Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 14/64/01. The protocol was agreed in December 2014. The assessment report began editorial review in July 2015 and was accepted for publication in October 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Shepherd et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the underlying health problem
Juvenile idiopathic arthritis (JIA) is an umbrella term that encompasses all forms of arthritis with onset before the age of 16 years and symptoms that persist for > 6 weeks for which the cause is unknown. 1,2 The role of infections (either bacterial or viral) in the development of JIA has been investigated but no unequivocal evidence to either support or rule out an association has been clearly demonstrated. 3 The term JIA has been in use since 1995 and was proposed by the International League of Associations for Rheumatology (ILAR) committee to replace the older terms ‘juvenile rheumatoid arthritis’ and ‘juvenile chronic arthritis’, which were the commonly used in the USA and in Europe, respectively. 4 JIA is characterised by joint pain, swelling and a limitation of movement which is caused by the inflammation of the synovial membrane of the affected joints. If untreated, this inflammation causes progressive erosive arthritis, which can lead to disability and growth retardation. 5 JIA is classified according to the Revised ILAR criteria6 into seven subtypes: systemic arthritis, oligoarthritis (subcategories persistent and extended), polyarthritis rheumatoid factor negative (RF–ve), polyarthritis rheumatoid factor positive (RF+ve), psoriatic arthritis (PA), enthesitis-related arthritis (ERA) and undifferentiated arthritis (Table 1); some forms of the disease are more likely to be associated with extra-articular features such as uveitis (inflammation of the middle layer of the eye).
| JIA classification6 and features7–12 | Included in NICE appraisal scope? |
|---|---|
| Oligoarthritis | |
|
|
| The ILAR classification recognises two subcategories: | |
|
No |
|
Yes |
| Polyarthritis | |
| Polyarthritis (RF+ve): accounts for about 4% of cases in the UK13 Polyarthritis (RF–ve): accounts for about 21% of cases in the UK13
|
Yes (all forms of polyarthritis) |
| ERA | |
|
Yes |
| PA | |
|
Yes |
| Systemic arthritis | |
|
Not active systemic onset JIA alone. Those who go on to have a form of JIA that is included (e.g. polyarthritis) do match the remit |
| Undifferentiated arthritis | |
|
Yes |
At onset, the particular subtype of JIA will be diagnosed according to the presenting features corresponding to one of the seven ILAR categories. As JIA progresses, more joints may become affected. For some, where JIA was classified at onset as oligoarthritis, problems with five or more joints develop after 6 months and the JIA type is then described as extended oligoarthritis (EO). Similarly, the term polyarthritis applies to patients who at a particular point in time 6 months or more after the onset of disease (JIA of any onset type) have five or more active joints. In this case they are said to have polyarticular-course JIA. The concept of polyarticular-course JIA has been used for clinical trials and can typically include RF+ve and RF–ve polyarthritis, EO, ERA, PA and undifferentiated arthritis. Systemic JIA may also be included in the definition of polyarticular-course JIA providing that there have been no active systemic symptoms during the previous 6 months. 14
Severity of disease and long-term outcome are variable both between different JIA subtypes and between different individuals with the same JIA subtype (Box 1). Analyses of historical cohorts of JIA patients (comprising a mix of JIA subtypes) have shown that > 50% of patients continued to have active disease for as long as 17 years after disease onset and such patients would require treatment into adulthood. 15,16 However, it should be noted that in historical studies the patients, particularly at disease onset, were unlikely to have been treated with methotrexate or biologic disease-modifying antirheumatic drugs (DMARDs), which were not available. Even when biologic DMARDs became available, they may not have been widely used. Consequently, for all types of JIA, outcomes in general are likely to have improved owing to more widespread use of the newer treatment strategies, particularly early in the disease course. Nevertheless, one-third or more of children will still require treatment for JIA in adult life. JIA that persists into adulthood is distinct from adulthood rheumatoid arthritis and should not be considered similar.
A recent systematic review of the prevalence and incidence of JIA in Europe17 found that rates varied greatly among published studies. Incidence rates ranged from 1.6 to 23 per 100,000 (33 studies) and prevalence rates from 3.8 to 400 per 100,000 (29 studies). The estimated annual incidence of JIA in England in the period 1989–91 was 11 per 100,000. 18 Prevalence in the UK has not been estimated since 1959 when a figure of 65 per 100,000 was reported. 18 The Children’s Chronic Arthritis Association website states that annual incidence is approximately 1 per 10,000 (i.e. 10 per 100,000) and prevalence is about 1 per 1000 (i.e. 1000 per 1,000,000). 19
-
Often mild and may resolve with little or no lasting damage to joints; has the best outlook of all the types of JIA.
-
Approximately half of children will have symptoms for at least 10 years, one-third or more of children will have arthritis continuing into adulthood.
-
Chronic anterior uveitis may cause blindness or visual loss if not detected and treated early enough.
-
Causes damage to joints so early treatment to minimise this is needed.
-
Can be destructive and disabling.
-
Approximately half of children will have symptoms for at least 10 years, one-third or more of children will have arthritis continuing into adulthood.
-
Chronic anterior uveitis may cause blindness or visual loss if not detected and treated early enough.
-
Approximately half of children will have symptoms for at least 10 years and at least one-third of children will have arthritis continuing into adulthood (most likely to be of the RF+ve type, which is more severe and can require more aggressive treatment).
-
Joints may become damaged if inflammation is not controlled, leading to the potential need for joint replacement or serious disability.
-
May evolve to ankylosing spondylitis in the adult years (especially in those with teenage onset) and may require long-term disease-modifying or biologic agents.
-
Although there are few long-term data, disease course may be similar to chronic arthritis (either oligoarthritis or polyarthritis) and is likely to continue into adulthood.
-
One-third of children will have one or two episodes that settle with treatment, one-third will have relapses and need intermittent treatment and one-third will require ongoing treatment into adulthood and are at risk of joint damage.
-
Although there are few long-term data, clinical advisors indicate that the long-term outcome is likely to depend on the predominant features of the arthritis and whether it is persistent oligoarthritis or polyarticular-course arthritis.
The sources of these data are not given; however, the same data are available in the Interim Clinical Commissioning Policy Statement for biological therapies for the treatment of JIA. 20 Based on the mid-2013 population estimates for those aged ≤ 17 years in England (approximately 11.5 million) and Wales (approximately 630,000),21 these incidence and prevalence values equate to an estimated incidence of 1150 cases a year in England and 63 cases a year in Wales, with an estimated 11,500 and 630 children with JIA overall in England and Wales, respectively.
A 2012 conference presentation13 presented data from a multicentre long-term prospective inception cohort study of children with newly diagnosed inflammatory arthritis (the Childhood Arthritis Prospective Study). This provides information on JIA subtypes classified using the ILAR criteria for 1014 newly diagnosed children [median disease duration 5.2 months; interquartile range (IQR) 2.5–10.9 months]. Among this cohort, EO and polyarticular-course JIA may be under-represented because median disease duration is < 6 months. Nevertheless, the proportions of each JIA subtype are similar to those reported by an older study22 for a smaller group of children (n = 521), as shown in Table 2.
| JIA classification6 | Newly diagnosed children, % (n = 1041)13 | From 17 centres within the UK, % | Children starting first-line antibiotics, % |
|---|---|---|---|
| Oligoarthritis | |||
| Persistent oligoarthritis EO |
48.2 (502/1041) 5.5 (57/1041) |
30.1 (157/521) 15.2 (79/521) |
12 (40/346) 17 (59/346) |
| Polyarthritis | |||
| RF+ve RF–ve |
3.6 (37/1041) 20.6 (214/1041) |
7.1 (37/521) 19.6 (102/521) |
29 (102/346) 8 (28/346) |
| ERA | 5.6 (58/1041) | 6.5 (34/521) | 6 (20/346) |
| PA | 7.0 (73/1041) | 7.1 (37/521) | 6 (20/346) |
| Systemic arthritis | 6.0 (62/1041) | 14.4 (75/521) | 16 (54/346) |
| Undifferentiated arthritis | 3.7 (38/1041) | NR | 2 (8/346) |
| Not recorded | N/A | N/A | 4 (15/346) |
Table 2 also reports the distribution of JIA subtypes from a dataset (n=346 children and young people) drawn from the British Society for Paediatric and Adolescent Rheumatology (BSPAR) etanercept cohort and the Biologics for Children with Rheumatic Diseases cohort. 23 These data are for patients starting a first-line biologic DMARD from 1 January 2010 to 28 August 2014, and although a smaller cohort, is more up to date than the other two cohorts. There are some differences in the distribution of subtypes, with a lower proportion of patients with persistent oligoarthritis and a higher proportion with EO. This may be because the children and young people in this cohort had longer disease duration (mean disease duration 2 years) and therefore more may have progressed from persistent to extended oligoarthritis (i.e. a total of more than four joints affected after the first 6 months of disease). Persistent oligoarthritis may be under-represented as it is a milder form of JIA and generally may be adequately managed without biologic DMARDs.
In addition to the immediate impacts of the joint pain, swelling and limitation of movement that characterise JIA, there are longer-term problems and other issues that may arise over time. Progressive joint damage can lead to permanent disability and eventually to a need for joint replacement. A retrospective review of 154 adolescents (aged 16–21 years) found that 14% had undergone a joint operation, with 30 separate surgeries (e.g. synovectomies, reconstructive finger or toe joint operations and one hip replacement) having been undertaken. 24 Growth impairment affects about 10–20% of patients with JIA (mainly those with systemic or polyarticular JIA and who require high doses of glucocorticoids),25 and decreased bone mass, which can lead to the development of osteoporosis, is also a recognised problem. 26
Juvenile idiopathic arthritis is associated with a range of extra-articular manifestations, including uveitis, inflammatory bowel disease and psoriasis. Uveitis commonly occurs in children with oligoarthritis and is less common in other subtypes of JIA. It is characterised by inflammation of the middle layer of the eye, the uveal tract. In severe cases that do not respond to treatment, uveitis can be associated with complications such as cataract, glaucoma and macular oedema, and can lead to sight impairment and blindness. Inflammatory bowel disease (e.g. Crohn’s disease and ulcerative colitis) is typically associated with ERA, whereas psoriasis is associated with PA.
The incidence of childhood uveitis in North America and Europe is estimated at 4.3–6 per 100,000 children and the prevalence at 30 per 100,000 children. 27 Between 20% and 25% of uveitis cases in children are associated with JIA. The prevalence of uveitis in JIA is between 8% and 30%, but in children with oligoarticular onset JIA it may be between 45% and 57%. 28 Uveitis in patients with JIA commonly occurs with the early onset of arthritis (mean age at onset 3–5 years). Presentation in younger children may be delayed owing to their inability to articulate symptoms. Screening for uveitis has therefore been implemented for children with JIA in England. 28 Complications are present in between 30% and 50% of children with JIA with uveitis at diagnosis. A total of 50–70% of children with severe uveitis will develop visual impairment. 29
A recent systematic review of qualitative studies that explored the experiences of children living with JIA highlighted the profound effect that JIA has on children’s lives. In particular, pain was a constant reminder of their disease and limited children’s abilities to participate in normal life, including social events and schooling. Their physical limitations meant that they had to look for alternative activities and potential career options which they would be able to pursue. Many children and adolescents felt misunderstood and some kept their illness a secret from their peers and others. 30
Measures of response to treatment and definition of remission
The aim of JIA treatment is to achieve clinical remission (complete absence of active disease). Aggressive early treatment aims to control inflammation and thus symptoms (e.g. joint pain); to decrease the number of actively affected joints in order to prevent joint damage, loss of function and disability; and to maintain or improve quality of life (QoL). Response to treatment is assessed in clinical trials by a validated core set of variables that were adopted by the American College of Rheumatology (ACR) in 1997. This definition of response is now known as the ACR Pediatric (ACR Pedi) definition of improvement. 31 The lowest level of improvement is known as ACR 30 (or ACR Pedi-30). The ACR Pedi-30 core variables are:
-
physician global assessment of disease activity (PGA) using a visual analogue scale (VAS) range from best score of 0 mm to worst score of 100 mm (although in some studies this was reported as 0–10 cm)
-
patient or parent global assessment of overall well-being using a VAS (range 0–100 mm, where 0 is the best score)
-
functional ability as measured by the patient or parent using the Childhood Health Assessment Questionnaire (CHAQ) (range 0–3, where 0 is the best score)
-
number of joints with active arthritis
-
number of joints with a limited range of motion
-
laboratory marker of inflammation [erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) level].
A response at the ACR Pedi-30 level is defined as an improvement in three of any six of the core variables by at least 30%, and a worsening of no more than one of the remaining variables by > 30%. In addition to the ACR Pedi-30, higher levels of response can also be defined: the ACR Pedi-50, -70, -90 and -100 levels of response require at least 50%, 70%, 90% or 100% improvement, respectively, in at least three of any six of the core set variables, with no more than one of the remaining variables worsening by > 30%. 14,32 It should be noted that according to expert advice, ACR Pedi-30 is no longer accepted as a response but is considered a non-response or inadequate response, with response levels of at least ACR Pedi-50 or -70 sought from a drug intervention.
More recently, in 2009, the Juvenile Arthritis Disease Activity Score (JADAS) was proposed and validated. 33 The JADAS is a composite score that can be quickly calculated because it is the arithmetic sum of the scores from the following four individual component measures:
-
PGA, measured on a 10-cm VAS (range 0 = no activity and 10 = maximum activity)
-
parent/patient global assessment of well-being, measured on a 10-cm VAS (range 0 = very well and 10 = very poor)
-
count of joints with active disease
-
ESR.
The component measures are also measures used in the ACR Pedi definition of improvement. 31
The count of joints with active disease in the JADAS is primarily based on a 27-reduced joint count (JADAS-27, total score range 0–57), although scores based on a full 71-joint count (JADAS-71, total score range 0–101) and a 10-joint count (JADAS-10, total score range 0–40) have also been validated. 33 Further studies have shown that a 3-item JADAS that does not use ESR data is also a robust measure,34,35 which is of particular benefit for children who do not need to provide a blood sample for routine medication monitoring. As the JADAS has become more widely used, further proposals have been made that would define low, medium and high disease activity35,36 and define improvement. 37 With these definitions in place, the future management goal would be to achieve minimal disease activity for all children with JIA. 38
Preliminary criteria to define clinical remission in oligoarticular (persistent and extended), RF+ve and RF–ve polyarticular and systemic JIA have also been developed. 39 Two levels of clinical remission have been proposed, namely clinical remission on medication and clinical medication off medication. The criteria for both types of clinical remission are based on achieving inactive disease, which is defined as:
-
no joints with active arthritis
-
no fever, rash, serositis, splenomegaly or generalised lymphadenopathy attributable to JIA
-
no active uveitis
-
normal ESR or CRP (or both normal if both tested)
-
a PGA that indicates no disease activity.
Clinical remission on medication is then proposed to have been achieved if all the criteria for inactive disease have been met for a minimum of 6 continuous months while the patient is on medication. Clinical remission off medication is proposed to have been achieved if all the criteria for inactive disease have been met for a minimum of 12 continuous months while the patient is off all antiarthritis and antiuveitis medications.
Since the original publication of the preliminary criteria to define clinical remission,39 validation of the criteria for defining clinical inactive disease in oligoarticular (persistent and extended), polyarticular (RF+ve and RF–ve) and systemic JIA has been undertaken. This has led to three changes: the addition of a definition for no active uveitis (as defined by the Standardization of Uveitis Nomenclature Working Group); clarification that the ESR or CRP level should be within the normal limits in the laboratory where tested or, if elevated, not attributable to JIA; and one additional criterion (duration of morning stiffness of 15 minutes or less). 40
In addition to definitions of response to treatment and clinical remission, some publications also report on the outcome of disease flare (periods in which symptoms worsen). A preliminary definition based on the ACR Pedi-30 core response variables was obtained from a single small study (n = 51). 41 This preliminary definition was worsening in any two of six core response variables by 40% or more without concomitant improvement of more than one of the remaining core response variables by 30% or more. However, other studies have used different flare definitions (e.g. a worsening of ≥ 30% in three of six ACR Pedi-30 variables). 42
Current service provision
There is currently no National Institute for Health and Care Excellence (NICE) clinical guideline on the treatment of JIA; however, there are two pieces of NICE guidance:
-
Guidance on the Use of Etanercept for the Treatment of Juvenile Idiopathic Arthritis [NICE Technology Appraisal (TA)35]43 (this assessment report will inform an update of this guidance)
-
Tocilizumab for the Treatment of Systemic Juvenile Idiopathic Arthritis (NICE TA238)44 (active systemic JIA is not included within this assessment report).
There are currently two interim commissioning statements: Biologic Therapies for the Treatment of Juvenile Idiopathic Arthritis20 and the draft NHS Clinical Commissioning Policy for severe refractory uveitis in paediatric patients. 29 The first interim clinical commissioning policy statement was published (January 2015) by NHS England Clinical Reference Group for Paediatric Medicine20 in the absence of NICE guidance for other biologic DMARDs and to cover more recent changes to the licensed indications to etanercept and is being consulted on. The purpose of the interim policy statement is to provide guidance for the use of biologic DMARDs in patients with JIA until the planned NICE guidance is published. The statement has a broader remit than the planned NICE guidance [Arthritis (juvenile idiopathic) – abatacept, adalimumab, etanercept and tocilizumab (including review of TA35)] as it includes all biologic DMARDs and all types of JIA (i.e. including persistent oligoarticular JIA and systemic JIA, which are not included in the NICE scope for the planned guidance). A summary of the key features of the drug treatment pathway is provided in Table 3.
| When/why and who | What | Notes |
|---|---|---|
| At diagnosis to induce disease remission, all patients | Corticosteroids: EITHER intra-articular to all affected joints OR systemic, preferably intravenous [owing to the side effects (e.g. effect on growth or increased risk of osteoporosis) of oral corticosteroids] |
In patients with mild disease limited to fewer than five joints, intra-articular steroids may induce remission of > 6 months, particularly if the long-acting corticosteroid triamcinolone hexacetonide is used |
| Patients with more severe disease may need intravenous steroids to induce remission, although intra-articular steroids are used in some patients as an alternative | ||
| To maintain remission, patients with arthritis affecting more than five joints or arthritis severely affecting crucial joints (e.g. spine, ankles, hips, wrists) | MTX | This accounts for around half of all children who develop JIA Effective in reducing the amount and severity or arthritis but only induces complete remission in 30–50% of patients |
| When JIA remains active despite optimal MTX dosing OR when patient is intolerant of MTX | Biologic DMARD (many given in coadministration with MTX to optimise their effect) | Estimated that one-third of all children who start treatment with MTX need to progress to a biologic DMARD |
Clinical advice to the authors of this assessment report (hereafter referred to as the assessment group) suggested that the interim statement largely reflects current practice. However, it was acknowledged that there would still be some variability across the country owing to differences in interpretation and limitation on access and prescribing.
According to the second interim clinical commissioning policy statement (the draft NHS Clinical Commissioning Policy for severe refractory uveitis in paediatric patients29), patients with JIA-associated uveitis may be managed initially with topical corticosteroids or systemic corticosteroids if required. In more severe cases, a DMARD can be used, with methotrexate being a standard treatment. If the disease is not controlled with DMARDs, the next line of treatment is use of a tumour necrosis factor (TNF) inhibitor (TNF-α is shown to be implicated in the pathogenesis of uveitis). TNF inhibitors include etanercept (Enbrel®, Pfizer), adalimumab (Humira®, AbbVie), infliximab (REMICADE®, Centocor Ortho Biotech Inc.), golimumab (SIMPONI®, Centocor Ortho Biotech Inc.) and certolizumab (Cimzia®, UCB Pharma S.A.), although the last two of these may not be easily available in the UK, and only etanercept and adalimumab are licensed for the treatment of JIA in children in Europe. For severe refractory uveitis in paediatric patients, the draft NHS Clinical Commissioning Policy states that etanercept is not suitable for use in JIA patients with uveitis or uveitis not associated with JIA. 29 Adalimumab is recommended where methotrexate does not control symptoms, with infliximab used in patients in whom adalimumab is not tolerated or not effective. 29
Description of the technology under assessment
Four biologic DMARDs are within the scope of the NICE appraisal and are therefore included in this assessment report: abatacept [Orencia®, Bristol-Myers Squibb (BMS)], adalimumab, etanercept and tocilizumab (RoActemra®, Roche). The licensed indication differs across these interventions (e.g. in terms of the age range of children and young people eligible for treatment, the previous treatment that they should have received and the subtype of JIA) as summarised in Table 4. The Interim Clinical Commissioning Policy Statement: Biologic Therapies for the Treatment of JIA20 provides a pragmatic estimate of 950 children with JIA in England who are currently receiving a biologic DMARD. This estimate is based on current data from the biologics databases in the UK which indicate that in England alone 890 children are receiving a biologic DMARD for JIA (most of which are NICE-approved biologic DMARDs). Clinical advice to the assessment group suggested that this figure may be an underestimate. An alternative estimate of 1500 was suggested by one clinician.
| Drug (chief mode of action) | Polyarthritis (polyarticular)a | ERA | PA | Systemic onset | ||
|---|---|---|---|---|---|---|
| RF+ve | RF–ve | EO | ||||
| Abatacept (prevents T-cell activation) | Yes | Yes | Yes | –b | – | – |
| With MTX. Patients ≥ 6 years of age with insufficient response to DMARDs including at least one TNF inhibitor | ||||||
| Adalimumab (TNF inhibitor) | Yes | Yes | Yes | Yes | – | – |
| With MTX unless not tolerated/not appropriate Patients ≥ 2 years of age, with inadequate response to 1 or more DMARDs |
Patients ≥ 6 years of age with inadequate response, to or intolerant of, conventional therapy | |||||
| Etanercept (TNF inhibitor) | Yes | Yes | Yes | Yes | Yes | – |
| Children and adolescents from 2 years of age with inadequate response to, or intolerant of, MTX | Adolescents from 12 years of age with inadequate response, or intolerant of, conventional therapy | Adolescents from 12 years of age with inadequate response, or intolerant of, MTX | ||||
| Tocilizumab (IL-6 inhibitor) | Yes | Yes | Yes | – | – | Yes |
| With MTX unless not appropriate. Patients ≥ 2 years of age who have responded inadequately to previous treatment with MTX | With MTX unless not appropriate. Patients ≥ 2 years of age with inadequate response to NSAIDs and systemic corticosteroids | |||||
As noted earlier in Current service provision, the interim clinical commissioning policy indicates that the initial biologic DMARDs to be considered for use would be a TNF inhibitor, which for the purposes of this assessment would be either adalimumab or etanercept (however, etanercept is not suitable for use in JIA patients with uveitis). If a treatment switch was required, the second-line biologic DMARD would initially be the alternative TNF inhibitor (i.e. a switch from adalimumab to etanercept or vice versa). If a further switch was necessary, the third-line biologic would be either abatacept or tocilizumab, and the final switch possible would be to change abatacept to tocilizumab or vice versa. However, in terms of the marketing authorisations, the licence for abatacept indicates that there should have been a prior insufficient response to at least one TNF inhibitor. There is no such indication in the licence for tocilizumab.
The summary of product characteristics (SPC) for each biologic DMARD should be consulted for the specific contraindications, special warnings and precautions for use; however, there are some aspects that are common to all biologic DMARDs, which are summarised here. 45–48 These drugs block aspects of normal immune system signalling and, consequently, it is recommended that all patients receiving a biologic DMARD carry an alert card to indicate that they are at increased risk of developing a serious infection. Patients are at risk not only of typical bacterial and viral infections but also of opportunistic infections including invasive fungal infections. Existing latent infections (e.g. latent hepatitis B, latent tuberculosis) could potentially reactivate. Consequently, if patients have an existing infection, treatment with a biologic DMARD is not recommended until the infection is treated. Patients should be screened for latent infections, and childhood vaccinations should be brought up to date prior to beginning therapy with a biologic DMARD.
The SPCs for each of the four biologic DMARDs included in the review do not explicitly specify license upper age limits for treatment. Clinical advisors have indicated that if adolescents are responding to treatment then this should be continued into adulthood as required. Furthermore, some JIA patients may need to restart a biologic DMARD in adulthood and some JIA patients may require a biologic DMARD for the first time in adulthood.
Abatacept
Abatacept in combination with methotrexate is indicated for the treatment of moderate to severe active polyarticular JIA in paediatric patients aged ≥ 6 years, who have had an insufficient response to other DMARDs including at least one TNF inhibitor. 45
Abatacept is a fusion protein produced by recombinant deoxyribonucleic acid (DNA) technology in Chinese hamster ovary cells. It inhibits T-cell activation by specifically binding to cluster differentiation (CD)80 and CD86, thereby selectively inhibiting a costimulatory pathway that is required for full activation of T lymphocytes. 45,49 Through this mechanism, abatacept modulates the downstream T lymphocyte-dependent antibody responses and inflammation that cause the symptoms of JIA.
Treatment should be initiated and supervised by specialist physicians experienced in the diagnosis and treatment of JIA at the appropriate dosage as indicated in Table 5. Abatacept is not recommended in combination with TNF inhibitors. 45
| Mode of administration and cost | Dose (children aged 6–17 years) | Notes | |
|---|---|---|---|
| Intravenous infusion given over a period of 30 minutes Cost: powder for reconstitution, net price for a 250-mg vial = £302.40 |
Body weight < 75 kg | 10 mg/kg, repeated at 2 weeks and 4 weeks after initial infusion, then every 4 weeks | Review treatment if no response within 6 months |
| Body weight 75–100 kg | 750 mg, repeated at 2 weeks and 4 weeks after initial infusion, then every 4 weeks | Dosing for patients weighing ≥ 75 kg follows the adult dosing regimen | |
| Body weight > 100 kg | 1 g, repeated at 2 weeks and 4 weeks after initial infusion, then every 4 weeks | ||
Adalimumab
Adalimumab in combination with methotrexate is indicated for the treatment of active polyarticular JIA in patients from the age of 2 years who have had an inadequate response to one or more DMARDs. Adalimumab can be given as monotherapy in the case of intolerance to methotrexate, or when continued methotrexate treatment is inappropriate. Adalimumab is also indicated for the treatment of active ERA in patients aged ≥ 6 years, who have had an inadequate response to, or who are intolerant of, conventional therapy. 46
Adalimumab is a fully human monoclonal antibody drug initially tested as a treatment for rheumatoid arthritis (hence the trade name Humira – HUman Monoclonal antibody In Rheumatoid Arthritis). It binds specifically to the inflammatory cytokine TNF, thereby neutralising its biological function46 and modifying the inflammatory disease process. The European Medicines Agency (EMA) therapeutic indication for adalimumab was extended to the treatment of JIA in July 2008.
Treatment should be initiated and supervised by specialist physicians experienced in the diagnosis and treatment of JIA at the appropriate dosage as indicated in Table 6. 46 The concomitant administration of adalimumab with other biologic DMARDs [e.g. anakinra (Kineret®, Swedish Orphan Biovitrum) and abatacept] or other TNF antagonists is not recommended. 46
| Mode of administration and cost | Dosage | Notes | |
|---|---|---|---|
| Polyarticular JIA | |||
| Subcutaneous injection given EOW (volume for injection is selected from a chart based on patient height and weight) Cost: net price for a 40-mg prefilled pen or prefilled syringe = £352.14; 40-mg/0.8-ml vial = £352.14 |
Patients aged 2 to < 4 years | 24 mg/m2 BSA up to a maximum single dose of 20 mg | A clinical response is usually achieved within 12 weeks of treatment. Continued therapy should be carefully reconsidered in a patient not responding within this time period Contraindicated in patients with moderate to severe heart failure (New York Heart Association class III/IV) |
| Patients aged 4–12 years | 24 mg/m2 BSA up to a maximum single dose of 40 mg adalimumab | ||
| Patients aged ≥ 13 years | 40 mg administered EOW regardless of body surface area | ||
| ERA | |||
| Patients ≥ 6 years of age | 24 mg/m2 BSA up to a maximum single dose of 40 mg | No indication for stopping treatment is provided | |
Etanercept
Etanercept (Enbrel®, Pfizer) is a fully humanised soluble TNF receptor fusion protein produced by recombinant DNA technology in Chinese hamster ovary cells. It is a dimer with two copies of the extracellular domain of the TNF receptor (p75) linked with the Fc component of human immunoglobulin 1, binding to TNF-α. 50 The mechanism of action of etanercept is thought to be its competitive inhibition of TNF binding to cell-surface TNF receptor, preventing TNF-mediated cellular responses by rendering TNF biologically inactive. Etanercept may also modulate biologic responses controlled by additional downstream molecules (e.g. cytokines, adhesion molecules or proteinases) that are induced or regulated by TNF. 47 The EMA therapeutic indication for etanercept in the treatment of JIA was extended in July 2012 to include:
-
treatment of polyarthritis (RF+ve or RF–ve) and EO in children and adolescents aged ≥ 2 years who have had an inadequate response to, or who have proved intolerant of, methotrexate
-
treatment of PA in adolescents aged ≥ 12 years who have had an inadequate response to, or who have proved intolerant of, methotrexate
-
treatment of ERA in adolescents aged ≥ 12 years who have had an inadequate response to, or who have proved intolerant of, conventional therapy.
The age for treating polyarticular disease has been reduced from 4 to 2 years of age and the upper age limit of 17 years has been removed.
Treatment should be initiated and supervised by specialist physicians experienced in the diagnosis and treatment of JIA at the appropriate dosage as indicated in Table 7. The combined use of etanercept and anakinra or etanercept and abatacept is not recommended.
| Mode of administration and cost | Dose for JIA | Notes |
|---|---|---|
| Subcutaneous injection Cost: net price of a 10-mg vial (with solvent) = £35.75 25-mg vial (with solvent) = £89.38 25-mg prefilled syringe = £89.38 50-mg prefilled pen or prefilled syringe = £178.75 |
0.4 mg/kg (up to a maximum of 25 mg per dose) given twice weekly with an interval of 3–4 days between doses OR 0.8 mg/kg (up to a maximum of 50 mg per dose) given once weekly |
Consider discontinuation in patients who show no response after 4 months |
Tocilizumab
Tocilizumab (RoActemra®, Roche) in combination with methotrexate is indicated for the treatment of juvenile idiopathic polyarthritis (RF+ve or RF–ve and EO) in patients ≥ 2 years of age who have responded inadequately to previous therapy with methotrexate. When the patient is intolerant to methotrexate or where continued treatment with methotrexate is inappropriate, tocilizumab can be given as monotherapy. 48 Tocilizumab is also indicated for the treatment of active systemic JIA but this indication is not included within the current NICE appraisal.
Tocilizumab is a humanised, monoclonal, antihuman interleukin-6 (IL-6) receptor (IL-6R) antibody that binds to membrane and soluble IL-6R, inhibiting IL-6-mediated signalling – a key cytokine in rheumatoid arthritis pathogenesis. 51 IL-6 is involved in causing inflammation and is found at high levels in patients with rheumatoid arthritis, systemic JIA and polyarticular JIA. By preventing IL-6 from attaching to its receptors, tocilizumab reduces the inflammation and other symptoms of these diseases. 48 The EMA was granted a licence for tocilizumab in the treatment of JIA in May 2011.
Treatment should be initiated by health-care professionals experienced in the diagnosis and treatment of JIA at the appropriate dosage as indicated in Table 8.
| Mode of administration and cost | Dose for polyarticular JIA in patients over 2 years of age | Notes | |
|---|---|---|---|
| Intravenous infusion over 1 hour Cost: net price for 4 ml (80-mg vial) = £102.40 10-ml (200-mg) vial = £256.00; 20-ml (400-mg vial) = £512.00 |
Body weight < 30 kg | 10 mg/kg once every 4 weeks | Dose interruptions (including discontinuation) are recommended for liver enzyme abnormalities, low absolute neutrophil count and low platelet count according to the tables provided in the SPC Clinical improvement is expected within 12 weeks of initiation of treatment. Continued therapy should be carefully reconsidered in a patient exhibiting no improvement within this time frame |
| Body weight ≥ 30 kg | 8 mg/kg once every 4 weeks | ||
Chapter 2 Definition of the decision problem
Decision problem
In line with the scope of the NICE appraisal, the clinical effectiveness and cost-effectiveness of abatacept, adalimumab, etanercept and tocilizumab for the treatment of JIA will be assessed.
The comparators for this assessment are: DMARDs (such as methotrexate), if DMARDs can be tolerated; best supportive care, if DMARDs are not tolerated; biologic DMARDs (etanercept, abatacept, adalimumab and tocilizumab) compared with each other within their licensed indications where appropriate.
The relevant population are children and young people with JIA diagnosed at onset either as polyarthritis (RF+ve and RF–ve) or EO, and those with other forms of polyarticular-course arthritis (e.g. ERA, PA or undifferentiated arthritis). Children/young people with JIA and uveitis are also relevant. The age of the children/young people may vary by intervention owing to differences in the licensed indications.
As specified in the NICE scope, the following clinical effectiveness outcome measures are relevant to the decision problem: disease activity, disease flares, physical function, joint damage, pain, reduced use of corticosteroids; occurrence of extra-articular manifestations (such as uveitis), changes in body weight and height, mortality, adverse effects of treatment and health-related quality of life (HRQoL).
Overall aims and objectives of the assessment
The aim of this multiple technology appraisal (MTA) is to assess the clinical effectiveness and cost-effectiveness of abatacept, adalimumab, etanercept and tocilizumab for treating JIA.
The objectives are:
-
to undertake systematic reviews of the clinical effectiveness and cost-effectiveness of abatacept, adalimumab, etanercept and tocilizumab for the treatment of JIA, and of the HRQoL of people with JIA
-
to critique the company submissions (CSs) to NICE from AbbVie (adalimumab), BMS (abatacept), Pfizer (etanercept) and Roche (tocilizumab), and to identify the strengths and weaknesses of the respective submissions
-
to conduct an economic evaluation to establish the cost-effectiveness of abatacept, adalimumab, etanercept and tocilizumab for the treatment of JIA.
Patients with systemic onset JIA exhibiting typical systemic features, such as spiking fever and rash, are excluded from this MTA but if those features are no longer present (no active systemic symptoms during the previous 6 months) and the patients have gone on to have polyarticular-course JIA, they will be included. Similarly, patients with undifferentiated arthritis, ERA and PA that has a polyarticular course will also be included.
Chapter 3 Methods
Note
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
The a-priori methods for systematically reviewing the evidence of clinical effectiveness and cost-effectiveness are described in a research protocol published on the NICE website and registered with the PROSPERO international prospective register of systematic reviews database (registration number CRD42015016459). The protocol was sent to our expert advisory group (see Acknowledgements) for comment. Minor amendments were made as appropriate. None of the comments that were received identified specific problems with the methods of the review.
Identification of studies
Sensitive search strategies were developed and refined by an experienced information specialist. Separate searches were conducted to identify studies of clinical effectiveness, cost-effectiveness and HRQoL.
The following databases were searched for published studies and ongoing research from inception to May 2015: The Cochrane Library, including the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, Centre for Reviews and Dissemination (University of York) Database of Abstracts of Reviews of Effects, the NHS Economic Evaluation Database and the Health Technology Assessment (HTA) database; MEDLINE (Ovid); EMBASE (Ovid); MEDLINE In-Process & Other Non-Indexed Citations (Ovid); Web of Science with Conference Proceedings: Science Citation Index Expanded and Conference Proceedings Citation Index – Science (ISI Web of Knowledge); Biosis Previews (ISI Web of Knowledge); Zetoc (Mimas); National Institute for Health Research (NIHR)-Clinical Research Network Portfolio; Clinical Trials.gov; International Standard Randomised Clinical Trial Number; UK Clinical Trials Gateway; and World Health Organization International Clinical Trials Research Platform. In addition, PsycINFO (EBSCOhost) was searched for HRQoL studies. Searches were not limited to particular trial designs and, although searches were not restricted by language, only full texts of English-language articles were retrieved during the study selection process. Cost-effectiveness and HRQoL searches were conducted from database inception to May 2015. References were downloaded into Reference Manager (Professional Edition Version 12, Thomson Reuters, New York, NY, USA) and deduplicated where necessary.
Bibliographies of included articles and systematic reviews were also searched. The CSs to NICE were searched for any additional studies that met the inclusion criteria (see Chapter 4, Review of clinical effectiveness in company submissions to the National Institute for Health and Care Excellence and Chapter 5, Review of cost-effectiveness in company submissions to the National Institute for Health and Care Excellence). Members of our advisory group were asked to identify additional published and unpublished evidence. Further details, including search dates for each database and an example search strategy, can be found in Appendix 1.
Inclusion and exclusion criteria
The following inclusion/exclusion criteria were applied to the clinical effectiveness review:
-
Interventions: etanercept, abatacept (with or without methotrexate), adalimumab (with or without methotrexate) and tocilizumab (with or without methotrexate). Each drug was evaluated within its licensed indication. Studies of treatment without methotrexate were permitted if patients were intolerant to methotrexate or for patients for whom treatment with methotrexate is inappropriate.
-
Comparators: DMARDs (such as methotrexate, which is the most common conventional treatment in the UK) if DMARDs can be tolerated and best supportive care if DMARDs are not tolerated. Etanercept, abatacept, adalimumab and tocilizumab compared with each other.
-
Population: patients with JIA including:
-
polyarthritis (RF+ve, RF–ve and EO, both onset and course)
-
ERA
-
PA.
-
Studies of patients with systemic JIA were not included, as this was the subject of a separate NICE appraisal (NICE TA 238). 44
-
Outcomes: studies reporting one or more of the following outcomes were included:
-
disease activity
-
disease flares
-
physical function
-
joint damage
-
pain
-
corticosteroid reducing regimens
-
extra-articular manifestations (such as uveitis)
-
body weight and height
-
mortality
-
adverse effects of treatment
-
HRQoL.
-
-
Study design: randomised controlled trials (RCTs). Non-randomised studies could be considered where RCT data were not available. Any relevant systematic reviews identified in the systematic review of clinical effectiveness were used as a source of references. Studies published as abstracts or conference presentations were only included if published from 2012 onwards and sufficient details were presented (or available elsewhere, e.g. in a full paper reporting on the same RCT) to allow an appraisal of the methodology and the assessment of results to be undertaken.
The inclusion/exclusion criteria for the cost-effectiveness and HRQoL studies are presented in Chapter 5, Systematic review of cost-effectiveness evidence and Systematic review of health-related quality-of-life studies, respectively.
Data extraction strategy
Reference screening
All studies were selected for inclusion through a two-stage process. Titles and abstracts were screened independently by two reviewers for potential eligibility, using a standardised and piloted eligibility selection worksheet (see Appendix 2) containing the inclusion/exclusion criteria detailed above.
Full-paper screening
Full texts for potentially relevant studies were obtained and screened using a standardised and piloted eligibility section worksheet (see Appendix 3) by one reviewer and checked by a second reviewer, and a final decision regarding inclusion was agreed. At each stage, any disagreements were resolved by discussion or with the involvement of a third reviewer when necessary.
Critical appraisal strategy
Clinical effectiveness studies were appraised using the Cochrane Risk of Bias criteria (e.g. selection bias, detection bias, performance bias, attrition bias and selective reporting bias). 52 Aspects of study quality, including statistical procedures, outcome measurement and generalisability, were also assessed.
A critical appraisal of the included clinical effectiveness and cost-effectiveness studies (Chapter 5, Systematic review of cost-effectiveness evidence) was conducted by one reviewer and checked by a second reviewer. Any disagreements were resolved by consensus or in consultation with a third reviewer where necessary.
Method of data synthesis
Details of the trial outcomes in the clinical effectiveness review were synthesised through narrative review with tabulation of the results of included studies. Quantitative pooling of outcomes across clinical effectiveness studies in a meta-analysis was not possible as the identified evidence included only one trial per biologic DMARD, all of which used placebo as the comparator. It was not considered appropriate to meta-analyse the four biologic DMARDs together owing to clinical heterogeneity.
An adjusted indirect comparison of the four biologic DMARDs was performed using the method described by Bucher and colleagues. 53 An indirect comparison refers to the synthesis of data from trials in which the technologies of interest have not been compared in head-to-head trials, but have been compared indirectly using data from a network of trials that compare the technologies with other interventions. A distinction is often made between adjusted and naive (unadjusted) indirect comparisons. In the adjusted indirect comparison, the comparison of the interventions of interest is adjusted by preserving the strength of randomisation. Unadjusted indirect comparisons are considered to be observational evidence and are, therefore, not recommended. 54,55
Chapter 4 Clinical effectiveness
Results
Quantity and quality of available research
Titles and, where available, abstracts of a total of 2651 references identified by searches (after deduplication) were screened and full copies of 60 references were retrieved. Of these, 29 articles were excluded after inspection of the full text as shown in Figure 1 and these are listed in Appendix 4. The most common reason for exclusion of a reference was an irrelevant study design (e.g. systematic reviews, which were used as a source of references, commentaries). One full text56 was of unclear relevance to the review because the type of JIA was not stated and it was not clear whether or not participants met the licensed indication for etanercept therapy in respect of having an inadequate response or intolerance to methotrexate. One full paper and eight conference abstracts relating to four ongoing studies that seemed to be relevant were tagged for inclusion in Ongoing trials (note that a further three ongoing studies were identified from a separate search specifically undertaken for ongoing studies, which is not represented in Figure 1; therefore, a total of seven ongoing studies are summarised in Ongoing trials).
FIGURE 1.
Flow chart for the identification of studies.

Nine full texts and 12 conference abstracts described four RCTs (each of which was described by at least one full paper) that met the inclusion criteria of the review (see Figure 1). As the full texts provided the most complete data, these were the primary source of information for this review.
One of the RCTs evaluated abatacept57–60 [the AWAKEN (Abatacept Withdrawal study to Assess efficacy and safety in Key Endpoints) trial], one RCT, by Lovell and colleagues, evaluated adalimumab,61–64 one RCT evaluated etanercept (Lovell et al. 42,65–67) and one RCT evaluated tocilizumab68–76 (the CHERISH trial). For the sake of brevity, generally only the key reference for each RCT is cited in the main text of this report. All four RCTs used placebo as the comparator; however, with the exception of the etanercept trial, the majority of the patients in the trials received methotrexate in addition to the biologic DMARD or placebo. The key characteristics of the trials are presented in Table 9, with the primary and secondary outcomes measured in trials summarised in Table 10. All studies were multicentre RCTs, with the number of centres ranging from 9 in the etanercept study42 to 58 in the tocilizumab study. 68 Locations of the studies included the USA (all four studies), Canada (one study42), Europe (three studies,57,61,68 with only the tocilizumab study68 including UK centres), Latin America (two studies57,68), Australia (one study68) and the Russian Federation (one study68). In each study, participants were initially treated in an open-label phase with the biologic DMARD under investigation and had to achieve at least an ACR Pedi-30 response to the biologic DMARD to be eligible for entry to the randomised double-blind withdrawal phase, with the number of participants randomised ranging from 51 in the etanercept study to 166 in the tocilizumab study. As each study investigated a different biologic DMARD, study-specific details are provided below by study drug.
| Characteristic | Abatacept | Adalimumab | Etanercept | Tocilizumab |
|---|---|---|---|---|
| Study details | AWAKEN, Ruperto et al. (2008),57 Ruperto et al. (2010),58 Ruperto et al. (2010),59 Lovell et al. (2012)60 Multicentre withdrawal RCT at 45 centres in Europe (not UK), Latin America and the USA |
Lovell et al. (2008),61 Lovell et al. (2012),62 Ruperto et al. (2013),63 Ruperto et al. (2014)64 Multicentre withdrawal RCT at 31 centres in Europe (not UK) and the USA |
Lovell et al. (2000),42 Lovell et al. (2003),65 Lovell et al. (2006),66 Lovell et al. (2008)67 Multicentre withdrawal RCT at nine centres in Canada and the USA |
CHERISH, Brunner et al. (2015),68 Brunner et al. (2014),69 Baildam et al. (2014),70 Brunner et al. (2013),71 De Benedetti et al. (2013),72 Baildam et al. (2013),73 De Benedetti et al. (2013),74 Brunner et al. (2012),75 Bharucha et al. (2014)76 Multicentre withdrawal RCT at 58 centres in Australia, Europe (including the UK), Latin America, Russia and the USA |
| Study phasesa | 16-week open-label lead-in 24-week randomised double-blind withdrawal OLE |
16-week randomised open-label 32-week randomised double-blind withdrawal OLE |
12-week open-label 16-week randomised double-blind withdrawal OLE |
16-week open-label lead-in 24-week randomised double-blind withdrawal OLE |
| Interventionb | Abatacept: n = 60 Abatacept 10 mg/kg at about 28-day intervals for 24 weeks or until disease flare |
Adalimumab: n = 38 Adalimumab 24 mg/m2 of BSA (to maximum of 40 mg) EOW for 32 weeks |
Etanercept: n = 25 Etanercept 0.4 mg/kg twice weekly until disease flare or for 16 weeks |
Tocilizumab: n = 82 10 mg/kg < 30 kg body weight, n = 16 8 mg/kg < 30 kg body weight, n = 11 8 mg/kg ≥ 30 kg body weight, n = 55 |
| Comparatorb | Placebo: n = 62 | Placebo: n = 37 Placebo ≥ 10 mg/m2 BSA/week |
Placebo: n = 26 | Placebo: n = 84 10 mg/kg < 30 kg body weight, n = 15 8 mg/kg < 30 kg body weight, n = 13 8 mg/kg ≥ 30 kg body weight, n = 56 |
| Key inclusion criteria | Age 6–17 years Activec JIA (extended oligoarticular, polyarticular, RF+ve or RF–ve, systemic without systemic manifestations) Inadequate response or intolerance to ≥ 1 DMARD including biologic agents ACR Pedi-30 for entry to randomised double-blind phase |
Age 4–17 years Activec polyarticular-course JIA (any onset type) Inadequate response to NSAIDs ACR Pedi-30 at week 16 for entry to double-blind withdrawal phase |
Age 4–17 years Activec JIA Inadequate response to NSAIDs and methotrexate at doses of ≤ 10 mg/m2 BSA/week |
Age 2–17 years Activec polyarticular-course or extended oligoarticular JIA (RF+ve or RF–ve) for ≥ 6 months Inadequate responses to or intolerant of methotrexate. Either never treated with biologics or use discontinued for a specified minimum period |
| Parameter | Abatacept | Adalimumab | Etanercept | Tocilizumab |
|---|---|---|---|---|
| Primary outcome | Time to disease flare | Proportion of participants not receiving methotrexate with disease flares (weeks 16–48) | Number of patients with disease flare | Proportion of patients in whom a JIA flare occurred during part 2 (up to and including week 40) compared with week 16 |
| Secondary outcomes | Proportion of patients at end of 6-month double-blind phase who had disease flare | AEs | Not specifically stated (ACR core variables, mortality and AEs among others reported) | JIA–ACR Pedi-30, -50, -70, -90 responses (week 40) |
| Changes from baseline in ACR core variables | Change from baseline in ACR core response variables (week 40) | |||
| Pain | Clinically inactive disease (week 40) | |||
| Assessment of safety and tolerability | ||||
| HRQoL |
Abatacept
The abatacept RCT57 was funded by BMS and consisted of three phases: a 4-month open-label lead-in phase (days 1–113); a 6-month double-blind randomised withdrawal phase (days 114–283); and an open-label extension (OLE) phase [up to day 1681 (5.5 years) for efficacy and up to 7 years for safety]. Enrolled participants all received abatacept intravenously (10 mg/kg to a maximum of 1000 mg) and were permitted to continue to take stable methotrexate during the 4-month lead-in phase. Those achieving an ACR Pedi-30 response were then eligible to be randomised in a 1 : 1 ratio to continued abatacept (n = 60) or placebo (n = 62). In the 6-month randomised withdrawal phase, abatacept was given at randomisation and at about 28-day intervals (see Table 9).
Patients were eligible for the trial if they were aged 6–17 years and had EO, polyarticular (RF+ve or RF–ve) or systemic JIA without systemic manifestations.
Participants were required to have at least five active joints (defined as swelling or, in the absence of swelling, limited range of motion, accompanied by either pain or tenderness), active disease (defined as at least two active joints and two joints with a limited range of motion) and an inadequate response to, or intolerance of, at least one DMARD, which could include biologic agents (e.g. etanercept, infliximab and adalimumab). Exclusion criteria included active uveitis, any major concurrent medical conditions and pregnancy or lactation.
The primary outcome measure was time to disease flare during the double-blind period. Disease flare was defined in three ways depending on the measure used: worsening of ≥ 30% in at least three of the six ACR core-response variables for JIA, and at least 30% improvement in no more than one variable during the double-blind period; a worsening of ≥ 20 mm on the 100-mm VAS if a global assessment by either a physician or a parent was used; worsening in two or more joints if the number of active joints or joints with limited range of motion was used. Clinical assessments preceded drug administration at each visit. Secondary outcomes included the proportion of patients at the end of the 6-month double-blind phase who had disease flare, changes from baseline in each of the six ACR core variables, pain, assessment of safety and tolerability and HRQoL.
Adalimumab
The Lovell and colleagues RCT61 was funded by a research grant from Abbott Laboratories and consisted of three phases: a 16-week randomised open-label phase, a 32-week randomised double-blind withdrawal phase and an OLE phase. Enrolled participants all received adalimumab subcutaneously (24 mg/m2 of body surface area, to a maximum of 40 mg) every other week and methotrexate (at least 10 mg/m2 of body surface area per week) during the 4-month lead-in phase. Those achieving an ACR Pedi-30 response were then eligible to be randomised in a 1 : 1 ratio to continued adalimumab plus methotrexate (n = 38) or placebo plus methotrexate (n = 37) (see Table 9). The trial included two further study arms (adalimumab only and placebo only), but because the majority of participants in these arms had never received methotrexate, they did not meet the licensed indication and are not included in this report.
Patients were eligible for the trial if they were aged 4–17 years and had polyarticular-course JIA of any onset type. If systemic onset, then patients had to be free of any systemic JIA manifestations for at least 3 months prior to study qualification. 77 Participants were required to have active disease (defined as five or more swollen joints and three or more joints with a limited range of motion), to have had an inadequate response to non-steroidal anti-inflammatory drugs (NSAIDs), and to have neither previously been treated with methotrexate nor, if previously treated with methotrexate, have had adverse events (AEs) or an inadequate response. Exclusion criteria included clinically significant deviations in haematological, hepatic or renal indicators; ongoing infection or a recent major infection that had required hospitalisation or intravenous antibiotics; and recent receipt of live or attenuated vaccines. Patients who had previously been treated with other biologic agents at any time or who had received recent treatment with intravenous immune globulin, cytotoxic agents, investigational agents, DMARDs (other than methotrexate) or corticosteroids administered by intra-articular, intramuscular or intravenous routes were also excluded from participation.
The primary outcome for the study (percentage of participants not receiving methotrexate who had a disease flare during the double-blind period) related to the two study arms that, as noted above, do not meet the licensed indication and are therefore not included in this report. Disease flare was reported for the two study arms relevant to this assessment and it was defined in different ways depending on the measure used: worsening of ≥ 30% in at least three of the six core criteria for JIA and at least 30% improvement in no more than one of the criteria during the double-blind period; an increase of > 30% on the 0–100mm VAS if a global assessment was used; an increase in the number of active joints to at least two when the patient had none or only one if the number of active joints was used, with the same approach used for defining flare using joints with loss of motion. Outcomes were assessed every 12 weeks. The occurrence of AEs was a secondary outcome.
Etanercept
The Lovell and colleagues RCT42,65–67 was funded by the Immunex Corporation and consisted of three phases: an open-label lead-in phase of up to 3 months; a 4-month double-blind randomised withdrawal phase; and an OLE phase. All enrolled participants received etanercept subcutaneously (0.4 mg/kg twice weekly) during the 4-month lead-in phase. Those who improved and achieved an ACR Pedi-30 response were then eligible to be randomised to continue to receive etanercept (n = 25) or placebo (n = 26) during the withdrawal phase (see Table 9).
Patients were eligible for the trial if they were aged 4–17 years and had active polyarticular JIA despite treatment with NSAIDs and methotrexate doses of at least 10 mg/m2 of body surface area per week. Active disease was defined as at least five swollen joints and at least three joints with limited motion with pain, tenderness or both. Exclusion criteria included any major concurrent medical conditions and pregnancy or lactation.
The primary outcome measure was the number of patients with disease flare during the double-blind withdrawal period. Disease flare was defined depending on the measure used: worsening of ≥ 30% in at least three of the six ACR core-response variables for JIA, at least 30% improvement in no more than one variable and a minimum of two active joints; a change of at least two units on a scale from 0 to 10 if a global assessment was used. Clinical assessments during the withdrawal phase took place on day 1, day 15 and at the end of each month. Secondary outcomes were not specifically listed.
Tocilizumab
The tocilizumab RCT68 consisted of three phases: a 16-week open-label lead-in phase; a double-blind randomised withdrawal phase (weeks 16–40); and an OLE phase (64 weeks). Some funding for manuscript preparation was provided by F. Hoffmann-La Roche Ltd.
Enrolled participants were permitted to receive methotrexate and all received tocilizumab intravenously (three groups). Those with a body weight of < 30 kg were randomised to either 10 mg/kg or 8 mg/kg every 4 weeks. Those with a body weight of 30 kg or more received 8 mg/kg every 4 weeks during the 16-week lead-in phase. Those achieving an ACR Pedi-30 response were then eligible to be randomised in a 1 : 1 ratio to continue tocilizumab (n = 82) or placebo (n = 84), given every 4 weeks until week 40 unless they experienced disease flare (see Table 9).
Patients were eligible for the trial if they were aged 2–17 years and had polyarticular-course or EO JIA that was either RF+ve or RF–ve for 6 months or more. Systemic JIA or any other categories of JIA were excluded from the trial. 78 Participants were required to have at least five active joints with a limited range of motion in at least three active joints and have an inadequate response, or intolerance, to methotrexate. If participants were taking methotrexate (10–20 mg/m2) or low-dose oral glucocorticoids (≤ 0.2 mg/kg/day, daily maximum 10 mg), the dose had to have been stable for 8 weeks or more (for methotrexate) or 4 weeks or more (for oral glucocorticoids). Patients had to be treatment-naive for biologics or had to have discontinued use for a specified minimum period. No other exclusion criteria were specified.
The primary outcome measure was the proportion of participants with disease flare during the double-blind period (up to and including week 40 compared with week 16). Disease flare was defined as worsening of ≥ 30% in at least three of the six ACR core-response variables for JIA and at least 30% improvement in no more than one variable during the double-blind period. Outcomes were assessed every 4 weeks. Secondary outcomes included the ACR Pedi-30, -50, -70, -90 responses, the change from baseline in JIA core response variables and clinically inactive disease (PGA indicating no disease activity plus the absence of all the following: joints with active arthritis, uveitis and ESR of > 20 mm/hour).
Overview of the participants in the withdrawal phases of the included studies
For three of the four trials (abatacept,57 adalimumab61 and etanercept42) baseline characteristics are provided for the participants who had achieved an ACR Pedi-30 response and who were randomised to the double-blind withdrawal phase of each trial. The tocilizumab trial publication,68 however, presented participant baseline characteristics for participants as randomised to the initial open-label lead-in phase, where three groups of participants all received the study drug (if body weight was < 30 kg then participants were randomised to either 10 mg/kg or 8 mg/kg every 4 weeks; if body weight was ≥ 30 kg then participants received 8 mg/kg every 4 weeks). Selected baseline characteristics are presented in Table 11, with the full set of characteristics available in the data extraction forms (see Appendix 5). The mean age of trial participants reflected the differing entry criteria for the trials. Participants in the abatacept trial57 (participants aged 6–17 years were eligible) had the highest mean age (12–13 years), whereas those in the adalimumab61 and etanercept trials42 (eligible ages were 4–17 years) had a slightly lower mean age (approximately 9–12 years) which was similar to those enrolled in the open-label phase of the tocilizumab study68 (eligible ages 2–17 years; mean age approximately 11 years). The majority of participants in all four studies were female (ranging from 67% in the etanercept study42 to 80% in the adalimumab study61) and of white ethnicity (73% in the etanercept study42 to 96% in the adalimumab study61). The proportion of patients across the subtypes of JIA were reported for only two of the trials (abatacept57 and etanercept42). In these two trials polyarthritis was the predominant subtype. In the abatacept trial just under 20% of patients had systemic JIA (without systemic manifestations),57 whereas in the etanercept trial around one-third had systemic JIA (with apparent systemic manifestations: spiking fever and rheumatoid rash). 42 The proportion of participants who were RF+ve ranged from 22% in the adalimumab study61 to 29% in the tocilizumab study,68 and the duration of JIA from just under 4 years in the abatacept study57 to approximately 6 years in the etanercept study. 42
| Baseline characteristics | Abatacept57 | Adalimumab61 | Etanercept42 | Tocilizumab68 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Abatacept (n = 60) | Placebo (n = 62) | Adalimumab (n = 38) | Placebo (n = 37) | Etanercept (n = 25) | Placebo (n = 26) | TCZ 8 mg/kg < 30 kg (n = 34)a | TCZ 10 mg/kg < 30 kg (n = 35)a | TCZ 10 mg/kg ≥ 30 kg (n = 119)a | |
| Age (years), mean (SD) | 12.6 (3) | 12.0 (3) | 11.7 (3.3) | 10.8 (3.4) | 8.9 | 12.2 | 7.6 (2.71) | 6.9 (3.02) | 13.1 (2.78) |
| Sex female, n (%) | 43 (72) | 45 (73) | 30 (79) | 30 (81) | 19 (76) | 15 (58) | 24 (71) | 30 (86) | 90 (76) |
| Ethnicity, n (%) | |||||||||
| White | 46 (77) | 49 (79) | 36 (95) | 36 (97) | 14 (56) | 23 (88) | NR | ||
| Black | 5 (8) | 4 (7) | 0 | 0 | 3 (12) | 1 (4) | |||
| Hispanic | NR | NR | NR | NR | 6 (24) | 2 (8) | |||
| Other | 9 (15) | 9 (15) | 2 (5) | 1 (3) | 2 (8) | 0 | |||
| Type of JIA, n (%) | |||||||||
| Pauciarticularb | 2 (8) | 1 (4) | Eligible patients had RF+ve or RF–ve polyarticular-course JIA or EO JIA but no further detail is provided | ||||||
| Persistent oligoarthritis | 0 | 2 (3) | Described as ‘polyarticular course’ but with no further detail (this older nomenclature could have included patients who would now be defined as having ERA or PA) | ||||||
| EO | 9 (15) | 7 (11) | |||||||
| Polyarthritis (RF+ve) | 14 (23) | 12 (19) | 14 (56) | 17 (65) | |||||
| Polyarthritis (RF–ve) | 26 (43) | 28 (45) | |||||||
| Systemic | 11 (18)c | 12 (19)c | 9 (36) | 8 (31) | |||||
| RF+ve, n (%) | 19 (32) | 12 (19) | 10/37 (27)d | 6/36 (17)d | 4 (16) | 8 (31) | 2 (6) | 4 (11) | 48 (40) |
| Duration of JIA (years), mean (SD) | 3.8 (3.7) | 3.9 (3.5) | 4.3 (4.1) | 4.0 (3.5) | 5.3 | 6.4 | 3.5 (2.57) | 3.4 (2.39) | 4.7 (4.16) |
The treatment groups in the abatacept study57 appear similar on most variables, although the placebo group had a smaller proportion of RF+ve patients than the abatacept group (19% vs. 32%). The adalimumab study report61 indicated that there were no significant differences in baseline characteristics between the placebo and adalimumab groups. Groups were described as well balanced in the etanercept study42 with the exceptions of age group (4–8 years: 52% etanercept vs. 19% placebo; p < 0.02), ethnicity (white ethnicity: 56% etanercept vs. 88% placebo; p < 0.02) and corticosteroid use (corticosteroid use at wash out: etanercept 24% vs. 50% placebo; p = 0.05). The tocilizumab study did not report baseline characteristics for those participants who entered the double-blind wash-out phase of this study.
Assessment of the risk of bias of included studies
The Cochrane risk of bias criteria52 focus on various aspects of study design, conduct and reporting which may help to gauge the internal validity (whether or not the study answered the research question in a manner that was free from bias) of the individual studies. The risk of bias in the included trials is summarised in Table 12 and further details are presented in the data extraction tables (see Appendix 5).
| Criteria | Abatacept | Adalimumab61 | Etanercept42 | Tocilizumab68 |
|---|---|---|---|---|
| Selection bias | ||||
| Random sequence generation | Yes | Unclear | Unclear | Unclear |
| Allocation concealment | Yes | Unclear | Unclear | Unclear |
| Performance bias | ||||
| Blinding of participants and personnel | Yes | Yes | Yes | Yes |
| Detection bias | ||||
| Blinding of outcome assessment | Yes | Yes | Yes | Yes |
| Attrition bias | ||||
| Incomplete outcome data addressed | ||||
| Non-HRQoL outcomes | Yes | Yes | Yes | Yes |
| HRQoL outcome | No | N/A | N/A | N/A |
| Reporting bias | ||||
| Selective reporting | Yes | Yes | Yes | Yes |
| Other bias | ||||
| Other sources | Unclear | Unclear | Unclear | Unclear |
Only the abatacept trial57 reported sufficient details of the methods for generating the random sequence (computer generated) and allocation concealment (interactive voice-randomisation system) to establish that there was a low risk of selection bias in this trial. In the three other trials (adalimumab,61 etanercept42 and tocilizumab68) the risk of selection bias associated with randomisation and allocation were unclear because either no details were reported or there was insufficient information to make a judgement. The randomised withdrawal phases of all four trials were described as double-blind with three of the trials providing some information to support this statement (e.g. placebo identical in appearance,57 indication of who was unaware of treatment assignment42,61). The risk of performance bias and detection bias was judged to be low for all four trials. Attrition bias (systematic differences in withdrawals between trial arms) was judged to be low for all outcomes in three trials42,61,68 (either because attrition was similar between groups or because incomplete data were addressed). In the abatacept trial,57 however, a larger proportion of patients dropped out of the placebo group in the double-blind phase (placebo 50%, abatacept 18%), with the main reason being a lack of efficacy.
