Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/44/01. The contractual start date was in August 2013. The draft report began editorial review in December 2014 and was accepted for publication in March 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Thurgar et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Introduction
Anogenital warts (AGWs) are the second most commonly diagnosed sexually transmitted infection (STI) in the UK. 1 In 2013, AGWs made up 16% (approximately 73,000 new cases) of all incident STI cases presenting in genitourinary medicine (GUM) clinics in England. 2 Typically occurring on the external genitalia, AGWs are usually painless but can be unsightly and physically uncomfortable, and affected people may experience psychological distress. 3 Additionally, recurrence of AGWs after initial clearance is common, with approximately half of patients experiencing the development of new AGWs within 1 year after clearance of lesions. 1
Aetiology and pathology
Anogenital warts are benign epithelial skin lesions and are caused by human papillomavirus (HPV) infection. Over 100 HPV types have been identified, of which about 30 have been found to infect genital epithelium. 4 AGWs are predominantly (approximately 90%) caused by HPV subtypes 6 and 11. 1 Many people who contract HPV do not develop AGWs and it can take some time after infection with HPV before AGWs appear;5,6 most people will develop AGWs between 3 weeks and 8 months after infection with HPV. 7 Consequently, people might not be aware that they are carrying the virus and could unknowingly pass on the infection. HPV can also be transmitted from a mother to her infant during labour (perinatal transmission), but this is rare. 8 Treatment of AGWs does not eliminate HPV infection, but most people whose lesions clear will become HPV deoxyribonucleic acid (DNA) negative. Cells that remain infected with HPV DNA can stay dormant (latent) for prolonged periods of time and a first episode or a recurrence of symptoms can occur months, or even years, after initial infection. Thus, those who do not become HPV DNA negative can also pass on the virus, even after treatment or clearance of lesions. 5
Like other papillomaviruses, HPV establishes productive infections in keratinocytes of the skin or mucous membrane. 9 HPV replicates only in the basal cell layer of surface tissues and infected areas are marked by a proliferation of viral DNA and the formation of AGWs. HPV is transferred by close skin-to-skin contact and so areas of skin that are traumatised during sexual intercourse are the sites on which AGWs are most likely to develop. 5,6 AGWs typically appear on the penis, scrotum, urethral meatus and perianal area in men and on the introitus (vaginal opening), vulva, perineum and perianal area in women. 10 AGWs rarely develop on the pubic area, upper thighs and inguinal folds in men and women or on the cervix and vaginal walls in women. Although AGWs most frequently appear on external genital locations, they can also develop in the anal canal5 and in the oral cavity, larynx, conjunctivae and nasal cavity. 3
An AGW can occur as a single lesion but cases of AGWs seen in clinical practice frequently consist of from five to 15 lesions of 1–10 mm in diameter. 10 Lesions can be condylomatous, papular, flat or keratotic in appearance. 11 Most AGWs present as soft cauliflower-like growths (condylomatous) of varying size, with flat, plaque-like and pigmented lesions seen less frequently. 12 Condylomatous and papular AGWs are usually soft and located on moist, non-keratinised, non-hair-bearing skin. By contrast, flat and keratotic AGWs are firm and located on dry, keratinised, hairy skin. 13 Soft, non-keratinised AGWs usually respond well to treatment with topical application of, for example, podophyllotoxin, imiquimod 5% cream (Aldara®, Meda Pharmaceuticals) and trichloroacetic acid (TCAA), whereas physical ablative methods are more effective for treating keratinised lesions. 5
Of those people with HPV who develop AGWs, most will notice painless lumps or growths in the anogenital area. 5,6 Depending on the number, size and location of the AGWs, a few people might experience local irritation, bleeding, discomfort or pain. Large AGWs can manifest with coincident maceration of the skin (softening of the skin caused by constant exposure to moisture), but this is rare;7 maceration of the skin increases the risk of secondary infection. 7
Risk factors for AGWs include a history of unprotected sexual intercourse, a history of STIs, smoking, the use of oral contraceptives and high parity (number of children). 14,15 Susceptibility to the development of AGWs is generally higher among patients who are immunocompromised, such as people who have undergone organ transplantation or those with human immunodeficiency virus (HIV) infection. 14,15 Hormonal factors and male circumcision have also been investigated as risk factors for genital HPV infection, with inconclusive results. 16,17
Diagnosis
Anogenital warts are typically diagnosed by visual examination and additional investigations are usually unnecessary. 5,6 HPV typing is not routine in the diagnosis of AGWs. European guidelines recommend against HPV typing as no additional information is gained. 6 AGWs with atypical features that are suggestive of precancerous or cancerous lesions should be biopsied. 6,11 When a person presents with perianal AGWs, or with irritation of or discharge from the anus, examination of the anal canal is recommended. 5,6
Application of acetic acid 5% can turn lesions white. 5,6 Some clinicians use acetic acid 5% to help visualise AGWs and to help diagnose subclinical HPV lesions. However, whitening of lesions is not specific to HPV-associated lesions and use of acetic acid in the diagnosis of AGWs remains controversial. 6
Prognosis
Anogenital warts can potentially clear without treatment, most likely in people who are immunocompetent. 11 On this basis, some people may prefer to wait a period of time before starting treatment. 10 However, there is uncertainty around the frequency of spontaneous resolution of lesions, with reports of rates of clearance without treatment ranging between 0% and 50% of people affected. 7,13,18,19 As well as increasing the risk of onward transmission, a delay in treatment could result in a worsening of AGWs, with increases in size or number of AGWs or the area affected, particularly in people who have impaired cellular immunity (e.g. as a result of pregnancy or infection with HIV). First-line treatment is not always successful in achieving complete clearance of AGWs and repeated treatments over a prolonged time period might be required to eradicate large or persistent AGWs. The recording of lesions on AGW maps at each visit affords an opportunity to monitor response or lack of response to treatment. 5
Treatment of AGWs does not necessarily eradicate the underlying HPV infection and the effect of treatment on reducing HPV infectivity is unclear. 20 Recurrence of AGWs after clearance is common. In 2013, GUM clinics diagnosed 73,418 new cases of AGWs and 62,873 cases of recurrent AGWs2 (an individual could have experienced more than one episode of recurrence). If a person has small, easy-to-treat AGWs, they are still likely to require multiple rounds of treatment. Additionally, it is often unclear whether recurrences are the result of recurrence of infection after a period of remission, a new infection or inadequate treatment during an active episode. 20
It is estimated that 20% of people with AGWs have a concurrent STI, including chlamydia, HIV infection and syphilis, and screening for other STIs should be discussed. 21–23 Tracing and notification of previous sexual partners is not recommended, but examination of current sexual partners should be considered. 5 There is no evidence to suggest that reinfection from an untreated current partner contributes to recurrence. 20 However, sexual partners may benefit from assessment for infection with HPV and the presence of AGWs and other STIs, and from the opportunity to discuss any concerns about AGWs and the prognosis for their partner. 5,24 From the perspective of the clinician, it would be valuable to convey to the current partner that they could be infected with HPV and that, if so, although they do not have visible AGWs, they could potentially infect another person.
Consistent condom use might be beneficial in preventing acquisition of HPV in those without HPV infection and is reported to reduce the infection rate by 30–60%. 5 However, the evidence base on the effectiveness of condoms in the prevention of transmission of HPV is of low quality. 11 Current UK guidance recommends that people with AGWs abstain from sex, including anal and oral sex, until their current AGWs have cleared. 5
Infection with some subtypes of HPV increases the risk of developing anogenital cancers. 25 HPV subtypes 6 and 11, which are the subtypes predominantly associated with AGWs, are classed as low-risk HPVs, that is, lesions resulting from subtypes 6 and 11 are rarely cancerous. 25 HPV subtypes associated with a high risk of cancer include subtypes 16 and 18. 25 Most high-risk HPV infections are transient. Persistent infection with a high-risk HPV subtype is a causal factor for the development of precancerous and cancerous lesions. 25 HPV subtypes 16 and 18 are associated with an increased risk of cervical cancer and are thought to have a causal role in vulval, vaginal and anal cancers;25 they account for an estimated 70% of all cervical cancers. 26 Co-infection with low- and high-risk HPV subtypes is common. 25
Epidemiology
Incidence and prevalence
Data from the GUM Activity Dataset [collated by the Health Protection Agency (HPA)] show a steady increase in the reported number of diagnoses of AGWs made in GUM clinics throughout England and Wales since records began in 1971. 27 Cases of first episodes and recurrent and re-registered persistent AGWs rose by 30% between 2000 and 2009 (from 70,414 in 2000 to 91,202 in 2009). 27 A small decline in reported diagnoses of first-episode AGWs has been observed in recent years but a similar reduction in recurrent episodes has not occurred (summarised in Table 1). 2 More men than women are diagnosed with AGWs, for both first-episode and recurrent cases (see Table 1). Moreover, younger men and women are more likely to present with first-episode AGWs, particularly those aged 20–24 years; trends in diagnoses of first-episode AGWs by age and gender are presented in Figure 1. 2 Men who have sex with men are at an increased risk of infection with HPV. In 2013, 8% of diagnoses of first-episode AGWs in men were in men who have sex with men compared with 6% in 2008. 2
| Episode | 2011 | 2012 | 2013 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Total | Men | Women | Total | Men | Women | Total | |
| First | 41,598 | 34,938 | 76,547a | 40,384 | 33,490 | 73,879a | 40,796 | 32,614 | 73,418a |
| Recurrent | 39,274 | 22,679 | 61,968a | 39,707 | 21,866 | 61,576a | 40,966 | 21,906 | 62,873a |
| Total | 80,872 | 57,617 | 138,245 | 80,091 | 55,356 | 135,455 | 81,762 | 54,520 | 136,291 |
The HPA notes that diagnoses reported by GUM clinics underestimate the total number of cases of AGWs. 27 Current estimates do not account for people with asymptomatic infections who do not attend a GUM clinic, people with symptomatic infections who either self-treat with over-the-counter preparations or receive treatment from their general practitioner (GP), or unrecognised cases of AGWs. Additionally, the HPA identified > 400 other sexual health services offering tests, diagnosis and treatment for STIs, which, as they are not associated with GUM clinics, would not report diagnoses of AGWs to the HPA. 27 Combining data from GUM clinics and other sexual health resources would more closely represent the population affected by AGWs.
Impact of the health problem
In the long term, HPV subtypes 6 and 11 are not associated with the development of chronic diseases. Although AGWs can cause discomfort, the detrimental impact of a diagnosis of AGWs on psychological health frequently outweighs the effect of AGWs on physical health. 28–31 People with AGWs often feel guilty and ashamed about their diagnosis and frequently experience a considerable degree of stigma at having contracted a STI. 29–34 Feelings of self-loathing, anger and depression are also common. 29–34 Additional psychological stress arises from anxiety about the risk of transmission of infection to others, uncertainty around the success of treatment, the time to clearance of lesions and the risk of recurrence. 31,33,35,36 A small quality-of-life (QoL) study in Denmark evaluating 10 people with AGWs identified that their principal concern was the negative effect of a diagnosis on their relationships and sex lives. 37,38 People with AGWs might be aware of HPV infection as a causal factor for both AGWs and anogenital malignancy but, without an in-depth knowledge of low- compared with high-risk subtypes, are likely to be anxious about the possibility of developing cancer. 37
Two vaccines against infection with HPVs are available for use in the UK. The bivalent vaccine Cervarix® (GlaxoSmithKline) protects against subtypes 16 and 18 and the quadrivalent vaccine Gardasil® (Sanofi Pasteur) additionally protects against subtypes 6 and 11. 39 Cervarix and Gardasil are licensed for use in female patients from the age of 9 years to protect against cervical cancer and precancerous lesions in the genital area (cervix, vulva or vagina). 40,41 Gardasil is additionally licensed (amendment granted in 2014) to protect against anal cancer, precancerous lesions in the anus and genital warts and can also be used in male patients from the age of 9 years. 41 At this time, the exact duration of the protective effect of Cervarix and Gardasil is unknown. In clinical trials, the vaccines afforded protection for up to 5 years. 42,43 In addition, there is some evidence that both vaccines might offer partial protection against other high-risk HPV subtypes that are not present in the vaccine. 43–45
In 2008, the Department of Health announced the introduction of a HPV immunisation programme for girls aged 12–13 years starting in September of that year. 46 After an economic evaluation of Cervarix and Gardasil, Cervarix was announced as the vaccine of choice for the first 3 years of the immunisation programme. 46,47 Subsequently, Cervarix was offered to all girls aged 12–13 years with an initial 2-year catch-up campaign for those aged up to 18 years. By 2010, > 84% of girls had received the scheduled three doses of vaccine (given at 0, 1 and 6 months) as part of the routine immunisation programme and > 47% of girls were vaccinated in the catch-up campaign. 48 Delivery of Cervarix was not expected to reduce the number of cases of AGWs presenting at GUMs. At the end of the initial 3-year immunisation schedule, the vaccine used in the programme was changed from Cervarix to Gardasil. Gardasil is delivered predominantly through secondary schools and was initially given as three injections over 12 months, with the dose reduced to two injections from September 2014. 49 It remains to be seen whether or not boys will be routinely vaccinated with Gardasil.
The effect of HPV vaccination on the incidence of cervical cancer caused by HPV subtypes 16 and 18 is likely to manifest in the long term. By contrast, based on the experience of other programmes, the benefits of vaccination with regard to the incidence of AGWs should be apparent in the near future. Australia introduced a national HPV vaccination programme using Gardasil in 2007, targeted at women aged ≤ 27 years. 50 One year after initiation of the programme the number of women aged ≤ 28 years presenting with AGWs at sexual health clinics declined by 25%. A marked reduction in the number of heterosexual, but not homosexual, men presenting with AGWs was also noted as a result of herd immunity. 50
The steadily increasing occurrence and high rate of recurrence of AGWs places a significant cost burden on the NHS in terms of disease management. A study based on data collated by the HPA from GUM clinics and primary care estimated the national cost of managing AGWs to be £52.4M in 2010 (£276 per treated AGW episode). 51 By contrast, another study estimated the annual cost of care per AGW episode in England to be £113, with a total cost of £16.8M. 3 With the introduction of a national vaccination programme, a fall in the incidence of AGWs is anticipated, particularly in women. However, no men, and not all women, receive the vaccine routinely. Therefore, a level of risk for contracting HPV infection, and developing AGWs, remains, as does the need for clinically effective and cost-effective treatments for AGWs.
Current service provision
In the UK, AGWs are managed predominantly at GUM clinics. Individuals might seek care directly or be referred to a GUM clinic by their GP. The goal of treatment is to reduce symptoms and visible lesions, not to treat the virus. The evidence base to direct first- and second-line treatment is limited. Although numerous randomised controlled trials (RCTs) are available, few studies compare active interventions against each other and those that do are typically small studies associated with an unclear or high risk of bias (summarised in Chapter 3).
Guidelines produced by the British Association for Sexual Health and HIV (BASHH) in 2007 on the management of AGWs5 recommend that GUM clinics develop their own treatment algorithms that accommodate local practice and the treatments available, a recommendation echoed by European guidelines. 6 Implementation of locally developed and monitored treatment algorithms is reported to improve the management of AGWs. 3 However, development of bespoke treatment pathways has led to variation in clinical practice across the UK in the treatment of AGWs.
An update to the BASHH guidelines became available subsequent to submission of this report. 7 The updated guidelines provide examples of treatment algorithms for the management of AGWs in women and men. The algorithms incorporate a review of treatment effectiveness every 4 weeks,5 which is also recommended in European guidelines. 6 Guidance from BASHH outlines that algorithms should encompass both initial treatment and the management of cases that do not respond promptly or relapse. The guidance goes on to recommend adoption of a continuous audit cycle to monitor the effectiveness of the algorithm and to ensure alteration of the algorithm if required, for example to incorporate new treatments.
Several treatments are available for the management of AGWs, with choice of treatment determined by morphology, number, localisation and distribution of warts and patient preference. 5,6 BASHH and European guidelines emphasise the importance of providing patients with information about their condition and discussing the treatment options available. 5,6 As noted earlier, not treating AGWs initially is also an option because some people will experience spontaneous clearance over a period of up to 6 months. Active treatments are divided into provider-applied (clinic-based) and patient-applied (home-based) therapy groups. Podophyllotoxin (available as a solution and a cream) and imiquimod 5% cream are the mainstay of the patient-applied therapies, having superseded interferons and 5-fluorouracil, which are no longer recommended for the routine management of AGWs because of their adverse effects (AEs). 5 Other topical treatment options applied by a clinician are TCAA and podophyllin, although the use of podophyllin is no longer recommended. 7 Destructive methods that require administration by a clinician, such as electrosurgery (cautery, hyfrecation), cryotherapy and laser therapy act to debulk the visible lesions. In some settings, topical treatments and ablative therapies might be used in combination. People who are immunocompromised typically have a poorer response to the treatments available. The topical antiviral cream cidofovir (Vistide®, Gilead), which is primarily used to treat cytomegalovirus retinitis, has been investigated as a potential treatment option for those with AGWs who are immunocompromised. As highlighted in BASHH guidance, treatments are associated with high rates of treatment failure and relapse. 5
When compared with guidance issued in 2007, the updated advice from BASHH provides firmer recommendations on preferred treatments for AGWs, as summarised in Box 1. 5,7
-
Soft non-keratinised AGWs respond well to podophyllotoxin and TCAA.
-
Keratinised lesions might be better treated with physical ablative methods, such as cryotherapy, excision, TCAA or electrocautery.
-
Imiquimod is a suitable treatment for both keratinised and non-keratinised warts.
-
People with a small number of low-volume warts, irrespective of type, can be treated with ablative therapy or topical treatment with podophyllotoxin from the outset.
-
Podophyllotoxin for 4 weeks or imiquimod for up to 16 weeks is suitable for home treatment by patients. The patient should be given a demonstration on lesion finding and treatment application.
-
Very large wart lesions, including Buschke–Löwenstein tumours, should be considered for surgical treatment.
-
Injectable local anaesthetic (e.g. 2% lidocaine) should be used before any surgical excision or ablative procedure. Topical anaesthetics [e.g. lidocaine cream (EMLA®, AstraZeneca)] can be used before local anaesthetic injection, or before cryotherapy, particularly when treating larger lesions.
-
Caution should be exercised when using any modality of treatment because of the danger of oedema and necrosis of surrounding tissue. This is most pronounced with agents such as TCAA but can also be seen with other treatments, including cryotherapy.
-
No treatment may be an option as approximately 30% of patients will experience spontaneous clearance of warts over a period of up to 6 months. However, most patients seek treatment for the discomfort, anxiety, distress or social unacceptability that warts cause.
Description of technologies under assessment
Several topical applications and ablative techniques are available for the treatment of AGWs. Topical treatments for AGWs are available that can be self-applied at home rather than attending a GUM clinic to be treated by a clinician. Patient-applied topical treatments are increasingly prescribed, particularly for mild, early lesions, because of the convenience of use and the increased privacy for the patient. However, only ablative techniques consistently achieve clearance rates approaching 100%. 6 No intervention has emerged as the most clinically effective treatment for AGWs and treatment choice is typically decided after discussion between the treating clinician and the person with AGWs.
Topical interventions
Topical interventions for the treatment of AGWs are available as creams and solutions. Although suitable for the treatment of penile AGWs, solutions are less practical for self-application to genital lesions in women and anal lesions in both men and women. Topical treatments evaluated in this project are those recommended in the BASHH guidelines available at the time of writing of the protocol for the project. 5 Based on clinical expert advice, although not licensed for the treatment of AGWs in the UK, cidofovir was also included because of the potential for use in the treatment of AGWs in people who are immunocompromised and who typically have a poorer response to other treatments. An update to the BASHH guidelines, which are under review at the time of writing, no longer lists podophyllin for the treatment of AGWs. 7 For completeness, and to adhere to the prespecified protocol, podophyllin has been included in the systematic review and analysis of clinical effectiveness.
Patient applied
Imiquimod
Imiquimod is an immunomodulator and acts by modifying the immune response, specifically the response of the innate immune system. 52 Binding of imiquimod to toll-like receptor 7 triggers the cellular release of cytokines. Studies report that treatment with imiquimod leads to increases in levels of the cytokines interferon-alpha, interleukin-1beta, interleukin-6 and tumour necrosis factor-alpha. 52–54 Cytokines act to boost the body’s defences by blocking multiplication of invading pathogens, including viruses.
Formulated as a 5% cream (12.5 mg of imiquimod in 250 mg of cream) for application by the patient, imiquimod is licensed by the European Medicines Agency (EMA)55 and the US Food and Drug Administration (FDA)56 for the topical treatment of:
-
external genital and perianal warts (condylomata acuminata) in adults
-
small superficial basal cell carcinomas in adults
-
clinically typical, non-hyperkeratotic, non-hypertrophic actinic keratoses on the face or scalp in immunocompetent adult patients when size or number of lesions limits the efficacy and/or acceptability of cryotherapy and other topical treatment options are contraindicated or less appropriate.
In addition, imiquimod 3.75% cream is also licensed by the FDA for the treatment of external AGWs. 57
According to the Summary of Product Characteristics (SmPC), when treating AGWs, imiquimod 5% cream should be applied topically three times per week on non-consecutive days (e.g. Monday, Wednesday and Friday or Tuesday, Thursday and Saturday) before normal sleeping hours. 58 It is advised that the cream be applied in a thin layer to clean skin involving lesions and, after smoothing completely into the skin, the cream should be left in place for 6–10 hours. After this period, the treated area should be washed with mild soap and water. Application of an excess of cream or prolonged contact with the skin might result in a severe application site reaction.
In studies evaluating the clinical effectiveness of imiquimod, local skin reactions were often the most common AE, with people experiencing erythema (61%), erosion (30%), excoriation/flaking/scaling (23%) and oedema (14%). 58 It is important that people understand that a degree of inflammation is to be expected and represents the local immune response, which is beneficial in clearing the infection. People also reported systemic AEs, including headache, nausea and myalgia.
Imiquimod is contraindicated in people who are hypersensitive to imiquimod or to any of the excipients in the formulation. 58 As imiquimod elicits an effect through stimulating the immune system, caution is advised when using imiquimod in the treatment of people who are receiving immunosuppressive treatments.
The British National Formulary (BNF)39 lists the net price of a pack of 12 sachets of Aldara as £48.60.
Podophyllotoxin
Podophyllotoxin is the most abundant lignan extracted from the resin podophyllin, which is itself isolated from species of the Podophyllum family. 59 Podophyllotoxin inhibits the action of topoisomerase II, an enzyme involved in DNA replication. Blocking topoisomerase II activity prevents cellular division and therefore multiplication of AGW cells. As AGW cells die, they are replaced by non-HPV-infected cells.
Podophyllotoxin has been evaluated in RCTs at various doses in gel, solution and cream formulations. 60–68 No preparation of podophyllotoxin is licensed by the EMA or FDA for the treatment of AGWs, but UK marketing authorisations have been granted for podophyllotoxin 0.5% solution (Condyline®, Takeda Pharmaceuticals Company Ltd; Warticon® solution, Stiefel Laboratories Ltd) and 0.15% cream (Warticon® cream, Stiefel Laboratories Ltd) preparations.
All podophyllotoxin preparations are for the treatment of external AGWs only. The SmPCs for the three podophyllotoxin-based treatments available in the UK indicate that, irrespective of formulation, the preparation should be applied directly to the AGWs twice daily for 3 consecutive days. 69–71 The SmPCs outline that, if required, the treatment schedule can be repeated at weekly intervals for a maximum of 4 weeks in the case of Warticon (cream and solution)70,71 and 5 weeks in the case of Condyline. 69 Because of the destructive effect of podophyllotoxin on cells, care should be taken to apply the preparation only to the affected area.
Preparations containing podophyllotoxin are contraindicated in people who:
-
are hypersensitive to podophyllotoxin or to any of the other ingredients
-
have open or bleeding lesions
-
are using another podophyllin- or podophyllotoxin-containing preparation. 69–71
Additionally, Condyline is contraindicated in pregnant or breastfeeding women and children aged < 12 years. 69
As with imiquimod 5% cream, the most common AEs associated with podophyllotoxin preparations are reactions at the application site, including erythema, pruritus and a skin-burning sensation. Skin erosion is also common with podophyllotoxin-based applications. 69–71
The BNF39 lists a net price for Condyline of £14.49 for a 3.5-ml bottle with applicators compared with £14.86 for 3.0 ml (with applicators) of Warticon solution. Podophyllotoxin 0.15% cream is available at a net cost of £17.83 for 5 g, together with a mirror to aid application.
Clinician applied
Cidofovir
Cidofovir is a monophosphate nucleotide analogue. 72 Conversion of cidofovir to the biphosphate form produces a metabolite that is a competitive inhibitor and an alternative substrate for viral DNA polymerases (DNA polymerase is an enzyme that is essential for DNA replication). Incorporation of the biphosphate form of cidofovir into the growing DNA chain in preference to the natural substrate of deoxycytidine triphosphate disrupts further elongation of the chain and thus viral replication. 72 As cidofovir acts directly on viral DNA, it has been proposed that topical cidofovir does not require a competent immune system to be effective and thus could potentially afford greater clinical benefit for people with HIV infection than with other treatments available. 73
At the time of writing, cidofovir is not licensed for the treatment of AGWs. Cidofovir is licensed by the EMA74 and FDA75 for intravenous use in the treatment of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome who do not have kidney disease (as cidofovir is associated with nephrotoxicity). As a potent inhibitor of viral DNA polymerase, there is considerable interest in the potential of cidofovir as a treatment for other conditions caused by viruses. Several in vivo and in vitro studies report the effectiveness of cidofovir against a range of DNA virus and retrovirus infections, including papillomavirus, adenovirus and herpes virus. 76 Additionally, small studies and case reports describe the effectiveness of topical and intralesional cidofovir in the treatment of virally induced skin conditions. 76 For the treatment of cutaneous disease, cidofovir has been formulated as a 1% gel and is applied topically to lesions overnight, three times a week for up to 16 weeks. 73
Podophyllin
Crude podophyllin is obtained as a powder. As noted earlier, the active metabolite in podophyllin is podophyllotoxin. The process to extract podophyllin from species of the Podophyllum family is not standardised and thus the concentration of podophyllotoxin can differ markedly from batch to batch of podophyllin. 59 Additionally, crude podophyllin has not been subject to rigorous investigation and the remaining constituents of the crude product have not been well characterised. Identification of the mutagenic flavenoids quercetin and kaempferol in crude podophyllin led to concern about the potential for exacerbation of oncogenic HPV-associated intraepithelial neoplasia77,78 (quercetin and kaempferol constitute 3% and 6% of the dry weight of podophyllin powder, respectively77,79,80). As a result of the concerns about toxicity and the varying concentration of podophyllotoxin, the use of podophyllin is no longer recommended. 6,7
For clinical use, crude podophyllin is added to a benzoin tincture to create a resin that is painted onto lesions. Podophyllin resin is not licensed by the EMA or FDA for the treatment of AGWs but has been approved by the Medicines and Healthcare products Regulatory Agency (the UK marketing authority) for the treatment of plantar warts and AGWs. 81 Because of the corrosive nature of the treatment, podophyllin must be applied by a clinician and care must be taken to avoid applying the resin to the surrounding skin; it is recommended that surrounding skin be covered with soft paraffin to protect against treatment. After application, the treated area should be covered with soft paraffin and left for a maximum of 6 hours, after which the podophyllin resin should be washed off. 81 Podophyllin is typically applied once weekly until complete clearance.
Podophyllin should not be used in women who are pregnant or breastfeeding and should not be used to treat facial warts. 81 Severe toxicity associated with absorption of podophyllin has been reported. Consequently, when a person presents with a large number of AGWs, it was recommended that only a few be treated at a time to reduce the risk of systemic toxicity. 81
Trichloroacetic acid
Trichloroacetic acid is a caustic agent used in various cosmetic treatments including facial peels and tattoo removal as well as for the treatment of AGWs. 82 Typically used at a concentration of 80–90% in the treatment of AGWs, TCAA destroys cellular proteins, which results in cell death. TCAA preparations are not licensed by the EMA or FDA for treatment of AGWs. Despite the lack of a licence, TCAA is recommended for the treatment of AGWs, particularly soft non-keratinised AGWs. 5 Incorrect application of TCAA can damage healthy skin and therefore it is not suitable for home application. If considered an appropriate treatment, TCAA is applied once weekly. Complete clearance of AGWs can occur after a single application, but most people will require multiple courses of treatment. The most common AEs of treatment are pain or burning during administration, with some people experiencing an intense burning sensation for 5–10 minutes after application. Ulceration after application of TCAA can also occur, which makes TCAA unsuitable for the treatment of AGWs of large volume. Despite the listed AEs, TCAA is thought to be the safest of the available topical treatments for use during pregnancy. 1
Physical ablative techniques
The four main physical ablative techniques used to treat AGWs are:
-
carbon dioxide (CO2) laser therapy
-
cryotherapy
-
electrotherapy
-
surgical excision.
Carbon dioxide laser therapy
Carbon dioxide laser therapy uses a concentrated beam of infrared light energy to heat and ultimately cauterise the affected area. Depending on the number and size of AGWs present, laser surgery can be carried out under either local or general anaesthetic. 5 CO2 laser therapy is particularly suitable for AGWs of a large volume or those that are located in anatomical sites that are difficult to access for other ablative techniques, such as AGWs deep inside the anal canal or urethra. After laser surgery, people are likely to experience soreness and irritation at the site of the AGWs. Other potential AEs include pain, bleeding and scarring at the site of treatment. Treatment can be repeated if necessary. CO2 laser surgery is more costly than other ablative techniques and is of limited availability. 5,82
Cryotherapy
Cryotherapy involves freezing AGWs using liquid nitrogen. Freezing causes permanent dermal and vascular damage, which triggers an immune response leading to the necrosis and clearance of the destroyed cells. 5,82 Cryotherapy is usually most effective for the treatment of multiple small AGWs and particularly those that develop on the shaft of the penis or on, or near, the vulva. 82 Treatment can be applied as a single freeze or a double freeze–thaw technique and is typically carried out once weekly for a maximum of 4 weeks. 5 Cryotherapy should be applied until complete freezing of the lesion is achieved and until a ‘halo’ of freezing is established a few millimetres around the treated lesion. 5 Achieving complete freezing of the lesion can take as long as 30 seconds, if not longer, and might not be possible if the person cannot tolerate the treatment.
Electrotherapy
Electrotherapy techniques use high-frequency electrical currents to cauterise lesions. There are two types of electrotherapy: electrocautery (also referred to as hyfrecation) and electrical surgery. In electrocautery, a direct or alternating electrical current is passed through a resistant metal wire electrode, which generates heat. Application of the heated electrode to the lesion cauterises the tissue. 83 Direct contact of the electrode with the skin causes electrodessication (coagulation and desiccation without carbonisation) of the lesion, whereas positioning the electrode above the skin with an air gap of 1–3 mm leads to electrofulguration (rapid heating and carbonisation) of the lesion. 83 In contrast to electrocautery, electrosurgery involves passing a high frequency alternating electrical current directly through the living tissue to destroy the lesions. Electrotherapy is particularly effective for treating smaller AGWs located on the shaft of the penis, the rectum or the vulva or for pedunculated lesions, but is not recommended for the treatment of larger AGWs because of the potential for permanent scarring. 82 Electrotherapy is often combined with excision to treat large AGWs that develop around the anus or vulva and which have failed to respond to topical treatments. 1 Undergoing electrotherapy can be painful and a local or general anaesthetic is usually required.
Surgical excision
Excision of AGWs under local anaesthetic is particularly effective for the removal of condylomatous AGWs and small hardened AGWs that are located in anatomically accessible sites. 5 The use of an anaesthetic cream before injection of local anaesthetic is recommended. Surgical excision can cause scarring and so might not be suitable for large AGWs. 1
Chapter 2 Definition of the decision problem
Scoping searches were carried out to gain an insight into current recommendations and the evidence base available for the interventions used in UK clinical practice for the treatment of AGWs. At the time of writing, UK guidelines5 recommend that treatment of AGWs be tailored to the requirements and preferences of the individual patient. From the clinician’s perspective, size, location and number of AGWs typically influence treatment recommendations. Interventions for clearing AGWs encompass a diverse range of topical pharmacological agents and ablative techniques and, on occasion, a combination of treatments. There is a marked difference in costs across the various interventions. The initial searches identified multiple studies comparing treatments against each other or placebo but no resource summarising a systematic evaluation of the comparative clinical effectiveness or cost-effectiveness of the various treatments available for clearing AGWs.
The protocol stipulated that studies evaluating any licensed dose or formulation of topical treatments would be eligible for inclusion. Of the topical treatments used for clearing AGWs, only imiquimod has a marketing authorisation for the treatment of AGWs (authorised doses of 5% and 3.75%). To enable comparison of imiquimod with the other prespecified topical interventions, the criterion of licensed dose or formulation was relaxed and studies of any dose or formulation of listed topical treatments were eligible. Studies have evaluated topical treatments in various settings (home vs. clinic) and using different formulations (solutions, creams and gels), doses and application schedules. It is not practical to evaluate all of the diverse treatment options available for clearing AGWs in a single head-to-head RCT. Thus, synthesis of the available clinical data could supplement expert opinion on which treatments are clinically effective. No resource reporting an indirect synthesis of clinical data was identified. To inform the decision problem in terms of clinical effectiveness and cost-effectiveness, and to build on the direct comparative data identified, a key objective of the project, if feasible, was to carry out a mixed-treatment comparison (MTC).
Decision problem
The eligibility criteria pertaining to population, intervention, comparators and outcomes are summarised in Table 2.
| PICO criteria | Criteria |
|---|---|
| Population | Patients aged ≥ 16 years with clinically diagnosed AGWs (irrespective of biopsy confirmation) |
| Intervention | Topical treatments evaluated: podophyllotoxin, imiquimod, podophyllin, TCAA and cidofovir. Physical ablation methods evaluated: cryotherapy (liquid nitrogen spray or cryoprobe), surgical excision (under local anaesthetic), electrotherapy (electrocautery, hyfrecator surgery) and laser therapy. Combination or sequential therapy (e.g. cryotherapy followed by podophyllotoxin) will also be included |
| Comparators | The interventions listed above compared with each other (either as monotherapy or combination therapy), placebo or no intervention |
| Outcomes | Clinical effectiveness (expressed in terms of clearance, recurrence and volume of AGWs), HRQoL and AEs (local and systemic). Specifically:
|
| Study design | RCTs |
Overall aims and objectives of the assessment
The objectives of this systematic review were to:
-
evaluate the clinical effectiveness of medical or surgical treatments for AGWs
-
evaluate the cost-effectiveness of medical or surgical treatments for AGWs
-
identify key areas for further primary and secondary research.
Adverse effects associated with the various treatments were also assessed and compared.
The review did not cover diagnostic tests or HPV typing as HPV typing is not routine in the diagnosis of AGWs. Omission of HPV typing is unlikely to influence treatment decisions as around 90% of AGWs are caused by HPV types 6 and 11. Health promotion (advice and counselling), prevention of transmission and screening for other STIs were also not addressed by this systematic review.
Interventions not recommended in the BASHH guidelines5,7 for routine management of AGWs and not typically used in NHS clinical practice were excluded from this review:
-
salicylic acid (not used on anogenital skin)
-
5-fluorouracil (rarely used in UK clinical practice because of associated severe ulceration after application)
-
interferon (rarely used in UK clinical practice; superseded by imiquimod)
-
sinecatechins 10% and 15% ointment (insufficient evidence to support use in treatment of AGWs).
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
Evidence on the clinical effectiveness of interventions to treat AGWs was identified by conducting a systematic review of the published research literature. The review was undertaken following the general principles published by the Centre for Reviews and Dissemination84 and the Cochrane Collaboration. 85 The protocol for the systematic review is registered on PROSPERO database (registration number CRD42013005457). 86
Identification of studies
To identify relevant studies, multiple electronic databases were searched:
-
Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE
-
Ovid EMBASE
-
The Cochrane Library [specifically Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects and Health Technology Assessment (HTA) database]
-
Web of Science.
Search strategies were designed to include medical subject headings (MeSH) and text terms for AGWs, including ‘condyloma acuminata’ (the medical term for AGWs). To maximise the number of potentially relevant studies retrieved, no MeSH or text terms were included for interventions of interest. Based on the results of the scoping search and clinical expert advice, it was anticipated that few RCTs meeting the eligibility criteria would be identified, despite the number of studies retrieved. Therefore, searches were simultaneously carried out for prospective observational studies (matched control studies, case series and case–control studies).
Search filters designed to retrieve reports by study design were identified through the InterTASC Information Specialists’ Sub-Group search filter resource. 87 Filters developed and validated by the Scottish Intercollegiate Guidelines Network were used to identify RCTs in MEDLINE and EMBASE. 88 Filters devised by Clinical Evidence (a collection of systematic overviews covering various conditions) were chosen to retrieve potentially relevant observational studies from MEDLINE and EMBASE. 89 Search terms for AGWs were tailored to the database searched.
Bibliographies of previous overviews, guidelines and retrieved articles were manually reviewed for additional studies. Clinical trial registries (World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov) were also searched to identify planned, ongoing and finalised clinical trials of interest. The website of the US FDA [see www.fda.gov/ (accessed 16 December 2015)] was also searched to identify unpublished data. In addition, clinical experts were contacted with a request for information on any additional studies of which they had knowledge.
No language restriction was applied to the searches. With the exception of Web of Science, electronic databases were searched from inception, with the initial search carried out on 30 August 2013. Search parameters for Web of Science were limited to a search period of 2000 to 1 September 2014, with study type restricted to article, meeting abstract, proceedings paper and corrections. Search results were uploaded into Reference Manager version 11.0 (Thomson ResearchSoft, San Francisco, CA, USA) and deduplicated. Update searches were carried out on 22 April and 1 September 2014. Full details of the search strategies are presented in Appendix 1.
Two researchers (SB and one of ET or CK) independently screened the titles and abstracts returned by the search strategy according to prespecified eligibility criteria (see Table 2). In cases in which consensus could not be achieved, the full texts of potentially relevant studies were ordered. During abstract appraisal, to facilitate discussion of whether or not sufficient evidence had been identified to restrict inclusion of study type to RCTs, potentially relevant studies were categorised as RCT, observational study or systematic review. Two reviewers (SB and CK) independently assessed full publications for inclusion, with studies classified as RCTs evaluated first. Discrepancies were resolved by discussion, with involvement of a third reviewer (SEd) if consensus could not be reached. After appraisal of full-text publications, the number of RCTs identified as eligible for inclusion in the review of clinical effectiveness and AEs led to the decision to limit reporting to RCTs.
Inclusion and exclusion criteria
Eligibility criteria for the review of clinical effectiveness were as specified in the decision problem (summarised in Table 2). The review included only RCTs, with systematic reviews and non-randomised studies excluded. The interventions of interest were topical treatments and ablative techniques, either alone or in combination. RCTs were included if the treatments were evaluated in a population with AGWs and compared with each other, placebo or no treatment. Studies were excluded if none of the outcomes of interest was reported.
Data abstraction
Because of the large number of RCTs identified, in the first instance two reviewers (SB and Victoria Wakefield) independently extracted data from only 10 studies onto a standardised data extraction form; a function of the initial 10 extractions was to pilot the suitability of the data extraction form. Subsequently, one reviewer (various) extracted data from the remaining studies onto a modified data extraction form, with validation of the data by a second reviewer (SB). Information extracted included details on study design and methodology, the baseline characteristics of the population and data on outcomes of interest, both clinical effectiveness outcomes and AEs. Discrepancies were resolved by discussion, with involvement of a third reviewer (SEd) when necessary. During data extraction, if the reviewer(s) identified areas with limited reporting (e.g. aspects of trial conduct) or discrepancies in reporting within the publication (e.g. in event rate), authors were contacted with a request for clarification. If a study was reported as a conference proceeding or an abstract only, study authors were contacted with a request for further details. Studies reporting data on an outcome of interest but for which insufficient methodological details were available to allow full critical appraisal of study quality, even after contact with authors, were included in sensitivity analyses (additional detail provided in Results). Data extraction forms for the included studies are provided in Appendix 2.
Critical appraisal strategy
Two reviewers independently assessed the quality of the clinical effectiveness studies. Discrepancies were resolved by discussion, with involvement of a third reviewer when necessary. Study quality was assessed according to recommendations of the Centre for Reviews and Dissemination84 and the Cochrane Handbook for Systematic Reviews of Interventions. 85 Study quality was recorded using the Cochrane risk of bias tool85,90 and was incorporated into the data extraction form (see Appendix 2).
Outcome-specific risk of bias was determined for the outcomes for which data were extracted. 90 The three bias assessment categories used were low, unclear and high. A study was deemed to be at low risk of bias when all key domains were associated with low risk of bias, at an unclear risk of bias when one or more key domains had an unclear risk of bias and at a high risk of bias when one or more key domains was thought to be at a high risk of bias.
Methods of data synthesis
Details of the clinical effectiveness results and quality assessment for each included study are presented in structured tables (see Appendix 2) and an overall assessment of study quality is provided as a narrative summary (see Quality assessment). The possible effects of study quality on the clinical effectiveness data and review findings are discussed where relevant.
Standard pair-wise meta-analysis was performed, where possible, to evaluate clinical effectiveness and was based on intention-to-treat analysis. Intention-to-treat analysis was defined as people being analysed in the treatment group to which they were allocated at randomisation, irrespective of whether they changed treatment, withdrew or were lost to follow-up. Dichotomous outcome data were meta-analysed using Mantel–Haenszel odds ratios (ORs) with 95% confidence intervals (CIs) and a fixed-effects model; meta-analysis with a random-effects model was carried out as a sensitivity analysis. To facilitate comparison and interpretation of estimates of effect across studies, when data from a single trial were available for a comparison of interest, and if appropriate, the trial data were analysed and presented as for meta-analysed data. Missing data were imputed and were analysed as a treatment failure for all outcomes (i.e. for complete clearance, people lost to follow-up were considered not to have achieved clearance and, for recurrence, people lost to follow-up were considered to have recurred).
Meta-analysis was carried out using Review Manager version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). Inconsistency among studies included in the meta-analysis was assessed using the I2 test and the level for statistically significant heterogeneity was set at p < 0.10. Levels of inconsistency were defined as follows: low level, I2 of 0–25%; moderate level, I2 of 26–50%; and high level, I2 of > 50%. In the presence of statistically significant heterogeneity (p < 0.10), possible sources were investigated, including differences in study populations, methods or interventions. The low number of studies included in each meta-analysis precluded the evaluation of publication bias and/or small study effects.
Additionally, the comparative clinical effectiveness of interventions was investigated using MTCs. The methods used for MTC followed the guidance described in the National Institute for Health and Care Excellence Decision Support Unit’s Technical Support Documents for Evidence Synthesis. 91,92 MTCs were conducted using a Bayesian Markov chain Monte Carlo simulation in WinBUGS (version 1.4.3; MRC Biostatistics Unit, Cambridge, UK). The following were implemented for each analysis:
-
uniform priors (also called ‘uninformed’ or ‘flat’ priors) were used
-
all outcomes were considered independent
-
to ensure convergence on the posterior distribution:
-
results for all clinical effectiveness outcomes analysed were based on 50,000 iterations after a ‘burn in’ of 150,000 iterations
-
results for all safety outcomes analysed had a ‘burn in’ of 30,000 iterations, with results based on 100,000 iterations
-
-
the OR was used as the summary effect estimate for all outcomes
-
a weighted average of the baseline treatment was used in combination with the relative treatment effect to calculate probabilities for all treatments included in the analysis of that outcome
-
any results taken forward into the economic model used the posterior sampling to retain the correlation between parameter estimates caused by their joint estimation from a single data set.
When a random-effects model was deemed the best fit, the extent of the between-study heterogeneity was investigated by evaluating the posterior mean of tau-squared.
The potential limitations of the MTC, together with the associated influence on the generated estimates of effect, are discussed in the strengths and limitations of the report (see Chapter 5).
Sensitivity analyses were carried out for the outcomes of complete clearance (at the end of treatment and at later time points) and recurrence of AGWs in both standard meta-analysis and the MTC. Sensitivity analyses included studies or individual outcomes deemed to be at an overall high risk of bias, together with studies in people with comorbid HIV infection and a cluster of differentiation 4+ cell count of < 200 cells/mm3.
As a consequence of the limited reporting of baseline characteristics in the included studies (discussed in greater detail in Quantity and quality of research available), planned subgroup analyses were not carried out. Planned analyses included:
-
soft, moist, non-keratinised AGWs
-
dry, keratinised AGWs
-
number of AGWs [to be grouped as single, few (two–five) or multiple (six or more)]
-
site of AGWs
-
no previous treatment for AGWs (‘first attack’ patients)
-
recurrent AGWs (return of AGW after a complete response to treatment)
-
persistent AGWs (treatment is continued for > 6 months)
-
immune status (immunosuppressed vs. not immunosuppressed).
Results
Quantity and quality of research available
Searches of electronic databases retrieved 4231 records (post deduplication) that were of possible relevance to the review (Figure 2). Manual searching identified one additional reference, giving a total of 4232 records screened for inclusion in the review. Full publications for 155 references were ordered. Of these, 13 publications were unobtainable. 93–105 Five studies published in Chinese were identified for which translations could not be obtained within the time frame of the project. 106–110 An evaluation of RCTs published in Chinese journals found that most studies described as randomised were not truly random. 111 Based on this report and translations obtained for this project for other retrieved studies published in Chinese, the authors of the project consider that most of the studies in Chinese are unlikely to be random and omission of these studies is unlikely to have influenced the assessment of clinical effectiveness. Of the 137 full articles evaluated, 70 publications describing 60 studies (full publications60–68,112–162) were relevant to the review. Citation details for conference abstracts related to full publications are provided only when additional information was available in the abstract. A list of publications screened but subsequently excluded (with reasons for exclusion) from the review is available in Appendix 3.
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
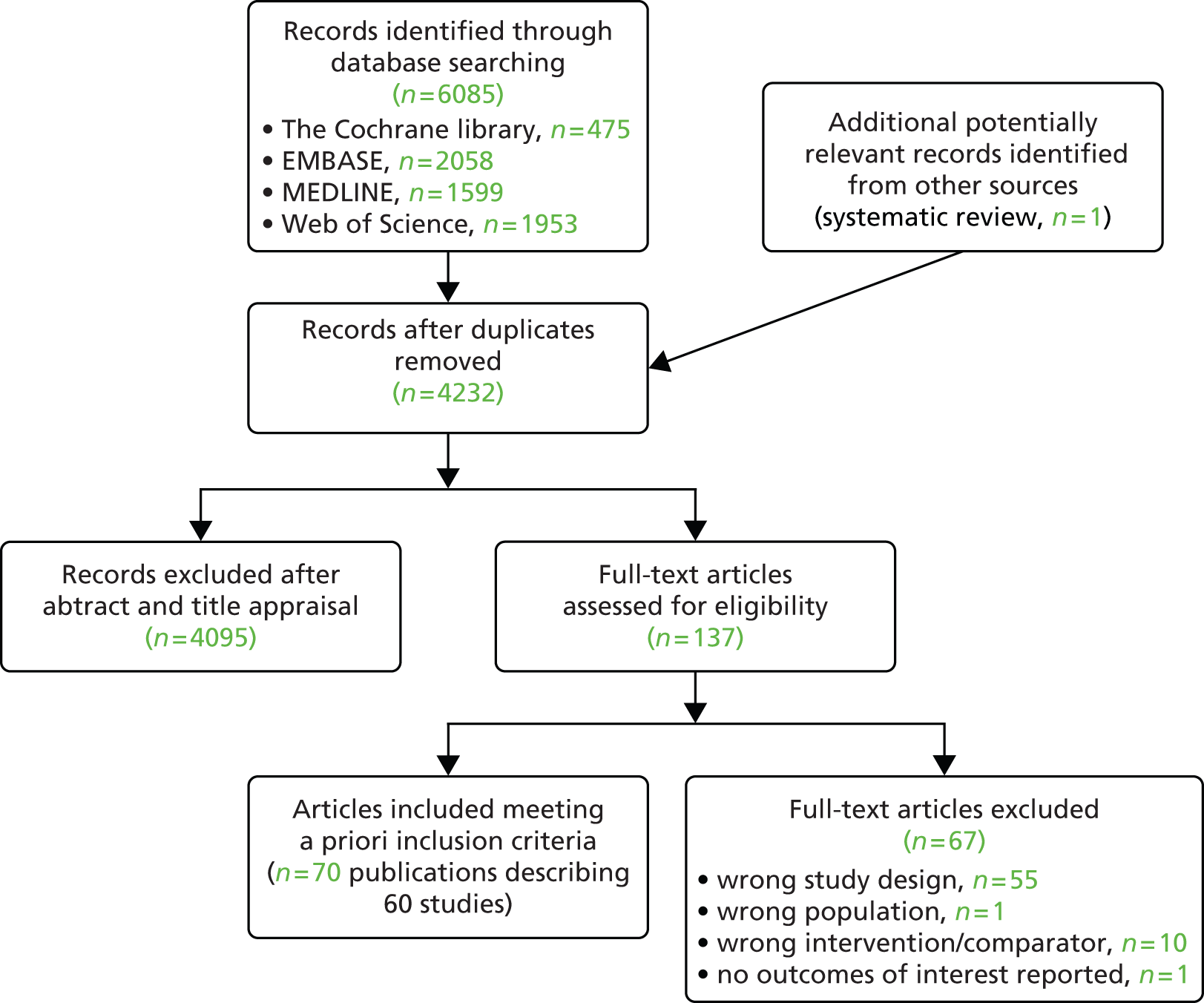
Summaries of the studies included in the review are presented by treatments evaluated (Table 3) and by key characteristics of studies (Table 4).
| Intervention | Identified studies evaluating intervention |
|---|---|
| Placebo | Arican 2004;112 Baker 2010;113 Benedetti Panici 1989;114 Beutner 1989;115 Beutner 1998;116 Beutner 1998;117 Edwards 1998;118 Gilson 1999;119 Greenberg 1991;62 Kirby 1990;61 Matteelli 2001;120 Snoeck 2001;121 Syed 1994;122 Syed 1995;123 Syed 1995;124 Tyring 1998;125 Tyring 1998;68 von Krogh 1992;126 von Krogh 1994127 |
| Topical interventions | |
| Cidofovir | Matteelli 2001;120 Orlando 2002;128 Snoeck 2001121 |
| Imiquimod | Arican 2004;112 Baker 2010;113 Beutner 1998;116 Beutner 1998;117 Edwards 1998;118 Fife 2001;129 Garland 2006;130 Gilson 1999;119 Komericki 2011;131 Padhiar 2006;132 Stefanaki 2008;133 Trofatter 2002;134 Tuncel 2005;135 Tyring 1998125 |
| Podophyllotoxin | Beutner 1989;115 Claesson 1996;65 Edwards 1988;136 Greenberg 1991;62 Handley 1992;67 Hellberg 1995;137 Kar 2003;138 Kinghorn 1993;63 Kirby 1990;61 Komericki 2011;131 Lacey 2003;64 Landthaler 1987;139 Lassus 1984;140 Mazurkiewicz 1990;60 Petersen 1995;141 Strand 1995;142 Syed 1993;143 Syed 1994;122 Syed 1995;123 Syed 1995;124 Tyring 1998;68 von Krogh 1992;126 von Krogh 1994;127 White 1997144 |
| Podophyllin | Edwards 1988;136 Gabriel 1983;145 Goh 1998;66 Handley 1992;67 Hellberg 1995;137 Jensen 1985;146 Kar 2003;138 Kinghorn 1993;63 Lacey 2003;64 Landthaler 1987;139 Lassus 1984;140 Maiti 1985;147 Mazurkiewicz 1990;60 Nath 1990;148 Padhiar 2006;132 Sherrard 2007;149 Simmons 1981;150 Stone 1990;151 Tabari 2010;152 White 1997144 |
| TCAA | Abdullah 1993;153 Godley 1987;154 Nath 1990;148 Sherrard 2007;149 Tabari 2010152 |
| Ablative therapies | |
| Argon plasma coagulation | Viazis 2007155 |
| CO2 laser therapy | Azizjalali 2012;156 Bar-Am 1993;157 Ferenczy 1995158 |
| Cryotherapy | Abdullah 1993;153 Azizjalali 2012;156 Gilson 2009;159 Godley 1987;154 Sherrard 2007;149 Simmons 1981;160 Stefanaki 2008;133 Stone 1990151 |
| Electrosurgery | Benedetti Panici 1989;114 Ferenczy 1995;158 Orlando 2002;128 Simmons 1981;160 Stone 1990151 |
| Surgical excision | Jensen 1985146 |
| Combination treatments | |
| Argon plasma coagulation plus imiquimod 5% cream | Viazis 2007155 |
| Cryotherapy plus imiquimod | Tuncel 2005135 |
| Cryotherapy plus podophyllin | Sherrard 2007149 |
| Cryotherapy plus podophyllotoxin | Gilson 2009159 |
| Electrocauterisation plus cidofovir | Orlando 2002128 |
| TCAA plus podophyllin | Gabriel 1983;145 Sherrard 2007149 |
| Study | Sample size (n) | Population | Intervention | Comparator | Duration of treatment and follow-up |
|---|---|---|---|---|---|
| Abdullah 1993153 | 86 | People with definite AGWs for the first time on clinical grounds | TCAA 95% | Cryotherapy | Maximum of six treatments with treatment given on a weekly basis. People with complete clearance of AGWs were followed up for 3 months after the end of treatment |
| Arican 2004112 | 45 | Aged ≥ 18 years, not received any therapies within the 3 months before enrolment and minimum of five AGWs at baseline | Imiquimod 5% cream | Placebo | Treatment period of 12 weeks followed by a treatment-free observation period of 6 months |
| Azizjalali 2012156 | 160 | Lesions with a diameter of ≥ 10 mm and located on the pubis, penis, scrotum, vulva or inguinal area | CO2 laser therapy | Cryotherapy | 3 months |
| Baker 2010113 | 981 (two trials) | Aged ≥ 12 years and from two to 30 external AGWs with a total AGW area of ≥ 10 mm2 | (1) Imiquimod 3.75% cream, (2) imiquimod 2.5% cream | Placebo | Initial treatment of up to 8 weeks with assessment for complete clearance continued for up to 8 weeks after treatment. Those achieving complete clearance were followed for 12 weeks |
| Bar-Am 1993157 | 148 | Women with benign vulvar and perineal HPV lesions. Men with disseminated foci of penile shaft condylomatous lesions | High-power CO2 laser | Low-power CO2 laser | Duration of treatment and follow-up unclear |
| Benedetti Panici 1989114 | 99 | Multiple condyloma lesions (two or more sites affected), aged 18–45 years, no previous therapy, informed written consent | Diathermocoagulation | No treatment | If required, diathermocoagulation was repeated at 3-week intervals. End of study was 6 months after completion of treatment. People with complete clearance of AGWs were also followed up at 12 months |
| Beutner 1989115 | 109 | Men aged ≥ 18 years with a clinical diagnosis of AGWs and with between two and 20 AGWs in an area not exceeding 10 cm2 | Podophyllotoxin 0.5% solution | Placebo | Treatment given for a minimum of 2 and a maximum of 4 weeks. People were also evaluated at 6, 12 and 16 weeks during a follow-up period (i.e. weeks 2, 8 and 12 after treatment) |
| Beutner 1998116 | 279 | Aged ≥ 18 years, seronegative for HIV infection, at least two but no more than 50 AGWs (defined as AGWs in the genital, anal, perineal or perianal area), a biopsy diagnostic or suggestive of condyloma acuminatum and a bidimensional AGW area of at least 10 mm2 | (1) Imiquimod 5% cream, (2) imiquimod 1% cream | Placebo | Treatment given for a maximum of 16 weeks. After treatment, people were followed up for 12 weeks during which time no treatment was given |
| Beutner 1998117 | 108 | Aged ≥ 18 years and seronegative for HIV infection | Imiquimod 5% cream | Placebo | Treatment given over 8 weeks. People achieving complete clearance of AGWs entered into a treatment-free follow-up period of 10 weeks or until recurrence occurred. People with partial response at the end of 8 weeks’ treatment were evaluated again at week 2 of follow-up to determine whether or not complete clearance had been achieved |
| Claesson 199665 | 180 | Men with condylomata acuminata located on the penis shaft and/or within the preputial cavity region. Women with vulval and/or perianal condylomata acuminata | (1) Podophyllotoxin 0.15% cream, (2) podophyllotoxin 0.3% cream | Podophyllotoxin 0.5% solution | Initial treatment period of up to 4 weeks. Those with complete clearance of AGWs were followed up at 16 weeks |
| Edwards 1988136 | 65 | Men with diagnoses, based on clinical appearance, of external penile AGWs | Podophyllotoxin 0.5% solution | Podophyllin 20% (clinician applied) | Initial 6-week treatment period with a subsequent 3-month follow-up period |
| Edwards 1998118 | 311 | Healthy men and women aged ≥ 18 years with a diagnosis of AGW, with a minimum of two but no more than 50 external AGWs and a total wart area of at least 10 mm2. People were enrolled only when deemed healthy based on medical history, physical examination and laboratory testing | (1) Imiquimod 5% cream, (2) imiquimod 1% cream | Placebo | Treatment period of 16 weeks followed by a treatment-free follow-up period of 12 weeks for those achieving complete clearance during treatment |
| Ferenczy 1995158 | 282 | Presence of vaginal and external anogenital condylomata (diagnosis verified by histology) and a total linear area of AGWs of ≥ 2 cm2 | Electrosurgery | CO2 laser | Treatment and then follow-up of at least 6 months (maximum 18 months, mean 8 months) after the last treatment received |
| Fife 2001129 | 110 | Men aged ≥ 18 years with from two to 50 AGWs confirmed by biopsy, and a total AGW area of 30–2000 mm2 after biopsy | (1) Imiquimod 5% cream, once daily, (2) imiquimod 5% cream, twice daily, (3) imiquimod 5% cream, three times daily | Imiquimod 5% cream three times per week | Treatment given over 16 weeks followed by a 4-week observation period for people whose lesions had not cleared by week 16 |
| Gabriel 1983145 | 73 | Men with AGWs | TCAA 50% plus podophyllin 25% | Podophyllin 25% | Treatment given over 6 weeks followed by a minimum follow-up of 3 months from beginning of treatment |
| Garland 2006130 | 120 | Women aged ≥ 16 years with from one to 50 visible external genital and/or perianal AGWs with an area of 10–2000 mm2 | (1) Imiquimod 5% cream for 4 weeks, (2) imiquimod 5% cream for 8 weeks, (3) imiquimod 5% cream for 12 weeks | Imiquimod 5% cream for 16 weeks | Initial treatment period of 4–16 weeks, dependent on treatment allocation. All women were followed up until the end of the study at 16 weeks |
| Gilson 1999119 | 100 | Aged ≥ 18 years with a clinical diagnosis of external AGWs and with a minimum of two AGWs with an area totalling at least 10 mm2. Laboratory-confirmed diagnosis for HIV infection (patients with AIDS were eligible if they had been clinically stable for 4 weeks before enrolment) and a CD4 T-lymphocyte count of ≥ 100 × 106 cells/l | Imiquimod 5% cream | Placebo | Treatment duration of 16 weeks. People experiencing > 80% but < 100% clearance of baseline AGWs continued on blinded treatment for an additional 8 weeks |
| Gilson 2009159 | 149 | Aged 18–70 years with at least two (maximum 30) external AGWs of a combined area of at least 10 mm2. AGWs were either previously untreated or had not been treated for at least 4 months | Cryotherapy plus podophyllotoxin 0.15% cream | Cryotherapy plus placebo | Initial 12-week treatment period. After 12 weeks treatment was given at the discretion of the clinician. People were followed up until 24 weeks after commencement of treatment |
| Godley 1987154 | 130 | Heterosexual men with penile AGWs | TCAA | Cryotherapy | Weekly treatment until disappearance of AGWs, for up to a maximum of 10 treatments. If complete AGW clearance was achieved, men were followed up for 2 months after the end of treatment |
| Goh 199866 | 45 | Men aged ≥ 16 years with penile AGWs | (1) Podophyllin 0.5% in ethanol (patient applied), (2) podophyllin 0.25% in ethanol (patient applied) | Podophyllin 25% (clinician applied) | Treatment groups were reviewed weekly for up to 6 weeks |
| Greenberg 199162 | 72 | Women with clinical diagnosis of exophytic vulvar condylomata and ≤ 25 lesions to be treated. AGWs had a total area of < 10 cm2 and occupied < 30% of the vulva | Podophyllotoxin 0.5% (solution and cream formulations) | Placebo | Treatment given for a maximum of 4 weeks, with patients followed up until week 10 after the start of treatment |
| Handley 199267 | 57 | Men with primary AGWs | Podophyllotoxin 0.5% solution | Podophyllin 0.5% solution (patient applied) | Initial treatment period of up to 5 weeks, with follow-up review at 3 months (unclear if this was final follow-up) |
| Hellberg 1995137 | 60 | Women with overt AGWs | Podophyllotoxin 0.5% cream | Podophyllin 20% (clinician applied) | Maximum duration of treatment of 4 weeks. Women with complete clearance were followed up at 3 months (unclear whether 3 months after the end of treatment or 3 months from initiation of trial) |
| Jensen 1985146 | 60 | First episode of AGWs and presence of perianal AGWs (the perianal region was defined as a circle of diameter 6 cm centring on the anus) | Podophyllin 25% (clinician applied) | Surgical excision | Initial assessment occurred 1 week after the final treatment. For podophyllin, treatment was repeated weekly for up to 6 weeks. People were followed up at 3, 6, 9 and 12 months |
| Kar 2003138 | 72 | Presence of AGWs as determined by visual inspection (without biopsy confirmation) | Podophyllotoxin 0.5% solution | Podophyllin 20% (clinician applied) | Initial treatment period of 6 weeks with a subsequent follow-up period of 6 months (unclear whether this is 6 months after the end of treatment or 6 months from start of treatment) |
| Kinghorn 199363 | 200 | External AGWs (condylomata acuminata) and aged ≥ 16 years | Podophyllotoxin 0.5% cream | Podophyllin 25% (clinician applied) | Treatment period of a maximum of 5 weeks followed by a final assessment at week 13 from the start of the study |
| Kirby 199061 | 38 | Men aged ≥ 18 years with from two to 20 external AGWs, excluding anal AGWs. Total AGW surface area of < 10 cm2 | Podophyllotoxin 0.5% solution | Placebo | Initial treatment period of 4 weeks with subsequent follow-up at 12 and 16 weeks for those categorised as cured at week 6 |
| Komericki 2011131 | 51 | Presence of untreated AGWs | Imiquimod 5% cream | Podophyllotoxin 0.5% solution | People evaluated at end of treatment: 4 weeks with podophyllotoxin 0.5% solution vs. 16 weeks with imiquimod 5% cream |
| Lacey 200364 | 354 | Aged 18–65 years and current episode of AGWs lasting ≤ 3 months and with no therapy in that time | (1) Podophyllotoxin 0.5% solution, (2) podophyllotoxin 0.15% cream | Podophyllin 25% (clinician applied) | Initial treatment period of a maximum of 4 weeks with follow-up at 12 weeks after trial entry for those with complete clearance at any time point during treatment |
| Landthaler 1987139 | 39 | Unclear | Podophyllotoxin 0.5% (solution for men and cream for women) | Podophyllin 25% (clinician applied) | AGWs were treated until clearance and patients were followed up at 4 weeks (or longer) after clearance. Patients received treatment for a minimum of 2 weeks. It is noted that treatment was stopped early in those not responding to treatment; the criteria used to determine non-response to treatment are unclear |
| Lassus 1984140 | 100 | Men with condylomata acuminata in the preputial cavity | Podophyllotoxin 0.5% solution | Podophyllin 20% solution | Initial treatment period of a maximum of 4 weeks with follow-up at 6 months after the start of treatment for those with complete clearance at the end of treatment |
| Maiti 1985147 | 100 | Male with penile AGWs who had not received treatment for their AGWs in the 6 months before enrolment | (1) Podophyllin 0.5% solution (patient applied), (2) podophyllin 1.0% solution (patient applied) | Podophyllin 2.0% solution (patient applied) | Initial treatment period of 1 week with follow-up at 3 weeks and 3 months for those with complete clearance at end of treatment |
| Matteelli 2001120 | 12 | Aged ≥ 18 years with a clinical diagnosis of external AGWs established by physical examination and a laboratory-confirmed diagnosis of HIV infection | Cidofovir 1% cream | Placebo | Initial treatment period of 2 weeks treatment followed by 2 weeks of observation |
| Mazurkiewicz 199060 | 54 | Presence of AGWs | (1) Podophyllotoxin 0.5% solution, (2) podophyllotoxin 0.5% cream | Podophyllin 20% (clinician applied) | Initial treatment period of up to 6 weeks with an observation period of 4 weeks after the end of treatment for those whose AGWs had completely cleared after treatment |
| Nath 1990148 | 100 | Presence of AGWs | Podophyllin 25% (clinician applied) | TCAA 50% (clinician applied) | Initial treatment period of 12 weeks with follow-up for 3 months for those categorised as ‘cured’ |
| Orlando 2002128 | 74 | Presence of AGWs and seropositive for HIV infection | Electrocauterisation plus cidofovir 1% | (1) Electrocauterisation, (2) cidofovir 1% (patient applied) | Initial assessment at the end of treatment (varies with allocated treatment) followed by 6 months’ follow-up for those who achieved complete clearance with treatment |
| Padhiar 2006132 | 60 | Aged 12–65 years with at least two but no more than 50 clinically diagnosed external AGWs. Seronegative for HIV infection | Imiquimod 5% cream | Podophyllin 20% (clinician applied) | Initial treatment period (16 weeks with imiquimod 5% vs. 6 weeks with podophyllin 20%) followed by a 6-month follow-up period for those with clearance of AGWs |
| Petersen 1995141 | 269 | Diagnosis of condyloma acuminatum. No topical, systemic antiviral or AGW treatment in the 4 weeks preceding the study | Podophyllotoxin 0.5% solution | Podophyllotoxin 0.5% cream | Initial treatment duration of 2–4 weeks with final follow-up at 12 weeks |
| Sherrard 2007149 | 409 | People with new or recurrent genital AGWs that had not been treated in the preceding 3 months | (1) TCAA plus podophyllin 25%, (2) cryotherapy plus podophyllin 25% | (1) Podophyllin 25% (clinician applied), (2) TCAA, (3) cryotherapy | 8 weeks (maximum treatment period); no follow-up period |
| Simmons 1981160 | 42 | Men with AGWs | Electrocautery | Cryotherapy | Up to two treatments at a 2-week interval plus a minimum follow-up for 3 months from start of the trial |
| Simmons 1981150 | 140 | Men with AGWs who had not received treatment in the preceding 3 months | Podophyllin 10% (clinician applied) | Podophyllin 25% (clinician applied) | Initial treatment period of 6 weeks with subsequent follow-up to 3 months after the start of treatment |
| Snoeck 2001121 | 30 | Biopsy-proven genital AGWs, perianal AGWs or both | Cidofovir 1% gel | Placebo | Up to 12 weeks’ treatment with subsequent follow-up for 4 weeks after completion of treatment or removal from the study. Those with a complete response were followed up for 6 months (unclear whether or not this is additional to the 4-week observation period) |
| Stefanaki 2008133 | 120 | Immunocompetent men with diagnosis of external genital or perianal AGWs and no previous treatment for AGWs | Imiquimod 5% cream | Cryotherapy | Initial treatment period of 3 months with subsequent follow-up at 6 and 12 months |
| Stone 1990151 | 450 | People with external AGWs that had not been treated in the month preceding trial entry | Podophyllin 25% (clinician applied) | (1) Cryotherapy, (2) electrofulguration | Initial treatment period of up to 6 weeks with follow-up after 3 months for those achieving complete clearance |
| Strand 1995142 | 90 | Men with genital AGWs (acuminata or papular) | (1) Podophyllotoxin 0.15% cream, (2) podophyllotoxin 0.3% cream | Podophyllotoxin 0.5% solution | Initial treatment period of up to 4 weeks with subsequent follow-up at 16 weeks after entry into the study |
| Syed 1993143 | 60 | Men aged 15–40 years with a clinical diagnosis of AGWs | (1) Podophyllotoxin 0.15% cream, (2) podophyllotoxin 0.3% cream | Podophyllotoxin 0.3% solution | Initial treatment for a maximum of 4 weeks with subsequent follow-up at 16 weeks after treatment for those classed as ‘cured’ during treatment |
| Syed 1994122 | 90 | Asian women between the age of 16 and 40 years with extravaginal AGWs | (1) Podophyllotoxin 0.3% cream, (2) podophyllotoxin 0.5% cream | Placebo | Initial treatment period of up to 4 weeks with subsequent follow-up at 16 weeks for those achieving complete clearance during treatment |
| Syed 1995123 | 40 | Men aged 18–40 years with genital AGWs (on the glans, shaft, corona sulcus or perianal area) and who were HIV negative at baseline | Podophyllotoxin 0.5% cream | Placebo | Initial treatment period of up to 4 weeks with follow-up at 16 weeks for those achieving complete clearance during treatment. Final follow-up was 1 year after the first day of treatment |
| Syed 1995124 | 40 | Women aged 18–40 years with AGWs and who were HIV negative | Podophyllotoxin 0.5% cream | Placebo | Initial treatment period of up to 4 weeks with follow-up at 16 weeks for those achieving complete clearance during treatment. Final follow-up was 1 year after the first day of treatment |
| Syed 1998161 | 60 | Women aged 18–45 years with AGWs | Imiquimod 2% cream | Placebo | Initial treatment period of 6 weeks with subsequent follow-up at 16 weeks from the start of the trial for those achieving complete clearance during treatment. Final follow-up was 11 months after the initial visit |
| Syed 2000162 | 60 | Men aged 18–50 years with AGWs | Imiquimod 2% cream | Placebo | Initial treatment period of 6 weeks with subsequent follow-up at 16 weeks from the start of the trial for those achieving complete clearance during treatment. Final follow-up was 11 months after the initial visit |
| Tabari 2010152 | 120 | People with a diagnosis of genital AGWs as evaluated by physical examination of the lesions | Podophyllin 25% (clinician applied) | TCAA 30% (clinician applied) | Total duration including follow-up of 6 months. Duration of treatment is unclear |
| Trofatter 2002134 | 90 | Women with histologically confirmed external AGWs | (1) Imiquimod 5% cream, once daily, (2) imiquimod 5% cream, twice daily | Imiquimod 5% cream three times per week | Initial treatment period of up to 16 weeks with a subsequent 4-week observational period |
| Tuncel 2005135 | 60 | Presence of recalcitrant AGWs (perianal and/or genital) that were refractory to at least one conventional therapy | Cryotherapy plus imiquimod 5% cream | (1) Imiquimod 5% cream, (2) cryotherapy | Initial treatment period of up to 16 weeks; unclear whether or not there was an additional follow-up period |
| Tyring 1998125 | 22 | Aged ≥ 18 years with a clinical diagnosis of external AGWs, with at least 10 but no more than 50 AGWs | Imiquimod 5% cream | Placebo | Initial treatment period of up to 16 weeks |
| Tyring 199868 | 326 | Immunocompetent people aged > 18 years with at least two distinct external AGWs (genital and/or perianal). Women had to have a negative pregnancy test | Podophyllotoxin 0.5% gel | Placebo | Initial treatment period of up to 8 weeks followed by assessment after a further 8 weeks to evaluate recurrence (depending on number of treatment cycles, total study time ranged from 9 to 16 weeks) |
| Viazis 2007155 | 49 | Intra-anal AGWs that had not been previously treated with any modality. Absence of AGWs, or previous elimination of AGWs, on the penis, groin, cervix, urethral meatus, vagina or pubis. People with simultaneous perianal AGWs were included | Argon plasma coagulation plus imiquimod 5% cream | Argon plasma coagulation | Argon laser treatment was repeated every 4 weeks until complete clearance of AGW was achieved. After elimination of AGWs, patients were followed up for a mean of 12 months (range 3–21 months) |
| von Krogh 1992126 | 60 | Women with vulvoanal condylomata (acuminata, popular or sessile) | Podophyllotoxin 0.5% cream | Placebo | Initial treatment period of 3 weeks with subsequent follow-up at 3 months for those categorised as cured at any point in the study |
| von Krogh 1994127 | 57 | Men attending sexually transmitted disease outpatient departments who had previously untreated penile AGWs | (1) Podophyllotoxin 0.5% solution, (2) podophyllotoxin 0.25% solution | Placebo | Initial treatment period of a maximum of 2 weeks with follow-up of up to 23 weeks for those considered to have complete clearance |
| White 1997144 | 315 | Men with first-episode, untreated penile AGWs | Podophyllotoxin 0.5% (formulation unclear) | (1) Podophyllin 0.5% (patient applied), (2) podophyllin 2% (patient applied) | Final follow-up occurred at 3 months (initial treatment period unclear) |
Study characteristics
Population
Inclusion criteria varied considerably across identified studies, with some studies reporting clinical diagnosis of AGWs as the sole criterion for eligibility (see Table 4). The size of studies was also wide-ranging, with the largest study randomising 450 people151 and the smallest randomising 12 people. 120
Few identified studies reported comprehensive baseline characteristics for the enrolled study population. Key characteristics of AGWs that potentially influence treatment choice and effectiveness include size, number and location of lesions, together with wart type (keratinised vs. non-keratinised). Of those studies reporting baseline characteristics, most studies included a mixed population in terms of AGW characteristics. AGW morphology was rarely specified as an inclusion criterion and only one study61 was identified that restricted inclusion by AGW type, specifying that AGWs should be non-keratinised. Most studies evaluated the treatment of external AGWs. One study155 was identified that evaluated treatment of intra-anal AGWs. Duration of AGWs and line of treatment also varied across studies. Some studies focused on people who were treatment naive or restricted inclusion to those who had not received treatment in a set time period before enrolment, typically the preceding 3 months. As well as varying across studies, duration of disease varied considerably within studies, for example ranging from 1 to 300 weeks in the study by Godley et al. 154
Several studies specified a minimum age for eligibility of 18 years and the reported mean age of people enrolled in studies ranged from 20 to 35 years. Although many studies included both genders, 1861,66,67,115,123,127,129,133,136,140,142,143,145,147,150,154,160,162 and eight62,122,124,126,130,134,137,161 studies focused on men and women respectively. Three studies evaluated treatments in people with HIV infection. 119,120,128
The generally limited reporting of baseline characteristics precludes discussion of the extent of clinical heterogeneity across studies. Given the limited information available, clinical experts were consulted about the potential disparity across studies in populations enrolled. Experts fed back that they considered the population across the studies likely to be representative of people with AGWs and who present to GUM clinics. Thus, the project team considered it appropriate to carry out a MTC.
Interventions and comparators
Studies evaluating all interventions of interest were identified (see Table 4). Topical treatments evaluated were the self-applied imiquimod 5% cream and podophyllotoxin, and the clinician-applied TCAA and podophyllin 20–25%. Various concentrations (e.g. podophyllotoxin 0.5% cream and 0.15% cream), formulations (e.g. podophyllotoxin 0.5% cream and podophyllotoxin 0.5% solution) and application schedules of podophyllotoxin were evaluated across studies. Additionally, the duration of treatment with imiquimod 5% cream varied across studies. The licence for imiquimod 5% cream indicates that treatment can be given for up to 16 weeks and recommends an application schedule of three times per week on non-consecutive days. 58 Studies were identified evaluating application of imiquimod 5% for 4, 8, 12 and 16 weeks130,133 and at application schedules of once daily, twice daily and three times daily. 129,134
After consultation with clinical experts on the potential influence of treatment duration, dose and formulation (relates to ease of application) on clinical effectiveness, the following assumptions were made for the purposes of carrying out meta-analysis, both direct and indirect:
-
Imiquimod 5% cream of any schedule (e.g. three times a week vs. once daily vs. twice daily) applied for 12 weeks is equivalent in clinical effectiveness to imiquimod 5% cream applied for 16 weeks; schedules of < 12 weeks’ duration were excluded from the analysis.
-
Clinician-applied podophyllin 20% and clinician-applied podophyllin 25% are clinically equivalent.
-
Different formulations of podophyllotoxin are of potentially sufficiently dissimilar clinical effectiveness to warrant analysis by preparation.
-
With the exception of podophyllotoxin 0.25% and 0.3%, doses of podophyllotoxin are potentially of sufficiently dissimilar clinical effectiveness to warrant analysis by dose.
Outcomes
The outcomes of interest to this review and reported in the included studies are listed in Table 5. No study reported data on the outcomes of relief of symptoms, malignancy or QoL. Although most studies presented results on the primary outcome of complete clearance of AGWs at the end of treatment, few studies presented data on complete clearance at subsequent time points. The definition of recurrence of AGWs differed slightly across studies. Most studies defined recurrence as the appearance of AGWs at a site previously cleared of AGWs, whereas some included appearance of AGWs at sites additional to those initially cleared.
| Study | Complete clearance at end of treatment | Complete clearance at other time points | Recurrence | Time to complete clearance | Volume of clearance | Appearance of new AGWs during treatment | AEs | Included in MTCa | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Complete clearance at end of treatment | Complete clearance at another time point | Recurrence | ||||||||
| Abdullah 1993153 | ✓ | ✓ | Primary | |||||||
| Arican 2004112 | ✓ | ✓ | ✓ | ✓ | ✓ | Primary | ||||
| Azizjalali 2012156 | ✓ | ✓ | ✓ | Primary | ||||||
| Baker 2010113 | ✓ | ✓ | ✓ | |||||||
| Bar-Am 1993157 | ✓ | |||||||||
| Benedetti Panici 1989114 | ✓ | ✓ | ✓ | ✓ | ✓ | Sensitivity | Sensitivity | |||
| Beutner 1989115 | ✓ | ✓ | ✓ | Primary | ||||||
| Beutner 1998116 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Primary | |||
| Beutner 1998117 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Claesson 199665 | ✓ | ✓ | ✓ | |||||||
| Edwards 1988136 | ✓ | ✓ | ✓ | Sensitivity | Sensitivity | |||||
| Edwards 1998118 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Primary | |||
| Ferenczy 1995158 | ✓ | ✓ | ✓ | |||||||
| Fife 2001129 | ✓ | ✓ | ✓ | ✓ | ||||||
| Gabriel 1983145 | ✓ | ✓ | ✓ | Primary | Primary | |||||
| Garland 2006130 | ✓ | ✓ | ||||||||
| Gilson 1999119 | ✓ | ✓ | ✓ | ✓ | Sensitivity | |||||
| Gilson 2009159 | ✓ | ✓ | ✓ | ✓ | ✓ | Primary | Sensitivity | |||
| Godley 1987154 | ✓ | ✓ | ✓ | Primary | ||||||
| Goh 199866 | ✓ | ✓ | Primary | |||||||
| Greenberg 199162 | ✓ | ✓ | ✓ | ✓ | ||||||
| Handley 199267 | ✓ | ✓ | ✓ | ✓ | Sensitivity | Sensitivity | ||||
| Hellberg 1995137 | ✓ | ✓ | ✓ | Sensitivity | Sensitivity | |||||
| Jensen 1985146 | ✓ | ✓ | ✓ | Primary | Primary | |||||
| Kar 2003138 | ✓ | ✓ | ✓ | ✓ | Primary | Primary | ||||
| Kinghorn 199363 | ✓ | ✓ | ✓ | Sensitivity | ||||||
| Kirby 199061 | ✓ | ✓ | ✓ | ✓ | ✓ | Primary | ||||
| Komericki 2011131 | ✓ | ✓ | Sensitivity | |||||||
| Lacey 200364 | ✓ | ✓ | ✓ | Sensitivity | Sensitivity | |||||
| Landthaler 1987139 | ✓ | ✓ | ✓ | |||||||
| Lassus 1984140 | ✓ | ✓ | ✓ | Primary | Primary | |||||
| Maiti 1985147 | ✓ | ✓ | ✓ | |||||||
| Matteelli 2001120 | ✓ | ✓ | ||||||||
| Mazurkiewicz 199060 | ✓ | ✓ | ✓ | Primary | ||||||
| Nath 1990148 | ✓ | ✓ | ✓ | Primary | Primary | |||||
| Orlando 2002128 | ✓ | ✓ | ✓ | Sensitivity | ||||||
| Padhiar 2006132 | ✓ | ✓ | ✓ | ✓ | ✓ | Primary | Primary | |||
| Petersen 1995141 | ✓ | |||||||||
| Sherrard 2007149 | ✓ | Primary | ||||||||
| Simmons 1981160 | ✓ | ✓ | Sensitivity | |||||||
| Simmons 1981150 | ✓ | ✓ | ✓ | |||||||
| Snoeck 2001121 | ✓ | ✓ | ✓ | ✓ | ✓ | Sensitivity | ||||
| Stefanaki 2008133 | ✓ | ✓ | ✓ | ✓ | Sensitivity | Sensitivity | ||||
| Stone 1990151 | ✓ | ✓ | ✓ | Sensitivity | Sensitivity | |||||
| Strand 1995142 | ✓ | ✓ | ✓ | Sensitivity | Sensitivity | |||||
| Syed 1993143 | ✓ | ✓ | ✓ | ✓ | Sensitivity | |||||
| Syed 1994122 | ✓ | ✓ | ✓ | Primary | ||||||
| Syed 1995123 | ✓ | ✓ | ✓ | Primary | ||||||
| Syed 1995124 | ✓ | ✓ | ✓ | Sensitivity | ||||||
| Syed 1998161 | ✓ | ✓ | ✓ | ✓ | ||||||
| Syed 2000162 | ✓ | ✓ | ✓ | ✓ | ||||||
| Tabari 2010152 | ✓ | ✓ | ✓ | |||||||
| Trofatter 2002134 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Tuncel 2005135 | ✓ | ✓ | ✓ | |||||||
| Tyring 1998125 | ✓ | ✓ | Sensitivity | |||||||
| Tyring 199868 | ✓ | ✓ | ✓ | Sensitivity | ||||||
| Viazis 2007155 | ✓ | ✓ | ✓ | ✓ | ||||||
| von Krogh 1992126 | ✓ | ✓ | ✓ | Primary | ||||||
| von Krogh 1994127 | ✓ | ✓ | ✓ | Primary | Primary | |||||
| White 1997144 | ✓ | ✓ | ||||||||
Quality assessment
No study was deemed to be at an overall low risk of bias, with the largest proportion of studies categorised as having an overall unclear risk of bias predominantly because of the limited reporting in the full publications (Table 6). In an attempt to supplement the information available, study authors were contacted with requests for additional detail on trial methodology. Only two authors replied by the prespecified deadline. Given that it is over 10 years since publication of most of the identified studies, the low response rate was to be expected.
| Study | Sources of bias relating to study characteristics | Outcome-specific sources of biasa | Overall assessment | |||||
|---|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Selective reporting | ‘Other bias’ | Blinding | Incomplete outcome data | |||
| Participants and personnel | Outcome assessment | |||||||
| Abdullah 1993153 | ? | ? | ? | ? | ? | ? | ? | ? |
| Arican 2004112 | ? | ? | ? | ? | ? | ? | ? | ? |
| Azizjalali 2012156 | ✓ | ? | ? | ? | ? | ✓ | ? | ? |
| Baker 2010113 | ✓ | ? | ✗ | ? | ✓ | ✓ | ? | ✗ |
| Bar-Am 1993157 | ✓ | ? | ✗ | ? | ? | ? | ? | ✗ |
| Benedetti Panici 1989114 | ? | ? | ? | ? | ? | ? | ✓ | ? |
| Beutner 1989115 | ? | ? | ? | ? | ? | ? | ? | ? |
| Beutner 1998116 | ? | ? | ✓ | ? | ? | ? | ✓ | ? |
| Beutner 1998117 | ? | ? | ✓ | ? | ? | |||
| AGW clearance at completion of treatment; recurrence of AGWs; time to complete clearance; and volume of wart clearance | ? | ? | ✓ | |||||
| Appearance of new AGWs during treatment and AEs | ? | ? | ✗ | |||||
| Claesson 199665 | ? | ? | ✗ | ? | ✗ | ✗ | ? | ✗ |
| Edwards 1988136 | ✓ | ? | ? | ? | ✗ | |||
| AGW clearance at completion of treatment and AEs | ✗ | ✗ | ? | |||||
| Recurrence of AGWs | ✗ | ✗ | ✗ | |||||
| Edwards 1998118 | ? | ? | ✓ | ? | ? | |||
| AGW clearance at completion of treatment and volume of AGW clearance | ? | ? | ✓ | |||||
| Time to complete clearance; appearance of new AGWs during treatment; and AEs | ? | ? | ? | |||||
| Recurrence of AGWs | ? | ? | ✗ | |||||
| Ferenczy 1995158 | ✓ | ? | ✗ | ? | ✗ | ✓ | ? | ✗ |
| Fife 2001129 | ✓ | ? | ? | ? | ? | ? | ? | ? |
| Gabriel 1983145 | ? | ✓ | ? | ? | ? | |||
| Recurrence of AGWs | ✓ | ✓ | ✓ | |||||
| AGW clearance at completion of treatment and AEs | ✓ | ✓ | ? | |||||
| Garland 2006130 | ? | ? | ✗ | ? | ✗ | ✗ | ? | ✗ |
| Gilson 1999119 | ? | ? | ? | ? | ? | |||
| AGW clearance at completion of treatment | ? | ? | ? | |||||
| Volume of AGW clearance and appearance of new AGWs during treatment | ? | ? | ✗ | |||||
| AEs | ? | ✗ | ✗ | |||||
| Gilson 2009159 | ? | ✓ | ? | ? | ? | |||
| Recurrence of AGWs | ? | ? | ✓ | |||||
| AGW clearance at completion of treatment and at other time points and AEs | ? | ? | ? | |||||
| Godley 1987154 | ? | ? | ? | ? | ? | |||
| Recurrence of AGWs | ? | ? | ✓ | |||||
| AGW clearance at completion of treatment and at other time points and AEs | ? | ? | ? | |||||
| Goh 199866 | ? | ? | ? | ? | ? | ? | ? | ? |
| Greenberg 199162 | ✓ | ? | ✗ | ? | ✗ | |||
| AGW clearance at other time points and AEs | ? | ? | ? | |||||
| Recurrence of AGWs and appearance of new AGWs during treatment | ? | ? | ✗ | |||||
| Handley 199267 | ? | ? | ? | ? | ? | ? | ? | ? |
| Hellberg 1995137 | ? | ? | ? | ? | ✗ | ✗ | ✓ | ✗ |
| Jensen 1985146 | ? | ? | ? | ? | ? | ? | ✓ | ? |
| Kar 2003138 | ? | ? | ? | ? | ? | ? | ? | ? |
| Kinghorn 199363 | ? | ? | ? | ? | ✗ | ✗ | ? | ✗ |
| Kirby 199061 | ? | ✓ | ? | ? | ✓ | ? | ✓ | ? |
| Komericki 2011131 | ? | ? | ? | ? | ✗ | ✗ | ✗ | ✗ |
| Lacey 200364 | ? | ? | ? | ? | ✗ | ✗ | ? | ✗ |
| Landthaler 1987139 | ? | ? | ✗ | ? | ? | ? | ? | ✗ |
| Lassus 1984140 | ? | ? | ? | ? | ? | ? | ? | ? |
| Maiti 1985147 | ? | ? | ? | ? | ✓ | ✓ | ? | ? |
| Matteelli 2001120 | ? | ? | ✗ | ? | ✗ | |||
| Volume of AGW clearance | ✗ | ✓ | ? | |||||
| AEs | ✗ | ✗ | ? | |||||
| Mazurkiewicz 199060 | ? | ? | ? | ? | ? | |||
| AEs | ? | ? | ✓ | |||||
| AGW clearance at completion of treatment and at other time points and recurrence of AGWs | ? | ? | ? | |||||
| Nath 1990148 | ? | ? | ? | ? | ? | ? | ? | ? |
| Orlando 2002128 | ? | ? | ✗ | ? | ✗ | |||
| AGW clearance at completion of treatment and AEs | ✗ | ✗ | ? | |||||
| Recurrence of AGWs | ✗ | ✗ | ✗ | |||||
| Padhiar 2006132 | ? | ? | ? | ? | ? | ? | ? | ? |
| Petersen 1995141 | ? | ? | ✗ | ? | ? | ? | ? | ✗ |
| Sherrard 2007149 | ? | ? | ? | ? | ? | ? | ? | ? |
| Simmons 1981160 | ✓ | ? | ✗ | ? | ✗ | ? | ? | ✗ |
| Simmons 1981150 | ✓ | ? | ✗ | ? | ? | ? | ? | ✗ |
| Snoeck 2001121 | ? | ? | ? | ? | ? | ? | ? | ? |
| Stefanaki 2008133 | ? | ? | ✗ | ? | ✗ | |||
| AGW clearance at completion of treatment and at other time points and AEs | ✗ | ✗ | ? | |||||
| Recurrence of AGWs | ✗ | ✗ | ✗ | |||||
| Stone 1990151 | ✓ | ? | ? | ✗ | ? | ? | ✓ | ✗ |
| Strand 1995142 | ? | ? | ? | ? | ✗ | ✗ | ? | ✗ |
| Syed 1993143 | ? | ? | ✗ | ? | ✗ | ✗ | ✓ | ✗ |
| Syed 1994122 | ? | ? | ? | ? | ? | ? | ✓ | ? |
| Syed 1995123 | ? | ? | ? | ? | ? | ? | ✓ | ? |
| Syed 1995124 | ? | ? | ✗ | ? | ✗ | |||
| AGW clearance at completion of treatment and at other time points | ? | ? | ✓ | |||||
| Recurrence of AGWs | ? | ? | ? | |||||
| Syed 1998161 | ? | ? | ? | ? | ? | ? | ✓ | ? |
| Syed 2000162 | ? | ? | ? | ? | ? | |||
| AGW clearance at completion of treatment and at other time points and recurrence of AGWs | ? | ? | ✓ | |||||
| AEs | ? | ? | ? | |||||
| Tabari 2010152 | ✓ | ? | ✗ | ? | ✗ | |||
| AGW clearance | ? | ? | ✓ | |||||
| Recurrence of AGWs and AEs | ? | ? | ? | |||||
| Trofatter 2002134 | ? | ? | ? | ? | ✗ | ✗ | ? | ✗ |
| Tuncel 2005135 | ? | ? | ✗ | ? | ✗ | ✗ | ? | ✗ |
| Tyring 1998125 | ? | ? | ? | ✗ | ? | ? | ? | ✗ |
| Tyring 199868 | ? | ? | ✗ | ? | ✗ | |||
| Recurrence of AGWs and AEs | ? | ? | ? | |||||
| AGW clearance at completion of treatment and at other time points | ? | ? | ✗ | |||||
| Viazis 2007155 | ✓ | ✓ | ? | ? | ? | ✗ | ✓ | ✗ |
| von Krogh 1992126 | ? | ? | ? | ? | ? | ? | ? | ? |
| von Krogh 1994127 | ? | ? | ? | ? | ? | ? | ✓ | ? |
| White 1997144 | ✓ | ✓ | ✗ | ? | ? | ? | ✗ | ✗ |
All studies were described as randomised but details on methods used to generate the randomisation sequence were rarely reported. Assessment of clearance and recurrence of AGWs was subjective and thus at risk of bias. Most studies involving topical applications were described as double blinded but, as for randomisation, the full publications provided little information on methods implemented to initially conceal allocation and to subsequently maintain masking of treatment from clinicians and participants. Additionally, for most studies described as double blind, it was unclear whether or not the outcome assessor was the treating clinician and, if not, if the outcome assessor was masked to treatment. Some studies evaluating topical treatments were described as open label in design and thus were categorised as being at high risk of bias for most outcomes reported (see Table 6). Differences in setting could make implementation of masking problematic in studies evaluating self-applied against clinician-applied treatments and topical against ablative therapies. However, masking in these studies could be achieved using sham treatments.
Follow-up at the end of treatment was generally high across studies, with several studies categorised as being at low risk of attrition bias for the outcomes evaluated. However, follow-up at later time points to evaluate recurrence was variable, with high rates of loss to follow-up reported in several studies. Authors of some studies suggested that the low rate of return for further assessments could be attributed to treatment success, that is, complete clearance of AGWs without recurrence had been achieved and people felt that they needed no additional treatment or monitoring.
Of the domains relating to study characteristics, selective reporting was the domain most frequently determined to be at a high risk of bias (see Table 6). In these cases, results for the primary clinical effectiveness outcomes for this project (complete clearance and recurrence) were either not reported, despite being listed in the publication as a primary outcome, or not reported in a way that facilitated incorporation of the data into meta-analysis.
Assessment of effectiveness
Complete clearance at the end of treatment
The comparative clinical effectiveness of achieving complete clearance at the end of treatment was evaluated through a MTC and standard pair-wise meta-analysis. It should be noted that, as a consequence of variation in the duration of treatment for active topical interventions, the duration of placebo treatment or no treatment differs across studies. Given the consistently small proportion of people achieving complete clearance without treatment in the included studies, the project team considers variation in duration of placebo treatment to have a minimal impact on relative estimates of comparative clinical effectiveness.
The primary network generated included 22 studies60,61,66,112,115,116,118,122,123,126,127,132,138,140,145,146,148,149,153,154,156,159 and provided information on 15 treatments; a list of the studies informing the MTC for complete clearance at the end of treatment is presented in Table 5. A prespecified sensitivity analysis included studies that (1) were deemed to be at high risk of bias, (2) enrolled people with AGWs who were seropositive for HIV infection and (3) were reported only as conference abstracts. The sensitivity analysis incorporated an additional 17 studies63,64,67,68,114,119,121,124,125,128,131,133,136,137,142,143,151 and provided information on four extra treatments. The networks of evidence are presented in Figure 3. Although no longer recommended as a treatment for AGWs, podophyllin 20–25% (clinician applied) was chosen as the baseline treatment for the MTCs because of the comparatively large number of studies available for analysis.
FIGURE 3.
Network diagrams of different interventions for complete clearance. The nodes represent the interventions. The thickness of the lines represents the number of studies informing the direct comparison. (a) Primary analysis; and (b) sensitivity analysis.

For the primary and sensitivity MTCs, analysis of model fit identified the random-effects model to be the best-fitting model in each case (Table 7). Additionally, the total residual deviance of the random-effects model in each analysis was closer to the number of data points included in the analysis (see Table 7). In the primary analysis, the model was a good fit for the data, with a residual deviance close to the number of unconstrained data points. There was evidence of heterogeneity in treatment effects across studies in the primary and sensitivity analyses (see Table 7). Codes for fixed- and random-effects models implemented in MTC analyses are supplied in Appendix 4.
| Characteristic | Primary analysis | Sensitivity analysis | ||
|---|---|---|---|---|
| Random effects | Fixed effects | Random effects | Fixed effects | |
| Deviance information criterion | 267.0 | 269.8 | 471.1 | 508.3 |
| Total residual deviance | 53.9 | 63.7 | 95.5 | 152.7 |
| Number of data points | 50 | 50 | 88 | 88 |
| Between study variance (95% CrI) | 0.72 (0.07 to 1.64) | NA | 1.12 (0.62 to 1.76) | NA |
Results from the MTCs indicate that ablative techniques, particularly CO2 laser therapy, have higher probabilities of being the best treatment than most of the topical treatments (summarised in Table 8). CO2 laser therapy is associated with the largest probability of achieving complete clearance at the end of treatment, with probabilities of 97.1% [95% credible interval (CrI) 84.8% to 99.9%] and 96.9% (95% CrI 81.6% to 99.9%) in the primary and sensitivity analyses, respectively. The probabilities of complete clearance at the end of treatment for the primary and sensitivity analyses are summarised in Figures 4 and 5, respectively. By contrast, placebo was associated with a probability of clearance of only 7.6% (95% CrI 1.1% to 20.9%) in the primary analysis and 7.1% (95% CrI 1.7% to 17.7%) in the sensitivity analysis, with the duration of treatment with placebo ranging from 2 weeks to 16 weeks. Of the topical treatments, podophyllotoxin 0.5% solution and podophyllotoxin 0.3% solution were associated with the highest probabilities of completely clearing AGWs by the end of treatment (see Figures 4 and 5). In the primary analysis, podophyllotoxin 0.5% solution had a marginally higher probability of achieving the outcome than podophyllotoxin 0.3% solution (see Figure 4), which was the reverse of the results generated by the sensitivity analysis (see Figure 5). Of the topical treatments, podophyllin (clinician applied), imiquimod 5% cream and podophyllotoxin creams were associated with no or a low probability of being the best treatment (see Table 8).
FIGURE 4.
Probability of complete clearance at the end of treatment: primary analysis. The square represents the mean probability of complete clearance for a particular treatment. The bar lines indicate the 95% CrI around the estimate of effect.
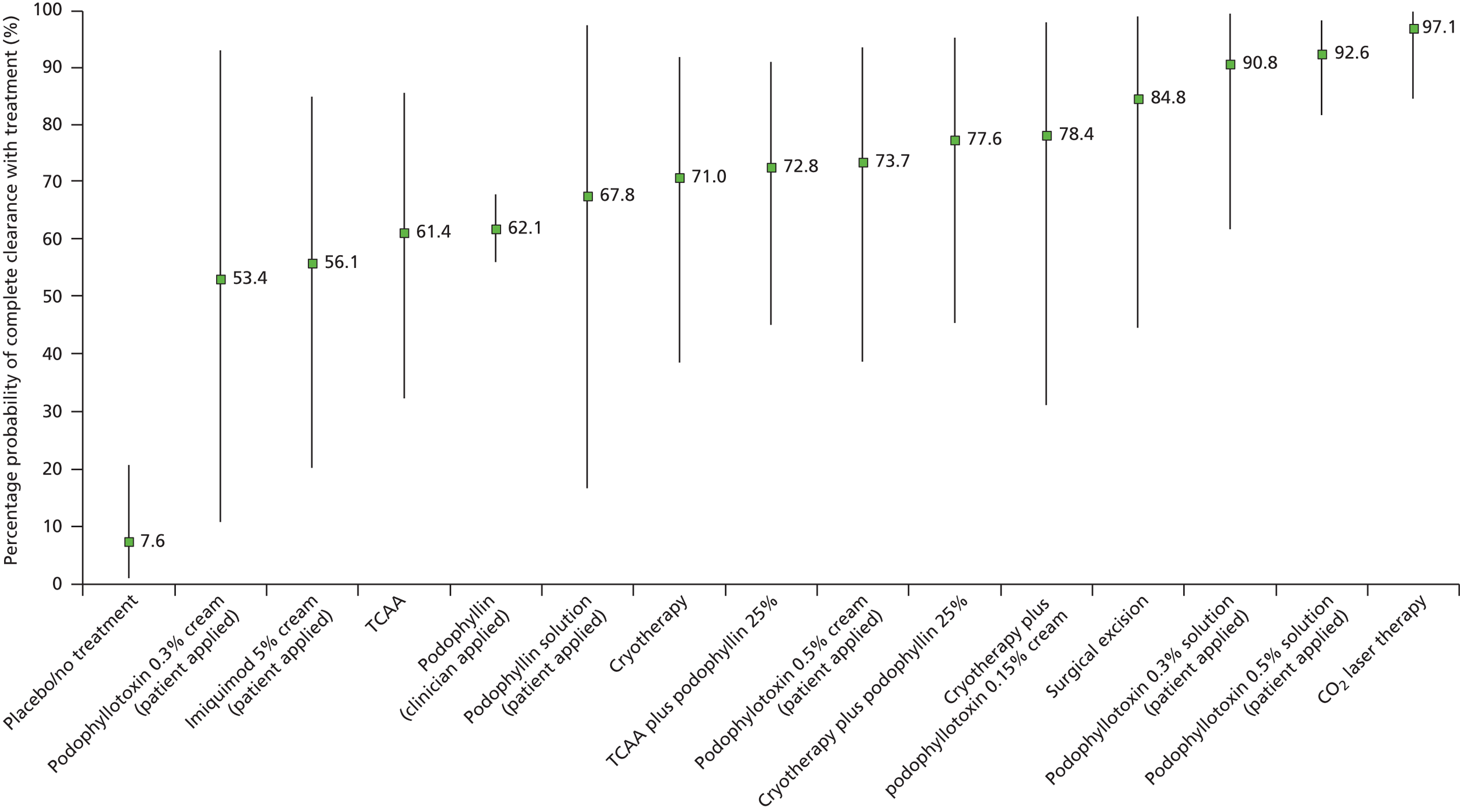
FIGURE 5.
Probability of complete clearance at the end of treatment: sensitivity analysis. The square represents the mean probability of complete clearance for a particular treatment. The bar lines indicate the 95% CrI around the estimate of effect.
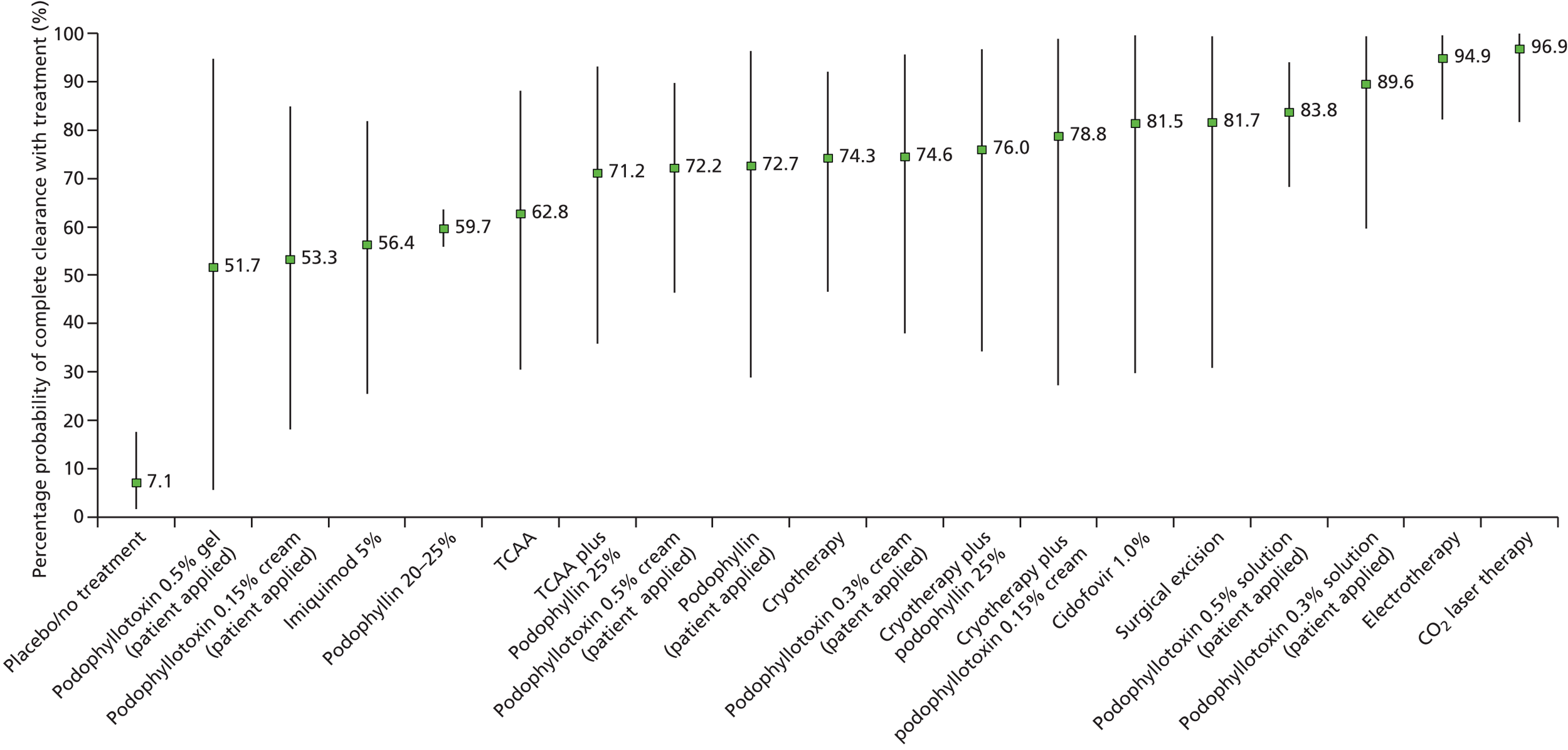
| Treatment | Mean probability (%) of treatment being best treatment (primary analysis) | Mean probability (%) of treatment being best treatment (sensitivity analysis) |
|---|---|---|
| Podophyllin 20–25% (clinician applied) | 0.0 | 0.0 |
| Placebo/no treatment | 0.0 | 0.0 |
| Imiquimod 5% cream (patient applied) | 0.0 | 0.0 |
| Podophyllotoxin 0.5% gel (patient applied) | NA | 0.5 |
| Podophyllotoxin 0.5% solution (patient applied) | 3.8 | 0.0 |
| Podophyllotoxin 0.3% solution (patient applied) | 14.3 | 8.8 |
| Podophyllotoxin 0.5% cream (patient applied) | 0.0 | 0.0 |
| Podophyllotoxin 0.3% cream (patient applied) | 0.0 | 0.2 |
| Podophyllotoxin 0.15% cream (patient applied) | NA | 0.0 |
| Podophyllin solution (patient applied) | 1.6 | 0.4 |
| TCAA | 0.0 | 0.0 |
| Cidofovir 1% | NA | 5.6 |
| Cryotherapy | 0.0 | 0.0 |
| Surgical excision | 6.7 | 6.5 |
| CO2 laser therapy | 71.8 | 62.1 |
| Electrotherapy | NA | 13.1 |
| TCAA plus podophyllin 25% | 0.0 | 0.0 |
| Cryotherapy plus podophyllotoxin 0.15% cream | 1.0 | 2.1 |
| Cryotherapy plus podophyllin 25% | 0 | 0.5 |
When compared with placebo or no treatment, in both the MTC primary and sensitivity analyses, all treatments evaluated were associated with a statistically significant improvement in complete clearance at the end of treatment (Figure 6) (full results of the MTC are presented in Appendix 5). Of the treatments evaluated, CO2 laser therapy was associated with the largest improvement over placebo or no treatment for this outcome, with an OR of 6533 (95% CrI 65.49 to 25,760) in the primary analysis. The results of the MTC are in agreement with findings from standard pairwise meta-analyses, in which all interventions analysed were found to be statistically significantly more effective than placebo at effecting complete clearance at the end of treatment (Figure 7). It should be noted that a high level of statistical heterogeneity was present in the pairwise analysis of imiquimod 5% cream (I2 = 53% in the primary analysis) and podophyllotoxin 0.5% cream (I2 = 61% in the primary analysis) compared with placebo. Forest plots for individual pairwise meta-analyses are available in Appendix 6.
FIGURE 6.
Forest plots presenting ORs and accompanying 95% CrIs for complete clearance of AGWs at the end of treatment for the comparison between the active interventions and placebo generated from the MTC. (a) Primary analysis; and (b) sensitivity analysis.
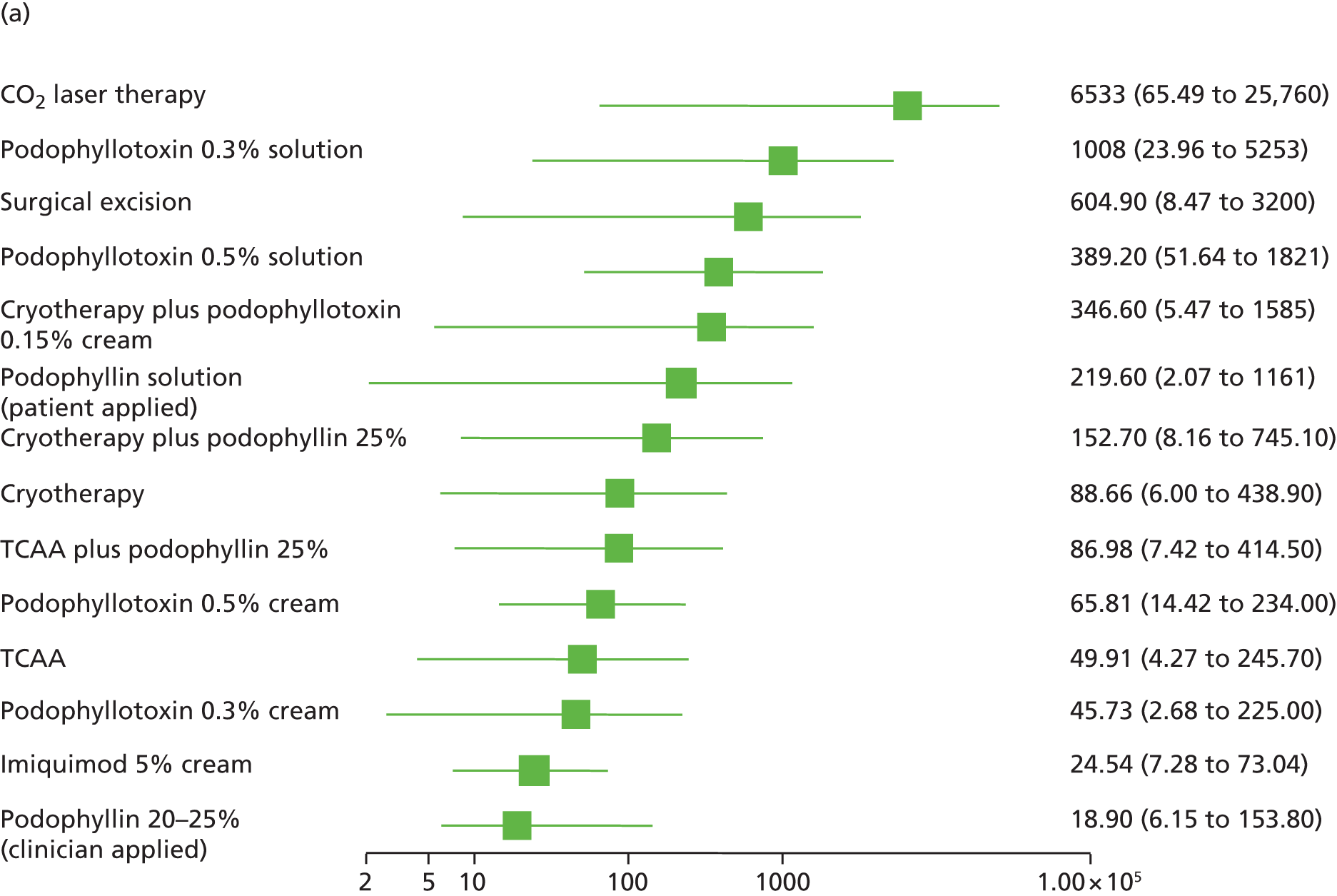
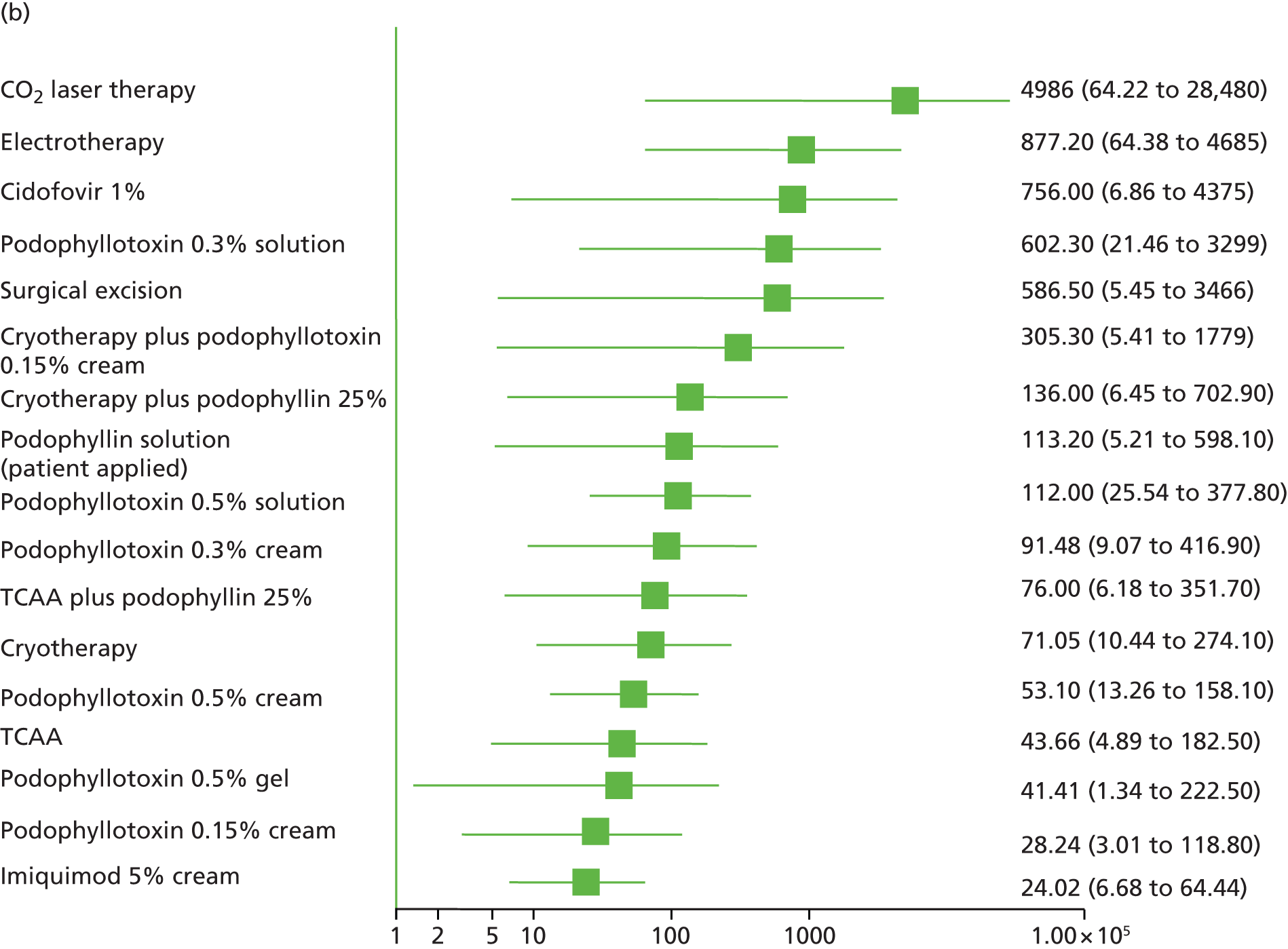
FIGURE 7.
Forest plots presenting ORs and accompanying 95% CIs for complete clearance of AGWs at the end of treatment for the comparison between the active interventions and placebo generated from standard pairwise meta-analysis.
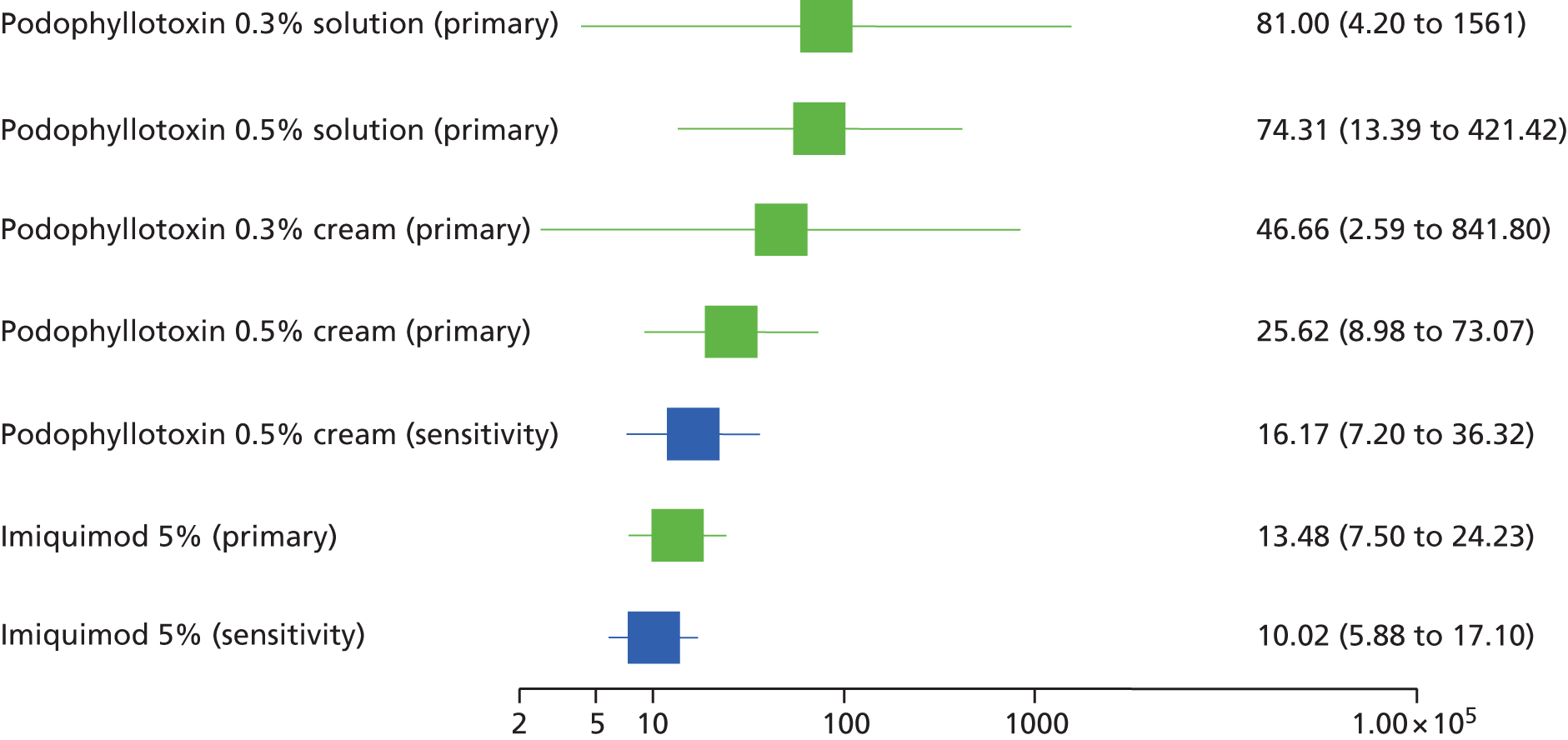
When compared with podophyllin 20–25%, the direction of the effect estimate favoured all other active interventions evaluated in primary and sensitivity analyses. However, most differences between treatments did not reach statistical significance (Figure 8). Of the treatments evaluated, CO2 laser therapy (OR 104.6, 95% CrI 3.35 to 505.2) and podophyllotoxin 0.5% solution (OR 11.65, 95% CrI 2.65 to 38.50) were statistically significantly more effective than podophyllin 20–25% at completely clearing AGWs at the end of treatment (primary and sensitivity analyses). Additionally, sensitivity analyses identified electrotherapy as statistically significantly more effective than podophyllin 20–25% at achieving the outcome (OR 32.72, 95% CrI 3.15 to 150.80). Results from the MTC are predominantly in agreement with findings from standard pairwise meta-analysis. Of the direct evidence available, with the exception of TCAA and podophyllotoxin 0.15% cream, all treatments were associated with a higher probability than podophyllin 20–25% of completely clearing AGWs at the end of treatment (Figure 9). Forest plots for individual pairwise meta-analyses are available in Appendix 6.
FIGURE 8.
Forest plots presenting ORs and accompanying 95% CrIs for complete clearance of AGWs at the end of treatment for the comparison between the interventions and podophyllin 20–25% generated from the MTC. (a) Primary analysis; and (b) sensitivity analysis.

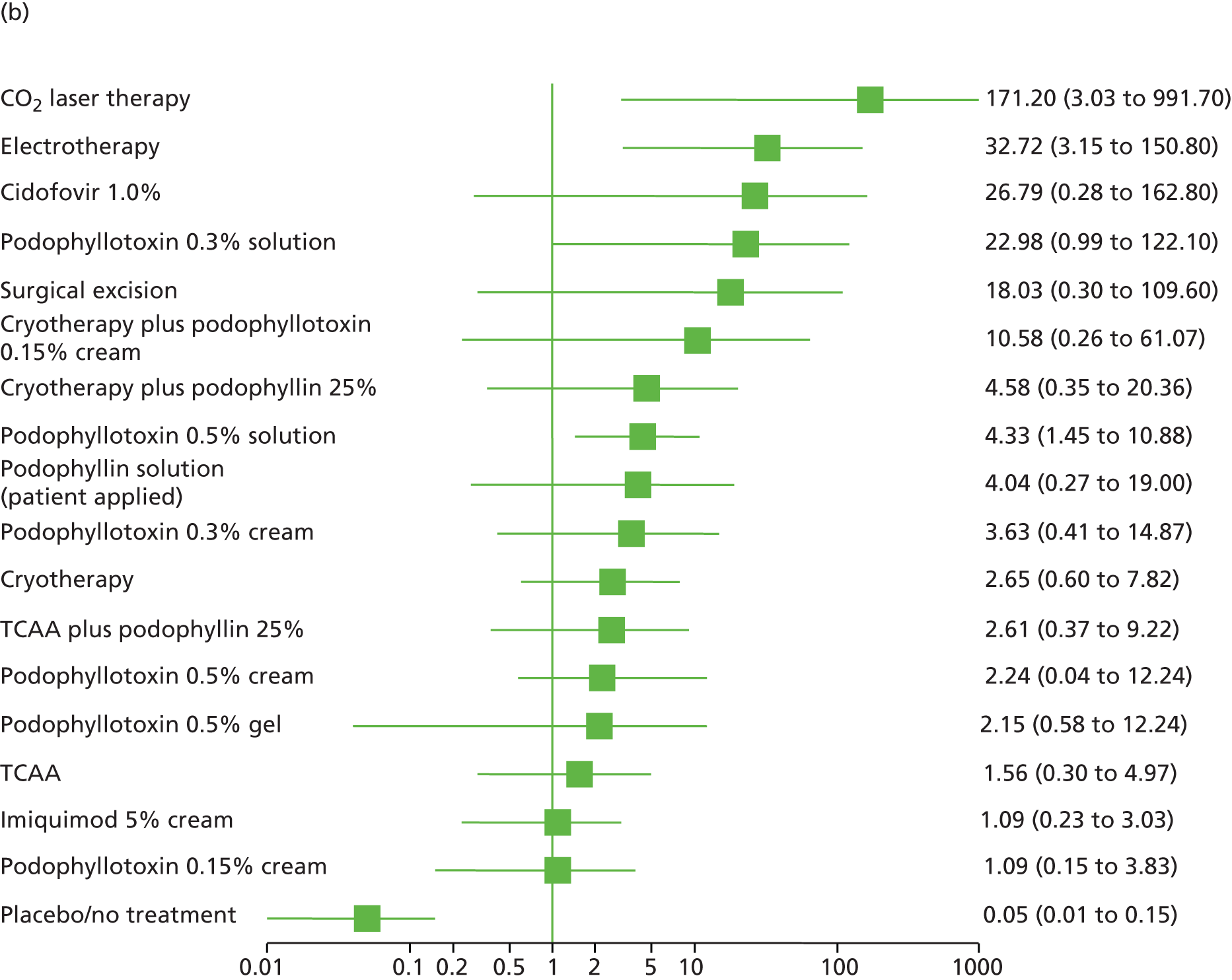
FIGURE 9.
Forest plots presenting ORs and accompanying 95% CIs for complete clearance of AGWs at the end of treatment for the comparison between the interventions and podophyllin 20–25% generated from standard pairwise meta-analysis.

