Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/118/03. The contractual start date was in June 2012. The draft report began editorial review in July 2015 and was accepted for publication in November 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Professor Christopher C Butler received fees for acting in an advisory capacity to Alere™ and is supporting a study on which he is the chief investigator with diagnostic devices in the form of an unconditional educational grant. He is also a National Institute for Health Research (NIHR) Efficacy and Mechanism Evaluation board member. Professor Kerenza Hood is a member of the NIHR Clinical Trials Unit standing committee. Dr Mandy Wootton has declared that she received a speakers honoraria from Nordic Pharma Ltd (who manufacture fosfomycin).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Francis et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Eczema is one of the most common disorders of childhood. 1,2 It affects > 20% of children in most developed countries,3 and up to 35% of children in the UK. 4 The prevalence appears to be increasing,4 particularly in the developing world. 2 Of those affected, 45% develop symptoms within the first 6 months of life and 85% by the age of 5 years. 5 The annual treatment cost for children in the UK with eczema aged 1–5 years was £47M in 1995–6. 6
Although not always recognised by health-care professionals as a serious medical condition,7 eczema has a significant impact on the quality of a child’s life and that of their family; the more severe the eczema, the greater the effect. 8 For the child, eczema can adversely influence their emotional and social development9 and may predispose them to psychological difficulties. 10 One study found that eczema resulted in a greater impairment of quality of life than other skin conditions, including urticaria and acne, and that generalised eczema resulted in greater impairment in quality of life than renal disease, cystic fibrosis, asthma, epilepsy and diabetes. 11
The predominant symptom of itching causes sleep disturbance in over 60% of children with eczema,12 and children with eczema have more sleep problems, a lower quality of life, and higher levels of attention deficit hyperactivity disorder and oppositional behaviour than children who do not have eczema. 13 Preliminary evidence suggests that the disease is associated with long-term behavioural and neurocognitive deficits, and that disturbed sleep may contribute to this. 14 Disturbed sleep also has an impact on other members of the family, and parents of children with eczema report stress and social isolation. 5 The application of eczema treatments can result in conflict between parents and their children, and this can also affect family relationships and drain the carers’ physical and emotional resources. 15,16 In addition, there are significant burdens for families of children suffering with eczema. For example, parents report having to take time off work and financial loss as a result of caring for their child. 6
Infected eczema
Eczema is a relapsing–remitting condition, yet there is considerable uncertainty about the cause of flares. 17–19 Eczema often results in skin that is dry, red, itchy, broken and sore, and can lead to a breakdown of the skin barrier. This makes the skin susceptible to trigger factors such as irritants and allergens, as well as microbial colonisation and infections. 20
Staphylococcus aureus has long been known to be more prevalent on the skin of patients with eczema. The organism can be isolated from up to 90% of patients with eczema21,22 compared with between 5% and 25% of healthy subjects. 21,23 Furthermore, more severe eczema is associated with higher densities of the organisms21,23,24 and more resistant strains. 25
There is evidence that a number of factors contribute to this propensity for colonisation and infection, including dysregulation of the adaptive immune response, reduced antimicrobial peptide levels, diminished recruitment of cells to the skin, toll-like receptor defects and epidermal barrier abnormalities. 26 The exact role of S. aureus in the maintenance or exacerbation of eczema is not clear. However, there is increasing evidence for the role of toxins with superantigenic properties (superantigens). 26
Use of antimicrobial treatments for eczema
Despite clear evidence for a relationship between eczema and the presence of S. aureus, there is a lack of clarity about what constitutes infection and when antibiotic treatments are likely to confer benefit. 27 A recently updated Cochrane review28 of antimicrobial interventions for people with eczema, which included 26 studies and 1229 participants, found that most studies were small and of poor quality, and that although there was evidence that interventions reduced the presence of S. aureus on the skin, none of the studies showed any meaningful clinical benefit from antibiotics or other antimicrobial interventions, for either clinically infected or non-infected eczema. The authors concluded that ‘Their continued use should be questioned in such situations, until better and longer-term studies show clear evidence of clinical benefit’. 28
This is important not only because of the need to identify effective treatments for children with eczema, but also to reduce the use of ineffective treatments currently being prescribed. Clinical experts estimate that children in the UK experience approximately 900,000 eczema flares a year, and that approximately 40% of them are treated with topical antibiotics. 29 Widespread use of antimicrobials is a key contributor to the development of antimicrobial resistance and exposes children to possible harms from adverse effects. Therefore, use of antibiotics is only justifiable where there is clear evidence of benefit. Topical antibiotics may be preferable to systemic treatment, as they maximise the effective doses at the site of infection while minimising the systemic effects. However, the prevalence of resistant strains of skin bacteria is steadily increasing and cases of allergy or skin sensitisation are not uncommon. 30,31 Fusidic acid resistance has been shown to be related to high levels of use, for example. 32 Therefore, once a decision is made to prescribe antibiotic treatment, it is unclear whether topical or oral antibiotics are most effective and which cause the least ‘collateral damage’ to the microbiome in terms of driving resistance.
Summary
Eczema has a negative impact on the quality of life of paediatric patients and their families. Oral and topical antibiotics are widely used to treat clinically infected eczema in primary care and yet there is insufficient evidence to be sure whether this established practice either helps or harms patients and, if antibiotics help, which route (topical or oral) does most good, causes least harm and is preferred by parents. Therefore, there is a clear need to identify whether or not oral and topical antibiotics confer meaningful benefit to children with clinically infected eczema. The ChildRen with Eczema, Antibiotic Management (CREAM) study aims to benefit patients with eczema (and their families), as well as helping to address the important issue of antibiotic use and resistance, by addressing this gap in the evidence.
Chapter 2 Methods
Summary of study design
The CREAM study was a three-arm, double-blind, randomised placebo-controlled trial which aims to determine the clinical effectiveness and cost-effectiveness of the most commonly used oral and topical antibiotics, in addition to topical corticosteroids (TCSs), in the management of clinically suspected infected eczema in children compared with placebo treatment. The CREAM study was based in the community, primarily in primary care, but also included four dermatology clinics that saw patients who could self-refer. Participating clinicians were asked to identify children (aged < 8 years) with clinically infected eczema. Children with clinically severe infections or significant comorbid illnesses were excluded. Eligible, consented children were randomly assigned to one of three treatment groups: (1) oral antibiotic and placebo topical cream; (2) topical antibiotic cream and placebo oral treatment; or (3) placebo oral and placebo topical cream treatment. In addition, all participants received standard advice about eczema care and were prescribed TCS creams and emollients.
At the baseline visit, a trained research nurse assessed the extent and severity of eczema, and parents reported quality of life, impact of the eczema on the family and health status, using established assessment tools. The research nurse conducted these assessments again, and enquired about side effects and health-care resource use, at 2 and 4 weeks after baseline. Parents were asked to complete a diary for the first 4 weeks following enrolment to record daily symptom severity, use of medication and health-care consultations. Participants were followed up at 3 months by a postal or telephone questionnaire and through a review of their primary care medical record in order to identify health-care consultations, medication usage and any subsequent episodes of infected eczema. Skin, mouth and nose swabs were collected at the baseline, 2 weeks and 3 months to assess for the presence of S. aureus on the skin (from the most severe site) and the impact of treatments on bacterial resistance.
The main analysis compared subjective eczema severity at week 2 in each of the two active treatment groups (oral and topical antibiotics) with the double placebo group.
The schedule of events and participant flow for the trial is summarised in Figure 1.
FIGURE 1.
Study schema and participant flow diagram. CDLQI, Children’s Dermatology Life Quality Index; DFI, Dermatitis Family Impact; EASI, Eczema Area and Severity Index; IDQoL, Infant’s Dermatology Quality of Life instrument; POEM, Patient-Orientated Eczema Measure.

Trial objectives
Primary objective
-
To assess the clinical effectiveness of oral and topical antibiotics compared with placebo on subjective eczema severity during the week following treatment (measured at 2 weeks).
Secondary objectives
-
To assess the short-term (up to 4 weeks) effectiveness of oral and topical antibiotics on subjective and objective severity, quality of life, impact on family and daily symptoms.
-
To assess the clinical effectiveness of oral and topical antibiotics on subjective and objective severity, and quality of life at 3 months.
-
To compare oral and topical antibiotic treatments in terms of short- and long-term effects, adverse effects, parent preference and effect on prevalence of colonisation/infection with resistant organisms.
-
To describe the prevalence of S. aureus isolates, and susceptibilities, in patients with suspected infected eczema, and describe the long-term prevalence of resistant isolates in those using oral antibiotics, topical antibiotics and placebo.
-
To describe the NHS resource used by the children recruited in the study and time off work taken by their parent or main carer (hereafter referred to as ‘parent’).
-
To carry out a validation exercise of the Atopic Dermatitis Quality of Life Index (ADQoL) preference-based index.
Setting
The study was community based and originally aimed to recruit patients only through general practices. However, in order to address a low recruitment rate, the study was opened in four dermatology sites.
Site recruitment
The study was open to participant recruitment from 12 July 2013 until 28 November 2014. The study was conducted in regional centres in Wales (Cardiff), England (Bristol) and Scotland (Dundee). A principal investigator led each region.
General practices and dermatology clinics (providing first point-of-contact dermatology services) were considered sites for the purpose of the study. The Primary Care Research Network in England and Scotland, and the National Institute for Social Care and Health Care Research Clinical Research Centre Network in Wales supported site recruitment.
General practitioner (GP) practices were invited to take part in the study either by letter or via a Primary Care Research Network newsletter. Interested practices were contacted initially by e-mail to provide further information about the study and were followed up by telephone or face-to-face visit with the practice manager or lead research GP to discuss the study in more detail.
Working with the local research networks in all three regions provided the study team with the knowledge of which practices had experience of recruiting to studies within primary care and also highlighted practices to approach.
Each region recruited one or more study research nurses and a local site pharmacy. All sites, research nurses, site pharmacies and research centres received training on study procedures and protocols.
Participant selection
Children were eligible to join the study if they were consulting in a participating NHS site for their routine care, and met the following inclusion criteria and did not meet any of the exclusion criteria.
Inclusion criteria
Children (aged 3 months to < 8 years) with atopic eczema (as defined by U.K. Working Party33) who presented with clinically suspected infected eczema. This could include children where:
-
the eczema was failing to respond to standard treatment with emollients and/or mild to moderate TCSs
-
there was a flare in the severity or extent of the eczema
-
there was weeping or crusting.
Exclusion criteria
Children were not eligible for inclusion if they had:
-
used oral or topical antibiotics to treat a skin infection within the past week
-
used potent or very potent TCSs within the past 2 days
-
features suggestive of eczema herpeticum (significant pain, punched out lesions)
-
known significant comorbid illness (e.g. significant immune compromise)
-
allergy to fusidic acid or both penicillin and erythromycin
-
contraindication to any study medication (penicillin, erythromycin, fusidic acid)
-
a treating clinician that believed the patient had a severe infection requiring immediate antibiotics or was arranging immediate hospitalisation or urgent (same or next day) dermatology referral because of the severity of the eczema or suspected infection.
Or if:
-
a parent/legal guardian was unable to provide written informed consent
-
a parent/legal guardian (or a person delegated by the parent/legal guardian) was not available for follow-up visits and who did not understand English well enough to complete verbal and written questionnaires.
Participant recruitment
Participating clinicians were asked to identify suitable children during routine consultations. Some clinicians were aided in the identification of suitable children through the use of a software package called Trial Torrent (Tay Dynamic Ltd, Dundee, UK) (TT; see Trial Torrent recruitment software).
Informing parents of potentially eligible children about the trial
Participating sites were asked to identify all children < 8 years of age who had a history of eczema recorded in their electronic medical record (EMR) and to write to their parent/legal guardian(s) (hereafter referred to as parent) to inform them about the study. The purpose of this letter was to provide parents of children with eczema with advanced notification and information about the study, including the potential risks and benefits of taking part. This information was designed to facilitate their decision about participation should their child develop a possible infection, while their practice was taking part in the study.
Identification of potentially eligible children
Participating clinicians identified potentially eligible children during routine consultations. Clinicians were asked to explain the study to the child’s parents and provided them with a written participant information sheet.
If parents were interested in taking part, clinicians would ask for written consent to pass parents’ contact details and information about the child’s current illness and penicillin allergy status, to the research team.
Clinicians recorded examination findings, working diagnosis, current treatment and penicillin allergy status on a paper-based or electronic case report form (CRF) depending on whether or not the practice was using the TT software. These data were used to record eligibility and need for non-penicillin treatment.
In addition to prescribing standard eczema treatment using normal prescribing procedures, clinicians completed a study prescription for study medication.
Trial Torrent recruitment software
A component of our recruitment strategy was to evaluate software that integrated with primary care clinical record software, to prompt clinicians to invite potential participants and to simplify data collection and transmission. We proposed to use TT software (formerly known as Scottish Acute Recruitment Multi-Agent),34 as this had been used previously and was designed and piloted to meet our objectives (see Appendix 1 for more details).
Trial Torrent is installed on the practice’s computers and links with the EMR system in order to identify when pre-programmed Read Codes (codes used in general practice computer systems to identify symptoms, diagnoses or medications) are used by a clinician. When relevant codes are entered the software produces a pop-up box that asks the clinician if they would like to consider discussing the trial with the patient. If a positive response is given then the software gathers further data and transmits this (and/or data from the EMR) securely to the research team. It also silently records the number of times that relevant codes are entered, and so can be used to measure numbers of potentially eligible patients consulting during the recruitment period.
The plan was to encourage all practices to use TT, but use of the software was not a requirement for participation. During the course of the study it became apparent that TT was not compatible with all software systems and so a standalone web portal (stand-alone TT) that allowed clinicians to collect relevant data and transmit them securely to the study team, was developed.
Unfortunately, TT software presented many problems for the study. This included problems with installation, training, function (pop-ups appearing up at the wrong time) and support. As a result, a decision was made to stop using TT and the formal evaluation of TT was abandoned. More detail about these problems, and their impact on the study, is provided in Chapter 5.
Instead of electronic referral via TT, clinicians faxed a paper CRF to the study team. The study team generated an electronic text message containing details of the referral, which was securely transmitted to mobile telephones provided to the research nurses.
Informed consent
Research nurses contacted parents that expressed an interest in participating, in order to arrange a baseline visit at their home or another mutually acceptable location within a maximum of 72 hours (usually 48 hours) of their initial consultation. During this visit the research nurse discussed study participation in detail, review eligibility and answer any questions. Parents were given as much time as they required for reading the study information and for asking questions. All study research nurses were trained in taking informed consent and would take written informed consent from the parent or legal guardian in order for the child to be enrolled in the study. Parents were notified that they could withdraw their consent for their child’s participation in the study at any time during the study period.
Randomisation, blinding and unblinding
Random allocation lists were prepared by the study statistician and were block randomised with randomly chosen balanced block sizes of six or nine. Each site pharmacy was provided with two randomisation lists, one for penicillin allergic patients and one for non-allergic patients. To ensure allocation concealment, treatment assignment was undertaken by each pharmacy.
As patients were recruited they were assigned the next vacant participant identification number. The randomisation list linked each unique participant identification number to a treatment group, denoted A, C or E for the non-penicillin allergic group, and B, D and F for the penicillin-allergic group. The pharmacist selected one or two (for older children) treatment packs for the relevant treatment arm based on the trial pack randomisation list. This was to ensure that trial pack identification numbers could not be used to identify treatment allocation.
At the point when a research nurse arranged to visit a potential participant, they informed their local site pharmacy who began to prepare the trial medication pack based on the faxed prescription received from the clinician and the randomisation list. Site pharmacists were not blinded, but the research nurses remained blinded at all times.
The research nurse collected the trial pack from the site pharmacy and transported the medication to the patient’s home. The research nurse only released the trial pack(s) once informed consent had been obtained and a consent form signed. If consent was not obtained then the medication was returned to the study pharmacy, logged and destroyed.
Blinding
Placebo products were matched to oral and topical antibiotic preparations. Participants, parents, clinicians and research nurses remained blinded to treatment allocation.
Unblinding
The treatments used in this study were all licensed products (or their placebos) used within their licensed indications, and the participants were provided with information about the medication they were prescribed which included information on unblinding. In the event of a request for emergency unblinding, either for clinical reasons or to facilitate monitoring of serious adverse events (SAEs), a 24-hour telephone service was available, and operated by the study team.
Withdrawal and loss to follow-up
Parents were informed that they had the right to withdraw consent for their child’s participation in any aspect of the study at any time. If a parent indicated that they wished to withdraw their child from the trial they were asked to give, though were not required to provide, a reason for withdrawal. The participants’ care was not affected by declining to participate or withdrawing from the study.
Trial interventions
The active medications being evaluated in this trial were well established and already widely used within their licensed indications. The active medications were not used outside their licensed indication during the course of this study.
Treatments
Antibiotic oral suspensions
The primary oral antibiotic used in the study was flucloxacillin suspension. We used a product manufactured by Crescent Pharma Ltd (Basingstoke, UK) and with a Medicines and Healthcare products Regulatory Agency (MHRA) marketing authorisation (PL 20416/0077). It was supplied as granules for reconstitution in 100 ml to provide a concentration of 250 mg/5 ml.
The oral antibiotic used for penicillin allergic children was erythromycin suspension. We used a product manufactured by Amdipharm UK Limited (London, UK) and with a MHRA marketing authorisation (PL 20072/0042). It was supplied as granules for reconstitution in 140 ml to provide a concentration of 250 mg/5 ml.
Placebo oral suspensions
The placebos for both flucloxacillin and erythromycin suspensions used in this study were manufactured by Tiofarma B.V. and supplied by Mawdsleys Brooks & Co Ltd (Salford, UK). Placebo to flucloxacillin was supplied as granules for reconstitution in 100 ml and placebo to erythromycin was supplied as granules for reconstitution in 140 ml. The placebos were also matched in terms of colour and taste to the active treatments.
Fusidic acid cream
The topical antibiotic used was 2% fusidic acid cream manufactured by Leo Laboratories Limited (Princes Risborough, UK) with a MHRA marketing authorisation (PL 00043/0065) was supplied by St Mary’s Pharmaceutical Unit (SMPU), Cardiff.
Placebo topical cream
The placebo antibiotic cream used in this study was manufactured to match the active treatment and supplied by SMPU.
Standard treatment for all patients
Participating clinicians were asked to prescribe clobetastone butyrate 0.05% (Eumovate®, GlaxoSmithKline) cream or ointment (the option of prescribing any other moderate-strength TCS had to be added part way through the trial because of a national shortage of Eumovate) for eczema on trunk and/or limbs and/or topical hydrocortisone 1% cream or ointment for eczema on face to all participants. The treating clinician could choose to prescribe creams or ointments, and was provided with guidance about best practice in relation to this decision. They were also asked to prescribe sufficient supply of emollient (choice according to clinician and parent preference) for the following 2 weeks, excluding those that contained antimicrobial agents.
Treatment arms
Participants were randomised to one of three treatment arms (Table 1). Each participant received one oral medication and one topical cream. All participants also received TCS treatment (hydrocortisone 1% for face and a moderate potency cream for trunk and/or limbs), were encouraged to use emollients (but not emollients with antimicrobial agents) and comprehensive verbal and written eczema care instructions.
| Group 1: control | Group 2: oral antibiotic | Group 3: topical antibiotic | |
|---|---|---|---|
| Oral treatment | Placebo oral treatment | Oral antibiotic | Placebo oral antibiotic |
| Topical treatment | Placebo topical cream | Placebo topical cream | Topical antibiotic cream |
Dosage
Oral antibiotic or placebo
-
For children aged 3 months to 2 years: 2.5 ml four times a day for 7 days.
-
For children aged > 2 years to < 8 years: 5 ml four times a day for 7 days.
Topical antibiotic or placebo
-
Cream applied to affected area(s) three times a day for 7 days.
Topical corticosteroid
-
Apply once daily for 14 days.
All investigational medicinal products (IMPs) were manufactured and reconciled into sealed and labelled ‘Trial Packs’ by SMPU in accordance with good manufacturing practice and in compliance with the Clinical Trial Regulations. 35 Trial materials were stored under the conditions specified by the manufacturer (or in the summary product characteristics) and stored in designated temperature monitored areas at site pharmacies.
Trial procedures
Training
All staff involved in the study, including clinicians and pharmacists at sites, were provided with study-specific training and written standard operating procedures prior to commencing the study.
The research nurses were provided training in all study-specific procedures during a study workshop, which included training in practical assessment of children with eczema, assessing eligibility, informed consent procedures, taking swabs and training parents in taking swabs; and data collection procedures (including administering the validated outcome measures).
Data collection
The schedule for timing, frequency and method of collection of all study data is summarised in Table 2. Assessments were performed as close as possible to the required time point.
| Data collected | Baseline: face to face | Follow-up period | |||
|---|---|---|---|---|---|
| 2 weeks: face to face | 4 weeks: face to face | 4-week diary: completed daily by parents | 3 months: questionnaire/postal swabs/medical notes search | ||
| Demographics, presenting features | ✗ | ||||
| Swab eczematous skin, nose and throat | ✗ | ✗ | ✗ | ||
| POEM | ✗ | ✗ | ✗ | ✗ | |
| EASI | ✗ | ✗ | ✗ | ||
| IDQoL/CDLQI | ✗ | ✗ | ✗ | ✗ | |
| DFI | ✗ | ✗ | ✗ | ✗ | |
| ADQoL: preference-based index | ✗ | ✗ | ✗ | ✗ | |
| Medication use | ✗a | ✗ | |||
| Parental preference for treatment | |||||
| Daily symptoms | ✗ | ||||
| Adverse effects | ✗ | ✗ | |||
| Resource use | ✗ | ✗ | ✗ | ✗ | |
| Consultations and antibiotic use for eczema | ✗ | ✗ | |||
| Parental preference for treatment | ✗ | ||||
Baseline assessments
Following referral into the study by a treating clinician, a research nurse contacted the parent to arrange an appointment for a baseline visit at their home (or other suitable location).
Once informed consent had been obtained the research nurse:
-
registered the participant and their parent to the trial
-
conducted a standardised interview with the parent
-
conducted a standardised examination of the child, including assessment of the Eczema Area and Severity Index (EASI) score
-
asked the parent to complete the validated outcome measures [Patient-Orientated Eczema Measure (POEM), Infant’s Dermatology Quality of Life instrument (IDQoL)/ Children’s Dermatology Life Quality Index (CDLQI), Dermatitis Family Impact (DFI)], the resource use and the ADQoL
-
took swab samples from the child’s infected eczema, nose and mouth
-
provided the parent with the study medication and instructions on use
-
provided the parent with standardised advice about caring for a child with eczema
-
gave the parent a 4-week symptom diary and provide them with instructions for diary completion
-
either photographed the infected areas of the child’s skin or, during the later stages of recruitment, completed a paper-based ‘Features of Infection’ assessment.
Research nurses arranged to revisit the participant and their parents at 2 and 4 weeks following their initial visit. Research nurses also advised parents that there would be telephone and postal follow-up at 3 months, and that they would be asked to take swab samples from the participant (infected eczema, nose and mouth) at 3 months.
Follow-up assessments
Week 2: research nurse visit
At 2 weeks following the baseline visit research nurses recorded medication use; collected any unused study medication; provided ongoing support for parents; collected data for the outcome measures (EASI, POEM, IDQoL/CDLQI, DFI and ADQoL); and collected the first 2 weeks’ data from the symptom diary. Research nurses provided parents with training in taking the swabs. Swabs were collected from the infected eczema, nose and mouth of the participating child and returned in postage-paid packaging.
