Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/41/02. The contractual start date was in March 2012. The draft report began editorial review in September 2013 and was accepted for publication in May 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Griffin et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Management of hip pain in young adults
Until recently, the management of hip pain in young adults has been largely conservative. A minority of such patients had established osteoarthritis, inflammatory arthritis, avascular necrosis or fractures, and their care sometimes included surgery. However, the majority had no specific diagnosis and received multidisciplinary non-operative care, provided by a combination of physiotherapists (probably the largest contribution), rheumatologists, orthopaedic surgeons, sport and exercise medicine physicians and general practitioners.
Femoroacetabular impingement
In the last few years there has been increasing recognition of the syndrome of femoroacetabular impingement (FAI), which seems to account for a large proportion of the previously undiagnosed cases of hip pain in young adults. 1,2 Subtle deformities of the hip combine to cause impingement between femoral neck and anterior rim of the acetabulum, most often in flexion and internal rotation. 2 The deformities may include asphericity of the femoral head, widening of the femoral neck, overcoverage of the anterosuperior acetabular wall and abnormal version of the femur or acetabulum. 2 Excess contact forces between the proximal femur and the acetabular rim during terminal motion of the hip lead to lesions of acetabular labrum and the adjacent acetabular cartilage. 2 FAI seems to be associated with progressive articular degeneration of the acetabulum, usually starting from the anterosuperior rim and accelerating medially and posteriorly. 1–3
Open surgery for femoroacetabular impingement
In 2001, Ganz et al. 4 described a surgical technique to dislocate a hip joint without damaging the blood supply to the femoral head. This allowed the development of surgical techniques to correct the shape abnormalities of FAI. The technique involved a major operation, with a trochanteric osteotomy and a prolonged period on crutches. Ganz et al. 4 described a total of 213 surgical hip dislocations, in 164 cases of which the indication was FAI. Subsequently, there was a gradual development of interest in the international orthopaedic community in the problem of hip pain in young adults and an increasing recognition of FAI. Improved imaging, especially magnetic resonance imaging (MRI)/magnetic resonance arthrography, allowed more confident diagnosis of FAI,5,6 and surgeons began to visit Ganz to learn the technique. Observational case series have been published describing good clinical results in terms of pain and function for surgical dislocation for FAI;7 however, only a few centres began to offer this treatment for patients, perhaps because the likely benefit, as perceived by surgeons and patients, was insufficient to justify the invasiveness and risks of such an extensive surgical procedure.
Arthroscopic surgery for femoroacetabular impingement
Since the early 1990s there has been a slowly developing interest in arthroscopic surgery in the hip. Confined to just a few centres around the world, it seemed to be only rarely indicated and of doubtful clinical usefulness. In the early 2000s, a few surgeons, including one of the authors of this report (DG), began to explore the possibilities of arthroscopic surgery in the management of FAI. Since then, numerous authors have published observational case series reporting favourable outcomes in terms of pain and function for the arthroscopic management of FAI. 8–10 Overall, a recent systematic review of hip arthroscopy judged the evidence to support this treatment of FAI only as ‘fair’. 11 The feasibility team have conducted a Cochrane Review on surgery for the treatment of FAI and found no published randomised controlled trials (RCTs) to help determine the efficacy and safety of surgery for FAI. 12 Four ongoing trials [including the Feasibility of Arthroscopic Surgery for Hip Impingement compared with Non-operative care (FASHIoN) study] were ongoing that when complete may be eligible for inclusion in the review and help determine the efficacy and safety of FAI surgery. The underlying message from systematic reviews is that better evidence for any sort of surgery is needed, ideally a well-designed RCT. With the majority of current literature focusing on the surgical management of FAI, there is little information about conservative (non-operative) treatment, which might form the natural comparator to surgery. 13
Likely difficulties in recruitment to a trial of treatment for femoroacetabular impingement
Pragmatic multicentre RCTs are acknowledged to be the best design for evaluating the effectiveness of health-care interventions as they provide robust evidence of effect, but they often encounter recruitment difficulties. 14–17 RCTs in surgery face particular challenges including that many surgeons have limited experience of RCTs, there are often learning curves for particular procedures, surgeons sometimes adopt idiosyncratic individual techniques, and the natural comparison might be a very different and more conservative type of management. 18,19 In order to participate in RCTs, all clinicians involved (surgeons and physiotherapists) need to accept at least collective uncertainty or equipoise between treatments, including the possibility that surgery is no more effective than best conservative care. For patients, the idea that there is uncertainty over the comparative effectiveness of surgical treatments and conservative care can be very difficult to accept. Lack of both clinician and patient equipoise could be major barriers to recruitment to a trial of surgery versus conservative care for FAI, especially as many patients may well feel that they have already had a period of conservative care. Trials comparing orthopaedic surgery with conservative care show widely varying recruitment rates.
-
A trial of surgery compared with conservative treatment for carpal tunnel syndrome found that 201 patients refused to enter any study and a further 207 refused to enter the trial but would enter an observational study. A total of 116 patients were randomised. Therefore, the recruitment rate for the trial was 22%. 20
-
A trial of vertebroplasty compared with conservative treatment in acute osteoporotic vertebral compression fractures recruited 202 of 479 eligible patients (42%). 21
-
A trial of arthroscopic surgery compared with physiotherapy for osteoarthritis of the knee recruited 188 patients out of 219 (86%). 22
Qualitative research methods can be used to understand recruitment difficulties and inform the development of strategies to improve recruitment to RCTs. 23–25 In the Health Technology Assessment (HTA)-funded ProtecT (Prostate testing for cancer and Treatment) study, for example, the findings from an integrated qualitative study led to the rate of randomisation of eligible participants rising from 30% to 70% over a 12-month period. 23 The ProtecT trial was expected to be challenging for recruitment for reasons including strong treatment preferences by patients and clinicians. The qualitative research integrated within a feasibility study26 enabled a nuanced understanding of the recruitment process from the perspectives of potential participants, clinicians and triallists, including reasons for treatment preferences and unexpected misinterpretations of information. 23 Strategies were developed that led to improvements in levels of randomisation and informed consent. The research methods used in the ProtecT study were then developed into a complex recruitment intervention,27 which has been applied to several other RCTs in different contexts, leading to insights about recruitment issues and the development of targeted recruitment strategies. 24,25 This research is a major theme of the Medical Research Council (MRC) Collaboration and Innovation in Difficult and Complex Randomised Controlled Trials methodology hub, which specialises in working with RCTs likely to be challenging for recruitment, such as this study.
Purpose of this study
Theoretical arguments have been made that surgery for FAI may prevent the development of osteoarthritis,1,4 but there is little evidence for this. Surgery might be indicated to relieve symptoms, but an equally strong argument can be made that a well-constructed regime of conservative care will reduce the symptoms of FAI.
Femoroacetabular impingement surgery, including arthroscopic surgery, has evolved quickly, more quickly than our understanding of the natural history of FAI,28–30 so it is now not clear whether or not surgery offers real benefit over conservative care. A RCT of arthroscopic surgery compared with conservative care for FAI is appropriate, prompting interest from the National Institute for Health Research (NIHR) HTA programme in funding such a trial.
However, this is a rapidly developing field, currently practised by relatively few surgeon innovators and specialists in conservative care; these groups may not be in equipoise, and this presents special problems in the performance of a trial. 18 In addition, patients are likely to have strong views about whether they would prefer surgical or conservative treatment, raising questions about their preparedness to be randomised.
The research question for this feasibility study was whether or not a substantive RCT of hip arthroscopy compared with conservative care for FAI could succeed and, if so, how best it might be designed. It included a pilot RCT to test the proposed processes of a substantive trial and, crucially, to estimate the rate of recruitment.
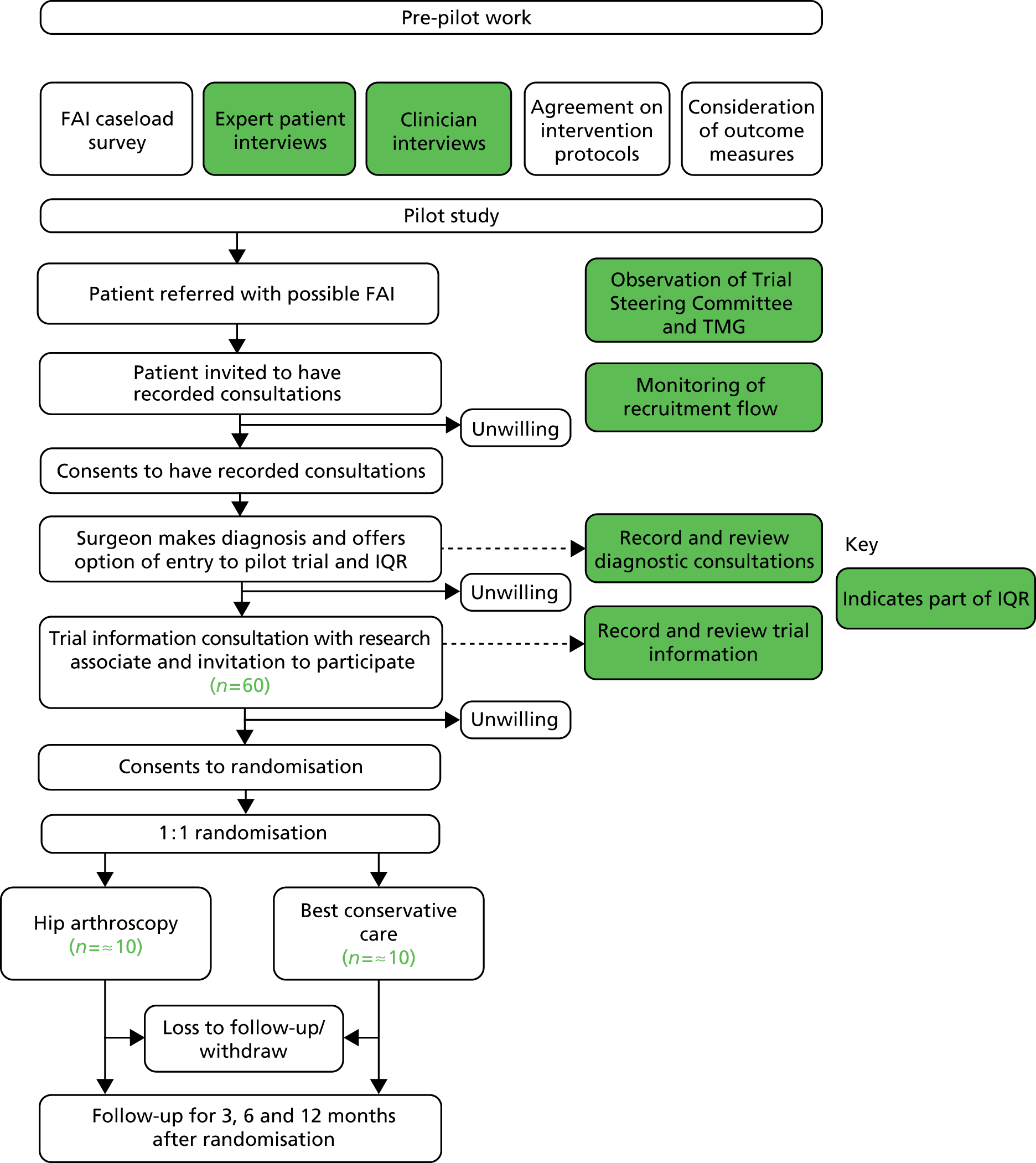
The research included several related studies, performed in two stages. Pre-pilot studies (see Chapter 2) estimated the number and distribution of patients available for a full trial in the UK, explored clinicians’ and patients’ attitudes towards a trial, developed a consensus among clinicians who manage patients with FAI for eligibility criteria, best conservative care and surgical protocols, examined possible outcome measures and estimated sample size for a full trial, and worked with patients to develop patient information for a trial. A pilot RCT (see Chapter 3) was then performed in order to estimate recruitment rates for a full trial. During the pilot RCT, an integrated qualitative study (see Chapter 4) of both patients and clinicians was performed to determine the barriers to, and develop solutions for, completing recruitment to a full RCT. The feasibility study design is summarised in Figure 1.
FIGURE 1.
Overview of the feasibility study design.
Chapter 2 Pre-pilot study
Objectives
The objectives of the pre-pilot study were to:
-
estimate the annual number of patients offered hip arthroscopy for FAI in the UK (see Hip arthroscopy for femoroacetabular impingement in the NHS in the UK: a workload survey)
-
explore clinicians’ (see Clinicians’ attitudes to randomisation of femoroacetabular impingement patients) and patients’ attitudes (see Develop eligibility criteria for a randomised controlled trial and design an operative protocol) to recruitment into a RCT of FAI treatments
-
develop a consensus for eligibility criteria for eligibility criteria, an operative care protocol and a best conservative care protocol among clinicians who manage patients with FAI (see Design of a best conservative care treatment protocol for femoroacetabular impingement and Possible outcome measures and sample size for a full randomised controlled trial)
-
consider possible outcome measures and estimate the sample size for a full trial (see Patients’ attitudes towards randomisation and design of patient information material for a randomised controlled trial)
-
develop trial procedures and patient information material to maximise recruitment rate to a RCT of arthrocopic surgery versus best conservative care for FAI section 2.7.
Hip arthroscopy for femoroacetabular impingement in the NHS in the UK: a workload survey
Introduction
The prevalence in the general population of symptomatic FAI (FAI hip shape morphology and concurrent hip symptoms) is not known. Similarly the proportion of these patients who require surgery is not known. The published literature has an overwhelming focus on the surgical management of FAI. 1,31,32 Obtaining an understanding of the amount of surgery that is being undertaken for FAI would help to understand the burden of symptomatic FAI and help to determine the likely pool of eligible patients for a RCT.
Objective
To estimate the frequency and types of FAI surgery being undertaken in the UK within the NHS.
Materials and methods
A list of all NHS hospital health boards and trusts within England, Northern Ireland, Scotland and Wales was compiled using the NHS online resource: www.nhs.uk. All hospital trusts and health boards were contacted by telephone to determine if they had an orthopaedic service and within each orthopaedic service the number of orthopaedic departments that made up that service. Clinical directors for these departments/services were then contacted by letter requesting the names and contact details of all FAI surgeons within their department/service. Each FAI surgeon identified received a letter requesting the following workload data for the financial year 2011/12:
-
hip arthroscopies performed within the NHS over the last 12 months
-
number of hip arthroscopies performed for FAI within the NHS over the last 12 months
-
number of open surgical procedures performed for FAI within the NHS over the last 12 months.
Clinical directors and surgeons were contacted repeatedly by letter, e-mail and telephone and through their secretaries. When a consultant’s practice spanned less than 12 months, the results were not rescaled; instead, conservative estimates were obtained for the full 12 months by keeping case numbers the same, no matter the period of practice. When consultants provided a range, this was recorded and final calculations were made based on the lowest figure. Each surgeon returning data was assigned a postcode for their NHS practice and this was used to create a choropleth map of the workload data based on regions within the UK. Prevalence rates for surgery were then calculated for each region per 100,000 population using mid-2010 population estimates from the Office for National Statistics (ONS). 33 Consultants who did not consider themselves as FAI specialty surgeons were removed from the database. Consultants who considered themselves to be FAI surgeons but were not currently performing this type of surgery within their NHS practice were kept on the database, with reasons for this recorded (e.g. no current funding for the procedure). Anyone who was considered a FAI surgeon but did not want to participate in the study was removed from the database. Data collection was undertaken over a 6-month period between May and October 2012.
In order to triangulate and validate the FAI surgical workload data obtained by survey, NHS Hospital Episode Statistics (HES) data were obtained for procedures undertaken in 2011/12 using Office of Population Censuses and Surveys Classification of Interventions and Procedures-Fourth Edition (OPCS-4) codes within England. OPCS is a procedural classification for the coding of operations, procedures and interventions performed during inpatient stays, day case surgery and some outpatient attendances in the NHS. OPCS-4 is an alphanumeric nomenclature, with a four-character code system. The code system can also be combined to provide further detail. Specific procedure codes for FAI surgery have not yet been established.
In the absence of any established OPCS-4 codes for FAI surgery, the codes currently agreed and applied to FAI surgery within University Hospitals Coventry and Warwickshire (UHCW) were used: Z843, which represents surgery on the hip joint, combined with W844, endoscopic decompression of joint, or W802, open debridement of joint.
Validation of the HES data was undertaken using an independently locally collected database for FAI surgery at UHCW.
International Business Machines (IBM) Statistical Product and Service Solutions (SPSS) Statistics (IBM Corporation, Armonk, NY, USA) version 21 for Windows was used for the statistical analysis. Summary statistics including mean [with confidence intervals (CIs)] and median (with interquartile ranges) values were reported for the data. Differences in workload data between arthroscopic and open surgery were analysed using a Student’s t-test. The level of statistical significance (p-value) was set at 0.05.
Results
There were a total of 193 NHS hospital health boards and trusts in the UK. Of these, 27 did not have an orthopaedic surgical department/service. A total of 2399 cases of surgery were undertaken for FAI over the 12 months. The breakdown of the workload data (both arthroscopic and open) for FAI surgeons who responded to the survey is shown in Table 1.
| Country | Hospital health boards and NHS trusts with a FAI surgeon | FAI surgeons | Surgeons not responding (%) | Hospital health board and NHS trusts with no funding for surgery | Open FAI surgery cases over 12 months | Arthroscopic FAI cases over 12 months |
|---|---|---|---|---|---|---|
| England | 69 | 110 | 17 | 6 (8 surgeons) | 444 | 1791 |
| Scotland | 2 | 2 | 0 | 0 | 38 | 62 |
| Wales | 2 | 6 | 3 | 0 | 9 | 55 |
| Northern Ireland | 2 | 2 | 0 | 2 | 0 | 0 |
| Total | 75 | 120 | 20 (17) | 8 | 491 | 1908 |
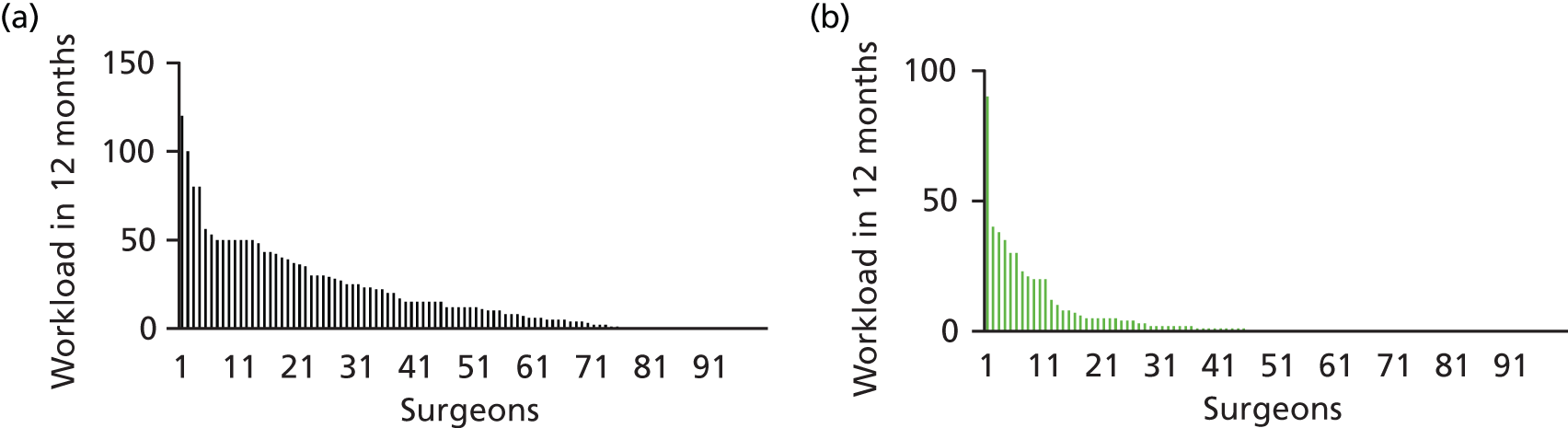
Of the 100 surgeons returning workload data, 25 did not perform any arthroscopic surgery over the 12-month period and 55 did not perform any open surgery over the 12-month period. The distributions of caseload by surgeon for open and arthroscopic surgery are shown in Figure 2 and the mean and median numbers of cases of FAI surgery per surgeon are shown in Table 2.
FIGURE 2.
Distributions of caseload by surgeon for open and arthroscopic surgery. (a) Individual surgeon workload for FAI treated arthroscopically; and (b) individual surgeon workload for FAI treated with open surgery.
| Summary statistic | Arthroscopic FAI workload per surgeon | Open FAI per surgeon | Total FAI surgery workload per surgeon |
|---|---|---|---|
| Mean (95% CI) | 19 (15 to 24) | 5 (7 to 3) | 24 (17 to 31) |
| Median (IQR) | 12 (0–30) | 0 (0–4) | 12 (0–34) |
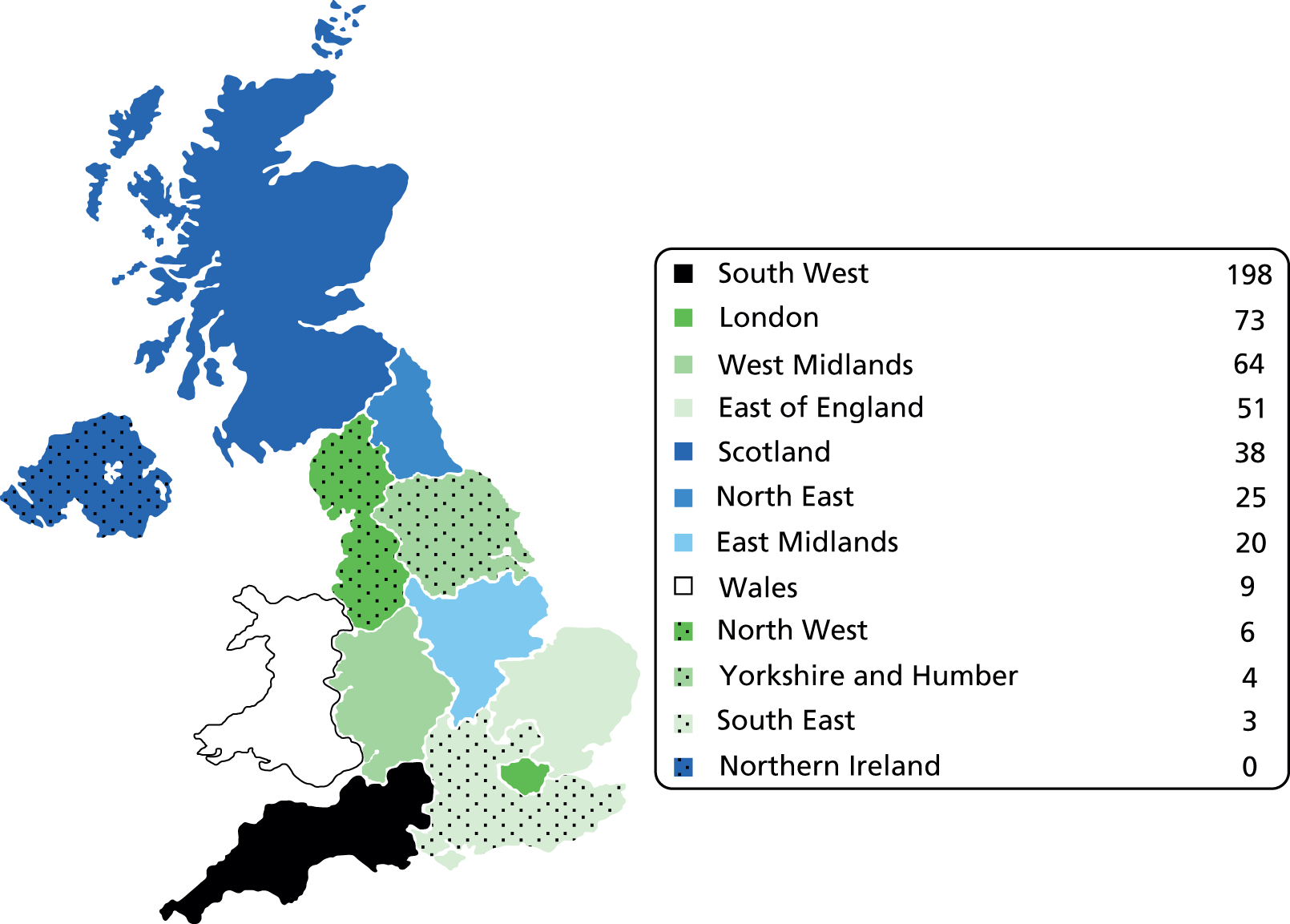
Each surgeon returning data was assigned a postcode for their NHS practice and this was used to create a choropleth map of the workload data based on regions within the UK. Figures 3 and 4 show the number of cases of FAI surgery performed by arthroscopic and open surgery, respectively, in regions of the UK.
FIGURE 3.
Choropleth map of arthroscopic surgery for FAI 2011/12. Number of cases of FAI surgery performed by arthroscopic surgery.
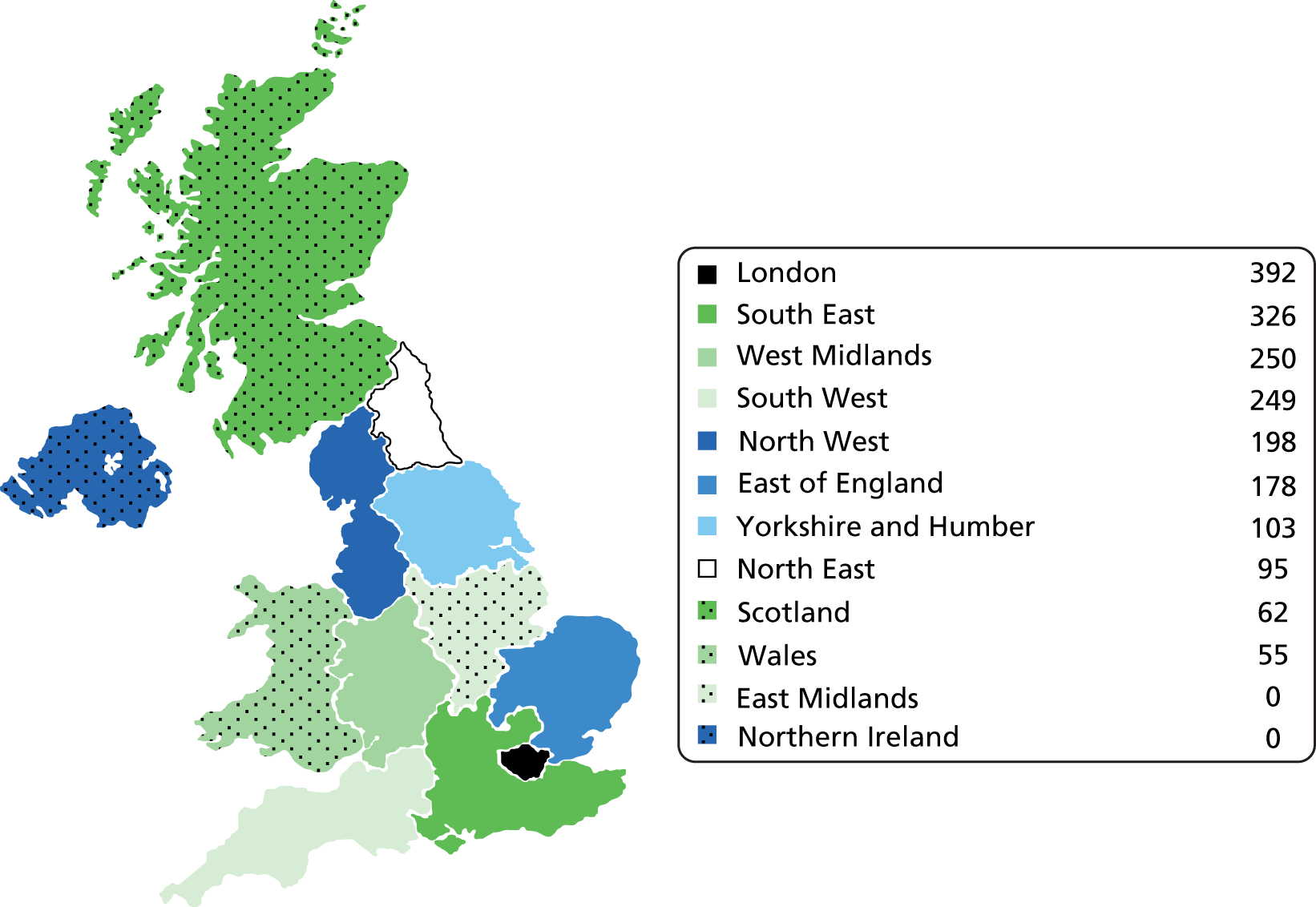
FIGURE 4.
Choropleth map of open surgery for FAI 2011/12. Number of cases of FAI surgery performed by open surgery.
