Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/52/03. The contractual start date was in July 2010. The draft report began editorial review in July 2015 and was accepted for publication in November 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Michael G Mythen reports grants from Smiths Medical Endowment and Deltrex Medical, personal fees from Edward Lifesciences and Fresenius-Kabi for speaking, consultation or travel expenses, and personal fees from AQIX (start-up company with novel crystalloid solution – pre-clinical), patent pending for the QUENCH pump and patent issued for Gastrotim outside the submitted work. Ella Segaran reports grants from Abbott Nutrition to attend a UK Intensive Care conference outside the submitted work. Danielle E Bear reports grants from the UK National Institute for Health Research Comprehensive Local Research Network, and personal fees/other support from Nestle Nutrition for speaker fees, Nutricia for speaker and consultancy fees plus payment of conference attendance, accommodation and travel expenses, Baxter for payment of course fees, travel and accommodation expenses and Corpak MedSystems UK for research support paid to Guy’s and St Thomas’ NHS Foundation Trust, outside the submitted work. Geoff Bellingan reports grants from the National Institute for Health Research for Selective Decontamination of the Digestive tract in critically ill patients treated in Intensive Care Unit (The SuDDICU study) (09/01/13) during the trial.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Harvey et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background and rationale
Malnutrition is a common problem in critically ill patients in UK NHS critical care units. 1 The consequences of malnutrition include vulnerability to complications, such as infection, which can lead to delays in recovery. Early nutritional support is therefore recommended for critically ill patients to address both deficiencies in nutritional state and related disorders in metabolism.
However, evidence is conflicting regarding the optimum route (parenteral or enteral) of delivery. 2–4 Meta-analyses of the trials comparing nutritional support via the enteral and parenteral route in critically ill patients have been published, but interpretation of their results is complicated by small sample size, poor methodological quality, select groups of critically ill patients studied, lack of standardised definitions for outcome measures and interventions combining more than one element of nutritional support, for example timing and route.
In 2003, Heyland et al. 2 reported no difference in mortality between patients given parenteral and enteral nutritional support, but enteral was associated with a significant reduction in infections. Safety, cost and feasibility led them to recommend enteral over parenteral in the critically ill adult patient. In 2004, Gramlich et al. 3 also found no difference in mortality but a significant reduction in infections with enteral nutrition (EN). 3 In addition, they reported no difference in length of unit stay or days on ventilation, but indicated that there were insufficient data to analyse these statistically. Using a different methodological approach to assessing quality of included studies (one less biased towards including the poorer-quality studies), Simpson and Doig,4 in 2005, found a significant reduction in mortality but a significant increase in infections with parenteral nutritional support compared with the enteral nutritional support. However, the significant mortality benefit with parenteral nutrition (PN) appeared to exist when compared with the provision of delayed, rather than early enteral nutritional support and thus this was not a like-for-like comparison. Similar time-based analyses for infections were not possible as a result of insufficient data.
All of the meta-analyses highlighted the problems of combining data from poor-quality studies conducted on heterogeneous patient populations (all were on select subgroups, such as head trauma, acute pancreatitis, etc.) plus variation in the timing of measurement of mortality and, perhaps more importantly, the nature and definitions for infections included and pooled (pneumonia, urinary tract, bacteraemia, wound, line sepsis, etc.). Owing to incomplete reporting, it was not possible to classify and combine infections based on risk of outcome (e.g. severe infection, moderate infection, subclinical infection).
The enteral route is the mainstay of nutritional support in critical care2,5,6 but it is frequently associated with gastrointestinal intolerance and underfeeding. 7,8 In contrast, the parenteral route though more invasive and expensive is more likely to secure delivery of the intended nutrition. 7 Historically, nutritional support via the parenteral route has been associated with more risks and complications (e.g. infectious complications) compared with the enteral route2–4 but recent improvements in the delivery, formulation and monitoring of PN justify further comparison and evaluation of these routes of nutritional support, particularly in the early phase of the illness. 9,10 Economic evidence surrounding the optimum route of delivery of nutritional support is largely based on evidence of effectiveness of questionable methodological quality and narrow focus on upfront acquisition costs, and full economic evaluation is lacking. 11
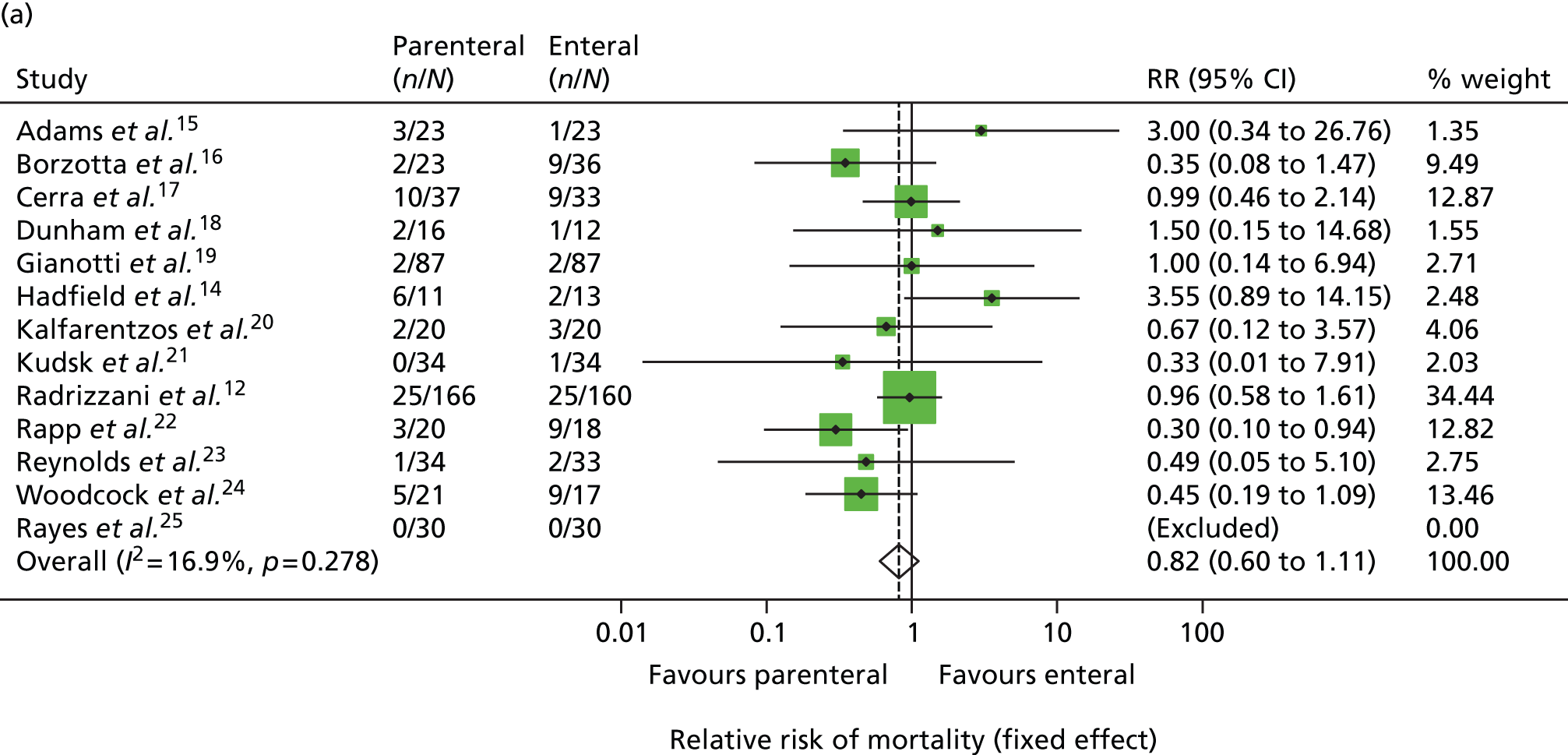
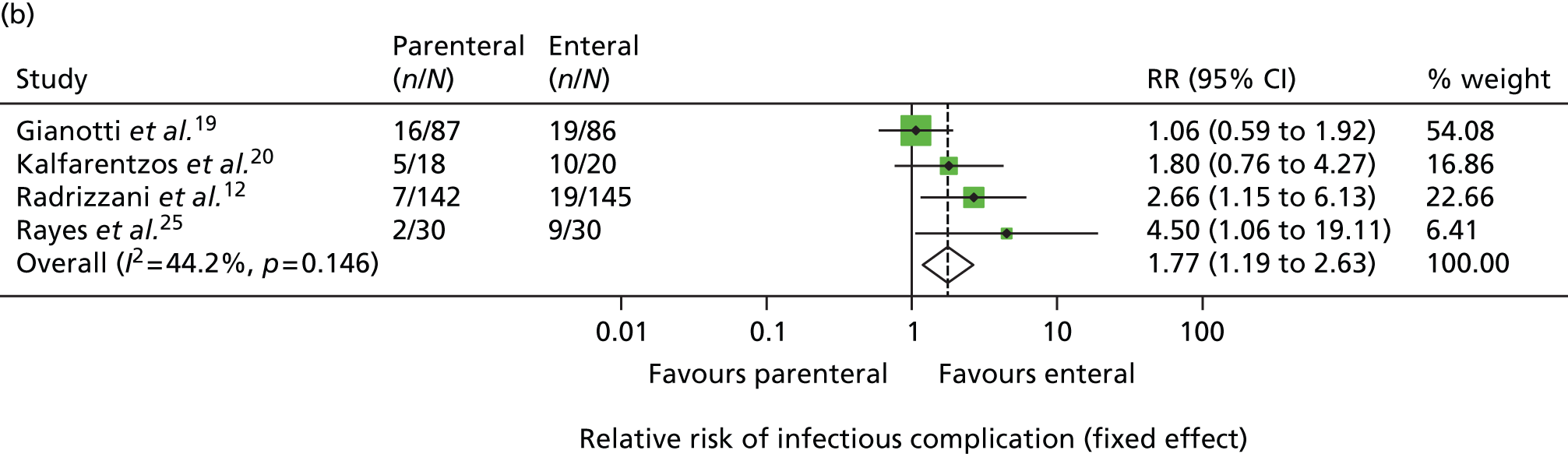
In view of this, in late 2007, the National Institute for Health Research (NIHR) Health Technology Assessment programme put out a call for a large pragmatic randomised controlled trial to be conducted to determine the optimal route of delivery of early nutritional support in critically ill adults. In response to this call, in 2008, we updated the most recent systematic review by Simpson and Doig (see Figure 1). Highly sensitive search criteria identified a further 570 potentially relevant studies since May 2003. Following detailed review of these studies, one additional randomised controlled trial comparing PN and EN was identified. 12 This paper reported the full results of a trial previously identified by Simpson and Doig as having only interim results reported,13 but was excluded from their meta-analysis, as the enteral nutritional support arm included immune-enhancing supplements. As the use of immune-enhancing supplements was to be permitted in our study, we repeated the meta-analysis to include studies with supplementation of either arm. This resulted in the inclusion of this trial and one additional randomised controlled trial excluded from the Simpson and Doig meta-analysis on this criterion. 14 The results of the updated meta-analysis, including a total of 13 studies,12,14–25 indicated a non-significant survival benefit for parenteral support [relative risk 0.82, 95% confidence interval (CI) 0.60 to 1.11] but an increased risk of infection (relative risk 1.77, 95% CI 1.19 to 2.63) compared with enteral nutritional support (Figure 1). Consequently, parenteral nutritional support in the critical care unit remained controversial and no clear evidence existed as to the optimum route for delivery of early nutritional support to critically ill patients.
FIGURE 1.
Updated meta-analysis of randomised trials comparing PN with EN. (a) Relative risk of mortality; and (b) relative risk of infectious complications. RR, risk ratio. Based on the criteria of Simpson and Doig,4 updated to 24 January 2008, and with exclusion criteria relaxed to include trials that had been excluded previously because of the use of immune-enhancing supplements.
Aim
The aim of the CALORIES trial was to compare the clinical effectiveness and cost-effectiveness of early nutritional support in critically ill patients, delivered via the parenteral compared with the enteral route.
Objectives
Primary
The primary objectives of the CALORIES trial were to estimate the:
-
effect of early (defined as within 36 hours of the date/time of original critical care unit admission) nutritional support via the parenteral route compared with early nutritional support via the enteral route on all-cause mortality at 30 days, and
-
incremental cost-effectiveness of early nutritional support via the parenteral route compared with early nutritional support via the enteral route at 1 year.
Secondary
The secondary objectives of the CALORIES trial were to compare early nutritional support via the parenteral and enteral routes for:
-
duration of specific and overall organ support in the critical care unit
-
infectious and non-infectious complications in the critical care unit
-
duration of critical care unit and acute hospital length of stay
-
duration of survival at 90 days and at 1 year
-
mortality at discharge from the critical care unit and from acute hospital
-
mortality at 90 days and at 1 year
-
nutritional and health-related quality of life at 90 days and at 1 year
-
resource use and costs at 90 days and at 1 year, and
-
estimated lifetime incremental cost-effectiveness.
Chapter 2 Methods
Trial design
The CALORIES trial was a pragmatic, open, multicentre, parallel-group, randomised controlled trial with an integrated economic evaluation.
The trial was nested in the Case Mix Programme, the national clinical audit of adult general critical care units in England, Wales and Northern Ireland, established in 1995 and co-ordinated by Intensive Care National Audit & Research Centre (ICNARC) (Scotland has its own separate national clinical audit). 26 The Case Mix Programme is listed in the Department of Health’s ‘Quality Accounts’ for 2013–14 as a recognised national audit by the National Advisory Group for Clinical Audit and Enquiries.
Nesting the CALORIES trial in the Case Mix Programme ensured an efficient design (with respect to participating units and data collection) and facilitated efficient management of the study, including monitoring recruitment.
Research governance
The trial was sponsored by ICNARC and co-ordinated by the ICNARC Clinical Trials Unit (CTU).
An ethics application was submitted to the North West London Research Ethics Committee on 28 October 2010 and the CALORIES trial received a favourable opinion on 16 December 2010 (reference number: 10/H0722/78). The protocol is available via www.nets.nihr.ac.uk/.
To ensure transparency, the trial was registered with the International Standard Randomised Controlled Trial Number (ISRCTN) Registry on 25 March 2009. Registration was confirmed on 9 April 2009 (ISRCTN17386141).
The NIHR Clinical Research Network (CRN) Portfolio details high-quality clinical research studies that are eligible for support from the NIHR CRN in England. The trial was adopted on to the NIHR CRN Portfolio on 24 March 2011 and was issued the NIHR CRN Portfolio number 10098.
Global NHS permissions were obtained from Central and East London Comprehensive Local Research Network (CLRN) on 10 March 2011 and local NHS permissions were obtained from each participating NHS trust. A clinical trial site agreement, based on the model agreement for non-commercial research in the health service, was signed by each participating NHS trust and the sponsor (ICNARC).
Following guidelines from the NIHR, a Trial Steering Committee (TSC), with a majority of independent members, was convened to oversee the trial on behalf of the funder (NIHR) and the sponsor (ICNARC). The TSC met at least annually during the trial and comprised an independent chairperson; independent lay members (representing patient perspectives); independent clinicians (specialising in critical care medicine); the chief investigator (KR); and the lead clinical investigator (MM) representing the Trial Management Group (TMG).
Additionally, an independent Data Monitoring and Ethics Committee (DMEC) was convened to monitor trial data and ensure the safety of trial participants. The DMEC met at least annually during the trial; it comprised two expert clinicians specialising in critical care medicine and was chaired by an experienced statistician.
Management of the trial
The trial manager was responsible for day-to-day management of the trial with support from the data manager, trial statistician and research assistant. The TMG, chaired by the ICNARC CTU manager (SH), was responsible for overseeing day-to-day management of the trial and comprised the chief investigator (KR), trial dietitians (DB, ES) and co-investigators (GB, RB, DH, RL and MM). The TMG met regularly throughout the trial to ensure adherence to the trial protocol and monitor the conduct and progress of the trial.
Network support
To maintain the profile of the trial, regular updates on trial progress were provided at quarterly meetings of the NIHR CRN Critical Care Specialty Group and at local CLRN meetings. In addition, updates were provided at national meetings, such as the Annual Meeting of the Case Mix Programme and the UK Critical Care Research Forum.
Design and development of the protocol
Clinicians – including doctors, nurses and dietitians, from NHS critical care units across the UK – were invited to a meeting in May 2010 to discuss the trial protocol. The purpose of the meeting was to provide a forum for clinicians who had expressed an interest in taking part in the trial to discuss the trial protocol in detail with the trial investigators. Following the meeting, minor changes were made to the trial protocol to ensure clarity.
Amendments to the trial protocol
Following receipt of a favourable opinion of the trial protocol from the Research Ethics Committee on 16 December 2010, four substantial amendments were submitted and received favourable opinion. In summary, these were:
-
Amendment 1 (13 May 2011) – the personal/professional consultee consent form was replaced with a personal/professional consultee agreement form to clarify that consultees were being asked for agreement (not consent) for patients to participate in the trial according to the Mental Capacity Act (2005)27 and the National Research Ethics Service Guidance for Researchers & Reviewers. The patient information sheets (prospective and retrospective for the patient and for the personal/professional consultee) and consent forms (patient consent form and retrospective patient consent form) had minor administrative changes. A personal/professional consultee telephone agreement form was produced to ensure that, in the situation that a personal/professional consultee was contacted via the telephone for their opinion, this contact was documented. The trial protocol was amended to clarify the aim of the trial – to compare early nutritional support delivered via the parenteral with early nutritional support delivered via the enteral route – and to incorporate the most up-to-date version of the EuroQoL 5-dimension (5-level version) questionnaire (EQ-5D-5L) to evaluate health-related quality of life at 90 days and at 1 year.
-
Amendment 2 (19 December 2011) – the letter to the patient’s general practitioner (GP) informing them of the patient’s participation in the trial was amended for use in cases when the patient was known to have died. The patient follow-up letters were amended to be specific to the follow-up time point, that is 90 days and 1 year post-randomisation, and minor semantic changes were made to the patient information sheets and consent/agreement forms.
-
Amendment 3 (4 October 2012) – following requests from research teams at sites, a CALORIES trial information leaflet for family and friends was produced. The leaflet was placed in the critical care unit relatives’ room, with the aim of providing relatives/friends of patients in critical care with a brief overview of the trial. The exclusion criterion ‘known to be participating in an interventional study’ was removed following review by the TMG; it was agreed that patients could be co-enrolled into two interventional studies if, after careful consideration, there were no concerns about patient safety, risk of biological interaction or the scientific integrity of the trial. Local principal investigators (PIs) were advised to contact the ICNARC CTU on a case-by-case basis to discuss co-enrolment of patients. In addition, minor semantic changes were made to the trial protocol and consent/consultee agreement forms.
-
Amendment 4 (23 October 2013) – a patient newsletter was added to the follow-up questionnaire pack that was sent to each patient at 90 days and 1 year post recruitment into the CALORIES trial.
NHS support costs
Trials in critical care are challenging and expensive to conduct. Unlike other areas of health care, such as oncology, recruitment cannot take place solely within usual office hours. Resources are needed to enable screening and recruitment 24 hours per day, 7 days per week. Patients with a critical illness can be admitted to the critical care unit at any time of day or night, including weekends. Another challenge of critical care research is the informed consent process, which often has to be completed within a short time frame, as treatments are often time limited. Furthermore, critically ill patients usually lack the mental capacity to be able to provide informed consent prior to randomisation, in which case it is necessary to involve a personal or professional consultee in accordance with the Mental Capacity Act. 27 Senior, experienced staff are needed to be able to assess the patient’s mental capacity and to be able to effectively communicate information about the trial to the patient and/or their relatives in a stressful situation.
To this end, resources equivalent to 0.5 whole-time equivalent (WTE) band 7 research nurse, 0.1 WTE critical care consultant, 0.1 WTE band 7 dietitian and 1.4 hours per week of a band 6 pharmacist were successfully agreed with the lead CLRN on 21 June 2011.
Resources were based on an estimated 175 eligible admissions per unit per year, of whom approximately 60 would be recruited and 30 randomised to receive early nutritional support via the parenteral route. Using these recommendations, participating sites, assisted by the TMG, negotiated resources required locally for the trial with their respective research and development departments and CLRNs.
Patient and public involvement
Engagement with patients was vital to the successful conduct of the trial. The original study proposal was reviewed and endorsed by Patients on Intravenous and Nasogastric Nutritional Therapy support group. Two former critical care patients were independent members of the TSC and they provided input into the conduct of the trial, including reviewing literature to be given to patients and their families (e.g. patient information sheets and patient newsletters).
Participants: sites
The trial aimed to recruit a representative sample of at least 20 adult, general critical care units from the UK. Adult, general critical care units were defined as intensive care units (ICUs) or combined intensive care/high-dependency units. Stand-alone high-dependency units and specialist critical care units (e.g. neurosciences, cardiothoracic, etc.) were not eligible for participation in the trial. The criteria for inclusion were:
-
active participation in the Case Mix Programme – defined as submission of data no later than 6 weeks after the end of each quarter and returning corrected data validation reports no later than 6 weeks after receipt
-
pre-existing, established protocols for PN and EN reflecting mainstream practice (reviewed and approved by the TMG)
-
pre-existing implementation of bundles as promoted by the NHS (NHS Saving Lives: reducing infection, delivering clean and safe care – ‘High Impact Intervention No. 1: Central venous catheter’ and ‘High Impact Intervention No. 5: Ventilator’) to prevent the development of bloodstream infection and ventilator-associated pneumonia28,29
-
pre-existing prophylaxis protocol for the prevention of venous thromboembolism
-
glycaemic control protocol in line with international guidelines30
-
agreement to incorporate the CALORIES trial into routine unit practice, including prior agreement – from all consultants in the unit – to adhere to the patient’s randomly allocated route (parenteral or enteral route) for delivery of early nutritional support
-
agreement to recruit all eligible patients into the CALORIES trial and to maintain a screening log of eligible patients who were not randomised, and patients who fulfilled the inclusion criteria but met one or more of the exclusion criteria
-
sign up from the unit clinical director, senior nurse manager, dietitian/clinical nutritionist and pharmacist, and
-
identification of a dedicated CALORIES trial research nurse.
All of the units actively participating in the Case Mix Programme were invited for expressions of interest to take part in the trial. In addition, the trial was promoted through presentations at relevant national meetings of professional organisations, such as the Intensive Care Society and at the UK Critical Care Research Forum.
A PI, who was responsible for the conduct of the trial locally, was identified at each participating site.
Site initiation
Site teams from all participating sites attended a site initiation meeting prior to the commencement of patient screening. Two site initiation meetings were held in London on 11 May 2011 and 8 June 2011, attended by staff from 22 critical care units. The purpose of these meetings was to present the background and rationale for the CALORIES trial and to discuss delivery of the protocol, including screening and recruiting patients, delivery of early nutritional support via the parenteral and enteral routes, data collection and validation, and safety monitoring. The operational challenges of conducting the trial at sites were discussed in detail, including strategies for ensuring effective communication within the critical care unit. The PI from each participating site was required to attend the meeting. If key research staff were unable to attend the meeting, or new staff came into post, additional site initiation meetings were conducted as required, either at sites or via teleconference. A standardised slide set from the site initiation meetings was circulated to facilitate internal training within a participating site.
Investigator site file
An investigator site file was provided to all participating sites. This contained all essential documents for the conduct of the trial and included the approved trial protocol; all relevant approvals (e.g. local NHS permissions); a signed copy of the clinical trial site agreement; the delegation of trial duties log; copies of the approved patient information sheets, patient consent form and personal/professional consultee agreement forms; and all standard operating procedures, for example for screening participants, for obtaining informed consent or consultee agreement, for randomising patients, for delivery of the intervention, and for collecting and entering data onto the secure, dedicated, electronic case report form. The site PI was responsible for maintaining the investigator site file. Responsible staff at sites were authorised to carry out trial duties (e.g. consenting, delivering the intervention) by the site PI on the delegation of trial duties log. This included a confirmation that the individual had been adequately training to carry out the specific duty.
Site management
Communication
The trial manager, with support from the data manager and research assistant, maintained close contact with the PI and trial team at participating sites by e-mail and telephone throughout the trial.
Teleconferences were held, initially every month then every 2 months, with trial teams at participating sites. The purpose of these was to provide updates on trial progress and to provide a forum for site teams to ask questions, discuss local barriers and challenges to the conduct of the trial, delivery of the intervention and to share successes and best practice. Notes, including ‘hints and tips’, from the teleconferences were distributed to all participating sites. The ICNARC CTU team facilitated further direct communication between sites via an e-mail forum for research nurses.
Teleconferences were also held with individual site teams, as required, to address site-specific issues in the conduct of the trial and/or to support training new staff.
Site monitoring visits
At least one routine monitoring visit was conducted at all participating sites during the trial. During the site visit, the investigator site file was checked for completeness, that is, that all essential documents were present; the patient consent forms and personal/professional consultee agreement forms were checked to ensure that the relevant correctly completed form was present for every patient recruited into the trial; and a random sample of patient case report forms were checked against the source data for accuracy and completeness. After the visit, the PI and site team were provided with a report summarising the documents that had been reviewed and actions required by the site team. The site PI was responsible for addressing the actions and reporting back to the ICNARC CTU. Additional visits were conducted on a risk-based approach, using recruitment rates, data quality and adherence to the protocol as central monitoring triggers.
Maintenance and motivation
During the trial, an e-mail was sent each month to site teams with an update on patient recruitment, and a newsletter was sent every quarter. These provided an opportunity to clarify any issues related to the conduct of the trial and to share ideas for maximising recruitment, as well as maintaining motivation and involvement through regular updates on progress.
To maintain the profile of the trial at participating sites, posters were displayed in staff areas and at relevant locations within the critical care unit, for example by the bedside or in EN and PN storage areas; pocket cards (summarising the eligibility criteria) and branded pens were distributed to staff; and certificates were given to clinical staff in recognition of their contribution to the trial.
Support
A 24 hours per day, 7 days per week, telephone support service was available to site teams for advice on screening and recruitment of patients and on delivery of the intervention. This ensured access to clinicians and dietitians for answering any queries on delivery of the intervention.
Collaborators’ meeting
A collaborators’ meeting was held on 17 January 2013 to provide an update on trial progress, provide a forum for site teams and investigators to discuss operational challenges to the trial and identify possible solutions, and to share successes and best practice.
Participants: patients
The trial procedures for recruitment and follow-up of patients are summarised in Figure 2.
FIGURE 2.
Summary of trial procedures for recruitment and follow-up of patients.