Week 4: research nurse visit
At 4 weeks following the baseline visit research nurses provided ongoing support for the parent; collected information for the outcome measures; collected the final 2 weeks of the 4-week symptom diary; and asked parents two short questions regarding any difficulties they may have had answering the ADQoL questions.
Month 3: telephone and postal follow-up
Three months following the recruitment of each participant, parents were sent a postal letter containing follow-up questionnaires, pre-paid return envelope and swabs, complete with full instructions on taking, handling and postal return of the swabs. The questionnaire involved completing information for outcome measures and resource use (health-care consultations, medications and time off work).
If the questionnaire and/or swab were not returned within 1 week, the research team conducted telephone interviews to collect the questionnaire information and also encourage collection of the 3-month swab samples.
Month 3: medical record search
Data were extracted from primary care medical records for each participant for the 3-month period following recruitment. GP practices were asked to review the participants’ medical records or, where necessary, were assisted to do so by a member of the research team, research nurse or a clinical research officer working for the local research network. The record search was used to identify health-care consultations (primary and secondary care), prescribed medications, subsequent episodes of infected eczema and use of antibiotics for the infected eczema.
Collection of swab samples
Swabs were collected at baseline, 2 weeks and 3 months. Swabs were taken from suspected infected eczema areas to identify S. aureus or group A Streptococcus associated with the suspected infection, nasal swabs for S. aureus carriage, and oral swabs to identify any change in frequency of penicillin or macrolide resistance in group A Streptococcus or meticillin- or fusidic acid-resistant staphylococci commensal flora. Research nurses collected the baseline and 2-week swabs and parents were asked to collect the swabs at 3 months. The samples were sent to the research laboratory (Specialist Antimicrobial Chemotherapy Unit) at the University Hospital of Wales, Cardiff.
Microbiology processing
Swabs were analysed for presence of S. aureus and β-haemolytic streptococci (including group A Streptococcus). Both nasal and eczema wound swabs were cultured onto non-selective media (Columbia blood agar, Oxoid, UK), S. aureus selective media (mannitol salt agar, Oxoid, UK) and streptococcus selective media [Columbia with colistin and nalidixic acid (CNA), Oxoid, UK]. Each swab was streaked to determine a semi-quantitative count (–+, +, ++, +++). Oropharyngeal (oral) swabs were cultured using a spiral plater (Whitley Automated Spiral Plater, Don Whitley, UK) to achieve accurate counts of S. aureus or β-haemolytic streptococci onto Columbia blood agar, mannitol salt agar, CNA plus CNA + 1 mg/l oxacillin, CNA + 1 mg/l erythromycin, CNA + 1 mg/l fusidic acid and CNA + 16 mg/l fusidic acid. The antimicrobial media was used to detect any penicillin-resistant streptococci, meticillin-resistant S. aureus, fusidic acid-resistant S. aureus and erythromycin-resistant S. aureus or streptococci.
The identity of all isolates were confirmed using the matrix-assisted laser desorption ionisation time-of-flight instrument and streptococcal groups confirmed using a latex agglutination Strep grouping kit (Pro-Lab Diagnostics, UK). For all S. aureus and streptococcal isolates, susceptibilities to oxacillin, erythromycin, clindamycin, fusidic acid, cefoxitin (Mefoxin®, Bioniche Pharma USA LLC) and tetracycline were determined by European Committee on Antimicrobial Susceptibility Testing disc testing.
Safety monitoring
Parents were asked to record non-SAEs or reactions or possible side effects and rate their severity in the participant diary up to the end of the fourth week of participation.
All research nurses and GP practice staff involved in the study were trained in reporting SAEs. A study-specific SAE form was to be completed and faxed to the study team at the South East Wales Trials Unit (SEWTU) within 24 hours of the research nurse or GP becoming aware of the event; additional information was to be sent within 5 days if the event was not resolved at the time of reporting.
Any SAE report received was to be reviewed by a designated clinical reviewer who was trained to assess reports for relatedness and expectedness. The study team were trained to notify the MHRA and main Research Ethics Committee of all serious unexpected suspected adverse reactions in accordance with statutory requirements. 36
Data management and monitoring
Data entry
Clinical data were entered into an online structured query language database built specifically for the CREAM study by SEWTU in-house database developers. It was originally intended that data would be directly entered into the database at the point of collection using iPads (Apple, Cupertino, CA, USA), but because of user acceptability issues it was, for the most part, collected on paper and then entered onto the database either by study team staff at SEWTU or locally by the research nurses (England only).
Data quality
Data monitoring was conducted throughout the study, and a routine data quality audit of the Bristol centre, as they had recruited the greatest proportion of participants, took place in November 2014. In addition, a 10% quality control of all data sets was undertaken after data collection ended, and a 100% quality control was also carried out for all POEM and EASI data sets and for the diary data sets. Further monitoring would be triggered if a > 1% error rate was detected.
Data cleaning
The CREAM structured query language database was built with internal validations and ranges, which ensured that the data entered into it were generally of high quality and required little cleaning. Queries that arose during data entry were referred back to the research nurse at site immediately. Where data between paper CRFs and the web-based database conflicted, the value on the paper CRF was deemed the true value (making the assumption that the database value was the result of a keying error) unless the paper CRF had already been appropriately annotated with a correction. Self-evident correction rules were developed during the course of the trial, in response to common errors of CRF completion.
Data storage and retention
All research data will be kept until the youngest participant has reached the age of 21 or 15 years after completion of the trial (whichever is longer), in line with Cardiff University’s Research and Development (R&D) Framework guidance for clinical research. Archiving and access to the archive will be managed in accordance with the standard operating procedures of the United Kingdom Clinical Research Collaboration registered SEWTU. Electronic data are stored confidentially on password-protected servers maintained on the Cardiff University network. Paper records are stored in appropriately labelled files in secure storage cabinets.
Research governance
This study had clinical trials authorisation from the UK Competent Authority, MHRA reference 21323/0035/001-0001 and was reviewed as risk category type B. Ethical approval was granted from a NHS Research Ethics Committee. The initial approval was granted by the National Research Ethics Service South Wales Ethics Committee on 17 July 2012, reference # 12/WA/0180. NHS R&D approval was sought from the respective NHS relevant organisations in Wales, England and Scotland.
The trial was assigned European Union Drug Regulating Authorities Clinical Trials (EudraCT) number 2011-003591-37 and the International Standard Randomised Controlled Trial Number (ISRCTN) 96705420.
Outcome measures
Primary outcome measure
The primary outcome was an assessment of subjective severity at 2 weeks as measured using the validated POEM. 37 The POEM has been shown to be valid and reliable, easy to complete, sensitive to change, and is recommended for use in trials of eczema. 38 It includes seven items and is based on symptoms over the previous week. Therefore, a POEM score at the end of week 2 measures symptom severity during the week following the experimental treatment, the period when a treatment effect is most likely. POEM scores can range from 0 to 28, and higher POEM scores represent worse eczema severity. Bandings for POEM scores have been determined, allowing POEM scores to be converted into a categorical variable (0–2 = clear/almost clear, 3–7 = mild, 8–16 = moderate, 17–24 = severe and 25–28 = very severe eczema). 39
We chose a subjective measure for our primary outcome in recognition of the importance of measuring effects that are of importance to patients and their parents.
Secondary outcome measures
Subjective eczema severity was measured using the POEM at 4 weeks and 3 months. Objective eczema severity was measured using the EASI,40 which was completed by the research nurses. The severity of each of four features (redness, thickness, scratching and lichenification) in a representative patch of eczema in each of four regions was rated, as well as the extent of the eczema, and these scores were used to calculate a score, as previously described. 40 The percentage area affected was calculated using a hand span as a measure. EASI scores range from 0 to 72, and higher scores represent more severe eczema.
Dermatology-specific quality of life was assessed using the IDQoL41 (for children aged 3 months to < 4 years) and the CDLQI42 (for children aged 4 years to < 8 years). These instruments have both been validated and are used extensively in dermatology research. Both instruments include 10 items and have scores that range from 0 to 30. For both instruments, higher scores represent more severe (worse) impact on quality of life. Impact on the family was measured using the DFI instrument,43 which includes 10 items each scored from 0 to 3. This results in a score from 0 to 30, with higher scores representing more severe impact on the family. Health utility was measured using the ADQoL instrument. 44 This is a relatively new instrument that includes four items and is a condition-specific, preference-based measure of health utility for children (see Appendix 2). 44 Use and validation of the ADQoL is described in detail in Chapter 4.
A diary designed to be completed daily was used to record symptom severity, medication use, parents’ preference for treatment (recorded at 2 weeks), and health-care resource use during the first 4 weeks. The diary recorded the following symptoms each day, using a severity scale of 0–6 (where 0 = normal/not affected, 6 = as worse as it could be): parent assessment of overall severity, itch, sleep disturbance, oozing or weeping, bleeding, fever and possible adverse effects (nausea, vomiting, diarrhoea, abdominal pain, joint pains and new rash). The diary was piloted with a sample of parents prior to use in the trial. Daily symptom diaries have been used previously in a number of studies, including 4-week diaries,45 and diaries completed by parents. 46,47 One study successfully collected daily diary data from the majority of parents for 8 weeks. 48 There was also evidence for the reliability and validity of diary data recorded by parents. 49
Statistical considerations
Sample size calculation
Because of the lack of indicative references when this trial was initialised, we used conservative estimates for the sample size calculation. Assuming a clinically important difference of 3 on the POEM score and a common standard deviation (SD) of 7, we estimated a meaningful effect size of 0.429. Therefore, using a significance of 0.025 (to allow for two comparisons), with 90% power, required 137 participants per treatment group (total 411). To allow for 20% loss to follow-up this was inflated to 517 participants. During the course of the study, a minimal clinically important difference (MCID) for POEM of 3.4 was published by Schram et al. ,50 and this was used in the interpretation of results.
In April 2014, we used the data from the first 69 participants (recruited until March 2014) to revisit the sample size calculation. Using the SD from the baseline POEM scores (SD 5.3) and the correlation between baseline and 2-week POEM scores (SD 0.27) and the same clinically important difference for POEM, we found that 75 patients per group are required to reach 90% power, giving a total of 225 required for analysis. Continuing to allow 20% loss to follow-up this resulted in a revised recruitment target of 282 participants.
Analysis
Owing to lower than expected recruitment rates the study was closed early, before the proposed reduced target sample size was reached. Because of this, the analysis focuses on estimating effect sizes and confidence intervals (CIs) rather than tests of significance, which would have been undertaken if the sample size had been achieved. Similarly, the cost-effectiveness analysis was replaced with a summary description of the NHS resource used and time off taken by the parent. This amended analysis plan was approved before database lock.
Main analysis
Our main (primary) analyses are intention-to-treat (ITT) analyses comparing POEM scores at 2 weeks in the oral antibiotic group with the control (placebo) group, and in the topical antibiotic group with the control (placebo) group, and using all participants who have baseline and 2-week POEM scores (i.e. not using imputation for the primary ITT analysis). This was conducted using the analysis of covariance (ANCOVA) approach, controlling for baseline POEM score.
Secondary analyses
The analysis of POEM scores at 4 weeks and 3 months, EASI scores at 2 and 4 weeks, IDQoL, CDLQI and DFI scores at 2 and 4 weeks and 3 months were also carried out using the ANCOVA approach. That is, we used these scores as dependent variables, controlling for baseline scores and treatment arms, where the placebo group was set as the reference category. As these scores (EASI, IDQoL, CDLQI and DFI) were positively skewed and contained a number of zeros, we took the natural log transformation of the scores plus one. Therefore, the results of these analyses are presented as the percentage differences of the scores between treatment groups.
Daily symptom scores
The daily symptom scores in the symptom diary were validated via Cronbach’s alpha and factor analysis. We investigated the correlation between daily symptom scores in the symptom diary and the POEM scores collected at the 2- and 4-week time points. The daily changes in children’s eczema severity in the three groups during the first 4 weeks of treatment were illustrated and compared using area under the curve analysis.
Parental views about use of treatments
At the 2-week visit, parents were asked to indicate which treatment (oral or topical) they found easier to administer, or they were able to indicate ‘no preference’ or ‘I do not know’. We present the proportion giving each response overall, and conducted a chi-squared test to assess for difference in preference for treatment between the allocated treatment groups.
Objective assessment of clinical features
This data came from two sources: (1) research nurses’ assessment of photographs of the child’s eczema, taken during the baseline visit; and (2) a questionnaire completed by the research nurses during the baseline assessment, that was introduced after the requirement for taking photographs of the affected areas of eczema was discontinued (see Summary of changes to study). All photographs were first assessed for quality (ability to make clinical assessments), and for patients with multiple photographs only the best quality and most representative were kept. Three research nurses who had been involved in assessing patients in the CREAM study then independently assessed each photograph for its quality and rated crusting, weeping, pustules and erythema as none, mild, moderate, severe or unable to assess (these were the same categories that were used on the questionnaire). Disagreement was resolved by two or more nurses reviewing each photograph and deciding by consensus on a final rating. Finally, for children with more than one photograph available, the highest (greatest severity) score for each feature was used. Using the combined photograph and questionnaire data set we describe the proportion of children with each severity rating for each feature (only four features were recorded on both the questionnaire and from the photographs).
Microbiology data
Baseline swabs culture results were used to describe the prevalence of S. aureus found on skin swabs, and baseline and follow-up swabs were used to describe sensitivities to flucloxacillin (meticillin), erythromycin and fusidic acid in S. aureus identified from the skin, nose or mouth, and sensitivities to penicillin and erythromycin in β-haemolytic streptococci cultured from the skin, nose and mouth swabs. These were described by treatment group and overall.
Adverse effects
The daily symptom diary included potential adverse effects (‘nausea’, ‘vomiting’, ‘diarrhoea’, ‘tummy pain’, ‘joint pains’ and ‘new rash’), with each symptom being rated from 0 to 6 (‘normal/not affected’ to ‘as bad as it could be’) by parents. In order to capture potential adverse effects related to treatment, we included the treatment period (the first 7 days) and the subsequent 2 days (i.e. the first 9 days). Patients were categorised as having that adverse event if any potential adverse symptom was rated as a ‘slight problem’ or worse (i.e. score of ≥ 2) in any of the 9 days from starting the study interventions. The proportion of patients experiencing each adverse effect was calculated, by treatment group and overall, and the odds of experiencing any adverse effect was compared between each of the treatment groups and the control group using logistic regression.
Economic analyses
The Health Technology Assessment monitoring committee that made the decision to close the study before target recruitment had been achieved indicated that because the study was likely to be underpowered, a full health economic evaluation was inappropriate and should not be conducted. Therefore, we have restricted our analyses to describing resource use and associated costs, but have not conducted an economic evaluation.
Resource use and costs
Parent-reported primary and secondary consultations in the first 4 weeks, in weeks 5–12 and over the whole 3-month follow-up period were reported by study arm and overall. Primary care prescriptions during the 3 month follow-up period were assessed from the notes review. Medications were classified into categories and those that related to eczema were presented. The statistical package PASW (v. 22; SPSS Inc., Chicago, IL, USA) was used to carry out these analyses.
Validation of the Atopic Dermatitis Quality of Life preference-based index
The methods for this are described in detail in Chapter 4.
Sensitivity analyses and process measures
Medication adherence
Adherence to oral and topical study medication (antibiotic or matched placebo) was calculated by comparing the number of doses taken/applied (recorded in the diary) with the total number of possible doses (four times a day × 7 days = 28 doses for the oral medication and three doses per day × 7 days = 21 doses for the topical medication). This was converted to a percentage of total doses and presented by study group for both oral and topical medication.
These data were then used to conduct a complier-average causal effect (CACE) analysis to assess the efficacy of the interventions, controlling for adherence. This approach is recommended over a per-protocol analysis. 51 We conducted a CACE analysis for the primary outcome (POEM at 2 weeks) and for most secondary analyses (POEM at 4 weeks and 3 months, EASI, DFI, IDQoL and CDLQI at each time point).
Use of topical corticosteroids
Participants recorded all use of medication during the first 4 weeks in the diary. All TCSs were identified and classified into one of the following categories using the classification described in the British National Formulary:52 (1) all hydrocortisone 1%; (2) all moderate-strength TCSs; (3) mild TCSs (other than hydrocortisone 1%); (4) potent TCSs; and (5) very potent TCSs. We then calculated the mean number of recorded doses per week for each category, by treatment group and overall.
Regional variation
An analysis of the primary outcome, controlling for regional effects (comparing England with Wales and Scotland) was conducted to assess regional variation due to the regional imbalance in recruitment.
Missing data
Considerable effort was devoted to minimising missing data. Research nurses were trained in data collection and the questionnaires filled out by them were designed to minimise the amount of missing information. Specific rules on missing data from the score questionnaires recommended by their developers had been used. For our primary outcome (POEM at 2-week follow-up), complete cases and those with missing follow-up data were compared by describing baseline data for each group. As the missing follow-up rate was just over 10% (12/113 = 89.4%), we implemented the multiple imputation approach as a sensitivity analysis of the primary outcome. Baseline demographics (age, sex and ethnicity) as well as baseline POEM scores were adopted as predictors to impute missing POEM scores at week 2. A total of 10 imputed data sets were generated for overall inference. Conventionally, 3–10 imputations are recommended. 53
Patient and public involvement
In the CREAM study we were lucky enough to benefit from an extremely experienced and able patient and public involvement representative. Amanda Roberts has eczema herself and has had many years of experience caring for her children with eczema. She is also an experienced patient and public involvement representative, having sat on a number of bodies including the patient panel at the Centre for Evidence Based Dermatology. She has also set up a support group for parents of children with eczema (Nottingham Support Group for Carers of Children with Eczema) and through this has had direct contact with many carers of children with eczema. Amanda was a co-applicant and joined the study as a member of the Trial Management Group. The Trial Management Group also had members in professional roles that had personal experience of children with eczema.
Some of the key areas that Amanda made important contributions to included reviewing parent and child information sheets, providing feedback on the study protocol, logo and randomisation process, and providing guidance on strategies for successful recruitment. The views of a wider audience were canvassed through Twitter (Twitter, Inc., San Francisco, CA, USA) as appropriate, together with input from other Trial Management Group members with relevant experience from a patient or parent perspective. This proved invaluable, particularly in providing advice on how to best inform potential participants about the trial and recruitment strategies. Recruitment was considered throughout the conduct of the study, with discussions at Trial Management Group meetings and e-mail contact in the interim periods. The acceptability of the study design from a public and patient perspective was reflected in the positive feedback from participants and the follow-up rates achieved.
The study has also benefited from Amanda’s contribution during the analysis and dissemination of study results and will continue to contribute with dissemination activities.
Summary of changes to the study
The main changes to the protocol that occurred during the conduct of the study are summarised below.
The taking of photographs of the effected eczema area was deemed too great a burden on both the families and also technically difficult for research nurses to take good-quality photos during the baseline visit, therefore this was replaced with a features of infection CRF completed by research nurses.
Originally a week 1 visit by research nurses was included for collection of the study medication, this was deemed as an additional burden to families and a logistical challenge for the research nurse and this visit was removed from the protocol, and study medication was collected at the week 2 visit. A telephone call was made at week 1 to parents by research nurses to remind them to discontinue the study medication as the prescribed course had been completed and to continue completing the diary as instructed.
As recruitment was slow, the inclusion of dermatology clinics in addition to primary care sites was included. The original plan included a 12-month primary care notes review, this was changed to a notes review conducted at 3 months.
The original plan also included a secondary objective to assess the short- and long-term cost-effectiveness of treating suspected atopic eczema with oral or topical antibiotics, in terms of cost per unit reduction in subjective eczema severity. This objective was dropped when a decision was made to close the trial early. We have restricted our analyses to describing resource use and associated costs only.
Chapter 3 Results
Sites
A breakdown of the number of sites approached, expressing an interest, agreeing to participate and actively recruiting, for each of the four study centres are presented in Figure 2.
FIGURE 2.
Site recruitment flow diagram. EOI, expression of interest. a, Five or more recruits was the target for initiated sites. This was achieved by 12% of initiated sites (n = 11).
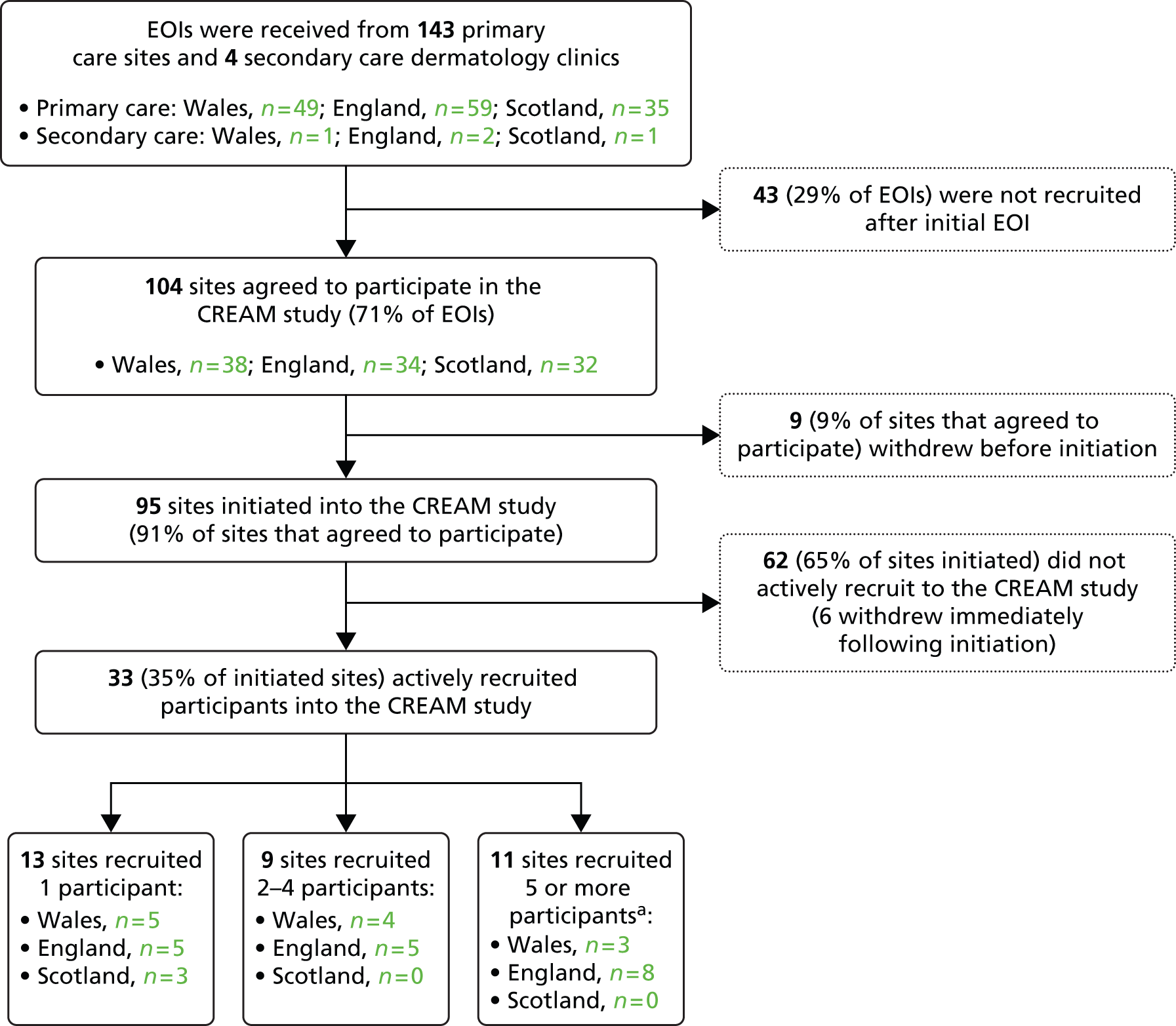
General practitioner practices and dermatology clinic sites were recruited between 9 July 2013 and 21 October 2014. A total of 147 sites (143 primary care and 4 dermatology clinics) expressed an interest in the study and 104 (71%) agreed to participate. Of the 95 sites that were initiated only 35% (32 GP sites and 1 dermatology clinic) actively recruited one or more participants into the study. Characteristics of recruiting and non-recruiting sites are given in Table 3.
| Characteristic | Recruiting practices (n = 32) | Non-recruiting practices (n = 58) |
|---|---|---|
| List size, mean (SD) | 9452 (3653) | 6451 (3777) |
| Number of full-time partners, median (IQR) | 7 (5–10) | 6 (4–8) |
| Proportion of list that are aged ≤ 8 years, median, % (IQR) | 10 (8–11) | 9 (7–11) |
| Proportion of children (aged ≤ 8 years) who have an eczema diagnosis, median, % (IQR) | 23 (20–31) | 25 (19–30) |
An explanation of the problems experienced by sites that agreed to participate but did not progress any further, and for those that did not recruit any participants, is presented in Chapter 5.