Although this was addressed for some outcomes (e.g. analysis of ACR variables), it was not addressed for HRQoL, where the analysis was based on available data at each time point, hence the risk of attrition bias is high for this outcome. Selective reporting bias was judged to be low for all the trials, as all outcomes were reported on. The only other uncertainty surrounding study biases was the risk of bias attributable to intercentre variability, which was not discussed in the adalimumab,61 etanercept42 and tocilizumab68 trials. In contrast, the abatacept study57 reported that training was in place for joint assessors from each centre who had specific and standardised joint assessment training.
Assessment of clinical effectiveness: biologic disease-modifying antirheumatic drugs versus placebo (with methotrexate where permitted)
Disease flare
The primary outcome for all four trials was disease flare, although there were some differences in the ways in which this outcome was reported. Data on disease flare from the trials contribute to the economic model in this assessment report (see Chapter 5, Data sources). The definitions for disease flare were broadly consistent between the studies (a worsening of at least 30% in three or more of the six core criteria for JIA and an improvement of ≥ 30% in no more than one of the criteria), with some studies also including flare definitions based on global assessments and the number of active joints. In all four studies, there were statistically significantly fewer arthritis flares in patients being treated with biologic DMARDs than in those patients receiving placebo, and in the three studies that reported time to disease flare this was statistically significantly longer in patients being treated with biologic DMARDs than in those receiving placebo (Table 13).
| Study (length: open-label, RCTa), outcome | Intervention | Comparator | Statistical significance |
|---|---|---|---|
| Abatacept57 (4-month open-label, 6-month RCT) | ABA (n = 60) | PBO (n = 62) | p-value |
| Time to flare (months), median | Not reached | 6 | 0.0002 |
| Disease flares, n (%) | 12 (20) | 33 (53) | 0.0003 |
| Disease flares, hazard ratio | 0.31 (95% CI 0.16 to 0.59) | NR | |
| Adalimumab61 (4-month open-label, 8-month RCT) | ADA (n = 38) | PBO (n = 37) | p-value |
| Disease flares, n/N (%) | 14/38 (37) | 24/37 (65) | 0.02 |
| Time to onset of disease flare (weeks) | > 32 | ≈ 20 | 0.03 |
| Etanercept42 (3-month open-label, 8-month RCT) | ETA (n = 25) | PBO (n = 26) | p-value |
| Disease flare, n (%) | 7 (28) | 21 (81) | 0.0031b |
| Corticosteroid use at baselinec | 0.05 | ||
| Yes | 3/6 (50) | 12/13 (92) | |
| No | 4/19 (21) | 9/13 (69) | |
| Time to flare (days), median | > 116 | 28 | < 0.001 |
| Tocilizumab68 (4-month open-label, 6-month RCTs) | TCZ (n = 82) | PBO (n = 81)d | Differencee TCZ vs. PBO (95% CI); p-value |
| Proportion with JIA flare, n (%) | 21 (25.6) | 39 (48.1) | –0.21 (–0.35 to –0.08); 0.0024 |
In the abatacept study,57 by the end of the RCT period, disease flare had occurred in 20% of patients receiving the study drug compared with 53% of patients receiving placebo (p = 0.0003). Median time to disease flare was 6 months for the placebo group and this was statistically significantly greater than in the abatacept group (p = 0.0002), but the authors state that insufficient events occurred in the abatacept group for this to be assessed. The risk of disease flare in patients randomised to continued abatacept during the RCT phase was just under one-third of that for those receiving placebo [hazard ratio 0.31, 95% confidence interval (CI) 0.16 to 0.95; no p-value reported].
Disease flare occurred in 37% of patients receiving adalimumab61 compared with 65% of those receiving placebo (p = 0.02) and the time to onset of disease flare was longer in the adalimumab group.
In the etanercept study, disease flare occurred in 28% of patients receiving etanercept42 compared with 81% receiving placebo (p = 0.003). The authors of the etanercept study state that after adjustment for the effects of baseline characteristics, the rates of flare remained significantly lower in the etanercept group (p < 0.001), with only corticosteroid use at baseline being a significant predictor of flare rates (p = 0.05). The median time to disease flare with etanercept was > 116 days compared with 28 days for the placebo group, with 13/25 patients still receiving etanercept at the end of the study (day 116) (p < 0.001).
In those participants receiving tocilizumab, disease flare occurred in 26% of patients compared with 48% receiving placebo (adjusted difference in flare rate −0.21, 95% CI −0.35 to −0.08; p = 0.0024), with authors stating that flares in the placebo group were evident as early as 28 days after randomisation.
American College of Rheumatology Pediatric responses
ACR Pedi-30, -50 and -70 responses were reported by all four studies, with all but the etanercept study42 also reporting ACR Pedi-90 responses. The abatacept and tocilizumab studies57,68 additionally report inactive disease, which was defined similarly in the two studies (no joints with active arthritis, normal ESR of ≤ 20 mm per hour, PGA < 10 on a 100-mm VAS,57 or PGA also < 10 on a 100-mm VAS), indicating no disease activity with the tocilizumab study,68 also including an absence of uveitis (patients with uveitis were excluded from the abatacept study). In all groups that continued to receive biologic DMARDs during the randomised withdrawal phase of the study, the proportion of participants with ACR Pedi responses of 30 or more were greater than in the placebo group and when a p-value was reported the differences were statistically significant in all but two instances (Table 14).
| Study (length: OL, RCT), outcome | Intervention | Comparator | Statistical significance |
|---|---|---|---|
| Abatacept57 (4-month OL, 6-month RCT) | |||
| ACR Pedi, n (%)a | ABA (n = 60) | PBO (n = 62) | p-value |
| 30 | 49 (82) | 43 (69) | 0.1712 |
| 50 | 46 (77) | 32 (52) | 0.0071 |
| 70 | 32 (53) | 19 (31) | 0.0185 |
| 90 | 24 (40) | 10 (16) | 0.0062 |
| Inactive diseaseb | 18 (30) | 7 (11) | 0.0195 |
| Adalimumab61 (4-month OL, 8-month RCT) | |||
| ACR Pedi, % | ADA (n = 38) | PBO (n = 37) | p-value |
| 30 | 63 | 38 | 0.03 |
| 50 | 63 | 38 | 0.03 |
| 70 | 63 | 27 | 0.002 |
| 90 | 42 | 27 | 0.17 |
| Etanercept42 (3-month OL, 4-month RCT) | |||
| ACR Pedi, n (%)c | ETA (n = 25) | PBO (n = 26) | p-value |
| 30 | 20 (80) | 9 (35) | p < 0.01 |
| 50 | 18 (72) | 6 (23) | NR |
| 70 | 11 (44) | 5 (19) | NR |
| Tocilizumab68 (4-month OL, 6-month RCT) | |||
| ACR Pedi, n (%) | TCZ (n = 82) | PBO (n = 81) | Differenced TCZ vs. PBO (95% CI); p-value |
| 30 | 61 (74.4) | 44 (54.3) | 0.09 (0.05 to 0.33); 0.0084 |
| 50 | 60 (73.2) | 42 (51.9) | 0.20 (0.06 to 0.34); 0.0050 |
| 70 | 53 (64.6) | 34 (42.0) | 0.22 (0.07 to 0.37); 0.0032 |
| 90 | 37 (45.1) | 19 (23.5) | 0.21 (0.07 to 0.35); NR |
| Inactive disease | 30 (36.6) | 14 (17.3) | 0.18 (0.05 to 0.32); NR |
Although more patients receiving abatacept (82%) achieved an ACR Pedi-30 response than those patients receiving placebo (69%), the difference was not statistically significant (p = 0.1712). 57 However, a statistically significantly greater proportion of patients in the abatacept treatment group achieved an ACR Pedi-50, -70 or -90 response than those receiving placebo (p = 0.0071, p = 0.0185 and p = 0.0062, respectively). In addition, statistically significantly more patients treated with abatacept (30%) than those receiving placebo (11%; p = 0.0195) were classified as having inactive disease.
A statistically significantly higher percentage of patients being treated with adalimumab achieved ACR Pedi-30, -50 and -70 responses compared with those receiving placebo (p = 0.03, p = 0.03 and p = 0.002, respectively). 61 The percentage of patients with ACR Pedi-90 response rates was also greater for adalimumab-treated patients than for patients receiving placebo (42 vs. 27 placebo); however, this difference was not statistically significant (p = 0.17).
In the etanercept study, ACR Pedi-30, -50 and -70 responses were achieved by a greater proportion of patients being treated with etanercept during the randomised withdrawal phase than those receiving placebo. However, a statistical comparison showing that this difference was statistically significant was reported only for ACR Pedi-30 (p < 0.01).
A statistically significantly higher proportion of tocilizumab-treated patients achieved ACR Pedi-30, -50 and -70 responses than those receiving placebo during the randomised withdrawal phase of the study (p = 0.0084, p = 0.0050 and p = 0.0032, respectively). Although the ACR Pedi-90 response was also higher in the tocilizumab group, no p-value was provided. The proportion of patients with inactive disease was 36.6% for the tocilizumab group compared with 17.3% for the placebo group (no p-value provided).
American College of Rheumatology Pediatric core variables
The adalimumab study61 did not report outcomes for the ACR Pedi core variables. The three remaining studies, however, reported data as mean (abatacept57), median (etanercept42) or an adjusted mean for change from baseline (tocilizumab68). In addition, the etanercept study reports additional joint and pain outcomes, whereas the abatacept and tocilizumab studies also report additional pain outcomes (see Joint-related outcomes and Pain).
Generally, the core-response variable outcomes were in favour of treatment with the biologic DMARDs compared with placebo. However, as can be seen in Table 15, there were some exceptions. For abatacept,57 differences in the adjusted mean percentage change (adjustment based on an analysis of covariance model with treatment as factor and baseline value as covariate) over the double-blind period (from day 113 to day 282) for the parent’s global assessment and ESR rates between the treatment groups were not significantly different (p = 0.6992 and p = 0.9562, respectively). The mean scores for the CHAQ disability index at the end of the double-blind withdrawal trial period (day 282) were the same for both groups (0.8). However, when the difference in the adjusted mean percentage change values for the CHAQ disability index from the start to the end of the double-blind period (day 113 to day 282) are compared, a statistically significant p-value is reported in favour of the abatacept group (p = 0.0388).
| Study (length: OL, RCT), outcome | Intervention | Comparator | Statistical significance |
|---|---|---|---|
| Abatacept57 (4-month OL, 6-month RCT) | ABA (n = 60) | PBO (n = 62) | p-valuea |
| Core-response variables, mean (SD)b | |||
| PGA (VAS: 100 mm) | 14.7 (18.9) | 23.2 (21.8) | 0.0004 |
| Parent’s global assessment (VAS: 100 mm) | 17.9 (22.2) | 23.9 (21.6) | 0.6992 |
| Physical function (CHAQ disability index: 0–3, best–worst) | 0.8 (0.9) | 0.8 (0.7) | 0.0388 |
| Number of active joints (number assessed not stated) | 4.4 (7.0) | 6.0 (5.8) | 0.0245 |
| Number of joints with LOM (number assessed not stated) | 8.8 (12.8) | 8.6 (12.0) | 0.0128 |
| ESR (mm/hour)c | 25.1 (26.4) | 30.7 (30.1) | 0.9562 |
| Etanercept42 (3-month OL, 4-month RCT) | ETA (n = 25) | PBO (n = 26) | p-value |
| JIA core set criteria, medianb | |||
| PGA of disease severity (0–10, best–worst) | 2 | 5 | NR |
| Patient/parent global assessment of overall well-being (0–10, best–worst) | 3 | 5 | NR |
| CHAQ scores (0–3, best–worst) | 0.8 | 1.2 | NR |
| Total number of active joints (out of 73 joints) | 7.0 | 13.0 | NR |
| Number of joints with LOM and with pain, tenderness, or both (out of 71 joints; 0–10, best–worst) | 1.0 | 4.5 | NR |
| ESR (normal ranges 1–30 mm/hour for females, 1–13 mm/hour for males)c | 18 | 30 | NR |
| Tocilizumab68 (4-month OL, 6-month RCT) | TCZ (n = 82) | PBO (n = 81) | Differenced TCZ vs. PBO (95% CI); p-value |
| JIA: core response variables, change from baseline – adjusted meanb | |||
| PGA of disease severity (0–100, 0 = inactive disease) | −45.2 | −35.2 | –9.9 (–16.5 to –3.4); 0.0031 |
| Patient global assessment of well-being (0–100, 0 = very poor) | −32.1 | −24.7 | –7.4 (–14.8 to 0.0); NR |
| CHAQ: disability index score (0–3, 0 = no disability) | −0.8 | −0.6 | –0.2 (–0.4 to 0.0); NR |
| Number of active joints (range 0–71) | −14.3 | −11.4 | –2.9 (–5.7 to –0.1); 0.0435 |
| Number of joints with LOM (range 0–67) | −9.5 | −7.7 | –1.8 (–4.1 to 0.5); 0.1229 |
| ESR (mm/hour) | −26.3 | −12.0 | –14.3 (–19.6 to –9.0); NR |
For etanercept,42 all core variable outcomes appear to be in favour of the etanercept group compared with placebo; however, no statistical comparisons between treatment groups were reported. For the tocilizumab study,68 differences in adjusted mean changes from baseline between treatment groups for PGA of disease and number of active joints are reported to be statistically significant in favour of tocilizumab (p-values of 0.0031 and 0.0435, respectively); no p-values for the remaining outcomes were reported.
Joint-related outcomes
None of the trials reported any radiographic outcomes. However, in addition to the ACR Pedi core variable outcomes that capture the numbers of active joints and joints with limited range of motion, the etanercept study42 presented data for some additional joint-related outcomes (see Table 16).
| Study (length: OL, RCT), outcome | Intervention | Comparator | Statistical significance |
|---|---|---|---|
| Etanercept42 (3-month OL, 4-month RCT) | ETA (n = 25) | PBO (n = 26) | p-value |
| Number of swollen joints (out of 66), median | 4.0 | 11.0 | NR |
| Number of joints with LOM (out of 71), mediana | 9 | 22 | NR |
| Articular severity score (0–962, best–worst), median | 38 | 66 | NR |
| Duration of morning stiffness (minutes), median | 5 | 38 | NR |
No statistical comparisons for these outcomes were reported between the etanercept and the placebo group; however, the median number of swollen joints (4.0 vs. 11.0 placebo), number of joints with limitation of motion (LOM) (9 vs. 22 placebo), articular severity score (38 vs. 66 placebo) and duration of stiffness (5 vs. 38 placebo) all favoured the etanercept treatment group (Table 16).
Pain
The adalimumab study61 did not report a pain outcome. All the other studies (abatacept,57 etanercept42 and tocilizumab68) report pain assessed on a VAS, and the data are reported differently (mean, median and mean change from baseline, respectively) (Table 17). The difference between the abatacept57 (mean pain 15 mm) and the placebo (mean pain 21 mm) treatment groups was not statistically significant (p = 0.105) and reported mean pain scores were lower for patients being treated with abatacept. The etanercept study42 did not report a statistical comparison between treatment groups; however, median pain scores for patients being treated with etanercept (VAS 1.5 cm) were less than half of those for patients receiving placebo (VAS 3.5 cm). The tocilizumab study68 reported the adjusted mean change from baseline, which, compared with the placebo group, was statistically in favour of the tocilizumab treatment group (p = 0.0076).
| Study (length: OL, RCT), outcome | Intervention | Comparator | Statistical significance |
|---|---|---|---|
| Abatacept57 (4-month OL, 6-month RCT) | ABA (n = 60) | PBO (n = 62) | p-value |
| Pain (parent global assessment of pain, CHAQ VAS: 100 mm), mean | 15a | 21a | 0.105 |
| Etanercept42 (3-month OL, 4-month RCT) | ETA (n = 25) | PBO (n = 26) | p-value |
| Pain (VAS: 0–10 cm, best–worst), median | 1.5 | 3.5 | NR |
| Tocilizumab68 (24 weeks) | TCZ (n = 82) | PBO (n = 81) | Differenceb TCZ vs. PBO (95% CI); p-value |
| Pain (VAS: no details reported), adjusted mean change from baseline | −32.4 | −22.3 | –10.2 (–17.6 to –2.7); 0.0076 |
Corticosteroid-reducing regimens
None of the included RCTs reported the effectiveness of biologic DMARDs on reducing the need for corticosteroids.
Extra-articular manifestations (such as uveitis)
None of the included RCTs reported outcomes for extra-articular manifestations. Of note, one of the trials (abatacept) excluded patients with active uveitis. 57
Height and body weight
None of the studies reported differences in height or body weight between the treatment groups for the double-blind, randomised controlled withdrawal phase of the trial.
Mortality
No deaths occurred in the adalimumab,61 etanercept42 and tocilizumab68 studies; this outcome was not reported in the abatacept study. 57
Quality of life: Child Health Questionnaire
The outcome measures for QoL in the abatacept study were summary physical scores, summary psychosocial scores (both measured on a 100-mm VAS) and 15 Child Health Questionnaire (CHQ) health concepts. 58 Differences between the abatacept and placebo treatment groups were not statistically significant for either the reported summary scores (p = 0.666 and p = 0.056, respectively), although there appears to be a positive trend for the latter (Table 18). Abatacept-treated patients (n = 52) had improved scores for 14 of the 15 subscales, and placebo-treated patients (n = 34) for 6 of the 15 CHQ subscales (p > 0.05 for abatacept versus placebo for all subscales; details not data extracted).
| Study (length: OL, RCT), outcome | Intervention | Comparator | Statistical significance |
|---|---|---|---|
| Abatacept57 (4-month OL, 6-month RCT) | ABA (n = 52)a | PBO (n = 34)a | p-value |
| CHQ: physical summary score | 43.6 | 41b | 0.666 |
| CHQ: psychosocial summary score | 51.7 | 47b | 0.056 |
Adverse events
A summary of AEs reported during the double-blind withdrawal trial phases is provided here with complete details for the AEs reported by each of the studies available in the data extraction forms (see Appendix 5). AEs reported during trial OLEs are presented below (see Adverse events open-label extension).
Abatacept57
During the 6-month double-blind withdrawal period, there were no statistically significant differences in AEs between the abatacept and placebo treatment groups. The total number of AEs (occurring in ≥ 5% of patients in the open-label and double-blind phase) was 62% for the abatacept and 55% for the placebo group, with two serious adverse events (SAEs) occurring in the placebo group but none in the abatacept group (Table 19). The most common class of AEs in both treatment groups were infections and infestations (44–45%). AEs were also reported under the headings of gastrointestinal disorders, general disorders and administration site conditions, nervous system disorders and respiratory, thoracic and mediastinal disorders (see Appendix 5).
| Study, outcome | Intervention | Comparator | Statistical significance |
|---|---|---|---|
| Abatacept57 (during 6-month double-blind period) | ABA (n = 60) | PBO (n = 62) | p-value |
| Total SAEs, n (%) | 0 | 2 (3) | 0.50 |
| Total AEs, n (%)a | 37 (62) | 34 (55) | 0.47 |
| Adalimumab61 (during 8-month double-blind period) | ADA (n = 38; 18.3 patient-years) | PBO (n = 37; 15 patient-years) | |
| Any AE, number of events (number of events per patient-year) | 234 (12.8) | 155 (10.3) | |
| SAEs, possibly related to study drug, n of events (n of events per patient-year)b | 0 | 1 (0.1) | |
| AEs leading to the discontinuation of the drug, n | 0 | 0 | |
| Etanercept42 (time period unclear unless stated below) | ETA (n = 25) | PBO (n = 26) | |
| Hospitalisation for SAEs, n | 2 | 0 | |
| Injection-site reactions during the 4-month double-blind period, n | 1 | 1 | |
| Most common AEs: injection-site reaction, number of events (number of events per patient-year) | 57 (3.8) | 73 (4.0) | |
| Tocilizumab68 (during 6-month double-blind period) | TCZ (n = 82)c | PBO (n = 81)c | |
| SAEs and AEs occurring in ≥ 5% of patients, n (%) | |||
| Duration in study (years) | 32.33 | 27.41 | |
| Patients with ≥ 1 AE | 58 (70.7) | 60 (74.1) | |
| Total number of AEsd | 147 | 141 | |
| Rate of AEs per 100 patient-years | 454.7 | 514.4 | |
| SAEs | |||
| Patients with ≥ 1 SAE | 3 (3.7) | 3 (3.7) | |
| Rate of SAEs per 100 patient-years | 9.3 | 10.9 | |
| Patients with ≥ 1 infectious SAE | 1 (1.2) | 0 | |
| Rates of infectious SAEs per 100 patient-years | 3.1 | 0 | |
| AEs leading to study drug discontinuation | 1 (1.2)e | 1 (1.2)f | |
Adalimumab61
There were 234 AEs in the adalimumab group (12.8 per patient-year) and 155 AEs in the placebo group (10.3 per patient-year) during the 8-month double-blind period. No statistical comparisons in AEs between treatment groups were reported. Only one SAE possibly related to the study drug was reported and this was gastroduodenitis, which occurred in one patient in the placebo group. The most common AEs were related to injection-site reactions [adalimumab: 73 events (4.0 events per patient-year); placebo: 57 events (3.8 events per patient-year)]. Other reported AEs were contusion, nasopharyngitis, upper respiratory tract infection, viral infection, vomiting and excoriation (see Appendix 5). No AEs led to the discontinuation of the treatment drug (see Table 19). Sixteen per cent of patients (27/171) had at least one positive test for antiadalimumab antibody during the open-label and double-blind phases [methotrexate: 5/85 (6%); no methotrexate: 22/86 (26%)], but this did not lead to a greater rate of discontinuation of the study drug, nor did it increase the incidence of SAEs. The study authors state that there was no occurrence of opportunistic infections, malignant conditions, demyelinating diseases or lupus-like reactions.
Etanercept42
Two patients who received etanercept needed hospitalisation for SAEs (one for depression and a personality disorder and the other for gastroenteritis-flu syndrome). It is not clear at what point in the trial these events occurred. One patient withdrew after the first dose of etanercept (presumably at the start of the open-label period) because of urticaria. There were only two reported injection-site reactions during the double-blind phase of the trial, one in each treatment group (see Table 19). All other AEs were reported to be of mild-to-moderate intensity, with no significant difference in the frequency of AEs between the treatment groups during the double-blind phase. There were no laboratory abnormalities requiring urgent treatment in the etanercept group. No patient had persistent elevations in autoantibodies or had signs or symptoms of another autoimmune disease. Two patients tested positive for non-neutralising antibody to etanercept.
Tocilizumab68
The safety population consisted of all patients who received more than one dose of study medication. During the double-blind period, the total number of patients with at least one AE was 58 in the tocilizumab group (454.7 AEs per 100 patient-years) and 60 in the placebo group (514.4 AEs per 100 patient-years). The most frequently reported AE in both treatment groups was nasopharyngitis (tocilizumab: 17%, placebo 11%). Other reported AEs were headache, upper respiratory infection, cough, pharyngitis, nausea, diarrhoea, rhinitis, vomiting, abdominal pain, oropharyngeal pain and rash (see Appendix 5). Two AEs led to drug discontinuation, one in each treatment group (tocilizumab: increased blood bilirubin level; placebo: gastroenteritis) and 3.7% of patients in each treatment group had more than one SAE. One patient in the tocilizumab group suffered with more than one infectious SAE. Rates of SAEs per 100 patient-years were similar between groups (tocilizumab: 9.3; placebo: 10.9), whereas the rate of infectious SAEs per 100 patient-years was 3.1 for the tocilizumab group. Other reported SAEs included pneumonia, upper limb fracture, uveitis, psychosomatic disease, enterocolitis and complicated migraine, with one case in each category and varying by treatment group (see Appendix 5).
Subgroup analyses
Only the tocilizumab study68 reported subgroup analyses, which reported on ACR Pedi-70 and ACR Pedi-90 responses at week 40 in three subgroups: patients with background treatment of methotrexate; background treatment of glucocorticoid; and previous biologic agent use at baseline. It is unclear if these analyses were pre-planned or post hoc. The trial authors also stated that no differences were observed in response to tocilizumab between patients who were RF+ve and those who were not, but no data in support of this statement were presented. No statistical comparisons between treatment groups were reported and it is therefore unclear if differences between the subgroups were statistically significant.
Background methotrexate
Patients receiving background methotrexate in both the tocilizumab and the placebo groups had higher ACR Pedi-70 and -90 response rates at the end of the double-blind RCT withdrawal phase than those who were not in receipt of background methotrexate (Table 20). However, patients receiving tocilizumab with or without background methotrexate had better response rates than patients in the corresponding placebo groups.
| Concomitant therapies and previous exposure to biologic agent | Response level | Tocilizumab68 (4-month OL, 6-month RCT)a | |||
|---|---|---|---|---|---|
| TCZ (n = 82), n/N (%) | PBO (n = 81), n/N (%) | ||||
| Yes | No | Yes | No | ||
| Background methotrexate | ACR Pedi-70 | 45/67 (67.2) | 8/15 (53.3) | 30/64 (46.9) | 4/17 (23.5) |
| ACR Pedi-90 | 32/67 (47.8) | 5/15 (33.3) | 18/64 (28.1) | 1/17 (5.9) | |
| Background glucocorticoid | ACR Pedi-70 | 23/33 (69.7) | 30/49 (61.2) | 4/38 (36.8) | 20/43 (46.5) |
| ACR Pedi-90 | 16/33 (48.5) | 21/49 (42.9) | 5/38 (13.2) | 14/43 (32.6) | |
| Previous biologic agent | ACR Pedi-70 | 13/27 (48.1) | 40/55 (72.7) | 2/23 (8.7) | 32/58 (55.2) |
| ACR Pedi-90 | 5/27 (18.5) | 32/55 (58.2) | 2/23 (8.7) | 17/58 (29.3) | |
Background glucocorticoid
At the end of the double-blind RCT phase (week 40) in the tocilizumab group, a slightly higher proportion of participants receiving background glucocorticoid achieved an ACR Pedi-70 and -90 response than those who were not in receipt of background glucocorticoid (see Table 20). However, among participants in the placebo group the opposite pattern was observed, with a lower proportion of those who were in receipt of background glucocorticoid achieving ACR Pedi-70 and -90 responses (see Table 20). Response rates for both ACR Pedi-70 and -90 were higher in subgroups of patients receiving tocilizumab than in subgroups of patients receiving placebo, regardless of whether or not patients received background glucocorticoid.
Previous biologic agent
Patients in either the tocilizumab or placebo groups who had received previous treatment with a biologic agent (primarily comprising antiTNF agents) had lower ACR Pedi-70 responses at the end of the double-blind RCT phase (week 40) than patients who had not previously been treated with a biologic agent (see Table 20). Patients receiving placebo who had not received previous treatment with a biologic agent had better ACR Pedi-70 and -90 response rates than patients on tocilizumab who had previous biologic agent experience (see Table 20).
Results: open-label extensions
All four studies included OLEs with some differences in which participants were eligible to enter and how data were presented. ACR Pedi results are presented in Table 21 and with additional outcomes presented either in the study data extraction forms (see Appendix 5) or published papers [adalimumab: minimal disease activity; abatacept: ACR Pedi component items, analysis according to prior exposure to biologic agents, ACR Pedi data for those in the OLE who had not taken part in the double-blind phase and information on antiabatacept and anticytotoxic T-lymphocyte-associated antibody production; etanercept: ACR Pedi component items, minimal disease activity; tocilizumab: ACR Pedi component items, minimal disease activity].
| Study (follow-up), outcome | Intervention | Comparator (during RCT phase) |
|---|---|---|
| Abatacept59 (OLE day 589) | ||
| ACR Pedi, n/N (%) | ABA (n = 51) | PBO (n = 47) |
| 30 | 46/51 (90) | 41/47 (87) |
| 50 | 45/51 (88) | 39/47 (83) |
| 70 | 38/51 (75) | 35/47 (75) |
| 90 | 29/51 (57) | 19/47 (40) |
| 100 | 20/51 (39) | 9/47 (19) |
| Inactive disease | 22/51 (43) | 11/47 (23) |
| Adalimumab61 (OLE week 104) | ||
| ACR Pedi, % | ADA (n = 128a) | PBO group from RCT phase not separately reported |
| 30 | 89 | |
| 50 | 86 | |
| 70 | 77 | |
| 90 | 59 | |
| 100 | 40 | |
| Etanercept67 (OLE up to 8 years) | ||
| ACR Pedi response, 8 years (LOCFb), n/N (%) | ETA (n = 58c) | PBO group from RCT phase not separately reported |
| 30 | 40/48 (83) | |
| 50 | 36/47 (77) | |
| 70 | 28/46 (61) | |
| 90 | 19/46 (41) | |
| 100 | 8/45 (18) | |
| Tocilizumab69,71,74 (104 weeks) | ||
| ACR Pedi, proportion of patients with improvement relative to baseline, n (%)69 | TCZ (n = 82) | PBO (n = 73d) |
| 70e | 71/82 (86.6) | NR |
| 90e | 58/82 (70.7) | NR |
| Proportion with inactive diseasef | 52/82 (63.4) | NR |
Abatacept59
The abatacept study reported ACR Pedi data separately for those who had been treated with abatacept continuously (lead-in, double-blind and OLE phases) and those whose abatacept had been interrupted by placebo during the double-blind-RCT phase. The OLE included 85% of the abatacept group and 76% of the placebo group from the double-blind phase. For those receiving continuous abatacept therapy, treatment length ranged from 31 to 52 months (participants who had entered the study earliest had been treated longest). Those who received placebo during the double-blind phase usually received abatacept for a shorter period (length not stated), but the ACR Pedi scores achieved were similar for ACR Pedi-30, -50 and -70 to those whose abatacept treatment had been continuous (see Table 21). The proportions of participants who had received placebo during the double-blind phase achieving ACR Pedi-90 and ACR Pedi-100 and having inactive disease are lower than those whose abatacept treatment had been continuous.
Adalimumab61
Results were reported for those who entered the OLE phase as a single group of participants (n = 128). This group included 35 of 38 (92%) participants who received adalimumab and methotrexate, and 36 of 37 (97%) participants who received placebo and methotrexate in the double-blind phase of the study. However, also within this group of 128 are 57 (45%) participants from two further study arms (adalimumab only and placebo only) who are not included in this report because the majority of participants in these arms had never received methotrexate and therefore do not meet the licensed indication. Through the first 104 weeks of the OLE phase there was no diminution of the ACR Pedi responses, such that after 104 weeks of open-label treatment in the extension phase, 40% of participants had an ACR Pedi-100 response (see Table 21).
Etanercept42
All 69 participants who began the open-label lead-in phase of the study (51 of whom took part in the double-blind randomised withdrawal phase) were eligible to enter the OLE phase but only 58 did so. Of the 58 who took part in the OLE, 26 entered the eight year of follow-up; therefore a last observation carried forward analysis was used to calculate the ACR Pedi responses reported in Table 21. These responses appear to have remained constant over the OLE. Although last observation carried forward analyses are commonly used in drug trials, this method can be prone to bias when used in progressive diseases such as JIA, and results should be interpreted with caution.
Tocilizumab68
Results from the OLE of the tocilizumab study68 are reported in conference abstracts. 69,71,74 Only participants who achieved at least ACR Pedi-30 during the open-label phase and who then continued into the double-blind RCT phase of the trial were eligible to enter the OLE, either after a JIA flare or when they completed the double-blind RCT phase. One-hundred and sixty (96%) of the 166 participants eligible to enter the OLE did so and 155 (97%) completed 104 weeks of follow-up (16-week open-label plus 24 weeks double-blind RCT plus 64 weeks OLE). ACR Pedi-70, ACR Pedi-90 and proportion with inactive disease are presented (see Table 21) only for the 82 participants who received continuous tocilizumab throughout the study and the proportion achieving each of these measures increased since the end of the double-blind phase (see Table 21).
Growth
Adalimumab61
Two abstracts62,63 report limited data for growth from a post hoc analysis of JIA patients who had taken part in any arm of the double-blind phase of the RCT and entered the OLE (this includes n = 58 who were not receiving methotrexate and who were therefore not receiving adalimumab treatment according to the licensed indication). All patients who received more than one dose of adalimumab +/– methotrexate were included in the analysis (n = 133). Patients were assigned by baseline weight into two groups: ≤ 33rd percentile (41%, n = 55) and > 33rd percentile (59%, n = 59) based on the US Centres for Disease Control and Prevention growth charts. Missing data were analysed using last observation carried forward. Those in the ≤ 33rd percentile baseline weight group had a higher mean percentile change from baseline in height at week 104 than those in the > 33rd percentile group (values for mean height percentile change from baseline estimated from graph by reviewer 5.5 and 3.3, respectively). 62 Similar patterns were stated to have been observed for weight and body mass index. At week 104 there were no statistically significant differences between the methotrexate and the non-methotrexate groups in mean changes from baseline in height, weight or body mass index (p > 0.26). Long-term adalimumab treatment appears to show improvement in growth for JIA patients who were in the ≤ 33rd percentile weight group at baseline receiving adalimumab with or without the addition of methotrexate. However, caution in the interpretation of the results is recommended owing to the limitations in the data presented and the absence of an appropriate control group.
Tocilizumab76
The Roche CS included data for growth and glucocorticoid treatment at week 104 based on a conference abstract76 from the CHERISH trial. 68 Most growth data came from a subset of patients (n = 123) with the highest growth potential, represented by patients with Tanner stage < 4 at baseline. The Tanner stages are based on a scale of physical development in children, adolescents and adults (boys: development of external genitalia; girls: breast development; boys and girls: pubic hair), with stage 5 being the final adult/mature stage. Growth measures included height standard deviation scores (SDSs) and height velocity. The mean height SDS of patients with polyarticular JIA and Tanner stage < 4 was below normal at baseline {–0.68 [standard deviation (SD) 1.23]} and rose to –0.19 (SD 1.14) at week 104 (n = 103) with the difference being statistically significant (p < 0.001 vs. baseline). Of these patients, 71.8% had an increased height SDS. The CS states (CS page 16)78 that there was no observed difference in patients who received placebo during the randomised phase of the trial (based on 154 patients of the growth population with height SDS data at both time points); however, fewer than half of the patients received placebo through the entire 24 weeks of the randomised phased of the trial, as most escaped to tocilizumab before week 40. For the entire growth population (n = 187; i.e. not restricted to those with Tanner stage < 4), the reported mean change in height SDS from baseline to week 104 was 0.25 (SD 0.54) (no p-value for comparison with baseline reported). The mean daily oral glucocorticoid dose decreased from baseline [0.05 mg/kg (SD 0.08 mg/kg)] to week 104 [(n = 103) (0.02 mg/kg (SD 0.05 mg/kg)]. A multiple linear regression analysis for the same 103 patients indicated that height velocity at week 52 was related to baseline age (p < 0.001) and oral glucocorticoid use at the end of week 52 (p = 0.0002). No data for week 104 were reported. Caution in the interpretation of the growth results is recommended owing to the limitations in the data presented and the absence of an appropriate control group.
Adverse events
This is a summary of OLE AEs presented in the published papers.
Abatacept: OLE to day 589 and year 7
In the abatacept study,57 common AEs (occurring in 10% or more of the total group, no data extracted) and common SAEs (occurring in 1% or more of the total group) were reported separately for those who had been in the abatacept group and those who had been in the placebo group during the double-blind period of the trial, and those who had not entered the double-blind phase because they did not achieve an ACR Pedi-30 response during initial open-label treatment. SAEs by day 589 (approximately 20–21 months) occurred in 23/153 patients (Table 22); the most common SAEs were arthritis flares (n = 6), arthralgia (n = 2), foot deformity (n = 2), pyrexia (n = 2) and vomiting (n = 2). The proportions of SAEs at day 589 were similar in the three groups. At 7-year follow-up (reported in an abstract60), 30/153 (19.6%) patients had SAEs. Most were unrelated and were primarily musculoskeletal or infectious events. The incidence rate (per 100 patient-years) of SAEs in the OLE at 7 years (5.6/100 patient-years) did not increase versus the 6-month double-blind rate (6.8/100 patient-years).
| Abatacept57 (OLE: day 589,59 7 years60) | |||
|---|---|---|---|
| SAEs | DB ABA (n = 58), n (%)a | DB PBO (n = 59), n (%)a | Patients with less than an ACR Pedi-30 response initially (n = 36), n (%) |
| Total SAEs, n/N (%) | 8/58 (14) | 8/59 (14) | 7/36 (19) |
| Most common SAEs | |||
| Arthritis flaresb | 3 (5.2) | 3 (8.3) | |
| Arthralgiab | 1 (1.7) | 1 (1.7) | 1 (2.8) |
| Foot deformityb | 1 (1.7) | 1 (1.7) | |
| Pyrexia | 1 (1.7) | 1 (1.7) | |
| Vomiting | 1 (1.7) | ||
| SAEs year 7, n/N (%) | 30/153 (19.6) | ||
Adalimumab: open-label extension ongoing
The OLE was ongoing at the time the key trial publication was published, and the time period for which events were reported is not clear. 61 SAEs possibly related to study drug occurred in seven patients during the OLE (a table in the published paper61 suggests none was receiving methotrexate, in which case they were not receiving adalimumab treatment according to the licensed indication). Three patients discontinued treatment owing to AEs during the OLE.
Etanercept: year 8
In the etanercept study42 OLE, the safety analyses captured SAEs, medically important infections (MIIs) and mortality, as well as some ‘events of interest’ (including opportunistic infections, tuberculosis, lupus, demyelinating disorders, malignancies and lymphomas). Non-SAEs were not recorded. 67 There were a total of 39 SAEs based on 318 patient-years of etanercept exposure (n = 69), with 26 patients entering their eighth year of etanercept treatment, equating to 0.12 events per patient-year (Table 23). There were nine MIIs resulting in the need for intravenous antibiotic therapy or hospitalisation, equating to 0.03 events per patient-years, with only one reported MII since 4-year follow-up (pyelonephritis). The most common new SAEs reported beyond 4 years of drug exposure were flares or worsening of disease, occurring in 6/9 patients (67%).
| Etanercept42 (OLE: up to 8 years67) | ||||
|---|---|---|---|---|
| Year of etanercept treatment from RCT (excluding gaps between RCT and OLE) | SAEa | MIIb | ||
| Number of events | Number of events/patient-year | Number of events | Number of events/patient-year | |
| 1 (n = 69; 57 patient-years of drug exposure) | 5 | 0.09 | 2 | 0.04 |
| 9 (n = 14; 4 patient-years of drug exposure) | 0 | 0 | 0 | 0 |
| Total for all years (n = 69; 318 patient-years of drug exposure) | 39 | 0.12 | 9 | 0.03 |
Tocilizumab: 104 weeks
Long-term AEs rates based on a safety population of 188 patients (307 patient-years) were 406.5 per 100 patient-years over 104 weeks (approximately 2 years) in patients receiving tocilizumab, based on an abstract only. 69 The equivalent SAEs rate was 11.1 per 100 patient-years (Table 24). Infections categorised into the most common AEs and SAEs were 151.4 and 5.2 per 100 patient-years, respectively. The study also reports AE safety population data for elevations of alanine aminotransferase and aspartate aminotransferase, grade 2/3/4 thrombocytopenia, grade-3 lowest neutrophil count and low-density lipoprotein cholesterol (see data extractions in Appendix 5).
Assessment of clinical effectiveness: biologic disease-modifying antirheumatic drugs versus each other (with methotrexate where permitted)
Background
None of the RCTs included in the systematic review of clinical effectiveness directly compared any of the biologic DMARDs with each other. It was therefore necessary to undertake an indirect comparison of the drugs to inform the assessment of comparative clinical effectiveness. One published indirect comparison was identified through literature searching, by Otten and colleagues. 79 This was a systematic review of RCTs that constructed two separate evidence networks: polyarticular-course JIA and systemic JIA. For each network, a series of pairwise indirect comparisons was conducted, with placebo as a common comparator, using the method described by Bucher and colleagues. 53
Three RCTs were included in Otten and colleagues’79 polyarticular-course JIA network,42,57,61 all of which have been included in this assessment report. However, this network did not include tocilizumab, as at that time no RCT evidence for that drug in polyarticular-course JIA was published. The network therefore included only comparisons of three of the four biologic DMARDs of relevance to the scope of this assessment (abatacept, adalimumab and etanercept). We have conducted a similar adjusted indirect comparison to Otten and colleagues79 including the recently published tocilizumab RCT by Brunner and colleagues (the CHERISH trial). 68 Figure 2 illustrates the design of the analysis, representing what is termed a star network. 80
FIGURE 2.
Indirect comparison of biologic DMARDs.
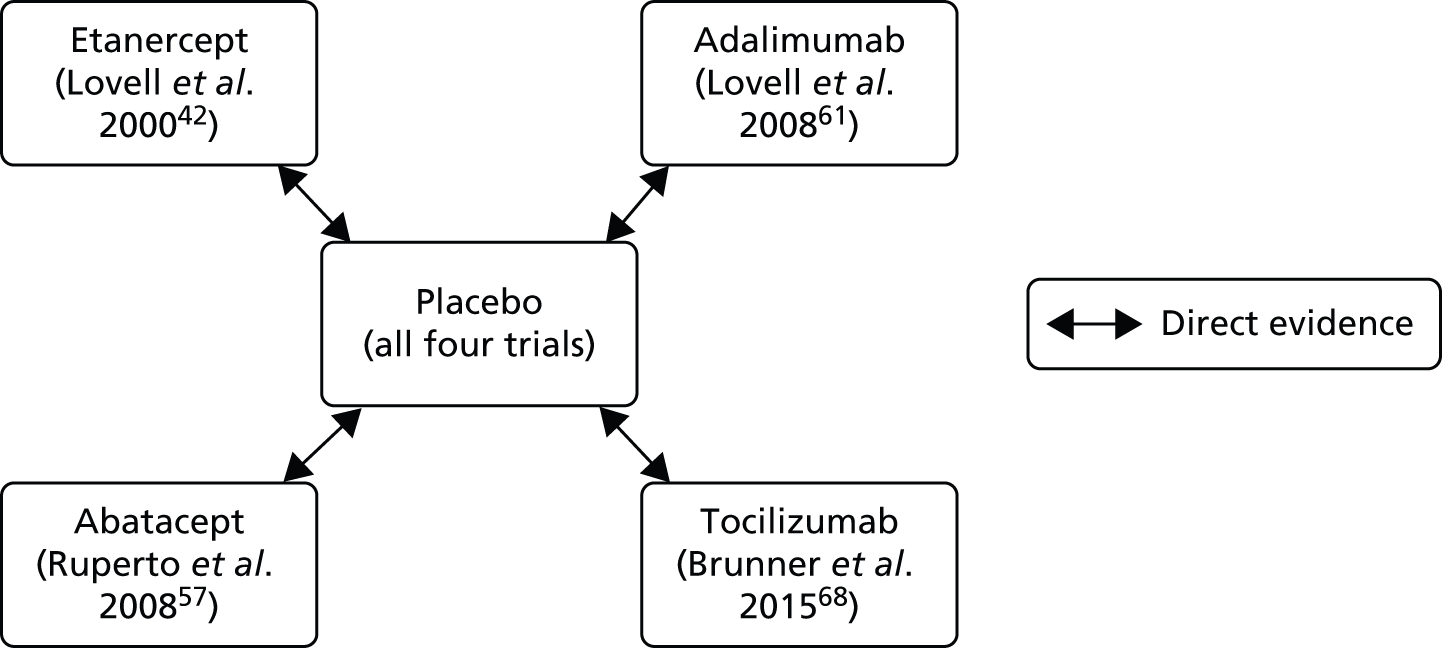
Otten and colleagues79 indirectly compared the drugs in relative risk (RR) of disease flare. We have similarly included disease flare as an outcome and, in addition, have chosen ACR Pedi-50 and -70 responses as an outcome. ACR Pedi-50 and -70 were chosen as opposed to ACR Pedi-30, as it was considered that a higher level would be a more clinically relevant level of treatment response. Furthermore, owing to the design of the RCTs, all patients who were randomised had achieved an ACR Pedi-30 response at the end of the open-label lead-in phase.
The adjusted indirect comparison should be considered to be exploratory rather than definitive owing to limitations in the evidence base and heterogeneity between the included trials.
-
There is only one trial available for each drug. Although the trials were considered to be of generally good methodological quality and low risk of bias, evidence networks are considered weaker if informed by small numbers of studies and small numbers of participants. 81 The number of patients in the trials was also relatively low (ranging from 51 to 163).
-
There is some variation in the proportion of subtypes of JIA in the included trials. Although the network is considered to be most applicable to polyarticular-course JIA, in one trial (etanercept42) around one-third of patients were classified as having systemic JIA with apparent systemic manifestations (which is outside the scope of the current appraisal). There was insufficient evidence from RCTs to construct a network for PA, or ERA because outcome data for these subtypes of JIA were not reported separately in trials, even though some cases with these subtypes may have been included.
-
The duration of JIA ranged from just under 4 years57 to approximately 6 years across the trials. 42 A study by the BSPAR etanercept cohort found that shorter disease duration was an independent predictor of achieving an excellent response to treatment (ACR Pedi-90) at one year. 82 Disease duration has also been found to be a predictor of response to etanercept83 and to methotrexate84 among patients from the German Biologika in der Kinderrheumatologie (BIKER) registry. Differences between the trials in disease duration may therefore potentially confound results.
-
Three of the four trials permitted patients to take methotrexate in addition to the biologic DMARD (in proportions of patients varying from 74% to 100%), whereas the fourth trial (etanercept)83 did not permit use of methotrexate.
-
Previous therapy with biologic DMARDs had been received by approximately one-third of participants who entered the initial open-label run-in of two trials (abatacept57 and tocilizumab68). Prior therapy with another biologic DMARD was an exclusion criterion for the adalimumab trial61 and was not mentioned for the earliest trial (etanercept42), presumably because no other biological therapies were available at the time. Currently, it is unclear whether or not prior biologic DMARD treatment influences the effectiveness of subsequent biologic treatment.
-
The mean age of patients across the trials varied from around 7.5 years to 13 years. Part of this variation may reflect the age ranges specified in the inclusion criteria of the trials and potentially the mix of JIA subtypes in the trials, which have a different mean age at onset. Age could be an effect modifier given the progressive nature of JIA.
-
The duration of the double-blind randomised treatment phase of the trials varied from 4 months42 to 8 months. 61 Treatment duration may affect outcomes that are time dependent, such as disease flare.
Results
Figure 3 illustrates the results of the four included RCTs comparing the biologic DMARDs with placebo (with background methotrexate where permitted) on the outcome of disease flare (the adalimumab trial61 stratified results according to whether or not patients received methotrexate background therapy, and we have included data only for patients who did receive methotrexate, in accordance with the licensed indication – this applies to disease flare and to ACR Pedi-50/-70). Treatment with each of the four DMARDs resulted in a statistically significant reduction in the RR of a disease flare, ranging from 0.38 to 0.57. (We have not presented a pooled RR given differences between the DMARDs and also heterogeneity between the trials.)
FIGURE 3.
Summary forest plot of biologic DMARDs vs. placebo: disease flare. M–H, Mantel–Haenszel.

Table 25 reports adjusted pairwise indirect comparisons for the four biologic DMARDs for the outcome of disease flare. The point estimate for risk of flare was lower for etanercept than the other three comparators. Abatacept had a lower risk of flare than adalimumab and tocilizumab. Tocilizumab had a lower risk of flare than adalimumab only. Adalimumab was associated with a higher risk of disease flare than the other three comparators. The ranking of treatments in terms of risk of flare was therefore etanercept, abatacept, tocilizumab and adalimumab. However, none of the comparisons demonstrated a statistically significant difference between the treatments being compared, with CIs crossing 1 in every case. The results of our analysis match those of Otten and colleagues,79 with the exception of the comparison with tocilizumab, which was not included in their polyarticular-course JIA trial network (as discussed above).
| Comparison | RR (95% CI) |
|---|---|
| Etanercept vs. adalimumab | 0.61 (0.27 to 1.38) |
| Etanercept vs. abatacept | 0.92 (0.39 to 2.18) |
| Etanercept vs. tocilizumab | 0.65 (0.30 to 1.43) |
| Adalimumab vs. abatacept | 1.51 (0.72 to 3.15) |
| Adalimumab vs. tocilizumab | 1.07 (0.56 to 2.04) |
| Abatacept vs. tocilizumab | 0.71 (0.35 to 1.43) |
Figure 4 illustrates the results of the four included RCTs comparing the biologic DMARDs with placebo (with background methotrexate where permitted) on the outcome of ACR Pedi-50 response. Treatment with each of the four DMARDs led to a statistically significant greater proportion of participants with ACR Pedi-50 response, with the RR ranging from 1.41 to 3.12.
FIGURE 4.
Summary forest plot of biologic DMARDs vs. placebo: ACR Pedi-50 response. M–H, Mantel–Haenszel.

Table 26 reports adjusted pairwise indirect comparisons for the four biologic DMARDs for the ACR Pedi-50 response outcome. Etanercept had a higher RR for treatment response than the other three comparators. Adalimumab had a higher RR for treatment response than abatacept and tocilizumab. Adalimumab had a higher RR for treatment response than tocilizumab. The ranking of treatments in terms of treatment response was therefore etanercept, adalimumab, abatacept and tocilizumab. With the exception of etanercept compared with tocilizumab, none of the comparisons indicated a statistically significant difference between the treatments being compared, with CIs crossing 1.
| Comparison | RR (95% CI) |
|---|---|
| Etanercept vs. adalimumab | 1.87 (0.77 to 4.53) |
| Etanercept vs. abatacept | 2.10 (0.95 to 4.64) |
| Etanercept vs. tocilizumab | 2.21 (1.01 to 4.84) |
| Adalimumab vs. abatacept | 1.12 (0.65 to 1.96) |
| Adalimumab vs. tocilizumab | 1.18 (0.69 to 2.02) |
| Abatacept vs. tocilizumab | 1.05 (0.72 to 1.53) |
Figure 5 illustrates the results of the four included RCTs comparing the biologic DMARDs with placebo (with background methotrexate where permitted) on the ACR Pedi-70 response outcome. Treatment with each of the four DMARDs led to a statistically significant greater proportion of participants with ACR Pedi-70 response, with the RR ranging from 1.54 to 2.34.
FIGURE 5.
Summary forest plot of biologic DMARDs vs. placebo: ACR Pedi-70 response. M–H, Mantel–Haenszel.
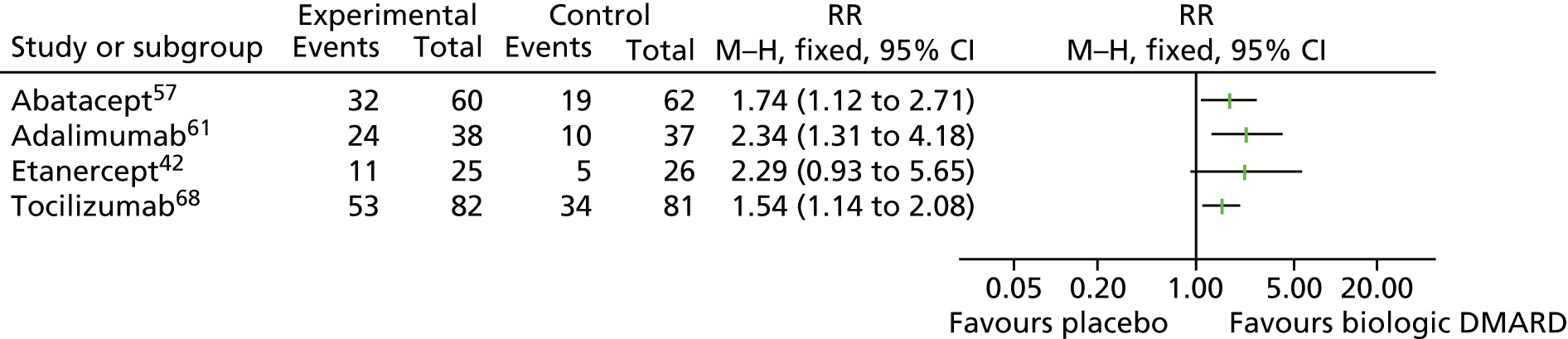
Table 27 reports adjusted pairwise indirect comparisons for the four biologic DMARDs for the outcome of ACR Pedi-70 response. Etanercept had a higher RR for treatment response than abatacept and tocilizumab but a slightly lower RR for treatment response than adalimumab. Adalimumab had a higher RR for treatment response than abatacept and tocilizumab. Abatacept had a higher RR for treatment response than tocilizumab. The ranking of treatments in terms of treatment response for ACR Pedi-70 (adalimumab, etanercept, abatacept and tocilizumab) was therefore different from the ACR Pedi-50. None of the comparisons indicated a statistically significant difference between the treatments being compared, with CIs crossing 1.
| Comparison | RR (95% CI) |
|---|---|
| Etanercept vs. adalimumab | 0.98 (0.33 to 2.87) |
| Etanercept vs. abatacept | 1.31 (0.48 to 3.60) |
| Etanercept vs. tocilizumab | 1.49 (0.57 to 3.85) |
| Adalimumab vs. abatacept | 1.34 (0.65 to 2.79) |
| Adalimumab vs. tocilizumab | 1.52 (0.79 to 2.92) |
| Abatacept vs. tocilizumab | 1.13 (0.66 to 1.93) |
The results of this exploratory analysis based on the limited evidence available currently (only one trial for each of the four biologic DMARDs) supports etanercept being more effective than the other three biologic DMARDs in terms of preventing disease flares and achieving a response to treatment based on a composite index (ACR Pedi-50), whereas the ACR Pedi-70 exploratory analysis shows adalimumab with a slight advantage over etanercept (although see comment below about CIs). Abatacept appeared to be superior to tocilizumab for all outcome measures. Adalimumab appeared to be less effective than abatacept and tocilizumab in terms of preventing disease flare but more effective in terms of ACR Pedi-50 and -70 responses. Therefore, there was no consistent ranking of treatment comparisons across these outcome measures. The indirect comparisons were generally not statistically significant and CIs were wide, so caution is advised in the interpretation of these results. Furthermore, the etanercept trial42 appears to have some differences from the other trials which may confound the results, namely the absence of methotrexate background therapy and the longer duration of JIA disease. There was also a noticeably higher rate of flares in the placebo arm of that trial than in the other three trials (81% compared with 48–65%) which may account for the bigger treatment effect seen. Taking the above limitations into account, an overall interpretation of the results of the indirect comparison is that, owing to the absence of statistically significant differences between the biologic DMARDs, they currently appear to be similar in treatment effectiveness. This accords with the conclusion reached by Otten and colleagues79 who suggested that the short-term efficacy of the biologic DMARDs in polyarticular-course JIA seem similar. Furthermore, the clinical advisors to the assessment group felt that these data generally reflect clinical experience in that when used for the same indication in the same population, effectiveness was likely to be similar. However, there was also a recognition that for individual patients, and potentially for particular subgroups of JIA patients, differential effects of each biologic DMARD might be apparent but these differential effects have not yet been captured by current trial data.
Summary of the systematic review of clinical effectiveness
-
Four multicentre RCTs, one each evaluating abatacept, adalimumab, etanercept and tocilizumab, met the inclusion criteria of this review. Only the tocilizumab RCT included UK patients. Seven additional RCTs (three for adalimumab and four for etanercept) are described as ongoing (details summarised in Ongoing trials, below).
-
Each RCT had three phases, an open-label lead-in period, a randomised withdrawal period and an OLE. The lengths of the lead-in and randomised phases varied between studies (open-label lead-in: 12–16 weeks; randomised double-blind withdrawal phase 16–32 weeks). In each study patients had to achieve an ACR Pedi-XX response during the initial open-label phase in order to be eligible for entry to the randomised double-blind withdrawal phase. Therefore, results are applicable only to patients who have already achieved an initial (low) degree of benefit from a biologic DMARD.
-
The quality of the included RCTs was reasonable overall, with a low risk of bias judged for most items, although some aspects were rated as unclear, mainly owing to a lack of reporting.
-
Disease flare: this was the primary outcome in all four RCTs, with definitions broadly consistent between the studies. Patients who continued to receive biologic DMARDs during the randomised withdrawal phase of the studies had statistically significantly fewer arthritis flares than those receiving placebo in all four studies, whereas time to disease flare reported in three studies (abatacept, adalimumab and etanercept) was statistically significantly longer in those treated with biologic DMARDs.
-
ACR Pedi: a greater proportion of patients receiving biologic DMARDs during the randomised withdrawal phase of the studies achieved ACR Pedi responses of ≥ 30 than placebo-treated patients, with differences statistically significant in all but two instances where p-values were reported. The proportion of biologic DMARD-treated patients with inactive disease was more than twice that of placebo-treated patients in the two studies (abatacept and tocilizumab) reporting this outcome.
-
ACR Pedi core variables: in the three studies reporting this outcome (abatacept, etanercept and tocilizumab) results were generally in favour of treatment with biologic DMARDs when compared with placebo.
-
Joint-related outcomes: one study (etanercept) reported additional joint outcomes without statistical comparisons. All outcomes favoured etanercept over placebo.
-
Pain: three studies reported pain (abatacept, etanercept and tocilizumab). A statistically significant difference in the mean change from baseline favoured the tocilizumab group. Although pain scores were lower for those receiving biologic DMARDs in the remaining two studies, differences between treatment groups were not statistically significant in the abatacept study and no statistical comparison reported in the etanercept study.
-
Mortality: no treatment-related deaths were reported in the three studies reporting this outcome (adalimumab, etanercept and tocilizumab).
-
Outcomes not reported by the included RCTs for the randomised withdrawal phase of the trials were corticosteroid-reducing regimens, extra-articular manifestations (such as uveitis), height and weight.
-
HRQoL: reported by the abatacept study only, with differences between treatment groups for the physical and psychosocial summary scores not statistically significant. Those treated with abatacept had improved scores for 14 of the 15 CHQ subscales compared with 6 out of 15 for placebo-treated patients.
-
AEs: during the randomised withdrawal phase of the trials, the proportions of AEs and SAEs were generally fairly similar between the biologic DMARDs and the placebo groups.
-
Subgroup analyses: the tocilizumab study reported data for subgroups but no statistical comparisons between treatment groups were reported.
-
OLE: all four studies included an OLE phase. There were differences in eligibility criteria between studies and in how data were presented. Only results for ACR Pedi, AEs and growth are included in this report.
-
OLE ACR Pedi:
-
Abatacept: the proportion of patients achieving ACR Pedi-30, -50 and -70 scores were similar for those with continuous abatacept therapy and those who received placebo during the double-blind phase, but were greater at achieving ACR Pedi-100 and inactive disease in abatacept-treated patients (ACR Pedi-100 abatacept 39%, placebo 19%; inactive disease abatacept 43%, placebo 23%).
-
Adalimumab: there was no diminution of ACR Pedi responses, and 40% of patients had an ACR Pedi-100 response after open-label treatment in the extension phase, but results included patients not meeting the licensed indication.
-
Etanercept: 26/58 (45%) patients who took part in the OLE entered the eighth year of follow-up. ACR Pedi responses appear to have remained constant over the OLE.
-
Tocilizumab: limited results were based on conference abstracts for 82 patients who received continuous tocilizumab throughout the study. The proportion of patients achieving ACR Pedi-70 and -90 increased since the end of the double-blind phase, with 63% having inactive disease.
-
-
OLE AEs:
-
Abatacept: at 7-year follow-up, 19.6% of patients had SAEs, with similar incidence rates between the OLE phase (5.6 per 100 patient-years) and the 6-month double-blind phase (6.8 per 100 patient-years).
-
Adalimumab: SAEs possibly related to study drug occurred in seven patients during the OLE, but would appear to be in patients not in line with licensed indication (OLE phase ongoing, time period unclear). Three patients discontinued treatment owing to AEs.
-
Etanercept: there were a total of 39 SAEs based on 318 patient-years of etanercept exposure, with 26/69 patients entering their eighth year of etanercept treatment (0.12 events per patient-year). Nine MIIs resulted in the need for intravenous antibiotic therapy or hospitalisation (0.03 events per patient-year).
-
Tocilizumab: AEs rates were 406.5 per 100 patient-years and the SAEs rate 11.1 per 100 patient-years over around 2 years, with the most common AEs and SAEs related to infections (151.4 and 5.2 per 100 patient-years, respectively).
-
-
Growth: limited data reported in abstracts at week 104 for adalimumab and tocilizumab appear to support the positive effect of these drugs on growth, but the use of different outcome measures prevents a comparison between the drugs.
-
An exploratory adjusted indirect comparison found that there was a lack of statistically significant differences between the four biologic DMARDs in terms of disease flare and ACR Pedi-50/-70 response, with wide CIs and clinical heterogeneity between the trials.
Review of clinical effectiveness in company submissions to the National Institute for Health and Care Excellence
Four companies made submissions in support of their drugs to NICE: BMS for abatacept, AbbVie for adalimumab, Pfizer Ltd for etanercept and Roche for tocilizumab. A review of the information presented about the economic evaluation of biologic DMARDs for treatment of JIA in the CSs can be found in Chapter 5, Review of cost-effectiveness in company submissions to National Institute for Health and Care Excellence.
Review of Bristol-Myers Squibb company evidence submission for abatacept
The company did not report a systematic review of clinical effectiveness of abatacept. 85 There is no indication that any databases were searched and no search strategies were supplied. Furthermore, there is no search or report for any ongoing studies. The majority of the clinical effectiveness information in the CS comes from published papers with a few details that are commercial-in-confidence (CiC) which come from the clinical study reports.
