Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/151/03. The contractual start date was in January 2015. The draft report began editorial review in May 2015 and was accepted for publication in December 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Fleeman et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Respiratory problems are one of the most common causes of morbidity in preterm infants,1 that is infants born before 37 completed weeks of gestation. Respiratory distress syndrome (RDS), also known as hyaline membrane disease, is a serious medical condition in which the lungs of a newborn baby lack surfactant and are not functioning at a level that is able to provide their body with enough oxygen. 2–4 It is a particular problem for preterm infants, as surfactant is usually produced between weeks 24 and 28 of pregnancy. European data for 2010 show an incidence of RDS of 92% at 24–25 weeks’ gestation, 88% at 26–27 weeks’ gestation, 76% at 28–29 weeks’ gestation and 57% at 30–31 weeks’ gestation. 4 The proportion of infants with RDS has been reported to fall to around one-tenth of those born at 34 weeks’ gestation2 (although the proportion with ‘respiratory problems’ at weeks 34 to 36 may be around three times higher).
Clinically, RDS presents with early respiratory distress comprising cyanosis, grunting, inter- and subcostal retractions and tachypnoea, and if left untreated it may result in death from progressive hypoxia and respiratory failure. 4 Consequences of RDS include:3
-
hypoxia, acidosis, hypothermia and hypotension
-
bronchopulmonary dysplasia (BPD) also commonly known as chronic lung disease
-
pulmonary haemorrhage
-
apnoea of prematurity/bradycardia
-
intraventricular haemorrhage (IVH).
Advances in care over the years have, however, resulted in significant decreases in mortality from RDS. 4,5 Although data on RDS mortality are not routinely collected in the UK, data from the USA show it has fallen from 2.89 per 1000 live births between 1969 and 19736 (or 2.6 per 1000 live births in 19707) to 0.37 per 1000 live births between 1987 and 19958 (or 0.4 per 1000 live births in 19947). This decrease in RDS is also reflected by a decrease in mortality from all causes as reported by a number of worldwide studies. 9
Epidemiology
According to the UK Office for National Statistics,10 there were 729,312 live births in England and Wales in 2012 and the gestational age was known and verified for 726,572 infants. Of these, 52,909 (7.3%) were born preterm, prior to 37 weeks. The majority (43,993, 83.1%) were born between 32 and 36 weeks, with 5693 (10.8%) born between 28 and 31 weeks, 2474 (4.7%) born between 24 and 27 weeks and 749 (1.4%) born before 24 weeks.
Birthweight is associated with gestational age. In England and Wales in 2012,10 the vast majority of infants born before 24 weeks or those born between 24 and 27 weeks weighed under 1500 g (99.5% and 96.2%, respectively). At between 28 and 31 weeks, 85.6% weighed 1000–2499 g and between 32 and 36 weeks 96.7% of those born weighed 1500–3999 g. 10
Infant mortality is associated with gestational age and birthweight, decreasing with advanced gestational age and increasing birthweight (Table 1). 10
| Gestational age | All | Birthweight (g) | ||||
|---|---|---|---|---|---|---|
| < 1000 | 1000–1499 | 1500–2499 | 2500–3999 | ≥ 4000 | ||
| All infants with known and verified gestational age | 3.9 | 316.6 | 55.9 | 9.3 | 1.3 | 0.9 |
| < 24 weeks | 877.2 | 885.1 | – | – | – | – |
| 24–27 weeks | 230.8 | 267.9 | 131.5 | 212.1 | – | – |
| 28–31 weeks | 48.3 | 110.7 | 49.3 | 28.2 | 20.0 | – |
| 32–36 weeks | 8.8 | 61.1 | 40.7 | 8.7 | 5.6 | – |
| Preterm to term | 23.6 | 215.9 | 56.4 | 10.4 | 5.7 | 13.7 |
| Term | 1.4 | 9.6 | 35.3 | 7.8 | 1.2 | 0.8 |
| Term to post-term | 0.9 | – | – | 27.8 | 0.6 | 1.0 |
Current treatment options for preterm infants
Over the years, several modalities for respiratory support have been developed. The treatments which have arguably had the largest impact in reducing mortality are the administration of surfactant5,7 and antenatal corticosteroids. 11 Improved methods of mechanical ventilation, regionalised perinatal care and continuous improvement in general neonatal care have also been highlighted as having an important impact, particularly in the period between 1970 and 1985, prior to the use of surfactant therapy in the 1990s. 5,7 Recently updated European Consensus Guidelines for the management of RDS in preterm infants4 highlight that, in many instances, the risk of a preterm birth is known and this should enable preterm infants at risk of RDS to be born in centres where appropriate facilities are available for stabilisation and ongoing respiratory support, including intubation and mechanical ventilation, following birth.
Once born, preterm infants require stabilisation. In practice, preterm infants who present with early respiratory distress may receive any one of the following interventions (described in more detail in the following sections):
-
mechanical endotracheal ventilation
-
nasal continuous positive airway pressure (NCPAP)
-
oxygen
-
nasal intermittent positive-pressure ventilation (NIPPV)
-
heated humidified high-flow nasal cannula (HHHFNC).
Mechanical endotracheal ventilation
Mechanical endotracheal ventilation assists breathing invasively via an endotracheal tube. This process is commonly referred to as intubation and was first introduced in the late 1950s. 5 Although this has increased survival, lung injury has been recognised as an associated complication. 5 Lung injury in the short term can lead to an air leak. 12 Air leaks and increased pressures used to ventilate infants may result in pneumothorax, pneumomediastinum and pneumopericardium. 3 Lung injury in the longer term may result in BPD. 1,12,13 Largely for these reasons, the European Consensus Guidelines4 recommend ventilation ‘for as short a time as possible’ for extremely preterm infants if antenatal steroids have not been given to the mother and also for infants who have not responded to NCPAP.
Nasal continuous positive airway pressure
Devices which generate NCPAP can broadly be divided into two categories: continuous flow or variable-flow devices. 14,15 Continuous flow devices include conventional ventilators, jet ventilation systems and bubble NCPAP. 14 Common features of all NCPAP devices are:12
-
a gas source, which provides a continuous supply of air and/or oxygen
-
a pressure generator, which creates positive pressure in the circuit
-
a patient interface, which connects the NCPAP circuit to the infant’s airway.
The most commonly used interfaces between the NCPAP circuit and the preterm infant are nasal prongs and/or nasal masks. 2,15 The results of a meta-analysis16 have shown that binasal prongs are more effective in preventing reintubation compared with either single nasal or nasopharyngeal prongs. Although there is evidence from meta-analyses that NCPAP may be more effective than head-box oxygen for reducing the incidence of respiratory failure (apnoea, respiratory acidosis and increased oxygen requirements) and the need for reintubation,17 there is no reliable evidence to suggest one NCPAP device is optimal over another NCPAP device.
Difficulties with the successful application of NCPAP are principally related to the relatively bulky interface with the infant, which can result in problems maintaining proper position. 15 If leaks around the nares and via the mouth occur, these can result in inconsistent airway pressure generation and respiratory instability with increased oxygen requirements. 15 In particular, the bulky nature of most NCPAP interfaces can predispose to nasal irritation and trauma,15,18 can restrict access to the head and face and have significant drawbacks with respect to integration of NCPAP with oral feeding. 19 Furthermore, face masks and standard nasal cannula associated with the prongs are uncomfortable and can cause irritation because of the use of dry, cold gas. 20 Finally, common to all variable-flow NCPAP systems is a significant noise level; it is currently unknown what effect the continuous exposure to such levels of noise has on the development of preterm infants. 12
Oxygen
Oxygen is the most widely used therapy in neonatology. 21 Aside from NCPAP, it may be administered via head-box, incubator or low-flow nasal cannula. The European Consensus Guidelines4 recommend a concentration of 21–30% oxygen to initiate stabilisation at resuscitation. Thereafter, in the neonatal intensive care unit (NICU) setting oxygen concentrations are closely monitored using oxygen saturation probes and targeting a narrow range of saturations to minimise effects of oxygen toxicity or hypoxia. As with ventilation, oxygen may lead to lung injury and the same short- and long-term effects.
Nasal intermittent positive-pressure ventilation
Nasal intermittent positive-pressure ventilation is a development in non-invasive ventilatory support, combining NCPAP with superimposed ventilator breathing at a set peak pressure. 12 NIPPV provides intermittent mandatory ventilation using nasal prongs22 and may be synchronised or non-synchronised NIPPV to the infant’s breathing efforts. 23 NIPPV has been reported to achieve better gaseous exchange than simple oxygen therapy, but has also been associated with significant head moulding, cerebral haemorrhage and gastric perforations. 24 Other complications related to nasal ventilation have been reported to be ‘essentially the same’ as those for infants on NCPAP. 25 Synchronised NIPPV is argued to be preferable over NIPPV in order to minimise gastrointestinal perforations. 25
The technology: heated humidified high-flow nasal cannula
A number of differently branded HHHFNC devices exist including the Vapotherm 2000i (Vapotherm Inc., Stevensville, MD, USA) and the Fisher & Paykel Healthcare (Auckland, New Zealand, and Irvine, CA, USA) devices. Three main features are common to any HHHFNC device:15
-
a respiratory circuit with a means to maintain the temperature and, by extension, the humidity of the delivered gas until the distal end of the circuit
-
a humidifier to effectively warm and humidify respiratory gases
-
a nasal cannula with adapter that connects to the delivery circuit and which should allow little or no excess tubing between the end of the delivery circuit and the actual nasal prongs, thereby minimising further any potential for gas cooling and precipitation.
In addition to HHHFNC, variations of this technology exist in which gas flow is provided at a high rate but not heated [high-flow nasal cannula (HFNC)]. Unheated gas cannot be adequately humidified even if it passes through a humidifier. 26
With regard to gas flow rate, no optimal level exists. 15 One early study reported that the flow rate should vary from infant to infant depending on weight. 27 It has also been stated that gas flow rate should be adjusted according to clinical response, generally being increased for increasing respiratory distress or oxygen requirement and decreased for improving respiratory distress or decreasing oxygen requirement. 15 Unlike the nasal prongs for NCPAP (which fit tightly in the nares), the nasal cannulae for HHHFNC are smaller and looser fitting. Nasal cannulae size varies from infant to infant, this being dictated by the size of the infant’s nares. 18,20
The HHHFNC is gaining popularity and is increasingly used in clinical practice in many units in the UK and other countries, particularly in North America and Australasia. 28 This is largely because of the perceived greater ease of use of such devices compared with NCPAP, allowing both practitioners and family members to handle and care for infants more easily. 15,20,29 In addition, it is considered that HHHFNC should improve patient tolerance and outcomes: heat and humidity should prevent airway water loss, airway cooling, thickened secretions and nasal irritation, allowing high-flow rates without nasal drying or bleeding while the comparably lighter and easier-to-apply interface may lessen nasal septal damage. 15,20 Other perceived advantages compared with NCPAP include a reduction in the number of ventilator days, an improvement in weight gain and being able to introduce oral feeding earlier. 18,20
However, there are concerns about the unpredictability of the positive airway pressures generated by HHHFNC and the potential for infection. Unless the infant’s mouth is closed and the leak around the nares minimised, it is unlikely that nasal cannulae deliver a clinically relevant level of positive airway pressure,15 while in the absence of an effective way of controlling distending pressure there is also the theoretical risk of lung overdistension and pneumothoraces;18 pressure appears to be related to gas flow, prong size and patient size. 15 The potential for infection was discovered in 2005 when instances of Gram-negative bacteria known as Ralstonia spp. were reported from Vapotherm devices in the USA. 30 This led to the recall of all devices in January 2006 but the product returned to the market with US Food and Drug Administration approval in January 2007, with new instructions for use including the recommendation to utilise only sterile water in the system. 15,30
Evidence for the effectiveness of heated humidified high-flow nasal cannula from previous reviews
In 2011, a Cochrane review related to heated and non-heated HFNC by Wilkinson et al. 31 concluded that there was ‘insufficient evidence to establish the safety or effectiveness of HFNC as a form of respiratory support in preterm infants’. Evidence was derived from two randomised controlled trials (RCTs)32,33 comparing HHHFNC with NCPAP (including one RCT that was unpublished and halted early when the equipment was recalled33), a RCT comparing two types of HHHFNC equipment (Vapotherm vs. Fischer & Paykel)34 and a crossover trial comparing HHHFNC with a non-humidified high-flow device. 35 A whole range of efficacy and safety outcomes were considered by this review, none of which could be pooled for a meta-analysis. More recently, a meta-analysis by Daish and Badurdeen,36 which included three RCTs37–39 that were published after the Cochrane review, examined the effects of HHHFNC on extubation failure (i.e. need for reintubation) and BPD. No significant differences were found between HHHFNC and NCPAP for either outcome. It is worth noting that one of the trials included in the meta-analysis (Yoder et al. 39) included both preterm and term infants.
Rationale for the current review
The wide variety of indications reported in studies included in systematic reviews,31,36 surveys28,29,40,41 and guidelines20,42 support the need for updated evidence of the effectiveness of HHHFNC for a variety of indications, not simply following ventilation. Although a recent meta-analysis has been published examining extubation failure and the incidence of BPD for HHHFNC compared with NCPAP,36 there is also the need for a review of the evidence for other relevant outcomes and comparators.
Clarification of research question and scope
The aim of this project was to answer the question: what is the clinical effectiveness and cost-effectiveness of HHHFNC compared with usual care for preterm infants? This was carried out by a systematic review of the available evidence and the subsequent assessment of the cost implications. We conducted a primary analysis of HHHFNC compared with usual care for preterm infants following ventilation and a secondary analysis of HHHFNC with usual care for preterm infants with no prior ventilation.
Chapter 2 Methods for synthesising clinical evidence
Evidence for the clinical effectiveness of HHHFNC compared with usual care for preterm infants was assessed by conducting a systematic review of published research evidence. The review was undertaken following the general principles published in the Centre for Reviews and Dissemination guidance for undertaking reviews in health care. 43
In order to ensure that adequate clinical input was obtained, an advisory panel comprising clinicians and a parent of children treated with a HHHFNC device was established. The role of this panel was to comment on the draft report and answer specific questions related to the care of preterm infants as the review progressed.
Search strategy
The following databases were searched for eligible studies:
-
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via OvidSP)
-
EMBASE (via OvidSP)
-
Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effectiveness, Health Technology Assessment database
-
ISI Web of Science – Science Citation Index Expanded and ISI Web of Science – Proceedings (Index to Scientific and Technical Proceedings)
-
PubMed (limited to the last 6 months).
Search terms included a combination of index terms (for the study population) and free-text words (for the technologies involved). No study design filters were applied. All databases were searched from 2000 to 8 September 2014, apart from PubMed which was searched from 1 March to 9 September 2014. The searches were then updated on 12 January 2015.
Details of the search strategies can be found in Appendix 1.
Trial and research registers were searched for ongoing trials and reviews including:
-
Clinicaltrials.gov
-
metaRegister of Controlled Trials and International Standard Randomised Controlled Trial Number Register
-
World Health Organization International Clinical Trials Registry Platform
-
PROSPERO systematic review register
-
National Institute for Health Research Clinical Research Network Co-ordinating Centre Portfolio Database
-
Turning Research into Practice Database Plus
-
US Food and Drug Administration.
Bibliographies of previous reviews and retrieved articles were searched for further studies.
Study selection
A decision was made by the review authors to trial the new and freely available web-based software platform developed for the production of systematic reviews, including Cochrane Reviews. The citations identified were independently assessed for inclusion through two stages by two reviewers (YD and RD). Initially the reviewers independently scanned all the titles and abstracts identified (and de-duplicated) through the searching exercise to identify the potentially relevant articles to be retrieved. Full-text copies of the selected studies were then subsequently obtained and assessed again for inclusion using the inclusion and exclusion criteria outlined in Table 2. Disagreements were resolved by discussion at each stage. There was no need to consult a third reviewer.
| Criteria | Included | Excluded |
|---|---|---|
| Study design | RCTs | Any study that is not a RCT |
| Patient population | Preterm infants requiring respiratory support | Not preterm infants |
| Interventions | HHHFNC of any type | A device not incorporating all elements associated with HHHFNC; for example, a HFNC device that is non-humidified |
| Comparators | Usual care Usual care was considered to be NCPAP, NIPPV or oxygen for the primary analysis and NCPAP, NIPPV, oxygen or mechanical ventilation for the secondary analysis |
Not usual care |
| Outcomes | Primary outcome: Failure of treatment as indicated by the need for reintubation (treated following ventilation), or need for intubation (no prior ventilation) as measured at three time points:
|
No study will be excluded based solely on outcomes measured |
Data extraction strategy
Data relating to study design and findings were extracted by one reviewer (VB) and independently checked for accuracy by a second reviewer (RD). Study details were extracted on pre-tested data extraction forms. Data from studies presented in multiple publications were extracted and reported as a single study with all other relevant publications listed. When studies included preterm and non-preterm infants, only data for preterm infants were extracted and study authors were contacted for missing data as necessary.
Assessing the risk of bias
The plan for the conduct of risk of bias of the individual studies was originally based on the Cochrane risk-of-bias criteria44 because the intention was to use the new and freely available web-based software platform developed for the production of systematic reviews, including Cochrane Reviews, for the entire review. However, it became clear that the data extraction tool used in this software did not allow us to easily produce tables for the review. We therefore opted to quality assess the included studies using criteria adapted from the Centre for Reviews and Dissemination at the University of York. 43 Criteria were assessed independently by one reviewer (VB) and then crosschecked by a second reviewer (YD). Disagreements were resolved through consensus and there was no need to consult a third reviewer.
Methods of analysis/synthesis
The results of the data extraction and quality assessment for each study were presented in structured tables and as a narrative summary for the primary analysis (preterm infants treated following ventilation) and secondary analysis (preterm infants with no prior ventilation). When data permitted, we conducted a meta-analysis of primary and secondary outcomes using an appropriate software package (RevMan; The Nordic Cochrane Centre, Copenhagen, Denmark). We also conducted subgroup analyses based on gestational age. We planned to use the categories < 30 weeks and ≥ 30 weeks (but the data did not permit us to use these specific thresholds once we had extracted the data). For dichotomous outcomes, we planned to use risk ratio (RR) and the corresponding 95% confidence intervals (CIs) to summarise results from each trial and for continuous outcomes, we planned to use the mean difference (or standardised mean difference when different scales are used). It was only possible to pool data for dichotomous outcomes.
