Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/151/02. The contractual start date was in November 2014. The draft report began editorial review in April 2015 and was accepted for publication in November 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Frampton et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the underlying health problem
Retinal conditions are diseases associated with the retina, that is, the part of the eye that collects light and converts it into electrical signals. Many different conditions can affect the retina, and these vary according to whether they are inherited or acquired, which parts of the retina they involve, whether they are acute or chronic, the extent to which they affect a person’s vision, whether they can be treated and at which ages they occur. Retinal conditions also vary according to whether or not they are limited to the retina or affect other parts of the body (the latter are referred to as retinal syndromes). Owing to the diversity of retinal conditions, some of which are very uncommon, it is not possible to describe them all in this report. Since the objective of this technology assessment is to evaluate the accuracy of fundus autofluorescence (FAF) imaging for diagnosing and monitoring retinal conditions, we describe those retinal conditions which, according to the project’s clinical advisors, could be amenable to diagnosis and/or monitoring using FAF imaging, or those where FAF imaging may already be in use. To help in understanding the terminology relating to retinal conditions and FAF imaging, we first explain the structures comprising the retina and related parts of the eye.
Overview of the retina and related parts of the eye
The interior surface at the back of the eye, opposite the lens, which can be viewed through the pupil with an ophthalmoscope, includes the retina, and is referred to as the ocular fundus. As well as the retina, the fundus includes the optic disc (where axons exit the eye to form the optic nerve), the macula (a yellow spot which includes the centre of the retina), the fovea (the central part of the macula responsible for sharp central vision) and the posterior pole (the posterior retina as seen with a slit lamp lens, including the macula, optic disc and area nasal to the disc). The left and right halves of the retina (i.e. either side of the fovea) are referred to as ‘temporal’ (the side towards the temple) or ‘nasal’ (the side towards the nose). Measurements of the retina are often expressed in degrees of visual angle. One degree of visual angle on the retina is equal to 288 µm, assuming no shrinkage.
When the eye functions well (i.e. without opacity due to cataracts or scars on the cornea, or other visual loss), light entering the eye is focused by the cornea and lens onto the retina. The retina is a highly complex structure comprising several layers and diaphanous membranes (Figure 1). Light entering the retina first passes through a layer of neurons before reaching light-sensitive photoreceptor cells (rods and cones), which convert the light energy into electrical signals. The electrical signals are then passed to neurons in the layer above the photoreceptor cells where they are then transferred, via a layer of nerve fibres near the surface of the retina, to the optic nerve, which connects directly with the brain. The photoreceptors are supported towards the outside by a layer of cells called the retinal pigment epithelium. The retinal pigment epithelium reduces light scatter within the eye, provides nourishment and growth factors for the maintenance and regeneration of the photoreceptors, and limits the passage of fluid and harmful substances into the retina. Beneath the retina are a vascular layer called the choroid, and the outside of the eye, known as the sclera. The innermost layer of the choroid is called Bruch’s membrane. Blood is supplied to the retina by the central retinal artery, which runs alongside the optic nerve and has four main branches that supply blood vessels in the choroid.
FIGURE 1.
Simplified schematic overview of retinal structure (not to scale).

There are two types of photoreceptors, rods and cones. Rods aid vision in low light levels, as well as providing peripheral vision and movement perception; they are found throughout the retina, but the very centre of the retina (1-degree diameter) is rod free with a high cone density. Cones in the macula enable detailed vision of objects directly in front of the viewer and the perception of colour. Cones are also located throughout the retina but their highest density is at the fovea.
Given the complexity of the retina, and the fact that any component of the retina and its surrounding structures can malfunction or become damaged, a diverse range of retinal conditions can occur.
Retinal conditions included in this review
In this report the term ‘retinal condition’ (or retinopathy) refers to any acquired or genetically determined disease of the retina. The protocol for the present research focused on the retinal conditions agreed by the project advisory group as being those for which the technology under review, FAF imaging, may be most useful. These are early and late age-related macular degeneration (AMD), geographic atrophy (GA), inherited retinal dystrophies and central serous chorioretinopathy (CSC). However, as explained in Chapter 3, Inclusion and exclusion criteria, the scope of the review was subsequently widened to include any retinal conditions (other than those caused by malignancy, major primary ocular conditions or trauma) in which the accuracy of FAF imaging as a diagnostic or monitoring test has been assessed. Where possible, descriptions of retinal conditions in the current report are based on the classification of Lois and Forrester. 1 Below we describe some common retinal conditions and those with significant impacts on patients; due to the wide variety of retinal conditions that exists, this list is not exhaustive.
Prevalence, natural history, symptoms and risk factors of retinal diseases
The estimated UK prevalence of different retinal diseases is summarised below, although this is difficult to compare accurately across the retinal conditions owing to the scarcity of epidemiological data on incidence and prevalence of visual impairment in the UK,2 and because of differences in how prevalence data have been calculated. As indicated in the sections on specific retinal conditions below, in some cases prevalence rates have been reported without clearly specifying the time periods they refer to. Overall, according to the Royal National Institute of Blind People (RNIB) Eye Health Data Summary 20143 (which does not include the less prevalent retinal conditions), based on data for 2010, early AMD is the most prevalent retinal condition in the UK (1,494,000 cases), followed by early diabetic retinopathy (748,000 cases) and late AMD (609,000 cases). Other retinal conditions affect fewer people but, in the case of the inherited retinal dystrophies, symptoms may appear and become debilitating in childhood or young adulthood, unlike in the more prevalent conditions. Where data are available for incidence and prevalence of retinal conditions these are reported in the sections on specific retinal conditions below. For some conditions only incidence or prevalence data are available, not both.
Age-related macular degeneration
Age-related macular degeneration is categorised as either early AMD or late AMD. In early AMD the vision is initially unaffected, but there are risk features for late AMD. Late AMD is classified into two types: wet AMD, which is also known as exudative or neovascular AMD (the term neovascular is used in this report); and dry AMD, which, in advanced cases, is also known as GA. AMD is the commonest cause of visual impairment, and results in progressive, irreversible damage to the macula, the retinal pigment epithelium and retinal photoreceptors. 4 Clinical and phenotypic variations have been identified, and increasing age and smoking are risk factors for the condition. 5 Although AMD usually affects both eyes, it rarely results in complete blindness, as the peripheral vision is not usually affected.
Early age-related macular degeneration
In the early stages of AMD, lipid and basal laminar waste products are deposited under the retinal pigment epithelium6 and in Bruch’s membrane,7 due to retinal pigment epithelium cells becoming less efficient as people age. 8 These deposits, called ‘drusen’, are thought to result from incomplete metabolism by retinal pigment epithelial cells. 6 Drusen gradually increase in number and size, although vision is not affected at first. However, central vision becomes less sharp as drusen become larger. Different types of drusen include hard discrete yellow drusen smaller than 50 µm in diameter; tiny whitish basal laminar drusen; soft yellowish drusen with poorly defined margins that often coalesce, and are usually larger than 50 µm; and discrete, calcific crystalline drusen, which look like yellow spots on the retina. 9 Drusen can spontaneously disappear in patients with AMD, often leaving atrophic lesions. 10 With advances in retinal imaging, reticular pseudodrusen have been identified which, unlike ‘conventional’ drusen, are located within the retinal pigment epithelium. 11,12 The retinal pigment epithelium also undergoes progressive changes in early AMD that appear as patches of hyperpigmentation and hypopigmentation. 7 As noted above, the prevalence of early AMD in the UK has been estimated (based on data from 2010) as 1,494,000 cases. 3
The sight of people with early AMD may deteriorate gradually over years, and, in some cases, the disease progresses to the more severe GA or neovascular AMD (see Late, neovascular AMD), with genetic and environmental risk factors playing a part. 13 About 250,000 older adults in the UK suffer from blindness due to late AMD,14 and the two main late AMD phenotypes, GA and neovascular AMD, account for two-thirds of registrations for visual impairment or blindness in the UK. 7
Late, dry age-related macular degeneration (geographic atrophy)
Advanced dry AMD involving atrophy of the retinal pigment epithelium as a result of cell death, is known as GA. 15 GA progresses slowly from early AMD; the evolution from high-risk large drusen to hypopigmentation to death of retinal pigment epithelial cells to legal blindness (Snellen visual acuity < 3/60) can take between 5 and 10 years,8 and varies considerably between patients. 16 Patients can experience loss of central vision and have difficulty reading small sizes of print, although this may not be noticeable if GA affects only one eye. 7 GA accounts for 12–20% of legal blindness in AMD, but some people retain visual acuity because the atrophy is away from the part of the retina where images are focused, as in foveal-sparing GA. 6 Increasing age, genetic factors and smoking have been reported as the most consistent factors leading to GA. 15 The UK annual incidence rate of GA is 2.4 per 1000 in women and 1.7 per 1000 in men. 17 In the UK, prevalence of dry AMD (based on data from 2010), has been estimated as 194,000 cases. 3 Prevalence rates of GA (based on data for 2007–9) have been reported as 1.3%, 2.6% and 6.7% in people aged over 50 years, over 65 years, and over 80 years, respectively. 17
Late, neovascular age-related macular degeneration
Neovascular AMD may result in rapid deterioration in vision. 18 Neovascular AMD is characterised by choroidal neovascularisation, which is the development of new blood vessels in the choroid. 7 With the development of new blood vessels, fluid accumulates leading to symptoms of visual distortion. If there is leakage of blood the vision will deteriorate significantly so treatment is needed before permanent damage occurs. As photoreceptors are displaced by fluid, vision becomes distorted and blurred and, without intervention, cell loss, fibrosis and eventual scarring are likely. 7 Patients may suddenly become unable to read, drive and see fine detail, such as facial expressions and features, because of haemorrhaging, fibrosis or scarring. 7 There are about 26,000 new cases of neovascular AMD per million each year in the UK,7 and women have slightly higher age-specific prevalence rates than men, although the greater number of older women in the UK results in sex differences in the number of prevalent and incident cases. 17 The UK prevalence of neovascular AMD (based on data from 2010) has been estimated at 415,000 cases. 3 Prevalence rates based on data for Wales from 2005–9 were reported as 1.2% among people aged over 50 years, 2.5% in those aged over 65 years and 6.3% in those aged over 80 years. 17
Diabetic retinopathy
Diabetic retinopathy is the leading cause of vision loss in people aged under 50 years in the developed world. Its prevalence is increasing,19 and it is the commonest cause of blindness certification in working age adults. 2 Diabetic retinopathy is a term that describes any pathological features that occur at the retina due to diabetes. This can range from minimal non-sight-threatening vascular features such as microaneurysms, to proliferative retinopathy, which is the growth of delicate new blood vessels that can bleed easily and may result in intraocular haemorrhage with sudden loss of vision. 20,21 Proliferative retinopathy may be transient if the haemorrhage clears, but may have features of advanced disease with an associated retinal detachment. Other types of diabetic retinopathy include fluid collecting at the macula (diabetic macular oedema) or loss of blood vessels at the macula, known as macular ischaemia, which can be associated with severe central visual loss depending on the extent of the loss of vascular perfusion. 22
The most common symptom of diabetic macular oedema is blurred vision. Other symptoms include metamorphopsia (distortion of the visual image); floaters (moving spots seen in the field of view); loss of contrast sensitivity; photosensitivity (intolerance to light); changes in colour vision; scotomas (where part of the visual field is missing); vision becoming blurred, hazy or fluctuating, or with the appearance of black lines or dots; periods of temporary ‘blackness’ caused by retinal haemorrhage; poor peripheral vision; and poor depth perception. 23 People with diabetic macular oedema may also be affected by a difference in sight between their two eyes. 22 Laser treatment of macular disease or proliferative retinopathy can also lead to visual symptoms including field loss and photosensitivity.
Estimates of UK prevalence of diabetic retinopathy vary owing to the range of different sources of data. Based on data from 2010, there is an estimated prevalence of 748,000 cases of early diabetic retinopathy in the UK, 85,000 cases of more advanced retinopathy and 188,000 cases of diabetic maculopathy. 3 In a study of people with diabetes in England (which also estimated prevalence data for 2010), 7.12% had diabetic macular oedema in one or both eyes, of which 39% had clinically significant diabetic macular oedema. 24
Central serous chorioretinopathy
Central serous chorioretinopathy is the fourth most common retinopathy after AMD, diabetic retinopathy and retinal vein occlusion. 25 It is characterised by fluid accumulating as a result of dysfunction of retinal pigment epithelial cells and/or the choroid. Usually, if the patient experiences symptoms, the fluid will have accumulated under the central macula. 26 Spontaneous visual recovery can occur within 2 or 3 months in the acute type,25 but chronic CSC, which is more common in older people, can lead to persistent or episodic blurred vision. In some cases of chronic CSC, disease progression is more severe, leading to retinal pigment epithelial atrophy,27 which may result in permanent visual loss. 25 There is some evidence that risk factors for CSC include smoking, hypertension or sympathetic responses (nervous system activation in response to stressors),27 steroid medications28 or genetic susceptibility. Symptoms of the condition include image size disparity between the eyes, blurred central vision, altered colour vision and, in chronic disease with atrophic change, a loss of visual acuity. 25
Men are more often affected by CSC than women, in a ratio of up to 8 : 1. 26 Although predominantly an adult disease, some cases of CSC have been reported in children. 26 People aged over 50 years are more likely to have the condition in both eyes, may develop choroidal neovascularisation25 and may have coincidental polypoidal choroidopathy. CSC recurs in about one-third of patients within around 1 year. 25 The peak incidence is around 40–45 years in men, but later in women, at about 51 years. 26 Reliable incidence and prevalence data are not available for the UK. According to a study in the USA (based on data for 1980–2002), the incidence of CSC is about 9.9 per 100,000 in men and 1.7 per 100,000 in women. 25
Inherited retinal dystrophies
Inherited retinal dystrophies are a broad group of genetically determined disorders that affect the retina and lead to progressive visual loss. These disorders can be generally classified according to whether they affect primarily the centre of the vision, the periphery or both. Further classification depends on whether rods or cones are primarily affected, whether or not both central and peripheral systems are involved and which is more affected (referred to as rod–cone or cone–rod). 29 Macular dystrophies may only involve central vision without affecting the visual fields, and the rods and cones may not be affected. However, progression may occur so that, for example, a disorder that was classified as macular dystrophy may, in time, develop cone and rod dysfunction. Terms used to describe these conditions depend on the appearance of the retina, which cells are involved, and (more recently) if the gene mutation(s) is/are known. Retinal dystrophies include, among others, retinitis pigmentosa, macular dystrophy, Stargardt disease (which primarily develops in childhood and adolescence) and Best disease.
The age of onset of retinal dystrophies varies, with early and late onset forms having different inheritance patterns, and mutations in over 200 different genes are known to be involved in different types, including syndromic retinal dystrophies. 30 Symptoms and disease progression also vary within each named condition. Symptoms may be apparent from birth or may appear at any age, depending on the type and the specific genetic variant inherited. The degree of central visual loss, peripheral field loss and difficulty with seeing in the dark depends on how well each group of photoreceptor cells is working, and the speed of sight loss varies with different genetic forms and between individuals. 31 In cases of severe rod–cone dystrophy with early onset, macular involvement may lead to early decline of visual acuity, and these cases may be more challenging to diagnose. 29
Although some inherited retinal dystrophies affect few people, overall, these disorders are stated as being a cause in 15.8% of certified blindness registrations and 10% of partial sight registrations in England and Wales, and are considered to be a significant burden in the working age population. 2 The annual incidence of hereditary retinal disorders in the UK has been estimated at around 1500 cases, and about 100 children and 480 adults of working age in the UK are registered as blind or partially sighted as a result of these conditions. 2 A study published in 201232 estimated that (based on data from 2006 to 2008) the annual UK incidence of childhood-onset retinal dystrophies, is 1.4 in 100,000 children (aged 0–15 years) and the cumulative incidence by age 16 years is 22.3 in 100,000 children.
Cystoid macular oedema
Macular oedema is the accumulation of fluid within the retina at the macular area, secondary to various retinal conditions. Depending on its cause, acute oedema may resolve spontaneously. 33 Cystoid macular oedema is the result of chronic oedema that persists for ≥ 4 months, leading to the formation of cystic honeycomb-like spaces in the retina,34 and can occur as a consequence of retinal dystrophies,35 inflammatory diseases (uveitis, scleritis, birdshot chorioretinopathy, toxoplasmosis),36 retinal vascular disease (retinal vein occlusions, idiopathic retinal telangiectasia, radiation retinopathy),34 diabetic retinopathy,34 cataract or other eye surgery, injury to the eye, choroidal tumours, or may be drug induced, for example with prostaglandins such as latanoprost. 37 As with other macular conditions, the main symptoms of cystoid macular oedema are blurred or decreased central vision, but peripheral vision is unaffected. Estimates of the incidence and prevalence of cystoid macular oedema vary considerably depending on the cause of the oedema. For example, the incidence of clinically significant cystoid macular oedema caused by uncomplicated cataract surgery has been estimated across studies as 0.6–2.6%,38 whereas the prevalence of cystoid macular oedema in patients with retinitis pigmentosa may be as high as 32–49% (unilateral) or 18–44% (bilateral). 39,40
Impact of retinal conditions
Significant distress results from developing visual loss,41 particularly when the loss is marked and/or of rapid onset. Depending on the condition, patients can experience a wide range of symptoms, as described above. Such symptoms can have a profound impact, for example, the loss of central vision associated with AMD affects patients’ ability to perform normal daily activities such as reading, writing and recognising faces. 18 People with diabetic retinopathy may experience a visual difference between their two eyes. 22
Visual loss adversely affects people’s ability to drive, which has a serious negative impact on work, social life, relationships, responsibilities and independence. 22,42 Visual loss also adversely affects self-care, increases the risk of falls, hip fractures and early admission to residential care, and adversely affects quality of life. 18 The psychological impact of changes caused by vision loss include alterations to the self-concept, life goals and social functioning. 43 Having to adapt to vision loss may result in emotional distress, which can lead to depression, anxiety and sleep problems. 18,43
Diagnosis and monitoring of retinal conditions may involve invasive procedures such as fluorescein or indocyanine green angiography (ICGA), which involve intravenous injections of dye. People with retinal conditions may require frequent treatments and regular monitoring of their response to treatment and disease progression, which may be uncomfortable and can cause anxiety and apprehension. 18 With the ageing population and recent treatment advances, demand for early identification of people who are at greatest risk of progressive disease is also increasing. 44 More accurate and less-invasive diagnostic procedures may therefore be helpful if they can improve the efficiency of diagnosis and/or monitoring of retinal conditions.
Measurement of disease: diagnostic features
Imaging techniques capture morphological patterns (appearance of structural features) at the back of the eye, as well as dynamic features such as transit of dye in blood vessels. These features are seen more or less clearly with different imaging modalities, for example, pooling, leakage or staining can be seen with fluorescein angiography. Grading systems have been proposed to classify different conditions, using different imaging modalities, although there is not always universal consensus about the most appropriate classification for each condition. For example, the Wisconsin age-related Maculopathy system consists of three sections: drusen, other lesions and other abnormalities, each of which includes subgroups of characteristics. 6
A classification by the International Fundus Autofluorescence Classification group describes patterns of fluorescence in early AMD and GA using FAF imaging. 45,46 With advances in imaging, reticular pseudodrusen have been identified, which unlike conventional drusen are located within the retinal pigment epithelium,11 and these are characteristic of AMD. 47 Determining the type and number of drusen present allows clinicians to better inform patients of their risk of developing AMD.
Geographic atrophy is defined as any sharply delineated round or oval area of hypopigmentation (paleness), or apparent absence of the retinal pigment epithelium and overlying photoreceptors, in which choroidal vessels are more visible than in surrounding areas, and the oval area is > 175 µm in diameter. 15
Diabetic macular oedema is characterised by macular thickening (focal, multifocal or diffuse) or hard exudates within 500 µm of the centre of the macula. Cystoid macular oedema is also characterised by retinal thickening and the development of honeycomb-like spaces in the retina on optical coherence tomography evaluation. 34
In inherited retinal dystrophies, clinical signs may indicate the type of dystrophy. For example, in retinitis pigmentosa, pigment changes (known as bone spicules because they look like bone under the microscope), pallor of the optic disc and narrowing of retinal blood vessels may be seen. Stargardt disease is characterised by white/yellow flecks at the back of the eye between the optic disc and the macula, seen by fundoscopy, colour imaging or autofluorescence, and can extend to cover a larger area of the retina. 48 In most patients some of the flecks will atrophy, leaving lesions with a beaten metal appearance on an electroretinogram. 48 Patients with cone–rod disorders may have pigment deposits in the retina, mainly in the macular region,29 or little or no pigment change but a ‘bull’s-eye maculopathy’ on fundus examination. 49 As diagnostic features of inherited retinal dystrophies overlap, for example Stargardt disease can be mistaken for pattern dystrophy and vice versa, genetic testing plays a helpful and necessary part in clarifying which disease is present.
Description of the technology under assessment
Fundus autofluorescence in relation to retinal health
The fundus of the human eye has a ‘natural’ or ‘background’ level of fluorescence, which is referred to as autofluorescence. This is caused by the presence of molecules with fluorescent properties (i.e. molecules that, when exposed to light of an appropriate wavelength, absorb the incident electromagnetic energy and re-emit this as light at wavelengths longer than those of the initial source). The fluorescent molecules, known as fluorophores, are potentially of clinical value in detecting age- or disease-related processes, since their density and distribution alters with ageing of the eye and with certain pathological conditions. Notably, lipofuscin is a fluorescent pigment that accumulates in the retinal pigment epithelium as a by-product of cell metabolism and can lead to the development of drusen. Lipofuscin deposition normally increases with age but its accumulation may also reflect cell dysfunction or metabolic abnormalities in the retinal pigment epithelium. Excessive lipofuscin deposition in the retinal pigment epithelium is considered pathologic and is associated with visual loss. 50 FAF imaging techniques have potential value as diagnostic tools, since the presence and distribution of fluorophores may correlate with disease activity and may provide an early indication of future disease development, progression or response to treatment. FAF imaging is also potentially valuable in detecting areas of reduced or absent autofluorescence (hypoautofluorescence), which can arise either through atrophy of retinal structures or blocking of FAF reflectance (e.g. by blood vessels).
Fundus autofluorescence imaging techniques
The intensity of light emitted during FAF is relatively weak (about two orders of magnitude lower than the background of a fluorescein angiogram at peak dye transit) and so specialist imaging techniques are required to enable FAF to be detected and mapped. FAF has been an area of interest in ophthalmic research for more than 40 years but it has only recently become clinically relevant, as a result of technological advances. 50 As outlined below, there are three main methods for assessing FAF: using a fundus camera, fundus spectrophotometry or confocal scanning laser ophthalmoscopy (cSLO).
The fundus camera uses a single flash to image the entire retinal area instantaneously. The acquired autofluorescence image is derived from all tissues in the light beam with fluorescent properties; light scattered anterior and posterior to the plane of interest can greatly influence the detected signal.
Fundus spectrophotometry was developed to measure FAF from small retinal areas (2 degrees of visual angular diameter). It incorporates an image intensifier diode array as a detector and beam separation in the pupil to minimise the contribution of autofluorescence from the crystalline lens. 51
In cSLO a focused low-power laser beam is swept across the fundus in a raster pattern (horizontal and vertical scanning across a grid) to provide the excitatory light source for fluorophores. 52 Different excitation wavelengths can be generated, depending on the type of laser used, including 488 nm (blue light) with a solid-state laser and 514 nm (green light) with an argon-ion laser. For near-infrared fundus autofluorescence (NIR-FAF), the excitation wavelength is 790 nm. The confocal nature of the optics reduces the detection of autofluorescence from structures anterior to the retina, such as the lens and cornea. Unlike fundus spectrophotometry, cSLO allows imaging of FAF over larger retinal areas [e.g. 55 degrees in Heidelberg Retina Angiograph-based systems (Heidelberg Engineering, Heidelberg, Germany)]. To reduce background noise and enhance image contrast, the mean image of several FAF images is obtained (usually based on up to 20 frames, after adjustments to correct for eye movement). In order to block the reflected light but permit autofluorescence light to pass, cSLO have barrier filters of 500 nm, 525 nm and 800 nm for excitation wavelengths of 488 nm, 514 nm and 790 nm, respectively. As well as the excitation and barrier filter wavelengths, image contrast and brightness settings also differ among cSLO devices and these differences must be taken into account when comparing the results of FAF imaging obtained from different cSLO devices. 16
Confocal scanning laser ophthalmoscopy is the most sensitive imaging approach for identifying autofluorescence that arises specifically from the fundus (i.e. minimising the detection of autofluorescence arising from other parts of the eye such as the lens). 16 According to clinical experts advising the review, cSLO is the current standard method employed for obtaining FAF images of retinal conditions, and is therefore the only type of scanning laser ophthalmoscopy permitted as an index test for assessing FAF in the current review.