The CS includes one phase-III double-blind randomised withdrawal study, the AWAKEN trial. Although not clearly summarised, the CS draws on two published papers,57,58 one conference presentation86 and the trial clinical study reports. The published papers57,58 met the inclusion criteria of this assessment report. One published paper59 relating to the AWAKEN trial that was identified in this assessment report was not cited by the CS but the data in this appear to have been superseded by the more recent conference presentation. 86 However, the more recent efficacy data are not presented according to the randomised groups in the double-blind period, whereas the safety summary data are. 86 Furthermore, there is limited detail regarding the length of follow-up, which is stated to be ≥ 56 months and up to 7 years of total follow-up. No critical appraisal is reported for any of the studies cited in the CS.
A summary of the AWAKEN trial is provided in the CS which is broadly similar to the information presented in the published papers. 57,58,86 Information from the OLE phase drawn from the conference presentation86 is more recent than the data from the published paper,59 which is included in the assessment report. Furthermore, an analysis was conducted (using Fisher’s exact test) to compare SAEs during the double-blind phases of trials of abatacept, adalimumab etanercept and tocilizumab (CS section 3.4.6). The CS highlights the lack of statistical power attributable to the low numbers of patients and event rates, which should be taken into account in the interpretation of their finding that the incidence of SAEs was likely to be similar between the biologic DMARDs.
The CS focuses on abatacept, with very little information provided regarding the other biologic DMARDs included in this MTA. However, information is presented in the CS on indirect pairwise comparisons for the four biologic DMARDs for the outcome of disease flare. The comparisons for abatacept, adalimumab and etanercept are taken from a published paper by Otten and colleagues79 and this is supplemented by new indirect pairwise comparisons with tocilizumab, taking data from a RCT68 that has been published since the Otten and colleagues study (the CHERISH trial. 68) The results of the indirect comparisons reported in the CS (tables 4 and 5) match those reported in the indirect comparisons conducted for this assessment report [see Assessment of clinical effectiveness: biologic disease-modifying antirheumatic drugs versus each other (with methotrexate where permitted)].
In summary, the CS has not conducted a systematic review of clinical effectiveness but has summarised data from the AWAKEN trial,57 presented indirect pairwise comparisons of the four biologic DMARDs included in this appraisal and conducted an analysis to compare SAEs during the double-blind phases of trials of the four biologic DMARDs. No additional RCTs were included in the CS that would have met the inclusion criteria of this assessment report.
Review of AbbVie company evidence submission for adalimumab
AbbVie submitted a report to NICE on adalimumab as a treatment for JIA. 77 The clinical effectiveness evidence has been briefly appraised.
The company did not conduct a formal systematic review of the clinical effectiveness evidence, but provided what they describe as ‘an iterative literature review’ (CS page 15). The company asserts that all RCTs of adalimumab in the treatment of JIA have been identified (CS page 15). It would appear that RCTs were identified chronologically from an adalimumab trial programme, and there is no mention that any databases were searched and no search strategies were provided. There is no search for or report of any ongoing studies, but the CS does contain information about a trial in progress, the SYCAMORE RCT. 87 This trial is evaluating the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate for the treatment of JIA-associated uveitis (further information on this trial is given Ongoing trials of this assessment report). Data from abstracts/conference proceedings are also presented in the CS.
The submission contains narrative summaries for the pivotal RCT by Lovell and colleagues,61 which formed the basis of the original marketing authorisation in 2008; an ongoing RCT of adalimumab treatment in patients with ERA by Burgos-Vargas and colleagues88–93 (see Ongoing trials of this assessment report for details of this study); two open-label single-arm studies94,95 and supporting data from an ongoing registry (STRIVE) funded by AbbVie. The multinational STRIVE registry is assessing the long-term safety and effectiveness of adalimumab in patients with moderate to severe polyarticular JIA. Some data from this registry are given in the CS, for efficacy outcomes up to 1 year, and safety outcomes for longer (mean duration of drug exposure was 643 days for methotrexate patients and 653 days for adalimumab and methotrexate patients). The registry does not appear to include patients from the UK. Other evidence such as case series, open-label trials, a systematic review96 and data from an Italian registry were included to provide evidence for the effectiveness of adalimumab in JIA-associated uveitis (see JIA-associated uveitis of this assessment report for details of these).
Based on the Lovell and colleagues RCT,61 the key outcomes of disease flares and ACR Pedi responses are the same in the CS and the assessment report.
The CS notes several methodological concerns that prevented the presentation of a network meta-analysis comparing the four biologic DMARDs. An indirect comparison was therefore not presented.
In summary, the CS has not conducted a systematic review of clinical effectiveness but has summarised data separately for RCTs and other non-randomised studies, as well as data from a registry. No indirect comparison of the biologic DMARDs was conducted by the company. No additional RCTs were included in the CS that met the inclusion criteria for systematic review in this assessment report; however, some of the non-randomised study evidence in the CS is presented in this assessment report for patient subgroups where randomised evidence is lacking (i.e. ERA and JIA-associated uveitis) (see Additional supporting evidence).
Review of Pfizer Ltd company evidence submission for etanercept
The company report a systematic review of clinical effectiveness of etanercept (in addition they report a systematic review of observational evidence on etanercept-associated innovation, caregiver burden and treatment adherence, plus a systematic review of HRQoL associated with etanercept). 97 Details of the literature search strategy are provided and the search appears to be comprehensive, up to date and reproducible. A search for ongoing studies was also conducted. A systematic process was followed to screen studies for inclusion, with titles, abstracts and full texts screened independently by two reviewers. The inclusion criteria are in keeping with the scope of the appraisal, with the exception that only studies of etanercept were included, not the other biologic DMARDs. A broad range of study designs were eligible, with the exception of case reports. The majority of the data are in the public domain, although some information is either academic-in-confidence (AiC) or CiC.
The review included 11 publications relating to five primary interventional studies and three extension studies (see CS table 7, CS page 44). It also included 41 observational studies (including registry studies) plus 2 unpublished studies (see CS table 18, CS page 81). Of the five included primary interventional studies, only one meets the inclusion criteria for the systematic review in this current report – Lovell and colleagues. 42 Of the remaining four studies, three were not relevant, as they were single-arm studies, and a fourth was a RCT reported in a conference abstract98 with only limited detail available. However, one of the single-arm studies – the CLIPPER study99 – is noteworthy as it focuses specifically on the JIA subtypes that were absent from the pivotal Lovell and colleagues trial,42 namely EO JIA, ERA and PA. Details are presented in Additional supporting evidence.
Of the three extension studies, only one was relevant to the inclusion criteria of this assessment report, namely the long-term follow-up publications65–67 of the Lovell and colleagues RCT,42 all of which have been included in the data extraction for this study (see Appendix 5). The other two extension studies included a Japanese open-label single-arm multicentre study followed by a double-blind, randomised dose-down extension study (two doses of etanercept – no comparator), and an open-label multicentre Phase-IIIb long-term safety and efficacy study of the CLIPPER study (reported in Additional supporting evidence). 99
A critical appraisal of the interventional studies is provided in CS section 4.7. The company’s appraisal of the Lovell and colleagues RCT42 is provided in CS table 13 (CS page 62). Our critical appraisal differs slightly from the company’s (see Quantity and quality of research available). Specifically, we did not consider that adequate details had been provided of the study’s randomisation method or concealment of allocation. We also note that there was a large imbalance in drop-outs between the randomised groups (see Table 12 and Appendix 5).
A narrative synthesis of the interventional and observational studies is provided in the CS, with detailed tabulation of study characteristics and results. A meta-analysis was not considered feasible or appropriate by the company, and an indirect comparison was not conducted as it was not considered feasible to conduct one owing to differences in respective marketing authorisations across biologic treatments, paucity of data and heterogeneity.
Of note, some of the observational studies of etanercept included in the CS reported (limited) data for outcomes relevant to the scope of the appraisal that were not included in the RCT by Lovell and colleagues,42 namely corticosteroid reduction, growth and disease activity according to the JADAS (see CS section 4.12.5).
In summary, the systematic review of clinical effectiveness reported in the CS appears to be of a good standard and no additional RCTs were included in the CS that met the inclusion criteria for the systematic review in this assessment report.
Review of Roche company evidence submission for tocilizumab
The company did not conduct a formal systematic review of the clinical effectiveness evidence, but provided ‘most relevant literature’ on the use of tocilizumab in patients with polyarticular JIA and EO (CS page 7). There is no evidence that searches were conducted and no search strategies were reported. The CS does state that a systematic literature review was completed for the indirect comparison presented in the submission but provides no further detail. The CS did not report searching conference proceedings or details of any ongoing trials, but data from abstracts/conference proceedings are included in the submission. CiC data are limited to the economic model.
The submission contains narrative summaries of two studies. One of the studies is a RCT comparing tocilizumab with placebo (CHERISH)68 linked to six additional conference publications/abstracts. 69,71–73,76,100 The CHERISH RCT68 met the inclusion criteria of this assessment report and was reported above in Results. Of the six conference publications/abstracts linked to the CHERISH RCT, only two were related to the randomised phase of the trial72,73 and the remaining four were related to the OLE phase. One of these four conference abstracts was not identified by searches for this assessment report, but if it had been, it would not have met the inclusion criteria as none of the outcomes reported was relevant. 100
The other study was a single-arm open-label study of efficacy, pharmacokinetics and safety of adalimumab in Japanese patients with polyarticular JIA. 95
The CS presents all the evidence separately for each study in the form of a narrative summary. Individual tables of baseline patient characteristics, as well as details of methods and design are reported for both the CHERISH trial68 and the open-label Japanese study. 95 No quality assessment of the studies is presented. The CS reports growth data from the OLE phase of the CHERISH trial at week 104, which have been included in this assessment report. The assessment report contains additional data for ACR Pedi-90 responses relative to baseline at week 40 and inactive disease from the CHERISH trial, both of which are not reported in the CS.
The CS includes a hierarchical Bayesian indirect treatment comparison of adalimumab and tocilizumab, conducted in WinBUGS version 1.4 software (Medical Research Council Biostatistics Unit, Cambridge, UK) and using methods described by Dias and colleagues. 101 Limited detail is provided on the specific methods used to conduct the indirect comparison (e.g. which adalimumab trial was used to compare against tocilizumab; this is likely to be Lovell and colleagues61 but is not explicitly stated). An indirect comparison with abatacept was not considered possible owing to the difference in trial design, the fact that it is not approved (appraised) by NICE, and also because of slight differences in licences (i.e. lower age for which treatment is indicated). We note the heterogeneity between the RCTs that increases uncertainties in any indirect comparison, although the fact that abatacept has not been appraised by NICE is not an adequate justification for not performing the comparison. The CS provides an additional analysis which assumes a class effect across antiTNF drugs (based on the indirect comparison with adalimumab which showed ‘overlapping ACR response rates’), permitting a comparison between tocilizumab with etanercept (CS section 5.17). The exploratory pairwise indirect comparisons of all four biologic DMARDS presented in this assessment report showed no statistically significant differences between the drugs [see Assessment of clinical effectiveness: biologic disease-modifying antirheumatic drugs versus each other (with methotrexate where permitted)].
Most of the AE and safety data are presented for the CHERISH RCT. 68 The AE data reported at week 40 (end of the randomised phase of the RCT) in the CS do not include any data for the placebo group, which is presented in this assessment report.
In summary, the CS has not conducted a systematic review of clinical effectiveness but has summarised data from the CHERISH trial and an open-label Japanese study, presented an indirect pairwise comparisons of two of the biologic DMARDs included in this appraisal (tocilizumab and adalimumab), as well as exploratory analysis comparing tocilizumab with etanercept. No additional RCTs were included in the CS that met the inclusion criteria of the assessment report.
Ongoing trials
As stated above (Quantity and quality of research available), citations relating to four ongoing RCTs were identified from the electronic bibliographic database literature search (see Figure 1) and a separate search specifically for ongoing studies identified a further three ongoing RCTs. Three trials are investigating adalimumab and four etanercept. Each trial is described, in turn, below, with preliminary results presented where possible. It should be noted that online clinical trial registers generally provide less information (and no outcome data) in comparison with published conference abstracts.
Adalimumab ongoing trial 1
This is a Phase-III, multicentre, randomised, double-blind study (NCT01166282) described by six conference abstracts (Burgos-Vargas and colleagues88–93) in children aged ≥ 6 to < 18 years with ERA based on ILAR criteria, with active disease not responsive to ≥ 1 NSAID and ≥ 1 DMARD. No full paper appears to have been published so far, and the six abstracts provide limited information, hence preventing a full assessment of the methodology and trial quality and risk of bias. In addition, baseline characteristics were reported only for the overall trial population and not separately for each randomised group. The estimated study completion date was December 2015; however, the study was published in full after the submission date of this report in July 2015. 102
Forty-six patients were randomised in a 2 : 1 ratio (adalimumab n = 31; placebo n = 15) to receive blinded adalimumab (24 mg/m2 body surface area up to 40 mg every other week) or placebo for 12 weeks followed by open-label adalimumab every other week for up to 144 weeks. It is unclear whether or not patients also received methotrexate. A table in one of the abstracts93 shows that 11/15 placebo patients and 21/31 adalimumab patients received DMARDs at baseline.
The primary endpoint of this study was per cent change from baseline in the number of active joints with arthritis (active joint count) at week 12 and secondary variables assessed included enthesitis count, tender and swollen joint counts, and ACR Pedi-30/-50/-70 responses. Active disease was defined as ≥ 3 active joints (swelling or loss of motion and pain/tenderness) and enthesitis in ≥ 1 location (past or present). Safety was assessed in terms of AEs, laboratory values and vital sign measurements. Some interim data were reported for 52 weeks including discontinuation of concomitant medication (at the discretion of the treating physician).
Authors state that no children discontinued the double-blind period, while at the same time reporting that seven children ‘escaped early’ to open-label adalimumab.
Results
At baseline, children had a mean age of 12.9 years, with 2.6 mean years of ERA symptoms, and a mean enthesitis count and active joint count of 8.1 and 7.8, respectively (Table 28). It is unclear if baseline characteristics between the treatment groups were balanced.
| Parameter, mean (SD)a | All children |
|---|---|
| Age, years | 12.9 (2.9) |
| ERA symptoms, years | 2.6 (2.3) |
| AJC | 7.8 (6.6) |
| EC | 8.1 (8.4) |
Only the primary outcome per cent change from baseline in the number of active joints with arthritis showed a statistically significantly greater improvement (p = 0.039) in the adalimumab treatment group (–62.6) than in the placebo group (–11.6) (Table 29). Secondary outcomes were reported to be mostly numerically greater in the adalimumab group, but none of the improvements was statistically significant.
| Primary outcomea | ADA (n = 31) | PBO (n = 15) | p-value |
|---|---|---|---|
| AJC, % change from baseline at week 12 | –62.6 (SD 59.5) (median % change –88.9%) | –11.6 (SD 100.5) (median % change –50.0%) | 0.039 |
| Secondary outcomes, change from baseline, mean (SD)b | |||
| Number of enthesitis sites (0–35) | –4.4 (6.2) | –2.7 (5.0) | NS |
| Tender joint count (0–72) | –7.9 (8.3) | –4.5 (9.0) | NS |
| Swollen joint count (0–8) | –3.5 (5.6) | –2.4 (4.7) | NS |
| ACR Pedi response, n (%)c | |||
| ACR Pedi-30 responder | 21 (67.7) | 10 (66.7) | NS |
| ACR Pedi-50 responder | 20 (64.5) | 7 (46.7) | NS |
| ACR Pedi-70 responder | 16 (51.6) | 4 (26.7) | NS |
Adverse events
Only one patient (in the adalimumab group) experienced a SAE (abdominal pain and headache). Around two-thirds of the children in the adalimumab treatment group and just over half in the placebo group experienced an AE, whereas nearly one-third of children in the adalimumab treatment group but only one-fifth in the placebo group experienced infectious AEs (Table 30).
| AEs | ADA (n = 31), % | PBO (n = 15), % |
|---|---|---|
| Any AE | 67.7 | 53.3 |
| SAE | 3.21 (1 patient) | 0 |
| Infectious AE | 29.0 | 20.0 |
Open-label week-52 results
The authors state that treatment response was maintained with continued adalimumab therapy up to 52 weeks [% change from baseline at week 52 in active joint court: –88.7 (SD 26.1)]. In those receiving at least one dose of adalimumab through to week 52, > 91% of children experienced an AE and > 76% experienced infectious AEs (Table 31). SAEs were reported in approximately 11% of children, with no reported deaths, tuberculosis or malignancies. Eight (19%) of the 43 participants who remained in the study at week 52 had completely discontinued concomitant ERA medication.
| AEs | ADA, %a |
|---|---|
| Any AEs | 91.3 |
| SAEs | 10.9 |
| Infectious AEs | 76.1 |
Adalimumab ongoing trial 2
The second adalimumab RCT (SYCAMORE, ISRCTN10065623)87 is funded by the NIHR HTA programme and Arthritis Research UK. The study is assessing adalimumab combined with methotrexate compared with placebo combined with methotrexate for JIA-associated uveitis in participants aged between 2 and 18 years. All participants will receive 18 months of treatment with a 3-year follow-up. The study will also include an assessment of cost-effectiveness. Originally expected to report findings in 2020, it has recently been announced that the trial has closed for recruitment early following interim analysis showing a favourable effect for adalimumab. Analysis of the primary outcome is under way and key findings will therefore be available earlier than expected. Collection and analysis of health economic data will continue as planned.
Adalimumab ongoing trial 3
This third adalimumab RCT [Effect of Adalimumab for the Treatment of Uveitis in Juvenile Idiopathic Arthritis (ADJUVITE); NCT01385826] is also currently in progress and is not expected to report findings until June 2016. The study is set in France (seven hospital ophthalmology departments) and assesses the efficacy of 2-month adalimumab treatment versus placebo treatment on reduction of ocular inflammation quantified by laser flare photometry in patients aged ≥ 4 years, with JIA-associated uveitis that is resistant to steroid therapy. The investigators plan to include 40 patients, follow-up appears to be 12 months and the final data collection date for the primary outcome measure is November 2015. The primary endpoint of this study is improvement of uveitis.
Etanercept ongoing trial 1
This is a RCT evaluating the efficacy of etanercept in 124 Chinese JIA patients (no clinical trial registration number has been found for this study). 98 No full paper appears to have been published so far and only one conference abstract was identified, which includes very limited information. The abstract states that a ‘randomised principle was applied’ to divide the JIA patients into a control and a treatment group. Although no baseline characteristics were reported, the authors of the abstract state that there were no significant differences of clinical classification and basic treatment between the groups.
Sixty-two patients in the treatment group (oligoarticular JIA n = 17, polyarticular JIA n = 15 and systemic JIA n = 30) received 0.8 mg/kg per week of subcutaneous etanercept for 6 months. No details for the control group are reported.
American College of Rheumatology Pedi-30, -50 and -70 responses were used to assess the clinical efficacy (primary outcome not stated) and adverse reactions were recorded.
Results
The authors state that the remission rates ‘of different cases’ (this is presumed to mean different types of JIA) in the treatment group differed at each time point (3- and 6-month time points are mentioned), with no obvious difference in ACR Pedi-30, -50 and -70 remissions for patients with oligoarticular and polyarticular JIA. Eighty per cent of these patients had ACR Pedi-50 remission after 6 months of treatment and > 50% had ACR Pedi-70 remission. The remission rate of systemic JIA cases was lower than the two other types (data not extracted). Although the differences between the randomised groups are said to be significant, no data were reported.
Adverse events
There were no reported AEs for patients with oligoarticular or polyarticular JIA. Details of AEs for the systemic JIA subgroup are reported (not extracted).
Etanercept ongoing trial 2
The second placebo-controlled etanercept RCT [Remission Induction by Etanercept in Enthesitis related Arthritis JIA-Patients (REMINDER) Study, EudraCT Number: 2010–020423–51] was identified from the search of ongoing clinical trials registers. The trial is set in Germany and has a start date of February 2016. This study has a withdrawal RCT design, with a 12-week open-label treatment phase prior to the controlled randomised double-blind phase The study will assess the safety and effectiveness of etanercept in patients diagnosed with ERA-JIA age ≥ 6 years and < 18 years having met all criteria for eligibility for treatment with etanercept according to SPC and local guidelines, with expectation of the requirement of a minimum of five affected joints. The online record does not provide the treatment time for the double-blind phase or report any follow-up period. The primary endpoint of the study will be inactive disease of ERA-JIA.
Etanercept ongoing trial 3
The third multicentre etanercept RCT was identified from a conference abstract103 which does not provide a clinical trial registration number. This trial appears to be set in Germany, has enrolled patients with ERA and has a withdrawal design, with a 24-week open-label etanercept treatment phase prior to the 24-week placebo-controlled double-blind withdrawal phase. Patients had to achieve at least an ACR Pedi-30 response in order to be randomised to the double-blind phase (terminated in case of a disease flare or at week 48, whichever occurs earlier). No details of the study’s inclusion or exclusion criteria are reported in the abstract.
Results
Forty-one patients entered the open-label phase, of whom two patients discontinued prematurely (one owing to intolerance and one owing to protocol deviation). Thirty-eight patients (93%) achieved at least an ACR Pedi-30 response and were randomised to the double-blind phase. As can be seen in Table 32, during the double-blind phase the majority of flares occurred in the placebo group, with 10 (56%) placebo-treated patients compared with 18 (90%) etanercept-treated patients reaching week 48 without a flare [odds ratio 7.2 placebo vs. etanercept (1.3 to 40.7; although not stated, this is presumed to be a 95% CI); p = 0.016].
| Time | ETA (n = 20) | PBO (n = 18) |
|---|---|---|
| Week 28 | 2 | 2 |
| Week 32 | 0 | 4 |
| Week 48 | 0 | 2 |
The authors state that in patients continuously treated with etanercept at week 48 (n = 20), JADAS 10 decreased to a mean of 3.4 (17.4 to 1.9 at week 24), with 12 (60%) patients reaching JADAS-minimal disease activity and 11 (55%) JADAS remission. The equivalent data for the placebo group are not reported.
Adverse events
There were 166 AEs in 39 patients and three SAEs. All were said to be considered unrelated and resolved without sequelae. It is unclear if these data are for the combined open-label and double-blind phases, but considering the number of patients reported (n = 39) this may indeed be the case.
Etanercept ongoing trial 4
The fourth multicentre etanercept RCT (set in the Netherlands) assessed when and in whom to stop etanercept after successful treatment of JIA (NCT01287715) and was identified from the search of ongoing clinical trials registers. Estimated enrolment was 50, the study completed in September 2013 and final data collection for the primary outcome measure is reported as September 2012 in the online record. No publication of data has been identified. Patients aged 4–17 years and in remission were selected from the National Arthritis and Biologicals in Children (ABC) register, an observational study including all Dutch JIA patients on etanercept therapy. The inclusion criteria states no or low dose of methotrexate and it is therefore unclear if patients were intolerant or had a previous inadequate response to methotrexate. All JIA subtypes were included in the study. Patients were randomised to a stop arm (discontinuation of etanercept – half of the dose for 3 months and discontinuation thereafter) or a control arm (etanercept continued for another 9 months and, if still meeting the eligibility criteria, discontinued thereafter). The primary outcome of the study was flare rate.
Additional supporting non-randomised evidence
This section includes additional non-randomised study evidence relating to aspects of JIA where adequate RCT data in the systematic review of clinical effectiveness were lacking. This evidence has been identified from the systematic review search itself, and from relevant studies included in the CSs to NICE (see Review of clinical effectiveness in company submissions to the National Institute for Health and Care Excellence). Evidence relating to two aspects is presented: JIA ERA and PA subtypes, and JIA-associated uveitis.
Enthesitis-related arthritis and psoriatic arthritis
The most informative study available for these subtypes is the CLIPPER study. 99
This is a single-arm, Phase-IIIb open-label, multicentre interventional study funded by Wyeth (subsequently acquired by Pfizer Inc.). The study was designed to assess the safety and efficacy of etanercept in children and adolescents with three JIA subtypes classified using the ILAR criteria: EO, ERA and PA. There are two parts to the study. Part 1 (which has been published99) has investigated the efficacy and safety of etanercept in the three JIA subtypes over an initial 12-week period with a primary endpoint of the percentage of patients achieving ACR Pedi-30 criteria at week 12. Part 2 of the study is a 96-week OLE, assessing the long-term safety and efficacy of etanercept in JIA subtypes, which is currently published in poster format only. 104 This study formed part of the evidence base supporting the licence extension for etanercept across the JIA subtypes in 2012. The assessment group has extracted 12-week data from the published paper99 on ACR Pedi response rates and inactive disease, change in CHAQ score, PGA of pain, number of active joints, number of joints with LOM and JIA category-specific assessments (data at week 12 compared with historical placebo data, historical active control data and data from a meta-analysis have not been data extracted). Data from the conference poster104 on ACR Pedi response rates and inactive disease have also been extracted and this summary is supplemented with some data presented in the Pfizer CS.
The study included 127 patients with the JIA subtypes of ERA (n = 38, age 12–17 years), EO (n = 60, age 2–17 years) and PA (n = 29, age 12–17 years) who received 0.8 mg/kg of etanercept once weekly (maximum dose 50 mg/week). Key inclusion criteria were ≥ 2 active joints (swollen or LOM accompanied by either pain or tenderness); history of intolerance or unsatisfactory response to at least a 3-month course of ≥ 1 DMARD or, for ERA only, unsatisfactory response to at least a 1-month course of ≥ 1 NSAID (i.e. for ERA, prior methotrexate treatment was not required). A stable dose of concomitant medication (only one DMARD, one oral corticosteroid and one NSAID) was permitted. The inclusion criteria extended below the threshold number of active joints for the classification of polyarticular disease. Key exclusion criteria included other rheumatic diseases, active uveitis within 6 months of baseline and any prior receipt of biologic DMARDs. A total of five patients failed to complete part 1 of the study (completed part 1: EO 97%, ERA 95% and PA 97%) and 13 patients part 2 (completed part 2: EO 90%, ERA 79% and PA 86%).
Key baseline characteristics can be seen in Table 33 (additional baseline characteristics are available in the published paper99).
| Parameter [mean (SD) unless stated otherwise] | All patients (n = 127) | EO (n = 60) | ERA (n = 38) | PA (n = 29) |
|---|---|---|---|---|
| Age, years | 11.7 (4.5) | 8.6 (4.6) | 14.5 (1.6) | 14.5 (2.0) |
| Female, % | 56.7 | 68.3 | 21.1 | 79.3 |
| JIA duration | 26.8 (26.4) | 31.6 (31.7) | 23.0 (19.8) | 21.8 (20.2) |
| Age at onset, months | 9.5 (4.8) | 6.1 (4.5) | 12.5 (2.1) | 12.6 (2.7) |
| Concomitant medication use, n (%) | ||||
| Any DMARD | 109 (85.8) | 54 (90.0) | 32 (84.2) | 23 (79.3) |
| Oral corticosteroid | 16 (12.6) | 7 (11.7) | 8 (21.1) | 1 (3.5) |
| Oral NSAID | 74 (58.3) | 32 (53.3) | 26 (68.4) | 16 (55.2) |
| Number of active joints | 6.7 (4.6) | 7.6 (5.1) | 5.2 (3.6) | 7.0 (4.3) |
| Number of joints with LOM | 5.7 (4.2) | 6.3 (4.4) | 4.8 (4.0) | 5.6 (4.1) |
| Number of painful joints | 6.4 (5.2) | 5.5 (4.1) | 6.7 (4.9) | 7.8 (7.0) |
| Number of swollen joints | 5.5 (4.2) | 6.5 (4.8) | 3.8 (2.8) | 5.6 (3.7) |
| CHAQ scorea | 0.8 (0.6) | 0.9 (0.7) | 0.7 (0.5) | 0.7 (0.6) |
| Parent global assessment pain (VAS) | 5.1 (2.5) | 4.8 (2.6) | 5.8 (25) | 4.6 (2.3) |
| JIA category-specific characteristics | ||||
| Tender entheseal score | 5.9 (9.4) | |||
| Overall back pain VAS, mm | 25.9 (28.0) | |||
| Nocturnal back pain VAS, mm | 16.4 (27.8) | |||
| Modified Schober’s test, cm | 15.0 (1.9) | |||
| Psoriasis BSA, % | 10.4 (13.4) | |||
| Parent global assessment of psoriasis | 1.8 (1.4) | |||
Patients with EO and PA had a higher number of active joints and number of joints with LOM [EO: mean 7.6 (SD 5.1) and 6.3 (SD 4.4), respectively; PA: mean 7.0 (SD 4.3) and 5.6 (SD 4.1), respectively] at baseline than ERA patients [mean 5.2 (SD 3.6) and 4.8 (SD 4.0), respectively]. The number of painful joints was highest in PA patients [mean 7.8 (SD 7.0)] compared with the other two subgroups, and the number of swollen joints was the lowest in ERA patients [mean 3.8 (SD 2.8)] (Table 34). Mean CHAQ subgroup scores ranged between 0.7 and 0.9, and the parent global assessment of pain VAS ranged between 4.6 and 5.8. Also reported are JIA category-specific assessments [ERA: tender entheseal score, back pain (VAS) and modified Schober’s test; PA: body surface area and PGA of psoriasis] at baseline. Limitations of the study noted by the authors were the difference in concomitant medication use at baseline which may have affected efficacy responses and the lower age limit of 12 years set for inclusion of EO patients (the licensed indication is from 2 years of age).
| Parameter | All patients (n = 127) | EO (n = 60) | ERA (n = 38) | PA (n = 29) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| Week 12 | ||||||||
| ACR Pedi-30 | CiC information has been removed | 88.6 (81.6 to 93.6) | CiC information has been removed | 89.7 (78.8 to 96.1) | CiC information has been removed | 83.3 (67.2 to 93.6) | CiC information has been removed | 93.1 (77.2 to 99.2) |
| ACR Pedi-50 | 81.1 (73.1 to 87.7) | CiC information has been removed | CiC information has been removed | CiC information has been removed | ||||
| ACR Pedi-70 | 61.5 (52.2 to 70.1) | |||||||
| ACR Pedi-90 | 29.8 (21.8 to 38.7) | |||||||
| Inactive disease | 12.1 (6.9 to 19.2) | 11.9 (4.9 to 22.9) | 16.7 (6.4 to 32.8) | 6.9 (0.8 to 22.8) | ||||
| Week 96a | All patients (n = 108), % (95% CI) | EOb (n = 53), % | ERAb (n = 30), % | PAb (n = 25), % | ||||
| ACR Pedi-30 | 99.1 (94.9 to 100) | 99 | 100 | 98 | ||||
| ACR Pedi-50 | 98.1 (93.5 to 99.8) | 99 | 97 | 98 | ||||
| ACR Pedi-70 | 92b | 94 | 87 | 98 | ||||
| ACR Pedi-90 | 65.4 (55.6 to 74.4) (n = 107) | 62 | 67 | 72 (n = 24) | ||||
| ACR Pedi-100 | 54b (n = 107) | 54 | 51 | 60 (n = 24) | ||||
| Inactive disease | 34 (25.0 to 43.8) (n = 106) | 37 | 29 (n = 28) | 29 | ||||
Results
At week 12, the overall ACR Pedi-30 response rate for patients was almost 89%, with response for the separate JIA disease type subgroups varying from around 83% to 93% (see Table 34). The overall ACR Pedi-90 response rate for patients was just under 30%, and 12% of patients had inactive disease. JIA disease subgroups varied in ACR Pedi-90 response rates between (CiC information has been removed). At week 12, 12%, 17% and 7% of EO, ERA and PA patients, respectively, had inactive disease, as can be seen in Table 34.
By week 96, around 99% of all patients achieved a ACR Pedi-30 response and > 65% an ACR Pedi-90 response. Thirty-four per cent of all patients had inactive disease. Overall, patients in the ERA subgroup appeared to have received the greatest benefit from etanercept therapy at 12 weeks, but by 96 weeks the subgroups achieved similar levels of ACR Pedi-90 (62% to 72%) and ACR Pedi-100 (51% to 60%) responses. Inactive disease at 96 weeks varied between 29% (ERA and PA) and 37% (EO). (CiC information has been removed.)
At week 12 mean change from baseline for CHAQ scores were similar for the subgroups (improvement of 51% to 58%), but there were differences in the parent global assessment of pain VAS. The lowest mean change in pain VAS occurred in patients with ERA and PA (decreases of 45% and 47%, respectively) compared with patients with EO (59% decrease). The mean decreases from baseline for the number of active joints ranged between 70% and 78% and between 64% and 72% for the number of joints with LOM (Table 35). It is unclear why details about changes from baseline for the number of painful and the number of swollen joints are not reported.
| Parameter | Overall (n = 123), mean (95% CI) [% change] | EO (n = 58), mean (95% CI) [% change] | ERA (n = 36), mean (95% CI) [% change] | PA (n = 29), mean (95% CI) [% change] |
|---|---|---|---|---|
| CHAQ | −0.5 (−0.6 to −0.4) [−53.6] | −0.5 (−0.7 to −0.4) [−52.2] | −0.5 (−0.7 to −0.3) [−57.8] | −0.4 (−0.6 to −0.2) [−51.3] |
| Parent global assessment of child’s pain VAS | −3.0 (−3.5 to −2.6) [−51.9] | −3.2 (−3.8 to −2.5) [−58.9] | −3.2 (−4.2 to −2.2) [−44.9] | −2.6 (−3.4 to −1.8) [−46.6] |
| Number of active joints | −5.1 (−5.8 to −4.3) [−73.0] | −5.5 (−6.7 to −4.2) [−69.8] | −4.3 (−5.4 to −3.1) [−77.7] | −5.2 (−6.8 to −3.6) [−73.8] |
| Number of joints with LOM | −4.1 (−4.8 to −3.4) [−66.9] | −4.5 (−5.6 to −3.3) [−64.1] | −3.4 (−4.1 to −2.6) [−67.4] | −4.3 (−5.7 to −2.9) [−71.7] |
| JIA category-specific assessments | ||||
| Tender entheseal score | −4.4 (−6.3 to −2.4) [−57.8] | |||
| Overall back pain VAS, mm | −12.5 (−21.3 to −3.7) [−21.2] | |||
| Nocturnal back pain VAS, mm | −8.9 (−16.7 to −1.2) [−6.8] | |||
| Modified Schober’s test, cm | 0.35a (−0.02 to 0.72) [9.7] (n = 35) | |||
| Psoriasis BSA, % | −6.7 (−10.6 to −2.9) [−48.2] | |||
| PGA of psoriasis | −1.0 (−1.4 to −0.6) [−39.6] (n = 28) | |||
The greatest improvements in JIA category-specific assessments was a 58% improvement from baseline in tender entheseal score at 12 weeks in patients with ERA, and for patients with PA a 48% improvement in body surface area of psoriasis and a 40% improvement in PGA of psoriasis (see Table 35).
Adverse events
The highest number of treatment-emergent AEs per patient-year of etanercept exposure occurred in the ERA subgroup (1.827; EO: 1.313; PA: 1.036), which also had the lowest number of treatment-emergent infections per patient-year of etanercept treatment (0.979; EO subgroup: 2.114; PA: 1.514). As Table 36 illustrates, patients with ERA appear to experience more treatment-related injection-site reactions than patients with either EO or PAA.
| All values are reported as number of events (events per patient-year of exposure to etanercept) unless otherwise stated | Overall (n = 127) EXP = 215.086 | EO (n = 60) EXP = 103.603 | ERA (n = 38) EXP = 61.298 | PA (n = 29) EXP = 50.185 |
|---|---|---|---|---|
| Treatment-emergent AEsa | 300 (1.395) | 136 (1.313) | 112 (1.827) | 52 (1.036) |
| Treatment-emergent infections | 355 (1.651) | 219 (2.114) | 60 (0.979) | 76 (1.514) |
| Treatment-emergent ISRs | 63 (0.293) | 22 (0.212) | 29 (0.473) | 12 (0.239) |
| Treatment-emergent AEs causing withdrawal, n (%)a | 3 (2.4) | 0 | 3 (7.9) | 0 |
| Treatment-emergent infections causing withdrawal, n (%) | 2 (1.6) | 1 (1.7) | 0 | 1 (3.4) |
| Serious treatment-emergent AEsa | 16 (0.074) | 2 (0.019) | 11 (0.179) | 3 (0.060) |
| Serious treatment-emergent infections | 10 (0.046) | 4 (0.039) | 3 (0.049) | 3 (0.060) |
| Opportunistic infectionsb | 1 (0.005) | 0 | 1 (0.016) | 0 |
| Infections considered preventable by vaccination in patients not previously vaccinated | 7 (0.033) | 5 (0.048) | 1 (0.016) | 1 (0.020) |
| Infections considered preventable by vaccination in patients previously vaccinated | 1 (0.005) | 1 (0.010)c | 0 | 0 |
| Treatment-emergent autoimmune disordersd | 4 (0.019) | 1 (0.010) | 2 (0.033) | 1 (0.020) |
Treatment-emergent AEs leading to patient withdrawal occurred only in the ERA subgroup (events 3, etanercept exposure 7.9), and there were two events of treatment emergent infections causing withdrawal (one each in the EO and PA groups). The rate of serious treatment-emergent AEs and serious treatment-emergent infections appears to be low in all three subgroups (see Table 36), as does the rate of treatment-emergent autoimmune disorder events. Of the two cases of uveitis (EO n = 1, PA n = 1), one was reported in a patient with EO after 7.8 months of etanercept plus methotrexate. This resolved and the patient completed the 96-week study. There were a total of three cases of Crohn’s disease in patients with ERA, of which two cases were considered to be unrelated to etanercept therapy.
Juvenile idiopathic arthritis-associated uveitis
As stated earlier, the effects of biologic DMARD on extra-articular manifestations such as uveitis were not assessed by the included RCTs. However, evidence from non-randomised studies is available, as summarised by systematic reviews.
A recently published systematic review by Simonini and colleagues96 assessed the effectiveness of antiTNF drugs for childhood uveitis. To be included, studies had to include patients with autoimmune uveitis refractory to topical and/or systemic steroids and at least one immunosuppressive therapy (e.g. methotrexate). The antiTNFα drugs of relevance to the review were etanercept, infliximab and adalimumab (infliximab is not within the scope of this NICE appraisal). The primary outcome was improvement in intraocular inflammation, with additional outcomes including tapering/stopping systemic steroid administration, improvement in visual acuity and treatment discontinuation among others. A number of bibliographic databases were searched from January 2000 to October 2012.
The review included 23 studies, mainly retrospective chart reviews with very small patient numbers. Of these 23 studies, only 7 were conducted exclusively in JIA uveitis patients (one RCT of etanercept; two retrospective studies of etanercept; two retrospective studies of infliximab; and two retrospective studies of adalimumab). Eleven studies comprised mixed study populations with uveitis associated with a range of conditions, including JIA. The remaining five studies included populations that did not include any children with JIA. It was not possible to analyse results separately by uveitis-associated condition. However, of the 229 children included across all the studies, 152 had chronic uveitis associated with JIA. The results can therefore be interpreted as being generally relevant to JIA uveitis.
A pooled analysis of the observational studies found that adalimumab and infliximab were more efficacious at improving intraocular inflammation than etanercept. The proportion of children with improved intraocular inflammation (responders) was 87% (95% CI 75% to 98%) for adalimumab, 72% (95% CI 64% to 79%) for infliximab, and 33% (95% CI 19% to 47%) for etanercept. There was no statistically significant difference in the proportion of responders between adalimumab and infliximab (p = 0.08), but there was a significant difference for both compared with etanercept (p = 0.001 for both comparisons).
Simonini and colleagues96 did not pool the results of the single RCT identified in the systematic review with the observational studies. 56 This was a small RCT (n = 12 children) of treatment with etanercept. The authors state that this study did not report substantial benefits for the biologic treatment. (Owing to limitations in reporting, this RCT was judged to be unclear for inclusion in our systematic review of clinical effectiveness, as it was not clear whether or not the etanercept was given within its licensed indication). Caution is advised in the interpretation of the findings of the Simonini and colleagues96 systematic review given the weaknesses of the study designs included.
The assessment group are aware of only one other recent systematic review of biologic DMARD treatment of children with uveitis, by Cordero-Coma and colleagues. 105 The most recent search date for literature was October 2011. This review had a broader inclusion criteria than that of Simonini and colleagues96 and a total of 61 studies was included. Again, much of the included evidence was from observational studies. A total of 14 studies assessed adalimumab, 11 assessed etanercept and 50 studies assessed infliximab [studies assessing certolizumab and golimumab were also included]. Of the 1093 patients included across the studies, 316 (30%) were classed as having JIA uveitis. The review does not provide any formal synthesis and quantification of the effectiveness of treatment, but provides a narrative conclusion for each biologic DMARD and a level of evidence. Adalimumab and infliximab were considered by the authors to be effective in autoimmune uveitis, both based on level-2b evidence (individual cohort study or low-quality RCT). Etanercept was judged ineffective, based on level-1b evidence (individual RCTs with narrow CIs).
The Abbvie CS to NICE77 provides narrative summaries of five selected studies published since the Simonini and colleagues96 systematic review. All of them were observational in design (three case series;106–108 one Italian registry-based study;109 one comparative cohort study110), and all assessed treatment with adalimumab (with one also assessing infliximab110 in uveitis patient populations with varying proportions of JIA uveitis. We have not performed an independent critical appraisal of these studies in this assessment report. From the summaries given it appears that adalimumab is associated with improvements in intraocular inflammation and visual acuity, and a decrease in use of corticosteroids. AEs appeared to be minor. The other CSs to NICE did not present much detail of studies of treatment of JIA uveitis with other biologic DMARDs.
In summary, the evidence from observational studies suggests that biologic DMARDs can improve uveitis symptoms in children with JIA, such as intraocular inflammation. Adalimumab and infliximab appear to be more effective than etanercept in improving uveitis. The effects of the treatments in terms of arthritis outcomes in JIA uveitis patients have not been reported. As noted above (see Ongoing trials), the UK-based SYCAMORE RCT87 has investigated adalimumab in the treatment of JIA uveitis patients, and the results of the trial (which will be available sooner than expected, although an exact date has not been specified) will provide more rigorous evidence for effectiveness than that currently available.
Chapter 5 Economic analysis
Introduction
The aim of the economic evaluation is to assess the cost-effectiveness of abatacept, adalimumab, etanercept and tocilizumab for people with JIA compared with alternative treatments. The economic evaluation comprises:
-
a systematic review of the cost-effectiveness of biologic DMARDs for people with JIA
-
a systematic review of studies of the HRQoL of people with JIA
-
a critical appraisal of the submissions from the relevant drug companies received as part of the NICE appraisal process
-
a de novo economic model and cost-effectiveness evaluation developed by Southampton Health Technology Assessments Centre (SHTAC) to inform the NICE appraisal.
Systematic review of cost-effectiveness evidence
Methods for the systematic review
A systematic literature search was undertaken to identify economic evaluations of the biologic DMARDs, within the NICE scope for this appraisal. Studies were included if they were full economic evaluations (cost-effectiveness, cost–utility, cost–consequence or cost–benefit analyses) conducted in children and young people with JIA that compared one or more biologics with a DMARD, such as methotrexate. Studies that were not reported in the English language or that did not provide sufficient information on the model structure, data and results were excluded. This systematic review aimed to summarise the currently available evidence and inform the construction of a de novo model.
Results of the systematic review
Searches for economic evaluations identified 387 potentially relevant references, and a further study was identified through ad hoc searching. The full texts for 17 papers were retrieved for further screening. A summary of the selection process and the reasons for exclusion are presented in Figure 6 and a list of excluded studies can be found in Appendix 6. Although seven studies reported as abstracts appeared to meet the a priori inclusion criteria, they did not contain sufficient information on the methods used and the results to permit formal data extraction or critical appraisal. 111–117 Five studies were found not to be economic evaluations. 118–122 Four studies were included, described in a total of five publications. 123–127 The characteristics of the four included economic evaluations are shown in Table 37. Data extraction forms for the studies can be found in Appendix 7.
FIGURE 6.
Flow chart of identification of studies for inclusion in the review of cost-effectiveness. a, including one study found through hand searching; b, the abstracts provided insufficient details of methods and results to allow inclusion in the systematic review.
| Characteristic | Cummins et al. (2002)123 | Prince et al. (2011)124 | Simpson et al. (2012)125 | Costa et al. (2010)126 and Ungar et al. (2011)127 |
|---|---|---|---|---|
| Country | UK | Netherlands | Russia | Canada |
| Funding source | UK HTA programme | Dutch Board of Health Insurance and Wyeth International | Not stated | Ontario Ministry of Health and Long-term Care Drug Innovation Fund |
| Analysis type | Cost–utility analysis | Cost–consequence analysis | Cost–utility analysis | Cost-effectiveness analysis |
| Perspective | Health-care system | Health-care system | Health-care system and societal | Societal |
| Study population | Children with polyarticular juvenile rheumatoid arthritis | Dutch JIA patients younger than 18 years eligible for treatment with etanercept; various types of JIA | Patients from adalimumab trial:61 children aged 4–17 years with JIA | Patients with JIA with a prior inadequate response to, or intolerance of, DMARDs |
| Intervention(s) | Etanercept | Etanercept | Adalimumab | Etanercept, adalimumab, abatacept and infliximab vs. methotrexate |
| Intervention effect | Effect size measured in terms of CHAQ and mortality. Cost per HAQ point | Six response variables measured, including overall assessment of well-being, CHAQ score and number of active joints. HUI3 also measured | CHAQ scores and active joint counts | Proportion of patients who had a reduction in symptoms at 1 year according to the ACR Pedi-30 criteria |
| Currency base | UK pounds (GBP, £) | Euros (EUR, €) | Russian roubles (RUB) | Canadian dollars (CAD, $) |
| Model type, health states | Not clear | None | Markov model | Decision-analysis model |
| Time horizon | Life course | 27 months | 7 years/lifetime | 1 year |
| Base-case results | Incremental cost £28,022; incremental effectiveness in terms of QALY 1.7; ICER £16,082 Sensitivity analysis ICER varied from £3900 to £34,000 |
HUI3 score increases from 0.53 to 0.78 after 28 months; total direct medical costs were €12,478 per patient-year after start of etanercept compared with €3720 before start | For a lifetime horizon, the incremental cost–utility ratio for adalimumab vs. conventional non-biologic therapy was 1,571,500 RUB/QALY | Costs per ACR Pedi-30 responder were $26,061, $46,711, $16,204 and $31,209 for etanercept, adalimumab, abatacept and infliximab, respectively, compared with methotrexate |
Critical appraisal of the studies
The cost-effectiveness studies were assessed using a critical appraisal checklist (Table 38). The checklist assessed study quality and generalisability to the UK. The checklist was adapted by the review authors from checklists by Philips and colleagues,128 Drummond and colleagues129 and methodological requirements stated in the NICE reference case. 130
| Item | Cummins et al. (2002)123 | Prince et al. (2011)124 | Simpson et al. (2012)125 | Costa et al. (2010)126 and Ungar et al. (2011)127 |
|---|---|---|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Yes | Yes | Yes | Yes |
| 2. Is the setting comparable to the UK? | Yes | Unclear | Unclear | No |
| 3. Is the analytical and modelling methodology appropriate? | No | Yesa | Yes | Yes |
| 4. Are all the relevant costs and consequences for each alternative identified? | No | Yes | Yes | Yes |
| 5. Are the data inputs for the model described and justified? | Yes | N/A | No | Yes |
| 6. Are health outcomes measured in QALYs? | Yes | No | Yes | No |
| 7. Is the time horizon considered appropriate? | Yes | No | Yes | No |
| 8. Are costs and outcomes discounted? | Yes | No | Yes | No |
| 9. Is an incremental analysis performed? | Yes | Uncleara | Yes | Yes |
| 10. Is uncertainty assessed? | Yes | Noa | Yes | Yes |
The Cummins and colleagues study was conducted in the UK,123 whereas the generalisability of the other studies to the NHS is unclear. The other studies were conducted in the Netherlands,124 Russia125 and Canada;127 used appropriate modelling methodology; and included relevant costs.
In terms of the analytical and modelling methodology used, the studies were generally considered appropriate, except for the model reported in Cummins and colleagues,123 which was based upon a number of questionable assumptions as a result of limitations in the data available at the time.
The data inputs for the model were clearly described and justified by two studies,123,127 but the description of some of the data inputs are missing from Simpson and colleagues. 125 Prince and colleagues124 conducted a cost–consequence analysis based on a prospective observational study that collected cost and utility data. The study did not measure quality-adjusted life-years (QALYs).
Two of the studies, Cummins and colleagues123 and Simpson and colleagues,125 used appropriate time horizons, measured the health outcomes in QALYs and discounted costs and outcomes. The model by Ungar and colleagues127 did not use QALYs in the model and used a 1-year time horizon, eliminating the need for discounting.
All three modelling studies analysed results incrementally and assessed uncertainty through sensitivity analyses. 123,125,127
In summary, the cost-effectiveness studies have certain limitations with regard to methodology, reporting of results or generalisability to the UK NHS (see Table 38).
Cummins and colleagues123
Approach
The Cummins and colleagues123 study consisted of a HTA conducted as part of the NICE appraisal of etanercept for JIA (NICE TA35). 131 The HTA includes a systematic review of clinical effectiveness and a critical appraisal of a CS to NICE from Wyeth Laboratories (manufacturer of etanercept). The HTA does not provide an independent economic model owing to considerable uncertainties in the available evidence for JIA at that time. The CS contained a cost–utility analysis of etanercept in patients with JIA, compared with other treatment options. The cost–utility model was based upon a model developed for rheumatoid arthritis in adults. The model used the results from the etanercept RCT by Lovell and colleagues. 42 The model assumed a positive linear relationship between the Health Assessment Questionnaire (HAQ) score and costs, modelling responders, non-responders and deaths at each time point.
The model used European Quality of Life-5 Dimensions (EQ-5D™) values derived from mapping HAQ values in adult rheumatoid arthritis patients. Mortality was related to HAQ values, with a 38% increase in mortality per unit change in HAQ. The model assumed a RR of mortality in JIA.
Estimation of effectiveness
The HAQ progression rate was 0 for responders for 0–4 years, 0.034 for responders after 4 years and 0.0669 for non-responders. No definition was given for response.
Cummins and colleagues123 reported the evidence limitations attributable to limited or non-existent long-term data on efficacy and lifelong impacts of the disease and treatment. The Juvenile Rheumatoid Arthritis-30 efficacy measure was assumed to be equivalent to ACR Pedi-20, HAQ and CHAQ were assumed equivalent, and utility and mortality were derived from an adult rheumatic arthritis trial. Owing to limited evidence on potential adverse effects, disease progression and long-term prognosis for treatment-resistant JIA, assumptions were made in the economic evaluation that are insufficiently supported. The authors of the review expressed concerns about the validity of the economic model and the assumptions made to extrapolate beyond the limited evidence base.
Estimation of quality-adjusted life-years
Utility values were derived from EQ-5D estimates for adults with rheumatoid arthritis, as there was limited evidence on HRQoL in JIA. The model assumed that the HAQ was equivalent to CHAQ and that adult values were therefore appropriate for children. The HAQ score for the placebo arm was 1.3 at baseline and 1.2 after 7 months, and the HAQ score for the etanercept arm was 1.6 at baseline and 0.8 at 7 months (lower scores indicate better health). In the base case, the model reported a 1.7 incremental QALY gain in favour of etanercept. However, this result was questioned by Cummins and colleagues123 owing to the limitations in the evidence for HRQoL.
Estimation of costs
Resource use was considered similar to that for the adult rheumatoid arthritis population. Information regarding resource use was not available from the JIA etanercept trial. 42 Costs were discounted at 6% per annum and benefits at 1% per annum. The cost offset per HAQ point was £860.
Results
The incremental QALYs were 1.74 for the patients on etanercept compared with placebo. The incremental cost-effectiveness ratio (ICER) was £16,082 per QALY gained in the base-case analysis, and in the sensitivity analyses ICERs ranged between £3900 (cost-offsets assumption changed to exclude nursing home and home help costs but to include indirect costs) and £34,000 (Short Form questionnaire-36 items regression used). Probabilistic results were not reported.
Key issues
-
There were concerns about the validity of the results owing to a lack of suitable evidence for model input parameters, particularly with regard to HRQoL.
Prince and colleagues124
Approach
Prince and colleagues124 reported a cost–consequence analysis of etanercept therapy in patients with JIA in the Netherlands, who had an insufficient response to the maximum dose of methotrexate. Forty-nine JIA patients were evaluated at start of treatment and after 3, 15 and 27 months of therapy from the National ABC register. For all included patients, data were collected on the use of etanercept, disease activity and HRQoL. Most of the patients had polyarticular JIA (45%), followed by EO (22%) and systemic JIA (22%). The remainder had ERA (4%) or juvenile arthritis psoriatica (6%). The median age of patients at the start of etanercept treatment was 11.6 years and median disease duration was 3.6 years.
Estimation of effectiveness
The outcome measure used to assess disease activity consisted of six response variables: (1) overall assessment of disease activity by the physician by means of the VAS; (2) CHAQ by the patient or parent; (3) overall assessment of well-being by the patient or parent through the VAS; (4) number of active joints (joints with swelling and/or limited motion with pain or tenderness); (5) number of joints with limited motion; and (6) a laboratory marker of inflammation, ESR.
After 3 months’ use of etanercept, the mean number of active joints decreased from 16.7 to 3.99 per person and the CHAQ score decreased from 1.70 to 1.00. These outcomes further improved at 27 months to 2.45 active joints per person and a CHAQ score of 0.50 (lower score indicates better outcome).
Estimation of quality-adjusted life-years
Health-related quality-of-life data were collected for patients in the study using the Health Utilities Index (HUI)-3. The questionnaire consists of eight health domains: vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain, each with five or six levels representing the range of functioning. HRQoL was collected by proxy by the parents of the study participants because children were considered unable to value health states. The HRQoL data are reported in more detail in the systematic review of HRQoL studies (see Systematic review of health-related quality-of-life studies).
Estimation of costs
Costs were collected for direct medical costs (i.e. medication, diagnostic and hospitalisation costs). The base year was 2008 for all costs, with costs retrieved from other years converted to 2008 euros using the general Dutch price index rate. Unit costs for medication were retrieved from the Pharmacotherapeutic Compass provided by the Dutch Board of Health Insurances, and treatment costs were calculated with the exact dose of medication and administration period as reported in the patients’ files. Prices for all hospital-related costs were based on real prices from the co-ordinating centre (Erasmus MC Sophia Children’s Hospital Rotterdam, the Netherlands). The etanercept unit cost was estimated at €10,478 per year.
Results
Mean total direct medical costs after the start of etanercept were, on average, €12,478 per patient-year compared with €3720 before the start of etanercept treatment. The utility for patients was 0.53 before start of etanercept treatment and increased to 0.78 over 27 months of etanercept treatment.
Key issues
-
The study does not report cost-effectiveness.
-
The study was based in the Netherlands, so unclear how generalisable results are to the NHS.
Simpson and colleagues125
Approach
Simpson and colleagues125 reported results from a Markov model developed to assess the cost-effectiveness of adalimumab relative to methotrexate for the treatment of JIA. Cost-effectiveness analyses were performed from the perspective of the Russian health-care system (base model) and society as a whole (secondary model). The base-case model reported outcomes for a cohort of 100 children with a mean age of 11 years. Sensitivity analyses assessed the variation in the age of children at treatment initiation. The model has two parts. The first part followed children from 11 to 18 years of age with 4-month long cycles. This part of the model used data derived from the adalimumab RCT (Lovell and colleagues61). Additional analyses followed patients aged 7 years at treatment initiation for a period of 11 years. The second part of the model was derived from the literature on adult rheumatoid arthritis and modelled the remaining lifetime of the patients (age > 18 years). The adalimumab RCT61 compared adalimumab plus methotrexate with placebo plus methotrexate for the treatment of JIA in children aged 4–17 years (further detail of this trial can be found in Chapter 4).
Disease activity was defined as mild, moderate or severe using the CHAQ scores and active joint counts from the adalimumab RCT. 61 Health states from the childhood model were used to capture the effects of joint damage and the need for hip replacement for the treatment of JIA in adulthood (> 18 years). The base model included five health states (remission no disease, remission disease, activity mild, activity moderate and activity severe). The remission disease group was introduced to capture the effect of joint damage and the need for joint replacement.
Estimation of effectiveness
Effectiveness estimates were based on observed changes from the adalimumab trial,61 assessed using the CHAQ. These effects within the model were translated to HUI2 utility values, using a mapping algorithm developed by the authors. 132
Estimation of quality-adjusted life-years
For the first part of the model (< 18 years), CHAQ items were transformed to HUI2 utility values, using the mapping algorithm. QALY estimates were based on these utility values. Utility values for the second part of the model were derived from the literature and were based on adult patients with rheumatoid arthritis. Mean predicted utility values varied from 0.56 to 0.98 (range 0.18 to 1.00).
Estimation of costs
Health-care costs were derived from a study by Yagudina and colleagues133 reporting the cost of JIA during a 15-month period in Russia. The costs were for the year 2011. Given that this study reported the cost of 1-month inpatient and 14-month outpatient treatments, the cost attributable to each health state was adjusted using this as starting point. Base-case costs were discounted at a rate of 3%, whereas additional sensitivity analysis used a discounting rate of 5%.
Results
Relative to conventional non-biologic therapy, adalimumab was assessed to be cost-effective when used to treat JIA patients whose disease severity was comparable to that of participants in the adalimumab RCT. 61 Adalimumab plus methotrexate was reported to be more effective and more costly than methotrexate with an incremental cost per QALY ratio of approximately 1,437,480 roubles (£16,974 at current exchange rate) for the base case (7 years) and 119,496 roubles (£1411) adopting a lifetime horizon.
Key issues
-
There was uncertainty relating to the predicted utility values used to estimate QALYs; a recent study indicated how using different algorithms to convert HAQ to utility values affects the cost-effectiveness and HTA results. 134
-
The lifelong model uses utility estimates derived from adult patients with rheumatoid arthritis. JIA that persists into adulthood has a different disease process to rheumatoid arthritis, and, therefore, assumptions of similarity between the two conditions are not valid.
-
Cost estimates may not be applicable to the UK.
-
Mortality rates are assumed to be equal to published rates for Russia; it is not clear if this refers to the general population or to JIA- and age-specific mortality rates.
Ungar and colleagues127
Approach
Ungar and colleagues127 developed a decision-analysis model for etanercept, infliximab, adalimumab and abatacept for polyarticular-course JIA patients in Canada, who had had an inadequate response or intolerance to DMARDs. The model had a 1-year time horizon and consisted of two consecutive 6-month cycles, with no discounting. The model incorporated the probabilities that patients would, based on their response at 6 months, either continue with the same treatment or switch to an alternative treatment. Patients switched as a result of lack of response, intolerance to therapy or AEs. Where data on switching biologic DMARDs were not available in paediatric studies, the RR of switching from biologic DMARDs owing to non-response or AEs was extrapolated from studies of rheumatoid arthritis in adults. Patients who switched from methotrexate were assumed to receive a biologic DMARD for the next 6 months, where the cost of the biologic was represented by the average cost of all the biologic DMARDs.
Estimation of effectiveness
The model compared each of the biologic DMARDs with methotrexate, but did not compare them with each other, given that head-to-head trials were not available and that the study populations differed by JIA onset type. The effectiveness measure was the proportion of patients who had a reduction in symptoms at 1 year in accordance with the ACR Pedi-30 criteria. To derive 6-month response rates for each biologic DMARD, data from the key RCTs (Lovell and colleagues42,61 and Ruperto and colleagues57,135) were combined with data from registry and observational studies in a meta-analysis. For the base-case analysis, the proportion of patients achieving ACR Pedi-30 at 6 months varied between 79% and 82% for the biologic DMARDs, with the assumption that 30% of patients treated with methotrexate would achieve ACR Pedi-30. Probabilities for switching owing to non-response and AEs were estimated from the RCTs and observational studies.
Estimation of quality-adjusted life-years
Health-related quality of life was not included in the analysis.
Estimation of costs
The model included the costs of medication, monitoring costs and costs associated with treating serious infections. Costs were in Canadian dollars and the price year was 2008. In the base-case analysis a 40-kg patient was assumed, based upon the mean weight of patients in the two paediatric trials that reported weight. The direct medical costs included drug acquisition costs for biologic DMARDs and methotrexate, concomitant drug costs, drug administration materials, nursing time, dispensing fees, physician assessments and laboratory tests. Unit prices of health resources were obtained from public sources, including the Quebec and Ontario provincial drug plan formularies for medications, and the Ontario Ministry of Health and Long-Term Care fee schedules (laboratory tests and physician fees).
Results
The model reports results as additional cost per additional ACR Pedi-30 responder at 1 year of $26,061, $46,711, $16,204 and $31,209 for etanercept, adalimumab, abatacept and infliximab versus methotrexate, respectively. Probabilistic sensitivity analyses were conducted for each treatment versus methotrexate, and cost-effectiveness acceptability curves were calculated. If a decision-maker was willing to pay no more than $30,000 per additional responder, then the probability that etanercept would demonstrate a net economic benefit would be 95%. The willingness-to-pay points at which the biologic DMARDs had a 50% probability of cost-effectiveness were $45,000, $17,000 and $27,500 for adalimumab, abatacept and infliximab, respectively.
Key issues
-
The time horizon was inadequate to model treatment of a long-term condition (only 1 year).
-
This was not a cost–utility study as no HRQoL data were included.
Summary of published economic evaluations
-
A systematic review of economic evaluations of biologic treatments included four studies, two of which were cost–utility analyses, one of which was a cost-effectiveness study and one of which was a cost–consequence study.