The decision to conduct a meta-analysis depended on there being sufficient data (at least two studies with the same interventions and comparators measuring the same outcome in the same way) and an assessment of heterogeneity. Heterogeneity was explored through consideration of the study populations (e.g. differences in gestational age), interventions (e.g. starting flow rate for HHHFNC or starting pressure for NCPAP), outcome definitions (e.g. different definitions for reintubation) and, in statistical terms, by the chi-squared test for homogeneity and the I2 statistic. 45 The I2 statistic, with a level of > 50%, was considered to indicate moderate levels of heterogeneity, and the chi-squared test of < 0.10 to indicate statistically significant heterogeneity. Based on these assessments, a decision was made on whether to combine the results using a fixed-effects model (in the case of minimal heterogeneity) or a random-effects model (in the case of substantial levels of heterogeneity).
If data had allowed, we would have conducted sensitivity analyses excluding trials deemed to be of low quality to assess the robustness of the findings. Had we included ≥ 10 studies in a meta-analysis an assessment of the risk of publication bias would have been conducted by constructing a funnel point and conducting a simple test of asymmetry to test for possible bias. 46
Chapter 3 Methods for synthesising evidence of cost-effectiveness
Scoping searches conducted in the preparation of the protocol identified no relevant published cost-effectiveness studies. The search strategy is reported in Appendix 2. We therefore did not conduct another search of the literature for published cost-effectiveness evidence but attempted to develop a de novo economic model if suitable data were available.
Modelling clinical pathway and outcomes
The definition of the patient pathway was determined through consultation of one of the authors who was a clinician (BS) and the economic modeller (JM). The pathway that was developed is shown in Figure 1. Data required to populate this patient pathway were taken from the studies included in the review (see Chapter 4, Included studies).
FIGURE 1.
Treatment pathway.
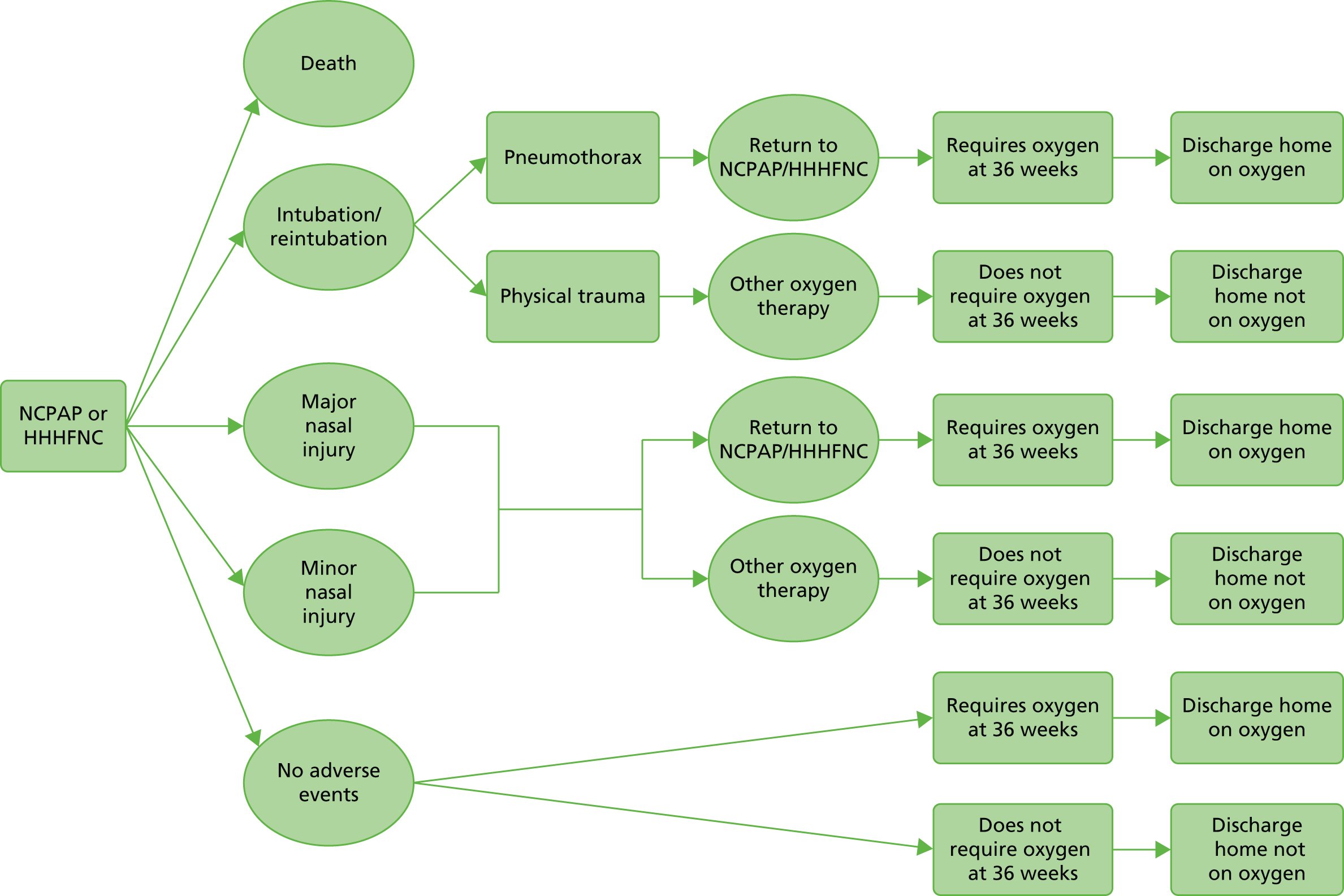
It was determined that the pathway was best modelled as a decision tree, as there is no long-term progression of disease over time. It is assumed that any loss in utility from the primary outcome is once and for all and that any short-term loss in utility from, for example, nasal injury, is a one-off utility decrement before a return to the long-term prior health state.
The model time horizon could, in theory, be lifetime provided, and there was evidence from the clinical review that the difference in outcomes between technologies had lifetime consequences.
Costs and utilities
Once the pathway and different clinical outcomes were determined, the appropriate treatment costs for the different technologies were identified through searching NHS Reference Costs: Financial Year 2013 to 201447 and the NHS Supply Chain48 when available and appropriate.
Costing of the outcomes in the pathway was not undertaken until the conclusion of the clinical review, such that only outcomes in which there was a difference identified in the review were costed. In all instances costs were to be taken from the perspective of the NHS.
When costs were not available from published sources, or when there was a menu of costs that could be chosen (such as from different manufacturers), then the costs were determined by resource use and costs in the neonatal units of the authors who are clinicians (BS and PS).
Patient-elicited health states, with societal preference weights applied to those health states, is the preferred method of utility derivation in health economics. Unfortunately, in preterm infants this approach was not possible. Should there be a difference in outcomes identified in the clinical literature review, in selecting utility weights for different health states, a pragmatic review of health–utility literature in preterm babies and the clinical outcomes (including complications) identified in the pathway was to be undertaken. This would include searching for cost–utility evaluations of other interventions for preterm babies to assess how utility values have been incorporated for this patient group by other researchers.
In the absence of any reliable utility information, provided there was published clinical evidence on differences in outcomes from using HHHFNC or NCPAP, then we planned to model the full cost implications of using the technology taking into account the improved outcomes. If HHHFNC or NCPAP improves outcomes at a lower cost than alternatives, then the absence of utility information would not then be important. If, on the other hand, the outcomes are improved with HHHFNC, but at a higher cost than with NCPAP, a cost-effectiveness analysis would be undertaken looking at ratios such as the cost per death averted.
A lack of evidence for difference in outcomes between HHHFNC and NCPAP would prevent undertaking of either cost–utility or cost-effectiveness analysis. If this was the case we planned to undertake a cost-minimisation analysis comparing HHHFNC with NCPAP. A cost-minimisation analysis looks at the overall costs of the technologies per patient by comparing the resources required in capital goods, consumables and clinician time to administer each technology with any evidence on adverse events and the resources required to treat these events. By applying suitable prices to these resources, the analysis looks to identify the least expensive of the options, in this case from the perspective of the NHS. For such an analysis in which there is no clinical difference in outcomes that can be identified between technologies, it is the least expensive of the technologies that is the most cost-effective.
Analysis of uncertainty
If a formal economic model could be constructed, appropriate sensitivity analyses were planned in order to assess the robustness of model results to realistic variations in the levels of the underlying data. When the overall results are sensitive to a particular variable, the sensitivity analysis would analyse the exact nature of the impact of variations.
Imprecision in the principal model cost-effectiveness results with respect to key parameter values was to be assessed by use of techniques compatible with the modelling methodology deemed appropriate to the research question and available evidence. This would include multiway sensitivity analysis and cost-effectiveness acceptability curves.
Chapter 4 Clinical effectiveness results
Initial searches and application of inclusion criteria
The results of the application of the study inclusion criteria are presented in Figure 2.
FIGURE 2.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. a, Ten papers report on seven separate studies.
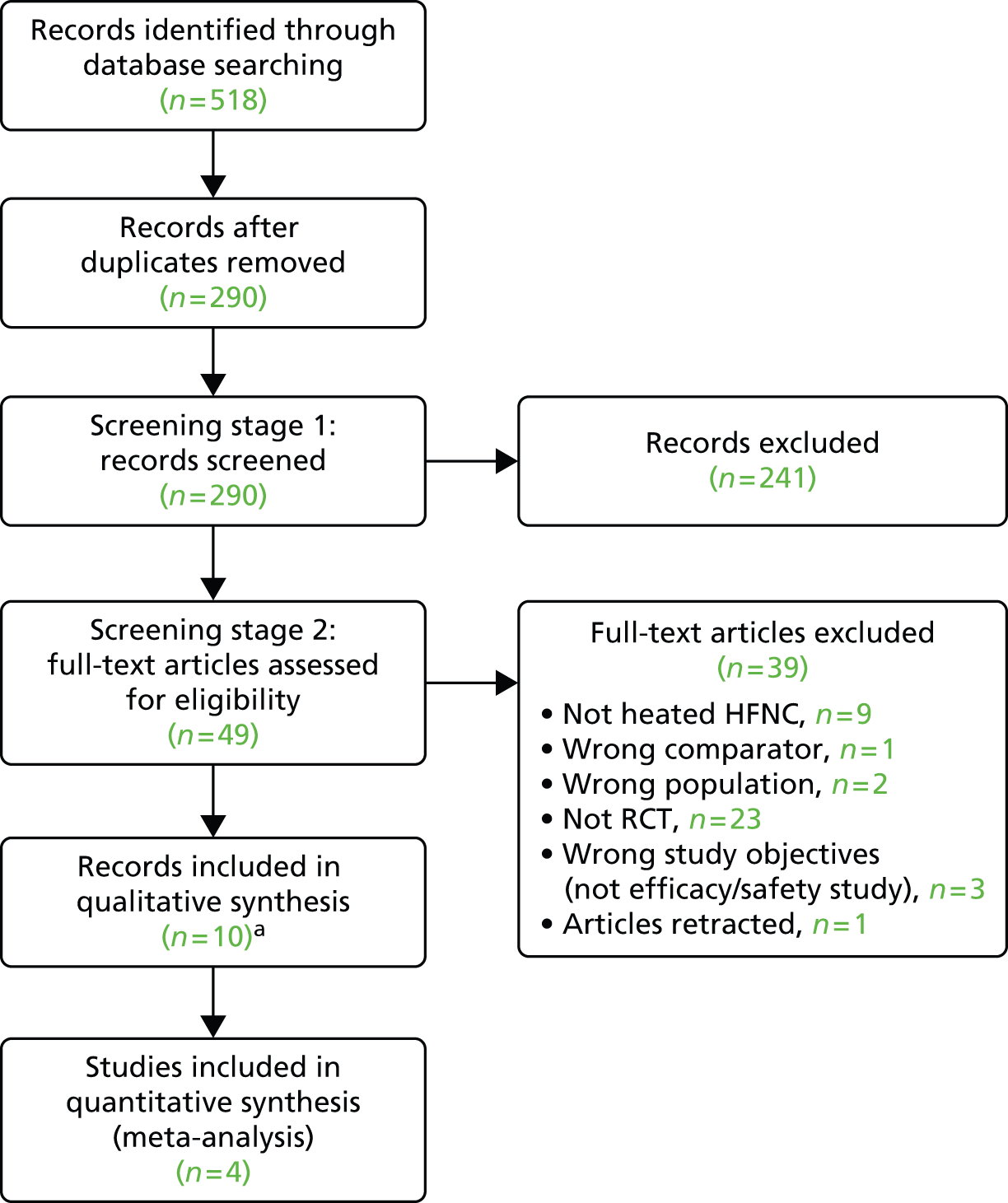
Included studies
In total, 10 records33,37–39,49–54 were included. These report on seven separate studies summarised in Table 3. For the remainder of this report, only the primary paper for each of the studies will be referred to. In one instance, this was only an abstract. 33
| Study | Primary paper | Secondary paper | Study sponsor |
|---|---|---|---|
| Primary analysis: preterm infants treated following ventilation | |||
| Collaborative Group, 2014 | Collaborative Group, 201449 (published in Chinese with English abstract) | Ma et al., 201453 (conference abstract) | Supported by grants from Hebei Provincial Health Bureau GL2012013 and Talents Training Project of Hebei Province 2012–334 |
| Collins et al., 2013 | Collins et al., 201337 | Collins et al., 201450 (substudy) | Medical Research Foundation for Women and Babies, Melbourne, VIC, Australia |
| Manley et al., 2013 | Manley et al., 201338 | Manley et al., 201354 | Programme grant and Centre for Clinical Research Excellence grant from the National Health and Medical Research Council |
| Yoder et al., 2013 | Yoder et al., 201339 | None | No external funding |
| Secondary analysis: infants who had received no prior ventilation | |||
| Klingenberg et al., 2014 | Klingenberg et al., 201451 | None | None stated |
| Kugelman et al., 2014 | Kugelman et al., 201452 | None | None. Equipment supplied by Vapotherm Inc. |
| Nair and Karna, 2005 | Nair and Karna, 200533 (abstract only) | None | Equipment support from Vapotherm Inc. |
Three studies37,38,49 reported on preterm infants that had been previously ventilated and were applicable for the primary analysis. An additional study,39 in which the majority of infants received prior ventilation (see Study characteristics), was also included in this primary analysis. The remaining three33,51,52 studies reported on preterm infants requiring respiratory support following no prior ventilation and were applicable for the secondary analysis.
Study quality assessment
A summary of the quality assessment conducted is presented in Table 4 and a more detailed assessment is presented in the sections following. Overall, the RCTs included in the review were of reasonable methodological quality, although it was not possible to blind administrators or participants in any study. Studies included in the primary analysis of HHHFNC compared with usual care for preterm infants following ventilation were generally of better quality than those in the secondary analysis of HHHFNC compared with usual care for preterm infants with no prior ventilation. One of the studies included in this latter analysis, by Nair and Karna,33 was not published but only presented as an abstract.
| Studies | Randomisation | Baseline comparability | Eligibility criteria specified | Cointerventions identified | Blinding | Withdrawals | Intention to treat | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Truly random | Allocation concealment | Number stated | Presented | Achieved | Assessors | Administrators | Participants | Procedure assessed | > 80% in final analysis | Reasons stated | ||||
| Primary analysis: preterm infants treated following ventilation | ||||||||||||||
| aCollaborative Group, 201449 | ✓ | ✓ | ✓ | ✓a | ✓a | ✓ | ✗ | ✗ | ✗ | ✗ | NA | ✗/✓a | NAa | ✓ |
| Collins et al., 201337 | ✓ | ✓ | ✓ | ✓ | ✗/✓ | ✓ | ✓ | ✓ | ✗ | ✗ | NS | ✓ | NA | ✓ |
| Manley et al., 201338 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | NA | ✓ | NA | ✓ |
| bYoder et al., 201339 | ✓ | ✓ | ✓ | ✓b | ✓b | ✓ | ✗ | ✗ | ✗ | ✗ | NA | ✗/✓b | NAb | ✓ |
| Secondary analysis: infants who had received no prior ventilation | ||||||||||||||
| Kingenberg et al., 201451 | NS | NS | ✓ | NA | NA | ✓ | ✗ | ✗ | ✗ | ✗ | NA | ✓ | ✓ | NS |
| Kugleman et al., 201452 | ✗/✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | NA | ✓ | NA | ✓ |
| cNair and Karna, 200533 | NS | NS | ✓ | ✓ | ✓ | ✗/✓ | ✗ | NS | NS | ✗ | NS | ✓ | NA | ✓ |
Quality assessment of studies included in primary analysis
All four studies37–39,49 were described as being randomised; however, for two studies39,49 preterm infants were a non-randomised subgroup. All studies37–39,49 provided information on treatment allocation. One study37 reported that assessors were blinded to treatment allocation.
Baseline comparability was provided for all four studies. 37–39,49 However, Collins et al. 37 did not report achievement of comparability for all characteristics.
All four studies37–39,49 reported 100% completion of study participants and, for all of these studies, analysis was conducted on an intention-to-treat basis.
All four studies37–39,49 provided details of eligibility criteria. However, two of the studies39,49 did not identify any cointerventions.
For all four studies,37–39,49 a number of outcomes were reported and all of these outcomes appeared to be specified in the study methods.
Quality assessment of studies included in secondary analysis
All three studies were described as randomised. 33,51,52 Two of the studies did not state the randomisation process33,51 and one study only partially described the method of randomisation. 52
Two of the studies presented and achieved baseline comparability. 33,52 The study by Klingenberg et al. 51 was a crossover trial, hence there was only one group. Baseline characteristics were therefore presented for all participants and comparability (and whether or not it is achieved) is not applicable.
Two of the studies reported 100% completion of study participants and reported using intention-to-treat analysis. 33,52 Klingenberg et al. 51 reported > 80% completion rate of participants and reasons for dropouts were reported; however, it was not stated whether or not intention-to-treat analysis was conducted.
One of the studies did not clearly identify their eligibility criteria,33 with only gestational age and requirement for respiratory support within the first 6 hours of life being specified. However, this study was only available as a conference abstract. Two of the studies did not identify any cointerventions. 33,51
For all studies,33,51,52 a number of outcomes were reported and all of these outcomes appeared to be specified in the study methods. One secondary outcome (salivary cortisol) in the study by Klingenberg et al. 51 was omitted from statistical comparisons because the study authors reported that they only managed to collect enough saliva for cortisol measurement in 11 out of 80 attempts. This outcome measure was not, however, a pre-specified outcome for our review.