Fundus autofluorescence for diagnosis and monitoring of disease
The quantitative accuracy of FAF imaging for the diagnosis and/or monitoring of retinal conditions (i.e. its sensitivity and specificity) is unclear. However, FAF imaging is considered helpful in a number of conditions to help establish a diagnosis and monitor treatment without the need for angiography,1 is relatively easy to accomplish and requires little time. 53 Studies have suggested the potential diagnostic value of FAF imaging as a more sensitive marker of retinal pathology than existing examinations alone, for example, in GA,54 choroidal neovascularisation development in AMD,55 retinal pigment epithelium alterations,56 Best disease,57 cystoid macular oedema58 and CSC. 59 Clinical advisors to the current review also suggested that FAF is useful for diagnosing any type of inherited retinal dystrophy, and has the potential to show pathologic features earlier than on fundoscopy. FAF imaging also appears promising for monitoring changes in a number of retinal conditions, either natural progression or responses to therapy. FAF imaging has been used, for example, for monitoring changes in GA;60–66 retinal pigment epithelium tear or loss;67–69 retinitis pigmentosa;70,71 Stargardt disease,72 CSC,73 diabetic macular oedema74 and retinal vasculitis. 75 Increasingly, FAF has been specified as an end point in clinical research studies of therapies for retinal conditions, for example, with antivascular endothelial growth factor drugs,68,76–78 sirolimus,65 lampalizumab,66 finasteride,73 photodynamic therapy79 and vitrectomy. 80
Reference standards
‘Reference standard’ refers to the current gold standard or best available method for accurately identifying a given retinal condition. A reference standard may consist of more than one retinal imaging test since different imaging modalities can provide different types of information which, when interpreted together, improve diagnosis. According to the paradigm for assessing test accuracy, the reference standard (against which any new tests will be compared) should have high (ideally 100%) sensitivity and high (ideally 100%) specificity for identifying the retinal condition, that is, the method should minimise false-positive and false-negative results. 81 The extent to which existing methods used in diagnosing retinal conditions fulfil these strict requirements is not always clear and there is not always agreement among clinicians on which method is the ‘best’. In early AMD, optical coherence tomography and fluorescein angiography are needed to distinguish between wet and dry AMD,82 but ICGA may be also used, for example if there is doubt about the presence of choroidal polyps or chorioretinal anastomoses.
Clinical advisors to the current review suggested that spectral-domain optical coherence tomography would be a standard approach for identification of reticular pseudodrusen in AMD, possibly also with fluorescein angiography and/or colour fundus photography. GA diagnosis is confirmed by clinical examination using a high-definition fundus lens for stereo biomicroscopy, as well as being noted on fluorescein angiography. 7 As noted above, FAF imaging and spectral-domain optical coherence tomography have made it easier to diagnose GA, particularly early signs of GA, as these imaging modalities can reveal areas of GA that may not be clinically visible on biomicroscopy. 7 The gold standard for diagnosing cystoid macular oedema has been specified as fluorescein angiography,38,83 although in practice optical coherence tomography is also important34,38 as diagnosis involves assessment of macular thickness as well as assessment of perfusion of the retinal vascular epithelium. Colour fundus photography, and more recently optical coherence tomography, are the reference standards for assessing diabetic macular oedema. 84 Fluorescein angiography may be used to identify leaking microaneurysms or capillaries and areas of macular ischaemia, as well as checking the rest of the retina for ischaemia or neovascularisation. Indocyanine green angiography is a standard approach for detecting choroidal abnormalities in CSC, and enhanced depth optical coherence tomography can provide three-dimensional information. 85 Diagnosis of retinitis pigmentosa involves visual field testing, electroretinography to measure the functional status of photoreceptors and stereo fundus biomicroscopy. Diagnosis of Stargardt disease may be based on visual acuity, fundus examination, electroretinography and fluorescein angiography, although optical coherence tomography and microperimetry may also be useful. 86
Current service provision
Although FAF imaging may be used on an ad-hoc basis in the NHS to support the diagnosis and monitoring of a range of retinal conditions, the extent to which it is used in practice is unclear. Clinical experts advising the current review suggested that the use of FAF imaging depends on the eye unit, its field of expertise and specialism. Although the role of FAF imaging is still evolving, the method is already widely used within the NHS for the assessment of inherited retinal dystrophies, such as retinitis pigmentosa and Stargardt disease, as high-resolution imaging of the distribution and levels of FAF correlate with pathogenesis in these conditions (e.g. indicating areas where the retina is dying). This is, however, in centres where there is provision of an inherited retinal dystrophy service and often a strong associated research unit. The extent to which FAF imaging is used for diagnosing and/or monitoring GA and CSC is unclear, but clinical experts have suggested that FAF imaging would mainly be used in specialised retinal clinics such as those seen in teaching hospitals. FAF imaging is unlikely to be used in isolation and would commonly be conducted together with other tests such as (depending on the retinal condition) optical coherence tomography, fundus photography, fluorescein angiography or indocyanine green angiography. FAF imaging would complement these tests rather than replace them, since the imaging modalities provide different information to help clinicians reach a diagnostic or therapeutic decision. An exception could be for the inherited retinal dystrophies where FAF imaging has potential to replace fluorescein angiography, for example, as the main test for diagnosing Stargardt disease (FAF could be an alternative indicator of disease by demonstrating flecks and atrophy, where previously fluorescein angiography was performed with the aim of confirming Stargardt disease if a dark choroid was identified). The clinical experts suggested that, for the diagnosis or monitoring of neovascular AMD, choroidal neovascularisation and macular oedemas (cystoid and diabetic), FAF imaging would provide little or no added value over optical coherence tomography and fluorescein angiography. This is because the accumulation and/or leakage of fluid in these conditions could mask the FAF signal, which might make the assessment of the distribution and intensity of autofluorescence unreliable, and also because dye leakage, as assessed using angiography, is necessary for confirming choroidal neovascularisation and macular oedema.
According to the Royal College of Ophthalmologists’ guidelines,7 FAF imaging along with spectral domain optical coherence tomography has made it easier to diagnose GA in AMD, but its role in diabetic retinopathy has yet to be fully elucidated. 20 Despite the potential value and existing use of FAF imaging in the inherited retinal dystrophies, no guidelines are currently available concerning the use of FAF imaging in diagnosing these retinal conditions.
Chapter 2 Definition of the decision problem
Decision problem
Fundus autofluorescence imaging appears to be a promising procedure for the diagnosis and/or monitoring of certain diseases of the retina. 50 Although FAF imaging is already employed (to an unclear extent) in the NHS, its accuracy for the diagnosis and monitoring of the different retinal conditions is uncertain. A systematic evaluation of both the quantity and the quality of the available evidence on the diagnostic and monitoring accuracy of FAF imaging is needed to inform best practice for retinal imaging in the NHS and to inform future research.
Population
The relevant population is patients of any age who are suspected to have, or have previously been diagnosed with, any retinal conditions, excluding those resulting from malignancy, other ocular diseases (such as glaucoma) or trauma. Note: this population is wider than that specified in the review protocol, as it is not limited to specific retinal conditions (see Chapter 3, Inclusion and exclusion criteria).
Index test
The relevant index tests are any FAF imaging approaches performed to assist diagnosis and/or monitoring of retinal conditions, using cSLO. As noted above, in some retinal conditions such as GA and Stargardt disease, FAF imaging may already contribute information to making the diagnosis. The use of FAF imaging as an index test has to be considered carefully because interpretation of its diagnostic or monitoring accuracy may be influenced by whether or not it is already part of the reference standard and by whether results of FAF imaging would normally be interpreted in isolation or in conjunction with other clinical information (e.g. results of other imaging tests). These issues are an important part of the critical appraisal of the available evidence and are considered in detail in this review when interpreting results (see Chapter 4).
Reference standard
To account for the wide range of retinal conditions eligible for inclusion in the review and differences in how reference standards have been defined and employed in various studies, fundus imaging performed using any clinically relevant method (e.g. fundus photography, fundus fluorescein angiography, indocyanine green angiography, optical coherence tomography or any combination of relevant tests) is eligible for inclusion in the current review.
Outcomes
The key outcome measures include: sensitivity and specificity; positive and negative likelihood ratios; positive and negative predictive values; and diagnostic odds ratios. The working definitions of each of these outcomes are:87
-
Sensitivity: true identification of the people with the condition of interest. It is also known as the true-positive rate. A high sensitivity implies that a negative result rules out a condition.
-
Specificity: also known as the true-negative rate, it indicates the true identification of people without the condition. A test with high specificity implies that a positive result confirms the condition.
-
Likelihood ratios: a positive likelihood ratio is the ratio of the true-positive rate to the false-positive rate, which is expressed as: sensitivity/(100 – specificity) whereas a negative likelihood ratio is the ratio of the false-negative rate to the true-negative rate, expressed as: (100 – sensitivity)/specificity. The positive likelihood ratio describes how many times more likely positive index test results are in the group with the retinal condition compared with those without the condition, and should be > 1 for the test to be informative. The negative likelihood ratio describes how many times more likely negative index test results are in the group with the retinal condition compared with those without the condition, and should be < 1 for the test to be informative.
-
Positive and negative predictive values: positive predictive value is the probability of the condition of interest among people with a positive test result. Negative predictive value is the probability of not having the condition among people with a negative test result.
-
Diagnostic odds ratio: this summarises the diagnostic accuracy of the index test in a single number that describes how many times higher the odds are of obtaining a positive test result in a patient with the retinal condition than in one without the condition.
Overall aims and objectives of the assessment
The aim of this project was to assess the accuracy of FAF imaging using cSLO for the diagnosis and monitoring of retinal conditions. Specific research objectives were:
-
for each retinal condition, to determine the diagnostic and monitoring accuracy of FAF imaging using cSLO, including monitoring of disease management
-
to identify future research needs and develop research recommendations.
Chapter 3 Methods for reviewing test performance
A review of the evidence for test accuracy was undertaken systematically and is reported systematically following the general principles outlined in the Centre for Reviews and Dissemination guidance,88 the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy87,89 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,90 taking into consideration specific aspects of methodology that are relevant to the synthesis of evidence of test accuracy.
The project was informed by an advisory group of three independent clinical experts (see Acknowledgements). Two of the experts were experienced ophthalmologists and the third represented RNIB, which is a national charity supporting people with sight problems.
Identification of studies
A comprehensive search strategy (see Appendix 1) was developed, tested and refined by an experienced information scientist. The search strategy aimed to identify studies on the diagnosis and/or monitoring of relevant retinal conditions based on the prespecified inclusion and exclusion criteria (see Inclusion and exclusion criteria). The search strategy comprised the following main elements:
-
searching electronic databases
-
searching internet pages of relevant organisations, meetings and trial registries
-
scrutiny of bibliographies of retrieved papers and relevant systematic reviews.
The following electronic databases were searched: MEDLINE (via Ovid); MEDLINE In-Process & Other Non-Indexed Citations; EMBASE; The Cochrane Library; Web of Science; Database of Abstracts of Reviews of Effectiveness (via the Centre for Reviews and Dissemination); Health Technology Assessment database; Medion database of diagnostic accuracy studies.
Internet pages of the following organisations were searched: American Academy of Ophthalmology, Association for Research in Vision and Ophthalmology; Cochrane Eyes and Vision Group; European Association for Vision and Eye Research; and Royal College of Ophthalmologists.
Searches for grey literature and research in progress included: the UK Clinical Research Network Portfolio Database; World Health Organization International Clinical Trials Registry Platform; Current Controlled Trials; Clinical Trials.gov; and the National Institute for Health Research Clinical Research Network Portfolio.
All databases were searched from 1990 (approximately 10 years prior to the likely publication of the earliest relevant evidence) to November 2014 and searches were limited to the English language.
Inclusion and exclusion criteria
The eligibility criteria for the systematic review are as specified in the review protocol,91 with two exceptions. First, the population has been widened to include patients with any retinal condition, but excluding those resulting from malignancy, major ocular conditions (e.g. glaucoma) or trauma. This change was made because the pilot screening process identified potentially relevant studies in which FAF imaging had been conducted on retinal conditions not specified in the protocol but did not appear to identify many studies on the prespecified conditions. Widening of the population inclusion criterion was considered acceptable on the basis that it was deemed unlikely to affect resource requirements for the review. To avoid any inconsistency and bias, the updated population eligibility criterion was applied to all of the bibliographic records screened. Second, the protocol specified that, if appropriate, prospective studies would be prioritised and retrospective studies would be included only if no prospective studies were available for a given retinal condition. However, this criterion was amended so that both prospective and retrospective studies would be included, owing to the limited number of available relevant studies. The eligibility criteria employed were therefore as follows:
-
Population: patients of any age who were suspected to have, or have previously been diagnosed with, any retinal conditions, excluding those resulting from malignancy, other ocular diseases (such as glaucoma) or trauma.
-
Index test: FAF imaging performed using cSLO.
-
Reference standard: fundus imaging performed using any clinically relevant method (e.g. fundus photography, fundus fluorescein angiography, indocyanine green angiography, optical coherence tomography, or any combination of relevant tests).
-
Primary outcomes: sensitivity and/or specificity (including any data from which these could be calculated) for the diagnosis or monitoring of retinal conditions.
-
Secondary outcomes (applicable only if primary outcomes were also reported): inter- and intraobserver agreement, adverse events, test acceptability to patients and clinicians, and test interpretability.
-
Study designs: any prospective or retrospective study design, provided that the index test(s) and reference standard were compared in the same patient group.
-
Studies were excluded if they had small sample sizes, that is, fewer than 10 study eyes. Based on published tables of sample sizes required to achieve a given level of diagnostic outcome precision,92 we considered a minimum sample size of 10 eyes per study appropriate to exclude highly imprecise evidence.
Study selection
Studies were selected for inclusion through a two-stage process using predefined and explicit criteria (as specified in Inclusion and exclusion criteria). First, two reviewers independently screened all titles and abstracts identified in the searches to identify bibliographic records that met the inclusion criteria, using a standard pilot-tested study selection worksheet (see Appendix 2). Second, full-text articles were retrieved for those bibliographic records judged to be relevant or unclear at the title and abstract screening step. If a study was reported in more than one article, all articles relating to the study were grouped together for assessment. One reviewer assessed eligibility of each study using the study selection worksheet and a second reviewer checked the decision. At each step of the selection process, any disagreements were resolved by discussion between the two reviewers or, if necessary, by involving a third reviewer.
Data extraction
Data extraction was undertaken by one reviewer and checked by a second reviewer using a predesigned and piloted data extraction form to minimise errors. Any disagreements between reviewers were resolved by consensus or, if necessary, arbitration by a third reviewer. Where sensitivity and specificity were reported in the primary studies with confidence intervals these were extracted and checked and, if necessary and possible, missing values were calculated. Positive and negative likelihood ratios, positive and negative predictive values and the diagnostic odds ratio were also checked and, if necessary, calculated where possible.
Critical appraisal
The methodological rigour of studies reporting diagnostic accuracy was assessed using the Cochrane adaptation81 of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool93 (which focuses on methodological rigour rather than quality of reporting) (see Appendix 3). For each of the included studies, judgements on study rigour were made by one reviewer using the QUADAS criteria and were checked by a second reviewer. Any disagreements between reviewers were resolved by consensus or, if necessary, arbitration by a third reviewer. The QUADAS tool93 asks 11 questions about the characteristics of the primary studies. These questions aim to identify potential threats to the validity of the study findings, and (in our classification) reflect 10 different types of bias that can be present in studies of test accuracy. The way these are interpreted in the current report is summarised in Table 1.
| QUADAS question | Type of bias | Explanation |
|---|---|---|
| 1 | Spectrum bias | The study population is not representative of those who will receive the index test (FAF imaging) in clinical practice |
| 2 | Verification bias | The reference standard does not accurately diagnose the target retinal condition |
| 3 | Disease progression bias | The time interval between index (FAF imaging) and reference standard tests is long enough that the two tests may not have measured the same retinal disease state |
| 4, 5 | Differential verification bias | Diagnosis is inaccurate because not all patients receive the same reference standard test |
| 6 | Incorporation bias | The index test (FAF imaging) is not independent of the reference standard (e.g. may be one of several tests used as the reference standard) |
| 7 | Diagnostic review bias | The index (FAF imaging) test result influences interpretation of the reference standard result |
| 8 | Test review bias | The reference standard result influences interpretation of the reference index test (FAF imaging) result |
| 9 | Clinical review bias | The information used when interpreting the index test (FAF imaging) does not reflect that likely to be available in clinical practice |
| 10 | Image classification bias | Incorrect inclusion or exclusion from the analysis of index test (FAF imaging) images classified as intermediate or of unclear quality may systematically influence sensitivity or specificity |
| 11 | Attrition bias | Exclusion of patients or eyes from analysis may systematically influence sensitivity or specificity if the reason for exclusion is linked to test performance, or if criteria for permitting exclusions differ between imaging tests, especially if the magnitude of attrition is unbalanced across the test methods |
The QUADAS question on ‘patient spectrum’ takes into consideration two elements: whether or not the population characteristics are likely to be representative of those found in actual clinical practice; and whether the primary study design is retrospective or prospective. In this sense the question combines elements of bias and applicability. The reason is that empirical evidence suggests that retrospectively conducted studies are at high risk of bias and as such this limits interpretation of the population relevance. 94
In addition to the modified QUADAS criteria, if necessary, a distinction was made between studies that included one or both eyes per individual so as to avoid any unit-of-analysis issues (e.g. using a subgroup analysis approach). 95
Method of data synthesis
Studies were synthesised through a structured narrative review with tabulation of results of included studies. Heterogeneity among studies and analyses of relevant subgroups was explored and presented using paired sensitivity and specificity forest plots. Paired forest plots differ from standard forest plots, which provide a measure of effect on the x-axis; one of the paired plots provides a measure of sensitivity on its x-axis and the other plot a measure of specificity on its x-axis. As such, visualising both plots together can illustrate how both sensitivity and specificity vary among a group of displayed studies. The x-axis in both cases ranges from 0 to 1, and in the ideal test both sensitivity and specificity would be 1. The analysis and synthesis followed good practice approaches as recommended by the Centre for Reviews and Dissemination (chapter 2: systematic reviews of clinical tests)88 and the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. 87,89
We planned (subject to the availability and suitability of the primary data) to conduct one or more meta-analyses of data on test sensitivity and specificity, in order to improve the precision of any estimates of the accuracy of FAF imaging for diagnosing or monitoring retinal conditions. The appropriateness of meta-analysis was determined by critical appraisal of the primary studies during the quality assessment step (see Critical appraisal). To account for correlation between sensitivity and specificity, and their dependence on the prevalence of retinal conditions, the planned pooling of sensitivity and specificity outcomes was based on appropriate hierarchical random-effects models [using statistical software such as Winbugs (MRC Biostatistics Unit, Cambridge, UK) or SAS (SAS Institute Inc., Cary, NC, USA)].
Chapter 4 Assessment of diagnostic and monitoring studies
This chapter presents the quantity of research available, including the number of studies, their designs, participant characteristics and the characteristics of the index tests and reference standards that they compared (see Quantity of research available). Critical appraisal of the included studies is then reported, including their risks of bias (see Quality of research available) before the assessment of diagnostic accuracy is presented (see Assessment of test accuracy), which takes into consideration the available evidence on diagnostic outcomes as well as any threats to validity highlighted in the preceding sections.
Quantity of research available
The selection of evidence for the systematic review is summarised in Figure 2. Searches yielded 2240 unique bibliographic records, the majority of which were excluded on inspection of the title and/or abstract because the record did not meet one or more of the specified criteria listed in the study selection worksheet (see Appendix 2). Full-text versions were obtained for 206 bibliographic records for further scrutiny where the title and abstract appeared to meet the inclusion criteria, or insufficient information was available to make a judgement on eligibility. Scrutiny of these 206 full-text articles revealed that the majority (198) were not relevant, primarily because they did not report (or provide sufficient data to enable us to calculate) sensitivity and specificity of FAF imaging. Reasons for excluding each of these 198 full-text articles are listed in Appendix 4. The remaining eight full-text articles (all peer-reviewed journal papers) met all of the specified eligibility criteria and are included in the current systematic review.
FIGURE 2.
Flow chart for the identification of studies.

Number and type of studies included
The eight included research papers describe eight unique primary studies. 83,84,96–101 Full details of these studies, including our assessment of their methodological rigour, are given in the data extraction forms (see Appendix 5).
Characteristics of the included studies
Key characteristics of the included studies are summarised in Table 2.
| Retinal condition | Study | Patient recruitment (for study inclusion criteria see Appendix 2) | Prospective research | Consecutive selection of patients (or images) | Diagnosis and/or monitoring | Interobserver agreement reported |
|---|---|---|---|---|---|---|
| AMD: choroidal neovascularisation | Cachulo et al.99 | Patients were actively recruited from one centre | Yes | Not reported | Diagnosis only | No |
| AMD: reticular pseudodrusen | Hogg et al.96 | Patients were actively recruited from three centres in three countries by invitation | Yes | Not reported | Diagnosis only | Yes, in one out of three study centres only |
| Smith et al.101 | FAF and CFP photograph pairs were selected from two databases in different countries | No | Not reported | Diagnosis only | No | |
| Ueda-Arakawa et al.97 | A series of patients with AMD was selected from one centre | No | Yes | Diagnosis only | Yes | |
| Cystoid macular oedema | McBain et al.100 | A series of patients was selected from an autofluorescence imaging database of one ophthalmology centre | No | Yes | Diagnosis only | No |
| Dinc et al.83 | Patients were selected from the ‘FAF database’ (no details provided) during a specified time period | Yesa | Not reported | Diagnosis only | No | |
| Diabetic macular oedema | Vujosevic et al.98 | Patients were recruited from diabetic retinopathy clinics (number not reported) during a specified time period | Unclear | Yes | Diagnosis only | No |
| Waldstein et al.84 | Chart review of all patients with diabetic retinopathy who underwent relevant imaging in one clinic during a specified time period | No | Yes | Diagnosis only | Yes |
Although FAF imaging may be used to detect autofluorescence in a wide range of retinal diseases (see Chapter 1), the eight included studies83,84,96–101 cover only three broad retinal conditions. These are AMD (four studies96,97,99,101) cystoid macular oedema (two studies83,100) and diabetic macular oedema (two studies84,98). Of four studies on AMD, three studies specifically investigated FAF imaging in the detection of reticular pseudodrusen96,97,101 and one study investigated FAF imaging in the diagnosis of choroidal neovascularisation. 99
The eight studies included in the systematic review provide information on diagnostic accuracy only (see Table 2). No studies of the use of FAF imaging for monitoring the natural progression of retinal conditions, or for monitoring the response of retinal conditions to therapy, met the inclusion criteria.
Four of the studies involved the analysis of retrospective data,84,97,100,101 two involved prospective recruitment of patients,96,99 one appeared to prospectively recruit patients, although this was not explicitly reported,83 and it is unclear whether the remaining study was retrospective or prospective98 (see Table 2). Four of the studies selected patients consecutively (i.e. in the chronological order in which they first presented to clinicians)84,97,98,100 and the remaining studies did not report whether selection was consecutive. 83,96,99,101 Retrospective studies, and those lacking consecutive patient recruitment, may be at greater risk of selection bias, and this is considered further below in relation to the overall assessment of the quality of evidence (see Quality of research available).
Most of the studies were conducted in Europe. The study by McBain and colleagues100 was exclusively in the UK and the study by Waldstein and colleagues84 also appears to have been conducted in the UK, although this was not explicitly reported. Two studies were conducted in multiple countries, which included the UK. Hogg and colleagues96 conducted studies in Italy, Portugal and the UK, and Smith and colleagues101 in the USA and UK. The remaining studies were conducted in Italy,98 Portugal,99 Turkey83 and Japan. 97 The studies were published between 2006 and 2014. The earliest reported participant recruitment was in 2004100 and the latest in November 201084,97 but dates of recruitment were not reported in three studies. 96,99,101
Both men and women were included in the studies. Where reported (in seven studies83,84,96–100), the proportion of men ranged from 50% to 69%. Age was not reported in one study on AMD,101 but in the remaining studies participants were older in the AMD studies (mean age 74–76 years)96,97,99 than in the studies on cystoid macular oedema (mean age 59–62 years)83,100 and diabetic macular oedema (mean age 49–67 years)84,98 (see Table 3).