-
The evaluations were published between 2002 and 2012 in the UK,123 the Netherlands,124 Russia125 and Canada. 127
-
The studies varied in design and structure. The time horizons varied between 1 year127 and lifetime. 123
-
The comparators differed between studies. One study compared etanercept with methotrexate,123 one compared adalimumab with methotrexate,125 one compared etanercept, adalimumab, abatacept and infliximab with methotrexate127 and the remaining study compared a cohort before and after receiving etanercept. 124
-
There were limitations in the methodological quality in all the studies identified, including limited reporting of model parameters and assumptions. The UK study123 is now considered out of date, and it is unclear how generalisable the results from the other studies are given the methodological limitations.
Systematic review of health-related quality-of-life studies
Methods for the systematic review
A systematic literature review was undertaken to assess the HRQoL of people with JIA treated with biologic DMARDs. The aim of the review was to provide data to populate the de novo economic model in this report with health-state utility values to calculate QALYs. The description of the search strategy is shown in Appendix 1. The inclusion criteria included primary studies that investigated HRQoL in people with JIA. To be eligible, the study should report health utility values using any generic preference-based HRQoL measure [e.g. EQ-5D, Short Form questionnaire-6 Dimensions (SF-6D)] or choice-based valuation methods (e.g. time trade-off, standard gamble). Studies that were not reported in English or did not provide sufficient information were excluded. The methodology used for searching and data extraction is outlined in Chapter 3 of this assessment report.
Results of the systematic review
The database searches identified 2249 references, with one further study retrieved by hand searching, making the total number of references identified 2250. Full-text papers for 28 references were retrieved, meeting the a priori inclusion criteria. Figure 7 presents a flow chart of the selection process and the excluded studies, with reasons for exclusion listed in Appendix 8. A total of 6 references were considered to have insufficient information on the study methods, population and results, 9 included an inappropriate population and 10 did not report a relevant outcome measure. Two studies, described in three publications, met the inclusion criteria and the characteristics of these studies are presented in Table 39. Data extraction forms for the included studies can be found in Appendix 9.
FIGURE 7.
Flow chart of identification of studies for inclusion in the review of QoL studies.
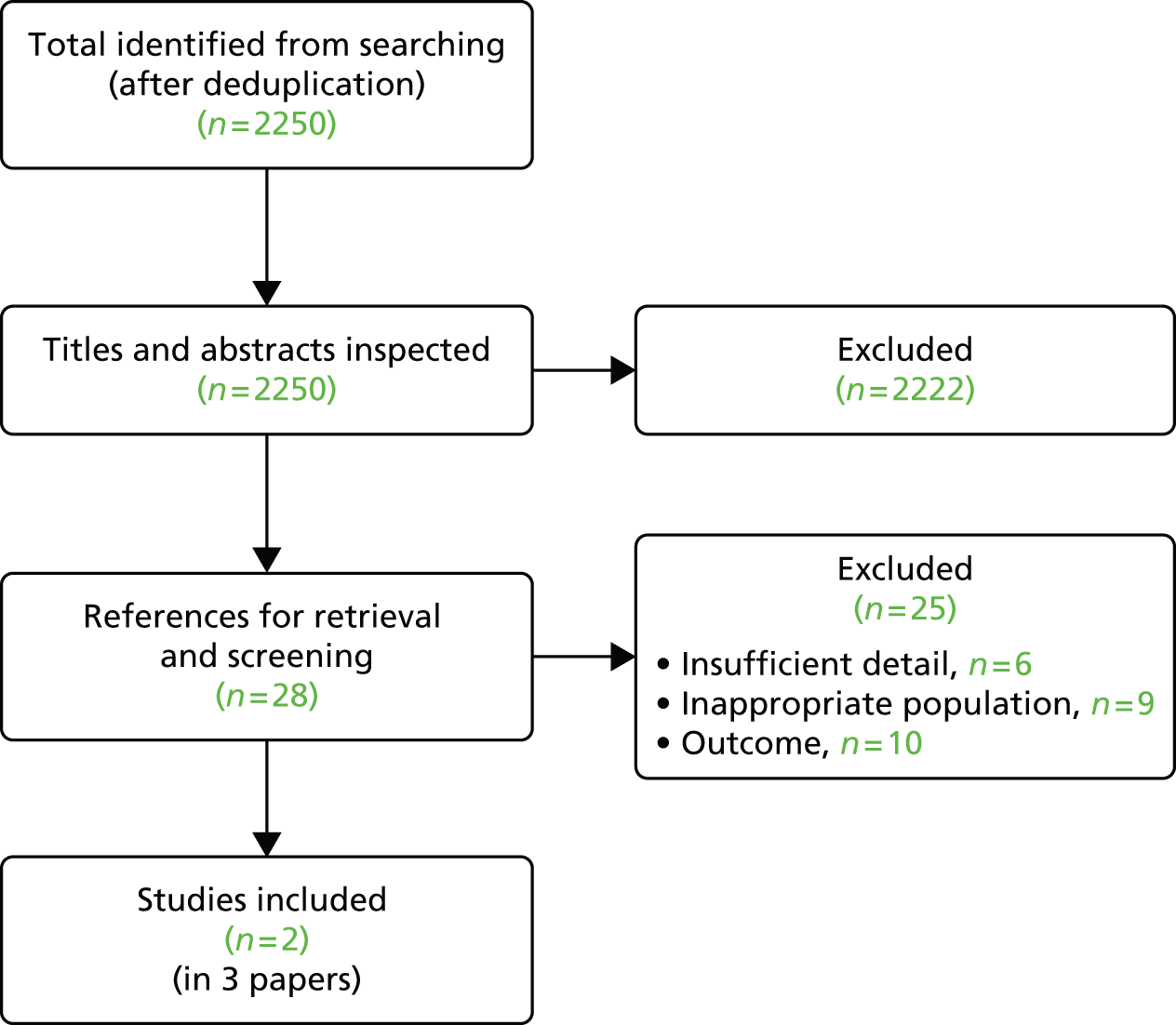
| Characteristic | Hendry et al. (2013)136 | Prince et al. (2011);124 Prince et al. (2010)137 |
|---|---|---|
| Country | UK | Netherlands |
| Study type | RCT | Prospective observational study |
| Study population | Children/adolescents with JIA and inflammatory joint disease affecting the foot/ankle (n = 44) | Children and adolescents with refractory JIA from the National ABC register (n = 49) |
| Study population age (mean) | 10 years old | 11.6 years old |
| Intervention(s) | Multidisciplinary foot care intervention informed by musculoskeletal ultrasound | Etanercept therapy |
| Comparator population | Standard care | No treatment |
| QoL instrument used | EQ-5D | HUI3 |
| Time period where HRQoL instruments administered | At baseline and 12 months | Baseline, 3 months, 15 months and 27 months |
| Methodology of collecting HRQoL data | EQ-5D was completed by patient (using EQ-5D-Y) and by proxy (using EQ-5D-3L) | The parents of the JIA patients completed the HUI3 |
| Results | EQ-5D was 0.57 and 0.69 for the intervention group at baseline for the self-reported and proxy groups. Results were similar at 12 months and in the control group | Utility was 0.53 at baseline and increased to 0.78 after 27 months |
The two HRQoL studies are each now described in more detail.
Hendry and colleagues136
Hendry and colleagues136 conducted an exploratory RCT to assess the effectiveness of a multidisciplinary foot care programme in children with JIA, and to investigate the methodological considerations of such a trial.
Children and adolescents with a definitive diagnosis of JIA and inflammatory joint disease affecting the foot/ankle were recruited at a single hospital (the Royal Hospital for Sick Children, Glasgow, UK). Participants were included if they satisfied at least one of the following criteria: (1) previously documented foot arthritis including small joints derived from medical case notes; (2) previously documented foot arthritis in one or more large joints derived from medical case notes; or (3) current widespread polyarthritis involving large and small foot joints derived from clinical examination by a consultant paediatric rheumatologist. Patients with an unconfirmed diagnosis of JIA, and/or only upper limb, jaw or neck involvement were excluded. Therefore, a subgroup of the JIA patient population of relevance to this assessment report (i.e. those whose disease had not affected the foot/ankle) was excluded from the study (Table 40).
| Characteristics | Multidisciplinary foot care | Standard care |
|---|---|---|
| Participants, n | 21 | 23 |
| Age (years), mean (SD) | 10.1 (4.22) | 10.0 (3.39) |
| Sex, n | ||
| Male | 7 | 6 |
| Female | 14 | 17 |
| Disease subtypes, n (%) | ||
| Persistent oligoarthritis | 7 (33) | 4 (17) |
| EO | 4 (19) | 5 (22) |
| Polyarthritis RF–ve | 6 (29) | 10 (43) |
| Polyarthritis RF+ve | 0 (0) | 2 (9) |
| PA | 2 (10) | 1 (4) |
| ERA | 2 (10) | 0 (0) |
| Undifferentiated | 0 (0) | 1 (4) |
| Pharmacological management, n (%) | ||
| Analgesics | 2 (9) | 3 (13) |
| NSAIDs | 2 (9) | 3 (13) |
| Methotrexate | 18 (86) | 16 (70) |
| Etanercept | 7 (33) | 5 (22) |
| Methotrexate and etanercept | 5 (24) | 5 (22) |
| Sulphasalazine | 1 (5) | 0 (0) |
| Rituximab | 0 (0) | 1 (4) |
| EQ-5D utility index at baseline | ||
| Self, mean (SD) | 0.57 (0.31) | 0.58 (0.35) |
| Self, median (IQR) | 0.62 (0.52–0.76) | 0.66 (0.52–0.75) |
| Proxy, mean (SD) | 0.69 (0.29) | 0.60 (0.33) |
| Proxy, median (IQR) | 0.69 (0.58–1) | 0.62 (0.55–0.82) |
| Change in EQ-5D utility index at 12 months, median (IQR) | ||
| Self | 0 (–0.1 to 0.01) | 0 (–0.04 to 0.04) |
| Proxy | 0 (0 to 0.11) | 0 (0 to 0.1) |
Enrolled participants (n = 44) were randomly allocated to the intervention group receiving multidisciplinary foot care (individualised care packages including foot orthoses and targeted home exercise programmes) or to the control group treated with standard care (normal outpatient medical care from their consultant paediatric rheumatologists). Treatment groups were similar in terms of pharmacological treatment and both had a proportion of patients receiving etanercept. There were small differences in proportions of JIA disease subtypes, but there were no statistically significant differences in baseline characteristics (see Table 40).
Patients’ HRQoL was collected at baseline and 12 months using the EQ-5D (Youth) (patients) and EQ-5D 3-Levels (parents/guardians). There were no significant differences in HRQoL between treatment groups at 12 months, and both self- and proxy-reported outcomes were similar (see Table 40).
Prince and colleagues124,137
Prince and colleagues124,137 evaluated changes in HRQoL in patients with refractory JIA who were being treated with etanercept, following an insufficient response to the maximum tolerated dose of methotrexate (This study was also discussed above; see Systematic review of existing cost-effectiveness evidence). Data were collected from Dutch patients registered at the National ABC) register, supplemented by prospectively collected additional data from patients who started etanercept treatment from 2003 until 2006. Three HRQoL questionnaires were used, one of which was the HUI3 preference-based HRQoL instrument. HRQoL questionnaires were completed at the start and after 3, 15 and 27 months of treatment.
Prince and colleagues124,137 report the results in two publications, and the results differ slightly between the publications. In the publication including costs,124 four fewer patients are included, as these patients did not continue treatment with etanercept for at least 27 months, whereas the publication reporting QoL only reports the results for all 53 patients. For the purposes of this assessment report, the smaller dataset is of more relevance,124 but results from both datasets are shown in Table 41.
| Characteristic | Prince et al. (2010)137 | Prince et al. (2011)124 |
|---|---|---|
| Participants, n | 53 | 49 |
| Age, median (IQR) | 11.9 (8.1–14.9) | 11.6 (7.9–14.6) |
| Sex, % | ||
| Male | 38 | 41 |
| Female | 62 | 59 |
| Proportion of sample with systemic JIA, % | 26 (14/53) | 22 (11/49) |
| Proportion of sample receiving methotrexate, % | 80 | 79 |
| HUI utility value (SD) baseline | 0.51 (0.04) | 0.53 (0.04) |
| HUI utility value (SD) follow-up (27 months) | 0.77 (0.08) | 0.78 (0.07) |
The results from the study indicated a statistically significant improvement in the HUI3 utility score from 0.53 at baseline to 0.78 at 27 months’ follow-up. Mean utility values were 0.69 at 3 months and 0.74 at 15 months’ follow-up. For the cohort with more patients, there was a mean utility improvement of 0.25 during the 27 months of treatment. 137 The baseline mean utility value was 0.51, and significant changes were observed in the domains of pain, ambulatory and dexterity.
Summary and conclusions of the health-related quality-of-life review
The included studies assessed the HRQoL of children and adolescents with JIA, applying EQ-5D and HUI3 preference-based utility measures. Although both studies reported utility values, they are not directly comparable. The study by Hendry and colleagues136 assessed the effectiveness of a foot care programme in a RCT, whereas Prince and colleagues124,137 conducted an observational study reporting HRQoL and costs from patients in the Dutch ABC registry to assess the effect of treating patients with etanercept. The mean utility values reported for baseline by Hendry and colleagues136 for the intervention and control group (0.57 and 0.58, respectively) are relatively similar to the baseline values reported by the Prince and colleagues study (0.51 and 0.53, respectively). 124,137 The HRQoL values may be higher for Hendry and colleagues136 owing to 23% of patients receiving etanercept, whereas no patients received etanercept at baseline in the Prince and colleagues study. 124,137 The sample size of both cohorts is considered relatively small, but it is reasonable given the population group. The cohort used in the Prince and colleagues study124,137 included patients with systemic JIA, who are outside the scope of the current review; however, the proportion of patients within the group with systemic JIA is relatively small (< 30%). Neither of the studies can be considered fully informative for the de novo economic evaluation in this assessment report. However, estimates provided by Prince and colleagues124,137 are considered reasonably appropriate for use in the economic evaluation, despite not being considered directly generalisable to the UK population.
Review of cost-effectiveness in company submissions to the National Institute for Health and Care Excellence
All four pharmaceutical companies submitted evidence to be considered for the NICE appraisal. Two of these submissions [by BMS (abatacept) and Roche (tocilizumab)]78,85 consisted of a written report and an electronic economic model, and the other two submissions [by AbbVie (adalimumab) and Pfizer (etanercept)]77,97 comprised only a written report.
A structured data extraction form was used by the assessment group to assess the CSs (see Appendix 10). A description and critique of each of the submissions in turn is provided in the following subsections. Greater description is provided for the Roche and BMS submissions as these conducted economic models. (a description and critique of the companies’ clinical effectiveness evidence is given in Chapter 4, Review of clinical effectiveness in company submissions to the National Institute for Health and Care Excellence).
Review of Bristol-Myers Squibb’s submission to the National Institute for Health and Care Excellence (abatacept)
The company submitted a de novo economic model that included all comparators specified in the NICE scope except for methotrexate monotherapy (i.e. abatacept, etanercept, adalimumab and tocilizumab). 85 The company states that methotrexate monotherapy was not included owing to inconsistency with the clinical effectiveness data (i.e. all patients in the RCTs either did not have sufficient response with methotrexate or were refractory to methotrexate). The scope reflects the licensed indication of abatacept, namely polyarticular JIA patients aged ≥ 6 years who have received at least one TNFα inhibitor (etanercept or adalimumab). All included drugs were assumed to be administered with subcutaneous methotrexate.
Modelling approach
The model presented in the CS is a cohort-based cost-minimisation model, in which all drugs were assumed to have identical efficacy. The base-case model presents a cohort of 12-year-old polyarticular JIA patients and follows them until aged 18 years in 4-week cycles. The model is essentially a one-state model. Patients gain weight and height as they age, but their disease does not change; only the costs associated with treating the disease increase owing to weight- and body surface area-based dosing among the drugs. The drug acquisition cost values within the model were appropriately derived from Monthly Index of Medical Specialties data. 138
Assumptions
The model contained a number of assumptions that appear reasonable: a 52-week year, a dosing regimen for methotrexate consistent between < 16-year-olds and > 16-year-olds, a constant weight after the age of 20 years, methotrexate dosing based on an algorithm for lowest cost when > 30 mg of methotrexate is necessary, a normal distribution for height and weight, a truncation of the model starting age in the probabilistic sensitivity analysis (PSA) to represent only between age 6 and 16 years as starting ages, assumptions for standard errors where they were unavailable and no vial sharing of drugs.
Critical appraisal of model
Although the comparators and population within the model were generally consistent with the NICE scope, the model does not adequately represent all the available evidence for the treatment pathway or natural history of the disease.
The model time horizon and structure are inadequate to capture long-term treatment effects or the treatment pathway of the disease, as many JIA patients continue to receive treatment into adulthood. The model does not allow for drug discontinuation or treatment switching, which is known to happen in clinical practice. The CS indicates that the model was validated by an internal reviewer but full details were not reported of this validation.
Estimation of effectiveness
The model did not include clinical effectiveness data to represent clinical outcomes or to represent events that incur costs such as disease flare, vision loss or joint surgery. The effectiveness of the biologic DMARDs was assumed to be equivalent as a justification for the use of cost-minimisation methods. The CS cites the systematic review and indirect comparison by Otten and colleagues79 for evidence of equivalent effectiveness (as discussed in Chapter 4).
Estimation of quality-adjusted life-years
Health-related quality of life was not assessed in the model and the CS indicates that this was due to uncertainty in the QoL values for JIA in the literature.
Estimation of costs
Intervention dosages and prices were derived from Monthly Index of Medical Specialties data,138 whereas costs for subcutaneous injection and infusion drug delivery methods were derived from a previously published HTA of biologic DMARDs in rheumatoid arthritis. 139 A confidential patient access scheme (PAS) was incorporated for abatacept. Sensitivity analyses were run for the price of tocilizumab using various assumed percentage price discounts, as a confidential PAS has been agreed for tocilizumab.
The costs were derived from appropriate sources and are clearly reported, but it is assumed that the drugs had identical AE costs, discontinuation rates and clinical effectiveness. The details for drug costs and dosages used in the BMS company model are shown in Table 42.
| Drug | Cost, £ | Dose, mg |
|---|---|---|
| Abatacept | 302.40 | 250 |
| Etanercept | 35.75 | 10 |
| 89.38 | 25 | |
| 178.75 | 50 | |
| Adalimumab | 352.14 | 40 |
| Tocilizumab | 102.40 | 80 |
| 256.00 | 200 | |
| 512.00 | 400 | |
| Methotrexate | 14.85 | 7.5 |
| 15.29 | 10 | |
| 16.50 | 12.5 | |
| 16.57 | 15 | |
| 17.50 | 17.5 | |
| 17.84 | 20 | |
| Administration method | ||
| Infusion | 154.00 | |
| Subcutaneous injection | 3.05 | |
Results
The model was a cost minimisation and analysed only costs, assuming equivalent clinical effectiveness for all biologic DMARDs. The results of the base-case analysis are shown in Table 43. The model results presented by the manufacturer include a PAS discount of (CiC information has been removed) for abatacept.
| Costs | Abatacept | Adalimumab | Etanercept | Tocilizumab |
|---|---|---|---|---|
| Drug costs | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Administration costs, £ | 11,797 | 871 | 871 | 11,646 |
| Total costs | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Cost savings with abatacept | CiC information has been removed | CiC information has been removed | CiC information has been removed | |
In the base case, the company found that abatacept was the least costly biologic DMARD. The CS states that abatacept has similar efficacy and safety to the other biologic DMARDs. Deterministic sensitivity analyses and PSA were conducted for a variety of scenarios. The findings were found to be robust to a wide range of scenarios.
The company undertook a number of deterministic and scenario sensitivity analyses:
-
A sensitivity analysis was undertaken wherein the infusion costs for tocilizumab were increased owing to the longer infusion time.
-
The starting age of patients in the model was varied between 6 and 16 years. In the biologic DMARD trials the mean age was 11 years at baseline, but the drug licences were for much younger ages.
-
The time horizon of the model was varied between 6 months and 20 years. Longer time horizons were meant to represent that one-third of children with JIA will have it into adulthood.
-
PAS discount for tocilizumab was tested for a range of percentages of list price reductions and calculated to show the tocilizumab discount with identical drug costs to abatacept.
-
Methotrexate was excluded from the etanercept arm.
There were no analyses that varied more than one parameter at a time. None of the one-way sensitivity analyses was accompanied by a probabilistic analysis that reassessed the probability that abatacept was the least costly biologic for second-line biologic DMARD therapy of polyarticular JIA. Given that the model was simple and that the number of simulations for the PSA was only 1000, these analyses would have been simple and quick to perform. It would have been especially informative to do this for the tocilizumab cost-sensitivity analyses. No analyses looked at subgroups of patients, and there was no discussion of potential subgroups.
The PSA results using 1000 simulations were within 5% of the base-case analysis results. Abatacept was the least costly option in 67% of simulations, whereas etanercept was the least costly option in the remaining 33% of simulations. Not all distributions used in the sensitivity analysis were appropriate. For the infusion costs and subcutaneous administration costs, a normal distribution was used for the cost data, which could lead to simulations with negative cost values; a gamma or log-normal distribution would be more appropriate. The drug cost data do not appear to have been subjected to uncertainty. Given that there is a PAS for tocilizumab, using a lower than average cost value for tocilizumab and subjecting it to PSA could have been an alternative approach to conducting only a deterministic analysis for tocilizumab. This would have given a more realistic estimates of the cost uncertainty between treatments.
Critique of the company’s submission
The company constructed a cost-minimisation model, assuming that there were no differences between the biologic DMARDs in clinical effectiveness, AEs and discontinuation rates. Patients do not discontinue or switch treatments, which does not reflect current clinical practice or the available evidence.
Overall, the model is limited for decision-making owing to factors such as inadequate time horizon and structural limitations. However, the methods for integrating variable dosing over time used within the model may be useful for building a more comprehensive model.
Review of the Roche submission to the National Institute for Health and Care Excellence (tocilizumab)
The CS includes an economic model and reports the total costs, the QALYs gained and cost-effectiveness of tocilizumab in the treatment of polyarticular JIA. 78 The model evaluates the lifetime costs and benefits for tocilizumab compared with adalimumab. The perspective of the analysis is that of the NHS and Personal Social Services (PSS).
Modelling approach
A de novo Markov state-transition decision model was developed in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) with three health states (uncontrolled disease/off treatment, on treatment and dead). The model has 6-month cycles and a time horizon of 25 years. Costs and benefits are discounted at 3.5%. Patients entering the model have active JIA and have previously experienced an inadequate response to, or were intolerant of, methotrexate. Patients in the model have a mean age of 11 years and are based on those in the CHERISH RCT. 68
Patients start with uncontrolled disease at cycle 0 then move to first-line biologic treatment. Once all lines of treatment are exhausted, patients move into the uncontrolled disease health state. Mortality is included in the model and assumes a 1% 6-month mortality rate across all years. The model includes the occurrence of SAEs. The mortality rate used in the model is about 100 times higher than the annual mortality rate for the general paediatric population of 0.02%. We consider that a lower mortality rate should be used in the model.
Assumptions
The CS states that, owing to differences in terms of trial design, patients, methods of imputation and quality, only adalimumab and tocilizumab could be compared. The CS states that an indirect comparison of safety was not possible and so the risk of SAEs was assumed to be the same for both biologic DMARDs. The model assumes that patients discontinue at a rate proportional to their ACR response [i.e. no response (ACR Pedi < 30), moderate response (ACR Pedi-30–ACR Pedi-70) and good response (ACR Pedi ≥ 70)].
Critical appraisal of model
The submission meets all of the requirements for methodological quality and generalisability, except that it did not fully explore uncertainty or provide any evidence that the economic model had been validated.
The evaluation provided a clear statement of the decision problem to be addressed, including the population, which appeared to follow the scope for the appraisal issued by NICE. The comparators included (adalimumab and tocilizumab) were appropriate, as these are being routinely used or considered for use within the NHS in England and Wales. The model also included etanercept in an exploratory analysis but did not include abatacept, and the CS states that this was not possible owing to differences in trial design, patients, methods of imputation and quality. The 25-year time horizon reflects the chronic nature of the disease and allows for all relevant costs and benefits to be included. The model structure was clearly presented with a description and justification of the key assumptions and data inputs used. Benefits for the model are measured in QALYs using the HUI3 for measuring utility. All benefits and costs are discounted at 3.5% as required by NICE. 130 The CS does not assess uncertainty in sensitivity analysis. It was unclear if the model had been fully validated, as no details were provided.
Estimation of effectiveness
The company reported a systematic review of biologic DMARDs in the treatment of JIA. The CS states that it was possible to compare adalimumab and tocilizumab only by indirect comparison, as these two treatments had greater similarities in trial design, patients, methods of imputation and quality. Results from each study were combined using a hierarchical Bayesian indirect treatment comparison, using an ordered probit model in WinBUGS software to estimate the relative treatment effects and achieving different levels of ACR response. The ACR response rates were estimated for the biologic DMARDs with and without methotrexate and are shown in Table 44. The response is generally similar for adalimumab and tocilizumab (both with methotrexate).
| Response rates | ACR Pedi-30, % | ACR Pedi-50, % | ACR Pedi-70, % | ACR Pedi-90, % | |
|---|---|---|---|---|---|
| Without methotrexate | Placebo | 31 | 28 | 25 | 12 |
| Tocilizumab | 62 | 59 | 54 | 35 | |
| Adalimumab | 52 | 49 | 44 | 26 | |
| With methotrexate | Methotrexate | 52 | 51 | 41 | 25 |
| Tocilizumab | 72 | 70 | 61 | 44 | |
| Adalimumab | 76 | 75 | 66 | 49 | |
The discontinuation rate used in the model was derived according to ACR response from the Dutch ABC register. 124 An exponential distribution was fitted to the data for no response (ACR Pedi response < 30), moderate response (ACR Pedi response > 30 and < 70) and good response (ACR Pedi response ≥ 70). The 6-month discontinuation rate was 0.126 for no response, 0.09 for moderate response and 0.042 for good response.
Estimation of quality-adjusted life-years
The company conducted a literature review that identified one study reporting utility values suitable for use in the model (Prince and colleagues124). This study reported utility scores obtained using the HUI3 questionnaire to JIA patients starting treatment with etanercept in the Dutch ABC register. Based on these data, the company used values at time 0 for the patients who are off treatment (utility of 0.53), and used values at time 1 year for patients on treatment (utility of 0.73).
The assessment group identified some errors in the Roche model with regard to estimation of QALYs. First, utility values for patients have been applied as if patients were on treatment for some time after finishing the first-line biologic treatment, when these patients should have been assigned the off-treatment utility. Second, utility values have been incorrectly calculated as the utility value for 1 year has been assigned to each cycle of 6 months. (For corrections to these errors, see Table 47.)
Estimation of costs
The costs associated with each health state was obtained from Prince and colleagues,124 who report costs data from the Dutch ABC register for the year before and after starting etanercept. The total 6-month health-state cost for patients on treatment is £912.33 and off treatment is £1591.43. Treatment unit costs and doses were reported. Tocilizumab was provided with a confidential PAS discount (CiC information has been removed). The model included costs for both intravenous infusion and for subcutaneous injection, as required by the treatment. The administration cost of an infusion (for tocilizumab) was £152.24, using inflated costs from Barton and colleagues. 140 The cost of an administration of a subcutaneous injection was £6.10 for children and £3.05 for a young person, assuming that a proportion of these patients would require nurse assistance.
Cost-effectiveness results
Tables 45 and 46 show the cost-effectiveness results from the CS for tocilizumab compared with adalimumab when used in combination with methotrexate or as a monotherapy. The CS states that the results indicate that both treatments are of similar clinical effectiveness and cost-effectiveness, regardless of whether they are used in combination with methotrexate or as a monotherapy. The company urges caution in the interpretation of the QALY estimates, but concludes that tocilizumab is less expensive and therefore represents better value to the NHS.
| Outcome | Adalimumab + methotrexate | Tocilizumab + methotrexate | Incremental difference | ICER (£ per QALY) |
|---|---|---|---|---|
| Total QALYs | 18.76 | 18.72 | –0.0303 | |
| Total cost, £ | 81,827 | 70,707 | –11,120 | South-west quadranta |
| Outcome | Adalimumab | Tocilizumab | Incremental difference | ICER (£ per QALY) |
|---|---|---|---|---|
| Total QALYs | 18.65 | 18.7 | 0.0455 | |
| Total cost, £ | 74,576 | 68,560 | –6015 | Dominant |
The CS does not include any sensitivity analyses. It includes an exploratory analysis with etanercept. This analysis assumed a class effect across antiTNFs in polyarticular JIA.
Critique of the company’s submission
There are some concerns over the reliability of the model results in the Roche submission owing to errors found by the assessment group in the calculation of QALYs.
The assessment group has corrected the errors in the Roche model by applying the off-treatment utility values when patients finished the first-line biologic treatment and assigning the 6-month utility value to each cycle. In addition, the mortality rate has been reduced to 0.03% per cycle to reflect that of the general population. The results for this analysis are shown in Table 47. The corrected results show reduced QALYs and increased costs for adalimumab and tocilizumab in combination with methotrexate compared with the base-case results. However, the incremental QALYs and costs between the tocilizumab and adalimumab are similar in the corrected results to the base-case results.
| Outcome | Adalimumab + methotrexate | Tocilizumab + methotrexate | Incremental difference | ICER (£ per QALY) |
|---|---|---|---|---|
| Total QALYs | 10.10 | 10.05 | –0.05 | |
| Total cost, £ | 95,761 | 83,593 | –12,168 | South-west quadranta |
Review of the AbbVie submission to the National Institute for Health and Care Excellence (adalimumab)
The company did not provide a systematic review of cost-effectiveness studies or an economic evaluation. 77 They discussed the interventions in the NICE scope: adalimumab, etanercept, abatacept, tocilizumab and methotrexate. In sections 5.8–5.10 of the CS, the company provides justifications for not conducting an economic evaluation. Other sections of the CS provide details on what the company would consider important in conducting an economic evaluation in JIA, including an evaluation of the costs associated with surgeries and vision loss.
The company states that an economic evaluation was not conducted owing to a lack of appropriate utility data for HRQoL, heterogeneity in study methods and populations between the interventions that complicated indirect comparisons, and a lack of long-term effectiveness data. The company identified one HRQoL study (Prince and colleagues124), which collected HUI3 utilities in addition to other JIA clinical variables, such as the CHAQ score. The data collected from Prince and colleagues124 were deemed unsuitable to map the CHAQ to HUI3 owing to insufficient sample size, but were considered the most suitable source of utility data by the company. The CS discusses the use of an algorithm by Khan and colleagues141 that mapped the Pediatric Quality of Life Inventory instrument to EQ-5D in secondary school pupils. The company notes the potential limitations in the use of this method in JIA, as Pediatric Quality of Life data were not collected in any of the JIA biologic DMARD RCTs. The company considers that the biologic DMARD trial populations and study methods were not sufficiently similar to allow indirect comparison through network meta-analysis. The CS concluded that using current data and methods would lead to ‘untenable’ uncertainty (CS page 90).
Review of the Pfizer submission to the National Institute for Health and Care Excellence (etanercept)
Pfizer did not submit any cost-effectiveness evidence. 97 The CS notes the limitations raised in the previous submissions for NICE of etanercept TA3543 and tocilizumab TA238. 44 These relate to the limitations in the HRQoL data and the limited evidence on the long-term outcomes and the effectiveness of the treatments. The CS states that any cost-effectiveness evidence would be associated with considerable and unresolvable uncertainty. The company submitted a cost analysis that compared the annual costs for the first year of treatment based on etanercept against adalimumab and tocilizumab in patients with polyarticular JIA. The CS states that the cost analysis showed that etanercept is the biologic DMARD with the lowest acquisition cost compared with list prices for tocilizumab and adalimumab.
Comparison of economic models in company submissions
The CSs differ in the approach to providing economic evidence for biologic DMARDs for JIA. Only one company (Roche) constructed an economic model for tocilizumab that included both costs and outcomes. Two companies (BMS for abatacept and Pfizer for etanercept) submitted cost analyses and assumed that the biologic DMARDs were equivalent, whereas the remaining company (AbbVie for adalimumab) considered there to be too many limitations with any potential analysis and, therefore, did not submit an economic analysis.
Although AbbVie has raised valid concerns about uncertainty in the data available for conducting an economic evaluation, we consider that concerns about uncertainty are an insufficient justification for not building an economic model. A model provides a representation of current knowledge in a subject and uncertainty is part of that current knowledge. A model, even an uncertain one, with limitations noted by the company, gives a more transparent description of available knowledge and enables more informed decision-making than simply presenting clinical trial data from trials that represent only a highly selected subgroup of the drug licences. Modelling also allows the exposure of the most valuable areas for future research enquiries.
Bristol-Myers Squibb and Pfizer consider that all of the treatments are equivalent. It is noted that the available evidence base consists of small trials that lack the statistical power to justify this assumption, and Otten and colleagues79 do not conclude that there is equivalent efficacy between the treatments, but that the treatments are similar.
Briggs and O’Brien142 argue that a cost-minimisation analysis should be conducted only when equivalence of comparators has been statistically demonstrated. Dakin and Wordsworth143 argue that the limitations of a cost-minimisation analysis do not allow for an appropriate assessment of uncertainty or for the value of future research and may lead to biased conclusions. It is also the case that equivalence of one clinical outcome does not mean equivalence of all clinical outcomes. Patients may have the same QoL on treatment but have different adherence and discontinuation or different AEs, for example. For these reasons, a cost-minimisation analysis is generally forgone in favour of a cost-effectiveness analysis and/or a cost–utility analysis.
Roche have provided a cost–utility analysis that compared two of the biologic DMARDs (adalimumab and tocilizumab). The model appears to be a reasonable attempt at modelling JIA, albeit in only two of the biologic DMARDs. However, we have noted errors in the calculation of QALYs in the model, which limit the credibility of the results.
Independent economic evaluation
The models described in our systematic review of economic evaluations (see Systematic review of cost-effectiveness evidence) had certain methodological limitations and were not wholly generalisable to the NHS. Furthermore, the economic evaluation used to inform the NICE appraisal of tocilizumab for systemic JIA (NICE TA238) was subject to a number of concerns from the Appraisal Committee, in particular with regard to the estimation of HRQoL. 44 Given the limitations of existing available models, we therefore constructed a de novo economic model to inform this current appraisal.
The model estimates the costs, benefits and cost-effectiveness of the four biologic DMARDs in patients with JIA and inadequate responses to, or intolerance of, methotrexate. The model compares the biologic DMARDs (in combination with methotrexate, where permitted) with a DMARD (e.g. methotrexate), as specified in the NICE scope. The model does not compare the biologic DMARDs with best supportive care (e.g. NSAIDS; corticosteroids) for patients who cannot tolerate a DMARD, as there are limited data available to make this comparison. Furthermore, patients who are intolerant to a DMARD such as methotrexate would be offered a biologic DMARD rather than best supportive care, particularly to avoid any potential adverse effects of long-term corticosteroid use. 144
The evidence used in the model was taken from data sources such as the RCTs of biologic DMARDS (in which a number of JIA subtypes were represented, with polyarticular-course JIA being the predominant subtype), and data sources such as registry studies comprising mixed JIA populations (primarily comprising polyarticular and oligoarthritis JIA patients, but also small proportions of patients with ERA, PA and systemic JIA). However, there was insufficient evidence available for all input parameters to permit a cost-effectiveness subgroup analysis for each of the respective types of JIA within the scope of the appraisal. Therefore, the modelled patient population is people with JIA, with the results of particular relevance to people with polyarticular course JIA (EO, and RF +ve and RF –ve polyarthritis). The biologic DMARDs are assessed in this report within their licensed indications (e.g. the cost-effectiveness estimates for some of the biologic DMARDs cannot be applied to JIA subtypes for which they are not licensed, such as abatacept and tocilizumab for the treatment of ERA and PA).
The model was populated with clinical effectiveness data from the included RCTs in our systematic review of clinical effectiveness (see Chapter 4), HRQoL data from our systematic review of HRQoL studies (see Systematic review of health-related quality-of-life studies) and cost data derived from published studies (where available), as well as national and local NHS unit costs.
The economic evaluation was from the perspective of the NHS and Personal Social Services, with only these direct costs included. The model estimates the long-term costs and benefits from each of the treatments. The costs and benefits were discounted at 3.5%, as recommended by NICE. 130 The base price year for the costs was 2014. The intervention effect, in terms of reducing disease flare, was derived from the systematic review of clinical effectiveness reported in Chapter 4. The outcome of the economic evaluation is reported as incremental cost per QALY gained.
Methods for independent economic analysis
A Markov model was developed in Microsoft Excel to assess the cost-effectiveness of the biologic DMARDs. The model contains health states for ‘on treatment’, ‘off treatment’, ‘remission off treatment’ and ‘death’. A diagram of the model is shown in Figure 8. The model uses 3-month cycles to be consistent with the usual time between outpatient appointments for JIA patients. A time horizon of 30 years was modelled as the base case, with shorter and longer horizons tested in sensitivity analyses. This time horizon was considered sufficiently long to capture the costs and effects of biologic DMARDs for paediatric patients, given the uncertainty around the long-term clinical outcomes for adults with JIA. The model structure is based upon the clinical pathway of patients who participated in the withdrawal RCTs, described in Chapter 4, the natural history of JIA described in registry data and discussions with clinical experts. The starting age of patients in the model is 11 years, based upon the mean age of patients in the RCTs. Patients’ heights and weights are assumed to be similar to those in the general population.
FIGURE 8.
Schematic of the SHTAC JIA model structure.

Patients treated with a biologic DMARD continue on treatment unless they die or withdraw from treatment owing to adverse effects, loss of efficacy or clinical remission off treatment. Patients with clinical remission who have their treatment discontinued may relapse and resume treatment with a biologic DMARD. Based on clinical advice, we assumed that clinicians would be reluctant to stop treatment with a biologic DMARD as a result of remission because of the risk of relapse, and so, for the base-case analysis we assume that no patients discontinue treatment owing to remission. We investigate discontinuation attributable to remission in more detail in a scenario analyses. Therefore, for the base-case analysis, no patients enter the ‘clinical remission off treatment’ health state and this is indicated in Figure 8 by dotted lines for this health state.
In the base-case analysis, patients treated with adalimumab, etanercept and tocilizumab receive only one line of biologic DMARD treatment. Patients treated with abatacept receive two lines of biologic DMARD treatment, as abatacept is licensed for use only after a preceding antiTNF. Following withdrawal from these biologic DMARDs, patients continue on a standard treatment regimen that does not contain a biologic DMARD.
In a scenario analysis (scenario V), we investigate multiple lines of treatment with biologic DMARDs, which reflects the range of strategies used in clinical practice. Patients continue on the subsequent treatments until they die or withdraw from treatment as a result of AEs, loss of efficacy or clinical remission. Following withdrawal from the final biologic DMARD, patients continue on a standard treatment regimen that does not contain a biologic DMARD.
Patients treated with methotrexate only (i.e. those not receiving a biologic DMARD) are assumed to continue on treatment unless they die or withdraw from treatment owing to AEs or loss of efficacy.
The model incorporates disease flares to estimate the clinical effectiveness of treatment. This was the primary outcome measure in the RCTs of the biologic DMARDs. Patients who have a disease flare continue in their current health state in the model but are allocated a HRQoL disutility and an additional health-care cost during that cycle.
The costs in the model comprise drug treatment, consultation and monitoring costs and costs for treating AEs. Costs used in the model are described in more detail in Data sources. HRQoL is estimated according to each health state.
In each cycle, the total costs and QALYs are calculated by multiplying the individual costs and HRQoL by the number of people in the cohort still alive for each of the treatments. The total long-term costs and QALYs are calculated by aggregating the costs and QALYs for all cycles. The total discounted QALY gain and cost of treatments are calculated and compared to give the cost-effectiveness of the treatments.
Assumptions are applied to all treatment options unless explicitly stated otherwise. These assumptions have been made owing to an absence of data and have been informed by discussion with our clinical advisors. The model includes the following main assumptions:
-
There are few studies for biologics, other than for etanercept, that report long-term discontinuation owing to AEs and inefficacy. The discontinuation rate is assumed to be similar for each of the biologic DMARDs.
-
Our clinical effectiveness review concluded that there is no evidence of a difference in efficacy between biologics and, therefore, we assumed that the QoL utility values are the same for all biologic DMARDs (‘on treatment’).
-
It is currently unclear whether or not the effectiveness of subsequent lines of biologic DMARDs would be reduced or remain the same. The effectiveness of the biologic DMARDs is assumed to be similar taken as a first- or subsequent-line biologic treatment. This applies to abatacept as a second-line biologic DMARD in the base case, and to the scenario analysis that models three lines of biologic DMARD treatment (scenario V).
Evaluation of uncertainty
The evaluation of the cost-effectiveness of JIA treatment is based on uncertain information about variables such as clinical effect, HRQoL and resource use. This uncertainty was evaluated using deterministic and PSA. One-way deterministic sensitivity analyses were conducted to evaluate the influence of individual parameters on the model results and to test the robustness of the cost-effectiveness results to variations in the structural assumptions and parameter inputs (see Results of the independent economic analysis). Where possible, the parameters were varied according to the ranges of the CIs of these parameters based on the published estimates. Where these data were not available an alternative range was chosen.
Multiparameter uncertainty in the model was addressed using PSA (Results of the independent economic analysis). 145 In the PSA, probability distributions are assigned to the parameter point estimates used in the base-case analysis. The model is run for 1000 iterations, with a different set of parameter values for each iteration by sampling parameter values at random from their probability distributions.
The uncertainty surrounding the cost-effectiveness of the treatment is represented on a cost-effectiveness acceptability curve according to the probability that the intervention will be cost-effective at a particular willingness-to-pay threshold. Appendix 11 reports the parameters included in the PSA, the form of distribution used for sampling each parameter and the upper and lower limits assumed for each variable.
Model validation
The model was validated by checking the model structure, calculations and data inputs for technical correctness by another researcher. The structure was reviewed by clinical experts from the advisory group for its appropriateness for the disease and its treatment. A senior health economist from the advisory group reviewed the methods and assumptions of the economic evaluation. The robustness of the model to changes in input values was tested using sensitivity analyses to ensure that any changes to the input values produced changes to the results of the expected direction and magnitude.
Data sources
Effectiveness data
Disease flare
The risk of disease flare was included in the model as a RR compared with methotrexate (Table 48), as derived from our systematic review of clinical effectiveness [Chapter 4, Assessment of clinical effectiveness: biologic disease-modifying antirheumatic drugs versus placebo (with methotrexate where permitted)]. The baseline risk of flare for methotrexate was a weighted average of the risk of flare estimates from the placebo arms of the abatacept, adalimumab and tocilizumab trials, converted to a 3-month cycle risk. For each biologic DMARD, the risk of flare was derived using the RR for that treatment compared with methotrexate from the relevant RCT, multiplied by the baseline risk.
Treatment discontinuation
Treatment discontinuation was assumed to be attributable to AEs, lack of efficacy or clinical remission. Estimates for treatment discontinuation were identified through a literature search of trial and registry data. The first model cycle has certain different treatment discontinuation parameters because it was designed to represent the open-label lead-in phase of the RCTs.
The estimates for the first model cycle were taken from the RCTs of the biologic DMARDs42,57,61,68 for the open-label lead-in period (Table 49). For the period after the treatment lead-in, the clinical trials of the biologic DMARDs also include other categories of withdrawal (such as patient/guardian consent or physician decision), but it is unclear whether these categories would also apply to clinical practice or were particular to the trials. As a result, the discontinuation rate after the first model cycle was not taken from these clinical trials but from Tynjala and colleagues146 who conducted a retrospective observational study on JIA patients in Finland taking etanercept or infliximab with a 4-year follow-up.
| Parameter | Abatacept57 | Adalimumab61 | Etanercept42 | Tocilizumab68 |
|---|---|---|---|---|
| AE, % | 0.5 | 1.8 | 1.4 | 1.6 |
| Loss of efficacy, % | 9.5 | 3.5 | 2.9 | 8.0 |
| Total discontinuation, % | 10.0 | 5.3 | 4.3 | 9.6 |
There were few long-term data on treatment discontinuation identified except for etanercept and, hence, we assumed that the discontinuation rate would remain constant over time and would be the same for all biologic DMARDs (based on data for from Tynjala and colleagues146). The discontinuation rate was 7% for AEs and 28% for inefficacy over 4 years. The discontinuation rate for inefficacy for methotrexate was taken from a retrospective analysis of the German Methotrexate Registry,147 which collected data on the efficacy and safety of methotrexate treatment since 2005. The discontinuation rate for inefficacy used in the model was 0.4% per cycle. The STRIVE registry (see Review of cost-effectiveness in company submissions to the National Institute for Health and Care Excellence) reported a methotrexate discontinuation rate for AEs of 2.3% per year and this was converted to a 3-month rate and used in the model. 77
Patients are assumed to have the same mortality rate as for the general population. Mortality was taken from age-related statistics from the Office for National Statistics. 21
Our systematic review of HRQoL studies identified two potentially relevant studies that reported generic preference-based HRQoL studies of people with JIA who received a biologic DMARD. 124,136 Furthermore, none of the clinical trials of the biologic DMARDs under review collected HRQoL data that could be used as health-state utility values. We investigated methods for mapping HRQoL to treatment response, for example from CHAQ or ACR Pedi to HUI3, but concluded that the data available were insufficient to provide a reliable fit for modelling. Therefore, the utility values used in the model were taken directly from the Dutch ABC Registry by Prince and colleagues,124 as this study was considered to be of most relevance from the available literature. This study is described in more detail above (see Systematic review of health-related quality-of-life studies). It consists of patients who have polyarticular-course JIA and in whom the response to the maximum dose of methotrexate is insufficient. The utility value for patients who had not yet received etanercept in that study is assumed to be representative of those patients with uncontrolled disease not currently receiving a biologic DMARD (i.e. those patients in the methotrexate-only arm and those patients who discontinue biologic DMARD therapy). In the study by Prince and colleagues,124 most of the patients were still receiving methotrexate. In the absence of any other utility data, we assumed that all biologic DMARDs would have the same utility values as each other and that this would increase over time, as seen in the Prince and colleagues study. 124 For simplicity, for the scenarios with additional lines of biologic treatments (scenario V), we assumed that treatment with the biologic DMARD would have a constant utility value of 0.74 (i.e. the value after 15 months of treatment for second- and third-line biologic DMARD treatment). The annual health-state utility values used are shown in Table 50.
| HRQoL utility values | Per year | Source |
|---|---|---|
| No treatment | 0.53 | Prince et al. (2011)124 |
| Treatment with first-line biologic, 0–3 months | 0.53 | Prince et al. (2011)124 |
| Treatment with first-line biologic, 3–15 months | 0.69 | Prince et al. (2011)124 |
| Treatment with first-line biologic, 15–27 months | 0.74 | Prince et al. (2011)124 |
| Treatment with first-line biologic, 27+ months | 0.78 | Prince et al. (2011)124 |
| Treatment with second- and third-line biologics | 0.74 | Prince et al. (2011)124 |
| Disutility for disease flare | 0.03 | Assumption |
Patients with a disease flare are assumed to have an associated disutility. We assumed that patients with a disease flare would have a similar HRQoL to patients with uncontrolled disease, but with appropriate treatment would recover from their disease flare within 3 months (one model cycle). Assuming that patients recovered HRQoL at a constant rate over the model cycle, the average HRQoL for these patients during that cycle would be 0.655, and converting this to an annual disutility would be equivalent to 0.03 per flare.
We conducted a literature search of studies reporting the quality-of-life impact on caregivers of patients with JIA and did not identify any studies that reported HRQoL as utility values. The precise quality-of-life impact on primary caregivers of patients with JIA, in terms of change in HRQoL utility values, is unclear. A study by Bruns and colleagues148 examined the HRQoL and disease burden of primary caregivers of 70 patients with JIA. They used the CHAQ, Short Form questionnaire-36 items and the psychiatric screening questionnaire (the Self-Reporting Questionnaire-20 items). The burden of disease on the caregivers was measured by the Caregiver Burden Scale. They concluded that there was a high prevalence of psychoemotional disturbance in JIA caregivers and that the burden of disease on the caregivers was primarily related to patients’ emotional status (rather than to their physical status).
In the absence of suitable HRQoL data for caregiver disutility, we assumed in the base-case analysis that there was no utility benefit for parents of children and young people and varied this assumption in a scenario analysis (scenario IV).
Estimation of costs
Drug costs
Drug unit costs and doses were based on the British National Formulary for Children 2015. 149 A summary of the dose and unit cost of treatment for each of the comparators is given in Table 51. The manufacturers of abatacept and tocilizumab have provided a confidential PAS. Cost-effectiveness results for these treatments presented in Results of the independent economic analysis are based on the drug list price, whereas a CiC separate appendix to this report available only to the NICE Appraisal Committee presents results with the confidential PAS discount applied. Patient height, weight and body surface area were taken from the British National Formulary and reflect the increase in children’s heights and weights as they grow older. 150 The administration costs for an intravenous infusion was £154 based on a HTA monograph of disease-modifying drugs in the treatment of rheumatoid arthritis (Stevenson and colleagues139). We assumed that for patients taking methotrexate, half would receive oral and half subcutaneous administration, based upon clinical advice.
| Parameter | Methotrexate | Abatacept | Adalimumab | Etanercept | Tocilizumab |
|---|---|---|---|---|---|
| Drug dose | 10–15 mg/m2 | 10 mg/kg | 24 mg/m2 (maximum 40 mg) aged 4–13 years 40 mg (aged 13–18 years) |
0.4 mg/kg (up to a maximum of 25 mg per dose) | 10 mg/kg for patients < 30 kg; 8 mg/kg for patients > 30 kg |
| Method | Subcutaneous injection/oral | Intravenous infusion | Subcutaneous injection | Subcutaneous injection | Intravenous infusion |
| Dosing schedule | Once weekly | Infusions given at weeks 0, 2, 4, 8, 12, 16 | Every other week | Twice weekly | Every 4 weeks |
| Unit cost | Oral: 2.5 mg 24-tab pack = £2.22; 28-tab pack = £2.60 Subcutaneous: Metoject (Medac GmbH, Wedel, Germany) pre-filled syringe. 50 mg/ml: 0.15 ml = £14.85; 0.2 ml = £15.29; 0.3 ml = £16.57; 0.4 ml = £17.84; 0.5 ml = £18.48; 0.6 ml = £18.95 |
250-mg vial = £302.40 | 40-mg prefilled pen or prefilled syringe = £352.14 | 10-mg vial = £35.75; 25-mg vial = £89.38; 25-mg prefilled syringe = £89.38 | 3 ml (80-mg vial) = £102.40; 10 ml (200-mg vial = £256; 20 ml (400-mg vial) = £512.00 |
| Administration cost, £ | 0 | 154 | 0 | 0 | 154 |
Patients taking biologic DMARDs also receive concomitant methotrexate treatment as shown in Table 52. These values have been taken from the RCTs or registries for these treatments. Patients receiving etanercept in the model do not also receive methotrexate, according to etanercept’s marketing authorisation. It was assumed that 20% of patients in the methotrexate comparator arm would be intolerant to methotrexate and therefore would not receive it. 124
| Drug use | Methotrexate only | Abatacept | Adalimumab | Etanercept | Tocilizumab |
|---|---|---|---|---|---|
| Methotrexate use, % | 80 | 80 | 69 | 0 | 82 |
Resource use
We conducted a literature search for costing studies in patients with JIA and identified two relevant studies. Thornton and colleagues153 examined the resources used and associated patient-based costs during the first year after diagnosis for JIA patients in the UK. Prince and colleagues124 analysed the costs of treatment for patients in the Dutch ABC Register before and after receiving etanercept. There are limitations to both studies: the patients in the Thornton and colleagues153 study are likely to have different resources and costs in the first year after diagnosis than the patients included in this assessment report; for example, they may have had less severe disease. The resources used by patients in the Prince and colleagues study124 are not reported and it is unclear how different Dutch health-care costs would be to the NHS. Our clinical experts commented that the resources for monitoring patients costs were not substantially different between the patients treated with methotrexate only or with a biologic DMARD, and were broadly similar to those in the Thornton and colleagues153 study. We therefore used the resources described by Thornton and colleagues in the base case and explored the costs used by Prince and colleagues124 in a scenario analysis (scenario II). The assumed resources used by patients are shown in Table 53. Blood tests consisted of the combined cost of full blood count, CRP, urea and electrolytes and a liver function test. Clinical imaging consisted of the combined cost of magnetic resonance imaging scan, dual-energy X-ray absorptiometry scan, ultrasound and radiography. The total health-care cost for patients on biological treatment and off biologic treatment using the resources shown in Table 53 was £724 per cycle.
| Resource per year | Resource use per year | Unit cost, £ | Reference | |
|---|---|---|---|---|
| Off biologic treatment | On biologic treatment | |||
| General practitioner visit | 10 | 10 | 46.00 | PSSRU (2013)151 |
| Hospital appointments | ||||
| Rheumatology paediatric consultant | 5.58 | 5.58 | 234.86 | National reference costs 2013/14152 (OP code 262) |
| Ophthalmologist | 2.69 | 2.69 | 114.73 | National reference costs 2013/14152 (OP code 216) |
| Specialist nurse | 7.00 | 7.00 | 40.00 | PSSRU 2013151 |
| Physiotherapist | 4.00 | 4.00 | 16.50 | PSSRU 2013151 |
| Occupational therapist | 0.65 | 0.65 | 16.50 | PSSRU 2013151 |
| Podiatry | 0.61 | 0.61 | 43.59 | National Reference Costs 2013/14152 (OP code 653) |
| Hospital tests | ||||
| Blood tests | 1 | 1 | 46.27 | Thornton et al. (2008),153 updated to 2013/14 values using PSSRU HCHS Index |
| Clinical imaging | 1 | 1 | 386.42 | |
| Disease flare | ||||
| Inpatient treatment per disease flare | 429.97 | National Reference Costs 2013/14152 (non-elective short stay. Weighted average codes HD23D–HD23JK) | ||
Patients who experienced a disease flare received one or more injections of intra-articular steroids and were treated as paediatric rheumatology inpatient cases at a cost of £429.97. 152
Adverse events
The database of studies from our systematic review was searched for studies reporting any AEs or discontinuation. In addition, the CSs were consulted for any relevant data. Although the types and frequencies of AEs were reported, no cost data were identified in any of the studies that reported SAEs or discontinuation rates, or in observational studies reported in the CSs. In order to identify data, previous NICE TAs were searched. Neither of the JIA technology appraisals, TA35 and TA238, contained data on the cost of SAEs. 43,44
Owing to the paucity of data relating to JIA, TAs of rheumatoid arthritis were also assessed. Of the six TA publications available on the NICE website,154–159 only one contained data for the cost of an AE, namely TA195: Adalimumab, Etanercept, Infliximab, Rituximab and Abatacept for the Treatment of Rheumatoid Arthritis after the Failure of a TNF Inhibitor. 158 A Pfizer CS provided the only relevant cost data in TA195. Pfizer assumed that a SAE involved two general practitioner visits, 7 days of hospitalisation and a utility decrement of 0.05, with a total cost of £1181. No specific AEs were identified by Pfizer. Further details on the types of SAE experienced by JIA patients are given in Chapter 4, Assessment of clinical effectiveness: biologic disease-modifying antirheumatic drugs versus placebo (with methotrexate where permitted) and in Appendix 2. The most common SAEs were serious infections and infestations, but SAEs also included autoimmune diseases and malignancies. All independent analyses in rheumatoid arthritis conducted by assessment groups to this point have been based on the Birmingham Rheumatoid Arthritis Model,140 which assigns a cost to increases in HAQ scores. In JIA, it is not possible to model SAE costs in this way owing to lack of HAQ in the RCTs.
In order to model the cost of SAEs, health-care resource group codes for intermediate and severe paediatric infections were consulted. In addition, a study in etanercept patients by Otten and colleagues160 indicated that the median length of hospitalisation for SAEs was 9 days (IQR 2–12). Given this, we estimated inpatient costs by averaging all spells for intermediate and major paediatric infections (£1532.87).
A summary of the input parameters used in the model are shown in Table 54.
| Parameter | Mean | Higher value | Lower value | Source |
|---|---|---|---|---|
| Starting age, years | 11 | 15 | 6 | Assumption, based on RCTs |
| Time horizon, years | 30 | 10 | 70 | Assumption |
| Discount rate (costs), % | 3.5 | 6 | 1.5 | NICE reference case130 |
| Discount rate (benefits), % | 3.5 | 6 | 1.5 | NICE reference case130 |
| Utility values, per cycle | ||||
| No treatment | 0.13 | 0.15 | 0.11 | Prince et al. (2011)124 |
| Treatment after 3 months | 0.17 | 0.20 | 0.15 | Prince et al. (2011)124 |
| Treatment after 15 months | 0.19 | 0.21 | 0.16 | Prince et al. (2011)124 |
| Treatment after 27 months | 0.20 | 0.23 | 0.16 | Prince et al. (2011)124 |
| Disease flare disutility | 0.03 | 0.04 | 0.02 | Assumption |
| Disease flare, per cycle | ||||
| Placebo | 0.25 | 0.34 | 0.16 | Ruperto et al. (2008),57 Lovell et al. (2008),61 Brunner et al. (2015)68 |
| Abatacept | 0.09 | 0.16 | 0.05 | Ruperto et al. (2008)57 |
| Adalimumab | 0.14 | 0.23 | 0.09 | Lovell et al. (2008)61 |
| Etanercept | 0.09 | 0.17 | 0.04 | Lovell et al. (2000)42 |
| Tocilizumab | 0.14 | 0.20 | 0.09 | Brunner et al. (2015)68 |
| AEs, first cycle, % | ||||
| Abatacept | 0.53 | 1.51 | 0.00 | Ruperto et al. (2008)57 |
| Adalimumab | 1.75 | 3.71 | 0.00 | Lovell et al. (2008)61 |
| Etanercept | 1.45 | 4.19 | 0.00 | Lovell et al. (2000)42 |
| Tocilizumab | 1.60 | 3.36 | 0.00 | Brunner et al. (2015)68 |
| Loss of efficacy, % | ||||
| Abatacept | 9.47 | 13.59 | 5.36 | Ruperto et al. (2008)57 |
| Adalimumab | 3.51 | 6.25 | 0.76 | Lovell et al. (2008)61 |
| Etanercept | 2.90 | 6.82 | 0.00 | Lovell et al. (2000)42 |
| Tocilizumab | 7.98 | 11.90 | 4.06 | Brunner et al. (2015)68 |
| Further-line treatment, % | ||||
| AEs, biologic DMARD | 0.43 | 0.82 | 0.04 | Tynjala et al. (2009)146 |
| Loss of efficacy biologic DMARD | 2.00 | 2.59 | 1.41 | Tynjala et al. (2009)146 |
| AEs, methotrexate | 0.58 | 0.82 | 0.34 | STRIVE (2015)77 |
| Loss of efficacy methotrexate | 0.42 | 0.79 | 0.05 | Klein et al. (2012)147 |
| Costs, £ | ||||
| On biologic DMARD cost | 724 | 940.92 | 506.65 | National Reference Costs 2013/14152 PSSRU 2013151 |
| Off biologic DMARD cost | 724 | 940.92 | 506.65 | |
| SAE cost | 1533 | 1993 | 1073 | National Reference Costs 2013/14152 |
| Disease flare cost | 430 | 301 | 559 | National Reference Costs 2013/14152 |
Results of the independent economic analysis
This section reports the cost-effectiveness results for a person with JIA who received treatment with a biologic DMARD in combination with methotrexate (where permitted) compared with those who received methotrexate only. Results for costs and QALYs are presented for each treatment, with costs and benefits discounted at 3.5%. The results are presented for biologic DMARDs licensed for use as a first-line biologic treatment (i.e. adalimumab, etanercept and tocilizumab) and are then presented for abatacept as a second-line biologic treatment following previous treatment with an antiTNF. The results shown in this section are for the drug list price, and the results with the confidential PAS discount for abatacept and tocilizumab are presented in a separate CiC appendix to this report available only to the NICE Appraisal Committee.
Licensed first-line biologics: adalimumab, etanercept and tocilizumab
The undiscounted summary results of the analyses for adalimumab, etanercept and tocilizumab compared with methotrexate for the treatment effects are shown in Tables 55–57. In the base case, total undiscounted QALYs vary between 14.98 for methotrexate and 17.99 for tocilizumab (see Table 55). Patients on methotrexate have higher QALYs only in the off-biologic-DMARD health state than the patients on biologics, as they spend more time in this health state. The summary results of the undiscounted drug costs are shown in Table 56. The total undiscounted drug acquisition cost of the biologic DMARDs varied between £103,497 and £128,071 for treatment first with etanercept and tocilizumab, respectively, compared with a total undiscounted cost of £7029 for patients treated with methotrexate only. The total patient costs varied between £107,299 and £225,797 for methotrexate only and tocilizumab, respectively. As noted earlier, patients taking etanercept do not receive methotrexate, which partially explains the lower costs for the etanercept regimen.
| Treatment | Health-state QALYs | |||
|---|---|---|---|---|
| On biologic DMARD | Off biologic DMARD | Disease flare | Total | |
| Methotrexate only | N/A | 15.9 | –0.9 | 14.98 |
| Adalimumab | 8.6 | 9.9 | –0.8 | 17.77 |
| Etanercept | 8.5 | 10.0 | –0.7 | 17.81 |
| Tocilizumab | 9.2 | 9.5 | –0.8 | 17.99 |
| Treatment | Total undiscounted costs, £ | ||||
|---|---|---|---|---|---|
| Medical | Drug | AEs | Flare | Total | |
| Methotrexate only | 86,938 | 7029 | 498 | 12,834 | 107,299 |
| Adalimumab | 86,938 | 114,701 | 248 | 10,805 | 212,693 |
| Etanercept | 86,938 | 103,497 | 254 | 9766 | 200,454 |
| Tocilizumab | 86,938 | 128,071 | 269 | 10,519 | 225,797 |
| Treatmenta | Costs, £ | QALYs | Incremental costs, £ | Incremental QALYs | ICER (£ per QALY gained) vs. methotrexateb |
|---|---|---|---|---|---|
| Methotrexate only | 67,426 | 9.35 | |||
| Adalimumab | 145,047 | 11.40 | 77,513 | 2.0 | 38,127 |
| Etanercept | 134,868 | 11.44 | 67,334 | 2.1 | 32,526 |
| Tocilizumab | 150,530 | 11.52 | 82,995 | 2.1 | 38,656 |
The base-case discounted cost-effectiveness results are shown in Table 57. Each of the biologic DMARDs is more expensive than methotrexate only, with the incremental cost ranging from £77,513 to £82,995 for etanercept and tocilizumab, respectively. The ICER versus methotrexate only for adalimumab, etanercept and tocilizumab is £38,127, £32,256 and £38,656 per QALY gained, respectively. The results are not presented as an incremental analysis of the biologic DMARDs, as the costs and QALYs for each biologic DMARD are generally similar and it has been previously discussed in Chapter 4 that the biologic DMARDs may be regarded as similar in effectiveness.