Prevalence rates for FAI surgery using mid-2010 population estimates from the ONS33 are shown Table 3.
| Region | Mid-2010 population estimate33 | Arthroscopic FAI surgery cases | Arthroscopic FAI surgery per 100,000 population | Open FAI surgery cases | Open FAI surgery per 100,000 population | Total FAI workload per 100,000 population |
|---|---|---|---|---|---|---|
| London | 7,825,000 | 392 | 5.0 | 73 | 0.9 | 5.9 |
| South East | 8,523,000 | 326 | 3.8 | 3 | 0.0 | 3.9 |
| West Midlands | 5,455,000 | 250 | 4.6 | 64 | 1.2 | 5.8 |
| South West | 5,274,000 | 249 | 4.7 | 198 | 3.8 | 8.5 |
| North West | 6,936,000 | 198 | 2.9 | 6 | 0.1 | 2.9 |
| East of England | 5,832,000 | 178 | 3.1 | 51 | 0.9 | 3.9 |
| Yorkshire and Humber | 5,301,000 | 103 | 1.9 | 4 | 0.1 | 2.0 |
| North East | 2,607,000 | 95 | 3.6 | 25 | 1.0 | 4.6 |
| Scotland | 5,222,000 | 62 | 1.2 | 38 | 0.7 | 1.9 |
| Wales | 3,006,000 | 55 | 1.8 | 9 | 0.3 | 2.1 |
| East Midlands | 4,481,000 | 0 | 0.0 | 20 | 0.4 | 0.4 |
| Northern Ireland | 1,799,000 | 0 | 0.0 | 0 | 0.0 | 0.0 |
| Total | 62,261,000 | 1908 | 3.1 | 491 | 0.8 | 3.9 |
Distribution of the numbers of cases per hospital trust is shown in Table 4, combining surgeons where they worked in the same trust.
| Summary statistic | FAI surgeons per hospital trust | Arthroscopy per hospital trust per 12 months | Arthroscopy for FAI per hospital trust per 12 months | Open surgery for FAI per hospital trust per 12 months |
|---|---|---|---|---|
| Mean | 1.3 | 37.3 | 25.4 | 6.5 |
| 95% CI | 1.2 to 1.5 | 25.1 to 50.1 | 17.6 to 33.3 | 2.5 to 10.5 |
| Median | 1 | 22 | 12 | 0 |
| Interquartile range | 1 | 45 | 41 | 5 |
| Minimum and maximum | 1 and 4 | 0 and 352 | 0 and 149 | 0 and 132 |
Trusts with a workload of ≥ 10 hip arthroscopies for FAI per 12 months are summarised in Table 5.
| Hospital trust | Number of surgeons | Hip arthroscopy per 12 months | Hip arthroscopy for FAI per 12 months | Open surgery for FAI per 12 months |
|---|---|---|---|---|
| Frimley Park Hospital NHS Foundation Trust | 2 | 159 | 149 | 1 |
| Guy’s and St Thomas’ | 2 | 333 | 144 | 9 |
| Barts and The London NHS Trust | 3 | 50 | 140 | 30 |
| South West London Elective Orthopaedic Centre | 4 | 135 | 129 | 1 |
| Royal Cornwall Hospitals NHS Trust | 3 | 157 | 100 | 132 |
| Addenbrookes Hospital | 2 | 143 | 91 | 0 |
| The Royal Orthopaedic Hospital Birmingham | 2 | 135 | 90 | 15 |
| Robert Jones and Agnes Hunt Hospital | 2 | 90 | 80 | 1 |
| South Tees Hospitals NHS Foundation Trust | 2 | 73 | 73 | 0 |
| Wrightington, Wigan and Leigh NHS Foundation Trust | 2 | 76 | 71 | 2 |
| Bangor Hospital | 3 | 59 | 55 | 9 |
| Buckinghamshire Healthcare NHS Trust | 1 | 53 | 53 | 1 |
| University College London Hospitals | 2 | 50 | 50 | 0 |
| Bolton NHS Foundation Trust | 1 | 51 | 50 | 0 |
| Harrogate and District NHS Foundation Trust | 1 | 50 | 50 | 0 |
| Royal National Orthopaedic Hospital NHS Trust | 1 | 50 | 50 | 30 |
| South London Healthcare NHS Trust | 1 | 50 | 50 | 0 |
| Oxford Radcliffe Hospitals NHS Trust | 2 | 50 | 45 | 0 |
| Great Western Hospitals NHS Foundation Trust | 3 | 47 | 43 | 7 |
| Sheffield Children’s NHS Foundation Trust | 1 | 102 | 43 | 0 |
| Royal Infirmary of Edinburgh | 1 | 48 | 42 | 0 |
| King’s College Hospital NHS Foundation Trust | 2 | 40 | 39 | 6 |
| Plymouth Hospitals NHS Trust | 1 | 35 | 35 | 0 |
| Yeovil District Hospital NHS Foundation Trust | 2 | 40 | 33 | 7 |
| Central Manchester University Hospitals | 1 | 40 | 30 | 0 |
| Northumbria Healthcare NHS Foundation Trust | 1 | 30 | 25 | 0 |
| The Princess Alexandra Hospital NHS Trust | 1 | 25 | 25 | 5 |
| Royal Exeter and Devon | 1 | 28 | 23 | 0 |
| Weston Area Health NHS Trust | 1 | 352 | 23 | 4 |
| Gateshead Health NHS Foundation Trust | 2 | 22 | 22 | 5 |
| Western Sussex Hospitals NHS Trust | 1 | 22 | 22 | 0 |
| Southern General Hospital – Glasgow | 1 | 38 | 20 | 38 |
| University Hospital of South Manchester NHS Trust | 1 | 17 | 17 | 0 |
| East Kent Hospitals University NHS Foundation Trust | 1 | 15 | 15 | 0 |
| Maidstone and Tunbridge Wells NHS Trust | 1 | 40 | 15 | 1 |
| UHCW | 1 | 60 | 15 | 0 |
| Wirral University Teaching Hospital | 1 | 30 | 15 | 0 |
| Blackpool, Fylde and Wyre Hospitals | 1 | 31 | 12 | 0 |
| Luton and Dunstable Hospitals NHS Trust | 1 | 15 | 12 | 0 |
| North Bristol NHS Trust | 1 | 52 | 12 | 20 |
| Royal Surrey County NHS Foundation Trust | 1 | 12 | 12 | 0 |
| South Warwickshire Hospital | 1 | 18 | 12 | 0 |
| Hull and East Yorkshire Hospitals | 1 | 29 | 10 | 4 |
| Poole Hospital | 1 | 20 | 10 | 0 |
From the initial survey there were 69 hospital trusts in England with at least one FAI surgeon. Of these 69 trusts, 53 had evidence of coded procedural activity from the HES data search. However, the data for both arthroscopic and open surgery were different by five or more procedures in 68 trusts when the two sources of data were compared. Local database results at UHCW showed that 12 arthroscopic and zero open surgeries were undertaken for FAI. The corresponding HES data reported 15 arthroscopic and more than one but fewer than five open surgeries for FAI.
Discussion
A minimum of 120 practising NHS FAI consultant surgeons collectively undertook at least 2399 FAI surgical procedures over a 12-month period. Of these, 1908 procedures (80%) were performed arthroscopically.
Although all health boards and trusts and corresponding clinical directors responded to our enquiries, it is possible that clinical directors are not always aware of all the expertise within their department. New FAI consultant surgeon appointments made after submission of data from the departmental clinical leads would not have been included in this data set, but the workload data for these surgeons are likely to be small given the short tenure and may already be accounted for in work being undertaken by existing surgeons.
The survey data rely heavily on surgeon recall, which is likely to have inaccuracies. However, the alternative of coded procedural data suffers from two major problems:
-
Coding of surgical procedures is known to be inaccurate. One study has reported accuracy of 47%. 34 The reason for this is that coding is frequently undertaken retrospectively by staff with no medical training and there are multiple ways of coding the same procedure. 34
-
Femoroacetabular impingement surgery has no specific procedural codes and is, therefore, coded using alternative combinations of the OPCS-4 coding system across NHS trusts.
For this reason, the HES data were used to triangulate/confirm locations of FAI surgery rather than provide any robust measure of the quantity of surgery.
Based on the ONS population data, the prevalence of surgery for FAI within the UK is 3.9 per 100,000. This figure represents a conservative estimate for prevalence of surgery for FAI within the UK because
-
a total of 20 (17%) surgeons did not provide data
-
the survey does not include surgery done outside the NHS.
In the absence of any formal operative coding for FAI, and with FAI surgical registries only recently commencing, this type of prevalence data has not been available to date through other sources. Although all health boards and trusts and corresponding clinical directors responded to our enquiries, it is possible that clinical directors are not always aware of all the expertise within their department. New FAI consultant surgeon appointments made after submission of data from the departmental clinical leads would not have been included in this data set; however, the workload of these surgeons is likely to be low given the short tenure and may all already be accounted for in work being undertaken by existing surgeons.
The results suggest the workload of FAI surgery is not spread evenly by region. There is a suggestion that more surgery is taking place in the South West, London and West Midlands regions per head of population, with marked variations in surgical workload across neighbouring regions, for example West Midlands and East Midlands (5.8 and 0.4 per 100,000, respectively). It is unlikely that these differences are due to regional variations in the prevalence of FAI; they and are more likely to represent regional variation in both surgical expertise and funding for FAI surgery. Surgical treatment of FAI is a relatively new and technically demanding procedure;35,36 therefore, the availability of surgeons with sufficient experience and expertise to undertake such surgery is likely to be limited. This is supported by the high workload among a small number of surgeons nationally. The data have highlighted marked regional variances in surgical workload and it is possible that some patients by the nature of their geography may not have easy access to the care that they would like.
The data presented provide epidemiological data on FAI surgery including those sites with a combined workload of 10 or more arthroscopic FAI procedures over 12 months. These sites may be particularly suited to become recruitment units for a RCT, provided the staff members involved in a RCT are in a position of equipoise.
The survey results provide a conservative estimate for both the number of FAI surgeons currently practising in the NHS and the prevalence of FAI surgery being undertaken. The results also highlight marked regional variances in surgical workload and it is possible that some patients by the nature of their geography may not have easy access to the care that they require. Finally, the results presented can be used to help plan a multicentre RCT based on the FAI surgical workload at each hospital trust.
Clinicians’ attitudes to randomisation of femoroacetabular impingement patients
Introduction
To participate in RCTs, clinicians need to accept at least collective uncertainty or equipoise between treatments, including the possibility that surgery does not work.
Objectives
To explore attitudes to randomisation of patients with FAI among a multidisciplinary sample of clinicians working in the centres performing hip arthroscopy.
Methods
We aimed to recruit a convenience sample of health-care professionals who have a special interest in hip conditions and were likely to see patients with FAI during their clinical practice. Semistructured interviews were carried out with a sample of orthopaedic surgeons, sport doctors and physiotherapists. To stimulate discussion, clinicians were provided with anonymous patient cases that cover the spectrum of patient presentations, including patient history (with duration of symptoms and previous treatments), examination findings and imaging (see Appendix 1). The clinicians were asked to ‘think aloud’ while considering the patient cases and the researcher used prompting questions to facilitate the clinician narrative of these processes. By introducing patient cases, we sought to deconstruct the cognitive processes involved when a clinician considers recruiting a patient to a clinical trial. This was done in order to elucidate the state of individual equipoise that may be influencing a recruitment decision (see Appendix 2).
The interviews were analysed thematically based on the Buckingham–Adams classification model of clinical decision-making. The analysis allowed to identify (1) relevant cues; (2) psychological representation of these clues (i.e. importance in diagnosis); (3) knowledge structures used (e.g. past clinical experience); (4) condition and treatment path inferences; and (5) potential outcomes associated with a particular treatment including introducing the trial. Key messages about what relevant cues to decision-making, information sources, clinical uncertainty and risks, and potential outcomes were considered by the clinicians were identified and discussed within the qualitative team. Recommendations about clinician recruitment and information provision for clinical teams involved in the trial were agreed and presented to the trial management team.
Results
A total of 28 clinicians were interviewed. Eighteen orthopaedic surgeons were identified from our workload survey (see Hip arthroscopy for femoroacetabular impingement in the NHS in the UK: a workload survey) as being high-volume arthroscopic FAI surgeons (> 20 procedures per year). Half of the physiotherapists (n = 6) were identified through a national survey (see Design of a best conservative care treatment protocol for femoroacetabular impingement), which requested the opinions of professionals who have a special interest in hip conditions. The other six physiotherapists and two sports physicians were interviewed when they attended a during an arthroscopic hip surgery conference. A detailed report of the recruitment processes and analysis of these interviews is presented in Appendix 3.
The results showed there was a lack of consensus about how best to treat FAI. The majority of clinicians accepted uncertainty between operative and non-operative treatments. Twenty-six clinicians (90%) believed that they were in equipoise and that a RCT was required to generate superior scientific evidence and guidelines for the care of patients with FAI. These were urgently needed, as they have witnessed increasing numbers of FAI patients in their routine practice. There were various statements supporting the trial; for example, a surgeon said:
The study is a very important one, it has not been done before and we need to do it to justify the role of surgery for this condition.
Surgeon 10
Despite this, five surgeons (36%) and two physiotherapists (10%) showed a lack of active clinical equipoise when faced with real-life case scenarios or discussing involvement with a pilot RCT. One surgeon has a fundamental disbelief in FAI, so that a trial of its treatment lacks relevance for them:
I’m not a massive believer that hip impingement exists, I think it’s over diagnosed.
Surgeon 3
Other clinicians are not in equipoise because they approach surgery for FAI with caution (n = 2); for example, a surgeon, referring to his practice, said:
I’m very careful that I counsel patients about the length of rehabilitation required after surgery, and that it’s definitely only for failure of conservative management.
Surgeon 6
Finally, some surgeons favoured surgery as the optimal treatment for FAI (n = 2), which is the case for the two physiotherapists who were not in equipoise; for example, one surgeon said:
I don’t believe there is any more a question of whether good surgery can relieve [FAI] symptoms.
Surgeon 9
There were seven surgeons who displayed both ‘theoretical’ and ‘active’ equipoise when assessing the case vignettes and these surgeons were invited to act as principal investigators (PIs) in the pilot RCT. Of these seven, there were three who were clearly of the strong belief that a RCT was the optimum solution to improving evidence and the remaining four were permissive.
A major concern for the surgeons (n = 10) was the duration of the trial, needing to balance sufficient length of follow-up to see changes with the potential for deterioration of a patient’s hip during conservative care; for example, one surgeon said:
The main concern with all of this is that if you’re delaying surgical treatment, are they going to progress essentially their arthritic symptoms or cartilage damage by introducing that delay.
Surgeon 13
They felt that the proposed duration of 12 months for the trial was pragmatic and, therefore, acceptable in this respect and would also be long enough to allow the patient to stabilise after either intervention. One surgeon indicated this agreement by saying ‘12 months may be a little long, but I can understand with what we are trying to come up’ (surgeon 11). The surgeons were anxious to know the mechanism by which the outcome of the trial would be assessed and were more comfortable with a patient-reported quality-of-life instrument rather than any other types of measure. All were familiar with and supported the use of either the Non-Arthritic Hip Score (NAHS) or the International Hip Outcome Tool (iHOT-33) as the primary outcome measure for a full trial.
The physiotherapists and sports physicians frequently mentioned two issues: (1) the need for more clarity of the eligibility criteria (i.e. patients who do not need surgery do not take part) and (2) the need for the new conservative care protocol to be more distinctive. For example, a physiotherapist said:
As long as conservative care is standardised and you’ve got a level of expertise by the people giving the conservative care, I think it is very valid.
Physiotherapist 5
The majority of clinicians that assumed patients would prefer surgery and were concerned about patient reactions to an invitation to participate in the study, which meant that patient expectations were not fulfilled. They also thought patient would respond negatively to being randomised. The following quotes illustrated these assumptions:
Patients get to the point where they’ve tried everything and sometimes they are looking for a surgical route, she had failed physiotherapy.
Physiotherapist 4
She’s going to be very unwilling not to have an operation, I would imagine.
Surgeon 3
Finally, clinicians reported potential regional sources of bias in the recruitment. For example, surgeons’ preferences for conservative or operative care could be at play. One surgeon said:
I think if it had been done 3–4 years ago, I think it would have been easier, it was less conviction and most surgeons that were doing it that it was the right thing to do.
Surgeon 14
Although another surgeon explained his own view as:
Of the majority of surgeons offering HA [hip arthroscopy] in the country, I see myself as being a little less invasive and more conservative [. . .] there are surgeons around the country who will operate in patients that I wouldn’t touch with a barge pole.
Surgeon 6
There are also perceived differences in physiotherapy provision. Clinicians reported their concern that local physiotherapy may not be of equal quality across the centres:
Are you suggesting a remote access physio who gives them a programme and liaises with the local physios? Because we don’t have specialist enthusiastic physios in our area which would be quite as knowledgeable as the ones you have in Coventry.
Surgeon 7
Discussion
Significant interest was evident in the clinical community for a RCT comparing hip arthroscopy and conservative care. Even though not all clinicians were in equipoise, almost all recognised uncertainty and felt that a RCT would help to guide their practice in future. Most surgeons were prepared to randomise patients.
The qualitative methods we described provided a novel approach to determining a more complete assessment of clinical uncertainty and equipoise. The results suggest that, while many clinicians might think they were in equipoise (theoretical equipoise) their actions and management decisions did not always support this position (active equipoise). It was likely that where there was a large discrepancy, recruitment of patients to a RCT was going to be challenging. Therefore, decisions about participating sites and PIs were informed by the results of this study. It also helped to provide guidance of study design features including follow-up duration, outcome measures and the need for distinctiveness of the best conventional care arm.
Develop eligibility criteria for a randomised controlled trial and design an operative protocol
Introduction
To date, no RCTs have been undertaken for FAI surgery and no previous eligibility criteria exist to guide a pilot. In addition, as a relatively new procedure, there are a variety of surgical techniques for arthroscopic FAI surgery and, therefore, it is necessary to develop and define a protocol of operative care in order to ensure that patients receive the same standard intervention and that the fidelity of this intervention can then be measured.
Objectives
-
Establish appropriate eligibility criteria for a RCT.
-
Establish a protocol of operative treatment for arthroscopic FAI surgery.
Methods
Researchers PW and DG developed draft eligibility criteria and an operative protocol based on their own experience of arthroscopic FAI surgery and available published literature. Sixteen hip arthroscopy surgeons, recognised as international experts in the field, were then individually invited to comment on these provisional documents. These surgeons were from the Multicenter Arthroscopy of the Hip Outcomes Research Network (MAHORN) (n = 12) and from a group who attended the American Academy of Orthopaedic Surgeons Research Symposium on Femoroacetabular Impingement in Chicago, IL, USA (n = 4). All surgeons approached agreed to participate. The feedback they provided was then used to modify the two documents. The eligibility criteria and protocol were recirculated to the 16 experts for further comment. The criteria and protocol were then discussed with a further sample of 14 UK specialist hip surgeons with experience of treating patients with FAI and likely collaborators for a full RCT. A final version of both documents was agreed and then used in the pilot RCT. The two documents continued to be evaluated in the light of feedback from the recruiting sites during the pilot RCT.
Results
The draft eligibility proposed that patients be included if:
-
they are aged 18–50 years
-
they have symptoms of hip pain: they may also have symptoms of clicking, catching or giving way
-
they show radiographic evidence of pincer- or cam-type FAI on plain radiographs and cross-sectional imaging37
-
the treating surgeon believes that they would benefit from arthroscopic FAI surgery
-
they are able to give written informed consent
-
they are able to participate fully in the interventions.
Patients would be excluded from participation in this study if:
-
they have previous significant hip pathology such as Perthes’ disease, slipped upper femoral epiphysis or avascular necrosis
-
they have had a previous hip injury such as acetabular fracture, hip dislocation or femoral neck fracture
-
they already have osteoarthritis, defined as Tönnis grade of > 1,38 or more than 2-mm loss of superior joint space width on anteroposterior pelvic radiograph8
-
there is evidence that the patient would be unable to participate fully in the interventions, adhere to trial procedures or complete questionnaires, such as cognitive impairment or intravenous drug abuse.
Initially, 11 out of the 16 surgeons agreed with these eligibility criteria. Others suggested the following modifications, which were agreed by the whole group:
-
Age range changed to include all patients ≥ 16 years. Some surgeons felt that patients between 16 and 18 years of age were an important part of their practice and that for these patients treatment effects would be similar to older adults. There was not thought to be any rationale for a maximum age limit; older patients may be, but need not necessarily be, excluded by the criterion ‘the treating surgeon believes that they would benefit from arthroscopic FAI surgery’.
-
Radiographic evidence of FAI was further defined to include an alpha angle of > 55 degrees or a lateral centre–edge (Wiberg) angle of > 40 degrees.
The draft operative protocol was:
-
general anaesthetic with muscle relaxation
-
supine or lateral patient positioning
-
operating table with facility for traction and allowing range of movement testing
-
arthroscopy of central compartment
-
arthroscopy of peripheral compartment working with one of the following: intact capsule, capsulotomy or capsulectomy
-
ability to undertake bony surgery to correct abnormalities on both the femoral head neck junction and acetabular side of the hip joint
-
ability to undertake soft tissue repair and/or debridement to the labrum and/or articular cartilage
-
ability to record with photos the intraoperative findings and solutions.
A total of 13 out of the 16 surgeons agreed with the draft operative protocol. Three surgeons proposed changes, which were agreed by the whole group:
-
the entire acetabular labrum should be examined
-
the entire articular surface should be examined
-
confirm that FAI has been relieved using either range of movement testing or an image intensifier
-
need to document intraoperative complication and their solutions.
Discussion
There was ready agreement on eligibility criteria for a trial, suggesting that surgeons have a clear idea who may benefit from hip arthroscopy for FAI. There was recognition that there are many subtleties of FAI morphology, but surgeons were happy to be pragmatic and ‘lump’ all of these into a single diagnosis. There may be differences in treatment effect according to type of FAI (some surgeons mentioned their sense that patients with cam-type FAI do better after surgery than those with pincer-type) and this raised the possibility of stratification or an a priori subgroup analysis in a full trial. These criteria define a generalisable sample representative of the patients currently receiving hip arthroscopy for FAI.
The operative protocol was broad enough to accommodate the variations of arthroscopic FAI surgical technique being used throughout the UK. However, the protocol retains the key steps regarded by the expert surgeons as essential to a successful hip arthroscopy for FAI.
We considered whether or not to protocolise the postoperative rehabilitation programme for the group randomised to surgery prior to the pilot trial. This is a pragmatic trial and we believe that the best option is for postoperative rehabilitation to reflect usual care as closely as possible. Postoperative rehabilitation varies, often from surgeon to surgeon and even within the same orthopaedic service. If we were to develop a postoperative rehabilitation programme that is similar to the personalised hip therapy (PHT) programme, this would change the question for the trial (and, therefore, it would no longer address the original commissioned call from the HTA programme). The question would then become ‘Does surgery provide additional benefit to a package of physiotherapy-led care?’ Our preference was to allow postoperative care to be offered as per usual practice for those randomised to surgery in the pilot trial and to measure that to allow for assessment of potential confounding.
Design of a best conservative care treatment protocol for femoroacetabular impingement
Introduction
Although conservative care is being used to treat patients with FAI,11 we had previously performed a systematic review that showed that detailed guidance and evidence on how this care should be delivered are not available. 13 This review suggested that non-operative care for FAI would typically be led by physiotherapists. Of 53 published articles, only four were empirical investigations of non-operative care for FAI and only one study39 provided both an experimental evaluation of treatment and an explicit description of the treatment protocol delivered by physiotherapists.
When there is a lack of evidence to guide care, it is appropriate to use a consensus-gathering approach in order to develop and rationalise best practice. 40 Murphy et al. 40 summarised three formal consensus-gathering techniques which have been used to guide health care in other subject areas:
-
Delphi method – involves participants receiving two, three or more sequential rounds of questionnaires and responding to ‘cues’, that is, statements that provoke decision-making based on responses from previous rounds. Results are aggregated and reviewed for agreement. 41
-
Nominal group technique (NGT) – involves a process of generating ideas which are then either accepted or rejected by group members. 42,43
-
Consensus Development Conference (CDC) – requires a group of individuals to attend a conference in which evidence is presented to them by experts. 44
Objectives
-
To develop a consensus on a best conservative care treatment protocol for patients with FAI that was both deliverable within the NHS and could be used within a RCT of best conservative care compared with hip arthroscopy surgery for FAI.
-
To agree, through patient involvement, the most appropriate name for the best conservative care treatment protocol.
Methods
The protocol proposed by Emara et al. 39 (Table 6) was used as the starting point for a best conservative care treatment protocol for FAI. This was developed using Delphi and NGT consensus-building methods, guided by available evidence and guidance from the MRC for developing a complex intervention. 45
| Initial assessment and treatment | Further assessment and treatment | ||
|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 |
| Avoidance of excessive physical activity and anti-inflammatory drugs for 2 to 4 weeks | Physiotherapy for 2 to 3 weeks in the form of stretching exercises to improve hip external rotation and abduction in extension and flexion | Assessment of the normal range of hip internal rotation and flexion after the acute pain has subsided | Modification of activities of daily living predisposing to FAI (e.g. hip internal rotation associated with flexion and adduction) |
A core study group was formed to oversee the development, evaluate information gathered and provide a layer of NGT consensus to support the Delphi process. The core study group comprised two senior musculoskeletal physiotherapists with an interest in managing patients with FAI (David Robinson and Ivor Hughes), a senior academic research physiotherapist (NF) and an orthopaedic surgeon (PW).
When we began this study, we did not know which physiotherapists were directly involved in the management of patients with FAI, nor did we think it likely to be efficient to simply sample the physiotherapy profession in the UK at random. We therefore took a targeted approach to sampling, using networks of physiotherapists most likely to be involved in the management of this patient group. National advertisements were placed in the orthopaedic, rheumatology, pain and manual therapy electronic networks through the interactive, electronic Chartered Society of Physiotherapy (CSP) communication system in the UK (iCSP) and in the CSP’s Frontline magazine (twice-monthly magazine posted to approximately 52,000 CSP members in the UK). The adverts invited UK physiotherapists to help develop a consensus for a best conservative care treatment protocol for FAI. Electronic invitations were also sent to physiotherapists in the USA and Australia known to members of the core study group through previous collaborative work on FAI. To encourage a process of ‘snowball sampling’ within the international community, these therapists were encouraged to invite colleagues with experience and interest in managing FAI to join in the consensus development process.
Each physiotherapist was given the first protocol39 with a questionnaire. The physiotherapists were asked whether they agreed or disagreed with the proposed programme for the conservative treatment of FAI patients and, when appropriate, to provide comments and suggestions for improvement.
Results from the each round of Delphi consensus were tabulated by the core study group, and additional comments and treatment strategies suggested by the respondents were grouped into themes. An agreement level of ≥ 50% for this Delphi consensus technique was used. If no consensus was evident from the survey the core study group refined the protocol in light of the available feedback using a NGT-type approach. The refined protocol was then recirculated to the physiotherapists taking part in the Delphi consensus process and the cycle repeated until a consensus of ≥ 50% was achieved.
This protocol was then discussed with our panel of expert patients (described in section Patients’ attitudes towards randomisation and design of patient information material for a randomised controlled trial) in individual interviews. They expressed the view that the name ‘best conservative care’ did not fully express (to patients) the intent and content of the protocol. Previous qualitative research has highlighted the importance of naming treatments in order to improve uptake and compliance, in particular when being used in RCTs. 46 The expert panel was asked to suggest a suitable name for this best conservative care treatment protocol.
All physiotherapists identified as likely to be best conservative care providers in the pilot RCT were asked to detail exercises that would allow them to deliver the protocol. The exercises were then ranked and the most popular were included as a database resource (exercise template) to be used alongside the best conservative care protocol. In the early phases of recruitment to the RCT, a workshop (CDC methodology) was held among the physiotherapists delivering care, to refine the protocol. The physiotherapists attending the workshop were asked to share their experiences of delivering the protocol and make any suggestions for further amendments.
In the pilot RCT, all physiotherapists delivering the protocol were asked to complete case report forms for each patient. This included details about the number, nature and duration of the patient contact, and details of the exercises prescribed to each patient were recorded.