As indicated in Table 3, three of the four studies on AMD excluded participants with ocular comorbidities,96,97,99 but this was not reported in the fourth study. 101 One study on cystoid macular oedema excluded participants with ocular comorbidities,83 while the other did not report exclusion criteria. 100 For diabetic macular oedema, one study excluded patients with significant media opacities98 while the other excluded patients with any macular comorbidity. 84
| Retinal condition | Study | Country | Number of patients | Number of eyes | Mean age (range), years | % male | Ocular comorbidities permitted |
|---|---|---|---|---|---|---|---|
| AMD: choroidal neovascularisation | Cachulo et al.99 | Portugal | 52a | 52 | 76 (56–92) | 50 | No |
| AMD: reticular pseudodrusen | Hogg et al.96 | Italy, Portugal, UK | 105 | 105 | 76 (52–93) | 50 | No |
| Smith et al.101 | UK, USA | 138 | 221 | Not reported | Not reported | Unclear | |
| Ueda-Arakawa et al.97 | Japan | 114 | 220 | 74 (52–92) | 69 | No | |
| Cystoid macular oedema | McBain et al.100 | UK | 34 | 34 | 59 (17–89) | 59 | Yes |
| Dinc et al.83 | Turkey | 55 | 67 | 62 (not reported) | 51 | Yes – apart from specific exclusions | |
| Diabetic macular oedema | Vujosevic et al.98 | Italy | 137 | 263 | T1D: 49 (28–64);b T2D: 67 (41–85)b | 64 | Yes – except significant media opacities |
| Waldstein et al.84 | UK? (not reported) | 71 | 125 | 63 (not reported) | 65 | No |
In all studies the unit of analysis was the eye, and the number of eyes included ranged from 34 to 263 (see Table 3). As shown in Table 4, the studies differed according to whether they included one eye per patient,96,99,100 both eyes,97 or a mixture of single eyes and both eyes. 83,84,98,101 In the study on AMD by Smith and colleagues,101 the results of two case series are reported (obtained from two databases – one in the USA and one in the UK) each with different inclusion criteria, one of which included both eyes, while the other included single eyes. However, the data on test accuracy from the study by Smith and colleagues101 relevant to the current review are only available for the pooled population.
| Retinal condition | Study | Eye(s) studied (one or both) and definition of the condition |
|---|---|---|
| AMD: choroidal neovascularisation | Cachulo et al.99 | Fellow eyes (defined as having early age-related maculopathy) of patients who had neovascular AMD in the non-study eye. Study eyes had: ≥ 5 intermediate (> 63 µm) or one large soft druse (> 125 µm), and/or confluent drusen within 3.0 µm of the foveal centre, with or without pigmentary changes |
| AMD: reticular pseudodrusen | Hogg et al.96 | Fellow eyes of patients who had advanced unilateral neovascular AMD in the non-study eye. Study eyes specifically had early AMD with no features of GA or neovascular AMD |
| Smith et al.101 | Mixture of 83 pairs of eyes which had bilateral large, soft drusen (with or without GA) but had no evidence of choroidal neovascularisation, and 55 single eyes which were the fellow eyes of patients who had unilateral choroidal neovascularisation | |
| Ueda-Arakawa et al.97 | Both eyes of patients newly diagnosed with early AMD, neovascular AMD or GA in at least one eye. Early AMD was defined as the presence of soft drusen (≥ 63 µm) or areas of hyper- or hypopigmentation in the retinal pigment epithelium, and GA was defined using colour fundus photography as a sharply delineated area (≥ 175 µm) of hypopigmentation, depigmentation or apparent absence of the retinal pigment epithelium in which choroidal vessels were clearly visible. Neovascular AMD was defined as neovascularisation detected using fluorescein or indocyanine green angiography | |
| Cystoid macular oedema | McBain et al.100 | Single eyes of patients with clinically suspected cystoid macular oedema secondary to cataract extraction, inherited retinopathies, inflammatory eye disease or idiopathic cases. In bilateral cases the left eye was arbitrarily chosen |
| Dinc et al.83 | Single eyes of 43 patients and both eyes of 12 patients whose cystoid macular oedema was secondary to diabetic retinopathy, retinal vein occlusions, uveitis, cataract surgery, epiretinal membrane formation or AMD | |
| Diabetic macular oedema | Vujosevic et al.98 | Both eyes of 126 patients and single eyes of 11 patients with diabetes mellitus (type 1 or 2) who had any stage of treated or untreated diabetic retinopathy |
| Waldstein et al.84 | Both eyes of 54 patients and single eyes of 17 patients with diabetic retinopathy with or without diabetic macular oedema |
Descriptions of the retinal conditions varied considerably in detail and the studies were generally heterogeneous in terms of the conditions they included (see Table 4). The three studies on reticular pseudodrusen in AMD96,97,101 differed according to the stage of AMD and whether it affected one or both eyes. The two studies on cystoid macular oedema83,100 differed in the primary conditions from which the cystoid macular oedema developed. The studies on diabetic macular oedema84,98 differed on whether they included ocular comorbidities.
In keeping with the inclusion criteria for the current review, all FAF imaging tests were conducted using confocal scanning ophthalmoscopy (Table 5). The studies (except one that did not specify the image acquisition equipment101) used variants of the Heidelberg Retina Angiograph (Heidelberg Engineering, Heidelberg, Germany). Where reported (six studies83,84,97–100), the excitation wavelength was 488 nm (blue) and, where reported (five studies83,97–100), the detection wavelength was > 500 nm. Two of the studies included an additional FAF imaging test with a higher excitation wavelength; these were at 514 nm (green) in a study of diabetic macular oedema84 and at 790 nm (near infrared) in a study of reticular pseudodrusen. 97 The field of view, which was specified in five studies,83,84,96,99,100 was 30 degrees and the number of frames from which the final (mean) FAF image was calculated (specified in all studies except one101) ranged from 9 to 20.
| Retinal condition | Study | Index test | Index test diagnostic criteria (full details in Appendix 5) | Reference standard |
|---|---|---|---|---|
| AMD: choroidal neovascularisation | Cachulo et al.99 | FAF: excitation 488 nm; detection > 500 nm (used HRA 2 cSLO) | Five types of FAF patterns. Images classified according to the International Fundus Autofluorescence Classification Group.45,46 No other details provided | FA – stated as being the gold standard for assuming the presence of conversion of early age-related maculopathy to AMD (diagnostic criteria not reported) |
| AMD: reticular pseudodrusen | Hogg et al.96 | FAF: excitation and detection wavelengths not stated (used Spectralis HRA + OCT cSLO) | Reticular hypoFAF pattern (identified using automated background levelling and segmentation): clusters of ill-defined hypoautofluorescent lesions interspersed against a background of mildly increased autofluorescence occurring in a regular and well-defined array | Positive diagnosis on one or more of five imaging modalities (CFP, RFP, IRP, FAF, OCT). Diagnostic criteria reported for each modality (see Appendix 5) |
| Smith et al.101 | FAF: excitation and detection wavelengths not stated (equipment not stated but cSLO implied) | Reticular hypoFAF pattern: stated only that definition excluded atrophic drusen patterns that were more central, darker and more scattered than the lower-contrast reticular autofluorescence that filled a whole region homogeneously | CFP (diagnostic criteria not explicitly reported – implied only that identification of drusen was based on areas of hypo- or hyperpigmentation) | |
| Ueda-Arakawa et al.97 | (1) FAF: excitation 488 nm; detection > 500 nm (2) NIR-FAF: excitation 790 nm; detection not stated (used Spectralis HRA + OCT cSLO) |
Reticular hypoFAF pattern: a group of ill-defined, hypofluorescent lesions against a background of mildly elevated autofluorescence | Positive diagnosis on two or more of seven imaging modalities (blue-channel CFP, IRR, FAF, NIR-FAF, CBR, ICGA, SD-OCT). Diagnostic criteria reported for each modality (see Appendix 5) | |
| Cystoid macular oedema | McBain et al.100 | FAF: excitation 488 nm; detection > 500 nm (used HRA cSLO) | Round or oval areas of FAF at the fovea, with an autofluorescence signal similar to background levels (normal eyes would have hypoautofluorescence at this location due to luteal pigment blocking) | FA – stated used as the reference test as it has been used routinely for diagnosis of CMO; diagnosed if late-phase FA gave a petaloid dye leakage pattern around the fovea |
| Dinc et al.83 | FAF: excitation 488 nm; detection > 500 nm (used HRA cSLO) | Not reported explicitly but mentioned post hoc (in results section) as being round and oval hyperFAF at the fovea | FA – diagnosis of CMO made if late-phase FA showed pathognomonic leakage of fluorescein at the fovea in a petaloid configuration with feathery margins | |
| Diabetic macular oedema | Vujosevic et al.98 | FAF: excitation 488 nm; detection > 500 nm (used HRA 2 cSLO) | Single or multiple spot hyper FAF, no other details provided | RM-SLO (diagnostic criteria not reported) |
| Waldstein et al.84 |
|
Not reported explicitly but mentioned post hoc (in results section) that DMO typically stands out as relatively bright, single or multiple, round or oval areas, mostly bordered by darker rims | SD-OCT – stated that this was considered the clinical standard for non-invasive diagnosis of DMO; only mentioned that signs of DMO on SD-OCT were intraretinal cysts, subfoveal neurosensory detachment and retinal thickening |
The reference standards reported in the primary studies were: fluorescein angiography for diagnosing choroidal neovascularistion99 and cystoid macular oedema;83,100 colour fundus photography101 or multiple imaging modalities96,97 for diagnosing reticular pseudodrusen; and either retromode scanning laser ophthalmology98 or spectral-domain optical coherence tomography84 for diagnosing diabetic macular oedema (see Table 5). In the two studies that employed multiple imaging modalities,96,97 the reference standard is somewhat unusual since diagnosis required a positive result on a specified minimum number of modalities. The clinical relevance of the reference standards is discussed further in our assessment of study quality (see Quality of research available).
Six of the studies reported that patients underwent an ophthalmic examination, which included assessments of visual acuity, intraocular pressure and/or slit lamp biomicroscopy83,84,96–99 but they did not specify whether or not information obtained from these examinations was necessary to assist any of the diagnostic decisions made using the index test or reference standard. Three studies employed further retinal imaging tests in addition to their index test and reference standard83,98,99 (these are listed as ‘comparators’ in Appendix 5). Two of these, by Cachulo and colleagues99 on choroidal neovascularisation (where the additional tests were colour fundus photography, indocyanine green angiography, optical coherence tomography and a retinal leakage analysis) and by Dinc and colleagues83 on cystoid macular oedema (where the additional test was optical coherence tomography), did not explain whether or not any information from the additional tests was taken into account when making diagnostic decisions using FAF imaging or the reference standard (fluorescein angiography). The third study, by Vujosevic and colleagues98 on diabetic macular oedema (where the additional tests were time-domain optical coherence tomography and fluorescein angiography), mentioned that images were graded in a masked fashion, suggesting that the additional tests would not have influenced any diagnostic decisions made using FAF imaging or the reference standard (retromode scanning laser ophthalmoscopy). One of the studies on diabetic macular oedema which employed both 488 nm and 514 nm wavelengths of FAF imaging used the results of both tests to calculate macular pigment optical density maps. 84 This approach was reported to have lower sensitivity and specificity for detecting diabetic macular oedema than either of the individual FAF imaging tests on which it was based (see Appendix 5) and as such is not considered as a separate index test in the current report.
The diagnostic criteria on FAF imaging for each of the retinal conditions were qualitative (i.e. descriptive) in all studies (see Table 5), and only one of the studies reported an objective (quantitative) approach for determining how abnormal (hypo or hyper) autofluorescence was defined. 101 The three studies on reticular pseudodrusen all specified that the diagnostic criterion was a reticular pattern of hypoautofluorescence, although there were slight differences in how this was described in each study. 96,97,101 The two studies on cystoid macular oedema83,100 both appeared to diagnose the condition as round or oval patterns of autofluorescence at the fovea, although this was not stated explicitly in one study. 83 The two studies on diabetic macular oedema84,98 only mentioned the diagnostic criteria briefly and differed in how these were described. Despite the subjective nature of the diagnostic criteria, only three of the eight studies investigated intergrader agreement in the classification of retinal images (see Table 2). 84,97,98 Limited information was provided about the expertise of the image graders, who were described as two independent ophthalmologists,97 two retinal specialists trained in image grading98 and two independent graders. 84
Quality of research available
This section presents an overview of study rigour as assessed using the Cochrane adaptation81 of the QUADAS tool. 93 Table 6 summarises the critical appraisal judgements for each of the eight included studies. 83,84,96–101 Supporting explanations for the judgements are given in the full versions of the data extraction forms (see Appendix 5). As explained above (see Table 1) each of the QUADAS questions relates to a different aspect of bias or applicability that could limit the validity of the study results. An overview of these risks of bias is provided at the end of the section.
| QUADAS question | CNV in AMD | Reticular pseudodrusen in AMD | Cystoid macular oedema | Diabetic macular oedema | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cachulo et al.99 | Smith et al.101 | Hogg et al.96 | Ueda-Arakawa et al.97 | Dinc et al.83 | McBain et al.100 | Vujosevic et al.98 | Waldstein et al.84 | ||
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | No | No | No | No | No | No | Unclear | No |
| 2 | Is the reference standard likely to classify the target condition correctly? | Yes | Yes | Unclear | Unclear | Yes | Yes | Unclear | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| 4 | Did the whole sample or a random selection of the sample receive verification using the intended reference standard? | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Unclear |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 10 | Were uninterpretable/intermediate test results reported? | Yesa | No | No | Yesb | No | Yesc | No | No |
| 11 | Were withdrawals from the study explained? | Yes | Not applicable | No | Yes | Not applicable | Yes | Not applicable | Not applicable |
Quality assessment of diagnostic accuracy studies question 1: applicability of the patient spectrum
The populations included in the primary studies were considered unlikely to be representative of those who would present in NHS clinical practice (the patient spectrum was considered ‘probably not representative’ in seven studies83,84,96,97,99,100,101 and unclear in one study98) (see Table 6). In the studies by Cachulo and colleagues,99 Hogg and colleagues96 and Smith and colleagues101 the case mix was considered atypical and unlikely to represent patients in the NHS as the retinal condition was required to differ between the two eyes. In the studies by McBain and colleagues,100 Dinc and colleagues,83 Ueda-Arakawa and colleagues97 and Waldstein and colleagues84 the case mix also appears to have limited relevance to current NHS practice, as McBain and colleagues100 and Dinc and colleagues83 required cystoid macular oedema to be specific to certain causal conditions, while Ueda-Arakawa and colleagues97 and Waldstein and colleagues84 excluded patients with ocular comorbidities. In addition, the studies by McBain and colleagues,100 Ueda-Arakawa and colleagues97 and Waldstein and colleagues84 selected patients retrospectively, which may further limit their applicability to current practice (as the case mix is potentially selective). 102 The study by Vusojevic and colleagues98 included patients with any stage of treated or untreated diabetic retinopathy and permitted ocular comorbidities and, as such, appears potentially relevant to the NHS; however, the study was conducted in Italy and it is unclear whether patients were selected prospectively or retrospectively.
Quality assessment of diagnostic accuracy studies question 2: applicability of reference standards
In five of the studies83,84,99–101 the reference standards employed for diagnosing the target retinal condition were judged to be appropriate in principle as they are accepted standard methods: fluorescein angiography was used for diagnosing choroidal neovascularisation99 and cystoid macular oedema;83,100 colour fundus photography was used for diagnosing reticular pseudodrusen;101 and spectral-domain optical coherence tomography was used for diagnosing diabetic macular oedema. 84 However, there are caveats around this interpretation because clinical experts advised that it is unlikely that individual imaging tests would be used in isolation, as appeared to be the case in these primary studies. For instance, fluorescein angiography would typically be employed in conjunction with optical coherence tomography when diagnosing cystoid macular oedema and diabetic macular oedema. 34,38 For the detection of reticular pseudodrusen, although colour fundus photography has been commonly used and is often considered a standard approach, this may not capture all the reticular pseudodrusen that could be identifiable using other imaging methods. 11,103 As noted in Table 6, for three studies we judged the ability of the reference standard to diagnose the target retinal condition as unclear. 96–98 This was either because the studies employed a suite of imaging modalities and required a positive result on a specified minimum number of these96,97 (i.e. a non-standard diagnostic approach) or because the reference standard employed in the primary study (retromode scanning laser ophthalmology) has not been previously explored as a diagnostic tool for the specified condition (diabetic macular oedema). 98 As such, these three studies would not be reflective of standard imaging approaches employed in the NHS.
Quality assessment of diagnostic accuracy studies question 3: test interval
The time period between the index and reference tests (QUADAS question 3) was unclear (not reported) in five of the studies83,96,97,99,101 and hence the risk of disease progression bias in these studies is unclear. In one study on cystoid macular oedema100 and both studies on diabetic macular oedema84,98 the time interval was considered short enough that both tests would have been assessing the same disease state and so these studies would be considered at low risk of disease progression bias.
Quality assessment of diagnostic accuracy studies question 4: population receiving the reference standard
In all studies except one96 all the patients included in each study received the reference standard. In the study by Hogg and colleagues96 on reticular pseudodrusen, multiple imaging methods were used, but it is unclear, according to the reported sample sizes, whether all participants received all tests and some data were then excluded, or whether some participants did not receive all tests.
Quality assessment of diagnostic accuracy studies question 5: same reference standard for all patients
In six studies all the patients included in each study received the same reference standard. 83,84,98–101 In the two studies where this was not the case, conducted by Hogg and colleagues96 and Ueda-Arakawa and colleagues,97 the combination of imaging modalities that contributed to a diagnosis (at least one of five tests96 or at least two of seven tests97) could have differed between the patients.
Quality assessment of diagnostic accuracy studies question 6: independence of index test and reference standard
In six studies the index text was independent of the reference standard. 83,84,98–101 In the two studies where this was not the case, conducted by Hogg and colleagues96 and Ueda-Arakawa and colleagues,97 the index tests were among five tests96 or seven tests97 that made up the reference standard.
False positives are not possible where FAF imaging is also part of the reference standard and, as would be expected, no false positives were reported for FAF imaging by Hogg and colleagues. 96 However, Ueda-Arakawa and colleagues did report false positives (9/217 for FAF imaging and 5/136 for near-infrared FAF imaging). These false positives appear to be an artefact of the diagnostic criterion for reticular pseudodrusen, which required a positive result on two or more of seven imaging modalities. The ‘false positives’ in this case indicate that, in nine and five eyes, FAF imaging and near-infrared FAF imaging, respectively, were the only tests out of seven in the reference standard that detected reticular pseudodrusen.
Quality assessment of diagnostic accuracy studies question 7: interpretation of reference standard independent of index test results
In all studies except one98 it was unclear (not reported) whether or not investigators interpreting the results of the reference standard test might have been aware of the results of the index test. In the study by Vusojevic and colleagues98 the order of tests was not reported but it was stated that images were independently graded in a masked fashion.
Quality assessment of diagnostic accuracy studies question 8: interpretation of index test independent of reference standard results
In six studies it was unclear (not reported) whether or not investigators interpreting the results of the index test might have been aware of the results of the reference standard. 84,96,97,99–101 In the study by Dinc and colleagues83 interpretation of the index test was independent of the reference standard as the index test was conducted first. In the study by Vusojevic and colleagues,98 the order of tests was not reported but it was stated that images were independently graded in a masked fashion.
Quality assessment of diagnostic accuracy studies question 9: clinical information available for test interpretation
None of the studies explicitly stated whether or not their index tests or reference standards were interpreted in conjunction with other clinical information (i.e. the results from standard ophthalmic examinations or other imaging tests) and this casts considerable doubt over whether or not any of the reference standard tests as employed in the primary studies would properly represent how the tests would be employed in clinical practice.
Quality assessment of diagnostic accuracy studies question 10: reporting of uninterpretable or intermediate test results
Five of the studies (two on reticular pseudodrusen,96,101 two on diabetic macular oedema84,98 and one on cystoid macular oedema83) did not report whether uninterpretable or intermediate test results were considered either in the calculation of diagnostic accuracy or as exclusion criteria, so it is unclear whether or not image quality might have limited the utility of any of the imaging tests in these studies. Three studies reported poor quality or uninterpretable images. 97,99,100 Cachulo and colleagues99 mentioned that the pattern of autofluorescence could not be determined for two eyes (4%) because of poor-quality images, but it is unclear whether or not these were included in the diagnostic accuracy calculation. Ueda-Arakawa and colleagues97 reported that 84 eyes (38%) were excluded from analysis because of poor image quality. McBain and colleagues100 stated that nine eyes (9%) were excluded because of poor FAF images.
Quality assessment of diagnostic accuracy studies question 11: study withdrawals explained
In four studies there were no withdrawals and so this question was answered as ‘not applicable’. 83,84,98,101 In three studies the authors provided explanations for the withdrawals. 97,99,100 Cachulo and colleagues99 reported these as death, withdrawal of informed consent, hospitalisation and loss to follow-up. McBain and colleagues100 reported these as no FAF, more than 2 weeks between tests, FAF imaging < 4 days after the reference standard (no explanation provided), poor FAF images related to media opacities and cystoid macular oedema related to other diseases (e.g. previous branch vein occlusion, diabetic retinopathy or AMD). Ueda-Arakawa and colleagues97 reported these as due to phthisis bulbi or poor image quality in three or more imaging modalities. One study, by Hogg and colleagues,96 explained that images were excluded from analysis because of poor image quality but did not provide this information specifically for the index test.
Summary of quality assessment in relation to risks of bias
Seven of the eight included studies appear to be at high risk of spectrum bias (QUADAS question 1)83,84,96,97,99–101 since their patient populations appeared not to be representative of those likely to be encountered in clinical practice, and in the remaining study98 the risk of spectrum bias is unclear. Although most (five) of the studies appear to have employed reference standards that are commonly used and therefore might be considered acceptable (QUADAS question 2),83,84,99–101 these studies appeared to employ single imaging tests as the reference standard whereas diagnostic decisions in clinical practice would more likely employ a combination of imaging tests. For this reason the risk of verification bias was considered as being unclear for these five studies and high for the remaining three studies96–98 where the reference standard was unlikely to have accurately diagnosed the target retinal condition. The risk of disease progression bias (assessed in QUADAS question 3) is largely unclear, except for one study on cystoid macular oedema100 and both studies on diabetic macular oedema,84,98 which were judged to be at low risk. The risk of differential verification bias, that is, bias arising as a result of participants not all receiving the same reference standard (assessed in QUADAS questions 4 and 5), was considered to be low for most (six)83,84,98–101 of the studies but high for two studies96,97 on reticular pseudodrusen owing an unorthodox design of reference standard (where diagnosis required a positive result on a specified minimum number of imaging modalities). These same two studies on reticular pseudodrusen were considered at high risk of incorporation bias (assessed in QUADAS question 6) since the index test was not independent of the reference standard; the remaining six studies were judged to have low risk of this bias. 83,84,98–101 The risk of diagnostic review bias, that is, the index test result influencing interpretation of the reference standard result (assessed in QUADAS question 7) was unclear for most (seven) studies but judged to be low for one study on diabetic macular oedema. 98 The risk of test review bias, that is, where results of the reference standard influence interpretation of the index test result (assessed in QUADAS question 8) was unclear in most (six) studies84,96,97,99–101 but judged to be low for one study on cystoid macular oedema83 and for one study on diabetic macular oedema. 98 The risk of clinical review bias (assessed in QUADAS question 9) is unclear since none of the included studies reported whether their index tests were interpreted together with other clinical information, such as the results of routine ophthalmic examinations or results of other imaging modalities they employed. The risk of bias from uninterpretable or intermediate test results (QUADAS question 10) is generally unclear. Risk of attrition bias (QUADAS question 11) was judged to be low in five studies since either there were no exclusions,83,84,98,101 or the exclusions were clearly explained and appeared unlikely to influence the analysis. 97 Two studies explained their withdrawals but presented discrepancies in the numbers they analysed99,100 (see Appendix 5), while one study did not explain withdrawals;96 these three studies were judged to have an unclear risk of attrition bias. Overall, taking into consideration the different risks of bias mentioned above, it is not possible to identify individual studies, or specific retinal conditions, for which the quality of the clinical evidence would be regarded as being adequately robust to directly inform clinical practice.
An aspect of study quality not captured by the QUADAS criteria is that studies that included both eyes of a patient did not take into account adjustment for the correlation between the patient’s eyes, and this might lead to underestimation of standard errors for sensitivity and specificity. 95 This applies to two studies of reticular pseudodrusen in AMD by Ueda-Arakawa and colleagues97 and Smith and colleagues,101 and to both studies of diabetic macular oedema, by Vusojevic and colleagues98 and Waldstein and colleagues. 84
Assessment of test accuracy
This section presents the best evidence available on the diagnostic accuracy of FAF imaging. Given the heterogeneity of study characteristics with respect to the retinal conditions included, whether they were assessed in one or both eyes and differences between studies in the reference standards employed (see Quantity of research available), it would be inappropriate to quantitatively pool diagnostic accuracy outcomes (i.e. sensitivity and specificity) across individual studies within each of the retinal conditions. Instead a narrative synthesis was undertaken, which provides sensitivity and specificity estimates for each retinal condition together with their confidence intervals, as well as likelihood ratios to illustrate the overall diagnostic results from each study and their uncertainty. Paired sensitivity and specificity forest plots are presented for illustrative purposes, without pooling the sensitivity or specificity measures across the studies. For brevity, likelihood ratios are discussed below only in cases where they suggest that the results of a given study might not be diagnostically informative (based on the criteria given in Chapter 2). Diagnostic odds ratios and positive and negative predictive values are also available for all studies (see Appendix 5). However, these statistics have to be interpreted with caution. 89 Given that there are concerns about the quality of the evidence in all studies (see Quality of research available), and in the absence of a quantitative meta-analysis, the predictive values and diagnostic odds ratio would not provide additional interpretational value in the assessment of diagnostic accuracy and as such they are not referred to below.
Choroidal neovascularisation in neovascular age-related macular degeneration
One study, by Cachulo and colleagues,99 assessed the diagnostic accuracy of FAF imaging (488 nm) against fluorescein angiography in detecting choroidal neovascularisation in AMD. The study authors reported sensitivity of 93% and specificity of 37%. However, using the data available from Cachulo and colleagues,99 we calculated sensitivity of 88% and specificity of 34% (Table 7) (for calculations see Appendix 5). This discrepancy might result from differences in categorising two (out of 52) eyes for which the pattern of FAF could not be determined because of poor-quality images. Confidence intervals for the estimates of sensitivity and specificity suggest moderate uncertainty around these values (Figure 3), but were not reported for the estimates calculated by the study authors.
| Study | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Positive likelihood ratio, % (95% CI) | Negative likelihood ratio, % (95% CI) |
|---|---|---|---|---|
| Cachulo et al., 201199 | 93 (not reported) | 37 (not reported) | Not reported | Not reported |
| Calculated by reviewer | 88.24 (63.52 to 98.20) | 34.29 (19.15 to 52.21) | 1.34 (1.00 to 1.80) | 0.34 (0.09 to 1.36) |
FIGURE 3.
