or a carer, to take action to maintain glucose levels. The insulin pump delivers continuous subcutaneous insulin according to a pre-programmed pattern, which can be adapted by the user or a carer in response to real-time glucose trends.
The MiniMed Paradigm Veo system appears to be unique in that it will actively suspend insulin delivery if it predicts a hypoglycaemic episode. This ‘low glucose suspend’ (LGS) function stops insulin delivery for 2 hours if there is no response to a low glucose warning.
Users of this system must perform regular (a minimum of two per day) capillary BG tests (such as a finger prick tests), as CGM measures interstitial fluid glucose levels, not capillary BG levels. Further finger prick tests are required to confirm a CGM value before making any adjustments to diabetes therapy.
The pump can be worn on a belt or in a pouch underneath clothes. Insulin is delivered through a small tube (or ‘infusion set’) placed under the skin. The transmitter is directly connected to the glucose sensor, which is inserted through the skin, usually in the stomach area. The manufacturer’s information for use document states that the infusion set should be replaced every 3 days.
The Vibe and G4 PLATINUM CGM system
The Vibe and G4 PLATINUM CGM system is a CGM-enabled insulin pump, integrated with the G4 PLATINUM sensor. It is similar to the MiniMed Paradigm Veo system in that the glucose sensor is placed under the skin and measures interstitial glucose levels rather than capillary BG levels. Confirmatory capillary BG tests are also required to confirm the value displayed by the continuous glucose monitor before making any adjustments to diabetes therapy. The sensor is approved for up to 7 days of wear.
The insulin pump in the Vibe and G4 PLATINUM CGM system also delivers insulin continuously from a refillable storage reservoir by means of a subcutaneously placed cannula and the pump can be programmed to deliver insulin at a basal rate throughout the day, with the option of triggering higher infusion rates at mealtimes, either as a bolus dose or over time. The pump can be programmed to deliver insulin at different basal rates at different times of the day and night.
The system produces glucose level readings in real time, alerts users of high or low readings, and glucose trend information. It does not have an automated LGS function.
Comparators
The scope, as defined by NICE, specifies the following comparator technologies:
-
CSII with SMBG by capillary blood testing (CSII + SMBG)
-
MDIs with SMBG by capillary blood testing (MDI + SMBG)
-
non-integrated, stand-alone CSII and CGM (CSII + CGM)
-
MDIs with CGM (MDI + CGM).
Non-integrated, stand-alone CSII and CGM require the simultaneous use, by patients, of both a continuous glucose monitor and a pump to deliver the insulin. The two interventions (Veo and Vibe) also both use a continuous glucose monitor and an insulin pump. However, for the non-integrated, stand-alone CSII and CGM, the two devices are supplied separately and for the Veo and Vibe interventions, these devices are supplied as a ‘system’, hence the term ‘integrated’. Although they may or may not differ in terms of monitoring and insulin delivery, this review will seek to find any differences with regard to their effectiveness and cost-effectiveness (see Chapter 2).
Within groups of comparator studies, there may be differences between studies (e.g. populations, interventions and outcomes). The possibility of pooling results from different trials will depend on the extent of these differences. In addition, the comparability of populations in studies evaluating insulin pumps and MDIs is a potential problem. Based on 2008 guidance,14 NICE recommends CSII as a potential treatment for children ≥ 12 years and adults, who have disabling hypoglycaemia (including anxiety about hypoglycaemia) when trying to attain HbA1c < 7.5%, or HbA1c is constantly > 8.5%, while undergoing multiple daily injection therapy (MDIT). Furthermore, CSII is recommended for children < 12 years when MDIT would not be practical. 14
In other words, insulin pumps are recommended for people with T1DM for whom MDIs are not suitable. Therefore, it might be problematic to find studies comparing insulin pumps (especially modern pumps such as the integrated systems) with MDIs in comparable populations.
Chapter 2 Objective
The overall objective of this project was to summarise the evidence on the clinical effectiveness and cost-effectiveness of the MiniMed Paradigm Veo system and the Vibe and G4 PLATINUM CGM system for the management of T1DM in adults and children.
The following research questions have been defined to address the review objective:
-
What is the clinical effectiveness of integrated insulin pump systems compared with:
-
CSII + SMBG
-
MDI + SMBG
-
CSII + CGM
-
MDI + CGM.
-
-
What is the cost-effectiveness of integrated insulin pump systems compared with:
-
CSII + SMBG
-
MDI + SMBG
-
CSII + CGM
-
MDI + CGM.
-
There are two interventions and four comparators. In this report, we will use the following descriptors for these interventions and comparators:
-
MiniMed Veo system An integrated CGM and insulin pump system with LGS function.
-
Integrated CSII + CGM Integrated CGM and insulin pump systems without LGS function (such as the Vibe and G4 PLATINUM CGM system). Although the only integrated system available in the UK is the Vibe and G4 PLATINUM CGM system, all integrated systems without a LGS function will be included in this category. This also includes integrated Medtronic systems (such as the Paradigm Revel™ and Paradigm REAL-Time systems).
-
CSII + CGM An insulin pump with stand-alone continuous glucose monitor.
-
CSII + SMBG An insulin pump with SMBG.
-
MDI + CGM MDIs with a continuous glucose monitor.
-
MDI + SMBG MDIs with SMBG.
Chapter 3 Assessment of clinical effectiveness
Systematic review methods for the assessment of clinical effectiveness
A systematic review was conducted to summarise the evidence on the clinical effectiveness of the MiniMed Paradigm Veo system and the Vibe and G4 PLATINUM CGM system for the management of T1DM in adults and children. Systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination guidance for undertaking reviews in health care,24 and the NICE Diagnostic Assessment Programme manual. 25
Inclusion and exclusion criteria
Participants
The study populations eligible for inclusion were adults, including pregnant women, and children with T1DM.
Setting
The relevant setting was self-use supervised by primary or secondary care.
Interventions
The main intervention technology for this appraisal was the MiniMed Paradigm Veo with CGM system and suspend function. In addition, we included fully integrated insulin pump systems as an alternative technology, including the only existing fully integrated system currently available in the UK: the Vibe and G4 PLATINUM CGM system.
Comparators
The scope, as defined by NICE, specified the following comparator technologies:
-
capillary blood testing with CSII
-
capillary blood testing with MDIs
-
CGM with CSII (non-integrated)
-
CGM with MDIs.
Studies comparing CSII with MDIs often use different types of monitoring (SMBG or CGM). Unless results were reported separately for the different types of monitoring, such studies were excluded from our review, because they do not allow a comparison of a relevant intervention with the comparators. The same applies to studies comparing CGM with SMBG, without specifying the way in which insulin was delivered (CSII or MDIs).
Outcomes
The main outcomes were:
-
HbA1c levels (i.e. change from baseline and the number of participants achieving a specified level of control)
-
the frequency of hyperglycaemic events and the number of hyperglycaemic episodes, stratified by severity into ‘mild’ or ‘severe’ if data were available.
-
the frequency of (nocturnal) hypoglycaemic events and the number of hypoglycaemic episodes, stratified by severity into ‘mild’ or ‘severe’ if data were available.
Possible secondary outcomes were:
-
mean BG levels, including fasting glucose levels
-
postprandial glucose levels
-
the amount of insulin being administered
-
episodes of diabetic ketoacidosis and the number of ketone tests
-
health-related quality of life (HRQoL)
-
long-term complications of diabetes and treatment, including retinopathy, neuropathy, cognitive impairment and end-stage renal disease
-
morbidity and mortality
-
adverse events from testing, false results, treatment and sequelae
-
the acceptability of the testing method and the method of insulin administration
-
anxiety about experiencing hypoglycaemia
-
costs, including the costs related to the support received from health professionals.
In pregnant women, additional T1DM-related clinical outcomes included:
-
premature birth
-
macrosomia (excessive birth weight)
-
respiratory distress syndrome in newborns.
Study design
Studies with the following types of study design were eligible for inclusion:
-
randomised controlled trials (RCTs) or, if no RCTs were available for comparisons of interventions and comparators, controlled clinical trials
-
observational studies for additional information with regard to interventions, if no RCTs were found.
Studies of < 6 weeks’ duration and cross-over studies were excluded.
Subgroup analyses
If the evidence and the structure of the cost-effectiveness model were to permit, the following subgroups would be explored:
-
pregnant women, and women planning pregnancy (but not including those with gestational diabetes)
-
people who need to self monitor their BG level > 10 times a day
-
people with T1DM who are having difficulty managing their condition; such difficulties include:
-
not being able to maintain the recommended HbA1c level of 8.5% (69.4 mmol/mol) or less
-
experiencing nocturnal hypoglycaemia
-
an impaired awareness of hypoglycaemia
-
experiencing severe hypoglycaemia, defined as having low BG levels that require assistance from another person to treat.
-
Search strategy
Systematic literature searches were conducted to identify studies of SAPT for T1DM (specifically the MiniMed Paradigm Veo system and the Vibe and G4 Platinum system), as well as RCTs and economic evaluations of insulin pump/infusion therapy and MDIs for T1DM. Search strategies were developed using the recommendations of the Centre for Reviews and Dissemination guidance for undertaking reviews in health care,24 and the Cochrane Handbook. 26 The search strategies used relevant search terms, comprising a combination of indexed keywords (e.g. from medical subject headings and the EMBASE thesaurus EMTREE) and free-text terms appearing in the titles and/or abstracts of database records. Search terms were identified through discussion among the review team, by scanning background literature and ‘key articles’ already known to the review team, and by browsing database thesauri. The search strategies were structured using search terms for ‘type 1 diabetes’ in combination with search terms for ‘sensor-augmented pump therapy’. Two further search term facets were included to capture ‘insulin infusion’ and ‘multiple daily injections’. In addition, the search strategy for clinical effectiveness studies included a sensitive methodological search filter designed to identify RCTs. The EMBASE search strategy was translated so that it could be run effectively in each of the databases searched. No date or language limits were applied. The main EMBASE search strategies were independently peer reviewed by a second information specialist using the Canadian Agency for Drugs and Technologies in Health peer review checklist. 27
Details of the full search strategies are presented in Appendix 1.
The following databases and resources were searched for relevant RCTs, systematic reviews and health technology assessments:
-
MEDLINE (via OvidSP): 1946–2014/Aug week 4
-
MEDLINE In-Process Citations and Daily Update (via OvidSP): up to 4 September 2014
-
PubMed (National Library of Medicine): up to 5 September 2014
-
EMBASE (via OvidSP): 1974–2014/week 34
-
Cochrane Database of Systematic Reviews (Wiley Online Library): issue 9/September 2014
-
Cochrane Central Register of Controlled Trials (Wiley Online Library): issue 8/August 2014
-
Database of Abstracts of Reviews of Effects (Wiley Online Library): issue 3/July 2014
-
Health Technology Assessment (HTA) Database (Wiley Online Library): issue 3/July 2014
-
Science Citation Index (Web of Science): 1988–29 August 2014
-
Latin American and Caribbean Health Sciences Literature (http://lilacs.bvsalud.org/en/): 1982–5 September 2014
-
National Institute for Health Research HTA Programme (www.hta.ac.uk/): up to 5 September 2014
-
PROSPERO (www.crd.york.ac.uk/prospero/): up to 5 September 2014
-
US Food and Drug Administration (www.fda.gov): up to 5 September 2014
-
Medicines and Healthcare products Regulatory Agency (www.mhra.gov.uk/): up to 5 September 2014
Completed and ongoing trials were identified by searches of the following trials registries:
-
US National Institutes of Health ClinicalTrials.gov (www.clinicaltrials.gov/): up to 2 September 2014
-
Current Controlled Trials (www.controlled-trials.com/): up to 5 September 2014
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/): up to 5 September 2014
Conference proceedings were also searched from the organisations: Diabetes UK, the European Association for the Study of Diabetes and the American Diabetes Association (see Appendix 1).
The bibliographies of identified research and review articles were checked for relevant studies. As a number of databases were searched, there was some degree of duplication. In order to manage this issue, the titles and abstracts of bibliographic records were downloaded and imported into EndNote X7 (Thomson Reuters, CA, USA) reference management software and duplicate records removed. Rigorous records were maintained as part of the searching process. Individual records within the Endnote reference libraries were tagged with searching information, such as searcher, date searched, database host, database searched, search strategy name and iteration, theme and search question. This enabled the information specialist to track the origin of each individual database record and its progress through the screening and review process.
Inclusion screening and data extraction
Two reviewers independently screened the titles and abstracts of all reports identified by searches and any discrepancies were discussed and resolved by consensus. Full-text copies of all studies deemed potentially relevant, after discussion, were obtained and the same two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus. Details of the studies excluded at the full-paper screening stage are presented in Appendix 2.
Data relating to study details, participants, intervention and comparator tests, and outcome measures were extracted by one reviewer, using a piloted, standard data extraction form. A second reviewer checked data extraction and any disagreements were resolved by consensus.
Quality assessment
The methodological quality of included studies was assessed using standard tools. 24 The assessment of the methodological quality of each included study was based on the Cochrane Collaboration quality assessment checklist,26 as detailed in Table 1.
| Domain | Item | Description |
|---|---|---|
| Sequence generation | Was the allocation sequence adequately generated? | The method used to generate the allocation sequence should be described in sufficient detail to allow an assessment of whether or not it should produce comparable groups |
| Allocation concealment | Was allocation adequately concealed? | The method used to conceal the allocation sequence should be described in sufficient detail to determine whether or not intervention allocations could have been foreseen in advance of, or during, enrolment |
| Blinding of participants, personnel and outcome assessors Assessments will be made for each main outcome (or class of outcomes) |
Was knowledge of the allocated intervention adequately prevented during the study? | All measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received, should be described. Any information relating to whether or not the intended blinding was effective should also be reported |
| Incomplete outcome data Assessments will be made for each main outcome (or class of outcomes) |
Were incomplete outcome data adequately addressed? | The completeness of outcome data for each main outcome should be described, including attrition and exclusions from the analysis. The authors should report any attrition and exclusions, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions and any re-inclusions in analyses |
| Other sources of bias | Was the study apparently free of other problems that could put it at a high risk of bias? | Overall, the study should be free from any important concerns about bias (i.e. bias from other sources not previously addressed by the other items) |
Each study was awarded a ‘yes’, ‘no’ or ‘unclear/unknown’ rating for each individual item in the checklist. Any additional clarifications or comments were also recorded.
Quality assessment was carried out independently by two reviewers. Any disagreements were resolved by consensus. The results of the quality assessment were used for descriptive purposes to provide an evaluation of the overall quality of the included studies and to provide a transparent method of recommendation for the design of any future studies. Based on the findings of the quality assessment, recommendations were made for the conduct of future studies.
Methods of analysis/synthesis
If meta-analysis was considered unsuitable for some or all of the data identified (e.g. because of the heterogeneity and/or small numbers of studies), we employed a narrative synthesis. Typically, this involves the use of text and tables to summarise data. These allow the reader to consider any outcomes in the light of differences in study designs and potential sources of bias for each of the studies being reviewed. Studies were organised according to which therapies were being compared.
The methods used to synthesise the data were dependent on the types of outcome data included, and the clinical effectiveness and statistical similarity of the studies. Possible methods of data synthesis include the types of analysis discussed in the following sections.
Dichotomous outcomes
Dichotomous data were analysed by calculating the relative risk (RR) for each trial using the fixed-effect method or the DerSimonian and Laird28 random-effects method and the corresponding 95% confidence intervals (CIs).
Continuous outcomes
Continuous data were analysed by calculating the weighted mean difference (WMD) between groups and the corresponding 95% CI. If the standard deviations (SDs) and means were not determinable, they were estimated from the data provided or using a representative value from other studies.
Systematic differences between studies (heterogeneity) were likely; therefore, the random-effects model was used for the calculation of RRs or WMDs if heterogeneity was moderate or high (I2 > 50%). Heterogeneity was initially assessed by measuring the degree of inconsistency in the studies’ results (I2). The I2 value describes the percentage of total variation across studies that was due to heterogeneity rather than chance. The value of I2 can lie between 0% and 100%. Low, moderate and high I2 values correspond to 25%, 50% and 75%, respectively.
If significant heterogeneity was identified, we planned to formally investigate this using metaregression. In particular, a model was planned to explore the possible modifying effects of the following pre-specified factors: methodological quality of the primary studies, underlying illness and different age groups. The coefficient describing the predictive value of each factor and the overall effect on the main outcome would be modelled, using a fixed-effects model. However, because of the limited number of studies for each comparison, this was not possible.
A funnel plot (plot of the logarithm value of the RR for efficacy against the precision of the logarithm value of the RR) would have been used to estimate potential asymmetry, and this would have been indicative of small study effects. HbA1c levels were chosen as an outcome since these are likely to be reported by the majority of included studies. In addition, the Egger regression asymmetry test29 would have been used to facilitate the prediction of potential publication biases. This test detects funnel plot asymmetry by determining whether or not the intercept deviates significantly from zero in a regression of the standardised effect estimates against their precision. However, because of the limited number of studies for each comparison, this was not possible.
Network meta-analysis methods
In the absence of RCTs directly comparing the MiniMed Veo system or an integrated CSII + CGM system (such as the Animas Vibe pump with Dexcom’s G4 continuous glucose monitor) with the comparators (i.e. CSII + CGM, CSII + SMBG, MDI + CGM or MDI + SMBG), indirect treatment comparisons were performed, if possible. As only limited networks could be formed, a mixed-treatment comparison was not possible. However, if possible, indirect comparisons were made. Although ‘head-to-head’ comparisons are preferred to indirect methods in health technology assessments, indirect methods are generally considered acceptable; for all methods, consideration of basic assumptions of homogeneity, similarity and consistency, as reported by Song et al. ,30 should be applied. For this assessment, where ‘head-to-head’ trials (i.e. ‘A’ vs. ‘B’) of the MiniMed Paradigm Veo with CGM System versus the comparators (CSII + CGM, CSII + SMBG, MDI + CGM or MDI + SMBG) were missing, the effect sizes (RR or mean difference) for ‘A’ versus ‘B’ were estimated using ‘indirect’ methods; for example, effect sizes for ‘A’ versus ‘B’ were estimated from ‘A’ versus ‘C’ and ‘B’ versus ‘C’, where ‘C’ was a common control group [e.g. CSII + CGM (i.e. CSII with a stand-alone CGM system)]. All indirect comparisons were consistent with International Society for Pharmacoeconomics and Outcomes Research taskforce recommendations for the conduct of direct and indirect meta-analysis and used the method described by Bucher et al. 31 A practical issue for indirect comparisons concerns the limitations in the availability of the same outcomes in the studies of interventions that are candidates for an indirect comparison. Only studies that provide the same outcome measures at the same follow-up time can be compared with each other, which may limit the availability of suitable trial networks. Depending on the data available, separate network analyses were performed for each of the subgroups specified in the protocol. Indirect meta-analyses were performed using Microsoft Excel® 2007 (Microsoft Corporation, Redmond, WA, USA), according to the method developed by Bucher et al. 31 Effect sizes with 95% CIs were calculated using results from the direct head-to-head meta-analyses. Direct head-to-head meta-analyses were performed using fixed-effect models in Stata™ for Windows, version 13 (StataCorp LP, College Station, TX, USA), unless significant heterogeneity was present, in which case we used random-effects models.
Results of the assessment of clinical effectiveness
Results of literature searches
The literature searches of bibliographic databases identified 9870 references. After initial screening of titles and abstracts, 555 were considered potentially relevant and were ordered for full-paper screening. Of the total of 555 publications considered potentially relevant, 29 could not be obtained within the time scale of this assessment. Most of these 29 unobtainable studies were published before 2000 or were conference abstracts; only four were possibly relevant trials published after 2000, but, based on their abstracts, it was unclear whether or not they fulfilled the inclusion criteria. Figure 1 shows the flow of studies through the review process and Appendix 2 provides details, with reasons for exclusions, of all the publications excluded at the full-paper screening stage.
FIGURE 1.
Flow of studies through the review process.

Based on the searches and inclusion screening described above, 54 publications resulting from 19 studies were included in the review. In addition, 19 publications of 18 ongoing studies were found (see Ongoing studies).
One study32 compared the MiniMed Veo system (with suspend function) with an integrated CSII + CGM system (MiniMed Veo with suspend function turned off) and another33 compared it with CSII + SMBG. Seven other studies compared an integrated CSII + CGM system with CSII + SMBG (three studies)34–36 or with MDI + SMBG (four studies). 37–40 The remaining 10 studies41–50 compared CSII + SMBG with MDI + SMBG. None of the studies included a treatment arm with CSII + CGM or MDI + CGM as a comparator (Table 2). Although several studies included an integrated CSII + CGM system as a treatment arm, it is important to note that none of these studies looked at the Vibe and G4 PLATINUM CGM system; in the included studies, the integrated CSII + CGM system was always a MiniMed Paradigm pump with an integrated CGM system.
| Study | Veo | Integrated CSII + CGM | CSII + CGM | CSII + SMBG | MDI + CGM | MDI + SMBG |
|---|---|---|---|---|---|---|
| Bergenstal et al., 2013 (ASPIRE in-home)32 | ✓ | ✓ | ||||
| Ly et al., 201333 | ✓ | ✓ | ||||
| Hirsch et al., 200834 | ✓ | ✓ | ||||
| O’Connell et al., 200935 | ✓ | ✓ | ||||
| Raccah et al., 2009 (RealTrend)36 | ✓ | ✓ | ||||
| Hermanides et al., 2011 (Eurythmics)37 | ✓ | ✓ | ||||
| Lee et al., 200738 | ✓ | ✓ | ||||
| Peyrot and Rubin, 200939 | ✓ | ✓ | ||||
| Bergenstal et al., 2010 (STAR-3)40 | ✓ | ✓ | ||||
| Bolli et al., 200941 | ✓ | ✓ | ||||
| DeVries et al., 200242 | ✓ | ✓ | ||||
| aNosadini et al., 198843 | ✓ | ✓ | ||||
| Brinchmann-Hansen et al., 1985 (OSLO)44 | ✓ | ✓ | ||||
| Thomas et al., 200745 | ✓ | ✓ | ||||
| Tsui et al., 200146 | ✓ | ✓ | ||||
| Weintrob et al., 200347 | ✓ | ✓ | ||||
| Thrailkill et al., 201148 | ✓ | ✓ | ||||
| Doyle et al., 200449 | ✓ | ✓ | ||||
| Nosari et al., 199350 | ✓ | ✓ |
Out of the 19 studies, eight were performed in North America32,34,38–40,46,48,49 and eight in Europe. 36,37,41–45,50 The remaining three studies were performed in Australia (two studies33,35) and Israel (one study47). Three out of the eight European studies included patients from the UK. 37,41,45
Twelve studies reported data for adults, five studies reported data for children and five studies reported data for mixed populations (adults and children). Two of these studies reported data for all three groups. One study included pregnant women (Table 3).
| Study | Population (age range, years) | n | Mean baseline age, years (SD) | Mean baseline HbA1c levels, % (SD) | Previous pump use, months | Follow-up, months |
|---|---|---|---|---|---|---|
| Bergenstal et al., 2013 (ASPIRE in-home)32 | A (16–70) | 247 | 43 (13) | 7.2 (0.7) | > 6 | 3 |
| Ly et al., 201333 | M (4–50) | 95 | 19 (12) | 7.5 (0.8) | > 6 | 6 |
| Hirsch et al., 200834 | M (12–72) | 146 | 33 (16) | 8.4 (0.7) | > 6 | 6 |
| A (18–72) | 8.3 (0.6) | > 6 | 6 | |||
| C (12–17) | 8.7 (0.9) | > 6 | 6 | |||
| O’Connell et al., 200935 | M (13–40) | 62 | 23 (8.4) | 7.4 (0.7) | > 3 | 3 |
| Raccah et al., 2009 (RealTrend)36 | M (2–65) | 132 | 28 (16) | 9.2 (1) | NR | 6 |
| Hermanides et al., 2011 (Eurythmics)37 | A (18–65) | 83 | 38 (11) | 8.6 (0.9) | Naive | 6 |
| Lee et al., 200738 | A (NR) | 16 | NR | 9 (0.9) | Naive | 3.5 |
| Peyrot and Rubin, 200939 | A (NR) | 29 | 47 (13) | 8.6 (1) | NR | 3.7 |
| Bergenstal et al., 2010 (STAR-3)40 | M (7–70) | 495 | 32 (17) | 8.3 (0.5) | Naive | 12 |
| A (19–70) | 41 (12) | Naive | 12 | |||
| C (7–18) | 12 (3) | Naive | 12 | |||
| Bolli et al., 200941 | A (18–70) | 58 | 40 (11) | 7.7 (0.7) | Naive | 6 |
| DeVries et al., 200242 | A (18–70) | 79 | 37 (10) | 9.4 (1.4) | Naive | 3.7 |
| Nosadini et al., 198843 | A (NR) | 96 | 34 (6) | NR | NR | 12 |
| Brinchmann-Hansen et al., 1985 (OSLO)44 | A (18–45) | 45 | 26 (21) | 8.5 (NR) | NR | 3, 6, 12 and 24 |
| Thomas et al., 200745 | A (NR) | 21 | 43 (10) | 8.5 (1.5) | NR | 4 and 6 |
| Tsui et al., 200146 | A (18–60) | 27 | 36 (11) | 8 (0.6) | Naive | 9 |
| Weintrob et al., 200347 | C (8–14) | 23 | 12 (1.5) | 8 (1) | NR | 3.5 |
| Thrailkill et al., 201148 | C (8–18) | 24 | 12 (3) | 11.5 (2.4) | Naive | 6 and 12 |
| Doyle et al., 200449 | C (8–21) | 32 | 13 (3) | 8.1 (1.2) | Naive | 3.7 |
| Nosari et al., 199350 | P (NR) | 32 | 26 (2.4) | NR | Naive | 9 |
Table 4 shows the inclusion criteria, regarding the HbA1c levels and hypoglycaemic events, used in the included studies. Further details of the characteristics of study participants and the interventions, comparators and results are reported in the data extraction tables presented in Appendix 3. It is clear from Table 3 that most studies included patients who had never used a pump before. However, both of the studies looking at the MiniMed Veo system (ASPIRE in-home32 and Ly et al. 33) included patients who had at least 6 months’ experience of using an insulin pump. In addition, baseline HbA1c levels differ considerably among studies. DeVries et al. 42 included patients with poor control at baseline who were pump-naive. The two studies looking at the MiniMed Veo system included patients with relatively good glycaemic control at baseline; however, that might have been because those patients had been using an insulin pump for at least 6 months. Other studies, such as Bolli et al. ,41 included patients with relatively good glycaemic control at baseline without any previous pump experience. Therefore, there is considerable heterogeneity among the study populations.
| Study | Inclusion criteria for HbA1c levels (%) | Inclusion/exclusion criteria with regard to hypoglycaemia |
|---|---|---|
| Bergenstal et al., 2013 (ASPIRE in-home)32 | 5.8–10 | Included if experienced two or more nocturnal hypoglycaemic events during the run in phase. Excluded if experienced more than one episode of severe hypoglycaemia in the previous 6 months |
| Ly et al., 201333 | ≤ 8.5 | Included those with an impaired awareness of hypoglycaemia (HUS ≥ 4). Mean HUS 6.2 (SD 1.5) |
| Hirsch et al., 200834 | ≥ 7.5 | There were no exclusions for hypoglycaemia unawareness |
| O’Connell et al., 200935 | ≤ 8.5 | Excluded those with any co-existent illness that otherwise predisposes to hypoglycaemia (e.g. adrenal insufficiency) or a history of severe hypoglycaemia while using insulin pump therapy |
| Raccah et al., 2009 (RealTrend)36 | > 8 | NR |
| Hermanides et al., 2011 (Eurythmics)37 | ≥ 8.2 | NR |
| Lee et al., 200738 | ≥ 7.5 | NR |
| Peyrot and Rubin, 200939 | NR | NR |
| Bergenstal et al., 2010 (STAR-3)40 | 7.4–9.5 | Excluded those with hypoglycaemia unawareness (two or more severe hypoglycaemic episodes without warning of low BG levels within the previous year) |
| Bolli et al., 200941 | 6.5–9 | Excluded those who had more than two severe hypoglycaemic events in the previous 6 months |
| DeVries et al., 200242 | ≥ 8.5 | NR |
| Nosadini et al., 198843 | NR | NR |
| Brinchmann-Hansen et al., 1985 (OSLO)44 | NR | NR |
| Thomas et al., 200745 | NR | Included those with long-standing T1DM complicated by at least one episode of severe hypoglycaemia within the preceding 6 months |
| Tsui et al., 200146 | NR | Excluded those who had a history of more than two severe hypoglycaemic episodes in the last year |
| Weintrob et al., 200347 | NR | NR |
| Thrailkill et al., 201148 | NR | NR |
| Doyle et al., 200449 | 6.5–11 | NR |
| Nosari et al., 199350 | NR | NR |
Most studies were rated as having a high risk of bias (11 out of 19), four studies were rated as having an unclear risk of bias and another four studies were rated as having a low risk of bias (see Appendix 2). The most problematic factor with regard to the risk-of-bias assessment was the lack of blinding (of participants, physicians and outcome assessors) in the included studies. For participants and physicians, it is almost impossible to perform a trial with true blinding with these types of interventions. Nevertheless, the fact that participants and physicians were not blinded may bias the results, and the outcome assessment for HbA1c measurement could be performed blinded. Selective outcome reporting was assessed as having a high risk of bias in only three trials. Incomplete data reporting was assessed as having a high risk of bias in 12 trials; this was rated as unclear in four trials. Overall, there was a high risk of bias in the included trials.
In the following chapters, we will discuss the results of the included studies by population (i.e. adults, children and pregnant women) and by follow-up time (i.e. 3 months, 6 months and 9 months or more).
Effectiveness of interventions in adults
We found 12 studies that reported data for adults. 32,34,37–46 As can be seen in Table 5, the age ranges differed considerably; therefore, we asked a panel of four expert committee members whether or not they thought that the results of these studies could be pooled. Three clinical experts agreed that the studies were similar enough to be pooled, as far as the differences in age ranges were concerned, and the fourth clinical expert did not respond.
| Study ID | Veo | Integrated CSII + CGM | CSII+ SMBG | MDI+ SMBG | Mean baseline age, years (SD); age range, years | Mean baseline HbA1c, % (SD) | Previous pump use, months | Follow-up, months |
|---|---|---|---|---|---|---|---|---|
| Bergenstal et al., 201332 | ✓ | ✓ | 43 (13); 16–70 | 7.2 (0.7) | > 6 | 3 | ||
| Hirsch et al., 200834 | ✓ | ✓ | 33 (16); 18–72 | 8.3 (0.6) | > 6 | 6 | ||
| Hermanides et al., 201137 | ✓ | ✓ | 38 (11); 18–65 | 8.6 (0.9) | Naive | 6 | ||
| Lee et al., 200738 | ✓ | ✓ | NR | 9 (0.9) | Naive | 3.5 | ||
| Peyrot and Rubin, 200939 | ✓ | ✓ | 47 (13); NR | 8.6 (1) | NR | 3.7 | ||
| Bergenstal et al., 201040 | ✓ | ✓ | 41 (12); 19–70 | 8.3 (0.5) | Naive | 12 | ||
| Bolli et al., 200941 | ✓ | ✓ | 40 (11); 18–70 | 7.7 (0.7) | Naive | 6 | ||
| DeVries et al., 200242 | ✓ | ✓ | 37 (10); 18–70 | 9.4 (1.4) | Naive | 3.7 | ||
| aNosadini et al., 198843 | ✓ | ✓ | 34 (6); NR | NR | NR | 12 | ||
| Brinchmann-Hansen et al., 198544 | ✓ | ✓ | 26 (21); 18–45 | 8.5 (NR) | NR | 3, 6, 12, 24 | ||
| Thomas et al., 200745 | ✓ | ✓ | 43 (10); NR | 8.5 (1.5) | NR | 4, 6 | ||
| Tsui et al., 200146 | ✓ | ✓ | 36 (11); 18–60 | 8 (0.6) | Naive | 9 |
Veo versus integrated CSII + CGM
One study compared the MiniMed Veo with an integrated CSII + CGM system at 3-month follow-up in adults (ASPIRE in-home). 32 The results of this study, for the head-to-head comparison of the MiniMed Veo system with an integrated CSII + CGM system, are reported in Table 6.
| Outcome | MiniMed Veo system (n = 121) | Integrated CSII + CGM (n = 126) | Difference at follow-up | ||
|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | ||
| Mean change in HbA1c levels, % (SD) | 7.26 (0.71) | 7.24 (0.67) | 7.21 (0.77) | 7.14 (0.77) | 0.05 (95% CI –0.05 to 0.15) |
| Nocturnal hypoglycaemic events per patient per week (glucose < 3.6 mmol/l) (SD) | 1.5 (1.0) | 2.2 (1.3) | NR; p < 0.001 | ||
| Day and night hypoglycaemic events per patient per week (glucose < 3.6 mmol/l) (SD) | 3.3 (2.0) | 4.7 (2.7) | NR; p < 0.001 | ||
| Nocturnal hypoglycaemic AUCa (SD) | 980 (1200) | 1568 (1995) | NR; p < 0.001 | ||
| Day and night hypoglycaemic AUCa (SD) | 798 (965) | 1164 (1590) | NR; p < 0.001 | ||
| Meter BG (previous 2 weeks, mg/dl) (SD) | 151.4 (24.3) | 167.5 (24.7) | 151.8 (23.6) | 163.9 (32.1) | NS |
| Insulin use (U per patient per day) (SD) | 47.8 (19.40) | 46.5 (21.66) | NS | ||
| DKA | 0 | 0 | No difference | ||
| EQ-5D | NR | NR | NR | NR | No difference |
| Device-related serious AEs | 0 | 0 | No difference | ||
| AE, death | 0 | 0 | No difference | ||
No results were found for the MiniMed Veo system versus any other treatment at follow-up of 6 months or more.
Nocturnal hypoglycaemic events occurred 31.8% less frequently in the MiniMed Veo group than in the integrated CSII + CGM group [1.5 (SD 1.0) vs. 2.2 (SD 1.3) events per patient per week, p < 0.001]. Similarly, the MiniMed Veo group had significantly lower weekly rates of combined daytime and night-time events than the integrated CSII + CGM group (p < 0.001).
The mean area under the curve (AUC) for nocturnal hypoglycaemic events was 37.5% lower in the MiniMed Veo group than in the integrated CSII + CGM group [980 mg/dl (SD 1200 mg/dl) or 54.4 mmol/l (SD 66.6 mmol/l) × minutes vs. 1568 mg/dl (SD 1995 mg/dl) or 87.0 mmol/l (SD 110.7 mmol/l) × minutes; p < 0.001]. The mean AUC for daytime and night-time hypoglycaemic events was also significantly lower in the threshold suspend group.
The other outcomes showed no significant differences between groups.
Veo versus integrated CSII + CGM, CSII + SMBG and MDI + SMBG
For two outcomes [change in HbA1c levels and diabetic ketoacidosis (DKA)], results of the MiniMed Veo system versus other treatments were available for 3-month follow-up in adults from more than one study,38,39 which could be combined in indirect comparisons. These two outcomes are reported below.
Change in glycated haemoglobin levels at 3-month follow-up
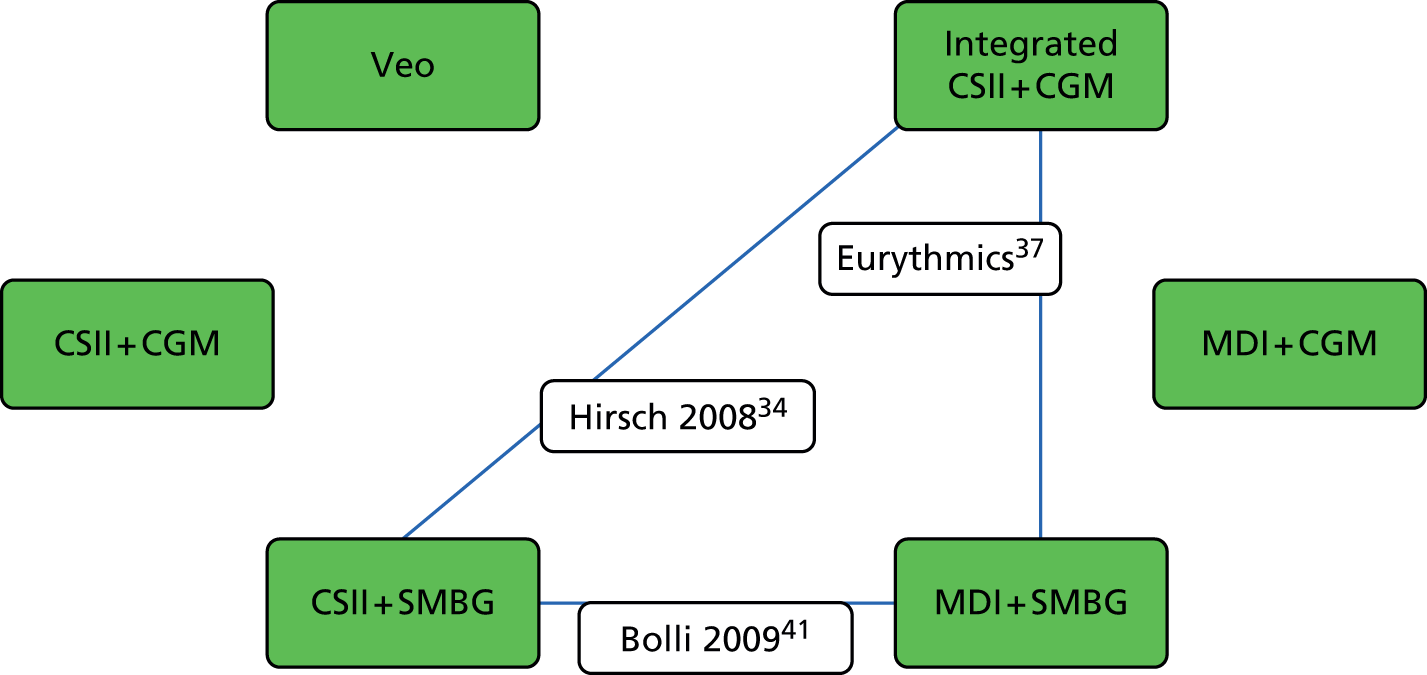
We found four studies32,38,39,42 comparing change in HbA1c levels at 3-month follow-up in adults, allowing a comparison of the MiniMed Veo system with an integrated CSII + CGM, CSII + SMBG and MDI + SMBG. Figure 2 shows the network linking these interventions and Table 7 shows the results.
FIGURE 2.
Network of studies comparing change in HbA1c levels and DKA at 3-month follow-up in adults. Note: green boxes represent the interventions; lines represent comparisons between interventions at 3-month follow-up; and transparent boxes represent studies in adults.

| Intervention | Integrated CSII + CGM, WMD (95% CI) | CSII + SMBG, WMD (95% CI) | MDI + SMBG, WMD (95% CI) |
|---|---|---|---|
| Veo | 0.04 (–0.07 to 0.15) | 0.41 (–0.31 to 1.13) | –0.43 (–0.95 to 0.10) |
| Integrated CSII + CGM | 0.37 (–0.34 to 1.08) | –0.47 (–0.98 to 0.04) | |
| CSII + SMBG | –0.84 (–1.33 to –0.35) |
The results of these indirect comparisons show that there are no significant differences between the MiniMed Veo system and any other intervention in change in HbA1c levels at 3-month follow-up. Similarly, there are no significant differences between the integrated CSII + CGM system and any other intervention in change in HbA1c levels at 3-month follow-up. The only significant difference found in this analysis was the difference between CSII + SMBG versus MDI + SMBG; in this regard, the results favour CSII + SMBG.
Diabetic ketoacidosis at 3-month follow-up
The same four studies32,38,39,42 provided data for DKA at 3-month follow-up in adults, allowing a comparison of the MiniMed Veo system with an integrated CSII + CGM system, CSII + SMBG and MDI + SMBG. However, the study that compared the MiniMed Veo system with the integrated CSII + CGM system (ASPIRE in-home)32 could not be included in the analysis as no events were reported in either arm. The results of the indirect comparisons for DKA are shown in Table 8.
| Intervention | Integrated CSII + CGM, RR (95% CI) | CSII + SMBG, RR (95% CI) | MDI + SMBG, RR (95% CI) |
|---|---|---|---|
| Veo | No events | No events | No events |
| Integrated CSII + CGM | 0.26 (0.01 to 8.53) | 0.32 (0.04 to 2.86) | |
| CSII + SMBG | 1.25 (0.08 to 19.22) |
The results of these indirect comparisons show that there are no significant differences with between the integrated CSII + CGM system and any other intervention with regard to DKA at 3-month follow-up. The comparison between CSII + SMBG and MDI + SMBG also showed no significant difference.
Integrated CSII + CGM versus CSII + SMBG
One study34 compared the integrated CSII + CGM system (Paradigm 722 System, Medtronic) with CSII + SMBG (Paradigm 715 Insulin Pump, Medtronic) at 6-month follow-up in adults.
At 6-month follow-up, results for the head-to-head comparison of the integrated CSII + CGM system versus CSII + SMBG were available for one outcome: change in HbA1c levels. Other outcomes were not reported separately for adults. The results for change in HbA1c levels are reported in Table 9.
| Outcome | Integrated CSII + CGM (n = 49) | CSII + SMBG (n = 49) | Difference at follow-up | ||
|---|---|---|---|---|---|
| Baseline (%) | Follow-up (%) | Baseline (%) | Follow-up (%) | ||
| Change in HbA1c levels (SD) | 8.37 (0.6) | 7.68 (0.84) | 8.30 (0.54) | 7.66 (0.67) | –0.0364 (SE 0.1412); p = 0.80 |
The results for the head-to-head comparison of the integrated CSII + CGM system versus CSII + SMBG at 6-month follow-up in adults showed no significant difference in HbA1c levels between groups.
Integrated CSII + CGM versus MDI + SMBG
Four studies37–40 compared the integrated CSII + CGM system (MiniMed Paradigm REAL-Time 722 System) with MDI + SMBG in adults. Two of these38,39 had results at 3 months, one37 at 6 months and one40 at 12-month follow-up.
At 3-month follow-up, results for the head-to-head comparison of the integrated CSII + CGM system versus MDI + SMBG were available for the following outcomes: change in HbA1c levels, hypoglycaemic events, DKA and adverse events. These results are reported in Table 10.
| Outcome/study | Integrated CSII + CGM | MDI + SMBG | Difference at follow-up | ||
|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | ||
| Three-month follow-up | |||||
| Change in HbA1c levels, % (SD) | |||||
| Peyrot and Rubin, 200939 (n = 27) | 8.87 (0.89), n = 14 | 7.16 (0.75) | 8.32 (1.05), n = 13 | 7.30 (0.92) | –0.69; p = 0.071 |
| Lee et al., 200738 (n = 16) | 9.45 (0.55), n = 8 | 7.40 (0.66) | 8.58 (1.30), n = 8 | 7.50 (1.01) | –0.97; p = 0.02 |
| Hypoglycaemic events (number of patients with events/total number of patients) | |||||
| Peyrot and Rubin, 200939 (n = 27) | NA | 0/14 | NA | 3/13 | NS |
| Lee et al., 200738 (n = 16) | NA | 0/8 | NA | 1/8 | NS |
| DKA (number of patients with DKA/total number of patients) | |||||
| Peyrot and Rubin, 200939 (n = 27) | NA | 0/14 | NA | 1/13 | NS |
| Lee et al., 200738 (n = 16) | NA | 0/8 | NA | 1/8 | NS |
| Serious AEs (number of patients with a serious AE/total number of patients) | |||||
| Lee et al., 200738 | NA | 0/8 | NA | 1/8 | NS |
| Six-month follow-up (Eurythmics37) | n = 41 | n = 36 | |||
| Change in HbA1c levels, % (SD) | 8.46 (0.95) | 7.23 (0.65) | 8.59 (0.82) | 8.46 (1.04) | –1.1, 95% CI –1.47 to –0.73 |
| Proportion achieving HbA1c levels of ≤ 7% (number of patients with HbA1c ≤ 7%/total number of patients) | NA | 14/41 | NA | 0/36 | p < 0.001 |
| Hypoglycaemic events, mean number of events (glucose levels of < 4.0 mmol/l) per day (SD) | NA | 0.7 (0.7) | NA | 0.6 (0.7) | 0.1, 95% CI –0.2 to 0.5 |
| Hyperglycaemic events, mean number of events (glucose levels of > 11.1 mmol/l) per day (SD) | NA | 2.1 (0.8) | NA | 2.2 (0.7) | –0.2, 95% CI –0.5 to 0.2 |
| Insulin use, total daily dose (SD) in units | NA | 46.7 (16.5) | NA | 57.8 (18.1) | –11.0, 95% CI –16.1 to –5.9; p < 0.001 |
| QoL: SF-36 Health Survey measuring general health, mean score (SD) | 55.5 (20.3) | 67.7 (21.6) | 59.8 (22.3) | 63.1 (19.1) | 7.9, 95% CI 0.5 to 15.3; p = 0.04 |
| Twelve-month follow-up (STAR-340) | n = 169 | n = 167 | |||
| Change in HbA1c levels, % (SD) | 8.3 (0.5) | 7.3 (NR) | 8.3 (0.5) | 7.9 (NR) | –0.6, 95% CI –0.8 to –0.4; p < 0.001 |
| Proportion achieving HbA1c levels of ≤ 7% (number of patients with HbA1c ≤ 7%/total number of patients) | NA | 57/166 | NA | 19/163 | p < 0.001 |
| Severe hypoglycaemia (patients with hypoglycaemic events/total patients) | NA | 17/169 | NA | 13/167 | NS |
| Severe hypoglycaemic event rate (per 100 person-years; HbA1c levels < 50 mg/dl) | NA | 15.31/169 | NA | 17.62/167 | p = 0.66 |
| Hypoglycaemic AUC (threshold of < 70 mg/dl) | NA | 0.25 (0.44) | NA | 0.29 (0.55) | p = 0.63 |
| Hyperglycaemic AUC (> 250 mg/dl) | NA | 3.74 (5.01) | NA | 7.38 (8.62) | p < 0.001 |
| Patients with DKA | NA | 2/169 | NA | 0/167 | NS |
| QoL | NA | NA | NA | NA | NA |
| SF-36 General Health | NA | Change: +2.7 (8.07) | NA | Change: –0.3 (7.13) | 3 (SD 7.75), 95% CI 1.36 to 4.64 |
| HFS | NA | Change: –9 (16.04) | NA | Change: –2.4 (15.88) | –6.5 (SD 16.0), 95% CI –9.76 to –3.27) |
At 6-month follow-up, results for the head-to-head comparison of the integrated CSII + CGM system versus MDI + SMBG were available for change in HbA1c levels, proportion achieving HbA1c levels of ≤ 7%, hypoglycaemic events, hyperglycaemic events, insulin use and quality of life. These results are also reported in Table 10, together with the results at 12-month follow-up for change in HbA1c levels, proportion achieving HbA1c levels of ≤ 7%, proportion with severe hypoglycaemia, rate of severe hypoglycaemic events, hypoglycaemic AUC, hyperglycaemic AUC, DKA and quality of life.
At 3-month follow-up, results were available from two small RCTs, with 2739 and 1638 adult respondents, respectively. With regard to change in HbA1c levels, the results from these RCTs favoured the integrated CSII + CGM system over MDI + SMBG, but this was not significant in one of the trials. 39 There were more hypoglycaemic events, DKA and serious adverse events in the MDI + SMBG groups at 3-month follow-up. None of these results was significant; however, the study sizes were small and the number of events was limited.
At 6-month follow-up, results were available from one small RCT with 77 adult respondents. 37 This trial showed a significant difference in HbA1c change scores favouring the integrated CSII + CGM system, with a significantly higher number of patients achieving HbA1c levels of ≤ 7%. Insulin use was significantly lower and quality of life was significantly higher in the integrated CSII + CGM group than in the MDI + SMBG group. The number of hypoglycaemic and hyperglycaemic events showed no differences between groups.
At 12-month follow-up, results were available from one RCT with 336 adult participants. 40 This trial also showed a significant difference in HbA1c change scores in favour of the integrated CSII + CGM system and a significantly higher number of patients achieving HbA1c levels of ≤ 7%. Hyperglycaemic AUC was significantly lower in the integrated CSII + CGM group, but hypoglycaemic AUC showed no significant difference. The results suggest that there were no significant differences between groups with regard to severe hypoglycaemia, nor was there any difference in the number of patients with DKA. Quality of life was more significantly improved in the integrated CSII + CGM group than in the MDI + SMBG group. The Hypoglycaemia Fear Survey (HFS) showed that there were significantly more reductions in fear in the integrated CSII + CGM group than in the MDI + SMBG group, for both worries and avoidant behaviour related to hypoglycaemia.
Integrated CSII + CGM versus CSII + SMBG and MDI + SMBG
Results at 3-month follow-up
The results of these indirect comparisons (Figure 3 and references 38, 39 and 42 therein) suggest that there are no significant differences between the integrated CSII + CGM system and any other intervention with regard to the ‘proportion of patients with severe hypoglycaemia’ at 3-month follow-up. The comparison between CSII + SMBG and MDI + SMBG also showed no significant difference. These findings are summarised in Table 11.
FIGURE 3.
Network of studies comparing ‘severe hypoglycaemia’ at 3-month follow-up in adults.

| Intervention | CSII + SMBG, RR (95% CI) | MDI + SMBG, RR (95% CI) |
|---|---|---|
| Integrated CSII + CGM | 0.33 (0.03 to 3.87) | 0.19 (0.02 to 1.51) |
| CSII + SMBG | 0.63 (0.17 to 2.31) |
Results at 6-month follow-up
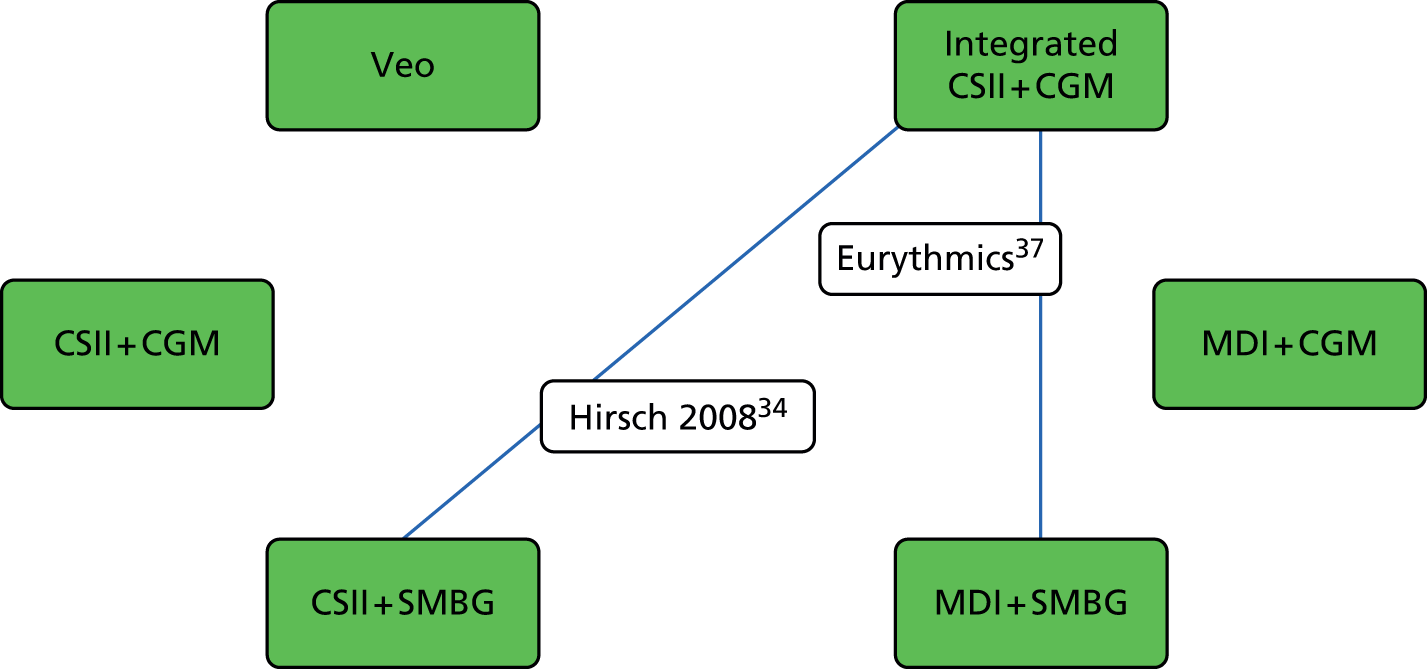
The results of these indirect comparisons (Figure 4 and references 34, 37 and 41 therein) suggest that there are no significant differences between the integrated CSII + CGM system and CSII + SMBG with regard to change in HbA1c levels at 6-month follow-up. The comparison between CSII + SMBG and MDI + SMBG also showed no significant difference. The comparison between the integrated CSII + CGM system and MDI + SMBG did show a significant difference, favouring the integrated CSII + CGM system. These findings are summarised in Table 12.
FIGURE 4.
Network of studies comparing change in HbA1c levels at 6-month follow-up in adults.
| Intervention | CSII + SMBG, WMD (95% CI) | MDI + SMBG, WMD (95% CI) |
|---|---|---|
| Integrated CSII + CGM | –0.05 (–0.31 to 0.21) | –1.10 (–1.46 to –0.74) |
| CSII + SMBG | –0.10 (–0.52 to 0.32) |
Results of these indirect comparisons (Figure 5 and references 34 and 37 therein) suggest that there are no significant differences between the integrated CSII + CGM system and CSII + SMBG with regard to ‘HbA1c levels < 7%’ at 6-month follow-up. However, the comparison between the integrated CSII + CGM system and MDI + SMBG did show a significant difference in favour of the integrated CSII + CGM system. Similarly, the comparison between CSII + SMBG and MDI + SMBG showed a significant difference in favour of CSII + SMBG. These findings are summarised in Table 13.
FIGURE 5.
Network of studies comparing ‘HbA1c levels < 7%’ at 6-month follow-up in adults.
| Intervention | CSII + SMBG, RR (95% CI) | MDI + SMBG, RR (95% CI) |
|---|---|---|
| Integrated CSII + CGM | 1.45 (0.74 to 2.84) | 25.55 (1.58 to 413.59) |
| CSII + SMBG | 17.56 (1.002 to 307.87) |
Different tools were used to measure HRQoL (Figure 6). Only those studies using the same questionnaire could be combined in the analysis. Two studies reported results at 6-month follow-up for the Diabetic Treatment Satisfaction Questionnaire (Eurythmics37 and Bolli et al. 41) using a scale from 0 to 36, with higher scores indicating more satisfaction with treatment. These findings are summarised in Table 14. Two studies reported results for the HFS (Eurythmics37 and Thomas et al. 45); however, these could not be analysed together as one reported only the worry subscale of the HFS, whereas the other reported the total score.
FIGURE 6.
Network of studies comparing ‘quality of life’ at 6-month follow-up in adults. DQOL, Diabetes Quality of Life questionnaire; DTSQ, Diabetic Treatment Satisfaction Questionnaire; SF-36, Short Form questionnaire-36 items.

| Intervention | CSII + SMBG, WMD (95% CI) | MDI + SMBG, WMD (95% CI) |
|---|---|---|
| Integrated CSII + CGM | 5.90 (2.22 to 9.58) | 8.60 (6.28 to 10.92) |
| CSII + SMBG | 2.70 (–0.16 to 5.56) |
The results of these indirect comparisons show that the integrated CSII + CGM system significantly improved the quality-of-life scores at 6-month follow-up when compared with CSII + SMBG or with MDI + SMBG. There was no significant difference between CSII + SMBG and MDI + SMBG.
Effectiveness of interventions in children
We found five studies34,40,47–49 that reported data for children. In addition, there was one study (Ly et al. 33) that included a mixed population of patients between 4 and 50 years old. Approximately 70% of patients were children (< 18 years).
We asked our panel of four expert committee members whether or not they thought that the results of these studies could be pooled, especially whether or not the study by Ly et al. 33 (age range of 4 to 50 years, with 70% of participants < 18 years) could be included as if it was a study in children. One clinical expert agreed that the six studies were similar enough, as far as the differences in age ranges were concerned, to be pooled. A second clinical expert agreed that five of the studies were similar enough, as far as the differences in age ranges were concerned, to be pooled, but given that approximately one-third of participants were aged 18–50, it would be difficult to include the Ly et al. 33 study in the analysis of the interventions in children (if the adult group had been a younger cohort, e.g. 18–25 years, this expert’s conclusion may have been different). The third clinical expert also thought the Ly et al. 33 study could not reasonably be included in analyses for either group (children or adults); this third expert also thought that teenage children behave in a different way from pre-teen children and that, therefore, the 8- to 14-year-old cohort may be significantly different and should perhaps have been excluded from analyses. The fourth clinical expert did not respond.
However, the study by Ly et al. 33 was the only study looking at the MiniMed Veo system in children; therefore, we will present the results from analyses that included this study as if it was a study in children. In addition, the study by Weintrob et al. ,47 with children aged 8 to 14 years old, is the only study with results at 6-month follow-up linking MDI + SMBG to the MiniMed Veo system and the integrated CSII + CGM system; therefore, we included this study in the analyses as well. The results of these analyses should be interpreted with great caution because of the differences in age ranges among the included studies, as shown in Table 15.
| Study | Veo | Integrated CSII + CGM | CSII+ SMBG | MDI+ SMBG | Mean baseline age, years (SD); age range, years | Mean baseline HbA1c, % (SD) | Previous pump use, months | Follow-up, months |
|---|---|---|---|---|---|---|---|---|
| Ly et al., 201333 | ✓ | ✓ | 19 (12); 4–50 | 7.5 (0.8) | > 6 | 6 | ||
| Hirsch et al., 200834 | ✓ | ✓ | 33 (16); 12–17 | 8.7 (0.9) | > 6 | 6 | ||
| Bergenstal et al., 201040 | ✓ | ✓ | 12 (3); 7–18 | 8.3 (0.5) | Naive | 12 | ||
| Weintrob et al., 200347 | ✓ | ✓ | 12 (1.5); 8–14 | 8 (1) | NR | 3.5 | ||
| Thrailkill et al., 201148 | ✓ | ✓ | 12 (3); 8–18 | 11.5 (2.4) | Naive | 6, 12 | ||
| Doyle et al., 200449 | ✓ | ✓ | 13 (3); 8–21 | 8.1 (1.2) | Naive | 3.7 |
Veo versus CSII + SMBG
One study33 compared the MiniMed Veo system with CSII + SMBG at 6-month follow-up in a mixed population of patients between 4 and 50 years old. Results were not reported separately for adults and children. However, approximately 70% of patients were children (< 18 years). As explained above, we have included this study as a study of children. The results of this study are summarised in Table 16.
| Outcome | MiniMed Veo system (n = 46) | CSII + SMBG (n = 49) | Difference at follow-up | ||
|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | ||
| Change in HbA1c levels, % (95% CI) | 7.6 (7.4 to 7.9) | 7.5 (7.3 to 7.7) | 7.4 (7.2 to 7.6) | 7.4 (7.2 to 7.7) | 0.07 (–0.2 to 0.3); p = 0.55 |
| Number of people with hypoglycaemic events | 0/41 | 6/45 | NS | ||
| Hypoglycaemic incidence ratea | 9.5 (95% CI 5.2 to 17.4) | 34.2 (95% CI 22.0 to 53.3) | IRR 3.6 (95% CI 1.7 to 7.5); p < 0.001 | ||
| HUSb | 5.9 (95% CI 5.5 to 6.4) | 4.7 (95% CI 4.0 to 5.1) | 6.4 (95% CI 5.9 to 6.8) | 5.1 (95% CI 4.5 to 5.6) | –0.2 (95% CI –0.9 to 0.5); p = 0.58 |
No results were found for the MiniMed Veo system versus any other treatment after 3 months, 9 months or longer follow-up.
As shown in Table 16, the only significant difference between treatment groups was the rate of moderate and severe hypoglycaemic events, which favoured the MiniMed Veo system. All other outcomes showed no significant differences between groups.
Veo versus integrated CSII + CGM and CSII + SMBG
Results at 6-month follow-up: change in glycated haemoglobin levels
The results of the indirect comparison, shown in Figure 7 and Table 17, demonstrate that there were no significant differences between any of the interventions with regard to changes in HbA1c levels at 6-month follow-up in children.
FIGURE 7.
Network of studies comparing change in HbA1c levels at 6-month follow-up in children.

| Intervention | Integrated CSII + CGM, WMD (95% CI) | CSII + SMBG, WMD (95% CI) |
|---|---|---|
| Veo | 0.38 (–0.16 to 0.92) | –0.04 (–0.26 to 0.18) |
| Integrated CSII + CGM | –0.42 (–0.92 to 0.08) |
Integrated CSII + CGM versus CSII + SMBG
One study compared the integrated CSII + CGM system with CSII + SMBG at 6-month follow-up in children. 34
At 6-month follow-up, results for the head-to-head comparison of the integrated CSII + CGM system with CSII + SMBG were available for one outcome: change in HbA1c levels. Other outcomes were not reported separately for children. The results for change in HbA1c levels are reported in Table 18.
| Outcome | Integrated CSII + CGM (n = 17) | CSII + SMBG (n = 23) | Difference at follow-up | ||
|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | ||
| Change in HbA1c levels, % (SD) | 8.82 (1.05) | 8.02 (1.11) | 8.59 (0.80) | 8.21 (0.97) | 0.4894 (SE 0.2899); p = 0.10 |
The results from the head-to-head comparison of the integrated CSII + CGM system with CSII + SMBG at 6-month follow-up in children showed no significant difference in HbA1c levels between groups.
Integrated CSII + CGM versus MDI + SMBG
One study compared the integrated CSII + CGM system with MDI + SMBG at 12-month follow-up in 159 children. 40
At 12-month follow-up, results from the head-to-head comparison of the integrated CSII + CGM system with MDI + SMBG were available for change in HbA1c levels, proportion achieving HbA1c levels of ≤ 7%, proportion with severe hypoglycaemia, rate of severe hypoglycaemic events, hypoglycaemic AUC, hyperglycaemic AUC, DKA and quality of life. These results are reported in Table 19.
| Outcome | Integrated CSII + CGM (n = 78) | MDI + SMBG (n = 81) | Difference at follow-up | ||
|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | ||
| Change in HbA1c levels, % (SD) | 8.3 (0.6) | 7.9 (NR) | 8.3 (0.5) | 8.5 (NR) | –0.5 (95% CI –0.8 to –0.2); p < 0.001 |
| Proportion achieving HbA1c levels of ≤ 7% (patients with HbA1c level ≤ 7%/total number of patients) | 10/78 | 4/78 | p = 0.15 | ||
| Number of people with severe hypoglycaemic events (patients with severe hypoglycaemic events/total number of patients) | 4/78 | 4/81 | NS | ||
| Severe hypoglycaemic event rate (per 100 person-years; HbA1c levels of < 50 mg/dl) | 8.98/78 | 4.95/81 | p = 0.35 | ||
| Hypoglycaemic (< 70 mg/dl) AUC (SD) | 0.23 (0.41) | 0.25 (0.41) | p = 0.79 | ||
| Hyperglycaemic (> 250 mg/dl) AUC (SD) | 9.2 (8.08) | 17.64 (14.62) | p < 0.001 | ||
| Patients with DKA | 1/78 | 1/81 | NS | ||
| QoL | |||||
| PedsQLa – psychosocial, mean score (SD) | 78.38 (14.59) | Change: 3.39 | 78.76 (10.27) | Change: 3.64 | NS |
| PedsQLa – physical, mean score (SD) | 86.99 (12.93) | Change: 2.53 | 88.37 (11.16) | Change: 1.41 | NS |
| HFSb – worry, mean score (SD) | 28.88 (9.74) | Change: –3.62 | 26.97 (8.06) | Change: –2.43 | NS |
| HFSb – avoidance, mean score (SD) | 30.60 (5.43) | Change: –4.01 | 29.70 (6.04) | Change: –2.25 | NS |
The trial showed a significant difference in HbA1c change scores in favour of the integrated CSII + CGM system, but no significant difference in the number of children achieving HbA1c levels of ≤ 7%. 40 The hyperglycaemic AUC was significantly lower in the integrated CSII + CGM group, but the hypoglycaemic AUC showed no significant difference. The results for severe hypoglycaemia showed no differences between groups; furthermore, there were no differences in the number of patients with DKA. Quality-of-life scores showed no significant differences between groups. The HFD showed that fear (as indicated by both worry and avoidance behaviour) was significantly reduced in both groups, but there was no difference between groups at 12-month follow-up.
Effectiveness of interventions in pregnant women
We found one RCT50 that reported data for pregnant women (Table 20). The study included 32 pregnancies in 31 different women. The number of pregnancies was the unit of analysis. The study compared CSII + SMBG with MDI + SMBG; as these are not the relevant interventions described by NICE, the results will not be further discussed in this chapter. Full results are reported in Appendix 3.
| Study | Veo | Integrated CSII + CGM | CSII+ SMBG | MDI+ SMBG | Mean baseline age, years (SD years); age range | Mean baseline HbA1c | Previous pump use | Follow-up, months |
|---|---|---|---|---|---|---|---|---|
| Nosari et al., 199350 | ✓ | ✓ | 26 (2.4); NR | NR | Naive | 9 |
Several non-RCTs (controlled clinical trials and observational studies) were identified; however, none of these looked at the MiniMed Veo system or an integrated CSII + CGM system. One ongoing study was identified; this is reported below (see Ongoing studies).
Additional analyses for the economic model
So far, we have adhered to the usual methods of meta-analyses, in accordance with which studies are combined in one analysis only if they compare similar interventions in similar populations at similar follow-up times, using similar outcomes.
We checked with clinical experts/committee members with regard to whether or not they agreed with these intended analyses and there was general agreement on the following points:
-
Age Studies in children and adults should be analysed separately and studies in mixed age groups (adults and children), if data are not reported separately by age group, should not be included in analyses for children or adults.
-
Follow-up Studies with results at 3-, 6- or 9-month follow-up should be analysed separately. Results from studies reporting outcomes at 2- to 4-month follow-ups can be pooled with results from studies reporting at 3-month follow-up; results from studies reporting at ≥ 9-month follow-up can be pooled in a ≥ 9-month follow-up group.
In cases in which the clinical experts disagreed with our suggested analyses, the clinical experts were always more cautious. For instance, it was suggested that Ly et al. 33 should not be treated as a study in children because one-third of participants were aged 18–50 years; therefore, it would be difficult to include this study with the analysis of children. If the adult age group in this study had been a younger cohort (e.g. 18–25 years) it may have been different. Similarly, teenage children were considered to behave in a different way from pre-teen children; therefore, the study by Weintrob et al. 47 (in which participants were aged 8 to 14 years) may be significantly different from the other studies in children (of up to 18 years) and perhaps should be excluded.
However, because of the lack of data, we have included the studies by Ly et al. 33 and Weintrob et al. 47 in the analyses for children. As a consequence, the results of these analyses are less reliable as a result of clinical heterogeneity between studies.
Despite trying to include as many studies as possible in the analyses for adults, we still have missing results for key comparisons for the economic model. Most importantly, results for comparisons of the MiniMed Veo system and the integrated CSII + CGM system with the comparators CSII + CGM, CSII + SMBG, MDI + CGM and MDI + SMBG are missing for the outcomes change in HbA1c levels and severe hypoglycaemic event rates. As can be seen in Table 2, none of the included studies looked at CSII + CGM and MDI + CGM. Therefore, a comparison between these comparators cannot be made. However, it is possible to calculate results for these outcomes (change in HbA1c and severe hypoglycaemic event rates) by comparing the MiniMed Veo system and the integrated CSII + CGM system with CSII + SMBG and with MDI + SMBG in a series of indirect comparisons, if we accept the following assumptions:
-
All studies can be pooled, irrespective of length of follow-up (3, 6 or ≥ 9 months).
-
Studies in mixed populations (including those of children and adults that do not report separate results by age group) can be pooled in one analysis. This means that we will include O’Connell et al. (30 adults and 32 children),35 RealTrend (81 adults and 51 children)36 and Hirsch et al. (98 adults and 40 children),34 in the analyses for adults. Ly et al. 33 (30 adults and 65 children) will still be excluded from these analyses.
-
For event rates, we assumed that if numbers of events were reported, the rate could be derived by assuming that all patients had been observed for the follow-up duration of the trial.
It should be taken into account that the following analyses, including any subsequent analyses, such as the economic model, are based on these assumptions and that the clinical experts advised against using these wide inclusion criteria for pooling studies in one analysis. The results of these analyses are therefore likely to be considerably less reliable because of higher levels of clinical heterogeneity between studies included in these analyses for adults.
Change in glycated haemoglobin levels
The results of the indirect comparison, as shown in Figure 8 and Table 21, demonstrate that there were no significant differences with regard to the change in HbA1c levels in adults (including mixed populations) between the MiniMed Veo system and the integrated CSII + CGM system. Similarly, there were no significant differences with regard to the change in HbA1c levels in adults (including mixed populations) between the MiniMed Veo system and the integrated CSII + CGM system on the one hand and CSII + SMBG on the other. There was a significant difference in the change in HbA1c levels in adults (including mixed populations) between the MiniMed Veo system and the integrated CSII + CGM system if both systems are compared with MDI + SMBG, favouring the MiniMed Veo system and the integrated CSII + CGM system over MDI + SMBG.
FIGURE 8.
Network of studies32,34–46 comparing change in HbA1c levels at all follow-up times in adults and mixed populations. Green boxes represent the interventions; lines represent comparisons between interventions at different follow-up times (blue line, 3 months; green line, 6 months; black line, ≥ 9 months); and transparent boxes represent studies (blue, mixed population; black, adult population).

| Intervention | Integrated CSII + CGM, WMD (95% CI) | CSII + SMBG, WMD (95% CI) | MDI + SMBG, WMD (95% CI) |
|---|---|---|---|
| Veo | 0.04 (–0.07 to 0.15) | –0.07 (–0.31 to 0.17) | –0.66 (–1.05 to –0.27) |
| Integrated CSII + CGM | –0.11 (–0.32 to 0.10) | –0.70 (–1.05 to –0.30) a | |
| CSII + SMBG | –0.46 (–1.18 to 0.27)b |
Overall, integrated systems (the MiniMed Veo system and the integrated CSII + CGM system) are superior to SMBG (with CSII or MDIs) in terms of HbA1c levels. However, as reported above, the reliability of the results of these analyses is reduced because of a relatively high level of heterogeneity between the studies included in the analyses. This is particularly true for the comparison between the MiniMed Veo system and CSII + SMBG, which is based not only on an indirect comparison (using data from the ASPIRE in-home trial,32 O’Connell et al. ,35 Hirsch et al. 34 and RealTrend36), but also on data from 3-month follow-up (ASPIRE in-home32 and O’Connell et al. 35) combined with data from 6-month follow-up (Hirsch et al. 34 and RealTrend36), and on data from adults (ASPIRE in-home32 and Hirsch et al. 34) and mixed populations (O’Connell et al. 35 and RealTrend36).
Severe hypoglycaemic event rate
The results of the indirect comparison, as shown in Figure 9 and Table 22, show that there were no significant differences in the severe hypoglycaemic event rate in adults (including mixed populations) between the MiniMed Veo system and any of the other treatments. Similarly, there were no significant differences in the change in severe hypoglycaemic event rate between the integrated CSII + CGM system and MDI + SMBG. There was a significant difference in the severe hypoglycaemic event rate between the integrated CSII + CGM system and CSII + SMBG, in favour of CSII + SMBG. However, as reported above, the reliability of the results of these analyses is reduced because of a relatively high level of heterogeneity between the studies included in the analyses. With regard to the significant difference in particular, it is important to point out that this result relies upon the data from three trials with different follow-up times (3 months for O’Connell et al. 35 and 6 months for Hirsch et al. 34 and RealTrend36), and that data from all three trials are from mixed populations, including adults and children.
FIGURE 9.
Network of studies32,34–46 comparing severe hypoglycaemic event rate at all follow-up times in adults and mixed populations. Green boxes represent the interventions; lines represent comparisons between interventions at different follow-up times (blue line, 3 months; green line, 6 months; black line, ≥ 9 months); transparent boxes represent studies (blue, mixed population; black, adult population; green, adults and all hypoglycaemic events).