Eligibility
Patients were eligible for inclusion in the trial who, on admission (but within a time frame to obtain patient consent/consultee agreement, randomise and start nutritional support within 36 hours of the date/time of original critical care unit admission), were:
-
an adult (defined as age ≥ 18 years)
-
an unplanned admission (including planned admissions becoming unplanned, e.g. unexpected postoperative complications)
-
expected to receive nutritional support for ≥ 2 days in the critical care unit, and
-
not planned to be discharged within 3 days (defined by clinical judgement) from the critical care unit.
Patients were excluded from the trial if they met any of the following criteria:
-
had been in a critical care unit for > 36 hours (i.e. from the date/time of original critical care unit admission)
-
had been previously randomised into the CALORIES trial
-
had pre-existing contraindications to PN or EN
-
had received PN or EN within the 7 days prior to admission to the critical care unit
-
had been admitted with a percutaneous endoscopic gastrostomy, percutaneous endoscopic jejunostomy, needle/surgical jejunostomy or nasojejunal tube in situ
-
had been admitted to the critical care unit for treatment of thermal injury (burns)
-
had been admitted to the critical care unit for palliative care
-
their expected stay in the UK was < 6 months, or
-
known to be pregnant.
During the trial, on the advice of the Research Ethics Committee, patients who were known to have a pre-existing condition, such as dementia, which would have precluded them from providing informed consent at any point during the trial, were also excluded.
Screening and recruitment
Following attendance at a site initiation meeting, screening and recruitment was commenced at participating units once the clinical trial site agreement had been signed and all of the necessary approvals were in place.
To promote awareness of the trial and facilitate recruitment, posters providing information about the CALORIES trial were displayed in the critical care unit and in family/visitor waiting rooms.
Potentially eligible patients were identified and approached by authorised members of staff about taking part in the trial. Information about the trial was provided to the patient, which included the purpose of the trial, the consequences of taking part or not, data security and funding of the trial. This information was also provided in a patient information sheet (see Appendix 1), along with the name and contact details of the local PI, which was given to the patient to read before making their decision to take part, or not, in the trial.
If the patient lacked mental capacity (because of their acute illness) to understand the information about the trial then, in accordance with the Mental Capacity Act,27 a personal consultee, who could be a relative or close friend, was identified with whom to discuss the patient’s participation in the trial. If there was no personal consultee available then the patient was provided with a professional consultee, for example an independent mental capacity advocate appointed by the NHS hospital trust, with whom to discuss the patient’s participation in the trial. The personal/professional consultee was provided with the same information as for patients (see Appendix 1) along with an explanation that they were being asked for their agreement for the patient to take part in the trial. Patients and personal/professional consultees were provided with an opportunity to ask questions before being invited to sign the consent form or personal/professional consultee agreement form, as appropriate.
Informed consent
Staff members, who had received training on the background, rationale and purpose of the CALORIES trial, and on the principles of the International Conference on Harmonisation Good Clinical Practice guidelines, were authorised by the PI to take informed consent from patients or informed agreement from a personal/professional consultee.
Once the staff member who was taking informed patient consent or consultee agreement was satisfied that the patient or personal/professional consultee had read and understood the patient information sheet, and that all of his/her questions about the trial had been answered, the patient or personal/professional consultee was invited to sign the patient consent form or personal/professional consultee agreement form, as appropriate.
For patients who had lacked mental capacity prior to randomisation, informed consent to continue participating in the trial was sought as soon as possible after the patient had regained mental capacity. If a patient did not regain mental capacity then, if possible, for patients entered via professional consultee agreement, agreement from a personal consultee was obtained for the patient to continue participating in the trial.
Randomisation and allocation procedure
Following informed consent from the patient or informed agreement from a personal/professional consultee, eligible patients were randomised via a central 24 hours per day, 7 days per week, telephone randomisation service hosted by Sealed Envelope Ltd. Patients were allocated, 1 : 1, to early nutritional support via either the parenteral route or the enteral route, by minimisation with a random component (each patient being allocated with 80% probability to the treatment group that would minimise imbalance). Minimisation was based on the following factors: site; age (< 65 years or ≥ 65 years); surgical status (surgery within 24 hours prior to unit admission or not); and malnutrition status (based on clinical judgement) (yes or no). A manual randomisation list (using permuted blocks, with block lengths of 4, 6 and 8) was prepared in advance of the trial by the trial statistician for use if the central telephone randomisation service was not available for any reason. Staff at participating sites were advised to call the 24 hours per day, 7 days per week, telephone support service if they experienced any problems with the central telephone randomisation service. Manual randomisation was carried out, as required, by the on-call member of the TMG. Details of any patients manually randomised were passed to the randomisation service for inclusion in the minimisation algorithm for subsequent allocations.
Screening log
To enable full and transparent reporting for the trial, brief details of all patients who met eligibility criteria or who met all of the inclusion criteria plus one or more of the exclusion criteria were recorded in the screening log. The reasons for eligible patients not being recruited were recorded, which included the patient declining the invitation to take part, the patient being excluded by the treating clinician, logistical reasons, etc. No patient identifiers were recorded in the screening log.
Treatment groups
As a pragmatic trial, the CALORIES trial did not dictate the use of specific nutritional products or protocols for delivery of nutritional support via the parenteral and enteral routes. However, existing established protocols for delivery of PN and EN at participating units were reviewed and approved by the TMG to ensure that they fell within common boundaries.
Early nutritional support was delivered via either the parenteral or enteral route for the 5-day (120 hours) intervention period, unless the patient transitioned to exclusive oral feeding or was discharged from the critical care unit before this time. Patients were able to start oral feeding if clinically indicated during the 5 days.
Early nutritional support via the parenteral route
For patients who were randomised to early nutritional support via the parenteral route, a central venous catheter, with a dedicated lumen, was inserted and positioned in accordance with NHS guidelines. 28 Patients received a standard parenteral feed, obtained from the unit’s usual supplier and used within the licence indication, which contained between 1365 and 2540 total kcal/bag and between 7.2 and 16.0 g of nitrogen/bag. Unit staff aimed to feed patients to a target of 25 kcal/kg/day (based on actual body weight) within 48–72 hours of starting the feed. There was no specific target for the amount of nitrogen to be given. Enteral ‘trickle feeding’ (‘trophic feeding’) was not permitted for the 5-day (120 hours) intervention period.
Local unit policy and practice was followed for delivery of nutritional support via the parenteral route and included provision for:
-
ensuring that the patient received a nutritionally complete feed
-
inclusion of additional micronutrients (made under appropriate pharmaceutically controlled environmental conditions) if clinically indicated, and as prescribed by the clinician and/or dietitian in accordance with National Institute for Health and Care Excellence (NICE) guidelines1
-
adjustment of total volume according to the patient’s fluid balance requirements
-
monitoring for specific nutritional-related complications
-
regular review of the patient for their ongoing nutritional support needs, and
-
energy requirements for patients with extremes of body mass index (BMI) (e.g. < 18.5 kg/m2 and > 30 kg/m2).
Early nutritional support via the enteral route
For patients who were randomised to early nutritional support via the enteral route, a nasogastric or nasojejunal tube was inserted and positioned in accordance with UK National Patient Safety Agency guidelines. 31,32 Patients received a standard enteral feed, obtained from the unit’s usual supplier, and used within the licence indication, which contained between 1365 and 2540 total kcal/day and between 7.2 and 16.0 g of nitrogen/day. There was no specific target for the amount of nitrogen to be given. Unit staff aimed to feed patients to a target of 25 kcal/kg/day (based on actual body weight) within 48–72 hours of starting the feed.
Local unit policy and practice was followed for delivery of nutritional support via the enteral route and included provision for:
-
ensuring that the patient received a nutritionally complete feed
-
adjustment of total volume according to the patient’s fluid balance requirements
-
monitoring for specific nutritional-related complications
-
regular review of patients for their ongoing nutritional support needs, and
-
energy requirements for patients with extremes of BMI (e.g. < 18.5 kg/m2 and > 30 kg/m2).
Outcome measures
The primary clinical effectiveness outcome was all-cause mortality at 30 days following randomisation and the primary cost-effectiveness outcome was the incremental net benefit (INB) gained at 1 year following randomisation, at a willingness-to-pay of £20,000 per quality-adjusted life-year (QALY).
The secondary outcomes were:
-
number of days alive and free from advanced respiratory support, advanced cardiovascular support, renal support, neurological support and gastrointestinal support up to 30 days following randomisation
-
number of new, treated, confirmed or strongly suspected infectious complications, classified according to clinical diagnosis, which occurred in the critical care unit
-
non-infectious complications of episodes of hypoglycaemia, elevated levels of liver enzymes, nausea requiring treatment, abdominal distension and vomiting, collected through adverse event reporting up to 30 days following randomisation, and new or significantly worsened pressure ulcers while in the critical care unit
-
duration of critical care unit stay (from dates and times) and acute hospital length of stay (in whole days) following randomisation
-
duration of survival at 90 days and at 1 year following randomisation
-
all-cause mortality at discharge from the critical care unit and from the acute hospital
-
all-cause mortality at 90 days and at 1 year following randomisation
-
nutritional and health-related quality of life at 90 days and at 1 year following randomisation
-
resource use and costs at 90 days and at 1 year following randomisation, and
-
estimated lifetime incremental cost-effectiveness (INB).
Safety monitoring
Patients were monitored for adverse events occurring between randomisation and 30 days following randomisation. Specified adverse events were defined as follows:
-
abdominal distension was defined as any new, clinically significant change in appearance; abdominal distension was considered severe if there was an acute obstruction
-
abdominal pain was defined as any new episode of abdominal pain, localised to the abdomen and requiring more than just simple analgesia; abdominal pain was considered severe if it was not controlled with opiates
-
episodes of electrolyte disturbance were defined as any new, clinically significant electrolyte disturbance requiring active monitoring or treatment
-
haemopneumothorax was defined as any new haemopneumothorax requiring insertion of a chest drain
-
hepatomegaly was defined as any new or increased hepatomegaly on clinical examination
-
hyperosmolar syndrome was defined as any new, clinically significant osmolar gap requiring active monitoring or treatment
-
hypersensitivity reaction (anaphylactic reaction) was defined as any new anaphylactic reaction
-
episodes of hypoglycaemia were defined as any new episode of clinically significant hypoglycaemia requiring active monitoring or treatment
-
ischaemic bowel was defined as any new episode of ischaemic bowel inferred on radiology or diagnosed visually, for example during surgery or by endoscopy
-
jaundice was defined as any new, clinically significant jaundice requiring active monitoring or treatment
-
nausea requiring treatment was defined as any new episode of nausea requiring treatment with anti-emetic drugs
-
pneumothorax was defined as any new pneumothorax requiring insertion of a chest drain
-
raised levels of liver enzymes were defined as any new, clinically significant rise in liver enzyme levels requiring active monitoring or treatment
-
regurgitation/aspiration was defined as any episode of regurgitation/aspiration
-
vascular catheter-related infection was defined as any new vascular catheter-related infection for which a vascular catheter, such as a central venous catheter, was identified as the primary source of infection and associated with signs and symptoms of infection requiring antimicrobial drugs and/or removal of catheter, and
-
vomiting was identified as any episode of vomiting.
Unspecified adverse events were defined as an unfavourable symptom or disease that was temporally associated with the use of the trial treatments, whether or not it was related to the trial treatment, which was not deemed to be a direct result of the patient’s medical condition and/or standard critical care treatment.
All adverse events were recorded in the electronic case report form and reported, as part of routine reporting throughout the trial, to the DMEC and research ethics committee. Adverse events that were assessed to be serious (i.e. prolonging hospitalisation or resulting in persistent or significant disability/incapacity), life-threatening or fatal – collectively termed serious adverse events – were reported to the ICNARC CTU and reviewed by a clinical member of the TMG. Serious adverse events that were unspecified and considered to be possibly, probably or definitely related to the trial treatment were reported to the research ethics committee within 15 calendar days of the event being reported.
Data collection
A secure, dedicated electronic case report form, hosted by ICNARC, was set up to enable trial data to be entered by staff at participating sites. The electronic case report form was accessible only to authorised users and access was approved centrally by the trial manager, data manager or research assistant (after cross-checking the site delegation of trial duties log). Each individual was provided with a unique username and password and had access to data only for patients recruited at their site.
The data set for the CALORIES trial included the minimum data required to confirm patient eligibility, to describe the patient population, to monitor and describe delivery of the intervention, to assess primary and secondary outcomes and to enable linkage to the ICNARC Case Mix Programme (see Appendix 2). 26
Randomisation
Data were collected to enable the patient to be randomised, and included confirmation that the patient met all of the inclusion criteria and none of the exclusion criteria (see Appendix 2).
Baseline
The following data were collected at baseline to enable follow-up and to describe the patient population:
-
full name and address of the patient and their GP
-
date of birth
-
gender, and
-
raw physiology data to enable calculation of the Sequential Organ Failure Assessment (SOFA) score (see Appendix 3). 33
Raw physiology data, to enable calculation of the Acute Physiology and Chronic Health Evaluation version II (APACHE II) and ICNARC scores and predicted risks of hospital death (see Appendix 3), were obtained from the Case Mix Programme Database. 34,35
Intervention period
Data were collected daily throughout the 5-day (120-hour) intervention period to monitor adherence to treatment allocation (early nutritional support via parenteral route or early nutritional support via enteral route) and to describe and cost delivery of early nutritional support via the parenteral and enteral routes. The data collected included:
-
nutritional support delivered during each calendar day, including mode of delivery of nutritional support, site of central venous catheter, site of enteral tube (nasogastric or nasojejunal), nutritional product type, volume of nutritional support delivered and any change to delivery of nutritional support
-
physiology, for example arterial oxygen pressure (PaO2), fraction of inspired oxygen (FiO2), blood pressure, Glasgow Coma Scale (GCS) score, blood glucose, urine output, and
-
interventions, for example mechanical ventilation, vasoactive drugs, systemic antibacterial drugs and/or antifungal drugs.
At critical care unit discharge
At the time of discharge from the critical care unit, the following data were collected:
-
route(s) of delivery of nutritional support in the critical care unit from day 7 following randomisation
-
interventions delivered in the critical care unit from day 7 following randomisation
-
new strongly suspected or confirmed infectious episodes, classified according to clinical diagnosis, which occurred in the critical care unit following randomisation
-
strongly suspected infection was defined as strongly suggestive evidence, for example evidence of gross purulence or evidence from radiological or other imaging techniques, and the commencement of antibacterials or antifungals for a suspected infection; strongly suggested evidence must have been documented in the case notes
-
confirmed infection was defined as laboratory/microbiological confirmation, including cultures, Gram stains and roentgenograms
-
-
organ support, as defined by the UK Department of Health Critical Care Minimum Dataset (CCMDS)36 (see Appendix 4) during the critical care unit stay, and
-
date and time of discharge from, or death in, the critical care unit.
At acute hospital discharge
At the time of discharge from the acute hospital, the following data were collected:
-
the locations of care during the patient’s stay in the acute hospital, for example critical care unit, ward
-
date that exclusive oral feeding commenced (if applicable) following the patient’s discharge from the critical care unit
-
date of discharge from, or death in, the acute hospital, and
-
discharge location, for example home, nursing home, other hospital.
At 30 days
All patients were followed up at 30 days following randomisation for the primary clinical effectiveness outcome (all-cause mortality) via the Health and Social Care Information Centre (HSCIC) Data Linkage and Extract Service.
Longer-term follow-up
Following randomisation, a letter was sent to the patient’s GP informing them of the patient’s participation in the trial and a request for assistance with follow-up, if required. All patients who survived to leave hospital were followed up at 90 days for the secondary outcomes (all-cause mortality, duration of survival, health-related quality of life and resource use), and at 1 year for secondary outcomes (all-cause mortality, duration of survival, health-related quality of life and resource use) and to calculate the primary cost-effectiveness outcome (INB).
Data linkage with death registration
Follow-up of patients was carefully monitored to prevent any potential distress to those who care for the patient receiving a letter addressed to a deceased relative, partner or friend. The follow-up process started at 75 days for the 90-day follow-up and at 350 days for the 1-year follow-up to allow for the administrative processes. Each week a list of all patients who had been discharged alive from hospital and who were either 75 days or 350 days post-randomisation was sent to the HSCIC Data Linkage and Extract Service to confirm their mortality status. Patients indicated as having died were logged and the follow-up process ended.
Follow-up procedure
Patients identified by the HSCIC Data Linkage and Extract Service as not having died started the follow-up process summarised in Figure 3. A questionnaire pack was sent from the ICNARC CTU, by post, to the patient. Following evidence-based practice for maximising responses to postal surveys,37 the questionnaire pack included a cover letter (see Appendix 5); the patient information sheet (see Appendix 1) or patient newsletter (which replaced the patient information sheet in November 2013); two questionnaires – the Health Questionnaire and the Health Services Questionnaire (see Appendix 6); a stamped-addressed return envelope; and a pen. The Health Questionnaire (see Appendix 6) included the required questions from the EQ-5D-5L to evaluate health-related quality of life38 and the Satisfaction with Food-related Life Questionnaire to evaluate the patient’s nutritional quality of life. 39 It is a measure of satisfaction developed by Grunert and the Food in Later Life team. The five items exhibit good reliability (as measured by Cronbach’s alpha), good temporal stability, convergent validity with two related measures, and construct validity as indicated by relationships with other indicators of quality of life. 39 The Health Services Questionnaire (see Appendix 6) included questions about the patient’s use of health services following discharge from the acute hospital and was used to cost subsequent use of health services. The cover of the questionnaires included a ‘do not wish to participate’ tick box.
FIGURE 3.
Patient follow-up process at 90 days and at 1 year.