In MTC analyses, there was no statistically significant difference between most treatments for complete clearance of AGWs at the end of treatment. Of those differences that reached statistical significance, most of the comparisons involved CO2 laser therapy or podophyllotoxin 0.5% or 0.3% solution. CO2 laser therapy was found to be significantly more effective than imiquimod 5% cream, TCAA and cryotherapy and the combinations of TCAA plus podophyllin and cryotherapy plus podophyllin. Analysis of direct evidence available for the comparison between CO2 laser therapy and cryotherapy indicated a statistically significant difference between treatments that favoured CO2 laser therapy (OR 22.08, 95 CI 7.37 to 66.16).
The MTC analysis indicated that podophyllotoxin 0.5% and 0.3% solution were both statistically significantly more effective at effecting complete clearance of AGWs than imiquimod 5% cream. However, analysis of direct evidence found no statistically significant difference between podophyllotoxin 0.5% solution and imiquimod 5% cream for this outcome (OR 1.50, 95% CI 0.47 to 4.76). Results from the MTC also demonstrated that podophyllotoxin 0.5% solution was associated with statistically significant improvements in complete clearance compared with podophyllotoxin 0.5% cream, podophyllotoxin 0.3% cream and TCAA. Full results of the MTC are presented in Appendix 5. A summary of the estimates of effect generated by the MTC and standard pairwise meta-analysis is presented in Table 9.
| Comparison | Pairwise meta-analysis, OR (95% CI) | MTC, OR (95% CrI) | ||
|---|---|---|---|---|
| Primary | Sensitivity | Primary | Sensitivity | |
| Topical treatments vs. each other | ||||
| Podophyllotoxin 0.5% solution vs. imiquimod 5% cream | – | 1.50 (0.47 to 4.76) | 18.97 (2.27 to 83.18) | 5.58 (1.16 to 19.2) |
| Podophyllin solution (patient applied) vs. podophyllotoxin 0.5% solution | – | 0.76 (0.20 to 2.86) | 0.61 (0.008 to 3.52) | 1.04 (0.07 to 4.69) |
| Podophyllotoxin 0.5% cream vs. podophyllotoxin 0.5% solution | 0.31 (0.08 to 1.22) | – | 0.30 (0.04 to 0.99) | 0.62 (0.13 to 1.74) |
| Podophyllotoxin 0.3% cream vs. podophyllotoxin 0.5% solution | – | 0.87 (0.23 to 3.22) | 0.19 (0.007 to 0.87) | 0.91 (0.11 to 3.44) |
| Podophyllotoxin 0.15% cream vs. podophyllotoxin 0.5% solution | – | 0.49 (0.14 to 1.68) | – | 0.28 (0.04 to 0.90) |
| Podophyllotoxin 0.3% solution vs. podophyllotoxin 0.5% solution | 1.00 (0.25 to 3.93) | – | 2.17 (0.13 to 10.08) | 5.32 (0.29 to 27.34) |
| Podophyllotoxin 0.3% cream vs. podophyllotoxin 0.3% solution | – | 1.00 | 0.31 (0.003 to 1.56) | 0.60 (0.02 to 3.29) |
| Podophyllotoxin 0.15% cream vs. podophyllotoxin 0.3% solution | – | 0.05 (0.00 to 1.04) | – | 0.18 (0.005 to 0.87) |
| Podophyllotoxin 0.3% cream vs. podophyllotoxin 0.5% cream | 0.23 (0.07 to 0.76) | – | 0.66 (0.06 to 2.9) | 1.96 (0.23 to 8.14) |
| Podophyllotoxin 0.15% cream vs. podophyllotoxin 0.3% cream | – | 0.33a (0.12 to 0.91) | – | 0.63 (0.07 to 2.49) |
| Ablative treatments vs. each other | ||||
| CO2 laser therapy vs. cryotherapy | 22.08 (7.37 to 66.16) | – | 44.61 (3.30 to 201.7) | 63.39 (1.88 to 343.1) |
| Electrotherapy vs. cryotherapy | – | 1.52 (0.97 to 2.39) | – | 15.79 (1.39 to 75.41) |
| Ablative treatments vs. topical treatments | ||||
| Cidofovir 1.0% vs. electrotherapy | – | 0.25 (0.05 to 1.35) | – | 8.71 (0.22 to 48.15) |
| Cryotherapy vs. imiquimod 5% | – | 0.77 (0.37 to 1.59) | 4.02 (0.28 to 18.73) | 3.36 (0.55 to 12.41) |
| Cryotherapy vs. TCAA | 1.67 (1.09 to 2.57) | – | 1.87 (0.55 to 4.73) | 2.19 (0.50 to 6.34) |
| Combination treatments vs. another active treatment | ||||
| TCAA plus podophyllin 20–25% vs. cryotherapy | 0.94 (0.47 to 1.89) | – | 1.53 (0.21 to 5.53) | 1.37 (0.14 to 5.42) |
| Cryotherapy plus podophyllin 20–25% vs. cryotherapy | 1.14 (0.54 to 2.38) | – | 2.37 (0.25 to 9.36) | 2.35 (0.14 to 10.98) |
| Cryotherapy plus podophyllotoxin 0.15% cream vs. cryotherapy | 1.76 (0.92 to 3.37) | – | 2.86 (0.27 to 11.39) | 3.89 (0.16 to 19.83) |
| Cryotherapy plus podophyllin 20–25% vs. TCAA | 2.76 (1.39 to 5.47) | – | 3.56 (0.46 to 13.2) | 4.16 (0.26 to 19.44) |
| Cryotherapy plus podophyllin 20–25% vs. TCAA plus podophyllin 20–25% | 1.21 (0.59 to 2.50) | – | 2.23 (0.22 to 8.83) | 3.15 (0.13 to 15.92) |
The project team acknowledges the considerable uncertainty around the results, as evidenced by the wide CrIs (see Figures 6 and 8).
Complete clearance at another time point
Few identified studies reported clinical effectiveness data for complete clearance without recurrence at time points after cessation of treatment. Additionally, some studies reported data on this outcome only for people achieving complete clearance at the end of treatment rather than the full study population. Complete clearance without recurrence is distinct from recurrence as the former outcome accounts for people who clear within a few days of completion of treatment. Here, results for complete clearance evaluated at least 1 month after the end of treatment are reported from those studies aiming to observe all those randomised. Of the 60 studies included in the review, seven studies (based on the clinical assumptions outlined in Quantity and quality of research available) presented results on complete clearance at ≥ 1 month. 114,129,133,134,150,159,160 Three studies were not suitable for the MTC as they evaluated various dosing schedules of the same treatment. 129,134,150 Only two114,159 of the four remaining studies were judged to be of unclear or low risk of bias, with the remaining studies deemed to be at high risk of bias because they were reported only as a conference abstract or enrolled people with HIV infection. Thus, a MTC was feasible only for the preplanned sensitivity analysis.
No study in the MTC evaluated podophyllin 20–25% and so cryotherapy was chosen as the baseline treatment because of the larger number of studies available. The fixed-effects model was the best-fitting model, with a deviance information criterion (DIC) of 47.9 (the DIC for the random-effects model was 48.1). The fixed-effects model was a good fit for the data, with a total residual deviance close to the number of data points analysed (residual deviance of 8.2 compared with eight unconstrained data points analysed).
Five interventions were indirectly compared in the MTC:
-
placebo or no treatment
-
imiquimod 5% cream (three times a week, patient applied)
-
cryotherapy
-
electrotherapy
-
cryotherapy plus podophyllotoxin 0.15% cream.
Of the five interventions analysed, electrotherapy was associated with the highest probability of achieving complete clearance without recurrence 3–6 months after the end of treatment (65.5%, 95% CrI 40.0% to 86.2%; Figure 10).
FIGURE 10.
Probability of complete clearance at another time point by treatment: sensitivity analysis.

Results of the MTC indicate that the four active interventions are more effective than placebo at improving complete clearance without recurrence, based on median OR (Table 10). Mean OR estimates generated for comparisons with placebo/no treatment were unstable and fell outside the 95% CrI. The instability is likely due to the low event rate for placebo informing the analysis (1 event on 48 patients at risk), which leads to extreme values of the ratio of the odds and produces distributions that are highly skewed.
| Intervention | Comparator, ORa (95% CrI) | ||||
|---|---|---|---|---|---|
| Cryotherapy | Placebo/no treatment | Imiquimod 5% cream | Electrotherapy | Cryotherapy plus podophyllotoxin 0.15% cream | |
| Cryotherapy | – | – | – | – | – |
| Placebo/no treatment | 0.09 (0.001 to 0.47) | – | – | – | – |
| Imiquimod 5% cream | 0.78 (0.35 to 1.51) | 1336 (1.42 to 489.9)b | – | – | – |
| Electrotherapy | 2.22 (0.53 to 6.49) | 4860 (5.90 to 970.5)c | 3.27 (0.60 to 10.85) | – | – |
| Cryotherapy plus podophyllotoxin 0.15% cream | 1.31 (0.64 to 2.41) | 2120 (2.38 to 811.9)d | 1.93 (0.63 to 4.62) | 0.89 (0.16 to 2.79) | – |
Results from the individual studies informing the MTC are in line with the findings of the MTC (Table 11).
| Study | Bias rating | Intervention | Comparator | OR (95% CI) |
|---|---|---|---|---|
| Benedetti Panici 1989114 | Unclear | Electrotherapy | Placebo/no treatment | 25.64 (3.26 to 201.60) |
| Gilson 2009159 | Unclear | Cryotherapy plus podophyllotoxin 0.15% cream | Cryotherapy | 1.23 (0.63 to 2.39) |
| Simmons 1981160 | High | Electrotherapy | Cryotherapy | 1.75 (0.51 to 6.01) |
| Stefanaki 2008133 | High | Imiquimod 5% cream | Cryotherapy | 0.73 (0.35 to 1.52) |
Of the three studies not included in the MTC, Fife et al. 129 and Trofatter et al. 134 compared various dosing schedules of imiquimod 5% cream and Simmons150 assessed different doses of clinician-applied podophyllin. Meta-analysis of the studies by Fife et al. 129 and Trofatter et al. 134 found no statistically significant differences between imiquimod 5% cream applied three times weekly, once daily or twice daily (all possible comparisons) (forest plot presented in Appendix 7).
Recurrence
Based on advice from clinical experts, for the analysis of clinical effectiveness, recurrence of AGWs has been analysed by period of follow-up. Durations of follow-up assessed are from 3 months up to, but not including, 6 months (hereafter referred to as < 6 months) and ≥ 6 months (maximum reported follow-up of 12 months). A separate analysis of recurrence carried out to inform the cost-effectivness analysis included all relevant studies and encompassed recurrence from 3 months onwards. The results of this analysis are not discussed in this section but are presented in Appendix 8.
Analyses of recurrence are based on all people reported to have achieved complete clearance of AGWs at the end of treatment. In cases in which fewer people were followed up than cleared their AGWs, a worst-case scenario was implemented and people lost to follow-up were assumed to have undergone recurrence of AGWs.
Many study groups receiving placebo treatment or no treatment included no people with completely cleared lesions at the end of treatment and, consequently, no person could experience recurrence. As such, it was not possible to include placebo treatment in the analysis of recurrence, which restricted the network.
Recurrence at < 6 months
Applying the clinical assumptions outlined earlier identified four studies127,140,145,148 to inform the primary analysis of recurrence occurring at < 6 months, which facilitated indirect comparison of:
-
podophyllin 20–25%
-
podophyllotoxin 0.5% solution
-
podophyllotoxin 0.25% solution
-
TCAA
-
TCAA plus podophyllin 20–25%.
The random- and fixed-effects models were similar in terms of goodness of fit (DIC 44.4 vs. 44.4, respectively) and had the same residual deviance (8.3 vs. 8.3, respectively), which was close to the number of unconstrained data points in the analysis (eight data points). However, because of the possibility of clinical heterogeneity in the populations of the trials combined in the network, the random-effects model was preferred.
There were no statistically significant differences between any comparisons for recurrence at < 6 months; results from the MTC are presented in Appendix 9. TCAA was associated with the lowest probability of recurrence (23.4%, 95% CrI 1.5% to 76.6%). By contrast, podophyllotoxin 0.25% solution had the highest probability of recurrence (66.9%, 95% CrI 5.2% to 99.5%). The probability of recurrence for all treatments is presented in Figure 11.
FIGURE 11.
Probability of recurrence at < 6 months by treatment: primary analysis.
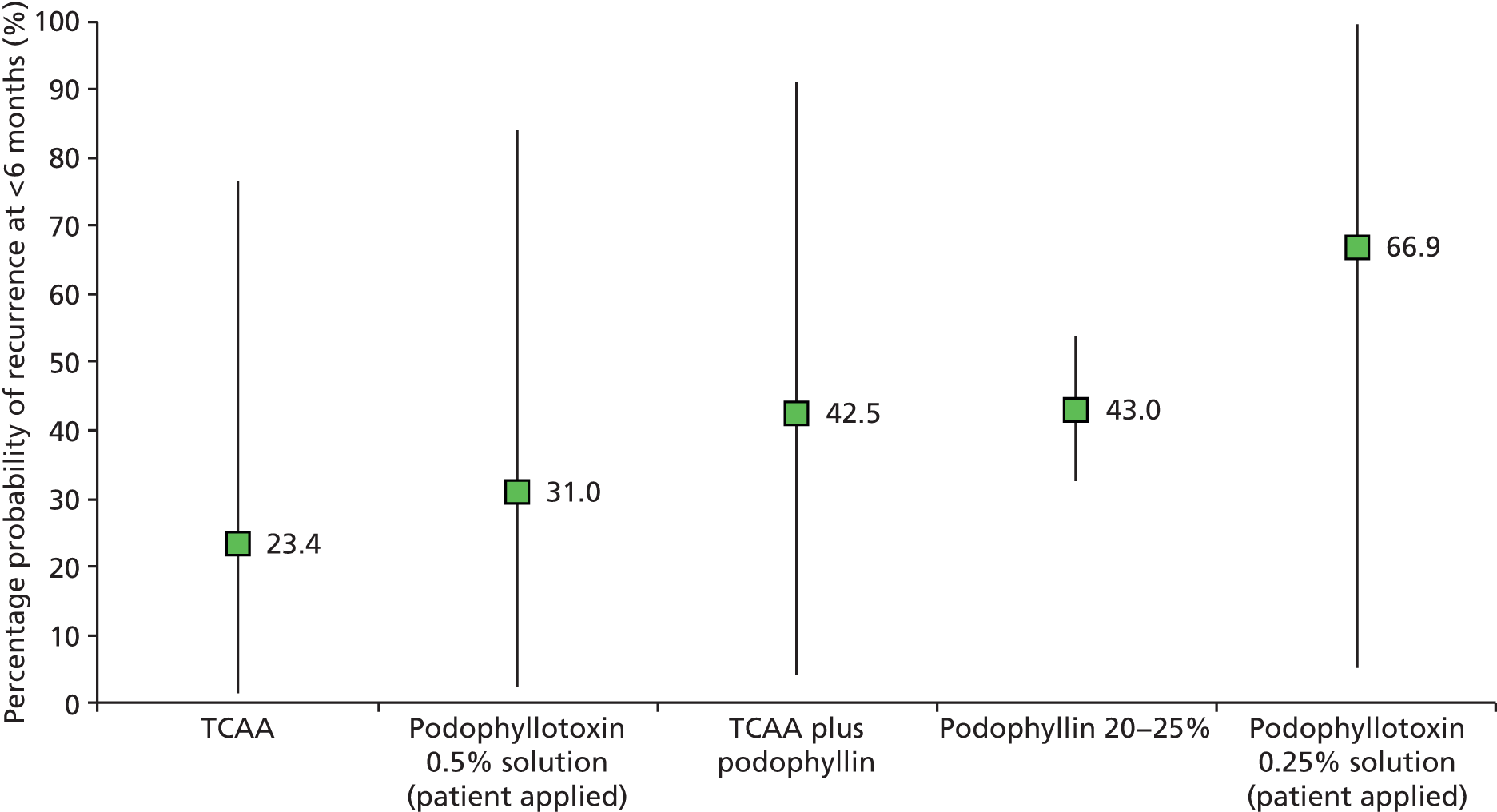
Sensitivity analysis of recurrence at < 6 months included an additional six studies64,67,136,137,142,151 and six other interventions:
-
podophyllotoxin 0.5% cream
-
podophyllotoxin 0.3% cream
-
podophyllotoxin 0.15% cream
-
podophyllin 0.5% solution (patient applied)
-
cryotherapy
-
electrotherapy.
The probability of recurrence for all treatments included in the sensitivity analysis is presented in Figure 12. Of the 11 interventions analysed, TCAA had the lowest probability of recurrence (24.7%, 95% CrI 8.7% to 46.9%). In marked contrast, podophyllotoxin 0.25% solution (83.3%, 95% CrI 49.5% to 98.5%) and podophyllin 0.5% solution (75.9%, 95% CrI 22.0% to 99.5%) had the highest probabilities of recurrence. The comparatively high probability of recurrence of the two topical treatments is reflected in the results of the MTC. As in the primary analysis, for most comparisons, the differences between interventions did not reach statistical significance. However, six comparisons were statistically significant, with four of these involving podophyllotoxin 0.3% solution and with the direction of effect favouring the comparator (podophyllotoxin 0.5% solution, TCAA, cryotherapy and electrotherapy). Additionally, TCAA was significantly more effective than podophyllin 20–25% (OR 0.35, 95% CrI 0.09 to 0.88) and podophyllotoxin 0.15% cream (OR 0.30, 95% CrI 0.06 to 0.90) at reducing recurrence at < 6 months. Full results of the MTC are presented in Appendix 9.
FIGURE 12.
Probability of recurrence at < 6 months by treatment: sensitivity analysis.
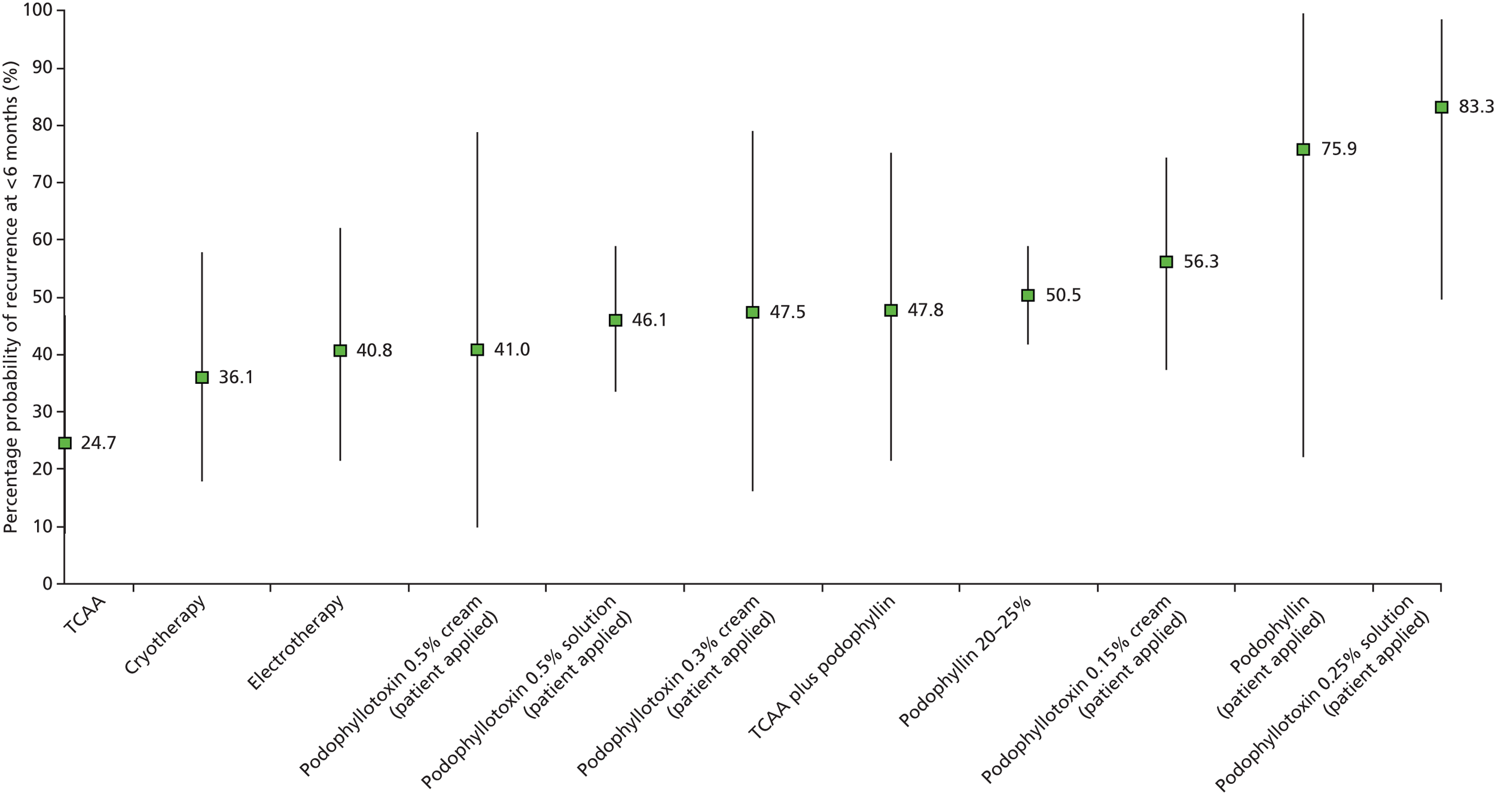
Recurrence at ≥ 6 months
Four studies132,138,146,155 reported recurrence at ≥ 6 months and informed the primary MTC, three132,138,146 of which were included in the MTC. No additional studies were identified for inclusion in a sensitivity analysis.
The random- and fixed-effects models had a similar goodness of fit (32.8 vs. 32.8, respectively). However, the fixed-effects model had a slightly lower residual deviance than the random-effects model (6.1 vs. 6.2, respectively), which is close to the number of unconstrained data points in the analysis (six data points).
The network generated evaluated four interventions:
-
podophyllin 20–25%
-
imiquimod 5% cream
-
podophyllotoxin 0.5% solution
-
surgical excision.
Of the comparisons evaluated, only two differences were statistically significant. Surgical excision was found to be statistically significantly more effective than podophyllin 20–25% (OR 0.16, 95% CrI 0.03 to 0.43) and podophyllotoxin 0.5% solution (OR 0.14, 95% CrI 0.02 to 0.50) at reducing recurrence at ≥ 6 months. Full results of the MTC are presented in Appendix 9. Surgical excision was also associated with the lowest probability of recurrence of the four treatments (15.4%, 95% CrI 4.7% to 33.5%; summarised in Table 12).
| Intervention | Probability (%) of recurrence (95% CrI) |
|---|---|
| Surgical excision | 15.4 (4.7 to 33.5) |
| Imiquimod 5% cream | 24.7 (6.4 to 53.2) |
| Podophyllin 20–25% | 55.9 (42.1 to 69.4) |
| Podophyllotoxin 0.5% solution (patient applied) | 62.1 (37.6 to 82.7) |
An additional study not included in the MTC155 investigated whether or not argon plasma coagulation in combination with imiquimod 5% cream was more effective than argon plasma coagulation alone in the treatment of intra-anal AGWs. Viazis et al. 155 found no statistically significant difference between the two treatments for recurrence of AGWs at a mean follow up of 6 months [5/22 people recurred with combination treatment vs. 8/23 people recurred with monotherapy; OR 0.55 (calculated by project authors), 95% CI 0.15 to 2.06].
Time to complete clearance of anogenital warts
No identified study reported time to complete clearance in a format that facilitated inclusion in a standard pairwise meta-analysis or MTC. Six studies116–118,132,134,155 presented results for time to complete clearance, but only one study155 reported an accompanying measure of variance and statistical evaluation.
Five studies evaluated imiquimod 5% cream (conventional regimen) compared with placebo,116–118 podophyllin 20%132 or alternative application schedules. 134 The median time to complete clearance for imiquimod 5% cream (conventional regimen) varied across the studies, ranging from 7 to 12 weeks. 116–118,132,134 Once daily and twice daily application of imiquimod 5% cream were associated with a median time to complete clearance of 6 and 8 weeks, respectively. 134 Padhiar et al. 132 found a median time to complete clearance of 4.85 weeks for podophyllin 20%. Time to complete clearance for placebo ranged from 10 to 12 weeks. 116–118
Viazis et al. 155 found that adding imiquimod 5% cream to argon plasma coagulation significantly reduced the time to complete clearance [62.5 days, standard error (SE) 5.4 days, with argon plasma coagulation plus imiquimod 5% cream vs. 91.2 days, SE 6.4 days, with argon plasma coagulation alone; p = 0.0016].
Volume of clearance of anogenital warts
Presented analyses are based on the subgroups reduction in baseline AGW volume by < 50% and reduction in baseline AGW volume by ≥ 50% at the end of treatment. Results for clearance by ≥ 50% exclude those achieving complete AGW clearance, which is evaluated as a separate outcome of interest. Applying the clinical assumptions outlined earlier led to analysis of data from three studies judged to be of unclear or low risk of bias reporting < 50% clearance of AGW volume112,121,132 and six studies reporting ≥ 50% clearance of AGW volume. 61,112,116,118,121,132 Twelve other studies reporting volume of AGW clearance were not suitable for inclusion in the MTC as they were judged to be at a high risk of bias, evaluated treatments not included in the analysis or did not present data in a format suitable for inclusion in a meta-analysis114,117,119,120,124,125,134,143,150,159,161,162 (see Table 5).
Clearance of anogenital warts of < 50%
Arican et al. 112 and Snoeck et al. 121 compared imiquimod 5% cream and cidofovir 1% gel, respectively, with placebo. The third study132 compared imiquimod 5% cream with podophyllin 20–25%.
The fixed-effects model was a slightly better fit than the random-effects model (DIC 28.6 vs. 28.7, respectively). The residual deviance of the fixed-effects model was also similar to the number of unconstrained data points analysed (residual deviance of 6.4 compared with six data points, respectively).
The MTC using the fixed-effects model indicated that the effect estimate for achieving < 50% clearance of AGWs was statistically significantly lower with imiquimod 5% cream than with placebo and cidofovir 1.0% gel (Table 13). For the comparison of imiquimod 5% cream and podophyllin 20–25%, the estimate of effect approached 1 and the difference between the treatments did not reach statistical significance.
| Intervention | Comparator, ORa (95% CrI) | |||
|---|---|---|---|---|
| Imiquimod 5% cream | Placebo/no treatment | Podophyllin 20–25% | Cidofovir 1% gel | |
| Imiquimod 5% cream | – | – | – | – |
| Placebo/no treatment | 1300 (15.29 to 5560) | – | – | – |
| Podophyllin 20–25% | 0.997 (0.12 to 3.66) | 0.01 (< 0.001 to 0.08) | – | – |
| Cidofovir 1% gel | 530.6 (2.28 to 2444) | 0.47 (0.04 to 1.86) | 1258 (2.11 to 5058) | – |
Clearance of anogenital warts of ≥ 50%
Five interventions were evaluated in the MTC:
-
placebo or no treatment
-
imiquimod 5% cream
-
podophyllotoxin 0.5% solution
-
podophyllin 20–25%
-
cidofovir 1% gel.
The fixed-effects model was a better fit than the random-effects model (DIC 64.2 vs. 65.3, respectively). The residual deviance of the fixed-effects model was also similar to the number of unconstrained data points in the analysis (11.7 vs. 12, respectively).
Based on the fixed-effects model, podophyllotoxin 0.5% solution was found to be significantly more effective than imiquimod 5% cream and placebo at reducing the volume of AGWs by ≥ 50% compared with the baseline volume (Table 14). No other difference between treatments achieved statistical significance.
| Intervention | Comparator, ORa (95% CrI) | ||||
|---|---|---|---|---|---|
| Imiquimod 5% cream | Placebo/no treatment | Podophyllotoxin 0.5% solution | Podophyllin 20–25% | Cidofovir 1% gel | |
| Imiquimod 5% cream | – | – | – | – | – |
| Placebo/no treatment | 0.66 (0.39 to 1.05) | – | – | – | – |
| Podophyllotoxin 0.5% solution | 109.2 (1.46 to 378.9) | 167.2 (2.40 to 566.2) | – | – | – |
| Podophyllin 20–25% | 1.80 (0.54 to 4.58) | 2.88 (0.75 to 7.84) | 0.27 (0.004 to 1.43) | – | – |
| Cidofovir 1% gel | 3.57 (0.30 to 16.9) | 5.35 (0.51 to 24.66) | 0.50 (0.004 to 3.24) | 2.67 (0.15 to 13.76) | – |
Appearance of new anogenital warts during treatment
Ten identified studies referred to recording the appearance of new AGWs during treatment. 61,62,112,116–119,121,129,138 Of the 10 studies, three were deemed to be at an unclear or low risk of bias, provided data in a format that could be incorporated into a meta-analysis and evaluated interventions meeting the assumptions outlined earlier. 61,116,118 As per the protocol, sensitivity analysis for this outcome was not planned.
The network generated evaluated imiquimod 5% cream, podophyllotoxin 0.5% solution and placebo or no treatment. Placebo was chosen as the baseline treatment because a placebo or no treatment group was involved in all studies in the MTC. The random-effects model was the best-fitting model, with a DIC of 38.9; the DIC for the fixed-effects model was 41.1. The random-effects model was a good fit for the data, with a total residual deviance close to the number of data points analysed (residual deviance of 6.3 compared with six data points analysed).
No statistically significant differences were found between any comparisons for the probability of developing new AGWs during treatment (Table 15). In comparison with placebo or no treatment, the effect estimate favoured imiquimod 5% cream but not podophyllotoxin 0.5% solution (i.e. placebo was favoured over podophyllotoxin 0.5% solution; see Table 15).
| Intervention | Comparator, ORa (95% CrI) | ||
|---|---|---|---|
| Placebo/no treatment | Imiquimod 5% cream | Podophyllotoxin 0.5% solution | |
| Placebo/no treatment | – | – | – |
| Imiquimod 5% cream | 0.57 (0.07 to 2.17) | – | – |
| Podophyllotoxin 0.5% solution | 2.05 (0.06 to 11.00) | 8.70 (0.09 to 45.35) | – |
Imiquimod 5% cream was associated with the lowest probability of new AGWs developing during treatment:
-
imiquimod 5% cream: 30.4% (95% CrI 6.7% to 68.5%)
-
podophyllotoxin 0.5% solution: 45.4% (95% CrI 5.8% to 91.1%)
-
placebo or no treatment: 49.8% (95% CrI 40.8% to 58.7%).
Adverse effects
Given the large number of studies identified, the decision was taken to restrict the comparison and reporting of AEs. After consultation with clinical experts, the project team focused on AEs highlighted as causing discomfort to the patient or being difficult to treat should they occur. The potential for a MTC was investigated for ulceration, blistering, erythema, oedema and itching. Sensitivity analyses for AEs were not planned and reporting of AEs is limited to data from studies deemed to be at low or unclear risk of bias. AEs data were extracted in full from individual studies and are presented in the data abstraction forms in Appendix 2.
Ulceration
Abdullah et al. 153 and Godley et al. 154 compared TCAA with cryotherapy and reported on the occurrence of ulceration. In both studies, a larger proportion of people in the TCAA group than in the cryotherapy group experienced ulceration. Standard pairwise meta-analysis using the fixed-effects model found that TCAA was associated with a significantly higher risk of ulceration than cryotherapy (OR 0.22, 95% CI 0.10 to 0.46; p < 0.0001); the forest plot is presented in Figure 13. However, the level of heterogeneity in the analysis was high (I2 = 68%). Using a random-effects model as a sensitivity analysis generated a non-statistically significant difference between treatments (OR 0.13, 95% CI 0.01 to 1.62; p = 0.11).
FIGURE 13.
Forest plot for the comparison between cryotherapy and TCAA for the outcome of ulceration. df, degrees of freedom; M–H, Mantel–Haenszel.
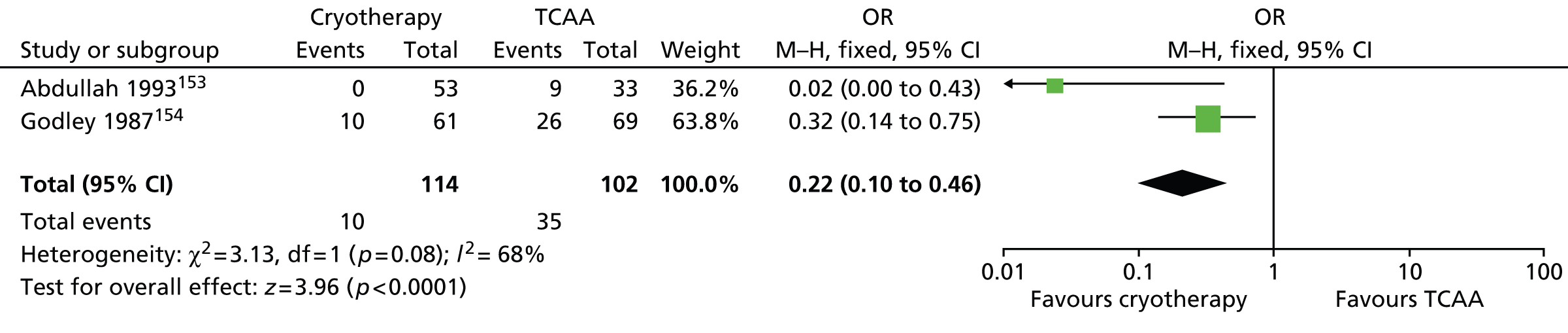
Blistering
In a comparison of cryotherapy and CO2 laser therapy, Azizjalali et al. 156 found a low occurrence of blistering in each treatment group, with two and no people in the cryotherapy and CO2 laser therapy groups, respectively, experiencing blistering (80 people were randomised to each group).
Erythema
Seven RCTs informed the MTC of erythema,60,61,112,116,118,123,132 generating a network incorporating five interventions:
-
placebo or no treatment
-
imiquimod 5% cream
-
podophyllin 20–25%
-
podophyllotoxin 0.5% solution
-
podophyllotoxin 0.5% cream.
The fixed-effects model was found to be a better fit than the random-effects model (DIC 75.0 vs. 76.2, respectively), with a similar residual deviance to the number of unconstrained data points (14.8 vs. 15, respectively).
Using the fixed-effects model with imiquimod 5% cream as baseline, the MTC found that all active interventions were associated with a statistically significant increase in risk of erythema compared with placebo (Table 16). However, no statistically significant differences in erythema were identified across the comparisons of active interventions (see Table 16).
| Intervention | Comparator, ORa (95% CrI) | ||||
|---|---|---|---|---|---|
| Imiquimod 0.5% cream | Placebo/no treatment | Podophyllin 20–25% | Podophyllotoxin 0.5% solution | Podophyllotoxin 0.5% cream | |
| Imiquimod 5% cream | – | – | – | – | – |
| Placebo/no treatment | 0.15 (0.10 to 0.24) | – | – | – | – |
| Podophyllin 20–25% | 0.59 (0.19 to 1.40) | 3.93 (1.17 to 9.83) | – | – | – |
| Podophyllotoxin 0.5% solution | 2.1 (0.29 to 7.87) | 13.9 (1.89 to 52.32) | 3.79 (0.66 to 13.07) | – | – |
| Podophyllotoxin 0.5% cream | 0.91 (0.17 to 3.08) | 6.00 (1.10 to 20.58) | 1.67 (0.37 to 5.20) | 0.59 (0.12 to 1.80) | – |
Of the five interventions compared, podophyllotoxin 0.5% solution was associated with the highest probability of occurrence of erythema:
-
placebo or no treatment: 19.1% (CrI 13.1% to 26.3%)
-
imiquimod 5% cream: 60.3% (95% CrI 52.6% to 67.7%)
-
podophyllin 20–25%: 44.5% (95% CrI 24.3% to 66.1%)
-
podophyllotoxin 0.5% solution: 66.8% (95% CrI 31.8% to 92.0%)
-
podophyllotoxin 0.5% cream: 50.9% (95% CrI 21.2% to 81.7%).
Oedema
The studies by Beutner et al. ,116 Edwards et al. 118 and Padhiar et al. 132 were included in the MTC of oedema, facilitating comparison of imiquimod 5% cream, podophyllin 20–25% and placebo or no treatment.
The best-fitting model was the fixed-effects model, with a DIC of 31.5 compared with 32.3 for the random-effects model. Additionally, the fixed-effects model was a good fit for the data (residual deviance of 5.1 compared with six unconstrained data points in the analysis).
Imiquimod 5% cream and podophyllin 20–25% both statistically significantly increased the risk of oedema compared with placebo:
-
imiquimod 5% cream: OR 0.05 (95% CrI 0.01 to 0.13; OR < 1 favours placebo)
-
podophyllin 20–25%: OR 316.2 (95% CrI 39.86 to 1304; OR > 1 favours placebo).
Additionally, podophyllin 20–25% was found to statistically significantly increase the risk of oedema compared with imiquimod 5% cream (OR 12.39, 95% CrI 2.74 to 40.21; OR > 1 favours imiquimod 5% cream).
Itching
Three studies informed the MTC of itching,61,116,132 enabling comparison of imiquimod 5% cream, podophyllin 20–25%, podophyllotoxin 0.5% solution and placebo or no treatment.
The random- and fixed-effects models were similar in terms of goodness of fit (DIC 35.2 vs. 35.2, respectively) and had the same residual deviance (6.1 vs. 6.1, respectively), which was close to the number of unconstrained data points in the analysis (six data points). However, because of the possibility of clinical heterogeneity in the populations of the trials combined in the network, the random-effects model was preferred.
There was no significant difference in the risk of itching between any of the active treatments compared with placebo (Table 17). Similarly, there was no significant difference between any of the active treatments compared with one another for this adverse event.
| Intervention | Comparator, ORa (95% CrI) | |||
|---|---|---|---|---|
| Imiquimod 0.5% cream | Placebo/no treatment | Podophyllotoxin 0.5% solution | Podophyllin 20–25% | |
| Imiquimod 0.5% cream | – | – | – | – |
| Placebo/no treatment | 1.19 (0.03 to 6.0) | – | – | – |
| Podophyllotoxin 0.5% solution | 18.0 (0.02 to 62.8) | 9.21 (0.14 to 52.2) | – | – |
| Podophyllin 20–25% | 6.8 (0.16 to 35.8) | 93.6 (0.12 to 230.5) | 111.4 (0.02 to 240.8) | – |
Of the active interventions, podophyllin 20–25% had the highest probability of being associated with itching (51.2%, 95% CrI 6.8% to 93.7%). Imiquimod 5% cream and podophyllotoxin 0.5% solution had similar probabilities of being associated with itching, at 30.7% (95% CrI 21.5% to 40.6%) and 39.9% (95% CrI 0.1% to 96.5%), respectively.
Summary of evidence synthesis
Analysis by MTC indicated that, in line with the conclusions outlined in European guidelines,6 ablative techniques, and in particular CO2 laser therapy, are generally associated with higher probabilities of complete clearance of AGWs at the end of treatment. There was considerable disparity in the probability of achieving complete clearance between podophyllotoxin 0.5% solution and imiquimod 5% cream, which are the mainstays of topical treatment. Podophyllotoxin 0.5% solution had a 92.6% (CrI 81.8% to 98.4%) probability of completely clearing lesions compared with 56.1% (CrI 20.3% to 85.0%) for imiquimod 5% cream. However, the wide CrIs indicate that there is considerable uncertainty associated with the results and the findings should be interpreted with caution.
In the primary MTC, there was no statistically significant difference between most of the treatments evaluated for complete clearance of AGWs at the end of treatment. Of those differences that reached statistical significance, most of the comparisons involved CO2 laser therapy or podophyllotoxin 0.5% or 0.3% solution.
CO2 laser therapy was found to be significantly more effective than:
-
imiquimod 5% cream: OR 247.0 (95% CrI 3.03 to 1087; OR > 1 favours CO2 laser therapy)
-
TCAA: OR 86.15 (95% CrI 4.05 to 415.3; OR > 1 favours CO2 laser therapy)
-
cryotherapy: OR 44.61 (95% CrI 3.30 to 201.7; OR > 1 favours CO2 laser therapy)
-
TCAA plus podophyllin: OR 0.13 (95% CrI 0.003 to 0.59; OR < 1 favours CO2 laser therapy)
-
cryotherapy plus podophyllin: OR 0.22 (95% CrI 0.004 to 0.94; OR < 1 favours CO2 laser therapy).
Podophyllotoxin 0.5% solution was associated with statistically significant improvements in complete clearance at the end of treatment compared with:
-
podophyllotoxin 0.5% cream: OR 0.30 (95% CrI 0.04 to 0.99; OR < 1 favours podophyllotoxin 0.5% solution)
-
podophyllotoxin 0.3% cream: OR 0.19 (95% CrI 0.007 to 0.874; OR < 1 favours podophyllotoxin 0.5% solution)
-
TCAA: OR 0.17 (95% CrI 0.02 to 0.63; OR < 1 favours podophyllotoxin 0.5% solution).
Limited reporting of data in available publications for other outcomes of interest led to restricted networks involving few interventions.
Chapter 4 Assessment of cost-effectiveness
Evidence on the cost-effectiveness of interventions to treat AGWs was identified by carrying out a systematic review of the published research literature (see Systematic review of existing cost and cost-effectiveness evidence) and through development of a de novo economic analysis (see Independent economic assessment: methods and Independent economic assessment: results).
Systematic review of existing cost and cost-effectiveness evidence
Search strategy
A systematic review was carried out in September 2013 to identify relevant published cost-effectiveness and costing studies on the treatment of AGWs. The following databases were searched from inception:
-
MEDLINE (Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE)
-
EMBASE (Ovid EMBASE)
-
HTA database (The Cochrane Library)
-
NHS Economic Evaluation Database (NHS EED; The Cochrane Library).
The search strategy combined terms to capture AGWs and terms to capture economic evaluation and costing studies. Full details of the search terms are presented in Appendix 1.
In addition to searching the above databases, reference lists of identified studies were reviewed for potentially relevant studies. No restrictions on language or setting were applied to any of the searches. Studies were assessed for inclusion based on the criteria outlined in Table 18.
| Search | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Economic evaluations | Study population with AGWs | Literature reviews |
| Any economic evaluation study: cost-effectiveness (including cost–utility) analyses, cost–benefit analyses, cost-minimisation analyses, cost–consequence analyses | ||
| Costing studies | Study population with AGWs |
The systematic review was updated in March 2014. The search strategy remained the same as outlined above; however, results were limited from 2013 to March 2014 to identify only additional relevant studies.
Search results
A total of 952 studies were identified from the September 2013 database search as being potentially relevant (Figure 14). Two health economists independently appraised the titles and abstracts of these studies; 270 studies were identified as duplicates and 626 studies were excluded on the basis of title and abstract. A total of 56 studies were therefore identified as potential economic evaluations or costing studies and were ordered for full review.
FIGURE 14.
Identified economic evaluation and costing studies, September 2013 search.
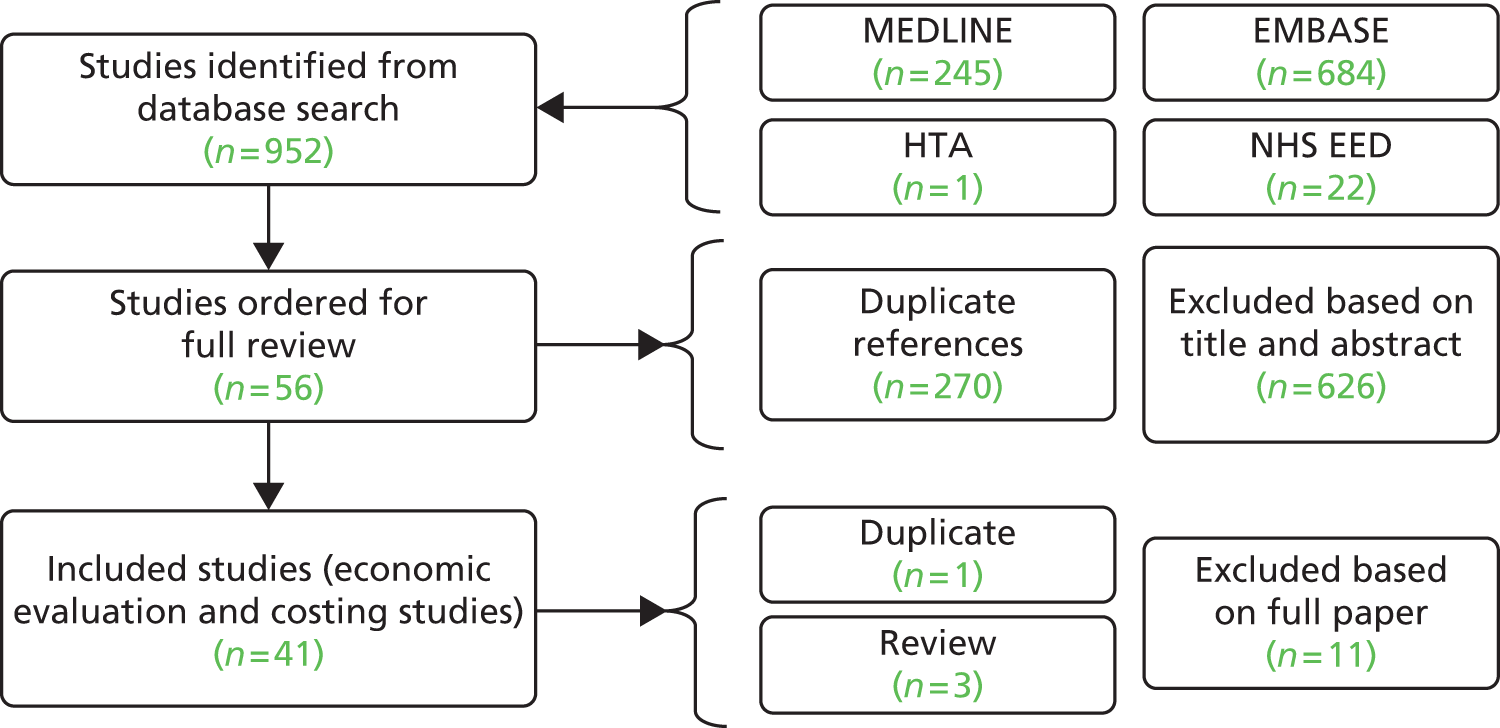
After review of the full papers, a total of 41 papers were included: 10 were economic evaluations,64,163–171 seven were UK costing studies3,51,172–176 and 24 were non-UK costing studies. 177–200 The remaining 15 studies64,201–214 were excluded for the following reasons:
-
duplicate paper (one study)64
-
not a costing study nor an economic evaluation (seven studies)202,204,206–210,213,214
The updated search carried out in March 2014 identified an additional 102 potentially relevant economic evaluation and costing studies. The title and abstracts of these studies were appraised independently by two health economists. A total of 61 studies were identified as duplicate studies, 40 studies were excluded based on title and abstract and one UK costing study was ordered for full review. This further UK costing study215 was included in the review (Figure 15).
FIGURE 15.
Identified economic evaluation and costing studies, March 2014 search.
Consequently, a total of 10 economic evaluation studies64,163–171 and eight UK costing studies3,51,172–176,215 were identified and reviewed; the results from these studies are summarised in the following sections.
Description of the identified cost-effectiveness studies
A total of 10 economic evaluation studies were identified from the September 2013 search. 64,163–171 A summary of the identified economic evaluations is presented in Table 19, with full data extraction tables presented in Appendix 2.
| Author, year, country | Interventions | Analysis, time horizon | Key outcome measure | ICER |
|---|---|---|---|---|
| Langley 2010,163 USA | Sinecatechins vs. imiquimod | Decision analysis, < 1 year | Sustained clearance of warts | NR |
| Walczak 2009,164 Poland | Imiquimod vs. podophyllotoxin | Decision analysis, < 1 year | Total clearing of warts | Incremental cost per total clearing of warts |
| Lacey 2003,64 UK | Clinic-applied 25% podophyllin, patient-applied 0.15% podophyllotoxin cream, patient-applied 0.5% podophyllotoxin solution | Trial-based analysis, < 1 year | Complete remission of warts at 12 weeks | Incremental cost per additional patient cured |
| Lafuma 2003,165 France | Imiquimod 5% vs. podophyllotoxin 0.5% first line followed by laser therapy | Decision analysis, < 1 year | Proportion of patients cured | Incremental cost per additional patient cured |
| Williams 2003,166 UK | Imiquimod vs. podophyllotoxin | Decision analysis, < 1 year | Sustained clearance of warts | Incremental cost per additional sustained clearance |
| Alam et al. 2001,167 USA | Podophyllin resin 25%, podophyllotoxin, TCAA, imiquimod, interferon-alpha-2b, cryotherapy, electrodessication, surgical excision and laser | Decision analysis, time horizon NR | Complete clearance | NR |
| Fagnani 2000,168 France | Imiquimod 5% vs. podophyllotoxin gel 0.5% first line followed by CO2 laser therapy | Decision analysis, < 1 year | Patients cured | NR |
| Langley 1999,169 USA | Analysis 1: imiquimod 5% first line followed by podophyllin, cryotherapy or TCAA | Decision analysis, < 1 year | Sustained clearance | NR |
| Analysis 2: podofilox (podophyllotoxin 0.5% solution) first line followed by podophyllin, cryotherapy or TCAA | ||||
| Analysis 3: cryotherapy first line followed by imiquimod 5% | ||||
| Langley 1999,170 USA | Analysis 1: imiquimod vs. podofilox (podophyllotoxin 0.5% solution) | Decision analysis, < 1 year | Sustained clearance | NR |
| Analysis 2: podophyllin, laser surgery, cryotherapy, TCAA second line | ||||
| Mohanty 1994,171 UK | Podophyllin 25% resin vs. podophyllotoxin 0.5% solution first line followed by cryotherapy, TCAA or electrocautery | NA, retrospective analysis of clinic data | Patients cured | NR |
In general, the analyses presented within the identified studies were non-comparative, with four of the 10 studies reporting an incremental cost-effectiveness ratio (ICER). 64,164–166 The remaining six studies reported the cost per benefit for each intervention but did not compare between therapies. 163,167–171 No studies reported a cost–utility analysis, that is, no economic evaluation used health-related quality of life (HRQoL) as the measure of outcome.
The stated study population varied within the studies and was reported alternatively as those with AGWs,166 condylomata acuminata,167 external AGWs,165 external genital warts,163,168–170 genital and perianal warts,164 and genital warts. 64,171 Following consultation with clinical experts, it was advised that the terms ‘genital’ and ‘anogenital’ warts are commonly used interchangeably, both within the literature and in clinical practice. Consequently, the distinction between the populations reported in each study is not considered to constitute a significant source of heterogeneity between studies.
Of the 10 identified economic evaluations, eight reported results calculated from simple decision analyses, including one UK study. 163–170 A critique of these analyses is presented in Appendix 2 using the Philips et al. 216 checklist for decision-analytical models. Models took the form of either a one-stage or a two-stage decision tree. The two remaining studies were UK based; one study carried out a trial-based economic evaluation64 and the remaining study analysed retrospective clinic data. 171 The time horizon for each identified economic evaluation was < 1 year; discounting was not applied to costs or benefits in any of the identified studies.
A one-stage decision tree was described within six of the studies. 163,164,166,167,169,170 In these studies, the cost and outcomes associated with one line of treatment for external genital warts were estimated. The key outcome for these models was complete clearance164,167 and sustained clearance163,166,169,170 of lesions. People entering each model were either clear or not clear of lesions at the end of the analysis.
Two-stage decision trees were described within four studies. 165,168–170 In these analyses, a second-line treatment was incorporated, allowing for non-response, relapse or recurrence after first-line treatment. Similar to the one-stage decision analyses, the key outcome for these models was sustained clearance169,170 and patients cured. 165,168 The two-stage sequential models were characterised by first-line treatment with patient-administered therapy followed by second-line provider-administered ablative therapies.
Of the 10 studies, three were UK based. 64,166,171 A narrative description is presented in Boxes 2–4 for the included economic evaluation studies adopting a UK perspective.
Results from a trial-based analysis were reported, assessing the cost and effectiveness of self-applied podophyllotoxin 0.5% solution, self-applied podophyllotoxin 0.15% cream and clinic-applied 25% podophyllin for the treatment of genital warts.
The authors carried out an open RCT across 11 sexually transmitted disease clinics in the UK. Patients were included if they were adults, were immunocompetent, had a current episode of AGWs of ≤ 3 months in duration and were not receiving current treatment. In total, 354 patients were analysed. Effectiveness and safety data were obtained from the clinical trial. Effectiveness was captured as complete remission of all warts at 12 weeks. The estimated complete remission rates at 12 weeks were 46.9% for clinic-applied podophyllin solution, 62.2% for patient-applied podophyllotoxin cream and 70.2% for podophyllotoxin solution.
The economic analysis was performed from a societal perspective. Neither costs nor benefits were discounted. The cost year of the analysis was 1998. Both direct and indirect costs were estimated and included the cost of visits to STD clinics (estimated as the average cost across the participating clinics); the cost of drug treatment; the cost associated with patient travel; the cost of treating AEs leading to discontinuation; and production losses when patients were absent from work (estimated using average incomes for women and men in the UK in 1998). The estimated total cost of 12 weeks’ treatment was £535 for clinic-applied podophyllin solution, £573 for patient-applied podophyllotoxin cream and £517 for podophyllotoxin solution.
Compared with clinic-applied podophyllin solution, patient-applied podophyllotoxin solution was estimated to be both less costly and more effective. The incremental cost per additional patient cured at 12 weeks for podophyllotoxin cream compared with podophyllin was estimated to be £246.73. The authors did not compare podophyllotoxin cream with podophyllotoxin solution. The authors noted that the conclusions of the analysis were limited because recurrence rates after individual treatments were not included.
The study provides a description of the cost-effectiveness of clinic-applied podophyllin solution and patient-applied podophyllotoxin solution but may be of limited use within this analysis because of its societal perspective, the use of ‘patient cured’ as the measure of outcome as opposed to quality-adjusted life-years, the lack of a reported sensitivity analysis and restriction to a within-trial analysis.
This study assessed the relative cost-effectiveness of podophyllotoxin (either cream or solution) and imiquimod for AGWs in HIV-negative people. A decision model was developed and the analysis was carried out from the perspective of the UK NHS.
The model was a simple decision tree that assessed the percentage of patients with sustained clearance of AGWs after treatment and follow-up. Follow-up was assumed to be 12 weeks in duration. Treatment duration varied between 4 weeks for podophyllotoxin and 16 weeks for imiquimod; therefore, the time horizon of the model was between 16 and 28 weeks. Clinical trial estimates of clearance during treatment were obtained from the literature. Identified estimates were pooled by simple summation, that is, no adjustments were made to account for differences in patient characteristics across studies. The sustained clearance percentage at the end of treatment and follow-up was calculated to be 35.1% for podophyllotoxin and 40.6% for imiquimod.
The analysis included costs of drug acquisition and GUM clinic attendance. Drug costs were obtained from the BNF (cost year 2002). GUM clinic costs were obtained from Lacey et al. 64 (cost year 1998). Costs were not discounted. The total cost was estimated to be £109.95 for treatment with podophyllotoxin and £245.83 for treatment with imiquimod.
The incremental cost per additional sustained clearance for imiquimod compared with podophyllotoxin was estimated to be £2477. The authors concluded that ‘the modest and statistically insignificant incremental effectiveness of imiquimod is thus purchased at enormous cost’.
A series of one-way analyses and probabilistic sensitivity analysis were carried out. In probabilistic analysis, the authors found that, in 9995 of the 10,000 iterations of the model, podophyllotoxin treatment was dominant when compared with imiquimod (i.e. was less costly and more effective). This conclusion implies that only five out of 10,000 simulations resulted in imiquimod having higher efficacy, despite having a higher base-case efficacy (40.6% vs. 35.1%); this seems an unlikely result.
The analysis carried out may be of limited use within this analysis. This is because of the lack of a systematic review to identify all relevant trials and meta-analyses to synthesise the identified effectiveness data, the use of ‘sustained clearance’ as the measure of outcome as opposed to quality-adjusted life-years and the counterintuitive probabilistic results.
Results from a retrospective cost-effectiveness analysis using GUM clinic data were reported. Notes from people aged ≥ 16 years who attended St Luke’s Hospital GUM clinic in 1991 were reviewed. In total, the notes from 6021 patients were reviewed. Of these people, 742 were diagnosed with genital warts. People who received and completed treatment with either podophyllin 25% resin or podophyllotoxin solution 0.5% first line for their genital warts were included in the analysis; this resulted in a sample size of 683 people. The cost of first- and second-line therapy was incorporated into the analysis. Second-line therapy was cryotherapy, TCAA or electrocautery; the choice of second-line therapy was dependent on the size and number of warts.
Effectiveness of therapy was assessed as ‘warts cured’; this was defined as complete clearance of warts by thorough clinical examination under bright light with no recurrence within 3 weeks of clearance. The percentage of patients cured was 34.6% for podophyllin 25% resin and 66% for podophyllotoxin solution 0.5%.
The analysis estimated the costs of drugs and staff associated with the treatment of each patient. The average cost per patient was estimated to be £14.95 for podophyllin 25% resin and £20.75 for podophyllotoxin solution 0.5%. Costs were not discounted.
Incremental cost-effectiveness was not reported. Instead, the authors presented a cost per patient cured of £27.15 for podophyllin 25% and £25.75 for podophyllotoxin solution 0.5%. The authors concluded that, although the cost per patient was higher for podophyllotoxin solution than for podophyllin, because the efficacy of podophyllotoxin was greater than that of podophyllin, the cost per patient cured was lower for podophyllotoxin solution. No sensitivity analysis was reported.
The data reported are of limited use within this analysis; this is because of the use of retrospective case note effectiveness data, a lack of incremental cost-effectiveness analysis and a lack of sensitivity analysis.
Description of the identified costing studies
A total of 32 costing studies were identified in the review (31 from the September 2013 search and one from the March 2014 search). Of these 32 studies, eight reported data from the UK. 3,51,172–176,215 UK data were considered to be the most relevant for this economic evaluation; therefore, these eight studies were extracted in full (see Appendix 2).
The cost of managing genital warts was reported both at a national level (total cost of treating genital warts)3,51,172,173,215 and at a micro level (cost per episode of care). 3,51,173–176,215 At a national level, four publications reported three separate analyses that estimated the UK total cost of genital wart treatment. Lanitis51 and Carroll et al. 173 reported the results of one analysis in which the annual UK cost associated with treating genital warts was estimated to be £52.4M. A second analysis, reported by Brown et al. ,172 estimated the annual UK cost to be £22.4M. Chapman et al. 215 presented results for the UK (£58.4M) and each of the devolved nations (England £41.72M, Scotland £1.90M, Wales £1.87M, Northern Ireland £0.95M). Finally, Desai et al. 3 reported total costs for England (£16.8M). The estimated average cost per episode of care ranged from £94,178 to £27,651 (Table 20).
| Study | Population | Cost year | Number of episodes (location of episode) | Cost per episode (£) | Total population cost (£) |
|---|---|---|---|---|---|
| Chapman 2013215 | UK and devolved nations | NR | 220,779 in the UK; 157,693 (England), 7461 (Scotland), 7091 (Wales), 3619 (Northern Ireland) | 265 | £58.42M UK; £41.72M (England), £1.90M (Scotland), £1.87M (Wales), £0.95M (Northern Ireland) |
| Lanitis 201251 | UK | 2010 | 173,077 (GUM clinic), 16,782 (primary care)a | 276 | £52.4M |
| Desai 20113 | England | 2008–10 | 141,770 (GUM clinic), 39,645 (primary care), 1978 (hospital) | 113 | £16.8M |
| Carroll 2011173 (linked to Lanitis 201251) | UK | 2010 | 173,077 (GUM clinic), 16,882 (primary care)a | 273 (women); 278 (men) | £52.4M |
| Woodhall 2011176 | Seven GUM clinics in the UK | 2010 | 370 (GUM clinic) | 94 | NR |
| Woodhall 2009175 | York GUM clinic | 2007 | 189 (GUM clinic) | 139 | NR |
| Brown 2006172 | UK | 2003 | 132,114 (GUM clinic) | NR | £22.4M |
| Langley 2004174 | Six GUM clinics in England and Wales | NR | 1200 chart reviews (GUM clinic) | 146.37 (women); 135.77 (men) | NR |
For studies reporting national-level cost estimates, the population included people attending GUM clinics,3,51,172,173,215 attending in primary care3,51,173,215 and receiving hospital treatment. 3 All studies described the patient population as people diagnosed with genital warts. No study identified people with AGWs as a separate population; however, clinical experts advised that the terms ‘genital warts’ and ‘anogenital warts’ are often used interchangeably, and the term ‘genital warts’ is considered appropriate to capture both genital warts and AGWs.
A narrative summary of the eight UK costing studies is provided in Boxes 5–11.
The authors present a conference abstract in which the cost of managing genital warts in the UK and each devolved nation was estimated. The cost per episode of genital warts was estimated to be £265. At a national level the annual UK cost of managing genital warts was estimated to be £58.42M, with costs of £41.72M for England, £1.9M for Scotland, £1.87M for Wales and £0.95M for Northern Ireland. Cost was calculated by multiplying the number of people with genital warts presenting at GUM clinics and primary care in the UK and devolved nations by the expected resource use for these patients (as estimated by GUM experts) and applying UK-specific unit costs.
The number of people with genital warts presenting at GUM clinics was estimated using HPA data for the UK and England and data from the Information Services Division for Scotland, the Communicable Disease Surveillance Centre for Wales and the Public Health Agency for Northern Ireland. The number of people with genital warts presenting in primary care was estimated using data from the Health Improvement Network database extrapolated using population statistics to estimate the number of genital wart cases at national level.
Both studies report the cost of managing genital warts in the UK estimated from the same analysis; Carroll et al. 173 is a conference abstract and Lanitis51 is the full paper publication. Both studies report an estimated UK cost of managing genital warts of £52.4M (cost year 2010). The average cost per episode was estimated to be £276. Cost was calculated by multiplying the number of people with genital warts in the UK by the expected resource use for these patients and applying UK-specific unit costs.
The number of people with genital warts in the UK was estimated by combining the number of people attending GUM clinics with the number of people attending primary care as a result of genital warts. GUM clinic data were obtained from the 2009 HPA surveillance report and primary care data were obtained from the Health Improvement Network database; both were projected to estimate 2010 figures. GUM clinic data were presented by episode type: first episode (96,278 people), recurrent episode (58,109 people) and persistent episode (18,690 people). Primary care data were not divided into categories. To avoid double counting across databases, patients not prescribed treatment by GPs in primary care were assumed to have been referred to a GUM clinic. The number of patients treated in primary care was estimated to be 16,782 in Lanitis51 and 16,882 in Carroll et al. 173 The total number of people with genital warts in GUM clinics and primary care in 2010 was estimated as 189,859.
To estimate treatment utilisation patterns, four GUM clinicians were surveyed and each provided an estimate of the number of visits required per patient, based on type of case (whether first episode, recurrent episode or persistent episode) and type of wart (keratinised or non-keratinised). Most genital wart episodes were found to require two visits, with persistent and hard-to-treat warts requiring three or more and eight to twelve visits, respectively. Keratinised warts were more likely to be treated with cryotherapy or combination therapy than with topical therapy alone, whereas non-keratinised warts were more likely to be treated with topical therapy. The authors considered that the proportion of patients treated with topical compared with ablative therapy ‘remained fairly consistent regardless of the episode’. 51
Unit costs of treatment were applied to the estimated number of episodes and the treatment patterns estimated by the four clinical advisors. A sensitivity analysis was carried out in which costs and patient characteristics were varied; the total cost estimate was most sensitive to the proportion of hard-to-treat patients.
The authors present an estimated cost of care in 2008 for people with genital warts in England presenting at GUM clinics, general practices and NHS hospitals. The estimated total cost was £16.8M (95% CI £15.5M to £18.0M), with an estimated average cost per episode of care of £113. Cost was calculated by multiplying the estimated number of people with genital warts in England with the expected resource use for these patients and applying UK-specific unit costs.
The number of people presenting with genital warts (new and recurrent cases) was obtained for GUM clinics, GP surgeries and NHS hospitals. The number of people attending GUM clinics for genital warts was obtained from HPA data; data from 2008 and 2009 were averaged to provide an estimate of 141,770 cases each year. To obtain GP surgery data, the General Practice Research Database (GPRD) was reviewed between 2006 and mid-2008; GPRD data were extrapolated to the total population under GP care in England, resulting in an estimated 39,645 episodes of care annually. Hospital data were obtained using Hospital Episode Statistics for people treated in a NHS hospital with a primary diagnosis of ‘anogenital (venereal) warts’. In total, 1978 episodes of care were recorded to this code in 2008. To account for overlap between GPRD and HPA data, an episode with a referral code or at least one diagnostic code in the referral table and an episode with no treatment recorded were assumed to have been referred to a GUM clinic. Costs presented by Woodhall et al. 176 were applied to the number of people with genital warts.
The authors present an estimated cost of care for people with genital warts in 2010 based on review of the treatment of patients presenting at seven sexual health clinics. The authors carried out a case note review of 370 people aged ≥ 16 years attending six sexual health clinics in England and one clinic in Northern Ireland. Patients were required to have a current diagnosis of genital warts (new or recurring episode) and to have attended the clinic between April and June 2007. Resources used in the care of each participant were recorded and costs were applied through a mixture of standard UK unit costs and clinic estimates of cost.
The case note review implied that patients attended for a mean of 2.5 visits per episode of care. An episode of care lasted for a weighted mean of 36 days (95% CI 27 to 46 days). The percentage of patients attending once was 45% for women and 55% for men. The authors also reported consultation times at clinic by staff involved in the visit. The estimated mean cost per episode of care was £94 without a STI screen and £146 with a STI screen (2010 cost year).
The authors present an estimated cost of care for adults with genital warts in 2007 based on review of the treatment of patients presenting at a York sexually transmitted disease clinic. The authors carried out a case note review of 189 patients registered at the clinic and diagnosed with genital warts (first or recurrent episode) and recorded the resources used by each patient. The time taken for each procedure was estimated through interviews with nine members of the clinical team.
UK costs were applied to the resources used; staff costs were taken from the Unit Costs for Health and Social Care 2007, published by the Personal Social Services Research Unit. The costs of treatment carried out in the clinic (cryotherapy, curettage, electrosurgery/hyfrecation, electrosurgery/diathermy, TCAA) were estimated from local costs of equipment and consumables. The cost of home treatment was estimated from the BNF 2007. The authors presented total costs in US dollars, converting from UK cost figures using an exchange rate of £1 = $2.0551. The mean cost of an episode of care was estimated to be $286 (£139).
The mean number of visits per episode of care (both first and recurrent) was estimated to be 2.8 (95% CI 2.4 to 3.2). Nearly half (46%) of all patients had one visit per episode of care; of these patients, > 80% had been provided with home treatment (either podophyllotoxin or imiquimod). The average length of an episode of care was 41 days, with 3% of cases having an episode of care > 6 months.
The authors found that the mean cost for first episodes was greater than the mean cost for recurrent episodes although this was not statistically significant (first episode $296 vs. recurrent episode $266; p = 0.43); this was a result of a lower average number of visits to a clinician (first episode 2.9 visits vs. recurrent episode 2.74 visits) and a shorter mean duration of episode of care (first episode 44 days vs. recurrent episode 36 days).
The authors report an estimated resource use and cost associated with screening and management of cervical dysplasia and cervical cancer, and treatment of genital warts in 2003. For the calculation of costs associated with the treatment of genital warts, the authors estimated the number of people with genital warts in the UK and multiplied this by the estimated resource use and associated costs to estimate a total cost of genital wart treatment in 2003. The total 2003 UK cost was estimated to be £22,402,330.
The number of people with genital warts (first or recurrent) presenting in GUM clinics was obtained from HPA surveillance data. The number of genital wart cases reported by GUM clinics in 2003 in the UK was 76,457 for incident cases, 38,902 for recurrent cases and 16,755 for persistent cases. Data for drug use, procedures and number of visits per episode was obtained from questionnaires sent to six GUM clinicians in Aberdeen, Liverpool, London (two clinicians), Nottingham and Southampton. Telephone interviews were conducted with each respondent to review and clarify responses. Responses were pooled and mean rates of events were used for costing. Length of visit was obtained from the study by Langley et al. ,174 which carried out a retrospective chart review. Physician and nurse costs were obtained from the Unit Costs for Health and Social Care 2001. For GUM clinic visits for diathermy, cryotherapy or combination therapy (procedure plus drug therapy), an all-inclusive payment was obtained from personal communication with a clinician. Costs of topical treatments were obtained from the BNF, accessed online in February 2006.
The authors report the results of a retrospective case note review of people with external genital warts, carried out in six GUM clinics in England and Wales in 2000. At each clinic, the case notes of 100 female and 100 male patients, each with a completed episode of care, were evaluated and costs were applied to the resources consumed. The treatment patterns observed in the audit were reported elsewhere. 209
The following costs were included in the analysis: labour costs, material costs, extra costs (i.e. STI screening) and clinic-related indirect costs. The sources of cost data were not provided within the study. Labour costs were described as being estimated using annual salaries. Indirect costs were described as non-labour expenses, non-patient care expenses and direct patient care costs.
The average total cost of an episode of care across the six study sites was estimated to be £146.37 for women and £135.77 for men. There was a high amount of variability in the average cost per episode of care across study sites; the cost for women varied between £96.78 and £265.31 and the cost for men varied between £66.57 and £195.58.
Summary of the systematic review of existing cost and cost-effectiveness evidence
A total of 10 economic evaluations and eight UK-based costing studies relevant to an economic analysis of treatments for AGWs were identified from the literature review.
Of the identified costing studies, four reported the total cost of treating genital warts in the UK51,172,173,215 and two reported the total cost of treating genital warts in England. 3,215 A cost per episode of care was reported in seven of the eight identified costing studies. 3,51,173–176,215 Chapman et al. 215 present, in an abstract, the results of an analysis in which the annual UK and devolved national costs of genital warts were estimated; the UK cost was estimated to be £58.42M. Lanitis51 and Carroll et al. 173 present the results of one analysis in which the annual UK cost associated with treating genital warts was estimated at £52.4M. A further analysis, reported by Brown et al. ,172 estimated the annual UK cost of treatment for AGWs at £22.4M. The estimated average cost per episode of care ranged from £94176 to £276. 51
Of the identified economic evaluation studies, none incorporated HRQoL as the measure of benefit; moreover, only four of the 10 studies reported a fully incremental analysis. 64,164–166 The outcome measure used in all analyses was related to complete clearance or complete curing of AGWs. Of the 10 identified studies, eight reported decision analyses, all of which were simple decision trees restricted to one or two lines of therapy. 163–170 None of the identified decision analyses incorporated costs associated with AEs, and uncertainty was not comprehensively assessed in any of the decision analyses.
Imiquimod and podophyllotoxin were the most widely assessed therapies and were assessed head-to-head in seven studies. 164–170 Three of these seven studies presented ICERs,164–166 and all reported that imiquimod was associated with an incremental cost and an incremental benefit compared with podophyllotoxin. Ablative therapies were generally assessed at second line of treatment.
Therefore, to obtain an analysis that included HRQoL estimates and assessed as fully as possible the scope of this review (see Chapter 2, Decision problem), a de novo economic model was developed; the methods are described in the following section, with the results presented in Independent economic assessment: results.
Independent economic assessment: methods
Scope
The scope of the independent economic assessment is described in Table 21 and reflects the decision problem as outlined in Chapter 2 (see Decision problem). When the economic assessment deviates from the decision problem, a rationale is provided within the table.
| Element | Overview | Comments | Reference section |
|---|---|---|---|
| Type of economic evaluation | Cost-effectiveness analysis; health benefit assessed using quality-adjusted life-years (i.e. cost–utility analysis) | – | Model structure |
| Population | People aged ≥ 16 years with clinically diagnosed AGWs, irrespective of biopsy confirmation | – | Population |
| Interventions | The following interventions were considered in the economic evaluation:
|
The following interventions were not considered in the economic evaluation because of a lack of any supporting clinical data:
|
Interventions |
| Model | |||
| Perspective | NHS and PSS | – | Model structure |
| Model type | Decision tree | – | |
| Time horizon | 58 weeks | – | |
| Discounting | No discounting | No discounting because of the short model time frame (approximately 1 year) | |
Model structure
A simple decision-tree model was developed from the perspective of the UK NHS and Personal Social Services to capture the key costs and consequences associated with alternative treatments for a single episode of AGWs. Costs captured included costs of interventions and resource use associated with AGWs. Consequences were assessed as quality-adjusted life-years (QALYs). The model structure is presented in Figure 16.
FIGURE 16.
De novo model structure. In the base case, complete clearance occurs at the mid-point of treatment and recurrence occurs at the mid-point of follow-up. This was tested in sensitivity analysis.
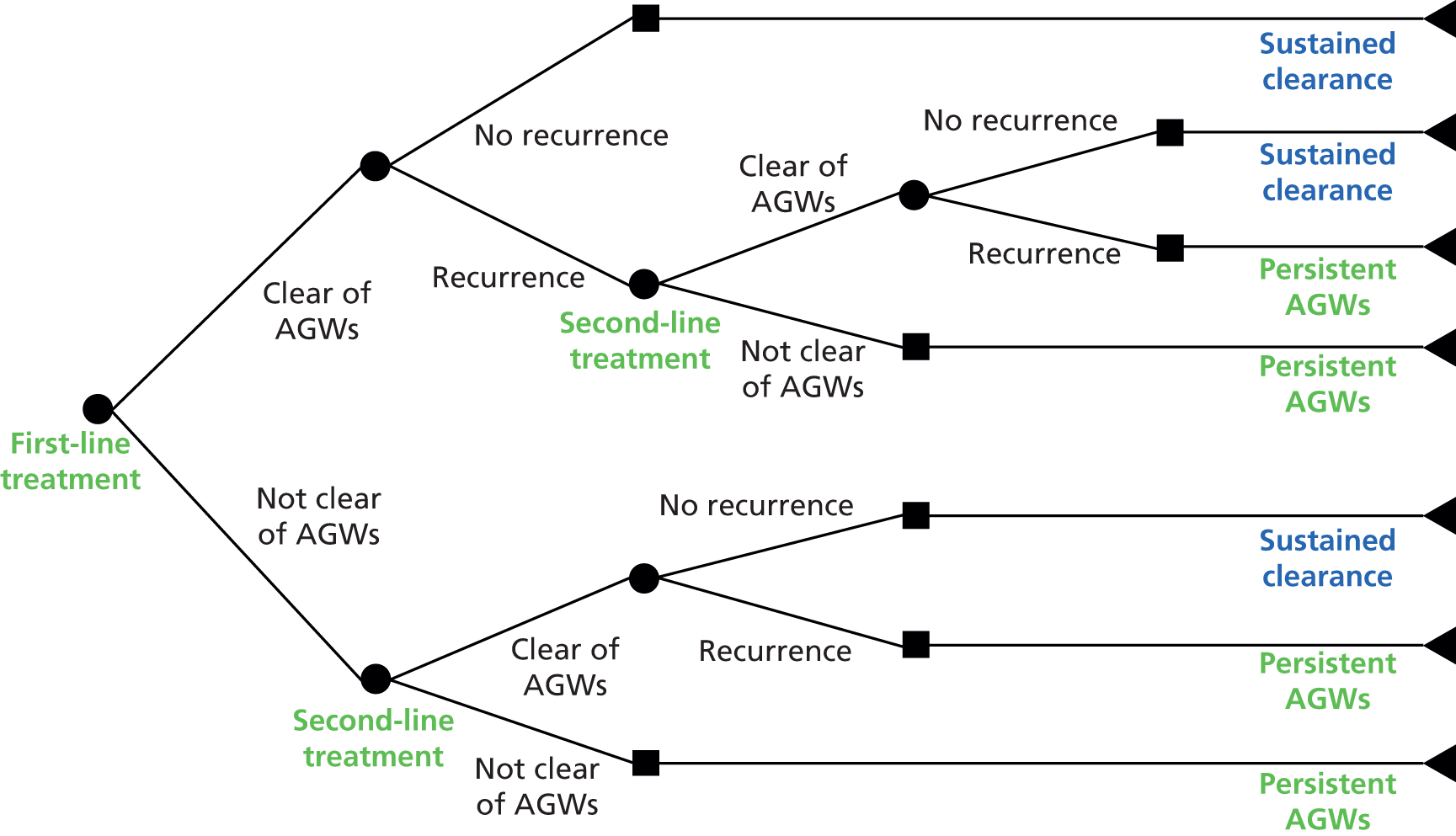
Within the model structure developed, people with AGWs are initially prescribed a first-line treatment. The aim of treatment is to completely clear AGWs. If AGWs are clear after first-line treatment, there is a probability of recurrence of AGWs within a 12-week period. If AGWs do not recur, the person remains clear of AGWs until the end of the modelled period.
If AGWs recur after first-line treatment, or if AGWs do not clear at the end of first-line treatment, a second-line treatment is prescribed. Again, AGWs either clear or do not clear at the end of second-line treatment. If AGWs clear, there is a probability of recurrence of AGWs within a 12-week period. If AGWs do not recur, the person remains clear of AGWs until the end of the modelled period.
If AGWs do not clear at the end of second-line treatment, or if AGWs recur after second-line treatment, the person is assumed to have persistent warts. A cost associated with further lines of therapy and a HRQoL decrement associated with continuing AGWs are applied.
The following key features of the model structure are described in greater detail in the following sections:
-
a single episode of AGWs rather than multiple episodes of AGWs was modelled
-
recurrence of AGWs after clearance is modelled
-
persistent warts were defined as AGWs that have not cleared after two lines of therapy
-
complete clearance and recurrence were assumed to occur half-way through follow-up.
A single episode of anogenital warts rather than multiple episodes of anogenital warts was modelled
Treatment for AGWs does not affect the underlying HPV infection; instead, treatment for AGWs is limited to treatment of the current presenting AGWs (see Chapter 1, Aetiology and pathology). Consequently, people who experience one episode of AGWs may experience future episodes of AGWs. For the purposes of this model, it was considered appropriate to capture the costs and QALYs associated with a single episode of AGWs rather than multiple AGW episodes within the economic analysis. This was for several reasons.
First, HRQoL and costs are not expected to differ between first and subsequent AGW episodes (Box 12). Therefore, regardless of whether the patient is experiencing a first or a later episode of AGWs, the model is considered appropriate to quantify the differences in costs and QALYs associated with each treatment.
Woodhall et al. 176 report HRQoL for 895 people with genital warts who presented at eight UK sexual health clinics between August 2009 and February 2010. Of these, 586 were attending for their first episode and 309 for a second or later episode. The authors found no significant difference in the estimate of disutility between people with a first episode of AGWs and those with a subsequent episode.
CostsWoodhall et al. 175 report data on the cost of treatment and duration of episodes of care from a retrospective case note review. The authors reviewed case notes from 189 patients attending the York sexually transmitted disease clinic during two 3-month periods in 2006 and 2007. In total, 127 patients presented for their first episode and 62 presented for a recurrent episode. Results were reported for all patients, by gender and by first or recurrent episode. The average number of visits per episode of care was similar for first and recurrent episodes [2.9 visits (95% CI 2.4 to 3.4 visits) for a first episode vs. 2.74 visits (95% CI 2.1 to 3.4 visits) for a recurrent episode]. The authors found no statistically significant difference in the mean cost between first ($296) and recurrent ($266) episodes (p = 0.43).
Second, there were insufficient data identified from the literature that were associated specifically with a first or a recurrent episode of AGWs (see Chapter 3, Results); therefore, it was not possible to model multiple episodes with differing efficacy. Moreover, clinical expert opinion suggested that, for a patient with the same characteristics, the response for a subsequent episode would be expected to be similar to the response for an initial episode.
Finally, previous cost-effectiveness analyses for AGWs have used short-term decision trees to model a single episode of AGWs (see Systematic review of existing cost and cost-effectiveness evidence).
Based on the proposed treatment pathway informed by clinical experts, the likely impact of an episode of AGWs and the limitations imposed by the available data from the literature, using a simple decision tree was determined as the most appropriate modelling approach. More complex modelling approaches, such as a state transition Markov model, could have been used but the additional complexity was unlikely to add any benefit in this economic evaluation. In addition, a decision tree model reflects the general approach adopted within the literature.
Recurrence of anogenital warts after clearance is modelled
In clinical practice, a proportion of patients may experience a recurrence of AGWs after initial clearance. Typically, based on consultation with clinical experts, recurrence up to 12 weeks after initial clearance is considered clinically to be an extension of the initial episode of AGWs rather than a new episode. Therefore, it was considered appropriate to include the treatment costs and QALYs for these patients in the economic model.
In addition, of the 10 identified cost-effectiveness analyses (see Description of the identified cost-effectiveness studies), one study used clearance of AGWs at the end of treatment as the outcome measure of interest. 167 The remainder (nine studies) incorporated some element of time between clearance at the end of treatment and assessment of clearance. 64,163–166,168–171 It is therefore considered that incorporation of recurrence within the model structure is in line with the majority of the identified economic evaluation studies on this topic.
The clinical data for AGW recurrence during follow-up were analysed using a MTC and are described in greater detail in Chapter 3 (see Assessment of effectiveness). A number of weaknesses associated with the clinical data for recurrence were identified, including a lack of data for some included treatments and differences in the time point at which recurrence was assessed.
Despite limitations of the data, it was considered clinically appropriate to model recurrence within the model structure. The data for recurrence were tested extensively in sensitivity analysis to establish the importance of this variable as a driver for cost-effectiveness. These issues are explored in greater detail in Recurrence of anogenital warts within 12 weeks of complete clearance.
Persistent warts were defined as anogenital warts that have not cleared after two lines of therapy
In the model, people with AGWs receive up to two lines of therapy, plus, for those people not clear of AGWs after two lines of therapy, a cost and HRQoL associated with treatment of persistent AGWs.
Although the definition of persistent warts is variable, as a simplifying assumption it was considered appropriate to limit the number of lines of therapy prior to treatment for persistent warts to two. Following the systematic review of the cost literature (see Systematic review of existing cost and cost-effectiveness evidence), evidence presented within the studies by Lanitis51 and Woodhall et al. 175,176 implied that the majority of people with AGWs require two or fewer appointments with a clinician whereas patients with persistent warts require additional appointments (Box 13).
Lanitis51 surveyed four clinical experts who observed that the majority of people with AGWs required two clinician visits and that people with persistent warts more commonly required three or more visits.
Woodhall et al. 176 reviewed the case notes for 370 patients; patients attended for a mean of 2.5 visits per episode of care, with the majority of patients (45% of women, 55% of men) attending for one visit.
Woodhall et al. 175 found that, following review of the case notes for 189 patients, patients attended for a mean of 2.8 visits per episode of care, with the majority (46%) attending for one visit per episode of care.
If at each appointment a new prescription was provided, this would imply that two lines of treatment within the model would capture the treatment pathway for the majority of people with AGWs. Moreover, this could be an overestimate of the number of lines of therapy prescribed in practice, as a proportion of patients may return to the clinician to verify complete clearance rather than receive a new prescription. For people requiring a greater number of appointments, the model captures the costs associated with additional treatment through the ‘persistent AGWs’ phase of the economic model.
Lanitis,51 Woodhall et al. 175 and Brown et al. 172 defined persistent AGWs as an episode of AGWs in which treatment continues for > 3 months. Alternative definitions include AGWs that last beyond two lines of therapy (clinical expert opinion) and AGWs that have not cleared despite numerous applications of multiple therapies after a period of approximately 1 year (clinical expert opinion). Within the protocol for this analysis, persistent AGWs were defined as AGWs that had remained for 6 months.
To reflect the varying interpretations of persistent warts present within clinical practice and the literature, within the model patients are assumed to have persistent AGWs after their AGWs have failed to completely clear with two lines of therapy. The duration of persistent AGWs is set at a minimum of 12 weeks after failure on two lines of therapy. Therefore, all people in the model who go on to have persistent AGWs are assumed to have AGWs for 58 weeks. This assumption is tested in sensitivity analysis.
Complete clearance and recurrence were assumed to occur half-way through follow-up
In the base case it was assumed that people achieving complete clearance of AGWs at the end of treatment were clear at the mid-point of the treatment duration. Similarly, it was assumed that any patient experiencing recurrence of AGWs experienced this at the mid-point of follow-up. This was a simplifying assumption, designed to reflect the fact that some patients would clear/recur immediately and some patients would clear/recur towards the end of treatment/follow-up. This assumption was tested in sensitivity analysis.
Time horizon and discounting
The time horizon of the model was 58 weeks. This reflects the maximum possible treatment and follow-up period (46 weeks; see Interventions) plus a minimum 12 weeks of persistent AGWs. Because of the short model time frame (approximately 1 year), costs and consequences were not discounted.
It was considered that a time horizon of 58 weeks (406 days) would capture the costs and consequences of the vast majority of AGW episodes, including episodes of persistent warts. This was based on the systematic review of existing cost and cost-effectiveness evidence. The average duration of an episode of AGWs has previously been estimated as 36 days176 and 41 days. 175 In the study by Woodhall et al. 176 it was estimated that the costs associated with the treatment of genital warts were incurred within 12 months for 98% of cases. In the study by Woodhall et al. ,175 it was reported that only 3% of patients had an episode lasting > 6 months. In the study by Desai et al. ,3 the average duration of an episode of care was analysed by number of consultations: for episodes requiring two consultations, the median duration of the episode of care was 12 days (range 2–57 days); for episodes requiring three or more consultations, the median duration of the episode of care was 48 days (range 4–331 days).
The time horizon was varied in sensitivity analysis.
Population
The population of interest for this economic assessment was people aged ≥ 16 years with clinically diagnosed AGWs, irrespective of biopsy confirmation.
Following a systematic search of the clinical literature, the data were not sufficient to analyse by subgroup (see Chapter 3, Quantity and quality of research available); therefore, subgroups were not considered within the economic analysis.
Interventions
Interventions included in the economic analysis
The interventions relevant for this economic assessment of treatments for AGWs were outlined in the protocol as the topical treatments podophyllotoxin, imiquimod, podophyllin, TCAA and cidofovir; the physical ablation methods cryotherapy, surgical excision, electrotherapy and laser therapy; and any combination or sequential treatment with either topical or physical ablation interventions.
Following review of the data identified in the systematic search of the clinical literature and presented in Chapter 3, the following interventions were included in the economic analysis:
-
placebo
-
imiquimod 5% cream
-
podophyllin 20–25%
-
podophyllotoxin 0.5% solution
-
TCAA
-
CO2 laser therapy
-
cryotherapy
-
surgical excision
-
cryotherapy plus podophyllin 25%
-
cryotherapy plus podophyllotoxin 0.15% cream
-
TCAA plus podophyllin 25%.
Data for ‘placebo’ were assumed to represent ‘no treatment’. This assumption was required because of a lack of clinical data around no treatment. Although acknowledging the weaknesses of this approach, it was considered, on balance, that this was appropriate because of the objective measures of treatment effectiveness used to assess clearance.
No data were obtained for cidofovir, electrotherapy or any sequential treatment sequences as set out in the protocol, nor were any further combinations of treatments identified. In addition, the following interventions identified from the search were not included in the base-case economic analysis:
-
podophyllin 0.25–0.5% (patient applied)
-
podophyllotoxin solution 0.25%
-
podophyllotoxin cream 0.3%
-
podophyllotoxin cream 0.5%.
These interventions were excluded from the economic base-case analysis because they are not routinely used or available in UK clinical practice. In sensitivity analysis, complete clearance data for podophyllotoxin cream 0.5% were used as a proxy for podophyllotoxin cream 0.15%, a concentration that is used in clinical practice (see Chapter 1, Current service provision); however, this was not presented in the base case.
Modelled treatment regimens for included interventions
Duration of treatment
The duration of treatment for trials included in the MTC varied (see Chapter 3, Quantity and quality of research available). Thus, the average duration of treatment seen in UK clinical practice was modelled based on consultation with clinical experts, ensuring comparability with the range of treatment durations investigated in the included clinical trials. The duration of treatment modelled in the base case for each intervention is presented in Table 22, alongside the duration of treatment analysed in the included clinical trials.
| Intervention | Number of trials included in the MTCa | Duration of treatment (weeks) analysed in clinical trials (range) | Modelled average duration of treatment (weeks) |
|---|---|---|---|
| No treatment | 9 | 4–16 | 6 |
| Imiquimod 5% cream | 4 | 12–16 | 16 |
| Podophyllin 20–25% | 9 | 4–12 | 6 |
| Podophyllotoxin 0.5% cream (sensitivity analysis only) | 4 | 3–6 | 4 |
| Podophyllotoxin 0.5% solution | 6 | 4–6 | 4 |
| TCAA | 4 | 6–12 | 6 |
| CO2 laser therapy | 1 | 6 | 6 |
| Cryotherapy | 5 | 6–12 | 6 |
| Surgical excision | 1 | NR; up to four visits, average of one visit | 6 |
| Cryotherapy plus podophyllin 25% | 1 | 8 | 6 |
| Cryotherapy plus podophyllotoxin 0.15% cream | 1 | 12 | 6 |
| TCAA plus podophyllin 25% | 2 | 8 | 6 |
It was therefore assumed that the treatment effect estimated from the MTC based on the included clinical trials was independent of treatment duration (see Chapter 3, Assessment of effectiveness). In addition, as CO2 laser therapy and surgery are typically carried out in one session, the average duration of treatment modelled is intended to reflect the typical waiting time for the procedure.
Because of the variability in treatment duration, the impact of uncertainty in these parameters was tested in a sensitivity analysis.
Duration of follow-up
Duration of follow-up was assumed to be 12 weeks for all interventions. This was based on clinical expert opinion and was selected to capture any recurrence of AGWs that would be considered part of the original episode of AGWs in clinical practice. This duration was tested in sensitivity analysis.
Treatment sequences
Within the base-case analysis, people with AGWs were assumed to receive up to two lines of treatment for their AGWs before being classed as having persistent AGWs. Complete clearance data at the end of the treatment were available for 11 interventions (see Complete clearance at the end of the treatment); thus, the total number of possible treatment sequences equalled 121. However, on the basis of the following assumptions, 84 possible treatment sequences were modelled (Table 23):
-
‘No treatment’ will be prescribed first line only, that is, an active intervention will always be prescribed second line.
-
No sequence of treatments will involve the same intervention twice, that is, if a patient fails on one intervention they will not be prescribed that intervention again. This is assumed to include combinations of therapy, for example a patient prescribed podophyllin first line would not receive cryotherapy in combination with podophyllin second line.
| First-line intervention | Second-line intervention | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No treatment | Imiquimod 5% cream | Podophyllin 20–25% | Podophyllotoxin 0.5% solution | TCAA acid | CO2 laser therapy | Cryotherapy | Surgical excision | Cryotherapy plus podophyllin 25% | Cryotherapy plus podophyllotoxin 0.15% cream | TCAA plus podophyllin 25% | |
| No treatment | ✗ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Imiquimod 5% cream | ✗ | ✗ | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
| Podophyllin 20–25% | ✗ | 20 | ✗ | 21 | 22 | 23 | 24 | 25 | ✗ | 26 | ✗ |
| Podophyllotoxin 0.5% solution | ✗ | 27 | 28 | ✗ | 29 | 30 | 31 | 32 | 33 | ✗ | 34 |
| TCAA | ✗ | 35 | 36 | 37 | ✗ | 38 | 39 | 40 | 41 | 42 | ✗ |
| CO2 laser therapy | ✗ | 43 | 44 | 45 | 46 | ✗ | 47 | 48 | 49 | 50 | 51 |
| Cryotherapy | ✗ | 52 | 53 | 54 | 55 | 56 | ✗ | 57 | ✗ | ✗ | 58 |
| Surgical excision | ✗ | 59 | 60 | 61 | 62 | 63 | 64 | ✗ | 65 | 66 | 67 |
| Cryotherapy plus podophyllin 25% | ✗ | 68 | ✗ | 69 | 70 | 71 | ✗ | 72 | ✗ | ✗ | ✗ |
| Cryotherapy plus podophyllotoxin 0.15% cream | ✗ | 73 | 74 | ✗ | 75 | 76 | ✗ | 77 | ✗ | ✗ | 78 |
| TCAA plus podophyllin 25% | ✗ | 79 | ✗ | 80 | ✗ | 81 | 82 | 83 | ✗ | 84 | ✗ |
These assumptions were verified through consultation with clinical experts.
Model inputs
A summary of all model inputs, the associated uncertainty and sources of data are presented in Table 24 and described in the remainder of this section. In addition, Table 25 summarises the key model assumptions.
| Input | Mean value | Source | Measure of variance | Lower value | Upper value | Distribution for probabilistic analysis | Comments |
|---|---|---|---|---|---|---|---|
| Duration of treatment (weeks) | |||||||
| No treatment | 6 | Clinical expert opinion | Upper and lower 20% of mean | 4.8 | 7.2 | Normal | – |
| Imiquimod 5% cream | 16 | 12.8 | 19.2 | Normal | |||
| Podophyllin 20–25% | 6 | 4.8 | 7.2 | Normal | |||
| Podophyllotoxin 0.5% cream (sensitivity analysis only) | 4 | 3.2 | 4.8 | Normal | |||
| Podophyllotoxin 0.5% solution | 4 | 3.2 | 4.8 | Normal | |||
| TCAA | 6 | 4.8 | 7.2 | Normal | |||
| CO2 laser therapy | 6 | 4.8 | 7.2 | Normal | |||
| Cryotherapy | 6 | 4.8 | 7.2 | Normal | |||
| Surgical excision | 6 | 4.8 | 7.2 | Normal | |||
| Cryotherapy plus podophyllin 25% | 6 | 4.8 | 7.2 | Normal | |||
| Cryotherapy plus podophyllotoxin 0.15% cream | 6 | 4.8 | 7.2 | Normal | |||
| TCAA plus podophyllin 25% | 6 | 4.8 | 7.2 | Normal | |||
| Probability (%) of complete clearance of AGWs at the end of treatment | |||||||
| No treatment | 7.6 | MTC (see Chapter 3, Assessment of effectiveness) | MTC CrIs (see Chapter 3, Assessment of effectiveness) | 1.1 | 20.9 | MTC CODA | The CODA output from WinBUGS provides a list of all values generated from the full posterior distribution. Therefore, for probabilistic analysis, rather than resampling from the posterior distribution, the output itself has been used |
| Imiquimod 5% cream | 56.1 | 20.3 | 85.0 | ||||
| Podophyllin 20–25% | 62.1 | 56.2 | 68.0 | ||||
| Podophyllotoxin 0.5% cream (sensitivity analysis only) | 73.7 | 38.9 | 93.8 | ||||
| Podophyllotoxin 0.5% solution | 92.6 | 81.9 | 98.4 | ||||
| TCAA | 61.4 | 32.5 | 85.8 | ||||
| CO2 laser therapy | 97.1 | 84.7 | 99.9 | ||||
| Cryotherapy | 71.0 | 38.8 | 92.1 | ||||
| Surgical excision | 84.8 | 44.9 | 99.1 | ||||
| Cryotherapy plus podophyllin 25% | 77.6 | 45.7 | 95.3 | ||||
| Cryotherapy plus podophyllotoxin 0.15% cream | 78.4 | 31.4 | 98.0 | ||||
| TCAA plus podophyllin 25% | 72.8 | 45.3 | 91.2 | ||||
| Duration of follow-up (weeks) | |||||||
| Duration of follow-up | 12 | Clinical expert opinion | Upper and lower 20% of mean | 9.6 | 14.4 | Normal | All treatments were assumed to be associated with the same duration of follow-up |
| Probability (%) of recurrence of AGWs after follow-up | |||||||
| Imiquimod 5% cream | 16.5 | MTC (see Appendix 8) | MTC CrIs (see Appendix 8) | 2.8 | 43.9 | CODA | The CODA output, from WinBUGS provides a list of all values generated from the full posterior distribution. Therefore, for probabilistic analysis, rather than resampling from the posterior distribution, the output itself has been used |
| Podophyllin 20–25% | 41.2 | 31.3 | 51.6 | CODA | |||
| Podophyllotoxin 0.5% solution | 34.6 | 20.0 | 51.4 | CODA | |||
| TCAA | 18.4 | 6.3 | 36.4 | CODA | |||
| Surgical excision | 9.7 | 2.2 | 24.7 | CODA | |||
| TCAA plus podophyllin 25% | 39.4 | 17.2 | 65.3 | CODA | |||
| No treatment | 26.6 | Clinical expert opinion | Upper and lower 20% of mean | 21.3 | 31.9 | Beta | Equal to the average value estimated from the MTC |
| Podophyllotoxin 0.5% cream (sensitivity analysis only) | 34.6 | Set equal to podophyllotoxin solution 0.5% | 20.0 | 51.4 | Beta | Set equal to values estimated for podophyllotoxin solution 0.5% | |
| CO2 laser therapy | 9.7 | Set equal to surgical excision | 2.2 | 24.7 | Beta | Set equal to values estimated for surgical excision | |
| Cryotherapy | 33.0 | Upper and lower 20% of mean | 26.4 | 39.6 | Beta | Clinical expert opinion | |
| Cryotherapy plus podophyllin 25% | 33.0 | Upper and lower 20% of mean | 26.4 | 39.6 | Beta | Clinical expert opinion | |
| Cryotherapy plus podophyllotoxin 0.15% cream | 26.6 | Upper and lower 20% of mean | 21.3 | 31.9 | Beta | Equal to the average value estimated from the MTC | |
| Structural inputs | |||||||
| Duration of persistent warts (weeks) | 12 | Assumption | Upper and lower 20% of mean | 9.6 | 14.4 | Normal | – |
| HRQoL | |||||||
| With AGWs | 0.87 | Woodhall et al.176 | 95% CI | 0.85 | 0.89 | Beta | – |
| Without AGWs | 0.926 | 0.899 | 0.953 | Beta | |||
| Price per item (£) | |||||||
| No treatment | 0.00 | BNF 6839 | Upper and lower 20% of mean | 0.00 | 0.00 | None | Intervention costs are known and are therefore not varied in probabilistic analysis |
| Podophyllotoxin solution 0.5% (Condyline) | 14.49 | 11.59 | 17.39 | None | |||
| Podophyllotoxin solution 0.5% (Warticon) | 14.86 | 11.89 | 17.83 | None | |||
| Podophyllotoxin cream 0.15% | 17.83 | 14.26 | 21.40 | None | |||
| Imiquimod 5% | 48.60 | 38.88 | 58.32 | None | |||
| Podophyllin 10 ml | 0.02 | 0.02 | 0.02 | None | |||
| TCAA | 0.32 | 0.26 | 0.38 | None | |||
| Cryotherapy | 4.27 | 3.42 | 5.12 | None | |||
| CO2 laser therapy | 125.49 | 100.39 | 150.59 | None | |||
| Surgical excision | 156.03 | 124.82 | 187.24 | None | |||
| Number of items per treatment period | |||||||
| Podophyllotoxin solution 0.5% (Condyline) | 1 | Clinical expert opinion | Upper and lower 20% of mean | 0.8 | 1.2 | Gamma | – |
| Podophyllotoxin solution 0.5% (Warticon) | 1 | 0.8 | 1.2 | Gamma | |||
| Podophyllotoxin cream 0.15% | 1 | 0.8 | 1.2 | Gamma | |||
| Imiquimod 5% | 4 | 3.2 | 4.8 | Gamma | |||
| Podophyllin 10 ml | 5 | 4 | 6 | Gamma | |||
| TCAA | 4 | 3.2 | 4.8 | Gamma | |||
| Cryotherapy | 4 | 3.2 | 4.8 | Gamma | |||
| CO2 laser therapy | 1 | 0.8 | 1.2 | Gamma | |||
| Surgical excision | 1 | 0.8 | 1.2 | Gamma | |||
| Cost of persistent warts (£) | |||||||
| Cost of persistent warts | 121.89 | Average cost estimated as twice the average cost of combination and ablative therapy | Upper and lower 20% of mean | 97.51 | 146.27 | None | – |
| Number of clinician appointments | |||||||
| To commence second-line treatment | 1 | Assumption | Upper and lower 20% of mean | 0.8 | 1.2 | Gamma | – |
| Number of additional clinician appointments | |||||||
| No treatment | 0 | Assumption | Upper and lower 20% of mean | 0 | 0 | Gamma | – |
| Imiquimod 5% cream | 0 | 0 | 0 | Gamma | |||
| Podophyllin 20–25% | 5 | 4 | 6 | Gamma | |||
| Podophyllotoxin 0.5% cream | 0 | 0 | 0 | Gamma | |||
| Podophyllotoxin 0.5% solution | 0 | 0 | 0 | Gamma | |||
| TCAA | 4 | 3.2 | 4.8 | Gamma | |||
| CO2 laser therapy | 1 | 0.8 | 1.2 | Gamma | |||
| Cryotherapy | 4 | 3.2 | 4.8 | Gamma | |||
| Surgical excision | 1 | 0.8 | 1.2 | Gamma | |||
| Cryotherapy plus podophyllin 25% | 5 | 4 | 6 | Gamma | |||
| Cryotherapy plus podophyllotoxin 0.15% cream | 4 | 3.2 | 4.8 | Gamma | |||
| TCAA plus podophyllin 25% | 5 | 4 | 6 | Gamma | |||
| For persistent warts | 2 | 1.6 | 2.4 | Gamma | |||
| Cost of appointments (per hour) (£) | |||||||
| Cost of doctor | 292.00 | Curtis217 | Upper and lower 20% of mean | 233.60 | 350.40 | Gamma | – |
| Cost of nurse | 58.00 | 46.40 | 69.60 | Gamma | |||
| Length of appointments (minutes): general (female) | |||||||
| Doctor time (no nurse involved) | 17 | Woodhall et al.176 | Upper and lower 20% of mean | 13.6 | 20.4 | Gamma | – |
| Doctor time (when nurse involved) | 18 | 14.4 | 21.6 | Gamma | |||
| Nurse time (when doctor involved) | 13 | 10.4 | 15.6 | Gamma | |||
| Nurse time (no doctor involved) | 14 | 11.2 | 16.8 | Gamma | |||
| Length of appointments (minutes): general (male) | |||||||
| Doctor time (no nurse involved) | 16 | Woodhall et al.176 | Upper and lower 20% of mean | 12.8 | 19.2 | Gamma | – |
| Doctor time (when nurse involved) | 16 | 12.8 | 19.2 | Gamma | |||
| Nurse time (when doctor involved) | 10 | 8 | 12 | Gamma | |||
| Nurse time (no doctor involved) | 14 | 11.2 | 16.8 | Gamma | |||
| Length of appointments (minutes): laser therapy | |||||||
| Doctor time (no nurse involved) | 38 | Woodhall et al.176 | Upper and lower 20% of mean | 30.4 | 45.6 | Gamma | – |
| Doctor time (when nurse involved) | 38 | 30.4 | 45.6 | Gamma | |||
| Nurse time (when doctor involved) | 45 | 36 | 54 | Gamma | |||
| Proportion of appointments with doctor/nurse: general | |||||||
| Doctor (no nurse involved) | 0.33 | Assumption | Upper and lower 20% of mean | 0.27 | 0.4 | Dirichlet | – |
| Doctor (with nurse) | 0.33 | 0.27 | 0.4 | Dirichlet | |||
| Proportion of appointments with doctor/nurse: laser therapy | |||||||
| Doctor (no nurse involved) | 0.50 | Assumption | Upper and lower 20% of mean | 0.4 | 0.6 | Dirichlet | – |
| Assumption | Justification | Associated sensitivity analysis (if applicable) |
|---|---|---|
| A single episode of AGWs was modelled rather than multiple episodes of AGWs | HRQoL and costs are not expected to differ between first and subsequent AGW episodes; therefore, regardless of whether the patient is experiencing a first or a later episode of AGWs, the model is considered appropriate to quantify the differences in costs and QALYs associated with each treatment There were insufficient data identified from the literature that were associated specifically with a first or recurrent episode of AGWs Previous cost-effectiveness analyses for AGWs have used short-term decision trees to model a single episode of AGWs; therefore, the approach taken reflects the general approach adopted within the literature |
NA |
| Persistent warts were defined as AGWs that have not cleared after two lines of therapy | The definition of persistent warts varies in the literature and in clinical practice | The duration of persistent warts was varied in one-way sensitivity analysis and in probabilistic analysis |
| People achieving complete clearance of AGWs were assumed to be clear at the mid-point of treatment | A simplifying assumption designed to reflect the fact that some patients will clear immediately and some patients will clear towards the end of treatment | In scenario analysis, complete clearance was alternatively set to occur at the start and the end of the treatment period |
| People recurring after complete clearance of AGWs were assumed to recur at the mid-point of follow-up | A simplifying assumption designed to reflect the fact that some patients will recur immediately and some patients will recur towards the end of follow-up | In scenario analysis, recurrence was alternatively set to occur at the start and the end of the follow-up period |
| Follow-up after complete clearance was assumed to be 12 weeks | This assumption was based on clinical expert opinion and was selected to capture any recurrence of AGWs that would be considered part of the original episode of AGWs in clinical practice | The duration of follow-up was varied in one-way sensitivity analysis and probabilistic analysis |
| Data for ‘placebo’ were assumed to represent ‘no treatment’ | This assumption was required because of a lack of clinical data around no treatment. Although acknowledging the weaknesses of this approach, it was considered, on balance, that this was appropriate because of the objective measures of treatment effectiveness used to assess clearance | NA |
| Treatment sequences: ‘no treatment’ will be prescribed first line only, that is, an active intervention will always be prescribed second line; no sequence of treatments will involve the same intervention twice, that is, if a patient fails on one intervention they will not be prescribed that intervention again | These assumptions were based on clinical expert opinion | NA |
| The probability of complete clearance or recurrence for second-line therapy is the same as the probability of complete clearance or recurrence for first-line therapy | This assumption was necessary because of a lack of clinical data available by line of therapy and was verified as reasonable based on clinical expert opinion | NA |
| The probability of complete clearance and recurrence was assumed to be independent of treatment duration | This assumption was necessary because of a lack of clinical data available by line of therapy and was verified as reasonable based on clinical expert opinion | NA |
| The duration of follow-up was assumed to be 12 weeks following complete clearance | This assumption was based on clinical expert opinion and was selected to capture any recurrence of AGWs that would be considered part of the original episode of AGWs in clinical practice | This duration was tested in one-way sensitivity analysis |
| The probabilities of recurrence for treatments not included in the MTC were assumed to be adequately represented by clinical expert opinion | This assumption was necessary because of a lack of clinical data available for a number of interventions | Recurrence rates were tested in scenario analysis, one-way sensitivity analysis and threshold analysis |
| It was assumed that all appointments take place within GUM clinics | This was a simplifying assumption that is not expected to impact on the model results. In clinical practice, the majority of people with AGWs are treated in GUM clinics; however, a proportion of patients may be treated by their GP or in hospital | NA |
| People treated with clinician-applied topical or ablative therapies, either as monotherapy or as part of a combination therapy, were assumed to require additional appointments at which the therapy is applied. The number of appointments required was assumed to be adequately captured using clinical expert opinion | This assumption was based on clinical expert opinion and was selected to capture resource costs associated with clinician-applied therapies | The number of appointments required was varied in one-way sensitivity analysis |
| It was assumed that the type of appointment in GUM clinics (doctor led, nurse led or doctor with nurse) was equally split | This was a simplifying assumption | The proportion of appointments by type was varied in one-way sensitivity analysis |
Complete clearance at the end of treatment
Data for complete clearance at the end of treatment were identified, extracted and synthesised as described in the primary analysis in Chapter 3 (see Results). The probability of complete clearance at the end of the treatment period for each included intervention obtained from these analyses are summarised in Table 26 and presented graphically in Figure 17.
| Intervention | Mean probability of complete clearance at the end of treatment (%) | CrI around the mean (%) | |
|---|---|---|---|
| Lower 2.5% CrI | Upper 97.5% CrI | ||
| No treatment | 7.6 | 1.1 | 20.9 |
| Imiquimod 5% cream | 56.1 | 20.3 | 85.0 |
| Podophyllin 20–25% (clinician applied) | 62.1 | 56.2 | 68.0 |
| Podophyllotoxin 0.5% cream (sensitivity analysis only) | 73.7 | 38.9 | 93.8 |
| Podophyllotoxin 0.5% solution | 92.6 | 81.9 | 98.4 |
| TCAA | 61.4 | 32.5 | 85.8 |
| CO2 laser therapy | 97.1 | 84.7 | 99.9 |
| Cryotherapy | 71.0 | 38.8 | 92.1 |
| Surgical excision | 84.8 | 44.9 | 99.1 |
| Cryotherapy plus podophyllin 25% | 77.6 | 45.7 | 95.3 |
| Cryotherapy plus podophyllotoxin 0.15% cream | 78.4 | 31.4 | 98.0 |
| TCAA plus podophyllin 25% | 72.8 | 45.3 | 91.2 |
FIGURE 17.
Probability of complete clearance at the end of treatment; random-effects MTC, base-case clinical analysis (lines indicate 95% CrIs).