| Intervention | Integrated CSII + CGM, rate ratio (95% CI) | CSII + SMBG, rate ratio (95% CI) | MDI + SMBG, rate ratio (95% CI) |
|---|---|---|---|
| Veo | 0.12 (0.01 to 2.14) | 0.39 (0.02 to 8.40) | 0.10 (0.01 to 1.93) |
| Integrated CSII + CGM | 3.23 (1.10 to 9.49) | 0.86 (0.51 to 1.46) | |
| CSII + SMBG | 0.67 (0.38 to 1.20) |
Overall, the main conclusion regarding the evidence for hypoglycaemic event rate, and change in HbA1c levels, in adults is that the evidence is limited and when all available evidence is combined, the results become highly unreliable.
Ongoing studies
We found 18 ongoing studies51–68 – 17 RCTs51–55,57–68 and one observational study56 looking at the use of a threshold suspend feature at home with a sensor-augmented insulin pump (SAP) (MiniMed 530G). Most ongoing studies are in children (12 out of 18 studies51,53,54,56,60–62,64–68), five are in a general population (adults or adults and children)52,55,57,59,63 and one study is in pregnant women. 58 Seven studies include the MiniMed Veo system51,52,54–56,59,64 and four studies include the integrated CSII + CGM system. 55,63,64,66 Details of ongoing studies are reported in Table 23.
| Study ID | Year | Intervention | RCT | Comment | Age |
|---|---|---|---|---|---|
| Lawson et al.51 | 2014 | Veo vs. CSII + SMBG | Yes | Complex design. Trial uses the Veo system. Patients are randomised to simultaneous initiation of pump and CGM vs. initiation of pump with CGM started 6 months later. Outcomes were measured after 6 and 12 months. Group B is pump + SMBG for 6 months then pump + CGM for the next 6 months | 5–18 years |
| Troub et al.52 | 2013 | Veo vs. CSII + CGM | Yes | General | |
| Blair et al.53 | 2010 | CSII + SMBG vs. MDI + SMBG | Yes | CSII compared with MDI regimens in children and young people at diagnosis of T1DM; protocol only | Children |
| Assistance Publique – Hôpitaux de Paris NCT0094922154 | 2012 | Veo vs. CSII + SMBG | Yes | Device: Medtronic’s Paradigm 754 Veo monitor with MiniLink REAL-Time transmitter (Conformité Européenne). 3 months and 9 months of SMBG vs. 12 months of using the Veo system | 2–18 years |
| Steno Diabetes Centre NCT0145470055 | 2012 | Veo vs. integrated CSII + CGM vs. MDI + SMBG | Yes | CSII plus CGM (Medtronic’s MiniMed Paradigm REAL-Time system or Veo) vs. MDIs | General |
| Medtronic Diabetes NCT0212079456 | 2014 | Veo | Obs | Use of threshold suspend feature at home with a SAP [MiniMed 530G (Medtronic)] in children with T1DM over 1 year | 2–15 years |
| Vastra Gotaland Region NCT0209205157 | 2014 | MDI + CGM vs. MDI + SMBG | Yes | CGM vs. SMBG in individuals with T1DM treated with MDIs | General |
| University of British Columbia NCT0206402358 | 2014 | CSII + SMBG vs. MDI + SMBG | Yes | Comparison of CSII with MDIs for the treatment of pregestational diabetes during pregnancy (T1DM and T2DM) | Pregnant |
| Sheffield Teaching Hospitals NHSFT (REPOSE Trial) NCT01616784EUCTR2010-023198–21-GB59 | 2013 | Veo vs. CSII + SMBG vs. MDI + SMBG | Yes | CSII (insulin pump) plus DAFNE versus MDI [insulin detemir (Levemir®, Novo Nordisk) and quick-acting insulin] plus DAFNE | > 18 years |
| Seattle Children’s Hospital NCT0087529060 | 2011 | CSII + CGM vs. CSII + SMBG | Yes | CSII alone vs. CSII + RTSA in infants with T1DM | 0–3 years |
| Nemours Children’s Clinic NCT0035789061 | 2012 | CSII + SMBG vs. MDI + SMBG | Yes | MDI vs. CSII in adolescents with newly diagnosed T1DM | 12–17 years |
| Addenbrooke’s NHS Trust EUCTR2005-004526-72-GB62 | 2006 | CSII + CGM vs. MDI + SMBG | Yes | CSII vs. MDI in preschool-aged children with T1DM | < 18 years |
| Medtronic Australasia ACTRN1260600004957263 | 2006 | Integrated CSII + CGM vs. CSII + SMBG | Yes | MiniMed Paradigm REAL-Time insulin pump and CGM system (MMT-722 pump) vs. pre-trial insulin pump device (no new intervention) | 13–39 years |
| Juvenile Diabetes Research Foundation ACTRN1261400051064064 | 2014 | Veo vs. integrated CSII + CGM | Yes | CSII with real-time CGM system and predictive LGS feature (Medtronic’s MiniMed 640G) vs. standard SAPT | 8–20 years |
| The Royal Children’s Hospital Melbourne ACTRN1261000060509965 | 2010 | CSII + SMBG vs. MDI + SMBG | Yes | CSII vs. MDIs in children and adolescents with T1DM | 9–16 years |
| Royal Children’s Hospital ACTRN1261100014293266 | 2011 | Integrated CSII + CGM vs. CSII + CGM | Yes | Patients’ own pump vs. a new integrated pump (unclear which type of monitoring was used with patients’ own pumps) | < 18 years |
| Alder Hey Children’s NHS Foundation Trust ISRCTN2925527567 | 2010 | CSII + SMBG vs. MDI + SMBG | Yes | CSII vs. MDIs in children with T1DM | 1–15 years |
| University of Schleswig-Holstein NCT0133892268 | 2011 | CSII + SMBG vs. MDI + SMBG | Yes | CSII vs. MDIs in children with T1DM | 6–16 years |
Summary of results
In this summary of results, we will describe the results by population (adults, children and pregnant women) and by comparison. First, we will describe comparisons between the MiniMed Veo system and other treatments, then comparisons between the integrated CSII + CGM system and other treatments, and, finally, we will describe the main remaining comparisons.
Nineteen trials were included:32–50 12 reported data for adults,32,34,37–46 six reported data for children33,34,40,47–49 and one trial reported data for pregnant women. 57 Four trials were in mixed populations (adults and children);34–36,40 two of these reported data separately for adults and children and are included in the 12 trials for adults and six trials for children. 34,40 Two trials (O’Connell et al. 35 and RealTrend36) did not report data separately for adults and children. Therefore, the results from these trials were not used in the main analyses. However, the data are reported in the data extraction tables in Appendix 3 and they are used in the additional analyses for the economic model (see Additional analyses for the economic model).
Studies in adults
Twelve studies were included in the analyses for adults. 32,34,37–46 Only one of these studies (Hirsch et al. 34) reported the change in HbA1c levels separately for adults. None of these studies looked at CSII or MDI + CGM. Table 5 shows an overview of these 12 studies, their comparisons and their baseline data. Further details are reported in Appendix 3.
MiniMed Veo system versus the integrated CSII + CGM system
Only one study (ASPIRE in-home32) with data for adults (n = 247) included the MiniMed Veo system as one of the treatment arms. This study compared the MiniMed Veo system with an integrated CSII + CGM system at 3-month follow-up. The results of this study showed that there was no significant difference in change in HbA1c levels at 3-month follow-up; however, both nocturnal hypoglycaemic event rates and day and night hypoglycaemic event rates were significantly reduced for patients using the MiniMed Veo system. There were no significant differences in any of the other reported outcomes (BG level at follow-up, insulin use, DKA, quality of life or adverse events). Therefore, the conclusion from this trial is that the MiniMed Veo system reduces hypoglycaemic events in adults more than the integrated CSII + CGM system does, without any differences in other outcomes, including the change in HbA1c levels.
MiniMed Veo system versus other treatments
Indirect evidence seems to suggest that that there are no significant differences between the MiniMed Veo system and CSII + SMBG or MDI + SMBG with regard to the change in HbA1c levels at 3-month follow-up.
However, if all studies are combined (see Additional analyses for the economic model), the MiniMed Veo system is significantly better than MDI + SMBG in terms of the change in HbA1c levels.
The integrated CSII + CGM system versus other treatments
Five studies compared the integrated CSII + CGM system with other treatments. 34,37–40 One of these compared the integrated CSII + CGM system with CSII + SMBG at 6-month follow-up (Hirsch et al. 34), but this study reported only the change in HbA1c levels separately for adults. The other four studies compared the integrated CSII + CGM system with MDI + SMBG at 3-month follow-up (Lee et al. ,38 and Peyrot and Rubin39), at 6-month follow-up (Eurythmics37) and at 12-month follow-up (STAR-340).
The results of the trial34 comparing the integrated CSII + CGM system with CSII + SMBG at 6-month follow-up in adults showed no significant difference in HbA1c levels between groups. Other outcomes in this trial were not reported separately for adults. 34 An indirect comparison showed that quality of life was significantly more improved in the integrated CSII + CGM group than in the CSII + CGM group. 37,41
For the comparison of the integrated CSII + CGM system with MDI + SMBG, the most reliable data, from the largest trial with 12-month follow-up (STAR-340), show that there is a significant difference in the change in HbA1c levels and in the proportion of patients achieving HbA1c levels of ≤ 7%, in favour of the integrated CSII + CGM system. With regard to hypoglycaemic event rates, none of the studies showed a significant difference between groups. Similarly, there were no significant differences in DKA between groups. Insulin use was significantly lower in patients using the integrated CSII + CGM system, and quality of life was significantly more improved in the integrated CSII + CGM group than in the CSII + SMBG group. Overall, the results show significant results in favour of the integrated CSII + CGM system over MDI + SMBG with regard to HbA1c levels and quality of life.
Continuous subcutaneous insulin infusion versus multiple daily insulin injections
We found six trials with data for adults comparing CSII + SMBG with MDI + SMBG. 41–46 No trials were found with data for adults comparing the treatments CSII + CGM and MDI + CGM.
In terms of the change in HbA1c levels, only one42 of the six trials showed a significant difference between CSII + SMBG and MDI + SMBG. DeVries et al. 42 found a significant difference in favour of CSII + CGM: at 16 weeks, the mean HbA1c level was 0.84% lower (mean = –0.84%, 95% CI –1.31% to –0.36%) in the CSII + SMBG group than in the MDI + SMBG group. Significance was not reported in the OSLO trial44 or in Nosadini et al. ,43 while the difference between groups was not significant in Bolli et al. ,41 Thomas et al. 45 or Tsui et al. 46
In terms of the number of severe hypoglycaemic events, three trials found no significant differences between groups (Bolli et al. ,41 DeVries et al. 42 and Thomas et al. 45), while this was not reported in the other three trials.
Studies in children
Six studies were included in the analyses for children. 33,34,40,47–49 One of these studies (Hirsch et al. 34) reported only the change in HbA1c levels separately for children. None of these studies looked at CSII or MDI + CGM. Table 15 shows an overview of these six studies, their comparisons and their baseline data. Further details are reported in Appendix 3.
MiniMed Veo system versus the integrated CSII + CGM system
None of the studies in children made a direct comparison between the MiniMed Veo system and the integrated CSII + CGM system.
An indirect comparison was possible, using data at 6-month follow-up from Ly et al. 33 and Hirsch et al. ,34 but only for HbA1c levels, which showed no significant difference between groups.
MiniMed Veo system versus other treatments
One study compared the MiniMed Veo system with CSII + SMBG at 6-month follow-up in a mixed population of patients between 4 and 50 years old (Ly et al. 33). No results were found for the MiniMed Veo system versus any other treatment at 3-month or ≥ 9-month follow-up.
The only significant difference between treatment groups was the rate of moderate and severe hypoglycaemic events, which favoured the MiniMed Veo system. All other outcomes showed no significant differences between groups.
The integrated CSII + CGM system versus other treatments
One study compared the integrated CSII + CGM system with CSII + SMBG at 6-month follow-up in children (Hirsch et al. 34). This trial found no significant difference in HbA1c levels between groups.
One study (STAR-340) compared the integrated CSII + CGM system with MDI + SMBG at 12-month follow-up in children. This trial showed a significant difference in HbA1c change scores in favour of the integrated CSII + CGM system, but no significant difference in the number of children achieving HbA1c levels of ≤ 7%. The hyperglycaemic AUC was significantly lower in the integrated CSII + CGM group, but the hypoglycaemic AUC showed no significant difference between groups. Other outcomes showed no significant differences between groups.
Studies in pregnant women
We found one RCT that reported data for pregnant women. 57 The study included 32 pregnancies in 31 different pregnant women. The number of pregnancies was the unit of analysis. The study compared CSII + SMBG with MDI + SMBG; therefore, the results are not relevant for comparisons with the MiniMed Veo system or the integrated CSII + CGM system.
Chapter 4 Assessment of cost-effectiveness
In this chapter, we explore the cost-effectiveness of integrated insulin pump systems in the management of T1DM in adults in the UK.
Review of the economic evaluations
Search methods
Literature searches were undertaken to identify published economic evaluations of the MiniMed Paradigm Veo system and the Vibe and G4 PLATINUM CGM system. The search strategy for economic evaluations included a filter designed to identify cost and economic studies in databases that are not health economics specific.
The following databases and resources were searched for relevant economic evaluations and cost studies:
-
NHS Economic Evaluation Database (Wiley Online Library): issue 3/July 2014
-
Health Economic Evaluations Database (Wiley Online Library): up to 5 September 2014
-
MEDLINE (via OvidSP): 1946–2014/August week 4
-
MEDLINE In-Process Citations and Daily Update (via OvidSP): up to 5 September 2014
-
PubMed (via National Library of Medicine): up to 5 September 2014
-
EMBASE (via OvidSP): 1974–2014/week 34
-
EconLit (EBSCOhost): 1969–1 August 2014
-
Cost-effectiveness Analysis Registry (www.cearegistry.org): up to 5 September 2014
-
Research Papers in Economics (http://repec.org/): up to 5 September 2014.
In addition, economic searches specifically for the MiniMed Paradigm Veo system, and Vibe and G4 PLATINUM CGM system were conducted using the same resources listed above.
The full search strategies are presented in Appendix 1.
Relevant studies were then identified in two stages. Titles and abstracts returned by the search strategy were examined independently by two researchers (Maiwenn Al and Isaac Corro Ramos) and screened for possible inclusion. Disagreements were resolved by discussion. Full texts of the identified studies were obtained. Two researchers (Maiwenn Al and Isaac Corro Ramos) examined these independently for inclusion or exclusion, and disagreements were resolved by discussion.
Inclusion criteria
The initial search identified a total of eight abstracts, six of which were of conference abstracts and were thus not included. Both of the full-text papers were identified as relevant to our review. These studies were by Kamble et al. 69 and Ly et al. 70 The study by Kamble et al. 69 evaluated integrated CSII + CGM versus MDI + SMBG in the USA, whereas the study by Ly et al. 70 evaluated the MiniMed Paradigm Veo system versus CSII + SMBG in Australia. The first evaluation69 showed that the integrated CSII + CGM system was not cost-effective compared with MDI + SMBG, despite taking all health effects into account through the IMS Centre for Outcomes Research and Effectiveness diabetes model (IMS CDM) version 8.5 (IMS Health, Danbury, CT, USA). On the other hand, the second study70 showed that the MiniMed Veo system was cost-effective compared with CSII + SMBG, if only the impact on the reduction of severe hypoglycaemic events was taken into account.
The characteristics of these studies are summarised in Table 24.
| Study, country | Summary of model | Intervention/comparator | Patient population; average age; HbA1c levels at baseline | QALYs (intervention; comparator) | Costs (intervention; comparator) | ICER (per QALY gained) | Sensitivity analyses |
|---|---|---|---|---|---|---|---|
| Kamble et al. (2012),69 USA |
|
Integrated CSII + CGM vs. MDI + SMBG | Adults with inadequately controlled T1DM; 41.3 years; 8.3% | 10.794; 10.418 | For 3-d sensor: US$253,493; US$167,170 For 6-d sensor: US$230,352; US$167,170 |
For 3-d sensor: US$229,675/QALY For 6-d sensor: US$168,104/QALY |
|
| Ly et al. (2014),70 Australia |
|
MiniMed Paradigm Veo system vs. CSII + SMBG | Patients with T1DM who have impaired awareness of hypoglycaemia (subgroup analysis for ≥ 12 years); 18.6 years; 7.5% | Outcome: severe hypoglycaemic events (all patients/patients ≥ 12 years); CSII + CGM + LGS: 0/0; CSII + SMBG: 0.08607/0.1052 Outcome: QALYs (using patients ≥ 12 years); CSII + CGM + LGS: 00.036650; CSII + SMBG: –0.00017 |
Total costs (intervention costs + other medical costs due to severe hypoglycaemic events) (all patients/patients ≥ 12 years); CSII + CGM + LGS: AU$4382/AU$4432; CSII + SMBG: AU$2867/AU$2929 | All patients: NA because utility data were available only for patients ≥ 12 years; patients ≥ 12 years: AU$40,803 | Sensitivity analysis indicated that ICER would only substantially increase (above AU$100,000) when the utility values were changed to 0.0075 |
Of the six (excluded) conference abstracts, one was an abstract that was later published as a full-text paper71 and was already included as one of the two selected full-text papers. 69 While we will not formally discuss the conference abstracts,72–76 their characteristics, as far as they can be found in these abstracts, are presented in Table 25.
| Study, country | Summary of model | Intervention/comparator | Patient population; average age; HbA1c levels at baseline | QALYs (intervention; comparator) | Costs (intervention; comparator) | ICER (per QALY gained) | Sensitivity analyses |
|---|---|---|---|---|---|---|---|
| Gomez et al. (2013),73 Colombia |
|
SAP/comparator not stated | Model population not described, trial population of 217 T1DM patients (average diabetes duration of 14 years); 34 years; 8.97% | QALYs not presented but life-year gain of 3.51 | No costs presented | COP44,889,916 (US$24,939) based on only direct costs | Extensive sensitivity analyses showed the robustness of the results |
| Gomez et al. (2014),72 Colombia | This study is the same as the study above,73 but reports only on effects, not costs | SAP vs. MDIs | The inputs were taken from a real-life Colombian clinical study of 217 T1DM patients on SAPT | QALYs not presented but life-year gain of 3.51; diabetes complications delayed by 1.74 years | |||
| Lindholm Olinder et al. (2014),74 Sweden | Abstract does not indicate if a model was used; systematic review to establish available evidence on effects of CGM and SAPT, in A, C and P, compared with SMBG | CGM and SAPT vs. SMBG | Patients (A, C and P) with T1DM | No QALYs presented | Calculations of costs demonstrated an increased cost of €3026 for CGM vs. SMBG and €4216 for SAP vs. MDI and SMBG | No ICER presented | |
| Roze et al. (2014),75 France |
|
Integrated CSII + CGM vs. CSII (the method for BG monitoring is not stated) | Adults with inadequately controlled T1DM; 36 years; 9% | 1.27 QALYs gained | Extra annual costs of €1258 per patient | €27,796 | Sensitivity analysis on key drivers confirmed robustness of results under a wide range of assumptions |
| Roze et al. (2014),76 UK |
|
Integrated CSII + CGM vs. CSII (the method for BG monitoring is not stated) | Adults with inadequately controlled T1DM; 27 years; 10% | 3.1 QALYs gained | Extra annual costs of £1143 per patient | £16,986 | Sensitivity analysis on key drivers confirmed robustness of results under a wide range of assumptions |
Quality assessment
A quality appraisal was carried out on the two studies,69,70 using the Drummond checklist. 77 A summary of the results are provided in Table 26.
| Criteria | Kamble et al. (2012)69 | Ly et al. (2014)70 |
|---|---|---|
| Study design | ||
| 1. Was the research question stated? | Yes | Yes |
| 2. Was the economic importance of the research question stated? | Yes | Yes |
| 3. Was/were the viewpoint(s) of the analysis clearly stated and justified? | Yes | Yes |
| 4. Was a rationale reported for the choice of the alternative programmes or interventions compared? | No, CEA based on clinical trial so alternative based on that | Yes |
| 5. Were the alternatives being compared clearly described? | Partially; not easy to find if glucose monitoring is CGM or SMBG | Yes |
| 6. Was the form of economic evaluation stated? | Yes | Yes |
| 7. Was the choice of form of economic evaluation justified in relation to the questions addressed? | Yes | Justification was given, but doubtful if choice is reasonable |
| Data collection | ||
| 8. Was/were the source(s) of effectiveness estimates used stated? | Yes | Yes |
| 9. Were details of the design and results of the effectiveness study given (if based on a single study)? | Yes; most details in separate paper | Yes; most details in separate paper |
| 10. Were details of the methods of synthesis or meta-analysis of estimates given (if based on an overview of a number of effectiveness studies)? | NA | NA |
| 11. Was/were the primary outcome measure(s) for the economic evaluation clearly stated? | Yes | Yes |
| 12. Were the methods used to value health states and other benefits stated? | Yes | Yes; however, after seeing QALY outcomes, explanation clearly insufficient |
| 13. Were the details of the subjects from whom valuations were obtained given? | NA; utilities from literature | Yes |
| 14. Were productivity changes (if included) reported separately? | NA | NA |
| 15. Was the relevance of productivity changes to the study question discussed? | NA | NA |
| 16. Were quantities of resources reported separately from their unit cost? | Yes for all treatment related costs; no for complication costs | Yes |
| 17. Were the methods for the estimation of quantities and unit costs described? | Yes | Yes |
| 18. Were currency and price data recorded? | Yes | Yes |
| 19. Were details of price adjustments for inflation or currency conversion given? | Yes | NA |
| 20. Were details of any model used given? | Yes | Yes |
| 21. Was there a justification for the choice of model used and the key parameters on which it was based? | No justification for why IMS CDM was used in the paper | A justification was given, i.e. the clinical trial was modelled and extrapolation was not considered of interest. Unlikely that only looking at hypoglycaemic events and not long-term complications is of interest for decision makers |
| Analysis and interpretation of results | ||
| 22. Was the time horizon of cost and benefits stated? | Yes | Yes |
| 23. Was the discount rate stated? | Yes | NA |
| 24. Was the choice of rate justified? | Yes | NA |
| 25. Was an explanation given if costs or benefits were not discounted? | NA | Yes |
| 26. Were the details of statistical test(s) and CIs given for stochastic data? | Yes | Yes |
| 27. Was the approach to sensitivity analysis described? | Yes | Yes |
| 28. Was the choice of variables for sensitivity analysis justified? | Yes | No justification given, but choices appear reasonable |
| 29. Were the ranges over which the parameters were varied stated? | Yes | Yes |
| 30. Were relevant alternatives compared? (That is, were appropriate comparisons made when conducting the incremental analysis?) | Yes | Yes |
| 31. Was an incremental analysis reported? | Yes | Yes |
| 32. Were major outcomes presented in a disaggregated as well as aggregated form? | Yes | Yes; this highlighted the lack of face validity: QALYs in both arms were 0.036650 and –0.00017, while perfect health would yield 0.5 per arm |
| 33. Was the answer to the study question given? | Yes | Yes |
| 34. Did conclusions follow from the data reported? | Yes | Yes |
| 35. Were conclusions accompanied by the appropriate caveats? | Yes | Not fully; authors did not discuss the impact of the intervention in the trial on HbA1c levels and how that would impact cost-effectiveness |
| 36. Were generalisability issues addressed? | No | Yes |
Results
Study design
Both studies69,70 were modelling studies, each based primarily on one clinical study. As a result, one of the studies69 did not explain why the comparator had been chosen. They both stated their research question and the approach to economic evaluation clearly.
In one study,70 results were presented both as cost per severe hypoglycaemic events avoided (all patients) and as costs per quality-adjusted life-year (QALY) gained (patients of ≥ 12 years of age). A clear rationale was provided [i.e. the European Quality of Life-5 Dimensions scale (EQ-5D) was administered to parents and carers on behalf of children aged < 12 years] with regard to why cost per QALY could only be estimated for patients of ≥ 12 years. The outcomes per severe hypoglycaemic events avoided are unlikely to be informative for decision makers who want to establish the cost-effectiveness from a health-care perspective.
Data
As mentioned above, both studies69,70 were based on a single clinical study. The current papers describe the details of the study design only briefly, but refer to the papers that specifically present the clinical results. The study69 based on the IMS CDM did not provide a rationale with regard to why the IMS CDM was chosen. The other study70 explained the choice of model by stating that this was a trial-based economic evaluation and so costs and effects were not extrapolated beyond the 6-month clinical trial period. This means that the long-term impact of the changes in HbA1c levels seen during the clinical study were not taken into consideration, and only the direct impact of avoiding severe hypoglycaemic events are accounted for.
For the study based on the IMS CDM,69 all utilities and costs of complications were taken from literature. Hence, in this paper, no information was available with regard to the subjects from whom valuations of quality of life were obtained, and resources for complications were not reported separately from their unit cost. The cost information relating to the technologies and insulin treatment did provide both resource-use and unit costs.
For the 6-month study,70 all details regarding utilities and resource use were clearly presented. However, once the results were presented, it became clear that an explanation for the calculation of utilities and QALYs was lacking. For example, the paper reported a QALY accumulation of –0.00017 for the standard pump group (CSII + SMBG), which would only be possible if patients had a health state of worse than death. A likely explanation is the definition of QALYs used in the paper, but this was not clarified.
Analysis and interpretation of results
Both studies69,70 were, in general, performed appropriately; however, the study by Kamble et al. 69 did not discuss any issues pertaining to generalisability.
In summary, only one study was found for the integrated CSII + CGM and one for the MiniMed Veo system, both with different comparators and for different countries. The latter study is of limited importance to the current diagnostic appraisal, given its short time horizon of 6 months and its very limited model structure. The study of integrated CSII + CGM by Kamble et al. 69 was better, given that all potentially relevant costs and effects were included. However, IMS Health has now published updated utility values that conform with the NICE standard (i.e. based on EQ-5D)78 and has also updated the IMS CDM several times. Thus, the value of the Kamble et al. paper69 mostly relates to its use for formulating scenarios and presenting a benchmark against which the validity of outcomes from the de novo cost-effectiveness analysis could be checked.
Model structure and methodology
This section describes the health economic model used to evaluate the cost-effectiveness of the MiniMed Paradigm Veo system (an integrated CGM and insulin pump system with LGS function) and the Vibe and G4 PLATINUM CGM system for the management of T1DM in adults in comparison with (1) CSII + CGM, (2) CSII + SMBG, (3) MDI + CGM and (4) MDI + SMBG.
The IMS CDM79 was chosen to perform the cost-effectiveness analyses in this assessment. The IMS CDM has been previously used in NICE- and NHS-related projects on T1DM. It is probably the most commonly used model in the literature and it has been validated extensively. It was used to assess the cost-effectiveness of CSII versus MDIs for T1DM patients in a HTA report from 2010. 80 In that report, the IMS CDM was deemed to be inappropriate for health economic outcomes in paediatric and adolescent populations. This was confirmed by the model developers who also mentioned that the model is not appropriate for pregnant women either. Therefore, these two subgroup populations were not included in the cost-effectiveness analyses. The IMS CDM has also been used in the current update of the NICE Guideline on T1DM (NG17). 81 The model’s time horizon was set to 80 years. Costs were estimated from the perspective of the NHS in England and Wales. Consequences were expressed in life-years gained and QALYs. All costs and effects were discounted by 3.5%. The uncertainty about model input parameters and the potential impact on the model results were explored through scenario analyses and probabilistic sensitivity analyses.
Model structure
The IMS CDM is an internet-based, interactive simulation model that predicts the long-term health outcomes and costs associated with the management of T1DM and T2DM. It is suitable for running cohort (bootstrap) and individual patient-level simulations. It was first developed by the Centre for Outcomes Research and Effectiveness and details of the first version were published by Palmer et al. in 2004. 79 It is widely used in diabetes cost-effectiveness research, both by health technology companies as well as those who pay for such technologies, and it has also been used in previous NICE technology assessments and clinical guidelines. 14,81–85 The model has been extensively validated. Since 1999, it has been examined at Mount Hood conferences, during which health economic models on diabetes are compared with each other in terms of their structure, performance and validity. 86–88 Two major validation papers on the IMS CDM have been published to date. 89,90 The latest one,90 from 2014, is the basis for the technical model description provided in this report. This description is consistent with the latest version of the model (version 8.5). Given the degree of validation of the model, and in order to be in line with the T1DM NICE guideline,81 it was deemed important not to use an alternative model or develop a de novo cost-effectiveness model for this evaluation.
The structure of the IMS CDM (from McEwan et al. 90) is shown in Figure 10. The IMS CDM comprises 17 interdependent submodels, which represent the most common diabetes-related complications: angina pectoris, myocardial infarction (MI), congestive heart failure (CHF), stroke, peripheral vascular disease (PVD), diabetic retinopathy, cataracts, hypoglycaemia, DKA, nephropathy, neuropathy, foot ulcer/amputation, macular oedema, lactic acidosis (T2DM only), (peripheral) oedema (T2DM only) and depression. A submodel for non-specific mortality is also included. Each of these submodels is a Markov model that includes different health states depicting the severity/stage of the complication. Transition probabilities in between the states of a complication submodel can be dependent on time, demographics, health state, physiological factors and diabetes type.
FIGURE 10.
IMS CDM model structure. Reprinted from Value in Health, 17/6, Phil McEwan, Volker Foos, James L. Palmer, Mark Lamotte, Adam Lloyd, David Grant, Validation of the IMS CORE Diabetes Model, Pages 714–724, Copyright (2014), with permission from Elsevier. 90 ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CHF, congestive heart failure; CVD, cardiovascular disease; Inc., including; MI, myocardial infarction; PVD, peripheral vascular disease.
In addition, the non-parametric bootstrapping approach provides additional information on the uncertainty surrounding the long-term outcomes provided by the model. In this approach, a cohort population (with a size that can be defined by the model user) is created. Each patient in this population is unique in the sense of its baseline characteristics (demographics, existing baseline complications, baseline physiological risk factors and other risk factors, e.g. the number of cigarettes smoked per day). Within the bootstrapping simulation approach, two types of analysis are possible: deterministic and probabilistic. In the deterministic simulation, the continuous input parameters (baseline age, diabetes duration, HbA1c levels, etc.) of each patient in the cohort that is created (e.g. 1000 patients) will be identical, but binary variables will differ (gender, presence of a diabetes-related complication, e.g. MI, etc.). In each iteration, one of the patients in this cohort is sampled with replacement and entered into the simulation (i.e. the complication submodels) until the patient dies. Applied treatment effects, utilities, costs and coefficients of cardiovascular disease (CVD) events will then be identical in each iteration. However, results will differ per iteration because of the differences in the binary input parameters in the baseline cohort and the way a patient progresses through the model (random walk). In the probabilistic simulation, all variables that are subjected to random sampling (i.e. cohort baseline parameters, treatment effects, coefficients of the CVD risk equations, health-state utilities/adverse event disutilities and costs) are randomly assigned at the beginning of the first iteration according to pre-defined probability distributions. Then all the patients in the cohort (e.g. 1000) are processed through the model while the parameters assigned at the start of the iteration are held constant. Those patients will only differ as a result of binary variables and random walk. When the model progresses to the next iteration, parameters are resampled again and the next 1000 patients are progressed though the model while parameters are held constant again. This process is repeated for all the bootstrap iterations.
However, it should be noted that because of computational time requirements, not all parameters in the model are subjected to random sampling. For instance, among the baseline risk factors, cigarette and alcohol consumption per day are not subjected to sampling. The same is true for minor and severe hypoglycaemia/ketoacidosis rates and coefficients from non-CVD-related risk adjustment equations.
Transition probabilities within each submodel (i.e. the annual probability of a change in health state) are dependent on baseline demographic and current physiological patient characteristics [HbA1c levels, body mass index (BMI), etc.], and the existence of other complications and concomitant treatments (e.g. angiotensin-converting enzyme inhibitor, statin or laser). Transition probabilities are further calculated based on established regression or risk adjustment functions from the literature. 91–93 State transitions of a cohort occur simultaneously in each submodel. Therefore, it is possible that a patient will develop multiple complications in 1 year. In the IMS CDM model, diabetes-specific mortality is assumed to be caused by the following complications: MI, stroke, CHF, nephropathy, foot ulcer/amputation, hypoglycaemia, DKA and lactic acidosis. However, non-specific mortality is based on UK life tables. 94 Additional details on the submodels of the IMS CDM are given in Appendix 5.
An important limitation of the model is that it is not suitable for modelling long-term outcomes for children or adolescent populations, because the background risk adjustment/risk factor progression equations (such as those based on the Framingham studies)93,95–97 are all based on adult populations. Hence, we had to limit all our analyses to the adult population.
Model input parameters
This section describes the input parameters used in the model for the base case and how their values were estimated. Six different input parameter databases can be distinguished in the IMS CDM: (1) cohort, (2) economics (including management costs, costs of complications and utilities), (3) treatment effects, (4) treatment costs, (5) other management and (6) clinical. Table 27 maps the IMS CDM input parameter databases into the conventional model input categories.
| IMS CDM input database | Conventional input parameter category |
|---|---|
| Cohort database | Demographics (age, diabetes duration, percentage male, racial profile) |
| Baseline physiological risk factors (e.g. HbA1c levels, SBP, T-CHOL, BMI, etc.) | |
| Baseline complications (proportion with MI history, proportion with cataract, etc.) | |
| Other risk factors (proportion that smoke, alcohol consumption, etc.) | |
| Economics database | Cost and effect discount rates |
| Sampling settings for PSA (for costs) | |
| Management costs (e.g. statin, aspirin, ACEI costs, screening costs for depression, foot ulcer, eye disease, etc.) | |
| Utilities/utility decrements for all relevant health states and adverse events | |
Direct costs for:
|
|
| Treatment database | Effect of the treatment on physiological parameters: |
Adverse events:
|
|
| Risk adjustments for concomitant medicines (e.g. ACEIs, statins) | |
| Treatment cost group database | Assigns treatment costs to the treatments for year 1 and afterwards |
| Management database | Percentage of patients on concomitant medication (e.g. statins, ACEIs) Percentage of patients on screening or patient management programmes (e.g. renal disease screening or foot ulcer prevention programme) Other:
|
| Clinical database | Risk adjustments for:
|
Risk multipliers for:
|
Given the degree of validation of the model, only those parameters that needed to be adapted to time (year 2015), place (the UK), population (T1DM patients eligible for a pump) and technologies to be compared were amended in the base case. Furthermore, for the sake of consistency, unless there was thought to be a more appropriate value, we chose to follow the approach from the latest diabetes NICE guideline81 (which also adopted the IMS CDM). In addition, many of the parameters were also validated by clinical experts. Further details on specific input parameters and their probability distributions are described below.
Baseline population characteristics
If possible, we estimated cohort baseline parameters based on the studies identified in our systematic review to properly reflect our base-case population (i.e. T1DM patients eligible for an insulin pump). In this case, only the study by Bergenstal et al. 32 provided reliable information for some patient characteristics. For the characteristics not reported in Bergenstal et al. ,32 we used those from the general T1DM population, as in the latest diabetes NICE guideline. 81 The cohort baseline characteristics used in our base-case analysis and their sources can be seen in Table 28. For the probabilistic sensitivity analysis (PSA) the input parameters age, duration of diabetes and baseline risk factors, for HbA1c levels, systolic blood pressure (SBP), BMI, total cholesterol and low-density lipoproteins, are sampled from a normal distribution; the means and SDs are given in Table 28. Baseline triglyceride and high-density lipoprotein levels are sampled from a gamma distribution with the following parameters: alpha = mean2/SD2 and beta = mean/SD2.
| Parameter | Mean | SD | Source |
|---|---|---|---|
| Patient demographics | |||
| Start age (years) | 41.6 | 12.8 | Bergenstal et al. (2013)32 |
| Duration of diabetes (years) | 27.1 | 12.5 | |
| Proportion male | 0.38 | NA | |
| Baseline risk factors | |||
| HbA1c (% points) | 7.26 | 0.71 | Bergenstal et al. (2013)32 |
| SBP (mmHg) | 128.27 | 16.07 | National Diabetes Audit99 |
| Total cholesterol (mg/dl) | 176.50 | 33.00 | Nathan et al. (2009)100 |
| HDL (mg/dl) | 50.25 | 13.00 | |
| LDL (mg/dl) | 109.75 | 29.00 | |
| Triglycerides (mg/dl) | 81.50 | 41.00 | |
| BMI (kg/m2) | 27.6 | 15.9 | Bergenstal et al. (2013)32 |
| eGFR (ml/min/1.73 m2) | 77.50 | 0 | Default IMS CDM value81 (not used in our analyses) |
| Haemoglobin (g/dl) | 14.50 | 0 | |
| White blood cell count (106/ml) | 6.80 | 0 | |
| Heart rate (b.p.m.) | 72 | 0 | |
| Proportion smoker | 0.22 | NA | National Diabetes Audit99 |
| Cigarettes/day | 12 | NA | Opinions and Lifestyle Survey, Smoking Habits Amongst Adults, 2012 101 |
| Alcohol consumption (oz/week) | 9a | NA | The WHO’s Global Status Report on Alcohol and Health (2011)102 |
| Racial characteristics | |||
| Proportion white | 0.92 | NA | National Diabetes Audit99 |
| Proportion black | 0.03 | NA | |
| Proportion Hispanic | 0.05 | NA | |
| Proportion Native American | 0 | NA | |
| Proportion Asian/Pacific Islander | 0 | NA | |
| Baseline CVD complications | |||
| Proportion MI | 0 | NA | Assumption |
| Proportion angina | 0.00298b | NA | England Health Survey (2011)103 |
| Proportion PVD | 0 | NA | Assumption |
| Proportion stroke | 0.00298c | NA | England Health Survey (2011)103 |
| Proportion heart failure | 0 | NA | Assumption |
| Proportion atrial fibrillation | 0 | NA | |
| Proportion left ventricular hypertrophy | 0 | NA | |
| Baseline renal complications | |||
| Proportion microalbuminuria | 0.181 | NA | National Diabetes Audit99 |
| Proportion gross proteinuria | 0 | NA | Assumption |
| Proportion end-stage renal disease | 0 | NA | |
| Baseline retinopathy complications | |||
| Proportion background diabetic retinopathy | 0 | NA | Assumption |
| Proportion proliferative diabetic retinopathy | 0 | NA | |
| Proportion severe vision loss | 0 | NA | |
| Baseline macular oedema | |||
| Proportion macular oedema | 0 | NA | Assumption |
| Baseline cataract | |||
| Proportion cataract | 0 | NA | Assumption |
| Baseline foot ulcer complications | |||
| Proportion uninfected ulcer | 0 | NA | Assumption |
| Proportion infected ulcer | 0 | NA | |
| Proportion healed ulcer | 0 | NA | |
| Proportion history of amputation | 0 | NA | |
| Baseline neuropathy | |||
| Proportion neuropathy | 0.049 | NA | Nathan et al. (2009)100 |
| Baseline depression | |||
| Proportion depression | 0.21 | NA | Hopkins et al. (2012)104 |
Costs
The direct costs included in the model are for:
-
management (for primary prevention of complications)
-
diabetes-related complications
-
the treatment of diabetes (this also includes the cost of the pump and/or glucose monitor)
-
other hospital costs.
Indirect costs parameters were set to zero in the model as these were not included in our analyses, given the perspective of the NHS. Treatment costs were not included in the PSA because this was not possible using the current version of the IMS CDM, as the model developers argue that the uncertainty around the pharmacy/treatment administration costs is very small.
All other direct costs can be included in the PSA. Although cost parameters are typically sampled from different distributions independently in other economic evaluations, in the IMS CDM all direct costs are multiplied by the same positive factor which is sampled from a log-normal distribution with a mean of 1 and a user-defined coefficient of variation. In line with the latest diabetes NICE guideline,81 for our analyses we assumed a 20% deviation from the mean as it is assumed that this would represent a reasonable range of variation. Detailed descriptions of all four direct cost categories are given in the following sections.
Disease management unit costs
Management costs include the costs of managing chronic conditions, performing screening procedures, administering concomitant medication, etc. All cost data were sourced from NG1781 and, if necessary, were further inflated to 2014 prices using the 2013/14 Hospital and Community Health Services (HCHS) index available from the Personal Social Services Research Unit (PSSRU). 105 The management costs used in our analyses can be seen in Table 29.
| Management type | Mean cost per year (£) | Source |
|---|---|---|
| ACEIs | 18.54a | NHS drug tariff (2014)106 |
| Statins | 38.22b | |
| Aspirin | 13.70c | |
| Screening for microalbuminuria | 3.12d | Lamb et al. (2009)107 |
| Screening for gross proteinuria | 2.94e | |
| Stopping ACEIs because of adverse events | 19.96f | NHS drug tariff (2014)106 |
| Eye screening | 35.38 | Assumptiong |
| Foot screening programme | 42.46h | NHS Reference Costs 2012–13 108 |
| Non-standard ulcer treatment [e.g. becaplermin (Regranex®, Smith & Nephew)] | 0 | Default value in IMS CDM81 |
| Antidepression treatment and management | 494.44 | NICE Guideline NG1781 |
| Screening for depression | 0 | Assumptioni |
Costs of diabetes-related complications
Both ongoing disease complications and acute events are considered in this section. The costs of ongoing complications are considered per year until the complication is resolved or the patient dies. The costs of acute events are assumed to occur at only the time of the event. The costs of diabetes-related complications were sourced from NICE Guideline NG1781 and, if necessary, were inflated to 2014 prices using the 2013/14 HCHS index available from the PSSRU. 105 These costs are shown in Table 30.
| Type of complication | Mean cost (£) | Source |
|---|---|---|
| CVD complications | ||
| MI, first year | 3731 | aNICE lipids clinical guideline (CG181)109,110 |
| MI, each subsequent year | 788 | |
| Angina, first year | 6406 | |
| Angina, each subsequent year | 288 | |
| CHF, first year | 3596 | |
| CHF, each subsequent year | 2597 | |
| Stroke, fatal (within 30 days) | 1174 | |
| Stroke, non-fatal first year | 4170 | |
| Stroke, each subsequent year | 155 | |
| PVD, first year | 952 | |
| PVD, each subsequent year | 529 | |
| Renal complications | ||
| Haemodialysis, each year | 30,819 | NICE peritoneal dialysis clinical guideline (CG125)111 |
| Peritoneal dialysis, each year | 24,793 | |
| Renal transplant, first year | 20,600 | |
| Renal transplant, each subsequent year | 7694 | |
| Acute events | ||
| Severe hypoglycaemic event (cost per event) | 439 | NICE Guideline NG1781 |
| Minor hypoglycaemic event (cost per event) | 0 | |
| DKA event (cost per event) | 0 | |
| Eye disease | ||
| Laser treatment | 705 | NHS Reference Costs 2012–13 (BZ24D: non-surgical ophthalmology with interventions)108 |
| Cataract operation | 1035 | Weighted NHS Reference Costs 2012/13: non-phacoemulsification cataract surgery, with complication score 0 (BZ03A) and score 1+ (BZ03B)108 |
| After cataract operation | 81 | NHS Reference Costs 2012–13 (WF01A: non-admitted face-to-face attendance, ophthalmology follow-up)108 |
| Blindness, year of onset | 5647 | NICE glaucoma clinical guideline (CG85)112,113 |
| Blindness, each subsequent year | 5456 | |
| Neuropathy/foot ulcer/amputation | ||
| Neuropathy, each year | 362 | MIMS, 2014 (online version):114 60 mg of duloxetine (Cymbalta®, Elli Lilly and Co.) daily (first-line treatment in NICE CG96)115 |
| Amputation, event based | 11,416 | NICE lower limb peripheral arterial disease clinical guideline (CG147)116,117 |
| Amputation with prosthesis, event based | 15,420 | |
| Gangrene treatment | 5483 | Ghatnekar et al. (2002)118 |
| Healed ulcer | 266 | |
| Infected ulcer | 7410 | NICE CG147116,117 and Kerr (2012)119 |
| Uninfected ulcer | 4115 | |
| Healed ulcer with history of amputation | 25,577 | NICE lower limb peripheral arterial disease clinical guideline (CG147)116,117 |
Treatment costs
Sensor-augmented pump therapy
In addition to the cost of the MiniMed Paradigm Veo System and the Vibe and G4 PLATINUM CGM system, a number of consumables are needed. These are cannulas, reservoirs and batteries for the insulin pump and sensors for the CGM device. The prices and expected lifetimes of these devices and consumables were reported by the relevant manufacturers. To estimate the equipment costs associated with these devices, the following assumptions were made:
-
insulin pumps have a 4-year lifetime
-
cannulas and reservoirs would be replaced every 3 days
-
the MiniMed Paradigm Veo requires one Energizer® AAA alkaline battery (Energizer® Holdings, Inc., St Louis, MO, USA) and the battery will be replaced every 8.5 days (the lifetime of the battery is dependent on the quality of the battery, the nature of the pump use, temperature, etc.)
-
the Vibe pump operates on one AA battery (lithium batteries are recommended) and the expected battery lifetime is 5 weeks (35 days) (continuous glucose monitor components are supplied with a rechargeable battery and a charger)
-
the MiniLink transmitter is replaced each year and the sensors are replaced every 6 days
-
the G4 PLATINUM monitor is replaced every 6 months and the sensors are replaced every 7 days.
Table 31 presents the estimated yearly equipment costs for the MiniMed Paradigm Veo system and the Vibe and G4 PLATINUM CGM system.
| Cost component | MiniMed Paradigm Veo system | Vibe/G4 Platinum CGM system |
|---|---|---|
| Insulin pump | £2679 | £2800 |
| Insulin pump cannula | £8.70 | £9.75 |
| Insulin pump reservoir | £2.68 | £2.46 |
| Insulin pump batteries | £0.49a | £1.77b |
| Continuous glucose monitor transmitter | £228.70 | £335.0 |
| Continuous glucose monitor sensor | £42.05 | £46.50 |
| Total device cost | £2961.62 | £3195.48 |
| Insulin pump | ||
| Years of use | 4 | 4 |
| Cannula, units/year (days of use) | 121.67 (3) | 121.67 (3) |
| Reservoir, units/year (days of use) | 121.67 (3) | 121.67 (3) |
| Batteries, units/year (days of use) | 42.94 (8.5) | 10.42 (35) |
| Continuous glucose monitor | ||
| Transmitter (years of use) | 1 | 0.5 |
| Sensor, units/year (days of use) | 60.83 (6) | 52.14 (7) |
| Total costs | ||
| Total cost per year | £4862.10 | £5298.65 |
Continuous subcutaneous insulin infusion (stand-alone insulin pumps)
The average price of a stand-alone insulin pump in the UK was sourced from a study from the London New Drugs Group in November 2013. 120 This was inflated to 2014 prices using the 2013/14 HCHS index available from the PSSRU105 and are shown in Table 32. An estimated market share for each brand was calculated based on White et al. 121 and data from Diabetes UK. 122 Based on this information, the estimated weighted average price for a stand-alone pump in the UK is £2173.54.
| Cost component (all costs net of VAT) | Insulin pump | ||||
|---|---|---|---|---|---|
| Accu-Chek® Spirit (Roche, Basel) | Dana (SOOIL, Seoul) | Animas Vibe | Medtronic Paradigm | mylife OmniPod (Ypsomed, Burgdorf) | |
| Insulin pump | £2523a | £1972a | £2831a | £2882a | £425a |
| Estimated annual non-consumables cost (based on 4 years of life) | £631 | £493 | £708 | £720 | |
| Estimated annual consumables cost | £1324 | £1400 | £1663 | £1282 | £3052 |
| Total cost per year | £1955 | £1893 | £2371 | £2002 | £3158 |
| Estimated UK market share (%)b | 30 | 3 | 23 | 35 | 9 |
| Average cost per year (based on market shares) | £2174 | ||||
Continuous glucose monitoring (stand alone)
We followed the approach in NICE Guideline NG1781 and considered the three main CGM technologies available in the UK: Dexcom G4 PLATINUM, Abbott (Chicago, IL) FreeStyle Navigator and Medtronic Guardian®. The items included were receivers, transmitters and sensors. The costs of the three receivers were sourced from NICE Guideline NG17. 81 Transmitter and sensor costs, and usage for the Dexcom G4 and the Medtronic Guardian, were assumed to be the same as for integrated systems (see Table 31), since this information was provided by the companies. For the Abbott FreeStyle Navigator, sensor costs (there is no transmitter) and usage were assumed to be the same as reported in NICE Guideline NG17. 81 Finally, a yearly weighted average cost, equal to £3087.75, was estimated based on the estimated market share from White et al. 121 and data from Diabetes UK. 122 This information is shown in Table 33.
| Continuous glucose monitor component | CGM device | ||
|---|---|---|---|
| Dexcom G4 | FreeStyle Navigator | Medtronic Guardian | |
| Receiver cost | £1750 | £950 | £1059 |
| Transmitter cost | £335 | £0 | £229 |
| Sensor cost | £47 | £48 | £42 |
| Total equipment cost | £2132 | £998 | £1330 |
| Receiver, years of use | 5 | 5 | 5 |
| Transmitter, years of use | 0.5 | 0 | 1 |
| Sensor, units/year (days of use) | 52.14 (7) | 60.83 (6) | 60.83 (6) |
| Total cost/year | £3445 | £3110 | £2999 |
| Estimated UK market share | 15% | 20% | 65% |
| Average cost per year (based on market shares) | £3088 | ||
Blood glucose tests costs
Blood glucose tests are needed in all interventions and comparators. Each time a BG test is conducted a lancet and a test strip are consumed. The estimated cost of a single BG test (computed as the average of all marketed lancets and test strips) is £0.29 according to NICE Guideline NG17. 81 We assumed that BG meters are supplied free of charge. The number of BG tests required for the different interventions and comparators depend on the method of monitoring glucose, whether it is manual (SMBG) or continuous (CGM). Our systematic review identified only two studies reporting the number of BG tests. 37,40 Based on these studies, we defined, on average, four BG tests per day for both SMBG and CGM for the base case. Based on clinical opinion, this choice seems to be somewhat counterintuitive as a higher number of tests would be expected for SMBG than for CGM. However, we believe that trial values are generally more valid and consistent within our analyses, given that the estimate of effectiveness comes from the trials and there is likely to be a correlation between frequency of monitoring and outcome. Nevertheless, since there was some uncertainty around these values, other options were explored in scenario analyses. Yearly costs associated with SMBG for the base case are shown in Table 34.
| Cost component | CGM and SMBG |
|---|---|
| Cost of single BG test | £0.29 |
| Number of tests per day | 4 |
| Total number of tests per year | 1460 |
| Total yearly cost | £423.40 |
Insulin costs
Both SAP and CSII therapies use short-acting insulin. Based on expert opinion, we assumed the same type and amount of short-acting insulin for both technologies. Following the approach in NICE Guideline NG17,81 only the cartridges and pre-filled pens were used to calculate the costs of short-acting insulin. For the base case, we assumed 48 units per day of short-acting insulin for pumps, as in Bergenstal et al. 32 and NICE Guideline NG17. 81 This choice was validated by clinical experts/committee members. The total insulin costs per year for patients on insulin pumps are shown in Table 35.
| Short-acting insulin | Cartridges and pens | Unit cost (£) | Cost per unit of insulin (£)a | Yearly cost per patient (£)b |
|---|---|---|---|---|
| Insulin aspart (NovoRapid®, Novo Nordisk) | 5 × 3-ml cartridges | 28.31 | 0.0188 | 330.66 |
| 5 × 3-ml FlexPen pre-filled (Novo Nordisk, Bagsværd, Denmark) | 30.60 | 0.0204 | 357.41 | |
| 5 × 3-ml FlexTouch pre-filled (Novo Nordisk, Bagsværd, Denmark) | 32.13 | 0.0214 | 375.28 | |
| Insulin glulisine (Apidra®, Sanofi-Aventis) | 5 × 3-ml cartridges | 28.30 | 0.0188 | 330.54 |
| 5 × 3-ml SoloStar pre-filled (Sanofi-Aventis, Paris, France) | 28.30 | 0.0188 | 330.54 | |
| Insulin lispro (Humalog®, Lilly) | 5 × 3-ml cartridges | 28.31 | 0.0188 | 330.66 |
| 5 × 3-ml KwikPen pre-filled (Eli Lilly, Indianapolis, IN, USA) | 29.46 | 0.0196 | 344.09 | |
| Average insulin costs | NA | 29.34 | 0.0196 | 342.74 |
Based on clinical opinion, we assumed that patients on MDIs would use a regimen with basal (long-acting) insulin once or twice daily, and bolus (short-acting) insulin with meals, three times per day. Furthermore, the conclusion from NICE Guideline NG1781 is that insulin detemir twice daily is the most cost-effective long-acting insulin regimen for people with T1DM. Therefore, we assumed this for the base case. Based on the information from our clinical experts, we also assumed that the number of insulin units would be split 50 : 50 between basal and bolus. For the base case, we also assumed 48 units per day for MDIs, as in NICE Guideline NG17. 81 Thus, we assumed 24 units per day of long-acting insulin and 24 units per day of short-acting insulin. The unit cost of the needles was assumed to be £0.11 as in NICE Guideline NG17. 81 This was calculated as a weighted average of the prices of the 10 most commonly used needles, according to data from Prescription Cost Analysis – England, 2012. 123 The annual cost of needles per patient was then calculated based on a frequency of five injections per day (long-acting twice daily and short-acting insulin three times per day) as mentioned above. The total insulin costs (including the costs of needles) per year for patients on MDIs are shown in Table 36.
| Cost item | Unit cost (£) | Cost per unit of insulin (£)a | Yearly cost per patient (£) |
|---|---|---|---|
| Long-acting insulin detemir | 42.00 | 0.0280 | 245.28b |
| Short-acting insulin | 29.34 | 0.0196 | 171.35b |
| Needles | 0.11 | NA | 200.75c |
| Total cost for MDIs | 617.38 |
There was some uncertainty around the assumption of equal amounts of insulin for pumps and MDIs. Clinical experts have different opinions about this; some experts expect that a lower amount of insulin would be used for pumps than would be used for MDIs (14% lower according to Cummins et al. 80). Therefore, we explored this in a separate scenario.
Other hospital costs
Outpatient care-related costs
Outpatient care-related costs (consultant and diabetic specialised nurse) were estimated based on clinical expert opinion. We assumed that in the first year during pump initiation, there would be seven appointments and three group sessions of 45 minutes each with diabetic specialist nurses in a 6-month period. After the pump initiation period, but still during the first year, we assumed two appointments of 45 minutes with a consultant and two appointments of 45 minutes with a diabetic specialised nurse. Therefore, in total, in the first year, we assumed that there would be nine appointments and three group sessions of 45 minutes with a diabetic specialised nurse, and two appointments of 45 minutes with a consultant. Each subsequent year we assumed that there would be two appointments of 45 minutes with a consultant and two appointments of 45 minutes with a diabetic specialised nurse. For patients on MDIs, we assumed two appointments of 45 minutes with a consultant and two appointments of 45 minutes with a diabetic specialised nurse every year. The NHS outpatient follow-up tariff is £99. 124 Total outpatient costs for the base case are shown in Table 37.
| Year | Insulin pump (£) | MDIs (£) |
|---|---|---|
| Year 1 | 1386.00 | 396.00 |
| Year 2 or more | 396.00 | 396.00 |
| Average yearly cost (based on a time horizon of 80 years) | 408.38 | 396.00 |
Glycated haemoglobin tests costs
The cost and frequency of HbA1c tests were also estimated based on clinical expert opinion. We assumed that, on average, this test would be performed three times a year. The cost of the test is dependent on the hospital, the lab, etc., in which the test is performed. Based on the average of three hospital prices, we assumed £3.14 as the average cost of a HbA1c test.
Summary of treatment and other hospital costs
A summary of treatment-related costs for the six technologies considered in this study is shown in Table 38.
| Technology | Equipment and consumables (£) | Blood glucose tests (£) | Insulin (£) | Outpatient (£) | HbA1c tests (£) | Total (£) |
|---|---|---|---|---|---|---|
| MiniMed Veo system | 4862.10 | 423.40 | 342.74 | 408.38 | 9.42 | 6046.04 |
| Integrated CSII + CGM (Vibe) | 5298.65 | 423.40 | 342.74 | 408.38 | 9.42 | 6482.59 |
| CSII + CGM | 5261.29 | 423.40 | 342.74 | 408.38 | 9.42 | 6445.22 |
| CSII + SMBG | 2166.13 | 423.40 | 342.74 | 408.38 | 9.42 | 3350.07 |
| MDI + CGM | 3288.50 | 423.40 | 416.63 | 396.00 | 9.42 | 4533.94 |
| MDI + SMBG | 200.75 | 423.40 | 416.63 | 396.00 | 9.42 | 1446.20 |
Utilities
Health benefits were expressed in terms of life-years and QALYs gained. If more than one complication occurs at a time, a multiplicative approach is applied. 125 For the PSA, utility and disutility values are sampled from a beta distribution. Means and SDs are inputs for the IMS CDM; these are parameterized into parameters a and b of the beta distribution as follows: a = ((mean2) × (1 – mean)/(SD2)); and b = (mean × (1 – mean)/(SD2)) – ((mean2) × (1 – mean)/(SD2)). The utilities used in the model are summarised in Table 39.
| Health state | Mean (dis)utility value | SE | Source |
|---|---|---|---|
| T1DM with no complications | 0.814 | 0.01 | Clarke et al. (2002)126 |
| MI, event year | –0.055 | 0.01 | Beaudet et al. (2014)78 |
| MI, after event | 0.759 | 0.01 | Equal to no complication minus event |
| Angina | 0.695 | 0.01 | Beaudet et al. (2014)78 |
| Chronic heart failure | 0.677 | 0.01 | |
| Stroke, event year | –0.164 | 0.01 | |
| Stroke, after event | 0.650 | 0.01 | Equal to no complication minus event |
| PVD | 0.724 | 0.01 | Beaudet et al. (2014)78 |
| Microalbuminuria | 0.814 | 0.01 | Equal to no complication |
| Gross proteinuria | 0.737 | 0.01 | Beaudet et al. (2014)78 |
| Haemodialysis | 0.621 | 0.03 | |
| Peritoneal dialysis | 0.581 | 0.03 | |
| Renal transplant | 0.762 | 0.12 | |
| Background diabetic retinopathy | 0.745 | 0.02 | |
| Background diabetic retinopathy, wrongly treated | 0.745 | 0.02 | |
| Proliferative diabetic retinopathy, laser treated | 0.715 | 0.02 | |
| Proliferative diabetic retinopathy, non-laser treated | 0.715 | 0.02 | |
| Macular oedema | 0.745 | 0.02 | |
| Severe vision loss | 0.711 | 0.01 | |
| Cataract | 0.769 | 0.02 | |
| Neuropathy | 0.701 | 0.01 | |
| Healed ulcer | 0.814 | 0.01 | Equal to no complication |
| Active ulcer | 0.615 | 0.01 | Beaudet et al. (2014)78 |
| Amputation, event year | –0.280 | 0.01 | |
| Amputation, after event | 0.534 | 0.01 | Equal to no complication minus event |
| Severe hypoglycaemic event | –0.012 | 0.00 | Currie et al. (2006)127 |
| Minor hypoglycaemic event | 0 | 0.00 | Assumption |
| Fear of hypoglycaemic event | 0 | 0.00 | Included in the disutility for severe hypoglycaemic event |
| DKA event | 0 | 0.00 | Assumption |
| Depression, not treated | 0.6059 | 0.00 | Goldney et al. (2004)128 |
| Depression, treated | 0.814 | 0.00 | Equal to no complication |
Treatment effects
We used the reduction in HbA1c baseline levels and the number of severe hypoglycaemic events as the outcomes to characterise treatment effectiveness. We considered using the number of minor hypoglycaemic and DKA events as well but not enough reliable data were found to make comparisons.
For HbA1c levels, a baseline value had to be established onto which the treatment effect could be applied [i.e. the value at the start of treatment (time zero)]. The mean baseline value was 7.26% (standard error 0.71%), based on the relevant population, as shown in Table 28. Treatment effects were then estimated as the mean reduction from the baseline value, determined from our systematic review. An indirect meta-analysis was conducted to estimate the WMD between the MiniMed Paradigm Veo system and integrated CSII + CGM (used to inform the Vibe and G4 PLATINUM CGM system), CSII + CGM, CSII + SMBG, MDI + CGM and MDI + SMBG. Because of a lack of published clinical data, MDI + CGM had to be excluded from the analysis (see Figure 8) and treatment effects of integrated CSII + CGM and non-integrated CSII + CGM were assumed to be identical (see Figure 8 and Table 21). After calculating the change in HbA1c levels from baseline in Bergenstal et al. 32 as –0.02, the change in HbA1c levels for other treatments could be found. These values are listed in Table 40.
| Treatment | Mean (SE) change in HbA1c levels compared with baseline, % |
|---|---|
| MiniMed Veo system | –0.02 (0.04) |
| Integrated CSII + CGM (Vibe) | –0.06 (0.05) |
| CSII + SMBG | 0.05 (0.12) |
| MDI + SMBG | 0.64 (0.19) |
| CSII + CGM | –0.06 (0.05) |
Since there is uncertainty and there are limitations in the indirect meta-analysis (because of heterogeneity and differences in baseline HbA1c levels), to explore the impact of different HbA1c change levels, we analysed a hypothetical situation in which the baseline HbA1c levels do not change after the initiation of treatment in a separate scenario. It should be noted that, in the IMS CDM, the change in HbA1c level is assumed to occur within the first 12 months. After this, an annual progression rate is applied. For the base case we followed the approach in NICE Guideline NG17,81 in which an annual progression of 0.045% (derived from the DCCT)92 was used.
For severe hypoglycaemic events, it is not necessary to set a baseline value since the IMS CDM assumes that this is a treatment-specific parameter. Treatment effects were estimated as the rate ratio of event rates per 100 patient-years obtained from our systematic review (see Figure 9 and Table 22). This was then applied to a reference value for integrated CSII + CGM, which was derived from a weighted average (by sample size) of the event rates observed in the CSII + CGM arms of the trials. These values are shown in Table 41.
| Treatment | Rate per 100 patient-years of severe hypoglycaemic episodes |
|---|---|
| MiniMed Veo system | 1.9584 |
| Integrated CSII + CGM (Vibe) | 16.32 |
| CSII + SMBG | 5.0215 |
| MDI + SMBG | 19.584 |
| CSII + CGM | 16.32 |
For the PSA, treatment effects on HbA1c levels at baseline are sampled from a beta distribution (mean and SD are converted into beta distribution-specific parameters, as explained in Utilities). The event rates of severe hypoglycaemic events are fixed in the IMS CDM and therefore they are not included in the PSA. In order to explore the uncertainty of the effects of severe hypoglycaemic episodes on long-term outcomes, several scenarios with different treatment-specific rates were analysed (see Treatment effects part II: severe hypoglycaemic event rates).
Disease management parameters
These parameters will determine the proportion of patients that will receive disease management regimens, such as preventative treatments or screening programmes. These parameters and their sources are shown in Table 42. With the exception of the proportion on the UK-specific foot ulcer prevention programme, for which we followed the approach in NICE Guideline NG17,81 the majority of the inputs are the default values from the IMS CDM and were also used in the latest diabetes NICE guideline. 81
| Parameter | Mean value | Source |
|---|---|---|
| Concomitant medication | ||
| Proportion using aspirin for primary prevention | 0.456 | Minshall et al. (2008)129 |
| Proportion using aspirin for secondary prevention | 0.755 | Gerstein et al. (2008)130 |
| Proportion using statins for primary prevention | 0.450 | Minshall et al. (2008)129 |
| Proportion using statins for secondary prevention | 0.878 | Gerstein et al. (2008)130 |
| Proportion using ACEIs for primary prevention | 0.500 | Minshall et al. (2008)129 |
| Proportion using ACEIs for secondary prevention | 0.708 | Gerstein et al. (2008)130 |
| Screening and patient management proportions | ||
| Proportion on foot ulcer prevention programme | 0.992 | National Diabetes Audit99 |
| Proportion screened for eye disease | 1.000 | No data |
| Proportion screened for renal disease | 1.000 | No data |
| Proportion receiving intensive insulin after MI | 0.877 | McMullin et al. (2004)131 |
| Proportion treated with extra ulcer treatment | 0.570 | Lyon (2008)132 |
| Proportion screened for depression with no complications | 0.830 | Jones and Doebbeling (2007)133 |
| Proportion screened for depression with complications | 0.830 | |
| Others | ||
| Reduction in incidence of foot ulcers with prevention programme | 0.310 | O’Meara et al. (2000)134 |
| Improvement in ulcer healing rate with extra ulcer treatment | 1.390 | Kantor and Margolis (2001)135 |
| Reduction in amputation rate with foot care | 0.340 | O’Meara et al. (2000)134 |
| Sensitivity of eye screening | 0.920 | Lopez-Bastida et al. (2007)136 |
| Specificity of eye screening | 0.960 | |
| Sensitivity of gross proteinuria screening | 0.830 | Cortes-Sanabria et al. (2006)137 |
| Sensitivity of microalbuminuria screening | 0.830 | |
| Specificity of microalbuminuria screening | 0.960 | |
Disease natural history parameters
These are the parameters that will determine the natural course of the disease. These parameters are either transition probabilities, that is the probability of each of the events (e.g. diabetic retinopathy or MI) or the (relative) risk of an event, given a particular risk factor; risk factors are based on physiological measures, such as HbA1c levels, BMI, SBP or characteristics like the presence of microalbuminuria. We considered the same values as in NICE Guideline NG17,81 most of which were the same as the IMS CDM default values. For that reason, and because the number of parameters is so large that it may distract the reader’s attention, we have decided to show these parameters in Appendix 6.
It should be noted that one of these parameters is the probability of death from a severe hypoglycaemic event. In line with NICE Guideline NG17,81 this was assumed to be zero for the base case. However, as deaths due to severe hypoglycaemic events have been reported,138,139 we expect that this parameter may have an impact on our results, as one of the key features of the MiniMed Paradigm Veo is the LGS function, which was shown to reduce the number of severe hypoglycaemic events, and thus the number of deaths caused by severe hypoglycaemia. Therefore, other options for this mortality rate were explored in additional scenarios.
Sensitivity and scenario analyses
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was used to explore the impact of statistical uncertainties regarding the model’s input parameters. PSA is an in-built feature of the IMS CDM, activated if the second order with sampling option is selected.
Probabilistic sensitivity analysis results were presented in the cost-effectiveness plane for all the treatments compared. Cost-effectiveness acceptability curves (CEACs) were used to describe the probability of a treatment being considered cost-effective given a threshold incremental cost-effectiveness ratio (ICER). The probability distributions used in the PSA are described throughout the Model input parameters section.
Scenario analyses
Scenario analyses were performed to explore the impact on costs and QALYs of using different assumptions on the baseline population characteristics, on the number of blood tests (finger prick tests) conducted per day, on the amount of insulin used, on the inclusion of HbA1c progression after year 1, on treatment effects (both in terms of HbA1c level change and in terms of the number of severe hypoglycaemic episodes per treatment), on the inclusion of a non-zero probability of death as a result of hypoglycaemia, on time horizon, on QALY estimation methods, on utility benefits associated with less fear of hypoglycaemia, and on the cost of the stand-alone insulin pump and CGM devices.
Baseline population characteristics
The base case assumed baseline population characteristics, as in the Bergenstal et al. 32 In this scenario, we considered the general T1DM population, as used in NICE Guideline NG17. 81 Table 43 shows the patient characteristics that were changed for this scenario.
| Parameter | Mean base case | Mean scenario | SD | Source |
|---|---|---|---|---|
| Patient demographics | ||||
| Start age (years) | 41.6 | 42.98 | 19.14 | Nathan et al. (2009)100 |
| Duration of diabetes (years) | 27.1 | 16.92 | 13.31 | National Diabetes Audit99 |
| Proportion male | 0.38 | 0.567 | NA | |
| Baseline risk factors | ||||
| HbA1c (% points) | 7.26 | 8.60 | 4.00 | National Diabetes Audit99 |
| BMI (kg/m2) | 28.27 | 27.09 | 5.77 | |
Number of blood glucose tests per day
In the base case, we assumed four BG tests (finger prick tests) for interventions containing CGM (the MiniMed Paradigm Veo system, integrated CSII + CGM and stand-alone CSII + CGM) and four BG tests for interventions containing SMBG (CSII + SMBG and MDI + SMBG). This assumption was based on the results from the systematic review, in which no significant differences in the number of tests between the CGM- and SMBG-containing treatments were observed. 32,37
In the sensitivity analysis, we followed the approach in NICE Guideline NG17 (appendix P of this guideline),81 and considered two tests per day (for calibration) for CGM-containing treatments and four tests per day for SMBG-containing treatments, since this is considered to be current practice. Moreover, we have included 8 (the most cost-effective frequency in the guideline) and 10 tests per day for SMBG-containing technologies versus 2 tests per day for CGM-containing technologies as scenarios in our analysis. Unlike the latest diabetes NICE guideline scenarios (appendix P of the guideline),81 we assumed in our analyses that the number of blood tests per day had no impact on the treatment effect, since such an effect (e.g. that more blood tests lead to a greater decrease in HbA1c levels) was not observed in our systematic review. Finally, we also explored a scenario based on the observational study by Lynch et al. ,140 which reports an average number of 4.35 BG tests per day for CGM and 7.11 for SMBG. The costs related to BG testing for the complete list of the scenarios conducted are given in Table 44.
| Cost component | CGM | SMBG |
|---|---|---|
| Cost of single BG test81 | 0.29 | 0.29 |
| Scenario 1 | ||
| Number of tests per day81 | 2 | 4 |
| Total number of tests per year (365 days) | 730 | 1460 |
| Total yearly cost (£) | 212 | 423 |
| Scenario 2 | ||
| Number of tests per day81 | 2 | 8 |
| Total number of tests per year (365 days) | 730 | 2920 |
| Total yearly cost (£) | 212 | 847 |
| Scenario 3 | ||
| Number of tests per day81 | 2 | 10 |
| Total number of tests per year (365 days) | 730 | 3650 |
| Total yearly cost (£) | 212 | 1058 |
| Lynch et al. (2012)140 scenario | ||
| Number of tests per day | 4.35 | 7.11 |
| Total number of tests per year (365 days) | 1588 | 2595 |
| Total yearly cost (£) | 460 | 753 |
Amount of insulin per day
For the base case, we assumed equal units of insulin per day for both MDI-containing interventions (MDI + SMBG) and insulin pump-containing interventions (the MiniMed Paradigm Veo system, integrated CSII + CGM, stand-alone CSII + CGM and CSII + SMBG). However, some of the clinical experts mentioned that they would expect a lower amount of insulin to be used for pumps than for MDIs. In addition, Cummins et al. 80 report a 14% reduction in insulin use with pumps compared with MDIs. From the findings of our systematic review, this seems to be a reasonable assumption. 37,41,141 Thus, for this scenario, we assumed 48 units per day of short-acting insulin for pump-containing treatments (which is the same as the insulin use assumption in the base case given in Table 35) and 55 units of insulin per day (14% more) for MDI + SMBG treatments. It was also assumed that the insulin used for MDI + SMBG is split 50 : 50 between basal and bolus (27.5 units per day of long-acting insulin and 27.5 units per day of short-acting insulin). The costs pertaining to the insulin use for this scenario analysis are given Table 45.
| Cost item | Unit cost (£) | Cost per unit of insulin (£)a | Yearly cost per patient (£) |
|---|---|---|---|
| Long-acting insulin detemir | 42.00 | 0.0280 | 281b |
| Short-acting insulin | 29.34 | 0.0196 | 196b |
| Needles | 0.11 | NA | 201c |
| Total cost for MDIs | 678 |
Glycated haemoglobin progression
In the base-case analysis, the IMS CDM default value for the annual progression in HbA1c levels after year 1 was used (0.045%). This value was based on the DCCT. 92 According to NICE Guideline NG17,81 the Guideline Development Group expects that HbA1c levels in T1DM patients will be more stable than in T2DM patients. Therefore, an alternative assumption of no annual progression in HbA1c levels (0%) was tested to gain insight into the effects of HbA1c progression rate on costs and QALYs gained after year 1.
Treatment effects part I: change in glycated haemoglobin levels in the first year
As explained above (see Treatment effects), treatment effects were estimated as the mean reduction from the baseline HbA1c value obtained from our systematic review. The baseline HbA1c value was taken from Bergenstal et al. 32 This value is lower than the average baseline HbA1c value of patients given in the National Diabetes Audit,99 which indicates that the patients in the Bergenstal et al. 32 study have better glycaemic control. As an alternative scenario, we assumed that the baseline HbA1c value is stable for one year and does not change with any of the treatments (0% change in HbA1c level in the first year).
Treatment effects part II: severe hypoglycaemic event rates
Treatment-specific severe hypoglycaemic event rates were derived from our systematic review, from which it was observed that the MiniMed Paradigm Veo system had fewer reported severe hypoglycaemic events than the other treatments. In the scenario analysis, we elaborate on this observation, and for all treatments other than the MiniMed Paradigm Veo system, we assumed a uniform event rate for severe hypoglycaemia (16.32 events per 100 patient-years) and applied different RR values (1, 0.5, 0.25 and 0.125) for the severe hypoglycaemic event rate for the MiniMed Paradigm Veo system. It should be noted that the value of 16.32 events per 100 patient-years is derived from the indirect comparison, as explained above (see Treatment effects), and is the weighted mean for the severe hypoglycaemic event rate for integrated CSII + CGM, which is chosen as a reference treatment in this case because the number of studies (n = 8)32,34–40 that the weighted average rate was based on is highest for integrated CSII + CGM; the Bergenstal et al. trial,32 from which the baseline population characteristics were derived, is one of these eight studies.
In addition, we conducted a scenario analysis in which the higher severe hypoglycaemic episode rate from Hirsch et al. 34 was taken as the baseline rate for integrated CSII + CGM, and the RRs from the indirect comparison in Treatment effects (base case) were applied for other treatments. Severe hypoglycaemic episode rates (number of events per 100 patient-years) are given in Table 46 for each scenario.
| Intervention | Number of events per 100 patient-years | ||||
|---|---|---|---|---|---|
| Scenario 1 (RR = 1) | Scenario 2 (RR = 0.5) | Scenario 3 (RR = 0.25) | Scenario 4 (RR = 0.125) | Scenario 5a | |
| MDI + SMBG | 16.32 | 16.32 | 16.32 | 16.32 | 38.37 |
| CSII + SMBG | 16.32 | 16.32 | 16.32 | 16.32 | 10.20 |
| CSII + CGM | 16.32 | 16.32 | 16.32 | 16.32 | 33 |
| MiniMed Veo system | 16.32 | 8.16 | 4.08 | 2.04 | 3.96 |
| Integrated CSII + CGM (Vibe) | 16.32 | 16.32 | 16.32 | 16.32 | 33 |
Non-zero probability of death resulting from severe hypoglycaemia
In the base case, the case fatality rate for severe hypoglycaemia was taken as zero. This assumption is in line with NICE Guideline NG1781 and systematic review results, since none of the included studies reported a death due to severe hypoglycaemia.
As an extreme scenario, as in NG17,81 we assumed a case fatality rate of 4.9%, derived from a study by Ben-Ami et al. 142 in which five patients were reported to die among 102 patients who had drug-induced hypoglycaemic coma.
Quality-adjusted life-year estimation method
In the base case, a multiplicative approach was applied for the QALY estimation. This approach, in which the utility values of multiple events are multiplied to derive an overall utility in cases of multiple events/complications, is considered to be appropriate for this condition because simultaneous complications often do develop. 125 As a scenario analysis, the minimum approach was used as an alternative QALY estimation method; for this approach, the minimum of the multiple health state utility values was applied for patients with a history of multiple events.
Different time horizons
In the base case, a lifetime analysis is achieved by selecting 80 years as the model time horizon. For scenario analyses, a 4-year time horizon (the average lifetime of an insulin pump) was selected and the effect of this time horizon on the results was explored.
Fear of hypoglycaemia unawareness
In the STAR-3 trial,40 patients using integrated CSII + CGM devices demonstrated more of an improvement compared with baseline values on the ‘worry’ subscale of the Hypoglycaemia Fear Survey143 than the MDI group. Subsequently, in Kamble et al. ,69 this improvement was translated into a utility increment of 0.0329 using the EQ-5D questionnaire index. As a scenario analysis, we applied this utility increment associated with less fear of hypoglycaemia throughout the remaining lifetimes of patients using integrated devices (the MiniMed Paradigm Veo system and integrated CSII + CGM). This benefit was not applied to non-integrated devices (CSII + CGM, CSII + SMBG and MDI + SMBG), as these devices do not give a warning or activate/stop the release of insulin automatically in response to low BG levels.
Cost of stand-alone insulin pumps and continuous glucose monitoring devices
In the base-case analysis, the yearly device cost (equipment + consumables) of the stand-alone CSII + CGM (£5261.29) was estimated based on the market share obtained from White et al. 121,122 As a scenario analysis, we considered the average costs without the market-share weighting. Therefore, in this scenario, the estimated yearly device cost is £2275.80 for the stand-alone insulin pump and £3184.39 for the stand-alone CGM device. Thus, when the other cost items are considered (insulin, BG tests, outpatient costs and HbA1c tests), the average yearly cost (without using any market share assumptions) of the stand-alone CSII + CGM is £6644.13. Hence, the cost of the stand-alone CSII + CGM combination is £198.90 higher than the base-case cost. In a similar manner, the yearly cost of CSII + SMBG is £102.26 higher than the base-case cost. Because of these higher costs, stand-alone CSII + CGM becomes more expensive than integrated CSII + CGM (Vibe) in this scenario. Since both technologies are assumed to have the same efficacy, integrated CSII + CGM (Vibe) will dominate stand-alone CSII + CGM.
Model assumptions
The main assumptions made in our analyses are summarised in Box 1.
-
For the base-case scenario, baseline population characteristics, as in Bergenstal et al. ,32 were assumed. In an additional scenario, we considered general T1DM population characteristics as in NICE Guideline NG17. 81
-
For the costs included in the PSA, 20% deviation from the mean was assumed. This is in line with NICE Guideline NG17. 81
-
Costs of initiation training for insulin pumps and CGM were covered by outpatient costs. This was based on clinical expert opinion.
-
A 4-year lifetime was assumed for insulin pumps.
-
Cannulas and reservoirs would be replaced every 3 days.
-
The MiniMed Paradigm Veo system requires one Energizer AAA alkaline battery. An estimated replacement time of 8.5 days was assumed.
-
The Vibe system pump operates on one AA lithium battery. The expected battery lifetime is 5 weeks (35 days) when used with CGM and 8 weeks when used without CGM.
-
We assumed the same percentage of increase in battery lifetime for the MiniMed Paradigm Veo system when used without CGM.
-
The MiniLink transmitter is replaced each year and the sensors are replaced every 6 days.
-
The G4 PLATINUM monitor is replaced every 6 months and the sensors are replaced every 7 days.
-
The assumptions made for integrated insulin pumps are also valid for stand-alone insulin pumps.
-
Transmitter and sensor costs, and usage for the Dexcom G4 and the Medtronic Guardian were assumed to be the same as for integrated systems. This was mentioned by the relevant companies.
-
For the Abbott FreeStyle Navigator, sensor costs and usage were assumed to be the same as reported in NICE Guideline NG17. 81
-
For the base case, we assumed, on average, four BG tests per day for both SMBG and CGM.
-
In the sensitivity analysis, we followed the approach in NICE Guideline NG1781 and considered two tests per day (for calibration) for CGM and four tests per day for SMBG, since this is considered current practice. Moreover, we have included eight tests per day (the most cost-effective frequency according to the guideline) and 10 tests per day for SMBG-containing technologies vs. two tests per day for CGM-containing technologies, as scenarios in our analysis.
-
We also explored a scenario based on the observational study by Lynch et al. 140 which reports an average number of 4.35 BG tests per day for CGM and 7.11 for SMBG.
-
We assumed that BG meters are supplied free of charge.
-
We assumed the same type and amount of short-acting insulin for both integrated and stand-alone insulin pumps. This was based on expert opinion.
-
For the base case, we assumed 48 units per day of short-acting for insulin pumps. This was based on Bergenstal et al. 32 and NICE Guideline NG17,81 and it was validated by clinical experts.
-
Based on clinical opinion, we assumed that patients on MDIs would use a regimen with basal (long-acting) insulin once or twice daily, and bolus (short-acting) insulin with meals, three times per day.
-
NICE Guideline NG1781 concluded that it is likely that insulin detemir twice daily is the most cost-effective long-acting insulin regimen for people with T1DM. Therefore, we assumed this for the base case.
-
Based on clinical opinion, we also assumed that the amount of daily insulin would split 50 : 50 between basal and bolus.
-
For the base case, we assumed 48 units per day for MDIs, as in NICE Guideline NG17. 81
-
In an additional scenario, we assumed 48 units per day of short-acting insulin for pumps, as in the base case, and 55 units per day (14% increase as reported in Cummins et al. 80) for MDIs.
-
The unit cost of the needles was assumed to be £0.11 as in NICE Guideline NG17. 81
-
The annual cost of needles per patient was then calculated based on a frequency of 5 injections per day (long-acting twice daily and short-acting insulin three times per day).
-
We assumed that, in the first year of pump initiation, there would be seven appointments and three group sessions of 45 minutes each with diabetic specialist nurses in a 6-month period.
-
After the pump initiation period, but still during the first year, we assumed two appointments of 45 minutes with a consultant and two appointments of 45 minutes with a diabetic specialised nurse.
-
Each subsequent year we assumed two appointments of 45 minutes with a consultant and two appointments of 45 minutes with a diabetic specialised nurse.
-
For patients on MDIs, we assumed two appointments of 45 minutes with a consultant and two appointments of 45 minutes with a diabetic specialised nurse every year.
-
We assumed that, on average, this test would be performed three times a year.
-
The cost of this test depends on the hospital, lab, etc., in which they are performed. Based on the average of three hospital prices, we assumed that the average price for a HbA1c test would be £3.14.
-
Treatment effects are estimated as the mean reduction from the baseline value from our systematic review. This reduction is assumed to occur for up to 12 months. After this, annual progression occurs. In the base case, we followed NICE Guideline NG17,81 which chose a T1DM trial, DCCT (annual progression of 0.045%), for the base case and no progression in sensitivity analysis.
-
In the absence of data, treatment effects of integrated CSII + CGM and non-integrated CSII + CGM were assumed to be identical.
-
The probability of death from severe hypoglycaemic events was assumed to be zero for the base case. 81 Other values were explored in separate scenarios.
Results of cost-effectiveness analyses
Base-case results
The base-case results from the full incremental analysis reported as cost per QALY gained (ICER) per technology for adult T1DM patients are summarised in Table 47.
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.4146 | 61,050 | – | – | – |
| CSII + SMBG | 11.9756 | 90,436 | 0.561 | 29,386 | 52,381 |
| MiniMed Veo system | 12.0412 | 138,357 | Extendedly dominateda by stand-alone CSII + CGM | ||
| CSII + CGM | 12.0604 | 146,476 | 0.0849 | 56,039 | 660,376 |
| Integrated CSII + CGM (Vibe) | 12.0604 | 147,150 | Dominated by stand-alone CSII + CGM | ||
First, it should be noted that since the same treatment effects were assumed for stand-alone and integrated CSII + CGM, the latter is dominated by the former (i.e. effectiveness is the same for the integrated as for the stand-alone technology, but the integrated technology is more expensive, as shown in Table 38). As expected, MDI + SMBG is the cheapest treatment but also the one that provides the lowest number of QALYs. The ICER of CSII + SMBG compared with MDI + SMBG is £52,381. MiniMed Paradigm Veo is extendedly dominated by stand-alone CSII + CGM. Essentially, this means that, in a full incremental analysis, where all the interventions and comparators are considered, CSII + CGM is better value for money than MiniMed Veo. This is because, from our systematic review, the decrease in HbA1c levels with respect to baseline was highest for stand-alone CSII + CGM; this decrease in HbA1c levels leads to a decrease in the number of complications that occur over a lifetime to such an extent that it compensates for the higher number of hypoglycaemic events. In any case, the ICER of stand-alone CSII + CGM compared with CSII + SMBG is £660,376. Thus, given the common threshold ICER of £30,000, it is clear that stand-alone CSII + CGM is not cost-effective.
Alternatively, we present the base-case ICERs for the two interventions against every comparator in Table 48. Integrated CSII + CGM (Vibe) is dominated by stand-alone CSII + CGM. It should be noted that when the MiniMed Veo system is compared with stand-alone CSII + CGM, the ICER obtained is high (£422,849) but that this results from both negative incremental QALYs and incremental costs (i.e. the ICER is in the south-west quadrant of the cost-effectiveness plane). In this case, the cost savings outweigh the loss in QALYs and therefore the MiniMed Veo system is more cost-effective than stand-alone CSII + CGM. This might not be immediately apparent when looking at the full incremental results in Table 47 because, in this table, the MiniMed Veo system is in position of extended dominance. The lowest ICERs are obtained when the interventions are compared with MDI + SMBG, but these are above £100,000 in the north-east quadrant of the cost-effectiveness plane. When the interventions are compared with CSII + SMBG, the highest ICERs are obtained (around £700,000 in the north-east quadrant of the cost-effectiveness plane). Thus, given the common threshold ICER of £30,000, the interventions are not cost-effective.
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.6266 | 77,307 | 123,375 |
| MiniMed Veo system | CSII + SMBG | 0.0656 | 47,921 | 730,501 |
| MiniMed Veo system | CSII + CGM | –0.0192 | 8119 | 422,849 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.6458 | 86,100 | 133,323 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.0849 | 56,713 | 668,789 |
| Integrated CSII + CGM (Vibe) | CSII + CGM | 0 | 674 | Undefined |
In the deterministic simulation, the cost-effectiveness results are very similar except that, in this simulation, MiniMed Veo is not extendedly dominated by stand-alone CSII + CGM. These results are shown in Table 24. Although overall cost and QALY estimates are higher than in the probabilistic simulation, the ICERs and the main conclusions from the simulation presented in Table 49 are similar to the conclusions drawn from the simulation presented in Table 47.
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 12.1450 | 62,927 | – | – | – |
| CSII + SMBG | 12.7258 | 93,433 | 0.5808 | 30,506 | 52,524 |
| MiniMed Veo system | 12.8087 | 143,309 | 0.0829 | 49,876 | 601,641 |
| CSII + CGM | 12.8223 | 151,671 | 0.0136 | 8,363 | 614,910 |
| Integrated CSII + CGM (Vibe) | 12.8223 | 152,372 | Dominated by CSII + CGM | ||
The base-case ICERs for the two interventions compared with every comparator in the deterministic simulation are shown in Table 50. These results are similar to those presented in Table 48 and so are the conclusions drawn.
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.6637 | 80,382 | 121,112 |
| MiniMed Veo system | CSII + SMBG | 0.0829 | 49,876 | 601,639 |
| MiniMed Veo system | CSII + CGM | –0.0136 | –8363 | 614,910 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.6773 | 89,445 | 132,061 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.0965 | 58,939 | 610,772 |
| Integrated CSII + CGM (Vibe) | CSII + CGM | 0 | 701 | Undefined |
When we looked at the breakdown of the total costs, we observed that treatment costs always represent the largest proportion of the total costs, independently of the treatment chosen. In Figure 11, the treatment costs constitute 79% of the total direct costs for the MiniMed Paradigm Veo system, and integrated and stand-alone CSII + CGM. For CSII + SMBG, treatment costs represent 66% of the total costs and for MDI + SMBG this is 41%. For each treatment, the foot ulcer/amputation/neuropathy cost category is the second largest, and eye diseases and renal diseases are the third and fourth largest cost categories, respectively. MDI + SMBG has higher complication incidences (CVD, ulcer, eye disease, etc.), whereas for the other four treatments these complication incidences are similar. Lifetime hypoglycaemic events were least reported for the MiniMed Paradigm Veo system (0.622 severe hypoglycaemic events per patient), and were most reported for MDI + SMBG (5.412 severe hypoglycaemic events per patient).
FIGURE 11.
Breakdown of costs per treatment: (a) breakdown of MiniMed Veo system costs; (b) breakdown of integrated CSII + CGM costs; (c) breakdown of stand-alone CSII + CGM costs; (d) breakdown of CSII + SMBG costs; and (e) breakdown of MDI + SMBG costs.

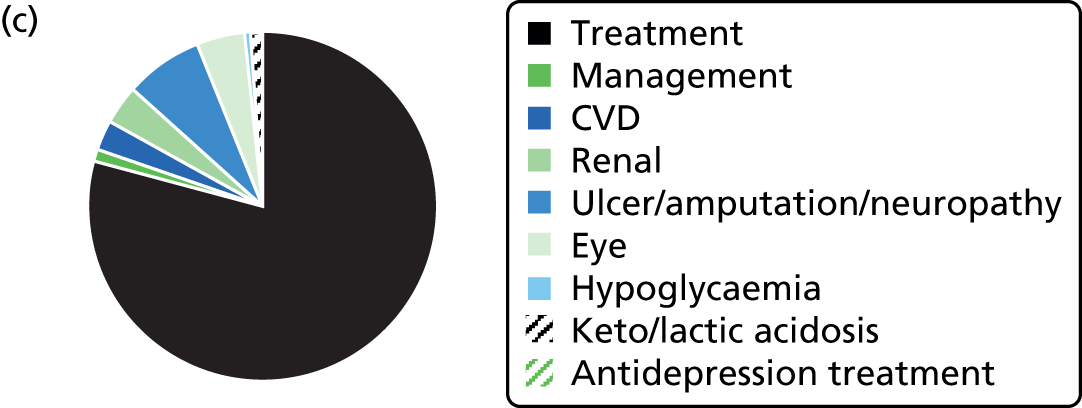


Results of the probabilistic sensitivity analyses
Statistical uncertainties in the model were investigated in the PSA. Since we compared five treatments simultaneously, the scatterplot of the PSA outcomes in the cost-effectiveness plane was not very informative (Figure 12). Nevertheless, we can observe a clear positive correlation between costs and QALYs and that the treatments including CGM are similarly scattered, showing that they are more expensive but also provide more QALYs.
FIGURE 12.
Cost-effectiveness plane with PSA outcomes for all treatments in T1DM patients.
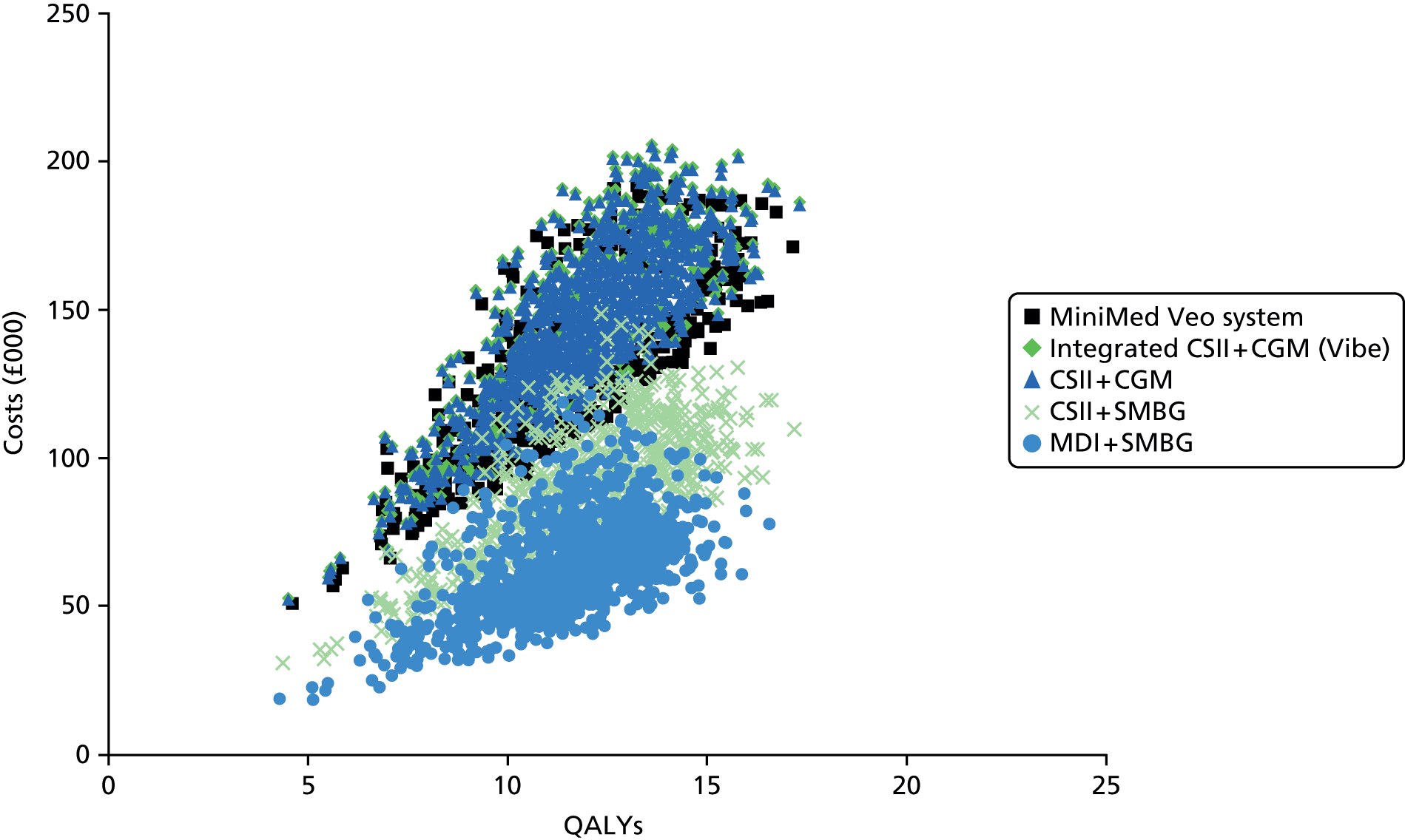
The CEACs for each treatment are shown in Figure 13. These CEACs confirm that only the treatments including SMBG are considered cost-effective. At ceiling ratio values of < £52,381, MDI + SMBG was the treatment with the highest probability of being cost-effective. When that threshold is exceeded, then CSII + SMBG was the treatment with the highest probability of being cost-effective. It should be noted that, for all three treatments including CGM, the cost-effectiveness probability was zero for all the ceiling ratios considered in the analysis. This was expected as the difference in costs between CGM treatments and SMBG treatments was too large to outweigh the additional QALYs gained by using CGM.
FIGURE 13.
Cost-effectiveness acceptability curves for all treatments in T1DM patients.

