Notes
Article history
This issue of Health Technology Assessment contains a project originally commissioned by the MRC but managed by the Efficacy and Mechanism Evaluation Programme. The EME programme was created as part of the National Institute for Health Research (NIHR) and the Medical Research Council (MRC) coordinated strategy for clinical trials. The EME programme is funded by the MRC and NIHR, with contributions from the CSO in Scotland and NISCHR in Wales and the HSC R&D, Public Health Agency in Northern Ireland. It is managed by the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC) based at the University of Southampton.
The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from the material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Featherstone et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Stroke is the major cause of acquired adult physical disability and is responsible for 12% of all deaths in the UK. In Europe alone, there are approximately one million new cases of stroke a year. Atherosclerotic stenosis of the carotid artery is an important cause of stroke, which may be heralded by a transient ischaemic attack (TIA) or minor stroke, which recovers without causing serious disability. The risk of recurrent stroke in recently symptomatic patients with severe carotid stenosis is as high as 28% over 2 years. The European Carotid Surgery Trial and the North American Symptomatic Carotid Endarterectomy Trial (NASCET) demonstrated that this risk was reduced significantly by carotid endarterectomy (CEA) compared with best medical treatment alone. 1–3 Carotid surgery has, therefore, become a standard treatment for these patients. However, the trials showed a significant risk of stroke or death resulting from surgery of between 6% and 8%. Surgery also caused significant morbidity from myocardial infarction (MI) during the general anaesthetic used in most centres and minor morbidity, including cranial nerve palsy and wound haematoma, from the incision.
The potential benefit of endovascular treatment (angioplasty with or without stenting) as an alternative to CEA was first highlighted by the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS). 4 This trial showed that endovascular treatment largely avoided the main complications of the endarterectomy incision (namely cranial nerve injury and severe haematoma). However, the rate of stroke or death within 30 days after treatment was high in both groups. Since completion of CAVATAS, stenting has largely replaced angioplasty, and stents and protection devices specifically designed for the carotid artery have been introduced.
At the time of the inception of the International Carotid Stenting Study (ICSS), stenting was a new method of treating carotid stenosis, which had evolved from the technique of percutaneous transluminal angioplasty. Stenting avoids some of the hazards of surgery and has become an established treatment for peripheral and coronary artery stenosis. Stenting is considered less invasive than CEA and has advantages in terms of patient comfort, because the procedure avoids an incision in the neck and is usually conducted under local anaesthesia. Hospital stay need only be for 24 hours after treatment if uncomplicated. When given the choice, stenting is preferred by many patients. However, stenting does not remove the atheromatous plaque, has not been shown to prevent stroke and might have an unacceptable incidence of restenosis.
Rationale
It would have been inappropriate to use the results of CAVATAS to propose the widespread introduction of percutaneous transluminal angioplasty for the treatment of carotid stenosis as an alternative to surgery, because the 95% confidence interval (CI) surrounding the 10% risk of any stroke within 30 days of treatment in the surgical and angioplasty groups was ± 5%. Nevertheless, the results supported the need for further randomised studies. The interventional technique used to treat carotid stenosis evolved during CAVATAS, from the use of simple inflatable balloon catheters at the beginning of the trial to the increasing use of stenting towards the end of the trial. Initially, stents were used only as a secondary procedure for inadequate results or complications of treatment after full balloon inflation. The desire to prevent these complications and superior early results in stented patients led to the increasing use of the technique of primary stenting, in which the intention is to deploy a stent in every patient before dilatation (but after pre dilatation if required to allow the atraumatic passage of the stent) of the artery. 5 Primary stenting is now accepted as best interventional practice6 and has become the radiological technique of choice for carotid stenosis, replacing balloon angioplasty. ICSS was initiated to provide randomised trial evidence on whether or not carotid artery stenting (CAS) was as effective as CEA in preventing recurrent stroke that is associated with symptomatic carotid artery stenosis.
Potential risks and benefits
Both surgical endarterectomy and stenting carry a risk of causing a stroke at the time of the treatment. Previous trials showed a significant risk of stroke or death at the time of surgery or stenting of between 6 and 10 in every 100 patients. However, patients were randomised to the study because the risk of strokes resulting from surgical or stenting treatment was believed to be less than leaving the carotid artery narrowing untreated. The majority of major strokes after carotid percutaneous transluminal angioplasty are the result of dissection of the carotid artery at the time of balloon inflation with subsequent thrombosis. It is believed that stenting is safer than simple balloon angioplasty because embolisation, dissection and closure of the carotid artery are less likely to occur. 5,6 The subgroup analysis of stented patients in CAVATAS was consistent with this suggestion. The adverse consequences of dissection are minimised because the stent maintains laminar flow across the stenosis and seals the site of dissection, preventing a free intimal flap. In addition, the stent mesh limits the size of any thrombus or atheromatous debris that may be dislodged from the plaque at the time of dilatation of the artery. Superior dilatation achieved by stenting compared with balloon angioplasty may also reduce the rate of stroke in the early post-treatment period. In the coronary circulation, stenting has been shown to produce superior outcomes compared with balloon angioplasty. 7,8 Individual case series suggested that carotid stenting has a similar rate of procedural stroke to that of carotid surgery. 5,6,9
Surgery also carries a risk of perioperative MI. Approximately 1 in 10 patients has temporary tongue or facial weakness as a result of cranial nerve palsy. A large blood clot (haematoma) may form at the site of incision, which may require removal. Surgery results in a permanent scar in the neck. Angiography and stenting may also result in bruising or haematoma at the site of injection (usually in the groin) and can cause temporary discomfort or pain in the neck. There is a small risk of allergic reactions to the contrast reagent used during angiography.
Although acceptable safety at the time of stenting had been suggested by the case series and registry data, at the time ICSS was initiated, stenting had not been subjected to a randomised trial in comparison with conventional surgical treatment and had not been demonstrated to prevent stroke, which is the aim of treatment. 10 Stenting does not remove the atheromatous plaque and stents may stimulate neointimal hyperplasia. In the long term, it is possible that the rate of restenosis would be greater after stenting than after carotid surgery, which could well result in an unacceptable rate of long-term stroke recurrence. There was, therefore, an important need to establish the efficacy of carotid stenting in comparison to surgery at a time when the technique was being widely introduced without adequate trial-based evidence for its safety and effectiveness.
Chapter 2 Methods
Objective
The objective of ICSS was to compare the risks, benefits and cost-effectiveness of a treatment policy of referral for carotid stenting compared with referral for carotid surgery.
Design
The ICSS was an international, multicentre, randomised controlled, open, prospective clinical trial comparing carotid surgery with carotid stenting. The trial was approved by the Northwest Multicentre Research Committee in the UK and by local ethics committees outside the UK. The full version of the protocol is available at www.cavatas.com. 11
Participants
Patients of either sex over the age of 40 years with symptomatic atherosclerotic stenosis of the carotid artery were included in the trial. The consent form and patient information form are shown in Appendices 1 and 2, respectively. The inclusion and exclusion criteria are outlined below.
Inclusion criteria
-
Symptomatic, extracranial, internal or bifurcation atheromatous carotid artery stenosis suitable for both stenting and surgery, and deemed by the randomising clinician to require treatment.
-
The severity of the stenosis of the randomised artery should be at least 50% (as measured by the NASCET method or non-invasive equivalent).
-
Symptoms must have occurred in the 12 months before randomisation. It was recommended that the time between symptoms and randomisation should be < 6 months, but patients with symptoms occurring between 6 months and 12 months could be included if the randomising physician considered that treatment was indicated.
-
The patient had to be clinically stable following their most recent symptoms attributable to the stenotic vessel.
-
Patients had to be willing to have either treatment, be able to provide informed consent and be willing to participate in follow-up.
-
Patients had to be able to undergo their allocated treatment as soon as possible after randomisation.
-
Any patient > 40 years of age could be included.
-
Patients could only be randomised if the investigator was uncertain which of the two treatments was best for that patient at that time.
Exclusion criteria
-
Patients refusing either treatment.
-
Patients unable or unwilling to give informed consent.
-
Patients unwilling or unable to participate in follow-up for whatever reason.
-
Patients who had previously had a major stroke with no useful recovery of function within the territory of the treatable artery.
-
Patients with a stenosis that was known to be unsuitable for stenting prior to randomisation because of one or more of:
-
tortuous anatomy proximal or distal to the stenosis
-
presence of visible thrombus
-
proximal common carotid artery stenotic disease
-
pseudo-occlusion (‘string sign’).
-
-
Patients not suitable for surgery owing to anatomical factors (e.g. high stenosis, rigid neck).
-
Patients in whom it was planned to carry out coronary artery bypass grafting or other major surgery within 1 month of carotid stenting or endarterectomy.
-
Carotid stenosis caused by non-atherosclerotic disease (e.g. dissection, fibromuscular disease or neck radiotherapy).
-
Previous CEA or stenting in the randomised artery.
-
Patients in whom common carotid artery surgery was planned.
-
Patients medically not fit for surgery.
-
Patients who had a life expectancy of < 2 years owing to a pre-existing condition (e.g. cancer).
Interventions
Stenting protocol
Stenting was carried out as soon as possible after randomisation using percutaneous transluminal interventional techniques from the femoral, brachial or common carotid artery by a designated interventional consultant using an appropriate stent. A cerebral protection system was used whenever the operator thought that one could be safely deployed. Stents and other devices used in the trial had to be Conformité Européenne (CE) marked and approved by the steering committee.
Pre-medication was at the discretion of the interventionist, although the protocol recommended the combination of aspirin and clopidogrel as the antiplatelet regime of choice to cover the period of stenting and for a minimum of 4 weeks afterwards. Intra-procedural heparin was mandatory at a dose determined by the operator; post-procedural heparin could be given according to clinical requirements.
The trial protocol stated that atropine, or a similar agent, must be administered prior to stent deployment to counteract any effects on the carotid artery baroreceptors, which could lead to severe bradycardia or asystole.
Angiographic images showing the stenosis at its most severe prior to stenting and the same view and any other view that demonstrated the maximum residual stenosis after stenting were collected by the trial office.
Details of the procedure, including all periprocedural complications, drug therapy and devices used in the procedure, were reported on the stenting and cerebral protection technical data sheet which was returned to the trial central office.
Endarterectomy protocol
Endarterectomy was carried out as soon as possible after randomisation by a designated consultant surgeon. It was carried out using whichever procedures were standard at the individual centre, including the use of local or general anaesthesia, shunts or patches as required by the operating surgeon. Standard or eversion endarterectomy could be performed. Details of the procedure, including all periprocedural complications, drug therapy and type of endarterectomy performed, were reported on the surgery technical data sheet which was returned to the trial central office.
Approval of surgeons and interventionists, and credentialing of less-experienced operatives
An accreditation committee decided if surgeons and interventionists at enrolling centres had the appropriate experience and expertise to join the study. Surgeons and interventionists were expected to show a stroke and death rate within 30 days of treatment, consistent with the centres in the European Carotid Surgery Trial who had an average rate of 7.0% with a 95% CI of 5.8% to 8.3%. 1 Surgeons were expected to have performed a minimum of 50 carotid operations with a minimum annual rate of at least 10 cases per year. Interventionists were expected to have performed a minimum of 50 stenting procedures, of which at least 10 should have been in the carotid territory. Centres that had little or no experience of carotid stenting were allowed to join ICSS for a probationary period in order to gain the minimum experience of 10 carotid stenting procedures required to join the trial fully. Stenting procedures carried out during the probationary period were proctored by an experienced carotid interventionist appointed by the trial steering committee, until the proctor was satisfied that the interventionist(s) at the centre could satisfactorily carry out procedures unproctored. Probationary interventionists became fully enrolled in ICSS when the proctor was satisfied that the interventionist could perform procedures unsupervised and the interventionist had 10 or more successfully completed cases in the trial, with an acceptable complication rate.
Reporting of suspected problems with surgical or stenting techniques at individual centres
The database manager at the trial office monitored periprocedural outcome events, and if there were two consecutive deaths or three consecutive major events at a single centre within 30 days of treatment in the same arm of the study, then assessment of the events was triggered. A blinded assessment of the relevant outcome events was submitted by the central office to the chairman of the data monitoring committee who had the power to recommend further action, such as suspending randomisation at the centre. A cumulative major event or death rate of more than 10% over 20 cases would also trigger careful assessment of the relevant outcome events.
Data collected at baseline
Baseline data collected at randomisation included demographic data; existing medical risk factors; neurological symptoms and an assessment of disability using the modified Rankin Scale (mRS); current antiplatelet therapy and blood pressure; and films and reports of pre-randomisation brain imaging and the results of duplex ultrasound (DUS).
Randomisation
Randomisation was performed by a telephone call or fax to a computerised service provided by the Oxford Clinical Trials Service Unit. It was stratified by centre with minimisation of the main risk factors and balanced between the arms. Patients who needed treatment of both carotid arteries could only be randomised for the carotid artery to be treated first. Two patients were randomised at the Service Unit by coin toss when the computerized service was not available.
Follow-up
Patients were followed up by a neurologist or a physician interested in stroke, who was not involved in the revascularisation procedure but who was not masked to group assignment, at the participating centres at 30 days after treatment, 6 months after randomisation and, then, annually after randomisation.
At each visit, levels of stroke-related disability were assessed using the mRS and any relevant outcome events notified to the central trial office. DUS examinations of the carotid arteries were carried out at each follow-up visit at centres with available facilities. Bilateral peak systolic and end diastolic velocities in the internal carotid artery and the peak systolic velocity in the common carotid artery were recorded. The data were collected on a follow-up form and an ultrasound report form (see Appendices 3 and 4), which were returned to the central trial office where the data were entered into a Microsoft Access (Microsoft Corporation, Redmond, WA, USA) database. In addition to the clinical data, patients were asked to complete a EuroQol European Quality of Life-5 Dimensions – 3 level response (EQ-5D-3L™) questionnaire (see www.euroqol.com) to assess quality of life and health status at baseline, after stenting or surgery at 1 month, and then at 6-month and annual follow-up visits.
Patients were followed up to the end of the trial in 2011 (a maximum of 10 years after randomisation). Patients reaching their 5-year follow-up before the end of the trial were asked if they were willing to carry on with follow-up, in which case they continued with annual follow-up until the end of the trial.
Outcomes
The following events were collected and analysed as trial outcome events:
-
any stroke or death
-
TIA
-
MI within 30 days of treatment
-
cranial nerve palsy within 30 days of treatment
-
haematoma caused by treatment requiring surgery, transfusion or prolonging hospital stay
-
stenosis ≥ 70% or occlusion during follow-up
-
further treatment of the randomised artery by interventional radiology techniques or surgery after the initial attempt
-
quality of life, health status and health service costs.
Outcome events included in the safety analysis or primary outcome (stroke, MI within 30 days of treatment, death) were documented in detail by the investigating centre, censored after receipt at the central office to remove clues as to the treatment allocated, and then adjudicated by a neurologist at the central office and by an independent external neurologist. If the external neurologist’s adjudication differed from the central office, a third independent neurologist reviewed the event and the majority opinion prevailed. The major event and death forms are shown in Appendices 5 and 6.
Centres were asked to supply the following information for adjudication, whenever possible:
-
a report of the event using the standard trial case report form
-
a film copy of a computerised tomography (CT) or magnetic resonance imaging (MRI) brain scan as soon as possible after the event, together with a film copy of the pre-randomisation scan (if done) and a report of the event
-
copies of discharge summaries, death certificates and post-mortem results (if relevant).
Disability after stroke and cranial nerve palsy was assessed 30 days after treatment or at onset using the mRS. Duration of symptoms was recorded and outcome events were classified as disabling if the mRS score was 3 or more at 1 month. If the patient was not seen at exactly 30 days after onset of the event, the investigator was asked to estimate the 30-day mRS.
The degree of carotid stenosis during follow-up was determined in the study central office based on flow velocity data using pre-defined criteria,12 masked to treatment allocation and date of ultrasound follow-up. Results of carotid imaging studies ordered outside regular follow-up at the discretion of the treating clinicians (e.g. for recurrent symptoms) were also included. The main outcome event of the restenosis analysis was defined as any severe (≥ 70%) residual or recurrent stenosis, or occlusion of the carotid artery during follow-up. No correction was made for the presence of a stent when measuring stenosis severity.
Statistical methods
All analyses were conducted according to the statistical analysis plan for the short-term (safety) analysis or the long-term analysis (see Appendix 7), which provides a detailed and comprehensive description of the main, pre-planned analyses for the study. Analyses were performed with Stata statistical software version 12.1 or earlier (StataCorp LP, College Station, TX, USA), except for the MRI substudy and the study on the effect of white-matter lesions on periprocedural stroke, which used SPSS statistical software version 17 (SPSS Inc., Chicago, IL, USA) and version 21 (IBM Corp., Armonk, NY, USA), respectively.
The main features of the analysis plan are summarised below.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is used to summarise representativeness of the study sample and patient throughput (see Figure 1). Baseline characteristics are presented by treatment group with continuous variables presented with means and standard error.
The analyses compare the treatment groups with respect to the length of time before treatment failure (i.e. occurrence of an outcome event) by means of the Mantel–Haenszel chi-squared test and Kaplan–Meier survival curves with a two-sided p-value of 0.05 (5% level) used to declare statistical significance with a 95% CI reported throughout. Secondary analyses compare the proportions of outcome events within 30 days of treatment. All analyses are adjusted for centre and pre-determined risk factors. Subgroup analyses examine risk factors for outcome events.
Cox proportional hazard models were used to calculate the hazard ratio (HR) and 95% CI with endarterectomy as the reference group using all available follow-up data. Log-rank tests were used to compare the two survival curves. Censoring was assumed to be non-informative.
As the restenosis outcome was interval-censored it was instead analysed using a generalised non-linear model which assumes proportional hazards and whose treatment effect parameter estimate can be interpreted as a log-HR. The treatment effect p-value for the restenosis outcome was calculated using a likelihood ratio test. Life-table analyses were used to estimate the cumulative incidences of restenosis at 1 year and 5 years after treatment.
Interaction tests were performed to investigate whether or not the relative treatment effect for the pre-defined primary long-term outcome, as well as for procedural stroke or death or ipsilateral stroke during follow-up, differed across various patient groups. Functional ability at the final follow-up or at death was compared between treatment groups across the entire range of the mRS at 1-year and 5-year follow-up using the permutation test described by Howard et al. 13 Drug treatments and blood pressure at 1-year and 5-year follow-up were compared using chi-squared tests and t-tests at each time point, respectively.
Sample size (original and revised)
At the commencement of recruitment in 2001 we planned to recruit 2000 patients, but this was revised shortly after the start of the trial in 2003 to 1500 in response to the initial funding period and taking into account the observed recruitment rate to that date. For 1500 patients, the 95% CI was the observed difference ± 3.0 percentage points for the outcome measure of 30-day stroke, MI and death rate and ± 3.3 percentage points for the outcome measure of death or disabling stroke over 3 years’ follow-up. The difference detectable with power 80% was 4.7 percentage points for 30-day outcome and 5.1 percentage points for survival free of disabling stroke. Similar differences were detectable for secondary outcomes. In 2007, the steering committee modified the protocol to emphasise that the sample size of 1500 patients should reflect only patients recruited at experienced centres, to ensure that the study would be adequately powered to compare outcomes of stenting performed by experienced interventionists with endarterectomy. An extension of funding was therefore obtained to allow the recruitment of a total of 1700 patients, anticipating that 200 of these would come from centres with probationary investigators.
Protocol amendments
The major amendments to the protocol during the course of the trial are detailed in Appendix 8. In brief, in addition to the modifications to the sample size described above, in 2003, clarification of the rules governing proctoring of probationary centres and the maximum permissible delays between symptoms and randomisation were added to the protocol. In 2007, an amendment was made to state that data from patients enrolled at probationary centres would be analysed separately from data from fully enrolled experienced centres. Subsequently, the steering committee decided after completion of recruitment and initial analysis of the results that the data from probationary and fully enrolled centres should be analysed together, because there was no significant difference in the results (indeed the results were slightly better at probationary centres).
The International Carotid Stenting Study–magnetic resonance imaging substudy: symptomatic and asymptomatic ischaemic and haemorrhagic brain injury following protected and unprotected stenting versus endarterectomy
Clinical follow-up of patients in ICSS was not masked to treatment allocation and, therefore, there was the possibility of potential bias in the ascertainment of non-disabling strokes. We therefore planned a substudy of ICSS at centres with sufficient neuroimaging facilities and capacity in which we would use multimodal MRI as an additional outcome measure of procedural cerebral ischaemia that could be analysed without knowledge of treatment allocation. We aimed to compare the risk of procedural ischaemia and persistent infarction on MRI between patients randomly allocated to receive stenting or endarterectomy. Moreover, diffusion weighted imaging (DWI), a modern MRI technique, may show ischaemic lesions after carotid interventions even in patients who do not experience symptoms. 14 In previous studies, new ischaemic lesions on DWI were detected more frequently after stenting than after endarterectomy. 15–21 DWI lesions were also more frequent after unprotected stenting than after protected stenting. 22,23 However, selection bias and the use of historical controls might account for the observed differences in these non-randomised comparisons. In addition, it was not clear how ischaemic lesions on DWI relate to the risk of clinically apparent cerebrovascular events (stroke or TIA) associated with the intervention. Larger studies with randomised treatment allocation were needed to gain further insight into the significance of asymptomatic DWI lesions and their potential role as surrogate markers of treatment risk.
Cerebral protection devices are used in stenting with the aim of reducing the risk of plaque embolisation during the procedure. Recently completed randomised trials comparing the safety of stenting and endarterectomy yielded conflicting results. 24,25 Concern that stenting without cerebral protection might be associated with an increased risk of stroke led to the abandonment of unprotected procedures in one trial,25 but in another trial, there was no difference in the risk of stenting with and without protection. 24 Although clear evidence that cerebral protection enhances treatment safety is lacking,26 protection devices were widely used, significantly contributing to the cost of carotid stenting. We therefore planned to carry out an exploratory analysis of the MRI data to investigate the effect of cerebral protection devices on the risk of ischaemia associated with stenting.
Objectives
The primary objective of this substudy was to compare the risk of ischaemic brain injury assessed on MRI in patients with symptomatic carotid artery stenosis undergoing stenting in comparison to those undergoing endarterectomy.
Secondary objectives were: to assess the effect of protection devices on the risk of ischaemic brain injury associated with stenting; to compare the risk of haemorrhagic brain injury assessed on MRI in stenting compared with endarterectomy; and to gain further insight into the usefulness of ischaemic and haemorrhagic brain lesions on MRI as surrogate markers of the risk of carotid interventions.
The ICSS–MRI substudy was designed to allow a randomised comparison of the procedural risk of symptomatic and asymptomatic ischaemic and haemorrhagic brain injury visible on MRI between stenting and endarterectomy. The use of cerebral protection devices in patients undergoing stenting was not subject to randomisation in ICSS. However, the participating centres systematically used either protected or unprotected stenting. The risk of brain injury associated with either stenting technique could, therefore, be compared with a randomised control group of patients undergoing endarterectomy.
Outcome measures and analyses were defined as follows:
Primary outcome measure: rate of symptomatic and asymptomatic ischaemic brain injury detectable on MRI after endarterectomy and stenting.
Secondary analyses:
-
interaction between the use of protection devices and ischaemic brain injury in patients undergoing stenting
-
rate of symptomatic and asymptomatic haemorrhagic brain injury detectable on MRI after endarterectomy and stenting
-
relation of brain injury on MRI to risk of stroke during procedure and follow-up.
Inclusion criteria
Patients were eligible to participate in the ICSS–MRI substudy if they were enrolled in the ICSS trial and separately provided written informed consent to undergo three MRI exams.