If no response was received after 2 weeks, a reminder letter was sent with another questionnaire pack. If no response was received after a further 2 weeks, the patient was telephoned, if contact details were available. Telephone calls were made at various times from Monday to Friday between 08.30 and 20.30 to maximise the chances of contacting the patient. Patients who were successfully contacted by telephone were asked if they had received the questionnaire pack and were invited to complete the questionnaires over the telephone, if it was convenient. In addition, patients were reminded about completing the questionnaire when they attended hospital follow-up appointments.
Follow-up ended on receipt of a completed (or blank) questionnaire; a questionnaire with a ticked ‘do not wish to participate’ box; notification to the ICNARC CTU by telephone or e-mail that the patient wished to withdraw from the trial; or if there was no response to telephone follow-up. For questionnaire packs returned indicating that the recipient was not known at the address, the contact details for the patient were checked with the recruiting hospital and/or GP.
For patients who were identified as either being a hospital inpatient, or resident in a care home or rehabilitation centre, the relevant institution was contacted to establish the status of the patient and the most appropriate way to proceed with follow-up. If the patient had mental capacity to consent but required assistance in reading and/or completing the questionnaire then health-care professionals usually assisted the patient. For patients who lacked mental capacity to consent, institutions advised on the most appropriate person to contact to complete the questionnaires.
If patients were identified as having no fixed abode but were registered with a GP or had regular contact with a homeless shelter then the questionnaire pack was sent to be passed (when appropriate) to them at their next appointment or visit.
Data linkage with the Case Mix Programme
Linkage of patient identifiable trial data to the Case Mix Programme Database provided information on the baseline characteristics of patients and subsequent admission to the critical care unit following discharge from the critical care unit.
Data for the Case Mix Programme are collected by trained data collectors to precise rules and definitions. The data then undergo extensive local and central validation for completeness, illogicalities and inconsistencies prior to pooling.
Data management
Data management was an ongoing process. Data entered by sites on to the electronic case report form were monitored and checked throughout the recruitment period to ensure that data were as complete and accurate as possible.
Two levels of data validation were incorporated into the electronic case report. The first was to prevent obviously erroneous data from being entered, for example entering a date of birth that occurred after the date of randomisation. The second level involved checks for data completeness and any unusual data entered, for example a physiological variable, such as blood pressure, which was outside of the predefined range. Site staff could generate data validation reports, listing all outstanding data queries, at any time via the electronic case report form. The site PI was responsible for ensuring that all of the data queries were resolved. Ongoing data entry and validation at sites was closely monitored by the data manager (JT) and any concerns were raised with the site PI.
The contact details for patients and their GPs (name and postal address) were checked weekly for completeness to avoid unnecessary delays in sending out questionnaire packs at 90 days and at 1 year.
Adherence to the trial protocol was closely monitored, including adherence to all elements of early nutritional support delivered via the parenteral and the enteral route. Any queries relating to adherence were generated in a separate monthly report, which was sent to the site PI. For each query, the PI was asked to explain the reason for any non-adherence to the protocol. If deemed necessary, a teleconference was arranged with the site to ensure that effective plans were put in place to improve future adherence.
Data received from completed Health and Health Services questionnaires were entered centrally into a secure database at the ICNARC CTU following a standard operating procedure. All identifiable information, such as names (e.g. of patients, family members or hospital staff members) were removed. All queries relating to data entry were reviewed by two members of the TMG (SH/BM) and any disagreement was reviewed and discussed with a third (KR).
To ensure that data were entered accurately, all questionnaire data entered into the database were cross-checked by a second member of the CTU team. Any errors that were found were logged and corrected on the database.
Sample size
Applying the trial entry criteria to over 500,000 admissions to adult, general critical care units in the Case Mix Programme Database, the 30-day mortality for unplanned, ventilated adult admissions staying ≥ 3 days was 32%. As the enteral route is the predominant choice for nutritional support, this mortality was used as the basis to estimate control group mortality.
A meta-analysis of existing randomised controlled trials of PN compared with EN indicated a potential relative risk reduction associated with PN of around 20% (see Figure 1). To have 90% power, with a type I error rate of 5% (two sided), to detect a 20% relative risk reduction (6.4% absolute risk reduction) from 32% in the enteral route group to 25.6% in the parenteral route group, requires a sample size of 1082 per treatment group (Stata/SE version 10.1, StataCorp LP, College Station, TX, USA). To allow 2% for crossover/protocol violation (in each direction) and 2% for loss to follow-up/withdrawal prior to 30 days (based on observed rates from the PAC-Man Study40), a sample size of 1200 per treatment group (2400 total) was required. No adjustment to the sample size calculation was made to account for subgroup analyses.
Interim analysis
Unblinded, comparative data on recruitment, withdrawal, adherence (to the allocated treatment) and serious adverse events were regularly reviewed by an independent DMEC.
Without specific analysis of the primary outcome, the DMEC reviewed data from the first 37 trial participants and continued to review data at least 6-monthly to assess potential safety issues and to review adherence. A single, planned, formal, interim analysis was performed at the point that 30-day outcome data for the first 1200 patients enrolled were available. A Haybittle–Peto stopping rule (p < 0.001) was used to guide recommendations for early termination as a result of harm. Following the planned interim analysis, the DMEC recommended that the trial continue with no changes.
Analysis principles
All analyses were based on the intention-to-treat principle. Patients were analysed according to the treatment group to which they were randomised, irrespective of whether or not the allocated treatment was received (i.e. regardless of whether they did or did not adhere to the allocated route). All tests were two-sided, with significance levels set at p < 0.05 and with no adjustment for multiplicity. All a priori subgroup analyses were carried out irrespective of whether or not there was strong evidence of a treatment effect associated with the primary outcome. As missing data for the clinical effectiveness primary outcome were anticipated to be minimal, a sensitivity approach was taken when the primary outcome was missing (see Secondary analyses of the primary outcome). Missing data for the cost-effectiveness analysis, as well as missing baseline data for adjusted analysis of clinical outcomes, were handled by multiple imputation.
Multiple imputation
Missing data in baseline covariates, resource use and health-related quality-of-life variables at 90 days and 1 year were handled with multivariate imputation by chained equations. 41 Under this approach, each variable was imputed conditional on fully observed baseline variables, such as age, sex, ICNARC Physiology Score, presence of cancer, length of stay in critical care and general medical wards, and all other imputed variables. When addressing the missing data, multiple imputation assumes that the data are missing at random, conditional on the observed data.
Patients who did not return or fully complete the EQ-5D-5L questionnaire administered at 90 days had their EQ-5D-5L utility scores imputed from those survivors who did fully complete the questionnaire. Similarly, for those eligible patients who did not return the Health Services Questionnaire, information on the use of outpatient services up to 90 days following randomisation was imputed from those patients who did complete this questionnaire. Health Services Questionnaire costs and quality-of-life end points were conditional on survival status; as such, the imputation was conducted in two stages. In the first stage, imputation models were specified for mortality at 90 days according to baseline covariates and auxiliary variables, including hospital length of stay of up to 90 days. In the second stage, for each of the imputed data sets from stage 1, imputation models were specified for Health Services Questionnaire costs and quality of life at 90 days for those patients who were missing these but were known to be alive at 90 days, or were predicted to be alive by the first stage imputation model.
Patients who did not return or fully complete the EQ-5D-5L questionnaire or the Health Services Questionnaire administered at 1 year also had their information imputed from those survivors who did fully complete the questionnaire, using a similar two-stage approach.
The resultant estimates were combined with Rubin’s rules, which recognise uncertainty both within and between imputations. 42 All multiple imputation models were implemented in the statistical package Stata/SE version 13.0 (StataCorp LP, College Station, TX, USA).
Statistical analysis: clinical effectiveness
Statistical analyses were conducted according to a pre-specified, published statistical analysis plan written prior to the interim analysis. 43 The final analyses were conducted using Stata/SE version 13.0.
Baseline characteristics
Baseline demographic and clinical data were summarised by treatment group. Statistical tests for differences between the groups were not reported, as these may be misleading. Discrete variables were summarised as numbers and percentages, which were calculated according to the number of patients for whom data were available; when values were missing, the denominator was reported. Continuous variables were summarised by standard measures of central tendency and dispersion, either mean and standard deviation (SD) and/or median and interquartile range (IQR) as specified below:
-
age, mean (SD) and median (IQR)
-
sex, n (%)
-
severe comorbidities (as defined by APACHE II34), n (%):
-
severe liver condition
-
severe renal condition
-
severe respiratory condition
-
severe cardiovascular condition
-
immunocompromised
-
-
acute severity of illness:
-
surgical status – surgery within 24 hours prior to critical care unit admission, n (%)
-
ventilation status – mechanical ventilation at admission to the critical care unit, n (%)
-
malnourished – yes/no (based on clinical judgement), n (%)
-
actual/estimated BMI, mean (SD) and median (IQR)
-
ulna length (cm), mean (SD) and median (IQR)
-
mid-upper arm circumference (cm), mean (SD) and median (IQR)
-
degree of malnutrition (high, BMI of < 18.5 kg/m2 or weight loss of > 10%; moderate, BMI of < 20 kg/m2 or weight loss of > 5%; no malnutrition) (based on NICE definitions1), n (%).
Adherence
Non-adherence with the allocated treatment was reported as the number and percentage of patients who:
-
did not receive any nutritional support
-
received first nutritional support via the opposite route to assigned
-
received initiation of nutritional support more than 36 hours after admission to critical care
-
received early nutritional support via assigned route and subsequently changed to opposite route during first 120 hours, or
-
received no nutritional support for at least a full 1-day period during the first 120 hours.
Delivery of care
Delivery of care was summarised by treatment group but not subjected to statistical testing. As with baseline characteristics, discrete variables were summarised as numbers and percentages. Percentages were calculated according to the number of patients for whom data were available; where values were missing, the denominator was reported. Continuous variables were summarised by mean (SD) and/or median (IQR), as specified below:
-
time from critical care unit admission to commencement of nutritional support (hours), median (IQR)
-
total calories and average calories per 24 hours received during intervention period (total calories and a breakdown of the total calories received via the enteral route, the parenteral route, intravenous (i.v.) glucose, propofol and oral feed), mean (SD)
-
total protein and average protein per 24 hours received during intervention period (total protein and a breakdown of the total protein received via the enteral and the parenteral route), mean (SD)
-
total gastric residual volume aspirated (ml) and average per 24 hours during intervention period, if fed via the enteral route, mean (SD)
-
total gastric residual volume replaced and average per 24 hours during intervention period, if fed via the enteral route, mean (SD)
-
patients receiving additives during intervention period (glutamine, selenium and fish oils), if fed via the parenteral route, n (%)
-
patients receiving prokinetics during intervention period, if fed via the enteral route, n (%)
-
patients receiving insulin, n (%), and total insulin received (IU), mean (SD), during intervention period
-
patients receiving vasoactive agents during intervention period, n (%)
-
incidence of diarrhoea and constipation, n (%)
-
time from randomisation to commencement of exclusive oral feeding (days), median (IQR)
-
daily SOFA score during the intervention period, median (IQR).
Primary outcome: clinical effectiveness
The number and percentage of deaths at 30 days following randomisation, due to any cause, were reported for each treatment group. The primary effect estimate was the relative risk of all-cause mortality at 30 days, reported with a 95% CI. The absolute risk reduction and 95% CI were also reported. Deaths at 30 days after randomisation were compared between the treatment groups, unadjusted, using Fisher’s exact test. A secondary analysis of the primary outcome, adjusted for baseline variables, was conducted using multilevel logistic regression. Baseline variables adjusted for in the multilevel logistic regression model were age, ICNARC Physiology Score, surgical status, degree of malnutrition and a site-level random effect. Baseline variables were selected for inclusion in the adjusted analysis a priori according to anticipated relationship with outcome. The results of the multilevel logistic regression model were reported as an adjusted odds ratio with 95% CI. The unadjusted odds ratio was presented for comparison.
Secondary outcomes: clinical effectiveness
The mean (SD) of the number of days alive and free from advanced respiratory support, advanced cardiovascular support, renal support, neurological support and gastrointestinal support, as defined by the CCMDS36 (see Appendix 4), up to 30 days, within each treatment group, were reported. Patients who died within the first 30 days were assigned zero days alive and free of each organ support. Days of organ support were recorded only while the patient was in a critical care unit; any days outside of a critical care unit were assumed to be free of organ support. Differences between the treatment groups were tested using the t-test, using the non-parametric bootstrap to account for anticipated non-normality in the distributions. 44 A total of 1000 bootstrap replications were taken, stratified by treatment group, with bias-corrected and accelerated CIs reported.
The mean (SD) number of treated infectious complications per patient and the number and percentage of patients with each infectious complication (chest infection, central venous catheter infection, other vascular catheter-related infection, bloodstream infection, infective colitis, urinary tract infection, surgical site infection, other infectious complication) and each non-infectious complication (episodes of hypoglycaemia, elevated levels of liver enzymes, nausea requiring treatment, abdominal distension, vomiting, new or substantially worsened pressure ulcers) within each treatment group were reported. Infectious complications and pressure ulcers were assessed while the patient was in the critical care unit; all other non-infectious complications were collected through adverse event reporting up to 30 days following randomisation. Differences between the treatment groups were tested using the t-test for means and Fisher’s exact test for percentages.
The median (IQR) of the length of stay in critical care and in acute hospital were reported for each treatment group. Differences in length of stay between the treatment groups were tested using the Wilcoxon rank-sum test, stratified by survival at critical care discharge and acute hospital discharge, respectively.
Kaplan–Meier curves by treatment group were plotted up to 90 days and 1 year after randomisation and compared using the log-rank test. An adjusted comparison was performed using a Cox proportional hazards model, which was adjusted for the same baseline variables as the primary outcome, including shared frailty within sites (gamma-distributed latent random effects). The appropriateness of the proportional hazards assumption was assessed graphically by plotting –log[−log(survival)] against log(time) within treatment groups. The number and percentage of deaths at critical care and acute hospital discharge, and by 90 days and 1 year post-randomisation, were reported for the treatment groups. Differences in all-cause mortality at each time point were compared, unadjusted, using Fisher’s exact test and, adjusted, using multilevel logistic regression, adjusted for the same baseline variables as the primary outcome.
Safety monitoring
The number and percentage of patients experiencing each serious adverse event (occurring between randomisation and 30 days) were reported for each treatment group. The total number of patients experiencing one or more serious adverse events was compared between treatment groups using Fisher’s exact test and summarised as a relative risk with 95% CI.
Subgroup analyses of the primary outcome
Subgroup analyses were conducted to test for a difference in treatment effect according to pre-specified subgroups. Differences in the primary outcome (30-day mortality) were analysed by age (in quartiles), degree of malnutrition (high/moderate or none), acute severity of illness (APACHE II34 and ICNARC model35 predicted risk of mortality – in quartiles), mechanical ventilation at admission to the critical care unit, presence of cancer and time from critical care unit admission to commencement of nutritional support (< 24 hours vs. ≥ 24 hours).
These analyses tested for an interaction between the subgroup categories and the treatment group in multilevel logistic regression models, adjusted for the same baseline variables as used in the primary analysis.
Secondary analyses of the primary outcome
Sensitivity analyses for missing data in the primary outcome
As the number of missing data was anticipated to be minimal, a sensitivity approach was taken when the primary outcome variable was missing. The primary analysis was repeated once, assuming that all patients in the enteral route group with missing outcomes survived and all patients in the parenteral route group with missing outcomes did not survive. The analysis was then repeated again with the opposite assumptions. This approach gives the absolute range of how much the results could change if all of the data were complete.
Adherence-adjusted analysis
Although the intention-to-treat analysis provides the best estimate of the clinical effectiveness of early nutritional support via the parenteral route compared with the enteral route, it was also of interest to estimate what the efficacy of early nutritional support delivered via the parenteral route would be compared with the enteral route, if delivered as intended. In a randomised controlled trial, the allocated treatment can be used as an ‘instrumental variable’, that is, a variable associated with receipt of the intervention and associated with the outcome only through its association with the intervention. 45 This relationship enables us to estimate what the treatment effect would be for patients who are adherent to the protocol. The primary analysis was repeated adjusting for adherence using a structural mean model with an instrumental variable of allocated treatment, assuming a linear relationship between the degree of adherence (duration of allocated treatment received) and treatment effect. 46
Cost-effectiveness analysis
A full cost-effectiveness analysis was undertaken to assess which route of delivery for early nutritional support in critically ill adults, parenteral compared with enteral, was most cost-effective. This analysis assessed whether or not the additional intervention costs of early nutritional support via the parenteral route compared with the enteral route were justified by any subsequent reductions in morbidity costs and/or improvements in patient outcomes. The cost-effectiveness analysis was reported for three time periods: randomisation to 90 days; randomisation to 1 year; and lifetime. For each time period the cost-effectiveness analysis took a health and personal health services perspective,47 using information on health-related quality of life collected at 90 days and 1-year follow-up, combined with information on vital status, to report QALY. Each QALY was valued using the NICE-recommended threshold of willingness to pay for a QALY gain (£20,000),47 in conjunction with the costs of each strategy to report the incremental net monetary benefits (INBs) of early nutritional support via the parenteral route compared with early nutritional support via the enteral route, overall and for the same pre-specified subgroups as for the evaluation of clinical effectiveness (see Subgroup analyses of the primary outcome, below).
The primary objective of the cost-effectiveness analysis was to compare incremental cost-effectiveness at 1 year of early nutritional support via the parenteral route compared with the enteral route. The secondary objectives were to:
-
compare health-related quality of life at 90 days and 1 year between the treatment groups
-
compare resource use and costs at 90 days and 1 year between the treatment groups
-
estimate the lifetime incremental cost-effectiveness between the treatment groups.
The main assumptions of the cost-effectiveness analysis were subjected to extensive sensitivity analyses.
Resource use
The resource-use categories considered were chosen a priori, for which differences between the treatment groups were judged as being possible and likely to drive incremental costs, and were reported for each treatment group. Data for interventions, staff time and acute hospital stay for the index hospital admission were collected as part of the CALORIES trial data set. Readmissions to acute hospital including a critical care stay were identified from the Case Mix Programme Database. Readmission to acute hospital not involving critical care, as well as hospital outpatient and community services use, were collected as part of the Health Services Questionnaires completed at 90 days and 1 year.
Interventions
Use of nutritional feeding as a part of the CALORIES trial interventions in critical care unit has been considered in economic evaluation. For each patient, total volume of nutritional support delivered within the first six calendar days of nutritional support (encompassing the 120-hour intervention period) was calculated according to route of feeding and type of nutritional products. The use of alternative nutritional feeding due to crossover was included in the costing, which followed the intention-to-treat principle, as per the analysis of clinical effectiveness.
The use of i.v. glucose, propofol and insulin within the first six calendar days of nutritional support was recorded daily on the trial case report form for each patient. The trial case report form recorded daily volume and concentration of i.v. glucose and propofol, and total units (IU) of insulin. Use of propofol was recorded because of its relatively high calorie content.
Nutritional support from day 7 following initiation of nutritional support to discharge from the critical care unit was reported using trial case report form data. From calendar day 7 up to discharge from the critical care unit, trial case report forms recorded the date of each change of route of nutritional support, and these were aggregated to calculate the number of days each patient received nutritional support via each route. Costs of nutritional support from calendar day 7 up to discharge from critical care unit were calculated according to number of days of nutritional support via each route.
Staff time
The cost-effectiveness analysis recognised that the delivery of nutritional support in critically ill adult patients via the parenteral route rather than the enteral route requires additional staff input. A multidisciplinary team with dietetic, nursing, pharmacy and medical expertise is required to provide PN in routine clinical practice. It was considered that critical care units have existing medical expertise but do not have a dedicated nutritional team to deliver nutritional support. The delivery of nutritional support required staff time for initial dietary assessment (dietitians’ time); prescribing and preparing PN (pharmacists’ time); daily monitoring and support of patients (dietitians’ and pharmacists’ time); and for changing gowns and bed sheets in the event of episodes of vomiting or diarrhoea (nurses’ time). The level of additional staff time for the delivery of nutritional support via the parenteral route was estimated according to expert opinion reflecting the most plausible assumption for routine practice in majority of the centres (Table 1), with alternative levels considered in the sensitivity analyses (see Table 3). The base-case analysis assumed that, compared with delivery of nutritional support via the enteral route, the delivery of nutritional support via the parenteral route required additional pharmacist’s time for initial assessment and set-up (30 minutes) and daily monitoring and support (30 minutes). It was recognised that the delivery of protocol would require additional staff time within the intervention period. To reflect routine critical care practice, it was considered that additional support from the nutritional team (dietitian and pharmacist) is available on weekdays and any additional nutritional bags over the weekends were prearranged during weekdays.
| Support requirement | Delivery of nutritional support via the: | |
|---|---|---|
| Parenteral route | Enteral route | |
| Initial dietary assessment and set-up of nutritional supporta | 30 minutes of dietitian’s time | 30 minutes of dietitian’s time |
| 30 minutes of pharmacist’s time | No additional pharmacist’s time | |
| Monitoring and support (daily)b | 20 minutes of dietitian’s time | 20 minutes of dietitian’s time |
| 30 minutes of pharmacist’s time | No additional pharmacist’s time | |
| Adverse events | ||
| Vomiting (episode) | Three nurses for 10 minutes | Three nurses for 10 minutes |
| Diarrhoea (episode)c | Three nurses for 10 minutes | Three nurses for 10 minutes |
Acute hospital length of stay
Length of stay in critical care and general medical wards within the index hospital admission (i.e. the hospital in which a patient was randomised to the trial) were reported. Each critical care stay was assigned a Healthcare Resource Group (HRG), applying the standard HRG grouper algorithm, according to the maximum number of organs supported during the stay. 48
An acute hospital readmission was defined as a further hospital admission, for any reason, following discharge home from the index admission. Length of stay in critical care and general medical wards within acute hospital readmissions were taken from the Case Mix Programme Database49 and the Health Services Questionnaires.
Hospital outpatient visits and community service use
The number of hospital outpatient visits and community service use following discharge from the index admission, for any reason, were reported. Items of community service use included visits to the GP (family doctor), nurse, health visitor, occupational therapist, dietitian, physiotherapist and psychologist. The levels of resource use were taken from responses to the Health Services Questionnaire.
Unit costs
The unit costs required for valuing the resource-use data listed in Table 2 were taken from four sources: the British National Formulary (BNF); manufacturers’ list prices; national unit cost databases; and local NHS prices. The unit costs of nutritional products were taken from the BNF50 and manufacturer’s list price. During the intervention period, the use of the top five high-volume nutritional products were costed individually; the rest were costed based on the weighted average of the costs for the top five high-volume products. Enteral nutritional products were costed using the weighted average of the costs of the top five high-volume products. The use of feeding after the intervention period up to discharge from the critical care unit was costed according to the number of days that a patient was receiving either PN or EN. The daily use of nutritional support after the intervention period was calculated from the weighted average of the number of bags of nutritional products which the patient had used during the intervention period. The unit costs of other interventions (glucose, propofol and insulin) were obtained from personal communication with local NHS finance department or from the BNF. The use of glutamine, selenium and fish oil in the parenteral group were not considered as separate items: their costs were included within the unit cost of a critical care bed-day according to the HRG definition.
| Items | Unit costs (£) | Source |
|---|---|---|
| Nutritional products | ||
| Parenteral | ||
| Kabiven® 7gN (1920 ml) | 50.00 | BNF50 |
| NuTRIflex® Lipid Peri (2500 ml) | 64.22 | BNF50 |
| Kabiven 9gN (2400 ml) | 64.00 | BNF50 |
| Kabiven 11gN (2053 ml) | 67.00 | BNF50 |
| Kabiven 5gN (1440 ml) | 35.00 | BNF50 |
| Weighted average unit cost of PN | 57.37 | BNF50 and CALORIESa |
| Enteral | ||
| Nutrison (1000 ml) | 8.25 | BNF50 |
| Nutrison Multi Fibre (1000 ml) | 9.54 | BNF50 |
| Fresubin® Original (1000 ml) | 7.96 | BNF50 |
| Nutrison Protein Plus (1000 ml) | 9.80 | BNF50 |
| Nutrison Low Sodium | 16.85 | Manufacturer’s list priceb |
| Weighted average unit cost of EN | 9.87 | BNF50 and CALORIESa |
| Other interventions | ||
| Glucose (4%): 500 ml | 0.93 | Local NHS finance department |
| Glucose (5%): 500 ml | 0.85 | Local NHS finance department |
| Glucose (10%): 500 ml | 0.70 | Local NHS finance department |
| Glucose (20%): 500 ml | 1.50 | Local NHS finance department |
| Glucose (50%): 500 ml | 2.00 | Local NHS finance department |
| Propofol (1%): 50 ml | 10.10 | Local NHS finance department |
| Propofol (2%): 50 ml | 21.30 | Local NHS finance department |
| Insulin: 1000 units | 7.48 | BNF50 |
| Staff time | ||
| Hospital dietitian | ||
| Band 6 (per hour) | 43 | PSSRU52 |
| Band 7 (per hour) | 53 | PSSRU52 |
| Hospital costs (bed-day) | ||
| Critical care bed-day | ||
| 0 organs supported | 696 | NHS reference costs51 |
| 1 organ supported | 932 | NHS reference costs51 |
| 2 organs supported | 1304 | NHS reference costs51 |
| 3 organs supported | 1479 | NHS reference costs51 |
| 4 organs supported | 1622 | NHS reference costs51 |
| 5 organs supported | 1692 | NHS reference costs51 |
| ≥ 6 organs supported | 1947 | NHS reference costs51 |
| General ward bed-day | 275 | NHS reference costs51 |
| Outpatient and community health services | ||
| Hospital outpatient (per visit) | 109 | PSSRU52 |
| GP practice visit (per visit) | 45 | PSSRU52 |
| GP home visit (per visit) | 114 | PSSRU52 |
| GP practice nursec | 13 | PSSRU52 |
| Hospital staff nursec | 13 | PSSRU52 |
| Health visitorc | 13 | PSSRU52 |
| Occupational therapistc | 9 | PSSRU52 |
| Psychologistc | 15 | PSSRU52 |
| Speech and language therapistc | 9 | PSSRU52 |
| Physiotherapistc | 9 | PSSRU52 |
| Dietitianc | 9 | PSSRU52 |
The unit costs associated with the additional staff time required to deliver nutritional support and address adverse events were taken from national sources. The costs per critical care bed-day, by HRG, and general medical bed-day were taken from the ‘Payment by Results’ database. 51 Unit costs for hospital outpatient visits and community service use were obtained from a recommended published source for Health and Social Care costs. 52 All unit costs were reported in 2013–14 prices.
Nutritional and health-related quality of life
The responses to the EQ-5D-5L questionnaire were used to report each patient’s described health, which was then valued according to health state preferences from the general population to calculate EQ-5D-5L utility scores, anchored on a scale from 0 (death) to 1 (perfect health). 53 The number and percentage of patients in each level of each dimension were reported by treatment group. The responses to the Satisfaction with Food-related Life questionnaire were used to evaluate the patient’s nutritional quality of life and were reported on a scale from 1 (worst possible satisfaction) to 7 (best possible satisfaction).
Cost-effectiveness at 90 days following randomisation
Mean EQ-5D-5L utility scores, QALYs, total costs and incremental net monetary benefits up to 90 days were reported for each treatment group. Unadjusted mean differences between the treatment groups, in quality of life, QALYs and incremental costs at 90 days were reported with 95% CIs. These were reported both overall and by each of the pre-specified subgroups (see Subgroup analyses of the primary outcome, below).
Total costs up to 90 days were calculated by combining the resource use with unit costs. For survivors at 90 days, QALYs were calculated by valuing each patient’s survival time by his/her health-related quality of life according to the ‘area under the curve’ approach,54 assuming an EQ-5D-5L utility score of zero at randomisation, and a linear interpolation of between randomisation and 90 days. Zero QALYs were assumed for decedents at between randomisation and 90 days. The differences in average costs and QALYs between the treatment groups were used to calculate the incremental net monetary benefit of delivery of early nutritional support via the parenteral route compared with the enteral route. The incremental QALY was valued according to the NICE-recommended threshold of willingness to pay for a QALY gain (£20,000) and the incremental cost was subtracted from this.
The uncertainty around the differences in average costs and QALYs between the treatment groups was illustrated on the cost-effectiveness plane. The incremental costs and QALYs were estimated with a seemingly unrelated regression model. 55 To express the uncertainty in the estimation of the incremental costs and QALYs, the estimates of the means, variances and the covariance from the regression model were used to generate 500 estimates of incremental costs and QALYs from the joint distribution of these end points, assuming asymptotic normality. A cost-effectiveness acceptability curve was also plotted, by calculating the probability that, compared with early nutritional support via the enteral route, early nutritional support via the parenteral route is cost-effective – given the data – at alternative levels of willingness to pay for a QALY gain.
The mean incremental net monetary benefit at 90 days with corresponding 95% CI was reported both overall and by each of the pre-specified subgroups (see Subgroup analyses of the primary outcome, below). As for the analysis of clinical effectiveness, the base-case analysis was repeated adjusting for adherence using a structural mean model with an instrumental variable of allocated treatment. 46
The cost-effectiveness analysis was repeated applying alternative assumptions in sensitivity analyses. The main assumptions made in the base-case scenario, and how each was relaxed in sensitivity analyses are detailed below and summarised in Table 3.
| Assumption | Base case | Sensitivity analysis |
|---|---|---|
| Costs of nutritional products | BNF price | Manufacturer’s list price |
| Dietitian’s time for daily monitoring and support | 20 minutes daily for both EN and PN | No additional dietitian’s time for daily monitoring and support of EN |
| Pharmacist’s time for daily monitoring and support | 30 minutes daily for PN | No additional pharmacist’s time for daily monitoring and support of PN |
| Additional staff time | Additional staff time included | No additional staff time to implement the interventions |
| Readmissions from Health Services Questionnaires | Included in the analysis | Excluded from the analysis |
| Baseline covariates | Unadjusted analysis | Adjusted for age, ICNARC model physiology score, surgical status and degree of malnutrition |
| Distributional assumptions | Costs and QALYs normally distributed | Costs and QALYs gamma distributed |
Costs of nutritional products
In the base case, unit costs for the nutritional products from the BNF were applied. In the sensitivity analysis, manufacturer’s list prices were applied.
Dietitian’s time during delivery of nutritional intervention
The intervention requires intensive monitoring and support of patients during the delivery of the nutritional support protocol (5 days/120 hours). In the base case, it was assumed that this monitoring and support would involve an additional 20 minutes of dietitian’s time per day for either type of nutritional support. In the sensitivity analysis, it was considered that daily monitoring and support of patients receiving EN by a dietitian would not be required.
Pharmacist’s time during delivery of nutritional intervention
In the base case, it was assumed that hospitals used custom-made PN bags and, therefore, pharmacist’s time for each day of nutritional support is required. In the sensitivity analysis, it was considered that daily monitoring and support of patients receiving PN by a pharmacist would not be required.
Additional time of dietitian, pharmacist and nurse
In the base case, it was assumed that any additional time of dietitian, pharmacist and nurse would be required to implement early nutritional support in critical care. In the sensitivity analysis, it was considered that the critical care unit has a dedicated nutritional team and, therefore, no additional support from an on-call nutritional team would be required.
Readmissions from Health Services Questionnaire
The base-case analysis included hospital readmissions, including a critical care stay recorded in the Case Mix Programme Database, but also hospital readmissions recorded from responses to the Health Services Questionnaire. To consider the possible impact of double-counting the same readmissions across both sources, in the sensitivity analysis only the readmissions from the Case Mix Programme Database were included.
Baseline covariates
The base-case analysis reported incremental costs and QALYs without any covariate adjustment, assuming that randomisation had ensured no imbalances in key prognostic factors such as age, ICNARC Physiology Score, ICNARC model predicted risk of death, surgical status and degree of malnutrition. In the sensitivity analysis, any chance imbalances in these factors were adjusted for using seemingly unrelated regression.
Distributional assumptions for costs and quality-adjusted life-years
The base-case analysis assumed that costs and QALYs were normally distributed when reporting the 95% CIs around incremental costs and QALYs. In sensitivity analyses the robustness of the cost-effectiveness results to alternative distributional assumptions about both outcomes were assessed. Following methodological guidance, the sensitivity analysis considered a gamma distribution for costs, as they had a right-skewed distribution. For QALYs, the sensitivity analysis also considered a gamma distribution because a large proportion of decedents had zero QALYs and the remainder of the distribution was again right skewed. In this sensitivity analysis, costs and QALYs were modelled as univariate regression models, assuming gamma distribution for each end point (i.e. ignoring possible correlation between the end points).
Cost-effectiveness at 1 year following randomisation (primary outcome)
Mean EQ-5D-5L utility scores, QALYs, total costs and incremental net monetary benefits up to 1 year were reported for each treatment group. Unadjusted mean differences between the treatment groups in quality of life, QALYs and incremental costs at 1 year were reported with 95% CIs. These were reported both overall and by each of the pre-specified subgroups.
Total costs up to 1 year were calculated by combining the resource use with unit costs. For survivors at 1 year, QALYs were calculated assuming an EQ-5D-5L utility score of zero at randomisation, and using the EQ-5D-5L utility scores at 90 days and 1 year, applying linear interpolation between each pair of time points. Zero QALYs were assumed for decedents at between randomisation and 90 days. For decedents at between 90 days and 1 year, a linear interpolation was applied between the EQ-5D-5L utility score at 90 days and the date of death, when an EQ-5D-5L utility score of zero was applied.
The incremental costs and QALYs at 1 year were plotted on the cost-effectiveness plane, and the cost-effectiveness acceptability curve was plotted. As for the cost-effectiveness analysis at 90 days, the mean INB at 1 year with corresponding 95% CI was reported overall, by each of the pre-specified subgroups, and adjusted for adherence.
For the 1-year cost-effectiveness analysis, sensitivity analyses considered the same scenarios as the 90-day cost-effectiveness analysis (see Table 3).
Lifetime incremental cost-effectiveness
The cost and outcome data reported at 1 year were used to estimate the effect of early nutritional support for critically ill adult patients via the parenteral route compared with the enteral route on longer-term costs and outcomes. A time horizon of 20 years was chosen to fully assess the relative impact alternative routes of early nutritional support. The maximum available survival data from the trial was used to plot Kaplan–Meier survival curves out to the date of censoring (17 March 2015). The patients who were observed to be alive at 1 year were extrapolated for years 2–20 by applying parametric extrapolations. The approach recommended by Latimer was used,56 considering alternative parametric survival curves and examining their relative fit to the observed data, but also the clinical plausibility of the ensuing extrapolations. In fitting these curves, survival data for the first 30 days were excluded, as the event rate during this early period was atypical and would not provide an appropriate basis for subsequent extrapolation. The relative fit of the alternative survival curves to the observed data was reported. In the base-case analysis, the survival curve that gave the most plausible extrapolation was applied according to previous literature and in comparison with age–gender-matched all-cause mortality. 56–58 Survival was then extrapolated according to the chosen parametric function for the duration of years that the parametric curve predicted excess mortality compared with the age–gender-matched general population, after which it was assumed that all-cause mortality rates were those of the age–gender-matched general population. The parametric extrapolation was combined with all-cause mortality rates to report life expectancy for each CALORIES trial patient who was observed to survive to 1 year.
To project lifetime costs attributable to the initial episode of critical illness, it was assumed that the average inpatient (general wards not critical care), outpatient and community service costs reported up to 1 year post-randomisation applied annually for the number of years (within the time horizon) over which the parametric survival curve predicted excess mortality compared with the age–gender-matched general population. After the period of excess mortality, it was assumed that there were no further costs attributable to the initial episode. Similarly, it was assumed that the quality-of-life decrement observed at 1 year compared with the age–gender-matched general population59 was maintained for the years of excess mortality, after which quality-of-life values for the age–gender-matched general population were applied. Lifetime QALYs were reported by combining life-years and health-related quality of life.
The lifetime INB was reported by valuing each QALY according to the NICE-recommended threshold of willingness to pay for a QALY gain (£20,000) and subtracting the incremental cost. All future costs and life-years were discounted at the recommended rate of 3.5%. 47 The mean lifetime INB with corresponding 95% CI was reported overall, by each of the pre-specified subgroups, and adjusted for adherence.
For the lifetime cost-effectiveness analysis, sensitivity analyses considered the scenarios from the 90-day and 1-year cost-effectiveness analyses (see Table 3) and also additional scenarios pertinent to the lifetime analysis. The main assumptions made in the base-case scenario, and how each was relaxed in the additional sensitivity analyses are detailed below and summarised in Table 4.
| Assumption | Base case | Sensitivity analysis |
|---|---|---|
| Parametric survival model | Selected model | Alternative model |
| Death rate years 2–20 | Parametric extrapolation applied over period of excess mortality | Age–gender-matched general population all-cause death rate applied for years 2–20 |
| Observed mortality data from the CALORIES trial applied to year 2, and age–gender-matched general population all-cause death rate applied for years 3–20 | ||
| Quality-of-life decrement | 16% decrement applied over years of excess mortality | 5% or 10% decrement |
Parametric survival model
In the base case, the parametric survival curve that gave the most plausible extrapolation was selected. In the sensitivity analysis, an alternative parametric survival model was applied.
Death rate for years 2–20
In the base case, the observed mortality data from the CALORIES trial were used for year 1 and the parametric extrapolation was applied for years 2–20 over the period of excess mortality. In a first sensitivity analysis, the age–gender-matched general population all-cause death rates were applied for years 2–20. In a second sensitivity analysis, the observed mortality data from the CALORIES trial were used for years 1 and 2, switching to age–gender-matched general population all-cause death rates for years 3–20.
Quality-of-life decrement
In the base case, a 16% decrement in quality of life was applied over the years of excess mortality. In the sensitivity analysis, this was reduced to a 5% or 10% decrement.
Chapter 3 Results: sites and patients
Participants: sites
Expressions of interests were received from 58 NHS adult, general critical care units in the UK. A total of 34 hospitals in England obtained local NHS permissions and opened to recruitment between 17 June 2011 and 14 October 2013. Twenty-one sites were opened within the first 3 months of the trial opening on 17 June 2011 (Figure 4).
FIGURE 4.
Sites open to recruitment during the trial recruitment period.
The median (IQR) time from local NHS permission to the trial opening at sites (i.e. start of screening) was 63 (23–88) days (Figure 5). Reasons for delays in opening were issues related to confirmation of NHS support costs from the CLRN and delays in local set-up of the trial, for example training staff.
FIGURE 5.
Time (in days) from local NHS permission to start of screening.
Overall, sites participated in the CALORIES trial for a median (IQR) of 31 (13–33) months. Of the 34 sites that opened, five were closed early because of poor recruitment (one unit did not recruit any patients) and one closed due to loss of eligibility to participate in the trial (no longer actively participating in the Case Mix Programme). There were 28 sites that remained open until the end of recruitment in March 2014 (Figure 6).
FIGURE 6.
Duration of participation of sites.
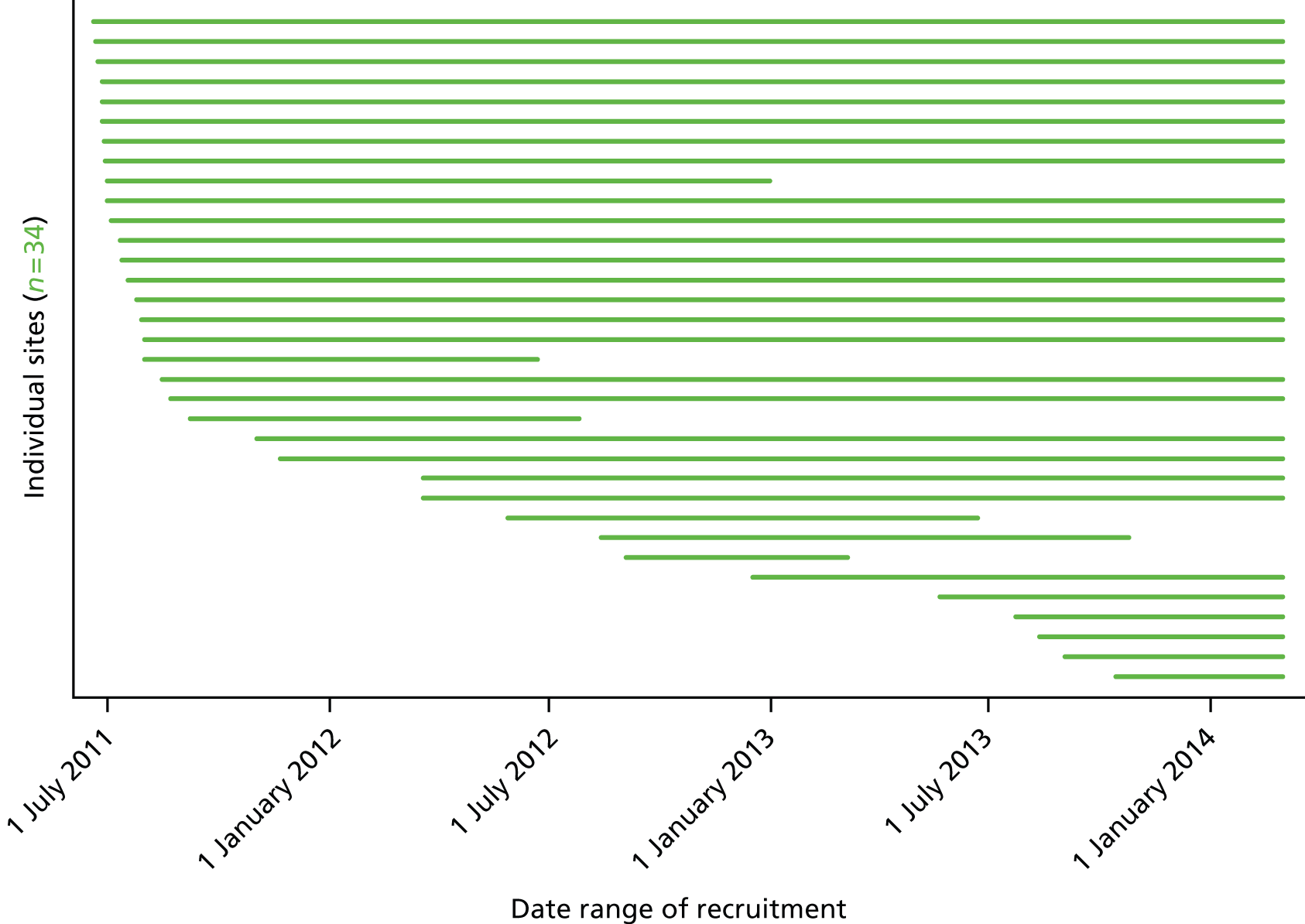
Characteristics of participating sites
The characteristics of the 33 adult general critical care units that participated in the CALORIES trial and recruited at least one patient compared with all adult general critical care units in the Case Mix Programme (n = 181) are presented in Table 5. Overall, a mix of university and non-university hospitals, geographically spread across England, took part in the trial. However, the proportions of units located in a university hospital and a hospital with a separate high-dependency unit were slightly higher in the CALORIES trial compared with the Case Mix Programme. There were also a higher proportion of large units (≥ 16 beds) and units with 1000 or more admissions per year that participated in the CALORIES trial compared with the Case Mix Programme. There was a higher proportion of units in the CALORIES trial, with > 60% of bed-days delivered at level 3 (intensive care), than in the Case Mix Programme (see Table 5).
| Critical care unit characteristic | Units in CALORIES (N = 33) | Units in Englanda (N = 181) |
|---|---|---|
| Region in England, n (%) | ||
| North | 5 (15.2) | 62 (34.3) |
| Midlands/East | 12 (36.4) | 47 (26.0) |
| London/South East | 9 (27.3) | 40 (22.1) |
| South West/South Central | 7 (21.2) | 32 (17.7) |
| Teaching status, n (%) | ||
| University teaching hospital | 12 (36.4) | 53 (29.3) |
| Non-university hospital | 21 (63.6) | 128 (70.7) |
| Size of critical care unit, n (%) | ||
| < 8 beds | 3 (9.1) | 39 (21.5) |
| 8–11 beds | 8 (24.2) | 62 (34.3) |
| 12–15 beds | 6 (18.2) | 33 (18.2) |
| ≥ 16 beds | 16 (48.5) | 47 (26.0) |
| Separate HDU in the same hospital, n (%) | ||
| No | 21 (63.6) | 133 (73.5) |
| Yes | 12 (36.4) | 48 (26.5) |
| Annual critical care unit admissions, n (%) | ||
| < 500 admissions | 5 (15.2) | 55 (30.4) |
| 500–749 admissions | 7 (21.2) | 60 (33.1) |
| 750–999 admissions | 8 (24.2) | 31 (17.1) |
| ≥ 1000 admissions | 13 (39.4) | 35 (19.3) |
| Percentage of bed-days delivered at level 3 (intensive care), n (%) | ||
| < 40% | 7 (21.2) | 35 (19.3) |
| 40–59.9% | 13 (39.4) | 100 (55.2) |
| ≥ 60% | 13 (39.4) | 46 (25.4) |
Participants: patients
In total, 11,108 patients were screened between 17 June 2011 and 2 March 2014. Of these, 6195 (55.8%) met one or more exclusion criteria. There were 2513 (22.6%) patients who, although eligible for inclusion in the trial, were not recruited. The most frequently reported reason for not recruiting eligible patients was that the treating clinician had refused to recruit the patient. Other reported reasons included: the patient (or their personal/professional consultee) declined to take part; the patient was unable to provide informed consent and a personal or professional consultee was unavailable or unable to provide agreement; or logistical issues, which were mainly due to no research staff being available, for example the patient was not screened for eligibility prior to commencement of early nutritional support (see Figure 7).
The required sample of 2400 (21.6%) patients was recruited between 22 June 2011 and 2 March 2014, with 1200 randomised to receive early nutritional support via the parenteral route and 1200 to receive early nutritional support via the enteral route (Figures 7 and 8). There was variation across the 33 sites in the rate of recruitment (Figure 9); the overall median (IQR) recruitment rate was 0.6 (0.4–0.8) patients per site per week, with the highest recruitment rate of 1.2 patients per week. Manual randomisation was required for two patients as a result of the telephone randomisation service being briefly unavailable.
FIGURE 7.
Screening, randomisation and follow-up. PEG, percutaneous endoscopic gastrostomy; PEJ, percutaneous endoscopic jejunostomy.

FIGURE 8.
Patient recruitment.

FIGURE 9.
Patient recruitment rate (patients per week).

Patients were generally recruited into the CALORIES trial during week days (Monday to Friday) and during usual office hours (Figures 10 and 11); most of the recruiting sites reported having insufficient resources to enable screening and recruitment at weekends and outside usual office hours.
FIGURE 10.
Randomisation by day of week.

FIGURE 11.
Randomisation by time of day.