Results
Consensus development for the best conservative care protocol
In total, 36 physiotherapists responded and agreed to take part in the consensus process; 24 from the UK, 10 from USA and two from Australia. All were senior musculoskeletal physiotherapists who had previously managed patients with FAI. Details of the initial round of consensus development received from 36 physiotherapists are summarised in Tables 7 and 8.
| Agreement | Stages 1 and 2 of the Emara et al.39 protocol (initial assessment and treatment); level of agreement, n (%) | Stages 3 and 4 of the Emara et al.39 protocol (further assessment and treatment); level of agreement, n (%) |
|---|---|---|
| Yes | 16 (47) | 9 (25) |
| No | 7 | 6 |
| Unsure | 13 | 21 |
| Total | 36 | 36 |
| Stages | Additional themed comments made | Number of comments | Origin of comments |
|---|---|---|---|
| Initial assessment and treatment (stages 1 and 2) | Core stability exercise and movement control | 21 | UK × 17, Australia × 2, USA × 2 |
| Muscle strengthening important | 7 | UK × 6, USA × 1 | |
| See patients more frequently/over a longer period | 5 | UK × 4, Australia × 1 | |
| Stretching exercise depending on what is limited | 4 | UK × 2, USA × 2 | |
| Soft tissue mobilisation to facilitate range of movement | 3 | UK × 2, USA × 1 | |
| Address flexion contractures | 2 | UK × 2 | |
| Massage to relieve tightness in hip muscles | 2 | UK × 1, Australia × 1 | |
| Avoid flexion stretching exercises during initial stages | 1 | UK × 1 | |
| Internal rotation stretching when pain free | 1 | USA × 1 | |
| Gentle exercise to mobilise the joint in all directions | 1 | UK × 1 | |
| Reduce overactive hamstring muscles | 1 | UK × 1 | |
| Work on active abduction and external rotation | 1 | UK × 1 | |
| Avoid excessive hip flexion | 1 | UK × 1 | |
| See patients less frequently | 1 | UK × 1 | |
| Prolonged follow-up often needed | 1 | UK × 1 | |
| Further assessment and treatment (stages 3 and 4) | Advice that cycling is acceptable with activity modification | 7 | UK × 6, Australia × 1 |
| Continue strengthening | 6 | UK × 6 | |
| Zigzag running no better than straight running | 4 | UK × 3, Australia × 1 | |
| Reassessment important | 2 | UK × 2 | |
| Using orthotics may help | 2 | UK × 2 | |
| Encourage hip capsule stretches | 2 | UK × 2 | |
| Stretches can be harmful | 2 | UK × 1, Australia × 1 | |
| Identify dysfunctional movement patterns to achieve long-term change | 2 | UK × 1, Australia × 1 | |
| More than twice-monthly supervision required | 2 | UK × 1, Australia × 1 | |
| Advice about lifestyle modification | 2 | UK × 1, USA × 1 | |
| Advice about alternative forms of exercise | 2 | UK × 1, Australia × 1 | |
| Advise to avoid deep squatting | 1 | UK × 1 | |
| Advice on return to sport specific training | 1 | Australia × 1 | |
| Strengthening of internal and external rotators of hip | 1 | UK × 1 | |
| Activity restriction on an individual basis | 1 | UK × 1 | |
| Modification of running on an individual basis | 1 | UK × 1 | |
| Ensure activities can be undertaken with minimal adduction/Internal rotation | 1 | UK × 1 |
The level of agreement with the Emara et al. 39 protocol (initial protocol) among the 36 physiotherapists was below the 50% threshold that had been set for the Delphi consensus method. However, using the additional comments made by the physiotherapists, available evidence and established theory, two further protocols were developed independently by NF and PW and presented at a core study group meeting. Using the two independent protocols presented, the core study group derived a second protocol based on a majority within the group (NGT methodology).
The second protocol that the core study group formulated had four core components and four optional components, which are described below with a justification for including each component.
-
Core component 1: patient assessment. Both independently developed protocols featured this component. 39,47 Although not formally a treatment and as such not specifically mentioned in the questionnaire feedback received, the core study felt that this component should be explicitly included in the protocol, as it would underpin the remainder of the best conservative care treatment protocol.
-
Core component 2: patient education and advice. Both independently developed protocols featured this component. Thirteen comments from questionnaire respondents had suggested that physiotherapists should provide patient specific education about FAI including advice on lifestyle modification, how to do different forms of exercise and how to undertake common activities. Advice on activity modification was a feature of the published literature, including the Emara et al. protocol39 and Hunt et al. protocol,47 and the core study group felt that education and advice would be regarded as a core component of best practice among physiotherapists managing any painful musculoskeletal condition. Both lifestyle and activity modification draw on relevant theory in that behavioural modifications that might lead to reduced functional impingement should result in reduced symptoms. 3
-
Core component 3: help with pain relief. Both independently developed protocols featured this component. It is a feature of the published literature, including the Emara et al. 39 protocol, with which 44% of the physiotherapists agreed. 39,47 Analgesia is an established treatment for musculoskeletal pain. 48,49 Controlling musculoskeletal pain associated with FAI with analgesia therefore follows MRC guidance that treatment draw on relevant theory.
-
Core component 4: exercise-based hip programme. Both independently developed protocols featured this component. Thirty-seven additional comments from questionnaire respondents endorsed both hip-specific and more general exercises for managing patients with FAI. Of these, core or stability exercises were the most common (21 additional comments). The feedback suggested that the exercise programme should be individualised to the patient and progressed over time from core stability exercise and stretching to strengthening exercises. Exercise was a predominant feature of the Emara et al. 39 protocol and the other published literature for managing FAI non-operatively. 39,47,50 Exercise is an effective treatment for many other musculoskeletal pain problems51,52 and exercise-based programmes can produce similar improvements in symptoms to surgery. 53 Therefore, including an exercise-based hip regime to help manage the symptoms of FAI follows MRC guidance that treatment draw on relevant theory.
The core study group proposed the inclusion of optional components which could be undertaken in addition to the core components in order to individualise treatment, at the discretion of the physiotherapist delivering care.
-
Option 1: additional symptoms that patients with FAI may present with can also be treated.
-
Option 2: orthotics can be used to aid the treatment of biomechanical abnormalities.
-
Option 3: corticosteroid hip joint injection may be used for patients who cannot engage with ‘core’ treatment owing to acute pain symptoms.
-
Option 4: manual therapy – hip joint mobilisations may be added if appropriate, for example distraction and trigger point work.
The Emara et al. 39 protocol suggested that physiotherapy should be offered over a period of between 2 and 3 weeks. The initial round of physiotherapist responses suggested patients should be seen over a longer period and more frequently in order to provide best care. Currently within the NHS the average number of treatment sessions given by physiotherapist to musculoskeletal pain patients is between three and four face-to-face contacts. There is evidence to suggest that better outcomes are achieved from exercise-based regimes when they are supervised and the contact between the supervisor and patient is increased. 54,55 In order to allow more contact between therapists and their patients without increasing the burden of having to travel to clinic appointments, non-face-to-face contacts (e.g. telephone and e-mail) were also allowed in order to progress the exercise programme and to support patients in their adherence to the recommended exercises. The core study group suggested that the agreed protocol should be delivered over at least a 12-week period and a minimum of six treatment sessions (of which at least three should be face to face). The duration of care was both in keeping with established theory that suggests physiological changes in muscle occur after a 12-week programme of exercise. 56
The core study group agreed on the following protocol exclusions:
-
Painful hard end stretches were excluded. Although mentioned by only two physiotherapists in the initial questionnaire responses, there is some evidence in the literature to suggest that painful hard end stretches and forceful manual techniques in a restricted range of movement may be harmful. Therefore, although stretching was permitted, hard end of range stretches were excluded. 30
-
Group-based treatment was excluded in order to ensure care was individualised.
-
Care delivered by a technical or student instructor was excluded in order to ensure that care was delivered by qualified musculoskeletal physiotherapists.
The second protocol was distributed to the original group of 36 physiotherapists. Thirty-five (97%) responded, 30 (86%) agreed with the second protocol and provided no additional suggestions for change, and five disagreed with elements of the second protocol and made suggestions for change. These points were discussed among the core study group and the following changes were made:
-
Two optional booster sessions that could be delivered between 12 weeks and 6 months were added to a revised protocol. This was in response to concerns that the initial 12-week programme could prove to be insufficient to correct what is likely to be a significant chronic biomechanical dysfunction. Booster sessions would also help with adherence to the programme.
-
Taping techniques to help with postural modification/reminding were added to the protocol. Although mentioned by only one physiotherapist, it was noted that taping was a feature of the published literature. 13
Given the level of agreement (83%) achieved with the second protocol, the core study group decided to use the second protocol with the modifications discussed above for implementation in the RCT. This final protocol is shown in Box 1.
Four core components. Each patient should receive all four core components over at least a 12-week programme with at least six patient contacts (of which at least three are face-to-face contacts). Up to a further four ‘booster’ follow-ups can be arranged between 12 weeks and 6 months.
Patient education and advice-
Education about FAI and available treatments.
-
Advice about posture, gait and lifestyle behaviour modifications to try to avoid FAI. These may include measures to encourage posterior pelvic tilt (reduce pelvic inclination); positioning when sitting, standing, sit to stand; positioning when sleeping; positioning when running/cycling when relevant.
-
Advice about activities of daily living to try to avoid FAI (reducing/avoiding deep flexion, adduction and internal rotation of hip).
-
Advice about relative rest (for acute pain where patients cannot engage with their exercise-based personal hip programme) given that soft tissues take at least 8–10 weeks to heal. In particular, relative rest in a specific ROM when pain in that particular ROM is likely to represent ongoing inflammation and damage.
-
Specific activity/sport technique advice and modification. Examples include running with a broader base to encourage abduction, cycling with less internal rotation on pedals, skiing with skis further apart and using knee flexion more than hip flexion to lower centre of gravity.
-
History, which should include (although not exclusively) history of presenting complaint; relieving and aggravating factors; past medical history; medications; previous treatments tried; social history including occupation; and patients’ concerns/fears/beliefs, individual requirements and expectations.
-
Examination, which should include (although not exclusively) the pain-free and passive range of movement of the hip; strength of the hip; and the anterior impingement test.
-
Advice about anti-inflammatory medication for 2–4 weeks if not already tried and simple analgesics if the patient does not respond well to anti-inflammatory medication.
-
Engagement in, and adherence to, an exercise programme that has the key features of individualisation, progression and supervision.
-
A phased exercise programme that begins with muscle control work and progresses to stretching and strengthening with increasing ROM and resistance.
-
Muscle control/stability exercise (targeting pelvic and hip stabilisation, gluteal and abdominal muscles).
-
Strengthening/resistance exercise firstly in available range (pain-free ROM).
-
Stretching exercise to improve hip external rotation and abduction in extension and flexion (but not vigorous stretching – no painful hard end stretches). Other muscles to be targeted if relevant.
-
Exercise progression in terms of intensity and difficulty, gradually progressing to activity or sport-specific exercise when relevant.
-
A personalised and written exercise prescription that is progressed and revised over treatment sessions.
-
Encourage motivation and adherence through the use of a patient exercise diary to review progress.
-
Patients to have to access to simple exercise equipment (e.g. resistance bands, exercise balls and exercise mats).
-
Additional symptoms that patients with FAI may present with can also be treated as per the treating physiotherapists’ preferred methods.
-
Manual therapy: hip joint mobilisations (e.g. distraction and rigger point work).
-
Hip joint injection: for patients who cannot engage with ‘core’ treatment owing to acute symptoms. Maximum of one steroid hip injection.
-
Orthotics: patients can be assessed for biomechanical abnormalities and have these corrected (e.g. referral to a podiatrist for custom-made insoles).
-
Taping: taping techniques are permissible, for example taping the thigh into external rotation and abduction to help with postural modification/reminding.
-
Forceful manual techniques in restricted range of movement. No painful hard end of range stretches.
-
Group-based treatment.
-
Care delivered by a student or technical instructor.
ROM, range of motion.
Naming the best conservative care protocol
Eighteen expert patients participated in consideration of the best name for this protocol. They were asked to choose between four potential names which had been suggested by the core study group, with the option to suggest a different name if they wished to do so. Eight patients opted for the name ‘personalised hip therapy’, four patients voted for ‘personalised hip programme’, one patient preferred the name ‘focused hip therapy’ and three offered their own suggestions (including ‘conservative hip rehabilitation programme’ and the inclusion of the word ‘non-invasive’). ‘Conservative’ and ‘non-invasive’ were disregarded because they appeared to have a value attached to them; for example, the term ‘conservative’ could be confused with terms used in politics. The word ‘personalised’ was preferred for most people, as exemplified by this quote from a patient: ‘I said the last two [personalised hip treatment and PHT] because it makes it a personal issue for that person . . . going down a non-operative route would require different treatment for every different patient’ (patient 3). Patients preferred the word ‘therapy’ to indicate that there was an effort to ‘solve’ or ‘cure’ the condition as opposed to ‘programme’: ‘Therapy from a psychological point of view, people understand therapy [. . .] with regards to clinical treatment rather than a programme which can relate to anything in life’ (patient 13).
The name ‘personalised hip therapy’ appealed to and conveyed a positive message to patients. This name emphasises that the protocol is an active intervention that differs from other regimes patients may have previously tried. Patients said that these two elements were very important to patients who are experiencing FAI and considering treatment options.
Developing an exercise template and testing the protocol
Twelve physiotherapists were initially identified as likely to be providers of the best conservative care protocol during the pilot RCT in their hospitals. They suggested suitable exercises for core component 4 and the most popular 21 exercises are shown in Appendix 4.
A workshop (CDC-type methodology) was held after 42 patients were recruited to the RCT and 21 patients were allocated to and receiving the PHT protocol. Eight physiotherapists (out of 12 physiotherapists participating in the RCT) from seven recruiting centres attended the workshop to review the content and delivery of the protocol. Collectively, the physiotherapists were treating 18 patients within the RCT. The physiotherapists agreed that the protocol worked well but felt that they wanted to change the number of treatment sessions and the overall duration of the protocol, in order to ensure that they were able to deliver best care. As a result, one change was made to the protocol, which allowed a minimum of six and a maximum of 10 contacts over a 6-month period. In addition, the physiotherapists recommended that three further exercises should be added to the selection of 21 within the exercise template (see Appendix 4). The physiotherapists agreed that no further amendments would be needed to the protocol.
Discussion
Despite the increase in attention and recognition of FAI as a source of hip pain in young adults, there has been very little published information about appropriate conservative treatment approaches. Our aim was to develop an agreed high-quality best conservative care and physiotherapy-led treatment protocol for patients with FAI that could be used within a RCT. We combined results from a systematic review, Delphi consensus surveys with FAI physiotherapists, NGT consensus methodology, relevant literature on effective conservative care for other musculoskeletal pain conditions and the experiences of physiotherapists treating FAI patients within a RCT (CDC methodology), in order to develop the agreed treatment protocol, referred to as PHT.
In summary, PHT comprises four ‘core’ elements designed to be offered over a maximum of 6 months: (1) patient assessment; (2) patient education and advice; (3) help with pain relief; and (4) engagement with a supervised individualised exercise-based hip programme. A minimum of six and a maximum of 10 treatment contacts should be provided by the supervising physiotherapist over the 6-month period.
The protocol followed MRC guidance for the development of complex interventions and is based on theory when possible. 45 Research has already shown that exercise is an effective treatment for many types of musculoskeletal pain51,52 and has identified that exercise-based programmes can produce similar improvements in symptoms to surgery. 53 The PHT protocol provides guidance to other clinicians and researchers in an area where evidence and guidance are very limited.
Possible outcome measures and sample size for a full randomised controlled trial
A variety of outcome measures have been used to study patients with FAI, especially patient-reported hip-specific pain and function scales. Some, such as the Western Ontario and McMaster Universities Osteoarthritis Index57 and the Harris Hip Score,58 were intended for older patients with symptoms of severe arthritis and are most suitable to measure the effect of hip replacement surgery. These measures tend to exhibit ceiling effects and are not sensitive to change after treatment in patients with FAI. 58,59 A review of hip-specific patient-reported instruments for FAI18 recommended that newer instruments specifically designed for young adults might be used to measure primary outcome in studies for the effectiveness of treatment of FAI.
Non-Arthritic Hip Score is a self-administered instrument to measure hip-related pain and function in younger patients without arthritis. The score is valid compared with other measures of hip performance, internally consistent and reproducible. 60 However, it is not patient-derived, raising concern that it may not measure what is most important to patients.
The iHOT is a patient-derived, hip-specific, patient-reported instrument that measures health-related quality of life in young, active patients with hip disorders. 59 It was developed in a 5-year study by a large international collaboration of patients and clinicians led by MAHORN: an academic group of highly experienced hip arthroscopists closely associated with the International Society for Hip Arthroscopy (www.isha.net). It comprises 33 items, each measured on a visual analogue scale, to assess functional limitations, sports activities, job-related and emotional concerns. Importantly, these items were generated and refined by patients, reflecting their most important concerns. The instrument generates a single score in the range 0–100. People with no hip complaints usually score ≥ 95; a diverse international population of younger adults with a variety of hip pathologies had a mean score of 66 with a standard deviation (SD) of 19.3. iHOT-33 has been validated for use in patients with FAI and is sensitive to change after treatment for FAI. The minimum clinically important difference (MCID) has been determined using an anchor and distribution-based approach in a group of 27 young active patients who were independent of the development population. Clinical change was determined using a global rating scale that asked patients whether their hip condition had improved, had deteriorated or had not changed since the previous assessment, using a single visual analogue scale. The MCID was 6.1 points. 61–63
The iHOT and European Quality of Life-5 Dimensions (EQ-5D) have been adopted as the principal outcome measures by the UK Non-Arthritic Hip Registry. This registry is led by the British Hip Society (BHS); its use in all patients having arthroscopic FAI surgery is required by the National Institute for Health and Care Excellence. 35
In our pilot RCT (see Chapter 3), we tested both NAHS and iHOT-33 as potential primary outcome measures and found both to be easy to use and acceptable to patients. We asked surgeons for their preference during our interviews with PIs, and all felt that either would be satisfactory; three suggested that the use of iHOT in the national Non-Arthritic Hip Registry made this preferable. The extensive patient involvement in item generation, the availability of an independently determined MCID and the use of iHOT as the principal outcome measure for the UK Non-Arthritic Hip Registry lead us to suggest iHOT-33 as the most appropriate primary outcome measure for a full trial.
Observational studies have measured clinical improvement after hip arthroscopy in FAI patients and a recent systematic review suggests that the effect size in these uncontrolled studies is between 0.67 and 2.95. 64 An effect size of 2.0 for hip arthroscopy in general has also been reported, but not specifically for patients with FAI. 59 However, these are descriptions of the mean change in a group of patients before and after one treatment rather than the difference between two groups of patients having two different treatments, and we expect that conventional care is likely to provide some benefit. Cochrane reviews have shown that good exercise-based regimes for patients with musculoskeletal conditions typically have a standardised effect in the order of 0.3. 53,55,65 We also expect these uncontrolled studies of hip arthroscopy to overestimate the real effect. We suggest that a realistic estimate of the effect size of arthroscopy compared with best conventional care in FAI might be 0.5.
The mean iHOT-33 score has been reported as 66 with a SD of 19.3 in a population of patients undergoing hip arthroscopy for a variety of conditions. 59 The baseline iHOT-33 data from our pilot RCT (see Chapter 3) suggest that the target population of patients being considered for hip arthroscopy for FAI in the UK have lower scores with less variability, with a mean of 33 points and SD of 16. This is consistent with an observational study over several years by one of our team (DG), which showed an SD of 14 iHOT-33 points among FAI patients who had no radiographic signs of arthritis.
Our sample size calculation is therefore based on a SD of 16 and MCID of 6.1 – a standardised effect difference between groups at 12 months of 0.38. Table 9 shows the expected sample size for scenarios with 80% and 90% power to detect an effect of this size, at a 5% significance level, assuming an approximately normal distribution of the iHOT-33 score. The table also shows sample sizes for small to moderate (0.32) and moderate (0.47) effects, which are typical of other pragmatic RCTs measuring clinical effectiveness.
| SD | Power | Standardised effect difference | |
|---|---|---|---|
| 80% | 90% | ||
| 13.3 | 144 | 192 | 0.47 |
| 16.0 | 218 | 292 | 0.38 |
| 19.3 | 316 | 422 | 0.32 |
For a full trial, we suggest a conservative approach, seeking to demonstrate only an effect difference between groups equal to the MCID. We propose to recruit sufficient patients to be able to analyse 292 participants at 12-month follow-up. Allowing for 15% loss to follow-up (a conservative estimate), we suggest that a full trial should recruit a sample of 344 participants (172 in each group). This will provide 90% power to detect a difference of 6.1 iHOT-33 units at 12-month follow-up, if that is the true difference.
Patients’ attitudes towards randomisation and design of patient information material for a randomised controlled trial
Introduction
Producing high-quality patient information sheets (PISs) and consent forms that are clear, address the relevant patient concerns and are presented in an easy to assimilate format is essential for successful recruitment to trials. In order to increase the numbers of patients randomised for the proposed trial, a qualitative study was set up to investigate concerns that patients may have and how best to inform patients of this trial.
Objectives
-
Explore patients’ views about operative and conservative treatments for FAI and the acceptability of randomisation between them.
-
Develop PISs for the RCT.
Methods
A panel of expert patients was established from among patients who had been treated for FAI at the UHCW NHS Trust. The panel was chosen by purposive sampling in order to include a range of age, sex, disease severity, activity and socioeconomic status as well as those who had received operative and conservative care. An invitation to be part of an expert patient panel was sent to patients and anyone who replied to the invitation and signed the consent form was asked if they were willing to participate in one-to-one interviews. Once the expert panel member declared their willingness to be interviewed, the researcher (AR) contacted them and explained the qualitative study. The PIS and consent form was sent by post and, when the consent form was returned signed by the participant, an appointment for the interview was set up, whether face to face at the hospital or by telephone.
A draft version of a PIS for a RCT comparing hip arthroscopy with best conservative care for FAI was prepared. Panel members were given this draft version and a questionnaire to complete about its readability. Panel members then had a semistructured interview (with AR), either face to face or over the telephone, about their experiences with FAI, the treatment they had received and the answers they gave to the PIS questionnaire (see Appendix 5).
Initially, we had planned to give questionnaires to 50 patients and to interview 15 of these. However, it emerged that the one-to-one interviews provided much richer in-depth information than administering surveys alone and that this allowed data saturation to be achieved.
Transcripts of interviews were prepared and thematic analysis was used to extract key messages about patient experiences of hip impingement and treatments, acceptability of the treatment options, the understanding of randomisation, and advice on how to improve the trial participant information sheet. AR coded the transcripts using NVivo (QSR International, Warrington, UK). Agreeing and contrasting views were identified for each area of interest (e.g. randomisation). After discussing the structure and content of the results, the qualitative recruitment intervention (QRI) team agreed on some recommendations which were presented to the trial management team. Further discussions took place in trial management meetings and, once agreement was reached regarding the recommendations, changes to the trial PIS and recruitment strategies to be tested in the pilot were implemented.
Results
A total of 18 FAI patients were part of the expert patient panel; 14 of them received operative treatment and four had managed the condition with physiotherapy and other non-operative interventions (e.g. steroid injections). A detailed report of the results of these interviews is presented in Appendix 2.
Patients’ experiences of hip impingement and treatments
The majority of these patients were young and physically active. Symptoms of FAI impacted on their recreational activities, working life and day-to-day activities, which led them to seek treatment. Patients were often misdiagnosed and sent to physiotherapy in an attempt to address the symptoms before they met the orthopaedic surgeon. Patients reported feeling relief once a diagnosis was made.
Members of the panel found the operative and conservative treatments for FAI acceptable. Some commented they were never offered a non-operative option and saw this as a positive addition to available FAI treatments. The majority of patients regarded surgery as a definite solution for a severe condition that they thought was mainly about abnormally shaped bones. On the other hand, conservative care was perceived as being attractive because it was less invasive. Intensity and history of pain were important reasons to consider a specific treatment; for example, if the pain was acute and the person had had the condition for a long time, members suggested operative treatments would be more suitable.
Patients’ views about participating in the research and randomisation
The benefit to the whole community and helping clinicians improve their knowledge and advice for future patients seemed to be important motivators for patients to participate in research.
However, when the design of the RCT was explained, patients felt that ‘randomisation’ or ’50 : 50 allocation’ was not acceptable. There was a perception that clinicians would be not looking after the best interest of the patients but distributing people according to the needs of the trial. Patients presumed that clinicians would know which was the best treatment for them. Therefore, random allocation was perceived as unnecessary or odd and they were left asking how and by whom the decisions would be carried out. They also perceived that there was an imbalance between the two treatments in which surgery was perceived as curative while the comparator, physiotherapy, was not. Two patients expressed concerns about waiting for 12 months, as it could have an impact on the options available after the study finished. Another patient asked about how the progress of the treatment would be assessed during the trial and what would happen if the care plan was not working.
Patients reported that randomisation would be more acceptable if patients were assured that the alternative treatment to what a patient was allocated would be available to them at the end of the study. It was also important for patients to feel their treatment plan responded to their individual needs and that the team would continue to provide health care during and after the study.
Patient information sheet assessment
The expert patient members stated that the PIS presented a balanced view of the two treatments that were being compared. The language and illustrations used in the document were appropriate and accessible. Furthermore, patients found and understood easily the information about why it is important to treat hip impingement, the purpose of the study and what operative care involves and its risks. One patient mentioned there was not much information on the risks of not treating hip impingement. Patients did not find satisfactory the information related to conservative care risks, what happens after the study finishes and 50 : 50 treatment allocations. These items were related to the explanations about the trial procedure.
Patients suggested that the PIS would benefit from adding a rationale for offering physiotherapy for a morphological condition such as FAI. They also commented that patients needed to be reassured they would continue with the same consultant and health-care team, and that they needed to be given more details about what happened after the end of the trial. Additionally, some members of the panel said patients tended to look up FAI and available treatments on the internet, so links to trusted websites were suggested. Information about follow-up, details about possible benefits of the best conservative treatment and trusted website links were added to the PIS. The words ‘trial’ and ’50 : 50’ were removed and replaced by ‘study’ and ‘allocated to one treatment’, respectively.
Finally, the expert patient panel emphasised the importance of making patients feel they are receiving personalised care for their hip. They said that being given enough time to ask questions should be a priority during the recruitment consultations and patients should be reassured they would receive adequate treatment independently of how it is chosen. They thought the name ‘personalised hip therapy’ corresponded to these patient needs.
A new version of the PIS was developed that addressed these comments. It was reviewed and approved by the patient and public involvement (PPI) member of our research team and is shown in Appendix 6.
Discussion
Patients were prepared to take part in research to identify the best treatment for FAI and were even enthusiastic about a trial. They expressed concerns about the language of random allocation and wanted to be assured that treatment would be personalised for them. Patients felt that these concerns could be addressed by modifications in the PIS, which we subsequently tested in the pilot RCT.
We recognise that this study was of expert patients who had already been treated for FAI. Their assertions that they would have been prepared to be randomised, when considering the question retrospectively, may not be representative of the behaviour of patients presented with a new diagnosis and making a decision that would actually affect their treatment. A prospective study of patients in this situation is presented in the pilot RCT. However, this was sufficiently reassuring to support performing that RCT, and comments from these patients substantially influenced the design of that pilot.
Chapter 3 Pilot study
Objectives
-
To estimate recruitment to a RCT of arthroscopic surgery compared with PHT for FAI.
-
To test trial procedures and to ensure acceptability of the trial to ethics and research and development (R&D) committees.
-
To test measures of treatment fidelity for both arthroscopy and PHT.
Methods
Design
A pragmatic multicentre, parallel group, pilot RCT of hip arthroscopy compared with best conservative care for FAI with a QRI was undertaken at 10 NHS centres within the UK.
Participants
Patients were eligible to participate in the study if:
-
they were aged ≥ 16 years
-
they had symptoms of hip pain – they may also have had symptoms of clicking, catching or giving way
-
they showed radiographic evidence of pincer- or cam-type FAI on plain radiographs and cross-sectional imaging37
-
the treating surgeon believed that they would benefit from arthroscopic FAI surgery
-
they were able to give written informed consent and to participate fully in the interventions.
Patients were excluded if:
-
they had previous significant hip pathology such as Perthes’ disease, slipped upper femoral epiphysis or avascular necrosis
-
they had a previous hip injury such as acetabular fracture, hip dislocation or femoral neck fracture
-
they had osteoarthritis, defined as Tönnis grade of > 1,38 or more than 2-mm loss of superior joint space width on anteroposterior pelvic radiograph8
-
there was evidence that the patient would be unable to participate fully in the interventions, adhere to trial procedures or complete questionnaires, such as cognitive impairment or intravenous drug abuse.
Participants were recruited from among patients presenting to young adult hip clinics in each of the following 10 NHS centres:
-
UHCW, Coventry, UK
-
Yeovil District Hospital, Yeovil, UK
-
Royal Devon and Exeter Hospital, Exeter, UK
-
Royal Orthopaedic Hospital, Birmingham, UK
-
Frimley Park Hospital, Frimley, UK
-
Royal Cornwall Hospitals, Truro, UK
-
Epsom and St Helier University Hospitals, Epsom, UK
-
Guy’s and St Thomas’ Hospitals, London, UK
-
St Bartholomew’s and Royal London Hospitals, London, UK
-
University College Hospitals, London, UK.
This was an increase in the number of sites originally planned and included some changes of sites. During the pre-pilot phase the surgeons from all of the eight potential sites listed in the protocol v 12.0 12/02/2012 were interviewed to determine their equipoise and willingness to take part in the pilot trial. The interviews suggested that some surgeons would not be suitable recruiters/PIs. However, interviews with surgeons from other potential sites were more promising and as a result they replaced some of the original sites.
One potential site declined participation as it wanted to take part in another study involving patients diagnosed with FAI and felt it could not offer both studies to patients.
Recruitment
Possible patients with FAI (adults with hip pain and without a prior diagnosis of osteoarthritis) were identified by collaborating surgeons from referral letters. These patients were then invited to a diagnostic consultation with one of the collaborating surgeons. Prior to their appointment, patients were approached to seek consent for audio recording of their clinic consultations.
Surgeons assessed patients as usual, taking a history, examining the patient and performing appropriate imaging investigations. Patients in whom a diagnosis of FAI was made, and who met the eligibility criteria, received a ‘diagnostic consultation’ including a description of the condition from their surgeon and an explanation that there are two possible treatments. This diagnostic consultation was recorded where consent had been given. Patients were provided with a PIS about FAI and the pilot RCT.
Patients were then invited to attend a ‘recruitment consultation’ by a trained research associate. Typically this occurred on the same day and during the same clinic. During the recruitment consultation, information was provided about FAI and its possible treatments and about the pilot RCT. Patients were given an opportunity to ask questions. This recruitment consultation was recorded where consent had been given. Patients were then invited to give their consent to become participants in the RCT. Patients who wished to take more time to consider were given an opportunity to do so. Those who agreed to take part completed baseline questionnaires and were then randomised to one of the two treatments.