Exclusion criteria
Patients with contraindications to MRI (e.g. pacemakers, metallic implants and claustrophobia) were excluded from the ICSS–MRI substudy.
Magnetic resonance imaging protocol
Patients enrolled in the ICSS–MRI substudy had three MRI investigations, at 1–3 days before, 1–3 days after and 30 ± 3 days after the intervention. The following sequences were performed in all three investigations:
-
DWI to detect acute ischaemic brain injury associated with the procedure
-
gradient echo T2 star-weighted sequences to detect haemorrhagic brain injury associated with the procedure
-
T1-weighted, T2-weighted and fluid-attenuated inversion recovery sequences were used to assess whether or not acute brain lesions detected on DWI after the procedure led to permanent scarring at 1 month.
Data acquisition
Baseline data (such as age, sex, medical risk factors, degree of carotid stenosis, etc.) were collected as part of ICSS. Two researchers, one a neurologist and one a neuroradiologist, with several years of experience in assessing brain scans in patients with cerebrovascular disease independently scored the presence, size and location (vascular territory) of ischaemic and haemorrhagic lesions on the MRI scans. A third experienced researcher reviewed the scans in case of disagreement. The scans were reported and scored blind to patient identifiers, treatment, date and time of the scans. Patients were clinically examined by a neurologist at the time of MRI examination and followed up after treatment as part of ICSS to determine outcome events, including TIA, stroke, MI and death.
Statistical analysis
The rates of ischaemic and haemorrhagic brain lesions were compared between patients undergoing endarterectomy and stenting using chi-squared tests2 and Fisher’s exact tests. Significance was declared at p < 0.05. Exploratory analyses were performed to test the interaction between the use of cerebral protection devices and the rate of DWI lesions after stenting.
Sample size calculation
Power calculations for this substudy were based on the primary outcome measure. The two largest series reported new ischaemic lesions on DWI after CEA in 17% and 34% of patients, respectively. 27,28 If a rate of new DWI lesions after endarterectomy of 25% is assumed, a total sample size of 200 patients would have a 90% power to detect a twofold increase in the DWI lesion rate associated with carotid stenting.
Effect of white-matter lesions on the risks of periprocedural stroke after carotid artery stenting versus endarterectomy
Leukoaraiosis was associated with a higher perioperative risk of stroke or death in patients assigned to CEA in the NASCET. 29 Patients with widespread white-matter changes allocated to the best medical management group also had an increased risk of stroke or death. To the best of our knowledge, the effect of white-matter lesions on the procedural risk of stroke and death in carotid stenting has hitherto not been investigated. We therefore investigated the effect of leukoaraiosis on the risk of procedural complications in a large group of patients with recently symptomatic carotid disease randomised in ICSS in a pre-specified analysis. 30 Brain imaging by CT or MRI was needed before revascularisation.
Methods
In this study of white-matter lesions, we included all patients enrolled in ICSS in whom copies of the baseline CT or MRI done before carotid stenting or endarterectomy were available. Patients were excluded if no baseline brain imaging was available or if the quality of the images was poor. Copies of baseline brain imaging were analysed by two investigators, one a neurology research fellow and one a neuroradiologist, who were both trained in the analysis of white-matter lesions and masked to treatment and clinical outcome, for the severity of white-matter lesions using the age-related white-matter changes (ARWMC) score. Differences were resolved by consensus. Patients were divided into two groups using the median ARWMC score. We analysed the risk of stroke within 30 days of revascularisation using a per-protocol analysis. A total of 1036 patients (536 randomly allocated to CAS, 500 to CEA) had baseline imaging available. The median ARWMC score was 7, and patients were dichotomised into those with a score of 7 or more and those with a score of < 7.
Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis
A cost–utility analysis with full incremental analysis was undertaken to compare the costs and outcomes associated with stenting and endarterectomy.
Methods
Outcome measure
The outcome measure was quality-adjusted life-years (QALYs), which combine length of life and quality of life; this is consistent with National Institute for Health and Care Excellence (NICE) recommendations. Cost-effectiveness was expressed as incremental net monetary benefits (NMBs). The analysis took a UK NHS and personal social services (PSS) perspective. 31 Costs are calculated in 2013–14 Great British pounds. The time horizon was 5 years, which was long enough to reflect all important differences in costs or outcomes between the two treatments. An annual discount rate of 3.5% was applied to costs and outcomes. 31
Resource use and costs
For every patient we calculated the cost of the index procedure and the cost of follow-up using resource-use data collected prospectively in the trial. The former included surgeon and radiologist time; operating theatre time, including nursing staff, drugs, consumables and overheads; anaesthesia; materials and devices including stents, shunts, patches, cerebral protection devices, catheters, wires and sheaths; and length of hospital stay in the intensive care unit (ICU) and inpatient ward. The latter included additional carotid artery procedures; complications within 30 days of index procedure (fatal and non-fatal MI, severe haematoma and disabling cranial nerve palsy); imaging tests; drug treatment; and non-disabling, disabling and fatal strokes.
Unit costs were obtained from published and local sources,32–35 inflated where appropriate32 and multiplied by resource use. Unit costs of surgeon, radiologist and operating theatre times were hourly costs applied to procedure durations collected during the trial. The choice of stents was at the discretion of the interventionist. In the base-case analysis each stent was assigned an acquisition cost of £840 based on the cost of the most commonly used stent, the Carotid WALLSTENT® (Boston Scientific, Marlborough, MA, USA) at the lead centre; this was varied in sensitivity analysis. Unit costs of hospital stays were daily costs applied to length-of-stay data collected in the trial. Length of stay on the ICU was not collected for individual patients, but mean values were collected by centre. From these data we assumed that where patients were admitted to the ICU post-operatively it was for 1 day. Unit costs of additional carotid artery procedures were assumed to be equal to the mean cost of the index procedures. Unit costs of drug treatment were monthly costs applied to treatment durations collected in the trial. Stroke events were recorded in the trial, but the costs of managing them were not. These were obtained from supplementary analyses of data from a contemporaneous UK population-based study of all strokes, the Oxford Vascular (OXVASC) study,36,37 which were used to predict care home and hospital care costs for each stroke patient as a function of their sex, age, disability before stroke, previous history of cardiovascular disease, initial stroke severity (non-disabling, disabling, fatal) and number of recurrent strokes (see Appendix 9).
Utilities and quality-adjusted life-years
Generic health status was described at baseline (randomisation), at 3 and 6 months, and at 1, 2, 3, 4 and 5 years post-randomisation using the EQ-5D-3L descriptive system (see www.euroqol.com), containing five dimensions (mobility, self-care, usual activities, pain and discomfort, anxiety and depression) with three levels in each dimension. Each EQ-5D-3L health state can be converted into a single summary index (utility value) by applying a formula that attaches weights to each of the levels in each dimension based on valuations by general population samples. Given the perspective of our analysis, we used a value set for the UK population to calculate utility values at each time point for every participant. 38 Utility values of 1 represent full health, values of 0 are equivalent to death, negative values represent states worse than death. Patients who died were assigned a utility value of 0 at their date of death. A utility profile was constructed for every patient assuming a straight line relation between their utility values at each measurement point. QALYs for every patient from baseline to 5 years were calculated as the area under the utility profile.
Dealing with missing data
Multiple imputation was used to impute missing data for the following variables: cost of surgeon and radiologist time; cost of operating theatre time; cost of anaesthesia; cost of stents; cost of patches; cost of cerebral protection devices; cost of other materials used in stenting; cost of length of hospital stay; cost of non-fatal MI; cost of imaging tests; costs of drug treatment; cost of strokes; total cost; utility values at baseline, 3 months and 6 months post-randomisation, and 1, 2, 3, 4 and 5 years post-randomisation; and total QALYs. The cost variables were unit costs multiplied by resource use. Age, sex, study centre and treatment allocation were included in the imputation models as additional explanatory variables. We used multivariate normal regression to impute missing values and generated 20 imputed data sets. We repeated the multiple imputation several times using different random number seeds to investigate if the conclusions of the analysis changed.
Statistical methods
Mean costs, outcomes and NMBs were compared between all patients randomly assigned to stenting and to endarterectomy, irrespective of which treatment was administered and whether or not they received additional carotid artery procedures of either type. We calculated differences in mean costs and QALYs and incremental NMBs between groups. NMBs for stenting and endarterectomy were calculated as the mean QALYs per patient multiplied by the maximum willingness to pay for a QALY minus the mean cost per patient. Incremental NMBs were calculated as the difference in mean QALYs per patient with stenting versus endarterectomy multiplied by the maximum willingness to pay for a QALY minus the difference in mean cost per patient. We used the cost-effectiveness threshold range recommended by NICE of £20,000 to £30,00031 as the lower and upper limits of the maximum willingness to pay for a QALY. If the incremental NMB is positive (negative) then stenting (endarterectomy) was preferred on cost-effectiveness grounds. The QALYs gained were adjusted for age, sex, study centre and baseline utility values using regression analysis; the incremental costs were adjusted for age, sex and study centre. For each of the 20 imputed data sets we ran 1000 bootstrap replications and combined the results using equations described by Briggs et al. 39 to calculate standard errors around mean values accounting for uncertainty in the imputed values, the skewed nature of the cost data and utility values, and sampling variation. Standard errors were used to calculate 95% CIs around point estimates.
Sensitivity and subgroup analyses
A cost-effectiveness acceptability curve40 showing the probability that stenting was cost-effective compared with endarterectomy at a range of values for the maximum willingness to pay for a QALY was generated based on the proportion of the bootstrap replications across all 20 imputed data sets with positive incremental NMBs. 41 The probability that stenting was cost-effective at a maximum willingness to pay for a QALY of £20,000 and £30,000 was reported, based on the proportion of bootstrap replications with positive incremental NMBs at these values. We undertook further sensitivity analyses to evaluate the impact of uncertainty in the following components: no adjustment for age, sex, study centre and baseline utility values; complete-case analysis without imputing missing values; univariate analyses of high- and low-cost values (unit costs multiplied by resource use) for anaesthesia, operating theatre time, surgeon and radiologist time, shunts, patches, stents, cerebral protection devices, other materials used in stenting, length of hospital stay, additional carotid artery procedures, imaging, severe haematoma, disabling cranial nerve palsy, fatal and non-fatal MI, drug treatment, treating strokes (values per patient were recalculated to be 50% higher and 50% lower than the base case); and discount rate (1.5%, 5%). No significant interactions were found in any subgroup analyses of the primary outcomes in ICSS. In post-hoc subgroup analyses we calculated cost-effectiveness results separately by sex and age (≥ 70 years, < 70 years). We completed a Consolidated Health Economic Evaluation Reporting Standards statement to ensure that the cost–utility analysis was reported appropriately (see Appendix 9).
Chapter 3 Results
The short-term and long-term outcomes of ICSS have been reported in the literature. 42,43
Recruitment
Figure 1 shows the CONSORT diagram of the flow of patients through the trial. Between May 2001 and October 2008, 1713 patients from 50 centres in the UK, mainland Europe, Australia, New Zealand and Canada were enrolled and randomised. The trial centres, together with the members of the trial committees, location of recruiting centres, number of patients recruited at each centre and the names of the investigators at each centre are detailed in Appendix 10. Three patients (two in the stenting group and one in the endarterectomy group) withdrew consent immediately after randomisation and were excluded from the intention-to-treat (ITT) analysis. In total, 1511 patients were enrolled at experienced centres and 202 at supervised probationary centres: 751 (88%) of 853 patients assigned to carotid stenting and 760 (89%) of 857 patients assigned to endarterectomy were randomised at centres classified as experienced. Most patients had their allocated treatment initiated (stenting group, n = 828; endarterectomy group, n = 821). Nine patients allocated to stenting crossed over to surgery without an attempt at the procedure and a further 16 had no attempted ipsilateral endarterectomy or stenting procedure (Figure 1). Fifteen patients allocated to endarterectomy crossed over to stenting without an attempt at endarterectomy and 21 had no attempted ipsilateral procedure.
FIGURE 1.
Consolidated Standards of Reporting Trials diagram for ICSS.

Monitoring of adverse events led to concern about the stenting results of two investigators at supervised centres. These investigators were stopped from treating further patients within the trial and their centres were suspended from randomisation. All the patients allocated to stenting (n = 11, five with disabling stroke or death) or endarterectomy during the same time period (n = 9, one with fatal stroke) at these centres were included in the analyses. One of the two centres subsequently restarted randomisation with a different investigator performing stenting.
Baseline characteristics
Table 1 shows baseline characteristics of study participants.
| Baseline patient characteristic | Stenting (n = 853) | Endarterectomy (n = 857) |
|---|---|---|
| Age (years), mean (SD) | 70 (9) | 70 (9) |
| Male sex, n (%) | 601 (70) | 606 (71) |
| Vascular risk factors | ||
| Treated hypertension, n (%) | 587 (69) | 596 (70) |
| Systolic blood pressure (mmHg), mean (SD) | 147 (24) | 146 (24) |
| Diastolic blood pressure (mmHg), mean (SD) | 79 (12) | 78 (13) |
| Cardiac failure, n (%) | 23 (3) | 47 (5) |
| Angina in last 6 months, n (%) | 83 (10) | 77 (9) |
| Previous MI, n (%) | 151 (18) | 156 (18) |
| Previous CABG, n (%) | 109 (13) | 116 (14) |
| Atrial fibrillation, n (%) | 57 (7) | 59 (7) |
| Other cardiac embolic source, n (%) | 19 (2) | 16 (2) |
| Diabetes mellitus, non-insulin dependent, n (%) | 134 (16) | 147 (17) |
| Diabetes mellitus, insulin dependent, n (%) | 50 (6) | 41 (5) |
| Peripheral artery disease, n (%) | 139 (16) | 136 (16) |
| Current smoker, n (%) | 205 (24) | 198 (23) |
| Ex-smoker, n (%) | 408 (48) | 424 (49) |
| Treated hyperlipidaemia, n (%) | 522 (61) | 563 (66) |
| Total serum cholesterol (mmol/l), mean (SD) | 4.8 (1.3) | 4.9 (1.3) |
| Degree of symptomatic carotid stenosis, n (%)a | ||
| 50–69% | 92 (11) | 76 (9) |
| 70–99% | 761 (89) | 781 (91) |
| Degree of contralateral carotid stenosis, n (%)a | ||
| < 50% | 565 (66) | 561 (65) |
| 50–69% | 128 (15) | 142 (17) |
| 70–99% | 105 (12) | 110 (13) |
| Occluded | 49 (6) | 37 (4) |
| Unknown | 6 (1) | 7 (1) |
| Most recent ipsilateral event, n (%)b | ||
| Ischaemic hemispheric stroke | 393 (46) | 376 (44) |
| TIA | 273 (32) | 303 (35) |
| Retinal infarction | 26 (3) | 23 (3) |
| Amaurosis fugax | 148 (17) | 142 (17) |
| Unknown | 13 (2) | 13 (2) |
| Event < 6 months prior to randomisation | 826 (97) | 816 (95) |
| Event 6–12 months prior to randomisationc | 27 (3) | 36 (4) |
| mRS at randomisation, n (%) | ||
| 0–2 | 756 (89) | 744 (87) |
| 3–5d | 81 (10) | 99 (12) |
Patient baseline characteristics (Table 1) and drug treatment during the trial (Table 2) were similar between the two treatment groups. At 1 year after randomisation, 97% of patients in both the stenting group and the endarterectomy group took any antiplatelet or anticoagulant; at 5 years, the percentages were 94% and 95% (Table 2). There were slightly more patients taking antihypertensive medications in the endarterectomy group at 1 year (71% vs. 75%; p = 0.088), but at 5 years the difference had reversed (83% vs. 76%; p = 0.017). However, this did not lead to any significant difference in systolic blood pressure or diastolic blood pressure (DBP) between groups at either time point. The majority of patients were treated with lipid-lowering medications with no significant difference between the groups (82% of patients in the CAS group vs. 84% in the CEA group at 1 year, and 87% of patients in the CAS group vs. 86% in the CEA group at 5 years).
| Drug treatment and blood pressure | 1 year | 5 years | ||
|---|---|---|---|---|
| Stenting | Endarterectomy | Stenting | Endarterectomy | |
| Drug treatment (number of patients with data) | 714 | 751 | 343 | 329 |
| Any antiplatelet, n (%) | 668 (94) | 688 (92) | 303 (88) | 284 (86) |
| Aspirin alone, n (%) | 401 (56) | 413 (55) | 197 (57) | 169 (51) |
| Clopidogrel alone, n (%) | 79 (11) | 79 (11) | 40 (12) | 46 (14) |
| Dipyridamole + aspirin or clopidogrel, n (%) | 130 (18) | 154 (21) | 48 (14) | 48 (15) |
| Aspirin + clopidogrel, n (%) | 55 (8) | 34 (5) | 14 (4) | 17 (5) |
| Anticoagulation: vitamin K antagonists, n (%) | 36 (5)a | 57 (8)a | 23 (7) | 33 (10) |
| Other anticoagulation or antiplatelet, n (%) | 3 (0) | 10 (1) | 5 (1) | 4 (1) |
| Any anticoagulation or antiplatelet, n (%) | 696 (97) | 731 (97) | 322 (94) | 313 (95) |
| Antihypertensive, n (%) | 510 (71) | 566 (75) | 286 (83)a | 250 (76)a |
| Lipid lowering, n (%) | 584 (82) | 629 (84) | 299 (87) | 282 (86) |
| Blood pressure (n patients with data) | 664b | 685b | 313b | 302b |
| Systolic blood pressure (mmHg), mean (SD) | 147 (22)a | 144 (22)a | 142 (22) | 143 (23) |
| Diastolic blood pressure (mmHg), mean (SD) | 79 (12)a | 78 (11)a | 77 (12) | 76 (12) |
Success of procedures and cross-overs
Figure 2 shows the delay from randomisation to first initiated ipsilateral treatment in the per-protocol analysis within the first 120 days after randomisation.
FIGURE 2.
Time between randomisation and treatment. Cumulative number of patients in whom allocated treatment was initiated per protocol plotted as a proportion (%) of the total number randomised in each treatment group (y-axis), against the delay in days between the dates of randomisation and treatment (x-axis). Only allocated per-protocol treatment dates were counted.
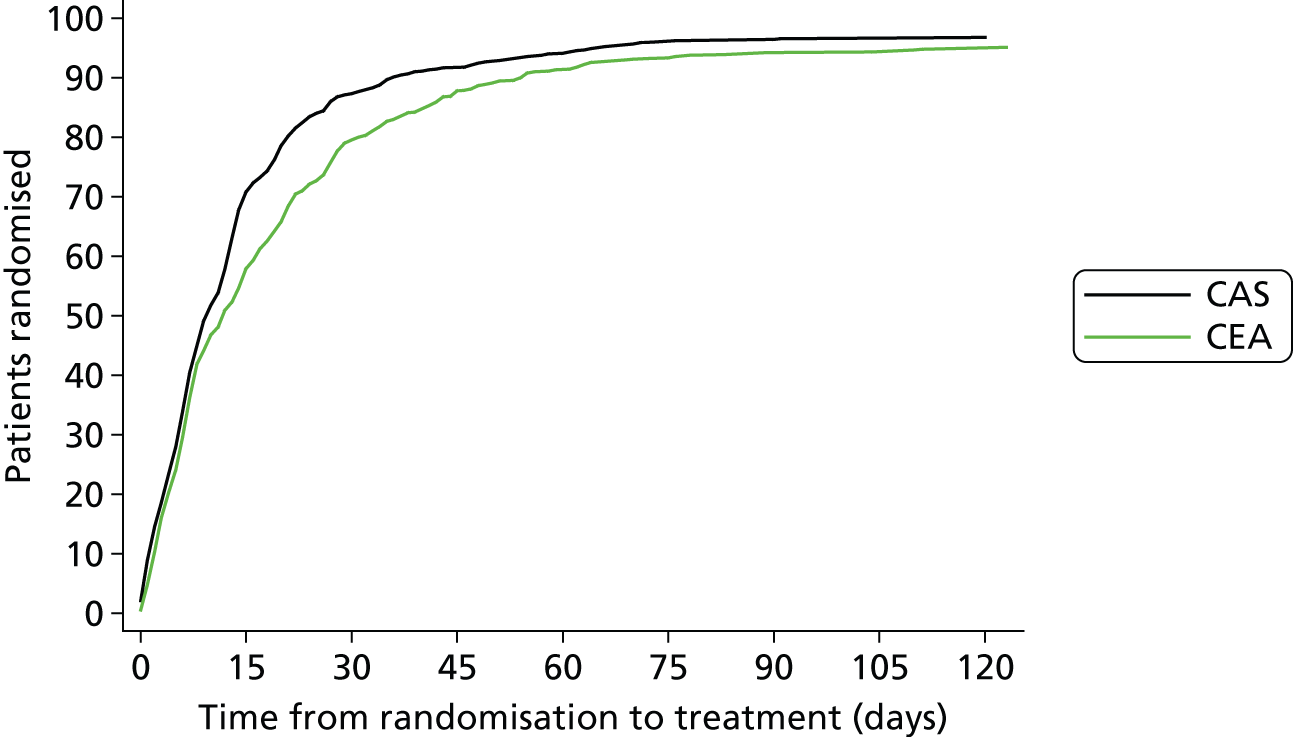
Median delay from randomisation to treatment was shorter in the stenting group than in the endarterectomy group, as was the delay from most recent ipsilateral event to treatment (Table 3).
| Stenting (n = 828) | Endarterectomy (n = 821) | p-valuea | |
|---|---|---|---|
| Time from randomisation to treatment (days), median (IQR) | 9 (5–17) | 11 (5–24) | < 0.001 |
| ≤ 14 days, n (%) | 578 (70) | 469 (57) | |
| > 14 days, n (%) | 250 (30) | 352 (43) | |
| Time from most recent event to treatment (days), median (IQR) | 35 (15–82) | 40 (18–87) | 0.013 |
| ≤ 14 days, n (%) | 205 (25) | 151 (18) | |
| > 14 days, n (%) | 623 (75) | 668 (81) |
Of the 828 patients in whom stenting was initiated as allocated, 64 (8%) had their procedure aborted before the insertion of a stent (38 procedures were aborted because of difficulty gaining access to the stenosis; 15 were aborted because of the finding of an occluded artery, one patient had a fatal stroke, one patient had a fatal MI before completion of treatment, two had other medical complications, and further investigation in seven patients showed the artery to be < 50% stenosed). Of the 62 patients whose stenting procedure was aborted after initiation and who did not have a fatal event, 37 went on to have an ipsilateral endarterectomy, whereas 25 continued with best medical care only. Only two of the 821 patients whose allocated endarterectomy was initiated had their procedure aborted (one patient had an allergic reaction during general anaesthesia; the other became distressed and the endarterectomy had to be abandoned). Both patients subsequently had ipsilateral stenting.
The following stents were each used in 10% or more of the 764 patients in whom stents were inserted: Carotid WALLSTENT® (Boston Scientific), Precision (Cordis®, Freemont, CA, USA), and Protege™ (EV3®, Dublin, Ireland). The following were each used in < 10% of patients: Acculink (Guidant™, Santa Clara, CA, USA), XACT® (Abbott™, Santa Clara, CA, USA), S.M.A.R.T. ® (Cordis®, Miami Lakes, FL, USA), Cristallo Ideale (Invatec, Roncadelle, Brescia, Italy), Exponent (Medtronic®, Minneapolis, MN, USA), Next Stent (Boston Scientific). Protection devices were known to have been used in 593 (72%) of 828 patients. The following protection devices were each used in 10% or more of the patients in whom stenting was attempted: FilterWire EZ™ (Boston Scientific), ANGIOGUARD® (Cordis), SpiderFX™ (EV3) and Emboshield® (Abbott). A range of other protection devices were used in < 5% of patients. In 27 patients, it was not clear whether or not a protection device was used.