For the majority of patients, agreement to participate in the trial was obtained from a personal consultee prior to randomisation (n = 2212, 92.2%). For the remaining patients, agreement was obtained from a professional consultee (6.1%) or informed consent was obtained from the patient prior to randomisation (1.7%) (Table 6). Twelve patients withdrew from the trial, requesting removal of all of their data from the analysis, resulting in data on 2388 patients for initial analysis (n = 1191 parenteral group; n = 1197 enteral group). Five patients were subsequently lost to follow-up before 30 days, resulting in data from 2383 patients for analysis of the primary clinical effectiveness outcome (n = 1188, 99.7% parenteral group; n = 1195, 99.8% enteral group) (see Figure 7). Four patients withdrew from follow-up (n = 3 parenteral group; n = 1 enteral group) and one was lost to follow-up (enteral group) before 90 days. A further two patients withdrew from follow-up (both parenteral group) and three were lost to follow-up (n = 2 parenteral group; n = 1 enteral group) before 1 year, resulting in final follow-up of 2372 patients for 1-year mortality (n = 1181, 99.2% parenteral group; n = 1192, 99.5% enteral group). Follow-up was completed on 23 March 2015.
| Type of consent/agreementa | All patients | Parenteral group | Enteral group | |||
|---|---|---|---|---|---|---|
| Patients, n (%) | Withdrawalsb | Patients, n (%) | Withdrawalsb | Patients, n (%) | Withdrawalsb | |
| Informed consent from patient prior to randomisation | 41 (1.7) | 0 | 20 (1.7) | 0 | 21 (1.8) | 0 |
| Agreement from a personal consultee | 2212 (92.2) | 10 | 1105 (92.1) | 8 | 1107 (92.3) | 2 |
| Agreement from a professional consultee | 147 (6.1) | 2 | 75 (6.3) | 1 | 72 (6.0) | 1 |
| Total | 2400 (100) | 12 | 1200 (100) | 9 | 1200 (100) | 3 |
Characteristics of patients at baseline
The groups were well matched at baseline (Table 7). The mean age of patients was similar in both groups (63.3 years parenteral group, 62.9 years enteral group) and more than half were male (57.9% parenteral group, 60.6% enteral group). The severity of illness of patients was similar in the two groups, with a median (IQR) ICNARC model predicted risk of death of 0.42 (0.22–0.65) in the parenteral group and 0.43 (0.23–0.650 in the enteral group, and mean (SD) SOFA scores of 9.5 (3.4) and 9.6 (3.3), respectively. The median (IQR) actual or estimated BMI was similar [26.2 kg/m2 (23.0–30.7 kg/m2) parenteral group and 26.8 kg/m2 (23.4–31.3 kg/m2) enteral group].
| Characteristic | Parenteral group (N = 1191) | Enteral group (N = 1197) |
|---|---|---|
| Age (years), mean (SD) | 63.3 (15.1) [1191] | 62.9 (15.4) [1197] |
| Age (years), median (IQR) | 66 (54–75) [1191] | 66 (54–74) [1197] |
| Age < 65 years,a n/N (%) | 530/1191 (44.5) | 540/1197 (45.1) |
| Male sex, n/N (%) | 689/1191 (57.9) | 725/1197 (60.6) |
| Severe co-existing illness, n/N (%): | ||
| Liver | 29/1181 (2.5) | 34/1193 (2.8) |
| Renal | 20/1181 (1.7) | 15/1193 (1.3) |
| Respiratory | 34/1181 (2.9) | 23/1193 (1.9) |
| Cardiovascular | 11/1181 (0.9) | 14/1193 (1.2) |
| Immunodeficiency | 78/1181 (6.6) | 95/1193 (8.0) |
| Surgery < 24 hours before ICU admission,a n/N (%) | 162/1191 (13.6) | 167/1197 (14.0) |
| APACHE II Acute Physiology score,b mean (SD) | 15.1 (6.2) [1191] | 15.2 (6.2) [1197] |
| APACHE II Score,c mean (SD) | 19.6 (6.9) [1191] | 19.6 (7.0) [1197] |
| APACHE II predicted risk of death,d median (IQR) | 0.34 (0.18–0.52) [1162] | 0.34 (0.18–0.52) [1173] |
| ICNARC Physiology Score,e mean (SD) | 25.6 (8.0) [1191] | 25.8 (7.8) [1197] |
| ICNARC model predicted risk of death,f median (IQR) | 0.42 (0.22–0.65) [1190] | 0.43 (0.23–0.65) [1197] |
| Mechanical ventilation, n/N (%) | 979/1178 (83.1) | 993/1185 (83.8) |
| SOFA score,g mean (SD) | 9.5 (3.4) [1191] | 9.6 (3.3) [1191] |
| Malnutrition status (based on clinical judgement),a n/N (%) | 151/1191 (12.7) | 152/1197 (12.7) |
| Actual or estimated BMIh (kg/m2), mean (SD) | 27.7 (7.4) [1177] | 28.2 (7.5) [1187] |
| Actual or estimated BMIh (kg/m2), median (IQR) | 26.2 (23.0–30.7) [1177] | 26.8 (23.4–31.3) [1187] |
| Ulna length (cm), mean (SD) | 26.4 (3.4) [1130] | 26.6 (3.7) [1121] |
| Ulna length (cm), median (IQR) | 26.0 (25.0–28.0) [1130] | 26.1 (25.0–28.0) [1121] |
| Mid-upper arm circumference (cm), mean (SD) | 30.5 (5.3) [1127] | 30.9 (5.4) [1123] |
| Mid-upper arm circumference (cm), median (IQR) | 30.0 (27.0–34.0) [1127] | 31.0 (27.0–34.0) [1123] |
| Degree of malnutrition, n/N (%): | ||
| High | 74/1152 (6.4) | 81/1161 (7.0) |
| Moderate | 8/1152 (0.7) | 10/1161 (0.9) |
| None | 1070/1152 (92.9) | 1070/1161 (92.2) |
Multiple imputation
Table 8 reports all the variables considered for multiple imputation, and for each variable, the number of missing values, and the imputation model chosen.
| Variable | Missing values,a n (%) | Imputation model |
|---|---|---|
| Baseline variables | ||
| Treatment group | 0 (0) | None required |
| Age | 0 (0) | None required |
| Sex | 0 (0) | None required |
| Surgery within 24 hours prior to admission to ICU | 0 (0) | None required |
| Presence of cancer | 0 (0) | None required |
| ICNARC Physiology Score | 0 (0) | None required |
| Height | 23 (1.0) | Predictive mean matching |
| Weight | 4 (0.2) | Predictive mean matching |
| Length of forearm (ulna) | 137 (5.7) | Predictive mean matching |
| BMI | 24 (1.0) | Predictive mean matching |
| APACHE II predicted risk of death | 53 (2.2) | Predictive mean matching |
| ICNARC model predicted risk of death | 52 (2.2) | Predictive mean matching |
| Mechanical ventilation | 25 (1.0) | Logistic regression |
| Degree of malnutrition | 75 (3.1) | Logistic regression |
| Extent of fluid retention | 44 (1.8) | Ordered logistic regression |
| Resource-use variables | ||
| Feeding days | 15 (0.6) | Predictive mean matching |
| Days in critical care: index admission | 0 (0) | None required |
| Days in critical care: readmission | 9 (0.4) | Predictive mean matching |
| Days in general ward: index admission | 0 (0) | None required |
| Days in general ward: readmission | 9 (0.4) | Predictive mean matching |
| Outpatient, primary and social care visits up to 90 days | 616 (41.9) | Predictive mean matching |
| Outpatient, primary and social care visits, 90 days to 1 year | 414 (31.0) | Predictive mean matching |
| Mortality and quality-of-life variables | ||
| Mortality at 90 days | 10 (0.4) | Logistic regression |
| EQ-5D-5L at 90 days | 368 (25.0) | Predictive mean matching |
| Mortality at 1 year | 15 (0.6) | Logistic regression |
| EQ-5D-5L at 1 year | 394 (29.5) | Predictive mean matching |
Chapter 4 Results: clinical effectiveness
Adherence to the protocol
Overall, adherence to delivery of nutritional support during the intervention period was high (Table 9). A similar number of patients in each treatment group received nutritional support throughout the intervention period. Any non-adherence to the protocol was reported for 150 (12.6%) patients in the parenteral group and 127 (10.6%) patients in the enteral group (Table 10).
| Days from initiation of nutritional supporta | Number receiving interventionb | Nutritional support received, n (%) | End of intervention, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Allocated route | Opposite route | Both | Neither | Death | Discharge | Oral feeding | 120 hours | ||
| Parenteral group | |||||||||
| Day 1 | 1167 | 1155 (99.0) | 11 (0.9) | 1 (0.1) | 0 (0.0) | 8 (0.7) | 1 (0.1) | 1 (0.1) | 0 (0.0) |
| Day 2 | 1157 | 1134 (98.0) | 11 (1.0) | 6 (0.5) | 6 (0.5) | 39 (3.4) | 21 (1.8) | 23 (2.0) | 0 (0.0) |
| Day 3 | 1074 | 1033 (96.2) | 15 (1.4) | 12 (1.1) | 14 (1.3) | 28 (2.6) | 35 (3.3) | 52 (4.8) | 0 (0.0) |
| Day 4 | 959 | 898 (93.6) | 22 (2.3) | 16 (1.7) | 23 (2.4) | 36 (3.8) | 34 (3.5) | 43 (4.5) | 0 (0.0) |
| Day 5 | 846 | 793 (93.7) | 22 (2.6) | 19 (2.2) | 12 (1.4) | 22 (2.6) | 30 (3.5) | 33 (3.9) | 0 (0.0) |
| Day 6 | 761 | 619 (81.3) | 39 (5.1) | 77 (10.1) | 26 (3.4) | 11 (1.4) | 11 (1.4) | 39 (5.1) | 700 (92.0) |
| Enteral group | |||||||||
| Day 1 | 1171 | 1167 (99.7) | 4 (0.3) | 0 (0.0) | 0 (0.0) | 16 (1.4) | 4 (0.3) | 5 (0.4) | 0 (0.0) |
| Day 2 | 1146 | 1120 (97.7) | 3 (0.3) | 2 (0.2) | 21 (1.8) | 44 (3.8) | 13 (1.1) | 42 (3.7) | 0 (0.0) |
| Day 3 | 1047 | 987 (94.3) | 7 (0.7) | 3 (0.3) | 50 (4.8) | 46 (4.4) | 29 (2.8) | 69 (6.6) | 0 (0.0) |
| Day 4 | 903 | 840 (93.0) | 7 (0.8) | 7 (0.8) | 49 (5.4) | 24 (2.7) | 24 (2.7) | 60 (6.6) | 0 (0.0) |
| Day 5 | 795 | 736 (92.6) | 9 (1.1) | 7 (0.9) | 43 (5.4) | 38 (4.8) | 31 (3.9) | 35 (4.4) | 0 (0.0) |
| Day 6 | 691 | 627 (90.7) | 12 (1.7) | 6 (0.9) | 46 (6.7) | 10 (1.4) | 6 (0.9) | 26 (3.8) | 649 (93.9) |
| Variable | Parenteral group (N = 1191) | Enteral group (N = 1197) |
|---|---|---|
| Any non-adherence to delivery of nutritional support during intervention period,a n/N (%) | 150/1191 (12.6) | 127/1197 (10.6) |
| Non-adherence, n/N (%) | ||
| Did not receive nutritional support | 24/1191 (2.0) | 26/1197 (2.2) |
| Received first nutritional support via the opposite route to assigned | 12/1191 (1.0) | 4/1197 (0.3) |
| Initiation of nutritional support > 36 hours after admission to critical care | 37/1191 (3.1) | 41/1197 (3.4) |
| Received early nutritional support via assigned route and subsequently changed to opposite route during first 120 hours | 81/1191 (6.8) | 18/1197 (1.5) |
| Received no nutritional support for at least a full one day period during the first 120 hours | 4/1191 (0.3) | 45/1197 (3.8) |
Ninety-seven per cent of patients received nutritional support via the assigned route, with 24 patients assigned to the parenteral route and 26 patients assigned to the enteral route receiving no nutritional support (Table 11). The primary reasons in both groups were due to withdrawal of treatment or death [11 (45.8%) in the parenteral group compared with 11 (42.3%) in the enteral group] and unexpected early extubation [six (25.0%) in the parenteral group vs. seven (26.9%) in the enteral group]. Twelve patients assigned to the parenteral route and four patients assigned to the enteral route received their first nutritional support via the opposite route to that assigned (Table 12). Initiation of nutritional support was delayed beyond 36 hours from original critical care unit admission for 37 patients assigned to the parenteral route and 41 patients assigned to the enteral route (Table 13). In the parenteral group, this was primarily due to delays in prescribing or obtaining PN (n = 12, 32.4%) and problems (i.e. insertion difficulties or delays) with the central line (n = 8, 21.6%). In the enteral group, over half of the delays were due to problems (i.e. insertion difficulties or delays) with the nasogastric tube (n = 24, 58.5%).
| Reason for non-adherence, n (%) | Parenteral group (N = 24) | Enteral group (N = 26) |
|---|---|---|
| Clinical decision | 1 (4.2) | 0 |
| New contraindication | 1 (4.2) | 1 (3.8) |
| Difficulty siting nasogastric tube | 0 | 3 (11.5) |
| Treatment withdrawn/death | 11 (45.8) | 11 (42.3) |
| Unexpected early extubation | 6 (25.0) | 7 (26.9) |
| Unanticipated inter-hospital transfer | 3 (12.5) | 2 (7.7) |
| Patient declined intervention | 1 (4.2) | 0 |
| No reason given | 1 (4.2) | 2 (7.7) |
| Reason for non-adherence, n (%) | Parenteral group (N = 12) | Enteral group (N = 4) |
|---|---|---|
| Clinical decision | 2 (16.7) | 1 (25.0) |
| New contraindication | 1 (8.3) | 0 |
| Did not want to insert new central line | 4 (33.3) | 0 |
| Difficulty siting nasogastric tube | 0 | 2 (50.0) |
| Delay prescribing/obtaining PN | 2 (16.7) | 0 |
| Staff not available | 1 (8.3) | 0 |
| Error by clinical team | 2 (16.7) | 1 (25.0) |
| Reason for non-adherence, n (%) | Parenteral group (N = 37) | Enteral group (N = 41) |
|---|---|---|
| Clinical decision | 1 (2.7) | 5 (12.2) |
| Vomiting/aspirates | 0 | 2 (4.9) |
| Agitation | 0 | 1 (2.4) |
| Difficulty inserting central line | 4 (10.8) | 0 |
| Delay inserting/confirming position of central line | 3 (8.1) | 0 |
| Problem with central line | 1 (2.7) | 0 |
| Did not want to insert new central line | 1 (2.7) | 0 |
| Difficulty siting nasogastric tube | 0 | 11 (26.8) |
| Delay inserting/confirming position of nasogastric tube | 0 | 8 (19.5) |
| Problem with nasogastric tube | 0 | 5 (12.2) |
| Delay prescribing/obtaining PN | 12 (32.4) | 0 |
| Problem with PN bag | 1 (2.7) | 0 |
| Equipment not available | 1 (2.7) | 0 |
| Delayed for surgery, scan or other intervention | 7 (18.9) | 7 (17.1) |
| Error by clinical team | 5 (13.5) | 1 (2.4) |
| No reason given | 1 (2.7) | 1 (2.4) |
Of patients who initially received nutrition via the assigned route, 81 assigned to the parenteral route and 18 assigned to the enteral route subsequently received nutritional support via the alternative route (i.e. ‘crossed over’) during the 120-hour intervention period (Table 14). However, the greatest proportion of these were towards the end of the 120-hour period, with 27 of the crossovers among those assigned to the parenteral route occurring in the final 6 hours of the 120-hour intervention period and 41 in the final 24 hours (Figure 12; see also Table 14). Reasons for these late crossovers were primarily due to an error by the clinical team regarding the precise timing of the end of the 120-hour period (n = 24 in final 24 hours).
FIGURE 12.
Timing of non-adherence according to treatment group. (a) The timing of crossovers in hours from initiation of nutritional support; and (b) days (from initiation of nutritional support) on which patients received no nutritional support for the full day. Seven patients received no nutritional support on more than 1 day.

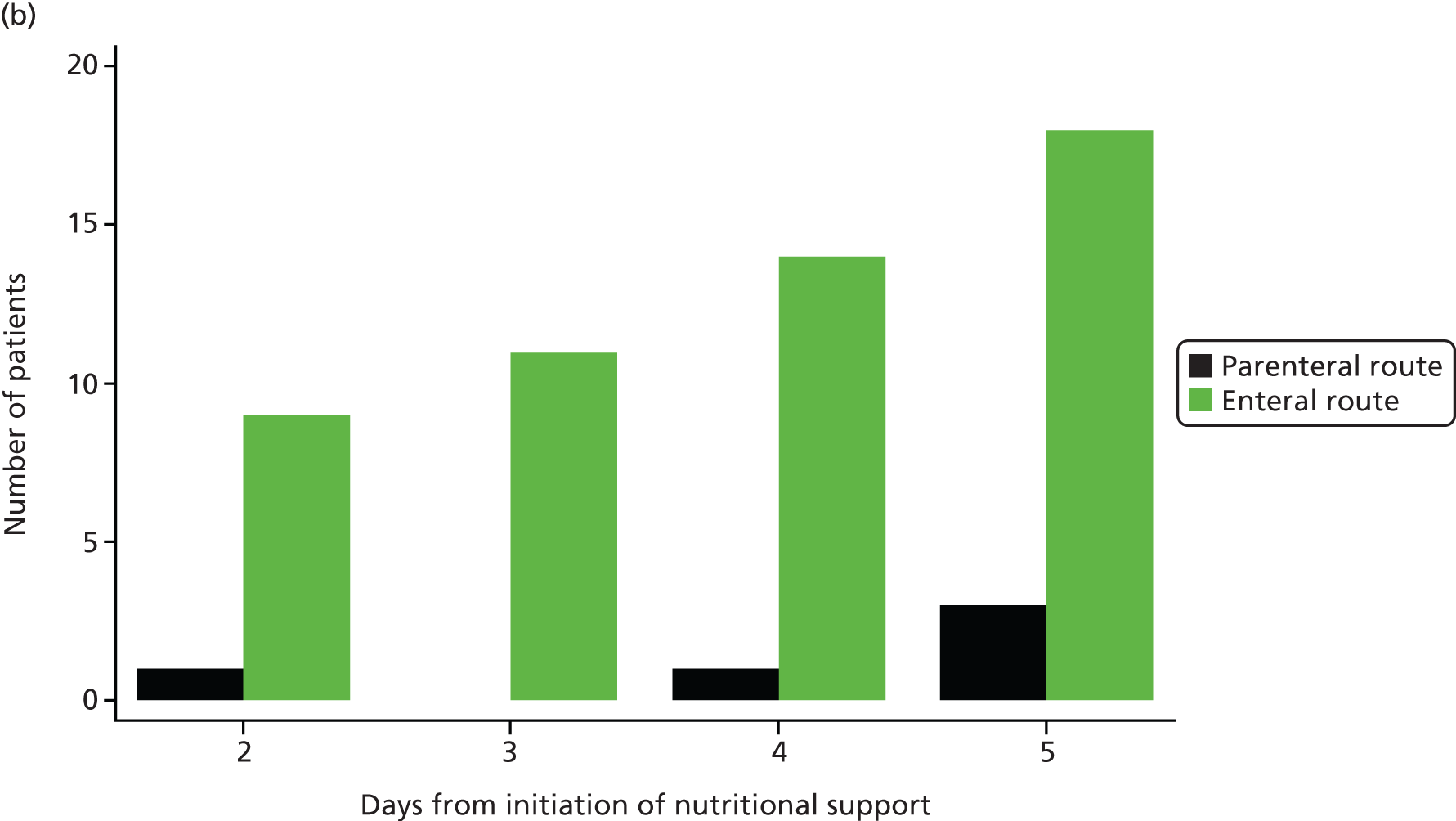
| Reason for non-adherence, n (%) | Parenteral group | Enteral group (N = 18) | ||
|---|---|---|---|---|
| 0–95 hours (N = 40) | 96–113 hours (N = 14) | 114–119 hours (N = 27) | ||
| Clinical decision | 8 (20.0) | 3 (21.4) | 0 | 6 (33.3) |
| New contraindication | 1 (2.5) | 0 | 0 | 4 (22.2) |
| Vomiting/aspirates | 0 | 0 | 0 | 3 (16.7) |
| Other complication | 4 (10.0) | 0 | 1 (3.4) | 1 (5.6) |
| Problem with central line | 10 (25.0) | 2 (14.3) | 1 (3.4) | 0 |
| Problem with nasogastric tube | 0 | 0 | 0 | 2 (11.1) |
| To facilitate discharge | 6 (15.0) | 1 (7.1) | 0 | 0 |
| EN started while weaning from PN | 0 | 0 | 2 (6.9) | 0 |
| Did not want to start new bag | 0 | 1 (7.1) | 6 (22.2) | 0 |
| Error by clinical team | 11 (27.5) | 7 (50.0) | 17a (63.0) | 2 (11.1) |
Patients assigned to the enteral route were more likely to have full days with no nutritional support; this was more likely to occur in the last 48 hours of the intervention period (see Figure 12; Table 15). Among those receiving nutritional support, the mean number of full days with no nutritional support was 0.004 for the parenteral group and 0.044 for the enteral group. Reasons for no nutritional support for a full day in the enteral group were primarily due to the patient vomiting or high volumes of aspirates via the nasogastric tube (n = 12). Nutritional support was stopped temporarily in eight patients for surgery, and a scan or another intervention and was stopped permanently in eight patients because treatment was withdrawn and/or the patient was receiving palliative care.
| Reason for non-adherence, n (%) | Parenteral group (N = 4) | Enteral group (N = 45) |
|---|---|---|
| Clinical decision | 0 | 1 (2.2) |
| New contraindication | 0 | 1 (2.2) |
| Vomiting/aspirates | 1a (25.0) | 12 (26.7) |
| Other complication | 0 | 3 (6.7) |
| Problem with nasogastric tube | 0 | 6 (13.3) |
| Treatment withdrawn/palliative care | 2 (50.0) | 8 (17.8) |
| Stopped for surgery, scan or other intervention | 0 | 8 (17.8) |
| No reason given | 1 (25.0) | 6 (13.3) |
Delivery of care by treatment group
In both treatment groups, the median time to initiation of nutritional support was within 24 hours of critical care unit admission [median (IQR) 23.5 (16.8–30.0) hours in the parenteral group and 21.8 (15.5–27.8) hours in the enteral group] (Table 16).
| Variable | Parenteral group (N = 1191) | Enteral group (N = 1197) |
|---|---|---|
| Time from ICU admission to initiation of early nutritional support (hours), median (IQR) | 23.5 (16.8–30.0) [1167] | 21.8 (15.5–27.8) [1171] |
| Calories received during intervention period (kcal/kg),a mean (SD) | ||
| Total | 88.7 (44.0) [1155] | 74.2 (44.2) [1175] |
| From parenteral feed | 76.2 (40.9) [1186] | 0.7 (7.1) [1196] |
| From enteral feed | 2.0 (12.1) [1188] | 63.5 (41.1) [1196] |
| From i.v. glucose | 1.6 (3.6) [1180] | 2.1 (4.8) [1189] |
| From propofol | 7.6 (8.0) [1180] | 7.1 (8.1) [1189] |
| From oral feed | 0.7 (3.7) [1165] | 0.5 (2.4) [1182] |
| Protein received during intervention period (g/kg), mean (SD) | ||
| Total | 2.9 (1.6) [1186] | 2.7 (1.8) [1196] |
| From parenteral feed | 2.8 (1.6) [1186] | 0.0 (0.3) [1196] |
| From enteral feed | 0.1 (0.5) [1188] | 2.6 (1.8) [1196] |
| Gastric residual volume (ml),b mean (SD) | ||
| Total aspirated during intervention period | 35.1 (264.7) [1191] | 958.3 (1312.1) [1197] |
| Total replaced during intervention period | 24.2 (170.1) [1191] | 617.8 (863.0) [1197] |
| Patients receiving prokinetic drugs during intervention period,b n/N (%) | 26/1191 (2.2) | 426/1197 (35.6) |
| Patients receiving vasoactive agents during intervention period, n/N (%) | 958/1184 (80.9) | 1007/1191 (84.6) |
| Patients receiving additives during intervention period,c n/N (%) | ||
| Glutamine | 50/1191 (4.2) | 0/1197 (0.0) |
| Selenium | 268/1191 (22.5) | 3/1197 (0.3) |
| Fish oils | 38/1191 (3.2) | 1/1197 (0.1) |
| Patients receiving insulin during intervention period, n/N (%) | 694/1184 (58.6) | 668/1191 (56.1) |
| Total insulin received during intervention period (IU), mean (SD) | 154 (223) [1184] | 122 (204) [1191] |
| Blood glucose during intervention period (mmol/l), mean (SD) | ||
| Daily lowest | 7.0 (1.4) [1181] | 6.5 (1.5) [1186] |
| Daily highest | 10.2 (2.4) [1176] | 10.0 (2.5) [1181] |
| Incidence of diarrhoea during intervention period,d n/N (%) | 192/1184 (16.2) | 250/1191 (21.0) |
| Incidence of constipation during intervention period,e n/N (%) | 726/1184 (61.3) | 643/1191 (54.0) |
| Time from randomisation to initiation of exclusive oral feeding (days), median (IQR) | 14 (5–36) [1189] | 13 (5–32) [1197] |
The mean total calories received during the 120-hour intervention period was higher in the parenteral group than the enteral group [mean (SD) 88.7 (44.0) kcal/kg in the parenteral group vs. 74.2 (44.2) kcal/kg in the enteral group] (see Table 16). On average, daily caloric intake was higher in patients assigned to the parenteral route [mean (SD) 21.3 (7.7) kcal/kg/day] than in those assigned to the enteral route [mean (SD) 18.5 (7.7) kcal/kg/day] (Table 17; Figure 13). However, in the majority of patients, the targeted delivery of 25 kcal/kg/day was not achieved irrespective of route of delivery (Figure 14).
| Variable | Parenteral group (N = 1191) | Enteral group (N = 1197) |
|---|---|---|
| Calories received per 24 hours during intervention period (kcal/kg), mean (SD) | ||
| Total | 21.3 (7.7) [1155] | 18.5 (7.7) [1175] |
| From parenteral feed | 18.2 (7.3) [1186] | 0.1 (1.4) [1196] |
| From enteral feed | 0.4 (2.5) [1188] | 15.5 (7.3) [1196] |
| From i.v. glucose | 0.5 (1.1) [1180] | 0.7 (1.5) [1189] |
| From propofol | 1.9 (2.1) [1180] | 1.9 (2.1) [1189] |
| From oral feed | 0.2 (0.9) [1165] | 0.2 (0.9) [1182] |
| Protein received per 24 hours during intervention period (g/kg), mean (SD) | ||
| Total | 0.7 (0.3) [1186] | 0.6 (0.3) [1196] |
| From parenteral feed | 0.7 (0.3) [1186] | 0.0 (0.1) [1196] |
| From enteral feed | 0.0 (0.1) [1188] | 0.6 (0.3) [1196] |
| Aspirates per 24 hours during intervention perioda (ml), mean (SD) | 7.6 (56.1) [1191] | 253.1 (321.4) [1197] |
| Aspirates put back per 24 hours during intervention perioda (ml), mean (SD) | 5.3 (37.0) [1191] | 158.3 (200.8) [1197] |
FIGURE 13.
Daily calories according to treatment group, showing the total calories received per kilogram of actual (or estimated) body weight for each day from days 1–6. Day 1 data are the values from the time of initiation of nutritional support to the end of the day of initiation of nutritional support. The horizontal lines within the boxes indicate medians, the lower and upper ends of the boxes indicate the 25th and 75th percentiles, respectively, and the lower and upper whiskers indicate the 1st and 99th percentiles, respectively.
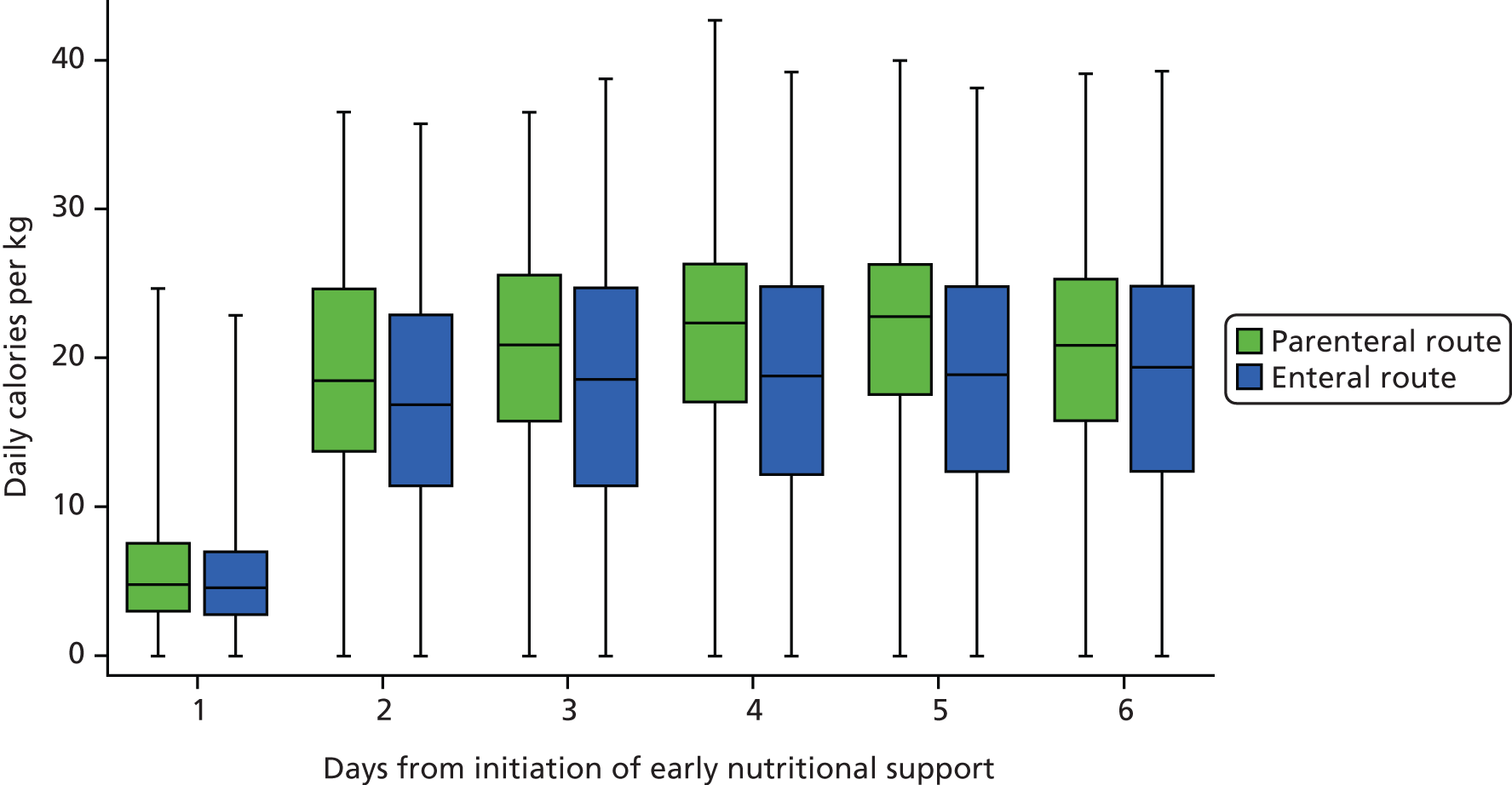
FIGURE 14.
Percentage of patients meeting daily energy target according to treatment group, showing the percentage of patients who met the daily energy target of 25 kcal/kg/day for each day from days 1–6. Day 1 data are the values from the time of initiation of nutritional support to the end of the day of initiation of nutritional support. The faded bars show the percentage of patients who met the target having adjusted for part-days of feeding (e.g. following the death of a patient).
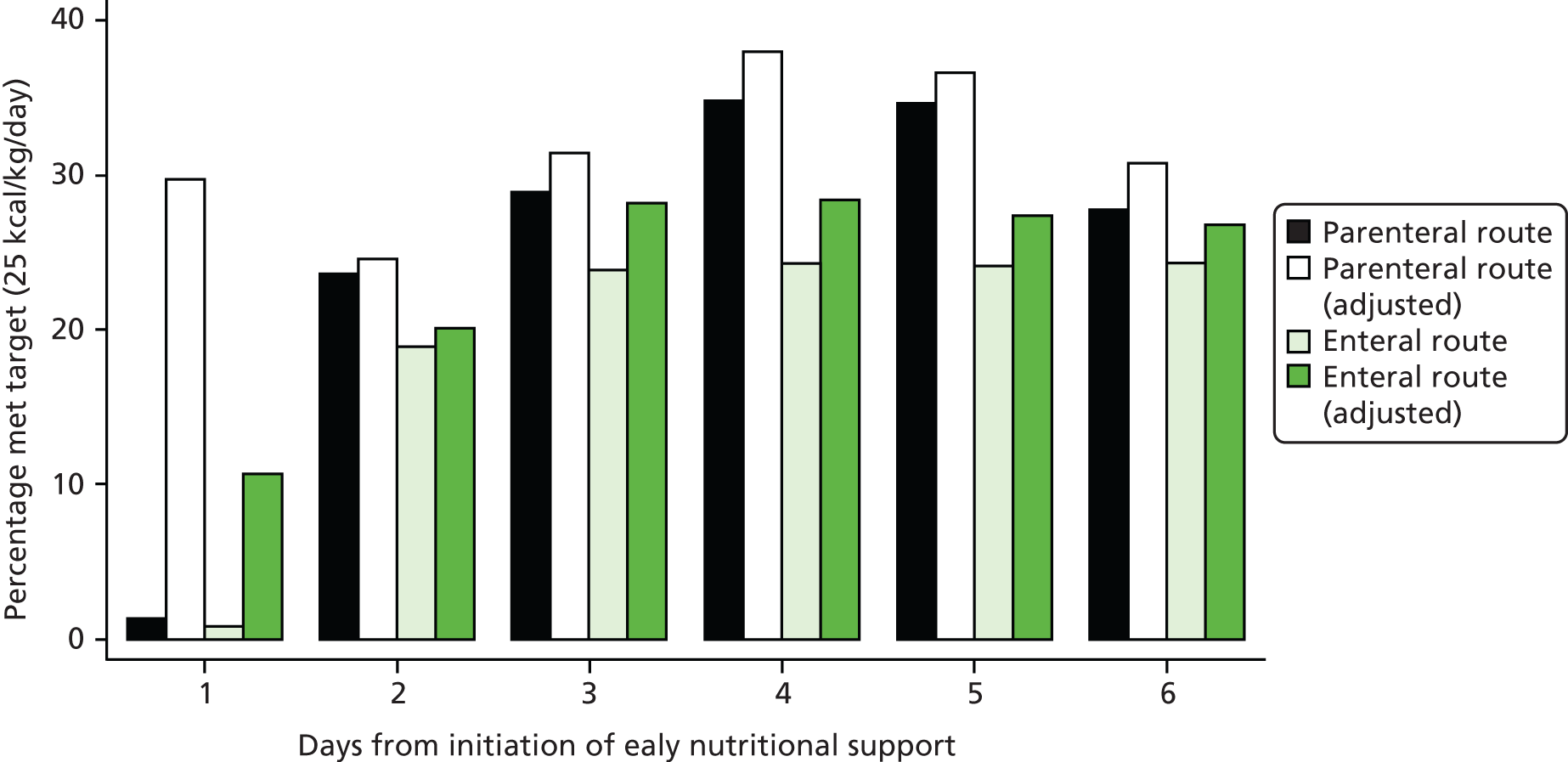
The mean total protein received during the 120-hour intervention period was similar in the two groups [mean (SD) 2.9 (1.6) g/kg in the parenteral group vs. 2.7 (1.8) g/kg in the enteral group] (see Table 16). The average daily protein received per day was also similar for the two groups [mean (SD) 0.7 (0.3) g/kg/day in the parenteral group vs. 0.6 (0.3) g/kg/day in the enteral group] (see Table 17; Figure 15).
FIGURE 15.
Daily protein according to treatment group. Shows the total protein received for each day from days 1–6. Day 1 data are the values from the time of initiation of nutritional support to the end of the day of initiation of nutritional support. The horizontal lines within the boxes indicate medians, the lower and upper ends of the boxes indicate the 25th and 75th percentiles, respectively, and the lower and upper whiskers indicate the 1st and 99th percentiles, respectively.
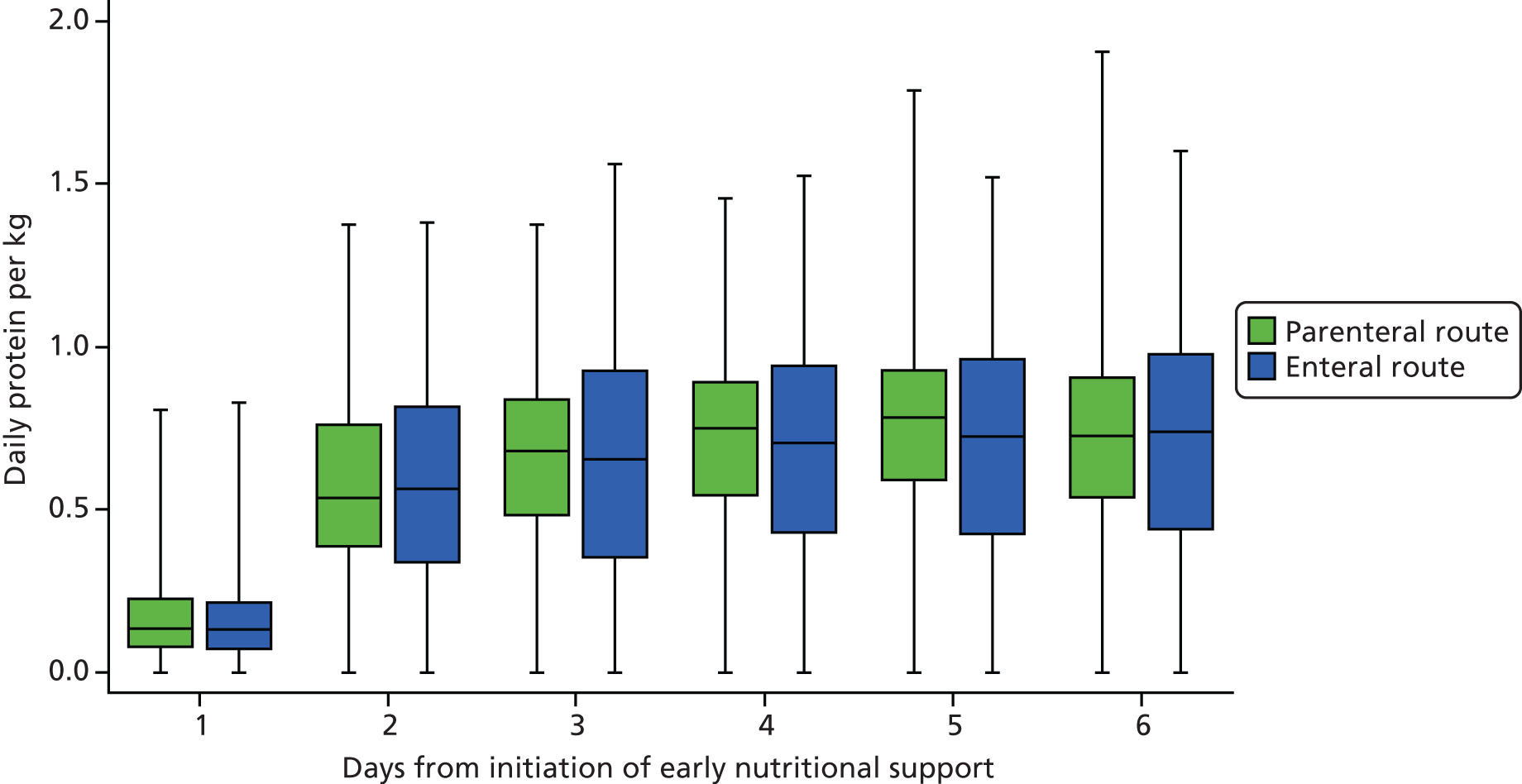
For patients assigned to the enteral route, the mean total volume of gastric aspirates during the intervention period was 958 ml (253 ml/day) and one-third received prokinetics (see Tables 16 and 17). These were recorded only for patients receiving EN.
For patients assigned to the parenteral route, the most frequent additive to PN during the intervention period was selenium (22.5%, compared with 4.2% for glutamine and 3.2% for fish oils). These were recorded only for patients receiving PN.
In both groups, a high proportion of patients received vasoactive agents, although this was slightly higher in the enteral group (84.6%) than in the parenteral group (80.9%). In both groups, more than half of the patients received insulin during the intervention period (58.6% in the parenteral group and 56.1% in the enteral group) (see Table 16). However, patients in the parenteral group received a higher total amount of insulin [mean (SD) 153.5 (223.0) IU] than patients in the enteral group [mean (SD) 122.3 (204.1) IU].
Median daily SOFA scores were similar in both groups, and decreased during the intervention period (Figure 16).
FIGURE 16.
Daily SOFA score according to treatment group. Shows the SOFA scores for the 24 hours prior to randomisation (baseline) and from days 1–6. Day 1 data are the values from the time of initiation of nutritional support to the end of the day of initiation of nutritional support. The horizontal lines within the boxes indicate medians, the lower and upper ends of the boxes indicate the 25th and 75th percentiles, respectively, and the lower and upper whiskers indicate the 1st and 99th percentiles, respectively. SOFA scores range from 0 to 24, with higher scores indicating a greater degree of organ failure.