Within the model, the same estimates of complete clearance at the end of treatment are used for first- and second-line treatment. It is therefore assumed that the probability of complete clearance at the end of treatment is the same, regardless of line of therapy. This assumption was necessary because of a lack of clinical data available by line of therapy and was verified as reasonable based on clinical expert opinion.
The results of the analysis imply that no treatment, as assessed through placebo treatment in the included clinical trials, is, on average, the least effective treatment for complete clearance of AGWs at the end of treatment. No treatment was associated with an average probability of complete clearance of 7.6% and was statistically significantly less effective at the 95% level when compared with all other interventions included within the analysis, with the exception of imiquimod 5% cream.
The most effective treatment for complete clearance at the end of treatment, on average, was found to be CO2 laser therapy. CO2 laser therapy was associated with an average probability of complete clearance of 97.1%. The probability of complete clearance for CO2 laser therapy was statistically significantly greater at the 95% level than for podophyllin 20–25% and no treatment.
The CrIs around the estimates of treatment effect were generally wide, with only one further statistically significant difference in treatment effect at the 95% level, with podophyllotoxin 0.5% solution found to be statistically significantly more effective than podophyllin 20–25%.
The findings of this analysis in relation to podophyllotoxin 0.5% solution and imiquimod are of particular interest. Imiquimod was found to be, on average, the second least effective treatment for complete clearance of AGWs at the end of treatment whereas podophyllotoxin 0.5% solution was found to be, on average, the second most effective treatment for complete clearance of AGWs at the end of treatment. These findings contradict the results of cost-effectiveness analyses identified in the cost-effectiveness literature review (see Systematic review of existing cost and cost-effectiveness evidence), which would more naturally imply the opposite finding, and the generally held view within clinical practice that imiquimod 5% cream is more effective than podophyllotoxin 0.5% solution.
The discrepancies between the findings of the clinical literature review and synthesis (see Chapter 3, Results) and the cost-effectiveness analyses identified from the systematic review of the economic literature (see Systematic review of existing cost and cost-effectiveness evidence) were investigated and are discussed below.
The following five cost-effectiveness analyses presented in Systematic review of existing cost and cost-effectiveness evidence compared the use of imiquimod with the use of podophyllotoxin for AGWs:
-
Walczak164 (Poland)
-
Lafuma165 (France)
-
Williams and von Krogh166 (UK)
-
Alam et al. 167 (USA)
-
Fagnani168 (France).
In four of these analyses, imiquimod was found to be more effective than podophyllotoxin at clearing AGWs by the end of treatment. 164–166,168 The estimated probability of complete clearance at the end of treatment for all five studies and the references used to inform these values are presented in Table 27.
| Study | Probability of complete clearance at the end of treatment (%) | Source | |
|---|---|---|---|
| Podophyllotoxin | Imiquimod | ||
| Walczak 2009164 | 19.6 | 42.9 | NR |
| Lafuma 2003165 | 37.0 | 49.5 | Podophyllotoxin: Tyring et al.,68 imiquimod: Edwards et al.118 |
| Williams 2003166 | 49.1 | 52.3 | Podophyllotoxin: average estimate from nine studies;61,62,115,122–127 imiquimod: average estimate from six studies68,115,118,125,161,162 |
| Alam 2001167 | 63 | 50 | A combination of data from two clinical literature reviews218,219 and three studies169,171,220 for podophylloxin and two studies170,220 for imiquimod |
| Fagnani 2000168 | 19.6 | 42.9 | Podophyllotoxin: Tyring et al.,68 imiquimod: Edwards et al.;118 using data for ‘clear 3 months after treatment’ |
It was not possible to verify the figures used in Walczak164 or Alam et al. ;167 in the study by Walczak164 the references were not provided and in the study by Alam et al. 167 the method of reaching a consensus estimate was not described. It is noted that the probabilities of complete clearance reported in Walczak164 for podophyllotoxin and imiquimod are identical to those presented in Fagnani;168 however, it is not possible to confirm that these data are from the same sources.
For the remaining studies, no study used systematic review methods to identify their data. In Lafuma165 and Fagnani168 two studies were selected without description of how they were identified, with their inclusion justified because the methodology in the two studies was determined to be similar. In the study by Williams and von Krogh,166 only placebo-controlled trials were identified.
The four studies providing references all included data that were excluded from the analysis described in Chapter 3. Data from Greenburg et al. 62 and Tyring et al. 68 were excluded because of an identified high risk of bias. Data from Syed et al. 122 were not included because these data related to podophyllotoxin cream rather than podophyllotoxin solution; a decision was made within this analysis (see Chapter 3, Quantity and quality of research available) to analyse the efficacy of podophyllotoxin cream separately. Data from Syed et al. 161,162 were not included because these data related to imiquimod 2% cream rather than imiquimod 5% cream. Data from four further studies169–171,220 were excluded because these studies were not prospective RCTs. No study included prospective RCT data that were not identified by the systematic review of the clinical literature reported in Chapter 3.
In addition, in the studies by Lafuma,165 Williams and von Krogh166 and Fagnani,168 the estimates of the probability of complete clearance were combined using ‘naive’ indirect comparison, that is, data were taken from a single arm for each trial and compared as if they were from the same trial. In the study by Williams and von Krogh,166 in which multiple data sources for one treatment were considered, data for the same treatment were pooled to produce a crude average. In the studies by Alam and Stiller167 and Walczak,164 no information was provided around how data were combined.
The differences in methodological approach and the included trials may explain the difference in results between this analysis and previous cost-effectiveness analyses. Specifically, for this analysis, a systematic review methodology was followed, data were excluded if they were subject to a high risk of bias and evidence was synthesised using meta-analytical techniques rather than through ‘naive’ indirect comparison.
Recurrence of anogenital warts within 12 weeks of complete clearance
Data for recurrence of AGWs within 12 weeks of complete clearance were identified, extracted and synthesised as described in Appendix 8. The probability of recurrence for each included intervention obtained from these analyses is summarised in Table 28 and presented graphically in Figure 18.
| Intervention | Mean probability of recurrence after 12 weeks (%) | Lower 2.5% CrI (%) | Upper 97.5% CrI (%) |
|---|---|---|---|
| Imiquimod 5% cream | 16.5 | 2.8 | 43.9 |
| Podophyllotoxin 0.5% solution | 34.6 | 20.0 | 51.4 |
| Podophyllin 20–25% | 41.2 | 31.3 | 51.6 |
| TCAA | 18.4 | 6.3 | 36.4 |
| TCAA plus podophyllin 25% | 39.4 | 17.2 | 65.3 |
| Surgical excision | 9.7 | 2.2 | 24.7 |
FIGURE 18.
Network meta-analysis results: probability of recurrence within 12 weeks of complete clearance of AGWs, fixed effects (lines indicate 95% CrIs).
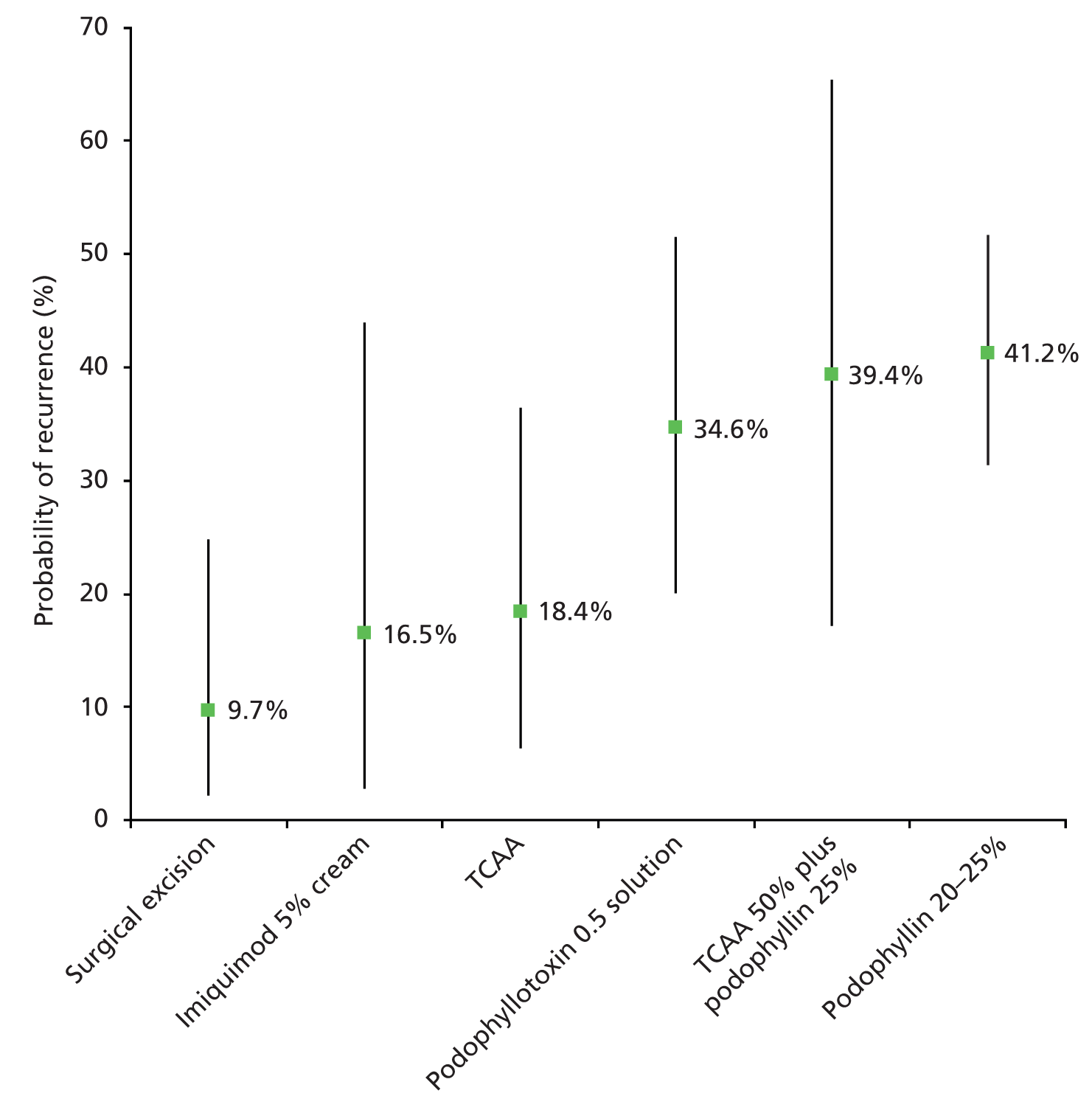
Similar to the results for complete clearance, the CrIs associated with the mean probabilities of recurrence were generally wide, indicating a high degree of uncertainty in the results. The estimated mean probability of recurrence of AGWs ranged from 9.7% (surgical excision) to 41.2% (podophyllin 20–25%). Surgical excision was associated with a statistically significantly (at the 95% confidence level) lower likelihood of recurrence compared with podophyllin 20–25%; no other differences between treatments were found to be statistically significant.
The data presented for recurrence within 12 weeks of complete clearance were subject to two key limitations:
-
data were not identified for all interventions included within the analysis
-
data analysed included follow-up periods of 3–12 months, in contrast to the required data for the model including follow-up for up to 3 months.
These issues are discussed in greater detail in the following sections.
Data were not identified for all interventions included within the analysis
The following interventions for which data were identified for the outcome of complete clearance had no data identified for the outcome of recurrence: no treatment, podophyllotoxin cream (sensitivity analysis only), CO2 laser therapy, cryotherapy, cryotherapy plus podophyllin 25% and cryotherapy plus podophyllotoxin 0.15% cream.
To include recurrence data within the base-case model for these interventions, clinical expert opinion was sought. The resulting probabilities of recurrence used within the base-case economic analysis are presented in Table 29.
| Intervention | Mean probability of recurrence 12 weeks after complete clearance of AGWs (%) | Lower 2.5% CrI (%) | Upper 97.5% CrI (%) | Source |
|---|---|---|---|---|
| No treatment | 26.6 | NA | Clinical opinion (average of values estimated from the MTC) | |
| Imiquimod 5% cream | 16.5 | 2.8 | 43.9 | MTC (see Appendix 8) |
| Podophyllin 20–25% | 41.2 | 31.3 | 51.6 | MTC (see Appendix 8) |
| Podophyllotoxin 0.5% cream (sensitivity analysis only) | 34.6 | NA | Clinical opinion (equal to value for podophyllotoxin solution 0.5%) | |
| Podophyllotoxin 0.5% solution | 34.6 | 20.0 | 51.4 | MTC (see Appendix 8) |
| TCAA | 18.4 | 6.3 | 36.4 | MTC (see Appendix 8) |
| CO2 laser therapy | 9.7 | NA | Clinical opinion (equal to value for surgical excision) | |
| Cryotherapy | 33.0 | NA | Clinical opinion | |
| Surgical excision | 9.7 | 2.2 | 24.7 | MTC (see Appendix 8) |
| Cryotherapy plus podophyllin 25% | 33.0 | NA | Clinical opinion | |
| Cryotherapy plus podophyllotoxin 0.15% cream | 26.6 | NA | Clinical opinion (average of values estimated from the MTC) | |
| TCAA plus podophyllin 25% | 39.4 | 17.2 | 65.3 | MTC (see Appendix 8) |
Data analysed included follow-up periods of 3–12 months, in contrast to the required data for the model including follow-up for up to 3 months
The data identified from the systematic review of the clinical literature for recurrence of AGWs were limited; thus, to provide a connected network from which data on recurrence could be estimated for use within the economic analysis, data from the assessment of recurrence at 3–12 months were combined in a MTC (see Appendix 8).
The baseline treatment effect was estimated using only data from 3 months. However, for the relative effects, it is necessary to assume that the relative difference in probability of recurrence between interventions will not differ over time. This is acknowledged as a weakness of the analysis and an area where further research is considered important.
To test the importance of recurrence data within the model, a number of sensitivity analyses were carried out:
-
setting the same probability of recurrence for all interventions:
-
setting the probability of recurrence to the average rate estimated from the MTC
-
setting the probability of recurrence to 0%
-
setting the probability of recurrence to 100%.
-
The results of these analyses are described in Sensitivity analysis.
Adverse events
Adverse events were not included in the economic analysis. This approach was taken after consultation with clinical experts, after review of previous approaches to cost-effectiveness analyses for AGWs and because of a lack of clinical data.
Clinical experts advised that adverse events during the treatment of AGWs are generally mild and reversible, and do not usually require prescribed treatment. In general, it is expected that the clinician would advise the patient experiencing an adverse event to cease using the medication and, if necessary, to apply a soothing cream (available over the counter) to the affected area.
Moreover, only 1 out of 10 cost-effectiveness studies identified from the systematic literature review included adverse events within the economic evaluation. 64 This economic evaluation by Lacey et al. 64 was trial based and used adverse event data collected within the trial. The authors concluded that side effects were not a limiting factor for treatments within the trial and accounted for approximately 0.6–6.7% of total direct costs for the treatments analysed within the study.
Finally, the reporting of adverse events within the trials identified in the systematic review of clinical effectiveness was poor (see Chapter 3, Assessment of effectiveness). Given the absence of reliable data for adverse events, it was considered appropriate to omit these from the economic model.
Health-related quality of life
Systematic review of existing health-related quality-of-life data
A systematic review was carried out in September 2013 to identify relevant published HRQoL evidence to populate the economic model. The following databases were searched from inception:
-
MEDLINE (Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE)
-
EMBASE (Ovid EMBASE)
-
CENTRAL (The Cochrane Library)
-
HTA database (The Cochrane Library)
-
NHS EED (The Cochrane Library).
The search strategy for all databases combined terms to capture AGWs and HRQoL. Full details of the search terms are presented in Appendix 1. In addition to searching the above databases, reference lists of identified studies were reviewed for any potentially relevant studies. No restrictions on language or setting were applied to any of the searches. Studies were assessed for inclusion based on the criteria outlined in Table 30.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
The systematic review was updated in March 2014. The search strategy remained the same as outlined above; however, results were limited from 2013 to March 2014 to identify only additional relevant studies.
In total, 468 studies were identified from the September 2013 database search (Figure 19). Two health economists reviewed all citations. Of these, 148 were identified as duplicates and 201 studies were excluded on the basis of title and abstract. A total of 119 papers were therefore identified as potentially relevant. Of these papers, 12 were identified from the abstract as either reporting condition-specific measures of HRQoL or generic non-preference-based measures of HRQoL, and 107 papers were identified as reporting possible generic, preference-based measures of HRQoL (Q1, see Table 30). If it was unclear which type of HRQoL measure was included in the study, the reviewer was inclusive and labelled the study as including a potential generic, preference-based measure of HRQoL.
FIGURE 19.
Identified HRQoL studies, September 2013 search.
The 12 studies identified as reporting either condition-specific measures of HRQoL or generic, non-preference-based measures of HRQoL were provisionally included, that is, these studies were not ordered in full in the first instance. However, as suitable studies reporting the use of generic, preference-based measures of HRQoL were identified, the 12 studies reporting either condition-specific measures of HRQoL or generic, non-preference-based measures of HRQoL were not included in the review. This is because a generic, preference-based measure of HRQoL, such as the European Quality of Life-5 Dimensions (EQ-5D), was considered preferable for use within an economic evaluation, based on the NICE Guide to the Methods of Technology Appraisal. 221 It was therefore considered appropriate to assess the suitability of condition-specific or generic non-preference-based measures of HRQoL, if, and only if, no suitable generic, preference-based measures of HRQoL were identified.
Following review of the 107 studies potentially reporting generic, preference-based measures of HRQoL, 10 studies were included in the review; seven studies32,176,222–226 were identified as reporting generic non-preference-based measures of QoL and three studies227–229 were identified as reporting condition-specific measures of QoL. In addition to the studies identified through the database search, three studies230–232 were identified through review of reference lists as reporting generic, preference-based measures of HRQoL.
A total of 88 studies were excluded. Of these, two were not retrievable; two were duplicate references; 57 did not contain any QoL data; and 27 were excluded on the basis that the study reported a measure of QoL from another study. The most commonly cited reference was a conference abstract by Myers et al. 233 The figures within the study were described as being elicited using time trade-off methods and therefore may constitute a generic, non-preference-based measure of HRQoL. The abstract was presented at the 21st International Papillomavirus Conference in Mexico in 2004. Unfortunately, it was not possible to retrieve the full paper for this reference as the study appears to be unpublished. Consequently, it was not possible to verify these QoL values.
A further 49 papers were identified from the updated search in March 2014. Of these, four papers were identified as potentially relevant and ordered for full review, of which three234–236 were excluded on the basis of the full paper and one237 was identified as including generic, preference-based HRQoL data.
A total of 14 studies from the September 2013 (13 studies32,176,222–232) and March 2014 (one study237) searches therefore reported relevant generic, preference-based HRQoL data. Information on the populations, health states, instruments and utility values reported in these studies is presented in Appendix 2; a summary of the HRQoL instrument used in each included study is presented in Table 31.
| Study | Country | Instrument |
|---|---|---|
| Dominiak-Felden 2013237 | UK | EQ-5D |
| Mennini 2013222 | Italy | EQ-5D |
| Shi 2012223 | China | EQ-5D |
| Drolet 2011227 | Canada | EQ-5D, SF-6D |
| Mennini 2011224 | Italy | EQ-5D |
| Senecal 2011225 | Canada | EQ-5D |
| Woodhall 2011176 | England, Northern Ireland | EQ-5D |
| Langley 2010228 | UK, France, Spain, Italy, Germany | SF-6D |
| Marra 2009229 | Canada | EQ-5D, SF-6D |
| Woodhall 200832 | England | EQ-5D |
| Brisson 2007226 | Canada | EQ-5D |
| Identified from review of reference lists of identified studies | ||
| Mennini 2010232 | Italy | Time trade-off |
| Fiander 2010230 | UK | EQ-5D |
| Fiander 2010231 | UK | EQ-5D |
Data from the UK were considered to be most relevant for this review in the first instance. Of the 14 identified studies, six contained HRQoL data for people from the UK. 32,176,228,230,231,237 These studies are described in further detail below.
Dominiak-Felden et al. 237 reported data from a large, multicentre, observational study carried out in the UK to investigate the impact of HPV-related genital diseases on QoL and psychosocial well-being. A total of 2502 individuals aged 18–64 years from 15 UK centres were screened for inclusion into the study between May 2008 and March 2009. Of those screened, 1512 people met the screening criteria and 1272 were included in the study after confirmation of diagnosis.
A subset of 186 people was found to have a current episode of genital warts. These participants were, on average, aged 28 years and 46% were female. It was not reported what proportion of patients were experiencing their first episode compared with a recurrent episode. People with a current episode of genital warts were invited to complete the EQ-5D questionnaire; the results are presented in Table 32.
| Estimate | Women | Men | ||||
|---|---|---|---|---|---|---|
| Sample size | Mean estimate | Standard deviation | Sample size | Mean estimate | Standard deviation | |
| EQ-5D index for people with genital warts, unweighted | 101 | 0.84 | 0.16 | 85 | 0.89 | 0.17 |
| Estimate | Women and men | |||||
| Sample size | Mean estimate | Standard deviation | ||||
| EQ-5D index for people with genital warts, weighted to reflect the age distribution of the UK general population | 186 | 0.9 | 0.13 | |||
| EQ-5D index for the general population | 2583 | 0.89 | NR | |||
| EQ-5D index for people with genital warts, weighted to reflect the age distribution of the UK general population, aged 18–24 years | NR | 0.86 | NR | |||
| EQ-5D index, mean for the general population aged 18–24 years | NR | 0.94 | NR | |||
| EQ-5D index for people with genital warts, weighted to reflect the age distribution of the UK general population, aged 25–34 years | NR | 0.87 | NR | |||
| EQ-5D index, mean for the general population aged 25–34 years | NR | 0.93 | NR | |||
The authors found that there was no significant difference in the overall EQ-5D score between people with genital warts and the general population when scores were weighted to match the age and sex profile of the general population. However, the authors found that people with genital warts reported, on average, statistically significantly greater issues with anxiety and depression than the age- and sex-matched general population (p < 0.001) and fewer issues with the remaining dimensions of the EQ-5D: mobility (p = 0.007), self-care (p = 0.083), usual activities (p = 0.005), pain and discomfort (p = 0.158).
The authors considered age subgroups (18–24 years and 25–34 years) and found statistically significant differences in HRQoL compared with the age- and sex-matched general population (see Table 32); no sample sizes were stated for these analyses.
Woodhall et al. 176 reported a study carried out in seven sexual health clinics in England (six clinics) and Northern Ireland (one clinic). Between August 2009 and February 2010, a total of 895 people aged ≥ 16 years with a current diagnosis of genital warts were invited to complete an EQ-5D questionnaire. The average age of respondents was 28 years (range 16–73 years) and 65% of people were attending for their first episode. EQ-5D scores from people with genital warts were compared with age- and sex-matched average scores from the UK population. The authors noted that HRQoL associated with genital warts differed between women and men; therefore, a utility score for a health state including genital warts was presented by gender, as was an estimate of disutility associated with genital warts (Table 33).
| Estimate | Women (n = 400)a | Men (n = 494)a | All (n = 895) |
|---|---|---|---|
| EQ-5D index, mean (95% CI) for people with genital warts | 0.87 (0.83 to 0.90) | 0.88 (0.86 to 0.90) | 0.87 (0.85 to 0.89) |
| Estimated disutility associated with genital warts, mean (95% CI) | 0.063 (0.029 to 0.097) | 0.043 (0.021 to 0.065) | 0.056 (0.038 to 0.074) |
Furthermore, Woodhall et al. 176 noted that no significant difference in HRQoL was observed between the first and recurrent episodes of AGWs; however, disutility was found to differ between age groups and this difference was statistically significant (p < 0.0001). The highest loss of QoL was seen in women aged 16–19 years and men aged 35–44 years. The numbers of patients in each of the different age groups were not presented.
Within the study, a further two UK studies reporting EQ-5D scores for people with AGWs were referenced. Both studies were conference abstracts published as part of the Eurogin 2010 Conference. 230,231 Neither study was identified in the original search; however, these studies were extracted in full and are presented in Appendix 2.
Fiander and Cohet230,231 present data relating to an observational, cross-sectional study in which 1264 subjects (women and men) aged 18–64 years with HPV-related diseases were recruited from 15 community and hospital health-care clinics; the first abstract reports results relating to women230 and the second reports results relating to men. 231 The study is related to the full paper by Dominiak-Felden et al. ,237 described above. The abstacts present EQ-5D data for a subset of the full study population, specifically, women and men aged 18–25 years with genital warts. The sample size for each analysis is not reported. The EQ-5D score for genital warts was estimated to be 0.83 and 0.89 for women and men aged 18–25 years, respectively. No estimates of uncertainty were presented.
Langley et al. 228 presented a conference abstract in which results from an internet-based survey carried out in the UK, France, Spain, Italy and Germany were published. A reported 53,524 people responded to the survey, with 521 reporting external genital warts. Respondents completed the Short Form questionnaire-6 Dimensions (SF-6D), from which the authors carried out a regression analysis controlling for presence or absence of genital warts, sociodemographic characteristics, health risk factors and the Charlson Comorbidity Index (an index that predicts the 10-year mortality rate for a person who may have a range of comorbid conditions). The disutility associated with external genital warts using the SF-6D (utilities scaled as 0–100) was consequently estimated as –2.47 (95% CI –3.58 to –1.36).
Woodhall et al. 32 reported a study of 81 adults attending the York GUM clinic with new, recurrent or persistent genital warts. Participants were invited to complete the EQ-5D questionnaire. EQ-5D scores from participants were compared with those in a control group of 1977 people in the same age range. Unadjusted EQ-5D scores were presented for people with and without genital warts. The authors estimated the mean EQ-5D score with genital warts (n = 81) to be 0.90 and the mean EQ-5D score without genital warts (n = 1977) to be 0.91. Adjusting for age and gender, the authors estimated a mean difference in EQ-5D score of 0.039 (95% CI 0.005 to 0.078) between people with and people without genital warts.
Health-related quality-of-life data selected for the economic analysis
Data from Woodhall et al. 176 were selected as the most relevant HRQoL data for the economic analysis. The baseline characteristics of the population presented in the study by Woodhall et al. 176 were considered to be reflective of the characteristics of the population seen in clinical practice in England and Wales. Moreover, the data presented in this study had a UK focus (England and Northern Ireland) and the study provided the largest reported sample of patients (895 vs. 186,237 8132 and 521228). In addition, the data were based on the most recent sample; although the data presented by Dominiak-Felden et al. 237 were published in 2013 (vs. 2011 for the study by Woodhall et al. 176), the questionnaires were completed in 2009–10 in the study by Woodhall et al. 176 and in 2008–9 in the study by Dominiak-Felden et al. 237
The population in Woodhall et al. 176 is described as people with ‘genital warts’. Although this differs from the focus for this analysis (i.e. both genital warts and AGWs), clinical experts advised that the terms ‘genital warts’ and ‘anogenital warts’ are often used interchangeably and the term ‘genital warts’ is considered appropriate to capture both genital warts and AGWs. It is therefore considered that the population is likely to include those with both genital warts and AGWs.
For the economic analysis, a mean estimate of HRQoL with and without AGWs was required; the estimates used within the economic analysis are presented in Table 34.
| Estimate | Mean | 95% CI | Source |
|---|---|---|---|
| Utility score with AGWs | 0.87 | 0.85 to 0.89 | Woodhall et al.,176 EQ-5D index for genital warts, women and men (n = 895) |
| Utility score without AGWs | 0.926 | 0.899 to 0.953 | Estimate of utility with genital warts from Woodhall et al.176 plus estimate of disutility associated with genital warts, also from Woodhall et al.176 |
In the sensitivity analysis, to test the impact of using alternative HRQoL data, a scenario focusing on young adults was carried out. Based on findings from Woodhall et al. 176 and Dominiak-Felden et al. ,237 HRQoL in young adults appears to be more greatly impacted by AGWs than HRQoL in other age groups. Data for this scenario were taken from the study by Dominiak-Felden237 for those aged 18–24 years (HRQoL 0.86 with AGWs vs. 0.94 without AGWs).
Costs overview
Costs of intervention acquisition and costs of resource use were incorporated in the analysis and inflated, when required, to 2013 prices; the cost of treating adverse events was not included in the analysis (see Model inputs, Adverse events). Table 35 provides a summary of the acquisition costs and resource costs per treatment course for first- and second-line interventions modelled in the base case. In addition, Table 35 presents the one-off intervention acquisition cost and resource use cost associated with persistent warts. The calculations are described in greater detail in the following sections.
| Intervention | Average acquisition cost per course of treatment (£) | Average resource use cost per course of treatment (£) | Average total cost per course of treatment (£) | ||
|---|---|---|---|---|---|
| First line | Second line | First line | Second line | ||
| No treatment | 0 | 0.00 | 62.12 | 0.00 | 62.12 |
| Imiquimod 5% cream | 194.40 | 0.00 | 62.12 | 194.40 | 256.52 |
| Podophyllin 20–25% | 0.11 | 310.60 | 372.72 | 310.71 | 372.83 |
| Podophyllotoxin 0.5% cream (sensitivity analysis only) | 17.83 | 0.00 | 62.12 | 17.83 | 79.95 |
| Podophyllotoxin 0.5% solution | 14.68 | 0.00 | 62.12 | 14.68 | 76.79 |
| TCAA | 1.38 | 248.48 | 310.60 | 249.86 | 311.98 |
| CO2 laser therapy | 135.07 | 206.68 | 268.80 | 341.75 | 403.87 |
| Cryotherapy | 18.38 | 248.48 | 310.60 | 266.86 | 328.98 |
| Surgical excision | 156.03 | 206.68 | 268.80 | 362.71 | 424.83 |
| Cryotherapy plus podophyllin 25% | 18.49 | 310.60 | 372.72 | 329.09 | 391.21 |
| Cryotherapy plus podophyllotoxin 0.15% cream | 36.21 | 248.48 | 310.60 | 284.69 | 346.81 |
| TCAA plus podophyllin 25% | 1.49 | 310.60 | 372.72 | 312.08 | 374.20 |
| Persistent warts | 121.89 | 413.37 | 535.26 | ||
Costs: intervention acquisition costs
No treatment
It is assumed that there is no acquisition cost associated with no treatment.
Patient-applied topical interventions
It is assumed that imiquimod 5% cream, podophyllotoxin 0.5% solution and podophyllotoxin 0.15% cream are applied by patients at home. The cost calculations for these treatments are presented in Table 36.
| Intervention | Brand name | Quantity per item | Number of items per treatment period | Total cost (£) | Source of cost data |
|---|---|---|---|---|---|
| Podophyllotoxin 0.5% solution | Condyline | 3.5 ml | 1 | 14.49 | BNF 6839 |
| Warticon | 3 ml | 1 | 14.86 | ||
| Imiquimod 5% cream | Aldara | 12 sachets | 4 | 194.40 | |
| Podophyllotoxin 0.15% cream | Warticon | 5 g | 1 | 17.83 |
It was assumed that one item for podophyllotoxin 0.5% solution and podophyllotoxin 0.15% cream would be sufficient for the full treatment period. For imiquimod, it was assumed that four items were required per treatment period. It is acknowledged that, in clinical practice, some patients may be prescribed fewer imiquimod items on the basis that a patient may use fewer sachets per week; however, in the base case, the full treatment regimen was modelled. These assumptions were verified with clinicians and varied in a one-way sensitivity analysis to test their impact on the model results.
Provider-applied topical interventions
Podophyllin and TCAA were assumed to be applied by a clinician at a clinic. The cost calculations for these treatments are presented in Table 37.
| Intervention | Cost per use (£) | Cost year | Number of uses per treatment period | Total cost in current prices (£) | Source of cost data |
|---|---|---|---|---|---|
| Podophyllin 10 ml | 0.02 | 2010 | 5 | 0.11 | Woodhall et al.176 |
| TCAA | 0.32 | 4 | 1.38 |
No published list price for podophyllin or TCAA was identified. Thus, the costs for these interventions were estimated using data extracted from the systematic review of the cost and cost-effectiveness literature (see earlier in this chapter).
A cost for podophyllin was reported in two UK cost-effectiveness studies64,171 and two UK costing studies. 172,176 The most recent study reporting a price for podophyllin was that by Woodhall et al. 176 In this study, a cost of £0.02 was estimated for each use of podophyllin. In the model, it was assumed that five sessions would typically be required to apply a course of treatment. This was based on consultation with a clinical expert, who estimated that a typical patient would receive between four and six sessions to apply a course of treatment with podophyllin. This was tested in sensitivity analysis. The cost estimated within the study by Woodhall et al. 176 was inflated to current prices using the Hospital and Community Health Services (HCHS) inflation index from the Unit Costs of Health and Social Care 2013217 and was varied in sensitivity analysis.
A cost for TCAA was reported in one UK cost-effectiveness study171 and one UK costing study. 176 The most recent study reporting a price for TCAA was again that by Woodhall et al. 176 In this study, a cost of £0.32 was estimated for each use of TCAA. In the model, it was assumed that four sessions would typically be required to apply a course of treatment. This was on the basis of consultation with a clinical expert, who estimated that a typical patient would receive between three and five sessions to apply a course of treatment with TCAA. As with podophyllin, the cost estimated within the study by Woodhall et al. 176 was inflated to current prices using the HCHS inflation index217 and was varied in sensitivity analysis.
Provider-applied ablative interventions
Cryotherapy, CO2 laser therapy and surgery were assumed to be carried out by a provider at a clinic. The cost calculations for these treatments are presented in Table 38.
| Intervention | Cost per use (£) | Cost year | Number of uses per treatment period | Total cost in current prices (£) | Source of cost data |
|---|---|---|---|---|---|
| Cryotherapy | 4.27 | 2010 | 4 | 18.38 | Woodhall et al.176 |
| CO2 laser therapy | 125.49 | 2010 | 1 | 135.07 | |
| Surgical excision | 156.03 | 2012/13 | 1 | 156.03 | Department of Health238 |
The costs applied for these interventions were estimated using data extracted from the systematic review of the cost and cost-effectiveness literature.
A cost for cryotherapy was reported in one UK cost-effectiveness study171 and two UK costing studies. 175,176 The most recent study reporting a price for cryotherapy was that by Woodhall et al. 176 In this study, a cost of £4.27 was estimated for each use of cryotherapy. In the model, it was assumed that four sessions would typically be required to apply a course of treatment. This was on the basis of consultation with a clinical expert, who estimated that a typical patient would receive between three and five sessions of cryotherapy. As before, the cost estimated within the study by Woodhall et al. 176 was inflated to current prices using the HCHS inflation index217 and was varied in sensitivity analysis.
A cost for CO2 laser therapy was reported in one UK costing study. 176 In this study, a cost of £125.49 was estimated per use. In the model, it was assumed that one treatment would typically be required per treatment period. This was on the basis of consultation with a clinical expert, who estimated that a typical patient would receive one treatment with CO2 laser therapy. As before, the cost was inflated to current prices using the HCHS inflation index217 and was varied in sensitivity analysis.
For this analysis, the cost of surgical excision was taken from NHS reference cost values for 2012/13. 238 Specifically, the average cost associated with the outpatient procedure codes MA22Z and MA23Z (‘Minor Lower Genital Tract procedures’), weighted by activity, was applied within the model (£156). In the model, it was assumed that one session would typically be required per treatment period. This was on the basis of consultation with a clinical expert. It was assumed that the cost of an appointment in a GUM clinic would also be required. Reference costs were selected in place of national tariff data because reference cost figures are based on the actual reported estimated costs of coded procedures, whereas national tariff data represent the payments received by providers from the Department of Health for the coded procedures.
Combination therapies
The costs applied for combination treatments were estimated as the sum of the costs of each individual element, as shown in Tables 36–38. A summary of the applied costs is provided in Table 39.
Persistent warts
The one-off intervention cost associated with persistent warts applied in the model was £121.89. The description of how this figure was estimated is provided below.
Following review of the cost papers identified from the economic literature review, it was noted that Lanitis51 reported data relating to the number of additional appointments required for people with persistent warts. Within the study, a total of four consultants were interviewed. These consultants estimated that people with persistent warts typically required two additional appointments with a clinician compared with those without persistent warts.
Within the model, it was assumed that a further two visits would result in an average of two further lines of prescribed therapy. This is a simplifying assumption based on lack of data, as it is acknowledged that some people with persistent warts may be prescribed more or fewer lines of therapy. Additionally, the therapies prescribed for persistent warts were assumed to consist of either combination treatments or ablative therapies. This was based on the review of cost studies identified in the literature review and on consultation with clinical experts. Two of the identified cost studies presented the results of physician interviews around the likely treatments prescribed for persistent warts. 51,172
In the study by Lanitis,51 four consultants were interviewed. These consultants estimated that, for keratinised persistent warts, approximately 37.5% of people would be treated with ablative or combination therapy, with the remainder receiving topical monotherapy. For non-keratinised warts, approximately 25% of people were expected to be treated with ablative or combination therapy, with the remainder receiving topical monotherapy.
In the study by Brown et al. ,172 six GUM physicians were interviewed. The expected treatment for people with persistent warts was estimated by the physicians to be:
-
cryotherapy (23%)
-
diathermy procedure (23%)
-
combination of a procedure and topical cream (22%)
-
surgery (6%)
-
topical cream (27%), of which imiquimod (98%) and podophyllotoxin (2%).
In addition, the clinical experts consulted for this project suggested that, in UK clinical practice, a patient with persistent warts would generally be treated with ablative therapy, most likely CO2 laser therapy.
To take into account the variation in reported treatment for people with persistent warts, an average cost associated with combination therapies (cryotherapy plus podophyllin 25%, cryotherapy plus podophyllotoxin 0.15% cream, TCAA plus podophyllin 25%) and ablative therapies (cryotherapy, CO2 laser therapy, surgical excision; for costs see Table 35) was used (£60.95) per additional appointment (two in the base case: £121.89). This figure was varied in sensitivity analysis.
Costs: resource use costs
Resource use
The resources modelled within the analysis specifically relate to appointments at GUM clinics. Table 40 provides a summary of the number of appointments applied within the economic analysis in the base case.
| Treatment pathway | Number of appointments | Included in model? | Comments |
|---|---|---|---|
| Person with AGWs presents to clinician for diagnosis | 1 | No | All patients experience this appointment and therefore there are no differences between treatment groups |
| Additional appointments required for treatment with: | |||
| No treatment | 0 | Yes | The number of additional appointments required depends on the number of administrations required |
| Imiquimod 5% cream | 0 | ||
| Podophyllin 20–25% | 5 | ||
| Podophyllotoxin 0.5% cream (sensitivity analysis only) | 0 | ||
| Podophyllotoxin 0.5% solution | 0 | ||
| TCAA | 4 | ||
| CO2 laser therapy | 1 | ||
| Cryotherapy | 4 | ||
| Surgical excision | 1 | ||
| Cryotherapy plus podophyllin 25% | 5 | ||
| Cryotherapy plus podophyllotoxin 0.15% cream | 4 | ||
| TCAA plus podophyllin 25% | 5 | ||
| Person is successfully cleared of AGWs following first-line treatment | |||
| After completion of treatment | 0 | Yes | No additional appointments required for people successfully treated for AGWs |
| After 12 weeks’ follow-up | 0 | Yes | |
| Person is not successfully cleared of AGWs following first-line treatment | |||
| After completion of treatment | 1 | Yes | An additional appointment is required to review a patient who is not clear of AGWs after first- or second-line treatment |
| After 12 weeks’ follow-up | 1 | Yes | |
| Person is prescribed second-line treatment | |||
| Additional appointments required for treatment with: | |||
| No treatment | 0 | Yes | The number of additional appointments required depends on the number of administrations required |
| Imiquimod 5% cream | 0 | ||
| Podophyllin 20–25% | 5 | ||
| Podophyllotoxin 0.5% cream (sensitivity analysis only) | 0 | ||
| Podophyllotoxin 0.5% solution | 0 | ||
| TCAA | 4 | ||
| CO2 laser therapy | 1 | ||
| Cryotherapy | 4 | ||
| Surgical excision | 1 | ||
| Cryotherapy plus podophyllin 25% | 5 | ||
| Cryotherapy plus podophyllotoxin 0.15% cream | 4 | ||
| TCAA plus podophyllin 25% | 5 | ||
| Person is successfully cleared of AGWs following second-line treatment | |||
| After completion of treatment | 0 | Yes | No additional appointments required for people successfully treated for AGWs |
| After 12 weeks’ follow-up | 0 | Yes | |
| Person is not successfully cleared of AGWs following second-line treatment | |||
| After completion of treatment | 2 | Yes | These patients were assumed to have persistent warts and require a further two appointments |
| After 12 weeks’ follow-up | 2 | Yes | |
Within the model it was assumed that all appointments take place within GUM clinics. This was a simplifying assumption that is not expected to impact on the model results. In clinical practice, the majority of people with AGWs are treated in GUM clinics; however, a proportion of patients may be treated by their GP or in hospital. The proportion of people with AGWs treated in GUM clinics is reported by Lanitis51 to be 91% and by Desai et al. 3 to be 77%. Clinical expert opinion considered it likely that approximately 85–90% of appointments take place in GUM clinics, with the vast majority of the remainder carried out at GP practices and a minority carried out in hospital. The clinical experts approached considered that the proportion of patients treated in GUM clinics compared with GP practices would not differ by intervention. Consequently, the assumption that all appointments will be carried out in GUM clinics was not considered to be a key point of difference between interventions and is unlikely to affect the model results.
In clinical practice, all people with AGWs will experience a first appointment with a clinician to diagnose their AGWs; however, this appointment has not been modelled within the analysis. This is because the cost of attendance for the initial appointment will be the same for all interventions modelled and therefore will not affect the incremental results.
People treated first line with clinician-applied topical or ablative therapies, either as monotherapy or as part of a combination therapy, were assumed to require additional appointments in which the therapy is applied; the number of appointments was assumed to directly correlate to the number of required administrations, see Model structure. People treated with patient-applied topical therapy, or those who receive no treatment, were assumed to incur no additional appointments for application of their therapy.
All people with recurrence of AGWs after clearance with first-line treatment, or with AGWs that do not clear after first-line treatment, were assumed to return to their clinician and to incur the cost of an appointment. In contrast to the initial appointment, the cost of this appointment has been included in the analysis, because the number of required appointments will vary by treatment because of differences in rates of complete clearance at the end of treatment and rates of recurrence.
As with first-line treatment, people treated second line with clinician-applied topical or ablative therapies, either as monotherapy or as part of a combination therapy, were assumed to require additional appointments in which the therapy is applied; the number of appointments was assumed to directly correlate with the number of required administrations, see Model structure. People treated with patient-applied topical therapy were assumed to incur no additional appointments for application of their therapy.
Successful treatment of AGWs was assumed to result in no further appointments. Following review of the studies identified in the systematic review of the cost literature, it was noted that Woodhall et al. 176 reported that 45% of women and 55% of men attended their physician once (i.e. the initial appointment). Following consultation with clinical experts, it is considered likely that a majority of patients attending only once at the initial appointment are clear of AGWs and therefore chose not to return to the physician.
It was assumed that people who experience a recurrence of AGWs after second-line treatment, or who do not clear after second-line treatment, had persistent warts and a further two appointments are applied. As described above, this assumption is based on review of the study by Lanitis. 51
Cost of resources
The cost of attendance at a GUM clinic for an appointment was estimated by multiplying the estimated time per appointment by the cost of clinician time. The estimated time per appointment was taken from the study by Woodhall et al. 176 and costs for clinician time were taken from the Unit Costs for Health and Social Care 2013217 for community-based staff.
The study by Woodhall et al. 176 reported the results of a case note review of 370 people aged ≥ 16 years attending six sexual health clinics in England and one clinic in Northern Ireland. The authors recorded the resources used in the care of each participant and reported consultation times at clinics by staff involved in the visit. The estimates of consultation time were presented separately in the online appendix for women and men, by type of staff involved in the visit, by first and follow-up appointments and by laser treatment compared with all other treatments.
In the model, data for follow-up appointments were used. This is because the first appointment at a GUM clinic was not modelled (see Model structure). In addition, rather than modelling women and men separately, an average appointment time was calculated, which was weighted by the number of women (n = 157) and men (n = 213) surveyed.
The duration of appointment was assumed to differ by treatment administered. Specifically, the duration of appointment reported for laser therapy in Woodhall et al. 176 was used for appointments related to laser therapy, for appointments related to persistent warts and for appointments related to surgical excision. The duration of appointment reported for all other therapies in Woodhall et al. 176 was used for the remaining clinician-administered topical and ablative therapies.
For simplicity, it was assumed that, except for CO2 laser therapy and surgical excision, the type of appointment (doctor led, nurse led or doctor with nurse) was equally split, that is, 33.3% doctor led, 33.3% doctor with nurse and 33.3% nurse led; for CO2 laser therapy and surgical excision the split was 50% doctor led and 50% doctor with nurse. This was varied in sensitivity analysis.
The cost applied for a doctor and a nurse in the base case was £292 and £58 per hour, respectively. 217 The estimated weighted average cost per consultation for CO2 laser therapy and surgical excision, and for appointments for persistent warts was £206.68. The estimated weighted average cost per consultation for all other treatments was £62.12. The consultation times and costs included in the analysis are presented in Table 41. Costs were varied in sensitivity analysis.
| Staff involved in visit | Average consultation time (minutes), females | Average consultation time (minutes), males | Weighted average consultation time (minutes) | Cost per consultation (£) | Assumed proportion of attendances (%) |
|---|---|---|---|---|---|
| For all interventions except CO2 laser therapy and surgical excision | |||||
| Doctor led | |||||
| Doctor time | 17 | 16 | 16.4 | 79.93 | 33.3 |
| Doctor with nurse | |||||
| Doctor time | 18 | 16 | 16.8 | 82.00 | 33.3 |
| Nurse time | 13 | 10 | 11.3 | 10.90 | |
| Nurse led | |||||
| Nurse time | 14 | 14 | 14.0 | 13.53 | 33.3 |
| Weighted average cost per consultation | 62.12 | ||||
| Staff involved in visit | Average consultation time (minutes) | Cost per consultation (£) | Assumed proportion of attendances (%) | ||
| For CO2 laser and surgical excision | |||||
| Doctor led | |||||
| Doctor time | 38 | 184.93 | 50 | ||
| Doctor with nurse | |||||
| Doctor time | 38 | 184.93 | 50 | ||
| Nurse time | 45 | 43.50 | |||
| Weighted average cost per consultation | 206.68 | ||||
It was considered appropriate to apply the duration of appointment related to laser therapy in Woodhall et al. 176 to appointments related to persistent warts to reflect the increased likelihood that clinician-applied ablative therapies would be prescribed at this stage. Similarly, it was considered appropriate to apply the duration of appointment related to laser therapy in Woodhall et al. 176 to appointments related to surgical excision because surgical excision was considered by the clinical experts consulted to require a similar length of appointment to that for laser therapy in clinical practice.
Two other UK cost papers identified from the systematic review recorded an estimated time per appointment. 174,175 Both of these papers were published before that by Woodhall et al. 176 and involved review of the case notes for fewer people with AGWs (Woodhall et al. :175 189 people, published 2009; Langley et al. :174 200 people, published 2004; Woodhall et al. :176 370 people, published 2011). For these reasons, data from Woodhall et al. 176 were considered most appropriate for use within the model in the base case.
Accounting for uncertainty
The impact of parameter uncertainty on the model results was investigated in both probabilistic and deterministic analyses.
Probabilistic analyses
Within the economic model, probabilistic analysis was used to investigate the simultaneous impact of parameter uncertainty on the cost-effectiveness results. Base-case probabilistic results are presented. Probability distributions were assigned to each parameter (except drug acquisition costs) used within the model, from which values were simultaneously sampled 1000 times. There was assumed to be zero uncertainty associated with drug acquisition costs. The type of distribution and rationale for selection of the distribution has previously been described in Model inputs.
Traditional head-to-head comparison of incremental results for each individual treatment sequence was considered to be unwieldy for this analysis because of the large number of treatment sequences considered in the base case (n = 84; see Interventions). Consequently, the results of the probabilistic analysis were presented using multiple cost-effectiveness acceptability curves (mCEACs) and the identified key comparisons investigated incrementally in sensitivity analysis.
Multiple cost-effectiveness acceptability curves enable the graphical presentation of the probability that a strategy will have the highest net benefit compared with all other strategies, for a given willingness to pay for an additional QALY gained. 239 Net benefit is estimated as the total benefit of a treatment strategy expressed in monetary terms less the cost of a treatment strategy. The net benefit is estimated as the QALY gain for the treatment strategy multiplied by the willingness to pay per additional QALY gained. For this analysis, the net benefit for every included treatment strategy was calculated for each of the 1000 simulations, for a range of willingness to pay values from £1000 to £50,000. For each simulation, the strategy with the highest net benefit was identified. These results were then plotted on the mCEAC graph.
Scenario analyses
A variety of assumptions have been made in the construction of the base-case model. Where possible, these have been tested in probabilistic scenario analysis. Table 42 lists the scenario analyses carried out, the parameters used to inform these scenarios and the rationale for each analysis. For each scenario, 1000 simulations were run and results captured using mCEACs.
| Scenario analysis | Parameter definition | Rationale |
|---|---|---|
| Timing of complete clearance of AGWs | In the base case, if complete clearance occurs, it is assumed to occur, on average, at the mid-point between commencing treatment and assessing response. Two further scenarios were investigated:
|
To investigate the impact of this assumption on model results |
| Timing of recurrence of AGWs | In the base case, if recurrence occurs, it is assumed to occur, on average, at the mid-point between completing treatment and assessing recurrence. Two further scenarios were investigated:
|
To investigate the impact of this assumption on model results |
| The probability of recurrence following complete clearance of AGWs | Setting the same probability of recurrence for all interventions to:
|
To investigate the importance of recurrence for the model results in light of a number of weaknesses associated with the clinical data for recurrence |
| Alternative HRQoL data | HRQoL for AGWs set to values for young adults aged 18–24 years from the study by Dominiak-Felden237 (HRQoL 0.86 with AGWs vs. 0.94 without AGWs) | To investigate the impact of AGWs on specific subgroups of patients |
| Podophyllotoxin 0.5% cream assessed as a proxy for podophyllotoxin 0.15% cream | Complete clearance data for podophyllotoxin 0.5% cream was used in a scenario analysis as a proxy for podophyllotoxin 0.15% cream | To investigate a scenario in which podophyllotoxin 0.15% cream, which is used in clinical practice, was assessed despite a lack of evidence identified from the clinical literature review |
Deterministic sensitivity analysis
One-way sensitivity analysis
The key head-to-head comparisons identified from the deterministic and probabilistic analyses were tested using one-way sensitivity analysis.
For each key treatment sequence, all model parameters were varied in a one-way sensitivity analysis. Parameters were assigned low and high values. When available, the low and high values used were the 95% CI or the 95% CrI. When these data were not available, an arbitrary upper and lower 20% value was used. The upper and lower values used have been previously described in Model inputs. The deterministic cost-effectiveness result was recorded for each one-way change in each parameter estimate. The variables associated with the greatest impact on the cost-effectiveness results are presented in tornado diagram format later in this chapter (see Figure 33).
Threshold analyses
Variables identified from the one-way sensitivity analysis as having the greatest impact on the model results were investigated in threshold analyses for key comparisons. The value for each variable was altered until each relevant ICER reached a threshold of £20,000 or £30,000 per additional QALY.
Independent economic assessment: results
Base-case results
Probabilistic analysis
The following probabilistic base-case results are presented:
-
the estimated average cost per patient per treatment strategy, with lower 2.5% and upper 97.5% values (Table 43)
-
the estimated average QALYs per patient per treatment strategy, with lower 2.5% and upper 97.5% values (Table 44)
-
a mCEAC presenting the probability of each treatment strategy being considered that with the highest net benefit for varying values for the willingness to pay for an additional QALY gained (from £1000 to £50,000, Figure 20)
-
a tabular summary of the mCEAC results for willingness-to-pay thresholds of £20,000 and £30,000 per additional QALY gained (Table 45).
| First line | Second line | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No treatment | Imiquimod 5% cream | Podophyllin 20–25% | Podophyllotoxin 0.5% solution | TCAA | CO2 laser therapy | Cryotherapy | Surgical excision | Cryotherapy plus podophyllin 25% | Cryotherapy plus podophyllotoxin 0.15% cream | TCAA plus podophyllin 25% | |
| No treatment | NA | 511 (335 to 736) | 674 (525 to 839) | 273 (185 to 392) | 550 (384 to 750) | 442 (334 to 592) | 577 (439 to 762) | 520 (381 to 739) | 614 (468 to 800) | 542 (406 to 740) | 636 (458 to 849) |
| Imiquimod 5% cream | NA | NA | 571 (360 to 819) | 347 (248 to 474) | 502 (323 to 745) | 441 (297 to 611) | 517 (334 to 755) | 485 (316 to 721) | 538 (346 to 800) | 498 (328 to 748) | 550 (349 to 828) |
| Podophyllin 20–25% | NA | 654 (494 to 838) | NA | 495 (380 to 638) | 682 (518 to 871) | 609 (478 to 757) | 700 (545 to 892) | 662 (516 to 841) | NA | 677 (521 to 861) | NA |
| Podophyllotoxin 0.5% solution | NA | 228 (130 to 370) | 297 (183 to 444) | NA | 244 (151 to 374) | 199 (128 to 300) | 256 (157 to 390) | 232 (141 to 368) | 272 (165 to 415) | NA | 280 (173 to 427) |
| TCAA | NA | 522 (355 to 745) | 610 (416 to 843) | 396 (290 to 524) | NA | 486 (346 to 658) | 562 (373 to 827) | 528 (368 to 756) | 579 (388 to 810) | 542 (368 to 791) | NA |
| CO2 laser therapy | NA | 407 (310 to 545) | 427 (316 to 602) | 377 (296 to 477) | 412 (312 to 561) | NA | 416 (313 to 570) | 408 (309 to 552) | 420 (315 to 586) | 411 (312 to 563) | 422 (315 to 593) |
| Cryotherapy | NA | 551 (411 to 757) | 643 (478 to 886) | 420 (316 to 544) | 577 (405 to 836) | 514 (389 to 686) | NA | 558 (421 to 750) | NA | NA | 622 (455 to 856) |
| Surgical excision | NA | 491 (360 to 717) | 532 (372 to 820) | 431 (337 to 571) | 501 (362 to 761) | 473 (353 to 663) | 508 (365 to 761) | NA | 517 (369 to 792) | 499 (365 to 743) | 522 (372 to 810) |
| Cryotherapy plus podophyllin 25% | NA | 591 (444 to 795) | NA | 470 (354 to 612) | 613 (448 to 834) | 556 (427 to 725) | NA | 596 (449 to 807) | NA | NA | NA |
| Cryotherapy plus podophyllotoxin 0.15% cream | NA | 514 (377 to 743) | 587 (426 to 841) | NA | 535 (374 to 790) | 485 (363 to 680) | NA | 519 (390 to 733) | NA | NA | 571 (413 to 835) |
| TCAA plus podophyllin 25% | NA | 614 (443 to 855) | NA | 473 (351 to 618) | NA | 574 (422 to 760) | 654 (469 to 919) | 620 (452 to 854) | NA | 633 (457 to 894) | NA |
| First line | Second line | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No treatment | Imiquimod 5% cream | Podophyllin 20–25% | Podophyllotoxin 0.5% solution | TCAA | CO2 laser therapy | Cryotherapy | Surgical excision | Cryotherapy plus podophyllin 25% | Cryotherapy plus podophyllotoxin 0.15% cream | TCAA plus podophyllin 25% | |
| No treatment | NA | 1.008 (0.905 to 1.115) | 1.006 (0.900 to 1.113) | 1.019 (0.914 to 1.127) | 1.011 (0.907 to 1.118) | 1.031 (0.925 to 1.142) | 1.011 (0.906 to 1.117) | 1.025 (0.916 to 1.135) | 1.014 (0.908 to 1.121) | 1.016 (0.911 to 1.123) | 1.010 (0.905 to 1.120) |
| Imiquimod 5% cream | NA | NA | 1.018 (0.914 to 1.126) | 1.024 (0.920 to 1.133) | 1.020 (0.918 to 1.128) | 1.028 (0.923 to 1.139) | 1.020 (0.916 to 1.129) | 1.026 (0.920 to 1.135) | 1.021 (0.918 to 1.130) | 1.022 (0.919 to 1.132) | 1.020 (0.915 to 1.129) |
| Podophyllin 20–25% | NA | 1.020 (0.916 to 1.129) | NA | 1.027 (0.921 to 1.138) | 1.022 (0.918 to 1.132) | 1.034 (0.927 to 1.146) | 1.022 (0.917 to 1.131) | 1.030 (0.924 to 1.142) | NA | 1.025 (0.919 to 1.134) | NA |
| Podophyllotoxin 0.5% solution | NA | 1.029 (0.924 to 1.140) | 1.029 (0.923 to 1.139) | NA | 1.031 (0.924 to 1.142) | 1.038 (0.930 to 1.150) | 1.031 (0.924 to 1.143) | 1.035 (0.928 to 1.147) | 1.032 (0.925 to 1.144) | NA | 1.031 (0.923 to 1.139) |
| TCAA | NA | 1.024 (0.920 to 1.134) | 1.023 (0.920 to 1.134) | 1.030 (0.924 to 1.141) | NA | 1.036 (0.927 to 1.148) | 1.026 (0.921 to 1.136) | 1.033 (0.925 to 1.144) | 1.027 (0.921 to 1.137) | 1.028 (0.921 to 1.138) | NA |
| CO2 laser therapy | NA | 1.038 (0.931 to 1.151) | 1.038 (0.931 to 1.151) | 1.039 (0.931 to 1.152) | 1.038 (0.931 to 1.151) | NA | 1.038 (0.931 to 1.151) | 1.040 (0.932 to 1.152) | 1.038 (0.932 to 1.151) | 1.039 (0.931 to 1.151) | 1.038 (0.931 to 1.150) |
| Cryotherapy | NA | 1.024 (0.919 to 1.133) | 1.023 (0.918 to 1.133) | 1.030 (0.923 to 1.141) | 1.025 (0.921 to 1.135) | 1.035 (0.927 to 1.147) | NA | 1.032 (0.926 to 1.143) | NA | NA | 1.025 (0.918 to 1.134) |
| Surgical excision | NA | 1.034 (0.927 to 1.145) | 1.033 (0.927 to 1.145) | 1.036 (0.929 to 1.148) | 1.034 (0.927 to 1.145) | 1.039 (0.931 to 1.151) | 1.034 (0.927 to 1.145) | NA | 1.035 (0.927 to 1.146) | 1.036 (0.928 to 1.146) | 1.034 (0.927 to 1.146) |
| Cryotherapy plus podophyllin 25% | NA | 1.025 (0.920 to 1.136) | NA | 1.031 (0.923 to 1.143) | 1.027 (0.920 to 1.136) | 1.036 (0.928 to 1.148) | NA | 1.033 (0.926 to 1.144) | NA | NA | NA |
| Cryotherapy plus podophyllotoxin 0.15% cream | NA | 1.027 (0.922 to 1.137) | 1.027 (0.920 to 1.136) | NA | 1.029 (0.921 to 1.139) | 1.036 (0.927 to 1.149) | NA | 1.034 (0.927 to 1.144) | NA | NA | 1.028 (0.920 to 1.135) |
| TCAA plus podophyllin 25% | NA | 1.023 (0.916 to 1.132) | NA | 1.029 (0.921 to 1.137) | NA | 1.035 (0.927 to 1.145) | 1.025 (0.917 to 1.134) | 1.031 (0.924 to 1.143) | NA | 1.027 (0.919 to 1.135) | NA |
FIGURE 20.
Multiple cost-effectiveness acceptability curves presenting the probability of each treatment strategy being considered that with the highest net benefit for varying values of willingness to pay for an additional QALY gained.
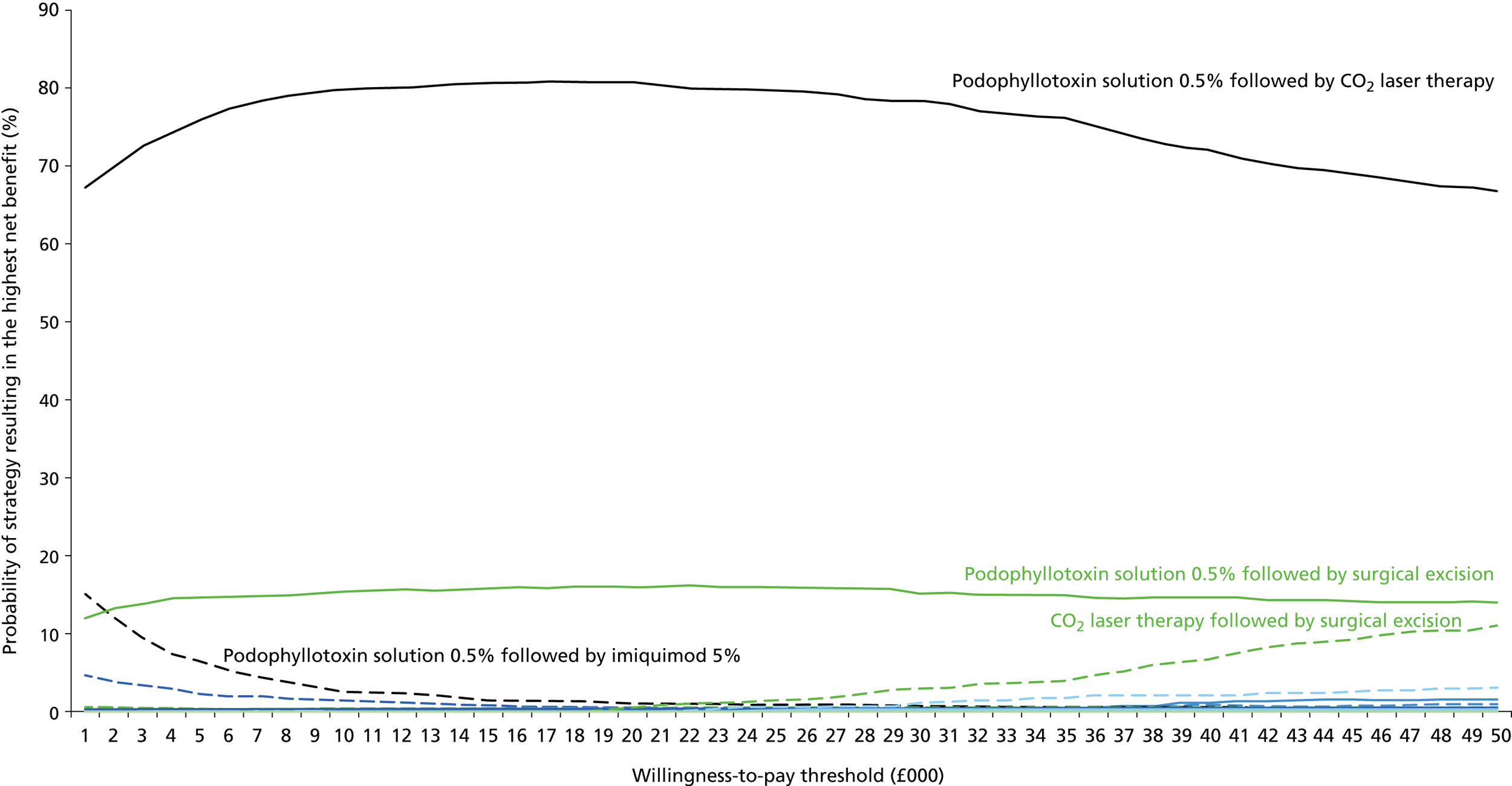
| Treatment strategy | Probability (%) of strategy resulting in the highest net benefit, based on a willingness to pay per additional QALY gained of | |
|---|---|---|
| £20,000 | £30,000 | |
| Podophyllotoxin 0.5% solution followed by CO2 laser therapy | 80.7 | 78.3 |
| Podophyllotoxin 0.5% solution followed by surgical excision | 15.9 | 15.1 |
| Podophyllotoxin 0.5% solution followed by imiquimod 5% cream | 1.1 | 0.7 |
| CO2 laser therapy followed by surgical excision | 0.6 | 3.0 |
| Podophyllotoxin 0.5% solution followed by TCAA | 0.5 | 0.5 |
| Podophyllotoxin 0.5% solution followed by TCAA plus podophyllin 25% | 0.4 | 0.4 |
| Surgical excision followed by cryotherapy plus podophyllotoxin 0.15% cream | 0.2 | 0.1 |
| Podophyllotoxin 0.5% solution followed by cryotherapy | 0.1 | 0.0 |
| Podophyllotoxin 0.5% solution followed by cryotherapy plus podophyllin 25% | 0.1 | 0.0 |
| CO2 laser therapy followed by cryotherapy plus podophyllotoxin 0.15% cream | 0.1 | 0.3 |
| CO2 laser therapy followed by TCAA plus podophyllin 25% | 0.1 | 0.1 |
| Surgical excision followed by CO2 laser therapy | 0.1 | 1.2 |
| Cryotherapy plus podophyllotoxin 0.15% cream followed by surgical excision | 0.1 | 0.1 |
| CO2 laser therapy followed by podophyllotoxin 0.5% solution | 0.0 | 0.2 |
Quality-adjusted life-years were calculated based on the 58-week time horizon and so the average QALYs per patient, irrespective of treatment sequence, were found to be > 1.
The average cost per treatment sequence was estimated to range between £199 (podophyllotoxin solution followed by CO2 laser therapy) and £700 (podophyllin 20–25% followed by cryotherapy) per patient. The average QALYs gained per patient per treatment sequence were estimated to range between 1.006 (no treatment followed by podophyllin 20–25%) and 1.040 (CO2 laser therapy followed by surgical excision) per patient. It is noted that the average cost per patient identified from the systematic review of the cost literature (see Description of the identified costing studies) ranged between £94176 and £276. 51 The results of this analysis imply a larger cost per treatment sequence. However, following consultation with clinical experts, it is noted that in clinical practice podophyllotoxin (solution or cream) is commonly prescribed first line because of its its relatively low cost. The average cost for strategies in which podophyllotoxin solution 0.5% was prescribed first line was estimated in this analysis to range from £199 to £297; thus, it is considered that the estimates of cost from this analysis are generally aligned with the higher end of the range identified from the systematic review of the cost literature.
The results of the mCEACs show that, at a willingness-to-pay threshold of £20,000 per additional QALY gained, podophyllotoxin solution 0.5% followed by CO2 laser therapy has a probability of being considered the strategy with the highest net benefit of approximately 80%. Similarly, at a willingness-to-pay threshold of £20,000 per additional QALY gained, podophyllotoxin 0.5% solution followed by surgical excision is associated with a probability of being considered the strategy with the second highest net benefit of approximately 16% (see Table 45). Results at a willingness-to-pay threshold of £30,000 per additional QALY gained were very similar (podophyllotoxin solution 0.5% followed by CO2 laser therapy: 78%; podophyllotoxin 0.5% solution followed by surgical excision: 15%).
These results imply that the treatment strategy of podophyllotoxin solution 0.5% followed by CO2 laser therapy is highly likely to be the strategy with the highest net benefit at typical UK willingness-to-pay thresholds.
Deterministic analysis
The estimated average costs per patient and average QALYs per patient according to the deterministic analysis are presented in Table 46 (costs) and Table 47 (QALYs).
| First line | Second line | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No treatment | Imiquimod 5% cream | Podophyllin 20–25% | Podophyllotoxin 0.5% solution | TCAA | CO2 laser therapy | Cryotherapy | Surgical excision | Cryotherapy plus podophyllin 25% | Cryotherapy plus podophyllotoxin 0.15% cream | TCAA plus podophyllin 25% | |
| No treatment | NA | 511 | 673 | 337 | 547 | 444 | 576 | 520 | 612 | 542 | 636 |
| Imiquimod 5% cream | NA | NA | 573 | 384 | 502 | 444 | 518 | 487 | 539 | 499 | 552 |
| Podophyllin 20–25% | NA | 654 | NA | 537 | 678 | 609 | 698 | 660 | NA | 675 | NA |
| Podophyllotoxin 0.5% solution | NA | 228 | 296 | NA | 243 | 200 | 255 | 232 | 270 | NA | 280 |
| TCAA | NA | 520 | 605 | 428 | NA | 484 | 554 | 524 | 573 | 536 | NA |
| CO2 laser therapy | NA | 408 | 429 | 386 | 413 | NA | 417 | 409 | 422 | 412 | 425 |
| Cryotherapy | NA | 550 | 640 | 454 | 570 | 513 | NA | 555 | NA | NA | 620 |
| Surgical excision | NA | 489 | 529 | 446 | 498 | 473 | 505 | NA | 514 | 497 | 520 |
| Cryotherapy plus podophyllin 25% | NA | 589 | NA | 501 | 607 | 555 | NA | 593 | NA | NA | NA |
| Cryotherapy plus podophyllotoxin 0.15% cream | NA | 514 | £587 | NA | 530 | 484 | NA | 518 | NA | NA | 570 |
| TCAA plus podophyllin 25% | NA | 614 | NA | 511 | NA | 574 | 652 | 619 | NA | £633 | NA |
| First line | Second line | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No treatment | Imiquimod 5% cream | Podophyllin 20–25% | Podophyllotoxin 0.5% solution | TCAA | CO2 laser therapy | Cryotherapy | Surgical excision | Cryotherapy plus podophyllin 25% | Cryotherapy plus podophyllotoxin 0.15% cream | TCAA plus podophyllin 25% | |
| No treatment | NA | 0.993 | 0.991 | 1.004 | 0.996 | 1.015 | 0.996 | 1.009 | 0.999 | 1.001 | 0.995 |
| Imiquimod 5% cream | NA | NA | 1.003 | 1.008 | 1.005 | 1.013 | 1.005 | 1.010 | 1.006 | 1.007 | 1.004 |
| Podophyllin 20–25% | NA | 1.004 | NA | 1.011 | 1.007 | 1.018 | 1.007 | 1.015 | NA | 1.010 | NA |
| Podophyllotoxin 0.5% solution | NA | 1.014 | 1.014 | NA | 1.015 | 1.022 | 1.015 | 1.020 | 1.016 | NA | 1.015 |
| TCAA | NA | 1.009 | 1.008 | 1.014 | NA | 1.020 | 1.011 | 1.017 | 1.012 | 1.013 | NA |
| CO2 laser therapy | NA | 1.022 | 1.022 | 1.023 | 1.023 | NA | 1.023 | 1.024 | 1.023 | 1.023 | 1.022 |
| Cryotherapy | NA | 1.008 | 1.008 | 1.014 | 1.010 | 1.019 | NA | 1.017 | NA | NA | 1.010 |
| Surgical excision | NA | 1.018 | 1.018 | 1.021 | 1.019 | 1.023 | 1.019 | NA | 1.019 | 1.020 | 1.019 |
| Cryotherapy plus podophyllin 25% | NA | 1.010 | NA | 1.015 | 1.012 | 1.020 | NA | 1.017 | NA | NA | NA |
| Cryotherapy plus podophyllotoxin 0.15% cream | NA | 1.012 | 1.011 | NA | 1.013 | 1.021 | NA | 1.018 | NA | NA | 1.013 |
| TCAA plus podophyllin 25% | NA | 1.007 | NA | 1.013 | NA | 1.019 | 1.009 | 1.016 | NA | 1.012 | NA |
The estimated average cost per treatment sequence ranged between £200 (podophyllotoxin 0.5% solution followed by CO2 laser therapy) and £698 (podophyllin 20–25% followed by cryotherapy) per patient. The estimated average QALYs per treatment sequence ranged between 0.991 (no treatment followed by podophyllin 20–25%) and 1.024 (CO2 laser therapy followed by surgical excision) per patient.
According to the results of the deterministic analysis, the majority of treatment strategies were dominated by three alternative treatment strategies (were more costly and less effective). Non-dominated (by strict dominance) treatment strategies are presented in Table 48.
| First-line treatment | Second-line treatment | Total cost per patient (£) | Total QALYs per patient | Incremental ICER (£) | Incremental ICER excluding extendedly dominated strategies (£) |
|---|---|---|---|---|---|
| Podophyllotoxin 0.5% solution | CO2 laser therapy | 199.96 | 1.022 | – | – |
| CO2 laser therapy | Podophyllotoxin 0.5% solution | 377.18 | 1.023 | 121,483 (extendedly dominated) | – |
| CO2 laser therapy | Surgical excision | 409.43 | 1.024 | 61,599 | 105,667 |
Similar to the results of the probabilistic analysis, podophyllotoxin 0.5% solution followed by CO2 laser therapy was found to be the least costly treatment strategy. According to the results of the deterministic analysis, no other treatment strategy provided a cost-effective alternative to podophyllotoxin 0.5% solution followed by CO2 laser therapy at a willingness to pay per additional QALY of £20,000–30,000.
Discussion of the results
The mean total costs and QALYs estimated from the deterministic and probabilistic analyses were found to be consistent and similar. For both the deterministic and probabilistic analyses, the least expensive treatment strategy was found to be podophyllotoxin 0.5% solution followed by CO2 laser therapy and the treatment strategy resulting in the greatest QALYs was CO2 laser therapy followed by surgical excision.
In addition, the main conclusion of the analyses was aligned, with both deterministic and probabilistic analyses implying that podophyllotoxin 0.5% solution followed by CO2 laser therapy was most likely to be the treatment strategy considered a cost-effective use of resources at a willingness to pay per additional QALY gained of £20,000–30,000.
Based on the results of both the deterministic analysis and the probabilistic analysis, the following comparisons were considered as key and were tested in sensitivity analysis:
-
podophyllotoxin 0.5% solution followed by surgery compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy
-
CO2 laser therapy followed by surgery compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy
-
CO2 laser therapy followed by podophyllotoxin 0.5% solution compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy.
Sensitivity analysis
Probabilistic analysis
This section presents the results of the incremental probabilistic analysis for each key comparison using the cost-effectiveness plane.
Podophyllotoxin 0.5% solution followed by surgery compared with podophyllotoxin 0.5% solution followed by carbon dioxide laser therapy
The results presented in Figure 21 indicate that the majority of simulations (78%) resulted in podophyllotoxin 0.5% solution followed by surgery being dominated by podophyllotoxin 0.5% solution followed by CO2 laser therapy, that is, being more costly and less effective. In 13% of simulations, podophyllotoxin 0.5% solution followed by surgery was dominant compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy, that is, less costly and more effective.
FIGURE 21.
Cost-effectiveness plane presenting the incremental costs and QALYs associated with podophyllotoxin 0.5% solution followed by surgery vs. podophyllotoxin 0.5% solution followed by CO2 laser therapy.
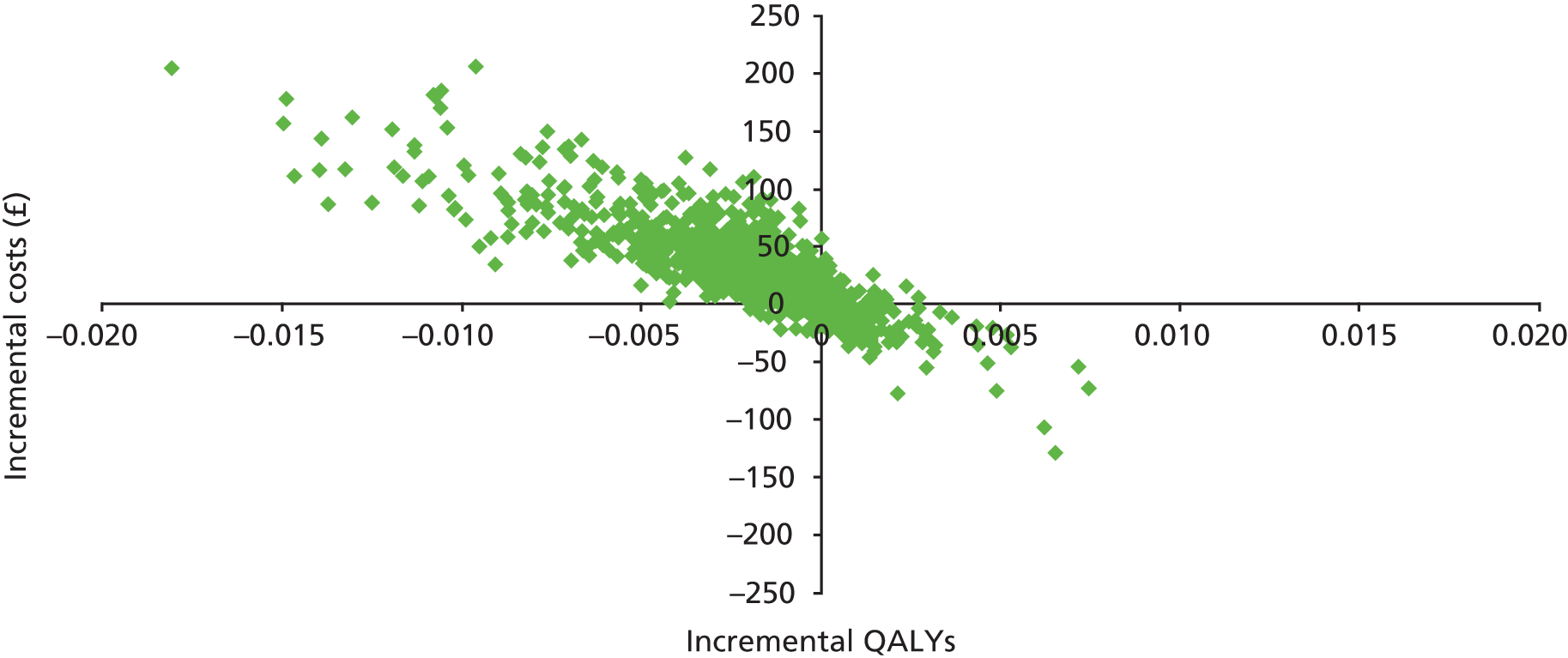
According to the results, the probability that podophyllotoxin 0.5% solution followed by surgery would be considered cost-effective compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy at a willingness-to-pay threshold of £20,000 and £30,000 per additional QALY gained was 15% and 16%, respectively.
Carbon dioxide laser therapy followed by surgery compared with podophyllotoxin 0.5% solution followed by carbon dioxide laser therapy
The results presented in Figure 22 indicate that the majority of simulations (93%) resulted in CO2 laser therapy followed by surgery being associated with a higher incremental cost and higher incremental QALYs compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy. The average ICER for these simulations was £368,128. No simulations fell into the south-east or south-west quadrants (i.e. lower incremental costs).
FIGURE 22.
Cost-effectiveness plane presenting the incremental costs and QALYs associated with CO2 laser therapy followed by surgery vs. podophyllotoxin 0.5% solution followed by CO2 laser therapy.
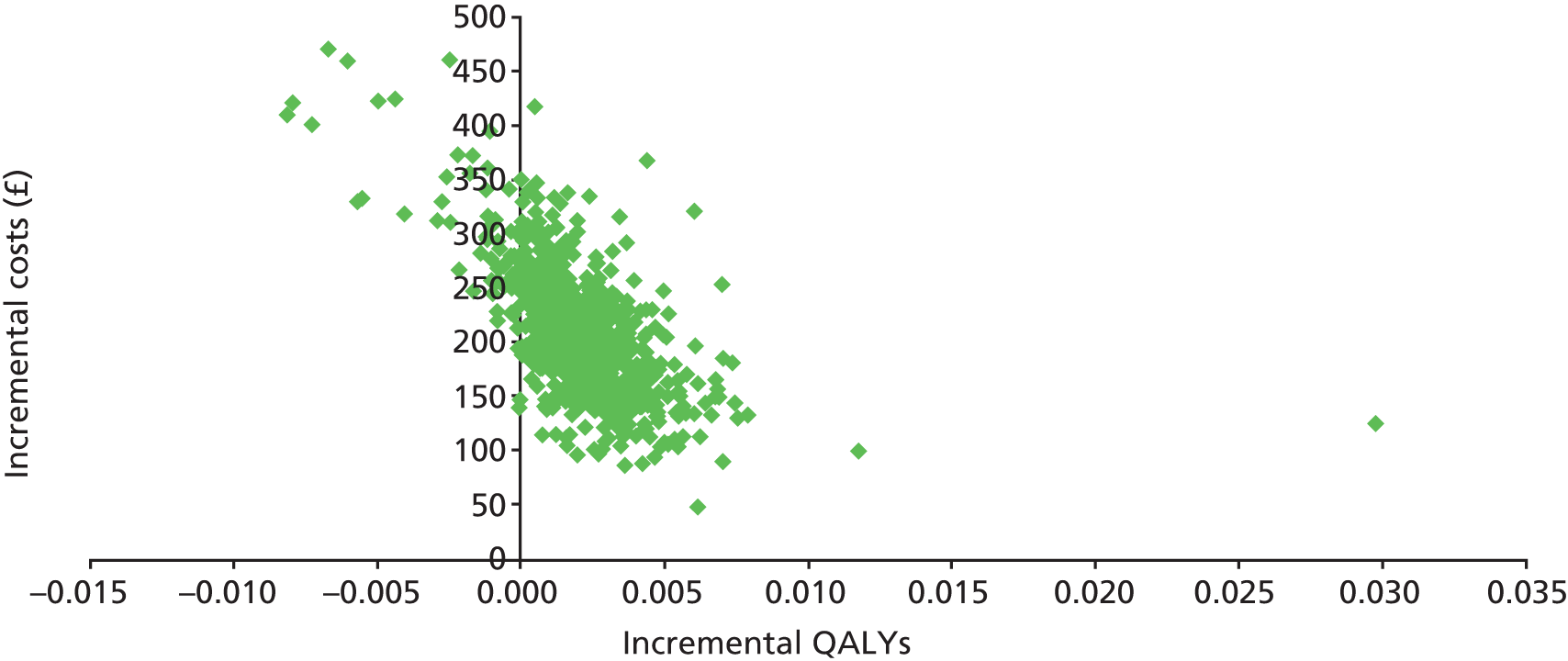
According to the results, the probability that CO2 laser therapy followed by surgery would be considered cost-effective compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy at a willingness-to-pay threshold of £20,000 and £30,000 per additional QALY gained was 1% and 5%, respectively.
Carbon dioxide laser therapy followed by podophyllotoxin 0.5% solution compared with podophyllotoxin 0.5% solution followed by carbon dioxide laser therapy
The results presented in Figure 23 indicate that the majority of simulations (93%) resulted in CO2 laser therapy followed by podophyllotoxin 0.5% solution being associated with a higher incremental cost and higher incremental QALYs compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy. The average ICER for these simulations was £490,895. No simulations fell into the south-east or south-west quadrants (i.e. lower incremental costs).
FIGURE 23.
Cost-effectiveness plane presenting the incremental costs and QALYs associated with CO2 laser therapy followed by podophyllotoxin 0.5% solution vs. podophyllotoxin 0.5% solution followed by CO2 laser therapy.
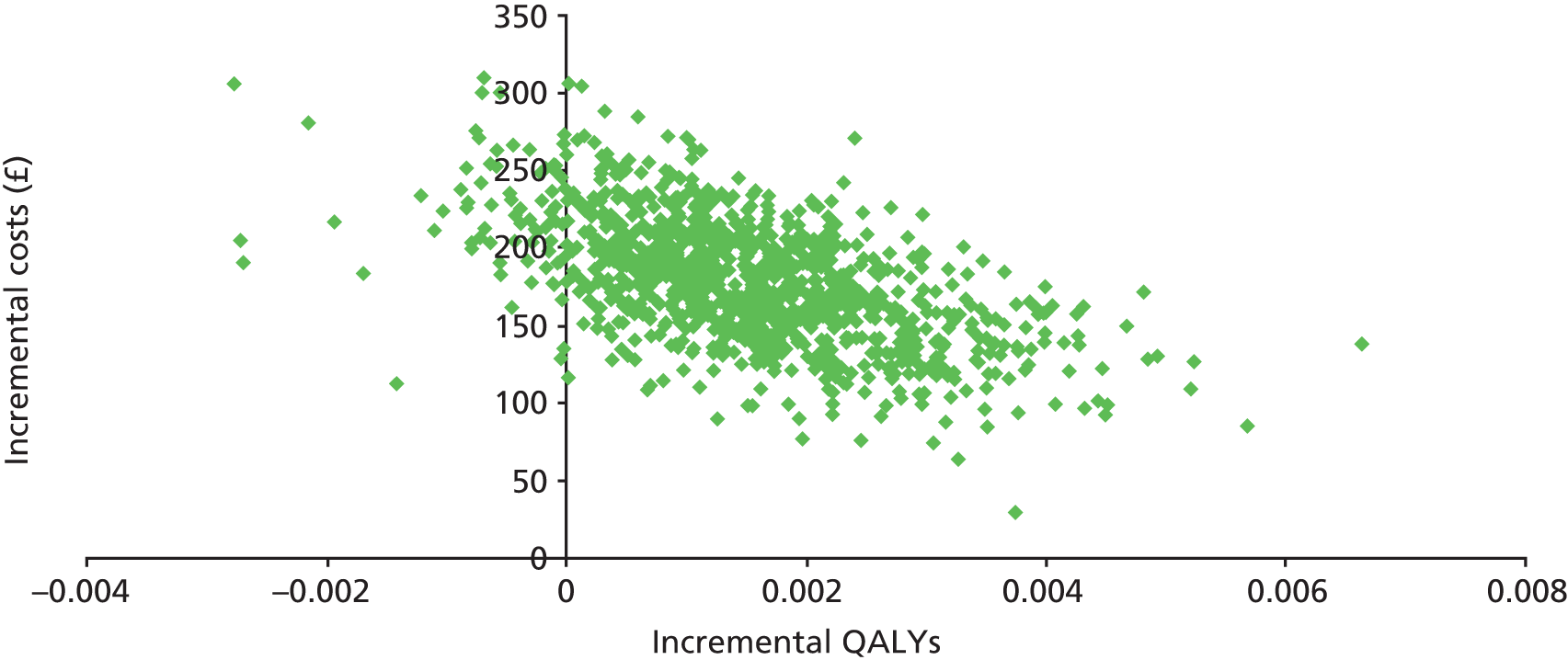
According to the results, the probability that CO2 laser therapy followed by podophyllotoxin 0.5% solution would be considered cost-effective compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy at a willingness-to-pay threshold of £20,000 and £30,000 per additional QALY gained was 0% and 2%, respectively.
Scenario analyses
As described in Accounting for uncertainty, scenario analyses were carried out on the probabilistic results, with 1000 simulations run for each scenario and the results presented as mCEACs (Figures 24–32).
FIGURE 24.
Multiple cost-effectiveness acceptability curves for the scenario analysis assuming that complete clearance occurs at the start of treatment.
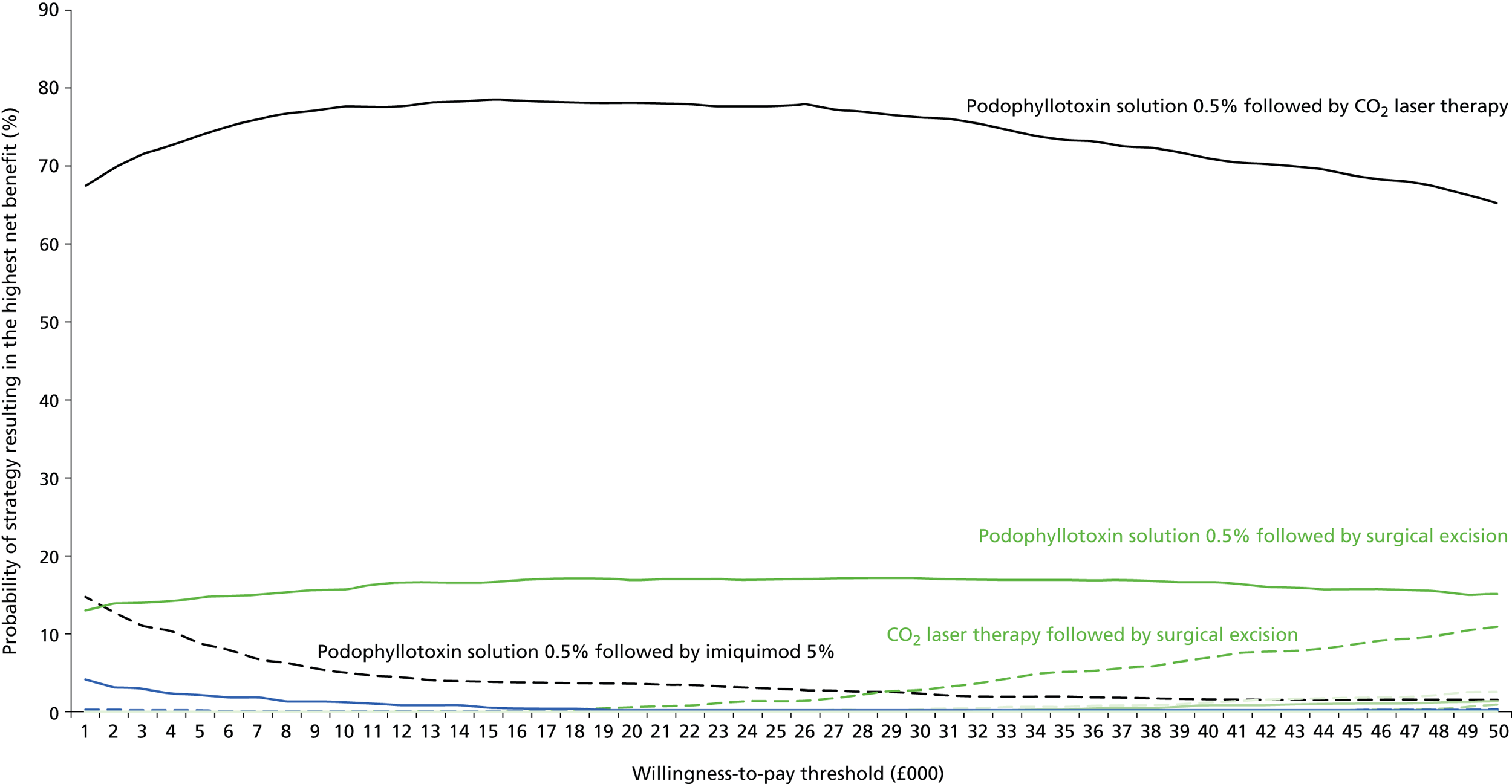
FIGURE 25.
Multiple cost-effectiveness acceptability curves for the scenario analysis assuming that complete clearance occurs at the end of treatment.
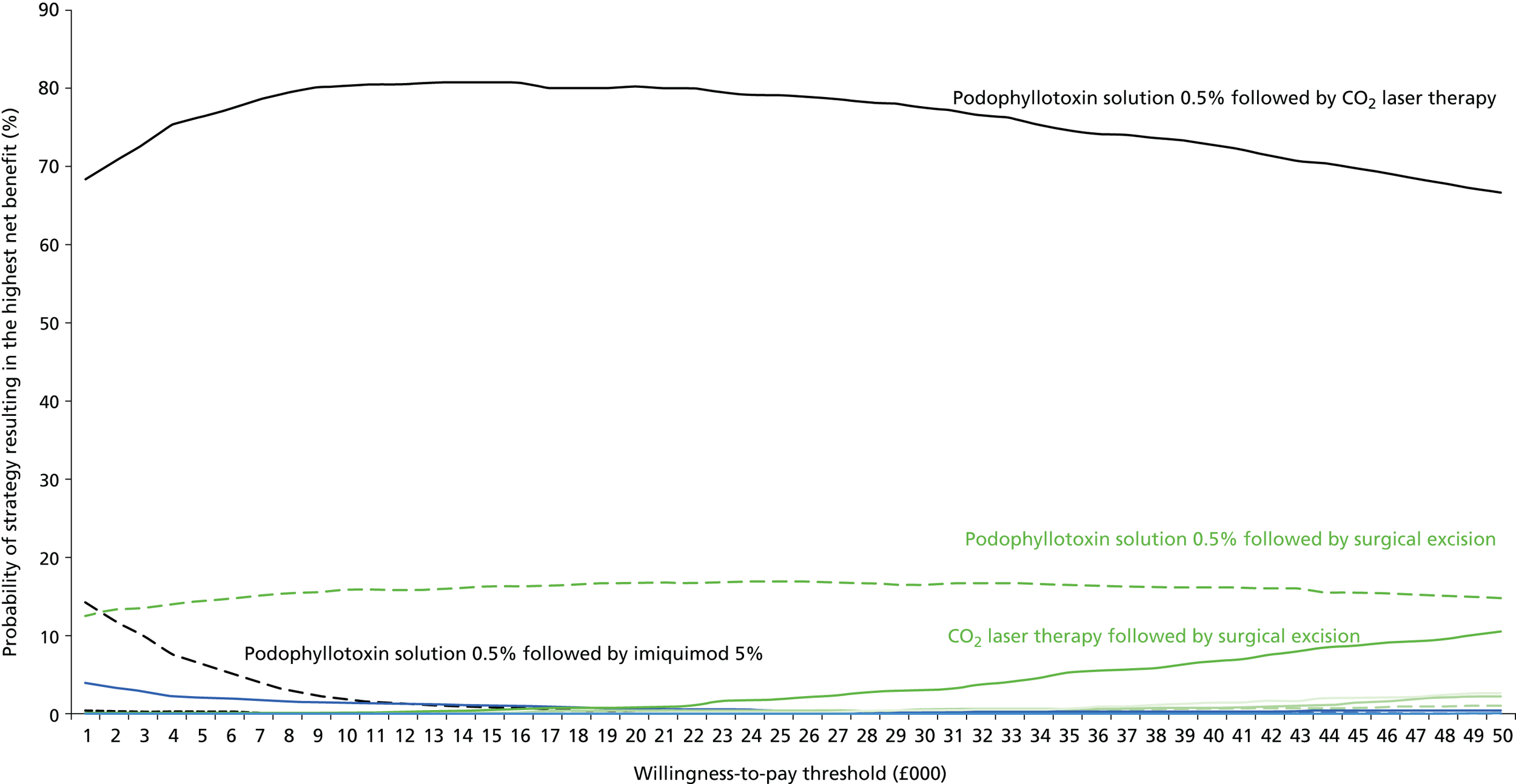
FIGURE 26.
Multiple cost-effectiveness acceptability curves for the scenario analysis assuming that recurrence occurs at the start of follow-up.

FIGURE 27.
Multiple cost-effectiveness acceptability curves for the scenario analysis assuming that recurrence occurs at the end of follow-up.

FIGURE 28.
Multiple cost-effectiveness acceptability curves for the scenario analysis assuming that the probability of recurrence was 26.6% for all interventions (the average recurrence figure from the MTC).
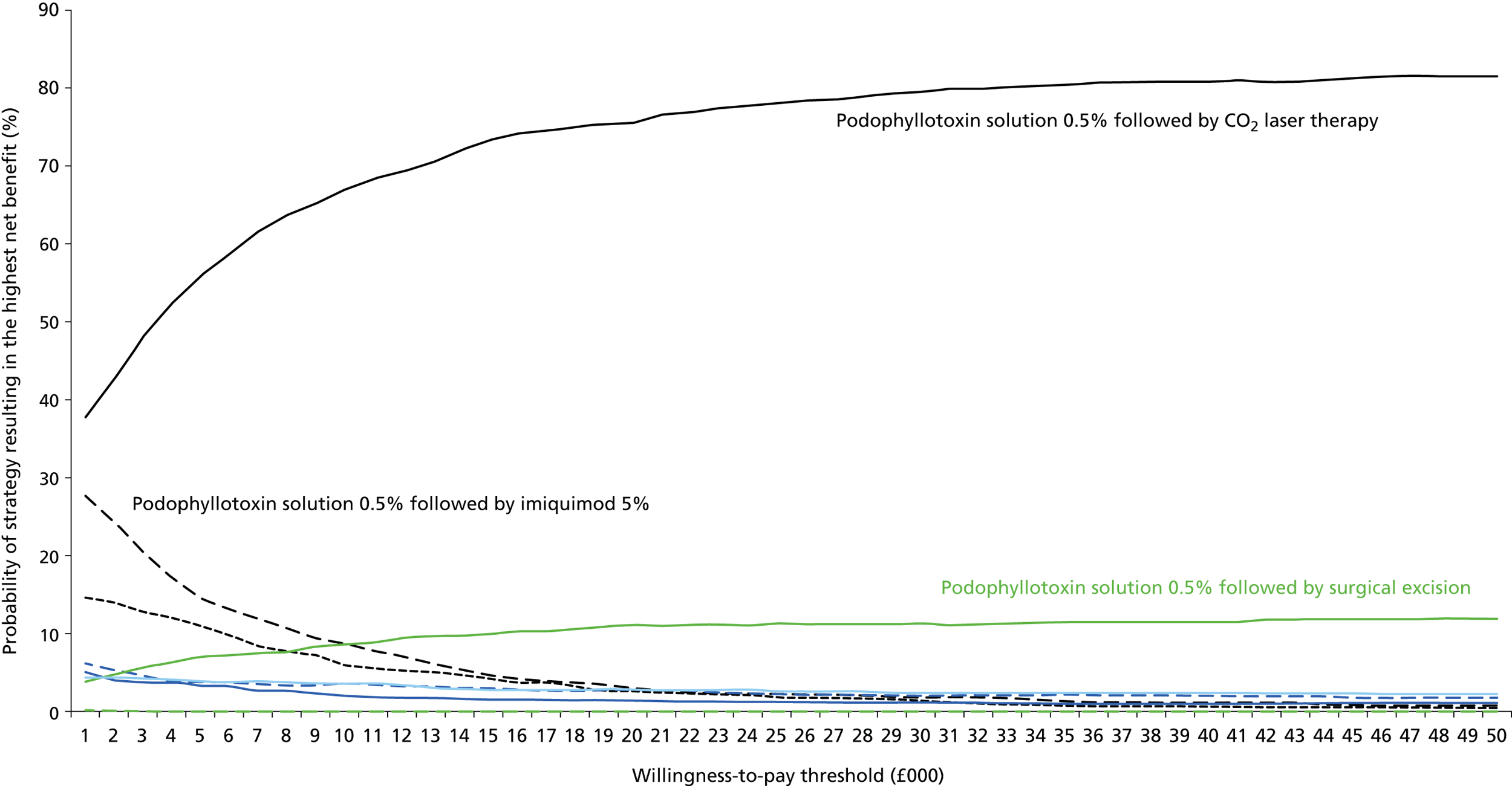
FIGURE 29.
Multiple cost-effectiveness acceptability curves for the scenario analysis assuming that the probability of recurrence was 0% for all interventions.

FIGURE 30.
Multiple cost-effectiveness acceptability curves for the scenario analysis assuming that the probability of recurrence was 100% for all interventions.
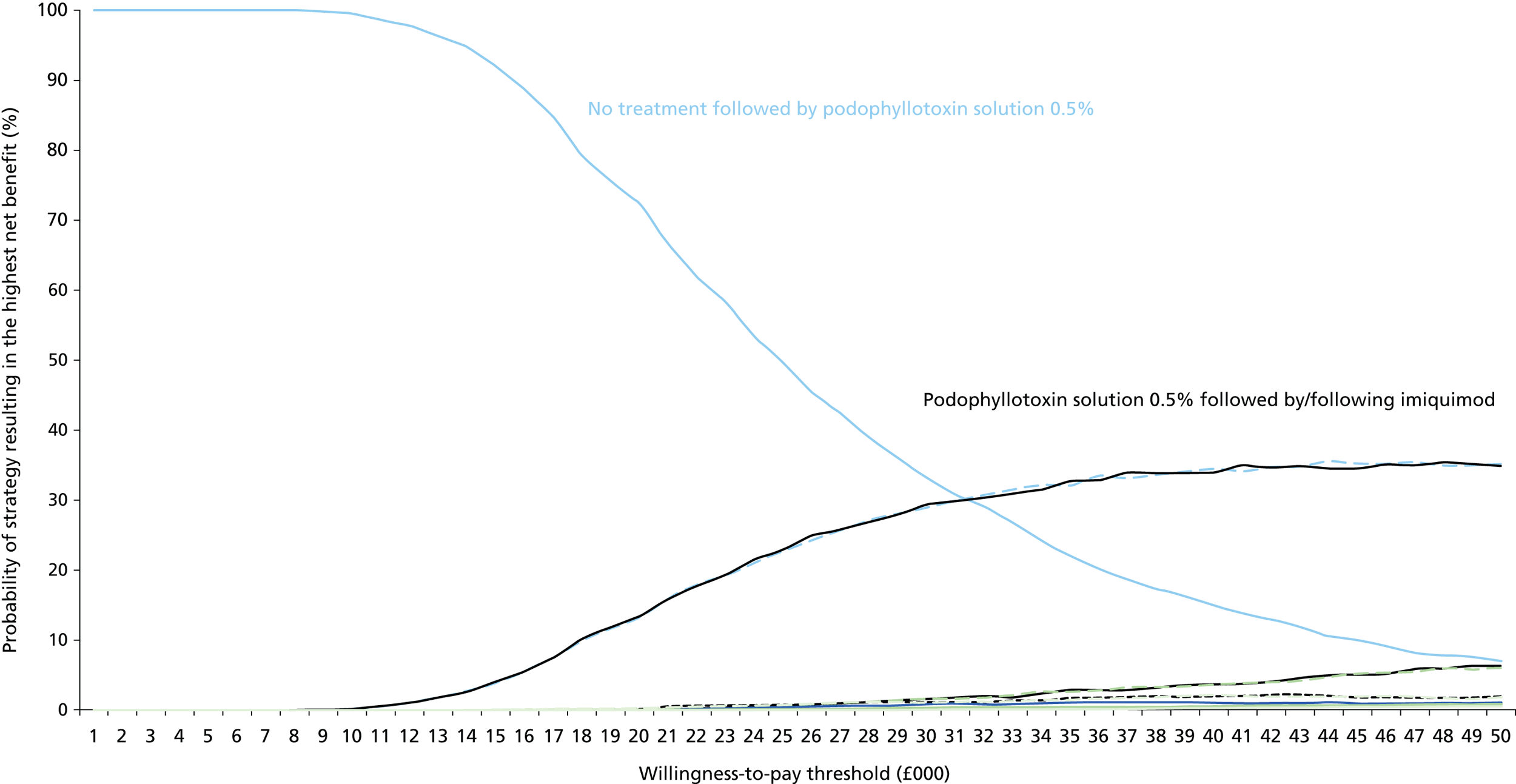
FIGURE 31.
Multiple cost-effectiveness acceptability curves for the scenario analysis using alternative HRQoL data to represent younger people with AGWs.
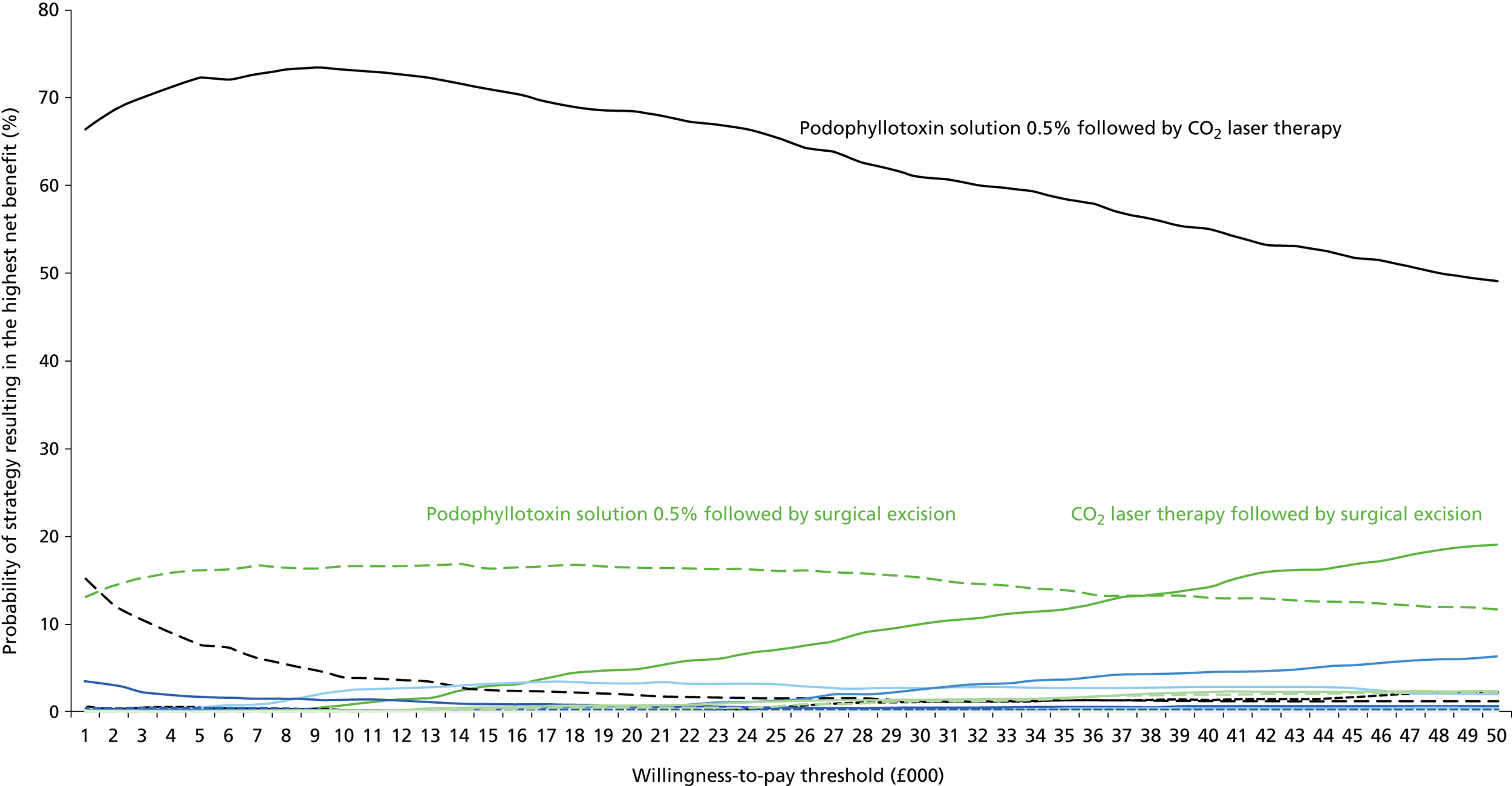
FIGURE 32.
Multiple cost-effectiveness acceptability curves for the scenario analysis including podophyllotoxin 0.5% cream as a proxy for podophyllotoxin 0.15% cream.

Only one scenario analysis had a significant impact on the model results. This was the scenario in which the probability of recurrence was set to 100% for all treatments. In this scenario, no treatment followed by treatment with podophyllotoxin 0.5% solution was estimated to be associated with a probability of approximately 73% of being considered the treatment with the highest net benefit at a willingness to pay of £20,000 per additional QALY gained and a 35% probability of being considered the treatment with the highest net benefit at a willingness to pay of £30,000 per additional QALY gained. Podophyllotoxin followed by/following imiquimod 5% cream was associated with a probability of approximately 30% of being considered the treatment with the highest net benefit at a willingness to pay of £30,000 per additional QALY gained.
The results for this scenario are not surprising; the assumption of 100% recurrence means that all people in the model go on to have persistent warts. It therefore is logical that the cheaper treatments are likely to be considered cost-effective, because the scenario automatically assumes that treatment will fail. A scenario in which 100% of people recur is not likely in clinical practice during a single episode (even if many people go on to experience a second or third episode) and therefore this scenario is likely to be extreme. Nevertheless, the results imply that recurrence values are an important driver of the model results.
For all other scenario analyses, the probability of podophyllotoxin 0.5% solution followed by CO2 laser therapy being considered the treatment strategy with the highest net benefit at a willingness to pay of £20,000 or £30,000 per additional QALY gained varied between approximately 63% and 88%. This implies that the model findings are extremely robust to the analysed scenarios.
One-way deterministic sensitivity analysis
Podophyllotoxin 0.5% solution followed by surgery compared with podophyllotoxin 0.5% solution followed by carbon dioxide laser therapy
In the deterministic base case, podophyllotoxin 0.5% followed by CO2 laser therapy strictly dominated podophyllotoxin 0.5% solution followed by surgery.
In the one-way sensitivity analysis, podophyllotoxin 0.5% solution followed by surgery remained dominated in all but three scenarios:
-
Using the lower CrI for the probability of complete clearance at the end of CO2 laser therapy (84.7%) resulted in podophyllotoxin 0.5% solution followed by surgery moving from being strictly dominated by podophyllotoxin 0.5% followed by CO2 laser therapy (more costly and less effective) to being comparatively more costly and more effective, with an ICER of £628,309.
-
Using the upper CrI for the probability of complete clearance at the end of surgery (99.1%) resulted in podophyllotoxin 0.5% solution followed by surgery moving from being strictly dominated by podophyllotoxin 0.5% followed by CO2 laser therapy (more costly and less effective) to being comparatively more costly and more effective, with an ICER of £12,925.
-
Using the upper value for the probability of recurrence at the end of follow-up following complete clearance with CO2 laser therapy (24.7%) resulted in podophyllotoxin 0.5% solution followed by surgery moving from being strictly dominated by podophyllotoxin 0.5% followed by CO2 laser therapy (more costly and less effective) to being comparatively more costly and more effective, with an ICER of £104,673.
Consequently, the parameter for which the results were most sensitive to change was found to be the probability of complete clearance at the end of surgery. No other parameters varied within the described upper and lower values (see Model inputs) affected the conclusions of the analysis.
Carbon dioxide laser therapy followed by surgery compared with podophyllotoxin 0.5% solution followed by carbon dioxide laser therapy
In the deterministic base case, CO2 laser therapy followed by surgery compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy was associated with an ICER of £105,667.
In the one-way sensitivity analysis, the top five parameters for which the model results were most sensitive were complete clearance at the end of treatment for surgical excision; the probability of recurrence after complete clearance with podophyllotoxin 0.5% solution; HRQoL without AGWs; duration of treatment with podophyllotoxin 0.5% solution; and duration of follow-up following complete clearance (Figure 33). No upper or lower parameter value reduced the ICER below £30,000 per additional QALY. The ICER was reduced to £32,969 with the use of the high value for the probability of recurrence after complete clearance with podophyllotoxin 0.5% solution (51.4%).
FIGURE 33.
Top five parameters for which the model results were most sensitive for the comparison of CO2 laser therapy followed by surgery with podophyllotoxin 0.5% solution followed by CO2 laser therapy.
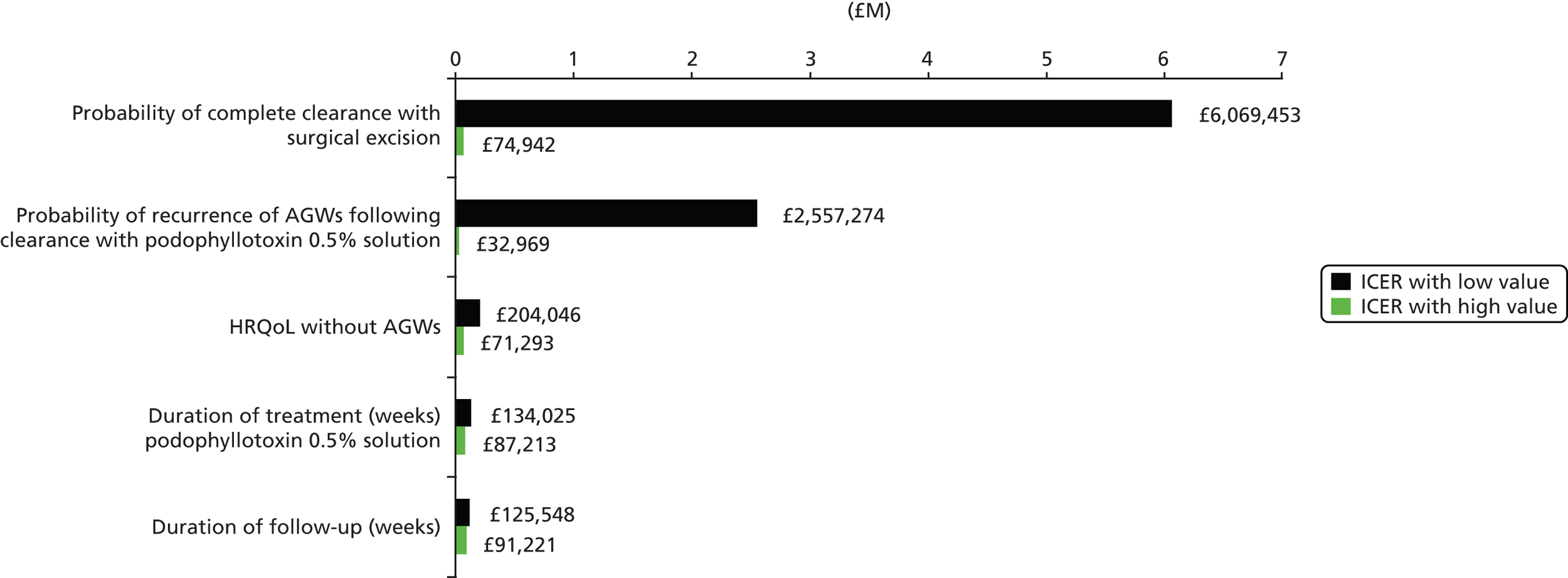
Consequently, the parameter for which the results were most sensitive to change was found to be the probability of recurrence after complete clearance with podophyllotoxin 0.5% solution. No other parameters varied within the described upper and lower values (see Model inputs) affected the conclusions of the analysis.
Carbon dioxide laser therapy followed by podophyllotoxin 0.5% solution compared with podophyllotoxin 0.5% solution followed by carbon dioxide laser therapy
In the deterministic base case, CO2 laser therapy followed by podophyllotoxin 0.5% solution compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy was associated with an ICER of £121,483.
In the one-way sensitivity analysis, the top five parameters for which the model results were most sensitive were recurrence of treatment after complete clearance with podophyllotoxin 0.5% solution; recurrence of treatment after complete clearance with CO2 laser therapy; HRQoL without AGWs; complete clearance at the end of CO2 laser therapy; and HRQoL with AGWs (Figure 34). No upper or lower parameter value reduced the ICER below £30,000 per additional QALY. The ICER was reduced to £38,895 with the use of the high value for the probability of recurrence after complete clearance with podophyllotoxin 0.5% solution (51.4%).
FIGURE 34.
Top five parameters for which the model results were most sensitive for the comparison of CO2 laser therapy followed by podophyllotoxin 0.5% solution with podophyllotoxin 0.5% solution followed by CO2 laser therapy.