Finally, it should be noted that one of the studies was halted early. 33 This was because of the temporary recall of Vapotherm devices as a result of reports external to this trial of Ralstonia spp. infections occurring with its use. This study has, to date, only been presented as an abstract.
Study characteristics
Study characteristics are presented in Tables 5 and 6. A total of 859 infants were involved in the seven trials and the trial sizes ranged from 2051 to 303 participants. 38
| Study | Study design, location and years conducted | Population studied | Excluded | Interventions | Outcomes |
|---|---|---|---|---|---|
| Collaborative Group, 201449 | Multicentre RCT China 2012–13 |
n = 150a Infants who were admitted to NICU within 7 days after birth and were planned to extubate to non-invasive ventilation after endotracheal ventilation. No limitation on GA or birthweight |
Life-threatening congenital anomaly Congenital anomalies requiring surgical intervention, for example CDH, TEF, gastroschisis or omphalocele Congenital airway abnormalities, for example Pierre Robin syndrome, mandibulofacial dysostosis, oculo–auriculo–vertebral dysplasia syndrome, cleft lip or palate Uncontrolled air leak |
HHHNFC (3–8 l/minute depending on birthweight) with heated humidifier (specific device not stated but provided by Fisher & Paykel, Auckland, New Zealand), Bird® Blender (CareFusion, Yorba Linda, CA, USA), compact air/oxygen gas mixing device and Optiflow™ (Fisher & Paykel, Auckland, New Zealand) nasal cannula, n = 79a NCPAP [devices included Infant Flow® NCPAP System (CareFusion, Yorba Linda, CA, USA) and Stephanie neonatal ventilation system (Fritz Stephan GmbH Medizintechnik, Gackenbach, Germany)] (6–10 l/minute, same PEEP with invasive ventilation), n = 71a |
Extubation failurea (reintubation within 7 days) |
| Collins et al., 201337 | Single-centre RCT Australia 2009–11 |
n = 132 GA < 32 weeks Previous endotracheal intubation with positive-pressure ventilation Ready for extubation |
Upper airway obstruction Congenital airway malformations Major cardiopulmonary malformations |
Vapotherm® HHHFNC with Sticky Whiskers® (Vapotherm Inc., Stevensville, MD, USA; Beevers Manufacturing & Supply, McMinville, OR, USA) (8 l/minute), n = 67 NCPAP via Hudson® binasal prongs (Hudson Respiratory Care Inc., Temecula, CA, USA) with either Sticky Whiskers® or Cannualaide® (Beevers Manufacturing & Supply, McMinville, OR, USA) PEEP of 8 cm water if FiO2 > 0.3 or a PEEP of 7 cm water if FiO2 < 0.3, n = 65 |
Extubation failure (composite outcomeb) in next 7 days Nasal trauma BPD Duration of respiratory support Duration of supplemental oxygen requirement Pneumothorax after extubation IVH NEC Death Days to reach full enteral feeds |
| Manley et al., 201338 | Multicentre non-inferiority RCT Australia 2010–12 |
n = 303 GA < 32 weeks Infants scheduled for extubation |
GA > 36 weeks Participation in concurrent study Major congenital anomalies |
HHHFNC (5–6 l/minute depending on prong size) with Fisher & Paykel Optiflow™ device, MR850 humidifier and binasal infant cannulae (Auckland, New Zealand), n = 152 NCPAP with binasal prongs (Fisher & Paykel, Auckland, New Zealand) or subnasal prongs (Hudson RCI®, Teleflex, Morrisville, NC, USA), mechanical ventilation or underwater ‘bubble’ at 7 cm of water, n = 151 |
Treatment failure (composite outcomeb) within 7 days Reintubation within 7 days Death before hospital discharge Require supplemental oxygen Duration of respiratory support Length of hospital admission Adverse events including BPD, nasal and septum trauma, NEC, IVH, nosocomial sepsis, gastrointestinal perforation and pneumothorax |
| Yoder et al., 201339 | Multicentre RCT USA 2007–12 |
n = 150a GA ≥ 28 weeks Birthweight ≥ 1000 g Intention to manage the infant with either non-invasive (no endotracheal tube) or mechanical ventilation (with an endotracheal tube) within first 24 hours of birth |
Weight < 1000 g GA < 28 weeks Presence of active air leak syndrome Concurrent participation in a study that prohibited HHHFNC Abnormalities of upper and lower airways Serious abdominal, cardiac or respiratory malformations including TEF, intestinal atresia, omphalocele, gastroschisis or diaphragmatic hernia |
HHHFNC (3–8 l/minute depending on birthweight) with various devices [Comfort Flo (Hudson RCI®, Teleflex, Morrisville, Research Triangle, NC, USA), Fisher & Paykel Healthcare (Irvine, CA, USA), and Vapotherm (Stevensville, MD, USA)], n = 75a NCPAP [various interfaces including bubble, Infant Flow® NCPAP System (CareFusion, Yorba Linda, CA, USA) and ventilator at 5–6 cm of water], n = 75a |
Extubation failurea (need for intubation within 72 hours) BPDa |
| Study | Study design, location and years conducted | Population studied | Excluded | Interventions | Outcomes |
|---|---|---|---|---|---|
| Klingenberg et al., 201451 | Single-centre crossover trial (2 × 24 hours) Norway 2012–13 |
n = 20 GA < 34 weeks Mild respiratory illness (treated with NCPAP for 72 hours) |
Congenital anomalies Required high oxygen levels or frequent blood samples because of infection or hypoglycaemia |
24-hour HHHFNC (Fisher & Paykel RT329 system, Auckland, New Zealand) (5–6 l/minute depending on birthweight), n = 10 24-hour NCPAP Infant Flow® SiPAP (CareFusion, San Diego, CA, USA) variable-flow driver (4–5 cm H2O), n = 10 |
Patient comfort (EDIN scale) Respiratory parameters Ambient noise Salivary cortisol Parental assessments |
| Kugelman et al., 201452 | Single-centre RCT Israel 2010–11 |
n = 76 GA < 35 weeks Birthweight > 1000 g Infants with RDS who need non-invasive respiratory support |
Significant morbidity | HHHFNC [Vapotherm Precision FlowTM or 2000i, Vapotherm, Inc., (Stevensville, MD, USA), at flows between 1.0 and 5.0 l/minute], n = 38 NIPPV SLE 2000 or 5000 (Specialized Laboratory Equipment Ltd, South Croydon, UK) via nasal prongs (INCA, Ackrad Labs, Berlin, Germany), n = 38 |
Reintubation Duration of nasal support Duration of endotracheal ventilation Time to full feeds Length of stay Air leaks Neonatal morbidities:
|
| Nair and Karna, 200533 | Single-centre RCT USA 2004 |
n = 28 GA 27–34 weeks Required NCPAP in first 6 hours |
No spontaneous respiration Major congenital anomalies Birth asphyxia (Apgar score of < 3) |
HHHNFC [Vapotherm 2000i (Stevensville, MD, USA), mean flow rate 1.8 l/minute], n = 13 Variable-flow NCPAP at 5–6 cm H2O, n = 15 |
Respiratory failure, two or more of:
|
Study characteristics of studies included in primary analysis
As per the inclusion criteria, the four included studies37–39,49 of infants who had been treated following ventilation were RCTs. A total of 735 infants were involved in the trials and the trial sizes ranged from 13237 to 303. 38
Three studies38,39,49 were multicentred; no study was carried out internationally, with two studies conducted in Australia,37,38 one in the USA39 and one in China. 49 The earliest study started enrolling participants in December 200739 and the most recent in 2012. 49 HHHNFC was compared with NCPAP in all four studies. 37–39,49
The length of the study follow-up was only explicitly stated by Collins et al. 37 in which it is stated that 132 infants were followed up for 7 days and 121 infants were followed up until their discharge home; reasons for loss to follow-up after 7 days are provided. Yoder et al. 39 also appear to have followed up infants until discharge, as they present a study flow chart presenting numbers of patients until discharge. It can be assumed that in the other two studies37,49 infants were followed up for a minimum of 7 days (as the primary outcome in each study required follow-up for 7 days).
Study participants were generally similar across the studies (in terms of inclusion and exclusion criteria), although the two Australian studies37,38 limited participation to infants with a gestational age of < 32 weeks and the US study39 to ≥ 28 weeks. The US and Chinese studies39,49 included preterm, term and post-term infants, but only data for preterm infants has been synthesised in the remainder of this report (56.6% of participants in the Chinese study49 and 32.4% with a gestational age of < 32 weeks in the US study39). In addition, the US study39 also included infants who had not received prior ventilation (32.4% of all participants, including term and post-term babies, the proportion of preterm infants being unknown). The type of HHHFNC device and flow rate varied across studies, as did the NCPAP devices and starting flow rates.
Study characteristics of studies included in secondary analysis
Regarding the studies of infants who had not received prior ventilation, again as per the inclusion criteria, the three included studies were RCTs, of which one was a crossover trial. 51 A total of 124 infants were involved in the trials and the trial sizes ranged from 2051 to 76. 52
All included studies33,51,52 were single-centre trials. One study was carried out in the USA,33 one in Norway51 and one was a pilot study conducted in Israel. 52 All studies were single-centre trials. The earliest study started enrolment from 2004,33 whereas the other two51,52 were from 2010 onwards. HHHNFC was compared with NCPAP33,51 and NIPPV. 52
The length of follow-up was not specified by any of the studies but may be assumed to be 48 hours (2 × 24 hours) in the crossover trial51 and a minimum of 7 days in Nair and Karna 2005,33 as the primary objective of this latter study was to compare the respiratory failure rate during the first 7 days of life. It is unclear how long preterm infants were followed up in the pilot study. 52
Study participants were generally similar across the studies (in terms of inclusion and exclusion criteria), with all infants with a gestational age of < 35 weeks. However, one study,51 which was the crossover study, included a minority (30%) of patients who had received prior ventilation. The type of HHHFNC device and flow rate varied across studies as did the NCPAP devices and starting flow rates.
Characteristics of the preterm infants included in the studies
Characteristics of the preterm infants that participated in the trials are presented in Table 7 (primary analysis of preterm infants treated following ventilation) and Table 8 (secondary analysis of preterm infants with no prior ventilation). There is a lack of data for two studies39,49 reporting on preterm infants treated following ventilation because both of these studies also included term and post-term infants and did not present baseline data for only preterm infants.
| Study | Arm | Race, white, n (%) | Gestational age (weeks), mean (SD) | Birthweight (g), mean (SD) | Male, n (%) | Prior mechanical ventilation, n (%) | Intubation in delivery room, n (%) | Antenatal/ pre-study steroids, n (%) | 5-minute Apgar score (range) |
|---|---|---|---|---|---|---|---|---|---|
| Collaborative Group, 201449 | HHHNFC (n = 79) | NR | NRa | NRa | NRa | 79 (100) | NR | NR | NR |
| NCPAP (n = 71) | NR | NRa | NRa | NRa | 71 (100) | NR | NR | NR | |
| Collins et al., 201337 | HHHNFC (n = 67) | NR | 27.9 (1.95) | 1123 (317) | 33 (49) | 67 (100) Median (range), hours: 46 (24–98) |
NR | 59 (88.1) | 7 (6–8) |
| NCPAP (n = 65) | NR | 27.6 (1.97) | 1105 (374) | 41 (63) | 65 (100) Median (range), hours: 57 (27–120) |
NR | 58 (89.2) | 8 (6–9) | |
| Manley et al., 201338 | HHHNFC (n = 152) | 127 (83.6) | 27.7 (2.1); n (%) < 26 weeks: 32 (21.1) | 1041 (338) | 89 (59) | 152 (100) Median (range), hours: 36 (19.5–101.5) |
102 (67.1) | 142 (93.4) | 7 (6–8) |
| NCPAP (n = 151) | 120 (79.5) | 27.5 (1.9); n (%) < 26 weeks: 31 (20.5) | 1044 (327) | 72 (48) | 151 (100) Median (range), hours: 36 (20–93) |
91 (60.3) | 143 (94.7) | 8 (6–8) | |
| Yoder et al., 201339 | HHHNFC (n = 75) | NR | NRa | NRa | NRa | NRa | NR | NRa | NR |
| NCPAP (n = 73) | NR | NRa | NRa | NRa | NRa | NR | NRa | NR |
| Study | Arm | Gestational age (weeks), mean (SD) | Birthweight (g), mean (SD) | Male, n (%) | Antenatal/pre-study steroids, n (%) | 5-minute Apgar score (range) |
|---|---|---|---|---|---|---|
| Klingenberg et al., 201451 | HHHNFC (n = 20)a | All infants: 29.3 (1.7)a | All infants: 1234 (353)a | All infants: 13 (65)a | NR | NR |
| NCPAP (n = 20)a | ||||||
| Kugelman et al., 201452 | HHHNFC (n = 38) | 31.8 (2.3) | 1759 (488) | 26 (68) | 19 (50) | 9 (6–10) |
| NIPPV (n = 38) | 32.0 (2.3) | 1835 (530) | 24 (63) | 19 (50) | 9 (7–10) | |
| Nair and Karna, 200533 | HHHNFC (n = 13) | 32 (0.5) | 1675 (139) | NR | NR | NR |
| NCPAP (n = 15) | 31 (0.5) | 1493 (64) | NR | NR | NR |
When data on birthweight were provided, birthweight was generally lower in those studies relevant to the primary analysis (mean < 1150 g) than those relevant to the secondary analysis (mean > 1490 g). Similarly, when data on mean gestational age were provided, this was generally lower in those studies relevant to the primary analysis (mean < 28 weeks) than those in the secondary analysis (mean ≥ 30 weeks). Prior steroid use was only reported in three studies37,38,52 and this was notably higher (≥ 88%) in the two studies relevant to the primary analysis37,38 than in the study52 included in the secondary analysis (50%). These differences in baseline findings suggest that infants in the primary analysis are heavier and have a shorter gestational age than those in the secondary analysis, which is not unexpected as these are the infants who tend to most need mechanical ventilation as soon as they are born.
Participant characteristics of studies included in primary analysis
The participant characteristics across all four trials were broadly similar (see Table 7).
Participant characteristics of studies included in secondary analysis
As evident from Table 8, infants in the Klingenberg et al. 51 study were notably lighter (< 1250 g) and slightly younger (≤ 29.3 weeks) than the other two studies33,52 included in the secondary analysis (≥ 1493 g and ≥ 31 weeks, respectively). This study51 did, in fact, include a minority (30%) of patients who had received prior ventilation unlike the other studies. 33,52 This may explain why mean birthweight and gestational age differed in this study compared with the other two studies,33,52 as the data may be being skewed by the inclusion of preterm infants who had been treated following ventilation.
Efficacy findings from primary analysis
For preterm infants treated following ventilation, it was possible to pool data in a meta-analysis for three outcomes: need for reintubation < 7 days, BPD and death. The primary outcome for our review was treatment failure as defined by the need for reintubation at < 72 hours, < 7 days or ever. For the primary analysis, three studies37,38,49 measured the need for reintubation within the first 7 days. The data for these three studies37,38,49 were pooled into a meta-analysis (Figure 3). Data were also pooled for BPD and death from three studies37–39 (Figures 4 and 5). For all analyses, a fixed-effects model was employed, as there was no evidence of statistical heterogeneity (or indeed clinical heterogeneity based on the data presented in Tables 5 and 7). The forest plots show that all the findings are in the direction of favouring HHHFNC. However, no statistically significant differences were reported between arms for any of the outcomes. No significant statistical heterogeneity between studies was noted in any of the three meta-analyses (I2 = 0% and chi-squared test, p ≥ 0.10).
FIGURE 3.
Meta-analysis for need for reintubation < 7 days. df, degrees of freedom; M–H, Mantel–Haenszel.

FIGURE 4.
Meta-analysis for BPD. df, degrees of freedom; M–H, Mantel–Haenszel.
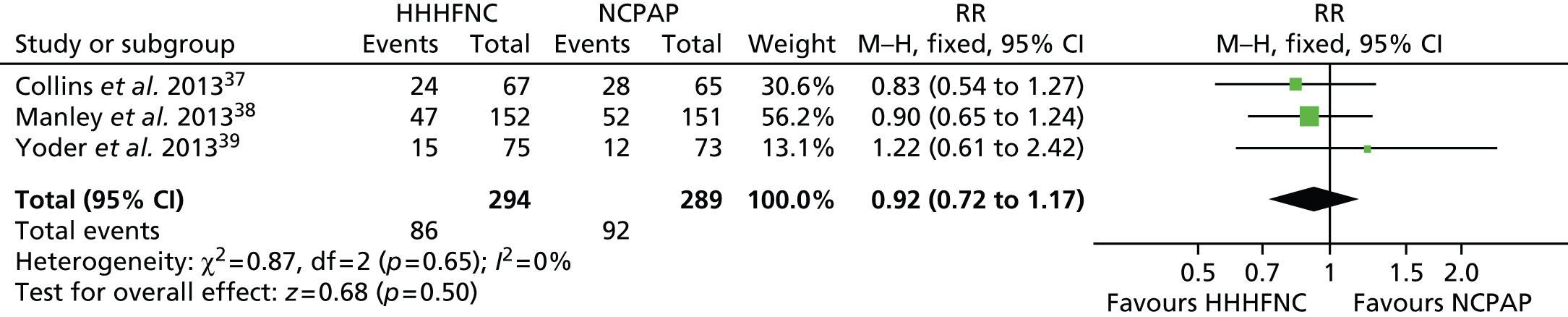
FIGURE 5.
Meta-analysis for death. df, degrees of freedom; M–H, Mantel–Haenszel.