Patients in the enteral group had a higher incidence of diarrhoea (21% vs. 16% in the parenteral group), whereas patients in the parenteral group had a higher incidence of constipation (61% vs. 54% in the enteral group) (see Table 16).
Median time from randomisation to exclusive oral feeding was about 2 weeks in both treatment groups [median (IQR) 14 (5–36) days in the parenteral group and 13 (5–32) days in the enteral group] (see Table 16).
Table 18 details the nutritional support delivered to patients, by treatment group, in the critical care unit after the 120-hour intervention period, that is, from 120 hours following initiation of nutritional support to critical care unit discharge or death. In both groups the most frequently used route for delivery of nutritional support was the enteral route either exclusively (30% for the parenteral group and 43% for the enteral group) or with oral feeding (22% and 35%, respectively) (see Table 18). However, those assigned to the parenteral route were more likely to continue to receive PN, either exclusively or with other routes, beyond 120 hours (37% compared with 7% for the enteral group).
| Nutritional support received between 120 hours and death or discharge,a n (%) | Parenteral group (N = 700) | Enteral group (N = 649) |
|---|---|---|
| No feed recorded | 38b (5.4) | 49c (7.6) |
| Enteral (exclusive) | 210 (30.0) | 276 (42.5) |
| Parenteral (exclusive) | 13 (1.9) | 2 (0.3) |
| Oral feeding (exclusive) | 40 (5.7) | 48 (7.4) |
| Enteral and parenteral feeding | 137 (19.6) | 25 (3.9) |
| Enteral and oral feeding | 154 (22.0) | 230 (35.4) |
| Parenteral and oral feeding | 26 (3.7) | 7 (1.1) |
| Enteral, parenteral and oral feeding | 82 (11.7) | 12 (1.9) |
Primary outcome: clinical effectiveness
At 30 days following randomisation, 393 (33.1%) patients assigned to receive nutritional support via the parenteral route had died compared with 409 (34.2%) patients assigned to receive nutritional support via the enteral route, corresponding to an absolute risk reduction of 1.15 percentage points (95% CI −2.65 to 4.94; p = 0.57) and a relative risk of 0.97 (95% CI 0.86 to 1.08). This difference remained non-significant after adjustment for baseline characteristics (odds ratio 0.95, 95% CI 0.79 to 1.13; p = 0.55) (Table 19).
| Outcome | Parenteral group (N = 1191) | Enteral group (N = 1197) | Effect estimate (95% CI) | p-value |
|---|---|---|---|---|
| All-cause mortality at 30 days, n/N (%) | 393/1188 (33.1) | 409/1195 (34.2) | 0.97 (0.86 to 1.08)a | 0.57e |
| 1.15 (−2.65 to 4.94)b | ||||
| 0.95 (0.80 to 1.13)c | ||||
| 0.95 (0.79 to 1.13)d | 0.55 |
Secondary outcomes: clinical effectiveness
The proportions of patients in the parenteral group who experienced episodes of hypoglycaemia (p = 0.006) and of vomiting (p < 0.001) were significantly lower than for patients in the enteral group. There were no significant differences between the groups in any of the other secondary outcomes, including duration of survival (log-rank test p = 0.056; adjusted Cox proportional hazards regression hazard ratio 0.90, 95% CI 0.80 to 1.00; p = 0.057) (Table 20; Figures 17 and 18).
| Outcome | Parenteral group (N = 1191) | Enteral group (N = 1197) | Effect estimate (95% CI) | p-value |
|---|---|---|---|---|
| Days alive and free from advanced respiratory support up to 30 days, mean (SD) | 14.3 (12.1) [1186] | 14.3 (12.2) [1195] | 0.04 (−0.94 to 1.01)a | 0.94 |
| Days alive and free from advanced cardiovascular support up to 30 days, mean (SD) | 18.9 (13.5) [1185] | 18.5 (13.6) [1195] | 0.41 (−0.63 to 1.53)a | 0.44 |
| Days alive and free from renal support up to 30 days, mean (SD) | 19.1 (13.9) [1186] | 18.8 (14.0) [1195] | 0.26 (−0.85 to 1.47)a | 0.66 |
| Days alive and free from neurological support up to 30 days, mean (SD) | 19.2 (13.8) [1186] | 18.9 (14.0) [1195] | 0.34 (−0.81 to 1.36)a | 0.57 |
| Days alive and free from gastrointestinal support up to 30 days, mean (SD) | 13.0 (11.7) [1186] | 13.2 (11.8) [1195] | −0.12 (−1.05 to 0.80)a | 0.81 |
| Number of treated infectious complications per patient, mean (SD) | 0.22 (0.60) [1191] | 0.21 (0.56) [1197] | 0.01 (−0.04 to 0.06)a | 0.72 |
| Treated infectious complications, n/N (%) | ||||
| Chest infection (pneumonia/lower respiratory tract infection) | 135/1191 (11.3) | 143/1197 (11.9) | 0.61 (−1.96 to 3.18)b | 0.66c |
| Central venous catheter infection | 11/1191 (0.9) | 9/1197 (0.8) | −0.17 (−0.90 to 0.56)b | 0.66c |
| Other vascular catheter infection | 4/1191 (0.3) | 3/1197 (0.3) | −0.09 (−0.52 to 0.35)b | 0.73c |
| Bloodstream infection | 27/1191 (2.3) | 21/1197 (1.8) | −0.51 (−1.64 to 0.61)b | 0.39c |
| Infective colitis | 8/1191 (0.7) | 4/1197 (0.3) | −0.34 (−0.91 to 0.23)b | 0.26c |
| Urinary tract infection | 16/1191 (1.3) | 15/1197 (1.3) | −0.09 (−1.00 to 0.82)b | 0.86c |
| Surgical site infection | 10/1191 (0.8) | 12/1197 (1.0) | 0.16 (−0.60 to 0.93)b | 0.83c |
| Other infectious complications | 18/1191 (1.5) | 24/1197 (2.0) | 0.49 (−0.56 to 1.55)b | 0.44c |
| Non-infectious complications, n/N (%) | ||||
| Episodes of hypoglycaemiad | 44/1191 (3.7)d | 74/1197 (6.2)e | 2.49 (0.75 to 4.22)b | 0.006c |
| Elevated liver enzymes | 212/1191 (17.8) | 179/1197 (15.0) | −2.85 (−5.81 to 0.12)b | 0.07c |
| Nausea requiring treatment | 44/1191 (3.7) | 53/1197 (4.4) | 0.73 (−0.85 to 2.32)b | 0.41c |
| Abdominal distension | 78/1191 (6.5) | 99/1197 (8.3) | 1.72 (−0.38 to 3.82)b | 0.12c |
| Vomiting | 100/1191 (8.4) | 194/1197 (16.2) | 7.81 (5.20 to 10.43)b | < 0.001c |
| New or substantially worsened pressure ulcers | 181/1190 (15.2) | 179/1195 (15.0) | −0.23 (−3.10 to 2.64)b | 0.91c |
| Length of stay in the ICU (days), median (IQR) | ||||
| All | 8.1 (4.0–15.8) [1190] | 7.3 (3.9–14.3) [1197] | – | 0.15 |
| Survivors | 8.8 (4.8–17.2) [872] | 8.2 (4.9–16.0) [845] | – | 0.62 |
| Non-survivors | 5.2 (2.3–11.3) [317] | 4.3 (1.9–10.2) [352] | – | 0.19 |
| Length of stay in acute hospital (days), median (IQR) | ||||
| All | 17 (8–34) [1185] | 16 (8–33) [1186] | – | 0.32 |
| Survivors | 24 (14–43) [753] | 24 (14–43) [736] | – | 0.87 |
| Non-survivors | 7 (3–16) [431] | 6 (2–14) [450] | – | 0.48 |
| All-cause mortality at ICU discharge, n/N (%) | 317/1190 (26.6) | 352/1197 (29.4) | 0.91 (0.80 to 1.03)f | 0.13c |
| 0.86 (0.71 to 1.04)g | 0.12 | |||
| All-cause mortality at acute hospital discharge, n/N (%) | 431/1185 (36.4) | 450/1186 (37.9) | 0.96 (0.86 to 1.06)f | 0.44c |
| 0.93 (0.78 to 1.11)g | 0.43 | |||
| All-cause mortality at 90 days, n/N (%) | 442/1185 (37.3) | 466/1193 (39.1) | 0.95 (0.86 to 1.06)f | 0.40c |
| 0.92 (0.77 to 1.10)g | 0.35 | |||
| All-cause mortality at 1 year, n/N (%) | 505/1181 (42.8) | 534/1192 (44.8) | 0.95 (0.87 to 1.05)f | 0.32c |
| 0.91 (0.76 to 1.08)g | 0.28 | |||
FIGURE 17.
Kaplan–Meier curves for survival to 90 days following randomisation.

FIGURE 18.
Kaplan–Meier curves for survival to 1 year following randomisation.

Safety monitoring
Fifty-eight patients in both the parenteral group (4.9%) and in the enteral group (4.8%) experienced one or more serious adverse events (Table 21). The most frequently reported serious adverse events were ischaemic bowel [n = 8 (0.7%) in the parenteral group and n = 11 (0.9%) in the enteral group], cardiac arrest [n = 9 (0.8%) in the parenteral group and n = 6 (0.5%) in the enteral group], electrolyte disturbance [n = 8 (0.7%) in the parenteral group and n = 5 (0.4%) in the enteral group], raised liver enzymes [n = 3 (0.3%) in the parenteral group and n = 7 (0.6%) in the enteral group] and gastrointestinal haemorrhage [n = 3 (0.3%) in the parenteral group and n = 8 (0.7%) in the enteral group]. There were eight episodes of hypoglycaemia [n = 5 (0.4%) in the parenteral group and n = 3 (0.3%) in the enteral group] that were reported as a serious adverse event (see Table 21).
| Serious adverse events,a n/N (%) | Parenteral group (N = 1191) | Enteral group (N = 1197) |
|---|---|---|
| Any | 58/1191 (4.9) | 58/1197 (4.8) |
| Specified | ||
| Abdominal distension | 1/1191 (0.1) | 2/1197 (0.2) |
| Abdominal pain | 0/1191 (0.0) | 0/1197 (0.0) |
| Electrolyte disturbance | 8/1191 (0.7) | 5/1197 (0.4) |
| Haemopneumothorax | 0/1191 (0.0) | 0/1197 (0.0) |
| Hepatomegaly | 0/1191 (0.0) | 0/1197 (0.0) |
| Hyperosmolar syndrome | 0/1191 (0.0) | 0/1197 (0.0) |
| Hypersensitivity reaction (anaphylactic reaction) | 0/1191 (0.0) | 0/1197 (0.0) |
| Hypoglycaemia | 5/1191 (0.4) | 3/1197 (0.3) |
| Ischaemic bowel | 8/1191 (0.7) | 11/1197 (0.9) |
| Jaundice | 1/1191 (0.1) | 1/1197 (0.1) |
| Nausea requiring treatment | 0/1191 (0.0) | 0/1197 (0.0) |
| Pneumothorax | 1/1191 (0.1) | 1/1197 (0.1) |
| Raised liver enzymes | 3/1191 (0.3) | 7/1197 (0.6) |
| Regurgitation or aspiration | 2/1191 (0.2) | 4/1197 (0.3) |
| Vascular catheter-related infection | 0/1191 (0.0) | 0/1197 (0.0) |
| Vomiting | 1/1191 (0.1) | 1/1197 (0.1) |
| Unspecified | ||
| Acidosis – metabolic | 1/1191 (0.1) | 0/1197 (0.0) |
| Air embolus | 1/1191 (0.1) | 0/1197 (0.0) |
| Anterolateral myocardial infarction | 1/1191 (0.1) | 0/1197 (0.0) |
| Bilateral pleural effusions | 1/1191 (0.1) | 0/1197 (0.0) |
| Bowel obstruction | 1/1191 (0.1) | 0/1197 (0.0) |
| Caecal perforation | 0/1191 (0.0) | 1/1197 (0.1) |
| Cardiac arrest | 9/1191 (0.8) | 6/1197 (0.5) |
| Cerebral infarction | 0/1191 (0.0) | 2/1197 (0.2) |
| Complete heart block | 1/1191 (0.1) | 0/1197 (0.0) |
| Deteriorating neurological condition | 0/1191 (0.0) | 1/1197 (0.1) |
| Extravasation of PN | 1/1191 (0.1) | 0/1197 (0.0) |
| Gastrointestinal haemorrhage or bleed | 3/1191 (0.3) | 8/1197 (0.7) |
| Hospital-acquired pneumonia leading to acute respiratory distress syndrome and acute lung injury | 1/1191 (0.1) | 0/1197 (0.0) |
| Hypotension | 0/1191 (0.0) | 1/1197 (0.1) |
| Hypoxia | 3/1191 (0.3) | 1/1197 (0.1) |
| Intracranial haemorrhage | 1/1191 (0.1) | 0/1197 (0.0) |
| Liver failure | 1/1191 (0.1) | 0/1197 (0.0) |
| Loss of vision | 1/1191 (0.1) | 0/1197 (0.0) |
| Lower limb ischaemia | 0/1191 (0.0) | 1/1197 (0.1) |
| Multiple organ failure | 0/1191 (0.0) | 2/1197 (0.2) |
| Other haemorrhage or bleed | 2/1191 (0.2) | 1/1197 (0.1) |
| Perforated duodenal ulcer | 1/1191 (0.1) | 1/1197 (0.1) |
| Popliteal artery thrombus | 0/1191 (0.0) | 1/1197 (0.1) |
| Pulmonary oedema | 2/1191 (0.2) | 0/1197 (0.0) |
| Renal failure | 4/1191 (0.3) | 1/1197 (0.1) |
| Respiratory distress | 1/1191 (0.1) | 0/1197 (0.0) |
| Sepsis or septic shock | 2/1191 (0.2) | 2/1197 (0.2) |
| Shock state | 0/1191 (0.0) | 1/1197 (0.1) |
| Sigmoid perforation | 0/1191 (0.0) | 1/1197 (0.1) |
| Stroke | 1/1191 (0.1) | 0/1197 (0.0) |
| Suspected bacterial peritonitis | 1/1191 (0.1) | 0/1197 (0.0) |
| Suspected intra-abdominal ischaemia | 0/1191 (0.0) | 1/1197 (0.1) |
There were five serious, unexpected adverse events that were deemed by the site investigator to be potentially related to the study treatment in four patients (one with ischaemic bowel and hypoglycaemia, and one each with upper gastrointestinal haemorrhage and anterolateral myocardial infarction in the parenteral group, and one with lower gastrointestinal haemorrhage in the enteral group).
Subgroup analyses of the primary outcome
There was no statistically significant interaction between the effect of treatment group on 30-day mortality and any of the pre-specified subgroups: age, degree of malnutrition, APACHE II predicted risk of death,34 ICNARC predicted risk of death,35 use of mechanical ventilation, presence of cancer and time from randomisation to start of feeding. The p-values ranged from 0.15 to 0.83 (Figure 19).
FIGURE 19.
Subgroup analyses of the primary outcome (30-day mortality). The p-values are for tests of interaction. The x-axis is presented on a log-scale. The solid line represents no difference between the groups and the dashed line represents the overall effect estimate.
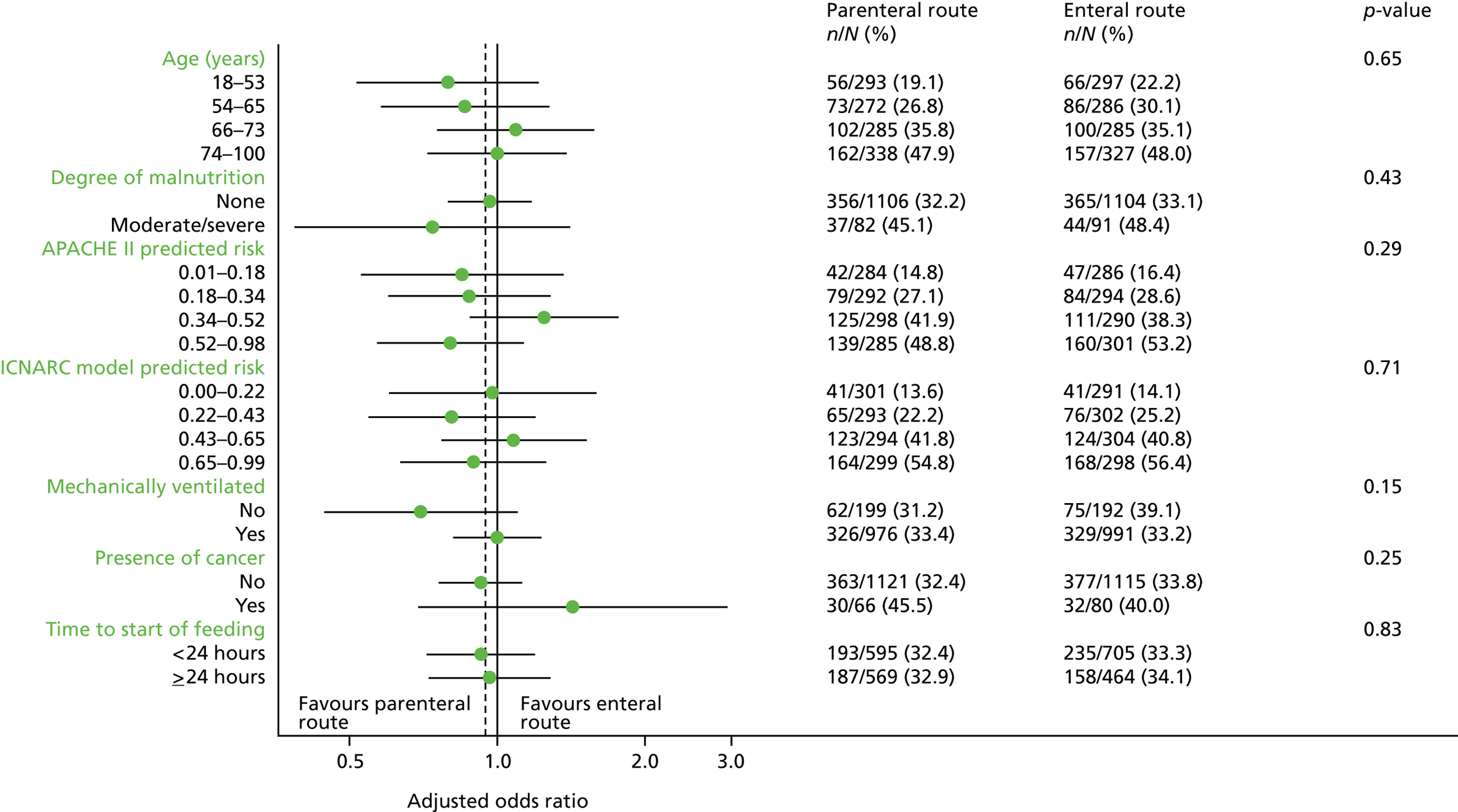
Secondary analyses of the primary outcome
Only five patients were lost to follow-up prior to 30 days and, therefore, making extreme assumptions for the outcomes of these patients made minimal difference to the result (relative risk of 30-day mortality between 0.96 and 0.97).
Adjusting for non-adherence also made minimal difference to the result (relative risk of 30-day mortality 0.96, 95% CI 0.85 to 1.09; p = 0.55).
Chapter 5 Results: cost-effectiveness
Cost-effectiveness at 90 days following randomisation
Resource use up to 90 days
Most patients followed their randomised route of early nutritional support, with only 81 (6.8%) and 18 (1.5%) patients crossing over in the parenteral route and enteral route groups, respectively (see Chapter 4, Adherence to protocol). The total volume of feeding per patient (including both parenteral and enteral products) was higher in the parenteral group (6776 ml) than in the enteral group (4446 ml) (Table 22). Patients in the enteral group received more additional energy sources than the parenteral group (except for insulin and propofol). The intervention required higher staff time for assessment and daily monitoring and support for the parenteral group, but the enteral group required slightly more staff time to address adverse events, such as diarrhoea and vomiting. After calendar day 6, the parenteral group had more days on feeding via both the parenteral and enteral routes than the enteral group. For the index hospital episode, the mean length of stay in critical care and on general medical wards was higher in the parenteral than the enteral group. The proportions of patients readmitted, and the average lengths of stay following readmission were similar between the treatment groups (see Table 22). The average total length of stay in acute hospital up to 90 days post-randomisation was 26.4 days in the parenteral group compared with 25.9 days in the enteral group.
| Resource-use category | Parenteral group (N = 1191) | Enteral group (N = 1197) |
|---|---|---|
| Interventions (from initiation of nutritional support to calendar day 6) | ||
| Nutritional products | ||
| PN (ml): | ||
| Kabiven® 7gN | 758 (2197) | 0 (0) |
| NuTRIflex® Lipid Peri | 918 (2455) | 22 (257) |
| Kabiven 9gN | 909 (2547) | 2 (35) |
| Kabiven 11gN | 873 (2441) | 7 (203) |
| Kabiven 5gN | 228 (791) | 3 (87) |
| Other parenteral nutrition productsa | 2935 (3302) | 25 (291) |
| EN (ml)b | 155 (626) | 4387 (2722) |
| Other interventions: | ||
| i.v. glucose (4%) (ml) | 78 (388) | 152 (634) |
| i.v. glucose (5%) (ml) | 592 (955) | 618 (918) |
| i.v. glucose (10%) (ml) | 9 (98) | 68 (433) |
| i.v. glucose (20%) (ml) | 5 (89) | 23 (185) |
| i.v. glucose (50%) (ml) | 7 (79) | 18 (222) |
| Propofol (1%) (ml) | 388 (609) | 396 (610) |
| Propofol (2%) (ml) | 192 (384) | 191 (392) |
| Insulin (IU) | 154 (224) | 123 (204) |
| Staff time: | ||
| Assessment and set-up (hours) | 0.8 (0.3) | 0.4 (0.2) |
| Monitoring and support (hours) | 2.3 (1.0) | 0.9 (0.5) |
| Addressing adverse events (hours) | 1.8 (2.3) | 2.1 (2.4) |
| Nutritional support in critical care unit between calendar day 7 and 90 days post-randomisation | ||
| PN (days) | 0.4 (2.8) | 0.2 (1.3) |
| EN (days) | 5.7 (11.1) | 5.4 (10.9) |
| Enteral and PN (days) | 0.7 (3.6) | 0.2 (2.3) |
| Hospital length of stay | ||
| Index admission: | ||
| Days in critical care | 11.9 (11.3) | 11.3 (10.9) |
| General medical bed-days | 13.4 (18.0) | 13.3 (18.7) |
| Readmissions: | ||
| Readmissions, n (%) | 124 (10) | 136 (11) |
| Days in critical care | 0.9 (4.5) | 0.9 (4.6) |
| General medical bed-days | 0.3 (3.2) | 0.3 (3.2) |
| Total length of stay up to 90 days | 26.4 (23.8) | 25.9 (23.8) |
Table 23 summarises the resource use reported from responses to the Health Services Questionnaire for all patients randomised. The mean number of inpatient days reported from hospital admissions, other than those involving critical care, were 5.1 days for the parenteral group and 4.7 for the enteral group. The enteral group had a higher average number of contacts with nurses and health visitors than the parenteral group. All other community care contacts up to 90 days were similar between the treatment groups. Patients in both groups reported low use of community health services over the 90 days following randomisation.
| Resource-use category | Parenteral group (n = 432)a | Enteral group (n = 422)a |
|---|---|---|
| Inpatient days (general medical) | 5.1 (12.8) | 4.7 (10.7) |
| Outpatient visits | 2.1 (3.6) | 2.4 (4.0) |
| GP contacts | 2.1 (2.5) | 2.2 (2.8) |
| Nurse contacts | 2.5 (6.9) | 3.0 (7.6) |
| Occupational therapist contacts | 0.8 (2.2) | 0.8 (2.2) |
| Health visitor contacts | 0.8 (4.7) | 1.6 (8.5) |
| Clinical psychologist contacts | 0.1 (1.0) | 0.2 (1.2) |
| Speech therapist contacts | 0.1 (0.7) | 0.1 (1.1) |
| Physiotherapist contacts | 1.3 (5.0) | 1.4 (4.3) |
| Dietitian contacts | 0.1 (0.6) | 0.1 (0.7) |
Total costs up to 90 days
Intervention costs were higher for the parenteral group, driven by both higher volume of feeding and higher unit costs of PN products relative to EN products. The net effect of the higher intervention, critical care and general medical ward costs was that the parenteral group had higher mean total costs per patient than the enteral group (£24,458 vs. £23,164) (Table 24).
| Resource-use category | Parenteral group (n = 1191) | Enteral group (n = 1197) |
|---|---|---|
| Interventions (from initiation of nutritional support to calendar day 6) | ||
| Nutritional product costs | 228 (107) | 51 (32) |
| Other nutritional intervention costs | 207 (189) | 206 (194) |
| Staff time costs | ||
| Assessment and set-up | 41 (16) | 17 (8) |
| Monitoring and support | 113 (51) | 38 (23) |
| Addressing adverse events | 75 (94) | 87 (100) |
| Nutritional support in critical care unit between calendar day 7 and 90 days post-randomisation | ||
| 122 (330) | 79 (222) | |
| Hospital costs | ||
| Index admission | ||
| Critical care | 17,384 (17,590) | 16,545 (16,698) |
| General medical ward | 3672 (4961) | 3670 (5131) |
| Readmission costsa | ||
| Critical care | 1181 (6327) | 1137 (6045) |
| General medical | 77 (886) | 91 (892) |
| Outpatient and community costsb,c | 1359 (3376) | 1244 (2728) |
| Total costs up to 90 daysa,b,c | 24,458 (21,400) | 23,164 (20,449) |
Nutritional and health-related quality of life at 90 days
The health status profiles reported from responses to the EQ-5D-5L questionnaires administered at 90 days post-randomisation are summarised by treatment group in Table 25. The distribution of health status profiles was similar between the treatment groups. At 90 days, the proportion of patients who reported ‘no problems’ for each dimension of the EQ-5D-5L in the parenteral group was no greater than for the enteral group. The resultant mean EQ-5D-5L utility scores and QALYs were also similar between the treatment groups (Table 26). The Satisfaction with Food-related Life questionnaire was very poorly completed. At 90 days, complete responses were available for only 405/743 (54.5%) eligible patients in the parenteral group and 378/727 (52.0%) in the enteral group. There was no significant difference in the mean response with a mean (SD) of 5.2 (1.6) in the parenteral group and 5.1 (1.7) in the enteral group (mean difference 0.10, 95% CI −0.14 to 0.33; p = 0.43).
| EQ-5D-5L component | Parenteral group (n = 558)a | Enteral group (n = 544)a |
|---|---|---|
| Mobility | ||
| No problems | 170 (30) | 168 (31) |
| Slight problems | 118 (21) | 108 (20) |
| Moderate problems | 135 (24) | 142 (26) |
| Severe problems | 75 (13) | 76 (14) |
| Extreme problems | 60 (11) | 50 (9) |
| Self-care | ||
| No problems | 299 (54) | 293 (54) |
| Slight problems | 106 (19) | 113 (21) |
| Moderate problems | 85 (15) | 72 (13) |
| Severe problems | 31 (6) | 29 (5) |
| Extreme problems | 37 (7) | 37 (7) |
| Usual activities | ||
| No problems | 131 (24) | 119 (22) |
| Slight problems | 123 (22) | 131 (24) |
| Moderate problems | 140 (25) | 130 (24) |
| Severe problems | 74 (13) | 67 (12) |
| Extreme problems | 90 (16) | 97 (18) |
| Pain/discomfort | ||
| No problems | 173 (31) | 178 (33) |
| Slight problems | 150 (27) | 163 (30) |
| Moderate problems | 162 (29) | 133 (24) |
| Severe problems | 56 (10) | 54 (10) |
| Extreme problems | 17 (3) | 16 (3) |
| Anxiety/depression | ||
| No problems | 242 (43) | 239 (44) |
| Slight problems | 158 (29) | 142 (26) |
| Moderate problems | 111 (20) | 114 (21) |
| Severe problems | 28 (5) | 35 (6) |
| Extreme problems | 19 (3) | 14 (3) |
Cost-effectiveness at 90 days
The incremental QALY gain for early nutritional support via the parenteral versus the enteral route was small and with a 95% CI that included zero (Table 27). The average costs were higher for the parenteral group, but this difference was not statistically significant. The INB for the parenteral route compared with the enteral route was negative at −£1263 (95% CI −£2952 to £426) (see Table 27).
| End point | Parenteral group (n = 1191) | Enteral group (n = 1197) | Incremental effect (unadjusted), mean (95% CI) | p-value |
|---|---|---|---|---|
| QALYsa | 0.051 (0.048) | 0.050 (0.049) | 0.002 (−0.002 to 0.006) | 0.46 |
| Costs (£)a | 24,458 (21,400) | 23,164 (20,449) | 1293 (−401 to 2988) | 0.14 |
| INB (£)a,b | −1263 (−2952 to 426) | 0.14 |
When the uncertainty in the incremental costs and QALYs is represented on the cost-effectiveness plane, the majority of the points are in the quadrant that shows that early nutritional support via the parenteral route has, on average, higher costs and improves QALYs, although the magnitude of these average QALY gains was small (Figure 20). The probability that early nutritional support via the parenteral route is more cost-effective at 90 days than via the enteral route – given the data – is never > 10%, irrespective of how much society is willing to pay for a QALY gain (Figure 21).
FIGURE 20.
Uncertainty in the mean costs (£) and QALY differences and their distribution for early nutritional support via the parenteral vs. the enteral route (within 90 days post-randomisation).