Short-term outcomes
In the ITT analysis, between randomisation and 120 days, there was no significant difference in the rate of disabling stroke or death between groups (stenting group, 4.0% vs. endarterectomy group, 3.2%; Table 4). The risk of stroke, death or procedural MI 120 days after randomisation was significantly higher in patients in the stenting group than in patients in the endarterectomy group (8.5% vs. 5.1%), representing an estimated 120-day absolute risk difference of 3.3% (95% CI 0.9% to 5.7%) with a HR in favour of surgery of 1.69 (1.16 to 2.45, log-rank p-value = 0.006) (Figure 3 and Table 4).
FIGURE 3.
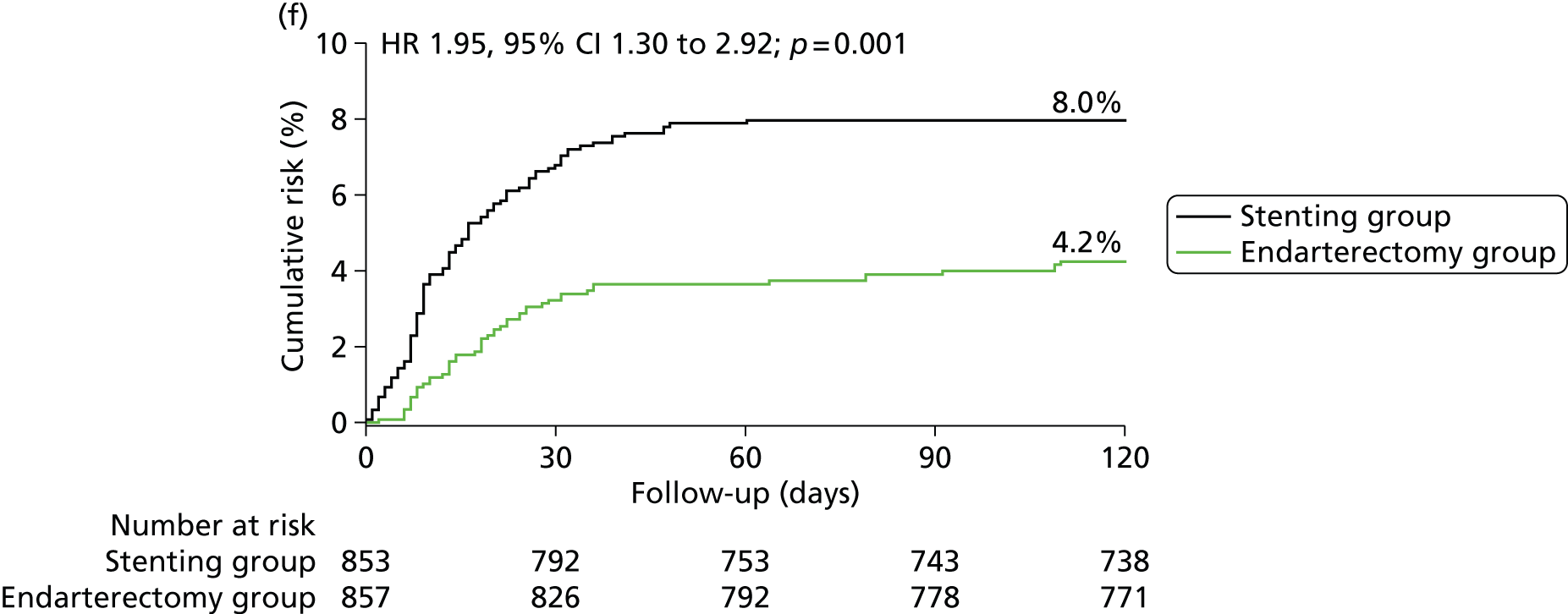
Kaplan–Meier estimates of cumulative incidence of main short-term outcome measures. Data are analysed by ITT. The numbers above the end of the lines are the incidence estimates at 120 days after randomisation. (a) Stroke, death or procedural MI (primary outcome measure); (b) any stroke; (c) stroke or death; (d) disabling stroke or death; (e) all-cause death and (f) any stroke or procedural death.

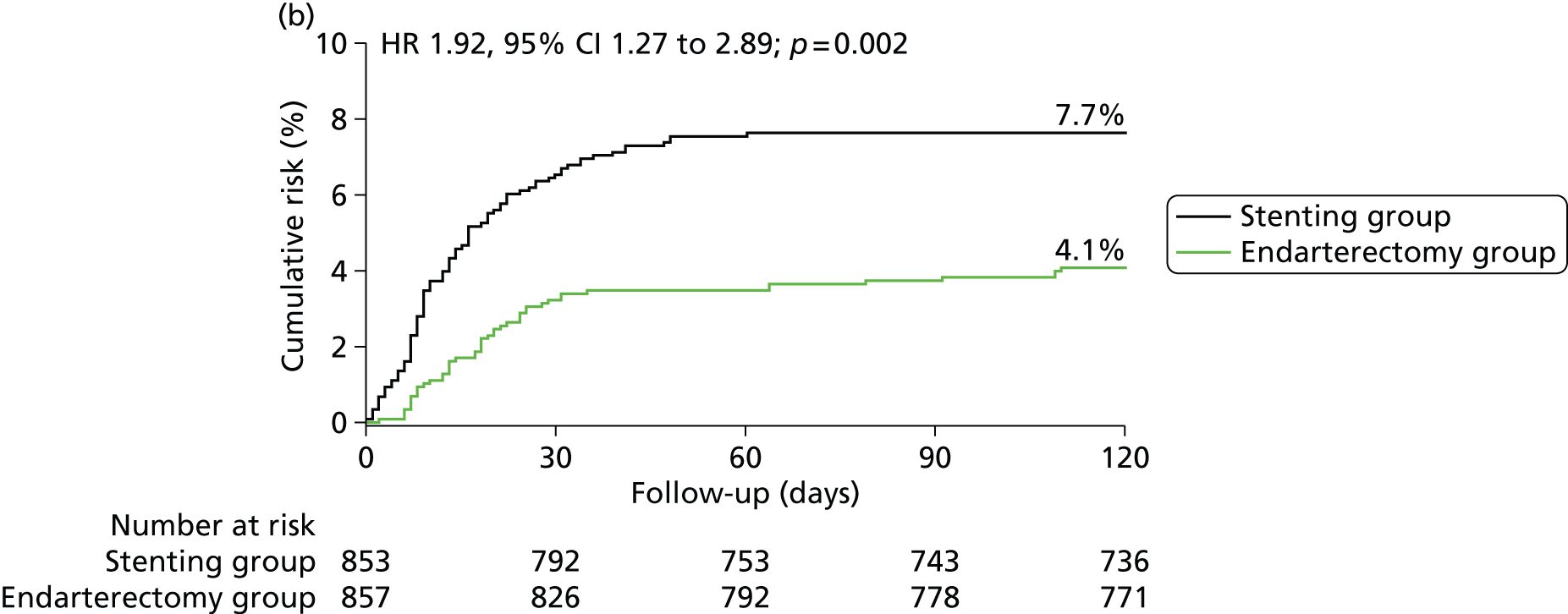
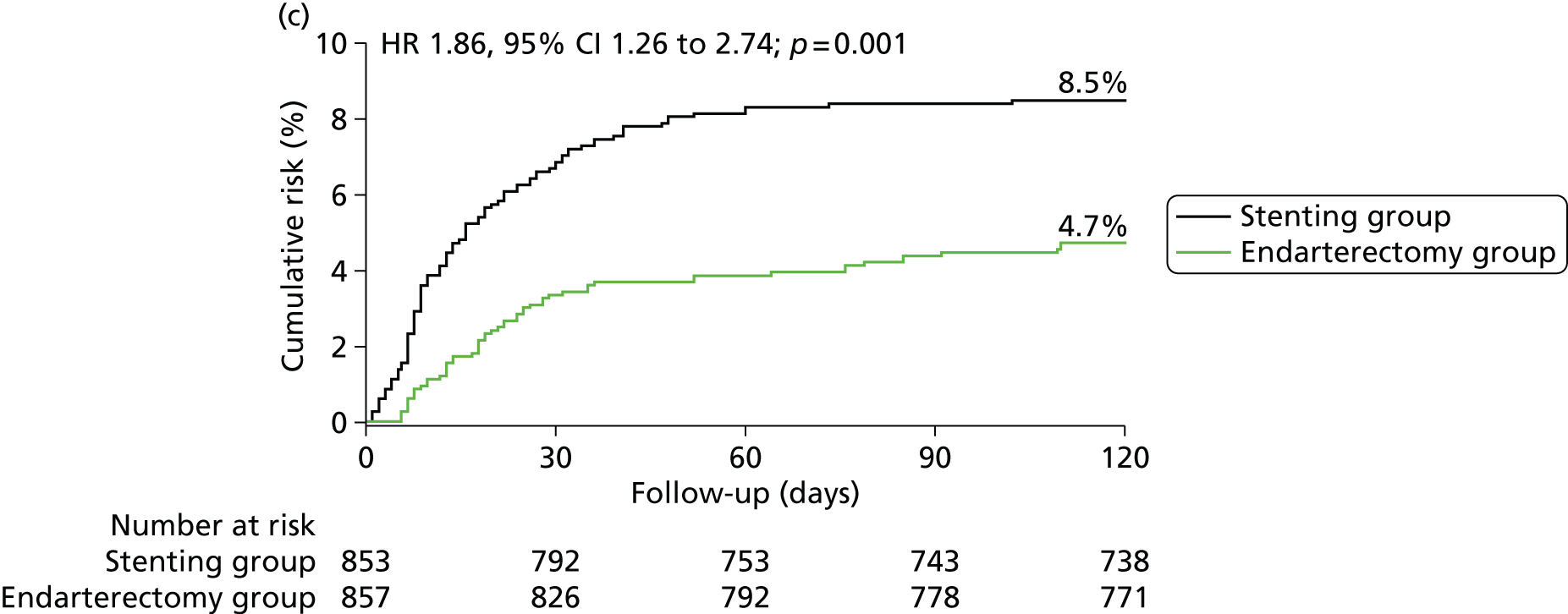

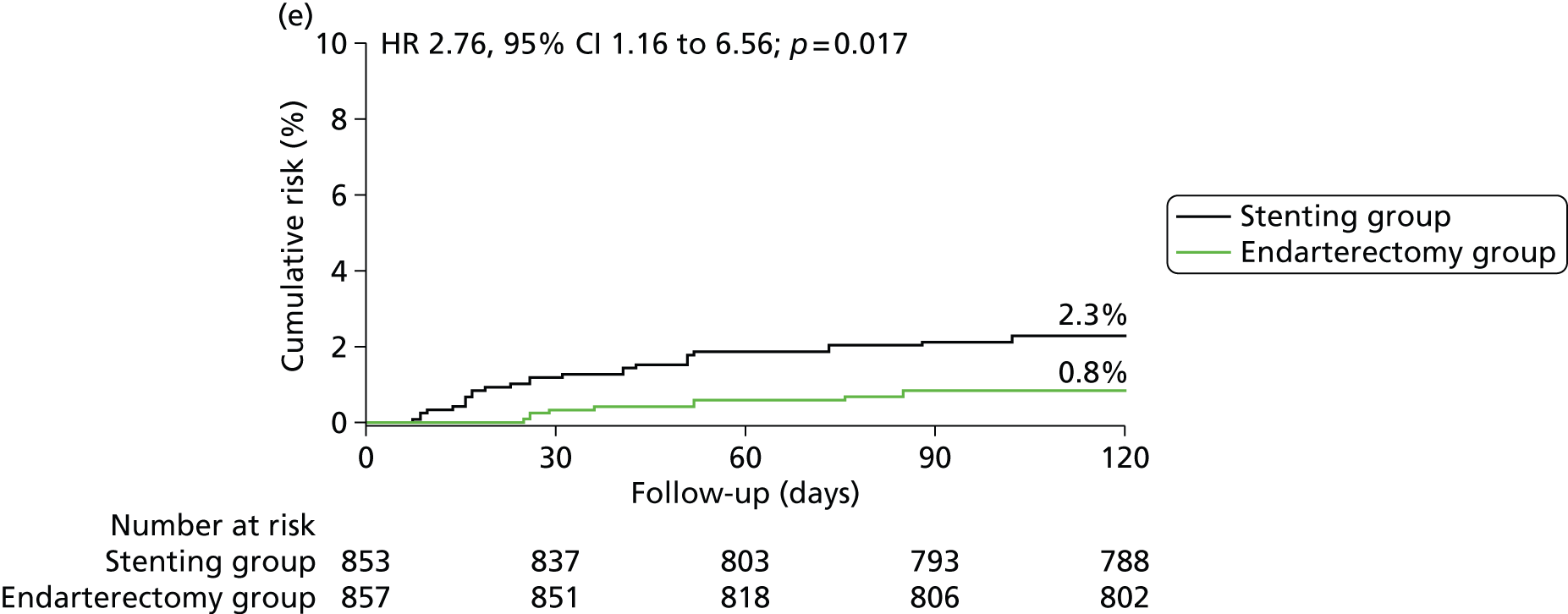
| Outcome measures | CAS N = 853, n (%) | CEA N = 857, n (%) | HR (95% CI) | RD (95% CI) | p-valuea |
|---|---|---|---|---|---|
| Main outcome | |||||
| Stroke, death or procedural MI | 72 (8.5) | 44 (5.2) | 1.69 (1.16 to 2.54) | 3.3 (0.9 to 5.7) | 0.006 |
| Secondary outcomes | |||||
| Any stroke | 65 (7.7) | 35 (4.1) | 1.92 (1.27 to 2.89) | 3.5 (1.3 to 5.8) | 0.002 |
| Any stroke or death | 72 (8.5) | 40 (4.7) | 1.86 (1.26 to 2.74) | 3.8 (1.4 to 6.1) | 0.001 |
| Any stroke or procedural death | 68 (8.0) | 36 (4.2) | 1.95 (1.30 to 2.92) | 3.8 (1.5 to 6.0) | 0.001 |
| Disabling stroke or death | 34 (4.0) | 27 (3.2) | 1.28 (0.77 to 2.11) | 0.8 (–0.9 to 2.6) | 0.34 |
| All-cause death | 19 (2.3) | 7 (0.8) | 2.76 (1.16 to 6.56) | 1.4 (0.3 to 2.6) | 0.017 |
Most outcome events, within 120 days of randomisation in the stent and endarterectomy groups occurred within 30 days of the first ipsilateral procedure (61 of 72 events vs. 31 of 44 events). A few events occurred after randomisation but before the date of treatment (two patients vs. one patient) in patients who had no attempted ipsilateral procedure (three patients vs. six patients), or more than 30 days after treatment but within 120 days of randomisation (six patients vs. six patients). Compared with endarterectomy, allocation to stenting had a greater 120-day risk of the outcome measures of any stroke, any stroke or death, any stroke or procedural death, and all-cause death (Table 4).
Most strokes within 120 days of randomisation were ipsilateral to the treated carotid artery and most were ischaemic (Table 5). There were very few haemorrhagic strokes, with only two patients in whom the cause of the stroke was uncertain.
| Outcome events | ITT analysis: events up to 120 days after randomisation, n (%) | Per-protocol analysis: events between 0 days and 30 days after treatment, n (%) | ||
|---|---|---|---|---|
| Stenting, (N = 853) | Endarterectomy, (N = 857) | Stenting, (N = 828) | Endarterectomy, (N = 821) | |
| Any stroke | 65 (7.6)a | 35 (4.1) | 58 (7.0)a | 27 (3.3) |
| Ipsilateral stroke | 58 (6.8) | 30 (3.5) | 52 (6.3) | 25 (3.0) |
| Ischaemic stroke | 63 (7.4) | 28 (3.3) | 56 (6.8) | 21 (2.6) |
| Haemorrhagic stroke | 3 (0.4) | 5 (0.6) | 2 (0.2) | 5 (0.6) |
| Uncertain pathology | 0 | 2 (0.2) | 0 | 1 (0.1) |
| Non-disabling stroke | 39 (4.6) | 14 (1.6) | 36 (4.6) | 11 (1.3) |
| Lasting < 7 days | 9 (1.1) | 5 (0.6) | 8 (1.0) | 5 (0.6) |
| Lasting > 7 days | 31 (3.6)b | 9 (1.1)c | 29 (3.5)b | 6 (0.7)c |
| Disabling stroke | 17 (2.0)d | 20 (2.3) | 14 (1.7) | 14 (1.7) |
| Fatal stroke | 9 (1.1) | 2 (0.2) | 8 (1.0) | 3 (0.4) |
| Procedural MI | 3 (0.4) | 4 (0.5) | 3 (0.4) | 5 (0.6) |
| Non-fatal MI | 0 | 4 (0.5) | 0 | 5 (0.6)e |
| Fatal MI | 3 (0.4) | 0 | 3 (0.4) | 0 |
| Non-stroke, non-MI death | 7 (0.8) | 5 (0.6) | 1 (1.0) | 1 (0.1) |
| Cranial nerve palsy | 1 (0.1)f | 45 (5.5) | 1 (1.0)f | 45 (5.5) |
| Disabling cranial nerve palsy | 1 (0.1)f | 1 (0.1) | 1 (1.0)f | 1 (0.1) |
| Haematoma | 31 (3.6) | 50 (5.8) | 30 (3.6) | 50 (6.0) |
| Severe haematomag | 9 (1.1) | 28 (3.3) | 8 (1.0) | 28 (3.4) |
The observed treatment effect was largely driven by the higher number of non-disabling strokes in the stenting group, most of which had symptoms lasting for more than 7 days. There was an excess of fatal strokes in the stenting group compared with the surgery group, but little difference in the number of patients with disabling stroke within 120 days of randomisation.
The per-protocol analysis included 1649 patients (stenting group, n = 828; endarterectomy group, n = 821). Results for 30-day procedural risk mirrored the results of the ITT analysis. Risk of stroke, death or procedural MI was higher in the stenting group than in the endarterectomy group (30-day risk 7.4% vs. 4.0%) [risk difference (RD) 3.3%, 95% CI 1.1% to 5.6%; risk ratio (RR) 1.83, 95% CI 1.21 to 2.77; χ2 p = 0.003] (Table 6). Risk of any stroke or death up to 30 days after treatment remained significantly higher in patients in whom stenting was initiated than in patients with surgery initiated, but there was no significant difference in the risk of disabling stroke or death between treatment groups. There were more fatal strokes in the stenting group than in the endarterectomy group (eight vs. three), but difference in the risk of death alone was no longer significant (see Table 5). Forty-three (74%) of 58 strokes in the stenting group and 12 (44%) of 27 strokes in the endarterectomy group occurred on the day of the procedure. There was no difference in the numbers of strokes occurring between day 2 and day 30 between the two treatments (15 vs. 15).
| Outcome measures | CAS N = 828, n (%) | CEA N = 821, n (%) | RR (95% CI) | RD (95% CI) | p-valuea |
|---|---|---|---|---|---|
| Main outcome | |||||
| Stroke, death or MI | 61 (7.4) | 33 (4.0) | 1.83 (1.21 to 2.77) | 3.3 (1.1 to 5.6) | 0.003 |
| Secondary outcomes | |||||
| Any stroke | 58 (7.0) | 27 (3.3) | 2.13 (1.36 to 3.33) | 3.7 (1.6 to 5.8) | 0.001 |
| Any stroke or death | 61 (7.4) | 28 (3.4) | 2.16 (1.40 to 3.34) | 4.0 (1.8 to 6.1) | 0.0004 |
| Disabling stroke or death | 26 (3.1) | 18 (2.2) | 1.43 (0.79 to 2.59) | 0.9 (–0.6 to 2.5) | 0.23 |
| Procedural death | 11b (1.3) | 4 (0.5) | 2.73 (0.87 to 8.53) | 0.8 (–0.1 to 1.8) | 0.072 |
Few procedural MIs were recorded (three in the stenting group, all of which were fatal, compared with five in the endarterectomy group). Cranial nerve palsies were almost completely avoided by stenting (RR 0.02, 95% CI 0.00 to 0.16; p < 0.0001) (see Table 5). The one cranial nerve palsy recorded in the stenting group occurred as a complication of an endarterectomy performed within 30 days of stenting. This patient and one additional patient in the endarterectomy group required percutaneous endoscopic gastrostomy feeding as a result of the cranial nerve palsies, which was classified as disabling. There were also fewer haematomas of any severity in the stenting group than in the endarterectomy group (RR 0.59, 95% CI 0.38 to 0.93; p = 0.0197), and fewer severe haematomas requiring surgical intervention, blood transfusion or extended hospital stay (RR 0.28, 95% CI 0.13 to 0.62; p = 0.0007) (see Table 5). A post-hoc sensitivity analysis was undertaken to examine if the results of the per-protocol analysis were affected by inclusion of patients in whom the allocated procedure was initiated but not completed. Exclusion of the 64 patients allocated to stenting and two patients allocated to endarterectomy in whom the procedures were aborted after initiation (i.e. including only patients in whom the allocated procedure was completed as planned) made little difference to the results (30-day risk of stroke, death or procedural MI of 7.6% in the stenting group vs. 4.0% in the endarterectomy group) (RD 3.6%, 95% CI 1.3% to 5.9%; RR 1.88, 95% CI 1.24 to 2.86; p = 0.002).
We undertook exploratory analyses of the composite outcome of stroke, death or procedural MI for pre-defined subgroups (Figure 4). These analyses suggested that carotid stenting might have a similar risk to endarterectomy in women, but that the intervention was more hazardous than endarterectomy in men. The difference was mainly caused by a higher risk of stroke, death or procedural MI in women assigned to endarterectomy than in men (7.6% vs. 4.2%). However, the difference between the HRs comparing the risk of stenting with endarterectomy in men and women only reached borderline significance (interaction p = 0.071). Stenting was more hazardous, and endarterectomy less hazardous, in patients not taking medication for hypertension at baseline than in patients taking medication for hypertension (see Figure 4). There was also a suggestion that patients who presented with multiple ipsilateral symptoms had a similar risk of stroke death, or procedural MI with stenting and endarterectomy. However, when compared with patients with only one event before randomisation, the difference in the HRs only reached borderline significance (interaction p = 0.055). There was no evidence that the relative increase in the hazard of an event in the stenting group compared with the endarterectomy group differed significantly across any other subgroups.
FIGURE 4.
Comparison of the short-term rate of stroke, death, or procedural myocardial infarction. Subgroups are defined according to baseline characteristics and analysed by intention to treat up to 120 days after randomisation, apart from time from event to treatment, which is analysed per protocol. p-values are associated with treatment-covariate interaction tests. a, Data are number of events of first stroke, death or procedural myocardial infarction within 120 days of randomisation/number of patients (Kaplan–Meier estimate at 120 days). b, Patients with missing information were excluded from the analysis. c, Time from the most recent ipsilateral event before randomisation to the date of treatment, analysed per protocol for 30-day procedural events only (results are relative risk and 95% CI at 30 days after treatment).

Duration of follow-up in the International Carotid Stenting Study
Figure 5 shows the number of patients remaining in follow-up in ICSS plotted against time from randomisation. Patients were followed up for a maximum of 10 years after randomisation with a median of 4.2 years and an interquartile range of 3.0–5.2 years. This amounted to 7355 patient-years of follow-up, without any difference between the two arms.
FIGURE 5.
Patients remaining in each arm of the study (per protocol) are plotted against year of follow-up. In total, there are 7354.45 patient-years of follow-up until time of last follow-up or death. CAS (n = 853): median follow-up = 4.2 years, interquartile range (IQR) 3.0–5.4 years (maximum = 10.0 years, 153 deaths); CEA (n = 857): median follow-up = 4.2 years, IQR 3.0–5.2 years (maximum = 9.6 years, 129 deaths).