Participants
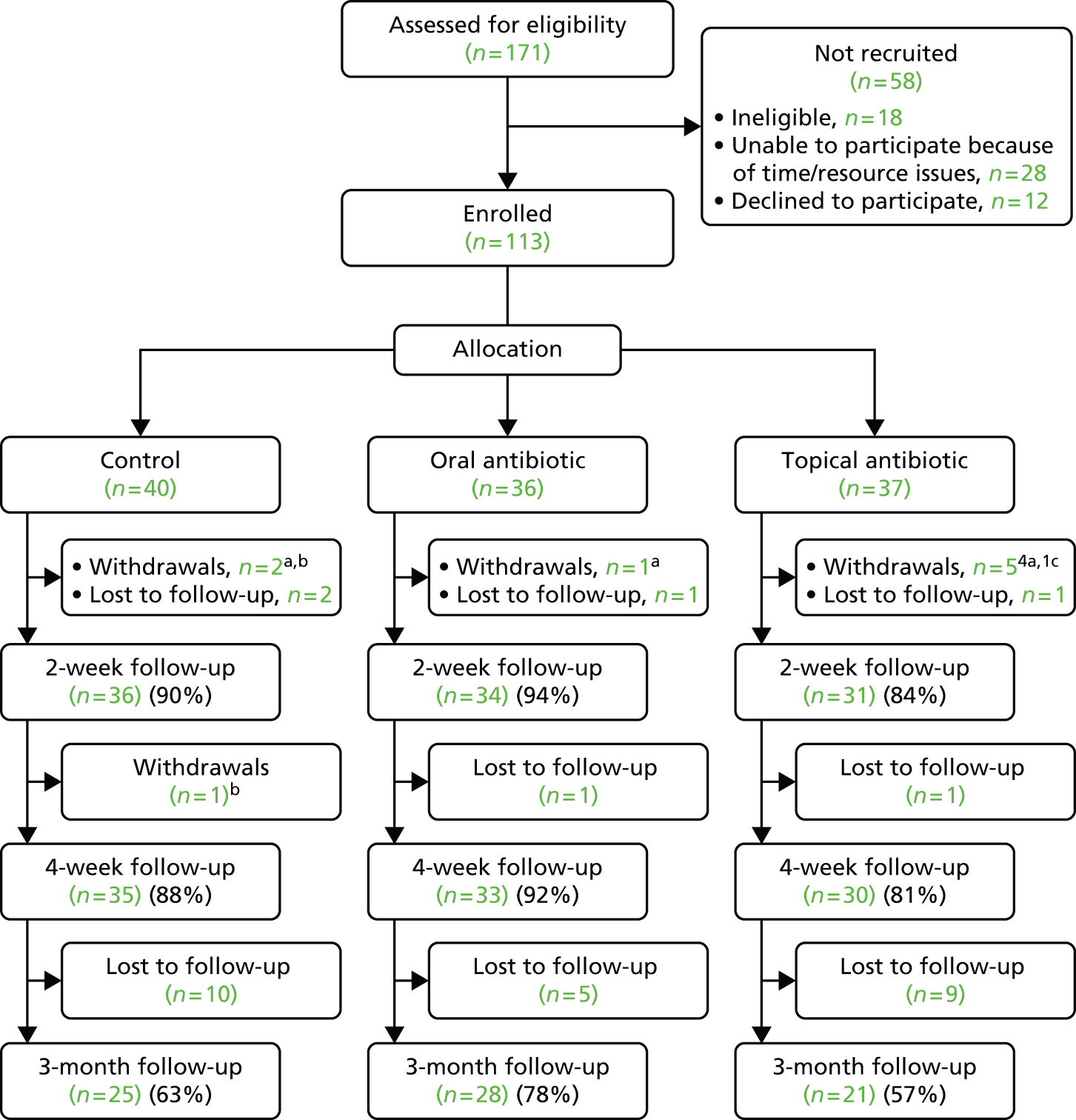
Participants were recruited between 16 July 2013 and 28 November 2014. Of the 171 children referred to the study team, 113 (35 from Wales, 71 from England, 3 from Scotland and 4 from dermatology clinics) were randomised. The numbers referred and recruited, the reasons for not recruiting and the numbers followed up in each arm are given in the consort flow diagram (Figure 3). One participant failed to provide a complete set of baseline data (including baseline POEM score) and withdrew 4 days later. Three children were recorded as having a penicillin allergy and were therefore randomised to erythromycin or its placebo. However, none of these children were randomised to the active erythromycin arm (two children were randomised to a topical antibiotic and once child to the control), so no child actually received oral erythromycin. Eight other children withdrew (two with worsened eczema, one who was intolerant to study medication and five who were unable or unwilling to follow the study protocol). A further four children were lost to follow-up prior to the 2-week follow-up point, giving us a follow-up rate for our primary outcome of 89.4% (n = 101). We were able to follow up 98 (86.7%) and 74 (65.5%) participants at 4 weeks and 3 months, respectively, and were able to review the medical records (at 3 months) of 97 (85.8%) participants.
FIGURE 3.
Consolidated Standards of Reporting Trials flow diagram. a, Condition worsened; b, unwilling/unable to follow protocol; c, intolerant to study medication.
Baseline characteristics
The three groups were similar in terms for age, sex and ethnicity, and similar in terms of prior duration of flare, severity of symptoms at baseline, previous treatment and bathing history (Table 4). Itch, dry skin, crusting, painful skin, hypersensitivity and disturbed sleep were the main symptoms present, with all having an average severity rating of 2 (‘slight problem’) or worse. Over 90% of children had one or more ‘classic’ signs of infection (weeping, crusting, pustules or painful skin), and 70% had S. aureus isolated from a skin swab.
| Characteristic | Control (n = 40) | Oral antibiotic (n = 36) | Topical antibiotic (n = 37) | Overall (n = 113) |
|---|---|---|---|---|
| Age, mean (SD) | 3.3 (2.2) | 2.9 (2.2) | 3.0 (2.1) | 3.1 (2.1) |
| Sex, n (%) | ||||
| Male | 17 (42.5) | 18 (50.0) | 17 (45.9) | 52 (46.0) |
| Female | 23 (57.5) | 18 (50.0) | 20 (54.1) | 61 (54.0) |
| Ethnicity, n (%) | ||||
| White | 33 (82.5) | 31 (86.1) | 27 (73.0) | 91 (80.5) |
| Mixed | 4 (10.0) | 1 (2.8) | 3 (8.1) | 8 (7.1) |
| Asian, Chinese or other | 1 (2.5) | 3 (8.3) | 3 (8.1) | 7 (6.2) |
| Black | 2 (5.0) | 0 (0.0) | 3 (8.1) | 5 (4.4) |
| Prefer not to answer | 0 (0.0) | 1 (2.8) | 1 (2.7) | 2 (1.8) |
| Duration of eczema flare, n (%) | ||||
| 1–3 days | 3 (12.5) | 3 (13.0) | 2 (10.0) | 8 (11.9) |
| 4–7 days | 10 (41.7) | 9 (39.1) | 4 (20.0) | 23 (34.3) |
| 8–14 days | 7 (29.2) | 7 (30.4) | 5 (25.0) | 19 (28.4) |
| 15–28 days | 4 (16.7) | 4 (17.4) | 9 (45.0) | 17 (25.4) |
| Indicators or infection, n (%) | ||||
| One or more of weeping, crusting, pustules or painful skin | 35 (89.7) | 33 (91.7) | 35 (94.6) | 103 (92.0) |
| Temperature (38 °C or higher) | 1 (2.6) | 2 (6.1) | 2 (5.7) | 5 (4.7) |
| Growth of S. aureus from skin swab | 16 (60.0) | 30 (83.3) | 24 (66.7) | 78 (69.6) |
| Bath/shower frequency, n (%) | ||||
| Daily | 23 (59.0) | 14 (38.9) | 18 (48.6) | 55 (49.1) |
| Less than daily | 16 (41.0) | 22 (61.1) | 19 (51.4) | 57 (50.9) |
Baseline symptom severity scores are described in Table 5.
| Baseline symptom | Number experiencing symptom, n (%) | Score, median (IQR) | |||
|---|---|---|---|---|---|
| Control (n = 40) | Oral antibiotic (n = 36) | Topical antibiotic (n = 37) | Overall (n = 113) | ||
| Weeping or oozing skin lesion | 61 (54.5) | 0.0 (0.0–2.0) | 1.5 (0.0–3.0) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) |
| Crusting | 83 (74.1) | 2.0 (0.0–4.0) | 3.0 (1.0–4.0) | 2.5 (0.5–3.0) | 2.0 (0.0–4.0) |
| Pustules | 34 (30.4) | 0.0 (0.0–2.0) | 0.0 (0.0–2.8) | 0.0 (0.0–0.0) | 0.0 (0.0–1.8) |
| Wheeze | 22 (19.6) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Fever during this illness | 23 (20.5) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Painful skin | 79 (70.5) | 2.0 (0.0–3.0) | 2.5 (0.0–3.8) | 2.0 (1.0–3.0) | 2.0 (0.0–3.0) |
| Hypersensitivity of skin | 80 (71.4) | 2.0 (0.0–4.0) | 3.0 (0.3–4.0) | 2.0 (0.0–3.5) | 2.0 (0.0–4.0) |
| Disturbed sleep | 79 (70.5) | 2.0 (0.0–5.0) | 3.0 (2.0–5.0) | 3.0 (0.0–4.0) | 3.0 (0.0–5.0) |
| Feeling generally unwell | 39 (34.8) | 0.0 (0.0–1.0) | 0.0 (0.0–2.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Interference in normal activities | 46 (41.4) | 0.0 (0.0–0.0) | 1.0 (0.0–2.0) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) |
Objective assessment of clinical features
A total of 100 participants had an objective assessment of the features of their infected eczema conducted by a research nurse at baseline (47 participants had photographs taken of their eczema and 53 participants had an ‘Objective Assessment of Clinical Features’ Questionnaire completed during the clinical examination). Of these, 30.0%, 10.1%, 6.8% and 53.0% had moderate or severe crusting, weeping, pustules and erythema, respectively (Table 6). Warmth, swelling and tenderness were only assessed in those who had a questionnaire completed, as these could not be assessed from photographs.
| Clinical feature | Photographs, n (%) | Questionnaire, n (%) | Combined, n (%) |
|---|---|---|---|
| Crusting | |||
| None | 13 (35.1) | 24 (45.3) | 37 (41.1) |
| Mild | 13 (35.1) | 13 (24.5) | 26 (28.9) |
| Moderate | 11 (29.7) | 14 (26.4) | 25 (27.8) |
| Severe | 0 (0.0) | 2 (3.8) | 2 (2.2) |
| Total | 37 (–) | 53 (–) | 90 (–) |
| Weeping | |||
| None | 31 (86.1) | 35 (66.0) | 66 (74.2) |
| Mild | 2 (5.6) | 12 (22.6) | 14 (15.7) |
| Moderate | 3 (8.3) | 4 (7.5) | 7 (7.9) |
| Severe | 0 (0.0) | 2 (3.8) | 2 (2.2) |
| Total | 36 (–) | 53 (–) | 89 (–) |
| Pustules | |||
| None | 35 (92.1) | 39 (73.6) | 74 (81.3) |
| Mild | 3 (7.9) | 6 (11.3) | 9 (9.8) |
| Moderate | 0 (0.0) | 8 (15.1) | 8 (8.7) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 38 (–) | 53 (–) | 91 (–) |
| Erythema | |||
| None | 1 (2.1) | 4 (7.5) | 5 (5.0) |
| Mild | 21 (44.7) | 21 (39.6) | 42 (42.0) |
| Moderate | 20 (42.6) | 24 (45.3) | 44 (44.0) |
| Severe | 5 (10.6) | 4 (7.5) | 9 (9.0) |
| Total | 47 (–) | 53 (–) | 100 (–) |
| Warmth | |||
| None | – | 29 (54.7) | 29 (54.7) |
| Mild | – | 18 (34.0) | 18 (34.0) |
| Moderate | – | 6 (11.3) | 6 (11.3) |
| Severe | – | 0 (0.0) | 0 (0.0) |
| Total | – | 53 (–) | 53 (–) |
| Swelling | |||
| None | – | 36 (67.9) | 36 (67.9) |
| Mild | – | 13 (24.5) | 13 (24.5) |
| Moderate | – | 4 (7.5) | 4 (7.5) |
| Severe | – | 0 (0.0) | 0 (0.0) |
| Total | – | 53 (–) | 53 (–) |
| Tenderness | |||
| None | – | 23 (43.4) | 23 (43.4) |
| Mild | – | 14 (26.4) | 14 (26.4) |
| Moderate | – | 13 (24.5) | 13 (24.5) |
| Severe | – | 3 (5.7) | 3 (5.7) |
| Total | – | 53 (–) | 53 (–) |
Primary outcomes
Mean baseline POEM scores were 13.42, 14.62 and 16.90 in the control (placebo/placebo), oral antibiotic (oral antibiotic/placebo topical) and topical antibiotic (topical antibiotic/oral placebo) groups, respectively (Table 7). These are all towards the upper end of the ‘moderately severe’ band of eczema severity. 39 By 2 weeks, the mean POEM score had reduced in all three groups (see Table 7). Patients with oral and topical antibiotics had non-significantly higher (worse severity) POEM scores at 2 weeks with mean differences from control of 1.52 (95% CI –1.35 to 4.40) and 1.49 (95% CI –1.55 to 4.53), respectively (see Table 7). The 95% CIs cross zero and so are compatible with harm, no effect or benefit. However, the lower bands of the CIs (–1.35 and –1.55) are less than the published MCID for POEM of 3.4. 50
| Outcome | Control | Oral antibiotic | Topical antibiotic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Follow-up, mean (SD) | n | Baseline, mean (SD) | Follow-up, mean (SD) | Intervention effect (95% CI) | n | Baseline, mean (SD) | Follow-up, mean (SD) | Intervention effect (95% CI) | |
| POEM | |||||||||||
| 2 weeks (primary outcome) | 36 | 13.42 (5.06) | 6.17 (5.97) | 34 | 14.62 (5.34) | 8.27 (7.33) | 1.52 (–1.35 to 4.40) | 31 | 16.90 (5.54) | 9.32 (6.17) | 1.49 (–1.55 to 4.53) |
| 4 weeks | 35 | 13.63 (4.97) | 8.03 (5.95) | 33 | 14.64 (5.42) | 8.36 (7.71) | –0.18 (–3.10 to 2.75) | 30 | 16.60 (5.37) | 9.53 (5.89) | 0.00 (–3.07 to 3.07) |
| 3 months | 25 | 13.68 (5.07) | 7.72 (5.52) | 28 | 14.43 (5.66) | 7.86 (6.09) | –0.21 (–3.12 to 2.70) | 21 | 16.38 (5.45) | 7.86 (5.85) | –1.13 (–4.32 to 2.06) |
| EASI | |||||||||||
| 2 weeks | 34 | 5.79 (4.98) | 2.50 (5.64) | 34 | 7.34 (6.09) | 3.09 (3.59) | 0.20 (–0.12 to 0.52) | 31 | 9.52 (9.66) | 4.91 (5.65) | 0.42 (0.09 to 0.75) |
| 4 weeks | 34 | 5.79 (4.98) | 4.01 (6.55) | 33 | 7.43 (6.16) | 3.23 (3.81) | –0.13 (–0.47 to 0.22) | 30 | 9.67 (9.79) | 4.98 (6.87) | 0.02 (–0.34 to 0.38) |
| DFI | |||||||||||
| 2 weeks | 35 | 5.31 (4.73) | 2.60 (4.76) | 34 | 6.50 (5.24) | 3.69 (4.42) | 0.17 (–0.18 to 0.53) | 31 | 8.19 (5.73) | 4.84 (5.35) | 0.21 (–0.15 to 0.58) |
| 4 weeks | 35 | 5.31 (4.73) | 3.11 (4.86) | 33 | 6.51 (5.32) | 3.52 (4.60) | –0.02 (–0.43 to 0.39) | 30 | 7.83 (5.46) | 4.23 (4.83) | –0.00 (–0.43 to 0.42) |
| 3 months | 24 | 6.11 (4.81) | 3.50 (4.28) | 25 | 6.65 (5.47) | 3.46 (4.40) | –0.04 (–0.56 to 0.49) | 20 | 6.55 (4.85) | 4.10 (5.52) | –0.01 (–0.57 to 0.55) |
| IDQoL | |||||||||||
| 2 weeks | 20 | 9.53 (2.62) | 6.07 (3.69) | 25 | 10.02 (3.81) | 6.74 (3.28) | 0.11 (–0.10 to 0.32) | 22 | 10.00 (4.06) | 7.19 (3.00) | 0.18 (–0.03 to 0.40) |
| 4 weeks | 20 | 9.53 (2.62) | 6.92 (3.74) | 24 | 10.11 (3.87) | 6.59 (3.23) | –0.04 (–0.28 to 0.21) | 22 | 10.00 (4.06) | 7.14 (2.96) | 0.05 (–0.20 to 0.30) |
| 3 months | 16 | 9.63 (2.63) | 7.25 (2.59) | 18 | 10.48 (4.07) | 6.01 (3.15) | –0.21 (–0.44 to 0.02) | 15 | 9.53 (3.94) | 6.67 (3.50) | –0.07 (–0.31 to 0.17) |
| CDLQI | |||||||||||
| 2 weeks | 14 | 7.61 (5.95) | 1.82 (1.98) | 9 | 9.80 (4.39) | 4.07 (3.04) | 0.43 (–0.16 to 1.02) | 9 | 9.65 (6.37) | 5.88 (6.25) | 0.70 (0.12 to 1.28) |
| 4 weeks | 14 | 7.61 (5.95) | 4.64 (5.89) | 9 | 9.80 (4.39) | 4.36 (4.79) | –0.15 (–0.84 to 0.54) | 8 | 7.99 (4.22) | 3.04 (2.22) | –0.17 (–0.87 to 0.53) |
| 3 months | 8 | 8.88 (7.07) | 6.18 (6.37) | 6 | 8.22 (4.32) | 5.57 (6.73) | –0.14 (–0.97 to 0.70) | 6 | 6.48 (3.40) | 4.61 (4.59) | –0.13 (–0.96 to 0.70) |
Secondary outcomes
Subjective severity at 4 weeks and 3 months
The effects of oral and topical antibiotics on POEM scores at 4 weeks were –0.18 (95% CI –3.10 to 2.75) and 0.00 (95% CI –3.07 to 3.07), respectively (Figure 4 and see Table 7). These point estimates suggest no (or minimal) effect, and although the CIs are slightly wider than at 2 weeks (because of slightly lower numbers with follow-up data) they are still smaller than the MCID and therefore exclude a clinically meaningful benefit. The effect sizes for oral and topical antibiotics at 3 months [–0.21 (95% CI –3.12 to 2.70) and –1.13 (95% CI –4.32 to 2.06), respectively] are negative (consistent with small benefit) and, again, include the possibility of a harmful effect, no effect or a beneficial effect. The CI around the effect size for topical antibiotics is –4.32, which is greater than the MCID and therefore does not exclude the possibility of a clinically meaningful beneficial effect (see Table 7).
FIGURE 4.
Baseline, week 2, week 4 and 3-month POEM scores for each treatment group.

Objective eczema severity
Eczema Area and Severity Index scores at follow-up are given in Table 7. The effects of oral antibiotics at 2 and 4 weeks were well below the MCID and the 95% CIs crossed zero [0.20 (95% CI –0.12 to 0.52) and –0.13 (95% CI –0.47 to 0.22), respectively], consistent with no clinically important benefit or harm (MCID = 6.6). 50 For topical antibiotics, the 2-week estimate suggested a small and clinically unimportant detrimental effect and the 4-week estimate is similar to those for oral antibiotics (CI bounds include null and exclude clinically important effects in either direction).
Impact on the family
The scores at baseline and each follow-up point are given in Table 7. All estimates of effect were close to null and all 95% CIs included zero.
Quality of life
The IDQoL was collected for participants aged 3 months to < 4 years, whereas the CDLQI was collected for children aged 4 years to < 8 years. No significant intervention effects were seen for either IDQoL or CDLQI (see Table 7).
Daily symptom scores
Cronbach’s alpha for the eight symptom scores in the daily diary was 0.985, indicating good reliability between these symptoms and suggesting that they could be combined into a single symptom score. The factor analysis also suggested that these symptoms could be used to form a single scale (see Appendix 3).
Table 8 shows the correlation between total symptom scores (sum of the scores of each of eight symptoms) and the POEM score at baseline, 2 weeks and 4 weeks. The high correlations demonstrate the validity of using the total symptom score to measure eczema severity. Figure 5 shows a plot of the mean daily total symptom score for each group.
| Time point | Pearson’s correlation | Spearman’s correlation |
|---|---|---|
| Baseline | 0.57** | 0.53** |
| 2 weeks | 0.61** | 0.65** |
| 4 weeks | 0.72** | 0.73** |
FIGURE 5.
Daily total symptom score for each treatment group over 4 weeks.