Treatment allocation
Participants were randomly allocated to arthroscopic surgery or best conservative care using 1 : 1 secure centralised telephone randomisation provided by Warwick Clinical Trials Unit. Patients were usually informed of their allocation at the recruitment consultation and plans for delivery of the intervention made at this point. Participants were allowed to withdraw from the trial at any time without prejudice. Participants who decided to have the treatment to which they were not randomised were followed up wherever possible and data collected as per the protocol.
Interventions
Participants were treated according to protocols summarised below.
Hip arthroscopy
Participants received the following protocol of care from a NHS orthopaedic consultant specialising in hip surgery:
-
Pre-operatively, patients underwent a routine pre-operative assessment of their general health and suitability for a general anaesthetic.
-
Perioperatively, patients underwent arthroscopic hip surgery under general anaesthesia in a lateral or supine position. Arthroscopic portals were established in the central and peripheral compartment under radiographic guidance in accordance with the surgeon’s usual practice. Shape abnormalities and consequent labral and cartilage pathology were addressed. Bony resection at the acetabular rim and at the head neck junction was ensured by intraoperative image intensifier radiographs and/or satisfactory impingement-free range of movement of the hip.
-
Post-operatively, patients were allowed home when they were ambulatory and safe with or without the aid of crutches. On discharge all patients were referred to outpatient-led physiotherapy services for a course of rehabilitation.
Best conservative care: ‘personalised hip therapy’
Participants received the following protocol of care from a substantive NHS physiotherapist specialising in musculoskeletal care who had attended a FASHIoN study PHT training workshop:
-
Pre-treatment – after allocation to PHT, patients received an information pack that provided details about PHT and what to expect during the course of their treatment.
-
Treatment – PHT was provided according to the protocol developed in the pre-pilot study and described above. In summary, participants had a detailed assessment of their condition, including an assessment of their pain, function and range of hip motion. Participants then received a further three core components of treatment: education about FAI and how to manage it; help with pain relief; and an exercise programme that had the key features of individualisation, progression and supervision. The protocol was delivered over at least 12 weeks with a minimum of six contacts. Some of the contacts were permissible using either telephone or e-mail in situations for which geographical distance prevented all contacts being carried out face to face.
-
Post-treatment booster sessions: after the 12-week treatment period, participants were offered additional support or guidance in up to four booster sessions with the physiotherapist up to 6 months after PHT began. A maximum of 10 treatment sessions in total was allowed.
Outcome assessment
Although the primary objective was to determine recruitment to a pilot RCT, patients were followed up as though they were part of a full RCT and the study conducted as though it was an internal pilot RCT. The following outcome measures were used:
-
hip pain and function – NAHS and the iHOT-33 (see Chapter 1)
-
activity level – University of California, Los Angeles (UCLA), activity score (a reliable and validated patient-reported activity grading system)66
-
health-related quality of life – Short Form questionnaire-12 items (SF-12)67 and EQ-5D68
-
a resource-use questionnaire.
These measures were taken at 3, 6 and 12 months after randomisation using a postal questionnaire (see Appendix 7). Questionnaires were posted and recipients were then followed up twice by telephone.
Concealment of treatment allocation
Patients, surgeons and physiotherapists were not blinded to the treatment allocation. The patient-reported functional outcome data were collected by post and entered onto the trial central database by a research assistant who was blinded to the treatment allocation. The statistical analysis was also performed blind.
Consent
Written informed consent for both consultation recording and involvement in the pilot RCT was undertaken by a clinical researcher or treating surgeon delegated and trained by the central research team.
Sample size
The pilot RCT was not powered to estimate a treatment effect, but rather to achieve a reasonable CI around the estimate of the recruitment rate, measured as the proportion of eligible patients who consent to participate and be randomised. Previous studies in orthopaedic surgery of operative compared with non-operative treatments have achieved recruitment rates of around 30%, so we modelled around this. Table 10 shows 95% CIs around various recruitment rates for a range of scenarios. Approaching 60 patients to invite them to take part in the trial would allow a 95% CI of 18% to 41% if the true recruitment rate was 30%. We judged this to be a good balance between cost and duration of the pilot RCT and the required precision of the estimate.
| Expected recruitment rate (%) | Sample size | ||||
|---|---|---|---|---|---|
| n = 15 | n = 30 | n = 60 | n = 90 | n = 120 | |
| 10 | 0.0 to 25.2 | 0.0 to 20.7 | 2.4 to 17.6 | 3.8 to 16.2 | 4.6 to 15.4 |
| 20 | 0.0 to 40.2 | 5.7 to 34.3 | 9.9 to 30.1 | 11.7 to 28.3 | 12.8 to 27.2 |
| 30 | 6.8 to 53.2 | 13.6 to 46.4 | 18.4 to 41.6 | 20.5 to 39.5 | 21.8 to 38.2 |
| 40 | 15.2 to 64.8 | 22.5 to 57.5 | 27.6 to 52.4 | 29.9 to 50.1 | 31.2 to 48.8 |
| 50 | 24.7 to 75.3 | 32.1 to 67.9 | 37.3 to 62.7 | 39.7 to 60.3 | 41.1 to 58.9 |
Therefore, we approached 60 eligible patients across 10 centres in order to estimate recruitment with sufficient precision to plan a full RCT.
Adverse events and serious adverse events
Adverse events (AEs) and serious adverse events (SAEs) not already identified locally and reported from the recruiting site were determined by a patient questionnaire at 6 weeks and 3, 6 and 12 months. Each recruitment site that discovered a SAE was obliged to report details by fax to the Clinical Trials Unit within 24 hours of the investigator becoming aware of them. Once received, causality and expectedness were confirmed by the chief investigator. All participants experiencing SAEs were followed up as per protocol until the end of the trial.
Fidelity of treatments
The quality of the evidence produced by a full RCT depends on the fidelity with which the two treatments being compared are implemented. Therefore, the following measures to determine treatment fidelity were tested in the pilot RCT.
Hip arthroscopy
For each participant, a vignette comprising an operative note describing the operation that had been performed, at least two intraoperative photos and a postoperative three-dimensional (3D) shape analysis from a single sequence magnetic resonance proton density volume acquisition were prepared. The 3D shape imaging was radially reformatted perpendicular to both the long axis of the femoral neck and the face of the acetabulum. Image slices for both the femoral head neck junction and acetabulum at 0 degrees (towards the patient’s head), 45 degrees and 90 degrees (directly anterior) were then included in the vignettes (six images in total) (see Appendix 8 for an example). Each vignette was then analysed by an international expert group comprising Mark Philippon (chairperson of the Research Committee of the International Society for Hip Arthroscopy; USA), Martin Beck (one of the investigators credited with developing the early understanding of FAI; Switzerland), John O’Donnell (a highly respected high-volume arthroscopic hip surgeon; Australia) and Professor Charles Hutchinson (an expert in musculoskeletal radiology; UK). Any disagreements were resolved by discussion and group consensus. When protocol deviations were noted, these were discussed with the surgeons involved in order to enhance compliance with the agreed intervention protocol.
Best conservative care: personalised hip therapy
For each participant, physiotherapists recorded full details of the advice and treatments, number and mode of treatment sessions, any non-attendance and any AEs for each patient on specifically designed case report forms. These case report forms were audited against the physiotherapist’s clinical notes to ensure accuracy and to determine protocol compliance by participating physiotherapists before collation by the research team and assessment by members of the core study group (see Chapter 2) in order to fully describe the interventions delivered. Any disagreements were resolved by discussion and group consensus. When protocol deviations were noted, these were discussed with the physiotherapists involved in order to enhance compliance with the agreed intervention protocol.
Economic analysis
One purpose of the feasibility study was to test measurement approaches and data collection systems that would facilitate an economic evaluation to be conducted as part of a full RCT. A patient-completed economic questionnaire was designed to capture health and social service resource use and any costs borne by the patient, including time and travel costs incurred in the receipt of care, out-of-pocket expenditures and time off work and usual activities. The economic questionnaire also included the EQ-5D and SF-12 health-related quality-of-life measures that can generate health utilities for economic evaluation purposes. The economic questionnaire was completed by study participants at baseline and, subsequently, at 3, 6 and 12 months. Information extracted from the resource-use component of the questionnaire was validated by cross-checking data provided by the first 10 patients concerning contacts with hospital services (inpatient and day case admissions and outpatient visits) at baseline in the lead site against electronic patient records available in the lead site. The validation process did not extend beyond local hospital services and, therefore, did not include contacts with primary care, other community services or contacts with other hospital services.
Statistical methods
Baseline characteristics (e.g. age and sex) are presented for both treatment groups (hip arthroscopy and PHT) to assess balance after randomisation; continuous data (e.g. age) were summarised by means and SDs and categorical (binary) data by counts and percentages. The distributional properties of the baseline scores were assessed visually using box plots. For the 42 patients recruited into the study, all data were complete (i.e. there were no missing data). Only 31 participants had completed 12 months of follow-up at the time of writing (February 2014), so only baseline scores are presented; outcome data will be described in future publications.
Summary of changes to the project protocol
Research Ethics Committee (REC) approval was received on 15 February 2012 for version 12.2 of the protocol. Following the original submission, a further three amendments have been made to the protocol and REC approval has been received for all three (listed below).
| Amendment | Protocol version, date | Summary of changes |
|---|---|---|
| 1 | 12.3, 19 June 2012 | The eligible age range was amended from 18–50 years to ≥ 16 years |
| The title ‘best conservative care’ was amended to ‘personalised hip therapy’ | ||
| SF-36 was replaced with SF-12 measure of quality of life | ||
| Addition of three new sites: | ||
| Yeovil District Hospital NHS Trust | ||
| Royal Devon & Exeter NHS Trust | ||
| Royal Cornwall Hospitals NHS Trust | ||
| Deletion of the following sites: | ||
| Cambridge University Hospitals NHS Foundation Trust | ||
| BMI The Meriden Hospital | ||
| 2 | 12.4, 30 October 2012 | Addition of UCLA activity score |
| Inclusion of operative photographs for the surgery quality review | ||
| Inclusion of a new section on international collaboration detailing four potential international sites | ||
| Addition of two new sites: | ||
| Frimley Park Hospital NHS Foundation Trust | ||
| Barts Health NHS Trust | ||
| Deletion of one site: | ||
| Royal Berkshire NHS Foundation Trust | ||
| 3 | 13.0, 30 January 2013 | The number of approaches was amended to ‘a minimum of 60 approaches’ |
Results
Trial approval and procedures
The trial was approved by National Research Ethics Committee (ref: 11/WM/0389) and by national and local R&D committees without changes and registered with the current controlled trials database under reference number ISRCTN 09754699. We identified several problems in obtaining local R&D approval and in setting up the trial across the 10 sites. These problems were different at each location, but were almost all solved. Further details are given in Appendix 9. Local R&D approval at one of the 10 sites was so delayed by local procedures that the centre opened only after target recruitment for the pilot RCT was completed. Once approval had been granted, we did not identify any major problems in the application of trial procedures at any of the sites.
Recruitment
A total of 60 eligible patients were approached for recruitment to the pilot RCT across nine centres during almost 10 months. One centre (site 10) was delayed in being set up and did not attempt recruitment before 60 approaches had already been made. Of these 60 patients, 42 consented to take part in the pilot RCT; therefore, recruitment was 70% (95% CI 58% to 81%). During this period a total of 151 patients were screened for the study. Overall recruitment activity is shown in Figure 5 and recruitment at individual centres is shown in Table 11. Note that centres opened for recruitment at different times and recruited for different periods. Reasons for these differences in uptake were explored in the QRI and they mainly related to logistical issues such as difficulties in setting up funding sources and assigning recruiters to attend clinics attended by potential participants (see Chapter 4).
FIGURE 5.
Recruitment during the pilot RCT.
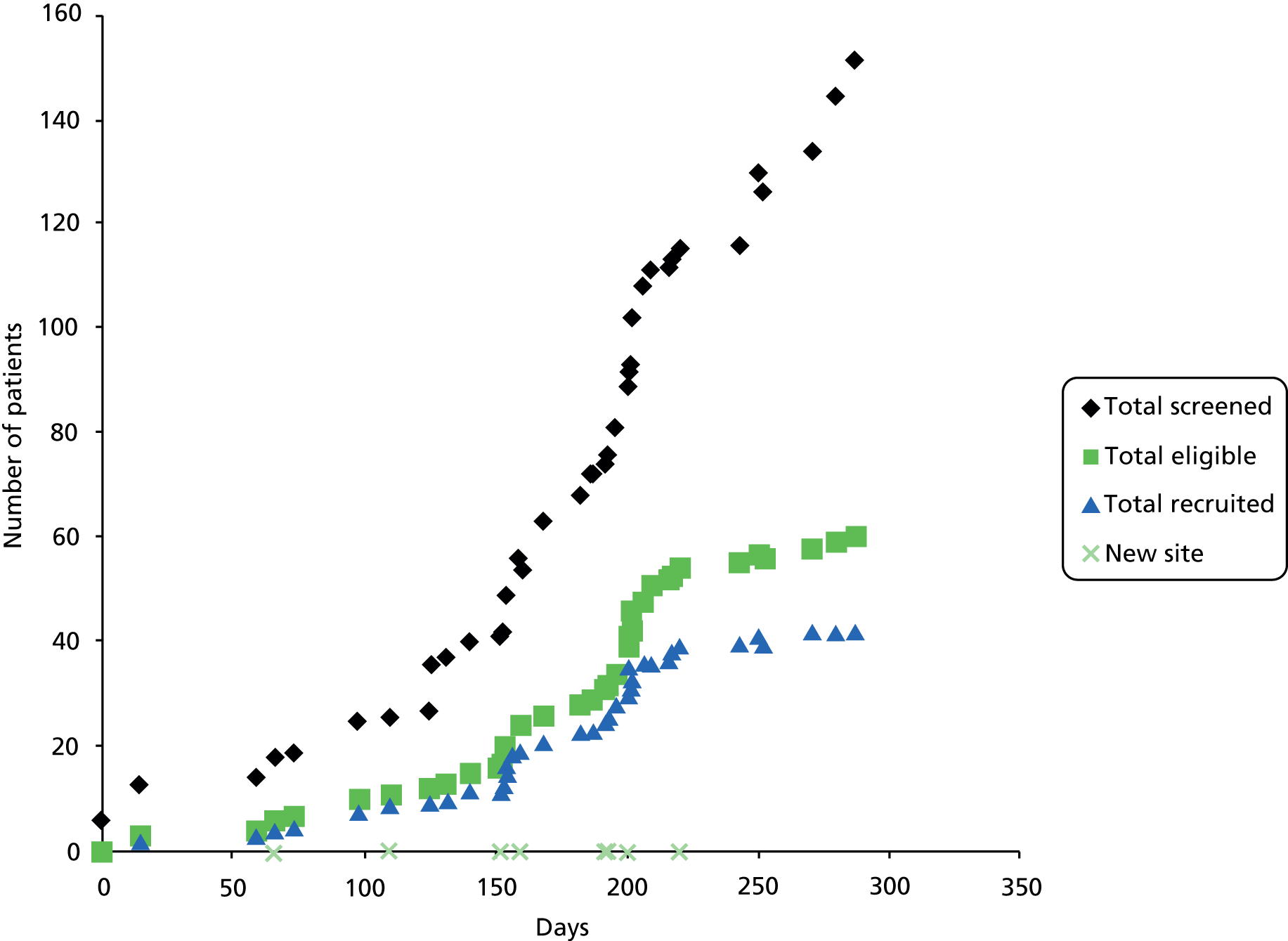
| Site | Recruitment duration (months) | Eligible patients | Recruited patients | Eligible patients/month | Recruited patients/month | Recruitment (%) |
|---|---|---|---|---|---|---|
| 1 | 9.3 | 24 | 19 | 2.6 | 2.1 | 79.2 |
| 2 | 7.1 | 7 | 3 | 1.0 | 0.4 | 42.9 |
| 3 | 4.4 | 3 | 2 | 0.7 | 0.5 | 66.7 |
| 4 | 5.0 | 6 | 3 | 1.2 | 0.6 | 50.0 |
| 5 | 4.1 | 4 | 4 | 1.0 | 1.0 | 100.0 |
| 6 | 3.1 | 4 | 4 | 1.3 | 1.3 | 100.0 |
| 7 | 3.0 | 1 | 1 | 0.3 | 0.3 | 100.0 |
| 8 | 2.8 | 10 | 5 | 3.6 | 1.8 | 50.0 |
| 9 | 2.2 | 1 | 1 | 0.5 | 0.5 | 100.0 |
| 10 | N/A | N/A | N/A | N/A | N/A | N/A |
| Mean | 4.6 | 6.7 | 4.7 | 1.5 | 1.0 | 70.0 (95% CI 58 to 81) |
Participant demographics were as expected, participants typically being young (mean age 35 years) and active (50% had a UCLA score of 1, 2 or 3, indicating regular participation in sport). Table 12 and Figure 6 show patient characteristics and baseline outcome scores by treatment group; there were no significant differences between those allocated to hip arthroscopy and those to PHT.
| Characteristics | PHT (n = 21) | Hip arthroscopy (n = 21) | All (n = 42) |
|---|---|---|---|
| Patient characteristics | |||
| Age (years)a | 33.4 (6.4) | 36.2 (9.8) | 34.8 (8.3) |
| Sex (female : male) | 6 : 15 (29%) | 10 : 11 (48%) | 16 : 26 (38%) |
| Side (left : right) | 11 : 10 (52%) | 5 : 16 (24%) | 16 : 26 (38%) |
| Smoker (no : yes) | 15 : 6 (71%) | 17 : 4 (81%) | 32 : 10 (76%) |
| Diabetic (no : yes) | 21 : 0 (100%) | 21 : 0 (100%) | 42 : 0 (100%) |
| CRF (no : yes) | 21 : 0 (100%) | 21 : 0 (100%) | 42 : 0 (100%) |
| Duration of symptoms (months)a | 30.9 (24.4) | 30.9 (28.5) | 30.9 (26.2) |
| Alcohol unitsa | 8.4 (9.4) | 8.9 (14.8) | 8.7 (12.2) |
| Scores | |||
| EQ-5Da | 0.58 (0.23) | 0.57 (0.30) | 0.57 (0.27) |
| NAHSa | 57.9 (18.9) | 52.9 (18.5) | 55.4 (18.6) |
| iHOT-33a | 31.4 (15.2) | 31.0 (17.3) | 31.2 (16.0) |
| SF-12 PCSa | 31.1 (14.8) | 28.6 (11.6) | 29.7 (13.3) |
| SF-12 MCSa | 46.4 (15.0) | 46.3 (11.8) | 46.3 (13.4) |
FIGURE 6.
Box plots of functional hip scores. (a) iHOT-33; and (b) NAHS, with means and 95% CIs.

Follow-up
This pilot study focused on estimating the recruitment rate and was not designed for estimation of a treatment effect. The majority of the participants had not reached final follow-up by the time this report was prepared. However, only 2 out of 42 participants did not receive their allocated treatment (one decided not to proceed with surgery and one asked to have surgery after beginning PHT), and follow-up rates were 98%, 94% and 100% at 3, 6 and 12 months, respectively. A Consolidated Standards of Reporting Trials (CONSORT) diagram for the pilot RCT is shown in Figure 7.
FIGURE 7.
The CONSORT diagram for pilot RCT. a, Follow-up rates reflect progress of patients as of 1 February 2014.

Procedures to ensure fidelity of interventions
Hip arthroscopy
Vignettes for the first seven participants to have hip arthroscopy were reviewed once the necessary post-operative MRI had been completed. Vignettes were provided by e-mail and the review was performed by teleconference. The panel felt that the balance, quantity and quality of the information presented was adequate to make a decisions about the fidelity of the surgery delivered. The panel resolved to classify each vignette as either demonstrating per-protocol care or not demonstrating per-protocol care. They discussed a category of borderline per-protocol care in circumstances for which they felt uncomfortable about one aspect of the surgery, but decided in the interests of a pragmatic trial to collapse this into per protocol care. Of the seven cases considered, the panel classified six as per protocol and one as not per protocol. This last patient had extensive cartilage damage at arthroscopy, which had not been identified during pre-operative assessment and was not sufficiently apparent on radiographs to warrant exclusion. The surgeon had decided not to proceed with reshaping and the panel considered this clinically appropriate, even though it deviated substantially from protocol, and suggested that such events be noted and considered in the analysis of a full trial.
Personalised hip therapy
Personalised hip therapy treatment logs and corresponding hospital notes were reviewed by the core study group for the first eight patients to complete PHT. After independent assessment, all members of the group agreed that the treatment logs accurately reflected and corroborated the corresponding patient’s notes and that these could be used to judge adherence to the protocol. Five of the eight participants were judged to have had per-protocol treatment. Deviations from protocol were for the first three patients in the trial and provided lessons on aspects of the protocol to be emphasised both in subsequent patient in the pilot trial and in the PHT training workshops for a full trial.
Economic analysis
We found a minimum of 98% completeness for all aspects of the economic questionnaires (encompassing resource use and health-related quality-of-life measures) at all follow-up points (Table 13). During the validation process, no discrepancies were found between use of hospital services reported by the patient and the corresponding electronic records.
| Resource-use questionnaire | EQ-5D | SF-12 | ||||||
|---|---|---|---|---|---|---|---|---|
| Time point | Number of forms | Overall | Time point | Number of forms | Overall | Time point | Number of forms | Overall |
| Baseline | 42 | 99% | Baseline | 42 | 99% | Baseline | 42 | 99% |
| 3 months | 39 | 98% | 3 months | 39 | 100% | 3 months | 39 | 100% |
| 6 months | 38 | 99% | 6 months | 38 | 100% | 6 months | 38 | 100% |
| 12 months | 31 | 99% | 12 months | 31 | 100% | 12 months | 31 | 100% |
Qualitative research found the economic questionnaire to be acceptable among patients at the lead site and that it could be completed by patients without any extra support from a research assistant.
We were able to establish estimates of unit costs for the majority of relevant resource inputs from a range of secondary sources, including national cost compendia such as the Patient Social Services Research Unit Costs of Health and Social Care and the British National Formulary. If unit costs were missing, these could be estimated using primary research methods in consultation with the lead site’s finance department.
Discussion
In this pilot RCT, we demonstrated that it was feasible to obtain ethics approval for this research question and to obtain R&D support in a variety of hospitals. Clinicians were prepared to take part, with surgeons agreeing to follow a defined operative protocol, and physiotherapists attending a training workshop and agreeing to deliver the PHT protocol.
We also demonstrated that that recruitment of study participants is possible across multiple sites. The recruitment rate (70%, 95% CI 58% to 81%) was encouraging when compared with similarly challenging RCTs that compare surgical and non-surgical interventions. The lead site (UHCW) was recruiting for the longest period (9.3 months) and achieved the highest recruitment rate among eligible patients, which is in keeping with published research suggesting that lead sites achieve the highest recruitment in RCTs. 44 Higher recruitment rates at the lead than other sites are probably a reflection of local enthusiasm for the RCT among investigators and the relative ease with which recruitment problems can be identified and resolved. When using the pilot data to plan a full RCT, it may be reasonable to exclude the lead site or make appropriate adjustments in order to more accurately predict recruitment rates across a multicentre study. Even excluding the lead site, eight other sites had a mean recruitment rate of 64%.
The number of potentially eligible patients identified in initial screening of clinic letters (n = 151) in the pilot trial was more than double the number of patients found to be eligible (n = 60), creating a substantial amount of work for research associates. Within hospitals, constraints on funding provided by the comprehensive local research network (CLRN) and the availability of research associates to recruit meant that not all clinics where potentially eligible patients could have been identified were able to be included. These two findings have implications for a full RCT, suggesting that considerable resources should be allocated for research associates to ensure identification of eligible patients at the fastest rate.
The two procedures to assess treatment fidelity that we tested within the pilot RCT worked well and could feasibly be implemented within a full RCT. This is important because, for the surgical protocol at least, we were not sure that clinicians would be comfortable with an independent panel assessing their surgical performance. Although the pilot RCT was pragmatic in design, all clinicians involved felt that some measure of treatment fidelity was important in order to ensure that an estimate of treatment effect was credible within the wider clinical and research community. Although the numbers were small, our assessment of fidelity suggests that clinicians and patients were able to follow the treatment protocols.
Participant compliance with treatment allocation was satisfactory: one participant allocated to PHT decided to have surgery (one crossover) and one participant who was allocated to surgery declined an operation and decided to be treated by a chiropractor. Follow-up rates were good and consistent with the 90–95% follow-up rate at 1 year that we have achieved in other orthopaedic surgical trials. Participants completed the questionnaire packs fully and expressed no concerns, confirming that the burden of the clinical effectiveness and cost-effectiveness instruments was not excessive.
An economic questionnaire encompassing a broad profile of resource-use questions and health-related quality-of-life measures that generate health utilities (e.g. EQ-5D, SF-12) was validated locally and shown to be acceptable to patients with high completion rates. These components, combined with the unit cost data we have estimated, will facilitate the economic evaluation planned as part of the full RCT.
In summary, the pilot RCT showed that clinicians and their patients with FAI are prepared to participate in a RCT comparing hip arthroscopy and best conservative care and that such a study is feasible.
Chapter 4 Qualitative recruitment intervention
Objectives
During the pilot RCT, we performed a QRI to understand how to optimise recruitment in a future full RCT of this question. The objectives were to:
-
understand the recruitment process so that any difficulties related to design or conduct can be identified and changes put in place
-
determine any staff training needs and develop a strategy to address these needs.
Overview of methods
Various methods were used to gather evidence to describe recruitment as it happened, which provided the basis for a plan of action to improve it. The aspects of the qualitative study of recruitment were not necessarily employed sequentially. The nature of the QRI meant that the research was moulded to fit the needs of the project and was completed when theoretical saturation was reached (that is, new data collection did not materially add to the findings). Qualitative data were collected at eight participating sites that recruited patients for the pilot RCT. Two sites were excluded from this report, as they had only just started recruitment at the time of submitting this report and needed more time before the teams could be interviewed.
Aspects of the qualitative study of recruitment are presented in the following sections, including, when appropriate, the data sample used, collection and analysis methods employed, and the corresponding summary of findings. A discussion of the overall results is presented in Discussion.
Patient pathways
Methods
A comprehensive process of logging of potential RCT participants through screening and eligibility phases was put in place in order to ensure compliance with the CONSORT checklist. These data were made available to the qualitative researcher (AR) on a regular basis to facilitate monitoring recruitment. These data and interviews with the different recruitment teams helped to develop a flow chart of the most likely patient pathways.
Results
Figure 8 presents a flow chart summarising the patient pathway. It was important to assess patient pathways in relation to their complexity and compliance with the protocol as well as variation between centres. This information provided indications of particular points where patients could potentially be ‘lost’ from the RCT.
FIGURE 8.
The FASHIoN pathway through eligibility and recruitment.
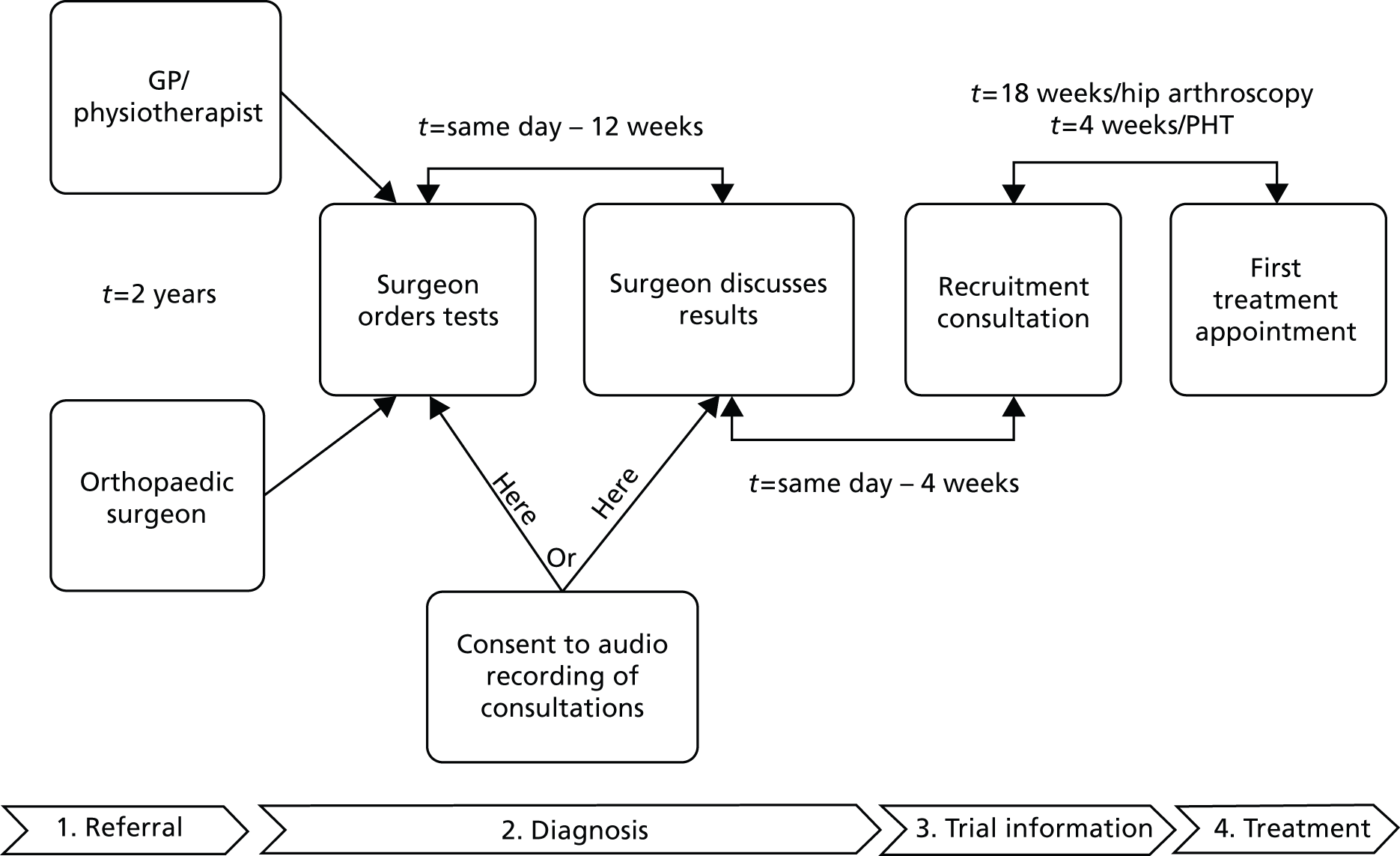
Referral
The participating centres varied in size, from medium-sized hospitals to highly specialised units in orthopaedic care within large institutions. Therefore, many patients arrived at the appointment after being referred by other orthopaedic surgeons or local physiotherapy services. On average, patients attended their first diagnostic consultation with an orthopaedic surgeon after 2 years of presenting symptoms.