Licensed second-line biologic: abatacept
Abatacept is licensed for use after at least one previous antiTNF biologic DMARD. The results are shown for abatacept, adalimumab, etanercept and tocilizumab compared with methotrexate. For each biologic comparator, patients are assumed to have been treated initially with etanercept as the first-line biologic. The summary results of the non-discounted treatment effects are shown in Table 58. In the base case, total undiscounted QALYs vary between 14.98 for methotrexate and 20.07 for abatacept. The summary results of the undiscounted costs are shown in Table 59. The total undiscounted drug acquisition cost of the DMARDs varied between £7029 for methotrexate only to £222,533 for abatacept. The total patient costs varied between £107,299 and £317,097 for methotrexate only and abatacept.
| Treatment | QALYs | |||
|---|---|---|---|---|
| On biologic DMARD | Off biologic DMARD | Disease flare | Total | |
| Methotrexate only | N/A | 15.9 | –0.9 | 14.98 |
| Abatacept | 15.8 | 4.8 | –0.5 | 20.07 |
| Adalimumab | 15.1 | 5.3 | –0.6 | 19.80 |
| Etanercept | 15.0 | 5.4 | –0.5 | 19.82 |
| Tocilizumab | 15.7 | 4.8 | –0.6 | 20.00 |
| Treatment | Costs, £ | ||||
|---|---|---|---|---|---|
| Medical | Drug | AEs | Flare | Total | |
| Methotrexate only | 86,938 | 7029 | 498 | 12,834 | 107,299 |
| Abatacept | 86,938 | 222,533 | 502 | 7124 | 317,097 |
| Adalimumab | 86,938 | 184,594 | 433 | 8118 | 280,082 |
| Etanercept | 86,938 | 179,686 | 440 | 7311 | 274,374 |
| Tocilizumab | 86,938 | 205,174 | 457 | 7840 | 300,409 |
The base-case discounted cost-effectiveness results are shown in Table 60. The costs and QALYs are different from those for the first-line biologic cost-effectiveness analysis (see Table 57) because this analysis includes the costs and QALYs of two lines of biologics. The cost-effectiveness of abatacept compared with methotrexate is £39,536 per QALY. The results are not presented as an incremental analysis, as the costs and QALYs for the biologic DMARDs are similar.
| Treatment | Costs, £ | QALYs | Incremental costs, £ | Incremental QALYs | ICER (£ per QALY gained) vs. methotrexatea |
|---|---|---|---|---|---|
| Methotrexate only | 67,534 | 9.37 | |||
| Abatacept | 203,276 | 12.80 | 135,742 | 3.4 | 39,536 |
| Adalimumab | 183,387 | 12.65 | 115,853 | 3.3 | 35,284 |
| Etanercept | 179,580 | 12.67 | 112,045 | 3.3 | 33,948 |
| Tocilizumab | 194,263 | 12.76 | 126,728 | 3.4 | 37,363 |
Sensitivity analysis
Deterministic sensitivity analysis
Tables 61–64 show the results of the deterministic sensitivity analyses for each of the biologic DMARDs versus methotrexate for the most influential parameters. Other parameters, such as time horizon, cost and frequency of disease flare, complete response rate and utility values were varied in the sensitivity analyses but were found to have only a negligible effect on the results. For each of the treatments, the models are most sensitive to the utility values chosen while on biologic DMARD treatment. They are also sensitive to the discount rate and the health-state costs.
| Adalimumab vs. methotrexate | High 95% CI, £ | Low 95% CI, £ | Range, £ |
|---|---|---|---|
| Base-case ICER: £38,127 | |||
| Utility treatment, long terma | 26,571 | 67,470 | 40,898 |
| Utility no treatment | 59,814 | 27,982 | 31,832 |
| Discount rate benefits | 45,936 | 32,123 | 13,813 |
| Discount rate costs | 31,919 | 45,016 | 13,097 |
| On biologic DMARD cost | 41,630 | 34,624 | 7006 |
| Off biologic DMARD cost | 34,624 | 41,630 | 7006 |
| Disease flare methotrexate | 35,871 | 40,598 | 4727 |
| AE adalimumab | 37,983 | 33,308 | 4675 |
| Etanercept vs. methotrexate | High 95% CI, £ | Low 95% CI, £ | Range, £ |
|---|---|---|---|
| Base-case ICER: £32,526 | |||
| Utility (treatment, long term)a | 22,886 | 56,196 | 33,310 |
| Utility (no treatment) | 50,511 | 23,986 | 26,525 |
| Discount rate (costs) | 26,909 | 38,783 | 11,874 |
| Discount rate (benefits) | 39,075 | 27,478 | 11,598 |
| Start age | 35,045 | 26,173 | 8873 |
| Off biologic DMARD cost | 29,118 | 35,934 | 6817 |
| On biologic DMARD cost | 35,934 | 29,118 | 6817 |
| Disease flare methotrexate | 30,566 | 34,668 | 4102 |
| Tocilizumab vs. methotrexate | High 95% CI, £ | Low 95% CI, £ | Range, £ |
|---|---|---|---|
| Base-case ICER: £38,656 | |||
| Utility (treatment, long term)a | 26,835 | 69,092 | 42,257 |
| Utility (no treatment) | 58,865 | 28,777 | 30,088 |
| Discount rate costs | 31,360 | 46,904 | 15,545 |
| Discount rate benefits | 47,140 | 32,196 | 14,943 |
| Start age | 42,589 | 32,993 | 9596 |
| On biologic DMARD cost | 42,130 | 35,182 | 6948 |
| Off biologic DMARD cost | 35,182 | 42,130 | 6948 |
| Disease flare methotrexate | 36,395 | 41,130 | 4735 |
| Abatacept vs. methotrexate | High 95% CI, £ | Low 95% CI, £ | Range, £ |
|---|---|---|---|
| Base-case ICER: £39,536 | |||
| Utility (treatment, long term)a | 31,529 | 52,995 | 21,467 |
| Discount rate costs | 30,512 | 50,137 | 19,625 |
| Utility treatment 15–27 months | 32,110 | 51,430 | 19,319 |
| Discount rate benefits | 49,908 | 31,906 | 18,002 |
| Utility no treatment | 50,345 | 32,549 | 17,796 |
| Start age | 42,187 | 33,234 | 8952 |
| On biologic DMARD cost | 43,094 | 35,978 | 7117 |
| Off biologic DMARD cost | 35,978 | 43,094 | 7117 |
The deterministic sensitivity results for adalimumab versus methotrexate are shown in Table 61 and varied between £26,571 and £67,470 per QALY gained.
The deterministic results for etanercept versus methotrexate only varied between £22,886 and £56,196 per QALY gained (see Table 62).
The deterministic sensitivity results for tocilizumab versus methotrexate only varied between £26,835 and £69,092 per QALY gained (see Table 63).
The deterministic sensitivity analysis results for abatacept versus methotrexate only varied between £31,259 and £52,995 per QALY gained (see Table 64).
Scenario analysis
We conducted several scenario analyses to investigate uncertainty for specific aspects of the modelling. The results of these analyses are presented for the first-line biologics.
Discontinuation of treatment owing to clinical remission (scenario I)
Patients with clinical remission off medication are at high risk of relapse. Baszis and colleagues161 conducted a retrospective chart review in a cohort of 171 patients with JIA (of a range of subtypes but predominantly polyarticular course) in the USA treated with TNFα antagonists. They found that 12 months after stopping treatment only 33% of patients still had clinical remission. Similarly, a retrospective chart review of 437 JIA patients from centres in the USA and Italy by Wallace and colleagues162 estimated that 6% of patients who had discontinued methotrexate therapy with clinical remission had persistent remission after 5 years off treatment.
The rate of discontinuation of biologic treatment varies between studies. In a retrospective observational study by Tynjala and colleagues,146 patients receiving etanercept were followed up for 4 years and 10% of patients had discontinued treatment owing to inactive disease. In the study by Baszis and colleagues,161 80% of patients discontinued TNFα antagonist treatment owing to inactive disease. We varied the discontinuation rate between that seen by Tynjala and colleagues146 (used in the base case) and that seen by Baszis and colleagues. 161
We assumed a relapse rate from Baszis and colleagues161 of 67% for that analysis and a 40% relapse rate as seen in Wallace and colleagues162 for the Tynjala and colleagues146 analysis. We assumed that no patients on the methotrexate-only arm would discontinue, as fewer patients on methotrexate would be in remission.
The results for the scenario with patients discontinuing treatment for clinical remission is shown in Table 65 for first-line biologics compared with methotrexate only. In the scenario with the highest discontinuation rate, the cost-effectiveness of the biologics improves from the base case by about £4000 per QALY.
Health-state costs from Prince and colleagues124 (scenario II)
The base-case analysis uses health-state costs estimated by a UK study by Thornton and colleagues153 of patients during the first year after diagnosis. However, as stated earlier, this may not necessarily reflect the patient group in this economic evaluation as patients in that study were newly diagnosed. The Roche CS cost-effectiveness analysis uses health-state costs based on the Prince and colleagues study. 124 Assuming that hospital admissions would be for disease flare only, the health-state costs per cycle are £589.51 and £408.91 for the off-treatment and on-treatment health states, respectively (compared with £724 in the base case). In this analysis, the biologic DMARDs are slightly more cost-effective and the ICER decreases by approximately £2900 per QALY compared with the base-case analysis (e.g. the ICER for adalimumab decreases to £35,214 per QALY) (Table 66).
| Treatment | Costs, £ | QALYs | Incremental costs, £ | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Methotrexate only | 57,306 | 9.37 | |||
| Adalimumab | 128,894 | 11.40 | 71,589 | 2.0 | 35,214 |
| Etanercept | 118,771 | 11.44 | 61,465 | 2.1 | 29,691 |
| Tocilizumab | 134,097 | 11.52 | 76,792 | 2.1 | 35,767 |
Discount rates used in the National Institute for Health and Care Excellence appraisal of etanercept (scenario III)
The previous NICE appraisal of etanercept (NICE TA3543) used a discount rate of 6% for costs and 1% for benefits123 (which were the recommended rates at the time). We ran the analysis for etanercept using those discount rates. Table 67 shows ICERs that are much reduced compared with the base case in the current assessment report, namely £21,718 per QALY. Using this discount rate, etanercept would be cost-effective at a willingness-to-pay threshold of £20,000–30,000 per QALY.
| Treatment | Costs, £ | QALYs | Incremental costs, £ | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Methotrexate only | 51,494 | 12.96 | |||
| Etanercept | 107,200 | 15.53 | 55,707 | 2.6 | 21,718 |
Caregiver benefit (scenario IV)
We were unable to find HRQoL utility values associated with caring for a child or young person with JIA and assumed in the base-case analysis no disutility benefit for parents and caregivers. A study by Kuhlthau and colleagues163 compared the well-being of parents of children with and without activity limitations. This list of conditions includes medical conditions that would commonly be considered disabling (e.g. paraplegia and blindness) as well as typically less disabling but chronic conditions (e.g. attention deficit hyperactivity disorder and asthma). They estimated the disutility for these parents to be 0.07 using the EQ-5D. Values from caregivers’ disutility for patients with multiple sclerosis, from a study by Gani and colleagues,164 indicate that the caregiver disutility is small (< 0.02) until patients reach a health state with significant mobility limitations. 164
Patients receiving a biologic DMARD have a better HRQoL than those eligible for a biologic who have not yet received one. It follows that this improvement in HRQoL may also improve the HRQoL of caregivers, although the magnitude of any improvement is unclear. In this scenario, we assume that the disutility of caregivers is half that for patients on a biologic DMARD compared with those on methotrexate only and vary the disutility according to the values in the studies by Kuhlthau and colleagues163 and Gani and colleagues. 164
The results for the scenario including a disutility for caregivers are shown in Table 68 for first-line biologics compared with methotrexate only. In the scenario with the highest disutility for caregivers, cost-effectiveness improves, with the ICER for etanercept reducing to £28,619 per QALY.
Three lines of biologic therapy (scenario V)
In the base-case analysis, patients treated with adalimumab, etanercept and tocilizumab received one line of biologic DMARD treatment, and those treated with abatacept received two lines of biologic DMARDs to account for the licensed indication for that drug. In this scenario, patients can receive three lines of biologic DMARDs to allow for treatment switching as happens in clinical practice. We included a scenario in which patients received etanercept as the first-line biologic, adalimumab as the second-line biologic and tocilizumab as the third-line biologic. These were the most common first-line and second-line treatments in an analysis of the UK Childhood Arthritis Prospective Study. 23 We also presented an alternative analysis with a third-line biologic of abatacept instead of tocilizumab.
The cost-effectiveness of the two scenarios varied between £36,982 and £38,152 per QALY (Table 69). The cost-effectiveness of three-line biologic therapy is similar to that seen in the base-case analysis for one line of biologic therapy (see Table 57).
| Treatment | Costs, £ | QALYs | Incremental costs, £ | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Methotrexate only | 67,534 | 9.37 | |||
| Etanercept, adalimumab, tocilizumab | 207,565 | 13.16 | 140,031 | 3.8 | 36,982 |
| Etanercept, adalimumab, abatacept | 212,562 | 13.17 | 145,028 | 3.8 | 38,152 |
Younger biologic disease-modifying anti-rheumatic drug starting age (scenario VI)
Adalimumab, etanercept and tocilizumab have a licensed indication from 2 years of age for patients with polyarticular arthritis, and abatacept has a licensed indication from ≥ 6 years. In this scenario we investigated the cost-effectiveness of the biologics with a starting age of 6 years old (in the base case the starting age is 11 years). The results of the analysis for first-line biologics are shown in Table 70. These indicate that there is minimal difference in the cost-effectiveness for adalimumab but a decrease of about £6000 in the cost-effectiveness of etanercept and tocilizumab.
| Treatment | Costs, £ | QALYs | Incremental costs, £ | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Methotrexate only | 67,492 | 9.39 | |||
| Adalimumab | 145,089 | 11.42 | 77,597 | 2.0 | 38,124 |
| Etanercept | 121,737 | 11.46 | 54,245 | 2.1 | 26,173 |
| Tocilizumab | 138,421 | 11.54 | 70,929 | 2.1 | 32,993 |
The results of the analysis for second-line biologics are shown in Table 71. These indicate a similar improvement in the cost-effectiveness and there is a reduction in the cost-effectiveness of abatacept of £6302 per QALY.
| Treatment | Costs, £ | QALYs | Incremental costs, £ | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Methotrexate only | 67,492 | 9.39 | |||
| Abatacept | 181,776 | 12.83 | 114,285 | 3.4 | 33,234 |
| Adalimumab | 170,364 | 12.68 | 102,872 | 3.3 | 31,283 |
| Etanercept | 163,006 | 12.69 | 95,514 | 3.3 | 28,895 |
| Tocilizumab | 176,066 | 12.78 | 108,575 | 3.4 | 31,961 |
Probabilistic sensitivity analyses
In the PSA, all parameters were sampled probabilistically from an appropriate distribution using similar ranges as used in the deterministic sensitivity analyses. The parameters sampled were: treatment effectiveness, discontinuation rate, health-state costs, disease flare parameters and HRQoL. The distribution assigned to each variable included in the PSA and the parameters of the distributions are reported in Appendix 11.
First-line biologics
A total of 1000 simulations were run. The PSA results are presented in Table 72 for first-line biologics and show similar results to the deterministic analyses (see Table 58). The cost-effectiveness for biologics versus methotrexate only varied between £32,554 and £38,744 per QALY for tocilizumab.
| Treatment | Costs, £ | QALYs | Incremental costs, £ | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Methotrexate only | 67,531 | 9.38 | |||
| Adalimumab | 145,933 | 11.43 | 78,402 | 2.05 | 38,181 |
| Etanercept | 135,803 | 11.48 | 68,272 | 2.10 | 32,554 |
| Tocilizumab | 151,800 | 11.55 | 84,269 | 2.18 | 38,744 |
The cost-effectiveness acceptability curve is shown in Figure 9 and indicates that at the £20,000 and £30,000 willingness-to-pay thresholds methotrexate has the highest probability of being cost-effective, at 0.98 and 0.62, respectively.
FIGURE 9.
Cost-effectiveness acceptability curve from the PSA for first-line biological treatments compared with methotrexate.
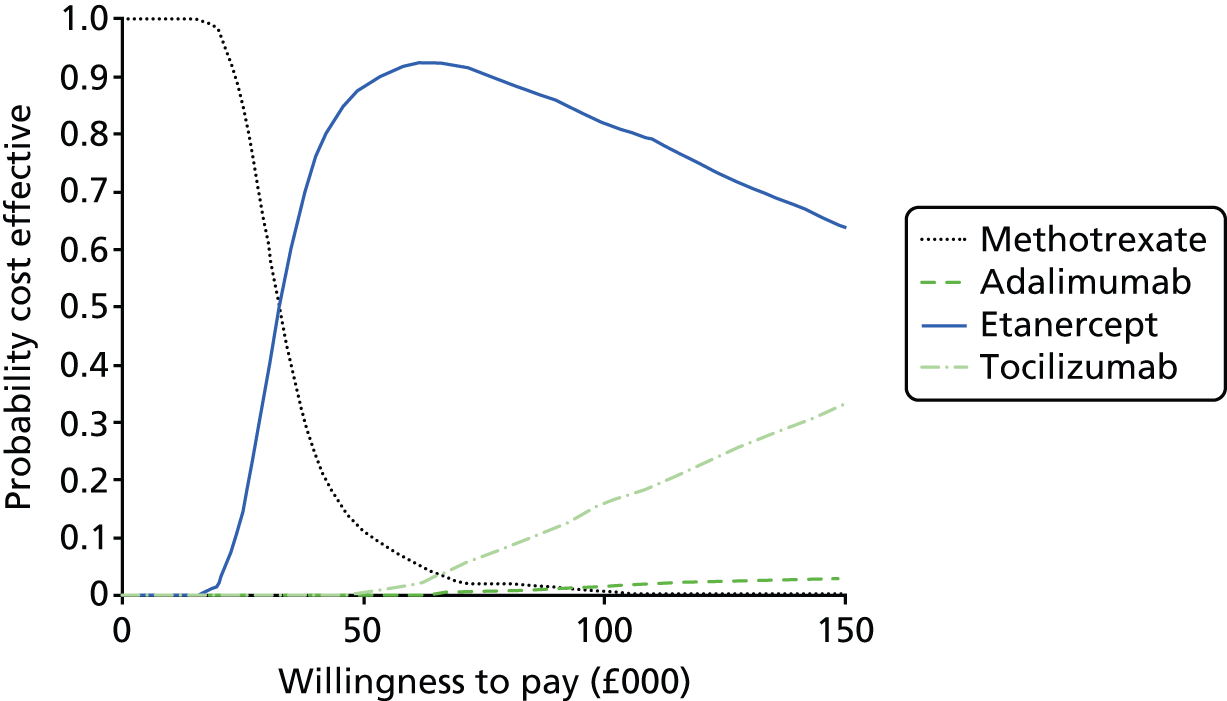
Second-line biologics
The PSA results are presented in Table 73 for second-line biologics and show similar results to the deterministic analyses (see Table 61). The cost-effectiveness of abatacept in the PSA is £39,608 per QALY.
| Treatment | Costs, £ | QALYs | Incremental costs, £ | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Methotrexate only | 67,168 | 9.35 | |||
| Abatacept | 203,396 | 12.81 | 136,041 | 3.43 | 39,608 |
| Adalimumab | 183,563 | 12.66 | 116,208 | 3.29 | 35,366 |
| Etanercept | 179,807 | 12.67 | 112,452 | 3.30 | 34,053 |
| Tocilizumab | 194,464 | 12.77 | 127,109 | 3.39 | 37,443 |
The cost-effectiveness acceptability curve is shown in Figure 10 and indicates that at the £20,000 and £30,000 willingness-to-pay thresholds methotrexate has the highest probability of being cost-effective, at 0.99 and 0.71, respectively.
FIGURE 10.
Cost-effectiveness acceptability curve from the PSA for second-line biological treatments compared with methotrexate.
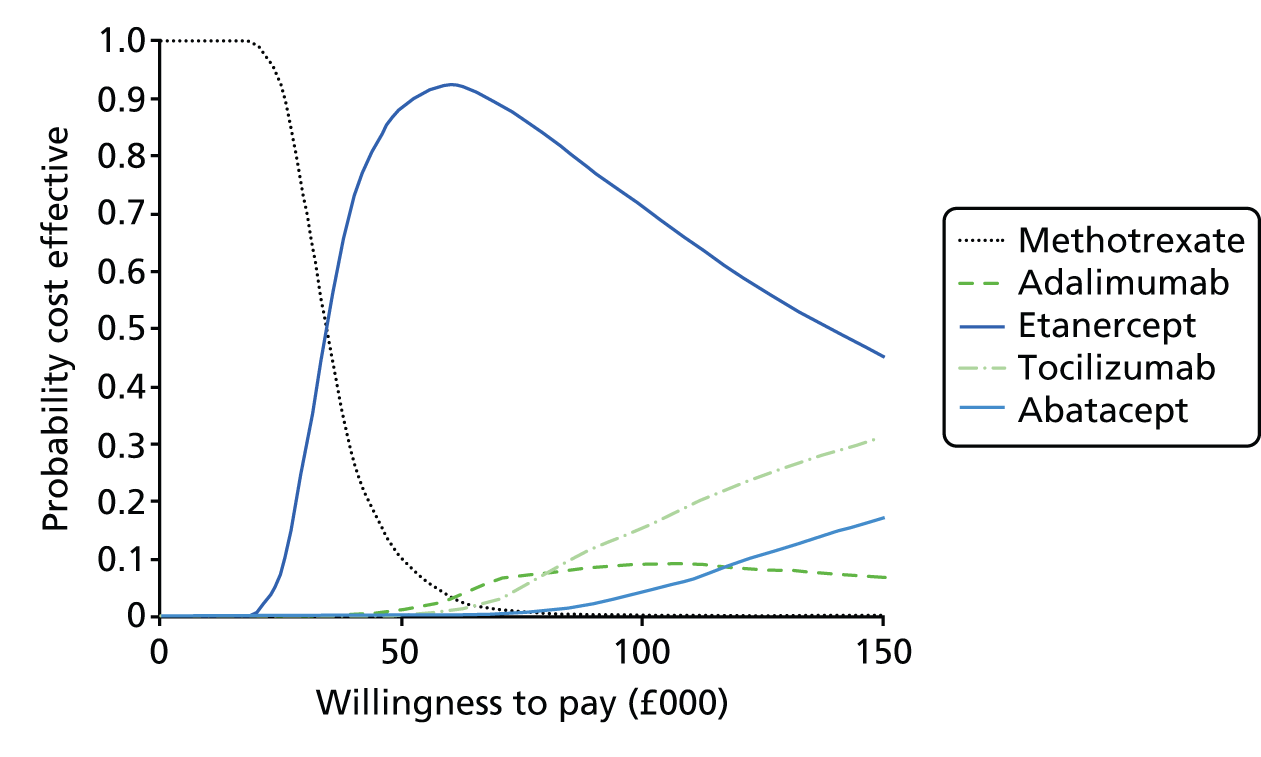
Subgroups
There are a number of potential subgroups that were within the NICE scope, including the subtypes of JIA (EO, polyarticular arthritis, ERA and PA) and patients with extra-articular manifestations such as uveitis. As stated earlier, subgroup analyses by subtype of JIA was not possible owing to insufficient evidence for input parameters to support modelling. The modelled patient population is therefore people with JIA, with the results of particular relevance to those with polyarticular-course JIA (EO, and RF+ve and RF–ve polyarthritis).
In considering the potential for modelling the clinical effectiveness and cost-effectiveness of biologic DMARDs in patients with JIA-associated uveitis, a draft NHS clinical commissioning policy on the use of antiTNFα agents in paediatric patients with severe refractory uveitis was consulted. 29 The policy discusses the cost-effectiveness of treatment and the elements of an economic evaluation are given, although the full results from such an economic evaluation have not been reported.
The report states that infliximab and adalimumab in combination with methotrexate are widely used worldwide for the treatment of refractory uveitis, and that etanercept is not recommended for use in this patient group. The report also cites evidence from a systematic review by Simonini and colleagues96 (see Chapter 4, Juvenile idiopathic arthritis-associated uveitis), which shows that, based on a pooled analysis of observational studies, the proportion of children with improved intraocular inflammation (responders) was 87% for adalimumab, 72% for infliximab and 33% for etanercept. Potential modelling of the clinical effectiveness and cost-effectiveness in JIA-associated uveitis in this report would therefore apply only to adalimumab, as this is the only one of the four biologic DMARDs within the scope of the appraisal recommended for treating this patient subgroup.
With regard to QoL, the clinical commissioning policy assumes that loss of vision causes detrimental effects on utility based on the results of a study of age-related macular degeneration by Reeves and colleagues. 165 This study measured HRQoL changes associated with loss of vision using data from the SF-6D and best corrected visual acuity. This population is quite different from JIA-associated uveitis, and the data do not capture aspects of JIA related to arthritic joints.
In our model, we have used utility data derived using the HUI3 generic preference instrument from the study by Prince and colleagues. 124 This instrument is appropriate for conditions that involve vision impairment, as it includes a domain for vision. HUI3 is not compatible with the SF-6D and the instruments will produce different QoL estimates. Attempting to combine data from Reeves and colleagues165 and Prince and colleagues124 would be inappropriate owing to the differences in the populations of the studies and the incompatibility of SF-6D and HUI3. Moreover, if it is assumed that adding vision loss to the other QoL decrements owing to advancing JIA even partially decreases patient QoL, then it follows that adalimumab will be more cost-effective in JIA patients with uveitis and joint inflammation than it is in JIA patients without uveitis.
Likewise, if most of the costs related to uveitis relate to the management of vision loss, as stated in the clinical commissioning policy,29 then any reduction of these costs attributable to improving vision would increase cost-effectiveness in the subgroup of JIA patients with uveitis. Any additional analysis of cost-effectiveness in a JIA uveitis population that is refractory to methotrexate, as is indicated in the licensing for adalimumab, is therefore likely to have predictable results.
As discussed Chapter 4, the SYCAMORE trial of adalimumab and methotrexate in JIA-associated uveitis patients has recently closed early following an interim analysis that showed a favourable effect for treatment. 87 The trial also includes a cost-effectiveness analysis, the results of which would be likely to concur with the logical implications discussed above.
Comparison of the economic models
The cost-effectiveness of biologic DMARDs estimated in this report varies between £30,000 and £40,000 per QALY gained compared with methotrexate only. This is higher than estimated by the previous NICE appraisal for etanercept in patients with JIA, which estimated an ICER of £16,082 per QALY gained. 123 The NICE Appraisal Committee accepted that the ICER for etanercept was likely to be in the region of £15,000–30,000 per QALY. 43 The model used in that NICE appraisal is not fully described and so it difficult to compare with the current model developed for this report. However, the discount rate for that appraisal was 6% for costs and 1% for benefits. Using these discount rates in the independent model in this assessment report gives cost-effectiveness estimates of between £20,000 and £30,000 per QALY gained.
A cost–consequence analysis conducted by Prince and colleagues124 in the Netherlands did not estimate the cost-effectiveness of etanercept. We have estimated the cost-effectiveness of etanercept compared with methotrexate from that study, by aggregating the costs and QALYs in each time period reported, to be £32,590 (€43,300).
Comparing the results from the independent model in this assessment report with those submitted by the companies was complicated by differences in structure between the models. Roche, who manufacture tocilizumab, was the only company that submitted a full economic analysis to NICE, including costs and QALYs and with a 25-year time horizon. BMS, who manufacturer abatacept, submitted a model with a 20-year time horizon with only drug and administration costs. In addition, in one company model, patients receive oral methotrexate (Roche), and in another company model, patients receive subcutaneous methotrexate (BMS). It was therefore only possible to compare drug costs between the three models with a 20-year time horizon and with discounting applied to allow a level comparison between the models independent of structural assumptions. Table 74 shows the comparison with the Roche model with patients using oral methotrexate, whereas Table 75 shows the comparison with the BMS model with patients using subcutaneous methotrexate.
| Treatment | Assessment report model (using oral methotrexate) | Roche model | ||
|---|---|---|---|---|
| Drug costs, £ | Total costs, £ | Drug costs, £ | Total costs, £ | |
| Methotrexate | 393 | 49,178 | CiC information has been removed | CiC information has been removed |
| Adalimumab | 71,992 | 119,269 | CiC information has been removed | CiC information has been removed |
| Etanercept | 65,396 | 111,941 | CiC information has been removed | CiC information has been removed |
| Tocilizumab | 74,578 | 121,725 | CiC information has been removed | CiC information has been removed |
| Treatment | Assessment report model (using subcutaneous methotrexate) | BMS model | ||
|---|---|---|---|---|
| Drug costs, £ | Total costs, £ | Drug costs, £ | Total costs, £ | |
| Methotrexate | 8012 | 56,798 | CiC information has been removed | CiC information has been removed |
| Adalimumab | 81,804 | 129,081 | CiC information has been removed | CiC information has been removed |
| Etanercept | 70,368 | 116,914 | CiC information has been removed | CiC information has been removed |
| Tocilizumab | 85,312 | 132,459 | CiC information has been removed | CiC information has been removed |
It should be noted that the Roche analysis has not compared the biologic DMARDs against methotrexate in its submission but has compared adalimumab with tocilizumab; however, this analysis was present in the economic model.
As can be seen, there was variation in costs between the models. The Roche model has lower drug costs and total costs than the assessment report model. This is because their model uses a higher discontinuation rate so patients remain on the biologic for a shorter duration, and with lower health-state costs. The BMS model does not include discontinuation for any cause, which explains why it has the highest drug costs of the above models. Overall, the differences between the model results may be explained by differences in model structures and choices with regard to discontinuation, AEs and other costs.
Discussion
-
A systematic search of the literature found four relevant economic evaluations of biologic DMARDs for patients with JIA. Two of the studies were presented as cost–utility studies,123,125 one was a cost-effectiveness study126 and the other was a cost–consequence study. 124 The evaluations were published between 2002 and 2012 in the UK, the Netherlands, Russia and Canada. One of the studies was the previous NICE appraisal of etanercept. 123 The studies varied in design and structure, time horizons and the comparators included. The limitations in the methodological quality in all the studies identified include limited reporting of model parameters and assumptions.
-
A systematic search of the literature found two HRQoL studies in children and adolescents with JIA. One study assessed the effectiveness of a foot care programme in a RCT setting,136 whereas the other evaluated QoL in a cohort of patients from the Dutch ABC Registry before and after treatment with etanercept. 124,137
-
Four pharmaceutical companies submitted evidence to NICE for consideration in this appraisal. Only one company (Roche) constructed a cost–utility analysis that included both costs and outcomes. 78 Two companies (BMS85 and Pfizer97) submitted cost analyses and assume that the biologic DMARDs were equivalent in effectiveness, whereas AbbVie77 did not submit an economic analysis owing to limitations identified with any potential analysis. Roche submitted a Markov state-transition model with health states for uncontrolled/off treatment, on treatment and dead. The model compared treatment with adalimumab to tocilizumab. The base-case results from the submission conclude that tocilizumab is of similar effectiveness and is less expensive than adalimumab.
-
We developed an independent cost–utility model comparing the biologic DMARDs to methotrexate alone. From this model, the incremental cost-effectiveness of adalimumab, etanercept and tocilizumab versus methotrexate only is estimated at £38,127, £32,526 and £38,656 per QALY gained, respectively. An analysis comparing second-line biologics with methotrexate only estimated a cost-effectiveness ratio of £39,536 per QALY gained. The model results are most sensitive to changes to the HRQoL utility values.
Chapter 6 Assessment of factors relevant to the NHS and other parties
Etanercept was recommended by NICE in 2002,131 and it is known that adalimumab, abatacept and tocilizumab are commonly used in practice (as well as infliximab – although not in the scope of this NICE appraisal). 144 It is unlikely that any positive NICE recommendations for the use of these biologic DMARDs will significantly increase the number of patients requesting treatment and thus affect budget impact.
Given that biologic DMARDs are currently used in the management of patients with JIA in the NHS, it is unlikely that substantial modifications will be needed to services, such as infrastructure development or increased staff training. However, a survey of services for children, young people and families living with JIA in the UK by the National Rheumatoid Arthritis Society found that, among the 13 specialist (tertiary) centres surveyed, there was a shortfall of staff to adequately cover the services required. 166 These included paediatric rheumatology consultants and clinical nurse specialists, clinical psychologists, occupational therapists and physiotherapists. Further recruitment and training of professionals to make up the multidisciplinary teams needed to provide effective treatment and care of JIA patients would seem to be necessary.
A long-term condition such as JIA can have a significant impact on children and young people’s education. They may need to miss lessons to attend health-care appointments and may be absent for longer periods of time while experiencing symptoms (including disease flares) or if joint or other surgery is required. This can have a negative impact on educational attainment and, in turn, on their ability to gain employment in adulthood. It may also affect their social and psychological health, through a reduced ability to participate in social and leisure activities and sport, and the general burden of a serious health condition during the sensitive period of adolescence. The effect of this may, therefore, widen socioeconomic and health inequalities in this group. Only one of the RCTs included in the systematic review of clinical effectiveness reported the impact of treatment (abatacept)58 on missed school days. This outcome was not formally included in our review, but it was found that treated patients experienced a statistically significantly higher increase in school days (1.9 days) than placebo patients (0.9 days). This indicates the potential for biologic DMARDs to improve education as well as health outcomes, although further evidence is required, particularly in a UK context.
Schools and health services are required to liaise to ensure appropriate care for children and young people with JIA. The National Rheumatoid Arthritis Society survey of 13 specialist centres found that all centres liaise with schools by letter or telephone, but fewer than half were unable to visit schools or provided only a limited service. 166 However, there were some examples of greater involvement, such as in one centre where the clinical nurse specialist will visit schools and give talks if required. Effective liaison between health services and schools is important to ensure that the needs of children with JIA receiving biologic DMARDs are adequately met.
The impact of JIA on parents and caregivers can also be significant. For example, they may have to pay for child care, take time away from work, or even cease employment altogether to provide care. This will negatively affect their income and may increase dependency on welfare benefits (where available). Again, this is likely to increase socioeconomic inequalities. The inability of parents and caregivers to work may have a negative impact on society and the economy, through reduced productivity, less income tax collection and, in some professions, a shortage of skilled workforce capacity. The impact of treating JIA on parents and caregivers was generally not assessed by the RCTs in the systematic review of clinical effectiveness. However, one of the RCTs (abatacept)58 reported improvements in the number of days of normal activity per month missed by parents, including work and non-work activities, compared with placebo. The number of days on which paid care was required remained stable in both trial arms (following an initial decline in the open-label lead-in phase with abatacept treatment). Further evidence on the impact of biologic DMARD treatment on parents and carers would be useful to gauge the full potential benefits of treatment beyond the patients themselves.
Chapter 7 Discussion
Statement of principal findings
Clinical effectiveness
The systematic review of clinical effectiveness conducted for this report found that biologic DMARDs are superior to placebo (with methotrexate where permitted) across a number of outcome measures in children with JIA (predominantly polyarticular course) and an insufficient response to previous DMARD treatment. With the exception of the etanercept trial, the majority of patients in the trials received methotrexate in addition to the biologic DMARD/placebo. Biologic DMARD-treated patients had fewer arthritis flares, a longer time to disease flare (applicable to abatacept, adalimumab and etanercept), were more likely to achieve a treatment response as defined by the ACR Pedi criteria and were more likely to have inactive disease (measured only in the abatacept and tocilizumab trials). This last outcome can be considered to be the most clinically significant, as absence of disease activity (e.g. no joints with active arthritis; PGA indicates no disease activity, etc.) is a key treatment goal. Treatment was associated with reduced pain scores, although this was reported as statistically significant in only one study (tocilizumab). HRQoL as measured by the CHAQ appeared to be higher for treated patients, although this was not always statistically significant.
The percentage of patients achieving ACR Pedi-30 in the open-label lead-in phases of the RCTs ranged from 65% to 94% across the trials. It should be acknowledged that owing to the withdrawal design of the RCTs, in which only patients achieving an ACR Pedi-30 response during the open-label lead-in phase are eligible for randomisation, the results of the double-blind randomised phase of the trials are therefore applicable only to patients who have achieved an initial degree of treatment benefit. The effects seen during the double-blind period in the placebo group may not necessarily be the same for a placebo group who had not received a biologic DMARD prior to randomisation. However, expert clinical opinion suggests that ACR Pedi-30 can be considered an inadequate or partial response threshold, and higher rates, such as ACR Pedi-70 or above, are considered more clinically significant. In this respect, the patients responding to ACR Pedi-30 in the open-label lead-in phase (and eligible to be randomised) may not necessarily be considered atypical of patients eligible for treatment in clinical practice, as both would have active disease.
The clinical significance of the ACR Pedi-30 results of the randomised phases of the trials may also be questioned. ACR Pedi-30 response rates varied from 63% to 80% across the trials and declined with increasing response thresholds. Nonetheless, at ACR Pedi-70 (the highest threshold for which data were available across all four RCTs), the response rate varied from 44% to 65% and remained higher in biologic DMARD-treated patients than placebo patients in all trials. Research is under way to further develop the JADAS tool as a clinically useful measurement tool,35–37 although clinical trials are continuing to use the ACR Pedi criteria, albeit with effectiveness judged at thresholds higher than ACR Pedi-30.
In the longer term, treatment effectiveness, in terms of ACR Pedi response, appears to be sustained, as reported in the observational OLE studies for all four included RCTs. The longest follow-up available is for etanercept, where ACR Pedi responses were maintained up to 8 years after treatment.
The occurrence of AEs was generally similar between biologic DMARD- and placebo-treated patients, based on reported non-statistically significant differences. A range of AEs were reported, including viral and upper respiratory tract infections, injection-site reactions and nasopharyngitis. Serious AEs were uncommon. Discontinuations attributable to AEs were also uncommon (< 3% patients). In the lead-in phase of the RCTs, discontinuations attributable to AEs were low, ranging from 0.5% to 1.8%. The incidence of AEs and SAEs during open-label long-term follow-up did not appear to be excessive. The safety profile of the biologic DMARDs, therefore, appears to be relatively favourable.
Subgroup analyses were reported in only one of the included RCTs (tocilizumab). 68 Patients receiving methotrexate background therapy had higher ACR Pedi response rates than those not receiving it, as did patients receiving background glucocorticoids. Patients who had received previous treatment with a biologic agent had lower ACR Pedi responses than those who were naive to biologic DMARDs. It is not clear whether these subgroup analyses were pre-planned or post hoc, so caution is advised in their interpretation.
Two recently published systematic reviews of the effectiveness of biologic DMARDs were identified during the production of this report. 167,168 Both of these included a range of biologic DMARDs, including the four relevant to the scope of this assessment. However, none of these reviews identified any additional RCT evidence to this assessment report. The only other relevant published systematic review of biologic DMARDs that we are aware of is by Otten and colleagues,79 (most recent search date January 2012). As discussed earlier in this report [see Chapter 4, Assessment of clinical effectiveness: biologic disease-modifying antirheumatic drugs versus each other (with methotrexate where permitted)], Otten and colleagues79 conducted an adjusted indirect comparison of adalimumab, abatacept and etanercept, using the same RCTs as included in this assessment report. We replicated the indirect comparison, extending it to include the tocilizumab RCT,68 which was not published during the timescale of the Otten and colleagues79 review. Our results and conclusions match those of Otten and colleagues,79 namely that the biologic DMARDs appear similar in effectiveness in polyarticular-course JIA, in terms of ACR Pedi response and preventing disease flares. Otten and colleagues79 also share some of the caveats made in this assessment report about the limitations of the data included in the indirect comparison, namely, the small number of trials (and patient numbers), and differences between the trials in key patient characteristics and in treatment duration.
The conclusion that biologic DMARDs may be similar in clinical effectiveness was supported by the expert advisers to this assessment report. In their experience, there is similarity in effects between the drugs at a population level. However, it is noted that interpatient variation in effects may occur, and comparative effectiveness of the biologic DMARDs may potentially vary between JIA subtypes. Currently, there are a lack of clinical trial data to confirm this. Experts suggested that future trials of biologic DMARDs should stratify by disease phenotype to assess the differential effects of each treatment.
As noted earlier in this report, the RCTs of the biologic DMARDs included a mixture of JIA subtypes, broadly under the classification of polyarticular-course JIA (including EO). The trials did not appear to include patients with ERA or PA; thus, we reviewed available evidence from trials in progress (see Chapter 4, Ongoing trials) and from non-randomised studies (see Chapter 4, Additional supporting evidence) to gauge the effectiveness of biologic DMARD treatment in these groups. Much of the evidence is for etanercept (licensed for ERA and PA) with some available for adalimumab (licensed for PA). A broad comparison of the results of these studies with those of the RCTs included in this assessment report suggests that effectiveness is generally similar between these JIA subtypes. For example, ACR Pedi-70 response rates for biologic DMARDs were in the range of 44–65% across the RCTs (see Table 14), compared with around (CiC information has been removed) across the JIA subtypes in the CLIPPER study of etanercept99 (see Table 34) (notwithstanding differences in study variables such as length of follow-up). Evidence from trials in progress will provide greater clarity regarding the efficacy and safety of biologic DMARDs in these JIA subtypes. At present, there do not appear to be any studies of the comparative effectiveness of biologic DMARDs in these subtypes (e.g. adalimumab vs. etanercept).
All of the RCTs were multinational, with only one specifying that it included patients from the UK. The distribution of JIA subtypes within the trials, as far as reported (see Table 11), appears reasonably similar to that seen in UK registry studies (see Table 2), although this comparison may be limited by different reporting classifications used between studies. In addition, clinical practice in the RCTs (e.g. the oldest one published in 200042) may not necessarily reflect current NHS care. The generalisability of the RCTs to the NHS is considered uncertain.
Cost-effectiveness
A systematic search of the literature found four economic evaluations of biologic DMARDs for patients with JIA. Two of the studies were presented as cost–utility studies, one was a cost-effectiveness study and the other was a cost–consequence study. The evaluations were published between 2002 and 2012 in the UK, the Netherlands, Russia and Canada. One of the studies was the assessment report which informed the previous NICE appraisal of etanercept (NICE TA35). 123 The studies varied in design and structure, time horizons and the comparators included. There were limitations in the methodological quality in all the studies identified, and limited reporting of model parameters and assumptions.
A systematic search of the literature found two HRQoL studies in children and adolescents with JIA. One study assessed the effectiveness of a foot care programme in a RCT setting, whereas the other evaluated the QoL in a cohort of patients from the Dutch ABC Registry before and after treatment with etanercept.
Four drug companies submitted evidence to be considered as part of the NICE appraisal. Only one company (Roche) constructed a cost–utility analysis that included both costs and outcomes. Two companies (BMS and Pfizer) submitted cost analyses and assumed that the biologic DMARDs were equivalent in effectiveness, whereas another company (AbbVie) did not submit an economic analysis owing to suggested methodological limitations with any potential analysis. Roche submitted a Markov state-transition model with health states for uncontrolled/off treatment, on treatment and dead. The model compared treatment with adalimumab to that with tocilizumab. The base-case results from the submission conclude that tocilizumab is of similar effectiveness and is less expensive than adalimumab.
We developed an independent cost–utility model comparing the biologic DMARDs to methotrexate only. From the model, the incremental cost-effectiveness versus methotrexate only for adalimumab, etanercept and tocilizumab is estimated at £38,127, £32,526 and £38,656 per QALY gained, respectively. The incremental cost-effectiveness for abatacept as a second-line biologic was £39,536 per QALY gained. The model results are most sensitive to changes to the HRQoL utility values.
The cost-effectiveness of biologic DMARDs estimated in this report is associated with some uncertainty owing to the limitations of the evidence base. For this reason, assumptions have had to be made to simplify the modelling. There was limited evidence on HRQoL, in particular with regard to disease progression. The HRQoL utility values were taken from a small Dutch registry study of patients receiving etanercept. The HRQoL values for patients treated with methotrexate were assumed to be constant over time. Patients with JIA who do not receive a biologic will experience disease progression and, therefore, their HRQoL will decline over time. In the model, we have assumed a constant HRQoL utility value for patients receiving methotrexate only and so the biologic DMARDs would be more cost-effective than estimated by the economic model.
The model has not considered the underlying disease progression in terms of joint damage for patients with JIA. These patients may have sustained permanent damage to one or more joints, thereby affecting their physical function and HRQoL into adulthood and potentially requiring joint surgery. The model has not considered the cost of this surgery and this assumption implies that biologic DMARDs have no impact on long-term disease progression in terms of joint damage. However, a prospective registry-based cohort study by Minden and colleagues169 showed improved long-term prognosis for adult JIA patients who received etanercept during childhood. Furthermore, the AbbVie CS suggests that the reduction in orthopaedic surgery in JIA patients has been attributable to the increase in the use of immunomodulatory agents among children in recent decades and so DMARDs and biologic agents may have successfully prevented end-stage joint damage, based upon historical data that have shown a reduction. Therefore, biologic DMARDs are likely to reduce long-term damage compared with treatment with methotrexate, and they would potentially be more cost-effective than estimated by the independent economic model.
The cost-effectiveness of biologic DMARD treatment of patients with JIA-associated uveitis has not been formally estimated in this economic evaluation, owing to a lack of suitable input parameter data. The current evidence base comprises mainly small retrospective observational studies and suggests that adalimumab and infliximab are clinically effective in terms of improving intraocular inflammation and vision impairment. 96 A US cohort study of children with JIA compared those with JIA-associated uveitis with those without JIA-associated uveitis. 170 It reported that vision-related HRQoL was worse in uveitis patients, but general HRQoL was similar to that of JIA patients without uveitis. 170 It can be assumed that biologic DMARD treatment in JIA-associated uveitis patients will result in bigger overall HRQoL improvement (including vision-related HRQoL) and therefore would be more cost-effective in this group than in JIA patients without uveitis.
It was also reported that significant predictors of uveitis were persistent oligoarthritis and younger age at JIA diagnosis. 170 As discussed in Chapter 1, persistent oligoarthritis accounts for up to 48% of JIA cases in the UK and is regarded as a milder form of JIA. In contrast, EO accounts for between 6% and 17% of JIA cases in the UK and results in more severe symptoms and disease progression. Only EO was explicitly included in the NICE scope for this appraisal, and, therefore, it can be considered that uveitis is less likely to affect the patient subtypes that are relevant to the appraisal.
The economic model does not include the wider societal costs associated with JIA, which are described in more detail in Chapter 6. In the base-case analysis we have not included caregiver benefits associated with biologic DMARD treatment. A scenario analysis showed an improvement in cost-effectiveness for the biologic DMARDs when incorporating a utility disutility for patient caregivers.
The base-case analysis includes only one line of biologic DMARD treatment; however, in clinical practice some patients may switch to second- or third-line DMARDs. A scenario analysis that included a sequence of biologic treatments that most resembles current clinical practice was performed. The cost-effectiveness of multiple lines of biologic therapy is similar to that seen in the base-case analysis for one line of biologic therapy. There are many other possible treatment sequences but these have not been modelled, as they were considered to be less likely to occur in clinical practice and the results for these sequences are similar to those presented. In clinical practice, infliximab is often used but this has not been included as a treatment in the economic model, as it is licensed for this indication.
The cost-effectiveness results in this report are consistent with those from an earlier NICE technology appraisal for etanercept for patients with JIA (NICE TA3543). The previous appraisal used a discount rate of 6% for costs and 1% for benefits. 123 We ran the analysis for etanercept using these discount rates and the cost-effectiveness of etanercept improved to £21,718 per QALY. Using these discount rates, etanercept would be cost-effective at a willingness-to-pay threshold of £30,000 per QALY.
Strengths and limitations of the assessment
The systematic reviews and economic evaluation in this report have been carried out independently of any competing interest, and the results are presented in a consistent and transparent manner.
The systematic reviews of clinical effectiveness, cost-effectiveness and HRQoL have been undertaken following an established methodology and principles for conducting a systematic review. 52 The methods used were reported in a research protocol, which defined the decision problem in line with the NICE scope and set out the inclusion and quality assessment criteria, data extraction process and the other methods to be employed during the evidence synthesis.
A multidisciplinary advisory group has informed the review from its initiation. The research protocol was informed by comments received from the advisory group. The group also commented on a draft of the final report.
A de novo economic model has been developed following recognised guidelines. The model structure and data inputs are clearly presented in this report. The economic model is based upon data identified from systematic searches for clinical effectiveness, cost-effectiveness and QoL evidence, and other best available data.
This report is subject to certain limitations. The lack of head-to-head trials meant performing an indirect comparison of the biologic DMARDs, which is subject to a number of caveats due to heterogeneity between the trials (e.g. patient characteristics, treatment duration).
Limited HRQoL data were available for children with JIA, with none of the RCTs of biologic DMARDs reporting health utility data. The model results were based upon one Dutch registry study for patients treated with etanercept. It was necessary to make assumptions about the QoL of patients treated with other biologic DMARDs. Owing to the scarcity of the HRQoL data, it was not possible to link effectiveness data from the RCTs, in terms of ACR Pedi or CHAQ score, to a HRQoL utility measure. Furthermore, no HRQoL data were identified to inform the estimate of disutility of disease flare or the caregiver burden.
There were limited data available for the long-term discontinuation rates for patients for some of the biologic DMARDs, and it was necessary to assume that the discontinuation rates for the biologic DMARDs were the same as each other.
The economic analysis has compared biologic DMARDs against methotrexate only, for patients with an insufficient response to previous methotrexate. The NICE scope also includes best supportive care (e.g. NSAIDs, corticosteroids) as a comparator in patients who cannot tolerate a DMARD (e.g. methotrexate), but this has not been included in the analysis owing to a lack of available data to make a comparison with best supportive care. Such patients would be likely to be offered a biologic DMARD rather than receiving best supportive care; therefore, this comparison is not necessarily clinically relevant.
The model consists of a simple structure that does not incorporate the natural history of the disease in terms of long-term disease progression. JIA causes joint disease that requires joint operations and is associated with other comorbidities. It is unclear from the current evidence how biologic DMARDs affect the natural history of the disease and the occurrence of these outcomes.
Uncertainties
The RCTs included in our systematic review of clinical effectiveness did not report the impact of treatment on extra-articular manifestations. Uveitis is the most common of these manifestations and, if not identified and adequately controlled, can lead to permanent vision loss. Current guidance is to treat JIA patients with uveitis who have not responded to steroids or methotrexate with either adalimumab or infliximab, both of which are antiTNF drugs (of these, only adalimumab is within the scope of this assessment). 29 The guidance29 states that etanercept is not suitable for the treatment of JIA patients with uveitis.
Furthermore, no HRQoL utility values for the impact of uveitis on the HRQoL in children with JIA were identified in our systematic review of QoL. The paucity of good-quality evidence for the effectiveness of biologic DMARDs means that the clinical effectiveness and cost-effectiveness of treating JIA patients with uveitis is currently uncertain. However, it could be assumed that if biologic DMARD treatment of uveitis is effective in reducing sight impairment in addition to improving general JIA symptoms, then the cost-effectiveness estimates generated in the independent economic evaluation in this assessment report would be improved. The SYCAMORE RCT of adalimumab in combination with methotrexate for JIA-associated uveitis (funded by the NIHR HTA programme and Arthritis Research UK) has recently completed recruitment and will include an assessment of cost-effectiveness. 87
The lack of available, suitable, published cost–utility models necessitated building a new model which aimed to resemble clinical practice but also to utilise the effectiveness data from the RCTs. The design of the RCTs does not necessarily represent clinical practice (e.g. there would not be a lead-in phase with a biologic DMARD).
The model has not incorporated the impact of biologic DMARD treatment on disease progression and assumes that the HRQoL of patients treated with methotrexate is constant over time. The results may, therefore, underestimate the cost-effectiveness of treatment.
The model has assumed that treatment is equally effective for subsequent lines of biologic DMARD treatment as for the first line of treatment. If effectiveness is seen to be reduced in subsequent lines of therapy for particular switching regimens, then cost effectiveness may be reduced compared with the results presented in this report (relating to abatacept as a second-line treatment, and the scenario analysis of three lines of treatment).
The model has been modelled with a 30-year time horizon in the base-case analysis. There are a lack of long-term outcome data for JIA patients. In addition, there are often differences in the management of JIA patients as adults, which may affect patient outcomes. However, there are few empirical data available on the management of adult patients with JIA.
Chapter 8 Conclusions
Implications for service provision
Given that biologic DMARDs are currently used in the treatment of JIA, any recommendation supporting their use is unlikely to have significant implications for service provision (e.g. in terms of changes to infrastructure and staff training). However, further recruitment and training of staff is required to address workforce capacity shortages in some specialist centres.
Suggested research priorities
Randomised head-to-head comparisons of biologic DMARDs are necessary to establish comparative effectiveness. Currently, they are assumed to be equivalent based on indirect comparisons of a small number of trials with relatively small patient numbers. Trials should be sufficiently powered, with long-term follow-up of safety and efficacy, and should include an economic evaluation to assess cost-effectiveness. Treatment response should be assessed at a threshold that is considered clinically significant (e.g. ACR Pedi-70 or higher) and should also include measures of disease inactivity. Additional instruments to the ACR Pedi criteria should be used, such as the JADAS instrument. 35–37 Future trials of biologic DMARDs should stratify by disease phenotype to assess the differential effects of each treatment. Where possible, trials should measure the impact of treatment on children’s educational and social outcomes, such as time away from school/college when experiencing symptoms and for health-care management, and the ability to participate in leisure and social activities.
Randomised controlled trials are also required for subtypes of JIA for which evidence is currently lacking, including ERA and PA. As mentioned, the SYCAMORE trial of adalimumab in patients with JIA-associated uveitis87 has recently closed for recruitment early, following interim analysis showing that adalimumab is favourable in the treatment of JIA-associated uveitis.
Further research is needed to establish the HRQoL benefits associated with biological treatment in children with JIA and their caregivers. Validated child-appropriate instruments should be used to assess HRQoL, including generic preference-based tools to enable utilities to be estimated to inform cost-effectiveness analyses.
Acknowledgements
We would like to thank members of our advisory group who provided expert advice and comments on the protocol and a draft of this report: Professor Michael Beresford, Professor in Child Health, University of Liverpool; Professor Rachel Elliot, Lord Trent Professor of Medicines and Health, University of Nottingham; Dr Kimme Hyrich, Reader in Rheumatic Disease Epidemiology, University of Manchester; Dr Alice Leahy, Paediatric Rheumatology Consultant, Southampton General Hospital; Sister Helen Strike, Nurse Specialist Paediatric and Adolescent Rheumatology, Bristol Royal Hospital for Children; and Professor Lucy Wedderburn, Professor of Paediatric Rheumatology, Institute of Child Health, London.
Thanks to Mrs Karen Welch, Information Specialist at SHTAC, for conducting the literature searches and to Dr Jeremy Jones, Principal Research Fellow at SHTAC, for reviewing a draft of the report. Thanks to Maria Chorozoglou (Senior Research Fellow at SHTAC) and Diana Mendes (independent health economist) for assisting with the systematic reviews of cost-effectiveness and HRQoL.
Contributions of authors
Jonathan Shepherd (Principal Research Fellow, evidence synthesis) project-managed the study, developed the research protocol, contributed to drafting the background section, assisted in the development of the search strategy, assessed studies for inclusion, performed data extraction and critical appraisal of included clinical effectiveness studies, synthesised evidence, assessed the company submissions, and drafted and edited the final report.
Keith Cooper (Senior Research Fellow, health economics) developed the research protocol, assessed cost-effectiveness and HRQoL studies for inclusion, synthesised evidence, led the development of the economic evaluation, assessed the company submissions and drafted the final report.
Petra Harris (Research Fellow, evidence synthesis) developed the research protocol, contributed to drafting the background section, assessed studies for inclusion, performed data extraction and critical appraisal of included clinical effectiveness studies, synthesised evidence, assessed the company submissions and drafted the final report.
Joanna Picot (Senior Research Fellow, evidence synthesis) developed the research protocol, contributed to drafting the background section, assessed studies for inclusion, performed data extraction and critical appraisal of included clinical effectiveness studies, synthesised evidence, assessed the company submissions and drafted the final report.
Micah Rose (Research Fellow, health economics) contributed to the development of the economic evaluation, assessed the company submissions and drafted the report.
Data sharing statement
All available data relating to the systematic reviews are included in this report and its appendices. Some confidential data were provided that are not in the public domain and this information has been redacted in this report and cannot be shared by the review authors.
The Assessment Group has presented the results of its economic modelling using the drug list prices for abatacept, adalimumab, etanercept and tocilizumab in this report. A separate confidential appendix reporting the results incorporating the confidential PASs for abatacept and tocilizumab has been prepared by the Assessment Group for NICE. The confidential appendix will not be released publicly. The economic model associated with this document is protected by intellectual property rights, which are owned by the University of Southampton. Anyone wishing to modify, adapt, translate, reverse engineer, decompile, dismantle or create derivative work based on the economic model must first seek the agreement of the property owners.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet 2011;377:2138-49. http://dx.doi.org/10.1016/S0140-6736(11)60244-4.
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767-78. http://dx.doi.org/10.1016/S0140-6736(07)60363-8.
- Rigante D, Bosco A, Esposito S. The etiology of juvenile idiopathic arthritis. Clin Rev Allergy Immunol 2015;49:253-61. http://dx.doi.org/10.1007/s12016-014-8460-9.
- Duffy CM, Colbert RA, Laxer RM, Schanberg LE, Bowyer SL. Nomenclature and classification in chronic childhood arthritis: time for a change?. Arthritis Rheum 2005;52:382-5. http://dx.doi.org/10.1002/art.20815.
- Moorthy LN, Peterson MG, Hassett AL, Lehman TJ. Burden of childhood-onset arthritis. Pediatr Rheumatol Online J 2010;8:20-8. http://dx.doi.org/10.1186/1546-0096-8-20.
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390-2.
- Arthritis Research UK . What Are the Different Types of JIA? 2014. www.arthritisresearchuk.org/arthritis-information/conditions/juvenile-idiopathic-arthritis/different-types.aspx (accessed October 2014).
- Arthritis Care . Arthritis in Children n.d. www.arthritiscare.org.uk/aboutarthritis/conditions/arthritisinchildren (accessed October 2014).
- My Child has Oligoarticular JIA. A Guide to the Condition and its Treatment. London: Arthritis Care; 2013.
- My Child has Polyarticular JIA. A Guide to the Condition and its Treatment. London: Arthritis Care; 2013.
- My Child has Systemic JIA. A Guide to the Condition and its Treatment. London: Arthritis Care; 2013.
- National Rheumatoid Arthritis Society . What Is JIA? 2014. www.nras.org.uk/information-on-jia-what-is-jia--81 (accessed October 2014).
- McErlane F, Beresford MW, Baildam EM, Thomson W, Hyrich K, Chieng A, et al. Validation of JADAS in all ILAR subtypes of juvenile idiopathic arthritis in the clinical setting (oral presentation). Rheumatology 2012;51:S27-S38. http://dx.doi.org/10.1093/rheumatology/kes119.
- Ruperto N, Giannini EH, Pistorio A, Brunner HI, Martini A, Lovell DJ. Is it time to move to active comparator trials in juvenile idiopathic arthritis? A review of current study designs. Arthritis Rheum 2010;62:3131-9. http://dx.doi.org/10.1002/art.27670.
- Minden K, Kiessling U, Listing J, Niewerth M, Doring E, Meincke J, et al. Prognosis of patients with juvenile chronic arthritis and juvenile spondyloarthropathy. J Rheumatol 2000;27:2256-63.
- Bertilsson L, Andersson-Gare B, Fasth A, Petersson IF, Forsblad-D’elia H. Disease course, outcome, and predictors of outcome in a population-based juvenile chronic arthritis cohort followed for 17 years. J Rheumatol 2013;40:715-24. http://dx.doi.org/10.3899/jrheum.120602.
- Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine 2014;81:112-17. http://dx.doi.org/10.1016/j.jbspin.2013.09.003.
- Parsons S, Ingram M, Clarke-Cornwell SM, Symmons DPM. A Heavy Burden. The Occurrence and Impact of Musculoskeletal Conditions in the United Kingdom Today. Manchester: The University of Manchester and Arthritis Research UK; 2011.
- Martin K. Juvenile Idiopathic Arthritis (JIA) Explained. 2014.
- Interim Clinical Commissioning Policy Statement: Biologic Therapies for the treatment of Juvenile Idiopathic Arthritis (JIA). 2015.
- Office for National Statistics . Population Estimates for UK, England and Wales, Scotland and Northern Ireland, Mid-2013 2014. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition = tcm%3A77–322718 (accessed April 2015).