FIGURE 21.
Cost-effectiveness acceptability curve, reporting the probability that early nutritional support via the parenteral route is cost-effective (within 90 days post-randomisation) at alternative willingness to pay for a QALY gain.
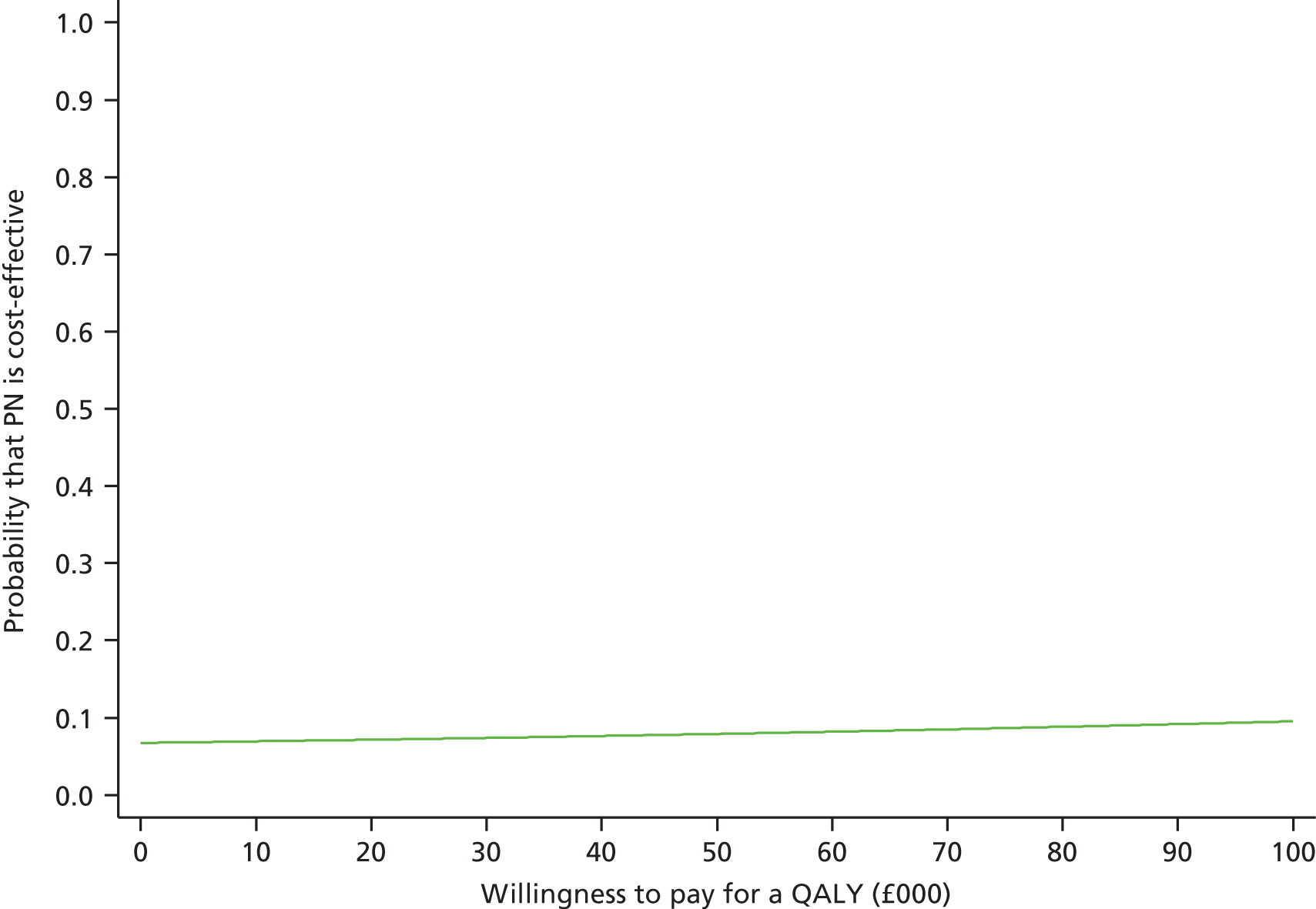
The estimated INBs were similar across all the scenarios considered in the sensitivity analyses (Figure 22). For example, the INB remains around −£1300 whether additional staff time is required to deliver nutritional support in the critical care or list prices of feeding products are considered. Similarly, excluding readmissions that were reported from responses to the Health Services Questionnaire – to avoid any risk of double-counting – had only a small impact on the mean INB (−£1147 vs. −£1263).
FIGURE 22.
Sensitivity analyses that report the mean (95% CI) INB (at £20,000 per QALY) within 90 days post-randomisation according to alternative assumptions compared with the base case. The vertical dashed line indicates INBs in the base-case analysis. The solid vertical line indicates no difference in net monetary benefits between comparator groups.
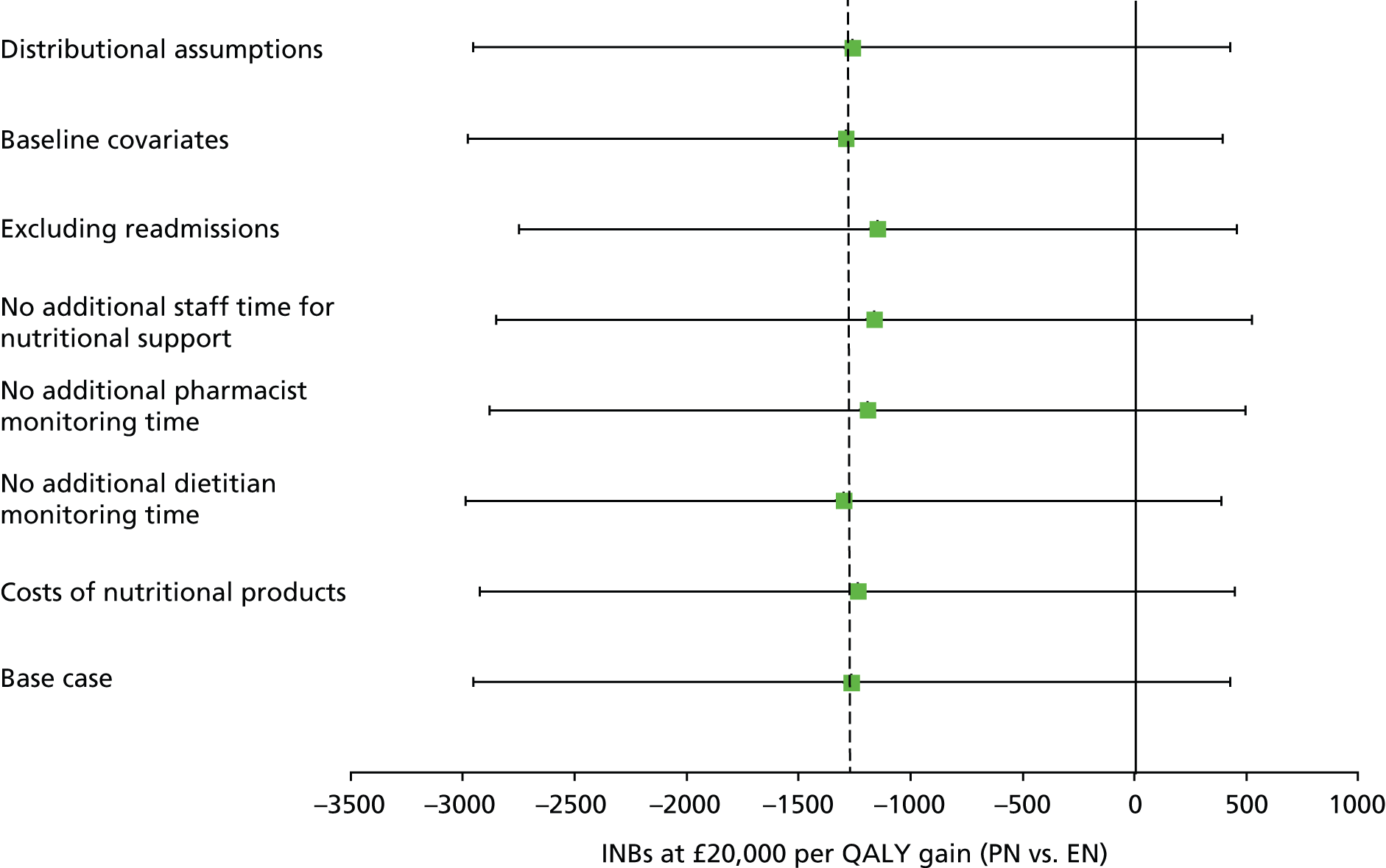
The results of the subgroup analyses are presented in Table 28, and show that the incremental QALYs were similar across all subgroups. Although there were some subgroups of patients for whom the incremental costs of early nutritional support via the parenteral route were negative, and hence the INBs were positive, the statistical uncertainty surrounding these findings was high. For patients with higher APACHE II predicted risk of death (0.52–0.98), early nutritional support via the parenteral route was more costly and the QALY gain was small, leading to significant negative INB. For all other subgroups, as for the overall results, the CIs around the INB included zero.
| Subgroup | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | INB (£) (95% CI) | |
|---|---|---|---|---|
| Age (years) | 18–53 | 764 (−2611 to 4140) | 0.002 (−0.006 to 0.01) | −729 (−4096 to 2638) |
| 54–65 | 2027 (−1453 to 5508) | 0.007 (−0.001 to 0.015) | −1893 (−5363 to 1577) | |
| 66–73 | 1625 (−1814 to 5065) | −0.002 (−0.01 to 0.006) | −1659 (−5090 to 1772) | |
| 74–100 | 1039 (−2149 to 4227) | 0.000 (−0.007 to 0.008) | −1030 (−4209 to 2149) | |
| Degree of malnutrition | None | 1171 (−590 to 2933) | 0.001 (−0.003 to 0.005) | −1148 (−2904 to 608) |
| Moderate/severe | 2683 (−3573 to 8939) | 0.004 (−0.011 to 0.02) | −2593 (−8830 to 3644) | |
| APACHE II predicted risk | 0.01–0.18 | −838 (−4253 to 2578) | 0.005 (−0.003 to 0.013) | 934 (−2469 to 4337) |
| 0.18–0.34 | 1669 (−1709 to 5047) | −0.002 (−0.01 to 0.006) | −1706 (−5075 to 1663) | |
| 0.34–0.52 | −429 (−3906 to 3047) | 0.002 (−0.006 to 0.01) | 460 (−3006 to 3926) | |
| 0.52–0.98 | 4494 (1182 to 7807) | 0.001 (−0.006 to 0.009) | −4469 (−7773 to −1165) | |
| ICNARC model predicted risk | 0.00–0.22 | −1520 (−4899 to 1859) | 0.005 (−0.003 to 0.013) | 1618 (−1751 to 4987) |
| 0.22–0.43 | 2735 (−651 to 6122) | −0.003 (−0.011 to 0.005) | −2798 (−6177 to 581) | |
| 0.43–0.65 | 1353 (−2024 to 4730) | 0.002 (−0.006 to 0.01) | −1311 (−4679 to 2057) | |
| 0.65–0.99 | 2606 (−757 to 5969) | 0.002 (−0.006 to 0.009) | −2572 (−5926 to 782) | |
| Mechanically ventilated | No | 2514 (−1666 to 6695) | 0.007 (−0.003 to 0.017) | −2372 (−6541 to 1797) |
| Yes | 1053 (−801 to 2907) | 0.000 (−0.004 to 0.005) | −1044 (−2892 to 804) | |
| Presence of cancer | No | 1378 (−370 to 3125) | 0.001 (−0.003 to 0.005) | −1356 (−3098 to 386) |
| Yes | −581 (−7453 to 6292) | 0.007 (−0.009 to 0.023) | 718 (−6132 to 7568) | |
| Time to start of feeding (hours) | < 24 | 1482 (−796 to 3759) | 0.001 (−0.004 to 0.006) | −1460 (−3731 to 811) |
| ≥ 24 | 741 (−1876 to 3358) | 0.002 (−0.005 to 0.008) | −704 (−3314 to 1906) | |
| Adherence adjusted | 1400 (−435 to 3235) | 0.002 (−0.003 to 0.006) | −1367 (−3196 to 462) | |
Cost-effectiveness at 1 year following randomisation (primary outcome)
Resource use up to 1 year
The resource use up to 1 year post-randomisation is presented in Table 29. The use of nutritional support between 90 days and 1 year post-randomisation was very low and similar between the treatment groups. The number of continuing index admissions and readmissions to hospital between 90 days and 1 year post-randomisation were also similar. Between 90 days and 1 year post-randomisation, the mean number of days in critical care was higher for the parenteral than the enteral group, but the mean number of days on general medical wards was lower. The average total hospital length of stay up to 1 year post-randomisation was 30.1 days in the parenteral group compared with 29.9 days in the enteral group.
| Resource use category | Parenteral group (N = 1191) | Enteral group (N = 1197) |
|---|---|---|
| Total hospital length of stay up to 90 days | 26.4 (23.8) | 25.9 (23.8) |
| Nutritional support between 90 days and 1 year: | ||
| PN (days) | 0.03 (1.1) | 0 (0) |
| EN (days) | 0.1 (2.4) | 0.1 (1.8) |
| Enteral and PN (days) | 0 (0) | 0 (0) |
| Hospital length of stay between 90 days and 1 year | ||
| Continuing index admission, n (%) | 56 (4.7) | 54 (4.5) |
| Days in critical carea | 0.4 (8.4) | 0.01 (0.2) |
| General medical bed-daysa | 2.3 (15.5) | 2.4 (15.1) |
| Readmissions, n (%) | 61 (5.1) | 57 (4.7) |
| Days in critical carea,b | 0.4 (2.2) | 0.4 (3.2) |
| General medical bed-daysa,b | 0.7 (6.6) | 1.2 (8.8) |
| Total hospital length of stay up to 1 year | 30.1 (36.7) | 29.9 (36.0) |
Table 30 reports results from responses to the Health Services Questionnaire administered at 1 year post-randomisation concerning resource use between 90 days and 1 year. The average number of inpatient days reported from admissions, which were not to critical care, were lower in the parenteral group (7.8 days) than in the enteral group (8.9 days). The enteral group had a slightly higher average number of outpatient visits and contact with health visitors. The parenteral group had slightly higher average number of outpatient visits and contacts with nurses, and a lower number of contacts with health visitors than the enteral group. All other community care contacts between 90 days and 1 year post-randomisation were similar between the treatment groups. Patients in both groups reported low use of community health services over 1 year following randomisation.
| Resource-use category | Parenteral group (n = 457)a | Enteral group (n = 463)a |
|---|---|---|
| Inpatient days (general medical) | 7.8 (24.9) | 8.9 (22.5) |
| Outpatient visits | 6.1 (9.8) | 5.6 (7.2) |
| GP contacts | 5.4 (7.5) | 5.5 (6.6) |
| Nurse contacts | 7.9 (26.7) | 7.3 (17.8) |
| Occupational therapist contacts | 1.4 (5.8) | 1.4 (6.5) |
| Health visitor contacts | 0.6 (4.4) | 3.9 (53.2) |
| Clinical psychologist contacts | 0.4 (1.8) | 0.5 (3.6) |
| Speech therapist contacts | 0.6 (3.2) | 0.4 (2.8) |
| Physiotherapist contacts | 1.7 (5.1) | 2.0 (5.9) |
| Dietitian contacts | 0.4 (1.4) | 0.3 (1.2) |
Total costs up to 1 year
Table 31 reports the total costs at 1 year, across all the resource use items recorded. At 1 year, the mean total costs per patient were £28,354 for the parenteral group and £26,775 for the enteral group.
| Resource-use category | Parenteral group (n = 1191) | Enteral group (n = 1197) |
|---|---|---|
| Total costs up to 90 daysa,b,c | 24,458 (21,400) | 23,164 (20,449) |
| Hospital costs between 90 days and 1 year: | ||
| Nutritional support | 3 (67.5) | 1 (17.7) |
| Ongoing index admissions: | ||
| Critical care costsa | 554 (13,434) | 7 (256) |
| General medical costsa | 622 (4274) | 650 (4163) |
| Readmissions: | ||
| Critical care costsa,c | 453 (3000) | 576 (4446) |
| General medical costsa,c | 197 (1827) | 337 (2412) |
| Outpatient and community costsb,c | 2069 (6574) | 2040 (5193) |
| Total costs up to 1 yeara–c | 28,354 (32,144) | 26,775 (26,273) |
Nutritional and health-related quality of life at 1 year
The health status profiles reported from responses to the EQ-5D-5L questionnaires administered at 1 year post-randomisation are summarised by treatment group in Table 32. At 1 year, the proportion of patients who reported ‘no problems’ for each dimension of the EQ-5D-5L in the parenteral group was no greater than for the enteral group. A lower proportion of patients in the parenteral group than in the enteral group reported ‘extreme problems’ for the mobility and usual activities dimensions of health. On the pain/discomfort and anxiety/depression dimensions, a higher proportion of patients in the parenteral group as compared with the enteral group reported ‘extreme problems’. The resultant mean EQ-5D-5L utility scores were similar between the treatment groups (parenteral group 0.684 vs. enteral group 0.683) (Table 33). At 1 year post-randomisation, a slightly higher proportion of patients in the parenteral group were alive than in the enteral group but the difference was not statistically significant (see Chapter 4, Secondary outcomes: clinical effectiveness) and the 1-year QALYs were similar between the treatment groups (see Table 33). At 1 year, complete responses to the Satisfaction with Food-related Life Questionnaire were available for 338 of 676 (50.0%) eligible patients in the parenteral group and 322 of 658 (48.9%) in the enteral group. There was no significant difference in the mean response, with a mean (SD) of 5.3 (1.6) in the parenteral group and 5.4 (1.6) in the enteral group (mean difference −0.10, 95% CI −0.35 to 0.14; p = 0.41).
| EQ-5D-5L component | Parenteral group (N = 467)a | Enteral group (N = 473)a |
|---|---|---|
| Mobility | ||
| No problems | 166 (36) | 172 (37) |
| Slight problems | 93 (20) | 90 (19) |
| Moderate problems | 114 (24) | 99 (21) |
| Severe problems | 65 (20) | 80 (17) |
| Extreme problems | 29 (6) | 32 (7) |
| Self-care | ||
| No problems | 280 (61) | 287 (61) |
| Slight problems | 87 (19) | 71 (15) |
| Moderate problems | 60 (13) | 71 (15) |
| Severe problems | 20 (4) | 24 (5) |
| Extreme problems | 20 (4) | 20 (4) |
| Usual activities | ||
| No problems | 151 (32) | 163 (34) |
| Slight problems | 110 (24) | 104 (22) |
| Moderate problems | 103 (22) | 99 (21) |
| Severe problems | 65 (14) | 62 (13) |
| Extreme problems | 38 (8) | 45 (10) |
| Pain/discomfort | ||
| No problems | 145 (31) | 159 (34) |
| Slight problems | 139 (30) | 136 (29) |
| Moderate problems | 111 (23) | 125 (26) |
| Severe problems | 54 (12) | 42 (9) |
| Extreme problems | 18 (4) | 11 (2) |
| Anxiety/depression | ||
| No problems | 218 (47) | 235 (50) |
| Slight problems | 109 (23) | 91 (19) |
| Moderate problems | 95 (20) | 95 (20) |
| Severe problems | 30 (6) | 41 (9) |
| Extreme problems | 15 (3) | 11 (2) |
Cost-effectiveness at 1 year
The incremental QALY gain for the parenteral group compared with the enteral group was positive, but with a 95% CI that included zero (Table 34). The mean total costs were higher in the parenteral group, with an incremental cost of £1580 (95% CI −£792 to £3951). Hence the INB for the parenteral group compared with the enteral group was negative at −£1320 (95% CI −£3709 to £1069).
| End point | Parenteral group (N = 1191) | Enteral group (N = 1197) | Incremental effect (unadjusted), mean (95% CI) | p-value |
|---|---|---|---|---|
| QALYsa | 0.348 (0.333) | 0.335 (0.332) | 0.013 (−0.014 to 0.040) | 0.35 |
| Costs (£)a | 28,354 (32,144) | 26,775 (26,273) | 1580 (−792 to 3951) | 0.19 |
| INB (£)a,b | −1320 (−3709 to 1069) | 0.28 |
When the uncertainty in the incremental costs and QALYs is represented on the cost-effectiveness plane, the majority of the points are in the quadrant that shows early nutritional support via the parenteral route has, on average, higher costs and improves QALYs, but again the magnitude of the average QALY gains was small (Figure 23). The cost-effectiveness acceptability curve (Figure 24) shows that at 1 year the probability that early nutritional support via the parenteral route is more cost-effective than via the enteral route – given the data – is < 20% at the £20,000 willingness-to-pay threshold stipulated by NICE and does not exceed 50% even at £100,000 per QALY.
FIGURE 23.
Uncertainty in the mean costs (£) and QALY differences and their distribution for early nutritional support via the parenteral route vs. the enteral route (within 1 year post-randomisation).
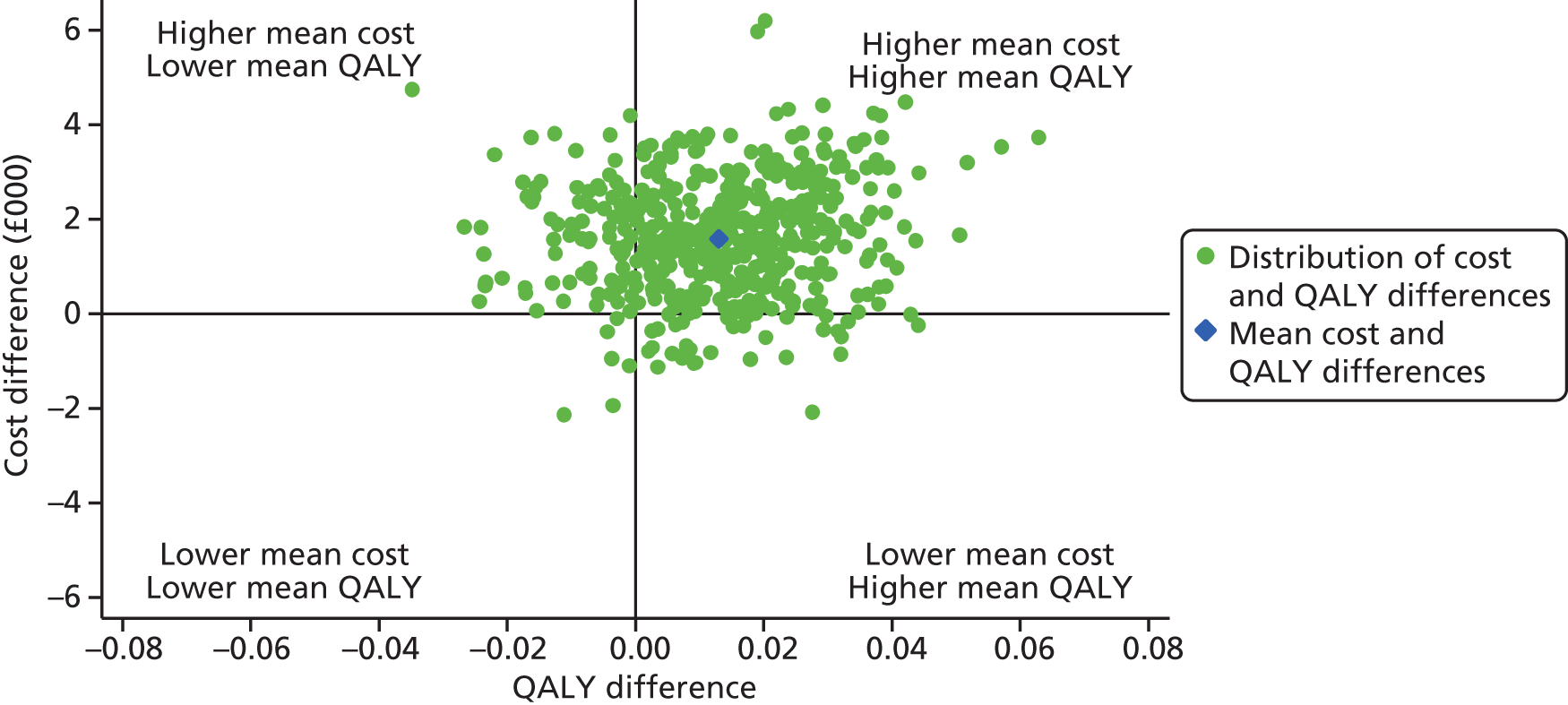
FIGURE 24.
Cost-effectiveness acceptability curve, reporting the probability that early nutritional support via the parenteral route is cost-effective (within 1 year post-randomisation) at alternative willingness to pay for a QALY gain.

The estimated INBs were similar across all of the scenarios considered in the sensitivity analyses (Figure 25). This shows that the base-case results are robust to alternative assumptions.
FIGURE 25.
Sensitivity analyses that report the mean (95% CI) INB (at £20,000 per QALY) within 1 year post-randomisation according to alternative assumptions compared with the base case. The vertical dashed line indicates INBs in the base-case analysis. The solid vertical line indicates no difference in net monetary benefits between comparator groups.