One trial39 only reported reintubation within 72 hours for preterm infants. As reported in Table 9, marginally fewer preterm infants required reintubation in the HHHFNC arm than in the NCPAP arm. However, the proportions were small and no statistically significant difference between arms was reported.
| Study | Arm | Reintubation, n (%) | BPD/death, n (%) | Time (hours) on mechanical support | Days on oxygen support | Length (days) of hospital stay | Days to full feeds, mean (SD)/weight gain (g) | Other, n (%) |
|---|---|---|---|---|---|---|---|---|
| Collaborative Group, 201449 | HHHNFC (n = 79) | < 7 days: 11 (13.9) | NRa | NRa | NRa | NRa | NRa | NRa |
| NCPAP (n = 71) | < 7 days: 11 (15.5) | NRa | NRa | NRa | NRa | NRa | NRa | |
| Collins et al., 201337 | HHHNFC (n = 67) | < 7 days: 7 (10.4) | BPD 24 (35.8); death 1 (1.5) | NR | NR | NR | Days to full feeds: 12.9 (0.73) | Failed extubation:b 15 (22.4) |
| Ever: 14 (20.9) | ||||||||
| NCPAP (n = 65) | < 7 days: 8 (12.3) | BPD 28 (43.1); death 3 (4.6) | NR | NR | NR | Days to full feeds: 12.3 (0.65) | Failed extubation:b 22 (33.8) | |
| Ever: 16 (24.6) | ||||||||
| Manley et al., 201338 | HHHNFC (n = 152) | < 7 days: 27 (17.8) | BPD 47 (30.9); death 5 (3.3) | Median 34 (range 7–55) | Median 38 (range 0–78) | Median 79 (range 63–105) | Weight gain: median 20 (range –42 to 79.5) | Treatment failure:c 52 (34.2) |
| NCPAP (n = 151) | < 7 days: 38 (25.2) | BPD 52 (34.4); death 6 (4. 0) | Median 38 (range 11–57) | Median 49 (range 8–83) | Median 84 (range 65–106) | Weight gain: median 10 (range –54 to 75) | Treatment failure:c 39 (25.8) | |
| Yoder et al., 201339 | HHHNFC (n = 75) | < 72 hours: 3 (4. 0) | BPD 15 (20.0); death 0 | NRa | NRa | NR | NRa | NRa |
| NCPAP (n = 73) | < 72 hours: 5 (6.7) | BPD 12 (16.4); death 2 (2.7) | NRa | NRa | NR | NRa | NRa |
All other outcomes reported in the trials are also presented in Table 9. No statistically significant differences were reported between arms in any of these studies37,38 and so no study reported the superiority of HHHFNC over usual care. However, it should be noted that the Manley et al. 38 study was a non-inferiority trial and so the aim of the trial was not to demonstrate superiority.
It should also be noted that the definition of extubation failure/treatment failure in two studies37,38 differed to that used for our review; both studies37,38 based failure on a composite outcome including apnoea, acidosis and increase in the fraction of inspired oxygen. In addition, Manley et al. 38 also included these three outcomes plus an urgent need for intubation in their composite outcome. Using these study definitions, it is noted that Manley et al. 38 reported a numerically higher rate of treatment failure with HHHFNC than NCPAP (but the opposite was the case with regard to need for reintubation). In contrast, Collins et al. 37 reported a numerically lower rate of extubation failure with HHHFNC (and reintubation rates were also numerically lower in the HHHFNC arm).
Hours on mechanical ventilation, days on oxygen support and length of hospital stay were reduced with HHHFNC than with NCPAP in the study by Manley et al. ;38 however, the differences were not statistically significant. In the same study,38 median weight gain also appeared to be higher in the HHHFNC arm than in the NCPAP arm, but again the difference was not statistically significant. Days to full feeds was reported only by Collins et al. 37 This was marginally higher in the HHHFNC arm by around half a day; the between-arm difference was not statistically significant.
A number of other secondary outcomes that we had planned to measure were not reported by any study, namely BPD/death (composite outcome), duration of respiratory support on NCPAP or HHHFNC, length of stay in NICU or measures of quality of care.
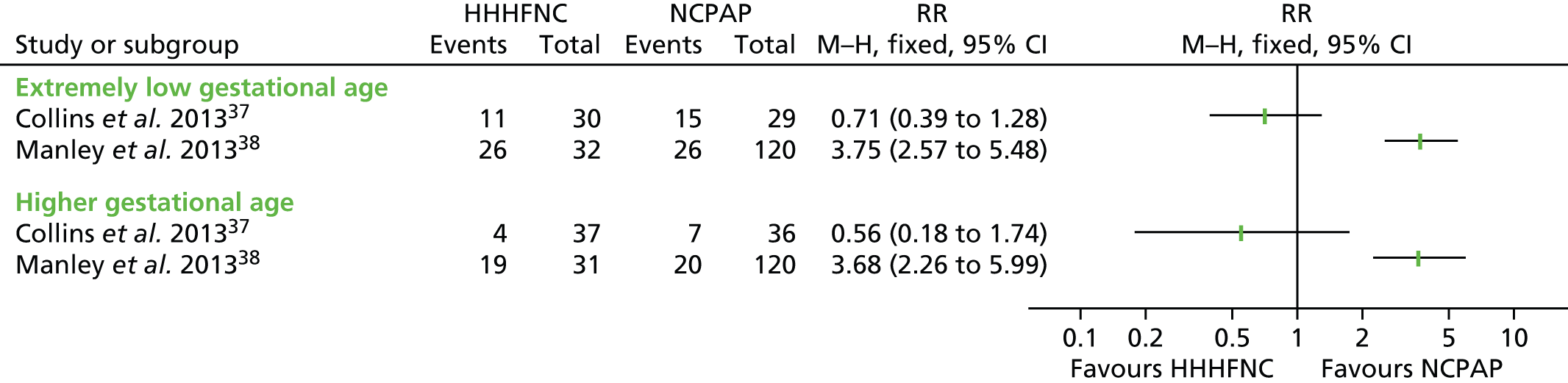
Exploratory subgroup analyses
Extubation failure/treatment failure (as defined, differentially, in the individual studies37,38) was considered by gestational age in two trials. 37,38 In Manley et al. ,38 it was considered in those infants born before 26 weeks of completed gestation and in those born from 26 weeks onwards, and in Collins et al. 37 it was considered in those born before/from 28 weeks. Unsurprisingly, the extubation failure/treatment failure rate was higher in infants with gestational ages below 26/28 weeks (extremely low gestational age) than in infants born later. As shown in Figure 6, and as noted above for the whole-trial population in Efficacy findings from primary analysis, the treatment effect was in opposite directions in the two included studies. 37,38
FIGURE 6.
Extubation failure/treatment failure by subgroup. Note that the extremely low gestational age subgroup was defined as < 28 weeks’ gestational age in Collins et al. 37 and < 26 weeks’ gestational age in Manley et al. 38 The higher gestational age subgroup was defined as ≥ 28 weeks’ gestational age in Collins et al. 37 and ≥ 26 weeks’ gestational age in Manley et al. 38 Collins et al. 37 defined extubation failure by composite criteria based on apnoea, acidosis and increase in fraction of inspired oxygen, whereas Manley et al. 38 defined treatment failure by composite criteria based on apnoea, acidosis, increase in fraction of inspired oxygen and urgent need for intubation. M–H, Mantel–Haenszel.
Reintubation rates were only presented by subgroup in one study. 37 As reported in Table 10, reintubation rates appeared to be higher in those treated with NCPAP than in those treated with HHHFNC, regardless of gestational age.
| Study | Arm | Gestational age | |
|---|---|---|---|
| < 28 weeks, n (%) | ≥ 28 weeks, n (%) | ||
| Collins et al., 201337 | HHHNFC | 5 (16.7) | 4 (10.8) |
| NCPAP | 7 (24.1) | 7 (19.4) | |
Adverse events reported for primary analysis
A summary of the adverse events reported in the included trials is presented in Table 11. Adverse event data were reported for preterm, term and post-term infants combined by the Collaborative Group49 and Yoder et al. ;39 therefore, these data are not presented here.
| Study | Arm | Air leak/pneumothorax, n (%) | Nasal trauma, n (%) | IVH (grade ≥ 3), n (%) | NEC, n (%) | Apnoea, n (%) | Acidosis, n (%) |
|---|---|---|---|---|---|---|---|
| Collaborative group, 201449 | HHHNFC (n = 79) | NRa | NRa | NR | NR | NRa | NRa |
| NCPAP (n = 71) | NRa | NRa | NR | NR | NRa | NRa | |
| Collins et al., 201337 | HHHNFC (n = 67) | Pneumothorax: 0 (0) | Leading to change of treatment: 0 (0.0)** | 2 (2.9) | 2 (2.9) | 14 (20.9) | 0 |
| Nasal trauma score, mean (SD): 3.1 (7.2) | |||||||
| NCPAP (n = 65) | Pneumothorax 1 (1.5) | Leading to change of treatment: 13 (20.0)** | 4 (6.2) | 5 (7.7) | 17 (26.2) | 3 (4.6) | |
| Nasal trauma score, mean (SD): 11.8 (10.7) | |||||||
| Manley et al., 201338 | HHHNFC (n = 152) | Pneumothorax: 0 (0.0) | Any documented: 60 (39.5)* | 3 (2.0) | Stage 2/3: 3 (2.0) | 32 (21.1) | 6 (11.5) |
| Leading to change of treatment: 8 (5.3)*** | |||||||
| Caused by the assigned treatment: 29 (19.1)**** | |||||||
| NCPAP (n = 151) | Pneumothorax 1 (0.7) | Any documented: 82 (54.3)* | 8 (5.3) | Stage 2/3: 7 (4.6) | 25 (16.6) | 2 (5.1) | |
| Leading to change of treatment: 27 (17.9)*** | |||||||
| Caused by the assigned treatment: 80 (53. 0)**** | |||||||
| Yoder et al., 201339 | HHHNFC (n = 75) | NRa | NRa | NR | NRa | NRa | NR |
| NCPAP (n = 73) | NRa | NRa | NR | NRa | NRa | NR |
Data were pooled into a meta-analysis for pneumothorax (Figure 7), nasal trauma leading to change of treatment (Figure 8), IVH (grade ≥ 3) (Figure 9), necrotising enterocolitis (NEC) (Figure 10), apnoea (Figure 11) and acidosis (Figure 12). With the exception of apnoea and acidosis, the forest plots show the findings are in the direction of favouring HHHFNC. Statistically significant differences were reported for nasal trauma leading to change of treatment, with fewer preterm infants changing treatment with HHHFNC than with NCPAP. No statistically significant differences between arms were reported for any other adverse events, although for IVH (grade ≥ 3) and NEC events were noticeably numerically fewer in the HHHFNC arm.
FIGURE 7.
Meta-analysis for pneumothorax. df, degrees of freedom; M–H, Mantel–Haenszel.

FIGURE 8.
Meta-analysis for nasal trauma leading to change of treatment. df, degrees of freedom; M–H, Mantel–Haenszel.
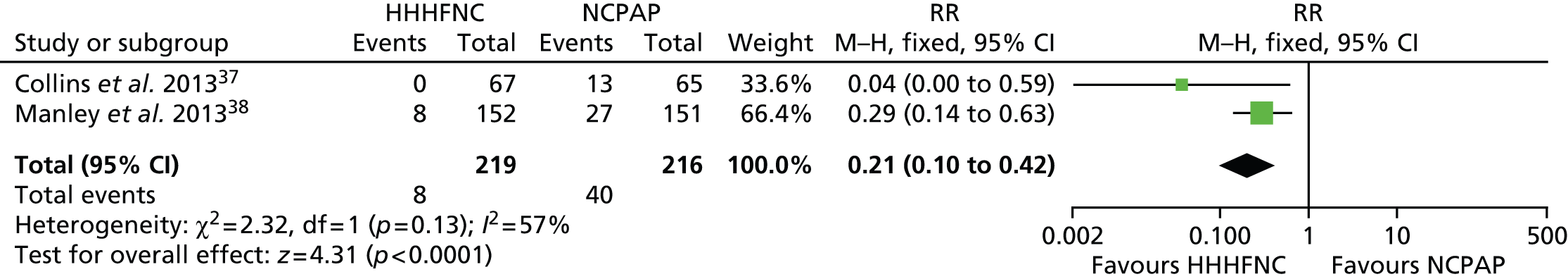
FIGURE 9.
Meta-analysis for IVH (grade ≥ 3). df, degrees of freedom; M–H, Mantel–Haenszel.

FIGURE 10.
Meta-analysis for NEC. df, degrees of freedom; M–H, Mantel–Haenszel.

FIGURE 11.
Meta-analysis for apnoea. df, degrees of freedom; M–H, Mantel–Haenszel.
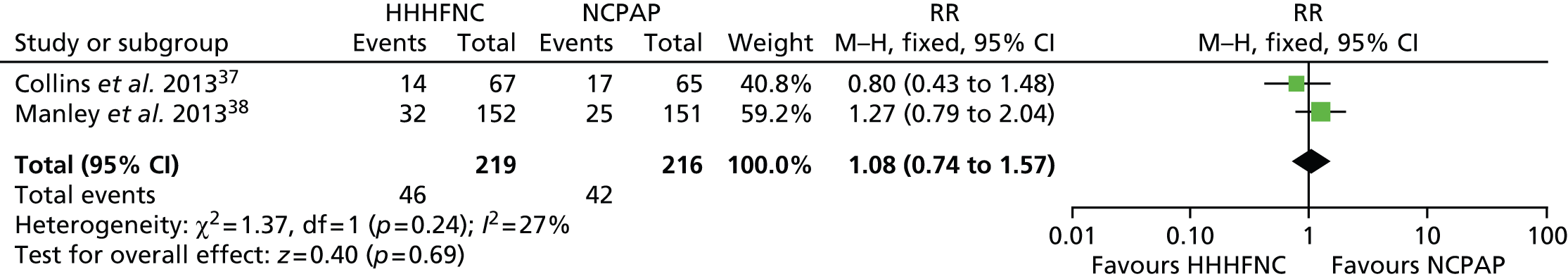
FIGURE 12.
Meta-analysis for acidosis. df, degrees of freedom; M–H, Mantel–Haenszel.
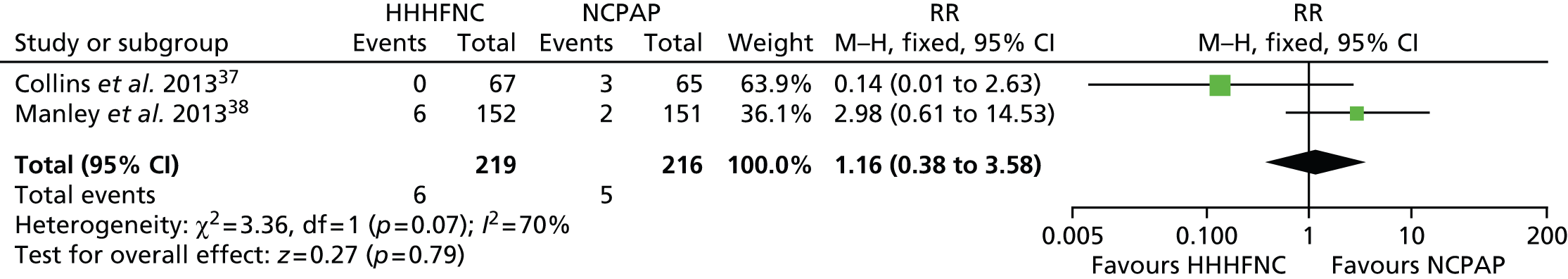
In addition to data that could be pooled, differences in the nasal trauma score were statistically different favouring HHHFNC in Collins et al. 37 In Manley et al. 38 the difference in the incidence of nasal trauma was statistically significant whether reported as any documented nasal trauma, nasal trauma leading to a change of treatment or nasal trauma caused by the assigned treatment. Manley et al. 38 was the only study to report on nosocomial sepsis and gastrointestinal perforation, both of which were numerically fewer in the HHHFNC arm than in the NCPAP arm (17.1% vs. 19.9% and 0.7% vs. 1.3%, respectively).
Efficacy findings from secondary analysis
Findings for infants who had not received prior ventilation are summarised in Table 12. The primary outcome of our review, treatment failure as defined by the need for intubation, was reported in one study. 52 Respiratory failure, defined by a composite outcome incorporating blood gas and another outcome such as fraction of inspired oxygen > 70% or frequent apnoea or bradycardia, was reported by one other. 33 Neither study33,52 reported a statistically significant difference between arms for treatment failure/respiratory failure for either HHHFNC compared with NIPPV52 or HHHFNC compared with NCPAP. 33
| Study | Arm | Treatment failure,a n (%) | BPD/death, n (%) | Time (days) on mechanical support, median (range) | Days on oxygen support, median (range) | Length (days) of hospital stay, median (range) | Days to full feeds, median (range) |
|---|---|---|---|---|---|---|---|
| Klingenberg et al., 201451 | HHHNFC (n = 20)b | NR | NR | NR | NR | NR | NR |
| NCPAP (n = 20)b | NR | NR | NR | NR | NR | NR | |
| Kugelman et al., 201452 | HHHNFC (n = 38) | 11 (28.9) | BPD, 1 (2.6); death 0 (0) | 3.0 (0.01–14) | 5.0 (0–69. 0) | 35 (8–91) | 13.0 (6–28) |
| NIPPV (n = 38) | 13 (34.2) | BPD, 2 (5.2); death, 0 (0) | 4.0 (0.5–16) | 3.0 (0–90.0) | 39.5 (9–113) | 11.0 (5–49) | |
| Nair and Karna, 200533 | HHHNFC (n = 13) | 2 (15.3) | NR | NR | NR | NR | NR |
| 4 (12.1)c | |||||||
| NCPAP (n = 15) | 2 (13.3) | NR | NR | NR | NR | NR | |
| 4 (11.8)c |
In the study by Kugelman et al. ,52 compared with NIPPV, time on mechanical ventilation and length of hospital stay were reduced with HHHFNC and days on oxygen support were increased; however, the differences between trial arms were not statistically significant. In the same study,52 days to full feeds also appeared to be greater in the HHHFNC arm than in the NIPPV arm, again the difference was not statistically significant. None of these outcomes was reported by either of the two other studies33,51 comparing HHHFNC with NCPAP. A number of the other secondary outcomes that we had planned to measure were not reported by any study at all, namely BPD/death (composite outcome), duration of respiratory support on NCPAP or HHHFNC or length of stay in NICU.
Adverse events reported for secondary analysis
The authors of the study by Kugelman et al. 52 reported adverse events for infants who had not received prior ventilation. These were numerically higher with HHHNFC than with NIPPV (except for apnoea), but no statistically significant differences were reported. The following adverse events were reported for HHHFNC compared with NIPPV: air leak (5.3% vs. 0.0%), nosocomial sepsis (10.5% vs. 7.8%), IVH (5.3% vs. 2.6%), NEC (5.3% vs. 0.0%) and apnoea (10.5% vs. 13.1%). There were no incidences of nasal trauma in either arm.