Long-term primary and secondary outcomes
In the ITT analysis, the primary outcome event, fatal or disabling stroke between randomisation and the end of follow-up, occurred in 52 patients in the stenting group, corresponding to a cumulative 5-year risk of 6.4%, and in 49 patients in the endarterectomy group (5-year risk n, 6.5%), without any evidence for a difference in time to first occurrence of an event (HR 1.06, 95% CI 0.72 to 1.57; p = 0.76) (Table 7 and Figure 6).
| Outcome events | Stenting (n = 853) | Endarterectomy (n = 857) | HRa (95% CI); p-value | Absolute risk difference, % (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| n eventsa | Cumulative 1-year risk, % (SE) | Cumulative 5-year risk, % (SE) | n eventsa | Cumulative 1-year risk, % (SE) | Cumulative 5-year risk, % (SE) | At 1 year | At 5 years | ||
| Fatal or disabling stroke (primary outcome measure) | 52 | 3.9 (0.7) | 6.4 (0.9) | 49 | 3.2 (0.6) | 6.5 (1.0) | 1.06 (0.72 to 1.57); 0.77 | 0.7 (–1.0 to 2.5) | –0.2 (–2.8 to 2.5) |
| Any stroke | 119 | 9.5 (1.0) | 15.2 (1.4) | 72 | 5.1 (0.8) | 9.4 (1.1) | 1.71 (1.28 to 2.30); 0.0003 | 4.4 (1.9 to 6.9) | 5.8 (2.4 to 9.3) |
| Procedural stroke or death, or ipsilateral stroke during follow-up | 95 | 9.0 (1.0) | 11.8 (1.2) | 57 | 4.7 (0.7) | 7.2 (0.9) | 1.72 (1.24 to 2.39); 0.001 | 4.2 (1.9 to 6.6) | 4.6 (1.6 to 7.6) |
| All-cause death | 153 | 4.9 (0.7) | 17.4 (1.5) | 129 | 2.3 (0.5) | 17.2 (1.5) | 1.17 (0.92 to 1.48); 0.19 | 2.6 (0.8 to 4.4) | 0.2 (–4.0 to 4.4) |
FIGURE 6.
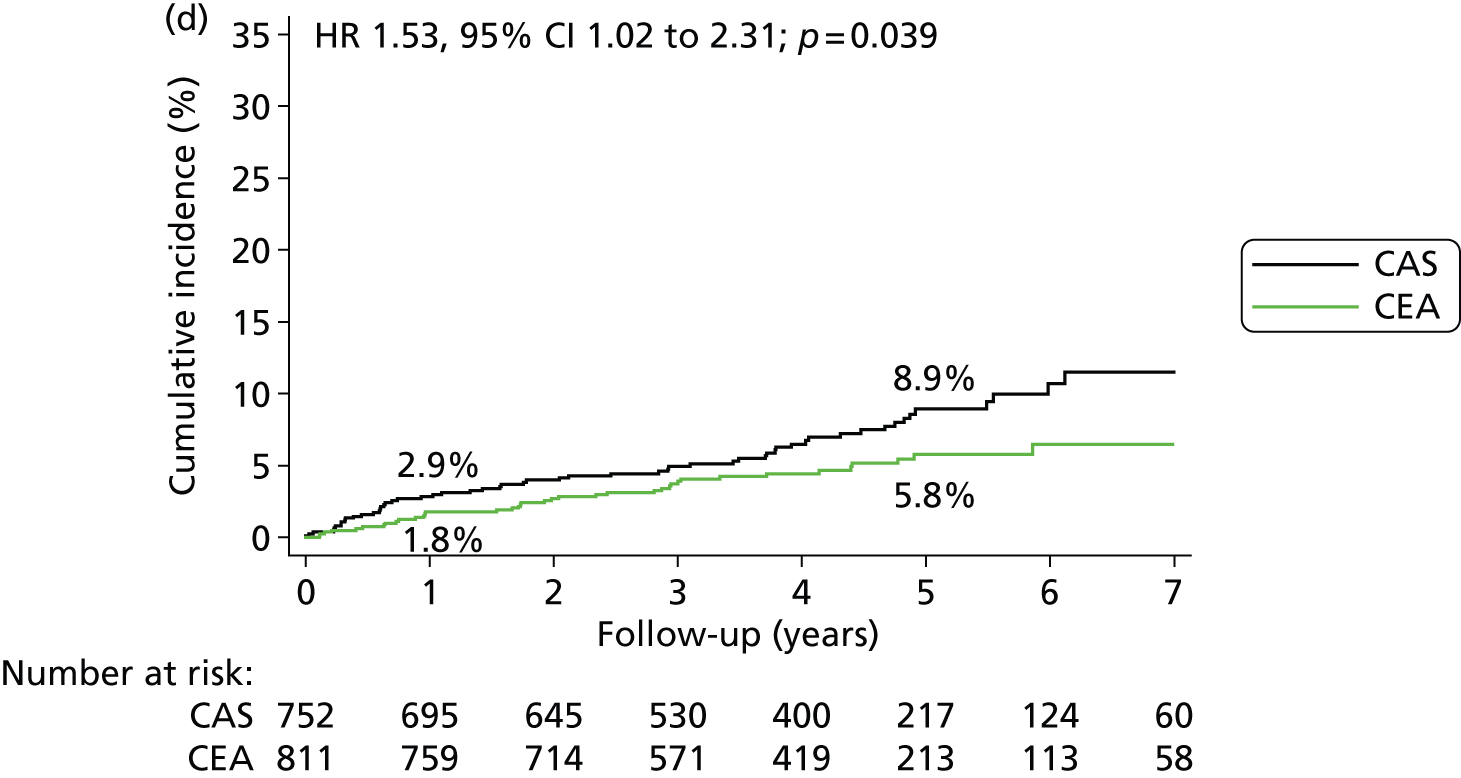
Kaplan–Meier estimates of cumulative incidence of major long-term outcome measures. (a) Fatal or disabling stroke; (b) any stroke; (c) procedural stroke or death, or ipsilateral stroke during follow up; (d) any stroke > 30 days after treatment; (e) ipsilateral stroke > 30 days after treatment; (f) contralateral carotid or vertebrobasilar stroke > 30 days after treatment; (g) ipsilateral carotid stenosis (≥ 70%) or occlusion during follow-up; and (h) all-cause death. Data were analysed by ITT from randomisation except for parts (d) to (f), which are analysed in the per-protocol population from 30 days post procedure, and part (g) which is analysed in the per-protocol population from treatment. The numbers on the lines are the estimated 1-year and 5-year cumulative incidences. The graphs have only been plotted to 7 years’ follow-up because the numbers with longer follow-up were < 100. However, the HRs were calculated using all relevant outcome events until the end of follow-up (maximum 10 years).
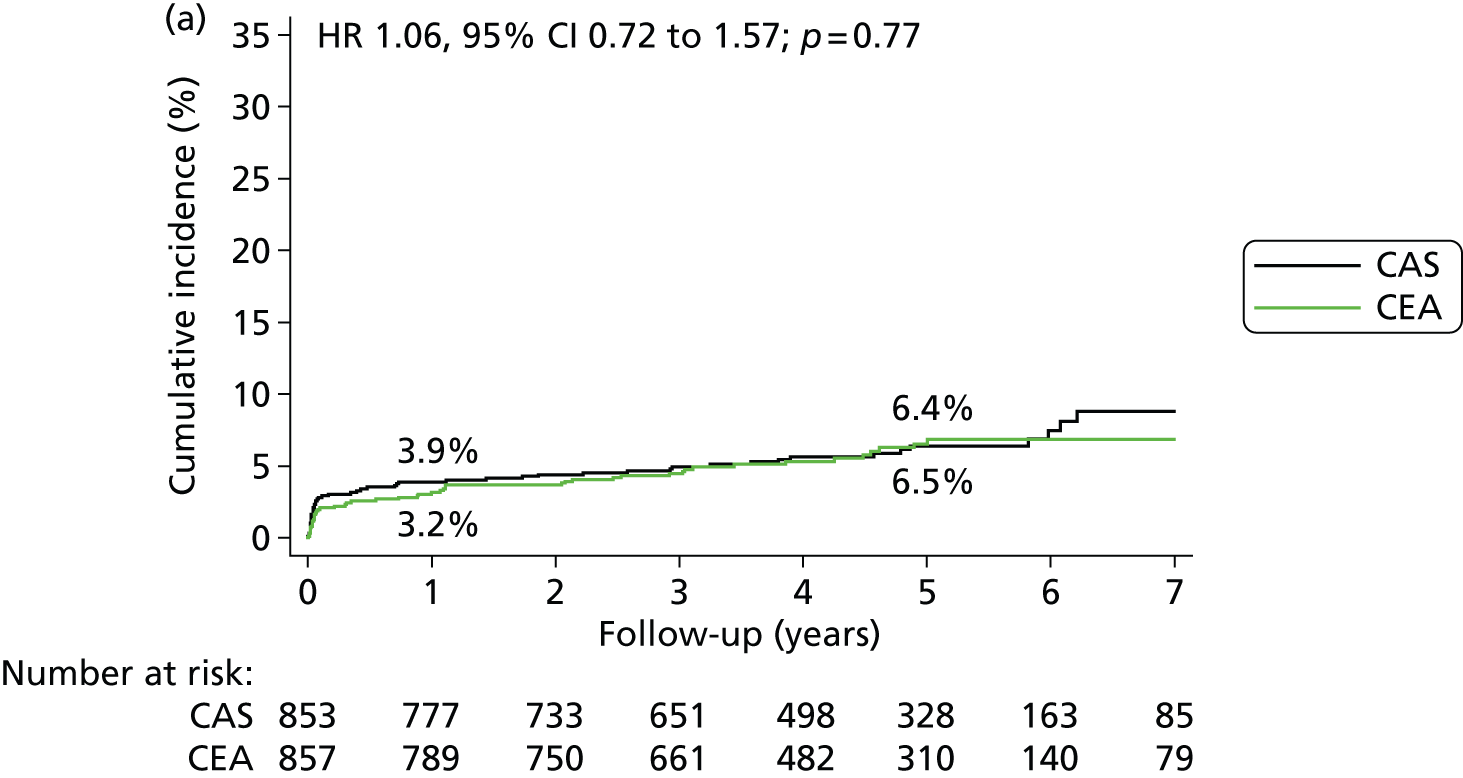

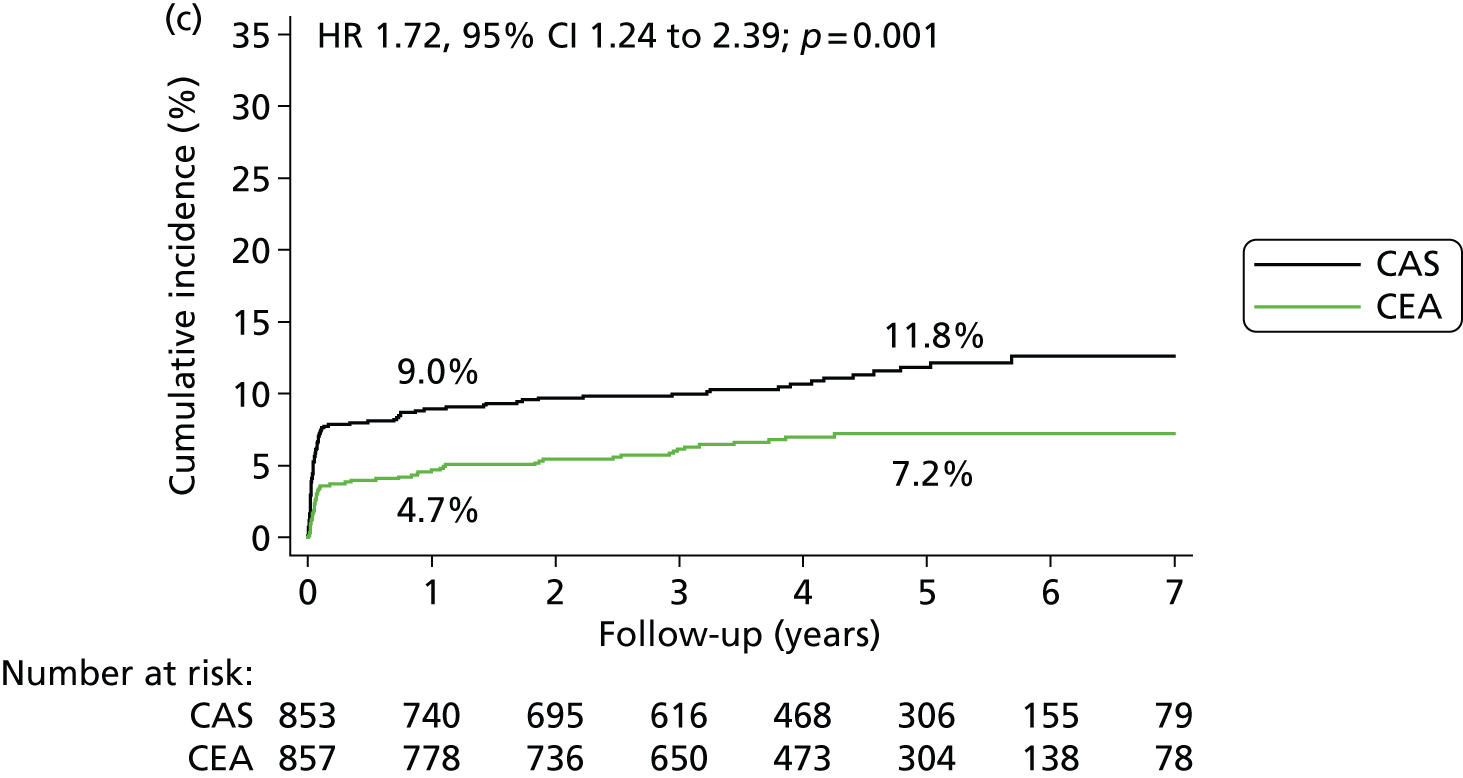



The following secondary outcome events occurred significantly more often in the stenting group in the ITT analysis between randomisation and the end of follow-up: any stroke (5-year risks 15.2% vs. 9.4%) (HR 1.71, 95% CI 1.28 to 2.30; p = 0.0003); any stroke or death (27.5% vs. 22.6%) (HR 1.34, 95% CI 1.11 to 1.63; p = 0.003); the combination of any procedural stroke, procedural death or ipsilateral stroke during follow-up (11.8% vs. 7.2%) (HR 1.72, 95% CI 1.24 to 2.39; p = 0.001). There was no difference in all-cause mortality between treatment groups (17.4% vs. 17.2%) (HR 1.17, 95% CI 0.92 to 1.48; p = 0.19).
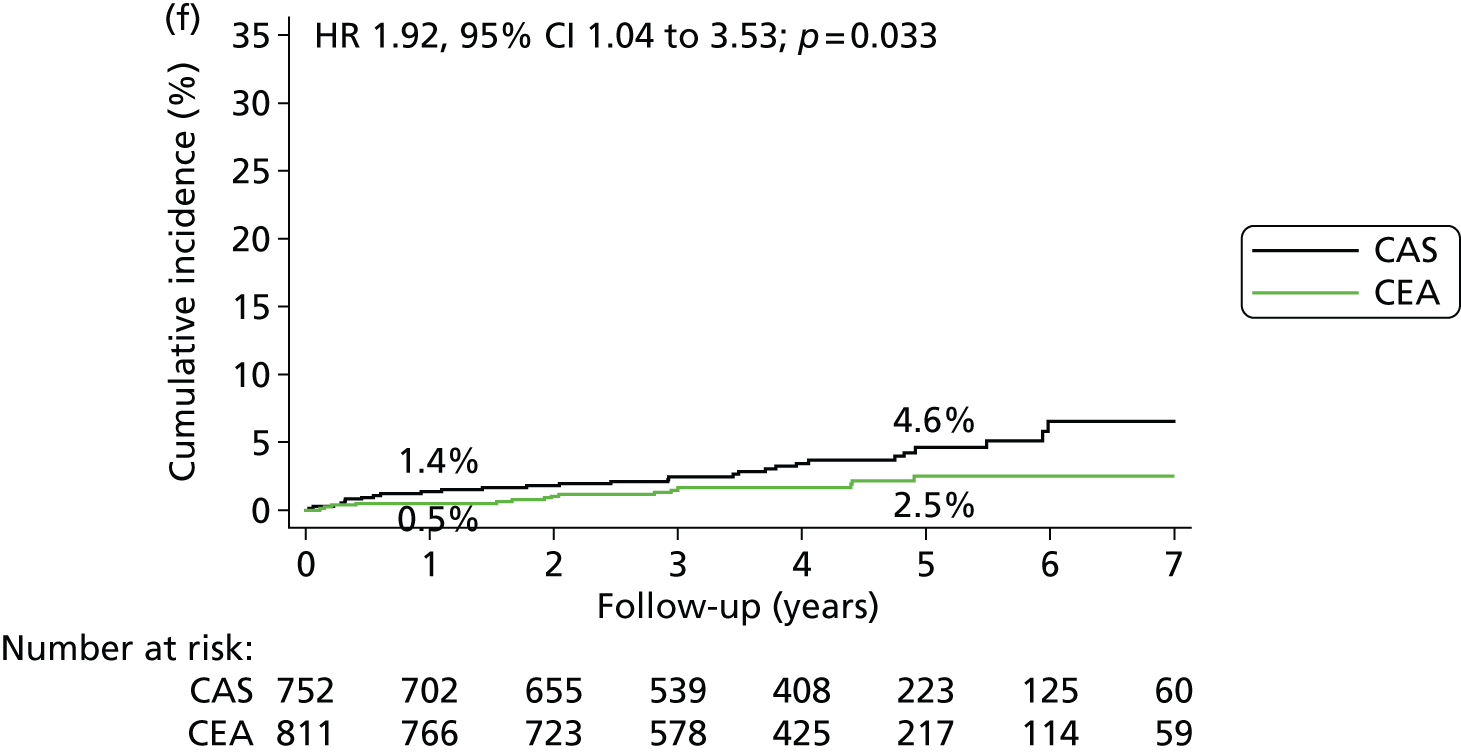
A total of 752 patients in the stenting group (88.2% of the ITT population) and 811 patients in the endarterectomy group (94.6%) were included in the per-protocol analysis of clinical outcome events. In the per-protocol analysis of events occurring more than 30 days after completed treatment up to the end of follow-up, there was no significant difference in the long-term risks of fatal and disabling stroke after stenting compared with endarterectomy (5-year risk 3.4% vs. 4.3%) (HR 0.93, 95% CI 0.53 to 1.60; p = 0.78) (Table 8). There was also no significant difference in the rates of ipsilateral stroke in the territory of the treated carotid artery (4.7% vs. 3.4%) (HR 1.29, 95% CI 0.74 to 2.24; p = 0.36). However, stroke of any severity occurred more often after stenting (8.9% vs. 5.8%) (HR 1.53, 95% CI 1.02 to 2.31; p = 0.039) (Figure 6 and Table 8). This difference was largely attributable to stroke occurring in the territory of the contralateral carotid artery or the vertebrobasilar circulation among patients treated with stents (5-year risks 4.6% vs. 2.5%) (HR 1.92, 95% CI 1.04 to 3.53; p = 0.033) and the majority were non-disabling.
| Outcome events | Stenting (n = 752) | Endarterectomy (n = 811) | Absolute risk difference, % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n eventsa | Cumulative 1-year risk, % (SE)a | Cumulative 5-year risk, % (SE)a | n events | Cumulative 1-year risk, % (SE)a | Cumulative 5-year risk, % (SE)a | HRb (95% CI); p-value | At 1 year | At 5 years | |
| Fatal or disabling stroke | 24 | 0.9 (0.4) | 3.4 (0.8) | 27 | 1.4 (0.4) | 4.3 (0.9)a | 0.93 (0.53 to 1.60); 0.78 | –0.5 (–1.5 to 0.6) | –0.9 (–3.2 to 1.4) |
| Any stroke | 56 | 2.9 (0.6) | 8.9 (1.2) | 39 | 1.8 (0.5) | 5.8 (1.0) | 1.53 (1.02 to 2.31); 0.039 | 1.1 (–0.4 to 2.6) | 3.1 (0.0 to 6.2) |
| Ipsilateral carotid stroke | 28 | 1.4 (0.4) | 4.7 (0.9) | 23 | 1.1 (0.4) | 3.4 (0.8) | 1.29 (0.74 to 2.24); 0.36 | 0.2 (–0.9 to 1.3) | 1.2 (–1.1 to 3.6) |
| Contralateral carotid or vertebrobasilar stroke | 29 | 1.4 (0.4) | 4.6 (0.9) | 16 | 0.5 (0.3) | 2.5 (0.7) | 1.92 (1.04 to 3.53); 0.033 | 0.9 (–0.1 to 1.8) | 2.1 (–0.2 to 4.3) |
| Severe carotid restenosis (≥ 70%) or occlusion | 72/737 | 6.9 (1.0) | 10.8 (1.3) | 62/793 | 5.3 (0.8) | 8.6 (1.1) | 1.25 (0.89 to 1.75); 0.20 | 1.7 (–0.8 to 4.1) | 2.2 (–1.1 to 5.4) |
A total of 737 (98.0%) patients in the stenting group and 793 (97.8%) in the endarterectomy group were followed up with carotid ultrasound for a median of 4.0 years (interquartile range, 2.3–5.0 years) after treatment. There was no significant difference in long-term rates of severe carotid restenosis (≥ 70%) or occlusion, which occurred in 72 patients in the stenting group (5-year risk 10.8%) and in 62 patients in the endarterectomy group (5-year risk 8.6%) (HR 1.25, 95% CI 0.89 to 1.75; p = 0.20; see Table 8 and Figure 6).
Exploratory subgroup analyses showed no significant modification of the HR of the primary outcome event (Figure 7), nor of the combined outcome of procedural death or stroke, or non-procedural ipsilateral stroke by any of the evaluated variables (Figure 8).
FIGURE 7.
Hazard ratios of fatal or disabling stroke between randomisation and end of follow-up in various patient subgroups. Subgroups are defined according to baseline characteristics and analysed by ITT for all available follow-up, apart from time from event to procedure, which is analysed per protocol. P-values are associated with treatment–covariate interaction tests. a, Data are number of events of first fatal or disabling stroke/number of patients, and Kaplan–Meier estimate of cumulative risk at 5 years. Patients with missing information were excluded from the analysis. b, Time from most recent ipsilateral event before randomisation to the date of treatment, analysed per protocol from the time of procedure. All subgroups for analysis were pre-specified except for treated hyperlipidaemia which was added post hoc. AFX, amaurosis fugax.

FIGURE 8.
Hazard ratios of procedural stroke, death or ipsilateral stroke between randomisation and end of follow-up in various patient subgroups. Subgroups are defined according to baseline characteristics and analysed by ITT for all available follow-up, apart from time from event to procedure, which is analysed per protocol. P-values are associated with treatment–covariate interaction tests. a, Data are number of events of first ipsilateral stroke or procedural stroke or death/number of patients, and Kaplan–Meier estimate of cumulative risk at 5 years. Patients with missing information were excluded from the analysis. b, Time from most recent ipsilateral event before randomisation to the date of treatment, analysed per protocol from the time of procedure. AFX, amaurosis fugax.