The mean (95% CI) of the area under the curve by treatment groups, which measures the accumulated daily symptom scores during the first 4 weeks of treatment, is shown in Table 9, and demonstrates that symptom recovery was similar in all three groups.
| Control | Oral antibiotic | Topical antibiotic | Overall | ||||
|---|---|---|---|---|---|---|---|
| n | Mean (95% CI) | n | Mean (95% CI) | n | Mean (95% CI) | n | Mean (95% CI) |
| 32 | 225.14 (–135.66 to 585.95) | 33 | 215.55 (–90.56 to 521.66) | 29 | 225.03 (–16.38 to 466.45) | 94 | 221.74 (–83.65 to 521.73) |
Microbiology
Staphylococcus aureus was identified from the skin of 69.6% (95% CI 61.0% to 78.3%) of participants at baseline. By 2 weeks and 3 months this had reduced to 44.4% (95% CI 34.5% to 54.4%) and 36.1% (24.7% to 47.5%), respectively (Table 10). No significant differences between the groups were observed in the proportion of patients from whom S. aureus was isolated.
| Trial arm | Baseline | Week 2 | 3 months | |||||
|---|---|---|---|---|---|---|---|---|
| n | % positive samples (95% CI) | n | % positive samples (95% CI) | Difference from baseline (95% CI) | n | % positive samples (95% CI) | Difference from baseline (95% CI) | |
| Control | 40 | 60.0 (44.1 to 75.9) | 34 | 44.4 (34.5 to 54.4) | –15.9 (–39.1 to 7.4) | 25 | 40.0 (19.4 to 60.6) | –20.0 (–45.4 to 5.4) |
| Oral antibiotic | 36 | 83.3 (70.5 to 96.1) | 34 | 52.9 (35.3 to 70.6) | –30.4 (–51.6 to –9.2) | 26 | 30.8 (11.8 to 49.8) | –52.6 (–74.1 to –31.0) |
| Topical antibiotic | 36 | 66.7 (50.5 to 82.8) | 31 | 35.5 (17.6 to 53.3) | –31.2 (–54.8 to –7.6) | 21 | 38.1 (15.4 to 60.7) | –28.6 (–55.3 to –1.9) |
| Overall | 112 | 69.6 (61.0 to 78.3) | 99 | 44.4 (34.5 to 54.4) | –25.2 (–38.2 to –12.1) | 72 | 36.1 (24.7 to 47.5) | –33.5 (–47.6 to –19.5) |
In S. aureus isolates cultured from the skin, nose and mouth exhibited low levels of resistance to flucloxacillin (overall < 10% at all time points and from all locations) and erythromycin [overall < 15% in skin and nose isolates at all time points and in 3/16 (18.8%) and 4/11 (36.4%) mouth isolates at baseline and 2 weeks, respectively]. No meticillin-resistant S. aureus isolates were identified and no resistance to clindamycin was detected. However, 26.9% of baseline skin S. aureus isolates were resistant to fusidic acid (Table 11). Resistant S. aureus isolates from nose and mouth swabs are shown in Tables 12 and 13. Fusidic acid resistance found in follow-up samples was greatest at the week 2 time point and greatest in the topical antibiotic group. However, the numbers were small and the differences between groups were not statistically significant. Only very small numbers of β-haemolytic streptococci were cultured from skin, nose and mouth swabs. None of these isolates were resistant to penicillin and only one isolate (cultured from skin and nose swabs) was found to be resistant to erythromycin (data not shown).
| Time point | Control | Oral antibiotic | Topical antibiotic | Overall | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Flucloxacillina | Erythromycinb | Fusidic acidc | n | Flucloxacillina | Erythromycinb | Fusidic acidc | n | Flucloxacillina | Erythromycinb | Fusidic acidc | n | Flucloxacillina | Erythromycinb | Fusidic acidc | |
| Baseline | 24 | 0 (0.0) | 2 (8.3) | 6 (25.0) | 30 | 1 (3.3) | 5 (16.7) | 7 (23.3) | 24 | 1 (4.2) | 2 (8.3) | 8 (33.3) | 78 | 2 (2.6) | 9 (11.5) | 21 (26.9) |
| Week 2 | 16 | 0 (0.0) | 2 (12.5) | 5 (31.2) | 18 | 0 (0.0) | 1 (5.6) | 1 (5.6) | 11 | 2 (18.2) | 0 (0.0) | 8 (72.7) | 45 | 2 (4.4) | 3 (6.7) | 14 (31.1) |
| 3 months | 10 | 0 (0.0) | 1 (10.0) | 2 (20.0) | 8 | 1 (12.5) | 1 (12.5) | 0 (0.0) | 8 | 1 (12.5) | 1 (12.5) | 2 (25.0) | 26 | 2 (7.7) | 3 (11.5) | 4 (15.4) |
| Time point | Control | Oral antibiotic | Topical antibiotic | Overall | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Flucloxacillina | Erythromycinb | Fusidic acidc | n | Flucloxacillina | Erythromycinb | Fusidic acidc | n | Flucloxacillina | Erythromycinb | Fusidic acidc | n | Flucloxacillina | Erythromycinb | Fusidic acidc | |
| Baseline | 16 | 0 (0.0) | 1 (6.2) | 5 (31.2) | 12 | 0 (0.0) | 3 (25.0) | 1 (8.3) | 19 | 1 (5.3) | 2 (10.5) | 6 (31.6) | 47 | 1 (2.1) | 6 (12.8) | 12 (25.5) |
| Week 2 | 9 | 0 (0.0) | 1 (11.1) | 4 (44.4) | 13 | 0 (0.0) | 1 (7.7) | 2 (15.4) | 13 | 2 (15.4) | 1 (7.7) | 7 (53.8) | 35 | 2 (5.7) | 3 (8.6) | 13 (37.1) |
| 3 months | 8 | 0 (0.0) | 0 (0.0) | 1 (12.5) | 11 | 0 (0.0) | 0 (0.0) | 2 (18.2) | 8 | 0 (0.0) | 1 (12.5) | 3 (37.5) | 27 | 0 (0.0) | 1 (3.7) | 6 (22.2) |
| Time point | Control | Oral antibiotic | Topical antibiotic | Overall | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Flucloxacillina | Erythromycinb | Fusidic acidc | n | Flucloxacillina | Erythromycinb | Fusidic acidc | n | Flucloxacillina | Erythromycinb | Fusidic acidc | n | Flucloxacillina | Erythromycinb | Fusidic acidc | |
| Baseline | 2 | 0 (0.0) | 1 (50.0) | 0 (0.0) | 6 | 0 (0.0) | 2 (33.3) | 1 (16.7) | 8 | 0 (0.0) | 0 (0.0) | 4 (50.0) | 16 | 0 (0.0) | 3 (18.8) | 5 (31.2) |
| Week 2 | 4 | 0 (0.0) | 0 (0.0) | 1 (25.0) | 4 | 0 (0.0) | 3 (75.0) | 2 (50.0) | 3 | 1 (33.3) | 1 (33.3) | 3 (100.0) | 11 | 1 (9.1) | 4 (36.4) | 6 (54.5) |
| 3 months | 5 | 0 (0.0) | 0 (0.0) | 3 (60.0) | 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 | 0 (0.0) | 0 (0.0) | 3 (27.3) |
Adverse effects
Data on adverse effects were obtained from the daily symptom diaries and were available for 97 (86%) participants. New rash, vomiting and diarrhoea were the most common adverse effects reported (Table 14). There were no notable or statistically significant differences by treatment group. No serious adverse events were reported in any of the treatment groups.
| Adverse event | Control (n = 35), n (%) | Oral antibiotic (n = 33), n (%) | Topical antibiotic (n = 29), n (%) | Overall (n = 97), n (%) |
|---|---|---|---|---|
| Nausea | 3 (8.6) | 2 (6.1) | 1 (3.4) | 6 (5.3) |
| Vomiting | 6 (17.1) | 4 (12.1) | 2 (6.9) | 12 (12.4) |
| Diarrhoea | 5 (14.3) | 5 (15.2) | 5 (17.2) | 15 (15.5) |
| Tummy pain | 2 (5.7) | 3 (9.1) | 3 (10.3) | 8 (8.2) |
| Joint pains | 0 (0.0) | 1 (3.0) | 2 (6.9) | 3 (3.1) |
| New rash | 8 (22.9) | 4 (12.1) | 5 (17.2) | 17 (17.5) |
Parental-reported NHS consultations
Table 15 shows the percentage of children whose parents reported that they were seen by a health professional during the 4 weeks and at 3 months after randomisation. The GP is the main health professional seen in primary care. There were a few hospital admissions and accident and emergency attendances. No significant differences were found between the three groups in the number of NHS consultations reported, either in primary or secondary care. The costs of health-care consultations in the first 4 weeks and from week 5 to week 12 are presented in Table 16.
| Health-care consultation | First 4 weeks, n (%) | Weeks 5–12, n (%)a | First 12 weeks, n (%)b | |||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 35) | Oral antibiotic (n = 33) | Topical antibiotic (n = 30) | Control (n = 25) | Oral antibiotic (n = 26) | Topical antibiotic (n = 21) | Overall (n = 72) | ||
| Primary care | ||||||||
| GPc | Consulted one or more time | 7 (20) | 10 (30) | 9 (26) | 11 (44) | 17 (65) | 10 (47) | 46 (64) |
| Median consultations of those who consulted | 2 | 1 | 1 | 2 | 1 | 1 | 1 | |
| Nurse | Consulted one or more time | 2 (6) | 4 (12) | 3 (10) | 3 (12) | 4 (15) | 3 (14) | 15 (21) |
| Pharmacist | Consulted one or more time | 1 (3) | 2 (6) | 1 (3) | 1 (4) | 2 (8) | 2 (9) | 9 (12) |
| Otherd | Consulted one or more time | 3 (8) | 4 (12) | 2 (7) | 4 (16) | 6 (23) | 3 (14) | 15 (21) |
| NHS Direct | Consulted one or more time | 2 (6) | 2 (6) | 1 (3) | 2 (8) | 2 (8) | 1 (5) | 8 (11) |
| Walk-in centre | Consulted one or more time | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (4) | 0 (0) | 2 (3) |
| Any primary care | Consulted one or more time | 8 (23) | 14 (42) | 12 (40) | 13 (52) | 18 (69) | 13 (62) | 53 (74) |
| Secondary care | ||||||||
| Outpatient | Consulted one or more time | 0 (0) | 0 (0) | 3 (10) | 2 (8) | 4 (15) | 2 (9) | 9 (12) |
| A&E | Consulted one or more time | 1 (3) | 1 (3) | 0 (0) | 1 (4) | 1 (4) | 0 (0) | 2 (3) |
| Inpatient | One or more admission | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 2 (3) |
| Any secondary care | One or more attendance | 1 (3) | 1 (3) | 3 (10) | 2 (8) | 4 (15) | 2 (9) | 13 (18) |
| NHS contact | First 4 weeks | Weeks 5–12a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 35), £ | Oral antibiotic (n = 33), £ | Topical antibiotic (n = 30), £ | Control (n = 25), £ | Oral antibiotic (n = 26), £ | Topical antibiotic (n = 21), £ | |||||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| GPb | 16.06 (36.23) | 0.00 (0.00–0.00) | 18.70 (37.52) | 0.00 (0.0–37.00) | 26.73 (61.14) | 0.00 (0.00–28.00) | 40.88 (70.00) | 0.00 (0.00–46.00) | 51.34 (58.64) | 46.00 (0.00–91.25) | 44.28 (83.60) | 0.00 (0.00–46.00) |
| Nurse | 0.78 (3.22) | 0.00 (0.00–0.00) | 1.43 (4.08) | 0.00 (0.00–0.00) | 1.37 (4.18) | 0.00 (0.00–0.00) | 4.86 (16.86) | 0.00 (0.00–0.00) | 4.81 (17.05) | 0.00 (0.00–0.00) | 2.61 (7.01) | 0.00 (0.00–0.00) |
| Pharmacist | 0.15 (0.92) | 0.00 (0.00–0.00) | 0.82 (3.87) | 0.00 (0.00–0.00) | 0.73 (2.36) | 0.00 (0.00–0.00) | 0.65 (3.26) | 0.00 (0.00–0.00) | 1.25 (3.54) | 0.00 (0.00–0.00) | 1.04 (2.78) | 0.00 (0.00–0.00) |
| NHS Direct | 1.66 (6.83) | 0.00 (0.00–0.00) | 2.64 (11.15) | 0.00 (0.00–0.00) | 0.97 (5.29) | 0.00 (0.00–0.00) | 2.32 (3.26) | 0.00 (0.00–0.00) | 2.23 (7.88) | 0.00 (0.00–0.00) | 2.76 (12.66) | 0.00 (0.00–0.00) |
| Walk-in centre | 0.00 (0.00) | 0.00 (0.00–0.00) | 0.00 (0.00) | 0.00 (0.00–0.00) | 0.00 (0.00) | 0.00 (0.00–0.00) | 1.69 (8.43) | 0.00 (0.00–0.00) | 1.62 (8.27) | 0.00 (0.00–0.00) | 0.00 (0.00) | 0.00 (0.00–0.00) |
| Otherc | 2.18 (7.44) | 0.00 (0.00–0.00) | 4.87 (13.99) | 0.00 (0.00–0.00) | 3.47 (11.81) | 0.00 (0.00–0.00) | 4.06 (14.26) | 0.00 (0.00–0.00) | 8.54 (21.98) | 0.00 (0.00–0.00) | 3.28 (10.47) | 0.00 (0.00–0.00) |
| Total primary care | 20.83 (48.85) | 0.00 (0.00–0.00) | 28.46 (50.90) | 0.00 (0.00–46.00) | 33.27 (72.81) | 0.00 (0.00–28.00) | 54.46 (84.44) | 13.69 (0.00–83.5) | 69.81 (77.45) | 46.00 (0.00–101.15) | 53.97 (96.23) | 29.64 (0.00–82.69) |
| Outpatient | 0.00 (0.00) | 0.00 (0.00–0.00) | 0.00 (0.00) | 0.00 (0.00–0.00) | 10.90 (33.26) | 0.00 (0.00–0.00) | 13.08 (47.92) | 0.00 (0.00–0.00) | 29.35 (78.95) | 0.00 (0.00–0.00) | 20.76 (55.78) | 0.00 (0.00–0.00) |
| A&E | 4.22 (24.95) | 0.00 (0.00–0.00) | 4.47 (25.69) | 0.00 (0.00–0.00) | 0.00 (0.00) | 0.00 (0.00–0.00) | 11.08 (40.87) | 0.00 (0.00–0.00) | 5.68 (78.95) | 0.00 (0.00–0.00) | 0.00 (0.00) | 0.00 (0.00–0.00) |
| Inpatient | 17.23 (101.92) | 0.00 (0.00–0.00) | 0.00 (0.00) | 0.00 (0.00–0.00) | 0.00 (0.00) | 0.00 (0.00–0.00) | 0.00 (0.00) | 0.00 (0.00–0.00) | 23.19 (118.26) | 0.00 (0.00–0.00) | 0.00 (0.00) | 0.00 (0.00–0.00) |
| Total secondary care | 21.45 (126.88) | 0.00 (0.00–0.00) | 4.47 (25.69) | 0.00 (0.00–0.00) | 10.90 (33.26) | 0.00 (0.00–0.00) | 24.89 (79.54) | 0.00 (0.00–0.00) | 58.21 (198.07) | 0.00 (0.00–0.00) | 20.76 (55.78) | 0.00 (0.00–0.00) |
| Total NHS contacts | 42.28 (165.21) | 0.00 (0.00–0.00) | 32.93 (72.54) | 0.00 (0.00–46.00) | 44.17 (100.125) | 0.00 (0.00–28.00) | 79.34 (165.20) | 46.00 (0.00–123.50) | 128.02 (253.26) | 46.00 (0.00–121.89) | 74.73 (131.67) | 29.64 (0.00–92.00) |
Medication use during follow-up
Table 17 indicates the number and proportion of children who had one or more prescriptions, and the total number of prescriptions recorded in the primary care medical record during the 3 months following randomisation for patients in each study group and overall. No significant differences in medication uses between the three groups were found.
| Prescription | Control, n (%) | Oral antibiotic, n (%) | Topical antibiotic, n (%) | Overall, n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Children (n = 33) | Items (n = 105) | Children (n = 33) | Items (n = 102) | Children (n = 33) | Items (n = 163) | Children (n = 97) | Items (n = 370) | |
| Steroid: mild | 12 (36.4) | 14 (13.3) | 10 (30.0) | 19 (18.6) | 14 (45.2) | 24 (14.7) | 36 (37.1) | 57 (15.4) |
| Steroid: moderate | 17 (51.5) | 19 (18.1) | 7 (21.2) | 15 (14.7) | 13 (41.9) | 20 (12.3) | 37 (38.1) | 54 (14.6) |
| Steroid: potent | 2 (6.1) | 2 (1.9) | 1 (3.0) | 1 (1.0) | 6 (19.3) | 9 (5.5) | 9 (9.3) | 12 (3.2) |
| Antifungal/steroid combination | 2 (6.1) | 2 (1.9) | 1 (3.0) | 1 (1.0) | 4 (12.9) | 4 (2.4) | 7 (7.2) | 7 (1.9) |
| Topical antibiotic/steroid combination | 3 (9.1) | 3 (2.8) | 8 (24.2) | 9 (8.8) | 3 (9.7) | 5 (3.1) | 14 (14.4) | 17 (4.6) |
| Antibiotic: oral | 2 (6.1) | 2 (1.9) | 6 (18.2) | 7 (6.8) | 7 (22.6) | 8 (4.9) | 15 (15.5) | 17 (4.6) |
| Antibiotic: topical | 2 (6.1) | 3 (2.8) | 1 (3.0) | 1 (1.0) | 2 (6.4) | 3 (1.8) | 5 (5.1) | 7 (1.9) |
| Antihistamine: oral | 0 (0) | 0 (0) | 4 (12.1) | 2 (1.9) | 2 (6.4) | 5 (3.1) | 6 (6.2) | 7 (1.9) |
| Emollient: leave on | 21 (63.6) | 40 (38.9) | 18 (54.4) | 35 (34.3) | 23 (74.2) | 63 (38.6) | 62 (63.9) | 138 (37.3) |
| Emollient: bath | 13 (33.4) | 18 (17.1) | 7 (21.2) | 11 (10.8) | 10 (32.2) | 17 (10.4) | 30 (30.9) | 46 (12.4) |
| Barrier cream | 1 (3.0) | 2 (1.9) | 0 (0) | 0 (0) | 1 (3.2) | 1 (0.6) | 2 (2.1) | 3 (1.0) |
| Bandages | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (6.4) | 3 (1.8) | 2 (2.1) | 3 (1.0) |
| Silk clothing | 0 (0) | 0 (0) | 1 (3.0) | 1 (1.0) | 1 (3.2) | 1 (0.6) | 2 (2.1) | 2 (0.5) |
The costs of medications prescribed in primary care over the 3 months following recruitment are presented by treatment group in Table 18.
| Control (n = 33) | Oral antibiotic (n = 33) | Topical antibiotic (n = 31) | |||
|---|---|---|---|---|---|
| Mean (SD), £ | Median (IQR), £ | Mean (SD), £ | Median (IQR), £ | Mean (SD), £ | Median (IQR), £ |
| 13.63 (13.19) | 12.81 (3.42–20.23) | 28.71 (67.80) | 17.07 (0.0–28.01) | 41.25 (62.29) | 18.78 (6.75–54.88) |
Time off work
Table 19 describes the cost of parental time off from work as a result of their child’s eczema. A total of 16.5 days were missed overall.
| First 4 weeks | Weeks 5–12a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 35), £ | Oral antibiotic (n = 33), £ | Topical antibiotic (n = 30), £ | Control (n = 24), £ | Oral antibiotic (n = 26), £ | Topical antibiotic (n = 21), £ | ||||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 12.83 (53.98) | 0.00 (0.00–0.00) | 0.00 (0.00) | 0.00 (0.00–0.00) | 14.97 (41.41) | 0.00 (0.00–0.00) | 3.74 (18.33) | 0.00 (0.00–0.00) | 19.00 (47.81) | 0.00 (0.00–0.00) | 6.41 (21.46) | 0.00 (0.00–0.00) |
Sensitivity analyses, process measures and post-hoc analyses
Medication adherence
In total, 97 (86%) participants provided data on use of study medication. The overall mean level of adherence [proportion of total recommended doses (28)] to oral antibiotic (or matched placebo) was 61.3%. Adherence in the oral antibiotic group was slightly higher (70.4%) although the differences between groups were not significant. Only 38 (39.2%) participants adhered to 80% or more of doses and 9 (9.3%) adhered to 100% of doses. The overall mean level of adherence [proportion of total recommended applications (21)] to topical antibiotic (or matched placebo) was 81.8%, and there was no significant difference by treatment group. A high proportion of participants, 73 (75.3%), adhered to 80% or more of recommended applications and 22 (22.7%) adhered to 100% of recommended applications (Table 20).
| Medication group | Adherence | Control, n (%) | Oral antibiotic, n (%) | Topical antibiotic, n (%) |
|---|---|---|---|---|
| Oral study medication | Mean adherence, % | 55.1 | 70.4 | 58.3 |
| 80% adherence | 12 (34.3) | 14 (42.4) | 12 (41.4) | |
| 100% adherence | 2 (5.7) | 4 (12.1) | 3 (10.3) | |
| Topical study medication | Mean adherence, % | 80.4 | 84.1 | 80.8 |
| 80% adherence | 24 (68.6) | 26 (78.8) | 23 (79.3) | |
| 100% adherence | 10 (28.6) | 8 (24.2) | 4 (13.8) |
A CACE analysis of the primary outcome resulted in intervention effects (adjusted for adherence) of 1.53 (95% CI –2.29 to 5.34) and 1.82 (95% CI –2.39 to 6.03) for oral and topical antibiotics, respectively. These are very similar to the ITT analyses, suggesting that medication adherence did not significantly influence the results (see Appendix 4 for all CACE analysis results).
The weights of the remaining IMP products were available for 94 participants (three of the participants were ‘penicillin allergic’ – two of these were randomised to topic antibiotics and one to placebo). Mean weights of remaining IMP are presented in Table 21. There were no significant differences between groups.
| Medication | Control | Oral antibiotic | Topical antibiotic | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Flucloxacillin or placebo weight (g) | 36 | 312 (191) | 30 | 243 (102) | 25 | 260 (110) | 91 | 275 (147) |
| Erythromycin or placebo weight (g) | 1 | 126 (NA) | 0 | NA (NA) | 2 | 160 (4) | 3 | 149 (20) |
| Fusidic acid or placebo weight (g) | 37 | 37 (20) | 30 | 36 (21) | 27 | 34 (20) | 94 | 36 (20) |
Parental views about use of treatment
A total of 104 parents provided data about which treatment they thought was easier to use at 2 weeks. Of those, 76 (73.1%) thought the topical treatment was easier to use, 12 (11.5%) thought the oral treatment was easier, 14 (13.5%) indicated that they had no preference and 2 (1.9%) said they did not know. There was no association between treatment preference and allocated treatment group.
Use of topical corticosteroids
During the first 2 weeks, 55 patients used a mild TCS and 70 patients used a moderate-strength TCS. In addition, seven patients used a potent topical corticosteroid. No patients reported using very potent TCSs. We found no difference in the average number of applications per week between the three groups for any strength of TCS (Table 22).
| TCS | Control (n = 36) | Oral antibiotic (n = 34) | Topical antibiotic (n = 30) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Most potent agent used, n (%) | Used one or more dose, n | Applications per week in those using one or more dose, mean (SD) | Most potent agent used, n (%) | Used one or more dose, n | Applications per week in those using one or more dose, mean (SD) | Most potent agent used, n (%) | Used one or more dose, n | Applications per week in those using one or more dose, mean (SD) | |
| None | 4 (11.1) | – | – | 1 (2.9) | – | – | 1 (3.3) | – | – |
| Mild | 8 (22.2) | 19 | 8.4 (6.6) | 11 (32.4) | 21 | 7.0 (4.8) | 5 (16.7) | 15 | 7.1 (4.5) |
| Moderate | 22 (61.1) | 24 | 7.0 (4.1) | 19 (55.9) | 22 | 6.9 (2.8) | 22 (73.3) | 24 | 7.3 (3.9) |
| Potent | 2 (5.6) | 2 | 4.0 (2.8) | 3 (8.8) | 3 | 6.2 (5.3) | 2 (6.7) | 2 | 8.0 (1.4) |
Regional variation
Baseline POEM scores were similar in all regions [mean 14.7 (SD 5.7), 13.3 (SD 4.2), 15.0 (SD 5.4), 18.5 (SD 3.3) in Wales, Scotland, England and dermatology clinic sites, respectively]. An analysis of the primary outcome, controlling for regional effects (comparing England with Wales and Scotland) resulted in similar effect sizes to the main analyses [2.08 (95% CI –0.84 to 4.99) and 1.91 (95% CI –1.21 to 5.02) for oral and topical antibiotics, respectively].