Multiple daily insulin injection-unsuitable subgroup
As mentioned in Chapter 1 (see Comparators), insulin pumps are recommended for people with T1DM for whom MDIs are not suitable. Therefore, we questioned the extent to which insulin pumps (especially modern pumps such as the integrated systems) and MDIs are used in similar populations. This seemed a reasonable question in light of the lack of studies found by our systematic review that compared these two treatments. If MDI + SMBG is not considered in the analysis, the ICERs from the full incremental analysis were the same as those reported in Table 47, but excluding the first row. It appears that CSII + SMBG is the strategy most likely to be cost-effective, independent of the ceiling ratio value (up to £100,000 per QALY), as shown in Figure 14.
FIGURE 14.
Cost-effectiveness acceptability curves for all non-MDI treatments in T1DM patients.
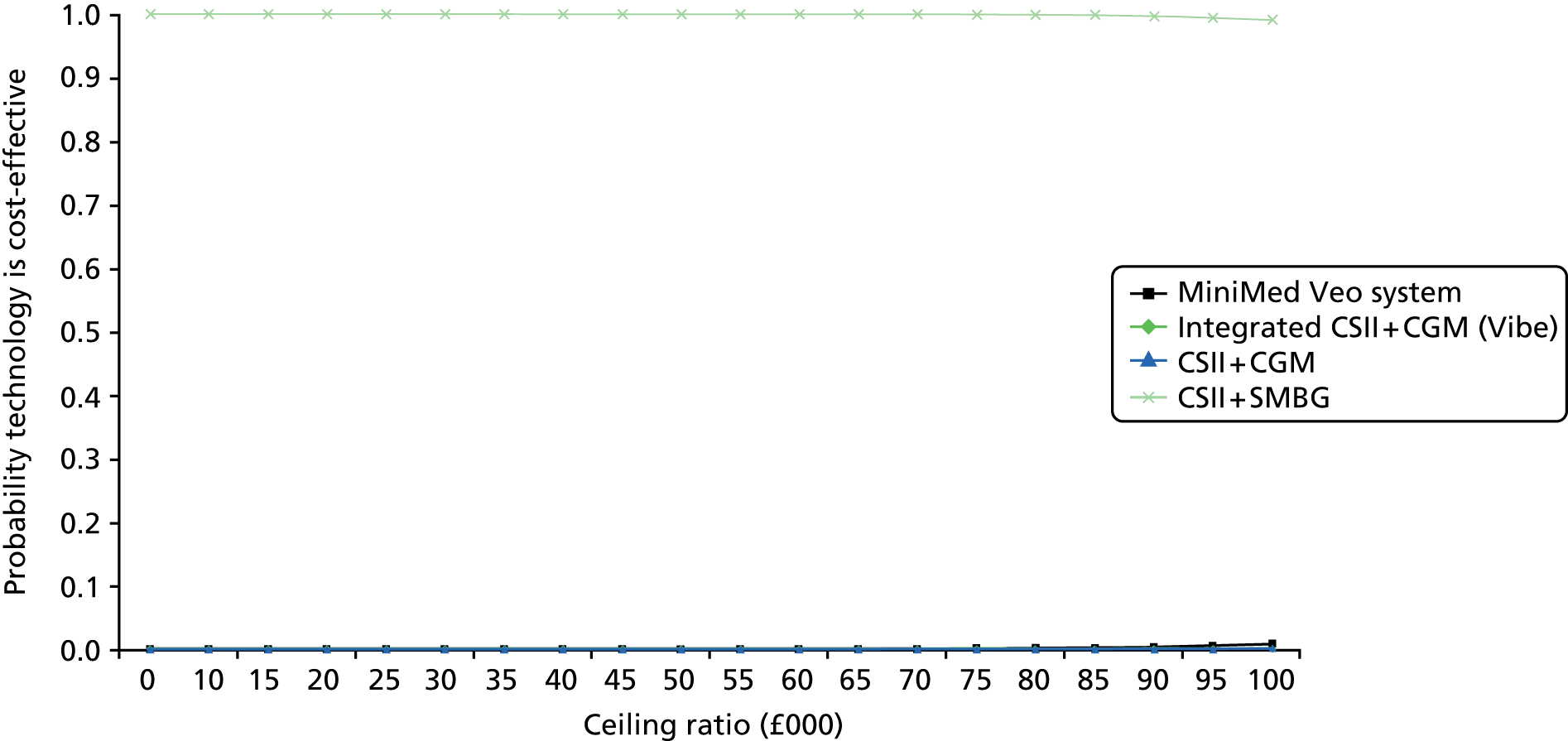
Continuous glucose monitoring-indicated/self-monitoring of blood glucose-unsuitable subgroup
In the analysis for the CGM-indicated/SMBG-unsuitable subgroup, we excluded SMBG-based treatment options from the analysis on the presumption that the most relevant population comprises those who find it difficult to perform SMBG often or adequately enough. In this situation, integrated CSII + CGM (Vibe) is dominated by stand-alone CSII + CGM, as shown in Table 47 and the only relevant comparison is the MiniMed Veo system with stand-alone CSII + CGM. The ICER is £422,849 (in the south-west quadrant of the cost-effectiveness plane), as shown in Table 48. The corresponding CEACs are shown in Figure 15. These CEACs indicate that the MiniMed Veo system is the CGM treatment most likely to be cost-effective for all the ceiling ratios considered in the analysis. However, as the ceiling ratio increases, the CEACs for the MiniMed Paradigm Veo system and stand-alone CSII + CGM seem to converge. As expected, the CEAC for integrated CSII + CGM was always zero for all the ceiling ratios considered in the analysis, since this was dominated by the stand-alone combination of CSII and CGM.
FIGURE 15.
Cost-effectiveness acceptability curves for CGM treatments in T1DM patients.

Results of scenario analyses
In the scenarios presented below, only the ICERs from the full incremental analysis are discussed. The ICERs for the two interventions against every comparator are shown in Appendix 7.
Baseline population characteristics
In the scenario analysis, in which the baseline population characteristics have been updated in accordance with NICE Guideline NG17,81 the main results are similar to the base-case results, as shown in Table 51.
| Interventions | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 9.6117 | 65,070 | – | – | – |
| CSII + SMBG | 10.0991 | 91,189 | 0.4874 | 26,119 | 53,588 |
| MiniMed Veo system | 10.1474 | 132,149 | Extendedly dominated by stand-alone CSII + CGM | ||
| CSII + CGM | 10.164 | 139,157 | 0.0649 | 47,967 | 738,593 |
| Integrated CSII + CGM (Vibe) | 10.164 | 139,733 | Dominated by stand-alone CSII + CGM | ||
The intervention with the lowest costs and the lowest gain in QALYs is MDI + SMBG. CSII + SMBG and stand-alone CSII + CGM are on the efficient frontier, with ICERs of £53,588 per QALY and £738,593 per QALY, respectively. Thus, given the common threshold ICER of £30,000, they are not cost-effective. The MiniMed Veo system and integrated CSII + CGM are extendedly dominated and dominated, respectively, by stand-alone CSII + CGM.
Number of blood glucose tests per day
All of the scenarios listed in Table 44 gave similar results. Compared with the base case, costs were lower in the scenarios for treatments that require fewer than four BG tests per day and, otherwise, were higher. Since all results were similar, in Table 52 we present only the full incremental cost-effectiveness results of the scenarios with two BG tests per day for CGM-containing treatments and eight BG tests per day for SMBG. Eight tests per day for SMBG represent the most cost-effective frequency, as was shown in NICE Guideline NG17. 81
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.4146 | 68,460 | – | – | – |
| CSII + SMBG | 11.9756 | 98,034 | 0.5610 | 29,574 | £52,717 |
| MiniMed Veo system | 12.0412 | 138,357 | Extendedly dominated by CSII + CGM | ||
| CSII + CGM | 12.0604 | 146,476 | 0.0849 | 48,441 | 570,844 |
| Integrated CSII + CGM (Vibe) | 12.0604 | 147,150 | Dominated by CSII + CGM | ||
The intervention with the lowest costs and the lowest gain in QALYs is MDI + SMBG. CSII + SMBG and stand-alone CSII + CGM are on the efficient frontier, with ICERs of £52,717 per QALY and £570,844 per QALY, respectively. Therefore, given the common threshold ICER of £30,000, they are not cost-effective. The MiniMed Veo system and integrated CSII + CGM are extendedly dominated and dominated, respectively, by stand-alone CSII + CGM.
Amount of insulin per day
In this scenario, the costs for MDI + SMBG were higher than in the base case; however, this had a very small impact on the cost-effectiveness results because all QALYs and the costs of the other treatments remained unchanged. Since the main conclusions of the cost-effectiveness analyses were the same in this scenario as in the base case, we have not presented these results in a separate table in this chapter, but these results are shown in Appendix 7.
Glycated haemoglobin progression
In this scenario, no HbA1c progression after year 1 was assumed for each treatment. Table 53 summarises the model results.
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.8715 | 58,520 | – | – | – |
| CSII + SMBG | 12.4558 | 88,663 | 0.5843 | 30,143 | 51,615 |
| MiniMed Veo system | 12.5228 | 137,739 | Extendedly dominated by CSII + CGM | ||
| CSII + CGM | 12.5398 | 146,076 | 0.0840 | 57,414 | 683,889 |
| Integrated CSII + CGM (Vibe) | 12.5398 | 146,767 | Dominated by CSII + CGM | ||
The intervention with the lowest costs and the lowest gain in QALYs is MDI + SMBG. CSII + SMBG and stand-alone CSII + CGM are on the efficient frontier, with ICERs of £51,615 per QALY and £683,889 per QALY, respectively. Therefore, they are not cost-effective given the common threshold ICER of £30,000. The MiniMed Veo system and integrated CSII + CGM (Vibe) are extendedly dominated and dominated, respectively, by stand-alone CSII + CGM.
Treatment effects part I: change in glycated haemoglobin levels in the first year
In this scenario analysis, we assumed that the baseline HbA1c value is stabilised for 1 year and that it does not change in any of the treatments (i.e. 0% change in HbA1c levels in the first year). The model results for this scenario are shown in Table 54.
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| CSII + CGM | 12.0006 | 146,632 | Dominated by MDI + SMBG | ||
| Integrated CSII + CGM (Vibe) | 12.0006 | 147,304 | Dominated by MDI + SMBG | ||
| MDI + SMBG | 12.0016 | 56,928 | – | – | – |
| CSII + SMBG | 12.0160 | 90,178 | 0.0144 | 33,250 | 2,309,028 |
| MiniMed Veo system | 12.0260 | 138,538 | 0.0099 | 48,360 | 4,871,356 |
The QALY expectations for all treatments are very similar. The minor differences in QALYs can be explained by the differences in severe hypoglycaemic episode rates. It should be noted that although the rate of severe hypoglycaemic events for MDI + SMBG was estimated to be higher than the rate for integrated CSII + CGM (see Treatment effects), MDI + SMBG resulted in a slightly higher gain in QALYs which could be due to randomness. CSII + CGM systems were dominated by MDI + SMBG. Furthermore, CSII + SMBG and the MiniMed Veo system are on the efficient frontier but with extremely high ICER values. As can be seen in the resulting CEACs in Figure 16, MDI + SMBG was the most cost-effective treatment for all the values of the ceiling ratio considered in the analysis.
FIGURE 16.
Cost-effectiveness acceptability curves for all treatments when there is no HbA1c treatment effect.

Treatment effects part II: severe hypoglycaemic event rates
When we used different RRs (0.125, 0.25, 0.5 and 1) for the severe hypoglycaemic episode rates for the MiniMed Veo system, the results did not deviate significantly from the base case. In all of the scenarios, MDI + SMBG was the lowest cost intervention, the MiniMed Veo system was extendedly dominated by stand-alone CSII + CGM and integrated CSII + CGM was dominated. Table 55 shows the results for the most extreme scenario, which is obtained if the RR value is 0.125. For this RR, the severe hypoglycaemia rates per 100 patient-years for all interventions are shown in Table 46.
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.4120 | 60,812 | – | – | – |
| CSII + SMBG | 11.9597 | 91,195 | 0.5477 | 30,383 | 55,474 |
| MiniMed Veo system | 12.0453 | 138,333 | Extendedly dominated by CSII + CGM | ||
| CSII + CGM | 12.0604 | 146,476 | 0.1007 | 55,281 | 549,080 |
| Integrated CSII + CGM (Vibe) | 12.0604 | 147,150 | Dominated by CSII + CGM | ||
Non-zero probability of death caused by severe hypoglycaemia
In this scenario, we assumed a mortality due to severe hypoglycaemia of 4.9%, as derived from Ben-Ami et al. 142 The model results are shown in Table 56.
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.1041 | 58,510 | – | – | – |
| CSII + CGM | 11.7701 | 142,215 | Dominated by CSII + SMBG | ||
| Integrated CSII + CGM (Vibe) | 11.7701 | 142,872 | Dominated by CSII + SMBG | ||
| CSII + SMBG | 11.8781 | 89,475 | 0.774 | 30,965 | 40,006 |
| MiniMed Veo system | 12.0071 | 137,801 | 0.129 | 8326 | 374,531 |
In this scenario, both integrated and stand-alone CSII + CGM were dominated by CSII + SMBG. The ICER of CSII + SMBG compared with MDI + SMBG was £40,006, and the ICER of MiniMed Veo compared with CSII + SMBG was £374,531. Thus, these treatments are not cost-effective given the common threshold ICER of £30,000. Both cost-effectiveness plane scatterplots and CEACs are similar to those for the base-case scenario and therefore they are not shown here. If only the CGM treatments were considered, the probability of the MiniMed Paradigm Veo system being cost-effective was equal to 1 for almost all the values of the ceiling ratio considered in the analysis; this is shown in Figure 17.
FIGURE 17.
Cost-effectiveness acceptability curves for only CGM treatments for the non-zero mortality due to severe hypoglycaemia scenario.

Quality-adjusted life-year estimation method
In this scenario, we assumed the minimum approach as an alternative QALY estimation method, in which the minimum of the multiple health-state utility values was applied for patients with a history of multiple events. The results of this scenario are shown in Table 57.
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 12.1327 | 61,050 | – | – | – |
| CSII + SMBG | 12.5861 | 90,436 | 0.4534 | 29,386 | 64,813 |
| MiniMed Veo system | 12.6408 | 138,357 | 0.0546 | 47,920 | 876,987 |
| CSII + CGM | 12.6462 | 146,476 | 0.0601 | 56,039 | 932,305 |
| Integrated CSII + CGM (Vibe) | 12.6462 | 147,150 | Dominated by CSII + CGM | ||
These results are similar to those obtained using the base-case scenario; however, in this scenario, the MiniMed Paradigm Veo system is not extendedly dominated by stand-alone CSII + CGM. All the ICERs are larger than £50,000 and therefore the different treatments are not cost-effective given the common threshold ICER of £30,000.
Different time horizon
In this scenario, we assumed a 4-year time horizon, which corresponds to the average lifetime of an insulin pump. These results are shown in Table 58.
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 2.7718 | 6706 | – | – | – |
| CSII + CGM | 2.7882 | 24,803 | Dominated by CSII + SMBG | ||
| Integrated CSII + CGM (Vibe) | 2.7886 | 24,939 | Dominated by CSII + SMBG | ||
| CSII + SMBG | 2.7906 | 13,365 | 0.0188 | 6659 | 354,202 |
| MiniMed Paradigm Veo | 2.7928 | 23,144 | 0.0022 | 9778 | 4,461,063 |
We observed that both stand-alone and integrated CSII + CGM are dominated by CSII + SMBG. Although the MiniMed Paradigm Veo system is the treatment with the highest number of QALYs gained, its high cost when compared with CSII + SMBG does not outweigh this gain in QALYs, and results in an ICER of £4,461,063. Therefore, for this scenario also, it is very unlikely that MiniMed Paradigm Veo will be deemed cost-effective, as illustrated by the corresponding CEACs in Figure 18.
FIGURE 18.
Cost-effectiveness acceptability curves for all treatments for the 4-year time horizon scenario.
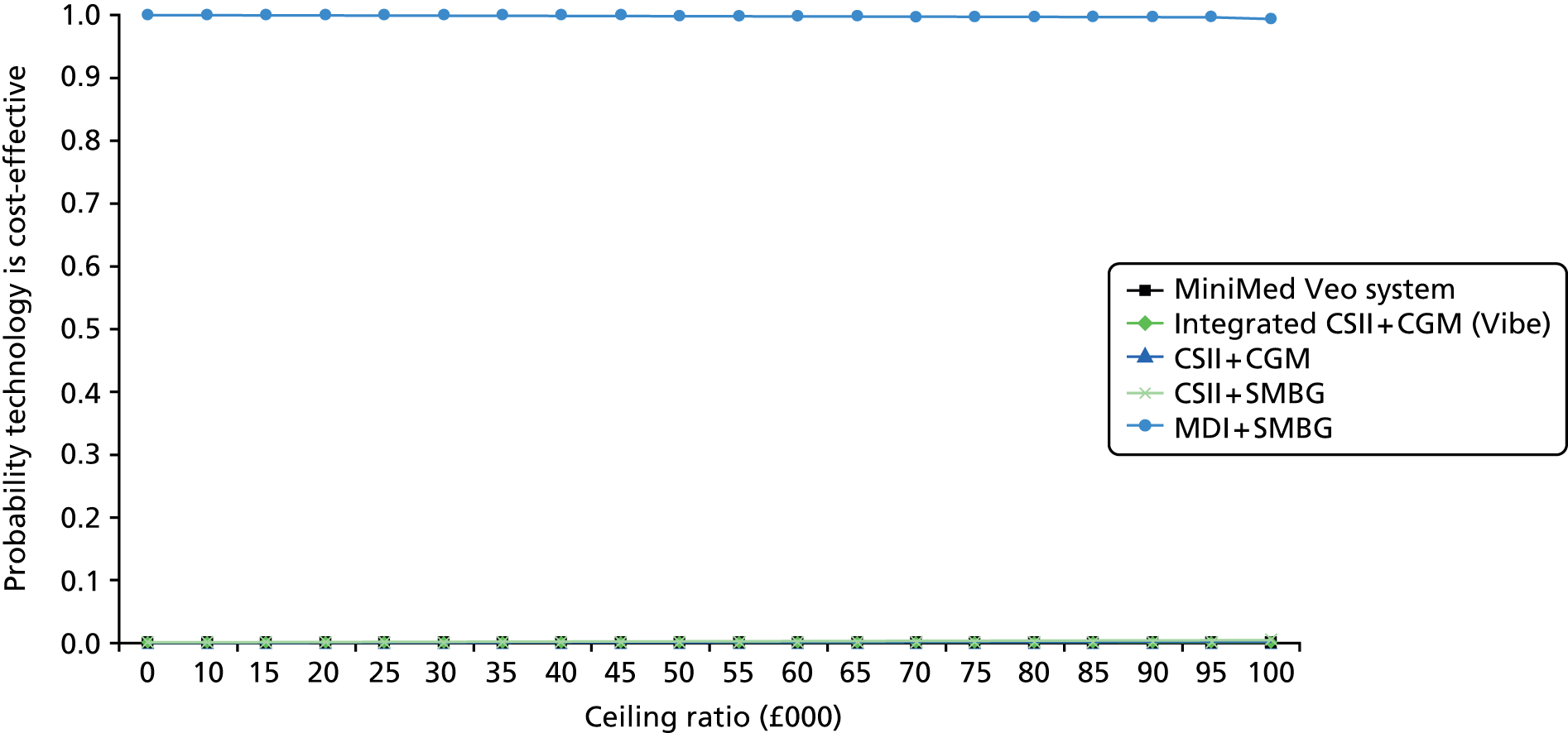
If only the CGM treatments are considered, the MiniMed Paradigm Veo system is clearly the treatment with the highest probability of being cost-effective, as shown in Figure 19.
FIGURE 19.
Cost-effectiveness acceptability curves for CGM treatments only: 4-year time horizon scenario.
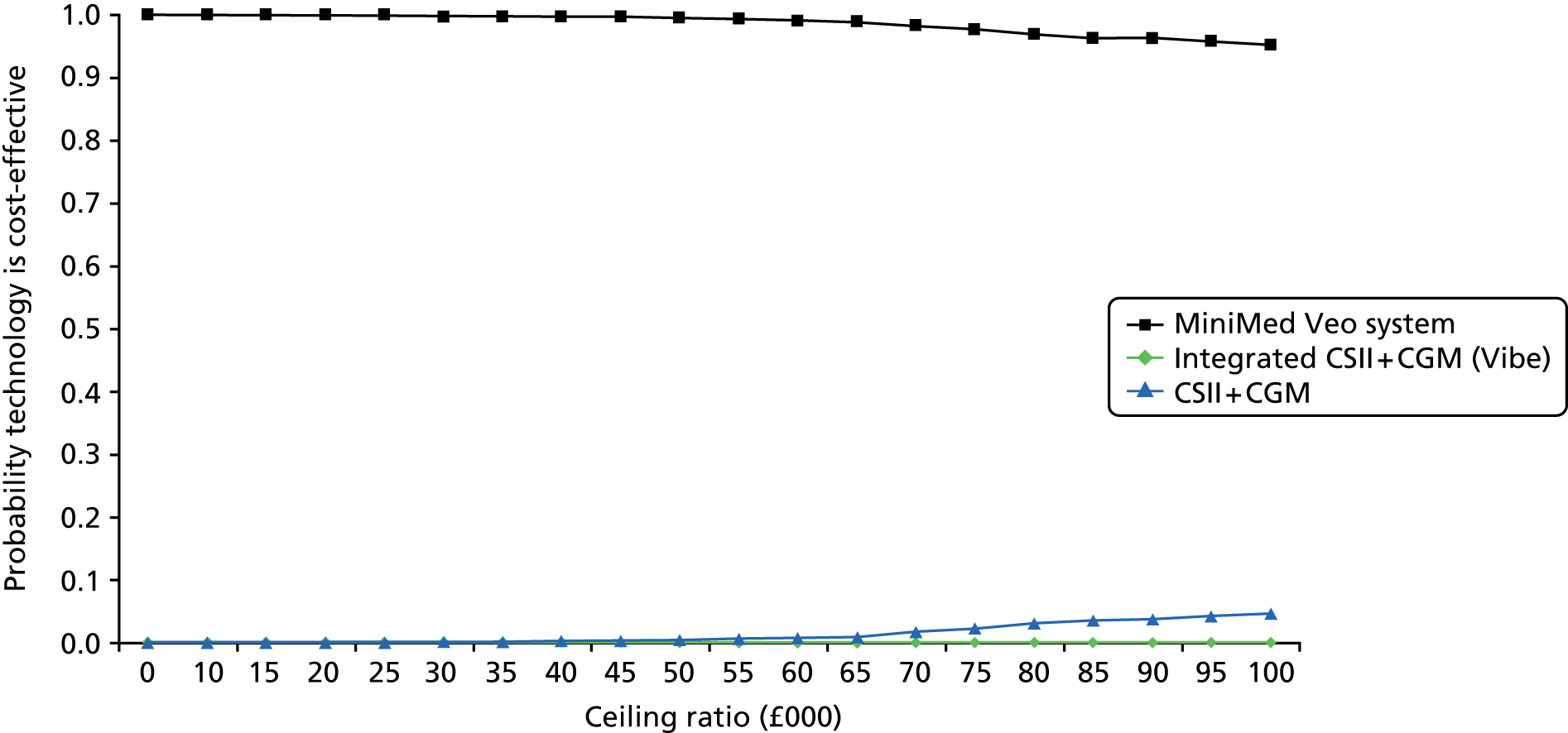
Fear of hypoglycaemia unawareness
Table 59 shows the results obtained when the utility increment (0.0329) from Kamble et al. 69 was used to represent the reduced fear of hypoglycaemia. We applied this utility increment throughout the remaining lifetimes of patients using integrated devices (the MiniMed Paradigm Veo system and integrated CSII + CGM). This benefit was not applied to non-integrated devices (stand-alone CSII + CGM, CSII + SMBG and MDI + SMBG), as these non-integrated devices do not give a warning or activate/stop the release of insulin automatically in response to low BG levels.
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.4146 | 61,050 | – | – | – |
| CSII + SMBG | 11.9756 | 90,436 | 0.5610 | 259,386 | 52,381 |
| CSII + CGM | 12.0604 | 146,476 | Extendedly dominated by MiniMed Veo system | ||
| MiniMed Veo system | 12.6224 | 138,357 | 0.6468 | 47,920 | 74,088 |
| Integrated CSII + CGM (Vibe) | 12.6429 | 147,150 | 0.0205 | 8792 | 428,595 |
For this scenario, the main difference with respect to the base-case scenario is that stand-alone CSII and stand-alone CGM devices is extendedly dominated by the MiniMed Paradigm Veo system, which has an ICER compared with CSII + SMBG of £74,088. Moreover, in this scenario, integrated CSII + CGM is not dominated by the corresponding stand-alone combination, as the utility increment for the integrated system led to a larger number of QALYs accumulated than the non-integrated options. Nevertheless, the ICER of integrated CSII + CGM compared with the MiniMed Paradigm Veo system is still very large (£428,595).
The scatterplot of the PSA outcomes in the CE plane is very similar to the one in the base-case scenario and therefore we decided not to show it here. The CEACs for each treatment are shown in Figure 20. These CEACs demonstrate that, compared with the base-case scenario, the probability of being cost-effective for CSII + SMBG starts decreasing at approximately £60,000. As the ceiling ratio increases, the probability of being cost-effective for the MiniMed Paradigm Veo system and integrated CSII + CGM systems also increases. At ceiling ratio values larger than (approximately) £75,000, the MiniMed Paradigm Veo system is the treatment with the highest probability of being cost-effective, followed by integrated CSII + CGM systems, at ceiling ratio values of more than (approximately) £90,000. It should be noted that for stand-alone CSII + CGM, the cost-effectiveness probability was zero for all of the ceiling ratios considered in the analysis.
FIGURE 20.
Cost-effectiveness acceptability curves for reduced fear of hypoglycaemia scenario.
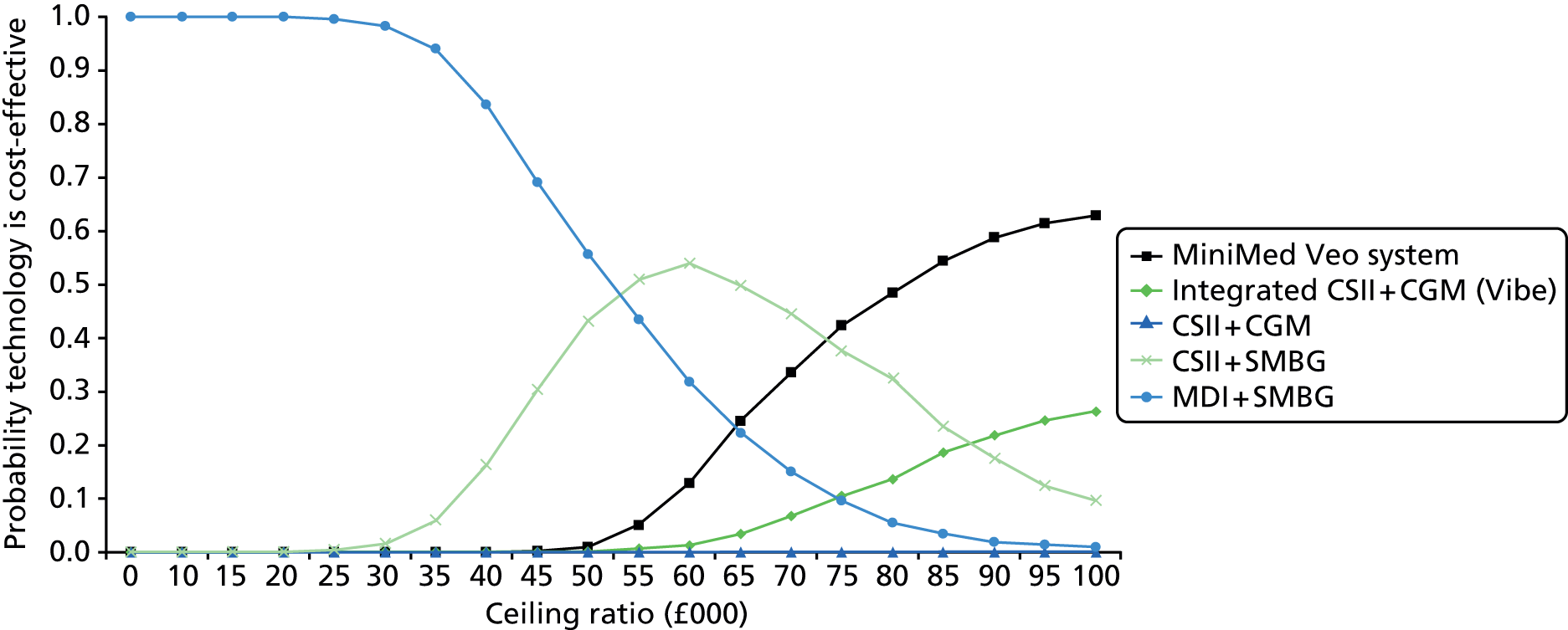
If only the CGM treatments were considered, we observed similar CEACs (Figure 21) to those observed for the base case (see Figure 14), but in this scenario the role of integrated and stand-alone CSII + CGM was interchanged in the CEAC.
FIGURE 21.
Cost-effectiveness acceptability curves for only CGM treatments for the fear of hypoglycaemia scenario.

Cost of stand-alone insulin pumps and continuous glucose monitoring devices
In this scenario, we assumed that the yearly cost of stand-alone CSII + CGM could be estimated from the average costs of the different stand-alone devices, as shown in Tables 32 and 33, but without the weighting for market share from White et al. 121,122 Therefore, in this scenario, the estimated yearly cost of stand-alone CSII + CGM was £5460. The results from this scenario are shown in Table 60.
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.4146 | 61,050 | – | – | – |
| CSII + SMBG | 11.9756 | 92,272 | 0.5610 | 31,222 | 55,654 |
| MiniMed Veo system | 12.0412 | 138,357 | Extendedly dominated by integrated CSII + CGM | ||
| Integrated CSII + CGM (Vibe) | 12.0604 | 147,150 | 0.0849 | 54,878 | 646,692 |
| CSII + CGM | 12.0604 | 150,063 | Dominated by integrated CSII + CGM | ||
The main difference in these results, with respect to the base-case scenario, was that, as expected, stand-alone CSII + CGM was more expensive than integrated CSII + CGM (Vibe). Since both technologies are assumed to have the same efficacy, integrated CSII + CGM (Vibe) dominated stand-alone CSII + CGM. The CEACs for each treatment are shown in Figure 22. These are very similar to those for the base-case scenario. The higher cost of stand-alone CSII + CGM had almost no impact on the cost-effectiveness probability since MDI + SMBG and CSII + SMBG are the only strategies that are considered cost-effective.
FIGURE 22.
Cost-effectiveness acceptability curves for cost of stand-alone CSII and stand-alone CGM devices CGM without market share scenario.