Quality of care
Klingenberg et al. 51 reported the results of a crossover study comparing HHHFNC with NCPAP for preterm infants who had received no prior ventilation (secondary analysis); this was the only study to report on outcomes relevant to quality of care (within two 24-hour periods). The primary outcome of the study was patient comfort, defined as a state free of prolonged pain by a validated neonatal pain and discomfort scale [the Echelle Douleur Inconfort Nouveau-Né (Neonatal Pain and Discomfort) scale]. 55 No statistically significant differences between arms were reported for this outcome or for noise of equipment (measured by a handheld audiometer). There were, however, statistically significant differences for all parental assessment measures (from a visual analogue scale rated 1–10) with parents preferring HHHFNC to NCPAP (Table 13). In addition, it was noted by the study authors that infants had significantly lower respiratory rates in the HHHFNC arm than in the NCPAP arm in this study51 but that all other respiratory parameters were similar.
| Study | Arm | EDIN score,a mean (SD) | Noise, dBA, mean (SD) | Parental assessment, mean (SD) | ||
|---|---|---|---|---|---|---|
| Child satisfiedb | Contact and interactionb | Participate in careb | ||||
| Klingenberg et al., 201451 | HHHNFC (n = 20)c | 10.7 (3.3) | 70 (10) | 8.6 (1.1)** | 9.0 (1.1)** | 9.1 (1.2)* |
| NCPAP (n = 20)c | 11.1 (3. 0) | 74 (10) | 6.9 (1.6)** | 6.7 (1.6)** | 8.0 (1.6)* | |
Chapter 5 Cost-effectiveness results
For the primary analysis of preterm infants treated following ventilation, there were no statistically significant differences in the primary outcome reported in the studies comparing HHHFNC and NCPAP that were included in the clinical review. The only difference identified was related to the rate of adverse events, notably in nasal injury in favour of HHHFNC. No long-term adverse events from nasal injury were identified from the studies included in the clinical review.
Given the absence of any differences in primary outcome or in long-term adverse events, the time horizon of the economic model was limited to the period during which a preterm infant received oxygen therapy. With the only difference in outcome being short-term nasal injury, this can be the only difference in quality of life for the patient.
Utility value derivation from preterm infants cannot be done directly and in this case would likely result in only very small quality-of-life decrements related to skin irritation and infection. Treatment is rapidly administered, and from the clinical experience of the authors who are clinicians (BS, PS) any irritation clears normally in 5–7 days. As such, any utility loss was thought to be so small as to be inconsequential to include in the analysis, although the treatment costs of this adverse event could be included. In the clinical experience of the authors who are clinicians (BS and PS), nasal trauma from NCPAP can be so severe as to require plastic surgery. As this event was thought to be very rare and there was no evidence in the available literature of this event occurring, it has not been included in the analysis.
Given the absence of any difference in primary outcome and utility between the technologies, a cost–utility analysis could not be undertaken.
In addition, in the absence of differences in primary outcome the only cost-effectiveness analysis that could be undertaken would be based on the use of secondary outcome data; in this case, the incremental cost-effectiveness ratio would be defined as the cost per case of nasal injury avoided. As this is not a primary outcome in any of the studies included in the clinical effectiveness review, in our opinion it is unlikely that such an analysis would be meaningful and so cost-effectiveness analysis was not undertaken based on any secondary outcome.
Given the inability to undertake cost–utility analysis or meaningful cost-effectiveness analysis, coupled with there being evidence for no statistically significant difference between treatment arms for the primary clinical outcome, the need for intubation, a cost-minimisation analysis for the primary analysis was undertaken comparing HHHFNC with NCPAP from the perspective of the NHS. For the secondary analysis of infants who had received no prior ventilation, there was an absence of evidence on the difference in the primary outcome, the need for intubation; only one completed small study52 examined this outcome whereas another, which was halted early,33 investigated a similar outcome (respiratory failure; which was a composite end point) and both compared HHHFNC with different devices (NIPPV52 and NCPAP33) and so we considered there to be an absence of evidence (as opposed to evidence of no difference from a meta-analysis for the primary outcome). Thus, while considered for the secondary analysis, a cost-minimisation analysis was potentially misleading, as it could lead decision-makers towards a cheaper technology which has unknown relative effectiveness.
Treatment resource use and costs
Resource use of treatment included capital equipment, consumable costs and clinician time taken to establish a preterm infant onto either HHHFNC or NCPAP. All prices are in 2015 GBP unless otherwise stated. Given the time horizon is the period up to discontinuation of NCPAP or HHHFNC, no discounting needed to be applied to costs.
Clinician time
From the clinical experience of two of the review authors (BS and PS), there is no difference in the time taken to set up a preterm infant on HHHFNC or on NCPAP and so this was not included in the analysis.
Capital equipment
Nasal continuous positive airway pressure can be delivered either through mechanical ventilators or through dedicated NCPAP equipment. It is the opinion of the authors who are clinicians (BS, PS) that the preference is to use dedicated NCPAP equipment, as this equipment is supposed to provide a nasal airflow that is more suitable for NCPAP than mechanical ventilation. In addition, the use of dedicated NCPAP equipment means that mechanical ventilators can be kept free for use elsewhere. Dedicated NCPAP equipment was therefore included as a resource in the evaluation rather than mechanical ventilators.
It is the opinion of the authors who are clinicians (BS and PS) that not only is there a range of manufacturers with different devices that can be used, the prices quoted by the manufacturer can vary depending on the volume purchased.
From NHS Supply Chain48 information the quoted price for a non-humidified NCPAP machine (the Maxblend NCPAP flow generator complete system by Armstrong Medical Ltd, Coleraine, Northern Ireland) was £6122. Although there may be other devices available, this appeared to be the only fixed (rather than portable) system that can be used specifically on preterm infants on NHS Supply Chain. 48 This compares with clinical experience of one the authors (BS) on the cost of a NCPAP machine being in the region of £5000 depending on make and volume purchased. As such, the £6122 figure for the Maxblend NCPAP machine seemed reasonable and was used in the analysis.
For HHHFNC, again there are several machines on the market that could potentially be used to deliver care. The Optiflow 850™ (Fisher & Paykel, Auckland, New Zealand) is used in the neonatal unit where one of the authors (BS) is based. The NHS Supply Chain48 cost of this device is £2755 and this figure was used in the economic analysis.
To provide a unit cost per infant of each machine, we have assumed that each machine lasts 5 years and that any service costs for machines are equal and so do not need to be included in analysis. We have then assumed that the devices are in use for 80% of the time and that each preterm infant requires oxygen support for 43.5 days, which is the mid-point of the medians for HHHFNC and NCPAP reported in Manley et al. 38
Putting these assumptions into a calculation suggests the unit cost of each machine per infant supported is equal to:
-
the cost of the machine (£6122 for NCPAP and £2755 for HHHFNC)
-
divided by 80% (the machine utilisation rate)
-
divided by 365.2 × 5 (the number of days in the 5-year lifespan on the machines)
-
multiplied by 43.5 (the number of days, on average, an infant requires use of NCPAP or HHHFNC).
This suggests a unit cost of £182 per preterm infant for a NCPAP machine and £82 per preterm infant for a HHHFNC machine.
Consumables
As was the case for capital equipment, there are a range of suppliers and potential prices available from the NHS Supply Chain48 for NCPAP and HHHFNC consumables (i.e. equipment that is required as part of the treatment but which is disposed of and cannot be reused, such as nasal canulae or tubing).
Given the variation in potential prices for different systems and the potential difference in quoted prices and prices paid, the weekly cost of consumables used in the economic analysis was provided directly by the neonatal unit that had provided information on the NCPAP and HHHFNC capital equipment (where BS is based). This approach was undertaken to ensure consistency and that any difference in the cost of consumables was that which was really experienced in a NHS setting.
For HHHFNC the total cost of all consumables was estimated to be £67 per week and for NCPAP it was estimated to be £55 per week.
Adverse events
The only evidence showing a statistically significant difference in the incidence of adverse events between infants on HHHFNC and NCPAP was nasal injury. There were no cases of nasal injury that were serious enough to require corrective surgery described in any of the studies included in the clinical review.
Based on the experience of two of the review’s authors who are clinicians (BS and PS), the majority, if not all, nasal injury would be relatively minor with no long-term consequences. One author was unaware of damage that had led to corrective surgery whereas another could think of only one case in 5 years in which nasal damage had resulted in the requirement for corrective surgery. Although it is recognised that there can be long-term aesthetic consequences from nasal injury, we are not aware of this as an issue nor are we aware of any literature that may point to this. As such, occurrences of serious and long-term nasal injury from either HHHFNC or NCPAP were not considered in the economic analysis, although the potential for long-term consequences from nasal injury should be considered as part of the overall analysis of the two technologies.
Treatment for nasal injury while the preterm infant is on oxygen therapy was described as being antiseptic/antibacterial cream two or three times a day for 5–7 days if it is ulcerated with rest to the infant’s septum.
As the preterm infant will be in a high-dependency care unit, Royal College of Nursing standards state a staff ratio of one nurse to two preterm infants will be required. 56 From a nurse time perspective, it is likely that application of the cream would form part of the care routine for a preterm infant and there is no real opportunity cost of the time taken to apply the cream, as the nurse would have to be on the unit in any event. As such, including the small amount of time it would take to apply the cream by a nurse is, in our opinion, not appropriate. The cost also of the antiseptic cream applied could vary by the preparation. It is assumed that the cream would contain chlorhexidine. Such creams are inexpensive even if bought privately. For example, 15 g of neomycin 0.5% chlorhexidine hydrochloride 0.1% cream can be purchased for £2.85. 57 With such low costs there is no need to be too precise when measuring the volume of cream used or on the exact cream used and price paid. As such, we have assumed that over the 5- to 7-day treatment period there is a £2 cost for the cream used.
Manley et al. 38 and Collins et al. 37 reported changes in treatment because of nasal injury. It is not clear whether or not changes in treatment protocol reflect routine clinical practice in the NHS. As a result of this, and as the changes in treatment did not result in longer lengths of stay (in Manley et al. 38) or statistically significantly higher reintubation rates (in Manley et al. 38 and Collins et al. 37), changes in treatment because of nasal injury are not considered as being economically important.
Resource and cost summary
The costs per preterm infant for HHHFNC and NCPAP are summarised in Table 14. The data support the clinical opinion of the authors who are clinicians (BS and PS) that there is not likely to be a statistically significant difference between the costs of therapy. The higher capital equipment costs of NCPAP are not outweighed by the higher consumable costs of HHHFNC, with HHHFNC estimated to cost £26.37 less than NCPAP per preterm infant treated.
| Resource | Cost per preterm infant (£) | Source | |
|---|---|---|---|
| HHHFNC | NCPAP | ||
| Capital equipment | 82.02 | 182.28 | NHS Supply Chain48 for machine cost assumption of 5-year lifespan of machine and 80% utilisation. Manley et al.38 for number of days per preterm infant on average on oxygen |
| Consumables | 416.36 | 341.79 | Clinical advice on weekly cost and Manley et al.38 for number of days per preterm infant on average on oxygen |
| Antiseptic cream for nasal injury | 0.38 | 1.06 | Assumption of £2 cost of cream with rates of nasal injury from Manley et al.38 |
| Total costs per preterm infant | 498.76 | 525.13 | |
Analysis of uncertainty
Ordinarily in an economic evaluation, scenario analysis and deterministic and probabilistic sensitivity analysis would be used to explore parameters when there was uncertainty in the economic model.
As we carried out a cost-minimisation analysis, this analysis has focused on the resources and costs associated with two treatments that the clinical evidence suggests are equally efficacious for the primary outcome of interest. The only notable difference between the treatments was in nasal injury as an adverse event and this has a very low cost per patient.
No distributions on any of the costs or resource use are available and so any probabilistic analysis of uncertainty is not possible. However, assumptions were made on the life expectancy of NCPAP and HHHFNC machines. As the cost saving for HHHFNC is driven by the greater capital cost of NCPAP, these assumptions were explored with sensitivity analysis.
Table 15 shows the two-way sensitivity analysis of the cost differential with HHHFNC compared with NCPAP as the utilisation rates vary between 20% and 100% and the lifespan varies between 2 and 10 years.
| Utilisation rates (%) | Machine lifespan (years) | |||||
|---|---|---|---|---|---|---|
| 2 | 4 | 5 | 6 | 8 | 10 | |
| 20 | £928.60 | £427.36 | £327.11 | £260.27 | £176.73 | £126.61 |
| 40 | £427.36 | £176.73 | £126.61 | £93.19 | £51.42 | £26.36 |
| 60 | £260.27 | £93.19 | £59.78 | £37.50 | £9.65 | –£7.06 |
| 80 | £176.73 | £51.42 | £26.36 | £9.65 | –£11.23 | –£23.77 |
| 100 | £126.61 | £26.36 | £6.31 | –£7.06 | –£23.77 | –£33.79 |
The threshold analysis shows that if the lifespan of the machines reaches 6.8 years then HHHFNC would no longer be cost saving compared with NCPAP. A machine lifespan above 6.8 years means that NCPAP becomes the less costly option.
Changes in machine lifespan and utilisation rate are positively related to the number of infants that can be used by each machine and therefore negatively related to the machine unit cost per infant (i.e. lower utilisation rates/machine lifespans lead to a lower number of infants that can use a machine over its lifespan, and therefore higher unit costs of the machine per infant). Although these changes in unit cost will be proportionally the same for each technology, the machine cost of NCPAP is higher than with HHHFNC. As such, the change in the absolute difference in unit cost per infant between the technologies is negatively related to the utilisation rate and machine lifespan (i.e. higher utilisation rates/machine lifespans lead to a smaller absolute difference in the machine unit costs per infant between NCPAP and HHHFNC).
It is also possible that different neonatal units pay different costs for consumables depending on the NCPAP and HHHFNC systems employed. However, what is important for our economic analysis is the size of the cost differential in consumables rather than the consumable costs per se. As costs can vary between units, a two-way sensitivity analysis was also undertaken to show how the differential in consumable costs together with the lifespan of the different machines change. The difference in consumable costs ± £24 (200%) is shown in Table 16; in the initial analysis there is a cost difference of –£12 per week (consumable cost with NCPAP is £55 and with HHHFNC is £67).
| Weekly consumable cost difference (NCPAP – HHHFNC) per preterm infant (£) | Machine lifespan (years) | |||||
|---|---|---|---|---|---|---|
| 2 | 4 | 5 | 6 | 8 | 10 | |
| –12 | £325.88 | £200.56 | £175.50 | £158.79 | £137.91 | £125.38 |
| 0 | £251.30 | £125.99 | £100.93 | £84.22 | £63.34 | £50.80 |
| 12 | £176.73 | £51.42 | £26.36 | £9.65 | –£11.24 | –£23.77 |
| 24 | £102.16 | –£23.15 | –£48.21 | –£64.92 | –£85.81 | –£98.34 |
| 36 | £27.59 | –£97.72 | –£122.78 | –£139.49 | –£160.38 | –£172.91 |
The results presented in Table 16 demonstrate that the main finding of the economic analysis, that is HHHFNC is cost saving compared with NCPAP, is relatively sensitive to changes in the difference in weekly consumable costs of the two technologies. Assuming a 5-year lifespan for equipment as in the initial analysis, if the difference in consumable prices rises approximately by 35% from £12 to £16.24 then HHHFNC will no longer be cost saving compared with NCPAP.
Chapter 6 Discussion
Principal findings
We have conducted a systematic review of the literature to summarise the clinical effectiveness of HHHFNC compared with usual care for preterm infants. Usual care was considered to consist of NCPAP, oxygen or NIPPV with five RCTs33,37–39,49,51 comparing HHHFNC with NCPAP and one RCT52 with NIPPV. Evidence was derived from four RCTs37–39,49 for effectiveness of treatment following ventilation (primary analysis) and three RCTs33,51,52 for clinical effectiveness following no prior ventilation (secondary analysis) including a crossover trial by Klingenberg et al. 51 The quality of the studies included in the primary analysis of treatment following ventilation could be considered to be superior to that of the studies included in the secondary analysis of treatment with no prior ventilation.
In the primary analysis, the primary outcome for our systematic review was treatment failure; we defined treatment failure to be the need for reintubation within 72 hours, within 7 days or ever (i.e. time period not specified). There were proportionally fewer cases of reintubation of preterm infants treated following ventilation in the HHHFNC arm than in the NCPAP arm in all four RCTs,37–39,49 although no statistically significant difference was found between treatment arms, either as reported in the individual studies37,38,49 or in the meta-analysis of these three trials reporting reintubation within 7 days. Two RCTs37,38 used composite outcomes to define extubation failure/treatment failure rather than simply defining it as the need for reintubation. Interestingly, despite the reintubation rate being lower for those treated with HHHFNC than those treated with NCPAP, the largest RCT by Manley et al. 38 reported a higher rate of treatment failure for HHHFNC compared with NCPAP.
Extubation failure/treatment failure was the only outcome that was considered in a subgroup analysis in which two trials37,38 considered this outcome by gestational age. In our review protocol, we had proposed conducting subgroup analyses of gestational age prior to and from 30 weeks but the included studies reported these prior to and from 26 weeks38 and prior to and from 28 weeks. 37 Unsurprisingly, infants with extremely low gestational age appeared to have higher rates of treatment failure in the individual studies,37,38 although the difference between subgroups was not statistically significant. The subgroup findings must be treated with extreme caution and can only be considered exploratory because different gestational age thresholds were used to define subgroups in the two studies and because extubation failure/treatment failure was also defined differently in the two studies;37,38 as discussed previously, these studies37,38 used composite outcomes, as opposed to our definition that was simply the need for reintubation.
In the secondary analysis, with regard to preterm infants who had received no prior ventilation, treatment failure was defined by the need for endotracheal ventilation52 or by a composite outcome. 33 Neither study33,52 reported a statistically significant difference in treatment failure rates for HHHFNC compared with NCPAP33 or HHHFNC compared with NIPPV. 52
Secondary efficacy outcomes for the comparison of HHHFNC compared with NCPAP were only reported in three studies;37–39 all three studies were included in the primary analysis of treatment following ventilation. Meta-analyses found that the findings for both outcomes are in the direction of favouring HHHFNC but no statistically significant differences were found. The majority of other relevant secondary outcome data (e.g. days on mechanical support and length of hospital stay) also suggested an improvement for HHHFNC over NCPAP but these were not reported by two or more trials and no statistically significant differences were reported between arms. 37,38
The authors of the study by Kugelman et al. 52 reported relevant secondary outcomes for HHHFNC compared with NIPPV. In this study, no preterm infant had received prior ventilation (secondary analysis). Although the findings from this small study52 appeared to marginally favour HHHFNC over NIPPV in terms of days on mechanical support and length of hospital stay and marginally favour NIPPV over HHHFNC in terms of days on oxygen support and days to full feeds, none of the between-arm differences was statistically significant.