- Thomson W, Barrett JH, Donn R, Pepper L, Kennedy LJ, Ollier WE, et al. Juvenile idiopathic arthritis classified by the ILAR criteria: HLA associations in UK patients. Rheumatology 2002;41:1183-9. http://dx.doi.org/10.1093/rheumatology/41.10.1183.
- Kearsley-Fleet L, Davies R, Baildam E, Beresford MW, Foster HE, . Factors associated with choice of biologic among children with juvenile idiopathic arthritis: results from two UK paediatric biologic registers [published online ahead of print January 4 2016]. Rheumatology 2016.
- Vidqvist KL, Malin M, Varjolahti-Lehtinen T, Korpela MM. Disease activity of idiopathic juvenile arthritis continues through adolescence despite the use of biologic therapies. Rheumatology 2013;52:1999-2003. http://dx.doi.org/10.1093/rheumatology/ket256.
- Bechtold S, Simon D. Growth abnormalities in children and adolescents with juvenile idiopathic arthritis. Rheumatol Int 2014;34:1483-8. http://dx.doi.org/10.1007/s00296-014-3022-2.
- Stagi S, Masi L, Capannini S, Cimaz R, Tonini G, Matucci-Cerinic M, et al. Cross-sectional and longitudinal evaluation of bone mass in children and young adults with juvenile idiopathic arthritis: the role of bone mass determinants in a large cohort of patients. J Rheumatol 2010;37:1935-43. http://dx.doi.org/10.3899/jrheum.091241.
- Heiligenhaus A, Heinz C, Edelsten C, Kotaniemi K, Minden K. Review for disease of the year: epidemiology of juvenile idiopathic arthritis and its associated uveitis: the probable risk factors. Ocul Immunol Inflamm 2013;21:180-91. http://dx.doi.org/10.3109/09273948.2013.791701.
- British Society for Paediatric and Adolescent Rheumatology . Guidelines for Screening for Uveitis in Juvenile Idiopathic Arthritis (JIA) 2006. www.bspar.org.uk/DocStore/FileLibrary/PDFs/BSPAR%20Guidelines%20for%20Eye%20Screening%202006.pdf (accessed April 2015).
- NHS England Specialised Services Clinical Reference Group for Specialised Ophthalmology . Clinical Commissioning Policy: Adalimumab (Humira) and Infliximab (Remicade) As Anti-TNF Alpha Treatment Options for Paediatric Patients With Severe Refractory Uveitis n.d.
- Tong A, Jones J, Craig JC, Singh-Grewal D. Children’s experiences of living with juvenile idiopathic arthritis: a thematic synthesis of qualitative studies. Arthritis Care Res 2012;64:1392-404. http://dx.doi.org/10.1002/acr.21695.
- Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997;40:1202-9. http://dx.doi.org/10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R.
- Prince FHM, Otten MH, van Suijlekom-Smit LWA. Diagnosis and management of juvenile idiopathic arthritis. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c6434.
- Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009;61:658-66. http://dx.doi.org/10.1002/art.24516.
- McErlane F, Beresford MW, Baildam EM, Chieng SE, Davidson JE, Foster HE, et al. Validity of a three-variable Juvenile Arthritis Disease Activity Score in children with new-onset juvenile idiopathic arthritis. Ann Rheum Dis 2013;72:1983-8. http://dx.doi.org/10.1136/annrheumdis-2012-202031.
- Consolaro A, Negro G, Chiara Gallo M, Bracciolini G, Ferrari C, Schiappapietra B, et al. Defining criteria for disease activity states in nonsystemic juvenile idiopathic arthritis based on a three-variable juvenile arthritis disease activity score. Arthritis Care Res 2014;66:1703-9. http://dx.doi.org/10.1002/acr.22393.
- Consolaro A, Ruperto N, Bracciolini G, Frisina A, Gallo MC, Pistorio A, et al. Defining criteria for high disease activity in juvenile idiopathic arthritis based on the juvenile arthritis disease activity score. Ann Rheum Dis 2014;73:1380-3. http://dx.doi.org/10.1136/annrheumdis-2013-204186.
- Horneff G, Becker I. Definition of improvement in juvenile idiopathic arthritis using the juvenile arthritis disease activity score. Rheumatology 2014;53:1229-34. http://dx.doi.org/10.1093/rheumatology/ket470.
- Consolaro A, Negro G, Lanni S, Solari N, Martini A, Ravelli A. Toward a treat-to-target approach in the management of juvenile idiopathic arthritis. Clin Exp Rheumatol 2012;30:S157-62.
- Wallace CA, Ruperto N, Giannini E, . Pediatric Rheumatology International Trials Organization . Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol 2004;31:2290-4.
- Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res 2011;63:929-36. http://dx.doi.org/10.1002/acr.20497.
- Brunner HI, Lovell DJ, Finck BK, Giannini EH. Preliminary definition of disease flare in juvenile rheumatoid arthritis. J Rheumatol 2002;29:1058-64.
- Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. New Engl J Med 2000;342:763-9. http://dx.doi.org/10.1056/NEJM200003163421103.
- National Institute for Health and Care Excellence . Guidance on the Use of Etanercept for the Treatment of Juvenile Idiopathic Arthritis 2002. www.nice.org.uk/guidance/ta35 (accessed October 2014).
- National Institute for Health and Care Excellence . Tocilizumab for the Treatment of Systemic Juvenile Idiopathic Arthritis 2011. www.nice.org.uk/guidance/ta238 (accessed October 2014).
- European Medicines Agency . Orencia: EPAR – Product Information 2016. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000701/human_med_000958.jsp&mid=WC0b01ac058001d124 (accessed October 2014).
- European Medicines Agency . Humira: EPAR – Product Information 2015. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000481/human_med_000822.jsp&mid=WC0b01ac058001d124 (accessed October 2014).
- European Medicines Agency . Etanercept: EPAR – Product Information 2014. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000262/human_med_000764.jsp&mid=WC0b01ac058001d124 (accessed October 2014).
- European Medicines Agency . Tocilizumab: EPAR – Product Information 2014. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000955/human_med_001042.jsp&mid=WC0b01ac058001d124 (accessed October 2014).
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Dec Making 1993;13:322-39. http://dx.doi.org/10.1177/0272989X9301300409.
- Berard RA, Laxer RM. Etanercept (Enbrel) in the treatment of juvenile idiopathic arthritis. Expert Opin Biol Ther 2013;13:1623-30. http://dx.doi.org/10.1517/14712598.2013.840580.
- Horizon Scanning Centre . Tocilizumab (RoActemra) for Active Polyarticular Juvenile Idiopathic Artheritis – Second Line 2012. www.hsc.nihr.ac.uk/topics/tocilizumab-roactemra-for-active-polyarticular-juv/ (accessed October 2014).
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011. www.cochrane-handbook.org (accessed June 2015).
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. http://dx.doi.org/10.1016/S0895-4356(97)00049-8.
- Donegan S, Williamson P, Gamble C, Tudur-Smith C. Indirect comparisons: a review of reporting and methodological quality. PLOS One 2010;5. http://dx.doi.org/10.1371/journal.pone.0011054.
- Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al. Indirect comparisons of competing interventions. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9260.
- Smith JA, Thompson DJ, Whitcup SM, Suhler E, Clarke G, Smith S, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum 2005;53:18-23. http://dx.doi.org/10.1002/art.20904.
- Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet 2008;372:383-91. http://dx.doi.org/10.1016/S0140-6736(08)60998-8.
- Ruperto N, Lovell DJ, Li T, Sztajnbok F, Goldenstein-Schainberg C, Scheinberg M, et al. Abatacept improves health-related quality of life, pain, sleep quality, and daily participation in subjects with juvenile idiopathic arthritis. Arthritis Care Res 2010;62:1542-51. http://dx.doi.org/10.1002/acr.20283.
- Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum 2010;62:1792-802. http://dx.doi.org/10.1002/art.27431.
- Lovell DJ, Ruperto N, Mouy R, Paz E, Rubio-Perez N, Silva CA, et al. Cumulative long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis: results up to 7 years of follow-up. Arthritis Rheum 2012;64:S717-18.
- Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. New Engl J Med 2008;359:810-20. http://dx.doi.org/10.1056/NEJMoa0706290.
- Lovell DJ, Ruperto N, Jarosova K, Nemcova D, Vargova V, Michels H, et al. The impact of adalimumab on growth in patients with juvenile idiopathic arthritis. Arthritis Rheum 2012;64:S329-30.
- Ruperto N, Lovell DJ, Jarosova K, Nemcova D, Vargova V, Michels H, et al. PReS-FINAL-2001: the impact of adalimumab on growth in patients with juvenile idiopathic arthritis. Paediatr Rheumatol 2013;11. http://dx.doi.org/10.1186/1546-0096-11-S2-O4.
- Ruperto N, Lovell D, Quartier P, Ravelli A, Karunaratne M, Kalabic J, et al. Treating to target of minimal disease activity and normal function in polyarticular juvenile idiopathic arthritis with adalimumab: analysis from a phase 3 clinical trial. Ann Rheum Dis 2014;73:130-1. http://dx.doi.org/10.1136/annrheumdis-2014-eular.1600.
- Lovell DJ, Giannini EH, Reiff A, Jones OY, Schneider R, Olson JC, et al. Long-term efficacy and safety of etanercept in children with polyarticular-course juvenile rheumatoid arthritis: interim results from an ongoing multicenter, open-label, extended-treatment trial. Arthritis Rheum 2003;48:218-26. http://dx.doi.org/10.1002/art.10710.
- Lovell DJ, Reiff A, Jones OY, Schneider R, Nocton J, Stein LD, et al. Long-term safety and efficacy of etanercept in children with polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum 2006;54:1987-94. http://dx.doi.org/10.1002/art.21885.
- Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Chon Y, Lin SL, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum 2008;58:1496-504. http://dx.doi.org/10.1002/art.23427.
- Brunner HI, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, et al. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis 2015;74:1110-17. http://dx.doi.org/10.1136/annrheumdis-2014-205351.
- Brunner H, Ruperto N, Zuber Z, Cuttica RJ, Xavier R, Calvo I, et al. A4: Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: 2-year data from CHERISH. Arthritis Rheumatol 2014;66:S5-6. http://dx.doi.org/10.1002/art.38415.
- Baildam E, Ramanan A, Woo P, Brunner H, Ruperto N, Zuber Z, et al. Efficacy and safety of tocilizumab in polyarticular-course juvenile idiopathic arthritis: 2 year data from cherish. Rheumatology 2014;53:S52-3.
- Brunner HI, Ruperto N, Zuber Z, Cuttica RJ, Xavier R, Calvo I, et al. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: 2-year data from Cherish. Arthritis Rheum 2013;65.
- De Benedetti F, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, et al. Efficacy and safety of tocilizumab in patients with polyarticular juvenile idiopathic arthritis: data from a phase 3 trial. Ann Rheum Dis 2013;72. http://dx.doi.org/10.1136/annrheumdis-2013-eular.265.
- Baildam E, Ruperto N, Brunner H, Zuber Z, Keane C, Harari O, et al. Efficacy and safety of tocilizumab in polyarticular juvenile idiopathic arthritis: cherish results at week 40. Rheumatology 2013;52.
- De Benedetti F, Ruperto N, Zuber Z, Cuttica R, Xavier R, Calvo I, et al. PReS-FINAL-2180: efficacy and safety of tocilizumab (TCZ) in patients with polyarticular-course juvenile idiopathic arthritis (pcJIA): 2-year data from CHERISH. Paediatr Rheumatol 2013;11. http://dx.doi.org/10.1186/1546-0096-11-S2-O15.
- Brunner H, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, et al. Efficacy and safety of tocilizumab in patients with polyarticular juvenile idiopathic arthritis: data from a phase 3 trial. Arthritis Rheum 2012;64.
- Bharucha KN, Brunner HI, Ruperto N, Cabral DA, Gedalia A, Gerloni V, et al. Growth During Tocilizumab Therapy for Polyarticular-Course Juvenile Idiopathic Arthritis: 2-Year Data from a Phase 3 Clinical Trial n.d.
- Abbvie . Adalimumab Multiple Technology Appraisal (MTA): Etanercept, Abatacept, Adalimumab and Tocilizumab for Treating Juvenile Idiopathic Arthritis (including Review of TA35) 2015.
- Roche . Tocilizumab (RoActemra®) for the Treatment of Juvenile Idiopathic Polyarthritis 2015.
- Otten MH, Anink J, Spronk S, Van Suijlekom-Smit LWA. Efficacy of biological agents in juvenile idiopathic arthritis: a systematic review using indirect comparisons. Ann Rheum Dis 2013;72:1806-12. http://dx.doi.org/10.1136/annrheumdis-2012-201991.
- Nikolakopoulou A, Chaimani A, Veroniki AA, Vasiliadis HS, Schmid CH, Salanti G. Characteristics of networks of interventions: a description of a database of 186 published networks. PLOS One 2014;9. http://dx.doi.org/10.1371/journal.pone.0086754.
- Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2914.
- Kearsley-Fleet L, Davies R, Lunt M, Southwood T, Hyrich K. Factors associated with improvement in disease activity following initiation of etanercept in children and young people with juvenile idiopathic arthritis: results from the British Society for Paediatric and Adolescent Rheumatology Etanercept Cohort Study (BSPAR-ETN) [published online ahead of print December 31 2015]. Rheumatology 2015.
- Geikowski T, Becker I, Horneff G. Predictors of response to etanercept in polyarticular-course juvenile idiopathic arthritis. Rheumatology 2014;53:1245-9. http://dx.doi.org/10.1093/rheumatology/ket490.
- Albarouni M, Becker I, Horneff G. Predictors of response to methotrexate in juvenile idiopathic arthritis. Pediatr Rheumatol Online J 2014;12. http://dx.doi.org/10.1186/1546-0096-12-35.
- Bristol-Myers Squibb . Bristol-Myers Squibb (BMS) Abatacept Submission for NICE Multiple Technology Appraisal (MTA) ID738: Abatacept, Adalimumab, Etanercept and Tocilizumab for Treating Juvenile Idiopathic Arthritis (including Review of TA35) 2015.
- Lovell D, Ruperto N, Mouy R, Paz E, Rubio-Pérez N, Silva CA, et al. Cumulative long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis: results up to 7 years of follow-up. American College of Rheumatology (ACR) Congress. Arthritis Rheum 2012;64.
- Ramanan AV, Dick AD, Benton D, Compeyrot-Lacassagne S, Dawoud D, Hardwick B, et al. A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate for the treatment of juvenile idiopathic arthritis associated uveitis (SYCAMORE Trial). Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-14.
- Burgos-Vargas R, Tse SM, Horneff G, Pangan AL, Unnebrink K, Anderson JK. A3: efficacy and safety of adalimumab in pediatric patients with enthesitis related arthritis. Arthritis Rheumatol 2014;66.
- Burgos-Vargas R, Tse S, Horneff G, Pangan A, Unnebrink K, Anderson J. Efficacy and safety of adalimumab in pediatric patients with enthesitis related arthritis. J Rheumatol 2014;41:1532-3.
- Burgos-Vargas R, Tse SML, Horneff G, Pangan AL, Unnebrink K, Anderson JK. Efficacy and safety of adalimumab in paediatric patients with enthesitis related arthritis. Rheumatology 2014;53:i51-2.
- Burgos-Vargas R, Tse SM, Horneff G, Pangan AL, Unnebrink K, Anderson JK. Efficacy and safety of adalimumab in pediatric patients with enthesitis related arthritis. Arthritis Rheum 2013;65.
- Burgos-Vargas R, Tse S, Horneff G, Pangan AL, Unnebrink K, Anderson JK. PReS-FINAL-2179: efficacy and safety of adalimumab in pediatric patients with enthesitis related arthritis. Pediatr Rheumatol 2013;11. http://dx.doi.org/10.1186/1546-0096-11-S2-O14.
- Tse SM, Burgos-Vargas R, Horneff G, Pangan AL, Kalabic J, Unnebrink K, et al. Discontinuation of concomitant medication for enthesitis-related arthritis during 52 weeks of treatment with adalimumab. Arthritis Rheumatol 2014;66.
- Kingsbury DJ, Bader-Meunier B, Patel G, Arora V, Kalabic J, Kupper H. Safety, effectiveness, and pharmacokinetics of adalimumab in children with polyarticular juvenile idiopathic arthritis aged 2 to 4 years. Clin Rheumatol 2014;33:1433-41. http://dx.doi.org/10.1007/s10067-014-2498-1.
- Imagawa T, Takei S, Umebayashi H, Yamaguchi K, Itoh Y, Kawai T, et al. Efficacy, pharmacokinetics, and safety of adalimumab in pediatric patients with juvenile idiopathic arthritis in Japan. Clin Rheumatol 2012;31:1713-21. http://dx.doi.org/10.1007/s10067-012-2082-5.
- Simonini G, Druce K, Cimaz R, Macfarlane GJ, Jones GT. Current evidence of anti-tumor necrosis factor alpha treatment efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach of individual drugs. Arthritis Care Res 2014;66:1073-84. http://dx.doi.org/10.1002/acr.22214.
- Pfizer Ltd . Multiple Technology Appraisal (MTA): Etanercept (Enbrel®) for Treating Juvenile Idiopathic Arthritis (including Review of TA35) 2015.
- Zeng H, Zeng P, Xie Y, Tang Y, Li F. A randomized controlled clinical trial to evaluate the efficacy of recombinant human tumor necrosis factor-a receptor type II fusion protein antibody in juvenile idiopathic arthritis. Allergy Eur J Allergy Clin Immunol 2013;68.
- Horneff G, Burgos-Vargas R, Constantin T, Foeldvari I, Vojinovic J, Chasnyk VG, et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann Rheum Dis 2014;73:1114-22. http://dx.doi.org/10.1136/annrheumdis-2012-203046.
- Ravelli A, Ruperto N, Pederzoli S, Burgos-Vargas R, Kobusinska K, Schmeling H, et al. Assessment of radiographic progression in patients with polyarticular-course juvenile idiopathic arthritis treated with tocilizumab: 2-year data from CHERISH. Arthritis Rheumatol 2014;66:S17-18. http://dx.doi.org/10.1002/art.38422.
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17. http://dx.doi.org/10.1177/0272989X12458724.
- Burgos-Vargas R, Tse SM, Horneff G, Pangan AL, Kalabic J, Goss S, et al. A randomized, double-blind, placebo-controlled, multicenter study of adalimumab in pediatric patients with enthesitis-related arthritis. Arthritis Care Res 2015;67:1503-12. http://dx.doi.org/10.1002/acr.22657.
- Foeldvari I, Horneff G, Minden K, Trauzeddel R, Kuemmerle-Deschner JB, Tenbrock K, et al. Remission induction by etanercept in enthesitis related arthritis JIA-patients (juvenile undifferentiated spondylarthropathy). Arthritis Rheumatol 2014;66.
- Constantin T, Foeldvari I, Vojinovic J, Horneff G, Burgos-Vargas R, Nikishina I, et al. Long-Term Safety and Efficacy of Etanercept in Paediatric Subjects With Extended Oligoarticular Juvenile Idiopathic Arthritis, Enthesitis-Related Arthritis, or Psoriatic Arthritis n.d.
- Cordero-Coma M, Yilmaz T, Onal S. Systematic review of anti-tumor necrosis factor-alpha therapy for treatment of immune-mediated uveitis. Ocul Immunol Inflamm 2013;21:12-20. http://dx.doi.org/10.3109/09273948.2012.723107.
- Garcia-De-Vicuna C, Diaz-Llopis M, Salom D, Bou R, Diaz-Cascajosa J, Cordero-Coma M, et al. Usefulness of adalimumab in the treatment of refractory uveitis associated with juvenile idiopathic arthritis. Mediators Inflamm 2013;2013. http://dx.doi.org/10.1155/2013/560632.
- Diaz-Llopis M, Salom D, Garcia-De-Vicuna C, Cordero-Coma M, Ortega G, Ortego N, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology 2012;119:1575-81. http://dx.doi.org/10.1016/j.ophtha.2012.02.018.
- Magli A, Forte R, Navarro P, Russo G, Orlando F, Latanza L, et al. Adalimumab for juvenile idiopathic arthritis-associated uveitis. Graefes Arch Clin Exp Ophthalmol 2013;251:1601-6. http://dx.doi.org/10.1007/s00417-013-2275-x.
- Zannin ME, Birolo C, Gerloni VM, Miserocchi E, Pontikaki I, Paroli MP, et al. Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year followup data from the Italian Registry. J Rheumatol 2013;40:74-9. http://dx.doi.org/10.3899/jrheum.120583.
- Simonini G, Taddio A, Cattalini M, Caputo R, de Libero C, Parentin F, et al. Superior efficacy of Adalimumab in treating childhood refractory chronic uveitis when used as first biologic modifier drug: adalimumab as starting anti-TNF-alpha therapy in childhood chronic uveitis. Pediatr Rheumatol Online J 2013;11. http://dx.doi.org/10.1186/1546-0096-11-16.
- Haapasaari JE, Kauppi M, Hakala MS, Kautiainen H. Economic evaluation of etanercept therapy in the treatment of re-fractory JIA. Arthritis Rheum 2002;46.
- Brodszky V, Pentek M, Majer I, Karpati K, Gulacsi L. Etanercept in Patients With Juvenile Idiopathic Arthritis: Systematic Review and Economic Evaluation 2006. http://hecon.uni-corvinus.hu/download/english/publ/hecon_research_20_eng.pdf (accessed November 2014).
- Prince FHM, de Bekker-Grob EW, Twilt M, Van Rossum MAJ, Hoppenreijs EPAH, ten Cate R, et al. An analysis of the costs and treatment success of etanercept in juvenile idiopathic arthritis. Clin Exp Rheumatol 2011;29. http://dx.doi.org/10.1093/rheumatology/keq432.
- Simpson K, Hubert MM, On PV, Cifaldi M, Shaw J. Long-term cost-effectiveness of adalimumab therapy in juvenile idiopathic arthritis: from a Canadian perspective. J Rheumatol 2012;39.
- Luca N, Burnett H, Ungar W, Beukelman T, Feldman BM, Schwartz G, et al. Cost-effectiveness analysis of early biologic treatment in polyarticular juvenile idiopathic arthritis. Arthritis Rheum 2012;64.
- Luca N, Burnett H, Ungar W, Beukelman T, Feldman B, Schwartz G, et al. Cost-effectiveness analysis of early biologic treatment in polyarticular juvenile idiopathic arthritis. Arthritis Rheumatol 2012;64.
- Chang S, Sawyer L, Dejonckheere F, van Suijlekom-Smit LW, Anink J, Diamantopoulos A. Tocilizumab in polyarticular juvenile idiopathic arthritis – a cost-utility model for the United Kingdom. Value Health 2013;16. http://dx.doi.org/10.1016/j.jval.2013.08.1496.
- All Wales Medicines Strategy Group . Adalimumab (Humira®) 2013. http://www.awmsg.org/.
- All Wales Medicines Strategy Group . Etanercept (Enbrel®) 2013. http://www.awmsg.org/.
- All Wales Medicines Strategy Group . Abatacept (Orencia®) 2014. http://www.awmsg.org/.
- All Wales Medicines Strategy Group . Tocilizumab (RoActemra®) 2014. http://www.awmsg.org/.
- Canadian Agency for Drugs and Technologies in Health . Tocilizumab (Actemra – Hoffmann-La Roche Limited) New Indication: Polyarticular Juvenile Idiopathic Arthritis 2014.
- Cummins C, Connock M, Fry-Smith A, Burls A. A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept. Health Technol Assess 2002;6. http://dx.doi.org/10.3310/hta6170.
- Prince FH, de Bekker-Grob EW, Twilt M, van Rossum MA, Hoppenreijs EP, ten Cate R, et al. An analysis of the costs and treatment success of etanercept in juvenile idiopathic arthritis: results from the Dutch Arthritis and Biologicals in Children register. Rheumatology 2011;50:1131-6.
- Simpson K, Marlow N, Shaw J, Rudakova A. Pharmacoeconomic issues of adalimumab therapy in juvenile idiopathic arthritis. Pediatr Pharmacol 2012;9:48-52. http://dx.doi.org/10.15690/pf.v9i4.390.
- Costa V, Ungar WJ, Hancock-Howard RL. The Use of Biologic Response Modifiers in Polyarticular-Course Juvenile Idiopathic Arthritis 2010. www.sickkids.ca/pdfs/Research/TASK/biologics/33870-biologics-report_JIA.pdf (accessed 6 November 2014).
- Ungar WJ, Costa V, Hancock-Howard R, Feldman BM, Laxer RM. Cost-effectiveness of biologics in polyarticular-course juvenile idiopathic arthritis patients unresponsive to disease-modifying antirheumatic drugs. Arthritis Care Res 2011;63:111-19. http://dx.doi.org/10.1002/acr.20337.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- National Institute for Health and Care Excellence . Guidance on the Use of Etanercept for the Treatment of Juvenile Idiopathic Arthritis 2002. www.nice.org.uk/guidance/ta35 (accessed 19 May 2015).
- Simpson K, Shaw J. Mapping the Childhood Health Assessment Questionnaire to the Health Utilities Index Mark 2 and Mark 3 in children with juvenile idiopathic arthritis. 19th Annual Conference of the International Society for Quality of Life Research. Quality Life Res 2012;21.
- Yagudina R, Kulikov A, Zinchuk I. PMS25 evaluation of direct costs for the treatment of active juvenile rheumatoid arthritis using biologics. ISPOR 14th annual European congress. Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.08.411.
- Pennington B, Davis S. Mapping from the Health Assessment Questionnaire to the EQ-5D: the impact of different algorithms on cost-effectiveness results. Value Health 2014;17:762-71. http://dx.doi.org/10.1016/j.jval.2014.11.002.
- Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum 2007;56:3096-106. http://dx.doi.org/10.1002/art.22838.
- Hendry GJ, Watt GF, Brandon M, Friel L, Turner DE, Lorgelly PK, et al. The effectiveness of a multidisciplinary foot care program for children and adolescents with juvenile idiopathic arthritis: an exploratory trial. J Rehabil Med 2013;45:467-76. http://dx.doi.org/10.2340/16501977-1130.
- Prince FH, Geerdink LM, Borsboom GJ, Twilt M, van Rossum MA, Hoppenreijs EP, et al. Major improvements in health-related quality of life during the use of etanercept in patients with previously refractory juvenile idiopathic arthritis. Ann Rheum Dis 2010;69:138-42. http://dx.doi.org/10.1136/ard.2009.111260.
- Monthly Index of Medical Specialties . Presciption Drug Database and Drug Prescribing Guide 2014. www.mims.co.uk/ (accessed June 2015).
- Stevenson M, Archer R, Tosh J, Simpson E, Everson-Hock E, Stevens J, et al. Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Tocilizumab and Abatacept for the Treatment of Rheumatoid Arthritis Not Previously Treated With Disease-Modifying Anti-Rheumatic Drugs and After the Failure of Conventional Diseasemodifying Anti-Rheumatic Drugs Only: Systematic Review and Economic Evaluation 2013. www.nice.org.uk/guidance/gid-tag313/documents/rheumatoid-arthritis-adalimumab-etanercept-infliximab-certolizumab-pegol-golimumab-abatacept-and-tocilizumab-review-assessment-report2 (accessed June 2015).
- Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8110.
- Khan KA, Petrou S, Rivero-Arias O, Walters SJ, Boyle SE. Mapping EQ-5D utility scores from the PedsQL generic core scales. Pharmacoeconomics 2014;32:693-706. http://dx.doi.org/10.1007/s40273-014-0153-y.
- Briggs AH, O’Brien BJ. The death of cost-minimization analysis?. Health Econ 2001;10:179-84. http://dx.doi.org/10.1002/hec.584.
- Dakin H, Wordsworth S. Cost-minimisation analysis versus cost-effectiveness analysis, revisited. Health Econ 2013;22:22-34. http://dx.doi.org/10.1002/hec.1812.
- British Society for Paediatric and Adolescent Rheumatology . Submission to NICE – MTA – Abatacept, Adalimumab, Etanercept and Tocilizumab for the Treatment of Juvenile Idiopathic Arthritis 2015.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press; 2006.
- Tynjala P, Vahasalo P, Honkanen V, Lahdenne P. Drug survival of the first and second course of anti-tumor necrosis factor agents in juvenile idiopathic arthritis. Ann Rheum Dis 2009;68:552-7. http://dx.doi.org/10.1136/ard.2007.087130.
- Klein A, Kaul I, Foeldvari I, Ganser G, Urban A, Horneff G. Efficacy and safety of oral and parenteral methotrexate therapy in children with juvenile idiopathic arthritis: an observational study with patients from the German Methotrexate Registry. Arthritis Care Res 2012;64:1349-56. http://dx.doi.org/10.1002/acr.21697.
- Bruns A, Hilario MO, Jennings F, Silva CA, Natour J. Quality of life and impact of the disease on primary caregivers of juvenile idiopathic arthritis patients. Joint Bone Spine 2008;75:149-54. http://dx.doi.org/10.1016/j.jbspin.2007.07.007.
- British National Formulary for Children (Online). BMJ Group, Pharmaceutical Press and RCPCH Publications; 2015.
- British National Formulary (Online). London: BMJ Group and Pharmaceutical Press; 2014.
- Curtis L. Unit Costs of Health and Social Care 2013 n.d. www.pssru.ac.uk/project-pages/unit-costs/2012/ (accessed July 2015).
- National Schedule of Reference Costs 2013–14 for NHS Trusts and NHS Foundation Trusts. London: Department of Health; 2014.
- Thornton J, Lunt M, Ashcroft DM, Baildam E, Foster H, Davidson J, et al. Costing juvenile idiopathic arthritis: examining patient-based costs during the first year after diagnosis. Rheumatology 2008;47:985-90. http://dx.doi.org/10.1093/rheumatology/ken039.
- Cummins C, Connock M, Fry-Smith A, Burls A. A Rapid Review of New Drug Treatments for Juvenile Idiopathic Arthritis: Etanercept [TA35 assessment report]. NICE; 2001.
- Riemsma R, Al Maiwenn J, Lhachimi SK, Armstrong N, Misso K, Manning N, et al. Tocilizumab for the Treatment of Systemic Juvenile Idiopathic Arthritis [TA238 ERG report]. NICE; 2011.
- Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A Systematic Review of the Effectiveness of Adalimumab, Etanercept and Infliximab for the Treatment of Rheumatoid Arthritis in Adults and an Economic Evaluation of their Cost-effectiveness [TA130 Assessment Report]. NICE; 2005.
- Connock M, Tubeuf S, Malottki K, Uthman A, Round J, Bayliss S, et al. Certolizumab Pegol (CIMZIA®) for the Treatment of Rheumatoid Arthritis [TA130 ERG Report]. NICE; 2009.
- Malottki K, Barton P, Tsourapas A, Uthman A, Liu Z, Routh K, et al. Adalimumab, Etanercept, Infliximab, Rituximab and Abatacept for the Treatment of Rheumatoid Arthritis After the Failure of a TNF Inhibitor [TA195 Assessment Report]. NICE; 2009.
- Lloyd Jones M, Stevenson M, Stevens J, Sutton A. Abatacept for the Treatment of Rheumatoid Arthritis after the Failure of Conventional Disease-Modifying Anti-rheumatic Drugs [TA247 Rapid Review of TA198, ERG Report]. NICE; 2015.
- Otten MH, Prince FH, Armbrust W, ten Cate R, Hoppenreijs EP, Twilt M, et al. Factors associated with treatment response to etanercept in juvenile idiopathic arthritis. JAMA 2011;306:2340-7. http://dx.doi.org/10.1001/jama.2011.1671.
- Baszis K, Garbutt J, Toib D, Mao J, King A, White A, et al. Clinical outcomes after withdrawal of anti-tumour necrosis factor alpha therapy in patients with juvenile idiopathic arthritis: a twelve-year experience. Arthritis Rheum 2011;63:3163-8. http://dx.doi.org/10.1002/art.30502.
- Wallace CA, Huang B, Bandeira M, Ravelli A, Giannini EH. Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum 2005;52:3554-62. http://dx.doi.org/10.1002/art.21389.
- Kuhlthau K, Kahn R, Hill KS, Gnanasekaran S, Ettner SL. The well-being of parental caregivers of children with activity limitations. Matern Child Health J 2010;14:155-63. http://dx.doi.org/10.1007/s10995-008-0434-1.
- Gani R, Giovannoni G, Bates D, Kemball B, Hughes S, Kerrigan J. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing–remitting multiple sclerosis in the UK. Pharmacoeconomics 2008;26:617-27. http://dx.doi.org/10.2165/00019053-200826070-00008.
- Reeves BC, Langham J, Walker J, Grieve R, Chakravarthy U, Tomlin K, et al. Verteporfin photodynamic therapy cohort study: report 2: clinical measures of vision and health-related quality of life. Ophthalmology 2009;116:2463-70. http://dx.doi.org/10.1016/j.ophtha.2009.10.031.
- National Rheumatoid Arthritis Society (NRAS) . A Focus on Juvenile Idiopathic Arthritis 2014. www.nras.org.uk/publications/a-focus-on-juvenile-idiopathic-arthritis-report (accessed May 2015).
- Ungar WJ, Costa V, Burnett HF, Feldman BM, Laxer RM. The use of biologic response modifiers in polyarticular-course juvenile idiopathic arthritis: a systematic review. Semin Arthritis Rheum 2013;42:597-618. http://dx.doi.org/10.1016/j.semarthrit.2012.10.006.
- Kemper A, Van Mater H, Coeytaux R, Williams J, Sanders G. Systematic review of disease-modifying antirheumatic drugs for juvenile idiopathic arthritis. BMC Pediatrics 2012;12. http://dx.doi.org/10.1186/1471-2431-12-29.
- Minden K, Niewerth M, Zink A, Seipelt E, Foeldvari I, Girschick H, et al. Long-term outcome of patients with JIA treated with etanercept, results of the biologic register JuMBO. Rheumatology 2012;51:1407-15. http://dx.doi.org/10.1093/rheumatology/kes019.
- Angeles-Han ST, McCracken C, Yeh S, Jenkins K, Stryker D, Rouster-Stevens K, et al. Characteristics of a cohort of children with juvenile idiopathic arthritis and JIA-associated uveitis. Pediatr Rheumatol Online J 2015;13. http://dx.doi.org/10.1186/s12969-015-0018-8.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Feeny D, Furlong W, Torrance GW, Goldsmith CH, Zhu Z, DePauw S, et al. Multiattribute and single-attribute utility functions for the Health Utilities Index Mark 3 System. Med Care 2002;40:113-28. http://dx.doi.org/10.1097/00005650-200202000-00006.
- Packham JC, Hall MA. Long-term follow-up of 246 adults with juvenile idiopathic arthritis: functional outcome. Rheumatology (Oxford) 2002;41:1428-35. http://dx.doi.org/10.1093/rheumatology/41.12.1428.
- Lundkvist J, Kastäng F, Kobelt G. The burden of rheumatoid arthritis and access to treatment: health burden and costs. Eur J Health Econ 2008;8:S49-60. http://dx.doi.org/10.1007/s10198-007-0088-8.
Appendix 1 Search dates and example MEDLINE search strategies for clinical effectiveness, cost-effectiveness and health-related quality of life
Databases searched for the systematic reviews of clinical effectiveness, cost-effectiveness and HRQoL are presented below. Clinical effectiveness searches were updated on 5 May 2015 and cost-effectiveness and HRQoL searches were updated on 6 May 2015.
| Database searched (host) | Clinical effectiveness searches | Cost-effectiveness searches HRQoL searches |
|---|---|---|
| BIOSIS Previews (Web of Science) | Searched to 29 October 2014 | 1956–11 November 2014 1956–2 December 2014 |
| Cochrane Central, CDSR, Cochrane DARE, Cochrane HTA and Cochrane Methods (The Cochrane Library) | Searched to 4 November 2014 | |
| Cochrane Central, Cochrane DARE, Cochrane Economic Evaluations and Cochrane Methods (The Cochrane Library) | HRQoL: searched to 9 December 2014 | |
| Centre for Reviews and Dissemination databases: DARE, HTA and NHS EED (CRD) | Searched to 4 November 2014 | All available years to 11 November 2014 All available years to 9 December 2014 |
| CPCI-S (Web of Science) | 1990–29 October 2014 | 1970–11 November 2014 1970–2 December 2014 |
| DELPHI | Costs: searched to 10 November 2014 | |
| EMBASE (Ovid) | All available years to 29 October 2014 | Searched to 10 November 2014 1974–1 December 2014 |
| MEDLINE (Ovid) | Searched to 29 October 2014 | 1946 to October week 5 2014 1946 to November week 2 2014 |
| MEDLINE In-Process & Other Non-Indexed Citations (Ovid) | Searched to 29 October 2014 | Searched to 10 November 2014 Searched to 25 November 2014 |
| PsycINFO (EBSCOhost) | HRQoL: 1954–9 December 2014 | |
| Science Citation Index Expanded (SCI-EXPANDED) (Web of Science) | 1970–29 October 2014 | 1970–11 November 2014 1970–2 December 2014 |
| Zetoc (Mimas) | Searched to 4 November 2014 |
National Institute for Health Research Clinical Research Network (NIHR CRN Portfolio, formally the UKCRN website).
Clinical trials.gov.
World Health Organization’ ICTRP.
ISRCTN.
MEDLINE search strategies for clinical effectiveness, cost-effectiveness and HRQoL are shown here. These were adapted for other databases and are available on request.
Clinical effectiveness MEDLINE search strategy
-
Arthritis, Juvenile/
-
JIA.tw.
-
exp Arthritis/
-
(arthriti* or oligoarthriti* or polyarthriti* or polyarticula*).tw.
-
Rheumatoid Factor/
-
“rheumatoid factor“.tw.
-
or/3-6
-
(juvenile* or child* or teen* or adolescen* or youth* or “young person” or “young people” or pediatric* or paediatric*).tw.
-
exp Child/ or Adolescent/
-
7 and (8 or 9)
-
1 or 2 or 10
-
(etanercept or enbrel).mp.
-
(abatacept or orencia).mp.
-
(adalimumab or humira).mp.
-
(tocilizumab or toclizumab or RoActemra).mp.
-
or/12-15
-
11 and 16
-
limit 17 to English language
-
limit 18 to humans
-
(letter or editorial or comment).pt.
-
19 not 20
Cost-effectiveness MEDLINE search strategy
-
Arthritis, Juvenile/
-
JIA.tw.
-
exp Arthritis/
-
(arthriti* or oligoarthriti* or polyarthriti* or polyarticula*).tw.
-
Rheumatoid Factor/
-
“rheumatoid factor”.tw.
-
or/3-6
-
(juvenile* or child* or teen* or adolescen* or youth* or “young person” or “young people” or pediatric* or paediatric*).tw.
-
exp Child/ or Adolescent/
-
7 and (8 or 9)
-
1 or 2 or 10
-
(etanercept or enbrel).mp.
-
(abatacept or orencia).mp.
-
(adalimumab or humira).mp.
-
(tocilizumab or toclizumab or RoActemra).mp.
-
or/12-15
-
11 and 16
-
limit 17 to English language
-
limit 18 to humans
-
(letter or editorial or comment).pt.
-
19 not 20
-
exp economics/
-
exp economics hospital/
-
exp economics pharmaceutical/
-
exp economics nursing/
-
exp economics medical/
-
exp “Costs and Cost Analysis”/
-
Cost Benefit Analysis/
-
exp models economic/
-
exp fees/ and charges/
-
exp budgets/
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic*).tw.
-
(value adj1 money).tw.
-
budget$.tw.
-
or/22-34
-
((energy or oxygen) adj cost).tw.
-
(metabolic adj cost).tw.
-
((energy or oxygen) adj expenditure).tw.
-
or/36-38
-
35 not 39
-
(letter or editorial or comment or historical article).pt.
-
40 not 41
-
21 and 42
Health-related quality-of-life MEDLINE search strategy
-
Arthritis, Juvenile/
-
JIA.tw.
-
exp Arthritis/
-
(arthriti* or oligoarthriti* or polyarthriti* or polyarticula*).tw.
-
Rheumatoid Factor/
-
“rheumatoid factor”.tw.
-
or/3-6
-
(juvenile* or child* or teen* or adolescen* or youth* or “young person” or “young people” or pediatric* or paediatric*).tw.
-
exp Child/ or Adolescent/
-
7 and (8 or 9)
-
1 or 2 or 10
-
CHAQ.tw.
-
childhood health assessment questionnaire.tw.
-
child health questionnaire.tw.
-
CHQ.tw.
-
CHU 9D.tw.
-
PedsQL.tw.
-
“Paediatric Quality of Life Inventory”.tw.
-
“Pediatric Quality of Life Inventory”.tw.
-
“juvenile arthritis disease activity score”.tw.
-
JADAS*.tw.
-
value of life/
-
quality adjusted life year/
-
quality adjusted life.ti,ab.
-
(qaly* or qald* or qale* or qtime*).tw.
-
disability adjusted life.ti,ab.
-
daly*.ti,ab.
-
health status indicators/
-
eq 5d 3l.tw.
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(hql or hqol or “h qol” or hrqol or “hr qol”).tw.
-
(hye or hyes).tw.
-
health* year* equivalen*.ti,ab.
-
health utilit*.ab.
-
(hui or hui1 or hui2 or hui3).ti,ab.
-
disutil*.ti,ab.
-
rosser.ti,ab.
-
“quality of well being”.tw.
-
“quality of wellbeing”.tw.
-
qwb.tw.
-
“willingness to pay”.tw.
-
“standard gamble*”.tw.
-
“time trade off”.tw.
-
“time tradeoff”.tw.
-
tto.tw.
-
(index adj2 “well being”).mp.
-
(quality adj2 “well being”).mp.
-
(health adj3 utilit*).mp.
-
((multiattribute* or “multi attribute*”) adj3 (“health ind*” or theor* or “health state*” or utilit* or analys*)).mp.
-
“quality adjusted life year*”.mp.
-
(15D or “15 dimension*”).mp.
-
(12D or “12 dimension*”).mp.
-
“rating scale*”.mp.
-
“linear scal*”.mp.
-
“linear analog”.mp.
-
“visual analog*”.mp.
-
(categor* adj2 scal*).mp.
-
or/12-57
-
11 and 58
-
(comment or editorial or letter).pt.
-
59 not 60
-
limit 61 to English language
Appendix 2 Screening phase 1: titles and abstracts for systematic review of clinical effectiveness
| Language | |
|---|---|
| Non-English language | Exclude |
| Intervention | |
|
Can be either with or without methotrexate (will check usage is as per licensed indication at full-paper screen) |
| Participants | |
JIA
|
For mixed populations (e.g. including systemic or oligoarthritis), include only if the proportion of the unwanted type(s) is < 33% (i.e. two-thirds of the population should meet the inclusion criteria) Exclude systemic arthritis (unless NO active systemic symptoms in the previous 6 months); exclude persistent oligoarthritis |
| Comparators | |
|
|
| Outcomes | |
One or more of:
|
Do not exclude at title and abstract screening stage on outcome. Get full paper to check |
| Design | |
| RCT | If NO but data may not be available from RCTs (e.g. long-term AEs, height and growth) |
| Systematic review | If YES (or possibly Yes) and cannot exclude on P, I or C, RETRIEVE for full-paper screen and possible reference list check if meets criteria |
| Abstracts/conference presentations | |
| Published 2011 or earlier | Exclude |
| Published 2012 or later: are sufficient details presented to allow appraisal of methodology and assessment of results? | If cannot definitely exclude on P, I, C or D RETRIEVE (for full-text screen and possible tie up with full papers or ongoing studies) |
Appendix 3 Screening phase 2: full papers for systematic review of clinical effectiveness
| Design | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCT | Yes ↓ Next Q |
Unclear ↓ Next Q |
No ↓ Exclude |
|||||||||||||||||
| Abstracts/conference presentations | ||||||||||||||||||||
| Published 2012 or later | Yes ↓ Next Q |
Unclear ↓ Next Q |
No ↓ Exclude |
|||||||||||||||||
| Intervention | Comments | |||||||||||||||||||
|
Yes (Y) ↓ To drug-specific section (row below) |
Unclear (U) ↓ Skip to comparator section |
No (N) ↓ Exclude |
Etanercept should be monotherapy (not with methotrexate) | ||||||||||||||||
| Abatacept (Orencia) | Adalimumab (Humira) | Etanercept (Enbrel) | Tocilizumab (RoActemra) | |||||||||||||||||
| JIA subtype polyarthritis with insufficient response to other DMARDs including at least one TNF inhibitor | JIA subtype polyarthritis with inadequate response to 1 or more DMARDs | JIA subtype ERA with inadequate response/intolerance to conventional treatment | JIA subtype polyarthritis (including RF+ve or RF–ve and EO) with inadequate response/intolerance to methotrexate | JIA subtype PA with inadequate response/intolerance to methotrexate | JIA subtype ERA with inadequate response/intolerance to conventional treatment | JIA subtype polyarthritis (including RF+ve or RF–ve and EO) in patients not responding to other NSAIDs or corticosteroids | ||||||||||||||
| Y ↓ Next Q |
U ↓ Next Q |
N ↓ Exclude |
Y ↓ Next Q |
U ↗ Next Q |
N ↗ Next Q |
Y ↓ Next Q |
U ↓ Age Qs |
N ↓ Exclude |
Y ↓ Next Q |
U ↗ Next Q |
N ↗ Next Q |
Y ↓ Next Q |
U ↗ Next Q |
N ↗ Next Q |
Y ↓ Next Q |
U ↓ Age Qs |
N ↓ Exclude |
Y ↓ Next Q |
U ↓ Next Q |
N ↓ Exclude |
| Participant age 6 years | Participant age 2 years | Participant age 6 years | Participant age 2 years | Participant age 12 years | Participant age 2 years | |||||||||||||||
| Y ↓ Comp |
U ↓ Comp |
N ↓ Ex |
Y ↓ Comp |
U ↓ Comp |
N ↓ Ex |
Y ↓ Comp |
U ↓ Comp |
N ↓ Ex |
Y ↓ Comp |
U ↓ Comp |
N ↓ Ex |
Y ↓ Comp |
U ↓ Comp |
N ↓ Ex |
Y ↓ Comp |
U ↓ Comp |
N ↓ Ex |
|||
| Comments: | Comments: | Comments: | Comments: | Comments: | Comments: | |||||||||||||||
| Comparators (Comp) | Comments | |||||||||||||||||||
|
Yes ↓ Next Q |
Unclear ↓ Next Q |
No ↓ Exclude |
Note what the comparator is | ||||||||||||||||
| Outcomes | ||||||||||||||||||||
| Any one or more from the list below: Disease activity; disease flares; physical function; joint damage; pain; corticosteroid-reducing regimens; extra-articular manifestations (e.g. uveitis); body weight and height; mortality; AEs of treatment; HRQoL |
Yes ↓ Next Q |
Unclear ↓ Next Q |
No ↓ Exclude |
|||||||||||||||||
| Abstracts/conference presentations | ||||||||||||||||||||
| Published with sufficient detail to allow appraisal of methodology and assessment of results | Yes ↓ Make final decision |
Unclear ↓ Make final decision |
No ↓ Exclude |
|||||||||||||||||
| Final decision | INCLUDE | UNCLEAR (discuss) | EXCLUDE | |||||||||||||||||
Appendix 4 Table of excluded and unclear studies from systematic review of clinical effectiveness
| Excluded study | Primary reason for exclusion (comments) |
|---|---|
| Amarilyo G, Tarp S, Foeldvari I, Cohen N, Pope TD, Woo JMP, et al. Efficacy and safety of biologic agents in patients with poly-articular juvenile idiopathic arthritis: network meta-analysis of randomized controlled withdrawal trials. Arthritis Rheum 2013;65:S922–3 | Design (NMA) |
| Anink J, Otten MH, Spronk S, van Suijlekom-Smit LW. Efficacy of biologic agents in juvenile idiopathic arthritis: a systematic review using indirect comparisons. Arthritis Rheum 2012;64:S490 | Design (SR and indirect comparison) |
| Canadian Agency for Drugs and Technologies in Health. Common Drug Review: Clinical Review Report for Tocilizumab (Actemra, intravenous) for the Treatment of Signs and Symptoms of Active Polyarticular Juvenile Idiopathic Arthritis. 2014. URL: www.cadth.ca/media/cdr/clinical/SR0343_Actemra%20pJIA_CL_Report_e.pdf (accessed May 2015) | Design (SR) |
| Cummins C, Connock M, Fry-Smith A, Burls A. A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept. Health Technol Assess 2002;6(17) | Design (SR and economic evaluation) |
| Decelle K, Horton ER. Tocilizumab for the treatment of juvenile idiopathic arthritis. Ann Pharmacother 2012;46:822–9 | Design (SR) |
| Foster CS, Tufail F, Waheed NK, Chu D, Miserocchi E, Baltatzis S, et al. Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate. Arch Ophthalmol 2003;121:437–40 | Population (adults) |
| Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN. Biologics for the treatment of juvenile idiopathic arthritis: a systematic review and critical analysis of the evidence. Clin Rheumatol 2008;27:67–76 | Design (SR) |
| Kemper AR, Van Mater HA, Coeytaux RR, Williams JW Jr, Sanders GD. Systematic review of disease-modifying antirheumatic drugs for juvenile idiopathic arthritis. BMC Pediatr 2012;12:29 | Design (SR) |
| Kingsbury D, Quartier P, Arora V, Kalabic J, Kupper H, Mozaffarian N. Safety and effectiveness of adalimumab in children with polyarticular juvenile idiopathic arthritis aged 2 to < 4 years or > = 4 years weighing < 15 kg. Ann Rheum Dis 2013;72:A729 | Design |
| Kingsbury D, Quartier P, Arora V, Kalabic J, Kupper H, Mozaffarian N. PReS-FINAL-2161: Safety and effectiveness of adalimumab in children with polyarticular juvenile idiopathic arthritis aged 2 to < 4 years or > = 4 years weighing < 15 kg. Pediatr Rheumatol 2013;11:P173 | Design |
| Kingsbury DJ, Quartier P, Arora V, Kalabic J, Kupper H, Mozaffarian N. Safety and effectiveness of adalimumab in children with polyarticular juvenile idiopathic arthritis aged 2 to < 4 years or > = 4 years weighing < 15 kg. Arthritis Rheum 2013;65:S117 | Design |
| Maneiro JR, Salgado E, Gomez-Reino JJ. Immunogenicity of monoclonal antibodies against tumor necrosis factor used in chronic immune-mediated Inflammatory conditions: systematic review and meta-analysis. JAMA Intern Med 2013;173:1416–28 | Design (SR and MA) |
| Martini A. Etanercept improves active polyarticular juvenile rheumatoid arthritis. Clin Exp Rheumatol 2001;19:122–4 | Design (commentary) |
| Mease P, Genovese MC, Gladstein G, Kivitz AJ, Ritchlin C, Tak PP, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum 2011;63:939–48 | Population (adults) |
| Mori M, Takei S, Imagawa T, Imanaka H, Nerome Y, Kurosawa R, et al. Etanercept in the treatment of disease-modifying anti-rheumatic drug (DMARD)-refractory polyarticular course juvenile idiopathic arthritis: experience from Japanese clinical trials. Mod Rheumatol 2011;21:572–8 | No comparator |
| Mori M, Takei S, Imagawa T, Imanaka H, Nerome Y, Higuchi R, et al. Safety and efficacy of long-term etanercept in the treatment of methotrexate-refractory polyarticular-course juvenile idiopathic arthritis in Japan. Mod Rheumatol 2012;22:720–6 | Design (open-label part) |
| Otten MH, Anink J, Spronk S, van Suijlekom-Smit LWA. Efficacy of biological agents in juvenile idiopathic arthritis: a systematic review using indirect comparisons. Ann Rheum Dis 2013;72:1806–12 | Design (review) |
| Pato E, Munoz-Fernandez S, Francisco F, Abad MA, Maese J, Ortiz A, et al. Systematic review on the effectiveness of immunosuppressants and biological therapies in the treatment of autoimmune posterior uveitis. Semin Arthritis Rheum 2011;40:314–23 | Design (review) |
| Sawyer L, Diamantopoulos A, Brunner HI, Benedetti F, Ruperto N, Dejonckheere F, et al. PReS-FINAL-2070: efficacy of biologic treatments in juvenile idiopathic arthritis with a polyarticular course: an indirect comparison. Pediatr Rheumatol 2013;11:P82 | Design (indirect comparison) |
| Sawyer L, Diamantopoulos A, Brunner H, De Benedetti F, Ruperto N, Dejonckheere F, et al. Efficacy of biologic treatments in juvenile idiopathic arthritis with a polyarticular course: an indirect comparison. Ann Rheum Dis 2013;72:740–1 | Design (indirect comparison) |
| Sawyer L, Diamantopoulos A, Brunner HI, De Benedetti F, Ruperto N, Dejonckheere F, et al. Efficacy of biologic treatments in juvenile idiopathic arthritis with a polyarticular course: an indirect comparison. Arthritis Rheum 2013;65:S119 | Design (indirect comparison) |
| Simonini G, Druce K, Cimaz R, Macfarlane GJ, Jones GT. Current evidence of anti-tumor necrosis factor alpha treatment efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach of individual drugs. Arthritis Care Res 2014;66:1073–84 | Design (review) |
| Simonini G, Katie D, Cimaz R, Macfarlane GJ, Jones GT. Does switching anti-TNFalpha biologic agents represent an effective option in childhood chronic uveitis: the evidence from a systematic review and meta-analysis approach. Semin Arthritis Rheum 2014;44:39–46 | Design (review) |
| Ungar WJ, Costa V, Burnett HF, Feldman BM, Laxer RM. The use of biologic response modifiers in polyarticular-course juvenile idiopathic arthritis: a systematic review. Semin Arthritis Rheum 2013;42:597–618 | Design (review) |
| Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O’Neil KM, Zeft AS, et al. The effects of early aggressive therapy in JIA: results of the TREAT study. Pediatr Rheumatol 2012;10:32 | Abstract (methods) |
| Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O’Neil KM, Zeft AS, et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum 2012;64:2012–21 | Unclear population |
| Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O’Neil KM, Zeft AS, et al. Predictors and sustainability of clinical inactive disease in polyarticular juvenile idiopathic arthritis given aggressive therapy very early in the disease course. Arthritis Rheum 2013;65:S334–5 | Abstract (methods) |
| Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O’Neil KM, Zeft AS, et al. Clinically inactive disease in a cohort of children with new-onset polyarticular juvenile idiopathic arthritis treated with early aggressive therapy: time to achievement, total duration, and predictors. J Rheumatol 2014;41:1163–70 | Unclear population |
| Wallace CA, Bonsack J, Spalding SJ, Brunner H, O’Neil KM, Milojevic D, et al. Results of a 24 month extension study in patients who participated in the trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum 2013;65:S116 | Abstract (methods) |
| Unclear studies |
|---|
| Smith JA, Thompson DJ, Whitcup SM, Suhler E, Clarke G, Smith S, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum 2005;53:18–23 |
Appendix 5 Clinical effectiveness data extraction tables
Data extraction: abatacept
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Study identifier: Ruperto et al. (2008),57 Ruperto et al. (2010),58 Ruperto et al. (2010),59 Lovell et al. (2012)60 Study acronym: AWAKEN Study design: withdrawal RCT (4-month open-label lead-in phase, 6-month double-blind randomised phase, OLE phase) Country or countries: Europe (none from the UK), Latin America, USA Number of centres: 45 Recruitment dates: February 2004 to June 2006 (date of last treatment, recruitment likely to be finished before then) Funding: BMS |
Intervention: 4-month open-label lead-in phase (days 1–113) ABA (10 mg/kg according to weight; maximum dose 1000 mg) on days 1, 15, 29, 57 and 85 Double-blind phase: ABA given at doses of 10 mg/kg at randomisation and at about 28-day intervals thereafter for 6 months (days 114–283) or until a flare of arthritis Comparator: matching placebo Other interventions used: all DMARDs except MTX (stable dose) withdrawn and prohibited during the trial (wash-out period of at least 4 weeks for any DMARD other than MTX, before the first dose of study medication). Oral corticosteroids were stabilised 4 weeks before enrolment NSAIDs or analgesics permitted for pain control Folinic acid or folic acid permitted. 140/190 (74%) received MTX concomitantly |
OL lead-in phase, number enrolled: n = 190. Those achieving ACR Pedi-30 response randomised in double-blind phase (limited data extracted for this phase) Double-blind withdrawal phase Number of randomised participants: ABA: n = 60 Placebo: n = 62 OLE study [up to day 1681 (year 5.5) efficacy, and up to 7 years safety]59,60 Non-responders to ABA during OL phase: n = 36 ABA treated patients in double-blind phase: n = 58 Placebo treated patients in double-blind phase: n = 59 Total in OLE: n = 153 Inclusion criteria: JIA (EO, polyarticular positive or negative for RF, or systemic without systemic manifestations) Aged 6–17 years At least five active joints (those with swelling or, in the absence of swelling, limited range of motion, accompanied by either pain or tenderness) and active disease (at least two active joints and two joints with a limited range of motion); inadequate response to, or intolerance to, at least one DMARD including biologic agents (e.g. etanercept, infliximab and adalimumab). (Previous anti-TNF therapy reported in 57/190 patients during OL lead in.) Exclusion criteria: active uveitis, major concurrent medical conditions, pregnant or lactating |
Primary outcome(s): time to disease flare Secondary outcomes: proportion of patients at the end of 6-month double-blind phase who had disease flare; changes from baseline in each of the six ACR core variables; pain; assessment of safety and tolerability; HRQoL (sleep and missed school days reported but not extracted here) Method of assessing outcomes: disease flare defined as worsening of 30% or more in at least three of the six ACR core-response variables for JIA, and at least 30% improvement in no more than one variable during the double-blind period. If a global assessment by either physician or parent was used, flare was defined as a worsening of 20 mm or more on the 100-mm VAS. If the number of active joints or joints with limited range of motion was used for assessment, it was defined as worsening in two or more joints Improvement defined as an improvement of 30% or more in at least three of six ACR core-response variables and at least 30% worsening in not more than one variable. Improvements were also defined by 50%, 70% and 90% improvements in the ACR paediatric criteria CHAQ used to assess physical, emotional and social aspects of HRQOL. Higher scores indicate better HRQoL, 0–100 scale. CHAQ disability index is scored 0–3, with a higher score indicating greater disability. CHQ used to assess pain on 100-mm VAS. Higher score indicates more severe pain Length of follow-up: end of double-blind period (day 169), plus assessments made for OLE at ≥ 21 months (day 589) (efficacy and safety), and at day 1681 (study year 5.5 – efficacy and safety, and study year 7 (safety). It is presumed that these time points are in relation to the start of the OL lead-in |
| Baseline characteristics (double-blind period) | ABA (n = 60) | Placebo (n = 62) | Comments |
| Age (mean), years (SD) | 12.6 (3) | 12.0 (3) | |
| Sex female, n (%) | 43 (72) | 45 (73) | |
| Ethnic origin, n (%) | |||
| White Black Other |
46 (77) 5 (8) 9 (15) |
49 (79) 4 (7) 9 (15) |
|
| Type of JIA | |||
| Persistent oligoarthritis EO Polyarthritis (RF+ve) Polyarthritis (RF–ve) Systemic |
0 9 (15) 14 (23) 26 (43) 11 (18) |
2 (3) 7 (11) 12 (19) 28 (45) 12 (19) |
|
| RF+ve, n (%) RF–ve, n (%) |
19 (32) 41 (68) |
12 (19) 50 (81) |
|
| Duration of JIA (mean), years (SD) | 3.8 (3.7) | 3.9 (3.5) | |
| Previous anti-TNF therapy discontinued, n (%) Lack of efficacy For financial reasons |
8 (13) 7 (12) 1 (2) |
13 (21) 11 (18) 2 (3) |
|
| Results (for double-blind period,57 unless otherwise stated) | |||
| Primary outcome | ABA (n = 60) | Placebo (n = 62) | p-value |
| Time to flare (median, months) | Not reached | 6 | 0.0002 |
| Comments: Kaplan–Meier survival curves are presented, but the survival probabilities can be read-off only from the curves and have not been extracted here. IQR could not be calculated for the placebo group as there were too few events | |||
| Secondary outcomes | ABA (n = 60) | Placebo (n = 62) | p-value |
| Disease activity, n (%)a | |||
| ACR Pedi-30 ACR Pedi-50 ACR Pedi-70 ACR Pedi-90 Inactive diseaseb |
49 (82) 46 (77) 32 (53) 24 (40) 18 (30) |
43 (69) 32 (52) 19 (31) 10 (16) 7 (11) |
0.1712 0.0071 0.0185 0.0062 0.0195 |
| Disease flares, n (%) | 12 (20) | 33 (53) | 0.0003 |
| Disease flares, hazard ratio | 0.31 (95% CI 0.16 to 0.59) | NR | |
| Core-response variables, mean (SD) | |||
| PGA (VAS: 100 mm) | 14.7 (18.9) | 23.2 (21.8) | 0.0004 |
| Parent’s global assessment (VAS: 100 mm) | 17.9 (22.2) | 23.9 (21.6) | 0.6992 |
| Physical function (CHAQ disability index: 0–3) | 0.8 (0.9) | 0.8 (0.7) | 0.0388 |
| Number of active joints | 4.4 (7.0) | 6.0 (5.8) | 0.0245 |
| Number of joints with limited range of motion | 8.8 (12.8) | 8.6 (12.0) | 0.0128 |
| ERS (mm per hour) | 25.1 (26.4) | 30.7 (30.1) | 0.9562 |
| CRT (mg/l) | 0.16 (0.25) | 0.29 (0.54) | 0.0255 |
| Pain (mean parent global assessment of pain, CHAQ 100-mm VAS) | 15c | 21c | 0.105 |
| Corticosteroid-reducing regimens | NR | ||
| Extra-articular manifestations | NR | ||
| Body weight and height | NR | ||
| Mortality | NR | ||
| HRQoL | |||
| CHQ physical summary score CHQ psychosocial summary score |
43.6 51.7 |
41d 47d |
0.666 0.056 |
| AEs (for double-blind period, unless otherwise stated) | |||
| Total SAEs, n (%) | 0 | 2 (3) | 0.50 |
| Total SAEs, OLE, n (%)e | 23 (15) | ||
| Total AEs, n (%)f | 37 (62) | 34 (55) | 0.47 |
| Infections and infestations, n (%) | 27 (45) | 27 (44) | 1.00 |
| Gastrointestinal disorders, n (%) | 10 (17) | 9 (15) | 0.81 |
| General disorders and administration site conditions, n (%) | 4 (7) | 9 (15) | 0.24 |
| Nervous system disorders, n (%) | 3 (5) | 2 (3) | 0.68 |
| Respiratory, thoracic and mediastinal disorders, n (%) | 6 (10) | 3 (5) | 0.32 |
| Results OLE | |||
|---|---|---|---|
| Original group sizes in double-blind phase | ABA (n = 60) | Placebo (n = 62) | |
| ACR Pedi outcomes OLE at day 58959 | Received ABA in double-blind phase (n = 51) | Received placebo in double-blind phase (n = 47) | |
| ACR Pedi-30 | 46/51 (90%) | 41/47 (87%) | |
| ACR Pedi-50 | 45/51 (88%) | 39/47 (83%) | |
| ACR Pedi-70 | 38/51 (75%) | 35/47 (75%) | |
| ACR Pedi-90 | 29/51 (57%) | 19/47 (40%) | |
| ACR Pedi-100 | 20/51 (39%) | 9/47 (19%) | |
| Inactive diseaseg | 22/51 (43%) | 11/47 (23%) | |
| Methodological comments: Allocation to treatment groups: patients randomly assigned (1 : 1) to receive either abatacept or placebo. The sequential number for each patient was allocated according to a computer-generated randomisation schedule Blinding: the main phase of the trial was described as double-blind. Responder and flare status were determined by independent blinded evaluators at the co-ordinating centres Comparability of treatment groups: appear similar on most variables, although placebo group had a greater proportion of RF–ve patients than ABA group (81% vs. 68%) Method of data analysis: Kaplan–Meier survival curves used to estimate the distribution of time to disease flare for each group in the 6-month double-blind phase. Log-rank test used to compare the time to disease flare between groups. A Cox proportional hazards model, with treatment as the only covariate, was used to compare the hazard ratio and 95% CIs for flare of arthritis between the two groups. Missing values in the double-blind phase imputed with the LOCF method in the analysis of the individual components of the six ACR paediatric response variables, the ACR responses and inactive disease status. HRQoL analysis (CHQ) based on available data at each time point Sample size/power calculation: estimated 200 patients needed in the open-label phase to have a sufficient sample size to compare the time to flare over 6 months between the abatacept and placebo groups (with two-sided log-rank tests at 5% significance). Assuming that 64% of patients would respond to treatment (based on experience with rheumatoid arthritis in adults), a sample size of 128 patients would yield 95% power to detect a difference of 35%, assuming a flare rate of 65% in placebo controls and a dropout rate of 10% for the double-blind phase. (The actual flare rate for placebo was 53% and the drop-out rate was 34%, with a difference of 33% between abatacept and placebo in percentage of patients experiencing a flare.) Attrition/drop-out: 42 (34%) patients discontinued during the double-blind period, 31 (50%) in the placebo group and 11 (18%) in the abatacept group); all but one (abatacept-treated patient) did so because the treatment was not effective. 8 patients (2 ABA, 6 placebo) did not receive treatment in accordance with protocol during the double-blind phase but were included in end-point analysis |
| General comments: Generalisability: results applicable to patients aged 6–17 years with JIA (EO, polyarthritis or systemic without systemic manifestations) with an inadequate response to, or intolerance to, at least one DMARD (including biologic agents), receiving background MTX Outcome measures: appear appropriate Intercentre variability: not reported, but to minimise variability in joint assessments each centre had at least two certified joint assessors who underwent specific and standardised joint assessment training Conflict of interests: the first two authors have received funding for research activity from a variety of pharmaceutical companies including BMS, although they have not received funding from companies as personal contribution for assistance during the trial. Three other authors are employees of BMS |
Quality criteria (Cochrane Risk of Bias tool) randomised controlled trials171
| Criteria | Judgementa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Yes | Computer-generated randomisation sequence |
| Allocation concealment (selection bias) | Yes | Centres were informed of the random allocation of patients by an interactive voice-randomisation system run by the central drug management group |
| Blinding of participants and personnel (performance bias) | Yes | Double-blind phase of the study (after open-label lead in) |
| Blinding of outcome assessment (detection bias) | Yes | Responder and flare status determined by independent blinded evaluators at the co-ordinating centres |
| Incomplete outcome data addressed (attrition bias) | Larger proportion of patients dropped out of the placebo group in the double-blind phase than the ABA group (50% vs. 18%). Main reason for drop-out was lack of efficacy | |
| ACR responses, inactive disease status | Yes | Missing values imputed with LOCF method |
| HRQoL58 | No | Analyses based on available data (observed analysis) |
| Selective reporting (reporting bias) | Yes | All outcomes reported on |
| Other sources of bias | Unclear | Intercentre variability not discussed |
Data extraction: adalimumab
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Study identifier: Lovell et al. (2008),61 Lovell et al. (2012),62 Ruperto et al. (2013),63 Ruperto et al. (2014)64 Study acronym: none Study design: medication-withdrawal RCT (16-week randomised open-label, 32-week double-blind randomised withdrawal phase, OLE phase) Country or countries: not specifically stated but appear to be Belgium, Czech Republic, France, Germany, Italy, Spain, Slovak Republic and the USA Number of centres: 31 (not specified by country) Recruitment dates: 19 September 2002 to 13 January 2005 Funding: research grant from Abbott Laboratories |
16-week open-label phase: 24 mg ADA per square metre (maximum of 40 mg) subcutaneously EOW Licence indication: polyarticular onset JIA: presumed to be the same as polyarticular-course JIA Double-blind phase: ADA: as per open-label phase for 32 weeks Comparator: placebo Other interventions used: MTX: ≥ 10 mg/m2 per week for 3-month prior screening, same dosage during open-label lead-in and double-blind phases No MTX: have never received MTX or had discontinued it ≥ 2 weeks prior to study drug Stable dosages of NSAIDs and low-dose corticosteroids (≤ 0.2 mg of prednisone or prednisone equivalent per kilogram of body weight per day to a maximum of 10 mg per day) were permitted. Pain medications were allowed except for the 12 hours preceding assessment of the joints |
First randomisation, open-label lead-in phase, number randomised: n = 171 MTX: n = 85 No-MTX: n = 86) (Limited data extracted for this phase.) Second randomisation, double-blind withdrawal phase: Number randomised: n = 133 MTX/placebo: n = 37 MTX/ADA: n = 38 No data for ADA (n = 30) and placebo (n = 28) group not receiving MTX extracted Loss to follow-up: n = 4 (5.3%) ADA: n = 3 (7.9%) Placebo: n = 1 (2.7%) Inclusion criteria: Aged 4–17 years Polyarticular-course JIA (with any type of onset) with active disease (≥ 5 swollen joints and ≥ 3 joints with LOM) and without adequate response to NSAIDs Either no previous treatment with MTX or previous treatment with MTX and AEs or an inadequate response Had to have an ACR Pedi-30 response at week 16 to enter double-blind phase Exclusion criteria: Clinically significant deviations in clinically hematologic, hepatic or renal indicators Ongoing infection or recent major infection requiring hospitalisation or intravenous antibiotics Recent live or attenuated vaccines Previously treated with other biologic agents at any time or recent treatment with intravenous immune globulin, cytotoxic agents, investigational agents, DMARDs other than MTX, or corticosteroids administered by the intra-articular, intramuscular or intravenous route |