Consequently, the parameter for which the results were most sensitive to change was found to be the probability of recurrence after complete clearance with podophyllotoxin 0.5% solution. No other parameters varied within the described upper and lower values (see Model inputs) affected the conclusions of the analysis.
Threshold analyses
Variables identified from one-way sensitivity analysis as having the greatest impact on the model results were investigated using threshold analyses for the key comparisons. The value for each parameter was altered until each relevant ICER reached a threshold of £20,000 or £30,000 per additional QALY. The results are presented in Tables 49–51.
| Parameter | Base-case parameter value | Approximate value to reach ICER of £20,000 | Approximate value to reach ICER of £30,000 |
|---|---|---|---|
| Base-case deterministic ICER (£) | Podophyllotoxin 0.5% followed by CO2 laser therapy dominates | ||
| Probability of complete clearance at the end of CO2 laser therapy (%) | 97.07 | 83.18 | 83.56 |
| Probability of complete clearance at the end of treatment by surgical excision (%) | 84.79 | 98.68 | 98.29 |
| Probability of recurrence at end of follow-up for CO2 laser (%) | 9.66 | 24.84 | 24.79 |
| Parameter | Base-case parameter value | Approximate value to reach ICER of £20,000 | Approximate value to reach ICER of £30,000 |
|---|---|---|---|
| Base-case deterministic ICER (£) | 105,667 | ||
| Probability of complete clearance at the end of treatment by surgical excision (%) | 84.8 | No solution | No solution |
| Probability of recurrence after complete clearance with podophyllotoxin 0.5% solution (%) | 34.6 | 59.1 | 52.9 |
| HRQoL without AGWs | 0.926 | No solution | No solution |
| Duration of treatment with podophyllotoxin 0.5% solution (weeks) | 4 | 19.6 | 13.2 |
| Duration of follow-up following complete clearance (weeks) | 12 | 79.6 | 50.2 |
| Parameter | Base-case parameter value | Approximate value to reach ICER of £20,000 | Approximate value to reach ICER of £30,000 |
|---|---|---|---|
| Base-case deterministic ICER (£) | 121,483 | ||
| Probability of recurrence after complete clearance with podophyllotoxin 0.5% solution (%) | 34.6 | 61.5 | 55.5 |
| Probability of recurrence after complete clearance with CO2 laser therapy (%) | 9.7 | No solution | No solution |
| HRQoL without AGWs | 0.926 | No solution | No solution |
| Complete clearance at the end of CO2 laser therapy (%) | 97.1 | No solution | No solution |
| HRQoL with AGWs | 0.87 | 0.586 | 0.699 |
For the comparison of podophyllotoxin 0.5% followed by CO2 laser therapy with podophyllotoxin 0.5% solution followed by surgery, threshold analyses indicated that the probability of complete clearance at the end of treatment by surgery would need to increase to approximately 98% to result in an ICER of £20,000–30,000. Similarly, the probability of complete clearance at the end of CO2 laser therapy would need to decrease to approximately 83% to result in an ICER of £20,000–30,000. It is noted that the probability of complete clearance following treatment by surgical excision required to reduce the ICER to £20,000–30,000 lies within the CrI estimated from the MTC.
Threshold analyses also demonstrated that the probability of recurrence at the end of follow-up for people treated with CO2 laser therapy would need to be > 24% for the deterministic ICER to reach £20,000–30,000. It is noted that no data were identified for recurrence following clearance with CO2 laser therapy; thus, this parameter was inferred using clinical expert opinion, with the value for recurrence following surgery used to inform the model parameter. It is therefore considered that the value of this variable is uncertain and this is noted as a potential area of uncertainty.
For the comparison of CO2 laser therapy followed by surgery with podophyllotoxin 0.5% solution followed by CO2 laser therapy, threshold analyses indicated that the duration of treatment with podophyllotoxin 0.5% solution and the duration of follow-up following complete clearance would need to increase to > 13 weeks and > 50 weeks, respectively, to reach an ICER of £30,000 per additional QALY. Given current clinical practice, these are unlikely scenarios.
In addition, threshold analyses indicated that the probability of recurrence after complete clearance with podophyllotoxin 0.5% solution would need to increase to approximately 53% from 34.6% to reach an ICER of £30,000 per additional QALY. It is noted that the estimated CrI for the probability of recurrence for podophyllotoxin solution was 20% to 51.4%; therefore this value exceeds the 95% CrI as estimated from the MTC (see Recurrence of anogenital warts within 12 weeks of complete clearance).
Threshold analyses indicated that the probability of recurrence after complete clearance with podophyllotoxin 0.5% solution would need to increase to approximately 55.5% from 34.6% to reach an ICER of £30,000 per additional QALY. It is noted that the estimated CrI for the probability of recurrence after complete clearance with podophyllotoxin 0.5% solution was 20–51.4%; therefore, this value exceeds the 95% CrI as estimated from the MTC (see Recurrence of anogenital warts within 12 weeks of complete clearance).
For the comparison of CO2 laser therapy followed by podophyllotoxin 0.5% solution with podophyllotoxin 0.5% solution followed by CO2 laser therapy, threshold analyses indicated that the HRQoL associated with AGWs would need to fall to 0.699 from 0.87 to reach an ICER of £30,000 per additional QALY gained. Of the UK studies identified that provided HRQoL data,32,176,228,237 none reported a HRQoL score for people with AGWs at such a value; thus, it is considered that a HRQoL estimate of 0.699 is unlikely in clinical practice.
Summary of the cost-effectiveness findings
Base-case results
Probabilistic analysis of the de novo economic model found that the treatment strategy of podophyllotoxin 0.5% solution followed by CO2 laser therapy had a probability of 80.7% of being considered the treatment strategy with the highest net benefit at a willingness to pay per additional QALY gained of £20,000. At a willingness to pay per additional QALY gained of £30,000, the probability reduced to 78.3%. The treatment strategy of podophyllotoxin 0.5% solution followed by CO2 laser therapy was therefore most likely to be considered a cost-effective treatment strategy compared with all other included treatment strategies.
The deterministic analysis of the model found that 81 of the 84 assessed treatment strategies were dominated in the base case, that is, were more expensive and less effective than the remaining three treatment strategies. The non-dominated treatment strategies were podophyllotoxin 0.5% solution followed by CO2 laser therapy, CO2 laser therapy followed by podophyllotoxin 0.5% solution and CO2 laser therapy followed by surgical excision. Of these three strategies, podophyllotoxin 0.5% solution followed by CO2 laser therapy was the least expensive. The remaining two treatment strategies were associated with ICERs well above the threshold of £20,000–30,000 per additional QALY compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy, at £121,483 (CO2 laser therapy followed by podophyllotoxin 0.5% solution) and £105,667 (CO2 laser therapy followed by surgical excision).
Consequently, the deterministic and probabilistic base-case results were aligned and showed that, given the model structure and model inputs used in this analysis, podophyllotoxin 0.5% solution followed by CO2 laser therapy was likely to be considered the most cost-effective use of resources at a willingness-to-pay threshold of £20,000–30,000 per additional QALY gained.
Results of the sensitivity analysis
Additional probabilistic analysis, one-way sensitivity analysis and threshold analyses were carried out on the key comparisons. The key comparisons were identified as:
-
podophyllotoxin 0.5% solution followed by surgery compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy
-
CO2 laser therapy followed by surgery compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy
-
CO2 laser therapy followed by podophyllotoxin 0.5% solution compared with podophyllotoxin 0.5% solution followed by CO2 laser therapy.
In the head-to-head probabilistic analysis, podophyllotoxin 0.5% solution followed by CO2 laser therapy continued to be the treatment strategy with the highest probability of being considered cost-effective at a willingness to pay for an additional QALY of £20,000–30,000 when compared individually with podophyllotoxin 0.5% solution followed by surgery, CO2 laser therapy followed by surgery and CO2 laser therapy followed by podophyllotoxin 0.5% solution.
In the scenario analysis, only one scenario significantly affected the base-case probabilistic results. This was the scenario in which it was assumed that 100% of people with AGWs recur following complete clearance. In this scenario, the treatment option of no treatment followed by podophyllotoxin 0.5% solution was estimated to be associated with a probability of approximately 73% of being considered the treatment strategy with the highest net benefit at a willingness to pay of £20,000 per additional QALY gained and with a 35% probability of being considered the treatment strategy with the highest net benefit at a willingness to pay of £30,000 per additional QALY gained. Podophyllotoxin followed by/following imiquimod 5% cream was associated with a probability of approximately 30% of being considered the treatment strategy with the highest net benefit.
In the one-way sensitivity analysis for the comparisons of CO2 laser therapy followed by surgery with podophyllotoxin 0.5% solution followed by CO2 laser therapy and CO2 laser therapy followed by podophyllotoxin 0.5% solution with podophyllotoxin 0.5% solution followed by CO2 laser therapy, no upper or lower value assigned to each variable resulted in an ICER below £30,000. In the comparison of podophyllotoxin 0.5% solution followed by surgery with podophyllotoxin 0.5% solution followed by CO2 laser therapy, one variable change resulted in an ICER of £12,925; this was use of the upper value for complete clearance at the end of surgery.
In the threshold analyses, the results were generally robust to changes in key parameters for the key comparisons of interest; however, the probability of the recurrence of AGWs following complete clearance with CO2 laser therapy was identified as a variable of possible interest.
Overall, it is considered that uncertainty in the effectiveness data is the key driver of the model results, in particular, the recurrence data.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
Analysis by MTC indicated that, in line with conclusions outlined in European guidelines,6 ablative techniques, and in particular CO2 laser therapy, are generally associated with higher probabilities of complete clearance at the end of treatment. CO2 laser therapy was associated with a probability of clearance of 97.1% (95% CrI 84.8% to 99.9%) in the primary MTC. By contrast, placebo was associated with a probability of clearance of only 7.6% (95% CrI 1.1% to 20.9%) in the primary MTC analysis.
Of the topical treatments evaluated, imiquimod 5% cream, podophyllotoxin 0.5% solution and podophyllotoxin 0.15% cream are the core treatments for AGWs that are suitable for people to self-apply in their home. Although these treatments are the mainstay of patient-applied treatments, the evidence to support their use is derived from predominantly small RCTs. Moreover, no study identified assessed the effectiveness of the three treatments in a head-to-head comparison. MTC analysis (primary) identified considerable disparity in the probability of achieving complete clearance between podophyllotoxin 0.5% solution and imiquimod 5% cream. Podophyllotoxin 0.5% solution had a 92.6% (95% CrI 81.8% to 98.4%) probability of completely clearing lesions whereas imiquimod 5% cream had a 56.1% (CrI 20.3% to 85.0%) probability of completely clearing lesions. However, the wide CrIs indicate that there is considerable uncertainty associated with the results and the findings should be interpreted with caution.
In the primary MTC there was no statistically significant difference in complete clearance of AGWs at the end of treatment between most of the treatments evaluated. Of those differences that reached statistical significance, most of the comparisons involved CO2 laser therapy or podophyllotoxin 0.5% solution.
Carbon dioxide laser therapy was found to be significantly more effective than:
-
imiquimod 5% cream (OR 247.0, 95% CrI 3.03 to 1087)
-
TCAA (OR 86.15, 95% CrI 4.05 to 415.3)
-
cryotherapy (OR 44.61, 95% CrI 3.30 to 201.7)
-
TCAA plus podophyllin (OR 0.13, 95% CrI 0.003 to 0.59; OR < 1 favours CO2 laser therapy)
-
cryotherapy plus podophyllin (OR 0.22, 95% CrI 0.004 to 0.94; OR < 1 favours CO2 laser therapy).
Podophyllotoxin 0.5% solution was associated with statistically significant improvements in complete clearance at the end of treatment compared with:
-
podophyllotoxin 0.5% cream (OR 0.30, 95% CrI 0.04 to 0.99; OR < 1 favours podophyllotoxin 0.5% solution)
-
podophyllotoxin 0.3% cream (OR 0.19, 95% CrI 0.007 to 0.874; OR < 1 favours podophyllotoxin 0.5% solution)
-
TCAA (OR 0.17, 95% CrI 0.02 to 0.63; OR < 1 favours podophyllotoxin 0.5% solution).
The MTC of recurrence between 3 and 6 months evaluated podophyllin 20–25%, podophyllotoxin 0.5% solution, podophyllotoxin 0.25% solution, TCAA and TCAA plus podophyllin 20–25%. There were no statistically significant differences in recurrence at < 6 months between any comparisons. TCAA was associated with the lowest probability of recurrence (23.4%, 95% CrI 1.5% to 76.6%). By contrast, podophyllotoxin 0.3% solution had the highest probability of recurrence (66.9%, 95% CrI 5.2% to 99.5%). Data for recurrence at ≥ 6 months facilitated comparison between podophyllin 20–25%, podophyllotoxin 0.5% solution, imiquimod 5% cream and surgical excision. Only one difference in the MTC was statistically significant. Surgical excision was found to be statistically more effective than podophyllin 20–25% at reducing recurrence at ≥ 6 months (OR 0.14, 95% CrI 0.02 to 0.50). Surgical excision was also associated with the lowest probability of recurrence out of the four treatments (15.4%, 95% CrI 4.7% to 33.5%).
Few identified studies reported clinical effectiveness data on complete clearance without recurrence at time points after the cessation of treatment. Additionally, some studies reported data for this outcome only for people achieving complete clearance at the end of treatment rather than the full study population. Complete clearance without recurrence at time points after the completion of treatment is distinct from recurrence as the former outcome accounts for people who clear within a few days of completion of treatment and who continue to be free of lesions. Five interventions were indirectly compared in a MTC: placebo or no treatment; imiquimod 5% cream (three times a week, patient applied); cryotherapy; electrotherapy; and cryotherapy plus podophyllotoxin 0.15% cream. Electrotherapy was associated with the highest probability of achieving complete clearance without recurrence at 3–6 months after the end of treatment (65.5%, 95% CrI 40.0% to 86.2%). Compared with placebo or no treatment, the four active interventions were associated with a statistically significant improvement in complete clearance without recurrence. However, there were no statistically significant differences between any of the active interventions.
Limited reporting of data for other outcomes of interest in available publications led to restricted networks involving few interventions. A network comprising five treatments evaluated the comparative effectiveness of treatments for clearing > 50% of the baseline volume of AGWs (excluding complete clearance). Analysis identified podophyllotoxin 0.5% solution as being significantly more effective than imiquimod 5% cream and placebo at reducing the volume of AGWs by ≥ 50% compared with baseline volume.
Evaluation of AEs focused on those that cause discomfort to the patient or that are difficult to treat should they occur: ulceration, blistering, erythema, oedema and itching. For ulceration, the results from two studies comparing TCAA with cryotherapy were meta-analysed, which indicated that TCAA was associated with a significantly higher risk of ulceration than cryotherapy (OR 0.22, 95% CI 0.10 to 0.46).
A MTC of four active interventions (imiquimod 5% cream, podophyllin 20–25%, podophyllotoxin 0.5% solution and podophyllotoxin 0.5% cream) enabled an evaluation of their association with an increased risk of erythema. All interventions statistically significantly increased the risk of erythema compared with placebo but not compared with each other.
In summary, the evidence base to inform first-line treatment of AGWs, albeit large, is limited in terms of the number and quality of reporting of studies providing data on the effectiveness of individual interventions. Analyses indicate that ablative techniques, and in particular CO2 laser therapy, are generally associated with higher probabilities of complete clearance at the end of treatment. Although topical treatments such as imiquimod 5% cream, podophyllotoxin 0.5% solution and podophyllotoxin 0.15% cream are the mainstay of patient-applied treatments, the evidence to support their use is limited, with analyses identifying considerable variation across topical treatments in the probability of achieving complete clearance.
Cost-effectiveness
The findings of the de novo economic analysis indicate that the treatment strategy of podophyllotoxin 0.5% solution followed by CO2 laser therapy is likely to be considered a cost-effective use of resources at a willingness to pay of £20,000–30,000 per additional QALY gained. This finding was robust to the majority of changes in the model parameters.
Despite a robust conclusion from the economic analysis, it is noted that there is uncertainty associated with the clinical data informing the model and therefore the results and conclusions. The main sources of uncertainty have previously been described in detail and include:
-
a concern regarding the quality of available clinical evidence, with no studies deemed to be at an overall low risk of bias
-
a lack of reporting of baseline characteristics and therefore uncertainty over the comparability of populations assessed in each clinical trial (e.g. size, number, location of lesions and wart type)
-
a lack of identified data for recurrence and therefore a reliance on assumptions.
With these concerns in mind, it is considered that the following general conclusions can be drawn from the economic analysis:
-
Cost-effectiveness finding 1. Podophyllotoxin 0.5% solution is an effective and relatively inexpensive treatment. It is therefore likely that prescription of this therapy first line would be considered a cost-effective use of resources.
-
Cost-effectiveness finding 2. No treatment and treatment with podophyllin are unlikely to be cost-effective treatment options for AGWs because of their relatively low rates of complete clearance and, in the case of podophyllin, higher estimated rates of recurrence, despite their low costs.
-
Cost-effectiveness finding 3. Highly effective treatments such as CO2 laser therapy or surgical excision are likely to represent a cost-effective treatment option at second line following failure to completely clear with podophyllotoxin solution, provided that these treatments are considered clinically appropriate. This is because, despite their relatively high initial costs, these treatments are likely to be effective and typically require only a single appointment with a clinician.
-
Cost-effectiveness finding 4. There is uncertainty around the cost-effectiveness of treatment with imiquimod, TCAA and cryotherapy at second line. In this economic analysis, these treatments were not found to offer cost-effective alternatives at second line because of their relatively lower rates of complete clearance compared with CO2 laser therapy and surgical excision. However, it is noted that the clinical systematic review reported uncertainty around treatment effects and rates of recurrence and, thus, clinical experience must be taken into account when using these treatments.
Strengths and limitations of the assessment
Clinical effectiveness
The evidence included in the report was identified through robust systematic review methodology. In addition, the evidence on clinical effectiveness facilitated carrying out a MTC and investigation of the comparative clinical effectiveness of the interventions of interest. However, the clinical evidence base identified was weak. The limited details available on methods implemented for randomisation and allocation concealment led to classification of most studies identified as being at unclear risk of bias. Additionally, few studies reported comprehensive baseline characteristics for the enrolled populations. Among those studies providing details on baseline characteristics, many enrolled a mixture of people who were treatment naive and people who had previously received treatment. It is thought that treatment of recurrent AGWs is more difficult than treatment of a first episode of AGWs. Based on feedback from clinical experts, the project team assumed that the populations enrolled are analogous and are representative of people with AGWs and attending GUM clinics. However, the uncertainty around the comparability of the study populations and therefore the generalisability of the results to clinical practice is acknowledged. Subgroup analyses were planned based on type, number and size of AGWs, all of which are factors thought to influence treatment effect. However, lack of data on clinical effectiveness based on these characteristics precluded analysis of the differential effects of treatments in the subgroups of interest. Determination of the effect of AGW morphology, if any, on treatment effect could contribute to the development of more clinically effective and cost-effective treatment algorithms for AGWs.
Despite identification of 60 studies, most comparisons in the MTC are informed by only one RCT. Additionally, a lack of head-to-head RCTs comparing key treatments, together with minimal reporting of results in some studies, precluded comprehensive analysis of all treatments for AGWs. As a consequence of these limitations in the available evidence, there is considerable uncertainty around the results generated, as evidenced by the wide CrIs. Because of time constraints it was not possible to assess separately the closed loops within the network, which would have helped to determine whether or not the results generated from ‘direct’ evidence aligned with the results generated from the ‘indirect’ evidence on introduction of the wider network. Additionally, it has been proposed that in MTCs of studies with variable periods of follow-up, the hazard ratio rather than the OR would be a more appropriate estimate of effect. 240 It is known that ORs change over time and use of a hazard ratio would account for the variable time horizons across the studies. 241 Given that the duration of treatment varied across the studies, particularly for the topical interventions, further analyses using the hazard ratio as the estimate of effect would be of interest.
Cost-effectiveness
Strengths of the analysis
The economic analysis has been carried out in accordance with the International Society for Pharmacoeconomics and Outcomes Research good modelling practice guidance. 242 The model structure was developed following review of the existing economic literature, and in conjunction with clinical experts.
Compared with existing analyses, this study analyses the greatest number of treatment options for AGW. Data sources for the economic model were systematically identified and standard UK sources of cost data were used. The clinical data included in the analysis were systematically identified and synthesised. This study incorporated HRQoL as the measure of benefit and is thus the first cost–utility analysis relating to treatments for AGWs.
The model results were tested extensively in sensitivity analysis including scenario analysis, one-way sensitivity analysis and threshold analysis.
Weaknesses of the analysis
The weaknesses of the analysis relate largely to the uncertainties in the clinical effectiveness data included within the model. Recurrence data were limited and a number of model assumptions were required for the base-case analysis. In particular, the following simplifying assumptions were made:
-
Complete clearance occurs at the mid-point of treatment.
-
Recurrence occurs at the mid-point of follow-up.
-
The number of lines of therapy prior to treatment for persistent warts is two.
-
All appointments take place within a GUM clinic.
-
The types of appointment in the GUM clinic (doctor led, nurse led or doctor with nurse) were equally split.
However, with the exception of all appointments taking place within a GUM clinic, which was not considered to be a key point of difference between interventions, these assumptions were comprehensively tested in sensitivity analysis.
Additionally, it was not possible to analyse data by characteristics of AGWs; thus, the findings of the analysis have limited applications to specific types of AGWs (e.g. keratinised vs. non-keratinised warts). In addition, based on the limited information provided in the clinical trials, it was not possible to specifically assess the effectiveness of treatments for those large and persistent AGWs requiring repeated treatments over a prolonged time period.
The economic analysis also omitted comparison of a number of treatment options of interest because of a lack of clinical data. These included cidofovir, electrotherapy, any combination of treatments in addition to those included and podophyllotoxin cream 0.15%.
Suggested research priorities
The evidence base to inform the first-line treatment of AGWs, albeit large, is limited in terms of the number of studies providing data on the effectiveness of individual interventions. A RCT evaluating the interventions predominantly used in clinical practice would go some way to clarifying the comparative clinical effectiveness of interventions. The inclusion of a simultaneous cost-effectiveness component would be beneficial. A search of the World Health Organization International Clinical Trials Registry Platform identified one trial, which, at the time of writing, is listed as having completed recruitment with a projected trial completion date of 31 March 2017. 243 The RCT is designed to evaluate whether imiquimod 5% cream or podophyllotoxin 0.15% cream is most effective at clearing AGWs and preventing recurrence. The trial will simultaneously evaluate whether or not HPV vaccine started at the time of initiating topical treatment increases the effectiveness of the cream in either clearing AGWs or preventing recurrence. A search of ClinicalTrials.gov identified no additional trials. Considering the findings from the MTC presented here, the project team considers that a RCT also including podophyllotoxin 0.5% solution would be warranted. In addition, people with either a first episode or a recurrent episode of AGWs are eligible for inclusion in the ongoing RCT. 243 A study stratifying by status of previous treatment would help to clarify whether or not the clinical effectiveness of an intervention varies according to whether it is used in the treatment of a first episode or a recurrent episode of AGWs. Assessment of a topical intervention in combination with an ablative technique would also help to inform the evidence base.
Limited data on recurrence were available. Given the propensity of AGWs to recur, clarification of the maintenance effect of interventions, if any, would be beneficial. Imiquimod 5% cream elicits an effect through modification of the immune system. One potential area for investigation is whether or not complete clearance achieved through an enhanced immune response after treatment with imiquimod 5% cream might be longer lasting than complete clearance using other interventions.
Acknowledgements
The project team thanks Dr Mayura Nathan and Dr Colm O’Mahony (Consultants in Genitourinary Medicine) for their input throughout the project and Dr Mark Lawton and Dr Gillian Dean (Consultants in Sexual Health and HIV) for reviewing the report. The project team also thanks Victoria Wakefield for her contributions to data abstraction and data validation for the review of clinical effectiveness. Thanks also to Fatima Salih for her contribution throughout the project, including devising and running the literature searches for studies of cost-effectiveness and costs, and drafting the corresponding sections of the report, and for carrying out data abstraction and quality assessment of cost-effectiveness studies.
The views expressed in this report are those of the authors and not necessarily those of the National Institute for Health Research (NIHR) HTA programme. Any errors are the responsibility of the authors. The British Medical Journal Technology Assessment Group (BMJ-TAG) and the editorial team of the BMJ work independently of one another. The views and opinions expressed in this report are those of the BMJ-TAG.
Contributions of authors
Elizabeth Thurgar provided project management; devised and carried out the literature searches for the review of cost-effectiveness and HRQoL; carried out the appraisal of abstracts retrieved from the clinical effectiveness, cost-effectiveness and HRQoL literature searches; carried out study selection and data extraction; designed and developed the economic model; wrote the sections of the report relating to the health economics and cost-effectiveness analysis; and contributed to the editing of the report.
Samantha Barton provided project management; devised and carried out the literature searches for the systematic review of clinical effectiveness; carried out the appraisal of abstracts retrieved from the literature searches; assessed full publications for inclusion; contributed to data extraction and validation; carried out meta-analysis; wrote the sections of the report relating to clinical effectiveness; and contributed to the editing of the report.
Charlotta Karner contributed to the appraisal of abstracts retrieved from the literature searches; assessed the full publications for inclusion; and contributed to the editing of the report.
Steven J Edwards contributed to the meta-analysis; contributed to the editing of the report; and was overall director of the project and guarantor of the report.
All authors read and commented on draft versions of the report.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- NHS Choices . Genital Warts 2012. www.nhs.uk/Conditions/Genital_warts/Pages/Introduction.aspx (accessed 7 September 2015).
- Health Protection Agency . Total Number of STI Diagnoses and Other Episodes of Care Seen at Genitourinary Medicine Clinics, UK &Amp; England 2014. www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1215589015024 (accessed 1 November 2014).
- Desai S, Wetten S, Woodhall SC, Peters L, Hughes G, Soldan K, et al. Genital warts and cost of care in England. Sex Transm Infect 2011;87:464-8. http://dx.doi.org/10.1136/sti.2010.048421.
- Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol 2005;32:S16-24. http://dx.doi.org/10.1016/j.jcv.2004.12.008.
- British Association for Sexual Health and HIV . United Kingdom National Guideline on the Management of Anogenital Warts 2007. www.bashh.org/documents/86/86.pdf (accessed 7 September 2015).
- Lacey CJ, Woodhall SC, Wikstrom A, Ross J. 2012 European guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol 2013;27:e263-70. http://dx.doi.org/10.1111/j.1468-3083.2012.04493.x.
- British Association for Sexual Health and HIV . UK National Guidelines on the Management of Anogenital Warts 2014. Draft Guidelines 2014. www.bashh.org/documents/UK%20national%20guideline%20on%20Warts%202015%20FINAL.pdf (accessed 30 November 2015).
- Smith EM, Parker MA, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP. Evidence for vertical transmission of HPV from mothers to infants. Infect Dis Obstet Gynecol 2010;2010. http://dx.doi.org/10.1155/2010/326369.
- Batista CS, Atallah AN, Saconato H, da Silva EM. 5-FU for genital warts in non-immunocompromised individuals. Cochrane Database Syst Rev 2010;4. http://dx.doi.org/10.1002/14651858.cd006562.pub2.
- Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserhiet JN, Corey L, et al. Sexually Transmitted Diseases. New York, NY: McGraw-Hill; 2008.
- Buck HW. Warts (Genital) 2015. http://clinicalevidence.bmj.com/x/systematic-review/1602/overview.html (accessed 7 September 2015).
- von Krogh G, Lacey CJ, Gross G, Barrasso R, Schneider A. European course on HPV associated pathology: guidelines for primary care physicians for the diagnosis and management of anogenital warts. Sex Transm Infect 2000;76:162-8. http://dx.doi.org/10.1136/sti.76.3.162.
- Patell H, Wagner M, Singhal P, Smita K. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis 2013;13. http://dx.doi.org/10.1186/1471-2334-13-39.
- Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis 2004;190:468-76. http://dx.doi.org/10.1086/421279.
- Burk RD, Ho GY, Beardsley L, Lempa M, Peters M, Bierman R. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. J Infect Dis 1996;174:679-89. http://dx.doi.org/10.1093/infdis/174.4.679.
- Mitrani-Rosenbaum S, Tsvieli R, Tur-Kaspa R. Oestrogen stimulates differential transcription of human papillomavirus type 16 in SiHa cervical carcinoma cells. J Gen Virol 1989;70:2227-32. http://dx.doi.org/10.1099/0022-1317-70-8-2227.
- Castellsagué X, Bosch FX, Muñoz N, Meijer CJ, Shah KV, de Sanjose S, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med 2002;346:1105-12. http://dx.doi.org/10.1056/NEJMoa011688.
- Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician 2004;70:2335-42.
- Wiley DJ, Douglas J, Beutner K, Cox T, Fife K, Moscicki AB, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis 2002;35:S210-24. http://dx.doi.org/10.1086/342109.
- Centers for Disease Control and Prevention . Sexually Transmitted Diseases Treatment Guidelines, 2006 2006. www.cdc.gov/mmwr/preview/mmwrhtml/rr5511a1.htm (accessed 7 September 2015).
- Carne CA, Dockerty G. Genital warts: need to screen for coinfection. BMJ 1990;300. http://dx.doi.org/10.1136/bmj.300.6722.459.
- Griffiths V, Cheung WH, Carlin EM, Ahmed-Jushuf I. Incidence of concurrent sexually transmitted infections in patients with genital warts. Int J STD AIDS 2006;17:413-14. http://dx.doi.org/10.1258/095646206777323328.
- Pakianathan MR, Ross JD, McMillan A. Characterizing patients with multiple sexually acquired infections: a multivariate analysis. Int J STD AIDS 1996;7:359-61. http://dx.doi.org/10.1258/0956462961918086.
- National Institute for Health and Care Excellence . Warts – Anogenital. Clinical Knowledge Summaries 2012. http://cks.nice.org.uk/warts-anogenital#!topicsummary (accessed 7 September 2015).
- World Health Organization International Agency for Research on Cancer . IARC Monograph on the Evaluation of Carcinogenic Risks to Humans. Volume 90: Human Papillomaviruses 2007. http://monographs.iarc.fr/ENG/Monographs/vol90/mono90.pdf (accessed 7 September 2015).
- Muñoz N, Bosch FX, de Sanjose S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348:518-27. http://dx.doi.org/10.1056/NEJMoa021641.
- Health Protection Agency . Health Protection Report. Trends in Genital Herpes and Genital Warts Infections, United Kingdom: 2000 to 2009 2011. www.hpa.org.uk/hpr/archives/2011/hpr1711.pdf (accessed 1 October 2014).
- Reitano M. Counseling patients with genital warts. Am J Med 1997;102:38-43. http://dx.doi.org/10.1016/S0002-9343(97)00182-4.
- Clarke P, Ebel C, Catotti DN, Stewart S. The psychosocial impact of human papillomavirus infection: implications for health care providers. Int J STD AIDS 1996;7:197-200. http://dx.doi.org/10.1258/0956462961917618.
- Jeynes C, Chung MC, Challenor R. ‘Shame on you’ – the psychosocial impact of genital warts. Int J STD AIDS 2009;20:557-60. http://dx.doi.org/10.1258/ijsa.2008.008412.
- Persson G, Dahlof LG, Krantz I. Physical and psychological effects of anogenital warts on female patients. Sex Transm Dis 1993;20:10-3. http://dx.doi.org/10.1097/00007435-199301000-00003.
- Woodhall S, Ramsey T, Cal C, Crouch S, Jit M, Birks Y, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect 2008;84:161-6. http://dx.doi.org/10.1136/sti.2007.029512.
- Ireland JA, Reid M, Powell R, Petrie KJ. The role of illness perceptions: psychological distress and treatment-seeking delay in patients with genital warts. Int J STD AIDS 2005;16:667-70. http://dx.doi.org/10.1258/095646205774357334.
- Waller J, Marlow LA, Wardle J. The association between knowledge of HPV and feelings of stigma, shame and anxiety. Sex Transm Infect 2007;83:155-9. http://dx.doi.org/10.1136/sti.2006.023333.
- Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS 1998;9:571-8. http://dx.doi.org/10.1258/0956462981921143.
- Badia X, Colombo JA, Lara N, Llorens MA, Olmos L, Sainz de los Terreros M, et al. Combination of qualitative and quantitative methods for developing a new health related quality of life measure for patients with anogenital warts. Health Qual Life Outcomes 2005;3. http://dx.doi.org/10.1186/1477-7525-3-24.
- Mortensen GL, Larsen HK. The quality of life of patients with genital warts: a qualitative study. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-113.
- Mortensen GL. Long-term quality of life effects of genital warts – a follow-study. Dan Med Bull 2010;57.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- European Medicines Agency . Cervarix 2014. www.ema.europa.eu/ema/index.jsp?curl = pages/medicines/human/medicines/000721/human_med_000694.jsp&mid = WC0b01ac058001d124 (accessed 1 October 2014).
- European Medicines Agency . Gardasil 2014. www.ema.europa.eu/ema/index.jsp?curl = pages/medicines/human/medicines/000703/human_med_000805.jsp&mid = WC0b01ac058001d124 (accessed 1 October 2014).
- Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions . N Engl J Med 2007;356:1915-27. http://dx.doi.org/10.1056/NEJMoa061741.
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a Phase III double-blind, randomised controlled trial. Lancet 2007;369:2161-70. http://dx.doi.org/10.1016/S0140-6736(07)60946-5.
- Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis 2009;199:926-35. http://dx.doi.org/10.1086/597307.
- Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis 2009;199:936-44. http://dx.doi.org/10.1086/597309.
- Department of Health . Introduction of Human Papillomavirus Vaccine into the National Immunisation Programme: Announcement of Vaccine to Be Used 2008. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_085581.pdf (accessed 7 September 2015).
- Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a769.
- Department of Health . Annual HPV Vaccine Coverage in England in 2009 2010 2010. www.gov.uk/government/uploads/system/uploads/attachment_data/file/215800/dh_123826.pdf (accessed 7 September 2015).
- NHS Choices . HPV Vaccine 2014. www.nhs.uk/Conditions/vaccinations/Pages/hpv-human-papillomavirus-vaccine.aspx (accessed 7 September 2015).
- Fairley CK, Hocking JS, Gurrin LC, Chen MY, Donovan B, Bradshaw CS. Rapid decline in presentations of genital warts after the implementation of a national quadrivalent human papillomavirus vaccination programme for young women. Sex Transm Infect 2009;85:499-502. http://dx.doi.org/10.1136/sti.2009.037788.
- Lanitis TC. The cost of managing genital warts in the UK. Int J STD AIDS 2012;23:189-94. http://dx.doi.org/10.1258/ijsa.2011.011218.
- Dockrell DH, Kinghorn GR. Imiquimod and resiquimod as novel immunomodulators. J Antimicrob Chemother 2001;48:751-5. http://dx.doi.org/10.1093/jac/48.6.751.
- Wagner TL, Horton VL, Carlson GL, Myhre PE, Gibson SJ, Imbertson LM, et al. Induction of cytokines in cynomolgus monkeys by the immune response modifiers, imiquimod, S-27609 and S-28463. Cytokine 1997;9:837-45. http://dx.doi.org/10.1006/cyto.1997.0239.
- Sick C, Schultz U, Munster U, Meier J, Kaspers B, Staeheli P. Promoter structures and differential responses to viral and nonviral inducers of chicken type I interferon genes. J Biol Chem 1998;273:9749-54. http://dx.doi.org/10.1074/jbc.273.16.9749.
- European Medicines Agency . Aldara 2014. www.ema.europa.eu/ema/index.jsp?curl = pages/medicines/human/medicines/000179/human_med_000635.jsp&mid = WC0b01ac058001d124 (accessed 1 October 2014).
- US Food and Drug Administration . Aldara: Clinical Executive Summary 2007. www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM162961.pdf (accessed 7 September 2015).
- US Food and Drug Administration . Zyclara: Clinical Review 2010. www.fda.gov/downloads/drugs/developmentapprovalprocess/developmentresources/ucm252892.pdf (accessed 7 September 2015).
- Electronic Medicines Compendium . Aldara 5% Cream 2014. www.medicines.org.uk/emc/medicine/8 (accessed 7 September 2015).
- Hartmann JT, Lipp HP. Camptothecin and podophyllotoxin derivatives: inhibitors of topoisomerase I and II – mechanisms of action, pharmacokinetics and toxicity profile. Drug Saf 2006;29:209-30. http://dx.doi.org/10.2165/00002018-200629030-00005.
- Mazurkiewicz W, Jablonska S. Clinical efficacy of Condyline (0.5% podophyllotoxin solution and cream) versus podophyllin in the treatment of external condylomata acuminata. J Dermatol Treat 1990;1:123-5. http://dx.doi.org/10.3109/09546639009086712.
- Kirby P, Dunne A, King DH, Corey L. Double-blind randomized clinical trial of self-administered podofilox solution versus vehicle in the treatment of genital warts. Am J Med 1990;88:465-9. http://dx.doi.org/10.1016/0002-9343(90)90424-C.
- Greenberg MD, Rutledge LH, Reid R, Berman NR, Precop SL, Elswick RK. A double-blind, randomized trial of 0.5% podofilox and placebo for the treatment of genital warts in women. Obstet Gynecol 1991;77:735-9.
- Kinghorn GR, McMillan A, Mulcahy F, Drake S, Lacey C, Bingham JS. An open, comparative, study of the efficacy of 0.5% podophyllotoxin lotion and 25% podophyllotoxin solution in the treatment of condylomata acuminata in males and females. Int J STD AIDS 1993;4:194-9.
- Lacey CJ, Goodall RL, Tennvall GR, Maw R, Kinghorn GR, Fisk PG, et al. Randomised controlled trial and economic evaluation of podophyllotoxin solution, podophyllotoxin cream, and podophyllin in the treatment of genital warts. Sex Transm Infect 2003;79:270-5. http://dx.doi.org/10.1136/sti.79.4.270.
- Claesson U, Lassus A, Happonen H, Hogstrom L, Siboulet A. Topical treatment of venereal warts: a comparative open study of podophyllotoxin cream versus solution. Int J STD AIDS 1996;7:429-34. http://dx.doi.org/10.1258/0956462961918400.
- Goh CL, Ang CB, Chan RK, Cheong WK. Comparing treatment response and complications between podophyllin 0.5%/0.25% in ethanol vs. podophyllin 25% in tincture benzoin for penile warts. Singapore Med J 1998;39:17-9.
- Handley J, Maw R, Horner T, Lawther H, Dinsmore W. Self-treatment of primary anogenital warts in men with podophyllin 0.5% in ethanol compared with podophyllotoxin (Condyline): a randomised double blind comparative study. Ir J Med Sci 1992;161.
- Tyring S, Edwards L, Cherry LK, Ramsdell WM, Kotner S, Greenberg MD, et al. Safety and efficacy of 0.5% podofilox gel in the treatment of anogenital warts. Arch Dermatol 1998;134:33-8. http://dx.doi.org/10.1001/archderm.134.1.33.
- Electronic Medicines Compendium . Condyline 2014. www.medicines.org.uk/emc/medicine/22861 (accessed 7 September 2015).
- Electronic Medicines Compendium . Warticon Solution 2014. www.medicines.org.uk/emc/medicine/7316 (accessed 7 September 2015).
- Electronic Medicines Compendium . Warticon Cream 2014. www.medicines.org.uk/emc/medicine/7322 (accessed 7 September 2015).
- Rodriguez M, Zachary KC. Cidofovir: An Overview 2014. www.uptodate.com/contents/cidofovir-an-overview (accessed 7 September 2015).
- Orlando G, Fasolo MM, Beretta R, Cargnel A. Diagnosis and Management of Genital Warts in HIV Disease 2000. www.medscape.com/viewarticle/410246_5 (accessed 1 October 2014).
- European Medicines Agency . Vistide 2014. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000121/human_med_001145.jsp&mid=WC0b01ac058001d124 (accessed 1 October 2014).
- FDA approves cidofovir for treatment of CMV retinitis . Food and Drug Administration. J Int Assoc Physicians AIDS Care 1996;2.
- De Clercq E. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin Microbiol Rev 2003;16:569-96. http://dx.doi.org/10.1128/CMR.16.4.569-596.2003.
- Petersen CS, Weismann K. Quercetin and kaempherol: an argument against the use of podophyllin?. Genitourin Med 1995;71:92-3. http://dx.doi.org/10.1136/sti.71.2.92.
- von Krogh G, Horenblas S. The management and prevention of premalignant penile lesions. Scand J Urol Nephrol Suppl 2000;205:220-9. http://dx.doi.org/10.1080/00365590050509959.
- Bjeldanes LF, Chang GW. Mutagenic activity of quercetin and related compounds. Science 1977;197:577-8. http://dx.doi.org/10.1126/science.327550.
- Pamukcu AM, Yalciner S, Hatcher JF, Bryan GT. Quercetin, a rat intestinal and bladder carcinogen present in bracken fern (Pteridium aquilinum). Cancer Res 1980;40:3468-72.
- Medicines and Healthcare products Regulatory Agency . Podophyllum Resin: Summary of Product Characteristics 2005. www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1369372854073.pdf (accessed 7 September 2015).
- Yanofsky VR, Patel RV, Goldenberg G. Genital warts: a comprehensive review. J Clin Aesthet Dermatol 2012;5:25-36.
- Leung L. Hyfrecation for recalcitrant nongenital warts. J Family Med Prim Care 2013;2:141-4. http://dx.doi.org/10.4103/2249-4863.117403.
- Centre for Reviews and Dissemination . CRD’s Guidance for Undertaking Reviews in Health Care 2011. www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm (accessed 7 September 2015).
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2009.
- Edwards S, Barton S, Karner C, Thurgar E, Trevor N. Clinical and Cost Effectiveness of Interventions for the Treatment of Anogenital Warts: Systematic Review and Economic Evaluation 2014. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013005457#.U-I9YPldV4U (accessed 1 October 2014).
- Information Specialists’ Sub-Group . The InterTASC Information Specialists’ Sub-Group Search Filter Resource 2013. https://sites.google.com/a/york.ac.uk/issg-search-filters-resource/methods-of-developing-search-filters (accessed 7 September 2015).
- Scottish Intercollegiate Guidelines Network . Search Filters 2013. www.sign.ac.uk/methodology/filters.html (accessed 1 August 2013).
- Clinical Evidence . Study Design Search Filters 2013. http://clinicalevidence.bmj.com/x/set/static/ebm/learn/665076.html (accessed 7 September 2015).
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. NICE; 2011.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: Introduction to Evidence Synthesis for Decision Making. NICE; 2013.
- Imiquimod – Aldara® – treatment of genital warts – oncolytic – immunomodulator . Drugs Future 2000;25.
- Biao ZS, Yue LS, Li ZG, Bai YG. The curative effect observation of home-made podophyllotoxin in external use for condyloma acuminatum. Chin J Dermatovenereol 1995;9:256-7.
- Cortés Gutiérrez EI, Witvrun Avila JN, Sánchez RG, Gaspar Belmonte JA, Hernández GF, Cerda Flores RM. Molecular detection of human papillomavirus in women with cervical condylomas treated with trichloroacetic acid. Ginecol Obstet Mex 2005;73:111-16.
- Alfonso-Trujillo I, Rodriguez Garcia MA, Rosa GA, Acosta MD, Navarro MM, Collazo CS. Trichloroacetic acid versus cryosurgery in the condyloma acuminata topical treatment. Piel 2009;24:176-80. http://dx.doi.org/10.1016/S0213-9251(09)70750-6.
- Alfonso-Trujillo I, Medina DA, Labrada MA, Quesada MR, Garcia MAR. Condyloma acuminate: efficacy of podophyllin alone vs. podophyllin associated with levamisole. Piel 2009;24:354-9. http://dx.doi.org/10.1016/S0213-9251(09)72017-9.
- Alfonso-Trujillo I, Pernas GA, Rodriguez Garcia MA, Perez AT, Toledo G, Collazo CS. Condyloma acuminata: comparative therapeutic efficacy of cryosurgery vs. cryosurgery associated with levamisole. Piel 2014;258:433-8.
- Liu CF, Li SP. Observation of 6 methods of treating condyloma acuminatum (genital warts). J Chin AIDS STD Prev Cont 1998;4:225-6.
- Mueller U, Philipp CM, Berlien HP. Laser therapy of human papilloma virus associated genital warts. Lasers Surg Med n.d.;52.
- Puri N. A study on the use of imiquimod for the treatment of genital molluscum contagiosum and genital warts in female patients. Indian J Sex Transm Dis 2009;30:84-8. http://dx.doi.org/10.4103/0253-7184.62763.
- Qian H, Shen SF, Zheng YB. The clinical observation of Podophyllinresin solution for condyloma acuminatum. Chin J Dermatovenereol 1995;9.
- Syed TA. Imiquimod 5% versus podophyllotoxin 0.5% in cream for the treatment of genital warts. A placebo-controlled, double-blind, comparative study. Ann Dermatol Venereol 2002.
- Wang MY, Tong ZQ. The comparative study of 3 kinds therapy methods in treating condyloma acuminatum. Chin J Dermatovenereol 2000;14.
- Wang MY, Zhang L, Lan HM, Yan RX. The curative effect observation of 30 cases home made Podphyllotoxin for Condyloma acuminatum. Chin J Dermatovenereol 1995;9.
- Podophyllotoxin 0.5% tincture in the treatment of 32 cases of condyloma acuminatum . Chin J Dermatovenereol 1995;28.
- Luo KP, Jiang H, Jiao Q, Wang GL, Zong YB, Ma LL. Treatment of multiple condyloma acuminatum of the coronary sulcus with sleeve circumcision of prepuce and coronary sulcus skin (a report of 35 cases). Zhonghua Nan Ke Xue 2006;12:240-6.
- Wu Y, Jin Z, Feng H, Zhang C, Ge M, Jia Q, et al. Clinical study on treatment of condyloma acuminata with 0.5% podophyllotoxin film. Chin Pharm J 2001;36:564-5.
- Xiao ZZ. The contrast of clinical efficacy of three ways for condyloma acuminatum. Chin J Dermatovenereol 1995;9.
- Yu TZ, Xie XJ. Effect analysis of three therapies for condyloma acuminatum. Chin J Dermatovenereol 1998;12:119-20.
- Wu T, Li Y, Bian Z, Liu G, Moher D. Randomized trials published in some Chinese journals: how many are randomized?. Trials 2009;10. http://dx.doi.org/10.1186/1745-6215-10-46.
- Arican O, Guneri F, Bilgic K, Karaoglu A. Topical imiquimod 5% cream in external anogenital warts: a randomized, double-blind, placebo-controlled study. J Dermatol 2004;31:627-31. http://dx.doi.org/10.1111/j.1346-8138.2004.tb00568.x.
- Baker D, Ferris DG, Tyring S, Ault K, Levy S, Liu T, et al. Imiquimod 2.5% and 3.75% applied daily for up to 8 weeks to treat external genital warts. Contraception 2010;82. http://dx.doi.org/10.1016/j.contraception.2010.04.129.
- Benedetti Panici P, Scambia G, Baiocchi G, Perrone L, Pintus C, Mancuso S. Randomized clinical trial comparing systemic interferon with diathermocoagulation in primary multiple and widespread anogenital condyloma. Obstet Gynecol 1989;74:393-7.
- Beutner KR, Conant MA, Friedman-Kien AE, Illeman M, Artman NN, Thisted RA, et al. Patient-applied podofilox for treatment of genital warts. Lancet 1989;1:831-4. http://dx.doi.org/10.1016/S0140-6736(89)92282-4.
- Beutner KR, Tyring SK, Trofatter KF, Douglas JM, Spruance S, Owens ML, et al. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother 1998;42:789-94.
- Beutner KR, Spruance SL, Hougham AJ, Fox TL, Owens ML, Douglas JM. Treatment of genital warts with an immune-response modifier (imiquimod). J Am Acad Dermatol 1998;38:230-9. http://dx.doi.org/10.1016/S0190-9622(98)70243-9.
- Edwards L, Ferenczy A, Eron L, Baker D, Owens ML, Fox TL, et al. Self-administered topical 5% imiquimod cream for external anogenital warts. HPV Study Group. Human PapillomaVirus. Arch Dermatol 1998;134:25-30. http://dx.doi.org/10.1001/archderm.134.1.25.
- Gilson RJ, Shupack JL, Friedman-Kien AE, Conant MA, Weber JN, Nayagam AT, et al. A randomized, controlled, safety study using imiquimod for the topical treatment of anogenital warts in HIV-infected patients. Imiquimod Study Group. AIDS 1999;13:2397-404. http://dx.doi.org/10.1097/00002030-199912030-00011.
- Matteelli A, Beltrame A, Graifemberghi S, Forleo MA, Gulletta M, Ciravolo G, et al. Efficacy and tolerability of topical 1% cidofovir cream for the treatment of external anogenital warts in HIV-infected persons. Sex Transm Dis 2001;28:343-6. http://dx.doi.org/10.1097/00007435-200106000-00007.
- Snoeck R, Bossens M, Parent D, Delaere B, Degreef H, Van RM, et al. Phase II double-blind, placebo-controlled study of the safety and efficacy of cidofovir topical gel for the treatment of patients with human papillomavirus infection. Clin Infect Dis 2001;33:597-602. http://dx.doi.org/10.1086/322593.
- Syed TA, Lundin S, Ahmad SA. Topical 0.3% and 0.5% podophyllotoxin cream for self-treatment of condylomata acuminata in women. A placebo-controlled, double-blind study. Dermatology 1994;189:142-5. http://dx.doi.org/10.1159/000246818.
- Syed TA, Cheema KM, Khayyami M, Ahmad SA, Ahmad SH, Ahmad S, et al. Human leukocyte interferon-alpha versus podophyllotoxin in cream for the treatment of genital warts in males. A placebo-controlled, double-blind, comparative study. Dermatology 1995;191:129-32. http://dx.doi.org/10.1159/000246530.
- Syed TA, Khayyami M, Kriz D, Svanberg K, Kahlon RC, Ahmad SA, et al. Management of genital warts in women with human leukocyte interferon-alpha vs. podophyllotoxin in cream: a placebo-controlled, double-blind, comparative study. J Mol Med (Berl) 1995;73:255-8.
- Tyring SK, Arany I, Stanley MA, Tomai MA, Miller RL, Smith MH, et al. A randomized, controlled, molecular study of condylomata acuminata clearance during treatment with imiquimod. J Infect Dis 1998;178:551-5. http://dx.doi.org/10.1086/517472.
- von Krogh G, Hellberg D. Self-treatment using a 0.5% podophyllotoxin cream of external genital condylomata acuminata in women. A placebo-controlled, double-blind study. Sex Transm Dis 1992;19:170-4. http://dx.doi.org/10.1097/00007435-199205000-00012.
- von Krogh G, Szpak E, Andersson M, Bergelin I. Self-treatment using 0.25%–0.50% podophyllotoxin-ethanol solutions against penile condylomata acuminata: a placebo-controlled comparative study. Genitourin Med 1994;70:105-9. http://dx.doi.org/10.1136/sti.70.2.105.
- Orlando G, Fasolo MM, Beretta R, Merli S, Cargnel A. Combined surgery and cidofovir is an effective treatment for genital warts in HIV-infected patients. AIDS 2002;16:447-50. http://dx.doi.org/10.1097/00002030-200202150-00017.
- Fife KH, Ferenczy A, Douglas JM, Brown DR, Smith M, Owens ML. Treatment of external genital warts in men using 5% imiquimod cream applied three times a week, once daily, twice daily, or three times a day. Sex Transm Dis 2001;28:226-31. http://dx.doi.org/10.1097/00007435-200104000-00007.
- Garland SM, Waddell R, Mindel A, Denham IM, McCloskey JC. An open-label phase II pilot study investigating the optimal duration of imiquimod 5% cream for the treatment of external genital warts in women. Int J STD AIDS 2006;17:448-52. http://dx.doi.org/10.1258/095646206777689161.
- Komericki P, Akkilic-Materna M, Strimitzer T, Aberer W. Efficacy and safety of imiquimod versus podophyllotoxin in the treatment of anogenital warts. Sex Transm Dis 2011;38:216-18. http://dx.doi.org/10.1097/OLQ.0b013e3181f68ebb.
- Padhiar BB, Karia UK, Aggarwal R, Shah BJ, Bilimoria FE. A comparative study of efficacy of imiquimod 5% versus podophyllin 20% in treatment of external and genital warts (60 patients). Indian J Sex Transm Dis 2006;27:67-9.
- Stefanaki C, Katzouranis I, Lagogianni E, Hadjivassiliou M, Nicolaidou E, Panagiotopoulos A, et al. Comparison of cryotherapy to imiquimod 5% in the treatment of anogenital warts. Int J STD AIDS 2008;19:441-4. http://dx.doi.org/10.1258/ijsa.2007.007196.
- Trofatter KF, Ferenczy A, Fife KH. Increased frequency of dosing of imiquimod 5% cream in the treatment of external genital warts in women. Int J Gynaecol Obstet 2002;76:191-3. http://dx.doi.org/10.1016/S0020-7292(01)00573-2.
- Tuncel A, Erbagci Z, Ozgoztasi O. An open-label comparative study to evaluate the efficacy and tolerability of imiquimod 5% cream alone and combined with cryotherapy in the treatment of recalcitrant anogenital warts. Eur Acad Dermatol Venereol 2005;19.
- Edwards A, Atma-Ram A, Thin RN. Podophyllotoxin 0.5% v podophyllin 20% to treat penile warts. Genitourin Med 1988;64:263-5. http://dx.doi.org/10.1136/sti.64.4.263.
- Hellberg D, Svarrer T, Nilsson S, Valentin J. Self-treatment of female external genital warts with 0.5% podophyllotoxin cream (Condyline) vs. weekly applications of 20% podophyllin solution. Int J STD AIDS 1995;6:257-61.
- Kar PK, Rajagopal R, Murthy PS. Topical podophyllin and podophyllotoxin for treatment of genital warts: a comparative study. Indian J Dermatol 2003;48:146-50.
- Landthaler M, Frosschl M. Zur behandlung von condylomata acuminata mit podophyllotoxin. Dt Dermatol 1987;11:1223-5.
- Lassus A, Haukka K, Forsstrom S. Podophyllotoxin for treatment of genital warts in males. A comparison with conventional podophyllin therapy. Eur J Sex Transm Dis 1984;2:31-3.
- Petersen CS, Agner T, Ottevanger V, Larsen J, Ravnborg L. A single-blind study of podophyllotoxin cream 0.5% and podophyllotoxin solution 0.5% in male patients with genital warts. Genitourin Med 1995;71:391-2. http://dx.doi.org/10.1136/sti.71.6.391.
- Strand A, Brinkeborn RM, Siboulet A. Topical treatment of genital warts in men, an open study of podophyllotoxin cream compared with solution. Genitourin Med 1995;71:387-90. http://dx.doi.org/10.1136/sti.71.6.387.
- Syed TA, Lundin S. Topical treatment of penile condylomata acuminata with podophyllotoxin 0.3% solution, 0.3% cream and 0.15% cream. A comparative open study. Dermatology 1993;187:30-3. http://dx.doi.org/10.1159/000247193.
- White DJ, Billingham C, Chapman S, Drake S, Jayaweera D, Jones S, et al. Podophyllin 0.5% or 2.0% v podophyllotoxin 0.5% for the self treatment of penile warts: a double blind randomised study. Genitourin Med 1997;73:184-7. http://dx.doi.org/10.1136/sti.73.3.184.
- Gabriel G, Thin RN. Treatment of anogenital warts. Comparison of trichloracetic acid and podophyllin versus podophyllin alone. Br J Vener Dis 1983;59:124-6. http://dx.doi.org/10.1136/sti.59.2.124.
- Jensen SL. Comparison of podophyllin application with simple surgical excision in clearance and recurrence of perianal condylomata acuminata. Lancet 1985;2:1146-8. http://dx.doi.org/10.1016/S0140-6736(85)92677-7.
- Maiti H, Haye KR. Self-treatment of condylomata acuminata with podophyllin resin. Practitioner 1985;229:37-9.
- Nath D, Kumar B, Sharma KV, Kaur I, Gupta R, Malhotra S. Comparison of podophyllin and trichloroacetic acid for the treatment of genital warts. Indian J Dermatol Venereol Leprol 1990;56:22-4.
- Sherrard J, Riddell L. Comparison of the effectiveness of commonly used clinic-based treatments for external genital warts. Int J STD AIDS 2007;18:365-8. http://dx.doi.org/10.1258/095646207781024711.
- Simmons PD. Podophyllin 10% and 25% in the treatment of ano-genital warts. A comparative double-blind study. Br J Vener Dis 1981;57:208-9. http://dx.doi.org/10.1136/sti.57.3.208.
- Stone KM, Becker TM, Hadgu A, Kraus SJ. Treatment of external genital warts: a randomised clinical trial comparing podophyllin, cryotherapy, and electrodesiccation. Genitourin Med 1990;66:16-9. http://dx.doi.org/10.1136/sti.66.1.16.
- Tabari ST, Javadian M, Barat S. The efficacy of podophylin 20% and thricholoroacetic acid 30% in the treatment of genital wart. Casp J Intern Med 2010;1:16-9.
- Abdullah AN, Walzman M, Wade A. Treatment of external genital warts comparing cryotherapy (liquid nitrogen) and trichloroacetic acid. Sex Transm Dis 1993;20:344-5. http://dx.doi.org/10.1097/00007435-199320060-00008.
- Godley MJ, Bradbeer CS, Gellan M, Thin RN. Cryotherapy compared with trichloroacetic acid in treating genital warts. Genitourin Med 1987;63:390-2. http://dx.doi.org/10.1136/sti.63.6.390.
- Viazis N, Vlachogiannakos J, Vasiliadis K, Theodoropoulos I, Saveriadis A, Karamanolis DG. Earlier eradication of intra-anal warts with argon plasma coagulator combined with imiquimod cream compared with argon plasma coagulator alone: a prospective, randomized trial. Dis Colon Rectum 2007;50:2173-9. http://dx.doi.org/10.1007/s10350-007-9041-2.
- Azizjalali M, Ghaffarpour G, Mousavifard B. CO2 laser therapy versus cryotherapy in treatment of genital warts; a randomized controlled trial (RCT). Iran J Microbiol 2012;4:187-90.
- Bar-Am A, Lessing JB, Niv J, Brenner SH, Peyser MR. High- and low-power CO2 lasers. Comparison of results for three clinical indications. J Reprod Med 1993;38:455-8.
- Ferenczy A, Behelak Y, Haber G, Wright TC, Richart RM. Treating vaginal and external anogenital condylomas with electrosurgery vs. CO2 laser ablation. J Gynecol Surg 1995;11:41-50. http://dx.doi.org/10.1089/gyn.1995.11.41.
- Gilson RJ, Ross J, Maw R, Rowen D, Sonnex C, Lacey CJ. A multicentre, randomised, double-blind, placebo controlled study of cryotherapy versus cryotherapy and podophyllotoxin cream as treatment for external anogenital warts. Sex Transm Infect 2009;85:514-19. http://dx.doi.org/10.1136/sti.2009.038075.
- Simmons PD, Langlet F, Thin RN. Cryotherapy versus electrocautery in the treatment of genital warts. Br J Vener Dis 1981;57:273-4. http://dx.doi.org/10.1136/sti.57.4.273.
- Syed TA, Ahmadpour OA, Ahmad SA, Ahmad SH. Management of female genital warts with an analog of imiquimod 2% in cream: a randomized, double-blind, placebo-controlled study. J Dermatol 1998;25:429-33. http://dx.doi.org/10.1111/j.1346-8138.1998.tb02429.x.
- Syed TA, Hadi SM, Qureshi ZA, Ali SM, Kwah MS. Treatment of external genital warts in men with imiquimod 2% in cream. A placebo-controlled, double-blind study. J Infect 2000;41:148-51. http://dx.doi.org/10.1053/jinf.2000.0709.
- Langley PC. A cost-effectiveness analysis of sinecatechins in the treatment of external genital warts. J Med Econ 2010;13:1-7. http://dx.doi.org/10.3111/13696990903451461.
- Walczak JN. Cost-effectiveness analysis of imiquimod versus podophyllotoxin in treatment of genital/perianal warts in Poland. Value Health 2009;12. http://dx.doi.org/10.1016/S1098-3015(10)75104-4.
- Lafuma AM. A model-based comparison of cost effectiveness of imiquimod versus podophyllotoxin for the treatment of external anogenital warts in France. Ann Dermatol Venereol 2003;130:731-6.
- Williams P, von Krogh G. The cost-effectiveness of patient-applied treatments for anogenital warts. Int J STD AIDS 2003;14:228-34. http://dx.doi.org/10.1258/095646203321264827.
- Alam M, Stiller M. Direct medical costs for surgical and medical treatment of condylomata acuminata. Arch Dermatol 2001;137:337-41.
- Fagnani F. Medicoeconomic analysis of the treatment of external condyloma acuminatum. Ann Dermatol Venereol 2000;127:3S27-9.
- Langley PC, Richwald GA, Smith MH. Modeling the impact of treatment options in genital warts: patient-applied versus physician-administered therapies. Clin Ther 1999;21:2143-55. http://dx.doi.org/10.1016/S0149-2918(00)87244-6.
- Langley PC, Tyring SK, Smith MH. The cost effectiveness of patient-applied versus provider-administered intervention strategies for the treatment of external genital warts. Am J Manag Care 1999;5:69-77.
- Mohanty KC. The cost effectiveness of treatment of genital warts with podophyllotoxin. Int J STD AIDS 1994;5:253-6.
- Brown RE, Breugelmans JG, Theodoratou D, Benard S, Brown RE, Breugelmans JG, et al. Costs of detection and treatment of cervical cancer, cervical dysplasia and genital warts in the UK. Curr Med Res Opin 2006;22:663-70. http://dx.doi.org/10.1185/030079906X99972.
- Carroll S, Charman F, Lanitis T, Brown R. Costs of managing genital warts in the UK. Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.08.222.
- Langley PC, White DJ, Drake SM. The costs of treating external genital warts in England and Wales: a treatment pattern analysis. Int J STD AIDS 2004;15:501-8. http://dx.doi.org/10.1258/0956462041558168.
- Woodhall SC, Jit M, Cai C, Ramsey T, Zia S, Crouch S, et al. Cost of treatment and QALYs lost due to genital warts: data for the economic evaluation of HPV vaccines in the United Kingdom. Sex Transm Dis 2009;36:515-21. http://dx.doi.org/10.1097/OLQ.0b013e3181a74c2c.
- Woodhall SC, Jit M, Soldan K, Kinghorn G, Gilson R, Nathan M, et al. The impact of genital warts: loss of quality of life and cost of treatment in eight sexual health clinics in the UK. Sex Transm Infect 2011;87:458-63. http://dx.doi.org/10.1136/sextrans-2011-050073.
- Annemans L, Remy V, Lamure E, Spaepen E, Lamotte M, Muchada JP, et al. Economic burden associated with the management of cervical cancer, cervical dysplasia and genital warts in Belgium. J Med Econ 2008;11:135-50. http://dx.doi.org/10.3111/13696990801961611.
- Baio G, Capone A, Marcellusi A, Mennini FS, Favato G. Economic burden of human papillomavirus-related diseases in Italy. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0049699.
- Castellsagué X, Cohet C, Puig-Tintoreé LM, Acebes LO, Salinas J, San Martin M, et al. Epidemiology and cost of treatment of genital warts in Spain. Eur J Public Health 2009;19:106-10. http://dx.doi.org/10.1093/eurpub/ckn127.
- Chesson HW, Ekwueme DU, Saraiya M, Watson M, Lowy DR, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine 2012;30:6016-19. http://dx.doi.org/10.1016/j.vaccine.2012.07.056.
- Dee A, Howell F, O’Connor C, Cremin S, Hunter K. Determining the cost of genital warts: a study from Ireland. Sex Transm Infect 2009;85:402-3. http://dx.doi.org/10.1136/sti.2008.033837.
- Dhitavat J, Charoenwatanachokchai A, Kongsin S, Kaewkungwal J, Ruengkris T, Bussaratid V, et al. PIN13 Burden and medical costs of anogenital warts in Bangkok, Thailand. Value Health 2010;7:A548-9. http://dx.doi.org/10.1016/S1098-3015(11)73293-4.
- Fox PA, Tung MY. Human papillomavirus: burden of illness and treatment cost considerations. Am J Clin Dermatol 2005;6:365-81. http://dx.doi.org/10.2165/00128071-200506060-00004.
- Herse F, Reissell E. The annual costs associated with human papillomavirus types 6, 11, 16, and 18 infections in Finland. Scand J Infect Dis 2011;43:209-15. http://dx.doi.org/10.3109/00365548.2010.541492.
- Hillemanns P, Breugelmans JG, Gieseking F, Benard S, Lamure E, Littlewood KJ, et al. Estimation of the incidence of genital warts and the cost of illness in Germany: a cross-sectional study. BMC Infect Dis 2008;8. http://dx.doi.org/10.1186/1471-2334-8-76.
- Hoy T, Singhal PK, Willey VJ, Insinga RP. Assessing incidence and economic burden of genital warts with data from a US commercially insured population. Curr Med Res Opin 2009;25:2343-51. http://dx.doi.org/10.1185/03007990903136378.
- Hu D, Goldie SJ. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol 2008;198. http://dx.doi.org/10.1016/j.ajog.2008.03.064.
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003;36:1397-403. http://dx.doi.org/10.1086/375074.
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005;23:1107-22. http://dx.doi.org/10.2165/00019053-200523110-00004.
- Low JJ, Ko Y, Ilancheran A, Zhang XH, Singhal PK, Tay SK, et al. Health and economic burden of HPV-related diseases in Singapore. Asian Pac J Cancer Prev 2012;13:305-8. http://dx.doi.org/10.7314/APJCP.2012.13.1.305.
- Lyalina LV, Tolkushin A, Akhmetzyanov IM, Nikolaevich MS. Cost of HPV associated diseases in Saint-Petersburg. Value Health 2011. http://dx.doi.org/10.1016/j.jval.2011.08.1144.
- Marra F, Ogilvie G, Colley L, Kliewer E, Marra CA. Epidemiology and costs associated with genital warts in Canada. Sex Transm Infect 2009;85:111-15. http://dx.doi.org/10.1136/sti.2008.030999.
- Merito M, Largeron N, Cohet C, Timelli L, Boselli F, Matteelli A, et al. Treatment patterns and associated costs for genital warts in Italy. Curr Med Res Opin 2008;24:3175-83. http://dx.doi.org/10.1185/03007990802485694.
- Monsonégo J, Breugelmans JG, Bouée S, Lafuma A, Bénard S, Rémy V. Anogenital warts incidence, medical management and costs in women consulting gynaecologists in France. Gynecol Obstet Fertil 2007;35:107-13. http://dx.doi.org/10.1016/j.gyobfe.2006.12.010.
- Owusu-Edusei K, Chesson HW, Gift TL, Tao G, Mahajan R, Ocfemia MC, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013;40:197-201. http://dx.doi.org/10.1097/OLQ.0b013e318285c6d2.
- Pirotta M, Stein AN, Conway EL, Harrison C, Britt H, Garland S. Genital warts incidence and healthcare resource utilisation in Australia. Sex Transm Infect 2010;86:181-6. http://dx.doi.org/10.1136/sti.2009.040188.
- Salo H, Leino T, Kilpi T, Auranen K, Tiihonen P, Lehtinen M, et al. The burden and costs of prevention and management of genital disease caused by HPV in women: a population-based registry study in Finland. Int J Cancer 2013;133:1459-69. http://dx.doi.org/10.1002/ijc.28145.
- Strauss MJ, Khanna V, Koenig JD, Downs SM, Goldberg SH, Manyak MJ, et al. The cost of treating genital warts. Int J Dermatol 1996;35:340-8. http://dx.doi.org/10.1111/j.1365-4362.1996.tb03635.x.
- Termrungruanglert, Havanond P, Khemapech N, Lertmaharit S, Pongpanich S, Khorprasert C, et al. PCN10 Estimation of the health and cost burden of HPV-related diseases in Thailand. Value Health 2010;13:A511-12. http://dx.doi.org/10.1016/S1098-3015(11)73103-5.
- van der Meijden WI, Notowicz A, Blog FB, Langley PC. A retrospective analysis of costs and patterns of treatment for external genital warts in the Netherlands. Clin Ther 2002;24:183-96. http://dx.doi.org/10.1016/S0149-2918(02)85014-7.
- Augustovski F, Pichon RA, Bardach A, Colantonio L, Ferrante D, Garcia Marti S, et al. Cryotherapy to Anogenital Warts. Informe Tecnico Breve No. 38. Buenos Aires: Institute for Clinical Effectiveness and Health Policy; 2009.
- Birley H, Clark F, Sparks R. Cost-effectiveness of imiquimod for anogenital warts. Int J STD AIDS 2002;13:647-8. http://dx.doi.org/10.1258/09564620260216371.
- Clemons RJ, Clemons-Miller A, Johnson SM, Williamson SK, Horn TD. Comparing therapy costs for physician treatment of warts. J Drugs Dermatol 2003;2:649-54.
- Dempsey AF, Koutsky LA. National burden of genital warts: a first step in defining the problem. Sex Transm Dis 2008;35:361-2. http://dx.doi.org/10.1097/OLQ.0b013e31816c5cda.
- Elbasha EH, Dasbach EJ, Insinga RP. A multi-type HPV transmission model. Bull Math Biol 2008;70:2126-76. http://dx.doi.org/10.1007/s11538-008-9338-x.
- Kong X, Gray RH, Moulton LH, Wawer M, Wang MC. A modeling framework for the analysis of HPV incidence and persistence: a semi-parametric approach for clustered binary longitudinal data analysis. Stat Med 2010;29:2880-9. http://dx.doi.org/10.1002/sim.4062.
- Koshiol JE, Laurent SA, Pimenta JM. Rate and predictors of new genital warts claims and genital warts-related healthcare utilization among privately insured patients in the United States. Sex Transm Dis 2004;31:748-52. http://dx.doi.org/10.1097/01.olq.0000145851.76025.ad.
- Kuwahara RT, Skinner RB, Tyring SK. Direct medical cost for surgical and medical treatment of condylomata acuminata. Arch Dermatol 2002;138:533-4.
- Langley PC, White DJ, Drake SM. Patterns of treatment and resource utilization in the treatment of external genital warts in England and Wales. Int J STD AIDS 2004;15:473-8. http://dx.doi.org/10.1258/0956462041211199.
- Lewis DA, Goldmeier D. The cost effectiveness of hospital-based 25% podophyllin vs home-based 0.5% podophyllotoxin in the treatment of anogenital warts. Int J STD AIDS 1995;6:224-5.
- Raymakers AJ, Sadatsafavi M, Marra F, Marra CA. Economic and humanistic burden of external genital warts. Pharmacoeconomics 2012;30:1-16. http://dx.doi.org/10.2165/11591170-000000000-00000.
- Scarbrough Lefebvre CD, Van Kriekinge G, Gonçalves MA, de Sanjose S. Appraisal of the burden of genital warts from a healthcare and individual patient perspective. Public Health 2011;125:464-75. http://dx.doi.org/10.1016/j.puhe.2011.01.016.
- Termrungruanglert W, Havanond P, Khemapech N, Lertmaharit S, Pongpanich S, Jirakorbchaipong P, et al. Model for predicting the burden and cost of treatment in cervical cancer and HPV-related diseases in Thailand. Eur J Gynaecol Oncol 2012;33:391-4.
- Van Enckevort P, Kongngamkam K, Van Kriekinge G. Costs and consequences of HPV vaccination in Thailand: results of a prevalence based model. Value Health 2011. http://dx.doi.org/10.1016/j.jval.2011.08.1163.
- Chapman R, Coles VAH, Lanitis T, Carroll SM. The costs of managing genital warts in the UK by devolved nation: England, Scotland, Wales and Northern Ireland. Value Health 2013;16. http://dx.doi.org/10.1016/j.jval.2013.08.163.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Beutner KR, Ferenczy A. Therapeutic approaches to genital warts. Am J Med 1997;102:28-37. http://dx.doi.org/10.1016/S0002-9343(97)00181-2.
- Mayeaux EJ, Harper MB, Barksdale W, Pope JB. Noncervical human papillomavirus genital infections. Am Fam Physician 1995;52:1137-50.
- Baker GE, Tyring SK. Therapeutic approaches to papillomavirus infections. Dermatol Clin 1997;15:331-40. http://dx.doi.org/10.1016/S0733-8635(05)70441-1.
- NICE Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Mennini FS, Marcellusi A, Baio G, Haeussler K, Favato G, Capone A. Loss of health utilities due to HPV-induced diseases in men and women: a multicenter Italian study. Value Health 2013;16. http://dx.doi.org/10.1016/j.jval.2013.03.219.
- Shi JF, Kang DJ, Qi SZ, Wu HY, Liu YC, Sun LJ, et al. Impact of genital warts on health related quality of life in men and women in mainland China: a multicenter hospital-based cross-sectional study. BMC Public Health 2012;12. http://dx.doi.org/10.1186/1471-2458-12-153.
- Mennini FS, Panatto D, Marcellusi A, Cristoforoni P, De Vincenzo R, Di Capua E, et al. Time trade-off procedure for measuring health utilities loss with human papillomavirus-induced diseases: a multicenter, retrospective, observational pilot study in Italy. Clin Ther 2011;33:1084-95. http://dx.doi.org/10.1016/j.clinthera.2011.06.012.
- Senecal MB. Loss of quality of life associated with genital warts: baseline analyses from a prospective study. Sex Transm Infect 2011;87:209-15. http://dx.doi.org/10.1136/sti.2009.039982.
- Brisson M, Van de Velde N, De Wals P, Boily MC. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine 2007;25:5399-408. http://dx.doi.org/10.1016/j.vaccine.2007.04.086.
- Drolet M, Brisson M, Maunsell E, Franco EL, Coutlee F, Ferenczy A, et al. The impact of anogenital warts on health-related quality of life: a 6-month prospective study. Sex Transm Dis 2011;38:949-56. http://dx.doi.org/10.1097/OLQ.0b013e3182215512.
- Langley PC, Freedman D, Wagner JS, Gupta S. The experience of external genital warts and genital herpes on quality of life. Value Health 2010;13. http://dx.doi.org/10.1016/S1098-3015(11)72661-4.
- Marra C, Ogilvie G, Gastonguay L, Colley L, Taylor D, Marra F, et al. Patients with genital warts have a decreased quality of life. Sex Transm Dis 2009;36:258-60. http://dx.doi.org/10.1097/OLQ.0b013e318191a55e.
- Fiander A, Cohet C. Psychosocial Impact of HPV-Related Diseases in Women in the UK: The PasQual Study n.d. www.eurogin.com/2010/EUROGIN2010_Abstracts.pdf (accessed 27 November 2015).
- Fiander A, Cohet C. Psychosocial Impact of HPV-Related Diseases in Men in the UK: The PasQual Study n.d. www.eurogin.com/2010/EUROGIN2010_Abstracts.pdf (accessed 27 November 2015).
- Mennini FS, Panatto D, Marcellusi A, Cristoforoni P, De Vincenzo R, Di Capua E, et al. The Time-Trade off Approach to Measure patients’ Preferences for Health States Associated With HPV-Related Pathologies: A Multicenter Study Performed in Italy n.d.
- Myers E, Green S, Lipkus I. Patient Preferences for Health States Related to HPV Infection: Visual Analog Scale Versus Time Trade-off Elicitation n.d.
- Bresse X, Goergen C, Prager B, Joura E. Universal vaccination with the quadrivalent HPV vaccine in Austria: impact on virus circulation, public health and cost-effectiveness analysis. Expert Rev 2014;14:269-81. http://dx.doi.org/10.1586/14737167.2014.881253.
- Drolet M, Laprise JF, Boily MC, Franco EL, Brisson M. Potential cost-effectiveness of the nonavalent human papillomavirus (HPV) vaccine. Int J Cancer 2014;134:2264-8. http://dx.doi.org/10.1002/ijc.28541.
- Khatibi M, Rasekh HR, Shahverdi Z, Jamshidi HR. Cost-effectiveness evaluation of quadrivalent human papilloma virus vaccine for HPV-related disease in Iran. Iran J Pharm Res 2014;13:225-34.
- Dominiak-Felden G, Cohet C, Atrux-Tallau S, Gilet H, Tristram A, Fiander A, et al. Impact of human papillomavirus-related genital diseases on quality of life and psychosocial wellbeing: results of an observational, health-related quality of life study in the UK. BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-1065.
- Department of Health . NHS Reference Costs 2012 to 2013 2013. www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013 (accessed 7 September 2015).
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Mavranezouli I, Meader N, Cape J, Kendall T. The cost effectiveness of pharmacological treatments for generalized anxiety disorder. Pharmacoeconomics 2013;31:317-33. http://dx.doi.org/10.1007/s40273-013-0031-z.
- Ades AE, Mavranezouli I, Dias S, Welton NJ, Whittington C, Kendall T. Network meta-analysis with competing risk outcomes. Value Health 2010;13:976-83. http://dx.doi.org/10.1111/j.1524-4733.2010.00784.x.
- Caro JJ, Briggs AH, Siebert U, Kuntz KM. ISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices – overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Value Health 2012;15:796-803. http://dx.doi.org/10.1016/j.jval.2012.06.012.
- International Standard Randomised Controlled Trial Number . Human Papillomavirus Infection: A Randomised Controlled Trial of Imiquimod Cream (5%) Versus Podophyllotoxin Cream (0.15%), in Combination With Quadrivalent Human Papillomavirus or Control Vaccination in the Treatment and Prevention of Recurrence of Anogenital Warts (HIPvac Trial) 2014. www.isrctn.com/ISRCTN32729817 (accessed 27 November 2015).
- Baker DA, Ferris DG, Martens MG, Fife KH, Tyring SK, Edwards L, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies. Infect Dis Obstet Gynecol 2011;2011. http://dx.doi.org/10.1155/2011/806105.
- ClinicalTrials.gov . Safety and Effectiveness Study of Imiquimod Creams in the Treatment of External Genital Warts 2011. http://clinicaltrials.gov/ct2/show/results/nct00674739 (accessed 1 December 2015).
- ClinicalTrials.gov . Phase 3 Study of Imiquimod Creams in the Treatment of External Genital Warts 2011. http://clinicaltrials.gov/ct2/show/study/nct00735462?sect=x01256 (accessed 1 December 2015).
- Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. Oxford: Blackwell Science Limited; 1971.
- Ferenczy A. Immune response modifiers: imiquimod. J Obstet Gynaecol 1998;18:S76-8. http://dx.doi.org/10.1080/01443619866606.
- Arany I, Tyring SK, Stanley MA, Tomai MA, Miller RL, Smith MH, et al. Enhancement of the innate and cellular immune response in patients with genital warts treated with topical imiquimod cream 5%. Antivir Res 1999;43:55-63. http://dx.doi.org/10.1016/S0166-3542(99)00033-9.
Appendix 1 Literature search strategies
Systematic review of the clinical literature
| Number | Search terms | Results |
|---|---|---|
| 1 | exp Condylomata Acuminata/ | 4578 |
| 2 | (genital$ adj3 wart$).tw. | 1931 |
| 3 | (anogenital$ adj3 wart$).tw. | 458 |
| 4 | (peni$ adj3 wart$).tw. | 67 |
| 5 | (venereal adj3 wart$).tw. | 89 |
| 6 | (condyloma$ adj3 acuminat$).tw | 2010 |
| 7 | (anal adj3 wart$).tw | 157 |
| 8 | or/1-7 | 6258 |
| 9 | exp cohort studies/ | 1,347,723 |
| 10 | cohort$.tw. | 274,812 |
| 11 | controlled clinical trial.pt. | 88,946 |
| 12 | epidemiologic methods/ | 30,812 |
| 13 | limit 12 to yr=1966-1989 | 11,256 |
| 14 | exp case-control studies/ | 652,799 |
| 15 | (case$ and control$).tw. | 330,295 |
| 16 | (case$ and series).tw. | 120,299 |
| 17 | or/9-11,13-16 | 1,945,365 |
| 18 | 8 and 17 | 1128 |
| 19 | Randomized Controlled Trials as Topic/ | 100,978 |
| 20 | randomized controlled trial/ | 383,304 |
| 21 | Random Allocation/ | 80,905 |
| 22 | Double Blind Method/ | 129,566 |
| 23 | Single Blind Method/ | 19,164 |
| 24 | clinical trial/ | 500,196 |
| 25 | clinical trial, phase i.pt | 15,918 |
| 26 | clinical trial, phase ii.pt | 26,494 |
| 27 | clinical trial, phase iii.pt | 9907 |
| 28 | clinical trial, phase iv.pt | 955 |
| 29 | controlled clinical trial.pt | 88,946 |
| 30 | randomized controlled trial.pt | 383,304 |
| 31 | multicenter study.pt | 178,695 |
| 32 | clinical trial.pt | 500,196 |
| 33 | exp Clinical Trials as topic/ | 292,888 |
| 34 | (clinical adj trial$).tw | 221,850 |
| 35 | ((singl$ or doubl$ or treb$ or tripl$) adj (blind$3 or mask$3)).tw | 132,283 |
| 36 | PLACEBOS/ | 33,402 |
| 37 | placebo$.tw | 165,785 |
| 38 | randomly allocated.tw | 16,922 |
| 39 | (allocated adj2 random$).tw | 19,489 |
| 40 | or/19-39 | 1,205,342 |
| 41 | case report.tw | 200,047 |
| 42 | letter/ | 819,925 |
| 43 | historical article/ | 298,131 |
| 44 | or/41-43 | 1,306,776 |
| 45 | 40 not 44 | 1,175,409 |
| 46 | 8 and 45 | 619 |
| 47 | 18 or 46 | 1536 |
| Number | Search terms | Results |
|---|---|---|
| 1 | exp condyloma acuminatum/ | 6357 |
| 2 | (genital$ adj3 wart$).tw. | 2376 |
| 3 | (anogenital$ adj3 wart$).tw. | 550 |
| 4 | (peni$ adj3 wart$).tw. | 81 |
| 5 | (venereal adj3 wart$).tw. | 89 |
| 6 | (condyloma$ adj3 acuminat$).tw | 2578 |
| 7 | (anal adj3 wart$).tw | 174 |
| 8 | or/1-7 | 8340 |
| 9 | exp cohort analysis/ | 157,222 |
| 10 | exp longitudinal study/ | 64,218 |
| 11 | exp prospective study/ | 248,369 |
| 12 | exp follow up/ | 740,825 |
| 13 | cohort$.tw. | 358,710 |
| 14 | exp case control study/ | 89,112 |
| 15 | (case$ and control$).tw. | 417,625 |
| 16 | exp case study/ | 21,092 |
| 17 | (case$ and series).tw. | 155,445 |
| 18 | or/9-17 | 1,764,753 |
| 19 | 8 and 18 | 1116 |
| 20 | Clinical trial/ | 892,502 |
| 21 | Randomized controlled trial/ | 357,457 |
| 22 | Randomization/ | 63,312 |
| 23 | Single blind procedure/ | 18,161 |
| 24 | Double blind procedure/ | 119,800 |
| 25 | Crossover procedure/ | 38,256 |
| 26 | Placebo/ | 237,167 |
| 27 | Randomi?ed controlled trial$.tw. | 93,520 |
| 28 | Rct.tw. | 12,481 |
| 29 | Random allocation.tw. | 1333 |
| 30 | Randomly allocated.tw. | 19,810 |
| 31 | Allocated randomly.tw. | 1942 |
| 32 | (allocated adj2 random).tw. | 813 |
| 33 | Single blind$.tw. | 14,112 |
| 34 | Double blind$.tw. | 146,330 |
| 35 | (treble or triple) adj (blind$).tw. | 351 |
| 36 | Placebo$.tw. | 199,821 |
| 37 | Prospective study/ | 248,369 |
| 38 | or/20-37 | 1,390,848 |
| 39 | Case study/ | 21,092 |
| 40 | Case report.tw. | 261,040 |
| 41 | Abstract report/ or letter/ | 902,640 |
| 42 | or/39-41 | 1,179,449 |
| 43 | 38 not 42 | 1,353,441 |
| 44 | 8 and 43 | 1094 |
| 45 | 19 or 44 | 1903 |
| Number | Search terms | Results |
|---|---|---|
| 1 | MeSH descriptor: [Condylomata Acuminata] explode all trees | 227 |
| 2 | genital* near/3 wart*:ti,ab,kw | 166 |
| 3 | anogenital* near/3 wart*:ti,ab,kw | 57 |
| 4 | peni* near/3 wart*:ti,ab,kw | 11 |
| 5 | venereal near/3 wart*:ti,ab,kw | 5 |
| 6 | condyloma* near/3 acuminat*:ti,ab,kw | 374 |
| 7 | anal near/3 wart*:ti,ab,kw | 6 |
| 8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 | 458 |
| Number | Search terms | Results |
|---|---|---|
| 1 | (condylom* acumin* or anogenital wart* or genital* wart*) | 1636 |
Economic evaluation and costing studies
| Number | Search terms | Results: 16 September 2013 | Results: 21 March 2014 |
|---|---|---|---|
| 1 | exp Condylomata Acuminata/ | 4583 | 4561 |
| 2 | (genital$ adj3 wart$).tw. | 1938 | 1899 |
| 3 | (anogenital$ adj3 wart$).tw. | 461 | 447 |
| 4 | (peni$ adj3 wart$).tw. | 68 | 67 |
| 5 | (venereal adj3 wart$).tw. | 89 | 83 |
| 6 | (condyloma$ adj3 acuminat$).tw | 2012 | 2021 |
| 7 | (anal adj3 wart$).tw | 158 | 150 |
| 8 | Health Economics | 2051 | 2086 |
| 9 | Economic evaluation | 5310 | 5139 |
| 10 | exp Costs and Cost Analysis/ | 41,817 | 41,415 |
| 11 | cost benefit analysis/ | 60,999 | 59,011 |
| 12 | exp models economic/ | 10,234 | 9945 |
| 13 | exp fees/ | 26,970 | 26,731 |
| 14 | exp budgets/ | 11,975 | 11,984 |
| 15 | (economic adj2 burden).tw. | 4407 | 4336 |
| 16 | (expenditure* not energy).tw. | 18,604 | 18,171 |
| 17 | Cost Effectiveness Analysis | 5727 | 5492 |
| 18 | (unit cost or unit-cost or unit-costs or unit costs or drug cost or drug costs or hospital costs or health-care costs or health care cost or medical cost or medical costs).tw. | 21,755 | 21,273 |
| 19 | Cost Minimization Analysis | 372 | 342 |
| 20 | (cost adj2 (util$ or effective$ or efficac$ or benefit$ or consequence$ or analys$ or minimi$ or allocation$ or control$ or illness$ or affordable$ or fee$ or charge$)).tw. | 98,718 | 96,427 |
| 21 | (decision adj1 (tree* or analys* or model*)).tw. | 8849 | 8599 |
| 22 | (econom* or price* or pricing or financ*or fee* or pharmacoeconomic* or pharmaeconomic* or pharmaco-economic*).tw. | 191,380 | 188,735 |
| 23 | ((value or values or valuation) adj2 (money or monetary or life or lives or costs or cost)).tw. | 4278 | 4206 |
| 24 | Markov*.tw | 14,411 | 13,331 |
| 25 | or/1-7 | 6272 | 6238 |
| 26 | or/8-24 | 393,780 | 387,425 |
| 27 | 25 and 26 | 245 | 229 |
| 28 | limit 27 to yr=”2013 -Current” | – | 22 |
| Number | Search terms | Results: 13 September 2013 | Results: 24 March 2014 |
|---|---|---|---|
| 1 | exp Condyloma Acuminatum/ | 6370 | 6253 |
| 2 | (genital$ adj3 wart$).tw. | 2379 | 2348 |
| 3 | (anogenital$ adj3 wart$).tw. | 552 | 548 |
| 4 | (peni$ adj3 wart$).tw. | 81 | 79 |
| 5 | (venereal adj3 wart$).tw. | 89 | 85 |
| 6 | (condyloma$ adj3 acuminat$).tw. | 2583 | 2499 |
| 7 | (anal adj3 wart$).tw. | 175 | 182 |
| 8 | exp health economics/ | 601,472 | 605,634 |
| 9 | exp economic evaluation/ | 204,598 | 207,979 |
| 10 | exp "Costs and Cost Analysis"/ | 246,649 | 250,939 |
| 11 | exp cost benefit analysis/ | 65,949 | 63,714 |
| 12 | exp models, economic/ | 108,598 | 99,561 |
| 13 | (fee or fees).tw. | 14,662 | 14,905 |
| 14 | budget$.tw. | 23,432 | 23,894 |
| 15 | (economic adj2 burden).tw. | 6188 | 6639 |
| 16 | (expenditure* not energy).tw. | 23,187 | 23,439 |
| 17 | Cost effectiveness analysis/ | 90,520 | 95,868 |
| 18 | (unit cost or unit-cost or unit-costs or unit costs or drug cost or drug costs or hospital costs or health-care costs or health care cost or medical cost or medical costs).tw. | 28,829 | 29,852 |
| 19 | Cost minimization analysis/ | 2297 | 2426 |
| 20 | (cost adj2 (util$ or effective$ or efficac$ or benefit$ or consequence$ or analys$ or minimi$ or allocation$ or control$ or illness$ or affordable$ or fee$ or charge$)).tw. | 124,323 | 126,576 |
| 21 | (decision adj2 (tree$ or analys$ or model$)).tw. | 14,571 | 14,966 |
| 22 | (econom$ or price$ or pharmacoeconomic$ or pharmaeconomic$ or pharmaco-economic$).tw. | 234,842 | 238,368 |
| 23 | (unit cost or unit-cost or unit-costs or unit costs or drug cost or drug costs or hospital costs or health-care costs or health care cost or medical cost or medical costs).tw. | 28,829 | 29,852 |
| 24 | ((value or values or valuation) adj2 (money or monetary or life or lives or costs or cost)).tw. | 5463 | 5530 |
| 25 | markov$.tw. | 14,987 | 15,397 |
| 26 | or/1-7 | 8355 | 8174 |
| 27 | or/8-25 | 984,178 | 982,805 |
| 28 | 26 and 27 | 684 | 699 |
| 29 | limit 28 to yr=”2013 -Current” | – | 77 |
| Number | Search terms | Results: 11 September 2013 | Results: 24 March 2014 |
|---|---|---|---|
| 1 | MeSH descriptor: [Condylomata Acuminata] explode all trees | 21 | 22 |
| 2 | genital* near/3 wart*:ti,ab,kw in Economic Evaluations | 5 | 5 |
| 3 | anogenital* near/3 wart*:ti,ab,kw in Economic Evaluations | 3 | 3 |
| 4 | peni* near/3 wart*:ti,ab,kw in Economic Evaluations | 1 | 1 |
| 5 | venereal near/3 wart*:ti,ab,kw in Economic Evaluations | 0 | 0 |
| 6 | condyloma* near/3 acuminat*:ti,ab,kw in Economic Evaluations | 21 | 22 |
| 7 | anal near/3 wart*:ti,ab,kw in Economic Evaluations | 0 | 0 |
| 8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 in Economic Evaluations | 22 | 22 |
| 9 | #1 or #2 or #3 or #4 or #5 or #6 or #7 Publication Date from 2013 to 2014 in Economic Evaluations | – | 1 |
| Number | Search terms | Results: 11 September 2013 | Results: 24 March 2014 |
|---|---|---|---|
| 1 | MeSH descriptor: [Condylomata Acuminata] explode all trees | 1 | 2 |
| 2 | genital* near/3 wart*:ti,ab,kw in Technology Assessments | 0 | 0 |
| 3 | anogenital* near/3 wart*:ti,ab,kw in Technology Assessments | 1 | 3 |
| 4 | peni* near/3 wart*:ti,ab,kw in Technology Assessments | 0 | 0 |
| 5 | venereal near/3 wart*:ti,ab,kw in Technology Assessments | 0 | 0 |
| 6 | condyloma* near/3 acuminat*:ti,ab,kw in Technology Assessments | 1 | 2 |
| 7 | anal near/3 wart*:ti,ab,kw in Technology Assessments | 0 | 0 |
| 8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 in Technology Assessments | 1 | 3 |
| 9 | #1 or #2 or #3 or #4 or #5 or #6 or #7 Publication Date from 2013 to 2014, in Technology Assessments | – | 2 |
Health-related quality of life
| Number | Search terms | Results: 11 September 2013 | Results: 24 March 2014 |
|---|---|---|---|
| 1 | exp Condylomata Acuminata/ | 4582 | 4561 |
| 2 | (genital$ adj3 wart$).tw. | 1936 | 1899 |
| 3 | (anogenital$ adj3 wart$).tw. | 461 | 447 |
| 4 | (peni$ adj3 wart$).tw. | 68 | 67 |
| 5 | (venereal adj3 wart$).tw. | 89 | 83 |
| 6 | (condyloma$ adj3 acuminat$).tw. | 2012 | 2021 |
| 7 | (anal adj3 wart$).tw. | 157 | 150 |
| 8 | Quality of Life/ | 118,500 | 114,259 |
| 9 | ((quality adj3 life) or life quality or QOL).ti,ab. | 154,139 | 150,091 |
| 10 | (HRQL or HRQOL or HRQol).ti,ab. | 9896 | 9514 |
| 11 | (value adj2 life).ti,ab. or Value of Life/ | 5944 | 5834 |
| 12 | (life adj2 qualit$3).tw. | 151,305 | 147,305 |
| 13 | (quality-adjusted life year$1 or QALY or QALYs or quality adjusted life year$1).ti,ab. or Quality-Adjusted Life Years/ | 10,465 | 10,009 |
| 14 | daly.ti,ab. | 807 | 781 |
| 15 | (disabilit$3 adj2 life).ti,ab. | 2106 | 2031 |
| 16 | Health Status Indicators/ | 20,775 | 19,646 |
| 17 | (sf36 or sf-36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).tw. | 16,478 | 15,787 |
| 18 | (sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. | 1343 | 1352 |
| 19 | (sf6d or sf 6d or sf-6d or short form 6d or shortform 6d or sf six dimension$1 or short form six dimension$1).tw | 434 | 424 |
| 20 | (sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve).tw. | 2843 | 2725 |
| 21 | (sf16 or sf 16 or sf-16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. | 23 | 22 |
| 22 | (sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty).tw. | 336 | 329 |
| 23 | (euroqol or euro qol or eq5d or eq 5d or eq-5d).tw. | 4102 | 4011 |
| 24 | (hye or hyes or health$ year$ equivalent$).tw. | 64 | 64 |
| 25 | hui$1.tw. | 1147 | 1099 |
| 26 | (willing$ adj2 pay).tw. | 2844 | 2805 |
| 27 | (willing$ adj2 accept).tw. | 1037 | 1031 |
| 28 | standard gamble$.tw. | 691 | 667 |
| 29 | (health adj3 (utilit$3 or value$2 or preference$2)).tw. | 7099 | 6938 |
| 30 | (visual analog$3 scale or VAS).tw. | 38,725 | 38,478 |
| 31 | patient preference$2.tw. | 4730 | 4547 |
| 32 | (person$ trade-off or person$ trade off or PTO).ti,ab. | 610 | 575 |
| 33 | (Contingent value or contingent valuation).ti,ab. | 407 | 412 |
| 34 | discrete choice.ti,ab. | 623 | 641 |
| 35 | health status.ti,ab. or Health Status/ | 85,172 | 82,068 |
| 36 | ((quality adj3 wellbeing index) or QWB).ti,ab. | 175 | 166 |
| 37 | (health utilities index or HUI).ti,ab. | 1080 | 1041 |
| 38 | (time trade off or time tradeoff or TTO or time trade-off).ti,ab. | 1202 | 1168 |
| 39 | (utility or utilities).ti,ab. | 121,289 | 116,788 |
| 40 | disutil$.ti,ab. | 227 | 215 |
| 41 | disability.tw. | 87,199 | 85,941 |
| 42 | (wellbeing or well-being or well being or qwb).ti,ab. | 44,695 | 44,284 |
| 43 | quality of well being.tw. | 358 | 328 |
| 44 | quality of wellbeing.tw. | 8 | 7 |
| 45 | or/1-7 | 6270 | 6239 |
| 46 | or/8-44 | 544,260 | 530,627 |
| 47 | 45 and 46 | 139 | 129 |
| 48 | limit 47 to yr=”2013-2014” | – | 9 |
| Number | Search terms | Results: 11 September 2013 | Results: 24 March 2014 |
|---|---|---|---|
| 1 | exp condyloma acuminatum/ | 6369 | 6252 |
| 2 | (genital$ adj3 wart$).tw. | 2379 | 2348 |
| 3 | (anogenital$ adj3 wart$).tw. | 552 | 548 |
| 4 | (peni$ adj3 wart$).tw. | 81 | 79 |
| 5 | (venereal adj3 wart$).tw. | 89 | 85 |
| 6 | (condyloma$ adj3 acuminat$).tw. | 2583 | 2498 |
| 7 | (anal adj3 wart$).tw. | 175 | 182 |
| 8 | exp Quality of Life/ | 246,790 | 258,667 |
| 9 | ((quality adj3 life) or life quality or QOL).ti,ab. | 208,667 | 216,752 |
| 10 | (HRQL or HRQOL or HRQol).ti,ab. | 12,942 | 13,580 |
| 11 | (value adj2 life).ti,ab. or exp Value of Life/ | 174,421 | 173,944 |
| 12 | (life adj2 qualit$3).tw. | 203,586 | 211,313 |
| 13 | (quality-adjusted life year$1 or QALY or QALYs or quality adjusted life year$1).ti,ab. or exp Quality-Adjusted Life Years/ | 14,706 | 15,315 |
| 14 | daly.ti,ab. | 951 | 963 |
| 15 | (disabilit$3 adj2 life).ti,ab. | 2361 | 2448 |
| 16 | exp Health Status Indicators/ | 3434 | 5418 |
| 17 | (sf36 or sf-36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).tw. | 21,489 | 22,280 |
| 18 | (sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. | 1507 | 1472 |
| 19 | (sf6d or sf 6d or sf-6d or short form 6d or shortform 6d or sf six dimension$1 or short form six dimension$1).tw | 604 | 667 |
| 20 | (sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve).tw. | 3737 | 4032 |
| 21 | (sf16 or sf 16 or sf-16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. | 33 | 36 |
| 22 | (sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty).tw. | 320 | 322 |
| 23 | (euroqol or euro qol or eq5d or eq 5d or eq-5d).tw. | 5968 | 6571 |
| 24 | (hye or hyes or health$ year$ equivalent$).tw. | 96 | 101 |
| 25 | hui$1.tw. | 1463 | 1521 |
| 26 | (willing$ adj2 pay).tw. | 3719 | 3931 |
| 27 | (willing$ adj2 accept).tw. | 1283 | 1321 |
| 28 | standard gamble$.tw. | 761 | 765 |
| 29 | (health adj3 (utilit$3 or value$2 or preference$2)).tw. | 8425 | 8686 |
| 30 | (visual analog$3 scale or VAS).tw. | 52,398 | 54,573 |
| 31 | patient preference$2.tw. | 5902 | 6132 |
| 32 | (person$ trade-off or person$ trade off or PTO).ti,ab. | 608 | 601 |
| 33 | (Contingent value or contingent valuation).ti,ab. | 526 | 537 |
| 34 | discrete choice.ti,ab. | 766 | 836 |
| 35 | health status.ti,ab. or exp Health Status/ | 148,028 | 151,822 |
| 36 | ((quality adj3 wellbeing index) or QWB).ti,ab. | 186 | 191 |
| 37 | (health utilities index or HUI).ti,ab. | 1296 | 1344 |
| 38 | (time trade off or time tradeoff or TTO or time trade-off).ti,ab. | 1476 | 1506 |
| 39 | (utility or utilities).ti,ab. | 145,629 | 148,499 |
| 40 | disutil$.ti,ab. | 327 | 355 |
| 41 | disability.tw. | 110,507 | 113,748 |
| 42 | (wellbeing or well-being or well being or qwb).ti,ab. | 54,684 | 56,406 |
| 43 | quality of well being.tw. | 366 | 367 |
| 44 | quality of wellbeing.tw. | 19 | 18 |
| 45 | or/1-7 | 8354 | 8172 |
| 46 | or/8-44 | 890,258 | 911,589 |
| 47 | 45 and 46 | 303 | 308 |
| 48 | limit 47 to yr=”2013 -Current” | – | 37 |
| Number | Search | Results: 11 September 2013 | Results: 24 March 2014 |
|---|---|---|---|
| 1 | MeSH descriptor: [Condylomata Acuminata] explode all trees | 21 | 22 |
| 2 | genital* near/3 wart*:ti,ab,kw in Economic Evaluations | 5 | 5 |
| 3 | anogenital* near/3 wart*:ti,ab,kw in Economic Evaluations | 3 | 3 |
| 4 | peni* near/3 wart*:ti,ab,kw in Economic Evaluations | 1 | 1 |
| 5 | venereal near/3 wart*:ti,ab,kw in Economic Evaluations | 0 | 0 |
| 6 | condyloma* near/3 acuminat*:ti,ab,kw in Economic Evaluations | 21 | 22 |
| 7 | anal near/3 wart*:ti,ab,kw in Economic Evaluations | 0 | 0 |
| 8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 in Economic Evaluations | 22 | 22 |
| 9 | #1 or #2 or #3 or #4 or #5 or #6 or #7 Publication Date from 2013 to 2014 in Economic Evaluations | – | 1 |
| Number | Search | Results: 11 September 2013 | Results: 24 March 2014 |
|---|---|---|---|
| 1 | MeSH descriptor: [Condylomata Acuminata] explode all trees | 1 | 2 |
| 2 | genital* near/3 wart*:ti,ab,kw in Technology Assessments | 0 | 0 |
| 3 | anogenital* near/3 wart*:ti,ab,kw in Technology Assessments | 1 | 3 |
| 4 | peni* near/3 wart*:ti,ab,kw in Technology Assessments | 0 | 0 |
| 5 | venereal near/3 wart*:ti,ab,kw in Technology Assessments | 0 | 0 |
| 6 | condyloma* near/3 acuminat*:ti,ab,kw in Technology Assessments | 1 | 2 |
| 7 | anal near/3 wart*:ti,ab,kw in Technology Assessments | 0 | 0 |
| 8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 in Technology Assessments | 1 | 3 |
| 9 | #1 or #2 or #3 or #4 or #5 or #6 or #7 Publication Date from 2013 to 2014, in Technology Assessments | – | 2 |
| Number | Search | Results: 11 September 2013 | Results: 24 March 2014 |
|---|---|---|---|
| 1 | MeSH descriptor: [Condylomata Acuminata] explode all trees | 194 | 229 |
| 2 | genital* near/3 wart*:ti,ab,kw in Trials | 148 | 159 |
| 3 | anogenital* near/3 wart*:ti,ab,kw in Trials | 46 | 48 |
| 4 | peni* near/3 wart*:ti,ab,kw in Trials | 10 | 10 |
| 5 | venereal near/3 wart*:ti,ab,kw in Trials | 2 | 2 |
| 6 | condyloma* near/3 acuminat*:ti,ab,kw in Trials | 339 | 352 |
| 7 | anal near/3 wart*:ti,ab,kw in Trials | 5 | 5 |
| 8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 in Trials | 416 | 434 |
| 9 | MeSH descriptor: [Quality of Life] explode all trees | 10,964 | 13,581 |
| 10 | MeSH descriptor: [Quality-Adjusted Life Years] explode all trees | 468 | 3524 |
| 11 | quality near/3 life:ti,ab,kw in Trials | 20,755 | 23,884 |
| 12 | qol:ti,ab,kw in Trials | 2639 | 3089 |
| 13 | hrqol or hr qol or hrql or hr ql:ti,ab,kw in Trials | 1179 | 1379 |
| 14 | QALY or quality adjusted life year or quality-adjusted life year:ti,ab,kw in Trials | 1140 | 1368 |
| 15 | SF 6d or SF-6d or sf6d or short form 6d or short form six dimension*:ti,ab,kw in Trials | 81 | 99 |
| 16 | SF 36 or SF-36 or SF36 or short form 36 or short form thirty six:ti,ab,kw in Trials | 3235 | 3754 |
| 17 | eq-5d or eq5d or eq 5d or euroqol:ti,ab,kw in Trials | 711 | 850 |
| 18 | hui or health utilities index:ti,ab,kw in Trials | 1985 | 2296 |
| 19 | standard gamble:ti,ab,kw in Trials | 70 | 71 |
| 20 | time trade off or TTO or time trade-off:ti,ab,kw in Trials | 115 | 124 |
| 21 | utilit*:ti,ab,kw in Trials | 3777 | 4315 |
| 22 | #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 in Trials | 26,949 | 30,940 |
| 23 | #8 and #22 in Trials | 3 | 3 |
| 24 | #8 and #22 Publication Date from 2013 to 2014, in Trials | – | 0 |
Appendix 2 Data abstraction tables and quality assessment
Clinical data abstraction and quality assessment
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Vicky Wakefield and Sam Barton | ||||
| Study ID | Abdullah 1993 | ||||
| Study details | Sex Trans Dis 1993;20:344–5 | ||||
| Language of publication | English | ||||
| Type of report | Full paper | ||||
| Section 2: Study information | |||||
| Location and number of sites | Department of Genitourinary Medicine at Coventry and Warwickshire Hospital (one site) | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | People attending the Department of Genitourinary Medicine were enrolled between November 1990 and June 1991 | ||||
| Trial design | RCT | ||||
| Trial duration | Maximum of six treatments with treatment given on a weekly basis. People with complete clearance of AGWs were followed up for 3 months after the end of treatment | ||||
| Line of therapy | First | ||||
| Inclusion criterion | People with definite AGWs for the first time on clinical grounds | ||||
| Exclusion criteria | Women who were pregnant and people with internal AGWs (cervical, vaginal and rectal) or Buschke–Lowenstein lesions | ||||
| All outcomes reported in paper | Complete clearance of AGWs by the end of up to six treatments; complete clearance of AGWs after more than six treatments; AEs (ulceration due to treatment) | ||||
| Subgroups evaluated | Gender (male vs. female) | ||||
| Stratification | Trial does not report that randomisation was stratified. It is stated that the area and size of AGWs were matched on clinical grounds as closely as possible to avoid bias in either treatment group | ||||
| Baseline measurement of disease | Not reported; people enrolled based on the presence of AGWs | ||||
| Treatment | TCAA 95% | Cryotherapy (liquid nitrogen) | |||
| Randomised, n | 33 | 53 | |||
| Withdrawals | 3 lost to follow-up (9.1%; reasons for withdrawals not reported) | 10 lost to follow-up (18.9%; reasons for withdrawals not reported) | |||
| Treatment regimen | TCAA 95% was applied by a clinician with a pointed plastic probe once a week | Cryotherapy using liquid nitrogen was administered by a clinician using a tapered cotton pledget on a wooden applicator stick. The pledget was applied for a period of time sufficient to freeze the AGW and a 1-mm margin of the surrounding skin. Individual AGWs were frozen twice, using different applicators and disposable small containers of liquid nitrogen for different people. Cryotherapy was applied weekly | |||
| Duration/number of administered treatment | Planned treatment schedule was to continue treatment either until all AGWs cleared or for a maximum of six treatments had been administered. Number of treatments applied not reported | ||||
| Baseline patient characteristics | TCAA 95% | Cryotherapy (liquid nitrogen) | p-value | ||
| Mean age (with SD/SE if given), years (range) | Not reported | ||||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | Not reported | ||||
| Type of AGWs, n (%) | Not reported | ||||
| Mean number of AGWs (with SD/SE if given) | Not reported | ||||
| Mean area of AGWs, mm2 | Not reported | ||||
| Sex, n (%) | |||||
| Men | 18 (54.5) | 30 (56.6) | Not reported | ||
| Women | 15 (45.5) | 23 (43.4) | Not reported | ||
| Any previous treatment, n (%) | 0 | 0 | Not reported | ||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Treatment given either until all AGWs cleared or for a maximum of six treatments had been administered, whichever occurred first. However, some people received more than six treatments (results not extracted for this group) | ||||
| AEs | Ulceration at site of treatment | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | TCAA 95%, n/N | Cryotherapy (liquid nitrogen), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | Up to six cycles of treatment | 21/33 | 37/53 | Not reported | |
| AEs: ulceration | Up to six cycles of treatment | 9/33 | 0/53 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation? | ? | It is stated that ‘people enrolled at random according to a randomisation schedule’ (p. 344). Further detail not provided | |||
| Allocation concealment | ? | No details provided | |||
| Selective reporting | ? | Information on prespecified outcomes not reported | |||
| ‘Other bias’ | ? | Insufficient information reported to assess other potential risks of bias | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | Details on level of masking not provided. Given the difference in the treatments administered, it could be envisaged that masking of key study personnel and participants might not be feasible. It is unclear whether the outcome assessor was masked to treatment. If the outcome assessor was masked to treatment, the probability of masking being broken is unclear | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | Number of people lost to follow-up was disclosed but reasons for loss to follow-up were not reported. It is stated that it had been ‘presumed that people lost to follow-up had moved out of the area, or their AGWs had cleared, or they had not tolerated the treatment’ (p. 345). Larger proportion of people lost to follow-up from the cryotherapy group. Impact of imbalance on results is unclear | |||
| AEs: ulceration | Blinding (participants and personnel) | ? | Details on level of masking not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients might not be feasible | ||
| Blinding of outcomes assessment | ? | Unclear whether clinician assessing ulceration was masked to treatment | |||
| Incomplete outcome data | ? | Number of people lost to follow-up was disclosed but reasons for loss to follow-up were not reported. It is stated that it had been ‘presumed that people lost to follow-up had moved out of the area, or their AGWs had cleared, or they had not tolerated the treatment’ (p. 345). Larger proportion of people lost to follow-up from the cryotherapy group. Ulceration is more likely to occur with TCAA, but influence of imbalance on rate of ulceration is unclear | |||
| Overall rating of bias | ? | Reflects limited information provided in full publication | |||
| Section 6: Additional comments | |||||
| Additional comments |
|
||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Vicky Wakefield and Sam Barton | ||||
| Study ID | Arican 2004 | ||||
| Study details | J Dermatol 2004;31:627–31 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Trial carried out in Turkey; number of sites unclear | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Unclear. It is stated that people enrolled in the trial were volunteers but no other details are provided | ||||
| Trial design | RCT | ||||
| Trial duration | Treatment period of 12 weeks followed by a treatment-free observation period of 6 months | ||||
| Line of therapy | Unclear. People enrolled had not received any therapies in the 3 months before enrolment | ||||
| Inclusion criteria | People with AGWs aged ≥ 18 years of age and who had not received any therapies within the 3 months before enrolment. Minimum number of AGWs at baseline was five | ||||
| Exclusion criteria | People were excluded if they had a pathological condition; were aged < 18 years; had an immunosuppressive or a serious systemic disorder; misused alcohol or drugs; had frequently recurrent genital herpes; or had undergone a local or systemic therapy within the previous 3 months | ||||
| All outcomes reported in paper | Clearance of AGWs at completion of treatment; volume of AGW clearance at completion of treatment (based on groups with AGW clearance of 0–10%, 11–50% and 51–99% of baseline volume); recurrence at 6 months’ follow-up; appearance of new AGWs during treatment; AEs | ||||
| Subgroups evaluated |
|
||||
| Stratification | Not reported | ||||
| Baseline measurement of disease | Regional lesions were determined and mapped at first examination (no other details provided) | ||||
| Treatment | Imiquimod 5% cream (patient applied) | Placebo (vaseline) | |||
| Randomised, n | 34 | 11 | |||
| Withdrawals, n (%) | 1 (2.9; reason for withdrawal not reported) | 1 (9.1; reason for withdrawal not reported) | |||
| Treatment regimen | Imiquimod 5% cream or placebo was applied by the patient three times a week (every other day in the evenings) using the tip of a stick. When AGWs had cleared, treatment was interrupted. The importance of applying the medication to a dry region, of adhering to the application schedule and of subsequent cleaning of the skin (in the evenings, 8 ± 2 hours after application) was stressed | ||||
| Duration/number of administered treatment | Details on the number of treatments administered not provided | ||||
| Baseline patient characteristics | Imiquimod 5% cream (patient applied) | Placebo (vaseline) | p-value | ||
| Age (years), mean ± SD (range) | 30.3 ± 6.1 (18–41) | 32.3 ± 6.8 (20–42) | Not reported | ||
| Duration of disease (months), mean ± SD | 11.9 ± 22.5 | 12.1 ± 24.2 | Not reported | ||
| Site of AGWs | |||||
| Women | |||||
| Vulva | 5 | 1 | Not reported | ||
| Perianal area | 5 | 1 | Not reported | ||
| Vulva and perianal area | 1 | 0 | Not reported | ||
| Men | |||||
| Perianal area | 7 | 1 | Not reported | ||
| Pubis | 3 | 2 | Not reported | ||
| Penis | 7 | 1 | Not reported | ||
| Penis and scrotum | 1 | 0 | Not reported | ||
| Penis, scrotum and perianal area | 1 | 1 | Not reported | ||
| Pubis and penis | 2 | 2 | Not reported | ||
| Scrotum, penis and pubis | 1 | 1 | Not reported | ||
| Type of AGWs, n (%) | Not reported | ||||
| Mean number of AGWs (with SD/SE if given) | Not reported | ||||
| Mean area of AGWs (mm2) | Not reported | ||||
| Sex, n/N (%) | |||||
| Men | 23/34 (67.6) | 9/11 (81.8) | Not reported | ||
| Women | 11/34 (32.4) | 2/11 (18.2) | Not reported | ||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | 100% clinical clearance of AGWs after 12 weeks of treatment | ||||
| Recurrence of AGWs | Appearance of new lesions or recurrence of AGWs in the 6-month follow-up period after the end of treatment. Details on methods to distinguish new lesions from existing lesions and to determine recurrence not reported | ||||
| Volume of wart clearance | Proportion of people with 0–10%, 11–50% and 51–99% clinical clearance of AGWs at the end of treatment | ||||
| Appearance of new warts during treatment | New AGWs appearing during treatment | ||||
| AEs | Definition of AEs not provided. Data presented for occurrence of erythema, erosion, burning sensation and itching | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Imiquimod 5% cream (patient applied), n/N | Placebo (vaseline), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 12 weeks | 23/33 | 1/10 | p < 0.001 | |
| Recurrence of AGW (includes new lesions) | 6 months after end of treatment | 6/23 | 1/1 | Not reported | |
| Volume of wart clearance | 12 weeks | ||||
| 0–10% | 0/33 | 8/10 | Not reported | ||
| 11–50% | 1/33 | 0/10 | Not reported | ||
| 51–99% | 9/33 | 1/10 | Not reported | ||
| Appearance of new warts during treatment | 12 weeks | 0/33 | Not reported | ||
| AEs | 12 weeks | ||||
| Mild-severity erythema | 3/33 | 2/10 | Not reported | ||
| Moderate-severity erythema | 4/33 | 0/10 | Not reported | ||
| Erosion | 1/33 | 0/10 | Not reported | ||
| Erythema and erosion | 6/33 | 0/10 | Not reported | ||
| Erythema and excoriation | 2/33 | 0/10 | Not reported | ||
| Burning sensation | 1/33 | 0/10 | Not reported | ||
| Itching | 0/33 | 2/10 | Not reported | ||
| Influenza-like symptoms | 1/33 | 0/10 | Not reported | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that the study was planned as a randomised trial but details on method of randomisation are not provided | |||
| Allocation concealment | ? | No details provided | |||
| Selective reporting | ? | Information on prespecified outcomes not reported | |||
| ‘Other bias’ | ? | Insufficient information reported to assess other potential risks of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | Described as double blinded but insufficient information provided to determine who was masked | ||
| Blinding of outcomes assessment | ? | Described as double blinded but insufficient information provided to determine who was masked | |||
| Incomplete outcome data | ? | Although number of withdrawals disclosed, reasons for withdrawal are not reported. Analysis of AGW clearance at end of treatment was carried out on a modified ITT population (two people, one from each group, not included in analysis) | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Described as double blinded but insufficient information provided to determine who was masked | ||
| Blinding of outcomes assessment | ? | Described as double blinded but insufficient information provided to determine who was masked | |||
| Incomplete outcome data | ? | Although number of withdrawals disclosed, reasons for withdrawal are not reported. Analysis of recurrence at end of follow-up was carried out on a modified ITT population (two people, one from each group, not included in analysis) | |||
| Volume of wart clearance | Blinding (participants and personnel) | ? | Described as double blinded but insufficient information provided to determine who was masked | ||
| Blinding of outcomes assessment | ? | Described as double blinded but insufficient information provided to determine who was masked | |||
| Incomplete outcome data | ? | Although number of withdrawals disclosed, reasons for withdrawal are not reported. Analysis of volume of AGW clearance was carried out on a modified ITT population (two people, one from each group, not included in analysis) | |||
| Appearance of new warts during treatment | Blinding (participants and personnel) | ? | Described as double blinded but insufficient information provided to determine who was masked | ||
| Blinding of outcomes assessment | ? | Described as double blinded but insufficient information provided to determine who was masked | |||
| Incomplete outcome data | ? | Although number of withdrawals disclosed, reasons for withdrawal are not reported. Analysis of appearance of new AGWs during treatment was carried out on a modified ITT population (two people, one from each group, not included in analysis) | |||
| AEs | Blinding (participants and personnel) | ? | Described as double blinded but insufficient information provided to determine who was masked | ||
| Blinding of outcomes assessment | ? | Described as double blinded but insufficient information provided to determine who was masked | |||
| Incomplete outcome data | ? | Although number of withdrawals disclosed, reasons for withdrawal are not reported. Analysis of AEs was carried out on a modified ITT population (two people, one from each group, not included in analysis) | |||
| Overall rating of bias | ? | Reflects limited description of methods and results in the full publication | |||
| Section 6: Additional comments | |||||
| Additional comments |
|
||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Vicky Wakefield and Sam Barton | ||||
| Study ID | Azizjalali 2012 | ||||
| Study details | Iran J Microbiol 2012;4:187–90 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Hazrat Rasool Akram Hospital, Tehran, Iran | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Between November 2009 and December 2010, all people with documented lower genital AGWs who visited the Hazrat Rasool Akram Hospital were eligible for inclusion | ||||
| Trial design | RCT | ||||
| Trial duration | 3 months | ||||
| Line of therapy | Unclear | ||||
| Inclusion criteria | People with lesions with a diameter of ≥ 10 mm located on the pubis, penis, scrotum, vulva or inguinal area | ||||
| Exclusion criteria | Exclusion criteria were history of immunosuppressive status; history of immune modulator drug use in the past 4 weeks; history of local antiviral agent use in the past 2 weeks; pregnancy; breastfeeding; destructive therapies; presence of any other concomitant sexually transmitted disease. In addition, the trial did not treat or evaluate vaginal and cervical lesions | ||||
| All outcomes reported in paper | Clearance of AGWs at completion of treatment; recurrence; AEs: postprocedure hypopigmentation and blistering | ||||
| Subgroups evaluated | None | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Dermatologist evaluation of location of AGWs and duration of disease | ||||
| Treatment | CO2 laser therapy | Cryotherapy | |||
| Randomised, n | 80 | 80 | |||
| Withdrawals, n (%) | Not reported | ||||
| Treatment regimen | After routine decontamination of the lesion area and local anaesthesia, the AGW and a 2-mm surrounding margin of normal skin were evaporated in focal distance of laser light using a unixel CO2 laser unit, 30-W fluence, with continuous mode wavelength of 10,600 nm with a fluency of 4.5 J/cm2 | The AGW and 2 mm of the normal surrounding margin were frozen using liquid nitrogen (–196°C) and open-spray mode in two freeze/thaw cycles. After lesion removal, tetracycline ointment was applied on the area for 24 hours | |||
| Duration/number of administered treatment | In both groups, lesions were evaluated after 2 weeks and then 3 months later. Second and third applications were performed every 2 weeks to completely clear the lesions | ||||
| Baseline patient characteristics | CO2 laser therapy | Cryotherapy | p-value | ||
| Age (years), mean | Not reported | ||||
| Duration of disease (months), n (%) | |||||
| ≤ 4 | 61 (76.3) | 56 (70.0) | |||
| ≥ 9 | 1 (1.3) | 1 (1.3) | |||
| Site of AGWs, n (%) | |||||
| Penis | 18 (22.5) | 14 (17.5) | |||
| Scrotum | 23 (28.8) | 28 (35.0) | |||
| Vulva | 22 (27.5) | 19 (23.8) | |||
| Inguinal | 17 (21.3) | 19 (23.8) | |||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | Not reported | ||||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex, n (%) | Not reported | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Complete eradication of AGWs (time frame unclear). AGWs were evaluated at 2 weeks and subsequently after a further 3 months. It is unclear whether complete clearance of AGWs refers to initial treatment at 2 weeks or after subsequent treatments, when appropriate | ||||
| Recurrence of AGWs | Recurrence rate reported but definition not provided. It is reported that AGWs were evaluated after 2 weeks and then 3 months later | ||||
| AEs | Postprocedure hypopigmentation and blistering | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | CO2 laser therapy, n/N | Cryotherapy, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | Unclear | 76/80 | 37/80 | p < 0.001 | |
| Recurrence of AGWs | 14 weeks | 4/76 | 7/37 | Not reported | |
| AEs | |||||
| Blistering | During treatment | 0/80 | 2/80 | p = 0.99 | |
| Postprocedure hypopigmentation | During treatment | Difference between groups reported to be not statistically different. Absolute numbers not reported | Not significant | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ✓ | It is stated that randomisation was ‘computerised’. Additional information not reported | |||
| Allocation concealment | ? | No details provided | |||
| Selective reporting | ? | Information on prespecified outcomes not reported | |||
| ‘Other bias’ | ? | Insufficient information reported to assess other potential risks of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | Unclear whether clinician administering treatment and/or patient were masked to treatment | ||
| Blinding of outcomes assessment | ✓ | It is stated that ‘the study group and the type of treatment has been blinded to the dermatologists who examined the patients after treatment to evaluate the lesions’ (p. 188) | |||
| Incomplete outcome data | ? | Loss to follow-up was not reported. Analysis of AGW clearance was based on all people randomised, but it is unclear whether there was an imbalance between the groups in the number of people who withdrew from the trial | |||
| Recurrence of AGW | Blinding (participants and personnel) | ? | Unclear whether clinician administering treatment and/or patient were masked to treatment | ||
| Blinding of outcomes assessment | ✓ | It is stated that ‘the study group and the type of treatment has been blinded to the dermatologists who examined the patients after treatment to evaluate the lesions’ (p. 188) | |||
| Incomplete outcome data | ? | Loss to follow-up was not reported. Analysis of recurrence seemed to be based on all people with complete clearance of AGWs, but it is unclear whether there was an imbalance between the groups in the number of people who withdrew from the trial | |||
| AEs | Blinding (participants and personnel) | ? | Unclear whether clinician administering treatment and/or patient were masked to treatment | ||
| Blinding of outcomes assessment | ✓ | It is stated that ‘the study group and the type of treatment has been blinded to the dermatologists who examined the patients after treatment to evaluate the lesions’ (p. 188) | |||
| Incomplete outcome data | ? | Loss to follow-up was not reported. Analysis of AEs seemed to be based on all people randomised, but it is unclear whether there was an imbalance between the groups in the number of people who withdrew from the trial | |||
| Overall rating of bias | ? | Reflects limited information presented in trial | |||
| Section 6: Additional comments | |||||
| Additional comments |
|
||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Sam Barton and Charlotta Karner | |||||
| Study ID | Baker 2010 | |||||
| Study details | Contraception 2010;82:211 Related publication in Infect Dis Obstet Gynecol 2011:806105 (presents results on clinical effectiveness in women);244 additional information on the two trials is available from ClinicalTrials.gov245,246 |
|||||
| Language of publication | English | |||||
| Type of report | Conference abstract | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Not reported | |||||
| Trial sponsor | Not reported | |||||
| Conflicts of interest | D Baker, D Ferris, M Martens, S Tyring, L Edwards, A Nelson, K Ault and K Trofatter have been consultants and/or advisory board members for Graceway Pharmaceuticals, LLC (trial sponsor). T Liu was an employee of Graceway and a consultant. S Levy and J Wu are employees of Graceway | |||||
| Patient enrolment | Not reported | |||||
| Trial design | RCT (publication describes the combined results from two RCTs) | |||||
| Trial duration | People were initially treated for up to 8 weeks, with assessment for complete clearance continued for up to 8 weeks after treatment. Those achieving complete clearance were followed for 12 weeks | |||||
| Line of therapy | Unclear | |||||
| Inclusion criteria | People were eligible for inclusion if they were aged ≥ 12 years and had 2–30 external AGWs with a total AGW area of ≥ 10 mm2 | |||||
| Exclusion criteria | Not reported in Baker et al.113 Exclusion criteria reported in Baker et al.:244 known HIV infection; infection, immunosuppression or other genital infections; allergy to imiquimod or cream excipients; history of high-risk-type HPV infection; high-grade pathology on Papanicolaou smear; pregnancy or lactation; imiquimod or HPV vaccination within 1 year; sinecatechins within 12 weeks; cytotoxics, immunomodulators/immunosuppressives, systemic antivirals (excluding oral antiherpes agents and oseltamivir), investigational therapies and any treatments procedures within the anogenital area within 4 weeks | |||||
| All outcomes reported in paper | Complete clearance at the end of treatment; recurrence; AEs | |||||
| Subgroups evaluated | Not reported | |||||
| Stratification | Not reported | |||||
| Baseline measurement of disease | AGWs were diagnosed clinically; histological confirmation was not required (taken from Baker et al.244) | |||||
| Treatment | Imiquimod 3.75% cream | Imiquimod 2.5% cream | Placebo | |||
| Randomised, n | It is stated that 981 people were enrolled and that randomisation was carried out in a 2 : 2 : 1 ratio (imiquimod 3.75% cream : imiquimod 2.5% cream : placebo). The number of people randomised to each group is not reported in the conference abstract. Details taken from ClinicalTrials.gov245,246 | |||||
| Study 1245 | 195 | 178 | 97 | |||
| Study 2246 | 204 | 202 | 105 | |||
| Withdrawals, n (%) | ||||||
| Non-completers | ||||||
| Study 1245 | 59 (30.3) | 57 (32.0) | 32 (33.0) | |||
| Study 2246 | 55 (27.0) | 63 (31.2) | 28 (26.7) | |||
| Discontinued early because of safety-related issues: both trials (%) | 1.5 | 1.6 | 0.5 | |||
| Treatment regimen | Allocated treatment was applied once daily until complete clearance of all (baseline and new) AGWs or for a maximum of 8 weeks, whichever occurred earlier | |||||
| Duration/number of administered treatment | Not reported | |||||
| Baseline patient characteristics | Imiquimod 3.75% cream | Imiquimod 2.5% cream | Placebo | p-value | ||
| Age (years), mean (SD) | ||||||
| Study 1245 | 32.5 (11.6) | 32.7 (11.3) | 30.5 (10.6) | |||
| Study 2246 | 32.8 (11.0) | 33.1 (10.1) | 33.3 (10.8) | |||
| Duration of disease | Mean duration of disease across all groups: 4.9 years; not reported separately by treatment group | |||||
| Site of AGWs, n (%) | Not reported | |||||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, mean (with SD/SE if given) | Mean number of AGWs across all groups: 8.7; not reported separately by treatment group | |||||
| Area of AGWs (mm2), mean | Mean AGW area across all groups: 158.8; not reported separately by treatment group | |||||
| Sex (M/F), n | ||||||
| Study 1245 | 95/100 | 83/95 | 47/50 | |||
| Study 2246 | 88/116 | 85/117 | 49/56 | |||
| Any previous treatment, n (%) | Not reported | |||||
| Ethnicity, n (%) | Not reported | |||||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Not defined | |||||
| Recurrence of AGWs | Those achieving complete clearance were followed and monitored for recurrence | |||||
| AEs | Data reported on severe local skin reactions | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Imiquimod 3.75% cream, n/N | Imiquimod 2.5% cream, n/N | Placebo, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment: absolute event rates not reported separately for the two trials | Up to 8 weeks | 102/399 | 74/380 | 13/202 | Imiquimod 3.75% and 2.5% vs. placebo: p < 0.001; imiquimod 3.75% vs. imiquimod 2.5%: p = 0.025 | |
| Recurrence of AGW | 12 weeks’ follow-up | 71/102 | 44/74 | 12/13 | Not reported | |
| AEs | ||||||
| Severe local skin reactions (%) | Up to 8 weeks | 16.3 | 15.0 | 1.0 | Not reported | |
| Local skin reactions | ||||||
| Study 1245 | Up to 8 weeks | 144/195 | 110/178 | 29/97 | Not reported | |
| Study 2246 | Up to 8 weeks | 148/205 | 123/201 | 41/105 | Not reported | |
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessment a | Comments | |||
| Random sequence generation | ✓ | The studies are described as randomised. Details on method used to generate random sequence not available in the conference abstract. In the related publication it is stated that women were randomised using a computer-generated sequence. It is assumed that men were subject to the same randomisation procedure | ||||
| Allocation concealment | ? | Details on method to conceal allocation not available | ||||
| Selective reporting | ✗ | Data on effectiveness for key clinical outcomes are not reported as absolute events rates in the identified publications or on ClinicalTrials.gov. Results presented cannot be incorporated in a meta-analysis | ||||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias | ||||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ✓ | In the related publication, it is stated that women received identically appearing prepackaged study kits. The treatment assignment was concealed from the participant, the investigators and their staff and the clinical research team | |||
| Blinding of outcomes assessment | ✓ | |||||
| Incomplete outcome data | ? | The proportion of people not completing the trial is reported in the entries on ClinicalTrials.gov; however, reasons for withdrawal are not specified. It is unclear whether there is an imbalance across the groups in withdrawal for a particular event | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ✓ | In the related publication it is stated that women received identically appearing prepackaged study kits. The treatment assignment was concealed from the participant, the investigators and their staff and the clinical research team | |||
| Blinding of outcomes assessment | ✓ | |||||
| Incomplete outcome data | ? | The proportion of people not completing the trial is reported in the entries on ClinicalTrials.gov. However, reasons for withdrawal are not specified. It is unclear whether there is an imbalance across the groups in withdrawal for a particular event | ||||
| AEs | Blinding (participants and personnel) | ✓ | In the related publication it is stated that women received identically appearing prepackaged study kits. The treatment assignment was concealed from the participant, the investigators and their staff and the clinical research team | |||
| Blinding of outcomes assessment | ✓ | |||||
| Incomplete outcome data | ? | The proportion of people not completing the trial is reported in the entries on ClinicalTrials.gov. However, reasons for withdrawal are not specified. It is unclear whether there is an imbalance across the groups in withdrawal for a particular event | ||||
| Overall rating of bias | ✗ | Reflects lack of reporting of the number of people randomised to each group and absolute event rates | ||||
| Section 6: Additional comments | ||||||
| Additional comments | It is stated that complete clearance was higher in women than in men. The related publication identified in the literature search244 discusses the results in women | |||||
| Further information that could be requested from authors | Methodological information | |||||
| Method of randomisation | ✓ | |||||
| Level of masking (if masked, who was masked) | ✓ | |||||
| Method for allocation concealment | ✓ | |||||
| Method for maintaining masking during the trial | ✓ | |||||
| Baseline characteristics | ||||||
| Mean and median age of people in each group (with accompanying measure of variation) | ✓ | |||||
| Breakdown by site of AGW in each group | ✓ | |||||
| Mean number and area of AGWs at baseline in each group | ✓ | |||||
| Proportion of people with (i) single, (ii) few (two to five) or (iii) multiple (six or more) AGWs at baseline in each group | ✓ | |||||
| Breakdown of type of AGWs (non-keratinised vs. keratinised) in each group | ✓ | |||||
| Immune status (immunosuppressed vs. not immunosuppressed) in each group | ✓ | |||||
| Any previous treatment | ✓ | |||||
| Ethnicity | ✓ | |||||
| Trial conduct | ||||||
| Was there a trial sponsor? | ✓ | |||||
| Did any of the authors have a conflict of interest? | ✓ | |||||
| When was complete clearance recorded? | ✓ | |||||
| Number of people lost to follow-up in each group | ✓ | |||||
| Number of people who withdrew from each group and reasons for withdrawal | ✓ | |||||
| Is the reported analysis based on an ITT population? | ✓ | |||||
| Any concomitant medications received in each group? | ✓ | |||||
| Did both groups receive the same care except for the allocated treatment? | ✓ | |||||
| Results | ||||||
| Complete clearance in each group based on subgroups of: | ||||||
| site of AGWs | ✓ | |||||
| number of AGWs at baseline [subgroups of few (two to five) and multiple (six or more)] | ✓ | |||||
| type of AGWs (non-keratinised vs. keratinised) | ✓ | |||||
| immune status (immunosuppressed vs. not immunosuppressed) | ✓ | |||||
| Miscellaneous | ||||||
| Item | Details | ||||||
|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||||
| Reviewers’ names | Shannon Amoils and Sam Barton | ||||||
| Study ID | Bar-Am 1993 | ||||||
| Study details | J Reprod Med 1993;38:455–8 | ||||||
| Language of publication | English | ||||||
| Type of report | Full publication | ||||||
| Section 2: Study information | |||||||
| Location and number of sites | Not reported | ||||||
| Trial sponsor | Not reported | ||||||
| Conflicts of interest | Not reported | ||||||
| Patient enrolment | Not reported | ||||||
| Trial design | RCT evaluating high- vs. low-power CO2 laser treatment in three distinct subgroups of people, only two of whom are relevant to this review; data extracted only for the relevant patient populations | ||||||
| Trial duration | Treatment duration unclear; it is possible that people undergo a single laser procedure. Duration of follow-up not reported; it is stated that ‘follow up visits were scheduled weekly until complete healing was observed’ (p. 456) | ||||||
| Line of therapy | Not reported | ||||||
| Inclusion criteria | Inclusion criteria for the two groups of interest to this review: women with benign vulvar and perineal HPV lesions; men with disseminated foci of penile shaft condylomatous lesions | ||||||
| Exclusion criteria | Not reported | ||||||
| All outcomes reported in paper |
|
||||||
| Subgroups evaluated | Randomisation was performed separately in the three distinct groups of people. No subgroups reported | ||||||
| Stratification | Not reported | ||||||
| Baseline measurement of disease | Not reported | ||||||
| Treatment | |||||||
| High-power CO2 laser treatment | Low-power CO2 laser treatment | ||||||
| Women | Men | Women | Men | ||||
| Randomised, n | Number of people randomised in each group of participants not reported. Results are based on number of people analysed | ||||||
| Number of people evaluated | 41 | 33 | 42 | 32 | |||
| Withdrawals, n (%) | Not reported | ||||||
| Treatment regimen | Laser beam with an output of 60 W and a spot size of 1–1.5 mm, creating high power density ranging between 3400 and 7640 W/cm2. Treatments were performed on an outpatient basis under local anaesthesia with 1–2% lidocaine injected intracervically or subcutaneously. Lesions were treated with laser vaporisation, including shallow ablation of 3–5 mm of the surrounding unaffected epithelium. Postprocedure care included application of 3% chloramphenicol skin ointment. All treatments were carried out by the same laser surgeon | Laser beam with an output of 20 W and a spot size of 1–1.5 mm, creating low power density ranging between 1136 and 2547 W/cm2. Treatments were performed on an outpatient basis under local anaesthesia with 1–2% lidocaine injected intracervically or subcutaneously. Lesions were treated with laser vaporisation, including shallow ablation of 3–5 mm of the surrounding unaffected epithelium. Postprocedure care included application of 3% chloramphenicol skin ointment. All treatments were carried out by the same laser surgeon | |||||
| Duration of treatment (minutes), mean (SD): | 12.1 (3.2) | 17.8 (2.7) | 13.5 (3.3) | 12.2 (13.2) | |||
| Baseline patient characteristics | |||||||
| High-power CO2 laser treatment | Low-power CO2 laser treatment | p-value | |||||
| Women | Men | Women | Men | ||||
| Age (years), mean | Not reported | ||||||
| Duration of disease | Not reported | ||||||
| Site of AGWs, n (%) | Not reported | ||||||
| Type of AGWs, n (%) | Not reported | ||||||
| Number of AGWs, mean (with SD/SE if given) | Not reported | ||||||
| Area of AGWs (mm2), mean | Not reported | ||||||
| Sex (M/F), n (%) | 100% female in one subgroup and 100% male in the second subgroup | ||||||
| Any previous treatment, n (%) | Not reported | ||||||
| Ethnicity, n (%) | Not reported | ||||||
| Section 3: Outcomes | |||||||
| Outcome | Definition | ||||||
| AEs | Local infection (no further details reported) | ||||||
| Section 4: Data extraction form | |||||||
| Outcome | Time frame | High-power CO2 laser treatment, n/N | Low-power CO2 laser treatment, n/N | Estimate of effect | p-value | ||
| Women | Men | Women | Men | ||||
| Dichotomous outcomes | |||||||
| AEs: local infection | Not reported | 3/41 | 1/33 | 2/42 | 2/32 | Difference between treatment groups reported to be not significant for both populations | |
| Section 5: Clinical trial quality | |||||||
| Outcome | Risk of bias | Risk assessment a | Comments | ||||
| Random sequence generation | ✓ | It is stated that groups were divided into two treatment groups according to a previously prepared computerised randomisation sequence | |||||
| Allocation concealment | ? | Detail on method used to conceal allocation not available | |||||
| Selective reporting | ✗ | Results for clinical effectiveness of treatments, such as complete clearance and recurrence, are not reported | |||||
| ‘Other bias’ | ? | Baseline characteristics for each population are not reported. It is unclear whether there are any imbalances between the treatment groups that would influence the estimates of effect | |||||
| AEs | Blinding (participants and personnel) | ? | Details on level of masking of personnel are not provided. It is reported that all laser treatments were carried out by the same laser surgeon and so masking of personnel administering laser treatment is not possible. It is unclear whether participants and the outcome assessor were masked to treatment | ||||
| Blinding of outcomes assessment | ? | ||||||
| Incomplete outcome data | ? | The numbers of people randomised to each group and withdrawing from each group (both by population and by treatment group) are not reported. It is unclear whether there is an imbalance in withdrawals from the groups that could influence assessment of local infection | |||||
| Overall rating of bias | ✗ | Reflects lack of reporting of clinical effectiveness outcomes | |||||
| Section 6: Additional comments | |||||||
| Additional comments | None | ||||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Vicky Wakefield and Sam Barton | ||||
| Study ID | Benedetti Panici 1989 | ||||
| Study details | Obstet Gynecol 1989;74:393–7 | ||||
| Language of publication | English | ||||
| Type of report | Full paper | ||||
| Section 2: Study information | |||||
| Location and number of sites | Department of Gynaecology, Catholic University, Rome, Italy | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Women attending the Department of Gynaecology were enrolled between September 1986 and August 1987. In total, 203 women entered the trial | ||||
| Trial design | Four-arm trial. Two arms evaluated two different schedules of interferon-alpha-2b and a third arm evaluated cauterisation. The fourth arm included people who received no treatment. Only the cauterisation and no treatment groups are of interest to this review | ||||
| Trial duration | End of study was 6 months after completion of treatment. People with complete clearance of AGWs were also followed up at 12 months | ||||
| Line of therapy | First (inclusion criterion that people had not received previous treatment) | ||||
| Inclusion criteria | Multiple condyloma lesions (two or more sites affected); aged 18–45 years; no previous therapy; informed written consent; feasible follow-up | ||||
| Exclusion criteria | Moderate or severe intraepithelial neoplasia of the cervix, vagina or vulva; pregnant or breastfeeding; systemic or immunological disease; anaemia (haemoglobin < 10 g/dl); leukopenia (< 4,000/µl); thrombocytopenia (< 100,000/µl); serum creatinine > 1 mg/dl); hepatic dysfunction | ||||
| All outcomes reported in paper | Overall clinical response (combination of complete response and partial response) at completion of treatment; complete response at end of treatment and other time points; partial response at end of treatment and other time points (evaluated by recording change in extension of areas affected by AGWs: ≥ 50% or < 50%); disease progression (increase of > 50% in diffusion of disease); recurrence of AGWs; AEs | ||||
| Subgroups evaluated | None | ||||
| Stratification | Not reported | ||||
| Baseline measurement of disease | Colposcopic evaluation of the lower anogenital tract (anus, vulva, vagina and cervix) was carried out. Cytological and histological examinations were also performed | ||||
| Treatment | Diathermocoagulation | No treatment | |||
| Randomised, n | 51 | 48 | |||
| Withdrawals, n (%) | 0 (0) | 0 (0) | |||
| Treatment regimen | Diathermocoagulation with bipolar electrodes. Procedure carried out under local anaesthetic. If required, repeat procedures were carried out at 3-week intervals | No treatment | |||
| Duration of administered treatment | 47 people required two or more sessions for apparent elimination of AGWs (median number of sessions 2; range 2–4) | No treatment given | |||
| Baseline patient characteristics | Diathermocoagulation | No treatment | p-value | ||
| Age (years), median | 25 | 26 | All differences between groups reported to be not significant; p-values not reported | ||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | |||||
| Vulva | 51 (100) | 48 (100) | |||
| Cervix | 32 (63) | 29 (58) | |||
| Urethra | 27 (53) | 29 (58) | |||
| Vagina | 24 (47) | 22 (46) | |||
| Perianal and canal | 18 (35) | 18 (37) | |||
| Number of sites affected, n (%) | |||||
| 2 | 5 (10) | 7 (15) | |||
| ≥ 2 | 46 (90) | 41 (85) | |||
| Type of AGWs, n (%) | |||||
| Warts | 20 (39) | 20 (42) | |||
| Flat | 22 (43) | 20 (42) | |||
| Spiked | 11 (22) | 9 (19) | |||
| Inverted | 1 (2) | 0 (0) | |||
| Mixed | 27 (53) | 23 (48) | |||
| Number of AGWs, mean (with SD/SE if given) | Not reported | ||||
| Area of AGWs (mm2), mean (with SD/SE if given) | Not reported | ||||
| Sex (M/F), n (%) | Not reported | ||||
| Any previous treatment, n (%) | 0 (0) | 0 (0) | |||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Clearance was evaluated by colposcopy, vaginovulvoscopy and anoscopy. Response was recorded at end of treatment | ||||
| AGW clearance at other time points | Response was evaluated by colposcopy, vaginovulvoscopy and anoscopy. Response was recorded at 1, 3 and 6 months after completion of treatment. Complete response was defined as total disappearance of condyloma for at least 4 weeks and had to be confirmed by three different colposcopists | ||||
| Recurrence | Recurrence of AGWs in people who had experienced a complete response to treatment (i.e. those people with a complete response 1 month after treatment), recorded at 3, 6 and 12 months after treatment | ||||
| Volume of wart clearance | Partial response was defined as a ≥ 50% reduction in condyloma extension. No change was defined as < 50% reduction in lesion extension | ||||
| AEs | Not defined for cauterisation technique | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Diathermocoagulation, n/N | No treatment, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | End of treatment | 51/51 | 0/48 | Not reported | |
| AGW clearance at other time points | 1 month after treatment | 31/51 | 0/48 | Not reported | |
| 3 months after treatment | 18/51 | 1/48 | Not reported | ||
| 6 months after treatment | 18/51 | 1/48 | p < 0.001 | ||
| Recurrence | 3 months after end of treatment | 15/31 | NA/NA | Not reported | |
| 12 months after end of treatment | 17/31 | NA/NA | Not reported | ||
| Volume of wart clearance | |||||
| ≥ 50% reduction in condyloma extension | After therapy | 0/51 | 0/48 | ||
| ≥ 50% reduction in condyloma extension | 1 month after treatment | 16/51 | 1/48 | ||
| ≥ 50% reduction in condyloma extension | 3 months after end of treatment | 25/51 | 3/48 | ||
| ≥ 50% reduction in condyloma extension | 12 months after end of treatment | 24/51 | 3/48 | ||
| < 50% reduction in condyloma extension | After therapy | 0/51 | 0/48 | ||
| < 50% reduction in condyloma extension | 1 month after treatment | 4/51 | 44/48 | ||
| < 50% reduction in condyloma extension | 3 months after end of treatment | 8/51 | 32/48 | ||
| < 50% reduction in condyloma extension | 12 months after end of treatment | 8/51 | 28/48 | ||
| AEs | |||||
| Local oedema and pain | Unclear | 17/51 | 0/48 | Not reported | |
| Dyspareunia | Unclear | 2/51 | 0/48 | Not reported | |
| Slow cicatrisation | Unclear | 9/51 | 0/48 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessment a | Comments | ||
| Random sequence generation | ? | Randomisation was carried out ‘using a simple randomisation method as outlined by Armitage’ (p. 394);247 no further details reported | |||
| Allocation concealment | ? | The paper comments that the investigator assessing response was masked to treatment given but details on method of allocation concealment not available | |||
| Selective reporting | ? | No details reported as to which outcomes were prespecified | |||
| ‘Other bias’ | ? | No details reported with regard to whether one surgeon performed all of the diathermocoagulation procedures. Variation in technique could potentially introduce performance bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | The paper comments that the investigator assessing response was masked to treatment given but details on the method of maintaining masking during the trial not provided. It is unclear whether the assessing clinician was an independent clinician or a member of the trial personnel. It might be difficult to mask clinicians to treatment given the variation in techniques (an ablative therapy vs. no treatment) | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ✓ | Losses to follow-up in the cauterisation and no treatment groups were reported (0 in each group) | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The paper comments that the investigator assessing response was masked to treatment given but details on the method of maintaining masking during the trial not provided. It is unclear whether the assessing clinician was an independent clinician or a member of the trial personnel. It might be difficult to mask clinicians to treatment given the variation in techniques (an ablative therapy vs. no treatment) | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ✓ | Analysis based on all people with complete clearance at end of treatment | |||
| Volume of wart clearance | Blinding (participants and personnel) | ? | The paper comments that the investigator assessing response was masked to treatment given but details on the method of maintaining masking during the trial not provided. It is unclear whether the assessing clinician was an independent clinician or a member of the trial personnel. It might be difficult to mask clinicians to treatment given the variation in techniques (an ablative therapy vs. no treatment) | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ✓ | Losses to follow-up in the cauterisation and no treatment groups were reported (0 in each group) | |||
| AEs | Blinding (participants and personnel) | ? | The paper comments that the investigator assessing response was masked to treatment given but details on the method of maintaining masking during the trial not provided. It is unclear whether the assessing clinician was an independent clinician or a member of the trial personnel. It might be difficult to mask clinicians to treatment given the variation in techniques (an ablative therapy vs. no treatment) | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ✓ | Losses to follow-up in the cauterisation and no treatment groups were reported (0 in each group) | |||
| Overall rating of bias | ? | ||||
| Section 6: Additional comments | |||||
| Additional comments |
|
||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Sam Barton and Vicky Wakefield | ||||
| Study ID | Beutner 1989 | ||||
| Study details | Lancet 1989;1:831–4 | ||||
| Language of publication | English | ||||
| Type of report | Full paper | ||||
| Section 2: Study information | |||||
| Location and number of sites | USA; number of sites unclear | ||||
| Trial sponsor | Oclassen Pharmaceuticals, Inc. | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Not reported | ||||
| Trial design | RCT | ||||
| Trial duration | Treatment could be given for a minimum of 2 weeks and a maximum of 4 weeks. Participants were also evaluated at 6, 12 and 16 weeks during a follow-up period (i.e. weeks 2, 8 and 12 after treatment) | ||||
| Line of therapy | Unclear | ||||
| Inclusion criteria | Men aged ≥ 18 years who had a clinical diagnosis of AGWs; number of warts between two and 20 in an area not exceeding 10 cm2 | ||||
| Exclusion criteria | People were excluded if they had untreated syphilis; had frequent genital herpes; had a history of bowenoid papulosis; gave unreliable answers to questions about history; had been treated for AGWs within a month of study entry; were thought to be immunocompromised based on history and physical examination | ||||
| All outcomes reported in paper | AGW clearance; reduction in AGW area; reduction in number of AGWs; recurrence; appearance of new AGWs, defined as a AGW that arose during the course of the study at a site distinct from that of the original AGWs (recorded during follow-up period after treatment, not during treatment); AEs | ||||
| Subgroups evaluated | Duration of present episode of AGWs (< 12 months vs. ≥ 12 months) | ||||
| Stratification | Randomisation prestratified by duration of present episode of AGWs | ||||
| Baseline measurement of disease | Site, number and size of AGWs before study entry were recorded | ||||
| Treatment | Podophyllotoxin 0.5% (patient applied) | Placebo | |||
| Randomised, n | 56 | 53 | |||
| Withdrawals | Not reported. Note: most patients who had new lesions or who had not responded to treatment were withdrawn from the study at the end of week 2. In addition, patients with recurrent or new AGWs at week 12 were excluded from further evaluations. All patients in the placebo group had withdrawn by week 12; reported that this was generally becase of non-response to therapy | ||||
| Treatment regimen | Podophyllotoxin (0.5%) in lactate-buffered USP alcohol. Treatment cycle consisted of application of podophyllotoxin (0.5%) by the patient twice daily (morning and evening) to external AGWs for 3 consecutive days followed by a 4-day period without treatment. At the end of the treatment cycle the patient returned to the investigator for evaluation and instruction about further treatment. The minimum number of treatment cycles administered was two, with a maximum number of allowed treatment cycles of four. Podophyllotoxin (0.5%) was applied only to intact lesions, avoiding adjacent uninvolved skin or bleeding or inflamed lesions | Placebo was vehicle alone | |||
| Mean number of treatment cycles | 3.2 | 3.3 | |||
| Baseline patient characteristics | Podophyllotoxin 0.5% (patient applied) | Placebo | p-value | ||
| Age (years), mean (SD) | 30.0 (0.9) | 31.7 (0.9) | Not reported | ||
| Duration of disease, n (%) | |||||
| ≤ 12 months | 29 (51.8) | 26 (49.1) | Not reported | ||
| > 12 months | 27 (48.2) | 27 (50.9) | Not reported | ||
| Site of AGWs (%) | |||||
| Penile shaft | 88.4 | 85.4 | Not reported | ||
| Other | 11.6 | 14.6 | Not reported | ||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean (SE) | 7.1 (0.7) | 7.8 (0.7) | |||
| Area of AGWs (mm2), mean (SE) | 87.4 (18.3) | 101.9 (21.0) | |||
| Sex (M/F), n (%) | All men | All men | NA | ||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Defined as complete clearance of AGWs at end of treatment (week 4). People who showed no change or showed progression at week 2 were considered to be treatment failures and were dropped from the study | ||||
| Recurrence of AGWs | AGW(s) that appeared near the site of an original baseline AGW after complete healing. Patients with recurrent or new AGWs at week 12 were not evaluated at week 16 | ||||
| AEs | Systemic safety and toxicity were evaluated by patient reports, by investigator reports of systemic AEs and by baseline and follow-up laboratory tests. Local adverse reactions (including pain, burning, inflammation, erosion and others to be specified by the investigator after examination of the patient) were classified as none, mild, moderate or severe. Evaluated at each visit during treatment (weeks 1–4) and at the first follow-up visit (week 6) | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllotoxin 0.5% (patient applied), n/N | Placebo, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | Week 4 | 25/56 | 0/53 | p < 0.001 | |
| Recurrence of AGWs | Week 12 | 15/25 | N/A | ||
| AEs | |||||
| Inflammation | Week 6 | 37/56 | 3/53 | Not reported | |
| Erosion | Week 6 | 35/56 | 2/53 | Not reported | |
| Burning | Week 6 | 33/56 | 19/53 | Not reported | |
| Pain | Week 6 | 26/56 | 7/53 | Not reported | |
| Other | Week 6 | 19/56 | 4/53 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessment a | Comments | ||
| Random sequence generation | ? | Described as randomised but no details on method of randomisation provided | |||
| Allocation concealment | ? | Details of method of allocation concealment not provided | |||
| Selective reporting | ? | Insufficient information to determine level of risk | |||
| ‘Other bias’ | ? | Insufficient information to assess whether an important risk of bias exists | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | Described as double blinded but insufficient information provided to determine who was masked | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | ITT analysis but unclear how many people were lost to follow-up and whether losses are balanced between the two trial arms | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Described as double blinded but insufficient information provided to determine who was masked | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | ITT analysis but unclear how many people were lost to follow-up and whether losses are balanced between the two trial arms | |||
| AEs | Blinding (participants and personnel) | ? | Described as double blinded but insufficient information provided to determine who was masked | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | ITT analysis but unclear how many people were lost to follow-up and whether losses are balanced between the two trial arms | |||
| Overall rating of bias | ? | Insufficient information provided to determine overall risk of bias | |||
| Section 6: Additional comments | |||||
| Additional comments |
|
||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Victoria Wakefield and Sam Barton | |||||
| Study ID | Beutner 1998a | |||||
| Study details | Antimirob Agents Chemother 1998;42:789–94 | |||||
| Language of publication | English | |||||
| Type of report | Full paper | |||||
| Section 2: Study information | ||||||
| Location and number of sites | USA; seven centres (number of sites not reported) | |||||
| Trial sponsor | Research was supported by a grant from 3M Pharmaceuticals | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | Not reported | |||||
| Trial design | RCT | |||||
| Trial duration | Treatment administered for a maximum of 16 weeks. After treatment, people were followed up over a 12-week period during which no treatment was given | |||||
| Line of therapy | Mixed population (approx. 30% of people were treatment naive) | |||||
| Inclusion criteria | Aged ≥ 18 years; seronegative for HIV infection; at least two but no more than 50 AGWs (defined as warts in the genital, anal, perineal or perianal area); had a biopsy diagnostic or suggestive of condyloma acuminatum and a bidimensional AGW area of at least 10 mm2 | |||||
| Exclusion criteria | People were excluded if they had received AGW therapy in the 4 weeks before treatment initiation. Women were excluded if they were pregnant or lactating; had not agreed to use effective birth-control measures; or had a prestudy Papanicolaou smear that showed presence of a high-grade squamous intraepithelial lesion | |||||
| All outcomes reported in paper | Complete clearance; proportion of people with at least a 50% reduction in AGW area; median time to complete clearance of baseline AGWs; appearance of new warts during treatment; recurrence; AEs | |||||
| Subgroups evaluated | Men vs. women | |||||
| Stratification | It is stated that ‘patients were randomised by study centre and by gender’ (p. 789) | |||||
| Baseline measurement of disease | At baseline, AGW assessment included photographs, measurement, count and location. The assessment was repeated at each evaluation visit during and after treatment | |||||
| Treatment | Imiquimod 5% cream | Imiquimod 1% cream | Placebo (vehicle cream) | |||
| Randomised, n | 94 | 90 | 95 | |||
| Withdrawals, n | 25 | 19 | 28 | |||
| Lost to follow-up, non-compliance or had an intercurrent illness, among other reasons, n | 24 | 11 | 20 | |||
| Treatment-related reasons, n | ||||||
| Local skin reactions | 1 | 1 | 0 | |||
| Lack of therapeutic effect | 0 | 4 | 2 | |||
| Clinically significant increase in wart area | 0 | 3 | 6 | |||
| Treatment regimen | Before bedtime, participants rubbed their allocated treatment into clean, dry AGW-area skin until the treatment disappeared. They were instructed to wash the area with soap and water 8 ± 2 hours after application. Evaluations took place weekly for the first 4 weeks and every 2 weeks thereafter for the remainder of the treatment period and the 12-week follow-up period. At any point during treatment, if all AGWs cleared, treatment was stopped and the participant entered the 12-week follow-up period | |||||
| Duration/number of administered treatment | Not reported. It was reported that, when local skin reactions made continued application of treatment difficult, participants were allowed to take rest periods of 1–7 days. The mean number of rest days per patient was 11 days (14 days for female patients vs. 9 days for male patients) | |||||
| Baseline patient characteristics | Imiquimod 5% cream | Imiquimod 1% cream | Placebo (vehicle cream) | p-value | ||
| Age (years), mean (SD) | 30 (10) | 33 (11) | 30 (9) | Not reported | ||
| Duration of current episode (months), median (range) | 7.3 (0.2–484.3) | 12.2 (0.3–388.1) | 8.7 (0.3–246.8) | Not reported | ||
| Site of AGWs, n (%)a | ||||||
| Women | ||||||
| Vulva | 35 (83) | 37 (90) | 38 (90) | Not reported | ||
| Perianal | 17 (40) | 21 (51) | 21 (50) | Not reported | ||
| Perineum | 1 (2) | 2 (5) | 1 (2) | Not reported | ||
| Other | 4 (9) | 2 (4) | 0 (0) | Not reported | ||
| Men | ||||||
| Penile | 50 (96) | 41 (84) | 47 (89) | Not reported | ||
| Perianal | 4 (8) | 12 (24) | 5 (9) | Not reported | ||
| Scrotum | 2 (4) | 3 (6) | 4 (8) | Not reported | ||
| Groin | 2 (4) | 7 (14) | 3 (6) | Not reported | ||
| Other | 4 (8) | 8 (16) | 4 (8) | Not reported | ||
| Type of AGWs (e.g. non-keratinsed, keratinised), n (%) | Not reported | Not reported | Not reported | |||
| Number of AGWs, median (range) | 7 (1–47) | 8 (1–50) | 8 (1–45) | Not reported | ||
| Area of AGWs (mm2), median (range) | 137 (2–9588) | 148 (10–13,461) | 121 (4–2603) | Not reported | ||
| Sex, n (%) | ||||||
| Male | 52 (55) | 49 (54) | 53 (56) | Not reported | ||
| Female | 42 (45) | 41 (46) | 42 (44) | Not reported | ||
| No previous treatment, n (%) | 31 (33) | 24 (27) | 23 (24) | Not reported | ||
| Ethnicity, n (%) | ||||||
| White | 89 (95) | 84 (93) | 93 (98) | Not reported | ||
| Other | 5 (5) | 6 (6) | 2 (2) | Not reported | ||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Primary outcome. Defined as complete clearance of baseline AGWs during the treatment period | |||||
| Recurrence of AGWs | Reappearance of AGWs during the follow-up period in people with complete clearance during treatment | |||||
| Time to complete clearance | Median time to complete clearance of baseline AGWs | |||||
| Volume of wart clearance | Proportion of people with a reduction in AGW area of at least 50% | |||||
| Appearance of new warts during treatment | Appearance of AGWs that were not present at initiation of treatment | |||||
| AEs | Local skin reactions were assessed by patients and study personnel using a 4-point scale from 0 (no reaction) to 3 (severe) | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Imiquimod 5% cream, n/N | Imiquimod 1% cream, n/N | Placebo (vehicle cream), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | At end of and during treatment | 49/94 | 13/90 | 3/95 | p < 0.001 for imiquimod 5% vs. imiquimod 1% and placebo | |
| Recurrence of AGWs | 12 weeks after end of treatment | 9/48 | 2/12 | 0/3 | Not reported | |
| Volume of wart clearance (proportion of patients with at least 50% reduction in wart area; includes those with 100% clearance) | End of treatment | 64/69 | 32/79 | 17/75 | Based on group of people not lost to follow-up | Not reported |
| Appearance of new warts during treatment | During treatment | 27/92 | 44/86 | 58/92 | Not reported | |
| AEs | ||||||
| Erythema | ||||||
| Mild | 15/92 | – | 26/92 | Not reported | ||
| Moderate | 40/92 | – | 8/92 | Not reported | ||
| Severe | 21/92 | – | 0/92 | Not reported | ||
| Excoriation or flaking | ||||||
| Mild | 20/92 | – | 10/92 | Not reported | ||
| Moderate | 16/92 | – | 3/92 | Not reported | ||
| Severe | 3/92 | – | 1/92 | Not reported | ||
| Erosion | ||||||
| Mild | 15/92 | – | 4/92 | Not reported | ||
| Moderate | 26/92 | – | 1/92 | Not reported | ||
| Severe | 3/92 | – | 0/92 | Not reported | ||
| Oedema | ||||||
| Mild | 19/92 | – | 2/92 | Not reported | ||
| Moderate | 13/92 | – | 1/92 | Not reported | ||
| Severe | 4/92 | – | 0/92 | Not reported | ||
| Scabbing | ||||||
| Mild | 14/92 | – | 5/92 | Not reported | ||
| Moderate | 12/92 | – | 0/92 | Not reported | ||
| Severe | 5/92 | – | 0/92 | Not reported | ||
| Induration | ||||||
| Mild | 17/92 | – | 3/92 | Not reported | ||
| Moderate | 5/92 | – | 0/92 | Not reported | ||
| Severe | 0/92 | – | 0/92 | Not reported | ||
| Ulceration | ||||||
| Mild | 3/92 | – | 0/92 | Not reported | ||
| Moderate | 8/92 | – | 0/92 | Not reported | ||
| Severe | 1/92 | – | 0/92 | Not reported | ||
| Vesicles | ||||||
| Mild | 4/92 | – | 0/92 | Not reported | ||
| Moderate | 3/92 | – | 0/92 | Not reported | ||
| Severe | 0/92 | – | 0/92 | Not reported | ||
| Most common application site reactions | ||||||
| Itching | 30/92 | 20/86 | 17/92 | p = 0.084 | ||
| Pain | 32/92 | 11/86 | 2/92 | p = 0.0001 | ||
| Burning | 15/92 | 12/86 | 1/92 | p = 0.0003 | ||
| Tenderness | 11/92 | 11/86 | 2/92 | p = 0.0120 | ||
| Other symptoms | ||||||
| Headache | 27/92 | 26/86 | 30/92 | p = 0.892 | ||
| Upper respiratory tract infection | 13/92 | 23/86 | 25/92 | p = 0.053 | ||
| Continuous outcomes | ||||||
| Time to complete clearance (weeks), median | During treatment | 9 (n = 49) | 7 (n = 13) | 12 (n = 3) | Not reported | |
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessment b | Comments | |||
| Random sequence generation | ? | Described as randomised but details of method of randomisation not provided | ||||
| Allocation concealment | ? | Details of method of allocation concealment not provided | ||||
| Selective reporting | ✓ | The study protocol is not available; however, the publication reports results for all expected clinical outcomes | ||||
| ‘Other bias’ | ? | Insufficient information to assess whether another important risk of bias exists | ||||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | Described as double blinded but details on who was masked to treatment and methods to maintain masking during the study not provided | |||
| Blinding of outcomes assessment | ? | Described as double blinded but details on who was masked to treatment not provided; unclear whether the physician assessing the outcome was also the treating physician | ||||
| Incomplete outcome data | ✓ | Missing outcome data balanced across intervention groups, with similar reasons for missing data across groups. In addition, analysis of complete clearance is based on all people randomised | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Described as double blinded but details on who was masked to treatment and methods to maintain masking during the study not provided | |||
| Blinding of outcomes assessment | ? | Described as double blinded but details on who was masked to treatment not provided; unclear whether the physician assessing the outcome was also the treating physician | ||||
| Incomplete outcome data | ✓ | Missing outcome data balanced across intervention groups, with similar reasons for missing data across groups. In addition, analysis of recurrence is based on all people with complete clearance of AGWs | ||||
| Time to complete clearance | Blinding (participants and personnel) | ? | Described as double blinded but details on who was masked to treatment and methods to maintain masking during the study not provided | |||
| Blinding of outcomes assessment | ? | Described as double blinded but details on who was masked to treatment not provided; unclear whether the physician assessing the outcome was also the treating physician | ||||
| Incomplete outcome data | ✓ | Missing outcome data balanced across intervention groups, with similar reasons for missing data across groups. In addition, analysis of time to complete clearance is based on all people randomised | ||||
| Volume of wart clearance | Blinding (participants and personnel) | ? | Described as double blinded but details on who was masked to treatment and methods to maintain masking during the study not provided | |||
| Blinding of outcomes assessment | ? | Described as double blinded but details on who was masked to treatment not provided; unclear whether the physician assessing the outcome was also the treating physician | ||||
| Incomplete outcome data | ✓ | Missing outcome data balanced across intervention groups, with similar reasons for missing data across groups. In addition, analysis of complete clearance is based on all people randomised | ||||
| Appearance of new warts during treatment | Blinding (participants and personnel) | ? | Described as double blinded but details on who was masked to treatment and methods to maintain masking during the study not provided | |||
| Blinding of outcomes assessment | ? | Described as double blinded but details on who was masked to treatment not provided; unclear whether the physician assessing the outcome was also the treating physician | ||||
| Incomplete outcome data | ✓ | Missing outcome data balanced across intervention groups, with similar reasons for missing data across groups | ||||
| AEs | Blinding (participants and personnel) | ? | Described as double blinded but details on who was masked to treatment and methods to maintain masking during the study not provided | |||
| Blinding of outcomes assessment | ? | Described as double blinded but details on who was masked to treatment not provided; unclear whether the physician assessing the outcome was also the treating physician | ||||
| Incomplete outcome data | ✓ | Missing outcome data balanced across intervention groups, with similar reasons for missing data across groups | ||||
| Overall rating of bias | ? | Reflects uncertainty around masking of treatment allocation and maintenance of masking during the study | ||||
| Section 6: Additional comments | ||||||
| Additional comments |
|
|||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Victoria Wakefield and Sam Barton | ||||
| Study ID | Beutner 1998b | ||||
| Study details | J Am Acad Dermatol 1998;38:230–9 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Three outpatient centres comprising one public health clinic, one university-based clinic and one private practice (carried out in the USA) | ||||
| Trial sponsor | Trial supported by a grant from 3M Pharmaceuticals | ||||
| Conflicts of interest | Not reported; three of six authors are listed as being based at 3M Pharmaceuticals | ||||
| Patient enrolment | Details on recruitment and dates of enrolment of participants not provided | ||||
| Trial design | RCT | ||||
| Trial duration | Treatment was carried out over 8 weeks. People who experienced complete clearance of AGWs entered into a treatment-free follow-up period of 10 weeks or until recurrence occurred. People with a partial response at the end of 8 weeks’ treatment were evaluated again at week 2 of follow-up to determine whether complete clearance had been achieved | ||||
| Line of therapy | Mixed; about 30% of patients were treatment naive | ||||
| Inclusion criteria | People aged ≥ 18 years who were HIV seronegative | ||||
| Exclusion criteria | Pregant and lactating women were excluded as were women with vaginal warts or low- or high-grade cervical squamous intraepithelial lesions | ||||
| All outcomes reported in paper | Complete clearance; recurrence; median time to complete clearance; reduction in volume of AGWs; appearance of new AGWs during treatment; AEs | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | Not clear | ||||
| Baseline measurement of disease | Diagnosis of AGWs was established by physical examination and confirmed by histopathology when indicated. Only clinically visible external AGWs were evaluated and treated. AGW area was determined by multiplying the two greatest perpendicular dimensions of each AGW. Total wart area was the sum of areas of individual AGWs | ||||
| Treatment | Imiquimod 5% | Placebo | |||
| Randomised, n | 51 | 57 | |||
| Withdrawals, n | 7 | 12 | |||
| Lost to follow-up | 4 | 6 | |||
| Discontinued because of local skin reaction | 2 | 0 | |||
| Discontinued because of increase/no change in AGW area | 1 | 5 | |||
| Personal reason | 0 | 1 | |||
| Treatment regimen | Treatment or placebo was applied by participants. A clinician supervised the first dose of treatment or placebo applied by the participant. Placebo was physically indistinguishable from imiquimod 5% cream. Participants were instructed to bathe or shower before drug application and to avoid bathing/showering for 24 hours when the cream was on the skin. Sufficient cream was applied to cover the AGW, with application three times per week (Monday, Wednesday and Friday or Tuesday, Thursday and Saturday). Treatment was carried out for 8 weeks. Participants were assessed at initiation of treatment and once per week thereafter | ||||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Imiquimod 5% | Placebo | p-value | ||
| Age (years), mean (SD) | 29 (8) | 30 (9) | p > 0.50 | ||
| Duration of disease (months since onset), mean (range) | 28 (2–181) | 14 (1–277) | p = 0.18 | ||
| Site of AGWs, n (%) | It is reported that, in men, the most frequent location of AGWs was the shaft of the penis (90–91%), with warts less commonly occurring on the perianal, scrotal, inguinal, pubic, thigh and perineal areas. In women, AGWs were located on the vulvar, perineal and perianal areas as well as the mons pubis and thigh. Some people had AGWs at multiple sites | Not reported | |||
| Type of AGWs | Not reported | ||||
| Number of AGWs, median (range) | 6 (1–29) | 7 (1–105) | p > 0.50 | ||
| Area of AGWs (mm2), median (range) | 47 (6–1785) | 63 (4–8784) | p = 0.14 | ||
| Sex: male, n (%) | 46 (90) | 52 (91) | p > 0.50 | ||
| Any previous treatment, n (%) | |||||
| None | 16 (31) | 18 (32) | p > 0.50 | ||
| Podophyllin resin | 26 (51) | 21 (37) | p = 0.18 | ||
| Cryotherapy | 18 (35) | 20 (35) | p > 0.50 | ||
| Surgical excision | 0 (0) | 1 (2) | p > 0.50 | ||
| Electrocautery | 4 (8) | 3 (5) | p > 0.50 | ||
| Laser therapy | 0 (0) | 1 (2) | p > 0.50 | ||
| TCAA | 4 (8) | 8 (14) | p = 0.37 | ||
| Condylox | 0 (0) | 2 (4) | p = 0.50 | ||
| Cornstarch | 0 (0) | 1 (2) | p > 0.50 | ||
| Unknown/other | 2 (4) | 4 (7) | p > 0.50 | ||
| Number of previous treatments, mean (SD) | 1.06 (0.91) | 1.05 (0.93) | p > 0.50 | ||
| Ethnicity, n (%) | p = 0.29 | ||||
| White | 48 (94) | 54 (95) | |||
| Black | 3 (6) | 1 (2) | |||
| Asian/Pacific islander | 0 (0) | 2 (4) | |||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | People with complete clearance during the treatment period. People with a partial response (> 0% to < 100% reduction) at the end of the 8-week treatment period were evaluated again at week 2 of the follow-up period to determine whether they had achieved complete clearance. Analysis of AGW clearance included people with complete clearance at this time point | ||||