If only the CGM treatments were considered, we observed similar CEACs (Figure 23) as those observed for the base-case scenario (see Figure 15) but, as expected, in this scenario the role of integrated CSII + CGM (Vibe) and stand-alone CSII + CGM was interchanged in the CEAC.
FIGURE 23.
Cost-effectiveness acceptability curves for CGM treatments only for the cost of stand-alone CSII and stand-alone CGM devices without market share scenario.
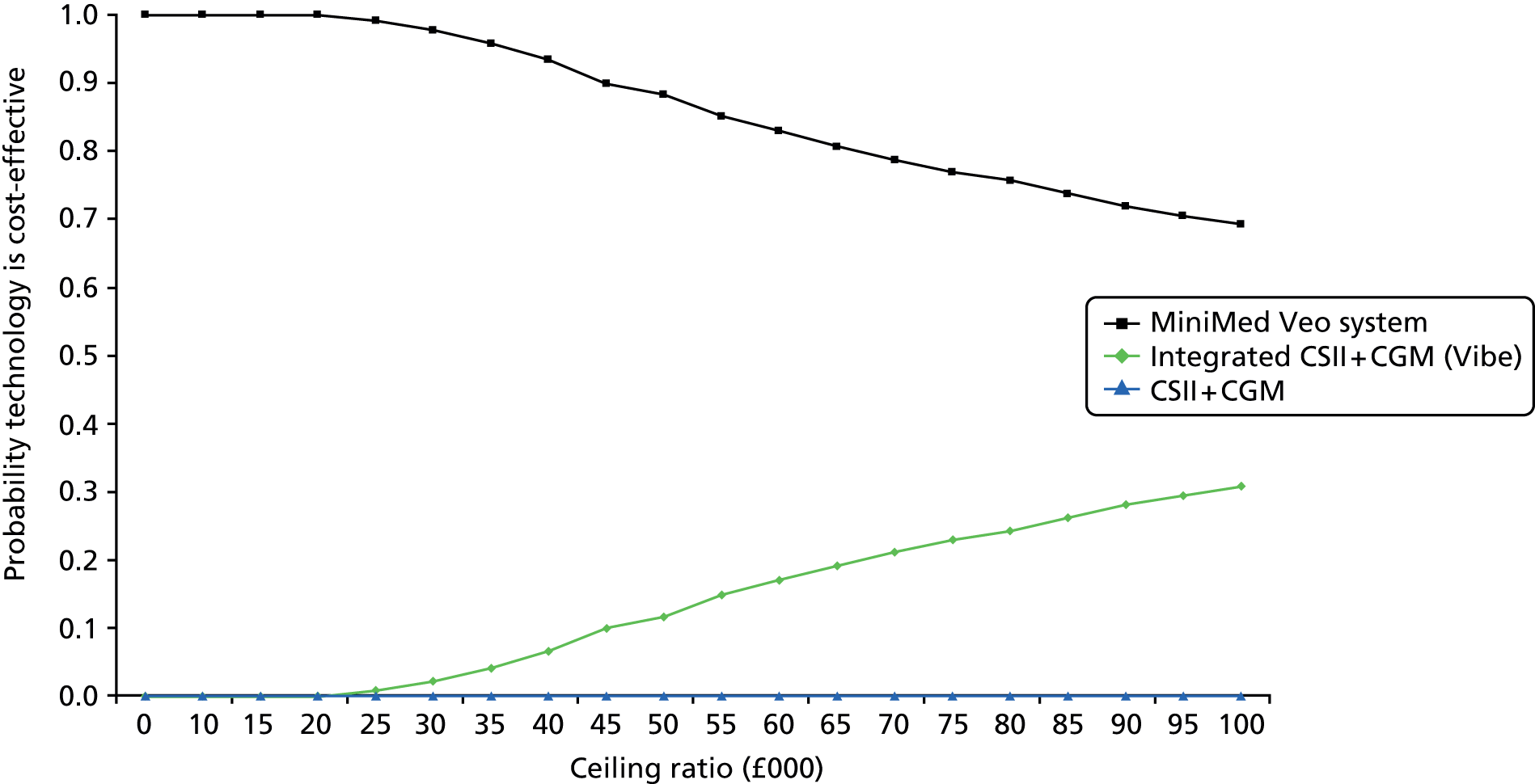
Extension of the health economic analysis to children and adolescents
In addition to the clinical effectiveness limitations with regard to the evidence for children and adolescent patients mentioned in Chapter 3 (see Effectiveness of interventions in children), the model employed to conduct the cost-effectiveness analyses, the IMS CDM, is not suitable for modelling long-term outcomes for children/adolescent populations, mostly because the background risk adjustment/risk factor progression equations are all based on adult populations.
Based on these limitations, it was deemed that there are too many crucial parameters with essentially no evidence specifically for these subgroups. This makes the reliability and validity of the results of conducting an economic evaluation for children and adolescents in this diagnostics assessment programme questionable. An overview of these parameters and reasons for the extreme uncertainty related to children and young adolescent patients is given in the following sections.
We have also reviewed the latest NICE guidelines (see Health economic analyses of type 1 diabetes for children and adolescent patients in other National Institute for Health and Care Excellence guidelines/assessment reports) in order to summarise how they have modelled with regard to children and further emphasise the limitations resulting from a lack of evidence.
Parameters subject to extreme uncertainty in the clinical effectiveness evidence for child and adolescent patients
These are all parameters for treatment effects on both HbA1c levels and hypoglycaemic event rates for all six treatment options (i.e. essentially 12 different parameters).
For the MiniMed Veo system, our systematic review identified only one study in children: Ly et al. 33 This study included patients between 4 and 50 years old, 70% of whom were children (4–18 years old). However, data were not reported separately by age group; therefore, we could use only the data for the total population and assume that it would apply to children.
However, our clinical experts advised us not to use this study as a study in children for two main reasons: (1) children behave differently to adults and, therefore, results for children are not the same as those for adults; and (2) pre-teen children behave differently from teenagers and, therefore, the 4- to 12-year-old age group would be different from a 12- to 18-year-old age group and the influence of parents on younger children would have to be taken into account. Indeed, this further subdivision of children essentially implies a doubling of the number of parameters for which there is no evidence of any treatment effect.
The only reason that we presented the data from this study in Chapter 3 (see Assessment of clinical effectiveness) is that, without it, there would have been no evidence at all with regard to the effectiveness of the MiniMed Veo system in children. Therefore, for the MiniMed Veo system (and the assessment of severe hypoglycaemic events), we have data from only one study and this does not properly apply to children.
In addition, we found two trials presenting evidence for the integrated CSII + CGM system versus CSII + SMBG34 and versus MDI + SMBG,40 and three trials comparing CSII + SMBG with MDI + SMBG. 47–49
However, these studies differed with regard to the age groups included (12–17 years,34 7–18 years,40 8–14 years,47 8–18 years48 and 8–21 years49), whether or not patients had pump experience, baseline HbA1c levels (8–11.5%), follow-up times (3, 6 and 12 months), hypoglycaemic status at baseline (in one study, patients with hypoglycaemia unawareness were excluded;40 in another study, only patients with impaired awareness of hypoglycaemia were included;33 and other studies had no exclusions34 or no information47–49), and country (Israel,47 the USA,34,48,49 and the USA and Canada40). None of the studies was performed in the UK. Therefore, there is considerable heterogeneity between the studies, which makes any pooling of results invalid.
Uncertainties around the parameters for disease progression and treatment within the IMS CDM for child and adolescent patients
Several additional modelling uncertainties with regard to using the IMS CDM for children and adolescents have been identified. Indeed, the CDM structure is limited in that it lacks crucial parameters to inform the long-term effects of hypoglycaemia. These uncertainties have been summarised in Table 61, along with those regarding the treatment effects on HbA1c levels and hypoglycaemic event rate.
| Category of parameter | Parameter | Possibility to include parameter in the current version of IMS CDM | Impact on CE results |
|---|---|---|---|
| Treatment-related adverse events | Long-term consequences of hypoglycaemia in young children are not included in the model, despite them being potentially relevant. Couper et al.,144 for example, indicate that there is greater concern about the consequences of hypoglycaemia in young children, given the rapid growth and development of the brain from birth to 7 years. In children who develop diabetes before 5 years of age, hypoglycaemia-related long-term adverse effects have been found, such as cognitive deficits, particularly in visuospatial tasks and lower IQ scores. In children who develop diabetes after 5 years, this impairment has not been found145 | No. The model structure is fixed | Long-term costs and QALYs associated with these complications would change. It is not possible to predict in which direction the CE results would change |
| Costs |
|
Partially (except for categories that only apply to children, if any). These costs could be averaged (together with the costs for the adult population) over the simulation time horizon | Costs 1, 2 and 5: no change in base-case incremental results, as these costs are the same for all treatments (unless there are categories that apply only to children) |
| Cost 3: results would be more favourable towards CSII technologies, as the difference in insulin costs with respect to MDI technologies would increase | |||
| Cost 4: it is not possible to predict in which direction the CE results would change | |||
| Utilities | It is uncertain how the different complications can affect quality of life in children compared with adults. If this differs, then at least two different utility values would be needed for each complication | No. The model only accepts one value per health state as input. Note also that the consequences of hypoglycaemic events in young children are not modelled | It is not possible to predict in which direction the CE results would change |
| We anticipate that utilities associated with severe hypoglycaemic events (including the fear of experiencing it) may be different, in particular for younger children, as hypoglycaemic events can cause serious long-term adverse events (e.g. brain damage) | |||
| Treatment effects: reduction in baseline HbA1c levels in the first 12 months | In the IMS CDM, the change in HbA1c levels is assumed to occur within the first 12 months. It is uncertain whether or not this is also applicable to children. It may take longer to observe the treatment benefits in children | Partially. The change in HbA1c levels can be an input in the model regardless of age. However, extending the treatment effect beyond 12 months is not possible | It is not possible to predict in which direction the CE results would change |
| Treatment effects: rate per 100 patient-years of severe hypoglycaemic episodes | The rate of severe hypoglycaemic events differs between children and adults146 | No. The model only accepts one value as input which is carried over the simulation time horizon | It is not possible to predict in which direction the CE results would change |
| HbA1c progression after year 1 | Annual HbA1c progression in children and adults is different;146 progression in children has been reported in the literature147 | Yes. This can be modelled, for example, as in NICE Guideline NG18 for children148 | It is not possible to predict in which direction the CE results would change |
| Disease management parameters | It is uncertain whether or not these parameters are the same for adults and children. If these are different then at least two values would be needed for each parameter | No. The model only accepts one value as input | It is not possible to predict in which direction the CE results would change |
| Disease natural history parameters | It is uncertain whether or not these parameters are the same for adults and children. If these are different then at least two values would be needed for each parameter | No. The model only accepts one value as input | It is not possible to predict in which direction the CE results would change |
| Transition probabilities/risk equations | All of these probabilities/equations are based on adult data. Therefore, it is uncertain to what extent these parameters are appropriate for modelling child populations. We anticipate that, for example, the reduction of the risk of MI or nephropathy for every 1% reduction in HbA1c levels or every 10 mmHg reduction in SBP would be different for children/younger patients than for adults because of differences in the accumulation of any depreciation with disease duration | No. The model only accepts one value as input | It is not possible to predict in which direction the CE results would change |
Health economic analyses of type 1 diabetes for children and adolescent patients in other National Institute for Health and Care Excellence guidelines/assessment reports
CG15 (2004)148 Type 1 Diabetes: Diagnosis and Management of Type 1 Diabetes in Children and Young People
This guideline was developed for the diagnosis and management of T1DM in adults and children/younger patients. In this guideline, no economic analysis was carried out for children or younger patients. 148 No explicit reasons for not conducting such economic analyses were mentioned in the guideline. In the introduction section of this guideline, it is stated that it was accepted that economic models utilising literature review data should be considered when there are resource implications with recommendations from guidelines, or when clinical effectiveness data from well-conducted studies were presented, or when guideline recommendations signified a policy amendment.
TA151 (2011)14 Continuous Subcutaneous Insulin Infusion for the Treatment of Diabetes Mellitus
No economic analysis was conducted for children in this assessment, because in the technology assessment report, it was stated that the IMS CDM (online software applied for the adult economic analysis) was not created to run with children and could not replicate childrens’ long-term outcomes. Therefore, the cost-effectiveness results for children/younger adults for CSII are not modelled in TA151. 80
NG18 (2015)148 Diabetes (Type 1 and Type 2) in Children and Young People: Diagnosis and Management
This guideline focuses on children and younger patients with T1DM as well as with T2DM.
In this guideline, two cost-effectiveness analyses for T1DM were conducted using the IMS CDM. The first analysis compared MDIs (four or more injections of insulin per day, matching insulin to food – also known as a basal–bolus regimen) with mixed insulin injections (less than four injections of insulin per day and no matching of insulin to food). The second analysis is a ‘what if’ analysis in which the intervention effects were based on an observational study and explored the possible cost-effectiveness of different frequencies of capillary BG monitoring.
For these analyses, a newly diagnosed cohort (i.e. with a disease duration of 0 years) with a baseline age of 12 years and an average baseline HbA1c value of 11.4% was used. Among the physical risk factors, only HbA1c progression was tailored by the Guideline Development Group (based on clinical advice) to represent progression in children. However, we anticipate that other risk factors and the risk adjustments for children/younger patients should also be adjusted: for example, the reduction of the risk of MI or nephropathy for every 1% reduction in HbA1c levels, or every 10 mmHg reduction in SBP would be different for children/younger patients than for adults because of differences in the accumulation of any depreciation with disease duration. In conclusion, some input parameters of the IMS CDM (such as the baseline HbA1c value and HbA1c progression) were adapted for the child population, but there are many other parameters that cannot be adjusted (see Table 61). It should be noted that it is not possible to predict the extent to which these non-adjusted parameters will affect the cost-effectiveness results; therefore, the use of the IMS CDM for these analyses of children/younger populations is questionable. No explicit discussion regarding the use of the IMS CDM in children/adolescents was given in this draft guideline.
Finally, it should also be noted that, in this draft guideline, it was mentioned that the clinical evidence was not sufficiently robust to support a recommendation for the routine use of CGM as a glucose monitoring strategy and therefore modelling was not used to aid recommendations. 81 In this regard, the conclusions of this draft guideline81 on the lack of clinical evidence are similar to those of our report, which are summarised in Parameters subject to extreme uncertainty in the clinical effectiveness evidence for children and adolescent patients.
Conclusion
The limited clinical effectiveness evidence (as discussed in Chapter 3, Effectiveness of interventions in children and Parameters subject to extreme uncertainty in the clinical effectiveness evidence for children and adolescent patients), the limitations of the model (summarised in Table 61), and the approaches followed in previous NICE clinical guidelines and assessment reports support our conclusion that it is not possible to conduct a reliable and valid economic evaluation for children/adolescent populations using the IMS CDM.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
Nineteen trials were included, 12 reported data for adults,32,34,37–46 six reported data for children33,34,40,47–49 and one trial reported data for pregnant women. 50 Four trials were in mixed populations (adults and children); two of these reported data separately for adults and children and are included in both the 12 trials for adults and the six trials for children. 34,40 Two trials did not report data separately for adults and children (O’Connell et al. 35 and RealTrend36). Therefore, the results from these trials were not used in the main analyses.
Twelve studies were included in the analyses for adults. 32,34,37–46 The main conclusion from these trials is that the MiniMed Paradigm Veo system reduces hypoglycaemic events in adults in comparison with the integrated CSII + CGM system, without any differences in other outcomes, including change in HbA1c levels. Nocturnal hypoglycemic events occurred 31.8% less frequently in the MiniMed Veo group than in the integrated CSII + CGM group [1.5 events (SD 1.0 event) vs. 2.2 events (SD 1.3 events) per patient per week; p < 0.001]. Similarly, the MiniMed Veo group had significantly lower weekly rates of combined daytime and night-time events than the integrated CSII + CGM group (p < 0.001). Indirect evidence seems to suggest that that there are no significant differences between the MiniMed Paradigm Veo system and CSII + SMBG or MDI + SMBG with regard to the change in HbA1c levels at 3-month follow-up. However, if all studies are combined (combining different follow-up times and including mixed populations), the MiniMed Paradigm Veo system is significantly better than MDI + SMBG in terms of HbA1c levels.
For the integrated CSII + CGM system (MiniMed Paradigm REAL-Time 722 System) versus other treatments, the results suggest a significant effect in favour of the integrated CSII + CGM system over MDI + SMBG for HbA1c levels, but not if compared with CSII + SMBG, and a significant effect in favour of the integrated CSII + CGM system over MDI + SMBG and CSII + SMBG with regard to quality of life.
With regard to comparisons of CSII and MDIs, only one of the six trials41–46 showed a significant difference between CSII + SMBG and MDI + SMBG in terms of change in HbA1c levels. DeVries et al. 42 found a significant difference in favour of CSII + CGM: at 16 weeks, mean HbA1c levels were 0.84% lower (mean = –0.84%, 95% CI –1.31% to –0.36%) in the CSII + SMBG group than the MDI + SMBG group. No differences were found in any trial with regard to the number of severe hypoglycaemic events.
Six studies were included in the analyses for children. 33,34,40,47–49 None of the studies in children made a direct comparison between the MiniMed Paradigm Veo system and the integrated CSII + CGM system. An indirect comparison was possible, using data from Ly et al. 33 and Hirsch et al. 34 at 6-month follow-up, but only for HbA1c levels, which showed no significant difference between groups.
One study33 compared the MiniMed Paradigm Veo system with CSII + SMBG. The only significant difference between treatment groups was the rate of moderate and severe hypoglycaemic events, which favoured the MiniMed Paradigm Veo system.
One study34 compared the integrated CSII + CGM system with CSII + SMBG; this trial found no significant difference in HbA1c levels between groups. One study40 compared the integrated CSII + CGM system with MDI + SMBG; this trial found a significant difference in HbA1c change scores in favour of the integrated CSII + CGM system, but no significant difference in the number of children achieving HbA1c levels of ≤ 7%. The hyperglycaemic AUC was significantly lower in the integrated CSII + CGM group, but the hypoglycaemic AUC showed no significant difference. Other outcomes showed no significant differences between groups.
For pregnant women, we found only one trial50 comparing CSII + SMBG with MDI + SMBG, which is not relevant to the decision problem.
Cost-effectiveness
We assessed the cost-effectiveness of the MiniMed Paradigm Veo system and the Vibe and G4 PLATINUM CGM system compared with stand-alone CSII + CGM, CSII + SMBG, MDI + CGM and MDI + SMBG for the management of T1DM in adults.
In addition to the literature limitations regarding the population subgroups of interest (i.e. children and pregnant women) mentioned above, the model employed to conduct the cost-effectiveness analyses, the IMS CDM, is not suitable for modelling long-term outcomes for child/adolescent or pregnant woman populations, because all of the background risk adjustment/risk factor progression equations are based on adult populations.
The comparator MDI + CGM was not included in the cost-effectiveness analyses as no relevant evidence for this comparator was found in the systematic review. Moreover, in the absence of data comparing the clinical effectiveness of integrated CSII + CGM systems with stand-alone CSII + CGM systems, we assumed, in our analyses, that both technologies would be equally effective, which seems to be plausible. The immediate consequence of this assumption was that stand-alone CSII + CGM systems dominated the integrated CSII + CGM systems since the stand-alone system was cheaper, according to our estimated cost, while being equally effective.
Overall, the cost-effectiveness results suggest that the technologies using SMBG (either with CSII or MDIs) are more likely to be cost-effective, since the higher quality of life provided by the technologies that use CGM does not outweigh their higher costs. This is in line with the findings in the currently updated T1DM guideline,81 in which CGM was compared with several SMBG setups and was found not to be cost-effective. In particular, the base-case results show that MDI + SMBG is the cheapest treatment, but also the one that provides the lowest number of QALYs. The ICER of CSII + SMBG compared with MDI + SMBG is £52,381. The MiniMed Paradigm Veo system is extendedly dominated by stand-alone CSII + CGM. This is mainly because, according to our systematic review, the decrease in HbA1c levels with respect to baseline was highest for integrated CSII + CGM, and this decrease in HbA1c leads to a decrease in the number of complications that occur over a lifetime to such an extent that it compensates for the higher number of severe hypoglycaemic events. In any case, the ICER of stand-alone CSII + CGM compared with CSII + SMBG was £660,376. Thus, given the common threshold ICER of £30,000, it is clear that stand-alone CSII + CGM would not be cost-effective.
We also considered two additional base-case analyses. Since insulin pumps are recommended for people with T1DM for whom MDIs are not suitable, we excluded MDI-containing technologies from the analysis. In this scenario, the CSII + SMBG appeared to be the strategy most likely to be cost-effective, with a cost-effectiveness probability equal to almost 1 for all of the ceiling ratios considered in the analysis. Following this, we also excluded SMBG treatments from the analysis in order to capture the effect of the LGS function of the MiniMed Paradigm Veo system, which is expected to have an influence on reducing the number of severe hypoglycaemic events, and thus on the number of QALYs gained. In this situation, the only relevant comparison was the MiniMed Veo system versus stand-alone CSII + CGM, since the Vibe and G4 PLATINUM CGM system was dominated by the stand-alone combination of CSII and CGM. The corresponding results showed that when the MiniMed Veo system was compared with stand-alone CSII + CGM, the ICER obtained was high (£422,849). However, this results from both negative incremental QALYs and incremental costs (i.e. the ICER is in the south-west quadrant of the cost-effectiveness plane). In this case, the higher the ICER, the better (i.e. any cost saving could be used on other patients in order to generate QALYs that could ‘outweigh’ the loss in QALYs). Therefore, at a ceiling ratio of £30,000 per QALY, the MiniMed Veo system would be more cost-effective than stand-alone CSII + CGM. This is demonstrated by the corresponding CEACs, since the MiniMed Paradigm Veo system is the CGM treatment most likely to be cost-effective for all of the ceiling ratios considered in the analysis. However, as the ceiling ratio increases, the CEACs for the MiniMed Paradigm Veo system and stand-alone CSII + CGM seem to converge. As expected, the PSA showed that, for the Vibe and G4 PLATINUM CGM system, the probability of this system being cost-effective is always zero for all of the ceiling ratios considered in the analysis.
The results of these different scenario analyses did not differ much from the base-case results. The scenario that was most favourable with regard to the MiniMed Paradigm Veo system was the one that considered an additional utility decrement associated with the fear of hypoglycaemia. In this scenario, the ICER of the MiniMed Paradigm Veo system compared with CSII + SMBG was equal to £74,088 (the lowest found in all analyses). However, given the common threshold ICER of £30,000, the MiniMed Paradigm Veo system would not be considered cost-effective. For the Vibe and G4 PLATINUM CGM system, when it was not (extendedly) dominated by other strategies, the lowest ICER obtained was £428,595 when compared with the MiniMed Paradigm Veo system. This was also the case for the scenario in which a utility increment associated with reducing the fear of hypoglycaemia was considered.
Strengths and limitations of the assessment
Clinical effectiveness
Overall, the evidence seems to suggest that the MiniMed Paradigm Veo system reduces hypoglycaemic events in comparison with other treatments, without any differences in other outcomes, including change in HbA1c levels. In addition, we found significant results in favour of the integrated CSII + CGM system in comparison with MDI + SMBG with regard to HbA1c levels and quality of life. However, the evidence base was poor. The quality of included studies was generally low and often there was only one study that compared treatments in a specific population and at a specific follow-up time. In particular, the evidence for the two interventions of interest was limited, with only one study comparing the MiniMed Paradigm Veo system with an integrated CSII + CGM system,32 and only one study, in a mixed population, comparing the MiniMed Paradigm Veo system with CSII + SMBG. 33 In addition, although several studies included the integrated CSII + CGM system as a treatment arm, it is important to note that none of these studies looked at the Vibe and G4 PLATINUM CGM system; in the included studies, the integrated CSII + CGM system was always a MiniMed Paradigm pump with integrated CGM system (MiniMed Paradigm REAL-Time 722 System). This also means that all of the studies that assessed the effectiveness of the integrated CSII + CGM system were performed in the USA. Overall, only 337,41,45 out of the 19 included studies included patients from the UK, and only one of these was completely performed in the UK (Thomas et al. ). 45 Interactions between patients and health-care providers may show considerable differences in different countries, which will affect patients’ behaviour and therefore the effectiveness of insulin pumps and monitors. Therefore, the results from the included studies may not be representative of the UK situation.
Unfortunately, many studies had to be excluded because they compared CSII with MDIs, without specifying the type of monitoring, or CGM with SMBG, without specifying the type of insulin delivery. Two studies149,150 with 2 × 2 factorial design, including CSII + CGM, CSII + SMBG, MDI + CGM and MDI + SMBG, had to be excluded because the results were reported for only CSII versus MDIs and CGM versus SMBG. One of these studies was in children (Mauras et al. 149) and one was in adults [Little et al. (HypoCOMPaSS trial. 150)] These studies were excluded because they could not be classified as one of the relevant comparators defined by NICE and they could not be compared with the MiniMed Paradigm Veo system or an integrated CSII + CGM system.
In addition, we had problems differentiating stand-alone and integrated CSII + CGM interventions because the interventions were often poorly described, making it difficult to be sure which type of intervention was used. Sometimes researchers indicated no differences between these two types of treatments and provided patients in the same treatment arm with stand-alone and integrated CSII + CGM systems (see Beck151).
Four of the included studies were in mixed populations (Ly et al. 33 used 65 children and 30 adults with an age range of 4–50 years; O’Connell et al. 35 used 32 children and 30 adults with an age range of 13–40 years; RealTrend36 used 51 children and 81 adults with an age range of 2–65 years; and Hirsch et al. 34 used 40 children and 98 adults with an age range of 12–72 years). The advice from clinical experts was not to combine results from adults and children and vice versa. Therefore, these studies were, in the first instance, excluded from our analyses. Only if results were reported separately for adults and children were results included in the analyses or if there would have been no data without using a mixed adult/child population study, as in the case of Ly et al. ,33 which was used as a study in children to make a comparison between the MiniMed Paradigm Veo system and other treatments.
As reported in Chapter 1 of this report (see Comparators), there is a problem with the comparability of populations in studies evaluating insulin pumps and MDIs. NICE recommended CSII as a potential treatment for children ≥ 12 years and adults, who have disabling hypoglycaemia (including anxiety about hypoglycaemia) when trying to attain HbA1c < 7.5%, or HbA1c is constantly > 8.5%, while undergoing MDIT. 14 In other words, insulin pumps are recommended for people with T1DM for whom MDIs are not suitable. Therefore, it was anticipated that it would be problematic finding studies comparing insulin pumps (especially modern pumps such as the integrated systems) with MDIs in similar populations.
Most studies comparing CSII with MDIs show no difference with regard to HbA1c levels. One trial found a significant difference in the change in HbA1c levels at follow-up (DeVries et al. 42). In this trial, patients with persistent poor control, defined as a mean of all HbA1c levels of ≥ 8.5% in the 6 months before the trial, were included. Partly based on this trial, NICE14 concluded that CSII would most likely be cost-effective in patients with poorly controlled diabetes. Our current systematic review shows that nothing has changed in the evidence base with regard to CSII versus MDIs. The trial by DeVries et al. 42 is still the only trial showing significant differences in HbA1c levels at follow-up between CSII + SMBG and MDI + SMBG. This highlights the problem with identifying the correct population for comparisons between the interventions relevant to this appraisal. For the comparison of the MiniMed Paradigm Veo system with the integrated CSII + CGM system, we have included a general population of T1DM patients. However, if we compare these interventions with CSII + SMBG or MDI + SMBG in general populations, we will obscure the differences that exist between CSII and MDIs in diabetes patients with poor control at baseline.
For the comparison of CSII with MDIs, it is important to differentiate between populations with good HbA1c control at baseline and populations with poor control. However, if we compare the MiniMed Paradigm Veo system with the integrated CSII + CGM system and with CSII + SMBG, all patients will be using a pump and, in most studies comparing different types of pumps, patients will have been using a pump for > 6 months. In such studies, baseline HbA1c levels will be relatively low because of long-term pump use. Therefore, it is difficult to assess how valid comparisons are between those patients and patients involved in trials comparing pump use with MDIs.
Given these problems resulting from the heterogeneity among RCT populations, we did not consider including any further observational studies, as these problems would be even more apparent if results from observational studies were compared.
For pregnant women, we found no studies looking at the MiniMed Paradigm Veo system or the integrated CSII + CGM system.
Cost-effectiveness
An important strength of the current cost-effectiveness evaluation is that we used a well-validated diabetes model (IMS CDM) that has been used for many assessments, including submissions for NICE. 14,81–85 In particular, this model was used to assess the cost-effectiveness of CSII versus MDIs for T1DM patients in a 2010 HTA report80 and in the current update of the NICE Guideline on T1DM (NG17). 81 Since 1999, the model has been used at Mount Hood conferences, during which health economic models on diabetes are compared with each other in terms of their structure, performance and validity. 86–88 Two major validation papers on the IMS CDM have been published to date. 89,90 The latest one,90 from 2014, is the basis for the technical model description provided in this report. This description is consistent with the latest version of the model (version 8.5). Given the degree of validation of the model, and in order to be in line with the currently updated T1DM guideline81 from which we sourced many input parameters, it was deemed important not to use an alternative model or develop a de novo cost-effectiveness model for this evaluation. The most recent unit cost data were obtained for the analyses, including detailed data on equipment costs obtained from the relevant companies.
Although many of our input parameters are the same as those described in NICE Guideline NG17,81 we have also considered interventions that were not assessed in the guideline. Furthermore, we have considered a large variety of scenarios and performed PSAs for all of them.
A major limitation of the model is that the IMS CDM is not appropriate for analysing health economic outcomes for paediatric/adolescent populations. This was reported in the 2010 assessment of CSII versus MDIs for T1DM patients80 and confirmed by the model developers, who also mentioned that the model is not appropriate for pregnant women either. Therefore, these two subgroup populations were not included in the cost-effectiveness analyses.
Another limitation of the IMS CDM is that not all input parameters can be included in a PSA because of the technical constraints of the model. It is likely that the most important parameter not included in the PSA was the rate of severe hypoglycaemic events, as this is considered to be one of the key drivers of the model results, especially with regard to the MiniMed Paradigm Veo system. As a consequence, the uncertainty regarding the ICERs is currently somewhat underestimated. However, the ICERs themselves are not influenced by this limitation.
Another major limitation is the lack of comparability of treatments and clinical trials to estimate the treatment effect for stand-alone CSII + CGM. In the current analysis, we had to assume equal effectiveness of integrated and stand-alone CSII + CGM, thus assuring that stand-alone CSII + CGM would always dominate integrated CSII + CGM. Moreover, it was difficult to determine the extent to which the effect of the LGS function of the MiniMed Paradigm Veo system was captured in the model results. Furthermore, we found no reliable data on minor hypoglycaemic events and DKA events. The impact of these parameters on the cost-effectiveness results is difficult to predict, but we expect them to have less of an impact than the other treatment effect parameters (e.g. reduction in HbA1c levels and rate of severe hypoglycaemic events).
Finally, information was limited for the estimation of the cost of the stand-alone insulin pump. Although we do not expect a large difference in our estimated costs, it may have a major implication for the comparison of stand-alone CSII + CGM versus the integrated Vibe and G4 PLATINUM CGM system, as both are equally effective. Thus, depending on the price, one of these two options will dominate the other.
Uncertainties
Clinical effectiveness
The main uncertainties with regard to clinical effectiveness are the general lack of data (especially for children and pregnant women) and the poor quality of the available data. In addition, there were problems with differentiating interventions (in particular integrated and stand-alone CSII + CGM systems) and with interpreting results from mixed populations (adults and children).
Because of inherent differences in patient characteristics at baseline, it was difficult to compare MDI + SMBG with any of the other interventions in this assessment.
Cost-effectiveness
The uncertainties described for clinical effectiveness also apply to the assessment of cost-effectiveness. In addition, it is uncertain how realistic it is to assume a continuous increase in HbA1c levels over the first year of treatment. It seems likely that, in clinical practice, efforts would be made to keep HbA1c levels as low as possible, so periods of increase may be followed by decreases. It is unclear at this moment what the most realistic scenario will be in the long term.
Chapter 6 Conclusions
Implications for service provision
Overall, the limited evidence seems to suggest that the MiniMed Paradigm Veo system reduces hypoglycaemic events in comparison with other treatments, without any differences in other outcomes, including change in HbA1c levels. In addition, we found significant results in favour of the integrated CSII + CGM system over MDI + SMBG with regard to HbA1c levels and quality of life. However, the evidence base was poor. The quality of included studies was generally low and there was often only one study to compare treatments in a specific population and at a specific follow-up time. In particular, the evidence for the two interventions of interest was limited, with only one study comparing the MiniMed Paradigm Veo system with an integrated CSII + CGM system, and only one study, in a mixed population, comparing the MiniMed Paradigm Veo system with CSII + SMBG.
Cost-effectiveness analyses indicated that MDI + SMBG is the option most likely to be cost-effective, given the current threshold of £30,000 per QALY gained, whereas integrated CSII + CGM systems and MiniMed Paradigm Veo are dominated and extendedly dominated, respectively, by stand-alone CSII + CGM. Scenario analyses, used to assess the potential impact of changing various input parameters, did not alter these conclusions. No cost-effectiveness modelling was conducted for children and pregnant women.
Suggested research priorities
In adults, a trial comparing the MiniMed Paradigm Veo system with CSII + SMBG is warranted. Similarly, a trial comparing the integrated CSII + CGM system with CSII + SMBG is warranted.
In children, a trial comparing the MiniMed Paradigm Veo system with the integrated CSII + CGM system is warranted. Similarly, a trial comparing the integrated CSII + CGM system with CSII + SMBG is warranted.
For pregnant women, trials comparing the MiniMed Paradigm Veo system and the integrated CSII + CGM system with other interventions are warranted.
Future trials should include longer-term follow-up and include EQ-5D (besides more disease-specific quality-of-life questionnaires) at various time points with a view to informing improved cost-effectiveness modelling.
Acknowledgements
The authors acknowledge the clinical advice and expert opinions provided by the following specialist Diagnostic Assessment Committee members:
-
Dr Karen Anthony, Consultant in Diabetes and Endocrinology, the Whittington Hospital NHS Trust
-
Mrs Joanne Buchanan, Diabetes Specialist Nurse, Portsmouth Hospital Trust
-
Dr Andrew Day, Consultant Medical Biochemist, University Hospitals Bristol NHS Foundation Trust.
In addition, the authors acknowledge the clinical advice and expert opinion provided by:
-
Dr Nick Oliver, Consultant Diabetologist, Imperial College Healthcare NHS Trust.
Contributions of authors
Rob Riemsma was involved in planning and performing this systematic review and interpreting the evidence.
Isaac Corro Ramos was involved in planning and performing the cost-effectiveness analyses and interpreting the results.
Richard Birnie was involved in planning and performing this systematic review and interpreting the evidence.
Nasuh Büyükkaramikli was involved in planning and performing the cost-effectiveness analyses and interpreting the results.
Nigel Armstrong contributed to the planning and interpretation of the cost-effectiveness analyses and the acquisition of input data for modelling.
Steve Ryder contributed to obtaining the input data for the modelling.
Steven Duffy devised and performed the literature searches and provided information support to the project.
Gill Worthy was involved in planning and performing this systematic review and interpreting the evidence.
Maiwenn Al was involved in planning and performing the cost-effectiveness analyses and interpreting the results.
Johan Severens provided senior advice and support to the assessment.
Jos Kleijnen provided senior advice and support to the assessment.
All of the authors were involved in drafting and/or commenting on the report.
Data sharing statement
Study characteristics and results of trials included in the systematic review of the effectiveness of interventions are provided in Appendix 3. Details of disease natural history parameters and transition probabilities are provided in Appendix 7, and results (full incremental and intervention vs. comparator) of base-case and scenario analyses are provided in Appendix 8. All data created during this research are available by request from the authors.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- National Institute for Health and Care Excellence . Type 1 Diabetes: Diagnosis And Management of Type 1 Diabetes in Adults. Final Scope 2012. www.nice.org.uk/guidance/gid-cgwaver122/resources/type-1-diabetes-update-final-scope2 (accessed 31 July 2014).
- NHS Choices . Diabetes 2012. www.nhs.uk/Conditions/Diabetes/Pages/Diabetes.aspx (accessed 31 July 2014).
- Yorkshire and Humber Health Intelligence . Diabetes Prevalence Model (APHO) 2010. www.yhpho.org.uk/default.aspx?RID=81090 (accessed 31 July 2014).
- National Institute for Health and Care Excellence . Clinical Knowledge Summaries. Diabetes – Type 1 2012. http://cks.nice.org.uk/diabetes-type-1 (accessed 31 July 2014).
- Type 2 Diabetes: Management of Type 2 Diabetes in Adults. Final Scope. London: NICE; 2012.
- Juvenile Diabetes Research Foundation Ltd . What Is the Optimal Blood Glucose (Sugar) Range? n.d. www.jdrf.org.uk/life-with-type-1-diabetes/faq-about-type-1-diabetes/what-is-the-optimal-blood-glucose-sugar-range (accessed 19 May 2015).
- The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86. http://dx.doi.org/10.1056/NEJM199309303291401.
- UK Prospective Diabetes Study Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53. http://dx.doi.org/10.1016/S0140-6736(98)07019-6.
- Ritz E, Rychlík I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis 1999;34:795-808. http://dx.doi.org/10.1016/S0272-6386(99)70035-1.
- Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA 2003;290:2057-60. http://dx.doi.org/10.1001/jama.290.15.2057.
- Diabetes UK . State of the Nation 2012: England 2012. www.diabetes.org.uk/Documents/Reports/State-of-the-Nation-2012.pdf (accessed 18 May 2015).
- Scottish Study Group for the Care of the Young with Diabetes . A longitudinal observational study of insulin therapy and glycaemic control in Scottish children with type 1 diabetes: DIABAUD 3. Diabet Med 2006;23. http://dx.doi.org/10.1111/j.1464-5491.2006.01962.x.
- The Healthcare Quality Improvement Partnership . National Paediatric Diabetes Audit Report 2011–12. Part 1 Care Processes and Outcomes 2013. www.rcpch.ac.uk/child-health/standards-care/clinical-audit-and-quality-improvement/national-paediatric-diabetes-au-1 (accessed 31 July 2014).
- National Institute for Health and Care Excellence . Continuous Subcutaneous Insulin Infusion for the Treatment of Diabetes Mellitus. NICE Technology Appraisal Guidance 151 n.d. www.nice.org.uk/Guidance/TA151 (accessed 8 July 2014).
- Heller S. Sudden death and hypoglycaemia. Diabetic Hypoglycemia 2008;1:2-7.
- Barnard K, Thomas S, Royle P, Noyes K, Waugh N. Fear of hypoglycaemia in parents of young children with type 1 diabetes: a systematic review. BMC Pediatr 2010;10. http://dx.doi.org/10.1186/1471-2431-10-50.
- DAFNE Study Group . Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325. http://dx.doi.org/10.1136/bmj.325.7367.746.
- Markowitz JT, Pratt K, Aggarwal J, Volkening LK, Laffel LM. Psychosocial correlates of continuous glucose monitoring use in youth and adults with type 1 diabetes and parents of youth. Diabetes Technol Ther 2012;14:523-6. http://dx.doi.org/10.1089/dia.2011.0201.
- Barnard KD, Wysocki T, Allen JM, Elleri D, Thabit H, Leelarathna L, et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diab Res Care 2014;2. http://dx.doi.org/10.1136/bmjdrc-2014-000025.
- Choudhary P, Shin J, Wang Y, Evans ML, Hammond PJ, Kerr D, et al. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care 2011;34:2023-5. http://dx.doi.org/10.2337/dc10-2411.
- Choudhary P. Insulin pump therapy with automated insulin suspension: toward freedom from nocturnal hypoglycemia. JAMA 2013;310:1235-6. http://dx.doi.org/10.1001/jama.2013.278576.
- Mensh BD, Wisniewski NA, Neil BM, Burnett DR. Susceptibility of interstitial continuous glucose monitor performance to sleeping position. J Diabetes Sci Technol 2013;7:863-70.
- Didangelos T, Iliadis F. Insulin pump therapy in adults. Diabetes Res Clin Pract 2011;93:109-13. http://dx.doi.org/10.1016/S0168-8227(11)70025-0.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009. www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm (accessed 8 July 2014).
- National Institute for Health and Clinical Excellence . Diagnostics Assessment Programme Manual 2011. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-diagnostics-guidance/Diagnostics-assessment-programme-manual.pdf (accessed 8 July 2014).
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [Updated March 2011] 2011. www.cochrane-handbook.org/ (accessed 8 July 2014).
- Canadian Agency for Drugs and Technologies in Health . CADTH Peer Review Checklist for Search Strategies 2013. www.cadth.ca/en/resources/finding-evidence-is (accessed 8 July 2014).
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. http://dx.doi.org/10.1016/0197-2456(86)90046-2.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. http://dx.doi.org/10.1136/bmj.315.7109.629.
- Song F, Loke YK, Walsh T, Glenny A, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b1147.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. http://dx.doi.org/10.1016/S0895-4356(97)00049-8.
- Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224-32. http://dx.doi.org/10.1056/NEJMoa1303576.
- Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA 2013;310:1240-7. http://dx.doi.org/10.1001/jama.2013.277818.
- Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, et al. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther 2008;10:377-83. http://dx.doi.org/10.1089/dia.2008.0068.
- O’Connell MA, Donath S, O’Neal DN, Colman PG, Ambler GR, Jones TW, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia 2009;52:1250-7. http://dx.doi.org/10.1007/s00125-009-1365-0.
- Raccah D, Sulmont V, Reznik Y, Guerci B, Renard E, Hanaire H, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care 2009;32:2245-50. http://dx.doi.org/10.2337/dc09-0750.
- Hermanides J, Nørgaard K, Bruttomesso D, Mathieu C, Frid A, Dayan CM, et al. Sensor-augmented pump therapy lowers HbA1c in suboptimally controlled type 1 diabetes; a randomized controlled trial. Diabet Med 2011;28:1158-67. http://dx.doi.org/10.1111/j.1464-5491.2011.03256.x.
- Lee SW, Sweeney T, Clausen D, Kolbach C, Hassen A, Firek A, et al. Combined insulin pump therapy with real-time continuous glucose monitoring significantly improves glycemic control compared to multiple daily injection therapy in pump naive patients with type 1 diabetes; single center pilot study experience. J Diabetes Sci Technol 2007;1:400-4. http://dx.doi.org/10.1177/193229680700100313.
- Peyrot M, Rubin RR. Patient-reported outcomes for an integrated real-time continuous glucose monitoring/insulin pump system. Diabetes Technol Ther 2009;11:57-62. http://dx.doi.org/10.1089/dia.2008.0002.
- Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311-20. http://dx.doi.org/10.1056/NEJMoa1002853.
- Bolli GB, Kerr D, Thomas R, Torlone E, Sola-Gazagnes A, Vitacolonna E, et al. Comparison of a multiple daily insulin injection regimen (basal once-daily glargine plus mealtime lispro) and continuous subcutaneous insulin infusion (lispro) in type 1 diabetes: a randomized open parallel multicenter study. Diabetes Care 2009;32:1170-6. http://dx.doi.org/10.2337/dc08-1874.
- DeVries JH, Snoek FJ, Kostense PJ, Masurel N, Heine RJ. Dutch Insulin Pump Study Group . A randomized trial of continuous subcutaneous insulin infusion and intensive injection therapy in type 1 diabetes for patients with long-standing poor glycemic control. Diabetes Care 2002;25:2074-80. http://dx.doi.org/10.2337/diacare.25.11.2074.
- Nosadini R, Velussi M, Fioretto P, Doria A, Avogaro A, Trevisan R, et al. Frequency of hypoglycaemic and hyperglycaemic-ketotic episodes during conventional and subcutaneous continuous insulin infusion therapy in IDDM. Diabetes Nutr Metab 1988;1:289-96.
- Brinchmann-Hansen O, Dahl-Jorgensen K, Hanssen KF, Sandvik L. Effects of intensified insulin treatment on various lesions of diabetic retinopathy. Am J Ophthalmol 1985;100:644-53. http://dx.doi.org/10.1016/0002-9394(85)90618-X.
- Thomas RM, Aldibbiat A, Griffin W, Cox MAA, Leech NJ, Shaw JAM. A randomized pilot study in type 1 diabetes complicated by severe hypoglycaemia, comparing rigorous hypoglycaemia avoidance with insulin analogue therapy, CSII or education alone. Diabet Med 2007;24:778-83. http://dx.doi.org/10.1111/j.1464-5491.2007.02196.x.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001;24:1722-7. http://dx.doi.org/10.2337/diacare.24.10.1722.
- Weintrob N, Benzaquen H, Galatzer A, Shalitin S, Lazar L, Fayman G, et al. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Pediatrics 2003;112:559-64. http://dx.doi.org/10.1542/peds.112.3.559.
- Thrailkill KM, Moreau CS, Swearingen C, Rettiganti M, Edwards K, Morales AE, et al. Insulin pump therapy started at the time of diagnosis: effects on glycemic control and pancreatic beta-cell function in type 1 diabetes. Diabetes Technol Ther 2011;13:1023-30. http://dx.doi.org/10.1089/dia.2011.0085.
- Doyle EA, Weinzimer SA, Steffen AT, Ahern JAH, Vincent M, Tamborlane WV. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care 2004;27:1554-8. http://dx.doi.org/10.2337/diacare.27.7.1554.
- Nosari I, Maglio ML, Lepore G, Cortinovis F, Pagani G. Is continuous subcutaneous insulin infusion more effective than intensive conventional insulin therapy in the treatment of pregnant diabetic women?. Diabetes Nutr Metab 1993;6:33-7.
- Lawson ML, Bradley B, McAssey K, Clarson C, Kirsch SE, Mahmud FH, et al. The JDRF CCTN CGM TIME trial: Timing of Initiation of continuous glucose Monitoring in Established pediatric type 1 diabetes: study protocol, recruitment and baseline characteristics. BMC Pediatr 2014;14. http://dx.doi.org/10.1186/1471-2431-14-183.
- Troub T, Shin J, Oroszlan G. The ASPIRE-2 Study of Automatic Insulin Suspension: Design, Methods, and Interim Baseline Characteristics. Paper presented at the 12th annual Diabetes Technology Meeting, 8–10 November 2012, Bethesda, USA, abstract no. 135. J Diabetes Sci Technol 2013;7.
- Blair J, Annan F, Didi M, Gamble C, Gregory J, Hughes D, et al. Randomised Controlled Trial of Continuous Subcutaneous Insulin Infusion Compared to Multiple Daily Injection Regimens in Children and Young People at Diagnosis of Type I Diabetes Mellitus 2010. www.nets.nihr.ac.uk/projects/hta/081439 (accessed 5 September 2014).
- Assistance Publique – Hôpitaux de Paris . Study of Insulin Therapy Augmented by Real Time Sensor in Type 1 Children and Adolescents (START-IN!) 2012. http://ClinicalTrials.gov/show/NCT00949221 (accessed 5 September 2014).
- Medtronic . Effect of CSII and CGM on Progression of Late Diabetic Complications 2011. http://ClinicalTrials.gov/show/NCT01454700 (accessed 5 September 2014).
- Threshold Suspend in Pediatrics at Home. 2014.
- DexCom Inc . CGM Treatment in Patients With Type 1 Diabetes Treated With Insulin Injections 2014. http://ClinicalTrials.gov/show/NCT02092051 (accessed 5 September 2014).
- University of British Columbia . Comparison of Insulin Pump and MDI for Pregestational Diabetes During Pregnancy 2014. http://ClinicalTrials.gov/show/NCT02064023 (accessed 5 September 2014).
- Harrogate & District NHS Foundation Trust . The REPOSE (Relative Effectiveness of Pumps Over MDI and Structured Education) Trial 2011. http://ClinicalTrials.gov/show/NCT01616784 (accessed 5 September 2014).
- Medtronic . The Effectiveness of Continuous Glucose Monitoring in Diabetes Treatment for Infants and Young Children (Gerber RTSA) 2011. http://ClinicalTrials.gov/show/NCT00875290 (accessed 5 September 2014).
- Nemours Children’s Clinic . Insulin Pump Therapy in Adolescents With Newly Diagnosed Type 1 Diabetes (T1D) 2015. http://ClinicalTrials.gov/show/NCT00357890 (accessed 5 September 2014).
- Addenbrooke’s NHS Trust . A Randomised Controlled Study of Continuous Subcutaneous Insulin Infusion (CSII) Therapy Compared to Conventional Bolus Insulin Treatment in Preschool Aged Children With Type 1 Diabetes 2006. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2005-004526-72 (accessed 5 September 2014).
- Medtronic Australasia . Does Real-Time Patient Analysis of Continuous Glucose Monitor Data Improve Glycaemic Control in Patients With Type 1 Diabetes on Insulin Pump Therapy? 2006. www.anzctr.org.au/ACTRN12606000049572.aspx (accessed 5 September 2014).
- Juvenile Diabetes Research Foundation . Predictive Low Glucose Management (PLGM) Trial: Comparing Insulin Pump Therapy With Predictive Low Glucose Suspend Feature Versus Standard Sensor Augmented Pump Therapy in Patients With Type 1 Diabetes 2014. www.anzctr.org.au/ACTRN12614000510640.aspx (accessed 5 September 2014).
- The Royal Children’s Hospital Melbourne . A Randomized Controlled Trial Comparing the Impact of Continuous Subcutaneous Insulin Infusion (CSII) Therapy and Multiple Daily Injection (MDI) Regimens Upon Indices of Behaviour, Cognition and Glycaemia in Children and Adolescents With Type 1 Diabetes 2010. www.anzctr.org.au/ACTRN12610000605099.aspx (accessed 5 September 2014).
- Roche Australia Pty Ltd . Integrated Blood Glucose Monitoring With Insulin Pumps Versus Standard Method – A Randomised Crossover Trial 2011. www.anzctr.org.au/ACTRN12611000142932.aspx (accessed 5 September 2014).
- Alder Hey Children’s NHS Foundation Trust . SCIPI-Subcutaneous Insulin: Pumps or Injections 2010. http://isrctn.org/ISRCTN29255275 (accessed 5 September 2014).
- University of Schleswig-Holstein . Psychosocial Issues in Insulin Pump Therapy in Children With Type 1 DM 2011. http://clinicaltrials.gov/show/NCT01338922 (accessed 5 September 2014).
- Kamble S, Schulman KA, Reed SD. Cost-effectiveness of sensor-augmented pump therapy in adults with type 1 diabetes in the United States. Value Health 2012;15:632-8. http://dx.doi.org/10.1016/j.jval.2012.02.011.
- Ly TT, Brnabic AJM, Eggleston A, Kolivos A, McBride ME, Schrover R, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health 2014;17:561-9. http://dx.doi.org/10.1016/j.jval.2014.05.008.
- Kamble S, Perry BM, Shafiroff J, Schulman KA, Reed SD. The cost-effectiveness of initiating sensor-augmented pump therapy versus multiple daily injections of insulin in adults with type 1 diabetes: evaluating a technology in evolution. Paper presented at the 16th annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), 21–25 May 2011, Baltimore, USA, abstract no. 82. Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.02.458.
- Gomez A, Alfonso-Cristancho R, Prieto-Salamanca D, Valencia JE, Lynch P, Roze S. Clinical impact of sensor-augmented insulin pump (SAP) therapy in type 1 diabetes long-term related complications in Colombia. Paper presented at the 7th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD), 5–8 February 2014, Vienna, Austria. Diabetes Technol Ther 2014;16:A67-A68.
- Gomez A, Alfonso-Cristancho R, Prieto-Salamanca D, Valencia JE, Lynch P, Roze S. Health economic benefits of sensor augmented insulin pump therapy in Colombia. Paper presented at ISPOR 4th Latin America Conference, 12–14 September 2013, Buenos Aires, Argentina. Value Health 2013;16. http://dx.doi.org/10.1016/j.jval.2013.08.2059.
- Lindholm Olinder A, Hanas R, Heintz E, Jacobson S, Johansson UB, Olsson PO, et al. CGM and SAP are valuable tools in the treatment of diabetes: a Swedish health technology assessment. Paper presented at the 7th International Conference on Advanced Technologies and Treatments for Diabetes, 5–8 February 2014, Vienna, Austria. Diabetes Technol Ther 2014;16.
- Roze S, Payet V, Debroucker F, de Portu S, Cucherat M. Projection of long term health-economic benefits of sensor augmented pump (SAP) versus pump therapy alone (CSII) in uncontrolled type 1 diabetes in France. Paper presented at ISPOR 17th Annual European Congress, 8–12 November 2014, Amsterdam, the Netherlands. Value Health 2014;17.
- Roze S, Cook M, Jethwa M, de Portu S. Projection of long term health-economic benefits of sensor augmented pump (SAP) versus pump therapy alone (CSII) in type 1 diabetes, a UK perspective. Paper presented at ISPOR 17th Annual European Congress, 8–12 November 2014, Amsterdam, the Netherlands. Value Health 2014;17.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health 2014;17:462-70. http://dx.doi.org/10.1016/j.jval.2014.03.003.
- Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20:5-26. http://dx.doi.org/10.1185/030079904X1980.
- Cummins E, Royle P, Snaith A, Greene A, Robertson L, McIntyre L, et al. Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14110.
- National Institute for Health and Care Excellence . Type 1 Diabetes in Adults: Diagnosis and Management. NICE Guideline (NG17) 2015. www.nice.org.uk/guidance/indevelopment/gid-cgwaver122/documents (accessed 15 January 2015).
- National Institute for Health and Care Excellence . Dapagliflozin in Combination Therapy for Treating Type 2 Diabetes. NICE Technology Appraisal Guidance 288 2013. www.nice.org.uk/Guidance/TA288 (accessed 26 January 2015).
- National Institute for Health and Care Excellence . Liraglutide for the Treatment of Type 2 Diabetes Mellitus. NICE Technology Appraisal Guidance 203 2014. www.nice.org.uk/Guidance/TA203 (accessed 26 January 2015).
- National Institute for Health and Care Excellence . Exenatide Prolonged-Release Suspension for Injection in Combination With Oral Antidiabetic Therapy for the Treatment of Type 2 Diabetes. NICE Technology Appraisal Guidance 248 2014. www.nice.org.uk/Guidance/TA248 (accessed 26 January 2015).
- National Institute for Health and Care Excellence . Canagliflozin in Combination Therapy for Treating Type 2 Diabetes. NICE Technology Appraisal Guidance 315 n.d. www.nice.org.uk/Guidance/TA315 (accessed 26 January 2015).
- The Mount Hood 4 Modeling Group . Computer modeling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diabetes Care 2007;30:1638-46. http://dx.doi.org/10.2337/dc07-9919.
- Brown JB, Palmer AJ, Bisgaard P, Chan W, Pedula K, Russell A. The Mt. Hood challenge: cross-testing two diabetes simulation models. Diabetes Res Clin Pract 2000;50:57-64. http://dx.doi.org/10.1016/S0168-8227(00)00217-5.
- Palmer AJ, Clarke P, Gray A, Leal J, Lloyd A, . Mount Hood 5 Modeling Group . Computer modeling of diabetes and its complications: a report on the Fifth Mount Hood challenge meeting. Value Health 2013;16:670-85. http://dx.doi.org/10.1016/j.jval.2013.01.002.
- Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin 2004;20:27-40. http://dx.doi.org/10.1185/030079904X2006.
- McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE Diabetes Model. Value Health 2014;17:714-24. http://dx.doi.org/10.1016/j.jval.2014.07.007.
- Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747-59. http://dx.doi.org/10.1007/s00125-004-1527-z.
- The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes 1996;45:1289-98. http://dx.doi.org/10.2337/diab.45.10.1289.
- D’Agostino RB, Russell MW, Huse DM, Ellison RC, Silbershatz H, Wilson PW, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J 2000;139:272-81. http://dx.doi.org/10.1016/S0002-8703(00)90236-9.
- World Health Organization . WHO Mortality Database 2014. www.who.int/healthinfo/mortality_data/en/ (accessed 26 January 2015).
- Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med 1999;159:1197-204. http://dx.doi.org/10.1001/archinte.159.11.1197.
- Kothari V, Stevens RJ, Adler AI, Stratton IM, Manley SE, Neil HA, et al. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke 2002;33:1776-81. http://dx.doi.org/10.1161/01.STR.0000020091.07144.C7.
- Murabito JM, D’Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation 1997;96:44-9. http://dx.doi.org/10.1161/01.CIR.96.1.44.
- Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671-9. http://dx.doi.org/10.1042/cs1010671.
- Health and Social Care Information Centre . National Diabetes Audit 2011–2012. Report 1: Care Processes and Treatment Targets 2013. https://catalogue.ic.nhs.uk/publications/clinical/diabetes/nati-diab-audi-11-12/nati-diab-audi-11-12-care-proc-rep.pdf (accessed 15 January 2015).
- Nathan DM, Zinman B, Cleary PA, Backlund J-YC, Genuth S, . Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005). Arch Intern Med 2009;169:1307-16. http://dx.doi.org/10.1001/archinternmed.2009.193.
- Office for National Statistics . Opinions and Lifestyle Survey, Smoking Habits Amongst Adults, 2012 2013. www.ons.gov.uk/ons/dcp171776_328041.pdf (accessed 16 January 2015).
- World Health Organization . Global Status Report on Alcohol and Health 2011. www.who.int/substance_abuse/publications/global_alcohol_report/msbgsruprofiles.pdf?ua=1 (accessed 16 January 2015).
- Health and Social Care Information Centre . Health Survey for England, 2011. Volume 1: Health, Social Care and Lifestyles: Cardiovascular Disease 2011. www.hscic.gov.uk/catalogue/PUB09300/HSE2011-Ch2-CVD.pdf (accessed 16 January 2015).
- Hopkins D, Lawrence I, Mansell P, Thompson G, Amiel S, Campbell M, et al. Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes: the U.K. DAFNE experience. Diabetes Care 2012;35:1638-42. http://dx.doi.org/10.2337/dc11-1579.
- Unit Costs of Health and Social Care. Canterbury: University of Kent; n.d.
- NHS England and Wales . Electronic Drug Tariff 2014. www.ppa.org.uk/edt/November_2014/mindex.htm (accessed 27 November 2014).
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured?. Ann Clin Biochem 2009;46:205-17. http://dx.doi.org/10.1258/acb.2009.009007.
- Department of Health . NHS Reference Costs 2012–2013 2013. www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013 (accessed 7 October 2014).
- National Institute for Health and Care Excellence . Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. NICE Guideline (CG181) 2014. http://guidance.nice.org.uk/CG181 (accessed 16 January 2015).
- National Clinical Guideline Centre . Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. Clinical Guideline: Methods, Evidence and Recommendations; July 2014 2014. www.nice.org.uk/guidance/cg181/evidence/cg181-lipid-modification-update-full-guideline3 (accessed 16 January 2015).
- National Institute for Health and Care Excellence . Peritoneal Dialysis: Peritoneal Dialysis in the Treatment of Stage 5 Chronic Kidney Disease. NICE Clinical Guideline 125 2011. http://guidance.nice.org.uk/CG125 (accessed 16 January 2015).
- National Institute for Health and Care Excellence . Glaucoma: Diagnosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension. NICE Clinical Guideline 85 2009. http://guidance.nice.org.uk/CG85 (accessed 16 January 2015).
- National Collaborating Centre for Acute Care . Glaucoma: Diagnosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension. Methods, Evidence and Recommendations 2009. www.nice.org.uk/guidance/cg85/resources/cg85-glaucoma-full-guideline2 (accessed 16 January 2015).
- Monthly Index of Medical Specialties . Duloxetine 2014. www.mims.co.uk/ (accessed 16 January 2015).
- National Institute for Health and Care Excellence . Neuropathic Pain: The Pharmacological Management of Neuropathic Pain in Adults in Non-Specialist Settings. NICE Clinical Guideline 96 2010. www.nice.org.uk/guidance/cg96 (accessed 16 January 2015).
- National Institute for Health and Care Excellence . Lower Limb Peripheral Arterial Disease: Diagnosis and Management. NICE Clinical Guideline 147 2012. http://guidance.nice.org.uk/CG147 (accessed 16 January 2015).
- National Clinical Guideline Centre . Lower Limb Peripheral Arterial Disease: Diagnosis and Management. NICE Clinical Guideline; Methods, Evidence and Recommendations. Draft for Consultation 2012. www.nice.org.uk/guidance/cg147/documents/lower-limb-peripheral-arterial-disease-guideline-review-full-guideline2 (accessed 16 January 2015).
- Ghatnekar O, Willis M, Persson U. Cost-effectiveness of treating deep diabetic foot ulcers with Promogran in four European countries. J Wound Care 2002;11:70-4. http://dx.doi.org/10.12968/jowc.2002.11.2.26675.
- Kerr M. Insight Health Economics . Foot Care for People With Diabetes: The Economic Case for Change 2012. www.diabetes.org.uk/Documents/nhs-diabetes/footcare/footcare-for-people-with-diabetes.pdf (accessed 16 January 2015).
- London New Drugs Group . Comparative Table of Insulin Pumps 2013. www.medicinesresources.nhs.uk/upload/documents/Evidence/Comparative%20table%20of%20insulin%20pumps.pdf (accessed 17 November 2015).
- White HD, Goenka N, Furlong NJ, Saunders S, Morrison G, Langridge P, et al. The U.K. service level audit of insulin pump therapy in adults. Diabet Med 2014;31. http://dx.doi.org/10.1111/dme.12325.
- Association of British Clinical Diabetologists . The United Kingdom Insulin Pump Audit – Service Level Data 2013. www.diabetes.org.uk/Documents/News/The_United_Kingdom_Insulin_Pump_Audit_May_2013.pdf (accessed 16 January 2015).
- Health and Social Care Information Centre . Prescription Cost Analysis – England, 2012 [NS] 2013. www.hscic.gov.uk/catalogue/PUB10610 (accessed 6 January 2015).
- Monitor, NHS England . National Tariff Payment System 2014 15 2013. www.gov.uk/government/publications/national-tariff-payment-system-2014-to-2015 (accessed 21 January 2015).
- Ara R, Brazier J. Comparing EQ-5D scores for comorbid health conditions estimated using 5 different methods. Med Care 2012;50:452-9. http://dx.doi.org/10.1097/MLR.0b013e318234a04a.
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9. http://dx.doi.org/10.1177/027298902400448902.
- Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006;22:1523-34. http://dx.doi.org/10.1185/030079906X115757.
- Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression, and quality of life: a population study. Diabetes Care 2004;27:1066-70. http://dx.doi.org/10.2337/diacare.27.5.1066.
- Minshall ME, Oglesby AK, Wintle ME, Valentine WJ, Roze S, Palmer AJ. Estimating the long-term cost-effectiveness of exenatide in the United States: an adjunctive treatment for type 2 diabetes mellitus. Value Health 2008;11:22-33. http://dx.doi.org/10.1111/j.1524-4733.2007.00211.x.
- Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, . Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545-59. http://dx.doi.org/10.1056/NEJMoa0802743.
- McMullin J, Brozek J, Jaeschke R, Hamielec C, Dhingra V, Rocker G, et al. Glycemic control in the ICU: a multicenter survey. Intensive Care Med 2004;30:798-803. http://dx.doi.org/10.1007/s00134-004-2242-4.
- Lyon KC. The case for evidence in wound care: investigating advanced treatment modalities in healing chronic diabetic lower extremity wounds. J Wound Ostomy Continence Nurs 2008;35:585-90. http://dx.doi.org/10.1097/01.WON.0000341471.41191.18.
- Jones LE, Doebbeling CC. Depression screening disparities among veterans with diabetes compared with the general veteran population. Diabetes Care 2007;30:2216-21. http://dx.doi.org/10.2337/dc07-0350.
- O’Meara S, Cullum N, Majid M, Sheldon T. Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration. Health Technol Assess 2000;4.
- Kantor J, Margolis DJ. Treatment options for diabetic neuropathic foot ulcers: a cost-effectiveness analysis. Dermatol Surg 2001;27:347-51. http://dx.doi.org/10.1097/00042728-200104000-00005.
- Lopez-Bastida J, Cabrera-Lopez F, Serrano-Aguilar P. Sensitivity and specificity of digital retinal imaging for screening diabetic retinopathy. Diabet Med 2007;24:403-7. http://dx.doi.org/10.1111/j.1464-5491.2007.02074.x.
- Cortes-Sanabria L, Martinez-Ramirez HR, Hernandez JL, Rojas-Campos E, Canales-Munoz JL, Cueto-Manzano AM. Utility of the Dipstick Micraltest II in the screening of microalbuminuria of diabetes mellitus type 2 and essential hypertension. Rev Invest Clin 2006;58:190-7.
- Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, . [Erratum published in N Engl J Med 2009;361:1914 . Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842-52. http://dx.doi.org/10.1056/NEJMoa066397.
- The Diabetes Control and Complications Trial Research Group . Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271-86. http://dx.doi.org/10.2337/diab.46.2.271.
- Lynch P, Attvall S, Persson S, Barsoe C, Gerdtham U. Routine use of personal continuous glucose monitoring system with insulin pump in Sweden. Paper presented at the 48th Annual Meeting of the European Association for the Study of Diabetes (EASD), 1–5 October 2012, Berlin, Germany. Diabetologia 2012;55.
- Dahl-Jorgensen K, Brinchmann-Hansen O, Hanssen KF, Ganes T, Kierulf P, Smeland E, et al. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. Br Med J 1986;293:1195-9. http://dx.doi.org/10.1136/bmj.293.6556.1195.
- Ben-Ami H, Nagachandran P, Mendelson A, Edoute Y. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med 1999;159:281-4. http://dx.doi.org/10.1001/archinte.159.3.281.
- Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617-21. http://dx.doi.org/10.2337/diacare.10.5.617.
- Couper JJ, Jones TW, Donaghue KC, Clarke CF, Thomsett MJ, Silink M. The Diabetes Control and Complications Trial. Implications for children and adolescents. Australasian Paediatric Endocrine Group. Med J Aust 1995;162:369-72.
- Rovet JF, Ehrlich RM, Hoppe M. Intellectual deficits associated with early onset of insulin dependent diabetes mellitus in children. Diabetes Care 1987;10:510-15. http://dx.doi.org/10.2337/diacare.10.4.510.
- Diabetes Control and Complications Trial Research Group . Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177-88. http://dx.doi.org/10.1016/S0022-3476(94)70190-3.
- Pinhas-Hamiel O, Hamiel U, Boyko V, Graph-Barel C, Reichman B, Lerner-Geva L. Trajectories of HbA1c levels in children and youth with type 1 diabetes. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0109109.
- National Institute for Health and Care Excellence . Diabetes (Type 1 and Type 2) in Children and Young People: Diagnosis and Management. NICE Guidelines (NG18) n.d. www.nice.org.uk/Guidance/NG18 (accessed 17 November 2015).
- Mauras N, Beck R, Xing DY, Ruedy K, Buckingham B, Tansey M, et al. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to < 10 years. Diabetes Care 2012;35:204-10. http://dx.doi.org/10.2337/dc11-1746.
- Little SA, Leelarathna L, Walkinshaw E, Tan HK, Chapple O, Lubina-Solomon A, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014;37:2114-22. http://dx.doi.org/10.2337/dc14-0030.
- Beck RW. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378-83. http://dx.doi.org/10.2337/dc09-0108.
- Lefebvre C, Manheimer E, Glanville J, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration; 2011.
- Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham study. Stroke 1991;22:312-18. http://dx.doi.org/10.1161/01.STR.22.3.312.
- Janghorbani MB, Jones RB, Allison SP. Incidence of and risk factors for cataract among diabetes clinic attenders. Ophthalmic Epidemiol 2000;7:13-25. http://dx.doi.org/10.1076/0928-6586(200003)711-2FT013.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975 through 1989. Neurology 1998;50:208-16. http://dx.doi.org/10.1212/WNL.50.1.208.
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341:1725-30. http://dx.doi.org/10.1056/NEJM199912023412303.
- Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology 2009;116:497-503. http://dx.doi.org/10.1016/j.ophtha.2008.10.016.
- Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, et al. Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol 2011;22:545-53. http://dx.doi.org/10.1681/ASN.2010040354.
- Grauslund J, Green A, Sjolie AK. Cataract surgery in a population-based cohort of patients with type 1 diabetes: long-term incidence and risk factors. Acta Ophthalmol 2011;89:25-9. http://dx.doi.org/10.1111/j.1755-3768.2009.01619.x.
- Lind M, Bounias I, Olsson M, Gudbjornsdottir S, Svensson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 2011;378:140-6. http://dx.doi.org/10.1016/S0140-6736(11)60471-6.
- Morioka T, Emoto M, Tabata T, Shoji T, Tahara H, Kishimoto H, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care 2001;24:909-13. http://dx.doi.org/10.2337/diacare.24.5.909.
- Wiesbauer F, Heinze G, Regele H, Horl WH, Schernthaner GH, Schwarz C, et al. Glucose control is associated with patient survival in diabetic patients after renal transplantation. Transplantation 2010;89:612-9. http://dx.doi.org/10.1097/TP.0b013e3181c6ffa4.
- Monami M, Vivarelli M, Desideri CM, Colombi C, Marchionni N, Mannucci E. Pulse pressure and prediction of incident foot ulcers in type 2 diabetes. Diabetes Care 2009;32:897-9. http://dx.doi.org/10.2337/dc08-1679.
- Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412-19. http://dx.doi.org/10.1136/bmj.321.7258.412.
- Malmberg K, Ryden L, Efendic S, Herlitz J, Nicol P, Waldenstrom A, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995;26:57-65. http://dx.doi.org/10.1016/0735-1097(95)00126-K.
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, . Antithrombotic Trialists’ (ATT) Collaboration . Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60. http://dx.doi.org/10.1016/S0140-6736(09)60503-1.
- Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: metaanalysis of randomised controlled trials. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b2376.
- Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623-30. http://dx.doi.org/10.1016/S0140-6736(02)11600-X.
- Heart Outcomes Prevention Evaluation (HOPE) Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253-9. http://dx.doi.org/10.1016/S0140-6736(99)12323-7.
- Sonke GS, Beaglehole R, Stewart AW, Jackson R, Stewart FM. Sex differences in case fatality before and after admission to hospital after acute cardiac events: analysis of community based coronary heart disease register. BMJ 1996;313:853-5. http://dx.doi.org/10.1136/bmj.313.7061.853.
- Antiplatelet Trialists’ Collaboration . Collaborative overview of randomised trials of antiplatelet therapy: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81-106. http://dx.doi.org/10.1136/bmj.308.6921.81.
- Stenestrand U, Wallentin L. Swedish Register of Cardiac Intensive Care (RIKS-HIA) . Early statin treatment following acute myocardial infarction and 1-year survival. JAMA 2001;285:430-6. http://dx.doi.org/10.1001/jama.285.4.430.
- Briel M, Schwartz GG, Thompson PL, de Lemos JA, Blazing MA, van Es GA, et al. Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes: a meta-analysis of randomized controlled trials. JAMA 2006;295:2046-56. http://dx.doi.org/10.1001/jama.295.17.2046.
- Gustafsson I, Torp-Pedersen C, Køber L, Gustafsson F, Hildebrandt P. Effect of the angiotensin-converting enzyme inhibitor trandolapril on mortality and morbidity in diabetic patients with left ventricular dysfunction after acute myocardial infarction. Trace Study Group. J Am Coll Cardiol 1999;34:83-9. http://dx.doi.org/10.1016/S0735-1097(99)00146-1.
- Amarenco P, Bogousslavsky J, Callahan A, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549-59. http://dx.doi.org/10.1056/NEJMoa061894.
- PROGRESS Collaborative Group . Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033-41. http://dx.doi.org/10.1016/S0140-6736(01)06178-5.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001;12:171-80. http://dx.doi.org/10.1159/000047700.
- Manktelow BN, Potter JF. Interventions in the management of serum lipids for preventing stroke recurrence. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.CD002091.pub2.
- Sandercock PAG, Counsell C, Gubitz GJ, Tseng MC. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002/14651858.CD000029.pub2.
- Chitravas N, Dewey HM, Nicol MB, Harding DL, Pearce DC, Thrift AG. Is prestroke use of angiotensin-converting enzyme inhibitors associated with better outcome?. Neurology 2007;68:1687-93. http://dx.doi.org/10.1212/01.wnl.0000261914.18101.60.
- Asberg S, Henriksson KM, Farahmand B, Asplund K, Norrving B, Appelros P, et al. Ischemic stroke and secondary prevention in clinical practice: a cohort study of 14,529 patients in the Swedish Stroke Register. Stroke 2010;41:1338-42. http://dx.doi.org/10.1161/STROKEAHA.110.580209.
- Ascenção R, Fortuna P, Reis I, Carneiro AV. Drug therapy for chronic heart failure due to left ventricular systolic dysfunction: a review. III. Angiotensin-converting enzyme inhibitors. Rev Port Cardiol 2008;27:1169-87.
- Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993;88:107-15. http://dx.doi.org/10.1161/01.CIR.88.1.107.
- Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, et al. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet 1998;351:28-31. http://dx.doi.org/10.1016/S0140-6736(97)06209-0.
- Penno G, Chaturvedi N, Talmud PJ, Cotroneo P, Manto A, Nannipieri M, et al. Effect of angiotensin-converting enzyme (ACE) gene polymorphism on progression of renal disease and the influence of ACE inhibition in IDDM patients: findings from the EUCLID Randomized Controlled Trial. EURODIAB Controlled Trial of Lisinopril in IDDM. Diabetes 1998;47:1507-11. http://dx.doi.org/10.2337/diabetes.47.9.1507.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993;329:1456-62. http://dx.doi.org/10.1056/NEJM199311113292004.
- MacIsaac RJ, Lee LY, McNeil KJ, Tsalamandris C, Jerums G. Influence of age on the presentation and outcome of acidotic and hyperosmolar diabetic emergencies. Intern Med J 2002;32:379-85. http://dx.doi.org/10.1046/j.1445-5994.2002.00255.x.
- Persson U, Willis M, Odegaard K, Apelqvist J. The cost-effectiveness of treating diabetic lower extremity ulcers with becaplermin (Regranex): a core model with an application using Swedish cost data. Value Health 2000;3:39-46. http://dx.doi.org/10.1046/j.1524-4733.2000.36027.x.
- Borkosky SL, Roukis TS. Incidence of re-amputation following partial first ray amputation associated with diabetes mellitus and peripheral sensory neuropathy: a systematic review. Diabet Foot Ankle 2012;3. http://dx.doi.org/10.3402/dfa.v3i0.12169.
- Ragnarson Tennvall G, Apelqvist J. Prevention of diabetes-related foot ulcers and amputations: a cost-utility analysis based on Markov model simulations. Diabetologia 2001;44:2077-87. http://dx.doi.org/10.1007/s001250100013.
- Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care 2005;28. http://dx.doi.org/10.2337/diacare.28.6.1339.
- Yoshida S, Hirai M, Suzuki S, Awata S, Oka Y. Neuropathy is associated with depression independently of health-related quality of life in Japanese patients with diabetes. Psychiatry Clin Neurosci 2009;63:65-72. http://dx.doi.org/10.1111/j.1440-1819.2008.01889.x.
- Whyte EM, Mulsant BH, Vanderbilt J, Dodge HH, Ganguli M. Depression after stroke: a prospective epidemiological study. J Am Geriatr Soc 2004;52:774-8. http://dx.doi.org/10.1111/j.1532-5415.2004.52217.x.
- United States Renal Data System . USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States 2010. www.usrds.org/atlas10.aspx (accessed 13 January 2015).
- Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA 2008;299:2751-9. http://dx.doi.org/10.1001/jama.299.23.2751.
- Valenstein M, Vijan S, Zeber JE, Boehm K, Buttar A. The cost-utility of screening for depression in primary care. Ann Intern Med 2001;134:345-60. http://dx.doi.org/10.7326/0003-4819-134-5-200103060-00007.
- Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ 2005;14:217-30. http://dx.doi.org/10.1002/hec.910.
- Herlitz J, Bång A, Karlson BW. Mortality, place and mode of death and reinfarction during a period of 5 years after acute myocardial infarction in diabetic and non-diabetic patients. Cardiology 1996;87:423-8. http://dx.doi.org/10.1159/000177131.
Appendix 1 Literature search strategies
Clinical effectiveness searches
EMBASE (via OvidSP)
Date range searched: 1974–2014/week 34.
Date searched: 5 September 2014.
Search strategy
-
insulin dependent diabetes mellitus/ (78,607)
-
exp diabetic ketoacidosis/ (7787)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ti,ab,ot,hw. (49,088)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ti,ab,ot,hw. (29,355)
-
((insulin$ adj2 depend$) or insulindepend$).ti,ab,ot,hw. (217,259)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,ot,hw. (20,038)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,ot,hw. (14,231)
-
hypoglycemia/ or hyperglycemia/ (108,615)
-
(hyperglyc?em$ or hypoglyc?em$).ti,ab,ot. (104,051)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ti,ab,ot,hw. (126,603)
-
or/1-10 (436,900)
-
(sensor$ adj3 (augment$ or pump$)).ti,ab,hw,ot. (598)
-
SAPT.ti,ab,ot,hw. (114)
-
(minimed or paradigmveo).ti,ab,ot,hw,dm,dv. (727)
-
(paradigm$ adj3 (veo or pump$)).ti,ab,hw,ot,dm,dv. (127)
-
(veo adj3 pump$).ti,ab,ot,hw,dm,dv. (38)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ti,ab,ot,hw,dm,dv. (25)
-
(g4 adj3 platinum).ti,ab,ot,hw,dm,dv. (27)
-
dexcom.ti,ab,ot,hw,dm,dv. (298)
-
or/12-19 (1674)
-
11 and 20 (1105)
-
insulin pump/ (3425)
-
insulin infusion/ (5096)
-
artificial pancreas/ (1433)
-
(insulin$ adj3 (pump$ or infus$ or deliver$ or catheter$)).ti,ab,ot,hw. (17,265)
-
(pump$ adj2 (therap$ or treatment$)).ti,ab,ot,hw. (3171)
-
((subcutaneous adj2 insulin$) or CSII).ti,ab,ot,hw. (4218)
-
(artificial adj3 (pancreas or beta cell$)).ti,ab,ot,hw. (2050)
-
(closed loop adj3 (pump$ or deliver$ or infus$ or therap$ or treatment$ or system$)).ti,ab,ot,hw. (1941)
-
(accu-chek or cellnovo or dana diabecare or omnipod).ti,ab,ot,hw,dm,dv. (529)
-
((integrat$ or dual or combined or unified) adj3 (system$ or device$)).ti,ab,ot,hw. (39,256)
-
or/22-31 (62,055)
-
insulin/ and exp injection/ (3392)
-
(multiple daily adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (1188)
-
(multiple dose adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (561)
-
(multiple adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (9358)
-
MDI.ti,ab,hw,ot. (3791)
-
(injection adj3 therapy).ti,ab,ot,hw. (4157)
-
((basal$ and bolus) adj3 (injection$ or regime$ or routine$ or system$)).ti,ab,hw,ot. (1491)
-
(short acting adj3 insulin).ti,ab,hw,ot. (1038)
-
rapid acting adj3 insulin).ti,ab,hw,ot. (864)
-
or/33-41 (22,079)
-
32 or 42 (81,787)
-
crossover-procedure/ or double-blind procedure/ or randomized controlled trial/ or single-blind procedure/ (397,683)
-
(random$ or factorial$ or crossover$ or cross over$ or cross-over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).ti,ab,ot,hw. (1,636,591)
-
44 or 45 (1,636,591)
-
11 and 43 and 46 (3628)
-
21 or 47 (4491)
-
animal/ (1,574,788)
-
animal experiment/ (1,795,287)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (5,694,449)
-
or/49-51 (5,694,449)
-
exp human/ (15,050,997)
-
human experiment/ (328,369)
-
53 or 54 (15,052,426)
-
52 not (52 and 55) (4,552,229)
-
(letter or editorial or note).pt. (1,874,995)
-
48 not (56 or 57) (4185)
The trials filter was based on terms suggested in chapter 6 of the Cochrane Handbook. 152
MEDLINE (via OvidSP)
Date range searched: 1946–2014/August week 4.
Date searched: 5 September 2014.
Search strategy
-
Diabetes Mellitus, Type 1/ (62,323)
-
Diabetic Ketoacidosis/ (5178)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ti,ab,ot,hw. (69,580)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ti,ab,ot,hw. (20,273)
-