Long-term functional outcome
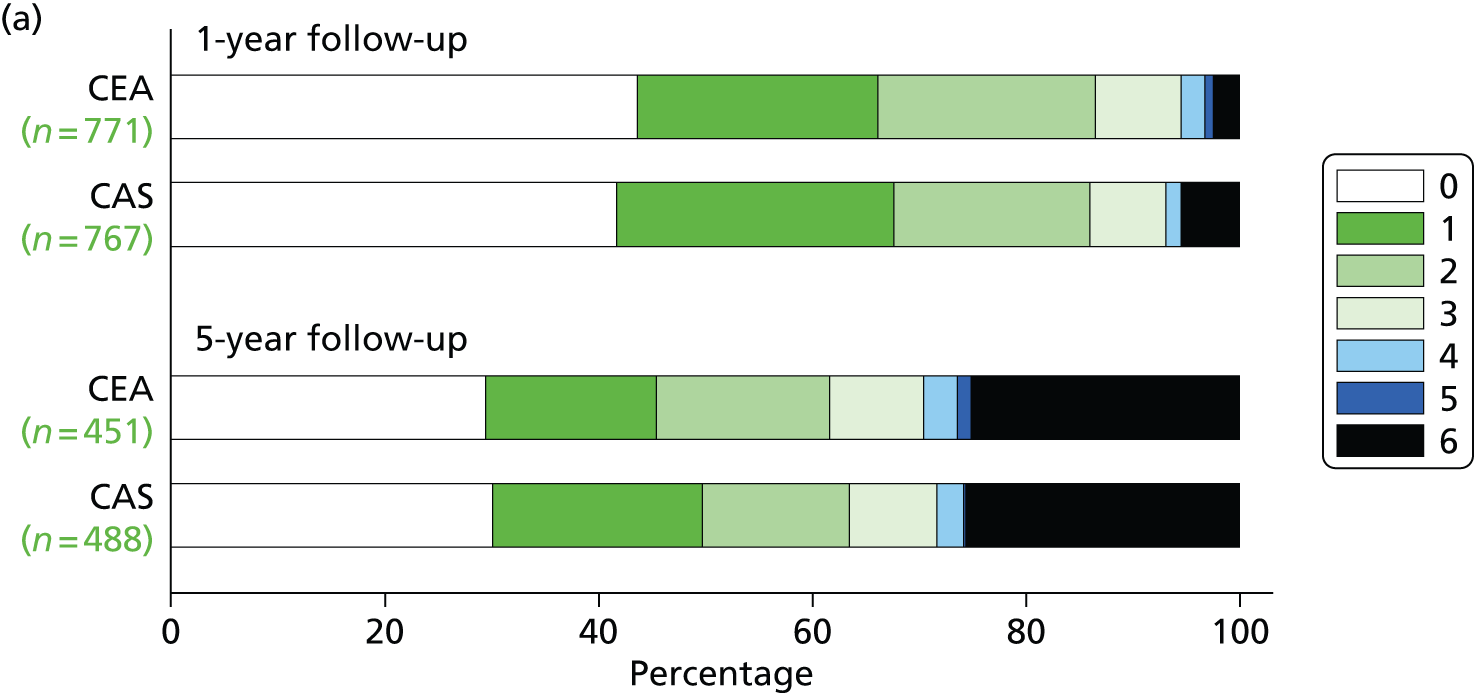
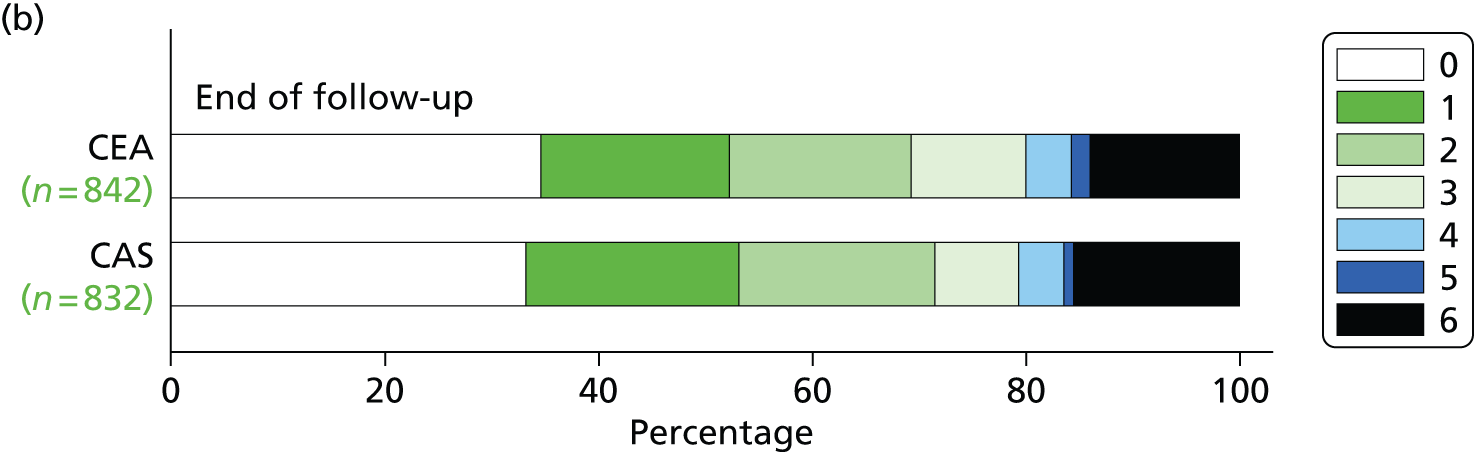
There was no difference in distribution of functional disability as measured by the mRS scores at the end of follow-up, nor was there any significant difference 1 or 5 years after randomisation (Figure 9).
FIGURE 9.
The distribution of scores on the mRS: (a) after 1-year and 5-year follow-up in patients allocated CAS or CEA using the Rankin scores in patients still surviving and in follow-up or who had died before at the indicated time points [permutation test comparing Rankin scores between the two groups at 1 year (unadjusted p = 0.70, adjusted for baseline mRS p = 0.11), at 5 years (unadjusted p = 0.54, adjusted for baseline mRS p = 0.98)13]; (b) at the last follow-up recorded for the patient, regardless of duration (unadjusted, p = 0.49; adjusted for baseline mRS score, p = 0.24).
Findings of the magnetic resonance imaging substudy
The MRI substudy has been previously published in detail. 44 A total of 231 patients (124 in the stenting group and 107 in the endarterectomy group) had MRI before and after treatment. Sixty-two (50%) of 124 patients in the stenting group and 18 (17%) of 107 patients in the endarterectomy group had at least one new DWI lesion detected on post-treatment scans done a median of 1 day after treatment [adjusted odds ratio (OR) 5.21, 95% CI 2.78 to 9.79; p < 0.0001]. At 1 month, there were changes on fluid-attenuated inversion recovery sequences in 28 (33%) of 86 patients in the stenting group and six (8%) of 75 in the endarterectomy group (adjusted OR 5.93, 95% CI 2.25 to 15.62; p = 0.0003). In patients treated at a centre with a policy of using cerebral protection devices, 37 (73%) of 51 in the stenting group and eight (17%) of 46 in the endarterectomy group had at least one new DWI lesion on post-treatment scans (adjusted OR 12.20, 95% CI 4.53 to 32.84), whereas in those treated at a centre with a policy of unprotected stenting, 25 of 73 patients (34%) in the stenting group and 10 of 61 (16%) in the endarterectomy group had new lesions on DWI (adjusted OR 2.70, 95% CI 1.16 to 6.24; interaction p = 0.019).
Studies on the predictors of risk of individual procedures
Findings of study on the effect of white-matter lesions on the risk of periprocedural stroke
This analysis has been published in detail elsewhere. 30 Patients were divided into two groups using the median ARWMC. We analysed the risk of stroke within 30 days of revascularisation using a per-protocol analysis. A total of 1036 patients (536 randomly allocated to CAS, 500 to CEA) had baseline imaging available. Median ARWMC score was 7, and patients were dichotomised into those with a score of 7 or more and those with a score of < 7. In patients treated with CAS, those with an ARWMC score of 7 or more had an increased risk of stroke compared with those with a score of < 7 (HR for any stroke 2.76, 95% CI 1.17 to 6.51; p = 0.021; HR for non-disabling stroke 3.00, 95% CI 1.10 to 8.36; p = 0.031). However, we did not see a similar association in patients treated with CEA (HR for any stroke 1.18, 95% CI 0.40 to 3.55; p = 0.76; HR for disabling or fatal stroke 1.41, 95% CI 0.38 to 5.26; p = 0.607). Carotid artery stenting was associated with a higher risk of stroke compared with CEA in patients with an ARWMC score of 7 or more (HR for any stroke 2.98, 95% CI 1.29 to 6.93; p = 0.011; HR for non-disabling stroke 6.34, 95% CI 1.45 to 27.71; p = 0.014), but there was no risk difference in patients with an ARWMC score of < 7.
Findings of the analysis of the effect of baseline characteristics on the risk of stenting
This analysis has been published in detail elsewhere. 45 We examined the influence of baseline patient characteristics influencing the risk of stroke, MI or death within 30 days of CAS in a regression model, including only patients allocated to stenting in whom the procedure was actually initiated (per-protocol analysis). Patients who crossed over to CAS, received CAS after an attempt at endarterectomy or received medical therapy instead of CAS were excluded. Risk factors were examined using binomial regression. A multivariable model was developed using a forward stepwise approach. Independent predictors of risk were age (RR 1.17 per 5 years of age, 95% CI 1.01 to 1.37), a right-sided procedure (RR 0.54, 95% CI 0.32 to 0.91), aspirin and clopidogrel prior to CAS (compared with any other antiplatelet regimen) (RR 0.59, 95% CI 0.36 to 0.98), smoking status and the severity of index event. In patients in whom a stent was deployed, use of an open-cell stent conferred higher risk than use of a closed-cell stent (RR 1.92, 95% CI 1.11 to 3.33). The use of a cerebral protection device did not modify the risk.
Incidence, impact and predictors of cranial nerve palsy and haematoma following carotid endarterectomy
This analysis has been published in detail elsewhere. 46 We analysed the effects of patient factors and surgical technique on the risk, and impact on disability, of cranial nerve palsy or haematoma in the surgical arm, including only patients allocated to endarterectomy in whom the procedure was actually initiated (per-protocol analysis). Patients who crossed over to CEA, received CEA after an attempt at CAS or received medical therapy instead of CAS were excluded. Forty-five of 821 (5.5%) patients undergoing CEA developed cranial nerve palsy, one instance of which was disabling (mRS of 3 at 1 month). Twenty-eight (3.4%) patients developed severe haematoma; 12 patients with haematoma also had cranial nerve palsy, a significant association (p < 0.01). Independent risk factors modifying the risk of cranial nerve palsy in the multivariate analysis were cardiac failure (RR 2.66, 95% CI 1.11 to 6.40), female sex (RR 1.80, 95% CI 1.02 to 3.20), the degree of contralateral carotid stenosis and time from randomisation to treatment > 14 days (RR 3.33, 95% CI 1.05 to 10.57). The risk of haematoma was increased in women, by the prescription of anticoagulant drugs pre-procedure and in patients with atrial fibrillation, and was decreased in patients in whom a shunt was used and in those with a higher baseline cholesterol level.
Findings of the analysis of the effect of baseline characteristics on the risks of procedural stroke, myocardial infarction or death after endarterectomy
This analysis has been published in detail elsewhere. 47 We examined the influence of baseline patient characteristics influencing the risk of stroke, MI or death within 30 days of endarterectomy in a regression model, including only patients allocated CEA in whom the procedure was actually initiated (per-protocol analysis). Patients who crossed over to CEA, received CEA after an attempt at CAS or received medical therapy instead of CAS were excluded. Demographic and technical risk factors for these procedural complications were analysed sequentially in a binomial regression analysis and, subsequently, in a multivariable model. The risk of stroke, MI or death within 30 days of CEA was higher in female patients (RR 1.98, 95% CI 1.02 to 3.87; p = 0.05), and with increasing baseline DBP (RR 1.30 for each 10 mmHg increase, 95% CI 1.02 to 1.66; p = 0.04). In a multivariable model, only DBP remained a significant predictor. The risk was not related to the type of surgical reconstruction, anaesthetic technique or perioperative medication regimen. A total of 21.2% of events occurred on or after the day of discharge.
Findings of the cost–utility analysis
Resource use and costs
The cost–utility economics analysis has been published elsewhere. 48 Mean index procedure duration was 107 minutes [standard deviation (SD) 47 minutes] in the endarterectomy group (n = 700) and 68 minutes (SD 33 minutes) in the stenting group (n = 691; see Appendix 9). Eighty-two per cent of endarterectomy patients (n = 794) had general anaesthetic compared with 0% of stenting patients (n = 853). Eighteen per cent of endarterectomy patients had local anesthetic compared with 100% of stenting patients. In the endarterectomy group a shunt was used in 40% of patients (n = 818) and a patch in 66% (n = 693). In the stenting group a stent was deployed in 92% of patients (n = 816) and a cerebral protection device was used in 71% (n = 824). Sixty-four per cent of endarterectomy patients (n = 813) were admitted to the ICU post-operatively versus 52% in the stenting group (n = 808). Length of stay on the ward was 5.7 days (SD 9.4 days) for endarterectomy (n = 803) and 5.1 days (SD 10.8 days) for stenting (n = 789). In patients randomised to endarterectomy the mean number of additional endarterectomies during follow-up was 0.039 (SD 0.193) and the mean number of stents was 0.023 (SD 0.159). In patients randomised to stenting, the figures were 0.066 (SD 0.257) and 0.028 (SD 0.172), respectively. No patients in the endarterectomy group had a fatal MI during the first 30 days after treatment, compared with three patients in the stenting group; five patients had a non-fatal MI in the endarterectomy group compared with none in the stenting group; 28 patients in the endarterectomy group had severe haematoma compared with eight patients in the stenting group; one patient in each group had disabling cranial nerve palsy. Patients in both groups underwent a range of imaging tests; ultrasound was the most common in the endarterectomy group (234 tests; n = 857) compared with intra-arterial angiography in the stenting group (352 tests; n = 853). Drug usage 1 month after treatment was similar for both endarterectomy (n = 785) and stenting (n = 781) groups. Seventy-one patients (8%) in the endarterectomy group had one or more strokes during the 5-year time horizon compared with 114 patients (13%) in the stenting group; a higher proportion of strokes in the stenting group were non-disabling (61% vs. 37%).
Accounting for missing data using multiple imputation, mean total costs per patient were £6762 (95% CI £6154 to £7369) in the endarterectomy group (n = 857) and £7351 (95% CI £6786 to £7915) in the stenting group (n = 853) (Table 9). In both groups, approximately two-thirds of the total costs were for the index procedure and one-third for follow-up. Values were similar for complete-case analysis (see Appendix 9).
| Variable | Endarterectomy (n = 857) | Stenting (n = 853) | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Cost of index procedure, £ | 4558 | 4319 to 4797 | 4787 | 4548 to 5026 |
| Cost of follow-up, £ | 2204 | 1696 to 2711 | 2563 | 2114 to 3013 |
| Total cost, £ | 6762 | 6154 to 7369 | 7351 | 6786 to 7915 |
| Utility values | ||||
| Baseline | 0.758 | 0.743 to 0.774 | 0.776 | 0.761 to 0.790 |
| 3 months | 0.779 | 0.763 to 0.795 | 0.777 | 0.759 to 0.795 |
| 6 months | 0.763 | 0.746 to 0.780 | 0.754 | 0.735 to 0.773 |
| 1 year | 0.739 | 0.721 to 0.758 | 0.737 | 0.718 to 0.757 |
| 2 years | 0.709 | 0.688 to 0.729 | 0.710 | 0.689 to 0.732 |
| 3 years | 0.677 | 0.655 to 0.699 | 0.674 | 0.650 to 0.698 |
| 4 years | 0.628 | 0.602 to 0.653 | 0.648 | 0.622 to 0.675 |
| 5 years | 0.594 | 0.563 to 0.625 | 0.609 | 0.578 to 0.641 |
| QALYs | 3.228 | 3.150 to 3.306 | 3.247 | 3.160 to 3.333 |
| NMB | ||||
| £20,000 | £57,793 | £55,994 to £59,592 | £57,580 | £55,699 to £59,461 |
| £30,000 | £90,070 | £87,520 to £92,621 | £90,046 | £87,329 to £92,762 |
Utility values and quality-adjusted life-years
Mean utility values at each follow-up point were similar for the two groups and there was a decline over time. Accounting for missing data, mean utility values per patient increased from 0.758 (95% CI 0.743 to 0.747) in the endarectomy group at baseline to 0.779 (95% CI 0.763 to 0.795) at 3 months and then declined to 0.594 (95% CI 0.563 to 0.625) at 5 years (Table 9). In the stenting group, the values were 0.776 (95% CI 0.761 to 0.790) at baseline, 0.777 (95% CI 0.759 to 0.795) at 1 month and 0.609 (95% CI 0.578 to 0.641) at 5 years. Mean total QALYs per patient were 3.228 (95% CI 3.150 to 3.306) in the endarterectomy group and 3.247 (95% CI 3.160 to 3.333) in the stenting group. Utility values and QALYs were similar for complete cases (see Appendix 9).
Cost–utility analysis
Mean NMBs for endarterectomy and stenting were £57,793 (95% CI £55,994 to £59,592) and £57,580 (95% CI £55,699 to £59,461) at a maximum willingness to pay for a QALY of £20,000, and £90,070 (95% CI £87,520 to £92,621) and £90,046 (95% CI £87,329 to £92,762) at a maximum willingness to pay for a QALY of £30,000 (Table 9). In the base-case analysis there were no significant differences in costs between the two groups (mean incremental costs for stenting versus endarterectomy £537, 95% CI –£238 to £1312) or in outcomes (mean QALYs gained –0.010, 95% CI –0.117 to 0.097; Table 10). The incremental NMB for stenting versus endarterectomy was not significantly different from zero at a maximum willingness to pay for a QALY of £20,000 (mean –£723, 95% CI –£3134 to £1670) or £30,000 (mean –£830, 95% CI –£4265 to £2605).
We repeated the analysis several times using alternative versions of the multiple imputation process using different random number seeds to investigate if the conclusions of the analysis changed; in every case the results were qualitatively the same (i.e. there were no significant differences between the two groups in terms of costs, QALYs and NMBs).
| Scenario | Incremental cost, £ | QALYs gained | Incremental NMB, £ | Probability stenting cost-effective | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | |||||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | £20,000 | £30,000 | |
| Base casea | 537 | –238 to 1312 | –0.010 | –0.117 to 0.097 | –732 | –3134 to 1670 | –830 | –4265 to 2605 | 0.27 | 0.31 |
| No adjustmentb | 589 | –202 to 1380 | 0.019 | –0.098 to 0.135 | –213 | –2821 to 2395 | –25 | –3761 to 3712 | 0.43 | 0.49 |
| Complete-case analysisc | 533 | –836 to 1902 | 0.006 | –0.194 to 0.206 | –415 | –4669 to 3840 | –355 | –6538 to 5827 | 0.42 | 0.45 |
| Subgroup analysesd | ||||||||||
| Men | 337 | –471 to 1145 | –0.055 | –0.185 to 0.076 | –1431 | –4278 to 1417 | –1977 | –6096 to 2142 | 0.17 | 0.18 |
| Women | 787 | –808 to 2382 | 0.103 | –0.098 to 0.304 | 1256 | –3334 to 5864 | 2291 | –4234 to 8815 | 0.71 | 0.75 |
| Age ≥ 70 years | 779 | –323 to 1881 | –0.061 | –0.219 to 0.097 | –1993 | –5474 to 1489 | –2599 | –7607 to 2408 | 0.13 | 0.16 |
| Age < 70 years | 143 | –713 to 999 | 0.057 | –0.094 to 0.208 | 992 | –2234 to 4219 | 1560 | –3139 to 6259 | 0.73 | 0.75 |
Sensitivity and subgroup analyses
The cost-effectiveness acceptability curve shows that at a maximum willingness to pay for a QALY of £20,000 the probability that stenting is cost-effective was 0.27; at a maximum willingness to pay for a QALY of £30,000 the probability that stenting is cost-effective was 0.31 (Table 10 and Figure 10).
FIGURE 10.
Cost-effectiveness acceptability curve. Cost-effectiveness acceptability curve showing the probability that stenting is cost-effective vs. endarterectomy at different values of the maximum willingness to pay for a QALY. The probability that endarterectomy is cost-effective is one minus the probability stenting is cost-effective at each value of the maximum willingness to pay for a QALY.
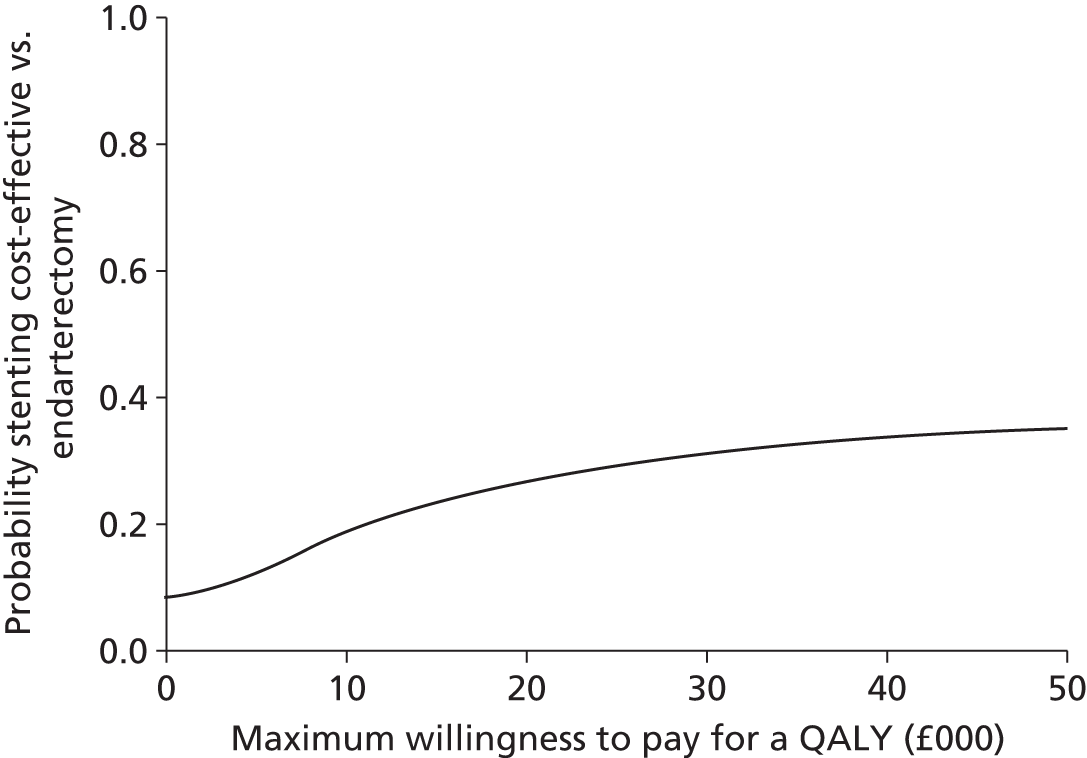
Incremental costs, QALYs gained and incremental NMBs for stenting versus endarterectomy remained not significantly different from zero when rerunning the base-case analysis without adjustment and using complete cases (see Table 10). At a maximum willingness to pay for a QALY of £20,000 the incremental NMB for stenting versus endarterectomy was most sensitive to the cost of stents, cost of operating theatre time, cost per hospital day and cost of treating stroke (Figure 11), but in every case the incremental NMB was not significantly different from zero. Similar findings were obtained using a maximum willingness to pay for a QALY of £30,000 (see Appendix 9). In women and patients aged < 70 years, the mean QALYs gained from stenting versus endarterectomy were positive, whereas in men and patients ≥ 70 years they were negative, but in all cases the differences were not significantly different from zero, neither were the incremental costs and the incremental NMBs.