| Recurrence of AGW | In people with complete clearance, reappearance of AGWs during the 10-week follow-up period | ||||
| Time to complete clearance | Median time to complete clearance, including those with initial partial clearance at the end of treatment and subsequent clearance at 2 weeks after the end of treatment | ||||
| Volume of wart clearance | Proportion of people with various reductions in baseline wart area (≥ 0%, ≥ 10%, ≥ 20%, ≥ 30%, ≥ 40%, ≥ 50%, ≥ 60%, ≥ 70%, ≥ 80%, ≥ 90%, 100%) | ||||
| Appearance of new warts during treatment | Not defined | ||||
| AEs | People were asked to quantify symptoms (itching, pain and burning at site of application or adjacent area) as mild, moderate or severe. Objective evidence of inflammation at site of application and adjacent sites was evaluated using a scale from 0 to 6 (0 = no visible reaction; 1 = equivocal response; 2 = mild erythema; 3 = moderate erythema; 4 = intense erythema; 5 = intense erythema with oedema; and 6 = intense erythema with oedema and vesicles). Skin irritation at wart site was reported by week of treatment rather than as the total number of people experiencing the event. In addition, although application-site reactions (e.g. pain, itching, erythema) were reported for the imiquimod 5% group, equivalent data were not reported for the placebo group. Data are not presented in a format that can be used in prespecified analysis | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Imiquimod 5%, n/N | Placebo, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 2 weeks after end of treatment | 19/51 | 0/57 | p < 0.001 | |
| Recurrence of AGW | 10 weeks after end of treatment | 3/16 | 0/0 | ||
| Volume of wart clearance (proportion of patients with ≥ 50% clearance; includes those with 100% clearance) | 2 weeks after end of treatment | 34/45 | 4/50 | p ≤ 0.001 | |
| Appearance of new warts during treatment | 8 weeks | 14/48 | 20/55 | p > 0.50 | |
| Continuous outcomes | |||||
| Time to complete clearance (weeks), median | 2 weeks after end of treatment | 7 (n = 18) | NA (n = 0) | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that ‘fifty-one patients were randomly selected to receive 5% imiquimod cream; 57 patients were randomly chosen to receive placebo cream’ (p. 233). Details on the method of randomisation are not provided | |||
| Allocation concealment | ? | Details on the method of allocation concealment are not reported | |||
| Selective reporting | ✓ | The study protocol is not available but the report provides data on all expected clinical outcomes | |||
| ‘Other bias’ | ? | Insufficient detail reported to evaluate risk of other bias | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | ||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing wart clearance is masked to treatment | |||
| Incomplete outcome data | ✓ | Loss to follow-up reported. AGW clearance is based on an ITT analysis | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | ||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing wart recurrence is masked to treatment | |||
| Incomplete outcome data | ✓ | Analysis of recurrence is based on all people with complete clearance at a defined time point | |||
| Time to complete clearance | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | ||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing wart clearance is masked to treatment | |||
| Incomplete outcome data | ✓ | Analysis of time to complete clearance is based on all people with complete clearance at a defined time point | |||
| Volume of wart clearance (proportion of patients with ≥ 50% clearance) | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | ||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing wart clearance is masked to treatment | |||
| Incomplete outcome data | ✓ | Number of people lost to follow-up is reported. Although proportion of people with reduction in wart clearance is not based on an ITT analysis, a similar proportion of people is excluded from each treatment group | |||
| Appearance of new warts during treatment | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | ||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing wart clearance is masked to treatment | |||
| Incomplete outcome data | ✗ | Although number of people lost to follow-up is reported, it is unclear how the number of people included as the denominator in this analysis has been derived (does not correspond to analysis outlined in the paper) | |||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | ||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing wart clearance is masked to treatment | |||
| Incomplete outcome data | ✗ | Absolute numbers of AEs not reported for the placebo group. Authors state that ‘Each symptom occurred significantly more frequently (p < 0.05) in imiquimod recipients than in placebo recipients’ (p. 235) | |||
| Overall rating of bias | ? | ||||
| Section 6: Additional comments | |||||
| Additional comments | The primary analysis reported in the paper excludes people who were lost to follow-up, who discontinued for a personal reason or who were judged to be non-compliant with dosing schedules. People who discontinued because of local skin reactions and for increase/no change in AGW area were considered treatment failures and were included in the analysis of efficacy | ||||
| Item | Details | |||||||
|---|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||||
| Reviewers’ names | Sjokvist Garcia-Stewart and Sam Barton | |||||||
| Study ID | Claesson 1996 | |||||||
| Study details | Int J STD AIDS 1996;7:429–34 | |||||||
| Language of publication | English | |||||||
| Type of report | Full publication | |||||||
| Section 2: Study information | ||||||||
| Location and number of sites | Study was carried out at multiple European sites: two sites in Sweden (Garnisonssjukhuset T2 and Vastadens Lakarmottgn); one site in Finland (Department of Dermatology and Venereology, University Hospital, Helsinki); one site in France (Institut Alfred Fournier, Paris) | |||||||
| Trial sponsor | Perstorp Pharma, Lund, Sweden | |||||||
| Conflicts of interest | None reported | |||||||
| Patient enrolment | Participants were recruited from those with AGWs attending the study sites. Dates of enrolment not reported | |||||||
| Trial design | RCT (three arms). Publication reports results from two RCTs, one involving men and one involving women | |||||||
| Trial duration | Initial treatment period of up to 4 weeks. Those with complete clearance were followed up at 16 weeks | |||||||
| Line of therapy | Unclear | |||||||
| Inclusion criteria | Inclusion criterion for the trial evaluating treatments in men: diagnosis of condylomata acuminata located on the penis shaft and/or within the preputial cavity region. Inclusion criterion for the trial evaluating treatments in women: diagnosis of vulval and/or perianal condylomata acuminata | |||||||
| Exclusion criteria | Exclusion criteria for both RCTs were aged < 18 years; presence of untreated gonorrhoea, syphilis, herpes and/or chlamydia infection; period of < 3 months elapsed since previous treatment for condylomata acuminata | |||||||
| All outcomes reported in paper | Response to treatment; complete clearance; recurrence; appearance of new AGWs during follow-up; AEs | |||||||
| Subgroups evaluated | None reported within the individual RCTs | |||||||
| Stratification | None reported | |||||||
| Baseline measurement of disease | Not reported | |||||||
| Treatment | Podophyllotoxin 0.15% cream (patient applied) | Podophyllotoxin 0.3% cream (patient applied) | Podophyllotoxin 0.5% solution (patient applied) | |||||
| Trial 1 (men) | Trial 2 (women) | Trial 1 (men) | Trial 2 (women) | Trial 1 (men) | Trial 2 (women) | |||
| Randomised, n | 30 | 30 | 30 | 30 | 30 | 30 | ||
| Withdrawals, n (%) | Not reported for either trial | |||||||
| Treatment regimen | Treatment schedules were the same in the two RCTs. Participants self-applied their allocated treatment (podophyllotoxin 0.15% cream, podophyllotoxin 0.3% cream or podophyllotoxin 0.5%) twice a day at home for 3 consecutive days. If a cure was not achieved, the treatment cycle was repeated with a 4-day break between cycles. The total number of applications did not exceed 24. Thus, treatment was repeated until complete clearance of AGWs was achieved or for a maximum of 4 weeks, whichever occurred earlier | |||||||
| Duration/number of administered treatment | Not reported | |||||||
| Baseline patient characteristics | Podophyllotoxin 0.15% cream (patient applied) | Podophyllotoxin 0.3% cream (patient applied) | Podophyllotoxin 0.5% solution (patient applied) | p-value | ||||
| Age (years), mean | 25.7 | 25 | 25.9 | 26.7 | 24.6 | 27.7 | Not reported | |
| Duration of disease (months), mean | 1.6 | 2 | 1.3 | 1.9 | 1.7 | 2.7 | Not reported | |
| Site of AGWs, n (%) | Men: penis shaft and/or within the preputial cavity region; women: vulval and/or perianal areas | |||||||
| Type of AGWs, n (%) | Not reported | |||||||
| Number of AGWs | ||||||||
| 1–5 | 9 | 13 | 12 | 10 | 10 | 12 | Not reported | |
| 6–10 | 2 | 3 | 6 | 6 | 7 | 5 | ||
| > 10 | 19 | 14 | 12 | 14 | 13 | 13 | ||
| Area of AGWs (mm2), mean | Not reported | |||||||
| Sex (M/F), n (%) | Trial 1: 100% men; trial 2: 100% women | |||||||
| Any previous treatment, n (%) | Not reported | |||||||
| Ethnicity, n (%) | Not reported | |||||||
| Section 3: Outcomes | ||||||||
| Outcome | Definition | |||||||
| AGW clearance at completion of treatment | Cure defined as elimination of all original warts. Evaluated during treatment | |||||||
| AGW clearance at other time points | Results presented for complete clearance at 1, 2 and 3 weeks of treatment | |||||||
| Recurrence of AGWs | In those with complete clearance, recurrence was defined as appearance of AGWs at the follow-up visit in an earlier treated and completely cured area | |||||||
| AEs | At follow-up visits, participants were asked by the clinician whether they had experienced itching, a burning sensation, tenderness, pruritus, erythema or erosion. Symptoms were evaluated numerically as not present (0), mild (1), moderate (2) and severe (3). An overall adverse symptom score was calculated to summarise the worst adverse symptoms recorded for each patient. Definitions of mild, moderate and severe were not available | |||||||
| Section 4: Data extraction form | ||||||||
| Outcome | Time frame | Podophyllotoxin 0.15% cream (patient applied), n/N | Podophyllotoxin 0.3% cream (patient applied), n/N | Podophyllotoxin 0.5% solution (patient applied), n/N | Estimate of effect | p-value | ||
| Dichotomous outcomes | ||||||||
| AGW clearance at completion of treatment | 4 weeks | No absolute numbers reported for complete clearance at any time point for the two trials. Response ratea reported and presented graphically. Number of people achieving complete clearance cannot be determined from graphs | Not reported | |||||
| AGW clearance at other time points | 1, 2 and 3 weeks | Not reported | ||||||
| Recurrence of AGWsb | 16 weeks after end of treatment | 6% | 8.6% | 8.6% | Not reported | |||
| AEs | 4 weeks | Absolute numbers not reported for mild, moderate or severe AEs for the two trials. Most data presented graphically | Not reported | |||||
| Moderate to severe recorded AEs in women (no further definition provided) | 4 weeks | 12/30 | 18/30 | 18/30 | Not reported | |||
| Section 5: Clinical trial quality | ||||||||
| Outcome | Risk of bias | Risk assessmentc | Comments | |||||
| Random sequence generation | ? | It is stated that men and women were randomised to treatment but details on the method used to generate the random sequence are not available | ||||||
| Allocation concealment | ? | Details of the method of allocation concealment not available | ||||||
| Selective reporting | ✗ | Data for most outcomes are reported or presented in such a way that precludes incorporation into a meta-analysis (percentages with no denominator or graphically). Results for complete clearance in both trials, although recorded, are not reported. Recurrence is reported as a percentage combining men and women, but the number of people with complete clearance is not available and so the number of people with recurrence cannot be back calculated | ||||||
| ‘Other bias’ | ? | Insufficient information to determine potential additional sources of bias. The study was carried out at multiple sites but randomisation was not stratified by site to investigate whether similar results were obtained at each site | ||||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence by lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | |||||
| Blinding of outcomes assessment | ✗ | |||||||
| Incomplete outcome data | ? | The number of people withdrawing or lost to follow-up is not reported. It is unclear whether there is an imbalance across the groups in the number of people withdrawing or lost to follow-up | ||||||
| Recurrence of AGWs | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AGW recurrence is likely to be subjective and open to influence by lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | |||||
| Blinding of outcomes assessment | ✗ | |||||||
| Incomplete outcome data | ? | The number of people withdrawing or lost to follow-up is not reported. The number of people returning for evaluation at 16 weeks is not reported. It is unclear whether there is an imbalance across the groups in the number of people withdrawing or lost to follow-up | ||||||
| AEs | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AEs is likely to be subjective and open to influence by lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | |||||
| Blinding of outcomes assessment | ✗ | |||||||
| Incomplete outcome data | ? | The number of people withdrawing or lost to follow-up is not reported. The number of people included in the evaluation of AEs is not reported. It is unclear whether there is an imbalance across the groups in the number of people withdrawing or lost to follow up | ||||||
| Overall rating of bias | ✗ | Reflects the open-label nature of the trial and the limited reporting of results for clinical outcomes and AEs | ||||||
| Section 6: Additional comments | ||||||||
| Additional comments | None | |||||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewer name | Sam Barton and Victoria Wakefield | ||||
| Study ID | Edwards 1988 | ||||
| Study details | Genitourin Med 1988;64:263–5 | ||||
| Language of publication | English | ||||
| Type of report | Full paper | ||||
| Section 2: Study information | |||||
| Location and number of sites | One site (St Thomas’ Hospital, London, UK) | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Not reported | ||||
| Trial design | RCT; participants randomised 2 : 1 to podophyllotoxin 0.5% and podophyllin 20% | ||||
| Trial duration | Initial 6-week treatment period with a subsequent 3-month follow-up period | ||||
| Line of therapy | Not reported | ||||
| Inclusion criterion | Men with a diagnosis, based on clinical appearance, of external penile AGWs | ||||
| Exclusion criteria | Men were excluded if they had had treatment for AGWs in the preceding 28 days or if the area to be treated exceeded 10 cm2 | ||||
| All outcomes reported in paper | Complete clearance of AGWs; recurrence; AEs | ||||
| Subgroups evaluated | None | ||||
| Stratification | Not reported | ||||
| Baseline measurement of disease | Not reported | ||||
| Treatment | Podophyllotoxin 0.5% (patient applied) | Podophyllin 20% (clinician applied) | |||
| Randomised, n | 42 | 23 | |||
| Withdrawals, n (%) | 10 (23.8) (reasons for withdrawal not reported). In addition, four patients whose AGWs had not resolved after 6 weeks were withdrawn from the trial and regarded as treatment failures | 4 (17.4) (reasons for withdrawal not reported). In addition, seven patients whose AGWs had not resolved after 6 weeks were withdrawn from the trial and regarded as treatment failures | |||
| Treatment regimen | Podophyllotoxin 0.5% solution was patient applied, with only the first treatment applied by a clinician. Participants were instructed to continue application morning and evening to complete 3 consecutive days of treatment and were asked to return a week later for further assessment. If AGWs were still present, they continued treatment each week for up to 6 weeks. Those experiencing side effects were advised to stop treatment until they were reviewed, at which time the clinician decided whether to continue treatment. If AGWs had not resolved after 6 weeks, they were withdrawn from the trial and classed as treatment failures | Podophyllin 20% in alcohol was applied by a doctor once a week for up to 6 weeks. If AGWs had not resolved after 6 weeks, participants were withdrawn from the trial and classed as treatment failures | |||
| Duration/number of administered treatment | Up to 6 weeks of treatment; further details not reported | ||||
| Baseline patient characteristics | Podophyllotoxin 0.5% (patient applied) | Podophyllin 20% (clinician applied) | p-value | ||
| Age (years), mean (range) | 27 (20–42) | 29 (19–45) | Not reported | ||
| Duration of disease | Not reported | Not reported | |||
| Site of AGWs, n (%) | Penile AGWs; no further details reported | Not reported | |||
| Type of AGWs, n (%) | Not reported | Not reported | |||
| Number of AGWs, mean (with SD/SE if given) | Not reported | Not reported | |||
| Area of AGWs (mm2), mean (with SD/SE if given) | Not reported | Not reported | |||
| Sex (M/F), n (%) | All men | All men | Not reported | ||
| Any previous treatment, n (%) | Not reported | Not reported | |||
| Ethnicity, n | |||||
| Black | 3 | 1 | Not reported | ||
| White | 36 | 23 | Not reported | ||
| Asian | 2 | 0 | Not reported | ||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Resolution was defined as the disappearance of the AGW(s) as assessed by a doctor at 6 weeks | ||||
| AGW clearance at other time points | Resolution (disappearance) of AGWs at 4 weeks and 3 months. Data for 3 months were not reported as follow-up was low at this time point, which the authors commented precluded comparison between treatments | ||||
| Recurrence of AGWs | Relapse was defined as resolution followed by reappearance of the AGW(s) during the 3 months of follow-up | ||||
| AEs | Not defined. AEs reported were side effects resulting in treatment disruption and transient side effects (trivial irritation, mild erythema) not requiring treatment discontinuation | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllotoxin 0.5% (patient applied), n/N | Podophyllin 20% (clinician applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 6 weeks | 28/32 | 12/19 | p < 0.05 | |
| AGW clearance at other time points | 4 weeks | 24/32 | 8/19 | p < 0.02 | |
| Recurrence of AGWs | 12 weeks after end of treatment | 5/13 | 4/8 | Not reported | |
| AEs | |||||
| AEs that interrupted treatment | 6 weeks | 2a/32 | 1a/19 | Not reported | |
| Transient side effects (trivial irritation and mild erythema) | 6 weeks | 21/32 | 15/19 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmentb | Comments | ||
| Random sequence generation | ✓ | ‘Patients were allocated treatment by means of a computer generated randomisation code’ (p. 263) | |||
| Allocation concealment | ? | Method of allocation concealment not described | |||
| Selective reporting | ? | Insufficient information available to evaluate whether all prespecified outcomes have been reported | |||
| ‘Other bias’ | ? | Insufficient information available to determine potential additional sources of bias | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ✗ | Described as an open comparison. Masking people to self-applied treatment three times per week vs. clinician-applied treatment once weekly could prove difficult | ||
| Blinding of outcomes assessment | ✗ | Assessing clinician not masked to treatment. Given the subjective nature of the assessment of AGW clearance, outcome measurement is likely to be influenced by lack of masking | |||
| Incomplete outcome data | ? | Number of people lost to follow-up from each group is reported but information on reasons for loss to follow-up is not available. A similar proportion of people is lost from each treatment group. Efficacy analysis not based on the ITT population | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ✗ | Described as an open comparison. Masking people to self-applied treatment three times per week vs. clinician-applied treatment once weekly could prove difficult | ||
| Blinding of outcomes assessment | ✗ | Assessing clinician not masked to treatment. Given the subjective nature of the assessment of AGW reappearance, outcome measurement is likely to be influenced by lack of masking | |||
| Incomplete outcome data | ✗ | Number of people lost to follow-up from each group is reported but information on reasons for loss to follow-up is not available. A large proportion of people did not return for assessment, with differences between groups in the proportion of people returning for reassessment. Analysis of recurrence is not based on all people with complete clearance of AGW | |||
| AEs | Blinding (participants and personnel) | ✗ | Described as an open comparison. Masking people to self-applied treatment three times per week vs. clinician-applied treatment once weekly could prove difficult | ||
| Blinding of outcomes assessment | ✗ | Assessing clinician not masked to treatment. Assessment of extent of irritation and other AEs is likely to be influenced by lack of masking | |||
| Incomplete outcome data | ? | Number of people available for analysis of safety is not reported | |||
| Overall rating of bias | ✗ | Reflects open-label nature of trial and incomplete outcome data for recurrence | |||
| Section 6: Additional comments | |||||
| Additional comments | Recurrence of AGWs: the authors comment that reattendance at 3 months’ follow-up was unsatisfactory and the default rate was too high to allow comparison | ||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Victoria Wakefield and Sam Barton | |||||
| Study ID | Edwards 1998. Related publication: Ferenczy248 | |||||
| Study details | Arch Dermatol 1998;134:25–30 | |||||
| Language of publication | English | |||||
| Type of report | Full paper | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Eleven ambulatory offices, including both private physician offices and referral medical centres | |||||
| Trial sponsor | Not reported | |||||
| Conflicts of interest | Not reported; study personnel included staff from 3M Pharmaceuticals, which is the developer of imiquimod | |||||
| Patient enrolment | Participants were recruited from the practices of investigators, referring physicians and advertisements | |||||
| Trial design | RCT; three-arm trial evaluating two doses of imiquimod cream (5% and 1%) vs. a placebo cream | |||||
| Trial duration | Treatment period of up to 16 weeks followed by a treatment-free follow-up period of up to 12 weeks for those with complete clearance of their AGWs during the treatment period | |||||
| Line of therapy | Unclear | |||||
| Inclusion criteria | Healthy men and women aged ≥ 18 years and with a diagnosis of AGWs. People were enrolled only when deemed to be healthy based on medical history, physical examination and laboratory testing, which included complete blood cell count; serum screening multiphasic chemistry panel; serum pregnancy test (women); and determination of HIV status. People had a minimum of two and a maximum of 50 external AGWs, with a total wart area no less than 10 mm2 | |||||
| Exclusion criteria | People were excluded if they were immunosuppressed by virtue of disease or use of medication; had current chemical or alcohol dependency; had treated their AGW within 4 weeks of enrolment (skin must have returned to normal after any previous therapy); had skin disease in the area to be treated, including frequently recurrent herpes simplex virus infection; or were using any local medications for any purpose, including topical corticosteroids, in the target area during the 2 weeks before enrolment. Women were excluded if they were found to have high-grade squamous intraepithelial lesions (greater than moderate dysplasia) after a Papanicolaou smear; were pregnant or lactating; or were not using contraception | |||||
| All outcomes reported in paper | Complete clearance; recurrence; proportion with ≥ 50% reduction in area of baseline AGWs; appearance of new AGWs during treatment; time to complete clearance of AGWs; AEs | |||||
| Subgroups evaluated | By gender (men vs. women) | |||||
| Stratification | Not reported | |||||
| Baseline measurement of disease | People underwent a skin biopsy test at baseline that was ‘interpreted as diagnostic or suggestive of AGWs and without evidence of dysplasia’ (p. 26). AGWs were photographed, measured and mapped at the initiation visit and subsequently every 2 weeks during the treatment and follow-up periods. AGW size was expressed as total area in square metres and determined by the product of the two longest perpendicular dimensions | |||||
| Treatment | Imiquimod 5% | Imiquimod 1% | Placebo (vehicle cream) | |||
| Randomised, n | 109 | 102 | 100 | |||
| Withdrawals, n (%) | 19 (17) | 31 (30) | 27 (27) | |||
| Discontinuation because of an AE or lack of therapeutic effect | 6 (6) | 8 (8) | 8 (8) | |||
| Withdrawal because of non-compliance, personal reasons or unavailability for follow-up | 13 (12) | 23 (23) | 19 (19) | |||
| Treatment regimen | Participants were instructed carefully in the use of the test medication and were asked to maintain a diary to record dosing and to ensure compliance. After cleaning and drying the area, the allocated treatment was applied to all external lesions in an amount that could be rubbed in until the cream disappeared. Participants were told to allow the cream to dry before dressing and to leave the medication on during their normal sleeping time. The cream was washed off with soap and water after an application time of 6–10 hours. Treatment was to be applied three times each week until all baseline AGWs were confirmed to have disappeared or for 16 weeks, whichever occurred first. Medication was to be applied every other day for three doses per week with individual applications separated by no less than 36 hours and no more than 96 hours. After the third dose, there was a 2-day pause (60–120 hours) before the next week’s dosing. No other topical preparations of any kind were allowed during the treatment period. At any time during the treatment phase, if AGWs were no longer visible, treatment was stopped and the person entered the follow-up phase of the study. Participants were seen weekly for the first 2 weeks, after which they were seen biweekly until their AGWs cleared or for the remainder of the 16-week treatment period | |||||
| Duration/number of administered treatment | Not reported | |||||
| Baseline patient characteristics | Imiquimod 5% | Imiquimod 1% | Placebo (vehicle cream) | p-value | ||
| Age (years), mean (SD) | 32 (12) | 30 (10) | 31 (10) | p > 0.50 | ||
| Duration of disease (months), median (range) | 4.2 (0.4–375) | 6.6 (0–182) | 5.8 (0–270) | p = 0.23 | ||
| Male | 6.7 (0.4–375) | 26.4 (0–182) | 7.9 (0.7–270) | p = 0.01 | ||
| Female | 3.4 (0.7–168) | 3.1 (0.2–90) | 4.4 (0–220) | p > 0.50 | ||
| Site of AGWs, n (%) | Not reported | |||||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, mean (with SD/SE if given) | Not reported | |||||
| Area of AGWs (mm2), median (range) | 69 (8–5525) | 74 (10–4271) | 77 (5–5000) | p > 0.50 | ||
| Male | 92 (8–5525) | 75 (10–2184) | 87 (10–5000) | p > 0.50 | ||
| Female | 58 (15–2294) | 58 (10–4271) | 71 (7–1468) | p > 0.50 | ||
| Sex, n (%) | ||||||
| Male | 63 (58) | 57 (56) | 60 (60) | p > 0.50 | ||
| Female | 46 (42) | 45 (44) | 40 (40) | p > 0.50 | ||
| Any previous treatment, n (%) | Not reported | |||||
| Ethnicity (%) | ||||||
| White | 85 | 81 | 83 | p > 0.50 | ||
| Black | 13 | 17 | 17 | |||
| Asian/Pacific Islander | 2 | 2 | 0 | |||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Complete clearance of baseline AGWs at any point during the 16-week treatment period | |||||
| Recurrence of AGWs | Recorded during the 12-week treatment-free follow-up period. Reappearance of one or more baseline AGWs in people with complete clearance of AGWs during the treatment period | |||||
| Time to complete clearance | Taken from Ferenczy;248 time to complete clearance of AGWs | |||||
| Volume of wart clearance | Proportion of patients with ≥ 50% reduction in area of baseline AGWs | |||||
| Appearance of new warts during treatment | New AGWs appearing during the treatment period. New AGWs could be treated with the study treatment but were tracked separately and were not evaluated in the clearance of baseline AGWs | |||||
| AEs | Local reactions were graded independently by the patient and the investigator using the following scale: none, mild (visible irritation with minimal or no discomfort that did not disrupt daily activity), moderate (caused considerable discomfort but did not disrupt normal activities) or severe (substantially interfered with the patient’s normal daily activities) | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Imiquimod 5%, n/N | Imiquimod 1%, n/N | Placebo (vehicle cream), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | 16 weeks | 54/109 | 21/102 | 11/100 | Imiquimod 5% vs. placebo: p < 0.001; imiquimod 1% vs. placebo: p = 0.08 | |
| Recurrence of AGWs | 12 weeks after end of treatment | 6/45 | 0/18 | 1/10 | Difference reported to be not significant | |
| Time to complete clearance | 16 weeks | Median time to clearance for those in the imiquimod 5% and placebo groups of 10 weeks (combined analysis; reported in Ferenczy248) | Not reported | |||
| Volume of wart clearance (proportion of patients with ≥ 50% reduction in baseline AGW area; includes those with 100% clearance) | 16 weeks | 83/109 | 36/102 | 28/100 | Imiquimod 5% vs. placebo: p < 0.001; imiquimod 1% vs. placebo: p = 0.29 | |
| Appearance of new warts during treatment | 16 weeks | 33/106 | 44/97 | 41a/100 | p = 0.20 | |
| AEsa (assessed by investigator) | ||||||
| Erythema | ||||||
| None | 16 weeks | 35/106 | 72/97 | 72/95 | ||
| Mild | 16 weeks | 29/106 | 21/97 | 20/95 | ||
| Moderate | 16 weeks | 36/106 | 4/97 | 3/95 | ||
| Severe | 16 weeks | 6/106 | 0/97 | 0/95 | ||
| Erosion | ||||||
| None | 16 weeks | 72/106 | 92/97 | 87/95 | ||
| Mild | 16 weeks | 22/106 | 4/97 | 6/95 | ||
| Moderate | 16 weeks | 11/106 | 1/97 | 2/95 | ||
| Severe | 16 weeks | 1/106 | 0/97 | 0/95 | ||
| Excoriation or flaking | ||||||
| None | 16 weeks | 80/106 | 93/97 | 93/95 | ||
| Mild | 16 weeks | 19/106 | 4/97 | 2/95 | ||
| Moderate | 16 weeks | 6/106 | 0/97 | 0/95 | ||
| Severe | 16 weeks | 1/106 | 0/97 | 0/95 | ||
| Oedema | ||||||
| None | 16 weeks | 89/106 | 94/97 | 94/95 | ||
| Mild | 16 weeks | 14/106 | 3/97 | 1/95 | ||
| Moderate | 16 weeks | 2/106 | 0/97 | 0/95 | ||
| Severe | 16 weeks | 1/106 | 0/97 | 0/95 | ||
| Scabbing | ||||||
| None | 16 weeks | 90/106 | 94/97 | 93/95 | ||
| Mild | 16 weeks | 11/106 | 1/97 | 2/95 | ||
| Moderate | 16 weeks | 5/106 | 1/97 | 0/95 | ||
| Severe | 16 weeks | 0/106 | 1/97 | 0/95 | ||
| Induration | ||||||
| None | 16 weeks | 97/106 | 93/97 | 92/95 | ||
| Mild | 16 weeks | 7/106 | 4/97 | 2/95 | ||
| Moderate | 16 weeks | 2/106 | 0/97 | 1/95 | ||
| Severe | 16 weeks | 0/106 | 0/97 | 0/95 | ||
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessmentb | Comments | |||
| Random sequence generation | ? | Described as a randomised study but details on method of randomisation not provided | ||||
| Allocation concealment | ? | Details on method of allocation concealment not provided | ||||
| Selective reporting | ✓ | The study protocol is not available but the report provides data on most expected clinical outcomes | ||||
| ‘Other bias’ | ? | Insufficient detail reported to evaluate risk of other bias | ||||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | |||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing wart clearance is masked to treatment | ||||
| Incomplete outcome data | ✓ | Number of people withdrawing from the trial, with reasons for withdrawal, are reported. Although a larger proportion of people withdrew from the imiquimod 1% (data not reported here) and placebo groups, the number of people withdrawing because of an AE or lack of therapeutic effect is similar for each group. AGW clearance is based on an ITT analysis | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | |||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing wart recurrence is masked to treatment | ||||
| Incomplete outcome data | ✗ | Number of people withdrawing from the trial, with reasons for withdrawal, is reported. Analysis of recurrence is not based on all people with complete clearance at a defined time point, and a larger proportion of people with complete clearance in the imiquimod 5% group was lost to follow-up for recurrence | ||||
| Time to complete clearance | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | |||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing wart clearance is masked to treatment | ||||
| Incomplete outcome data | ? | Results for time to complete clearance reported as a combined analysis for only two of the treatment groups (imiquimod 5% and placebo) | ||||
| Volume of wart clearance (proportion of patients with ≥ 50% reduction in baseline AGW area) | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | |||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing wart clearance is masked to treatment | ||||
| Incomplete outcome data | ✓ | Number of people withdrawing from the trial, with reasons for withdrawal, is reported. Although a larger proportion of people withdrew from the imiquimod 1% (data not reported here) and placebo groups, the number of people withdrawing because of an AE or lack of therapeutic effect is similar for each group. Volume of AGW clearance is based on an ITT analysis | ||||
| Appearance of new warts during treatment | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | |||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing wart clearance is masked to treatment | ||||
| Incomplete outcome data | ? | Number of people withdrawing from the trial, with reasons for withdrawal, is reported. Appearance of new warts during treatment is not based on an ITT analysis, but proportion of people excluded from analysis is low | ||||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blinded but details on who is masked to treatment are unclear | |||
| Blinding of outcomes assessment | ? | The study is described as double blinded but it is unclear whether the person assessing AEs is masked to treatment | ||||
| Incomplete outcome data | ? | Number of people withdrawing from the trial, with reasons for withdrawal, is reported. Reported AEs are not based on an ITT population, but proportion of people excluded from analysis is low | ||||
| Overall rating of bias | ? | |||||
| Section 6: Additional comments | ||||||
| Additional comments | None | |||||
| Item | Details | ||||||
|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||||
| Reviewers’ names | Shannon Amoils and Sam Barton | ||||||
| Study ID | Ferenczy 1995 | ||||||
| Study details | J Gynecol Surg 1995;11:41–50 | ||||||
| Language of publication | English | ||||||
| Type of report | Full publication | ||||||
| Section 2: Study information | |||||||
| Location and number of sites | Study carried out at two sites in Montreal (Sir Mortimer B Davis–Jewish General Hospital and Reddy Memorial Hospital), Canada | ||||||
| Trial sponsor | Not reported | ||||||
| Conflicts of interest | Not declared | ||||||
| Patient enrolment | All patients attending the colposcopy/androscopy clinics of the two locations who were eligible and who gave written consent were enrolled in the study. Enrolment dates not reported | ||||||
| Trial design | RCT in which each patient acted as the internal control (randomisation of ‘side’ to be treated with technique rather than randomisation of people) | ||||||
| Trial duration | Treatment and then follow-up of at least 6 months (maximum 18 months, mean 8 months) after the last treatment received | ||||||
| Line of therapy | Not reported | ||||||
| Inclusion criteria | Presence of vaginal and external anogenital condylomata (diagnosis verified by histology); AGW total linear area of ≥ 2 cm2 | ||||||
| Exclusion criteria | Pregnanc; aged ≤ 18 years | ||||||
| All outcomes reported in paper | Time to complete ablation (minutes); proportion of people with complete response by number of treatments; recurrence; healing; complications | ||||||
| Subgroups evaluated | Men vs. women, but results for clinical outcomes not reported by treatment group | ||||||
| Stratification | None reported | ||||||
| Baseline measurement of disease | Total linear area of AGWs at baseline was measured by recording the length and width of each condyloma and calculating the total area. Clinical and colposcopic impressions were verified histologically. Measuring the volume of AGWs was attempted but abandoned because of the considerable observed variation in height | ||||||
| Treatment | Electrosurgery | Continuous-wave CO2 laser therapy | |||||
| Randomised, na | 282 | 282 | |||||
| Withdrawals, n (%) | 74 (26) | ||||||
| Treatment regimen | Electrosurgery consisted of thin wire electrosurgical excision and fulguration procedures. A square-shaped loop electrode was used for excising larger lesions from the vagina, external anogenital skin and anal canal. A 5-mm ball electrode was used for fulgurating smaller lesions and bleeding sites. Patients with extensive disease (two-thirds of the vagina or external anogenital skin) and those with intra-anal involvement were treated under general anaesthesia at one site, whereas those with less extensive disease were treated under local anaesthesia at the other site | ‘CO2 laser densities ranged from 350 W/cm2 to 1000 W/cm2 of continuous wave’ (p. 42). Patients with extensive disease (two-thirds of the vagina or external anogenital skin) and those with intra-anal involvement were treated under general anaesthesia at one site, whereas those with less extensive disease were treated under local anaesthesia at the other site | |||||
| Duration/number of administered treatment | Mean time to complete treatment: 12 minutes when data controlled for total linear area treated (cm2); mean time to complete treatment of lesions ≥ 1 cm2 (controlled for total areas treated and proportion of lesions excised): 7 (2.5–36) minutes | Mean time to complete treatment: 6 minutes when data controlled for total linear area treated (cm2); mean time to complete treatment of lesions ≥ 1 cm2 (controlled for total areas treated and proportion of lesions excised): 9 (4–31) minutes | |||||
| Baseline patient characteristics | Electrosurgery | Continuous-wave CO2 laser therapy | p-value | ||||
| Age (years), mean | Men 27.5; women 23.6 | ||||||
| Duration of disease | Not reported | ||||||
| Site of AGWs, n (%) | |||||||
| Women | |||||||
| Vagina only | 8 (4) | ||||||
| Vulva only | 89 (47) | ||||||
| Anus only | 16 (9) | ||||||
| All | 75 (40) | ||||||
| Men | |||||||
| Penis only | 46 (49) | ||||||
| Anus only | 26 (28) | ||||||
| Both | 22 (23) | ||||||
| Type of AGWs, n (%) | Not reported | ||||||
| Number of AGWs, mean | Not reported | ||||||
| Area of AGWs (mm2), mean | Not reported | ||||||
| Sex, n (%) | Men 94 (33); women 188 (64) | ||||||
| Any previous treatment, n (%) | Not reported | ||||||
| Ethnicity, n (%) | Not reported | ||||||
| Section 3: Outcomes | |||||||
| Outcome | Definition | ||||||
| AGW clearance | Not defined. Number of men and women achieving ‘complete response’ is reported by number of treatments required to achieve complete response. Evaluation was by physical examination, including colposcopy | ||||||
| Recurrence of AGWs | Reported as proportion of people with recurrence; ‘recurrence’ not further defined | ||||||
| AEs | Pain–discomfort level was assessed using Melzack’s McGill Pain Questionnaire. Participants were instructed to record their discomfort level using numerical headings: 0 = no pain; 1 = mild pain; 2 = moderate pain; and 3 = severe pain. Physician’s assessment of pain was based on the level of vocalisation and perineal movements (score range 0–3) and was recorded independently at the third and sixth post-treatment visits. Participants’ and physicians’ scores were averaged and analysed separately. No other AEs were reported by treatment group | ||||||
| Section 4: Data extraction form | |||||||
| Outcome | Time frame | Electrosurgery, n/N | Continuous-wave CO2 laser therapy, n/N | Estimate of effect | p-value | ||
| Dichotomous outcomes | |||||||
| AGW clearanceb | Unclear | Not reported | |||||
| Recurrence of AGWsc | Unclear | Not reported | |||||
| AEs | |||||||
| Pain: physician assessment | Unclear | ||||||
| Mild | 106/208 | 114/208 | Differences between groups reported to be non-significant | ||||
| Moderate | 83/208 | 79/208 | |||||
| Severe | 19/208 | 15/208 | |||||
| Pain: participant assessment | Unclear | ||||||
| Mild | 101/208 | 110/208 | Differences between groups reported to be non-significant | ||||
| Moderate | 84/208 | 76/208 | |||||
| Severe | 23/208 | 22/208 | |||||
| Section 5: Clinical trial quality | |||||||
| Outcome | Risk of bias | Risk assessment d | Comments | ||||
| Random sequence generation | ✓ | It is stated that people were randomised by computer-generated numbers as to which side was treated with electrosurgery or CO2 laser therapy. The same randomisation schedule was applied to intra-anal condylomata | |||||
| Allocation concealment | ? | Details on method used to conceal allocation not available | |||||
| Selective reporting | ✗ | Data on effectiveness for key clinical outcomes are not reported as absolute events rates by treatment group. Results presented cannot be used in a meta-analysis | |||||
| ‘Other bias’ | ? | Insufficient information reported to determine presence of additional sources of bias | |||||
| AGW clearance | Blinding (participants and personnel) | ✗ | Participants and personnel are not masked to treatment. Given that the techniques evaluated are surgical and the participant undergoes both techniques, it might not be feasible to mask key study personnel and participants to treatment. AGW clearance is a subjective outcome and is liable to bias by lack of masking | ||||
| Blinding of outcomes assessment | ✓ | It is reported that ‘the assessors were blinded at follow-up to the method of treatment used in a specific area in each patient. Each patient was assessed by a physician who was not directly involved in the treatment of that patient’ (p. 43). Given that the participant is treated with both electrosurgery and CO2 laser therapy, it is unlikely that masking of the outcome assessor would be broken | |||||
| Incomplete outcome data | ? | Number of participants lost to follow-up is reported. However, it is unclear whether any participants withdrew from treatment but continued to be followed up. Reasons for loss to follow-up are not reported | |||||
| Recurrence of AGWs | Blinding (participants and personnel) | ✗ | Participants and personnel are not masked to treatment. Given that the techniques evaluated are surgical and the participant undergoes both techniques, it might not be feasible to mask key study personnel and participants to treatment. AGW recurrence is a subjective outcome and is liable to bias by lack of masking | ||||
| Blinding of outcomes assessment | ✓ | It is reported that ‘the assessors were blinded at follow-up to the method of treatment used in a specific area in each patient. Each patient was assessed by a physician who was not directly involved in the treatment of that patient’ (p. 43). Given that the participant is treated with both electrosurgery and CO2 laser therapy, it is unlikely that masking of the outcome assessor would be broken | |||||
| Incomplete outcome data | ? | Number of participants lost to follow-up is reported. However, it is unclear whether any participants withdrew from treatment but continued to be followed up. Reasons for loss to follow-up are not reported | |||||
| AEs | Blinding (participants and personnel) | ✗ | Participants and personnel are not masked to treatment. Given that the techniques evaluated are surgical and the participant undergoes both techniques, it might not be feasible to mask key study personnel and participants to treatment. Evaluation of pain is a subjective outcome and is liable to bias by lack of masking | ||||
| Blinding of outcomes assessment | ✓ | It is reported that ‘the assessors were blinded at follow-up to the method of treatment used in a specific area in each patient. Each patient was assessed by a physician who was not directly involved in the treatment of that patient’ (p. 43). Given that the participant is treated with both electrosurgery and CO2 laser therapy, it is unlikely that masking of the outcome assessor would be broken. It is unlikely that a person would be able to discriminate between pain generated from two areas of treatment | |||||
| Incomplete outcome data | ? | Number of people lost to follow-up is reported. However, it is unclear whether any participants withdrew from treatment but continued to be followed up. Reasons for loss to follow-up are not reported | |||||
| Overall rating of bias | ✗ | Reflects lack of masking of key study personnel and participants and limited reporting of key clinical outcomes | |||||
| Section 6: Additional comments | |||||||
| Additional comments | None | ||||||
| Item | Details | ||||||
|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||||
| Reviewers’ names | Fatima Salih and Sam Barton | ||||||
| Study ID | Fife 2001 | ||||||
| Study details | Sex Transm Dis 2001;28:226–31 | ||||||
| Language of publication | English | ||||||
| Type of report | Full publication | ||||||
| Section 2: Study information | |||||||
| Location and number of sites | Nine clinical sites in the USA and Canada | ||||||
| Trial sponsor | 3M Pharmaceuticals | ||||||
| Conflicts of interest | None reported | ||||||
| Patient enrolment | Details on how people were recruited at the clinical sites not available | ||||||
| Trial design | RCT | ||||||
| Trial duration | 16-week treatment period followed by a 4-week observation period for people whose lesions had not cleared by week 16 | ||||||
| Line of therapy | Mixed population; 70% of men enrolled had received previous treatment for AGWs | ||||||
| Inclusion criteria | Male gender; healthy except for AGWs; aged ≥ 18 years; presence of 2–50 AGWs confirmed by biopsy; total AGW area of 30–2000 mm2 after biopsy. To be eligible for enrolment, participants had to have AGWs that were either previously untreated or, if treated, had not been treated with other methods for at least 4 weeks before study entry | ||||||
| Exclusion criterion | People previously treated with imiquimod were excluded | ||||||
| All outcomes reported in paper | Complete clinical clearance of AGWs at end of treatment; decrease in volume of AGWs; time to complete clearance; appearance of new lesions during treatment; AEs | ||||||
| Subgroups evaluated | Not reported | ||||||
| Stratification | Not reported | ||||||
| Baseline measurement of disease | Biopsy of a single representative AGW was carried out to confirm clinical diagnosis. At baseline, AGWs were enumerated, measured and photographed | ||||||
| Treatment | Imiquimod 5% three times per week | Imiquimod 5% once daily | Imiquimod 5% twice daily | Imiquimod 5% three times a day | |||
| Randomised, n | 26 | 29 | 29 | 26 | |||
| Withdrawals, n (%) | A total of 35 people (31.8%) withdrew from the trial; data for each group not reported separately. It is reported that there was no difference across the treatment groups in the proportion of people failing to complete the study, with the exception of withdrawal because of an AE | ||||||
| AEs, n (%) | 0 | 1 (3) | 0 | 3 (12) | |||
| Lost to follow-up, n (%) | 24 (21.8%) across the four groups; data for each group not reported separately | ||||||
| Treatment regimen | It is stated that patients were given detailed instructions on how to apply the imiquimod 5% cream; further details not available. Men were assigned to apply imiquimod 5% cream three times a week, once daily, twice daily or three times a day for a maximum of 16 weeks or until complete clearance, whichever occurred earlier | ||||||
| Duration/number of administered treatment | Not reported | ||||||
| Baseline patient characteristics | Imiquimod 5% three times per week | Imiquimod 5% once daily | Imiquimod 5% twice daily | Imiquimod 5% three times a day | p-value | ||
| Age (years), mean (range) | 32 (19–53) | 32 (18–50) | 31 (20–47) | 32 (19–67) | Not reported | ||
| Duration of disease (months), median (range) | 25.9 (1.5–182) | 35.6 (1.1–423) | 32.9 (0.8–225) | 21.6 (0.3–117) | Not reported | ||
| Site of AGWs, n (%) | Not reported | ||||||
| Type of AGWs, n (%) | Not reported | ||||||
| Number of AGWs, median (range) | 10 (3–29) | 9 (2–41) | 12 (2–34) | 11(2–39) | Not reported | ||
| Area of AGWs (mm2), median (range) | 83 (31–1830) | 225 (12–1699) | 122 (31–3656) | 88 (31–1886) | Not reported | ||
| Sex (M/F), n (%) | All men | ||||||
| Any previous treatment, n (%) | 19 (73) | 22 (76) | 20 (69) | 16 (62) | Not reported | ||
| Ethnicity: white, n (%) | 25 (96) | 25 (86) | 24 (83) | 22 (85) | Not reported | ||
| Section 3: Outcomes | |||||||
| Outcome | Definition | ||||||
| AGW clearance at completion of treatment | Patients were assessed at the end of weeks 1, 2, 3 and 4 then every other week through to week 16 (maximum duration of treatment) or until they experienced complete clinical clearance of AGWs | ||||||
| AGW clearance at other time points | Complete clearance at 4 weeks after the end of treatment (16 weeks) in people whose lesions had not cleared by week 16 | ||||||
| Time to complete clearance | Median time until complete AGW clearance during the 16-week treatment period or 4-week observation period | ||||||
| Appearance of new warts during treatment | Development of new lesions | ||||||
| AEs | Specific local reactions (erythema, oedema, induration, vesicles, erosion, ulceration, excoriation/flaking and scabbing), application site reactions (pain, burning and itching) and systemic AEs were assessed at each visit. Local skin reactions were clinician assessed using a scale from 0 (none) to 3 (severe). Patient-assessed severity of each local skin reaction was based on three categorisations: mild = reaction with little or no discomfort and no effect on normal activities; moderate = causing considerable discomfort, but not disrupting normal activities; severe = sufficient discomfort to interfere with normal activities | ||||||
| Section 4: Data extraction form | |||||||
| Outcome | Time frame | Imiquimod 5% three times per week, n/N | Imiquimod 5% once daily, n/N | Imiquimod 5% twice daily, n/N | Imiquimod 5% three times a day, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||||
| AGW clearance at completion of treatment | 16 weeks | 9/26 | 8/29 | 7/29 | 7/26 | Difference among groups reported to be not significant | |
| AGW clearance at other time points | 4 weeks after end of treatment | 10/26 | 10/29 | 8/29 | 7/26 | ||
| Appearance of new warts during treatment | 16 weeks | 39 men developed new AGWs during treatment; data not reported separately for each group | |||||
| AEs | |||||||
| Application site reactions | 16 weeks | 15/26 | 18/29 | 21/29 | 20/26 | p = 0.43 | |
| Systemic AEs attributed to interferon | 16 weeks | Reported to be 3.4–26.9% of men in the different treatment groups; data not reported separately for the individual treatment groups | Not reported | ||||
| Continuous outcomes | |||||||
| Time to complete clearance (weeks), median | Up to 20 weeks | 10 (n = 26) | 9 (n = 29) | 7 (n = 29) | 10 (n = 26) | Not reported | |
| Section 5: Clinical trial quality | |||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||||
| Random sequence generation | ✓ | It is stated that ‘Eligible patients were assigned consecutive study numbers according to order of enrolment. A randomisation table assigning each study number to one of the four treatments was generated before study initiation’ (p. 227) | |||||
| Allocation concealment | ? | It is stated that ‘treatment assignments were kept in sealed envelopes until each study number was assigned’ (p. 227). It is unclear whether the envelopes were opaque | |||||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | It is stated that ‘No attempt was made to blind the assigned treatment regimens, but patients were told about the uncertainty of the optimal treatment regimen’ (p. 227). It is unclear whether the statement about masking also applies to clinical personnel or outcome assessors | ||||
| Blinding of outcomes assessment | ? | ||||||
| Incomplete outcome data | ? | Number of people withdrawing from the study is reported, with data by treatment group available for those withdrawing because of an AE. It is unclear whether there is an imbalance across the groups in the proportion of people withdrawing from treatment | |||||
| Time to complete clearance | Blinding (participants and personnel) | ? | It is stated that ‘No attempt was made to blind the assigned treatment regimens, but patients were told about the uncertainty of the optimal treatment regimen’ (p. 227). It is unclear whether the statement about masking also applies to clinical personnel or outcome assessors | ||||
| Blinding of outcomes assessment | ? | ||||||
| Incomplete outcome data | ? | Number of people withdrawing from the study is reported, with data by treatment group available for those withdrawing because of an AE. It is unclear whether there is an imbalance across the groups in the proportion of people withdrawing from treatment | |||||
| Appearance of new warts during treatment | Blinding (participants and personnel) | ? | It is stated that ‘No attempt was made to blind the assigned treatment regimens, but patients were told about the uncertainty of the optimal treatment regimen’ (p. 227). It is unclear whether the statement about masking also applies to clinical personnel or outcome assessors | ||||
| Blinding of outcomes assessment | ? | ||||||
| Incomplete outcome data | ? | Number of people withdrawing from the study is reported, with data by treatment group available for those withdrawing because of an AE. It is unclear whether there is an imbalance across the groups in the proportion of people withdrawing from treatment | |||||
| AEs | Blinding (participants and personnel) | ? | It is stated that ‘No attempt was made to blind the assigned treatment regimens, but patients were told about the uncertainty of the optimal treatment regimen’ (p. 227). It is unclear whether the statement about masking also applies to clinical personnel or outcome assessors | ||||
| Blinding of outcomes assessment | ? | ||||||
| Incomplete outcome data | ? | Number of people withdrawing from the study is reported, with data by treatment group available for those withdrawing because of an AE. It is unclear whether there is an imbalance across the groups in the proportion of people withdrawing from treatment | |||||
| Overall rating of bias | ? | ||||||
| Section 6: Additional comments | |||||||
| Additional comments |
|
||||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Fatima Salih and Sam Barton | ||||
| Study ID | Gabriel 1983 | ||||
| Study details | Br J Vener Dis 1983;9:124–6 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Department of Genital Medicine at St Bartholomew’s Hospital, London, UK | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Men attending the Department of Genital Medicine were considered for entry into the trial | ||||
| Trial design | RCT | ||||
| Trial duration | Treatment was administered over 6 weeks. Follow-up occurred for a minimum period of 3 months from the beginning of treatment | ||||
| Line of therapy | Unclear | ||||
| Inclusion criteria | Male gender and presence of AGWs | ||||
| Exclusion criterion | Receipt of treatment for AGWs in the 3 months before the initial visit | ||||
| All outcomes reported in paper | Complete clearance of AGWs; recurrence; AEs | ||||
| Subgroups evaluated | Not reported | ||||
| Stratification | Not reported | ||||
| Baseline measurement of disease | Not reported | ||||
| Treatment | TCAA 50% plus podophyllin 25% | Podophyllin 25% | |||
| Randomised, n | 35 | 38 | |||
| Withdrawals, n (%) | 4 (11.4); reasons for withdrawal not reported | 9 (23.7); reasons for withdrawal not reported | |||
| Treatment regimen | Men were treated weekly for up to 6 weeks with TCAA 50% plus podophyllin 25% in industrial methylated spirits saturated with a brown inert dye | Men were treated weekly for up to 6 weeks with podophyllin 25% in industrial methylated spirits | |||
| In both groups, treatment was carried out by one clinician. Treatment was applied with an orange stick and care was taken to ensure that the application was strictly limited to AGWs and that surrounding skin was avoided. Each application was allowed to air dry for 5 minutes out of sight of the treating clinician, who also assessed clearance of AGWs. If AGWs persisted after 6 weeks, treatment was changed to TCAA 100% or cryocautery on a non-trial basis | |||||
| Duration/number of administered treatment | Number of treatments to clear AGWs at 6 weeks, mean (SD): 2.9 (1.1) | Number of treatments to clear AGWs at 6 weeks, mean (SD): 4.0 (1.6) | |||
| Difference between groups in mean number of treatments required to clear AGWs is statistically significant (0.02 > p > 0.01) | |||||
| Baseline patient characteristics | TCAA 50% plus podophyllin 25% | Podophyllin 25% | p-value | ||
| Age (years), mean (with SD/SE if given) | Not reported | ||||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | Not reported | ||||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean (with SD/SE if given) | Not reported | ||||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | All men | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | AGW clearance at completion of treatment (6 weeks) | ||||
| Recurrence of AGWs | Recurrence is not defined within the study. Data are reported for the proportion of men who ‘remained clear’ at 3 months after the initiation of treatment. The number of men not remaining clear has been assumed to be the number of men who experience recurrence of AGWs | ||||
| AEs | Not defined | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | TCAA 50% plus podophyllin 25%, n/N | Podophyllin 25%, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 6 weeks | 21/35 | 20/38 | p > 0.5 | |
| Recurrence of AGW | 3 months | 11/21 | 11/20 | p > 0.5 | |
| AEs | |||||
| Superficial ulceration at site of treatment | 6 weeks | 3/35 | 0/38 | Not reported | |
| Excessive soreness a day or two after treatment | 6 weeks | 2/35 | 0/38 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that ‘patients were allocated by means of a random numbers table’ (p. 124), but additional information on method of randomisation is not available | |||
| Allocation concealment | ✓ | Both treatment solutions were prepared by one pharmacy and dispensed in stock 10-ml bottles labelled A and B. The pharmacy alone held the code key, which was not revealed until the end of the trial | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ✓ | It is reported that the study was double blind. The key difficulty encountered with maintaining masking in this study was described as the characteristic white appearance of AGWs after treatment with TCAA. In the study, the pharmacy preparing the solutions added an inert brown dye to the TCAA plus podophyllin solution, which masked the white appearance of AGWs after treatment with TCAA and made the solution the same colour as podophyllin solution | ||
| Blinding of outcomes assessment | ✓ | The treating clinician also assessed clinical outcomes. The treating clinician was masked to treatment allocation | |||
| Incomplete outcome data | ? | The number of people withdrawing from each group is reported, but reasons for withdrawal are not provided. There is an imbalance in withdrawal between the groups (11.4% with TCAA plus podophyllin vs. 23.4% with podophyllin). As reasons for withdrawal are not reported, the potential impact of the imbalance on the relative treatment effect is unclear | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ✓ | It is reported that the study was double blind. The key difficulty encountered with maintaining masking in this study was described as the characteristic white appearance of AGWs after treatment with TCAA. In the study, the pharmacy preparing the solutions added an inert brown dye to the TCAA plus podophyllin solution, which masked the white appearance of AGWs after treatment with TCAA and made the solution the same colour as podophyllin solution | ||
| Blinding of outcomes assessment | ✓ | The treating clinician also assessed clinical outcomes. The treating clinician was masked to treatment allocation | |||
| Incomplete outcome data | ✓ | Analysis of recurrence is based on all people with complete clearance at the end of treatment | |||
| AEs | Blinding (participants and personnel) | ✓ | It is reported that the study was double blind. The key difficulty encountered with maintaining masking in this study was described as the characteristic white appearance of AGWs after treatment with TCAA. In the study, the pharmacy preparing the solutions added an inert brown dye to the TCAA plus podophyllin solution, which masked the white appearance of AGWs after treatment with TCAA and made the solution the same colour as podophyllin solution | ||
| Blinding of outcomes assessment | ✓ | The treating clinician also assessed clinical outcomes. The treating clinician was masked to treatment allocation | |||
| Incomplete outcome data | ? | The number of people withdrawing from each group is reported, but reasons for withdrawal are not provided. There is an imbalance in withdrawal between the groups (11.4% with TCAA plus podophyllin vs. 23.4% with podophyllin). As reasons for withdrawal are not reported, the potential impact of the imbalance on AEs is unclear | |||
| Overall rating of bias | ? | ||||
| Section 6: Additional comments | |||||
| Additional comments | Baseline characteristics were not reported in the full publication but it is stated that ‘the two groups of patients were statistically comparable in age range, country of origin, sexual preference, and sites of warts present’ (p. 125) | ||||
| Item | Details | ||||||
|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||||
| Reviewers’ names | Victoria Wakefield and Sam Barton | ||||||
| Study ID | Garland 2006 | ||||||
| Study details | Int J STD AIDS 2006;17:448–52 | ||||||
| Language of publication | English | ||||||
| Type of report | Full publication | ||||||
| Section 2: Study information | |||||||
| Location and number of sites | Study carried out at nine public sexual health clinics in Australia | ||||||
| Trial sponsor | Not reported | ||||||
| Conflicts of interest | Not reported | ||||||
| Patient enrolment | Only women enrolled; no details provided on methods used to identify and recruit women or dates of enrolment | ||||||
| Trial design | Four-arm RCT | ||||||
| Trial duration | Initial treatment period of 4–16 weeks, dependent on treatment allocation. All women were followed up until the end of the study at 16 weeks. Women could stop treatment earlier than the allocated treatment period if AGWs had completely cleared | ||||||
| Line of therapy | Mixed: 88/120 (73.3%) women had received previous treatment for their AGWs (cryotherapy in 73 women and podophyllotoxin in 49 women) | ||||||
| Inclusion criteria | Female gender; aged ≥ 16 years; presence of 1–50 visible external genital and/or perianal AGWs with an area of 10–2000 mm2 | ||||||
| Exclusion criteria | Pregnant or lactating; HIV-positive status; internal vaginal or anal lesions requiring treatment; presence of psoriasis or other skin disease (e.g. herpes) that may confound examination; moderate or severe dysplasia [cervical intraepithelial neoplasia (CIN) 2 or 3]; carcinoma in situ; presence of squamous cell carcinoma; receipt of organ transplant; use of other therapies for AGWs or topical immunomodulators (including imiquimod) in the 4 weeks preceding entry to the study or corticosteroids within 2 weeks of study entry | ||||||
| All outcomes reported in paper | Complete clearance of AGWs at 4, 8, 12 and 16 weeks; AEs | ||||||
| Subgroups evaluated | None reported | ||||||
| Stratification | None reported | ||||||
| Baseline measurement of disease | The size, number and location of AGWs were recorded and AGWs were photographed twice | ||||||
| Treatment | Imiquimod 5% cream for 4 weeks (patient applied) | Imiquimod 5% cream for 8 weeks (patient applied) | Imiquimod 5% cream for 12 weeks (patient applied) | Imiquimod 5% cream for 16 weeks (patient applied) | |||
| Randomised, n | 30 | 31 | 28 | 31 | |||
| Withdrawals, n (%) | 13 (43) | 6 (19) | 8 (29) | 8 (26) | |||
| It is stated that the main reasons for discontinuation were non-compliance and loss to follow-up. Two women withdrew because of the severity of local skin reactions and one woman withdrew because of nausea, fatigue and vagueness. The number of women excluded because of non-compliance and the number lost to follow-up not reported for overall trial population or individual treatment groups | |||||||
| Treatment regimen | Imiquimod 5% cream was applied by women three times a week (every other night followed by 2 days without treatment) for the allocated period of time (4, 8, 12 or 16 weeks) or until complete clearance of AGWs, whichever occurred earlier. No additional treatment was allowed | ||||||
| Duration/number of administered treatment | Not reported | ||||||
| Baseline patient characteristics | Imiquimod 5% cream for 4 weeks (patient applied) | Imiquimod 5% cream for 8 weeks (patient applied) | Imiquimod 5% cream for 12 weeks (patient applied) | Imiquimod 5% cream for 16 weeks (patient applied) | p-value | ||
| Age (years), mean (range) | Overall trial population: 26.5 (17–67); not reported separately by treatment group | Not reported | |||||
| Duration of disease (months), median (range) | 3.2 (0.6–93.3) | 3.3 (0.2–25.0) | 3.8 (0.8–24.3) | 3.1 (0.4–89.7) | p = 0.889 | ||
| Site of AGWs (%) | Overall trial population: 69.2% vulvar; 59.2% labial; 45.8% perianal; 34.2% perineal; not reported separately by treatment group | Not reported | |||||
| Type of AGWs, n (%) | Not reported | Not reported | |||||
| Number of AGWs, median (range) | 12.5 (1–43) | 13.0 (1–43) | 18.0 (3–43) | 12.0 (2–52) | p = 0.336 | ||
| Area of AGWs (mm2), median (range) | 168.5 (10–1706) | 86.0 (18–577) | 153.0 (41–1983) | 109.0 (38–860) | p = 0.403 | ||
| Sex (M/F), n (%) | All women | ||||||
| Any previous treatment, n (%) | 19 (63.3) | 24 (77.4) | 23 (82.1) | 22 (71.0) | p > 0.15 | ||
| Ethnicity, n (%) | Trial population was reported to be 117 (97.5%) white: ethnicity not reported separately by treatment group and no further details reported on ethnicity of remaining 2.5% of study population | Not reported | |||||
| Section 3: Outcomes | |||||||
| Outcome | Definition | ||||||
| AGW clearance at completion of treatment | Complete clearance of AGWs at 16 weeks: additional details not available | ||||||
| AGW clearance at other time points | Complete clearance of AGWs at 4, 8 and 12 weeks; additional details not available. Absolute numbers not reported (results presented graphically in the full publication) | ||||||
| AEs | Assessed by spontaneous reporting of application site reactions (symptoms at treatment site) and other AEs, in addition to local skin reactions (the most common signs at the treatment site) | ||||||
| Section 4: Data extraction form | |||||||
| Outcome | Time frame | Imiquimod 5% cream for 4 weeks (patient applied), n/N | Imiquimod 5% cream for 8 weeks (patient applied), n/N | Imiquimod 5% cream for 12 weeks (patient applied), n/N | Imiquimod 5% cream for 16 weeks (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||||
| AGW clearance at completion of treatment | 16 weeks | 12/30 | 15/31 | 11/28 | 16/31 | p = 0.724 | |
| AGW clearance at completion at other time points | 4, 8 and 12 weeks | Absolute numbers not reported; results presented graphically in the full publication | |||||
| AEs (%) | |||||||
| Pain | 16 weeks | 0 | 19 | 4 | 13 | p = 0.026 | |
| Local skin reactions | 16 weeks | 67 | 87 | 96 | 87 | p = 0.021 | |
| Section 5: Clinical trial quality | |||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||||
| Random sequence generation | ? | It is stated that the study was randomised but details on the method of randomisation are not available | |||||
| Allocation concealment | ? | Information on method of allocation concealment not provided | |||||
| Selective reporting | ✗ | Complete clearance at various time points is presented graphically, with no absolute numbers reported. In addition, results for AEs are not reported separately for each treatment group. Data reported for key outcomes cannot be entered in a meta-analysis | |||||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence by lack of masking | ||||
| Blinding of outcomes assessment | ✗ | Study is described as ‘open label’. Assessment of AGW clearance is likely to be subjective and open to influence by lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | |||||
| Incomplete outcome data | ? | Although number of withdrawals and loss to follow-up are reported for each treatment group, accompanying reasons are not available. In addition, there is an imbalance across the groups in the proportion of women withdrawing or lost to follow-up (19–43%). The effect of this imbalance on estimates of effect is unclear | |||||
| AEs | Blinding (participants and personnel) | ✗ | Study is described as ‘open label’. Assessment of AEs is likely to be subjective and open to influence by lack of masking | ||||
| Blinding of outcomes assessment | ✗ | Study is described as ‘open label’. Assessment of AEs is likely to be subjective and open to influence by lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | |||||
| Incomplete outcome data | ? | Although number of withdrawals and loss to follow-up are reported for each treatment group, accompanying reasons are not available. In addition, there is an imbalance across the groups in the proportion of women withdrawing or lost to follow-up (19–43%). The effect of this imbalance on estimates of effect is unclear | |||||
| Overall rating of bias | ✗ | Reflects open-label nature of trial and limited reporting on key clinical outcomes (complete clearance at various time points and occurrence of AEs) | |||||
| Section 6: Additional comments | |||||||
| Additional comments |
|
||||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Sjokvist Garcia-Stewart and Sam Barton | ||||
| Study ID | Gilson 1999 | ||||
| Study details | AIDS 1999;13:2397–404 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study carried out in the UK (eight sites) and USA (five sites) | ||||
| Trial sponsor | 3M Healthcare Ltd, UK, and 3M Pharmaceuticals, USA | ||||
| Conflicts of interest | None reported | ||||
| Patient enrolment | Information on recruitment of patients and dates of enrolment not available | ||||
| Trial design | RCT | ||||
| Trial duration | Treatment duration of 16 weeks. People experiencing > 80% but < 100% clearance of baseline AGWs continued on blinded treatment for an additional 8 weeks | ||||
| Line of therapy | Mixed; proportion of people receiving previous treatment unclear | ||||
| Inclusion criteria | Aged ≥ 18 years; clinical diagnosis of external AGWs; laboratory-confirmed diagnosis of HIV infection (patients with AIDS were eligible if they had been clinically stable for 4 weeks before enrolment); minimum of two AGWs with an area totalling at least 10 mm2; CD4 T-lymphocyte count of ≥ 100 × 106 cells/l; haemoglobin ≥ 10 g/dl; granulocytes ≥ 1.5 × 109 cells/l; platelet count ≥ 75 × 109 cells/l; total bilirubin ≤ 26 µmol/l; aspartate aminotransferase and alanine transaminase less than three times the upper limit of normal; creatinine ≤ 130 µmol/l; minimum Karnofsky score of 70 | ||||
| Exclusion criteria | Previous treatment with imiquimod; sexual or household partner currently being treated with imiquimod; pregnancy; lactating or < 3 months post partum or post abortion; presence of class 2 or greater vaginal, vulvar or cervical intraepithelial neoplasia; presence of psoriasis or other dermatological disease at the AGW site; experience of more than six outbreaks per year of herpes genitalis; receipt of interferon (IFN), IFN inducers, cytotoxic or investigational drugs, immunomodulators or topical acyclovir; receipt of chemical or surgical AGW therapy in the 4 weeks preceding study initiation | ||||
| All outcomes reported in paper | Safety [primary objective was to evaluate safety of imiquimod (local skin reactions, other AEs, vital signs, laboratory tests (including CD4 T lymphocyte counts) and serum pregnancy tests]; complete clearance; reduction in AGW area; appearance of new AGWs during treatment | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | Gender | ||||
| Baseline measurement of disease | Not reported | ||||
| Treatment | Imiquimod 5% cream (patient applied) | Placebo (vehicle) cream (patient applied) | |||
| Randomised, n | 65 | 35 | |||
| Withdrawals, n (%) | 27 (42) | 20 (57) | |||
| Local skin reaction | 1 (2)a | 1 (3) | |||
| Lack of therapeutic effect | 5 (8) | 8 (23) | |||
| Intercurrent disease | 4 (6) | 0 (0) | |||
| Personal | 6 (9) | 2 (6) | |||
| Non-compliance | 2 (3) | 0 (0) | |||
| Lost to follow-up | 5 (8) | 7 (20) | |||
| Other | 4 (6) | 2 (6) | |||
| Treatment regimen | Imiquimod 5% cream or placebo cream was self-applied for 8 ± 2 hours three times per week (prior to normal sleeping hours) every other day, followed by two consecutive days without treatment. The treatment cycle was repeated until complete AGW clearance was achieved or for a maximum of 16 weeks, whichever occurred earlier. New AGWs that appeared during treatment were eligible for treatment but were analysed separately. Extended blinded treatment for 8 weeks was allowed for those people experiencing > 80% but < 100% clearance of their baseline AGWs. Lamivudine, saquinavir, ritonavir and indinavir were approved during the conduct of the study and were permitted as pre-study or concomitant medication | ||||
| Duration/number of administered treatment | Median amount of imiquimod 5% cream applied during treatment period (16 weeks): 2476 mg (containing 123.8 mg of imiquimod) | Median amount of vehicle cream applied during treatment period (16 weeks): 2450 mg | |||
| Baseline patient characteristics | Imiquimod 5% cream (patient applied) | Placebo (vehicle) cream (patient applied) | p-value | ||
| Age (years), mean (SD) (range) | 35 (6.9) (20–52) | 33 (8.3) (21–58) | p = 0.023 (not considered to be a clinically meaningful difference) | ||
| Duration of disease since onset (months), median (range) | 12.0 (0.3–173.9) | 12.3 (0.5–195.8) | p = 0.715 | ||
| Site of AGWs, n (%) | Not reported | ||||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, median (range) | 5 (1–28) | 5 (1–14) | p = 0.558 | ||
| Area of AGWs (mm2), median (range) | 48.0 (11–3612) | 60.5 (10–2304) | p = 0.299 | ||
| Sex, n (%) | Men: 62 (95); women: 3 (5%) | Men: 35 (100) | p = 0.550 | ||
| Any previous treatment, n (%) | |||||
| Podophyllin | 40 (62) | 16 (46) | Difference between groups reported to be non-significant | ||
| Cryotherapy | 35 (54) | 19 (54) | |||
| Electrocautery | 18 (28) | 5 (14) | |||
| Ethnicity, n (%) | |||||
| White | 59 (91) | 31 (89) | p = 0.737 | ||
| Black | 3 (5) | 3 (9) | |||
| Asian | 3 (5) | 1 (3) | |||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Not defined. Total clearance was reported, based on a maximum of 16 weeks’ treatment | ||||
| Volume of wart clearance (proportion of patients with > 50% clearance in wart area) | Proportion of people experiencing a > 50% reduction in total AGW area | ||||
| Appearance of new warts during treatment | Appearance of AGWs during the treatment period that were not present at the initiation visit | ||||
| AEs | Safety was evaluated through the incidence and severity of local skin reactions: erythema, erosion and ulceration at the AGW site and at remote sites (non-treated areas near the AGW site where study cream may have come into contact with the skin). AEs were graded as mild, moderate or severe (definitions of mild, moderate and severe not available) | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Imiquimod 5% cream (patient applied), n/N | Placebo (vehicle) cream (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 16 weeks | ||||
| ITT | 7/65 | 2/35 | p = 0.488 | ||
| PP | 7/53 | 2/25 | p = 0.710 | ||
| Volume of wart clearance (> 50% reduction in total wart area) | 16 weeks | ||||
| ITT | 25/65 | 5/35 | p = 0.013 | ||
| PP | 25/53 | 5/25 | p = 0.026 | ||
| Appearance of new warts during treatment | 16 weeks | 12/62 | 7/30 | p = 0.784 | |
| AEs | |||||
| Mild skin reaction at AGW site | 16 weeks | ||||
| Erythema | 16/62 | 8/30 | Differences between groups reported to be non-significant | ||
| Erosion | 6/62 | 3/30 | |||
| Ulceration | 4/62 | 1/30 | |||
| Anyb | 16/62 | 11/30 | |||
| Moderate skin reaction at AGW site | 16 weeks | ||||
| Erythema | 9/62 | 0/30 | Differences between groups reported to be non-significant | ||
| Erosion | 3/62 | 0/30 | |||
| Ulceration | 0/62 | 0/30 | |||
| Anyb | 10/62 | 0/30 | |||
| Severe skin reaction at AGW site | 16 weeks | ||||
| Erythema | 1/62 | 0/30 | Differences between groups reported to be non-significant | ||
| Erosion | 1/62 | 0/30 | |||
| Ulceration | 1/62 | 0/30 | |||
| Anyb | 2/62 | 0/30 | |||
| Application site reactionc | 16 weeks | 10/65 | 7/35 | Difference between groups reported to be non-significant | |
| Diarrhoeac | 16 weeks | 12/65 | 2/35 | ||
| Herpes simplexc | 16 weeks | 8/65 | 3/35 | ||
| Number of people reporting at least one event | 16 weeks | 45/65 | 23/35 | ||
| Number of people reporting at least one moderate or severe event | 16 weeks | 34/65 | 23/35 | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmentd | Comments | ||
| Random sequence generation | ? | It is stated that eligible people were randomised to treatment group. Additional details on random number sequence generation not available | |||
| Allocation concealment | ? | Information on method of allocation concealment not available | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | The study is described as double blind but information on who was masked or how masking was achieved is not available | ||
| Blinding of outcomes assessment | ? | Although an independent safety review board, not otherwise involved in the study, reviewed AE data, it is unclear whether the clinician assessing clearance was masked to treatment | |||
| Incomplete outcome data | ? | Number of withdrawals and reasons for withdrawals reported. There is an imbalance between the groups in the number of withdrawals, with a larger proportion of people not available for evaluation in the placebo group. A larger proportion of people withdrew from the placebo group as a result of lack of therapeutic effect and a larger proportion of this group was also lost to follow-up. The greater loss to follow-up observed for the placebo group may also be attributed to the lack of therapeutic effect, but this cannot be stated with certainty. This difference is likely to influence the effect estimate | |||
| Volume of wart clearance | Blinding (participants and personnel) | ? | The study is described as double blind but information on who was masked or how masking was achieved is not available | ||
| Blinding of outcomes assessment | ? | Although an independent safety review board, not otherwise involved in the study, reviewed AE data, it is unclear whether the clinician assessing clearance was masked to treatment | |||
| Incomplete outcome data | ✗ | Number of withdrawals and reasons for withdrawals reported. There is an imbalance between the groups in the number of withdrawals, with a larger proportion of people not available for evaluation in the placebo group. A larger proportion of people withdrew from the placebo group as a result of lack of therapeutic effect and a larger proportion of this group was also lost to follow-up. The greater loss to follow-up observed for the placebo group may also be attributed to the lack of therapeutic effect, but this cannot be stated with certainty. The impact of the imbalance on the effect estimate is unclear | |||
| Appearance of new warts during treatment | Blinding (participants and personnel) | ? | The study is described as double blind but information on who was masked or how masking was achieved is not available | ||
| Blinding of outcomes assessment | ? | Although an independent safety review board, not otherwise involved in the study, reviewed AE data, it is unclear whether the clinician assessing AGW appearance was masked to treatment | |||
| Incomplete outcome data | ✗ | Number of withdrawals and reasons for withdrawals reported. There is an imbalance between the groups in the number of withdrawals, with a larger proportion of people not available for evaluation in the placebo group. A larger proportion of people withdrew from the placebo group as a result of lack of therapeutic effect and a larger proportion of this group was also lost to follow-up. The greater loss to follow-up observed for the placebo group may also be attributed to the lack of therapeutic effect, but this cannot be stated with certainty | |||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blind but information on who was masked or how masking was achieved is not available | ||
| Blinding of outcomes assessment | ✓ | It is stated that an independent safety review board, not otherwise involved in the study, reviewed individual patient data in a blinded manner to assess the relationship of AEs to study drug, underlying HIV disease or associated treatment | |||
| Incomplete outcome data | ✗ | Number of withdrawals and reasons for withdrawals reported. There is an imbalance between the groups in the number of withdrawals, with a larger proportion of people not available for evaluation in the placebo group. A larger proportion of people withdrew from the placebo group as a result of lack of therapeutic effect and a larger proportion of this group was also lost to follow-up. The greater loss to follow-up observed for the placebo group may also be attributed to the lack of therapeutic effect, but this cannot be stated with certainty | |||
| Overall rating of bias | ? | ||||
| Section 6: Additional comments | |||||
| Additional comments | Study was not powered to show statistically significant differences between treatment groups in defined safety and efficacy outcomes | ||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Shannon Amoils and Sam Barton | ||||
| Study ID | Gilson 2009 | ||||
| Study details | Sex Trans Infect 2009;85:514–19 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | United Kingdom, five sexual health clinics: Camden Primary Care Trust, London; Whittall Street Clinic, Birmingham; Royal Victoria Hospital, Belfast; Royal South Hants Hospital, Southampton; and Addenbrooke’s Hospital, Cambridge | ||||
| Trial sponsor | Investigator-led study funded by Stiefel International R&D | ||||
| Conflicts of interest | None declared | ||||
| Patient enrolment | Information on methods used to identify and recruit patients not available. Patients enrolled 2005–6 | ||||
| Trial design | RCT | ||||
| Trial duration | Initial 12-week treatment period. After 12 weeks, treatment was given at the discretion of the clinician. Participants were followed up until 24 weeks after commencement of treatment | ||||
| Line of therapy | Mixed population; 70% of people enrolled were experiencing their first episode of AGW | ||||
| Inclusion criteria | Aged 18–70 years; at least two (maximum 30) external AGWs with a combined area of at least 10 mm2; AGWs could be appropriately treated with cryotherapy with or without podophyllotoxin cream, based on opinion of the investigator; previously untreated AGW or AGWs that had not been treated for at least 4 months | ||||
| Exclusion criteria | AGW treated in the past 4 months before enrolment; known to be HIV positive (HIV testing not required); concurrent internal AGWs; individual AGWs with an area > 4 cm2 | ||||
| All outcomes reported in paper | Primary end points: clearance of all AGWs at 4 and 12 weeks. Secondary endpoints: complete clearance at 24 weeks; recurrence at 12 weeks in those cleared at 4 weeks; recurrence at 24 weeks in those cleared at 12 weeks | ||||
| Subgroups evaluated | It is noted that the subgroup analyses (gender, history of AGWs and type of AGW) are only exploratory and interaction effects would not be investigated | ||||
| Stratification | Randomisation stratified by gender and history of AGWs (blocks of four) | ||||
| Baseline measurement of disease | Baseline evaluation included documentation of number, size (area judged against a calibration template), location and type (hyperkeratotic, non-keratotic, mixed) of AGWs | ||||
| Treatment | Cryotherapy plus podophyllotoxin 0.15% cream | Cryotherapy plus placebo | |||
| Randomised, n | 74 | 75 | |||
| All results and baseline characteristics are based on 140 people (70 people per group). Data were reported for people who received at least one treatment with cryotherapy and who attended at least one follow-up assessment | |||||
| Total withdrawals, n (%)a | 19 (27) | 24 (34) | |||
| Perceived condition cured | 3 (4) | 2 (3) | |||
| Personal reasons | 0 | 2 (3) | |||
| Lost to follow-up | 14 (20) | 16 (23) | |||
| Withdrew consent | 1 (1.4) | 2 (3) | |||
| Other | 1 (1.4) | 2 (3) | |||
| Treatment regimen | Cryotherapy was applied using a liquid nitrogen spray (Cry-Ac; Brymill Cryogenic Systems, Basingsstoke, UK) to obtain a minimum of a 45-s freeze (from start of application), which was repeated after a thaw in a standardised manner. Cryotherapy was repeated weekly for up to 12 weeks. Starting the day after cryotherapy, podophyllotoxin 0.15% cream was applied twice daily for 3 consecutive days and repeated weekly for up to 4 weeks or until all AGWs had cleared, whichever occurred first. If further treatment after week 4 was required, only cryotherapy was used and this was repeated weekly between week 4 and week 12. From weeks 12–24, treatment was at the discretion of the clinician (who remained blinded to the earlier treatment allocation) | Cryotherapy was applied using a liquid nitrogen spray (Cry-Ac; Brymill Cryogenic Systems, Basingsstoke, UK) to obtain a minimum of a 45-s freeze (from start of application), which was repeated after a thaw in a standardised manner. Cryotherapy was repeated weekly for up to 12 weeks. Starting the day after cryotherapy, placebo cream was applied twice daily for 3 consecutive days and repeated weekly for up to 4 weeks or until all AGW had cleared, whichever occurred first. If further treatment was required after week 4, only cryotherapy was used and this was repeated weekly between week 4 and week 12 if required. From weeks 12–24, treatment was at the discretion of the clinician (who remained blinded to the earlier treatment allocation) | |||
| Duration/number of administered treatment | Not reported. Use of podophyllotoxin cream declined over the masked phase of the treatment protocol (first 4 weeks) to 31.1% | Not reported. Use of the placebo cream declined over the masked phase of the treatment protocol (first 4 weeks) to 54.8% | |||
| Baseline patient characteristics | Cryotherapy plus podophyllotoxin 0.15% cream | Cryotherapy plus placebo | p-value | ||
| Note: Baseline characteristics are based on 70 people in each group (those who were randomised, received at least one cryotherapy treatment and attended at least one follow-up assessment) | |||||
| Age (years), median (range) | 26 (18–58) | 24.5 (18–43) | Not reported | ||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | |||||
| Anal only | 7 (10.0) | 4 (5.7) | Not reported | ||
| Non-anal only | 52 (74.3) | 55 (78.6) | Not reported | ||
| Both anal and non-anal | 11 (15.7) | 11 (15.7) | Not reported | ||
| Type of AGWs, n (%) | |||||
| Keratotic | 25 (35.7) | 12 (17.1) | Not reportedb | ||
| Non-keratotic | 29 (41.4) | 38 (54.3) | Not reported | ||
| Mixed | 16 (22.9) | 20 (28.6) | Not reported | ||
| Number of AGWs, median (range) | 7 (2–30) | 6.5 (2–34) | Not reported | ||
| Total area of AGWs (mm2), median (range) | 49.5 (10–400) | 38.0 (12–464) | Not reportedb | ||
| Sex: male, n (%) | 47 (67.1) | 44 (62.9) | Not reported | ||
| Any previous treatment, n (%) | |||||
| First episode | 49 (70.0) | 52 (74.3) | Not reported | ||
| Previous history | 21 (30.0) | 18 (25.7) | Not reported | ||
| Ethnicity, n (%) | |||||
| White | 61 (87.1) | 63 (90.0) | Not reported | ||
| Black | 5 (7.1) | 6 (8.6) | Not reported | ||
| Other | 4 (5.7) | 1 (1.4) | Not reported | ||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Complete clearance of AGWs at weeks 4 (end of podophyllotoxin treatment) and 12 (end of cryotherapy), including AGWs that had been present at baseline and any AGWs that may have appeared, or recurred, during follow-up. Analysis based on last observation carried forward. Also, if patients did not attend but were contactable, self-assessed clearance was recorded. Self-assessment was documented on a proforma returned by the patient to the clinic or by the investigator after a telephone interview | ||||
| AGW clearance at other time points | Complete clearance of AGWs at 24 weeks | ||||
| Recurrence of AGWs | Recurrence or new AGWs at 12 weeks in those with complete clearance at 4 weeks and recurrence or new AGWs at 24 weeks in those with complete clearance at 12 weeks | ||||
| Volume of wart clearance | It is stated that more patients receiving cryotherapy in combination with podophyllotoxin had at least a 50% reduction in AGW area by weeks 4 and 12, but the difference was not statistically significant (no other details reported) | ||||
| AEs | Not defined | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Cryotherapy plus podophyllotoxin 0.15% cream, n/N | Cryotherapy plus placebo, n/N | Estimate of effect | CI and p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 4 weeks | 42/70 | 32/70 | RR 1.31 | 0.95 to 1.81 |
| Unadjusted OR 1.78 | 0.91 to 3.48 | ||||
| Adjusted OR 1.99c | 0.91 to 4.36 | ||||
| 12 weeks | 42/70 | 32/70 | RR 1.31 | 0.95 to 1.81 | |
| Unadjusted OR 1.78 | 0.91 to 3.48 | ||||
| Adjusted OR 1.94c | 0.95 to 3.97 | ||||
| AGW clearance at other time points | 24 weeks | 48/70 | 45/70 | RR 1.07 | 0.84 to 1.35 |
| Unadjusted OR 1.21 | 0.60 to 2.45 | ||||
| Adjusted OR 1.18c | 0.56 to 2.49 | ||||
| Recurrence of AGWs | 12 weeks | 12/42 | 11/32 | p = 0.67 | |
| Recurrence of AGWs | 12–24 weeks | 7/42 | 6/32 | Not reported | |
| AEs | |||||
| Any AE | Unclear | 51/70 | 49/70 | p = 0.85 | |
| Application site events (all) | Unclear | 45/70 | 31/70 | p = 0.027 | |
| Type of application site event: pain | Unclear | 24/70 | 13/70 | p = 0.055 | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessment d | Comments | ||
| Random sequence generation | ? | It is stated that people were ‘randomly assigned in equal numbers to each group’ (p. 515). Additional details on random number sequence generation not available | |||
| Allocation concealment | ✓ | It is stated that ‘treatment packs were supplied prelabelled by the clinical trials unit at the manufacturer (Stiefel). Study numbers and corresponding treatment packs were then allocated in sequence by site investigators, against a local register. Blocks were reallocated between centres if required by the rate of recruitment’ (p. 515) | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | Information on level of masking is not available. For all outcomes, the authors relied on self-report for those people not attending follow-up clinics, which, if masking had not been maintained, could introduce bias in favour of the combination therapy | ||
| Blinding of outcomes assessment | ? | The outcome assessors were masked to treatment groups. However, for patients who could not attend follow-up clinics, self-report from patients was used to determine AGW clearance. As the authors note, self-report is unreliable and may overestimate AGW clearance. At week 4, more people in the combination group self-reported than in the cryotherapy alone group [9 (13%) vs. 4 (6%)]. Self-report was also more frequent in the combination group than in the cryotherapy alone group at 12 weeks (14% vs. 10%). The results of self-report might also be influenced by the person’s assessment of whether they have received active treatment. It was unclear how closely the placebo matches the active treatment in physical appearance. Self-report may be more favourable to the combination group | |||
| Incomplete outcome data | ? | The number of people withdrawing from each group is reported, together with reasons for withdrawal. However, the numbers include people with more than one reason for withdrawal and it is unclear how many people had multiple reasons for withdrawal. In addition, reported analyses are not based on all those randomised. Imputed data are based on last observation carried forward | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Information on level of masking is not available. For all outcomes, the authors rely on self-report for those people not attending follow-up clinics, which, if masking had not been maintained, could introduce bias in favour of the combination therapy | ||
| Blinding of outcomes assessment | ? | The outcomes assessors were masked to treatment groups. However, for patients who could not attend follow-up clinics, self-report from patients was used. As the authors note, self-report is unreliable and may overestimate AGW clearance. At week 4, more people in the combination group self-reported than in the cryotherapy alone group [9 (13%) vs. 4 (6%)]. Self-report was also more frequent in the combination group than in the cryotherapy alone group at 12 weeks (14% vs. 10%). The results of self-report might also be influenced by the person’s assessment of whether they have received active treatment. It was unclear how closely the placebo matches the active treatment in physical appearance. Self-report may be more favourable to the combination group | |||
| Incomplete outcome data | ✓ | Analysis of recurrence is based on all those with complete clearance | |||
| AEs | Blinding (participants and personnel) | ? | Information on level of masking is not available. For all outcomes, the authors rely on self-report for those people not attending follow-up clinics, which, if masking had not been maintained, could introduce bias in favour of the combination therapy | ||
| Blinding of outcomes assessment | ? | The outcome assessors were masked to treatment groups. However, for patients who could not attend follow-up clinics, self-report from patients was used. As the authors note, self-report is unreliable and may overestimate AGW clearance. At week 4, more people in the combination group self-reported than in the cryotherapy alone group [9 (13%) vs. 4 (6%)]. Self-report was also more frequent in the combination group than in the cryotherapy alone group at 12 weeks (14% vs. 10%). The results of self-report might also be influenced by the person’s assessment of whether they have received active treatment. It was unclear how closely the placebo matches the active treatment in physical appearance. Self-report may be more favourable to the combination group | |||
| Incomplete outcome data | ? | Number of people withdrawing from each group is reported, together with reasons for withdrawal. However, the numbers include people with more than one reason for withdrawal and it is unclear how many people had multiple reasons for withdrawal. In addition, reported analyses are not based on all those randomised. Imputed data are based on last observation carried forward | |||
| Overall rating of bias | ? | ||||
| Section 6: Additional comments | |||||
| Additional comments | If patients did not attend for follow-up assessments they were contacted by letter and telephone and encouraged to reattend. If this failed, self-assessed clearance was recorded, together with information on any treatment received outside of the trial. Self-assessment was documented on a proforma returned by the patient to the clinic or by the investigator after a telephone interview Sample size calculation – the sample size for the study was based on an estimate of a 45% response rate in the cryotherapy alone arm and a 70% response in the podophyllotoxin combination arm. Sixty-eight patients in each group would have provided 80% power to detect such a difference, or 85 patients after allowing for a 20% loss to follow-up (170 patients in total) Analysis – in the event of an observation being missing (because of non-attendance), the analysis was based on the last observation carried forward process to impute the status at relevant time points In a multivariate analysis of factors associated with clearance of AGWs, there were associations with history of AGWs and gender |
||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Shannon Amoils and Sam Barton | ||||
| Study ID | Godley 1987 | ||||
| Study details | Genitourin Med 1987;63:390–2 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Single site: St Thomas’ Hospital, London, UK | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Heterosexual men with penile AGWs attending the Department of Genitourinary Medicine at St Thomas’ Hospital from May 1983 to July 1985 were invited to join the study | ||||
| Trial design | RCT | ||||
| Trial duration | Weekly treatment until disappearance of AGWs for up to a maximum of 10 treatments. If complete AGW clearance was achieved, men were followed up for 2 months after the end of treatment | ||||
| Line of therapy | Of 106 men who completed the study, the authors noted that the study interventions were first-line therapy for 61 men (data for individual groups not reported separately) and recurrent therapy for 45 men (data for individual groups not reported separately). Data on previous lines of treatment not available for 24 men who failed to complete the trial | ||||
| Inclusion criteria | Heterosexual men with penile AGWs | ||||
| Exclusion criteria | Men were excluded if they had received treatment for AGWs in the previous 8 weeks; had AGWs at sites other than the penile shaft, prepuce or glans; had intrameatal AGWs | ||||
| All outcomes reported in paper | Complete regression of AGWs; number of treatments until complete regression of AGWs; recurrence within 2 months of treatment; AEs | ||||
| Subgroups evaluated | First episode of AGWs; unclear whether this analysis was prespecified | ||||
| Stratification | Not reported | ||||
| Baseline measurement of disease | Presence and site of AGWs were examined at baseline | ||||
| Treatment | TCAA | Cryotherapy | |||
| Randomised, n | 69 | 61 | |||
| Withdrawals, n (%) | |||||
| Withdrawn from the trial after randomisation because of ‘failure to attend regularly’ | 12 (17.4) | 11 (18.0) | |||
| AEs, n (%) | 0 (0) | 1 (1.6) | |||
| Treatment regimen | TCAA was applied to the AGW with an orange stick followed by starch talc application to protect adjacent skin | Liquid nitrogen was applied to the AGW using a fine nozzle spray gun (Cryak Unit, Alcon Laboratories, Camberley, UK). Each AGW was frozen for 15 seconds, allowed to thaw out and refrozen for 15 seconds, if tolerated. No local anaesthetic or lubricant was used | |||
| Duration/number of administered treatment | Mean (SD) number of treatments to complete resolution of AGWs: 4.0 (2.3) | Mean (SD) number of treatments to complete resolution of AGWs: 3.0 (2.2) | |||
| Baseline patient characteristics | TCAA | Cryotherapy | p-value | ||
| Note: Baseline characteristics were recorded for only the 106 (57 in the TCAA group and 49 in the cryotherapy group) men of the 130 randomised who completed the study | |||||
| Age (years), median (range) | 24 (18–64) | 24 (19–44) | Difference reported to be non-significant | ||
| Duration of disease (weeks), median (range) | 6 (1–300) | 5 (1–125) | Difference reported to be non-significant | ||
| Site of AGWs, n (%) | |||||
| Prepuce | 35 (61) | 26 (53) | Differences reported to be non-significant | ||
| Glans | 1 (2) | 0 (0) | |||
| Shaft | 14 (25) | 9 (18) | |||
| Prepuce and glans | 3 (5) | 10 (20) | |||
| Prepuce and shaft | 4 (7) | 3 (6) | |||
| Shaft and glans | 0 (0) | 1 (2) | |||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, median (range) | 6 (1–32) | 6 (1–60) | Difference reported to be non-significant | ||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | All men | ||||
| Any previous treatment, n (%) | 23 (40%) had previous history of AGWs, but previous treatment received not reported | 22 (45%) had previous history of AGWs, but previous treatment received not reported | Difference reported to be non-significant | ||
| Ethnicity, n (%) | |||||
| White | 51 (89) | 40 (82) | Differences reported to be non-significant | ||
| Black | 5 (9) | 8 (16) | |||
| Asian | 1 (2) | 1 (2) | |||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Defined as complete resolution of warts at end of treatment (up to 10 treatments) | ||||
| Recurrence of AGWs | For those with complete clearance during treatment, presence of AGWs at 2 months after the end of treatment | ||||
| AEs | AEs reported were local discomfort, ulceration and scab formation | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | TCAA, n/N | Cryotherapy, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | Up to 10 weeks | 46/57 | 43/49 | Difference reported to be non-significant | |
| Recurrence of AGWs | 2 months after the end of treatment | 14/39 | 15/38 | Not reported | |
| AEs | |||||
| Mild | 3/57 | 9/49 | Not reported | ||
| Moderate (ulceration/scabbing) | 26/57 | 10/49 | Not reported | ||
| Severe (withdrawn from trial) | 0/57 | 1/49 | Not reported | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that people were ‘randomly allocated to treatment with either TCAA or cryotherapy’ (p. 390). Additional details on method of randomisation not available | |||
| Allocation concealment | ? | Detail on method used to conceal allocation not available | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible. It is stated that ‘the patients were reviewed at weekly intervals by an independent observer who had no knowledge of the treatment given’ (p. 390). It is unclear whether masking could have been broken (e.g. participants might have inadvertently revealed treatment allocated) | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | The number of people not included in the analysis is reported and a similar proportion of people was withdrawn from each group. Reason for withdrawal given as failure to attend regularly. Unclear how many people were lost to follow-up rather than withdrew from treatment and whether there is an imbalance between the groups with regard to irregular attendance | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible. It is stated that ‘the patients were reviewed at weekly intervals by an independent observer who had no knowledge of the treatment given’ (p. 390). It is unclear whether masking could have been broken (e.g. participants might have inadvertently revealed treatment allocated) | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ✓ | All people with complete clearance were evaluated for recurrence | |||
| AEs | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible. It is stated that ‘the patients were reviewed at weekly intervals by an independent observer who had no knowledge of the treatment given’ (p. 390). It is unclear whether masking could have been broken (e.g. participants might have inadvertently revealed treatment allocated) | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | The number of people not included in the analysis is reported and a similar proportion of people was withdrawn from each group. Reason for withdrawal given as failure to attend regularly. Unclear how many people were lost to follow-up rather than withdrew from treatment and whether there is an imbalance between the groups with regard to irregular attendance | |||
| Overall rating of bias | ? | ||||
| Section 6: Additional comments | |||||
| Additional comments | Baseline characteristics for men with a first episode of AGWs are reported separately | ||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | |||||
| Study ID | Goh 1998 | |||||
| Study details | Singapore Med J 1998;39:17–19 | |||||
| Language of publication | English | |||||
| Type of report | Full publication | |||||
| Section 2: Study information | ||||||
| Location and number of sites | One site (clinic situated within the Department of STD Control) in Singapore | |||||
| Trial sponsor | Not reported | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | All consecutive male patients presenting with penile AGWs to the Department of STD Control clinic were eligible for entry into the trial; dates of enrolment not reported | |||||
| Trial design | RCT (three arm) | |||||
| Trial duration | Treatment groups were reviewed weekly for up to 6 weeks | |||||
| Line of therapy | 15% of men had a past history of penile AGWs | |||||
| Inclusion criteria | Male gender; age > 16 years; presence of penile AGWs | |||||
| Exclusion criteria | More than five penile AGWs; receipt of treatment in the 2 weeks before trial entry; keratinised penile AGWs; HIV-positive status or immunocompromised | |||||
| All outcomes reported in paper | Complete clearance; AEs | |||||
| Subgroups evaluated | None | |||||
| Stratification | None | |||||
| Baseline measurement of disease | On the first visit, demographic data were collected and the number and size of AGWs were carefully described and recorded | |||||
| Treatment | Podophyllin 25% solution (in tincture benzoin) (clinician applied) | Podophyllin 0.5% in ethanol (patient applied) | Podophyllin 0.25% in ethanol (patient applied) | |||
| Randomised, n | It is stated that 45 men were recruited into the study. The number of men randomised into each treatment group was not reported. Of the 35 men for whom data were available, it is stated that 11 men were treated with podophyllin 0.5%, six with podophyllin 0.25% and 18 with podophyllin 25% | |||||
| Withdrawals | Overall, 10/45 (22%) men were lost to follow-up. Withdrawals and loss to follow-up were not reported separately by treatment group | |||||
| Treatment regimen | Podophyllin 25% solution in tincture benzoin was applied by a doctor or nurse at the STD clinic twice weekly. The solution was washed off 4 hours after application | Podophyllin 0.5% or 0.25% in ethanol was applied by the patient at home twice a day for 3 consecutive days. If AGWs persisted, patients were instructed to repeat the application 1 week later | ||||
| Duration/number of administered treatment | Not reported | |||||
| Baseline patient characteristics | Podophyllin 25% solution (in tincture benzoin) (clinician applied) | Podophyllin 0.5% in ethanol (patient applied) | Podophyllin 0.25% in ethanol (patient applied) | p-value | ||
| Age (years), mean (with SD/SE if given) | The age range of men in the study was 20–71 years. It is stated that most men were aged from 20 to 39 years (93.3% in the podophyllin 25% group and 100% in the podophyllin 0.5% and 0.25% groups). Age range not reported separately for the individual treatment groups. Mean or median age not reported | It is stated that ‘there were no significant differences between treatment groups in terms of age or racial composition’ (p. 18) | ||||
| Duration of disease | It is reported that 68% of men had AGWs of < 1 months’ duration. The duration of disease was not reported separately for the individual treatment groups | Not reported | ||||
| Site of AGWs, n (%) | Sites of AGWs in the groups receiving podophyllin 0.5% and 0.25% in ethanol were reported to be the corona (43.5%) and the glans (26.1%). The remainder were located on the frenulum, prepuce, meatus and shaft (decreasing order of frequency; proportions not given). Men in the group receiving podophyllin 25% in tincture benzoin had a similar distribution, with the exception that AGWs were found more commonly on the prepuce than the frenulum. The site of AGWs was not reported separately for the individual treatment groups | Not reported | ||||
| Type of AGWs, n (%) | 100% non-keratinised (keratinised AGWs excluded) | |||||
| Number of AGWs, mean (with SD/SE if given) | Not reported | |||||
| Area of AGWs (mm2), mean | Not reported | |||||
| Sex (M/F), n (%) | 100% male | |||||
| Any previous treatment, n (%) | 15% of men had a past history of penile AGWs; previous history not reported separately for the individual treatment groups. Type of previous treatment not reported | Not reported | ||||
| Ethnicity, n (%) | The racial distribution of the trial was Chinese 29/35 (82.9%); Indian 5/35 (14.3%); and other 1/35 (2.9%). Breakdown by treatment group not reported | It is stated that ‘there were no significant differences between treatment groups in terms of age or racial composition’ (p. 18) | ||||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Clearance at 6 weeks; no further details on ‘clearance’ provided | |||||
| AGW clearance at other time points | Clearance at 1 week; no further details on ‘clearance’ provided | |||||
| AEs | Skin irritation | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Podophyllin 25% solution (in tincture benzoin) (clinician applied), n/N | Podophyllin 0.5% in ethanol (patient applied), n/N | Podophyllin 0.25% in ethanol (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | 6 weeks | 15/17 | 9/11 | 6/6 | Reported to be not significant | |
| AGW clearance at other time points | 1 week | 6/18 | 4/11 | 4/6 | Reported to be not significant | |
| AEs: skin irritation | 6 weeks | 7/18 | 8/11 | 0/6 | Reported to be not significant | |
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessment a | Comments | |||
| Random sequence generation | ? | It is stated that men were randomly assigned. Details on method of randomisation not available | ||||
| Allocation concealment | ? | Details not provided | ||||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | ||||
| ‘Other bias’ | ? | Baseline characteristics not reported separately for treatment groups; thus, it is unclear whether the groups are comparable at baseline | ||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | Details on whether the authors made attempts to mask patients and personnel not provided. Given the two different settings in which treatments were administered (clinic vs. home), it might be impractical to mask patients and key personnel to treatment. In addition, it is unclear whether the clinician assessing clearance was masked to treatment allocation. If the assessor was not masked to treatment, assessment of complete clearance could be considered to be a subjective assessment and at risk of bias | |||
| Blinding of outcomes assessment | ? | |||||
| Incomplete outcome data | ? | Overall, 10/45 (22%) men were lost to follow-up. It is unclear how many men were randomised to each group. Withdrawals and loss to follow-up were not reported separately by treatment group and so it is unclear whether there is an imbalance among groups in the proportion of men lost to follow-up or who withdrew | ||||
| AEs | Blinding (participants and personnel) | ? | It might not be feasible to mask treatment between patient-applied and physician-applied treatment. Details on whether the authors attempted to mask patients and personnel are not provided. Also, it is unclear whether the clinician assessing clearance was masked to treatment allocation. If the assessor was not masked to treatment, assessment of AEs could be considered to be a subjective assessment and at risk of bias | |||
| Blinding of outcomes assessment | ? | |||||
| Incomplete outcome data | ? | Overall, 10/45 (22%) men were lost to follow-up. It is unclear how many men were randomised to each group. Withdrawals and loss to follow-up were not reported separately by treatment group and so it is unclear whether there is an imbalance among groups in the proportion of men lost to follow-up or who withdrew | ||||
| Overall rating of bias | ? | Reflects limited details reported in full publication | ||||
| Section 6: Additional comments | ||||||
| Additional comments | None | |||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Orla Ní Ógáin and Sam Barton | ||||
| Study ID | Greenberg 1991 | ||||
| Study details | Obstet Gynecol 1991;77:735–9 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Single-centre study; location not specified, but all authors based in USA | ||||
| Trial sponsor | It is stated that the trial was supported by Oclassen Pharmaceuticals, San Rafael, CA, USA | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Details on patient recruitment and dates of enrolment not available | ||||
| Trial design | RCT | ||||
| Trial duration | Treatment given for a maximum of 4 weeks, with patients followed up until week 10 after the start of treatment | ||||
| Line of therapy | Not reported | ||||
| Inclusion criteria | Women with a clinical diagnosis of exophytic vulvar condyloma; ≤ 25 lesions to be treated; total area of involvement of < 10 cm2 and occupying < 30% of the vulva | ||||
| Exclusion criteria | Women were excluded if they had received treatment for AGWs within 1 month of study entry or might have been immunocompromised. The study does not explicitly state that pregnancy was an exclusion criterion but it is stated that ‘all women were given a serologic test for pregnancy before starting drug therapy, and all were cautioned to use a reliable method of contraception during the 10-week study period’ (p. 736). Concurrent use of topical or systemic medication for condylomata was not permitted during the study | ||||
| All outcomes reported in paper | Treatment response (two analyses, with the first analysis evaluating clearance by patient and the second evaluating clearance based on individual AGWs); recurrence; appearance of new AGWs during treatment; AEs (local and systemic complications) | ||||
| Subgroups evaluated | None | ||||
| Stratification | None | ||||
| Baseline measurement of disease | At the baseline visit, clinicians performed a general health history and physical, concentrating on any known previous genital AGW infections. Sites of clinically overt condylomata were mapped out on a diagram and a total AGW count recorded | ||||
| Treatment | Podophyllotoxin 0.5% (solution and cream formulations; patient applied) | Placebo (patient applied) | |||
| Randomised, n | 48; women were allocated to podophyllotoxin 0.5% solution (n = 24) and podophyllotoxin 0.5% cream (n = 24) | 24; women were allocated to placebo solution and placebo cream; the numbers allocated to the different formulations of placebo were not reported | |||
| Withdrawals, n (%) | Not reported | Three (12.5%) women did not return after their baseline visit. The reasons for withdrawal were not reported, but authors propose that withdrawals are likely to be because of an inadequate response | |||
| Analyses are based on 69 women; they exclude the three women who did not return after their baseline visit | |||||
| Treatment regimen | Patients applied their allocated treatment (either podophyllotoxin 0.5% or placebo) twice daily for 3 days followed by a 4-day rest period. Patients were required to undergo a minimum of two treatment cycles, with up to two more cycles if baseline AGWs were not totally cleared after two treatments. Patients were instructed on the correct method of application, treating only external lesions and avoiding application to adjacent normal skin or areas of skin that were bleeding, inflamed or ulcerated | ||||
| Duration/number of administered treatment | Not specified | Not specified | |||
| Baseline patient characteristics | Podophyllotoxin 0.5% (solution and cream formulations; patient applied) | Placebo (patient applied) | p-value | ||
| Age (years), mean (range) | Not reported | ||||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | Not reported. It is stated that ‘over one-third of the warts in 60% of the patients were located in the perianal area’ (p. 737) | ||||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | 9.29 | 9.62 | p = 0.8485 | ||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | Women 100% | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at other time points | Number/percentage of people completely healed at any time during the study. Investigator clinical assessment was based on a 5-point rating scale: progression of disease; no change; some improvement; marked improvement; and complete cure. Complete clearance was reported separately | ||||
| Recurrence of AGWs | No definition reported | ||||
| Appearance of new warts during treatment | New AGWs were defined as condylomata developing in sites that were anatomically remote from the baseline AGWs | ||||
| AEs | Reported as local and systemic complications. AEs were graded on 4-point scale ranging from none to severe. Local AEs were patient report of pain, burning or itching and clinician assessment of inflammation and erosion. Systemic complications were identified through haematological and biochemical testing (further details on tests not available) and from patient reports | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllotoxin 0.5% (solution and cream formulations; patient applied), n/N | Placebo (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at other time points | Unclear; assumed 10 weeks | 24/48 | 5/21 | Not reported | |
| Analysis by individual AGWs | 332/446 | 37/202 | p < 0.05 | ||
| Recurrence of AGWs | Unclear; assumed 10 weeks | Recurrence not reported by group. Reported that, of people who responded completely, relapse rate was 33% (8/24) | |||
| Appearance of new warts during treatment | Unclear; assumed 10 weeks | 19 people (77 new AGWs)/not reported | 8 people (23 new AGWs)/not reported | ||
| AEs | |||||
| Inflammation | Unclear; assumed 10 weeks | Podophyllotoxin 0.5% solution: 12/24 | Placebo solution: 1/10 | Not reported | |
| Podophyllotoxin 0.5% cream: 10/24 | Placebo cream: 0/11 | ||||
| Erosion | Unclear; assumed 10 weeks | Podophyllotoxin 0.5% solution: 11/24 | Placebo solution: 3/10 | Not reported | |
| Podophyllotoxin 0.5% cream: 8/24 | Placebo cream: 10/11 | ||||
| Pain | Unclear; assumed 10 weeks | Podophyllotoxin 0.5% solution: 15/24 | Placebo solution: 4/10 | Not reported | |
| Podophyllotoxin 0.5% cream: 13/24 | Placebo cream: 10/11 | ||||
| Burning | Unclear; assumed 10 weeks | Podophyllotoxin 0.5% solution: 19/24 | Placebo solution: 7/10 | Not reported | |
| Podophyllotoxin 0.5% cream: 18/24 | Placebo cream: 0/11 | ||||
| Itching | Unclear; assumed 10 weeks | Podophyllotoxin 0.5% solution: 15/24 | Placebo solution: 5/10 | Not reported | |
| Podophyllotoxin 0.5% cream: 17/24 | Placebo cream: 5/11 | ||||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ✓ | It is stated that ‘patients were sequentially assigned by a random code to either an active (solution or cream) or placebo (solution or cream) preparation in an active-to-placebo ratio of 2 : 1’ (p. 736). It is stated that the randomised list was computer generated | |||
| Allocation concealment | ? | Details on allocation concealment not available | |||
| Selective reporting | ✗ | It is stated that complete clearance at various time points was measured, as was recurrence. However, the reported event rates are incompletely reported and cannot be entered in a meta-analysis. In addition, the time frame over which the reported events have been reported is unclear | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at other time points | Blinding (participants and personnel) | ? | It is stated that the study is a double-blinded study and a placebo has been implemented. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | Unclear whether the clinician assessing clinical outcomes was masked to treatment allocation | |||
| Incomplete outcome data | ? | The number of people not included in the analysis is reported. Reasons for withdrawal not reported. Unclear how many people were lost to follow-up rather than withdrew from treatment and whether there is an imbalance between the groups | |||
| Recurrence of AGW | Blinding (participants and personnel) | ? | It is stated that study is a double-blinded study and a placebo has been implemented. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | Unclear whether the clinician assessing clinical outcomes was masked to treatment allocation | |||
| Incomplete outcome data | ✗ | Data not presented separately by treatment group. The number of people not included in the analysis is reported. Reasons for withdrawal not reported. Unclear how many people were lost to follow-up rather than withdrew from treatment and whether there is an imbalance between the groups | |||
| Appearance of new warts during treatment | Blinding (participants and personnel) | ? | It is stated that study is a double-blinded study and a placebo has been implemented. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | Unclear whether the clinician assessing clinical outcomes was masked to treatment allocation | |||
| Incomplete outcome data | ✗ | Data not presented separately by treatment group. The number of people not included in the analysis is reported. Reasons for withdrawal not reported. Unclear how many people were lost to follow-up rather than withdrew from treatment and whether there is an imbalance between the groups | |||
| AEs | Blinding (participants and personnel) | ? | It is stated that study is a double-blinded study and a placebo has been implemented. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | Unclear whether the clinician assessing clinical outcomes was masked to treatment allocation | |||
| Incomplete outcome data | ? | The number of people not included in the analysis is reported. Reasons for withdrawal not reported. Unclear how many people were lost to follow-up rather than withdrew from treatment and whether there is an imbalance between the groups | |||
| Overall rating of bias | ✗ | A key domain has been determined to be at a high risk of bias | |||
| Section 6: Additional comments | |||||
| Additional comments | AGWs developing on internal mucosal surfaces treated with ablative or destructive methods. By contrast, new lesions developing on cutaneous surfaces could be treated with podophyllotoxin, provided that the person was still enrolled in the active phase of the study. Missing data were imputed based on the last observation carried forward method | ||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Orla Ní Ógáin and Sam Barton | ||||
| Study ID | Handley 1992 | ||||
| Study details | Irish J Med Sci 1992;161:56 | ||||
| Language of publication | English | ||||
| Type of report | Conference abstract | ||||
| Section 2: Study information | |||||
| Location and number of sites | Not specified; author affiliation listed as Department of Genitourinary Medicine, Royal Victoria Hospital, Belfast, Northern Ireland | ||||
| Trial sponsor | Not specified | ||||
| Conflicts of interest | Not specified | ||||
| Patient enrolment | Not reported | ||||
| Trial design | RCT | ||||
| Trial duration | Initial treatment period of up to 5 weeks, with follow-up review at 3 months (unclear whether this was final follow-up) | ||||
| Line of therapy | Not specified | ||||
| Inclusion criteria | Men with primary AGWs: no other details provided in conference abstract | ||||
| Exclusion criteria | Not reported | ||||
| All outcomes reported in paper | Complete clearance at 5 weeks and 3 months; recurrence at 3 months in men who were AGW free at 5 weeks; systemic AEs | ||||
| Subgroups evaluated | Men with perianal AGWs. It is stated that ‘multiple warts at diagnosis were associated with an adverse prognosis’ (p. 56); further details not reported in abstract | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Not reported | ||||
| Treatment | Podophyllin 0.5% solution (patient applied) | Podophyllotoxin 0.5% solution (patient applied) | |||
| Randomised, n | Number randomised not reported | ||||
| 29 treated | 28 treated | ||||
| Withdrawals, n (%) | Not reported. Analysis at 3 months included 21 men, which infers that eight men withdrew or were lost to follow-up | Not reported. Analysis at 3 months included 20 men, which infers that eight men withdrew or were lost to follow-up | |||
| Treatment regimen | Men self-applied allocated treatment (podophyllin 0.5% or podophyllotoxin 0.5% solution) twice daily for the same 3 consecutive days per week for 5 weeks | ||||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Podophyllin 0.5% solution (patient applied) | Podophyllotoxin 0.5% solution (patient applied) | p-value | ||
| Age (years), mean | Not reported | ||||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | Not reported | ||||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | Not reported | ||||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | 100% male | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Data reported for number of men ‘clinically wart free’ at 5 weeks (end of treatment). No further details available | ||||
| AGW clearance at other time points | Data reported for number of men ‘clinically wart free’ at 3 months’ follow-up. No further details available | ||||
| Recurrence of AGWs | Data reported for ‘recurrence in patients wart free’. No further details available | ||||
| AEs | Number of events reported for systemic side effects and local irritation (moderate to severe). No further details available | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllin 0.5% solution (patient applied), n/N | Podophyllotoxin 0.5% solution (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 5 weeks | 5/22a | 6/23a | Difference reported to be not significant | |
| AGW clearance at other time points | 3 months | 1/21 | 3/20 | Difference reported to be not significant | |
| Recurrence of AGWs | 3 months | 3/4 | 0/3 | Difference reported to be not significant | |
| AEs | |||||
| Systemic side effects | 0/29 | 0/28 | Not reported | ||
| Local irritation (moderate to severe) | 2/29 | 2/28 | Not reported | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmentb | Comments | ||
| Random sequence generation | ? | Title of abstract describes the study as randomised. Details on method used to generate random sequence not available | |||
| Allocation concealment | ? | Details on method used to conceal allocation not available | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | Title describes study as double blind. Details on masking not available and it is unclear who was masked to treatment | ||
| Blinding of outcomes assessment | ? | Title describes study as double blind. Details on masking not available and it is unclear whether the outcomes assessor was masked to treatment | |||
| Incomplete outcome data | ? | Number of men randomised to each group not reported. Number of withdrawals and loss to follow-up not reported. It is unclear whether there was an imbalance between the groups in the proportion of men withdrawing or lost to follow-up | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Title describes study as double blind. Details on masking not available and it is unclear who was masked to treatment | ||
| Blinding of outcomes assessment | ? | Title describes study as double blind. Details on masking not available and it is unclear whether the outcomes assessor was masked to treatment | |||
| Incomplete outcome data | ? | Number of men randomised to each group not reported. Number of withdrawals and loss to follow-up not reported. It is unclear whether there was an imbalance between the groups in the proportion of men withdrawing or lost to follow-up | |||
| AEs | Blinding (participants and personnel) | ? | Title describes study as double blind. Details on masking not available and it is unclear who was masked to treatment | ||
| Blinding of outcomes assessment | ? | Title describes study as double blind. Details on masking not available and it is unclear whether the outcomes assessor was masked to treatment | |||
| Incomplete outcome data | ? | Number of men randomised to each group not reported. Number of withdrawals and loss to follow-up not reported. It is unclear whether there was an imbalance between the groups in the proportion of men withdrawing or lost to follow-up | |||
| Overall rating of bias | ? | Reflects limited reporting in conference abstract | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reviewers’ names | Shannon Amoils and Sam Barton | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID | Hellberg 1995 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Int J STD AIDS 1995;6:257–61 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Language of publication | English | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of report | Full publication | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 2: Study information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Location and number of sites | Sweden; number of sites not stated | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trial sponsor | Yamanouchi Europe BV | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Conflicts of interest | None declared | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patient enrolment | No details available on how people were recruited or the dates of enrolment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trial design | RCT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trial duration | Maximum duration of treatment time 4 weeks. Women with complete clearance were followed up at 3 months (unclear whether 3 months after the end of treatment or 3 months from initiation of trial) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Line of therapy | Unclear. It is stated that 24 women (40.6%) had a history of AGWs but details of any previous treatments received not available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inclusion criteria | Women with overt AGWs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclusion criteria | Current pregnancy; not using adequate contraception; immunosuppressive disease; vaginal, cervical or rectal AGWs; AGW exceeding 5 mm in size; treatment for AGWs in the previous month | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All outcomes reported in paper | Primary clearance (complete clearance at end of treatment); final clearance (complete clearance at 3 months’ follow-up); reduction in the total number of AGWs after each treatment cycle; recurrence; AEs (special attention paid to local tenderness, burning, pain, erythema, erosion and oedema) graded as slight, moderate or severe | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Subgroups evaluated | Performed a subgroup analysis comparing treatment effects between the groups based on site of AGWs (analysis based on reduction in number of AGWs rather than the proportion of women with complete clearance) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stratification | None reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline measurement of disease | At baseline, size of AGWs was estimated and the location (labiae, vulva or perianal) and number affecting each area were recorded | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Podophyllotoxin 0.5% cream (patient applied) | Podophyllin 20% solution (clinician applied) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Randomised, n | 30 | 30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Withdrawals, n (%) | 2 (6.7%). Women failed to return to one or more check-ups and were considered dropouts. No reasons given for failure to return to check-ups | 3 (10%). Women failed to return to one or more check-ups and were considered dro outs. No reasons given for failure to return to check-ups | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment regimen | Self-treatment with podophyllotoxin 0.5% cream twice daily for 3 consecutive days in weekly intervals, for a maximum of 4 weeks. Women were instructed how to locate individual AGWs with one finger and apply cream with another finger | Weekly application of podophyllin 20% by a health-care professional for a maximum of 4 weeks. Women were instructed to wash off the solution 4 hours after application | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Duration/number of administered treatment | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline patient characteristics | Podophyllotoxin 0.5% cream (patient applied) | Podophyllin 20% solution (clinician applied) | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | 24.5 (17–45) | 24.8 (17–58) | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Duration of disease | Not recorded separately for the different treatment groups. Noted that mean duration of AGWs was 9.3 months overall | Difference between groups reported to be not significant (p-value not reported) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Site of AGWs | Reported as number of AGWs at various sites (not number of women) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vulva | 11 | 16 | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labia | 24 | 17 | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Perianal | 9 | 9 | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of AGWs, n (%) | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Number of AGWs, mean | 9.0 (total 251 AGWs) | 11.3 (total 305 AGWs) | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Area of AGWs (mm2), mean | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex (M/F), n (%) | All women | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Any previous treatment, n (%) | Not recorded separately for the different treatment groups. Noted that 24 women (40.6%) had a history of AGWs | Difference between groups reported to be not significant (p-value not reported) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ethnicity, n (%) | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 3: Outcomes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome | Definition | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at completion of treatment | Referred to as ‘primary clearance’ in the full publication. Defined as complete clearance at a maximum of four cycles of treatment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at other time points | Referred to as ‘final clearance’ in the full publication. Defined as no relapse at 3 months’ follow-up | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recurrence of AGWs | Not defined but can be calculated at 3 months based on AGW clearance at 3 months | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AEs | Focused on occurrence of local tenderness, burning, pain, erythema, erosion and oedema. AEs graded as slight, moderate or severe: slight = symptoms have no impact on the woman’s daily life; moderate = symptoms that have prevented some actions of the woman’s daily life; severe = symptoms that led to absence from work | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 4: Data extraction form | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome | Time frame | Podophyllotoxin 0.5% cream (patient applied), n/N | Podophyllin 20% solution (clinician applied), n/N | Estimate of effect | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dichotomous outcomess | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at completion of treatment | 4 weeks | 23/28 | 16/27 | p < 0.05 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at other time points | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| One treatment cycle | 1 week | 7/28 | 2/27 | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Two treatment cycles | 2 weeks | 18/28 | 12/27 | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Three treatment cycles | 3 weeks | 21/28 | 15/27 | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recurrence of AGWs | 3 months | 3/23 | 3/16 | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AEs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tenderness | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mild | 4 weeks | 6/28 | 11/27 | Difference between groups reported to be not significant for all comparisons | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Moderate | 4 weeks | 11/28 | 9/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severe | 4 weeks | 1/28 | 0/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Burning | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mild | 4 weeks | 8/28 | 9/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Moderate | 4 weeks | 14/28 | 10/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severe | 4 weeks | 0/28 | 0/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pain | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mild | 4 weeks | 7/28 | 7/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Moderate | 4 weeks | 11/28 | 10/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severe | 4 weeks | 0/28 | 0/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Erythema/erosion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mild | 4 weeks | 2/28 | 1/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Moderate | 4 weeks | 5/28 | 0/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severe | 4 weeks | 0/28 | 0/27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 5: Clinical trial quality | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome | Risk of bias | Risk assessment a | Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Random sequence generation | ? | It is stated that ‘The women were randomly allocated’ (p. 258). Information on the method used to generate the random sequence is not available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allocation concealment | ? | Information on method of allocation concealment not provided | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Incomplete outcome data | ✓ | Although the reasons for loss to follow-up are not specified, few people have been lost to follow-up and missing outcome data are balanced between the groups | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recurrence of AGWs | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of recurrence of AGWs is likely to be subjective and open to influence from lack of masking | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of recurrence of AGWs is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Incomplete outcome data | ✓ | Although the reasons for loss to follow-up are not specified, few people have been lost to follow-up and missing outcome data are balanced between the groups | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AEs | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AEs is likely to be subjective and open to influence from lack of masking | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AEs is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Incomplete outcome data | ✓ | Although the reasons for loss to follow-up are not specified, few people have been lost to follow-up and missing outcome data are balanced between the groups | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall rating of bias | ✗ | Reflects open-label nature of trial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 6: Additional comments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional comments | It is noted that 7/60 (11.7%) women had cellular atypias at baseline OutcomePodophyllotoxin 0.5% creamPodophyllin 20% solutionp-valueNumber of AGWsNumber of AGWs at baselineNumber of AGWsNumber of AGWs at baselineMean number of AGWsOne treatment cycle151251144305Two treatment cycles4525184305Three treatment cycles2725166305Four treatment cycles1425178305p < 0.001nNnNMean number of AGWs by locationa,bOne treatment cycle Vulva5111516 Labiae17241317 Perianal7969Two treatment cycles Vulva111816 Labiae724917 Perianal5939Three treatment cycles Vulva211716 Labiae324817 Perianal2929Four treatment cycles Vulva211716 Labiae224717 Perianal1929Primary cure rate by number of AGWs> 8912613< 814161014aSum of denominators is larger than the trial population as some women had AGWs at more than one site.bDifferences between groups stated to be non-significant for vulval and perianal AGWs. Podophyllotoxin 0.5% was found to be significantly more efficient than podophyllin 20% in eliminating labial AGWs (p = 0.03). |
Outcome | Podophyllotoxin 0.5% cream | Podophyllin 20% solution | p-value | Number of AGWs | Number of AGWs at baseline | Number of AGWs | Number of AGWs at baseline | Mean number of AGWs | One treatment cycle | 151 | 251 | 144 | 305 | Two treatment cycles | 45 | 251 | 84 | 305 | Three treatment cycles | 27 | 251 | 66 | 305 | Four treatment cycles | 14 | 251 | 78 | 305 | p < 0.001 | n | N | n | N | Mean number of AGWs by locationa,b | One treatment cycle | Vulva | 5 | 11 | 15 | 16 | Labiae | 17 | 24 | 13 | 17 | Perianal | 7 | 9 | 6 | 9 | Two treatment cycles | Vulva | 1 | 11 | 8 | 16 | Labiae | 7 | 24 | 9 | 17 | Perianal | 5 | 9 | 3 | 9 | Three treatment cycles | Vulva | 2 | 11 | 7 | 16 | Labiae | 3 | 24 | 8 | 17 | Perianal | 2 | 9 | 2 | 9 | Four treatment cycles | Vulva | 2 | 11 | 7 | 16 | Labiae | 2 | 24 | 7 | 17 | Perianal | 1 | 9 | 2 | 9 | Primary cure rate by number of AGWs | > 8 | 9 | 12 | 6 | 13 | < 8 | 14 | 16 | 10 | 14 | aSum of denominators is larger than the trial population as some women had AGWs at more than one site.bDifferences between groups stated to be non-significant for vulval and perianal AGWs. Podophyllotoxin 0.5% was found to be significantly more efficient than podophyllin 20% in eliminating labial AGWs (p = 0.03). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome | Podophyllotoxin 0.5% cream | Podophyllin 20% solution | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Number of AGWs | Number of AGWs at baseline | Number of AGWs | Number of AGWs at baseline | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean number of AGWs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| One treatment cycle | 151 | 251 | 144 | 305 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Two treatment cycles | 45 | 251 | 84 | 305 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Three treatment cycles | 27 | 251 | 66 | 305 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Four treatment cycles | 14 | 251 | 78 | 305 | p < 0.001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n | N | n | N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean number of AGWs by locationa,b | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| One treatment cycle | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vulva | 5 | 11 | 15 | 16 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labiae | 17 | 24 | 13 | 17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Perianal | 7 | 9 | 6 | 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Two treatment cycles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vulva | 1 | 11 | 8 | 16 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labiae | 7 | 24 | 9 | 17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Perianal | 5 | 9 | 3 | 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Three treatment cycles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vulva | 2 | 11 | 7 | 16 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labiae | 3 | 24 | 8 | 17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Perianal | 2 | 9 | 2 | 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Four treatment cycles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vulva | 2 | 11 | 7 | 16 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labiae | 2 | 24 | 7 | 17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Perianal | 1 | 9 | 2 | 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Primary cure rate by number of AGWs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| > 8 | 9 | 12 | 6 | 13 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 8 | 14 | 16 | 10 | 14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aSum of denominators is larger than the trial population as some women had AGWs at more than one site.bDifferences between groups stated to be non-significant for vulval and perianal AGWs. Podophyllotoxin 0.5% was found to be significantly more efficient than podophyllin 20% in eliminating labial AGWs (p = 0.03). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Orla Ní Ógáin and Sam Barton | ||||
| Study ID | Jensen 1985 | ||||
| Study details | Lancet 1985;2:1146–8 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Not specified; it is inferred that the trial was carried out at one site in Denmark | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Patients were enrolled from June 1979 to December 1983. Details on method of recruitment not available | ||||
| Trial design | RCT | ||||
| Trial duration | Initial assessment was 1 week after the final treatment. People were followed up at 3, 6, 9 and 12 months;12 months was the final follow-up | ||||
| Line of therapy | First (first episode) | ||||
| Inclusion criteria | People were included if they were experiencing their first episode of AGWs and had perianal AGWs and the clinical appearance of lesions made the diagnosis obvious. The perianal region was defined as a circle of diameter 6 cm centring on the anus | ||||
| Exclusion criteria | Not explicit; patients were examined at baseline to exclude the presence of other sexually transmitted diseases | ||||
| All outcomes reported in paper | Complete clearance; recurrence; median number of visits required to achieve complete clearance; AEs | ||||
| Subgroups evaluated | None | ||||
| Stratification | Not reported | ||||
| Baseline measurement of disease | At the initial visit, the clinical appearance of AGWs was documented and a full proctological examination carried out to determine the extent of AGWs. Patients with genital warts also underwent urethroscopy | ||||
| Treatment | Podophyllin 25% (clinician applied) | Surgical excision | |||
| Randomised, n | 30 | 30 | |||
| Withdrawals, n (%) | 0 (0) | 0 (0) | |||
| Treatment regimen | Local application of a podophyllin 25% tincture of benzoin by a health-care professional. Lesions were painted, avoiding the adjacent skin and mucosa. Participants were instructed to wash the lesions 6 hours after each application. Treatment was repeated weekly for up to 6 weeks if required | Participants underwent simple surgical excisions, under local anaesthetic (lidocaine with noradrenaline). If there were too many AGWs to be removed in one procedure, AGWs were removed in two procedures, with an interval of 2 weeks. Participants were allowed home within 30 minutes, with aspirin tablets and a dry dressing on the wounds | |||
| Duration/number of administered treatment | Median number of visits to complete clearance: 5 (range 1–6) | Median number of visits to complete clearance: 1 (range 1–4) | |||
| Difference between groups in the median number of visits required to achieve complete clearance was statistically significant, favouring surgical excision (p < 0.01) | |||||
| Baseline patient characteristics | Podophyllin 25% (clinician applied) | Surgical excision | p-value | ||
| Age (years), median (range) | 24 (19–38) | 26 (17–44) | Differences between groups reported to be non-significant | ||
| Duration of disease (weeks), median (range) | 8 (2–20) | 7 (3–24) | |||
| Site of AGWs, n (%) | |||||
| Perianal alone | 4 (13) | 6 (20) | |||
| Perianal plus | |||||
| Anal canal | 12 (40) | 10 (33) | |||
| Anal canal, genitalia | 5 (17) | 6 (20) | |||
| Anal canal, genitalia, rectum | 1 (3) | 0 (0) | |||
| Genitalia | 6 (20) | 5 (17) | |||
| Rectum | 2 (7) | 3 (10) | |||
| Two people were identified as having urethral AGWs, which were removed by a urologist | |||||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, median (range) | 7 (3–31) | 9 (3–27) | Difference reported to be non-significant | ||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex, n (%) | |||||
| Men | 24 (80) | 21 (70) | Difference reported to be non-significant | ||
| Women | 6 (20) | 9 (30) | |||
| Any previous treatment, n (%) | All first episode | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | It is stated that the ‘short-term effect of both regimens was evaluated 1 week after the final treatment’ (p. 1146). No further details reported. The publication includes data on recurrence in people with complete clearance. Based on the numbers reported, it has been assumed that data for short-term effect are complete clearance at 1 week after treatment | ||||
| Recurrence of AGWs | Not defined. Data on recurrent AGWs requiring further treatment are reported | ||||
| AEs | Not defined. Data on pain, bleeding, burns and soiling are reported | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllin 25% (clinician applied), n/N | Surgical excision, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 1 week after treatment | 23/30 | 28/30 | Not reported | |
| Recurrence of AGWs | 12 months | 17/23 | 8/28 | p < 0.01 | |
| AEs | During treatment | ||||
| Skin burns | 3/30 | 0/30 | |||
| Minor bleeding | 4/30 | 0/30 | |||
| Bleeding | 0/30 | 11/30 | |||
| Soiling | 4/30 | 0/30 | |||
| Pain | |||||
| Requiring analgesics | 3/30 | 12/30 | |||
| Slight | 4/30 | 9/30 | |||
| Severe | 0/30 | 4/30 | |||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | The study is described as a randomised controlled study but further details on method of randomisation not available | |||
| Allocation concealment | ? | No details provided | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | Level of masking is not described. Given that one of the treatments is surgical excision, it is likely not to have been feasible to mask participants and key study personnel to treatment. It is stated that follow-up was carried out by an independent observer who was masked to treatment. It is unclear whether the independent observer also evaluated short-term effect (reporting suggests not, but does not state that this is the case). If the outcome assessor was masked to treatment, the probability of masking being broken is unclear | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ✓ | No missing outcome data | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Level of masking is not described. Given that one of the treatments is surgical excision, it is likely not to have been feasible to mask participants and key study personnel to treatment. It is stated that follow-up was carried out by an independent observer who was masked to treatment. It is unclear whether the independent observer also evaluated short-term effect (reporting suggests not, but does not state that this is the case). If the outcome assessor was masked to treatment, the probability of masking being broken is unclear | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ✓ | No missing outcome data | |||
| AEs | Blinding (participants and personnel) | ? | Level of masking is not described. Given that one of the treatments is surgical excision, it is likely not to have been feasible to mask participants and key study personnel to treatment. It is stated that follow-up was carried out by an independent observer who was masked to treatment. It is unclear whether the independent observer also evaluated short-term effect (reporting suggests not, but does not state that this is the case). If the outcome assessor was masked to treatment, the probability of masking being broken is unclear | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ✓ | No missing outcome data | |||
| Overall rating of bias | ? | Reflects the limited reporting in the trial | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Orla Ní Ógáin and Sam Barton | ||||
| Study ID | Kar 2003 | ||||
| Study details | Indian J Dermatol 2003;48:146–50 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Location was STD treatment centres located in hospitals for military, serving personnel; although not explicitly stated, authors’ affiliation suggests trial carried out in India. Number of sites not reported | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Men attending STD treatment centres in hospitals for military, serving personnel were enrolled between January 1995 and December 2001 | ||||
| Trial design | RCT | ||||
| Trial duration | Initial treatment period of 6 weeks with a subsequent follow-up period of 6 months (unclear whether this is 6 months after the end of treatment or 6 months from start of treatment) | ||||
| Line of therapy | Not reported | ||||
| Inclusion criterion | Presence of AGWs as determined by visual inspection (without biopsy confirmation) | ||||
| Exclusion criteria | Presence of untreated syphilis; frequent genital herpes; presence of bowenoid papulosis; allergy to podophyllin 20% in 1% benzoin tincture; received treatment for AGWs within a month of study entry; immunocompromised (by history or clinical examination) | ||||
| All outcomes reported in paper | Treatment response/cure; recurrence of AGWs or formation of new lesions; occurrence of local adverse reactions | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Presence of AGWs at baseline was determined by visual inspection. The site, number, and size of AGWs were recorded at baseline and at subsequent follow-up visits | ||||
| Treatment | Podophyllin 20% (in 1% tincture of benzoin; clinician applied) | Podophyllotoxin 0.5% solution (patient applied) | |||
| Randomised, n | 35 | 37 | |||
| Withdrawals, n (%) | Not reported. It is stated that ‘no patient withdrew from any treatment because of the side effects’ (p. 148). Analyses are based on ITT population | ||||
| Treatment regimen | Podophyllin 20% in 1% tincture of benzoin was applied with a cotton swab once a week by a health-care professional in a clinic. The surrounding skin was covered with Vaseline to avoid chemical injury to normal skin. Men were instructed to keep the area open for 30 minutes to allow the treatment to dry and to wash the area with soap and water 4 hours after application. Treatment was repeated weekly for up to 6 weeks or until there was no visible AGW tissue. If there was an incomplete response after 6 weeks, treatment was discontinued | The men were instructed to apply podophyllotoxin 0.5% solution twice a day (every 12 hours) for 3 consecutive days and then to withhold application for 4 consecutive days. Treatment was repeated weekly for up to 6 weeks or until there was no visible AGW tissue. If there was an incomplete response after 6 weeks, treatment was discontinued | |||
| Duration/number of administered treatment | Mean number of treatments (presumed to be to complete clearance): 4.8 (SD/SE not reported) | Mean number of treatments (presumed to be to complete clearance): 4.6 (SD/SE not reported) | |||
| Baseline patient characteristics | Podophyllin 20% (in 1% tincture of benzoin; clinician applied) | Podophyllotoxin 0.5% solution (patient-applied) | p-value | ||
| Age (years) | Mean and median age not reported for either the full trial population or by treatment group. Overall age distribution in study: < 20 years: 7 (9.7%), 21–25 years: 15 (20.8%), 26–30 years: 18 (25%), 31–35 years: 16 (22.2%), 36–40 years: 9 (12.5%), 41–50 years: 5 (6.9%), > 50 years: 2 (2.7%) | Not reported | |||
| Duration of disease (months) | 4.8 (unclear whether this is mean or median) | 5.7 (unclear whether this is mean or median) | Not reported | ||
| Site of AGWs, n (%) | |||||
| Glans penis | 7 (20) | 8 (21.6) | Not reported | ||
| Coronal sulcus | 27 (77.1) | 29 (78.3) | Not reported | ||
| Urethral meatus | 2 (5.7) | 1 (2.7) | Not reported | ||
| Prepuce | 7 (20) | 5 (13.5) | Not reported | ||
| Shaft of penis | 2 (5.7) | 3 (8.1) | Not reported | ||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | 4 | 5 | Not reported | ||
| Size of AGWs (mm), mean [size given in mm not area (mm2)] | 4.8 | 3.5 | Not reported | ||
| Sex (M/F), n (%) | 100% male | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | It is reported that complete resolution was recorded at the end of treatment (6 weeks) | ||||
| AGW clearance at other time points | It is reported that complete resolution was also recorded at the end of weeks 1, 2, 3, 4 and 5 during treatment and at weeks 4, 8, 12 and 24 of follow-up. Men were categorised as clinically ‘cured’ at the end of 24 weeks’ follow-up | ||||
| Recurrence of AGWs | Recurrence of AGWs was recorded by the investigator. It is unclear whether data for recurrence include new lesions appearing during or after treatment | ||||
| Appearance of new warts during treatment | It is stated that formation of new AGWs was recorded by the investigator. A new AGW was defined as one that arose at a site distant from that of the original AGWs. Data for the appearance of new AGWs either during treatment or during the post-treatment follow-up period are not reported separately. It is unclear whether data for recurrence include new lesions appearing during or after treatment | ||||
| AEs | Local adverse reactions (pain, burning, inflammation and erosion) were reported and categorised as none, mild, moderate or severe (definitions of the individual categories not available) | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllin 20% (in 1% tincture of benzoin; clinician applied), n/N | Podophyllotoxin 0.5% solution (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 6 weeks | 29/35 | 33/37 | Not reported | |
| AGW clearance at other time pointsa | 1 week | 0/35 | 0/37 | Not reported | |
| 2 weeks | 0/35 | 0/37 | Not reported | ||
| 3 weeks | 0/35 | 0/37 | Not reported | ||
| 4 weeks | 16/35 | 18/37 | Not reported | ||
| 5 weeks | 22/35 | 27/37 | Not reported | ||
| Recurrence of AGWs | 6 months | 8/29 | 11/33 | Not reported | |
| Appearance of new warts during treatment | Not reported separately; unclear whether data have been recorded with ‘recurrence of warts’ | ||||
| AEs | |||||
| Pain | |||||
| None | 6 weeks | 25/35 | 23/37 | Not reported | |
| Mild | 6 weeks | 6/35 | 7/37 | ||
| Moderate | 6 weeks | 3/35 | 6/37 | ||
| Severe | 6 weeks | 1/35 | 1/37 | ||
| Burning | |||||
| None | 6 weeks | 22/35 | 22/37 | Not reported | |
| Mild | 6 weeks | 9/35 | 8/37 | ||
| Moderate | 6 weeks | 4/35 | 6/37 | ||
| Severe | 6 weeks | 0/35 | 1/37 | ||
| Erosion | |||||
| None | 6 weeks | 23/35 | 21/37 | Not reported | |
| Mild | 6 weeks | 7/35 | 10/37 | ||
| Moderate | 6 weeks | 4/35 | 4/37 | ||
| Severe | 6 weeks | 1/35 | 1/37 | ||
| Inflammation | |||||
| None | 6 weeks | 21/35 | 21/37 | Not reported | |
| Mild | 6 weeks | 10/35 | 9/37 | ||
| Moderate | 6 weeks | 3/35 | 5/37 | ||
| Severe | 6 weeks | 1/35 | 2/37 | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmentb | Comments | ||
| Random sequence generation | ? | It is stated that men were randomly divided into groups but details on method of randomisation not available | |||
| Allocation concealment | ? | No details provided | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting. In the paper, there is a description of the assessment of the formation of new AGWs, but data are not presented. Unclear whether formation of new AGWs is captured within recurrence | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | Details on whether the authors made attempts to mask patients and personnel not provided. Given the two different settings in which treatments were administered (clinic vs. home), it might be impractical to mask patients and key personnel to treatment. Also, it is unclear whether the clinician assessing clearance was masked to treatment allocation. If assessor not masked to treatment, assessment of complete clearance could be considered to be a subjective assessment and at risk of bias | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | Although the analysis is based on the ITT population, the number of people lost to follow-up or withdrawing from the trial is not reported. There might be an imbalance between the groups in the number of people for whom data are not available | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Details on whether the authors made attempts to mask patients and personnel not provided. Given the two different settings in which treatments were administered (clinic vs. home), it might be impractical to mask patients and key personnel to treatment. Also, it is unclear whether the clinician assessing clearance was masked to treatment allocation. If assessor not masked to treatment, assessment of complete clearance could be considered to be a subjective assessment and at risk of bias | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | Although the analysis is based on all people with complete clearance, the number of people lost to follow-up or withdrawing from the trial during the observation period is not reported. There might be an imbalance between the groups in the number of people for whom data are not available | |||
| AEs | Blinding (participants and personnel) | ? | Details on whether the authors made attempts to mask patients and personnel not provided. Given the two different settings in which treatments were administered (clinic vs. home), it might be impractical to mask patients and key personnel to treatment. Also, it is unclear whether the clinician assessing clearance was masked to treatment allocation. If assessor not masked to treatment, assessment of complete clearance could be considered to be a subjective assessment and at risk of bias | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | Although the analysis is based on the ITT population, the number of people lost to follow-up or withdrawing from the trial is not reported. There might be an imbalance between the groups in the number of people for whom data are not available | |||
| Overall rating of bias | ? | Reflects limited reporting in full publication | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID | Kinghorn 1993 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Int J STD AIDS 1993;4:194–9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Language of publication | English | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of report | Full publication | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 2: Study information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Location and number of sites | Carried out at six clinics in the UK and Eire: Royal Hallamshire Hospital, Sheffield; St James’s Hospital, Dublin; Royal Infirmary, Edinburgh; Coventry and Warwickshire Hospital, Coventry; Middlesex Hospital, London; and General Infirmary, Leeds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trial sponsor | Brocades Pharma supplied the study treatments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Conflicts of interest | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patient enrolment | Patients were recruited from people presenting to the six genitourinary clinics listed above; dates of enrolment not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trial design | RCT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trial duration | Treatment period of a maximum of 5 weeks followed by a final assessment at week 13 from the start of the study | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Line of therapy | 31% of people had received previous treatment for AGWs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inclusion criteria | Presence of external AGWs (condylomata acuminata); aged ≥ 16 years | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclusion criteria | People were not eligible if they had or were at high risk of having HIV infection; had any other immunosuppressive disorder; had other untreated STD; had received any AGW treatment within 1 month of study entry; had condylomata of the vagina, cervix or anus; were a pregnant or lactating woman | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All outcomes reported in paper | Complete clearance; recurrence; AEs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Subgroups evaluated | Gender; location of lesion (presented by lesions rather than patients) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stratification | Unclear. Baseline characteristics and results are presented by gender, but it is unclear whether randomisation was stratified by gender | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline measurement of disease | At the initial visit, the number and sites of AGWs were described and recorded. Details on the morphological types of AGW were not routinely collected | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Podophyllotoxin 0.5% cream (patient applied) | Podophyllin 25% solution (clinician applied) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Randomised, n | Number of people randomised unclear; 138 available for follow-up | Number of people randomised unclear; 62 available for follow-up | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Withdrawals, n (%) | Overall, 52/252 (21%) people were lost to follow-up by the 5-week assessment (number of people lost to follow-up not reported separately for each treatment group). At the 13-week follow-up, of men and women with complete clearance, 75 out of 157 (47.8%) returned for assessment of relapse | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment regimen | Podophyllotoxin 0.5% cream was self-applied twice daily for 3 consecutive days each week, followed by a 4-day treatment-free period. The treatment cycle was repeated until AGWs were eradicated or for a maximum of 5 weeks, whichever occurred first | Podophyllin 25% solution was applied by a physician or nurse twice weekly. The patient was instructed to wash the area 4 hours after application. The treatment cycle was repeated until AGWs were eradicated or for a maximum of 5 weeks, whichever occurred first | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Duration/number of administered treatment | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline patient characteristics | Podophyllotoxin 0.5% cream (patient applied) | Podophyllin 25% solution (clinician applied) | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All baseline characteristics were reported by gender within each treatment group rather than for the full group | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean (range) | Men 25 (16–46); women 22 (18–30) | Men 25 years (18–46); women 23 years (17–37) | Difference between groups reported to be not significant | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Duration of disease (weeks), mean | Men 16; women 9 | Men 24; women 14 | Difference between groups reported to be not significant | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Site of AGWs, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mena | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prepuce | 29 | 13 | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Glans | 14 | 7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shaft | 21 | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other | 51 | 21 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Womena | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labia majora | 18 | 11 | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labia minora | 15 | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Introitus | 14 | 16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other | 18 | 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of AGWs, n (%) | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Number of AGWs, mean (range) | Men 8.8 (1–35); women 9.3 (1–40) | Men 8.8 (1–26); women 13.4 (range 2–48) | Difference between groups reported to be not significant | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Area of AGWs (mm2), mean | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | 97 (70.3) | 36 (58.1) | Not significant | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | 41 (29.7) | 26 (41.9) | Not significant | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Any previous treatment, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | 34 (35.1) | 14 (38.9) | Difference between groups reported to be statistically significant for women | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | 13 (31.7) | 1 (3.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ethnicity, n (%) | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 3: Outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome | Definition | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at completion of treatment | Clearance of all AGWs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at other time points | Clearance of all AGWs at 1 week after start of treatment | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recurrence of AGWs | Referred to as relapse in the full publication; not further defined | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AEs | Subjective effects defined as tenderness, burning and pain. Objective effects defined as erythema, oedema and erosions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 4: Data extraction form | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome | Time frame | Podophyllotoxin 0.5% cream (patient applied), n/N | Podophyllin 25% solution (clinician applied), n/N | Estimate of effect | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dichotomous outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at completion of treatment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | 5 weeks | 83/97 | 26/36 | p = 0.08 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | 32/41 | 16/26 | p = 0.14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 115/138 | 42/62 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at other time points | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | 1 week | 51/97 | 7/36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | 15/41 | 5/26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 66/138 | 12/62 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recurrence of AGWs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | 13 weeks | 7/37 | 5/10 | p = 0.015 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | 5/21 | 3/7 | p = 0.35 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 12/58 | 8/17 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AEs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Subjective: tenderness, burning and pain | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | 1 week | 57/97 | 1/36 | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | 18/41 | 3/26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 75/138 | 4/62 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Objective: erythema, oedema and erosions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | 1 week | 48/97 | 4/36 | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | 10/41 | 2/26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 58/138 | 6/62 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 5: Clinical trial quality | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome | Risk of bias | Risk assessmentb | Comments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Random sequence generation | ? | It is stated that patients were randomly allocated in strict sequence. Additional information on method used to generate random sequence not available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allocation concealment | ? | It is stated that patients were randomly allocated in strict sequence by numbered sealed envelopes. It is unclear whether the envelopes were opaque | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ‘Other bias’ | ? | Imbalance between treatment groups in the proportion of women who had received previous treatment for AGWs. The group with a higher proportion of women who had received previous treatment could be more resistant to treatment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ✗ | Described as an open, unblinded trial. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blinding of outcomes assessment | ✗ | The personnel assessing the outcomes were not independent observers. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Incomplete outcome data | ? | Number of people randomised to each group not reported. Loss to follow-up at 5 weeks not reported by treatment group but for the overall population. As number of people randomised to each group was not reported, unclear whether there is an imbalance between groups in number of people lost to follow-up | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recurrence of AGWs | Blinding (participants and personnel) | ✗ | Described as an open, unblinded trial. Assessment of AGW relapse is likely to be subjective and open to influence from lack of masking | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blinding of outcomes assessment | ✗ | The personnel assessing the outcomes were not independent observers. Assessment of AGW relapse is likely to be subjective and open to influence from lack of masking | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Incomplete outcome data | ? | Number of people randomised to each group not reported. A large proportion of people was lost to follow-up during the observation phase. The proportion returning for assessment was similar for the two groups. It is unclear what effect the large loss to follow-up will have on the effect estimate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AEs | Blinding (participants and personnel) | ✗ | Described as an open, unblinded trial. Assessment of AEs is likely to be subjective and open to influence from lack of masking | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blinding of outcomes assessment | ✗ | The personnel assessing the outcomes were not independent observers. Assessment of AEs is likely to be subjective and open to influence from lack of masking | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Incomplete outcome data | ? | Number of people randomised to each group not reported. Loss to follow-up at 5 weeks not reported by treatment group but for the overall population. As the number of people randomised to each group was not reported, unclear whether there is an imbalance between groups in number of people lost to follow-up | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall rating of bias | ✗ | Reflects open-label nature of trial | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 6: Additional comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional comments | SitePodophyllotoxin 0.5% creamPodophyllin 25% solutionNumber of sites cleared, n (%)Number of sites treated, nNumber of sites cleared, n (%)Number of sites treated, nMenPrepuce27 (93.1)2912 (92.3)13Glans11 (78.6)145 (71.4)7Shaft16 (76.2)211 (12.5)8Other44 (86.3)5113 (61.9)21Totala98 (85.2)11531 (63.3)49WomenLabia majora12 (66.7)185 (45.5)11Labia minora11 (73.3)155 (62.5)8Introitus10 (71.4)1410 (62.5)16Other14 (77.8)187 (63.6)11Totala47 (72.3)6527 (58.7)46ap-values for the difference between treatments in healing at 5 weeks for all sites treated were < 0.002 and 0.10 for men and women respectively. | Site | Podophyllotoxin 0.5% cream | Podophyllin 25% solution | Number of sites cleared, n (%) | Number of sites treated, n | Number of sites cleared, n (%) | Number of sites treated, n | Men | Prepuce | 27 (93.1) | 29 | 12 (92.3) | 13 | Glans | 11 (78.6) | 14 | 5 (71.4) | 7 | Shaft | 16 (76.2) | 21 | 1 (12.5) | 8 | Other | 44 (86.3) | 51 | 13 (61.9) | 21 | Totala | 98 (85.2) | 115 | 31 (63.3) | 49 | Women | Labia majora | 12 (66.7) | 18 | 5 (45.5) | 11 | Labia minora | 11 (73.3) | 15 | 5 (62.5) | 8 | Introitus | 10 (71.4) | 14 | 10 (62.5) | 16 | Other | 14 (77.8) | 18 | 7 (63.6) | 11 | Totala | 47 (72.3) | 65 | 27 (58.7) | 46 | ap-values for the difference between treatments in healing at 5 weeks for all sites treated were < 0.002 and 0.10 for men and women respectively. | ||||||||||||||||||