Diagnostic accuracy of FAF imaging for choroidal neovascularisation in neovascular AMD. CI, confidence interval; FN, false negative; FP, false positive; TN, true negative; TP, true positive.
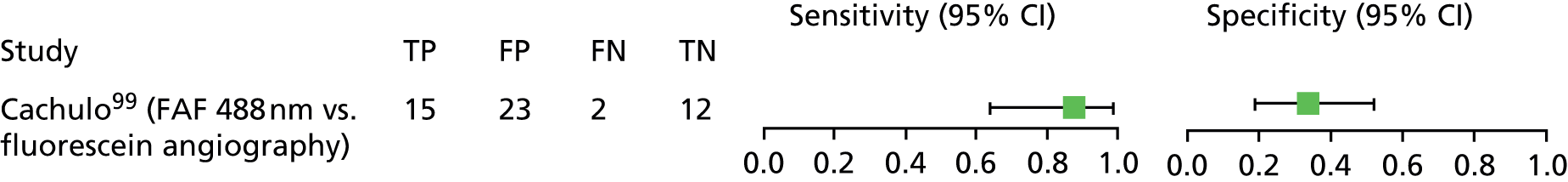
Cachulo and colleagues99 did not report any information on interobserver or intraobserver agreement in image grading, test acceptability to patients or clinicians, or adverse events that might influence the interpretation of diagnostic outcomes.
As noted above (see Quality of research available), the single available study on diagnosis of choroidal neovascularisation in AMD99 was judged to be at high risk of spectrum bias (owing to an unrepresentative case mix); in addition the risks of several types of bias that could arise in relation to the sequence and timing of the index and reference tests, as well as the risk of bias in the interpretation of these tests, were unclear. Taking these considerations into account, it is not possible to draw a rigorous clinically relevant conclusion about the diagnostic accuracy of FAF 488 nm imaging as compared against fluorescein angiography in this retinal condition.
Reticular pseudodrusen in age-related macular degeneration (different stages)
Three studies, by Hogg and colleagues,96 Smith and colleagues101 and Ueda-Arakawa and colleagues,97 assessed the accuracy of FAF imaging for detecting reticular pseudodrusen in AMD.
Two of the studies did not report the FAF imaging excitation wavelength,96,101 while Ueda Arakawa and colleagues97 used FAF 488 nm and infrared FAF 790 nm (NIR-FAF) as index tests. The studies used various reference standards. Smith and colleagues101 used colour fundus photography, Hogg and colleagues96 required a positive result on one or more of five imaging modalities, and Ueda-Arakawa and colleagues97 required a positive result on two or more of seven imaging modalities. Each study aimed to detect reticular pseudodrusen in a different stage of AMD (see Table 4). Hogg and colleagues96 studied fellow eyes of patients with neovascular AMD in the non-study eye, Ueda-Arakawa and colleagues97 studied both eyes of patients newly diagnosed with early AMD, neovascular AMD or GA, and Smith and colleagues101 included a mixture of both eyes of patients without any evidence of choroidal neovascularisation and fellow eyes of patients with unilateral choroidal neovascularisation in the non-study eye.
By taking together the appropriate reference standards for diagnosing reticular pseudodrusen (i.e. colour fundus photography and optical coherence tomography) and the other reference standards nominated in the primary studies, data are available for six diagnostic accuracy comparisons for FAF imaging (Table 8). Although the reference standard reported by Hogg and colleagues96 involves a positive result on one or more tests, individual comparisons of FAF imaging against colour fundus photography and FAF imaging against optical coherence tomography were also reported.
| Study | Index text | Reference standard | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Positive likelihood ratio, % (95% CI) | Negative likelihood ratio, % (95% CI) |
|---|---|---|---|---|---|---|
| Smith et al.101 | FAFa | CFP | 93.33 (77.89 to 98.99) | 97.91 (94.72 to 99.41) | 44.57 (16.82 to 118.08) | 0.07 (0.02 to 0.26) |
| Hogg et al.96 | FAFa | Spectralis OCT | 87.88 (71.78 to 96.52) | 84.21 (72.13 to 92.30) | 5.57 (3.02 to 10.27) | 0.14 (0.06 to 0.36) |
| CFP | 100 (78.03 to 100.00) | 66.67 (55.08 to 76.94) | 3.00 (2.19 to 4.11) | 0.00 (not calculable) | ||
| Positive on one of more of five imaging modalities (CFP, RFP, IRP, FAF, OCT) | 95.35 (84.16 to 99.30) | 100 (92.82 to 100.00) | Not calculable | 0.05 (0.01 to 0.18) | ||
| Ueda-Arakawa et al.97 | FAF 488 nm | Positive on two or more of seven imaging modalities (CFP, IRR, FAF, NIR-FAF, CBR, ICGA, SD-OCT) | 86.49 (71.21 to 95.41) | 95.00 (90.72 to 97.68) | 17.30 (9.04 to 33.11) | 0.14 (0.06 to 0.32) |
| FAF 790 nm (NIR-FAF) | 32.14 (15.91 to 52.35) | 95.37 (89.52 to 98.46) | 6.94 (2.53 to 19.08) | 0.71 (0.55 to 0.92) |
FAF imaging based on a 488 nm excitation wavelength had relatively high (86–100%) sensitivity for detecting reticular pseudodrusen when compared against the various reference standards (Figure 4). However, near-infrared FAF imaging had low (32%) sensitivity (see Figure 4). In contrast, both FAF and NIR-FAF imaging had high (84–100%) specificity compared against the various reference standards, except for a lower specificity (67%) when FAF imaging was compared against colour fundus photography in the study by Hogg and colleagues. 96 A major limitation of the results for near-infrared FAF imaging reported by Ueda-Arakawa and colleagues97 is that 38% of the eyes were excluded owing to poor image quality.
FIGURE 4.
Diagnostic accuracy of FAF imaging for reticular pseudodrusen in AMD. CFP, colour fundus photography; CI, confidence interval; FN, false negative; FP, false positive; OCT, optical coherence tomography; TN, true negative; TP, true positive. a, Versus two or more imaging modalities.

For most of the studies the 95% confidence intervals around the specificity estimates are relatively narrow. However, confidence intervals for specificity reported by Hogg and colleagues,96 and those reported for sensitivity by all studies are wider, meaning that these estimates may be less reliable (see Figure 4). However, it is possible that the width of the confidence intervals reported by Smith and colleagues101 and Ueda-Arakawa and colleagues97 might have been underestimated, as the analyses did not account for any intrapatient correlations in cases where both eyes of the same patient were analysed.
Two of the studies, by Hogg and colleagues96 and Ueda-Arakawa and colleagues,97 provided information on intergrader agreement in diagnosing reticular pseudodrusen using FAF imaging. Kappa values indicate actual agreement compared with that which would occur by chance. A value of 1 indicates perfect agreement and a value of 0 indicates agreement equivalent to chance. Kappa values were 0.563 (n = 35, p < 0.001) (84.2% agreement)97 and 0.94 (sample size, p-value and percentage agreement not reported). 96 These kappa values could be interpreted as ‘moderate’ and ‘almost perfect’ agreement, respectively. 104 Although intergrader agreement was almost perfect in Hogg and colleagues’ study,96 it was reported for only one of their three study centres and so it is unclear whether or not the ‘best’ agreement was selectively presented or whether or not the reported agreement is reflective of that across all the study centres. None of the studies on reticular pseudodrusen provided any information on intragrader variation in diagnosis, the acceptability of the imaging modalities to patients or clinicians, or on whether any adverse events of any tests were observed.
In summary, the diagnostic outcomes suggest that FAF imaging with an excitation wavelength of 488 nm could potentially contribute to the diagnosis of reticular pseudodrusen but near-infrared FAF imaging (790 nm) does not appear adequately sensitive to be of diagnostic value. However, there is considerable uncertainty around these findings, owing to the limited number of studies and comparisons available and shortcomings in the study designs. As noted above (see Quality of research available), the three available studies on diagnosis of reticular pseudodrusen in AMD96,97,101 were judged to be at high risk of spectrum bias (owing to an unrepresentative case mix), while the risks of several types of bias that could arise in relation to the sequence and timing of the FAF imaging tests in relation to those of the reference standards, and in the interpretation of these tests, were unclear. In addition, in the studies by Hogg and colleagues96 and Ueda-Arakawa and colleagues,97 the FAF imaging index tests also formed part of the reference standard, which complicates interpretation of any diagnostic outcomes. Furthermore, Ueda-Arakawa and colleagues97 had to exclude over one-third of the eyes from their analysis of near-infrared FAF imaging because good image quality could not be obtained with this method. Taking these considerations into account, it is not possible to draw a rigorous clinically relevant conclusion about the diagnostic accuracy of FAF imaging for diagnosing reticular pseudodrusen in AMD.
Cystoid macular oedema secondary to various conditions
Two studies, by Dinc and colleagues83 and McBain and colleagues,100 reported the diagnostic accuracy of 488 nm FAF imaging for identifying cystoid macular oedema secondary to various conditions. Both studies employed fluorescein angiography as the reference standard, which would partly reflect current clinical practice (optical coherence tomography and possibly also colour fundus imaging would be used in addition to fluorescein angiography to reach a diagnosis in clinical practice). The study by Dinc and colleagues83 indicated high (98%) sensitivity with a narrow confidence interval suggesting good precision of the estimate, while that of McBain and colleagues100 indicated moderate sensitivity (81%) but with high uncertainty (Table 9 and Figure 5). However, the former study had 0% specificity (reflecting a lack of true negatives in the data) and hence, as the likelihood ratios suggest, the study is not diagnostically informative. The latter study, by McBain and colleagues,100 is limited by the uncertainty around its sensitivity and specificity estimates which, according to the lower confidence limits, could have been as low as 58% and 39%, respectively.
| Study | Index text | Reference standard | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Positive likelihood ratio, % (95% CI) | Negative likelihood ratio, % (95% CI) |
|---|---|---|---|---|---|---|
| Dinc et al.83 | FAF 488 nm | FA | 98.46 (91.69 to 99.74) | 0.00 (0.00 to 80.71) | 0.98 (0.96 to 1.01) | Not calculable |
| McBain et al.100 | FAF 488 nm | FA | 80.95 (58.08 to 94.44) | 69.23 (38.61 to 90.72) | 2.63 (1.13 to 6.10) | 0.28 (0.11 to 0.71) |
Neither of the two studies on cystoid macular oedema83,100 reported any information on interobserver or intraobserver agreement in image grading, test acceptability to patients or clinicians, or adverse events of any tests that might influence the interpretation of diagnostic outcomes.
FIGURE 5.
Diagnostic accuracy of FAF imaging for cystoid macular oedema. CI, confidence interval; FA, fluorescein angiography; FN, false negative; FP, false positive; TN true negative; TP, true positive.
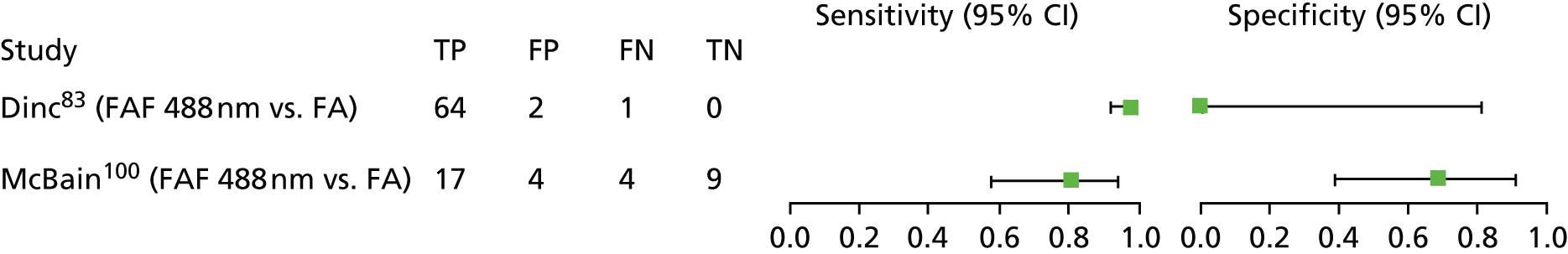
As noted above (see Quality of research available), both studies on diagnosis of cystoid macular oedema83,100 were judged to be at high risk of spectrum bias, owing to an unrepresentative case mix. Although Dinc and colleagues83 interpreted results of the index test (FAF imaging) without knowledge of the results of the reference standard (fluorescein angiography), indicating a low risk of test review bias, the risks of diagnostic review bias and clinical review bias were unclear in both studies. Taking these considerations into account, noting that one study appears to be diagnostically uninformative83 while the other has high uncertainty of its diagnostic outcomes,100 it is not possible to draw a rigorous clinically relevant conclusion about the accuracy of FAF imaging for diagnosing cystoid macular oedema.
Diabetic macular oedema
Two studies, by Vujosevic and colleagues98 and Waldstein and colleagues,84 reported the diagnostic accuracy of FAF imaging for diabetic macular oedema. Vujosevic and colleagues98 compared 488 nm FAF imaging against a reference standard of retromode scanning laser ophthalmoscopy. Waldstein and colleagues84 compared 488 nm and 514 nm FAF imaging against a reference standard of spectral-domain optical coherence tomography. For diabetic macular oedema, optical coherence tomography would be a clinically relevant reference standard (as acknowledged by Vusojevic and colleagues98), although, according to clinical experts consulted during the present review, it would likely be combined in clinical practice with colour fundus imaging and, if macular ischaemia is suspected, also with fluorescein angiography.
Diagnostic outcomes are shown in Table 10 and Figure 6. Sensitivity of FAF imaging (488 nm) for diagnosing diabetic macular oedema was highest (92%) when compared against retromode scanning laser ophthalmoscopy, although this is unlikely to be used routinely in clinical practice. Sensitivity of FAF imaging when compared against spectral-domain optical coherence tomography was moderate (81%) for the shorter-wavelength imaging (488 nm) but relatively low (55%) for the longer-wavelength method (514 nm). In contrast, specificity was relatively high for both wavelengths of FAF imaging when compared against spectral-domain optical coherence tomography (90% to 95%) but lower when 488 nm FAF imaging was compared against retromode scanning laser ophthalmoscopy (85%). The estimates of sensitivity and specificity have relatively narrow confidence intervals, suggesting they are reasonably precise, although it is possible that the width of the confidence intervals for both studies might have been underestimated, since the analyses did not account for any intrapatient correlations where both eyes of the same patient were analysed.
| Study | Index text | Reference standard | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Positive likelihood ratio, % (95% CI) | Negative likelihood ratio, % (95% CI) |
|---|---|---|---|---|---|---|
| Vusojevic et al.98 | FAF 488 nm | RM-SLO | 92.42 (87.98 to 95.60) | 84.62 (71.91 to 93.10) | 6.01 (3.17 to 11.38) | 0.09 (0.06 to 0.15) |
| Waldstein et al.84 | FAF 488 nm | SD-OCT | 80.60 (69.11 to 89.24) | 89.66 (78.82 to 96.08) | 7.79 (3.62 to 16.77) | 0.22 (0.13 to 0.36) |
| FAF 514 nm | 55.22 (42.58 to 67.39) | 94.83 (85.60 to 98.86) | 10.68 (3.47 to 32.82) | 0.47 (0.36 to 0.62) |
FIGURE 6.
Diagnostic accuracy of FAF imaging for diabetic macular oedema. CI, confidence interval; FN, false negative; FP, false positive; RM-SLO, retromode scanning laser opthalmoscopy; SD-OCT, spectral domain optical coherence tomography; TN, true negative; TP, true positive.

Vusojevic and colleagues98 did not report intergrader agreement on interpreting FAF images. Waldstein and colleagues84 reported kappa values (but not sample size, p-value or percentage reviewer agreement) for intergrader agreement in interpretation of the FAF images, which were 0.84 for 488 nm FAF imaging and 0.63 for 514 nm FAF imaging. These could be interpreted as ‘almost perfect agreement’ and ‘substantial agreement’, respectively. 104 Neither study reported intraobserver agreement for image grading, test acceptability to patients or clinicians, or adverse events of any tests that might influence the interpretation of diagnostic outcomes.
As noted above (see Quality of research available), the study by Waldstein and colleagues84 was judged to be at high risk of spectrum bias (owing to an unrepresentative and retrospectively selected case mix) while the risks of various types of bias associated with the timing of the index test and reference standard, and interpretation of these tests, were judged to be unclear. The study by Vujosevic and colleagues98 was considered to be at unclear risk of spectrum bias because although the case mix appears potentially reflective of patients likely to receive retinal imaging in clinical practice (diabetic retinopathy patients with comorbidities permitted) it is unclear whether the patients were selected prospectively or retrospectively. This study98 was the only one of the eight included in the systematic review that was deemed to be at low risk of both test review bias and diagnostic review bias. However, as noted above, the reference standard employed by Vusojevic and colleagues98 is unlikely to reflect clinical practice. Taking these considerations into account, it is not possible to draw a rigorous clinically relevant conclusion about the accuracy of FAF imaging for diagnosing diabetic macular oedema, although results from the study by Waldstein and colleagues84 suggest that the longer-wavelength FAF imaging method (514 nm) was less sensitive for diagnosing diabetic macular oedema than the standard wavelength approach (488 nm).
Summary of diagnostic accuracy assessment
The eight studies83,84,96–101 included in the systematic review suggest qualitatively that FAF imaging may have a potential role in supporting the diagnosis of choroidal neovascularisation in AMD, reticular pseudodrusen in AMD, cystoid macular oedema or diabetic macular oedema. However, after taking into account a number of quality issues concerning the study designs, none of the studies were found to provide convincing quantitative information about the diagnostic accuracy of FAF imaging of relevance to current clinical practice. In two cases where long and short excitation wavelengths of FAF imaging could be compared, the longer wavelength appeared less diagnostically sensitive: 719 nm was less sensitive than 488 nm for detecting reticular pseudodrusen97 while 514 nm was less sensitive than 488 nm for detecting diabetic macular oedema. 84 However, these differences are based on only two studies that had methodological limitations and, as such, are illustrative only.
Chapter 5 Discussion
Statement of principal findings
-
There is considerable primary research interest in FAF imaging of retinal conditions (of 206 full-text papers retrieved for inspection, 199 papers reported on primary studies that had employed FAF imaging to investigate retinal conditions). However, most studies have been descriptive, providing reports of how FAF patterns reflect the pathology of retinal conditions (e.g. 105–107), rather than quantitatively assessing diagnostic or monitoring accuracy.
-
Eight primary research studies met the inclusion criteria for the current review. 83,84,96–101 These investigated the accuracy of FAF imaging in diagnosing choroidal neovascularisation (one study99), reticular pseudodrusen in various stages of AMD (three studies96,97,101), cystoid macular oedema (two studies83,100) and diabetic macular oedema (two studies84,98).
-
No studies have assessed the accuracy of FAF imaging for monitoring the progression of retinal conditions or their response to therapy.
-
Most of the studies included in the current review provided very limited information on the diagnostic criteria they used and these were qualitative (descriptive); most studies did not check for intergrader agreement in reading FAF images, despite the potential subjectivity of the descriptive diagnostic criteria; and none of the studies reported the clinical information used by researchers when interpreting FAF images.
-
In most of the included studies that used an excitation wavelength of 488 nm, the sensitivity of FAF imaging was high (range 81–100%), although it was lower in two studies that used longer excitation wavelengths (32% in one study of reticular pseudodrusen in AMD which used an excitation wavelength of 790 nm,97 and 55% in one study of diabetic macular oedema, which used an excitation wavelength of 514 nm84). The specificity of FAF imaging across all studies ranged from 34% to 100% and was not clearly related to the excitation wavelength. However, owing to the relative paucity of reliable data, and limitations in experimental rigour, it is unclear whether FAF imaging would be accurate in diagnosing retinal conditions or in monitoring their progression or response to therapy in clinical practice.
The current review has identified several evidence gaps, including:
-
Robust data on the monitoring accuracy of FAF imaging for assessing the progression of retinal conditions or their response to therapy do not currently exist.
-
Limited data are available on the diagnostic accuracy of FAF imaging but these are for four retinal conditions and suffer from methodological limitations. As such, they are effectively qualitative rather than reliably quantitative.
-
No diagnostic or monitoring accuracy studies of FAF imaging were found for the inherited retinal dystrophies, early AMD, GA or CSC. According to the review advisory group, these are conditions where FAF imaging might be most useful and is already being used (to an unclear extent) in the NHS.
Methodological challenges
A challenge in the current review was to identify studies of the diagnostic accuracy of FAF imaging based on screening titles and abstracts. It was difficult to determine, based on titles and abstracts alone, whether or not studies reported sensitivity and specificity, or data from which these could be calculated. As a result it was necessary to retrieve and check 206 full-text records. Nevertheless, only eight studies finally met the inclusion criteria. 83,84,96–101 A difficulty is that most of these eight studies that provided relevant diagnostic outcomes were not primarily diagnostic studies and as such they were rated relatively poorly against the QUADAS criteria for risks of bias. Ideally, future studies that wish to explore the diagnostic accuracy of FAF imaging should be based on appropriate designs and methods compatible with the paradigm of diagnostic accuracy assessment,108,109 and should include a clear statement in the abstract as to the availability of quantitative diagnostic outcomes. 110
Patient and public involvement
This review did not formally involve patients or the general public. However, the draft protocol and report were provided to the advisory group, which included the RNIB, for comment. The RNIB did not identify any major issues of equitability in the use of FAF imaging from the perspective of patients and the general public. However, it should be noted that the technology is relatively new and the extent to which patients have access to the technology is currently unclear.
Strengths and limitations of the assessment
Strengths
-
The review was based on a prespecified peer-reviewed protocol.
-
A comprehensive literature search was conducted based on a wide range of prespecified evidence sources.
-
All steps of the systematic review process were conducted by at least two reviewers, minimising the risks of errors and bias.
-
The primary evidence was assessed critically using accepted criteria for the critical appraisal of test accuracy studies.
-
All steps of the evidence synthesis are reported transparently; worksheets for study selection and data extraction were pilot tested and are presented with this report; all studies excluded at full-text screening are listed in Appendix 4, stating reasons for exclusion.
-
An independent advisory group informed the review.
Limitations
-
Interpretation of the primary research is hampered by clinical heterogeneity among the studies and limitations in methodological rigour, which in all studies led to judgements of high risk of bias. Owing to the small number of studies available it was not possible to assess the impact of methodological rigour on observed diagnostic outcomes.
-
The primary research studies had an inappropriate (or in one study unclear98) patient spectrum (i.e. a case mix unlikely to be reflective of that presenting for retinal imaging in current NHS practice).
-
Some studies included both eyes of patients in analyses, which (because of intrasubject correlations) may have led to underestimation of standard errors for sensitivity and specificity (and hence also underestimation of the width of the confidence intervals). 95 As there is no formally agreed approach for analysing such data in evidence syntheses,95 the current review does not investigate their impact on the precision of diagnostic outcomes. However, such cases are clearly identified in the review and any inaccuracy of confidence intervals in these cases would not have markedly altered the review conclusions.
-
The systematic review was limited to English-language studies.
Uncertainties
-
The current use of FAF imaging for diagnosing and/or monitoring retinal conditions in clinical practice is unclear.
-
The relevance of FAF imaging for monitoring retinal conditions is uncertain. Numerous studies suggest FAF imaging appears to have potential value as a monitoring tool (e.g. detecting the progression of the GA area in dry AMD, or the presence and distribution of autofluorescence and development of atrophy in different inherited retinal dystrophies), but so far all studies that have assessed FAF imaging as a monitoring tool have lacked a reference standard.
-
The information required to adequately interpret FAF images in primary studies is unclear, as it was not reported in any study whether FAF images were interpreted in isolation or in conjunction with other clinical information. All studies were therefore judged to be at unclear risk of clinical review bias. This is important as diagnostic tests should ideally be interpreted using the same information that would be available in clinical practice.
-
It is uncertain how variability between methods (different models of cSLO with different software and image acquisition protocols) may have affected diagnostic outcomes as this level of detail was not consistently reported in the included studies.
-
It is uncertain whether or not diagnostic criteria used for FAF imaging are reliable (interobserver reliability of diagnostic criteria was assessed in only three out of eight studies and only in limited detail84,96,97). The recently updated classification of early AMD and GA using FAF, by the International Fundus Autofluorescence Classification group,45,46 was used in one of the included studies. 99 However, the classification consists of descriptions of patterns seen on retinal imaging rather than quantifiable characteristics, and, as such, is open to subjective interpretation. There appears to be a lack of internationally identified and agreed classifications for other conditions, although these may be needed to allow meaningful interpretation in future diagnostic accuracy studies of FAF imaging.
-
Although it was not an objective of the current review to assess costs or cost-effectiveness of FAF imaging, it was noted during the inspection of full-text articles that none of the studies that have assessed the diagnostic accuracy of FAF imaging provided any information on costs or resources, such as the time taken to acquire images or any resources used for image processing and quality control.
Chapter 6 Conclusions
It is not possible to give a clear indication of the diagnostic or monitoring accuracy of FAF imaging for retinal conditions based on existing research. Although some studies reported relatively high sensitivity, these had various methodological limitations, which hinder the interpretation of test accuracy. There is an indication that standard wavelength FAF imaging (488 nm) may be more sensitive than longer-wavelength approaches but this is based on only two studies, involving 790-nm imaging for detecting reticular pseudodrusen97 and 514-nm imaging for detecting diabetic macular oedema. 84 Owing to the relative paucity of reliable data, further studies are required. In particular, prospective studies would be helpful in inherited retinal dystrophies, early AMD, GA and CSC, and the studies should be designed according to the paradigm for the quantitative assessment of test accuracy.