FIGURE 11.
Univariate sensitivity analysis. All analyses are as for the base-case analysis with univariate adjustment of the parameters listed. Results are point estimates of the incremental NMB of stenting vs. endarterectomy (circles) and 95% CIs (capped spikes). The incremental NMB is calculated at a maximum willingness to pay for a QALY of £20,000 (see Figure 12 in Appendix 9 for results calculated at a maximum willingness to pay for a QALY of £30,000).

Future planned analyses
In addition to the data presented in this report further analyses planned include:
-
analysis of the carotid artery ‘in-stent’ stenosis measurements with DUS versus computerised tomography angiography substudy
-
analysis of the relation between restenosis and recurrent stroke
-
a more detailed analysis of the mRS as an index of disability during follow-up.
Chapter 4 Discussion
Main findings
Carotid artery stenting has a higher short-term (periprocedural) risk than CEA in terms of stroke, but has a lower rate of MI and severe haematoma, and avoids injury to cranial nerves during endarterectomy. The additional short-term risk associated with CAS is largely attributable to non-disabling strokes, and the absolute difference in the risk of any stroke during the whole of follow-up in ICSS was small, with the 47 additional strokes in the stenting group translating to one extra stroke (typically non-disabling) for every 156 patient-years of follow-up. The primary analysis of the trial showed that stenting is equivalent to endarterectomy in preventing fatal or disabling stroke up to 10 years after treatment. Severe restenosis or occlusion of the treated carotid artery was rare, with no difference between treatment groups. Stenting also appeared to be as effective as endarterectomy in preventing ipsilateral stroke occurring during follow-up after the 30-day procedural period. Importantly, there was no difference in functional outcome of patients allocated stenting compared with endarterectomy as assessed by the distribution of mRS scores at 1 year, 5 years or the end of follow-up. Moreover, there were no differences in costs or QALYs between the treatments.
Comparison with other trials
Previous data on prevention of strokes in patients with symptomatic carotid stenosis comparing stenting with endarectomy have been available from three trials reporting near- and mid-term follow-up only, two of which had stopped recruitment before reaching the full sample size. 49,50
The Endarterectomy versus Stenting in Patients with Symptomatic Severe Carotid Stenosis trial (EVA-3S) was stopped early because of a significantly lower rate of periprocedural stroke or death in the endarterectomy group than in the stenting group. 25,49 However, it showed no significant differences in cumulative 4-year rates of fatal or disabling stroke between stenting (6.3%) and endarterectomy (4.0%). 51 The Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) trial in symptomatic patients was stopped early on grounds of cost and futility, but did not show non-inferiority of stenting compared with endarterectomy within 30 days after treatment. 50 In SPACE, ipsilateral disabling stroke within 2 years, or death or disabling stroke in any territory within 30 days of treatment, was recorded in 5.7% patients randomised to stent treatment and in 4.7% of patients allocated to surgery. 52
In the Carotid Revascularisation Endarterectomy versus Stenting Trial (CREST), there was a trend of an increased risk for major ipsilateral stroke in the stent group (1.4%) compared with the endarterectomy group (0.5%) occurring up to 4 years after treatment (p = 0.05), and including both patients with symptomatic and asymptomatic carotid stenosis. 53 This inclusion of asymptomatic patients in CREST probably also accounts for the lower overall event rates reported in that trial. The data from ICSS support these findings; the risk of having a severe stroke remains low after endarterectomy or stenting even after the first 2–4 years of follow-up and, in ICSS, does not differ between stenting and endarterectomy up to 10 years after randomisation.
ICSS is also in agreement with other randomised trials that have previously reported increased risks in procedure-related strokes that did not lead to disability associated with stenting,49,50,53 and in the MRI substudy of ICSS there was a higher incidence of cerebral infarction 1 month after stenting compared with endarterectomy, even where this was not associated with a clinical event. 44 This excess of periprocedural non-disabling strokes accounts for the difference in the combined outcome measure of procedural stroke or death, or stroke in the ipsilateral carotid territory thereafter, in favour of surgery. However, if we consider all strokes occurring in the territory of the treated carotid artery after the procedural period, these were no more frequent after stenting than endarterectomy. It is these strokes that the procedure is designed to prevent and, in these terms, stenting can be considered to be as effective as surgery. However, we observed a small increase in risk in non-procedural stroke occurring in the contralateral carotid or vertebrobasilar territory in the stenting group compared with the endarterectomy group. It is possible that endarterectomy has a beneficial effect in preventing strokes occurring outside the territory of the revascularised artery, possibly through improvement in collateral flow. However, the most likely explanation is that the difference in non-ipsilateral strokes between CAS and CEA in ICSS represents a chance finding.
No other trials have reported the impact of stenting versus endarterectomy on long-term functional outcome measured using the mRS. We have previously reported that the impact of the excess of non-disabling stroke on the mRS scores at 30 days after stenting in ICSS patients was balanced by the impact of the excess of MI, cranial nerve palsy and haematoma on mRS scores after endarterectomy (data not shown). The fact that there was no long-term difference in the distribution of mRS scores at 1 year, 5 years and at the end of follow-up implies that the differences in the various outcomes, including stroke, between the two treatments also balance each other in the long term.
Secondary outcomes and substudies
Restenosis
The SPACE trial reported higher cumulative rates of severe restenosis 2 years after stenting (10.7%) compared with the endarterectomy group (4.6%). 52 In contrast, there was no significant difference in severe restenosis between stenting and endarterectomy in the EVA-3S trial at 3 years (3.3% vs. 2.8%), nor in CREST at 2 years (6.0% vs. 6.3%). 54,55 ICSS with cumulative 5-year rates of 11.8% and 8.6% for CAS and CEA, respectively, supports the view that completed stent treatment is as effective as endarterectomy in preventing residual or recurrent narrowing or occlusion of the carotid artery.
White-matter lesions and periprocedural stroke risk
Carotid artery stenting was associated with a higher risk of stroke compared with CEA in patients with an ARWMC score of 7 or more, but there was no risk difference in patients with an ARWMC score of < 7. 30 This implies that the presence of white-matter lesions on brain imaging should be taken into account when selecting patients for CAS, which should be avoided in patients with extensive white-matter lesions, but might be an acceptable alternative to CEA in patients with less-extensive lesions.
Magnetic resonance imaging substudy
Approximately three times more patients in the stenting group than in the endarterectomy group had new ischaemic lesions on DWI on post-treatment scans. 44 This suggests that the difference in periprocedural clinical stroke risk in ICSS is not likely to have been caused by ascertainment bias. In fact, there is a higher incidence of ischaemic lesions seen on MRI than of recorded clinical events (stroke/TIA). Whether these excess ‘silent’ lesions have any functional consequence for the patient (e.g. cognitive impairment or increased susceptibility to vascular dementia) is an issue not addressed by ICSS, but the fact that there was no difference in functional outcome between the two treatment groups makes it unlikely that the excess of ‘silent’ lesions had any long-term consequences.
Subgroup analysis of procedural events
An exploratory analyses of the composite outcome of stroke, death or procedural MI for pre-defined subgroups suggested that carotid stenting might have a similar risk to endarterectomy in women, but that the intervention was more hazardous than endarterectomy in men. However, the difference between the HRs comparing the risk of stenting with endarterectomy in men and women only reached borderline significance (interaction p = 0.071). Stenting was more hazardous, and endarterectomy less hazardous, in patients not taking medication for hypertension at baseline than in patients taking medication for hypertension.
Subgroup analysis: long-term primary outcome
Exploratory subgroup analyses showed no significant modification of the HR of the primary outcome event or of the combined outcome of procedural death or stroke or non-procedural ipsilateral stroke by any of the evaluated variables.
Cost–utility analysis
Our economic analysis showed that stenting and endarterectomy had similar costs (index procedure costs, follow-up costs and total costs) and outcomes (utility values, QALYs). This was despite the finding in the trial of higher rates of non-disabling strokes in the stenting group. Comprehensive sensitivity analyses showed little uncertainty in this finding. Non-significant differences in utility values and QALYs mirror differences in mRS scores and all-cause mortality found in the trial. The findings mean that there is no reason to prefer either stenting or endarterectomy on the basis of differences in quality of life or on economic grounds; other factors should be taken into account when deciding which option to use to treat patients with symptomatic carotid stenosis (e.g. the age of the patient or imaging features).
Strengths, weaknesses and generalisability
Because of the nature of the interventions in ICSS, it is not possible for either patients or researchers to be blinded to allocated treatment. However, the Chief Investigator and the steering committee remained blinded to the cumulative event rate throughout the trial and the outcome events that were independently assessed according to the following procedure: the events were documented in detail by the investigating centre, censored after receipt at the central office to remove clues as to the treatment allocated, and then adjudicated by an independent neurologist. Furthermore, the results of the imaging substudies were all assessed blinded to treatment.
A range of imaging techniques, including DUS, was allowed in the trial to assess the degree of carotid stenosis prior to randomisation, according to standard practice at the specific centre. Bilateral DUS scan alone was allowed by the protocol only if it was standard practice to treat on the basis of ultrasound alone in individual centres. The number of patients not having a procedure initiated because initial imaging appeared to have misclassified the severity of the stenosis was low (three patients in the CAS arm and one in the CEA arm were found to have < 50% stenosis after randomisation). Fourteen patients did not have a procedure because the stenosis was found to have progressed to occlusion after randomisation (five patients in the CAS arm and nine in the CEA arm). A number of initiated stenting procedures were aborted because the stenosis was found to be < 50% (seven cases) or occlusion was found to be present (15 cases) when angiography was performed immediately prior to attempting to place the stent. Obviously such cases would not have been detected in surgical patients, where the procedure would have gone ahead without knowledge of the exact degree of stenosis immediately prior to the surgery. We can assume, therefore, that the total rate of stenosis found to be inappropriate at procedure in the trial is accurately reflected by the figures for the stenting arm [i.e. a total of 10 cases found to have < 50% stenosis (1.2% of 853 patients randomised to stenting) and perhaps also the 20 cases of occlusion (2.3%)]. However, the proportion of patients who developed occlusion after randomisation (and who were therefore appropriately randomised) is unknown. Thus, despite the leeway allowed in the approach to measuring baseline stenosis in ICSS, at least 96.5% of patients were correctly identified in terms of their degree of stenosis as being suitable for inclusion in the trial (50–99%).
Inevitably in ICSS, which started when stenting was a relatively new treatment, the experience of the interventionists in carotid stenting was less than that of the surgeons in CEA. However, the risk of outcome events associated with stenting was lower in inexperienced, supervised centres than in more experienced centres (see Figure 4) and there was no significant difference in the excess hazard of stenting compared with endarterectomy between supervised and experienced centres, or between centres recruiting more or less than 50 patients; therefore, inexperience is not a major determinant of our results. However, the HR was lower among patients treated at the larger centres than in the smaller centres, which might indicate some effect of procedural volume on technical expertise. A pooled analysis of stenting outcomes by the Carotid Stenting Trialists’ Collaboration showed lower procedural stroke or death risks among patients treated by interventionists with higher annual in-trial procedure volumes, indicating that regular practice in carrying out the procedure matters more than individual total experience or centre volumes. 56
The trial was an international, multicentre study, so patients were included at centres with a range of experience (above the minimum necessary for trial entry) and standards of practice, and a variety of stents and protection devices were used in the trial. Overall, this should mean that the results of ICSS may be taken as being widely applicable in patients and at centres who match the criteria for inclusion in the study.
In terms of the cost–utility analysis, the main strength is that it is based on a large, international, multicentre, randomised trial with detailed information on resource use, utility values and mortality for a median follow-up period of 4.2 years. There are several limitations. First, data on costs of managing strokes were not collected in the trial. However, rather than simply extrapolating from averaged external cost data for stroke, as is done in the vast majority of health economic analyses of large trial cohorts, given the specific nature of the strokes occurring in our particular population, we used individual patient data from the OXVASC study to predict these costs at the patient-level. These were, therefore, detailed contemporaneous UK-specific costs matched to patients in the trial according to significant cost drivers, and blinded as to treatment allocation, but they do not reflect the actual costs incurred by each patient. Nevertheless, when we adjusted these costs in sensitivity analyses the results did not change appreciably. Second, the analysis took a UK NHS/PSS perspective on costs, and utility values were calculated using a UK population value set. Results may differ for other countries depending on the relative value of unit costs and the value set used to generate utility values. Third, a wider perspective (e.g. societal) could have been taken to measure costs, including impacts on the rest of society, including patients, families and businesses. Given that the trial found no differences in mortality or disability, it is unlikely that this would affect the incremental costs, although total costs associated with each treatment may change. Fourth, the time horizon was 5 years. We could have taken a longer time horizon, but there were no differences in costs or benefits between groups at this point, so this would not have affected the results of the incremental analyses.
Implications for health care
Stroke is the major cause of acquired adult physical disability and is responsible for 12% of all deaths in the UK. Atherosclerotic stenosis of the carotid artery is an important cause of stroke, and the risk of recurrent stroke in recently symptomatic patients with severe carotid stenosis is as high as 28% over 2 years. Therefore, revascularisation offers an important means to reduce the burden of stroke in the population. The results of ICSS taken with those of other trials demonstrate that CAS is as effective as endarterectomy in preventing ipsilateral stroke during long-term follow-up. Carotid stenting carries a higher risk of periprocedural stroke, which is largely accounted for by an excess of non-disabling stroke, and more patients in the stenting group than in the endarterectomy group had new ischaemic lesions on post-treatment MRI scans. This excess of non-disabling stroke and new lesions associated with stenting does not translate into a significant difference between stenting and endarterectomy in long-term functional outcomes as assessed by the mRS, quality of life as assessed by the EQ-5D-3L, or a difference in costs as assessed by the economics analysis. The mRS is not a precise measure of the level of independence, and we cannot rule out subtle differences in cognitive outcome or subjective perception of wellbeing between the two treatment groups not captured by these scales. However, it is notable that a substudy of ICSS, carried out at two ICSS centres in which 177 patients enrolled in ICSS had detailed neuropsychological examination, showed no significant difference in cognition 6 months after stenting compared with endarterectomy, despite double the number of new ischaemic lesions on MRI after stenting. 57 Thus, the evidence from ICSS indicates that any impact of the excess of non-disabling stroke and asymptomatic infarction associated with stenting is limited and short-lasting.
We have shown that the severity of white-matter disease is an important modifier of risk with stenting, but not with endarterectomy. In a separate analysis restricted to ICSS patients who were randomised to and completed stenting treatment, age was an independent predictor of the risk of stroke, MI or death within 30 days of stenting. In an analysis of CREST, age also significantly modified the risk of stenting versus endarterectomy,53 whereas a pooled analysis of the data from ICSS with data from EVA-3S and SPACE, as well as a meta-analysis of all the existing trials, have confirmed that patients over the age of 70 years have a higher risk of stroke or death with stenting, but patients below the age of 70 have a similar risk with stenting compared with endarterectomy. 58,59
The analysis of the risk of baseline and procedural-related factors in ICSS showed that the use of an open-cell stent conferred higher risk than use of a closed-cell stent (RR 1.92, 95% CI 1.11 to 3.33), but the use of a cerebral protection device did not modify the risk. Although this was not a randomised comparison, the findings are in keeping with results from the SPACE study and large observational studies. 60,61 This effect of stent design may reflect the benefit of closed-cell stents providing greater coverage of the atheromatous lesion and implies that, in general, closed-cell stents should be preferred to open-cell stents.
The multivariate analysis showed that DBP at baseline was a significant predictor of the risk of stroke, MI or death within 30 days of CEA, implying that control of hypertension pre-operatively is an important aspect of ensuring the safety of surgery. Our analysis confirmed that cranial nerve palsy remains an important complication of endarterectomy, but cranial nerve palsy recovered in almost all patients. Thus, the risk of cranial nerve injury should not influence the choice between CAS and CEA in patients who have not had previous carotid surgery, unless other features favour stenting.
We therefore conclude that the data from ICSS together with data from the other randomised trials show that the choice between stenting and endarterectomy should take into account the different procedural risks of these treatments related to individual patient characteristics. Endarterectomy remains the treatment of choice for older patients and those with extensive white-matter disease, but stenting is an appropriate treatment alternative for patients with symptomatic carotid stenosis if the risk of periprocedural stroke is low, for example in younger patients and those with lower levels of pre-existing white-matter disease. Such patients should be offered stenting after informed consent giving full consideration of the overall periprocedural risks. Considerations of cost and cost-effectiveness should not affect the decision about which of these two treatments to use. In addition to taking into account clinical and imaging features, treatment decisions should take into account patient preferences, with reference to the differing nature of the risks with the two procedures.
Implications for research
The impact of the treatments on patients’ cognitive function was not assessed as part of the main protocol in ICSS (or any of the other reported trials). Given the effect of stenting on silent infarction noted in ICSS MRI substudy, measurement of cognitive function should be an important part of any future study of stenting and/or CEA as this might be an important consideration in choosing between stenting and endarterectomy.
Other important areas for future studies include assessing the morphology and stability of the carotid plaque using modern imaging techniques prior to intervention, and identifying the clinical characteristics of patients that determine how likely they are to benefit from revascularisation in the context of modern optimised medical therapy in lower-risk symptomatic and asymptomatic patients.
Acknowledgements
The ICSS Trial Steering Committee wishes to express its gratitude to all of the principal investigators and researchers (listed in Appendix 9), and the patients and their families whose hard work and commitment were vital to the success of ICSS. Professor Peter Rothwell and Dr Ramon Luengo-Fernandez kindly contributed to the cost-effectiveness evaluation using information from the OXVASC study.
This study was funded by grants from the Medical Research Council (MRC), the Stroke Association, Sanofi-Synthélabo, and the European Union. The funding from the MRC was managed by the National Institute for Health Research (NIHR) on behalf of the MRC–NIHR partnership. MMB’s Chair in Stroke Medicine is supported by the Reta Lila Weston Trust for Medical Research. JE and RLF were supported by a grant from the MRC. LHB was supported by grants from the Swiss National Science Foundation (PBBSB-116873) and the University of Basel. This work was supported by researchers at the NIHR University College London Hospitals Biomedical Research Centre.
Contributions of authors
Roland L Featherstone is the trial manager of ICSS and maintained the trial database with day-to-day responsibility for data collection and completeness. He prepared the data for analysis.
Joanna Dobson, the trial statistician, contributed to the design of the study, its conduct and analysis.
Jörg Ederle contributed to the data analysis and had particular responsibility for the white-matter analysis.
David Doig contributed to the data analysis and interpretation.
Leo H Bonati contributed to analysis and interpretation of data, and led the MRI substudy.
Stephen Morris and Nishma V Patel were responsible for the cost–utility analysis.
Martin M Brown led the development of the protocol and had ongoing oversight and management of the study; he had the final responsibility for the analyses and the manuscript content as the chief investigator of ICSS.
All the authors contributed to drafting the manuscript or revising it critically for important intellectual content.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Publications
ICSS, investigators, Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HR, et al. Carotid artery stenting compared with endarteretomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 2010;375:985–97.
Ederle J, Davagnanam I, van der Worp HB, Venables GS, Lyrer PA, Featherstone RL, et al. Effect of white-matter lesions on the risk of periprocedural stroke after carotid artery stenting versus endarterectomy in the International Carotid Stenting Study (ICSS): a prespecified analysis of data from a randomised trial. Lancet Neurol 2013;12:866–72.
Bonati LH, Jongen LM, Haller S, Flach HZ, Dobson J, Nederkoorn PJ, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol 2010;9:353–62.
Carotid Stenting Trialists’ Collaboration, Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010;376:1062–73.
Bonati LH, Dobson J, Featherstone RL, Ederle J, van der Worp HB, de Borst GJ, et al. Long-term outcome after stenting versus endarterectomy for treatment of symptomatic carotid stenosis in the International Carotid Stenting Study (ICSS): primary analysis of a randomized randomised trial. Lancet 2015;385:529–38. http://dx.doi.org/10.1016/S0140-6736(14)61184-3
Doig D, Turner EL, Dobson J, Featherstone RL, Lo RTH, Gaines PA, et al. Predictors of stroke, myocardial infarction, or death within 30 days of carotid artery stenting: results from the International Carotid Stenting Study. Eur J Vasc Endovasc Surg 2016;51:327–34. http://dx.doi.org/10.1016/j.ejvs.2015.08.013
Doig D, Turner EL, Dobson J, Featherstone RL, de Borst GJ, Brown MM, et al. Incidence, impact, and predictors of cranial nerve palsy and haematoma following carotid endarterectomy in the International Carotid Stenting Study. Eur J Vasc Endovasc Surg 2014;48:498–504. http://dx.doi.org/10.1016/j.ejvs.2014.08.002
Doig D, Turner EL, Dobson J, Featherstone RL, de Borst GJ, Brown MM, et al. Risk factors for stroke, myocardial infarction, or death following carotid endarterectomy: results from the International Carotid Stenting Study. Eur J Vasc Endovasc Surg 2015;50:688–94. http://dx.doi.org/10.1016/j.ejvs.2015.08.006
Morris S, Patel NV, Dobson J, Featherstone RL, Richards T, Luengo-Fernandez R, et al. Cost–utility analysis of stenting versus endarterectomy in the International Carotid Stenting Study [published online ahead of print on 15 February 2016]. Int J Stroke 2016; in press. http://dx.doi.org/10.1177/1747493016632237
Calvet D, Mas JL, Algra A, Becquemin JP, Bonati LH, Dobson J, et al. Carotid stenting: is there an operator effect? A pooled analysis from the carotid stenting trialists’ collaboration. Stroke 2014;45:527–32. http://dx.doi.org/10.1161/STROKEAHA.113.003526
Altinbas A, van Zandvoort MJ, van den Berg E, Jongen LM, Algra A, Moll FL, et al. Cognition after carotid endarterectomy or stenting: a randomized comparison. Neurology 2011;77:1084–90. http://dx.doi.org/10.1212/WNL.0b013e31822e55b9
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC NETSCC, the HTA programme the EME programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme the EME programme or the Department of Health.
References
- European Carotid Surgery Trialists’ Collaborative Group . Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379-87. http://dx.doi.org/10.1016/S0140-6736(97)09292-1.
- Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson CG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415-25. http://dx.doi.org/10.1056/NEJM199811123392002.
- Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107-16. http://dx.doi.org/10.1016/S0140-6736(03)12228-3.
- CAVATAS investigators . Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet 2001;357:1729-37. http://dx.doi.org/10.1016/S0140-6736(00)04893-5.
- Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg 1996;3:42-6. http://dx.doi.org/10.1583/1074-6218(1996)003<0042:SITCAI>2.0.CO;2.
- Yadav JS, Roubin GS, Iyer S, Vitek J, King P, Jordan WD, et al. Elective stenting of the extracranial carotid arteries. Circulation 1997;95:376-81. http://dx.doi.org/10.1161/01.CIR.95.2.376.
- Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx H, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in-patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994;331:489-95. http://dx.doi.org/10.1056/NEJM199408253310801.
- Fischman DL, Leon MB, Baim D, Schatz RA, Savage MP, Penn I, et al. A randomised comparison of coronary stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med 1994;331:496-501. http://dx.doi.org/10.1056/NEJM199408253310802.
- Wholey MH, Wholey M, Bergeron P, Diethrich FB, Henry M, Laborde JC, et al. Current global status of carotid artery stent placement. Cathet Cardiovasc Diagn 1998;44:1-6. http://dx.doi.org/10.1002/(SICI)1097-0304(199805)44:1<1::AID-CCD1>3.0.CO;2-B.
- Brown MM. Carotid artery stenting: the need for randomised trials. Cerebrovasc Dis 2004;18:57-61. http://dx.doi.org/10.1159/000078750.