The estimated INBs were similar across all pre-specified subgroups (Table 35). As for the results at 90 days, although there were some subgroups of patients for whom early nutritional support via the parenteral route was cost-saving and hence their INBs were positive, there was high statistical uncertainty around these findings. For patients with higher APACHE II predicted risk of death (0.52–0.98), early nutritional support via the parenteral route was more costly and the INB was negative, and both of these end points were statistically significant for this subgroup. For all other subgroups, as for the overall results, the CIs around the INBs included zero.
| Subgroup | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | INB (£) (95% CI) | |
|---|---|---|---|---|
| Age (years) | 18–53 | 2017 (−2743 to 6777) | 0.016 (−0.037 to 0.07) | −1691 (−6509 to 3127) |
| 54–65 | 2396 (−2491 to 7283) | 0.033 (−0.022 to 0.088) | −1731 (−6671 to 3209) | |
| 66–73 | 983 (−3843 to 5808) | 0.005 (−0.049 to 0.06) | −880 (−5754 to 3994) | |
| 74–100 | 1283 (−3189 to 5756) | 0.005 (−0.045 to 0.056) | −1177 (−5700 to 3346) | |
| Degree of malnutrition | None | 1308 (−1157 to 3774) | 0.011 (−0.017 to 0.039) | −1086 (−3570 to 1398) |
| Moderate/severe | 4735 (−4052 to 13521) | 0.025 (−0.078 to 0.128) | −4235 (−13103 to 4633) | |
| APACHE II predicted risk | 0.01–0.18 | −1793 (−6581 to 2995) | 0.041 (−0.013 to 0.094) | 2611 (−2210 to 7432) |
| 0.18–0.34 | 1776 (−2959 to 6512) | −0.01 (−0.064 to 0.043) | −1985 (−6770 to 2800) | |
| 0.34–0.52 | −442 (−5308 to 4423) | 0.006 (−0.048 to 0.06) | 564 (−4333 to 5461) | |
| 0.52–0.98 | 6380 (1745 to 11014) | 0.013 (−0.04 to 0.065) | −6129 (−10810 to −1448) | |
| ICNARC model predicted risk | 0.00–0.22 | −2692 (−7441 to 2058) | 0.034 (−0.018 to 0.086) | 3370 (−1430 to 8170) |
| 0.22–0.43 | 4059 (−701 to 8818) | −0.01 (−0.063 to 0.043) | −4260 (−9078 to 558) | |
| 0.43–0.65 | 612 (−4155 to 5379) | 0.009 (−0.043 to 0.061) | −428 (−5238 to 4382) | |
| 0.65–0.99 | 4272 (−443 to 8988) | 0.015 (−0.037 to 0.066) | −3977 (−8736 to 782) | |
| Mechanically ventilated | No | 3114 (−2746 to 8973) | 0.056 (−0.012 to 0.123) | −1998 (−7910 to 3914) |
| Yes | 1280 (−1320 to 3880) | 0.005 (−0.025 to 0.034) | −1187 (−3806 to 1432) | |
| Presence of cancer | No | 1743 (−702 to 4189) | 0.009 (−0.02 to 0.037) | −1573 (−4037 to 891) |
| Yes | −1850 (−11479 to 7779) | 0.065 (−0.045 to 0.174) | 3142 (−6565 to 12,849) | |
| Time to start of feeding | < 24 hours | 2766 (−443 to 5974) | 0.015 (−0.022 to 0.051) | −2473 (−5704 to 758) |
| ≥ 24 hours | −90 (−3778 to 3598) | 0.01 (−0.033 to 0.052) | 282 (−3441 to 4005) | |
| Adherence adjusted | 1710 (−858 to 4277) | 0.014 (−0.015 to 0.044) | −1428 (−4014 to 1158) | |
Lifetime incremental cost-effectiveness
Long-term survival
The Kaplan–Meier survival curves show that when the time horizon was extended beyond 1 year, for those with survival data available, the probability of survival was higher for the parenteral group than the enteral group (Figure 26). The survival difference between the treatment groups was maintained over time, even though a large proportion of cases were censored beyond year 2 following randomisation.
FIGURE 26.
Kaplan–Meier survival curves.

To calculate QALYs over 20 years, the long-term survival for each patient was estimated by combining the observed survival for each patient up to 1 year with their predicted survival for years 2–20. Alternative parametric extrapolation approaches were compared with predict the longer-term survival of patients recruited to the CALORIES trial. Figure 27 considers alternative parametric extrapolations for CALORIES trial patients, using the observed survival data after day 30. The survival data were pooled across the treatment groups, given that there was no statistically significant evidence of treatment effect on survival at 1 year and beyond. The parametric models predict excess mortality in patients recruited to the CALORIES trial compared with the age–gender-matched general population. Of the alternative survival functions, the log-normal distribution appears to fit the observed data best, in that it reports the lowest Akaike information criterion (AIC) and Bayesian information criterion (BIC) (Table 36). It also offers the most plausible projections of future survival (see Figure 27 and Table 36), for which the excess mortality of the CALORIES trial patients is maintained over the time horizon of the study. In the base case, death rates were applied according to the most plausible parametric model (i.e. log-normal) for years 2–20. The logistic model also provides a reasonable fit to the observed data, but the excess mortality is higher than that of the base-case parametric model. Therefore, a sensitivity analysis extrapolating survival according to the logistic function was run.
FIGURE 27.
Comparison of alternative parametric survival functions applied to CALORIES data after day 30.
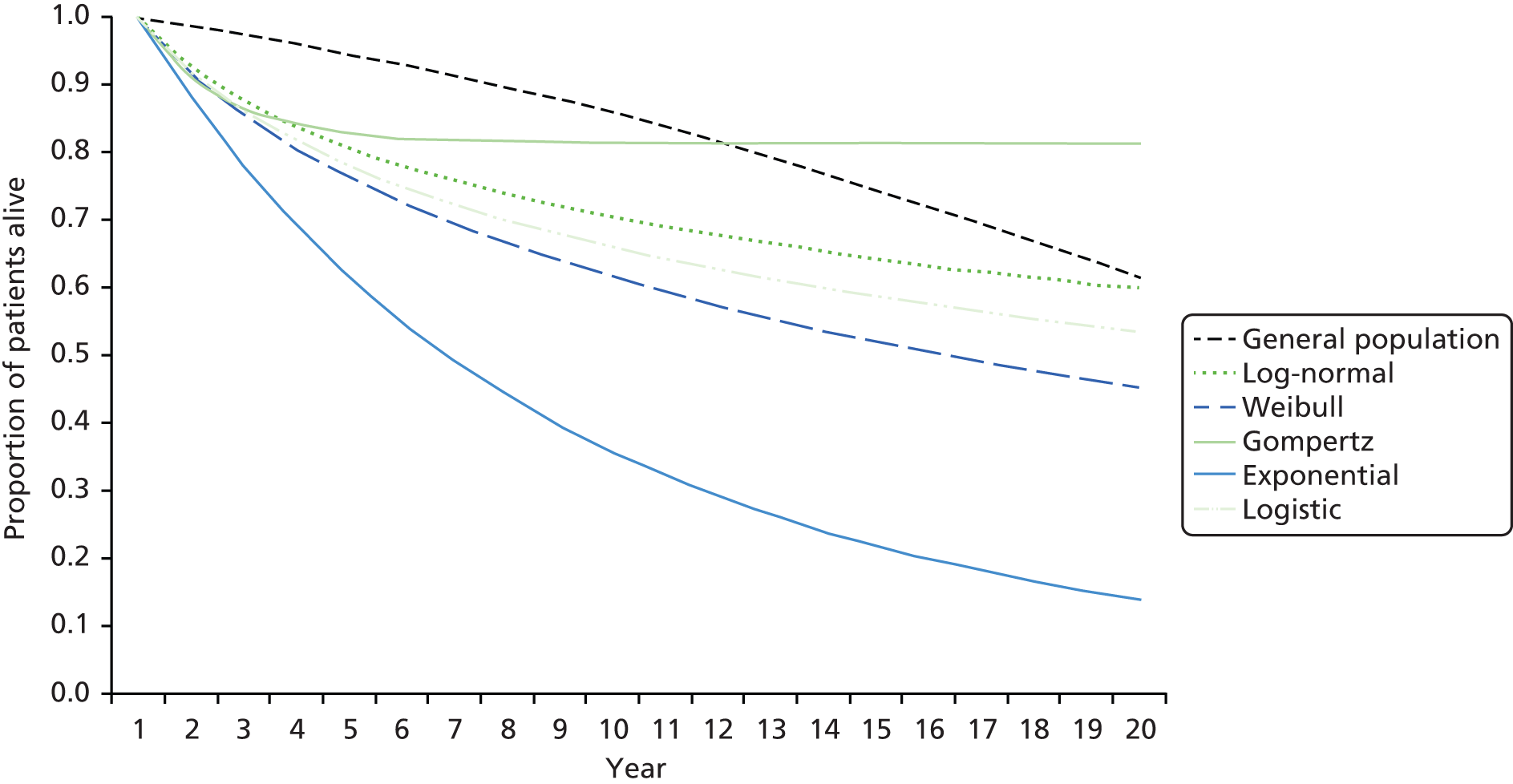
| Distribution | AIC | BIC |
|---|---|---|
| Gompertz | 2930.062 | 2962.260 |
| Log-normal | 2862.885 | 2895.083 |
| Logistic | 2867.942 | 2900.140 |
| Weibull | 2870.448 | 2902.647 |
| Exponential | 3003.800 | 3030.632 |
Long-term health-related quality of life
The lifetime cost-effectiveness analysis required quality of life to be estimated over time. Quality of life from the general population at the age of 64 years (median age of the CALORIES trial 1-year survivors) was used to predict the long-term health-related quality of life of patients recruited to the CALORIES trial. In the base case, general population quality of life for years 2–20 with a decrement of 16% to allow for the lower average quality of life of the CALORIES trial patients (who had survived a critical care episode) was applied.
Long-term costs
To project lifetime costs attributable to the initial critical care episode, mean inpatient, outpatient and community costs up to 1 year estimated from the Health Services Questionnaires were considered.
The mean costs for each treatment group were calculated for those patients who survived at least up to 1 year. These mean costs were used to impute mean costs for years 2–20. For each group, these mean costs were similar (£5629 for the parenteral group and £5625 for the enteral group).
Lifetime incremental cost-effectiveness
Table 37 presents the resultant lifetime QALYs, costs and INB according to the base case assumptions. Overall, at the NICE-stipulated threshold of £20,000 per QALY, the INB was positive (£440) but with a wide 95% CI that included zero.
| End point | Parenteral group (n = 1191) | Enteral group (n = 1197) | Incremental effect (unadjusted), mean (95% CI) | p-value |
|---|---|---|---|---|
| QALYsa | 3.996 (3.432) | 3.849 (3.448) | 0.147 (−0.129 to 0.423) | 0.30 |
| Costs (£)a | 53,100 (42,282) | 50,595 (37,968) | 2505 (−733 to 5744) | 0.13 |
| INB (£)a,b | 440 (−3586 to 4466) | 0.83 |
Figure 28 presents the uncertainties in costs and QALYs extrapolated to the lifetime. There are considerable uncertainties around both costs and QALYs, with the majority of the points in the quadrant which shows that early nutritional support via the parenteral route has, on average, higher costs and improves QALYs. The cost-effectiveness acceptability curve shows that that the probability of early nutritional support via the parenteral route being most cost-effective is around 60% at the NICE-stipulated threshold of £20,000 per QALY, increasing to around 80% at higher willingness to pay (Figure 29).
FIGURE 28.
Uncertainty in the mean costs (£) and QALY differences and their distribution for early nutritional support via the parenteral vs. the enteral route (at lifetime).

FIGURE 29.
Cost-effectiveness acceptability curve, reporting the probability that early nutritional support via the parenteral route is cost-effective (at lifetime) at alternative willingness to pay for a QALY gain.
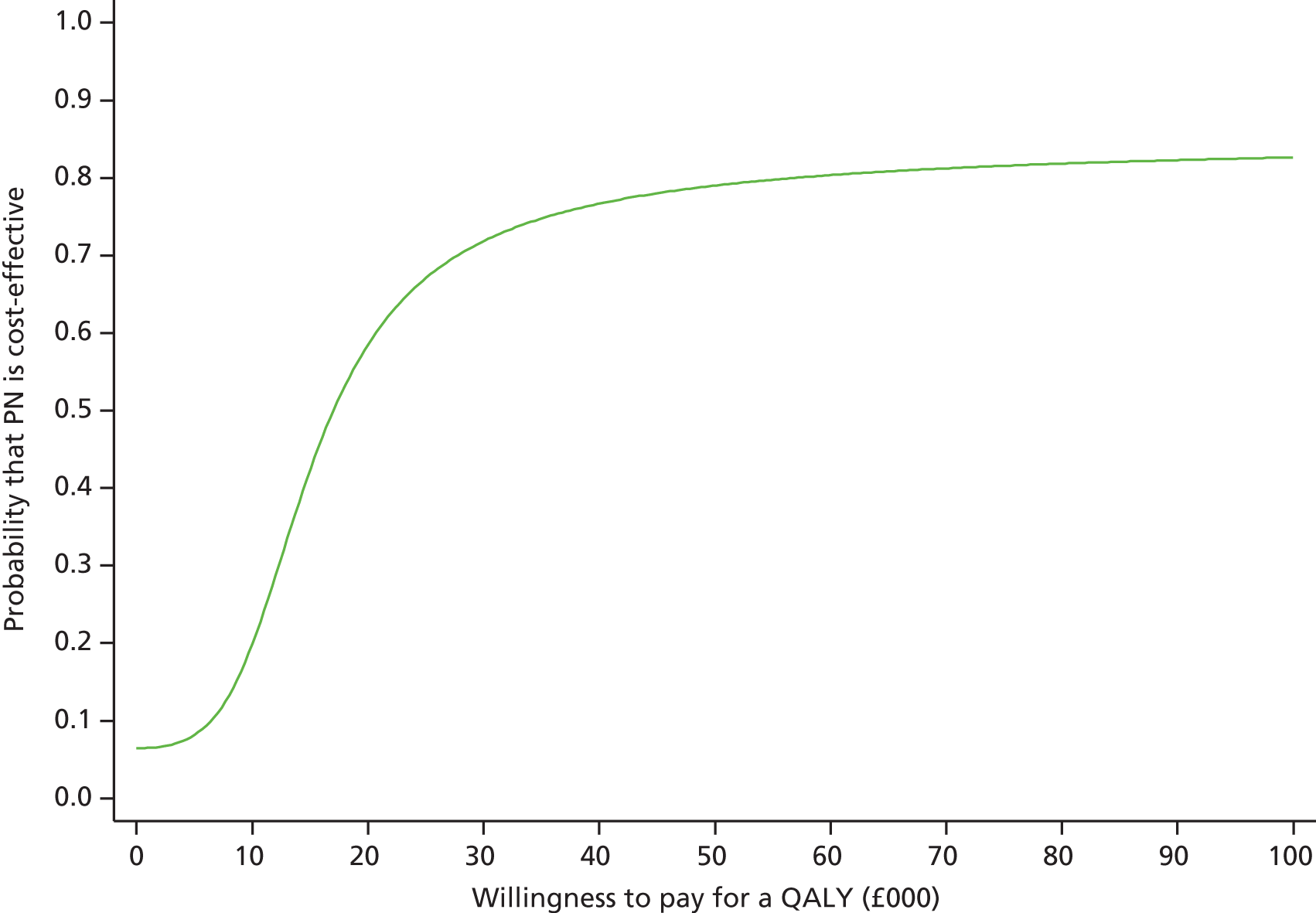
The sensitivity analyses on the lifetime results suggest that these findings are robust to alternative assumptions, including those applied to extrapolation of long-term survival and to quality of life for survivors (Figure 30). For example, alternative assumptions for survival extrapolation had small effect on the mean INB. Similarly, a large decrement compared with a smaller decrement in quality of life had only marginal impact on the mean INB.
FIGURE 30.
Sensitivity analyses that report the mean (95% CI) INB (at £20,000 per QALY) at lifetime according to alternative assumptions compared with the base case. The vertical dashed line indicates INBs in the base-case analysis. The solid vertical line indicates no difference in net monetary benefits between comparator groups.