| Site | Podophyllotoxin 0.5% cream | Podophyllin 25% solution | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Number of sites cleared, n (%) | Number of sites treated, n | Number of sites cleared, n (%) | Number of sites treated, n | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prepuce | 27 (93.1) | 29 | 12 (92.3) | 13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Glans | 11 (78.6) | 14 | 5 (71.4) | 7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shaft | 16 (76.2) | 21 | 1 (12.5) | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other | 44 (86.3) | 51 | 13 (61.9) | 21 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Totala | 98 (85.2) | 115 | 31 (63.3) | 49 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labia majora | 12 (66.7) | 18 | 5 (45.5) | 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labia minora | 11 (73.3) | 15 | 5 (62.5) | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Introitus | 10 (71.4) | 14 | 10 (62.5) | 16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other | 14 (77.8) | 18 | 7 (63.6) | 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Totala | 47 (72.3) | 65 | 27 (58.7) | 46 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ap-values for the difference between treatments in healing at 5 weeks for all sites treated were < 0.002 and 0.10 for men and women respectively. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | ||||
| Study ID | Kirby 1990 | ||||
| Study details | Am J Med 1990;88:465–9 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study carried out in Washington, USA. Number of sites not specified; study possibly carried out a single site | ||||
| Trial sponsor | It is stated that the study was part funded by grants from Oclassen Pharmaceuticals, Inc. and the National Institutes of Health | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Details not available | ||||
| Trial design | RCT | ||||
| Trial duration | Initial treatment period of 4 weeks with subsequent follow-up at 12 and 16 weeks for those categorised as cured at week 6 | ||||
| Line of therapy | 25/38 (65.8%) men had received previous treatment for AGWs | ||||
| Inclusion criteria | Male gender; aged ≥ 18 years; presence of 2–20 external AGWs, excluding anal AGWs; total AGW surface area of < 10 cm2 | ||||
| Exclusion criteria | Presence of untreated syphilis; previous AGW therapy within 1 month of enrolment; possible immunocompromise; active genital herpes simplex infection; history of bowenoid papulosis; unreliable history | ||||
| All outcomes reported in paper | Complete clearance; proportion of men with AGWs resistant to treatment; volume reduction in area of AGWs; recurrence; AEs | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | Stratified based on duration of AGWs: ≤ 12 months vs. > 12 months | ||||
| Baseline measurement of disease | Examination of AGWs included recording of size, location and number together with circumcision status. At baseline, AGWs were characterised as keratotic or non-keratotic. Each lesion was assigned a number and a diagram was made to facilitate monitoring | ||||
| Treatment | Podophyllotoxin 0.5% solution (patient applied) | Placebo solution (patient applied) | |||
| Randomised, n | 19 | 19 | |||
| Withdrawals, n (%) | 1 (5.3%). Patient withdrew because of AEs and was subsequently lost to follow-up | 0 | |||
| Treatment regimen | Men applied podophyllotoxin 0.5% solution or placebo solution twice daily for 3 consecutive days a week, for a minimum of 2 weeks and a maximum of 4 weeks. Solutions were applied using a swab. Men were instructed not to treat inflamed or bleeding lesions | ||||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Podophyllotoxin 0.5% solution (patient applied) | Placebo solution (patient applied) | p-value | ||
| Age (years), mean (SE) | 28.8 (1.66) | 29.8 (1.71) | Not significant | ||
| Duration of disease, n (%) | Mean duration not reported | ||||
| ≤ 12 months | 9 (47.4) | 10 (52.6) | Not significant | ||
| > 12 months | 10 (52.6) | 9 (47.4) | Not significant | ||
| Site of AGWs, n (%) | Not reported | Not significant | |||
| Type of AGWs, n (%) | 100% non-keratinised | 100% non-keratinised | Not significant | ||
| Number of AGWs, mean (SE) (range) | 8.1 (1.09) (1–20) | 9.5 (1.34) (2–20) | Not significant | ||
| Area of AGWs (mm2), mean (SE) (range) | 86.2 (19.43) (7–331) | 118.2 (47.97) (8–942) | Not significant | ||
| Sex (M/F), n (%) | 100% male | 100% male | Not significant | ||
| Any previous treatment, n (%) | |||||
| Podophyllin | 10 (52.6) | 7 (36.8) | Not significant | ||
| Liquid nitrogen | 11 (57.9) | 9 (47.4) | Not significant | ||
| Electrocautery | 2 (10.5) | 2 (10.5) | Not significant | ||
| Laser | 0 (0) | 0 (0) | |||
| 5-Fluoruracil | 0 (0) | 0 (0) | |||
| Any previous genital wart therapy | 15 (78.9) | 10 (52.6) | Not significant | ||
| Ethnicity, n (%) | |||||
| Caucasian | 16 (84.2) | 18 (94.7) | Not significant | ||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Free of visible genital AGWs at 4 weeks | ||||
| Recurrence of AGWs | Men judged to have resolution of genital AGWs at the week 6 visit returned at weeks 12 and 16 for evaluation of recurrence | ||||
| Volume of wart clearance | Various measures of reduction in volume of AGWs were used: at least 50% reduction in original AGW area; percentage of original AGW area visible; and percentage of original AGW count present | ||||
| Appearance of new warts during treatment | New lesions that appeared during 4 weeks of treatment | ||||
| AEs | Men were questioned about the presence of AEs and the investigator examined the men for the presence of erosion, inflammation and other findings. The investigator graded AEs as mild, moderate or severe; severity not further defined. Effects evaluated were burning, erosions, pain, inflammation, itching, dryness, erythema, irritation and nausea | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllotoxin 0.5% solution (patient applied), n/N | Placebo solution (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 4 weeks | 11/19 | 0/19 | p < 0.01 | |
| Recurrence of AGWs | 16 weeks | 10/11 | 0/0 | – | |
| Volume of wart clearance (at least 50% reduction in original wart area) | 2 weeks | 19/19 | 1/19 | Not reported | |
| 4 weeks | 19/19 | 1/19 | |||
| 6 weeks | 17/19 | 1/19 | |||
| Appearance of new warts during treatment | 4 weeks | 8/19 | 9/19 | Not reported | |
| AEs | |||||
| Burning | 4 weeks | 14/19 | 1/19 | p < 0.01 | |
| Erosions | 11/19 | 0/19 | Not reported | ||
| Pain | 11/19 | 1/19 | Not reported | ||
| Inflammation | 9/19 | 0/19 | Not reported | ||
| Itching | 6/19 | 3/19 | Not reported | ||
| Dryness | 2/19 | 1/19 | Not reported | ||
| Erythema | 1/19 | 0/19 | Not reported | ||
| Irritation | 1/19 | 0/19 | Not reported | ||
| Nausea | 1/19 | 0/19 | Not reported | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that men were randomly assigned to treatment. Details on method of randomisation not available | |||
| Allocation concealment | ✓ | Men were given coded vials that were identical in appearance. The code for the double-blind portion of the trial was not broken until the conclusion of the placebo-controlled and open phases of the study | |||
| Selective reporting | ? | Insufficient information reported to assess level of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ✓ | Described as a double-blind study. Given that identical vials were used and the randomisation code was not broken until after conclusion of the open phase of the trial, it is likely that masking has been maintained | ||
| Blinding of outcomes assessment | ? | It is stated that a single investigator performed all assessments. Although the study is described as double blinded and it is likely that the investigator was masked to treatment and masking has been maintained, which personnel have been masked to treatment is not explicitly stated | |||
| Incomplete outcome data | ✓ | Number of people lost to follow-up reported. Only one person lost to follow-up from the study during the treatment phase. An additional person lost to follow-up from the phase evaluating recurrence | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ✓ | Described as a double-blind study. Given that identical vials were used and the randomisation code was not broken until after conclusion of the open phase of the trial, it is likely that masking has been maintained | ||
| Blinding of outcomes assessment | ? | It is stated that a single investigator performed all assessments. Although the study is described as double blinded and it is likely that the investigator was masked to treatment and masking has been maintained, which personnel have been masked to treatment is not explicitly stated | |||
| Incomplete outcome data | ✓ | Number of people lost to follow-up reported. Only one person lost to follow-up from the study during the treatment phase. An additional person lost to follow-up from the phase evaluating recurrence | |||
| Volume of wart clearance (proportion of patients with at least 50% clearance) | Blinding (participants and personnel) | ✓ | Described as a double-blind study. Given that identical vials were used and the randomisation code was not broken until after conclusion of the open phase of the trial, it is likely that masking has been maintained | ||
| Blinding of outcomes assessment | ? | It is stated that a single investigator performed all assessments. Although the study is described as double blinded and it is likely that the investigator was masked to treatment and masking has been maintained, which personnel have been masked to treatment is not explicitly stated | |||
| Incomplete outcome data | ✓ | Number of people lost to follow-up reported. Only one person lost to follow-up from the study during the treatment phase. An additional person lost to follow-up from the phase evaluating recurrence | |||
| AEs | Blinding (participants and personnel) | ✓ | Described as a double-blind study. Given that identical vials were used and the randomisation code was not broken until after conclusion of the open phase of the trial, it is likely that masking has been maintained | ||
| Blinding of outcomes assessment | ? | It is stated that a single investigator performed all assessments. Although the study is described as double blinded and it is likely that the investigator was masked to treatment and masking has been maintained, which personnel have been masked to treatment is not explicitly stated | |||
| Incomplete outcome data | ✓ | Number of people lost to follow-up reported. Only one person lost to follow-up from the study during the treatment phase. An additional person lost to follow-up from the phase evaluating recurrence | |||
| Overall rating of bias | ? | ||||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | ||||
| Study ID | Komericki 2011 | ||||
| Study details | Sex Trans Dis 2011;38:216–18 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study carried out at the Medical University of Graz, Austria. Number of sites not specified; study likely to have been carried out a single site | ||||
| Trial sponsor | It is stated that the study was not funded by any drug manufacturer | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Consecutive patients presenting with untreated AGWs over a 2-year period (date not stated) were eligible. Further details not available | ||||
| Trial design | RCT | ||||
| Trial duration | Study focused on the treatment period: 4 weeks of treatment with podophyllotoxin 0.5% solution vs. 16 weeks of treatment with imiquimod 5% cream | ||||
| Line of therapy | First line | ||||
| Inclusion criterion | Untreated AGWs | ||||
| Exclusion criteria | Age < 18 years; lack of informed consent; known immunosuppression; pregnancy; breastfeeding; involvement of the anal canal; severe disease requiring surgery | ||||
| All outcomes reported in paper | Complete clearance; AEs | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Initial evaluation of AGWs included recording of location of AGWs (anal, genital or anogenital) together with an assessment of severity, which was graded as mild (area of involvement 0–100 mm2), moderate (area of involvement 100–200 mm2) or severe (area of involvement > 200 mm2) | ||||
| Treatment | Podophyllotoxin 0.5% solution (patient applied) | Imiquimod 5% cream (patient applied) | |||
| Randomised, n | 26 | 25 | |||
| Withdrawals, n (%) | 1 (3.8%) lost to follow-up (reason not reported) | 5 (20%) lost to follow-up (reasons not reported) | |||
| Treatment regimen | Podophyllotoxin 0.5% solution was applied by the patient twice daily for 3 consecutive days per week either until complete clearance of AGWs or for a maximum of 4 weeks, whichever occurred first | Imiquimod 5% cream was applied by the patient three times a week either until complete clearance of AGW or for a maximum of 16 weeks, whichever occurred first | |||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Podophyllotoxin 0.5% solution (patient applied) | Imiquimod 5% cream (patient applied) | p-value | ||
| Note: Baseline characteristics are reported for the people completing the study rather than all people randomised | |||||
| Age (years), mean | 30.0 | 30.8 | Not reported | ||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | |||||
| Genital | 14 (56.0) | 13 (65.0) | Differences between groups reported to be not significant | ||
| Anal | 5 (20.0) | 4 (20.0) | |||
| Anogenital | 6 (24.0) | 3 (15.0) | |||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean (with SD/SE if given) | Not reported | ||||
| Mean area of AGWs, n (%) | |||||
| Mild (0–100 mm2) | 9 (36.0) | 8 (40.0) | Differences between groups reported to be not significant | ||
| Moderate (100–200 mm2) | 9 (36.0) | 7 (35.0) | |||
| Severe (> 200 mm2) | 7 (28.0) | 5 (25.0) | |||
| Sex, n (%) | |||||
| Men | 22 (88.0) | 16 (80.0) | Differences between groups reported to be not significant | ||
| Women | 3 (12.0) | 4 (20.0) | |||
| Any previous treatment, n (%) | None; all AGWs were untreated | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Complete clearance of AGWs at 5 weeks after start of podophyllotoxin 0.5% or 16 weeks after start of imiquimod 5% | ||||
| AEs | At the individual assessments, for AEs, patients were assigned to one of no side effects; erythema/inflammation; erosions; or erythema/inflammation plus erosions | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllotoxin 0.5% solution (patient applied), n/N | Imiquimod 5% cream (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | Treatment dependent | 18/25 | 15/20 | p = 1 | |
| AEs | |||||
| Erythema/inflammation | Treatment dependent | 10/25 | 7/20 | Differences between groups reported to be not significant | |
| Erosion | Treatment dependent | 5/25 | 5/20 | ||
| Erythema/inflammation plus erosion | Treatment dependent | 6/25 | 8/20 | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that ‘Allocation to treatment groups was done according to a ranking, which was created by block randomisation. Each block contained 4 items (2 × imiquimod and 2 × podophyllotoxin) to assure a balanced design with equal group sizes’ (p. 216). Details on method of sequence generation not available | |||
| Allocation concealment | ? | Information on allocation concealment not reported | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking | ||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | |||
| Incomplete outcome data | ✗ | The number of people lost to follow-up from each group is reported, but reasons for loss to follow-up are not available. There is an imbalance between the groups in the proportion of people randomised and subsequently lost to follow-up (3.8% podophyllotoxin vs. 20.0% imiquimod). This imbalance is likely to influence the effect estimate | |||
| AEs | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AEs is likely to be subjective and open to influence from lack of masking | ||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AEs is likely to be subjective and open to influence from lack of masking | |||
| Incomplete outcome data | ✗ | The number of people lost to follow-up from each group is reported, but reasons for loss to follow-up are not available. There is an imbalance between the groups in the proportion of people randomised and subsequently lost to follow-up (3.8% podophyllotoxin vs. 20.0% imiquimod). Unclear what impact this imbalance could have on analysis of AEs. This imbalance is likely to influence the effect estimate | |||
| Overall rating of bias | ✗ | Reflects open-label nature of the study | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | |||||
| Study ID | Lacey 2003 | |||||
| Study details | Sex Transm Infect 2003;79:270–5 | |||||
| Language of publication | English | |||||
| Type of report | Full publication | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Carried out at 11 STD clinics in the UK | |||||
| Trial sponsor | Perstorp Pharma, Lund, Sweden | |||||
| Conflicts of interest | Dr Lacey has acted as a consultant to 3M Pharmaceuticals, GlaxoSmithKline, Merck and Xenova. Dr Maw has acted as an adviser to Perstorp Pharma, 3M Pharmaceuticals and Stiefel. None of the other authors has acted as a consultant for Stiefel and none has a financial interest in Stiefel or podophyllotoxin | |||||
| Patient enrolment | Details not available | |||||
| Trial design | RCT | |||||
| Trial duration | Initial treatment period of a maximum of 4 weeks. Those with complete clearance at any time point during treatment were followed up at 12 weeks after trial entry | |||||
| Line of therapy | Not reported | |||||
| Inclusion criteria | Aged 18–65 years; current AGWs with a history of ≤ 3 months and no therapy in that time | |||||
| Exclusion criteria | Known HIV infection or immunosuppression; homosexual male with perianal AGWs; total lesional area > 400 mm2; any individual lesion with an area of > 100 mm2; presence of intrameatal or vaginal AGWs or ulcerative or inflammatory STDs of the anogenital region; pregnancy | |||||
| All outcomes reported in paper | Complete clearance at end of treatment; recurrence; AEs; cost-effectiveness | |||||
| Subgroups evaluated | None reported | |||||
| Stratification | Gender and number of AGWs at baseline (< 10 AGWs vs. ≥ 10 AGWs) | |||||
| Baseline measurement of disease | The number and location of AGWs were recorded | |||||
| Treatment | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.15% cream (patient applied) | Podophyllin 25% (clinician applied) | |||
| Randomised, n | 120 | 118 | 116 | |||
| Withdrawals, n (%) | 24 (20.0) | 36 (30.5) | 18 (15.5) | |||
| Data reported for withdrawals are the number of people reported not to have completed the trial as per the trial protocol. Reasons for non-completion of the trial are not available. Number of people lost to follow-up during treatment not reported. It should be noted that a large proportion of people with complete clearance after treatment failed to attend follow-up at 12 weeks [103/177 (58.2%)] | ||||||
| Treatment regimen | Podophyllotoxin 0.5% solution and podophyllotoxin 0.15% cream were self-applied twice daily for 3 consecutive days, followed by 4 treatment-free days. Treatment was applied for a maximum of 4 weeks or until complete clearance of AGWs, whichever occurred earlier | Podophyllin 25% in tincture of compound benzoin was applied twice weekly by a health-care professional. Treatment was applied for a maximum of 4 weeks or until complete clearance of AGWs, whichever occurred earlier | ||||
| Duration/number of administered treatment | Not reported | |||||
| Baseline patient characteristics | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.15% cream (patient applied) | Podophyllin 25% (clinician applied) | p-value | ||
| Age (years), mean (range) | Not reported | |||||
| Duration of disease | Not reported | |||||
| Site of AGWs, n (%) | Not reported | |||||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, mean | Not reported | |||||
| Number of people with < 10 AGWs, n (%) | 79 (66) | 84 (71) | 82 (71) | |||
| Number of people with ≥ 10 AGWs, n (%) | 41 (34) | 34 (29) | 34 (29) | |||
| Area of AGWs (mm2), mean | Not reported | |||||
| Sex, n (%) | ||||||
| Men | 62 (52) | 60 (51) | 60 (52) | |||
| Women | 58 (48) | 58 (49) | 56 (48) | |||
| Any previous treatment, n (%) | Not reported | |||||
| Ethnicity, n (%) | ||||||
| White | 114 (95) | 111 (94) | 110 (95) | |||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Complete clearance of original AGWs (those identified at baseline) during treatment up to a maximum of four cycles of treatment. Authors based primary analysis on the per-protocol population but also carried out ‘best case’ and ‘worst case’ analyses in which missing data were imputed as all excluded people being cured or not cured respectively. Separate analysis of all AGWs (including new AGWs appearing during treatment) was also reported | |||||
| Recurrence of AGWs | For those with complete clearance after treatment, recurrence at 12 weeks after study entry | |||||
| AEs | AEs were not defined. Data were reported for occurrence of local side effects and ulceration. Unclear whether analysis is based on per-protocol population, people receiving at least one dose of treatment or ITT population | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Podophyllotoxin 0.5% solution (patient applied), n/N | Podophyllotoxin 0.15% cream (patient applied), n/N | Podophyllin 25% (clinician applied), n/N | Estimate of effect | CI and p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | 4 weeks | 72/96 | 53/82 | 52/98 | Podophyllotoxin solution significantly better than the other interventions (p-value not reported) | |
| Recurrence of AGWs | 12 weeks | 15/33 | 12/22 | 5/19 | ||
| AEs | ||||||
| Local side effects | 4 weeks | 32/96 | 20/82 | 17/98 | Not reported | |
| Ulceration | 17/96 | 10/82 | 10/98 | Not reported | ||
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | |||
| Random sequence generation | ? | It is stated that men were randomly allocated to treatment. Details on method used to generate random sequence not available | ||||
| Allocation concealment | ? | Details on method used to conceal allocation not available | ||||
| Selective reporting | ? | Insufficient information available to assess potential bias in selective reporting | ||||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias | ||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ✗ | Described as an open-label trial. Given the variation in settings in which the treatments were applied (home vs. clinic), it might be impractical to mask to treatment allocation. AGW clearance is a subjective outcome that could potentially be influenced by patients and key study personnel | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | The analyses reported are based on the per-protocol population. The number of people who withdrew from the trial, together with reasons for withdrawal, are not reported. There might be an imbalance across the groups in reasons for withdrawal that could influence the effect estimate | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ✗ | Described as an open-label trial. Given the variation in settings in which the treatments were applied (home vs. clinic), it might be impractical to mask to treatment allocation. AGW recurrence is a subjective outcome that could potentially be influenced by patients and key study personnel | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AGW recurrence is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | A large proportion of people with complete clearance during the treatment phase of the study failed to return for subsequent assessment. Reasons for loss to follow-up are not reported. There might be an imbalance across the groups in reasons for withdrawal that could influence the effect estimate | ||||
| AEs | Blinding (participants and personnel) | ✗ | Described as an open-label trial. Given the variation in settings in which the treatments were applied (home vs. clinic), it might be impractical to mask to treatment allocation. Interpretation of subjective AEs (such as pain) could potentially be influenced by patients and key study personnel | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AEs is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | It is unclear whether the results reported are based on the per-protocol population, those receiving one dose of allocated treatment or the ITT population | ||||
| Overall rating of bias | ✗ | Reflects the open-label nature of the study | ||||
| Section 6: Additional comments | ||||||
| Additional comments | None | |||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Sam Barton and Charlotta Karner | ||||
| Study ID | Landthaler 1987 | ||||
| Study details | Dt Dermatol 1987;11:1223–5 | ||||
| Language of publication | German | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Unclear | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | People were enrolled from October 1985 to August 1986. Further details on how people were recruited are not available | ||||
| Trial design | RCT | ||||
| Trial duration | It was initially planned that people would receive treatment over a period of 6 weeks and would then be followed up 4 weeks after the end of treatment. However, the authors decided that this schedule was impractical. Instead, AGWs were treated until clearance and people were followed up at 4 weeks (or longer) after clearance. All patients were treated for a minimum of 2 weeks. It is noted that treatment was stopped early in those not responding to treatment; the criteria used to determine non-response to treatment are unclear | ||||
| Line of therapy | Not reported | ||||
| Inclusion criteria | Not reported | ||||
| Exclusion criteria | Not reported | ||||
| All outcomes reported in paper | Complete clearance; recurrence; AEs | ||||
| Subgroups evaluated | Not reported | ||||
| Stratification | Not reported | ||||
| Baseline measurement of disease | Not reported | ||||
| Treatment | Podophyllotoxin 0.5% (patient applied) | Podophyllin 20% (clinician applied) | |||
| Randomised, n | 22 | 17 | |||
| Withdrawals | 5 (22.7%) people were classified as dropouts, with 3 people lost to follow-up | 6 (35.3%) people were classified as dropouts, with 4 people lost to follow-up | |||
| Treatment regimen | People self-applied podophyllotoxin 0.5% in the morning and again in the evening for 3 consecutive days (Monday, Tuesday and Wednesday) each week until complete clearance or classification of non-response, whichever occurred earlier. Of the 22 people receiving podophyllotoxin 0.5%, 19 men applied the treatment as a solution and three women as a cream | People receiving podophyllin 20% were treated once a week by a clinician in the outpatient clinic. Treatment continued until complete clearance or classification of non-response, whichever occurred earlier | |||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Podophyllotoxin 0.5% (patient applied) | Podophyllin 20% (clinician applied) | p-value | ||
| Age (years), mean (SD) (range) | 30 (9) (20–45) | 26 (6) 19–43 | Difference reported to be not significant | ||
| Duration of disease (months), mean (SD) (range) | 6.8 (6.2) (1–14) | 5.3 (4.9) (1–18) | Difference reported to be not significant | ||
| Site of AGWs, n (%)a | Not reported | ||||
| Genital | 17 | 14 | Difference reported to be not significant | ||
| Perianal | 5 | 4 | Difference reported to be not significant | ||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean (SD) (range) | 9.3 (5.2) (2–20) | 9.5 (5.7) (2–17) | Difference reported to be not significant | ||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex, n (%) | |||||
| Male | 19 (86.4) | 17 (100) | Difference reported to be not significant | ||
| Female | 3 (13.6) | 0 | |||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Complete clearance of AGWs | ||||
| Recurrence of AGWs | Not defined | ||||
| Time to complete clearance | Mean time to complete clearance of AGWs | ||||
| AEs | AEs not defined. Limited data reported on occurrence of AEs | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllotoxin 0.5% (patient applied), n/N | Podophyllin 20% (clinician applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | At least 2 weeks | 13/17 | 6/11 | Difference reported to be not significant | |
| Recurrence of AGWsb | At least 4 weeks after complete clearance | 2/13 | 3/11 | Difference reported to be not significant | |
| AEs: ‘toxic dermatitis with erythema, swelling, blistering, and subsequent scaly crusts’ | At least 2 weeks | 4/17 | 3/11 | ||
| All patients experienced a moderate reddening of the treated areas of the skin and a slight burning sensation | |||||
| Continuous outcomes | |||||
| Time to complete clearance (weeks), mean (SD/SE) | At least 2 weeks | 2.6 (1.1) (n = 17) | 3.4 (3.2) (n = 11) | Difference reported to be not significant | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessment c | Comments | ||
| Random sequence generation | ? | Described as a randomised study but details on method of randomisation not available | |||
| Allocation concealment | ? | Details on method used to conceal allocation not available | |||
| Selective reporting | ✗ | Clear definitions of duration of treatment regimens, outcome assessment and time at which outcomes were reported are not available. Lack of reporting of duration of treatment and time points for key clinical outcomes makes it difficult to compare the study with other trials in a meta-analysis | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible. It is unclear whether the clinician assessing clinical outcomes was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | The numbers of people withdrawing and lost to follow-up are reported. There is a slight imbalance across the groups in the proportion of people withdrawing. The impact on the estimate of effect is unclear | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible. It is unclear whether the clinician assessing clinical outcomes was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | The numbers of people withdrawing and lost to follow-up are reported. There is a slight imbalance across the groups in the proportion of people withdrawing. The impact on the estimate of effect is unclear | |||
| Time to complete clearance | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible. It is unclear whether the clinician assessing clinical outcomes was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | The numbers of people withdrawing and lost to follow-up are reported. There is a slight imbalance across the groups in the proportion of people withdrawing. The impact on the estimate of effect is unclear | |||
| AEs | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible. It is unclear whether the clinician assessing clinical outcomes was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | The numbers of people withdrawing and lost to follow-up are reported. There is a slight imbalance across the groups in the proportion of people withdrawing. The impact on the estimate of effect is unclear | |||
| Overall rating of bias | ✗ | Reflects the limited reporting in the study around the duration of treatment and clinical outcomes evaluated | |||
| Section 6: Additional comments | |||||
| Additional comments |
|
||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | ||||
| Study ID | Lassus 1984 | ||||
| Study details | Eur J Sex Transm Dis 1984;2:31–3 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study carried out at the University Central Hospital, Helsinki, Finland | ||||
| Trial sponsor | Study podophyllotoxin 0.5% solution was donated by Produktkontroll AB, Sweden | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Men attending the Outpatient Department for Venereal Diseases at the University Central Hospital were enrolled between January 1981 and February 1982 | ||||
| Trial design | RCT | ||||
| Trial duration | Initial treatment period of a maximum of 4 weeks. Those with complete clearance at the end of treatment were followed up for up to 6 months after the start of treatment | ||||
| Line of therapy | Not reported | ||||
| Inclusion criteria | Men with condylomata acuminata in the preputial cavity | ||||
| Exclusion criteria | Not reported | ||||
| All outcomes reported in paper | Complete clearance; recurrence; AEs | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | Not reported | ||||
| Baseline measurement of disease | Not reported. Based on presented baseline characteristics, number of AGWs and number of sites affected in the preputial cavity were recorded | ||||
| Treatment | Podophyllotoxin 0.5% solution (patient applied) | Podophyllin 20% solution (clinician applied) | |||
| Randomised, n | 48 | 52 | |||
| Withdrawals, n (%) | Not reported | ||||
| Treatment regimen | Podophyllotoxin 0.5% solution was self-applied twice daily for 3 days for up to four weekly treatments or until complete clearance, whichever occurred earlier | Podophyllin 20% solution was applied in clinic by a clinician or a nurse. Men were instructed to wash off the solution 6 hours after application. Treatment was repeated weekly for up to 4 weeks or until complete clearance, whichever occurred earlier | |||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Podophyllotoxin 0.5% solution (patient applied) | Podophyllin 20% solution (clinician applied) | p-value | ||
| Age (years), n (%) | Mean age not reported; data reported based on two age groups | ||||
| 18–30 | 39 (81.3) | 46 (88.5) | Difference between groups reported to be not significant | ||
| ≥ 31 | 9 (18.7) | 6 (11.5) | |||
| Duration of disease (months), n (%) | Mean duration of disease not reported | ||||
| < 1 month | 5 (10.4) | 4 (7.7) | Difference between groups reported to be not significant | ||
| 1–2 months | 24 (50.0) | 26 (50.0) | |||
| > 2 months | 19 (39.6) | 22 (42.3) | |||
| Site of AGWs, n (%) | All AGWs located in the preputial cavity. Data reported for number of sites affected | ||||
| One site | 19 (39.6) | 21 (40.4) | Difference between groups reported to be not significant | ||
| Two sites | 8 (16.7) | 13 (25.0) | |||
| Three or four sites | 21 (43.8) | 18 (34.6) | |||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, n (%) | Mean number of AGWs not reported. Data reported based on three categories of number of AGWs at baseline | ||||
| < 5 | 12 (25.0) | 13 (25.0) | Difference between groups reported to be not significant | ||
| 5–15 | 27 (56.3) | 24 (46.2) | |||
| > 15 | 9 (18.8) | 15 (28.8) | |||
| Area of AGWs (mm2) | Mean area of AGWs not reported. It was stated that all AGWs were 1–6 mm in size | ||||
| Sex (M/F), n (%) | 100% men | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Not defined. Treatment failure was defined as follows: ‘if the patient still had warts after four treatments, he was transferred to other therapy, mainly surgery’ (p. 32). For this review, data on complete clearance after three or four cycles of treatment have been used for complete clearance at the end of treatment; data not reported separately for three and four treatments | ||||
| AGW clearance at other time points | Not defined. Data on complete clearance after one and two cycles of treatment are reported in the full publication | ||||
| Recurrence of AGWs | Recurrence at 3 months’ follow-up from initiation of treatment | ||||
| AEs | AEs were not defined. Data are reported on occurrence of local reactions, categorised as mild, moderate or severe; definition of mild, moderate and severe not available | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllotoxin 0.5% solution (patient applied), n/N | Podophyllin 20% solution (clinician applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | Three to four treatments | 48/48 | 37/52 | p < 0.001 | |
| AGW clearance at other time points | One treatment | 45/48 | 15/52 | Not reported | |
| Two treatments | 47/48 | 24/52 | |||
| Recurrence of AGWs | 3 months | 11/48 | 14/37 | Not reported | |
| AEs: local reactions (mild, moderate and severe) | 4 weeks | 8/48 | 13/52 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that men were randomly allocated to treatment. Details on method used to generate random sequence not available | |||
| Allocation concealment | ? | Details on method used to conceal allocation not available | |||
| Selective reporting | ? | Insufficient information available to assess potential bias in selective reporting | |||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | Given the variation in settings in which the treatments were applied (home vs. clinic), it might be impractical to mask to treatment allocation. AGW clearance is a subjective outcome that could potentially be influenced by patients and key study personnel. However, it is not explicitly stated that the trial is open label and it is unclear whether attempts were made to mask patients and personnel | ||
| Blinding of outcomes assessment | ? | It is unclear whether attempts were made to mask the outcome assessor to treatment allocation | |||
| Incomplete outcome data | ? | Analysis of complete clearance is based on all men randomised. However, it is not stated that no men were lost to follow-up or withdrew from the trial | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Given the variation in settings in which the treatments were applied (home vs. clinic), it might be impractical to mask to treatment allocation. AGW recurrence is a subjective outcome that could potentially be influenced by patients and key study personnel. However, it is not explicitly stated that the trial is open label and it is unclear whether attempts were made to mask patients and personnel | ||
| Blinding of outcomes assessment | ? | It is unclear whether attempts were made to mask the outcome assessor to treatment allocation | |||
| Incomplete outcome data | ? | Analysis of recurrence is based on all men randomised. However, it is not stated that no men were lost to follow-up or withdrew from the trial | |||
| AEs | Blinding (participants and personnel) | ? | Given the variation in settings in which the treatments were applied (home vs. clinic), it might be impractical to mask to treatment allocation. AGW clearance is a subjective outcome that could potentially be influenced by patients and key study personnel. However, it is not explicitly stated that the trial is open label and it is unclear whether attempts were made to mask patients and personnel | ||
| Blinding of outcomes assessment | ? | It is unclear whether attempts were made to mask the outcome assessor to treatment allocation | |||
| Incomplete outcome data | ? | Analysis of AEs is based on all men randomised. However, it is not stated that no men was lost to follow-up or withdrew from the trial | |||
| Overall rating of bias | ? | Reflects limited reporting on methods in the full publication | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | |||||
| Study ID | Maiti 1985 | |||||
| Study details | Practitioner 1985;229:37–9 | |||||
| Language of publication | English | |||||
| Type of report | Full publication | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Study carried out at St Luke’s Clinic, Manchester, UK | |||||
| Trial sponsor | Not reported | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | Men were recruited from those attending St Luke’s Clinic. Dates of enrolment not reported | |||||
| Trial design | Blind controlled study | |||||
| Trial duration | Initial treatment period of 1 week. Those who had complete clearance after 1 week were followed up at 3 weeks and 3 months | |||||
| Line of therapy | Unclear; inclusion criterion of no treatment in preceding 6 months | |||||
| Inclusion criteria | Men with penile AGWs who had not received treatment for their AGWs in the 6 months before enrolment | |||||
| Exclusion criteria | Not reported | |||||
| All outcomes reported in paper | Complete clearance; recurrence; AEs | |||||
| Subgroups evaluated | None reported | |||||
| Stratification | None reported | |||||
| Baseline measurement of disease | Not reported | |||||
| Treatment | Podophyllin 0.5% solution (patient applied) | Podophyllin 1.0% solution (patient applied) | Podophyllin 2.0% solution (patient applied) | |||
| Randomised, n | It is stated that 100 men were allocated to treatment but number of men randomised to each group not reported. Results are reported for those men attending the 3-month follow-up visit (84 men) | |||||
| Attended 3-month follow-up | 28 | 27 | 29 | |||
| Withdrawals | 16 men in total did not attend the 3-month follow-up. Reasons for withdrawal and number of men withdrawing from each treatment group not reported | |||||
| Treatment regimen | All solutions were applied by the patient twice daily for 3 consecutive days using sticks supplied by the investigators. Men were instructed to wash the afflicted area before application of the solution and to not wash the site after application. Treatment regimen was not repeated | |||||
| Duration/number of administered treatment | Not applicable; treatments were evaluated over only 1 week | |||||
| Baseline patient characteristics | Podophyllin 0.5% solution (patient applied) | Podophyllin 1.0% solution (patient applied) | Podophyllin 2.0% solution (patient applied) | p-value | ||
| Age (years), mean (range) | Not reported | |||||
| Duration of disease | Not reported | |||||
| Site of AGWs, n (%) | Not reported | |||||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, mean | Not reported | |||||
| Area of AGWs (mm2), mean | Not reported | |||||
| Sex (M/F), n (%) | 100% male | |||||
| Any previous treatment, n (%) | Not reported | |||||
| Ethnicity, n (%) | Not reported | |||||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Free of AGWs at 1 week | |||||
| Recurrence of AGWs | Recurrence of AGWs at 3 months in those who were AGW free at 1 week | |||||
| AEs | Not defined. Data on general AEs reported | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Podophyllin 0.5% solution (patient applied), n/N | Podophyllin 1.0% solution (patient applied), n/N | Podophyllin 2.0% solution (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | 1 week | 23/28 | 24/27 | 27/29 | Not reported | |
| Recurrence of AGWs | 3 months | 6/23 | 6/24 | 8/27 | Not reported | |
| AEs: anya | 1 week | 0/28 | 5/27 | 10/29 | Not reported | |
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessment b | Comments | |||
| Random sequence generation | ? | It is stated that men were allocated to treatment. Unclear whether a random sequence has been used to allocate treatment | ||||
| Allocation concealment | ? | It is stated that the podophyllin solutions were stored in numbered bottles as prepared by the pharmacy. It is unclear whether treatment allocation could have been broken | ||||
| Selective reporting | ? | Insufficient information available to assess potential bias in selective reporting | ||||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias. Baseline characteristics have not been reported; unclear whether there is an imbalance across groups in patient characteristics | ||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ✓ | The podophyllin solutions were prepared by the pharmacy in a hospital. Solutions (2 ml) were stored in numbered bottles. The solution concentration in the bottle was known only to the pharmacist and was not revealed until completion of the study | |||
| Blinding of outcomes assessment | ✓ | It is not stated that the clinician assessing AGW clearance was masked to treatment but the reviewers consider it likely that the assessor was masked to treatment as only the pharmacist was aware of the solution concentrations | ||||
| Incomplete outcome data | ? | Number of men randomised to each group is not reported. In total, 16 men did not attend the 3-month follow-up. It is unclear whether the 100 men randomised were treated or whether the 16 men withdrew before treatment and whether there is an imbalance between groups in the number of men withdrawing. Reasons for withdrawal are not reported. The impact of withdrawal on the effect estimate is unclear | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ✓ | The podophyllin solutions were prepared by the pharmacy in a hospital. Solutions (2 ml) were stored in numbered bottles. The solution concentration in the bottle was known only to the pharmacist and was not revealed until completion of the study | |||
| Blinding of outcomes assessment | ✓ | It is not stated that the clinician assessing AGW recurrence was masked to treatment but the reviewers consider it likely that the assessor was masked to treatment as only the pharmacist was aware of the solution concentrations | ||||
| Incomplete outcome data | ? | Number of men randomised to each group is not reported. It is unclear whether there were any additional losses to follow-up during the follow-up period. The analysis is based on all people with complete clearance | ||||
| AEs | Blinding (participants and personnel) | ✓ | The solutions of podophyllin were prepared by the pharmacy in a hospital. Solutions (2 ml) were stored in numbered bottles. The solution concentration in the bottle was known only to the pharmacist and was not revealed until completion of the study | |||
| Blinding of outcomes assessment | ✓ | It is not stated that the clinician assessing AEs was masked to treatment but the reviewers consider it likely that the assessor was masked to treatment as only the pharmacist was aware of the solution concentrations | ||||
| Incomplete outcome data | ? | Number of men randomised to each group is not reported. In total, 16 men did not attend the 3-month follow-up. It is unclear whether the 100 men randomised were treated or whether the 16 men withdrew before treatment and whether there is an imbalance between groups in the number of men withdrawing. Reasons for withdrawal are not reported. The impact of withdrawal on the effect estimate is unclear | ||||
| Overall rating of bias | ? | Reflects limited reporting in the full publication | ||||
| Section 6: Additional comments | ||||||
| Additional comments | None | |||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | ||||
| Study ID | Matteelli 2001 | ||||
| Study details | Sex Transm Dis 2001;28:343–6 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study carried out at one site in Italy | ||||
| Trial sponsor | Study drug provided complementarily by Pharmacia & Upjohn | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Not reported | ||||
| Trial design | Crossover RCT. People initially receiving placebo were crossed over to cidofovir at 4 weeks in an open-label extension phase. Pre- and post-crossover data are reported in the publication. Only pre-crossover data have been extracted | ||||
| Trial duration | Initial treatment period of 2 weeks followed by 2 weeks of observation | ||||
| Line of therapy | Most people enrolled were experiencing recurrence of AGWs after surgery | ||||
| Inclusion criteria | Men and women were eligible for enrolment if they aged ≥ 18 years; had a laboratory-confirmed diagnosis of HIV infection; had a clinical diagnosis of external AGWs established by physical examination; were able to give signed, informed consent; were able to use condoms during the treatment and follow-up period; were free of signs and symptoms associated with other HIV-related opportunistic infections and neoplasms at the time of enrolment | ||||
| Exclusion criteria | Men and women were ineligible if they had impaired renal function; were pregnant; had received chemical or surgical therapy for their AGWs in the preceding 2 months | ||||
| All outcomes reported in paper | Volume of AGW clearance; AEs | ||||
| Subgroups evaluated | By gender (pre- and post-crossover results combined for cidofovir) and by AGW location | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | A clinical examination was performed at baseline, but, because most patients had very extensive lesions, the total AGW area and total number of AGWs were not recorded | ||||
| Treatment | Cidofovir 1% cream | Placebo | |||
| Randomised, n | 6 | 6 | |||
| Withdrawals, n (%) | Not reported. It is stated that 4 out of 12 (33%) people discontinued cidofovir 1% after the first week of therapy because of severe mucosal erosions. It is unclear how many of the four people discontinuing were included among the six people allocated to cidofovir 1% in the blinded treatment phase and how many were from the open-label phase | ||||
| Treatment regimen | Cidofovir 1% cream or placebo was applied once daily on all external AGWs at bedtime 5 days a week for 2 weeks | ||||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Cidofovir 1% cream | Placebo | p-value | ||
| Age (years), mean | 33.8 | 31.8 | Difference between groups reported to be not significant | ||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | |||||
| Perianal | 5 (83.3) | 3 (50.0) | Difference between groups reported to be not significant | ||
| Vulvar | 3 (50.0) | 2 (33.3) | |||
| Perineal | 1 (16.7) | 3 (50.0) | |||
| Penile | 1 (16.7) | 2 (33.3) | |||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | Not reported | ||||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex, n (%) | |||||
| Male | 3 (50.0) | 3 (50.0) | Difference between groups reported to be not significant | ||
| Female | 3 (50.0) | 3 (50.0) | |||
| Any previous treatment, n (%) | 5 (83.3) | 4 (66.7) | Difference between groups reported to be not significant | ||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| Volume of wart clearance | AGW clearance at 4 weeks was assessed by comparison between baseline and post-treatment (day 28) photographic documentation of the lesions by three independent clinicians who were masked to treatment. Treatment outcome was categorised as no modification or worsening of the total AGW area between baseline and end of the follow-up period; a reduction in volume of < 25%; a reduction in volume between 25% and 50%; a reduction in volume of ≥ 50%. Data for complete clearance were not reported separately | ||||
| AEs | Signs (oedema, erythema and erosion) and symptoms (itching, pain and burning) of local reactions were recorded | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Cidofovir 1% cream, n/N | Placebo, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| Volume of wart clearance | |||||
| No reduction | 4 weeks | 1/6 | 4/6 | p = 0.02 | |
| Reduction < 25% | 4 weeks | 1/6 | 2/6 | ||
| Reduction 25–50% | 4 weeks | 1/6 | 0/6 | ||
| Reduction > 50% | 4 weeks | 3/6 | 0/6 | ||
| AEs | |||||
| Itching | 2 weeks | 0/6 | 0/6 | Not reported | |
| Pain | 2 weeks | 1/6 | 0/6 | ||
| Burning | 2 weeks | 3/6 | 0/6 | ||
| Oedema | 2 weeks | 1/6 | 0/6 | ||
| Erythema | 2 weeks | 3/6 | 0/6 | ||
| Erosion | 2 weeks | 2/6 | 0/6 | ||
| Any | 2 weeks | 5/6 | 0/6 | p < 0.001 | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that people were randomly allocated to treatment. Details on method used to generate random sequence not available | |||
| Allocation concealment | ? | Details on method used to conceal allocation not available | |||
| Selective reporting | ✗ | Given that volume of AGW clearance has been evaluated, it could be expected that the number of people with complete clearance would have been reported | |||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias | |||
| Volume of wart clearance | Blinding (participants and personnel) | ✗ | Described as a single-blind study (independent clinicians assessing outcomes are masked to treatment). Lack of masking of study personnel and patients could introduce bias and potentially risk breaking of masking of outcome assessors | ||
| Blinding of outcomes assessment | ✓ | AGW clearance was assessed by three independent clinicians who were masked to treatment. Although patients and study personnel were aware of treatment allocation, which could influence assessment of AGW clearance, it is thought that assessment by three independent clinicians minimises the risk of bias | |||
| Incomplete outcome data | ? | The number of people withdrawing from the study is not reported. It is stated that four people withdrew from cidofovir treatment because of AEs, but results are combined from the treatment and open-label phase. It is unclear how many people withdrew from each group during the treatment phase | |||
| AEs | Blinding (participants and personnel) | ✗ | Described as a single-blind study (independent clinicians assessing outcomes are masked to treatment). Lack of masking of study personnel and patients could introduce bias and potentially risk breaking of masking of outcome assessors | ||
| Blinding of outcomes assessment | ✗ | AGW clearance was assessed by three independent clinicians who were masked to treatment. Unclear whether same clinicians evaluated AEs. Patients were aware of treatment allocation, which could influence reporting of symptoms associated with treatment | |||
| Incomplete outcome data | ? | The number of people withdrawing from the study is not reported. It is stated that four people withdrew from cidofovir treatment because of AEs, but results are combined from the treatment and open-label phase. It is unclear how many people withdrew from each group during the treatment phase | |||
| Overall rating of bias | ✗ | Reflects the single-blind nature of the trial and limited reporting on clinical outcomes | |||
| Section 6: Additional comments | |||||
| Additional comments |
|
||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | |||||
| Study ID | Mazurkiewicz 1990 | |||||
| Study details | J Dermatol Treat 1990;1:123–5 | |||||
| Language of publication | English | |||||
| Type of report | Full publication | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Study was carried out at the Department of Dermatology and Venereology, Warsaw School of Medicine, Poland | |||||
| Trial sponsor | Not reported | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | Not reported | |||||
| Trial design | RCT | |||||
| Trial duration | Initial treatment period of up to 6 weeks followed by an observation period of 4 weeks after the end of treatment for those whose AGWs had completely cleared | |||||
| Line of therapy | Mixed; patients who had received previous treatment for AGWs were included | |||||
| Inclusion criteria | Males or females with condylomata acuminata | |||||
| Exclusion criteria | People were ineligible for enrolment if they were aged < 12 years; were female and pregnant, lactating or not taking adequate contraception; had a total AGW surface area of > 10 cm2; had received treatment for their AGWs within the 2 weeks preceding study entry | |||||
| All outcomes reported in paper | Complete clearance at various time points; appearance of new AGW during treatment; relapse; AEs | |||||
| Subgroups evaluated | None reported | |||||
| Stratification | None reported | |||||
| Baseline measurement of disease | At baseline, an investigator counted the number of AGWs and recorded AGW diameter | |||||
| Treatment | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.5% cream (patient applied) | Podophyllin 20% solution (clinician applied) | |||
| Randomised, n | 16 | 22 | 16 | |||
| Withdrawals, n (%) | 2 (12.5) | 6 (27.3) | 3 (18.8) | |||
| Lost to follow-up | 0 | 4 (18.2) | 2 (12.5) | |||
| Other | 2 (12.5%) (one dropped out because of a head injury and one because of marked pruritus) | 2 (9.1%) (dropped out because of swelling of foreskin) | 1 (6.3%) (withdrawn because of enlargement of papule on prepuce) | |||
| Treatment regimen | Podophyllotoxin 0.5% solution or 0.5% cream was self-applied twice daily for 3 consecutive days per week for up to 6 weeks or until complete clearance, whichever occurred earlier | Podophyllin 20% solution was applied once a week by a physician. Podophyllin solution was washed off after 2 hours after the first application, after 4 hours for the second application and after 6 hours for subsequent applications. Treatment was repeated for up to 6 weeks or until complete clearance, whichever occurred earlier | ||||
| Duration/number of administered treatment | Mean number of treatments not reported | |||||
| Baseline patient characteristics | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.5% cream (patient applied) | Podophyllin 20% solution (clinician applied) | p-value | ||
| Age (years), mean (SD) (range) | 29.2 (4.9) (21–38) | 26.6 (5.4) (18–36) | 31.8 (11.6) (17–54) | Difference across groups reported to be not significant | ||
| Duration of disease (months), n (%) | Mean duration of disease not reported. Data presented based on categories of duration | |||||
| < 2 | 4 (25.0) | 5 (22.7) | 6 (37.5) | Difference across groups reported to be not significant | ||
| 2–4 | 10 (62.5) | 7 (31.8) | 7 (43.8) | |||
| > 4 | 2 (12.5) | 10 (45.5) | 3 (18.8) | |||
| Site of AGWs, n (%) | ||||||
| Males | ||||||
| Glans | 0 (0) | 0 (0) | 1 (6.3) | Difference across groups reported to be not significant | ||
| Prepuce | 4 (25.0) | 6 (27.3) | 5 (31.2) | |||
| Shaft | 1 (6.3) | 0 (0) | 0 (0) | |||
| Perianal | 0 (0) | 0 (0) | 1 (6.3) | |||
| Other | 0 (0) | 0 (0) | 1 (6.3) | |||
| More than one site | 9 (56.3) | 13 (59.1) | 6 (37.5) | |||
| Females | ||||||
| Labia majora | 0 (0) | 1 (4.5) | 0 (0) | |||
| Introitus | 0 (0) | 0 (0) | 1 (6.3) | |||
| More than one site | 2 (12.5) | 2 (9.1) | 1 (6.3) | |||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, n (%) | Mean number of AGWs at baseline not reported. Data presented based on categories of number of AGWs | |||||
| < 15 | 6 (37.5) | 7 (31.8) | 10 (62.5) | Difference across groups reported to be not significant | ||
| 16–30 | 6 (37.5) | 7 (31.8) | 3 (18.8) | |||
| > 30 | 4 (25.0) | 8 (36.4) | 3 (18.8) | |||
| Area of AGWs (mm3), mean | Mean area of AGWs at baseline not reported. Data presented based on categories of area of AGWs | |||||
| < 40 | 7 (43.8) | 4 (18.2) | 10 (62.5) | p = 0.01 | ||
| 41–80 | 4 (25.0) | 8 (36.4) | 1 (6.3) | |||
| > 80 | 5 (31.2) | 10 (45.5) | 5 (31.2) | |||
| Sex, n (%) | ||||||
| Male | 14 (87.5) | 19 (86.4) | 14 (87.5) | Difference across groups reported to be not significant | ||
| Female | 2 (12.5) | 3 (13.6) | 2 (12.5) | |||
| Any previous treatment, n (%) | Not reported | |||||
| Ethnicity, n (%) | Not reported | |||||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Complete clearance at end of treatment not defined. Data on complete clearance are reported at various time points. End of treatment has been taken to be 6 weeks, which is the maximum number of treatments permitted | |||||
| AGW clearance at other time points | Complete clearance reported at 1–5 weeks of treatment | |||||
| Recurrence of AGWs | Not defined. It is stated that people with complete clearance were followed up for a further 4 weeks after the end of treatment. Data on recurrence have been presumed to be reported after 4 weeks of observation | |||||
| AEs | Local AEs were reported: oedema or swelling, pain, burning, erythema, erosion, enlarging of papule and marked pruritus | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Podophyllotoxin 0.5% solution (patient applied), n/N | Podophyllotoxin 0.5% cream (patient applied), n/N | Podophyllin 20% solution (clinician applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | 6 weeks | 11/14 | 9/16 | 5/13 | Not reported | |
| AGW clearance at other time points (cumulative on a weekly basis) | 1 week | 1/14 | 2/16 | 0/13 | Not reported | |
| 2 weeks | 3/14 | 3/16 | 1/13 | Not reported | ||
| 3 weeks | 7/14 | 7/16 | 1/13 | Not reported | ||
| 4 weeks | 8/14 | 7/16 | 2/13 | Not reported | ||
| 5 weeks | 8/14 | 8/16 | 3/13 | Not reported | ||
| Recurrence of AGWs | 4 weeks after end of treatment | 2/11 | 0/9 | 0/5 | Note: it is stated that one person experiencing relapse had new lesions in a different location. Unclear whether lesions also developed in same location | |
| AEs | ||||||
| Oedema/swelling | 6 weeks | 1/16 | 6/22 | 0/16 | AEs reported as occurring more frequently in podophyllotoxin group (especially cream) but p-values not reported | |
| Pain | 6 weeks | 1/16 | 5/22 | 0/16 | ||
| Burning | 6 weeks | 0/16 | 4/22 | 1/16 | ||
| Erythema | 6 weeks | 7/16 | 6/22 | 4/16 | ||
| Erosion | 6 weeks | 9/16 | 14/22 | 4/16 | ||
| Enlarging of papule | 6 weeks | 0/16 | 0/22 | 1/16 | ||
| Marked pruritus | 6 weeks | 1/16 | 0/22 | 0/16 | ||
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | |||
| Random sequence generation | ? | It is stated that people were randomly allocated to treatment. Details on method used to generate random sequence not available | ||||
| Allocation concealment | ? | Details on method used to conceal allocation not available | ||||
| Selective reporting | ? | Insufficient information available to determine risk of selective reporting | ||||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias. It is noted that there was a significant difference in total size of AGW at baseline, with the difference potentially favouring the podophyllin 20% group. The extent of influence on the estimate of effect is unclear | ||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | It is stated that the study is single blind. Information on who is masked is not reported. Given the different settings for application of treatment (home vs. clinic), it could be considered impractical to mask patients and key personnel to treatment | |||
| Blinding of outcomes assessment | ? | It is unclear whether the investigator assessing clinical outcomes was masked to treatment | ||||
| Incomplete outcome data | ? | The number of people withdrawing (with accompanying reasons) and lost to follow-up is reported. There is an imbalance across the groups in the proportion of people withdrawing. The impact on the estimate of effect size for complete clearance is unclear | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | It is stated that the study is single blind. Information on who is masked is not reported. Given the different settings for application of treatment (home vs. clinic), it could be considered impractical to mask patients and key personnel to treatment | |||
| Blinding of outcomes assessment | ? | It is unclear whether the investigator assessing clinical outcomes was masked to treatment | ||||
| Incomplete outcome data | ? | The number of people withdrawing (with accompanying reasons) and lost to follow-up is reported. There is a slight imbalance across the groups in the proportion of people withdrawing. The impact on the estimate of effect size for recurrence is unclear | ||||
| AEs | Blinding (participants and personnel) | ? | It is stated that the study is single blind. Information on who is masked is not reported. Given the different settings for application of treatment (home vs. clinic), it could be considered impractical to mask patients and key personnel to treatment | |||
| Blinding of outcomes assessment | ? | It is unclear whether the investigator assessing clinical outcomes was masked to treatment | ||||
| Incomplete outcome data | ✓ | The number of people withdrawing (with accompanying reasons) and lost to follow-up is reported. There is a slight imbalance across the groups in the proportion of people withdrawing but all but one person has been included in the analysis of AEs | ||||
| Overall rating of bias | ? | Reflects limited reporting in the full publication | ||||
| Section 6: Additional comments | ||||||
| Additional comments | None | |||||
| Item | Details | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||||||||||||||||||||||||||||||||||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | ||||||||||||||||||||||||||||||||||||||
| Study ID | Nath 1990 | ||||||||||||||||||||||||||||||||||||||
| Study details | Indian J Dermatol Venereol Leprol 1990;56:22–4 | ||||||||||||||||||||||||||||||||||||||
| Language of publication | English | ||||||||||||||||||||||||||||||||||||||
| Type of report | Full publication | ||||||||||||||||||||||||||||||||||||||
| Section 2: Study information | |||||||||||||||||||||||||||||||||||||||
| Location and number of sites | Authors based at the Departments of Dermatology and Obstetrics and Gynaecology, Postgraduate Institute of Medical Education and Research, India; number of sites involved not reported | ||||||||||||||||||||||||||||||||||||||
| Trial sponsor | Not reported | ||||||||||||||||||||||||||||||||||||||
| Conflicts of interest | Not reported | ||||||||||||||||||||||||||||||||||||||
| Patient enrolment | Patients were recruited from people with AGWs attending the STD clinic between 1986 and 1988 | ||||||||||||||||||||||||||||||||||||||
| Trial design | RCT, with the exception that pregnant women were allocated to TCAA | ||||||||||||||||||||||||||||||||||||||
| Trial duration | Initial treatment period of 12 weeks. People who achieved a ‘cure’ were followed up for an additional 3 months | ||||||||||||||||||||||||||||||||||||||
| Line of therapy | Not reported | ||||||||||||||||||||||||||||||||||||||
| Inclusion criterion | Presence of AGWs | ||||||||||||||||||||||||||||||||||||||
| Exclusion criterion | Receipt of any treatment for AGWs in the preceding 2 months | ||||||||||||||||||||||||||||||||||||||
| All outcomes reported in paper | Complete clearance; recurrence | ||||||||||||||||||||||||||||||||||||||
| Subgroups evaluated | Duration of AGWs (< 6 months vs. > 6 months); number of lesions at baseline (< 5 vs. ≥ 5); location (moist vs. dry and keratotic areas) | ||||||||||||||||||||||||||||||||||||||
| Stratification | None reported | ||||||||||||||||||||||||||||||||||||||
| Baseline measurement of disease | Initial evaluation involved recording site and morphology of AGWs | ||||||||||||||||||||||||||||||||||||||
| Treatment | Podophyllin 25% solution (clinician applied) | TCAA 50% solution (clinician applied) | |||||||||||||||||||||||||||||||||||||
| Randomised, n | It is stated that 100 people were randomised to treatment. Number randomised to each group not reported. Clinical results based on the 95 people who were followed up during the treatment period | ||||||||||||||||||||||||||||||||||||||
| 47 | 48 | ||||||||||||||||||||||||||||||||||||||
| Withdrawals | Overall, five people ‘defaulted’ and were lost to follow-up from the initial 100 people randomised. Reasons for loss to follow-up not reported | ||||||||||||||||||||||||||||||||||||||
| Treatment regimen | Podophyllin 25% solution was applied weekly with a swab stick and allowed to dry in air for 2–3 minutes. People washed off the solution with tap water 2 hours after application. Treatment was repeated weekly for up to a maximum of 12 weeks or until complete clearance, whichever occurred earlier | TCAA 50% was applied weekly with a swab stick and allowed to dry in air for 2–3 minutes. Treatment was repeated weekly for up to a maximum of 12 weeks or until complete clearance, whichever occurred earlier | |||||||||||||||||||||||||||||||||||||
| Duration/number of administered treatment | Mean number of treatments required to achieve complete clearance: 3 | Mean number of treatments required to achieve complete clearance: 3.8 | |||||||||||||||||||||||||||||||||||||
| Difference between groups not statistically significant (p > 0.05) | |||||||||||||||||||||||||||||||||||||||
| Baseline patient characteristics | Podophyllin 25% solution (clinician applied) | TCAA 50% solution (clinician applied) | p-value | ||||||||||||||||||||||||||||||||||||
| Age (years), mean (range | Overall trial population: 25.9 (17–43); mean age for individual treatment groups not reported | Reported to be ‘statistically comparable’ | |||||||||||||||||||||||||||||||||||||
| Duration of disease | Not reported | Not reported | |||||||||||||||||||||||||||||||||||||
| Site of AGWs, n (%) | Not reported | Reported to be ‘statistically comparable’ | |||||||||||||||||||||||||||||||||||||
| Type of AGWs, n (%) | |||||||||||||||||||||||||||||||||||||||
| Hyperplastic | 35 (74.5) | 34 (70.8) | Not reported | ||||||||||||||||||||||||||||||||||||
| Flat | 4 (8.5) | 4 (8.3) | |||||||||||||||||||||||||||||||||||||
| Verruca vulgaris | 3 (6.4) | 6 (12.5) | |||||||||||||||||||||||||||||||||||||
| Pigmented | 3 (6.4) | 4 (8.3) | |||||||||||||||||||||||||||||||||||||
| Giant | 2 (4.3) | 0 (0) | |||||||||||||||||||||||||||||||||||||
| Number of AGWs, mean | Not reported | ||||||||||||||||||||||||||||||||||||||
| Area of AGWs (mm2), mean | Not reported | ||||||||||||||||||||||||||||||||||||||
| Sex, n (%) | |||||||||||||||||||||||||||||||||||||||
| Male | 44 (93.6) | 44 (91.7) | Not reported | ||||||||||||||||||||||||||||||||||||
| Female | 3 (6.4) (no women were pregnant) | 4 (8.3) (all women were pregnant) | Not reported | ||||||||||||||||||||||||||||||||||||
| Any previous treatment, n (%) | Not reported | ||||||||||||||||||||||||||||||||||||||
| Ethnicity, n (%) | Not reported | ||||||||||||||||||||||||||||||||||||||
| Section 3: Outcomes | |||||||||||||||||||||||||||||||||||||||
| Outcome | Definition | ||||||||||||||||||||||||||||||||||||||
| AGW clearance at completion of treatment | Free of AGWs at 3 months (end of maximum number of permitted treatments) | ||||||||||||||||||||||||||||||||||||||
| Recurrence of AGWs | Recurrence of AGWs within 3 months of clearance | ||||||||||||||||||||||||||||||||||||||
| AEs | Absolute numbers not reported. It is stated that ‘Local soreness was experienced by 8.5% cases. There was no systemic toxicity’ (p. 23) | ||||||||||||||||||||||||||||||||||||||
| Section 4: Data extraction form | |||||||||||||||||||||||||||||||||||||||
| Outcome | Time frame | Podophyllin 25% solution (clinician applied), n/N | TCAA 50% solution (clinician applied), n/N | Estimate of effect | p-value | ||||||||||||||||||||||||||||||||||
| Dichotomous outcomes | |||||||||||||||||||||||||||||||||||||||
| AGW clearance at completion of treatment | 3 months | 38/47 | 39/48 | Not reported | |||||||||||||||||||||||||||||||||||
| Recurrence of AGWs | 3 months after clearance | 14/38 | 6/39 | Not reported | |||||||||||||||||||||||||||||||||||
| Section 5: Clinical trial quality | |||||||||||||||||||||||||||||||||||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||||||||||||||||||||||||||||||||||||
| Random sequence generation | ? | It is stated that people were randomly allocated to treatment. However, women were allocated by pregnancy status, not randomly. The rationale for this decision is not reported. Only seven women (7% of full trial population) were included. The impact of this decision on the estimate of effect is unclear. Details on method used to generate random sequence not available | |||||||||||||||||||||||||||||||||||||
| Allocation concealment | ? | Details on method used to conceal allocation not available | |||||||||||||||||||||||||||||||||||||
| Selective reporting | ? | Insufficient information available to determine risk of selective reporting. Limited information reported on AEs (no absolute numbers) but authors of the paper acknowledge that both treatments are associated with recognised AEs; unclear whether this is selective reporting or whether presence of AEs was not a prespecified outcome | |||||||||||||||||||||||||||||||||||||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias | |||||||||||||||||||||||||||||||||||||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | Level of masking of study personnel and patients is not reported. It is stated that the treatments were applied by the same observer. It is unclear whether the observer was masked to treatment | ||||||||||||||||||||||||||||||||||||
| Blinding of outcomes assessment | ? | It is unclear whether the investigator assessing clinical outcomes was masked to treatment | |||||||||||||||||||||||||||||||||||||
| Incomplete outcome data | ? | The number of people randomised to each group is not reported. The number of people lost to follow-up during the treatment period is reported, but it is unclear whether these were the only people withdrawing from treatment and whether there is a potential imbalance across the groups | |||||||||||||||||||||||||||||||||||||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Level of masking of study personnel and patients is not reported. It is stated that the treatments were applied by the same observer. It is unclear whether the observer was masked to treatment | ||||||||||||||||||||||||||||||||||||
| Blinding of outcomes assessment | ? | It is unclear whether the investigator assessing clinical outcomes was masked to treatment | |||||||||||||||||||||||||||||||||||||
| Incomplete outcome data | ? | The number of people randomised to each group is not reported. The number of people lost to follow-up during treatment is reported, but it is unclear whether these were the only people withdrawing from treatment and whether other people withdrew during the follow-up phase | |||||||||||||||||||||||||||||||||||||
| Overall rating of bias | ? | Reflects limited reporting of methods in full publication | |||||||||||||||||||||||||||||||||||||
| Section 6: Additional comments | |||||||||||||||||||||||||||||||||||||||
| Additional comments |
Type of AGWNumber (%) of people cleared of AGW at 3 monthsMean (SD) number of treatments required to clear AGW at 3 monthsPodophyllin 25%TCAA 50%Podophyllin 25%TCAA 50%Hyperplastic31/35 (88)30/34 (88)3.4 (1.88)3.7 (1.9)Flat3/4 (75)3/4 (75)4.0 (1.84)5.0 (2.1)Verruca vulgaris2/3 (67)4/6 (67)4.0 (1.76)5.0 (2.12)Pigmented2/3 (67)2/4 (50)4.2 (1.67)4.4 (1.82)Giant0/2––– |
Type of AGW | Number (%) of people cleared of AGW at 3 months | Mean (SD) number of treatments required to clear AGW at 3 months | Podophyllin 25% | TCAA 50% | Podophyllin 25% | TCAA 50% | Hyperplastic | 31/35 (88) | 30/34 (88) | 3.4 (1.88) | 3.7 (1.9) | Flat | 3/4 (75) | 3/4 (75) | 4.0 (1.84) | 5.0 (2.1) | Verruca vulgaris | 2/3 (67) | 4/6 (67) | 4.0 (1.76) | 5.0 (2.12) | Pigmented | 2/3 (67) | 2/4 (50) | 4.2 (1.67) | 4.4 (1.82) | Giant | 0/2 | – | – | – | ||||||
| Type of AGW | Number (%) of people cleared of AGW at 3 months | Mean (SD) number of treatments required to clear AGW at 3 months | |||||||||||||||||||||||||||||||||||||
| Podophyllin 25% | TCAA 50% | Podophyllin 25% | TCAA 50% | ||||||||||||||||||||||||||||||||||||
| Hyperplastic | 31/35 (88) | 30/34 (88) | 3.4 (1.88) | 3.7 (1.9) | |||||||||||||||||||||||||||||||||||
| Flat | 3/4 (75) | 3/4 (75) | 4.0 (1.84) | 5.0 (2.1) | |||||||||||||||||||||||||||||||||||
| Verruca vulgaris | 2/3 (67) | 4/6 (67) | 4.0 (1.76) | 5.0 (2.12) | |||||||||||||||||||||||||||||||||||
| Pigmented | 2/3 (67) | 2/4 (50) | 4.2 (1.67) | 4.4 (1.82) | |||||||||||||||||||||||||||||||||||
| Giant | 0/2 | – | – | – | |||||||||||||||||||||||||||||||||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | |||||
| Study ID | Orlando 2002 | |||||
| Study details | AIDS 2002;16:447–50 | |||||
| Language of publication | English | |||||
| Type of report | Full publication | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Study carried out at the Department of Infectious Diseases, STD Service, L Sacco Hospital, Milan, Italy | |||||
| Trial sponsor | Not reported | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | Patients were enrolled from people referred to the Department of Infectious Diseases, STD Service, L Sacco Hospital, from January 2000 to March 2001 | |||||
| Trial design | RCT (three arms) | |||||
| Trial duration | Initial assessment at the end of treatment (varies with allocated treatment) followed by 6 months’ follow-up for those who achieved complete clearance with treatment | |||||
| Line of therapy | Not reported | |||||
| Inclusion criteria | HIV-positive people with genital AGWs were eligible for enrolment | |||||
| Exclusion criteria | Not reported | |||||
| All outcomes reported in paper | Complete clearance; recurrence | |||||
| Subgroups evaluated | None reported | |||||
| Stratification | None reported | |||||
| Baseline measurement of disease | At baseline, lesion size, number and location were recorded, the sum of which was defined as the ‘lesion score’. For the full trial population, at baseline, the mean lesion score was 76.57 (range 5–292). Difference between groups reported to be not significant | |||||
| Treatment | Electrocauterisation | Cidofovir 1% (patient applied) | Electrocauterisation plus cidofovir 1% | |||
| Randomised, n | 29 | 26 | 19 | |||
| Withdrawals, n (%) | Not reported | Not reported | Not reported | |||
| It is stated that three people definitively stopped application of cidofovir 1%. It is unclear from which treatment group or groups people withdrew | ||||||
| Treatment regimen | Surgical excision by electrocautery; further details not reported | Topical cidofovir 1% gel was self-applied for 1–2 hours for 5 days per week. Treatment was repeated weekly for a maximum of 6 weeks or until complete clearance, whichever occurred earlier | After initial electrocauterisation, cidofovir 1% gel was self-applied for 1–2 hours for 5 days per week for 2 weeks. Application of cidofovir 1% commenced within 1 month of surgical treatment | |||
| Duration/number of administered treatment | Not applicable | Not reported | ||||
| Baseline patient characteristics | Electrocauterisation | Cidofovir 1% (patient applied) | Electrocauterisation plus cidofovir 1% | p-value | ||
| Age (years), mean (range) | Full trial population 33.4 (24–41); mean age not reported for individual treatment groups | Difference between groups reported to be not significant | ||||
| Duration of disease | Not reported | |||||
| Site of AGWs, n (%) | It is stated that ‘warts lesions were located in the perianal area (15 patients), the vulval area (15 patients), on the perineum (three patients) and on the penis (42 patients)’ (p. 448). Site of AGWs not reported by treatment group | |||||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, mean | Not reported. Mean lesion score for full trial population was 76.57 (range 5–292) | Difference between groups reported to be not significant | ||||
| Area of AGWs (mm2), mean | Not reported | |||||
| Sex (M/F), n (%) | Full trial population consisted of 53 men (71.6%) and 21 (28.4%) women; proportions not reported for individual treatment groups | |||||
| Any previous treatment, n (%) | Not reported | |||||
| Ethnicity, n (%) | Not reported | |||||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Complete response was defined as an end of treatment lesion score of 0% of the baseline score. Lesion score was calculated by summing the size (mm), number and anatomic localisation of lesions | |||||
| Recurrence of AGWs | Recurrence was defined as the reappearance of lesions in the same anatomical area after a complete response | |||||
| AEs | Not defined. Occurrence of mild local erosion was reported | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Electrocauterisation, n/N | Cidofovir 1% (patient applied), n/N | Electrocauterisation plus cidofovir 1%, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | Treatment dependent | 27/29 | 20/26 | 19/19 | p = 0.033 | |
| Recurrence of AGWs | 6 months after end of treatment | 14/19 | 6/17 | 3/11 | p = 0.018 | |
| AEs: mild local erosions | End of treatment | – | 11/26 | 6/19 | ||
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | |||
| Random sequence generation | ? | It is stated that men were randomly allocated to treatment. Details on method used to generate random sequence not available | ||||
| Allocation concealment | ? | Details on method used to conceal allocation not available | ||||
| Selective reporting | ✗ | It is stated that partial response (defined as an end of treatment score of < 50% of baseline score) was recorded but data on this outcome are not reported | ||||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias. Unclear whether any imbalances in baseline characteristics are present as few details reported | ||||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ✗ | Described as an open-label trial. Given that two of the treatment groups involve a surgical intervention, it might be impractical to mask to treatment allocation. Assessment of AGW clearance is typically subjective and could potentially be influenced by patients and key study personnel | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence by lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | The number of people withdrawing during the treatment period because of an AE of cidofovir is reported, but it is unclear whether these were the only people withdrawing during treatment and whether there is a potential imbalance across the groups | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ✗ | Described as an open-label trial. Given that two of the treatment groups involve a surgical intervention, it might be impractical to mask to treatment allocation. Assessment of AGW recurrence is typically subjective and could potentially be influenced by patients and key study personnel | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AGW recurrence is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ✗ | The number of people with complete clearance returning for assessment at 6 months is reported. There is an imbalance across groups in the proportion of people lost to follow-up for this assessment. The large variation in proportion of missing outcomes could introduce clinically relevant bias in the evaluation of the effect estimate | ||||
| AEs | Blinding (participants and personnel) | ✗ | Described as an open-label trial. Given that two of the treatment groups involve a surgical intervention, it might be impractical to mask to treatment allocation. Assessment of AEs could be subjective and potentially influenced by patients and key study personnel | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AEs is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | The number of people withdrawing during the treatment period because of an AE of cidofovir is reported, but it is unclear whether these were the only people withdrawing during treatment and whether there is a potential imbalance across the groups | ||||
| Overall rating of bias | ✗ | Reflects limited reporting on clinical outcomes and the open-label nature of the trial | ||||
| Section 6: Additional comments | ||||||
| Additional comments | Mean CD4 count at baseline was 264.9 mm3; not reported by treatment group | |||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Vicky Wakefield and Sam Barton | ||||
| Study ID | Padhiar 2006 | ||||
| Study details | Indian J Sex Transmit Dis 2006;27:67–9 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | STD clinic at the Civil Hospital, Ahmedabad, India | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | People enrolled from the STD clinic at the Civil Hospital, Ahmedabad, India. Additional details not provided | ||||
| Trial design | RCT | ||||
| Trial duration | Initial treatment period (16 weeks with imiquimod 5% vs. 6 weeks with podophyllin 20%) followed by a 6-month follow-up period after clearance of AGWs | ||||
| Line of therapy | Not reported | ||||
| Inclusion criteria | Aged 12–65 years; clinically diagnosed external AGWs with at least two but no more than 50 AGWs at baseline; HIV-negative status | ||||
| Exclusion criteria | Not reported | ||||
| All outcomes reported in paper | Clearance of AGWs at end of treatment; recurrence; time to complete clearance; volume of AGW clearance; AEs | ||||
| Subgroups evaluated | None | ||||
| Stratification | Not reported | ||||
| Baseline measurement of disease | At baseline, a detailed assessment of AGWs was carried out, including evaluation of number, area and location of AGWs | ||||
| Treatment | Imiquimod 5% | Podophyllin 20% | |||
| Randomised, n | 30 | 30 | |||
| Withdrawals, n (%) | Not reported | ||||
| Treatment regimen | After cleaning and drying the skin, participants applied the cream before going to bed to all external lesions in an amount that could be rubbed into the skin until the cream disappeared. The skin was cleaned 6–10 hours after application. Imiquimod 5% was applied three times a week until all baseline AGWs had disappeared or for a maximum of 16 weeks, whichever occurred first | Perilesional skin was covered with petroleum jelly. After cleaning and drying the AGWs, podophyllin 20% was applied with a swab stick. The skin was cleaned 4–6 hours after application. Podophyllin 20% was applied once a week until all baseline AGWs had disappeared or for a maximum of 6 weeks, whichever occurred first. Not reported whether podophyllin was applied by the patient or clinician; assumed to be the clinician | |||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Imiquimod 5% | Podophyllin 20% | p-value | ||
| Age (years), mean (with SD/SE if given) (range) | Not reported [reports that largest proportion of people were in the age range 26–35 years: 27 (45%) people] | ||||
| Duration of disease (months), median | 5 | ||||
| Site of AGWs, n (%) | Not reported | ||||
| Type of AGWs, n (%) | Not reported | ||||
| Total number of AGWs (mean number not reported) | 105 | 90 | Not reported | ||
| Total area of AGWs (mm2) (mean area not reported) | 2100 | 2500 | Not reported | ||
| Sex (M/F), n (%) | 54 males and 6 females randomised | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Complete clearance by the end of the treatment period (16 weeks with imiquimod 5% vs. 6 weeks with podophyllin 20%) | ||||
| Recurrence of AGWs | Recurrence at 6 months’ follow-up | ||||
| Time to complete clearance | Median duration in weeks until complete clearance | ||||
| Volume of wart clearance | Proportion of people with a decrease in area of lesions corresponding to < 50% or 50–99% of baseline lesion area at end of treatment | ||||
| AEs | Not defined. Data presented for erythema, oedema, scabbing, itching, systemic signs and symptoms, headache, flu-like symptoms, diarrhoea and tingling | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Imiquimod 5%, n/N | Podophyllin 20%, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at end of treatment | Treatment dependent | 16/30 | 14/30 | Not reported | |
| Recurrence of AGWs | 6 months after end of treatment | 5/16 | 9/14 | Not reported | |
| Volume of wart clearance (%) | |||||
| < 50 | End of treatment | 4/30 | 3/30 | Not reported | |
| 50–99 | End of treatment | 10/30 | 13/30 | Not reported | |
| AEs | |||||
| Erythema | End of treatment | 21/30 | 16/30 | Not reported | |
| Oedema | End of treatment | 4/30 | 17/30 | Not reported | |
| Scabbing, induration, ulceration, and vesiculation | End of treatment | 6/30 | 20/30 | Not reported | |
| Itching | End of treatment | 9/30 | 15/30 | Not reported | |
| Systemic signs and symptoms (not further defined) | End of treatment | 3/30 | 10/30 | Not reported | |
| Headache | End of treatment | 2/30 | 4/30 | Not reported | |
| Flu-like symptoms | End of treatment | 1/30 | 0/30 | Not reported | |
| Diarrhoea | End of treatment | 0/30 | 3/30 | Not reported | |
| Tingling | End of treatment | 0/30 | 3/30 | Not reported | |
| Continuous outcomes | |||||
| Time to complete clearance (weeks), median | 8 (n = 16) | 4.85 (n = 14) | Not reported | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessment a | Comments | ||
| Random sequence generation | ? | Described as a randomised study but details on method of randomisation not available | |||
| Allocation concealment | ? | No details provided | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | Information on masking not available | ||
| Blinding of outcomes assessment | ? | Unclear whether clinician assessing clearance was masked to treatment | |||
| Incomplete outcome data | ? | Withdrawals and loss to follow-up were not disclosed. Although analysis of AGW clearance at end of treatment is based on an ITT analysis, unclear whether there is an imbalance in withdrawals between the groups | |||
| Recurrence of AGW | Blinding (participants and personnel) | ? | Information on masking not available | ||
| Blinding of outcomes assessment | ? | Unclear whether clinician assessing recurrence was masked to treatment | |||
| Incomplete outcome data | ? | Withdrawals and loss to follow-up were not disclosed. Unclear whether there is an imbalance in withdrawals between the groups | |||
| Time to complete clearance | Blinding (participants and personnel) | ? | Information on masking not available | ||
| Blinding of outcomes assessment | ? | Unclear whether clinician assessing clearance was masked to treatment | |||
| Incomplete outcome data | ? | Withdrawals and loss to follow-up were not disclosed. Unclear whether there is an imbalance in withdrawals between the groups | |||
| Volume of wart clearance (e.g., proportion of patients with 50% clearance) | Blinding (participants and personnel) | ? | Information on masking not available | ||
| Blinding of outcomes assessment | ? | Unclear whether clinician assessing clearance was masked to treatment | |||
| Incomplete outcome data | ? | Withdrawals and loss to follow-up were not disclosed. Unclear whether there is an imbalance in withdrawals between the groups | |||
| AEs | Blinding (participants and personnel) | ? | Information on masking not available | ||
| Blinding of outcomes assessment | ? | Unclear whether clinician assessing AEs was masked to treatment | |||
| Incomplete outcome data | ? | Withdrawals and loss to follow-up were not disclosed. Unclear whether there is an imbalance in withdrawals between the groups | |||
| Overall rating of bias | ? | Reflects limited reporting in full publication | |||
| Section 6: Additional comments | |||||
| Additional comments | Reports that the most common location of AGWs was the penis in men and the vulva in women (50%) | ||||
| Item | Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reviewers’ names | Fatima Salih and Sam Barton | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID | Petersen 1995 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Genitourin Med 1995;71:391–2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Language of publication | English | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of report | Full publication | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 2: Study information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Location and number of sites | Study carried out at three dermato-venereological centres at the University of Copenhagen: Bispebjerg Hospital, Rigshospitalet and Gentofte Hospital | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trial sponsor | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Conflicts of interest | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patient enrolment | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trial design | RCT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trial duration | Initial treatment duration of 2–4 weeks, with final follow-up at 12 weeks | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Line of therapy | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inclusion criteria | Diagnosis of condyloma acuminatum; general good health; no topical or systemic antiviral or AGW treatment in the 4 weeks preceding the study | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclusion criteria | No additional exclusion criteria reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All outcomes reported in paper | Complete clearance; AEs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Subgroups evaluated | None reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stratification | None reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline measurement of disease | Not reported. It is stated that ‘clinical efficacy was determined by quantitative assessment of the treated AGWs. The two larger perpendicular dimensions of the AGWs were measured and the AGW area was defined as the product of these two measurements’ (p. 391). It is inferred that this measurement was also carried out at baseline | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.5% cream (patient applied) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Randomised, n | Number of people randomised to each group not reported. It is reported that 36 people were enrolled (in batches of 14, 12 and 10 at the three individual sites) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Number of lesions randomised | 133 | 136 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Withdrawals, n (%) | Not reported. It is stated that ‘Only one patient treated with podophyllotoxin cream 0.5% did not complete the trial for reasons not associated with therapy’ (p. 392); no further details available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment regimen | Podophyllotoxin 0.5% in alcoholic solution or podophyllotoxin 0.5% cream was self-applied twice daily for 3 days and the cycle repeated after a 4-day interval for a minimum of two and a maximum of four cycles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Duration/number of administered treatment | Not reported. It is stated that the number of applications of podophyllotoxin cream or solution was not statistically significantly different between the two treatment groups | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline patient characteristics | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.5% cream (patient applied) | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years), mean | 24 | 22 | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Duration of disease (months), mean | 2.5 | 3.5 | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Site of AGWs, n (number of AGWs at sites) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Penile | 122 | 126 | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Perianal | 5 | 7 | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Urethral | 4 | 3 | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of AGWs, n (%) | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Number of AGWs, median | 7 | 8 | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Area of AGWs (mm2), mean (SD) | 92.3 (7.5) | 87.7 (8.4) | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex (M/F), n (%) | 100% men | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Any previous treatment (%) | 30 (absolute numbers not reported) | 28 (absolute numbers not reported) | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ethnicity, n (%) | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 3: Outcomes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome | Definition | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at other time points | Not defined. Results for number of AGWs remaining are presented at various time points (extracted into additional comments); no data specified as results at ‘end of treatment’. Proportion of men with complete clearance (expressed as a percentage) is reported for 2 weeks after the end of treatment and for week 12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AEs | Safety was assessed by questioning and examining patients for local reactions indicating intolerance of the treatment. It is stated that tenderness, burning, pain, erythema, erosions and oedema were noticed and recorded as mild, moderate or severe (definitions not available) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 4: Data extraction form | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome | Time frame | Podophyllotoxin 0.5% solution (patient applied), n/N | Podophyllotoxin 0.5% cream (patient applied), n/N | Estimate of effect | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Dichotomous outcomes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at other time points | 2 weeks after end of treatment | 95% | Not reported | 63% | Not reported | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 weeks (end of study) | 63% | Not reported | 63% | Not reported | Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||
| AEs: mild to moderate | During treatment | 35% | Not reported | 40% | Not reported | Separate analyses of individual AEs identified no statistically significant differences between treatment groups | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 5: Clinical trial quality | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome | Risk of bias | Risk assessment a | Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Random sequence generation | ? | It is stated that men were randomly allocated to treatment. Details on method used to generate random sequence not available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allocation concealment | ? | Details on method used to conceal allocation not available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selective reporting | ✗ | Complete clearance at various time points is reported by number of AGWs cleared and percentage of men with complete clearance, with no absolute numbers reported. Data reported cannot be entered in a meta-analysis. In addition, data for clearance at end of treatment are not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGW clearance at various time points | Blinding (participants and personnel) | ? | Study is described as single blind. It is unclear who was masked to treatment (men participating, key study personnel or outcome assessor). Given the difference in the formulations of podophyllotoxin 0.5% (cream vs. solution), it is likely not to have been feasible to mask participants and key study personnel to treatment. It is unclear whether the outcome assessor was masked to treatment. If the outcome assessor was masked to treatment, the probability of masking been broken is unclear | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blinding of outcomes assessment | ? | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Incomplete outcome data | ? | Number of people randomised to each group and number of people evaluated in each group not reported. It is stated that only one person withdrew for a non-treatment-related reason; not reported from which group the person withdrew. Number of people withdrawing for treatment-related reasons or lost to follow-up not reported. Unclear whether there is an imbalance between groups in those excluded from analysis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AEs | Blinding (participants and personnel) | ? | Study is described as single blind. It is unclear who was masked to treatment (men participating, key study personnel or outcome assessor). Given the difference in the formulations of podophyllotoxin 0.5% (cream vs. solution), it might be difficult to mask participants to treatment, which could jeopardise masking of study personnel or outcome assessor | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blinding of outcomes assessment | ? | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Incomplete outcome data | ? | Number of people randomised to each group and number of people evaluated in each group not reported. It is stated that only one person withdrew for a non-treatment-related reason; not reported from which group the person withdrew. Number of people withdrawing for treatment-related reasons or lost to follow-up not reported. Unclear whether there is an imbalance between groups in those excluded from analysis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall rating of bias | ✗ | Reflects limited reporting of clinical effectiveness results in full publication | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Section 6: Additional comments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional comments | Week of studyPodophyllotoxin 0.5% solutionPodophyllotoxin 0.5% creamNumber of lesionsMean (SD) AGW area (mm2)Number of lesionsMean (SD) AGW area (mm2)013392.3 (7.5)13687.7 (8.4)18559.7 (6.7)a12276.0 (5.6)27247.0 (4.8)a10266.4 (8.7)33825.2 (3.2)b8052.2 (7.2)a5129.2 (0.8)b5133.6 (4.2)a654.5 (0.5)b4429.2 (3.2)b122921.5 (2.8)b3220.6 (2.7)bap < 0.05 (significant reduction from baseline).bp < 0.01 (significant reduction from baseline). | Week of study | Podophyllotoxin 0.5% solution | Podophyllotoxin 0.5% cream | Number of lesions | Mean (SD) AGW area (mm2) | Number of lesions | Mean (SD) AGW area (mm2) | 0 | 133 | 92.3 (7.5) | 136 | 87.7 (8.4) | 1 | 85 | 59.7 (6.7)a | 122 | 76.0 (5.6) | 2 | 72 | 47.0 (4.8)a | 102 | 66.4 (8.7) | 3 | 38 | 25.2 (3.2)b | 80 | 52.2 (7.2)a | 5 | 12 | 9.2 (0.8)b | 51 | 33.6 (4.2)a | 6 | 5 | 4.5 (0.5)b | 44 | 29.2 (3.2)b | 12 | 29 | 21.5 (2.8)b | 32 | 20.6 (2.7)b | ap < 0.05 (significant reduction from baseline).bp < 0.01 (significant reduction from baseline). | ||||||||||||
| Week of study | Podophyllotoxin 0.5% solution | Podophyllotoxin 0.5% cream | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Number of lesions | Mean (SD) AGW area (mm2) | Number of lesions | Mean (SD) AGW area (mm2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0 | 133 | 92.3 (7.5) | 136 | 87.7 (8.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | 85 | 59.7 (6.7)a | 122 | 76.0 (5.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 72 | 47.0 (4.8)a | 102 | 66.4 (8.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 38 | 25.2 (3.2)b | 80 | 52.2 (7.2)a | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 12 | 9.2 (0.8)b | 51 | 33.6 (4.2)a | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 5 | 4.5 (0.5)b | 44 | 29.2 (3.2)b | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | 29 | 21.5 (2.8)b | 32 | 20.6 (2.7)b | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| ap < 0.05 (significant reduction from baseline).bp < 0.01 (significant reduction from baseline). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Item | Details | |||||||
|---|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | |||||||
| Study ID | Sherrard 2007 | |||||||
| Study details | Int J STD AIDS 2007;18:365–8 | |||||||
| Language of publication | English | |||||||
| Type of report | Full publication | |||||||
| Section 2: Study information | ||||||||
| Location and number of sites | Study carried out in UK; number of sites involved is unclear | |||||||
| Trial sponsor | Not reported | |||||||
| Conflicts of interest | Not reported | |||||||
| Patient enrolment | People presenting with new or recurrent AGWs were eligible for enrolment; dates of enrolment not reported | |||||||
| Trial design | RCT (five treatment groups) | |||||||
| Trial duration | 8 weeks (maximum treatment period); no follow-up observation period | |||||||
| Line of therapy | Mixed | |||||||
| Inclusion criteria | People with new or recurrent AGWs that had not been treated in the preceding 3 months | |||||||
| Exclusion criteria | People were excluded if they were aged < 16 years; were pregnant women or women not using effective contraception; had atypical lesions where malignancy suspected; were unable to complete 8 weeks’ treatment at the department; refused a specific treatment modality; were immunosuppressed, including HIV infevtion | |||||||
| All outcomes reported in paper | Complete clearance | |||||||
| Subgroups evaluated | By number of AGWs (< 4 vs. 5–9 vs. ≥ 10) | |||||||
| Stratification | None reported | |||||||
| Baseline measurement of disease | Number and surface area of AGWs were assessed at baseline | |||||||
| Treatment | Podophyllin 25% (clinician applied) | TCAA | Cryotherapy | TCAA plus podophyllin 25% | Cryotherapy plus podophyllin 25% | |||
| Randomised, n | 79 | 88 | 81 | 85 | 76 | |||
| Withdrawals, n (%) | 23 (29.1) | 30 (34.1) | 15 (18.5) | 20 (23.5) | 17 (22.4) | |||
| Withdrawals for various reasons, n (%) | 4 (5.1) | 3 (3.4) | 4 (4.9) | 4 (4.7) | 4 (5.3) | |||
| Reasons for withdrawal were reported to be difficulty attending for follow up (seven people); wanting to try to conceive (two women); fear of cryotherapy machine (three people); reaction to podophyllin (one person); cryotherapy too painful to tolerate (one person); cryotherapy machine out of order (one person). Other reasons not reported. Reasons for withdrawal not reported by treatment group | ||||||||
| Loss to follow-up (defined as ‘did not attend’), n (%) | 19 (24.1) | 27 (30.7) | 11 (12.5) | 16 (18.8) | 13 (17.1) | |||
| Treatment regimen | Podophyllin 25% | TCAA (concentration not reported) | Cryotherapy | TCAA followed by podophyllin 25% | Cryotherapy followed by podophyllin 25% | |||
| Treatment regimens were applied on a weekly basis until AGW clearance or for a maximum of eight treatments, whichever occurred earlier. It is stated that a standard assessment and treatment proforma was used and that all doctors and nurses in the department were formally instructed in the use of each modality. When combined treatments were given, treatments were applied together to each wart, with initial application of the destructive method (i.e. cryotherapy or TCAA) followed by podophyllin 25% | ||||||||
| Median number of treatments to wart clearance (based on ‘on-treatment’ population) | 2.6 | 2.6 | 1.7 | 1.5 | 1.3 | |||
| Baseline patient characteristics | Podophyllin 25% (clinician applied) | TCAA | Cryotherapy | TCAA plus podophyllin 25% | Cryotherapy plus podophyllin 25% | p-value | ||
| Age (years), mean | 25.4 | 25.4 | 26.8 | 26.0 | 25.7 | Not reported | ||
| Duration of disease | Not reported | |||||||
| Site of AGWs, n (%) | Not reported | |||||||
| Type of AGWs, n (%) | Not reported | |||||||
| Number of AGWs, median | 4.2 | 4.8 | 4.1 | 4.7 | 4.4 | Not reported | ||
| Area of AGWs (mm2), mean | Not reported | |||||||
| Sex, n (%) | n calculated by review authors; proportion of men and women in each group presented as ratios in full publication | |||||||
| Male | 41 (51.9) | 46 (52.3) | 42 (51.8) | 46 (54.1) | 41 (53.9) | Not reported | ||
| Female | 38 (49.1) | 42 (47.7) | 39 (49.2) | 39 (45.9) | 35 (46.1) | Not reported | ||
| Any previous treatment, n (%) | Not reported | |||||||
| Ethnicity, n (%) | Not reported | |||||||
| Section 3: Outcomes | ||||||||
| Outcome | Definition | |||||||
| AGW clearance at completion of treatment | AGW clearance after a maximum of eight treatments. Analysis based on ITT population, in which defaulters were counted as treatment failures | |||||||
| AGW clearance at other time points | Percentage of AGWs cleared after two treatments (stated to represent ‘clinical situation’) | |||||||
| Section 4: Data extraction form | ||||||||
| Outcome | Time frame | Podophyllin 25% (clinician applied), n/N | TCAA, n/N | Cryotherapy, n/N | TCAA plus podophyllin 25%, n/N | Cryotherapy plus podophyllin 25%, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||||
| AGW clearance at completion of treatment | Up to 8 weeks | 46/79 | 49/88 | 61/81 | 63/85 | 59/76 | ||
| AGW clearance at other time points (number of events calculated by review authors) | After two treatments | 22/79 | 22/88 | 41/81 | 42/85 | 42/76 | Based on Figure 1; assumed reported percentages were based on proportion of people with complete clearance rather than percentage of AGWs cleared. p-value not reported | |
| Section 5: Clinical trial quality | ||||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | |||||
| Random sequence generation | ? | It is stated that people were randomly allocated to treatment. Details on method used to generate random sequence not available | ||||||
| Allocation concealment | ? | Details on method used to conceal allocation not available | ||||||
| Selective reporting | ? | Results are presented only for complete clearance. No information is reported for AEs. It is stated that the objective of the study was ‘to assess effectiveness of the five most commonly used clinic-based wart treatments’ (p. 365). Protocol is not available. Unclear whether there are prespecified outcomes that have not been reported | ||||||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias | ||||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | Level of masking is not described. Given the variation in the treatment types (topical vs. surgical vs. combination), it is likely not to have been feasible to mask participants and key study personnel to treatment. It is unclear whether the outcome assessor was masked to treatment. If the outcome assessor was masked to treatment, the probability of masking been broken is unclear | |||||
| Blinding of outcomes assessment | ? | |||||||
| Incomplete outcome data | ? | Number of withdrawals and loss to follow-up is reported. Reasons for withdrawal are not reported for all withdrawals. There is a slight variation across groups in the proportion of people who withdrew or were lost from each group (from 18.5% to 34.1%) and the impact of this variation on the effect estimate is unclear | ||||||
| Overall rating of bias | ? | Reflects limited reporting on methods in full publication | ||||||
| Section 6: Additional comments | ||||||||
| Additional comments | None | |||||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | ||||
| Study ID | Simmons 1981a | ||||
| Study details | Br J Vener Dis 1981;57:273–4 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study was carried out in the UK; number of sites and location unclear | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Not reported | ||||
| Trial design | RCT | ||||
| Trial duration | Up to two treatments at a 2-week interval plus a minimum follow-up for 3 months from start of the trial | ||||
| Line of therapy | Not reported | ||||
| Inclusion criteria | Men with AGWs who had given informed consent | ||||
| Exclusion criteria | Not reported | ||||
| All outcomes reported in paper | Complete clearance | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Not reported | ||||
| Treatment | Cryotherapy | Electrocautery | |||
| Randomised, n | 24 | 18 | |||
| Withdrawals, n (%) | 8 (33.3%) (at 3 months’ follow-up); reasons for withdrawal not reported | 7 (38.9%) (at 3 months’ follow-up); reasons for withdrawal not reported | |||
| Treatment regimen | Cryotherapy with nitrous oxide (no local anaesthetic). The cryoprobe produced ice balls 1–2 mm larger than the diameter of the AGW to be treated (no electrolyte or lubricant applied). Treatment could be repeated once, 2 weeks later (based on assessment by a clinician who was masked to treatment) | Electrocautery with 2% lidocaine as a local anaesthetic. No further details reported. Treatment could be repeated once, 2 weeks later (based on assessment by a clinician who was masked to treatment) | |||
| Duration/number of administered treatment | Mean number of treatments required for ‘cure’: 2.6 | Mean number of treatments required for ‘cure’: 1.4 | |||
| Baseline patient characteristics | Cryotherapy | Electrocautery | p-value | ||
| Age (years), mean | Not reported | Reported to be comparable between treatment groups | |||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | Not reported | ||||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | Not reported | Reported to be comparable between treatment groups | |||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | 100% men | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | Reported to be comparable between treatment groups | |||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at other time points | Complete clearance at 3 months’ follow-up | ||||
| AEs | Reporting of AEs was minimal. It was stated that ‘No ulceration occurred after cryotherapy and no patient required local anaesthesia’ (p. 274). It is also reported that no patient complained of pain | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Cryotherapy, n/N | Electrocautery, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at other time points | 3 months | 10/16 | 10/11 | p > 0.05 | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessment a | Comments | ||
| Random sequence generation | ✓ | It is stated that men were randomly allocated to treatment using a random number table | |||
| Allocation concealment | ? | Details on method used to conceal allocation not available | |||
| Selective reporting | ✗ | Complete clearance at end of treatment is not reported, although results are reported for clearance at 3 months’ follow-up. Limited information available on AEs | |||
| ‘Other bias’ | ? | It is stated that treatment groups were comparable in terms of age, country of origin, marital state, sexual orientation, previous STDs and number of warts present at baseline, but no baseline characteristics are reported. Insufficient information reported to evaluate presence of other sources of bias | |||
| AGW clearance | Blinding (participants and personnel) | ✗ | The trial is described as single blind and it is reported that the clinician assessing clearance was masked to treatment. Given that the treatments evaluated are both surgical, it is likely that masking of personnel and participants was not feasible, but this could introduce bias. It is stated that the assessor evaluated participants after the first round of treatment. It is unclear whether the same assessor evaluated clearance at 3 months and also whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | The number of people not evaluated at 3 months is reported, but reasons for withdrawal are not available. It is unclear whether there is an imbalance between the groups in reasons for not returning for evaluation at 3 months | |||
| Overall rating of bias | ✗ | Reflects limited reporting of outcomes and lack of masking of participants and study personnel | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | ||||
| Study ID | Simmons 1981b | ||||
| Study details | Br J Vener Dis 1981;57:208–9 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study carried out in the UK at two clinical sites (Departments of Genital Medicine at St Bartholomew’s Hospital and the Prince of Wales Hospital, London) | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Men were recruited from those with AGWs attending the Departments of Genital Medicine at the study sites | ||||
| Trial design | RCT | ||||
| Trial duration | Initial treatment period of 6 weeks with subsequent follow-up to 3 months after the start of treatment | ||||
| Line of therapy | Not reported | ||||
| Inclusion criteria | Men with AGWs who had not received treatment for AGWs in the preceding 3 months | ||||
| Exclusion criteria | No additional criteria reported | ||||
| All outcomes reported in paper | Complete clearance | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Not reported | ||||
| Treatment | Podophyllin 25% (clinician applied) | Podophyllin 10% (clinician applied) | |||
| Randomised, n | 140 men were randomised; number of men randomised to each group not reported. Results are based on the men attending for assessment at 3 months’ follow-up (55 in the podophyllin 25% group vs. 54 in the podophyllin 10% group) | ||||
| Withdrawals, n (%) | 31 (22.1%) men withdrew before follow-up at 3 months. Reasons for withdrawal and number of withdrawals from individual treatment groups not reported | ||||
| Treatment regimen | Podophyllin 25% and 10% solutions were applied with cotton-wool swabs by one doctor once weekly for 6 weeks. External AGWs were washed 4, 6, 8, 12 and 24 hours after application at the first through to fifth attendance for treatment | ||||
| Duration/number of administered treatment | Number of mean treatments required for complete clearance: 3.7 | Number of mean treatments required for complete clearance: 4.3 | |||
| Baseline patient characteristics | Podophyllin 25% (clinician applied) | Podophyllin 10% (clinician applied) | p-value | ||
| Age (years), mean | Not reported | Reported to be statistically comparable between treatment groups | |||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | Not reported | ||||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | Not reported | Reported to be statistically comparable between treatment groups | |||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | 100% men | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | Reported to be statistically comparable between treatment groups | |||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at other time points | Complete clearance at 3 months’ follow-up | ||||
| Volume of wart clearance | Number of patients with ≤ 10% of original AGW volume at 6 weeks | ||||
| AEs | Reporting of AEs was minimal. It was stated that ‘neither hypersensitivity nor chemical ulceration occurred with either concentration of podophyllin’ (p. 209) | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllin 25% (clinician applied), n/N | Podophyllin 10% (clinician applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at other time points | 3 months | 12/55 | 12/54 | Not reported | |
| Volume of wart clearance (proportion of people with ≤ 10% of original AGW volume remaining, i.e. ≥ 90% reduction in AGW volume) | 6 weeks | 17/55 | 16/54 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ✓ | It is stated that men were randomly allocated to treatment using a random number table | |||
| Allocation concealment | ? | Podophyllin solutions were prepared in one pharmacy and dispensed in stock bottles labelled A and B. The pharmacy alone held the code key, which was broken only on completion of the trial. It is unclear whether the bottles were sequentially numbered or of identical appearance | |||
| Selective reporting | ✗ | Complete clearance at end of treatment is not reported, although results are reported for clearance at 3 months’ follow-up. Limited information available on AEs | |||
| ‘Other bias’ | ? | It is stated that treatment groups were comparable in terms of age, country of origin, marital state, sexual orientation, previous STDs and number of warts present at baseline, but no baseline characteristics are reported. Insufficient information reported to evaluate presence of other sources of bias | |||
| AGW clearance | Blinding (participants and personnel) | ? | The study is described as double blind but it is unclear who was masked to treatment. From the full publication it could be inferred that the patients and treating clinician are masked. Only one doctor applied the podophyllin solution in both groups. It is unclear whether the treating clinician also evaluated clinical outcomes and, if not, whether the outcome assessor was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | The number of people randomised to each group is not reported. The number of people not evaluated at 3 months is reported, but reasons for withdrawal are not available. It is unclear whether there is an imbalance between the groups in reasons for not returning for evaluation at 3 months | |||
| Volume of wart clearance (e.g. proportion of patients with 50% clearance) | Blinding (participants and personnel) | ? | The study is described as double blind but it is unclear who was masked to treatment. From the full publication it could be inferred that the patients and treating clinician are masked. Only one doctor applied the podophyllin solution in both groups. It is unclear whether the treating clinician also evaluated clinical outcomes and, if not, whether the outcome assessor was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | The number of people randomised to each group is not reported. The number of people included in the analysis of volume of wart clearance at 6 weeks is not reported. It is unclear whether there is an imbalance between the groups in the number of people withdrawn or lost to follow-up at 6 weeks | |||
| Overall rating of bias | ✗ | Reflects the limited reporting of clinical outcomes in the full publication | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | ||||
| Study ID | Snoeck 2001 | ||||
| Study details | Clin Infect Dis 2001;33:597–602 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study carried out at four centres in Belgium: Erasme Hospital, Brussels; Saint-Luc Hospital, Brussels; St Rafael Hospital, Leuven; and Etterbeek-Ixelles Hospital Centre | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Participants were enrolled from people attending the listed centres. Dates of enrolment not reported | ||||
| Trial design | RCT | ||||
| Trial duration | Up to 12 weeks’ treatment with subsequent follow-up for 4 weeks after completion of treatment or removal from the study. Those with a complete response were followed up for 6 months (unclear whether this is additional to the 4-week observation period or whether those with a complete response were followed up for a further 5 months to give a total of 6 months) | ||||
| Line of therapy | Mixed | ||||
| Inclusion criteria | External biopsy-proven genital AGWs, perianal AGWs or both; use of an adequate means of birth control during the study (partners were asked to use barrier contraception) | ||||
| Exclusion criteria | Any AGW > 10 mm in height or > 20 AGWs; presence of any other dermatological condition in the anogenital area; history of significant renal, hepatic or hematological abnormalities or of substance abuse within the previous 12 months; serum creatinine level > 2 mg/dl; women with current evidence of vulvar or cervical intraepithelial neoplasia grade II or III; presence of internal warts requiring immediate treatment; seropositive for HIV or a history of underlying immunodeficiency; treatment within the previous 4 weeks with any drug of known or potential anti-HPV activity; treatment within the previous 8 weeks with interferon; women who were pregnant, lactating or planning to become pregnant | ||||
| All outcomes reported in paper | Complete clearance (defined as complete response); volume of AGW clearance [partial response (≥ 50% decrease in total surface area) and no change (< 50% decrease or < 25% increase in total surface area)]; AEs | ||||
| Subgroups evaluated | Total lesion surface area (< 50 mm2 vs. ≥ 50 mm2) | ||||
| Stratification | Stratified by total lesion surface area at screening (< 50 mm2 vs. ≥ 50 mm2) | ||||
| Baseline measurement of disease | Baseline evaluation not described. Based on reporting, it can be inferred that the area and number of AGWs were recorded at baseline | ||||
| Treatment | Cidofovir 1% | Placebo | |||
| Randomised, n | 19 | 11 | |||
| It is reported that a total of 31 adult patients were included in the study. One person was determined to be ineligible for the study after 1 week of treatment because of a lack of biopsy confirmation of condylomata. Data from this person were excluded from further analysis and the patient was replaced by a newly randomised patient. However, presented results are based on a total of 30 people rather than 31 | |||||
| Withdrawals, n (%) | 4 (21.1) | Not reported | |||
| Lost to follow-up | 2 (10.5) | – | |||
| Treatment discontinuation | 2 (10.5) | – | |||
| Treatment regimen | Cidofovir 1% gel or placebo was applied once daily at bedtime for 5 consecutive days every other week for a maximum of six cycles (a cycle was defined as 1 week of gel application followed by 1 week of observation). All external AGWs, including new AGWs that developed after the baseline assessment, were treated. The treated areas, particularly those occluded by skin folds or foreskin, were washed the next morning to remove residual gel. The first administration was made under the supervision of a doctor. Later applications were self-administered on an outpatient basis. The gel was applied with a cotton-tipped swab or a rubber glove in a thin layer sufficient to cover the wart area and to extend beyond the edge of each AGW by a margin of 5 mm. If a complete response was achieved any time during the 12 weeks of treatment, the patient continued treatment through one additional 2-week cycle and then proceeded to follow-up | ||||
| Duration/number of administered treatment | For people with a complete response in the cidofovir group, the median duration of therapy was 43 days | ||||
| Baseline patient characteristics | Cidofovir 1% | Placebo | p-value | ||
| Age (years), median (range) | 27 (20–51) | 27 (21–41) | Reported to be similar in each group | ||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | Not reported | ||||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, median (range) | 9 (1–18) | 7 (2–20) | Reported to be similar in each group | ||
| Area of AGWs (mm2), median (range) | 56.4 (8.4–1259.2) | 55.7 (15.3–1756.1) | Reported to be similar in each group | ||
| Sex, n (%) | |||||
| Male | 8 (42.1) | 5 (45.4) | Not reported | ||
| Female | 11 (57.9) | 6 (54.5) | Not reported | ||
| Any previous treatment, n (%) | 8 (42.1) | 5 (45.4) | Reported to be similar in each group | ||
| Ethnicity, n (%) | |||||
| White | 15 (78.9) | 10 (90.9) | Not reported | ||
| African | 1 (5.3) | 1 (9.1) | |||
| Asian | 1 (5.3) | 0 (0) | |||
| Other | 2 (10.5) | 0 (0) | |||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Complete response by 12 weeks, where ‘complete response’ was defined as total healing. New lesions appearing after the baseline assessment were treated and quantified but were not included as part of the evaluation of complete response | ||||
| Recurrence of AGWs | Recurrence not defined; assessed in those achieving a complete response | ||||
| Volume of wart clearance | Definitions of response, other than complete response, were as follows: partial response: ≥ 50% decrease in total surface area; no change: < 50% decrease or < 25% increase in total surface area; progression: > 25% increase in total surface area | ||||
| Appearance of new warts during treatment | It is stated that new lesions appearing after the baseline assessment were treated and quantified. The number of new lesions appearing during treatment in each group was not reported | ||||
| AEs | AEs were not defined. Data were reported for pain, pruritus and rash at the application site and for erosion or ulceration | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Cidofovir 1%, n/N | Placebo, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 12 weeks | 9/19 | 0/11 | p = 0.006 | |
| Recurrence of AGWs | Median follow-up period of 168 days (range 77–217 days) | 1/9 | 0/0 | Not reported | |
| Volume of wart clearance | |||||
| ≥ 50% decrease in total surface area | 12 weeks | 7/19 | 2/11 | p = 0.001 | |
| < 50% decrease or < 25% increase in total surface area | 12 weeks | 3/19 | 4/11 | Not reported | |
| AEs | |||||
| Pain, pruritus and rash at the application site | 12 weeks | 13/19 | 7/11 | p = 1.0 | |
| Erosion or ulceration | 12 weeks | 6/19 | 5/11 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that ‘randomisation was performed by permuted blocks of size 3 within strata. Each investigator was assigned blocks’ (p. 598). Methods used to generate sequence not available | |||
| Allocation concealment | ? | Details on method used to conceal allocation not available | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information to assess whether an important risk of bias exists | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | The study is described as double blind but it is unclear who was masked to treatment. From the full publication, it could be inferred that the patients and treating clinician are masked. It is unclear whether the treating clinician also evaluated clinical outcomes and, if not, whether the outcome assessor was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | Analysis is based on 30 people. A total of 31 people were randomised. One person withdrew and was replaced by a newly randomised person. Withdrawals from the cidofovir group with accompanying reasons are reported but it is unclear whether anyone was lost to follow-up or withdrew from the placebo group | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The study is described as double blind but it is unclear who was masked to treatment. From the full publication, it could be inferred that the patients and treating clinician are masked. It is unclear whether the treating clinician also evaluated clinical outcomes and, if not, whether the outcome assessor was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | Analysis of recurrence is based on all people with complete clearance but it is unclear whether anyone was lost to follow-up during the observation period | |||
| Volume of wart clearance (e.g. proportion of patients with 50% clearance) | Blinding (participants and personnel) | ? | The study is described as double blind but it is unclear who was masked to treatment. From the full publication, it could be inferred that the patients and treating clinician are masked. It is unclear whether the treating clinician also evaluated clinical outcomes and, if not, whether the outcome assessor was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | Analysis is based on 30 people. A total of 31 people were randomised. One person withdrew and was replaced by a newly randomised person. Withdrawals from the cidofovir group with accompanying reasons are reported but is unclear whether anyone was lost to follow-up or withdrew from the placebo group | |||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blind but it is unclear who was masked to treatment. From the full publication, it could be inferred that the patients and treating clinician are masked. It is unclear whether the treating clinician also evaluated clinical outcomes and, if not, whether the outcome assessor was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | Analysis is based on 30 people. A total of 31 people were randomised. One person withdrew and was replaced by a newly randomised person. Withdrawals from the cidofovir group with accompanying reasons are reported but is unclear whether anyone was lost to follow-up or withdrew from the placebo group | |||
| Overall rating of bias | ? | Reflects limited reporting on methods in full publication | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Fatima Salih and Sam Barton | ||||
| Study ID | Stefanaki 2008 | ||||
| Study details | Int J STD AIDS 2008;19:441–4 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study carried out at one centre in Greece (the Sexually Transmitted Disease Unit of Andreas Sygros University Hospital for Skin and Sexually Transmitted Diseases) | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Not reported | ||||
| Trial design | RCT | ||||
| Trial duration | Initial treatment period of 3 months with subsequent follow-up at 6 and 12 months | ||||
| Line of therapy | First | ||||
| Inclusion criteria | Male gender; immunocompetency; diagnosis of external genital or perianal AGWs; no previous treatment for AGWs | ||||
| Exclusion criteria | Presence of Bowenoid papulosis; presence of a severe medical condition [haematological, hepatic (hepatitis B or C), neurological, renal, endocrine, collagen and gastrointestinal]; drug or alcohol dependency; HIV infection or syphilis; recent treatment with corticosteroids, cytotoxic drugs and interferon | ||||
| All outcomes reported in paper | Complete clearance; recurrence; AEs | ||||
| Subgroups evaluated | Time to cure was evaluated by baseline number of lesions and total AGW area but results were not presented separately by treatment group | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | At the initial visit prior to treatment, AGWs were photographed and measured in cm2 and the exact number of AGWs and their location, morphology, colour and duration were recorded | ||||
| Treatment | Cryotherapy (liquid nitrogen) | Imiquimod 5% (patient applied) | |||
| Randomised, n | 70 | 50 | |||
| Number of men randomised calculated by review authors based on number of men evaluated and number of withdrawals reported for each group | |||||
| Withdrawals, n (%) | 25 (35.7) | 15 (30.0) | |||
| Reasons for withdrawal not reported. It is stated that men were ‘either lost from follow-up or did not provide analysable data’ (p. 442); no further details available | |||||
| Treatment regimen | Cryotherapy with liquid nitrogen was applied once every 3 weeks for three consecutive sessions or until complete clearance of AGWs, whichever occurred earlier. Using a spray gun, AGWs were frozen for 10–20 seconds or until the AGWs and a small margin of surrounding normal skin were frozen. Larger lesions underwent two freeze cycles | Men applied imiquimod 5% cream ‘3 times a week for 6–10 hours’ (p. 441). Treatment was repeated weekly for 3 months or until complete clearance of AGWs, whichever occurred earlier. Men were allowed to take a rest period of 7–10 days if they developed severe local reactions | |||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Cryotherapy (liquid nitrogen) | Imiquimod 5% (patient applied) | p-value | ||
| Note: Baseline characteristics are based on the 80 men evaluated (45 in the cryotherapy group vs. 35 in the imiquimod 5% group) not the 120 men randomised | |||||
| Age (years), mean (unclear whether SD or SE) | 30.7 (12.2) | 31.8 (10.8) | p = 0.859 | ||
| Duration of disease (months), n (%) | |||||
| < 3 | 26 (57.8) | 28 (79.5) | p = 0.03 | ||
| 3–6 | 3 (6.6) | 5 (14.3) | |||
| 7–12 | 9 (20) | 1 (2.9) | |||
| > 12 | 6 (13.3) | 1 (2.9) | |||
| Site of AGWs, n (%)a | |||||
| 1 | 8 (17.8) | 7 (20) | p = 0.101 | ||
| 2 | 11 (24.4) | 13 (37.1) | |||
| 3 | 3 (6.6) | 7 (20) | |||
| 1 + 2 | 20 (44.4) | 6 (17.1) | |||
| 1 + 3 | 1 (2.2) | 0 (0) | |||
| 2 + 3 | 0 (0) | 1 (2.9) | |||
| 1 + 2 + 3 | 2 (4.4) | 1 (2.9) | |||
| Type of AGWs, n (%) | |||||
| Pedunculated | 9 (20) | 20 (57.1) | p = 0.001 | ||
| Papular | 18 (40) | 3 (8.6) | |||
| Flat | 0 (0) | 1 (2.9) | |||
| Pedunculated and papular | 18 (40) | 11 (31.4) | |||
| Number of AGWs, n (%) | |||||
| 1 | 4 (8.9) | 5 (14.3) | p = 0.533 | ||
| 2–5 | 13 (28.9) | 6 (17.1) | |||
| 6–10 | 12 (26.7) | 9 (25.7) | |||
| 11–20 | 8 (17.8) | 5 (14.3) | |||
| > 20 | 8 (17.8) | 10 (28.6) | |||
| Total area of AGWs (mm2) | |||||
| < 0.5 | 1 (2.2) | 6 (17.1) | p = 0.067 | ||
| 0.6–1 | 9 (20) | 11 (31.4) | |||
| 1.1–2 | 9 (20) | 4 (11.4) | |||
| 2.1–3 | 8 (17.8) | 4 (11.4) | |||
| 3.1–4 | 4 (8.9) | 5 (14.3) | |||
| > 4 | 14 (31.1) | 5 (14.3) | |||
| Sex (M/F), n (%) | 100% men | ||||
| Any previous treatment (%) | 0 (men who had received previous treatment were excluded) | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Not defined. Results are presented for complete clearance at 3 months, which is the end of treatment | ||||
| AGW clearance at other time points | Complete clearance at 1 and 2 months during treatment and at 6 months after treatment | ||||
| Recurrence of AGWs | Results for recurrence reported at 12 months. Absolute numbers are not reported and it is unclear whether results are based on all men evaluated (80 men) or just those with complete clearance at any point (i.e. more men had complete clearance at 6 months) | ||||
| AEs | Not defined. Local or adverse systemic reactions were recorded (erythema, oedema, erosions, ulceration, fever and pain). AEs were graded as mild, moderate or severe; definitions of mild, moderate and severe not available | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Cryotherapy (liquid nitrogen), n/N | Imiquimod 5% (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 12 weeks | 29/45 | 24/35 | Not reported | |
| AGW clearance at other time points (cumulative) | 4 weeks | 9/45 | 10/35 | Not reported | |
| 8 weeks | 19/45 | 20/35 | |||
| 6 months | 39/45 | 24/35 | |||
| Recurrence of AGWs (%) | 12 months | 59 | 41 | p = 0.138 | |
| AEs | |||||
| Number of men experiencing at least one AE | 12 weeks | 45/45 | 19/35 | p = 0.034 | |
| Pain | 12 weeks | 38/45 | 9/35 | p < 0.0001 | |
| Painful ulcerations | 12 weeks | 7/45 | 8/35 | Not reported | |
| Fever | 12 weeks | 0/45 | 1/35 | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmentb | Comments | ||
| Random sequence generation | ? | It is stated that men were randomly allocated to treatment. Details on method used to generate random sequence not available | |||
| Allocation concealment | ? | Details on method used to conceal allocation not available | |||
| Selective reporting | ✗ | Insufficient information is reported on recurrence to facilitate entry into a meta-analysis | |||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ✗ | Described as an open-label trial. Given the variation in settings in which the treatments were applied (home vs. clinic), it might be impractical to mask to treatment allocation. AGW clearance is a subjective outcome that could potentially be influenced by patients and key study personnel. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||
| Blinding of outcomes assessment | ✗ | ||||
| Incomplete outcome data | ? | The number of men randomised to each group is not reported. The analyses reported are based on men for whom evaluable data were available. The number of men excluded from the analysis is reported, but reasons for withdrawal are not reported. There might be an imbalance across the groups in reasons for withdrawal that could influence the effect estimate | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ✗ | Described as an open-label trial. Given the variation in settings in which the treatments were applied (home vs. clinic), it might be impractical to mask to treatment allocation. AGW recurrence is a subjective outcome that could potentially be influenced by patients and key study personnel. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||
| Blinding of outcomes assessment | ✗ | ||||
| Incomplete outcome data | ✗ | Data are presented as percentages and it is unclear whether the analysis is based on all men randomised or those with complete clearance of AGWs at some point during the study | |||
| AEs | Blinding (participants and personnel) | ✗ | Described as an open-label trial. Given the variation in settings in which the treatments were applied (home vs. clinic), it might be impractical to mask to treatment allocation. Evaluation of pain and local AEs is subjective and could potentially be influenced by patients and key study personnel. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||
| Blinding of outcomes assessment | ✗ | ||||
| Incomplete outcome data | ? | The number of men randomised to each group is not reported. The analyses reported are based on men for whom evaluable data were available. The number of men excluded from the analysis is reported, but reasons for withdrawal are not reported. There might be an imbalance across the groups in reasons for withdrawal that could influence the effect estimate | |||
| Overall rating of bias | ✗ | Reflects open-label nature of trial and lack of reporting of number of men experiencing recurrence | |||
| Section 6: Additional comments | |||||
| Additional comments |
|
||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Jacoby Patterson and Victoria Wakefield | |||||
| Study ID | Stone 1990 | |||||
| Study details | Genitourin Med 1990;66:16–19 | |||||
| Language of publication | English | |||||
| Type of report | Full publication | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Study carried out at one centre (number of sites not reported) in the USA | |||||
| Trial sponsor | Not reported | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | People were recruited between September 1984 and May 1986. Anyone with external AGWs not treated in the month before recruitment was eligible for the trial | |||||
| Trial design | RCT | |||||
| Trial duration | Initial treatment period of up to 6 weeks with follow-up after 3 months for those achieving complete clearance. People were asked to return sooner if AGWs reappeared | |||||
| Line of therapy | Not reported | |||||
| Inclusion criteria | People with external AGWs that had not been treated in the month preceding trial entry | |||||
| Exclusion criteria | Pregnancy; age < 18 years; allergy to podophyllin, tincture of benzoin compound or lidocaine; presence of obvious internal (cervical, vaginal or anal) warts or Buschke–Lowenstein lesions; contraindications for electrosurgical procedures (such as presence of a cardiac pacemaker) | |||||
| All outcomes reported in paper | Complete clearance at various time points; recurrence; post-treatment pain; local infection | |||||
| Subgroups evaluated | Gender, male sexual preference (heterosexual vs. gay/bisexual), volume of AGWs (≤ 50 mm3 vs. > 50 mm3), duration of AGWs (≤ 30 days vs. > 30 days) and site (anal vs. genital) | |||||
| Stratification | None reported | |||||
| Baseline measurement of disease | Not reported | |||||
| Treatment | Podophyllin 25% (clinician applied) | Cryotherapy | Electrofulguration | |||
| Randomised, n | 144 | 154 | 152 | |||
| Withdrawals, n (%) | Total number of withdrawals for individual treatment groups and for the overall trial population not reported. Information is available on the number of patients lost to follow-up and the number not completing the therapy as per the protocol. It is unclear whether there were any other withdrawals | |||||
| Did not complete therapy (non-compliant) | 81 (56.3) | 68 (44.2) | 64 (42.1) | |||
| Lost to follow-up (defined as non-compliant patients) | 10 (6.9) | 26 (16.9) | 37 (24.3) | |||
| Treatment regimen | Podophyllin 25% in tincture of benzoin compound was applied using a cotton swab. Patients were instructed to wash off the podophyllin 25% 2 hours after the first treatment. If the preceding application did not produce excessive irritation, the interval between application and washing was extended by 2 hours with each successive treatment to a maximum of 12 hours. A maximum surface area of 4 cm2 was treated in any visit | Cryotherapy was administered using liquid nitrogen applied with a tapered cotton pledget on the end of a wooden applicator stick. The pledget was applied long enough to freeze the AGW and a 1-mm margin of surrounding skin, with each AGW being frozen once. A maximum surface area of 4 cm2 was treated in any visit | Electrofulguration is a modification of electrodessication in which the electrode does not touch the tissue. After anaesthetising the area to be treated with subcutaneous 1% lidocaine, electrofulguration was performed with an electrosurgical apparatus using a point electrode and standard settings for electrodessication. A maximum surface area of 4 cm2 was treated in any visit | |||
| All treatments were administered weekly until complete clearance of AGWs or for a maximum of 6 weeks, whichever occurred earlier | ||||||
| Duration/number of administered treatment | Mean number of treatments required to achieve complete clearance: 3.4 | Mean number of treatments required to achieve complete clearance: 3.2 | Mean number of treatments required to achieve complete clearance: 1.3 | |||
| Baseline patient characteristics | Podophyllin 25% (clinician applied) | Cryotherapy | Electrofulguration | p-value | ||
| Note: Baseline characteristics are based on those patients who were classed as ‘compliant’, that is, those who completed treatment and were followed up at the end of treatment | ||||||
| n | 53 | 60 | 51 | |||
| Age (years), mean | 26.7 | 27.3 | 26.9 | Difference across groups reported to be not significant | ||
| Duration of disease | Not reported | Reported to be similar across groups | ||||
| Site of AGWs, n (%) | Not reported | |||||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, mean | Not reported | |||||
| Area of AGWs (mm2), mean | Not reported | Reported to be similar across groups | ||||
| Sex, n (%) | ||||||
| Male | 38 (72) | 43 (72) | 38 (75) | Difference across groups reported to be not significant | ||
| Female | 15 (28) | 17 (28) | 13 (25) | |||
| Any previous treatment, n (%) | Not reported | |||||
| Ethnicity, n (%) | ||||||
| White | 33 (62) | 31 (52) | 29 (57) | Difference across groups reported to be not significant | ||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | AGW free within six treatments | |||||
| Recurrence of AGWs | Recurrence at 3 months | |||||
| AEs | AEs were not defined. Occurrence of local infection and post-treatment pain were reported. It is stated that similar proportions (17%) of people in each treatment group experienced post-treatment pain (absolute event numbers not reported) | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Podophyllin 25% (clinician applied), n/N | Cryotherapy, n/N | Electrofulguration, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | 6 weeks | 26/63 | 68/86 | 83/88 | Electrotherapy was more effective than cryotherapy (p = 0.003), which in turn was more effective than podophyllin 25% (p < 0.0001) | |
| Recurrence of AGWs | 3 months | 7/16 | 9/42 | 10/46 | p = 0.17 | |
| AEs: local infection | 6 weeks | 0/63 | 1/86 | 0/88 | Note: denominator not reported in publication; assumed to be same as that used in the efficacy analyses | |
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessment a | Comments | |||
| Random sequence generation | ✓ | It is stated that randomisation was carried out using a computer-generated randomisation schedule | ||||
| Allocation concealment | ? | Details on method used to conceal allocation not available | ||||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | ||||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | ||||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible. It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Blinding of outcomes assessment | ? | |||||
| Incomplete outcome data | ✗ | The total number of withdrawals is not reported. The numbers of people lost to follow-up (7–24%) and withdrawing from treatment are reported (42–56%). The high number of people withdrawing from treatment and the noted imbalance across the groups in loss to follow-up and withdrawal is likely to introduce clinically relevant bias in the estimate of effect | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible. It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Blinding of outcomes assessment | ? | |||||
| Incomplete outcome data | ✗ | The total number of withdrawals is not reported. The numbers of people lost to follow-up (7–24%) and withdrawing from treatment are reported (42–56%). Follow-up of those who achieved complete clearance was also low (55–62%). The high number of people withdrawing from treatment and the noted imbalance across the groups in loss to follow-up and withdrawal is likely to introduce clinically relevant bias in the estimate of effect | ||||
| AEs | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible. It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Blinding of outcomes assessment | ? | |||||
| Incomplete outcome data | ✗ | The total number of withdrawals is not reported. The numbers of people lost to follow-up (7–24%) and withdrawing from treatment are reported (42–56%). The high number of people withdrawing from treatment and the noted imbalance across the groups in loss to follow-up and withdrawal is likely to introduce clinically relevant bias in the estimate of effect | ||||
| Overall rating of bias | ✗ | Reflects the imbalance across the groups in the combined high rate of withdrawal and loss to follow-up | ||||
| Section 6: Additional comments | ||||||
| Additional comments |
|
|||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Jacoby Patterson and Victoria Wakefield | |||||
| Study ID | Strand 1995 | |||||
| Study details | Genitourin Med 1995;71:387–90 | |||||
| Language of publication | English | |||||
| Type of report | Full publication | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Study carried out at three sites (two in Sweden and one in France) | |||||
| Trial sponsor | Not reported | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | Enrolment occurred at the STD clinics of the Akademiska Hospital, Uppsala and the Military Hospital, Enköping, Sweden, and the Institut Alfred Fournier, Paris, France. Men were enrolled between January 1990 and January 1991. No further details on recruitment reported | |||||
| Trial design | RCT (three arms) | |||||
| Trial duration | Initial treatment period of up to 4 weeks with subsequent follow-up at 16 weeks after entry into the study | |||||
| Line of therapy | Mixed; proportion of men having received previous treatment not reported | |||||
| Inclusion criteria | Men with genital AGWs (acuminata or papular) | |||||
| Exclusion criteria | HIV-positive status; age < 18 years; duration of present lesion > 3 months; presence of intra-anal or anal warts; receipt of treatment within the 3 months preceding the study | |||||
| All outcomes reported in paper | Complete clearance at end of treatment and other time points; relapse; AEs | |||||
| Subgroups evaluated | Site of lesion | |||||
| Stratification | None reported | |||||
| Baseline measurement of disease | Location and number of warts | |||||
| Treatment | Podophyllotoxin 0.15% cream (patient applied) | Podophyllotoxin 0.3% cream (patient applied) | Podophyllotoxin 0.5% solution (patient applied) | |||
| Randomised, n | 30 | 31 | 29 | |||
| Withdrawals, n (%) | Overall, 11 men were lost to follow up at 16 weeks; loss to follow-up not reported by individual treatment group | |||||
| Treatment regimen | Men self-applied podophyllotoxin 0.15% or 0.3% cream using a finger and podophyllotoxin 0.5% solution using an applicator. All treatments were applied twice a day for 3 consecutive days followed by a 4-day no treatment period (defined as a treatment cycle). The cream preparations of podophyllotoxin consisted of an oil phase blended into a purified water phase. The podophyllotoxin 0.5% solution contained 5.0 mg of podophyllotoxin. Men were treated until complete clearance or for a maximum of 4 weeks (four treatment cycles), whichever occurred earlier. Non-achievement of complete clearance after 4 treatment cycles was regarded as a treatment failure | |||||
| Duration/number of administered | Not reported | |||||
| Baseline patient characteristics | Podophyllotoxin 0.15% cream (patient applied) | Podophyllotoxin 0.3% cream (patient applied) | Podophyllotoxin 0.5% solution (patient applied) | p-value | ||
| Age (years), mean (range) | 25.7 (18–44) | 27.2 (20–48) | 27.5 (20–43) | Not reported | ||
| Duration of disease (months), mean (range) | 2.5 (0.4–5.5) | 2.5 (0.7–5.6) | 2.6 (0.6–4.9) | Not reported | ||
| Site of AGWs, number of lesions | ||||||
| Prepuce | 129 | 137 | 139 | Not reported | ||
| Glans | 43 | 61 | 73 | Not reported | ||
| Shaft | 34 | 22 | 8 | Not reported | ||
| Other | 11 | 0 | 9 | Not reported | ||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, mean (range) | 6.9 (1–23) | 7.0 (1–32) | 7.9 (1–27) | Not reported | ||
| Area of AGWs (mm2), mean | Not reported | |||||
| Sex (M/F), n (%) | 100% male | |||||
| Any previous treatment, n (%) | 5 (16.6) | 5 (16.1) | 6 (20.7) | Not reported | ||
| Ethnicity | Overall, the study included 88 white and two black men; numbers not reported for individual treatment groups | Not reported | ||||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Complete clearance at 4 weeks | |||||
| AGW clearance at other time points | Complete clearance at 1, 2 or 3 weeks | |||||
| Recurrence of AGWs | Recurrence at 16 weeks | |||||
| AEs | AEs were recorded as itching, burning sensation, tenderness, pruritus, erythema, erosion and others. It is reported that most AEs were mild to moderate, with 59 men experiencing an AE (number of events in each treatment group not reported separately); definitions for mild, moderate and severe not available | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Podophyllotoxin 0.15% cream (patient applied), n/N | Podophyllotoxin 0.3% cream (patient applied), n/N | Podophyllotoxin 0.5% solution (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | 4 weeks | 21/30 | 25/31 | 24/29 | Not significant | |
| AGW clearance at other time points | 1 week | 11/30 | 14/31 | 15/29 | Not significant | |
| 2 weeks | 15/30 | 23/31 | 23/29 | Reported to be significantly lower in the 0.15% cream group | ||
| 3 weeks | 20/30 | 24/31 | 23/29 | Not significant | ||
| Recurrence of AGWs (recurrence in areas similar to those of original AGWs) | 16 weeks | 4/21 | 4/25 | 4/24 | Not reported | |
| Recurrence of AGWs (includes recurrence in locations other than those of original AGWs) | 16 weeks | 4/21 | 6/25 | 5/24 | Not significant | |
| Severe AEs | 16 weeks | 2/30 | 5/31 | 5/29 | Not significant | |
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | |||
| Random sequence generation | ? | Study is described as randomised. Details on method used to generate random sequence not provided | ||||
| Allocation concealment | ? | Details on method used to conceal allocation not provided | ||||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | ||||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | ||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | Absolute numbers for loss to follow-up and withdrawal are not reported for individual treatment groups. Reasons for loss to follow-up not reported. It is unclear whether there is an imbalance across treatment groups in numbers lost to follow-up or withdrawing | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of recurrence is likely to be subjective and open to influence from lack of masking | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of recurrence is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | Absolute numbers for loss to follow-up and withdrawal are not reported for individual treatment groups. Reasons for loss to follow-up not reported. It is unclear whether there is an imbalance across treatment groups in numbers lost to follow-up or withdrawing | ||||
| AEs | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AEs is likely to be subjective and open to influence from lack of masking | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AEs is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | Absolute numbers for loss to follow-up and withdrawal are not reported for individual treatment groups. Reasons for loss to follow-up not reported. It is unclear whether there is an imbalance across treatment groups in numbers lost to follow-up or withdrawing | ||||
| Overall rating of bias | ✗ | Reflects open-label nature of trial | ||||
| Section 6: Additional comments | ||||||
| Additional comments |
|
|||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Sjokvist Garcia-Stewart and Sam Barton | |||||
| Study ID | Syed 1993 | |||||
| Study details | Dermatology 1993;187:30–3 | |||||
| Language of publication | English | |||||
| Type of report | Full publication | |||||
| Section 2: Study information | ||||||
| Location and number of sites | The study was carried out at three clinics in Pakistan (clinics located in Karachi, Lahore and Peshawar) | |||||
| Trial sponsor | Not reported | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | Not reported | |||||
| Trial design | RCT (three arms) | |||||
| Trial duration | Initial treatment period of a maximum of 4 weeks with subsequent follow-up at 16 weeks after treatment for those classed as achieving cure during treatment | |||||
| Line of therapy | Unclear | |||||
| Inclusion criteria | Male gender; age between 15 and 40 years; clinical diagnosis of AGWs | |||||
| Exclusion criteria | Not reported | |||||
| All outcomes reported in paper | Complete clearance; partial response or no response (based on percentage regression of baseline AGWs); response rate after 1, 2, 3 and 4 weeks of treatment; relapse; AEs | |||||
| Subgroups evaluated | None reported | |||||
| Stratification | Not reported | |||||
| Baseline measurement of disease | Diagnosis based on clinical examination with androscopy and painting with 5% acetic acid at the initial visit. At baseline, the location, number, type and size in diameter of each AGW were evaluated and recorded. A representative biopsy specimen was taken from each patient for the detection of HPV lesions | |||||
| Treatment | Podophyllotoxin 0.3% cream (patient applied) | Podophyllotoxin 0.15% cream (patient applied) | Podophyllotoxin 0.3% solution (patient applied) | |||
| Randomised, n | 20 | 20 | 20 | |||
| Withdrawals, n (%) | 0 (0) | 0 (0) | 0 (0) | |||
| Treatment regimen | Men applied podophyllotoxin 0.3% cream, podophyllotoxin 0.15% cream or podophyllotoxin 0.3% solution at home, twice daily, for 3 consecutive days. If complete clearance was not achieved, the treatment cycle was repeated after 4 treatment-free days. The cycle was repeated until complete clearance was achieved or for a maximum of four treatments, whichever occurred earlier. A maximum of 24 applications was permitted. Men were taught the basic steps and precautions to be observed, including how to wash, dry and carefully paint the affected area with the provided cotton swab, avoiding excess spreading to adjacent tissue. The painted area was to be left to dry for at least 1 minute. Trial preparations were dispensed in identical test tubes containing 5 ml of active preparation per period of 3-day treatment | |||||
| Duration/number of administered treatment | Not reported | |||||
| Baseline patient characteristics | Podophyllotoxin 0.3% cream (patient applied) | Podophyllotoxin 0.15% cream (patient applied) | Podophyllotoxin 0.3% solution (patient applied) | p-value | ||
| Age (years), mean (range) | Overall population 19.5 (15–40). Mean age for individual treatment groups not reported | |||||
| Duration of disease | Not reported | |||||
| Site of AGWs, n (%) | For overall population, 39 (65.0%) men had lesions of the penile shaft and perianal area and 21 (35.0%) men had lesions on the penile shaft alone. Sites for individual treatment groups not reported | |||||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, mean (with SD/SE if given) | For overall population, 6.5 at baseline; not reported for individual treatment groups. Of the overall trial population, five (8.3%) men had one to two AGWs, 30 (50.0%) men had three to five AGWs and the remainder [25 men (41.7%)] had > 10 AGWs | |||||
| area of AGWs (mm2), mean | Mean area of baseline AGWs not reported. Range of diameter of AGWs for overall trial population reported to be between 1 and 8 mm, with a mean diameter of 1.9 mm (not reported for individual treatment groups) | |||||
| Sex (M/F), n (%) | 100% male | |||||
| Any previous treatment, n (%) | Not reported | |||||
| Ethnicity, n (%) | 100% Asian | |||||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Complete eradication of venereal AGWs. Achievement of complete cure was recorded as 100% regression of baseline AGWs. Complete clearance at various time points is reported. For the purposes of this review, end of treatment has been taken as 4 weeks (maximum number of permitted treatments) | |||||
| AGW clearance at other time points | Complete clearance after 1, 2 and 3 weeks | |||||
| Recurrence of AGWs | For those achieving complete clearance, reappearance of AGWs at a previously treated site at 16 weeks’ follow-up | |||||
| Volume of wart clearance | Men with ≥ 50% regression of baseline AGWs were classified as partially cured and those with < 50% regression were categorised as having ‘no response’ | |||||
| AEs | It is stated that AEs were graded as mild, moderate or severe or none; definitions of mild, moderate and severe not available. Duration of AEs was noted. AEs monitored were pain, pruritus, burning sensation, tenderness, erythema and erosion | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Podophyllotoxin 0.3% cream (patient applied), n/N | Podophyllotoxin 0.15% cream (patient applied), n/N | Podophyllotoxin 0.3% solution (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | 4 weeks | 20/20 | 14/20 | 20/20 | Not reported | |
| AGW clearance at other time points | 1 week | 0/20 | 0/20 | 0/20 | NR; inferred to be zero | Not reported |
| 2 weeks | NR/20 | NR/20 | 16/20 | |||
| 3 weeks | 15/20 | NR/20 | 20/20 | |||
| Recurrence of AGWs | 16 weeks after treatment | Of the 54 men with complete clearance, three experienced recurrence; recurrence by treatment group not reported | ||||
| Volume of wart clearance (≥ 50% regression from baseline) | 4 weeks | 0/20 | 6/20 | 0/20 | Not reported | |
| AEs | AEs not reported separately by treatment group. It is reported that six (30.0%) men in the podophyllotoxin 0.3% solution group experienced localised mild erythema and burning sensation. In the groups receiving the cream preparations, 18 men (45.0%), predominantly in the 0.3% cream group, reported mild to moderate erythema, pruritus and burning sensation | Not reported | ||||
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | |||
| Random sequence generation | ? | Trial described as a randomised trial. Details on methods used to generate random sequence not available | ||||
| Allocation concealment | ? | Methods implemented to conceal allocation not reported | ||||
| Selective reporting | ✗ | Data for key clinical outcomes (recurrence and AEs) are not reported as absolute events rates by treatment group. Results presented cannot be used in a meta-analysis | ||||
| ‘Other bias’ | ? | Insufficient information reported to determine presence of additional sources of bias | ||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ✗ | Participants and personnel are not masked to treatment. Given that the techniques evaluated are topical applications, it is feasible to mask key study personnel and participants to treatment. AGW clearance is a subjective outcome and is liable to bias because of lack of masking | |||
| Blinding of outcomes assessment | ✗ | |||||
| Incomplete outcome data | ✓ | All people randomised were reported to be followed up | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ✗ | Participants and personnel are not masked to treatment. Given that the techniques evaluated are topical applications, it is feasible to mask key study personnel and participants to treatment. AGW recurrence is a subjective outcome and is liable to bias because of lack of masking | |||
| Blinding of outcomes assessment | ✗ | |||||
| Incomplete outcome data | ✓ | All people randomised were reported to be followed up | ||||
| Volume of wart clearance (≥ 50% regression) | Blinding (participants and personnel) | ✗ | Participants and personnel are not masked to treatment. Given that the techniques evaluated are topical applications, it is feasible to mask key study personnel and participants to treatment. AGW clearance is subjective and liable to bias from lack of masking | |||
| Blinding of outcomes assessment | ✗ | |||||
| Incomplete outcome data | ✓ | All people randomised were reported to be followed up | ||||
| AEs | Blinding (participants and personnel) | ✗ | Participants and personnel are not masked to treatment. Given that the techniques evaluated are topical applications, it is feasible to mask key study personnel and participants to treatment. AE outcomes are subjective outcomes and are liable to bias because of lack of masking | |||
| Blinding of outcomes assessment | ✗ | |||||
| Incomplete outcome data | ✓ | All people randomised were reported to be followed up | ||||
| Overall rating of bias | ✗ | Reflects the limited reporting of results for outcomes of interest and the open-label nature of the trial | ||||
| Section 6: Additional comments | ||||||
| Additional comments | None | |||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Jacoby Patterson and Victoria Wakefield | |||||
| Study ID | Syed 1994 | |||||
| Study details | Dermatology 1994;189:142–5 | |||||
| Language of publication | English | |||||
| Type of report | Full publication | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Multiple centres (number of sites not reported) in Pakistan | |||||
| Trial sponsor | Not reported | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | Participants were recruited from Asian women attending clinics in Pakistan during the summer of 1991 | |||||
| Trial design | RCT | |||||