Missing data
Baseline data for complete cases and those with missing week 2 follow-up data are presented in Table 23. The estimated intervention effects for the primary outcome, using multiple imputation for missing POEM scores at 2 weeks, were 1.26 (95% CI –1.61 to 4.12) and 1.40 (95% CI –1.51 to 4.31) for oral and topical antibiotics respectively.
| Baseline characteristic | Complete cases | Missing 2-week POEM scores | Overall | ||
|---|---|---|---|---|---|
| n | Mean (SD) or % | n | Mean (SD) or % | Mean (SD) or % | |
| Patients | |||||
| Age | 101 | 3.09 (2.65) | 11 | 2.25 (1.39) | 3.01 (2.10) |
| POEM (baseline) | 101 | 14.89 (5.44) | 11 | 15.91 (4.87) | 14.99 (5.38) |
| Sex | |||||
| Male | 59 | 58.4 | 2 | 18.2 | 54.5 |
| Female | 42 | 41.6 | 9 | 81.8 | 45.5 |
| Ethnicity | |||||
| White | 80 | 79.2 | 11 | 100 | 81.3 |
| Mixed | 8 | 7.9 | 0 | 0.0 | 7.1 |
| Asian | 5 | 5.0 | 0 | 0.0 | 4.5 |
| Black | 5 | 5.0 | 0 | 0.0 | 4.5 |
| Chinese or other | 1 | 1.0 | 0 | 0.0 | 0.9 |
| Prefer not to answer | 2 | 2.0 | 0 | 0.0 | 1.8 |
Subgroup analysis by presence of Staphylococcus aureus on the skin at baseline
We conducted a post-hoc (unplanned) analysis of the primary outcome (POEM score at 2 weeks) comparing participants who did and did not have S. aureus cultured from a skin swab taken at baseline. The results are consistent with our primary analysis, confirming that in those with a positive skin culture for S. aureus any effect of oral and topical antibiotics is unlikely to include a clinically meaningful benefit (Table 24).
| Intervention | S. aureus skin swab culture | Intervention | Control | Intervention effect adjusted for baseline POEM (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline mean (SD) | Follow-up mean (SD) | n | Baseline mean (SD) | Follow-up mean (SD) | |||
| Oral antibiotic | Positive | 28 | 14.93 (5.67) | 8.93 (7.75) | 22 | 14.86 (4.80) | 6.68 (5.83) | 2.20 (–1.06 to 5.50) |
| Negative | 6 | 13.17 (3.43) | 5.17 (4.02) | 14 | 11.14 (4.74) | 5.36 (6.33) | –1.14 (–6.78 to 4.49) | |
| Topical antibiotic | Positive | 24 | 17.75 (5.67) | 9.83 (6.53) | 22 | 14.86 (4.80) | 6.68 (5.83) | 1.79 (–1.67 to 5.25) |
| Negative | 7 | 14.00 (4.20) | 7.57 (4.72) | 14 | 11.14 (4.74) | 5.36 (6.33) | 0.87 (–4.50 to 6.24) | |
Chapter 4 Validation of the Atopic Dermatitis Quality of Life preference-based index
This chapter describes the testing of the construct validity and the responsiveness of a new preference-based index (ADQoL) developed by the Sheffield Health Economics Group for use in children with eczema.
Introduction
NHS resources are always finite and often scarce, whereas demand on these resources can seem to be unlimited and is certainly far in excess of the budget allocation in the UK. Health economists have developed instruments to help identify the optimal decision around the distribution of these finite resources – quality-adjusted life-years (QALYs) is one such measure. 54 Cost–utility analysis is the economic evaluation of alternative treatment options assessed in terms of costs per QALY and, as such, the QALY provides a common metric to compare different treatments for the same condition and also across conditions. In the UK, the National Institute for Health and Care Excellence (NICE) recommends the use of QALYs as a measure of health benefits and the use of the generic preference-based health-related quality of life (HRQoL) measure European Quality of Life-5 Dimensions (EQ-5D)55 to determine health status. 56
National Institute for Health and Care Excellence guidelines recognise that there might be population subgroups (i.e. children) for which using EQ-5D to derive QALYs might not be appropriate ‘when EQ-5D is not appropriate for the condition or effects of treatment, the valuation methods should be fully described and comparable to those used for EQ-5D’ (i.e. preference-based HRQoL measure). 56 In relation to paediatric cost–utility analyses, 2013 NICE guidelines56 state that ‘when necessary, consideration should be given to alternative standardised and validated preference-based measures of health-related quality of life that have been designed specifically for use in children’. Nonetheless, the guidelines do not advocate the use of any specific instrument, leaving practitioners with no definite guidance.
In 2014 Adlard et al. 57 carried out a systematic review to assess within the UK how paediatric cost–utility analyses have evolved over time and the extent to which practice adhered to NICE guidelines. The authors identified 43 papers. Most used adult measures and none used children-specific instruments such as Child Health Utility Instrument – 9 Dimensions. 58 More recently, Thorrington and Eames59 carried out a systematic review to assess the methods used by researchers and health economists to estimate health utilities for children and adolescents. They concluded that researchers were left to use instruments not specifically designed for children because specific instruments are still being developed and validated. In relation to very young children and toddlers, Griebsch et al. 60 concluded from their systematic review that there was a lack of health-state classification instruments for use in children and infants who are younger than 5 years, and there is a need to understand the role of proxies for measuring and valuing child health.
The aim of this work is to use the children recruited into the CREAM study to validate the condition-specific preference-based HRQoL measure for children with eczema developed by Steven et al. ,44 known as the ADQoL, and from which QALYs can be generated. The ADQoL includes four dimensions and each dimension is covered by two options: (a) activities (‘Your child cannot join in some activities with other children’, ‘Your child is not limited in activities with other children’); (b) mood (‘Your child is very moody’, ‘Your child is not very moody’); (c) being settled (‘Your child cannot be comforted’, ‘Your child is quite settled’); and (d) sleep pattern (‘Your child sleeps badly most nights’, ‘Generally, your child sleeps well’) (see Appendix 2).
The ADQoL (a preference-based index) uses the parent as proxy to assess the quality of life of the child and there is no age limit to the child. In the CREAM study the majority of the children and toddlers are 5 years old or younger. This cohort, therefore, produced a unique sample for this validation exercise. Testing the construct validity of the instrument constitutes an important step towards the wider implementation of this preference-based outcome measure. To the best of our knowledge this instrument has never been applied to a trial population.
Methods
Study population
In the CREAM study the ADQoL was answered by the parent of the child.
Data collection
The ADQoL questionnaire was administered on paper at baseline, 2 weeks, 4 weeks and 3 months after randomisation (see Chapter 2). At 4 weeks the questionnaire included additional questions to assess the feasibility, face validity and content validity of the instrument. (The ADQoL questionnaire can be found in Appendix 2.) Parents completed it for all children. Data entry and cleaning follow up the same processes as other data collected in the study.
Hypotheses testing
In order to assess if the preference-based ADQoL reflects the quality of life of children aged between 3 months and 8 years with eczema, we a priori identified the following hypotheses:
-
The preference-based ADQoL scores should increase if the subjective perceived measure of severity of the condition (measured using POEM) decreases. 8
-
The preference-based ADQoL scores should increase if the objective perceived measure of severity of the condition (measured using EASI) decreases. 8
-
The preference-based ADQoL scores should increase if the infant quality of life (measured using IDQoL) increases. 8
-
The preference-based ADQoL scores increase over time in accordance with resolution from an acute flare of the illness.
Statistical analysis
Patterns of missing data by item were assessed over time to see if they were related to age or sex.
Spearman’s correlation was used to assess the strength of the linear relationship between the preference-based ADQoL scores and the POEM (subjective severity), the EASI (objective severity), the ADQoL and the CDLQI scores. Hinkle et al. 61 guidelines were used to interpret the coefficient of correlation. Discriminant validity of the preference-based ADQoL was assessed by measuring how the change by 3 or more units in the POEM scores was reflected in the preference-based ADQoL. The standardised response mean (SRM) was used to assess how sensitive the preference-based ADQoL scores were to change over time. 62
In order to assess the content validity of the questionnaire, respondents were asked if they thought the questionnaire was describing their child’s quality of life. Categorical and thematic analysis was used to study the carers’ narratives around these questions. 63
Results
Table 25 describes the available data collected at baseline and follow-up time points at 2 weeks, 4 weeks and 3 months.
| Questionnaire Item | Baseline, n (%) | 2 weeks, n (%) | 4 weeks, n (%) | 3 months, n (%) |
|---|---|---|---|---|
| Joining in activities with other children | ||||
| Your child is not limited in joining in activities with other children | 87 (77.0) | 85 (75.2) | 91 (80.5) | 65 (57.5) |
| Your child cannot join in some activities with other children | 23 (20.4) | 15 (13.3) | 7 (6.2) | 7 (6.2) |
| Missing | 3 (2.7) | 13 (11.5) | 15 (13.3) | 41 (36.3) |
| Your child is very moody/your child is not very moody | ||||
| Your child is not very moody | 76 (67.3) | 87 (77.0) | 79 (69.9) | 61 (54.0) |
| Your child is very moody | 34 (30.1) | 13 (11.5) | 18 (15.9) | 10 (8.8) |
| Missing | 3 (2.7) | 13 (11.5) | 16 (14.2) | 42 (37.2) |
| Your child cannot be comforted/your child is quite settled | ||||
| Your child is quite settled | 91 (80.5) | 93 (82.3) | 87 (77.0) | 66 (58.4) |
| Your child cannot be comforted | 19 (16.8) | 7 (6.2) | 11 (9.7) | 5 (4.4) |
| Missing | 3 (2.7) | 13 (11.5) | 15 (13.3) | 42 (37.2) |
| Your child sleeps badly most nights/generally, your child sleeps very well | ||||
| Generally, your child sleeps very well | 57 (50.4) | 73 (64.6) | 74 (65.5) | 56 (49.6) |
| Your child sleeps badly most nights | 53 (46.9) | 27 (23.9) | 24 (21.2) | 16 (14.2) |
| Missing | 3 (2.7) | 13 (11.5) | 15 (13.3) | 41 (36.3) |
The children with missing data were not dissimilar from the children with available data in terms of age but carers of girls were less likely to answer the questionnaire (Table 26).
| Missing data | Female, n (%) | Male, n (%) |
|---|---|---|
| Baseline | 3 (5.8) | 0 (0.0) |
| 2 weeks | 11 (21.2) | 2 (3.3) |
| 4 weeks | 13 (25.0) | 3 (4.9) |
| 3 months | 28 (53.8) | 14 (23.0) |
The developers of the instrument did not design a set of rules for imputing missing data and as a result it was decided that we would not impute any missing data for the purpose of this validation exercise.
The box plot graph (Figure 6) shows the level of dispersion of the data. Most observations are close to the maximum value possible for the health state with a series of observations tailing towards the minimum possible value for the health state (i.e. negative skewed distribution).
FIGURE 6.
Box plot of ADQoL at each time point.
Table 27 lists the key summary statistics of the ADQoL scores at each data collection point, minimum and maximum reflects the possible range of values for the measure.
| ADQoL scores | n | Minimum | Maximum | Mean | SD | Median | IQR |
|---|---|---|---|---|---|---|---|
| Baseline | 110 | 0.36 | 0.84 | 0.7268 | 0.11 | 0.7440 | 0.19 |
| 2 weeks | 100 | 0.36 | 0.84 | 0.7805 | 0.09 | 0.8410 | 0.10 |
| 4 weeks | 97 | 0.49 | 0.84 | 0.7848 | 0.08 | 0.8410 | 0.10 |
| 3 months | 71 | 0.36 | 0.84 | 0.7889 | 0.10 | 0.8410 | 0.10 |
Correlation between scores
The correlation between the ADQoL and the subjective (POEM) and objective (EASI) measures of severity was low to moderate, and in the right direction (ADQoL improving with reducing severity) and statistically significant (Table 28). A similar pattern is reflected in the correlation between the ADQoL and the IDQoL and the CDLQl measures (Tables 29 and 30), whereas the correlation with the CDLQI is stronger although the numbers for CDLQI are small.
| Time point | ADQoL | POEM | EASI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 weeks | 4 weeks | 3 months | Baseline | 2 weeks | 4 weeks | 3 months | Baseline | 2 weeks | 4 weeks | |
| Baseline, n | 1 | 0.472** | 0.382** | 0.425** | –0.422** | –0.286** | –0.153 | –0.273* | –0.325** | –0.268** | –0.236* |
| 110 | 100 | 97 | 71 | 110 | 101 | 98 | 74 | 109 | 100 | 98 | |
| 2 weeks, n | 1 | 0.513** | 0.445** | –0.279** | –0.509** | –0.475** | –0.088 | –0.295** | –0.436** | –0.402** | |
| 100 | 97 | 71 | 100 | 100 | 98 | 74 | 99 | 100 | 98 | ||
| 4 weeks, n | 1 | 0.471** | –0.315** | –0.346** | –0.500** | –0.045 | –0.195 | –0.262** | –0.345** | ||
| 97 | 71 | 97 | 97 | 97 | 74 | 96 | 97 | 97 | |||
| 3 months, n | 1 | –0.215 | –0.317** | –0.329** | –0.388** | –0.174 | –0.15 | –0.309** | |||
| 71 | 71 | 71 | 71 | 71 | 70 | 71 | 71 | ||||
| Time point | ADQoL scores | IDQoL scores | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 2 weeks | 4 weeks | 3 months | Baseline | 2 weeks | 4 weeks | 3 months | |
| Baseline, n | 1 | 0.472** | 0.382** | 0.425** | –0.436** | –0.297* | –0.137 | –0.299** |
| 110 | 100 | 97 | 71 | 76 | 68 | 67 | 50 | |
| 2 weeks, n | 1 | 0.513** | 0.445** | –0.213 | –0.477** | –0.428** | –0.058 | |
| 97 | 71 | 67 | 68 | 67 | 50 | |||
| 4 weeks, n | 1 | 0.471** | –0.180 | –0.249* | –0.435** | –0.086 | ||
| 71 | 65 | 66 | 66 | 50 | ||||
| 3 months, n | 1 | –0.340* | –0.293* | –0.294* | –0.526** | |||
| 51 | 52 | 52 | 50 | |||||
| Time point | ADQoL scores | CDLQI scores | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 2 weeks | 4 weeks | 12 weeks | Baseline | 2 weeks | 4 weeks | 12 weeks | |
| Baseline, n | 1 | 0.472** | 0.382** | 0.425** | –0.509** | –0.444* | –0.362* | –0.601** |
| 100 | 97 | 71 | 33 | 32 | 31 | 20 | ||
| 2 weeks, n | 1 | 0.534** | 0.439** | –0.603** | –0.798** | –0.470** | –0.566** | |
| 97 | 71 | 32 | 32 | 31 | 20 | |||
| 4 weeks, n | 1 | 0.541** | –0.558** | –0.664** | –0.758** | –0.742** | ||
| 71 | 31 | 31 | 31 | 20 | ||||
| 3 months, n | 1 | –0.420 | –0.662** | –0.569* | –0.800** | |||
| 19 | 19 | 19 | 19 | |||||
Discriminant validity of the preference-based Atopic Dermatitis Quality of Life Index
At 2 weeks, 75 children showed a clinically significant improvement (reduction in POEM score of 3 or more) in their subjective eczema. This was reflected in the difference in preference-based ADQoL scores at 2 weeks (Table 31).
| No clinically significant improvement in subjective eczema severity | Clinically significant improvement in subjective eczema severity | Mean difference | 95% CI | p-valuea | ||
|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | n (%) | Mean (SD) | |||
| 26 (25.7) | 0.7207 (0.1382) | 75 (74.3) | 0.8005 (0.0606) | –0.0798 | –0.1194 to –0.0402 | 0.000 |
Change of preference-based Atopic Dermatitis Quality of Life Index scores over time
Figure 7 shows the change of the preference-based scores at each time point and, as expected, the health scores suggest that the biggest improvement to the child’s health takes place between baseline and 2 weeks. This is reflected in the sensitiveness of the preference-based outcome measured by the SRM (Table 32).
FIGURE 7.
Preference-based ADQoL scores change over time.
| Time window | Mean difference | SD | SRM |
|---|---|---|---|
| Baseline to 2 weeks | 0.0525 | 0.1083 | 0.485 |
| 2 weeks to 4 weeks | –0.0022 | 0.0848 | 0.026 |
| 4 weeks to 3 months | 0.0062 | 0.0939 | 0.066 |
The SRM suggests a low to moderate response for the first change and very low sensitiveness to the changes after 2 weeks. Although given the lack of evidence of any ‘real’ change on other measures after 2 weeks it is unlikely that there is any change occurring for the SRM to detect.
Participants’ feedback on the preference-based ADQoL questionnaire
In terms of face validity, 87 parents completed the questions about the ADQoL at 4 weeks. Of these, 76 (67.3%) parents found the questionnaire easy to complete and only two participants found it very difficult to complete (Table 33).
| Ease of completion | n | % |
|---|---|---|
| Easy | 76 | 67.3 |
| Neither easy nor difficult | 2 | 1.8 |
| Somewhat difficult | 7 | 6.2 |
| Very difficult | 2 | 1.8 |
| Total | 87 | 77.0 |
| Missing | 26 | 23.0 |
| Total | 113 | 100.0 |
A total of 98 parents answered the question about whether or not the ADQoL questionnaire was describing their child’s quality of life, and 80 (82%) parents thought that the questionnaire did reflect their child’s quality of life whereas 18 (18%) did not agree with this statement.
Parents who felt that the questionnaire did not describe their child’s quality of life were asked to list the aspects that they thought were relevant and not captured in the questionnaire. Comments were noted by 17 out of 18 parents.
The narratives to these questions seem to suggest three main points:
-
The respondents felt that the questionnaire was not relevant to them because the child was too young. This comment is reflected in the children’s age; this group of children were younger compared with the rest of the cohort, mean age of 26 and 40 months, respectively.
-
Some of the respondents felt that some additional dimensions should have been added (e.g. food and diet).
-
Some of the respondents felt that the dimensions could have benefited from additional response options to accommodate health status in between those currently presented in the questionnaire.
Discussion
Preference-based outcome measures are key to helping policy-makers support the implementation of the health-care interventions that are most valued by the population. In the UK the EQ-5D is the generic preference-based HRQoL measure accepted by NICE; however, this generic measure might not be sensitive enough to capture the effect on quality of life for specific conditions (e.g. eczema) and/or specific patient groups (e.g. very young children). Steven et al. 44 developed a condition-specific preference-based HRQoL measure for children with atopic dermatitis, which is capable of generating QALYs and, as far as we are aware, has not been previously validated alongside a randomised controlled trial. The aim of this study was to validate this specific preference-based HRQoL (ADQoL preference-based index) on the patients recruited in the CREAM study.
Non-response to questionnaires and individual items are often used as rough indicators of the ease of completion of the questionnaire. In the CREAM study only one participant did not fully complete the 3-month questionnaire and the cohort of the non-responders to the questionnaire overlapped almost completely with the participants who did not complete the other questionnaires. This would suggest that the likelihood of answering the questionnaire was not linked to the questionnaire itself. Furthermore, only 9 (12%) participants reported that the questionnaire was somewhat difficult or very difficult to complete. However, although most participants found the questionnaire reasonably easy to complete, 17 participants (15%) thought that the questionnaire was not fully describing the effect of the condition on their child’s life. Not surprisingly, those who found that the questionnaire was not fully capturing their child’s quality of life tended to find it more difficult to answer. Reasons reported for this included the young age of the child, the absence of important health dimensions and the lack of enough levels attached to each health dimension.
Correlations with health outcome measures used in the study were significant and in the right direction and of moderate strength. The instrument showed good discriminate validity at 2 weeks and similarly the sensitivity to change was moderate for the change between baseline and 2 weeks.
The instrument was designed to allow parents of older children to directly ask the questions to the child and for younger children to act as a proxy. As the CREAM study mainly recruited children younger than 4 years, most questionnaires were completed by the parent acting as proxy. This did not allow separation of the performance of the instrument between these two groups.
Conclusions
If preference-based outcome measures are to be used to inform policy decisions it is paramount that they are validated and reflect the true values that the population attaches to the health states of a patient and that any change in the health status is captured. Our evaluation of the construct validity and performance characteristics of this preference-based HRQoL measure are encouraging; however, some of the respondents felt the questionnaire was not relevant to them because the child was too young, whereas others commented that each dimension could have benefited from additional response options to accommodate health status in between those currently presented in the questionnaire.
Chapter 5 Study challenges
A number of challenges were encountered in study set-up and recruitment of study participants. Qualitative analysis did not form part of the study design; however, an exploration of the challenges facing the research team and research sites was undertaken to inform recruitment strategies. Qualitative data on these challenges were collected through a combination of semistructured audio-recorded telephone interviews with a small sample of participating GPs, written responses by GPs to questionnaires, research nurse field notes, regular documented meetings and opportunistic feedback from GPs and other stakeholders. Data were thematically analysed to identify key themes.
Issues encountered prior to the recruitment phase of the study, including delays in manufacture of IMP and contracting and gaining statutory approvals, impacted on the opening of the study. However, the most significant challenges encountered were related to participant recruitment. Clinicians reported that recruitment was difficult because infected eczema in children was not common. In addition, the lack of a standard, clear definition of infected eczema, and unclear clinical equipoise, made recruitment challenging. There appeared to be a narrow margin of clinical equipoise for the inclusion of children: clinicians sometimes felt that the suspicion of infection was too weak to support the use of antibiotics, and sometimes felt that the severity of the infection was too great to risk exposure to a placebo control (treatment without either oral or topical antibiotics). These challenges, together with the time required for clinicians to refer children to the study and the loss of potential participants during the period between referral and recruitment had a significant impact on the number of participants recruited.
Manufacture and supply of trial treatments
Significant problems were encountered in sourcing the pharmaceutical products required for the study. Extensive negotiations with a number of pharmaceutical manufacturers and suppliers were required to secure the supply of the required oral and topical active antibiotics, and matched oral and topical placebo products, with sufficient assurances in terms of liability protection and provision of necessary good manufacturing practice documentation (summary product characteristics, IMP dossier, stability data). This ultimately required contracting with a pharmaceutical service provider for the supply of the matched placebo antibiotic suspensions (manufactured by a third party) and a separate pharmaceutical manufacturing unit for the supply of the active antibiotic suspensions, the active and placebo antibiotic creams, and the supply and packaging of the trial packs. Unfortunately this solution, including the protracted contracting process, the process for matching taste and appearance of the oral placebo product and required pharmaceutical stability testing, resulted in significant time delays. The additional costs for contracting with a number of pharmaceutical providers, along with changes in the market value of the active antibiotics and relatively short shelf life of the pharmaceuticals, resulted in an increase in the cost of pharmaceuticals that exceeded the original funded costs.
Contracts and research and development approvals
Initial delays in contracting processes had a knock-on effect that prolonged the set-up phase of the study and ultimately significantly delayed the start of site and participant recruitment. This impacted on other processes, such as NHS approvals as GP and pharmacy agreements need to be reviewed by NHS R&D officers prior to issuing local approvals.
The delays in obtaining NHS approvals meant that recruitment of sites and pharmacies could not progress, and therefore the initiation and training required before the study could open to recruitment of participants was delayed. The study involved 91 primary care sites and four dermatology clinic sites across England, Wales and Scotland, which required NHS approvals through three systems for co-ordinating permissions (Central Permissions Unit, National Institute of Social Care and Health Research – Permissions Coordinating Unit and NHS Research Scotland respectively). Delays were encountered due to multiple submissions of documentation and local checks being carried out at various stages and not necessarily in parallel to the global checks initially undertaken by the National Institute of Social Care and Health Research – Permissions Coordinating Unit. Responding to queries from multiple individual R&D offices, often using a variety of different processes, rather than a single centralised body or uniform system, was a significant burden on staff time during this period.