Adverse event data for the comparison of HHHFNC compared with NCPAP were only available from two studies37,38 that were included in the primary analysis (preterm infants treated following ventilation). Importantly, nasal trauma was statistically significantly lower in the HHHFNC arm in the largest study by Manley et al. 38 Meta-analysis of nasal trauma leading to change of treatment also showed statistically significantly fewer infants changing treatment from HHHFNC than with NCPAP. With the exception of apnoea and acidosis, where mixed results were reported in the individual studies, pneumothorax, IVH (grade ≥ 3) and NEC appeared to be less common with HHHFNC than NCPAP but differences were not statistically significant.
Adverse event data for the comparison of HHHFNC with NIPPV were only available from one study52 that was included in the secondary analysis. Generally the adverse event profile appeared to marginally favour NIPPV over HHHFNC, but there were no between-arm statistically significant differences.
Klingenberg et al. 51 reported outcomes from the smallest RCT included in our review (n = 20) and was the only study to report quality-of-care outcomes. Although there were no statistically significant differences between arms in terms of noise or neonatal pain and discomfort, there were statistically significant differences between study arms in terms of parental preferences for HHHFNC over NCPAP. Parental preferences were based on the belief that (1) child satisfaction, (2) contact and interaction and (3) opportunities to take part in care were all improved with HHHFNC compared with NCPAP. In this study,51 preterm infants were not supposed to have been treated following ventilation, although a minority of infants had, in fact, received prior ventilation (n = 7, 30%).
In summary, therefore, following ventilation (primary analysis), there is a lack of convincing evidence for a difference in the need for reintubation, BPD or death between HHHFNC and NCPAP; there is, however, some evidence for a decrease in nasal trauma. For preterm infants with no prior ventilation (secondary analysis), there is some suggestive evidence for parental preferences for HHHFNC over NCPAP but overall an absence of any consistent evidence to suggest that HHHFNC is superior or inferior to usual care.
The lack of evidence supporting the clinical effectiveness of HHHFNC compared with usual care, or vice versa, precluded us from being able to conduct a cost–utility or cost-effectiveness analysis for either HHHFNC compared with usual care following ventilation or HHHFNC compared with usual care with no prior ventilation. Instead, we were only able to conduct a cost-minimisation analysis. Given the absence of evidence for infants who had no prior ventilation, a cost-minimisation analysis was only performed for infants who had been treated following ventilation.
The results of our cost-minimisation analysis suggest that HHHFNC would be cost saving over NCPAP for infants who have been treated following ventilation. However, the results of our economic analysis are sensitive to both the size of the machine lifespan and utilisation of equipment. When estimating and valuing resources for these two items in the analysis, it was necessary to make assumptions and so there is a degree of uncertainty associated with the results. If the HHHFNC and NCPAP machines last, on average, longer than 6.8 years and assuming an 80% utilisation rate for equipment, NCPAP is likely to become the less costly of the two technologies. Although the cost differential of consumables has a higher degree of certainty than the lifespan of the machines, as costs have been derived from an individual neonatal unit, it is not known how representative this difference might be across units in the UK. If HHHFNC consumables cost £16.24 or more than NCPAP consumables per week, then NCPAP will become the less costly of the two technologies. Hence, while the best estimate from the economic analysis is that HHHFNC will cost just over £26 less per infant than NCPAP, the cost saving could be as high as £326 per infant with HHHFNC over NCPAP or NCPAP could save £173 compared with HHHFNC, depending on differences in the lifespan of machines, utilisation rates and cost differences in consumables.
In reality, the actual total cost differential between infants on either technology is relatively insignificant compared with the cost per day in a neonatal intensive care ward, regardless of the assumptions employed in the analysis. The NHS Reference Cost47 for a day in a neonatal high-dependency unit in 2013/14 was £839 per day or just under £36,500 for a 43.5 day stay. The cost of either treatment with HHHFNC or NCPAP during this period therefore costs less than 2% of the total care while the infant requires oxygen. The economic analysis therefore shows that cost does not seem to be a paramount consideration when deciding between the two technologies.
Similarities and differences with previous systematic reviews and meta-analyses
We are aware of two other published meta-analyses of HHHFNC compared with NCPAP; one published alongside a systematic review by Daish and Badurdeen36 and another which accompanies a review of the literature by DeMauro et al. 58 Both of these meta-analyses include the same three trials. 37–39 In addition, we are aware of an unpublished ‘pooled analysis’ of HFNC compared with NCPAP which has only been presented as an abstract by Rotta et al. 59 and which includes four trials; as data have only been presented in abstract form for this unpublished analysis59 it is unclear which trials were included and if the HFNC described in all four trials is heated.
All analyses reported no statistically significant differences between arms for extubation failure, although the RR exceeded one, suggesting that the treatment effect may be in favour of NCPAP (RR 1.12, 95% CI 0.85 to 1.4736 and RR 1.05, 95% CI 0.79 to 1.3958 in the published meta-analyses; RR 1.17, 95% CI 0.90 to 1.5159 in the unpublished analysis59). Although our meta-analysis also reported no statistically significant differences, the RR was less than one suggesting that the treatment effect may be in favour of HHHFNC (RR 0.76, 95% CI 0.54 to 1.09).
The reasons for marginal differences in results arise from including different studies in the meta-analyses and from differences in how data were pooled in each of the meta-analyses. It is unclear from the unpublished abstract which four trials were included in the pooled analysis by Rhotta et al. 59 However, both the published meta-analyses36,58 and our meta-analysis reported on three trials, including the same two Australian RCTs. 37,38 Whereas Daish and Badurdeen36 and DeMauro et al. 58 also included the US study by Yoder et al. ,39 we excluded this study from our meta-analysis as extubation failure reported for the subgroup of preterm infants was for reintubation within 72 hours. Data from a subgroup analysis of preterm infants from the Chinese study49 were also included in our meta-analysis but not in the other studies.
Crucially, we also used a standard definition of treatment failure across all studies included in our meta-analysis (reintubation rates within 7 days). The other two published meta-analyses,36,58 however, used the original study definitions of treatment failure/extubation failure which differed across all three studies37–39 and, importantly, were measured over different time points (within 7 days in the two Australian studies37,38 and within 72 hours in the study by Yoder et al. ). 39 Finally, our meta-analysis included data describing only preterm infants who had received treatment following ventilation; the inclusion of the study by Yoder et al. 39 in the other two published meta-analyses36,58 resulted in a mixed population of infants, some of whom had received treatment following ventilation and some of whom had received no prior ventilation.
Differences in the choice of studies that were included in the meta-analyses and differences in how the data were pooled and analysed are reflected in the measures of statistical heterogeneity reported. A moderate level of statistical heterogeneity was identified for the meta-analysis of treatment failure (I2 = 56% and χ2 = 4.5; p = 0.11) by Daish and Badurdeen. 36 Greater and statistically significant (p < 0.10) levels of heterogeneity (I2 = 59.5%; p = 0.085) were reported in the meta-analysis by DeMauro et al. 58 Our meta-analysis reported no statistical heterogeneity at all (I2 = 0% and χ2 = 0.34; p = 0.84).
As per our meta-analysis, Daish and Badurdeen36 also pooled data for BPD and found no statistically significant differences between treatment arms. Data were pooled from the same three RCTs37–39 in both our meta-analysis and in the analysis conducted by Daish and Badurdeen. 36 Hence, the findings of the meta-analyses were identical (RR 0.92, 95% CI 0.72 to 1.17) with no statistical heterogeneity evident (I2 = 0% and χ2 = 0.87; p = 0.65). In this instance, it should be noted that the inclusion of the study by Yoder et al. 39 did result in a mixed population of infants in our meta-analysis, some of whom had received treatment following ventilation and some of whom had received no prior ventilation.
No previous meta-analysis of death has been previously published. The meta-analysis we conducted again found no statistically significant differences between arms. However, as perhaps expected from a meta-analysis of only two trials with few events, CIs were wide (RR 0.56, 95% CI 0.22 to 1.44). No significant statistical heterogeneity between studies was reported (I2 = 0% and χ2 = 1.21; p = 0.23).
Prior to the publication of the meta-analyses by Daish and Badudeen36 and DeMauro et al. 58 a Cochrane review31 included narrative results from a systematic review of the effectiveness of HFNC (as opposed to HHHFNC) from four RCTs. 32–35 Four different analyses were presented, with one study included in each analysis: HFNC compared with NCPAP for preterm infants who had received prior ventilation,32 HFNC compared with NCPAP with no prior ventilation,33 HHHFNC compared with ‘standard’ HFNC35 and a comparison of two different brands of equipment for HHHFNC. 34 It was not possible to conduct meta-analyses given each analysis only included one study. The review authors concluded that there was insufficient evidence to establish the safety or effectiveness of HFNC and that HFNC may be associated with a higher rate of reintubation than NCPAP when used after ventilation. It should be noted that this latter conclusion was drawn from one study32 comparing HFNC (not HHHFNC) to NCPAP and reporting a significantly worse outcome for HFNC (RR 4.0, 95% CI 1.33 to 12.05). No statistical differences were reported for HHHFNC compared with HFNC in the study by Woodhead et al. 35 which examined reintubation rates within the first 24 hours. However, the study was small (n = 30) and only two infants who received standard HFNC as opposed to no infants who received HHHFNC required reintubation; the data therefore suggest that HHHFNC may be superior to HFNC. Furthermore, infants were statistically significantly more likely to have a normal appearance of their nasal mucosa in the HHHFNC arm than in the HFNC arm in this study35 (p < 0.0005). This arguably highlights the importance of distinguishing between HHHFNC and HFNC.
Strengths and limitations
One of the strengths of our systematic review is that we have limited the inclusion of our evidence to RCTs in which evidence has been presented for only preterm infants, as opposed to a mixed population of preterm, term and post-term infants. We have also limited our review to only include studies when it was clear that the intervention was HHHFNC; HFNC that is neither heated or humidified is now considered by many review authors36,58 to be inconsistent with clinical practice. Finally, we have considered the clinical effectiveness of HHHFNC compared with usual care both following ventilation and in preterm infants who have received no prior ventilation. This distinction is of importance given that the European Consensus Guidelines4 recommend that NCPAP should be the preferred option for the stabilisation of preterm infants when possible, ventilation being preferred for less mature infants.
While we consider that limiting the inclusion of studies to only those in which it was clear HFNC was heated to be a strength, this approach may also be considered to be a limitation; study authors do not always explicitly state that the interventions they are studying are heated. Therefore, it is possible we have excluded some studies that we should have included. Certainly, we have excluded three abstracts by Collins60 and Collins et al. 61,62 that report on the same study that we have included. 37 This is because it was not stated in these three abstracts60–62 that the intervention was heated. Excluding these abstracts was, however, of no importance because we did include the fully published study with the relevant results. It does, however, suggest that there is a need for common and consistent terminology when describing whether HFNC is heated or humidified. Of the other six papers that we excluded for not being heated,27,32,63–66 three27,32,63 explicitly stated that they were unheated, meaning that three other papers64–66 (of two studies, one64 reported only as an abstract) may actually have been studies of HHHFNC.
An advantage of the data available for our primary analysis of infants who have received treatment following ventilation is that there is an element of consistency in how outcomes have so far been reported. This is particularly true for reintubation, which has been reported within 7 days, enabling comparisons across trials, and also for BPD and death. However, it still remains unclear if reintubation within 7 days is the optimal outcome and, arguably, reintubation should be reported at three different time points, within 72 hours, within 7 days and ever. The only study we are aware of that has reported reintubation at different time points is the study by Yoder et al. 39 Unfortunately, the findings at these two time periods are for a mixed population of preterm, term and post-term infants. This study39 did, however, provide a subgroup analysis for some, but not all, preterm infants [gestational age < 32 weeks (34.7% of the study population) as opposed to < 37 weeks] but only for reintubation within 72 hours. We contacted the principal author of the Yoder et al. 39 study to request further information about all preterm infants but we have not received a reply. It should also be noted that the study by Yoder et al. 39 also included infants who had received no prior ventilation alongside those who had been ventilated. However, it is unclear how many of the preterm infants had received prior ventilation.
A limitation of our review is the lack of evidence regarding the quality of care delivered in the clinical studies. It is often cited that HHHFNC is preferred over NCPAP by staff and parents of preterm infants, as it enables infants to be more easily handled and cared for than does NCPAP. 15,20,29 Only one of the RCTs51 we identified examined outcomes relating to quality of care, and data were available from only 20 participants and hence the generalisability of the findings should be treated with caution. Nevertheless, this study51 did report that parents preferred HHHFNC to NCPAP. In terms of neonatal pain and discomfort and noise, there were no statistically significant differences between HHHFNC and NCPAP. However, RCTs are not necessarily the best types of study to evaluate such outcomes, with qualitative studies and surveys probably being better suited to studying such outcomes. For example, it would be illustrative to know whether or not improved parental contact which was reported with HHHFNC over NCPAP by Klingenberg et al. 51 included an increase in the amount of time spent in ‘Kangaroo Care’. ‘Kangaroo Care’ entails skin-to-skin care between mother and infant. Previous studies have reported this practice to be beneficial to the development of infants67,68 and to reduce mortality. 69,70 Nonetheless, the inclusion of outcomes such as those measuring parental preferences as secondary outcomes in RCTs is informative.
Another limitation of the evidence base is that it was not possible for investigators to blind health-care staff or study participants to the treatment that they delivered or received in any of the RCTs. This is commonly cited as a major weakness of clinical trials44 but when comparing an intervention such as HHHFNC to an intervention such as NCPAP, such blinding would be impossible to employ; realistically, only those responsible for the analysis of the results could be blinded. Only one study (included in the primary analysis) reported that assessors were blinded to treatment allocation. 37
Arguably the largest limitation of our review, however, is the lack of published RCT data from relatively large-sized populations in which HHHFNC is compared with usual care. The lack of evidence is perhaps most stark when we present the secondary analysis of our review, assessing the clinical effectiveness of interventions in preterm infants with no prior ventilation. As discussed, there were only 124 preterm infants from the three relevant trials33,51,52 in the secondary analysis (although as also highlighted, seven of the participants in one trial51 had in fact received treatment following ventilation); this figure (n = 124) is smaller number than the number of participants in the smallest trial (n = 132)37 of preterm infants in the primary analysis. However, even for the primary analysis of those who received treatment following ventilation, more RCT evidence is required.
Finally, the lack of evidence describing treatment failure across trials and from our meta-analysis has also precluded us from being able to conduct a cost-effectiveness or cost–utility analysis, another limitation of our research. Instead we have only been able to conduct a cost-minimisation analysis which has levels of uncertainties around the costs and lifespan of different HHHFNC and NCPAP devices and associated consumables.
Uncertainty in the evidence base is evident from comparing the (statistically non-significant) findings for treatment failure from our meta-analysis to those of other authors;36,58,59 the results of our meta-analysis suggest that the treatment effect may be in favour of HHHFNC over NCPAP, whereas other authors36,58,59 suggest the opposite effect. However, as discussed, other authors use different definitions of treatment failure and include mixed populations, whereas we have limited the data in our meta-analysis to reintubation within 7 days in a population limited to preterm infants who have previously been ventilated.
Chapter 7 Conclusions
There is a lack of convincing clinical evidence to suggest that HHHFNC is superior or inferior to usual care, in particular NCPAP. This is true for preterm infants who have received treatment following ventilation and for those who have received no prior ventilation. The results of one small trial suggest that parents do, however, prefer HHHFNC to NCPAP.
There is also uncertainty regarding whether or not HHHFNC can be considered cost-effective because the lack of clinical evidence precluded us from conducting a cost–utility or cost-effectiveness analysis. The results of our cost-minimisation analysis suggest that HHHFNC may cost less than NCPAP, but there is much uncertainty around the assumptions employed and it is quite possible that HHHFNC costs more than NCPAP. As the overall cost of either HHHFNC or NCPAP is small compared with the cost of preterm neonatal care as a whole, and the potential cost differences between the systems are even smaller, the financial case for HHHFNC over NCPAP or vice versa is not compelling.
More RCT evidence comparing HHHFNC with usual care (in particular, NCPAP) is required to inform the evidence base for both the clinical effectiveness and cost-effectiveness for HHHFNC. Ideally, a large and adequately powered trial is required to compare HHHFNC to NCPAP in preterm infants previously ventilated and for preterm infants who have not received prior ventilation. Based on available evidence from a meta-analysis suggesting that the majority of outcomes (including reintubation, BPD, death and many important adverse events) are in the direction of favouring HHHFNC, it is possible that further research could include evidence derived from a non-inferiority trial.
Recommendations for future research
Based on the available evidence, the following research recommendations are made:
-
There is a need for more RCT evidence comparing HHHFNC with usual care including, but not limited to, a comparison with NCPAP. End points should include (re)intubation, BPD, death and adverse events. In particular, there is a need for research into the need for (re)intubation at both 72 hours and 7 days, both outcomes which should ideally be measured in individual trials. This is because trials have utilised both outcome measures, and results with respect to efficacy may differ at different follow-up times (as preterm infants may remain extubated for the first 72 hours but then be reintubated at 7 days).
-
Ideally, studies should only include preterm infants, and when infants may have received either previous ventilation or no prior ventilation RCTs should be stratified for these factors and subgroup analyses conducted.
-
Given the evidence has not shown HHHFNC to be statistically superior to NCPAP but the direction of the treatment effect appears to favour HHHFNC over NCPAP, a non-inferiority trial may be of particular value. As the primary outcome, BPD may be particularly clinically important and meaningful, as it has been shown to be associated with long-term disability and morbidities. The sample size for such a trial would then depend on the significance level and desired statistical power as well as the rate of BPD and preferred non-inferior margin, as detailed in Appendix 4, Table 23.
-
There is also a need for more research on quality of care in terms of staff and parental preferences, and infant comfort. Although these outcomes are arguably best investigated via qualitative studies and surveys, including such outcomes in future RCTs will be informative.
Acknowledgements
The authors would like to thank to Gareth Jones [Liverpool Reviews and Implementation Group (LRiG)] for administrative support, Siyuan Jiang (Clinical Fellow in Neonatology at Hospital for Sick Children, Toronto and Fudan University, China) for translating one of the papers into English and Angela Boland (LRiG) for reading and commenting on the final draft report.