Implications for service provision
Owing to a lack of studies addressing the appropriate populations and employing appropriate imaging methods there remains uncertainty whether or not FAF imaging is accurate for the diagnosis and monitoring of retinal conditions in clinical practice. As noted in the specific recommendations below, research is needed to address these knowledge gaps.
The current extent of use of FAF imaging for different retinal conditions in the NHS is unclear. An audit or survey of current practice would be helpful to clarify how the method is used and whether or not its usage is appropriate, so as to inform future guidance for NHS practice. The review advisory group suggested that although the role of FAF imaging in clinical practice is still developing it may already be used by specialists in the NHS to assist with diagnosis and monitoring of inherited retinal dystrophies such as Stargardt disease, and may also have a role in the diagnosis and/or monitoring of early AMD and GA.
There seems to be lack of consensus on whether or not FAF imaging may have a role in diagnosing wet AMD or macular oedemas. Clinical experts advising the current review suggested that the oedema and fluid leakage in these conditions would obscure the FAF signal, meaning that both fluorescein angiography and optical coherence tomography would be more appropriate imaging modalities for making a diagnosis (since blood perfusion and leakage, and macular thickness are key diagnostic aspects of these conditions). However, some researchers have suggested that FAF imaging could have a role for diagnosing cystoid macular oedema107 and retinal pigment epithelium loss in wet AMD,67 while the studies on choroidal neovascularisation,99 cystoid macular oedema83,100 and diabetic macular oedema84,98 that were included in the current review highlight that there is research interest in the use of FAF imaging in detecting and diagnosing, or monitoring these conditions.
Given that FAF imaging is non-invasive, there might be benefits to both patients and the NHS if FAF imaging could, in some cases, replace fluorescein angiography, which is the most frequently used invasive retinal imaging test. Fluorescein angiography carries a risk of complications (adverse reaction to dye), side effects (ranging from mild nausea or hives to anaphylaxis and death) and may impact on patients’ quality of life (e.g. fear of needles), as well as requiring specialist clinical facilities (including a sterile clinical environment and staff qualified in venous access). Where complications occur or patients experience diminished quality of life this may require additional therapy, which would impact on health resources. None of the studies included in the current review assessed patients’ perceptions of the test procedures or reported whether or not the angiography reference standard was associated with any adverse events. Further evidence would therefore be helpful to clarify the magnitude of benefits or disadvantages to patients and the NHS of any switch from fluorescein angiography to FAF imaging.
Any future research into the accuracy of FAF imaging (see Suggested research priorities) will need to consider whether FAF imaging is intended to supplement or replace existing imaging modalities. 111 According to clinical experts (see Chapter 1, Current service provision), FAF could potentially replace fluorescein angiography for diagnosing inherited retinal dystrophies, such as retinitis pigmentosa and Stargardt disease. FAF imaging has also been proposed as a replacement for fluorescein angiography in certain monitoring applications, for example, in monitoring the progression of Best’s disease,57 verifying the results of laser therapy in retinal oedema,112 or monitoring outcomes of macular hole surgery,113 but evidence of whether FAF imaging can completely replace angiography in these roles is currently lacking. In the majority of retinal conditions FAF imaging is likely to be applied as an additional test, and would probably accompany optical coherence tomography and other tests, such as angiography or fundus photography, as needed.
Review of the primary evidence suggests that guidance on how to standardise FAF imaging is limited and this would need to be considered before formal recommendation of the use of FAF imaging could be made. It is unclear which guidance, if any, is being currently followed in NHS practice for classifying and interpreting FAF images. Potentially relevant methods that might serve as a starting point for further development and evaluation include those of the International Fundus Autofluorescence Classification group,45,46 although the classification of FAF patterns is currently descriptive. Development of quantitative diagnostic criteria based on FAF patterns may help to reduce subjectivity of interpretation. 114
Suggested research priorities
-
Prospective studies that conform to the paradigm for test accuracy assessments (i.e. those that include a clearly specified population, index test, reference standard and diagnostic outcomes) would be helpful to evaluate the diagnostic accuracy of FAF imaging in the inherited retinal dystrophies, early AMD, GA and CSC.
-
Prospective studies that conform to the paradigm for test accuracy assessments would be helpful to evaluate the accuracy of FAF imaging in monitoring the progression of retinal conditions and their response to therapy, alongside current best practice, in the inherited retinal dystrophies, early AMD, GA and CSC.
-
Future test accuracy studies for FAF imaging should:
-
recruit participants who are representative of those likely to present for retinal screening in the NHS
-
employ all relevant components of currently used reference standards
-
clearly report the clinical information required to interpret FAF images in order to reach diagnostic and/or therapeutic decisions
-
consider carefully whether FAF imaging is appropriate as an ancillary test or as a replacement for an existing test
-
assess intergrader and intragrader agreement
-
assess other aspects of test acceptability (e.g. patient acceptability, adverse events).
-
-
Future test accuracy studies for FAF imaging should also report clearly the duration of imaging and any resources associated with the acquisition, processing, quality assurance and interpretation of FAF images. This would assist any evaluations of the cost-effectiveness of FAF imaging that are conducted for the NHS.
-
A survey or audit of the current use of FAF imaging in NHS practice would be helpful to clarify current practice and any limitations and research requirements associated with it. In particular, a survey or audit of the extent to which FAF imaging is currently used for different retinal conditions and which standards are currently employed for ensuring consistency of test results would assist prioritisation of areas where NHS resources may be needed to support the future development of FAF imaging.
-
Research would be helpful to clarify which guidance, if any, is currently being followed in NHS practice for classifying and interpreting FAF images.
Acknowledgements
We thank the following experts who kindly provided comments on the protocol and/or the draft report: Professor Susan Downes, Consultant Ophthalmic Surgeon, Oxford Eye Hospital and John Radcliffe Hospital; Professor Andrew Lotery, Consultant Ophthalmologist, Faculty of Medicine, University of Southampton and Southampton University Hospitals NHS Foundation Trust; and Steve Winyard, Royal National Institute for Blind People. We also thank: Karen Welch, Information Scientist, Southampton Health Technology Assessments Centre, for generating and running the literature searches; and Dr Jo Picot, Southampton Health Technology Assessments Centre, for acting as an internal editor for the report.
Contributions of authors
Dr Geoff K Frampton (Senior Research Fellow, evidence synthesis) developed the research protocol, assessed studies for inclusion, extracted data from and critically appraised the included studies, synthesised the evidence, drafted and edited the final report and project managed the review.
Neelam Kalita (Research Fellow, health economics) developed the research protocol, assessed studies for inclusion, extracted data from and critically appraised the included studies, synthesised the evidence and drafted the report.
Dr Liz Payne (Research Fellow, evidence synthesis) developed the research protocol, assessed studies for inclusion, extracted data from and critically appraised the included studies and drafted the report.
Dr Jill Colquitt (Senior Research Fellow, evidence synthesis) assessed studies for inclusion, extracted data from and critically appraised the included studies and commented on the draft report.
Dr Emma Loveman (Senior Research Fellow, evidence synthesis) developed the research protocol, assessed studies for inclusion, extracted data from and critically appraised the included studies, commented on the draft report and acted as the project guarantor.
Data sharing statement
All data relevant to this technology assessment report are provided in the accompanying appendices.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Lois N, Forrester V. Fundus Autofluorescence. Philadelphia, PA: Lippincott Williams and Wilkins; 2009.
- Moore T, Burton H. Genetic Ophthalmology in Focus: A Needs Assessment and Review of Specialist Services for Genetic Eye Disorders: Report for the United Kingdom Genetic Testing Network. Cambridge: PHG Foundation; 2008.
- Slade J. Eye Health Data Summary: A Review of Published Data in England. London: RNIB; 2014.
- Velez-Montoya R, Oliver SC, Olson JL, Fine SL, Quiroz-Mercado H, Mandava N. Current knowledge and trends in age-related macular degeneration: genetics, epidemiology, and prevention. Retina 2013;33:1487-502. http://dx.doi.org/10.1097/IAE.0b013e318271f265.
- Saksens NT, Kersten E, Groenewoud JM, van Grinsven MJ, van de Ven JP, Sanchez CI, et al. Clinical characteristics of familial and sporadic age-related macular degeneration: differences and similarities. Invest Ophthalmol Vis Sci 2014;55:7085-92. http://dx.doi.org/10.1167/iovs.14-14659.
- Alfaro V, Liggett PE, Mieler WF. Age-Related Macular Degeneration: A Comprehensive Textbook. Philadelphia, PA: Lippincott Williams and Wilkins; 2006.
- Age-related Macular Degeneration: Guidelines for Management. London: Royal College of Ophthalmologists; 2013.
- Chopdar A, Chakravarthy U, Verma D. Age related macular degeneration. BMJ 2003;326:485-8. http://dx.doi.org/10.1136/bmj.326.7387.485.
- Parodi MB, Virgili G, Evans JR. Laser treatment of drusen to prevent progression to advanced age-related macular degeneration. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.cd006537.pub2.
- Bressler NM, Munoz B, Maguire MG, Vitale SE, Schein OD, Taylor HR. Five-year incidence and disappearance of drusen and retinal pigment epithelial abnormalities: Waterman study. Arch Ophthalmol 1995;113:301-8. http://dx.doi.org/10.1001/archopht.1995.01100030055022.
- Alten F, Eter N. Current knowledge on reticular pseudodrusen in age-related macular degeneration. Br J Ophthalmol 2015;99:712-22.
- Hogg RE. Reticular pseudodrusen in age-related macular degeneration. Optom Vis Sci 2014;91:854-9. http://dx.doi.org/10.1097/OPX.0000000000000287.
- Smith W, Assink J, Klein R, Mitchell P, Klaver C, Klein BE, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 2001;108:697-704. http://dx.doi.org/10.1016/S0161-6420(00)00580-7.
- Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K. Clinical classification of age-related macular degeneration. Ophthalmology 2013;120:844-51. http://dx.doi.org/10.1016/j.ophtha.2012.10.036.
- Vaz F. Geographic Atrophy 2014. www.amdbook.org/content/geographic-atrophy-0 (accessed 7 March 2016).
- Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina 2008;28:385-409. http://dx.doi.org/10.1097/IAE.0b013e318164a907.
- Owen CG, Jarrar Z, Wormald R. The estimated prevalence and incidence of late stage age-related macular degeneration in the UK. Br J Ophthalmol 2012;96:752-6. http://dx.doi.org/10.1136/bjophthalmol-2011-301109.
- Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C. Experiences of patients undergoing anti-VEGF treatment for neovascular age-related macular degeneration: a systematic review. Psychol Health Med 2015;20:296-310. http://dx.doi.org/10.1080/13548506.2014.936886.
- Klein R, Klein BE, Moss SE. The Wisconsin Epidemiologic Study of diabetic retinopathy. XIV. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol 1994;112:1217-28. http://dx.doi.org/10.1001/archopht.1994.01090210105023.
- Diabetic Retinopathy Guidelines. London: Royal College of Ophthalmologists; 2012.
- Frank RN. Diabetic retinopathy. N Engl J Med 2004;350:48-5. http://dx.doi.org/10.1056/NEJMra021678.
- Fenwick EK, Pseudovs K, Khadka J, Dirani M, Rees G, Wong TY, et al. The impact of diabetic retinopathy on quality of life: qualitative findings from an item bank development project. Qual Life Res 2012;21:1771-82. http://dx.doi.org/10.1007/s11136-012-0110-1.
- Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev 2014;10. http://dx.doi.org/10.1002/14651858.cd007419.pub4.
- Minassian DC, Owens DR, Reidy A. Prevalence of diabetic macular oedema and related health and social care resource use in England. Br J Ophthalmol 2012;96:345-9. http://dx.doi.org/10.1136/bjo.2011.204040.
- Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Optom 2013;41:201-14. http://dx.doi.org/10.1111/j.1442-9071.2012.02848.x.
- Liegl R, Ulbig MW. Central serous chorioretinopathy. Ophthalmologica 2014;232:65-76. http://dx.doi.org/10.1159/000360014.
- Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye 2010;24:1743-56. http://dx.doi.org/10.1038/eye.2010.130.
- Koyama M, Mizota A, Igarashi Y, Adachi-Usami E. Seventeen cases of central serous chorioretinopathy associated with systemic corticosteroid therapy. Ophthalmologica 2004;218:107-10. http://dx.doi.org/10.1159/000076145.
- Hamel C. Cone rod dystrophies. Orphan J Rare Dis 2007;2. http://dx.doi.org/10.1186/1750-1172-2-7.
- Daiger SP, Bowne SJ, Sullivan LS. Genes and mutations causing autosomal dominant retinitis pigmentosa. Cold Spring Harbor Perspect Med 2015;5. http://perspectivesinmedicine.cshlp.org/content/early/2014/10/10/cshperspect.a017129.abstract (accessed 7 March 2016).
- Flynn MF, Fishman GA, Anderson RJ, Roberts DK. Retrospective longitudinal study of visual acuity change in patients with retinitis pigmentosa. Retina 2001;21:639-46. http://dx.doi.org/10.1097/00006982-200112000-00012.
- Hamblion EL, Moore AT, Rahi JS. British Childhood Onset Hereditary Retinal Disorders Network . Incidence and patterns of detection and management of childhood-onset hereditary retinal disorders in the UK. Br J Ophthalmol 2012;96:360-5. http://dx.doi.org/10.1136/bjo.2010.201178.
- Sivaprasad S, Bunce C, Crosby NR. Non-steroidal anti-inflammatory agents for treating cystoid macular oedema following cataract surgery. Cochrane Database Syst Rev 2012;2. http://dx.doi.org/10.1002/14651858.cd004239.pub3.
- Rotsos T, Moschos M. Cystoid macular edema. Clin Ophthalmol 2008;2:919-30. http://dx.doi.org/10.2147/OPTH.S4033.
- Salvatore S, Fishman G, Genead M. Treatment of cystic macular lesions in hereditary retinal dystrophies. Surv Ophthalmol 2013;58:560-84. http://dx.doi.org/10.1016/j.survophthal.2012.11.006.
- Cho H, Madu A. Etiology and treatment of the inflammatory causes of cystoid macular edema. J Inflamm Res 2009;2:37-43. http://dx.doi.org/10.2147/JIR.S5706.
- Agange N, Mosaed S. Prostaglandin-induced cystoid macular edema following routine cataract extraction. J Ophthalmol 2010;2010. http://dx.doi.org/10.1155/2010/690707.
- Vukicevic M, Gin T, Al-Queshi S. Prevalence of optical coherence tomography-diagnosed postoperative cystoid macular oedema in patients following uncomplicated phaco-emulsification cataract surgery. Clin Experiment Ophthalmol 2012;40:282-7. http://dx.doi.org/10.1111/j.1442-9071.2011.02638.x.
- Hajali M, Fishman GA. The prevalence of cystoid macular oedema on optical coherence tomography in retinitis pigmentosa patients without cystic changes on fundus examination. Eye 2009;23:915-19. http://dx.doi.org/10.1038/eye.2008.110.
- Adackapara CA, Sunness JS, Dibernado CW, Melia BM, Dagnelie G. Prevalence of cystoid macular oedema and stability in OCT retinal thickness in eyes with retinitis pigmentosa during a 48-week lutein trial. Retina 2008;28:103-10. http://dx.doi.org/10.1097/IAE.0b013e31809862aa.
- Interim Guidelines for Management of Retinal Vein Occlusion. London: Royal College of Ophthalmologists; 2010.
- Owsley C, McGwin G. Driving and age-related macular degeneration. J Vis Impair Blind 2008;102:621-35.
- Senra H, Barbosa F, Ferreira P, Vieira CR, Perrin PB, Rogers H, et al. Psychologic adjustment to irreversible vision loss in adults: a systematic review. Ophthalmology 2015;122:851-61. http://dx.doi.org/10.1016/j.ophtha.2014.10.022.
- Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol 2010;10. http://dx.doi.org/10.1186/1471-2415-10-31.
- Bindewald A, Bird AC, Dandekar SS, Dolar-Szczasny J, Dreyhaupt J, Fitzke FW, et al. Classification of fundus autofluorescence patterns in early age-related macular disease. Invest Ophthalmol Vis Sci 2005;46:3309-14. http://dx.doi.org/10.1167/iovs.04-0430.
- Bindewald A, Schmitz-Valckenberg S, Jorzik JJ, Dolar-Szczasny J, Sieber H, Keilhauer C, et al. Classification of abnormal fundus autofluorescence patterns in the junctional zone of geographic atrophy in patients with age related macular degeneration. Br J Ophthalmol 2005;89:874-8. http://dx.doi.org/10.1136/bjo.2004.057794.
- Sohrab M, Smith RT, Salehi-Had H, Sadda SR, Fawzi AA. Image registration and multimodal imaging of reticular pseudodrusen. Invest Ophthalmol Vis Sci 2011;52:5743-8. http://dx.doi.org/10.1167/iovs.10-6942.
- Walia S, Fishman GA. Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet 2009;30:63-8. http://dx.doi.org/10.1080/13816810802695550.
- Michaelides M, Hardcastle AJ, Hunt DM, Moore AT. Progressive cone and cone-rod dystrophies: phenotypes and underlying molecular genetic basis. Surv Ophthalmol 2006;51:232-58. http://dx.doi.org/10.1016/j.survophthal.2006.02.007.
- Sepah YJ, Akhtar A, Sadiq MA, Hafeez Y, Nasir H, Perez B, et al. Fundus autofluorescence imaging: fundamentals and clinical relevance. Saudi J Ophthalmol 2014;28:111-6. http://dx.doi.org/10.1016/j.sjopt.2014.03.008.
- Delori FC. Spectrophotometer for non-invasive measurement of intrinsic fluorescence and reflectance of the ocular fundus. Appl Optics 1994;33:7429-52. http://dx.doi.org/10.1364/AO.33.007439.
- Webb RH, Hughes GW, Delori FC. Confocal scanning laser ophthalmoscope. Appl Optics 1987;26:1492-9. http://dx.doi.org/10.1364/AO.26.001492.
- Holz FG, Steinberg JS, Gobel A, Fleckenstein M, Schmitz-Valckenberg S. Fundus autofluorescence imaging in dry AMD: 2014 Jules Gonin lecture of the Retina Research Foundation. Graefes Arch Clin Exp Ophthalmol 2015;253:7-16. http://dx.doi.org/10.1007/s00417-014-2858-1.
- Bearelly S, Cousins SW. Fundus autofluorescence imaging in age-related macular degeneration and geographic atrophy. Adv Exp Med Biol 2010;664:395-402. http://dx.doi.org/10.1007/978-1-4419-1399-9_45.
- Dandekar SS, Jenkins SA, Peto T, Scholl HP, Sehmi KS, Fitzke FW, et al. Autofluorescence imaging of choroidal neovascularization due to age-related macular degeneration. Arch Ophthalmol 2005;123:1507-13. http://dx.doi.org/10.1001/archopht.123.11.1507.
- Kellner U, Kellner S, Weinitz S. Fundus autofluorescence (488 nm) and near-infrared autofluorescence (787 nm) visualize different retinal pigment epithelium alterations in patients with age-related macular degeneration. Retina 2010;30:6-15. http://dx.doi.org/10.1097/IAE.0b013e3181b8348b.
- Jarc-Vidmar M, Kraut A, Hawlina M. Fundus autofluorescence imaging in Best’s vitelliform dystrophy. Klin Monatsbl Augenheilkd 2003;220:861-7. http://dx.doi.org/10.1055/s-2003-812555.
- Kellner S, Weinitz S, Farmand G, Kellner U. Cystoid macular oedema and epiretinal membrane formation during progression of chloroquine retinopathy after drug cessation. Br J Ophthalmol 2014;98:200-6. http://dx.doi.org/10.1136/bjophthalmol-2013-303897.
- Framme C, Walter A, Gabler B, Roider J, Sachs HG, Gabel VP. Fundus autofluorescence in acute and chronic-recurrent central serous chorioretinopathy. Acta Ophthalmol Scand 2005;83:161-7. http://dx.doi.org/10.1111/j.1600-0420.2005.00442.x.
- Simader C, Sayegh RG, Montuoro A, Azhary M, Koth AL, Baratsits M, et al. A longitudinal comparison of spectral-domain optical coherence tomography and fundus autofluorescence in geographic atrophy. Am J Ophthalmol 2014;158:557-66. http://dx.doi.org/10.1016/j.ajo.2014.05.026.
- Lee JY, Lee DH, Lee JY, Yoon YH. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:7812-18. http://dx.doi.org/10.1167/iovs.13-12284.
- Schmitz-Valckenberg S, Bindewald-Wittich A, Dolar-Szczasny J, Dreyhaupt J, Wolf S, Scholl HP, et al. Correlation between the area of increased autofluorescence surrounding geographic atrophy and disease progression in patients with AMD. Invest Ophthalmol Vis Sci 2006;47:2648-54. http://dx.doi.org/10.1167/iovs.05-0892.
- Jeong YJ, Hong IH, Chung JK, Kim KL, Kim HK, Park SP. Predictors for the progression of geographic atrophy in patients with age-related macular degeneration: fundus autofluorescence study with modified fundus camera. Eye 2014;28:209-18. http://dx.doi.org/10.1038/eye.2013.275.
- Pilotto E, Guidolin F, Convento E, Spedicato L, Vujosevic S, Cavarzeran F, et al. Fundus autofluorescence and microperimetry in progressing geographic atrophy secondary to age-related macular degeneration. Br J Ophthalmol 2013;97:62-6. http://dx.doi.org/10.1136/bjophthalmol-2012-302633.
- Petrou PA, Cunningham D, Shimel K, Harrington M, Hammel K, Cukras CA, et al. Intravitreal sirolimus for the treatment of geographic atrophy: results of a phase I/II clinical trial. Invest Ophthalmol Vis Sci 2014;56:330-8. http://dx.doi.org/10.1167/iovs.14-15877.
- Hoffmann-La Roche . A Study of Lampalizumab Intravitreal Injections Administered Every Two Weeks or Every Four Weeks to Patients With Geographic Atrophy. Clinical Trials.Gov NCT02288559 n.d. https://clinicaltrials.gov/ct2/show/NCT02288559?term=faf&rank=9 (accessed 7 March 2016).
- Kumar N, Mrejen S, Fung AT, Marsiglia M, Loh BK, Spaide RF. Retinal pigment epithelial cell loss assessed by fundus autofluorescence imaging in neovascular age-related macular degeneration. Ophthalmology 2013;120:334-41. http://dx.doi.org/10.1016/j.ophtha.2012.07.076.
- Asao K, Gomi F, Sawa M, Nishida K. Additional anti-vascular endothelial growth factor therapy for eyes with a retinal pigment epithelial tear after the initial therapy. Retina 2014;34:512-18. http://dx.doi.org/10.1097/IAE.0b013e31829f73eb.
- Clemens CR, Alten F, Baumgart C, Heiduschka P, Eter N. Quantification of retinal pigment epithelium tear area in age-related macular degeneration. Retina 2014;34:24-31. http://dx.doi.org/10.1097/IAE.0b013e3182947811.
- Robson AG, Lenassi E, Saihan Z, Luong VA, Fitzke FW, Holder GE, et al. Comparison of fundus autofluorescence with photopic and scotopic fine matrix mapping in patients with retinitis pigmentosa: 4- to 8-year follow-up. Invest Ophthalmol Vis Sci 2012;53:6187-95. http://dx.doi.org/10.1167/iovs.12-10195.
- Aizawa S, Mitamura Y, Hagiwara A, Sugawara T, Yamamoto S. Changes of fundus autofluorescence, photoreceptor inner and outer segment junction line, and visual function in patients with retinitis pigmentosa. Clin Exp Optom 2010;38:597-604. http://dx.doi.org/10.1111/j.1442-9071.2010.02321.x.
- Scholl H. A Natural History of the Progression of Stargardt Disease: Retrospective and Prospective Studies (ProgSTAR) n.d. https://clinicaltrials.gov/ct2/show/NCT01977846?term=faf&rank=2 (accessed 7 March 2016).
- Meyerle C. Extension Study for the Evaluation of Finasteride in the Treatment of Chronic Central Serous Chorioretinopathy (CSC-Ext) n.d. https://clinicaltrials.gov/ct2/show/NCT01227993?term=faf&rank=5 (accessed 7 March 2016).
- Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 2010;30:908-16. http://dx.doi.org/10.1097/IAE.0b013e3181c96986.
- Mesquida M, Llorenc V, Fontenla JR, Navarro MJ, Adan A. Use of ultra-wide-field retinal imaging in the management of active Behcet retinal vasculitis. Retina 2014;34:2121-7. http://dx.doi.org/10.1097/IAE.0000000000000197.
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. SEVEN-UP Study Group . Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 2013;120:2292-9. http://dx.doi.org/10.1016/j.ophtha.2013.03.046.
- Mehta H, Davidson A, Devary S, Egan C, Hykin P, Moore A, et al. Autofluorescence and spectral-domain OCT findings in an atrophic maculopathy associated with pseudoxanthoma elasticum. Clin Experiment Ophthalmol 2012;40:49-50.