The results of the subgroup analyses presented in Table 38 show that there were some differences in the direction of mean incremental effects, but high statistical uncertainty surrounds these findings. For each subgroup, as for the overall results, there was high statistical uncertainty surrounding INBs and all 95% CIs included zero.
| Subgroup | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | INB (£) (95% CI) | |
|---|---|---|---|---|
| Age (years) | 18–53 | 4246 (−2125 to 10,617) | 0.342 (−0.191 to 0.875) | 2596 (−5317 to 10,509) |
| 54–65 | 4558 (−1990 to 11,106) | 0.35 (−0.2 to 0.899) | 2434 (−5720 to 10,588) | |
| 66–73 | 1192 (−5285 to 7668) | 0.034 (−0.51 to 0.578) | −514 (−8568 to 7540) | |
| 74–100 | 1057 (−4942 to 7056) | −0.03 (−0.533 to 0.474) | −1649 (−9104 to 5806) | |
| Degree of malnutrition | None | 2094 (−1269 to 5458) | 0.125 (−0.162 to 0.411) | 400 (−3781 to 4581) |
| Moderate/severe | 6654 (−5318 to 18,626) | 0.306 (−0.723 to 1.335) | −529 (−15,589 to 14,531) | |
| APACHE II predicted risk | 0.01–0.18 | 304 (−6116 to 6724) | 0.347 (−0.187 to 0.881) | 6636 (−1259 to 14,531) |
| 0.18–0.34 | 1958 (−4391 to 8307) | 0.014 (−0.516 to 0.544) | −1679 (−9521 to 6163) | |
| 0.34–0.52 | −1810 (−8362 to 4742) | −0.198 (−0.743 to 0.348) | −2142 (−10,160 to 5876) | |
| 0.52–0.98 | 8776 (2562 to 14,991) | 0.364 (−0.155 to 0.884) | −1488 (−9170 to 6194) | |
| ICNARC model predicted risk | 0.00–0.22 | −1038 (−7359 to 5282) | 0.275 (−0.251 to 0.8) | 6529 (−1310 to 14,368) |
| 0.22–0.43 | 5481 (−867 to 11,829) | 0.197 (−0.329 to 0.723) | −1539 (−9359 to 6281) | |
| 0.43–0.65 | −246 (−6592 to 6101) | −0.119 (−0.643 to 0.404) | −2141 (−9927 to 5645) | |
| 0.65–0.99 | 5438 (−853 to 11,730) | 0.185 (−0.334 to 0.705) | −1730 (−9447 to 5987) | |
| Mechanically ventilated | No | 6324 (−1658 to 14,305) | 0.527 (−0.153 to 1.207) | 4217 (−5726 to 14,160) |
| Yes | 1762 (−1783 to 5308) | 0.074 (−0.229 to 0.376) | −292 (−4705 to 4121) | |
| Presence of cancer | No | 2421 (−908 to 5750) | 0.106 (−0.177 to 0.39) | −298 (−4439 to 3843) |
| Yes | 1016 (−12,078 to 14,109) | 0.486 (−0.628 to 1.6) | 8700 (−7597 to 24,997) | |
| Time to start of feeding | < 24 hours | 4014 (−345 to 8373) | 0.196 (−0.175 to 0.568) | −85 (−5524 to 5354) |
| ≥ 24 hours | 394 (−4614 to 5401) | 0.079 (−0.348 to 0.505) | 1181 (−5070 to 7432) | |
| Adherence adjusted | 2711 (−794 to 6217) | 0.159 (−0.14 to 0.458) | 476 (−3881 to 4833) | |
Chapter 6 Discussion and conclusions
Principal findings
Among adults with an unplanned critical care unit admission for whom early nutritional support could be provided through either the parenteral or the enteral route, there was no significant difference in mortality at 30 days according to the route of delivery. In addition, there was no significant interaction on the basis of age, degree of malnutrition, severity of illness or timing of the initiation of nutritional support. The enteral route was associated with significantly more episodes of hypoglycaemia and vomiting, but there were no significant differences between treatment groups in the duration of organ support, infectious complications, critical care unit or hospital length of stay, or duration of survival up to 1 year. The energy target of 25 kcal/kg/day was not reached in a majority of patients in each treatment group.
Providing nutritional support to critically ill adult patients via the parenteral route compared with the enteral route is unlikely to be cost-effective. At the primary end point for the cost-effectiveness analysis at 1 year post-randomisation, on average, early nutritional support via the parenteral route had higher intervention and morbidity costs, similar QALYs and a negative INB than the enteral route. Cost-effectiveness results for the pre-specified subgroups were similar to the overall results and the sensitivity analyses indicated that the conclusions were robust to alternative assumptions to those made in the base-case analysis. The lifetime analysis indicated that early nutritional support via the parenteral route had higher mean lifetime QALY at higher additional mean costs, leading to a positive INB but with a wide 95% CI that included zero.
Interpretation
The CALORIES trial was conducted in a sample of adult general critical care units in the NHS in England that had pre-existing, established protocols for nutritional support, prevention of infection and for glycaemic control – reflecting good mainstream practice. The characteristics of participating units were broadly representative (with a slight preponderance of larger units located in university hospitals unlikely to jeopardise the generalisability of our findings) and, as a pragmatic effectiveness study, probably represents the reality of current, NHS nutritional practice in critical care.
There is debate not only about the route, but also about the timing, dose, duration, delivery (continuous vs. intermittent) and type of nutritional support for critically ill patients. The aim of the CALORIES trial was to address solely the question about the optimal route. Our pragmatic trial had two major findings. First, there was no significant difference in outcomes between the two groups, other than the perhaps unsurprising increase in the incidence of vomiting and hypoglycaemia in patients receiving early nutritional support via the enteral route. Specifically, we observed neither the trend towards decreased mortality nor the increase in infectious complications previously reported for patients fed via the parenteral route. 2–4 It is possible that the lack of an infectious burden from feeding via the parenteral route is because of improvements in central venous catheter management (CALORIES data indicate a low incidence of catheter-related and bloodstream infections);60 delivery and composition of feed; and avoidance of overfeeding and hyperglycaemia.
Second, there was only a very small difference in the energy delivered. In both groups, a majority of patients did not reach caloric targets. Although enteral feeding is commonly associated with a failure to reach nutritional targets,7,8 there is a widespread assumption that the parenteral route should be more reliable in guaranteeing delivery. 61,62 Although we do not know exactly why target was not achieved in those fed via the parenteral route, we do know that participation in the CALORIES trial required units to deliver nutritional support via the parenteral route to more patients than would be their usual practice. Other possible factors include the lack of availability of feed out-of-hours (nights/weekends); the use of commercially available products with fixed energy content rather than individualised feeds (with selection of fixed energy products to under- rather than overprovide energy); interruptions in delivery to allow other critical care-related procedures to occur; and transfer out of the critical care unit for other procedures. Another contributory factor, derived from adherence data from the CALORIES trial, indicates that there was a reluctance to continue delivery via the parenteral route towards the end of the 5-day (120 hours) intervention period if this necessitated insertion of a new central venous catheter and/or commencement of a new bag of feed. Finally, there may have been clinical preference for a lower dose. The similar energy intake between the groups, however, reinforced the evaluation and interpretation of our findings on the route of delivery, unconfounded by dose.
Although there was a low probability of early nutritional support via the parenteral route being cost-effective at the primary end point of 1 year, extrapolation to the lifetime resulted in positive INB. At 1 year, the parenteral route group had higher mean survival than the enteral route group, but survival difference between the groups was not statistically significant. The lifetime analysis allowed for the non-significant gains observed in survival at 1 year but did not assume that any gains in mortality were maintained after 1 year. The projected lifetime results indicated QALY gain and higher net monetary benefits for the parenteral group compared with the enteral route group. However, considerable uncertainty surrounded the lifetime cost-effectiveness results. The results of the subgroup analysis suggested that the point estimate of the INB was positive for some subgroups, negative for others, but that the CIs around each of these estimates were wide and included zero. In interpreting these findings, it should be recognised that this study was not powered to detect whether there were subgroup by treatment interactions for either the clinical effectiveness or cost-effectiveness end points, and hence the subgroup results should be regarded as exploratory.
Strengths and limitations
The CALORIES trial is the largest trial addressing the question of the optimal route of nutritional support in critical care. In comparison, of trials included in the Canadian Clinical Practice Guidelines Committee’s meta-analysis of the use of EN versus PN, no trial published prior to the year 2000 contained more than 100 critically ill patients, and those published since 2000 contribute a total of 207 critically ill patients. 63 The CALORIES trial was rigorously conducted, with randomised treatment groups that were well balanced at baseline and with early initiation of nutritional support, as intended. Protocol compliance was high and loss to follow-up was extremely low.
Our understanding of the consequences of critical illness is much greater than when we designed this trial back in 2007. The primary outcome measure of mortality, although objective and accurate, does not recognise the other health states, in particular the consequences of muscle wasting and fatigue, experienced by many survivors of critical illness. It may also have not been sufficiently sensitive to detect meaningful differences between the groups.
Blinding of nutritional support was deemed to be impractical and, although the primary outcome was objective, some of the secondary outcomes, although defined and objectively assessed, may have been more vulnerable to observer bias. Given the very large number of participating critical care units and investigators, it seems improbable that any resulting bias could have been systematic. A relatively large number of secondary outcomes was evaluated and no formal statistical approach was taken to control for the multiple analyses; caution should therefore be taken in interpreting statistically significant results on the secondary outcomes.
Although the trial was not designed specifically to recruit malnourished patients, the small number of these patients recruited limits the relevant subgroup analysis, thus we cannot rule out a difference in outcome according to route for this group of patients. The results of the CALORIES trial should not be generalised to other types of units, other patient groups (from those studied) or other timing, dose (target) or duration of nutritional support. In addition, a large proportion of eligible but not randomised patients (28%) were excluded by the clinician, which may limit the generalisability of the results.
The CALORIES trial included the detailed measurement of resource use within a prospectively designed cost-effectiveness analysis with the collection of resource use data from index and readmissions beyond the trial intervention period. Three sources (trial case report forms, linked data from the Case Mix Programme and responses to Health Service Questionnaires) provided detailed resource use measurement for those events that were anticipated to be the key drivers of the incremental costs of early nutritional support via the parenteral and enteral routes. Costs of the interventions were calculated to represent routine NHS critical care practice and extensive sensitivity analyses were performed. Unlike previous cost-effectiveness analyses, our study has made a direct comparison of early nutritional support via the parenteral route compared with the enteral route, based on data from a large trial with projected lifetime cost-effectiveness results using appropriate methods. The study has assessed quality of life at two time points allowing comparison of changes over time and comparison with the age–gender-matched general population. Quality of life was measured with the EQ-5D-5L; this version of the instrument was anticipated to be sensitive to differences in health status between the treatment groups. Nutritional quality of life was measured by Satisfaction with Food-related Life Questionnaire; it ended up being poorly completed and thus limited the reliability of the results. To address missing data, we undertook a recommended approach for multiple imputation and imputed missing values, conditional on all the information observed.
Inevitably, assumptions – in particular, about mortality, quality of life and costs – were required to be made beyond the observed data. The cost-effectiveness analysis made maximum use of available data from the CALORIES trial and followed a recommended approach to inform these assumptions. These, and other requisite structural assumptions, were made transparent and were subjected to extensive sensitivity analyses. The cost-effectiveness analysis presented results for the same pre-specified subgroups as for the clinical effectiveness analysis.
Results in context
How do our findings compare with those of other recent trials on nutritional support in the critically ill?
In the Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically Ill Patients (EPaNIC) trial,61 which was conducted in two hospitals (involving seven different ICUs) with patients recruited to receive early or late parenteral supplementation of enteral feeding (if the enteral route alone was not meeting their nutritional target). The investigators found an association between supplemental PN delivered within 48 hours after admission (compared with supplemental PN delayed until after 8 days) and an increased number of new infectious episodes and days of mechanical ventilation. These differences were found both for the large subgroup of cardiac surgical patients and for other critically ill patients. However, the need of many patients for nutritional support, the high target energy intake and the practice of using tight glycaemic control have all been questioned, and make the generalisability of these findings to NHS critical care potentially challenging. Post hoc analysis suggested a dose–response relationship between an increased amount of parenteral supplementation and an increased rate of infectious episodes. 64 Despite important differences between the CALORIES and the EPaNIC trials (research question evaluated, patients studied, nutrition and other care practices), our results potentially support the hypothesis that among patients receiving early PN/supplementation, the dose administered may be more associated with harm than the route of delivery.
In a trial conducted at two ICUs, Heidegger et al. 62 found no difference in the rate of infection between day 8 and day 28 among patients receiving individually optimised PN to supplement inadequate enteral intake on day 4 and patients receiving EN only. In a trial conducted at 31 ICUs, Doig et al. 65 studied patients with relative contraindications to early enteral feeding and found no differences in 60-day mortality or the incidence of infection but fewer days of mechanical ventilation in patients receiving early PN than those with standard care. However, in the standard care group, 27% received early PN and 41% received no nutritional support.
There are two major and contradictory perspectives when it comes to how much to feed critically ill patients in the early phase. One perspective maintains that overfeeding is potentially harmful,61,66 a second that underfeeding is potentially harmful67–69 and a third that there is no difference between underfeeding and standard feeding. 70–72 Still others argue that any effect of nutritional support is likely to be seen only in selected patients who are at greater nutritional risk as a result either of pre-existing malnutrition, obesity or of the nature of their presenting illness. In the CALORIES trial, in both groups, the amount of nutrition delivered was below target but similar to that seen in previous studies in which nutritional targets were also commonly not met. 67,73,74 This suggests that there are substantial practical and organisational obstacles for both routes of feeding. Other research, more recently, has suggested that it is adequacy of protein intake, rather than simply energy intake, which requires to be supported in critical illness. 70
Previous economic analyses report cost savings with the use of the enteral route, rather than the parenteral route, in critically ill patients. 3,75,76 However, these results need to be interpreted with caution, as no incremental cost-effectiveness results were provided. A few economic analyses performed only cost-minimisation analysis because there were no differences in outcome between the parenteral and enteral routes. These studies have suggested that the parenteral route may significantly reduce total costs of hospital care. 65,77 A few other studies have performed economic analysis of nutritional support in critically ill patients but the treatment comparators were different from those in the CALORIES trial. For example, use of early PN compared with late PN in critically ill patients was associated with higher costs and no additional clinical benefit. 78 Early nutrition compared with standard enteral nutrition was found cost-effective for patients admitted to ICUs. 79
Compared with previous economic analyses, our analysis undertook an integrated, full economic evaluation to provide a direct comparison of early nutritional support via the parenteral route compared with the enteral route and extrapolated to lifetime cost-effectiveness results. A key advantage of the integrated economic evaluation, undertaken as part of the CALORIES trial, is that individual-level data on quality of life and resource use were collected prospectively. The quality-of-life data were collected at 90 days and at 1 year post-randomisation with the EQ-5D-5L version. 38 Hence the cost-effectiveness analysis was able to incorporate any quality-of-life differences between the treatment groups into the final measures of cost-effectiveness. The quality-of-life results also showed that, for both treatment groups and time points, patients’ average quality of life (which was between 0.65 and 0.68) was substantially lower than that for the age–gender-matched general population (approximately 0.81)59 and similar to previous estimates for general ICU survivors. 80 In the CALORIES trial, at 1 year post-randomisation, about 30–40% of responders reported ‘severe’ or ‘extreme’ problems with mobility and/or undertaking usual activities indicating substantial ongoing morbidity for this patient group.
The findings of our study support previous analyses that nutritional support via the parenteral route is more costly. The results of Doig et al.,65 that early PN is cost saving, are in contrast with both our integrated economic analysis and another recent costing analysis, by Vanderheyden et al. ,78 reporting increased treatment costs attributable to PN. However, clinical indication and population groups are different across these studies. The CALORIES trial compared costs and QALYs in critically ill adults for whom either route was indicated. Doig et al. 65 addressed the financial consequences of administering PN to patients who were unable to receive early enteral nutrition because of short-term relative contraindications and Vanderheyden et al. 78 assessed costs of early PN compared with late PN to critically ill patients who were able to receive enteral nutrition.
In drawing any comparisons between nutrition studies, it must always be noted that the CALORIES trial asked a different research question in a different population of critically ill patients to other studies. the CALORIES trial does, however, suggest that modern, early nutritional support via the parenteral route, as typically utilised in critical care units in the NHS, is neither more harmful nor more beneficial than via the enteral route and is unlikely to be cost-effective.
Implications for health care
Providing nutritional support to critically ill patients (who are typically unable to eat and therefore require artificial feeding) is an accepted fundamental element of providing critical care.
Guidelines
As outlined in Chapter 1, evidence is conflicting regarding the optimum route (parenteral or enteral) of delivery. 2–4 Interpretation of the existing, published meta-analyses of trials comparing nutritional support via the enteral and parenteral routes in critically ill patients was complicated by small sample sizes; poor methodological quality; selected groups of critically ill patients studied; lack of standardised definitions for outcome measures; and interventions combining more than one element of nutritional support, for example timing and route. CALORIES, as the largest trial addressing the question of optimal route for early nutritional support in critical care, has substantially added to, and improved, the evidence base. The results from the CALORIES trial have already been incorporated into the Canadian Clinical Practice Guidelines Committee’s meta-analysis of the use of EN versus PN63 and it is envisaged that wider incorporation into ongoing/updated meta-analyses and into national and international guidelines will continue.
Practice
The results of the CALORIES trial support the continuation of current, widespread practice in NHS critical care units of delivering early nutritional support via the enteral route as both clinically effective and cost-effective. However, they also challenge concerns about possible harm from delivering early nutritional support via the parenteral route when such delivery is clinically indicated.
Recommendations for research
Recommendation 1
Evaluation of the longer-term outcomes for patients recruited to the CALORIES trial should be extended beyond 1 year.
The CALORIES trial indicated considerable uncertainty for both the long-term survival analysis and the lifetime cost-effectiveness results. Both of these analyses made use of the maximum available observed data, with the cost-effectiveness analysis making certain plausible assumptions to project longer-term mortality, quality of life and costs. The cohort of patients recruited to the CALORIES trial provides the opportunity to obtain ethics approval for a follow-on study to obtain longer-term clinical and economic outcomes beyond 1 year – the final end point for the CALORIES trial. These data would reduce the uncertainty in the survival and cost-effectiveness evidence.
Recommendation 2
Following evaluation of the route for delivery of early nutritional support (CALORIES), a study employing rigorous consensus methods is required to establish future priorities for research on optimal nutritional support for all/groups of critically ill patients.
Nutritional support is standard for critically ill patients. The CALORIES trial delivered nutritional support, early, for 5 days, to a broadly defined patient group, and largely excluded an effect of route of feeding on clinical outcomes. Nutritional support, however, is a complex combination of other elements – timing, dose, duration, delivery and type – all of which may affect outcomes and costs. Recent findings from other contemporaneous, large-scale randomised controlled trials have led to considerable changes in the understanding of the metabolic response to critical illness and various aspects of nutritional management and support. Conflicting evidence and controversies remain regarding the optimum provision of nutritional support to critically ill patients, including timing, duration, optimal calorie and protein intake, the incidence and management of re-feeding syndrome, the role of gastric residual volume monitoring, the place of supplemental PN when enteral feeding is deemed insufficient, the role of indirect calorimetry, and potential indications for several pharmaconutrients. 81 There is a need to engage rigorous consensus methods, involving all stakeholders, to establish the future priorities for basic and clinical research in this area.
Acknowledgements
We wish to thank the NIHR Health Technology Assessment programme for funding this trial. We also wish to thank all of the patients and staff from all of the sites that participated in the trial.
A thank you also to all of the staff at ICNARC, with special thanks to Catrina Adams, Rahi Jahan, Alvin Richards-Belle, Steven Saunders and Emma Walmsley for their assistance with patient follow-up.
Research staff at participating sites
We acknowledged that there have been many other individuals who made a contribution within the participating units. It is impossible to thank everyone personally; however, we would like to thank the following research staff:
Addenbrooke’s Hospital (Jacobus Preller, Petra Polgarova); Blackpool Victoria Hospital (Jason Cupitt, Sally Baddeley); Bradford Royal Infirmary (Stephen Fletcher, Martin Northey, Linda Kenny); Bristol Royal Infirmary (Jeremy Bewley, Kathryn Pollock, Katie Sweet); Dorset County Hospital (Andy Ball, Sarah Moreton, Stephanie Jones); Furness General Hospital (Andrzej Szymczakowski, Carol McArthur, Kimberley Pallister); Kettering General Hospital (Philip Watt, Parizade Raymode, Nigel Dunk); King’s College Hospital (Philip Hopkins, Daniel Hadfield, Fiona Wade-Smith); Medway Maritime Hospital (Paul Hayden, Lucy Cooper, Claire Pegg); Musgrove Park Hospital (Richard Innes, Pippa Richards, Angela Locke, Dawn Bayford); New Cross Hospital (Shameer Gopal, Jagtar Singh Pooni, Hazel Spencer); Norfolk and Norwich University Hospital (Steve Hutchinson, Melissa Rosbergen, Georgina Glister); Northern General Hospital (Sarah Irving, Rachel Walker, Angela Pinder); Papworth Hospital (Stephen Webb, Fiona Bottrill); Poole Hospital (Henrik Reschreiter, Julie Camsooksai, Helena Barcraft-Barnes); Queen Alexandra Hospital (David Pogson, Steve Rose, Nicola Lamb, Johanna Mouland); Royal Free Hospital (Daniel Martin, Paula Meale, Sarah James); Royal Hampshire County Hospital (Arthur Goldsmith, Dawn Trodd, Jane Martin); Royal Shrewsbury Hospital (Robin Hollands, Mandy Carnahan); Royal Surrey County Hospital (Ben Creagh-Brown, Justin Kirk-Bayley, Peter Carvalho); Southampton General Hospital (Susan Tanser, Clare Bolger, Kim Golder, Karen Salmon); Southend University Hospital (David Higgins, Sarah Martin); St Mary’s Hospital, London (Richard Leonard, Verena Hauer, Adaeze Ochelli-Okpue); St Richard’s Hospital (Mike Margarson, Yolanda Baird, Justin Dickens); St Thomas’ Hospital (Richard Beale, Katie Lei, John Smith); Stafford Hospital (Moses Chikungwa, Garud Chandan, Clare Jackson); The Ipswich Hospital (Robert Lewis, Stephanie Bell, Heather Blaylock); The Princess Alexandra Hospital (Dev Dutta, Jackie Power, Carol Keel); The Queen Elizabeth Hospital, King’s Lynn (Parvez Moondi, Kate Wong, Katharine Draper); The Royal Blackburn Hospital (Anton Krige, Lynne Bullock, Donna Harrison-Briggs, Martin Bland); University College Hospital (David Brealey, Jung Ryu, Georgia Bercades); University Hospital Lewisham (Marthin Mostert, Clair Harris, David Grannell); and University Hospital of North Staffordshire (Bryan Carr, Ruth Salt, Claire Matthews).
Contributions of authors
Dr Sheila E Harvey (CTU Manager and Senior Research Fellow) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Francesca Parrott (Statistician) contributed to the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Dr David A Harrison (Senior Statistician and Honorary Senior Lecturer in Medical Statistics) contributed to the design of the trial, the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Dr M Zia Sadique (Lecturer in Health Economics) contributed to the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Professor Richard D Grieve (Professor of Health Economics Methodology) contributed to the design of the trial, the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Ruth R Canter (Research Assistant) assisted in the management of the trial, contributed to the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Blair KP McLennan (Trial Manager) managed the trial, contributed to the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Jermaine CK Tan (Trials Data Manager) contributed to the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Ms Danielle E Bear (Principal Critical Care Dietitian) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Ms Ella Segaran (Advanced Dietitian for Critical Care) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Dr Richard Beale (Clinical Director/Consultant, Intensive Care Unit) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Professor Geoff Bellingan (Divisional Clinical Director/Consultant, Critical Care and Theatres) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Dr Richard Leonard (Clinical Director/Consultant, Intensive Care Unit) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Professor Michael G Mythen (Professor of Anaesthesia & Critical Care) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Professor Kathryn M Rowan (Director of Scientific & Strategic Development/CTU Director and Honorary Professor) conceived and designed the trial, contributed to acquisition, analysis and interpretation of the data, and drafted and critically revised the manuscript.
Publications
Harvey SE, Parrott F, Harrison DA, Mythen M, Rowan KM. The CALORIES trial: statistical analysis plan. Crit Care Resusc 2014;16:248–54.
Harvey SE, Parrott F, Harrison DA, et al. Trial of the route of early nutritional support in critically ill adults. N Engl J Med 2014;371:1673–84.
Trial Management Team
Kimberley Anderson (previous Research Assistant); Ruth Canter (Research Assistant); Louise Clement (previous Trial Manager); Sarah Corlett (previous Trial Administrator); Dr David Harrison (Senior Statistician); Dr Sheila Harvey (CTU Manager/Senior Research Fellow); Blair McLennan (Trial Manager); Hannah Muskett (previous Assistant Trial Manager); Francesca Parrott (Trial Statistician); Krishna Patel (previous Trial Statistician); Professor Kathryn Rowan (Chief Investigator); Dr Rachael Scott (previous Senior Trial Manager); and Jermaine Tan (Data Manager).
Trial Management Group
Professor Richard Beale (Co-Investigator); Danielle Bear (Trial Dietitian); Geoff Bellingan (Co-Investigator); Dr David Harrison (Senior Statistician); Dr Sheila Harvey (CTU Manager/Senior Research Fellow); Dr Richard Leonard (Co-Investigator); Professor Michael Mythen (Lead Clinical Investigator); Professor Kathryn Rowan (Chief Investigator); and Ella Segaran (Trial Dietitian).
Trial Steering Committee
Professor Michael Stroud (independent, chairperson); Professor Peter Emery (independent); Professor Alastair Forbes (Co-Investigator); Peter Gibb (independent, patient representative); Dr George Grimble (Co-Investigator, observer); Carys Jones (independent); Professor Hugh Montgomery (Co-Investigator); Janet Myers (previous independent, patient representative); Dr Dewi Williams (independent); Professor Michael Mythen (Lead Clinical Investigator); and Professor Kathryn Rowan (Chief Investigator).
Data Monitoring and Ethics Committee
Dr Elizabeth Allen (chairperson), Professor Peter Andrews and Professor Steve Webb.
Data sharing statement
Data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- National Institute for Health and Care Excellence . Nutrition Support in Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition 2006. www.nice.org.uk/guidance/cg32 (accessed 6 August 2014).
- Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr 2003;27:355-73. http://dx.doi.org/10.1177/0148607103027005355.
- Gramlich L, Kichian K, Pinilla J, Rodych NJ, Dhaliwal R, Heyland DK. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition 2004;20:843-8. http://dx.doi.org/10.1016/j.nut.2004.06.003.
- Simpson F, Doig GS. Parenteral vs. enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intensive Care Med 2005;31:12-23. http://dx.doi.org/10.1007/s00134-004-2511-2.
- Martindale RG, McClave SA, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med 2009;37:1757-61. http://dx.doi.org/10.1097/CCM.0b013e3181a40116.
- Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, et al. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr 2009;28:387-400. http://dx.doi.org/10.1016/j.clnu.2009.04.024.
- De Jonghe B, Appere-De-Vechi C, Fournier M, Tran B, Merrer J, Melchior JC, et al. A prospective survey of nutritional support practices in intensive care unit patients: what is prescribed? What is delivered?. Crit Care Med 2001;29:8-12. http://dx.doi.org/10.1097/00003246-200101000-00002.
- Engel JM, Muhling J, Junger A, Menges T, Karcher B, Hempelmann G. Enteral nutrition practice in a surgical intensive care unit: what proportion of energy expenditure is delivered enterally?. Clin Nutr 2003;22:187-92. http://dx.doi.org/10.1054/clnu.2002.0622.
- Boitano M, Bojak S, McCloskey S, McCaul DS, McDonough M. Improving the safety and effectiveness of parenteral nutrition: results of a quality improvement collaboration. Nutr Clin Pract 2010;25:663-71. http://dx.doi.org/10.1177/0884533610385349.
- Naylor CJ, Griffiths RD, Fernandez RS. Does a multidisciplinary total parenteral nutrition team improve patient outcomes? A systematic review. JPEN J Parenter Enteral Nutr 2004;28:251-8. http://dx.doi.org/10.1177/0148607104028004251.
- Pritchard C, Duffy S, Edington J, Pang F. Enteral nutrition and oral nutrition supplements: a review of the economics literature. JPEN J Parenter Enteral Nutr 2006;30:52-9. http://dx.doi.org/10.1177/014860710603000152.
- Radrizzani D, Bertolini G, Facchini R, Simini B, Bruzzone P, Zanforlin G, et al. Early enteral immunonutrition vs. parenteral nutrition in critically ill patients without severe sepsis: a randomized clinical trial. Intensive Care Med 2006;32:1191-8. http://dx.doi.org/10.1007/s00134-006-0238-y.
- Bertolini G, Iapichino G, Radrizzani D, Facchini R, Simini B, Bruzzone P, et al. Early enteral immunonutrition in patients with severe sepsis: results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med 2003;29:834-40.
- Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med 1995;152:1545-8. http://dx.doi.org/10.1164/ajrccm.152.5.7582291.
- Adams S, Dellinger EP, Wertz MJ, Oreskovich MR, Simonowitz D, Johansen K. Enteral versus parenteral nutritional support following laparotomy for trauma: a randomized prospective trial. J Trauma 1986;26:882-91. http://dx.doi.org/10.1097/00005373-198610000-00004.
- Borzotta AP, Pennings J, Papasadero B, Paxton J, Mardesic S, Borzotta R, et al. Enteral versus parenteral nutrition after severe closed head injury. J Trauma 1994;37:459-68. http://dx.doi.org/10.1097/00005373-199409000-00022.
- Cerra FB, McPherson JP, Konstantinides FN, Konstantinides NN, Teasley KM. Enteral nutrition does not prevent multiple organ failure syndrome (MOFS) after sepsis. Surgery 1988;104:727-33.
- Dunham CM, Frankenfield D, Belzberg H, Wiles C, Cushing B, Grant Z. Gut failure: predictor of or contributor to mortality in mechanically ventilated blunt trauma patients?. J Trauma 1994;37:30-4. http://dx.doi.org/10.1097/00005373-199407000-00007.
- Gianotti L, Braga M, Vignali A, Balzano G, Zerbi A, Bisagni P, et al. Effect of route of delivery and formulation of postoperative nutritional support in patients undergoing major operations for malignant neoplasms. Arch Surg 1997;132:1222-9. http://dx.doi.org/10.1001/archsurg.1997.01430350072012.
- Kalfarentzos F, Kehagias J, Mead N, Kokkinis K, Gogos CA. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg 1997;84:1665-9. http://dx.doi.org/10.1002/bjs.1800841207.
- Kudsk KA, Minard G, Wojtysiak SL, Croce M, Fabian T, Brown RO. Visceral protein response to enteral versus parenteral nutrition and sepsis in patients with trauma. Surgery 1994;116:516-23.
- Rapp RP, Young B, Twyman D, Bivins BA, Haack D, Tibbs PA, et al. The favorable effect of early parenteral feeding on survival in head-injured patients. J Neurosurg 1983;58:906-12. http://dx.doi.org/10.3171/jns.1983.58.6.0906.
- Reynolds JV, Kanwar S, Welsh FK, Windsor AC, Murchan P, Barclay GR, et al. 1997 Harry M. Vars Research Award. Does the route of feeding modify gut barrier function and clinical outcome in patients after major upper gastrointestinal surgery?. JPEN J Parenter Enteral Nutr 1997;21:196-201. http://dx.doi.org/10.1177/0148607197021004196.
- Woodcock NP, Zeigler D, Palmer MD, Buckley P, Mitchell CJ, MacFie J. Enteral versus parenteral nutrition: a pragmatic study. Nutrition 2001;17:1-12. http://dx.doi.org/10.1016/S0899-9007(00)00576-1.
- Rayes N, Hansen S, Seehofer D, Muller AR, Serke S, Bengmark S, et al. Early enteral supply of fiber and Lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition 2002;18:609-15. http://dx.doi.org/10.1016/S0899-9007(02)00811-0.
- Harrison DA, Brady AR, Rowan K. Case mix, outcome and length of stay for admissions to adult, general critical care units in England, Wales and Northern Ireland: the Intensive Care National Audit & Research Centre Case Mix Programme Database. Crit Care 2004;8:R99-111. http://dx.doi.org/10.1186/cc2834.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Saving Lives: Reducing Infection, Delivering Clean and Safe Care. DH: Central Venous Catheter Care Bundle; 2007.
- Saving Lives: Reducing Infection, Delivering Clean and Safe Care. DH: Care Bundle for Ventilated Patients; 2007.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. http://dx.doi.org/10.1097/CCM.0b013e31827e83af.
- Reducing the Harm Caused by Misplaced Nasogastric Feeding Tubes – Patient Safety Alert. National Patient Safety Agency; 2005.
- How to Confirm the Correct Position of Nasogastric Feeding Tubes in Infants, Children and Adults – Interim advice for Healthcare Staff. National Patient Safety Agency; 2005.
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. http://dx.doi.org/10.1007/BF01709751.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. http://dx.doi.org/10.1097/00003246-198510000-00009.
- Harrison DA, Parry GJ, Carpenter JR, Short A, Rowan K. A new risk prediction model for critical care: the Intensive Care National Audit & Research Centre (ICNARC) model. Crit Care Med 2007;35:1091-8. http://dx.doi.org/10.1097/01.CCM.0000259468.24532.44.
- Critical Care Minimum Data Set Full Specification, Version 8. Information Standards Board for Health and Social Care; 2010.
- Edwards PJ, Roberts I, Clarke MJ, Diguiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.mr000008.pub4.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. http://dx.doi.org/10.1007/s11136-011-9903-x.
- Grunert KG, Dean M, Raats MM, Nielsen NA, Lumbers M. A measure of satisfaction with food-related life. Appetite 2007;49:486-93. http://dx.doi.org/10.1016/j.appet.2007.03.010.
- Harvey SE, Elbourne D, Ashcroft J, Jones CM, Rowan K. Informed consent in clinical trials in critical care: experience from the PAC-Man Study. Intensive Care Med 2006;32:2020-5. http://dx.doi.org/10.1007/s00134-006-0358-4.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. http://dx.doi.org/10.1002/sim.4067.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: J Wiley & Sons; 1987.
- Harvey SE, Parrott F, Harrison DA, Mythen M, Rowan KM. The CALORIES trial: statistical analysis plan. Crit Care Resusc 2014;16:248-54.
- Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed?. BMJ 2000;320:1197-200. http://dx.doi.org/10.1136/bmj.320.7243.1197.
- Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol 2000;29:722-9. http://dx.doi.org/10.1093/ije/29.4.722.
- Fischer K, Goetghebeur E, Vrijens B, White IR. A structural mean model to allow for noncompliance in a randomized trial comparing 2 active treatments. Biostatistics 2011;12:247-57. http://dx.doi.org/10.1093/biostatistics/kxq053.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Critical Care Minimum Data Set. Leeds: HSCIC; 2012.
- Harrison DA, D’Amico G, Singer M. Case mix, outcome, and activity for admissions to UK critical care units with severe acute pancreatitis: a secondary analysis of the ICNARC Case Mix Programme Database. Crit Care 2007;11. http://dx.doi.org/10.1186/cc5682.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- NHS Reference Costs 2012–13. London: DH; 2013.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2012.
- Van Hout B, Devlin N. An EQ-5D-5L Value Set for England: Final Model Results. London: Office of Health Economics; 2014.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. http://dx.doi.org/10.1002/hec.843.
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials: extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making 2013;33:743-54. http://dx.doi.org/10.1177/0272989X12472398.
- Interim Life Tables, 2008–2010. Newport: ONS; 2011.
- Soares MO, Welton NJ, Harrison DA, Peura P, Shankar-Hari M, Harvey SE, et al. An evaluation of the feasibility, cost and value of information of a multicentre randomised controlled trial of intravenous immunoglobulin for sepsis (severe sepsis and septic shock): incorporating a systematic review, meta-analysis and value of information analysis. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16070.
- Ara R, Brazier JE. populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Bion J, Richardson A, Hibbert P, Beer J, Abrusci T, McCutcheon M, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf 2013;22:110-23. http://dx.doi.org/10.1136/bmjqs-2012-001325.
- Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 2011;365:506-17. http://dx.doi.org/10.1056/NEJMoa1102662.
- Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet 2013;381:385-93. http://dx.doi.org/10.1016/S0140-6736(12)61351-8.
- Canadian Clinical Practice Guidelines Committee . 2015 Canadian Clinical Practice Guidelines. 1.0. The Use of Enteral Nutrition Vs. Parenteral Nutrition 2015. www.criticalcarenutrition.com/index.php?option=com_content&view=article&id=337:2015cpgintro&catid=25:cpgs2015&Itemid=109 (accessed 1 June 2015).
- Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med 2013;187:247-55. http://dx.doi.org/10.1164/rccm.201206-0999OC.
- Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA 2013;309:2130-8. http://dx.doi.org/10.1001/jama.2013.5124.
- Casaer MP, Wilmer A, Van den Berghe G. Supplemental parenteral nutrition in critically ill patients. Lancet 2013;381. http://dx.doi.org/10.1016/S0140-6736(13)61068-5.
- Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med 2009;35:1728-37. http://dx.doi.org/10.1007/s00134-009-1567-4.
- Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux RNM, Delarue J, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr 2005;24:502-9. http://dx.doi.org/10.1016/j.clnu.2005.03.006.
- Dvir D, Cohen J, Singer P. Computerized energy balance and complications in critically ill patients: an observational study. Clin Nutr 2006;25:37-44. http://dx.doi.org/10.1016/j.clnu.2005.10.010.
- Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, et al. Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. N Engl J Med 2015;372:2398-408. http://dx.doi.org/10.1056/NEJMoa1502826.
- Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, Sakkijha MH, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr 2011;93:569-77. http://dx.doi.org/10.3945/ajcn.110.005074.
- Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med 2011;39:967-74. http://dx.doi.org/10.1097/CCM.0b013e31820a905a.
- Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care 2011;15. http://dx.doi.org/10.1186/cc10546.
- Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland DK. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the ‘modified NUTRIC’ nutritional risk assessment tool. Clin Nutr 2015;35:158-62. http://dx.doi.org/10.1016/j.clnu.2015.01.015.
- Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Di Carlo V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med 2001;29:242-8. http://dx.doi.org/10.1097/00003246-200102000-00003.
- Abunnaja S, Cuviello A, Sanchez JA. Enteral and parenteral nutrition in the perioperative period: state of the art. Nutrients 2013;5:608-23. http://dx.doi.org/10.3390/nu5020608.
- Louie BE, Noseworthy T, Hailey D, Gramlich LM, Jacobs P, Warnock GL. 2004 MacLean-Mueller prize enteral or parenteral nutrition for severe pancreatitis: a randomized controlled trial and health technology assessment. Can J Surg 2005;48:298-306.
- Vanderheyden S, Casaer MP, Kesteloot K, Simoens S, De Rijdt T, Peers G, et al. Early versus late parenteral nutrition in ICU patients: cost analysis of the EPaNIC trial. Crit Care 2012;16. http://dx.doi.org/10.1186/cc11361.
- Doig GS, Chevrou-Severac H, Simpson F. Early enteral nutrition in critical illness: a full economic analysis using US costs. Clinicoecon Outcomes Res 2013;5:429-36. http://dx.doi.org/10.2147/CEOR.S50722.
- Cuthbertson BH, Scott J, Strachan M, Kilonzo M, Vale L. Quality of life before and after intensive care. Anaesthesia 2005;60:332-9. http://dx.doi.org/10.1111/j.1365-2044.2004.04109.x.
- Preiser JC, van Zanten AR, Berger MM, Biolo G, Casaer MP, Doig GS, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care 2015;19. http://dx.doi.org/10.1186/s13054-015-0737-8.
Appendix 1 Patient information sheet
Appendix 2 Case report form
Appendix 3 Severity of illness scores
Acute Physiology and Chronic Health Evaluation version II
The APACHE II Acute Physiology score consists of weightings for 12 physiological parameters to give a total score ranging from 0 to 60, with higher scores indicating greater severity of illness. 34 The 12 physiological parameters are as follows:
-
temperature
-
mean arterial pressure
-
heart rate
-
respiratory rate
-
alveolar–arterial gradient (if FiO2 ≥ 0.5) or PaO2 (if FiO2 < 0.5)
-
arterial pH (or serum bicarbonate if no arterial blood gas recorded)
-
serum sodium
-
serum potassium
-
serum creatinine (with double weighting for acute renal failure)
-
haematocrit (estimated from haemoglobin)
-
white blood cell count, and
-
GCS score (assumed to be normal for patients sedated or paralysed).
The APACHE II Score comprises the Acute Physiology score plus additional weightings for age and severe comorbidities in the past medical history to give a total score ranging from 0 to 71. Severe comorbidities must have been present and documented in the past medical history within the 6 months prior to presentation at hospital and are defined as follows:
-
Severe liver condition – presence of portal hypertension, biopsy-proven cirrhosis or hepatic encephalopathy.
-
Severe cardiovascular condition – presence of fatigue, claudication, dyspnoea or angina at rest (New York Heart Association Functional Class IV).
-
Severe respiratory condition – presence of permanent shortness of breath with light activity as a result of pulmonary disease, or on home ventilation.
-
Severe renal condition – receiving chronic renal replacement therapy (haemodialysis, haemofiltration or peritoneal dialysis) for irreversible end-stage renal disease.
-
Immunological condition – receiving chemotherapy, radiotherapy or daily high-dose steroid treatment (0.3 mg/kg, or greater, prednisolone or equivalent) for 6 months, or diagnosis of human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), lymphoma, acute or chronic myelogenous/lymphocytic leukaemia, multiple myeloma or active metastatic disease.
The APACHE II predicted risk of death combines the APACHE II Score with additional weightings for admission following emergency surgery and for diagnostic categories from the primary reason for admission to the critical care unit to estimate the predicted risk of death before ultimate discharge from an acute hospital.
The APACHE II severity of illness scores and predicted risk of mortality were calculated from raw data using standardised computer algorithms. Severity of illness scores were based on the most extreme (highest or lowest) values of physiological parameters recorded during the first 24 hours following admission to the critical care unit. Coefficients for the APACHE II risk prediction model were taken from the most recent (2013) UK recalibration of the model, based on 242,450 admissions to 207 UK ICUs participating in the Case Mix Programme between 1 January 2011 and 31 December 2012.
Intensive Care National Audit & Research Centre model
The ICNARC Physiology Score consists of objectively defined weightings for 12 physiological parameters to give a total score ranging from 0 to 100, with higher scores indicating greater severity of illness. 35 The 12 physiological parameters are as follows:
-
heart rate
-
systolic blood pressure
-
temperature
-
respiratory rate
-
PaO2/FiO2 ratio (weighted differently, depending on whether the patient was ventilated at any time during the first 24 hours in the unit, or the entire stay if < 24 hours)
-
arterial pH
-
serum urea
-
serum creatinine
-
serum sodium
-
urine output
-
white blood cell count, and
-
GCS score (plus additional weightings for patients sedated or paralysed and sedated for the whole of the first 24 hours in the unit, or the entire stay if < 24 hours).
The ICNARC model predicted risk of death combines the ICNARC Physiology Score with additional weightings for age, cardiopulmonary resuscitation within 24 hours prior to admission to the critical care unit, location prior to admission to the critical care unit, urgency of surgery (for admissions from theatre), primary reason for admission to the critical care unit, and interactions between the Physiology Score and primary reason for admission to estimate the predicted risk of death before ultimate discharge from an acute hospital.
The ICNARC Physiology Score and predicted risk of mortality were calculated from raw data using standardised computer algorithms. The Physiology Score was based on the most extreme (highest or lowest) values of physiological parameters recorded during the first 24 hours following admission to the critical care unit. Coefficients for the ICNARC risk prediction model were taken from the most recent (2013) UK recalibration of the model based on 242,450 admissions to 207 UK ICUs participating in the Case Mix Programme between 1 January 2011 and 31 December 2012.
Sequential Organ Failure Assessment
The SOFA score consists of weightings for six organ systems to give a total score ranging from 0 to 24, with higher scores indicating a greater degree of organ failure. 33 The organ failure assessments are as follows:
-
respiratory dysfunction, based on lowest PaO2/FiO2
-
cardiovascular dysfunction, based on vasopressor use and lowest mean arterial pressure
-
renal dysfunction, based on highest creatinine
-
neurological dysfunction, based on lowest (or last pre-sedation) GCS score
-
hepatic dysfunction, based on highest bilirubin, and
-
coagulation dysfunction, based on lowest platelet count.
The SOFA score was calculated from raw physiology and treatment data from the 24 hours prior to randomisation.
Appendix 4 Critical Care Minimum Dataset
Definitions
Duration of organ support in the critical care unit was defined as the number of days alive and free from support of each of the following organ systems, as defined by the UK Department of Health CCMDS during the first 30 days following randomisation. 36 Patients who died within the first 30 days were assigned zero days alive and free from organ support. Organ support definitions were as follows:
-
Advanced respiratory – indicated by one or more of invasive mechanical ventilatory support through a translaryngeal tube or tracheostomy; bilevel positive airway pressure through a translaryngeal tube or tracheostomy; continuous positive airway pressure through a translaryngeal tube; or extracorporeal respiratory support.
-
Advanced cardiovascular – indicated by one or more of receipt of multiple intravenous and/or rhythm controlling drugs (of which at least one must be vasoactive) when used simultaneously to support or control arterial pressure, cardiac output or organ/tissue perfusion; continuous observation of cardiac output and derived indices; an intra-aortic balloon pump or other assist device; or temporary cardiac pacemaker.
-
Renal – indicated by receipt of acute renal replacement therapy (e.g. haemodialysis, hemofiltration, etc.); or receipt of renal replacement therapy for chronic renal failure when other acute organ support is received.
-
Neurological – indicated by one or more of central nervous system depression that was sufficient to prejudice the airway and protective reflexes (except when caused by sedation prescribed to facilitate mechanical ventilation or by poisoning, e.g. deliberate or accidental self-administered overdose, alcohol, drugs, etc.); receipt of invasive neurological monitoring or treatment (e.g. intracranial pressure monitoring, jugular bulb sampling, external ventricular drain, etc.); receipt of continuous intravenous medication to control seizures and/or for continuous cerebral monitoring; or receipt of therapeutic hypothermia using cooling protocols or devices.
-
Gastrointestinal – indicated by receipt of PN or EN (i.e. any method of feeding other than normal oral intake).
Appendix 5 Patient follow-up cover letter
Appendix 6 Patient follow-up questionnaire
List of abbreviations
- AIC
- Akaike information criterion
- APACHE II
- Acute Physiology and Chronic Health Evaluation version II
- BIC
- Bayesian information criterion
- BMI
- body mass index
- BNF
- British National Formulary
- CCMDS
- Critical Care Minimum Dataset
- CI
- confidence interval
- CLRN
- Comprehensive Local Research Network
- CRN
- Clinical Research Network
- CTU
- clinical trials unit
- DMEC
- Data Monitoring and Ethics Committee
- EN
- enteral nutrition
- EQ-5D-5L
- EuroQol 5-dimension (5-level version) questionnaire
- FiO2
- fraction of inspired oxygen
- GCS
- Glasgow Coma Scale
- GP
- general practitioner
- HRG
- Healthcare Resource Group
- HSCIC
- Health and Social Care Information Centre
- ICNARC
- Intensive Care National Audit & Research Centre
- ICU
- intensive care unit
- INB
- incremental net benefit
- IQR
- interquartile range
- ISRCTN
- International Standard Randomised Controlled Trial Number
- i.v.
- intravenous
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PaO2
- arterial oxygen pressure
- PI
- principal investigator
- PN
- parenteral nutrition
- QALY
- quality-adjusted life-year
- SD
- standard deviation
- SOFA
- Sequential Organ Failure Assessment
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- WTE
- whole-time equivalent