Contributions of authors
Nigel Fleeman (Review Co-ordinator, LRiG) drafted the review protocol, managed process of study selection and provided input to the final report.
James Mahon (External Economic Consultant, Coldingham Economics) commented on the protocol, conducted assessment economic costs and implications, and provided input to the final report.
Vickie Bates (Reviewer, LRiG) commented on the protocol, conducted data extraction and quality assessment, and provided input to the final report.
Rumona Dickson (Director, LRiG) commented on the protocol, conducted study selection, checked data for accuracy and provided input to the final report.
Yenal Dundar (Reviewer, LRiG) contributed to the initial search strategy, conducted study selection and quality assessment, and provided input to the final report.
Kerry Dwan (Statistician, LRiG) contributed to the proposed statistical approach.
Laura Ellis (parent of premature infants) commented on the review protocol and draft of final report.
Eleanor Kotas (Information Specialist, LRiG) conducted literature searches for studies and costs, and gave input to the final report.
Marty Richardson (Statistician, LRiG) conducted the statistical analyses.
Prakesh Shah (Clinical Consultant, Mount Sinai Hospital and Departments of Paediatrics and of Health Policy, Management and Evaluation, University of Toronto) reviewed the protocol and provided input to the final report.
Ben NJ Shaw (Clinical Consultant, Consultant in Neonatal and Respiratory Paediatrics Neonatal Unit, Liverpool Women’s Hospital) reviewed the protocol and provided input to final report.
Data sharing statement
The RevMan file used for conducting the data analyses can be obtained from the corresponding author on request.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- de Winter JP, de Vries MA, Zimmermann LJ. Clinical practice: noninvasive respiratory support in newborns. Eur J Pediatr 2010;169:777-82. http://dx.doi.org/10.1007/s00431-010-1159-x.
- Miall L, Wallis S. The management of respiratory distress in the moderately preterm newborn infant. Arch Dis Child Educ Pract Ed 2011;96:128-35. http://dx.doi.org/10.1136/adc.2010.189712.
- Pramanik A. Respiratory Distress Syndrome 2012. http://emedicine.medscape.com/article/976034-overview#aw2aab6b2b4aa (accessed 3 December 2014).
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants – 2013 update. Neonatology 2013;103:353-68. http://dx.doi.org/10.1159/000349928.
- Kamath BD, MacGuire ER, McClure EM, Goldenberg RL, Jobe AH. Neonatal mortality from respiratory distress syndrome: lessons for low-resource countries. Pediatrics 2011;127:1139-46. http://dx.doi.org/10.1542/peds.2010-3212.
- Malloy MH, Hartford RB, Kleinman JC. Trends in mortality caused by respiratory distress syndrome in the United States, 1969–83. Am J Public Health 1987;77:1511-14. http://dx.doi.org/10.2105/AJPH.77.12.1511.
- Lee K, Khoshnood B, Wall SN, Chang Y, Hsieh HL, Singh JK. Trend in mortality from respiratory distress syndrome in the United States, 1970–1995. J Pediatr 1999;134:434-40. http://dx.doi.org/10.1016/S0022-3476(99)70200-3.
- Malloy MH, Freeman DH. Respiratory distress syndrome mortality in the United States, 1987 to 1995. J Perinatol 2000;20:414-20. http://dx.doi.org/10.1038/sj.jp.7200420.
- Marlow N. Keeping up with outcomes for infants born at extremely low gestational ages. JAMA Pediatr 2015;169:207-8. http://dx.doi.org/10.1001/jamapediatrics.2014.3362.
- Office for National Statistics . Gestation-Specific Infant Mortality, 2012 2014. www.ons.gov.uk/ons/rel/child-health/gestation-specific-infant-mortality-in-england-and-wales/2012/index.html (accessed 9 December 2014).
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2006;3. http://dx.doi.org/10.1002/14651858.cd004454.pub2.
- Mahmoud RA, Roehr CC, Schmalisch G. Current methods of non-invasive ventilatory support for neonates. Paediatr Respir Rev 2011;12:196-205. http://dx.doi.org/10.1016/j.prrv.2010.12.001.
- Kugelman A. Optimal management of neonatal lung diseases using current technologies. Pediatr Pulmonol 2014;49:S26-8.
- Chowdhury O, Wedderburn CJ, Duffy D, Greenough A. CPAP review. Eur J Pediatr 2012;171:1441-8. http://dx.doi.org/10.1007/s00431-011-1648-6.
- de Klerk A. Humidified high-flow nasal cannula: is it the new and improved CPAP?. Adv Neonat Care 2008;8:98-106. http://dx.doi.org/10.1097/01.ANC.0000317258.53330.18.
- De Paoli AG, Davis PG, Faber B, Morley CJ. Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.cd002977.pub2.
- Davis PG, Henderson-Smart DJ. Nasal continuous positive airways pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Database Syst Rev 2003;2. http://dx.doi.org/10.1002/14651858.cd000143.
- Armfield M, West G. Use of Vapotherm for respiratory support with neonates. Paediatr Nurs 2009;21:27-30. http://dx.doi.org/10.7748/paed2009.02.21.1.27.c6909.
- Pillow JJ. Which continuous positive airway pressure system is best for the preterm infant with respiratory distress syndrome?. Clin Perinatol 2012;39:483-96. http://dx.doi.org/10.1016/j.clp.2012.06.007.
- NHS Forth Valley Women & Children’s Unit . Humidified High Flow Nasal Cannulae Guideline, Version 1 2011. www.nhsforthvalley.com/__documents/qi/ce_guideline_wcdneonatal/humidifiedhighflownasalcannulaeguideline.pdf (accessed 2 December 2014).
- Cherian S, Morris I, Evans J, Kotecha S. Oxygen therapy in preterm infants. Paediatr Respir Rev 2014;15:135-41. http://dx.doi.org/10.1016/j.prrv.2012.12.003.
- Bhandari V. The potential of non-invasive ventilation to decrease BPD. Semin Perinatol 2013;37:108-14. http://dx.doi.org/10.1053/j.semperi.2013.01.007.
- Bhandari V. Nasal intermittent positive pressure ventilation in the newborn: review of literature and evidence-based guidelines. J Perinatol 2010;30:505-12. http://dx.doi.org/10.1038/jp.2009.165.
- Garg S, Sinha S. Non-invasive ventilation in premature infants: based on evidence or habit. J Clin Neonatol 2013;2:155-9. http://dx.doi.org/10.4103/2249-4847.123082.
- Bhandari V. Noninvasive respiratory support in the preterm infant. Clin Perinatol 2012;39:497-511. http://dx.doi.org/10.1016/j.clp.2012.06.008.
- Dani C, Pratesi S, Migliori C, Bertini G. High flow nasal cannula therapy as respiratory support in the preterm infant. Pediatr Pulmonol 2009;44:629-34. http://dx.doi.org/10.1002/ppul.21051.
- Sreenan C, Lemke RP, Hudson-Mason A, Osiovich H. High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics 2001;107:1081-3. http://dx.doi.org/10.1542/peds.107.5.1081.
- Ojha S, Gridley E, Dorling J. Use of heated humidified high-flow nasal cannula oxygen in neonates: a UK wide survey. Acta Paediatr 2013;102:249-53. http://dx.doi.org/10.1111/apa.12090.
- Hough JL, Shearman AD, Jardine LA, Davies MW. Humidified high flow nasal cannulae: current practice in Australasian nurseries, a survey. J Paediatr Child Health 2012;48:106-13. http://dx.doi.org/10.1111/j.1440-1754.2011.02070.x.
- Morbidity and Mortality Weekly Report . Update: Public health notification regarding Ralstonia associated with Vapotherm© respiratory gas administration devices – United States, 2005. MMWR 2005;54:1286-7.
- Wilkinson D, Andersen C, O’Donnell CPF, De Paoli AG. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev 2011;5. http://dx.doi.org/10.1002/14651858.cd006405.pub2.
- Campbell DM, Shah PS, Shah V, Kelly EN. Nasal continuous positive airway pressure from high flow cannula versus infant flow for preterm infants. J Perinatol 2006;26:546-9. http://dx.doi.org/10.1038/sj.jp.7211561.
- Nair G, Karna P. Comparison of the effects of Vapotherm and nasal CPAP in respiratory distress in preterm infants. PAS 2005;57.
- Miller SM, Dowd SA. High-flow nasal cannula and extubation success in the premature infant: a comparison of two modalities. J Perinatol 2010;30:805-8. http://dx.doi.org/10.1038/jp.2010.38.
- Woodhead DD, Lambert DK, Clark JM, Christensen RD. Comparing two methods of delivering high-flow gas therapy by nasal cannula following endotracheal extubation: a prospective, randomized, masked, crossover trial. J Perinatol 2006;26:481-5. http://dx.doi.org/10.1038/sj.jp.7211543.
- Daish H, Badurdeen S. Question 2: humidified heated high flow nasal cannula versus nasal continuous positive airway pressure for providing respiratory support following extubation in preterm newborns. Arch Dis Child 2014;99:880-2. http://dx.doi.org/10.1136/archdischild-2014-306617.
- Collins CL, Holberton JR, Barfield C, Davis PG. A randomized controlled trial to compare heated humidified high-flow nasal cannulae with nasal continuous positive airway pressure postextubation in premature infants. J Pediatr 2013;162:949-54. http://dx.doi.org/10.1016/j.jpeds.2012.11.016.
- Manley BJ, Owen LS, Doyle LW, Andersen CC, Cartwright DW, Pritchard MA, et al. High-flow nasal cannulae in very preterm infants after extubation. N Engl J Med 2013;369:1425-33. http://dx.doi.org/10.1056/NEJMoa1300071.
- Yoder BA, Stoddard RA, Li M, King J, Dirnberger DR, Abbasi S. Heated, humidified high-flow nasal cannula versus nasal CPAP for respiratory support in neonates. Pediatrics 2013;131:e1482-90. http://dx.doi.org/10.1542/peds.2012-2742.
- Desai P, Shetty S, Singh N, Kennea N. Current practice regarding the use of humidified high flow nasal cannulae (HHFNC) in UK neonatal units. Arch Dis Child 2012;97. http://dx.doi.org/10.1136/archdischild-2012-302724.0396.
- Hochwald O, Osiovich H. The use of high flow nasal cannulae in neonatal intensive care units: is clinical practice consistent with the evidence?. J Neonatal Perinatal Med 2010;3:187-91.
- Liverpool Women’s NHS Foundation Trust . High Flow Therapy (HFT) (Version 1 – NICU204) 2014. www.liverpoolwomens.nhs.uk/Library/health_professionals/Neonatal_policy_library/High_Flow_Oxygen_Guideline.pdf (accessed 2 December 2014).
- CRD’s Guidance for Undertaking Reviews in Healthcare: Systematic Reviews (3rd Edition). York: CRD, University of York; 2008.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2011. www.cochrane-handbook.org (accessed 17 December 2014).
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. http://dx.doi.org/10.1136/bmj.327.7414.557.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. http://dx.doi.org/10.1136/bmj.315.7109.629.
- NHS Reference Costs: Financial Year 2013 to 2014. London: Department of Health; 2015.
- NHS . NHS Supply Chain n.d. www.supplychain.nhs.uk/ (accessed 16 April 2015).
- Collaborative Group for the Multicenter Study on Heated Humidified High-flow Nasal Cannula Ventilation . Efficacy and safety of heated humidified high-flow nasal cannula for prevention of extubation failure in neonates. Zhonghua Er Ke Za Zhi 2014;52:271-6.
- Collins CL, Barfield C, Horne RS, Davis PG. A comparison of nasal trauma in preterm infants extubated to either heated humidified high-flow nasal cannulae or nasal continuous positive airway pressure. Eur J Pediatr 2014;173:181-6. http://dx.doi.org/10.1007/s00431-013-2139-8.
- Klingenberg C, Pettersen M, Hansen EA, Gustavsen LJ, Dahl IA, Leknessund A, et al. Patient comfort during treatment with heated humidified high flow nasal cannulae versus nasal continuous positive airway pressure: a randomised cross-over trial. Arch Dis Child Fetal Neonatal Ed 2014;99:F134-7. http://dx.doi.org/10.1136/archdischild-2013-304525.
- Kugelman A, Riskin A, Said W, Shoris I, Mor F, Bader D. A randomized pilot study comparing heated humidified high-flow nasal cannulae with NIPPV for RDS. Pediatr Pulmonol 2014;50:576-83. http://dx.doi.org/10.1002/ppul.23022.
- Ma L, Liu CQ, Gu XH, Liu XJ. The efficacy and safety of heated humidified high-flow nasal cannula for prevention of extubation failure in neonates. J Matern Fetal Neonatal Med 2014;27:208-9.
- Manley BJ, Owen LS, Doyle LW, Andersen CC, Cartwright DW, Pritchard MA, et al. High-flow nasal cannulae vs. nasal CPAP for post-extubation respiratory support of very preterm infants: a multicentre, randomised non-inferiority trial. J Paediatr Child Health 2013;49.
- Debillon T, Zupan V, Ravault N, Magny J, Dehan M, Abu-Saad H. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Arch Dis Child Fetal Neonatal 2001;85:F36-41. http://dx.doi.org/10.1136/fn.85.1.F36.
- Defining Staffing Levels for Children and Young People’s Services. London: Royal College of Nursing; 2013.
- Clarity Pharmacy . Neomycin 0.5% Chlorhexidine Hydrochloride 0.1% Cream n.d. www.claritypharmacy.com/product.php/14923/86/neomycin_0_5____chlorhexidine_hydrochloride_0_1__cream (accessed 16 April 2015).
- DeMauro SB, Millar D, Kirpalani H. Noninvasive respiratory support for neonates. Curr Opin Pediatr 2014;26:157-62. http://dx.doi.org/10.1097/MOP.0000000000000066.
- Rotta A, Speicher R, Shein S, Speicher D. Crit Care Med 2014;42. http://dx.doi.org/10.1097/01.ccm.0000458250.60840.9f.
- Collins C. High flow nasal cannulae cause less nasal trauma compared to nasal continuous positive airway pressure in preterm infants. Arch Dis Child 2012;97:A116-17. http://dx.doi.org/10.1136/archdischild-2012-302724.0397.
- Collins C, Holberton JR, Barfield C, Davis PG. High flow nasal cannulae (HFNC) or nasal continuous positive airway pressure (NCPAP) post-extubation in premature infants? A randomised controlled trial. Arch Dis Child 2012;97:A38-9. http://dx.doi.org/10.1136/archdischild-2012-302724.0137.
- Collins CL, Holberton JR, Barfield C, Davis PG. Randomised controlled trial of high flow nasal cannulae (HFNC) versus nasal continuous positive airway pressure (NCPAP) post-extubation in preterm infants < 32 weeks’ gestation. J Paediatr Child Health 2012;48:66-7.
- Campbell DM, Shah P, Shah V, Kelly E. High flow nasal cannula CPAP versus infant flow nasal CPAP in newly-extubated neonates < 1250 g. Pediatr Res 2004;56. http://dx.doi.org/10.1203/00006450-200409000-00070.
- Hua C, McEwan A, Callander I. Hiflow compared to CPAP from 30 weeks gestation at Liverpool hospital. J Paediatr Child Health 2013;49.
- Iranpour R, Sadeghnia A, Hesaraki M. High-flow nasal cannula versus nasal continuous positive airway pressure in the management of respiratory distress syndrome. J Isfahan Med Sch 2011;29:761-72.
- Iranpour R, Sadeghnia A, Hesaraki M. 393 High-flow nasal cannula versus nasal continuous positive airway pressure in the management of respiratory distress syndrome. Arch Dis Child 2012;97:115-16. http://dx.doi.org/10.1136/archdischild-2012-302724.0393.
- Lawn JE, Mwansa-Kambafwile J, Horta BL, Barros FC, Cousens S. ‘Kangaroo mother care’ to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol 2010;39:144-54. http://dx.doi.org/10.1093/ije/dyq031.
- Conde-Agudelo A, Belizan JM, Diaz-Rossello J. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev 2011;3. http://dx.doi.org/10.1002/14651858.cd002771.pub2.
- Vesel L, Bergh AM, Kerber KJ, Valsangkar B, Mazia G, Moxon SG, et al. Kangaroo mother care: a multi-country analysis of health system bottlenecks and potential solutions. BMC Pregn Childbirth 2015;15. http://dx.doi.org/10.1186/1471-2393-15-S2-S5.
- Ashish KC, Wrammert J, Nelin V, Ewald U, Clark R, Malqvist M. Level of mortality risk for babies born preterm or with a small weight for gestation in a tertiary hospital of Nepal. BMC Public Health 2015;15. http://dx.doi.org/10.1186/s12889-015-2232-1.
- Al-Alaiyan S. A randomized controlled trial to compare heated humidified high-flow nasal cannulae with nasal continuous positive airway pressure postextubation in premature infants. J Clin Neonatol 2013;2.
- Andaya SC, Ramanathan R, Sardesai S, Cayabyab R, Garingo A, Seri I. Nasal respiratory support in preterm infants: a novel means of delivering time-cycled, pressure and flow limited intermittent mandatory ventilation via nasal cannula. J Invest Med 2010;58.
- Archer N, Cottis H, Ball S, Mills K, Hilliard T. A Pilot Randomised Controlled Study of Oxygen Delivery Via Vapotherm in Infants With Moderately Severe Acute Bronchiolitis 2009. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/731/CN-00758731/frame.html (accessed 12 January 2015).
- Beltramo F, Romero R, Chandler B, Soliz A. Successful Extubation in Low Birth Weight Infants: A Comparison of Continuous Positive Airway Pressure (CPAP) Versus Vapotherm n.d. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/469/CN-00838469/frame.html (accessed 12 January 2015).
- Bushell T, McHugh C, Meyer MP. A comparison of two nasal continuous positive airway pressure interfaces – a randomized crossover study. J Neonatal Perinatal Med 2013;6:53-9. http://dx.doi.org/10.3233/NPM-1363612.
- Ciuffini F, Colnaghi M, Lavizzari A, Mercadante D, Musumeci S, Mosca F. Therapy with high-flow nasal intubation in preterm infants. Pediatr Med Chir 2013;35:118-24.
- Daish H, Badurdeen S. Heated humidified high-flow nasal cannula versus nasal continuous positive airway pressure for postextubation ventilatory support in neonates: a meta-analysis. J Matern Fetal Neonatal Med 2014;27.