| Trial duration | Initial treatment period of up to 4 weeks with subsequent follow-up for 12 weeks (i.e. total study duration of 16 weeks) for those achieving complete clearance during treatment | |||||
| Line of therapy | Not reported | |||||
| Inclusion criteria | Asian women between the ages of 16 and 40 years with extravaginal condylomata | |||||
| Exclusion criteria | Heart, renal or liver insufficiency; untreated classical STDs; pregnancy or lactation; duration of condylomata > 6 months; receipt of medication for AGWs in the 3 months preceding study entry | |||||
| All outcomes reported in paper | Complete clearance; relapse; AEs | |||||
| Subgroups evaluated | None reported | |||||
| Stratification | None reported | |||||
| Baseline measurement of disease | Location and number of warts recorded at baseline visit | |||||
| Treatment | Podophyllotoxin 0.3% cream (patient applied) | Podophyllotoxin 0.5% cream (patient applied) | Placebo cream (patient applied) | |||
| Randomised, n | 30 | 30 | 20 | |||
| Withdrawals, n (%) | 0 | 0 | 0 | |||
| Treatment regimen | Women self-applied their allocated treatment (podophyllotoxin 0.3% cream, podophyllotoxin 0.5% cream or placebo) twice daily for 3 consecutive days per week. Treatment cycle was repeated until complete clearance was achieved or for a maximum of 4 weeks (24 topical applications), whichever occurred earlier. Women were instructed to avoid excessive spreading to surrounding tissue and to allow the cream to dry for at least 1 minute without washing the treated area | |||||
| Duration/number of administered treatment | Not reported | |||||
| Baseline patient characteristics | Podophyllotoxin 0.3% cream (patient applied) | Podophyllotoxin 0.5% cream (patient applied) | Placebo cream (patient applied) | p-value | ||
| Age (years), mean | 22.4 | 24.0 | 24.8 | Not reported | ||
| Duration of disease | Not reported | |||||
| Site of AGWs, number of lesions | ||||||
| Total number of lesions | 206 | 214 | 124 | |||
| Labia majora | 41 | 45 | 33 | Not reported | ||
| Labia minora | 39 | 48 | 31 | Not reported | ||
| Introitus | 44 | 53 | 28 | Not reported | ||
| Clitoris | 10 | 12 | 5 | Not reported | ||
| Perineum | 29 | 27 | 9 | Not reported | ||
| Perianal area | 31 | 16 | 10 | Not reported | ||
| Anal | 12 | 13 | 8 | Not reported | ||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, mean | 6.86 | 7.13 | 6.2 | Not reported | ||
| Area of AGWs (mm2), mean | Not reported | |||||
| Sex (M/F), n (%) | 100% women | |||||
| Any previous treatment, n (%) | Not reported | |||||
| Ethnicity, n (%) | 100% Asian | |||||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Women achieving complete clearance at 4 weeks. Number of AGWs cleared at 4 weeks also reported | |||||
| Recurrence of AGWs | Relapse at 16 weeks (i.e. 12 weeks after end of treatment) in those achieving complete clearance of AGWs | |||||
| AEs | AEs recorded were pruritus, burning sensation, tenderness, erythema, erosion and any other symptoms | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Podophyllotoxin 0.3% cream (patient applied), n/N | Podophyllotoxin 0.5% cream (patient applied), n/N | Placebo cream (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment (analysis based on number of women) | 4 weeks | 16/30 | 25/30 | 0/20 | Podophyllotoxin 0.3% or 0.5% cream vs. placebo: p < 0.001; podophyllotoxin 0.3% vs. 0.5% cream: p < 0.01 | |
| AGW clearance at completion of treatment (analysis based on number of AGWs) | 4 weeks | 144/206 | 205/214 | 0/124 | Not reported | |
| Recurrence of AGWs | 16 weeks | 3/16 | 1/25 | 0/0 | Not reported | |
| AEs | ||||||
| Any | 4 weeks | 9/30 | 22/30 | 6/20 | Not reported | |
| Tenderness | 4 weeks | 5/30 | 12/30 | 6/20 | Not reported | |
| Burning | 4 weeks | 4/30 | 10/30 | 0/20 | Not reported | |
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | |||
| Random sequence generation | ? | It is stated that people were randomly allocated to treatment. Additional details on method of randomisation not available | ||||
| Allocation concealment | ? | Detail on method used to conceal allocation not available | ||||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | ||||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | ||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication test tube preparations were similar. However, it is unclear who was masked to treatment and whether masking could have been broken | |||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | ||||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication test tube preparations were similar. However, it is unclear who was masked to treatment and whether masking could have been broken | |||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | ||||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | ||||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication test tube preparations were similar. However, it is unclear who was masked to treatment and whether masking could have been broken | |||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | ||||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | ||||
| Overall rating of bias | ? | Reflects limited reporting on methods in full publication | ||||
| Section 6: Additional comments | ||||||
| Additional comments | None | |||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Victoria Wakefield | ||||
| Study ID | Syed 1995a | ||||
| Study details | Dermatology 1995;191:129–32 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Multiple sites (number not stated) in the Punjab region of Pakistan | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Study was carried out between March 1992 and February 1993; details on patient recruitment not available | ||||
| Trial design | RCT (three arms; third arm evaluated interferon, which is not an intervention of interest for this review) | ||||
| Trial duration | Initial treatment period of up to 4 weeks with follow-up at 16 weeks for those achieving complete clearance during treatment. Final follow-up was 1 year after the first day of treatment | ||||
| Line of therapy | Not reported | ||||
| Inclusion criteria | Men aged 18–40 years with genital AGWs (on the glans, shaft, corona sulcus or perianal area) and who were HIV negative at baseline | ||||
| Exclusion criteria | Untreated classical STDs; duration of genital AGWs > 6 months; intake of immunosuppressive drugs or use of any topical antiviral medication or any type of therapy for genital AGWs in the 4 weeks preceding study entry | ||||
| All outcomes reported in paper | Complete clearance; relapse; AEs | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Location, size and number of AGWs recorded at the baseline visit | ||||
| Treatment | Podophyllotoxin 0.5% cream (patient applied) | Placebo | |||
| Randomised, n | 20 | 20 | |||
| Withdrawals, n (%) | 0 | 0 | |||
| Treatment regimen | Podophyllotoxin 0.5% in a hydrophilic cream or matching placebo cream was self-applied three times daily for 3 consecutive days (maximum of nine applications per week) until either complete clearance was achieved or for 4 weeks, whichever occurred earlier | ||||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Podophyllotoxin 0.5% cream (patient applied) | Placebo | p-value | ||
| Age (years), mean | 24.4 | 25.0 | Groups reported to be ‘comparable’ | ||
| Duration of disease | Not reported | ||||
| Site of AGWs, initial number of lesions | |||||
| Corona sulcus | 30 | 27 | Groups reported to be ‘comparable’ | ||
| Glans and shaft | 62 | 59 | |||
| Perianal | 39 | 43 | |||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | 6.5 | 6.4 | Groups reported to be ‘comparable’ | ||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | 100% male | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | 100% Asian | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Complete elimination of lesions defined as disappearance of all visible lesions by colposcopy and absence of HPV DNA and cellular atypia confirmed by Southern dot blot at 4 weeks. Analysis based on number of AGWs cured at 4 weeks also reported | ||||
| Recurrence of AGWs | Relapse after 10 months | ||||
| AEs | Data were reported on occurrence of erythema, burning sensation, itching (pruritus) and fever plus headache plus itching | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllotoxin 0.5% cream (patient applied), n/N | Placebo, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment (patients) | 4 weeks | 11/20 | 3/20 | p < 0.0098 | |
| AGW clearance at completion of treatment (number of warts) | 4 weeks | 83/131 | 15/129 | Not reported | |
| Recurrence of AGWs | 10 months | 2/11 | 0/3 | Not reported | |
| AEs | |||||
| Erythema | 4 weeks | 4/20 | 1/20 | Not reported | |
| Burning | 4 weeks | 3/20 | 1/20 | Not reported | |
| Itching | 4 weeks | 1/20 | 0/20 | Not reported | |
| Fever plus headache plus itching | 4 weeks | 0/20 | 0/20 | Not reported | |
| Any | 4 weeks | 8/20 | 2/20 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that people were randomly assigned to treatment. Additional details on method of randomisation not available | |||
| Allocation concealment | ? | Detail on method used to conceal allocation not available | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication test tube preparations were similar. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication test tube preparations were similar. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | |||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication test tube preparations were similar. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | |||
| Overall rating of bias | ? | Reflects limited reporting in full publication | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Victoria Wakefield | ||||
| Study ID | Syed 1995b | ||||
| Study details | J Mol Med (Berl) 1995;73:255–8 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Multiple sites (number not stated) in the Punjab region of Pakistan | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Participants enrolled from March 1992 to February 1993; details on how patients were recruited not available | ||||
| Trial design | RCT [three arms; only two arms of interest to this review (third arm evaluated interferon)] | ||||
| Trial duration | Initial treatment period of 4 weeks with subsequent follow-up at 16 weeks from the start of the trial for those achieving complete clearance during treatment. Final follow-up was 1 year after the initial visit | ||||
| Line of therapy | Not reported | ||||
| Inclusion criteria | Women aged 18–40 years with AGWs and who were HIV negative | ||||
| Exclusion criteria | Pregnancy; breastfeeding; receipt of any therapy for AGWs in the 4 weeks preceding trial entry; concurrent cardiac, renal, hepatic, pulmonary, gastrointestinal or haematological disorder or untreated classical STD; AGWs with a duration > 6 months; receiving any immunosuppressive or topical antiviral drugs | ||||
| All outcomes reported in paper | Clearance (complete, partial and no response); relapse | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Location, size and number of AGWs were recorded at the baseline visit | ||||
| Treatment | Podophyllotoxin 0.5% cream (patient applied) | Placebo cream (patient applied) | |||
| Randomised, n | 20 | 20 | |||
| Withdrawals, n (%) | 0 | 0 | |||
| Treatment regimen | Women self-applied podophyllotoxin 0.5% cream or placebo cream three times daily for 3 consecutive days per week until complete AGW clearance or for up to 4 weeks, whichever occurred earlier. Maximum number of treatments allowed (as per the protocol) was 36 applications | ||||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Podophyllotoxin 0.5% cream (patient applied) | Placebo cream (patient applied) | p-value | ||
| Age (years), mean | 22.9 years for full trial population (three treatment groups); not reported separately by treatment group | Not reported | |||
| Duration of disease | Not reported | ||||
| Site of AGWs | Overall for full trial population (three treatment groups; locations not reported by treatment group): 26 women had 138 lesions on the labia majora or minora; 13 women had 66 lesions on the introitus; 10 women had 51 lesions on the perianal area; seven women had 40 lesions on the perineum; four women had 25 lesions on the clitoris | Not reported | |||
| Type of AGWs, n (%) | Not reported | ||||
| Baseline number of AGWs | Overall for full trial population (three treatment groups; number of AGWs not reported by treatment group): one to five AGWs: 33 women; six to eight AGWs: 27 women | Not reported | |||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | 100% women | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | 100% Asian | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Biopsy-confirmed total elimination of lesion; analysed by number of patients with complete clearance and number of AGWs cleared | ||||
| AGW clearance at other time points | Biopsy-confirmed total elimination of lesions at 1 week | ||||
| Recurrence of AGWs | Relapse after 9 months. It is reported that two out of 34 cured women achieving complete clearance of AGWs across the three treatment groups experienced relapse. However, data are not reported by treatment group | ||||
| Volume of wart clearance (proportion of women with ≥ 50% clearance) | Proportion of women with ≥ 50% reduction in the surface area of baseline lesions (referred to as partial response) | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllotoxin 0.5% cream (patient applied), n/N | Placebo cream (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment (number of women; note, includes women with partial clearance) | 4 weeks | 12/20 | 4/20 | Not reported | |
| AGW clearance (number of lesions) | 4 weeks | 87/108 | 16/104 | Not reported | |
| AGW clearance (number of women) | 1 week | 0/20 | 0/20 | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that people were randomly allocated to treatment. Additional details on method of randomisation not available | |||
| Allocation concealment | ? | Detail on method used to conceal allocation not available | |||
| Selective reporting | ✗ | It is stated that partial response was recorded but data are not reported separately from data for complete clearance. Recurrence is not reported separately for each treatment group and thus data reported cannot be entered in a meta-analysis | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication test tube preparations were similar. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication test tube preparations were similar. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ? | It is unclear whether there were any additional losses to follow-up during the follow-up period. The analysis is based on all people with complete clearance | |||
| Overall rating of bias | ✗ | Reflects limited reporting of clinical effectiveness results in full publication | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Victoria Wakefield | ||||
| Study ID | Syed 1998 | ||||
| Study details | J Dermatol 1998;25:429–33 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Multiple centres (number not stated) in the Punjab region of Pakistan | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Participants enrolled from February 1994 to July 1995; details on how patients were recruited not available | ||||
| Trial design | RCT | ||||
| Trial duration | Initial treatment period of 6 weeks with subsequent follow-up at 16 weeks from the start of the trial for those achieving complete clearance during treatment. Final follow-up was 11 months after the initial visit | ||||
| Line of therapy | Not reported | ||||
| Inclusion criteria | Women aged 18–45 years | ||||
| Exclusion criteria | History or presence of malignancy; Papanicolaou smear cervical intraepithelial neoplasia positivity; HIV-positive status; abnormal cardiac, hepatic, pulmonary, gastrointestinal or renal function; concomitant STD; pregnant or breastfeeding; using immunosuppressive or topical antiviral drugs; receipt of any therapy for AGWs in the 8 weeks preceding trial entry | ||||
| All outcomes reported in paper | Clearance (complete, partial or no response); recurrence; AEs (nausea, tenderness, erythema and burning sensation) | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Location, size and number of AGWs were recorded at the baseline visit | ||||
| Treatment | Imiquimod 2% cream (self-applied) | Placebo cream (self-applied) | |||
| Randomised, n | 30 | 30 | |||
| Withdrawals, n (%) | 0 | 0 | |||
| Treatment regimen | Women self-applied imiquimod 2% cream or placebo cream twice daily for 5 consecutive days per week until complete clearance or for up to 6 weeks, whichever occurred earlier. Maximum number of treatments allowed (as per the protocol) was 60 applications | ||||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Imiquimod 2% cream (self-applied) | Placebo cream (self-applied) | p-value | ||
| Age (years), mean | 24.2 | 24.4 | Not reported | ||
| Duration of disease | Not reported | ||||
| Site of AGWs, initial number of AGWs | |||||
| Labia majora | 62 | 55 | Not reported | ||
| Labia minora | 54 | 65 | |||
| Introitus | 31 | 34 | |||
| Clitoris | 10 | 11 | |||
| Perineum | 21 | 19 | |||
| Perianal area | 23 | 26 | |||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | 6.8 | 6.9 | Not reported | ||
| Area of AGWs (mm2), mean | Not reported | Not reported | |||
| Sex (M/F), n (%) | 100% female | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Total elimination of AGWs, as confirmed by colposcopy; analysed by number of patients with complete clearance and number of AGWs cleared | ||||
| Recurrence of AGWs | Relapse after 11 months | ||||
| Volume of wart clearance (proportion of women with ≥ 50% clearance) | Proportion of women with ≥ 50% reduction in the surface area of baseline lesions (referred to as partial response) | ||||
| AEs | Data reported on occurrence of nausea, burning, tenderness and erythema | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Imiquimod 2% cream (self-applied), n/N | Placebo cream (self-applied) | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment (number of women) | 6 weeks | 22/30 | 1/30 | Not reported | |
| AGW complete or partial clearance (number of AGWs) | 6 weeks | 172/204 | 4/207 | Reported as significant | |
| Recurrence of AGWs | 11 months | 4/25a | 1/1 | Not reported | |
| Volume of wart clearance (proportion of women with ≥ 50% clearance) | 6 weeks | 3/30 | 0/30 | Not reported | |
| AEs | |||||
| Nausea | 6 weeks | 2/30 | 0/30 | Not reported | |
| Burning | 6 weeks | 1/30 | 0/30 | Not reported | |
| Tenderness | 6 weeks | 3/30 | 0/30 | Not reported | |
| Erythema | 6 weeks | 2/30 | 0/30 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmentb | Comments | ||
| Random sequence generation | ? | It is stated that people were randomly assigned to treatment. Additional details on method of randomisation not available | |||
| Allocation concealment | ? | Detail on method used to conceal allocation not available | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication tube preparations were similar. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication tube preparations were similar. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | |||
| Volume of wart clearance (proportion of women with ≥ 50% clearance) | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication tube preparations were similar. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | |||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medication tube preparations were similar. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | |||
| Overall rating of bias | ? | Reflects limited reporting in full publication | |||
| Section 6: Additional comments | |||||
| Additional comments |
|
||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Victoria Wakefield | ||||
| Study ID | Syed 2000 | ||||
| Study details | J Infect 2000;41:148–51 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Multiple sites (number not stated) in the Punjab region of Pakistan | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Participants were selected from those attending family planning clinics, public health centres and government-affiliated municipal dispensaries as well as from those referred by physicians who were aware of the study. The trial was carried out between February 1995 and July 1996; it is unclear over what time period people were recruited | ||||
| Trial design | RCT | ||||
| Trial duration | Initial treatment period of up to 4 weeks, with follow-up at 16 weeks after trial initiation for those achieving complete clearance during treatment. Final follow-up took place at 18 months after the first day of treatment | ||||
| Line of therapy | 17/60 men had previously used podophyllotoxin cream 0.5% and had experienced resistance or treatment failure | ||||
| Inclusion criteria | Men aged 18–50 years | ||||
| Exclusion criteria | Concurrent STD; past or current malignancy; HIV-positive status; allergy to imidazoquinoline; abnormal cardiac, renal, pulmonary, gastrointestinal or hepatic function; use of immunosuppressive drugs or any topical antiviral compound for external AGWs in the 8 weeks preceding trial entry | ||||
| All outcomes reported in paper | Clearance (complete, partial or no response); recurrence; AEs | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Location, size and number of AGWs were recorded at the baseline visit | ||||
| Treatment | Imiquimod 2% cream (patient applied) | Placebo cream (patient applied) | |||
| Randomised, n | 30 | 30 | |||
| Withdrawals, n (%) | 0 | 0 | |||
| Treatment regimen | Men self-applied imiquimod 2% or placebo cream once daily at bedtime for 3 consecutive days per week until complete clearance or for up to 4 weeks, whichever occurred earlier. Maximum number of treatments allowed (as per the protocol) was 12 applications | ||||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Imiquimod 2% cream (patient applied) | Placebo cream (patient applied) | p-value | ||
| Age (years), mean | 24.7 | 23.7 | Not reported | ||
| Duration of disease | Not reported | ||||
| Site of AGWs | Of the overall trial population, 45 men (75%) had lesions on the penile shaft and corona sulcus and 15 men (25%) had lesions confined to the perianal area. Site of AGWs not reported separately by treatment group | Not reported | |||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | 9.6 | 9.0 | Not reported | ||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | 100% male | ||||
| Any previous treatment | 17/60 (28.3%) men had previously used podophyllotoxin 0.5% cream and had encountered resistance or treatment failure; number of men in each treatment group undergoing previous treatment with podophyllotoxin 0.5% not reported | Not reported | |||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Total elimination of AGWs, as confirmed by colposcopy; analysed by number of patients with complete clearance and number of AGWs cleared | ||||
| Recurrence of AGWs | Recurrence after 14 months | ||||
| Volume of wart clearance | Proportion of men with ≥ 50% reduction in the surface area of baseline lesions (referred to as partial response). Number of men achieving a partial response in each treatment group not reported. Data on number of AGWs partially eliminated by treatment are available | ||||
| AEs | AEs were scored as mild, moderate, severe or none with respect to duration in days; further definitions of mild, moderate or severe not available. Data reported on occurrence of burning sensation, itching, pain, erythema, erosion and oedema | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Imiquimod 2% cream (patient applied), n/N | Placebo (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment (number of men) | 4 weeks | 21/30 | 3/30 | p < 0.0001 | |
| AGW clearance (number of AGWs) | 4 weeks | 250/288 | 28/270 | p < 0.0001 | |
| Recurrence of AGWs | 14–18 months | 1/21 | 2/3 | Not reported | |
| Volume of wart clearance (proportion of women with ≥ 50% clearance) | 4 weeks | It is reported that seven men experienced partial regression of 18 lesions. Results not reported by treatment group | |||
| AEs | |||||
| Erythema | 4 weeks | 4/30 | 0/30 | Not reported | |
| Erosiona | 4 weeks | 4/30 | 1/30 | Not reported | |
| Oedema | 4 weeks | 2/30 | 0/30 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmentb | Comments | ||
| Random sequence generation | ? | It is reported that people were randomly allocated to treatment. Additional details on method of randomisation not available | |||
| Allocation concealment | ? | Detail on method used to conceal allocation not available | |||
| Selective reporting | ? | It is stated that partial response was recorded but data are not reported in a way that they can be entered in a meta-analysis. Volume of AGW clearance has not been defined as a primary outcome for this review. Although data for this outcome cannot be entered into a meta-analysis, data on the primary outcomes of interest are available | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medications were packed in identical precoded tubes. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medications were packed in identical precoded tubes. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ✓ | No withdrawals or missing outcome data | |||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blind and it is reported that the study medications were packed in identical precoded tubes. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||
| Incomplete outcome data | ? | Minor discrepancy in the numbers reported in the text and results table for the outcome of erosion | |||
| Overall rating of bias | ? | Reflects limited reporting in full publication | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Shannon Amoils and Sam Barton | ||||
| Study ID | Tabari 2010 | ||||
| Study details | Casp J Intern Med 2010;1:16–19 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study carried out at a single site in Iran (Babol University of Medical Sciences) | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | It is stated that people were recruited from ‘outpatient genital wart cases’ (p. 17) attending the outpatient dermatology clinic from June 2005 to June 2008 | ||||
| Trial design | RCT | ||||
| Trial duration | Total duration including follow-up is 6 months. Duration of treatment is unclear. Participants were followed up at 1 month, 2 months and 6 months | ||||
| Line of therapy | Not reported | ||||
| Inclusion criteria | People with a diagnosis of genital AGWs as evaluated by physical examination of the lesions were eligible | ||||
| Exclusion criteria | Pregnant women were excluded | ||||
| All outcomes reported in paper | Complete clearance; recurrence after treatment; complications | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Not reported. Participants were included on the basis of a ‘clinical examination of the lesions’ (p. 17) to confirm diagnosis of genital warts. The presence of multiple lesions was noted. No other baseline measurements on the lesions were carried out | ||||
| Treatment | Podophyllin 20% (clinician applied) | TCAA 30% (clinician applied) | |||
| Randomised, n | 60 | 60 | |||
| Withdrawals, n | 0 | 0 | |||
| It is stated that no patient interrupted treatment because of complications | |||||
| Treatment regimen | Podophyllin 20% was ‘applied on the affected area topically twice a week and was washed after 20 minutes of application’ (p. 17) | TCAA 30% was applied ‘with topical cotton soap’ every other day and was washed off 1 minute after application’ (p. 17) | |||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Podophyllin 20% (clinician applied) | TCAA 30% (clinician applied) | p-value | ||
| Age (years), mean (SD) | 32.0 (17.0) | 31.8 (16.2) | Not reported | ||
| Duration of disease | Not reported | ||||
| Site of AGWs, n (%) | Not reported | ||||
| Type of AGWs, n (%) | Not reported | ||||
| Presence of multiple lesions, n (%) | 42 (70.0) | 45 (75.0) | Reported as no significant difference between treatment groups; p-value not reported | ||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | Not reported | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance | Proportion of people who had complete clearance is reported. However, description of assessment of complete clearance is not available and it is unclear for which follow-up time point the data are reported (i.e. 1, 2 or 6 months). In addition, duration of treatment is unclear; thus, it is unclear whether reported data are for end of treatment or some other time point | ||||
| Recurrence of AGWs | Not defined. It is unclear how recurrence was determined. It is stated that recurrence was observed ‘three months later’ (p. 17). It is unclear whether this is 3 months after study initiation, after the end of treatment or after diagnosis of complete clearance of AGWs | ||||
| AEs | Not defined. Data are reported for the number of people reporting a burning sensation | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllin 20% (clinician applied), n/N | TCAA 30% (clinician applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance (referred to as ‘completely treated’) | Unclear | 56/60 | 56/60 | Not reported | |
| Recurrence of AGWs | Unclear | 4/56 | 0/56 | Not reported | |
| AEs; burning sensation | Not reported | 35/60 | 28/60 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ✓ | It is stated that ‘For the selection of patients in each arm, we prepared 120 cards and wrote the regimen podophyllin (60 cards) and regimen trichloroacetic acid (60 cards) on it. For every patient, a card was drawn and the regimen therapy noted on it was administered’ (p. 17) | |||
| Allocation concealment | ? | Insufficient information to determine risk of bias. It is stated that ‘For every patient, a card was drawn and the regimen therapy noted on it was administered’ (p. 17). It is unclear whether the cards were concealed in any way. It could be inferred that the personnel randomising participants were aware of allocation concealment | |||
| Selective reporting | ✗ | Clear definitions of duration of treatment regimens, outcome assessment and time at which outcomes were reported are not available. Lack of reporting of duration of treatment and time points for key clinical outcomes makes it difficult to compare the study with other trials in a meta-analysis | |||
| ‘Other bias’ | ? | Insufficient information reported to evaluate presence of other potential sources of bias | |||
| AGW clearance | Blinding (participants and personnel) | ? | Level of masking of participants and key study personnel not reported. It is unclear whether the person evaluating AGW clearance was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ✓ | It is stated that ‘all cases were followed’ (p. 17) at 1 month, 2 months and 6 months | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Level of masking of participants and key study personnel not reported. It is unclear whether the person evaluating AGW clearance was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | Rate of recurrence is reported for people receiving podophyllin 20%. The reported text infers that no person receiving TCAA experienced recurrence, but this is not explicitly stated | |||
| AEs | Blinding (participants and personnel) | ? | Level of masking of participants and key study personnel not reported. It is unclear whether the person evaluating AGW clearance was masked to treatment | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | Number of people evaluated for AEs unclear. Reporting suggests that all people randomised have been included but denominator has not been reported | |||
| Overall rating of bias | ✗ | Reflects unclear reporting as to duration of treatment and time point for reported clinical data | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Shannon Amoils and Sam Barton | |||||
| Study ID | Trofatter 2002 | |||||
| Study details | Int J Gynaecol Obstet 2002;76:191–3 | |||||
| Language of publication | English | |||||
| Type of report | Full publication | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Not reported | |||||
| Trial sponsor | Study supported by a research grant from 3M Pharmaceuticals | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | Not reported | |||||
| Trial design | RCT (Phase II, dose-ranging study) | |||||
| Trial duration | Initial treatment period of up to 16 weeks with a subsequent 4-week observational period | |||||
| Line of therapy | Not reported | |||||
| Inclusion criteria | Women with histologically confirmed external AGWs | |||||
| Exclusion criteria | Not reported | |||||
| All outcomes reported in paper | Complete clearance; proportion of women with > 50% reduction in baseline AGW area; median time to clearance; AEs: application site reactions | |||||
| Subgroups evaluated | None reported | |||||
| Stratification | None reported | |||||
| Baseline measurement of disease | Not reported | |||||
| Treatment | Imiquimod 5% cream twice daily (patient applied) | Imiquimod 5% cream once daily (patient applied) | Imiquimod 5% cream three times weekly (patient applied) | |||
| Randomised, n | 32 | 32 | 26 | |||
| Withdrawals, n (%) | Not reported | |||||
| Treatment regimen | Women self-applied imiquimod 5% cream twice daily, once daily or three times weekly until complete resolution of baseline AGWs was confirmed or up to a maximum of 16 weeks, whichever occurred earlier. Women who developed an intolerable reaction were given the option to either discontinue treatment or take a rest period, during which treatment was deferred (for up to 14 consecutive days) until the reaction had subsided | |||||
| Duration/number of administered treatment | Not reported | |||||
| Baseline patient characteristics | Imiquimod 5% cream twice daily (patient applied) | Imiquimod 5% cream once daily (patient applied) | Imiquimod 5% cream three times weekly (patient applied) | p-value | ||
| Age (years), mean (SD | 29 (9.1) | 30 (12.3) | 30 (13.0) | p = 0.928 | ||
| Duration of disease (months), median (range) | ||||||
| Time since onset | 11.0 (2.0–248) | 7.3 (1.1–368) | 10.5 (0.1–361) | p = 0.438 | ||
| Duration of current outbreak | 4.1 (0.3–248) | 2.6 (0.7–368) | 3.7 (0.2–361) | p = 0.306 | ||
| Site of AGWs, n (%) | Not reported | |||||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, median (range) | 10 (2–50) | 11 (2–50) | 13 (2–49) | p = 0.801 | ||
| Area of AGWs (mm2), median (range) | 260 (34–1995) | 138 (13–1975) | 121 (31–1231) | p = 0.211 | ||
| Sex (M/F), n (%) | 100% female | |||||
| Any previous treatment, n (%) | Not reported | |||||
| Ethnicity, n (%) | ||||||
| Caucasian | 29 (90.6) | 28 (87.5) | 23 (88.5) | p = 1.000 | ||
| African American | 3 (9.4) | 4 (12.5) | 3 (11.5) | |||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Not defined. Results are presented for the proportion of women with a 100% reduction in AGW area during the treatment and observation periods. Number of women achieving 100% reduction during the observation period noted. Number of women achieving clearance at end of treatment calculated by review authors | |||||
| AGW clearance at other time points | Not defined. Results are presented for the proportion of women with a 100% reduction in AGW area during the treatment and observation periods (4 weeks after the end of treatment) | |||||
| Time to complete clearance | Reported as ‘median time to clearance’ (p. 192) | |||||
| Volume of wart clearance (> 50% clearance) | Number of women with a > 50% reduction in AGW area during the treatment and observation periods is reported. Presented results include those with 100% clearance. Proportion achieving > 50% but < 100% reduction in AGW area calculated by review authors | |||||
| AEs | AEs not defined. Data reported for occurrence of application site reactions and patient-reported AEs of burning, itching and pain at the AGW site. Occurrence of investigator-observed local skin reactions (erythema, ulceration and erosion) presented graphically | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Imiquimod 5% cream twice daily (patient applied), n/N | Imiquimod 5% cream once daily (patient applied), n/N | Imiquimod 5% cream three times weekly (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | Up to 16 weeks | 18/32 | 23/32 | 16/26 | Not reported | |
| AGW clearance at other time points (cumulative) | 4 weeks after end of treatment | 20/32 | 23/32 | 16/26 | p = 0.3 | |
| Volume of wart clearance (> 50% clearance) | Up to 20 weeks | 9/32 | 7/32 | 6/26 | p = 0.61 | |
| AEs | ||||||
| Burning | Up to 16 weeks | 7/31 | 10/31 | 6/28 | p > 0.50 | |
| Itching | Up to 16 weeks | 11/31 | 9/31 | 12/28 | p = 0.40 | |
| Pain | Up to 16 weeks | 8/31 | 7/31 | 3/28 | p = 0.48 | |
| Severe erythema | Up to 16 weeks | Numerator and denominator not reported for severe erythema; number of women evaluated unclear | p = 0.01 | |||
| 25% | 10% | 4% | ||||
| Erythema or erosion or ulceration | Up to 16 weeks | Results presented graphically; unable to report numbers accurately | Not reported | |||
| Continuous outcomes | ||||||
| Time to complete clearance (weeks), median | 8 (n not reported) | 6 (n not reported) | 12 (n not reported) | Not reported | ||
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | |||
| Random sequence generation | ? | It is stated that women were randomised to treatment but details on method of randomisation not available | ||||
| Allocation concealment | ? | Details on method used to conceal allocation not available | ||||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | ||||
| ‘Other bias’ | ? | Insufficient information to determine risk of bias from other potential sources | ||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | The number of people withdrawing or lost to follow-up is not reported. Data are reported for the ITT population but it is unclear whether there is an imbalance across the groups in the number of people withdrawing or lost to follow-up | ||||
| Time to complete clearance | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | The number of people withdrawing or lost to follow-up is not reported. Data are reported for the ITT population but it is unclear whether there is an imbalance across the groups in the number of people withdrawing or lost to follow-up | ||||
| Volume of wart clearance (> 50% clearance) | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | The number of people withdrawing or lost to follow-up is not reported. Data are reported for the ITT population but it is unclear whether there is an imbalance across the groups in the number of people withdrawing or lost to follow-up | ||||
| AEs | Blinding (participants and personnel) | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AEs is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | The number of people withdrawing or lost to follow-up is not reported. It is unclear whether there is an imbalance across the groups in the number of people withdrawing or lost to follow-up. Data reported for AEs are not based on the same number of women in each group as reported for clinical outcomes. Reasons for the difference in number of women evaluated not discussed | ||||
| Overall rating of bias | ✗ | Reflects open-label nature of the study | ||||
| Section 6: Additional comments | ||||||
| Additional comments | None | |||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Victoria Wakefield and Sam Barton | |||||
| Study ID | Tuncel 2005 | |||||
| Study details | Eur Acad Dermatol Venereol 2005;19(Suppl. 2):361 | |||||
| Language of publication | English | |||||
| Type of report | Conference abstract | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Study carried out in Turkey; number of sites not reported | |||||
| Trial sponsor | Not reported | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | Not reported | |||||
| Trial design | Three-arm RCT | |||||
| Trial duration | Initial treatment period of up to 16 weeks; unclear whether there was an additional follow-up period | |||||
| Line of therapy | At least second line: inclusion criterion of persistent AGWs that were refractory to at least one conventional therapy | |||||
| Inclusion criteria | Presence of recalcitrant AGWs (perianal and/or genital) that were refractory to at least one conventional therapy | |||||
| Exclusion criteria | None reported | |||||
| All outcomes reported in paper | Results at end of study (presumed to be complete clearance); recurrence; AEs | |||||
| Subgroups evaluated | Gender (male vs. female) | |||||
| Stratification | None reported | |||||
| Baseline measurement of disease | Not reported | |||||
| Treatment | Imiquimod 5% cream (self-applied) | Cryotherapy plus imiquimod 5% cream (self-applied) | Cryotherapy | |||
| Randomised, n | 20 | 20 | 20 | |||
| Withdrawals, n (%) | Not reported | |||||
| Treatment regimen | Imiquimod 5% cream was self-applied to all external AGWs overnight three times a week until AGW clearance or for up to 16 weeks, whichever occurred earlier | Cryotherapy was administered at 3-week intervals until AGW clearance or for up to 16 weeks. Concomitantly, imiquimod 5% cream was self-applied to all external AGWs overnight three times a week until AGW clearance or for up to 16 weeks, whichever occurred earlier | Cryotherapy was administered at 3-week intervals until AGW clearance or for up to 16 weeks, whichever occurred earlier | |||
| Duration/number of administered treatment | Not reported | |||||
| Baseline patient characteristics | Imiquimod 5% cream (self-applied) | Cryotherapy plus imiquimod 5% cream (self-applied) | Cryotherapy | p-value | ||
| Age (years), mean | Not reported | |||||
| Duration of disease | Not reported | |||||
| Site of AGWs, n (%) | Not reported | |||||
| Type of AGWs, n (%) | Not reported | |||||
| Number of AGWs, mean | Not reported | |||||
| Area of AGWs (mm2), mean | Not reported | |||||
| Sex (M/F), n (%) | Not reported | |||||
| Any previous treatment, n (%) | Not reported | |||||
| Ethnicity, n (%) | Not reported | |||||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Results at end of study are discussed in the abstract; no further details available | |||||
| Recurrence of AGWs | Recurrence rates are discussed in the abstract; no further details available | |||||
| AEs | Local and systemic AEs are discussed in the abstract. AEs were graded as mild, moderate or severe; no further details available | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Imiquimod 5% cream (self-applied), n/N | Cryotherapy plus imiquimod 5% cream (self-applied), n/N | Cryotherapy, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment | 16 weeks | Absolute numbers not reported. It is stated that ‘statistically significant differences were found among the results in the groups at the end of the study’ (p. 361) (p < 0.05). In addition, it is stated that better results were seen with imiquimod 5% cream plus cryotherapy than with imiquimod 5% cream monotherapy, but the difference did not reach statistical significance (p > 0.05) | ||||
| Recurrence of AGWs | 16 weeks | Absolute numbers not reported. It is stated that recurrence rates were lower with both imiquimod 5% cream (p < 0.05) and imiquimod 5% cream (p < 0.01) plus cryotherapy than with cryotherapy alone | ||||
| AEs | 16 weeks | Absolute numbers not reported. It is stated that ‘home application of imiquimod was better tolerated and associated with fewer side effects than combination therapy’ (p. 361) | ||||
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | |||
| Random sequence generation | ? | The study is described as randomised but details on the method of randomisation are not reported | ||||
| Allocation concealment | ? | Insufficient information available to assess whether allocation concealment has been implemented and, if so, the risk of bias associated with the method | ||||
| Selective reporting | ✗ | Absolute numbers are not reported for any outcome assessed and thus data cannot be evaluated in a meta-analysis | ||||
| ‘Other bias’ | ? | Insufficient information available to assess presence of other potential sources of bias | ||||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ✗ | Study is described as open label. Given the difference in the types of treatment administered (topical vs. ablative), it might be impractical to mask personnel and patients to treatment. AGW clearance is a subjective outcome and at risk of bias | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | Number of withdrawals not reported | ||||
| Recurrence of AGWs | Blinding (participants and personnel) | ✗ | Study is described as open label. Given the difference in the types of treatment administered (topical vs. ablative), it might be impractical to mask personnel and patients to treatment. Recurrence of AGWs is a subjective outcome and at risk of bias | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of recurrence is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | Number of withdrawals not reported | ||||
| AEs | Blinding (participants and personnel) | ✗ | Study is described as open label. Given the difference in the types of treatment administered (topical vs. ablative), it might be impractical to mask personnel and patients to treatment. AEs associated with the treatments under evaluation are likely to be subjective and thus at risk of bias | |||
| Blinding of outcomes assessment | ✗ | Study is described as open label. Assessment of AEs is likely to be subjective and open to influence from lack of masking. Although it is unclear whether the outcome assessor was masked to treatment, given that patients and other key study personnel were not, it is likely that masking would be broken | ||||
| Incomplete outcome data | ? | Number of withdrawals not reported | ||||
| Overall rating of bias | ✗ | Reflects open-label nature of study and lack of numerical data for use in a meta-analysis | ||||
| Section 6: Additional comments | ||||||
| Additional comments | None | |||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Shannon Amoils and Sam Barton | ||||
| Study ID | Tyring 1998a. Related publications: Arany et al.249 | ||||
| Study details | J Infect Dis 1998;178:551–5 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Not reported | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | It is stated that ‘patients were enrolled after study procedures were explained, and they signed formal written consent’ (p. 57).249 Date of enrolment not reported | ||||
| Trial design | RCT | ||||
| Trial duration | Up to 16 weeks (treatment was administered until complete clearance or for a maximum of 16 weeks, whichever occurred earlier) | ||||
| Line of therapy | Unclear. It is stated that people entering the trial were receiving treatment with imiquimod for the first time | ||||
| Inclusion criteria | Aged ≥ 18 years; histologically confirmed diagnosis of condylomata acuminata; presence of at least 10 but no more than 50 AGWs prior to prestudy biopsy | ||||
| Exclusion criteria | Known positive status for HIV infection; presence of high-grade cervical intraepithelial lesions; previous treatment with imiquimod; receipt of interferon, an interferon inducer, an immunomodulator, oral or topical antiviral drugs, cytotoxic or investigational drugs or chemical and/or surgical AGW treatment within the 4 weeks preceding study entry; receipt of topical non-AGW treatment to the AGW site or oral or inhaled corticosteroids (1000 µg/day) within the 2 weeks preceding study entry | ||||
| All outcomes reported in paper | Complete clearance of AGWs (reported in Tyring et al.126); volume of AGW clearance (proportion of people with ≥ 75% clearance in AGW area from baseline); cytokine mRNA expression (changes from baseline); immune cell surface marker mRNA expression (changes from baseline); markers of viral infection (changes from baseline); keratinocyte markers (changes from baseline); cell cycle markers (changes from baseline) | ||||
| Subgroups evaluated | None reported | ||||
| Stratification | Study drug was randomised at a ratio of 4 : 1 for each gender such that, for every four patients who received imiquimod 5% cream, one received vehicle | ||||
| Baseline measurement of disease | A biopsy of the target AGW area was obtained at baseline for confirmation of diagnosis and to establish a baseline for biological markers | ||||
| Treatment | Imiquimod 5% cream (patient applied) | Vehicle (placebo) cream (patient applied) | |||
| Randomised, n | Number randomised to each group not reported. It is stated that 22 people were randomised and three people were lost to follow-up. Loss to follow-up not reported by treatment group | ||||
| 16 included in analysis | Three included in analysis | ||||
| Withdrawals | In total, three (13.6%) people were lost to follow-up. Reasons for withdrawal and loss by treatment group not reported | ||||
| Treatment regimen | People self-applied either imiquimod 5% cream or vehicle cream to AGWs overnight (for 8 ± 2 hours) three times per week on non-consecutive days. Treatment was repeated weekly until clearance of AGWs or for a maximum of 16 weeks, whichever occurred earlier | ||||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Imiquimod 5% cream (patient applied) | Vehicle (placebo) cream (patient applied) | p-value | ||
| Age (years), mean | Not reported | ||||
| Time since onset of disease (months), median (range) (reported in Tyring et al.126) | 60 (3.5–204) | 19.7 (6.6–100.3) | Not reported | ||
| Site of AGWs, n (%) | Not reported | ||||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean (with SD/SE if given) | Not reported | ||||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | In total, 12 men and 10 women were randomised. Ratio of genders in each treatment group not reported separately | ||||
| Any previous treatment, n (%) | Not reported | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Data reported on complete clearance of baseline AGWs at any point during the 16-week treatment period (Tyring et al.126). Clinical evaluation of clearance not described | ||||
| Volume of wart clearance (proportion of patients with ≥ 75% clearance) | Data reported on proportion of people with ≥ 75% clearance in total AGW area from baseline (Tyring et al.126 and Arany et al.249); data reported include those with 100% clearance. Number of people with clearance between ≥ 75% and < 100% calculated by review authors | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Imiquimod 5% cream (patient applied), n/N | Vehicle (placebo) cream (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | Up to 16 weeks | 7/16 | 1/3 | Not reported | |
| Volume of wart clearance (proportion of people with ≥ 75% clearance) | Up to 16 weeks | 9/16 | 0/3 | Not reported | |
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||
| Random sequence generation | ? | It is stated that people were randomly allocated to treatment. Details on method used to generate random sequence not available | |||
| Allocation concealment | ? | Details on method used to conceal allocation not available | |||
| Selective reporting | ? | The objectives of the study seem to be to assess cellular response to imiquimod 5% cream by measuring viral load and the molecular immune response to treatment. The outcomes reported do not pertain to a clinical outcomes study and appropriate molecular and immunological outcomes are reported. Protocol is not available and it is unclear whether clinical outcomes were prespecified | |||
| ‘Other bias’ | ✗ | In this small study of 22 people, the authors randomised participants in a ratio of 4 : 1 (study drug : placebo). Reasons for unequal randomisation were not available. Unequal randomisation reduces the power of the study to detect a true difference between treatment groups. Based on the 22 participants randomised, at most, five people would receive placebo, which is likely to be too small a number to provide clinically meaningful results. In addition, although limited details on baseline characteristics are reported, there is a marked difference between groups in the time since onset of disease, with a median time since onset of 60 months in the group treated with imiquimod and 19.7 months in the group treated with placebo | |||
| AGW clearance at completion of treatment | Blinding (participants and personnel) | ? | Described as a double-blinded trial. Details on who was masked to treatment are not available. In addition, it is unclear whether attempts were made to mask the outcome assessor to treatment allocation | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | The number of people randomised to each group is not reported. The analyses reported are based on those for whom evaluable data were available. The number of people excluded from the analysis is reported, but reasons for withdrawal are not. Although the number of people withdrawing is low, because of the small size of the trial the proportion of people withdrawing is high | |||
| Volume of wart clearance | Blinding (participants and personnel) | ? | Described as a double-blinded trial. Details on who was masked to treatment are not available. In addition, it is unclear whether attempts were made to mask the outcome assessor to treatment allocation | ||
| Blinding of outcomes assessment | ? | ||||
| Incomplete outcome data | ? | The number of people randomised to each group is not reported. The analyses reported are based on those for whom evaluable data were available. The number of people excluded from the analysis is reported, but reasons for withdrawal are not. Although the number of people withdrawing is low, because of the small size of the trial, the proportion of people withdrawing is high | |||
| Overall rating of bias | ✗ | Reflects the considerable imbalance in the randomisation ratio between groups in a small study and imbalance in available baseline characteristics | |||
| Section 6: Additional comments | |||||
| Additional comments | None | ||||
| Item | Details | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||||||||||||||||||||||||||
| Reviewers’ names | Jacoby Patterson and Vicky Wakefield | ||||||||||||||||||||||||||||
| Study ID | Tyring 1998b | ||||||||||||||||||||||||||||
| Study details | Arch Dermatol 1998;134:33–8 | ||||||||||||||||||||||||||||
| Language of publication | English | ||||||||||||||||||||||||||||
| Type of report | Full publication | ||||||||||||||||||||||||||||
| Section 2: Study information | |||||||||||||||||||||||||||||
| Location and number of sites | Study carried out at multiple sites (number not reported) in the USA | ||||||||||||||||||||||||||||
| Trial sponsor | Supported by a grant from Oclassen Pharmaceuticals Inc., San Rafael | ||||||||||||||||||||||||||||
| Conflicts of interest | Not reported | ||||||||||||||||||||||||||||
| Patient enrolment | People were recruited from private dermatology practices, university clinics (dermatology, gynaecology and infectious diseases) and contract research organisations and were enrolled between December 1992 and March 1994 | ||||||||||||||||||||||||||||
| Trial design | RCT | ||||||||||||||||||||||||||||
| Trial duration | Initial treatment period of up to 8 weeks followed by assessment after a further 8 weeks to evaluate recurrence (depending on number of treatment cycles, total study time ranged from 9 to 16 weeks) | ||||||||||||||||||||||||||||
| Line of therapy | It is reported that approximately 20% of patients had received previous treatment for their current AGWs; no further details available | ||||||||||||||||||||||||||||
| Inclusion criteria | Age > 18 years; immunocompetent; at least two distinct external AGWs (genital and/or perianal); negative pregnancy test for women of child-bearing potential; women who were not breastfeeding and used an approved method of birth control during the study | ||||||||||||||||||||||||||||
| Exclusion criteria | People were excluded if they had received treatment for AGWs within the month preceding trial entry | ||||||||||||||||||||||||||||
| All outcomes reported in paper | Complete clearance; number of AGWs remaining each week; change in AGW surface area; change in individual AGW assessment scores; physician assessment of overall response; recurrence; AEs (including pain, burning, inflammation, itching, erosion and bleeding) | ||||||||||||||||||||||||||||
| Subgroups evaluated | Location (external AGWs vs. perianal AGWs) and gender (male vs. female) | ||||||||||||||||||||||||||||
| Stratification | None reported | ||||||||||||||||||||||||||||
| Baseline measurement of disease | Location, number, size, morphology and total area of AGWs were recorded at the baseline visit | ||||||||||||||||||||||||||||
| Treatment | Podophyllotoxin 0.5% gel (self-applied) | Placebo gel (self-applied) | |||||||||||||||||||||||||||
| Randomised, n | 219 | 107 | |||||||||||||||||||||||||||
| Withdrawals, n (%) | 40 (18.3) | 66 (61.7) | |||||||||||||||||||||||||||
| Not compliant with protocol (excluded from efficacy analysis) | 16 | 8 | |||||||||||||||||||||||||||
| Discontinuation because of inadequate response | 17 (8.6) (three people had discontinued by week 4) | 58 (61.1) (35 people had discontinued by week 4) | |||||||||||||||||||||||||||
| Discontinuation because of drug-related reactions | 7 (3.2) | 0 (0) | |||||||||||||||||||||||||||
| Treatment regimen | Podophyllotoxin 0.5% gel or placebo gel were self-applied using a finger or applicator twice daily for 3 consecutive days, followed by 4 treatment-free days. The treatment cycle was repeated until complete clearance or for a maximum of eight cycles, whichever occurred earlier. People underwent a minimum of two cycles. If a local reaction was experienced, treatment was postponed for up to 1 week | ||||||||||||||||||||||||||||
| Duration/number of administered treatment | Not reported | ||||||||||||||||||||||||||||
| Baseline patient characteristics | Podophyllotoxin 0.5% gel (self-applied) | Placebo gel (self-applied) | p-value | ||||||||||||||||||||||||||
| n | Note: baseline characteristics are based on those patients who returned after the first visit | ||||||||||||||||||||||||||||
| 213 | 103 | ||||||||||||||||||||||||||||
| Age (years), mean | 31.5 | 30.6 | Difference between groups reported to be not significant | ||||||||||||||||||||||||||
| Duration of disease (months), mean | 27.3 | 24.0 | Difference between groups reported to be not significant | ||||||||||||||||||||||||||
| Site of AGWs, n/N (%) | Note: characteristics reported for those included in the efficacy analysis | ||||||||||||||||||||||||||||
| External genital only | 158/197 (80.2) | 79/95 (83.2) | Differences between groups reported to be not significant | ||||||||||||||||||||||||||
| Perianal only | 12/197 (6.1) | 11/95 (11.6) | |||||||||||||||||||||||||||
| Both | 27/197 (13.7) | 5/95 (5.3) | |||||||||||||||||||||||||||
| Type of AGWs, n (%) | Not reported | Difference between groups reported as not significant for AGW morphology; further details not available | |||||||||||||||||||||||||||
| Number of AGWs, mean | 5.5 | 5.4 | Difference between groups reported to be not significant | ||||||||||||||||||||||||||
| Area of AGWs (mm2), mean | 159.2 | 141.8 | Difference between groups reported to be not significant | ||||||||||||||||||||||||||
| Sex, n (%) | |||||||||||||||||||||||||||||
| Male | 127 (59.6) | 62 (60.2) | Difference between groups reported to be not significant | ||||||||||||||||||||||||||
| Female | 86 (40.4) | 41 (39.8) | |||||||||||||||||||||||||||
| Any previous treatment | It is reported that about 20% of people had received previous treatment; number of people receiving previous treatment not reported by treatment group. The most frequent previous therapies administered for AGWs, in both treatment groups, were podophyllum resin (10–12%) and cryotherapy (9–10%) | Difference between groups reported to be not significant | |||||||||||||||||||||||||||
| Ethnicity | For the overall population, 80% white, 12% black. Ethnicity not reported by treatment group | Difference between groups reported to be not significant | |||||||||||||||||||||||||||
| Section 3: Outcomes | |||||||||||||||||||||||||||||
| Outcome | Definition | ||||||||||||||||||||||||||||
| AGW clearance at completion of treatment | Total disappearance of all treated AGWs at week 8 (last observation carried forward) | ||||||||||||||||||||||||||||
| AGW clearance at other time points | Total disappearance of all treated AGWs at week 4 (last observation carried forward) | ||||||||||||||||||||||||||||
| Recurrence of AGWs | Recurrence of at least one AGW within 12 weeks of successful treatment of all AGWs | ||||||||||||||||||||||||||||
| AEs | Severity of local AEs in the treated area (including pain, burning, inflammation, itching, erosion and bleeding) was assessed and categorised as mild, moderate or severe; definitions of mild, moderate and severe not available | ||||||||||||||||||||||||||||
| Section 4: Data extraction form | |||||||||||||||||||||||||||||
| Outcome | Time frame | Podophyllotoxin 0.5% gel (self-applied), n/N | Placebo gel (self-applied), n/N | Estimate of effect | p-value | ||||||||||||||||||||||||
| Dichotomous outcomes | |||||||||||||||||||||||||||||
| AGW clearance at completion of treatmenta | 8 weeks | 81/181 | 4/93 | p < 0.001 | |||||||||||||||||||||||||
| AGW clearance at other time pointsa | 4 weeks | 62/167 | 2/86 | p < 0.001 | |||||||||||||||||||||||||
| Recurrence of AGWs | 12 weeks | 25/81 | Not assessed/4 | Not reported | |||||||||||||||||||||||||
| AEs (%)b | |||||||||||||||||||||||||||||
| Burning | 8 weeks | 76.1 | 45.6 | Not reported | |||||||||||||||||||||||||
| Inflammation | 8 weeks | 71.4 | 11.7 | ||||||||||||||||||||||||||
| Itching | 8 weeks | 58.7 | 25.2 | ||||||||||||||||||||||||||
| Erosion | 8 weeks | 54.0 | 2.9 | ||||||||||||||||||||||||||
| Pain | 8 weeks | 54.0 | 5.8 | ||||||||||||||||||||||||||
| Bleeding | 8 weeks | 29.1 | 1.9 | ||||||||||||||||||||||||||
| Headache | 8 weeks | 9.4 | 5.8 | ||||||||||||||||||||||||||
| Any non-local AE | 8 weeks | 38 | 24 | p = 0.02 | |||||||||||||||||||||||||
| Section 5: Clinical trial quality | |||||||||||||||||||||||||||||
| Outcome | Risk of bias | Risk assessmentc | Comments | ||||||||||||||||||||||||||
| Random sequence generation | ? | It is reported that people were randomised 2 : 1 to treatment groups. Additional details on method of randomisation not available | |||||||||||||||||||||||||||
| Allocation concealment | ? | Detail on method used to conceal allocation not available | |||||||||||||||||||||||||||
| Selective reporting | ✗ | Number of people included in analysis of AGW clearance differs from that specified in the statistical analysis. Number of people experiencing recurrence in the placebo group is not reported. AEs are reported as percentages, with no indication of how many people were included in the analysis. As presented, data for recurrence and AEs cannot be entered in a meta-analysis | |||||||||||||||||||||||||||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||||||||||||||||||||||||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | The study is described as double blind. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||||||||||||||||||||||||||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||||||||||||||||||||||||||
| Incomplete outcome data | ✗ | It is unclear why the number of people included in the analysis of AGW clearance differs from that specified in the statistical analysis. In addition, the number of people analysed at week 4 differs from the number analysed at week 8. This potential discrepancy is not discussed. The number of people withdrawing from treatment and lost to follow-up is reported. A significantly larger proportion of people in the placebo group withdrew from treatment as a result of treatment inefficacy than in the podophyllotoxin 0.5% gel group. Although the analysis is based on last observation carried forward, and as such people withdrawing from treatment will be considered treatment failures, the imbalance in withdrawals is likely to induce clinically relevant bias in intervention effect estimates | |||||||||||||||||||||||||||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The study is described as double blind. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||||||||||||||||||||||||||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||||||||||||||||||||||||||
| Incomplete outcome data | ? | All people achieving complete clearance were reported to have been followed up. However, it is unclear what influence the high rate of withdrawal from treatment will have on the estimate of effect for recurrence | |||||||||||||||||||||||||||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blind. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||||||||||||||||||||||||||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||||||||||||||||||||||||||
| Incomplete outcome data | ? | It is unclear how many people are included in the analysis and therefore unclear whether a large proportion of people have been excluded and whether there is an imbalance in the number of people analysed in each group | |||||||||||||||||||||||||||
| Overall rating of bias | ✗ | Reflects limited reporting of some key outcomes and high rate of withdrawal from the study | |||||||||||||||||||||||||||
| Section 6: Additional comments | |||||||||||||||||||||||||||||
| Additional comments |
Complete clearance by location of AGW (external vs. perianal) is presented in the following table Treatment weekExternal AGWs, n/NPerianal AGWs, n/NPodophyllotoxin 0.5%PlaceboPodophyllotoxin 0.5%Placebo460/157a0/75a11/362/15877/170a1/82a16/383/16ap < 0.001. |
Treatment week | External AGWs, n/N | Perianal AGWs, n/N | Podophyllotoxin 0.5% | Placebo | Podophyllotoxin 0.5% | Placebo | 4 | 60/157a | 0/75a | 11/36 | 2/15 | 8 | 77/170a | 1/82a | 16/38 | 3/16 | ap < 0.001. | ||||||||||
| Treatment week | External AGWs, n/N | Perianal AGWs, n/N | |||||||||||||||||||||||||||
| Podophyllotoxin 0.5% | Placebo | Podophyllotoxin 0.5% | Placebo | ||||||||||||||||||||||||||
| 4 | 60/157a | 0/75a | 11/36 | 2/15 | |||||||||||||||||||||||||
| 8 | 77/170a | 1/82a | 16/38 | 3/16 | |||||||||||||||||||||||||
| ap < 0.001. | |||||||||||||||||||||||||||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Victoria Wakefield | ||||
| Study ID | Viazis 2007 | ||||
| Study details | Dis Colon Rectum 2007;50:2173–9 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study carried out at one centre in Greece | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Patients with intra-anal AGWs who were referred to the gastroenterology unit for treatment of AGWs between October 2002 and March 2005 were offered entry into the study | ||||
| Trial design | RCT | ||||
| Trial duration | Argon laser treatment was repeated every 4 weeks until complete clearance of AGWs was achieved. After elimination of AGWs, participants were followed up for a mean of 12 months (range 3–21 months) | ||||
| Line of therapy | First line of treatment for intra-anal AGWs; people were excluded if they had received previous treatment for intra-anal warts or had a history of any warts on the penis, groin, cervix, urethral meatus, vagina or pubis | ||||
| Inclusion criteria | No previous treatment of intra-anal AGWs with any modality; absence of AGWs or previous elimination of AGWs on the penis, groin, cervix, urethral meatus, vagina or pubis. People with simultaneous perianal AGWs were included | ||||
| Exclusion criteria | People were excluded if they had AGWs in sites other than the anal area | ||||
| All outcomes reported in paper | Time to elimination of all intra-anal AGWs; recurrence of intra-anal AGWs; AEs | ||||
| Subgroups evaluated | HIV status (positive vs. negative) | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Not reported | ||||
| Treatment | Argon plasma coagulation plus imiquimod 5% cream (self-applied) | Argon plasma coagulation | |||
| Randomised, n | 24 | 25 | |||
| Withdrawals, n (%) | 2 (8.3) (lost to follow-up) | 2 (8.0) (lost to follow-up) | |||
| Treatment regimen | Argon plasma coagulation was repeated every 4 weeks until elimination of intra-anal AGWs. Using a finger, imiquimod 5% cream was self-applied to intra-anal AGWs at bedtime three times a week. Perianal AGWs, if present, were treated with imiquimod 5% cream at bedtime three times a week. Treatment with imiquimod 5% cream commenced just after the first treatment with argon laser and continued until the elimination of intra-anal AGWs | Argon plasma coagulation was repeated every 4 weeks until elimination of intra-anal AGWs. Perianal AGWs, if present, were treated with imiquimod 5% cream at bedtime three times a week | |||
| Duration/number of administered treatment | Mean number of sessions of argon plasma coagulation required to achieve complete clearance: 2.1 ± 0.2 | Mean number of sessions of argon plasma coagulation required to achieve complete clearance: 3 ± 0.2 | |||
| Difference between groups reported to be statistically significant, favouring the combination treatment (p = 0.0016) | |||||
| Baseline patient characteristics | Argon plasma coagulation plus imiquimod 5% cream (self-applied) | Argon plasma coagulation | p-value | ||
| Age (years), mean (unclear whether SD or SE) | 32.3 (11.5) | 30.4 (12.4) | Not reported | ||
| Duration of disease | Not reported | ||||
| Site of AGWs | 100% intra-anal. Note: two people also had perianal warts | 100% intra-anal. Note: three people also had perianal warts | |||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | Not reported | ||||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex, n (%) | |||||
| Male | 20 (83.3) | 22 (88) | Not reported | ||
| Female | 4 (16.7) | 3 (12) | Not reported | ||
| Any previous treatment, n | 0 for intra-anal AGWs | 0 for intra-anal AGWs | |||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Treatments were administered until all intra-anal AGWs in each person had been eliminated. Maximum number of treatments not specified and so time frame for completion of treatment not specified | ||||
| Recurrence of AGWs | Recurrence of intra-anal AGWs during the follow-up period after complete clearance (mean follow-up was 12 months) | ||||
| Time to complete clearance | Time to complete elimination of intra-anal AGWs in all people | ||||
| AEs | Occurrence of AEs of argon plasma coagulation and imiquimod 5% cream reported for itching/burning, erythema in anal canal and pain/bleeding | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Argon plasma coagulation plus imiquimod 5% cream (self-applied), n/N | Argon plasma coagulation, n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| Complete clearance of AGWs at end of treatment | End of treatment | 22/22 | 23/23 | – | |
| Recurrence of AGWs | Mean follow-up of 12 months | 5/22 | 8/23 | Not significant | |
| AEs | |||||
| Itching/burninga | Unclear | 16/24 | 0/25 | Not reported | |
| Mild erythema in anal canal | Unclear | 10/24 | 0/25 | Not reported | |
| Pain/bleeding | Unclear | 0/24 | 0/25 | Not reported | |
| Continuous outcomes | |||||
| Time to complete clearance (days), mean (SE) | 62.5 (5.4) (n = 24) | 91.2 (6.4) (n = 25) | p = 0.0016 | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmentb | Comments | ||
| Random sequence generation | ✓ | It is stated that randomisation was based on a table of random numbers | |||
| Allocation concealment | ✓ | It is stated that opaque, serially numbered envelopes were used to allocate treatment | |||
| Selective reporting | ? | Insufficient information provided to determine risk of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible | ||
| Blinding of outcomes assessment | ✗ | The endoscopist assessing the outcomes was not an independent observer. Assessment of AGW recurrence is likely to be subjective and open to influence from lack of masking | |||
| Incomplete outcome data | ✓ | Few people were lost to follow-up and missing outcome data are balanced in numbers between the groups | |||
| Time to complete clearance | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible | ||
| Blinding of outcomes assessment | ✗ | The endoscopist assessing the outcomes was not an independent observer. Assessment of AGW clearance is likely to be subjective and open to influence from lack of masking | |||
| Incomplete outcome data | ✓ | Few people were lost to follow-up and missing outcome data are balanced in numbers between the groups | |||
| AEs | Blinding (participants and personnel) | ? | Details on level of masking of patients and personnel not provided. Given the difference in the treatments administered, it could be envisaged that masking of patients and personnel might not be feasible | ||
| Blinding of outcomes assessment | ✗ | The endoscopist assessing the outcomes was not an independent observer. Assessment of AEs is likely to be subjective and open to influence from lack of masking | |||
| Incomplete outcome data | ✓ | Few people were lost to follow-up and missing outcome data are balanced in numbers between the groups | |||
| Overall rating of bias | ✗ | Reflects the potential bias associated with outcome assessment | |||
| Section 6: Additional comments | |||||
| Additional comments |
|
||||
| Item | Details | ||||
|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | ||||
| Study ID | von Krogh 1992 | ||||
| Study details | Sex Transm Dis 1992;19:170–4 | ||||
| Language of publication | English | ||||
| Type of report | Full publication | ||||
| Section 2: Study information | |||||
| Location and number of sites | Study carried out at the Department of Obstetrics and Gynaecology, Falu Hospital, Sweden | ||||
| Trial sponsor | Not reported | ||||
| Conflicts of interest | Not reported | ||||
| Patient enrolment | Patients were enrolled at the Department of Obstetrics and Gynaecology, Falu Hospital. Details on methods used to recruit patients and dates of enrolment not available | ||||
| Trial design | RCT | ||||
| Trial duration | 3 weeks of treatment with subsequent follow-up at 3 months for those categorised as cured at any point in the trial | ||||
| Line of therapy | Overall, 25/60 (41.7%) participants had received previous treatment for AGWs in the previous 6 months | ||||
| Inclusion criteria | Women with vulvoanal condylomata (acuminata, popular or sessile) | ||||
| Exclusion criteria | Age < 18 years; presence of AGWs in the vagina, cervix or anus (as evaluated through colposcopy and proctoscopy); pregnancy or no safe contraceptive method; receipt of treatment for AGWs within the preceding 4 weeks | ||||
| All outcomes reported in paper | Complete clearance; recurrence; AEs | ||||
| Subgroups evaluated | Number of lesions (< 10 vs. ≥ 10), mean size of lesion (< 3 mm vs. ≥ 3 mm), site (outer vulva, inner vulva or perianal) | ||||
| Stratification | None reported | ||||
| Baseline measurement of disease | Details of baseline assessment not reported. Based on subgroups reported, it is inferred that location, size and number of AGWs were recorded | ||||
| Treatment | Podophyllotoxin 0.5% cream (patient applied) | Placebo (patient applied) | |||
| Randomised, n | 48 | 12 | |||
| Withdrawals, n (%) | 4 (8.3) | 0 (0) | |||
| Ineligible | 1 (2.1; intra-anal condylomata detected) | 0 (0) | |||
| Lost to follow-up | 2 (4.2) | 0 (0) | |||
| AEs | 1 (2.1) | 0 (0) | |||
| Treatment regimen | Podophyllotoxin 0.5% cream or placebo cream was applied by the patient twice daily for 3 days per week for up to 3 weeks. Participants were instructed how to locate individual condyloma with one finger and how to apply the cream with another finger | ||||
| Duration/number of administered treatment | Not reported | ||||
| Baseline patient characteristics | Podophyllotoxin 0.5% cream (patient applied) | Placebo (patient applied) | p-value | ||
| Note: baseline characteristics in the podophyllotoxin 0.5% cream group are based on the 44 women for whom data were available rather than the 48 randomised to treatment | |||||
| Age (years), mean | 26.3 | 25.5 | Difference between groups reported to be not significant | ||
| Duration of disease (months), mean | 6.9 | 6.0 | Difference between groups reported to be not significant | ||
| Site of AGWs, n (%)a | |||||
| Outer vulvab | 8 | Not reported | Not reported | ||
| Inner vulvab | 35 | Not reported | |||
| Perianal | 20 | Not reported | |||
| Type of AGWs, n (%) | Not reported | ||||
| Number of AGWs, mean | 11.7 | Not reported | |||
| Area of AGWs (mm2), mean | Not reported | ||||
| Sex (M/F), n (%) | 100% female | ||||
| Any previous treatment | Overall, 25/60 (41.7%) women had been treated for AGWs in the preceding 6 months; data not reported separately by treatment group | ||||
| Ethnicity, n (%) | Not reported | ||||
| Section 3: Outcomes | |||||
| Outcome | Definition | ||||
| AGW clearance at completion of treatment | Complete clearance of AGWs at 3 weeks | ||||
| AGW clearance at other time points | Complete clearance of AGWs also recorded after 1 week and 2 weeks | ||||
| Recurrence of AGWs | Recurrence of AGWs in treated location at 3 months’ follow-up. Authors corrected for instances of ‘reoccurrences’, which were defined as development of AGWs during follow-up on locations other than those initially identified as afflicted by AGWs; three women in the podophyllotoxin 5% group were identified as having ‘reoccurrence’ at follow-up | ||||
| AEs | AEs were not defined. Local AEs, including burning, pain/tenderness and erosion, were evaluated | ||||
| Section 4: Data extraction form | |||||
| Outcome | Time frame | Podophyllotoxin 0.5% cream (patient applied), n/N | Placebo (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | |||||
| AGW clearance at completion of treatment | 3 weeks | 40/44 | 1/12 | Not reported | |
| AGW clearance at other time points | 1 week | 18/44 | 0/12 | Not reported | |
| 2 weeks | 29/44 | 0/12 | |||
| Recurrence of AGWs (does not include reoccurrences) | 3 months | 3/40 | 0/0 | Not reported | |
| AEs | |||||
| Burning | 3 weeks | 33/44 | 4/12 | Not reported | |
| Pain/tenderness | 3 weeks | 24/44 | 0/12 | ||
| Erosion | 3 weeks | 21/44 | 0/12 | ||
| Section 5: Clinical trial quality | |||||
| Outcome | Risk of bias | Risk assessmentc | Comments | ||
| Random sequence generation | ? | It is stated that women were randomly selected for treatment. Additional details on the method of randomisation not reported | |||
| Allocation concealment | ? | Details not provided | |||
| Selective reporting | ? | Insufficient information reported to assess level of selective reporting | |||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | |||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | The study is described as double blind and it is stated that ‘all test tube preparations had an identical appearance’ (p. 171). Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | Unclear whether the clinician assessing clinical outcomes was masked to treatment allocation | |||
| Incomplete outcome data | ? | Loss to follow-up and reasons for withdrawal reported. Not all women randomised evaluated in their allocated treatment group. Although loss to follow-up is low, there is an imbalance between the groups in the proportion of women withdrawing. The effect of this minor imbalance on the estimate of effect is unclear | |||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The study is described as double blind and it is stated that ‘all test tube preparations had an identical appearance’ (p. 171). Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | Unclear whether the clinician assessing clinical outcomes was masked to treatment allocation | |||
| Incomplete outcome data | ? | It is unclear whether there were any additional losses to follow-up during the follow-up period. The analysis is based on all people with complete clearance | |||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blind and it is stated that ‘all test tube preparations had an identical appearance’ (p. 171). Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | ||
| Blinding of outcomes assessment | ? | Unclear whether the clinician assessing clinical outcomes was masked to treatment allocation | |||
| Incomplete outcome data | ? | Loss to follow-up and reasons for withdrawal reported. Not all women randomised were evaluated in their allocated treatment group. Although loss to follow-up is low, there is an imbalance between the groups in the proportion of women withdrawing. The effect of this minor imbalance on the results is unclear | |||
| Overall rating of bias | ? | Reflects limited reporting on methodology in the full publication | |||
| Section 6: Additional comments | |||||
| Additional comments |
|
||||
| Item | Details | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | |||||||||||||||||||||||||||||
| Reviewers’ names | Jacoby Patterson and Sam Barton | ||||||||||||||||||||||||||||
| Study ID | von Krogh 1994 | ||||||||||||||||||||||||||||
| Study details | Genitourin Med 1994;70:105–9 | ||||||||||||||||||||||||||||
| Language of publication | English | ||||||||||||||||||||||||||||
| Type of report | Full publication | ||||||||||||||||||||||||||||
| Section 2: Study information | |||||||||||||||||||||||||||||
| Location and number of sites | Study carried out at Department of Dermatovenereology, South Hospital, Stockholm, Sweden; study indicates that only one site involved | ||||||||||||||||||||||||||||
| Trial sponsor | Not reported | ||||||||||||||||||||||||||||
| Conflicts of interest | Not reported | ||||||||||||||||||||||||||||
| Patient enrolment | Patients were recruited from men attending STD outpatient department for previously untreated penile warts. Dates of enrolment not reported | ||||||||||||||||||||||||||||
| Trial design | RCT | ||||||||||||||||||||||||||||
| Trial duration | Initial treatment period of a maximum of 2 weeks with follow-up of up to 23 weeks for those considered to have complete clearance | ||||||||||||||||||||||||||||
| Line of therapy | First line | ||||||||||||||||||||||||||||
| Inclusion criteria | Men attending STD outpatient department who had previously untreated penile AGWs | ||||||||||||||||||||||||||||
| Exclusion criteria | It is stated that at the first visit ‘concurrent syphilis was ruled out’ (p. 106); it is not stated that men with concurrent syphilis were excluded | ||||||||||||||||||||||||||||
| All outcomes reported in paper | Complete clearance; recurrence; AEs | ||||||||||||||||||||||||||||
| Subgroups evaluated | Location of AGWs | ||||||||||||||||||||||||||||
| Stratification | None reported | ||||||||||||||||||||||||||||
| Baseline measurement of disease | Four categories of AGW were recorded and monitored based on anatomical location: urinary meatus, preputial cavity, transitional area between the inner and outer aspect of the foreskin and the penile shaft. Each site was monitored for the number of AGWs during the trial. No additional details on baseline assessment available | ||||||||||||||||||||||||||||
| Treatment | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.25% solution (patient applied) | Placebo solution (patient applied) | ||||||||||||||||||||||||||
| Randomised, n | 19 | 19 | 19 | ||||||||||||||||||||||||||
| Withdrawals, n (%) | 3 (15.8) (lost to follow-up) | 1 (5.3) (lost to follow-up) | 2 (10.5) (lost to follow-up) | ||||||||||||||||||||||||||
| Loss to follow-up reported for men lost during the treatment period. Two more men from the podophyllotoxin groups were lost to follow-up during the treatment-free period | |||||||||||||||||||||||||||||
| Treatment regimen | Using swabs, men applied their allocated treatment (podophyllotoxin 0.5% solution, podophyllotoxin 0.25% solution or placebo solution) at home twice daily for 3 days. If complete clearance was not achieved after the first round of treatment, men were instructed to repeat the treatment cycle against residual AGWs. The second cycle was to start at day 8–10 after the initiation of the first cycle but could be initiated ‘when required within another week or so’ (p. 106). It is stated that the mean number of days to start of the second round of treatment was 9.5 (SD 0.6; range 6–28) | ||||||||||||||||||||||||||||
| Duration/number of administered treatment | Not reported | ||||||||||||||||||||||||||||
| Baseline patient characteristics | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.25% solution (patient applied) | Placebo solution (patient applied) | p-value | |||||||||||||||||||||||||
| Age (years), mean (with SD/SE if given) (range) | Full trial population 25.4 (17–48). Range of mean age across groups was 23.2–27.2 years; mean age not reported for individual treatment groups | It is stated that, across the groups, ages were ‘fairly well matched’ (p. 107) | |||||||||||||||||||||||||||
| Duration of disease (months), mean | Full trial population 4.1 (1–24). Range of mean duration of disease across groups was 3.7–4.6 months; mean duration of disease not reported for individual treatment groups | Difference across groups reported to be not significant | |||||||||||||||||||||||||||
| Site of AGWs, number of lesions | |||||||||||||||||||||||||||||
| Preputial cavity | 74 | 109 | 39 | Not reported | |||||||||||||||||||||||||
| Transition inner/outer part of foreskin | 58 | 104 | 94 | ||||||||||||||||||||||||||
| Other | 3 | 4 | 0 | ||||||||||||||||||||||||||
| Type of AGWs, n (%) | Not reported | ||||||||||||||||||||||||||||
| Number of AGWs, mean | 8.4 | 12.1 | 7.8 | p = 0.27 | |||||||||||||||||||||||||
| Area of AGWs (mm2), mean | Not reported | ||||||||||||||||||||||||||||
| Sex (M/F), n (%) | 100% male | ||||||||||||||||||||||||||||
| Any previous treatment, n (%) | No (100% previously untreated AGWs) | ||||||||||||||||||||||||||||
| Ethnicity, n (%) | Not reported | ||||||||||||||||||||||||||||
| Section 3: Outcomes | |||||||||||||||||||||||||||||
| Outcome | Definition | ||||||||||||||||||||||||||||
| AGW clearance at completion of treatment | Not defined. Defined here as complete clearance after a maximum of two cycles of treatment | ||||||||||||||||||||||||||||
| AGW clearance at other time points | Not defined. Defined here as complete clearance after one cycle of treatment | ||||||||||||||||||||||||||||
| Recurrence of AGWs | Reappearance of AGWs in people considered to be AGW free after treatment. Recurrence also includes men who experienced a reoccurrence (defined as AGWs occurring on previously untreated sites) | ||||||||||||||||||||||||||||
| AEs | AEs were classified as mild, moderate and pronounced; definitions of the individual categories not available. AEs monitored included itching, stinging, burning, erythema, tenderness or erosion. Data on individual AEs not reported separately for the two podophyllotoxin groups | ||||||||||||||||||||||||||||
| Section 4: Data extraction form | |||||||||||||||||||||||||||||
| Outcome | Time frame | Podophyllotoxin 0.5% solution (patient applied), n/N | Podophyllotoxin 0.25% solution (patient applied), n/N | Placebo solution (patient applied), n/N | Estimate of effect | p-value | |||||||||||||||||||||||
| Dichotomous outcomes | |||||||||||||||||||||||||||||
| AGW clearance at completion of treatment | Two treatment cycles | 13/16 | 13/18 | 0/17 | p < 0.001 for podophyllotoxin (combined) vs. placebo; no significant difference between 0.5% and 0.25% podophyllotoxin solutions | ||||||||||||||||||||||||
| AGW clearance at other time points | One treatment cycle | 9/16 | 9/18 | 0/17 | |||||||||||||||||||||||||
| Recurrence of AGWs | 20–23 weeks after treatment | 1/8 | 3/5 | 0/0 | Not reported | ||||||||||||||||||||||||
| AEs | |||||||||||||||||||||||||||||
| Mild | Maximum of two cycles of treatment | 9/16 | 9/18 | 3/17 | Not reported | ||||||||||||||||||||||||
| Moderate | 2/16 | 2/18 | 1/17 | ||||||||||||||||||||||||||
| Pronounced | 3/16 | 3/18 | 0/17 | ||||||||||||||||||||||||||
| Section 5: Clinical trial quality | |||||||||||||||||||||||||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | ||||||||||||||||||||||||||
| Random sequence generation | ? | It is stated that men were randomly allocated to treatment. Details on method used to generate random sequence not available | |||||||||||||||||||||||||||
| Allocation concealment | ? | Details on method used to conceal allocation not available | |||||||||||||||||||||||||||
| Selective reporting | ? | Insufficient information available to assess potential bias in selective reporting | |||||||||||||||||||||||||||
| ‘Other bias’ | ? | Insufficient information available to evaluate other potential sources of bias | |||||||||||||||||||||||||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | The study is described as double blind but, from the information available, it is unclear whether the patients and key study personnel were masked to treatment. It is stated that the test bottles given to patients were of identical appearance but it is unclear whether there was the potential for masking to be broken | ||||||||||||||||||||||||||
| Blinding of outcomes assessment | ? | It is stated that ‘patients who considered themselves as cured after the first three-day cycle of home treatment were instructed to return for a follow-up visit two weeks after initiation of therapy’ (p. 106). Although patients are likely to be masked to treatment, self-assessment could introduce bias. In addition, it is unclear whether the clinician assessing AGW clearance and recurrence was masked to treatment | |||||||||||||||||||||||||||
| Incomplete outcome data | ✓ | A small number of participants were lost to follow-up and a similar proportion of participants were lost from the three treatment groups. It is unlikely that the exclusion of these participants from the analyses will have a clinically relevant impact on the effect estimate | |||||||||||||||||||||||||||
| Recurrence of AGWs | Blinding (participants and personnel) | ? | The study is described as double blind but, from the information available, it is unclear whether the patients and key study personnel were masked to treatment. It is stated that the test bottles given to patients were of identical appearance but it is unclear whether there was the potential for masking to be broken | ||||||||||||||||||||||||||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing AGW recurrence was masked to treatment | |||||||||||||||||||||||||||
| Incomplete outcome data | ✓ | The number of people lost to follow-up during the observation period is reported and is low | |||||||||||||||||||||||||||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blind but, from the information available, it is unclear whether the patients and key study personnel were masked to treatment. It is stated that the test bottles given to patients were of identical appearance but it is unclear whether there was the potential for masking to be broken | ||||||||||||||||||||||||||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | |||||||||||||||||||||||||||
| Incomplete outcome data | ✓ | A small number of participants were lost to follow-up and a similar proportion of participants were lost from the three treatment groups. It is unlikely that the exclusion of these participants from the analyses will have a clinically relevant impact on the effect estimate | |||||||||||||||||||||||||||
| Overall rating of bias | ? | Reflects limited reporting in the full publication | |||||||||||||||||||||||||||
| Section 6: Additional comments | |||||||||||||||||||||||||||||
| Additional comments |
SiteNumber of AGWs remaining after treatment/number of AGWs identified at site prior to treatmentPodophyllotoxin 0.5% solution (patient applied)Podophyllotoxin 0.25% solution (patient applied)Placebo solution (patient applied)Preputial cavity0/7410/10936/39Transition inner/outer part of foreskin4/5822/10489/94Other1/31/40/0Total number of warts eradicated130/135184/2178/133 |
Site | Number of AGWs remaining after treatment/number of AGWs identified at site prior to treatment | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.25% solution (patient applied) | Placebo solution (patient applied) | Preputial cavity | 0/74 | 10/109 | 36/39 | Transition inner/outer part of foreskin | 4/58 | 22/104 | 89/94 | Other | 1/3 | 1/4 | 0/0 | Total number of warts eradicated | 130/135 | 184/217 | 8/133 | |||||||
| Site | Number of AGWs remaining after treatment/number of AGWs identified at site prior to treatment | ||||||||||||||||||||||||||||
| Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.25% solution (patient applied) | Placebo solution (patient applied) | |||||||||||||||||||||||||||
| Preputial cavity | 0/74 | 10/109 | 36/39 | ||||||||||||||||||||||||||
| Transition inner/outer part of foreskin | 4/58 | 22/104 | 89/94 | ||||||||||||||||||||||||||
| Other | 1/3 | 1/4 | 0/0 | ||||||||||||||||||||||||||
| Total number of warts eradicated | 130/135 | 184/217 | 8/133 | ||||||||||||||||||||||||||
| Item | Details | |||||
|---|---|---|---|---|---|---|
| Section 1: Reviewer and study information | ||||||
| Reviewers’ names | Jacoby Patterson and Victoria Wakefield | |||||
| Study ID | White 1997 | |||||
| Study details | Genitourin Med 1997;73:184–7 | |||||
| Language of publication | English | |||||
| Type of report | Full publication | |||||
| Section 2: Study information | ||||||
| Location and number of sites | Study carried out at two centres in the UK (Birmingham General Hospital and Coventry and Warwickshire Hospital) | |||||
| Trial sponsor | Former United Birmingham Hospitals trust fund | |||||
| Conflicts of interest | Not reported | |||||
| Patient enrolment | Men presenting with a first episode of untreated penile AGWs at the Departments of Genitourinary Medicine at Birmingham General Hospital and the Coventry and Warwickshire Hospital were eligible for the study. Recruitment occurred between September 1991 and October 1992 | |||||
| Trial design | RCT (three arms) | |||||
| Trial duration | Final follow-up occurred at 3 months (period of initial treatment unclear) | |||||
| Line of therapy | First line | |||||
| Inclusion criteria | Men with first-episode, untreated penile AGWs were eligible | |||||
| Exclusion criteria | Age < 16 years; presence of non-penile AGWs requiring separate treatment; any substantial risk for HIV infection; another painful penile condition; presence of intrameatal AGWs; receipt of any treatment for AGWs in the 12 months preceding trial entry | |||||
| All outcomes reported in paper | Complete clearance; AEs; patient satisfaction with treatment | |||||
| Subgroups evaluated | None reported | |||||
| Stratification | None reported | |||||
| Baseline measurement of disease | At baseline visit, the duration of AGWs was recorded. In addition, the number, size and morphology of AGWs was assessed and recorded | |||||
| Treatment | Podophyllin 0.5% (patient applied) | Podophyllin 2% (patient applied) | Podophyllotoxin 0.5% (patient applied) | |||
| Randomised, n | 103 | 106 | 106 | |||
| Withdrawals | 86 men were eligible for assessment according to the protocol. Total number of withdrawals not reported | 81 men were eligible for assessment according to the protocol. Total number of withdrawals not reported | 77 men were eligible for assessment according to the protocol. Total number of withdrawals not reported | |||
| Side effects, n | 0 | 4 | 0 | |||
| Excluded from analysis because of protocol violations/not meeting entry criteria, n | 17 | 25 | 29 | |||
| Number of protocol-eligible men failing to attend follow-up at 5 weeks, n | 46 | 42 | 51 | |||
| Treatment regimen | Allocated treatment [podophyllin 0.5% or 2.0% or podophyllotoxin 0.5% (formulations not reported)] was self-applied twice daily for 3 consecutive days per week using 1.0 µl soft plastic microbiological loops. Men were instructed that, if soreness occurred, they should stop applying the medication but could recommence the following week. If soreness/side effects proved unacceptable, the men left the study. Number of treatment cycles permitted is unclear. Podophyllin was prepared in the Birmingham General Hospital pharmacy, from Podophyllum hexandrum (emodi)-derived podophyllin resin powder in 90% industrial methylated spirits | |||||
| Duration/number of administered treatment | Not reported | |||||
| Baseline patient characteristics | Podophyllin 0.5% (patient applied) | Podophyllin 2% (patient applied) | Podophyllotoxin 0.5% (patient applied) | p-value | ||
| Age (years), mean | Not reported | |||||
| Duration of disease, n (%) | ||||||
| Unknown | 11 (10.7) | 19 (17.9) | 22 (20.8) | Reported as not significant | ||
| < 1 month | 27 (26.2) | 31 (29.2) | 24 (22.6) | |||
| 1–3 months | 45 (43.7) | 33 (31.1) | 36 (34.0) | |||
| > 3 months | 20 (19.4) | 23 (21.7) | 24 (22.6) | |||
| Site of AGWs, n (%) | Not reported | |||||
| Type of AGWs, n (%) | ||||||
| Unknown | 19 (18.4) | 25 (23.6) | 28 (26.4) | Reported as not significant | ||
| Acuminata | 38 (36.9) | 29 (27.4) | 40 (37.7) | |||
| Sessile | 41 (39.8) | 47 (44.3) | 30 (28.3) | |||
| Mixed | 5 (4.9) | 5 (4.7) | 8 (7.5) | |||
| Number of AGWs, n (%) | ||||||
| Unknown | 17 (16.5) | 20 (18.9) | 22 (20.8) | Reported as not significant | ||
| 1–5 | 54 (52.4) | 55 (51.9) | 45 (42.5) | |||
| 5–10 | 20 (19.4) | 24 (22.6) | 28 (26.4) | |||
| > 10 | 12 (11.7) | 7 (6.6) | 11 (10.4) | |||
| Area of AGWs (mm2), mean | Not reported | |||||
| Sex (M/F), n (%) | 100% male | |||||
| Any previous treatment, n (%) | 100% untreated | |||||
| Ethnicity, n (%) | Not reported | |||||
| Section 3: Outcomes | ||||||
| Outcome | Definition | |||||
| AGW clearance at completion of treatment | Complete clearance. Follow-up was carried out at week 5 and at 3 months after commencing treatment. In the full publication, results are reported only for week 5 | |||||
| AEs | AEs were categorised as mild/none, moderate or severe. Definitions not reported for mild, moderate and severe and type of AEs experienced not reported | |||||
| Section 4: Data extraction form | ||||||
| Outcome | Time frame | Podophyllin 0.5% (patient applied), n/N | Podophyllin 2% (patient applied), n/N | Podophyllotoxin 0.5% (patient applied), n/N | Estimate of effect | p-value |
| Dichotomous outcomes | ||||||
| AGW clearance at completion of treatment (healed completely on clinical examination) | Week 5 | 28/40 | 28/39 | 18/26 | p = 0.97 | |
| AGW clearance at completion of treatment (healed completely on clinical examination or patient self-assessment via postal questionnaire) | Week 5 | 41/106 | 42/103 | 38/106 | Not reported | |
| AEs | ||||||
| Unevaluable | Week 5 | 7/103 | 3/106 | 5/106 | Not reported | |
| Mild/none | Week 5 | 33/103 | 32/106 | 22/106 | Not reported | |
| Moderate | Week 5 | 2/103 | 5/106 | 2/106 | Not reported | |
| Severe | Week 5 | 2/103 | 0/106 | 0/106 | Not reported | |
| Severe/withdrawn | Week 5 | 0/103 | 4/106 | 0/106 | Not reported | |
| Section 5: Clinical trial quality | ||||||
| Outcome | Risk of bias | Risk assessmenta | Comments | |||
| Random sequence generation | ✓ | It is stated that a computer-generated random allocation list was used | ||||
| Allocation concealment | ✓ | Treatment was dispensed by the hospital pharmacy in sealed boxes with obscured contents labels | ||||
| Selective reporting | ✗ | Formulations of included interventions and duration of treatment are not reported, which precludes comparison with results from other studies | ||||
| ‘Other bias’ | ? | Insufficient information provided to determine presence of additional sources of bias | ||||
| AGW clearance at completion of treatment and at other time points | Blinding (participants and personnel) | ? | The study is described as double blind. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | |||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | ||||
| Incomplete outcome data | ✗ | High number of withdrawals with no reasons reported and an imbalance in number of withdrawals across treatment groups. The high withdrawal rate and imbalance in withdrawal rate is likely to influence the estimate of effect | ||||
| AEs | Blinding (participants and personnel) | ? | The study is described as double blind. Limited details on methods are reported and it is unclear who was masked to treatment and whether masking could have been broken | |||
| Blinding of outcomes assessment | ? | It is unclear whether the clinician assessing clinical outcomes was masked to treatment | ||||
| Incomplete outcome data | ✗ | High number of withdrawals with no reasons reported and imbalance in number of withdrawals between treatment groups. The high withdrawal rate and imbalance in withdrawal rate is likely to influence the estimate of effect | ||||
| Overall rating of bias | ✗ | Reflects limited reporting in full publication and high loss to follow-up | ||||
| Section 6: Additional comments | ||||||
| Additional comments | A total of 19 patients who did not conform to the entry criteria and a further 52 classified as ‘protocol violators’ had follow-up entries set to ‘unknown results’. Only 105 of 277 protocol-eligible patients attended for clinical examination at week 5. Only 76 patients attended at 3 months for review. As a consequence of the low numbers attending follow-up, analyses were not carried out | |||||
Economic data abstraction
| Author, year, country | Overview | Population | Intervention | Costs | Outcomes | ICER | Uncertainty |
|---|---|---|---|---|---|---|---|
| Langley 2010,163 USA | Cost-effectiveness analysis. A one-stage decision model (first-line therapies only) estimating cost-effectiveness. The decision model developed was based on the model described in a previous analysis by the same author. The analysis was from a US health-care perspective with a time horizon of treatment duration of 16 weeks. In addition, a two-stage model was reported; this model considered costs associated with the addition of a second line of therapy | People with external genital warts | First-line: sinecatechins, imiquimod | Costs captured: drug costs, physician costs and procedure costs Source: average wholesale price data and for procedures 2009 Medicare fee schedule Costs were not discounted Average cost of first-line therapy $774 for sinecatechins and $930 for imiquimod |
Percentage sustained clearance (estimated as initial clearance multiplied by one minus recurrence rate) obtained from the published literature. Sustained clearance for sinecatechins was estimated by pooling data from two clinical trials Sustained clearance: 51.9% sinecatechins, 40.6% imiquimod |
Incremental cost-effectiveness was not reported. Cost per sustained clearance was estimated to be $1492 and $2289 for sinecatechins and imiquimod respectively | The authors found that use of sinecatechins first line was the dominant strategy. Scenario analysis was carried out; the results of the model were robust to the scenarios modelled |
| Walczak 2009,164 Poland | A cost-effectiveness analysis conducted from a payer (patient and health-care provider) perspective. A decision model was built with a time horizon of 28 weeks (duration of imiquimod clinical trial) | Adults with genital/perianal warts | Imiquimod, podophyllotoxin | Costs of medications and clinic visits were included. Costs were not discounted. Total costs estimated to be 1121.34 PLN for imiquimod and 218.35 PLN for podophyllotoxin | Total clearing of warts based on published literature. Effects were not discounted. Probability of total clearing of warts 0.429 imiquimod, 0.196 podophyllotoxin | The ICER was incremental cost per total clearing of warts. The ICER for imiquimod vs. podophyllotoxin was PLN 3865 | Authors concluded that imiquimod was more effective and more expensive compared with podophyllotoxin. No sensitivity analysis was reported |
| Lacey 2003,64 UK | A UK trial-based cost-effectiveness analysis carried out from a societal perspective. Time horizon was 12 weeks | 354 immunocompetent women and men, aged 18–65 years with current genital warts and receiving no therapy | Clinic-applied 25% podophyllin, patient-applied 0.15% podophyllotoxin cream, patient-applied 0.5% podophyllotoxin solution | Cost year 1998. Costs captured included treatment costs, cost of physician visits, costs of AEs, costs resulting from absence from work and travelling costs. Costs for physician visits were obtained from six units participating in the study. Costs resulting from absence from work were estimated using average incomes for women and men in the UK in 1998. Costs were not discounted. The estimated total cost at 12 weeks was £535 for clinic-applied podophyllin solution, £573 for patient-applied podophyllotoxin cream and £517 for podophyllotoxin solution | Measure of effectiveness was complete remission of all warts at 12 weeks; data were obtained from the clinical trial. The probability of an AE was taken from the clinical trial. Outcomes were not discounted. The estimated complete remission rate at 12 weeks was 46.9% for clinic-applied podophyllin solution, 62.2% for patient-applied podophyllotoxin cream and 70.2% for podophyllotoxin solution | The ICER was incremental cost per additional patient cured. The ICER reported for podophyllotoxin cream vs. podophyllin was £246.73. Podophyllin vs. podophyllotoxin solution was assessed to be dominated (more costly and less effective) | No sensitivity analysis was reported; CIs reflect the uncertainty around the effectiveness data |
| Lafuma 2003,165 France | A cost-effectiveness analysis carried out from the perspective of the French national health insurance scheme. A decision tree was developed, incorporating two lines of treatment. At first line, people had a probability of being cured or not. At second line, people who were cured had a probability of relapse; people who were not initially cured had a probability of being cured with second-line treatment. The time horizon of the model was the initial treatment duration (4–16 weeks) followed by 3 months’ follow-up | People with external AGWs | Imiquimod 5% (16 weeks) followed by CO2 laser therapy for people with treatment failure or recurrence following clearance; podophyllotoxin 0.5% (4 weeks) followed by CO2 laser therapy for people with treatment failure or recurrence following clearance | Direct medical-related costs were included. Resource use was estimated from a survey of 21 French physicians with experience of treating sexually transmitted diseases. Costs were obtained from national sources | The proportion of patients cured and the probability of relapse for imiquimod and podophyllotoxin was obtained from reanalysis of data published in two clinical trials.68,118 The proportion of patients cured with laser therapy was obtained through a review of the literature | The ICER calculated was incremental cost per patient cured at the end of all treatments. The estimated incremental cost per additional patient cured for imiquimod vs. podophyllotoxin was €603 | Scenario analyses were carried out, varying the number of sessions of podophyllotoxin (to 8 weeks) and effectiveness (clearance and recurrence). The authors reported the ICER to be robust to changes |
| Williams 2003,166 UK | A cost-effectiveness analysis carried out from a UK NHS perspective. A decision model was developed with a time horizon of between 16 and 28 weeks | People with AGWs who were HIV negative | Imiquimod, podophyllotoxin | Costs included were UK drug acquisition costs obtained from the BNF 42 (2002); GUM clinic costs were obtained from Lacey et al.64 Costs were not discounted. Total cost was estimated to be £109.95 for treatment with podophyllotoxin and £245.83 for treatment with imiquimod | Sustained clearance percentage was estimated by combining estimates of clearance at the end of clinical trials and estimates of recurrence in a 12-week follow-up period. Clinical trial estimates of clearance were obtained from a simple search (for podophyllotoxin) and use of a recent quantitative review (for imiquimod). Estimates identified were pooled by summation between trials and 95% CIs were calculated. Sustained clearance was estimated as the multiple of the pooled clearance rate and the pooled complement of the recurrence rate. Sustained clearance was estimated to be 35.1% for podophyllotoxin and 40.6% for imiquimod | Incremental cost-effectiveness (incremental cost per sustained clearance) for imiquimod vs. podophyllotoxin was £2477 per additional sustained clearance | A series of one-way and probabilistic sensitivity analyses were carried out. In probabilistic analysis, the authors found that, in 9995 of the 10,000 iterations of the model, podophyllotoxin treatment was dominant compared with imiquimod (i.e. was less costly and more effective). This conclusion implies that only five out of 10,000 simulations resulted in imiquimod having higher efficacy, despite having a higher base-case efficacy (40.6% vs. 35.1%) |
| Alam 2001,167 USA | A cost-effectiveness analysis carried out from a health-care provider perspective | Adults with no presenting complaints other than condylomata acuminata | Surgical excision, loop electrosurgical excision, electrodesiccation, CO2 laser therapy, podofilox, pulsed-dye laser therapy, cryotherapy, TCAA, imiquimod, podophyllum resin 25%, interferon-alpha-2b. All interventions were assessed as monotherapy | Costs of physician visits, procedures, medications and medical devices were included (direct medical costs). Office visits and physician-administered treatment costs were estimated from the November 1999 Medicare fee schedule; costs of medications and medical devices were estimated as average wholesale prices quoted in the 2000 Red Book Drug Topics. Drug usage was estimated from published guidelines or published empirical data if more recent | Short-term efficacy rates taken from a review of the literature were used to weight treatment costs | ICERs were not reported. Mean medical costs per complete clearance of simple and extensive condylomata were $285 surgical excision, $316 loop electrosurgical excision, $347 electrodesiccation, $416 CO2 laser therapy, $424 podofilox, $479 pulsed-dye laser therapy, $951 cryotherapy, $986 TCAA, $1255 imiquimod, $1632 podophyllum resin and $6665 interferon-alpha-2b. Surgical excision was associated with the lowest cost per complete clearance | No sensitivity analysis was reported |
| Fagnani 2000,168 France | A cost-effectiveness analysis from a French national health system perspective. A decision model was developed in which people had a maximum of two lines of therapy and were either cured or not cured at each line; people could relapse at either line of therapy | People with external AGWs | First-line treatment: imiquimod 5%, podophyllotoxin gel 0.5%; second-line treatment: CO2 laser therapy | Treatment-related direct costs were included. Costs were not discounted. The average cost per patient was estimated to be 2733 francs for imiquimod followed by CO2 laser therapy and 2120 francs for podophyllotoxin followed by CO2 laser therapy | Patients cured and relapse rates, based on published literature. Percentage of disease cured after imiquimod strategy: 62.4%; percentage of disease cured after podophyllotoxin strategy: 46.9% | Incremental cost-effectiveness was not reported. Cost per patient cured was 4383 francs for the imiquimod strategy and 4519 francs for the podophyllotoxin strategy | A scenario analysis was reported in which fewer sessions with podophyllotoxin was assessed |
| Langley 1999,169 USA | A description of three cost-effectiveness analyses carried out from a health-care payer perspective. Decision-analytical models were developed for the analyses. All models considered two lines of treatment, with second-line treatment a result of first-line treatment failure | People with external genital warts | Analysis 1: imiquimod 5% first line followed by podophyllin, cryotherapy or TCAA Analysis 2: podofilox (podophyllotoxin 0.5% solution) first line followed by podophyllin, cryotherapy or TCAA Analysis 3: cryotherapy first line followed by imiquimod 5% |
Cost year 1998. Direct treatment-related costs were used. Provider-administered costs were based on published literature whereas patient-applied therapy costs were based on average wholesale price costs. No discounting was applied to costs | Sustained clearance based on published literature | ICERs were not reported Analysis 1: cost per sustained clearance for imiquimod followed by podophyllin $1456, cryotherapy $1367, TCAA, $1237 Analysis 2: cost per sustained clearance for podophyllotoxin followed by podophyllin $1737, cryotherapy $1508, TCAA $1239 Analysis 3: cost per sustained clearance for cryotherapy followed by imiquimod $1368 |
A scenario analysis was carried out using lower bounds of clearance for the following combinations: imiquimod followed by cryotherapy, podofilox followed by cryotherapy and cryotherapy followed by imiquimod. Cost per sustained clearance decreased such that podofilox followed by cryotherapy became the least favourable option. Imiquimod followed by cryotherapy and cryotherapy followed by imiquimod had nearly identical costs per sustained clearance |
| Langley 1999,170 USA | A description of three cost-effectiveness analyses carried out from a health-care payer perspective, using decision tree models. Analysis 1 evaluated patient-applied therapies (12-week time horizon); analysis 2 evaluated provider-administered ablative therapies (time horizon not stated); analysis 3 evaluated a two-stage approach in which patient-applied therapies were used initially followed by provider-administered therapies for failures (time horizon not stated) | People with external genital warts | Analysis 1 (patient-applied therapies): imiquimod, podofilox (podophyllotoxin 0.5% solution) Analysis 2 (provider-administered therapies): podophyllin, laser surgery, cryotherapy, TCAA Analysis 3 (two-stage model): patient-applied therapies followed by provider-administered therapies for treatment failures |
Direct treatment costs were included (costs of physician visits and drug therapy). The cost estimates were based on a 1992 study by Strauss.198 Costs were inflated to 1997 prices. Costs were not discounted | A literature search was carried out using MEDLINE. Studies containing sustained clearance data at or close to 12 weeks for monotherapy were reviewed by an expert panel. ITT clearance rates were extracted from the included papers. People who discontinued therapy were assumed to be non-responders | ICERs were not reported. Analysis 1: cost per sustained clearance was $1150 for imiquimod and $990 for podofilox Analysis 2: cost per sustained clearance was $2508 for podophyllin, $1120 for laser surgery, $1833 for cryotherapy and $1300 for TCAA Analysis 3: Cost per sustained clearance when assuming clearance rate for ablative treatment was 30% (the average sustained clearance rate for provider-administered therapies) was $1263 and $1304 for imiquimod and podofilox respectively |
A scenario analysis was carried out using alternative sustained clearance rates for provider treatments (10% and 20%). The authors found that, regardless of the assumed sustained clearance rate for provider treatments, imiquimod had a lower cost per sustained clearance |
| Mohanty 1994,171 UK | A retrospective cost-effectiveness analysis using GUM clinic data. The perspective of the analysis was a health-care perspective | 683 people aged ≥ 16 years who attended St Luke’s Hospital GUM clinic in 1991, were diagnosed with genital warts and who received and completed treatment with either podophyllin 25% resin or 0.5% podophyllotoxin solution first line | Podophyllin 25% vs. podophyllotoxin 0.5% solution as first-line therapy followed by cryotherapy, TCAA or electrocautery; the choice of second-line therapy was dependent on the size and number of warts | Direct costs of treatment were included (staff and drug costs). An average cost associated with a doctor was applied to reflect the varying grades of doctors present at the GUM clinic. The cost of a receptionist was included in the analysis. Costs were not discounted. The average cost per patient was £14.95 for podophyllin 25% and £20.75 for podophyllotoxin 0.5% solution | Patients cured (complete clearance of warts and no recurrence after 3 weeks of clearance). The proportion of patients cured was estimated based on retrospective analysis of clinic data. The percentage of patients cured was 34.6% for podophyllin 25% and 66% for podophyllotoxin 0.5% solution | Incremental cost-effectiveness not reported. Cost per patient cured was £27.15 for podophyllin 25% and £25.73 for podophyllotoxin 0.5% solution. The authors concluded that, although the cost per patient was higher for podophyllotoxin solution than for podophyllin, because the efficacy of podophyllotoxin was greater than that of podophyllin the cost per patient cured was lower for podophyllotoxin | No sensitivity analysis was reported |
| Author, year, country | Population | Methods | Health states | Instrument (valuation) | Utility results | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dominiak-Felden 2013,237 UK | 186 patients aged 18–64 years with a current episode of genital warts | Patients completed the EQ-5D along with 43 patients with vulval intraepithelial neoplasia. Patients were recruited at 15 centres across the UK between May 2008 and March 2009. Their scores were compared with the UK general population normal values | With genital warts, without genital warts | EQ-5D | With genital warts: women 0.84 (SD 0.16), unweighted; men 0.89 (SD 0.17), unweighted; all 0.90 (SD 0.13), weighted; all (aged 18–24 years) 0.86, weighted; all (aged 25–34 years) 0.93, weighted Without genital warts: all 0.89; all (aged 18–24 years) 0.94; all (aged 25–34 years) 0.93 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mennini 2013, Italy222 | 421 patients with HPV-related diseases in Italy; number of patients with AGWs specifically was not reported | Patients completed a QoL questionnaire | With AGWs | EQ-5D (time trade-off) | Mean utility value with AGWs 0.58 (SD 0.31) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shi 2012, China223 | A convenience sample of 1358 genital wart patients from 18 centres in seven geographical regions of China | Patients completed QoL and demographic questionnaires | EQ-5D scores for multiple covariates including gender, region, urban/rural, marital status, education, income, insurance coverage, smoking status, number of sexual partners and clinical status. In addition, overall EQ-5D score was available for Japanese, US and UK valuations | EQ-5D (time trade-off), using Japanese valuations | Preference weightsMeanSDJapan0.8430.129UK0.8260.201USA0.8590.145 | Preference weights | Mean | SD | Japan | 0.843 | 0.129 | UK | 0.826 | 0.201 | USA | 0.859 | 0.145 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Preference weights | Mean | SD | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Japan | 0.843 | 0.129 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UK | 0.826 | 0.201 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| USA | 0.859 | 0.145 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drolet 2011,227 Canada | 272 patients with first or recurrent AGWs in Canada | Between September 2006 and February 2008, patients with a first or recurrent episode of AGWs were recruited from the clinical practices of 42 physicians across Canada. QoL assessed at recruitment, month 2 and month 6. Canadian norms for the EQ-5D were also presented | QoL presented at recruitment, at follow-up dependent on clearance or not and by gender | EQ-5D (time trade-off), SF-6D (standard gamble) | Utility measurePopulation norm, meanAGWs at recruitment6 months, with clearance of warts6 months, no clearance of wartsMean95% CIMean95% CIMean95% CIWomenEQ-5D88.677.474.0 to 80.889.384.6 to 94.079.673.4 to 85.7SF-6DNA7169.0 to 73.076.773.8 to 79.471.567.8 to 75.2MenEQ-5D89.18177.4 to 84.586.179.8 to 92.383.878.5 to 89.1SF-6DNA74.2(2.0 to 76.577.573.2 to 81.873.870.3 to 77.4NA, not applicable. | Utility measure | Population norm, mean | AGWs at recruitment | 6 months, with clearance of warts | 6 months, no clearance of warts | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Women | EQ-5D | 88.6 | 77.4 | 74.0 to 80.8 | 89.3 | 84.6 to 94.0 | 79.6 | 73.4 to 85.7 | SF-6D | NA | 71 | 69.0 to 73.0 | 76.7 | 73.8 to 79.4 | 71.5 | 67.8 to 75.2 | Men | EQ-5D | 89.1 | 81 | 77.4 to 84.5 | 86.1 | 79.8 to 92.3 | 83.8 | 78.5 to 89.1 | SF-6D | NA | 74.2 | (2.0 to 76.5 | 77.5 | 73.2 to 81.8 | 73.8 | 70.3 to 77.4 | NA, not applicable. | ||||||||||||||||||||||||
| Utility measure | Population norm, mean | AGWs at recruitment | 6 months, with clearance of warts | 6 months, no clearance of warts | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EQ-5D | 88.6 | 77.4 | 74.0 to 80.8 | 89.3 | 84.6 to 94.0 | 79.6 | 73.4 to 85.7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-6D | NA | 71 | 69.0 to 73.0 | 76.7 | 73.8 to 79.4 | 71.5 | 67.8 to 75.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EQ-5D | 89.1 | 81 | 77.4 to 84.5 | 86.1 | 79.8 to 92.3 | 83.8 | 78.5 to 89.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-6D | NA | 74.2 | (2.0 to 76.5 | 77.5 | 73.2 to 81.8 | 73.8 | 70.3 to 77.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NA, not applicable. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mennini 2011,224 Italy | 36 women with histologically confirmed diagnosis of high-grade cervical intraepithelial neoplasias in Italy | 42 patients were screened at three clinical research centres in Italy. A QoL questionnaire was administered | AGWs by study centre and overall | EQ-5D (time trade-off) | Mean EQ-5D for AGWs overall: 0.71 (SD 0.35) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Senecal 2011,225 Canada | 273 adults with initial occurrence or recurrence of genital warts in Canada | 42 physicians across Canada recruited adults with genital warts. Patients completed a questionnaire collecting QoL and demographic data three times over a 6-month period (baseline, 2 months, 6 months). QoL scores were compared with Canadian population norms from a 1997 study of 3594 residents of Alberta. A further comparative analysis was carried out using US data | Disutility associated with genital warts for all patients and by covariates: gender, age, number of recurrences, time since onset of first genital warts episode, patient assessment of severity, physician assessment of severity | EQ-5D (time trade-off) | EQ-5D scores for covariates presented within the paper. Overall EQ-5D loss (mean percentage points) for genital warts: 9.9 (95% CI 7.3 to 12.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Woodhall 2011,176 England and Northern Ireland | A convenience sample of 895 people aged ≥ 16 years with a current diagnosis of genital warts from seven sexual health clinics in England and one in Northern Ireland | Patients were invited to respond to demographic, clinical, behavioural and QoL questions from August 2009 to February 2010. QoL scores were compared with age group- and sex-matched average scores from the UK reference population. Consenting patients received a second questionnaire 2 weeks after their baseline visit | Utility associated with and without genital warts, by gender | EQ-5D (time trade-off) | EQ-5D score genital warts (women and men): 0.87 (95% CI 0.85 to 0.89), disutility: 0.056 (95% CI 0.038 to 0.074); EQ-5D score genital warts (women): 0.87 (95% CI 0.83 to 0.90), disutility: 0.063 (95% CI 0.029 to 0.097); EQ-5D score genital warts (men): 0.88 (95% CI 0.86 to 0.90), disutility: 0.043 (95% CI 0.021 to 0.065) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fiander 2010,230 UK | Data from an observational cross-sectional study in which 1264 subjects (women and men) aged 18–64 years were recruited from 15 community and hospital health-care clinics | Women with genital warts aged 18–25 years recruited into the observational cross-sectional study. Number of patients not reported | Utility associated with genital warts | EQ-5D (time trade-off) | EQ-5D score associated with genital warts in women aged 18–25 years: 0.83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fiander 2010,231 UK | Data from an observational cross-sectional study of HPV-related disease in which 1264 subjects (women and men) aged 18–64 years were recruited from 15 community and hospital health-care clinics | Men with genital warts aged 18–25 years recruited into the observational cross-sectional study. Number of patients not reported | Utility associated with genital warts | EQ-5D (time trade-off) | EQ-5D score associated with genital warts in men aged 18–25 years: 0.89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Langley 2010,228 UK, France, Spain, Italy and Germany | Internet-based survey carried out in the UK, France, Spain, Italy and Germany; 53,524 respondents in total, with 521 respondents with external genital warts | Regression analysis on SF-6D scores from respondents controlling for presence/absence of external genital warts, sociodemographic characteristics, health risk factors and Charlson Comorbidity Index | Disutility associated with external genital warts | SF-6D (standard gamble) | Disutility associated with external genital warts: –2.47 (95% CI –3.58 to –1.36) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mennini 2011,224 Italy | 276 patients with HPV-related diseases (including genital warts) and 75 age-matched female healthy control subjects in Italy | Female patients with HPV-related diseases that were detected and managed in the preceding 18 months completed a computerised algorithm | Mean utility with and without genital warts | Time trade-off | Utility with genital warts: 0.69 ± 0.29; utility for healthy control subjects: 0.92 ± 0.19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Marra 2009, Canada229 | 75 participants with a history of AGWs who were recruited through a newspaper advertisement in Vancouver, Canada | Participants were asked questions about their sociodemographic and health status and filled in two QoL questionnaires. Participants were asked to consider the health state they were experiencing when they had AGWs | Utility scores with AGWs | EQ-5D (time trade-off), SF-6D (standard gamble) | EQ-5D score for genital warts: 0.76 (SD 0.19); SF-6D score for genital warts: 0.74 (SD 0.13) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Woodhall 2008,32 England | 81 adults attending the York GUM clinic with new, recurrent or persistent genital warts | Consenting participants completed questionnaires on demographic status and QoL. An EQ-5D control group was considered that included 1977 people in the same age range as the sample with genital warts | QoL with and without genital warts | EQ-5D (time trade-off) | Unadjusted: mean EQ-5D score with genital warts (n = 81): 0.90, mean EQ-5D without genital warts (n = 1977): 0.91; adjusted for age and gender, mean EQ-5D difference between people with genital warts and people without genital warts: 0.039 (95% CI 0.005 to 0.078) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brisson 2007, Canada226 | 31 women presenting at a physician’s office with genital warts | The QoL estimates from the 31 women with genital warts presenting at a physician’s office were compared with average population QoL estimates, matched on age and gender | Disutility associated with genital warts | EQ-5D (time trade-off) | Disutility associated with genital warts: 0.10 (95% CI 0.05 to 0.15) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Author, year | Studied population | Methods overview | Incidence/prevalence data | Cost year | Therapy costs | Resource use | Resource use costs | Cost per episode | Cost per population |
|---|---|---|---|---|---|---|---|---|---|
| Chapman 2013215 | UK and devolved nations | Cost was calculated by multiplying the estimated number of people with genital warts in the UK by the expected resource use for these patients and applying UK-specific unit costs | Cases of genital warts at GUM clinics estimated using data from the HPA for the UK and England, the Information Services Division for Scotland, the Communicable Disease Surveillance Centre for Wales and the Public Health Agency for Northern Ireland. Cases of genital warts in primary care estimated using data from the Health Improvement Network database extrapolated using population statistics to estimate the number of genital wart cases at national level | Not stated | Therapy use estimated through discussions with GUM experts. Costs from the BNF were applied | Resource use estimated through discussions with GUM experts | Costs taken from the ‘most recent’ NHS payment by results tariffs and data from the Unit Costs for Health and Social Care | The cost per episode of genital warts was estimated to be £265 (UK) | £58.42M UK, £41.72M England, £1.9M Scotland, £1.87M Wales and £0.95M Northern Ireland |
| Lanitis 201151 | UK | Cost was calculated by multiplying the estimated number of people with genital warts in the UK by the expected resource use for these patients and applying UK-specific unit costs | Number of episodes estimated for GUM clinics and GP surgeries and projected to 2010. GUM clinics: number of cases estimated from HPA data; GP surgeries: number of cases estimated from Health Improvement Network data. Estimates for 2010: 173,077 GUM clinics (96,278 first episodes, 58,109 recurrent episodes and 18,690 persistent episodes); 16,782 primary care. On average, the four clinical experts felt that 2% (range 0.5– 8%) of patients were ‘hard to treat’ | 2010 | Therapy use estimated through discussions with four GUM experts. Average values were used in the base-case analysis. Costs from the BNF were applied | To estimate treatment utilisation patterns, four GUM clinicians were surveyed and each provided an estimate of the number of visits required per patient, based on the type of case (whether first episode, recurrent episode or persistent episode) and the type of wart (keratinised or non-keratinised). Most genital wart episodes were found to require two visits whereas persistent and hard-to-treat warts required three or more and eight to twelve visits respectively | Cost of attendance at GUM clinic taken from WF01B first attendance single professional, 360 GUM outpatient attendance tariff. £133 WF01A follow-up attendance single professional, 360 GUM, outpatient attendance tariff. £101 GP visit from the Unit Costs of Health and Social Care: £36 | £276 | £52.4M |
| Desai 20113 | England | Cost was calculated by multiplying the estimated number of people with genital warts in England with the expected resource use for these patients and applying UK-specific unit costs | Number of people presenting with genital warts (new and recurrent cases) was obtained for GUM clinics, GP surgeries and NHS hospitals. GUM clinics: data obtained from HPA data; data from 2008 and 2009 were averaged to provide an estimate of 141,770 cases each year. GP surgeries: the GPRD was reviewed between 2006 and mid-2008; GPRD data were extrapolated to the total population under GP care in England, resulting in an estimated 39,645 episodes of care annually. Hospital data were obtained using HES for people treated in a NHS hospital with a primary diagnosis of ‘anogenital (venereal) warts’, denoted by ICD-10 code A630; 1978 episodes of care were recorded to this code in 2008. To account for overlap between GPRD and HPA data, an episode with a referral code or at least one diagnostic code in the referral table and an episode with no treatment recorded were assumed to have been referred to a GUM clinic | 2008–10 | Costs presented by Woodhall et al.176 were applied to the number of people with genital warts | Not reported | Costs presented by Woodhall et al.176 were applied to the number of people with genital warts | The average cost per episode of care resolved by a GP was £79 and per episode seen by both GP and GUM clinicians was £138. The average cost per hospital care was £718. The estimated cost per episode of care for all settings was £113 (95% CI £104 to £121) | The annual cost of care in England for GP and GUM clinics combined was approximately £15.3M. The annual cost of hospital care in England was £1.4M. The total cost of care in England was £16.8M |
| Carroll 2011173 (linked to Lanitis 201251) | UK | Cost was calculated by multiplying the estimated number of people with genital warts in the UK by the expected resource use for these patients and applying UK-specific unit costs | Numbers of episodes estimated for GUM clinics and GP surgeries and projected to 2010. GUM clinics: number of cases estimated from HPA data. GP surgeries: number of cases estimated from Health Improvement Network data. Estimates for 2010: 173,077 GUM clinics (33.5% recurrent, 11% persistent), 16,882 primary care | 2010 | Therapy use estimated through discussions with GUM experts. Costs from the BNF applied | Number of visits required per episode estimated through discussions with GUM experts for standard and hard-to-treat patients | Resource use multiplied by UK-specific costs. NHS payment by results tariffs used for GUM costs. GP visit costs taken from Unit Costs of Health and Social Care | £273 female, £278 male | £52.4M |
| Woodhall 2011176 | Seven GUM clinics in UK | Estimated cost of care for people with genital warts in 2010 based on review of the treatment of patients presenting at seven sexual health clinics. The authors carried out a case note review of 370 people aged ≥ 16 years attending six sexual health clinics in England and one clinic in Northern Ireland. Patients were required to have a current diagnosis of genital warts (new or recurrent episode) and have attended the clinic between April and June 2007. The authors recorded the resources used in the care of each participant and applied costs to the resource use using a mixture of standard UK unit costs and clinic estimates of cost | Not applicable | 2010 | Unit costs per use: podophyllotoxin cream £14.86 (source electronic drug tariff), podophyllotoxin solution £12.38 (source BNF), imiquimod £51.32 (source electronic drug tariff), cryotherapy £4.27 (clinic costing), TCAA £0.32 (clinic costing), podophyllin £0.02 (clinic costing), eutectic mixture of local anaesthetic cream £1.73 (BNF), hyfrecation £5.63 (clinic costing), curettage £4.66 (clinic costing), diode laser £143.50 (clinic costing), CO2 laser therapy £125.49 (clinic costing) | Duration of episode of care from case note review: women 37 days, men 35 days, average 36 days. Average number of visits per episode of care from case note review: 2.5. The percentage of patients attending once was 45% for women and 55% for men | Not reported | The estimated mean cost per episode of care was £94 without a STI screen and £146 with a STI screen | Not reported |
| Woodhall 2009175 | 189 patients with genital warts presenting at York GUM clinic | Case note review of 189 patients registered at the York clinic who were diagnosed with genital warts (first or recurrent episode). Resources used by each patient during an episode were recorded. The time taken for each procedure was estimated through interviews with nine members of the clinical team. UK costs were applied to the resources used; staff costs were taken from the Unit Costs for Health and Social Care 2007 whereas the costs of treatments carried out in the clinic (cryotherapy, curettage, electrosurgery/hyfrecation, electrosurgery/diathermy, TCAA) were estimated from local costs of equipment and consumables | Not applicable | 2007 | The costs of home treatments were estimated from the BNF (2007): podophyllotoxin solution (males) $26.47, podophyllotoxin cream (females) $31.77, imiquimod $105.47. The costs of clinic treatments were taken from local costs of equipment and consumables (2007): cryotherapy $8.27, curettage $6.04, electrosurgery/hyfrecation $9.70, electrosurgery/diathermy $8.48, TCAA ‘negligable’ | Number of appointments taken from case notes. The mean number of visits per episode of care (both first and recurrent) was estimated to be 2.8 (95% CI 2.4 for 3.2), with first episode mean of 2.9 and recurrent mean of 2.74. Nearly half (46%) of patients had one visit per episode of care. The average length of an episode of care was 41 days, with 3% of cases having an episode of care > 6 months. The time taken for each procedure was estimated through interviews with nine members of the clinical team. Clinician time for the first visit was estimated to be 19.2 (SD 1.9) minutes (n = 9) Clinician time for the follow-up visits was estimated to be 9.7 (SD 0.94) minutes (n = 9) | Staff costs were taken from the Unit Costs for Health and Social Care 2007. Staff included specialist physician ($359.64 per hour), associate specialist ($85.58 per hour), trainee doctor ($84.26 per hour), band 5 nurse ($53.43 per hour), band 6 nurse ($61.65 per hour), band 7 nurse ($78.09 per hour), band 2 (chaperone) ($26.72 per hour) | Mean cost of an episode of care estimated to be $286 (£139; 95% CI $246 to $327) | Not reported |
| Brown 2006172 | UK | For the calculation of the costs associated with the treatment of genital warts, the authors estimated the number of people with genital warts in the UK and multiplied this by the estimated resource use and associated costs to estimate a total cost of genital wart treatment in 2003 | The number of people with genital warts (first or recurrent) presenting in GUM clinics was obtained from HPA surveillance data. The number of genital wart cases reported by GUM clinics in 2003 in the UK was 76,457 for incident cases, 38,902 for recurrent cases and 16,755 for persistent cases | 2003 | Data for drug use and procedures were obtained from questionnaires sent to six GUM clinicians in Aberdeen, Liverpool, London (n = 2), Nottingham and Southampton. Telephone interviews were conducted with each respondent to review and clarify responses. For GUM clinic visits for diathermy, cryotherapy or combination treatment (procedure plus drug therapy), an all-inclusive payment was obtained from personal communication with a clinician. Costs for topical treatments were obtained from the BNF, accessed online in February 2006. GUM clinic procedures included physician time £70, loop electrosurgical excision procedure excision £280, imiquimod 5% £55.18, podophyllotoxin 5-g tube £16.62, podophyllin 10 ml £3.00 | Data for number of visits per episode were obtained from questionnaires sent to six GUM clinicians in Aberdeen, Liverpool, London (n = 2), Nottingham and Southampton. Telephone interviews were conducted with each respondent to review and clarify responses. Responses were pooled and mean rates of events were used for costing. Length of visit was obtained from Langley et al.,174 a study that carried out a retrospective chart review | GUM clinic procedures, including physician time £70 | Not reported | The total annual cost for genital warts was £22.4M. When using individual physician treatment patterns the cost varied between £18M and £25.3M |
| Langley 2004174 | Six GUM clinics in England and Wales | Retrospective case note review of people with external genital warts, carried out in six GUM clinics in England and Wales in 2000. At each clinic, the case notes of 100 female and 100 male patients, each with a completed episode of care, were evaluated and costs were applied to the resources consumed | Not applicable | Not reported | Not reported | Average contact time and type of professional was taken from the case note review. Initial treatment visit (minutes): doctor 16.2 males, 20.5 females; nurse 10.3 males and females; health advisor 11.5 males and females. Subsequent treatment visit (minutes): doctor 10.5 males and females; nurse 7.9 males and females; health advisor 10.3 males and females | The source of cost data was not provided within the study. Labour costs were described as estimated using annual salaries. Indirect costs were described as non-labour expenses, non-patient care expenses and direct patient care costs | Average total cost £135.77 males, £146.37 females | Not reported |
Quality assessment of included economic evaluation studies
| Dimension of quality | Studies | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Langley 2010163 | Walczak 2009164 | Lafuma 2003165 | Williams 2003166 | Alam 2001167 | Fagnani 2000168 | Langley 1999169 | Langley 1999170 | |||||||||
| Structure | ||||||||||||||||
| S1: Statement of decision problem/objective | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated |
| S2: Statement of scope/perspective | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated |
| S3: Rationale for structure | ✓ | Stated | ✗ | Not stated | ✗ | Not stated | ✗ | Not stated | ✗ | Not stated | ✗ | Not stated | ✓ | Stated | ✓ | Stated |
| S4: Structural assumptions | ✓ | Stated | ✗ | Not stated | ✗ | Not stated | ✗ | Not stated | ✗ | Not stated | ✗ | Not stated | ✓ | Stated | ✓ | Stated |
| S5: Strategies/comparators | ? | Did not include the full range of comparators. Included: imiquimod | ? | Did not include the full range of comparators. Included: imiquimod, podophyllotoxin | ? | Did not include the full range of comparators. Included: imiquimod, podophyllotoxin, CO2 laser therapy | ? | Did not include the full range of comparators. Included: imiquimod, podophyllotoxin | ? | All comparators were included with the exception of cidofovir | ? | Did not include the full range of comparators. Included imiquimod, podophyllotoxin, CO2 laser therapy | ? | Did not include the full range of comparators. Included imiquimod, podophyllin, cryotherapy, TCAA | ? | Did not include the full range of comparators. Included imiquimod, podofilox, podophyllin, laser surgery, cryotherapy, TCAA |
| S6: Model type | ✓ | Decision analysis | ✓ | Decision analysis | ✓ | Decision analysis | ✓ | Decision analysis | ✓ | Stated | ✓ | Decision analysis | ✓ | Decision analysis | ✓ | Decision analysis |
| S7: Time horizon | ? | The time horizon for analysis 1 (one line of treatment) was 16 weeks. For analysis 2 (two lines of therapy) there was no fixed time frame | ✓ | 28 weeks | ✓ | 4–16 weeks | ✓ | 16–28 weeks | ✗ | Not stated | ✗ | Not stated | ✗ | Not stated | ? | Time horizon was stated for only one of the three analyses conducted |
| S8: Disease states/pathways | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated | ✓ | Stated |
| S9: Cycle length | – | NA | – | NA | – | NA | – | NA | – | NA | – | NA | – | NA | – | NA |
| Data | ||||||||||||||||
| D1: Data identification | ✓ | Reported | ✗ | Not reported | ✓ | Reported | ✓ | Reported | ✓ | Reported | ✓ | Reported | ✓ | Reported | ✓ | Reported |
| D2: Pre-model data analysis | ✓ | Reported | ✗ | Not reported | ✓ | Reported | ✓ | Reported | ✗ | Not reported | ✓ | Reported | ✗ | Not reported | ✓ | Reported |
| D2a: Baseline data | ✓ | Reported | ✗ | Not reported | ✓ | Reported | ✓ | Reported | ✗ | Not reported | ✓ | Reported | ✓ | Reported | ✓ | Reported |
| D2b: Treatment effects | ✓ | Reported | ✓ | Reported | ✓ | Reported | ✓ | Reported | ✓ | Reported | ✓ | Reported | ✓ | Reported | ✓ | Reported |
| D2c: Costs | ✓ | Reported; discounting was not discussed | ✗ | Not reported | ✓ | Reported; discounting was not discussed | ✓ | Reported; discounting was not discussed | ✓ | Reported; discounting was not discussed | ✓ | Reported | ✓ | Reported; discounting was not discussed | ✓ | Reported; discounting was not discussed |
| D2d: QoL weights (utilities) | ✗ | QoL not considered | ✗ | QoL not considered | ✗ | QoL not considered | ✗ | QoL not considered | ✗ | QoL was not incorporated | ✗ | QoL was not incorporated | ✗ | QoL was not incorporated | ✗ | QoL was not incorporated |
| D3: Data incorporation | ✓ | Reported | ✗ | Not reported | ✓ | Reported | ✓ | Stated | ✓ | Reported | ✓ | Reported | ✓ | Reported | ✓ | Reported |
| D4: Assessment of uncertainty | ? | Scenario analysis was carried out | ✗ | Not reported | ? | Scenario analysis was carried out | ✓ | One-way and probabilistic analysis | ✗ | Not reported | ? | Partial | ? | Partial | ? | Partial |
| D4a: Methodological | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported |
| D4b: Structural | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported |
| D4c: Heterogeneity | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported |
| D4d: Parameter | ? | Scenario analysis reported | ✗ | Not reported | ? | Scenario analysis reported | ✓ | One-way and probabilistic sensitivity analysis | ✗ | Not reported | ✓ | Scenario analysis was carried out | ✓ | Scenario analysis was carried out | ✓ | Scenario analysis was conducted |
| Consistency | ||||||||||||||||
| C1: Internal consistency | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported |
| C2: External consistency | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported | ✓ | Results were compared with those of previous cost-effectiveness analyses | ✓ | Comparison was made with results from previous economic evaluation studies | ✗ | Not reported | ✗ | Not reported | ✗ | Not reported |
Appendix 3 Excluded studies
| Excluded study | Reason for exclusion |
|---|---|
| Clinical observation of treatments for condyloma acuminatum. Chin J Dermatol 1989;22:114–15 | Not a RCT |
| Genital warts reduced with imiquimod. Aids Patients Care STDS 1998;12:409 | Not a RCT |
| Imiquimod (Aldara) for the treatment of external genital and perianal warts. Geneesmiddelenbulletin 2000;34:148–9 | Not a RCT |
| Imiquimod cream for genital warts. Am Fam Phys 1997;55:2348 | Not a RCT |
| Aigner F, Conrad F, Widschwendter A, Zangerle R, Zelger B, Haidenberger A, et al. [Anal HPV infections.] Wien Klin Wochenschr 2008;120:631–41 | Not a RCT |
| Audisio T, Roca FC, Piatti C. Topical imiquimod therapy for external anogenital warts in pregnant women. Int J Gynaecol Obstet 2008;100:275–6 | Not a RCT |
| Augustovski F, Pichon RA, Bardach A, Colantonio L, Ferrante D, Garcia MS, et al. Cryotherapy to anogenital warts. Health Technol Assess Database 2009;3. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32009100592/frame.html (accessed 21 December 2015) | Not a RCT |
| Bashi SA. Cryotherapy versus podophyllin in the treatment of genital warts. Int J Dermatol 1985;24:535–6 | Not a RCT |
| Batista CS, Atallah AN, Saconato H, da Silva EM. 5-FU for genital warts in non-immunocompromised individuals. Cochrane Database Syst Rev 2010;4:CD006562 | Not a RCT |
| Bianco V, Erba P, Remotti G. [Florid vulvar condylomatosis. Comparison of therapeutic schedules.] Ann Ostet Ginecol Med Perinat 1991;112:247–56 | Not a RCT |
| Billingham RP, Lewis FG. Laser versus electrical cautery in the treatment of condylomata acuminata of the anus. Surg Gynecol Obstet 1982;155:865–7 | Not a RCT |
| Chen HF. Photodynamic therapy with aminolevulinic acid (ALA-PDT) for urethral condyloma acuminatum: a clinical observation. J Clin Dermatol 2009;38:193–4 | Not an intervention of interest (photodynamic therapy plus aminolaevulinic acid) |
| Chen K, Chang BZ, Ju M, Zhang XH, Gu H. Comparative study of photodynamic therapy vs CO2 laser vaporization in treatment of condylomata acuminata: a randomized clinical trial. Br J Dermatol 2007;156:516–20 | Not an intervention of interest (photodynamic therapy plus aminolaevulinic acid) |
| Chopra K, Lee P, Tyring SK, Arany I, Mcdemott D. Vehicle-controlled study investigating the mechanism of action of 5% imiquimod cream applied three times a week for the treatment of patients with genital/perianal warts. Australas J Dermatol 1997;1320:113–14 | No outcomes of interest reported |
| Coremans G, Margaritis V, Snoeck R, Wyndaele J, de Clercq E, Geboes K. Topical cidofovir (HPMPC) is an effective adjuvant to surgical treatment of anogenital condylomata acuminata. Dis Colon Rectum 2003;46:1103–8 | Not a RCT |
| Coremans G, Wyndaele J, Dockx S, Vandenbussche FG, Geboes K, de Clercq E, et al. Eradication of intra-anal condylomata acuminata and histologically dysplasia with combined intralaesional cidofovir and coagulations. Gastroenterology 2008;134:A318 | Not a RCT |
| Damstra RJ, van Vloten WA. Cryotherapy in the treatment of condylomata acuminata: a controlled study of 64 patients. J Dermatol Surg Oncol 1991;17:273–6 | Not a RCT |
| de Luca, Kharaeva Z, Raskovic D, Pastore P, Luci A, Korkina L. Coenzyme q(10), vitamin E, selenium, and methionine in the treatment of chronic recurrent viral mucocutaneous infections. Nutrition 2012;28:509–14 | Not an intervention of interest |
| Desai A, Saple DG, Baliga V. Assessment of efficacy, safety, and tolerability of imiquimod cream 5% in adult patients with external genital warts: a first Indian study. J Am Acad Dermatol 2006;54:135 | Not a RCT |
| Di Stefano, Facchini D, de Paulis AL, Cappa F, Moscarini M. [Vulvar lesions caused by papillomavirus (HPV): effectiveness of thymopentin (syntomoduline). First results and experiences.] Minerva Ginecol 1991;43:53–6 | Not interventions of interest |
| Dominguez GJ, Simon RD, Abreu DA, Zlenkova H. Effectiveness of glycyrrhizinic acid (glizigen) and an immunostimulant (viusid) to treat anogenital warts. ISRN Dermatol 2012;2012:863692 | Not interventions of interest |
| Duus BR, Philipsen T, Christensen JD, Lundvall F, Sondergaard J. Refractory condylomata acuminata: a controlled clinical trial of carbon dioxide laser versus conventional surgical treatment. Genitourin Med 1985;61:59–61 | Not a comparator of interest (comparator is conventional surgery and includes a mixture of electrocautery and surgical excision; results are not reported separately for the individual techniques) |
| Duus BR, Dahl JC, Philipsen T. [Surgery and laser treatment of podophyllin-resistant condylomata acuminate.] Ugeskr Laeger 1986;148:1212–14 | Not a RCT |
| Einarson A, Costei A, Kalra S, Rouleau M, Koren G. The use of topical 5% imiquimod during pregnancy: a case series. Reprod Toxicol 2006;21:1–2 | Not a RCT |
| Ferenczy A. Laser treatment of patients with condylomata and squamous carcinoma precursors of the lower female genital tract. CA Cancer J Clin 1987;37:334–47 | Not a RCT |
| French LN. What is the most effective treatment for external genital warts? J Fam Pract 2014;51:313 | Not a RCT |
| Goldmeier D, Madden P, Lacey C, Legg K, Tamm N, Cowen M. Complementary therapy and genital warts. Sex Transm Infect 2005;81:360 | Not a RCT |
| Gollnick H, Barasso R, Jappe U, Ward K, Eul A, Carey-Yard M, et al. Safety and efficacy of imiquimod 5% cream in the treatment of penile genital warts in uncircumcised men when applied three times weekly or once per day. Int J STD AIDS 2001;12:22–8 | Not a RCT |
| Gori J, Castano R, Dominguez J, Puga A. Laser vaporization, cryotherapy and trichloroacetic application in the therapy of ectocervical condylomatous lesions: a comparison of clinical results. Cervix Low Fem Genit Tract 1992;10:217–19 | Not a RCT |
| Graversen PH, Bagi P, Rosenkilde P. Laser treatment of recurrent urethral condylomata acuminata in men. Scand J Urol Nephrol 1990;24:163–6 | Not a RCT |
| Greenberg MD. A double-blind, randomized trial of 0.5% podoflox and placebo for the treatment of genital warts in women. Genitourin Med 1991;67:359 | Not a RCT |
| Heim K, Krause P, Huter O, Wartusch B, Holbock E, Conrad F, et al. [Laser therapy of condylomata of the female genitals.] Gynakol Rundsch 1989;29(Suppl. 2):96–100 | Not a RCT |
| Jensen SL. Comparison of podophyllin application with simple surgical excision in clearance and recurrence of perianal condylomata acuminata. Genitourin Med 1986;62:212 | Not a RCT |
| Jiang RF, Shi TN. [123 cases curative effect observation of podophyllin resin solution improved for external use in treating condyloma acuminatum.] Chin J Dermatovenereol 1991;15:191 | Not a RCT |
| Johnson R, Stockfleth E. Imiquimod 5% cream for the treatment of cutaneous lesions in immunocompromised patients. Acta Derm Venereol Suppl (Stockh) 2003;214:23–7 | Not a RCT |
| Khawaja HT. Podophyllin versus scissor excision in the treatment of perianal condylomata acuminata: a prospective study. Br J Surg 1989;76:1067–8 | Not a RCT |
| Kressenstein S. Treatment of condyloma acuminata by patient-applied cream. Arztliche Praxis Dermatol 2000;5:37 | Not a RCT |
| Lassus A. Comparison of podophyllotoxin and podophyllin in treatment of genital warts. Lancet 1987;2:512–13 | Not a RCT |
| Lewis MI. Treatment of extensive condyloma acuminata of the anal canal. Int Surg 1973;58:412–14 | Not a RCT |
| Li CH, Lu ZZ. [The different ways of CO2 laser or electric burn therapy for condyloma acuminatum.] Chin J Dermatovenereol 1994;8:98 | Not a RCT |
| Lim KB, Lee CT, Koh YL, Yeo WL, Tan T. Self-application of podophyllin resin for penile condylomata acuminata. Ann Acad Med Singapore 1987;16:167–9 | Not a RCT |
| Lwegaba A, Phillips A, Kiraru R. Silver nitrate may be far superior to podophyllin in clearing HPV external anogenital warts. West Indian Med J 2008;57:63–5 | Not a RCT |
| Maier H, Donath P, Cabaj A, Honigsmann H. Successful pulsed dye laser treatment of genital warts. 24th Annual Meeting of the American Society for Laser Medicine and Surgery, Dallas, TX, 31 March 31–4 April 2004. Abstract 66 | Not a RCT |
| Mazurkiewicz W, Jablonska S. [Comparative studies between 0.5 percent podophyllotoxin preparations (condyline) and 20 percent podophyllin dissolved in alcohol, in the therapy of raised condylomas.] Z Hautkr 1986;61:1387–95 | Not a RCT |
| Orlando G, Fasolo MM, Beretta R, Cargnel A. Combined surgical–medical treatment of genital warts in HIV positive patients. Tumori 2001;87:s11–12 | Not a RCT |
| Potocnik M, Bartenjev I. Genital warts treatment – ultrapulse CO2 or argon laser. Australas J Dermatol 1997;38(Suppl. 2):30–1 | Not a RCT |
| Renziehausen K. [Cryotherapy of condylomata acuminata and other benign vulvar neoplasms.] Zentralbl Gynakol 1974;96:1135–9 | Not a RCT |
| Sait MA, Garg BR. Treatment of warts – a study of one hundred and six cases. Indian J Dermatol Venerol Leprol 1985;51:96–8 | Not a RCT |
| Schofer H, Van Ophoven A, Henke U, Lenz T, Eul A. Randomized, comparative trial on the sustained efficacy of topical imiquimod 5% cream versus conventional ablative methods in external anogenital warts. Eur J Dermatol 2006;16:642–8 | Not an intervention of interest (all ablative therapies analysed together) |
| Seo JY, Park SC, Oh SJ, Rim JS. Urethroscopic ND:YAG laser therapy for urethral condyloma acuminata in men. Int J Androl 2005;28:100 | Not a RCT |
| Song W. [Combination therapy in the treatment of 68 cases of condyloma acuminatum.] Chin J Dermatol 1994;27:173 | Not a RCT |
| Stefanaki C, Katzouranis I, Lagogianni E, Hadjivassiliou M, Nicolaidou E, Panagiotopoulos A, et al. Comparison of cryotherapy to imiquimod 5% in the treatment of anogenital warts. Int J STD AIDS 2008;19:722 | Not a RCT (erratum) |
| Stefanaki C, Hadjivassiliou M, Katzouranis I, Bethimoutis G, Nicolaidou E, Anyfantakis V, et al. Prognostic factors for the response to treatment in males with genital warts. J Eur Acad Dermatol Venereol 2009;23:1156–60 | Not a RCT |
| Stragier I, Snoeck R, De Clercq E, Van den Oord JJ, Van Ranst M, De Greef H. Local treatment of HPV-induced skin lesions by cidofovir. J Med Virol 2002;67:241–5 | Not a RCT |
| Thivolet J. Treatment of warts. Concours Med 1982;104:4957–66 | Not a RCT |
| Usman N, Udayashankar K Subramanian S, Thyagarajan SP. Autoimplantation technique in the treatment of anogenital warts: a clinico-immunological study. Int J STD AIDS 1996;7:55–7 | Not a RCT |
| Van Ophoven A, Schofer H, Henke U, Lenz T, Eul A. Randomized, comparative trial on the sustained efficacy of topical imiquimod 5% cream versus conventional ablative methods in external anogenital warts. J Urol 2007;177:36 | Not a RCT |
| Von Krogh G. Topical treatment of penile condylomata acuminata with podophyllin, podophyllotoxin and colchicine. A comparative study. Acta Derm Venereol 1978;58:163–8 | Not a RCT |
| Von Krogh G. Podophyllotoxin for condylomata acuminata eradication. Clinical and experimental comparative studies on podophyllum lignans, colchicine and 5-fluorouracil. Acta Derm Venereol Suppl (Stockh) 1981;98:1–48 | Not a RCT |
| Von Krogh G. Topical self-treatment of penile warts with 0.5% podophyllotoxin in ethanol for four or five days. Sex Transm Dis 1987;14:135–40 | Not a RCT |
| Ward BG, Thomas IL. Randomized prospective intervention study of human cervical wart virus infection. Aust N Z J Obstet Gynaecol 1994;34:182–5 | Not a population of interest (no AGWs) |
| Webb DGK. Management of external genital warts: a comparison of podophyllin and podophyllotoxin. Pharm J 2014;252:291–3 | Not a RCT |
| Weinberg JM, Stewart A, Stern JO. Successful treatment of extensive condyloma acuminata of the inguinal area and thigh with topical imiquimod cream. Acta Derm Venereol 2001;81:76–7 | Not a RCT |
| Wiltz OH, Torregrosa M, Wiltz O. Autogenous vaccine: the best therapy for perianal condyloma acuminata? Dis Colon Rectum 1995;38:838–41 | Not a RCT |
| Xie FM, Zeng K, Chen ZL, Li Gf, Lin ZF, Zhu XL, et al. [Treatment of recurrent condyloma acuminatum with solid lipid nanoparticle gel containing podophyllotoxin: a randomized double-blinded, controlled clinical trial.] Nan Fang Yi Ke Da Xue Xue Bao 2007;27:657–9 | Not an intervention of interest |
| Zeng K, Li GF, Xu CY, Chen ZL, Wang ZF. A double-blind randomized controlled trial of podophyllotoxin liposomes ointments in the treatment of condylomata acuminata. J First Military Med Univ 1998;18:246 | Not an intervention of interest |
| Zhou SJ, Li ZW, Li B, Cheng P. [Observation on effects of 27 cases of condyloma acuminatum treated by combined therapy.] Chin J Leprosy Skin Dis 2014;16:61 | Not an intervention of interest |
Appendix 4 Codes for fixed-effects and random-effects models in the mixed-treatment comparisons
Fixed effects
model{
for(i in 1:ns){
delta[i,t[i,1]]<-0
mu[i] ∼ dnorm(0,.0001)
for (k in 1:na[i]) {
r[i,t[i,k]] ∼ dbin(p[i,t[i,k]],n[i,t[i,k]])
logit(p[i,t[i,k]])<-mu[i] + delta[i,t[i,k]]
rhat[i,t[i,k]]<- p[i,t[i,k]] * n[i,t[i,k]]
resdev[i,k]<- 2 * (r[i,t[i,k]] * (log(r[i,t[i,k]]) - log(rhat[i,t[i,k]])) + (n[i,t[i,k]] - r[i,t[i,k]]) * (log(n[i,t[i,k]] - r[i,t[i,k]]) - log(n[i,t[i,k]] - rhat[i,t[i,k]])))
}
sumdev[i]<-sum(resdev[i,1:na[i]])
for (k in 2:na[i]) {
delta[i,t[i,k]] <- d[t[i,k]] - d[t[i,1]] # trial-specific LOR
}
}
sumdevtot<- sum(sumdev[])
d[1]<-0
for (k in 2:nt){
d[k] ∼ dnorm(0,.0001)
}
for (i in 1:ns) {
mu1[i] <- mu[i] * equals(t[i,1],1)
}
for (k in 1:nt) {
logit(T[k])<- sum(mu1[])/nb +d[k]
}
for (k in 1:nt) {
rk[k]<-nt+1 - rank(T[],k)
best[k]<-equals(rk[k],1)
}
for (c in 1:(nt-1)) { for (k in (c+1):nt) { or[c,k] <- exp(d[k] - d[c] ) }}
}
Random effects
model{
for(i in 1:ns){
w[i,1] <-0
delta[i,t[i,1]]<-0
mu[i] ∼ dnorm(0,.0001)
for (k in 1:na[i]) {
r[i,t[i,k]] ∼ dbin(p[i,t[i,k]],n[i,t[i,k]])
logit(p[i,t[i,k]])<-mu[i] + delta[i,t[i,k]]
rhat[i,t[i,k]]<- p[i,t[i,k]] * n[i,t[i,k]]
resdev[i,k]<- 2 * (r[i,t[i,k]] * (log(r[i,t[i,k]]) - log(rhat[i,t[i,k]])) + (n[i,t[i,k]] - r[i,t[i,k]]) * (log(n[i,t[i,k]] - r[i,t[i,k]]) - log(n[i,t[i,k]] - rhat[i,t[i,k]])))
}
sumdev[i]<-sum(resdev[i,1:na[i]])
for (k in 2:na[i]) {
delta[i,t[i,k]] ∼ dnorm(md[i,t[i,k]],taud[i,t[i,k]])
md[i,t[i,k]] <- d[t[i,k]] - d[t[i,1]] + sw[i,k]
taud[i,t[i,k]] <- tau*2*(k-1)/k
w[i,k] <- (delta[i,t[i,k]] - d[t[i,k]] + d[t[i,1]])/(k-1)
sw[i,k] <-sum(w[i,1:k-1])/(k-1)
}
}
sumdevtot<- sum(sumdev[])
d[1]<-0
for (k in 2:nt){
d[k] ∼ dnorm(0,.0001)
}
sd∼dunif(0,2)
tau<-1/pow(sd,2)
for (i in 1:nt) {
mu1[i] <- mu[i] * equals(t[i,1],1)
}
for (k in 1:nt) {
logit(T[k])<- sum(mu1[])/nb +d[k]
}
for (k in 1:nt) {
rk[k]<-nt+1 - rank(T[],k)
best[k]<-equals(rk[k],1)
}
for (c in 1:(nt-1)) { for (k in (c+1):nt) { or[c,k] <- exp(d[k] - d[c] ) }}
for (c in 1:(nt-1)) { for (k in (c+1):nt) { lor[c,k] <- (d[k] - d[c] ) }}
Appendix 5 Results of mixed-treatment comparison for complete clearance at end of treatment
| Intervention | Comparator, OR (95% CrI)a | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Podophyllin 20–25% (clinician applied) | Placebo/no treatment | Imiquimod 5% cream for 12 or 16 weeks (patient applied) | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.3% solution (patient applied) | Podophyllotoxin 0.5% cream (patient applied) | Podophyllotoxin 0.3% cream (patient applied) | Podophyllin solution (patient applied) | TCAA | Cryotherapy | Surgical excision | CO2 laser therapy | TCAA plus podophyllin 25% | Cryotherapy plus podophyllotoxin 0.15% cream | Cryotherapy plus podophyllin 25% | |
| Podophyllin 20–25% (clinician applied) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Placebo/no treatment | 0.053 (0.007 to 0.1626) | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Imiquimod 5% cream for 12 or 16 weeks (patient applied) | 1.07 (0.15 to 3.45) | 24.54 (7.28 to 73.04) | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.5% solution (patient applied) | 11.65 (2.65 to 38.50) | 389.2 (51.6 to 1821) | 18.97 (2.27 to 83.18) | – | – | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.3% solution (patient applied) | 28.50 (0.97 to 143.4) | 1008 (23.96 to 5253) | 51.02 (1.07 to 243.7) | 2.17 (0.13 to 10.08) | – | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.5% cream (patient applied) | 2.68 (0.37 to 9.19) | 65.81 (14.42 to 234) | 3.48 (0.51 to 13.03) | 0.30 (0.04 to 0.99) | 0.45 (0.01 to 2.29) | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.3% cream (patient applied) | 1.84 (0.07 to 8.35) | 45.73 (2.68 to 225) | 2.33 (0.10 to 11.55) | 0.19 (0.007 to 0.874) | 0.31 (0.003 to 1.56) | 0.66 (0.06 to 2.9) | – | – | – | – | – | – | – | – | – |
| Podophyllin solution (patient applied) | 4.57 (0.11 to 25.49) | 219.6 (2.07 to 1161) | 9.59 (0.10 to 50.16) | 0.61 (0.008 to 3.52) | 1.23 (0.004 to 5.50) | 3.77 (0.04 to 20.59) | 16.46 (0.06 to 77.65) | – | – | – | – | – | – | – | – |
| TCAA | 1.23 (0.30 to 3.56) | 49.91 (4.27 to 245.7) | 2.30 (0.20 to 10.2) | 0.17 (0.02 to 0.63) | 0.37 (0.005 to 1.40) | 0.94 (0.07 to 4.12) | 3.99 (0.09 to 19.0) | 2.18 (0.03 to 11.86) | – | – | – | – | – | – | – |
| Cryotherapy | 2.11 (0.39 to 6.90) | 88.66 (6.00 to 438.9) | 4.02 (0.28 to 18.73) | 0.29 (0.02 to 1.15) | 0.54 (0.008 to 2.46) | 1.67 (0.10 to 7.29) | 7.16 (0.13 to 33.6) | 3.69 (0.05 to 20.3) | 1.87 (0.55 to 4.73) | – | – | – | – | – | – |
| Surgical excision | 13.08 (0.48 to 70.51) | 604.9 (8.47 to 3200) | 26.07 (0.41 to 142.2) | 1.82 (0.03 to 9.90) | 3.28 (0.014 to 16.97) | 11.92 (0.16 to 59.99) | 54.34 (0.24 to 229.9) | 23.98 (0.09 to 128.7) | 16.53 (0.36 to 93.16) | 11.35 (0.20 to 61.91) | – | – | – | – | – |
| CO2 laser therapy | 104.6 (3.35 to 505.2) | 6533 (65.49 to 25,760) | 247.0 (3.03 to 1087) | 14.9 (0.24 to 72.79) | 39.21 (0.10 to 123.0) | 103.1 (1.11 to 430.7) | 412.3 (1.62 to 1735) | 211.3 (0.61 to 986.4) | 86.15 (4.05 to 415.3) | 44.61 (3.30 to 201.7) | 78.35 (0.22 to 254.9) | – | – | – | – |
| TCAA plus podophyllin 25% | 2.14 (0.50 to 6.16) | 86.98 (7.42 to 414.5) | 3.99 (0.33 to 17.76) | 0.30 (0.03 to 1.08) | 0.56 (0.009 to 2.43) | 1.62 (0.12 to 7.11) | 7.35 (0.15 to 33.3) | 3.80 (0.05 to 19.95) | 2.32 (0.39 to 7.86) | 1.53 (0.21 to 5.53) | 1.01 (0.02 to 4.75) | 0.13 (0.003 to 0.59) | – | – | – |
| Cryotherapy plus podophyllotoxin 0.15% cream | 6.63 (0.28 to 29.42) | 346.6 (5.47 to 1585) | 15.2 (0.25 to 66.81) | 0.96 (0.02 to 4.13) | 2.96 (0.008 to 7.30) | 5.97 (0.09 to 26.41) | 37.92 (0.13 to 104.9) | 13.85 (0.05 to 59.66) | 5.76 (0.33 to 24.0) | 2.86 (0.27 to 11.39) | 4.10 (0.02 to 15.09) | 0.26 (0.004 to 1.10) | 4.23 (0.14 to 19.05) | – | – |
| Cryotherapy plus podophyllin 25% | 3.42 (0.51 to 12.05) | 152.7 (8.16 to 745.1) | 6.79 (0.37 to 31.24) | 0.47 (0.03 to 1.91) | 0.88 (0.01 to 3.90) | 2.72 (0.14 to 12.72) | 13.28 (0.18 to 55.53) | 6.06 (0.06 to 33.04) | 3.56 (0.46 to 13.2) | 2.37 (0.25 to 9.36) | 1.80 (0.02 to 8.29) | 0.22 (0.004 to 0.94) | 2.23 (0.22 to 8.83) | 2.91 (0.07 to 11.3) | – |
| Intervention | Comparator, OR (95% CrI)a | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Podophyllin 20–25% (clinician applied) | Placebo/no treatment | Imiquimod 5% cream for 12 or 16 weeks (patient applied) | Podophyllotoxin 0.5% gel (patient applied) | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.3% solution (patient applied) | Podophyllotoxin 0.5% cream (patient applied) | Podophyllotoxin 0.3% cream (patient applied) | Podophyllotoxin 0.15% cream (patient applied) | Podophyllin solution (patient applied) | TCAA | Cidofovir 1.0% | Cryotherapy | Surgical excision | CO2 laser therapy | Electrotherapy | TCAA plus podophyllin 25% | Cryotherapy plus podophyllotoxin 0.15% cream | Cryotherapy plus podophyllin 25% | |
| Podophyllin 20–25% (clinician applied) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Placebo/no treatment | 0.05 (0.01 to 0.15) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Imiquimod 5% cream for 12 or 16 weeks (patient applied) | 1.09 (0.23 to 3.03) | 24.02 (6.68 to 64.4) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.5% gel (patient applied) | 2.15 (0.04 to 12.24) | 41.41 (1.34 to 222.5) | 2.45 (0.05 to 13.77) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.5% solution (patient applied) | 4.33 (1.45 to 10.88) | 112.0 (25.54 to 377.8) | 5.58 (1.16 to 19.20) | 17.11 (0.31 to 99.45) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.3% solution (patient applied) | 22.98 (0.99 to 122.1) | 602.3 (21.46 to 3299) | 31.13 (0.97 to 168.5) | 98.1 (0.42 to 558.9) | 5.32 (0.29 to 27.34) | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.5% cream (patient applied) | 2.24 (0.58 to 5.95) | 53.1 (13.26 to 158.1) | 2.80 (0.53 to 9.41) | 7.90 (0.16 to 44.5) | 0.62 (0.13 to 1.74) | 0.42 (0.01 to 2.12) | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.3% cream (patient applied) | 3.63 (0.41 to 14.87) | 91.48 (9.07 to 416.9) | 4.77 (0.40 to 21.66) | 15.88 (0.15 to 87.83) | 0.91 (0.11 to 3.44) | 0.60 (0.02 to 3.29) | 1.96 (0.23 to 8.14) | – | – | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.15% cream (patient applied) | 1.09 (0.15 to 3.84) | 28.24 (3.01 to 118.8) | 1.42 (0.14 to 6.05) | 4.37 (0.05 to 24.91) | 0.28 (0.04 to 0.90) | 0.18 (0.005 to 0.87) | 0.63 (0.07 to 2.49) | 0.49 (0.05 to 1.83) | – | – | – | – | – | – | – | – | – | – | – |
| Podophyllin solution (patient applied) | 4.04 (0.27 to 19.00) | 113.2 (5.21 to 598.4) | 5.54 (0.24 to 29.03) | 17.62 (0.10 to 107.2) | 1.04 (0.07 to 4.69) | 0.75 (0.01 to 4.52) | 2.47 (0.12 to 12.57) | 2.33 (0.07 to 12.45) | 6.91 (0.23 to 37.19) | – | – | – | – | – | – | – | – | – | – |
| TCAA | 1.56 (0.30 to 4.98) | 43.66 (4.89 to 182.5) | 2.08 (0.25 to 8.51) | 6.85 (0.08 to 39.64) | 0.46 (0.05 to 1.63) | 0.32 (0.007 to 1.75) | 0.98 (0.11 to 3.88) | 0.97 (0.05 to 4.53) | 2.88 (0.19 to 13.18) | 1.26 (0.04 to 6.82) | – | – | – | – | – | – | – | – | – |
| Cidofovir 1.0% | 26.79 (0.29 to 162.8) | 756.0 (6.86 to 4375) | 37.02 (0.30 to 224.9) | 175.3 (0.16 to 612.3) | 7.86 (0.07 to 43.84) | 5.40 (0.01 to 29.88) | 16.77 (0.14 to 100.8) | 16.41 (0.08 to 95.29) | 57.72 (0.26 to 307.2) | 24.65 (0.07 to 132.9) | 25.95 (0.19 to 165.5) | – | – | – | – | – | – | – | – |
| Cryotherapy | 2.65 (0.60 to 7.83) | 71.05 (10.44 to 274.1) | 3.36 (0.55 to 12.41) | 11.39 (0.15 to 63.8) | 0.77 (0.11 to 2.63) | 0.53 (0.01 to 2.86) | 1.62 (0.23 to 6.03) | 1.61 (0.10 to 7.21) | 4.79 (0.38 to 21.58) | 2.14 (0.08 to 11.38) | 2.19 (0.50 to 6.34) | 1.33 (0.11 to 8.04) | – | – | – | – | – | – | – |
| Surgical excision | 18.03 (0.30 to 109.6) | 586.5 (5.45 to 3466) | 27.05 (0.26 to 166.3) | 130.5 (0.13 to 477.3) | 5.30 (0.06 to 32.21) | 3.68 (0.01 to 23.75) | 12.14 (0.13 to 71.5) | 11.96 (0.07 to 70.48) | 36.41 (0.24 to 209.5) | 15.87 (0.06 to 92.61) | 19.51 (0.17 to 121.9) | 10.73 (0.01 to 63.98) | 10.56 (0.10 to 66.12) | – | – | – | – | – | – |
| CO2 laser therapy | 171.2 (3.03 to 991.7) | 4986 (64.22 to 28,480) | 222.2 (3.18 to 1344) | 821.2 (1.49 to 4056) | 49.81 (0.66 to 284.3) | 34.11 (0.12 to 207.8) | 111.2 (1.36 to 638.5) | 103.6 (0.75 to 629.4) | 329.5 (2.59 to 1920) | 147.4 (0.66 to 855.7) | 144.2 (2.51 to 838.1) | 88.94 (0.12 to 516.4) | 63.39 (1.88 to 343.1) | 145.8 (0.16 to 636.9) | – | – | – | – | – |
| Electrotherapy | 32.72 (3.15 to 150.08) | 877.2 (64.38 to 4685) | 44.47 (2.91 to 239.5) | 168.7 (1.16 to 898.3) | 9.30 (0.70 to 44.14) | 6.51 (0.10 to 38.37) | 20.12 (1.38 to 102.5) | 20.18 (0.73 to 108.2) | 64.83 (2.82 to 338.4) | 27.8 (0.57 to 160.5) | 32.23 (2.02 to 166.0) | 8.71 (0.22 to 48.15) | 15.79 (1.39 to 75.41) | 25.87 (0.11 to 137.7) | 2.03 (0.01 to 10.53) | – | – | – | – |
| TCAA plus podophyllin 25% | 2.61 (0.37 to 9.22) | 76.0 (6.18 to 351.7) | 3.68 (0.31 to 16.71) | 11.65 (0.10 to 71.06) | 0.78 (0.07 to 3.12) | 0.55 (0.008 to 3.09) | 1.66 (0.14 to 7.18) | 1.66 (0.07 to 8.15) | 4.92 (0.26 to 24.35) | 2.22 (0.06 to 12.11) | 2.45 (0.22 to 9.98) | 1.51 (0.008 to 9.23) | 1.37 (0.14 to 5.42) | 1.72 (0.01 to 9.87) | 0.15 (0.001 to 0.84) | 0.21 (0.007 to 0.97) | – | – | – |
| Cryotherapy plus podophyllotoxin 0.15% cream | 10.58 (0.23 to 61.07) | 305.3 (5.41 to 1779) | 14.38 (0.26 to 82.42) | 56.03 (0.12 to 273.9) | 3.06 (0.06 to 17.57) | 2.60 (0.01 to 13.28) | 6.77 (0.11 to 39.36) | 6.48 (0.06 to 38.94) | 21.41 (0.22 to 120.4) | 9.04 (0.06 to 52.16) | 8.81 (0.20 to 49.16) | 5.50 (0.01 to 32.31) | 3.89 (0.16 to 19.83) | 9.04 (0.01 to 41.94) | 0.44 (0.002 to 2.51) | 0.70 (0.008 to 4.13) | 7.83 (0.10 to 43.69) | – | – |
| Cryotherapy plus podophyllin 25% | 4.58 (0.35 to 20.36) | 136.0 (6.45 to 702.9) | 6.53 (0.31 to 33.15) | 23.76 (0.12 to 126.9) | 1.36 (0.07 to 6.41) | 0.97 (0.01 to 5.71) | 2.89 (0.14 to 14.73) | 2.86 (0.07 to 15.36) | 8.69 (0.27 to 47.89) | 3.76 (0.06 to 22.37) | 4.16 (0.26 to 19.44) | 2.49 (0.009 to 16.29) | 2.35 (0.14 to 10.98) | 3.63 (0.01 to 18.04) | 0.28 (0.001 to 1.47) | 0.35 (0.008 to 1.84) | 3.15 (0.13 to 15.92) | 3.27 (0.03 to 18.34) | – |
Appendix 6 Results of standard pairwise meta-analysis for complete clearance at end of treatment
Imiquimod 5% compared with placebo
Primary analysis
df, degrees of freedom; M–H, Mantel–Haenszel.