Primary outcome(s): percentage of patients not receiving MTX with disease flares (week 16–48) Secondary outcomes: AEs Method of assessing outcomes: every 12 weeks Disease flare (ACR Pedi responses): worsening of ≥ 30% in more than three of the six core criteria for JIA and an improvement of ≥ 30% in one or fewer criteria. If the number of joints with active arthritis was used as a criterion of flare, an increase in the number of active joints to two or more was required if there were no initial active joints or only one active joint. The same approach was used if the number of joints with loss of motion was used as a criterion of flare. If either of the global assessments was used as a criterion of flare, any increase of > 30% in the VAS of 0 to 100 was sufficient and no minimum clinically important increase was required (e.g. an increase of 2–4 would qualify for use of that criterion in the determination of flare) ACR Pedi criteria: physician’s and patient’s/parent’s global assessment of overall well-being (both measured with a 100-mm VAS: 0 = no disease activity or ‘very well’ for overall well-being, 100 = most disease activity or ‘very poor’ for overall well-being) the number of joints with active arthritis (defined as joints with swelling not caused by deformity or joints, in the absence of swelling, with limitation of passive motion accompanied by pain tenderness, or both), the number of joints with limitation of passive motion, physical function measured by the CHAQ-DI, and a laboratory assessment of inflammation (CRP concentrations) ACR Pedi-50, -70, -90 and -100 levels of response were evaluated, defined as improvements of 50% or more, 70% or more, 90% or more and 100%, respectively, in three or more of the six core criteria for JIA, with worsening of 30% or more in only one criterion Safety: physical examinations, laboratory results, vital signs and AEs Post hoc analysis: Clinical outcomes: 27-joint JADAS27 based on C-creative protein; functional outcome: CHAQ-DI. MDA defined as JADAS-27 < 3.8 and normal function defined as CHAQ-DI < 0.5. Higher scores indicate higher disease activity Length of follow-up: 70 days after last dose for AEs for all patients who discontinued study medication. Those enrolled in the double-blind phase were eligible to receive open-label treatment with ADA in an extension phase of the study (duration not specified) |
| Baseline characteristics | ADA (n = 38) | Placebo (n = 37) | Comments |
|---|---|---|---|
| Age, mean years (SD) | 11.7 (3.3) | 10.8 (3.4) | |
| Age group, n (%) | |||
| 4–8 years 9–12 years 13–17 years |
6 (16) 17 (45) 15 (40) |
12 (32) 10 (27) 15 (41) |
|
| Sex: female, n (%) | 30 (79) | 30 (81) | |
| Ethnicity, n (%) | |||
| White Black Other |
36 (95) 0 2 (5) |
36 (97) 0 1 (3) |
Determined by the patient or parent |
| Body weight (mean), kg (SD) | 42.1 (17.9) | 44.3 (18.9) | |
| Type of JIA | Reported as poly-articular-course JIA | ||
| RF–ve, n/N (%) | 27/37 (73) | 30/36 (83) | |
| Duration of JIA (mean), years (SD) | 4.3 (4.1) | 4.0 (3.5) | |
| Previous medication use, n (%) | |||
| MTX Other DMARDs Methylprednisolone |
38 (100) 1 (3) 2 (5) |
37 (100) 7 (19) 2 (5) |
|
| Results (double-blind phase, weeks 16–48) | |||
| Primary outcome | ADA (n = 38) | Placebo (n = 37) | p-value |
| Disease flares, n/N (%) | 14/38 (37) | 24/37 (65) | p = 0.02 |
| Comments: | |||
| Secondary outcomes | ADA (n = 38) | Placebo (n = 37) | p-value |
| Disease activity | |||
| ACR Pedi response week 48 (%)a 30 50 70 90 |
63 63 63 42 |
38 38 27 27 |
p = 0.03 p = 0.03 p = 0.002 p = 0.17 |
| Physical function | NR | NR | |
| Joint damage | NR | NR | |
| Pain | NR | NR | |
| Corticosteroid reducing regimens | NR | NR | |
| Extra-articular manifestations (such as uveitis) | NR | NR | |
| Body weight and height | NR | NR | |
| Mortality | See comments AEs | ||
| HRQoL | NR | NR | |
| AEs, number of events (number of events per patient-year) | ADA (n = 38) (18.3 patient-years) | Placebo (n = 37) (15 patient-years) | p-value |
|---|---|---|---|
| Any AE | 234 (12.8) | 155 (10.3) | |
| Most frequently reported AEs | |||
| Related to injection-site reaction | 73 (4.0) | 57 (3.8) | |
| Contusion | 12 (0.7) | 7 (0.5) | |
| Nasopharyngitis | 5 (0.3) | 6 (0.4) | |
| Upper respiratory tract infection | 6 (0.3) | 5 (0.3) | |
| Viral infection | 7 (0.4) | 3 (0.2) | |
| Vomiting | 4 (0.2) | 2 (0.1) | |
| Excoriation | 10 (0.6) | 1 (0.1) | |
| Serious AEs, possibly related to study drugb | 0 | 1 (0.1) – gastroduodenitis | |
| AEs leading to the discontinuation of the drug | 0 | 0 | |
| First 104 weeks of OLE phase61 | |
|---|---|
| ACR Pedi at 104 weeks of OLE, % | OLE phase (n = 128)c |
| 30 | 89%d |
| 50 | 86%d |
| 70 | 77%d |
| 90 | 59%d |
| 100 | 40% |
| Post hoc analysis: OLE,64 n (%) | ADA | Placebo | p-valuee | ||||
|---|---|---|---|---|---|---|---|
| week 48 | week 88 | week 48 | week 88 | ||||
| Minimal disease activityf | 19 (76) | 26 (83.9) | 15 (62.5) | 14.0 (50.0) | |||
| Minimal disease activityf with normal functiong | 17 (68.0) | 24 (77.4) | 15 (62.5) | 14 (50.0) | |||
| Methodological comments: |
| Allocation to treatment groups: randomisation at a 1 : 1 ratio within patients’ previous respective strata (stratified according to MTX use), no further details reported Blinding: double-blind (investigators, study co-ordinators, assessors, patients and parents were unaware of the treatment assignment during the double-blind phase of the study) Comparability of treatment groups: states no significant differences in baseline characteristics between the placebo and the ADA group within either stratum (MTX or no MTX) – no statistical comparison between the ADA–MTX and placebo–MTX group reported. There were some baseline differences between the later treatment groups: the ADA group had a higher percentage of children in the 9- to 12-year age group than the placebo group; conversely, mean body weight was slightly lower. Mean negative for RF was 10% lower in this group compared with placebo, duration of JIA was slightly lower and previous medication use for other DMARDs was 16% lower than the placebo group Method of data analysis: efficacy analyses ITT; however, this was defined as all patients who received a ≥ 1 dose of the study drug during the phase of the study for which the analysis was being conducted. For the primary efficacy end point and for all secondary analyses of disease flare, missing values were treated as disease flares. For secondary analyses of ACR Pedi-30, -50, -70 and -90 responses during the open-label lead-in and double-blind phases, missing values were imputed as non-responses. In addition, patients in whom a flare occurred according to the protocol definition during the double-blind phase were classified as having no response (ACR Pedi < 30) at week 48, regardless of their actual ACR Pedi responses Sample size/power calculation: assumption of a 70% response rate to ADA reported, requiring 42 patients in the open-label lead-in phase to yield the 29 patients needed for each group in the double-blind phase. This estimate was based on a 40% difference in the rate of flare between the placebo and the ADA groups and provided a power of 80% at an alpha level of 0.05. However, it is stated that the study was not statistically powered to detect differences between patients receiving and those not receiving MTX Attrition/drop-out: Double-blind: n = 4 (5.3%); ADA, n = 3 (withdrew for other reasons, no further details); placebo, n = 1 (withdrew consent). [Loss to follow-up, total: n = 43 (25%); open-label, all: n = 38 (22%); double-blind all n = 5 (3.8%)] |
| General comments: Generalisability: limited to polyarticular-course JIA patients aged 4 to 17 years, who have previously received 16 weeks’ ADA treatment and treated with MTX, and had an ACR Pedi-30 response at week 16 Outcome measures: appear to be appropriate Intercentre variability: not discussed Conflict of interests: authors received various financial support and/or unrestricted continued medical education grants from various pharmaceutical companies including the drug manufacturer. States ‘no other potential conflict of interests relevant to this article was reported’. Individuals at JK Associates and Abbott Laboratories provided editorial support, and individuals at Abbott Laboratories helped with data management and statistical analysis |
Quality criteria (Cochrane Risk of Bias tool) randomised controlled trials171
| Criteria | Judgementa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Unclear | No details reported |
| Allocation concealment (selection bias) | Unclear | No details reported |
| Blinding of participants and personnel (performance bias) | Yes | Double-blind, details reported |
| Blinding of outcome assessment (detection bias) | Yes | Double-blind, details reported |
| Incomplete outcome data addressed (attrition bias) | Yes | Details reported |
| Selective reporting (reporting bias) | Yes | All outcomes stated were reported |
| Other sources of bias | Unclear | Intercentre variability not discussed |
Data extraction: etanercept
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Study identifier: Lovell et al.(2000),42 Lovell et al. (2003),65 Lovell et al. (2006)66 and Lovell et al. (2008)67 Study acronym: none Study design: medication-withdrawal RCT (3 months open-label lead-in phase, 4-month double-blind randomised withdrawal phase, OLE phase) Country or countries: Canada and the USA Number of centres: not specifically stated, but appears to be 965 Recruitment dates: not reported Funding: Immunex Corporation171 |
Intervention: 0.4 mg of etanercept per kilogram subcutaneously twice weekly until disease flare occurred or 4 months elapsed (includes 33% of patients with systemic onset JIA) Comparator: placebo Other interventions used: stable doses of NSAIDs, low doses of corticosteroids (≤ 0.2 mg of prednisone per kilogram per day, with a maximum of 10 mg per day) or both. Pain medications were allowed except during the 12 hours before a joint assessment MTX discontinued 14 days and other DMARDs 28 days before receipt of etanercept OLE: 0.4 mg/kg etanercept twice weekly (maximum dose 25 mg per injection) or 0.8 mg/kg once weekly (maximum dose of 50 mg/week) subcutaneously |
(3 months open- label (OL) lead-in phase: n = 69) (limited data extracted for this phase) Double-blind withdrawal phase Number of randomised participants: n = 51 Etanercept: n = 25 Placebo: n = 26 OLE: n = 58 Loss to follow-up: [Part 1: n = 5 (7%)] Part 2-RCT: Etanercept: n = 6 (24%); Placebo: n = 19 (73%) Part 3: OLE: n = 38 (66%) Inclusion criteria: children aged 4–17 years with JIA, with active disease (five or more swollen joints and three or more joints with LOM and pain, tenderness, or both) despite treatment with NSAIDs and with MTX at doses of at least 10 mg/m2 of body-surface area per week Exclusion criteria: No intra-articular and soft-tissue corticosteroid injections for 1 month before the trial; patients with major concurrent medical conditions; Pregnant and lactating patients |
Primary outcome(s): number of patients with disease flare Secondary outcomes: not specifically stated Method of assessing outcomes for Part 2: physical examinations, measures of disease activity and laboratory tests (hematologic analysis, serum chemical analysis, and urinalysis) on day 1 (before etanercept or placebo) and day 15 and at the end of each month. Final safety assessments 30 days after discontinuation of study drug for withdrawals or at next scheduled visit if withdrawal is attributable to disease flare. Serum at the end of 7 months for testing for autoantibodies (antinuclear antibodies, antibodies to double-stranded DNA, IgG and IgM anticardiolipin antibodies and antibodies to extractable nuclear antigens), and on day 1 before the administration of the study drug and at the end of months 7 for testing for antibodies to etanercept Physician’s global assessment of disease severity 0–10 (best-worst); patient’s or parent’s global assess of overall well-being: 0–10 (best-worst); CHAQ: 0–3 (best–worst); ESR: normal ranges 1–30 mm per hour for females and 1–13 mm per hour for males; articular severity score: 0 (best) to 962 (worst); pain: VAS 0 cm (best) to 10 cm (worst); CRP: normal range is 0–0.79 mg per decilitre. Other: 73 joints were evaluated for the total active-joint count; 71 for LOM with pain, tenderness or both; 66 for swollen joints; and 71 for LOM Definition of disease flare: change in the core set of response variables from the beginning of the double-blind study: worsening of ≥ 30% in three of six response variables and a minimum of two active joints. Could also have improvement of ≥ 30% in no more than one of six response variables. Global assessments, if used to define flare, had to change by at least 2 units on a scale from 0 to 10 Definition of improvement of disease response was based on changes from baseline values at enrolment, whereas flare was measured from beginning of the double-blind study. For example, 28 active joints at baseline, but only 2 active joints at the time of randomisation – a change to 3 active joints would be considered a flare (at least 30% worse than the condition at the time of randomisation) but would also still be considered improvement (at least 30% improved from baseline) Length of follow-up: 4 months (double-blind only); OLE: 8 years67 |
| Baseline characteristics: double-blind study | Etanercept (n = 25) | Placebo (n = 26) | Comments |
|---|---|---|---|
| Mean age, year | 8.9 | 12.2 | |
| Age group, n (%) | |||
| 4–8 years 9–12 years 13–17 years |
13 (52) 5 (20) 7 (28) |
5 (19) 4 (15) 17 (65) |
p < 0.02 |
| Sex, n (%) | |||
| Female Male |
19 (76) 6 (24) |
15 (58) 11 (42) |
|
| Ethnicity, n (%) | |||
| White Black Hispanic Other |
14 (56) 3 (12) 6 (24) 2 (8) |
23 (88) 1 (4) 2 (8) 0 |
p < 0.02 |
| Type of JIA, n (%) | |||
| Pauciarticular Polyarticular Systemic |
2 (8) 14 (56) 9 (36) |
1 (4) 17 (65) 8 (31) |
|
| RF+ve, n (%) | 4 (16) | 8 (31) | |
| Mean duration of JIA, year | 5.3 | 6.4 | |
| Previous medication, n (%) | |||
| MTX | 25 (100) | 26 (100) | |
| DMARDs at washout, n (%) MTX Hydroxychloroquine |
16 (64) 16 (64) 2 (8) |
19 (73) 18 (69) 7 (27) |
|
| Concomitant therapy at washout, n (%) | |||
| Corticosteroids NSAIDs |
6 (24) 25 (100) |
13 (50) 24 (92) |
|
| Mean dose of corticosteroids, mg/day | 6.5 | 5.5 | |
| Results: double-blind study | |||
| Primary outcome | Etanercept (n = 25) | Placebo (n = 26) | p-value |
| Disease flare, n (%) Corticosteroid use at baselineb Yes No |
7 (28) 3/6 (50) 4/19 (21) |
21 (81) 12/13 (92) 9/13 (69) |
p = 0.003a p = 0.05 |
| Time to flare, median days | > 116 | 28 | p < 0.001 |
| Secondary outcomes at 7 months, median | Etanercept (n = 25) | Placebo (n = 26) | p-value |
|---|---|---|---|
| 30% improvement, n (%) 50% improvement, n (%) 70% improvement, n (%) |
20 (80) 18 (72) 11 (44) |
9 (35) 6 (23) 5 (19) |
p < 0.01 |
| JIA core set criteria, median | |||
| Total number of active joints (out of 73 joints) | 7.0 | 13.0 | |
| Number of joints with LOM and with pain, tenderness, or both (out of 71 joints) | 1.0 | 4.5 | |
| PGA of disease severity | 2 | 5 | |
| Patient/parent’s global assess. of overall well-being | 3 | 5 | |
| Score on CHAQ (disability domain) | 0.8 | 1.2 | |
| ESR | 18 | 30 | |
| Articular severity score, median | 38 | 66 | |
| Duration of stiffness (minimum), median | 5 | 38 | |
| Pain (on a VAS), median | 1.5 | 3.5 | |
| CRP, median | 0.4 | 3.0 | |
| Number of swollen joints, median | 4.0 | 11.0 | |
| Number of joints with LOM, median | 9 | 22 | |
| Corticosteroid reducing regimens | NR | NR | |
| Extra-articular manifestations (such as uveitis) | NR | NR | |
| Body weight and height | NR | NR | |
| Mortality | See AEs | ||
| AEs | Etanercept (n = 25) | Placebo (n = 26) | p-value |
|---|---|---|---|
| Death, n | 0 | 0 | |
| Urticaria, n | 1c | 0 | |
| Hospitalisation for serious AEs, n | |||
| Depression and personality disorder Gastroenteritis-flu syndrome |
1 1 |
0 0 |
|
| Injection-site reactions, n | 1 | 1 | |
| Tested positive for non-neutralising antibody to etanercept, n | 2 | N/A |
| Baseline characteristics: OPE, 8-year follow-up67 | OLE (n = 58) | Eighth year of OLE (n = 26) |
|---|---|---|
| Age, mean years (SD) | 10.4 (3.8) | 10.8 (3.9) |
| JRA onset type, n (%) | ||
| Pauciarticular Polyarticular Systemic |
5 (9) 34 (58) 19 (33) |
2 (8) 19 (73) 5 (19) |
| Duration of JRA, mean years (SD) | 5.9 (3.2) | 6.4 (3.4) |
| RF+ve, n (%) | 13 (23) (n = 56) | 6 (24) (n = 25) |
| Concomitant therapy at enrolment, n (%) | ||
| NSAIDs Corticosteroids Corticosteroid dosage, mg/day mean (SD) |
56 (97) 22 (38) 5.7 (3.2) |
25 (96) 8 (31) 4.1 (2.3) |
| Results: OLE year 8, mean (SEM)d | Completed year 8 (n = 16) |
| Total number of joints with active arthritis (n = 11) | 2.2 (0.9) |
| Total number of joints with LOM and tenderness and/or pain on motion (n = 11) | 0 |
| Total number of joints with LOM (n = 11) | 11.8 (4.4) |
| PGA | 1.6 (0.3) |
| Patient’s/parent’s global assessment | 2.0 (0.6) |
| Pain score | 1.8 (0.5) |
| CHAQ score (n = 11) | 0.6 (0.2) |
| CRPe | 1.1 (0.5) |
| ACR Pedi response, 8 years (LOCF), % (n/N) | ||||
|---|---|---|---|---|
| ACR Pedi-30 | 83 (40/48) | |||
| ACR Pedi-50 | 77 (36/47) | |||
| ACR Pedi-70 | 61 (28/46) | |||
| ACR Pedi-90 | 41 (19/46) | |||
| ACR Pedi-100 | 18 (8/45) | |||
| Year of etanercept treatment from RCT (excluding gaps between RCT and OLE) | SAEf | MIIg | ||
| Number of events | Number of events/patient-year | Number of events | Number of events/patient-year | |
| 1 (n = 69; 57 patient-years of drug exposure) | 5 | 0.09 | 2 | 0.04 |
| 9 (n = 14; 4 patient-years of drug exposure) | 0 | 0 | 0 | 0 |
| Total for all years (n = 69; 318 patient-years of drug exposure) | 39 | 0.12 | 9 | 0.03 |
| Methodological comments: Allocation to treatment groups: a blocked randomisation scheme with stratification according to study centre and number of active joints (≤ 2 vs. > 2) at the end of month 3 (in the open-label study) was used to assign patients to their treatment group Blinding: double-blind (no further details) Comparability of treatment groups: authors state that the groups were well balanced in the double-blind study, except for age group and ethnicity (p < 0.02) and corticosteroid use at baseline (p = 0.05) and that the unequal randomisation did not affect the study results. The etanercept group had a significantly higher number of younger patients than the placebo group (4- to 8-year olds: etanercept 52% vs. 19% placebo) and a greater ethnic mix (white: etanercept 56% vs. 88% placebo), whereas the placebo group had a significantly larger use of corticosteroid use at washout (placebo 50% vs. 24% E) Method of data analysis: statistical methods employed were reported. All tests were two-sided, with a significance level of 0.05. Patients who withdrew early without disease flare were counted in the analysis with those who continued to have a response; a LOCF approach was used for missing data and visits and for early termination. To evaluate any bias introduced by the withdrawal assumption in the primary analysis, an analysis of time to flare (by the log-rank test) was undertaken in which data on patients who withdrew without flare were censored at the time of withdrawal. The effect of baseline characteristics on flare rates was assessed by main-effects logistic regression OLE: data from patients who reached the age of 18 and discontinued the study and who therefore no longer had valid childhood efficacy measures were not included in efficacy analysis (summary of the last visit using the LOCF method). Adult-specific measures of disease for patients ≥ 18 years of age were not included in analyses (n = 5 each at years 7 and 8) Sample size/power calculation: none reported Attrition/drop-out: part 1 OL: 64/69 (93%) urticaria with the first dose of etanercept n = 1; refusal of treatment n = 2; lack of response n = 2. Part 2 – RCT: etanercept 6/25 (24%): disease flare n = 6; placebo: 19/26 (73%): parental refusal to allow continuation n = 1, disease flare n = 18 Part 3 OLE: 38/58 (66%): lack of efficacy n = 7 (12%); AEs n = 4 (7%); physician decision n = 5 (9%); protocol issue n = 3 (5%); lost to follow-up n = 3 (5%); patient/guardian refusal n = 5 (9%); other n = 8 (14%). 36% patients (n = 21) discontinued during the first 4 years |
| General comments: Generalisability: limited to pauciarticular, polyarticular and systemic onset JIA patients aged 4 to 17 years, who did not tolerate or had an inadequate response to MTX and had received 3 months of etanercept treatment Outcome measures: appear to be appropriate Intercentre variability: not discussed Conflict of interests: two authors had served as ad hoc consultants to Immunex |
Quality criteria (Cochrane Risk of Bias tool) randomised controlled trials171
| Criteria | Judgementa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Unclear | Blocked randomisation scheme with stratification, no details about how randomisation was performed |
| Allocation concealment (selection bias) | Unclear | No details reported |
| Blinding of participants and personnel (performance bias) | Yes | Double-blind phase of study (after open-label lead in) |
| Blinding of outcome assessment (detection bias) | Yes | Paper states that study site-staff who were not involved in patient assessments constituted the contents of the vials (etanercept or placebo) |
| Incomplete outcome data addressed (attrition bias) | Yes | Details reported, but drop-outs are nearly three times higher in the placebo group. Incomplete data appears to have been address with the LOCF method (used for missing data/visits and early terminations) |
| Selective reporting (reporting bias) | Yes | All outcomes reported on |
| Other sources of bias | Unclear | Intercentre variability not discussed |
Data extraction: tocilizumab
| Reference and design | Intervention and Comparator | Participants | Outcome measures | |
|---|---|---|---|---|
| Study identifier: Brunner et al. (2015),68 Brunner et al. (2014),69 Baildam et al. (2014),70 Brunner et al. (2013),71 De Benedetti et al. (2013),72 Baildam et al. (2013),73 De Benedetti et al. (2013),74 and Brunner et al. (2012)75 Study acronym: CHERISH Study design: medication-withdrawal RCT (16-week randomised open-label, 24-week double-blind randomised withdrawal phase, OLE phase) Country or countries: Australia, Canada, Europe, Latin America, Russia and the USA Number of centres: 58 Recruitment dates: 14 October 2009 to 31 January 2011 Funding: funding for manuscript preparation by F. Hoffmann-La Roche Ltd |
Intervention: intravenous TCZ at 8 mg/kg (8 mg/kg for < 30 kg group) or 10 mg/kg (10 mg/kg for < 30 kg group) every 4 weeks (based on pharmacokinetic modelling and simulation, doses of 10 mg/kg for patients weighing < 30 kg achieved TCZ exposure comparable to that of 8 mg/kg for patients weighing ≥ 30 kg) Comparator: placebo Other interventions used: stable doses of NSAIDs and low-dose glucocorticoids (≤ 0.2 mg/kg/day prednisone; daily maximum 10 mg) and MTX (10–20 mg/m2 body surface area/week) |
Randomised, OL lead-in phase: TCZ every 4 weeks until week 16, n = 188 – patients randomised to < 30 kg TCZ (n = 69) or ≥ 30 kg TCZ (n = 119) (Limited data extracted for this phase.) Double-blind withdrawal phase Number of randomised participants: n = 166 TCZ: n = 82: 10 mg/kg < 30 kg body weight: n = 16 8 mg/kg < 30 kg body weight: n = 11 8 mg/kg ≥ 30 kg body weight: n = 55 Placebo: n = 84: 10 mg/kg < 30 kg body weight: n = 15 8 mg/kg < 30 kg body weight: n = 13 8 mg/kg ≥ 30 kg body weight: n = 56 Loss to follow-up: OL: n = 22/188 (11.7%) RCT: TCZ n = 3/82 (3.7%) Placebo: 3/84 (3.6%). OLE: n = 5/160 (3.1%) Inclusion criteria: 2- to 17-year olds; Diagnoses of RF+ve or RF–ve Polyarticular-course JIA or EO JIA Disease duration ≥ 6months; Inadequate responses to or intolerant of MTX Five or more active joints, with LOM present in three or more of the active joints No MTX ≥ 4 weeks before and including baseline visit or had been taking MTX ≥ 12 weeks immediately before and including baseline visit and on stable dose of 10–20 mg/m2 for ≥ 8 weeks before and including baseline visit together with either folic acid or folinic acid No oral glucocorticoids at baseline visit or had been taking oral glucocorticoids at a stable dose for ≥ 4 weeks before and including baseline visit (n ≤ 10 mg/day or 0.2 mg/kg/day) No NSAIDs at baseline or more than one type of NSAID at a stable dose (less than or equal to the recommended daily dose) ≥ 2 weeks before and including the baseline visit Never been treated with biologics or had been previously treated with biologics and discontinued them for at least the following periods: anakinra: 1 week; etanercept: 2 weeks; rilonacept: 5 weeks; infliximab or adalimumab: 8 weeks; abatacept: 12 weeks; canakinumab:, 20 weeks, before and including the baseline visit Exclusion criteria: none in addition to the above |
Primary outcome(s): proportion of patients in whom a JIA-flare occurred during part 2 (up to and including week 40) compared with week 16 Secondary outcomes week 40: JIA–ACR 30/50/70/90 responses, change from baseline in JIA- CRVs and clinically inactive disease Method of assessing outcomes: 4-weekly assessments JIA–ACR-30 response: defined as ≥ 30% improvement of three or more of six JIA core response variables (JIA–CRVs) without > 30% worsening in one or more of the remaining JIA-CRVs compared with baseline (part 1: patients who had at least one JIA–ACR-30 response entered part 2) Active joints: defined as the presence of swollen joints (or, in the absence of swelling, joints with LOM plus pain on motion and/or tenderness with palpation) Clinically inactive disease was defined as PGA indicating no disease activity plus absence of all the following: joints with active arthritis, uveitis and ESR > 20 mm/h Serious infections were defined in accordance with the definition of SAEs in the International Conference on Harmonisation guidelines (reference provided) PGA of disease activity: VAS 0–100 (0 = inactive disease); assessment of patient overall well-being: VAS 0–100 (0 = very poor); physical function measured by the CHAQ-DI: 0–3 (0 = no disability) Patients continued RCT until week 40 unless JIA-flare (> 30% worsening in three of six JIA–CRVs without > 30% improvement in one or more of the remaining JIA–CRVs) compared with week 16. They then entered the OLE study Length of follow-up: OL 16 weeks; RCT 24 weeks; total 40 weeks. OLE 64 weeks (total 104 weeks) |
|
| Baseline characteristicsa | TCZ 8 mg/kg < 30 kg (n = 34) |
TCZ 10 mg/kg < 30 kg (n = 35) |
TCZ 10 mg/kg 30 kg (n = 119) |
|
| Age, years | 7.6 (2.71) | 6.9 (3.02) | 13.1 (2.78) | |
| Sex, females n (%) | 24 (71) | 30 (86) | 90 (76) | |
| Ethnicity | NR | |||
| Type of JIA | NR | |||
| Weight (kg) | 22.4 (5.3) | 20.7 (5.7) | 50.0 (12.6) | |
| RF+ve, n (%) | 2 (6) | 4 (11) | 48 (40) | |
| Duration of JIA, years | 3.5 (2.57) | 3.4 (2.39) | 4.7 (4.16) | |
| Previous medication, n (%) | ||||
| DMARD Biologic agentb |
26 (76) 6 (18) |
21 (60) 8 (23) |
87 (73) 47 (39) |
|
| Joints with active arthritis, n | 21.2 (13.6) | 23.9 (18.3) | 18.9 (13.0) | |
| Joints with LOM, n | 17.3 (13.3) | 23.1 (19.2) | 16.0 (12.7) | |
| Assessment of patient overall well-being, VAS | 59.1 (26.2) | 51.5 (26.9) | 51.6 (24.1) | |
| PGA of JIA activity, VAS | 64.7 (18.5) | 64.7 (20.5) | 59.4 (21.3) | |
| CRP (mg/l) (standard reference range 0–10 mg/l) | 26.6 (33.6) | 21.8 (32.3) | 22.8 (38.8) | |
| CHAQ-DI score | 1.8 (0.68) | 1.7 (0.71) | 1.2 (0.69) | |
| ESR (mm/h) (standard reference range 0–18 mm/h) | 36.6 (23.0) | 35.1 (24.1) | 34.2 (26.7) | |
| Concurrent MTX use, n (%) Dose (mg/m2/week) |
30 (88) 13.8 (2.9) |
29 (83) 16.5 (11.1) |
89 (75) 11.6 (2.7) |
|
| Concurrent glucocorticoid use, n (%)c Dose (mg/kg/day)c |
18 (53) 0.15 (0.038) |
15 (43) 0.15 (0.033) |
54 (45) 0.12 (0.052) |
|
| Results, week 40 double-blind study68,73,75 | |||
|---|---|---|---|
| Primary outcome | TCZd (n = 82) | Placebo (n = 81) | Differenced TCZ vs. placebo (95% CI); p-value |
| Proportion with JIA-ACR30 flare (compared with week 16), n (%) | 21 (25.6%) | 39 (48.1) | –0.21 (–0.35 to –0.08); 0.0024 |
| Secondary outcomes | TCZd (n = 82) | Placebo (n = 81) | Differenced TCZ vs. placebo (95% CI); p-value |
| Proportion of patients with JIA–ACR-30 improvement relative to baseline, n (%) | 61 (74.4) | 44 (54.3) | 0.09 (0.05 to 0.33); 0.0084 |
| Proportion of patients with JIA–ACR-50 improvement relative to baseline, n (%) | 60 (73.2) | 42 (51.9) | 0.20 (0.06 to 0.34); 0.0050 |
| Proportion of patients with JIA–ACR-70 improvement relative to baseline, n (%) | 53 (64.6) | 34 (42.0) | 0.22 (0.07 to 0.37); 0.0032 |
| Change from baseline in number of active joints, adjusted mean | −14.3 | −11.4 | –2.9 (–5.7 to –0.1); 0.0435 |
| Change from baseline in PGA (VAS), adjusted mean | −45.2 | −35.2 | –9.9 (–16.5 to –3.4); 0.0031 |
| Change from baseline in the pain (VAS), adjusted mean | −32.4 | −22.3 | –10.2 (–17.6 to –2.7); 0.0076 |
| Change from baseline in number of joints with LOM, adjusted mean | −9.5 | −7.7 | –1.8 (–4.1 to 0.5); 0.1229 |
| Change from baseline in ESR (mm/h), adjusted mean | −26.3 | −12.0 | –14.3 (–19.6 to –9.0)e |
| CHAQ-disability score | −0.8 | −0.6 | –0.2 (–0.4 to 0.0)e |
| Proportion with JIA-ACR90 improvement, n (%) | 37 (45.1) | 19 (23.5) | 0.21 (0.07 to 0.35)e |
| Proportion with inactive disease, n (%) | 30 (36.6) | 14 (17.3) | 0.18 (0.05 to 0.32)e |
| Corticosteroid reducing regimens | NR | ||
| Extra-articular manifestations (such as uveitis) | NR | ||
| Body weight and height | NR | ||
| Mortality | 0 | 0 | |
| Change from baseline in patient global assessment of well-being adjusted mean | −32.1 | −24.7 | –7.4 (–14.8 to 0.0)e |
| Proportion of patients in the ITT population with JIA–ACR-70 and JIA–ACR-90 response at week 40 by background MTX, glucocorticoid and previous biologic agent use at baselinef | |||||
|---|---|---|---|---|---|
| Concomitant therapies and previous exposure to biologic agent, n/N (% N) | Response level | TCZ (n = 82) | Placebo (n = 81) | ||
| Yes | No | Yes | No | ||
| Background MTX | JIA-ACR70 JIA-ACR90 |
45/67 (67.2) 32/67 (47.8) |
8/15 (53.3) 5/15 (33.3) | 30/64 (46.9) 18/64 (28.1) | 4/17 (23.5) 1/17 (5.9) |
| Background glucocorticoid | JIA-ACR70 JIA-ACR90 |
23/33 (69.7) 16/33 (48.5) |
30/49 (61.2) 21/49 (42.9) | 4/38 (36.8) 5/38 (13.2) | 20/43 (46.5) 14/43 (32.6) |
| Previous biologic agent | JIA-ACR70 JIA-ACR90 |
13/27 (48.1) 5/27 (18.5) |
40/55 (72.7) 32/55 (58.2) | 2/23 (8.7) 2/23 (8.7) | 32/58 (55.2) 17/58 (29.3) |
| AEs and SAEs | |||
|---|---|---|---|
| SAEs and AEs occurring ≥ 5% of patients, n (%) | TCZg (n = 82) | Placebog (n = 81) | p-value |
| Duration in study (years) | 32.33 | 27.41 | |
| Patients with ≥ 1 AE | 58 (70.7) | 60 (74.1) | |
| Total number of AEsh | 147 | 141 | |
| Rate of AEs per 100 patient-years | 454.7 | 514.4 | |
| Most frequent AEs | |||
| Nasopharyngitis | 14 (17.1) | 9 (11.1) | |
| Headache | 3 (3.7) | 0 | |
| Upper respiratory infection | 4 (4.9) | 2 (2.5) | |
| Cough | 2 (2.4) | 1 (1.2) | |
| Pharyngitis | 3 (3.7) | 3 (3.7) | |
| Nausea | 2 (2.4) | 2 (2.5) | |
| Diarrhoea | 2 (2.4) | 3 (3.7) | |
| Rhinitis | 2 (2.4) | 1 (1.2) | |
| Vomiting | 3 (3.7) | 1 (1.2) | |
| Abdominal pain | 2 (2.4) | 2 (2.5) | |
| Oropharyngeal pain | 1 (1.2) | 5 (6.2) | |
| Rash | 4 (4.9) | 1 (1.2) | |
| SAEs | |||
| Patients with ≥ 1 SAE | 3 (3.7) | 3 (3.7) | |
| Rate of SAEs per 100 patient-years | 9.3 | 10.9 | |
| Patients with ≥ 1 infectious SAE | 1 (1.2) | 0 | |
| Rates of infectious SAEs per 100 patient-years | 3.1 | 0 | |
| Pneumonia | 1 (1.2) | 0 | |
| Upper limb fracture | 1 (1.2) | 0 | |
| Uveitis | 0 | 1 (1.2) | |
| Psychosomatic disease | 1 (1.2) | 0 | |
| Enterocolitis | 0 | 1 (1.2) | |
| Complicated migraine | 0 | 1 (1.2) | |
| AEs leading to study drug discontinuation | |||
| Increased blood bilirubin leveli | 1 (1.2) | ||
| Gastroenteritis | 1 (1.2)j | ||
| Results for OLE, 104 weeks | ||||
|---|---|---|---|---|
| kEfficacy endpoints and percentage change from baseline in JIA ACR components (continuous TCZ, n = 82)69,71,74 | Baseline | Week 40 | Week 104 (n = 160) | Change from baseline to week 104, % |
| JIA ACR-70 responders, n (%)l | 65 (79.3) | 71 (86.6) | ||
| JIA ACR-90 responders, n (%)l | 41 (50.0) | 58 (70.7) | ||
| Active joints (0–71), mean (SD) | 19.7 (14.0) | 4.7 (9.1) | 3.3 (9.1) | –87.7 (27.1) |
| Joints with LOM (0–67), mean (SD) | 16.5 (13.8) | 5.6 (10.1) | 3.6 (7.3) | –81.3 (31.7) |
| Patient global (VAS 0–100 mm), mean (SD)m | 45.5 (23.1) | 12.2 (19.0) | 9.1 (18.4) | –75.4 (43.8) |
| PGA (VAS 0–100 mm), mean (SD) | 57.8 (20.3) | 8.8 (10.9) | 5.0 (10.5) | –89.7 (23.7) |
| CHAQ-DI (0–3), mean (SD) | 1.2 (0.7) | 0.4 (0.5) | 0.2 (0.4) | –76.7 (34.7) |
| ESR (mm/h), mean (SD) | 31.7 (22.9) | 5.4 (6.3) | 5.1 (5.6) | –76.2 (27.3) |
| Inactive disease, n (%)n | 33 (40.2) | 52 (63.4) | ||
| Remission, n (%)o | 5 (6.1) | 31 (37.8) | ||
| Minimal disease activity (JADAS-71 < 3.8), n (%) | 0 (0) | 49 (59.8) | 60 (73.2) | |
| Inactive disease (JADAS-71 < 1), n (%) | 0 (0) | 24 (29.3) | 48 (58.5) | |
| AEs and SAEs69–71,74 | Safety population = 188 with 307 patient-years |
|---|---|
| AEs, rates/100 patient-years | 406.5 |
| SAEs, rates/100 patient-years | 11.1 |
| Most common AE: infections | 151.4 |
| Infections: SAE | 5.2 |
| ALT elevations ≥ 3× upper limit of normal, % | 6.4 |
| AST elevations ≥ 3× upper limit of normal, % | 2.7 |
| Grade 3 lowest neutrophil count, % | 5.9 |
| Grade 2/3/4 thrombocytopenia, % | 1.6 |
| Low-density lipoprotein cholesterol ≥ 110 mg/dl, % | 16.2 |
| Methodological comments: | |
| Allocation to treatment groups: randomly assigned 1 : 1, stratified by background use of MTX and oral glucocorticoid use. OL (Part 1): patients weighing < 30 kg were randomly assigned 1 : 1 to receive intravenous TCZ 8 mg/kg or 10 mg/kg, whereas patients weighing ≥ 30 kg received 8 mg/kg. Double-blind RCT (Part 2): each of the three previous groups was randomised to receive either the existing dose of TCZ or placebo, equating to six groups in total. No further details about randomisation procedure were reported Blinding: double-blind, no further details of procedure reported. States that JIA ACR response rates and clinically inactive disease status were performed in real time by independent masked evaluators at the co-ordinating centres of the Pediatric Rheumatology International Trials Organisation and Pediatric Rheumatology Collaborative Study Group, according to validated criteria Comparability of treatment groups: no baseline characteristics for Part 2 (RCT) reported. States that disease characteristics at baseline for the OL (Part 1) were generally similar across the 3 groups with the exception of body weight based dosing regime, but no details are reported for the six groups in the RCT Part 2 Method of data analysis: ITT, however 3/166 patients from the placebo group were excluded as they discontinued without receiving the study drug therefore modified ITT. To control for the type-1 error rate, secondary endpoints were tested in a hierarchical fixed-sequence approach provided the primary endpoint was found to be statistically significant. The robustness of the results of the statistical procedure used for the primary endpoint analysis was assessed by logistic regression analysis of the proportion of patients with JIA–flare in the ITT during Part 2, showed a statistically significant treatment difference in favour of TCZ and was consistent with the primary analysis Primary endpoint analysis was conducted with the Cochran–Mantel–Haenszel test (also used for secondary endpoints), adjusted for stratification factors; patients who withdrew or for whom the endpoint could not be determined were considered to have experienced JIA flare. Patients who escaped or withdrew or for whom the end point could not be determined were considered non-responders. Continuous variables were evaluated using analysis of variance, adjusted for baseline differences between groups and stratification variables Ad hoc analysis was conducted in patients continuously treated with TCZ up to week 40, including those who escaped from blinded to OL TCZ, using an ITT approach Sample size/power calculation: sample size estimation was reported. States that recruitment was planned to ensure that a sufficient number of patients were available for randomisation in Part 2, needing 60 patients in each group to achieve 80% power to detect a significant difference in assumed JIA flare rates (35% TCZ, 65% placebo) between groups using a two-sided significance test with α = 0.05. For the results, the three TCZ groups were combined and so were the three placebo groups, giving each combined group sufficient power Attrition/drop-out: Part 1 lead in – states 10.6% discontinued (n = 20), but flow chart shows n = 22 (11.7%): lack of JIA ACR-30 response n = 15, withdrew consent, n = 3, AEs n = 3, failure to return n = 1 Part 2 RCT: TCZ 3/82 [3.7% (10 mg/kg < 30 kg body weight group: n = 1; 8 mg/kg ≥ 30 kg body weight: n = 2) – AEs n = 1, insufficient therapeutic response n = 1, withdrew consent n = 1. Placebo n = 3/84 (3.6%) – AEs n = 2, insufficient therapeutic response n = 1] OLE: 5/160 (3.1%) – reasons not reported69 |
|
| General comments: Generalisability: to patients aged 2 to 17 years, with diagnoses of RF+ve or RF–ve polyarticular-course JIA or EO JIA, with a minimum disease duration of at least 6 months and had inadequate responses to or were intolerant of MTX, and experienced at least one (JIA ACR-30) response to TCZ Outcome measures: appear to be appropriate Intercentre variability: not discussed Conflict of interests: various authors received funding/support from a variety of pharmaceutical companies |
|
Quality criteria (Cochrane Risk of Bias tool) randomised controlled trials171
| Criteria | Judgementa | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Unclear | Insufficient information |
| Allocation concealment (selection bias) | Unclear | Insufficient information |
| Blinding of participants and personnel (performance bias) | Yes | Double-blind |
| Blinding of outcome assessment (detection bias) | Yes | JIA-ACR response rates and clinically inactive disease status were performed in real time by independent masked evaluators at two co-ordinating centres according to validated criteria |
| Incomplete outcome data addressed (attrition bias) | Yes | Details reported and similar between groups |
| Selective reporting (reporting bias) | Yes | All outcomes reported on |
| Other sources of bias | Unclear | Intercentre variability not discussed |
Appendix 6 Table of excluded studies for systematic review of cost-effectiveness
| Excluded study | Primary reason for exclusion |
|---|---|
| Haapasaari JE, Kauppi M, Hakala MS, Kautiainen H. Economic evaluation of etanercept therapy in the treatment of re-fractory JIA. Arthritis Rheum 2002;46:S480 | Abstract |
| Brodszky V, Pentek M, Majer I, Karpati K, Gulacsi L. [Etanercept in patients with juvenile idiopathic arthritis: systematic review and economic evaluation.] Budapest: Unit of Health Economics and Technology Assessment in Health Care; 2006 | Abstract |
| Prince FHM, de Bekker-Grob EW, Twilt M, Van Rossum MAJ, Hoppenreijs EPAH, ten Cate R, et al. An analysis of the costs and treatment success of etanercept in juvenile idiopathic arthritis. Clin Exp Rheumatol 2011;29:443 | Abstract |
| Simpson K, Hubert MM, On PV, Cifaldi M, Shaw J. Long-term cost-effectiveness of adalimumab therapy in juvenile idiopathic arthritis: from a Canadian perspective. J Rheumatol 2012;39:1712 | Abstract |
| Luca N, Burnett H, Ungar W, Beukelman T, Feldman BM, Schwartz G, et al. Cost-effectiveness analysis of early biologic treatment in polyarticular juvenile idiopathic arthritis. Arthritis Rheum 2012;64:S501 | Abstract |
| Luca N, Burnett H, Ungar W, Beukelman T, Feldman B, Schwartz G, et al. Cost-effectiveness analysis of early biologic treatment in polyarticular juvenile idiopathic arthritis. J Rheumatol 2013;40:988 | Abstract |
| Chang S, Sawyer L, Dejonckheere F, van Suijlekom-Smit LW, Anink J, Diamantopoulos A. Tocilizumab in polyarticular juvenile idiopathic arthritis – a cost–utility model for the United Kingdom. Value Health 2013;16:A564 | Abstract |
| All Wales Medicines Strategy Group. Adalimumab (Humira®). 2013. URL: www.awmsg.org/ | Not economic evaluation |
| All Wales Medicines Strategy Group. Etanercept (Enbrel®). 2013. URL: www.awmsg.org/ | Not economic evaluation |
| All Wales Medicines Strategy Group. Abatacept (Orencia®). 2014. URL: www.awmsg.org/ | Not economic evaluation |
| All Wales Medicines Strategy Group. Tocilizumab (RoActemra®). 2014. URL: www.awmsg.org/ | Not economic evaluation |
| Canadian Agency for Drugs and Technologies in Health. Tocilizumab (Actemra – Hoffmann-La Roche Limited) New Indication: Polyarticular Juvenile Idiopathic Arthritis. 2014 | Not economic evaluation |
Appendix 7 Cost-effectiveness studies: data extraction forms
| 1 | Study | Cummins et al. (2002)123 | ||||||||||||||||
| 2 | Research question | To provide background info and systematic review of JIA, including economic evidence of etanercept compared with other treatment options | ||||||||||||||||
| 3 | Country/setting | Not stated | ||||||||||||||||
| 4 | Funding source | Not stated | ||||||||||||||||
| 5 | Analysis type | Cost–utility analysis | ||||||||||||||||
| 6 | Study type | Industry submission cost–utility model using results from one JIA trial (Lovell et al.42). The model assumes response related to health assessment (HAQ) and mortality | ||||||||||||||||
| 7 | Perspective | Health-care system | ||||||||||||||||
| 8 | Time horizon | Model cycle length: 3 months, 6 months and 1 year, then yearly intervals over the life-course | ||||||||||||||||
| 9 | Model assumptions | It is an adaptation of a rheumatoid arthritis model for adults using strong and questionable assumptions, related to health assessment, utility, mortality and costs | ||||||||||||||||
| 10 | Discounting (rate) | Yes, costs 6% per annum and benefits 1% | ||||||||||||||||
| 11 | Costing year, currency | 2001, £ | ||||||||||||||||
| 12 | Population | Etanercept in children with polyarticular juvenile rheumatoid arthritis Definition of condition: JIA, heterogeneous group of painful conditions involving persistent swelling of the joints with variable presentation and course |
||||||||||||||||
| 13 | Intervention(s), comparator(s) | Etanercept vs. placebo (placebo effect assumed to last 3 months) | ||||||||||||||||
| 14 | Intervention effect | Effect size measured in terms of CHAQ and mortality Cost offset per HAQ point £860 38% increase in mortality per point change in HAQ Relative risk of mortality in JIA was 2.98 Placebo and etanercept HAQ progression: responders 0–4 years 0, responders > 4 years 0.034, non-responders 0.0669 Annual withdrawal from responders to non-responders: placebo 50%, etanercept 13% |
||||||||||||||||
| 15 | Health-state utilities | EQ-5D adults | ||||||||||||||||
| 16 | Intervention cost | Adult cost | ||||||||||||||||
| 17 | Indirect costs | N/A | ||||||||||||||||
| 18 | Results Discounted/undiscountedInterventionComparatorIncrementalICER, £Costs, £40,62412,60228,022QALY15.013.31.716,082 |
Discounted/undiscounted | Intervention | Comparator | Incremental | ICER, £ | Costs, £ | 40,624 | 12,602 | 28,022 | QALY | 15.0 | 13.3 | 1.7 | 16,082 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discounted/undiscounted | Intervention | Comparator | Incremental | ICER, £ | ||||||||||||||
| Costs, £ | 40,624 | 12,602 | 28,022 | |||||||||||||||
| QALY | 15.0 | 13.3 | 1.7 | 16,082 | ||||||||||||||
| 19 | Sensitivity analysis Sensitivity analysis varied from £3900 to £34,000 (SF-36 regression used) |
|||||||||||||||||
| 20 | Author’s conclusions | Insufficient data to construct a model for JIA, and little is known about HRQoL in JIA. The ICER should be viewed with caution | ||||||||||||||||
| 21 | Reviewer’s comments | Limited relevance in cost/utilities; adult rheumatoid arthritis cannot be assumed to be the same for JIA in children | ||||||||||||||||
Critical appraisal checklist for economic evaluations (based on Drummond et al.)129
| Item | Y/N? |
|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Y |
| 2. Is the setting comparable to the UK? | Y |
| 3. Is the analytical and modelling methodology appropriate? | N |
| 4. Are all the relevant costs and consequences for each alternative identified? | N |
| 5. Are the data inputs for the model described and justified? | Y |
| 6. Are health outcomes measured in QALYs? | Y |
| 7. Is the time horizon considered appropriate? | Y |
| 8. Are costs and outcomes discounted? | Y |
| 9. Is an incremental analysis performed? | Y |
| 10. Is uncertainty assessed? | Y |
| Comments Out of date, not children-specific, relying on very strong assumptions due to lack of evidence. Informative in general terms but not relevant |
| 1 | Study | Prince et al. (2011)124 | ||||||||||||
| 2 | Research question | To analyse and report the costs and effects of etanercept therapy in patients with JIA | ||||||||||||
| 3 | Country/setting | Netherlands/national ABC register | ||||||||||||
| 4 | Funding source | The Dutch Board of Health Insurances and Wyeth International | ||||||||||||
| 5 | Analysis type | Cost–consequence analysis | ||||||||||||
| 6 | Study type | Trial-based: prospective etanercept effectiveness and safety add-on study with JIA patients in 7 of 9 Dutch paediatric rheumatology centres | ||||||||||||
| 7 | Perspective | Health-care system | ||||||||||||
| 8 | Time horizon | 27 months | ||||||||||||
| 9 | Model assumptions | N/A | ||||||||||||
| 10 | Discounting (rate) | No | ||||||||||||
| 11 | Costing year, currency | 2008, € | ||||||||||||
| 12 | Population | Dutch JIA patients younger than 18 years of age are eligible for treatment with etanercept if the disease has a polyarticular course and the response to the maximum (tolerated) dose of MTX) is not sufficient Onset subtype JIA (n = 49):
|
||||||||||||
| 13 | Intervention(s), comparator(s) | Intervention: etanercept (add-on to conventional treatment) Comparator: conventional treatment with synthetic DMARDs, mostly MTX, if required accompanied by anti-inflammatories or systemic glucocorticoids |
||||||||||||
| 14 | Intervention effect | Effect size measured in the study in terms of change in disease activity response variables of the JIA core set and HUI3 | ||||||||||||
| 15 | Health-state utilities | HUI3: preference-based HRQoL measure completed by the parents of study participants (eight domains in 15-item parent questionnaire) Valuation using value scores obtained by Feeny et al.172 from the Canadian general population |
||||||||||||
| 16 | Intervention cost | Unit costs for medication were retrieved from the Pharmacotherapeutic Compass provided by the Dutch Board of Health Insurances, and treatment costs were calculated with the exact dose of medication and administration period as reported in the patients’ files Etanercept unit cost ≈€10,478/year |
||||||||||||
| 17 | Indirect costs | N/A | ||||||||||||
| 18 | Results: UndiscountedInterventionComparatorIncrementalCosts (€) (27 months)28,075837019,705Utility0.78 (27 months)0.53 (0 months)0.25 |
Undiscounted | Intervention | Comparator | Incremental | Costs (€) (27 months) | 28,075 | 8370 | 19,705 | Utility | 0.78 (27 months) | 0.53 (0 months) | 0.25 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Undiscounted | Intervention | Comparator | Incremental | |||||||||||
| Costs (€) (27 months) | 28,075 | 8370 | 19,705 | |||||||||||
| Utility | 0.78 (27 months) | 0.53 (0 months) | 0.25 | |||||||||||
| 19 | Sensitivity analysis: N/A | |||||||||||||
| 20 | Author’s conclusions | ‘Although etanercept is expensive, the major utility gain justifies the costs’ | ||||||||||||
| 21 | Reviewer’s comments | Sound trial-based evaluation of costs and consequences (including disease activity improvement and utility) associated to adding etanercept to conventional care Full incremental cost-effectiveness/utility analysis not performed and there is no indication of the variation from the mean estimates reported nor assessment of uncertainty |
||||||||||||
Critical appraisal checklist for economic evaluations (based on Drummond et al.)129
| Item | Y/N? |
|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Y |
| 2. Is the setting comparable to the UK? | ? |
| 3. Is the analytical and modelling methodology appropriate? | Ya |
| 4. Are all the relevant costs and consequences for each alternative identified? | Y |
| 5. Are the data inputs for the model described and justified? | N/A |
| 6. Are health outcomes measured in QALYs? | N |
| 7. Is the time horizon considered appropriate? | N |
| 8. Are costs and outcomes discounted? | N |
| 9. Is an incremental analysis performed? | ?a |
| 10. Is uncertainty assessed? | N |
| 1 | Study | Simpson et al. (2012)125 | ||||||||||||||||
| 2 | Research question | To evaluate the cost-effectiveness of adalimumab vs. non-biologic therapy for the treatment of JIA in Russian children and adolescents | ||||||||||||||||
| 3 | Country/setting | Russia/health-care system | ||||||||||||||||
| 4 | Funding source | Not stated | ||||||||||||||||
| 5 | Analysis type | Cost–utility analysis | ||||||||||||||||
| 6 | Study type | Markov model; mutually exclusive health states:
|
||||||||||||||||
| 7 | Perspective | Health-care system and society | ||||||||||||||||
| 8 | Time horizon | Lifetime. Model cycle length: 4 months | ||||||||||||||||
| 9 | Model assumptions |
|
||||||||||||||||
| 10 | Discounting (rate) | Yes (3% per annum, costs and outcomes) | ||||||||||||||||
| 11 | Costing year, currency | 2011, Russian roubles | ||||||||||||||||
| 12 | Population | Trial name: randomised double-blinded placebo-controlled trial DE038 (adalimumab + methotrexate vs. placebo + methotrexate) Children aged 4–17 years with JIA |
||||||||||||||||
| 13 | Intervention(s), comparator(s) | Intervention: adalimumab + methotrexate Comparator: placebo + methotrexate |
||||||||||||||||
| 14 | Intervention effect | Intervention effect was incorporated in terms of HRQoL and the estimated number of person-years spent in each health state. The HRQoL associated to each health state was obtained from the CHAQ responses in trial DE038 | ||||||||||||||||
| 15 | Health-state utilities | For the base model, HUI2 utilities for each health state were mapped from CHAQ responses from parents of children participating in DE038. HUI2 valuation was derived from survey to the UK general population For the second part of the model, utilities were derived from the literature on adult patients with rheumatoid arthritis174 |
||||||||||||||||
| 16 | Intervention cost | Cost of treatment with adalimumab was derived from its expected cost in the List of Vital and Essential Medicinal Products (58,100 roubles for two 40-mg syringes after adjustment for VAT and 10% trade mark-up) | ||||||||||||||||
| 17 | Indirect costs | A secondary analysis included value of time lost from work to provide care for a sick child. Value and source not reported | ||||||||||||||||
| 18 | Results for base-case lifetime time horizon NHS perspective DiscountedInterventionComparatorIncrementalICERCosts (roubles)4,116,2312,753,9541,362,277–QALY24.8020.044.76286,267 |
Discounted | Intervention | Comparator | Incremental | ICER | Costs (roubles) | 4,116,231 | 2,753,954 | 1,362,277 | – | QALY | 24.80 | 20.04 | 4.76 | 286,267 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discounted | Intervention | Comparator | Incremental | ICER | ||||||||||||||
| Costs (roubles) | 4,116,231 | 2,753,954 | 1,362,277 | – | ||||||||||||||
| QALY | 24.80 | 20.04 | 4.76 | 286,267 | ||||||||||||||
| 19 | Sensitivity analysis: Deterministic univariate lifetime time horizon Parameter/scenarioValueICER, £Age of treatment initiation7 years of age229,744Discounting rate0%119,4965%428,236Note: results for 11-year time horizon and societal perspective also reported |
Parameter/scenario | Value | ICER, £ | Age of treatment initiation | 7 years of age | 229,744 | Discounting rate | 0% | 119,496 | 5% | 428,236 | ||||||
| Parameter/scenario | Value | ICER, £ | ||||||||||||||||
| Age of treatment initiation | 7 years of age | 229,744 | ||||||||||||||||
| Discounting rate | 0% | 119,496 | ||||||||||||||||
| 5% | 428,236 | |||||||||||||||||
| 20 | Author’s conclusions | Adalimumab seems to be cost-effective relative to conventional non-biologic therapy. ICERs estimated in the base-case lifetime analyses did not exceed the per-capita gross domestic product for Russia (≈380,000 roubles) | ||||||||||||||||
| 21 | Reviewer’s comments | Transition probabilities not reported; poor reporting of sources for the estimates used and their derivation; limited sensitivity analysis without indication of most influential parameters | ||||||||||||||||
Critical appraisal checklist for economic evaluations (based on Drummond et al.)129
| Item | Y/N? |
|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Y |
| 2. Is the setting comparable to the UK? | ? |
| 3. Is the analytical and modelling methodology appropriate? | Y |
| 4. Are all the relevant costs and consequences for each alternative identified? | Y |
| 5. Are the data inputs for the model described and justified? | N |
| 6. Are health outcomes measured in QALYs? | Y |
| 7. Is the time horizon considered appropriate? | Y |
| 8. Are costs and outcomes discounted? | Y |
| 9. Is an incremental analysis performed? | Y |
| 10. Is uncertainty assessed? | Y |
| Comments: Limited assessment of uncertainty |
| 1 | Study | Ungar et al. (2011)127 | |||||||||||||||||||||
| 2 | Research question | To determine the incremental costs of biologics per additional responder compared with conventional treatment (methotrexate) | |||||||||||||||||||||
| 3 | Country/setting | Canada, secondary care | |||||||||||||||||||||
| 4 | Funding source | Ontario Ministry of Health and Long-term Care Drug Innovation Fund | |||||||||||||||||||||
| 5 | Analysis type | CEA | |||||||||||||||||||||
| 6 | Study type | Decision-analysis model | |||||||||||||||||||||
| 7 | Perspective | Societal | |||||||||||||||||||||
| 8 | Time horizon | 1-year time horizon with two consecutive 6-month cycles | |||||||||||||||||||||
| 9 | Model assumptions | Model incorporated probabilities that patients would, based on their response at 6 months, either continue with the same treatment or switch | |||||||||||||||||||||
| 10 | Discounting (rate) | Not included | |||||||||||||||||||||
| 11 | Costing year, currency | 2008 Canadian dollars | |||||||||||||||||||||
| 12 | Population | In the base case, a 40-kg patient was assumed, similar to the mean weight in two paediatric RCTs. Patients had JIA with a prior inadequate response or intolerance to DMARDs | |||||||||||||||||||||
| 13 | Intervention(s), comparator(s) | Etanercept, adalimumab, abatacept and infliximab vs. MTX | |||||||||||||||||||||
| 14 | Intervention effect | The effectiveness measure was the proportion of patients who had a reduction in symptoms at 1 year according to the ACR Pedi-30 criteria Effect size were taken from RCTs: etanercept (Lovell et al., 200042), adalimumab (Lovell et al., 200861), infliximab (Ruperto et al., 2007135) and abatacept (Ruperto et al., 200857) For the base case, patients achieving ACR Pedi-30, % TimeEtanerceptAdalimumabAbataceptInfliximabDTX6 months798082803012 months7963827930 |
Time | Etanercept | Adalimumab | Abatacept | Infliximab | DTX | 6 months | 79 | 80 | 82 | 80 | 30 | 12 months | 79 | 63 | 82 | 79 | 30 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Etanercept | Adalimumab | Abatacept | Infliximab | DTX | ||||||||||||||||||
| 6 months | 79 | 80 | 82 | 80 | 30 | ||||||||||||||||||
| 12 months | 79 | 63 | 82 | 79 | 30 | ||||||||||||||||||
| 15 | Health-state utilities | No utility values included | |||||||||||||||||||||
| 16 | Intervention cost | Total annual costs for treatment were: abatacept ($14,733), infliximab ($17,259), etanercept ($18,966), adalimumab ($18,654), methotrexate ($952). Treatment costs included medication costs, preparation and administration costs and concomitant medications | |||||||||||||||||||||
| 17 | Indirect costs | The costs for abatacept and infliximab included parental time losses of $1875 and $1071. There were no indirect costs for the other treatments | |||||||||||||||||||||
| 18 | Results Intervention vs. MTX UndiscountedEtanerceptAdalimumabAbataceptInfliximabIncremental costs, $11,09013,1077,87312,167Incremental effectiveness, %47.629.449.443.2ICER, £26,06146,71116,20431,209 |
Undiscounted | Etanercept | Adalimumab | Abatacept | Infliximab | Incremental costs, $ | 11,090 | 13,107 | 7,873 | 12,167 | Incremental effectiveness, % | 47.6 | 29.4 | 49.4 | 43.2 | ICER, £ | 26,061 | 46,711 | 16,204 | 31,209 | ||
| Undiscounted | Etanercept | Adalimumab | Abatacept | Infliximab | |||||||||||||||||||
| Incremental costs, $ | 11,090 | 13,107 | 7,873 | 12,167 | |||||||||||||||||||
| Incremental effectiveness, % | 47.6 | 29.4 | 49.4 | 43.2 | |||||||||||||||||||
| ICER, £ | 26,061 | 46,711 | 16,204 | 31,209 | |||||||||||||||||||
| 19 | Sensitivity analysis Deterministic analysis was performed for extreme efficacy with biologic high efficacy and MTX low efficacy and vice versa Probabilistic sensitivity analyses were conducted for each treatment vs. MTX and cost-effectiveness acceptability curves were calculated. If a decision-maker was willing to pay no more than $30,000 to gain a responder, then the probability that etanercept would demonstrate a net economic benefit would be 95%. The willingness to pay points at which the biologic had a 50% probability of cost-effectiveness were $45,000, $17,000 and $27,500 for adalimumab, abatacept and infliximab respectively |
||||||||||||||||||||||
| 20 | Author’s conclusions | JIA patients with a prior suboptimal response or intolerance to MTX may benefit from treatment with biologic for at least 1 year | |||||||||||||||||||||
| 21 | Reviewer’s comments | Results not present in QALYs, which makes results difficult to interpret. Short time horizon used (1 year). Unclear how ICERs are calculated | |||||||||||||||||||||
Critical appraisal checklist for economic evaluations (based on Drummond et al.129)
| Item | Y/N? |
|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Y |
| 2. Is the setting comparable to the UK? | N |
| 3. Is the analytical and modelling methodology appropriate? | Y |
| 4. Are all the relevant costs and consequences for each alternative identified? | Y |
| 5. Are the data inputs for the model described and justified? | Y |
| 6. Are health outcomes measured in QALYs? | N |
| 7. Is the time horizon considered appropriate? | N |
| 8. Are costs and outcomes discounted? | N |
| 9. Is an incremental analysis performed? | Y |
| 10. Is uncertainty assessed? | Y |
Appendix 8 Table of excluded studies for systematic review of health-related quality of life
| Identified studies from titles/abstracts and full papers | Reason for exclusion |
|---|---|
| Anink J, Prince FHM, Dijkstra M, Otten H, Twilt M, Ten CR, et al. Long term functional outcome and quality of life of patients with refractory juvenile idiopathic arthritis treated with etanercept: results of the Dutch arthritis and biologicals in children register. Pediatr Rheumatol 2014;12(Suppl. 1):P28 | Abstract |
| Duarte-Salazar C, Guzman-Vazquez S, Soto-Molina H, Chaidez-Rosales P, Ilizaliturri-Sanchez V, Nieves-Silva J, et al. Disability impact on quality of life in Mexican adults with juvenile idiopathic arthritis and juvenile ankylosing spondylitis. Clin Exp Rheumatol 2007;25:922–7 | No utilities reported |
| Hendry GJ, Gardner-Medwin J, Turner DE, Woodburn J, Lorgelly PK. Self-vs Proxy-Reported Health-Related Quality of Life of Patients with Juvenile Idiopathic Arthritis: Implications for a Cost-Utility Analysis of Multidisciplinary Foot Care. 2011. URL: http://rheumatology.oxfordjournals.org/content/50/suppl_1/i2.full.pdf+html (accessed March 2015) | Abstract |
| Hendry GJ, Gardner-Medwin J, Steultjens MPM, Woodburn J, Sturrock RD, Turner DE. Frequent discordance between clinical and musculoskeletal ultrasound examinations of foot disease in juvenile idiopathic arthritis. Arthritis Care Res 2012;64:441–7 | No utilities reported |
| Janse AJ, Uiterwaal CS, Gemke RJ, Kimpen JL, Sinnema G. A difference in perception of quality of life in chronically ill children was found between parents and pediatricians. J Clin Epidemiol 2005;58:495–502 | Irrelevant population |
| Janse AJ, Sinnema G, Uiterwaal CS, Kimpen JL, Gemke RJ. Quality of life in chronic illness: perceptions of parents and paediatricians. Arch Dis Child 2005;90:486–91 | Irrelevant population |
| Janse A, Sinnema G, Uiterwaal C, Kimpen J, Gemke R. Quality of life in chronic illness: children, parents and paediatricians have different, but stable perceptions. Acta Paediatr 2008;97:1118–24 | Irrelevant population |
| Angeles-Han ST, Griffin KW, Lehman TJA, Rutledge JR, Lyman S, Nguyen JT, et al. The importance of visual function in the quality of life of children with uveitis. J Am Assoc Pediatr Opthalmol Strabismus; 2010;14:163–8 | Irrelevant population |
| Cespedes-Cruz A, Gutierrez-Suarez R, Pistorio A, Ravelli A, Loy A, Murray KJ, et al. Methotrexate improves the health-related quality of life of children with juvenile idiopathic arthritis. Ann Rheum Dis 2008;67:309–14 | No utilities reported |
| Feinstein AB, Forman EM, Masuda A, Cohen LL, Herbert JD, Moorthy LN, et al. Pain intensity, psychological inflexibility, and acceptance of pain as predictors of functioning in adolescents with juvenile idiopathic arthritis: a preliminary investigation. J Clin Psychol Med Settings 2011;18:291–8 | No utilities reported |
| Maetzel A, Strand V, Tugwell P, Wells G, Bombardier C. Economic comparison of leflunomide and methotrexate in patients with rheumatoid arthritis: an evaluation based on a 1-year randomised controlled trial. Pharmacoeconomics 2002;20:61–70 | Irrelevant population |
| Matza LS, Boye KS, Feeny DH, Johnston JA, Bowman L, Jordan JB. Impact of caregiver and parenting status on time trade-off and standard gamble utility scores for health state descriptions. Health Qual Life Outcomes 2014;12:48 | Irrelevant population |
| McTaggart-Cowan HM, Brazier JE, Tsuchiya A. Clustering Rasch results: a novel method for developing rheumatoid arthritis states for use in valuation studies. Value Health 2010;13:787–95 | Irrelevant population |
| Medrare L, Ngeuleu A, Rkain M, Bouaddi I, Znat F, El KS, et al. Is there any relationship between the children health assessment questionnaire (CHAQ) and the european quality of life (EUROQOL) in children suffering from chronic haemophilic arthropathy? Ann Rheum Dis 2014;73:1099 | Irrelevant population |
| Mo F, Choi BC, Li FC, Merrick J. Using Health Utility Index (HUI) for measuring the impact on health-related quality of life (HRQL) among individuals with chronic diseases. Scientific World J 2004;4:746–57 | Irrelevant population |
| Nordvag B-Y, Bernklev T, Slevolden E, Myhr K-M, Stensland E. Norwegian quality registry for biological drugs: The NOKBIL project. Scand J Rheumatol 2012;41(Suppl. 126):50 | Abstract |
| Osnes-Ringen H, Kvien TK, Henriksen JE, Mowinckel P, Dagfinrud H. Orthopaedic surgery in 255 patients with inflammatory arthropathies: longitudinal effects on pain, physical function and health-related quality of life. Ann Rheum Dis 2009;68:1596–601 | Irrelevant population |
| Osnes-Ringen H, Kvien TK, Henriksen JE, Dagfinrud H. Patients with inflammatory arthropathies undergo feet surgery later in the disease course than hand surgery. Clin Exp Rheumatol 2010;28:702–7 | Irrelevant population |
| Osnes-Ringen H, Kvamme MK, Kristiansen IS, Thingstad M, Henriksen JE, Kvien TK, et al. Cost-effectiveness analyses of elective orthopaedic surgical procedures in patients with inflammatory arthropathies. Scand J Rheumatol 2011;40:108–15 | Irrelevant population |
| Shelepina TA, Stepanenko NY, Fedorov ES. Comparative characteristic of quality of life with patients suffering from juvenile idiopathic arthritis (JIA), attending school and taught at home. Pediatr Rheumatol 2011;9:106 | Abstract |
| Simpson K, Hubert MM, On PV, Cifaldi M, Shaw J. Long-term cost-effectiveness of adalimumab therapy in juvenile idiopathic arthritis: from a Canadian perspective. J Rheumatol 2012;39:1712 | Abstract |
| Solari N, Viola S, Pistorio A, Magni-Manzoni S, Vitale R, Ruperto N, et al. Assessing current outcomes of juvenile idiopathic arthritis: a cross-sectional study in a tertiary center sample. Arthritis Rheum 2008;59:1571–9 | No utilities reported |
| Sparsa L, Job DC, Quartier P, Kahan A, Wipff J. Quality of life of juvenile idiopathic arthritis cohort at adulthood in a transition program. Ann Rheum Dis 2013;71(Suppl. 3):432 | Abstract |
| Wade AG, Crawford GM, Pumford N, Koscielny V, Maycock S, McConnachie A. Baseline characteristics and patient reported outcome data of patients prescribed etanercept: web-based and telephone evaluation. BMC Med Res Methodol 2011;11:91 | No utilities reported |
| Wang H-M, Beyer M, Gensichen J, Gerlach FM. Health-related quality of life among general practice patients with differing chronic diseases in Germany: cross sectional survey. BMC Public Health 2008;8:246 | Irrelevant population |
Appendix 9 Health-related quality-of-life systematic review: data extraction forms
Reference
Hendry et al. (2013)136
Study characteristics
Research question
What are the stated objectives of the study?