Diagnosis
Usually further tests were requested before a FAI diagnosis could be confirmed. The waiting time between ordering the tests and confirming the diagnosis varied from a few hours on the same day to 12 weeks. The patient referral letters were screened prior to the start of the clinic and patients who could potentially have a FAI diagnosis were identified and approached in the waiting room. The recruiter sought consent from them to have their consultations audio recorded. The recruiter then facilitated the recording of the diagnostic consultation with the surgeon. Every so often a patient with suspected FAI needed further investigations before the diagnosis could be confirmed. According to the protocol, these patients were not eligible for the trial at this point and therefore should not be approached about the study. These situations had a tendency to confuse surgeons and recruiters because the patients might not be eligible to take part in the trial once the results were in, and yet they had been introduced to the audio-recording parts of the research. Further clarification of the protocol process and guidelines for this particular aspect of recruitment were developed and given to all the PIs and recruiters.
Successful recruitment experiences were common in larger centres where the PIs were able to run targeted young people’s hip clinics where the research associate could attend and carry out the trial recruitment processes immediately after the diagnostic consultation. However, this was not always possible, commonly because:
-
patients with a variety of diagnoses were being seen in the clinics, which had to respond to service demands and time constraints
-
not enough patients with FAI were being referred to the participating site or
-
limited research associate time made it difficult to assign a research associate for every appropriate clinic or a potential participant diagnostic consultation.
Once patients were told about their FAI diagnosis, the surgeon was meant to introduce the trial briefly and invite the patient to talk to the research associate. The PIs were aware of the division between diagnostic and recruitment consultations and the requirement of not discussing treatment options with the patients. Nevertheless, surgeons varied in the amount of information they gave to the patients about the trial in their introduction; for example, one surgeon opted for stopping at the point of discussing interventions and sending patients to talk to the recruiters directly, as illustrated by the following extract:
I’m quite happy that the diagnosis is femoral acetabular impingement, you may well have a bit of back pain going on as well, but there’s certainly enough, I think, symptoms around your hip to identify that.
OK.
So move things forward, is it all right if I leave you with [research nurse] to discuss the options that we’ve got for treatment, because the whole purpose of the research we’re doing is to obviously get to the bottom of that.
SN008: diagnostic consultation
By contrast, other clinicians discussed the treatment options with the patient at length. This situation was connected with unsuccessful recruitment consultations as explained later in this report. Some clinicians actually invited the patient to take part in the trial, which seemed to facilitate the subsequent recruitment consultation; for example, surgeons used phrases such as ‘I think you would potentially be suitable to enter the trial, should you wish’ (SN014).
Trial information
Patients were handed the PIS and were left to consider it. The time lapse between the diagnostic and recruitment consultations varied from a few minutes to weeks, depending on the clinic set-up at the particular site. Success in recruiting patients seemed independent of when the recruitment consultation happened; however, more recruitment appointments were carried out directly after the diagnostic consultation than when the recruiter and patient met on a different day. Patients who decided to become participants were asked to sign a consent form and recruiters then called the randomisation centre. Participants were told what intervention they would have and the contact details of the person in charge of making appointments. Some centres offered written information about the surgery to participants allocated to this group.
Treatment
The waiting list for the non-operative arm of the trial, PHT, tended to be shorter in most sites than the operative arm. Waiting times also varied across the centres and clinicians found it difficult to make an accurate estimate of waiting times during the interviews. Access to treatment centres was difficult in rural areas, especially for physiotherapy, as it was not readily available in the same sites where the surgery would have taken place. This was perceived as a disadvantage to those participants allocated to PHT and a concern for the recruiters at these sites.
Recommendations derived from the patient pathway analysis were incorporated into feedback given to the Trial Management Group (TMG) and site teams (see Recordings of diagnostic and recruitment consultations).
In-depth interviews with members of the Trial Management Group
Methods
In-depth, semistructured face-to-face interviews were conducted with members of the TMG, including the chief investigator and those most closely involved in the design, management, leadership and co-ordination of the trial.
Informants were asked about the background, development and purpose of the RCT; their knowledge of the evidence and their own opinion or equipoise; their role in the trial; and their expectation of the pathway through eligibility and recruitment. They were also asked to provide a short verbal summary of the RCT for the interviewer, as if AR was a patient.
Interview topic guides were used to ensure similar areas were covered in each interview, based on those used in previous studies,27 but also encouraging the informants to express their own views about the RCT and any recruitment challenges expected or experienced (see Appendix 10).
Transcripts of the interviews were analysed thematically by AR, using techniques of constant comparison and case study approaches. This involved detailed coding and then comparing emerging themes looking for shared or disparate views among TMG members. The coding was carried out using the qualitative data analysis software NVivo. The initial coding (AR) was checked by two other QRI researchers (AA and JD) and inconsistencies resolved by discussion. Detailed descriptive accounts of the themes and cases were produced.
Results
Ten interviews were conducted with members of the TMG. Analysis of these and observation of the TMG meetings showed the group was formed of four subteams with specific functions:
-
orthopaedic surgeons and senior physiotherapists in charge of designing the interventions, recruiting participants and offering a critical view of the research procedures
-
the trial administration and finance control group dedicated to data management, liaison with other agencies, and finance planning and expenditure
-
expert statisticians in charge of designing and planning statistical analysis
-
qualitative researchers in charge of the QRI.
Members of each subgroup had at least three different levels of involvement with the research. The first one was with the day-to-day running of the trial; at a second level, senior members supervised tasks and made major decisions (e.g. TMG meetings); and, finally, some members of the team remained distant from the trial procedures and provided a critical assessment of the trial and/or consultancy in particular aspects of the research (e.g. statistical analysis).
The team members showed an adequate knowledge of the trial protocol according to their level of involvement. Clinicians were the most knowledgeable as well as those involved with everyday activities, which was as expected. Task delegation within this multidisciplinary team prevented recurrent role conflicts and helped the team to develop ‘collective equipoise’. This term refers to the tendency of the team members to challenge each other’s expectations about the results of the RCT without favouring either of the two treatments tested. For example, although it was likely the orthopaedic surgeons running this trial tended to advocate for the operative treatment of FAI, they had a strong background in research and, therefore, were able to suspend clinical judgement and achieved a balanced view of the treatments that were compared, as illustrated by the following quote:
I distinguish there between my sort of gut feeling and my instinct. My instinct is that it works, surgery works [. . .] However, I do recognise that I have also seen it not work and I recognise that the evidence that supports it working, even the evidence I’ve generated myself, is methodologically weak and therefore I think that a scientific and objective perspective of the evidence [. . .] I am therefore an equipoise so I’m able to hold two views on it. And obviously, I mean the equipoise view is the one that I use when I’m involved in a trial.
TMG01
As a result, the team sought collaboration with physiotherapists at national and international levels and developed a credible conservative care protocol. Additionally, the TMG included professionals without expertise on hip arthroscopy or physiotherapy that facilitated equipoise.
A major concern for the TMG was the multicentre aspect of the pilot. They mentioned challenges such as difficulties implementing and overseeing procedures in other centres, delays in setting up, low numbers of eligible patients and lack of equipoise in research teams. The following quotes exemplified these concerns:
I know that the start of some of the sites has been a bit delayed but that is not unusual nor is it surprising given the complexities of setting up research in different sites.
TMG05
I’ve got major doubts [the study will be successful]. I think it was entirely the right thing to do it as a feasibility study, I have serious concerns that we won’t be able to recruit outside of this centre [. . .] Finding that surgeons and patients at the same time have both got equipoise is just going to be very difficult.
TMG02
Among the suggestions collected from the group to address the concerns were:
-
regular visits to the centres by the PI and other TGM members to keep momentum
-
delivery of a slick and easy-to-implement recruitment process in order to be the least disruptive to routine clinical practice
-
providing frequent and comprehensive training to recruiters helping them to develop skills and confidence (e.g. role playing, FAQ, peer support, etc.)
-
modifying the support to teams in other centres according to their research experience
-
setting recruitment targets and engendering a healthy competition between centres
-
sending regular newsletters providing information about recruitment.
In-depth interviews with surgeons, physiotherapists and research associates at participating sites
Methods
In-depth, semistructured face-to-face interviews were conducted with clinicians and research associates tasked with recruiting patients in visits to sites soon after they had started to recruit patients for the pilot RCT.
Informants were asked questions about their knowledge of the evidence for the treatment of FAI and their personal views about equipoise; the recruitment pathway; how they feel the protocol fits their clinical setting; and any adjustments they thought were needed to the trial procedures. They were also asked about the audio recording of their consultations with patients, with a view to discussing any discomfort or perceived difficulty with this.
Again, interview topic guides were used (see Appendix 10) and informants were encouraged to express their own views about the RCT and any recruitment challenges expected or experienced.
Analysis was as for the TMG interviews, but themes were also explored to examine differences or similarities between the TMG members and specialist clinicians and recruiters, and within or between centres or clinical specialties.
Results
Twenty-one interviews of clinicians and research associates were performed at eight participating sites. Common concerns and good practice examples were identified across the transcripts and were organised in the following themes.
Patient preferences and reactions
Various participating sites (n = 5) were pleasantly surprised by the acceptability of the trial to patients; for example, one PI reported:
I’ve been surprised at how willing people have been to go into the trial.
PI 3
This encouraged clinicians to continue approaching patients, despite their concerns about patient reactions and preferences at the start of the trial, which eased after they approached a few patients who consented to the study enthusiastically. PIs have found patients who were willing to be advised about a course of treatment (i.e. without set preferences) were generally happy with them suggesting they take part in the trial. One surgeon mentioned concerns about discussing uncertainty with patients because it could be detrimental to creating trust in their relationship:
I think the patients wouldn’t trust me. They would wonder what I was up to if I’d had one conversation and then reversed the conversation the second time around. And if there’s anything you need it’s for this kind of patients to trust you for this condition.
PI 6
However, this was not a majority view; surgeons and recruiters (n = 18) involved in this trial mentioned being comfortable explaining uncertainties within the context of the research, because they explained surgical uncertainties routinely in their clinical practice, e.g. one PI said:
I don’t have a problem with [explaining uncertainty]. Many of the conditions that we treat, there is no certainty about the results of treatment
PI 4
Contextual aspects of patient care
Principal investigators and recruiters commented on how external circumstances concerning the services they provided or patient-specific situations may have an impact on who might approach prospective candidates and how they would do it. For example, various sites expressed concern about patients being referred for ‘surgery’ instead of ‘treatment’. One site took the initiative of writing to the local referring professionals to make them aware of the trial and to ask for the referral letter to the patients to be changed so patients would expect to receive ‘treatment’ and not ‘surgery’. Some centres use a conservative approach and, therefore, patients tend to go for physiotherapy first before arriving at a surgeon appointment, as exemplified by the following quotes:
The other problem we’ve got is that patients who are referred from the other centres where they might have had extensive physiotherapy.
PI 3
Up here, prior to the trial starting, I’d always wanted patients to be seen by physio first to see if they could be improved by conservative means anyway.
P5
Recruiters said they would find it difficult to approach these patients or to feel confident they would agree to take part in the trial. The same applied to patients who would have to travel long distances in rural areas.
Accord with clinical practice
Clinicians concurred that the trial procedures and paperwork were not disruptive of routine clinical practice because they were clearly explained during the first visit and the study file was well organised. Research associates had a key role taking the burden of research procedures from the PIs and consultants during busy clinics; for example, a recruiting surgeon said:
So it’s a pressurised environment but with the research nurse it seemed to have run without causing too many time delays or any problems.
PI 6
This proved successful in that clinicians were less likely to disengage from the research procedure in order to complete their workload for the day. Nevertheless, teams experienced issues such as remembering to approach patients at each possible opportunity, or the need not to discuss surgery before diagnosis was confirmed. These issues happened more often when research associates were not available to attend the clinics. Some teams reported that communication between research associates and consultant surgeons about upcoming recruitment opportunities could be better in order to arrange the attendance of the research associates. The person in charge of the clinician’s diary or clinic appointments allocation at the site was in a privileged position to facilitate the communication between these two groups of professionals. Some research associates expressed their concern about talking to patients about the audio recording of the consultation. Patients were asked to give consent for the audio recordings at the beginning of their clinic but at times they were not eligible for the trial. Research associates did not know how to explain the recordings without telling patients about FAI and the FASHIoN study and felt uncomfortable to ask for consent from people not eligible to the trial.
Confidence of research associates about approaching patients
The research associates found the initial training received during the opening site visit was particularly useful because they had an opportunity to watch an experienced recruiter discuss the trial with a patient; for example, a recruiter said:
[Research fellow] came down and went through things with both of us on the first day and we’d had the material to read, you know, in the site file and things. So I’d say that the training on this study was better than I’ve had for a lot of studies.
R6
Research associates shared their concerns about not being able to answer patient questions and obtain consent without a surgeon or other senior clinician signing the form for them. For example, a research associate recalled a particular ‘bad recruitment’ experience when a patient did not understand how a computer could select their treatment and preferred the surgeon to choose a treatment for them instead, and another research associate wished a doctor to be present during the recruitment consultation. Alternative ways of explaining randomisation were collected and shared in the feedback to research associates. In addition, misconceptions that patients are taking a greater risk by consenting to the trial than by making a decision about their treatment were examined in training. Long periods between recruitment clinics represented a challenge for these research associates to maintain confidence and knowledge about the UK FASHIoN trial. The majority of them worked on different projects and gaps in recruitment meant it was likely they would forget the knowledge acquired in the previous recruitment appointments; for example, a recruiter said:
The gaps can be quite big between the patients so I go back to my notes and reread everything again just before I’m going to see them so it’s fresh in my mind because otherwise you’re likely to forget.
R3
Concise information about the trial and treatments as well as the six-step recruitment consultation model were made available to recruiters as quick reminders of the trial procedures prior to each recruitment opportunity.
Team familiarity with femoroacetabular impingement treatments and trial procedures
Principal investigators and recruiters recognised the importance of familiarising themselves with the material and were confident that more recruitment experiences would help them become effective at giving information. The majority of these clinicians were enthusiastic about their potential contribution to answering a relevant and valid scientific question through methods they found acceptable. However, some surgeons did not know or did not want to know about the non-operative arm and some divergent views were expressed among these professionals; for example, two surgeons thought (1) the conservative care arm did not seem an appropriate comparator:
I share the concerns and doubts that many of the patients do, i.e. that it won’t work and it’s difficult to sell a treatment when you yourself don’t really believe it’s going to make any difference.
PI 4
and (2) enough evidence of the benefit of hip arthroscopy for treating FAI was already available:
I spent 20 years learning how to treat patients with certain problems and I’ve gradually learned over the years which ones I can help and which ones I’m less effective in helping.
PI 7
These views were difficult to reconcile with their commitment to participate in the trial. Surgeons explained their participation out of a sense of duty to professional collaboration between peers; for example, one of the surgeons said:
My aim is to help Damian Griffin with his study and one day I may ask him to help me with a study that he thinks is awful.
PI 7.a
These themes were shared with the TMG and were used to design action plans for supporting recruitment at the different sites (see Evidence base for the trial).
Recordings of diagnostic and recruitment consultations
Methods
The chief investigator and TMG decided that the meeting in which randomisation was discussed, the ‘recruitment consultation’, as well as the prior appointment when the patient was told their diagnosis of FAI, ‘diagnostic consultation’, represented pivotal points of the recruitment to this trial and, therefore, sites were asked to record these two consultations for each potential trial participant.
The importance of audio recording discussions about RCT recruitment was emphasised at site initiation visit. All participating centres in the pilot RCT were asked to audio record recruitment and diagnostic consultations when appropriate and feasible. One main point of contact (usually the research associate) was identified per centre and digital audio recorders were provided. PISs and consent forms for audio recording and instructions for the operation of the recorder, dictation of patient/recruiter/recording identifiers, naming and transferring of the recording to the computer and then to the QRI team were provided to centres. Clinicians were assured the feedback to them was going to be confidential and positive (not critical). Prior to their consultation with the surgeon, patients were approached and the PIS for the audio recording of consultations was given to them to read. Once their questions were answered and if they agreed to the audio recording, patients were then asked to sign a consent form.
Audio recordings of consultations were analysed following thematic analysis and some of the techniques of focused conversation analysis pioneered in previous studies (Donovan et al. 2003;26 Donovan et al. 200927). The analytical techniques were used to identify and document aspects of informed consent and information provision that were unclear or disrupted or which hindered recruitment. The content of the appointments was evaluated, including what basic content was covered, the order of presentation of RCT arms and other treatment options, time spent on interventions and time spent describing both the RCT design and the randomisation process. Furthermore, an assessment was made as to whether or not the recruiter listened to patients’ concerns and addressed them and also the degree to which there was evidence that the participant understood the key issues of equipoise, randomisation, participation in the RCT, the option to choose their treatment and the option to withdraw from the research at any time.
AR documented these details and provided an account for JD and AA. When at least three recordings per recruiter had been analysed, the QRI researchers decided what confidential feedback would be given in order to promote research associates’ communication skills development and recruitment effectiveness. Issues to be fed back to the RCT chief investigator/TMG, or to be used anonymously in training programmes, were discussed and defined.
Results
Eighty-seven diagnostic and recruitment consultations relating to 60 individual patients were recorded and analysed. A subset of eight successful and 13 unsuccessful recruitment consultations were studied in detail in order to understand communication patterns that were linked to improved recruitment rates and which could be repeated in future patient approaches. The remaining audio recordings were used to validate the best recruitment practice model (see Figure 9) and create personalised feedback for recruiters who had submitted the recordings.
Qualitative recruitment intervention support focused on three sites where recruitment was comparatively low (42–66%).
Common problems identified were:
Unbalanced presentations of treatment options, for which surgery has been presented at greater length and more favourably than either choosing conservative care or participating in the RCT (surgeons tend to talk most about what they are most familiar with). The following quote illustrated this information unbalance:
There are other centres and other people have shown physiotherapy to be very useful. It has to be quite specialist, quite bespoke physiotherapy and the chap here from W [Warwick] University today has actually come down to recruit patients to a trial. The overall sort of duration of therapy is roughly around sort of 6 months for both of them to have their benefit, so it’s a similar timescale. Obviously the operation has small risks attached to it. There’s a very small risk of serious nerve injury, I mean it’s less than half a per cent, but, you know that’s the main concern with keyhole hip surgery, because we have to put the leg on traction in order to get in to the hip joint. And sometimes people’s nerves do not like being pulled upon, but that is very rare. We’ve had 2 cases at this hospital in around about 1000, so you know it’s a rare complication. A little bit of wound infection is a possibility, a little bit of bleeding, blood clots, things like this. They are all theoretical risks; they all seem to be quite low for keyhole surgery, so nerve injury is the only sort of serious problem that we’ve experienced. You can get a bit of numbness of the outside of the thigh because of the skin nerve that can get pranged because it’s near one of the portals. So these are the kind of downsides of surgery. So you know those are the two options available and I think you’re a good candidate for either one.
PI8
Graphic descriptions of surgery that may have put patients off randomisation; for example, one clinician explained:
There’s always a risk from the traction that it may stretch the nerves down the leg, so that could leave you with some numbness. If you’re very unlucky it could leave you with a little bit of weakness there.
PI4
Presenting trial information in an order that is confusing for patients, which is illustrated in the following quote:
OK, and I’ll probably bring [surgeon] in to explain more about the pros and cons of each of the treatments. Mr [local surgeon] would do the arthroscopy, but for the purpose of the study, we ask people if they’re happy to do it, and either of the treatments is chosen for you. So a specialised programme, a computer programme will choose the treatment of your . . . that you will receive.
SN004
Surgeons going beyond their protocol brief, to explain the trial rather than referring patients on to the trial recruiter for this information; this is illustrated in the following quote in which the surgeon has already discussed and agreed on a treatment before calling the researcher:
So I’m going to recommend that we (drilling continues) err, at least consider looking inside your hips.
Yes.
If you’ve got a moment, I would appreciate if you have a chat with P, who’s the researcher.
OK.
And just see if the study appeals to you. But you don’t have to, there’s no pressure, you don’t have to be enrolled in the study.
SN008
Conversely, surgeon endorsement of trial participation to patients appeared to have a positive impact on recruitment.
Previous research has shown that, in general, one in four people decline to participate in a RCT despite high-quality recruitment practices because of strong patient preferences or treatment refusal. The recruitment rate for the FASHIoN study was 70%, which suggested processes of informed consent and participant self-selection were working well. Therefore, identifying what worked in the recruitment appointments was relevant in order to replicate recruitment practices in the full trial.
A model of a good FASHIoN recruitment consultation was developed based on communication patterns identified in the observation of successful recruiters and the comparison and contrast of these patterns with the ones observed in unsuccessful recruitment consultations. Aspects such as sequence of presentation, balancing the time and level of detail of the treatment discussions and the sufficiency of explanations about the trial procedures were addressed. Figure 9 shows the stepwise approach to a good a recruitment consultation that has been developed based on the analysis of the audio recordings collected during the FASHIoN trial.
FIGURE 9.
The FASHIoN model of a good recruitment consultation: a six-step approach.

The main principle underpinning the model is that recruitment consultations are different from clinical consultations in that they should enable patients to understand uncertainty and lack of clinical research evidence. In routine clinical consultations, clinicians and patients may be dealing with many uncertainties at the beginning, but these are soon resolved through the interaction and when a decision about treatment options is made. The direction of the clinical consultation is from uncertainty towards decision-making, while in recruitment consultations the sequence of information sharing starts from stating what is currently known about the treatments and moves towards uncertainty. During the observation of successful recruitment consultations, a logical sequence emerged which achieved this purpose and is explained next.
Step 1: explain what femoroacetabular impingement is to the patient
The first step in the recruitment process is the diagnostic consultation. In successful recruitment consultations, patients received an explanation about FAI that was easy to understand. Clinicians tended to use lay terms to illustrate what happens to the body because of this condition (e.g. ‘shape abnormality’, ‘egg shape’, ‘extra little piece of bone’, etc.). They also made use of metaphors from common everyday experiences, for example piston heads in a car, as illustrated by the following quote:
If we imagine a car has a piston that’s not smooth, yours, your hip joint is like a, sort of a joint that’s not completely smooth and so when you try to move it, it’s causing damage to and pain in your hip joint.
SN034
These attempts to make sure the patient understands what it is happening to their bodies are important investments in the relationship and helped patients to feel confident in the care they were receiving. In addition to the ‘shape abnormality’ explanation of FAI, successful recruiters also introduced the role of muscle control in the diagnosis of FAI. This information permitted patients to make a logical link between the condition and the PHT as a plausible treatment, which could be utilised later in the discussion. Many recruiters expressed their concern about not being able to justify why physiotherapy could be effective. This explanation addressed this concern; for example, a surgeon summarised it as:
My idea on this is if you’ve got the egg shape, and your muscles are not good at supporting it, then you run into trouble.
SN053
Surgeons also introduced the common incidence of this condition in the population, which supported the sense of urgency about answering the research questions and increasing the available knowledge about FAI.
Step 2: reassure the patient that they will receive treatment
Patients who agreed to participate in the trial were reassured their diagnosis was confirmed. They were also reassured they would receive the best treatment to meet their individual needs, whether they agreed to be in the trial or not, as illustrated in the following quote:
You’re getting a bit of extra rubbing. And that rubbing is causing the pain, so there’s not really any mystery, we know what the problem is [. . .] my suggestion is that we treat this problem; I don’t think we should just leave it alone, I think we need to try and make you better.
SN034
This was important because many patients waited for a long time to reach the appointment with the surgeon and may have been previously misdiagnosed. Patients may arrive at the clinic dissatisfied with the treatment they received so far. Often patients who declined to be part of the trial have experienced these difficulties. Being told that finally they have the right diagnosis, that someone is confident about it, generates trust and openness to the invitation to help improving care for other patients like themselves. This step addressed the previously identified concern that the treatment allocated to them is not taking into consideration their personal circumstances and their individual needs for care. A direct invitation from the consultant surgeon to the patient about participating in the trial seems particularly effective in getting the person to consider the trial and to listen to the recruiter; an example is presented in the following extract:
And in a minute, one of my colleagues, if you agree, will come and talk to you about how that particular study is working. And, if you would like to, I would very much like to include you in that study?
SN053
Step 3: explain that there is uncertainty about which treatment is the best
Steps 1 and 2 promote certainty, whereas step 3 is about introducing uncertainty. In successful recruitment consultations, uncertainty was mentioned earlier on and by the consultant, and then it was reinforced repeatedly by the recruiter during their appointment with the patient. Even in effective recruitment consultations, recognising uncertainty may be difficult for patients. Effective recruiters enabled the patient to understand that the two treatments that were being compared are effective in their own right. This argument helps to compensate for the uneasiness of not knowing which treatment is best. The following extract of a diagnostic consultation exemplifies this step:
I think we can offer you some treatment for it, but the trouble is we’ve only really just started to understand this disease [. . .] we’ve got two treatments that we know can help, but we just don’t know which is the better one.
What we’re doing is, as [the consultant] has explained, we don’t know which is best, we’re not sure. [Consultant], myself and any of the other consultants that specialise in hips here, can’t tell you. For you, I think the best thing to do is X or Y because we don’t know.
You don’t know what the best treatment is?
Well, we need people like you to say, ‘OK, I’m happy to take part, and whichever treatment I get then I should, should get a little bit better’, but one will be better than the other probably.
SN034
Step 4: explain the purpose of the study
Once uncertainty was been explained, the purpose of the study follows logically. Recruiters stated how the findings of the research would help clinicians to advise patients like them in the future. At this point, successful recruiters mentioned the need for evidence, implying the real contribution to advancing science and health care that patients could make. Recruiters were successful at harmonising the message that FAI is a condition that clinicians are interested in knowing more about how to treat and the message that patients would be valued, respected and cared for during the trial. An example is presented below:
[The recruiter] can explain the study to you, and explain how we try and find out the answer to the question and how you might get involved in that. One thing though, is whatever we do, and I’m going to do everything I can to make you better.
SN006
Step 5: give the patient a balanced view about the pros and cons of each of the two treatments
In some cases, the recruitment consultation was introduced as a conversation about the options available to patients. For example, one surgeon said:
[Recruiter] is doing a study looking at the different solutions and he could talk to you about the options if we got to the point where you said you’d like to do something about it.
SN007
Instead, some successful recruiters and clinicians avoided the word ‘options’ when referring to the treatment arms of the RCT because it implies there is a decision to be made between the two treatments, whereas the patient has to decide between being part of the trial or not. Alternative expressions such as ‘available treatments’ or ‘ways to treat FAI’ were used, providing a clearer message. In effective recruitment consultations, the treatment arms were presented as two ways of dealing with the same condition. Recruiters spent similar amounts of time explaining each one of the arms. They tended to start with the non-operative arm and the balance between the two treatments was maintained when talking in detail about the benefits and risks of each arm (e.g. number of visits to hospital for each arm, duration of the intervention). For example, a recruiter discussed the balance between arms as:
The kind of use of your time is equal, whichever one you go for, whichever one you’re allocated to . . . The reason I say that is because if you have an operation you have to come for a pre-operative assessment, you have to have the operation and you have to have some rehab. If you’re allocated to the personalised hip therapy, you’ll have to come here on three, at least three occasions, and then they’ll give you specific exercises to practise at home.
SN034
Effective recruiters also emphasised the benefits of the two treatments in order to assure patients they were not taking any more risks than if they were making the choice themselves. After step 5, patients usually were not sure what treatment to choose. They may have thought about having one treatment at the start of the conversation, but at this point, they expressed either the opposite choice or simply uncertainty about which one to have. Patient equipoise was thus achieved.