- Fung A, Kumar N, Mrejen S, Marsiglia M, Loh B, Spaide R. Retinal pigment epithelial cell loss assessed by fundus autofluorescence imaging in patients with neovascular age-related macular degeneration. Clin Experiment Ophthalmol 2012;40.
- Ozmert E, Batioglu F. Fundus autofluorescence before and after photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmologica 2009;223:263-8. http://dx.doi.org/10.1159/000210386.
- Brito PN, Gomes NL, Vieira MP, Faria PA, Fernandes AV, Rocha-Sousa A, et al. Possible role for fundus autofluorescence as a predictive factor for visual acuity recovery after epiretinal membrane surgery. Retina 2014;34:273-80. http://dx.doi.org/10.1097/IAE.0b013e3182999a02.
- Reitsma JB, Rutjes AWS, Whiting P, Vlassov VV, Leeflang MMG, Deeks JJ, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 1.0.0. London: The Cochrane Collaboration; 2009.
- Ilginis T, Clarke J, Patel PJ. Ophthalmic imaging. Br Med Bull 2014;111:77-88. http://dx.doi.org/10.1093/bmb/ldu022.
- Dinc UA, Tatlipinar S, Yenerel M, Gorgun E, Ciftci F. Fundus autofluorescence in cystoid macular edema: can it be an ancillary test?. Retina 2010;18:12-7.
- Waldstein SM, Hickey D, Mahmud I, Kiire CA, Charbel IP, Chong NV. Two-wavelength fundus autofluorescence and macular pigment optical density imaging in diabetic macular oedema. Eye 2012;26:1078-85. http://dx.doi.org/10.1038/eye.2012.100.
- Laviers H, Zambarakji H. Enhanced depth imaging-OCT of the choroid: a review of the current literature. Graefes Arch Clin Exp Ophthalmol 2014;252:1871-3. http://dx.doi.org/10.1007/s00417-014-2840-y.
- Testa F, Melillo P, Di Iorio V, Orrico A, Attanasio M, Rossi S, et al. Macular function and morphologic features in juvenile Stargardt disease: longitudinal study. Ophthalmology 2014;121:2399-405. http://dx.doi.org/10.1016/j.ophtha.2014.06.032.
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y, Deeks JJ, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 1.0. London: The Cochrane Collaboration; 2010.
- Systematic reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: York Publishing Services Ltd, CRD; 2009.
- Bossuyt P, Davenport C, Deeks J, Hyde C, Leeflang M, Scholten R, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 0.9. London: The Cochrane Collaboration; 2013.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2535.
- Southampton Health Technology Assessments Centre (SHTAC) . The Use of Fundus Autofluorescence Imaging for Retinal Conditions (systematic Review Protocol) 2014. www.nets.nihr.ac.uk/__data/assets/pdf_file/0019/134713/PRO-14-151-02.pdf (accessed 7 March 2016).
- Flahault A, Cadilhac M, Thomas G. Sample size calculation should be performed for design accuracy in diagnostic test studies. J Clinical Epidemiol 2005;58:859-62. http://dx.doi.org/10.1016/j.jclinepi.2004.12.009.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3. http://dx.doi.org/10.1186/1471-2288-3-25.
- Viswanathan M, Berkman ND, Dryden DM, Hartling L. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item Bank. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013.
- Virgili G, Menchini F, Murro V, Peluso E, Rosa F, Casazza G. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev 2011;7. http://dx.doi.org/10.1002/14651858.cd008081.pub2.
- Hogg RE, Silva R, Staurenghi G, Murphy G, Santos AR, Rosina C, et al. Clinical characteristics of reticular pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degeneration. Ophthalmology 2014;121:1748-55. http://dx.doi.org/10.1016/j.ophtha.2014.03.015.
- Ueda-Arakawa N, Ooto S, Tsujikawa A, Yamashiro K, Oishi A, Yoshimura N. Sensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patients. Retina 2013;33:490-7. http://dx.doi.org/10.1097/IAE.0b013e318276e0ae.
- Vujosevic S, Trento B, Bottega E, Urban F, Pilotto E, Midena E. Scanning laser ophthalmoscopy in the retromode in diabetic macular oedema. Acta Opthalmol 2012;90:e374-80. http://dx.doi.org/10.1111/j.1755-3768.2012.02410.x.
- Cachulo L, Silva R, Fonseca P, Pires I, Carvajal-Gonzalez S, Bernardes R, et al. Early markers of choroidal neovascularization in the fellow eye of patients with unilateral exudative age-related macular degeneration. Ophthalmologica 2011;225:144-9. http://dx.doi.org/10.1159/000321064.
- McBain VA, Forrester JV, Lois N. Fundus autofluorescence in the diagnosis of cystoid macular oedema. Br J Ophthalmol 2008;92:946-9. http://dx.doi.org/10.1136/bjo.2007.129957.
- Smith RT, Chan JK, Busuoic M, Sivagnanavel V, Bird AC, Chong NV. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest Ophthalmol Vis Sci 2006;47:5495-504. http://dx.doi.org/10.1167/iovs.05-1318.
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration; 2011.
- Finger RP, Wu Z, Luu CD, Kearney F, Ayton LN, Lucci LM, et al. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularisation. Ophthalmology 2014;121:1252-6. http://dx.doi.org/10.1016/j.ophtha.2013.12.034.
- Viera AJ, Garrett JD. Understanding interobserver agreement: the kappa statistic. Fam Med 2005;37:360-3.
- Batioglu F, Demirel S, Ozmert E, Oguz YG, Ozyol P. Autofluorescence patterns as a predictive factor for neovascularization. Optom Vis Sci 2014;91:950-5. http://dx.doi.org/10.1097/OPX.0000000000000321.
- Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S, et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol 2007;143:463-72. http://dx.doi.org/10.1016/j.ajo.2006.11.041.
- Peng X-J, Su L-P. Characteristics of fundus autofluorescence in cystoid macular edema. Chin Med J 2011;124:253-7.
- Bossuyt PM, Irwig LM, Craig J, Glasziou P. Comparative accuracy: assessing new tests against existing diagnostic pathways. BMJ 2006;332:1089-92. http://dx.doi.org/10.1136/bmj.332.7549.1089.
- Knotterus JA, van Weel C, Muris JW. Evaluation of diagnostic procedures. BMJ 2002;324:477-80. http://dx.doi.org/10.1136/bmj.324.7335.477.
- Bossuyt P, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM. The STARD Statement for Reporting Studies of Diagnostic Accuracy: explanation and elaboration. Ann Intern Med 2003;138:W1-12. http://dx.doi.org/10.7326/0003-4819-138-1-200301070-00010.
- Ahn SJ, Ahn J, Park KH, Woo SJ. Multimodal imaging of occult macular dystrophy. JAMA Ophthalmol 2013;131:880-90. http://dx.doi.org/10.1001/jamaophthalmol.2013.172.
- Framme C, Brinkmann R, Birngruber R, Roider J. Autofluorescence imaging after selective RPE laser treatment in macular diseases and clinical outcome: a pilot study. Br J Ophthalmol 2002;86:1099-106. http://dx.doi.org/10.1136/bjo.86.10.1099.
- Framme C, Roider J. Fundus autofluorescence in macular hole surgery. Ophthalmic Surg Lasers 2001;32:383-90.
- Schachar IH, Zahid S, Comer GM, Stem M, Schachar AG, Saxe SJ, et al. Quantification of fundus autofluorescence to detect disease severity in nonexudative age-related macular degeneration. JAMA Ophthalmol 2013;131:1009-15. http://dx.doi.org/10.1001/jamaophthalmol.2013.4014.
Appendix 1 Search strategy
| Database, host, years searched, date of search | Literature search strategy | Results |
|---|---|---|
| MEDLINE(R), via Ovid, 1990 to November week 1 2014, searched 13 November 2014 | 1 (autofluorescen* and (fundus or fundi or fundal)).tw. (892) 2 FAF.tw. (569) 3 1 or 2 (1180) 4 topcon.tw. (407) 5 ‘confocal scanning laser ophthalmoscop*’.tw. (572) 6 CSLO.tw. (163) 7 optos.tw. (52) 8 spectralis.tw. (337) 9 imagenet*.tw. (79) 10 bluepeak.tw. (2) 11 ‘heidelberg retina angiograph’.tw. (92) 12 ‘heidelberg engineering’.tw. (365) 13 ‘AO SLO’.tw. (22) 14 ‘spectral domain OCT’.tw. (447) 15 optovue.tw. (77) 16 ‘carl zeiss meditec’.tw. (653) 17 or/4-16 (2552) 18 (fluorescen* or autofluorescen*).tw. (316,708) 19 Fluorescence/ (32,554) 20 or/18-19 (325,649) 21 17 and 20 (287) 22 3 or 21 (1287) 23 (image* or imaging).tw. (668,379) 24 (camera* or photograph* or laser* or infrared or ophthalmoscop* or instrument*).tw. (418,062) 25 Tomography, Optical Coherence/ (15,722) 26 Fluorescein Angiography/ (18,783) 27 Optical Imaging/ (1622) 28 Electroretinography/ (14,273) 29 Microscopy, Confocal/ (44,481) 30 (diagnos* or electrodiagnos*).tw. (1,620,715) 31 Diagnosis, Computer-Assisted/ (20,766) 32 Lasers/du, is [Diagnostic Use, Instrumentation] (4259) 33 Image Processing, Computer-Assisted/ (100,139) 34 (automat* adj5 (detect* or captur* or quantif*)).tw. (10,352) 35 or/23-34 (2,559,128) 36 22 and 35 (992) 37 exp Retinal Diseases/ (102,626) 38 (retina* or retinitis or retinopath* or epiretina* or subretina* or preretina* or posterioretina* or intraretina* or chorioretinopath* or vitreoretinopath*).tw. (167,490) 39 (macula* or maculopath* or ‘wet AMD’ or ‘dry AMD’ or ‘exud* AMD’).tw. (38,891) 40 ((fundus or fundi or fundal) adj5 (change* or impair* or disease* or disorder* or detect* or diagnos*)).tw. (2365) 41 (geographical adj atroph*).tw. (11) 42 hyperfluorescen*.tw. (822) 43 (RVO or CRVO or BRVO).tw. (1256) 44 (cone*1 adj2 dystroph*).tw. (850) 45 or/37-44 (212,953) 46 36 and 45 (929) 47 (comment or editorial or letter).pt. (1,326,748) 48 46 not 47 (922) 49 limit 48 to english language (855) |
855 |
| MEDLINE(R) In-Process & Other Non-Indexed Citations, via Ovid, searched from 1990 to 12 November 2014, searched on 13 November 2014 | As per MEDLINE | 112 |
| EMBASE, via Ovid, searched from 1990 to 12 November 2014, searched 13 November 2014 | 1 (autofluorescen* and (fundus or fundi or fundal)).tw. (1043) 2 FAF.tw. (673) 3 1 or 2 (1384) 4 topcon.tw. (486) 5 ‘confocal scanning laser ophthalmoscop*’.tw. (582) 6 CSLO.tw. (169) 7 optos.tw. (56) 8 spectralis.tw. (479) 9 imagenet*.tw. (84) 10 bluepeak.tw. (1) 11 ‘heidelberg retina angiograph’.tw. (104) 12 ‘heidelberg engineering’.tw. (424) 13 ‘AO SLO’.tw. (29) 14 ‘spectral domain OCT’.tw. (603) 15 optovue.tw. (93) 16 ‘carl zeiss meditec’.tw. (695) 17 or/4-16 (3004) 18 (fluorescen* or autofluorescen*).tw. (379,356) 19 Fluorescence/ (89,241) 20 or/18-19 (393,399) 21 17 and 20 (344) 22 3 or 21 (1517) 23 (image* or imaging).tw. (906,976) 24 (camera* or photograph* or laser* or infrared or ophthalmoscop* or instrument*).tw. (556,941) 25 optical coherence tomography/ (20,952) 26 Fluorescein Angiography/ (14,527) 27 fluorescence imaging/ (6870) 28 Electroretinography/ (11,726) 29 confocal microscopy/ (40,822) 30 (diagnos* or electrodiagnos*).tw. (2,271,388) 31 computer assisted diagnosis/ (31,733) 32 Laser/ and Diagnosis/ (1798) 33 image processing/ (49,197) 34 (automat* adj5 (detect* or captur* or quantif*)).tw. (13,596) 35 or/23-34 (3,455,191) 36 22 and 35 (1154) 37 autofluorescence imaging/ (1207) 38 36 or 37 (2000) 39 exp Retinal Disease/ (184,028) 40 chorioretinopathy/ (1483) 41 retina* vein occlusion/ (3370) 42 retina macula degeneration/ or retina macula age related degeneration/ or retina maculopathy/ or retina degeneration/ or subretinal neovascularization/ (32,777) 43 diabetic retinopathy/ (28,376) 44 (retina* or retinitis or retinopath* or epiretina* or subretina* or preretina* or posterioretina* or intraretina* or chorioretinopath* or vitreoretinopath*).tw. (193,702) 45 (macula* or maculopath* or ‘wet AMD’ or ‘dry AMD’ or ‘exud* AMD’).tw. (47,694) 46 ((fundus or fundi or fundal) adj5 (change* or impair* or disease* or disorder* or detect* or diagnos*)).tw. (3129) 47 (geographical adj atroph*).tw. (11) 48 hyperfluorescen*.tw. (1120) 49 (RVO or CRVO or BRVO).tw. (1782) 50 (cone*1 adj2 dystroph*).tw. (951) 51 or/39-50 (296,820) 52 38 and 51 (1294) 53 (autofluorescence and fund* and imag*).tw. (765) 54 (FAF and imag*).tw. (275) 55 53 or 54 (776) 56 52 or 55 (1359) 57 limit 56 to (human and yr=‘1990 -Current’) (1248) 58 limit 57 to english language (1152) |
1152 |
| SCI-E and CPCI-S, Web of Science, searched from 1990–2014, searched on 13 November 2014 | # 1 (TS=(FAF and imag*)) AND LANGUAGE: (English) (233) # 2 (TS=((autofluorescen* and imag*) and (fundus or fundi or fundal))) (732) # 3 (TS=(‘confocal scanning laser ophthalmoscop*’)) (592) # 4 (TS=(Topcon or optos or spectralis or bluepeak or ‘Heidelberg retina angiograph’ or ‘AO SLO’ or ‘spectral domain OCT’ or optovue)) (1404) # 5 (TS=(image* or imaging or electroretinography)) AND LANGUAGE: (English) (1,325,626) # 6 #5 AND #4 (860) # 7 (TS=(autofluorescenc* and (‘fundus camera*’ or ‘fundus spectrophotometry’ or ‘confocal scan*’ or ‘laser ophthalmoscop*’ or CsLO))) (311) # 8 (TS=(retina* or retinitis or retinopath* or epiretina* or subretina* or preretina* or posterioretina* or intraretina* or chorioretinopath* or vitreoretinopath*)) (150,838) # 9 (TS=(macula* or maculopath* or ‘wet AMD’ or ‘dry AMD’ or ‘exud* AMD’)) (45,128) # 10 (TS=((fundus or fundi or fundal) NEAR (change* or impair* or disease* or disorder* or detect* or diagnos*))) (3652) # 11 (TS=(geographical adj atroph*)) (80) # 12 (TS=(hyperfluorescen*)) (548) # 13 (TS=(RVO or CRVO or BRVO)) (1097) # 14 (TS=(cone* NEAR dystroph*)) (1301) # 15 #7 OR #6 OR #3 OR #2 OR #1 (2053) # 16 #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 (175,649) # 17 #16 AND #15 (1669) # 18 (TI=(‘fundus autofluorescence imaging’)) (38) #19 #18 OR #17 (1661) |
1661 |
| The Cochrane Library, all years, searched 13 November 2014. Results found only in CDSR. Issue 11 of 12, November 2014 and CENTRAL Issue 10 of 12, October 2014 (nothing in HTA, NHS Economic Evaluation Database, DARE Issue 4 October 2014) (Also nothing unique in Cochrane Eyes and Vision group) |
#1 (autofluorescen* and (fundus or fundi or fundal)) (25) #2 FAF (31) #3 #1 or #2 (44) #4 topcon (74) #5 ‘confocal scanning laser ophthalmoscop*’ (30) #6 cSLO (8) #7 optos (6) #8 spectralis (17) #9 imagenet (13) #10 bluepeak (0) #11 ‘Heidelberg retina* angiograph*’ (12) #12 ‘Heidelberg Engineering’ (46) #13 ‘AO SLO’ (0) #14 ‘spectral domain OCT’ (16) #15 optovue (6) #16 ‘carl zeiss meditec’ (73) #17 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 (240) #18 (fluorescen* or autofluorescen*) (2613) #19 MeSH descriptor: [Fluorescence] this term only (185) #20 #18 or #19 (2613) #21 #17 and #20 (19) #22 #3 or #21 (53) #23 (image* or imaging) (27,896) #24 (camera* or photograph* or laser* or infrared or ophthalmoscop* or instrument*) (43,198) #25 MeSH descriptor: [Tomography, Optical Coherence] this term only (527) #26 MeSH descriptor: [Fluorescein Angiography] this term only (545) #27 MeSH descriptor: [Optical Imaging] explode all trees (593) #28 MeSH descriptor: [Electroretinography] explode all trees (140) #29 MeSH descriptor: [Microscopy, Confocal] explode all trees (164) #30 (diagnos* or electrodiagnos*) (101,633) #31 MeSH descriptor: [Diagnosis, Computer-Assisted] explode all trees (1610) #32 MeSH descriptor: [Lasers] explode all trees and with qualifier(s): [Diagnostic use - DU] (150) #33 MeSH descriptor: [Image Processing, Computer-Assisted] explode all trees (2935) #34 (automat* N/5 (detect* or captur* or quantif*)) (124) #35 #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 (148,379) #36 #22 and #35 (37) |
37 (4 CDSR, 33 CENTRAL) |
| CRD databases: DARE, HTA, NHS EED. Searched from 1990 to 12 November 2014, searched on 13 November 2014 | 1 ((autofluorescen* and (fundus or fundi or fundal)) ) FROM 1990 TO 2014 (0) 2 (FAF) FROM 1990 TO 2014 (0) 3 (#1 or #2) FROM 1990 TO 2014 (0) 4 (topcon) FROM 1990 TO 2014 (2) 5 ((‘confocal scanning laser ophthalmoscop*’)) FROM 1990 TO 2014 (2) 6 (cslo) FROM 1990 TO 2014 (1) 7 (optos) FROM 1990 TO 2014 (0) 8 (spectralis) FROM 1990 TO 2014 (0) 9 (imagenet) FROM 1990 TO 2014 (0) 10 (bluepeak) FROM 1990 TO 2014 (0) 11 ((heidelberg retina* angiograph*)) FROM 1990 TO 2014 (0) 12 (heidelberg engineering) FROM 1990 TO 2014 (0) 13 (‘AO SLO’) FROM 1990 TO 2014 (0) 14 (‘spectral domain’) AND (OCT) FROM 1990 TO 2014 (1) 15 (optovue) FROM 1990 TO 2014 (0) 16 (carl zeiss meditec) FROM 1990 TO 2014 (1) 17 (#4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16) FROM 1990 TO 2014 (5) 18 ((fluorescen* or autofluorescen*)) FROM 1990 TO 2014 (141) 19 MeSH DESCRIPTOR Fluorescence EXPLODE ALL TREES (16) 20 (#18 or #19) FROM 1990 TO 2014 (141) 21 (#17 and #20) FROM 1990 TO 2014 (0) 22 ((image* or imaging)) FROM 1990 TO 2014 (2889) 23 ((camera* or photograph* or laser* or infrared or ophthalmoscop* or instrument*)) FROM 1990 TO 2014 (4177) 24 MeSH DESCRIPTOR Tomography, Optical Coherence EXPLODE ALL TREES (20) 25 MeSH DESCRIPTOR Tomography, Optical EXPLODE ALL TREES (21) 26 MeSH DESCRIPTOR Fluorescein Angiography EXPLODE ALL TREES (11) 27 MeSH DESCRIPTOR Optical Imaging EXPLODE ALL TREES (29) 28 MeSH DESCRIPTOR Electroretinography EXPLODE ALL TREES (3) 29 MeSH DESCRIPTOR Microscopy, Confocal EXPLODE ALL TREES (9) 30 ((diagnos* or electrodiagnos*)) FROM 1990 TO 2014 14978 31 MeSH DESCRIPTOR Diagnosis, Computer-Assisted EXPLODE ALL TREES (114) 32 MeSH DESCRIPTOR lasers EXPLODE ALL TREES WITH QUALIFIER DU (5) 33 MeSH DESCRIPTOR Image Processing, Computer-Assisted EXPLODE ALL TREES (168) 34 ((automat* NEAR (detect* or captur* or quantif*))) FROM 1990 TO 2014 (11) 35 (#22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34) FROM 1990 TO 2014 (18,561) 36 (#4 or #5 or #6 or #14 or #16) FROM 1990 TO 2014 (5) 37 (#35 and #36) FROM 1990 TO 2014 (5) |
Two NHS EED (three results from HTA were all glaucoma – agreed not to download these ones; zero from DARE) |
Appendix 2 Study selection worksheet
Appendix 3 Critical appraisal worksheet
Critical appraisal criteria (based on Reitsma and colleagues81 adaptation of the QUADAS Tool93)
| Item | Description | Judgement (yes/no/unclear) | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | ||
| 2 | Is the reference standard likely to classify the target condition correctly? | ||
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | ||
| 4 | Did the whole sample or a random selection of the sample receive verification using the intended reference standard? | ||
| 5 | Did patients receive the same reference standard irrespective of the index test result? | ||
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | ||
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | ||
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | ||
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | ||
| 10 | Were uninterpretable/ intermediate test results reported? | ||
| 11 | Were withdrawals from the study explained? |
Appendix 4 Table of excluded studies with rationale
Note that some studies may have been excluded for more than one reason; the primary reason reported here is the first reason agreed by reviewers when following the study selection worksheet (see Appendix 2).