((insulin$ adj2 depend$) or insulindepend$).ti,ab,ot,hw. (30,469)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,ot,hw. (13,085)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,ot,hw. (9331)
-
Hyperglycemia/ (20,833)
-
Hypoglycemia/ (21,743)
-
(hyperglyc?em$ or hypoglyc?em$).ti,ab,ot. (72,656)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ti,ab,ot,hw. (94,623)
-
or/1-11 (24,5714)
-
(sensor$ adj3 (augment$ or pump$)).ti,ab,hw,ot. (312)
-
SAPT.ti,ab,ot,hw. (93)
-
(minimed or paradigmveo).ti,ab,ot,hw. (197)
-
(paradigm$ adj3 (veo or pump$)).ti,ab,hw,ot. (34)
-
(veo adj3 pump$).ti,ab,ot,hw. (5)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ti,ab,ot,hw. (7)
-
(g4 adj3 platinum).ti,ab,ot,hw. (3)
-
dexcom.ti,ab,ot,hw. (44)
-
or/13-20 (645)
-
12 and 21 (297)
-
Insulin Infusion Systems/ (3988)
-
Pancreas, Artificial/ (402)
-
(insulin$ adj3 (pump$ or infus$ or deliver$ or catheter$)).ti,ab,ot,hw. (11,972)
-
(pump$ adj2 (therap$ or treatment$)).ti,ab,ot,hw. (1810)
-
((subcutaneous adj2 insulin$) or CSII).ti,ab,ot,hw. (2474)
-
(artificial adj3 (pancreas or beta cell$)).ti,ab,ot,hw. (1203)
-
(closed loop adj3 (pump$ or deliver$ or infus$ or therap$ or treatment$ or system$)).ti,ab,ot,hw. (1310)
-
(accu-chek or cellnovo or dana diabecare or omnipod).ti,ab,ot,hw. (150)
-
((integrat$ or dual or combined or unified) adj3 (system$ or device$)).ti,ab,ot,hw. (32,573)
-
or/23-31 (47,787)
-
Insulin/ and Injections, Subcutaneous/ (2134)
-
(multiple daily adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (624)
-
(multiple dose adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (452)
-
(multiple adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (6795)
-
MDI.ti,ab,hw,ot. (2372)
-
(injection adj3 therapy).ti,ab,ot,hw. (2858)
-
((basal$ and bolus) adj3 (injection$ or regime$ or routine$ or system$)).ti,ab,hw,ot. (1015)
-
(short acting adj3 insulin).ti,ab,hw,ot. (466)
-
(rapid acting adj3 insulin).ti,ab,hw,ot. (468)
-
or/33-41 (15,196)
-
32 or 42 (61,325)
-
randomized controlled trial.pt. (387,461)
-
controlled clinical trial.pt. (89,748)
-
randomized.ab. (283,558)
-
placebo.ab. (150,467)
-
randomly.ab. (200,457)
-
trial.ab. (294,684)
-
groups.ab. (1,279,172)
-
or/44-50 (1,878,983)
-
exp Animals/ not (exp Animals/ and Humans/) (4,007,023)
-
51 not 52 (1,535,840)
-
12 and 43 and 53 (2750)
-
22 not 52 (291)
-
54 or 55 (2966)
Based on trials filter from box 6.4.c of the Cochrane Handbook. 152
MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE Daily Update (via OvidSP)
Date searched: 5 September 2014.
Search strategy
-
Diabetes Mellitus, Type 1/ (36)
-
Diabetic Ketoacidosis/ (3)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ti,ab,ot,hw. (2614)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ti,ab,ot,hw. (1105)
-
((insulin$ adj2 depend$) or insulindepend$).ti,ab,ot,hw. (701)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,ot,hw. (884)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,ot,hw. (430)
-
Hyperglycemia/ (20)
-
Hypoglycemia/ (10)
-
(hyperglyc?em$ or hypoglyc?em$).ti,ab,ot. (5462)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ti,ab,ot,hw. (7457)
-
or/1-11 (14909)
-
(sensor$ adj3 (augment$ or pump$)).ti,ab,hw,ot. (59)
-
SAPT.ti,ab,ot,hw. (83)
-
(minimed or paradigmveo).ti,ab,ot,hw. (13)
-
(paradigm$ adj3 (veo or pump$)).ti,ab,hw,ot. (4)
-
(veo adj3 pump$).ti,ab,ot,hw. (1)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ti,ab,ot,hw. (0)
-
(g4 adj3 platinum).ti,ab,ot,hw. (3)
-
dexcom.ti,ab,ot,hw. (7)
-
or/13-20 (164)
-
12 and 21 (40)
-
Insulin Infusion Systems/ (2)
-
Pancreas, Artificial/ (2)
-
(insulin$ adj3 (pump$ or infus$ or deliver$ or catheter$)).ti,ab,ot,hw. (504)
-
(pump$ adj2 (therap$ or treatment$)).ti,ab,ot,hw. (189)
-
((subcutaneous adj2 insulin$) or CSII).ti,ab,ot,hw. (172)
-
(artificial adj3 (pancreas or beta cell$)).ti,ab,ot,hw. (61)
-
(closed loop adj3 (pump$ or deliver$ or infus$ or therap$ or treatment$ or system$)).ti,ab,ot,hw. (343)
-
(accu-chek or cellnovo or dana diabecare or omnipod).ti,ab,ot,hw. (16)
-
((integrat$ or dual or combined or unified) adj3 (system$ or device$)).ti,ab,ot,hw. (4137)
-
or/23-31 (5154)
-
Insulin/ and Injections, Subcutaneous/ (3)
-
(multiple daily adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (66)
-
(multiple dose adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (9)
-
(multiple adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (492)
-
MDI.ti,ab,hw,ot. (161)
-
(injection adj3 therapy).ti,ab,ot,hw. (206)
-
((basal$ and bolus) adj3 (injection$ or regime$ or routine$ or system$)).ti,ab,hw,ot. (51)
-
(short acting adj3 insulin).ti,ab,hw,ot. (29)
-
(rapid acting adj3 insulin).ti,ab,hw,ot. (59)
-
or/33-41 (937)
-
32 or 42 (6014)
-
randomized controlled trial.pt. (809)
-
controlled clinical trial.pt. (53)
-
randomized.ab. (24,330)
-
placebo.ab. (8979)
-
randomly.ab. (21,647)
-
trial.ab. (25,986)
-
groups.ab. (122,705)
-
or/44-50 (163,158)
-
exp Animals/ not (exp Animals/ and Humans/) (1565)
-
51 not 52 (162,926)
-
12 and 43 and 53 (178)
-
22 not 52 (40)
-
54 or 55 (203)
Based on trials filter from box 6.4.c of the Cochrane Handbook. 152
PubMed (via the National Library of Medicine)
URL: www.ncbi.nlm.nih.gov/pubmed/
Date range searched: from inception until 5 September 2014.
Date searched: 5 September 2014.
Search strategy
#63 Search (#61 and #62) (99)
#62 Search (pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb]) (1,815,003)
#61 Search (#57 not #60) (1862)
#60 Search ((#58 not (#58 and #59))) (2,730,690)
#59 Search human*[tiab] (2,017,079)
#58 Search (rat[tiab] or rats[tiab] or mouse[tiab] or mice[tiab] or murine[tiab] or rodent[tiab] or rodents[tiab] or hamster[tiab] or hamsters[tiab] or pig[tiab] or pigs[tiab] or porcine[tiab] or rabbit[tiab] or rabbits[tiab] or animal[tiab] or animals[tiab] or dogs[tiab] or dog[tiab] or cats[tiab] or cow[tiab] or bovine[tiab] or sheep[tiab] or ovine[tiab] or monkey[tiab] or monkeys[tiab]) (3,335,539)
#57 Search (#30 or #56) (1967)
#56 Search (#20 and #54 and #55) (1778)
#55 Search (#38 or #46) (19531)
#54 Search (#47 or #48 or #49 or #50 or #51 or #52 or #53) (2,074,509)
#53 Search groups [tiab] (1,413,274)
#52 Search trial [tiab] (369,610)
#51 Search randomly [tiab] (219,790)
#50 Search placebo [tiab] (160,018)
#49 Search randomized [tiab] (324,067)
#48 Search controlled clinical trial [pt] (87,768)
#47 Search randomized controlled trial [pt] (371,691)
#46 Search (#39 or #40 or #41 or #42 or #43 or #44 or #45) (9426)
#45 Search (“short acting insulin”[tiab] OR “rapid acting insulin”[tiab]) (810)
#44 Search (basal*[tiab] AND bolus[tiab] AND (injection*[tiab] OR regime*[tiab] OR routine*[tiab] OR system*[tiab])) (1549)
#43 Search “injection therapy”[tiab] (2098)
#42 Search MDI[tiab] (2524)
#41 Search “multiple injection”[tiab] or “multiple injections”[tiab] or “multiple insulin”[tiab] or “multiple regime”[tiab] or “multiple regimes”[tiab] or “multiple routine”[tiab] or “multiple routines”[tiab] (2414)
#40 Search “multiple dose injection”[tiab] or “multiple dose injections”[tiab] or “multiple dose insulin”[tiab] or “multiple dose regime”[tiab] or “multiple dose regimes”[tiab] or “multiple dose routine”[tiab] or “multiple dose routines”[tiab] (48)
#39 Search “multiple daily injection”[tiab] or “multiple daily injections”[tiab] or “multiple daily insulin”[tiab] or “multiple daily regime”[tiab] or “multiple daily regimes”[tiab] or “multiple daily routine”[tiab] or “multiple daily routines”[tiab] (603)
#38 Search (#31 or #32 or #33 or #34 or #35 or #36 or #37) (10,964)
#37 Search “integrated system”[tiab] or “integrated systems”[tiab] “integrated device”[tiab] or “integrated devices”[tiab] or “dual system”[tiab] or “dual systems”[tiab] or “dual device”[tiab] or “dual devices”[tiab] or “combined system”[tiab] or “combined systems”[tiab] or “combined device”[tiab] or “combined devices”[tiab] or “unified system”[tiab] or “unified systems”[tiab] or “unified device”[tiab] or “unified devices”[tiab] (1317)
#36 Search (accu-chek[tiab] or cellnovo[tiab] or “dana diabecare”[tiab] or omnipod[tiab]) (159)
#35 Search “closed loop pump”[tiab] or “closed loop pumps”[tiab] or “closed loop delivery”[tiab] or “closed loop infusion”[tiab] or “closed loop infusions”[tiab] or “closed loop therapy”[tiab] or “closed loop treatment”[tiab] or “closed loop treatments”[tiab] or “closed loop system”[tiab] or “closed loop systems”[tiab] (812)
#34 Search “artificial pancreas”[tiab] or “artificial beta cell”[tiab] (822)
#33 Search “subcutaneous insulin”[tiab] or CSII[tiab] (2385)
#32 Search “pump therapy”[tiab] or “pump therapies”[tiab] or “pump treatment”[tiab] or “pump treatments”[tiab] (920)
#31 Search “insulin pump”[tiab] or “insulin pumps”[tiab] or “insulin infusion”[tiab] or “insulin infuse”[tiab] or “insulin infused”[tiab] or “insulin deliver”[tiab] or “insulin delivery”[tiab] (7485)
#30 Search (#20 and #29) (273)
#29 Search (#21 or #22 or #23 or #25 or #26 or #27 or #28) (928)
#28 Search dexcom (54)
#27 Search (animas or vibe) AND (pump* or infus* or system*) (81)
#26 Search “veo pump” or “veo pumps” (15)
#25 Search (paradigm* AND (veo or pump*)) (350)
#23 Search minimed or paradigmveo (216)
#22 Search SAPT[tiab] (184)
#21 Search “sensor augmented”[tiab] or “sensor augment”[tiab] or “sensor pump”[tiab] or “pump sensor”[tiab] or “sensor pumps”[tiab] (91)
#20 Search (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19) (126,788)
#19 Search “high glycohemoglobin”[tiab] or “higher glycohemoglobin”[tiab] or “low glycohemoglobin”[tiab] or “lower glycohemoglobin”[tiab] or “increase glycohemoglobin”[tiab] or “increased glycohemoglobin”[tiab] or “increases glycohemoglobin”[tiab] or “decrease glycohemoglobin”[tiab] or “decreasedcglycohemoglobin”[tiab] or “decreases glycohemoglobin”[tiab] or “deficient glycohemoglobin”[tiab] or “sufficient glycohemoglobin”[tiab] or “insufficient glycohemoglobin”[tiab] or “reduce glycohemoglobin”[tiab] or “reduced glycohemoglobin”[tiab] or “glycohemoglobin reduction”[tiab] or “fallen glycohemoglobin”[tiab] or “falling glycohemoglobin”[tiab] or “glycohemoglobin threshold”[tiab] or “safe glycohemoglobin”[tiab] (17)
#18 Search “high haemoglobin”[tiab] or “higher haemoglobin”[tiab] or “low haemoglobin”[tiab] or “lower haemoglobin”[tiab] or “increase haemoglobin”[tiab] or “increased haemoglobin”[tiab] or “increases haemoglobin”[tiab] or “decrease haemoglobin”[tiab] or “decreasedchaemoglobin”[tiab] or “decreases haemoglobin”[tiab] or “deficient haemoglobin”[tiab] or “sufficient haemoglobin”[tiab] or “insufficient haemoglobin”[tiab] or “reduce haemoglobin”[tiab] or “reduced haemoglobin”[tiab] or “haemoglobin reduction”[tiab] or “fallen haemoglobin”[tiab] or “falling haemoglobin”[tiab] or “haemoglobin threshold”[tiab] or “safe haemoglobin”[tiab] (1110)
#17 Search “high hemoglobin”[tiab] or “higher hemoglobin”[tiab] or “low hemoglobin”[tiab] or “lower hemoglobin”[tiab] or “increase hemoglobin”[tiab] or “increased hemoglobin”[tiab] or “increases hemoglobin”[tiab] or “decrease hemoglobin”[tiab] or “decreasedchemoglobin”[tiab] or “decreases hemoglobin”[tiab] or “deficient hemoglobin”[tiab] or “sufficient hemoglobin”[tiab] or “insufficient hemoglobin”[tiab] or “reduce hemoglobin”[tiab] or “reduced hemoglobin”[tiab] or “hemoglobin reduction”[tiab] or “fallen hemoglobin”[tiab] or “falling hemoglobin”[tiab] or “hemoglobin threshold”[tiab] or “safe hemoglobin”[tiab] (3476)
#16 Search “high a1c”[tiab] or “higher a1c”[tiab] or “low a1c”[tiab] or “lower a1c”[tiab] or “increase a1c”[tiab] or “increased a1c”[tiab] or “increases a1c”[tiab] or “decrease a1c”[tiab] or “decreasedca1c”[tiab] or “decreases a1c”[tiab] or “deficient a1c”[tiab] or “sufficient a1c”[tiab] or “insufficient a1c”[tiab] or “reduce a1c”[tiab] or “reduced a1c”[tiab] or “a1c reduction”[tiab] or “fallen a1c”[tiab] or “falling a1c”[tiab] or “a1c threshold”[tiab] or “safe a1c”[tiab] (291)
#15 Search ((“high hba1”[tiab] or “higher hba1”[tiab] or “low hba1”[tiab] or “lower hba1”[tiab] or “increase hba1”[tiab] or “increased hba1”[tiab] or “increases hba1”[tiab] or “decrease hba1”[tiab] or “decreasedchba1”[tiab] or “decreases hba1”[tiab] or “deficient hba1”[tiab] or “sufficient hba1”[tiab] or “insufficient hba1”[tiab] or “reduce hba1”[tiab] or “reduced hba1”[tiab] or “hba1 reduction”[tiab] or “fallen hba1”[tiab] or “falling hba1”[tiab] or “hba1 threshold”[tiab] or “safe hba1”[tiab])) (76)
#14 Search “high hb a1”[tiab] or “higher hb a1”[tiab] or “low hb a1”[tiab] or “lower hb a1”[tiab] or “increase hb a1”[tiab] or “increased hb a1”[tiab] or “increases hb a1”[tiab] or “decrease hb a1”[tiab] or “decreasedchb a1”[tiab] or “decreases hb a1”[tiab] or “deficient hb a1”[tiab] or “sufficient hb a1”[tiab] or “insufficient hb a1”[tiab] or “reduce hb a1”[tiab] or “reduced hb a1”[tiab] or “hb a1 reduction”[tiab] or “fallen hb a1”[tiab] or “falling hb a1”[tiab] or “hb a1 threshold”[tiab] or “safe hb a1”[tiab] (0)
#13 Search “high hba1c”[tiab] or “higher hba1c”[tiab] or “low hba1c”[tiab] or “lower hba1c”[tiab] or “increase hba1c”[tiab] or “increased hba1c”[tiab] or “increases hba1c”[tiab] or “decrease hba1c”[tiab] or “decreasedchba1c”[tiab] or “decreases hba1c”[tiab] or “deficient hba1c”[tiab] or “sufficient hba1c”[tiab] or “insufficient hba1c”[tiab] or “reduce hba1c”[tiab] or “reduced hba1c”[tiab] or “hba1c reduction”[tiab] or “fallen hba1c”[tiab] or “falling hba1c”[tiab] or “hba1c threshold”[tiab] or “safe hba1c”[tiab] (1271)
#12 Search “high sugar”[tiab] or “higher sugar”[tiab] or “low sugar”[tiab] or “lower sugar”[tiab] or “increase sugar”[tiab] or “increased sugar”[tiab] or “increases sugar”[tiab] or “decrease sugar”[tiab] or “decreasedcsugar”[tiab] or “decreases sugar”[tiab] or “deficient sugar”[tiab] or “sufficient sugar”[tiab] or “insufficient sugar”[tiab] or “reduce sugar”[tiab] or “reduced sugar”[tiab] or “sugar reduction”[tiab] or “fallen sugar”[tiab] or “falling sugar”[tiab] or “sugar threshold”[tiab] or “safe sugar”[tiab] (1539)
#11 Search (“high glucose”[tiab] or “higher glucose”[tiab] or “low glucose”[tiab] or “lower glucose”[tiab] or “increase glucose”[tiab] or “increased glucose”[tiab] or “increases glucose”[tiab] or “decrease glucose”[tiab] or “decreasedcglucose”[tiab] or “decreases glucose”[tiab] or “deficient glucose”[tiab] or “sufficient glucose”[tiab] or “insufficient glucose”[tiab] or “reduce glucose”[tiab] or “reduced glucose”[tiab] or “glucose reduction”[tiab] or “fallen glucose”[tiab] or “falling glucose”[tiab] or “glucose threshold”[tiab] or “safe glucose”[tiab]) (16,645)
#10 Search (hyperglycemia[tiab] or hypoglycaemia[tiab] or hyperglycemic[tiab] or hypoglycaemic[tiab]) (44,267)
#9 Search ketoacidosis[tiab] or acidoketosis[tiab] or “keto acidosis”[tiab] or ketoacidemia[tiab] or ketosis[tiab] (7293)
#8 Search dm1[tiab] or “dm 1”[tiab] or t1dm[tiab] or “t1 dm”[tiab] or t1d[tiab] or iddm[tiab] (13,131)
#7 Search “insulin dependent”[tiab] or insulindepend*[tiab] (27,550)
#6 Search “brittle diabetic”[tiab] or “diabetic juvenile”[tiab] or “diabetic pediatric”[tiab] or “diabetic paediatric”[tiab] or “diabetic early”[tiab] or “diabetic labile”[tiab] or “diabetic acidosis”[tiab] or “diabetic sudden onset”[tiab] (348)
#5 Search “diabetic brittle”[tiab] or “juvenile diabetic”[tiab] or “pediatric diabetic”[tiab] or “paediatric diabetic”[tiab] or “early diabetic”[tiab] or “labile diabetic”[tiab] or “acidosis diabetic”[tiab] or “sudden onset diabetic”[tiab] (1122)
#4 Search “brittle diabetes”[tiab] or “diabetes juvenile”[tiab] or “diabetes pediatric”[tiab] or “diabetes paediatric”[tiab] or “diabetes early”[tiab] or “diabetes ketosis”[tiab] or “diabetes labile”[tiab] or “diabetes acidosis”[tiab] or “diabetes sudden onset”[tiab] (264)
#3 Search “diabetes brittle”[tiab] or “juvenile diabetes”[tiab] or “pediatric diabetes”[tiab] or “paediatric diabetes”[tiab] or “early diabetes”[tiab] or “ketosis diabetes”[tiab] or “labile diabetes”[tiab] or “acidosis diabetes”[tiab] or “sudden onset diabetes”[tiab] (2238)
#2 Search “diabetic type 1”[tiab] OR “type 1 diabetic”[tiab] OR “diabetic type i”[tiab] OR “type i diabetic”[tiab] OR “diabetic type1”[tiab] OR “type1 diabetic”[tiab] OR “diabetic typei”[tiab] OR “typei diabetic”[tiab] (6044)
#1 Search ((“diabetes type 1”[tiab] OR “type 1 diabetes”[tiab] OR “diabetes type i”[tiab] OR “type i diabetes”[tiab] OR “diabetes type1”[tiab] OR “type1 diabetes”[tiab] OR “diabetes typei”[tiab] OR “typei diabetes”[tiab])) (28,884)
Cochrane Database of Systematic Reviews (via Wiley Online Library), Cochrane Central Register of Controlled Trials (via Wiley Online Library), Database of Abstracts of Reviews of Effects (via Wiley Online Library) and Health Technology Assessment Database (via Wiley Online Library)
Cochrane Database of Systematic Reviews: issue 9 of 12, September 2014.
Cochrane Central Register of Controlled Trials: issue 8 of 12, August 2014.
Database of Abstracts of Reviews of Effects: issue 3 of 4, July 2014.
Health Technology Assessment Database: issue 3 of 4, July 2014.
Date searched: 5 September 2014.
Search strategy
#1 MeSH descriptor: [Diabetic Mellitus, Type 1] this term only
#2 MeSH descriptor: [Diabetic Ketoacidosis] this term only
#3 (diabet* near/3 (typ* next 1 or typ* next i or type1 or typei or typ* next one)):ti,ab,kw
#4 (diabet* near/3 (britt* or juvenil* or pediatric or paediatric or early or keto* or labil* or acidos* or autoimmun* or auto next immun* or sudden next onset)):ti,ab,kw
#5 ((insulin* near/2 depend*) or insulindepend*):ti,ab,kw
#6 (dm1 or dm next 1 or dmt1 or dm next t1 or t1dm or t1 next dm or t1d or iddm):ti,ab,kw
#7 (ketoacidosis or acidoketosis or keto next acidosis or ketoacidemia or ketosis):ti,ab,kw
#8 MeSH descriptor: [Hyperglycemia] this term only
#9 MeSH descriptor: [Hypoglycemia] this term only
#10 (hyperglyc?em* or hypoglyc?em*):ti,ab,kw
#11 ((high or higher or low or lower or increas* or decreas* or deficien* or sufficien* or insufficien* or reduce* or reduction* or fluctuat* or fallen or falling or threshold or safe) near/3 (glucose* or sugar* or hba1c or hb next a1 or hba1 or a1c or h?emoglob* or glycoh?emoglob*)):ti,ab,kw
#12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11
#13 (sensor* near/3 (augment* or pump*))
#14 SAPT:ti,ab,kw
#15 minimed or paradigmveo
#16 (paradigm* near/3 (veo or pump*))
#17 (veo near/3 pump*)
#18 ((animas or vibe) near/3 (pump* or infus* or system*))
#19 dexcom
#20 #13 or #14 or #15 or #16 or #17 or #18 or #19
#21 MeSH descriptor: [Insulin Infusion Systems] this term only
#22 MeSH descriptor: [Pancreas, Artificial] this term only
#23 (insulin* near/3 (pump* or infus* or deliver* or catheter*)):ti,ab,kw
#24 (pump* near/2 (therap* or treatment*)):ti,ab,kw
#25 ((subcutaneous near/2 insulin*) or CSII):ti,ab,kw
#26 (artificial near/3 (pancreas or beta next cell*)):ti,ab,kw
#27 (closed next loop near/3 (pump* or deliver* or infus* or therap* or treatment* or system*)):ti,ab,kw
#28 accu-chek or cellnovo or dana next diabecare or omnipod
#29 ((integrat* or dual or combined or unified) near/3 (system* or device*)):ti,ab,kw
#30 #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29
#31 MeSH descriptor: [Insulin] this term only
#32 MeSH descriptor: [Injections, Subcutaneous] this term only
#33 #31 and #32
#34 “multiple daily” near/3 (inject* or insulin* or regime* or routine*):ti,ab,kw
#35 “multiple dose” near/3 (inject* or insulin* or regime* or routine*):ti,ab,kw
#36 multiple near/3 (inject* or insulin* or regime* or routine*):ti,ab,kw
#37 MDI:ti,ab,kw
#38 injection near/3 therapy:ti,ab,kw
#39 (basal* and bolus) near/3 (inject* or regime* or routine* or system*):ti,ab,kw
#40 (“short acting” near/3 insulin) or (“rapid acting” near/3 insulin):ti,ab,kw
#41 #34 or #35 or #36 or #37 or #38 or #39 or #40
#42 #12 and (#20 or #30 or #41)
Cochrane Database of Systematic Reviews: 14.
Database of Abstracts of Reviews of Effects: 25.
Cochrane Central Register of Controlled Trials: 1910.
HTA: 19.
Science Citation Index Expanded (Web of Science)
Date range searched: 1988–29 August 2014.
Date searched: 5 September 2014.
Search strategy
# 40 4,012 #38 not #39
# 39 3,123,359 TS=(rat or rats or mouse or mice or murine or hamster or hamsters or animal or animals or dogs or dog or pig or pigs or cats or bovine or cow or sheep or ovine or porcine or monkey)
# 38 5,027 #37 OR #18
# 37 4,914 #36 AND #33 AND #8
# 36 4,219,275 #35 OR #34
# 35 4,185,460 TS=((clinic* SAME trial*) OR (placebo* OR random* OR control* OR prospectiv*))
# 34 194,182 TS=((singl* or doubl* or trebl* or tripl*) SAME (blind* or mask*))
# 33 126,955 #32 OR #26
# 32 11,323 #31 OR #30 OR #29 OR #28 OR #27
# 31 837 TS=(“short acting” NEAR/3 insulin) or TS=(“rapid acting” NEAR/3 insulin)
# 30 5,207 TS=(injection NEAR/3 therapy)
# 29 4,652 TS=MDI
# 28 332 TS=(“multiple dose” NEAR/3 (inject* or insulin* or regime* or routine*))
# 27 774 TS=(“multiple daily” NEAR/3 (inject* or insulin* or regime* or routine*))
# 26 116,578 #19 or #20 or #21 or #22 or #23 or #24 or #25
# 25 91,258 TS=((integrat* or dual or combined or unified) NEAR/3 (system* or device*))
# 24 165 TS=(accu-chek or cellnovo or “dana diabecare” or omnipod)
# 23 11,130 TS=(“closed loop” NEAR/3 (pump* or deliver* or infus* or therap* or treatment* or system*))
# 22 851 TS=(artificial NEAR/3 (pancreas or “beta cell*”))
# 21 3,017 TS=((subcutaneous NEAR/2 insulin*) or CSII)
# 20 3,696 TS=(pump* NEAR/2 (therap* or treatment*))
# 19 10,301 TS=(insulin* NEAR/3 (pump* or infus* or deliver* or catheter*))
# 18 260 #8 and #17
# 17 1,375 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
# 16 38 TS=dexcom
# 15 7 TS=(g4 NEAR/3 platinum)
# 14 13 TS=((animas or vibe) NEAR/3 (pump* or infus* or system*))
# 13 4 TS=(veo NEAR/3 pump*)
# 12 38 TS=(paradigm* NEAR/3 (veo or pump*))
# 11 154 TS=(minimed or paradigmveo)
# 10 396 TS=SAPT
# 9 765 TS=(sensor* NEAR/3 (augment* or pump*))
# 8 226,312 #1 or #2 or #3 or #4 or #5 or #6 or #7
# 7 109,659 TS=((high or higher or low or lower or increas* or decreas* or deficien* or sufficien* or insufficien* or reduce* or reduction* or fluctuat* or fallen or falling or threshold or safe) NEAR/3 (glucose* or sugar* or hba1c or “hb a1” or hba1 or a1c or hemoglob* or glycohemoglob* or haemoglob* or glycohaemoglob*))
# 6 68,183 TS=(hyperglycem* or hypoglycem* or hyperglycaem* or hypoglycaem*)
# 5 5,944 TS=(ketoacidosis or acidoketosis or “keto acidosis” or ketoacidemia or ketosis)
# 4 17,145 TS=(dm1 or “dm 1” or dmt1 or “dm t1” or t1dm or “t1 dm” or t1d or iddm)
# 3 25,575 TS=((insulin* NEAR/2 depend*) or insulindepend*)
# 2 17,654 TS=(diabet* NEAR/3 (britt* or juvenil* or pediatric or paediatric or early or keto* or labil* or acidos* or autoimmun* or “auto immun*” or “sudden onset”))
# 1 40,584 TS=(diabet* NEAR/3 (“typ* 1” or “typ* i” or type1 or typei or “typ* one”))
Latin American and Caribbean Health Sciences Literature (LILACS)
URL: http://lilacs.bvsalud.org/en/
Date range searched: 1982–5 September 2014.
Date searched: 5 September 2014.
Search strategy
((MH:C18.452.394.750.124 or MH:C18.452.076.176.652.500 or MH:C18.452.394.952 or MH:C18.452.394.984 or “diabetes type 1” or “diabetes type i” or “diabetes type1” or “diabetes typei” or “diabetes type one” or “type 1 diabetes” or “type I diabetes” or “type1 diabetes” or “typei diabetes” or “type one diabetes” or “diabetes tipo 1” or “diabetes tipo i” or “diabetes tipo1” or “diabetes tipoi” or “tipo 1 diabetes” or “tipo I diabetes” or “tipo1 diabetes” or “tipoi diabetes” or “brittle diabetes” or “juvenile diabetes” or “pediatric diabetes” or “paediatric diabetes” or “early diabetes” or “labile diabetes” or “autoimmune diabetes” or “auto immune diabetes” or “sudden onset diabetes” or “diabetes autoimune” or “diabetes inestable” or “diabetes instável” or “insulin dependent” or insulindependent or “insulin dependiente” or insulinodependiente or “insulin dependente” or insulinodependente or dm1 or “dm 1” or dmt1 or “dm t1” or t1dm or “t1 dm” or t1d or iddm or dmid or ketoacidosis or acidoketosis or “keto acidosis” or ketoacidemia or ketosis or cetoacidosis or cetoacidose or hyperglycem$ or hyperglycaem$ or hiperglucem$ or hiperglicem$ or hypoglycem$ or hypoglycaem$ or hipoglucem$ or hipoglicem$) AND (MH:E02.319.300.508 or “insulin pump” or “insulin pumps” or “insulin infusion” or “insulin infusions” or “insulin delivery” or “insulin catheter” or “insulin catheters” or “pump therapy” or “pump therapies” or “pump treatment” or “pump treatments” or “insulina sistemas” or “sistemas insulina” or “insulina infusion” or “infusion insulina” or “insulina infusions” or “infusion insulinas” or “infusão de insulina” or “subcutaneous insulin” or CSII or “artificial pancreas” or “artificial beta cell” or “célula beta artificial” or “páncreas endocrino artificial” or “integrated system” or “integrated systems” or “integrated devices” or “dual system” or “dual systems” or “dual devices” or “combined system” or “combined systems” or “combined devices” or “unified system” or “unified systems” or “unified devices” or (MH: D06.472.699.587.200.500.625 and MH; E02.319.267.530.620) or “multiple daily injection” or “multiple daily injections” or “multiple daily insulin” or “multiple dose injection” or “multiple dose injections” or “multiple injection” or “multiple injections” or MDI or “injection therapy” or “inyecciones terapia” or “injeções terapia” or “short acting insulin” or “rapid acting insulin”)) or (“sensor augmented pump” or “sensor augmented pumps” or “sensor augmented insulin” or SAPT or minimed or paradigmveo or “paradigm veo” or “paradigm pump” or “veo pump” or “veo pumps” or “animas pump” or “animas pumps” “animas system” or “vibe pump” or “vibe pumps” or “vibe system” or dexcom)
Retrieved: 58.
NIHR Project Portfolio and NIHR Journals Library
NIHR Project Portfolio URL: www.nets.nihr.ac.uk/projects/
NIHR Journals Library URL: www.journalslibrary.nihr.ac.uk/
Date range searched: from inception until 5 September 2014.
Date searched: 5 September 2014.
| Search terms | NIHR Project Portfolio | NIHR Journals Library |
|---|---|---|
| “sensor augmented pump” | 0 | 0 |
| “sensor augmented pumps” | 0 | 0 |
| “sensor augmented insulin” | 0 | 0 |
| SAPT | 0 | 0 |
| minimed | 1 | 6 |
| paradigmveo | 0 | 0 |
| “paradigm veo” | 0 | 0 |
| “veo pump” | 0 | 0 |
| “veo pumps” | 0 | 0 |
| animas | 0 | 4 |
| vibe | 0 | 0 |
| dexcom | 0 | 1 |
| “insulin pump” | 5 | 14 |
| “insulin pumps” | 5 | 12 |
| “continuous subcutaneous insulin infusion” [Journals Library limit: ICD-10; E10-E14 Diabetes mellitus*] |
4 | 17* |
| “artificial pancreas” | 0 | 2 |
| “multiple daily injection” | 3 | 7 |
| “multiple daily injections” | 3 | 11 |
| Total | 21 (14 duplicates) | 72 (47 duplicates) |
| Total after removal of duplicates | 32 (61 duplicates) | |
PROSPERO
URL: www.crd.york.ac.uk/prospero/
Date range searched: up to 5 September 2014.
Date searched: 5 September 2014.
Search: combine phrase/terms with ‘OR’; five search boxes in ‘All fields’.
| Terms searched | Records |
|---|---|
| sensor augmented pump* OR sensor augmented insulin* OR SAPT OR minimed OR paradigmveo | 2 |
| paradigm veo OR veo pump* OR animas OR vibe OR dexcom | 0 |
| insulin pump* OR insulin infusion* OR insulin therapy OR subcutaneous insulin OR CSII | 14 (1 duplicate) |
| artificial pancreas | 0 |
| multiple daily injection* OR multiple daily insulin* | 1 |
| MDI [review title] | 0 |
| Total | 17 (2 duplicates) |
| Total after removal of duplicates | 15 |
US Food and Drug Administration
URL: www.fda.gov/ (includes links to approval/summary of safety and effectiveness).
Date range searched: from inception up to 5 September 2014.
Date searched: 5 September 2014.
Search strategy for medical devices
| Search terms | Records |
|---|---|
| minimed | 6 |
| animas | 5 |
| Vibe | 0 |
| “g4 platinum” | 3 |
| “multiple daily injection” | 0 |
| “multiple daily injections” | 0 |
| “multiple daily insulin” | 1 |
| Total | 15 |
Medicines and Healthcare Products Regulatory Agency
URL: www.mhra.gov.uk
Date range searched: from inception up to 5 September 2014.
Date searched: 5 September 2014.
Search strategy
| Search terms | Records |
|---|---|
| minimed | 7 |
| Animas + insulin + pump | 16 |
| Animas + vibe | 5 |
| “g4 platinum” | 2 |
| “multiple daily injection” | 0 |
| “multiple daily injections” | 0 |
| “multiple daily insulin” | 0 |
| Total | 30 a |
National Institutes of Health (US) ClinicalTrials.gov
URL: http://clinicaltrials.gov/ct2/search/advanced
Date range searched: from inception up to 2 September 2014.
Date searched: 2 September 2014.
Advanced search option.
Search strategy
| Search terms | Results |
|---|---|
| Search terms: (“sensor augmented pump” OR “sensor augmented insulin” OR SAPT OR minimed or paradigmveo OR paradigm* OR veo OR animas OR vibe OR dexcom OR “G4 platinum”) Conditions: Type 1 Diabetes Mellitus OR Hyperglycemia OR Hypoglycemia |
84 |
| Search terms: “insulin pump” OR “insulin pumps” OR “insulin infusion” OR “insulin delivery” OR “pump therapy” OR “subcutaneous insulin” OR CSII OR “artificial pancreas” OR “artificial beta cell” Conditions: Type 1 Diabetes Mellitus OR Hyperglycemia OR Hypoglycemia |
454 |
| Search terms: “closed loop” OR accu-chek OR cellnovo OR “dana diabecare” OR omnipod Conditions: Type 1 Diabetes Mellitus OR Hyperglycemia OR Hypoglycemia |
136 |
| Search terms: “integrated system” OR “integrated device” OR “integrated systems” OR “integrated devices” OR “dual system” OR “dual device” OR “dual systems” OR “dual devices” OR “combined system” OR “combined device” OR “combined systems” OR “combined devices” Conditions: Type 1 Diabetes Mellitus OR Hyperglycemia OR Hypoglycemia |
1 |
| Search terms: “multiple daily injection” OR “multiple daily injections” OR “multiple daily insulin” OR “multiple dose injection” OR “multiple dose injections” Conditions: Type 1 Diabetes Mellitus OR Hyperglycemia OR Hypoglycemia |
42 |
| Search terms: MDI OR “multiple dose insulin” OR “multiple injection” OR “multiple injections” OR “multiple insulin” OR “injection therapy” Conditions: Type 1 Diabetes Mellitus OR Hyperglycemia OR Hypoglycemia |
46 |
| Total | 763 |
| Total after removal of duplicates | 496 |
metaRegister of Controlled Trials
URL: www.controlled-trials.com/
Date range searched: from inception up to 5 September 2014.
Date searched: 5 September 2014.
National Institutes of Health (US) Clinical Trials register option not ticked as already searched separately.
Search strategy
| Search terms | Results |
|---|---|
| (“sensor augmented pump” OR “sensor augmented insulin” OR SAPT OR minimed or paradigmveo OR paradigm* OR veo OR animas OR vibe OR dexcom OR “G4 platinum”) AND (Diabetes OR Hyperglycemia OR Hypoglycemia) | 2 |
| (“insulin pump” OR “insulin pumps” OR “insulin infusion” OR “insulin delivery” OR “pump therapy” OR “subcutaneous insulin” OR CSII OR “artificial pancreas” OR “artificial beta cell”) AND (Diabetes OR Hyperglycemia OR Hypoglycemia) | 4 |
| (“closed loop” OR accu-chek OR cellnovo OR “dana diabecare” OR omnipod) AND (Diabetes OR Hyperglycemia OR Hypoglycemia) | 0 |
| (“integrated system” OR “integrated device” OR “integrated systems” OR “integrated devices”) AND (Diabetes OR Hyperglycemia OR Hypoglycemia) | 0 |
| (“dual system” OR “dual device” OR “dual systems” OR “dual devices” OR “combined system” OR “combined device” OR “combined systems” OR “combined devices”) AND (Diabetes OR Hyperglycemia OR Hypoglycemia) | 0 |
| (“multiple daily injection” OR “multiple daily injections” OR “multiple daily insulin” OR “multiple dose injection” OR “multiple dose injections”) AND (Diabetes OR Hyperglycemia OR Hypoglycemia) | 0 |
| (MDI OR “multiple dose insulin” OR “multiple injection” OR “multiple injections” OR “multiple insulin” OR “injection therapy”) AND (Diabetes OR Hyperglycemia OR Hypoglycemia) | 3 |
| Total | 9 |
| Total after removal of duplicates | 7 |
WHO International Clinical Trials Register Platform
Date range searched: from inception up to 5 September 2014.
Date searched: 5 September 2014.
Standard search option.
Search strategy
| Search terms | Results |
|---|---|
| sensor augmented pump* OR SAPT OR minimed OR paradigmveo OR paradigm veo OR animas vibe OR dexcom OR G4 platinum | 70 for 65 trials |
| type 1 diabetes mellitus AND insulin pump* OR insulin infusion* OR pump therapy OR subcutaneous insulin* OR CSII OR artificial pancreas | 317 for 297 trials |
| type 1 diabetes mellitus AND closed loop* OR accu-chek OR cellnovo OR dana diabecare OR omnipod | 115 for 115 trials |
| type 1 diabetes mellitus AND integrated system* OR integrated device* OR dual system* OR dual device* | 1 |
| type 1 diabetes mellitus AND multiple daily injection* OR multiple dose injection* OR multiple daily insulin* OR multiple injection* | 75 for 50 trials |
| type 1 diabetes mellitus AND MDI OR multiple insulin OR injection therapy | 95 for 78 trials |
| Total | 606 |
| Total after removal of duplicates | 475 |
Diabetes UK Professional Conference
URL: www.diabetes.org.uk/diabetes-uk-professional-conference/
Date range searched: 2010–14.
Date searched: 10 September 2014.
Abstracts were not available from the Diabetes UK website; proceedings were published in the journal Diabetic Medicine. It was not possible to search the proceedings from the Diabetic Medicine search screen. Available PDFs were scanned for 2014 and 2013. Previous conference proceedings (2010, 2011 and 2012) were only available for purchase online, so could not be scanned.
Abstracts of the Diabetes UK Professional Conference 2014
Abstracts of the Diabetes UK Professional Conference 2014, Arena and Convention Centre, Liverpool, UK, 5–7 March 2014. Diabet Med 2014;31(Suppl. 1):1–184. URL: http://onlinelibrary.wiley.com/doi/10.1111/dme.2014.31.issue-s1/issuetoc (accessed 10 September 2014).
Basic and clinical science posters.
Clinical care and other categories posters.
Hypoglycaemia.
Children, young people and emerging adulthood.
Abstracts of the Diabetes UK Professional Conference 2013
Abstracts of the Diabetes UK Professional Conference 2013. Manchester, UK, 13–15 March 2013. Diabet Med 2013;30(Suppl. 1):1–213, E1–10. URL: http://onlinelibrary.wiley.com/doi/10.1111/dme.2013.30.issue-s1/issuetoc (accessed 10 September 2014).
Basic and clinical science posters.
Clinical care and other categories posters.
Abstracts of Diabetes UK Professional Conference 2012
Abstracts of Diabetes UK Professional Conference 2012. Glasgow, UK, 7–9 March 2012. Diabet Med 2012;29(Suppl. 1):1–187. URL: http://onlinelibrary.wiley.com/doi/10.1111/dme.2012.29.issue-s1/issuetoc (accessed 10 September 2014).
Not available online. Purchase access only.
Abstracts of Diabetes UK Professional Conference 2011
Abstracts of Diabetes UK Annual Professional Conference 2011. London, UK, 30 March 30–1 April 2011. Diabet Med 2011;28(Suppl. 1):1–214. URL: http://onlinelibrary.wiley.com/doi/10.1111/dme.2012.29.issue-s1/issuetoc (accessed 10 September 2014).
Not available online. Purchase access only.
Abstracts of Diabetes UK Professional Conference 2010
Abstracts of Diabetes UK Annual Professional Conference. Liverpool, UK. 3–5 March 2010. Diabet Med 2010;27(Suppl. 1):1–188. URL: http://onlinelibrary.wiley.com/doi/10.1111/dme.2010.27.issue-s1/issuetoc (accessed 10 September 2014).
Not available online. Purchase access only.
Search results
| Terms scanned | Abstracts identified |
|---|---|
| sensor augmented | 2014 = 0 2013 = 1 |
| SAPT | 2014 = 0 2013 = 0 |
| minimed | 2014 = 0 2013 = 0 |
| paradigmveo | 2014 = 0 2013 = 0 |
| paradigm veo | 2014 = 0 2013 = 0 |
| animas | 2014 = 0 2013 = 0 |
| dexcom | 2014 = 0 2013 = 0 |
| Total | 1 |
European Association for the Study of Diabetes annual meeting
URL: www.easd.org
Date searched: 10 September 2014.
Advanced search
Session type = ALL Keyword = ALL.
Searched in presentation title and abstract body.
Meetings searched
The 50th European Association for the Study of Diabetes (EASD) Annual Meeting, 15–19 September 2014, Vienna, Austria.
The 49th EASD Annual Meeting, 23–27 September 2013, Barcelona, Spain (URL: www.abstractsonline.com/plan/start.aspx?mkey={7E87E03A-5554-4497-B245-98ADF263043C}).
The 48th EASD Annual Meeting, 1–5 October 2012, Berlin, Germany (URL: www.abstractsonline.com/plan/ViewSession.aspx?mID=1668&skey=8e40db00-2d48-40da-891e-e4c9db8d9378&mKey={2DBFCAF7-1539-42D5-8DDA-0A94ABB089E8}).
The 47th EASD Annual Meeting, 12–16 September 2011, Lisbon, Portugal (URL: www.abstractsonline.com/plan/start.aspx?mkey={BAFB2746-B0DD-4110-8588-E385FAF957B7}).
The 46th EASD Meeting. 20–24 September 2010, Stockholm, Sweden (URL: www.abstractsonline.com/plan/AdvancedSearch.aspx?mkey={10A86782-07E4-4A2D-9100-F660E5D752A9}).
The 45th EASD Meeting. 29 September-2 October 2009, Vienna, Austria (URL: www.abstractsonline.com/plan/start.aspx?mkey={B3E385FB-2CC7-4F7C-8766-2F743C19F069}).
Search results
| Terms | Hits in title | Hits in abstract body |
|---|---|---|
| “sensor augmented pump” | 2013 = 0 2012 = 0 2011 = 1 2010 = 0 2009 = 1 |
2013 = 0 2012 = 1 2011 = 0 2010 = 2 |
| “sensor augmented pumps” | 2013 = 0 2012 = 0 2011 = 0 2010 = 0 2009 = 0 |
2013 = 0 2012 = 0 2011 = 0 2010 = 0 |
| SAPT | 2013 = 0 2012 = 0 2011 = 0 2010 = 0 2009 = 0 |
2013 = 0 2012 = 0 2011 = 0 2010 = 0 |
| minimed | 2013 = 0 2012 = 0 2011 = 0 2010 = 0 2009 = 0 |
2013 = 5 2012 = 5 2011 = 2 2010 = 5 |
| paradigmveo | 2013 = 0 2012 = 0 2011 = 0 2010 = 0 2009 = 0 |
2013 = 0 2012 = 0 2011 = 0 2010 = 0 |
| “paradigm veo” | 2013 = 0 2012 = 0 2011 = 0 2010 = 0 2009 = 0 |
2013 = 0 2012 = 0 2011 = 2 2010 = 0 |
| “veo pump” | 2013 = 0 2012 = 0 2011 = 0 2010 = 0 2009 = 0 |
2013 = 0 2012 = 0 2011 = 0 2010 = 0 |
| animas | 2013 = 0 2012 = 0 2011 = 0 2010 = 0 2009 = 0 |
2013 = 0 2012 = 1 2011 = 2 2010 = 3 |
| vibe | 2013 = 0 2012 = 0 2011 = 0 2010 = 0 2009 = 0 |
2013 = 0 2012 = 0 2011 = 1 2010 = 0 |
| dexcom | 2013 = 0 2012 = 1 2011 = 0 2010 = 0 2009 = 0 |
2013 = 4 2012 = 7 2011 = 1 2010 = 2 |
| “insulin pump” | 2013 = 7 2012 = 8 2011 = 8 2010 = 5 2009 = 3 |
2013 = 18 2012 = 20 2011 = 18 2010 = 16 |
| “insulin pumps” | 2013 = 4 2012 = 0 2011 = 0 2010 = 0 2009 = 2 |
2013 = 8 2012 = 6 2011 = 6 2010 = 5 |
| “continuous subcutaneous insulin infusion” | 2013 = 3 2012 = 1 2011 = 4 2010 = 7 2009 = 6 |
2013 = 8 2012 = 8 2011 = 11 2010 = 13 |
| CSII | 2013 = 3 2012 = 2 2011 = 2 2010 = 3 2009 = 4 |
2013 = 15 2012 = 23 2011 = 17 2010 = 20 |
| “artificial pancreas” | 2013 = 2 2012 = 3 2011 = 0 2010 = 0 2009 = 0 |
2013 = 5 2012 = 7 2011 = 3 2010 = 1 |
| “multiple daily injection” | 2013 = 1 2012 = 0 2011 = 0 2010 = 4 2009 = 0 |
2013 = 6 2012 = 1 2011 = 2 2010 = 6 |
| “multiple daily injections” | 2013 = 0 2012 = 0 2011 = 0 2010 = 2 2009 = 3 |
2013 = 7 2012 = 2 2011 = 6 2010 = 7 |
| MDI | 2013 = 0 2012 = 2 2011 = 1 2010 = 0 2009 = 1 |
2013 = 13 2012 = 12 2011 = 8 2010 = 13 |
| Total | 94 | 354 |
| Overall total | 448 | |
| Overall total after removal of duplicates | 196 | |
American Diabetes Association Scientific Sessions
URL: www.diabetes.org/
Date searched: 10 September 2014.
Sessions searched
74th American Diabetes Association Scientific Sessions, 13–17 June 2014, San Francisco, CA (URL: www.abstractsonline.com/plan/start.aspx?mkey={40FC5C61-819A-4D1B-AABA-3705F7D0EA76}).
73rd American Diabetes Association Scientific Sessions, 21–25 June 2013, Chicago, IL (URL: www.abstractsonline.com/plan/start.aspx?mkey={89918D6D-3018-4EA9-9D4F-711F98A7AE5D}).
72nd American Diabetes Association Scientific Sessions, 8–12 June 2012, Philadelphia, PA (URL: www.abstractsonline.com/plan/start.aspx?mkey={0F70410F-8DF3-49F5-A63D-3165359F5371}).
Search results
| Terms | Hits in abstract title |
|---|---|
| “sensor augmented pump” | 2014 = 0 2013 = 0 2012 = 0 |
| “sensor augmented pumps” | 2014 = 0 2013 = 0 2012 = 0 |
| SAPT | 2014 = 0 2013 = 0 2012 = 0 |
| minimed | 2014 = 0 2013 = 2 2012 = 0 |
| paradigmveo | 2014 = 0 2013 = 0 2012 = 0 |
| “paradigm veo” | 2014 = 0 2013 = 0 2012 = 0 |
| “veo pump” | 2014 = 0 2013 = 0 2012 = 0 |
| animas | 2014 = 0 2013 = 0 2012 = 0 |
| vibe | 2014 = 0 2013 = 0 2012 = 0 |
| dexcom | 2014 = 1 2013 = 0 2012 = 1 |
| “insulin pump” | 2014 = 16 2013 = 7 2012 = 12 |
| “insulin pumps” | 2014 = 6 2013 = 0 2012 = 1 |
| “continuous subcutaneous insulin infusion” | 2014 = 5 2013 = 6 2012 = 3 |
| CSII | 2014 = 8 2013 = 7 2012 = 5 |
| “artificial pancreas” | 2014 = 13 2013 = 7 2012 = 4 |
| “multiple daily injection” | 2014 = 1 2013 = 0 2012 = 0 |
| “multiple daily injections” | 2014 = 1 2013 = 0 2012 = 0 |
| MDI | 2014 = 2 2013 = 6 2012 = 1 |
| Total | 115 |
| Total after removal of duplicates | 91 |
Cost-effectiveness searches
NHS Economic Evaluation Database (via Wiley Online Library)
Issue searched: 3 of 4, July 2014.
Date searched: 5 September 2014.
Search strategy
#1 MeSH descriptor: [Diabetes Mellitus, Type 1] this term only
#2 MeSH descriptor: [Diabetic Ketoacidosis] this term only
#3 (diabet* near/3 (typ* next 1 or typ* next i or type1 or typei or typ* next one)):ti,ab,kw
#4 (diabet* near/3 (britt* or juvenil* or pediatric or paediatric or early or keto* or labil* or acidos* or autoimmun* or auto next immun* or sudden next onset)):ti,ab,kw
#5 ((insulin* near/2 depend*) or insulindepend*):ti,ab,kw
#6 (dm1 or dm next 1 or dmt1 or dm next t1 or t1dm or t1 next dm or t1d or iddm):ti,ab,kw
#7 (ketoacidosis or acidoketosis or keto next acidosis or ketoacidemia or ketosis):ti,ab,kw
#8 MeSH descriptor: [Hyperglycemia] this term only
#9 MeSH descriptor: [Hypoglycemia] this term only
#10 (hyperglyc?em* or hypoglyc?em*):ti,ab,kw
#11 ((high or higher or low or lower or increas* or decreas* or deficien* or sufficien* or insufficien* or reduce* or reduction* or fluctuat* or fallen or falling or threshold or safe) near/3 (glucose* or sugar* or hba1c or hb next a1 or hba1 or a1c or h?emoglob* or glycoh?emoglob*)):ti,ab,kw
#12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11
#13 (sensor* near/3 (augment* or pump*))
#14 SAPT:ti,ab,kw
#15 minimed or paradigmveo
#16 (paradigm* near/3 (veo or pump*))
#17 (veo near/3 pump*)
#18 ((animas or vibe) near/3 (pump* or infus* or system*))
#19 dexcom
#20 #13 or #14 or #15 or #16 or #17 or #18 or #19
#21 MeSH descriptor: [Insulin Infusion Systems] this term only
#22 MeSH descriptor: [Pancreas, Artificial] this term only
#23 (insulin* near/3 (pump* or infus* or deliver* or catheter*)):ti,ab,kw
#24 (pump* near/2 (therap* or treatment*)):ti,ab,kw
#25 ((subcutaneous near/2 insulin*) or CSII):ti,ab,kw
#26 (artificial near/3 (pancreas or beta next cell*)):ti,ab,kw
#27 (closed next loop near/3 (pump* or deliver* or infus* or therap* or treatment* or system*)):ti,ab,kw
#28 accu-chek or cellnovo or dana next diabecare or omnipod
#29 ((integrat* or dual or combined or unified) near/3 (system* or device*)):ti,ab,kw
#30 #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29
#31 MeSH descriptor: [Insulin] this term only
#32 MeSH descriptor: [Injections, Subcutaneous] this term only
#33 #31 and #32
#34 “multiple daily” near/3 (inject* or insulin* or regime* or routine*):ti,ab,kw
#35 “multiple dose” near/3 (inject* or insulin* or regime* or routine*):ti,ab,kw
#36 multiple near/3 (inject* or insulin* or regime* or routine*):ti,ab,kw
#37 MDI:ti,ab,kw
#38 injection near/3 therapy:ti,ab,kw
#39 (basal* and bolus) near/3 (inject* or regime* or routine* or system*):ti,ab,kw
#40 (“short acting” near/3 insulin) or (“rapid acting” near/3 insulin):ti,ab,kw
#41 #34 or #35 or #36 or #37 or #38 or #39 or #40
#42 #12 and (#20 or #30 or #41)
NHS Economic Evaluation Database (NHS EED) records retrieved: 16 records.
Health Economic Evaluations Database (via Wiley Online Library)
Date range searched: from inception up to 5 September 2014.
Date searched: 5 September 2014.
Search strategy
AX=‘sensor augmented’ or sensor-augmented or SAPT (1)
AX=minimed or paradigmveo or ‘paradigm veo’ or ‘paradigm pump’ or ‘veo pump’ or ‘animas pump’ or ‘animas infusion’ or ‘vibe pump’ or ‘vibe infusion’ or ‘g4 platinum’ or dexcom (0)
CS=1 or 2 (1)
AX=diabetes or dm1 or ‘dm 1’ or dmt1 or ‘dm t1’ or t1dm or ‘t1 dm’ or t1d or iddm (2289)
AX=ketoacidosis or acidoketosis or ‘keto acidosis’ or ketoacidemia or ketosis (28)
AX=hyperglycemia or hypoglycemia or hyperglycaemia or hypoglycaemia (146)
CS=4 or 5 or 6 (2321)
AX=‘insulin pump’ or ‘insulin pumps’ or ‘insulin infusion’ or ‘insulin infusions’ or ‘insulin delivery’ (46)
AX=‘pump therapy’ or ‘subcutaneous insulin’ or CSII or ‘artificial pancreas’ or ‘artificial beta-cell’ (41)
AX=‘closed loop’ or accu-chek or cellnovo or ‘dana diabecare’ or omnipod (1)
AX=‘integrated system’ or ‘integrated systems’ or ‘integrated device’ or ‘integrated devices’ or ‘dual system’ or ‘dual systems’ or ‘dual device’ or ‘dual devices’ (7)
AX=‘multiple daily injection’ or ‘multiple daily injections’ or ‘multiple daily insulin’ or ‘multiple dose injection’ or ‘multiple dose injections’ or ‘multiple dose insulin’ or AX=‘multiple injection’ or ‘multiple injections’ or ‘multiple insulin’ OR MDI (45)
CS=8 or 9 or 10 or 11 or 12 (86)
CS=7 and 12 (52)
CS=3 or 14 (52)
EMBASE (via OvidSP)
Date range searched: 1974–2014/week 34.
Date searched: 5 September 2014.
Search strategy
-
insulin dependent diabetes mellitus/ (78,607)
-
exp diabetic ketoacidosis/ (7787)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ti,ab,ot,hw. (49,088)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ti,ab,ot,hw. (29,355)
-
((insulin$ adj2 depend$) or insulindepend$).ti,ab,ot,hw. (217,259)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,ot,hw. (20,038)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,ot,hw. (14,231)
-
hypoglycemia/ or hyperglycemia/ (108,615)
-
(hyperglyc?em$ or hypoglyc?em$).ti,ab,ot. (104,051)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ti,ab,ot,hw. (126,603)
-
or/1-10 (436,900)
-
(sensor$ adj3 (augment$ or pump$)).ti,ab,hw,ot. (598)
-
SAPT.ti,ab,ot,hw. (114)
-
(minimed or paradigmveo).ti,ab,ot,hw,dm,dv. (727)
-
(paradigm$ adj3 (veo or pump$)).ti,ab,hw,ot,dm,dv. (127)
-
(veo adj3 pump$).ti,ab,ot,hw,dm,dv. (38)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ti,ab,ot,hw,dm,dv. (25)
-
(g4 adj3 platinum).ti,ab,ot,hw,dm,dv. (27)
-
dexcom.ti,ab,ot,hw,dm,dv. (298)
-
or/12-19 (1674)
-
insulin pump/ (3425)
-
insulin infusion/ (5096)
-
artificial pancreas/ (1433)
-
(insulin$ adj3 (pump$ or infus$ or deliver$ or catheter$)).ti,ab,ot,hw. (17,265)
-
(pump$ adj2 (therap$ or treatment$)).ti,ab,ot,hw. (3171)
-
((subcutaneous adj2 insulin$) or CSII).ti,ab,ot,hw. (4218)
-
(artificial adj3 (pancreas or beta cell$)).ti,ab,ot,hw. (2050)
-
(closed loop adj3 (pump$ or deliver$ or infus$ or therap$ or treatment$ or system$)).ti,ab,ot,hw. (1941)
-
(accu-chek or cellnovo or dana diabecare or omnipod).ti,ab,ot,hw,dm,dv. (529)
-
((integrat$ or dual or combined or unified) adj3 (system$ or device$)).ti,ab,ot,hw. (39,256)
-
or/21-30 (62,055)
-
insulin/ and exp injection/ (3392)
-
(multiple daily adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (1188)
-
(multiple dose adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (561)
-
(multiple adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (9358)
-
MDI.ti,ab,hw,ot. (3791)
-
(injection adj3 therapy).ti,ab,ot,hw. (4157)
-
((basal$ and bolus) adj3 (injection$ or regime$ or routine$ or system$)).ti,ab,hw,ot. (1491)
-
(short acting adj3 insulin).ti,ab,hw,ot. (1038)
-
(rapid acting adj3 insulin).ti,ab,hw,ot. (864)
-
or/32-40 (22,079)
-
20 or 31 or 41 (82,594)
-
11 and 42 (18,536)
-
health-economics/ (33,789)
-
exp economic-evaluation/ (214,699)
-
exp health-care-cost/ (207,493)
-
exp pharmacoeconomics/ (168,062)
-
or/44-47 (484,055)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (620,526)
-
(expenditure$ not energy).ti,ab. (24,446)
-
(value adj2 money).ti,ab. (1422)
-
budget$.ti,ab. (24,740)
-
or/49-52 (645,088)
-
48 or 53 (918,375)
-
letter.pt. (853,934)
-
editorial.pt. (454,769)
-
note.pt. (566,292)
-
or/55-57 (1,874,995)
-
54 not 58 (830,092)
-
(metabolic adj cost).ti,ab. (913)
-
((energy or oxygen) adj cost).ti,ab. (3189)
-
((energy or oxygen) adj expenditure).ti,ab. (20,605)
-
or/60-62 (23,877)
-
59 not 63 (824,949)
-
exp animal/ (19,314,568)
-
exp animal-experiment/ (1,798,176)
-
nonhuman/ (4,359,920)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (4,850,843)
-
or/65-68 (20,707,342)
-
exp human/ (15,050,997)
-
exp human-experiment/ (328,369)
-
70 or 71 (15,052,426)
-
69 not (69 and 72) (5,655,873)
-
64 not 73 (761,307)
-
43 and 74 (1027)
The economics terms were based on the following costs filter:
Centre for Reviews and Dissemination. Search strategies: NHS EED EMBASE using OvidSP (economics filter). York: Centre for Reviews and Dissemination; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedembase (accessed 2 June 2014).
MEDLINE (via OvidSP)
Date range searched: 1946–2014/August week 4.
Date searched: 5 September 2014.
-
Diabetes Mellitus, Type 1/ (62,323)
-
Diabetic Ketoacidosis/ (5178)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ti,ab,ot,hw. (69,580)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ti,ab,ot,hw. (20,273)
-
((insulin$ adj2 depend$) or insulindepend$).ti,ab,ot,hw. (30469)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,ot,hw. (13,085)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,ot,hw. (9331)
-
Hyperglycemia/ (20,833)
-
Hypoglycemia/ (21,743)
-
(hyperglyc?em$ or hypoglyc?em$).ti,ab,ot. (72,656)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ti,ab,ot,hw. (94,623)
-
or/1-11 (245,714)
-
(sensor$ adj3 (augment$ or pump$)).ti,ab,hw,ot. (312)
-
SAPT.ti,ab,ot,hw. (93)
-
(minimed or paradigmveo).ti,ab,ot,hw. (197)
-
(paradigm$ adj3 (veo or pump$)).ti,ab,hw,ot. (34)
-
(veo adj3 pump$).ti,ab,ot,hw. (5)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ti,ab,ot,hw. (7)
-
(g4 adj3 platinum).ti,ab,ot,hw. (3)
-
dexcom.ti,ab,ot,hw. (44)
-
or/13-20 (645)
-
Insulin Infusion Systems/ (3988)
-
Pancreas, Artificial/ (402)
-
(insulin$ adj3 (pump$ or infus$ or deliver$ or catheter$)).ti,ab,ot,hw. (11,972)
-
(pump$ adj2 (therap$ or treatment$)).ti,ab,ot,hw. (1810)
-
((subcutaneous adj2 insulin$) or CSII).ti,ab,ot,hw. (2474)
-
(artificial adj3 (pancreas or beta cell$)).ti,ab,ot,hw. (1203)
-
(closed loop adj3 (pump$ or deliver$ or infus$ or therap$ or treatment$ or system$)).ti,ab,ot,hw. (1310)
-
(accu-chek or cellnovo or dana diabecare or omnipod).ti,ab,ot,hw. (150)
-
((integrat$ or dual or combined or unified) adj3 (system$ or device$)).ti,ab,ot,hw. (32,573)
-
or/22-30 (47,787)
-
Insulin/ and Injections, Subcutaneous/ (2134)
-
(multiple daily adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (624)
-
(multiple dose adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (452)
-
(multiple adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (6795)
-
MDI.ti,ab,hw,ot. (2372)
-
(injection adj3 therapy).ti,ab,ot,hw. (2858)
-
((basal$ and bolus) adj3 (injection$ or regime$ or routine$ or system$)).ti,ab,hw,ot. (1015)
-
(short acting adj3 insulin).ti,ab,hw,ot. (466)
-
(rapid acting adj3 insulin).ti,ab,hw,ot. (468)
-
or/32-40 (15,196)
-
21 or 31 or 41 (61,753)
-
12 and 42 (10,730)
-
economics/ (27,125)
-
exp “costs and cost analysis”/ (184,746)
-
economics, dental/ (1867)
-
exp “economics, hospital”/ (19,806)
-
economics, medical/ (8680)
-
economics, nursing/ (3985)
-
economics, pharmaceutical/ (2574)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (431,861)
-
(expenditure$ not energy).ti,ab. (17,649)
-
(value adj1 money).ti,ab. (23)
-
budget$.ti,ab. (17,373)
-
or/44-54 (557,969)
-
((energy or oxygen) adj cost).ti,ab. (2704)
-
(metabolic adj cost).ti,ab. (788)
-
((energy or oxygen) adj expenditure).ti,ab. (16,809)
-
or/56-58 (19,580)
-
55 not 59 (553,698)
-
letter.pt. (826,900)
-
editorial.pt. (346,911)
-
historical article.pt. (306,574)
-
or/61-63 (1,465,388)
-
60 not 64 (525,046)
-
43 and 65 (327)
The economics terms were based on the following costs filter:
Centre for Reviews and Dissemination. Search strategies: NHS EED MEDLINE using OvidSP (economics filter). York: Centre for Reviews and Dissemination; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedmedline (accessed 2 June 2014).
MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE Daily Update (via OvidSP); 4 September 2014
Date searched: 5 September 2014.
Search strategy
-
Diabetes Mellitus, Type 1/ (36)
-
Diabetic Ketoacidosis/ (3)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ti,ab,ot,hw. (2614)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ti,ab,ot,hw. (1105)
-
((insulin$ adj2 depend$) or insulindepend$).ti,ab,ot,hw. (701)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,ot,hw. (884)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,ot,hw. (430)
-
Hyperglycemia/ (20)
-
Hypoglycemia/ (10)
-
(hyperglyc?em$ or hypoglyc?em$).ti,ab,ot. (5462)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ti,ab,ot,hw. (7457)
-
or/1-11 (14909)
-
(sensor$ adj3 (augment$ or pump$)).ti,ab,hw,ot. (59)
-
SAPT.ti,ab,ot,hw. (83)
-
(minimed or paradigmveo).ti,ab,ot,hw. (13)
-
(paradigm$ adj3 (veo or pump$)).ti,ab,hw,ot. (4)
-
(veo adj3 pump$).ti,ab,ot,hw. (1)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ti,ab,ot,hw. (0)
-
(g4 adj3 platinum).ti,ab,ot,hw. (3)
-
dexcom.ti,ab,ot,hw. (7)
-
or/13-20 (164)
-
Insulin Infusion Systems/ (2)
-
Pancreas, Artificial/ (2)
-
(insulin$ adj3 (pump$ or infus$ or deliver$ or catheter$)).ti,ab,ot,hw. (504)
-
(pump$ adj2 (therap$ or treatment$)).ti,ab,ot,hw. (189)
-
((subcutaneous adj2 insulin$) or CSII).ti,ab,ot,hw. (172)
-
(artificial adj3 (pancreas or beta cell$)).ti,ab,ot,hw. (61)
-
(closed loop adj3 (pump$ or deliver$ or infus$ or therap$ or treatment$ or system$)).ti,ab,ot,hw. (343)
-
(accu-chek or cellnovo or dana diabecare or omnipod).ti,ab,ot,hw. (16)
-
((integrat$ or dual or combined or unified) adj3 (system$ or device$)).ti,ab,ot,hw. (4137)
-
or/22-30 (5154)
-
Insulin/ and Injections, Subcutaneous/ (3)
-
(multiple daily adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (66)
-
(multiple dose adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (9)
-
(multiple adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,ot,hw. (492)
-
MDI.ti,ab,hw,ot. (161)
-
(injection adj3 therapy).ti,ab,ot,hw. (206)
-
((basal$ and bolus) adj3 (injection$ or regime$ or routine$ or system$)).ti,ab,hw,ot. (51)
-
(short acting adj3 insulin).ti,ab,hw,ot. (29)
-
(rapid acting adj3 insulin).ti,ab,hw,ot. (59)
-
or/32-40 (937)
-
21 or 31 or 41 (6140)
-
12 and 42 (543)
-
economics/ (0)
-
exp “costs and cost analysis”/ (103)
-
economics, dental/ (0)
-
exp “economics, hospital”/ (10)
-
economics, medical/ (0)
-
economics, nursing/ (0)
-
economics, pharmaceutical/ (0)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (51,540)
-
(expenditure$ not energy).ti,ab. (1501)
-
(value adj1 money).ti,ab. (5)
-
budget$.ti,ab. (2211)
-
or/44-54 (53,783)
-
((energy or oxygen) adj cost).ti,ab. (294)
-
(metabolic adj cost).ti,ab. (80)
-
((energy or oxygen) adj expenditure).ti,ab. (1183)
-
or/56-58 (1507)
-
55 not 59 (53,348)
-
letter.pt. (30,310)
-
editorial.pt. (18,730)
-
historical article.pt. (112)
-
or/61-63 (49,132)
-
60 not 64 (52,805)
-
43 and 65 (35)
The economics terms were based on the following costs filter:
Centre for Reviews and Dissemination. Search strategies: NHS EED MEDLINE using OvidSP (economics filter). York: Centre for Reviews and Dissemination; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedmedline (accessed 2 June 2014).
PubMed (via the National Library of Medicine)
URL: www.ncbi.nlm.nih.gov/pubmed/
Date range searched: from inception up to 5 September 2014.
Date searched: 5 September 2014.
Search strategy
| #59 | Search (#57 and #58) | 20 |
| #58 | Search (pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb]) | 18,150,03 |
| #57 | Search (#46 and #56) | 188 |
| #56 | Search (#51 not #55) | 498,516 |
| #55 | Search (#52 or #53 or #54) | 20,445 |
| #54 | Search “energy expenditure”[tiab] or “oxygen expenditure”[tiab] | 17,356 |
| #53 | Search “metabolic cost”[tiab] | 879 |
| #52 | Search “energy cost”[tiab] or “oxygen cost”[tiab] | 2972 |
| #51 | Search (#47 or #48 or #49 or #50) | 503,197 |
| #50 | Search budget*[tiab] | 19,728 |
| #49 | Search “value for money” | 928 |
| #48 | Search (expenditure*[tiab] not energy[tiab]) | 19,130 |
| #47 | Search (economic*[tiab] or cost[tiab] or costs[tiab] or costly[tiab] or costing[tiab] or price[tiab] or prices[tiab] or pricing[tiab] or pharmacoeconomic*[tiab]) | 482,242 |
| #46 | Search (#20 and #45) | 5237 |
| #45 | Search (#28 or #36 or #44) | 20,242 |
| #44 | Search (#37 or #38 or #39 or #40 or #41 or #42 or #43) | 9426 |
| #43 | Search (“short acting insulin”[tiab] OR “rapid acting insulin”[tiab]) | 810 |
| #42 | Search (basal*[tiab] AND bolus[tiab] AND (injection*[tiab] OR regime*[tiab] OR routine*[tiab] OR system*[tiab])) | 1549 |
| #41 | Search “injection therapy”[tiab] | 2098 |
| #40 | Search MDI[tiab] | 2524 |
| #39 | Search “multiple injection”[tiab] or “multiple injections”[tiab] or “multiple insulin”[tiab] or “multiple regime”[tiab] or “multiple regimes”[tiab] or “multiple routine”[tiab] or “multiple routines”[tiab] | 2414 |
| #38 | Search “multiple dose injection”[tiab] or “multiple dose injections”[tiab] or “multiple dose insulin”[tiab] or “multiple dose regime”[tiab] or “multiple dose regimes”[tiab] or “multiple dose routine”[tiab] or “multiple dose routines”[tiab] | 48 |
| #37 | Search “multiple daily injection”[tiab] or “multiple daily injections”[tiab] or “multiple daily insulin”[tiab] or “multiple daily regime”[tiab] or “multiple daily regimes”[tiab] or “multiple daily routine”[tiab] or “multiple daily routines”[tiab] | 603 |
| #36 | Search (#29 or #30 or #31 or #32 or #33 or #34 or #35) | 10,964 |
| #35 | Search “integrated system”[tiab] or “integrated systems”[tiab] “integrated device”[tiab] or “integrated devices”[tiab] or “dual system”[tiab] or “dual systems”[tiab] or “dual device”[tiab] or “dual devices”[tiab] or “combined system”[tiab] or “combined systems”[tiab] or “combined device”[tiab] or “combined devices”[tiab] or “unified system”[tiab] or “unified systems”[tiab] or “unified device”[tiab] or “unified devices”[tiab] | 1317 |
| #34 | Search (accu-chek[tiab] or cellnovo[tiab] or “dana diabecare”[tiab] or omnipod[tiab]) | 159 |
| #33 | Search “closed loop pump”[tiab] or “closed loop pumps”[tiab] or “closed loop delivery”[tiab] or “closed loop infusion”[tiab] or “closed loop infusions”[tiab] or “closed loop therapy”[tiab] or “closed loop treatment”[tiab] or “closed loop treatments”[tiab] or “closed loop system”[tiab] or “closed loop systems”[tiab] | 812 |
| #32 | Search “artificial pancreas”[tiab] or “artificial beta cell”[tiab] | 822 |
| #31 | Search “subcutaneous insulin”[tiab] or CSII[tiab] | 2385 |
| #30 | Search “pump therapy”[tiab] or “pump therapies”[tiab] or “pump treatment”[tiab] or “pump treatments”[tiab] | 920 |
| #29 | Search “insulin pump”[tiab] or “insulin pumps”[tiab] or “insulin infusion”[tiab] or “insulin infuse”[tiab] or “insulin infused”[tiab] or “insulin deliver”[tiab] or “insulin delivery”[tiab] | 7485 |
| #28 | Search (#21 or #22 or #23 or #24 or #25 or #26 or #27) | 928 |
| #27 | Search dexcom | 54 |
| #26 | Search (animas or vibe) AND (pump* or infus* or system*) | 81 |
| #25 | Search “veo pump” or “veo pumps” | 15 |
| #24 | Search ((paradigm* AND (veo or pump*))) | 350 |
| #23 | Search minimed or paradigmveo | 216 |
| #22 | Search SAPT[tiab] | 184 |
| #21 | Search “sensor augmented”[tiab] or “sensor augment”[tiab] or “sensor pump”[tiab] or “pump sensor”[tiab] or “sensor pumps”[tiab] | 91 |
| #20 | Search (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19) | 126,838 |
| #19 | Search “high glycohemoglobin”[tiab] or “higher glycohemoglobin”[tiab] or “low glycohemoglobin”[tiab] or “lower glycohemoglobin”[tiab] or “increase glycohemoglobin”[tiab] or “increased glycohemoglobin”[tiab] or “increases glycohemoglobin”[tiab] or “decrease glycohemoglobin”[tiab] or “decreased glycohemoglobin”[tiab] or “decreases glycohemoglobin”[tiab] or “deficient glycohemoglobin”[tiab] or “sufficient glycohemoglobin”[tiab] or “insufficient glycohemoglobin”[tiab] or “reduce glycohemoglobin”[tiab] or “reduced glycohemoglobin”[tiab] or “glycohemoglobin reduction”[tiab] or “fallen glycohemoglobin”[tiab] or “falling glycohemoglobin”[tiab] or “glycohemoglobin threshold”[tiab] or “safe glycohemoglobin”[tiab] | 17 |
| #18 | Search (“high haemoglobin”[tiab] or “higher haemoglobin”[tiab] or “low haemoglobin”[tiab] or “lower haemoglobin”[tiab] or “increase haemoglobin”[tiab] or “increased haemoglobin”[tiab] or “increases haemoglobin”[tiab] or “decrease haemoglobin”[tiab] or “decreased haemoglobin”[tiab] or “decreases haemoglobin”[tiab] or “deficient haemoglobin”[tiab] or “sufficient haemoglobin”[tiab] or “insufficient haemoglobin”[tiab] or “reduce haemoglobin”[tiab] or “reduced haemoglobin”[tiab] or “haemoglobin reduction”[tiab] or “fallen haemoglobin”[tiab] or “falling haemoglobin”[tiab] or “haemoglobin threshold”[tiab] or “safe haemoglobin”[tiab]) | 1161 |
| #17 | Search “high hemoglobin”[tiab] or “higher hemoglobin”[tiab] or “low hemoglobin”[tiab] or “lower hemoglobin”[tiab] or “increase hemoglobin”[tiab] or “increased hemoglobin”[tiab] or “increases hemoglobin”[tiab] or “decrease hemoglobin”[tiab] or “decreasedchemoglobin”[tiab] or “decreases hemoglobin”[tiab] or “deficient hemoglobin”[tiab] or “sufficient hemoglobin”[tiab] or “insufficient hemoglobin”[tiab] or “reduce hemoglobin”[tiab] or “reduced hemoglobin”[tiab] or “hemoglobin reduction”[tiab] or “fallen hemoglobin”[tiab] or “falling hemoglobin”[tiab] or “hemoglobin threshold”[tiab] or “safe hemoglobin”[tiab] | 3476 |
| #16 | Search “high a1c”[tiab] or “higher a1c”[tiab] or “low a1c”[tiab] or “lower a1c”[tiab] or “increase a1c”[tiab] or “increased a1c”[tiab] or “increases a1c”[tiab] or “decrease a1c”[tiab] or “decreasedca1c”[tiab] or “decreases a1c”[tiab] or “deficient a1c”[tiab] or “sufficient a1c”[tiab] or “insufficient a1c”[tiab] or “reduce a1c”[tiab] or “reduced a1c”[tiab] or “a1c reduction”[tiab] or “fallen a1c”[tiab] or “falling a1c”[tiab] or “a1c threshold”[tiab] or “safe a1c”[tiab] | 291 |
| #15 | Search (((“high hba1”[tiab] or “higher hba1”[tiab] or “low hba1”[tiab] or “lower hba1”[tiab] or “increase hba1”[tiab] or “increased hba1”[tiab] or “increases hba1”[tiab] or “decrease hba1”[tiab] or “decreasedchba1”[tiab] or “decreases hba1”[tiab] or “deficient hba1”[tiab] or “sufficient hba1”[tiab] or “insufficient hba1”[tiab] or “reduce hba1”[tiab] or “reduced hba1”[tiab] or “hba1 reduction”[tiab] or “fallen hba1”[tiab] or “falling hba1”[tiab] or “hba1 threshold”[tiab] or “safe hba1”[tiab]))) | 76 |
| #14 | Search “high hb a1”[tiab] or “higher hb a1”[tiab] or “low hb a1”[tiab] or “lower hb a1”[tiab] or “increase hb a1”[tiab] or “increased hb a1”[tiab] or “increases hb a1”[tiab] or “decrease hb a1”[tiab] or “decreasedchb a1”[tiab] or “decreases hb a1”[tiab] or “deficient hb a1”[tiab] or “sufficient hb a1”[tiab] or “insufficient hb a1”[tiab] or “reduce hb a1”[tiab] or “reduced hb a1”[tiab] or “hb a1 reduction”[tiab] or “fallen hb a1”[tiab] or “falling hb a1”[tiab] or “hb a1 threshold”[tiab] or “safe hb a1”[tiab] | 0 |
| #13 | Search “high hba1c”[tiab] or “higher hba1c”[tiab] or “low hba1c”[tiab] or “lower hba1c”[tiab] or “increase hba1c”[tiab] or “increased hba1c”[tiab] or “increases hba1c”[tiab] or “decrease hba1c”[tiab] or “decreasedchba1c”[tiab] or “decreases hba1c”[tiab] or “deficient hba1c”[tiab] or “sufficient hba1c”[tiab] or “insufficient hba1c”[tiab] or “reduce hba1c”[tiab] or “reduced hba1c”[tiab] or “hba1c reduction”[tiab] or “fallen hba1c”[tiab] or “falling hba1c”[tiab] or “hba1c threshold”[tiab] or “safe hba1c”[tiab] | 1271 |
| #12 | Search “high sugar”[tiab] or “higher sugar”[tiab] or “low sugar”[tiab] or “lower sugar”[tiab] or “increase sugar”[tiab] or “increased sugar”[tiab] or “increases sugar”[tiab] or “decrease sugar”[tiab] or “decreasedcsugar”[tiab] or “decreases sugar”[tiab] or “deficient sugar”[tiab] or “sufficient sugar”[tiab] or “insufficient sugar”[tiab] or “reduce sugar”[tiab] or “reduced sugar”[tiab] or “sugar reduction”[tiab] or “fallen sugar”[tiab] or “falling sugar”[tiab] or “sugar threshold”[tiab] or “safe sugar”[tiab] | 1539 |
| #11 | Search (“high glucose”[tiab] or “higher glucose”[tiab] or “low glucose”[tiab] or “lower glucose”[tiab] or “increase glucose”[tiab] or “increased glucose”[tiab] or “increases glucose”[tiab] or “decrease glucose”[tiab] or “decreasedcglucose”[tiab] or “decreases glucose”[tiab] or “deficient glucose”[tiab] or “sufficient glucose”[tiab] or “insufficient glucose”[tiab] or “reduce glucose”[tiab] or “reduced glucose”[tiab] or “glucose reduction”[tiab] or “fallen glucose”[tiab] or “falling glucose”[tiab] or “glucose threshold”[tiab] or “safe glucose”[tiab]) | 16,645 |
| #10 | Search (hyperglycemia[tiab] or hypoglycaemia[tiab] or hyperglycemic[tiab] or hypoglycaemic[tiab]) | 44,267 |
| #9 | Search ketoacidosis[tiab] or acidoketosis[tiab] or “keto acidosis”[tiab] or ketoacidemia[tiab] or ketosis[tiab] | 7293 |
| #8 | Search dm1[tiab] or “dm 1”[tiab] or t1dm[tiab] or “t1 dm”[tiab] or t1d[tiab] or iddm[tiab] | 13,131 |
| #7 | Search “insulin dependent”[tiab] or insulindepend*[tiab] | 27,550 |
| #6 | Search “brittle diabetic”[tiab] or “diabetic juvenile”[tiab] or “diabetic pediatric”[tiab] or “diabetic paediatric”[tiab] or “diabetic early”[tiab] or “diabetic labile”[tiab] or “diabetic acidosis”[tiab] or “diabetic sudden onset”[tiab] | 348 |
| #5 | Search “diabetic brittle”[tiab] or “juvenile diabetic”[tiab] or “pediatric diabetic”[tiab] or “paediatric diabetic”[tiab] or “early diabetic”[tiab] or “labile diabetic”[tiab] or “acidosis diabetic”[tiab] or “sudden onset diabetic”[tiab] | 1122 |
| #4 | Search “brittle diabetes”[tiab] or “diabetes juvenile”[tiab] or “diabetes pediatric”[tiab] or “diabetes paediatric”[tiab] or “diabetes early”[tiab] or “diabetes ketosis”[tiab] or “diabetes labile”[tiab] or “diabetes acidosis”[tiab] or “diabetes sudden onset”[tiab] | 264 |
| #3 | Search “diabetes brittle”[tiab] or “juvenile diabetes”[tiab] or “pediatric diabetes”[tiab] or “paediatric diabetes”[tiab] or “early diabetes”[tiab] or “ketosis diabetes”[tiab] or “labile diabetes”[tiab] or “acidosis diabetes”[tiab] or “sudden onset diabetes”[tiab] | 2238 |
| #2 | Search “diabetic type 1”[tiab] OR “type 1 diabetic”[tiab] OR “diabetic type i”[tiab] OR “type i diabetic”[tiab] OR “diabetic type1”[tiab] OR “type1 diabetic”[tiab] OR “diabetic typei”[tiab] OR “typei diabetic”[tiab] | 6044 |
| #1 | Search (((“diabetes type 1”[tiab] OR “type 1 diabetes”[tiab] OR “diabetes type i”[tiab] OR “type i diabetes”[tiab] OR “diabetes type1”[tiab] OR “type1 diabetes”[tiab] OR “diabetes typei”[tiab] OR “typei diabetes”[tiab]))) | 28,884 |
The economics terms were based on the following costs filter:
Centre for Reviews and Dissemination. Search strategies: NHS EED MEDLINE using OvidSP (economics filter). York: Centre for Reviews and Dissemination; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedmedline (accessed 2 June 2014).
American Economic Association’s electronic bibliography EconLit (via EBSCOhost)
Date range searched: 1969 to 1 August 2014.
Date searched: 5 September 2014.
Search strategy
S28 S7 AND S27 (1)
S27 (S11 OR S19 OR S26) (2379)
S26 S20 OR S21 OR S22 OR S23 OR S24 OR S25 (174)
S25 TI (“short acting” N3 insulin or “rapid acting” N3 insulin) or AB (“short acting” N3 insulin or “rapid acting” N3 insulin (0)
S24 TI (((basal* and bolus) N3 injection*) or ((basal* and bolus) N3 regime*) or ((basal* and bolus) N3 routine*) or ((basal* and bolus) N3 system*)) or AB (((basal* and bolus) N3 injection*) or ((basal* and bolus) N3 regime*) or ((basal* and bolus) N3 routine*) or ((basal* and bolus) N3 system*)) (0)
S23 TI (MDI or injection N3 therapy) or AB (MDI or injection N3 therapy) (11)
S22 TI (multiple N3 inject* or multiple N3 insulin* or multiple N3 regime* or multiple N3 routine*) or AB (multiple N3 inject* or multiple N3 insulin* or multiple N3 regime* or multiple N3 routine*) (163)
S21 TI (“multiple dose” N3 inject* or “multiple dose” N3 insulin* or “multiple dose” N3 regime* or “multiple dose” N3 routine*) or AB (“multiple dose” N3 inject* or “multiple dose” N3 insulin* or “multiple dose” N3 regime* or “multiple dose” N3 routine*) (0)
S20 TI (“multiple daily” N3 inject* or “multiple daily” N3 insulin* or “multiple daily” N3 regime* or “multiple daily” N3 routine*) or AB (“multiple daily” N3 inject* or “multiple daily” N3 insulin* or “multiple daily” N3 regime* or “multiple daily” N3 routine*) (0)
S19 S12 or S13 or S14 or S15 or S16 or S17 or S18 (2,206)
S18 TI (integrat* N3 system* or integrat* N3 device* or dual N3 system* or dual N3 device* or combined N3 system* or combined N3 device* or unified N3 system* or unified N3 device) or AB (integrat* N3 system* or integrat* N3 device* or dual N3 system* or dual N3 device* or combined N3 system* or combined N3 device* or unified N3 system* or unified N3 device) (2,187)
S17 TI (accu-chek or cellnovo or “dana diabecare” or omnipod) or AB (accu-chek or cellnovo or “dana diabecare” or omnipod) (0)
S16 TI (“closed loop” N3 pump* or “closed loop” N3 deliver* or “closed loop” N3 infus* or “closed loop” N3 therap* or “closed loop” N3 treatment* or “closed loop” N3 system*) or AB (“closed loop” N3 pump* or “closed loop” N3 deliver* or “closed loop” N3 infus* or “closed loop” N3 therap* or “closed loop” N3 treatment* or “closed loop” N3 system*) (18)
S15 TI (artificial N3 pancreas or artificial N3 “beta cell*” or artificial N2 beta-cell*) or AB (artificial N3 pancreas or artificial N3 “beta cell*” or artificial N3 beta-cell*) (0)
S14 TI (subcutaneous N2 insulin* or CSII) or AB (subcutaneous N2 insulin* or CSII (2)
S13 TI (pump* N3 therap* or pump* N3 treatment*) or AB (pump* N3 therap* or pump* N3 treatment*) (1)
S12 TI (insulin* N3 pump* or insulin* N3 infus* or insulin* N3 deliver* or insulin N3 catheter*) or AB (insulin* N3 pump* or insulin* N3 infus* or insulin* N3 deliver* or insulin N3 catheter*) (1)
S11 S8 or S9 or S10 (0)
S10 TI (animas N3 pump* or animas N3 infus* or animas N3 system* or vibe N3 pump* or vibe N3 infus* or vibe N3 system* or g4 N3 platinum or dexcom) or AB (animas N3 pump* or animas N3 infus* or animas N3 system* or vibe N3 pump* or vibe N3 infus* or vibe N3 system* or g4 N3 platinum or dexcom) (0)
S9 TI (minimed or paradigmveo or paradigm* N3 veo or paradigm* N3 pump* or veo N3 pump*) or AB (minimed or paradigmveo or paradigm* N3 veo or paradigm* N3 pump* or veo N3 pump*) (0)
S8 TI (sensor* N3 augment* or sensor* N3 pump* or sensor-augment* or SAPT) or AB (sensor* N3 augment* or sensor* N3 pump* or sensor-augment* or SAPT) (0)
S7 S1 or S2 or S3 or S4 or S5 or S6 (26)
S6 TI (hyperglycem* or hypoglycem* or hyperglycaem* or hypoglycaem*) or AB (hyperglycem* or hypoglycem* or hyperglycaem* or hypoglycaem*) (5)
S5 TI (ketoacidosis or acidoketosis or “keto acidosis” or ketoacidemia or ketosis) or AB (ketoacidosis or acidoketosis or “keto acidosis” or ketoacidemia or ketosis) (0)
S4 TI (dm1 or “dm 1” or dmt1 or “dm t1” or t1dm or “t1 dm” or t1d or iddm) or AB (dm1 or “dm 1” or dmt1 or “dm t1” or t1dm or “t1 dm” or t1d or iddm) (2)
S3 TI (insulin* N2 depend* or insulindepend*) or AB (insulin* N2 depend* or insulindepend*) (5)
S2 TI (diabet* N3 britt* or diabet* N3 juvenil* or diabet* N3 pediatric or diabet* N3 paediatric or diabet* N3 early or diabet* N3 keto* or diabet* N3 labil* or diabet* N3 acidos* or diabet* N3 autoimmun* or diabet* N3 “auto immune*” or diabet* N3 “sudden onset”) or AB (diabet* N3 britt* or diabet* N3 juvenil* or diabet* N3 pediatric or diabet* N3 paediatric or diabet* N3 early or diabet* N3 keto* or diabet* N3 labil* or diabet* N3 acidos* or diabet* N3 autoimmun* or diabet* N3 “auto immune*” or di ... (2)
S1 TI (diabet* N3 “typ* 1” or diabet* N3 “typ* i” or diabet* N3 type1 or diabet* N3 typei or diabet* N3 “typ* one”) or AB (diabet* N3 “typ* 1” or diabet* N3 “typ* i” or diabet* N3 type1 or diabet* N3 typei or diabet* N3 “typ* one”) (14)
Cost-effectiveness Analysis Registry
URL: www.cearegistry.org
Date range searched: from inception up to 5 September 2014.
Date searched: 5 September 2014.
Seven records retrieved.
Search strategy
sensor augmented
sensor-augmented
SAPT
minimed
paradigmveo
paradigm veo
paradigm-veo
veo pump
animas
vibe pump
vibe infusion
vibe system
vibe systems
g4 platinum
dexcom
insulin pump
insulin pumps
insulin infusion
insulin delivery
pump therapy
pump treatment
pump treatments
subcutaneous insulin
CSII
artificial pancreas
artificial beta cell
artificial beta-cell
closed loop
integrated system
integrated systems
integrated device
integrated devices
multiple daily injection
multiple daily injections
multiple dose injection
multiple dose injections
multiple daily insulin
multiple dose insulin
multiple injection
multiple injections
MDI
injection therapy
basal bolus
short acting insulin
rapid acting insulin
RePEc Research Papers in Economics
URL: http://repec.org/
Date range searched: from inception up to 5 September 2014.
Date searched: 5 September 2014.
IDEAS search interface.
Search strategy
(“diabetes mellitus type 1” | “diabetes type 1” | “diabetes mellitus type1” | “diabetes type1” | “diabetes mellitus type I” | “diabetes type I” | “diabetes mellitus typeI” | “diabetes typeI” | “diabetes mellitus type one” | “diabetes type one” | dm1 | “dm 1” | dmt1 | “dm t1” | t1dm | “t1 dm” | t1d | iddm | ketoacidosis) + (“sensor augmented” | sensor-augmented | SAPT | minimed | paradigmveo | “paradigm veo” | “paradigm pump” | “veo pump” | animas | vibe | “g4 platinum” | dexcom)
Records retrieved: 0.
(“brittle diabetes” | “juvenile diabetes” | “pediatric diabetes” | “paediatric diabetes” | “early diabetes” | “autoimmune diabetes” | “auto immune diabetes” | “sudden onset diabetes”) + (“sensor augmented” | sensor-augmented | SAPT | minimed | paradigmveo | “paradigm veo” | “paradigm pump” | “veo pump” | animas | vibe | “g4 platinum” | dexcom)
Records retrieved: 0.
(hyperglycemia | hypoglycemia | hyperglycaemia | hypoglycaemia) + (“sensor augmented” | sensor-augmented | SAPT | minimed | paradigmveo | “paradigm veo” | “paradigm pump” | “veo pump” | animas | vibe | “g4 platinum” | dexcom)
Records retrieved: 0.
(“diabetes mellitus type 1” | “diabetes type 1” | “diabetes mellitus type1” | “diabetes type1” | “diabetes mellitus type I” | “diabetes type I” | “diabetes mellitus typeI” | “diabetes typeI” | “diabetes mellitus type one” | “diabetes type one” | dm1 | “dm 1” | dmt1 | “dm t1” | t1dm | “t1 dm” | t1d | iddm | ketoacidosis) + (“insulin pump” | “insulin pumps” | “insulin infusion” | “insulin delivery” | “pump therapy” | “pump treatment” | “pump treatments”)
Records retrieved: 1.
(“diabetes mellitus type 1” | “diabetes type 1” | “diabetes mellitus type1” | “diabetes type1” | “diabetes mellitus type I” | “diabetes type I” | “diabetes mellitus typeI” | “diabetes typeI” | “diabetes mellitus type one” | “diabetes type one” | dm1 | “dm 1” | dmt1 | “dm t1” | t1dm | “t1 dm” | t1d | iddm | ketoacidosis) + (“subcutaneous insulin” | CSII | “artificial pancreas” | “artificial beta cell” | “artificial beta-cell” | “artificial beta cells” | “artificial beta-cells” | “closed loop” | closed-loop | “integrated system” | “integrated systems | “dual system” | “dual systems” | “integrated device” | “integrated devices | “dual device” | “dual devices”)
Records retrieved: 11.
(“brittle diabetes” | “juvenile diabetes” | “pediatric diabetes” | “paediatric diabetes” | “early diabetes” | “autoimmune diabetes” | “auto immune diabetes” | “sudden onset diabetes”) + (“insulin pump” | “insulin pumps” | “insulin infusion” | “insulin delivery” | “pump therapy” | “pump treatment” | “pump treatments”)
Records retrieved: 0.
(“brittle diabetes” | “juvenile diabetes” | “pediatric diabetes” | “paediatric diabetes” | “early diabetes” | “autoimmune diabetes” | “auto immune diabetes” | “sudden onset diabetes”) + (“subcutaneous insulin” | CSII | “artificial pancreas” | “artificial beta cell” | “artificial beta-cell” | “artificial beta cells” | “artificial beta-cells” | “closed loop” | closed-loop | “integrated system” | “integrated systems | “dual system” | “dual systems” | “integrated device” | “integrated devices | “dual device” | “dual devices”)
Records retrieved: 0.
(hyperglycemia | hypoglycemia | hyperglycaemia | hypoglycaemia) + (“insulin pump” | “insulin pumps” | “insulin infusion” | “insulin delivery” | “pump therapy” | “pump treatment” | “pump treatments”)
Records retrieved: 0.
(hyperglycemia | hypoglycemia | hyperglycaemia | hypoglycaemia) + (“subcutaneous insulin” | CSII | “artificial pancreas” | “artificial beta cell” | “artificial beta-cell” | “artificial beta cells” | “artificial beta-cells” | “closed loop” | closed-loop | “integrated system” | “integrated systems | “dual system” | “dual systems” | “integrated device” | “integrated devices | “dual device” | “dual devices”)
Records retrieved: 0.
(“diabetes mellitus type 1” | “diabetes type 1” | “diabetes mellitus type1” | “diabetes type1” | “diabetes mellitus type I” | “diabetes type I” | “diabetes mellitus typeI” | “diabetes typeI” | “diabetes mellitus type one” | “diabetes type one” | dm1 | “dm 1” | dmt1 | “dm t1” | t1dm | “t1 dm” | t1d | iddm | ketoacidosis) + (“multiple daily injection” | “multiple daily injections” | “multiple dose injection” | “multiple dose injections” | “multiple daily insulin” | “multiple dose insulin” | “multiple injection” | “multiple injections” | MDI | “injection therapy” | “basal bolus” | basal-bolus | basalbolus | “short acting insulin” | “rapid acting insulin”)
Records retrieved: 11.
(“brittle diabetes” | “juvenile diabetes” | “pediatric diabetes” | “paediatric diabetes” | “early diabetes” | “autoimmune diabetes” | “auto immune diabetes” | “sudden onset diabetes”) + (“multiple daily injection” | “multiple daily injections” | “multiple dose injection” | “multiple dose injections” | “multiple daily insulin” | “multiple dose insulin” | “multiple injection” | “multiple injections” | MDI | “injection therapy” | “basal bolus” | basal-bolus | basalbolus | “short acting insulin” | “rapid acting insulin”)
Records retrieved: 0.
(hyperglycemia | hypoglycemia | hyperglycaemia | hypoglycaemia) + (“multiple daily injection” | “multiple daily injections” | “multiple dose injection” | “multiple dose injections” | “multiple daily insulin” | “multiple dose insulin” | “multiple injection” | “multiple injections” | MDI | “injection therapy” | “basal bolus” | basal-bolus | basalbolus | “short acting insulin” | “rapid acting insulin”)
Records retrieved: 1.
Records retrieved in total: 24.
Records retrieved after removal of duplicates: 11.
Key:
| OR
+ AND
” “ phrase search
Specific economic searches (MiniMed and Animas Vibe only)
NHS Economic Evaluation Database (via Wiley Online Library)
Issue searched: 3 of 4, July 2014.
Date searched: 2 October 2014.
Search strategy
#1 (sensor* near/3 (augment* or pump*))
#2 SAPT:ti,ab,kw
#3 minimed or paradigmveo
#4 (paradigm* near/3 (veo or pump*))
#5 (veo near/3 pump*)
#6 ((animas or vibe) near/3 (pump* or infus* or system*))
#7 dexcom
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
NHS EED: 4.
Health Economic Evaluations Database (via Wiley Online Library)
Date range searched: from inception up to 2 October 2014.
Date searched: 2 October 2014.
Search strategy
AX=‘sensor augmented’ or sensor-augmented or SAPT (1)
AX=minimed or paradigmveo or ‘paradigm veo’ or ‘paradigm pump’ or ‘veo pump’ or ‘animas pump’ or ‘animas infusion’ or ‘vibe pump’ or ‘vibe infusion’ or ‘g4 platinum’ or dexcom (0)
CS=1 or 2 (1)
EMBASE (via OvidSP)
Date range searched: 1974–2014/week 39.
Date searched: 2 October 2014.
Search strategy
-
insulin dependent diabetes mellitus/ (79,725)
-
exp diabetic ketoacidosis/ (7880)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ti,ab,ot,hw. (50,200)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ti,ab,ot,hw. (29,720)
-
((insulin$ adj2 depend$) or insulindepend$).ti,ab,ot,hw. (221,115)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,ot,hw. (20,641)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,ot,hw. (14,385)
-
hypoglycemia/ or hyperglycemia/ (110,120)
-
(hyperglyc?em$ or hypoglyc?em$).ti,ab,ot. (105,704)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ti,ab,ot,hw. (128,520)
-
or/1-10 (442,805)
-
(sensor$ adj3 (augment$ or pump$)).ti,ab,hw,ot. (611)
-
SAPT.ti,ab,ot,hw. (114)
-
(minimed or paradigmveo).ti,ab,ot,hw,dm,dv. (746)
-
(paradigm$ adj3 (veo or pump$)).ti,ab,hw,ot,dm,dv. (134)
-
(veo adj3 pump$).ti,ab,ot,hw,dm,dv. (41)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ti,ab,ot,hw,dm,dv. (29)
-
(g4 adj3 platinum).ti,ab,ot,hw,dm,dv. (29)
-
dexcom.ti,ab,ot,hw,dm,dv. (314)
-
or/12-19 (1730)
-
11 and 20 (1156)
-
health-economics/ (33,844)
-
exp economic-evaluation/ (215,823)
-
exp health-care-cost/ (208,556)
-
exp pharmacoeconomics/ (168,747)
-
or/22-25 (486,347)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (625,347)
-
(expenditure$ not energy).ti,ab. (24,608)
-
(value adj2 money).ti,ab. (1430)
-
budget$.ti,ab. (24,869)
-
or/27-30 (650,042)
-
26 or 31 (924,348)
-
letter.pt. (856,710)
-
editorial.pt. (456,641)
-
note.pt. (570,035)
-
or/33-35 (1,883,386)
-
32 not 36 (835,648)
-
(metabolic adj cost).ti,ab. (924)
-
((energy or oxygen) adj cost).ti,ab. (3207)
-
((energy or oxygen) adj expenditure).ti,ab. (20,769)
-
or/38-40 (24,065)
-
37 not 41 (830,473)
-
exp animal/ (19,415,638)
-
exp animal-experiment/ (1,804,426)
-
nonhuman/ (4,376,931)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (4,869,940)
-
or/43-46 (20,812,704)
-
exp human/ (15,138,243)
-
exp human-experiment/ (329,281)
-
48 or 49 (15,139,672)
-
47 not (47 and 50) (5,673,989)
-
42 not 51 (766,321)
-
21 and 52 (73)
The economics terms were based on the following costs filter:
Centre for Reviews and Dissemination. Search strategies: NHS EED EMBASE using OvidSP (economics filter). York: Centre for Reviews and Dissemination; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedembase (accessed 2 June 2014).
MEDLINE (via OvidSP)
Date range searched: 1946–2014/September week 4.
Date searched: 2 October 2014.
Search strategy
-
Diabetes Mellitus, Type 1/ (62,498)
-
Diabetic Ketoacidosis/ (5186)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ti,ab,ot,hw. (69,786)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ti,ab,ot,hw. (20,339)
-
((insulin$ adj2 depend$) or insulindepend$).ti,ab,ot,hw. (30,496)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,ot,hw. (13,154)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,ot,hw. (9345)
-
Hyperglycemia/ (20,917)
-
Hypoglycemia/ (21,796)
-
(hyperglyc?em$ or hypoglyc?em$).ti,ab,ot. (72,929)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ti,ab,ot,hw. (95,034)
-
or/1-11 (246,558)
-
(sensor$ adj3 (augment$ or pump$)).ti,ab,hw,ot. (313)
-
SAPT.ti,ab,ot,hw. (93)
-
(minimed or paradigmveo).ti,ab,ot,hw. (198)
-
(paradigm$ adj3 (veo or pump$)).ti,ab,hw,ot. (34)
-
(veo adj3 pump$).ti,ab,ot,hw. (5)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ti,ab,ot,hw. (7)
-
(g4 adj3 platinum).ti,ab,ot,hw. (4)
-
dexcom.ti,ab,ot,hw. (45)
-
or/13-20 (648)
-
12 and 21 (300)
-
economics/ (27,132)
-
exp “costs and cost analysis”/ (185,352)
-
economics, dental/ (1867)
-
exp “economics, hospital”/ (19,852)
-
economics, medical/ (8682)
-
economics, nursing/ (3987)
-
economics, pharmaceutical/ (2577)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (434,246)
-
(expenditure$ not energy).ti,ab. (17,736)
-
(value adj1 money).ti,ab. (23)