Step 6: explain the study procedures
Once uncertainty and equipoise were established, the allocation of treatments by randomisation seemed more acceptable. Randomisation was mentioned later on in the conversation and recruiters spent a lot less time explaining it than the time they spent on the other steps. Randomisation was usually presented as an allocation or being invited to join one of the groups, which should allow a fair comparison. The following quote exemplified this explanation:
But in order to make it a fair test and in order to make sure that we’re truly, truly testing which one’s better, we have to go through a process whereby we allocate you with a treatment. So I don’t choose and [Professor] doesn’t choose and we don’t choose which, which treatment you have . . . it’s randomly allocated to you
SN003
The questionnaires and the reassessment after a year were constructed as part of a closer follow-up of participants in the trial than for people going through regular care. This was part of the efforts of the recruiters to emphasise that patients would be treated with respect and receive personalised care.
Two more tasks were added to the model. They are not a fixed part of the six-step sequence; instead they happen at different points throughout the most successful consultations. These two tasks appeared to be essential and determinant in the successful of a recruitment consultation.
Task 1: respond to patients’ concerns and questions
The majority of patient questions occurred after the two treatments were explained. Often patients wanted to know about the details of each arm and the rationale for the non-operative arm. Successful recruiters stopped delivering information and answered the questions or listened to patient concerns before moving onto the next step. The conversations in the most successful recruitment consultations were highly patient-centred, with evidence the clinicians were responding to the patient’s concerns and doubts about the trial. Patients asked more questions than in unsuccessful recruitment appointments. Recruiters confirmed that patient participation was voluntary and that patients would receive treatment regardless of their involvement with the study, which encouraged trust.
Task 2: show confidence and a relaxed manner
Effective recruiters appeared confident and relaxed when talking about the study. This was based on having a good working knowledge about the condition and the treatments or taking actions towards achieving it. In addition, successful recruiters used a set of ready-made answers to common patient questions prepared by the TMG and worked closely with the clinicians in order to deal with tricky questions.
The model structure is identical to the approach used in the PIS. Therefore, giving the patient the opportunity to read this document at the beginning of recruitment could save time dedicated to information sharing. Instead, this time can be used to respond to patients’ concerns, showing the balance between risks and benefits between the two trial arms, reassuring patients about participating in a trial, explaining the condition in more detail or discussing uncertainty. Feedback to the sites was prepared based on this model and presented to the recruiters and PIs (see Evidence base for the trial). Further data collection continued to be used to develop and validate the model.
Study documentation
Patient information sheets were compared with the findings from the interviews and recorded diagnostic and recruitment consultations, to identify any disparities or improvements that could be made. Careful preparation of the study documentation was carried out during the pre-pilot phase of the study as reported in previous sections. The pilot trial showed this documentation fulfilled its purpose well and would be suitable for a full trial.
Evidence base for the trial
During the interviews and recorded appointments, the QRI team checked for any relevant and emergent evidence that could support or threaten the RCT. Surgeons in participating sites referred to case studies of successful FAI treatment with hip arthroscopy as the highest level of evidence. However, the clinician group in this RCT has been actively involved in the assessment of the scientific literature for operative and non-operative FAI treatments and recently published a systematic review of best conservative treatment for FAI. The evidence is still that a RCT is required.
Qualitative recruitment intervention feedback to chief investigator and trial management group
The TMG met regularly and aspects of the recruitment and eligibility criteria as well as the day-to-day running of the pilot trial were discussed. The QRI research team attended the TMG meetings providing an update on recruitment progress, raising issues discovered during the QRI and suggesting training or advice about recruitment. The QRI team prepared and presented various reports (e.g. Appendices 4 and 7) identifying any aspects of the study design or conduct that could be hindering recruitment with the supporting evidence. These findings were the basis for action plans that included issues with patient pathways and logistical challenges in particular centres. Agreed actions with the chief investigator and TMG to address these issues were:
-
Feedback to PIs and recruiters should be given through personalised letters. These were prepared and sent to the centres (see Appendix 11 for examples).
-
Specific centres and PIs needed follow-up with text messages and newsletter reminders about the need to recruit.
-
Contacts between research and clinical departments about recruitment opportunities should be encouraged.
-
Training materials should be prepared and disseminated. These included:
-
a document explaining the FASHIoN recruitment consultation model
-
an example of successful recruitment consultation
-
patients’ frequently asked questions and answers document and
-
treatment protocols.
-
Overall, the recruitment rates have remained stable over the course of the pilot. The qualitative study of recruitment therefore showed there are no major concerns about the feasibility of patient recruitment for this trial. The work with the three centres with lower rates continues and their improvement will be assessed shortly; however, owing to the pilot time scale, these results had not been received by the time this report was completed.
Discussion
The pilot phase of this RCT has shown the recruitment of patients is feasible and acceptable. The qualitative analysis of recruitment highlighted good practices that can continue to be promoted in a full trial. There is evidence the separation between diagnostic and recruitment consultations led by research associate recruiters can be implemented and have a positive impact on recruitment rates. These research associates contribute to making the research process less disruptive of clinical practice and promote equipoise when presenting the available treatments to patients. Clinic attendance and communication between the research associates and personnel in charge of scheduling appointments has been shown to be relevant. Therefore, it is important sufficient resources be allocated to these aspects of the research in a full RCT.
The study documentation was well organised and performed as expected. The initial visits to the sites included training sessions modelling good recruitment consultations, which were valued by research associates, as they helped them to develop their understanding and skills about recruitment for this particular RCT. Creating a video and material that they can access easily before each recruitment opportunity arises may be an ideal way to disseminate and standardise recruitment practices across participating sites.
We had been concerned that clinicians and research associates would be unfamiliar with audio recording and, even if they agreed to it, might resist making successful recordings. However, there was no evidence that this was the case in this pilot RCT. The audio recordings of consultations were introduced to the sites at the site initiation visits and adequate information about the recording and transfer of files was developed. Further clarity about the consent process for the audio recording will be required to avoid misunderstandings within the research teams at participating sites.
Two qualitative analytical methods described in the protocol were not used in the pilot study. We proposed to transcribe and analyse the TMG and TSG meetings. Recruitment difficulties were expected at the early stages of the RCT and the analysis was set up to foster discussions between team members. However, the QRI team attended these meetings and observed the groups were generally in agreement about the way forwards and hence functioning sufficiently well. Consequently, it was decided that no further intervention was necessary.
The second method we did not use was the Roter Interaction Analysis System (RIAS) system. Initially RIAS was going to be applied to diagnostic and recruitment consultations that failed to recruit patients to the trial. The RIAS system works based on the frequencies of certain target behaviours, which then can be analysed statistically. However, there were few unsuccessful recruitment consultations, which were likely to register low frequencies in RIAS. As a result, this quantitative method was not viable. The QRI team decided instead to focus on the qualitative analysis of successful consultations using focused conversation analysis, a method developed and tested by JD in previous research. 22 The method is suitable for small samples and has the advantage of uncovering subtleties in communication that can be modified. The analyses highlighted and illustrated key messages for successful recruitment practice, providing data that helped underpin the training materials developed.
Some challenges to recruitment success emerged from this research connected to logistical issues. Strategies for reminding research teams, and especially surgeons, about identifying and approaching potential participants for the trial should be implemented. These could be electronic remainders sent on the day of clinics, regular visits to the site and newsletters to maintain momentum and engagement at participating sites. It is also important to consider how the TMG can facilitate awareness of the trial at referring centres and among orthopaedic surgeons who are not directly involved in the trial. This is a concern for the PIs and research associates who perceive they may need support in dealing with patient expectations. Based on the evidence collected in this qualitative study of recruitment, the TMG has shown a high level of organisation and effectiveness in delivering an acceptable research study for patients with FAI and clinicians dedicated to its treatment.
Chapter 5 Discussion and conclusion
We have shown that a RCT of arthroscopic surgery for hip impingement compared with best conservative care is feasible.
The feasibility study included (1) a pre-pilot phase including patient and clinician surveys and interviews; (2) a workload survey of hip arthroscopy for FAI; (3) development of best conventional care and arthroscopic surgery protocols; (4) a pilot RCT to measure recruitment rate; and (5) an integrated programme of qualitative research (QRI) to understand and optimise recruitment.
The feasibility study specifically addressed the following aspects of the design of a full-scale RCT.
-
Define eligibility criteria: we have defined these by international consensus and confirmed that they are applicable in a pilot RCT.
-
Define a protocol for hip arthroscopy for FAI: we have defined this and shown that it can be applied in several centres and by several surgeons in the context of a RCT. We also developed a method to measure fidelity by intraoperative photographs and postoperative MRI, assessed by a panel of independent international experts. We showed that this approach was acceptable to surgeons and demonstrated complete adherence to protocol in six out of seven operations at the first panel conference.
-
Define a protocol for best conventional care (comparator): we have developed a physiotherapy-led, four-component protocol through national and international consensus. We used a patient focus group to choose the most acceptable name for this protocol of best conventional care; they selected ‘personalised hip therapy (PHT)’. In the development of PHT we struck a balance between the need for a meaningful comparator for hip arthroscopy, the need to ensure PHT is different from previous physiotherapy that FAI patients may have experienced and the need for PHT to be deliverable in the NHS outside a trial. We tested the protocol and a logbook approach to assessing fidelity in 21 participants randomised to PHT in the pilot trial. The protocol was acceptable to patients and physiotherapists and we demonstrated complete adherence in five of the first eight participants.
-
Define willingness of centres and patients to be recruited to a RCT: we have demonstrated that around 2000 patients have arthroscopic surgery for FAI each year in the UK and that most of these are treated in relatively few high-volume centres. Most (17/18) of the surgeons in these centres expressed a preparedness to randomise patients. All (18/18) of a sample of patients who had been treated for FAI said that they would have been prepared to take part in a trial if it had been offered to them, but expressed concerns about how the trial and treatment might be explained adequately.
-
Understand and optimise recruitment: we identified structural features associated with successful recruitment, such as running targeted clinics, having a dedicated research associate in attendance and ensuring referred patients arrived with expectations of receiving treatment for FAI rather than being told they had been referred for surgery. We identified clinician and research associate behaviours that were associated with successful recruitment and developed training packages to correct common problems. Analysis of 87 consultations with 60 patients led to the development of a six-step model for presentation of trial information (see Figure 1) to optimise recruitment.
-
Estimate recruitment rate: we have demonstrated a recruitment rate of 70% in a pilot RCT, with a lower limit 95% CI of 54%. This suggests that it would be reasonable to conservatively plan for a 50% recruitment rate in a full trial.
-
Select appropriate outcome measures: we have shown that iHOT-33 and NAHS are both easy to use and acceptable to patients. The extensive patient involvement in item generation, the availability of an independently determined MCID and the use of iHOT as the principal outcome measure for the UK Non-Arthritic Hip Registry lead us to suggest iHOT-33 as the most appropriate primary outcome measure for a full trial.
-
Estimate the SD and effect size of the primary outcome measure: in our pilot study, the baseline iHOT-33 data of patients who were suitable for inclusion had a SD of 16.0, which is consistent with other studies. There is little information with which to estimate the likely effect of hip arthroscopy compared with best conventional care, but an analysis of observational studies suggests a standardised effect size of > 0.5. iHOT-33 has a reported MCID of 6.1 points, so a RCT using this instrument should be able to demonstrate a standardised effect size as low as 0.5.
-
Develop and test trial procedures: we designed protocols, eligibility criteria, patient information material and case report forms and found that they performed well in the pilot RCT. REC and national R&D approvals were granted for the pilot trial promptly and without any significant concerns.
-
Estimate follow-up rates: the pilot RCT was designed to estimate recruitment rate. As of 1 February 2014, 32 patients have reached the full 12 months of follow-up. We have achieved 93% (39/42) follow-up at 3 months, 90% (38/42) follow-up at 6 months and 97% (31/32) follow-up at 12 months.
-
Calculate sample size for a definitive study: we estimate that 292 participants would be required for a full trial to have 90% power to detect an effect difference equal to or more than the MCID at 12-month follow-up, between treatment arms, in iHOT-33 (6.1 points) at 5% significance. Assuming 85% follow-up at 1 year, this suggests a total sample size of 344 participants. With a 50% recruitment rate, we estimate that this could be achieved in 25 of the higher-volume centres in the UK over 20 months.
-
Public and patient involvement: patients made a very large contribution to this study. A patient was part of the research team from the outset and is one of the authors of this report. A panel of expert patients made key contributions to the pre-pilot work, particularly in expressing enthusiasm for a trial, explaining how patients would feel about being approached to be randomised and designing the patient information for the pilot RCT. Patients told us that we had to be careful to design a credible best conventional care comparator for hip arthroscopy and chose the name for it. Our patient collaborator helped us to develop our ethics submission and then participated in the Trial Steering Committee. In subsequent work to plan a full RCT, a second patient has joined our collaboration and we have created a panel of patients and members of the public drawn from the University of Warwick University/User Teaching and Research Action Partnership (UNTRAP) PPI programme. They are guiding us in marketing the trial and ensuring dissemination methods such as Twitter (San Francisco, CA, USA) and Facebook (Cambridge, MA, USA) that are suitable for our target patient population.
In conclusion, it is clear that a full RCT of hip arthroscopy compared with best conventional care for FAI is feasible. The technology of hip arthroscopy is sufficiently mature and in sufficiently widespread use for a pragmatic trial to be done. Our findings were that equipoise is not universal, but that there are still enough patients and surgeons with enough uncertainty for a trial to be completed now. The design and structure of a RCT was developed and tested in a pilot RCT, which can be used as an internal pilot for a full RCT. Recruitment was challenging, largely because the two interventions under scrutiny are so very different. Despite this, a QRI helped us to achieve a recruitment rate of 70% recruitment across the sites. A full RCT involving more sites will need to continue to use a QRI to ensure successful recruitment.
The window of opportunity for a trial of this technology is open now and we have developed a study design that we have demonstrated to be feasible. We propose that a full trial be performed.
Acknowledgements
The authors wish to acknowledge funding for this study from NIHR HTA, support from hospital Trusts, CLRNs and collaborating surgeons, physiotherapists and research associates, and the work of Ceri Jones, R&D manager at the lead site, UHCW NHS Trust. Finally we thank the patients who gave so generously of their time in the expert patient panel, and those who agreed to participate in the pilot RCT.
Contributions of the authors
Professor Damian Griffin, Professor of Trauma and Orthopaedic Surgery, led the research as chief investigator. He wrote the proposal, designed the study, supervised the research and wrote the final report.
Mr Peter Wall, Clinical Research Fellow in Trauma and Orthopaedic Surgery, helped to plan the study, developed the surgery and PHT protocols, performed the FAI surgery workload study, supported the centres during the pilot trial and wrote a first draft of the final report.
Dr Alba Realpe, Qualitative Research Fellow, conducted and analysed interviews with patients, clinicians and researchers, edited patient information and developed the six-step guide to a good recruitment consultation.
Dr Ann Adams, Principal Research Fellow, helped to design and analyse the QRI and supervised Alba Realpe.
Dr Nick Parsons, Medical Statistician, helped to design the study and performed the quantitative analyses.
Mrs Rachel Hobson, Trial Manager, was responsible for the day-to-day running of the study.
Dr Juul Achten, Research Manager, provided senior management support.
Mr Jeremy Fry, Patient representative, reviewed the original proposal, was a member of the Trial Steering Committee and helped to design the PPI components.
Professor Matthew Costa, Professor of Trauma and Orthopaedic Surgery, helped to design the study.
Professor Stavros Petrou, Professor of Health Economics, developed the economic analysis.
Professor Nadine Foster, NIHR Professor of Musculoskeletal Health in Primary Care, led the development of the PHT and implementation of that protocol.
Professor Jenny Donovan, Professor of Social Medicine, co-led the QRI with Dr Adams.
All of the authors were involved in drafting the final report and approved the final version.
Publications
Wall PDH, Fernandez M, Griffin DR, Foster NE. Non-operative treatment for femoroacetabular impingement: a systematic review of the literature. PM R 2013;5:418–26. doi: 10.1016/j.pmrj.2013.02.005
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock K. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003;417:112-20.
- Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br 2005;87:1012-18. http://dx.doi.org/10.1302/0301-620X.87B7.15203.
- Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The aetiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res 2008;466:264-72. http://dx.doi.org/10.1007/s11999-007-0060-z.
- Ganz R, Gill TJ, Gautier E, Ganz K, Krugel N, Berlemann U. Surgical dislocation of the adult hip a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br 2001;83:1119-24. http://dx.doi.org/10.1302/0301-620X.83B8.11964.
- Beaule P. The Young Adult with Hip Pain. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007.
- Ito K, Minka MA, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect: a MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br 2001;83:171-6. http://dx.doi.org/10.1302/0301-620X.83B2.11092.
- Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res 2004;418:67-73. http://dx.doi.org/10.1097/00003086-200401000-00012.
- Philippon MJ, Briggs KK, Yen YM, Kuppersmith DA. Outcomes following hip arthroscopy for femoroacetabular impingement with associated chondrolabral dysfunction: minimum two-year follow-up. J Bone Joint Surg Br 2009;91:16-23. http://dx.doi.org/10.1302/0301-620X.91B1.21329.
- Bardakos NV, Vasconcelos JC, Villar RN. Early outcome of hip arthroscopy for femoroacetabular impingement: the role of femoral osteoplasty in symptomatic improvement. J Bone Joint Surg Br 2008;90:1570-5. http://dx.doi.org/10.1302/0301-620X.90B12.21012.
- Schilders E, Dimitrakopoulou A, Bismil Q, Marchant P, Cooke C. Arthroscopic treatment of labral tears in femoroacetabular impingement: a comparative study of refixation and resection with a minimum two-year follow-up. J Bone Joint Surg Br 2011;93:1027-32. http://dx.doi.org/10.1302/0301-620X.93B8.26065.
- Stevens MS, Legay DA, Glazebrook MA, Amirault D. The evidence for hip arthroscopy: grading the current indications. Arthroscopy 2010;26:1370-83. http://dx.doi.org/10.1016/j.arthro.2010.07.016.
- Wall PD, Brown JS, Parsons N, Buchbinder R, Costa ML, Griffin D. Surgery for treating hip impingement (femoroacetabular impingement). The Cochrane Library 2014.
- Wall PDH, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R 2013;5:418-26. http://dx.doi.org/10.1016/j.pmrj.2013.02.005.
- Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C. Choosing between randomised and non-randomised studies: a systematic review. Health Technol Assess 1998;2.
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999;52:1143-56. http://dx.doi.org/10.1016/S0895-4356(99)00141-9.
- Mapstone J, Elbourne D, Roberts I. Strategies to improve recruitment to research studies. Cochrane Database Syst Rev 2007;18.
- Treweek S, Mitchell E, Pitkethly M, Cook J, Kjeldstrom M, Taskila T, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev 2010;20.
- McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ 2002;324:1448-51. http://dx.doi.org/10.1136/bmj.324.7351.1448.
- Ergina PL, Cook JA, Blazeby JM, Boutron I, Clavien PA, Reeves BC, et al. Challenges in evaluating surgical innovation. Lancet 2009;374:1097-104. http://dx.doi.org/10.1016/S0140-6736(09)61086-2.
- Jarvik JG, Comstock BA, Kliot M, Turner JA, Chan L, Heagerty PJ, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet 2009;374:1074-81. http://dx.doi.org/10.1016/S0140-6736(09)61517-8.
- Klazen CA, Lohle PN, de Vries J, Jansen FH, Tielbeek AV, Blonk MC, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet 2010;376:1085-92. http://dx.doi.org/10.1016/S0140-6736(10)60954-3.
- Kirkley A, Birmingham TB, Litchfield RB, Giffin JR, Willits KR, Wong CJ, et al. A randomised trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2008;359:1097-107. http://dx.doi.org/10.1056/NEJMoa0708333.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70. http://dx.doi.org/10.1136/bmj.325.7367.766.
- de Salis I, Tomlin Z, Toerien M, Donovan J. Qualitative research to improve RCT recruitment: issues arising in establishing research collaborations. Contemp Clin Trials 2008;29:663-70. http://dx.doi.org/10.1016/j.cct.2008.03.003.
- Howard L, de Salis I, Tomlin Z, Thornicroft G, Donovan J. Why is recruitment to trials difficult? An investigation into recruitment difficulties in an RCT of supported employment in patients with severe mental illness. Contemp Clin Trials 2009;30:40-6. http://dx.doi.org/10.1016/j.cct.2008.07.007.
- Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al. Prostate Testing for Cancer and Treatment (ProtecT) feasibility study. Health Technol Assess 2003;7. http://dx.doi.org/10.3310/hta7140.
- Donovan JL, Lane JA, Peters TJ, Brindle L, Salter E, Gillatt D, et al. Development of a complex intervention improved randomisation and informed consent in a randomised controlled trial. J Clin Epidemiol 2009;62:29-36. http://dx.doi.org/10.1016/j.jclinepi.2008.02.010.
- Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am 2010;92:1162-9. http://dx.doi.org/10.2106/JBJS.H.01674.
- Hack K, Di Primio G, Rakhra K, Beaule PE. Prevalence of cam-type femoroacetabular impingement morphology in asymptomatic volunteers. J Bone Joint Surg Am 2010;92:2436-44. http://dx.doi.org/10.2106/JBJS.J.01280.
- Allen D, Beaule PE, Ramadan O, Doucette S. Prevalence of associated deformities and hip pain in patients with cam-type femoroacetabular impingement. J Bone Joint Surg Br 2009;91:589-94. http://dx.doi.org/10.1302/0301-620X.91B5.22028.
- Lavigne M, Parvizi J, Beck M, Siebenrock KA, Ganz R, Leunig M. Anterior femoroacetabular impingement: part I. Techniques of joint preserving surgery. Clin Orthop Relat Res 2004;418:61-6. http://dx.doi.org/10.1097/00003086-200401000-00011.
- Clohisy JC, St John LC, Schutz AL. Surgical treatment of femoroacetabular impingement: a systematic review of the literature. Clin Orthop Relat Res 2010;468:555-64. http://dx.doi.org/10.1007/s11999-009-1138-6.
- Annual Mid-year Population Estimates, 2010. Newport: ONS; 2010.
- Mitra I, Malik T, Homer JJ, Loughran S. Audit of clinical coding of major head and neck operations. Ann R Coll Surg Engl 2009;91:245-8. http://dx.doi.org/10.1308/003588409X391884.
- Arthroscopic Femoro-Acetabular Surgery for Hip (IPG 408) Impingement Syndrome. London: National Institute for Health and Care Excellence; 2011.
- Open Femoro-Acetabular Surgery for Hip Impingement Syndrome (IPG403). London: National Institute for Health and Care Excellence; 2011.
- Beall DP, Sweet CF, Martin HD, Lastine CL, Grayson DE, Ly JQ, et al. Imaging findings of femoroacetabular impingement syndrome. Skeletal Radiol 2005;34:691-70. http://dx.doi.org/10.1007/s00256-005-0932-9.
- Tonnis D, Heinecke A. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am 1999;81:1747-70.
- Emara K, Samir W, Motasem EL, Ghafar KA. Conservative treatment for mild femoroacetabular impingement. J Orthop Surg (Hong Kong) 2011;19:41-5.
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2.
- Dalkey NC, Helmer O. An experimental application of the Delphi method to the use of experts. Manage Sci 1963;9:458-67. http://dx.doi.org/10.1287/mnsc.9.3.458.
- Delbecq AL, Van de Ven AH. A group process model for problem identification and program planning. J Appl Behav Sci 1971;7:466-92. http://dx.doi.org/10.1177/002188637100700404.
- Van de Ven AH, Delbecq AL. The nominal group as a research instrument for exploratory health studies. Am J Public Health 1972;62:337-42. http://dx.doi.org/10.2105/AJPH.62.3.337.
- Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Publ Health 1984;74:979-83. http://dx.doi.org/10.2105/AJPH.74.9.979.
- Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council; 2008.
- Featherstone K, Donovan JL. Random allocation or allocation at random? Patients’ perspectives of participation in a randomised controlled trial. BMJ 1998;317:1177-80. http://dx.doi.org/10.1136/bmj.317.7167.1177.
- Hunt D, Prather H, Hayes MH, Clohisy JC. Clinical outcomes analysis of conservative and surgical treatment of patients with clinical indications of prearthritic, intra-articular hip disorders. PM R 2012;4:479-87. http://dx.doi.org/10.1016/j.pmrj.2012.03.012.
- Moulin DE. Systemic drug treatment for chronic musculoskeletal pain. Clin J Pain 2001;17:S86-93. http://dx.doi.org/10.1097/00002508-200112001-00017.
- Bergman S. Management of musculoskeletal pain. Best Pract Res Clin Rheumatol 2007;21:153-66. http://dx.doi.org/10.1016/j.berh.2006.10.001.
- Feeley BT, Powell JW, Muller MS, Barnes RP, Warren RF, Kelly BT. Hip injuries and labral tears in the national football league. Am J Sports Med 2008;36:2187-95. http://dx.doi.org/10.1177/0363546508319898.
- The Care and Management of Osteoarthritis in Adults [CG59]. London: National Institute for Health and Care Excellence; 2008.
- Low Back Pain [CG88]. London: National Institute for Health and Care Excellence; 2009.
- Frost H, Lamb SE, Robertson S. A randomised controlled trial of exercise to improve mobility and function after elective knee arthroplasty: feasibility, results and methodological difficulties. Clin Rehabil 2002;16:200-9. http://dx.doi.org/10.1191/0269215502cr483oa.
- Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev 2008;4.
- Hayden JA, van Tulder MW, Malmivaara AV, Koes BW. Meta-analysis: exercise therapy for nonspecific low back pain. Ann Intern Med 2005;142:765-75. http://dx.doi.org/10.7326/0003-4819-142-9-200505030-00013.
- Holm L, Reitelseder S, Pedersen TG, Doessing S, Petersen SG, Flyvbjerg A, et al. Changes in muscle size and MHC composition in response to resistance exercise with heavy and light loading intensity. J Appl Physiol 2008;105:1454-61. http://dx.doi.org/10.1152/japplphysiol.90538.2008.
- Quintana JM, Escobar A, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after hip joint replacement. Osteoarthritis Cartilage 2005;13:1076-83. http://dx.doi.org/10.1016/j.joca.2005.06.012.
- Wamper KE, Sierevelt IN, Poolman RW, Bhandari M, Haverkamp D. The Harris hip score: do ceiling effects limit its usefulness in orthopedics?. Acta Orthop 2010;81:703-7. http://dx.doi.org/10.3109/17453674.2010.537808.
- Mohtadi NG, Griffin DR, Pedersen ME, Chan D, Safran MR, Parsons N, et al. Multicenter Arthroscopy of the Hip Outcomes Research Network. The development and validation of a self-administered quality-of-life outcome measure for young, active patients with symptomatic hip disease: the International Hip Outcome Tool (iHOT-33). Arthroscopy 2012;28:595-60.
- Christensen CP, Althausen PL, Mittleman MA, Lee JA, McCarthy JC. The nonarthritic hip score: reliable and validated. Clin Orthop Relat Res 2003;406:75-83. http://dx.doi.org/10.1097/00003086-200301000-00013.
- Phillips L, Mohtadi N, Chan D. The responsiveness and Minimal Clinically Important Difference (MCID) of the Mahorn Quality of Life Tool. J Arthrosc Relat Res 2011;27. http://dx.doi.org/10.1016/j.arthro.2010.11.053.
- Mohtadi N. Prospective Quality of Life Outcome Comparing IHOT-33 and IHOT-12 n.d.
- Harris-Hayes M, McDonough CM, Leunig M, Lee CB, Callaghan JJ, Roos EM. Clinical outcomes assessment in clinical trials to assess treatment of femoroacetabular impingement: use of patient reported outcome measures. J Am Acad Orthop Surg 2013;21:S39-46. http://dx.doi.org/10.5435/JAAOS-21-07-S39.
- Ng VY, Arora N, Best TM, Pan X, Ellis TJ. Efficacy of surgery for femoroacetabular impingement: a systematic review. Am J Sports Med 2010;38:2337-45. http://dx.doi.org/10.1177/0363546510365530.
- Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Does land-based exercise reduce pain and disability associated with hip osteoarthritis? A meta-analysis of randomised controlled trials. Osteoarthritis Cartilage 2010;18:613-20. http://dx.doi.org/10.1016/j.joca.2010.01.003.
- Naal FD, Impellizzeri FM, Leunig M. Which is the best activity rating scale for patients undergoing total joint arthroplasty?. Clin Orthop Relat Res 2009;467:958-65. http://dx.doi.org/10.1007/s11999-008-0358-5.
- Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12v2® Health Survey (with a Supplement Documenting SF-12® Health Survey). Boston, MA: QualityMetric Incorporated; 2002.
- Janssen MF, Birnie E, Haagsma JA, Bonsel GJ. Comparing the standard EQ-5D three-level system with a five-level version. Value Health 2008;11:275-84. http://dx.doi.org/10.1111/j.1524-4733.2007.00230.x.
- Adamso J, Gooberman-Hill R, Woolhead G, Donovan J. ‘Questerviews’: using questionnaires in qualitative interviews as a method of integrating qualitative and quantitative health service research. J Health Serv Res Policy 2004;9:139-45.
Appendix 1 Anonymous patient cases and clinician interview schedule
Appendix 2 Detailed results of patient interviews
Integrative qualitative study of recruitment pre-pilot report: patients’ views
Method
Semistructured interviews were conducted with a sample of patients who have received treatment for FAI. The interviews explored patients’ perspectives on their hip problems, previous experiences with treatments, views about arthroscopy surgery and conservative care and the acceptability of randomisation between the interventions. Fourteen patients interviewed were also asked to complete the expert patient survey while talking to the researcher.