- Dani C. High-flow nasal cannulae in very preterm infants after extubation. N Engl J Med 2014;370. http://dx.doi.org/10.1056/NEJMc1314238#SA4.
- Dutta S. High-flow nasal cannula versus nasal continuous positive airway pressure in the management of apnea of prematurity. Pediatrics 2002;109:718-19. http://dx.doi.org/10.1542/peds.109.4.718.
- Gagliardi L, Rusconi F. High-flow nasal cannulae in very preterm infants after extubation. N Engl J Med 2014;370:384-5. http://dx.doi.org/10.1056/NEJMc1314238#SA2.
- Ignacio L, Alfaleh K. A randomized controlled trial to compare heated humidified high-flow nasal cannulae with nasal continuous positive airway pressure postextubation in premature infants. J Clin Neonatol 2013;2:75-7. http://dx.doi.org/10.4103/2249-4847.116405.
- Ignacio L, Alfaleh K. High-flow nasal cannulae in very preterm infants after extubation. J Clin Neonatol 2014;3:11-3. http://dx.doi.org/10.4103/2249-4847.128719.
- Kugelman A. NCPAP vs. NIPPV vs. heated humidified high-flow nasal cannula (HHHFNC) for the treatment of premature infants with RDS. J Matern Fetal Neonatal Med 2014;27.
- Lavizzari A, Ciuffini F, Colnaghi M, Mercadante D, Vendettuoli V, Pierro M. High Flow Nasal Cannula Versus Nasal CPAP in the Management of Respiratory Distress Syndrome: Preliminary Data n.d. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/965/CN-00997965/frame.html (accessed 12 January 2015).
- Lavizzari A, Veneroni C, Colnaghi M, Ciuffini F, Zannin E, Fumagalli M, et al. Respiratory mechanics during NCPAP and HHHFNC at equal distending pressures. Arch Dis Child Fetal Neonatal 2014;99:F315-20. http://dx.doi.org/10.1136/archdischild-2013-305855.
- Lee EH, Park KH, Park C, Hwang MJ, Choi BM, Hong YS. Comparing Humidified High Flow Nasal Cannula Versus Nasal Continuous Positive Airway Pressure As Respiratory Supports After Extubation in Preterm Infants n.d. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/098/CN-00832098/frame.html (accessed 12 January 2015).
- Nagar S, Nanan R, Liu A. High-flow nasal cannulae in very preterm infants after extubation. N Engl J Med 2014;370. http://dx.doi.org/10.1056/NEJMc1314238#SA1.
- Park KH, Lee EH, Chung BH, Choi YO, Lee JH, Choi BM, et al. Comparing Usefulness of Humidified High-Flow Nasal Cannula and Nasal Continuous Positive Airway Pressure for Neonatal Respiratory Diseases in Preterm Infants n.d. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/960/CN-00831960/frame.html (accessed 12 January 2015).
- Phadtare R, Joshi R, Rajhans A, Patil S, Dominic S, Devaskar U. High flow nasal cannula oxygen (Vapotherm) in premature neonates with respiratory distress syndrome: is it better than the conventional nasal continuous positive airway pressure (NCPAP)?. Perinatology 2009;11:1-8.
- Roberts SA, Mitchell S, Victor S. High-flow nasal cannulae in very preterm infants after extubation. N Engl J Med 2014;370. http://dx.doi.org/10.1056/NEJMc1314238#SA3.
- Saslow JG, Aghai ZH, Nakhla TA, Hart JJ, Lawrysh R, Stahl GE, et al. Work of breathing using high-flow nasal cannula in preterm infants. J Perinatol 2006;26:476-80. http://dx.doi.org/10.1038/sj.jp.7211530.
- Saslow JG, Aghai ZH, Nakhla TA, Hart JJ, Lawrysh R, Stahl GE, et al. Comparison of work of breathing (WOB) and changes in end-distending pressure (PD) using nasal continuous positive airway pressure (NCPAP) and vapotherm (VAPO). PAS 2006;26:476-80.
- Shetty S, Greenough A. Review finds insufficient evidence to support the routine use of heated, humidified high-flow nasal cannula use in neonates. Acta Paediatr 2014;103:898-903. http://dx.doi.org/10.1111/apa.12695.
Appendix 1 Search strategies for evidence of clinical effectiveness
A draft search strategy for MEDLINE was prepared and run on 8 September 2014 as part of the scoping searches. The search was updated on 12 January 2015 alongside a search of additional databases. The search strategies for each database are reported in Tables 17–20.
| Search terms | |
|---|---|
| 1 | ((heat* or hot* or humid* or high-flow or “high flow” or highflow or “higher flow”) adj5 (nasal adj3 (cannul* or prong*))). mp. |
| 2 | ((high-flow or “high flow” or highflow or “higher flow”) adj4 (therap* or treat*)). mp. |
| 3 | HFT. mp. |
| 4 | HHHFNC. mp. |
| 5 | HFNC. mp. |
| 6 | Fisher &Paykel Healthcare HHHFNC. mp. |
| 7 | Vapotherm 2000i. mp. |
| 8 | vapotherm*. mp. |
| 9 | “fisher and paykel”. mp. |
| 10 | “fisher&paykel”. mp. |
| 11 | or/1-10 |
| 12 | exp Oxygen Inhalation Therapy/ |
| 13 | (oxygen* adj4 inhalat* adj4 (therap* or deliver*)). mp |
| 14 | ((low flow or low-flow) adj5 (nasal adj3 (prong* or cannul*))). mp. |
| 15 | exp Continuous Positive Airway Pressure/ |
| 16 | exp Administration, Inhalation/ |
| 17 | NCPAP. mp. |
| 18 | NCPAP. mp. |
| 19 | LFNC. mp. |
| 20 | exp High-Frequency Ventilation/ |
| 21 | exp Positive-Pressure Respiration/ |
| 22 | ((oxygen* or high-freq*) adj4 (inhalat* or ventilat* or deliver* or admin*)). mp. |
| 23 | (continu* adj4 positiv* adj4 air* adj4 press*). mp. |
| 24 | (posit* adj4 press* adj4 (end-expirat* or respirat*)). mp. |
| 25 | or/12-24 |
| 26 | exp Infant, Premature/ |
| 27 | (infant* or child* or bab* or birth* or newborn* or neonat* or preterm* or prematur* or preterm*). mp. |
| 28 | infant/ or infant, newborn/ or infant, low birth weight/ |
| 29 | infant care/ or intensive care, neonatal/ |
| 30 | Infant, Newborn, Diseases/ |
| 31 | Infant, Premature, Diseases/ |
| 32 | or/26-31 |
| 33 | 11 and 25 and 32 |
| Search terms | |
|---|---|
| #1 | ((heat* or hot* or humid* or high-flow or “high flow” or highflow or “higher flow”)) AND (nasal adj3 (cannul* or prong*) |
| #2 | (((((HFT) OR HHHFNC) OR HFNC) OR fisher &paykel) OR (fisher and paykel)) OR vapotherm |
| #3 | (#1 or #2) |
| #4 | ((oxygen*) AND inhalat*) AND (therap* or deliver*) |
| #5 | (((low flow or low-flow)) AND nasal) AND (prong* or cannul*) |
| #6 | ((NCPAP) OR NCPAP) OR LFNC |
| #7 | ((oxygen* or high-freq*)) AND (inhalat* or ventilat* or deliver* or admin*) |
| #8 | (((continu*) AND positiv*) AND air*) AND press* |
| #9 | ((posit*) AND press*) AND (end-expirat* or respirat*) |
| #10 | (#4 or #5 or #6 or #7 or #8 or #9) |
| #11 | (infant* or child* or bab* or birth* or newborn* or neonat* or preterm* or prematur* or preterm*) |
| #12 | (#3 and #10 and #11) |
| #13 | (“2014/03/01”[Date - Entrez]: “2014/09/09” [Date Entrez]) |
| #14 | (#12 and #13) |
| Search terms | |
|---|---|
| 1 | ((heat* or hot* or humid* or high-flow or “high flow” or highflow or “higher flow”) adj5 (nasal adj3 (cannul* or prong*))). mp. |
| 2 | ((high-flow or “high flow” or highflow or “higher flow”) adj4 (therap* or treat*)). mp. |
| 3 | (HFT or HHHFNC or HFNC). mp. |
| 4 | (Vapotherm 2000i or vapotherm*). mp. |
| 5 | (“fisher&paykel” or “fisher and paykel”). mp. |
| 6 | or/1-5 |
| 7 | exp oxygen therapy/ |
| 8 | (oxygen* adj4 inhalat* adj4 (therap* or deliver*)). mp. |
| 9 | ((low flow or low-flow) adj5 (nasal adj3 (prong* or cannul*))). mp. |
| 10 | exp positive end expiratory pressure/ |
| 11 | exp inhalational drug administration/ |
| 12 | (NCPAP or NCPAP or LFNC). mp. |
| 13 | exp high frequency ventilation/ |
| 14 | ((oxygen* or high-freq*) adj4 (inhalat* or ventilat* or deliver* or admin*)). mp. |
| 15 | (continu* adj4 positiv* adj4 air* adj4 press*). mp. |
| 16 | (posit* adj4 press* adj4 (end-expirat* or respirat*)). mp. |
| 17 | or/7-16 |
| 18 | exp prematurity/ |
| 19 | (infant* or child* or bab* or birth* or newborn* or neonat* or preterm* or prematur* or preterm*). mp. |
| 20 | exp low birth weight/ or exp extremely low birth weight/ or exp small for date infant/ or exp very low birth weight/ |
| 21 | newborn disease/ |
| 22 | newborn intensive care/ |
| 23 | or/18-22 |
| 24 | and/6, 17, 23 |
| Search terms | |
|---|---|
| #1 | ((heat* or hot* or humid* or high-flow or “high flow” or highflow or “higher flow”) near/5 (nasal near/3 (cannul* or prong*))) |
| #2 | ((high-flow or “high flow” or highflow or “higher flow”) near/4 (therap* or treat*)) |
| #3 | HFT |
| #4 | HHHFNC |
| #5 | HFNC |
| #6 | Fisher &Paykel Healthcare HHHFNC |
| #7 | Vapotherm 2000i |
| #8 | vapotherm* |
| #9 | “fisher and paykel” |
| #10 | “fisher &paykel” |
| #11 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 |
| #12 | MeSH descriptor: [Oxygen Inhalation Therapy] explode all trees |
| #13 | (oxygen* near/4 inhalat* near/4 (therap* or deliver*)) |
| #14 | ((low flow or low-flow) near/5 (nasal near/3 (prong* or cannul*))) |
| #15 | MeSH descriptor: [Continuous Positive Airway Pressure] explode all trees |
| #16 | MeSH descriptor: [Administration, Inhalation] explode all trees |
| #17 | NCPAP |
| #18 | NCPAP |
| #19 | LFNC |
| #20 | MeSH descriptor: [High-Frequency Ventilation] explode all trees |
| #21 | MeSH descriptor: [Positive-Pressure Respiration] explode all trees |
| #22 | ((oxygen* or high-freq*) near/4 (inhalat* or ventilat* or deliver* or admin*)) |
| #23 | (continu* near/4 positiv* near/4 air* near/4 press*) |
| #24 | (posit* near/4 press* near/4 (end-expirat* or respirat*)) |
| #25 | #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 |
| #26 | MeSH descriptor: [Infant, Premature] explode all trees |
| #27 | (infant* or child* or bab* or birth* or newborn* or neonat* or preterm* or prematur* or preterm*) |
| #28 | MeSH descriptor: [Infant] explode all trees |
| #29 | MeSH descriptor: [Infant, Newborn] explode all trees |
| #30 | MeSH descriptor: [Infant, Low Birth Weight] explode all trees |
| #31 | MeSH descriptor: [Infant Care] explode all trees |
| #32 | MeSH descriptor: [Intensive Care, Neonatal] explode all trees |
| #33 | MeSH descriptor: [Infant, Premature, Diseases] explode all trees |
| #34 | MeSH descriptor: [Infant, Newborn, Diseases] explode all trees |
| #35 | #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 |
| #36 | #11 and #25 and #35 |
Appendix 2 Search strategies for evidence of cost-effectiveness
As part of the scoping searches, the following databases were searched to identify cost-effectiveness studies:
-
MEDLINE (via OvidSP)
-
MEDLINE In-Process Citations & Other Non-Indexed Citations (via OvidSP)
-
EMBASE (via OvidSP)
-
NHS Economic Evaluation Database (via The Cochrane Library)
-
Heath Economics Evaluation Database (via Wiley Online Library).
The searches were run on 5 December 2014. The search strategy is reported in Table 21.
| Search terms | |
|---|---|
| 1 | ((heat* or hot* or humid* or high-flow or “high flow” or highflow or “higher flow”) adj5 (nasal adj3 (cannul* or prong*))). mp. |
| 2 | ((high-flow or “high flow” or highflow or “higher flow”) adj4 (therap* or treat*)). mp. |
| 3 | HFT. mp. |
| 4 | HHHFNC. mp. |
| 5 | HFNC. mp. |
| 6 | Fisher &Paykel Healthcare HHHFNC. mp. |
| 7 | Vapotherm 2000i. mp. |
| 8 | vapotherm*. mp. |
| 9 | “fisher and paykel”. mp. |
| 10 | “fisher&paykel”. mp. |
| 11 | or/1-10 |
| 12 | Economics/ |
| 13 | “costs and cost analysis”/ |
| 14 | Cost allocation/ |
| 15 | Cost-benefit analysis/ |
| 16 | Cost control/ |
| 17 | Cost savings/ |
| 18 | Cost of illness/ |
| 19 | Cost sharing/ |
| 20 | “deductibles and coinsurance”/ |
| 21 | Medical savings accounts/ |
Appendix 3 Table of excluded studies with rationale
The list of citations excluded at stage 2 with reasons is presented in Table 22.
| Study | Reason for exclusion |
|---|---|
| Al-Alaiyan, 201371 | Article retracted (RCT) |
| Andaya et al., 201072 | Wrong population (mixed preterm, term and post-term) |
| Archer et al., 200973 | Wrong population (acute bronchiolitis) |
| Beltramo et al., 200874 | Wrong study design (not a RCT) |
| Bushell et al., 201375 | Not efficacy/safety study (mechanics of devices) |
| Campbell et al., 200463 | Not heated HFNC (abstract) |
| Campbell et al., 200632 | Not heated HFNC (RCT) |
| Chowdhurry et al., 201214 | Wrong study design (review) |
| Ciuffini et al., 201376 | Wrong study design (not a RCT) |
| Collins, 201260 | Not heated HFNC [RCT (abstract)]a |
| Collins et al., 201261 | Not heated HFNC [RCT (abstract)]a |
| Collins et al., 201262 | Not heated HFNC [RCT (abstract)]a |
| Daish and Badurdeen, 201477 | Wrong study design (review) |
| Daish and Badurdeen, 201436 | Wrong study design (review) |
| Dani, 201478 | Wrong study design (letter) |
| Dani et al., 200926 | Wrong study design (review) |
| DeMauro et al., 201458 | Wrong study design (review) |
| Dutta, 200279 | Wrong study design (letter) |
| Gagliardi and Rusconi, 201480 | Wrong study design (letter) |
| Hua et al., 201364 | Not heated HFNC [RCT (abstract)] |
| Ignacio and Alfaleh, 201381 | Synopsis of another RCT (Collins et al., 201337) |
| Ignacio and Alfaleh, 201482 | Synopsis of another RCT (Manley et al., 201338) |
| Iranpour et al., 201165 | Not heated HFNC [RCT (abstract)] |
| Iranpour et al., 201266 | Not heated HFNC (RCT) |
| Kugelman, 201483 | Wrong study design (review) |
| Lavizzari et al., 201384 | Not efficacy/safety study (mechanics of devices) |
| Lavizzari et al., 201485 | Not efficacy/safety study (mechanics of devices) |
| Lee et al., 201186 | Wrong study design (not a RCT) |
| Nagar et al., 201487 | Wrong study design (letter) |
| Park et al., 201188 | Wrong study design (not a RCT) |
| Phadtare et al., 200989 | Wrong study design (not a RCT) |
| Roberts et al., 201490 | Wrong study design (letter) |
| Rotta et al., 201459 | Wrong study design [review (abstract)] |
| Saslow et al., 200691 | Wrong study design (not a RCT) and not efficacy/safety study (mechanics of devices) |
| Saslow et al., 200692 | Wrong study design (not a RCT) and not efficacy/safety study (mechanics of devices) |
| Shetty and Greenough, 201493 | Wrong study design (review) |
| Sreenan et al., 200127 | Not heated HFNC (RCT) |
| Wilkinson et al., 201131 | Wrong study design (review) |
| Woodhead et al., 200635 | Wrong comparator (HFNC, not usual care) |
Appendix 4 Required sample size for a non-inferiority trial
A research recommendation of this review is to conduct a non-inferiority trial, with BPD as the primary outcome. Table 23 shows the different sample sizes that would be required to conduct such a trial, always assuming a significance level (α) of 5% and statistical power (1 – β) of 90%, but with differences in the assumptions about the rate of BPD (which is always assumed to be equal in both arms of the trial) and desired non-inferiority margin.
| Non-inferiority margin (%) | Rate of BPDb (%) | Total sample size requiredc |
|---|---|---|
| 10 | 25 | 644 |
| 30 | 720 | |
| 35 | 780 | |
| 7.5 | 25 | 1084 |
| 30 | 1280 | |
| 35 | 1388 | |
| 5 | 25 | 2572 |
| 30 | 2880 | |
| 35 | 3120 |
The sample sizes have been calculated from the Sealed Envelope™ website at www.sealedenvelope.com/power/binary-noninferior/ (accessed 24 November 2015).
The formula for the sample size calculation is:
where πs and πe are the true per cent ‘success’ in the standard and experimental treatment group, respectively;
and Φ–1 is the cumulative distribution function of a standardised normal deviate.
List of abbreviations
- BPD
- bronchopulmonary dysplasia
- CI
- confidence interval
- HFNC
- high-flow nasal cannula
- HHHFNC
- heated humidified high-flow nasal cannula
- IVH
- intraventricular haemorrhage
- NCPAP
- nasal continuous positive airway pressure
- NEC
- necrotising enterocolitis
- NICU
- neonatal intensive care unit
- NIPPV
- nasal intermittent positive-pressure ventilation
- RCT
- randomised controlled trial
- RDS
- respiratory distress syndrome
- RR
- risk ratio