Delays in obtaining local R&D approvals were compounded by problems in obtaining required documentation from, and arranging visits with, sites. In some cases sites reported that the delays had had an effect on motivation. The impact of time taken to gain statutory approvals for research sites has previously been reported. 64,65 Despite the large number of sites recruited the majority did not recruit any participants.
The study team also encountered some difficulties in obtaining excess treatment costs in England. There are differences in the assessment and handling of excess treatment costs between England and Wales, and R&D offices in different regions in England did not have a uniform approach to the assessment or handling of excess treatment costs. This caused delay in getting approvals for excess treatment costs, and resulted in delays in recovering costs for the active treatments.
Recruitment of sites
A number of challenges were experienced in the recruitment of primary care sites. A key decision in the design of the trial had been to have research nurses visit potential participants at home, in order to minimise the burden for GP practices. However, as a result, we were limited to practices that were within travelling distance of research nurse support. This was a major challenge, both in terms of recruiting our original cohort of practices and attempts to expand once it was clear that recruitment was below target. Another key factor was distance from pharmacy sites. Our initial plan had been for GP practices to store and dispense IMP; however, given the need for reconstitution and temperature monitoring the study sponsor (Cardiff University) assessed the risk as being too great, and therefore we contracted a hospital pharmacy site in each region to store and dispense IMP. However, this led to further challenges for the study team as the research nurses’ travel time was increased by having to attend a hospital pharmacy before visiting a potential participant and, again, it made it more challenging to try expanding the study to other regions. Alternatives to dispensing by hospital pharmacy departments were considered at various times during implementation of the study, but were deemed unfeasible due to the limitations for controlled temperature storage, reconstitution of IMP and the short shelf life of IMP once reconstituted (7 days) that required same day delivery to study participants. There is a growing role for the involvement of community pharmacies in research (Royal Pharmaceutical Research Ready Accreditation Scheme – URL: www.rpharms.com/research-ready/benefits-of-research-ready.asp). However, there were no participating pharmacies that had appropriate good clinical practice and blinded IMP monitoring or accountability processes to undertake the study in the required geographical locations. Therefore, hospital clinical trials pharmacies, who generally experience high volumes of workload and long waiting times for dispensing, were required to dispense study medication.
Delays in recruitment of sites, due to the problems described above as well as other practice demands (e.g. Quality and Outcomes Framework, holiday seasons, etc.) meant that we did not have a full complement of practices (we had 60 out of a target of 90) when the study opened to recruitment in July 2013. Further practices were recruited during the study, with a total of 90 practices opened to recruitment. The number of practices open at any given time fluctuated in response to a temporary lack of research nurse support in the South East England network, and the decision to close the network in Scotland. Overall, the number of practices open to recruitment was not clearly associated with participant recruitment rates.
After 6 months of participant recruitment the number of children recruited was significantly below target and therefore a number of strategies were introduced to try and improve recruitment.
Actions taken to improve recruitment at site level
November 2013 (4 months into the study recruitment)
Feedback from some of the primary care sites indicated that participant recruitment was being limited by an increase in the proportion of children with eczema that were directly accessing secondary care dermatology (primarily nurse-led) services. Practices reported that children who were referred for assessment by dermatology specialist services were continuing to be managed by these services in the event of subsequent eczema flares. These patients would contact dermatology services directly instead of presenting in primary care general practice.
The decision was made to extend recruitment of sites to include community based dermatology services and dermatology research networks, such as the Dermatology Clinical Trials Network, were asked to inform members about the study and invite expressions of interest from interested dermatology clinicians and community dermatology services. Research nurse availability and pharmacy support were limiting factors in a number of regions where clinicians expressed an interest in participating. In addition, a number of potential sites indicated that the majority of their patient caseload were likely to be ineligible because of existing use of more potent corticosteroids. Feasibility assessments were undertaken with sites expressing an interest in participating in order to ensure that necessary resources and an appropriate case mix were in place to support the study. Consequently, four dermatology services (one in Cardiff, one in Dundee and two in the West of England) were recruited as additional study sites.
April 2014 (9 months into the study recruitment)
Following a review of recruitment across the participating regions, a decision was made to close the GP practice network in Dundee and Grampian in order to focus resources on regions where referral and recruitment rates were higher. The lack of recruitment in Scotland was attributed by GPs to the model of care in place there. In recent years children with eczema who had attended a dermatology clinic in Scotland were provided access to outpatient clinics if their eczema flared. Balancing availability of research nurse support and rates of referrals across participating regions was challenging. The research nurse was at capacity in the West of England, with no additional support available from the research networks. The situation was improved by removing the 1-week visit and clinical photography (November 2013), but insufficient research nurse capacity for conducting all necessary assessments within mandated time scales continued to be a problem in the West of England region.
June 2014 (11 months into the study recruitment)
Research networks outside of the participating regions (including National Institute for Health Research Clinical Research Network Dermatology Specialty group and UK Dermatology Clinical Trials Network) were asked to invite expressions of interest from GPs nationally as part of the recruitment extension. GP practices willing to participate were asked to assess the feasibility of the study by reviewing patient lists for numbers of children aged 0–8 years with a history of eczema, and the availability of a dispensing pharmacy and research nurse support. The requirement for all of these resources to be available before the study was viable in a region was challenging, particularly as this period coincided with the re-organisation of the National Institute for Health Research Clinical Research Network, which resulted in uncertainty about future network capacity and support. Expressions of interest were received from practices dispersed throughout the UK, often without available research nurse support able to be identified. A large research active GP practice in Cumbria with research nurse support within the practice and nearby clinical trial pharmacy support was subsequently recruited as a site. A further two GP practices were recruited in South West Wales following the decision to extend recruitment of sites in neighbouring areas and the identification of an additional pharmacy to provide dispensing services.
The additional time and resources required to identify and set up additional sites, including approvals for the inclusion of secondary care dermatology services and initiation and training of additional personnel, impacted on the conduct of the study. Unplanned costs were incurred as a result of set-up costs for additional pharmacies and equipment for sites.
Incidence of infected eczema
Feedback from participating sites was that the key challenge to recruitment was that eligible patients were not consulting very frequently. Prior to study funding, an assessment was conducted of recruitment potential by analysing data from 70 general practices in Scotland. The incidence of eczema in children aged < 5 years (the age range of children set in the commissioning brief) during a 1-year period was calculated. Then an estimate of the incidence of ‘infected eczema’ was obtained by calculating the proportion of these patients with eczema who had received a prescription for an antibiotic typically used to treat skin infections (flucloxacillin or a topical antibiotic). Using these data it was estimated that an average-sized practice (6000 patient list size) would see one or two patients per month with infected eczema. It was therefore estimated that the study would be able to meet recruitment targets in a 12-month period with 90 GP practices. This was supported by a reported 19% increase in primary care consultations for skin conditions in children and a 64% increase in prescription of flucloxacillin (used for S. aureus infections) between 1997 and 2006. 66
In response to below-anticipated recruitment and the feedback received from participating practices, we conducted some further assessments of the incidence of children presenting with infected eczema in primary care. Practices were asked to conduct a review of their medical notes using eczema-specific Read Codes, and the reported incidence was lower than anticipated.
Infected eczema represents a common and important problem across the UK, but a relatively low incidence makes it difficult for practices to identify eligible children. General practices are busy places with many service delivery demands that vary from day to day and month to month. Practices may well have had every intention of approaching all eligible children, but if they were only presenting once every month or two then it would have been harder to stay alert to the study. In addition, many practices tried to filter potential research participants to one or two clinicians who were involved in the study. With a low-frequency condition this was more difficult and patients were more likely to be missed.
We found considerable practice-level variation in coding for eczema. Practices were asked to send letters to children on their list with a Read Code for eczema in their record (using a standardised set of Read Codes), and we found significant variation in the number of letters sent out, despite relatively similar list sizes. There were also differences between practices in terms of the age of the patient populations that they served. A number of practices had a smaller than average proportion of children registered with the practice and served a local population that was relatively elderly. Some GPs reported that children with a history of recurrent infected eczema may receive antibiotics on repeat (or repeat acute) prescription so would rarely present at the surgery to be recruited and were likely to be excluded due to recent use of antimicrobial agents if they were seen in the practice. As GPs did not reliably record details of potentially eligible children screened/reviewed in their practices, there are no data regarding children who consulted their GP for infected eczema and were not referred because they were ineligible at the time of consultation. Some GPs reported they were more reluctant to prescribe antibiotics in recent years because of their concerns about antimicrobial resistance and general public awareness of antibiotic stewardship campaigns. GPs also reported that the incidence of infected eczema might be lower than previously reported due to improved eczema care such as prescription and use of emollients.
Practices with GPs with a special interest in dermatology were more likely to agree to participate in the study, but were more likely to provide an enhanced level of dermatology care overall. It is possible this may have resulted in better treatment and adherence, and therefore patients at these practices may have experienced fewer eczema flares, and/or less use of antibiotics than patients from other practices with lower levels of dermatology expertise.
Defining eligibility
There is a well-described relationship between the isolation and culture of S. aureus on the skin and the presence of eczema. The organism can be isolated from over 90% of atopic eczema lesions. 21,67–69 The presence of bacteria, particularly staphylococcus expressing exotoxins, has been shown to be associated with more severe eczema,70–72 and higher concentrations of S. aureus are associated with increasing clinical severity. 73 Therefore, it might seem reasonable to assume that (if not all) flares of eczema are probably infected. However, there is no clear, widely accepted definition for infected eczema, and guidelines often refer to more overt features such as pain, oozing, crusting, asymmetry, extensive disease, fever and malaise. 74 The presence of S. aureus on eczematous lesions does not prove a causal relationship, or that treating S. aureus will improve eczema – hence the need for the study.
We took the pragmatic approach of including children with eczema (as defined by U.K. Working Party33) who were suspected of having infected eczema by their treating clinician. This could include where:
-
the eczema is failing to respond to standard treatment with emollients and/or mild–moderate TCSs
-
there is a flare in the severity or extent of the eczema
-
there is weeping or crusting.
However, despite lack of evidence about which children will benefit from antibiotic treatment, some clinicians reported during qualitative interviews, written responses to questionnaires and informal discussion that they felt uncomfortable with entering all children with ‘suspected infection’ into the trial. A number of the clinicians reported that it was unethical to include children with more pronounced signs suggestive of infection into a trial where they may not receive any antimicrobial treatment, and others reporting unease at including children with a flare but no specific signs of infection into a trial where they may receive systemic antibiotics.
Discussions with GPs participating in the trial attempted to tackle these concerns about equipoise by highlighting the current lack of evidence to guide treatment. The definition of an eczema ‘flare’ is, in itself, subject to extensive variation and lacking in validity. 17
Nevertheless, for some clinicians there were a limited number of patients with eczema flares who they would consider entering into the study, which limited potential recruitment.
Attrition between identification and recruitment
Of patients referred to the study by participating clinicians, 33% (58/171) were not recruited. Of these, 31% (18/58) were identified by the research nurse as not meeting the study inclusion criteria, and 21% (12/58) decided that they did not wish to participate, sometimes because their child’s condition had improved by the time they were seen by the research nurse, but more frequently because parents did not have a complete understanding of the requirements of the study when they consented to the referral being made. One-quarter of those who did not wish to take part cited antibiotic or placebo use.
Another 48% (28/58) were not recruited because of difficulties in arranging a baseline visit within the study parameters. This included cases where parents were either not contactable or were unavailable for a baseline visit (22%, 13/58). Reasons stated for unavailability of the parent included work commitments and the child being in school or childcare during the period for a baseline visit. Reasons for unavailability for the research nurse to arrange the visit within the study parameters were conflict between study appointments, or the time required for dispensing and collection of IMP prior to the visit (14%, 8/58). Twelve per cent (7/58) were not recruited because of a lack of time or resources, but the exact reasons were not adequately documented. In response to the challenges experienced in trying to recruit within tight time frames, a decision was taken by the study team (and agreed by the funder and Trial Steering Committee) to increase the maximum time allowed between referral and baseline assessment from 48 (usually 24 hours) to 72 hours. Although the research nurses endeavoured to contact parents at the earliest opportunity, it was left to GP discretion whether or not it was considered safe/appropriate to refer a child on a Friday, knowing they would not be seen by a research nurse over the weekend. Difficulties remained if the child was of school age, and well enough to attend school, due to the length of time needed for a baseline visit (up to 2 hours) and the need to assess the child’s skin and take swabs during the visit.
In retrospect, the decision to use research nurses to recruit participants in the days following identification in general practice may not have been the most effective strategy. Although it helped to address the issue of general practice clinical staff not having time or flexibility to be able to conduct an unplanned 2-hour baseline assessment, it resulted in loss between identification and recruitment, deterred some parents from participating and led to difficulties in expanding the study. It would not have been possible to conduct the baseline appointment within most GP surgeries, given the time required to complete all the assessments. A scaled down appointment might have been possible and, if combined with local GP storage of trial treatments, dispensed to parents at the practice, this may have enabled us to roll out the study on a much wider scale.
Clinicians and research nurses reported that parent preference either for antibiotic use or against antibiotic use was an important factor in parents’ decisions about participation. Four parents indicated that they did not want their child to participate because they did not want their child to receive antibiotics and one parent indicated that they did not want their child to participate because they wanted antibiotic treatment and did not want to risk receiving placebo. It has been reported that there has been a shift from parents’ expectation and demand for antibiotics when their child is unwell to concerns regarding antibiotic resistance related to unnecessary prescribing. 75 This may be a result of awareness campaigns about the links between antimicrobial use and antimicrobial resistance. Future research to evaluate parental treatment preferences in terms of the perceived efficacy of antibiotics, adherence to standard eczema care and impact on quality of life may be warranted.
The study protocol required that all children referred into the study to be prescribed a mild and/or moderate potency TCS for application to affected areas on the face and body respectively, as part of standard eczema care. This was to be used concurrently with trial medication for 1 week and continued for a second week after the trial medication had stopped. A number of referrals were received where prescriptions for concurrent moderate corticosteroid medications were absent. In most cases the prescriber was contacted and a prescription for moderately potent corticosteroids obtained. Some GPs reported hesitation in prescribing moderately potent corticosteroids for infants; however, it is not known if this had any impact on recruitment.
Amendment of inclusion and exclusion criteria
In addition to supporting clinicians to maintain an awareness and interest in the study, and to continue to refer potentially eligible children, amendments were made to the inclusion and exclusion criteria in order to try and increase recruitment potential while maintaining the validity of the research question.
The eligible age range was increased from 0–5 years (as set in the commissioning brief) to 0–8 years. This was done prior to opening to recruitment and with full consent from the study funders.
The inclusion criteria were simplified in November 2013 by removing renal or hepatic impairment as an excluding factor, allowing more potent topical steroids to have been used up until 2 days prior to recruitment and the previous use of antibiotics changed from any ‘use of oral antibiotics’ to only ‘use of antibiotics for a skin infection’ as an exclusion criterion.
Recruitment barriers at site
Recruitment and retention in trials in the demanding world of primary care is extremely challenging. 76 Unique barriers to recruitment were encountered during this study.
Trial Torrent
The study design included determining the effectiveness of the use of a novel software package that aimed to assist practices in identifying patients seen during routine consultation that may be eligible for research studies open to recruitment at the practice. TT software is installed on GP workstations that should integrate with the EMR system in order to both prompt clinicians when potentially eligible patients are seen, and facilitate the transfer of data from the clinical record to the research team, thus simplifying processes for general practice staff. Given the relatively low incidence of infected eczema, the prospect of using this system was greeted with enthusiasm by most practices. However, we encountered difficulties with installation and operation of the software. Unfortunately, TT was not compatible with all practice EMR systems and therefore a non-integrated webportal had to be developed so that practices that were not able to use the integrated version were still able to use it for referring patients. While setting up the study, 42% (32/77) of practices had EMR systems that were suitable for installing TT, 26% (20/77) were due to use the non-integrated version, and 32% (25/77) were due to use faxed referrals only. However, the web-based portal did not perform as required and therefore all practices required installation of either the integrated or non-integrated version of the software on all workstations that might be used by referring clinicians. Installation required approval and co-operation from several bodies (e.g. Clinical Commissioning Groups, GP practice, NHS information technology managers). In order to install the software, a technician had to remotely dial in and this meant co-ordinating with the surgery in order to allow remote access to each workstation (for each participating clinician). There was often a need for telephone guidance to support this. This often resulted in considerable delays in installation, and this, along with problems in the actual operation of TT (such as pop-ups occurring inappropriately or not at all), led to a number of practices losing confidence in the system. As such, a decision to discontinue use of TT and make all referrals on paper was made in March 2014. However, the negative impact of this failure had a persisted effect for some practices, with them citing this as a reason for poor recruitment even after its use had been discontinued.
Consultation time
Time constraints, level of remuneration and workforce shortages are well-known barriers to effective primary care research,77 and these factors were commonly reported during interviews and informal feedback by clinicians and practice managers participating in this study as reasons for poor recruitment. Opportunistic recruitment was necessary because of the unpredictable nature of eczema flares. Although GPs did not have to obtain written consent from parents, distribute IMP or complete baseline data forms, they did have to describe and discuss the study, check eligibility, obtain consent to pass the child’s details to the study team, prescribe emollients and suitable TCSs (as per protocol), complete a prescription for study medication, and fax forms to the study team (participant details) and the site pharmacy (prescription). This placed additional burdens on clinician consultation time. The process seemed to work better in practices where reception staff were actively engaged with the study, where research nurses were employed at the practice or where a nurse practitioner was actively involved with recruitment.
Challenges to assessment and data collection
A review of the recruitment process was undertaken to identify issues that may be impacting on lower than anticipated recruitment rates, with feedback invited from participating research nurses and networks. Research nurses reported during documented meetings that the baseline and follow-up assessment visits were challenging at times, which impacted on their availability to conduct a baseline visit at short notice within a fixed time frame. The main factors contributing to this were the length of time required, having to deal with young children, who could be upset or unco-operative, and having to conduct the assessment in a non-clinical setting. Conducting visits in the participant’s home had advantages such as reducing the need for travelling arrangements; however, it also presented challenges such as interruptions from other family members, conflicting childcare or domestic duties, and also required lone-working arrangements. Clinical photography was reported to be particularly challenging, with compliance by children, adequate lighting and technical skills in use of the camera being the main challenges. Clinical photography was therefore dropped in November 2013. Undressing children sufficiently for a comprehensive examination was sometimes challenging. Electronic data collection using tablet devices presented problems on some occasions. The tablet devices required an internet connection and this was often achieved through the mobile phone network. However, network coverage was poor in many areas, particularly in North Wales, and entering data onto the online database could be frustrating and time-consuming because of slow connections and intermittent drop out. This added to the time required for an already lengthy baseline visit and could be particularly challenging in the context of a home visit with a young, unwell child who is demanding of their parent’s attention. As a result, most data collection was done using paper-based CRFs, which were later entered onto the online database on return to base. Some regions discontinued the use of electronic data collection entirely and completed paper-based CRFs only. Tablet devices were not introduced when additional regions were set up as a result of these difficulties.
Enabling factors
Research nurse support and advice
Some GPs reported that they were motivated to refer patients into the study because of the nurse support that was available through participation in the study. Participants received a consultation with a study research nurse in their own home within 72 hours of referral. GPs often do not have sufficient time to educate parents on correct application of emollient and corticosteroid therapy and may lack the skills of practical dermatology care and support. 78 Specialist dermatology nurse resources, on the other hand, are overstretched and there may be long waiting lists for dermatology services. Lack of understanding and support in the use of emollient and corticosteroid therapy for parents, which includes prescription labels on corticosteroids advising parents to ‘apply sparingly’, may result in undertreatment and subsequent ‘flare-ups’. However, TCSs are recommended as first-line treatment for flare-ups. 79 The research nurses in CREAM were able to provide additional advice and support on standard eczema care including correct dosing of corticosteroids (using the finger-tip unit measure to ensure parents understood the need to optimise treatment and not undertreat), and emollient application to optimise effect and minimise the risk of cross-infection.
Record of treatment use and adherence
Parents were asked to complete a daily symptom diary over 4 weeks, documenting doses of prescribed and over-the-counter medication as well as a rating of the severity of eczema symptoms. Overall, the diary was well received. Some parents reported that the diary was useful because it allowed them to see cause and effect relationships in treatment and flare-ups, as well as being a simple reminder to give treatment. The diary helped them to understand and take control of the eczema, and some parents’ were keen to carry on with the diary after 4 weeks.
General practitioners and research nurses reported positive feedback from parents regarding the level of support and advice given to participants and their parents. Parents were asked by the research nurse to complete an exit questionnaire at the week-4 visit that invited feedback from parents regarding their decision to participate and their experience of taking part in the study. Exit questionnaires were completed and returned by 70 parents. An improved understanding of their child’s condition and/or support for their child’s treatment was a common positive experience reported by parents. Of those completing the questionnaire, 39% (27/70) state that they benefited in terms of improved knowledge, understanding, advice, learning or support from participating in the study.
Strategies employed to try and improve recruitment
A number of strategies were used to try and improve recruitment. Regular communication with practices and principal investigators was essential. Communication strategies included monthly CREAM study newsletters, and frequent practice visits by members of the research team, with a particular aim of raising awareness among the reception staff. Practices that took a co-ordinated approach to actively identifying potential participants were generally more successful at recruiting than those with a single GP participating without support from the wider practice team.
As part of the study set-up, GP practices sent information about the study to those identified on a search of patient records as aged < 8 years with a history of consultation for eczema. Where practices had not recruited a participant for a long period of time, practices were encouraged to rescreen and resend out the information to parents of children with eczema, as there may have been new children identified since the original mailshot and it would also raise awareness about the study where this had lapsed since the previous letter. This resulted in a small number of additional potentially eligible children being identified.
When contacting practices to chase prescription faxes or to verify patient contact information, it became apparent that reception staff in some practices were not familiar with the study. A laminated prompt for reception staff to position next to their booking-in screens was created and distributed to practices by research nurses when visiting for notes reviews. The simple visual aid was intended to raise awareness and encourage everyone to get involved in the study. The prompt sheet encouraged reception staff to allocate potentially eligible patients to doctors or nurses who were involved in the study.
We consider that the most effective strategy was researcher visits to the GP practices. Members of the research team contacted and visited GP practices where there had been lower levels of eligible children reported or low levels of recruitment activity identified. It was often reported at this contact that few eligible children had been seen during recent consultations. However, this contact, while time and resource intensive, raised the profile of the study in the practice and identified staff turnover at some sites, such as practice managers. This provided opportunities to inform new practice personnel about the study and provide study-specific training as required, and some visits were followed by a subsequent increase in recruitment.
Conclusion
The CREAM study experienced delays in set-up, followed by below anticipated recruitment rates. Set-up delays were mainly due to problems with IMP procurement and site recruitment and approvals, and the main factors contributing to slow recruitment appear to be a low rate of infected eczema, problems in defining infection and clinicians and parents having equipoise, problems integrating the study into busy general practice and loss of potential participants before they could be seen by a study nurse. Many strategies were implemented to try and simplify the study and improve recruitment rates; however, the design of the study did not allow for easy expansion to other sites, and the low numbers made it very difficult for practices to remain alert and ‘catch’ potential participants when they did present.