Procedure
An invitation to be part of an expert patient panel was sent to patients who have been diagnosed and treated for FAI at the UHCW NHS Trust. Patients who replied to the invitation and signed the consent form were asked if they were willing to participate in one-to-one interviews. Initially, we had planned to give questionnaires to 50 patients and to interview 15 of these. However, it emerged that the one-to-one interviews provided much richer in-depth information than administering surveys alone and that this allowed data saturation to be achieved. Participants were purposively chosen to include a range of age, sex, disease severity, activity and socioeconomic status as well as those who had received operative and conservative care in the patient sample.
Once the expert panel member declared their willingness to be interviewed, the researcher (AR) contacted them and explained the qualitative study. The participant information sheet and consent form was sent by post and, when the consent form was returned signed by the participant, an appointment for the interview was set up, whether face to face at the hospital or by telephone. The participant was also asked to read and assess the draft trial PIS prior to the interview. The expert patient questionnaire was given to the participant in order to facilitate the assessment of the trial PIS. During the interview, open-ended questions were used to guide the conversation and the participant’s answers to the questionnaire were discussed. The participants did not receive any monetary or other reward for their participation. The study was approved by the ethics committee at University of Warwick and NHS.
Data analysis
Transcripts of the interviews were prepared and thematic analysis was used to extract key messages about patient experiences of hip impingement and treatments, acceptability of the treatment options, the understanding of randomisation and advice on how to improve the trial participant information sheet. AR coded the transcripts using NVivo. Agreeing and contrasting views were identified for each area of interest (e.g. randomisation). After discussing the structure and content of the results, the QRI team agreed on some recommendations which were presented to the trial management team. Further discussions took place in trial management meetings and, once agreement was reached regarding the recommendations, changes to the trial PIS and recruitment strategies to be tested in the pilot were implemented.
Findings
Sample
Eight female and 10 male patients who were members of the expert patient panel answered the invitation to a one-to-one interview. The conversation was focused on their experience with hip impingement and the RCT proposed in UK FASHIoN. Five patients were currently undergoing treatment for FAI, one was scheduled to have the surgery in the next few months and the other four were about to start a course of non-operative treatment. The remaining 13 patients have had hip arthroscopy. The surgery had taken place from a few months earlier to over the past 8 years.
Patients’ experiences of hip impingement and treatments
The patients in this sample tended to be generally fit and healthy. They were involved in various sporting activities at different levels of competence or they have physically demanding jobs (e.g. military, farming):
I’m member of my local football team, I play for the first, I’m captain, and it’s important to me that I’m involved, I’d love playing over the weekend, I play once a week, it’s been great that I can continue to do that.
EP 4
I’ve always been, through my school life, been very active, doing lots of sports, sky, horse riding when I was younger, and for quite a long time now I’ve gone to my local gym and all kinds of running, cross training, row machine.
EP 5
However, the condition was not exclusively developed by people who did sports:
I’ve never been terribly active . . . I don’t take regular exercise apart from being the mother of three and two grandchildren, so I’m on the go all the time.
EP 10
About half of the patients in this sample reported at first feeling acute pain after physical activity, which did not have a great impact in other activities of everyday life. Other common symptoms reported were discomfort and lack of sleep owing to pain. Some have had pain over a few years before they sought treatment:
During the course of playing golf, it wasn’t that bad, it was afterwards [. . .] it was very painful, it was bad enough to stop me sleeping and things like that because I couldn’t feel comfortable in bed.
EP 7
I had pain for 2–3 years not knowing what it was, thinking it was some kind of strain.
EP 5
The pain increased with time and started to interfere in patients’ lives, which led to seeking a solution to their discomfort. A patient, who is in the military, explained that he had been involved in many different treatments wasting time and opportunities for career advancement (EP 2). For other patients, seeking treatment was related to their lifestyle and coping mechanisms, as demonstrated by the following quote:
It started like a general discomfort and then it became more of impact in our day-to-day lives, me and my husband like long distance walking and that became a problem . . . when you are in pain it gets you down after a while.
EP11
Patients were often misdiagnosed and sent to physiotherapy in an attempt to address the symptoms before they met the orthopaedic surgeon:
I did get sent to a lot of physiotherapy, it was only when I pushed it ‘look this isn’t right, this is not how it should be’ the hip specialist in a private hospital, put me through to [orthopaedic surgeon].
EP 4
Because I originally went to the doctor with groin pain, they thought it was perhaps a strain, they thought my back wasn’t aligned or something.
EP 5
As a consequence, they experienced different types of physiotherapy with little results. This was reported often by those who underwent hip arthroscopy:
The symptoms came and went, I did a lot of climbing, a lot of twisting, stuff like that [. . .] I’ve got to the point where I was referred to a physio, that didn’t do any good and then it was suggested I went private.
EP 9
As the majority of patients have private health insurance, it appeared that their waiting time between their first complaint and the consultation with the surgeon was shorter than those on the public health service:
I do have private health care which allowed me to go down this route and used [orthopaedic surgeon].
EP 4
The patients who had hip arthroscopy reported the recovery time was acceptable and, although some still experience occasional pain, they were in general very satisfied with the outcome:
It was great, the first one I’ve done, the recovery was really fast, I was in the clinic that day I had the surgery, I went through heavy intensive physiotherapy for 2 months after, but within 3 months I was back running again.
EP 4
The two patients who were managing the condition conservatively reported being pleased with not having to go through surgery, one because of an unsuccessful operation that led to more complications and the other because it would involve more time off work following a previous health complaint. However, the non-operative care plan is perceived as a temporary measure:
I like the approach, we know what it is now, let’s try the cortisones, I’m surprised about having a second cortisone, but on the basis it worked quite well last time, who knows if it may work this time, I think we are all sort of expecting seeing me back here again in a few months’ time.
EP 1
Acceptability of treatment options
This section summarises the different ideas that patients shared regarding the two treatment options that have been compared.
Hip arthroscopy surgery
The majority of patients regarded surgery as a ‘point of last resort’ (EP 1) and a definite solution for a severe condition that is mainly morphological:
I was a bit shocked to realise that I would need surgery to remove part of the bone, it was probably more serious than I thought it was going to be.
EP 5
With the exception of doing nothing, some patients did not know of, or were not offered, any other treatment option at the time than surgery:
I didn’t know there was a decision to be made . . . I had a problem and that’s how you corrected it, so it wasn’t like there was a choice and needed an explanation.
EP 4
Patients perceived hip arthroscopy as an acceptable option and those who had this treatment decided to go ahead because they trusted the surgeon and had the expectation of a shorter recovery time than other surgical options such as open surgery:
Because of my partner had the hip surfacing I thought that was going to be the course of action . . . then when there was a lesser intrusive operation available I was completely happy to attend that and I realise if and when that no longer works for me then I have a surfacing after that so it was weighting up the logical course of treatment.
EP 10
Another advantage of the surgery was the possibility of preventing the progression of arthritis and the need for a total hip replacement later in life:
This is just feeling, not proven, I can see if I didn’t do anything now in 20 years’ time I’d probably need a hip replacement surgery.
EP 1
One patient who was having conservative care at the time expressed concerns about nerve damage, surgery complications or ending with a limp and visible scars. Furthermore, this patient worried about the postoperative care:
Because you have to be out work and in crutches, and it would have implications for me for childcare if I have it, I don’t know how immobile you’d be.
EP 3
However, it appeared that for those patients who have undergone surgery the benefits outweighed the risks and they tended to recommend this option even though they may not have experienced total recovery:
Being through the arthroscopy myself and the successful nature of it, or nearly successful nature, let’s remember I still feel a little bit of pain in my right hip [. . .] it’s not 100% resolved or solved, I do feel it has a big impact there.
EP 4
The explanations giving by the consultant were particularly useful for patients to decide to go ahead with the surgical treatment:
[surgeon] actually went through everything in a precise way, he showed me the scans in the laptop and he showed me what he needed to do in order to alleviate my problem that certainly thinking in a mechanical way in a laptop was very good.
EP 11
Conservative care
Conservative care was perceived as a less invasive procedure acceptable for people who experience less serious FAI symptoms or were at risk of complications with surgery:
Try to resolve it in a more considerate more gently fashion, as opposed to going to surgery.
EP 1
Therapy in some situations would be a better way of treating this problem, because there is no surgery involved and the risks that come with that.
EP 4
One patient mentioned the funding may have been a consideration in the treatment that was offered:
I just think if you don’t get many symptoms, they are not going to use the funding to operate.
EP 3
A patient in conservative treatment made sense of the physiotherapy as an opportunity to retrain the muscles:
The idea is that the physiotherapy will teach me to my muscles to work in a way that doesn‘t impinge in my hip. If that can happen, great.
EP 1
However, for the majority of patients, non-operative care was perceived as a provisional solution or as a first step treatment plan because it was not clear how physiotherapy alone can repair the damage. Understanding how conservative treatment could cure the condition was a major concern for patients in this sample.
Various patients expressed fears of further damage of the tissue or eventually developing arthritis if the intervention was conservative:
Physio may improve a bit but I can’t see how physio can resolve the problem if it is hip impingement. I, it can tear again if I get active, and if I’ve got arthritis, I don’t see how it can get better.
EP 3
Additionally, attending physiotherapy appointments may be troublesome for some, especially for those with previous experience of conservative care who did not obtain good results. A patient ‘was not keen on physiotherapy’ as he was involved in a 7- to 8-hour workout to relieve pain, which in his view was completely impractical. Therefore, it seemed to be a less optimal treatment option:
I think I would have been a bit cynical and assumed that the operative choice would work for me so if the pain was not improving significantly, I would have probably lost patience quite quickly.
EP 10
The idea of personalised care that includes different treatments may be more appealing:
Different treatment for every different patient that was involved, someone would react better to steroid injections, some would react better to acupuncture, some wouldn’t, so I think a personalised programme, or therapy, would be good because people would realise the treatment would be specific to them and their particular situation.
EP 4
However, as previous experiences with treatment seemed important in deciding future care paths, the non-operative treatment should be presented as a different alternative to just physiotherapy. For example, a regime that addresses the particular issue of FAI could be perceived more positively:
In terms of physio[therapy] on my hip, the only physio[therapy] I’ve done is the post-operation physio[therapy], so certainly I would be interested in [physiotherapy], something that focus and look the area and work with [the hip muscles].
EP 8
Alternatively, more explanation about what can be achieved with this treatment may help to convince patients to join the trial:
I’d say because I had physio and have not have had much success with it I would be less willing to go into the clinical trial where I’d end with more physio, that would need to be what else is other than physiotherapy, which in this trial is because you are talking about injections, massages and acupuncture . . . that there are alternatives, what the steroid injections do, why is that done and what they are hoping to achieve with that.
EP 11
[physiotherapy] didn’t help me, but it wasn’t in combination [. . .] perhaps therapy rather than the surgery would include steroid injections and things like that, so I don’t know, maybe in combination it may help with this.
EP 9
Acceptability of randomisation
Various patients shared their thoughts about the importance of being involved in research. The benefit to the whole community and helping clinicians improve their knowledge and advice for future patients seemed important motivators; hence, they responded to the invitation to participate in the one-to-one interviews:
I understand why it’s been done, and it sounds like it probably needs to be done, so medical teams and authorities know the best course of action for hip impingement, so that’s where we are in reality, and I could see in terms of the benefit of the whole, taking a holistic approach to it for future patients having that sample undertaking.
EP 1
However, when confronted with the design of the RCT, patients felt the 50 : 50 allocation was not acceptable. They gave various reasons summarised below.
The first issue with the allocation was the perception that clinicians would be not looking after the best interest of the patients but distributing people according to the needs of the trial:
It is not ethical if it’s random [. . .] It makes you wonder 50 : 50 if you’re just going to be put in one or the other without looking at how your symptoms are or how you are, it sounds worrying.
EP 3
You’re going to take part on our study and we will tell you which treatment you are having, whether it’s the right necessary for you, that’s how it come across.
EP 5
Connected to this issue, patients presumed that the health-care providers would know what is the best line of treatment for them. Therefore, allocation was perceived as unnecessary or odd and they were left asking who would make the decisions and how. Patients described randomisation or 50 : 50 allocations as ‘a toss of a coin’, ‘bingo’, ‘being a guinea pig’, ‘everybody is put into a hat’, ‘a lottery’:
I think it ought to be dependent on your particular situation rather than on a 50 : 50 toss of a coin which way you went. I’m sure some issues, and some hip injuries, are probably more suited to one route rather than other.
EP 4
The word ‘allocated’, I think it is the wrong word to use, your consultant would decide which treatment is best for you, but allocated makes me feel that actually they are not really taking my medical complaint seriously or the best to what it would make it better.
EP 5
The third issue mentioned was the imbalance between the two treatments when one is perceived as curative and the other is not:
I don’t know to be honest, I don’t know if that feels the right way to do it, and having pain that I had for so long, would I like to have go and have an injection and wait for another few months, and then have to have an operation, that’s probably a hard decision.
EP 5
If they have the symptoms the same as somebody else and the other person’s having surgery to correct it, the person who’s having physio would think ‘how can it be corrected without the surgery?’
EP 3
Two patients expressed concerns about waiting for 12 months, as it could have an impact on the options available after the study finishes:
It’s about if the problem was left and treated by physiotherapy there was the possibility of something else being affected during the physiotherapy that then the surgery couldn’t cure, which could have been cured straight away.
EP 6
Similarly, another patient asked about how the progress of the treatment would be assessed during the trial and what would happen if the care plan was not working:
Do you see the consultant at any point during the year? I presume you see him at the beginning, but how is it assessed during the trial as whether it is not actually getting worse? Because that’s not in the information, is there? . . . it may be worth thinking about putting at the end because it doesn’t say . . . how the year progresses, how am I improving? So it may be nice for the patients to actually think 3 months, 6 months actually it is getting worse.
EP 11
Finally, patients felt that comparing surgery with injections and medication treatments would be more acceptable, leaving physiotherapy for the postoperative care. Alternatively, by making sure that the operative option is available at the end of the trial:
Surgery for most people has got to be the last option, now if they can sort it out with physio, and after 12 months of physio doesn’t work, OK let’s do the surgery, I think that’s acceptable to me, I won’t have a problem with that, it is information that I’d like to have.
EP 13
Patient recommendations to improve the participant information sheet and study in general
Time to ask questions with a specialist
Patients valued the opportunity to talk to a specialist without being rushed and the ability to ask questions about their particular situation:
It’s always about explaining the options, being opened, honest with the patient, [surgeon] had time to talk about the options and explained the option, so great!
EP 4
I have complete faith in the [surgeon] because he was incredibly good at explaining everything, and very reassuring, he showed the diagrams, the pictures and explain everything and how the picture relates to my hip, but he provide an environment where you feel very comfortable to ask questions, he also explains things in lay terms, he doesn’t baffle you with medical terminology.
EP 12
Continuity of care
People trusted their surgeon and wished to continue under his care, in contrast to the experience of a patient who previously had consulted with up to 15 different doctors, which caused delays in the treatment:
If you are comfortable with your consultant, the manner of the consultation and the consultant would go a long way to give you confidence that that’s the best treatment.
EP 10
Trusted information sources
The majority of people were satisfied with the explanations received during the consultation with the surgeon and did not wish to have a second professional opinion. However, they looked up FAI and available treatments in the internet and found the information confusing, wrong and worrisome.
When I went to the internet I found things that people had had done but also the internet could be quite a scary thing and you may get worried.
EP 5
In particular, one patient suggested that links to trusted websites could be added to the PIS for those who wanted more details about the condition and its treatments.
Others commented that having read the PIS for the RCT has clarified and enriched their understanding of the condition. One patient said:
Perhaps if someone’s been diagnosed with this problem, then given the [PIS] document and then ask to have a discussion with a consultant. That would work for me!
EP 4
Patients suggested that more information could be added specifically about the long-term risks of hip impingement (i.e. arthritis), the risks of non-operative care and details of the postoperative care (i.e. what happens soon after the surgery):
I’d probably I would have liked more information on after I had it done, what happens and how would I feel? And what I can/can’t do, and I’m talking immediately after, the day you wake up after the operation, what can you do? What pains are you going to feel? I remember the first time I had it done, I had terrible pins and needles, and my leg was going numb.
EP 5
Detailed follow-up/physiotherapy protocol
A few patients appreciated the level of detail of the rehabilitation guide after the surgery. It seems that, despite it being a demanding programme, people were able to follow it and remain motivated:
That’s something I would highlight, it was amazing, how detailed and supportive that follow-up programme was. I was giving a 12-week programme, basically week 1, these are the exercises you should be doing, then week 2 progress to this, so that was very informative and helpful, because you have that guidance it made me very diligent.
EP 12
I suppose I’m the sort of person that asks a lot of questions at the start. I, it was useful to know how much the rehab programme was going to be, there was a lot in it, and it was useful to have that beforehand.
EP 13
Trial patient information sheet review by patient panel
The purpose of the expert patient panel was to involve patients in the revision and redesign of the PIS that would be used in the pilot work and eventually in a full RCT. Initially we provided an online and on-paper questionnaire and targeted 50 patients who have been diagnosed with FAI and received treatment at UHCW. However, in order to obtain a richer source of information about how patients perceived and interpreted the PIS, the majority of the expert patients who agreed to be interviewed also answered the questionnaire with the researcher (AR). This method has been used in another RCT by a member of the team (JD). 69 It allowed us to access the thinking process behind the answers given by the participants. The QRI team decided that the questionnaire in combination with the interview was a more effective way of obtaining the key messages required to change the PIS. Therefore, the collection of questionnaires was stopped before we could complete the target sample size.
Findings
Sample
Fourteen questionnaires were filled in by participants during the interview, one questionnaire was returned by post and one was filled in online.
Feedback
The patients received the PIS positively because the wording was easy to understand and the information clear:
I think the patient information sheet was very readable . . . I gave it to my husband and he was quite happy, even my children read it, my boys are 11 and said ‘Mum, what are you reading?’, and I gave it to them and they understood it and they asked me ‘Mummy, why are you having another operation?’, but they understood what a clinical trial was at the end of it.
EP 11
Patients also appreciated the hip diagram at the front and the flow chart at the end of the PIS. They tended to refer often to those graphs when discussing their experiences. Two patients suggested one diagram of an abnormal hip with FAI.
Perhaps some more detail in terms of photos/images of damage and typical damage, because there is a big [picture] about the socket area and a normal hip joint and it would be interesting to see what it isn’t [. . .] to suggest what the problems look like and why they don’t know what the best fixers are.
EP 10
Concretely, patients found and understood easily the information for (1) why it is important to treat hip impingement, (2) the purpose of the study and (3) what operative care involves and its risks. One patient mentioned there was not much information of the risks of not treating hip impingement.
Patients did not find satisfactory the information related to (1) non-operative care risks, (2) what happens after the study finishes and (3) 50 : 50 treatment allocations. These items are related to the explanations about the trial procedure.
Other patients wanted to know if the same clinician would look after them and if they would have access to an alternative treatment after the study:
There is nothing in the information sheet that says actually if it doesn’t work you can then go on to discuss further options which may involve surgery.
EP 11
[About the flow chart] the last box doesn’t make it entirely clear that your treatment plan may alter after the trial, maybe that bit can be elaborated to clarify that.
EP 9
Some patients requested more information about the postoperative care and others wanted further information about what risks are involved with the non-operative care (i.e. further tears to the labrum). Finally, people were unclear about how the decisions to allocate patients were made and if the patient’s clinical needs were considered in this procedure.
The patients in this sample were reluctant to make a decision to enter a RCT and the reasons were explained in the section above. They reported that the PIS presented the options in a balanced way; a couple of patients said it was a useful document and wished it had been available when they were first diagnosed. Some patients made suggestions about the level of information it contained, also discussed in the previous section.
The preferred name for the conservative care was discussed and in general people opted for the word ‘personalised’:
I said the last two [personalised hip treatment and personalised hip therapy] because it makes it a personal issue for that person . . . going down a non-operative route would require different treatment for every different patient.
EP 3
I liked the word ‘personalised’ rather than ‘focused’ because if you are going to have something done, it is nice to feel that it is personal to you.
EP 11
One patient expressed a preference for the word ‘therapy’ as it indicates an effort to ‘solve’ or ‘cure’ the condition, as opposed to ‘programme’, which does not have that connotation.
Therapy from a psychological point of view, people understand therapy [. . .] with regards to clinical treatment rather than a programme which can relate to anything in life.
EP 13
The name of the conservative arm was decided on the basis of these patients’ responses; eight patients voted for the name ‘personalised hip therapy’, four preferred ‘personalised hip programme’, one voted for ‘focused hip therapy’ and three offered other suggestions, such as conservative hip rehabilitation programme or the inclusion of the word ‘non-invasive’.
Recommendations
Recruitment
-
Be aware of patients’ previous treatment history, as past experiences with either of the treatments seemed to have great value in the decisions made afterwards.
-
Allow time for patients to consider the PIS and then ask questions.
-
Use open communication and clear explanations of the condition, as these are linked to patient satisfaction and compliance. In contrast, if the patient feels rushed and overwhelmed by the information provided, this creates anxiety and mistrust, and the patient is probably less willing to participate or stay in the trial.
-
The non-operative arm of the trial should be called ‘personalised hip therapy’ in accordance with patient preferences.
Patient information sheet
The following points need to be given greater emphasis:
-
The patient should have the opportunity to talk to a researcher for longer and should be able to ask questions and raise concerns.
-
Success rates of both treatments should be included. Patients need to feel that treatment will solve the problem. It may be useful to include some patient testimonies describing positive experiences of both treatments.
-
The differences between any past physiotherapy regime and this new programme should be highlighted. ‘Personalised’ seemed to be a preferred term.
-
Continuity of care with the surgeon and team should be assured. Patients care about the reputation of their clinicians and do not like to be referred to another doctor. In addition, the PSI should point out that participation in the study may result in extra care and attention and this can be a selling point for some patients.
-
Altruistic motivations, such as the need for research and the personal contribution to the whole community, should be emphasised.
-
It should be made clear that participants are guaranteed access to treatment at the end of the trial, that is that the non-operative treatment offered is a precursor to surgery.
The following additional information is required:
-
a logical explanation or reasons for considering physiotherapy/conservative care as an acceptable treatment option within the context of FAI
-
details about the postoperative recovery
-
details about the non-operative arm: purpose, expectations, time commitment (i.e. required on a regular basis) and risks associated with allocation to this arm in terms of aggravation of symptoms
-
details of the 50 : 50 allocation procedure, including how patients’ clinical needs will be met, and by whom, as well as how the decisions will be made and whether the patient’s condition will be assessed regularly
-
details of trusted websites that the patient can consult or else create a new site in which the balance between operative and non-operative care can be explored further.
Appendix 3 Detailed results of clinician interviews
Integrative qualitative study of recruitment pre-pilot report: clinicians’ views
Clinicians’ interviews
The purpose of this activity was to explore attitudes to randomisation of FAI patients of a multidisciplinary sample of clinicians working in the centres performing hip arthroscopy. Semistructured interviews were carried out and, to stimulate discussion, clinicians were provided with anonymous patient cases that cover the spectrum of patient presentations, including patient history (with duration of symptoms and previous treatments), examination findings and imaging (for examples and interview schedule please see Appendix 1). By introducing patient cases we sought to deconstruct the cognitive processes involved when a clinician considers recruiting a patient to a clinical trial. This was done in order to elucidate the state of individual equipoise that may be influencing a recruitment decision.
Procedure
We aimed to recruit a convenience sample of health-care professionals who have a special interest in hip conditions and were likely to see patients with FAI during their clinical practice (i.e. orthopaedic surgeons, physiotherapist and sports doctors). We invited clinicians attending to two national events, the BHS annual meeting and the 2012 Hip Surgery Conference. We also contacted physiotherapists who participated in a national survey that contributed to the development of the PHT protocol. The clinicians were contacted by the researcher (AR) during the conferences or by e-mail. Those contacted during the event were invited to have an interview in a private room. The rest of the clinicians were interviewed at their offices or over the phone. After consenting to take part, the clinicians were presented with the two anonymous patient cases. The researcher used the interview schedule and materials presented in Appendix 5. The clinicians were asked to ‘think aloud’ while considering the patient cases and the researcher used prompting questions to facilitate the clinician narrative of these processes. The interviews were audio recorded. The study received the relevant ethics approval from the West Midlands Ethics Committee.
Data analysis
Transcripts of the interviews were prepared and coded using NVivo by AR. The coding was based on the Buckingham–Adams classification model of clinical decision-making, which allowed identification of (1) relevant cues; (2) psychological representation of these clues (i.e. importance in diagnosis); (3) knowledge structures used (e.g. past clinical experience); (4) condition inferences generated as a result of these processes; and (5) consideration of the potential outcomes associated with a particular treatment including introducing the trial. Key messages about what relevant cues to decision-making, information sources, clinical uncertainty and risks and potential outcomes considered by the clinicians were discussed with AA and JD. Recommendations about clinician recruitment and information provision for clinical teams involved in the trial were agreed and presented to the trial management team.
Findings
Sample
The majority of the surgeons (n = 12) interviewed were recruited during the BHS annual meeting and are currently treating patients with FAI. Of the total sample of surgeons, two do not operate on the hip and one carries out open surgery; these surgeons tend to refer patients to another colleague who does hip arthroscopy. Surgeons who specialise in arthroscopy have more expertise than those who are still in training.
Six physiotherapists were identified through a national survey that requested the opinions of professionals who have a special interest in hip conditions. The other six physiotherapists and two sports doctors were interviewed during the Hip Surgery Conference 2012, an event dedicated to health professionals who also have interest in hip issues and rehabilitation and a high level of expertise.
Patient’s’ characteristics and their psychological representation in clinical decision-making
Patients’ age was mentioned by the majority of surgeons and physiotherapists. In general, they reported that patients with FAI are young and active. When the patients were relatively older, clinicians were mindful of distinguishing FAI from early arthritis onset.
Between mid to late 40s even early 50s, that are borderline [. . .] they don’t have full on arthritis but they have mechanical symptoms with relatively early onset.
Surgeon 3
A surgeon and a physiotherapist also mentioned certain patient personality traits that may influence their decisions. One surgeon described the ideal patient as ‘a highly motivated and intelligent patient’ (surgeon 3) with a good prognosis. According to one physiotherapist ‘again it depends on the state of mind, some patients are very driven, they are going to get better’ (physiotherapist 1).
Clinicians were also interested in the type and level of patients’ physical activity and the impact the symptoms had on patients’ lives. This seemed to be important when assessing the level of severity of the condition and the need for treatment:
I would like to know if he’s able to do his job or not because that’d be important, or whether it is just wicket keeping that precipitates his symptoms.
Sports doctor 1
Will surgery enable him to continue the sporting activity that he wishes to undertake? Are there other activities that he can’t undertake at the moment? So it’s not interfering with his work, it’s purely recreation.
Surgeon 9
In terms of patients’ medical history, various surgeons and physiotherapists mentioned that patients with FAI have typically experienced pain for a few years. They also remarked on the type of impingement suggested by the tests.
He has pain, positive clinical examinations and positive radiographic findings a triad you showed me in each one that is consistent with a diagnosis of FAI, this guy has labrum tear as well I see.
Surgeon 14
The gap between first experiencing the symptoms and seeking treatment was reported to be shorter when the patients are private:
Obviously being the private sector, we tend to see them [patients] sooner rather than later.
Physiotherapist 2
Finally, the treatment history of the patient seemed to be particularly concerning for a group of clinicians, especially when deciding what treatment they should recommend. The general perception is that the majority of patients may have already had a course of conservative care and are seeking advice from the consultants to obtain a more definite solution, which in most cases involves a surgical procedure:
Patients come when [they have] already had a course of physiotherapy, that is patients have often come [after] non-operative treatments, and they look at you as if you are mad if you say ‘I want to send you back to physiotherapy for more treatment’.
Surgeon 7
Recommended treatment in case studies
The section above showed the aspects that clinicians tend to focus on when suggesting a course of treatment for patients with FAI. In general, no one disputed the diagnosis in any of the fictional cases we presented, although some surgeons and some physiotherapists remarked on the need for further examinations.
Other thing would just want to know about any other issues that she’s had, whether it’s lower back pain, chronic pain problems, yellow flags that [she] may have an issue.
Physiotherapist 3
The physiotherapists suggested that activity modification in most cases would help to alleviate the symptoms, along with exercises to stabilise the hip joint. Three physiotherapists were of the opinion that the fictional patients were ‘suitable for operable or non-operable treatment’ (physiotherapist 3). Others mentioned that, in their experience, patients with this pathology started with a course of physiotherapy:
If he didn’t have any labrum or chondral changes then we would automatically send him to physiotherapy first, purely to see if we can upload symptoms with conservative measures [. . .] if they can’t do the physio because of the pain, we would inject them surgical local anaesthetic, partly to confirm that what we’re seeing on the scan is to sort out their symptoms.
Physiotherapist 7
The group of surgeons generally agreed the fictional cases portrayed patients to whom they offered hip arthroscopic surgery. In the case of a young farmer who had experienced pain for 5 years, seven surgeons were happy to offer operative or non-operative care. However, one of them was cautious about intervening:
He is someone I would not rush into surgery with him. I would ask him to see my physio. I would ask her possibly in the course of being on a waiting list to see this patient.