- International Carotid Stenting Study . International Carotid Stenting Study Protocol. 2007. www.cavatas.com (accessed 3 November 2015).
- Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis. Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003;229:340-6. http://dx.doi.org/10.1148/radiol.2292030516.
- Howard G, Waller JL, Voeks JH, Howard VJ, Jauch EC, Lees KR, et al. A simple, assumption-free, and clinically interpretable approach for analysis of modified Rankin outcomes. Stroke 2012;43:664-9. http://dx.doi.org/10.1161/STROKEAHA.111.632935.
- Bendszus M, Stoll G. Silent cerebral ischaemia: hidden fingerprints of invasive medical procedures. Lancet Neurol 2006;5:364-72. http://dx.doi.org/10.1016/S1474-4422(06)70412-4.
- Flach HZ, Ouhlous M, Hendriks JM, Van Sambeek MR, Veenland JF, Koudstaal PJ, et al. Cerebral ischemia after carotid intervention. J Endovasc Ther 2004;11:251-7. http://dx.doi.org/10.1583/03-1128.1.
- Poppert H, Wolf O, Resch M, Theiss W, Schmidt-Thieme T, Graefin von Einsiedel H, et al. Differences in number, size and location of intracranial microembolic lesions after surgical versus endovascular treatment without protection device of carotid artery stenosis. J Neurol 2004;251:1198-203. http://dx.doi.org/10.1007/s00415-004-0502-4.
- Garcia-Sanchez S, Millan-Torne M, Capellades-Font J, Muchart J, Callejas-P JM, Vila-Moriente N. Ischemic brain lesions following carotid revascularisation procedures: a comparative study using diffusion-weighted magnetic resonance imaging. Rev Neurol 2004;38:1013-17.
- Iihara K, Murao K, Sakai N, Yamada N, Nagata I, Miyamoto S. Outcome of carotid endarterectomy and stent insertion based on grading of carotid endarterectomy risk: a 7-year prospective study. J Neurosurg 2006;105:546-54. http://dx.doi.org/10.3171/jns.2006.105.4.546.
- Gossetti B, Gattuso R, Irace L, Faccenna F, Venosi S, Bozzao L, et al. Embolism to the brain during carotid stenting and surgery. Acta Chir Belg 2007;107:151-4.
- Lacroix V, Hammer F, Astarci P, Duprez T, Grandin C, Cosnard G, et al. Ischemic cerebral lesions after carotid surgery and carotid stenting. Eur J Vasc Endovasc Surg 2007;33:430-5. http://dx.doi.org/10.1016/j.ejvs.2006.11.012.
- Roh HG, Byun HS, Ryoo JW, Naa DG, Moona W-J, Leeb BB, et al. Prospective analysis of cerebral infarction after carotid endarterectomy and carotid artery stent placement by using diffusion-weighted imaging. Am J Neuroradiol 2005;26:376-84.
- Cosottini M, Michelassi MC, Puglioli M, Lazzarotti G, Orlandi G, Marconi F, et al. Silent cerebral ischemia detected with diffusion-weighted imaging in patients treated with protected and unprotected carotid artery stenting. Stroke 2005;36:2389-93. http://dx.doi.org/10.1161/01.STR.0000185676.05358.f2.
- Kastrup A, Nagele T, Groschel K, Schmidt F, Vogler E, Schulz J, et al. Incidence of new brain lesions after carotid stenting with and without cerebral protection. Stroke 2006;37:2312-16. http://dx.doi.org/10.1161/01.STR.0000236492.86303.85.
- Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, . SPACE Collaborative Group . 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet 2006;368:1239-47. http://dx.doi.org/10.1016/S0140-6736(06)69122-8.
- Mas JL, Chatellier G, Beyssen B. EVA-3S Investigators . Carotid angioplasty and stenting with and without cerebral protection: clinical alert from the Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) trial. Stroke 2004;35:e18-20.
- Brown MM, Featherstone RL, Coward LJ. Carotid artery stenting with and without cerebral protection. Stroke 2004;35:2434-5. http://dx.doi.org/10.1161/01.STR.0000143726.33139.6c.
- Wolf O, Heider P, Heinz M, Poppert H, Schmidt-Thieme T, Sander D, et al. Frequency, clinical significance and course of cerebral ischemic events after carotid endarterectomy evaluated by serial diffusion weighted imaging. Eur J Vasc Endovasc Surg 2004;27:167-71. http://dx.doi.org/10.1016/j.ejvs.2003.11.002.
- Muller M, Reiche W, Langenscheidt P, Hassfeld J, Hagen T. Ischemia after carotid endarterectomy: comparison between transcranial Doppler sonography and diffusion-weighted MR imaging. Am J Neuroradiol 2000;21:47-54.
- Streifler JY, Eliasziw M, Benavente OR, Alamowitch S, Fox AJ, Hachinski VC, et al. North American Symptomatic Carotid Endarterectomy Trial Group. Prognostic importance of leukoaraiosis in patients with symptomatic internal carotid artery stenosis. Stroke 2002;33:1651-5. http://dx.doi.org/10.1161/01.STR.0000018010.38749.08.
- Ederle J, Davagnanam I, van der Worp HB, Venables GS, Lyrer PA, Featherstone RL, et al. Effect of white-matter lesions on the risk of periprocedural stroke after carotid artery stenting versus endarterectomy in the International Carotid Stenting Study (ICSS): a prespecified analysis of data from a randomised trial. Lancet Neurol 2013;12:866-72. http://dx.doi.org/10.1016/S1474-4422(13)70135-2.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU, University of Kent; 2011.
- Gomes M, Soares MO, Dumville JC, Lewis SC, Torgerson DJ, Bodenham AR, et al. Cost-effectiveness analysis of general anaesthesia versus local anaesthesia for carotid surgery (GALA Trial). Br J Surg 2010;97:1218-25. http://dx.doi.org/10.1002/bjs.7110.
- National Schedule of Reference Costs Year 2011–12. NHS Trusts and NHS Foundation Trusts: NHS Own Costs. London: Department of Health; 2012.
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11140.
- Luengo-Fernandez R, Gray AM, Rothwell PM. Oxford Vascular Study . A population-based study of hospital care costs during 5 years after transient ischemic attack and stroke. Stroke 2012;43:3343-51. http://dx.doi.org/10.1161/STROKEAHA.112.667204.
- Luengo-Fernandez R, Paul NL, Gray AM, Pendlebury ST, Bull LM, Welch SJ, et al. A population-based study of disability and institutionalisation after TIA and stroke: 10-year results of the Oxford Vascular Study. Stroke 2013;44:28-54. http://dx.doi.org/10.1161/STROKEAHA.113.001584.
- Dolan P. Modelling valuations for health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Briggs A, Clark T, Wolstenholme J, Clarke P, Clark T. Missing . . . presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. http://dx.doi.org/10.1002/hec.766.
- Briggs AH, Gray AM. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess 1999;3.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. J Med Decis Making 1998;18:S64-80.
- Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, . International Carotid Stenting Study investigators . Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 2010;375:985-97. http://dx.doi.org/10.1016/S0140-6736(10)60239-5.
- Bonati LH, Dobson J, Featherstone RL, Ederle J, van der Worp HB, de Borst GJ, et al. Long-term outcome after stenting versus endarterectomy for treatment of symptomatic carotid stenosis in the International Carotid Stenting Study (ICSS): primary analysis of a randomised trial. Lancet 2015;385:529-38. http://dx.doi.org/10.1016/S0140-6736(14)61184-3.
- Bonati LH, Jongen LM, Haller S, Flach HZ, Dobson J, Nederkoorn PJ, et al. ICSS MRI study group . New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol 2010;4:353-62. http://dx.doi.org/10.1016/S1474-4422(10)70057-0.
- Doig D, Turner EL, Dobson J, Featherstone RL, Lo RTH, Gaines PA, et al. Predictors of stroke, myocardial infarction, or death within 30 days of carotid artery stenting: results from the International Carotid Stenting Study. Eur J Vasc Endovasc Surg 2016;51:327-34. http://dx.doi.org/10.1016/j.ejvs.2015.08.013.
- Doig D, Turner EL, Dobson J, Featherstone RL, de Borst GJ, Brown MM, et al. Incidence, impact, and predictors of cranial nerve palsy and haematoma following carotid endarterectomy in the International Carotid Stenting Study. Eur J Vasc Endovasc Surg 2014;48:498-504. http://dx.doi.org/10.1016/j.ejvs.2014.08.002.
- Doig D, Turner EL, Dobson J, Featherstone RL, de Borst GJ, Brown MM, et al. Risk factors for stroke, myocardial infarction, or death following carotid endarterectomy: results from the International Carotid Stenting Study. Eur J Vasc Endovasc Surg 2015;50:688-94. http://dx.doi.org/10.1016/j.ejvs.2015.08.006.
- Morris S, Patel NV, Dobson J, Featherstone RL, Richards T, Luengo-Fernandez R, et al. Cost–utility analysis of stenting versus endarterectomy in the International Carotid Stenting Study. Int J Stroke 2016. http://dx.doi.org/10.1177/1747493016632237.
- Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin J-P, et al. For the EVA-3S Investigators. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med 2006;355:1660-71. http://dx.doi.org/10.1056/NEJMoa061752.
- Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, et al. For the SPACE Collaborative Group. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet 2006;368:1239-47. http://dx.doi.org/10.1016/S0140-6736(06)69122-8.
- Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol 2008;7:885-92. http://dx.doi.org/10.1016/S1474-4422(08)70195-9.
- Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol 2008;7:893-902. http://dx.doi.org/10.1016/S1474-4422(08)70196-0.
- Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W, et al. For the CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010;363:11-23. http://dx.doi.org/10.1056/NEJMoa0912321.
- Arquizan C, Trinquart L, Touboul PJ, Long A, Feasson S, Terriat B, et al. Restenosis is more frequent after carotid stenting than after endarterectomy: the EVA-3S study. Stroke 2011;42:1015-20. http://dx.doi.org/10.1161/STROKEAHA.110.589309.
- Lal BK, Beach KW, Roubin GS, Lutsep HL, Moore WS, Malas MB, et al. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol 2012;11:755-63. http://dx.doi.org/10.1016/S1474-4422(12)70159-X.
- Calvet D, Mas JL, Algra A, Becquemin JP, Bonati LH, Dobson J, et al. Carotid stenting: is there an operator effect? A pooled analysis from the carotid stenting trialists’ collaboration. Stroke 2014;45:527-32. http://dx.doi.org/10.1161/STROKEAHA.113.003526.
- Altinbas A, van Zandvoort MJ, van den Berg E, Jongen LM, Algra A, Moll FL, et al. Cognition after carotid endarterectomy or stenting: a randomized comparison. Neurology 2011;77:1084-90. http://dx.doi.org/10.1212/WNL.0b013e31822e55b9.
- Carotid Stenting Trialists’ Collaboration . Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010;376:1062-73. http://dx.doi.org/10.1016/S0140-6736(10)61009-4.
- Bonati LH, Lyrer P, Ederle J. Percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev 2012;9. http://dx.doi.org/10.1002/14651858.cd000515.pub4.
- Jansen O, Fiehler J, Harmann M, Brückmann H. Protection or non-protection in carotid artery angioplasty: the influence of interventional techniques on outcome data from the SPACE trial. Stroke 2009;40:841-6. http://dx.doi.org/10.1161/STROKEAHA.108.534289.
- Bosiers M, de Donato G, Deloose K, Verbist J, Peeters P, Castriota F, et al. Does free cell area influence the outcome in carotid artery stenting?. Eur J Vasc Endovasc 2007;33:135-43. http://dx.doi.org/10.1016/j.ejvs.2006.09.019.
- British National Formulary. 66 ed. London: BMJ Group and Pharmaceutical Press; 2013.
Appendix 1 Consent form
Appendix 2 Patient information sheet
Appendix 3 International Carotid Stenting Study follow-up form
Appendix 4 International Carotid Stenting Study ultrasound form
Appendix 5 Major event report
Appendix 6 Death report
Appendix 7 Statistical analysis plan
Appendix 8 Protocol amendment details
Original ethical approval for ICSS was received on 4 October 2000 and randomisation commenced in May 2001 using protocol version 2.00.
Protocol Version 3.1 was introduced after receiving ethical approval on 31 October 2003. The main changes from version 2.00 were:
-
The rules governing proctoring and probationary centres, in particular under what conditions a centre may make the transition from probationary to full, were made more explicit.
-
The inclusion criteria were clarified (making it explicit that it is atheromatous stenosis being studied) and modified (the degree of stenosis warranting treatment set at 50% or greater by the NASCET criteria) to reflect current generally accepted practice in the assessment and treatment of stenosis.
-
The nature of symptomatic disease in the inclusion criteria was defined so that: ‘Symptoms must have occurred in the 12 months before randomisation. It is recommended that the time between symptoms and randomisation should be less than 6 months, but patients with symptoms occurring between 6 and 12 months may be included if the randomising physician considers treatment indicated.’
-
Exclusion criteria were extended to include non-atheromatous disease, previously treated artery, planned major surgery (especially CABG) within 1 month of proposed carotid intervention, or planned common carotid surgery.
-
Procedures for dealing with suspected problems with surgical or stenting technique at individual centres were included in the protocol.
-
The projected sample size was reduced from 2000 to 1500.
-
For the primary outcome measure the wording was altered to clarify that non-stroke deaths will be censored in the primary outcome analysis.
-
The EQ-5D-3L became the only measure of quality of life – the Short Form questionnaire-36 items (SF-36) was dropped. Details of the arrangements for payments to centres and the responsibilities of centres with regard to indemnity were included.
Protocol Version 3.2 was introduced after receiving ethical approval on 22 November 2007. The main changes from version 3.10 were:
-
Sample size: the protocol was clarified to make it plain that 1500 patients will be enrolled at full centres – data from patients enrolled at probationary centres to be analysed separately.
-
The duration of follow-up will now exceed 5 years for some patients: at the 5-year follow-up, patients will be asked if they are willing to continue follow-up, in which case annual follow-up will continue up to a maximum of 10 years from randomisation.
-
Two substudies were appended to the main protocol: a restenosis substudy involving an additional investigation (computerised tomography angiography of the carotid) at 1 year after randomisation and a MRI substudy involving three MRI investigations (one before and two after treatment). Consent and patient information forms for these substudies were included with this notification.
Appendix 9 Further details of the cost–utility analysis
-
Prediction of hospital and care home costs in ICSS stroke patients using OXVASC study data.
-
Summary of data used in cost–utility analysis.
-
Additional results for univariate sensitivity analysis.
-
Consolidated Health Economic Evaluation Reporting Standards statement.
Prediction of hospital and care home costs in the International Carotid Stenting Study stroke patients using Oxford Vascular study data
Hospital and care home costs for patients who had one or more strokes in ICSS were predicted using the results from multivariate regression analyses aimed at determining the predictors of hospital and care home costs in patients enrolled in the OXVASC study. 36,37
Oxford Vascular study
The OXVASC study population comprises over 91,000 patients registered in nine general practices across Oxfordshire, UK. Briefly, patient registration began on April 2002 and is ongoing. Only consenting TIA or stroke patients recruited from 1 April 2002 to 31 March 2007 were included in this analysis.
Patients were followed up from the first TIA or stroke in the study period for which the patient sought medical attention, referred to here as the index event. Surviving patients were followed up by a research nurse at 1, 6, 12, 24 and 60 months after the event. Data were collected on patients’ living arrangements, risk factor changes and disability (measured using the mRS). Patients were also followed up via their general practitioner and hospital records, recurrent vascular events were identified by ongoing ascertainment, and all patients had mortality follow-up.
Impairment was measured using the National Institutes of Health Stroke Scale (NIHSS), which was used to categorise event severity. Non-disabling events were defined as NIHSS scores of ≤ 3, and disabling events were those with scores of ≥ 4. Case fatality was defined as death within 30 days of index event. Long-term institutionalisation was defined as admission into a nursing or residential care home. Patients’ hospital records from the Oxford Radcliffe Hospitals NHS Trust were reviewed for any accident and emergency visit, emergency transport, outpatient care visit, day case or hospitalisation from the date of first TIA or stroke within the OXVASC study period (i.e. index event) and for up to 5 years.
Costs
Long-term institutionalisation was costed as the cost per week in a private nursing home, which in 2013 was £750 per week. 32 All hospital care resource use was priced using reference costs for NHS trusts and NHS trust financial returns for the year 2009. 34 These costs were then updated to 2013 prices using the Hospital and Community Health Services pay and price inflation index. 32
Statistical analysis
Nursing/residential care home admission
A Cox proportional hazards model was used to assess the predictors of 6-month admission into a nursing or residential care home. We only assessed admission within 6 months of first stroke to avoid over-estimation of costs owing to non-related conditions. The analysis excluded all patients who had died within 30 days of index event, as no patient with a case fatal event was admitted into a care home in OXVASC study. Independent variables included in the analysis were age at time of event; sex; previously disabled before the event; previous history of MI, angina, TIA, stroke or atrial fibrillation (AF); index event type (TIA or stroke); index event severity (non-disabling or disabling); and number of recurrent strokes up to 5 years after the index event (Table 11).
| Characteristic | HR | p-value | 95% CI |
|---|---|---|---|
| Age | 1.088 | < 0.001 | 1.044 to 1.134 |
| Sex | |||
| Female | Reference | ||
| Male | 0.687 | 0.245 | 0.365 to 1.293 |
| Previously disabled | 3.514 | < 0.001 | 1.866 to 6.620 |
| History of: | |||
| MI | 0.515 | 0.212 | 0.182 to 1.458 |
| TIA | 0.319 | 0.037 | 0.109 to 0.935 |
| Stroke | 0.687 | 0.460 | 0.667 to 2.448 |
| Angina | 1.222 | 0.634 | 0.535 to 2.791 |
| AF | 2.567 | 0.003 | 1.374 to 4.797 |
| Index event | |||
| TIA | Reference | ||
| Stroke | 1.138 | 0.794 | 0.432 to 2.996 |
| Index event severity | |||
| Non-disabling | Reference | ||
| Disabling | 4.590 | < 0.001 | 2.164 to 9.735 |
| Number of recurrent strokes | 1.372 | 0.149 | 0.893 to 2.107 |
| Observations | 1046 | ||
A 6-month risk of admission into a care home in ICSS stroke patients was predicted based on the results of the regression analyses presented in Table 11. We multiplied this predicted risk by £19,740, which was the mean 1-year care home cost for a stroke patient admitted within 6 months to a nursing or residential care home. Given that no OXVASC study patient was admitted into a care home after a case fatal event, ICSS patients with a fatal event as their first stroke were assigned a cost of £0.
Hospital care
A generalised gamma linear model assuming a log identity was used to determine the predictors of 5-year hospital care costs. Independent variables included in the analysis were: age at time of event; sex; previously disabled before the event; previous history of each of MI, angina, peripheral vascular disease (PVD), hypertension, TIA, stroke or atrial fibrillation; index event type (TIA or stroke); index event severity (non-disabling, disabling, fatal); and, number of recurrent strokes up to 5 years after the index event (Table 12).
| Predictors | Coefficient | p-value | 95% CI |
|---|---|---|---|
| Age | 0.019 | < 0.001 | 0.013 to 0.026 |
| Sex | |||
| Female | Reference | ||
| Male | –0.207 | 0.010 | –0.364 to –0.050 |
| Previously disabled | 0.229 | < 0.001 | 0.034 to 0.424 |
| History of: | |||
| MI | 0.037 | 0.777 | –0.220 to 0.294 |
| TIA | –0.224 | 0.036 | –0.433 to –0.014 |
| Stroke | 0.083 | 0.388 | –0.105 to 0.270 |
| PVD | 0.335 | 0.012 | 0.734 to 0.598 |
| Angina | 0.045 | 0.708 | –0.191 to 0.282 |
| Hypertension | 0.017 | 0.838 | –0.144 to 0.177 |
| AF | 0.159 | 0.116 | –0.040 to 0.358 |
| Index event | |||
| TIA | Reference | ||
| Stroke | 0.203 | 0.049 | 0.001 to 0.406 |
| Index event severity | |||
| Non-disabling | Reference | ||
| Disabling | 0.676 | < 0.001 | 0.486 to 0.865 |
| Fatal | –1.608 | < 0.001 | –1.912 to –1.304 |
| Number of recurrent strokes | 0.309 | < 0.001 | 0.200 to 0.419 |
| Constant | 7.988 | < 0.001 | 7.427 to 8.550 |
| Observations | 1205 | ||
The 5-year costs estimated using this model were assumed to be distributed across each year using the proportions reported in Luengo-Fernandez et al. 36 We applied these proportions and matched the resulting cost profile to the stroke patients in the ICSS data set based on the year of follow-up in which the event occurred, omitting costs that occurred outside the 5-year time horizon of the economic evaluation.