Sensitivity analysis
df, degrees of freedom; M–H, Mantel–Haenszel.

Podophyllotoxin 0.5% solution compared with placebo
Primary analysis
df, degrees of freedom; M–H, Mantel–Haenszel.

Podophyllotoxin 0.5% cream compared with placebo
Primary analysis
df, degrees of freedom; M–H, Mantel–Haenszel.

Sensitivity analysis
df, degrees of freedom; M–H, Mantel–Haenszel.
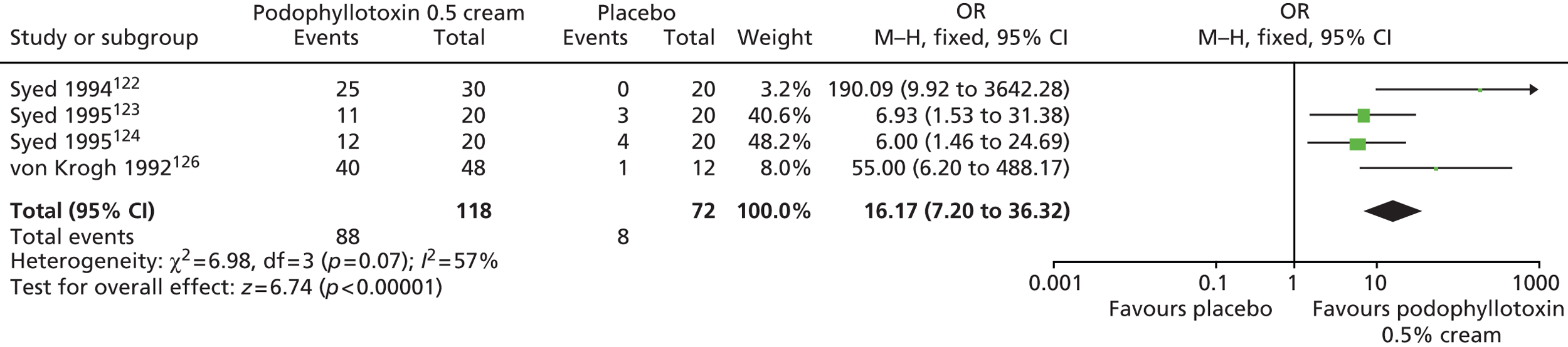
Trichloroacetic acid compared with podophyllin 20–25%
Primary analysis
df, degrees of freedom; M–H, Mantel–Haenszel.
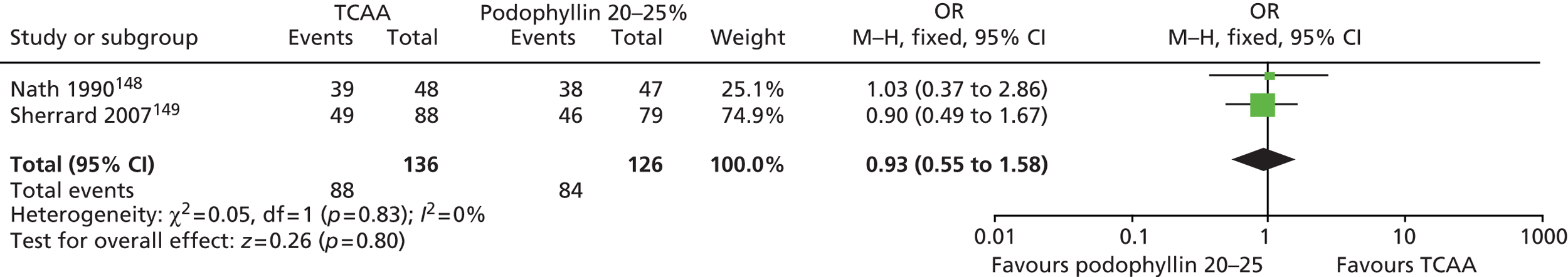
Podophyllotoxin 0.5% solution compared with podophyllin 20–25%
Primary analysis
df, degrees of freedom; M–H, Mantel–Haenszel.

Sensitivity analysis
df, degrees of freedom; M–H, Mantel–Haenszel.

Podophyllotoxin 0.5% cream compared with podophyllin 20–25%
Sensitivity analysis
df, degrees of freedom; M–H, Mantel–Haenszel.
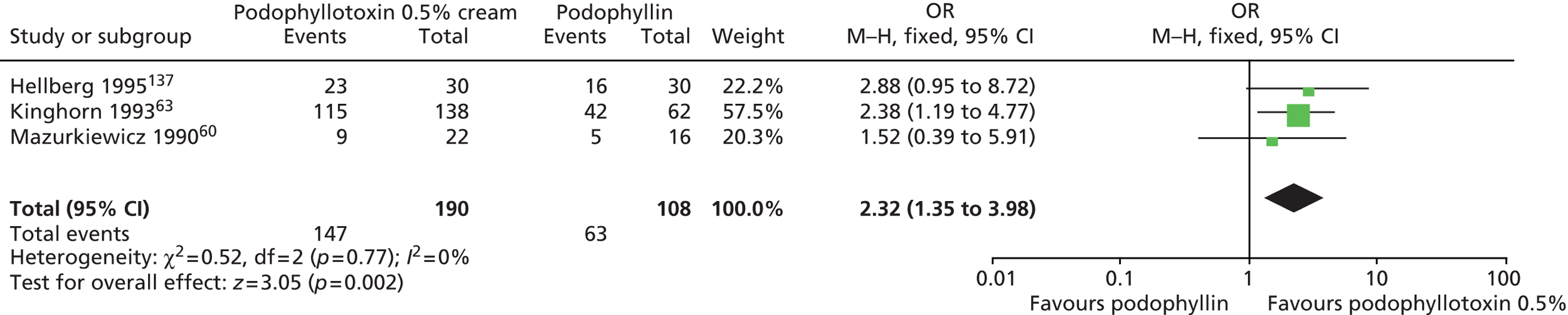
Cryotherapy compared with podophyllin 20–25%
Sensitivity analysis
df, degrees of freedom; M–H, Mantel–Haenszel.
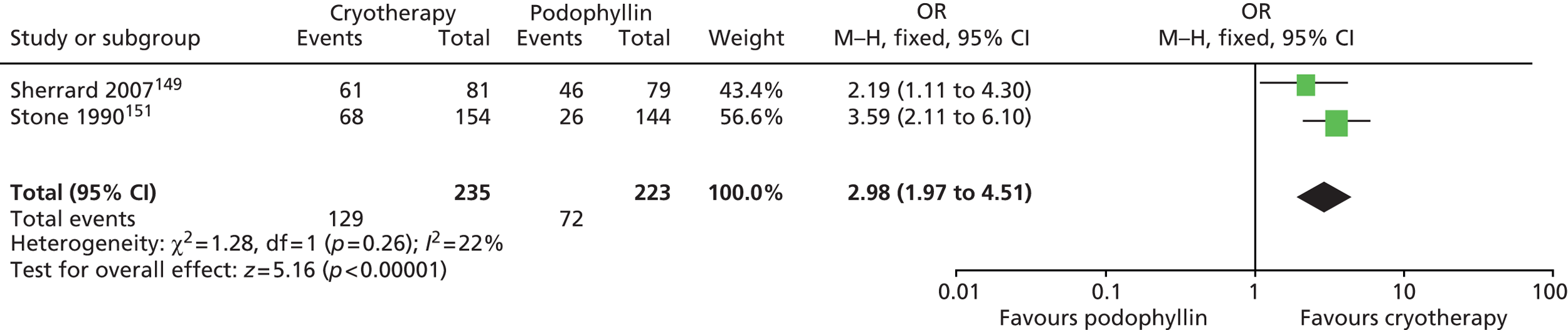
Trichloroacetic acid plus podophyllin 20–25% compared with podophyllin 20–25% alone
Sensitivity analysis
df, degrees of freedom; M–H, Mantel–Haenszel.
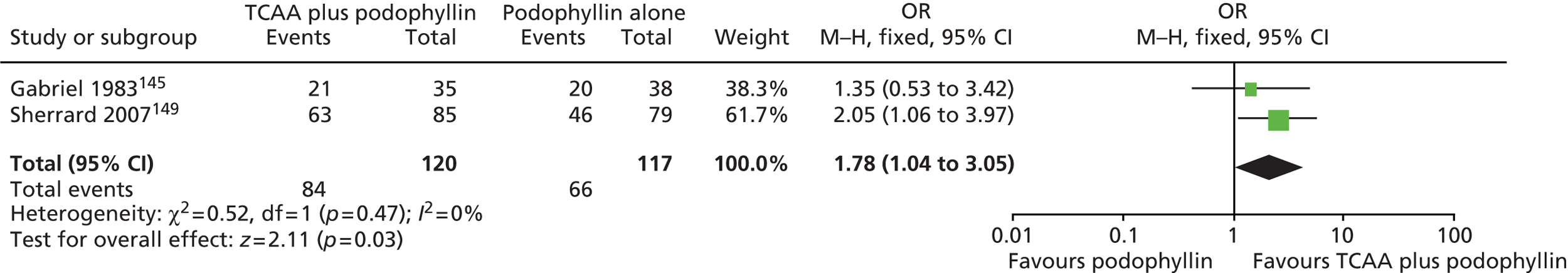
Podophyllotoxin 0.15% cream compared with podophyllotoxin 0.3% cream
Sensitivity analysis: fixed effects
df, degrees of freedom; M–H, Mantel–Haenszel.
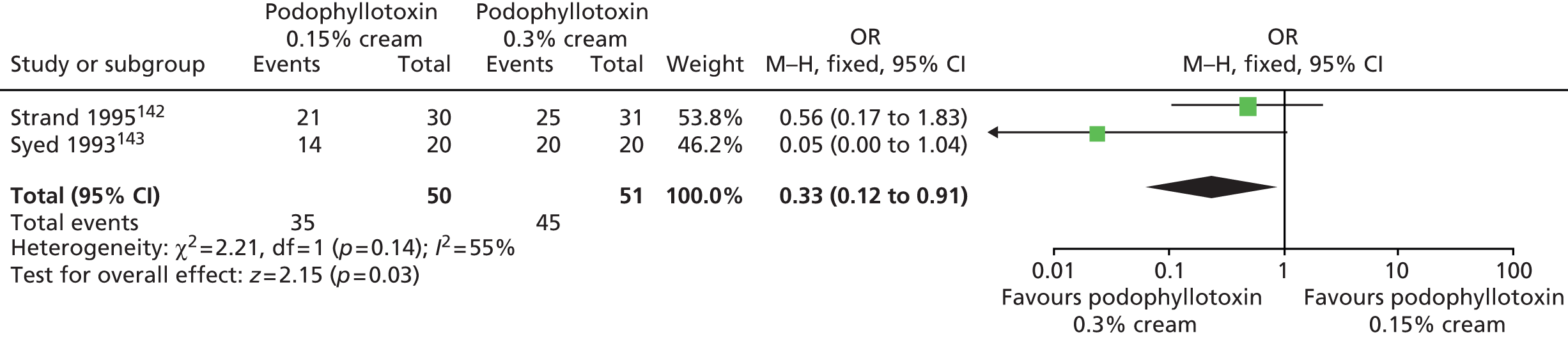
Sensitivity analysis: random effects
df, degrees of freedom; M–H, Mantel–Haenszel.

Cryotherapy compared with trichloroacetic acid
Primary analysis
df, degrees of freedom; M–H, Mantel–Haenszel.
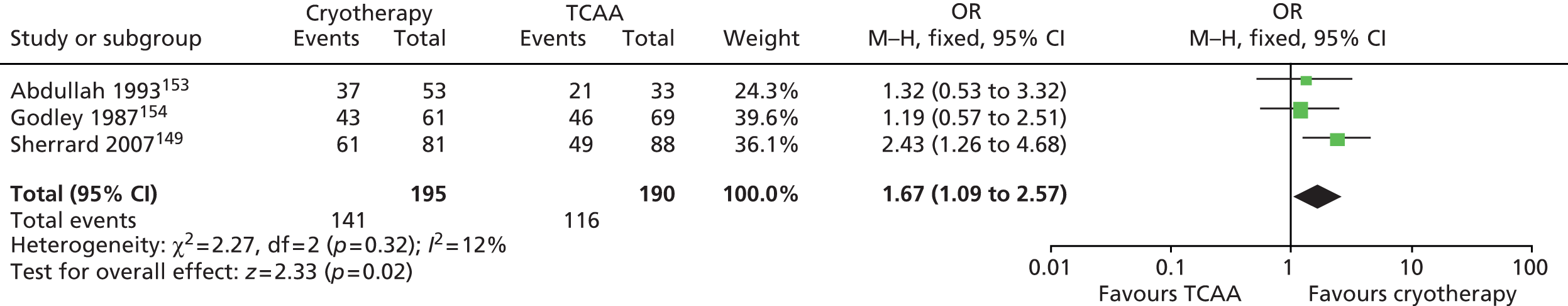
Appendix 7 Results of standard pairwise meta-analysis for complete clearance at another time point
Imiquimod 5% cream applied three times weekly, once daily or twice daily
df, degrees of freedom; M–H, Mantel–Haenszel.

Appendix 8 Data on clinical effectiveness used to inform the cost-effectiveness analysis
| Intervention | Podophyllin 20–25% (comparator), OR (95% CrI)a | Probability of recurrence (95% CrI) (%)b |
|---|---|---|
| Podophyllin 20–25% | NA | 41.2 (31.3 to 51.6) |
| Imiquimod 5% cream | 0.3 (0.04 to 1.07) | 16.5 (2.8 to 43.9) |
| Podophyllotoxin 0.5% solution (patient applied) | 0.8 (0.4 to 1.5) | 34.6 (20.0 to 51.4) |
| Podophyllotoxin 0.3% solution (patient applied) | 12.3 (0.8 to 60.6) | 76.2 (36.1 to 97.7) |
| TCAA | 0.3 (0.09 to 0.87) | 18.4 (6.3 to 36.4) |
| Surgical excision | 0.2 (0.03 to 0.44) | 9.7 (2.2 to 24.7) |
| TCA 50% plus podophyllin 25% | 1.1 (0.3 to 3.1) | 39.4 (17.2 to 65.3) |
Appendix 9 Results of mixed-treatment comparison for recurrence
| Intervention | Comparator, OR (95% CrI)a | ||||
|---|---|---|---|---|---|
| Podophyllin 20–25% (clinician applied) | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.3% solution (patient applied) | TCAA | TCAA 50% plus podophyllin 25% | |
| Podophyllin 20–25% (clinician applied) | – | – | – | – | – |
| Podophyllotoxin 0.5% solution (patient applied) | 1.37 (0.03 to 7.15) | – | – | – | – |
| Podophyllotoxin 0.3% solution (patient applied) | 67.8 (0.07 to 261.5) | 36.15 (0.39 to 208.7) | – | – | – |
| TCAA | 0.81 (0.02 to 4.45) | 5.31 (0.01 to 26.05) | 3.43 (< 0.001 to 8.91) | – | – |
| TCAA 50% plus podophyllin 25% | 2.63 (0.05 to 16.64) | 18.03 (0.04 to 83.35) | 14.35 (0.001 to 31.02) | 35.71 (0.06 to 145.3) | – |
| Intervention | Comparator, OR (95% CrI)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Podophyllin 20–25% (clinician applied) | Podophyllotoxin 0.5% solution (patient applied) | Podophyllotoxin 0.3% solution (patient applied) | Podophyllotoxin 0.5% cream (patient applied) | Podophyllotoxin 0.3% cream (patient applied) | Podophyllotoxin 0.15% cream (patient applied) | Podophyllin 0.5% solution (patient applied) | TCAA | Cryotherapy | Electrotherapy | TCAA 50% plus podophyllin 25% | |
| Podophyllin 20–25% (clinician applied) | – | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.5% solution (patient applied) | 0.87 (0.47 to 1.49) | – | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.3% solution (patient applied) | 13.8 (0.94 to 65.21) | 15.71 (1.25 to 74.3) | – | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.5% cream (patient applied) | 1.02 (0.09 to 4.22) | 1.27 (0.11 to 5.51) | 0.26 (0.005 to 1.46) | – | – | – | – | – | – | – | – |
| Podophyllotoxin 0.3% cream (patient applied) | 1.16 (0.19 to 3.77) | 1.35 (0.24 to 4.22) | 0.27 (0.009 to 1.37) | 2.88 (0.12 to 14.81) | – | – | – | – | – | – | – |
| Podophyllotoxin 0.15% cream (patient applied) | 1.40 (0.57 to 2.92) | 1.64 (0.74 to 3.22) | 0.33 (0.02 to 1.44) | 3.46 (0.25 to 15.5) | 1.93 (0.37 to 6.42) | – | – | – | – | – | – |
| Podophyllin 0.5% solution (patient applied) | 62.5 (0.27 to 202.2) | 73.95 (0.35 to 236.0) | 15.64 (0.02 to 45.73) | 127.8 (0.25 to 468.0) | 95.13 (0.24 to 282.2) | 52.68 (0.21 to 165.9) | – | – | – | – | – |
| TCAA | 0.35 (0.09 to 0.88) | 0.44 (0.10 to 1.21) | 0.09 (0.003 to 0.43) | 0.87 (0.05 to 4.13) | 0.54 (0.05 to 2.26) | 0.30 (0.06 to 0.90) | 0.21 (0.001 to 1.33) | – | – | – | – |
| Cryotherapy | 0.61 (0.20 to 1.41) | 0.76 (0.21 to 1.98) | 0.15 (0.007 to 0.72) | 1.51 (0.10 to 7.03) | 0.94 (0.11 to 3.75) | 0.52 (0.12 to 1.48) | 0.37 (0.002 to 2.33) | 2.41 (0.42 to 7.92) | – | – | – |
| Electrotherapy | 0.74 (0.25 to 1.68) | 0.93 (0.26 to 2.37) | 0.19 (0.008 to 0.88) | 1.85 (0.13 to 8.41) | 1.15 (0.13 to 4.57) | 0.64 (0.15 to 1.81) | 0.46 (0.003 to 2.87) | 2.96 (0.53 to 9.69) | 1.30 (0.65 to 2.36) | – | – |
| TCAA 50% plus podophyllin 25% | 1.10 (0.25 to 3.13) | 1.37 (0.27 to 4.21) | 0.27 (0.01 to 1.37) | 2.71 (0.15 to 13.22) | 1.70 (0.15 to 7.4) | 0.93 (0.16 to 3.09) | 0.67 (0.004 to 4.34) | 4.35 (0.56 to 16.28) | 2.29 (0.34 to 8.01) | 1.85 (0.28 to 6.49) | – |
| Intervention | Comparator, OR (95% CRI)a | |||
|---|---|---|---|---|
| Podophyllin 20–25% (clinician applied) | Imiquimod 5% cream | Podophyllotoxin 0.5% solution (patient applied) | Surgical excision | |
| Podophyllin 20–25% (clinician applied) | – | – | – | – |
| Imiquimod 5% cream | 0.31 (0.04 to 1.06) | – | – | – |
| Podophyllotoxin 0.5% solution (patient applied) | 1.56 (0.46 to 4.08) | 9.64 (0.89 to 42.07) | – | – |
| Surgical excision | 0.16 (0.03 to 0.43) | 0.97 (0.07 to 4.34) | 0.14 (0.02 to 0.50) | – |
Glossary
- Cost-effectiveness acceptability curve
- A graphical representation of the probability of an intervention being considered cost-effective over a range of monetary values for society’s willingness to pay for an additional unit of health gain.
- Cost-effectiveness analysis
- A form of economic evaluation comparing the costs and outcomes of two or more courses of action.
- Cost-effectiveness plane
- A diagrammatic presentation of incremental costs and outcomes from the economic evaluation of two interventions, consisting of four quadrants.
- Cost–utility analysis
- A special kind of cost-effectiveness analysis in which outcomes are measured as quality-adjusted life-years.
- Electrotherapy
- Ablative technique in which an electrical current is used to transect or vaporise tissue.
- Incremental cost-effectiveness ratio
- An expression of the additional cost of health gain associated with an intervention relative to an appropriate comparator. Expressed as the difference in mean costs (relative to the comparator) divided by the difference in mean effects.
- Innate immune system
- First line of defence against invading pathogens. The cells of the innate system recognise and respond to pathogens in a generic way. In contrast to the adaptive immune system, the innate immune system does not confer protection against re-exposure to the same pathogen.
- Multiple cost-effectiveness acceptability curve
- A graphical representation of the probability that a single treatment strategy will have the highest net benefit compared with two or more additional treatment strategies for a given willingness to pay for an additional quality-adjusted life-year gained.
- Quality-adjusted life-year
- A measure of disease burden, capturing both the quality and quantity of life.
- Quality of life
- A concept incorporating all of the factors that might impact on an individual’s life, including factors such as the absence of disease or infirmity as well as other factors that might affect his or her physical, mental and social well-being.
List of abbreviations
- AE
- adverse effect
- AGW
- anogenital wart
- BASHH
- British Association for Sexual Health and HIV
- BNF
- British National Formulary
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CO2
- carbon dioxide
- CrI
- credible interval
- DIC
- deviance information criterion
- DNA
- deoxyribonucleic acid
- EMA
- European Medicines Agency
- EQ-5D
- European Quality of Life-5 Dimensions
- FDA
- Food and Drug Administration
- GP
- general practitioner
- GUM
- genitourinary medicine
- HCHS
- Hospital and Community Health Services
- HIV
- human immunodeficiency virus
- HPA
- Health Protection Agency
- HPV
- human papillomavirus
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- mCEAC
- multiple cost-effectiveness acceptability curve
- MeSH
- medical subject heading
- MTC
- mixed-treatment comparison
- NHS EED
- NHS Economic Evaluation Database
- OR
- odds ratio
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SE
- standard error
- SF-6D
- Short Form questionnaire-6 Dimensions
- SmPC
- Summary of Product Characteristics
- STI
- sexually transmitted infection
- TCAA
- trichloroacetic acid