Chapter 6 Discussion and conclusions
Summary of main results
The CREAM study randomised 113 children and over 90% had one or more ‘classical’ signs or symptoms of infection, and approximately 70% had S. aureus cultured from a skin swab. Treatment with oral and topical antibiotics was associated with non-significantly higher (worse severity) POEM scores at 2 weeks than placebo, after controlling for baseline severity. The CIs included zero, but the lower bands were substantially less than the published MCID, suggesting that these interventions are either associated with no effect or harmful effect, while ruling out a beneficial effect. POEM scores at 4 weeks and 3 months, and objective eczema severity (EASI), family impact (DFI), quality of life (IDQoL and CDLQI) and daily symptom scores all showed small effect sizes with 95% CIs that included zero. Adherence to oral antibiotic (or placebo) was moderate, with overall average adherence at 61.3% of treatment doses and adherence in the oral antibiotic group at 70.4%. Adherence to topical antibiotic was better, with 81.8% of applications being applied overall (80.8% in the topical antibiotic group). Sensitivity analyses, including adjusting for compliance and missing data and a subgroup analysis with those who had S. aureus cultured from skin swabs, were all consistent with the main findings. Flucloxacillin and erythromycin non-susceptibility in isolated pathogens was low at baseline and follow-up. However, 27% of S. aureus skin isolates were resistant to fusidic acid at baseline, and at 2 weeks this increased to 31% overall and 73% in the topical antibiotic group. By 3 months non-susceptibility rates had reverted to 15% overall and 25% in the topical antibiotic group. However, the numbers were small and the differences not statistically significant.
Strengths and limitations
The CREAM study is the largest trial to evaluate the effectiveness of oral and topical antibiotic treatment for clinically infected eczema in children, and the only trial to be conducted in primary care, where most people with eczema are treated. We used pragmatic inclusion criteria, based around clinical suspicion of infection, and interventions that are commonly used in routine clinical practice (flucloxacillin and fusidic acid are the most common oral and topical antibiotics prescribed for skin infections in the UK and are commonly used around the world). Randomisation was conducted independently, we used matched placebos and there was no indication of breaches in allocation concealment. All participants were prescribed TCSs and we measured use of all medications, including antibiotics, TCSs and other medications for eczema. In addition, we measured quality of life, impact on family, symptoms and skin pathogens. We found no evidence of differential use of medications, including TCSs, and a CACE analysis that adjusted for compliance did not alter our findings. Blinded outcome assessors used well-validated instruments to assess subjective and objective eczema severity at baseline and at follow-up. We achieved high rates of follow-up. Our primary analysis was by ITT. Analysis of longer-term follow-up and analyses controlling for missing data were all consistent with our main findings of no additional clinically important benefit from either topical or oral antibiotics.
The main weakness is that we failed to reach our recruitment target, and this limits the power of the study, particularly for longer-term follow-up and for subgroup analyses. Nevertheless, the 95% CIs around our primary outcome, difference in POEM score at 2 weeks, exclude a clinically meaningful benefit from either intervention. Other secondary findings are largely consistent with the estimate of our primary end-point analysis.
Almost a third of the children from whom we cultured S. aureus in the topical antibiotic group (but only 16% of the whole group) had organisms that were resistant to fusidic acid, and therefore this could be postulated as a reason for the lack of effectiveness of antibiotic treatments. However, resistance to flucloxacillin was only identified in one child in the oral antibiotic group (3.3% of those with S. aureus and 2.8% of all children) and yet there was a similar lack of effect in this group. Although we found no differential rate of TCSs between groups, we have few data about how use of TCSs changed following inclusion into the study or whether or not TCS use in this population that had relatively intensive education differs from that found in normal clinical practice. Nevertheless, the underlying message that antibiotics do not confer benefit in addition to optimal TCS treatment remains.
Generalisability
Although we used broad inclusion criteria and, in terms of infection severity, only excluded children with ‘severe infection’ or who, ‘required immediate admission to hospital’, our research staff received anecdotal reports from clinicians that for some patients with ‘clear infection’, either they or the child’s parent felt unhappy about including them in a trial where they risked receiving no antibiotic treatment. Most clinicians did not accurately record recruitment logs and therefore it is difficult to quantify the number of children falling into this category. Nevertheless, all children included in the study were deemed to have suspected infected eczema and over 90% had ‘classical’ signs of infection (weeping, crusting, pustules or painful skin). Significant improvement in outcome measures (symptom scores, POEM and EASI) during the follow-up period in all three groups indicates that this was a population experiencing a flare in their eczema. We identified S. aureus from the skin of 70% of participants, which is lower than the proportion reported in some studies of patients with eczema and without a clinical diagnosis of infection. 21 However, these studies are primarily based in secondary care settings, used more complicated procedures for collecting samples (e.g. adhesion of Film Stamp Check) and immediately processed samples, while our study relied on swabs being taken in patients’ homes by research nurses and sent in the post to the laboratory. Therefore, we may have failed to identify some pathogens because of the sampling procedure and transportation processes. The proportion of children in whom S. aureus was cultured from the skin decreased from baseline to follow-up in all three groups, which also suggests resolution regardless of antibiotic treatment.
Nevertheless, there was a strong feeling among clinicians and parents that there were some clinical presentations that should be treated with antibiotics, or at least a belief that the risks of no treatment were so great that including them in a trial where they might be randomised to no antibiotic treatment would be unethical. Therefore, there were children who had ‘clear evidence of infection’ who were not included in the trial, and it was difficult to clearly define this boundary. We have described our population as clearly as possible, and cannot draw conclusions about the likely effects in children with more extensive and severe disease than those included in the trial.
The participants in our study all received a visit from a nurse who had training in eczema care and a booklet with standardised advice about eczema care. As such, we have compared ‘good advice about eczema care’ with ‘good advice about eczema care’ plus antibiotics, and our results may not be generalisable to a comparison with standard eczema care in primary care. Nevertheless, all patients should be receiving good advice about eczema care, and our results suggest that, in the population studied, antibiotic treatment does not confer meaningful benefit over this standard level of care.
More than two-thirds of our participants were recruited by one centre (Bristol) and only one in three research sites (practices or dermatology clinics) that were initiated actually recruited any participants, and this may have affected generalisability. However, we have no reason to believe that patients in Scotland or Wales are likely to respond differently from those in the Bristol area, and the characteristics of recruiting and non-recruiting sites were similar.
Interpretation
The CIs for our primary effect sizes suggest that both oral and topical antibiotics may be associated with no effect or a small harmful effect, but are not associated with clinically important benefit. We conclude this as the lower bands of the 95% CIs around our estimates of the POEM scores are less than the published MCID of 3.4. This MCID was derived in a secondary care population with higher baseline POEM scores, and may not be relevant to a primary care setting like this. A MCID for primary care has not formally been established, but a recent primary care trial, in which children had lower baseline POEM scores than in the CREAM trial, assumed a MCID of 2,80 and a recent secondary care trial has assumed a MCID of 3. 81 However, even if we adopt the most conservative MCID of 2, our results still suggest no clinically important benefit at 2 weeks from either oral or topical antibiotics for clinically suspected infected flares of eczema in children. The 95% CIs for the effect sizes at 4 weeks and 3 months do include the possibility of a POEM score that is lower (beneficial) in both intervention arms by more than 3, and the estimate of effect of oral antibiotic at 3 months on the POEM score had a 95% CI that went down to –4.3. However, the point estimates for these effects were close to zero and it is likely that the lower bands included potential beneficial effect only because of the smaller sample sizes at these points. It seems unlikely that antibiotics would have no beneficial (and potentially harmful) effects during the week of treatment but then be beneficial later on.
Although, overall, resistance to antibiotics in S. aureus isolated from eczema lesions was low and had mostly returned to baseline by 3 months, we did find an increased risk of carriage of fusidic acid-resistant S. aureus from treatment with topical fusidic acid. Effect on resistance requires further assessment in larger studies.
The results of our study, the largest and most rigorous study of antibiotics for clinically infected eczema in children, are largely in keeping with previously published research in this area. Only one previous study has assessed the effects of antibiotics in children (not combined with adults) with clinically infected eczema. 82 Weinberg et al. 82 showed that oral cefadroxil (a first-generation cephalosporin) was associated with no statistically significant difference in eczema activity (measured using the Hanifin/Rajka activity scale) at follow-up when compared with placebo in a trial of 33 children (aged 6 months to 12 years) with ‘S. aureus superinfected atopic dermatitis’. Both groups showed improved eczema severity scores at follow-up, and there were similar within-group improvement but lack of between-group differences for eczema signs and symptoms (pruritus, erythema, peeling, lichenification, induration and ulceration). The only between-group differences were the proportion with ‘positive’ culture results at follow-up [4/13 (30.8%) in the cefadroxil group and 14/17 (82.4%) in the placebo group; p < 0.0001] and the proportion with, ‘clinically apparent superinfection’ at follow-up [0/13 in the cefadroxil group and 9/17 (52.9%) in the placebo group]. However, this trial is at high risk of bias because of a lack of data about blinding, no clearly defined primary outcome, small sample size and no ITT analysis (three patients in the cefadroxil group were withdrawn because of side effects, non-compliance and presence of a resistant organism). The only other trial of antibiotics for clinically infected eczema that included children (as well as adults and with no subgroup analysis for just the children) was a three-arm randomised controlled trial comparing topical betamethasone combined with gentamycin, topical betamethasone alone, or topical gentamycin alone. 83 In this trial, 83 patients were enrolled and 79 were included in the analysis. No indication is given of the number of children included or the average age of participants. Investigator-graded severity improved in all three groups, but with significantly better final (day 22) total score in the betamethasone combined with gentamycin group when compared with either agent on its own. The study was reported to be ‘double-blind’, but no information was given about allocation concealment or matching of treatments. Given the subjective nature of the outcomes measured, and no pre-specified primary outcome, the risk of bias is also high.
Three studies have evaluated the effect of oral antibiotics in children without clinically infected eczema84,85 or in a mixed group that included both infected and uninfected and only excluded severe infection. 86 A parallel group randomised controlled trial84 and a cross-over study85 evaluated flucloxacillin and cefuroxime, respectively, and both used placebo as a comparator, and the final study used a before-and-after design to evaluate cloxacillin or erythromycin (depending on the sensitivity of the organisms identified) and made within-group comparisons over time. 86 The only one of these studies to report clinical improvement associated with the use of antibiotic was Boguniewicz et al. ’s non-blinded before-and-after study. 85 Neither of the randomised controlled trials found a significant clinical benefit associated with antibiotic treatments. Indeed, the trial of flucloxacillin found worse clinical outcomes (pooled efficacy scores) in the intervention group, which is similar to findings from our study. 84
Low levels of adherence to flucloxacillin could be a reason for the lack of effectiveness that we found (only 42.4% of patients in the oral antibiotic arm adhered to 80% or more of the recommended doses). Flucloxacillin is well known to have an unpleasant bitter taste which is likely to affect adherence. 87 However, adherence in clinical practice is likely to be as bad or even worse (without nurse monitoring and a diary to complete) and therefore our results reflect what is likely to be seen in clinical practice. Furthermore, adherence to topical antibiotic was much better (79.3% of those in the topical antibiotic group adhered to 80% or more of the recommended applications), but resulted in a similar lack of clinically important benefit.
Our evaluation of the construct validity and performance characteristics of the ADQoL are encouraging and suggest that it may be a good instrument for measuring QALYs in children with eczema. However, 15% of parents thought it was not reflecting the effect of the illness on their child, and it may be that it is not appropriate for younger children or that more items need to be added.
We found that a smaller proportion of the parents of girls completed the questions about quality of life than did the parents of boys. This may be a chance finding or may represent a genuine difference between the way that parents of girls and boys respond, although it is difficult to think of a rational explanation for such a difference.
Conclusions
Implications for health care
Our results provide the clearest evidence to date that neither topical nor oral antibiotics are likely to benefit children with mild clinically infected eczema. However, it is difficult to evaluate the applicability of these findings to children with more severe infected eczema. Clinicians were asked to include children with ‘infected eczema’, but some children with more severe signs of infection were excluded. The children in the study were clearly children with a flare in their eczema (POEM, EASI and symptom severity scores reduced significantly over the first week), and the majority had at least some degree of crusting and painful skin, and most had S. aureus cultured from their skin swabs. However, objective assessments found that only just over 20% and 16% had weeping and pustules, respectively, and therefore results need to be interpreted with caution in children with these features, especially if the eczema is severe. Nevertheless, although this trial was about clinically infected eczema, and did not seek to evaluate these interventions in those with no signs of infected eczema, the lack of benefit in children with mild or minimal ‘classic’ signs of infection would suggest that children who have a flare of eczema without classic signs of infection are unlikely to benefit from antibiotics.
Recommendations for research
One of the key needs is for more research into the diagnosis, and especially the prognosis, of ‘infection’ in children with eczema. Although our data provide some evidence about the limited benefit of antibiotics in children with ‘less severe’ signs of infection, without a greater understanding of how different eczema flare phenotypes are defined and behave, it is difficult to provide clear messages about when antibiotics should and should not be used. There are not even standard definitions of what constitutes an eczema ‘flare’, and this hampers eczema research. 17 Further research could involve identifying characteristics that are associated with better recovery and/or lower counts of S. aureus cultured from the skin. If successful, this may allow parents and clinicians to feel it is more acceptable to include children (who are not high risk) to be included in a subsequent trial evaluating the effects of antibiotics. Although there is a need for more trial evidence, we found that some clinicians and parents were reluctant to include children with eczema that was clinically infected into a randomised controlled trial where they might not receive any antibiotics. The publication of this trial, showing a lack of benefit in those without signs of severe infection, may shift the balance towards willingness to include children in a placebo-controlled trial, but experience from negative trials of antibiotics for other conditions suggest that it can take a long time for opinions to shift. Other types of design may be possible, such as an adaptive trial that increases the chances of being randomised to an arm where there is a beneficial effect.
Qualitative research to evaluate parental treatment preferences and parent and clinician beliefs about antibiotics and the management of eczema in general, would help give a clearer understanding of likely barriers to implementing the results of this study and conducting further research in this area.
Children in all three of our treatment groups had a substantial improvement in the severity of their eczema over the first week following randomisation. Although there may have been some spontaneous resolution, recovery is likely to have been substantially facilitated by use of standard treatments, and in particular, TCSs, which have a substantial evidence base. However, despite good evidence of effectiveness, these agents are often not used and therefore research should be conducted into how to increase uptake of these effective interventions, including how to combat ‘steroid phobia’. 88 Further understanding of the poor adherence to oral flucloxacillin that was found in the study is also important.
Although our evaluation of the ADQoL suggested reasonable construct and face validity, we believe that further research into its performance (e.g. sensitiveness to change) is needed before it can be widely recommended for use.
Acknowledgements
Contributions of authors
Nick A Francis (GP and Reader in Primary Care and Public Health) was the lead applicant and co-chief investigator for the CREAM study. He had overall responsibility for the overall study design and implementation, and the writing of the background, methods, results and discussion sections of the report, with final approval of the report submission.
Matthew J Ridd (GP and Consultant Senior Lecturer in Primary Health Care) was a co-investigator for the study and contributed to the overall study design, study implementation and approved the final version of the report.
Emma Thomas-Jones (Research Fellow) was the lead for study management, contributed to the study design and implementation, and to the writing of the methods and results sections of the report. She also was responsible for co-ordinating the compilation, formatting, proofreading and final approval of the report.
Victoria Shepherd (Research Associate/Research Nurse) contributed to the study management, study design and study implementation, and to the writing of the challenges section of the report. She contributed to the proofreading of the report and final approval of the report.
Christopher C Butler (GP, Professor of Primary Care Medicine) was a co-investigator for the study and contributed to the overall study design, study implementation, interpretation of findings and approved the final version of the report.
Kerenza Hood (Professor in Statistics, Director of Centre for Trials Research, Cardiff University) was a co-investigator and contributed to the overall study design, study implementation, supervision of the statistical and economic analyses, and approved the final version of the report.
Chao Huang (Statistician) contributed to the study design, conducted the statistical data analysis, and to writing the methods and results sections, and final approval of the report.
Katy Addison (Research Assistant) contributed to the design of CRFs and to the data management of the study, methods and results sections, and to proofreading the report.
Mirella Longo (Health Economist) conducted the statistical analysis and reporting of the validation exercise of the preference-based ADQoL and of patient NHS resource use, and final approval of the report.
Charis Marwick (Senior Clinical Academic Fellow and Consultant in Infectious Diseases) was a co-investigator and contributed to conduct of the study, critical review of drafts and approval of the final version of the report.
Mandy Wootton (Lead Scientist) was responsible for the integrity of the microbiology data and contributed to the overall study design, data analysis and writing of the methods and results section of the report, with respect to microbiology.
Robin Howe (Consultant Microbiologist and Head of the Wales Public Health Laboratory Reference Laboratory) was a co-investigator, lead microbiologist and contributed to the overall study design and final approval of the report.
Amanda Roberts (Public and Patient Representative) contributed to the study design, study implementation in terms of public and patient involvement, and review of the report.
Mohammed Inaam-ul Haq (Trial Manager) contributed to the study design, study implementation and review of the report.
Vishnu Madhok (GP) contributed to the study design, study implementation and review of the report.
Frank Sullivan (GP and Professor of Primary Care) was a co-chief investigator for the study and contributed to the overall study design, study implementation and approved the final version of the report.
Other members of the study team
We would like to thank and acknowledge the input from Professor David Cohen (Health Economist) and Manju Kalavala (Dermatologist), who contributed to the study design.
In addition, we would also like to thank Dr Eleri Owen-Jones (Trial Manager), Dr Natasha Kalebic (Data Manager), Mr Miquel Cossio (Database Developer) and Ms Jane Woodhead (Data Manager) for their contribution to the study implementation and management.
We would also like to thank the following research nurses for their contribution to the conduct of the study: Nicola Ball, Anna Gilbertson, Rebecca Elliott-Jones, Lyn Liddiard, Pauline Jones, Linda Phillips, Melissa Van Der Bijl, Karen Jones, Mandy Cook, Mat Williams, Hayley Prout, Susan Macfarlane, Debbie Rice, Fiona Treanor and Fiona Gammie.
Finally, we would like to thank Jennifer Richards and Leanne Davies (Specialist Antimicrobial Chemotherapy Unit Laboratory, Cardiff), who contributed to the acquisition of microbiology data.
Independent members of the Study Steering Committee and Data Monitoring Committee
We would like to thank our Trial Steering Committee for their continued support. Dr Anthony Harden (Trial Steering Committee Chairperson, University of Oxford), Dr Kim Thomas (Professor of Applied Dermatology Research and Co-Director of the Centre of Evidence Based Dermatology, University of Nottingham) and Therese Gladys Hingco (Patient and Public Representative), and also our Independent Data Monitoring Committee, Dr Sonia Saxena (Independent Data Monitoring Committee Chairperson, Clinical Senior Lecturer and GP, Imperial College London), Dr Miriam Santer (Clinical Research Fellow and GP, University of Southampton) and Mark Mullee (Senior Lecturer in Medical Statistics, University of Southampton).
Administrative team
We would like to thank all the administrative team across the centres, namely Kerry Fuery (Research Administrator, Cardiff University), Sian Dawes (Research Administrator, Cardiff University), Julia Carver (Study Administrator, University of Bristol) and Janice Broomhall (Project Manager, University of Dundee).
Research networks
We also wish to thank the providers of nursing/clinical studies officer support in all three centres. These include staff at the National Institute for Social Care and Health Research, Clinical Research Centre in Wales [all three regions (North Wales, South West Wales and South East Wales)]. In Bristol the Primary Care Research Network in the South West, and in Scotland the Scottish Primary Care Research Network.
Recruitment sites
We would like to thank all our recruiting sites across the three regions.
In Wales:
-
Ely Bridge Surgery
-
Practice of Health Surgery
-
Highlight Park Medical Practice
-
Avicenna Medical Centre
-
Cardiff Road Surgery
-
Stanwell Surgery
-
Pen Y Cae Surgery
-
Meddygfa Gelligaer Surgery
-
Clifton Surgery
-
Llandaff North Medical Centre
-
Plas Menai Surgery
-
Clarence House Surgery.
In Scotland:
-
North Glen Medical Practice
-
Linburn Medical Practice
-
Ravenswood Surgery.
In West England:
-
Bradgate Surgery
-
Malago Surgery
-
Air Balloon Surgery
-
Clevedon Riverside Group
-
Wellspring Surgery
-
Backwell & Nailsea Medical Centre
-
Frome Valley Medical Centre
-
Portishead Medical Centre
-
Chew Medical Practice
-
Southville Surgery
-
Oldfield Surgery
-
Yorkleigh Surgery
-
Portland Practice
-
St Chad’s Surgery
-
Courtside Surgery
-
Gloucester Road Medical Centre
-
Kingswood Health Centre
-
Yeovil District Hospital.
And finally we would also like to thank the children and families who participated in the study, without whom this study would not have been possible.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Taieb A. The natural history of atopic dermatitis. J Am Acad Dermatol 2001;45:S4-5.
- Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups . Is eczema really on the increase worldwide?. J Allergy Clin Immun 2008;121:947-54. http://dx.doi.org/10.1016/j.jaci.2007.11.004.
- Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 2014;69:3-16. http://dx.doi.org/10.1111/all.12270.
- McNeill G, Tagiyeva N, Aucott L, Russell G, Helms PJ. Changes in the prevalence of asthma, eczema and hay fever in pre-pubertal children: a 40-year perspective. Paediatr Perinat Ep 2009;23:506-12. http://dx.doi.org/10.1111/j.1365-3016.2009.01057.x.
- Levy ML. Atopic dermatitis: understanding the disease and its management. Curr Med Res Opin 2007;23:3091-103. http://dx.doi.org/10.1185/030079907X242593.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children?. Br J Dermatol 2001;144:514-22. http://dx.doi.org/10.1046/j.1365-2133.2001.04077.x.
- Atopic Eczema in Children. London: NICE; 2007.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol 2004;150:284-90. http://dx.doi.org/10.1111/j.1365-2133.2004.05776.x.
- Barnetson RS, Rogers M. Childhood atopic eczema. BMJ 2002;324:1376-9. http://dx.doi.org/10.1136/bmj.324.7350.1376.
- Absolon CM, Cottrell D, Eldridge SM, Glover MT. Psychological disturbance in atopic eczema: the extent of the problem in school-aged children. Br J Dermatol 1997;137:241-5. http://dx.doi.org/10.1046/j.1365-2133.1997.18121896.x.
- Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol 2006;155:145-51. http://dx.doi.org/10.1111/j.1365-2133.2006.07185.x.
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract 2006;60:984-92. http://dx.doi.org/10.1111/j.1742-1241.2006.01047.x.
- Camfferman D, Kennedy JD, Gold M, Martin JA, Winwood P, Lushington K. Eczema, sleep, and behavior in children. J Clin Sleep Med 2010;6:581-8.
- Camfferman D, Kennedy JD, Gold M, Martin JA, Lushington KA. Eczema and sleep and its relationship to daytime functioning in children. Sleep Med Rev 2010;6:359-69. http://dx.doi.org/10.1016/j.smrv.2010.01.004.
- Lawton S. Atopic eczema: the current state of clinical research. Br J Nurs 2014;23. http://dx.doi.org/10.12968/bjon.2014.23.20.1061.
- Santer M, Burgess H, Yardley L, Ersser SJ, Lewis-Jones S, Muller I, et al. Managing childhood eczema: qualitative study exploring carers’ experiences of barriers and facilitators to treatment adherence. J Adv Nurs 2013;69:2493-501. http://dx.doi.org/10.1111/jan.12133.
- Langan SM, Schmitt J, Williams HC, Smith S, Thomas KS. How are eczema ‘flares’ defined? A systematic review and recommendation for future studies. Br J Dermatol 2014;170:548-56. http://dx.doi.org/10.1111/bjd.12747.
- Langan SM, Silcocks P, Williams HC. What causes flares of eczema in children?. Br J Dermatol 2009;161:640-6. http://dx.doi.org/10.1111/j.1365-2133.2009.09320.x.