| Study | Primary reason for exclusion |
|---|---|
| Ahn SJ, Ahn J, Park KH, Woo SJ. Multimodal imaging of occult macular dystrophy. JAMA Ophthalmol 2013;131:880–90 | Sensitivity of FAF imaging not reported, not calculable |
| Aizawa S, Mitamura Y, Hagiwara A, Sugawara T, Yamamoto S. Changes of fundus autofluorescence, photoreceptor inner and outer segment junction line, and visual function in patients with retinitis pigmentosa. Clin Exp Optom 2010;38:597–604 | Sensitivity of FAF imaging not reported, not calculable |
| Alten F, Heiduschka P, Clemens CR, Eter N. Multifocal electroretinography in eyes with reticular pseudodrusen. Invest Ophthalmol Vis Sci 2012;53:6263–70 | Sensitivity of FAF imaging not reported, not calculable |
| Alten F, Clemens CR, Heiduschka P, Eter N. Characterisation of reticular pseudodrusen and their central target aspect in multi-spectral, confocal scanning laser ophthalmoscopy. Graefes Arch Clin Exp Ophthalmol 2014;252:715–21 | Sensitivity of FAF imaging not reported, not calculable |
| Asao K, Gomi F, Sawa M, Nishida K. Additional anti-vascular endothelial growth factor therapy for eyes with a retinal pigment epithelial tear after the initial therapy. Retina 2014;34:512–18 | Sensitivity of FAF imaging not reported, not calculable |
| Ayata A, Tatlipinar S, Kar T, Unal M, Ersanli D, Bilge AH. Near-infrared and short-wavelength autofluorescence imaging in central serous chorioretinopathy. Br J Ophthalmol 2009;93:79–82 | Sensitivity of FAF imaging not reported, not calculable |
| Batioglu F, Demirel S, Ozmert E, Oguz YG, Ozyol P. Autofluorescence patterns as a predictive factor for neovascularization. Optom Vis Sci 2014;91:950–5 | Sensitivity of FAF imaging not reported, not calculable |
| Bessho K, Gomi F, Harino S, Sawa M, Sayanagi K, Tsujikawa M, et al. Macular autofluorescence in eyes with cystoid macula edema, detected with 488 nm-excitation but not with 580 nm-excitation. Graefes Arch Clin Exp Ophthalmol 2009;247:729–34 | Sensitivity of FAF imaging not reported, not calculable |
| Bessho K, Rodanant N, Bartsch DU, Cheng L, Koh HJ, Freeman WR. Effect of subthreshold infrared laser treatment for drusen regression on macular autofluorescence in patients with age-related macular degeneration. Retina 2005;25:981–8 | No appropriate reference standard |
| Bonnet C, Querques G, Zerbib J, Oubraham H, Garavito RB, Puche N, et al. Hyperreflective pyramidal structures on optical coherence tomography in geographic atrophy areas. Retina 2014;34:1524–30 | Sensitivity of FAF imaging not reported, not calculable |
| Boon CJF. SD-OCT and fundus autofluorescence imaging for differential diagnosis of macular and retinal dystrophies. Ophthalmologica 2013;230:15–16 (meeting abstract) | Sensitivity of FAF imaging not reported, not calculable |
| Bottoni F, Carmassi L, Cigada M, Moschini S, Bergamini F. Diagnosis of macular pseudoholes and lamellar macular holes: is optical coherence tomography the ‘gold standard’? Br J Ophthalmol 2008;92:635–9 | Sensitivity of FAF imaging not reported, not calculable |
| Bottoni F, Eandi CM, Pedenovi S, Staurenghi G. Integrated clinical evaluation of type 2A idiopathic juxtafoveolar retinal telangiectasis. Retina 2010;30:317–26 | Sensitivity of FAF imaging not reported, not calculable |
| Brar M, Kozak I, Cheng L, Bartsch DU, Yuson R, Nigam N, et al. Correlation between spectral-domain optical coherence tomography and fundus autofluorescence at the margins of geographic atrophy. Am J Ophthalmol 2009;148:439–44 | Sensitivity of FAF imaging not reported, not calculable |
| Carreno E, Portero A, Herreras JM, Lopez MI. Assesment of fundus autofluorescence in serpiginous and serpiginous-like choroidopathy. Eye 2012;26:1232–6 | Sensitivity of FAF imaging not reported, not calculable |
| Chen Z, Song Y. Functional and structural changes after pattern scanning laser photocoagulation in diabetic retinopathy. Doc Ophthalmol 2013;127:37 (meeting abstract) | Sensitivity of FAF imaging not reported, not calculable |
| Chen FK, Patel PJ, Coffey PJ, Tufail A, Da Cruz CL. Increased fundus autofluorescence associated with outer segment shortening in macular translocation model of neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2010;51:4207–12 | Sensitivity of FAF imaging not reported, not calculable |
| Chhablani JK, Narayanan R. Fundus autofluorescence patterns in type 2A idiopathic juxtafoveolar retinal telangiectasis. Eur J Ophthalmol 2012;22:398–403 | Sensitivity of FAF imaging not reported, not calculable |
| Chia A, Tan A, Boon KL, Cheung G. Autofluorescence imaging in children with retinal pathology identified with visual electrophysiology. Doc Ophthalmol 2013;127:9 (meeting abstract) | No appropriate reference standard |
| Chung H, Park B, Shin HJ, Kim HC. Correlation of fundus autofluorescence with spectral-domain optical coherence tomography and vision in diabetic macular edema. Ophthalmology 2012;119:1056–65 | Sensitivity of FAF imaging not reported, not calculable |
| Clemens CR, Alten F, Baumgart C, Heiduschka P, Eter N. Quantification of retinal pigment epithelium tear area in age-related macular degeneration. Retina 2014;34:24–31 | No appropriate reference standard |
| Cuba J, Gomez-Ulla F. Fundus autofluorescence: applications and perspectives. Arch Soc Esp Oftalmol 2013;88:50–5 | Sensitivity of FAF imaging not reported, not calculable |
| De Bats F, Wolff B, Mauget-Faysse M, Meunier I, Denis P, Kodjikian L. Association of reticular pseudodrusen and early onset drusen. ISRN Ophthalmol 2013;2013 | Sensitivity of FAF imaging not reported, not calculable |
| de Laat P, Smeitink JA, Janssen MC, Keunen JE, Boon CJ. Mitochondrial retinal dystrophy associated with the m.3243A>G mutation. Ophthalmology 2013;120:2684–96 | Sensitivity of FAF imaging not reported, not calculable |
| Deli A, Moetteli L, Ambresin A, Mantel I. Comparison of fundus autofluorescence images acquired by the confocal scanning laser ophthalmoscope (488 nm excitation) and the modified Topcon fundus camera (580 nm excitation). Int Ophthalmol 2013;33:635–43 | Sensitivity of FAF imaging not reported, not calculable |
| Dinc UA, Tatlipinar S, Yenerel M, Gorgun E, Ciftci F. Fundus autofluorescence in acute and chronic central serous chorioretinopathy. Clin Exp Optom 2011;94:452–7 | Sensitivity of FAF imaging not reported, not calculable |
| Dinc UA, Tatlipinar S, Gorgun E, Yenerel M. Fundus autofluorescence in optic disc drusen: comparison of confocal scanning laser ophthalmoscope and standard fundus camera. Neuroophthalmology 2009;33:318–21 | Sensitivity of FAF imaging not reported, not calculable |
| Duncker T, Tabacaru MR, Lee W, Tsang SH, Sparrow JR, Greenstein VC. Comparison of near-infrared and short-wavelength autofluorescence in retinitis pigmentosa. Invest Ophthalmol Vis Sci 2013;54:585–91 | Sensitivity of FAF imaging not reported, not calculable |
| Duncker T, Greenberg JP, Ramachandran R, Hood DC, Smith RT, Hirose T, et al. Quantitative fundus autofluorescence and optical coherence tomography in Best vitelliform macular dystrophy. Invest Ophthalmol Vis Sci 2014;55:1471–82 | Sensitivity of FAF imaging not reported, not calculable |
| Einbock W, Moessner A, Schnurrbusch UE, Holz FG, Wolf S, FAM study group. Changes in fundus autofluorescence in patients with age-related maculopathy. Correlation to visual function: a prospective study. Graefes Arch Clin Exp Ophthalmol 2005;243:300–5 | Sensitivity of FAF imaging not reported, not calculable |
| Ergun E, Hermann B, Wirtitsch M, Unterhuber A, Ko TH, Sattmann H, et al. Assessment of central visual function in Stargardt’s disease/fundus flavimaculatus with ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci 2005;46:310–16 | Sensitivity of FAF imaging not reported, not calculable |
| Erol MK, Ozdemir O, Coban DT, Ceran BB, Bulut M. Ranibizumab treatment for choroidal neovascularization secondary to causes other than age-related macular degeneration with good baseline visual acuity. Semin Ophthalmol 2014;29:108–13 | Sensitivity of FAF imaging not reported, not calculable |
| Finger RP, Charbel IP, Schmitz-Valckenberg S, Holz FG, Scholl HN. Long-term effectiveness of intravitreal bevacizumab for choroidal neovascularization secondary to angioid streaks in pseudoxanthoma elasticum. Retina 2011;31:1268–78 | Sensitivity of FAF imaging not reported, not calculable |
| Finger RP, Charbel IP, Ladewig M, Gotting C, Holz FG, Scholl HP. Fundus autofluorescence in Pseudoxanthoma elasticum. Retina 2009;29:1496–505 | Sensitivity of FAF imaging not reported, not calculable |
| Finger RP, Charbel IP, Ladewig M, Holz FG, Scholl HP. Intravitreal bevacizumab for choroidal neovascularisation associated with pseudoxanthoma elasticum. Br J Ophthalmol 2008;92:483–7 | Sensitivity of FAF imaging not reported, not calculable |
| Forte R, Querques G, Querques L, Leveziel N, Benhamou N, Souied EH. Multimodal evaluation of foveal sparing in patients with geographic atrophy due to age-related macular degeneration. Retina 2013;33:482–9 | Sensitivity of FAF imaging not reported, not calculable |
| Forte R, Querques G, Querques L, Massamba N, Le Tien V, Souied EH. Multimodal imaging of dry age-related macular degeneration. Acta Opthalmol 2012;90:e281–7 | Sensitivity of FAF imaging not reported, not calculable |
| Framme C, Brinkmann R, Birngruber R, Roider J. Autofluorescence imaging after selective RPE laser treatment in macular diseases and clinical outcome: a pilot study. Br J Ophthalmol 2002;86:1099–106 | Sensitivity of FAF imaging not reported, not calculable |
| Framme C, Walter A, Gabler B, Roider J, Sachs HG, Gabel VP. Fundus autofluorescence in acute and chronic-recurrent central serous chorioretinopathy. Acta Ophthalmol Scand 2005;83:161–7 | Sensitivity of FAF imaging not reported, not calculable |
| Framme C, Bunse A, Sofroni R, Thalhammer T, Walter A, Sachs HG, et al. Fundus autofluorescence before and after photodynamic therapy for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2006;37:406–14 | Sensitivity of FAF imaging not reported, not calculable |
| Fujiwara T, Imamura Y, Giovinazzo VJ, Spaide RF. Fundus autofluorescence and optical coherence tomographic findings in acute zonal occult outer retinopathy. Retina 2010;30:1206–16 | Sensitivity of FAF imaging not reported, not calculable |
| Furino C, Boscia F, Cardascia N, Sborgia L, Sborgia C. Fundus autofluorescence, optical coherence tomography and visual acuity in adult-onset foveomacular dystrophy. Ophthalmologica 2008;222:240–4 | Sensitivity of FAF imaging not reported, not calculable |
| Gajdzik-Gajdecka U, Dorecka M, Nita E, Michalska A, Miniewicz-Kurowska J, Romaniuk W. Indocyanine green angiography in chronic central serous chorioretinopathy. Med Sci Monit 2012;18:CR51–7 | Sensitivity of FAF imaging not reported, not calculable |
| Garcia CR, Rivero ME, Bartsch DU, Ishiko S, Takamiya A, Fukui K, et al. Oral fluorescein angiography with the confocal scanning laser ophthalmoscope. Ophthalmology 1999;106:1114–18 | No appropriate index test |
| Gendy MG, Fawzi AA, Wendel RT, Pieramici DJ, Miller JA, Jampol LM. Multimodal imaging in persistent placoid maculopathy. JAMA Ophthalmol 2014;132:38–49 | Sensitivity of FAF imaging not reported, not calculable |
| Giani A, Pellegrini M, Carini E, Peroglio DA, Bottoni F, Staurenghi G. The dark atrophy with indocyanine green angiography in Stargardt disease. Invest Ophthalmol Vis Sci 2012;53:3999–4004 | Sensitivity of FAF imaging not reported, not calculable |
| Gili P, Flores-Rodriguez P, Yanguela J, Herreros Fernàndez ML. Using autofluorescence to detect optic nerve head drusen in children. J AAPOS 2013;17:568–71 | No appropriate index test |
| Gillies MC, Zhu M, Chew E, Barthelmes D, Hughes E, Ali H, et al. Familial asymptomatic macular telangiectasia type 2. Ophthalmology 2009;116:2422–9 | Sensitivity of FAF imaging not reported, not calculable |
| Giuliari G, Hinkle DM, Foster CS. The spectrum of fundus autofluorescence findings in birdshot chorioretinopathy. J Ophthalmol 2009;2009 | Sensitivity of FAF imaging not reported, not calculable |
| Golchet PR, Jampol LM, Mathura JR Jr, Daily MJ. Torpedo maculopathy. Br J Ophthalmol 2010;94:302–6 | Inadequate sample size (< 10 eyes) |
| Gomes NL, Greenstein VC, Carlson JN, Tsang SH, Smith RT, Carr RE, et al. A comparison of fundus autofluorescence and retinal structure in patients with Stargardt disease. Invest Ophthalmol Vis Sci 2009;50:3953–9 | Sensitivity of FAF imaging not reported, not calculable |
| Gomes NL, Corcostegui I, Fine HF, Chang S. Subfoveal pigment changes in patients with longstanding epiretinal membranes. Am J Ophthalmol 2009;147:865–8 | Sensitivity of FAF imaging not reported, not calculable |
| Greenberg JP, Sherman J, Zweifel SA, Chen RW, Duncker T, Kohl S, et al. Spectral-domain optical coherence tomography staging and autofluorescence imaging in achromatopsia. JAMA Ophthalmol 2014;132:437–45 | Sensitivity of FAF imaging not reported, not calculable |
| Greenstein VC, Santos RA, Tsang SH, Smith RT, Barile GR, Seiple W. Preferred retinal locus in macular disease: characteristics and clinical implications. Retina 2008;28:1234–40 | Sensitivity of FAF imaging not reported, not calculable |
| Greenstein VC, Duncker T, Holopigian K, Carr RE, Greenberg JP, Tsang SH, et al. Structural and functional changes associated with normal and abnormal fundus autofluorescence in patients with retinitis pigmentosa. Retina 2012;32:349–57 | Sensitivity of FAF imaging not reported, not calculable |
| Haas P, Esmaeelpour M, Ansari-Shahrezaei S, Drexler W, Binder S. Choroidal thickness in patients with reticular pseudodrusen using 3D 1060-nm OCT maps. Invest Ophthalmol Vis Sci 2014;55:2674–81 | Sensitivity of FAF imaging not reported, not calculable |
| Heimes B, Lommatzsch A, Zeimer M, Gutfleisch M, Spital G, Bird AC, et al. Foveal RPE autofluorescence as a prognostic factor for anti-VEGF therapy in exudative AMD. Graefes Arch Clin Exp Ophthalmol 2008;246:1229–34 | No appropriate reference standard |
| Helb HM, Charbel IP, van der Veen RL, Berendschot TT, Scholl HP, Holz FG. Abnormal macular pigment distribution in type 2 idiopathic macular telangiectasia. Retina 2008;28:808–16 | Sensitivity of FAF imaging not reported, not calculable |
| Helb HM, Charbel IP, Fleckenstein M, Schmitz-Valckenberg S, Scholl HP, Meyer CH, et al. Clinical evaluation of simultaneous confocal scanning laser ophthalmoscopy imaging combined with high-resolution, spectral-domain optical coherence tomography. Acta Opthalmol 2010;88:842–9 | Sensitivity of FAF imaging not reported, not calculable |
| Henderson RH, Mackay DS, Li Z, Moradi P, Sergouniotis P, Russell-Eggitt I, et al. Phenotypic variability in patients with retinal dystrophies due to mutations in CRB1. Br J Ophthalmol 2011;95:811–17 | Sensitivity of FAF imaging not reported, not calculable |
| Heussen FM, Tan CS, Sadda SR. Prevalence of peripheral abnormalities on ultra-widefield greenlight (532 nm) autofluorescence imaging at a tertiary care center. Invest Ophthalmol Vis Sci 2012;53:6526–31 | Sensitivity of FAF imaging not reported, not calculable |
| Heussen FMA, Fawzy NF, Joeres S, Lux A, Maaijwee K, Meurs JC, et al. Autologous translocation of the choroid and RPE in age-related macular degeneration: 1-year follow-up in 30 patients and recommendations for patient selection. Eye 2008;22:799-807 | Sensitivity of FAF imaging not reported, not calculable |
| Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S, et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol 2007;143:463–72 | No appropriate reference standard |
| Hu Z, Medioni GG, Hernandez M, Hariri A, Wu X, Sadda SR. Segmentation of the geographic atrophy in spectral-domain optical coherence tomography and fundus autofluorescence images. Invest Ophthalmol Vis Sci 2013;54:837–83 | Sensitivity of FAF imaging not reported, not calculable |
| Imamura Y, Fujiwara T, Spaide RF. Fundus autofluorescence and visual acuity in central serous chorioretinopathy. Ophthalmology 2011;118:700–5 | No appropriate reference standard |
| Iriyama A, Yanagi Y. Fundus autofluorescence and retinal structure as determined by spectral domain optical coherence tomography, and retinal function in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol 2012;250:333–9 | Sensitivity of FAF imaging not reported, not calculable |
| Issa PC, Finger RP, Holz FG, Scholl HPN. Multimodal imaging including spectral domain OCT and confocal near infrared reflectance for characterization of outer retinal pathology in pseudoxanthoma elasticum. Invest Ophthalmol Vis Sci 2009;50:5913–18 | Sensitivity of FAF imaging not reported, not calculable |
| Jarc-Vidmar M, Kraut A, Hawlina M. Fundus autofluorescence imaging in Best’s vitelliform dystrophy. Klin Monatsbl Augenheilkd 2003;220:861–7 | Sensitivity of FAF imaging not reported, not calculable |
| Jeong YJ, Hong IH, Chung JK, Kim KL, Kim HK, Park SP. Predictors for the progression of geographic atrophy in patients with age-related macular degeneration: fundus autofluorescence study with modified fundus camera. Eye 2014;28:209–18 | Sensitivity of FAF imaging not reported, not calculable |
| Jung JJ, Khan S, Mrejen S, Gallego-Pinazo R, Cunningham ET Jr, Freund KB, et al. Idiopathic multifocal choroiditis with outer retinal or chorioretinal atrophy. Retina 2014;34:1439–50 | Sensitivity of FAF imaging not reported, not calculable |
| Kaya M, Yaman A, Oner FH, Saatci AO. Autofluorescence imaging in eyes with various types of retinal artery occlusion: case report. Turk Klin J Med Sci 2011;31:1283–7 | Inadequate sample size (< 10 eyes) |
| Kellner U, Weinitz S, Kellner S. Visualizing the onset of retinitis pigmentosa. Doc Ophthalmol 2010;121:17–18 (meeting abstract) | Sensitivity of FAF imaging not reported, not calculable |
| Kellner S, Neuhann TM, Abicht A, Wissinger B, Renner AB, Weinitz S, et al. Combined confocal near-infrared reflectance (815 nm) and macular spectral domain OCT identify optic atrophy in patients with bilateral unexplained visual loss. Doc Ophthalmol 2013;127:28 (meeting abstract) | Not a retinal condition |
| Khanifar AA, Lederer DE, Ghodasra JH, Stinnett SS, Lee JJ, Cousins SW, et al. Comparison of color fundus photographs and fundus autofluorescence images in measuring geographic atrophy area. Retina 2012;32:1884–91 | Sensitivity of FAF imaging not reported, not calculable |
| Kim SK, Kim SW, Oh J, Huh K. Near-infrared and short-wavelength autofluorescence in resolved central serous chorioretinopathy: association with outer retinal layer abnormalities. Am J Ophthalmol 2013;156:157–64 | Sensitivity of FAF imaging not reported, not calculable |
| Kim SW, Oh J, Huh K. Correlations among various functional and morphological tests in resolved central serous chorioretinopathy. Br J Ophthalmol 2012;96:350–5 | Sensitivity of FAF imaging not reported, not calculable |
| Koizumi H, Pozzoni MC, Spaide RF. Fundus autofluorescence in birdshot chorioretinopathy. Ophthalmology 2008;115:e15–20 | Sensitivity of FAF imaging not reported, not calculable |
| Kojima H, Otani A, Ogino K, Nakagawa S, Makiyama Y, Kurimoto M, et al. Outer retinal circular structures in patients with Bietti crystalline retinopathy. Br J Ophthalmol 2012;96:390–3 | Sensitivity of FAF imaging not reported, not calculable |
| Kolomeyer AM, Baumrind BR, Szirth BC, Shahid K, Khouri AS. Fundus autofluorescence and colour fundus imaging compared during telemedicine screening in patients with diabetes. J Telemed Telecare 2013;19:209–12 | Sensitivity of FAF imaging not reported, not calculable |
| Kolomeyer AM, Nayak NV, Szirth BC, Khouri AS. Fundus autofluorescence imaging in an ocular screening program. Int J Telemed Appl 2012;2012 | Inadequate sample size (< 10 eyes) |
| Kramer M, Priel E. Fundus autofluorescence imaging in multifocal choroiditis: beyond the spots. Ocul Immunol Inflamm 2014;22:349–55 | Inadequate sample size (< 10 eyes) |
| Kumar N, Mrejen S, Fung AT, Marsiglia M, Loh BK, Spaide RF. Retinal pigment epithelial cell loss assessed by fundus autofluorescence imaging in neovascular age-related macular degeneration. Ophthalmology 2013;120:334–41 | Sensitivity of FAF imaging not reported, not calculable |
| Kurz-Levin MM, Halfyard AS, Bunce C, Bird AC, Holder GE. Clinical variations in assessment of bull’s-eye maculopathy. Arch Ophthalmol 2002;120:567–75 | Inadequate sample size (< 10 eyes) |
| Lai WW, Leung GY, Chan CW, Yeung IY, Wong D. Simultaneous spectral domain OCT and fundus autofluorescence imaging of the macula and microperimetric correspondence after successful repair of rhegmatogenous retinal detachment. Br J Ophthalmol 2010;94:311–18 | Sensitivity of FAF imaging not reported, not calculable |
| Landa G, Rosen RB, Pilavas J, Garcia PM. Drusen characteristics revealed by spectral-domain optical coherence tomography and their corresponding fundus autofluorescence appearance in dry age-related macular degeneration. Ophthalmic Res 2012;47:81–6 | Sensitivity of FAF imaging not reported, not calculable |
| Lee CS, Lee AY, Forooghian F, Bergstrom CS, Yan J, Yeh S. Fundus autofluorescence features in the inflammatory maculopathies. Clin Ophthalmol 2014;8:2001–12 | No appropriate reference standard |
| Lee JE, Lim DW, Bae HY, Park HJ. Photoreceptor layer map using spectral-domain optical coherence tomography. Optom Vis Sci 2009;86:E1320–27 | Sensitivity of FAF imaging not reported, not calculable |
| Lee JY, Lee DH, Lee JY, Yoon YH. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:7812–18 | Sensitivity of FAF imaging not reported, not calculable |
| Lee M, Yoon J, Ham DI. Clinical features of reticular pseudodrusen according to the fundus distribution. Br J Ophthalmol 2012;96:1222–6 | Sensitivity of FAF imaging not reported, not calculable |
| Lee MY, Yoon J, Ham DI. Clinical characteristics of reticular pseudodrusen in Korean patients. Am J Ophthalmol 2012;153:530–5 | Sensitivity of FAF imaging not reported, not calculable |
| Lee MY, Ham DI. Subretinal drusenoid deposits with increased autofluorescence in eyes with reticular pseudodrusen. Retina 2014;34:69–76 | Sensitivity of FAF imaging not reported, not calculable |
| Lee TJ, Hwang JC, Chen RW, Lima LH. The role of fundus autofluorescence in late-onset retinitis pigmentosa (LORP) diagnosis. Ophthalmic Genet 2014;35:170–9 | No appropriate reference standard |
| Lenassi E, Troeger E, Wilke R, Hawlina M. Correlation between macular morphology and sensitivity in patients with retinitis pigmentosa and hyperautofluorescent ring. Invest Ophthalmol Vis Sci 2012;53:47–52 | Sensitivity of FAF imaging not reported, not calculable |
| Lenassi E, Troeger E, Wilke R, Tufail A, Hawlina M, Jeffery G, et al. Laser clearance of drusen deposit in patients with autosomal dominant drusen (p.Arg345Trp in EFEMP1). Am J Ophthalmol 2013;155:190–8 | Sensitivity of FAF imaging not reported, not calculable |
| Leon PE, Saviano S, Zanei A, Pastore MR, Guaglione E, Mangogna A, et al. Spontaneous or secondary to intravitreal injections of anti-angiogenic agents retinal pigment epithelial tears in age-related macular degeneration. Int J Ophthalmol 2014;7:681–5 | Sensitivity of FAF imaging not reported, not calculable |
| Levinson RD, Monnet D. Imaging in birdshot chorioretinopathy. Int Ophthalmol Clin 2012;52:191–8 | Not primary research |
| Lima LH, Laud K, Freund KB, Yannuzzi LA, Spaide RF. Acquired vitelliform lesion associated with large drusen. Retina 2012;32:647–51 | Sensitivity of FAF imaging not reported, not calculable |
| Lima LH, Greenberg JP, Greenstein VC, Smith RT, Sallum JM, Thirkill C, et al. Hyperautofluorescent ring in autoimmune retinopathy. Retina 2012;32:1385–94 | Inadequate sample size (< 10 eyes) |
| Lima LH, Cella W, Greenstein VC, Wang NK, Busuioc M, Smith RT, et al. Structural assessment of hyperautofluorescent ring in patients with retinitis pigmentosa. Retina 2009;29:1025–31 | Sensitivity of FAF imaging not reported, not calculable |
| Lima LH, Burke T, Greenstein VC, Chou CL, Cella W, Yannuzzi LA, et al. Progressive constriction of the hyperautofluorescent ring in retinitis pigmentosa. Am J Ophthalmol 2012;153:718–27 | Sensitivity of FAF imaging not reported, not calculable |
| Lindner E, Weinberger A, Kirschkamp T, El-Shabrawi Y, Barounig A. Near-infrared autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Ophthalmologica 2012;227:34–8 | Sensitivity of FAF imaging not reported, not calculable |
| Liu DN, Liu Y, Meng XH, Yin ZQ. The characterization of functional disturbances in Chinese patients with Bietti’s crystalline dystrophy at different fundus stages. Graefes Arch Clin Exp Ophthalmol 2012;250:191–200 | Sensitivity of FAF imaging not reported, not calculable |