To evaluate the effectiveness of multidisciplinary foot-care, and to evaluate the methodological considerations of a trial of multidisciplinary care in JIA.
Describe the type of study and study design.
Exploratory randomised controlled trial
Was the sample from (1) the general population; (2) patients with the disease of interest; (3) individuals with knowledge of the disease; (4) other?
Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that may be relevant (e.g. > 80 years)?
The sample was drawn from patients with the disease of interest (i.e. children and adolescents with a definitive diagnosis of JIA and inflammatory joint disease affecting the foot/ankle).
The inclusion/exclusion criteria were clearly stated; however, might exclude a proportion of individuals with the disease of interest but whose disease has not affected the foot/ankle.
Patients were included if they satisfied at least one of the following: (1) previously documented arthritis in the foot including small joints derived from medical case notes; (2) previously documented foot arthritis in one or more large joints derived from medical case notes; or (3) current widespread polyarthritis involving large and small foot joints derived from clinical examination by a consultant paediatric rheumatologist. Patients with an unconfirmed diagnosis of JIA, and/or only upper limb, jaw, or neck involvement were excluded.
What are the characteristics of the baseline cohort for the evaluation?
| Age, years, mean (SD) | |
| Intervention arm | 10.1(4.22) |
| Control arm | 10.0(3.39) |
| Male/female, n | |
| Intervention arm | 7/14 |
| Control arm | 6/17 |
| Race (if appropriate) | NR |
| Disease subtypes, n (%) | |
| Intervention arm | |
| Persistent oligoarthritis | 7 (33) |
| EO | 4 (19) |
| Polyarthritis RF–ve | 6 (29) |
| Polyarthritis RF+ve | 0 (0) |
| Psoriatic arthritis | 2 (10) |
| ERA | 2 (10) |
| Undifferentiated | 0 (0) |
| Control arm | |
| Persistent oligoarthritis | 4 (17) |
| EO | 5 (22) |
| Polyarthritis RF–ve | 10 (43) |
| Polyarthritis RF+ve | 2 (9) |
| Psoriatic arthritis | 1 (4) |
| ERA | 0 (0) |
| Undifferentiated | 1 (4) |
| Sample size, n | |
| Intervention arm | 21 |
| Control arm | 23 |
| Pharmacological management, n (%) | |
| Intervention arm | |
| Analgesics | 2 (9) |
| NSAIDs | 18 (86) |
| Methotrexate | 7 (33) |
| Etanercept | 1 (5) |
| Sulphasalazine | 0 (0) |
| Rituximab | 5 (24) |
| Control arm | |
| Analgesics | 3 (13) |
| NSAIDs | 16 (70) |
| Methotrexate | 5 (22) |
| Etanercept | 0 (0) |
| Sulphasalazine | 1 (4) |
| Rituximab | 5 (22) |
| Combination methotrexate and etanercept | – |
| QoL instrument | EQ-5D-Y (patients) and EQ-5D-3L (parents/guardians) questionnaires |
| Utility values (Y/N) | Y |
| Treatment effect, if reported | Both the treatment groups appeared to improve by one point on the JAFI impairment scale between baseline and 12 months follow up, however, the differences between groups for change scores did not reach statistical significance |
Country/setting
What is the country and setting for the evaluation?
Royal Hospital for Sick Children, Glasgow, UK.
Data sources
Effectiveness
Were the QoL data derived from: a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
This single exploratory RCT.
Results
Summarise the results.
There were no significant differences between treatment groups for secondary outcomes at final follow-up.
| Intervention arm | Control arm | |
|---|---|---|
| Baseline | ||
| Self EQ-5D utility index, mean (SD) | 0.57 (0.31) | 0.58 (0.35) |
| Self EQ-5D utility index, median (IQR) | 0.62 (0.52–0.76) | 0.66 (0.52–0.75) |
| Proxy EQ-5D utility index, mean (SD) | 0.69 (0.29) | 0.60 (0.33) |
| Proxy EQ-5D utility index, median (IQR) | 0.69 (0.58–1) | 0.62 (0.55–0.82) |
| Change at 12 months | ||
| Self EQ-5D utility index, median (IQR) | 0 (–0.1 to 0.01) | 0 (–0.04 to 0.04) |
| Proxy EQ-5D utility index, median (IQR) | 0 (0–0.11) | 0 (0–0.1) |
Were the methods for deriving these data adequately described (give sources if using data from other published studies)? (Was a valid preference based instrument used to describe health states, such as EQ-5D? Was the valuation of health states from the UK general population?)
A valid preference-based instrument was used: EQ-5D-Y and EQ-5D-3L.
Are the levels of missing data reported? How are they dealt with?
For missing data identified at the end of the study, a sensitivity analysis was performed in order to identify the most appropriate method to address this problem (LOCF, mean value imputation, maximum value imputation, minimum value imputation and random value imputation). LOCF was found to be the most conservative method while being less labour intensive; therefore, it was subsequently used to impute all missing data at final follow-up.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
Not applicable.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
Integrated multidisciplinary foot care did not result in a significant reduction in disease-related foot impairments and disability.
What are the implications of the study for the model?
In both arms, a proportion of participants received etanercept, so the utility values reported cannot be used in the model for baseline HRQoL with standard of care.
Reference
Prince et al. (2011),124 Prince et al. (2010)137
Study characteristics
Research question
What are the stated objectives of the study?
To evaluate changes in HRQoL in patients with refractory JIA who are being treated with etanercept.
Describe the type of study and study design.
Prospective study.
Was the sample from: (1) the general population; (2) patients with the disease of interest; (3) individuals with knowledge of the disease; (4) other?
Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that may be relevant (e.g. > 80 years)?
JIA patients younger than 18 years treated with etanercept.
What are the characteristics of the baseline cohort for the evaluation?
| Study | Prince et al. (2010)137 | Prince et al. (2011)124 |
|---|---|---|
| Age | 11.9 years (IQR 8.1–14.9) | 11.6 years (IQR 7.9–14.9) |
| Sex, n (%) | ||
| Male Female |
20 (38) 33 (62) |
20 (41) 33 (59) |
| Ethnicity (if appropriate) | ||
| Indication/disease, n (%) | Systemic 14 (26) Polyarticular RF+ve 5 (9) Polyarticular RF–ve 18 (34) EO 11 (21) ERA 2 (4) Juvenile PA 3 (6) |
Systemic 11 (22) Polyarticular RF+ve 4 (8) Polyarticular RF–ve 18 (37) EO 11 (22) ERA 2 (4) Juvenile PA 3 (6) |
| Other characteristics (sample size) | Sample size 53 Median disease duration JIA (years) at start of etanercept 3.0 |
Sample size 49 Median disease duration JIA (years) at start of etanercept 3.6 |
| Quality-of-life instrument | HUI3 | HUI3 |
| Utility values (Y/N) | Yes | Yes |
| Treatment effect, if reported | Significant improvements were shown after 3 months and these continued at least up to 27 months | Significant improvements were shown after 3 months and these continued at least up to 27 months |
Country/setting
What is the country and setting for the evaluation?
The Netherlands.
Data sources
Effectiveness
Were the QoL data derived from: a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
Single prospective study.
Results
Summarise the results.
| Prince et al. (2010)137 | Baseline | 3 months | 15 months | 27 months |
|---|---|---|---|---|
| HUI3 mean (SE) | 0.53 (0.04) | 0.69 (0.05) | 0.74 (0.06) | 0.78 (0.07) |
Were the methods for deriving these data adequately described (give sources if using data from other published studies)? (Was a valid preference based instrument used to describe health states, such as EQ-5D? Was the valuation of health states from the UK general population?)
Yes.
Are the levels of missing data reported? How are they dealt with?
Not reported.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
Mapping was not used.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
This study shows that the HRQoL of patients with refractory JIA can be substantially improved by the use of etanercept.
What are the implications of the study for the model?
This is a potential source of HRQoL for the SHTAC economic model.
Appendix 10 Cost-effectiveness data extraction forms for the company submissions
Company submission from Abbvie
Reference
AbbVie (2015)77
Health technology
Adalimumab
Interventions and comparators
What interventions/strategies were included?
No economic evaluation was conducted; however, reasons for not conducting an economic evaluation were discussed and included the following interventions: adalimumab, etanercept, abatacept, tocilizumab and methotrexate.
Was a no treatment/supportive care strategy included?
No.
Describe interventions/strategies
p. 13 CS: ‘The aim of drug therapy in JIA patients is to induce and maintain remission of symptoms, and thus allow a child to achieve normal growth, development, and allow full participation in school, career, sport and all other aspects of normal life. The initial aim is induction of complete disease remission using corticosteroids – either intravenously (IV) or intra-articular. Oral corticosteroids are avoided where possible to avoid side effects (can affect growth or increase risk of osteoporosis) but may be needed for short time periods.’
p. 14 EMA licence: ‘Adalimumab in combination with methotrexate is indicated for the treatment of active polyarticular juvenile idiopathic arthritis, in patients from the age of 2 years who have had an inadequate response to one or more disease-modifying anti-rheumatic drugs (DMARDs). Adalimumab can be given as monotherapy in case of intolerance to methotrexate or when continued treatment with methotrexate is inappropriate. Adalimumab has not been studied in patients aged less than 2 years.’
Adalimumab is delivered as 24 mg/m2 body surface area (with varying maximum doses dependent on weight and study protocol) subcutaneous injection with concomitant methotrexate for 24 weeks or more. Patients were allowed to take NSAIDs and prednisone or equivalents to prednisone.
Research question
What are the stated objectives of the evaluation?
No economic evaluation was conducted.
Study type: cost-effectiveness/cost–utility/cost–benefit analysis?
No economic evaluation was conducted.
Study population
What definition was used for [condition]? What are the characteristics of the baseline cohort for the evaluation?
From pp. 9–10 of the CS: ‘Juvenile Idiopathic Arthritis (JIA) is the most common rheumatic disease of childhood and describes a group of conditions that involve joint inflammation which lasts for more than 6 weeks in people under 16 years of age. . . . JIA is an “umbrella” term which covers a number of different subtypes listed below that were proposed by the International League of Associations for Rheumatology (ILAR) in 1995 for the classification of JIA:
-
Oligoarticular JIA – Oligoarthritis is the most common type of JIA, accounting for up to 50% of new diagnoses in Europe each year. It is diagnosed when four or fewer joints are affected in the first 6 months of disease.
-
Extended Oligoarticular JIA – If oligoarthritis progresses and affects more than four joints during the first 6 months, it is called extended oligoarthritis.
-
Poly-Articular JIA (RF –ve or RF +ve) – Polyarticular JIA is diagnosed when five or more joints are affected at presentation, and can be further divided into rheumatoid factor positive arthritis and rheumatoid factor negative disease.
-
Systemic-Onset JIA – Systemic JIA accounts for 5–10% of new diagnoses and is diagnosed when arthritis is part of a general illness involving features such as fever, lymphadenopathy, hepatosplenomegaly and serositis. This patient group was not included in the NICE Scope for this project.
-
Psoriatic JIA – Psoriatic arthritis accounts for 2–15% of new diagnoses and is diagnosed when there is joint swelling associated with psoriasis, or a family history of psoriasis.
-
Enthesitis-Related Arthritis (ERA) – ERA accounts for 2–10% of new diagnoses and is diagnosed in the presence of arthritis or inflammation of tendon attachments to the bones (entheses), in association with two or more other features of spondyloarthropathy.’
-
No economic evaluation was conducted, so there is no relevant baseline cohort.
Institutional setting: where is/are the intervention(s) being evaluated usually provided?
Paediatric secondary care.
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
No economic evaluation was conducted.
Funding source
AbbVie.
Analytical perspective
What is the perspective adopted for the evaluation (health service, health and personal social services, third party payer, societal (i.e. including costs borne by individuals and lost productivity)?
No economic evaluation was conducted.
Effectiveness
Were the effectiveness data derived from: a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation.
No economic evaluation was conducted.
Intervention costs
Were the cost data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)? List the direct intervention costs and other direct costs used in the evaluation: include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used.
No economic evaluation was conducted.
Indirect costs (costs attributable to lost productivity, unpaid inputs to patient care)
Were indirect costs included?
No economic evaluation was conducted.
Health-state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion. Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
No economic evaluation was conducted.
List the utility values used in the evaluation
No economic evaluation was conducted.
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported: list them if reported.
No modelling was undertaken.
Extract transition probabilities for (natural history/disease progression) model and show sources (or refer to table in text)
No modelling was undertaken.
What is the model time horizon?
No modelling was undertaken.
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
No modelling was undertaken.
If no economic evaluation was conducted, state the manufacturer’s reasons for this
No economic evaluation was conducted owing to heterogeneity in study methods and populations between the interventions that complicated indirect comparisons, a lack of appropriate utility data for HRQoL and lack of long-term data.
Results/analysis
What measure(s) of benefit were reported in the evaluation?
No economic evaluation was undertaken.
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation
No economic evaluation was undertaken.
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
No economic evaluation was undertaken.
Synthesis of costs and benefits: are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results
No economic evaluation was undertaken.
Give results of any statistical analysis of the results of the evaluation
No economic evaluation was undertaken.
Was any sensitivity analysis performed: if yes, what type(s) [i.e. deterministic (one-way, two-way, etc.) or probabilistic]?
No economic evaluation was undertaken.
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, quality of life or disease progression rates)?
No economic evaluation was undertaken.
Give a summary of the results of the sensitivity analysis: did they differ substantially from the base-case analysis. If so, what were the suggested causes?
No economic evaluation was undertaken.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
No economic evaluation was undertaken.
What are the implications of the evaluation for practice?
No economic evaluation was undertaken.
Southampton Health Technology Assessments Centre commentary
Selection of comparators
Although no economic evaluation was conducted, the comparators listed by the manufacturer were appropriate and in accordance with the NICE scope.
Validity of estimate of measure of benefit
No economic evaluation was undertaken.
Validity of estimate of costs
No economic evaluation was undertaken.
Company submission from BMS
Reference
BMS (2015)85
Health technology
Abatacept.
Interventions and comparators
What interventions/strategies were included?
Abatacept, adalimumab, etanercept and tocilizumab.
Was a no treatment/supportive care strategy included?
No.
Describe interventions/strategies
Abatacept is a biologic DMARD that prevents T-cell activation, thus down-regulating the immune response of inflammatory disease. (p. 9). Abatacept is administered intravenously.
p. 9 CS: ‘The recommended dose of abatacept for polyarticular JIA patients aged 6 to 17 years (who weigh less than 75 kg) is 10 mg/kg, calculated based on the patient’s body weight at each administration. Paediatric patients weighing 75 kg or more should follow the abatacept adult dosing regimen and should not exceed a maximum dose of 1,000 mg. Abatacept should be administered as a 30-minute intravenous infusion. Following the initial administration, abatacept should be given at 2 and 4 weeks after the first infusion, and every 4 weeks thereafter.’
Etanercept is a biologic DMARD that inhibits TNF activation. It is administered subcutaneously. For patients aged 2–18 400 µg/kg (maximum 25 mg twice a week) or 800 µg/kg (maximum 50 mg once a week) was administered. For patients over 18 years of age the dose was 50 mg.
Adalimumab is a biologic DMARD that inhibits TNF activation. It is administered subcutaneously. For patients aged 4–13 24 mg/m2 (maximum 40 mg) was administered EOW. For patients aged 13 and above the dose was 40 mg.
Tocilizumab is a biologic DMARD. It is a humanised monoclonal antibody that inhibits the cytokine IL-6. It is administered by intravenous infusion. Tocilizumab dose was based on weight. For patients weighing < 30 kg the dose was 10 mg/kg. For patients 30 kg and above the dose was 8 mg/kg (maximum 800 mg). Doses were administered every 4 weeks. All tocilizumab patients were older than 2 years of age.
All drugs were administered with subcutaneous methotrexate. BMS indicated that methotrexate was given every 4 weeks at a dosage of 13.5 mg/m2.
Research question
What are the stated objectives of the evaluation?
From CS model: ‘The model evaluates the cost-effectiveness of abatacept against other biological disease modifying anti rheumatic drugs (bDMARDs) in moderate-to-severe active polyarthritis in paediatric patients from the age of 6 years and who have shown insufficient response to other DMARDs, including at least one anti-TNF.’
BMS reports that this is not in perfect agreement with the NICE Scope, but is in accord with the drug licence. The NICE Scope is broader with no specifications for patient age, or insufficient response on other DMARDs (including failure of at least one TNF inhibitor). The NICE Scope also includes ERA.
Study type: cost-effectiveness/cost–utility/cost–benefit analysis?
BMS has conducted a cost-minimisation analysis with an assumption that there is no difference between the biologic DMARDs in effectiveness. BMS indicated that effectiveness evidence was not considered because it ‘would lead to uncertainty within the model’.
Study population
What definition was used for [condition]? What are the characteristics of the baseline cohort for the evaluation?
‘JIA encompasses all forms of arthritis of unknown aetiology that persist for at least 6 weeks and begin in patients younger than 16 years.1 JIA comprises several heterogeneous subtypes (oligoarthritis, polyarthritis, systemic, psoriatic, enthesitis-related and undifferentiated), all presenting with different clinical signs and symptoms. 1,3,14 Overall, JIA is characterised by persistent joint swelling, pain and limitation of movement and has an estimated incidence in the UK of 1 per 10,000 children and a prevalence in the order of 1 per 1,000 children. 2 Polyarticular JIA (classifiable as polyarthritis [rheumatoid factor-positive or -negative]) is characterised by arthritis affecting five or more joints during the first 6 months of the disease,1,3,14,15 and it affects 13%–37% of patients with JIA. 3
JIA causes functional impairment due to joint and back pain, heel pain, swelling of joints and morning stiffness, contractures, pain and anterior uveitis leading to blindness. 16 This leads to suboptimal health-related quality of life (HRQL) in patients and parents or carers alike. 17,18 Moreover, as JIA patients reach adulthood, they face possible continuing disease activity, medication-associated morbidity, life-long disability and the risk of emotional and social dysfunction. 16’
CS p. 8. Reference citations have been reproduced from the CS as supplied
The baseline cohort population was defined as 12-year old ‘moderate-to-severe active polyarticular JIA [patients] who have had an insufficient response to other DMARD, including at least one TNF inhibitor’ in the decision problem stated by BMS.
Institutional setting: where is/are the intervention(s) being evaluated usually provided?
The institutional setting appears to be paediatric secondary care, but this is not entirely clear. The delivery environment is not specifically referenced.
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
The country setting given is the UK. Costs are expressed in pounds sterling (£). Costs were derived from the MIMS database (accessed November 2014). The price year was not explicitly stated.
Funding source
BMS.
Analytical perspective
What is the perspective adopted for the evaluation [health service, health and personal social services, third party payer, societal (i.e. including costs borne by individuals and lost productivity)]?
The model reports that it adopts an NHS and PSS perspective; however, it appears that only drug and administration costs have been included in the model. The NHS and PSS perspective generally includes costs associated with the disease. This would routinely include hospitalisation costs, costs for physician visits and nurse time, as well as costs for managing AEs. This could also include reductions in costs attributable to temporary dose reductions and interruptions. The NHS and PSS perspectives presented by BMS are much more limited than those commonly presented in NHS economic evaluations.
Effectiveness
Were the effectiveness data derived from: a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation.
No effectiveness data were used.
Intervention costs
Were the cost data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)? List the direct intervention costs and other direct costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used.
Intervention costs were derived from MIMS. A PAS was incorporated for abatacept (CiC information has been removed). Sensitivity analyses were run for the price of tocilizumab using various percentage price discounts as a CiC PAS has been agreed for tocilizumab.
| Drug | Cost, £ | Dose | PAS discount | PAS cost |
|---|---|---|---|---|
| Abatacept | 302.40 | 250 mg | (CiC information has been removed) | (CiC information has been removed) |
| Etanercept | 35.75 | 10 mg | ||
| 89.38 | 25 mg | |||
| 178.75 | 50 mg | |||
| Adalimumab | 352.14 | 40 mg | ||
| Tocilizumab | 102.40 | 80 mg | ||
| 256.00 | 200 mg | |||
| 512.00 | 400 mg | |||
| Methotrexate | 14.85 | 7.5 mg | ||
| 15.29 | 10 mg | |||
| 16.50 | 12.5 mg | |||
| 16.57 | 15 mg | |||
| 17.50 | 17.5 mg | |||
| 17.84 | 20 mg |
| Administration method | Costs, £ |
|---|---|
| Infusion | 154.00 |
| Subcutaneous injection | 3.05 |
Indirect costs (costs attributable to lost productivity, unpaid inputs to patient care)
Were indirect costs included:
No.
Health-state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion. Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
No health valuations were undertaken.
List the utility values used in the evaluation
No health valuations were undertaken.
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported? List them if reported.
The model is essentially a one-state model where child height and weight change as they age which affects only the cost of drug doses. There are no health states.
Extract transition probabilities for (natural history/disease progression) model and show sources (or refer to table in text)
There was no natural history modelling.
What is the model time horizon?
The base-case model has a 6-year time-horizon. The time-horizon is user adjustable within the model by setting different exit ages.
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
A discount rate of 3.5% annually has been applied to costs, in accordance with the NICE Reference Case.
If no economic evaluation was conducted, state the manufacturer’s reasons for this
A cost-minimisation analysis was conducted.
Results/analysis
What measure(s) of benefit were reported in the evaluation?
No benefit measure was evaluated.
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation
No clinical outcomes nor benefit measures were evaluated.
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
Discounted results for the base case (table 13 in CS)
| Results for the base-case model (12 year olds, 6-year time horizon, from Excel model) | ||||
|---|---|---|---|---|
| Abatacept | Adalimumab | Etanercept | Tocilizumab | |
| Drug costs | (CiC information has been removed) | (CiC information has been removed) | (CiC information has been removed) | (CiC information has been removed) |
| Administration costs, £ | 11,797 | 871 | 871 | 11,646 |
| Total costs | (CiC information has been removed) | (CiC information has been removed) | (CiC information has been removed) | (CiC information has been removed) |
| Cost savings with abatacept | (CiC information has been removed) | (CiC information has been removed) | (CiC information has been removed) | |
Undiscounted results for the base case (table 12 in CS)
| Results for the base-case model (12 year olds, 6-year time horizon, from Excel model) | ||||
|---|---|---|---|---|
| Abatacept | Adalimumab | Etanercept | Tocilizumab | |
| Drug costs | (CiC information has been removed) | (CiC information has been removed) | (CiC information has been removed) | (CiC information has been removed) |
| Administration costs, £ | 13,040 | 964 | 964 | 12,889 |
| Total costs | (CiC information has been removed) | (CiC information has been removed) | (CiC information has been removed) | (CiC information has been removed) |
| Cost savings with abatacept | (CiC information has been removed) | (CiC information has been removed) | (CiC information has been removed) | |
Synthesis of costs and benefits: are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results
A cost minimisation analysis was undertaken, so there was no synthesis of costs and benefits.
Give results of any statistical analysis of the results of the evaluation
There were no statistical analyses of the results of the evaluation.
Was any sensitivity analysis performed – if yes, what type(s) [i.e. deterministic (one-way, two-way, etc.) or probabilistic]
A PSA was undertaken and scenario analyses were undertaken.
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, quality of life or disease progression rates)?
A sensitivity analysis was undertaken wherein the infusion costs for tocilizumab were increased owing to the longer infusion time. This evaluates parameter uncertainty.
The starting age of patients in the model was varied between 6 and 16 years. This related to the structural assumption of starting age. In the biologic DMARD trials, the mean age was 11 years at baseline, but the drug licences were for much younger ages.
The time horizon of the model was varied between 6 months and 20 years. Longer time horizons were meant to represent that one-third of children with JIA will have it continue into adulthood. It was unclear why shorter time horizons were tested.
PAS discount for tocilizumab. This represents parameter uncertainty in the cost of tocilizumab.
Exclude methotrexate from the etanercept arm. This scenario dabbles across all the types of uncertainty. NICE specifies that etanercept probably benefits from methotrexate being given concurrently, but the licence is not for etanercept plus methotrexate, so there is some uncertainty in the appropriateness of methodology recommended by NICE. Changing a comparator is a structural modification and requires new parameter estimates.
Give a summary of the results of the sensitivity analysis: did they differ substantially from the base-case analysis. If so, what were the suggested causes?
Adjusting the starting age of patients downwards favoured etanercept with a starting age of 10 or under resulting in etanercept being cost saving compared with abatacept. Applying a PAS discount of (CiC information has been removed) to tocilizumab makes the costs for the two drugs identical. Excluding methotrexate costs from the etanercept arm made etanercept cost-saving compared with abatacept. There were no suggested causes for any of the analyses, only a statement of the analysis results.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
In the base case, abatacept is the least costly bDMARD and has similar efficacy and safety to other bDMARDs. These results remain stable for a wide range of scenarios.
What are the implications of the evaluation for practice?
There are no implications, because the economic evaluation did not evaluate practice, it only evaluated drug pricing. The model assumes that the drugs will have identical discontinuation rates, AEs and onset of effectiveness and duration of effectiveness. These assumptions are unlikely to be true. Even in an analysis that assumes there is no difference in effectiveness, differences in how the drugs behave in practice should be reflected in the costs.
Southampton Health Technology Assessments Centre commentary
Selection of comparators:
The comparators were consistent with the NICE Scope.
Validity of estimate of measure of benefit:
The assumption of equivalent efficacy was of unclear validity. Although BMS provided justification for assuming equivalence, the nature of the data available may not justify this approach. The trials were small, but generally share many characteristics, and indirect comparisons were referenced by BMS and conducted by BMS. A full evaluation of AEs using data beyond the clinical trials was not undertaken, and no comparisons of discontinuation rates were undertaken. The data from the trials were of insufficient quantity to make equivalency assumptions on event rates over time.
Given that there is a large amount of uncertainty in the effectiveness data, it may have been more appropriate to conduct a full economic evaluation with that uncertainty incorporated.
Validity of estimate of costs:
The costs were derived from appropriate sources, but BMS has assumed that the drugs will have identical AE costs and discontinuation rates (and identical everything else), both of which would lead to costs that have not been captured here. The strong assumption that there are no differences in the behaviour of the drugs in spite of different licences and mechanisms of action lacks face validity.
Critical appraisal checklist of economic evaluation (questions in this checklist based on Philips et al. 128 and Drummond et al. 129)
| Item | MS 1 | |
|---|---|---|
| 1 | Is there a clear statement of the decision problem? | Yes |
| 2 | Is the comparator routinely used in UK NHS? | Yes |
| 3 | Is the patient group in the study similar to those of interest in UK NHS? | Yes |
| 4 | Is the health-care system comparable to UK? | Yes |
| 5 | Is the setting comparable to the UK? | Yes |
| 6 | Is the perspective of the model clearly stated? | Yes |
| 7 | Is the study type appropriate? | No |
| 8 | Is the modelling methodology appropriate? | No |
| 9 | Is the model structure described and does it reflect the disease process? | ?a |
| 10 | Are assumptions about model structure listed and justified? | Yes |
| 11 | Are the data inputs for the model described and justified? | Yes |
| 12 | Is the effectiveness of the intervention established based on a systematic review? | N/A |
| 13 | Are health benefits measured in QALYs? | N/A |
| 14 | Are health benefits measured using a standardised and validated generic instrument? | N/A |
| 15 | Are the resource costs described and justified? | Yes |
| 16 | Have the costs and outcomes been discounted? | Nob |
| 17 | Has uncertainty been assessed? | Yes |
| 18 | Has the model been validated? | Yes |
Company submission from Pfizer
Reference
Pfizer (2015)97
Health technology
Etanercept.
Interventions and comparators
What interventions/strategies were included?
No economic evaluations were conducted; however, a cost analysis comparing etanercept, adalimumab and tocilizumab was conducted.
Was a no treatment/supportive care strategy included?
No.
Describe interventions/strategies
No economic evaluation was conducted. The cost analysis included etanercept, adalimumab and tocilizumab. Etanercept is administered by subcutaneous injection at a recommended dose of 0.4 mg/kg (up to a maximum of 25 mg per dose), given twice weekly as a subcutaneous injection with an interval of 3–4 days between doses or 0.8 mg/kg (up to a maximum of 50 mg per dose) given once weekly.
Research question
What are the stated objectives of the evaluation?
No economic evaluation was conducted.
Study type: cost-effectiveness/cost–utility/cost–benefit analysis?
No economic evaluation was conducted, however a cost analysis was conducted.
Study population
What definition was used for (condition)? What are the characteristics of the baseline cohort for the evaluation?
The cost analysis was undertaken for a cohort with polyarticular JIA.
Institutional setting: where is/are the intervention(s) being evaluated usually provided?
NHS outpatient setting.
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
No economic evaluation. The cost analysis was conducted in pounds sterling (£) but does not state the price year.
Funding source
Pfizer.
Analytical perspective
What is the perspective adopted for the evaluation (health service, health and personal social services, third party payer, societal (i.e. including costs borne by individuals and lost productivity)?
No economic evaluation was conducted.
Effectiveness
Were the effectiveness data derived from: a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation.
No economic evaluation was conducted.
Intervention costs
Were the cost data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)? List the direct intervention costs and other direct costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used.
No economic evaluation was conducted. The cost analysis used drug costs and administration costs for first year of treatment for different patient ages and weights. Cost sources are not given.
Indirect costs (costs attributable to lost productivity, unpaid inputs to patient care)
Were indirect costs included:
No economic evaluation was conducted.
Health-state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion. Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
No economic evaluation was conducted.
List the utility values used in the evaluation
No economic evaluation was conducted.
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported – list them if reported.
No economic evaluation was conducted.
Extract transition probabilities for (natural history/disease progression) model and show sources (or refer to table in text)
No economic evaluation was conducted.
What is the model time horizon?
No economic evaluation was conducted.
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
No economic evaluation was conducted.
If no economic evaluation was conducted, state the manufacturer’s reasons for this
The CS notes the limitation raised in previous NICE submission TA35 and TA238. These relate to the limitations in the HRQoL data and the limited evidence on the long term outcomes and the effectiveness of the treatments. The company states that any cost-effectiveness evidence would be associated with considerable and unresolvable uncertainty and have therefore not submitted a cost-effectiveness model for this appraisal.
Results/analysis
What measure(s) of benefit were reported in the evaluation?
No economic evaluation was conducted.
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation
No economic evaluation was conducted.
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
No economic evaluation was conducted; the cost analysis shows the costs were similar between etanercept, adalimumab and tocilizumab.
| Etanercepta | Adalimumab | Tocilizumabb | |
|---|---|---|---|
| 2 years | £1,859.00 | £9,155.64 | £5,000.19 |
| 3 years | £3,718.00 | £9,155.64 | £5,000.19 |
| 4 years | £3,718.00 | £9,155.64 | £5,665.79 |
| 5 years | £3,718.00 | £9,155.64 | £5,665.79 |
| 6 years | £3,718.00 | £9,155.64 | £6,331.39 |
| 7 years | £3,718.00 | £9,155.64 | £6,331.39 |
| 8 years | £3,718.00 | £9,155.64 | £6,996.99 |
| 9 years | £4,647.76 | £9,155.64 | £6,996.99 |
| 10 years | £4,647.76 | £9,155.64 | £6,996.99 |
| 11 years | £9,295.00 | £9,155.64 | £6,996.99 |
| 12 years | £9,295.00 | £9,155.64 | £8,328.19 |
| 13 years | £9,295.00 | £9,155.64 | £8,993.79 |
| 14 years | £9,295.00 | £9,155.64 | £8,993.79 |
| 15 years | £9,295.00 | £9,155.64 | £8,993.79 |
| 16 years | £9,295.00 | £9,155.64 | £10,324.99 |
| 17 years | £9,295.00 | £9,155.64 | £10,324.99 |
To reflect clinical practice and avoidance of drug wastage, doses were rounded down to the nearest available combination of vial strengths to a maximum of 10% variation from estimated dose.
Synthesis of costs and benefits: are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results
No economic evaluation was conducted.
Give results of any statistical analysis of the results of the evaluation
No economic evaluation was conducted.
Was any sensitivity analysis performed: if yes, what type(s) (i.e. deterministic (one-way, two-way, etc.) or probabilistic)
No economic evaluation was conducted.
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, quality of life or disease progression rates)?
No economic evaluation was conducted.
Give a summary of the results of the sensitivity analysis: did they differ substantially from the base-case analysis. If so, what were the suggested causes?
No economic evaluation was conducted.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
No economic evaluation was conducted.
What are the implications of the evaluation for practice?
No economic evaluation was conducted.
Southampton Health Technology Assessments Centre commentary
Selection of comparators:
No economic evaluation was conducted, the cost analysis did not include abatacept.
Validity of estimate of measure of benefit:
No economic evaluation was conducted.
Validity of estimate of costs:
No economic evaluation was conducted. Costs used in the cost analysis appear reasonable.
Company submission from Roche
Reference
Roche (2015)78
Health technology
Tocilizumab.
Interventions and comparators
What interventions/strategies were included?
Tocilizumab vs. adalimumab.
Was a no treatment/supportive care strategy included?
No.
Describe interventions/strategies
Tocilizumab + MTX vs. adalimumab + MTX.
Tocilizumab only vs. adalimumab only.
Research question
What are the stated objectives of the evaluation?
To demonstrate the cost-effectiveness of tocilizumab when used in patients with pJIA who had an inadequate response to DMARDs.
Study type: cost-effectiveness/cost–utility/cost–benefit analysis?
Cost utility.
Study population
What definition was used for (condition)? What are the characteristics of the baseline cohort for the evaluation?
Patients entering the model have active JIA and have previously experienced an inadequate response to, or were intolerant of methotrexate. The modelled population is in line with the CHERISH trial population.
Institutional setting: where is/are the intervention(s) being evaluated usually provided?
NHS outpatient care.
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
UK. Costs have been taken from sources from year 2011–15 with some costs taken from the Netherlands. Costs have not been inflated to a common base year.
Funding source
Roche.
Analytical perspective
What is the perspective adopted for the evaluation (health service, health and personal social services, third party payer, societal (i.e. including costs borne by individuals and lost productivity)?
UK NHS and PSS.
Effectiveness
Were the effectiveness data derived from: a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation.
The company completed a systematic review of biologics in the treatment of JIA. The effectiveness data were derived from a WinBUGS indirect comparison with an order probit model. The results were in terms of level of ACR response.
| JIA ACR-30 | JIA ACR-50 | JIA ACR-70 | JIA ACR-90 | ||
|---|---|---|---|---|---|
| Without MTX | Placebo | 31% | 28% | 25% | 12% |
| Tocilizumab | 62% | 59% | 54% | 35% | |
| Adalimumab | 52% | 49% | 44% | 26% | |
| With MTX | MTX | 52% | 51% | 41% | 25% |
| Tocilizumab | 72% | 70% | 61% | 44% | |
| Adalimumab | 76% | 75% | 66% | 49% |
Intervention costs
Were the cost data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)? List the direct intervention costs and other direct costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used.
The costs associated with each health state was obtained from Prince et al. ,124 who report costs data from the Dutch ABC register for the year before and after starting etanercept. The total 6-month health-state cost for patients on treatment is £912.33 and off treatment is £1591.43.
The source of the treatment acquisition costs was not stated (assumed to be BNF).
| Treatment | Dose 1 | Frequency | Unit cost (list price), £ |
|---|---|---|---|
| Adalimumab (40 mg) | 40 mg (assume wastage and all children receive 40-mg vial) | Every 2 weeks | 352.14 |
| Etanercept (10 mg) | 0.4 mg/kg (maximum 25 mg) | Twice a week | 35.75 |
| Etanercept (25 mg) | 89.38 | ||
| Methotrexate (10 mg, oral) | 10 mg | Every week | 0.56 |
| Tocilizumab (80 mg) | 10 mg/kg for patients < 30 kg 8 mg/kg for patients ≥ 30 kg |
Every 4 weeks | 102.40 |
| Tocilizumab (200 mg) | 256.00 | ||
| Tocilizumab (400 mg) | 512.00 |
Indirect costs (costs attributable to lost productivity, unpaid inputs to patient care)
Were indirect costs included:
Indirect costs are not included.
Health-state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion. Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
The company conducted a literature review that identified one study that reported utility values suitable for use in the model (Prince et al. 124) This study reported utility scores obtained using the HUI3 questionnaire to JIA patients starting treatment with etanercept in the Dutch ABC register.
Based on these data, the company used values at time 0 for the patients who are off treatment, and used values at time 1 year for patients on treatment.
List the utility values used in the evaluation
On treatment: 0.7275.
Off treatment: 0.53.
Dead: 0.
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported – list them if reported.
A de novo Markov state transition model with three health states (uncontrolled disease/off treatment, on treatment and dead) was developed. The model has 6-month cycles. Patients start with uncontrolled disease at cycle 0 then move to first-line treatment. Patients discontinue from treatment at a rate proportional to their response. Once all lines of treatment are exhausted, patients move into uncontrolled disease health state.
The model uses a 1% 6-month mortality rate.
Extract transition probabilities for (natural history/disease progression) model and show sources (or refer to table in text)
The 6-month discontinuation rate is 0.126 for no response, 0.09 for moderate response and 0.042 for good response.
What is the model time horizon?
25-year time frame. The company states that this reflects the chronic nature of the disease and allows for all relevant costs and benefits to be included in the analysis.
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
3.5% for costs and benefits.
If no economic evaluation was conducted, state the manufacturer’s reasons for this
Not applicable.
Results/analysis
What measure(s) of benefit were reported in the evaluation?
Cost per QALY gained.
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation
Combination therapy
| Adalimumab + MTX | Tocilizumab + MTX | |
|---|---|---|
| Total QALYs | 18.76 | 18.72 |
Monotherapy
| Adalimumab + MTX | Tocilizumab + MTX | |
|---|---|---|
| Total QALYs | 18.65 | 18.7 |
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
Combination therapy
| Adalimumab + MTX | Tocilizumab + MTX | |
|---|---|---|
| Total cost, £ | 81,827 | 70,707 |
Monotherapy
| Adalimumab + MTX | Tocilizumab + MTX | |
|---|---|---|
| Total cost, £ | 74,576 | 68,560 |
Synthesis of costs and benefits: are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results
| Combination therapy | Monotherapy | |
|---|---|---|
| Incremental QALYs | 0.03 | 0.0455 |
| Incremental cost, £ | 11,120 | 6015 |
| Incremental ICER, £ | 280,370 | Tocilizumab dominant |
Give results of any statistical analysis of the results of the evaluation
None reported.
Was any sensitivity analysis performed. If yes, what type(s) [i.e. deterministic (one-way, two-way, etc.) or probabilistic]
None reported.
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, quality of life or disease progression rates)?
An exploratory analysis has been performed for tocilizumab versus etanercept. The analysis assumes a class effect across TNFs in pJIA. The analysis found that tocilizumab was a cost-effective alternative to etanercept.
Give a summary of the results of the sensitivity analysis: did they differ substantially from the base-case analysis. If so, what were the suggested causes?
None reported.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
Adalimumab and tocilizumab have similar outcomes for patients with JIA, however tocilizumab is a less expensive alternative to adalimumab.
What are the implications of the evaluation for practice?
None.
Southampton Health Technology Assessments Centre commentary
Selection of comparators:
Results not presented for tocilizumab compared with methotrexate only.
Validity of estimate of measure of benefit:
Based on only utility estimates available for this population.
Validity of estimate of costs:
Based on relevant dataset of costs for patients on etanercept in the Netherlands. May be differences in costs between countries.
Critical appraisal checklist of economic evaluation (questions in this checklist based on Philips et al. 128 and Drummond et al. 129).
| Item | Roche | |
|---|---|---|
| 1 | Is there a clear statement of the decision problem? | Yes |
| 2 | Is the comparator routinely used in UK NHS? | Yes |
| 3 | Is the patient group in the study similar to those of interest in UK NHS? | Yes |
| 4 | Is the health-care system comparable to UK? | ?a |
| 5 | Is the setting comparable to the UK? | Yes |
| 6 | Is the perspective of the model clearly stated? | Yes |
| 7 | Is the study type appropriate? | Yes |
| 8 | Is the modelling methodology appropriate? | Yes |
| 9 | Is the model structure described and does it reflect the disease process? | Yes |
| 10 | Are assumptions about model structure listed and justified? | Yes |
| 11 | Are the data inputs for the model described and justified? | Yes |
| 12 | Is the effectiveness of the intervention established based on a systematic review? | Yes |
| 13 | Are health benefits measured in QALYs? | Yes |
| 14 | Are health benefits measured using a standardised and validated generic instrument? | Yes |
| 15 | Are the resource costs described and justified? | Yes |
| 16 | Have the costs and outcomes been discounted? | Yes |
| 17 | Has uncertainty been assessed? | Nob |
| 18 | Has the model been validated? | No |
Appendix 11 Parameters used in the independent model probabilistic sensitivity analysis
| Parameter | Mean | Higher CI | Lower CI | Standard error | Distribution |
|---|---|---|---|---|---|
| Utility values | |||||
| No treatment | 0.13 | 0.15 | 0.11 | 0.010 | Beta |
| Treatment 3 months | 0.17 | 0.20 | 0.15 | 0.013 | Beta |
| Treatment 15-month phase | 0.19 | 0.21 | 0.16 | 0.015 | Beta |
| Treatment long term 27+ months | 0.20 | 0.23 | 0.16 | 0.018 | Beta |
| Disease flare disutility | 0.03 | 0.04 | 0.02 | 0.006 | Beta |
| Disease flare | |||||
| Placebo | 0.25 | 0.34 | 0.16 | 0.046 | Beta |
| Abatacept | 0.09 | 0.16 | 0.05 | 0.021 | Beta |
| Adalimumab | 0.14 | 0.23 | 0.09 | 0.028 | Beta |
| Etanercept | 0.09 | 0.17 | 0.04 | 0.021 | Beta |
| Tocilizumab | 0.14 | 0.20 | 0.09 | 0.025 | Beta |
| AEs first cycle, % | |||||
| Abatacept | 0.53 | 1.51 | 0.00 | 0.005 | Beta |
| Adalimumab | 1.75 | 3.71 | 0.00 | 0.010 | Beta |
| Etanercept | 1.45 | 4.19 | 0.00 | 0.014 | Beta |
| Tocilizumab | 1.60 | 3.36 | 0.00 | 0.009 | Beta |
| Loss of efficacy, % | |||||
| Abatacept | 9.47 | 13.59 | 5.36 | 0.021 | Beta |
| Adalimumab | 3.51 | 6.25 | 0.76 | 0.014 | Beta |
| Etanercept | 2.90 | 6.82 | 0.00 | 0.020 | Beta |
| Tocilizumab | 7.98 | 11.90 | 4.06 | 0.020 | Beta |
| Further-line treatment, % | |||||
| AEs biologic DMARD | 0.43 | 0.82 | 0.04 | 0.002 | Beta |
| Loss of efficacy biologic DMARD | 2.00 | 2.59 | 1.41 | 0.003 | Beta |
| AEs MTX | 0.58 | 0.82 | 0.34 | 0.001 | Beta |
| Loss of efficacy MTX | 0.42 | 0.79 | 0.05 | 0.002 | Beta |
| Costs, £ | |||||
| On biologic DMARD cost | 724 | 724 | 941 | 507 | Gamma |
| Off biologic DMARD cost | 724 | 724 | 941 | 507 | Gamma |
| SAE cost | 1,533 | 1,533 | 1,993 | 1,073 | Gamma |
| Disease flare cost | 430 | 430 | 559 | 301 | Gamma |
List of abbreviations
- ABC
- Arthritis and Biologicals in Children
- ACR
- American College of Rheumatology
- ACR Pedi-20,-30,-50,-70,-90,-100
- American College of Rheumatology Pediatric response level 20, 30, 50, 70, 90, 100
- AE
- adverse event
- AiC
- academic in confidence
- AWAKEN
- Abatacept Withdrawal study to Assess efficacy and safety in Key Endpoints
- BMS
- Bristol-Myers Squibb
- BSPAR
- British Society for Paediatric and Adolescent Rheumatology
- CHAQ
- Childhood Health Assessment Questionnaire
- CHQ
- Child Health Questionnaire
- CI
- confidence interval
- CiC
- commercial in confidence
- CRP
- C-reactive protein
- CS
- company submission
- DMARD
- disease-modifying antirheumatic drug
- DNA
- deoxyribonucleic acid
- EMA
- European Medicines Agency
- EO
- extended oligoarthritis
- EQ-5D™
- European Quality of Life-5 Dimensions
- ERA
- enthesitis-related arthritis
- ESR
- erythrocyte sedimentation rate
- HAQ
- Health Assessment Questionnaire
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- HUI
- Health Utilities Index
- ICER
- incremental cost-effectiveness ratio
- IL-6
- interleukin-6
- ILAR
- International League of Associations for Rheumatology
- IQR
- interquartile range
- JADAS
- Juvenile Arthritis Disease Activity Score
- JIA
- juvenile idiopathic arthritis
- LOM
- limitation of motion
- MII
- medically important infection
- MTA
- multiple technology appraisal
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NSAID
- non-steroidal anti-inflammatory drug
- OLE
- open-label extension
- PA
- psoriatic arthritis
- PAS
- patient access scheme
- PGA
- physician global assessment of disease activity
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RF−ve
- rheumatoid factor negative
- RF+ve
- rheumatoid factor positive
- RR
- relative risk
- SAE
- serious adverse event
- SD
- standard deviation
- SDS
- standard deviation score
- SF-6D
- Short Form questionnaire-6 Dimensions
- SHTAC
- Southampton Health Technology Assessments Centre
- SPC
- summary of product characteristics
- TA
- technology appraisal
- TNF
- tumour necrosis factor
- VAS
- visual analogue scale
This monograph is based on the Technology Assessment Report produced for the National Institute for Health and Care Excellence (NICE). The full report contained a considerable number of data that were deemed academic-in-confidence and commercial-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of academic-in-confidence and commercial-in-confidence data removed and replaced by the statement ‘academic-in-confidence information (or data) removed’ or ‘commercial-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.