- Langan SM, Williams HC. What causes worsening of eczema? A systematic review. Br J Dermatol 2006;155:504-14. http://dx.doi.org/10.1111/j.1365-2133.2006.07381.x.
- Cork M, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, et al. Epidermal barrier dysfunction in atopic dermatitus. J Invest Dermatol 2009;129:1892-908. http://dx.doi.org/10.1038/jid.2009.133.
- Higaki S, Morohashi M, Yamagishi T, Hasegawa Y. Comparative study of staphylococci from the skin of atopic dermatitis patients and from healthy subjects. Int J Dermatol 1999;38:265-9. http://dx.doi.org/10.1046/j.1365-4362.1999.00686.x.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol 1974;90:525-30. http://dx.doi.org/10.1111/j.1365-2133.1974.tb06447.x.
- Goh CL, Wong JS, Giam YC. Skin colonization of Staphylococcus aureus in atopic dermatitis patients seen at the National Skin Centre, Singapore. Int J Dermatol 1997;36:653-7. http://dx.doi.org/10.1046/j.1365-4362.1997.00290.x.
- Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, Bi ZG, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol 2006;155:680-7. http://dx.doi.org/10.1111/j.1365-2133.2006.07410.x.
- Gomes PL, Malavige GN, Fernando N, Mahendra MH, Kamaladasa SD, Seneviratne JK, et al. Characteristics of Staphylococcus aureus colonization in patients with atopic dermatitis in Sri Lanka. Clin Exp Dermatol 2011;36:195-200. http://dx.doi.org/10.1111/j.1365-2230.2010.03962.x.
- Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immun 2010;125:4-5. http://dx.doi.org/10.1016/j.jaci.2009.11.027.
- Birnie AJ, Bath-Hextall FJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002/14651858.cd003871.pub2.
- Bath-Hextall FJ, Birnie AJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dermatol 2010;163:12-26. http://dx.doi.org/10.1111/j.1365-2133.2010.09743.x.
- National Costing Report: Atopic Eczema in Children. London: NICE; 2008.
- Hon KL, Wang SS, Lee KK, Lee VW, Leung TF, Ip M. Combined antibiotic/corticosteroid cream in the empirical treatment of moderate to severe eczema: friend or foe?. J Drugs Dermatol 2012;11:861-4.
- Heng YK, Tan KT, Sen P, Chow A, Leo YS, Lye DC, et al. Staphyloccus aureus and topical fusidic acid use: results of a clinical audit on anti microbial resistance. Int J Dermatol 2013;52:876-81. http://dx.doi.org/10.1111/j.1365-4632.2012.05747.x.
- Ravenscroft JC, Layton A, Barnham M. Observations on high levels of fusidic acid resistant Staphylococcus aureus in Harrogate, North Yorkshire, UK. Clin Exp Dermatol 2000;25:327-30. http://dx.doi.org/10.1046/j.1365-2230.2000.00654.x.
- Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, et al. The U.K. Working Party’s diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994;131:383-96. http://dx.doi.org/10.1111/j.1365-2133.1994.tb08530.x.
- Treweek S, Pearson E, Smith N, Neville R, Sargeant P, Boswell B, et al. Desktop software to identify patients eligible for recruitment into a clinical trial: using SARMA to recruit to the ROAD feasibility trial. Inform Prim Care 2010;18:51-8. http://dx.doi.org/10.14236/jhi.v18i1.753.
- The Medicines for Human Use (Clinical Trials) Regulations. London: The Stationery Office; 2004.
- legislation.gov.uk . The Medicine for Human Use (Clinical Trials) Regulations 2004 – Statutory Instrument 2004 1031 n.d. www.legislation.gov.uk/uksi/2004/1031/pdfs/uksi_20041031_en.pdf.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004;140:1513-19. http://dx.doi.org/10.1001/archderm.140.12.1513.
- Schmitt J, Langan S, Williams HC. European Dermato-Epidemiology Network . What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immun 2007;120:1389-98. http://dx.doi.org/10.1016/j.jaci.2007.08.011.
- Charman CR, Venn AJ, Ravenscroft JC, Williams HC. Translating patient-oriented eczema measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol 2013;169:1326-32. http://dx.doi.org/10.1111/bjd.12590.
- Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI evaluator group. Exp Dermatol 2001;10:11-8. http://dx.doi.org/10.1034/j.1600-0625.2001.100102.x.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The infants’ dermatitis quality of life index. Br J Dermatol 2001;144:104-10. http://dx.doi.org/10.1046/j.1365-2133.2001.03960.x.
- Lewis-Jones MS, Finlay AY. The children’s dermatology life quality index (CDLQI): initial validation and practical use. Br J Dermatol 1995;132:942-9. http://dx.doi.org/10.1111/j.1365-2133.1995.tb16953.x.
- Lawson V, Lewis-Jones MS, Finlay AY, Reid P, Owens RG. The family impact of childhood atopic dermatitis: the dermatitis family impact questionnaire. Br J Dermatol 1998;138:107-13. http://dx.doi.org/10.1046/j.1365-2133.1998.02034.x.
- Steven KJ, Brazier JE, McKenna SP, Doward LC, Cork MJ. The development of a preference-based measure of health in children with atopic dermatitis. Br J Dermatol 2005;153:372-7. http://dx.doi.org/10.1111/j.1365-2133.2005.06736.x.
- Butler CC, Hood K, Verheij TJ, Little P, Melbye H, Nuttall J, et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b2242.
- Butler CC, Robling M, Prout H, Hood K, Kinnersley P. Management of suspected acute viral upper respiratory tract infection in children with intranasal sodium cromoglicate: a randomised controlled trial. Lancet 2002;359:2153-8. http://dx.doi.org/10.1016/S0140-6736(02)09091-8.
- Hay AD, Costelloe C, Redmond NM, Montgomery AA, Fletcher M, Hollinghurst S, et al. Paracetamol plus ibuprofen for the treatment of fever in children (PITCH): randomised controlled trial. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1302.
- Hensley MJ, Chalmers A, Clover K, Gibson PG, Toneguzzi R, Lewis PR. Symptoms of asthma: comparison of a parent-completed retrospective questionnaire with a prospective daily symptom diary. Pediatr Pulm 2003;36:509-13.
- Ely B, Dampier C, Gilday M, O’Neal P, Brodecki D. Caregiver report of pain in infants and toddlers with sickle cell disease: reliability and validity of a daily diary. J Pain 2002;3:50-7. http://dx.doi.org/10.1054/jpai.2002.xb30064.
- Schram MSPI, Leeflang MMG, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2011;67:99-106. http://dx.doi.org/10.1111/j.1398-9995.2011.02719.x.
- Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al. Contamination in trials of educational interventions. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11430.
- BNF for Children [2014–2015]. London: BMJ Group, Pharmaceutical Press and RCPCH Publications; 2014.
- Rubin DB. Multiple Imputation for Non-Response in Surveys. New York, NY: John Wiley; 1987.
- Weinstein MC, Torrance G, McGuire A. QALYs: the basics. Value Health 2009;12:S5-9. http://dx.doi.org/10.1111/j.1524-4733.2009.00515.x.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. http://dx.doi.org/10.1136/bmj.316.7133.736.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Adlard N, Kinghorn P, Frew E. Is the UK NICE ‘reference case’ influencing the practice of pediatric quality-adjusted life-year measurement within economic evaluations?. Value Health 2014;17:454-61. http://dx.doi.org/10.1016/j.jval.2014.02.007.
- Stevens K. Developing a descriptive system for a new preference-based measure of health-related quality of life for children. Qual Life Res 2009;18:1105-13. http://dx.doi.org/10.1007/s11136-009-9524-9.
- Thorrington D, Eames K. Measuring health utilities in children and adolescents: a systematic review of the literature. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0135672.
- Griebsch I, Coast J, Brown J. Quality-adjusted life-years lack quality in pediatric care: a critical review of published cost-utility studies in child health. Pediatrics 2005;115:e600-14. http://dx.doi.org/10.1542/peds.2004-2127.
- Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences. Boston, MA: Houghton Mifflin; 2003.
- Norman GR, Wyrwich KW, Patrick DL. The mathematical relationship among different forms of responsiveness coefficients. Qual Life Res 2007;16:815-22. http://dx.doi.org/10.1007/s11136-007-9180-x.
- Miles MH, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. London: Sage; 1994.
- Snook H, Hutchings H, Seagrove A, Stewart-Brown S, Williams J, Russell I. Bureaucracy stifles medical research in Britain: a tale of three trials. BMC Med Res Methodol 2012;12. http://dx.doi.org/10.1186/1471-2288-12-122.
- Fudge N, Redfern J, Wolfe C, McKevitt C. Streamlined research governance: are we there yet?. BMJ n.d.;341. http://dx.doi.org/10.1136/bmj.c4625.
- Saxena S, Thompson P, Birger R, Bottle A, Spyrridis N, Wong I, et al. Increasing skin infections and Staphylococcus aureus complications in children, England, 1997–2006. Emerg Infect Dis 2010;16:500-33. http://dx.doi.org/10.3201/eid1603.090809.
- Leyden JJ, Kligman AM. The case for steroid – antibiotic combinations. Br J Dermatol 1977;96:179-87. http://dx.doi.org/10.1111/j.1365-2133.1977.tb12541.x.
- Breuer K, Haussler S, Kapp A, Werfel T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol 2002;147:55-61. http://dx.doi.org/10.1046/j.1365-2133.2002.04872.x.
- Roll A, Cozzio A, Fischer B, Schmid-Grendelmeier P. Microbial colonization and atopic dermatitis. Curr Opin Allergy Clin Lmmunol 2004;4:373-8. http://dx.doi.org/10.1097/00130832-200410000-00008.
- Ong P, Leung DY. The infectious aspects of atopic dermatitis. Immunol Allergy Clin 2010;30:309-21. http://dx.doi.org/10.1016/j.iac.2010.05.001.
- Cardona ID, Cho SH, Leung DYM. Role of bacterial superantigens in atopic dermatitis: implications for future therapeutic strategies. Am J Clin Dermatol 2006;7:273-9. http://dx.doi.org/10.2165/00128071-200607050-00001.
- Leung AD, Schiltz AM, Hall CF, Liu AH. Severe atopic dermatitis is associated with a high burden of environmental Staphylococcus aureus. Clin Exp Allergy 2008;38:789-93. http://dx.doi.org/10.1111/j.1365-2222.2008.02964.x.
- Williams RE, Gibson AG, Aitchison TC, Lever R, Mackie RM. Assessment of a contact-plate sampling technique and subsequent quantitative bacterial studies in atopic dermatitis. Br J Dermatol 1990;123:493-501. http://dx.doi.org/10.1111/j.1365-2133.1990.tb01455.x.
- Atopic Eczema in Under 12s: Diagnosis and Management. London: NICE; 2007.
- Finkelstein JA, Dutta-Linn M, Meyer R, Goldman R. Childhood infections, antibiotics and resistance: what are parents saying now?. Clin Pediatr 2014;53:145-50. http://dx.doi.org/10.1177/0009922813505902.
- Bower P, Wallace P, Ward E, Graffy J, Miller J, Delaney B, et al. Improving recruitment to health research in primary care. Fam Pract 2009;26:391-7. http://dx.doi.org/10.1093/fampra/cmp037.
- Yallop JJ, McAvoy BR, Croucher JL, Tonkin A, Piterman L. Primary health care research – essential but disadvantaged. Med J Aust 2006;185:118-20.
- Kernick D, Cox A, Powell R, Reinhold D, Sawkins J, Warin A. A cost consequence study of the impact of a dermatology-trained practice nurse on the quality of life of primary care patients with eczema and psoriasis. Br J Gen Pract 2000;50:555-8.
- Frequency of Application of Topical Corticosteroids for Atopic Eczema. NICE Technology Appraisal Guidance TA81. London: NICE; 2004.
- Santer M, Muller I, Yardley L, Burgess H, Selinger H, Stuart BL, et al. Supporting self-care for families of children with eczema with a web-based intervention plus health care professional support: pilot randomized conrolled trial. J Med Internet Res 2014;16. http://dx.doi.org/10.2196/jmir.3035.
- Harrison EF, Haines RH, Cowdell F, Sach TH, Dean T, Pollock I, et al. A multi-centre, parallel group superiority trial of silk therapeutic clothing compared to standard care for the management of eczema in children (CLOTHES trial): study protocol for a randomised controlled trial. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-0921-9.
- Weinberg E, Fourie B, Allmann B, Toerien A. The use of cefadroxil in superinfected atopic dermatitis. CTR 1992;52:671-6. http://dx.doi.org/10.1016/s0011-393x(05)80509-0.
- Wachs GN, Maibach HI. Co-operative double-blind trial of an antibiotic/corticoid combination in impetiginized atopic dermatitis. Br J Dermatol 1976;95:323-8. http://dx.doi.org/10.1111/j.1365-2133.1976.tb07021.x.
- Ewing CI, Ashcroft C, Gibbs AC, Jones GA, Connor PJ, David TJ. Flucloxacillin in the treatment of atopic dermatitis. Br J Dermatol 1998;138:1022-9. http://dx.doi.org/10.1046/j.1365-2133.1998.02271.x.
- Boguniewicz M, Sampson H, Leung SB, Harbeck R, Leung DY. Effects of cefuroxime axetil on Staphylococcus aureus colonization and superantigen production in atopic dermatitis. J Allergy Clin Immun 2001;108:651-2. http://dx.doi.org/10.1067/mai.2001.118598.
- Dhar S, Kanwar AJ, Kaur S, Sharma P, Ganguly NK. Role of bacterial flora in the pathogenesis and management of atopic dermatitis. Indian J Med Res 1992;95:234-8.
- Baguley D, Lim E, Bevan A, Pallet A, Faust SN. Prescribing for children – taste and palatability affect adherence to antibiotics: a review. Arch Dis Child 2012;97:293-7. http://dx.doi.org/10.1136/archdischild-2011-300909.
- Hon KL, Kam WY, Leung TF, Lam MC, Wong KY, Lee KC, et al. Steroid fears in children with eczema. Acta Paediatr 2006;95:1451-5. http://dx.doi.org/10.1080/08035250600612298.
Appendix 1 Trial Torrent
Following the request from the Health Technology Assessment to evaluate use of TT we developed a protocol for randomising practices to use TT or not. Practices that were randomised to no TT prompts would still have TT installed, in order to collect data about number of children with relevant Read Codes seen and so that it could still be used for collecting and transmitting data, but the prompts would be turned off.
Evaluation of the Trial Torrent software
General practitioner practices that were willing to use TT were randomly assigned to receive TT prompts or not. Randomisation occurred at the time of practice enrolment and was minimised by list size (< 6000, > 6000) and whether or not the practice had been research active in the past 12 months.
The plan was to compare the mean recruitment rate (recruited patients per month recruiting per whole-time equivalent participating clinician) for GP practices in each arm (of the TT evaluation) at 3 and 6 months (from the start of patient recruitment). In addition, at 3 months from the start of recruitment, to compare the time to first patient recruited in the two groups. A significant improvement in either the mean recruitment rate or time to first patient recruited at 3 months or in mean recruitment rate at 6 months would prompt the evaluation to stop and TT to be rolled out to the rest of the practices. As the analysis of the mean recruitment rate at 3 and 6 months were interim analyses, the significance level for those tests needed to be adjusted if we were to retain an overall significance level of 5%. The Pocock method was used implying a significance level of 0.022 and was used for all three analyses. With 90 practices we would have a power of 84% for detecting a hazard ratio of 0.5 with an estimated SD of 0.5 and at the 2.2% significance level.
Appendix 2 Atopic Dermatitis Quality of Life Index: preference-based index questionnaire
Appendix 3 Factor analysis on total daily symptoms score
| Component | Initial eigenvalues | Extraction sums of squared loadings | ||||
|---|---|---|---|---|---|---|
| Total | % of variance | Cumulative % | Total | % of variance | Cumulative % | |
| 1 | 7.314 | 91.427 | 91.427 | 7.314 | 91.427 | 91.427 |
| 2 | 0.200 | 2.505 | 93.932 | |||
| 3 | 0.142 | 1.781 | 95.713 | |||
| 4 | 0.116 | 1.456 | 97.168 | |||
| 5 | 0.084 | 1.052 | 98.221 | |||
| 6 | 0.069 | 0.865 | 99.085 | |||
| 7 | 0.044 | 0.544 | 99.629 | |||
| 8 | 0.030 | 0.371 | 100.000 | |||
| Clinical feature | Component 1 |
|---|---|
| Skin redness | 0.970 |
| Skin cracking | 0.960 |
| Skin soreness | 0.973 |
| Skin itching | 0.969 |
| Sleep disturbance | 0.923 |
| Oozing or weeping from skin | 0.942 |
| Bleeding from skin | 0.949 |
| Fever | 0.962 |
Appendix 4 Complier-average causal effect analysis for key secondary outcomes
| Trial arm | Week 4 | 3 months | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline POEM, mean (SD) | Follow-up POEM, mean (SD) | Intervention effect (95% CI)a | n | Baseline POEM, mean (SD) | Follow-up POEM, mean (SD) | Intervention effect (95% CI)a | |
| Control | 35 | 13.63 (4.97) | 8.03 (5.95) | – | 25 | 13.68 (5.07) | 7.72 (5.52) | – |
| Oral antibiotic | 33 | 14.64 (5.42) | 8.36 (7.71) | –0.35 (–4.37 to 3.68) | 28 | 14.43 (5.66) | 7.86 (6.09) | –0.29 (–3.84 to 3.27) |
| Topical antibiotic | 29 | 16.41 (5.36) | 8.93 (4.97) | –0.27 (–4.46 to 3.92) | 21 | 16.38 (5.45) | 7.86 (5.85) | –1.22 (–4.80 to 2.36) |
| Trial arm | Week 2 | Week 4 | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline EASI, mean (SD) | Follow-up EASI, mean (SD) | Intervention effect (95% CI)a | n | Baseline EASI, mean (SD) | Follow-up EASI, mean (SD) | Intervention effect (95% CI)a | |
| Control | 34 | 5.79 (4.98) | 2.50 (5.64) | – | 34 | 5.79 (4.98) | 4.01 (6.55) | – |
| Oral antibiotic | 33 | 7.43 (6.16) | 3.10 (3.65) | 0.27 (–0.18 to 0.72) | 33 | 7.43 (6.16) | 3.23 (3.81) | –0.19 (–0.66 to 0.27) |
| Topical antibiotic | 29 | 9.70 (9.96) | 4.27 (4.65) | 0.55 (0.02 to 1.09) | 29 | 9.70 (9.96) | 4.47 (6.40) | –0.06 (–0.66 to 0.53) |
| Trial arm | Week 2 | Week 4 | 3 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline DFI, mean (SD) | Follow-up DFI, mean (SD) | Intervention effect (95% CI)a | n | Baseline DFI, mean (SD) | Follow-up DFI, mean (SD) | Intervention effecta | n | Baseline DFI, mean (SD) | Follow-up DFI, mean (SD) | Intervention effect (95%CI)a | |
| Control | 35 | 5.31 (4.73) | 2.60 (4.76) | – | 35 | 5.31 (4.73) | 3.11 (4.86) | – | 24 | 6.11 (4.81) | 3.50 (4.28) | – |
| Oral antibiotic | 33 | 6.51 (5.32) | 3.71 (4.49) | 0.28 (–0.25 to 0.80) | 33 | 6.51 (5.32) | 3.52 (4.60) | –0.01 (–0.58 to 0.56) | 25 | 6.65 (5.47) | 3.46 (4.40) | –0.04 (–0.74 to 0.66) |
| Topical antibiotic | 29 | 7.79 (5.55) | 4.17 (4.54) | 0.17 (–0.26 to 0.61) | 29 | 7.79 (5.55) | 4.07 (4.83) | –0.05 (–0.58 to 0.48) | 20 | 6.55 (4.85) | 4.10 (5.52) | –0.06 (–0.62 to 0.50) |
| Trial arm | Week 2 | Week 4 | 3 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline IDQoL, mean (SD) | Follow-up IDQoL, mean (SD) | Intervention effect (95% CI)a | n | Baseline IDQoL, mean (SD) | Follow-up IDQoL, mean (SD) | Intervention effecta | n | Baseline IDQoL, mean (SD) | Follow-up IDQoL, mean (SD) | Intervention effect (95%CI)a | |
| Control | 20 | 9.53 (2.62) | 6.07 (3.69) | – | 20 | 9.53 (2.62) | 6.92 (3.74) | – | 16 | 9.63 (2.63) | 7.25 (2.59) | – |
| Oral antibiotic | 24 | 10.01 (3.87) | 6.60 (3.28) | 0.12 (–0.18 to 0.42) | 24 | 10.11 (3.87) | 6.59 (3.23) | –0.05 (–0.38 to 0.27) | 18 | 10.48 (4.07) | 6.01 (3.15) | –0.28 (–0.56 to 0.00) |
| Topical antibiotic | 21 | 10.10 (4.13) | 6.96 (2.87) | 0.19 (–0.07 to 0.44) | 21 | 10.10 (4.13) | 6.95 (2.91) | 0.03 (–0.26 to 0.32) | 15 | 9.53 (3.94) | 6.67 (3.50) | –0.08 (–0.33 to 0.17) |
| Trial arm | Week 2 | Week 4 | 3 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline CDLQI, mean (SD) | Follow-up CDLQI, mean (SD) | Intervention effect (95% CI)a | n | Baseline CDLQI, mean (SD) | Follow-up CDLQI, mean (SD) | Intervention effecta | n | Baseline CDLQI, mean (SD) | Follow-up CDLQI, mean (SD) | Intervention effect (95%CI)a | |
| Control | 14 | 7.61 (5.95) | 1.82 (1.98) | – | 14 | 7.61 (5.95) | 4.64 (5.89) | – | 8 | 8.88 (7.07) | 6.18 (6.37) | – |
| Oral antibiotic | 9 | 9.80 (4.39) | 4.07 (3.04) | 0.67 (–0.19 to 1.53) | 9 | 9.80 (4.39) | 4.36 (4.79) | –0.20 (–1.19 to 0.79) | 6 | 8.22 (4.32) | 5.57 (6.73) | –0.18 (–1.07 to 0.72) |
| Topical antibiotic | 8 | 7.99 (4.22) | 3.97 (2.71) | 0.77 (0.11 to 1.43) | 8 | 7.99 (4.22) | 3.04 (2.22) | –0.23 (–0.93 to 0.47) | 6 | 6.48 (3.40) | 4.61 (4.59) | –0.18 (–1.13 to 0.77) |
List of abbreviations
- ADQoL
- Atopic Dermatitis Quality of Life Index
- ANCOVA
- analysis of covariance
- CACE
- complier-average causal effect
- CDLQI
- Children’s Dermatology Life Quality Index
- CI
- confidence interval
- CNA
- colistin and nalidixic acid
- CREAM
- ChildRen with Eczema, Antibiotic Management
- CRF
- case report form
- DFI
- Dermatitis Family Impact
- EASI
- Eczema Area and Severity Index
- EMR
- electronic medical record
- EQ-5D
- European Quality of Life-5 Dimensions
- GP
- general practitioner
- HRQoL
- health-related quality of life
- IDQoL
- Infant’s Dermatology Quality of Life instrument
- IMP
- investigational medicinal product
- ITT
- intention to treat
- MCID
- minimal clinically important difference
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NICE
- National Institute for Health and Care Excellence
- POEM
- Patient-Orientated Eczema Measure
- QALY
- quality-adjusted life-year
- R&D
- research and development
- SAE
- serious adverse event
- SD
- standard deviation
- SEWTU
- South East Wales Trials Unit
- SMPU
- St Mary’s Pharmaceutical Unit
- SRM
- standardised response mean
- TCS
- topical corticosteroid
- TT
- Trial Torrent