| Lois N, Owens SL, Coco R, Hopkins J, Fitzke FW, Bird AC. Fundus autofluorescence in patients with age-related macular degeneration and high risk of visual loss. Am J Ophthalmol 2002;133:341–9 | Sensitivity of FAF imaging not reported, not calculable |
| Lois N, McBain V, Abdelkader E, Scott NW, Kumari R. Retinal pigment epithelial atrophy in patients with exudative age-related macular degeneration undergoing anti-vascular endothelial growth factor therapy. Retina 2013;33:13–22 | Sensitivity of FAF imaging not reported, not calculable |
| Lorenz B, Wabbels B, Wegscheider E, Hamel CP, Drexler W, Preising MN. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology 2004;111:1585–94 | Sensitivity of FAF imaging not reported, not calculable |
| Luttrull JK, Sramek C, Palanker D, Spink CJ, Musch DC. Long-term safety, high-resolution imaging, and tissue temperature modeling of subvisible diode micropulse photocoagulation for retinovascular macular edema. Retina 2012;32:375–86 | Sensitivity of FAF imaging not reported, not calculable |
| MacLaren RE, Uppal GS, Balaggan KS, Tufail A, Munro PMG, Milliken AB, et al. Autologous transplantation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degeneration. Ophthalmology 2007;114:561–70 | Sensitivity of FAF imaging not reported, not calculable |
| Makiyama Y, Ooto S, Hangai M, Takayama K, Uji A, Oishi A, et al. Macular cone abnormalities in retinitis pigmentosa with preserved central vision using adaptive optics scanning laser ophthalmoscopy. PLOS ONE 2013;8:e79447 | Sensitivity of FAF imaging not reported, not calculable |
| Margolis R, Mukkamala SK, Jampol LM, Spaide RF, Ober MD, Sorenson JA, et al. The expanded spectrum of focal choroidal excavation. Arch Ophthalmol 2011;129:1320–5 | Sensitivity of FAF imaging not reported, not calculable |
| Marsiglia M, Boddu S, Bearelly S, Xu L, Breaux BE Jr, Freund KB, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:7362–9 | No appropriate reference standard |
| Maruko I, Iida T, Ojima A, Sekiryu T. Subretinal dot-like precipitates and yellow material in central serous chorioretinopathy. Retina 2011;31:759–65 | Sensitivity of FAF imaging not reported, not calculable |
| Mathew R, Papavasileiou E, Sivaprasad S. Autofluorescence and high-definition optical coherence tomography of retinal artery occlusions. Clin Ophthalmol 2010;4:1159-63 | Inadequate sample size (< 10 eyes) |
| Matsumoto H, Kishi S, Sato T, Mukai R. Fundus autofluorescence of elongated photoreceptor outer segments in central serous chorioretinopathy. Am J Ophthalmol 2011;151:617–23. | Sensitivity of FAF imaging not reported, not calculable |
| Maurizio BP, Pierluigi I, Stelios K, Stefano V, Marialucia C, Ilaria Z, et al. Retro-mode imaging and fundus autofluorescence with scanning laser ophthalmoscope of retinal dystrophies. BMC Ophthalmol 2012;12:8 | No appropriate reference standard |
| McBain VA, Townend J, Lois N. Fundus autofluorescence in exudative age-related macular degeneration. Br J Ophthalmol 2007;91:491–6 | Sensitivity of FAF imaging not reported, not calculable |
| McBain VA, Kumari R, Townend J, Lois N. Geographic atrophy in retinal angiomatous proliferation. Retina 2011;31:1043–52 | Sensitivity of FAF imaging not reported, not calculable |
| Mehta H, Davidson A, Devary S, Egan C, Hykin P, Moore A, et al. Autofluorescence and spectral-domain OCT findings in an atrophic maculopathy associated with pseudoxanthoma elasticum. Clin Experiment Ophthalmol 2012;40(Suppl. 1):49–50 (meeting abstract) | Sensitivity of FAF imaging not reported, not calculable |
| Meleth AD, Mettu P, Agron E, Chew EY, Sadda SR, Ferris FL, et al. Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Invest Ophthalmol Vis Sci 2011;52:1119–26 | Sensitivity of FAF imaging not reported, not calculable |
| Mendis R, Lois N. Healing of the retinal pigment epithelium (RPE) imaged ‘in vivo’ in patients. Clin Exp Ophthalmol 2011;39(Suppl. 1):69–70 (meeting abstract) | Sensitivity of FAF imaging not reported, not calculable |
| Mesquida M, Llorenc V, Fontenla JR, Navarro MJ, Adan A. Use of ultra-wide-field retinal imaging in the management of active Behcet retinal vasculitis. Retina 2014;34:2121–7 | Sensitivity of FAF imaging not reported, not calculable |
| Meyerle CB, Smith RT, Barbazetto IA, Yannuzzi LA. Autofluorescence of basal laminar drusen. Retina 2007;27:1101–6 | Inadequate sample size (< 10 eyes) |
| Midena E, Vujosevic S, Convento E, Manfre’ A, Cavarzeran F, Pilotto E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol 2007;91:1499–1503 | Sensitivity of FAF imaging not reported, not calculable |
| Mones J, Biarnes M, Trindade F. Hyporeflective wedge-shaped band in geographic atrophy secondary to age-related macular degeneration: an underreported finding. Ophthalmology 2012;119:1412–19 | Sensitivity of FAF imaging not reported, not calculable |
| Muqit MM, Gray JC, Marcellino GR, Henson DB, Young LB, Charles SJ, et al. Fundus autofluorescence and fourier-domain optical coherence tomography imaging of 10 and 20 millisecond Pascal retinal photocoagulation treatment. Br J Ophthalmol 2009;93:518–25 | Sensitivity of FAF imaging not reported, not calculable |
| Murakami T, Akimoto M, Ooto S, Suzuki T, Ikeda H, Kawagoe N, et al. Association between abnormal autofluorescence and photoreceptor disorganization in retinitis pigmentosa. Am J Ophthalmol 2008;145:687–94 | Sensitivity of FAF imaging not reported, not calculable |
| Oh J, Kim SW, Kwon SS, Oh IK, Huh K. Correlation of fundus autofluorescence gray values with vision and microperimetry in resolved central serous chorioretinopathy. Invest Ophthalmol Vis Sci 2012;53:179–84 | Sensitivity of FAF imaging not reported, not calculable |
| Ojima A, Iida T, Sekiryu T, Maruko I, Sugano Y. Photopigments in central serous chorioretinopathy. Am J Ophthalmol 2011;151:94–152 | Sensitivity of FAF imaging not reported, not calculable |
| Olsen TW. The Minnesota Grading System using fundus autofluorescence of eye bank eyes: a correlation to age-related macular degeneration (an AOS thesis). Trans Am Ophthalmol Soc 2008;106:383–401 | Sensitivity of FAF imaging not reported, not calculable |
| Ooto S, Ellabban AA, Ueda-Arakawa N, Oishi A, Tamura H, Yamashiro K, et al. Reduction of retinal sensitivity in eyes with reticular pseudodrusen. Am J Ophthalmol 2013;156:1184–91 | Sensitivity of FAF imaging not reported, not calculable |
| Pang CE, Shah VP, Sarraf D, Freund KB. Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am J Ophthalmol 2014;158:362–71 | Sensitivity of FAF imaging not reported, not calculable |
| Pang CE, Freund KB. Ghost maculopathy: an artifact on near-infrared reflectance and multicolor imaging masquerading as chorioretinal pathology. Am J Ophthalmol 2014;158:171–8 | Not a retinal condition |
| Park B, Kim J, Chung H, Kim HC. Correlation of fundus autofluorescence with foveal microstructures and vision in branch retinal vein occlusion. Retina 2014;34:531–8 | Sensitivity of FAF imaging not reported, not calculable |
| Park SP, Siringo FS, Pensec N, Hong IH, Sparrow J, Barile G, et al. Comparison of fundus autofluorescence between fundus camera and confocal scanning laser ophthalmoscope-based systems. Ophthalmic Surg Lasers Imaging Retina 2013;44:536–43 | Sensitivity of FAF imaging not reported, not calculable |
| Parodi MB, Iacono P, Ravalico G. Fundus autofluorescence in subfoveal choroidal neovascularisation secondary to Pathological Myopia. Br J Ophthalmol 2009;93:771–4 | Sensitivity of FAF imaging not reported, not calculable |
| Pece A, Isola V, Holz F, Milani P, Brancato R. Autofluorescence imaging of cystoid macular edema in diabetic retinopathy. Ophthalmologica 2010;224:230–5 | Sensitivity of FAF imaging not reported, not calculable |
| Peng Q, Dong Y, Zhao PQ. Fundus autofluorescence in exudative age-related macular degeneration. Genet Mol Res 2013;12:6140–8 | Sensitivity of FAF imaging not reported, not calculable |
| Peng X-J, Su L-P. Characteristics of fundus autofluorescence in cystoid macular edema. Chin Med J 2011;124:253–7 | Sensitivity of FAF imaging not reported, not calculable |
| Pichi F, Morara M, Veronese C, Nucci P, Ciardella AP. Multimodal imaging in hereditary retinal diseases. J Ophthalmol 2013;2013 | Sensitivity of FAF imaging not reported, not calculable |
| Pilotto E, Sportiello P, Alemany-Rubio E, Vujosevic S, Segalina S, Fregona I, et al. Confocal scanning laser ophthalmoscope in the retromode imaging modality in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2013;251:27–34 | Sensitivity of FAF imaging not reported, not calculable |
| Pilotto E, Vujosevic S, Grgic VA, Sportiello P, Convento E, Secchi AG, et al. Retinal function in patients with serpiginous choroiditis: a microperimetry study. Graefes Arch Clin Exp Ophthalmol 2010;248:1331–7 | Inadequate sample size (< 10 eyes) |
| Pilotto E, Guidolin F, Convento E, Spedicato L, Vujosevic S, Cavarzeran F, et al. Fundus autofluorescence and microperimetry in progressing geographic atrophy secondary to age-related macular degeneration. Br J Ophthalmol 2013;97:62–6 | No appropriate reference standard |
| Pilotto E, Vujosevic S, Melis R, Convento E, Sportiello P, Alemany-Rubio E, et al. Short wavelength fundus autofluorescence versus near-infrared fundus autofluorescence, with microperimetric correspondence, in patients with geographic atrophy due to age-related macular degeneration. Br J Ophthalmol 2011;95:1140–4 | No appropriate reference standard |
| Pryds A, Larsen M. Foveal function and thickness after verteporfin photodynamic therapy in central serous chorioretinopathy with hyperautofluorescent subretinal deposits. Retina 2013;33:128–35 | Sensitivity of FAF imaging not reported, not calculable |
| Puche N, Querques G, Blanco-Garavito R, Zerbib J, Gherdaoui F, Tilleul J, et al. En face enhanced depth imaging optical coherence tomography features in adult onset foveomacular vitelliform dystrophy. Graefes Arch Clin Exp Ophthalmol 2014;252:555–62 | No appropriate index test |
| Querques G, Zerbib J, Georges A, Massamba N, Forte R, Querques L, et al. Multimodal analysis of the progression of Best vitelliform macular dystrophy. Mol Vis 2014;20:575–92 | Sensitivity of FAF imaging not reported, not calculable |
| Querques G, Leveziel N, Benhamou N, Voigt M, Soubrane G, Souied EH. Analysis of retinal flecks in fundus flavimaculatus using optical coherence tomography. Br J Ophthalmol 2006;90:1157–62 | Sensitivity of FAF imaging not reported, not calculable |
| Querques G, Querques L, Forte R, Massamba N, Blanco R, Souied EH. Precursors of type 3 neovascularization: a multimodal imaging analysis. Retina 2013;33:1241–8 | Sensitivity of FAF imaging not reported, not calculable |
| Querques L, Querques G, Forte R, Souied E. Microperimetric correlations of autofluorescence and optical coherence tomography imaging in dry age-related macular degeneration. Am J Ophthalmol 2012;153:1110–15 | Sensitivity of FAF imaging not reported, not calculable |
| Querques G, Guigui B, Leveziel N, Querques L, Coscas G, Soubrane G, et al. Insights into pathology of cuticular drusen from integrated confocal scanning laser ophthalmoscopy imaging and corresponding spectral domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 2011;249:1617–25 | Sensitivity of FAF imaging not reported, not calculable |
| Querques G, Querques L, Martinelli D, Massamba N, Coscas G, Soubrane G, et al. Pathologic insights from integrated imaging of reticular pseudodrusen in age-related macular degeneration. Retina 2011;31:518–26 | Sensitivity of FAF imaging not reported, not calculable |
| Querques G, Atmani K, Bouzitou-Mfoumou R, Leveziel N, Massamba N, Souied EH. Preferential hyperacuity perimeter in best vitelliform macular dystrophy. Retina 2011;31:959–66 | Sensitivity of FAF imaging not reported, not calculable |
| Renner AB, Kellner U, Cropp E, Preising MN, MacDonald IM, van den Hurk JA, et al. Choroideremia: variability of clinical and electrophysiological characteristics and first report of a negative electroretinogram. Ophthalmology 2006;113:2066e.1–10 | Sensitivity of FAF imaging not reported, not calculable |
| Renner AB, Kellner U, Fiebig B, Cropp E, Foerster MH, Weber BH. ERG variability in X-linked congenital retinoschisis patients with mutations in the RS1 gene and the diagnostic importance of fundus autofluorescence and OCT. Doc Ophthalmol 2008;116:97–109 | Sensitivity of FAF imaging not reported, not calculable |
| Reznicek L, Seidensticker F, Stumpf C, Kampik A, Thurau S, Kernt M, et al. Systematic analysis of wide-field fundus autofluorescence (FAF) imaging in posterior uveitis. Curr Eye Res 2014;39:164–71 | Sensitivity of FAF imaging not reported, not calculable |
| Reznicek L, Dabov S, Haritoglou C, Kampik A, Kernt M, Neubauer AS. Green-light fundus autofluorescence in diabetic macular edema. Int J Ophthalmol 2013;6:75–80 | Sensitivity of FAF imaging not reported, not calculable |
| Reznicek L, Dabov S, Kayat B, Liegl R, Kampik A, Ulbig M, et al. Scanning laser ‘en face’ retinal imaging of epiretinal membranes. Saudi J Ophthalmol 2014;28:134–8 | Sensitivity of FAF imaging not reported, not calculable |
| Reznicek L, Wasfy T, Stumpf C, Kampik A, Ulbig M, Neubauer AS, et al. Peripheral fundus autofluorescence is increased in age-related macular degeneration. Invest Ophthalmol Vis Sci 2012;53:2193–8 | No appropriate reference standard |
| Robson AG, Lenassi E, Saihan Z, Luong VA, Fitzke FW, Holder GE, et al. Comparison of fundus autofluorescence with photopic and scotopic fine matrix mapping in patients with retinitis pigmentosa: 4- to 8-year follow-up. Invest Ophthalmol Vis Sci 2012;53:6187–95 | Sensitivity of FAF imaging not reported, not calculable |
| Robson AG, Tufail A, Fitzke F, Bird AC, Moore AT, Holder GE, et al. Serial imaging and structure-function correlates of high-density rings of fundus autofluorescence in retinitis pigmentosa. Retina 2011;31:1670–9 | Sensitivity of FAF imaging not reported, not calculable |
| Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 2013;120:2292–9 | Sensitivity of FAF imaging not reported, not calculable |
| Roisman L, Lavinsky D, Magalhaes F, Aggio FB, Moraes N, Cardillo JA, et al. Fundus autofluorescence and spectral domain OCT in central serous chorioretinopathy. J Ophthalmol 2011;2011 | Sensitivity of FAF imaging not reported, not calculable |
| Sallo FB, Rechtman E, Peto T, Stanescu-Segall D, Vogt G, Bird AC, et al. Functional aspects of drusen regression in age-related macular degeneration. Br J Ophthalmol 2009;93:1345–50 | Sensitivity of FAF imaging not reported, not calculable |
| Sarks J, Arnold J, Ho I-V, Sarks S, Killingsworth M. Evolution of reticular pseudodrusen. Br J Ophthalmol 2011;95:979–85 | Sensitivity of FAF imaging not reported, not calculable |
| Sayegh RG, Simader C, Scheschy U, Montuoro A, Kiss C, Sacu S, et al. A systematic comparison of spectral-domain optical coherence tomography and fundus autofluorescence in patients with geographic atrophy. Ophthalmology 2011;118:1844–51 | Sensitivity of FAF imaging not reported, not calculable |
| Schachar IH, Zahid S, Comer GM, Stem M, Schachar AG, Saxe SJ, et al. Quantification of fundus autofluorescence to detect disease severity in nonexudative age-related macular degeneration. JAMA Ophthalmol 2013;131:1009–15 | Sensitivity of FAF imaging not reported, not calculable |
| Schmitz-Valckenberg S, Fleckenstein M, Gobel AP, Sehmi K, Fitzke FW, Holz FG, et al. Evaluation of autofluorescence imaging with the scanning laser ophthalmoscope and the fundus camera in age-related geographic atrophy. Am J Ophthalmol 2008;146:183–92 | No appropriate reference standard |
| Schmitz-Valckenberg S, Bultmann S, Dreyhaupt J, Bindewald A, Holz FG, Rohrschneider K. Fundus autofluorescence and fundus perimetry in the junctional zone of geographic atrophy in patients with age-related macular degeneration.[Erratum published in Invest Ophthalmol Vis Sci 2005;46:7]. Invest Ophthalmol Vis Sci 2004;45:4470–6 | No appropriate reference standard |
| Schmitz-Valckenberg S, Fleckenstein M, Gobel AP, Hohman TC, Holz FG. Optical coherence tomography and autofluorescence findings in areas with geographic atrophy due to age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:1–6 | Sensitivity of FAF imaging not reported, not calculable |
| Schmitz-Valckenberg S, Jorzik J, Unnebrink K, Holz FG, FAM study group. Analysis of digital scanning laser ophthalmoscopy fundus autofluorescence images of geographic atrophy in advanced age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2002;240:73–8 | No appropriate reference standard |
| Schmitz-Valckenberg S, Bindewald-Wittich A, Dolar-Szczasny J, Dreyhaupt J, Wolf S, Scholl HP, et al. Correlation between the area of increased autofluorescence surrounding geographic atrophy and disease progression in patients with AMD. Invest Ophthalmol Vis Sci 2006;47:2648–54 | No appropriate reference standard |
| Schmitz-Valckenberg S, Alten F, Steinberg JS, Jaffe GJ, Fleckenstein M, Mukesh BN, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:5009–15 | No appropriate reference standard |
| Schutze C, Bolz M, Sayegh R, Baumann B, Pircher M, Gotzinger E, et al. Lesion size detection in geographic atrophy by polarization-sensitive optical coherence tomography and correlation to conventional imaging techniques. Invest Ophthalmol Vis Sci 2013;54:739–45 | Sensitivity of FAF imaging not reported, not calculable |
| Sekiryu T, Iida T, Maruko I, Saito K, Kondo T. Infrared fundus autofluorescence and central serous chorioretinopathy. Invest Ophthalmol Vis Sci 2010;51:4956–62 | Sensitivity of FAF imaging not reported, not calculable |
| Shah VP, Shah SA, Mrejen S, Freund KB. Subretinal hyperreflective exudation associated with neovascular age-related macular degeneration. Retina 2014;34:1281–8 | Sensitivity of FAF imaging not reported, not calculable |
| Shen Y, Xu X, Liu K. Fundus autofluorescence characteristics in patients with diabetic macular edema. Chin Med J 2014;127:1423–8 | Sensitivity of FAF imaging not reported, not calculable |
| Silva R, Cachulo ML, Fonseca P, Bernardes R, Nunes S, Vilhena N, et al. Age-related macular degeneration and risk factors for the development of choroidal neovascularisation in the fellow eye: a 3-year follow-up study. Ophthalmologica 2011;226:110–18 | Sensitivity of FAF imaging not reported, not calculable |
| Simader C, Sayegh RG, Montuoro A, Azhary M, Koth AL, Baratsits M, et al. A longitudinal comparison of spectral-domain optical coherence tomography and fundus autofluorescence in geographic atrophy. Am J Ophthalmol 2014;158:557–66 | Sensitivity of FAF imaging not reported, not calculable |
| Smith RT, Sohrab MA, Busuioc M, Barile G. Reticular macular disease. Am J Ophthalmol 2009;148:733–43 | No appropriate reference standard |
| Spaide RF, Klancnik JM Jr. Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology 2005;112:825–33 | Sensitivity of FAF imaging not reported, not calculable |
| Suzuki M, Gomi F, Sawa M, Ueno C, Nishida K. Changes in fundus autofluorescence in polypoidal choroidal vasculopathy during 3 years of follow-up. Graefes Arch Clin Exp Ophthalmol 2013;251:2331–7 | Sensitivity of FAF imaging not reported, not calculable |
| Tan CS, Heussen F, Sadda SR. Peripheral autofluorescence and clinical findings in neovascular and non-neovascular age-related macular degeneration. Ophthalmology 2013;120:1271–7 | Sensitivity of FAF imaging not reported, not calculable |
| Teke MY, Elgin U, Nalcacioglu-Yuksekkaya P, Sen E, Ozdal P, Ozturk F. Comparison of autofluorescence and optical coherence tomography findings in acute and chronic central serous chorioretinopathy. Int J Ophthalmol 2014;7:350–4 | Sensitivity of FAF imaging not reported, not calculable |
| Toju R, Iida T, Sekiryu T, Saito M, Maruko I, Kano M. Near-infrared autofluorescence in patients with idiopathic submacular choroidal neovascularization. Am J Ophthalmol 2012;153:314–19 | Sensitivity of FAF imaging not reported, not calculable |
| Toy BC, Krishnadev N, Indaram M, Cunningham D, Cukras CA, Chew EY, et al. Drusen regression is associated with local changes in fundus autofluorescence in intermediate age-related macular degeneration. Am J Ophthalmol 2013;56:532–42 | Sensitivity of FAF imaging not reported, not calculable |
| Tsakonas GD, Kotsolis AI, Koutsandrea C, Georgalas I, Papaconstantinou D, Ladas ID. Multiple spots of photodynamic therapy for the treatment of severe chronic central serous chorioretinopathy. Clin Ophthalmol 2012;6:1639–44 | Sensitivity of FAF imaging not reported, not calculable |
| Vaclavik V, Vujosevic S, Dandekar SS, Bunce C, Peto T, Bird AC. Autofluorescence imaging in age-related macular degeneration complicated by choroidal neovascularization: a prospective study. Ophthalmology 2008;115:342–6 | Sensitivity of FAF imaging not reported, not calculable |
| Vidinova CN, Gouguchkova PT, Vidinov KN. Fundus autofluorescence in dry AMD – impact on disease progression. Klin Monatsbl Augenheilkd 2013;230:1135–41 | Non-English language |
| von Ruckmann A, Fitzke FW, Fan J, Halfyard A, Bird AC. Abnormalities of fundus autofluorescence in central serous retinopathy. Am J Ophthalmol 2002;133:780–6 | Sensitivity of FAF imaging not reported, not calculable |
| von Ruckmann A, Fitzke FW, Bird AC. In vivo fundus auto fluorescence in macular dystrophies. Arch Ophthalmol 1997;115:609–15 | Sensitivity of FAF imaging not reported, not calculable |
| Vujosevic S, Vaclavik V, Bird AC, Leung I, Dandekar S, Peto T. Combined grading for choroidal neovascularisation: colour, fluorescein angiography and autofluorescence images. Graefes Arch Clin Exp Ophthalmol 2007;245:1453–60 | Sensitivity of FAF imaging not reported, not calculable |
| Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 2010;30:908–16 | Sensitivity of FAF imaging not reported, not calculable |
| Vujosevic S, Casciano M, Pilotto E, Boccassini B, Varano M, Midena E. Diabetic macular edema: fundus autofluorescence and functional correlations. Invest Ophthalmol Vis Sci 2011;52:442–8 | Sensitivity of FAF imaging not reported, not calculable |
| Wakabayashi T, Sawa M, Gomi F, Tsujikawa M. Correlation of fundus autofluorescence with photoreceptor morphology and functional changes in eyes with retinitis pigmentosa. Acta Opthalmol 2010;88:e177–83 | Sensitivity of FAF imaging not reported, not calculable |
| Wang Q, Jiang L. Fundus autofluorescence imaging of acute zonal occult outer retinopathy. Doc Ophthalmol 2013;127:25 (meeting abstract) | Sensitivity of FAF imaging not reported, not calculable |
| Weinberger AW, Lappas A, Kirschkamp T, Mazinani BA, Huth JK, Mohammadi B, et al. Fundus near infrared fluorescence correlates with fundus near infrared reflectance. Invest Ophthalmol Vis Sci 2006;47:3098–108 | Sensitivity of FAF imaging not reported, not calculable |
| Wolf-Schnurrbusch UE, Enzmann V, Brinkmann CK, Wolf S. Morphologic changes in patients with geographic atrophy assessed with a novel spectral OCT-SLO combination. Invest Ophthalmol Vis Sci 2008;49:3095–9 | Sensitivity of FAF imaging not reported, not calculable |
| Wong WT, Forooghian F, Majumdar Z, Bonner RF, Cunningham D, Chew EY. Fundus autofluorescence in type 2 idiopathic macular telangiectasia: correlation with optical coherence tomography and microperimetry. Am J Ophthalmol 2009;148:573–83 | Sensitivity of FAF imaging not reported, not calculable |
| Yoshitake S, Murakami T, Horii T, Uji A, Ogino K, Unoki N, et al. Qualitative and quantitative characteristics of near-infrared autofluorescence in diabetic macular edema. Ophthalmology 2014;121:1036–44 | Sensitivity of FAF imaging not reported, not calculable |
Appendix 5 Data extraction tables
List of abbreviations
- AF
- autofluorescence
- AMD
- age-related macular degeneration
- CBR
- confocal blue reflectance
- CFP
- colour fundus photography
- CMO
- cystoid macular oedema
- CSC
- central serous chorioretinopathy
- cSLO
- confocal scanning laser ophthalmoscopy
- DMO
- diabetic macular oedema
- DR
- diabetic retinopathy
- FA
- fluorescein angiography
- FAF
- fundus autofluorescence
- GA
- geographic atrophy
- ICGA
- indocyanine green angiography
- IR
- infrared reflectance
- MPOD
- macular pigment optical density
- NIR-FAF
- near-infrared fundus autofluorescence
- NPV
- negative predictive value
- OCT
- optical coherence tomography
- PPV
- positive predictive value
- QUADAS
- Quality Assessment of Diagnostic Accuracy Studies instrument
- RF
- red-free (photography)
- RM-SLO
- retromode scanning laser ophthalmoscopy
- RNIB
- Royal National Institute of Blind People
- RPD
- reticular pseudodrusen
- RPE
- retinal pigment epithelium
- SD-OCT
- spectral domain optical coherence tomography
- SLO
- scanning laser ophthalmoscopy
- TD-OCT
- time domain optical coherence tomography