-
budget$.ti,ab. (17,453)
-
or/23-33 (560,640)
-
((energy or oxygen) adj cost).ti,ab. (2713)
-
(metabolic adj cost).ti,ab. (793)
-
((energy or oxygen) adj expenditure).ti,ab. (16,876)
-
or/35-37 (19,659)
-
34 not 38 (556,354)
-
letter.pt. (829,485)
-
editorial.pt. (348,438)
-
historical article.pt. (307,377)
-
or/40-42 (1,470,234)
-
39 not 43 (527,602)
-
22 and 44 (8)
The economics terms were based on the following costs filter:
Centre for Reviews and Dissemination. Search strategies: NHS EED MEDLINE using OvidSP (economics filter). York: Centre for Reviews and Dissemination; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedmedline (accessed 2 June 2014).
MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE Daily Update (via OvidSP)
Date searched: 2 October 2014.
Search strategy
-
Diabetes Mellitus, Type 1/ (64)
-
Diabetic Ketoacidosis/ (5)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ti,ab,ot,hw. (2660)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ti,ab,ot,hw. (1112)
-
((insulin$ adj2 depend$) or insulindepend$).ti,ab,ot,hw. (712)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,ot,hw. (879)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,ot,hw. (440)
-
Hyperglycemia/ (32)
-
Hypoglycemia/ (27)
-
(hyperglyc?em$ or hypoglyc?em$).ti,ab,ot. (5503)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ti,ab,ot,hw. (7549)
-
or/1-11 (15,088)
-
(sensor$ adj3 (augment$ or pump$)).ti,ab,hw,ot. (61)
-
SAPT.ti,ab,ot,hw. (86)
-
(minimed or paradigmveo).ti,ab,ot,hw. (12)
-
(paradigm$ adj3 (veo or pump$)).ti,ab,hw,ot. (4)
-
(veo adj3 pump$).ti,ab,ot,hw. (1)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ti,ab,ot,hw. (0)
-
(g4 adj3 platinum).ti,ab,ot,hw. (3)
-
dexcom.ti,ab,ot,hw. (7)
-
or/13-20 (167)
-
12 and 21 (39)
-
economics/ (3)
-
exp “costs and cost analysis”/ (243)
-
economics, dental/ (0)
-
exp “economics, hospital”/ (22)
-
economics, medical/ (3)
-
economics, nursing/ (3)
-
economics, pharmaceutical/ (1)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (52,040)
-
(expenditure$ not energy).ti,ab. (1513)
-
(value adj1 money).ti,ab. (5)
-
budget$.ti,ab. (2216)
-
or/23-33 (54,328)
-
((energy or oxygen) adj cost).ti,ab. (303)
-
(metabolic adj cost).ti,ab. (83)
-
((energy or oxygen) adj expenditure).ti,ab. (1206)
-
or/35-37 (1538)
-
34 not 38 (53,879)
-
letter.pt. (30,601)
-
editorial.pt. (18,927)
-
historical article.pt. (188)
-
or/40-42 (49,699)
-
39 not 43 (53,316)
-
22 and 44 (3)
The economics terms were based on the following costs filter:
Centre for Reviews and Dissemination. Search strategies: NHS EED MEDLINE using OvidSP (economics filter). York: Centre for Reviews and Dissemination; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedmedline (accessed 2 June 2014).
PubMed (via National Library of Medicine)
URL: www.ncbi.nlm.nih.gov/pubmed/
Date range searched: from inception up to 5 September 2014.
Date searched: 5 September 2014.
Search strategy
| #42 | Search (#41 and #42) | 0 |
| #41 | Search (pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb]) | 18,267,75 |
| #40 | Search (#35 not #39) | 501,673 |
| #39 | Search ((#36 or #37 or #38)) | 20,549 |
| #38 | Search “energy expenditure”[tiab] or “oxygen expenditure”[tiab] | 17,441 |
| #37 | Search “metabolic cost”[tiab] | 888 |
| #36 | Search “energy cost”[tiab] or “oxygen cost”[tiab] | 2986 |
| #35 | Search ((#31 or #32 or #33 or #34)) | 506,382 |
| #34 | Search budget*[tiab] | 19,827 |
| #33 | Search “value for money” | 934 |
| #32 | Search (expenditure*[tiab] not energy[tiab]) | 19,227 |
| #31 | Search (economic*[tiab] or cost[tiab] or costs[tiab] or costly[tiab] or costing[tiab] or price[tiab] or prices[tiab] or pricing[tiab] or pharmacoeconomic*[tiab]) | 485,328 |
| #30 | Search (#20 and #29) | 276 |
| #29 | Search (#21 or #22 or #23 or #24 or #25 or #26 or #27 or #28) | 937 |
| #28 | Search “g4 platinum” | 10 |
| #27 | Search dexcom | 56 |
| #26 | Search (animas or vibe) AND (pump* or infus* or system*) | 81 |
| #25 | Search “veo pump” or “veo pumps” | 15 |
| #24 | Search ((paradigm* AND (veo or pump*))) | 354 |
| #23 | Search minimed or paradigmveo | 217 |
| #22 | Search SAPT[tiab] | 187 |
| #21 | Search “sensor augmented”[tiab] or “sensor augment”[tiab] or “sensor pump”[tiab] or “pump sensor”[tiab] or “sensor pumps”[tiab] | 92 |
| #20 | Search ((#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19)) | 127,385 |
| #19 | Search “high glycohemoglobin”[tiab] or “higher glycohemoglobin”[tiab] or “low glycohemoglobin”[tiab] or “lower glycohemoglobin”[tiab] or “increase glycohemoglobin”[tiab] or “increased glycohemoglobin”[tiab] or “increases glycohemoglobin”[tiab] or “decrease glycohemoglobin”[tiab] or “decreased glycohemoglobin”[tiab] or “decreases glycohemoglobin”[tiab] or “deficient glycohemoglobin”[tiab] or “sufficient glycohemoglobin”[tiab] or “insufficient glycohemoglobin”[tiab] or “reduce glycohemoglobin”[tiab] or “reduced glycohemoglobin”[tiab] or “glycohemoglobin reduction”[tiab] or “fallen glycohemoglobin”[tiab] or “falling glycohemoglobin”[tiab] or “glycohemoglobin threshold”[tiab] or “safe glycohemoglobin”[tiab] | 17 |
| #18 | Search (“high haemoglobin”[tiab] or “higher haemoglobin”[tiab] or “low haemoglobin”[tiab] or “lower haemoglobin”[tiab] or “increase haemoglobin”[tiab] or “increased haemoglobin”[tiab] or “increases haemoglobin”[tiab] or “decrease haemoglobin”[tiab] or “decreased haemoglobin”[tiab] or “decreases haemoglobin”[tiab] or “deficient haemoglobin”[tiab] or “sufficient haemoglobin”[tiab] or “insufficient haemoglobin”[tiab] or “reduce haemoglobin”[tiab] or “reduced haemoglobin”[tiab] or “haemoglobin reduction”[tiab] or “fallen haemoglobin”[tiab] or “falling haemoglobin”[tiab] or “haemoglobin threshold”[tiab] or “safe haemoglobin”[tiab]) | 1167 |
| #17 | Search “high hemoglobin”[tiab] or “higher hemoglobin”[tiab] or “low hemoglobin”[tiab] or “lower hemoglobin”[tiab] or “increase hemoglobin”[tiab] or “increased hemoglobin”[tiab] or “increases hemoglobin”[tiab] or “decrease hemoglobin”[tiab] or “decreasedchemoglobin”[tiab] or “decreases hemoglobin”[tiab] or “deficient hemoglobin”[tiab] or “sufficient hemoglobin”[tiab] or “insufficient hemoglobin”[tiab] or “reduce hemoglobin”[tiab] or “reduced hemoglobin”[tiab] or “hemoglobin reduction”[tiab] or “fallen hemoglobin”[tiab] or “falling hemoglobin”[tiab] or “hemoglobin threshold”[tiab] or “safe hemoglobin”[tiab] | 3497 |
| #16 | Search “high a1c”[tiab] or “higher a1c”[tiab] or “low a1c”[tiab] or “lower a1c”[tiab] or “increase a1c”[tiab] or “increased a1c”[tiab] or “increases a1c”[tiab] or “decrease a1c”[tiab] or “decreasedca1c”[tiab] or “decreases a1c”[tiab] or “deficient a1c”[tiab] or “sufficient a1c”[tiab] or “insufficient a1c”[tiab] or “reduce a1c”[tiab] or “reduced a1c”[tiab] or “a1c reduction”[tiab] or “fallen a1c”[tiab] or “falling a1c”[tiab] or “a1c threshold”[tiab] or “safe a1c”[tiab] | 294 |
| #15 | Search ((((“high hba1”[tiab] or “higher hba1”[tiab] or “low hba1”[tiab] or “lower hba1”[tiab] or “increase hba1”[tiab] or “increased hba1”[tiab] or “increases hba1”[tiab] or “decrease hba1”[tiab] or “decreasedchba1”[tiab] or “decreases hba1”[tiab] or “deficient hba1”[tiab] or “sufficient hba1”[tiab] or “insufficient hba1”[tiab] or “reduce hba1”[tiab] or “reduced hba1”[tiab] or “hba1 reduction”[tiab] or “fallen hba1”[tiab] or “falling hba1”[tiab] or “hba1 threshold”[tiab] or “safe hba1”[tiab])))) | 76 |
| #14 | Search “high hb a1”[tiab] or “higher hb a1”[tiab] or “low hb a1”[tiab] or “lower hb a1”[tiab] or “increase hb a1”[tiab] or “increased hb a1”[tiab] or “increases hb a1”[tiab] or “decrease hb a1”[tiab] or “decreasedchb a1”[tiab] or “decreases hb a1”[tiab] or “deficient hb a1”[tiab] or “sufficient hb a1”[tiab] or “insufficient hb a1”[tiab] or “reduce hb a1”[tiab] or “reduced hb a1”[tiab] or “hb a1 reduction”[tiab] or “fallen hb a1”[tiab] or “falling hb a1”[tiab] or “hb a1 threshold”[tiab] or “safe hb a1”[tiab] | 0 |
| #13 | Search “high hba1c”[tiab] or “higher hba1c”[tiab] or “low hba1c”[tiab] or “lower hba1c”[tiab] or “increase hba1c”[tiab] or “increased hba1c”[tiab] or “increases hba1c”[tiab] or “decrease hba1c”[tiab] or “decreasedchba1c”[tiab] or “decreases hba1c”[tiab] or “deficient hba1c”[tiab] or “sufficient hba1c”[tiab] or “insufficient hba1c”[tiab] or “reduce hba1c”[tiab] or “reduced hba1c”[tiab] or “hba1c reduction”[tiab] or “fallen hba1c”[tiab] or “falling hba1c”[tiab] or “hba1c threshold”[tiab] or “safe hba1c”[tiab] | 1287 |
| #12 | Search “high sugar”[tiab] or “higher sugar”[tiab] or “low sugar”[tiab] or “lower sugar”[tiab] or “increase sugar”[tiab] or “increased sugar”[tiab] or “increases sugar”[tiab] or “decrease sugar”[tiab] or “decreasedcsugar”[tiab] or “decreases sugar”[tiab] or “deficient sugar”[tiab] or “sufficient sugar”[tiab] or “insufficient sugar”[tiab] or “reduce sugar”[tiab] or “reduced sugar”[tiab] or “sugar reduction”[tiab] or “fallen sugar”[tiab] or “falling sugar”[tiab] or “sugar threshold”[tiab] or “safe sugar”[tiab] | 1551 |
| #11 | Search (“high glucose”[tiab] or “higher glucose”[tiab] or “low glucose”[tiab] or “lower glucose”[tiab] or “increase glucose”[tiab] or “increased glucose”[tiab] or “increases glucose”[tiab] or “decrease glucose”[tiab] or “decreasedcglucose”[tiab] or “decreases glucose”[tiab] or “deficient glucose”[tiab] or “sufficient glucose”[tiab] or “insufficient glucose”[tiab] or “reduce glucose”[tiab] or “reduced glucose”[tiab] or “glucose reduction”[tiab] or “fallen glucose”[tiab] or “falling glucose”[tiab] or “glucose threshold”[tiab] or “safe glucose”[tiab]) | 16,743 |
| #10 | Search (hyperglycemia[tiab] or hypoglycaemia[tiab] or hyperglycemic[tiab] or hypoglycaemic[tiab]) | 44,476 |
| #9 | Search ketoacidosis[tiab] or acidoketosis[tiab] or “keto acidosis”[tiab] or ketoacidemia[tiab] or ketosis[tiab] | 7314 |
| #8 | Search dm1[tiab] or “dm 1”[tiab] or t1dm[tiab] or “t1 dm”[tiab] or t1d[tiab] or iddm[tiab] | 13,200 |
| #7 | Search “insulin dependent”[tiab] or insulindepend*[tiab] | 27,576 |
| #6 | Search “brittle diabetic”[tiab] or “diabetic juvenile”[tiab] or “diabetic pediatric”[tiab] or “diabetic paediatric”[tiab] or “diabetic early”[tiab] or “diabetic labile”[tiab] or “diabetic acidosis”[tiab] or “diabetic sudden onset”[tiab] | 348 |
| #5 | Search “diabetic brittle”[tiab] or “juvenile diabetic”[tiab] or “pediatric diabetic”[tiab] or “paediatric diabetic”[tiab] or “early diabetic”[tiab] or “labile diabetic”[tiab] or “acidosis diabetic”[tiab] or “sudden onset diabetic”[tiab] | 1125 |
| #4 | Search “brittle diabetes”[tiab] or “diabetes juvenile”[tiab] or “diabetes pediatric”[tiab] or “diabetes paediatric”[tiab] or “diabetes early”[tiab] or “diabetes ketosis”[tiab] or “diabetes labile”[tiab] or “diabetes acidosis”[tiab] or “diabetes sudden onset”[tiab] | 264 |
| #3 | Search “diabetes brittle”[tiab] or “juvenile diabetes”[tiab] or “pediatric diabetes”[tiab] or “paediatric diabetes”[tiab] or “early diabetes”[tiab] or “ketosis diabetes”[tiab] or “labile diabetes”[tiab] or “acidosis diabetes”[tiab] or “sudden onset diabetes”[tiab] | 2243 |
| #2 | Search “diabetic type 1”[tiab] OR “type 1 diabetic”[tiab] OR “diabetic type i”[tiab] OR “type i diabetic”[tiab] OR “diabetic type1”[tiab] OR “type1 diabetic”[tiab] OR “diabetic typei”[tiab] OR “typei diabetic”[tiab] | 6061 |
| #1 | Search ((((“diabetes type 1”[tiab] OR “type 1 diabetes”[tiab] OR “diabetes type i”[tiab] OR “type i diabetes”[tiab] OR “diabetes type1”[tiab] OR “type1 diabetes”[tiab] OR “diabetes typei”[tiab] OR “typei diabetes”[tiab])))) | 29,036 |
The economics terms were based on the following costs filter:
Centre for Reviews and Dissemination. Search strategies: NHS EED MEDLINE using OvidSP (economics filter). York: Centre for Reviews and Dissemination; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedmedline (accessed 2 June 2014).
American Economic Association’s electronic bibliography EconLit (via EBSCOhost)
Date range searched: 1969–2014.
Date searched: 2 October 2014.
Search strategy
S4 S1 or S2 or S3 (0)
S3 TI (animas N3 pump* or animas N3 infus* or animas N3 system* or vibe N3 pump* or vibe N3 infus* or vibe N3 system* or g4 N3 platinum or dexcom) or AB (animas N3 pump* or animas N3 infus* or animas N3 system* or vibe N3 pump* or vibe N3 infus* or vibe N3 system* or g4 N3 platinum or dexcom) (0)
S2 TI (minimed or paradigmveo or paradigm* N3 veo or paradigm* N3 pump* or veo N3 pump*) or AB (minimed or paradigmveo or paradigm* N3 veo or paradigm* N3 pump* or veo N3 pump*) (0)
S1 TI (sensor* N3 augment* or sensor* N3 pump* or sensor-augment* or SAPT) or AB (sensor* N3 augment* or sensor* N3 pump* or sensor-augment* or SAPT) (0)
Cost-effectiveness Analysis Registry
URL: www.cearegistry.org
Date range searched: from inception up to 5 September 2014.
Date searched: 2 October 2014.
1 record retrieved.
Search strategy
sensor augmented
sensor-augmented
SAPT
minimed
paradigmveo
paradigm veo
paradigm-veo
veo pump
animas
vibe pump
vibe infusion
vibe system
vibe systems
g4 platinum
dexcom
RePEc:Research Papers in Economics
URL: http://repec.org/
Date range searched: from inception up to 2 October 2014.
Date searched: 2 October 2014.
IDEAS search interface.
Search strategy
(“diabetes mellitus type 1” | “diabetes type 1” | “diabetes mellitus type1” | “diabetes type1” | “diabetes mellitus type I” | “diabetes type I” | “diabetes mellitus typeI” | “diabetes typeI” | “diabetes mellitus type one” | “diabetes type one” | dm1 | “dm 1” | dmt1 | “dm t1” | t1dm | “t1 dm” | t1d | iddm | ketoacidosis) + (“sensor augmented” | sensor-augmented | SAPT | minimed | paradigmveo | “paradigm veo” | “paradigm pump” | “veo pump” | animas | vibe | “g4 platinum” | dexcom)
Records retrieved: 0.
(“brittle diabetes” | “juvenile diabetes” | “pediatric diabetes” | “paediatric diabetes” | “early diabetes” | “autoimmune diabetes” | “auto immune diabetes” | “sudden onset diabetes”) + (“sensor augmented” | sensor-augmented | SAPT | minimed | paradigmveo | “paradigm veo” | “paradigm pump” | “veo pump” | animas | vibe | “g4 platinum” | dexcom)
Records retrieved: 0.
(hyperglycemia | hypoglycemia | hyperglycaemia | hypoglycaemia) + (“sensor augmented” | sensor-augmented | SAPT | minimed | paradigmveo | “paradigm veo” | “paradigm pump” | “veo pump” | animas | vibe | “g4 platinum” | dexcom)
Records retrieved: 0.
Records retrieved in total: 0.
Key:
| OR
+ AND
” “ phrase search
Appendix 2 List of excluded studies with rationale
The following table lists the studies that were excluded at the full-paper screening stage of the review, along with the reasons for their exclusion.
| Reason for exclusion | Number of excluded studies |
|---|---|
| Population | 8 |
| Intervention | 86 |
| Outcomes | 109 |
| Study design | 206 |
| Systematic review/meta-analysis | 36 |
| Background | 3 |
| Duplicate | 5 |
| Not found | 29 |
| Total | 482 |
| Excluded study | Reason for exclusion |
|---|---|
| Conference: 11th Annual Diabetes Technology Meeting San Francisco, CA, USA, 27–29 October 2011. J Diabetes Sci Technol 2012;6:453–A202 | Study design |
| Conference: 4th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). London, UK, 16–19 February 2011. Diabetes Technol Ther 2011;13:S1–108 | Study design |
| Abraham M, Davey R, Paramalingam N, Keenan B, Ambler G, Fairchild J, et al. Prevention of hypoglycaemia with predictive low glucose management system: comparison of hypoglyclaemia induction with exercise and subcutaneous bolus. Diabetes Technol Ther 2014;16:A43. Conference: 7th International Conference on Advanced Technologies and Treatments for Diabetes, (ATTD). Vienna, Austria, 5–8 February 2014 | Study design |
| Conference: 7th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Vienna, Austria, 5–8 February 2014. Diabetes Technol Ther 2014;16:A1–162 | Study design |
| ACTRN12607000198426. The Australian Sensor-Augmented Pump Algorithm Study. 2007. URL: www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12607000198426 (accessed 11 January 2016) | Study design |
| ACTRN12614000035628. The Performance of an Artificial Pancreas at Home in People with Type 1 Diabetes. 2014. URL: https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000035628 (accessed 11 January 2016) | Study design |
| ACTRN12614000482662. Closed Loop Insulin Delivery and Glucose Control for Type 1 Diabetes, Seven Days and Nights, Hospital to Home. 2014. URL: www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366247 (accessed 11 January 2016) | Study design |
| Agrawal P, Kannard B, Shin J, Huang S, Welsh JB, Kaufman FR. Improvement in glycemic parameters with use of the low glucose suspend feature of the veo insulin pump. Diabetes 2012;61:A229–30. Conference: 72nd Scientific Sessions of the American Diabetes Association. Philadelphia, PA, USA, 8–12 June 2012 | Study design |
| Agrawal P, Welsh JB, Kannard B, Askari S, Yang Q, Kaufman FR. Usage and effectiveness of the low glucose suspend feature of the Medtronic Paradigm Veo insulin pump. J Diabetes Sci Technol 2011;5:1137–41 | Outcomes |
| Agrawal P, Welsh JB, Kaufman FR. Use of the low glucose suspend (LGS) feature results in significant reduction in hypoglycemia in pediatric and adult patients with type 1 diabetes. Pediatr Diabetes 2012;13:116. Conference: 38th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Istanbul, Turkey, 10–13 October 2012 | Study design |
| Alemzadeh R, Palma-Sisto P, Parton E, Holzum M, Kichler J. Insulin pump therapy attenuated glycemic instability without improving glycemic control in a one-year study of preschool children with type 1 diabetes. Diabetes 2006;55:A97. Paper presented at 66th Annual Meeting of the American Diabetes Association. Washington, DC, USA, 9–13 June 2006 | Not found |
| Alemzadeh R, Palma-Sisto P, Parton EA, Holzum MK. Continuous subcutaneous insulin infusion and multiple dose of insulin regimen display similar patterns of blood glucose excursions in pediatric type 1 diabetes. Diabetes Technol Ther 2005;7:587–96 | Study design |
| Allen TJ, Cao Z, Youssef S, Hulthen UL, Cooper ME. High-dose intravenous insulin infusion versus intensive insulin treatment in newly diagnosed IDDM. Diabetes 1997;46:1612–18 | Population |
| Ambrosino JM, Weinzimer SA, Steffen AT, Ruedy K. Short-term psychosocial impact of sensor-augmented pump therapy within three months of diagnosis of type 1 diabetes. Diabetes 2012;61:A586. Conference: 72nd Scientific Sessions of the American Diabetes Association Philadelphia, PA, USA, 8–12 June 2012 | Outcomes |
| Conference: 72nd Scientific Sessions of the American Diabetes Association Philadelphia, PA, USA, 8–12 June 2012. Diabetes 2012;61:A1–722 | Study design |
| Arias P, Kerner W, Zier H, Navascues I, Pfeiffer EF. Incidence of hypoglycemic episodes in diabetic patients under continuous subcutaneous insulin infusion and intensified conventional insulin treatment: assessment by means of semiambulatory 24-hour continuous blood glucose monitoring. Diabetes Care 1985;8:134–40 | Study design |
| Bailey TS, Weiss R, Bode BW, Garg S, Ahmann AJ, Welsh JB, et al. Hypoglycemia reduction and changes in A1C in the aspire in-home study. Diabetes 2014;63:A60. Conference: 74th Scientific Sessions of the American Diabetes Association San Francisco, CA, USA, 13–17 June 2014 | Outcomes |
| Bak JF, Nielsen OH, Pedersen O, Beck-Nielsen H. Multiple insulin injections using a pen injector versus insulin pump treatment in young diabetic patients. Diabetes Res 1987;6:155–8 | Outcomes |
| Bangstad HJ, Kofoed-Enevoldsen A, Dahl-Jorgensen K, Hanssen KF. Glomerular charge selectivity and the influence of improved blood glucose control in type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia 1992;35:1165–9 | Population |
| Bangstad HJ, Osterby R, Dahl-Jorgensen K, Berg KJ, Hartmann A, Hanssen KF. Improvement of blood glucose control in IDDM patients retards the progression of morphological changes in early diabetic nephropathy. Diabetologia 1994;37:483–90 | Study design |
| Barcelo-Rico F, Luis Diez J, Vehi J, Ampudia-Blasco FJ, Rossetti P, Bondia J. Evaluation of a local-model-based calibration algorithm for continuous glucose monitoring in subjects with type 1 diabetes. J Diabetes Sci Technol 2013;7:A5. Conference: 12th Annual Diabetes Technology Meeting. Bethesda, MD, USA, 8–10 November 2012 | Study design |
| Battelino T, Conget I, Olsen B, Schutz-Fuhrmann I, Hommel E, Hoogma R, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia 2012;55:3155–62 | Outcomes |
| Battelino T, Conget I, Olsen B, Schutz-Fuhrmann I, Hommel E, Hoogma R, et al. The SWITCH study: continuous glucose monitoring in type 1 diabetes. Pediatr Diabetes 2011;12:30. Conference: 37th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Miami Beach, FL, USA, 19–22 October 2011 | Outcomes |
| Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care 2011;34:795–800 | Study design |
| Beck RW, Raghinaru D, Wadwa RP, Chase HP, Maahs DM, Buckingham BA, In Home Closed Loop Study Group. Frequency of morning ketosis after overnight insulin suspension using an automated nocturnal predictive low glucose suspend system. Diabetes Care 2014;37:1224–9 | Study design |
| Beck RW. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–83 | Intervention |
| Bell PM, Hayes JR, Hadden DR. A comparison of continuous subcutaneous insulin infusion (CSII) and conventional therapy in insulin dependent diabetes mellitus (IDDM). Ir J Med Sci 1984;153:116 | Intervention |
| Berg TJ, Nourooz-Zadeh J, Wolff SP, Tritschler HJ, Bangstad HJ, Hanssen KF. Hydroperoxides in plasma are reduced by intensified insulin treatment. A randomized controlled study of IDDM patients with microalbuminuria. Diabetes Care 1998;21:1295–300 | Intervention |
| Bergenstal RM, Dupre J, Lawson PM, Rizza RA, Rubenstein AH. Observations on C-peptide and free insulin in the blood during continuous subcutaneous insulin infusion and conventional insulin therapy. Diabetes 1985;34(Suppl. 3):31–6 | Intervention |
| Bergenstal RM, Lee SW, Welsh JB, Shin J, Kaufman FR. Prevention of hypoglycemia in the aspire in-home study. Diabetes Technol Ther 2014;16:A107. Conference: 7th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Vienna, Austria, 5–8 February 2014 | Outcomes |
| Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Sensor-augmented pump therapy for A1C reduction (STAR 3) study: results from the 6-month continuation phase. Diabetes Care 2011;34:2403–5 | Study design |
| Bergenstal RM. Sensor-augmented insulin-pump therapy in type 1 diabetes. REPLY. N Engl J Med 2010;363:2071 | Study design |
| Berhe T, Innocenti M. Insulin pump therapy as a routine care for children with type 1 diabetes: improvement in glycemic control using insulin pump therapy with intermittent higher basal rate in adolescents with type 1 diabetes who have a previous history of poor glyaemic control (HbA1c > 10%). Diabetes 2008;57:A748. Paper presented at 68th Annual Meeting of the American Diabetes Association. San Francisco, USA, 6–10 June 2008 | Not found |
| Blair J, Gregory JW, Peak M. Insulin delivery by multiple daily injections or continuous subcutaneous insulin infusion in childhood: addressing the evidence gap. Practical Diabetes 2012;29:47–8 | Study design |
| Blue Cross Blue Shield Association. Artificial pancreas device systems. Technol Eval Cent Assess Program 2014;28:122 | Systematic review/meta-analysis |
| Bode B, Gross K, Rikalo N, Schwartz S, Wahl T, Page C, et al. Alarms based on real-time sensor glucose values alert patients to hypo- and hyperglycemia: the guardian continuous monitoring system. Diabetes Technol Ther 2004;6:105–13 | Study design |
| Bode B, Lee SW, Kaufman FR. Predictors of hypoglycemia during the run-in period of the aspire-2 study. Diabetes Technol Ther 2013;15:A35. Conference: 6th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Paris, France, 27 February–2 March 2013 | Outcomes |
| Bode B, Shelmet J, Gooch B, Hassman DR, Liang J, Smedegaard JK, et al. Patient perception and use of an insulin injector/glucose monitor combined device. Diabetes Educ 2004;30:301–9 | Outcomes |
| Bode BW, Lee SW, Kaufman FR. Predictors of nocturnal hypoglycemia during the run-in period of the ASPIRE-2 study. Diabetes 2013;62:A252. Conference: 73rd Scientific Sessions of the American Diabetes Association. Chicago, IL USA, 21–25 June 2013 | Outcomes |
| Bode BW, Steed RD, Davidson PC. Reduction in severe hypoglycemia with long-term continuous subcutaneous insulin infusion in type I diabetes. Diabetes Care 1996;19:324–7 | Study design |
| Bode BW, Steed RD, Schleusener DS, Strange P. Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open-label study using a continuous glucose monitoring system. Endocr Pract 2005;11:157–64 | Study design |
| Bolli GB, Capani F, Home PD, Kerr D, Thomas R, Torlone E, et al. Comparison of a multiple daily injection regimen with once-daily insulin glargine basal insulin and mealtime lispro, to continuous subcutaneous insulin infusion: a randomised, open, parallel study. Diabetes 2004;53:A107–8. Paper presented at 64th Annual Meeting of the American Diabetes Association. Orlando, USA, 4–8 June 2004 | Intervention |
| Bonfanti R, Meschi F, Viscardi M, Rigamonti A, Biffi V, Frontino G, et al. Insulin pump therapy versus multiple injections in young children with diabetes: comparison of long-term efficacy. Pediatr Diabetes 2010;11:100. Conference: 36th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Buenos Aires, Argentina, 27–30 October 2010 | Study design |
| Bonfanti R, Meschi F, Viscardi M, Rigamonti A, Biffi V, Frontino G, et al. Long-term efficacy of insulin pump therapy in young children with diabetes. Diabetologia 2010;53:S372. Conference: 46th Annual Meeting of the European Association for the Study of Diabetes (EASD). Stockholm, Sweden, 20–24 September 2010 | Study design |
| Bonnemaison E, Hasselmann C, Dieckmann K, Perdereau S, Marques C, Faure N, et al. Observational study: continuous glucose monitoring in children under 7 years old. Pediatr Diabetes 2011;12:132. Conference: 37th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Miami Beach, FL, USA, 19–22 October 2011 | Study design |
| Boston University, Massachusetts General Hospital, Juvenile Diabetes Research Foundation. Closed-loop Glucose Control for Automated Management of Type 1 Diabetes. NCT00811317 2010. URL: https://clinicaltrials.gov/ct2/show/NCT00811317 (accessed 12 November 2015) | Intervention |
| Botta RM, Sinagra D, Angelico MC, Bompiani GD. [Comparison of intensified traditional insulin therapy and micropump therapy in pregnant women with type 1 diabetes mellitus.] Minerva Med 1986;77:657–61 | Not found |
| Bragd J, Adamson U, Lins PE, Von Dobeln A, Oskarsson P. Basal insulin substitution with glargine or CSII in adult type I diabetes patients: a randomized controlled trial. Diabetes 2009;58:A60–1. Paper presented at 69th Annual Meeting of the American Diabetes Association. New Orleans, USA, 5–9 June 2009 | Not found |
| Bratina N. The switch study: the impact of continuous glucose monitoring on health care resource utilization. Diabetes Technol Ther 2013;15:A3. Conference: 6th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Paris, France, 27 February 2013–2 March 2013 | Outcomes |
| Brazg R, Garg S, Bailey T, Buckingham B, Slover R, Klonoff D, et al. Interim analysis of an in-clinic, randomized, crossover study to assess efficacy of the low glucose suspend feature of the Paradigm Veo system with hypoglycemic induction from exercise. J Diabetes Sci Technol 2012;6:A19. Conference: 11th Annual Diabetes Technology Meeting. San Francisco, CA, USA, 27–29 October 2011 | Study design |
| Brazg RL, Bailey TS, Garg S, Buckingham BA, Slover RH, Klonoff DC, et al. The ASPIRE study: design and methods of an in-clinic crossover trial on the efficacy of automatic insulin pump suspension in exercise-induced hypoglycemia. J Diabetes Sci Technol 2011;5:1466–71 | Study design |
| Brinchmann-Hansen O, Dahl-Jorgensen K, Hanssen KF, Sandvik L. The response of diabetic retinopathy to 41 months of multiple insulin injections, insulin pumps, and conventional insulin therapy. Arch Ophthalmol 1988;106:1242–6 | Outcomes |
| Bruttomesso D, Bonomo M, Costa S, Dal Pos M, Di Cianni G, Pellicano F, et al. Type 1 diabetes control and pregnancy outcomes in women treated with continuous subcutaneous insulin infusion (CSII) or with insulin glargine and multiple daily injections of rapid-acting insulin analogues (glargine-MDI). Diabetes Metab 2011;37:426–31 | Study design |
| Bruttomesso D, Crazzolara D, Maran A, Costa S, Dal Pos M, Girelli A, et al. In type 1 diabetic patients with good glycaemic control, blood glucose variability is lower during continuous subcutaneous insulin infusion than during multiple daily injections with insulin glargine. Diabet Med 2008;25:326–32 | Intervention |
| Buckingham B, Beck RW, Ruedy KJ, Cheng P, Kollman C, Weinzimer SA, et al. Effectiveness of early intensive therapy on beta-cell preservation in type 1 diabetes. Diabetes Care 2013;36:4030–5 | Intervention |
| Buckingham B, Nakamura K, Benassi K, Realsen J, Liljenquist D, Chase P. Effectiveness and safety study of the prototype 4th generation seven day continuous glucose monitoring system in youth with type 1 diabetes mellitus. Paper presented at 47th Annual Meeting of the European Association for the Study of Diabetes (EASD). Lisbon, Portugal, 12–16 September 2011 | Study design |
| Buckingham B, Ruedy K, Chase HP, Weinzimer S, DiMeglio L, Russell W, et al. Does intensive metabolic control at the onset of diabetes followed by one year of sensor augmented pump therapy improve C-peptide levels one year post diagnosis? Diabetes Technol Ther 2013;15:A137. Conference: 6th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Paris, France, 27 February–2 March 2013 | Study design |
| Buckingham BA, Cameron F, Calhoun P, Maahs DM, Wilson DM, Chase HP, et al. Outpatient safety assessment of an in-home predictive low-glucose suspend system with type 1 diabetes subjects at elevated risk of nocturnal hypoglycemia. Diabetes Technol Ther 2013;15:622–7 | Study design |
| Buckingham BA, Cheng P, Beck RW, Kollman C, Ruedy K, Weinzimer SA, et al. Relationship of glycemic control and c-peptide levels 2 years following diagnosis of T1D. Diabetes 2014;63:A392. Conference: 74th Scientific Sessions of the American Diabetes Association San Francisco, CA, USA, 13–17 June 2014 | Outcomes |
| Buckingham BA, Tanner JP. Factors predictive of continuous glucose monitoring (CGM) use and benefit in the JDRF CGM RCT. Diabetes 2009;58. Conference: 69th Annual Meeting of the American Diabetes Association. New Orleans, LA, USA, 5–9 June 2009 | Study design |
| Bukara-Radujkovic G, Zdravkovic D, Lakic S. Short-term use of continuous glucose monitoring system adds to glycemic control in young type 1 diabetes mellitus patients in the long run: a clinical trial. Vojnosanit Pregl 2011;68:650–4 | Study design |
| Burkart W, Hanker JP, Schneider HP. Complications and fetal outcome in diabetic pregnancy. Intensified conventional versus insulin pump therapy. Gynecol Obstet Invest 1988;26:104–12 | Population |
| Buse JB, Kudva YC, Guthrie RA, Laffel L, Battelino T, Shin J, et al. Assessment of glycemic variability and CD40 ligand in the STAR 3 study. Diabetes 2011;60:A252. Conference: 71st Scientific Sessions of the American Diabetes Association. San Diego, CA, USA, 24–28 June 2011 | Outcomes |
| Butcher B, Jones T. Safety, Efficacy and Quality of Life Associated with Continuous Glucose Monitoring in People with Diabetes. PROSPERO: CRD42014013270; 2014. URL: www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014013270 (accessed 16 November 2015) | Systematic review/meta-analysis |
| Callaghan BC, Little AA, Feldman EL, Hughes RAC. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev 2012;6:CD007543 | Study design |
| Cander S, Oz Gul O, Deligonul A, Un OK, Kiyici S, Tuncel E, et al. Weight gain in type 1 diabetic patients with insulin pump therapy. Obesity Rev 2011;12:214. Conference: 18th European Congress on Obesity (ECO). Istanbul, Turkey, 25–28 May 2011 | Outcomes |
| Capel I, Rigla M, Garcia-Saez G, Rodriguez-Herrero A, Pons B, Subias D, et al. Artificial pancreas using a personalized rule-based controller achieves overnight normoglycemia in patients with type 1 diabetes. Diabetes Technol Ther 2014;16:172–9 | Study design |
| Carta Q, Meriggi E, Trossarelli GF, Catella G, Dal Molin V, Menato G, et al. Continuous subcutaneous insulin infusion versus intensive conventional insulin therapy in type I and type II diabetic pregnancy. Diabetes Metab 1986;12:121–9 | Not found |
| Centre d’Etudes et de Recherche pour l’Intensification du Traitement du D, Abbott. Are the Continuous Glucose Monitoring Systems Able to Improve Long Term Glycaemic Control in Type 1 Diabetic Patients? NCT00726440 2012. URL: https://clinicaltrials.gov/ct2/show/NCT00726440 (accessed 12 November 2015) | Outcomes |
| Chase HP, Beck R, Tamborlane W, Buckingham B, Mauras N, Tsalikian E, et al. A randomized multicenter trial comparing the GlucoWatch Biographer with standard glucose monitoring in children with type 1 diabetes. Diabetes Care 2005;28:1101–6 | Study design |
| Chase HP, Beck RW, Xing D, Tamborlane WV, Coffey J, Fox LA, et al. Continuous glucose monitoring in youth with type 1 diabetes: 12-month follow-up of the juvenile diabetes research foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther 2010;12:507–15 | Intervention |
| Chase HP, Kim LM, Owen SL, MacKenzie TA, Klingensmith GJ, Murtfeldt R, et al. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics 2001;107:222–6 | Intervention |
| Chase HP. A randomized trial of a home system to reduce nocturnal hypoglycemia in type 1 diabetes. Diabetes Technol Ther 2014;16:A2. Conference: 7th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Vienna, Austria, 5–8 February 2014 | Study design |
| Chatelais L, Voinot C, Robine A, Gatelais F, Dufresne S, Bouhours-Nouet N, et al. Continuous subcutaneous insulin infusion in type 1 diabetic adolescents with poor glycemic control under multiple daily injections: 1-year evaluation of HbA1c and acceptability. Horm Res 2009;72:182. Paper presented at LWPES/ESPE 8th Joint Meeting Global Care in Pediatric Endocrinology in collaboration with APEG, APPES, JSPE and SLEP. New York, NY, USA, 9–12 September 2009 | Study design |
| Chen R, Yogev Y, Weissman-Brenner A, Ben-Haroush A, Hod M. Level of glycemic control and pregnancy outcome in type-1 diabetes: a comparison between multiple daily injections (MDI) and continuous subcutaneous insulin infusions (CSII). Diabetes 2007;56:A703. Paper presented at 67th Annual Meeting of the American Diabetes Association. Chicago, USA, 22–26 June 2007 | Not found |
| Chen R, Yogev Y, Weissman-Brenner A, Haroush AB, Hod M. Level of glycemic control and pregnancy outcome in type-1 diabetes: a comparison between multiple daily injections (MDI) and continuous subcutaneous insulin infusions (CSII). Am J Obstet Gynecol 2006;195:S132. Paper presented at 27th Annual Meeting of the Society of Maternal Fetal Medicine. San Francisco, USA, 5–10 February 2007 | Study design |
| Chen Y, Ben-Haroush A, Weismann-Brenner A, Melamed N, Hod M, Yogev Y. Level of glycemic control and pregnancy outcome in type 1 diabetes: a comparison between multiple daily insulin injections and continuous subcutaneous insulin infusions. Am J Obstet Gynecol 2007;197:e1–5. [Erratum published in Am J Obstet Gynecol 2008;198:610] | Study design |
| Chevremont A, Collet-Gaudillat C, Duvezin-Caubet P, Franc S, Gouet D, Jan P, et al. [Insulin pump Paradigm Veo with automated insulin suspension function: results of a pilot study in type 1 diabetic patients at high hypoglycemic risk.] Medecine des Maladies Metaboliques 2012;6:531–8 | Study design |
| Chiasson JL, Ducros F, Poliquin-Hamet M, Lopez D, Lecavalier L, Hamet P. Continuous subcutaneous insulin infusion (Mill-Hill Infuser) versus multiple injections (Medi-Jector) in the treatment of insulin-dependent diabetes mellitus and the effect of metabolic control on microangiopathy. Diabetes Care 1984;7:331–7 | Study design |
| Chico A, Saigi I, Garcia-Patterson A, Santos MD, Adelantado JM, Ginovart G, et al. Glycemic control and perinatal outcomes of pregnancies complicated by type 1 diabetes: influence of continuous subcutaneous insulin infusion and lispro insulin. Diabetes Technol Ther 2010;12:937–45 | Study design |
| Chico A, Vidal-Rios P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003;26:1153–7 | Population |
| Choudhary P, Shin J, Wang Y, Evans ML, Hammond PJ, Kerr D, et al. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care 2011;34:2023–5 | Study design |
| Christensen CK, Christiansen JS, Christensen T, Hermansen K, Mogensen CE. The effect of six months continuous subcutaneous insulin infusion on kidney function and size in insulin-dependent diabetics. Diabet Med 1986;3:29–32 | Not found |
| Christensen CK, Christiansen JS, Schmitz A, Christensen T, Hermansen K, Mogensen CE. Effect of continuous subcutaneous insulin infusion on kidney function and size in IDDM patients: a 2 year controlled study. J Diabet Complications 1987;1:91–5 | Intervention |
| Christiansen JS, Ingerslev J, Bernvil SS, Christensen CK, Hermansen K, Schmitz A. Near normoglycemia for 1 year has no effect on platelet reactivity, factor VIII, and von Willebrand factor in insulin-dependent diabetes mellitus: a controlled trial. J Diabet Complications 1987;1:100–6 | Intervention |
| Churchill JN, Ruppe RL, Smaldone A. Use of continuous insulin infusion pumps in young children with type 1 diabetes: a systematic review. J Pediatr Health Care 2009;23:173–9 | Systematic review/meta-analysis |
| Ciavarella A, Vannini P, Flammini M, Bacci L, Forlani G, Borgnino LC. Effect of long-term near-normoglycemia on the progression of diabetic nephropathy. Diabetes Metab 1985;11:3–8 | Not found |
| Cinar A, Turksoy K, Quinn L, Littlejohn E. An integrated hypoglycemia early alarm and adaptive control system for artificial pancreas. Diabetes Technol Ther 2014;16:A103. Paper presented at 7th International Conference on Advanced Technologies & Treatments for Diabetes. Vienna, Austria, 5–8 February 2014 | Study design |
| Clarke WL, Anderson S, Breton M, Patek S, Kashmer L, Kovatchev B. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: the Virginia experience. J Diabetes Sci Technol 2009;3:1031–8 | Study design |
| Cobry E, Chase HP, Burdick P, McFann K, Yetzer H, Scrimgeour L. Use of CoZmonitor in youth with type 1 diabetes. Pediatr Diabetes 2008;9:148–51 | Study design |
| Cohen D, Weintrob N, Benzaquen H, Galatzer A, Fayman G, Phillip M. Continuous subcutaneous insulin infusion versus multiple daily injections in adolescents with type I diabetes mellitus: a randomized open crossover trial. J Pediatr Endocrinol 2003;16:1047–50 | Intervention |
| Cohen N, Minshall ME, Sharon-Nash L, Zakrzewska K, Valentine WJ, Palmer AJ. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin: economic comparison in adult and adolescent type 1 diabetes mellitus in Australia. Pharmacoeconomics 2007;25:881–97 | Outcomes |
| Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technol Assess 2004;8(43) | Systematic review/meta-analysis |
| Conget I, Battelino T, Gimenez M, Gough H, Castaneda J, Bolinder J, et al. The SWITCH study (sensing with insulin pump therapy to control HbA(1c)): design and methods of a randomized controlled crossover trial on sensor-augmented insulin pump efficacy in type 1 diabetes suboptimally controlled with pump therapy. Diabetes Technol Ther 2011;13:49–54 | Study design |
| Conget I, Battelino T, Gimenez M, Gough H, Castaneda J, Bolinder J. The SWITCH study (Sensing with insulin pump therapy to control HbA1c). Design and methods of a randomized controlled cross-over trial on sensor-augmented insulin pump efficacy in type 1 diabetes suboptimally controlled with pump therapy. Pediatr Diabetes 2010;11:105. Conference: 36th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Buenos Aires, Argentina, 27–30 October 2010 | Outcomes |
| Cooke D, Hurel SJ, Casbard A, Steed L, Walker S, Meredith S, et al. Randomized controlled trial to assess the impact of continuous glucose monitoring on HbA(1c) in insulin-treated diabetes (MITRE Study). Diabet Med 2009;26:540–7 | Study design |
| Corabian P, Guo B, Harstall C, Chuck A, Yan C. Insulin Pump Therapy for Type 1 Diabetes. Edmonton, AB: Institute of Health Economics, 2012 | Systematic review/meta-analysis |
| Cordua S, Secher AL, Ringholm L, Damm P, Mathiesen ER. Real-time continuous glucose monitoring during labour and delivery in women with type 1 diabetes – observations from a randomized controlled trial. Diabet Med 2013;30:1374–81 | Intervention |
| Cosson E, Hamo Tchatchouang E, Dufaitre Patouraux L, Attali JR, Pariès J, Schaepelynck-Bélicar P. Multicentre, randomised, controlled study of the impact of continuous sub cutaneous glucose monitoring (GlucoDay) on glycaemic control in type 1 and type 2 diabetes patients. Diabetes Metab 2009;35:312–18 | Study design |
| Coustan DR, Reece EA, Sherwin RS, Rudolf MC, Bates SE, Sockin SM, et al. A randomized clinical trial of the insulin pump vs intensive conventional therapy in diabetic pregnancies. JAMA 1986;255:631–6 | Intervention |
| Crepaldi C, Nosadini R, Bruttomesso D, Fioretto P, Fedele D, Segato T, et al. The effect of continuous insulin infusion as compared with conventional insulin therapy in the evolution of diabetic retinal ischaemia. Two years report. Diabetes Nutr Metab Clin Exp 1989;2:209–18 | Intervention |
| Cummins E, Royle P, Snaith A, Greene A, Robertson L, McIntyre L, et al. Clinical and Cost-effectiveness of Continuous Subcutaneous Infusion for Diabetes: Updating Review. A Technology Assessment Report Commissioned by the HTA Programme on behalf of NICE. HTA reference 06/61. London: NICE; 2007. URL: www.nice.org.uk/guidance/ta151/resources/diabetes-insulin-pump-therapy-assessment-report2 (accessed 8 July 2014) | Systematic review/meta-analysis |
| Cummins E, Royle P, Snaith A, Greene A, Robertson L, McIntyre L, et al. Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation. Health Technol Assess 2010;14(11) | Systematic review/meta-analysis |
| Cyganek K, Hebda-Szydło A, Katra B, Klupa T, Kaim I, Skupien J, et al. Efficacy and safety of continuous subcutaneous insulin infusion therapy in pregnancy complicated by type 1 diabetes. Paper presented at 45th Annual Meeting of the European Association for the Study of Diabetes (EASD). Vienna, Austria, 30 September–2 October 2009 | Study design |
| Cyganek K, Hebda-Szydlo A, Katra B, Skupien J, Klupa T, Janas I, et al. Glycemic control and selected pregnancy outcomes in type 1 diabetes women on continuous subcutaneous insulin infusion and multiple daily injections: the significance of pregnancy planning. Diabetes Technol Ther 2010;12:41–7 | Outcomes |
| Cyganek K, Hebda-Szydlo A, Katra B, Skupien J, Klupa T, Janas I, et al. Pregnancy planning improves glycemic control and pregnancy outcomes in type 1 diabetes women on CSII and MDI. Eur J Clin Invest 2010;40:8. Paper presented at 44th Annual Scientific Meeting of the European Society for Clinical Investigation. Bari, Italy, 24–27 February 2010 | Study design |
| Cypryk K, Kosinski M, Kaminska P, Kozdraj T, Lewinski A. Diabetes control and pregnancy outcomes in women with type 1 diabetes treated during pregnancy with continuous subcutaneous insulin infusion or multiple daily insulin injections. Pol Arch Med Wewn 2008;118:339–44 | Study design |
| Dahl-Jorgensen K, Hanssen KF, Aagenaes O, Larsen S. [New methods for subcutaneous insulin administration. A year’s experience with the insulin pump and multiple insulin injection therapy.] Tidsskr Nor Laegeforen 1984;104:856–61 | Not found |
| Dahl-Jorgensen K, Hanssen KF, Kierulf P, Bjoro T, Sandvik L, Aagenaes O. Reduction of urinary albumin excretion after 4 years of continuous subcutaneous insulin infusion in insulin-dependent diabetes mellitus. The Oslo Study. Acta Endocrinol 1988;117:19–25 | Outcomes |
| Dahl-Jorgensen K. Blood glucose control and progression of diabetic neuropathy: eight years results from the Oslo study. Diabetologia 1992;35:A15. Paper presented at 28th Annual Meeting of the European Association for the Study of Diabetes (EASD). Prague, Czech Republic, 8–11 September 1992 | Not found |
| Dahl-Jorgensen K. Near-normoglycemia and late diabetic complications. The Oslo Study. Acta Endocrinol 1987;284:1–38 | Not found |
| Damiano ER, McKeon K, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A comparative effectiveness analysis of three continuous glucose monitors: the Navigator, G4 Platinum, and Enlite [published online ahead of print 21 April 2014]. J Diabetes Sci Technol 2014 | Intervention |
| Danne T, Kordonouri O, Holder M, Haberland H, Golembowski S, Remus K, et al. [LGS system cuts hypoglycaemia excursion frequency in children on SAP therapy.] Diabetes Stoffwechsel Herz 2012;21:157–63 | Study design |
| Danne T, Kordonouri O, Holder M, Haberland H, Golembowski S, Remus K, et al. Prevention of hypoglycemia by using low glucose suspend function in sensor-augmented pump therapy. Diabetes Technol Ther 2011;13:1129–34 | Study design |
| Danne T, Kordonouri O, Remus K, Blasig S, Holder M, Wadien T, et al. The Low Glucose Suspend (LGS) function in sensor-augmented pump therapy prevents hypoglycaemia in children. Diabetes 2011;60:A41. Conference: 71st Scientific Sessions of the American Diabetes Association. San Diego, CA, USA, 24–28 June 2011 | Study design |
| Danne T, Kordonouri O, Remus K, Holder M, Wadien T, Haberland H, et al. Prevention of hypoglycaemia by using low glucose suspend (LGS) function in sensor-augmented pump therapy. Diabetes Technol Ther 2011;13:217. Conference: 4th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). London, UK, 16–19 February 2011 | Study design |
| Danne T. Predictive low glucose management with sensor augmented CSII in response to exercise. Diabetes Technol Ther 2014;16:A2. Conference: 7th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Vienna, Austria, 5–8 February 2014 | Study design |
| Daskalaki E, Norgaard K, Prountzou A, Zuger T, Diem P, Mougiakakou S. Alarm system for the early warning of hypo- and hyperglycemic events based on online adaptive models. Diabetes Technol Ther 2013;15:A77–8. Conference: 6th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Paris, France, 27 February–2 March 2013 | Study design |
| Dauber A, Corcia L, Safer J, Agus MSD, Einis S, Steil GM. Closed-loop insulin therapy improves glycemic control in children aged > 7 years. Diabetes Technol Ther 2014;16(Suppl. 1):23–4 | Study design |
| Davies AG, Price DA, Houlton CA, Burn JL, Fielding BA, Postlethwaite RJ. Continuous subcutaneous insulin infusion in diabetes mellitus. A year’s prospective trial. Arch Dis Child 1984;59:1027–33 | Intervention |
| Davis EA, Siafarikas A, Ratnam N, Loveday J, Baker V, Marangou D, et al. The initiation of intensive pump therapy at diagnosis of type 1 diabetes mellitus in adolescents: a randomised trial. Diabetes 2007;56:A53. Paper presented at the 67th Annual Meeting of the American Diabetes Association. Chicago, IL, USA, 22–26 June 2007 | Intervention |
| de Beaufort CE, Bruining GJ, Aarsen RS, den Boer NC, Grose WF. Does continuous subcutaneous insulin infusion (CSII) prolong the remission phase of insulin-dependent diabetic children? Preliminary findings of a randomized prospective study. Neth J Med 1985;28(Suppl. 1):53–4 | Not found |
| de Beaufort CE, Houtzagers CM, Bruining GJ, Aarsen RS, den Boer NC, Grose WF, et al. Continuous subcutaneous insulin infusion (CSII) versus conventional injection therapy in newly diagnosed diabetic children: two-year follow-up of a randomized, prospective trial. Diabet Med 1989;6:766–71 | Not found |
| De Bock MI, Dart J, George CE, Abraham M, Cooper M, Paramalingam N, et al. Performance of a predictive insulin pump suspension algorithm for prevention of overnight hypoglycaemia. Diabetes 2014;63:A240–1. Conference: 74th Scientific Sessions of the American Diabetes Association. San Francisco, CA, USA, 13–17 June 2014 | Study design |
| De Portu S, Castaneda J, Hommel E, Olsen BS, Battelino T, Conget I, et al. The switch study: the impact of continuous glucose monitoring on health care resource utilization. Value Health 2012;15:A357. Conference: ISPOR 15th Annual European Congress. Berlin, Germany, 3–7 November 2012 | Outcomes |
| Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care 2006;29:2730–2 | Intervention |
| Deiss D, Hartmann R, Schmidt J, Kordonouri O. Results of a randomized controlled cross-over trial on the effect of continuous subcutaneous glucose monitoring (CGMS) on glycemic control in children and adolescents with type 1 diabetes. Exp Clin Endocrinol Diabetes 2006;114:63–7 | Intervention |
| DeLuca FC, Timoshin A, Bamji N, Ferraro G, Himel A, Noto J, et al. The effect of insulin pump therapy on the diabetes control of children and adolescents with IDDM-1. Pediatr Res 2004;55:136A. Paper presented at the Annual Meeting of the Pediatric Academic Societies, 4 May 2004, San Francisco, USA. | Study design |
| Derosa G, Maffioli P, D’Angelo A, Salvadeo SAT, Ferrari I, Fogari E, et al. Effects of insulin therapy with continuous subcutaneous insulin infusion (CSII) in diabetic patients: comparison with multi-daily insulin injections therapy (MDI). Endocr J 2009;56:571–8 | Population |
| DeSalvo DJ, Keith-Hynes P, Peyser T, Place J, Caswell K, Wilson DM, et al. Remote glucose monitoring in cAMP setting reduces the risk of prolonged nocturnal hypoglycemia. Diabetes Technol Ther 2014;16:1–7 | Study design |
| DeVries JH. Health-economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of type 1 diabetes in the UK (letter). Diabet Med 2006;23:709 | Outcomes |
| DexCom Inc. Effectiveness and Safety Study of the DexCom™ G4 Continuous Glucose Monitoring System in Children and Adolescents With Type 1 Diabetes Mellitus. NCT01185496 2011. URL: https://clinicaltrials.gov/ct2/show/NCT01185496 (accessed 12 November 2015) | Outcomes |
| DexCom Inc. Efficacy of Continuous Glucose Monitoring in Subjects With Type 1 Diabetes Mellitus on Multiple Daily Injections (MDI) or Continuous Subcutaneous Insulin Infusion (CSII) Therapy. NCT01104142 2010. URL: https://clinicaltrials.gov/ct2/show/NCT01104142 (accessed 12 November 2015) | Study design |
| Diabetes Research in Children Network Study Group, Weinzimer S, Xing D, Tansey M, Fiallo-Scharer R, Mauras N, et al. Prolonged use of continuous glucose monitors in children with type 1 diabetes on continuous subcutaneous insulin infusion or intensive multiple-daily injection therapy. Pediatr Diabetes 2009;10:91–6 | Study design |
| The Kroc Collaborative Study Group. Diabetic retinopathy after two years of intensified insulin treatment. Follow-up of the Kroc Collaborative Study. JAMA 1988;260:37–41 | Intervention |
| DiMeglio LA, Pottorff TM, Boyd SR, France L, Fineberg N, Eugster EA. A randomized, controlled study of insulin pump therapy in diabetic preschoolers. J Pediatr 2004;145:380–4 | Intervention |
| Edelmann E, Walter H, Biermann E, Schleicher E, Bachmann W, Mehnert H. Sustained normoglycemia and remission phase in newly diagnosed type I diabetic subjects. Comparison between continuous subcutaneous insulin infusion and conventional therapy during a one year follow-up. Horm Metab Res 1987;19:419–21 | Intervention |
| Elleri D, Allen JM, Nodale M, Wilinska ME, Acerini CL, Dunger DB, et al. Suspended insulin infusion during overnight closed-loop glucose control in children and adolescents with type 1 diabetes. Diabet Med 2010;27:480–4 | Study design |
| Ellery B, Mundy L, Hiller JE. Closed-Loop Insulin Delivery System (‘Artificial Pancreas’) for Management of Hypoglycaemia in Type 1 Diabetics. Adelaide, SA: Adelaide Health Technology Assessment on behalf of National Horizon Scanning Unit; 2010 | Systematic review/meta-analysis |
| Emelyanov A, Kuraeva T, Peterkova V. CSII with real time continuous glucose monitoring versus traditional CSII: the comparative results. Pediatr Diabetes 2009;10:101. Conference: 35th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Ljubljana, Slovenia, 2–5 September 2009 | Study design |
| Emelyanov A, Kuraeva T, Peterkova V. CSII with real time continuous glucose monitoring vs. traditional CSII: two year comparative results. Hormone Res Paediatr 2010;74:57. Conference: 49th Annual Meeting of the European Society for Paediatric Endocrinology (ESPE). Prague, Czech Republic, 22–25 September 2010 | Study design |
| Enander R, Adolfsson P, Bergdahl T, Forsander G, Gundevall C, Karlsson AK, et al. Intensive subcutaneous insulin therapy and intravenous insulin infusion at onset of T1DM preserve beta-cell function equally well in children. Diabetes 2011;60:A336. Conference: 71st Scientific Sessions of the American Diabetes Association San Diego, CA, USA. 24–28 June 2011 | Intervention |
| Enander R, Bergdahl T, Adolfsson P, Forsander G, Gundevall C, Karlsson AK, et al. Intensive subcutaneous insulin therapy and intravenous insulin infusion at onset of diabetes preserve beta-cell function equally well in children. Pediatr Diabetes 2011;12:69–70. Conference: 37th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes, ISPAD Miami Beach, FL, USA, 19–22 October 2011 | Intervention |
| Erasmus Medical Center. Comparison Between Insulin Pump Treatment and Multiple Daily Insulin Injections in Diabetic Type 1 Children. NCT00462371 2007. URL: https://clinicaltrials.gov/ct2/show/NCT00462371 (accessed 12 November 2015) | Outcomes |
| Esvant A, Guilhem I, Jouve A, Leguerrier AM, Poirier JY. Real-time continuous monitoring in brittle diabetes: a 6-month observational study. Diabetes Technol Ther 2013;15:A61. Conference: 6th International Conference on Advanced Technologies and Treatments for Diabetes, ATTD 2013 Paris, France 27 February–2 March 2013 | Study design |
| Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Randomized Trial to Assess Efficacy and Safety of Continuous Glucose Monitoring in Children 4-< 10 Years With T1DM. NCT00760526 2014. URL: https://clinicaltrials.gov/ct2/show/NCT00760526 (accessed 12 November 2015) | Outcomes |
| Farrar D, Tuffnell DJ, West J. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2007;3:CD005542 | Systematic review/meta-analysis |
| Fatourechi MM, Kudva YC, Murad MH, Elamin MB, Tabini CC, Montori VM. Clinical review: hypoglycemia with intensive insulin therapy: a systematic review and meta-analyses of randomized trials of continuous subcutaneous insulin infusion versus multiple daily injections. J Clin Endocrinol Metab 2009;94:729–40 | Systematic review/meta-analysis |
| Feldt-Rasmussen B, Mathiesen ER, Jensen T, Lauritzen T, Deckert T. Effect of improved metabolic control on loss of kidney function in type 1 (insulin-dependent) diabetic patients: an update of the Steno studies. Diabetologia 1991;34:164–70 | Intervention |
| Fendler W, Baranowska AI, Mianowska B, Szadkowska A, Mlynarski W. Three-year comparison of subcutaneous insulin pump treatment with multi-daily injections on HbA1c, its variability and hospital burden of children with type 1 diabetes. Acta Diabetol 2012;49:363–70 | Intervention |
| Fiallo-Scharer R. Eight-point glucose testing versus the continuous glucose monitoring system in evaluation of glycemic control in type 1 diabetes. J Clin Endocrinol Metab 2005;90:3387–91 | Intervention |
| Flores d’Arcais A, Morandi F, Beccaria L, Meschi F, Chiumello G. Metabolic control in newly diagnosed type 1 diabetic children. Effect of continuous subcutaneous infusion. Horm Res 1984;19:65–9 | Intervention |
| Fortwaengler K, Rautenberg T, Caruso A. Short term health-economic outcomes of continuous subcutaneous insulin infusion (CSII) in type 1 diabetes: a cost comparison analysis. Value Health 2012;15:A350. Conference: ISPOR 15th Annual European Congress. Berlin, Germany, 3–7 November 2012 | Outcomes |
| Fox L, Englert K, Mauras N. Effects of continuous subcutaneous insulin infusion (CSII) in adolescents with newly-diagnosed type 1 diabetes (T1D) on insulin resistance and s-cell function: a pilot study. Diabetes 2009;58:S1–700. Conference: 69th Annual Meeting of the American Diabetes Association. New Orleans, LA, USA, 5–9 June 2009 | Intervention |
| Fox LA, Buckloh LM, Smith S, Wysocki T, Mauras N. A randomized trial of insulin pump therapy in toddlers and preschool age children with type 1 diabetes (DM1). Pediatr Res 2004;55:136A. Paper presented at the Annual Meeting of the Pediatric Academic Societies. San Francisco, USA, 4 May 2004 | Intervention |
| Fox LA, Buckloh LM, Smith SD, Wysocki T, Mauras N. A randomized controlled trial of insulin pump therapy in young children with type 1 diabetes. Diabetes Care 2005;28:1277–81 | Study design |
| Fox LA, Wilkinson K, Buckloh L, Wysocki T, Mauras N. A randomized trial of insulin pump therapy in preschool age children with type 1 diabetes mellitus: preliminary results. Diabetes 2002;51(Suppl. 2):A426. Paper presented at the 62nd Annual Meeting of the American Diabetes Association. San Francisco, CA, USA, 14–18 June 2002 | Outcomes |
| Frandsen CSS, Kristensen PL, Beck-Nielsen H, Nørgaard K, Perrild H, Christiansen JS, et al. Patients with Type 1 Diabetes Treated with Insulin Pumps do not Experience a Reduced Risk of Severe Hypoglycaemia in a Real Life Setting. Paper presented at the 49th Annual Meeting of the European Association for the Study of Diabetes (EASD). Barcelona, Spain, 23–27 September 2013 | Study design |
| Frias JP, Gottlieb PA, Mackenzie T, Chillara B, Ashley M, Garg SK. Better glycemic control and less severe hypoglycemia in pregnant women with type 1 diabetes treated with continuous subcutaneous insulin infusion. Diabetes 2002;51(Suppl. 2):A431. Paper presented at the 62nd Annual Meeting of the American Diabetes Association. San Francisco, CA, USA, 14–18 June 2002 | Study design |
| Gane J, White B, Christie D, Viner R. Systematic review and meta-analysis of insulin pump therapy in children and adolescents with type 1 diabetes. Arch Dis in Child 2010;95:A94. Conference: Royal College of Paediatrics and Child Health Annual Conference (RCPCH). Coventry, UK, 20–22 April 2010 | Systematic review/meta-analysis |
| Garg S, Bode BW, Bergenstal R, Klonoff DC, Mao M, Weiss R, et al. Characteristics and predictors of nocturnal hypoglycemia in the run-in phase of the aspire in-home study. Diabetes 2014;63:A242. Conference: 74th Scientific Sessions of the American Diabetes Association. San Francisco, CA, USA, 13–17 June 2014 | Outcomes |
| Garg S, Brazg RL, Bailey TS, Buckingham BA, Klonoff DC, Shin J, et al. Automatic insulin pump suspension for induced hypoglycemia: the ASPIRE study. Diabetes 2012;61:A59. Conference: 72nd Scientific Sessions of the American Diabetes Association. Philadelphia, PA, USA, 8–12 June 2012 | Study design |
| Garg S, Brazg RL, Bailey TS, Buckingham BA, Klonoff DC, Shin J, et al. The order effect of the in-clinic ASPIRE study: hypoglycemia begets hypoglycemia. Diabetes 2012;61:A58–9. Conference: 72nd Scientific Sessions of the American Diabetes Association. Philadelphia, PA, USA, 8–12 June 2012 | Study design |
| Garg S, Brazg RL, Bailey TS, Buckingham BA, Slover RH, Klonoff DC, et al. Reduction in duration of hypoglycemia by automatic suspension of insulin delivery: the in-clinic ASPIRE study. Diabetes Technol Ther 2012;14:205–9 | Outcomes |
| Garg S, Brazg RL, Bailey TS, Buckingham BA, Slover RH, Klonoff DC, et al. Reduction in duration of hypoglycemia by automatic suspension of insulin delivery: the in-clinic ASPIRE study. Diabetes Technol Ther 2013;15(Suppl. 1):17–18 | Study design |
| Garg S, Ellis SL, Beatson C, Gottlieb P, Gutin R, Bookout T, et al. Improved glycaemic control in intensively treated subjects with type 1 diabetes using Accu-Chek* advisor insulin guidance software. Diabetologia 2007;50(Suppl. 1):116–17. Paper presented at the 43rd Annual Meeting of the European Association for the Study of Diabetes (EASD). Amsterdam, the Netherlands, 18–21 September 2007 | Intervention |
| Garg SK, Brazg RL, Bailey TS, Buckingham BA, Klonoff DC, Shin J, et al. Reduction of hypoglycaemia with insulin pump suspension and role of antecedent hypoglycaemia on future hypoglycaemic inductions: ASPIRE study. Diabetologia 2012;55:S258–9. Conference: 48th Annual Meeting of the European Association for the Study of Diabetes (EASD). Berlin, Germany, 1–5 October 2012 | Study design |
| Garg SK, Brazg RL, Bailey TS, Buckingham BA, Slover RH, Klonoff DC, et al. Hypoglycemia begets hypoglycemia: the order effect in the ASPIRE in-clinic study. Diabetes Technol Ther 2014;16:125–30 | Study design |
| Garg SK, Crew LB, Moser EG, Voelmle MK, Beatson CR. Effect of continuous glucose monitoring on glycemic control in subjects with type 1 diabetes (T1D) delivering insulin via pump or multiple daily injections (MDI): a prospective study. Diabetes 2010;59:A33–4. Paper presented at the 70th Annual Meeting of the American Diabetes Association. Orlando, USA, 25–29 June 2010 | Study design |
| Garg SK, Voelmle MK, Beatson CR, Miller HA, Crew LB, Freson BJ, et al. Use of continuous glucose monitoring in subjects with type 1 diabetes on multiple daily injections versus continuous subcutaneous insulin infusion therapy: a prospective 6-month study. Diabetes Care 2011;34:574–9 | Study design |
| Garg SK, Weiss R, Shah A, Mao M, Kaufman FR. Change in A1c and reduction in hypoglycemia with threshold suspend in the aspire in-home study. Diabetes Technol Ther 2014;16:A107. Conference: 7th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Vienna, Austria, 5–8 February 2014 | Outcomes |
| Giacomet AC. [Efficacy of the monitoring of the glycemias and insulin pump in the control of diabetes mellitus type I.] Rev AMRIGS 1984;28:303–9 | Not found |
| Gimenez M, Conget I, Nicolau J, Pericot A, Levy I. Outcome of pregnancy in women with type 1 diabetes intensively treated with continuous subcutaneous insulin infusion or conventional therapy. A case–control study. Acta Diabetol 2007;44:34–7 | Study design |
| Goicolea I, Hernández I, Fombellida J, Vázquez JA. Evolution of GFR and other renal function parameters in insulin-dependent diabetic patients treated with subcutaneous insulin infusion. Comparison against an optimized standard therapy: 1 year follow-up effects. An Med Internal 1988;5:169–72 | Not found |
| Goicolea Opacua I, Hernandez Colau I, Vazquez Garcia JA. [Comparative study between the subcutaneous continuous insulin infusion pump and optimized conventional treatment. Effects at 6 months.] Rev Clin Esp 1986;179:3–7 | Intervention |
| Golden SH, Brown T, Yeh HC, Maruthur N, Ranasinghe P, Berger Z, et al. Methods for Insulin Delivery and Glucose Monitoring: Comparative Effectiveness. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012. Report No: 12-EHC036-EF. URL: www.ncbi.nlm.nih.gov/books/NBK99217/ (accessed 16 November 2015) | Systematic review/meta-analysis |
| Gomez A, Alfonso-Cristancho R, Prieto-Salamanca D, Valencia JE, Lynch P, Roze S. Health economic benefits of sensor augmented insulin pump therapy in Colombia. Value Health 2013;16:A690. Conference: ISPOR 4th Latin America Conference. Buenos Aires, Argentina, 12–14 September 2013 | Outcomes |
| Gonzalez-Romero S, Gonzalez-Molero I, Fernandez-Abellan M, Dominguez-Lopez ME, Ruiz-de-Adana S, Olveira G, et al. Continuous subcutaneous insulin infusion versus multiple daily injections in pregnant women with type 1 diabetes. Diabetes Technol Ther 2010;12:263–9 | Study design |
| Gottlieb PA, Crew LB, Moser EG, Voelmle MK, Beatson CR, Gutin RS, et al. Effects of continuous glucose monitoring on glycaemic control in subjects with type 1 diabetes delivering insulin via pump or multiple daily injections: a prospective study. Diabetologia 2010;53:S25. Conference: 46th Annual Meeting of the European Association for the Study of Diabetes (EASD). Stockholm, Sweden, 20–24 September 2010 | Study design |
| Gough H, Castaneda J, Hommel E, Olsen BS, Battelino T, Conget I, et al. The switch study: the impact of continuous glucose monitoring on quality of life and treatment satisfaction. Value Health 2012;15:A359. Conference: ISPOR 15th Annual European Congress. Berlin, Germany, 3–7 November 2012 | Outcomes |
| Greene SA, Smith MA, Baum JD. Clinical application of insulin pumps in the management of insulin dependent diabetes. Arch Dis Child 1983;58:578–81 | Study design |
| Guerci B, Meyer L, Delbachian I, Kolopp M, Ziegler O, Drouin P. Blood glucose control on Sunday in IDDM patients: intensified conventional insulin therapy versus continuous subcutaneous insulin infusion. Diabetes Res Clin Pract 1998;40:175–80 | Outcomes |
| Guilmin-Crepon S, Scornet E, Couque N, Sulmont V, Salmon AS, Le Tallec C, et al. Could clinical parameters at initiation of continuous glucose monitoring (CGM) predict efficacy on HbA1c in type 1 diabetes (T1D) pediatric patients at 3 months? Preliminary results in a prospective study of 141 patients (Start-In!). Pediatr Diabetes 2012;13:117. Conference: 38th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Istanbul, Turkey, 10–13 October 2012 | Study design |
| Haakens K, Hanssen KF, Dahl-Jorgensen K, Vaaler S, Aagenaes O, Mosand R. Continuous subcutaneous insulin infusion (CSII), multiple injections (MI) and conventional insulin therapy (CT) in self-selecting insulin-dependent diabetic patients. A comparison of metabolic control, acute complications and patient preferences. J Intern Med 1990;228:457–64 | Study design |
| Haardt MJ, Selam JL, Slama G, Bethoux JP, Dorange C, Mace B, et al. A cost–benefit comparison of intensive diabetes management with implantable pumps versus multiple subcutaneous injections in patients with type I diabetes. Diabetes Care 1994;17:847–51 | Study design |
| Haidar A, Legault L, Dallaire M, Alkhateeb A, Coriati A, Messier V, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ 2013;185:297–305 | Study design |
| Halvorson M, Carpenter S, Kaiserman K, Kaufman FR. A pilot trial in pediatrics with the sensor-augmented pump: combining real-time continuous glucose monitoring with the insulin pump. J Pediatr 2007;150:103–5 | Study design |
| Hanaire-Broutin H, Melki V, Bessieres-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. The Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000;23:1232–5 | Outcomes |
| Hanas R, Lindholm Olinder A, Olsson PO, Johansson UB, Jacobson S, Heintz E, et al. CSII and SAP valuable tools in the treatment of diabetes; a Swedish health technology assesment. Diabetes Technol Ther 2014;16:A56. Conference: 7th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Vienna, Austria, 5–8 February 2014 | Systematic review/meta-analysis |
| Hanssen KF, Dahl-Jorgensen K, Brinchmann-Hansen O. The influence of strict control on diabetic complications. Acta Endocrinol 1985;272(Suppl.):57–60 | Not found |
| Haugstvedt A, Wentzel-Larsen T, Graue M, Sovik O, Rokne B. Fear of hypoglycaemia in mothers and fathers of children with type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population-based study. Diabet Med 2010;27:72–8 | Study design |
| Hayes Inc. MiniMed paradigm REAL-Time Closed-Loop Continuous Insulin Infusion and Blood Glucose Monitoring System (Medtronic MiniMed Inc.). Hayes, Inc; 2010. URL: www.crd.york.ac.uk/crdweb/ShowRecord.asp?LinkFrom=OAI&ID=32010000975 (accessed 2 February 2015) | Systematic review/meta-analysis |
| Health Quality Ontario. Continuous glucose monitoring for patients with diabetes: an evidence-based analysis. Ont Health Technol Assess Ser 2011;11:1–29 | Systematic review/meta-analysis |
| Health Quality Ontario. Continuous subcutaneous insulin infusion (CSII) pumps for type 1 and type 2 adult diabetic populations: an evidence-based analysis. Ont Health Technol Assess Ser 2009;9:1–58 | Systematic review/meta-analysis |
| Helve E, Koivisto VA, Lehtonen A, Pelkonen R, Huttunen JK, Nikkila EA. A crossover comparison of continuous insulin infusion and conventional injection treatment of type I diabetes. Acta Med Scand 1987;221:385–93 | Intervention |
| Helve E, Laatikainen L, Merenmies L, Koivisto VA. Continuous insulin infusion therapy and retinopathy in patients with type I diabetes. Acta Endocrinol 1987;115:313–19 | Not found |
| Hermanides J, DeVries JH. Sensor-augmented insulin pump more effective than multiple daily insulin injections for reducing HbA1c in people with poorly controlled type 1 diabetes. Evid Based Med 2011;16:46–8 | Study design |
| Hermanides J, Norgaard K, Bruttomesso D, Mathieu C, Frid A, Dayan CM, et al. Sensor augmented pump therapy substantially lowers HbA1c; a randomized controlled trial. Diabetologia 2009;52:S43. Conference: 45th EASD Annual Meeting of the European Association for the Study of Diabetes. Vienna, Austria, 30 September–2 October 2009 | Study design |
| Hermanns N, Kulzer B, Gulde C, Eberle H, Pradler E, Patzelt-Bath A, et al. Short-term effects on patient satisfaction of continuous glucose monitoring with the glucoday with real-time and retrospective access to glucose values: a crossover study. Diabetes Technol Ther 2009;11:275–81 | Study design |
| Hermansen K, Moller A, Christensen CK, Christiansen JS, Schmitz O, Orskov H, et al. Diurnal plasma profiles of metabolite and hormone concentration in insulin-dependent diabetic patients during conventional insulin treatment and continuous subcutaneous insulin infusion. A controlled study. Acta Endocrinol 1987;114:433–9 | Not found |
| Hermansen K, Schmitz O, Boye N, Christensen CK, Christiansen JS, Alberti KG, et al. Glucagon responses to intravenous arginine and oral glucose in insulin-dependent diabetic patients during six months conventional or continuous subcutaneous insulin infusion. Metabolism 1988;37:640–4 | Intervention |
| Hiéronimus S, Cupelli C, Bongain A, Durand-Réville M, Berthier F, Fénichel P. [Pregnancy in type 1 diabetes: insulin pump versus intensified conventional therapy.] Gynecol Obstet Fertil 2005;33:389–94 | Study design |
| Hirsch IB, Bode BW, Garg S, Lane WS, Sussman A, Hu P, et al. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005;28:533–8 | Study design |
| Hoeks L, Greven WL, de Valk HW. Real-time continuous glucose monitoring system for treatment of diabetes: a systematic review. Diabet Med 2011;28:386–94 | Systematic review/meta-analysis |
| Hoffmann-La R. A Study Comparing Continuous Subcutaneous Insulin Infusion With Multiple Daily Injections With Insulin Lispro and Glargine. NCT00468754; 2014. URL: https://clinicaltrials.gov/ct2/show/NCT00468754 (accessed 12 November 2015) | Outcomes |
| Hoffmann-La R. European, Open-label, Prospective, Multinational, Multicenter Study in Adult Subjects With Type 1 or Type 2 Diabetes Previously on MDI or CSII Therapy. Subjects Home Setting is Considered Routine Practice. NCT02105103; 2014. URL: https://clinicaltrials.gov/ct2/show/NCT02105103 (accessed 12 November 2015) | Study design |
| Holder M, Kordonouri O, Haberland H, Golembowski S, Zierow S, Remus K, et al. The low glucose suspend function in sensor-augmented pump therapy prevents hypoglycaemia in children. Diabetologia 2011;54:S400. Conference: 47th Annual Meeting of the European Association for the Study of Diabetes (EASD). Lisbon, Portugal, 12–16 September 2011 | Study design |
| Hollander AS, White NH. Continuous subcutaneous insulin infusion (CSII) reduces severe hypoglycemia (SH) in children with type 1 diabetes mellitus (T1DM) without compromising overall glycemic control. Pediatr Res 2000;47:132A. Paper presented at the Pediatric Academic Societies and the American Academy of Pediatrics joint meeting. Boston, USA, 12–16 May 2000 | Study design |
| Home PD, Capaldo B, Burrin JM, Worth R, Alberti KG. A crossover comparison of continuous subcutaneous insulin infusion (CSII) against multiple insulin injections in insulin-dependent diabetic subjects: improved control with CSII. Diabetes Care 1982;5:466–71 | Study design |
| Hommel E, Olsen B, Battelino T, Conget I, Schutz-Fuhrmann I, Hoogma R, et al. Impact of continuous glucose monitoring on quality of life, treatment satisfaction, and use of medical care resources: analyses from the SWITCH study. Acta Diabetol 2014;51:845–51 | Outcomes |
| Hoogma R, Hoekstra JB, Michels BP, Levi M. Comparison between multiple daily insulin injection therapy (MDI) and continuous subcutaneous insulin infusion therapy (CSII), results of the five nations study. Diabetes Res Clin Pract 2006;74:S144–7. Paper presented at International Symposium on New Technologies for Insulin Replacement. Assisi, Italy, 28 April–1 May 2005 | Study design |
| Hoogma R, Spijker AJM, van Doorn-Scheele M, van Doorn TT, Michels RPJ, van Doorn RG, et al. Quality of life and metabolic control in patients with diabetes mellitus type I treated by continuous subcutaneous insulin infusion or multiple daily insulin injections. Neth J Med 2004;62:383–7 | Study design |
| Hoogma RP, Hammond PJ, Gomis R, Kerr D, Bruttomesso D, Bouter KP, et al. Comparison of the effects of continuous subcutaneous insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and quality of life: results of the 5-nations trial. Diabet Med 2006;23:141–7 | Intervention |
| Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–51 | Study design |
| Hovorka R, Elleri D, Thabit H, Allen JM, Leelarathna L, El-Khairi R, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2014;37:1204–11 | Study design |
| Huang ES, O’Grady M, Basu A, Winn A, John P, Lee J, et al. The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care 2010;33:1269–74 | Outcomes |
| Husted SE, Nielsen HK, Bak JF, Beck-Nielsen H. Antithrombin III activity, von Willebrand factor antigen and platelet function in young diabetic patients treated with multiple insulin injections versus insulin pump treatment. Eur J Clin Invest 1989;19:90–4 | Outcomes |
| Ignatova N, Arbatskaya N, Melnikova E. Continuous subcutaneous insulin infusion (CSII) reduces the rate of hypoglycaemic episodes throughout pregnancy. Diabetologia 2007;50(Suppl. 1):383–4 | Outcomes |
| In Home Closed Loop Study Group. Outpatient Reduction of Nocturnal Hypoglycemia by Using Predictive Algorithms and Pump Suspension in Children. NCT01823341; 2014. URL: https://clinicaltrials.gov/ct2/show/NCT01823341 (accessed 12 November 2015) | Study design |
| In Home Closed Loop Study Group. An Outpatient Pump Shutoff Pilot Feasibility and Safety Study. NCT01736930; 2014. URL: https://clinicaltrials.gov/ct2/show/NCT01736930 (accessed 12 November 2015) | Study design |
| In Home Closed Loop Study Group. Outpatient Pump Shutoff Pilot Feasibility and Efficacy Study. NCT01591681; 2014. URL: https://clinicaltrials.gov/ct2/show/NCT01591681 (accessed 12 November 2015) | Study design |
| Indiana University, Juvenile Diabetes Research Foundation. Prospective Study of the Impact of Insulin Pump Therapy in Young Children With Type 1 Diabetes. NCT00727220; 2012. URL: https://clinicaltrials.gov/ct2/show/NCT00727220 (accessed 12 November 2015) | Study design |
| ISRCTN01687353. Standardized Procedure for the Assessment of New-to-Market Continuous Glucose Monitoring Systems. 2012. URL: www.controlled-trials.com/ISRCTN01687353 (accessed 11 January 2016) | Study design |
| ISRCTN05450731. Paediatric Onset Study to Assess the Efficacy of Insulin Pump Therapy using the MiniMed Paradigm® REAL-Time System during the First Year of Diabetes in Children and Adolescents with Type 1 Diabetes. 2008. URL: www.controlled-trials.com/ISRCTN05450731 (accessed 11 January 2016) | Study design |
| ISRCTN28387915. Utility of Continuous Glucose Monitoring (CGMS) in Children with Type 1 Diabetes on Intensive Treatment Regimens. URL: www.controlled-trials.com/ISRCTN28387915 (accessed 11 January 2016) | Outcomes |
| ISRCTN33678610. A Randomised Controlled Trial (RCT) to Compare Minimally Invasive Glucose Monitoring Devices to Conventional Monitoring in the Management of Insulin Treated Diabetes Mellitus. URL: www.controlled-trials.com/ISRCTN33678610 (accessed 11 January 2016) | Study design |
| ISRCTN33678610. A Randomised Controlled Trial (RCT) to Compare Minimally Invasive Glucose Monitoring Devices to Conventional Monitoring in the Management of Insulin Treated Diabetes Mellitus. 2003. URL: www.controlled-trials.com/ISRCTN33678610 (accessed 11 January 2016) | Intervention |
| ISRCTN37153662. Comparison Between Continuous Subcutaneous Insulin Infusion with Multiple Basal Lispro Infusion Rates and Multiple Daily Insulin Injection with Lispro And Glargine. 2007. URL: www.controlled-trials.com/ISRCTN37153662 (accessed 11 January 2016) | Intervention |
| ISRCTN52164803. Prevention of Recurrent Severe Hypoglycaemia: Optimised Multiple Daily Insulin Injection (MDI) versus Continuous Subcutaneous Insulin Infusion (CSII) with or without Adjunctive Real-Time Continuous Glucose Monitoring. 2009. URL: www.controlled-trials.com/ISRCTN52164803 (accessed 11 January 2016) | Outcomes |
| ISRCTN62034905. Comparison of Two Artificial Pancreas Systems for Closed Loop Blood Glucose Control Versus Open Loop Control in Patients with Type 1 Diabetes. 2011. URL: www.controlled-trials.com/ISRCTN62034905 (accessed 11 January 2016) | Study design |
| ISRCTN64351161. Comparison in Metabolic Control and Treatment Satisfaction with Continuous Subcutaneous Insulin Infusion and Multiple Daily Injections in Children at Onset of Type 1 Diabetes Mellitus. 2007. URL: www.controlled-trials.com/ISRCTN64351161 (accessed 11 January 2016) | Outcomes |
| ISRCTN77773974. A Randomised Study of Continuous Subcutaneous Insulin Infusion (CSII) Therapy Compared to Conventional Bolus Insulin Treatment in Preschool Aged Children with Type 1 Diabetes. URL: www.controlled-trials.com/ISRCTN77773974 (accessed 11 January 2016) | Outcomes |
| Jakisch BI, Wagner VM, Heidtmann B, Lepler R, Holterhus PM, Kapellen TM, et al. Comparison of continuous subcutaneous insulin infusion (CSII) and multiple daily injections (MDI) in paediatric type 1 diabetes: a multicentre matched-pair cohort analysis over 3 years. Diabet Med 2008;25:80–5 | Study design |
| JDRF Artificial Pancreas Project. Randomized Study of Real-Time Continuous Glucose Monitors (RT-CGM) in the Management of Type 1 Diabetes. NCT00406133; 2010. URL: https://clinicaltrials.gov/ct2/show/NCT00406133 (accessed 12 November 2015) | Outcomes |
| Jeha GS, Karaviti LP, Anderson B, Smith EOB, Donaldson S, McGirk TS, et al. Insulin pump therapy in preschool children with type 1 diabetes mellitus improves glycemic control and decreases glucose excursions and the risk of hypoglycemia. Diabetes Technol Ther 2005;7:876–84 | Study design |
| Jeitler K, Horvath K, Berghold A, Gratzer TW, Neeser K, Pieber TR, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia 2008;51:941–51 | Systematic review/meta-analysis |
| Jenkins AJ, Krishnamurthy B, Best JD, Cameron FJ, Colman PG, Hamblin PS, et al. An algorithm guiding patient responses to real-time-continuous glucose monitoring improves quality of life. Diabetes Technol Ther 2011;13:105–9 | Intervention |
| Jennings AM, Lewis KS, Murdoch S, Talbot JF, Bradley C, Ward JD. Randomized trial comparing continuous subcutaneous insulin infusion and conventional insulin therapy in type II diabetic patients poorly controlled with sulfonylureas. Diabetes Care 1991;14:738–44 | Population |
| Jiang L, Jiang S, Ma Y, Zhang M, Feng X. Real-time Continuous Glucose Monitoring vs. Conventional Glucose Monitoring in Critically Ill Patients. PROSPERO: CRD42014013488; 2014. URL: www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014013488 (accessed 16 November 2015) | Systematic review/meta-analysis |
| Jimenez M, Hernaez R, Conget I, Alonso A, Yago G, Pericot A, et al. Metabolic control, maternal and perinatal outcomes in type 1 diabetic pregnancies intensively treated with conventional insulin therapy vs. continuous subcutaneous insulin infusion. Diabetologia 2005;48(Suppl. 1):A315. Paper presented at 41st Annual Meeting of the European Association for the Study of Diabetes (EASD). Athens, Greece, 10–15 September 2005 | Study design |
| Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Group, Beck RW, Lawrence JM, Laffel L, Wysocki T, Xing D, et al. Quality-of-life measures in children and adults with type 1 diabetes: Juvenile Diabetes Research Foundation Continuous Glucose Monitoring randomized trial. Diabetes Care 2010;33:2175–7 | Intervention |
| Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–76 | Intervention |
| Kamble S, Perry BM, Shafiroff J, Schulman KA, Reed SD. The cost-effectiveness of initiating sensor-augmented pump therapy versus multiple daily injections of insulin in adults with type 1 diabetes: evaluating a technology in evolution. Value Health 2011;14:A82. Conference: 16th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Baltimore, MD, USA, 21–25 May 2011 | Outcomes |
| Kamble S, Schulman KA, Reed SD. Cost-effectiveness of sensor-augmented pump therapy in adults with type 1 diabetes in the USA. Value Health 2012;15:632–8 | Outcomes |
| Kamble S, Weinfurt KP, Perry BM, Schulman KA, Reed SD. Patient time and indirect costs associated with sensor-augmented insulin pump therapy in type 1 diabetes. Value Health 2011;14:A824. Conference: 16th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Baltimore, MD, USA, 21–25 May 2011 | Outcomes |
| Kamble S, Weinfurt KP, Schulman KA, Reed SD. Patient time costs associated with sensor-augmented insulin pump therapy for type 1 diabetes: results from the STAR 3 randomized trial. Med Decis Making 2013;33:215–24 | Outcomes |
| Kapellen T, Kordonouri O, Pankowska E, Rami B, Coutant R, Hartmann R, et al. Sensor-augmented pump therapy from the onset of type 1 diabetes in children and adolescents – results of the Pediatric ONSET Study after 12 months of treatment. Horm Res Paediatr 2010;74:58. Conference: 49th Annual Meeting of the European Society for Paediatric Endocrinology (ESPE). Prague, Czech Republic, 22–25 September 2010 | Study design |
| Kaufman F, Shin J, Yang Q. Differences in measures of glycemic variability between the multiple daily injection therapy and sensor-augmented pump therapy groups in the star 3 study. Diabetes Technol Ther 2011;13:186. Conference: 4th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). London, UK, 16–19 February 2011 | Outcomes |
| Kaufman FR, Agrawal P, Askari S, Kannard B, Welsh JB. Effectiveness of the low glucose suspend feature of the medtronic paradigm Veo insulin pump in children and adolescents. Pediatr Diabetes 2011;12:30–31. Conference: 37th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Miami Beach, FL, USA, 19–22 October 2011 | Study design |
| Kaufman FR, Agrawal P, Lee SW, Kannard B. Characterization of the low glucose suspend feature of the medtronic minimed paradigm veo insulin pump system and events preceding its activation. Diabetes 2011;60:A249. Conference: 71st Scientific Sessions of the American Diabetes Association. San Diego, CA, USA, 24–28 June 2011 | Study design |
| Kaufman FR, Austin J, Neinstein A, Jeng L, Halvorson M, Devoe DJ, et al. Nocturnal hypoglycemia detected with the continuous glucose monitoring system in pediatric patients with type 1 diabetes. J Pediatr 2002;141:625–30 | Study design |