Surgeon 6
When the fictional case of female patient was presented, the majority of surgeons would advise this patient to undergo arthroscopy on the hip; however, one surgeon and one physiotherapist disagreed:
This particular patient in my practice and also speaking to a lot of the colleagues, they all know about this condition, they don’t do well by the surgical intervention.
Surgeon 11
If she was my patient I’d be keen to go down the conservative route, just because reading it, it doesn’t sound that the symptoms are bothering her that much.
Physiotherapist 2
Another surgeon raised the question about the effect of sex in the natural history of FAI: ‘maybe the literature has moved on, the last time I looked the difference between men and women having hip impingement was not that clear’ (surgeon 8).
The clinicians’ hypothetical decisions are linked to their level of certainty about the usefulness of operative and non-operative routes for treating FAI. They are explored in the following sections.
Certainty of conservative care
A subgroup of clinicians agreed that conservative care can have a positive impact in relieving FAI symptoms. Seven physiotherapists cited examples from their own practice in which they have seen satisfactory recovery after a course of treatment. One of them explained it like this:
Some people have and we’ve never heard from them again because with activity moderation and changes in their lifestyle that’s been enough.
Physiotherapist 2
Physiotherapists explained how the non-conservative care could improve outcomes:
[Physiotherapy will be an advantage] in individuals who are poorly conditioned, the muscles around the hip are in poor condition and they are weak, more improvements can be made there and their control on the hip and the lumbar area(?) [. . .] Other sources of pain which are related to the hip, as it is not always the hip pathology that is causing the pain, there are other things that can trigger it.
Physiotherapist 5
However, they were aware that this line of treatment did not address morphological issues, and physiotherapists tended to agree that success may be related to the severity of the symptoms: ‘but I’d guess with those that the impingement and the damage was not as bad as it can be in some’ (physiotherapist 9) and ‘the ones with the most middle signs maybe if they just have a pincer or a cam rather than mixed on the radiographic findings’ (physiotherapist 11).
Eight surgeons in this sample expressed the opinion that there may be a group of patients who could gain relief from conservative care:
There is a small cohort of patients [. . .] whom maybe benefit from physiotherapy. These patients are those who have small cam lesions and who are not going to strain their hip to the extent that is going to cause that impingement, so modification of activity for them may be the treatment they required.
Surgeon 2
However, it is not clear what type of patients would benefit the most, as two other surgeons suggested that patients with pincer impingement may respond better to this treatment. This is because they usually see mixed results after surgery with patients with pincer impingement. Additionally, some surgeons have noted that patients may get better when there is nothing done to them.
You won’t surprise me if it turns out this is a condition where the pain resolves with time, because we know a lot of people with meniscus tear, for instance, the pain would get better despite the tear being there and also a lot of musculoskeletal pain is episodic.
Surgeon 8
Because we didn’t have access to [a surgeon] for 3 years, we just got on with it, and did realise that people settle down. We only had one last year that needed referral out of six [patients].
Sports doctor 2
There is also a general concern about the quality of the physiotherapy offered, which seemed to affect the outcomes, as expressed in the following quotes:
Depending on the type of physio and the quality, the physiotherapist understanding of the pathology [. . .] I think if the right people are involved with the right level of skill, the outcomes will be a lot better.
Physiotherapist 5
But has he had proper physiotherapy? Do you think? Has he had proper activity modification? No, the answer generally will be no, he needs to give it a good shot, it’s my feeling.
Surgeon 11
Physiotherapy has to be carefully considered as how it is going to be delivered in the local hospitals . . . huge variations in the quality.
Surgeon 3
Several clinicians suggested that non-operative treatment would not work. The physiotherapists who shared this opinion mentioned that some patients do get better when not participating in the usual activities that cause them pain but that symptoms return once they resume those activities. Some surgeons also reported this experience:
We’ve seen in elite sports people who have access to very good therapy despite all that still they don’t get any better.
Physiotherapist 2
I don’t think conservative care works very well. I, it does in some people, but my experience is that conservative care means they go away for 6 months and come back.
Surgeon 14
Another argument to doubt the success of conservative care is that the non-operative treatment ‘would not help the morphological abnormality’ (surgeon 12). This surgeon continued to explain that sending someone to physiotherapy may be a breach of ethics, as they know the condition could worsen.
How can you possibly subject that patient to physiotherapy with higher deflection exercises, whatever they may be, with the possibility of the morphological abnormality increasing that tear?
Surgeon 12
This is also a concern for other surgeons, who believe that their patients are seeking a solution to their problem:
People come to me because their life is no longer working satisfactory, they tried the conservative treatments and they haven’t worked.
Surgeon 9
Certainty of hip arthroscopy
Various clinicians, including physiotherapists, expressed the opinion that surgery is a curative treatment for FAI. The main reason given is the morphological description of the condition:
Young men like [patient] have very specific symptoms and have often cam impingements and big bumps on the front femur and have slightly restricted movements but otherwise they are fit and well. And have good muscle control and that a group who do well with surgery.
Physiotherapist 3
Some surgeons were satisfied with the amount of case evidence series collected in the scientific community which, according to them, has shown surgery is successful in relieving FAI symptoms:
I don’t believe there is any more a question of whether good surgery can relieve symptoms.
Surgeon 9
The literature by and large shows 80% of the people get good results, as far as symptoms go with surgical treatment. There is no evidence anywhere that conservative treatment will work.
Surgeon 8
However, the expressed views showed an inherent debate between surgeons who believe that surgery stops the advance of hip arthritis and those who think that operative care has limited benefits, which is demonstrated in the quotes below:
[FAI] is probably the result of a morphological problem, therefore, simplistically by dealing with the morphological problem, hopefully you should limit or slow the disease progression [. . .] I’ve done a lot of research with regards to impingement and firmly believe that it does cause arthritis.
Surgeon 8
I am reasonably convinced of that FAI surgery in carefully selected patients has a short-term functional benefits but I’m not convinced at all that we have the evidence that it is the cure for progression to arthritis.
Surgeon 2
Furthermore, a number of clinicians have spoken of the risks and side effects of surgery, which may outweigh the clinical benefits of undergoing hip arthroscopy:
There are some patients who would be worse after this operation, they need to be told that so it’s not all benefit if you have surgery because there are some real downsides.
Surgeon 5
It’s only now in the last five-ten years that a lot of operations are useless . . . for knees, that’s been shown not just being useless but also counterproductive for knee pain . . . I suspect some of those cases will get better conservatively.
Sports doctor 2
A surgeon also mentioned that hip abnormality is found in the normal population, which would suggest that some of these operations may be unnecessary:
Because the uncertainties about the long-term outcome, about the problems about the same imaging findings within the normal population and the uncertainties whether treating these imaging findings is going to result in improvement.
Surgeon 7
Equipoise
Up to 10 surgeons, the physiotherapists and the sports doctors seemed to agree with the need to gather scientific evidence that could answer the question about what FAI treatment performs better and in which circumstances:
I think it’ll be excellent if there was one and it’d put the whole issue to bed.
Surgeon 3
We don’t have enough details of the natural history of FAI that we could say that doing nothing is not reasonable.
Surgeon 2
Some clinicians feel it is a matter of finding which cohort of patients benefits more from operative and non-operative treatment, as they have witnessed different outcomes in their practice:
My feeling would be there may be subgroups of patients who do well with one or the other.
Physiotherapist 3
I don’t have strong opinions about the outcome of the surgery. M, my feeling is that it works in certain people and doesn’t in some of them.
Surgeon 14
Other clinicians think that finding out the evidence for or against surgical intervention could prove to have further consequences in their practice:
You look at the parallel hip situation, one side they have pain, the other side they don’t have pain. I, if you are the school of thought that you need to prevent arthritis, you should do both sides.
Surgeon 11
If we had the study then it should provide us with the justification that we need [to commissioners] or it’ll educate us into thinking we shouldn’t be doing the surgery.
Surgeon 10
However, some opinions that challenged the equipoise of the treatments on the base of previous experience and case evidence:
As far I am concerned, there isn’t a position of equipoise for consent on this, because there is enough data in the literature, which admittedly, isn’t high-quality data, particularly with cam impingement, we get good results with surgery in terms of symptom relief.
Surgeon 8
Another surgeon was particularly vocal in relation to this point, arguing that the most pressing issue is to answer the questions of whether [surgery] can significantly change the natural history of what it would happen to the hip over time (surgeon 9) rather than which treatment works better.
Potential outcomes of a randomised controlled trial
This section is dedicated to exploring a number of concerns the clinicians expressed in relation to the RCT that has been proposed. The differences between the treatments, the length of time of the trial, patient preferences, surgeon attitudes and the importance of clear eligibility criteria were the most recurrent themes.
Differences between the treatments that are being compared
Clinicians seemed concerned about sending patients to the trial who may need surgery, as they may be in the non-operative arm of the trial. This is understood as wasting another year, as conservative care is not perceived as curative:
I feel a bit guilty, ‘Have I given them the best advice’, you know?
Physiotherapist 1
Similarly, other clinicians are worried that people who may not need to go through the operative route can be rushed into it owing to their participation in the trial and they are also worried about the risks associated with surgery:
I suppose if the trial is there, you still want to make sure they didn’t have surgery earlier than you normally want to.
Sports doctor 2
I think the trial only kicks in if he is eligible for surgery not otherwise, if he’s not eligible for surgery, the trial doesn’t kick in.
Surgeon 11
There are significant risks in people who undertake surgery compared to those that don’t, that’s where it is awkward because these two treatments are so very different.
Surgeon 2
Those surgeons who believe that operative treatment does cure the condition and stops the deterioration of the hip joint would find difficult to send a patient to the trial:
So you’ve got the data so in that setting randomising patients to physiotherapy, i.e. not surgery, I don’t think it’s ethically correct.
Surgeon 13
If the findings are really clear of specific impingement, then it is a bit hard to convince them to undergo conservative treatment.
Surgeon 7
Other potential sources of bias in the recruitment were that the patient may have had physiotherapy before:
If you get referrals from me, and I think all the sport docs will be the same, we would have done to death the therapy.
Sports doctor 1
that the selected patients would have only milder symptoms:
He’s only symptomatic in his recreational activity so therefore it is not impinging in his activities of daily living and he is possibly the candidate on who the study might be safe for but it is not the candidate that needs my help badly.
Surgeon 9
and that the local physiotherapy may not be of equal quality across the centres:
Are you suggesting a remote access physio who gives them a programme and liaises with the local physios, because we don’t have specialist enthusiastic physios in our area who would be quite as knowledgeable as the ones you have in Coventry.
Surgeon 7
Duration of the trial
The duration of the trial is a major concern for the clinicians that were interviewed. They felt that delaying the recommended treatment could compromise the outcome of future surgical interventions:
The main concern with all of this is that if you’re delaying surgical treatment, are they going to progress essentially their arthritic symptoms or cartilage damage by introducing that delay.
Surgeon 13
I feel as it is, by the time they are referred to me it’s too late, if I actually send them for another year of physiotherapy, it’ll be a failing.
Surgeon 12
This concern was expressed in the context of finding damage to the joint and signs of deterioration. This is because, according to some surgeons, the opportunity to successfully treat FAI with hip arthroscopy depends on the level of cartilage damage. In addition, they wondered if there was going to be enough time to recover from surgery:
One of the thing [that] is potentially an issue is how advanced the degenerative change may be that you can pick up on your MRI.
Surgeon 10
Whether you’re going to follow up for only 12 months, I wonder if that gives enough time for recovery from surgery.
Surgeon 13
The same clinician suggested that it would be important to feed back this information to the patient:
If I was a patient and I wasn’t aware that there is a possibility that things can progress during that interval, I’d feel I’m not being fully informed about going into the trial.
Surgeon 13
On the other hand, other clinicians thought it was not long enough to answer the question about the natural history of the condition:
Follow-up of 12 months is interesting, I am also interested in the long-term outcome, because the question we really want to know is ‘are we influencing the natural history of the condition?’, which is going to take a lot longer to know.
Surgeon 7
However, they were aware of the restrictions of a RCT:
Generally I do wait for 6 months, 12 months maybe a little long, but I can understand what we are trying to [do].
Surgeon 11
Importance of the eligibility criteria
Seven clinicians reported that they wanted to have clear inclusion and exclusion criteria before they would consider sending patients to the RCT.
A physiotherapist explained that an age range would be useful, as well as criteria that are specific to their specialty:
I would want some more information or an assessment [of] the neuromuscular problems and the pain problems.
Physiotherapist 3
Surgeons suggested that eligibility requirements should include accurate classification of morphology and degenerative changes. They also mention the difficulties in determining the levels of activity and discomfort, which are subjective. The criteria would also assist the distinction between similar diagnoses:
I suppose dysplastic patients we need to be careful about, making sure those patients who have acetabula dyspraxia are not confused as having FAI.
Surgeon 13
Two surgeons asked about the outcome measures that would be used to assess the performance of the two treatments. They suggested that patient outcomes such as ‘time off work, period of mobility, complications [. . .] very important if you go into the operative group’ (surgeon 7) would provide useful information for the comparison of treatments.
Patient preferences
Up to 13 clinicians expressed concerns about how the trial would be perceived by their patients. Once the patient is willing to have some treatment, clinicians seem to worry how to present the non-operative arm of the trial, especially to those patients who have already had physiotherapy in the past. Some clinicians expected that patients would not agree to participate because it would be perceived as the same as they had already tried or because it would require much more effort:
I think that’s the problem with randomisation in that there may be not many patients who will be willing to be randomised because if they perceived they’ve already been through a trial of physio and other non-operative treatments they would not want to go into that arm of the trial.
Surgeon 10
I think we probably have to get her to buy in to it because it’d probably be hard work.
Physiotherapist 12
Another concern is that patients may be already set on having a particular intervention; specifically, those who are more seriously affected by the condition may want to have surgery:
[Surgeons] who have a special interest in [hip arthroscopy] may [have] a bias against randomization because some patients come through saying ‘I want surgery’- and this is the sort of patients who would do that - because they are keen sportsmen and have had pain for 12 months.
Surgeon 10
Other clinicians were concerned that patients might drop out of the trial if they were allocated to the non-operative arm and failed to see results within a few months.
Some surgeons mentioned that the way in which the non-operative treatment is presented would have a huge influence on whether or not patients agreed to enter the trial:
The problem is recruiting the patients, I think if you couldn’t come up with a logical alternative method of treatment, I think it is more likely to be accepted.
Surgeon 8
It can’t be seen as just physiotherapy, it needs to be a complete management plan, not just a group of exercises to strengthen [. . .] so it is a whole life style change, medication, physiotherapy intervention, the whole package, maybe even psychological interventions. Who knows?
Surgeon 1
Surgeon attitudes
A few surgeons in this sample spoke about how the attitudes towards surgery and being involved in a RCT vary across the scientific community, which may have an impact on the levels of recruitment to this specific trial.
The first obstacles could be described as the lack of equipoise in the community and the preference for surgical intervention:
I think if it had been done 3–4 years ago, I think it would have been easier. There was less conviction and most surgeons that were doing it [thought] that it was the right thing to do.
Surgeon 14
Of the majority of surgeons offering HA [hip arthroscopy] in the country, I see myself as being a little less invasive and more conservative [. . .] there are surgeons around the country who will operate in patients that I wouldn’t touch with a barge pole.
Surgeon 6
The second obstacle is the lack of awareness or willingness to be involved in research despite the institutional requirement in health services to do so:
I am an atypical hip surgeon who sees these types of patients. I am heavily involved in clinical trials.
Surgeon 10
I mean I have a quite strong research background. I, if you’re recruiting people potentially to this trial they need to be adequately informed.
Surgeon 2
Finally, there may be a bias introduced by vested interests of practising surgeons:
Trial is very important because FAI is expensive [. . .] however, I think it is going to be a very difficult trial [. . .] it is practised predominantly by a group of specialist surgeons that have vested interests in the success of the FAI over conservative care.
Surgeon 1
Recommendations
-
Clinicians asked for clear inclusion and exclusion criteria. These should include requirements that can be interpreted by a multidisciplinary team. Surgeons, physiotherapists and sport doctors tend to work together.
-
Surgeons are concerned about accurate classification of morphology and degenerative changes in order to exclude similar conditions. Physiotherapists would like more information about neuromuscular problems and other examination parameters.
-
Reaching agreements about the age range, level of activity, severity of symptoms and even psychological assessment of patients will be useful, as they are important cues in deciding what treatment to suggest.
-
Concerns about NHS patients who usually have tried non-operative care plans and may ask for the surgical treatment need to be addressed. The non-operative arm has to be presented as a different option from past care plans, to the clinicians as well as to the patients.
-
Information about outcome measures and how the results will be disseminated can be a motivational tool.
-
Clarification about how the fact that patients have had past therapy will affect the results of the trial may be necessary.
-
In the process of choosing the centres where the trial will be run, and the surgeons who will take part, there has to be awareness about possible vested interests in showing that the surgery works better, willingness to participate in research and quality of the local physiotherapy.
-
Addressing the concern of deterioration during the 12-month period would be helpful, for instance by explaining the procedure of regular and continuous assessment of patients’ condition and also showing that patients will be informed about the risks of delaying treatment.
-
It may be advisable to provide more information about how the non-operative arm will be presented to patients and how patients’ expectations will be managed to minimise drop-out rates owing to lack of results.
Appendix 4 Database of exercises to accompany personalised hip therapy protocol
Appendix 5 Pre-pilot patient interview schedule and patient information sheet questionnaire
Appendix 6 Final patient information sheet
Appendix 7 Outcome questionnaire
Appendix 8 Hip arthroscopy surgery vignettes
Appendix 9 Site research and development approval
The diversity of sites, from a small district hospital to a large London teaching hospital, provided valuable information on the processes required in order to gain site R&D approval. For all sites, significant improvement in the time taken to approval was achieved following the site visit (Figure 10). Ensuring that a member of the R&D department was able to attend the site visit was key in order to clarify queries about the study.
FIGURE 10.
UK FASHIoN: time in days to R&D approval.
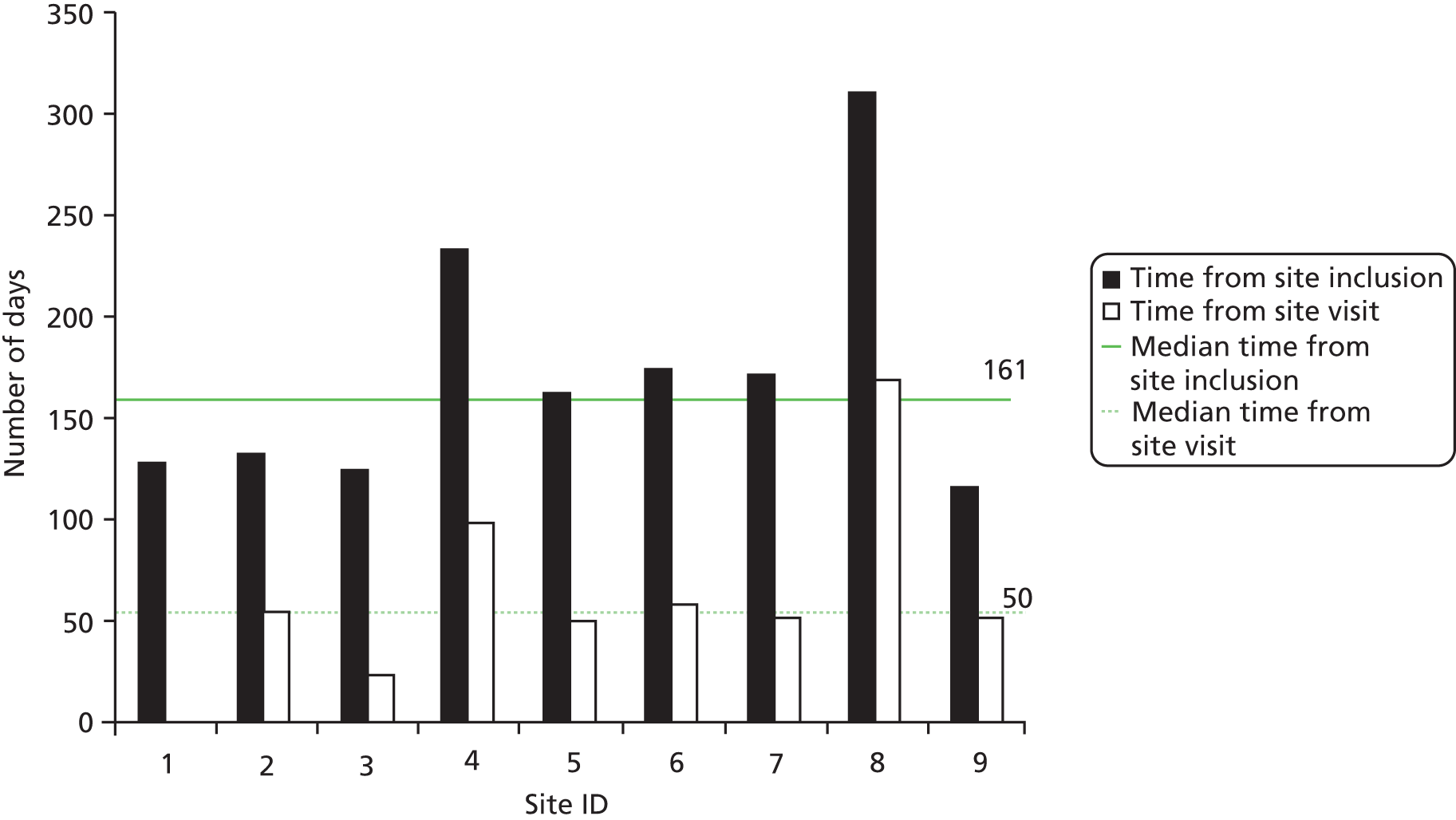
Problems encountered in time taken to achieve R&D approval
Good clinical practice (GCP) training: for two sites (8 and 10) delays occurred in submission to R&D as at the time it was thought that the PI required GCP training. Identification of GCP training within each of the site Trusts was proving difficult. Combining this with the PI’s schedule made finding a suitable time virtually impossible. Both Trust R&D departments then confirmed that in fact no GCP training was required as this was not a trial involving an investigational product.
Ionising Radiation (Medical Exposure) Regulations (IRMER) review: two sites (5 and 9) experienced delays in obtaining IRMER sign-off. At site 5, reviewers were just busy while, at another site, confusion was caused by the R&D project information submitted to the Integrated Research Application System.
Peer review: one site (9) experienced delays in obtaining peer review. The trust in question required all studies to be peer-reviewed. One of the reviewers was too busy to complete. The PI had to ask for another peer reviewer to be found.
CSP: at two sites (6 and 7) delays due to central updates of the system meant delay in the transference of site-specific information forms. The R&D department require these forms in order to progress local approvals.
Appendix 10 Pilot: qualitative recruitment intervention study – trial management group and recruiter interview schedule
Pre-pilot clinicians interviews schedule: UK FASHIoN
-
Greeting
-
Thank you for agreeing to take part in this study. As you know, we are seeking the advice of expert clinicians like yourself, about randomising patients to clinical trials. We would like to talk to you about this.
-
-
Verbal consent
-
Will it be OK with you if we audio record this conversation?
-
-
Explaining the trial
-
The context for this request is a new research project – the UK feasibility study of a trial of arthroscopic surgery for hip impingement compared with non-operative care (the UK FASHIoN project).
-
We would like you to imagine that there is a randomised controlled trial currently taking place comparing hip arthroscopy versus best conservative care (physiotherapy) for femoroacetabular impingement.
-
The trial design is outlined in this information sheet.
-
As a recruiting clinician, it is up to you whether to send a patient to the randomised trial or continue with usual care. If the patient is referred to be part of the trial, a researcher will discuss the trial with the patient and obtain informed consent to the randomisation.
-
-
Case study introduction
-
These are the notes of a patient who may have hip impingement. I would like you to think aloud and tell me what is going through your mind as you read this case. I am particularly interested in thoughts you may have when considering an intervention including referring the patient to the randomised control trial. So, can you tell me what it is in your mind about this patient?
-
Prompts:
-
What other interventions are you considering in this case?
-
What concerns do you have about sending the patient to randomisation?
-
What would make you decide one way or another?
-
What would you do if the trial was not running?
-
Will you opt for operative or non-operative care?
-
Which treatment do you think will help this patient more?
-
What do you think about the control condition, conservative care?
-
Are there any patients you would prefer not to randomise?
-
-
-
Next case
-
‘Please have a look [at] the next case and just as before I would like you to think aloud and tell me what is going through your mind as you read this case. I am particularly interested in thoughts you may have when considering an intervention including referring the patient to the randomised control trial. So, can you tell me what it is in your mind about this patient?’
-
(Prompts)
-
-
Closing
-
Thank you very much! Any other comments or thoughts?
-
Your contribution is much appreciated and extremely valuable to us. It will help us to improve the information available to clinicians deciding whether or not to participate in the UK FASHIoN study in the future.
-
Background
-
How long have you recruited patients to RCTs?
-
How did you become a recruiter?
-
Have you recruited to other RCTs, before this one?
-
How did you come to be working on this RCT?
-
Have you received any training (a) as a recruiter; (b) for this RCT?
-
When did you start recruiting to this trial?
-
Please describe how recruitment has been for you in this RCT.
Introducing/explaining the trial
-
How do you introduce/explain the trial to patients?
-
Why do you think this RCT is needed?
-
What is involved in taking part in this RCT; and in each arm?
-
What happens to patients if they agree to take part?
Randomisation
-
How do you explain to patients how the decision is made in the trial about which treatment they will receive?
-
How easy do you think the concept of randomisation is for patients to understand? Note: If this seems threatening, acknowledge that this is a difficult and confusing concept for everybody and then explore again.
-
Does randomisation seem sensible and reasonable to you?
Uncertainty
-
How do patients react to the idea that the specialist doctor does not know what the best treatment is?
-
How do you describe the uncertainty?
-
How do you feel about explaining uncertainty?
-
Do you ever have a feeling during an appointment that a patient should really have one treatment rather than another? Probe: Why? What do you do about that?
Difficulties during recruitment
-
What would you say are the main difficulties you face as a recruiter?
-
Can you describe specific examples of ‘good’ and ‘bad’ recruitment experiences?
-
Is recruitment organised well? Do other people explain the RCT to patients?
-
Are there difficulties with any particular arm?
-
Do patients have strong preferences?
-
Do you think this RCT is the right thing for these patients?
Finally – improving recruitment
-
What might, in your opinion, improve the recruitment appointments?
-
Do you think you (or others) need more support or training?
OPTIONAL
Recruitment pathway
-
How are patients recruited into this RCT? Is there a ‘recruitment’ appointment?
-
Who undertakes the assessment of patient eligibility for this RCT? Are you involved in eligibility assessment?
-
How do you find out which patients to approach about the RCT?
Informed consent
-
How easy do you think it is to obtain fully informed consent for patients participating in this RCT? (Probe: what is informed consent; how do you know when you have reached it?)
-
Are you ever concerned about the possibility of coercing patients into taking part in the trial?
Treatment preferences of patients
-
Do patients express preferences for particular treatments?
-
(Probe – what are their preferences; do you know why they express preferences?)
-
What do you do when a patient expresses a preference?
-
(Probe – do you accept it – what happens next? Or do you ever explore or challenge their preference?)
-
What other reasons (not preferences) have patients given for not taking part in this RCT?
-
What do you do when a patient gives a reason for not wanting to take part?
Personal views
-
Do you think your work as a recruiter is similar or different from your usual work/practice? Probe: In what ways it is (a) similar, (b) different?
-
Do you think your work as a recruiter has changed the way you practise outside research?
-
How do you feel about being a recruiter? Probe: Do you see yourself as a doctor/nurse/clinician, scientist, or researcher (if nurse also add ‘patient advocate’); or all of these? Are there benefits or problems that arise from these different roles?
-
Do you know (or have a hunch) about what the outcome of the RCT will be?
-
If you were a patient in this position today, would you agree to be recruited to the RCT and be randomised? Or would you choose a treatment? Which one?
Appendix 11 Examples of feedback letters to principal investigators and research associates
List of abbreviations
- 3D
- three-dimensional
- AE
- adverse event
- BHS
- British Hip Society
- CDC
- Consensus Development Conference
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CSP
- Chartered Society of Physiotherapy
- EQ-5D
- European Quality of Life-5 Dimensions
- FAI
- femoroacetabular impingement
- FASHIoN
- Feasibility of Arthroscopic Surgery for Hip Impingement compared with Non-operative care
- GCP
- good clinical practice
- HES
- Hospital Episode Statistics
- HTA
- Health Technology Assessment
- IBM
- International Business Machines
- iHOT-33
- International Hip Outcome Tool
- MAHORN
- Multicenter Arthroscopy of the Hip Outcomes Research Network
- MCID
- minimum clinically important difference
- MRC
- Medical Research Council
- MRI
- magnetic resonance imaging
- NAHS
- Non-Arthritic Hip Score
- NGT
- nominal group technique
- NIHR
- National Institute for Health Research
- ONS
- Office for National Statistics
- OPCS-4
- Office of Population Censuses and Surveys Classification of Interventions and Procedures-Fourth Edition
- PHT
- personalised hip therapy
- PI
- principal investigator
- PIS
- patient information sheet
- PPI
- patient and public involvement
- QRI
- qualitative recruitment intervention
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RIAS
- Roter Interaction Analysis System
- SAE
- serious adverse event
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- TMG
- Trial Management Group
- UCLA
- University of California, Los Angeles
- UHCW
- University Hospitals Coventry and Warwickshire