Given that stroke is associated with old age and often occurs in patients with other comorbidities, such patients are likely to consume substantial health-care resources regardless of event onset. Therefore, the predicted costs that can be estimated from Table 12 include costs incurred owing to conditions unrelated to stroke. As a result, to better quantify the impact of stroke on costs, we estimated the pre-morbid annual costs (i.e. costs before the initial stroke) and subtracted these from the hospital care costs incurred at each year of follow-up. A similar regression model to that presented in Table 12 was used to estimate pre-morbid costs each year (Table 13). However, we assumed that all costs associated with a fatal event were attributable to the initial stroke. The data used in the cost-utility analysis are summarised in Table 14. The additional results for univariate sensitivity analysis calculated at a maximum willingness to pay for a QALY of £30,000 are shown in Figure 12. The Consolidated Health Economic Evaluation Reporting Standards statement is given in Table 15.
| Predictors | Coefficient | p-value | 95% CI |
|---|---|---|---|
| Age | 0.019 | 0.004 | 0.006 to 0.032 |
| Sex | |||
| Female | Reference | ||
| Male | –0.090 | 0.590 | –0.416 to 0.236 |
| Previously disabled | 1.880 | < 0.001 | 1.425 to 2.333 |
| History of: | |||
| MI | 0.436 | 0.074 | –0.043 to 0.914 |
| TIA | 0.150 | 0.546 | –0.337 to 0.637 |
| Stroke | –0.091 | 0.682 | –0.528 to 0.345 |
| PVD | 0.624 | 0.041 | 0.026 to 1.221 |
| Angina | 0.591 | 0.008 | 0.156 to 1.028 |
| Hypertension | –0.099 | 0.564 | –0.436 to 0.238 |
| AF | 0.512 | 0.006 | 0.144 to 0.880 |
| Index event | |||
| TIA | Reference | ||
| Stroke | 0.226 | 0.169 | –0.096 to 0.548 |
| Index event severity | |||
| Non-disabling | Reference | ||
| Disabling | 0.253 | 0.266 | –0.192 to 0.698 |
| Constant | 4.813 | < 0.001 | 3.769 to 5.856 |
| Observations | 1096 | ||
Summary of data used in cost–utility analysis
| Variable | Endarterectomy | Stenting | Unit cost/source | ||
|---|---|---|---|---|---|
| Value (£) | N | Value (£) | N | ||
| Procedure duration (minutes), mean (SD) | 107 (47) | 700 | 68 (33) | 691 | |
| Consultant vascular surgeon, n | 1 | 0 | £140 per hour/reference32 | ||
| Consultant interventional radiologist, n | 0 | 1 | £140 per hour/reference32 | ||
| Surgical registrar, n | 1 | 0 | £59 per hour/reference32 | ||
| Consultant anaesthetist, na | 1 | 1 | £140 per hour/reference32 | ||
| Theatreb | £743 per hour/reference32 | ||||
| Type of anaesthesia, % | 794 | 853 | |||
| Local anaesthetic | 18 | 100 | £9/reference33 | ||
| General anaesthetic | 82 | 0 | £30/reference33 | ||
| Shunt used, % | 40 | 818 | £38/University College London Hospitals NHS Foundation Trust | ||
| Patch used, % | 66 | 693 | £96/University College London Hospitals NHS Foundation Trust | ||
| Stent deployed, % | 92 | 816 | £840/University College London Hospitals NHS Foundation Trust | ||
| Cerebral protection device used, % | 71 | 824 | £780/University College London Hospitals NHS Foundation Trust | ||
| Other materials used in stentingc | £232/University College London Hospitals NHS Foundation Trust | ||||
| Admitted to ICU post-operatively, % | 64 | 813 | 52 | 808 | |
| Length of stay on ICU if admitted (days) | 1 | 1 | £661 per day/reference34 | ||
| Length of stay on ward (days), mean (SD) | 5.7 (9.4) | 803 | 5.1 (10.8) | 789 | Endarterectomy £339 per day, stenting £301 per day/reference34 |
| Additional carotid artery procedures, mean (SD) | 857 | 853 | |||
| Endarterectomy | 0.039 (0.193) | 0.066 (0.257) | Mean cost of index procedure | ||
| Stenting | 0.023 (0.159) | 0.028 (0.172) | Mean cost of index procedure | ||
| Fatal MI in first 30 days, n (%) | 0 (0) | 857 | 3 (0.35) | 853 | £1485/reference35 |
| Non-fatal MI in first 30 days, n (%) | 5 (1) | 857 | 0 (0) | 853 | £5665 in first year, £218 each year thereafter/reference35 |
| Severe haematoma in first 30 days, n (%)d | 28 (3) | 857 | 8 (1) | 853 | £9302/reference34 |
| Disabling cranial nerve palsy in first 30 days, n (%) | 1 (0) | 857 | 1 (0) | 853 | £6964/reference34 |
| Imaging tests, ne | 857 | 853 | |||
| CEMRA | 14 | 13 | £208/reference34 | ||
| CT scan | 156 | 171 | £88/reference34 | ||
| CTA | 54 | 93 | £110/reference34 | ||
| Intra arterial angiography | 43 | 352 | £112/reference34 | ||
| Intravenous DSA | 4 | 16 | £150/reference34 | ||
| MRA | 17 | 23 | £157/reference34 | ||
| MRA/CTA | 34 | 48 | £267/reference34 | ||
| MRI | 123 | 119 | £157/reference34 | ||
| Ultrasound | 234 | 192 | £67/reference34 | ||
| Drug treatment at 1 month after treatment, n (%) | 785 | 781 | |||
| Aspirin | 653 (83) | 698 (89) | £2.57 per month/reference62 | ||
| Clopidogrel | 136 (17) | 426 (55) | £1.83 per month/reference62 | ||
| Dipyridamole | 161 (21) | 94 (12) | £5.29 per month/reference62 | ||
| Antihypertensive | 556 (71) | 479 (61) | £9.61 per month/reference62 | ||
| Statin | 634 (81) | 601 (77) | £2.12 per month/reference62 | ||
| Anticoagulant (vitamin K antagonist) | 50 (6) | 37 (5) | £0.96 per month/reference62 | ||
| Stroke during 5 year follow-up, n (%) | 71 (8) | 857 | 114 (13) | 853 | |
| Stroke severity, n (% strokes) | 71 | 114 | |||
| Non-disabling | 26 (37) | 70 (61) | |||
| Disabling | 35 (49) | 27 (24) | |||
| Fatal | 10 (14) | 17 (15) | |||
| Recurrent strokes, n (% strokes) | 71 | 114 | |||
| 0 | 62 (87) | 102 (89) | |||
| 1 | 7 (10) | 10 (9) | |||
| 2 | 1 (1) | 2 (2) | |||
| 3 | 1 (1) | 0 (0) | |||
| Cost per patient of treating stroke, mean (SD) | 71 | 114 | |||
| First year | 7281 (5627) | 5792 (5726) | Mean predicted costs/OXVASC Study | ||
| Second year | 1398 (1398) | 1079 (1628) | Mean predicted costs/OXVASC Study | ||
| Third year | 2050 (1929) | 1602 (2034) | Mean predicted costs/OXVASC Study | ||
| Fourth year | 1522 (1615) | 1178 (1702) | Mean predicted costs/OXVASC Study | ||
| Fifth year | 1831 (1795) | 1426 (1894) | Mean predicted costs/OXVASC Study | ||
| Cost of index procedure, mean (SD) | 4501 (3570) | 586 | 4724 (3293) | 660 | |
| Cost of follow-up, mean (SD) | 2187 (4522) | 389 | 2401 (5115) | 424 | |
| Total costs, mean (SD) | 6851 (7403) | 274 | 6994 (7913) | 340 | |
| Utility values, mean (SD) | |||||
| Baseline | 0.758 (0.231) | 846 | 0.775 (0.212) | 841 | |
| 3 months | 0.779 (0.233) | 805 | 0.779 (0.255) | 810 | |
| 6 months | 0.768 (0.247) | 764 | 0.759 (0.279) | 774 | |
| 1 year | 0.745 (0.272) | 775 | 0.743 (0.296) | 777 | |
| 2 years | 0.720 (0.302) | 741 | 0.720 (0.305) | 744 | |
| 3 years | 0.685 (0.333) | 722 | 0.675 (0.341) | 735 | |
| 4 years | 0.619 (0.361) | 589 | 0.635 (0.363) | 620 | |
| 5 years | 0.564 (0.396) | 461 | 0.575 (0.393) | 498 | |
| QALYs | 3.189 (1.204) | 329 | 3.164 (1.364) | 362 | |
Additional results for univariate sensitivity analysis
FIGURE 12.
Univariate sensitivity analysis: results calculated at a maximum willingness to pay for a QALY of £30,000. All analyses are as for the base-case analysis with univariate adjustment of the parameters listed. Results are point estimates of the incremental NMB of stenting vs. endarterectomy (circles) and 95% CIs (capped spikes). The incremental NMB is calculated at a maximum willingness to pay for a QALY of £30,000.
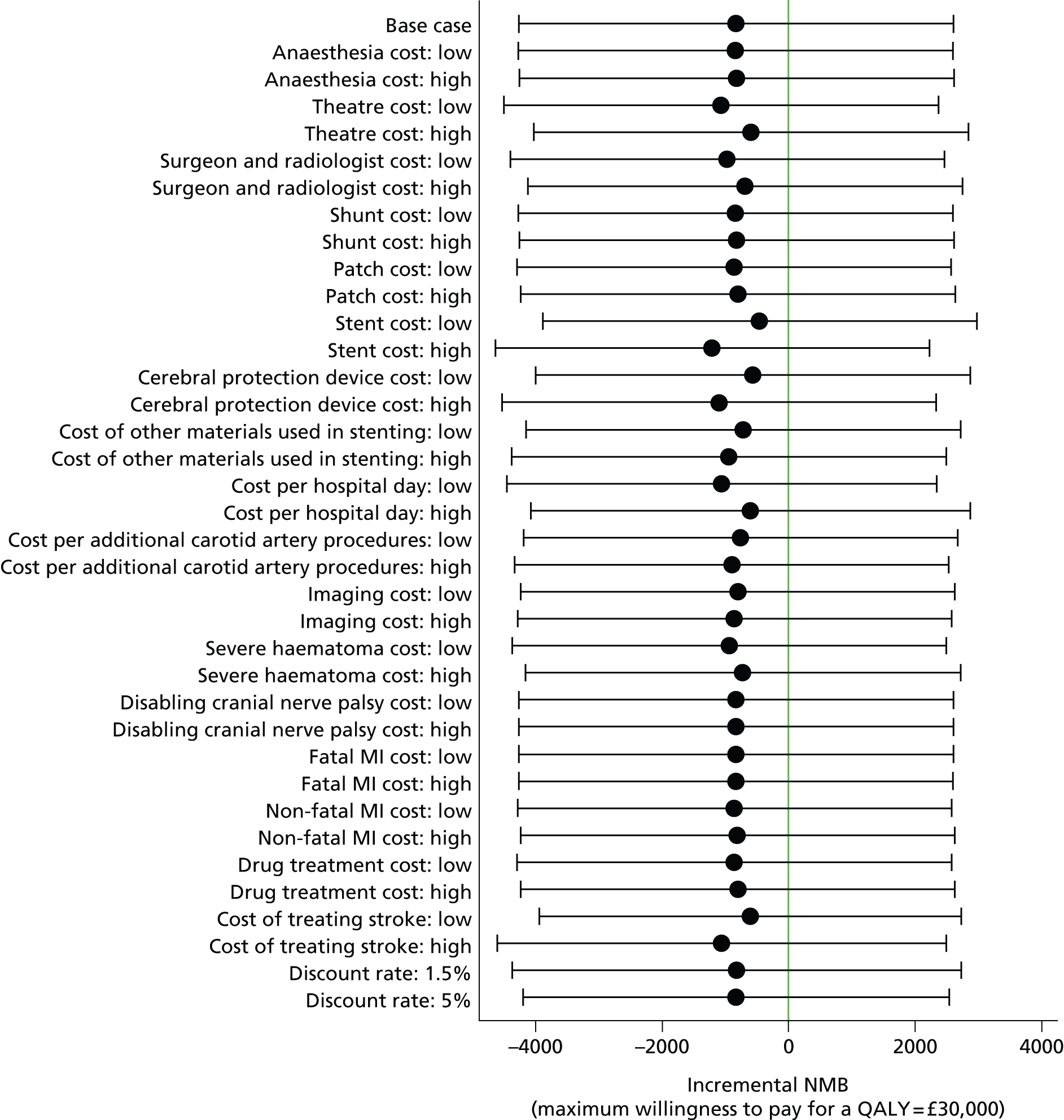
Consolidated Health Economic Evaluation Reporting Standards statement
| Section | Item number | Reported on |
|---|---|---|
| Title and abstract | ||
| Title | 1 | The title of Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis identifies the study as an economic analysis and describes the interventions being evaluated |
| Abstract | 2 | A structured summary is provided in the Scientific summary |
| Introduction | ||
| Background and objectives | 3 | The broader context for the study, and the research question and its rationale, are described in Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis |
| Methods | ||
| Target population and subgroups | 4 | The target population is described in Chapter 2 |
| Setting and location | 5 | The setting and location of the trial are described in Chapter 2 |
| Study perspective | 6 | The study perspective is described in the first part of Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis |
| Comparators | 7 | The comparators are described and justified in the first part of Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis |
| Time horizon | 8 | The time horizon is described and justified in the first part of Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis |
| Discount rate | 9 | The discount rate for costs and outcomes is described and justified in the first part of Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis |
| Choice of health outcomes | 10 | The outcome measure is described and justified in the first part of Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis |
| Measurement of effectiveness | 11a | The clinical trial used to measure effectiveness is described in Chapter 2 |
| Measurement and valuation of preference-based outcomes | 12 | Methods used to measure and value preference-based outcomes are described in the Utilities and QALYs subsection of Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis |
| Estimating resources and costs | 13a | Methods used to estimate resources and costs are described in the Resource use and costs subsection of Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis |
| Currency, price date, and conversion | 14 | Currency, price date and conversion are described in the first part of Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis |
| Choice of model | 15 | We explain that extrapolation beyond the end of the trial using decision–analytical modelling was not undertaken in the first part of Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis |
| Assumptions | 16 | All assumptions used in the analysis are described throughout Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis and Appendix 9 |
| Analytical methods | 17 | Analytical methods are described in the Dealing with missing data and Statistical methods subsections in Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis |
| Results | ||
| Study parameters | 18 | The main study parameters are in Table 9 in Findings of the cost–utility analysis and Appendix 9 |
| Incremental costs and outcomes | 19 | Incremental costs and outcomes are reported in Tables 9 and 10 and Appendix 9 and discussed throughout Findings of the cost–utility analysis |
| Characterising uncertainty | 20a | Methods used in the sensitivity analyses are described in the Sensitivity and subgroup analyses subsection in Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis. The results are presented in Table 10 and Figures 10 and 11, and discussed in the Sensitivity and subgroup analyses subsection in Findings of the cost–utility analysis |
| Characterising heterogeneity | 21 | Methods used in the subgroup are described in the Sensitivity and subgroup analyses subsection in the Cost–utility analysis of stenting versus endarterectomy for treatment of symptomatic carotid stenosis. The results are presented in Table 10 and discussed in the Sensitivity and subgroup analyses subsection in Findings of the cost–utility analysis |
| Discussion | ||
| Study findings, limitations, generalisability and current knowledge | 22 | Study findings, limitations, generalisability and comparisons with current knowledge are discussed in Chapter 4 |
| Other | ||
| Source of funding | 23 | The funding source is in the Acknowledgements. The role of the funder is described in the Role of the funding source |
| Conflicts of interest | 24 | Conflicts of interests are described in the Competing interests |
Appendix 10 International Carotid Stenting Study investigators and recruiting centres
Steering Committee
A Algra, J Bamford (chairperson), J Beard, M Bland, AW Bradbury, MM Brown (chief investigator), A Clifton, P Gaines, W Hacke, A Halliday, I Malik, JL Mas, AJ McGuire, P Sidhu and G Venables.
Credentialling Committee
A Bradbury, MM Brown, A Clifton and P Gaines.
Data Monitoring Committee
R Collins, A Molyneux, R Naylor and C Warlow (chairperson).
Outcome Event Adjudication Committee
JM Ferro and D Thomas.
Central office staff at University College London Institute of Neurology
LH Bonati, L Coward, J Dobson (trial statistician), D Doig, J Ederle, RF Featherstone (trial manager), F Kennedy, H Tindall, E Turner, DJH McCabe and A Wallis.
Location of International Carotid Stenting Study recruiting centres
The numbers of patients recruited at each centre (in square brackets) and investigators at each centre are recorded; PI, local principal investigator.
Australia
Austin Health, Heidelberg [46]: M Brooks, B Chambers (PI), A Chan, P Chu, D Clark, H Dewey, G Donnan, G Fell, M Hoare, M Molan, A Roberts and N Roberts.
Box Hill Hospital (Monash University), Melbourne [25]: B Beiles, C Bladin (PI), C Clifford, G Fell, M Grigg and G New.
Monash Medical Centre, Clayton [26]: R Bell, S Bower, W Chong, M Holt, A Saunder and PG Than (PI).
Princess Alexandra Hospital, Brisbane [48]: S Gett, D Leggett, T McGahan (PI), J Quinn, M Ray, A Wong and P Woodruff.
Repatriation General Hospital, Daw Park, Adelaide [6]: R Foreman, D Schultz (PI), R Scroop and B Stanley.
Royal Melbourne Hospital, Melbourne [57]: B Allard, N Atkinson, W Cambell, S Davies (PI), P Field, P Milne, P Mitchell, B Tress and B Yan.
The Royal Hobart Hospital, Hobart [18]: A Beasley, D Dunbabin, D Stary and S Walker (PI).
Belgium
Antwerp University Hospital, Antwerp [10]: P Cras, O d’Archambeau, JMH Hendriks (PI) and P Van Schil.
AZ St Blasius, Dendermonde [5]: M Bosiers (PI), K Deloose and E van Buggenhout.
AZ Sint Jan Brugge-Oostende, Campus Brugge, Brugges [18]: J De Letter, V Devos, J Ghekiere and G Vanhooren (PI).
Cliniques Universitaires St Luc, Bruxelles [1]: P Astarci, F Hammer, V Lacroix, A Peeters (PI) and R Verhelst.
Imelda Ziekenhuis, Bonheiden [3]: L DeJaegher (PI), A Peeters and J Verbist.
Canada
Centre hospitalier de l’université de Montréal/Notre-Dame Hospital, Montreal [30]: J-F Blair, JL Caron, N Daneault, M-F Giroux, F Guilbert, S Lanthier, L-H Lebrun, V Oliva, J Raymond, D Roy (PI), G Soulez and A Weill.
Foothills Medical Centre, Calgary [4]: M Hill (PI), W Hu, M Hudion, W Morrish, G Sutherland and J Wong.
Finland
Helsinki University Central Hospital, Helsinki [33]: A Albäck, H Harno, P Ijäs, M Kaste (PI), M Lepäntalo, S Mustanoja, T Paananen, M Porras, J Putaala, M Railo, T Sairanen, L Soinne, A Vehmas and P Vikatmaa.
Germany
Otto von Guericke University, Magdeburg [9]: M Goertler (PI), Z Halloul and M Skalej.
Ireland
Beaumont Hospital, Dublin [4]: P Brennan, C Kelly, A Leahy, J Moroney (PI) and J Thornton.
New Zealand
Auckland City Hospital, Auckland [40]: PA Barber, R Bourchier, A Hill, A Holden and J Stewart (PI).
Norway
Rikshospitalet University Hospital, Oslo [16]: SJ Bakke (PI), K Krohg-Sørensen, M Skjelland and B Tennøe.
Poland
Institute of Psychiatry and Neurology (2nd Department of Neurology and Department of Neuroradiology) and Medical University of Warsaw (2nd Department of General, Vascular and Oncological Surgery), Warsaw [20]: P Bialek, Z Biejat, W Czepiel, A Czlonkowska (PI), A Dowzenko, J Jedrzejewska, A Kobayashi, M Lelek and J Polanski.
Slovenia
University Medical Centre, Ljubljana [12]: J Kirbis, Z Milosevic and B Zvan (PI).
Spain
Hospital Clinic, Barcelona [18]: J Blasco, A Chamorro (PI), J Macho, V Obach, V Riambau and L San Roman.
Parc Taulí Sabadell Hospital, Barcelona [33]: J Branera, D Canovas (PI), Jordi Estela, A Gimenez Gaibar and J Perendreu.
Sweden
Malmö University Hospital, Malmö [67]: K Björses, A Gottsater (PI), K Ivancev, T Maetzsch and B Sonesson.
Sodersjukhuset, Stockholm [55]: B Berg, M Delle, J Formgren, P Gillgren, T-B Kall, P Konrad (PI), N Nyman and R Takolander.
The Karolinska Institute, Stockholm [5]: T Andersson, J Malmstedt, M Soderman, C Wahlgren and N Wahlgren (PI).
Switzerland
Centre Hospitalier Universitaire Vaudois, Lausanne [12]: S Binaghi, L Hirt, P Michel (PI) and P Ruchat.
University Hospital Basel, Basel [94]: LH Bonati, ST Engelter, F Fluri, L Guerke, AL Jacob, E Kirsch, PA Lyrer (PI), E-W Radue, P Stierli, M Wasner and S Wetzel.
University Hospital of Geneva, Geneva [16]: C Bonvin, A Kalangos, K Lovblad, N Murith, D Ruefenacht and R Sztajzel (PI).
The Netherlands
Academic Medical Centre, Amsterdam [56]: M Koelemaij, PJ Nederkoorn (PI), J Reekers and YB Roos.
Erasmus Medical Centre, Rotterdam [75]: JM Hendriks, PJ Koudstaal (PI), PMT Pattynama, A van der Lugt, LC van Dijk, MRHM van Sambeek, H van Urk and HJM Verhagen.
The Haga Teaching Hospitals, The Hague [45]: CMA Bruijninckx, SF de Bruijn, R Keunen, B Knippenberg, A Mosch (PI), F Treurniet, L van Dijk, H van Overhagen and J Wever.
Isala Klinieken, Zwolle [14]: FC de Beer, JSP van den Berg (PI), BAAM van Hasselt and DJ Zeilstra.
Medical Centre Haaglanden, The Hague [3]: J Boiten (PI), JCA de Mol van Otterloo, AC de Vries, GJ Lycklama a Nijeholt and BFW van der Kallen.
UMC St Radboud, Nijmegen [13]: JD Blankensteijn, FE De Leeuw, LJ Schultze Kool (PI) and JA van der Vliet.
University Medical Centre, Utrecht [270]: GJ de Borst, GAP de Kort, LJ Kapelle (PI), TH Lo, WPThM Mali, F Moll, HB van der Worp and H Verhagen.
UK
Addenbrookes Hospital, Cambridge [5]: N Higgins, PJ Kirkpatrick, P Martin (PI) and K Varty.
Birmingham Heartlands Hospital, Birmingham [11]: D Adam, J Bell, AW Bradbury, P Crowe, M Gannon, MJ Henderson, D Sandler, RA Shinton (PI), JM Scriven and T Wilmink.
Lancashire Teaching Hospitals NHS Trust, Preston [2]: S D’Souza, A Egun, R Guta, S Punekar, DM Seriki (PI) and G Thomson.
Liverpool Royal Infirmary [21] and The Walton Centre, Liverpool [7]: JA Brennan, TP Enevoldson, G Gilling-Smith (PI), DA Gould, PL Harris, RG McWilliams, H-C Nasser and R White.
Manchester Royal Infirmary, Manchester [2]: KG Prakash, F Serracino-Inglott, G Subramanian (PI), JV Symth and MG Walker.
Newcastle Acute Hospitals NHS Foundation Trust, Newcastle-Upon-Tyne [108]: M Clarke, M Davis, SA Dixit, P Dorman (PI), A Dyker, G Ford, A Golkar, R Jackson, V Jayakrishnan, D Lambert, T Lees, S Louw, S Macdonald, AD Mendelow, H Rodgers, J Rose, G Stansby and M Wyatt.
North Bristol NHS Trust, Frenchay Hospital, Bristol [13]: T Baker, N Baldwin (PI), L Jones, D Mitchell, E Munro and M Thornton.
Royal Free Hospital, London [1]: D Baker, N Davis, G Hamilton (PI), D McCabe, A Platts and J Tibballs.
Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield [151]: J Beard, T Cleveland, D Dodd, P Gaines, R Lonsdale, R Nair, A Nassef, S Nawaz and G Venables (PI).
St George’s University of London and St George’s NHS Healthcare Trust, London [58]: A Belli, A Clifton, G Cloud, A Halliday, H Markus (PI), R McFarland, R Morgan, A Pereira and A Thompson.
St Mary’s Hospital, Imperial College Healthcare NHS Trust, London [13]: J Chataway (PI), N Cheshire, R Gibbs, M Hammady, M Jenkins, I Malik and J Wolfe.
University College London Hospitals NHS Foundation Trust, London [51]: M Adiseshiah, C Bishop, S Brew, J Brookes, MM Brown (PI), R Jäger and N Kitchen.
University Hospital of South Manchester, Wythenshawe, Manchester [58]: R Ashleigh, S Butterfield, GE Gamble, C McCollum (PI), A Nasim, P O’Neill and J Wong.
Western Infirmary, Glasgow [5]: RD Edwards, KR Lees, AJ MacKay, J Moss (PI) and P Rogers.
List of abbreviations
- ARWMC
- age-related white-matter changes
- CAS
- carotid artery stenting
- CAVATAS
- Carotid and Vertebral Artery Transluminal Angioplasty Study
- CEA
- carotid endarterectomy
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CREST
- Carotid Revascularisation Endarterectomy versus Stenting Trial
- CT
- computerised tomography
- DBP
- diastolic blood pressure
- DUS
- duplex ultrasound
- DWI
- diffusion weighted imaging
- EQ-5D-3L™
- European Quality of Life-5 Dimensions – 3 level response
- EVA-3S
- Endarterectomy versus Stenting in Patients with Symptomatic Severe Carotid Stenosis trial
- HR
- hazard ratio
- ICSS
- International Carotid Stenting Study
- ICU
- intensive care unit
- ITT
- intention to treat
- MI
- myocardial infarction
- MRI
- magnetic resonance imaging
- mRS
- modified Rankin Scale
- NASCET
- North American Symptomatic Carotid Endarterectomy Trial
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- OR
- odds ratio
- OXVASC
- Oxford Vascular
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- RR
- risk ratio
- SD
- standard deviation
- SPACE
- Stent-Protected Angioplasty versus Carotid Endarterectomy
- TIA
- transient ischaemic attack