| Kaufman FR, Gibson LC, Halvorson M, Carpenter S, Fisher LK, Pitukcheewanont P. A pilot study of the continuous glucose monitoring system: clinical decisions and glycemic control after its use in pediatric type 1 diabetic subjects. Diabetes Care 2001;24:2030–4 | Study design |
| Kaufman FR, Halvorson M, Kim C, Pitukcheewanont P. Use of insulin pump therapy at nighttime only for children 7–10 years of age with type 1 diabetes. Diabetes Care 2000;23:579–82 | Study design |
| Keenan DB, Cartaya R, Mastrototaro JJ. Accuracy of a new real-time continuous glucose monitoring algorithm. J Diabetes Sci Technol 2010;4:111–18 | Study design |
| Keenan DB, Mastrototaro JJ, Zisser H, Cooper KA, Raghavendhar G, Lee SW, et al. Accuracy of the Enlite 6-day glucose sensor with guardian and Veo calibration algorithms. Diabetes Technol Ther 2012;14:225–31 | Study design |
| Kernaghan D, Farrell T, Hammond P, Owen P. Fetal growth in women managed with insulin pump therapy compared to conventional insulin. Eur J Obstet Gynecol Reprod Biol 2008;137:47–9 | Intervention |
| Khalil S, Wright T, Field A, Hand J, Dyer P, Karamat MA. Does continuous subcutaneous insulin infusion (CSII) provide an effective method of controlling diabetes in pregnant women with type 1 diabetes? Diabet Med 2013;30(Suppl. 1):170. Paper presented at Diabetes UK Professional Conference. Manchester, UK, 13–15 March 2013 | Study design |
| King Abdullah International Medical Research Centre. Incidence of Hypoglycemia During Ramadan in Patients With Type 1 Diabetes on Insulin Pump Versus Multi Dose Injection. NCT01941238; 2013. URL: https://clinicaltrials.gov/ct2/show/NCT01941238 (accessed 12 November 2015) | Study design |
| Kordonouri O, Hartmann R, Lauterborn R, Barnekow C, Hoeffe J, Deiss D. Age-specific advantages of continuous subcutaneous insulin infusion as compared with multiple daily injections in pediatric patients: one-year follow-up comparison by matched-pair analysis. Diabetes Care 2006;29:133–4 | Intervention |
| Kordonouri O, Hartmann R, Pankowska E, Rami B, Kapellen T, Coutant R, et al. Follow-up of patients with sensor-augmented pump therapy during the first year of diabetes-pediatric onset study. Pediatr Diabetes 2011;12:29. Conference: 37th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Miami Beach, FL, USA, 19–22 October 2011 | Study design |
| Kordonouri O, Hartmann R, Pankowska E, Rami B, Kapellen T, Coutant R, et al. Sensor augmented pump therapy from onset of type 1 diabetes: late follow-up results of the Pediatric ONSET Study. Diabetologia 2011;54:S41. Conference: 47th Annual Meeting of the European Association for the Study of Diabetes (EASD). Lisbon, Portugal, 12–16 September 2011 | Study design |
| Kordonouri O, Hartmann R, Pankowska E, Rami B, Kapellen T, Coutant R, et al. Sensor augmented pump therapy from onset of type 1 diabetes: late follow-up results of the Pediatric Onset Study. Pediatr Diabetes 2012;13:515–18 | Study design |
| Kordonouri O, Pankowska E, Rami B, Kapellen T, Coutant R, Hartmann R, et al. Sensor-augmented pump therapy from the diagnosis of childhood type 1 diabetes: results of the Paediatric Onset Study (ONSET) after 12 months of treatment. Diabetologia 2010;53:2487–95 | Study design |
| Kordonouri O. Pumps and sensors from the onset of diabetes. Pediatr Diabetes 2010;11:6. Conference: 36th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Buenos Aires, Argentina, 27–30 October 2010 | Study design |
| Kovatchev BP. Safety and efficacy of outpatient closed-loop control – results from randomized crossover trials of a wearable artificial pancreas. Paper presented at 74th Scientific Sessions of the American Diabetes Association. San Francisco, CA, USA, 13–17 June 2014 | Study design |
| Kracht T, Kordonouri O, Datz N, Scarabello C, Walte K, Blaesig S, et al. Reducing glycaemic variability and HbA1c with the Dexcom Seven.2 continuous glucose monitoring system in children and young adults with type 1 diabetes (T1D). Pediatr Diabetes 2009;10:104. Conference: 35th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Ljubljana, Slovenia, 2–5 September 2009 | Intervention |
| Kruger J, Brennan A. The cost of type 1 diabetes mellitus in the United Kingdom: a review of cost-of-illness studies. Eur J Health Econ 2013;14:887–99 | Outcomes |
| Laatikainen L, Teramo K, Hieta-Heikurainen H, Koivisto V, Pelkonen R. A controlled study of the influence of continuous subcutaneous insulin infusion treatment on diabetic retinopathy during pregnancy. Acta Med Scand 1987;221:367–76 | Intervention |
| Laffel L, Buckingham B, Chase P, Bailey T, Liljenquist D, Daniels M, et al. Performance of a continuous glucose monitoring system (CGM) and CGM glucose ranges in youth ages 2–17 yr old. Pediatr Diabetes 2013;14:47–48. Conference: 39th Annual Conference of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Gothenburg, Sweden, 16–19 October 2013 | Intervention |
| Lagarde WH, Barrows FP, Davenport ML, Kang M, Guess HA, Calikoglu AS. Continuous subtaneous glucose monitoring in children with type 1 diabetes mellitus: a single-blind, randomized, controlled trial. Pediatr Diabetes 2006;7:159–64 | Outcomes |
| Laguna AJ, Rossetti P, Ampudia-Blasco FJ, Vehi J, Bondia J. Postprandial performance of Dexcom SEVEN PLUS and Medtronic Paradigm Veo: modeling and statistical analysis. Biomed Signal Process Control 2014;10:322–31 | Study design |
| Lange K, Coutant R, Danne T, Kapellen T, Pankowska E, Rami B, et al. High quality of life in children and psychological wellbeing in mothers 12 month after diabetes onset: results of the paediatric onset-trial of sensor-enhanced CSII. Pediatr Diabetes 2010;11:101. Conference: 36th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Buenos Aires, Argentina, 27–30 October 2010 | Study design |
| Langeland LB, Salvesen O, Selle H, Carlsen SM, Fougner KJ. Short-term continuous glucose monitoring: effects on glucose and treatment satisfaction in patients with type 1 diabetes mellitus; a randomized controlled trial. Int J Clin Pract 2012;66:741–7 | Study design |
| Langendam M, Luijf YM, Hooft L, DeVries JH, Mudde AH, Scholten RJPM. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev 2012; 1:CD008101 | Systematic review/meta-analysis |
| Lapolla A, Dalfra MG, Masin M, Bruttomesso D, Piva I, Crepaldi C, et al. Analysis of outcome of pregnancy in type 1 diabetics treated with insulin pump or conventional insulin therapy. Acta Diabetol 2003;40:143–9 | Study design |
| Lauritzen T, Frost-Larsen K, Larsen HW, Deckert T. Two-year experience with continuous subcutaneous insulin infusion in relation to retinopathy and neuropathy. Diabetes 1985;34(Suppl. 3):74–9 | Intervention |
| Lawson ML, Bradley B, McAssey K, Clarson C, Kirsch S, Curtis JR, et al. Timing of initiation of continuous glucose monitoring in established pediatric diabetes: recruitment and baseline characteristics in the CGM time trial. Diabetes Technol Ther 2014;16:A73–4. Conference: 7th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Vienna, Austria, 5–8 February 2014 | Outcomes |
| Lawson ML, Olivier P, Huot C, Richardson C, Nakhla M, Romain J. Simultaneous vs. delayed initiation of Real-Time Continuous Glucose Monitoring (RT-CGM) in children and adolescents with established type 1 diabetes starting insulin pump therapy: a pilot study. Pediatr Diabetes 2011;12:126–7. Conference: 37th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Miami Beach, FL, USA, 19–22 October 2011 | Outcomes |
| Lawson ML, Richardson C, Muileboom J, Evans K, Landry A, Cormack L. Development of a standardized approach to initiating continuous glucose monitoring in amulticentre pediatric study. Diabetes Technol Ther 2014;16:A73. Conference: 7th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Vienna, Austria, 5–8 February 2014 | Outcomes |
| Lawson P, Home PD, Bergenstal R. Observations on blood lipid and intermediary metabolite concentrations during conventional insulin treatment or CSII. Diabetes 1985;34(Suppl. 3):27–30 | Intervention |
| Lebenthal Y, Lazar L, Benzaquen H, Shalitin S, Phillip M. Patient perceptions of using OmniPod System compared with conventional insulin pumps in young adults with type 1 diabetes. Pediatr Diabetes 2011;12:131–2. Conference: 37th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Miami Beach, FL, USA, 19–22 October 2011 | Intervention |
| Lebenthal Y, Lazar L, Benzaquen H, Shalitin S, Phillip M. Patient perceptions of using the OmniPod system compared with conventional insulin pumps in young adults with type 1 diabetes. Diabetes Technol Ther 2012;14:411–17 | Intervention |
| Lecavalier L, Havrankova J, Hamet P, Chiasson JL. Effects of continuous subcutaneous insulin infusion versus multiple injections on insulin receptors in insulin-dependent diabetics. Diabetes Care 1987;10:300–5 | Study design |
| Lee SW, Welsh JB, Green JB, Joyce C, Tamborlane WV, Kaufman FR. Successful transitions from MDI therapy to sensor-augmented pump therapy in the STAR 3 study: system settings and behaviours. Diabetologia 2011;54:S395–6. Conference: 47th Annual Meeting of the European Association for the Study of Diabetes (EASD). Lisbon, Portugal, 12–16 September 2011 | Outcomes |
| Leelarathna L, Little SA, Walkinshaw E, Tan HK, Lubina-Solomon A, Kumareswaran K, et al. Restoration of self-awareness of hypoglycemia in adults with long-standing type 1 diabetes: hyperinsulinemic-hypoglycemic clamp substudy results from the HypoCOMPaSS trial. Diabetes Care 2013;36:4063–70 | Study design |
| Legacy Health System, Juvenile Diabetes Research Foundation. Sensor-Augmented Insulin Delivery: Insulin Plus Glucagon Versus Insulin Alone. 2011. URL: http://ClinicalTrials.gov/show/NCT00797823 (accessed 23 February 2016) | Study design |
| Lepore G, Dodesini AR, Nosari I, Trevisan R. Both continuous subcutaneous insulin infusion and a multiple daily insulin injection regimen with glargine as basal insulin are equally better than traditional multiple daily insulin injection treatment. Diabetes Care 2003;26:1321–2 | Study design |
| Lepore G, Dodesini AR, Nosari I, Trevisan R. Effect of continuous subcutaneous insulin infusion vs. multiple daily insulin injection with glargine as basal insulin: an open parallel long-term study. Diabetes Nutr Metab 2004;17:84–9 | Not found |
| Leveno KJ, Fortunato SJ, Raskin P, Williams ML, Whalley PJ. Continuous subcutaneous insulin infusion during pregnancy. Diabetes Res Clin Pract 1988;4:257–68 | Intervention |
| Li A, Tsang CH. The Effectiveness of Continuous Subcutaneous Insulin Infusion on Quality of Life of Families and Glycaemic Control Among Children with Type 1 Diabetes: A Systematic Review. PROSPERO: CRD42012002029; 2012. URL: www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42012002029 (accessed 16 November 2015) | Systematic review/meta-analysis |
| Li XL. Multiple daily injections versus insulin pump therapy in patients with type 1 diabetes mellitus: a meta analysis. J Clin Rehabil Tissue Engineering Res 2010;14:8722–5 | Systematic review/meta-analysis |
| Lindholm Olinder A, Hanas R, Heintz E, Jacobson S, Johansson UB, Olsson PO, et al. CGM and SAP are valuable tools in the treatment of diabetes; a swedish health technology assessment. Diabetes Technol Ther 2014;16:A74. Conference: 7th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Vienna, Austria, 5–8 February 2014 | Systematic review/meta-analysis |
| Liouri E, Koutsovasilis A, Kounenou K, Kamaratos A, Koukouli M-P, Nikolaou A, et al. Intensified insulin therapy vs CSII: the influence on family cohesion and adaptability of type 1 diabetics. Paper presented at 45th EASD Annual Meeting of the European Association for the Study of Diabetes. Vienna, Austria, 30 September–2 October 2009 | Outcomes |
| Little S, Chadwick T, Choudhary P, Brennand C, Stickland J, Barendse S, et al. Comparison of Optimised MDI versus Pumps with or without Sensors in Severe Hypoglycaemia (the Hypo COMPaSS trial). BMC Endocr Disord 2012;12:33 | Outcomes |
| Little SA, Leelarathna L, Walkinshaw E, Kai Tan H, Chapple O, Barendse S, et al. A definitive multicenter RCT to restore hypoglycemia awareness and prevent recurrent severe hypoglycemia in adults with long- standing type 1 diabetes: Results from the hypocompass trial. Diabetes 2013;62:A98. Conference: 73rd Scientific Sessions of the American Diabetes Association. Chicago, IL, USA, 21–25 June 2013 | Outcomes |
| Little SA, Leelarathna L, Walkinshaw E, Tan HK, Chapple O, Lubina-Solomon A, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014;37:2114–22 | Outcomes |
| Littlejohn E, Turksoy K, Quinn LT, Cinar A. Integrated multivariable artificial pancreas control systems work as well as operator controlled systems. Paper presented at 74th Scientific Sessions of the American Diabetes Association. San Francisco, CA, USA, 13–17 June 2014 | Intervention |
| Litton J, Rice A, Friedman N, Oden J, Lee MM, Freemark M. Insulin pump therapy in toddlers and preschool children with type 1 diabetes mellitus. J Pediatr 2002;141:490–5 | Study design |
| Logtenberg SJ, Kleefstra N, Groenier KH, Gans RO, Bilo HJ. Use of short-term real-time continuous glucose monitoring in type 1 diabetes patients on continuous intraperitoneal insulin infusion: a feasibility study. Diabetes Technol Ther 2009;11:293–9 | Intervention |
| Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics 2003;111:933–8 | Intervention |
| Luijf YM, De Vries JH, Mader JK, Doll W, Place J, Renard E, et al. Accuracy and reliability of current continuous glucose monitoring systems: a direct comparison. J Diabetes Sci Technol 2013;7:A83. Conference: 12th Annual Diabetes Technology Meeting. Bethesda, MD, USA, 8–10 November 2012 | Outcomes |
| Luijf YM, DeVries JH, Mader JK, Doll W, Place J, Renard E, et al. Accuracy and reliability of current CGM systems: a direct comparison. Diabetes Technol Ther 2013;15:A13–14. Conference: 6th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Paris, France, 27 February–2 March 2013 | Outcomes |
| Luijf YM, DeVries JH, Zwinderman K, Leelarathna L, Nodale M, Caldwell K, et al. Day and night closed-loop control in adults with type 1 diabetes: a comparison of two closed-loop algorithms driving continuous subcutaneous insulin infusion versus patient self-management. Diabetes Care 2013;36:3882–7 | Study design |
| Luijf YM, Mader JK, Doll W, Pieber T, Farret A, Place J, et al. Accuracy and reliability of continuous glucose monitoring systems: a head-to-head comparison. Diabetes Technol Ther 2013;15:721–6 | Study design |
| Ly TT, Keenan DB, Spital G, Roy A, Grosman B, Cantwell M, et al. Portable glucose control with daytime treat-to-range and overnight proportionalintegral-derivative control in adolescents with type 1 diabetes. Diabetes Technol Ther 2013;15:A14. Conference: 6th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Paris, France, 27 February–2 March 2013 | Outcomes |
| Ly TT, Nicholas JA, Davis EA, Jones TW. Initial experience of automated low glucose insulin suspension using the medtronic paradigm veo system. Diabetes 2011;60:A112. Conference: 71st Scientific Sessions of the American Diabetes Association. San Diego, CA, USA, 24–28 June 2011 | Study design |
| Ly TT, Nicholas JA, Retterath A, Davis EA, Jones TW. Analysis of glucose responses to automated insulin suspension with sensor-augmented pump therapy. Diabetes Care 2012;35:1462–5 | Outcomes |
| Maahs DM, Calhoun P, Buckingham BA, Chase HP, Hramiak I, Lum J, et al. A randomized trial of a home system to reduce nocturnal hypoglycemia in type 1 diabetes. Diabetes Care 2014;37:1885–91 | Study design |
| Maahs DM, Chase HP, Westfall E, Slover R, Huang S, Shin JJ, et al. The effects of lowering nighttime and breakfast glucose levels with sensor-augmented pump therapy on hemoglobin A1c levels in type 1 diabetes. Diabetes Technol Ther 2014;16:284–91 | Study design |
| Maiorino MI, Bellastella G, Petrizzo M, Improta MR, Brancario C, Castaldo F, et al. Treatment satisfaction and glycemic control in young type 1 diabetic patients in transition from pediatric health care: CSII versus MDI. Endocrine 2014;46:256–62 | Study design |
| Manfrini S, Crino A, Fredrickson L, Pozzilli P. CSII versus intensive insulin therapy at onset of type 1 diabetes: the IMDIAB 8 two-year randomised trial. Diabetes 2002;51(Suppl. 2):A4. Paper presented at 62nd Annual Meeting of the American Diabetes Association. San Francisco, CA, USA, 14–18 Jun 2002 | Study design |
| Maran A, Crazzolara D, Nicoletti M, Costa S, dal Pos M, Tiengo A, et al. A randomized crossover study to compare continuous subcutaneous insulin infusion (CSII) with multiple daily injection (MDI) in type 1 diabetic patients previously treated with CSII. Diabetologia 2005;48(Suppl. 1):A328. Paper presented at 41st Annual Meeting of the European Association for the Study of Diabetes (EASD). Athens, Greece, 10–15 September 2005 | Outcomes |
| Mauras N, Beck R, Xing D, Ruedy K, Buckingham B, Tansey M, et al. A randomized controlled trial (RCT) to assess the efficacy and safety of real-time continuous glucose monitoring (CGM) in the management of type 1 diabetes (T1D) in young children. Pediatr Diabetes 2011;12:30. Conference: 37th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Miami Beach, FL, USA, 19–22 October 2011 | Intervention |
| Mauras N, Beck R, Xing D, Ruedy K, Buckingham B, Tansey M, et al. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to < 10 years. Diabetes Technol Ther 2013;15(Suppl. 1):S14–15 | Study design |
| Mauras N, Beck R, Xing DY, Ruedy K, Buckingham B, Tansey M, et al. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to < 10 years. Diabetes Care 2012;35:204–10 | Study design |
| McCoy R, Smith S. Insulin pumps with a sensor and threshold-suspend reduced nocturnal hypoglycemia in type 1 diabetes. Ann Intern Med 2013;159:JC7 | Study design |
| McCoy R. Insulin pumps with a sensor and threshold-suspend reduced nocturnal hypoglycemia in type 1 diabetes. Ann Intern Med 2013;159:JC7 | Study design |
| Medtronic Diabetes. Feasibility Study for Training Pump Naive Subjects To Use The Paradigm® System And Evaluate Effectiveness. NCT00530023; 2011. URL: https://clinicaltrials.gov/ct2/show/NCT00530023 (accessed 12 November 2015) | Intervention |
| Medtronic. SWITCH – Sensing With Insulin Pump Therapy to Control HbA1c. 2010. URL: http://ClinicalTrials.gov/show/NCT00598663 (accessed 16 November 2015) | Study design |
| Melki V, Hanaire-Broutin H, Bessieres-Lacombe S, Tauber JP. CSII versus MDI in IDDM patients treated with insulin lispro: results of a randomised, cross-over trial. Diabetologia 1999;42(Suppl. 1):A17. Paper presented at 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October 1999 | Outcomes |
| Mello G, Biagioni S, Ottanelli S, Nardini C, Tredici Z, Serena C, et al. Continuous subcutaneous insulin infusion (CSII) versus multiple daily injections (MDI) of rapid-acting insulin analogues and detemir in type 1 diabetic (T1D) pregnant women. J Matern Fetal Neonatal Med 2014;28:276–80 | Study design |
| Mello G, Parretti E, Tondi F, Riviello C, Borri P, Scarselli G. Impact of two treatment regimens with insulin lispro in post-prandial glucose excursion patterns and fetal fat mass growth in type 1 diabetic pregnant women. Am J Obstet Gynecol 2005;193(Suppl. 6):S36. Paper presented at 26th Annual Meeting of the Society for Maternal–Fetal Medicine: the Pregnancy Meeting, 30 January–4 February 2006, Miami, FL, USA | Outcomes |
| Meschi F, Beccaria L, Vanini R, Szulc M, Chiumello G. Short-term subcutaneous insulin infusion in diabetic children. Comparison with three daily insulin injections. Acta Diabetol Lat 1982;19(4371–5) | Not found |
| Meyer L, Boullu-Sanchis S, Boeckler P, Sibenaler A, Treger M, Pinget M, et al. Comparison of glycemic control in 3 groups of type 1 diabetic patients treated with multiinjections and lispro (MDI), continuous subcutaneous insulin infusion with lispro (CSII) or continuous peritoneal insulin infusion (CPII): data of continuous subcutaneous glucose sensing (CGMS). Diabetes 2002;51(Suppl. 2):A124–5. Paper presented at 62nd Annual Meeting of the American Diabetes Association. San Francisco, CA, USA, 14–18 June 2002 | Study design |
| Micossi P, Raggi U, Dosio F. Open-loop device microjet MC 2 improves unstable diabetes, lowers the daily insulin requirement and reduces the excursions of plasma free insulin levels: comparison with a traditional intensive treatment. J Endocrinol Invest 1983;6:189–94 | Intervention |
| Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev 2010; 1:CD005103 | Systematic review/meta-analysis |
| Moller A, Rasmussen L, Ledet T, Christiansen JS, Christensen CK, Mogensen CE, et al. Lipoprotein changes during continuous subcutaneous insulin infusion in insulin-dependent diabetic patients. Scand J Clin Lab Invest 1986;46:471–5 | Intervention |
| Monami M, Lamanna C, Marchionni N, Mannucci E. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in type 1 diabetes: a meta-analysis. Acta Diabetol 2010;47(Suppl. 1):77–81 | Systematic review/meta-analysis |
| Monnier LH, Rodier M, Gancel A, Crastes de Paulet P, Colette C, Piperno M, et al. Plasma lipid fatty acids and platelet function during continuous subcutaneous insulin infusion in type I diabetes. Diabetes Metab 1987;13:210–16 | Intervention |
| Moreno-Fernandez J, Gomez FJ, Gazquez M, Pedroche M, Garcia-Manzanares A, Tenias JM, et al. Real-time continuous glucose monitoring or continuous subcutaneous insulin infusion, what goes first? Results of a pilot study. Diabetes Technol Ther 2013;15:596–600 | Study design |
| Mukhopadhyay A, Farrell T, Fraser RB, Ola B. Continuous subcutaneous insulin infusion vs. intensive conventional insulin therapy in pregnant diabetic women: a systematic review and metaanalysis of randomized, controlled trials. Am J Obstet Gynecol 2007;197:447–56 | Systematic review/meta-analysis |
| Murphy HR, Kumareswaran K, Elleri D, Allen JM, Caldwell K, Biagioni M, et al. Safety and efficacy of 24-h closed-loop insulin delivery in well-controlled pregnant women with type 1 diabetes: a randomized crossover case series. Diabetes Care 2011;34:2527–9. [Erratum appears in Diabetes Care 2012;35:191] | Study design |
| Myers SJ, Uhrinak AN, Kaufman FR, Lee SW, Yusi J, Huang S, et al. Retrospective analysis of events preceding low glucose suspend activation in pediatric subjects on the Paradigm Veo system. J Diabetes Sci Technol 2012;6:A125. Conference: 11th Annual Diabetes Technology Meeting. San Francisco, CA, USA, 27–29 October 2011 | Study design |
| Nabhan ZM, Kreher NC, Greene DM, Eugster EA, Kronenberger W, DiMeglio LA. A randomized prospective study of insulin pump vs. insulin injection therapy in very young children with type 1 diabetes: 12-month glycemic, BMI, and neurocognitive outcomes. Pediatr Diabetes 2009;10:202–8 | Intervention |
| Nahata L. Insulin therapy in pediatric patients with type I diabetes: continuous subcutaneous insulin infusion versus multiple daily injections. Clin Pediatr (Phila) 2006;45:503–8 | Study design |
| Nathan DM, Lou P, Avruch J. Intensive conventional and insulin pump therapies in adult type I diabetes. A crossover study. Ann Intern Med 1982;97:31–6 | Study design |
| Nemours Children’s Clinic. Insulin Pump Therapy in Adolescents With Newly Diagnosed Type 1 Diabetes (T1D). NCT00357890; 2006. URL: https://clinicaltrials.gov/ct2/show/NCT00357890 (accessed 12 November 2015) | Population |
| JRDF Artificial Pancreas Project. Randomized Study of Real-Time Continuous Glucose Monitors (RT-CGM) in the Management of Type 1 Diabetes. NCT00406133; 2006. URL: https://clinicaltrials.gov/ct2/show/NCT00406133 (accessed 12 November 2015) | Outcomes |
| Erasmus Medical Center. Comparison Between Insulin Pump Treatment and Multiple Daily Insulin Injections in Diabetic Type 1 Children. NCT00462371; 2007. URL: https://clinicaltrials.gov/ct2/show/NCT00462371 (accessed 12 November 2015) | Outcomes |
| Hoffman-La Roche. A Study Comparing Continuous Subcutaneous Insulin Infusion With Multiple Daily Injections With Insulin Lispro and Glargine. NCT00468754; 2007. URL: https://clinicaltrials.gov/ct2/show/NCT00468754 (accessed 12 November 2015) | Outcomes |
| Medtronic Diabetes. Feasibility Study for Training Pump Naive Subjects To Use The Paradigm® System And Evaluate Effectiveness. NCT00530023; 2007. URL: https://clinicaltrials.gov/ct2/show/NCT00530023 (accessed 12 November 2015) | Duplicate |
| Novo Nordisk A/S. Efficacy and Safety of Insulin Aspart in MDI or CSII in Children Below 7 Years of Age With Type 1 Diabetes. NCT00571935; 2007. URL: https://clinicaltrials.gov/ct2/show/NCT00571935 (accessed 12 November 2015) | Outcomes |
| Arkansas Children’s Hospital Research Institute. Preservation of Pancreatic Beta Cell Function Through Insulin Pump Therapy. NCT00574405; 2007. URL: https://clinicaltrials.gov/ct2/show/NCT00574405 (accessed 12 November 2015) | Outcomes |
| Medtronic. SWITCH – Sensing With Insulin Pump Therapy to Control HbA1c. NCT00598663; 2007. URL: https://clinicaltrials.gov/ct2/show/NCT00598663 (accessed 12 November 2015) | Study design |
| Boston University. Closed-loop Glucose Control for Automated Management of Type 1 Diabetes. NCT00811317; 2008. URL: https://clinicaltrials.gov/ct2/show/NCT00811317 (accessed 12 November 2015) | Intervention |
| Seattle Children’s Hospital. The Effectiveness of Continuous Glucose Monitoring in Diabetes Treatment for Infants and Young Children. NCT00875290; 2009. URL: https://clinicaltrials.gov/ct2/show/NCT00875290 (accessed 12 November 2015) | Duplicate |
| DexCom Inc. Effectiveness and Safety Study of the DexCom™ G4 Continuous Glucose Monitoring System in Children and Adolescents With Type 1 Diabetes Mellitus. NCT01185496; 2010. URL: https://clinicaltrials.gov/ct2/show/NCT01185496 (accessed 12 November 2015) | Study design |
| Steen Andersen. Effect of CSII and CGM on Progression of Late Diabetic Complications. NCT01454700; 2011. URL: https://clinicaltrials.gov/ct2/show/NCT01454700 (accessed 12 November 2015) | Duplicate |
| In Home Closed Loop Study Group. Outpatient Pump Shutoff Pilot Feasibility and Efficacy Study. NCT01591681; 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01591681 (accessed 12 November 2015) | Study design |
| DexCom Inc. Effectiveness and Safety of the Dexcom™ G4 Continuous Glucose Monitoring System in Pediatric Subjects With Diabetes Mellitus. NCT01667185; 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01667185 (accessed 12 November 2015) | Study design |
| In Home Closed Loop Study Group. An Outpatient Pump Shutoff Pilot Feasibility and Safety Study. NCT01736930; 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01736930 (accessed 12 November 2015) | Study design |
| In Home Closed Loop Study Group. Outpatient Reduction of Nocturnal Hypoglycemia by Using Predictive Algorithms and Pump Suspension in Children. NCT01823341; 2013. URL: https://clinicaltrials.gov/ct2/show/NCT01823341 (accessed 12 November 2015) | Study design |
| Medtronic Diabetes. Post Approval Study of the Threshold Suspend Feature With the Medtronic MiniMed® 530G Insulin Pump. NCT02003898; 2013. URL: https://clinicaltrials.gov/ct2/show/NCT02003898 (accessed 12 November 2015) | Study design |
| Vastra Gotaland Region. CGM Treatment in Patients With Type 1 Diabetes Treated With Insulin Injections. NCT02092051; 2014. URL: https://clinicaltrials.gov/ct2/show/NCT02092051 (accessed 12 November 2015) | Duplicate |
| Medtronic Diabetes. Threshold Suspend in Pediatrics at Home. NCT02120794; 2014. URL: https://clinicaltrials.gov/ct2/show/NCT02120794 (accessed 12 November 2015) | Duplicate |
| University Hospital, Montpellier. Hybrid Artificial Pancreas in Home Setting. NCT02153190; 2014. URL: https://clinicaltrials.gov/ct2/show/NCT02153190 (accessed 12 November 2015) | Intervention |
| University of Ljubljana, Faculty of Medicine. Prevention of Hypoglycaemia With Predictive Insulin Suspend Using Sensor Augmented Insulin Pump in Children. NCT02179281; 2014. URL: https://clinicaltrials.gov/ct2/show/NCT02179281 (accessed 12 November 2015) | Study design |
| Neeser K, Kocher S, Weber C, Heister F. CSII compared to MDI: a health economic analysis in the German health care setting. Value Health 2009;12:A407. Conference: ISPOR 12th Annual European Congress. Paris, France, 24–27 October 2009 | Outcomes |
| Neff K, McCarthy A, Forde R, Foley M, Coulter-Smith S, Daly S, et al. Intensive glycaemic control in type 1 diabetic pregnancy: a comparison of continuous subcutaneous insulin infusion and multiple daily injection therapy. Diabetologia 2010;53(Suppl. 1):S433. Paper presented at 46th Annual Meeting of the European Association for the Study of Diabetes (EASD). Stockholm, Sweden, 20–24 September 2010 | Study design |
| Nemours Children’s Clinic. Insulin Pump Therapy in Adolescents With Newly Diagnosed Type 1 Diabetes (T1D). NCT00357890; 2012. URL: https://clinicaltrials.gov/ct2/show/NCT00357890 (accessed 12 November 2015) | Population |
| New J, Ajjan R, Pfeiffer AFH, Freckmann G. Impact of alarm functions with real time continuous glucose monitoring (CGM). Diabetes Technol Ther 2013;15:A8–9. Conference: 6th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Paris, France, 27 February–2 March 2013 | Outcomes |
| Newman SP, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al. A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE). Health Technol Assess 2009;13(28) | Intervention |
| Neylon OM, O’Connell MA, Donath S, Cameron FJ. Can integrated technology improve self-care behavior in youth with type 1 diabetes? A randomized crossover trial of automated pump function. Pediatr Diabetes 2013;14:46. Conference: 39th Annual Conference of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Gothenburg, Sweden, 16–19 October 2013 | Outcomes |
| Ng Tang Fui S, Pickup JC, Bending JJ, Collins AC, Keen H, Dalton N. Hypoglycemia and counterregulation in insulin-dependent diabetic patients: a comparison of continuous subcutaneous insulin infusion and conventional insulin injection therapy. Diabetes Care 1986;9:221–7 | Study design |
| Nimri R, Miller S, Muller I, Atlas E, Fogel A, Bratina N, et al. The home use of MD-logic closed-loop system during the nights significantly improves daytime glycemic control in subjects with type 1 diabetes. Diabetes 2014;63:A243. Conference: 74th Scientific Sessions of the American Diabetes Association. San Francisco, CA, USA, 13–17 June 2014 | Intervention |
| Nimri R, Muller I, Atlas E, Miller S, Fogel A, Bratina N, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care 2014;37:3025–32 | Intervention |
| Nixon R, Pickup JC. Fear of hypoglycemia in type 1 diabetes managed by continuous subcutaneous insulin infusion: is it associated with poor glycemic control? Diabetes Technol Ther 2011;13:93–8 | Study design |
| Norgaard K, Sohlberg A, Goodall G. [Cost-effectiveness of continuous subcutaneous insulin infusion therapy for type 1 diabetes.] Ugeskr Laeger 2010;172:2020–5 | Not found |
| Hermanides J. Randomized, Controlled, Multinational, Multi-center, Clinical Trial to Examine Whether HbA1c Can Improve in Type 1 Diabetes Patients who Continuously Use the Paradigm® REAL-Time System with Alarm Function as Compared to Patients on Multiple Injection Therapy Receiving One Six-Day Period of Continuous Glucose Monitoring – Without Alarm Function (Guardian® REAL-Time Clinical). NTR863; 2007. URL: www.trialregister.nl/trialreg/admin/rctview.asp?TC=863 (accessed 11 January 2016) | Intervention |
| Nuboer R, Borsboom GJJM, Zoethout JA, Koot HM, Bruining J. Effects of insulin pump vs. injection treatment on quality of life and impact of disease in children with type 1 diabetes mellitus in a randomized, prospective comparison. Pediatr Diabetes 2008;9:291–6 | Intervention |
| O’Connell R, Oroszlan G, Hamer G, Yusi J, Kaufman F, Welsh J, et al. Efficacy of low glucose suspend and low predictive alert: data analysis using the Medtronic carelink therapy management software database. Diabetes Technol Ther 2011;13:244. Conference: 4th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). London, UK, 16–19 February 2011 | Study design |
| O’Grady MJ, Retterath AJ, Keenan DB, Kurtz N, Cantwell M, Spital G, et al. The use of an automated, portable glucose control system for overnight glucose control in adolescents and young adults with type 1 diabetes. Diabetes Care 2012;35:2182–7 | Study design |
| Olivier P, Lawson ML, Huot C, Richardson C, Nakhla M, Romain J. Lessons learned from a pilot RCT of simultaneous versus delayed initiation of continuous glucose monitoring in children and adolescents with type 1 diabetes starting insulin pump therapy. J Diabetes Sci Technol 2014;8:523–8 | Outcomes |
| Opipari-Arrigan L, Fredericks EM, Burkhart N, Dale L, Hodge M, Foster C. Continuous subcutaneous insulin infusion benefits quality of life in preschool-age children with type 1 diabetes mellitus. Pediatr Diabetes 2007;8:377–83 | Intervention |
| Pankowska E, Blazik M, Dziechciarz P, Szypowska A, Szajewska H. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta-analysis of randomized control trials. Pediatr Diabetes 2009;10:52–8 | Systematic review/meta-analysis |
| Patrakeeva EM, Zalevskaya AG, Shlyakhto EV. Fear of hypoglycemia in relatives of young type 1 diabetes mellitus (T1DM) patients on MDI and CSII therapy. Paper presented at 74th Scientific Sessions of the American Diabetes Association. San Francisco, CA, USA, 13–17 June 2014 | Study design |
| Perkins BA, Halpern EM, Orszag A, Weisman A, Houlden RL, Bergenstal RM, et al. Sensor-augmented pump and multiple daily injection therapy in the USA and Canada: post-hoc analysis of a randomized controlled trial. Can J Diabetes 2015;39:50–4 | Outcomes |
| Petkova E, Petkova V, Konstantinova M, Petrova G. Economic evaluation of continuous subcutaneous insulin infusion for children with diabetes – a pilot study: CSII application for children – economic evaluation. BMC Pediatr 2013;13:155 | Outcomes |
| Petkova E, Petkova V, Konstantinova M, Petrova G. Economic evaluation of continuous subcutaneous insulin infusion for children with diabetes – part II. Modern Economy 2013;4:9–13 | Outcomes |
| Petkova V, Petrova G, Petkova E. Comparative analysis of the cost and metabolic control in diabetic children using insulin pumps. Value Health 2013;16:A437. Conference: ISPOR 16th Annual European Congress. Dublin, Ireland, 2–6 November 2013 | Outcomes |
| Petrovski G, Jovanovska B, Bitovska I, Ahmeti I. Constant or intermittent glucose monitoring: evaluation on pregnancy and glycemic outcome in type 1 diabetics on insulin pump. Diabetes 2013;62:A684. Conference: 73rd Scientific Sessions of the American Diabetes Association. Chicago, IL, USA, 21–25 June 2013 | Intervention |
| Phillip M, Battelino T, Atlas E, Kordonouri O, Bratina N, Miller S, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368:824–33 | Study design |
| Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ 2011;343:d3805 | Systematic review/meta-analysis |
| Pickup JC. The evidence base for diabetes technology: appropriate and inappropriate meta-analysis. J Diabetes Sci Technol 2013;7:1567–74 | Background |
| Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr 2013;5:39 | Systematic review/meta-analysis |
| Pozzilli P, Crino A, Schiaffini R, Manfrini S, Fioriti E, Coppolino G, et al. A 2-year pilot trial of continuous subcutaneous insulin infusion versus intensive insulin therapy in patients with newly diagnosed type 1 diabetes (IMDIAB 8). Diabetes Technol Ther 2003;5:965–74 | Intervention |
| Price D, Nakamura K, Christiansen M, Bailey T, Watkins E, Liljenquist D, et al. Accuracy and reliability of a next generation continuous glucose monitoring system: the Dexcom G4 platinum pivotal trial results. Diabetes Technol Ther 2013;15:A70–1. Conference: 6th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Paris, France, 27 February–2 March 2013 | Study design |
| Price DA, Peyser T, Simpson P, Nakamura K, Mahalingam A. Impact of study design and analytic techniques on the reported accuracy of Continuous Glucose Monitoring (CGM) systems. Diabetes 2012;61:A1. Conference: 72nd Scientific Sessions of the American Diabetes Association. Philadelphia, PA, USA, 8–12 June 2012 | Background |
| Price DA, Peyser TA, Graham C. Challenges with systematic reviews and meta-analyses of real-time continuous glucose monitoring (CGM). Diabetes 2013;62:A644. Conference: 73rd Scientific Sessions of the American Diabetes Association. Chicago, IL, USA, 21–25 June 2013 | Background |
| Quiroz M, Machado F, Shafiroff J, Gill M, Molina M, Gonzalez P. Insulin pump cost–utility analysis compared to multiple daily injection in type 1 diabetic patients in the Mexican social security institute, 21st century hospital. Value Health 2012;15:A69. Conference: 17th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Washington, DC, USA, 2–6 June 2012 | Outcomes |
| Rabin Medical Center. Treatment Satisfaction of Using OmniPod System Compared With Conventional Insulin Pump in Adults With Type 1 Diabetes. 2012. URL: http://ClinicalTrials.gov/show/NCT00935129 (accessed 12 November 2015) | Intervention |
| Radermecker RP, Saint Remy A, Scheen AJ, Bringer J, Renard E. Continuous glucose monitoring reduces both hypoglycaemia and HbA1c in hypoglycaemia-prone type 1 diabetic patients treated with a portable pump. Diabetes Metab 2010;36:409–13 | Outcomes |
| Ranasinghe P, Maruthur N, Yeh HC, Brown T, Suh Y, Wilson L, et al. Comparative effectiveness of continuous subcutaneous insulin infusion with multiple daily injections among pregnant women with diabetes mellitus: a systematic review. J Hos Med 2012;7:S52. Conference: 2012 Annual Meeting of the Society of Hospital Medicine (SHM). San Diego, CA, USA, 1–4 April 2012 | Systematic review/meta-analysis |
| Reid SM, Lawson ML. Comparison of continuous subcutaneous insulin infusion versus conventional treatment of type 1 diabetes with respect to metabolic control, quality of life and treatment satisfaction. Pediatr Res 2002;51(Suppl. 4):122A–3A. Paper presented at Pediatric Academic Societies’ annual meeting. Baltimore, MD, USA, 4–7 May 2002 | Intervention |
| Riveline J-P, Schaepelynck P, Chaillous L, Renard E, Sola-Gazagnes A, Penfornis A, et al. Assessment of patient-led or physician-driven continuous glucose monitoring in patients with poorly controlled type 1 diabetes using basal-bolus insulin regimens: a 1-year multicenter study. Diabetes Care 2012;35:965–71 | Intervention |
| Robinson-Vincent KA. Systematic review of the effects of continuous glucose monitoring on metabolic control in children and adolescents with type 1 diabetes. Can J Diabetes 2013;37:S21. Conference: 16th Annual Canadian Diabetes Association/Canadian Society of Endocrinology and Metabolism Professional Conference and Annual Meetings. Montreal, QC, Canada, 17–19 October 2013 | Systematic review/meta-analysis |
| Roy A, Kaufman FR, Spital G, Clark B, Grosman B, Parikh N, et al. An in-silico study of predictive low glucose management algorithm for minimizing hypoglycemia. Diabetes Technol Ther 2013;15:A81–2. Conference: 6th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD). Paris, France, 27 February–2 March 2013 | Study design |
| Roze S, Demessinov A, Zeityn M, Toktarova N, Abduakhassova G, Sissemaliev R, et al. Health-economic comparison of continuous subcutaneous insulin infusion versus multiple daily injections for the treatment of type 1 diabetes in Kazakhstan children. Value Health 2013;16:A439–40. Conference: ISPOR 16th Annual European Congress. Dublin, Ireland, 2–6 November 2013 | Outcomes |
| Roze S, Lynch P, Cook M. Projection of long term health-economic benefits of Continuous Glucose Monitoring (CGM) versus self monitoring of blood glucose in type 1 diabetes, a UK perspective. Diabetologia 2012;55:S427. Conference: 48th Annual Meeting of the European Association for the Study of Diabetes (EASD). Berlin, Germany, 1–5 October 2012 | Outcomes |
| Roze S, Valentine WJ, Zakrzewska KE, Palmer AJ. Health-economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of type 1 diabetes in the UK. Diabet Med 2005;22:1239–45 | Outcomes |
| Rubin RR, Peyrot M. Patient-reported outcomes in the sensor-augmented pump therapy (SAPT) for A1c reduction (STAR) 3 trial. Diabetes 2011;60:A82. Conference: 71st Scientific Sessions of the American Diabetes Association. San Diego, CA, USA, 24–28 June 2011 | Outcomes |
| Rys PM, Mucha A, Koprowski M, Nowicki M, Malecki MT. Efficacy and safety of continuous glucose monitoring systems vs. self-monitoring blood glucose in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes 2011;60:A244. Conference: 71st Scientific Sessions of the American Diabetes Association. San Diego, CA, USA, 24–28 June 2011 | Not found |
| Sadri H, Bereza BG, Longo CJ. Cost-consequence analysis of CSII vs. MDI: a Canadian perspective. Value Health 2010;13:A290. Conference: ISPOR 13th Annual European Congress. Prague, Czech Republic, 6–9 November 2011 | Outcomes |
| Sahin SB, Cetinkalp S, Ozgen AG, Saygili F, Yilmaz C. The importance of anti-insulin antibody in patients with type 1 diabetes mellitus treated with continuous subcutaneous insulin infusion or multiple daily insulin injections therapy. Acta Diabetol 2010;47:325–30 | Study design |
| Saigí I, Chico A, Santos L, Aulinas A, Adelantado J, Ginovart G, et al. Glycaemic control and perinatal outcomes of pregnancies complicated by type 1 diabetes: multiple daily injections vs. continuous subcutaneous insulin infusion. Paper presented at 45th EASD Annual Meeting of the European Association for the Study of Diabetes. Vienna, Austria, 30 September–2 October 2009 | Outcomes |
| Saigi I, Chico A, Santos L, Aulinas A, Adelantado J, Ginovart G, et al. Glycaemic control and perinatal outcomes of pregnancies complicated by type 1 diabetes: multiple daily injections vs. continuous subcutaneous insulin infusion. Diabetologia 2009;52(Suppl. 1):S46. Paper presented at 45th Annual Meeting of the European Association for the Study of Diabetes (EASD). Vienna, Austria, 29 September–2 October 2009 | Study design |
| Saraiva J, Paiva S, Ruas L, Barros L, Baptista C, Melo M, et al. Type 1 diabetes and pregnancy: continuous subcutaneous insulin infusion systems versus multiple daily injection therapy. Paper presented at 49th Annual Meeting of the European Association for the Study of Diabetes (EASD). Barcelona, Spain, 23–27 September 2013 | Study design |
| Saurbrey N, Arnold-Larsen S, Moller-Jensen B, Kuhl C. Comparison of continuous subcutaneous insulin infusion with multiple insulin injections using the NovoPen. Diabet Med 1988;5:150–3 | Not found |
| Scaramuzza A, De Angelis L, Bosetti A, Gazzarri A, Platerote F, Redaelli F, et al. Evaluation of three bolus calculators in children with type 1 diabetes using insulin pump therapy. Pediatr Diabetes 2011;12:128. Conference: 37th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Miami Beach, FL, USA, 19–22 October 2011 | Study design |
| Scaramuzza AE, De Angelis L, Gazzarri A, Bosetti A, Platerote F, Redaelli F, et al. Evaluation of 3 bolus calculators in children and adolescents with type 1 diabetes using insulin pump therapy. Diabetologia 2011;54:S352. Conference: 47th Annual Meeting of the European Association for the Study of Diabetes (EASD). Lisbon, Portugal, 12–16 September 2011 | Study design |
| Schaepelynck P, Rocher L, Hanaire H, Chaillous L, Renard E, Sola A, et al. Patient- or physician-driven continuous glucose monitoring (CGM) improves control and quality of life (QoL) in poorly-controlled type 1 diabetic patients on intensified insulin therapy: a one-year multicenter study. Diabetes 2011;60:A65. Conference: 71st Scientific Sessions of the American Diabetes Association San Diego, CA, USA, 24–28 June 2011 | Outcomes |
| Schaepelynck-Belicar P, Vague P, Simonin G, Lassmann-Vague V. Improved metabolic control in diabetic adolescents using the continuous glucose monitoring system (CGMS). Diabetes Metab 2003;29:608–12 | Study design |
| Schiaffini R, Patera PI, Bizzarri C, Ciampalini P, Cappa M. Basal insulin supplementation in type 1 diabetic children: a long-term comparative observational study between continuous subcutaneous insulin infusion and glargine insulin. J Endocrinol Invest 2007;30:572–7 | Intervention |
| Schiel R, Burgard D, Bambauer R, Perenthaler T, Kramer G. [Differences between intensified insulin therapy using multiple insulin injections (ICT) or continuous subcutaneous insulin infusion using pumps (CSII) in children and adolescents with type 1 diabetes mellitus.] Diabetol Stoffwechs 2013;8:380–6 | Not found |
| Schiffrin A, Belmonte MM. Comparison between continuous subcutaneous insulin infusion and multiple injections of insulin. A one-year prospective study. Diabetes 1982;31:255–64 | Study design |
| Schiffrin A, Desrosiers M, Moffatt M, Belmonte MM. Feasibility of strict diabetes control in insulin-dependent diabetic adolescents. J Pediatr 1983;103:522–7 | Outcomes |
| Schiffrin AD, Desrosiers M, Aleyassine H, Belmonte MM. Intensified insulin therapy in the type 1 diabetic adolescent: a controlled trial. Diabetes Care 1984;7:107–13 | Outcomes |
| Schmidt S, Norgaard K. Long-term effects of sensor-augmented pump therapy in type 1 diabetes: a 3-year follow-up study. Diabetes 2012;61:A3. Conference: 72nd Scientific Sessions of the American Diabetes Association. Philadelphia, PA, USA, 8–12 June 2012 | Study design |
| Schmidt S, Norgaard K. Sensor-augmented pump therapy at 36 months. Diabetes Technol Ther 2012;14:1174–7 | Study design |
| Schmitz A, Christiansen JS, Christensen CK, Hermansen K, Mogensen CE. Effect of pump versus pen treatment on glycaemic control and kidney function in long-term uncomplicated insulin-dependent diabetes mellitus (IDDM). Dan Med Bull 1989;36:176–8 | Outcomes |
| Schottenfeld-Naor Y, Galatzer A, Karp M. Comparison of metabolic and psychological parameters during continuous subcutaneous insulin infusion and intensified conventional insulin treatment in type I diabetic patients. Isr J Med Sci 1985;21:822–8 | Not found |
| Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care 2013;36:1877–83 | Intervention |
| Selam JL, Haardt MJ, Slama G, Bethoux JP. A randomized cross-over cost–benefits comparison of intensive insulin therapy with intraperitoneal infusion via implantable pumps vs multiple subcutaneous injections in patients with type-I diabetes. Diabetes 1994;43(Suppl. 1):A167. Paper presented at 54th Annual Meeting of the American Diabetes Association. New Orleans, LA, USA, 11–14 June 1994 | Outcomes |
| Selam JL, Raccah D, Jean-Didier N, Lozano JL, Waxman K, Charles MA. Randomized comparison of metabolic control achieved by intraperitoneal insulin infusion with implantable pumps versus intensive subcutaneous insulin therapy in type I diabetic patients. Diabetes Care 1992;15:53–8 | Intervention |
| Self-monitoring of blood glucose. Int J Clin Pract 2012;66(Suppl. 175):2–93 | Study design |
| Sequeira PA, Montoya L, Ruelas V, Xing D, Chen V, Beck R, et al. Continuous glucose monitoring pilot in low-income type 1 diabetes patients. Diabetes Technol Ther 2013;15:855–8 | Outcomes |
| Shehadeh N, Battelino T, Galatzer A, Naveh T, Hadash A, de Vries L, et al. Insulin pump therapy for 1–6 year old children with type 1 diabetes. Isr Med Assoc J 2004;6:284–6 | Study design |
| Sherr J, Carria LR, Weyman K, Zgorski M, Steffen AT, Tichy EM, et al. Effect of 2-hr suspensions of basal insulin on elevating nighttime sensor glucose concentrations. Diabetes 2013;62:A249. Conference: 73rd Scientific Sessions of the American Diabetes Association. Chicago, IL, USA, 21–25 June 2013 | Study design |
| Sherr J, Collazo PM, Caria L, Steffen A, Weyman K, Zgorski M, et al. Safety of nighttime 2-hour suspension of basal insulin in pump-treated type 1 diabetes (T1D) even in absence of low glucose. Diabetes Technol Ther 2013;15:A22. Conference: 6th International Conference on Advanced Technologies and Treatments for Diabetes. Paris, France, 27 February–2 March 2013. | Study design |
| Sherr JL, Collazo MMP, Carria LR, Steffen AT, Zgorski M, Weyman K, et al. Safety of nighttime 2-hour suspensions of basal insulin in pump-treated type 1 diabetes (T1D) even in absence of low glucose. Diabetes 2012;61:A226–7. Conference: 72nd Scientific Sessions of the American Diabetes Association. Philadelphia, PA, USA, 8–12 June 2012 | Study design |
| Sherr JL, Collazo PM, Cengiz E, Michaud C, Carria L, Steffen AT, et al. Safety of nighttime 2-hour suspension of basal insulin in pump-treated type 1 diabetes even in the absence of low glucose. Diabetes Care 2014;37:773–9 | Study design |
| Skogsberg L, Fors H, Hanas R, Chaplin JE, Lindman E, Skogsberg J. Improved treatment satisfaction but no difference in metabolic control when using continuous subcutaneous insulin infusion vs. multiple daily injections in children at onset of type 1 diabetes mellitus. Pediatr Diabetes 2008;9:472–9 | Intervention |
| Skogsberg L, Skogsberg J, Fors H. Improved treatment satisfaction using continuous subcutaneous insulin infusion compared to multiple daily injections in children at onset of type 1 diabetes mellitus – a five-year follow-up study. Pediatr Diabetes 2010;11:S14. Conference: 36th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes. Buenos Aires, Argentina, 27–30 October 2010 | Outcomes |
| Slover R, Daniels MW, Foster CM, Wood MA, Kaufman FR, Welsh JB, et al. Insulin pump adjustments and glycemic outcomes in the pediatric cohort of the STAR 3 study. Diabetes 2011;60:A254. Conference: 71st Scientific Sessions of the American Diabetes Association. San Diego, CA, USA, 24–28 June 2011 | Outcomes |
| Slover RH, Buckingham BA, Garg S, Brazg RL, Bailey TS, Klonoff DC, et al. Efficacy of automatic insulin pump suspension in youth with type 1 diabetes. Pediatr Diabetes 2012;13:40–1. Conference: 38th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes. Istanbul, Turkey, 10–13 October 2012 | Study design |
| Slover RH, Tamborlane WV, Battelino T, Criego A, Daniels M, Foster C, et al. Glucose excursions in children and adolescents in the STAR 3 study: a 1-year randomized controlled trial comparing sensor-augmented pump therapy to multiple daily injections. Pediatr Diabetes 2010;11(Suppl.14):33. Conference: 36th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes. Buenos Aires, Argentina, 27–30 October 2010 | Outcomes |
| St Charles M, Lynch P, Graham C, Minshall ME. A cost-effectiveness analysis of continuous subcutaneous insulin injection versus multiple daily injections in type 1 diabetes patients: a third-party US payer perspective. Value Health 2009;12:674–86 | Outcomes |
| St Charles ME, Sadri H, Minshall ME, Tunis SL. Health economic comparison between continuous subcutaneous insulin infusion and multiple daily injections of insulin for the treatment of adult type 1 diabetes in Canada. Clin Ther 2009;31:657–67 | Outcomes |
| Szypowska A, Dżygało K, Ramotowska A, Lipka M, Procner-Czaplińska M, Trippenbach-Dulska H. The benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes mellitus started at diabetes recognition. A 7 year follow-up. Paper presented at 46th Annual Meeting of the European Association for the Study of Diabetes, 20–24 September 2010, Stockholm, Sweden | Study design |
| Szypowska A, Ramotowska A, Dzygalo K, Golicki D. Beneficial effect of real-time continuous glucose monitoring system on glycemic control in type 1 diabetic patients: systematic review and meta-analysis of randomized trials. Eur J Endocrinol 2012;166:567–74 | Systematic review/meta-analysis |
| Tamborlane W, Buse J, Slover R, Green J, Kaufman F, Shin J. Comparison of insulin pump settings and insulin usage patterns in adult and pediatric subjects in the star 3 study. Diabetes Technol Ther 2011;13:173–293. Conference: 4th International Conference on Advanced Technologies and Treatments for Diabetes. London, UK, 16–19 February 2011 | Outcomes |
| Tamborlane WV, Batas SE, Rudolf MC. Comparison of continuous subcutaneous insulin infusion versus multiple daily injections in adolescents with insulin-dependent diabetes. Adv Diabetol 1989;2(Suppl. 1):24–7 | Not found |
| Tamborlane WV, Ruedy KJ, Wysocki T, O’Grady M, Kollman C, Block J, et al. JDRF randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther 2008;10:310–21 | Intervention |
| Tanenberg R, Bode B, Lane W, Levetan C, Mestman J, Harmel AP, et al. Use of the continuous glucose monitoring system to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc 2004;79:1521–6 | Intervention |
| Tanenberg RJ, Houlden RL, Tildesley HD, Kaufman FR, Welsh JB, Shin J. Insulin pump adjustments and glycemic outcomes in the adult cohort of the STAR 3 study. Diabetes 2011;60:A253–4. Conference: 71st Scientific Sessions of the American Diabetes Association. San Diego, CA, USA, 24–28 June 2011 | Outcomes |
| Tanenberg RJ, Welsh JB. Patient behaviors associated with optimum glycemic outcomes with sensor-augmented pump therapy: insights from the STAR 3 study. Endocr Pract 2015;21:41–5 | Outcomes |
| Thabit H, Lubina-Solomon A, Stadler M, Leelarathna L, Walkinshaw E, Pernet A, et al. Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol 2014;2:701–9 | Study design |
| Thabit H, Lubina-Solomon A, Stadler M, Leelarathna LT, Walkinshaw E, Pernet A, et al. Four weeks’ home use of overnight closed-loop insulin delivery in adults with type 1 diabetes: a multicentre, randomised, crossover study. Diabetes 2014;63:A61. Conference: 74th Scientific Sessions of the American Diabetes Association. San Francisco, CA, USA, 13–17 June 2014 | Intervention |
| Thomas LE, Kane MP, Bakst G, Busch RS, Hamilton RA, Abelseth JM. A glucose meter accuracy and precision comparison: the freestyle flash versus the Accu-Chek Advantage, Accu-Chek Compact Plus, Ascensia Contour, and the BD Logic. Diabetes Technol Ther 2008;10:102–10 | Intervention |
| Trossarelli GF, Cavallo-Perin P, Meriggi E, Menato G, Dolfin G, Carta Q, et al. Metabolic and obstetrical results in type 1 (insulin-dependent) diabetic pregnancy: pump versus optimized conventional insulin therapy. Diabetologia 1984;27:340A | Not found |
| Tsioli C, Remus K, Blaesig S, Datz N, Schnell K, Marquardt E, et al. The predictive low glucose management system in youth with type 1 diabetes during exercise-data from the Pilgrim study. Pediatr Diabetes 2013;14:48. Conference: 39th Annual Conference of the International Society for Pediatric and Adolescent Diabetes. Gothenburg, Sweden, 16–19 October 2013 | Study design |
| Tumminia A, Crimi S, Sciacca L, Buscema M, Frittitta L, Squatrito S, et al. Efficacy of REAL-Time continuous glucose monitoring on glycaemic control and glucose variability in type 1 diabetic patients treated with either insulin pumps or multiple insulin injection therapy: a randomised controlled cross-over trial. Diabetes Metab Res Rev 2015;31:61–8 | Outcomes |
| Uhrinak AN, Myers SJ, Kaufman FR, Lee SW, Yusi J, Huang S, et al. Retrospective analysis of events preceding low glucose suspend activation in adult subjects on the paradigm veo system. J Diabetes Sci Technol 2012;6;A182. Conference: 11th Annual Diabetes Technology Meeting. San Francisco, CA, USA 27–29 October 2011 | Study design |
| Ulf S, Ragnar H, Arne WP, Johnny L. Do high blood glucose peaks contribute to higher HbA1c? Results from repeated continuous glucose measurements in children. World J Pediatr 2008;4:215–21 | Intervention |
| University of Ljubljana, Faculty of Medicine. Prevention of Hypoglycaemia With Predictive Insulin Suspend Using Sensor Augmented Insulin Pump in Children. NCT02179281; 2014. URL: https://clinicaltrials.gov/ct2/show/NCT02179281 (accessed 12 November 2015) | Study design |
| US Food and Drug Administration. Dexcom G4 PLATINUM (Pediatric) Continuous Glucose Monitoring System – P120005/S002. US Food and Drug Administration; 2014. URL: www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm386985.htm (accessed 5 September 2014) | Study design |
| US Food and Drug Administration. Dexcom G4 PLATINUM (Pediatric) Continuous Glucose Monitoring System. FDA Summary of Safety and Effectiveness Data. US Food and Drug Administration; 2014. URL: www.accessdata.fda.gov/cdrh_docs/pdf12/P120005S002b.pdf (accessed 5 September 2014) | Study design |
| US Food and Drug Administration. MiniMed 530G System – P120010. US Food and Drug Administration; 2014. URL: www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm372176.htm (accessed 5 September 2014) | Study design |
| Volpe L, Pancani F, Aragona M, Lencioni C, Battini L, Ghio A, et al. Continuous subcutaneous insulin infusion and multiple dose insulin injections in type 1 diabetic pregnant women: a case–control study. Gynecol Endocrinol 2010;26:193–6 | Study design |
| von Hagen C, Bechtold S, Temme K, Tremml S, Wex S, Schwarz HP. [Metabolic control and quality of life in adolescents with type 1 diabetes: insulin pump therapy versus multiple daily injections.] Diabetol Stoffwechs 2007;2:238–47 | Not found |
| Voormolen DN, DeVries JH, Evers IM, Mol BWJ, Franx A. The efficacy and effectiveness of continuous glucose monitoring during pregnancy: a systematic review. Obstet Gynecol Surv 2013;68:753–63 | Systematic review/meta-analysis |
| Weinstock RS, Bergenstal RM, Garg S, Bailey TS, Thrasher J, Mao M, et al. Reduction in hypoglycemia across a range of definitions in the aspire in-home study. Diabetes 2014;51(Suppl. 2):A240. Conference: 74th Scientific Sessions of the American Diabetes Association. San Francisco, CA, USA, 13–17 June 2014 | Outcomes |
| Weintrob N, Benzaquen H, Galatzer A, Shalitin S, Lazar L, Fayman G, et al. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Paper presented at 62nd Annual Meeting of the American Diabetes Association, 14–18 June 2002, San Francisco, USA. Diabetes 2002;51(Suppl. 2):A479 | Outcomes |
| Weintrob N, Schechter A, Benzaquen H, Shalitin S, Lilos P, Galatzer A, et al. Glycemic patterns detected by continuous subcutaneous glucose sensing in children and adolescents with type 1 diabetes mellitus treated by multiple daily injections vs continuous subcutaneous insulin infusion. Arch Pediatr Adolesc Med 2004;158:677–84 | Study design |
| Weintrob N, Schechter A, Bezaquen H, Shalitin S, Lilos P, Galatzer A, et al. Glycemic patterns detected by continuous subcutaneous glucose sensing in children with type 1 diabetes treated by MDI or CSII. Diabetes 2003;52(Suppl. 1):A100 | Outcomes |
| Weinzimer SA, Ahern JH, Doyle EA, Vincent MR, Dziura J, Steffen AT, et al. Persistence of benefits of continuous subcutaneous insulin infusion in very young children with Type 1 diabetes: a follow-up report. Pediatrics 2004;114:1601–5 | Study design |
| Weiss R, Bailey TS, Schwartz FL, Garg S, Ahmann AJ, Thrasher J, et al. Time spent (%) in hypoglycemia following automatic threshold suspend activation in the aspire in-home study. Diabetes 2014;63:A241. Conference: 74th Scientific Sessions of the American Diabetes Association. San Francisco, CA, USA, 13–17 June 2014 | Outcomes |
| Weiss R, Schwartz FL, Weinstock RS, Bode BW, Bailey TS, Ahmann AJ, et al. Bolus insulin dosing and nocturnal hypoglycemia in the aspire in-home study. Diabetes 2014;63:A601. Conference: 74th Scientific Sessions of the American Diabetes Association. San Francisco, CA, USA, 13–17 June 2014 | Outcomes |
| Wender-Ozegowska E, Zawiejska A, Ozegowska K, Wroblewska-Seniuk K, Iciek R, Mantaj U, et al. Multiple daily injections of insulin versus continuous subcutaneous insulin infusion for pregnant women with type 1 diabetes. Aust N Z J Obstet Gynaecol 2013;53:130–5 | Study design |
| Wilson DC, Halliday HL, Reid M, McClure G, Dodge JA. Continuous insulin infusion in hyperglycaemic extremely low birthweight infants? A randomized trial. Proceedings of 14th European Congress of Perinatal Medicine, 14th European Congress. Helsinki, Finland, 5–8 June 1994 | Not found |
| Wilson DM, Buckingham BA, Kunselman EL, Sullivan MM, Paguntalan HU, Gitelman SE. A two-center randomized controlled feasibility trial of insulin pump therapy in young children with diabetes. Diabetes Care 2005;28:15–19 | Intervention |
| Wiseman MJ, Saunders AJ, Keen H, Viberti G. Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin-dependent diabetes. N Engl J Med 1985;312:617–21 | Intervention |
| Wojciechowski P, Rys P, Lipowska A, Gaweska M, Malecki MT. Efficacy and safety comparison of continuous glucose monitoring and self-monitoring of blood glucose in type 1 diabetes: systematic review and meta-analysis. Pol Arch Med Wewn 2011;121:333–43 | Systematic review/meta-analysis |
| Yates K, Hasnat Milton A, Dear K, Ambler G. Continuous glucose monitoring-guided insulin adjustment in children and adolescents on near-physiological insulin regimens: a randomized controlled trial. Diabetes Care 2006;29:1512–17 | Outcomes |
| Yeh HC, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012;157:336–47 | Systematic review/meta-analysis |
| Yogev Y, Chen R, Ben-Haroush A, Phillip M, Jovanovic L, Hod M. Continuous glucose monitoring for the evaluation of gravid women with type 1 diabetes mellitus. Obstet Gynecol 2003;101:633–8 | Study design |
| Ziegler D, Dannehl K, Koschinsky T, Toeller M, Gries FA. Comparison of continuous subcutaneous insulin infusion and intensified conventional therapy in the treatment of type I diabetes: a two-year randomized study. Diabetes Nutr Metab Clin Exp 1990;3:203–13 | Intervention |
| Zisser HC, Dassau E, Bevier W, Harvey RA, Jovanovic L, Doyle FJ III. Clinical evaluation of a fully-automated artificial pancreas using zone-model predictive control with health monitoring system. Paper presented at 72nd Scientific Sessions of the American Diabetes Association. Philadelphia, PA, USA, 8–12 June 2012 | Study design |
| Zucchini S, Scipione M, Maltoni G, Rollo A, Balsamo C, Zanotti M, et al. Comparison between sensor-augmented insulin therapy with either insulin pump (CSII) or multiple daily injections (MDI) in everyday life: analysis of glucose variability and sensor reliability. Horm Res Paediatr 2011;76:157–8. Conference: 50th Annual Meeting of the European Society for Paediatric Endocrinology. Glasgow, UK, 25–28 September 2011 | Study design |
Appendix 3 Data extraction tables
| Follow-up, months | Study | Countries | Inclusion | Intervention | Number analysed for efficacy per arm |
|---|---|---|---|---|---|
| 3 | Bergenstal et al., 201332 | USA | Age: 16–70 years; HbA1c: 5.8–10%; CSII experience: 6 months prior CSII treatment; number of hypoglycaemic events: > 1; episode of severe hypoglycaemia in the previous 6 months: excluded; and ≥ 2 nocturnal hypoglycaemic events in the run-in period required | CSII + CGM + suspend: Paradigm Veo pump + Enlite sensor (Medtronic) | 121 |
| CSII + CGM integrated: Paradigm Revel 2.0 pump + Enlite sensor | 126 | ||||
| 3.45 | Lee et al., 200738 | USA | Age: adults; HbA1c: ≥ 7.5%; CSII experience: CSII naive; number of hypoglycaemic events: NR | Integrated CSII + CGM: MiniMed Paradigm REAL-Time 722 system as adjunct to SMBG (Paradigm Link™ glucose meter)a | 8 |
| MDI + SMBG: SMBG (Paradigm Link glucose meter) | 8 | ||||
| 3.69 | Peyrot and Rubin, 200939 | USA | Age: adults; HbA1c: NR; CSII experience: CSII naive; number of hypoglycaemic events: NR | Integrated CSII + CGM: Paradigm 722 System (smart CSII pump with real-time CGM and CareLink™ data management software) as adjunct to SMBG [Becton Dickinson (Franklin Lakes, NJ) meters and strips] | 14 |
| MDI + SMBG: SMBG (Becton Dickinson meters and strips) with CareLink™ data management software | 13 | ||||
| 3.69 | DeVries et al., 200242 | The Netherlands | Age: 18–70 years; HbA1c: ≥ 8.5%; CSII experience: NR; number of hypoglycaemic events: NR | CSII + SMBG: Disetronic H-TRONplus insulin pump; Glucotouch or One Touch Profile memory glucose meter (LifeScan, Inc., Milpitas, CA) | 32 |
| MDI + SMBG: Glucotouch or One Touch Profile memory glucose meter (LifeScan) | 40 | ||||
| 6 | Bolli et al., 200941 | Europe | Age: 18–70 years; HbA1c: 6.5–9%; CSII experience: CSII naive; number of hypoglycaemic events: ≥ 2; episodes of severe hypoglycaemia in the previous 6 months: excluded | CSII + SMBG: MiniMed 508 with SMBG | 24 |
| MDI + SMBG: NR | 26 | ||||
| 6 | Hermanides et al., 201137 | Denmark; Switzerland; Sweden; the Netherlands; France; UK; Belgium; Italy | Age: 18–65 years; HbA1c: ≥ 8.2%; CSII experience: CSII in the previous 6 months excluded; number of hypoglycaemic events: NR | Integrated CSII + CGM: Paradigm REAL-Time system with SMBG (meter not described) | 41 |
| MDI + SMBG: SMBG meter not described | 36 | ||||
| 6 | Hirsch et al., 200834 | USA | Age: 18–80 years; HbA1c: ≥ 7.5%; CSII experience: ≥ 6 months prior CSII treatment; number of hypoglycaemic events: NR | Integrated CSII + CGM: Paradigm 722 System | 17 |
| CSII + SMBG: SMBG and a Paradigm 715 Insulin Pump (Medtronic) | 23 | ||||
| 6 | Thomas et al., 200745 | UK | Age: adults; HbA1c: NR; CSII experience: NR; number of hypoglycaemic events: ≥ 1 episode of severe hypoglyaemia in the previous 6 months | CSII + SMBG: Medtronic 508 with SMBG | 7 |
| MDI + SMBG: NR | 7 | ||||
| 9 | Tsui et al., 200146 | Canada | Age: 18–60 years; HbA1c: NR; CSII experience: CSII naive; number of hypoglycaemic events: ≥ 2; episodes of severe hypoglycaemia in the previous year excluded | CSII + SMBG: MiniMed 507 insulin infusion pump; Advantage meter (Roche Diagnostics, Bale, Switzerland) | 12 |
| MDI + SMBG: Advantage meter (Roche Diagnostics) | 14 | ||||
| 12 | Nosadini et al., 198843 | Italy | Age: NR; HbA1c: NR; CSII experience: NR; number of hypoglycaemic events: NR | CSII + SMBG: Betatron II (Firenze, Italy) + SMBG (CSII-HOR) | 10 |
| CSII + SMBG: Microjet Mc 20 (Miles-Ames, Cavenago, Italy) + SMBG (CSII-FBR) | 19 | ||||
| MDI + SMBG: NR (ICIT) | 15 | ||||
| 12 | Bergenstal et al., 201040 | USA; Canada | Age: 7–70 years; HbA1c: 7.4–9.5%; CSII experience: CSII naive or no CSII in the last 3 years; number of hypoglycaemic events: ≥ 2 episodes of severe hypoglycaemia in the previous year excluded | Integrated CSII + CGM: MiniMed Paradigm REAL-Time system | 166 |
| MDI + SMBG: Guardian REAL-Time Clinical | 163 | ||||
| 84 | Brinchmann-Hansen et al., 198544 | Norway | Age: 18–45 years; HbA1c: NR; CSII experience: NR; number of hypoglycaemic events: NR | CSII + SMBG: Nordisk Infuser (n = 3) or AutoSyringe AS6C (n = 12) | 15 |
| MDI + SMBG: NR | 15 |
| Follow-up, months | Study | Countries | Inclusion | Intervention | Number analysed for efficacy per arm |
|---|---|---|---|---|---|
| 3.5 | Weintrob et al., 200347 | Israel | Age: 8–14 years; HbA1c: NR; CSII experience: NR; number of hypoglycaemic events: NR | CSII + SMBG: programmable external pump (MiniMed 508) using insulin lispro | 11 |
| MDI + SMBG: NR | 12 | ||||
| 3.69 | Doyle et al., 200449 | USA | Age: 8–21 years; HbA1c: 6.5–11%; CSII experience: CSII naive; number of hypoglycaemic events: NR | CSII + SMBG: Medtronic MiniMed 508 or Paradigm 511; LifeScan InDuo™ glucose meter | 16 |
| MDI + SMBG: MDI; LifeScan InDuo glucose meter | 16 | ||||
| 6 | Hirsch et al., 200834 | USA | Age: 12 to < 18 years; HbA1c: ≥ 7.5%; CSII experience: ≥ 6 months prior CSII treatment; number of hypoglycaemic events: NR | Integrated CSII + CGM: Paradigm 722 System (Medtronic) | 49 |
| CSII + SMBG: SMBG and a Paradigm 715 Insulin Pump (Medtronic) | 49 | ||||
| 12 | Bergenstal et al., 201040 | USA; Canada | Age: 7–70 years; HbA1c: 7.4–9.5%; CSII experience: CSII naive or no CSII in the last 3 years; number of hypoglycaemic events: ≥ 2 episodes of severe hypoglycaemia in the previous year excluded | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System(Medtronic) | 78 |
| MDI + SMBG: Medtronic Guardian REAL-Time Clinical | 78 | ||||
| 12 | Thrailkill et al., 201148 | USA | Age: 8–18 years; HbA1c: NR; CSII experience: NR; number of hypoglycaemic events: NR | CSII + SMBG: Animas pump model IR 1250; OneTouch Ultra blood glucose meter (LifeScan) | NR |
| MDI + SMBG: MDI; OneTouch Ultra blood glucose meter (LifeScan) | NR |
| Follow-up, months | Study | Countries | Inclusion | Intervention | Number analysed for efficacy per arm |
|---|---|---|---|---|---|
| 3 | O’Connell et al., 200935 | Australia | Age: 13–40 years; HbA1c: ≤ 8.5%; CSII experience: > 3 months experience with CSII; number of hypoglycaemic events: history of severe hypoglycaemia while using CSII excluded | Integrated CSII + CGM: MiniMed Paradigm REAL-Time system | 26 |
| CSII + SMBG: NR; continue their usual insulin pump therapy and SMBG regimen | 29 | ||||
| 6 | Hirsch et al., 200834 | USA | Age: 12–72 years; HbA1c: ≥ 7.5%; CSII experience: ≥ 6 months prior CSII treatment; number of hypoglycaemic events: NR | Integrated CSII + CGM: Paradigm 722 System | 66 |
| CSII + SMBG: SMBG and a Paradigm 715 insulin pump | 72 | ||||
| 6 | Ly et al., 201333 | Australia | Age: 4–50 years; HbA1c: ≤ 8.5%; CSII experience: ≥ 6 months prior CSII treatment; number of hypoglycaemic events: NR | CSII + CGM + Suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | 46 |
| CSII + SMBG: continue using their insulin pump | 49 | ||||
| 6 | Raccah et al., 200936 | France | Age: 2–65 years; HbA1c: > 8%; CSII experience: NR; number of hypoglycaemic events: NR | Non-integrated CSII + CGM: insulin pump with Holter-type CGM device | 55 |
| CSII + SMBG: Paradigm 512/712 with SMBG | 60 | ||||
| 12 | Bergenstal et al., 201040 | USA; Canada | Age: 7–70 years; HbA1c: 7.4–9.5%; CSII experience: CSII naive or no CSII in the last 3 years; number of hypoglycaemic events: ≥ 2 episodes of severe hypoglycaemia in the previous year excluded | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System | 244 |
| MDI + SMBG: Guardian REAL-Time Clinical | 241 |
| Follow-up, months | Study | Country | Inclusion | Intervention | Number analysed for efficacy per arm |
|---|---|---|---|---|---|
| NR (9 months) | Nosari et al., 199350 | Italy | Age: adults; HbA1c: NR; CSII experience: NR; number of hypoglycaemic events: NR | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps (Disetronic Medical Systems, Inc., FL, USA) | 16 |
| MDI + SMBG: NR | 16 |
| Follow-up, months | Study | Intervention | Total (N) | Age, years (SD) | Gender, n (%) | Duration of diabetes, years (SD) | BMI, kg/m2 (SD) | Weight, kg (SD) | HbA1c% (SD) |
|---|---|---|---|---|---|---|---|---|---|
| 3 | Bergenstal et al., 201332 | CSII + CGM+ Suspend: Paradigm Veo pump with Enlite sensor | 121 | 41.6 (12.8) | Male: 46 (38.0) Female: 75 (62.0) | 27.1 (12.5) | 27.6 (4.6) | 79.6 (15.9) | 7.26 (0.7) |
| Integrated CSII + CGM: Paradigm Revel 2.0 pump with Enlite sensor | 126 | 44.8 (13.8) | Male: 50 (39.7) Female: 76 (60.3) | 26.7 (12.7) | 27.1 (4.3) | 79.1 (15.1) | 7.21 (0.8) | ||
| 3.45 | Lee et al., 200738 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time 722 System as adjunct to SMBG (Paradigm Link glucose meter) | 8 | NR (NR) | Male: NR (NR) Female: NR (NR) | NR (NR) | NR (NR) | NR (NR) | 9.45 (0.6) |
| MDI + SMBG: SMBG (Paradigm Link glucose meter) | 8 | NR (NR) | Male: NR (NR) Female: NR (NR) | NR (NR) | NR (NR) | NR (NR) | 8.58 (1.3) | ||
| 3.69 | Peyrot and Rubin, 200939 | Integrated CSII + CGM: Paradigm 722 System (smart CSII pump with real-time CGM and CareLink data management software) as adjunct to SMBG (Becton Dickinson meters and strips) | 14 | NR (NR) | Male: NR (NR) Female: NR (NR) | NR (NR) | NR (NR) | 77.69 (18.7) | 8.87 (0.9) |
| MDI + SMBG: SMBG (Becton Dickinson meters and strips) with CareLink data management software | 13 | NR (NR) | Male: NR (NR) Female: NR (NR) | NR (NR) | NR (NR) | 82.61 (16.0) | 8.32 (1.1) | ||
| 3.69 | DeVries et al., 200242 | CSII + SMBG: Disetronic H-TRONplus insulin pump; Glucotouch or One Touch Profile memory glucose meter (LifeScan) | 32 | 36.2 (10.3) | Male: 21 (54.0) Female: 18 (46.0) | 17.6 (9.8) | NR (NR) | 77.3 (13.6) | 9.27 (1.4) |
| MDI + SMBG: Glucotouch or One Touch Profile memory glucose meter (LifeScan) | 40 | 37.3 (10.6) | Male: 21 (53.0) Female: 19 (47.0) | 18 (9.4) | NR (NR) | 79.8 (13.5) | 9.25 (1.4) | ||
| 6 | Bolli et al., 200941 | CSII + SMBG: MiniMed 508 with SMBG | 24 | 37.6 (12.3) | Male: 13 (54.2) Female: 11 (45.8) | 18.5 (8.4) | 23.8 (2.7) | 70.1 (11.6) | 7.7 (0.7) |
| MDI + SMBG: NR | 26 | 42.4 (9.9) | Male: 14 (53.8) Female: 12 (46.2) | 20.9 (10.6) | 24.3 (1.9) | 70.8 (10.5) | 7.8 (0.6) | ||
| 6 | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic MiniMed Inc.) with SMBG (meter not described) | 41 | 39.3 (11.9) | Male: 22 (50.0) Female: 22 (50.0) | 16.9 (10.7) | NR (NR) | NR (NR) | 8.47 (0.9) |
| MDI + SMBG: SMBG (meter not described) | 36 | 37.3 (10.7) | Male: 21 (53.8) Female: 18 (46.2) | 21 (9.4) | NR (NR) | NR (NR) | 8.64 (0.9) | ||
| 6 | Hirsch et al., 200834 | Integrated CSII + CGM: Paradigm 722 System | 17 | NR (NR) | Male: NR (NR) Female: NR (NR) | NR (NR) | NR (NR) | NR (NR) | 8.37 (0.6) |
| CSII + SMBG: SMBG and a Paradigm 715 insulin pump | 23 | NR (NR) | Male: NR (NR) Female: NR (NR) | NR (NR) | NR (NR) | NR (NR) | 8.3 (0.5) | ||
| 6 | Thomas et al., 200745 | CSII + SMBG: Medtronic 508 with SMBG | 7 | NR (NR) | Male: NR (NR) Female: NR (NR) | NR (NR) | NR (NR) | 72.5 (8.6) | 8.5 (1.9) |
| MDI + SMBG: NR | 7 | NR (NR) | Male: NR (NR) Female: NR (NR) | NR (NR) | NR (NR) | 78 (15.2) | 8.6 (1.1) | ||
| 9 | Tsui et al., 200146 | CSII + SMBG: MiniMed 507 insulin infusion pump; Advantage meter (Roche Diagnostics) | 12 | 36 (12.0) | Male: 8 (62.0) Female: 5 (38.0) | 17 (10.0) | 27 (4.0) | NR (NR) | 7.7 (0.6) |
| MDI + SMBG: Advantage meter (Roche Diagnostics) | 14 | 36 (10.0) | Male: 10 (71.0) Female: 4 (29.0) | 15 (9.0) | 26 (3.0) | NR (NR) | 8.2 (0.7) | ||
| 12 | Nosadini et al., 198843 | CSII + SMBG: Betatron II + SMBG (CSII-HOR) | 10 | 34 (3.0) | Male: 6 (60.0) Female: 4 (40.0) | 7 (3.0) | NR (NR) | 70 (7.0) | NR (NR) |
| CSII + SMBG: Microjet Mc 20 + SMBG (CSII-FBR) | 19 | 36 (6.0) | Male: 11 (57.9) Female: 8 (42.1) | 8 (3.0) | NR (NR) | 77 (7.0) | NR (NR) | ||
| MDI + SMBG: NR (ICIT) | 15 | 32 (9.0) | Male: 11 (73.3) Female: 4 (26.7) | 7 (4.0) | NR (NR) | 71 (6.0) | NR (NR) | ||
| 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System | 166 | 41.9 (12.3) | Male: 94 (57.0) Female: 72 (43.0) | 20.2 (12.2) | 27.4 (4.4) | 80.8 (15.9) | 8.3 (0.5) |
| MDI + SMBG: Guardian REAL-Time Clinical | 163 | 40.6 (12.0) | Male: 93 (57.0) Female: 70 (43.0) | 20.2 (11.7) | 28.4 (5.7) | 85.1 (18.5) | 8.3 (0.5) | ||
| 84 | Brinchmann-Hansen et al., 198544 | CSII + SMBG: Nordisk Infuser (n = 3) or AutoSyringe AS6C (n = 12) | 15 | 26 (19.8) | Male: 7 (46.7) Female: 8 (53.3) | 12.75 (NR) | NR (NR) | 68.6 (NR) | 8.7 (NR) |
| MDI + SMBG: NR | 15 | 26 (22.7) | Male: 7 (46.7) Female: 8 (53.3) | 12.83 (NR) | NR (NR) | 71.7 (NR) | 8.3 (NR) |
| Follow-up, months | Study | Intervention | Total (N) | Age, years (SD) | Gender, n (%) | Duration of diabetes, years (SD) | BMI, kg/m2 (SD) | Weight, kg (SD) | HbA1c% (SD) |
|---|---|---|---|---|---|---|---|---|---|
| 3.5 | Weintrob et al., 200347 | CSII + SMBG: programmable external pump (MiniMed 508) using insulin lispro | 11 | 11.9 (1.4) | Male: 4 (36.4) Female: 7 (63.6) | 5.3 (1.9) | NR (NR) | NR (NR) | 7.9 (1.3) |
| MDI + SMBG: NR | 12 | 11.6 (1.5) | Male: 6 (50.0) Female: 6 (50.0) | 6.3 (2.6) | NR (NR) | NR (NR) | 8.6 (0.8) | ||
| 3.69 | Doyle et al., 200449 | CSII + SMBG: Medtronic MiniMed 508 or Paradigm 511; LifeScan InDuo glucose meter | 16 | 12.5 (3.2) | Male: 6 (37.5) Female: 10 (62.5) | 6.8 (3.8) | NR (NR) | NR (NR) | 8.1 (1.2) |
| MDI + SMBG: MDI; LifeScan InDuo glucose meter | 16 | 13 (2.8) | Male: 8 (50.0) Female: 8 (50.0) | 5.6 (4.0) | NR (NR) | NR (NR) | 8.2 (1.1) | ||
| 6 | Hirsch et al., 200834 | Integrated CSII + CGM: Paradigm 722 System | 49 | NR (NR) | Male: NR (NR) Female: NR (NR) | NR (NR) | NR (NR) | NR (NR) | 8.82 (1.1) |
| CSII + SMBG: SMBG and a Paradigm 715 insulin pump | 49 | NR (NR) | Male: NR (NR) Female: NR (NR) | NR (NR) | NR (NR) | NR (NR) | 8.59 (0.8) | ||
| 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System | 78 | 11.7 (3.0) | Male: 46 (59.0) Female: 32 (41.0) | 4.7 (3.1) | 20.2 (3.8) | 49 (17.9) | 8.3 (0.6) |
| MDI + SMBG: Guardian REAL-Time Clinical | 78 | 12.7 (3.1) | Male: 41 (53.0) Female: 37 (47.0) | 5.4 (3.7) | 20.6 (4.5) | 51.6 (19.3) | 8.3 (0.5) | ||
| 12 | Thrailkill et al., 201148 | CSII + SMBG: Animas pump model IR 1250; OneTouch Ultra blood glucose meter (LifeScan) | NR | 12.1 (3.6) | Male: 5 (41.7) Female: 7 (58.3) | 0 (NA) | 19.56 (4.1) | 40.56 (13.6) | 11.2 (2.1) |
| MDI + SMBG: MDI; OneTouch Ultra blood glucose meter (LifeScan) | NR | 12.1 (2.5) | Male: 6 (50.0) Female: 6 (50.0) | 0 (NA) | 18.82 (3.4) | 46.53 (12.6) | 11.7 (2.6) |
| Follow-up, months | Study | Intervention | Total (N) | Age, years (SD) | Gender, n (%) | Duration of diabetes, years (SD) | BMI, kg/m2 (SD) | Weight, kg (SD) | HbA1c% (SD) |
|---|---|---|---|---|---|---|---|---|---|
| 3 | O’Connell et al., 200935 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time system | 26 | 23.4 (8.6) | Male: 9 (29.0) Female: 22 (71.0) | 11.1 (7.6) | NR (NR) | NR (NR) | 7.3 (0.6) |
| CSII + SMBG: NR; continue their usual insulin pump therapy and SMBG regimen | 29 | 23 (8.1) | Male: 9 (29.0) Female: 22 (71.0) | 9.2 (7.2) | NR (NR) | NR (NR) | 7.5 (0.7) | ||
| 6 | Hirsch et al., 200834 | Integrated CSII + CGM: Paradigm 722 System | 66 | 33 (14.6) | Male: 32 (48.5) Female: 34 (51.5) | 20.8 (12.4) | 26.9 (5.5) | 76.8 (19.3) | 8.49 (0.8) |
| CSII + SMBG: SMBG and a Paradigm 715 insulin pump | 72 | 33.2 (16.4) | Male: 28 (39.9) Female: 44 (61.1) | 16.7 (10.5) | 26.3 (5.1) | 75.4 (18.0) | 8.39 (0.6) | ||
| 6 | Ly et al., 201333 | CSII + CGM + Suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | 46 | 17.4 (10.6) | Male: 26 (56.5) Female: 20 (43.5) | 9.8 (7.4) | NR (NR) | NR (NR) | 7.6 (0.9) |
| CSII + SMBG: continue using their insulin pump | 49 | 19.7 (12.9) | Male: 21 (42.9) Female: 28 (57.1) | 12.1 (10.0) | NR (NR) | NR (NR) | 7.4 (0.7) | ||
| 6 | Raccah et al., 200936 | Non-integrated CSII + CGM: insulin pump with Holter-type CGM device | 55 | 28.1 (15.1) | Male: 30 (54.5) Female: 25 (45.5) | 11.2 (9.0) | 23.5 (4.1) | 65.7 (17.4) | 9.11 (1.3) |
| CSII + SMBG: Paradigm 512/712 with SMBG | 60 | 28.8 (16.7) | Male: 34 (56.7) Female: 26 (43.3) | 12.3 (8.8) | 22.5 (4.4) | 62.6 (18.6) | 9.28 (1.2) | ||
| 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System | 244 | 32.2 (17.5) | Male: 140 (57.0) Female: 104 (43.0) | 15.2 (12.5) | 25.3 (6.0) | 71.9 (25.3) | 8.3 (0.5) |
| MDI + SMBG: Guardian REAL-Time Clinical | 241 | 31.5 (16.5) | Male: 134 (56.0) Female: 107 (44.0) | 15.4 (12.0) | 25.6 (5.6) | 73 (21.8) | 8.3 (0.5) |
| Study | Intervention | Total (N) | Age, years (SD) | Gender, n (%) | Duration of diabetes, years (SD) | BMI, kg/m2 (SD) | Weight, kg (SD) | HbA1c% (SD) |
|---|---|---|---|---|---|---|---|---|
| Nosari et al., 199350 | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps | 16 | 25.5 (1.8) | Male: 0 (0) Female: 16 (100.0) | NR (NR) | 21.8 (0.4) | NR (NR) | NR (NR) |
| MDI + SMBG: NR | 16 | 27 (3.0) | Male: 0 (0) Female: 16 (100.0) | NR (NR) | 21.6 (0.6) | NR (NR) | NR (NR) |
| Follow-up, months | Study | Intervention | Number analysed | Change in HbA1c levels from baseline, % (SD) |
|---|---|---|---|---|
| 2 | Thomas et al., 200745 | CSII + SMBG: Medtronic 508 with SMBG | 7 | Baseline: 8.5 (1.9); follow-up: 7.3 (0.67); change from baseline: NR (NR) |
| MDI + SMBG: NR | 7 | Baseline: 8.6 (1.1); follow-up: 8.3 (1); change from baseline: NR (NR) | ||
| 3 | Bergenstal et al., 201332 | CSII + CGM + suspend: Paradigm Veo pump with Enlite sensor | 121 | Baseline: 7.26 (0.71); follow-up: 7.24 (0.67); change from baseline: 0 (0.44) |
| Integrated CSII + CGM: Paradigm Revel 2.0 pump with Enlite sensor | 126 | Baseline: 7.21 (0.77); follow-up: 7.14 (0.77); change from baseline: –0.04 (0.42) | ||
| 3 | Brinchmann-Hansen et al., 198544 | CSII + SMBG: NR | 15 | Baseline: 10.1 (NR); follow-up: 8.9 (NR); change from baseline: NR (NR) |
| MDI + SMBG: NR | 15 | Baseline: 9.4 (NR); follow-up: 8.7 (NR); change from baseline: NR (NR) | ||
| 3.45 | Lee et al., 200738 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time 722 system as adjunct to SMBG (Paradigm Link glucose meter) | 8 | Baseline: 9.45 (0.55); follow-up: 7.4 (0.66); change from baseline: –2.05 (NR) |
| MDI + SMBG: SMBG (Paradigm Link glucose meter) | 8 | Baseline: 8.58 (1.3); follow-up: 7.5 (1.01); change from baseline: –1.08 (NR) | ||
| 3.68 | Peyrot and Rubin, 200939 | Integrated CSII + CGM: Paradigm 722 system (smart CSII pump with real-time CGM and CareLink data management software) as adjunct to SMBG (Becton Dickinson meters and strips) | 14 | Baseline: 8.87 (0.89); follow-up: 7.16 (0.75); change from baseline: –1.71 (NR) |
| MDI + SMBG: SMBG (Becton Dickinson meters and strips) with CareLink™ data management software | 13 | Baseline: 8.32 (1.05); follow-up: 7.3 (0.92); change from baseline: –1.02 (NR) | ||
| 3.69 | DeVries et al., 200242 | CSII + SMBG: Disetronic H-TRONplus insulin pump; Glucotouch or One Touch Profile memory glucose meter (LifeScan) | 32 | Baseline: 9.27 (1.4); follow-up: NR (NR); change from baseline: –0.91 (1.28) |
| MDI + SMBG: Glucotouch or One Touch Profile memory glucose meter (LifeScan) | 40 | Baseline: 9.25 (1.4); follow-up: NR (NR); change from baseline: –0.07 (0.7) | ||
| 4 | Thomas et al., 200745 | CSII + SMBG: Medtronic 508 with SMBG | 7 | Baseline: 8.5 (1.9); follow-up: 7.4 (1.16); change from baseline: NR (NR) |
| MDI + SMBG: NR | 7 | Baseline: 8.6 (1.1); follow-up: 8 (0.9); change from baseline: NR (NR) | ||
| 6 | Bolli et al., 200941 | CSII + SMBG: MiniMed 508 + glucose monitor. Glucose monitor: NR | 24 | Baseline: 7.7 (0.7); follow-up: 7 (0.8); change from baseline: –0.7 (0.7) |
| MDI + SMBG: insulin glargine (Lantus®, Sanofi-Aventis) plus mealtime insulin lispro. Glucose monitor: NR | 26 | Baseline: 7.8 (0.6); follow-up: 7.2 (0.7); change from baseline: –0.6 (0.8) | ||
| 6 | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic MiniMed Inc.) with SMBG (meter not described) | 41 | Baseline: 8.46 (0.95); follow-up: 7.23 (0.65); change from baseline: –1.23 (1.01) |
| MDI + SMBG: SMBG (meter not described) | 36 | Baseline: 8.59 (0.82); follow-up: 8.46 (1.04); change from baseline: –0.13 (0.56) | ||
| 6 | Hirsch et al., 200834 | Integrated CSII + CGM: Paradigm 722 System | 49 | Baseline: 8.37 (0.6); follow-up: 7.68 (0.84); change from baseline: –0.69 (0.73) |
| CSII + SMBG: SMBG and a Paradigm 715 insulin pump | 49 | Baseline: 8.3 (0.54); follow-up: 7.66 (0.67); change from baseline: –0.64 (0.57) | ||
| 6 | Brinchmann-Hansen et al., 198544 | CSII + SMBG: NR | 15 | Baseline: 10.1 (NR); follow-up: 9.1 (NR); change from baseline: NR (NR) |
| MDI + SMBG: NR | 15 | Baseline: 9.4 (NR); follow-up: 8.8 (NR); change from baseline: NR (NR) | ||
| 6 | Thomas et al., 200745 | CSII + SMBG: Medtronic 508 + SMBG | 7 | Baseline: 8.5 (1.9); follow-up: 7.4 (1); change from baseline: NR (NR) |
| MDI + SMBG: NR | 7 | Baseline: 8.6 (1.1); follow-up: 7.6 (0.7); change from baseline: NR (NR) | ||
| 9 | Tsui et al., 200146 | CSII + SMBG: MiniMed 507 insulin infusion pump; Advantage meter (Roche Diagnostics) | 12 | Baseline: 7.73 (0.6); follow-up: 7.38 (NR); change from baseline: NR (NR) |
| MDI + SMBG: Advantage meter (Roche Diagnostics) | 14 | Baseline: 8.16 (0.7); follow-up: 7.56 (NR); change from baseline: NR (NR) | ||
| 12 | Nosadini et al., 198843 | CSII + SMBG: Betatron II + SMBG (CSII-HOR) | 10 | Baseline: NR (NR); follow-up: 6.1 (0.9); change from baseline: NR (NR) |
| CSII + SMBG: Microjet Mc 20 + SMBG (CSII-FBR) | 19 | Baseline: NR (NR); follow-up: 6.3 (0.7); change from baseline: NR (NR) | ||
| MDI + SMBG: NR (ICIT) | 15 | Baseline: NR (NR); follow-up: 7.1 (0.9); change from baseline: NR (NR) | ||
| 12 | Brinchmann-Hansen et al., 198544 | CSII + SMBG: NR | 15 | Baseline: 10.1 (NR); follow-up: 8.5 (NR); change from baseline: NR (NR) |
| MDI + SMBG: NR | 15 | Baseline: 9.4 (NR); follow-up: 8.5 (NR); change from baseline: NR (NR) | ||
| 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System | 166 | Baseline: 8.3 (0.5); follow-up: NR (NR); change from baseline: –1 (0.7) |
| MDI + SMBG: Guardian REAL-Time Clinical | 163 | Baseline: 8.3 (0.5); follow-up: NR (NR); change from baseline: –0.4 (0.8) | ||
| 24 | Brinchmann-Hansen et al., 198544 | CSII + SMBG: NR | 15 | Baseline: 10.1 (NR); follow-up: 8.7 (NR); change from baseline: NR (NR) |
| MDI + SMBG: NR | 15 | Baseline: 9.4 (NR); follow-up: 9.1 (NR); change from baseline: NR (NR) |
| Follow-up, months | Study | Intervention | Number analysed | Change in HbA1c levels from baseline, % (SD) |
|---|---|---|---|---|
| 3.5 | Weintrob et al., 200347 | CSII + SMBG: programmable external pump (MiniMed 508) using insulin lispro | 11 | Baseline: 7.9 (1.3); follow-up: 7.9 (0.7); change from baseline: NR (NR) |
| MDI + SMBG: NR | 12 | Baseline: 8.6 (0.8); follow-up: 8.2 (0.8); change from baseline: NR (NR) | ||
| 3.69 | Doyle et al., 200449 | CSII + SMBG: Medtronic MiniMed 508 or Paradigm 511; LifeScan InDuo glucose meter | 16 | Baseline: 8.1 (1.2); follow-up: 7.2 (1); change from baseline: NR (NR) |
| MDI + SMBG: MDIs and LifeScan InDuo glucose meter | 16 | Baseline: 8.2 (1.1); follow-up: 8.1 (1.2); change from baseline: NR (NR) | ||
| 6 | Hirsch et al., 200834 | Integrated CSII + CGM: Paradigm 722 System (Medtronic) | 17 | Baseline: 8.82 (1.05); follow-up: 8.02 (1.11); change from baseline: –0.79 (0.65) |
| CSII + SMBG: SMBG and a Paradigm 715 insulin pump (Medtronic) | 23 | Baseline: 8.59 (0.8); follow-up: 8.21 (0.97); change from baseline: –0.37 (0.95) | ||
| 6 | Thrailkill et al., 201148 | CSII + SMBG: Animas pump model IR 1250; OneTouch Ultra blood glucose meter (LifeScan) | NR | Baseline: 11.2 (2.1); follow-up: 6.34 (0.7); change from baseline: NR (NR) |
| MDI + SMBG: MDIs and OneTouch Ultra blood glucose meter (LifeScan) | NR | Baseline: 11.7 (2.6); follow-up: 7 (1.1); change from baseline: NR (NR) | ||
| 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System (Medtronic) | 78 | Baseline: 8.3 (0.6); follow-up: NR (NR); change from baseline: –0.4 (0.9) |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 78 | Baseline: 8.3 (0.5); follow-up: NR (NR); change from baseline: 0.2 (1) | ||
| 12 | Thrailkill et al., 201148 | CSII + SMBG: Animas pump model IR 1250; OneTouch Ultra blood glucose meter (LifeScan) | NR | Baseline: 11.2 (2.1); follow-up: 6.9 (0.7); change from baseline: NR (NR) |
| MDI + SMBG: MDIs and OneTouch Ultra blood glucose meter (LifeScan) | NR | Baseline: 11.7 (2.6); follow-up: 6.9 (0.9); change from baseline: NR (NR) |
| Follow-up, months | Study | Intervention | Number analysed | Change from baseline in HbA1c (%) |
|---|---|---|---|---|
| 3 | O’Connell et al., 200935 | CSII + CGM Integrated: MiniMed Paradigm REAL-Time system (Medtronic) | 26 | Baseline: 7.3 (0.6); follow-up: 7.1 (0.8); change from baseline: NR (NR) |
| CSII + SMBG: continue their usual insulin pump therapy and SMBG regimen | 29 | Baseline: 7.5 (0.7); follow-up: 7.8 (0.9); change from baseline: NR (NR) | ||
| 6 | Hirsch et al., 200834 | Integrated CSII + CGM: Paradigm 722 system (Medtronic) | 66 | Baseline: 8.39 (0.64); follow-up: 7.77 (0.92); change from baseline: –0.71 (0.71) |
| CSII + SMBG: SMBG and a Paradigm 715 insulin pump (Medtronic) | 72 | Baseline: 8.49 (0.76); follow-up: 7.84 (0.81); change from baseline: –0.56 (0.72) | ||
| 6 | Ly et al., 201333 | CSII + CGM + Suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | 46 | Baseline: 7.6 (NR); follow-up: 7.5 (NR); change from baseline: −0.1 (NR) |
| CSII + SMBG: continue using their insulin pump | 49 | Baseline: 7.4 (NR); follow-up: 7.4 (NR); change from baseline: −0.06 (NR) | ||
| 6 | Raccah et al., 200936 | Non-integrated CSII + CGM: insulin pump with Holter-type CGM device | 55 | Baseline: 9.11 (1.28); follow-up: NR (NR); change from baseline: –0.81 (1.09) |
| CSII + SMBG: Paradigm 512/712 + SMBG | 60 | Baseline: 9.28 (1.19); follow-up: NR (NR); change from baseline: –0.57 (0.94) | ||
| 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System (Medtronic) | 244 | Baseline: 8.3 (0.5); follow-up: 7.5 (NR); change from baseline: –0.8 (0.84) |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 241 | Baseline: 8.3 (0.5); follow-up: 8.1 (NR); change from baseline: –0.2 (0.89) |
| Study | Follow-up, pregnancy trimester | Intervention | Number analysed | Change in HbA1c levels from baseline, % (SD) |
|---|---|---|---|---|
| Nosari et al., 199350 | First | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps | 16 | Baseline: NR (NR); follow-up: 6 (NR); change from baseline: NR (NR) |
| MDI + SMBG: NR | 16 | Baseline: NR (NR); follow-up: 6.2 (NR); change from baseline: NR (NR) | ||
| Second | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps | 16 | Baseline: NR (NR); follow-up: 6.8 (NR); change from baseline: NR (NR) | |
| MDI + SMBG: NR | 16 | Baseline: NR (NR); follow-up: 6.1 (NR); change from baseline: NR (NR) | ||
| Third | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps | 16 | Baseline: NR (NR); follow-up: 6.3 (NR); change from baseline: NR (NR) | |
| MDI + SMBG: NR | 16 | Baseline: NR (NR); follow-up: 6.2 (NR); change from baseline: NR (NR) |
| Follow-up, months | Study | Intervention | Proportion achieving HbA1c levels of ≤ 7%, n (%) | Total number analysed |
|---|---|---|---|---|
| 6 | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic) with SMBG (meter not described) | 14 (34) | 41 |
| MDI + SMBG: SMBG (meter not described) | 0 (0) | 36 | ||
| 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System (Medtronic) | 57 (34) | 166 |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 19 (12) | 163 |
| Follow-up, months | Study | Intervention | Proportion achieving HbA1c levels of ≤ 7%, n (%) | Total number analysed |
|---|---|---|---|---|
| 3.69 | Doyle et al., 200449 | CSII + SMBG: Medtronic MiniMed 508 or Paradigm 511; LifeScan InDuo glucose meter | 8 (50) | 16 |
| MDI + SMBG: MDIs and LifeScan InDuo glucose meter | 2 (12.5) | 16 | ||
| 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time system (Medtronic) | 10 (13) | 78 |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 4 (5) | 78 |
| Follow-up, months | Study | Intervention | Proportion achieving HbA1c levels of ≤ 7%, n (%) | Total number analysed |
|---|---|---|---|---|
| 3 | O’Connell et al., 200935 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time system (Medtronic) | 14 (56) | 26 |
| CSII + SMBG: continue their usual insulin pump therapy and SMBG regimen | 5 (17) | 29 | ||
| 6 | Hirsch et al., 200834 | Integrated CSII + CGM: Paradigm 722 System (Medtronic) | 16 (24.2) | 66 |
| CSII + SMBG: SMBG and a Paradigm 715 insulin pump (Medtronic) | 12 (19.4) | 72 | ||
| 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time system (Medtronic) | 67 (27) | 244 |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 23 (10) | 241 |
| Population | Severity | Follow-up, months | Study | Intervention | Number of people with hypoglycaemia/number analysed (%) | Number of hypoglycaemic events/number of people analysed |
|---|---|---|---|---|---|---|
| Adults | Any | 6 | Bolli et al., 200941 | CSII + SMBG: MiniMed 508 and glucose monitor (NR) | 2/28 (7.14) | NR |
| MDI + SMBG: insulin glargine plus mealtime insulin lispro; glucose monitor NR | 2/29 (6.90) | NR | ||||
| Mild | 6 | Thomas et al., 200745 | CSII + SMBG: Medtronic 508 with SMBG | NR | 141/7 | |
| MDI + SMBG: NR | NR | 75/7 | ||||
| NR | 3.45 | Lee et al., 200738 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time 722 System as adjunct to SMBG (Paradigm Link glucose meter) | 0/8 (0.00) | NR | |
| MDI + SMBG: SMBG (Paradigm Link glucose meter) | 1/8 (12.50) | NR | ||||
| Severe | 3.68 | DeVries et al., 200242 | CSII + SMBG: Disetronic H-TRONplus insulin pump; Glucotouch or One Touch Profile memory glucose meter (LifeScan) | 3/32 (9.40) | NR | |
| MDI + SMBG: Glucotouch or One Touch Profile memory glucose meter (LifeScan) | 6/40 (15.00) | NR | ||||
| Severe | 3.68 | Peyrot and Rubin, 200939 | Integrated CSII + CGM: Paradigm 722 System (smart CSII pump with real-time CGM and CareLink data management software) as adjunct to SMBG (Becton Dickinson meters and strips) | NR | 0/14 | |
| MDI + SMBG: SMBG (Becton Dickinson meters and strips) with CareLink data management software | NR | 3/13 | ||||
| Severe | 6 | Bolli et al., 200941 | CSII + SMBG: MiniMed 508 with glucose monitor (NR) | 23/28 (82.14) | NR | |
| MDI + SMBG: insulin glargine plus mealtime insulin lispro; glucose monitor NR | 27/29 (93.10) | NR | ||||
| Thomas et al., 200745 | CSII + SMBG: Medtronic 508 with SMBG | NR | 3/7 | |||
| MDI + SMBG: NR | NR | 2/7 | ||||
| Severe | 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time system (Medtronic) | 17/169 (10.10) | NR | |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 13/167 (7.80) | NR | ||||
| Children | Severe | 3.68 | Doyle et al., 200449 | CSII + SMBG: Medtronic MiniMed 508 or Paradigm 511; LifeScan InDuo glucose meter | 2/16 (12.50) | 2/16 |
| MDI + SMBG: MDIs with LifeScan InDuo glucose meter | 4/16 (25.00) | 5/16 | ||||
| Severe | 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System (Medtronic) | 4/78 (5.10) | NR | |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 4/81 (4.90) | NR | ||||
| Severe | 12 | Thrailkill et al., 201148 | CSII + SMBG: Animas pump model IR 1250; OneTouch Ultra blood glucose meter (LifeScan) | 0/0 (0.00) | NR | |
| MDI + SMBG: MDIs with OneTouch Ultra blood glucose meter (LifeScan) | 0/0 (0.00) | NR | ||||
| Mixed | Moderate | 6 | Ly et al., 201333 | CSII + CGM + suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | 35/41 (85.37) | NR |
| CSII + SMBG: continue using their insulin pump | 13/45 (28.89) | NR | ||||
| Moderate and severe | 6 | Ly et al., 201333 | CSII + CGM + suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | 35/41 (85.37) | NR | |
| CSII + SMBG: continue using their insulin pump | 13/45 (28.89) | NR | ||||
| Severe | 6 | Hirsch et al., 200834 | Integrated CSII + CGM: Paradigm 722 System (Medtronic) | 8/66 (NR) | 11/66 | |
| CSII + SMBG: SMBG and a Paradigm 715 insulin pump (Medtronic) | 3/72 (NR) | 3/72 | ||||
| Severe | 6 | Ly et al., 201333 | CSII + CGM + suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | 0/41 (0.00) | NR | |
| CSII + SMBG: continue using their insulin pump | 6/45 (13.33) | NR | ||||
| Severe | 12 | Bergenstal et al.,40 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System (Medtronic) | 21/247 (8.50) | NR | |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 17/248 (6.90) | NR | ||||
| Pregnant | Severe | NR | Nosari et al., 199350 | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps | NR | 3/NR |
| MDI + SMBG: NR | NR | 1/NR |
| Population | Severity | Follow-up, months | Event rate definition | Study | Intervention | Hypoglycaemic event rate | Hyperglycaemic event rate | Total number analysed |
|---|---|---|---|---|---|---|---|---|
| Adults | Mild | 3.69 | Number of mild hypoglycaemic episodes per patient-week | DeVries et al., 200242 | CSII + SMBG: Disetronic H-TRONplus insulin pump; Glucotouch or One Touch Profile memory glucose meter (LifeScan) | 0.98 (2.0) | NR | 32 |
| MDI + SMBG: Glucotouch or One Touch Profile memory glucose meter (LifeScan) | –0.02 (1.2) | NR | 40 | |||||
| Mild | 6 | Mild symptomatic hypoglycaemic events per patient-year | Thomas et al., 200745 | CSII + SMBG: Medtronic 508 with SMBG | 40 (NR) | NR | 7 | |
| MDI + SMBG: NR | 21 (NR) | NR | 7 | |||||
| Mild | 12 | Hypoglycaemic events per patient per year | Nosadini et al., 198843 | CSII + SMBG: Betatron II with SMBG (CSII-HOR) | 30 (11.0) | NR | 10 | |
| CSII + SMBG: Microjet Mc 20 with SMBG (CSII-FBR) | 36 (10.0) | NR | 19 | |||||
| MDI + SMBG: NR (ICIT) | 59 (12.0) | NR | 15 | |||||
| NR | 3 | Day and night hypoglycaemia: events per patient-week | Bergenstal et al., 201332 | CSII + CGM + suspend: Paradigm Veo pump with Enlite sensor | 3.3 (2.0) | NR | 121 | |
| Integrated CSII + CGM: Paradigm Revel 2.0 pump with Enlite sensor | 4.7 (2.7) | NR | 126 | |||||
| NR | 3 | Nocturnal hypoglycaemia: events per patient-week | Bergenstal et al., 201332 | CSII + CGM + Suspend: Paradigm Veo pump with Enlite sensor | 1.5 (1.0) | NR | 121 | |
| Integrated CSII + CGM: Paradigm Revel 2.0 pump with Enlite sensor | 2.2 (1.3) | NR | 126 | |||||
| NR | 6 | Number of hyperglycaemic events standardised per day | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic) with SMBG (meter not described) | NR | 2.1 (0.8) | 40 | |
| MDI + SMBG: SMBG (meter not described) | NR | 2.2 (0.7) | 31 | |||||
| NR | 6 | Number of hypoglycaemic events standardised per day | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic) with SMBG (meter not described) | 0.7 (0.7) | NR | 40 | |
| MDI + SMBG: SMBG (meter not described) | 0.6 (0.7) | NR | 31 | |||||
| NR | 12 | Hyperglycaemic events per patient per year | Nosadini et al., 198843 | CSII + SMBG: Betatron II with SMBG (CSII-HOR) | NR | 17 (4.0) | 10 | |
| CSII + SMBG: Microjet Mc 20 with SMBG (CSII-FBR) | NR | 18 (5.0) | 19 | |||||
| MDI + SMBG: NR (ICIT) | NR | 20 (3.0) | 15 | |||||
| NR | 24 | Symptomatic hypoglycaemic episodes per patient per week | Brinchmann-Hansen et al., 198544 | CSII + SMBG: NR | 1.7 (NR) | NR | 15 | |
| MDI + SMBG: NR | 1.2 (NR) | NR | 15 | |||||
| Severe | 6 | Severe hypoglycaemic events per patient-year | Thomas et al., 200745 | CSII + SMBG: Medtronic 508 with SMBG | 0.9 (NR) | NR | 7 | |
| MDI + SMBG: NR | 0.6 (NR) | NR | 7 | |||||
| Severe | 12 | Hypoglycaemic events per patient per year | Nosadini et al., 198843 | CSII + SMBG: Betatron II + SMBG (CSII-HOR) | 0.16 (0.1) | NR | 10 | |
| CSII + SMBG: Microjet Mc 20 + SMBG (CSII-FBR) | 0.14 (0.1) | NR | 19 | |||||
| MDI + SMBG: NR (ICIT) | 0.42 (0.2) | NR | 15 | |||||
| Severe | 12 | Severe hypoglycaemic event rate per 100 person-years | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System (Medtronic) | 15.31 (NR) | NR | 169 | |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 17.62 (NR) | NR | 167 | |||||
| Children | Severe | 12 | Severe hypoglycaemic event rate per 100 person-years | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time system (Medtronic) | 8.98 (NR) | NR | 78 |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 4.95 (NR) | NR | 81 | |||||
| Mixed | Moderate | 6 | Rate of hypoglycaemic events: 6-month rate per 100 patient-months | Ly et al., 201333 | CSII + CGM + suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | 28.5 (NR) | NR | 46 |
| CSII + SMBG: continue using their insulin pump | 9.6 (NR) | NR | 49 | |||||
| Moderate | 6 | Rate of hypoglycaemic events: incidence rate per 100 patient-months | Ly et al., 201333 | CSII + CGM + suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | 9.6 (NR) | NR | 41 | |
| CSII + SMBG: continue using their insulin pump | 26.3 (NR) | NR | 45 | |||||
| Moderate + severe | 6 | Rate of hypoglycaemic events: 6-month rate per 100 patient-months | Ly et al., 201333 | CSII + CGM + suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | 28.4 (NR) | NR | 46 | |
| CSII + SMBG: continue using their insulin pump | 11.9 (NR) | NR | 49 | |||||
| Moderate + severe | 6 | Rate of hypoglycaemic events: incidence rate per 100 patient-months | Ly et al., 201333 | CSII + CGM + suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | 9.5 (NR) | NR | 41 | |
| CSII + SMBG: continue using their insulin pump | 34.2 (NR) | NR | 45 | |||||
| Severe | 6 | Rate of hypoglycaemic events: 6-month rate per 100 patient-months | Ly et al., 201333 | CSII + CGM + suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | 0 (NR) | NR | 46 | |
| CSII + SMBG: continue using their insulin pump | 2.2 (NR) | NR | 49 | |||||
| Severe | 6 | Rate of hypoglycaemic events: incidence rate per 100 patient-months | Ly et al., 201333 | CSII + CGM + suspend: sensor-augmented pump (Medtronic Paradigm Veo System, Medtronic MiniMed) with automated insulin suspension | NR (NR) | NR | 41 | |
| CSII + SMBG: continue using their insulin pump | NR (NR) | NR | 45 | |||||
| Severe | 12 | Severe hypoglycaemia event rate per 100 person-years | Bergenstal et al., 201040 | CSII + CGM integrated: MiniMed Paradigm REAL-Time System, Medtronic | 13.31 (NR) | NR | 247 | |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 13.48 (NR) | NR | 248 |
| HRQoL scale | Follow-up, months | Study | Intervention | Number analysed | HRQoL score (SD) |
|---|---|---|---|---|---|
| Diabetes QOL | 6 | Thomas et al., 200745 | CSII + SMBG: Medtronic 508 with SMBG | 7 | Baseline: 69 (19); follow-up: 74 (20); change from baseline: NR (NR) |
| MDI + SMBG: NR | 7 | Baseline: 47 (20); follow-up: 70 (11); change from baseline: NR (NR) | |||
| Diabetes QOL; diabetic worry | 9 | Tsui et al., 200146 | CSII + SMBG: MiniMed 507 insulin infusion pump; Advantage meter (Roche Diagnostics) | 12 | Baseline: NR (NR); follow-up: 85.2 (NR); change from baseline: NR (NR) |
| MDI + SMBG: Advantage meter (Roche Diagnostics) | 14 | Baseline: NR (NR); follow-up: 79.8 (NR); change from baseline: NR (NR) | |||
| Diabetes QOL; global health | 9 | Tsui et al., 200146 | CSII + SMBG: MiniMed 507 insulin infusion pump; Advantage meter (Roche Diagnostics) | 12 | Baseline: NR (NR); follow-up: 68.2 (NR); change from baseline: NR (NR) |
| MDI + SMBG: Advantage meter (Roche Diagnostics) | 14 | Baseline: NR (NR); follow-up: 67.3 (NR); change from baseline: NR (NR) | |||
| Diabetes QOL; impact | 9 | Tsui et al., 200146 | CSII + SMBG: MiniMed 507 insulin infusion pump; Advantage meter (Roche Diagnostics) | 12 | Baseline: NR (NR); follow-up: 69.9 (NR); change from baseline: NR (NR) |
| MDI + SMBG: Advantage meter (Roche Diagnostics) | 14 | Baseline: NR (NR); follow-up: 68.4 (NR); change from baseline: NR (NR) | |||
| Diabetes QOL; satisfaction | 9 | Tsui et al., 200146 | CSII + SMBG: MiniMed 507 insulin infusion pump; Advantage meter (Roche Diagnostics) | 12 | Baseline: NR (NR); follow-up: 75.6 (NR); change from baseline: NR (NR) |
| MDI + SMBG: Advantage meter (Roche Diagnostics) | 14 | Baseline: NR (NR); follow-up: 68.3 (NR); change from baseline: NR (NR) | |||
| Diabetes QOL; social worry | 9 | Tsui et al., 200146 | CSII + SMBG: MiniMed 507 insulin infusion pump; Advantage meter (Roche Diagnostics) | 12 | Baseline: NR (NR); follow-up: 89.6 (NR); change from baseline: NR (NR) |
| MDI + SMBG: Advantage meter (Roche Diagnostics) | 14 | Baseline: NR (NR); follow-up: 94 (NR); change from baseline: NR (NR) | |||
| SF-36; bodily pain | 6 | Hermanides et al.,37 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic) with SMBG (meter not described) | 42 | Baseline: 78.9 (25.4); follow-up: 79.9 (24.4); change from baseline: 1 (NR) |
| MDI + SMBG: SMBG (meter not described) | 33 | Baseline: 78.7 (23); follow-up: 78.7 (22.6); change from baseline: 0 (NR) | |||
| SF-36; general health | 6 | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic) with SMBG (meter not described) | 42 | Baseline: 55.5 (20.3); follow-up: 67.7 (21.6); change from baseline: 12.2 (NR) |
| MDI + SMBG: SMBG (meter not described) | 33 | Baseline: 59.8 (22.3); follow-up: 63.1 (19.1); change from baseline: 3.3 (NR) | |||
| 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time system (Medtronic) | 153 | Baseline: NR (NR); follow-up: NR (NR); change from baseline: 2.7 (8.07) | |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 151 | Baseline: NR (NR); follow-up: NR (NR); change from baseline: –0.3 (7.13) | |||
| SF-36; general health | 3.68 | DeVries et al., 200242 | CSII + SMBG: Disetronic H-TRONplus insulin pump; Glucotouch or One Touch Profile memory glucose meter (LifeScan) | NR | Baseline: 59.8 (37); follow-up: NR (NR); change from baseline: –1.2 (NR) |
| MDI + SMBG: G Rucotouch or One Touch Profile memory glucose meter (Lifescan) | Baseline: 61.4 (20.5); follow-up: NR (NR); change from baseline: 5.9 (NR) | ||||
| SF-36; mental composite score | 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System (Medtronic) | 166 | Baseline: 49.86 (9.64); follow-up: NR (NR); change from baseline: 0.05 (NR) |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 168 | Baseline: 49.5 (9.09); follow-up: NR (NR); change from baseline: –1.26 (NR) | |||
| SF-36; mental health | 6 | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic) with SMBG (meter not described) | 42 | Baseline: 72.6 (14.8); follow-up: 79.2 (12.5); change from baseline: 6.6 (NR) |
| MDI + SMBG: SMBG (meter not described) | 33 | Baseline: 77.9 (20.2); follow-up: 76.8 (16.5); change from baseline: –1.1 (NR) | |||
| SF-36; mental health | 3.68 | DeVries et al., 200242 | CSII + SMBG: Disetronic H-TRONplus insulin pump; Glucotouch or One Touch Profile memory glucose meter (LifeScan) | NR | Baseline: 78 (NR); follow-up: NR (NR); change from baseline: –0.6 (NR) |
| Baseline: 80 (NR); follow-up: NR (NR); change from baseline: 5.2 (NR) | |||||
| SF-36; physical composite score | 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time System (Medtronic) | 166 | Baseline: 50.61 (7.12); follow-up: NR (NR); change from baseline: 1.22 (NR) |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 168 | Baseline: 50.97 (7.86); follow-up: NR (NR); change from baseline: 0.26 (NR) | |||
| SF-36; physical functioning | 6 | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic) with SMBG (meter not described) | 42 | Baseline: 89.4 (14.5); follow-up: 92.7 (11.2); change from baseline: 3.3 (NR) |
| MDI + SMBG: SMBG (meter not described) | 33 | Baseline: 90.5 (14.3); follow-up: 91.4 (12.7); change from baseline: 0.9 (NR) | |||
| SF-36; role – emotional | 6 | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic) with SMBG (meter not described) | 42 | Baseline: 84.9 (20.4); follow-up: 87.1 (19.6); change from baseline: 2.2 (NR) |
| MDI + SMBG: SMBG (meter not described) | 33 | Baseline: 89.6 (16.7); follow-up: 88 (16); change from baseline: –1.6 (NR) | |||
| SF-36; role – physical | 6 | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic with SMBG (meter not described) | 42 | Baseline: 76.8 (23.8); follow-up: 85.7 (20.7); change from baseline: 8.9 (NR) |
| MDI + SMBG: SMBG (meter not described) | 33 | Baseline: 84.4 (19.3); follow-up: 87.3 (20.4); change from baseline: 2.9 (NR) | |||
| SF-36; social functioning | 6 | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic) with SMBG (meter not described) | 42 | Baseline: 81.5 (20.3); follow-up: 89.3 (16); change from baseline: 7.8 (NR) |
| MDI + SMBG: SMBG (meter not described) | 33 | Baseline: 86.4 (21); follow-up: 82.2 (25.2); change from baseline: –4.2 (NR) | |||
| SF-36; vitality | 6 | Hermanides et al., 201137 | Integrated CSII + CGM: Paradigm REAL-Time System (Medtronic) with SMBG (meter not described) | 42 | Baseline: 53.9 (20); follow-up: 66.7 (20.2); change from baseline: 12.8 (NR) |
| MDI + SMBG: SMBG (meter not described) | 33 | Baseline: 61 (23.7); follow-up: 65.2 (19.3); change from baseline: 4.2 (NR) |
| Outcome definition | Population | Follow-up, months | Study | Intervention | Number with AE (%) | Total number analysed |
|---|---|---|---|---|---|---|
| All serious AEs | Adults | 3.45 | Lee et al., 200738 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time 722 System as adjunct to SMBG (Paradigm Link glucose meter) | 0 (0.0) | 8 |
| MDI + SMBG: SMBG (Paradigm Link glucose meter) | 1 (12.5) | 8 | ||||
| Mixed | 6 | Raccah et al., 200936 | Non-integrated CSII + CGM: insulin pump + Holter-type CGM device | 3 (NR) | NR | |
| CSII + SMBG: Paradigm 512/712 + SMBG | 7 (NR) | NR | ||||
| 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time system (Medtronic) | 32 (13.0) | 247 | ||
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 30 (12.1) | 248 | ||||
| Death | Adults | 3 | Bergenstal et al., 201332 | Integrated CSII + CGM: Paradigm Revel 2.0 pump with Enlite sensor | 0 (0.0) | 126 |
| CSII + CGM + suspend: Paradigm Veo pump with Enlite sensor | 0 (0.0) | 121 | ||||
| Device-related serious AEs | Adults | 3 | Bergenstal et al., 201332 | Integrated CSII + CGM: Paradigm Revel 2.0 pump with Enlite sensor | 0 (0.0) | 126 |
| CSII + CGM + suspend: Paradigm Veo pump with Enlite sensor | 0 (0.0) | 121 | ||||
| Hypoglycaemic coma | Adults | 24 | Brinchmann-Hansen et al., 198544 | MDI + SMBG: NR | 6 (40.0) | 15 |
| CSII + SMBG: NR | 2 (13.3) | 15 | ||||
| Subcutaneous abscess | Adults | 24 | Brinchmann-Hansen et al., 198544 | MDI + SMBG: NR | 0 (0.0) | 15 |
| CSII + SMBG: NR | 6 (40.0) | 15 | ||||
| Total AEs, not including serious AEs | Mixed | 12 | Bergenstal et al., 201040 | Integrated CSII + CGM: MiniMed Paradigm REAL-Time system (Medtronic) | 96 (38.9) | 247 |
| MDI + SMBG: Guardian REAL-Time Clinical (Medtronic) | 49 (19.8) | 248 | ||||
| Treatment emergent AE | Adults | 6 | Bolli et al., 200941 | CSII + SMBG: MiniMed 508 with glucose monitor (NR) | 18 (64.3) | 28 |
| MDI + SMBG: insulin glargine plus mealtime insulin lispro glucose monitor (NR) | 22 (75.9) | 29 |
| Outcome definition | Follow-up, months | Study ID | Intervention | Number with AE (%) | Total number analysed |
|---|---|---|---|---|---|
| Fetal respiratory distress syndrome | NR | Nosari et al., 199350 | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps | 1 (6.3) | 16 |
| MDI + SMBG: NR | 0 (0.0) | 16 | |||
| Intrauterine death | NR | Nosari et al., 199350 | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps | 2 (12.5) | 16 |
| MDI + SMBG: NR | 1 (6.3) | 16 | |||
| Large for gestational age | NR | Nosari et al., 199350 | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps | 1 (6.3) | 16 |
| MDI + SMBG: NR | 0 (0.0) | 16 | |||
| Neonatal hypoglycaemia (plasma glucose of < 30 mg/dl) | NR | Nosari et al., 199350 | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps | 1 (6.3) | 16 |
| MDI + SMBG: NR | 1 (6.3) | 16 | |||
| Premature birth of a viable fetus | NR | Nosari et al., 199350 | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps | 0 (0.0) | 16 |
| MDI + SMBG: NR | 1 (6.3) | 16 | |||
| Small for gestational age | NR | Nosari et al., 199350 | CSII + SMBG: Microjet MC 20 and Dahedi B.V. portable battery-powered syringe infusion pumps | 0 (0.0) | 16 |
| MDI + SMBG: NR | 2 (12.5) | 16 |
Appendix 4 Risk-of-bias assessment results
| Study ID | Random sequence generation | Allocation concealment | Participant blinding | Care staff blinding | Outcome assessor blinding | Selective outcome reporting | Incomplete data | Overall |
|---|---|---|---|---|---|---|---|---|
| Bergenstal et al., 201332 | Unclear | Unclear | High | High | High | Low | High | High |
| Bolli et al., 200941 | Low | Low | High | High | High | Low | High | High |
| DeVries et al., 200242 | Low | Low | High | High | High | Low | High | High |
| Doyle et al., 200449 | Low | Low | High | High | High | High | Low | Low |
| Hermanides et al., 201137 | Low | Low | High | High | High | Low | Unclear | Low |
| Hirsch et al., 200834 | Unclear | Unclear | High | High | High | Low | High | High |
| Lee et al., 200738 | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| Ly et al., 201333 | Low | Unclear | High | High | High | Low | High | High |
| Nosadini et al., 198843 | Unclear | Low | High | High | High | High | High | High |
| Nosari et al., 199350 | Unclear | Unclear | High | High | High | Low | High | High |
| O’Connell et al., 200935 | Low | Unclear | High | High | High | Low | High | High |
| Brinchmann-Hansen et al., 198544 | Low | Unclear | High | High | High | Low | Low | Low |
| Peyrot and Rubin, 200939 | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| Raccah et al., 200936 | Unclear | Unclear | High | High | High | High | High | High |
| Bergenstal et al., 201040 | Unclear | Low | High | High | High | Low | High | High |
| Thomas et al., 200745 | Unclear | Unclear | High | High | High | Low | Unclear | Unclear |
| Thrailkill et al., 201148 | Low | Unclear | High | High | High | Low | High | High |
| Tsui et al., 200146 | Low | Low | Unclear | Unclear | Unclear | Low | High | Unclear |
| Weintrob et al., 200347 | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | Low |
Appendix 5 Conversion tables for glycated haemoglobin and glucose values
| HbA1c ‘old’ | HbA1c ‘new’ | HbA1c ‘old’ | HbA1c ‘new’ |
|---|---|---|---|
| 4.0 | 20 | 9.1 | 76 |
| 4.1 | 21 | 9.2 | 77 |
| 4.2 | 22 | 9.3 | 78 |
| 4.3 | 23 | 9.4 | 79 |
| 4.4 | 25 | 9.5 | 80 |
| 4.5 | 26 | 9.6 | 81 |
| 4.6 | 27 | 9.7 | 83 |
| 4.7 | 28 | 9.8 | 84 |
| 4.8 | 29 | 9.9 | 85 |
| 4.9 | 30 | 10.0 | 86 |
| 5.0 | 31 | 10.1 | 87 |
| 5.1 | 32 | 10.2 | 88 |
| 5.2 | 33 | 10.3 | 89 |
| 5.3 | 34 | 10.4 | 90 |
| 5.4 | 36 | 10.5 | 91 |
| 5.5 | 37 | 10.6 | 92 |
| 5.6 | 38 | 10.7 | 93 |
| 5.7 | 39 | 10.8 | 95 |
| 5.8 | 40 | 10.9 | 96 |
| 5.9 | 41 | 11.0 | 97 |
| 6.0 | 42 | 11.1 | 98 |
| 6.1 | 43 | 11.2 | 99 |
| 6.2 | 44 | 11.3 | 100 |
| 6.3 | 45 | 11.4 | 101 |
| 6.4 | 46 | 11.5 | 102 |
| 6.5 | 48 | 11.6 | 103 |
| 6.6 | 49 | 11.7 | 104 |
| 6.7 | 50 | 11.8 | 105 |
| 6.8 | 51 | 11.9 | 107 |
| 6.9 | 52 | 12.0 | 108 |
| 7.0 | 53 | 13.0 | 119 |
| 7.1 | 54 | 13.1 | 120 |
| 7.2 | 55 | 13.2 | 121 |
| 7.3 | 56 | 13.3 | 122 |
| 7.4 | 57 | 13.4 | 123 |
| 7.5 | 58 | 13.5 | 124 |
| 7.6 | 60 | 13.6 | 125 |
| 7.7 | 61 | 13.7 | 126 |
| 7.8 | 62 | 13.8 | 127 |
| 7.9 | 63 | 13.9 | 128 |
| 8.0 | 64 | 14.0 | 130 |
| 8.1 | 65 | 14.1 | 131 |
| 8.2 | 66 | 14.2 | 132 |
| 8.3 | 67 | 14.3 | 133 |
| 8.4 | 68 | 14.4 | 134 |
| 8.5 | 69 | 14.5 | 135 |
| 8.6 | 70 | 14.6 | 136 |
| 8.7 | 72 | 14.7 | 137 |
| 8.8 | 73 | 14.8 | 138 |
| 8.9 | 74 | 14.9 | 139 |
| 9.0 | 75 |
| mg/dl to mmol/l | mmol/l to mg/dl | ||
|---|---|---|---|
| mg/dl | mmol/l | mmol/l | mg/dl |
| 40 | 2.2 | 2.0 | 36 |
| 45 | 2.5 | 2.5 | 45 |
| 50 | 2.8 | 3.0 | 54 |
| 55 | 3.1 | 3.5 | 63 |
| 60 | 3.3 | 4.0 | 72 |
| 65 | 3.6 | 4.5 | 81 |
| 70 | 3.9 | 5.0 | 90 |
| 75 | 4.2 | 5.5 | 99 |
| 80 | 4.4 | 6.0 | 108 |
| 85 | 4.7 | 6.5 | 117 |
| 90 | 5.0 | 7.0 | 126 |
| 95 | 5.3 | 7.5 | 135 |
| 100 | 5.6 | 8.0 | 144 |
| 110 | 6.2 | 8.5 | 153 |
| 120 | 6.7 | 9.0 | 162 |
| 130 | 7.2 | 9.5 | 171 |
| 140 | 7.8 | 10.0 | 180 |
| 150 | 8.3 | 10.5 | 189 |
| 160 | 8.9 | 11.0 | 198 |
| 170 | 9.4 | 11.5 | 207 |
| 180 | 10.0 | 12.0 | 216 |
| 190 | 10.6 | 12.5 | 225 |
| 200 | 11.1 | 13.0 | 234 |
| 220 | 12.2 | 13.5 | 243 |
| 240 | 13.3 | 14.0 | 252 |
| 260 | 14.4 | 14.5 | 261 |
| 280 | 15.5 | 15.0 | 270 |
| 300 | 16.7 | 16.0 | 288 |
| 320 | 17.8 | 17.0 | 306 |
| 340 | 18.9 | 18.0 | 324 |
| 360 | 20.0 | 19.0 | 342 |
| 380 | 21.1 | 20.0 | 360 |
| 400 | 22.2 | 21.0 | 378 |
| 420 | 23.3 | 22.0 | 396 |
| 440 | 24.4 | 23.0 | 414 |
| 460 | 25.5 | 24.0 | 432 |
Appendix 6 Detailed description of the IMS core diabetes model
The IMS CDM is a multilayer internet application linked to a mathematical calculation model and structured query language (SQL) database sited on a central server. Online access to the IMS CDM software is available under license from IMS, the developers of the model. The structure is based on four separate elements: the user interface, the input databases, the data processor and the output databases. Figure 24 outlines the overview of the IMS CDM software structure.
FIGURE 24.
IMS CDM software model structure.
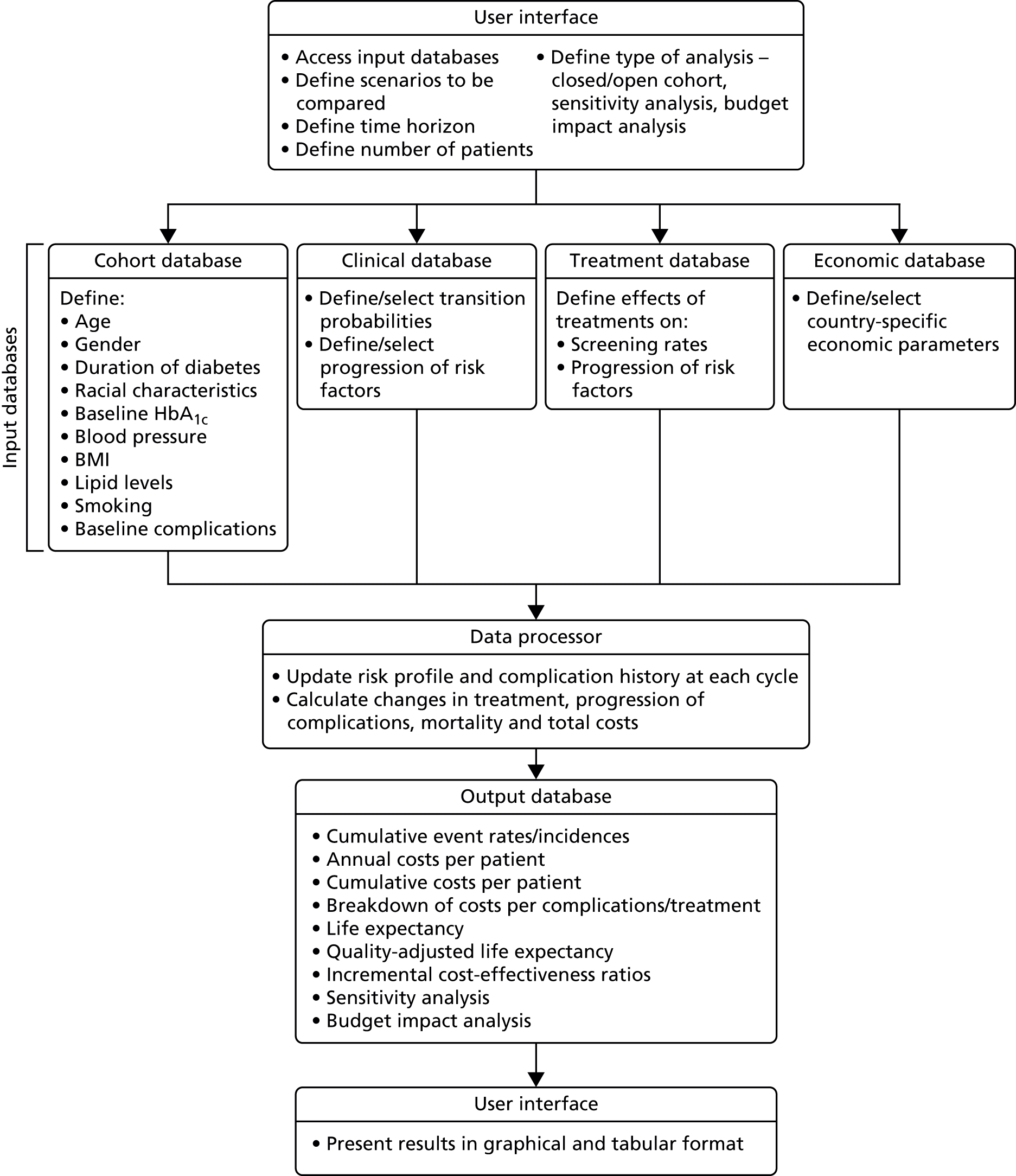
Complication submodels
The myocardial infarction submodel
The MI submodel is made up of three states: no history of MI, history of MI and death following MI. Transition probabilities between the states can be taken from the UK Prospective Diabetes Study (UKPDS) risk engine,98 Framingham93 or the UKPDS outcomes model. 91 In our calculations, Framingham92 was chosen as it is the only one that is based on T1DM only.
Unstable angina submodel
The unstable angina submodel is made up of two states: no history of angina and history of angina. Transition probabilities between the states are derived from Framingham. 93 They are adjusted according to HbA1c levels and renal function.
Congestive heart failure submodel
The CHF submodel is composed of three states: no CHF, history of CHF and death following CHF. A logistic regression based on Framingham95 generates the risk profile and includes the following risk factors: age, sex, left ventricular hypertrophy, heart rate, SBP, congenital heart disease, valve disease, presence of diabetes, BMI, presence of diabetes and valve disease jointly.
Stroke submodel
The stroke submodel is composed of three states: no stroke, history of stroke and death following stroke. Transition probabilities between the states can be taken from the UKPDS risk engine,96 Framingham153 or the UKPDS outcomes model. 91 In our calculations, Framingham was chosen as it is the only one that is based on T1DM only.
Peripheral vascular disease submodel
The PVD submodel is made up of two states: no PVD and PVD. Transition probabilities are the same as T1DM and T2DM. A logistic regression based on Framingham97 is used to generate the risk for PVD, including the following risk factors: age, sex, blood pressure (normal–high), stage 1 hypertension (yes/no), stage 2 hypertension (yes/no), presence of diabetes, number of cigarettes per day, cholesterol level and heart failure history.
Neuropathy submodel
The neuropathy submodel is made up of two states: no neuropathy and neuropathy. Transition probabilities for T1DM are derived from DCCT. 92 Transition probabilities are indexed by diabetes duration and are adjusted for HbA1c levels, SBP and angiotensin-converting enzyme inhibitor (ACEI) use.
Foot ulcer/amputation submodel
This submodel consists of nine states: (1) no foot ulcer; (2) uninfected ulcer; (3) infected ulcer; (4) healed ulcer; (5) uninfected recurrent ulcer; (6) infected recurrent ulcer; (7) gangrene; (8) history of amputation; and (9) death resulting from foot ulcer. Transition probabilities are the same for T1DM and T2DM. Unlike other submodels, this submodel runs in monthly cycles. Therefore, patients may have multiple foot ulcers in a single year.
Diabetic retinopathy submodel
This submodel is composed of 10 states: (1) no retinopathy and not screened; (2) no retinopathy and screened; (3) background diabetic retinopathy (BDR) and not screened; (4) BDR and screened; (5) BDR and wrongly diagnosed as proliferative; (6) diabetic retinopathy and laser (retinal photocoagulation) treated; (7) proliferative diabetic retinopathy (PDR), not screened and no laser treatment; (8) PDR, screened, detected and laser treated; (9) PDR, screened and not detected; and (10) severe vision loss.
Severe vision loss is a terminal state. Transition probabilities for T1DM are derived from DCCT,92 and are adjusted for HbA1c levels, SBP and ACEI use.
Macular oedema submodel
The macular oedema submodel consists of six states: (1) no macular oedema and not screened; (2) no macular oedema and screened; (3) macular oedema, not screened and no laser treatment; (4) macular oedema, screened and not detected; (5) macular oedema, screened, detected and laser treated; and (6) severe vision loss.
Severe vision loss is a terminal state. Transition probabilities for T1DM are derived from DCCT,92 and are adjusted for HbA1c levels, SBP and ACEI use.
Cataract submodel
The cataract submodel is composed of three states: no cataract, first cataract with operation and second cataract with operation. Transition probabilities are the same for T1DM and T2DM and are taken from a study in diabetes outpatients in the UK published by Janghorbani et al. 154
Nephropathy submodel
This submodel is composed of 13 states: (1) no renal complications and no treatment with ACEI; (2) no renal complications and treated with ACEI; (3) no renal complications after ACEI side effects; (4) microalbuminuira and no treatment with ACEI; (5) microalbuminuira, screened, detected and treated with ACEI; (6) microalbuminuira after ACEI side effects; (7) gross proteinuria and no treatment with ACEI; (8) gross proteinuria, screened, detected and treated with ACEI; (9) gross proteinuria after ACEI side effects; (10) end-stage renal disease, treated with haemodialysis; (11) end-stage renal disease, treated with peritoneal dialysis; (12) end-stage renal disease, treated with renal transplant; and (13) end-stage renal disease death.
Data on the cumulative incidence of progression of microalbuminuria and gross proteinuria were taken from the DCCT,92 probabilities for the progression from gross proteinuria to end-stage renal disease are based on cumulative incidence data for T2DM patients in the Rochester population. 155 It is assumed that the probability of progression from gross proteinuria to end-stage renal disease is the same for T1DM and T2DM. The probability of progression from end-stage renal disease states to death is dependent on treatment and ethnic group (Wolfe et al. 156). Transition probabilities are adjusted according to patient HbA1c levels, SBP and concomitant ACEI treatment
Hypoglycaemia submodel
The hypoglycaemia submodel is a state in which the minor and severe hypoglycaemic episodes are counted. Minor hypoglycaemic events are calculated on a daily basis (cycle length = 1 day). For the simulation of severe hypoglycaemic events, the submodel runs four times for each year of simulation. All rates (defined as number of events per 100 patient-years) are adjusted to the 1-day or 3-month cycle length. Therefore, patients can have multiple hypoglycaemic episodes in a single year. The patients may die after a severe hypoglycaemic episode. The definition of severe and minor hypoglycaemia can be refined by the user according to the available data. In our analysis, hypoglycaemic episode rates are treatment specific and any hypoglycaemic episode that required assistance from a third party is considered as severe. It should be noted that in our base-case analysis the probability of death as a result of a severe hypoglycaemic episode was assumed to be zero.
Ketoacidosis submodel
The ketoacidosis submodel has two states: alive and dead (as a result of ketoacidosis). There are no probability adjustments in the ketoacidosis submodel.
Depression submodel
The depression submodel has three states: no depression, depression receiving antidepression programme and depression not receiving antidepression programme. The onset probability of depression is the same for T1DM and T2DM, and is dependent on gender.
Lactic acidosis submodel
This submodel is relevant for T2DM only.
Peripheral oedema submodel
This submodel is relevant for T2DM only.
Non-specific mortality submodel
This submodel consists of two states: alive or dead. The transition probabilities are indexed by age, sex and ethnicity, and reflect the UK life tables. 94
Appendix 7 Results (full incremental and intervention vs. comparator) of base-case and scenario analyses
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.4146 | 61,050 | – | – | – |
| CSII + SMBG | 11.9756 | 90,436 | 0.561 | 29,386 | 52,381 |
| MiniMed Veo system | 12.0412 | 138,357 | Extendedly dominateda by stand-alone CSII + CGM | ||
| Stand-alone CSII + CGM | 12.0604 | 146,476 | 0.0849 | 56,039 | 660,376 |
| Integrated CSII + CGM (Vibe) | 12.0604 | 147,150 | Dominated by stand-alone CSII + CGM | ||
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.6266 | 77,307 | 123,375 |
| MiniMed Veo system | CSII + SMBG | 0.0656 | 47,921 | 730,501 |
| MiniMed Veo system | Stand-alone CSII + CGM | –0.0192 | –8119 | 422,849 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.6458 | 86,100 | 133,323 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.0849 | 56,713 | 668,789 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0 | 674 | Undefined |
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 12.1450 | 62,927 | – | – | – |
| CSII + SMBG | 12.7258 | 93,433 | 0.5808 | 30,506 | 52,524 |
| MiniMed Veo system | 12.8087 | 143,309 | 0.0829 | 49,876 | 601,641 |
| Stand-alone CSII + CGM | 12.8223 | 151,671 | 0.0136 | 8363 | 614,910 |
| Integrated CSII + CGM (Vibe) |
12.8223 | 152,372 | Dominated by stand-alone CSII+ CGM | ||
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.6637 | 80,382 | 121,112 |
| MiniMed Veo system | CSII + SMBG | 0.0829 | 49,876 | 601,639 |
| MiniMed Veo system | Stand-alone CSII + CGM | –0.0136 | –8363 | 614,910 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.6773 | 89,445 | 132,061 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.0965 | 58,939 | 610,772 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0 | 701 | Undefined |
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 9.6117 | 65,070 | – | – | – |
| CSII + SMBG | 10.0991 | 91,189 | 0.4874 | 26,119 | 53,588 |
| MiniMed Veo system | 10.1474 | 132,149 | Extendedly dominated by stand-alone CSII + CGM | ||
| Stand-alone CSII + CGM | 10.164 | 139,157 | 0.0649 | 47,967 | 738,593 |
| Integrated CSII + CGM (Vibe) | 10.164 | 139,733 | Dominated by stand-alone CSII + CGM | ||
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.5357 | 67,079 | 125,217 |
| MiniMed Veo system | CSII + SMBG | 0.0483 | 40,960 | 848,028 |
| MiniMed Veo system | Stand-alone CSII + CGM | –0.0166 | –7008 | 422,148 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.5523 | 74,663 | 135,186 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.0649 | 48,543 | 747,971 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0 | 576 | Undefined |
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.4146 | 68,460 | – | – | – |
| CSII + SMBG | 11.9756 | 98,034 | 0.561 | 29,574 | 52,717 |
| MiniMed Veo system | 12.0412 | 138,357 | Extendedly dominated by stand-alone CSII + CGM | ||
| Stand-alone CSII + CGM | 12.0604 | 146,476 | 0.0849 | 48,441 | 570,844 |
| Integrated CSII + CGM (Vibe) | 12.0604 | 147,150 | Dominated by stand-alone CSII + CGM | ||
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.6266 | 69,897 | 111,550 |
| MiniMed Veo system | CSII + SMBG | 0.0656 | 40,323 | 614,683 |
| MiniMed Veo system | Stand-alone CSII + CGM | –0.0192 | –8119 | 422,849 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.6458 | 78,690 | 121,849 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.0849 | 49,116 | 579,194 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0 | 674 | Undefined |
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.4146 | 62,114 | – | – | – |
| CSII + SMBG | 11.9756 | 90,437 | 0.5610 | 28,323 | 50,487 |
| MiniMed Veo system | 12.0412 | 138,358 | Extendedly dominated by stand-alone CSII + CGM | ||
| Stand-alone CSII + CGM | 12.0604 | 146,476 | 0.0849 | 56,040 | 660,376 |
| Integrated CSII + CGM (Vibe) | 12.0604 | 147,150 | Dominated by stand-alone CSII + CGM | ||
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.6266 | 76,244 | 121,679 |
| MiniMed Veo system | CSII + SMBG | 0.0656 | 47,921 | 730,501 |
| MiniMed Veo system | Stand-alone CSII + CGM | –0.0192 | –8119 | 422,849 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.6458 | 85,036 | 131,675 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.0848 | 56,713 | 668,789 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0 | 674 | Undefined |
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.8715 | 58,520 | – | – | – |
| CSII + SMBG | 12.4558 | 88,663 | 0.5843 | 30,143 | 51,615 |
| MiniMed Veo system | 12.5228 | 137,739 | Extendedly dominated by stand-alone CSII + CGM | ||
| Stand-alone CSII + CGM | 12.5398 | 146,076 | 0.0840 | 57,414 | 683,889 |
| Integrated CSII + CGM (Vibe) | 12.5398 | 146,767 | Dominated by stand-alone CSII + CGM | ||
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.6513 | 79,219 | 121,632 |
| MiniMed Veo system | CSII + SMBG | 0.067 | 49,076 | 732,483 |
| MiniMed Veo system | Stand-alone CSII + CGM | –0.017 | –8337 | 490,424 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.6683 | 88,247 | 132,047 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.084 | 58,104 | 691,715 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0 | 690 | Undefined |
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| Stand-alone CSII + CGM | 12.0006 | 146,632 | Dominated by MDI + SMBG | ||
| Integrated CSII + CGM (Vibe) | 12.0006 | 147,304 | Dominated by MDI + SMBG | ||
| MDI + SMBG | 12.0016 | 56,928 | – | – | – |
| CSII + SMBG | 12.016 | 90,178 | 0.0144 | 33,250 | 2,309,028 |
| MiniMed Veo system | 12.026 | 138,538 | 0.0099 | 48,360 | 4,871,356 |
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.0244 | 81,610 | 3,344,672 |
| MiniMed Veo system | CSII + SMBG | 0.0099 | 48,360 | 4,871,356 |
| MiniMed Veo system | Stand-alone CSII + CGM | 0.0254 | –8093 | –318,634 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | –0.0009 | 90,376 | –100,417,778 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | –0.0154 | 57,126 | –3,709,460 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0 | 672 | Undefined |
| Hypo MiniMed Veo system RR | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.412 | 60,812 | – | – | – |
| CSII + SMBG | 11.9597 | 91,195 | 0.5477 | 30,383 | 55,474 |
| MiniMed Veo system | 12.0453 | 138,333 | Extendedly dominated by stand-alone CSII + CGM | ||
| Stand-alone CSII + CGM | 12.0604 | 146,476 | 0.1007 | 55,281 | 549,080 |
| Integrated CSII + CGM (Vibe) | 12.0604 | 147,150 | Dominated by stand-alone CSII + CGM | ||
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.6333 | 77,521 | 122,408 |
| MiniMed Veo system | CSII + SMBG | 0.0856 | 47,138 | 550,675 |
| MiniMed Veo system | Stand-alone CSII + CGM | –0.0151 | –8143 | 539,295 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.6484 | 86,338 | 133,155 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.1007 | 55,955 | 555,659 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0 | 674 | Undefined |
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.1041 | 58,510 | – | – | – |
| Stand-alone CSII + CGM | 11.7701 | 142,215 | Dominated by CSII + SMBG | ||
| Integrated CSII + CGM (Vibe) | 11.7701 | 142,872 | Dominated by CSII + SMBG | ||
| CSII + SMBG | 11.8781 | 89,475 | 0.774 | 30,965 | 40,006 |
| MiniMed Veo system | 12.0071 | 137,801 | 0.129 | 8326 | 374,531 |
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.9029 | 79,291 | 87,818 |
| MiniMed Veo system | CSII + SMBG | 0.1290 | 48,327 | 374,626 |
| MiniMed Veo system | Stand-alone CSII + CGM | 0.2369 | –4413 | –18,622 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.6659 | 84,362 | 126,689 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | –0.1079 | 53,397 | –494,418 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0 | 657 | Undefined |
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 12.1327 | 61,050 | |||
| CSII + SMBG | 12.5861 | 90,436 | 0.4534 | 29,386 | 64,813 |
| MiniMed Veo system | 12.6408 | 138,357 | 0.0546 | 47,920 | 876,987 |
| Stand-alone CSII + CGM | 12.6462 | 146,476 | 0.0601 | 56,039 | 932,305 |
| Integrated CSII + CGM (Vibe) | 12.6462 | 147,150 | Dominated by stand-alone CSII + CGM | ||
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.5081 | 77,307 | 152,149 |
| MiniMed Veo system | CSII + SMBG | 0.0547 | 47,921 | 876,067 |
| MiniMed Veo system | Stand-alone CSII + CGM | –0.0054 | –8119 | 1,503,465 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.5135 | 86,100 | 167,673 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.0601 | 56,713 | 943,649 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0 | 674 | Undefined |
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 2.7718 | 6706 | – | – | – |
| Stand-alone CSII + CGM | 2.7882 | 24,803 | Dominated by CSII + SMBG | ||
| Integrated CSII + CGM (Vibe) | 2.7886 | 24,939 | Dominated by CSII + SMBG | ||
| CSII + SMBG | 2.7906 | 13,365 | 0.0188 | 6659 | 354,202 |
| MiniMed Veo system | 2.7928 | 23,144 | 0.0022 | 9778 | 4,461,063 |
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.0210 | 16,438 | 782,762 |
| MiniMed Veo system | CSII + SMBG | 0.0022 | 9779 | 4,445,000 |
| MiniMed Veo system | Stand-alone CSII + CGM | 0.0046 | –1659 | –360,652 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.0168 | 18,233 | 1,085,298 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | –0.0020 | 11,574 | –5,787,000 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0.0004 | 136 | 340,000 |
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.4146 | 61,050 | – | – | – |
| CSII + SMBG | 11.9756 | 90,436 | 0.5610 | 29,386 | 52,381 |
| Stand-alone CSII + CGM | 12.0604 | 146,476 | Extendedly dominated by MiniMed Veo system | ||
| MiniMed Veo system | 12.6224 | 138,357 | 0.6468 | 47,920 | 74,088 |
| Integrated CSII + CGM (Vibe) | 12.6429 | 147,150 | 0.0205 | 8792 | 428,595 |
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 1.2077 | 77,307 | 64,012 |
| MiniMed Veo system | CSII + SMBG | 0.6468 | 47,921 | 74,088 |
| MiniMed Veo system | Stand-alone CSII + CGM | 0.5619 | –8119 | –14,448 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 1.2282 | 86,100 | 70,103 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.6468 | 47,921 | 74,089 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0.5824 | 674 | 1157 |
| Intervention | QALYs | Cost (£) | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| MDI + SMBG | 11.4146 | 61,050 | – | – | – |
| CSII + SMBG | 11.9756 | 92,272 | 0.561 | 31,222 | 55,654 |
| MiniMed Veo system | 12.0412 | 138,357 | Extendedly dominated by Integrated CSII + CGM | ||
| Integrated CSII + CGM (Vibe) | 12.0604 | 147,150 | 0.0849 | 54,878 | 646,692 |
| Stand-alone CSII + CGM | 12.0604 | 150,063 | Dominated by integrated CSII + CGM | ||
| Intervention | Comparator | Incremental QALY | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|
| MiniMed Veo system | MDI + SMBG | 0.6266 | 77,307 | 123,375 |
| MiniMed Veo system | CSII + SMBG | 0.0656 | 46,086 | 702,530 |
| MiniMed Veo system | Stand-alone CSII + CGM | –0.0192 | –11,705 | 609,635 |
| Integrated CSII + CGM (Vibe) | MDI + SMBG | 0.6458 | 86,100 | 133,323 |
| Integrated CSII + CGM (Vibe) | CSII + SMBG | 0.0848 | 54,878 | 647,146 |
| Integrated CSII + CGM (Vibe) | Stand-alone CSII + CGM | 0 | –2913 | Undefined |
Appendix 8 Disease natural history parameters and transition probabilities
The parameters that will determine the natural course of the disease and their corresponding sources can be seen in Table 113. We considered the same values as in NICE Guideline NG17. 81
| Parameter | Mean value | Source |
|---|---|---|
| HbA1c adjustments | ||
| Risk reduction of BDR with 10% lower HbA1c | 39% | DCCT92 |
| Risk reduction of proliferative diabetic retinopathy with 10% lower HbA1c | 43% | DCCT92 |
| Risk reduction of sever vision loss with 10% lower HbA1c | 0% | No data |
| Risk reduction of macular oedema with 10% lower HbA1c | 13% | Klein et al., 2009157 |
| Risk reduction of microalbuminuria with 10% lower HbA1c | 28% | DCCT92 |
| Risk reduction of gross proteinuria with 10% lower HbA1c | 37% | DCCT92 |
| Risk reduction of end-stage renal disease with 10% lower HbA1c | 21% | Rosolowsky et al., 2011158 |
| Risk reduction of neuropathy with 10% lower HbA1c | 32% | DCCT92 |
| Risk reduction of MI with 1% lower HbA1c | 20% | DCCT92 |
| Risk reduction of cataract with 1% lower HbA1c | 0% | Grauslund et al., 2011159 |
| Risk reduction of heart failure with 1% lower HbA1c | 23% | Lind et al., 2011160 |
| Risk reduction of stroke with 1% lower HbA1c | 20% | DCCT92 |
| Risk reduction of angina with 1% lower HbA1c | 20% | DCCT92 |
| Risk reduction of haemodialysis mortality with 1% lower HbA1c | 12% | Morioka et al., 2001161 |
| Risk reduction of peritoneal dialysis mortality with 1% lower HbA1c | 12% | Morioka et al., 2001161 |
| Risk reduction of renal transplant mortality with 1% lower HbA1c | 0% | Wiesbauer et al., 2010162 |
| Risk reduction of first ulcer with 1% lower HbA1c | 17% | Monami et al., 2009163 |
| SBP adjustments | ||
| Risk reduction of microalbuminuria with 10 mmHg lower SBP | 13% | Adler et al., 2000164 |
| Risk reduction of severe vision loss with 10 mmHg lower SBP | 0% | No data |
| MI adjustments | ||
| Proportion with MI having an initial CHD event, female | 0.361 | D’Agostino et al., 200093 |
| Proportion with MI having an initial CHD event, male | 0.522 | D’Agostino et al., 200093 |
| Proportion with MI having a subsequent CHD event MI, female | 0.474 | D’Agostino et al., 200093 |
| Proportion with MI having a subsequent CHD event MI, male | 0.451 | D’Agostino et al., 200093 |
| RR of MI if microalbuminuria is present | 1 | No data |
| RR of MI if gross proteinuria is present | 1 | No data |
| RR of MI if end-stage renal disease is present | 1 | No data |
| RR of recurrent MI if DIGAMI165 intensive control is used | 1 | No data |
| RR of MI mortality if DIGAMI165 intensive control is used | 1 | No data |
| RR of MI if aspirin used for primary prevention | 0.82 | Baigent et al., 2009166 |
| RR of MI if aspirin used for secondary prevention | 0.80 | Baigent et al., 2009166 |
| RR of MI if statins used for primary prevention | 0.70 | Brugts et al., 2009167 |
| RR of MI if statins used for secondary prevention | 0.81 | Shepherd et al., 2002168 |
| RR of MI if ACEIs used for primary prevention | 0.78 | HOPE Study Investigators, 2000169 |
| RR of MI if ACEIs used for secondary prevention | 0.78 | D’Agostino et al., 200093 |
| MI mortality | ||
| Probability of sudden death after first MI, male | 0.393 | Sonke et al., 1996170 |
| Probability of sudden death after first MI, female | 0.364 | Sonke et al., 1996170 |
| Probability of sudden death after recurrent MI, male | 0.393 | Sonke et al., 1996170 |
| Probability of sudden death after recurrent MI, female | 0.364 | Sonke et al., 1996170 |
| RR of 12-month mortality after MI conventional treatment | 1.45 | Malmberg et al., 1995165 |
| RR of mortality first year after MI aspirin treatment | 0.88 | Antiplatelet Triallists’ Collaboration, 1994171 |
| RR of mortality each subsequent year after MI aspirin treatment | 0.88 | |
| RR of mortality first year after MI statin treatment | 0.75 | Stenestrand et al., 2001172 |
| RR of mortality each subsequent year after MI statin treatment | 1.00 | No data |
| RR of sudden death after MI aspirin treatment | 1.00 | No data |
| RR of sudden death after MI statin treatment | 1.00 | Briel et al., 2006173 |
| RR of sudden death after MI ACEI treatment | 1.00 | No data |
| RR of long-term mortality after MI using ACEIs | 0.64 | Gustafsson et al., 1999174 |
| RR 12-month mortality after MI using ACEIs | 0.64 | Sonke et al., 1996170 |
| Stroke | ||
| RR of stroke with microalbuminuria | 1.00 | No data |
| RR of stroke with gross proteinuria | 1.00 | No data |
| RR of stroke with end-stage renal disease | 1.00 | No data |
| RR of first stroke if aspirin used | 0.86 | Baigent et al., 2009166 |
| RR of second stroke if aspirin used | 0.78 | Baigent et al., 2009166 |
| RR of first stroke if statins used | 0.81 | Brugts et al., 2009167 |
| RR of second stroke if statins used | 0.84 | Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) investigators, 2006175 |
| RR of first stroke if ACEIs used | 0.67 | HOPE Study investigators, 2000169 |
| RR of recurrent stroke if ACEIs used | 0.72 | PROGRESS Collaborative Group, 2001176 |
| Stroke mortality | ||
| Probability of 30-day death after first stroke | 0.124 | Eriksson and Olsson, 2001177 |
| Probability of 30-day death after recurrent stroke | 0.422 | |
| RR of mortality after stroke if aspirin used | 0.84 | Antiplatelet Triallists’ Collaboration, 1994171 |
| RR of mortality if statins used | 1.00 | Manktelow and Potter, 2009178 |
| RR of sudden death after stroke if aspirin used | 0.95 | Sandercock et al., 2008179 |
| RR of sudden death after stroke if statins used | 1.00 | Briel et al., 2006173 |
| RR of sudden death after stroke if ACEIs used | 0.49 | Chitravas et al., 2007180 |
| RR of long-term mortality after stroke using ACEIs | 1.000 | Asberg et al., 2010181 |
| RR of 12-month mortality after stroke using ACEIs | 1.000 | Eriksson and Olsson, 2001177 |
| Angina adjustments | ||
| Proportion with angina having first CHD event, female | 0.621 | D’Agostino et al., 200093 |
| Proportion with angina having first CHD event, male | 0.420 | D’Agostino et al., 200093 |
| Proportion with angina having subsequent CHD event, female | 0.359 | D’Agostino et al., 200093 |
| Proportion with angina having subsequent CHD event, male | 0.301 | D’Agostino et al., 200093 |
| RR of angina with microalbuminuria | 1.00 | No data |
| RR of angina with gross proteinuria | 1.00 | No data |
| RR of angina with end-stage renal disease | 1.00 | No data |
| CHF adjustments | ||
| RR of heart failure with microalbuminuria | 1.00 | No data |
| RR of heart failure with gross proteinuria | 1.00 | No data |
| RR of heart failure with end-stage renal disease | 1.00 | No data |
| RR of heart failure if aspirin used | 1.00 | No data |
| RR of heart failure if statins used | 1.00 | No data |
| RR of heart failure if ACEIs used | 0.80 | HOPE Study Investigators, 2000169 |
| RR of heart failure death if ACEIs used | 0.80 | Ascenção et al., 2008182 |
| RR of heart failure death diabetic, male | 1.00 | Ho et al., 1993183 |
| RR of heart failure death diabetic, female | 1.70 | Ho et al., 1993183 |
| ACEI adjustments for micro-vascular complications | ||
| RR of BDR if ACEIs used | 0.75 | Chaturvedi et al., 1998184 |
| RR of proliferative diabetic retinopathy if ACEIs used | 0.19 | Chaturvedi et al., 1998184 |
| RR of macular oedema if ACEIs used | 1.00 | No data |
| RR of severe vision loss if ACEIs used | 1.00 | No data |
| RR of worsening microalbuminuria if ACEIs used, no complications | 0.79 | Penno et al., 1998185 |
| RR of worsening gross proteinuria if ACEIs used, with microalbuminuria | 0.41 | Penno et al., 1998185 |
| RR of worsening end stage renal disease if ACEIs used, with gross proteinuria | 0.63 | Lewis et al., 1993186 |
| RR of neuropathy if ACEIs used | 1.00 | No data |
| Side effects of ACEIs | ||
| Probability stopping ACEIs because of side effects first year | 0 | Assumption |
| Probability stopping ACEIs because of side effects each subsequent year | 0 | Assumption |
| Adverse events | ||
| Probability death from severe hypoglycaemic event | 0 | Assumption |
| Probability death from severe ketoacidosis event | 0.027 | MacIsaac et al., 2002187 |
| RR of hypoglycaemic events with ACEIs | 1.00 | No data |
| Foot ulcer and amputation | ||
| Probability gangrene to amputation | 0.181800 | Persson et al., 2000188 |
| Probability gangrene to healed amputation | 0.308200 | Persson et al., 2000188 |
| Probability death following onset gangrene | 0.009800 | Persson et al., 2000188 |
| Probability death with history amputation | 0.004000 | Persson et al., 2000188 |
| Probability death following healed ulcer | 0.004000 | Persson et al., 2000188 |
| Probability developing recurrent uninfected ulcer | 0.039300 | Persson et al., 2000188 |
| Probability amputation following infected ulcer | 0.003700 | Persson et al., 2000188 |
| Probability infected ulcer after amputation healed | 0.044500 | Persson et al., 2000188 |
| Probability of death from infected ulcer | 0.009800 | Persson et al., 2000188 |
| Probability of gangrene from infected ulcer | 0.007500 | Persson et al., 2000188 |
| Probability of infected ulcer from uninfected ulcer | 0.139700 | Persson et al., 2000188 |
| Probability of recurrent amputation | 0.008451 | Borkosky et al., 2012189 |
| Probability of death from uninfected ulcer | 0.004000 | Persson et al., 2000188 |
| Probability of uninfected ulcer from infected ulcer | 0.047300 | Persson et al., 2000188 |
| Probability of healed ulcer from uninfected ulcer | 0.078700 | Persson et al., 2000188 |
| Probability developing ulcer with neither neuropathy or PVD | 0.000250 | Ragnarson et al., 2001190 |
| Probability developing ulcer with either neuropathy or PVD | 0.006092 | Ragnarson et al., 2001190 |
| Probability developing ulcer with both neuropathy or PVD | 0.006092 | Persson et al., 2000188 |
| Depression | ||
| RR for all-cause death if depression | 1.33 | Egede et al., 2005191 |
| RR for CHF if depression | 1.00 | No data |
| RR for MI if depression | 1.00 | No data |
| RR for depression if neuropathy | 3.10 | Yoshida et al., 2009192 |
| RR for depression if stroke | 6.30 | Whyte et al., 2004193 |
| RR for depression if amputation | 1.00 | No data |
| Other | ||
| Probability of severe vision loss from BDR | 0.015 | CORE default, 200481 |
| Probability of reversal of neuropathy | 0.000 | No data |
Transition probabilities values were provided by the IMS CDM developers and were not changed in our analyses given the high degree of validation of the model. These were UK specific if possible and based on relevant sources (e.g. DCCT trial). 92 In Table 114 we report these sources. We do not report the complete set of probabilities as we believe this would be too extensive and not very informative because of the complexity of the model.
| Parameter | Dependent on | Source |
|---|---|---|
| Renal disease | ||
| Probability of onset of microalbuminuria | Duration of diabetes | DCCT92 |
| Probability of worsening from microalbuminuria to gross proteinuria | Duration of diabetes | DCCT92 |
| Probability of worsening from gross proteinuria to end-stage renal disease | Duration of gross proteinuria | Rosolowsky et al., 2011158 |
| Proportion of end-stage renal disease with haemodialysis, peritoneal dialysis or renal transplant | Current age | US Renal Data System (USRDS), 2010194 |
| Probability of death end-stage renal disease under haemodialysis, peritoneal dialysis or renal transplant | Current age | US Renal Data System (USRDS), 2010194 |
| Eye disease | ||
| Probability of onset BDR, proliferative diabetic retinopathy, macular oedema or severe vision loss | Duration of diabetes | DCCT92 |
| Probability of onset of cataract extraction; male, female | Current age | Janghorbani et al., 2000154 |
| Probability of recurrent cataract extraction; male, female | Current age | Janghorbani et al., 2000154 |
| Neuropathy | ||
| Probability of onset of neuropathy | Duration of diabetes | DCCT92 |
| Heart failure | ||
| Probability of heart failure long-term mortality, per gender and age range | Time since onset of heart failure | Ho et al., 1993183 |
| MI | ||
| Probability of death within 12 months of first/recurrent MI; male, female | Current age | Malmberg et al., 1995165 |
| Probability of post-MI long-term mortality; male, female | Time since first MI | Malmberg et al., 1995165 |
| Stroke | ||
| Probability of death within 12 months of first/recurrent stroke; male, female | Current age | Eriksson and Olsson, 2001177 |
| Probability of post-stroke long-term mortality; male, female | Time since first stroke | Eriksson and Olsson, 2001177 |
| Probability of recurrent stroke; male, female | Time since first stroke | Eriksson and Olsson, 2001177 |
| Depression | ||
| Probability of onset of depression; male, female | Time of simulation | Golden et al., 2008195 |
| Probability of depression reversal for patients receiving/not receiving anti-depression programme | Time of simulation | Valenstein et al., 2001196 |
| Non-specific mortality | ||
| Probability of non-specific mortality per ethnicity and gender | Current age | UK life tables94 |
| Physiological parameter progression tables | ||
| HbA1c progression | Time of simulation | DCCT92 |
| BMI, haemodialysis, LDL, SBP, T-CHOL, triglyceride progression | Time of simulation | CORE default, 200481 |
| Other adjustment factors | ||
| Quality of life adjustment based on current BMI | BMI | Bagust and Beale, 2005197 |
| Age adjustment for MI mortality | Current age | Herlitz et al., 1996198 |
Appendix 9 Guidance relevant to the treatment of type 1 diabetes
Published National Institute for Health and Care Excellence guidance
NICE Pathway. Diabetes Overview. 2013. URL: http://pathways.nice.org.uk/pathways/diabetes (accessed 15 February 2016).
NICE Pathway. Preventing Type 2 Diabetes. June 2013. URL: http://pathways.nice.org.uk/pathways/preventing-type-2-diabetes (accessed 15 February 2016).
NICE Clinical Guideline CG15. Diagnosis and Management of Type 1 Diabetes in Children, Young People and Adults. 2004. URL: www.nice.org.uk/CG15 (accessed 15 February 2016). Date for review: reviewed in August 2011 and decision was made to update the guideline. Update scheduled to be published in 2015.
NICE Clinical Guideline CG119. Diabetic Foot: Inpatient Management of People with Diabetic Foot Ulcers and Infection. 2011. URL: http://guidance.nice.org.uk/CG119 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Clinical Guideline CG66. Type 2 Diabetes: The Management of Type 2 Diabetes (update). 2008. URL: http://guidance.nice.org.uk/CG66 (accessed 15 February 2016). Date for review: following a review in 2011 an update of this guideline is currently in the process of being scheduled into the work programme.
NICE Clinical Guideline CG10. Type 2 Diabetes: Prevention and Management of Foot Problems. 2004. URL: http://guidance.nice.org.uk/CG10 (accessed 15 February 2016). Date for review: an update of this guideline is under way to coincide with publication of the four diabetes guidelines currently being updated.
NICE Clinical Guideline CG87. Type 2 Diabetes: Newer Agents (Partial Update of CG66) (CG87). 2009. URL: http://guidance.nice.org.uk/CG87 (accessed 15 February 2016). Date for review: following the recent review recommendation, an update of this guideline is in progress.
NICE Clinical Guideline. Diabetes in Pregnancy: Management of Diabetes and its Complications from Pre-conception to the Postnatal Period. 2008. URL: http://guidance.nice.org.uk/CG63 (accessed 15 February 2016). Date for review: this guideline is currently being updated. Further information can be found on the Diabetes in Pregnancy guideline in development page.
NICE Clinical Guideline CG173. Neuropathic Pain – Pharmacological Management: the Pharmacological Management of Neuropathic Pain in Adults in Non-specialist Settings. 2013. URL: http://guidance.nice.org.uk/CG173 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Clinical Guideline CG130. Hyperglycaemia in Acute Coronary Syndrome. 2011. URL: www.nice.org.uk/guidance/CG130 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Technology Appraisal Guidance TA53. The Clinical Effectiveness and Cost-effectiveness of Long Acting Insulin Analogues for Diabetes. 2002. URL: www.nice.org.uk/guidance/TA53 (accessed 15 February 2016). Date for review: the recommendations in this technology appraisal relating to type 2 diabetes have been replaced by recommendations in the Diabetes: Type 2 (update) clinical guideline published in May 2008. Please note that the recommendations in this technology appraisal relating to type 1 diabetes have not changed.
NICE Technology Appraisal Guidance TA151. Continuous Subcutaneous Insulin Infusion for the Treatment of Diabetes Mellitus (Review). 2008. URL: http://guidance.nice.org.uk/TA151 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Technology Appraisal Guidance TA301. Fluocinolone Acetonide Intravitreal Implant for Treating Chronic Diabetic Macular Oedema After an Inadequate Response to Prior Therapy (Rapid Review of Technology Appraisal Guidance 271). 2013. URL: http://guidance.nice.org.uk/TA301 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Technology Appraisal Guidance TA288. Dapagliflozin in Combination Therapy for Treating Type 2 Diabetes. 2013. URL: http://guidance.nice.org.uk/TA288 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Technology Appraisal Guidance TA274. Ranibizumab for the Treatment of Diabetic Macular Oedema (Rapid Review of TA237). 2013. URL: http://guidance.nice.org.uk/TA274 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Technology Appraisal Guidance TA248. Exenatide Prolonged-release Suspension for Injection in Combination with Oral Antidiabetic Therapy for the Treatment of Type 2 Diabetes: 2012. URL: http://guidance.nice.org.uk/TA248 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Technology Appraisal Guidance TA203. Liraglutide for the Treatment of Type 2 Diabetes Mellitus. 2010. URL: http://guidance.nice.org.uk/TA203 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Technology Appraisal Guidance TA60. The Clinical Effectiveness and Cost-effectiveness of Patient Education Models for Diabetes. 2003. URL: http://guidance.nice.org.uk/TA60 (accessed 15 February 2016). Date for review: in December 2005, following consultation, the Institute proposed that the guidance be updated as part of the reviews of the guidelines on type 1 and type 2 diabetes. The recommendations in this technology appraisal relating to type 2 diabetes have been replaced by recommendations in the Diabetes: Type 2 (update) clinical guideline published in May 2008. Please note that the recommendations in this technology appraisal relating to type 1diabetes have not changed.
NICE Technology Appraisal TA288. Dapagliflozin in Combination Therapy for Treating Type 2 Diabetes. 2013. URL: http://guidance.nice.org.uk/TA288 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Technology Appraisal TA271. Fluocinolone Acetonide Intravitreal Implant for the Treatment of Chronic Diabetic Macular Oedema After an Inadequate Response to Prior Therapy. 2013. URL: http://guidance.nice.org.uk/TA271 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Interventional Procedure IPG257. Allogenic Pancreatic Islet Cell Transplantation for Type 1 Diabetes Mellitus. 2008. URL: http://guidance.nice.org.uk/IPG257 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Interventional Procedure IPG274. Autologous Pancreatic Islet Cell Transplantation for Improved Glycaemic Control After Pancreatectomy. 2008. URL: http://guidance.nice.org.uk/IPG274 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Interventional Procedure IPG316. Extracorporeal Albumin Dialysis for Acute Liver Failure. 2009. URL: http://guidance.nice.org.uk/IPG316 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Public Health Guidance PH38. Preventing Type 2 Diabetes: Risk Identification and Interventions for Individuals at High Risk. 2012. URL: http://guidance.nice.org.uk/PH38 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Public Health Guidance PH35. Preventing Type 2 Diabetes: Population and Community-level Interventions in High-risk Groups and the General Population. 2011. URL: www.nice.org.uk/guidance/PH35 (accessed 15 February 2016). Date for review: May 2014.
NICE Evidence Summaries: New Medicines ESNM20. Type 2 Diabetes: Alogliptin. 2013. URL: http://publications.nice.org.uk/esnm20-type-2-diabetes-alogliptin-esnm20 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Evidence Summaries: New Medicines ESNM26. Type 2 Diabetes: Lixisenatide. 2013 URL: http://publications.nice.org.uk/esnm26type-2-diabetes-lixisenatide-esnm26 (accessed 15 February 2016). Date for review: to be confirmed.
NICE Evidence Summaries: New Medicines ESNM5. Type 1 Diabetes: Insulin Degludec. 2012. URL: www.nice.org.uk/mpc/evidencesummariesnewmedicines/ESNM5.jsp (accessed 15 February 2016). Date for review: to be confirmed.
NICE Evidence Summaries: New Medicines ESNM4. Type 2 Diabetes: Insulin Degludec. 2012. URL: www.nice.org.uk/mpc/evidencesummariesnewmedicines/ESNM4.jsp (accessed 15 February 2016). Date for review: to be confirmed.
NICE Quality Standard QS6. Diabetes in Adults. 2011. URL: http://guidance.nice.org.uk/QS6. In a statement dated August 2015, NICE explains that this quality standard was updated to make sure it was aligned with new NICE guidance (NG17 and NG19) for diabetes and diabetic foot problems, which superseded some previous development sources for the quality standard.
National Institute for Health and Care Excellence guidance under development
Diabetes in children and young people (update); NICE clinical guideline (publication expected August 2015).
Type 1 Diabetes (update); NICE clinical guideline (publication expected August 2015).
Type 2 Diabetes (update); NICE clinical guideline (publication expected August 2015).
Diabetes in Pregnancy (update); NICE clinical guideline (publication expected February 2015).
Diabetic Foot Problems (update); NICE clinical guideline (publication expected June 2015).
National Institute for Health and Care Excellence pathways
The guidance Type 1 Diabetes: Integrated Sensor-augmented Pump Therapy Systems for Managing Blood Glucose Levels (the MiniMed Paradigm Veo System and the Vibe and G4 PLATINUM CGM system) will be included in the NICE diabetes pathway.
Relevant guidance from other organisations
Scottish Intercollegiate Guidelines Network guideline 116. Management of Diabetes. 2010. URL: www.sign.ac.uk/guidelines/fulltext/116/ (accessed 15 February 2016).
Diabetes UK. The Hospital Management of Hypoglycaemia in Adults with Diabetes Mellitus. 2010. URL: www.diabetologists-abcd.org.uk/JBDS/JBDS_IP_Hypo_Adults.pdf (accessed 15 February 2016).
Diabetes UK. State of the Nation: England 2013. 2013. URL: www.diabetes.org.uk/Documents/About%20Us/What%20we%20say/0160b-state-nation-2013-england-1213.pdf (accessed 15 February 2016).
Diabetes UK. Use of Analogue Insulins. 2012. URL: www.diabetes.org.uk/Documents/Position%20statements/Analogue-insulin-pos-statement.2012.pdf (accessed 15 February 2016).
Diabetes UK. End of Life Diabetes Care. 2013. URL: www.diabetes.org.uk/upload/Position%20statements/End-of-life-care-Clinical-recs111113.pdf (accessed 15 February 2016).
Diabetes UK. Recommendations for the Provision of Services in Primary Care for People with Diabetes. 2005. URL: www.diabetes.org.uk/documents/professionals/primary_recs.pdf (accessed 15 February 2016).
Joint Royal Colleges Ambulance Liaison Committee. Glycaemic Emergencies in Children. 2006. URL: www.swast.nhs.uk/Downloads/SWASFT%20campaigns/clinical_guidelines_2006.pdf (accessed 15 February 2016). (See Part 3 – Paediatric Guidelines; Section 1: Emergencies in Children – Glycaemic emergencies in children.)
National Metabolic Biochemistry Network. Guidelines for the Investigation of Hypoglycaemia in Infants and Children. 2012. URL: www.metbio.net/docs/MetBio-Guideline-GARU968012-23-01-2012.pdf (accessed 15 February 2016).
British Inherited Metabolic Diseases Group. Recurrent Hypoglycaemia. 2013. URL: www.bimdg.org.uk/store/guidelines/Hypoglycaemiav1-2-461185-22-05-2013.pdf (accessed 15 February 2016).
British Inherited Metabolic Diseases Group. Ketotic Hypoglycaemia. 2008. URL: www.bimdg.org.uk/store/guidelines/ER-KH-v3_616477_18032015.pdf (accessed 15 February 2016).
British Inherited Metabolic Diseases Group. Management of Surgery in Children at Risk of Hypoglycaemia. 2013. URL: www.bimdg.org.uk/store/guidelines/Management-of-surgery-in-those-at-risk-of-hypoglycaemiav4-755756-22-05-2013.pdf (accessed 15 February 2016).
Joint Royal Colleges Ambulance Liaison Committee. Glycaemic Emergencies in Adults. 2006. URL: www.swast.nhs.uk/Downloads/SWASFT%20campaigns/clinical_guidelines_2006.pdf (accessed 15 February 2016). (See Part 2 – Adult Guidelines, Section 3: Specific Treatment Options, Glycaemic emergencies in adults.)
Driver and Vehicle Licensing Agency. DVLA’s Current Medical Guidelines for Professionals – Conditions D to F. 2013. URL: www.gov.uk/guidance/current-medical-guidelines-dvla-guidance-for-professionals-conditions-d-to-f (accessed 15 February 2016).
Driver and Vehicle Licensing Agency. DVLA’s Current Medical Guidelines for Professionals – Conditions G to I. 2013. URL: www.gov.uk/guidance/current-medical-guidelines-dvla-guidance-for-professionals-conditions-g-to-i (accessed 15 February 2016).
Royal College of Nursing. Children and Young People with Diabetes: RCN Guidance for Newly-appointed Nurse Specialists. 2013. URL: www2.rcn.org.uk/__data/assets/pdf_file/0009/78633/002474.pdf (accessed 15 February 2016).
Royal College of Nursing. Supporting Children and Young People with Diabetes. 2013. URL: www2.rcn.org.uk/__data/assets/pdf_file/0008/267389/003_318.pdf (accessed 15 February 2016).
Royal College of Nursing. Specialist Nursing Services for Children and Young People with Diabetes. 2006. URL: www2.rcn.org.uk/__data/assets/pdf_file/0009/78687/003015.pdf (accessed 15 February 2016).
Royal College of Nursing. Starting Injectable Treatment in Adults with Type 2 Diabetes. 2012. URL: www2.rcn.org.uk/__data/assets/pdf_file/0009/78606/002254.pdf (accessed 15 February 2016).
Glossary
- Cost-effectiveness analysis
- An economic analysis that converts effects into health benefits and describes the costs for additional health gains.
- Decision modelling
- A mathematical construct that allows the comparison of the relationship between costs and outcomes for alternative health-care interventions.
- Diabetic ketoacidosis
- Occurs when the body is unable to use blood glucose because of inadequate insulin. Instead, fat is broken down as an alternative source of fuel; this process leads to the build-up of by-products called ketones.
- False negative
- Incorrect negative test result (e.g. the number of diseased persons with a negative test result).
- False positive
- Incorrect positive test result (e.g. the number of non-diseased persons with a positive test result).
- Glycated haemoglobin test
- The glycated haemoglobin test measures diabetes management over 2–3 months.
- Hyperglycaemic and hypoglycaemic area under the curve
- The area under the curve is the product of the magnitude and duration of the sensor-measured glucose level above or below a specified cut-off level. Higher values for this calculation indicate more numerous, severe or protracted glycaemic events.
- Hypocalcaemia
- Low blood calcium level.
- Hypomagnesaemia
- Low levels of magnesium in the blood.
- Impaired awareness of hypoglycaemia
- When people with diabetes, usually type 1 diabetes, are frequently unable to notice when they have low blood sugar.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test whose performance is being evaluated.
- Integrated CSII + CGM
- An integrated continuous glucose monitoring and insulin pump system intended to aid the effective management of diabetes, without a low glucose suspend function.
- Ketonaemia
- The presence of an abnormally high concentration of ketone bodies in the blood.
- Ketonuria
- The presence of abnormally high amounts of ketones and ketone bodies (by-products of the breakdown of cells) in the urine. Ketonuria is a sign seen in badly controlled diabetes.
- Low glucose suspend function
- Stops insulin delivery for 2 hours if there is no response to a low glucose warning.
- Markov model
- An analytical method particularly suited to modelling repeated events or the progression of a chronic disease over time.
- Meta-analysis
- Statistical techniques used to combine the results of two or more studies and obtain a combined estimate of effect.
- Meta-regression
- Statistical technique used to explore the relationship between study characteristics and study results.
- MiniMed® Paradigm™ Veo System (Medtronic Inc., Northridge, CA, USA)
- An integrated continuous glucose monitoring and insulin pump system intended to aid the effective management of diabetes, with added insulin suspend function intended to prevent hypoglycaemia, including nocturnal hypoglycaemia.
- Opportunity costs
- The costs of forgone outcomes that could have been achieved through alternative investments.
- Polycythaemia
- An abnormally increased concentration of haemoglobin in the blood, as a result of either a reduction in plasma volume or an increase in red blood cell numbers.
- Publication bias
- Bias arising from the preferential publication of studies with statistically significant results.
- Quality-adjusted life-year
- A measure of health gain, used in economic evaluations, in which survival duration is weighted or adjusted by a patient’s quality of life during the survival period.
- Quality of life
- An individual’s emotional, social and physical well-being, and their ability to perform the ordinary tasks of living.
- Receiver operating characteristic curve
- A graph which illustrates the trade-offs between sensitivity and specificity which result from varying the diagnostic threshold.
- Reference standard
- The best currently available diagnostic test, against which the index test is compared.
- Retinopathy
- Diabetic retinopathy is a common complication of diabetes. It occurs when high blood sugar levels damage the cells at the back of the eye (known as the retina). If it is not treated, it can cause blindness.
- Sensitivity
- Proportion of people with the target disorder who have a positive test result.
- Specificity
- Proportion of people without the target disorder who have a negative test result.
- True negative
- Correct negative test result (i.e. the number of non-diseased persons with a negative test result).
- True positive
- Correct positive test result (i.e. the number of diseased persons with a positive test result).
- Type 1 diabetes mellitus
- A condition in which the body does not produce insulin.
- Vibe™ (Animas® Corporation, West Chester, PA, USA) and Dexcom G4® PLATINUM (Dexcom Inc., San Diego, CA, USA) system
- An integrated continuous glucose monitoring and insulin pump system intended to aid the effective management of diabetes, without a low glucose suspend function.
List of abbreviations
- AUC
- area under the curve
- BG
- blood glucose
- BMI
- body mass index
- CEAC
- cost-effectiveness acceptability curve
- CGM
- continuous glucose monitoring
- CHF
- congestive heart failure
- CI
- confidence interval
- CSII
- continuous subcutaneous insulin infusion
- CSII + CGM
- non-integrated, stand-alone continuous subcutaneous insulin infusion and continuous glucose monitoring
- CSII + SMBG
- continuous subcutaneous insulin infusion with self-monitoring of blood glucose by capillary blood testing
- CVD
- cardiovascular disease
- DCCT
- Diabetes Control and Complications Trial
- DKA
- diabetic ketoacidosis
- EQ-5D
- European Quality of Life-5 Dimensions
- HbA1c
- glycated haemoglobin
- HCHS
- Hospital and Community Health Services
- HFS
- Hypoglycaemia Fear Survey
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IMS CDM
- IMS Centre for Outcomes Research and Effectiveness diabetes model (IMS Health, Danbury, CT, USA)
- LGS
- low glucose suspend
- MD
- mean difference
- MDI
- multiple daily insulin injection
- MDI + CGM
- multiple daily insulin injections with continuous monitoring of blood glucose
- MDI + SMBG
- multiple daily insulin injections with self-monitoring of blood glucose by capillary blood testing
- MI
- myocardial infarction
- NICE
- National Institute for Health and Care Excellence
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- PVD
- peripheral vascular disease
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SAP
- sensor-augmented insulin pump
- SAPT
- sensor-augmented pump therapy
- SBP
- systolic blood pressure
- SD
- standard deviation
- SMBG
- self-monitoring of blood glucose
- T1DM
- type 1 diabetes mellitus
- T2DM
- type 2 diabetes mellitus
- WMD
- weighted mean difference